User login
Two-drug dolutegravir treatment noninferior to 3/4 drug regimen
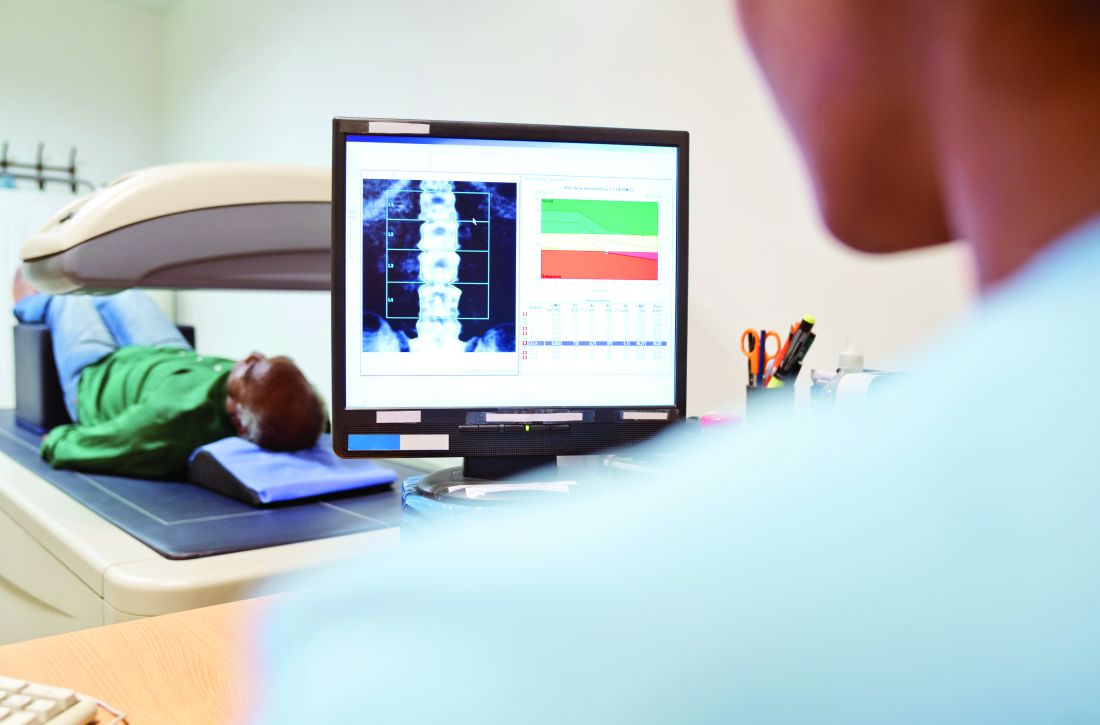
A two-drug fixed-dose tablet therapy of dolutegravir/lamivudine (Dovato, ViiV Healthcare; DTG/3TC) shows noninferiority in viral suppression among people with HIV-1 who switch from any type of three- or four-drug antiretroviral (ART) regimens. But, presented at the virtual meeting of the International AIDS Society.
The results on the switch to DTG/3TC are from the phase 3 SALSA trial, which compared patients with HIV-1 who either remained on any current three- or four-drug ART regimen or who switched to the two-drug dolutegravir option.
For the primary endpoint, rates of virologic failure at 48 weeks were noninferior in the DTG/3TC group versus the three- or four-drug regimen (.4% vs. 1.2; adjusted difference: –.8% [95% confidence interval, –2.4%, .8%]).
In addition, rates of virologic suppression at week 48 were noninferior, with 94.3% of patients achieving HIV-1 RNA < 50 c/mL in the DTG/3TC group versus 92.7% in the three- or four-drug regimen (adjusted difference: 1.6% [95% CI, –2.8%, 5.9%).
“These data build upon the previous TANGO study and support DTG/3TC as a robust switch option with high levels of efficacy, good safety and tolerability, and a high barrier of resistance,” first author Josep M. Llibre, MD, PhD, consultant, infectious diseases department, Germans Trias i Pujol University Hospital, Barcelona, said in presenting the findings.
The two-drug dolutegravir-based regimen had previously been shown in the phase 3 GEMINI-1 and GEMINI-2 trials to have virologic noninferiority and safety compared with three- or four-drug DTG plus tenofovir/emtricitabine (TDF/FTC) ART regimens in treatment-naive individuals, and, in the subsequent TANGO trial, the regimen was also noninferior versus tenofovir alafenamide–based regimens among treatment-experienced patients, at 144 weeks in both studies.
Trial details
The new SALSA trial, designed to broaden the comparison to treatment with any current three- or four-drug ART regimen, involved 493 patients at 120 study sites in 17 countries.
All patients were initially on a three- or four-drug regimen, with HIV-1 RNA of less than 50 c/mL for more than 6 months, and without prior virologic failure or nucleoside reverse transcriptase inhibitors or dolutegravir resistance-associated mutations.
The participants were randomized 1:1 to remain on their current regimen (n = 247) or to switch to the once-daily, fixed-dose tablet two-drug combination of dolutegravir 50 mg/lamivudine 300 mg (n = 246) for 52 weeks.
In addition to the noninferior virologic outcomes, there were no serious drug-related adverse events, no confirmed virologic withdrawals, and no resistance mutations in either group.
Of note, weight increase was higher in the DTG/3TC group (8%; n = 20) versus the current ART arm (2%; n = 5), as has been observed in previous studies. The adjusted mean change in weight from baseline to week 48 in the DTG arm was 2.1 kg versus 0.6 kg in the current ART arm.
Dr. Llibre pointed out that many of the participants who switched were discontinuing regimens such as TDF and efavirenz that are associated with weight loss, “so discontinuation could be more related to weight gain than the introduction of dolutegravir, but this deserves further study,” he noted.
There were no significant differences in changes in eGFR and fasting lipids, or in changes in inflammatory biomarkers between the groups.
Bone and renal biomarkers were more favorable in the dolutegravir two-drug arm, suggesting that bone and renal function was either maintained or even improved with the drug switch, Dr. Llibre noted.
Commenting on the research, Alexandra Calmy, PhD, of the HIV/AIDS Unit and LIPO & metabolism group, infectious disease division, Geneva University Hospitals, said that data on quality of life and patient satisfaction measures would be of particular interest.
“Indeed, it is not absolutely clear how a two-in-one versus a three-in-one pill may really influence treatment satisfaction and/or quality of life,” she said in an interview. “Validated scales and patient-reported outcomes with regards to treatment satisfaction would have been an added value to the study.”
Dr. Calmy coauthored a previous study looking at weight change and pharmacokinetic parameters in patients with HIV who switched to DTG-based regimens, and also found weight changes were increased with the DTG regimens. However, the weight gain was not associated with DTG plasma levels, thus supporting Dr. Llibre’s suggestion of possible withdrawal effects from other drugs.
STAT trial: Feasibility of two-drug DTG/3TC as first-line treatment
In further findings presented at the meeting on the STAT trial, researchers evaluated the feasibility not of switching to, but of initiating patients on, the two-drug DTG treatment as a first-line therapy, within 14 days of HIV-1 diagnosis.
The “test-and-treat” approach counters common belief that the regimen should be started only after the traditional three-drug regimens, because of the potential of transmitted resistance and baseline hepatitis B virus coinfection.
In the study of 131 patients, at week 48, 82% (107/131) of all participants and 97% (107/110) of those with available data achieved HIV-1 RNA levels of < 50 c/mL.
While two participants had confirmed virologic failure in the study, there were no treatment-emergent resistance-associated mutations, and neither patient discontinued the two-drug DTG treatment. There were low rates of drug-related adverse events (8%) and they were not serious.
“The STAT data is important as it shows us, for the first time, that in patients where potentially very little is known prior to treatment initiation, DTG/3TC can be very effectively used as a first-line regimen in a ‘test-and-treat’ approach without compromising on patient safety,” first author Charlotte-Paige Rolle, MD, director of research operations, Orlando (Fla.) Immunology Center, said in an interview.
Dr. Rolle added that “with careful monitoring of test results in the first weeks of therapy, we can appropriately and safely adjust therapy from DTG/3TC to a three-drug regimen if needed for patients that have transmitted drug resistance to DTG or 3TC, or hepatitis B coinfection, with both of these occurring at very low rates regardless.”
The SALSA and STAT studies received funding from ViiV Healthcare. Dr. Llibre has received honoraria or consultation fees from ViiV Healthcare, Gilead Sciences and Janssen-Cilag. Dr. Rolle has received grants from and served on advisory boards/speakers bureaus for ViiV Healthcare, Gilead, and Janssen. Dr. Calmy had no disclosures to report.
A two-drug fixed-dose tablet therapy of dolutegravir/lamivudine (Dovato, ViiV Healthcare; DTG/3TC) shows noninferiority in viral suppression among people with HIV-1 who switch from any type of three- or four-drug antiretroviral (ART) regimens. But, presented at the virtual meeting of the International AIDS Society.
The results on the switch to DTG/3TC are from the phase 3 SALSA trial, which compared patients with HIV-1 who either remained on any current three- or four-drug ART regimen or who switched to the two-drug dolutegravir option.
For the primary endpoint, rates of virologic failure at 48 weeks were noninferior in the DTG/3TC group versus the three- or four-drug regimen (.4% vs. 1.2; adjusted difference: –.8% [95% confidence interval, –2.4%, .8%]).
In addition, rates of virologic suppression at week 48 were noninferior, with 94.3% of patients achieving HIV-1 RNA < 50 c/mL in the DTG/3TC group versus 92.7% in the three- or four-drug regimen (adjusted difference: 1.6% [95% CI, –2.8%, 5.9%).
“These data build upon the previous TANGO study and support DTG/3TC as a robust switch option with high levels of efficacy, good safety and tolerability, and a high barrier of resistance,” first author Josep M. Llibre, MD, PhD, consultant, infectious diseases department, Germans Trias i Pujol University Hospital, Barcelona, said in presenting the findings.
The two-drug dolutegravir-based regimen had previously been shown in the phase 3 GEMINI-1 and GEMINI-2 trials to have virologic noninferiority and safety compared with three- or four-drug DTG plus tenofovir/emtricitabine (TDF/FTC) ART regimens in treatment-naive individuals, and, in the subsequent TANGO trial, the regimen was also noninferior versus tenofovir alafenamide–based regimens among treatment-experienced patients, at 144 weeks in both studies.
Trial details
The new SALSA trial, designed to broaden the comparison to treatment with any current three- or four-drug ART regimen, involved 493 patients at 120 study sites in 17 countries.
All patients were initially on a three- or four-drug regimen, with HIV-1 RNA of less than 50 c/mL for more than 6 months, and without prior virologic failure or nucleoside reverse transcriptase inhibitors or dolutegravir resistance-associated mutations.
The participants were randomized 1:1 to remain on their current regimen (n = 247) or to switch to the once-daily, fixed-dose tablet two-drug combination of dolutegravir 50 mg/lamivudine 300 mg (n = 246) for 52 weeks.
In addition to the noninferior virologic outcomes, there were no serious drug-related adverse events, no confirmed virologic withdrawals, and no resistance mutations in either group.
Of note, weight increase was higher in the DTG/3TC group (8%; n = 20) versus the current ART arm (2%; n = 5), as has been observed in previous studies. The adjusted mean change in weight from baseline to week 48 in the DTG arm was 2.1 kg versus 0.6 kg in the current ART arm.
Dr. Llibre pointed out that many of the participants who switched were discontinuing regimens such as TDF and efavirenz that are associated with weight loss, “so discontinuation could be more related to weight gain than the introduction of dolutegravir, but this deserves further study,” he noted.
There were no significant differences in changes in eGFR and fasting lipids, or in changes in inflammatory biomarkers between the groups.
Bone and renal biomarkers were more favorable in the dolutegravir two-drug arm, suggesting that bone and renal function was either maintained or even improved with the drug switch, Dr. Llibre noted.
Commenting on the research, Alexandra Calmy, PhD, of the HIV/AIDS Unit and LIPO & metabolism group, infectious disease division, Geneva University Hospitals, said that data on quality of life and patient satisfaction measures would be of particular interest.
“Indeed, it is not absolutely clear how a two-in-one versus a three-in-one pill may really influence treatment satisfaction and/or quality of life,” she said in an interview. “Validated scales and patient-reported outcomes with regards to treatment satisfaction would have been an added value to the study.”
Dr. Calmy coauthored a previous study looking at weight change and pharmacokinetic parameters in patients with HIV who switched to DTG-based regimens, and also found weight changes were increased with the DTG regimens. However, the weight gain was not associated with DTG plasma levels, thus supporting Dr. Llibre’s suggestion of possible withdrawal effects from other drugs.
STAT trial: Feasibility of two-drug DTG/3TC as first-line treatment
In further findings presented at the meeting on the STAT trial, researchers evaluated the feasibility not of switching to, but of initiating patients on, the two-drug DTG treatment as a first-line therapy, within 14 days of HIV-1 diagnosis.
The “test-and-treat” approach counters common belief that the regimen should be started only after the traditional three-drug regimens, because of the potential of transmitted resistance and baseline hepatitis B virus coinfection.
In the study of 131 patients, at week 48, 82% (107/131) of all participants and 97% (107/110) of those with available data achieved HIV-1 RNA levels of < 50 c/mL.
While two participants had confirmed virologic failure in the study, there were no treatment-emergent resistance-associated mutations, and neither patient discontinued the two-drug DTG treatment. There were low rates of drug-related adverse events (8%) and they were not serious.
“The STAT data is important as it shows us, for the first time, that in patients where potentially very little is known prior to treatment initiation, DTG/3TC can be very effectively used as a first-line regimen in a ‘test-and-treat’ approach without compromising on patient safety,” first author Charlotte-Paige Rolle, MD, director of research operations, Orlando (Fla.) Immunology Center, said in an interview.
Dr. Rolle added that “with careful monitoring of test results in the first weeks of therapy, we can appropriately and safely adjust therapy from DTG/3TC to a three-drug regimen if needed for patients that have transmitted drug resistance to DTG or 3TC, or hepatitis B coinfection, with both of these occurring at very low rates regardless.”
The SALSA and STAT studies received funding from ViiV Healthcare. Dr. Llibre has received honoraria or consultation fees from ViiV Healthcare, Gilead Sciences and Janssen-Cilag. Dr. Rolle has received grants from and served on advisory boards/speakers bureaus for ViiV Healthcare, Gilead, and Janssen. Dr. Calmy had no disclosures to report.
A two-drug fixed-dose tablet therapy of dolutegravir/lamivudine (Dovato, ViiV Healthcare; DTG/3TC) shows noninferiority in viral suppression among people with HIV-1 who switch from any type of three- or four-drug antiretroviral (ART) regimens. But, presented at the virtual meeting of the International AIDS Society.
The results on the switch to DTG/3TC are from the phase 3 SALSA trial, which compared patients with HIV-1 who either remained on any current three- or four-drug ART regimen or who switched to the two-drug dolutegravir option.
For the primary endpoint, rates of virologic failure at 48 weeks were noninferior in the DTG/3TC group versus the three- or four-drug regimen (.4% vs. 1.2; adjusted difference: –.8% [95% confidence interval, –2.4%, .8%]).
In addition, rates of virologic suppression at week 48 were noninferior, with 94.3% of patients achieving HIV-1 RNA < 50 c/mL in the DTG/3TC group versus 92.7% in the three- or four-drug regimen (adjusted difference: 1.6% [95% CI, –2.8%, 5.9%).
“These data build upon the previous TANGO study and support DTG/3TC as a robust switch option with high levels of efficacy, good safety and tolerability, and a high barrier of resistance,” first author Josep M. Llibre, MD, PhD, consultant, infectious diseases department, Germans Trias i Pujol University Hospital, Barcelona, said in presenting the findings.
The two-drug dolutegravir-based regimen had previously been shown in the phase 3 GEMINI-1 and GEMINI-2 trials to have virologic noninferiority and safety compared with three- or four-drug DTG plus tenofovir/emtricitabine (TDF/FTC) ART regimens in treatment-naive individuals, and, in the subsequent TANGO trial, the regimen was also noninferior versus tenofovir alafenamide–based regimens among treatment-experienced patients, at 144 weeks in both studies.
Trial details
The new SALSA trial, designed to broaden the comparison to treatment with any current three- or four-drug ART regimen, involved 493 patients at 120 study sites in 17 countries.
All patients were initially on a three- or four-drug regimen, with HIV-1 RNA of less than 50 c/mL for more than 6 months, and without prior virologic failure or nucleoside reverse transcriptase inhibitors or dolutegravir resistance-associated mutations.
The participants were randomized 1:1 to remain on their current regimen (n = 247) or to switch to the once-daily, fixed-dose tablet two-drug combination of dolutegravir 50 mg/lamivudine 300 mg (n = 246) for 52 weeks.
In addition to the noninferior virologic outcomes, there were no serious drug-related adverse events, no confirmed virologic withdrawals, and no resistance mutations in either group.
Of note, weight increase was higher in the DTG/3TC group (8%; n = 20) versus the current ART arm (2%; n = 5), as has been observed in previous studies. The adjusted mean change in weight from baseline to week 48 in the DTG arm was 2.1 kg versus 0.6 kg in the current ART arm.
Dr. Llibre pointed out that many of the participants who switched were discontinuing regimens such as TDF and efavirenz that are associated with weight loss, “so discontinuation could be more related to weight gain than the introduction of dolutegravir, but this deserves further study,” he noted.
There were no significant differences in changes in eGFR and fasting lipids, or in changes in inflammatory biomarkers between the groups.
Bone and renal biomarkers were more favorable in the dolutegravir two-drug arm, suggesting that bone and renal function was either maintained or even improved with the drug switch, Dr. Llibre noted.
Commenting on the research, Alexandra Calmy, PhD, of the HIV/AIDS Unit and LIPO & metabolism group, infectious disease division, Geneva University Hospitals, said that data on quality of life and patient satisfaction measures would be of particular interest.
“Indeed, it is not absolutely clear how a two-in-one versus a three-in-one pill may really influence treatment satisfaction and/or quality of life,” she said in an interview. “Validated scales and patient-reported outcomes with regards to treatment satisfaction would have been an added value to the study.”
Dr. Calmy coauthored a previous study looking at weight change and pharmacokinetic parameters in patients with HIV who switched to DTG-based regimens, and also found weight changes were increased with the DTG regimens. However, the weight gain was not associated with DTG plasma levels, thus supporting Dr. Llibre’s suggestion of possible withdrawal effects from other drugs.
STAT trial: Feasibility of two-drug DTG/3TC as first-line treatment
In further findings presented at the meeting on the STAT trial, researchers evaluated the feasibility not of switching to, but of initiating patients on, the two-drug DTG treatment as a first-line therapy, within 14 days of HIV-1 diagnosis.
The “test-and-treat” approach counters common belief that the regimen should be started only after the traditional three-drug regimens, because of the potential of transmitted resistance and baseline hepatitis B virus coinfection.
In the study of 131 patients, at week 48, 82% (107/131) of all participants and 97% (107/110) of those with available data achieved HIV-1 RNA levels of < 50 c/mL.
While two participants had confirmed virologic failure in the study, there were no treatment-emergent resistance-associated mutations, and neither patient discontinued the two-drug DTG treatment. There were low rates of drug-related adverse events (8%) and they were not serious.
“The STAT data is important as it shows us, for the first time, that in patients where potentially very little is known prior to treatment initiation, DTG/3TC can be very effectively used as a first-line regimen in a ‘test-and-treat’ approach without compromising on patient safety,” first author Charlotte-Paige Rolle, MD, director of research operations, Orlando (Fla.) Immunology Center, said in an interview.
Dr. Rolle added that “with careful monitoring of test results in the first weeks of therapy, we can appropriately and safely adjust therapy from DTG/3TC to a three-drug regimen if needed for patients that have transmitted drug resistance to DTG or 3TC, or hepatitis B coinfection, with both of these occurring at very low rates regardless.”
The SALSA and STAT studies received funding from ViiV Healthcare. Dr. Llibre has received honoraria or consultation fees from ViiV Healthcare, Gilead Sciences and Janssen-Cilag. Dr. Rolle has received grants from and served on advisory boards/speakers bureaus for ViiV Healthcare, Gilead, and Janssen. Dr. Calmy had no disclosures to report.
FROM IAS 2021
Vertebral fractures still a risk with low-dose oral glucocorticoids for RA
Patients with rheumatoid arthritis currently being treated with low doses of oral glucocorticoids (GCs) had a 59% increased risk of sustaining a vertebral fracture when compared with past users, results of a retrospective cohort study have shown.
Although the overall risk of an osteoporotic fracture was not increased when comparing current and past GC users, with a hazard ratio of 1.14 (95% confidence interval, 0.98-1.33), the HR for sustaining a spinal fracture was 1.59 (95% CI, 1.11-2.29).
“Clinicians should be aware that, even in RA patients who receive low daily glucocorticoid doses, the risk of clinical vertebral fracture is increased,” Shahab Abtahi, MD, of Maastricht (the Netherlands) University and coauthors reported in Rheumatology.
This is important considering around a quarter of RA patients are treated with GCs in the United Kingdom in accordance with European recommendations, they observed.
Conflicting randomized and observational findings on whether or not osteoporotic fractures might be linked to the use of low-dose GCs prompted Dr. Abtahi and associates to see if there were any signals in real-world data. To do so, they used data one of the world’s largest primary care databases – the Clinical Practice Research Datalink (CPRD), which consists of anonymized patient data from a network of primary care practices across the United Kingdom.
Altogether, the records of more than 15,000 patients with RA aged 50 years and older who were held in the CRPD between 1997 and 2017 were pulled for analysis, and just half (n = 7,039) were receiving or had received GC therapy. Low-dose GC therapy was defined as a prednisolone equivalent dose (PED) of 7.5 mg or less per day.
The use of low-dose GCs use during three key time periods was considered: within the past 6 months (current users), within the past 7-12 months (recent users), and within the past year (past users).
The analyses involved time-dependent Cox proportional-hazards models to look for associations between GC use and all types of osteoporotic fracture, including the risk for incident hip, vertebral, humeral, forearm, pelvis, and rib fractures. They were adjusted for various lifestyle parameters, comorbidities, and the use of other medications.
“Current GC use was further broken down into subcategories based on average daily and cumulative dose,” Dr. Abtahi observed. As might be expected, doses even lower than 7.5 mg or less PED did not increase the chance of any osteoporotic fracture but there was an increased risk for some types with higher average daily doses, notably at the hip and pelvis, as well as the spine.
“Low-dose oral GC therapy was associated with an increased risk of clinical vertebral fracture, while the risk of other individual OP fracture sites was not increased,” said the team, adding that the main results remained unchanged regardless of short- or long-term use.
“We know that vertebral fracture risk is markedly increased in RA, and it is well known that GC therapy in particular affects trabecular bone, which is abundantly present in lumbar vertebrae,” Dr. Abtahi wrote.
“Therefore, we can hypothesize that the beneficial effect of low-dose GC therapy on suppressing the background inflammation of RA could probably be enough to offset its negative effect on bone synthesis in most fracture sites but not in vertebrae,” they suggested.
One of the limitations of the study is that the researchers lacked data on the disease activity of the patients or if they were being treated with biologic therapy. This means that confounding by disease severity might be an issue with only those with higher disease activity being treated with GCs and thus were at higher risk for fractures.
“Another limitation was a potential misclassification of exposure with oral GCs, as we had only prescribing information from CPRD, which is roughly two steps behind actual drug use by patients,” the researchers conceded. The average duration of GC use was estimated at 3.7 years, which is an indication of actual use.
A detection bias may also be involved with regard to vertebral fractures, with complaints of back pain maybe being discussed more often when prescribing GCs, leading to more referrals for possible fracture assessment.
Dr. Abtahi and a fellow coauthor disclosed receiving research and other funding from several pharmaceutical companies unrelated to this study. All other coauthors had no conflicts of interest.
Patients with rheumatoid arthritis currently being treated with low doses of oral glucocorticoids (GCs) had a 59% increased risk of sustaining a vertebral fracture when compared with past users, results of a retrospective cohort study have shown.
Although the overall risk of an osteoporotic fracture was not increased when comparing current and past GC users, with a hazard ratio of 1.14 (95% confidence interval, 0.98-1.33), the HR for sustaining a spinal fracture was 1.59 (95% CI, 1.11-2.29).
“Clinicians should be aware that, even in RA patients who receive low daily glucocorticoid doses, the risk of clinical vertebral fracture is increased,” Shahab Abtahi, MD, of Maastricht (the Netherlands) University and coauthors reported in Rheumatology.
This is important considering around a quarter of RA patients are treated with GCs in the United Kingdom in accordance with European recommendations, they observed.
Conflicting randomized and observational findings on whether or not osteoporotic fractures might be linked to the use of low-dose GCs prompted Dr. Abtahi and associates to see if there were any signals in real-world data. To do so, they used data one of the world’s largest primary care databases – the Clinical Practice Research Datalink (CPRD), which consists of anonymized patient data from a network of primary care practices across the United Kingdom.
Altogether, the records of more than 15,000 patients with RA aged 50 years and older who were held in the CRPD between 1997 and 2017 were pulled for analysis, and just half (n = 7,039) were receiving or had received GC therapy. Low-dose GC therapy was defined as a prednisolone equivalent dose (PED) of 7.5 mg or less per day.
The use of low-dose GCs use during three key time periods was considered: within the past 6 months (current users), within the past 7-12 months (recent users), and within the past year (past users).
The analyses involved time-dependent Cox proportional-hazards models to look for associations between GC use and all types of osteoporotic fracture, including the risk for incident hip, vertebral, humeral, forearm, pelvis, and rib fractures. They were adjusted for various lifestyle parameters, comorbidities, and the use of other medications.
“Current GC use was further broken down into subcategories based on average daily and cumulative dose,” Dr. Abtahi observed. As might be expected, doses even lower than 7.5 mg or less PED did not increase the chance of any osteoporotic fracture but there was an increased risk for some types with higher average daily doses, notably at the hip and pelvis, as well as the spine.
“Low-dose oral GC therapy was associated with an increased risk of clinical vertebral fracture, while the risk of other individual OP fracture sites was not increased,” said the team, adding that the main results remained unchanged regardless of short- or long-term use.
“We know that vertebral fracture risk is markedly increased in RA, and it is well known that GC therapy in particular affects trabecular bone, which is abundantly present in lumbar vertebrae,” Dr. Abtahi wrote.
“Therefore, we can hypothesize that the beneficial effect of low-dose GC therapy on suppressing the background inflammation of RA could probably be enough to offset its negative effect on bone synthesis in most fracture sites but not in vertebrae,” they suggested.
One of the limitations of the study is that the researchers lacked data on the disease activity of the patients or if they were being treated with biologic therapy. This means that confounding by disease severity might be an issue with only those with higher disease activity being treated with GCs and thus were at higher risk for fractures.
“Another limitation was a potential misclassification of exposure with oral GCs, as we had only prescribing information from CPRD, which is roughly two steps behind actual drug use by patients,” the researchers conceded. The average duration of GC use was estimated at 3.7 years, which is an indication of actual use.
A detection bias may also be involved with regard to vertebral fractures, with complaints of back pain maybe being discussed more often when prescribing GCs, leading to more referrals for possible fracture assessment.
Dr. Abtahi and a fellow coauthor disclosed receiving research and other funding from several pharmaceutical companies unrelated to this study. All other coauthors had no conflicts of interest.
Patients with rheumatoid arthritis currently being treated with low doses of oral glucocorticoids (GCs) had a 59% increased risk of sustaining a vertebral fracture when compared with past users, results of a retrospective cohort study have shown.
Although the overall risk of an osteoporotic fracture was not increased when comparing current and past GC users, with a hazard ratio of 1.14 (95% confidence interval, 0.98-1.33), the HR for sustaining a spinal fracture was 1.59 (95% CI, 1.11-2.29).
“Clinicians should be aware that, even in RA patients who receive low daily glucocorticoid doses, the risk of clinical vertebral fracture is increased,” Shahab Abtahi, MD, of Maastricht (the Netherlands) University and coauthors reported in Rheumatology.
This is important considering around a quarter of RA patients are treated with GCs in the United Kingdom in accordance with European recommendations, they observed.
Conflicting randomized and observational findings on whether or not osteoporotic fractures might be linked to the use of low-dose GCs prompted Dr. Abtahi and associates to see if there were any signals in real-world data. To do so, they used data one of the world’s largest primary care databases – the Clinical Practice Research Datalink (CPRD), which consists of anonymized patient data from a network of primary care practices across the United Kingdom.
Altogether, the records of more than 15,000 patients with RA aged 50 years and older who were held in the CRPD between 1997 and 2017 were pulled for analysis, and just half (n = 7,039) were receiving or had received GC therapy. Low-dose GC therapy was defined as a prednisolone equivalent dose (PED) of 7.5 mg or less per day.
The use of low-dose GCs use during three key time periods was considered: within the past 6 months (current users), within the past 7-12 months (recent users), and within the past year (past users).
The analyses involved time-dependent Cox proportional-hazards models to look for associations between GC use and all types of osteoporotic fracture, including the risk for incident hip, vertebral, humeral, forearm, pelvis, and rib fractures. They were adjusted for various lifestyle parameters, comorbidities, and the use of other medications.
“Current GC use was further broken down into subcategories based on average daily and cumulative dose,” Dr. Abtahi observed. As might be expected, doses even lower than 7.5 mg or less PED did not increase the chance of any osteoporotic fracture but there was an increased risk for some types with higher average daily doses, notably at the hip and pelvis, as well as the spine.
“Low-dose oral GC therapy was associated with an increased risk of clinical vertebral fracture, while the risk of other individual OP fracture sites was not increased,” said the team, adding that the main results remained unchanged regardless of short- or long-term use.
“We know that vertebral fracture risk is markedly increased in RA, and it is well known that GC therapy in particular affects trabecular bone, which is abundantly present in lumbar vertebrae,” Dr. Abtahi wrote.
“Therefore, we can hypothesize that the beneficial effect of low-dose GC therapy on suppressing the background inflammation of RA could probably be enough to offset its negative effect on bone synthesis in most fracture sites but not in vertebrae,” they suggested.
One of the limitations of the study is that the researchers lacked data on the disease activity of the patients or if they were being treated with biologic therapy. This means that confounding by disease severity might be an issue with only those with higher disease activity being treated with GCs and thus were at higher risk for fractures.
“Another limitation was a potential misclassification of exposure with oral GCs, as we had only prescribing information from CPRD, which is roughly two steps behind actual drug use by patients,” the researchers conceded. The average duration of GC use was estimated at 3.7 years, which is an indication of actual use.
A detection bias may also be involved with regard to vertebral fractures, with complaints of back pain maybe being discussed more often when prescribing GCs, leading to more referrals for possible fracture assessment.
Dr. Abtahi and a fellow coauthor disclosed receiving research and other funding from several pharmaceutical companies unrelated to this study. All other coauthors had no conflicts of interest.
FROM RHEUMATOLOGY
Resistant TB: Adjustments to BPaL regimen reduce AEs, not efficacy
Lower doses of linezolid in the BPaL drug regimen (bedaquiline, pretomanid, and linezolid) significantly reduce the adverse events associated with the treatment for patients with highly drug-resistant tuberculosis (TB) without compromising its high efficacy, new research shows.
“The ZeNix trial shows that reduced doses and/or shorter durations of linezolid appear to have high efficacy and improved safety,” said first author Francesca Conradie, MB, BCh, of the clinical HIV research unit, faculty of health sciences, University of Witwatersrand, Johannesburg, South Africa, in presenting the findings at the virtual meeting of the International AIDS Society conference.
As recently reported in the pivotal Nix-TB trial, the BPaL regimen yielded a 90% treatment success rate among people with highly drug-resistant forms of TB.
However, a 6-month regimen that included linezolid 1,200 mg resulted in toxic effects: 81% of patients in the study experienced peripheral neuropathy, and myelosuppression occurred in 48%. These effects often led to dose reductions or treatment interruption.
Adjustments in the dose of linezolid in the new ZeNix trial substantially reduced peripheral neuropathy to 13% and myelosuppression to 7%, with no significant reduction in the treatment response.
Importantly, the results were similar among patients with and those without HIV. This is of note because TB is the leading cause of death among patients with HIV.
“In the ZeNix trial, only 20% of patients were HIV infected, but in the [previous] Nix-TB trial, 30% were infected, so we have experience now in about 70 patients who were infected, and the outcomes were no different,” Dr. Conradie said in an interview.
Experts say the findings represent an important turn in the steep challenge of tackling highly resistant TB.
“In our opinion, these are exciting results that could change treatment guidelines for highly drug-resistant tuberculosis, with real benefits for the patients,” said Hendrik Streeck, MD, International AIDS Society cochair and director of the Institute of Virology and the Institute for HIV Research at the University Bonn (Germany), in a press conference.
Payam Nahid, MD, MPH, director of the Center for Tuberculosis at theUniversity of California, San Francisco, agreed.
“The results of this trial will impact global practices in treating drug-resistant TB as well as the design and conduct of future TB clinical trials,” Dr. Nahid said in an interview.
ZeNix trial
The phase 3 ZeNix trial included 181 patients with highly resistant TB in South Africa, Russia, Georgia, and Moldova. The mean age of the patients was 37 years; 67.4% were men, 63.5% were White, and 19.9% were HIV positive.
All patients were treated for 6 months with bedaquiline 200 mg daily for 8 weeks followed by 100 mg daily for 18 weeks, as well as pretomanid 200 mg daily.
The patients were randomly assigned to receive one of four daily doses of linezolid: 1,200 mg for 6 months (the original dose from the Nix-TB trial; n = 45) or 2 months (n = 46), or 600 mg for 6 or 2 months (45 patients each).
Percentages of patients with HIV were equal among the four groups, at about 20% each.
The primary outcomes – resolution of clinical disease and a negative culture status after 6 months – were observed across all linezolid dose groups. The success rate was 93% for those receiving 1,200 mg for 6 months, 89% for those receiving 1,200 mg for 2 months, 91% for those receiving 600 mg for 6 months, and 84% for those receiving 600 mg for 2 months.
With regard to the key adverse events of peripheral neuropathy and myelosuppression, manifested as anemia, the highest rates were among those who received linezolid 1,200 mg for 6 month, at 38% and 22%, respectively, compared with 24% and 17.4% among those who received 1,200 mg for 2 months, 24% and 2% among those who received 600 mg for 6 months, and 13% and 6.7% among those who received 600 mg for 2 months.
Four cases of optic neuropathy occurred among those who received 1,200 mg for 6 months; all cases resolved.
Patients who received 1,200 mg for 6 months required the highest number of linezolid dose modifications; 51% required changes that included reduction, interruption, or discontinuation, compared with 28% among those who received 1,200 mg for 2 months and 13% each in the other two groups.
On the basis of these results, “my personal opinion is that 600 mg at 6 months [of linezolid] is most likely the best strategy for the treatment of this highly resistant treatment population group,” Dr. Conradie told this news organization.
Findings represent ‘great news’ in addressing concerns
Dr. Nahid further commented that the results are highly encouraging in light of the ongoing concerns about the effects of linezolid in the BPaL regimen.
“This is great news,” he said. “The ZeNix trial addresses a key concern that providers and patients have had regarding the safety and tolerability of taking 6 months of linezolid at 1200 mg/d as part of the BPaL regimen.
“The findings that doses lower and durations shorter than the current 1,200 mg linezolid daily for 6 months will significantly expand the usability of the BPaL regimen worldwide.”
The inclusion of patients with HIV was essential in the trial, he noted.
“There are drug-drug interactions to be considered, among other factors that impact drug exposure,” Dr. Nahid said.
“Inclusion of patients living with HIV in this study means that any modifications to the BPaL regimen considered by the WHO [World Health Organization] and other policy decision makers will include data from this key population,” he said. “Of course, more data are needed on safety, tolerability, and efficacy on BPaL in general, and there are international cohorts and demonstration projects underway that will enhance our understanding of the regimen in HIV and in other special populations.”
The authors, Dr. Streeck, and Dr. Nahid have disclosed no relevant financial relationships.
This article was updated 7/21/21.
Lower doses of linezolid in the BPaL drug regimen (bedaquiline, pretomanid, and linezolid) significantly reduce the adverse events associated with the treatment for patients with highly drug-resistant tuberculosis (TB) without compromising its high efficacy, new research shows.
“The ZeNix trial shows that reduced doses and/or shorter durations of linezolid appear to have high efficacy and improved safety,” said first author Francesca Conradie, MB, BCh, of the clinical HIV research unit, faculty of health sciences, University of Witwatersrand, Johannesburg, South Africa, in presenting the findings at the virtual meeting of the International AIDS Society conference.
As recently reported in the pivotal Nix-TB trial, the BPaL regimen yielded a 90% treatment success rate among people with highly drug-resistant forms of TB.
However, a 6-month regimen that included linezolid 1,200 mg resulted in toxic effects: 81% of patients in the study experienced peripheral neuropathy, and myelosuppression occurred in 48%. These effects often led to dose reductions or treatment interruption.
Adjustments in the dose of linezolid in the new ZeNix trial substantially reduced peripheral neuropathy to 13% and myelosuppression to 7%, with no significant reduction in the treatment response.
Importantly, the results were similar among patients with and those without HIV. This is of note because TB is the leading cause of death among patients with HIV.
“In the ZeNix trial, only 20% of patients were HIV infected, but in the [previous] Nix-TB trial, 30% were infected, so we have experience now in about 70 patients who were infected, and the outcomes were no different,” Dr. Conradie said in an interview.
Experts say the findings represent an important turn in the steep challenge of tackling highly resistant TB.
“In our opinion, these are exciting results that could change treatment guidelines for highly drug-resistant tuberculosis, with real benefits for the patients,” said Hendrik Streeck, MD, International AIDS Society cochair and director of the Institute of Virology and the Institute for HIV Research at the University Bonn (Germany), in a press conference.
Payam Nahid, MD, MPH, director of the Center for Tuberculosis at theUniversity of California, San Francisco, agreed.
“The results of this trial will impact global practices in treating drug-resistant TB as well as the design and conduct of future TB clinical trials,” Dr. Nahid said in an interview.
ZeNix trial
The phase 3 ZeNix trial included 181 patients with highly resistant TB in South Africa, Russia, Georgia, and Moldova. The mean age of the patients was 37 years; 67.4% were men, 63.5% were White, and 19.9% were HIV positive.
All patients were treated for 6 months with bedaquiline 200 mg daily for 8 weeks followed by 100 mg daily for 18 weeks, as well as pretomanid 200 mg daily.
The patients were randomly assigned to receive one of four daily doses of linezolid: 1,200 mg for 6 months (the original dose from the Nix-TB trial; n = 45) or 2 months (n = 46), or 600 mg for 6 or 2 months (45 patients each).
Percentages of patients with HIV were equal among the four groups, at about 20% each.
The primary outcomes – resolution of clinical disease and a negative culture status after 6 months – were observed across all linezolid dose groups. The success rate was 93% for those receiving 1,200 mg for 6 months, 89% for those receiving 1,200 mg for 2 months, 91% for those receiving 600 mg for 6 months, and 84% for those receiving 600 mg for 2 months.
With regard to the key adverse events of peripheral neuropathy and myelosuppression, manifested as anemia, the highest rates were among those who received linezolid 1,200 mg for 6 month, at 38% and 22%, respectively, compared with 24% and 17.4% among those who received 1,200 mg for 2 months, 24% and 2% among those who received 600 mg for 6 months, and 13% and 6.7% among those who received 600 mg for 2 months.
Four cases of optic neuropathy occurred among those who received 1,200 mg for 6 months; all cases resolved.
Patients who received 1,200 mg for 6 months required the highest number of linezolid dose modifications; 51% required changes that included reduction, interruption, or discontinuation, compared with 28% among those who received 1,200 mg for 2 months and 13% each in the other two groups.
On the basis of these results, “my personal opinion is that 600 mg at 6 months [of linezolid] is most likely the best strategy for the treatment of this highly resistant treatment population group,” Dr. Conradie told this news organization.
Findings represent ‘great news’ in addressing concerns
Dr. Nahid further commented that the results are highly encouraging in light of the ongoing concerns about the effects of linezolid in the BPaL regimen.
“This is great news,” he said. “The ZeNix trial addresses a key concern that providers and patients have had regarding the safety and tolerability of taking 6 months of linezolid at 1200 mg/d as part of the BPaL regimen.
“The findings that doses lower and durations shorter than the current 1,200 mg linezolid daily for 6 months will significantly expand the usability of the BPaL regimen worldwide.”
The inclusion of patients with HIV was essential in the trial, he noted.
“There are drug-drug interactions to be considered, among other factors that impact drug exposure,” Dr. Nahid said.
“Inclusion of patients living with HIV in this study means that any modifications to the BPaL regimen considered by the WHO [World Health Organization] and other policy decision makers will include data from this key population,” he said. “Of course, more data are needed on safety, tolerability, and efficacy on BPaL in general, and there are international cohorts and demonstration projects underway that will enhance our understanding of the regimen in HIV and in other special populations.”
The authors, Dr. Streeck, and Dr. Nahid have disclosed no relevant financial relationships.
This article was updated 7/21/21.
Lower doses of linezolid in the BPaL drug regimen (bedaquiline, pretomanid, and linezolid) significantly reduce the adverse events associated with the treatment for patients with highly drug-resistant tuberculosis (TB) without compromising its high efficacy, new research shows.
“The ZeNix trial shows that reduced doses and/or shorter durations of linezolid appear to have high efficacy and improved safety,” said first author Francesca Conradie, MB, BCh, of the clinical HIV research unit, faculty of health sciences, University of Witwatersrand, Johannesburg, South Africa, in presenting the findings at the virtual meeting of the International AIDS Society conference.
As recently reported in the pivotal Nix-TB trial, the BPaL regimen yielded a 90% treatment success rate among people with highly drug-resistant forms of TB.
However, a 6-month regimen that included linezolid 1,200 mg resulted in toxic effects: 81% of patients in the study experienced peripheral neuropathy, and myelosuppression occurred in 48%. These effects often led to dose reductions or treatment interruption.
Adjustments in the dose of linezolid in the new ZeNix trial substantially reduced peripheral neuropathy to 13% and myelosuppression to 7%, with no significant reduction in the treatment response.
Importantly, the results were similar among patients with and those without HIV. This is of note because TB is the leading cause of death among patients with HIV.
“In the ZeNix trial, only 20% of patients were HIV infected, but in the [previous] Nix-TB trial, 30% were infected, so we have experience now in about 70 patients who were infected, and the outcomes were no different,” Dr. Conradie said in an interview.
Experts say the findings represent an important turn in the steep challenge of tackling highly resistant TB.
“In our opinion, these are exciting results that could change treatment guidelines for highly drug-resistant tuberculosis, with real benefits for the patients,” said Hendrik Streeck, MD, International AIDS Society cochair and director of the Institute of Virology and the Institute for HIV Research at the University Bonn (Germany), in a press conference.
Payam Nahid, MD, MPH, director of the Center for Tuberculosis at theUniversity of California, San Francisco, agreed.
“The results of this trial will impact global practices in treating drug-resistant TB as well as the design and conduct of future TB clinical trials,” Dr. Nahid said in an interview.
ZeNix trial
The phase 3 ZeNix trial included 181 patients with highly resistant TB in South Africa, Russia, Georgia, and Moldova. The mean age of the patients was 37 years; 67.4% were men, 63.5% were White, and 19.9% were HIV positive.
All patients were treated for 6 months with bedaquiline 200 mg daily for 8 weeks followed by 100 mg daily for 18 weeks, as well as pretomanid 200 mg daily.
The patients were randomly assigned to receive one of four daily doses of linezolid: 1,200 mg for 6 months (the original dose from the Nix-TB trial; n = 45) or 2 months (n = 46), or 600 mg for 6 or 2 months (45 patients each).
Percentages of patients with HIV were equal among the four groups, at about 20% each.
The primary outcomes – resolution of clinical disease and a negative culture status after 6 months – were observed across all linezolid dose groups. The success rate was 93% for those receiving 1,200 mg for 6 months, 89% for those receiving 1,200 mg for 2 months, 91% for those receiving 600 mg for 6 months, and 84% for those receiving 600 mg for 2 months.
With regard to the key adverse events of peripheral neuropathy and myelosuppression, manifested as anemia, the highest rates were among those who received linezolid 1,200 mg for 6 month, at 38% and 22%, respectively, compared with 24% and 17.4% among those who received 1,200 mg for 2 months, 24% and 2% among those who received 600 mg for 6 months, and 13% and 6.7% among those who received 600 mg for 2 months.
Four cases of optic neuropathy occurred among those who received 1,200 mg for 6 months; all cases resolved.
Patients who received 1,200 mg for 6 months required the highest number of linezolid dose modifications; 51% required changes that included reduction, interruption, or discontinuation, compared with 28% among those who received 1,200 mg for 2 months and 13% each in the other two groups.
On the basis of these results, “my personal opinion is that 600 mg at 6 months [of linezolid] is most likely the best strategy for the treatment of this highly resistant treatment population group,” Dr. Conradie told this news organization.
Findings represent ‘great news’ in addressing concerns
Dr. Nahid further commented that the results are highly encouraging in light of the ongoing concerns about the effects of linezolid in the BPaL regimen.
“This is great news,” he said. “The ZeNix trial addresses a key concern that providers and patients have had regarding the safety and tolerability of taking 6 months of linezolid at 1200 mg/d as part of the BPaL regimen.
“The findings that doses lower and durations shorter than the current 1,200 mg linezolid daily for 6 months will significantly expand the usability of the BPaL regimen worldwide.”
The inclusion of patients with HIV was essential in the trial, he noted.
“There are drug-drug interactions to be considered, among other factors that impact drug exposure,” Dr. Nahid said.
“Inclusion of patients living with HIV in this study means that any modifications to the BPaL regimen considered by the WHO [World Health Organization] and other policy decision makers will include data from this key population,” he said. “Of course, more data are needed on safety, tolerability, and efficacy on BPaL in general, and there are international cohorts and demonstration projects underway that will enhance our understanding of the regimen in HIV and in other special populations.”
The authors, Dr. Streeck, and Dr. Nahid have disclosed no relevant financial relationships.
This article was updated 7/21/21.
FROM IAS 2021
Large remdesivir study finds no COVID-19 survival benefit
A lack of consensus in the evidence regarding the antiviral remdesivir (Veklury) to treat people with COVID-19 continues, leaving clinicians without clear direction on one of the few treatments for the illness approved under U.S. Food and Drug Administration emergency use authorization.
The latest research comes from Michael Ohl, MD, MSPH, and colleagues, who studied a large group of VA patients hospitalized with COVID-19. Compared with a matched group of veterans who did not receive the antiviral, remdesivir did not significantly improve survival.
The percentages were close: 12.2% of patients in the remdesivir group died within 30 days compared with 10.6% of those in the control group.
At the same time, the retrospective cohort study showed remdesivir was associated with more days in the hospital.
“There is still uncertainty about the role of remdesivir in treatment for people hospitalized with COVID-19,” Dr. Ohl told this news organization.
“It is reasonable to follow the CDC and Infectious Diseases Society of America guidelines for remdesivir use, “but clinicians should avoid admitting people or keeping people in the hospital solely to receive remdesivir if they do not meet other criteria for hospitalization,” said Dr. Ohl, lead author and an infectious disease specialist at the Center for Access & Delivery Research and Evaluation, Iowa City Veterans Affairs (VA) Health Care System.
The study was published online July 15 in JAMA Network Open.
Sticking with the official protocol?
The longer a hospital stays associated with remdesivir, a median 6 days versus 3 days, could be a result of treating people for 5 or 10 days with the antiviral agent. In other words, it is “possible that clinicians were not discharging patients who otherwise met the criteria for hospital discharge until the remdesivir course was completed,” Dr. Ohl and colleagues note.
Not doing so, they add, could have resulted in “increased used of scarce hospital beds during the pandemic.”
“The recommended remdesivir treatment course is a somewhat arbitrary 5 or 10 days depending on illness severity, and remdesivir is currently available only as an intravenous formulation for use in health care settings,” they add.
This is the “most likely explanation,” notes Gio J. Baracco, MD, in an invited commentary accompanying the study.
At the time of the study, use of remdesivir also required patient consent, close adverse event monitoring, and ongoing testing, Dr. Baracco notes.
He added that an option to discharge patients earlier if they responded to treatment might have been lost in translation from clinical trial protocol to real-world use in the VA system.
While a large clinical trial protocol called for the remdesivir infusions to be stopped early if the patient met the primary outcome and was ready to be discharged, “this detail was not adequately translated to the clinicians treating these patients,” added Dr. Baracco, who’s with the Division of Infectious Diseases at the University of Miami Miller School of Medicine and the Miami VA Healthcare System.
Conflicting evidence
Another large study, the World Health Organization Solidarity Trial, found remdesivir was not associated with shorter hospital stays or improved survival compared with standard of care. For this reason, the WHO recommends against use of remdesivir.
In contrast, the double-blind, randomized Adaptive COVID-19 Treatment Trial (ACTT-1) linked remdesivir treatment to shorter stays in the hospital, a median 10 days versus 15 days in a placebo group.
The FDA included the 2020 ACTT-1 in its consideration for remdesivir emergency use authorization. The FDA issued the EUA in May 2020, followed by full approval as the first treatment indicated for COVID-19 in October.
ACTT-1 lead author John H. Beigel, MD, and colleagues also looked at the death rates for remdesivir versus placebo.
By day 15, the proportion of people who died was 6.7% in the remdesivir group versus 11%. By day 29, the rate was 11.4% among those who received the antiviral versus 15.2% among those who did not.
When asked why the VA and ACTT-1 studies yielded different results, Dr. Beigel cited two reasons. The timing was different, with the VA study starting after the remdesivir EUA was issued, and ACTT-1 findings were announced.
“So at that point, clinicians understood those populations most likely to benefit from remdesivir. The use of remdesivir likely did not occur at random; it was likely to be more commonly used in those who were sicker or at higher risk for poor outcomes,” said Dr. Beigel, associate director for clinical research in the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases (NIAID).
In addition, the studies evaluated very different populations, he said. The differences in median duration of hospitalization between the trials reflects this, Dr. Beigel added.
Furthermore, when asked if he thinks the new evidence should affect clinical use of remdesivir, Dr. Beigel replied, “No. Observational studies, even with adjustments such as propensity score matching, are not equivalent levels of proof compared to randomized trials.”
Study details
Dr. Ohl and colleagues identified patients admitted to one of 123 VA hospitals for the first time for COVID-19 from May 1 to Oct. 8, 2020. Each had a PCR-confirmed SARS-CoV-2 infection. The researchers then compared 1,172 patients receiving remdesivir to another 1,172 patients not receiving the agent.
Those receiving remdesivir were more likely to be older, White, have chronic obstructive pulmonary disease and have more severe COVID-19. A total 94% of the remdesivir group were men.
“Over 90% of the people included in VA study were men, mostly over the age of 60,” Dr. Ohl said when asked how generalizable the findings would be to a non-VA population.
“There is no obvious biological reason that remdesivir should have different effects in men and women, but we should be cautious about extrapolating study findings to women and younger individuals,” he added.
Limitations of the study include its observational design, which makes unadjusted confounding based on illness severity a possibility. In addition, the investigators were unable to identify specific subgroups that might benefit from remdesivir treatment.
The data did suggest that remdesivir was more effective earlier in the course of disease when patients required supplemental oxygen and before need for mechanical ventilation.
Dr. Baracco pointed out the contradictory findings in his commentary: “The real-life application of a drug promising to hasten discharge from the hospital as its primary beneficial outcome must include an assessment of how easy it is to do so and make it clear that once a patient reaches that point, they can discontinue the drug.”
“The paradoxical findings in the study by Dr. Ohl et al. compared with the study used for its authorization illustrate this point very clearly,” he adds.
Dr. Ohl reported receiving grants from Veterans Affairs Health Services Research and Development during the conduct of the study and consulting for Gilead Pharmaceuticals outside the submitted work. Dr. Baracco reported receiving salary support from the U.S. Department of Veterans Affairs. Dr. Beigel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A lack of consensus in the evidence regarding the antiviral remdesivir (Veklury) to treat people with COVID-19 continues, leaving clinicians without clear direction on one of the few treatments for the illness approved under U.S. Food and Drug Administration emergency use authorization.
The latest research comes from Michael Ohl, MD, MSPH, and colleagues, who studied a large group of VA patients hospitalized with COVID-19. Compared with a matched group of veterans who did not receive the antiviral, remdesivir did not significantly improve survival.
The percentages were close: 12.2% of patients in the remdesivir group died within 30 days compared with 10.6% of those in the control group.
At the same time, the retrospective cohort study showed remdesivir was associated with more days in the hospital.
“There is still uncertainty about the role of remdesivir in treatment for people hospitalized with COVID-19,” Dr. Ohl told this news organization.
“It is reasonable to follow the CDC and Infectious Diseases Society of America guidelines for remdesivir use, “but clinicians should avoid admitting people or keeping people in the hospital solely to receive remdesivir if they do not meet other criteria for hospitalization,” said Dr. Ohl, lead author and an infectious disease specialist at the Center for Access & Delivery Research and Evaluation, Iowa City Veterans Affairs (VA) Health Care System.
The study was published online July 15 in JAMA Network Open.
Sticking with the official protocol?
The longer a hospital stays associated with remdesivir, a median 6 days versus 3 days, could be a result of treating people for 5 or 10 days with the antiviral agent. In other words, it is “possible that clinicians were not discharging patients who otherwise met the criteria for hospital discharge until the remdesivir course was completed,” Dr. Ohl and colleagues note.
Not doing so, they add, could have resulted in “increased used of scarce hospital beds during the pandemic.”
“The recommended remdesivir treatment course is a somewhat arbitrary 5 or 10 days depending on illness severity, and remdesivir is currently available only as an intravenous formulation for use in health care settings,” they add.
This is the “most likely explanation,” notes Gio J. Baracco, MD, in an invited commentary accompanying the study.
At the time of the study, use of remdesivir also required patient consent, close adverse event monitoring, and ongoing testing, Dr. Baracco notes.
He added that an option to discharge patients earlier if they responded to treatment might have been lost in translation from clinical trial protocol to real-world use in the VA system.
While a large clinical trial protocol called for the remdesivir infusions to be stopped early if the patient met the primary outcome and was ready to be discharged, “this detail was not adequately translated to the clinicians treating these patients,” added Dr. Baracco, who’s with the Division of Infectious Diseases at the University of Miami Miller School of Medicine and the Miami VA Healthcare System.
Conflicting evidence
Another large study, the World Health Organization Solidarity Trial, found remdesivir was not associated with shorter hospital stays or improved survival compared with standard of care. For this reason, the WHO recommends against use of remdesivir.
In contrast, the double-blind, randomized Adaptive COVID-19 Treatment Trial (ACTT-1) linked remdesivir treatment to shorter stays in the hospital, a median 10 days versus 15 days in a placebo group.
The FDA included the 2020 ACTT-1 in its consideration for remdesivir emergency use authorization. The FDA issued the EUA in May 2020, followed by full approval as the first treatment indicated for COVID-19 in October.
ACTT-1 lead author John H. Beigel, MD, and colleagues also looked at the death rates for remdesivir versus placebo.
By day 15, the proportion of people who died was 6.7% in the remdesivir group versus 11%. By day 29, the rate was 11.4% among those who received the antiviral versus 15.2% among those who did not.
When asked why the VA and ACTT-1 studies yielded different results, Dr. Beigel cited two reasons. The timing was different, with the VA study starting after the remdesivir EUA was issued, and ACTT-1 findings were announced.
“So at that point, clinicians understood those populations most likely to benefit from remdesivir. The use of remdesivir likely did not occur at random; it was likely to be more commonly used in those who were sicker or at higher risk for poor outcomes,” said Dr. Beigel, associate director for clinical research in the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases (NIAID).
In addition, the studies evaluated very different populations, he said. The differences in median duration of hospitalization between the trials reflects this, Dr. Beigel added.
Furthermore, when asked if he thinks the new evidence should affect clinical use of remdesivir, Dr. Beigel replied, “No. Observational studies, even with adjustments such as propensity score matching, are not equivalent levels of proof compared to randomized trials.”
Study details
Dr. Ohl and colleagues identified patients admitted to one of 123 VA hospitals for the first time for COVID-19 from May 1 to Oct. 8, 2020. Each had a PCR-confirmed SARS-CoV-2 infection. The researchers then compared 1,172 patients receiving remdesivir to another 1,172 patients not receiving the agent.
Those receiving remdesivir were more likely to be older, White, have chronic obstructive pulmonary disease and have more severe COVID-19. A total 94% of the remdesivir group were men.
“Over 90% of the people included in VA study were men, mostly over the age of 60,” Dr. Ohl said when asked how generalizable the findings would be to a non-VA population.
“There is no obvious biological reason that remdesivir should have different effects in men and women, but we should be cautious about extrapolating study findings to women and younger individuals,” he added.
Limitations of the study include its observational design, which makes unadjusted confounding based on illness severity a possibility. In addition, the investigators were unable to identify specific subgroups that might benefit from remdesivir treatment.
The data did suggest that remdesivir was more effective earlier in the course of disease when patients required supplemental oxygen and before need for mechanical ventilation.
Dr. Baracco pointed out the contradictory findings in his commentary: “The real-life application of a drug promising to hasten discharge from the hospital as its primary beneficial outcome must include an assessment of how easy it is to do so and make it clear that once a patient reaches that point, they can discontinue the drug.”
“The paradoxical findings in the study by Dr. Ohl et al. compared with the study used for its authorization illustrate this point very clearly,” he adds.
Dr. Ohl reported receiving grants from Veterans Affairs Health Services Research and Development during the conduct of the study and consulting for Gilead Pharmaceuticals outside the submitted work. Dr. Baracco reported receiving salary support from the U.S. Department of Veterans Affairs. Dr. Beigel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A lack of consensus in the evidence regarding the antiviral remdesivir (Veklury) to treat people with COVID-19 continues, leaving clinicians without clear direction on one of the few treatments for the illness approved under U.S. Food and Drug Administration emergency use authorization.
The latest research comes from Michael Ohl, MD, MSPH, and colleagues, who studied a large group of VA patients hospitalized with COVID-19. Compared with a matched group of veterans who did not receive the antiviral, remdesivir did not significantly improve survival.
The percentages were close: 12.2% of patients in the remdesivir group died within 30 days compared with 10.6% of those in the control group.
At the same time, the retrospective cohort study showed remdesivir was associated with more days in the hospital.
“There is still uncertainty about the role of remdesivir in treatment for people hospitalized with COVID-19,” Dr. Ohl told this news organization.
“It is reasonable to follow the CDC and Infectious Diseases Society of America guidelines for remdesivir use, “but clinicians should avoid admitting people or keeping people in the hospital solely to receive remdesivir if they do not meet other criteria for hospitalization,” said Dr. Ohl, lead author and an infectious disease specialist at the Center for Access & Delivery Research and Evaluation, Iowa City Veterans Affairs (VA) Health Care System.
The study was published online July 15 in JAMA Network Open.
Sticking with the official protocol?
The longer a hospital stays associated with remdesivir, a median 6 days versus 3 days, could be a result of treating people for 5 or 10 days with the antiviral agent. In other words, it is “possible that clinicians were not discharging patients who otherwise met the criteria for hospital discharge until the remdesivir course was completed,” Dr. Ohl and colleagues note.
Not doing so, they add, could have resulted in “increased used of scarce hospital beds during the pandemic.”
“The recommended remdesivir treatment course is a somewhat arbitrary 5 or 10 days depending on illness severity, and remdesivir is currently available only as an intravenous formulation for use in health care settings,” they add.
This is the “most likely explanation,” notes Gio J. Baracco, MD, in an invited commentary accompanying the study.
At the time of the study, use of remdesivir also required patient consent, close adverse event monitoring, and ongoing testing, Dr. Baracco notes.
He added that an option to discharge patients earlier if they responded to treatment might have been lost in translation from clinical trial protocol to real-world use in the VA system.
While a large clinical trial protocol called for the remdesivir infusions to be stopped early if the patient met the primary outcome and was ready to be discharged, “this detail was not adequately translated to the clinicians treating these patients,” added Dr. Baracco, who’s with the Division of Infectious Diseases at the University of Miami Miller School of Medicine and the Miami VA Healthcare System.
Conflicting evidence
Another large study, the World Health Organization Solidarity Trial, found remdesivir was not associated with shorter hospital stays or improved survival compared with standard of care. For this reason, the WHO recommends against use of remdesivir.
In contrast, the double-blind, randomized Adaptive COVID-19 Treatment Trial (ACTT-1) linked remdesivir treatment to shorter stays in the hospital, a median 10 days versus 15 days in a placebo group.
The FDA included the 2020 ACTT-1 in its consideration for remdesivir emergency use authorization. The FDA issued the EUA in May 2020, followed by full approval as the first treatment indicated for COVID-19 in October.
ACTT-1 lead author John H. Beigel, MD, and colleagues also looked at the death rates for remdesivir versus placebo.
By day 15, the proportion of people who died was 6.7% in the remdesivir group versus 11%. By day 29, the rate was 11.4% among those who received the antiviral versus 15.2% among those who did not.
When asked why the VA and ACTT-1 studies yielded different results, Dr. Beigel cited two reasons. The timing was different, with the VA study starting after the remdesivir EUA was issued, and ACTT-1 findings were announced.
“So at that point, clinicians understood those populations most likely to benefit from remdesivir. The use of remdesivir likely did not occur at random; it was likely to be more commonly used in those who were sicker or at higher risk for poor outcomes,” said Dr. Beigel, associate director for clinical research in the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases (NIAID).
In addition, the studies evaluated very different populations, he said. The differences in median duration of hospitalization between the trials reflects this, Dr. Beigel added.
Furthermore, when asked if he thinks the new evidence should affect clinical use of remdesivir, Dr. Beigel replied, “No. Observational studies, even with adjustments such as propensity score matching, are not equivalent levels of proof compared to randomized trials.”
Study details
Dr. Ohl and colleagues identified patients admitted to one of 123 VA hospitals for the first time for COVID-19 from May 1 to Oct. 8, 2020. Each had a PCR-confirmed SARS-CoV-2 infection. The researchers then compared 1,172 patients receiving remdesivir to another 1,172 patients not receiving the agent.
Those receiving remdesivir were more likely to be older, White, have chronic obstructive pulmonary disease and have more severe COVID-19. A total 94% of the remdesivir group were men.
“Over 90% of the people included in VA study were men, mostly over the age of 60,” Dr. Ohl said when asked how generalizable the findings would be to a non-VA population.
“There is no obvious biological reason that remdesivir should have different effects in men and women, but we should be cautious about extrapolating study findings to women and younger individuals,” he added.
Limitations of the study include its observational design, which makes unadjusted confounding based on illness severity a possibility. In addition, the investigators were unable to identify specific subgroups that might benefit from remdesivir treatment.
The data did suggest that remdesivir was more effective earlier in the course of disease when patients required supplemental oxygen and before need for mechanical ventilation.
Dr. Baracco pointed out the contradictory findings in his commentary: “The real-life application of a drug promising to hasten discharge from the hospital as its primary beneficial outcome must include an assessment of how easy it is to do so and make it clear that once a patient reaches that point, they can discontinue the drug.”
“The paradoxical findings in the study by Dr. Ohl et al. compared with the study used for its authorization illustrate this point very clearly,” he adds.
Dr. Ohl reported receiving grants from Veterans Affairs Health Services Research and Development during the conduct of the study and consulting for Gilead Pharmaceuticals outside the submitted work. Dr. Baracco reported receiving salary support from the U.S. Department of Veterans Affairs. Dr. Beigel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Metformin use may curb BCC risk
in Iceland.
“In addition to general anticarcinogenic effects, metformin has also been shown to directly inhibit the sonic hedgehog pathway, a key pathway in basal cell carcinoma (BCC) pathogenesis,” Jonas A. Adalsteinsson, MD, of the University of Iceland, Reykjavik, and colleagues wrote. “The relationship between metformin and keratinocyte carcinoma has not been well-characterized but is of importance considering that metformin is a commonly prescribed medication.”
They added that the hedgehog pathway inhibitors vismodegib (Erivedge) and sonidegib (Odomzo), approved for treating BCC, “are highly effective for BCC prevention, but their broad use for BCC prophylaxis is limited due to numerous side effects.”
In the study, published in the Journal of the American Academy of Dermatology, the researchers identified 6,880 first-time cancer patients with BCC, squamous cell carcinoma in situ (SCCis), or invasive SCC, and 69,620 population controls using data from the Icelandic Cancer Registry and the Icelandic Prescription Medicine Register between 2003 and 2017. Metformin exposure was defined as having filled at least one prescription of metformin more than 2 years prior to cancer diagnosis. They used grams and daily dose units of metformin in their analysis; one DDU of metformin, “or its average daily maintenance dose when used for its primary indication, is 2 grams,” they noted.
Overall, metformin use was associated with a significantly lower risk of developing BCC, compared with nonuse (adjusted odds ratio, 0.71; 95% confidence interval, 0.61-0.83).
The reduced risk occurred similarly across age and gender subgroups, with the exception of individuals younger than 60 years, the researchers said. “This might signify that metformin has less of a protective effect in younger individuals, but we might also have lacked power in this category.” The association with reduced BCC risk remained significant at all three cumulative dose levels measured: 1-500 DDUs, 501-1,500 DDUs, and more than 1,500 DDUs.
Metformin use was not significantly associated with reduced risk of invasive SCC (aOR, 1.01) and in most cases of SCCis. However, the 501-1,500 DDU dose category was associated with a slight increase in risk of SCCis (aOR, 1.40; 95% CI, 1.00-1.96), “showing a possible increased risk of SCCis,” the authors wrote.
The decrease in BCC risk was seen across all metformin dosing levels, but the reason for this remains unclear, and might be related to a confounding factor that was not considered in this study, the researchers said. “It could also be that metformin’s BCC risk-lowering effect is immediate, with only a low dose being needed to see a clinical benefit.”
The study findings were limited by several factors, including the retrospective design and the inability to adjust for factors including ultraviolet exposure, Fitzpatrick skin type, and comorbidities. The frequent use of metformin by people with type 2 diabetes suggests diabetes itself or other diabetes medications could be possible confounding factors, the researchers wrote.
However, the results were strengthened by the large study population, and the data suggest an association between reduced risk of first-time BCC and metformin use, they added.
“Randomized, prospective trials are required to fully understand the effect metformin has on BCC and SCC risk,” the researchers concluded.
“There is a dire need to reduce incidence of skin cancers in general, and consequently a need for new non-surgical treatment options for keratinocytic nonmelanoma skin cancers,” Amor Khachemoune, MD, a dermatologist at the State University of New York, Brooklyn, and the department of dermatology of the Veteran Affairs NY Harbor Healthcare System, also in Brooklyn, said in an interview.
Dr. Khachemoune, who was not involved with the study, said that he was not surprised by the findings. “Like other well-studied sonic hedgehog inhibitors, vismodegib and sonidegib, metformin has a demonstrated effect on this pathway. The medical community outside of dermatology has extensive experience with the use of metformin for a host of other indications, including its role as anticarcinogenic, so it seemed natural that one would consider widening its use to quell the ever-expanding cases of basal cell carcinomas.”
However, complications from long-term use, though likely rare, could be a limitation in using metformin as a chemoprotective agent, Dr. Khachemoune said. Metformin-associated lactic acidosis is one example of a rare, but potentially life-threatening adverse event.
“Finding the right dosage and having an algorithm for follow up monitoring of side effects would certainly need to be put in place in a standardized way,” he emphasized. “As stated by the authors of this study, more inclusive research involving other groups with nonkeratinocytic malignancies in larger cohorts is needed.”
The study received no outside funding. The researchers and Dr. Khachemoune had no financial conflicts to disclose.
in Iceland.
“In addition to general anticarcinogenic effects, metformin has also been shown to directly inhibit the sonic hedgehog pathway, a key pathway in basal cell carcinoma (BCC) pathogenesis,” Jonas A. Adalsteinsson, MD, of the University of Iceland, Reykjavik, and colleagues wrote. “The relationship between metformin and keratinocyte carcinoma has not been well-characterized but is of importance considering that metformin is a commonly prescribed medication.”
They added that the hedgehog pathway inhibitors vismodegib (Erivedge) and sonidegib (Odomzo), approved for treating BCC, “are highly effective for BCC prevention, but their broad use for BCC prophylaxis is limited due to numerous side effects.”
In the study, published in the Journal of the American Academy of Dermatology, the researchers identified 6,880 first-time cancer patients with BCC, squamous cell carcinoma in situ (SCCis), or invasive SCC, and 69,620 population controls using data from the Icelandic Cancer Registry and the Icelandic Prescription Medicine Register between 2003 and 2017. Metformin exposure was defined as having filled at least one prescription of metformin more than 2 years prior to cancer diagnosis. They used grams and daily dose units of metformin in their analysis; one DDU of metformin, “or its average daily maintenance dose when used for its primary indication, is 2 grams,” they noted.
Overall, metformin use was associated with a significantly lower risk of developing BCC, compared with nonuse (adjusted odds ratio, 0.71; 95% confidence interval, 0.61-0.83).
The reduced risk occurred similarly across age and gender subgroups, with the exception of individuals younger than 60 years, the researchers said. “This might signify that metformin has less of a protective effect in younger individuals, but we might also have lacked power in this category.” The association with reduced BCC risk remained significant at all three cumulative dose levels measured: 1-500 DDUs, 501-1,500 DDUs, and more than 1,500 DDUs.
Metformin use was not significantly associated with reduced risk of invasive SCC (aOR, 1.01) and in most cases of SCCis. However, the 501-1,500 DDU dose category was associated with a slight increase in risk of SCCis (aOR, 1.40; 95% CI, 1.00-1.96), “showing a possible increased risk of SCCis,” the authors wrote.
The decrease in BCC risk was seen across all metformin dosing levels, but the reason for this remains unclear, and might be related to a confounding factor that was not considered in this study, the researchers said. “It could also be that metformin’s BCC risk-lowering effect is immediate, with only a low dose being needed to see a clinical benefit.”
The study findings were limited by several factors, including the retrospective design and the inability to adjust for factors including ultraviolet exposure, Fitzpatrick skin type, and comorbidities. The frequent use of metformin by people with type 2 diabetes suggests diabetes itself or other diabetes medications could be possible confounding factors, the researchers wrote.
However, the results were strengthened by the large study population, and the data suggest an association between reduced risk of first-time BCC and metformin use, they added.
“Randomized, prospective trials are required to fully understand the effect metformin has on BCC and SCC risk,” the researchers concluded.
“There is a dire need to reduce incidence of skin cancers in general, and consequently a need for new non-surgical treatment options for keratinocytic nonmelanoma skin cancers,” Amor Khachemoune, MD, a dermatologist at the State University of New York, Brooklyn, and the department of dermatology of the Veteran Affairs NY Harbor Healthcare System, also in Brooklyn, said in an interview.
Dr. Khachemoune, who was not involved with the study, said that he was not surprised by the findings. “Like other well-studied sonic hedgehog inhibitors, vismodegib and sonidegib, metformin has a demonstrated effect on this pathway. The medical community outside of dermatology has extensive experience with the use of metformin for a host of other indications, including its role as anticarcinogenic, so it seemed natural that one would consider widening its use to quell the ever-expanding cases of basal cell carcinomas.”
However, complications from long-term use, though likely rare, could be a limitation in using metformin as a chemoprotective agent, Dr. Khachemoune said. Metformin-associated lactic acidosis is one example of a rare, but potentially life-threatening adverse event.
“Finding the right dosage and having an algorithm for follow up monitoring of side effects would certainly need to be put in place in a standardized way,” he emphasized. “As stated by the authors of this study, more inclusive research involving other groups with nonkeratinocytic malignancies in larger cohorts is needed.”
The study received no outside funding. The researchers and Dr. Khachemoune had no financial conflicts to disclose.
in Iceland.
“In addition to general anticarcinogenic effects, metformin has also been shown to directly inhibit the sonic hedgehog pathway, a key pathway in basal cell carcinoma (BCC) pathogenesis,” Jonas A. Adalsteinsson, MD, of the University of Iceland, Reykjavik, and colleagues wrote. “The relationship between metformin and keratinocyte carcinoma has not been well-characterized but is of importance considering that metformin is a commonly prescribed medication.”
They added that the hedgehog pathway inhibitors vismodegib (Erivedge) and sonidegib (Odomzo), approved for treating BCC, “are highly effective for BCC prevention, but their broad use for BCC prophylaxis is limited due to numerous side effects.”
In the study, published in the Journal of the American Academy of Dermatology, the researchers identified 6,880 first-time cancer patients with BCC, squamous cell carcinoma in situ (SCCis), or invasive SCC, and 69,620 population controls using data from the Icelandic Cancer Registry and the Icelandic Prescription Medicine Register between 2003 and 2017. Metformin exposure was defined as having filled at least one prescription of metformin more than 2 years prior to cancer diagnosis. They used grams and daily dose units of metformin in their analysis; one DDU of metformin, “or its average daily maintenance dose when used for its primary indication, is 2 grams,” they noted.
Overall, metformin use was associated with a significantly lower risk of developing BCC, compared with nonuse (adjusted odds ratio, 0.71; 95% confidence interval, 0.61-0.83).
The reduced risk occurred similarly across age and gender subgroups, with the exception of individuals younger than 60 years, the researchers said. “This might signify that metformin has less of a protective effect in younger individuals, but we might also have lacked power in this category.” The association with reduced BCC risk remained significant at all three cumulative dose levels measured: 1-500 DDUs, 501-1,500 DDUs, and more than 1,500 DDUs.
Metformin use was not significantly associated with reduced risk of invasive SCC (aOR, 1.01) and in most cases of SCCis. However, the 501-1,500 DDU dose category was associated with a slight increase in risk of SCCis (aOR, 1.40; 95% CI, 1.00-1.96), “showing a possible increased risk of SCCis,” the authors wrote.
The decrease in BCC risk was seen across all metformin dosing levels, but the reason for this remains unclear, and might be related to a confounding factor that was not considered in this study, the researchers said. “It could also be that metformin’s BCC risk-lowering effect is immediate, with only a low dose being needed to see a clinical benefit.”
The study findings were limited by several factors, including the retrospective design and the inability to adjust for factors including ultraviolet exposure, Fitzpatrick skin type, and comorbidities. The frequent use of metformin by people with type 2 diabetes suggests diabetes itself or other diabetes medications could be possible confounding factors, the researchers wrote.
However, the results were strengthened by the large study population, and the data suggest an association between reduced risk of first-time BCC and metformin use, they added.
“Randomized, prospective trials are required to fully understand the effect metformin has on BCC and SCC risk,” the researchers concluded.
“There is a dire need to reduce incidence of skin cancers in general, and consequently a need for new non-surgical treatment options for keratinocytic nonmelanoma skin cancers,” Amor Khachemoune, MD, a dermatologist at the State University of New York, Brooklyn, and the department of dermatology of the Veteran Affairs NY Harbor Healthcare System, also in Brooklyn, said in an interview.
Dr. Khachemoune, who was not involved with the study, said that he was not surprised by the findings. “Like other well-studied sonic hedgehog inhibitors, vismodegib and sonidegib, metformin has a demonstrated effect on this pathway. The medical community outside of dermatology has extensive experience with the use of metformin for a host of other indications, including its role as anticarcinogenic, so it seemed natural that one would consider widening its use to quell the ever-expanding cases of basal cell carcinomas.”
However, complications from long-term use, though likely rare, could be a limitation in using metformin as a chemoprotective agent, Dr. Khachemoune said. Metformin-associated lactic acidosis is one example of a rare, but potentially life-threatening adverse event.
“Finding the right dosage and having an algorithm for follow up monitoring of side effects would certainly need to be put in place in a standardized way,” he emphasized. “As stated by the authors of this study, more inclusive research involving other groups with nonkeratinocytic malignancies in larger cohorts is needed.”
The study received no outside funding. The researchers and Dr. Khachemoune had no financial conflicts to disclose.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Do patients with cancer need a third shot of COVID vaccine?
Patients with cancer have shown varying responses to COVID-19 vaccination, with good responses in patients with solid tumors (even while on systemic therapy) and poor responses in patients with blood cancers, particularly those on immunosuppressive therapies.
The data are evolving to show factors associated with a poor response but are not strong enough yet to recommend booster shots, say researchers.
The work is defining who will likely need a COVID vaccine booster when they become available. “It’s definitely not all cancer patients,” said Dimpy Shah, MD, PhD, a cancer epidemiologist at the Mays Cancer Center, University of Texas, San Antonio.
Public anxiously awaiting boosters
Boosters aren’t recommended in the United States at the moment, in large part because the Emergency Use Authorization under which the vaccines are being administered allows for only two shots of the Pfizer and Moderna vaccines and one shot of the Johnson & Johnson vaccine.
Even so, regulators and policymakers are “keenly aware that physicians and patients alike are anxious to get going and start doing boosters,” Dr. Shah said. There’s concern that antibody response might wane over time, perhaps even more quickly in patients with cancer.
Pfizer is already in talks with the U.S. Food and Drug Administration to authorize a third dose of its vaccine in the United States. Guidelines could very well change in coming months, said Ghady Haidar, MD, a specialist in infectious diseases and cancer at the University of Pittsburgh.
However, it’s still early in the game, and it’s not clear yet if boosters are necessary in cancer, Dr. Haidar said in an interview.
For one thing, it’s unknown if poor antibody response really means that patients aren’t protected, he explained. The vaccines elicit T-cell responses that could protect patients regardless of antibody levels. It’s also unclear if antibody titer levels are clinically relevant, and there hasn’t been much indication yet that less-than-robust vaccine responses translate to worse COVID outcomes in patients with cancer.
Those and other questions are areas of active investigation by Dr. Shah, Dr. Haidar, and others. Dozens of clinical trials are investigating vaccine response in patients with cancer, including the use of boosters.
Meanwhile, some cancer patients aren’t waiting around for more study results. “I get many, many emails a day” about booster shots, Dr. Haidar said. “We recommend against” them for now but some people bend the rules and get an extra shot anyway. “I get it. People are apprehensive.”
Three COVID deaths despite full vaccination
The vaccine clinical trials had fewer patients with cancer, so researchers are moving fast to backfill the data. Although there is some variation in what’s being reported, an overall picture is slowly emerging.
Dr. Shah and her team reported on responses to the mRNA COVID vaccines from Pfizer and Moderna and found a 94% seroconversion rate in 131 patients with cancer 3-4 weeks after their second dose of vaccine. They also found good responses among patients on cytotoxic chemotherapy within 6 months of their first vaccine dose, although their antibody titer levels were significantly lower than seen in other patients with cancer.
Investigators from Montefiore Medical Center in New York City also recently reported a 94% seroconversion rate among 200 patients with cancer, including 98% seroconversion in patients with solid tumors. Rates were lower in patients with blood cancers but were still 85% overall, with 70% conversion among patients on anti-CD20 therapies and 73% among stem cell transplant patients.
Dr. Haidar’s group reported a seroconversion rate of 82.4% among patients with solid tumors but only 54.7% among those with blood cancer. Risk factors for poor response included treatment with antimetabolites and anti-CD20 therapies, and, in the solid tumor group, radiation therapy, likely because of its overall toxicity and impact on lymphocyte function.
Israeli investigators reported in May a 90% seroconversion rate after two doses of the Pfizer vaccine among 102 patients with solid tumors on active treatment, which compared favorably to the 100% conversion rate in healthy controls, but they noted that antibody titers were considerably lower in patients with cancer.
The only variable associated with lower titer levels was combined use of chemotherapy and immunotherapy, they noted. There were also three women on dose-dense chemotherapy for breast cancer who did not produce any antibodies.
In a study limited to patients with blood cancers, a Lithuanian team recently reported that among 885 patients, those on Bruton tyrosine kinase inhibitors, ruxolitinib (Jakafi), venetoclax (Venclexta), or anti-CD20 therapies mounted almost no antibody response to the Pfizer vaccine.
The Lithuanian group also reported nine breakthrough COVID infections among their fully vaccinated blood cancer patients, including three deaths.
A team from the Icahn School of Medicine at Mount Sinai, New York reported that more than 15% of 260 patients with multiple myeloma also had no response to the Pfizer or Moderna vaccine; they were on BCMA-targeted therapy or anti-CD38 monoclonal antibody therapy at the time of vaccination, but a few had undergone CAR-T cell therapy more than 3 months beforehand.
Heated debate about antibody testing
Despite these reports of some patients with cancer having poorer responses, there’s some uncertainty over the benefit of giving a third (booster) shot.
There’s the question about the clinical relevance of antibody titer levels, and very little work has been done to date on cellular T-cell immunity from the vaccines.
“Right now, we are using titer levels like they actually mean something when they might not,” said Ravi Parikh, MD, a genitourinary and thoracic oncologist at the University of Pennsylvania, Philadelphia, who co-wrote an editorial that accompanies the Israeli report.
That’s one of the reasons why the FDA and others do not currently recommend antibody tests for COVID vaccine decisions outside of a clinical trial, but not everyone agrees with that position.
There’s been “a lot of heated debate in the medical community” over the issue, Dr. Haidar said.
The Icahn team, for instance, said that their results “underscore the need for routine serological monitoring of [multiple myeloma] patients following COVID-19 vaccination” to see if they might still need to mask-up and socially distance.
There is precedence, too, for vaccine boosters in cancer. As Dr. Parikh noted in his editorial, guidelines recommend revaccination after stem cell transplant for meningococcus, tetanus, and varicella, and other infections.
In France, COVID booster shots are already standard care for patients on dialysis and those on anti-CD20 agents, as well as for solid organ transplant recipients, for whom the literature supporting the benefit of COVID boosters is much more evolved than in cancer.
Israel has also authorized vaccine boosters for immunocompromised patients, including those with cancer, according to news reports.
It is also almost certain that the FDA will grant a formal approval for the COVID vaccines, at which point doctors will be free to administer boosters as they see fit.
“People are going to have to think really hard about what to do with them” if guidance hasn’t changed by then, Dr. Haidar said.
As the story unfolds, Dr. Haidar and others said in an interview that the take-home message for oncologists remains largely what it has been – namely to get patients vaccinated but also to consider masks and social distancing afterward for those at risk of a poor response.
Dr. Shah, Dr. Haidar, and Dr. Parikh have disclosed no relevant financial relationships. Dr. Parikh is a regular contributor to Medscape Oncology.
A version of this article first appeared on Medscape.com.
Patients with cancer have shown varying responses to COVID-19 vaccination, with good responses in patients with solid tumors (even while on systemic therapy) and poor responses in patients with blood cancers, particularly those on immunosuppressive therapies.
The data are evolving to show factors associated with a poor response but are not strong enough yet to recommend booster shots, say researchers.
The work is defining who will likely need a COVID vaccine booster when they become available. “It’s definitely not all cancer patients,” said Dimpy Shah, MD, PhD, a cancer epidemiologist at the Mays Cancer Center, University of Texas, San Antonio.
Public anxiously awaiting boosters
Boosters aren’t recommended in the United States at the moment, in large part because the Emergency Use Authorization under which the vaccines are being administered allows for only two shots of the Pfizer and Moderna vaccines and one shot of the Johnson & Johnson vaccine.
Even so, regulators and policymakers are “keenly aware that physicians and patients alike are anxious to get going and start doing boosters,” Dr. Shah said. There’s concern that antibody response might wane over time, perhaps even more quickly in patients with cancer.
Pfizer is already in talks with the U.S. Food and Drug Administration to authorize a third dose of its vaccine in the United States. Guidelines could very well change in coming months, said Ghady Haidar, MD, a specialist in infectious diseases and cancer at the University of Pittsburgh.
However, it’s still early in the game, and it’s not clear yet if boosters are necessary in cancer, Dr. Haidar said in an interview.
For one thing, it’s unknown if poor antibody response really means that patients aren’t protected, he explained. The vaccines elicit T-cell responses that could protect patients regardless of antibody levels. It’s also unclear if antibody titer levels are clinically relevant, and there hasn’t been much indication yet that less-than-robust vaccine responses translate to worse COVID outcomes in patients with cancer.
Those and other questions are areas of active investigation by Dr. Shah, Dr. Haidar, and others. Dozens of clinical trials are investigating vaccine response in patients with cancer, including the use of boosters.
Meanwhile, some cancer patients aren’t waiting around for more study results. “I get many, many emails a day” about booster shots, Dr. Haidar said. “We recommend against” them for now but some people bend the rules and get an extra shot anyway. “I get it. People are apprehensive.”
Three COVID deaths despite full vaccination
The vaccine clinical trials had fewer patients with cancer, so researchers are moving fast to backfill the data. Although there is some variation in what’s being reported, an overall picture is slowly emerging.
Dr. Shah and her team reported on responses to the mRNA COVID vaccines from Pfizer and Moderna and found a 94% seroconversion rate in 131 patients with cancer 3-4 weeks after their second dose of vaccine. They also found good responses among patients on cytotoxic chemotherapy within 6 months of their first vaccine dose, although their antibody titer levels were significantly lower than seen in other patients with cancer.
Investigators from Montefiore Medical Center in New York City also recently reported a 94% seroconversion rate among 200 patients with cancer, including 98% seroconversion in patients with solid tumors. Rates were lower in patients with blood cancers but were still 85% overall, with 70% conversion among patients on anti-CD20 therapies and 73% among stem cell transplant patients.
Dr. Haidar’s group reported a seroconversion rate of 82.4% among patients with solid tumors but only 54.7% among those with blood cancer. Risk factors for poor response included treatment with antimetabolites and anti-CD20 therapies, and, in the solid tumor group, radiation therapy, likely because of its overall toxicity and impact on lymphocyte function.
Israeli investigators reported in May a 90% seroconversion rate after two doses of the Pfizer vaccine among 102 patients with solid tumors on active treatment, which compared favorably to the 100% conversion rate in healthy controls, but they noted that antibody titers were considerably lower in patients with cancer.
The only variable associated with lower titer levels was combined use of chemotherapy and immunotherapy, they noted. There were also three women on dose-dense chemotherapy for breast cancer who did not produce any antibodies.
In a study limited to patients with blood cancers, a Lithuanian team recently reported that among 885 patients, those on Bruton tyrosine kinase inhibitors, ruxolitinib (Jakafi), venetoclax (Venclexta), or anti-CD20 therapies mounted almost no antibody response to the Pfizer vaccine.
The Lithuanian group also reported nine breakthrough COVID infections among their fully vaccinated blood cancer patients, including three deaths.
A team from the Icahn School of Medicine at Mount Sinai, New York reported that more than 15% of 260 patients with multiple myeloma also had no response to the Pfizer or Moderna vaccine; they were on BCMA-targeted therapy or anti-CD38 monoclonal antibody therapy at the time of vaccination, but a few had undergone CAR-T cell therapy more than 3 months beforehand.
Heated debate about antibody testing
Despite these reports of some patients with cancer having poorer responses, there’s some uncertainty over the benefit of giving a third (booster) shot.
There’s the question about the clinical relevance of antibody titer levels, and very little work has been done to date on cellular T-cell immunity from the vaccines.
“Right now, we are using titer levels like they actually mean something when they might not,” said Ravi Parikh, MD, a genitourinary and thoracic oncologist at the University of Pennsylvania, Philadelphia, who co-wrote an editorial that accompanies the Israeli report.
That’s one of the reasons why the FDA and others do not currently recommend antibody tests for COVID vaccine decisions outside of a clinical trial, but not everyone agrees with that position.
There’s been “a lot of heated debate in the medical community” over the issue, Dr. Haidar said.
The Icahn team, for instance, said that their results “underscore the need for routine serological monitoring of [multiple myeloma] patients following COVID-19 vaccination” to see if they might still need to mask-up and socially distance.
There is precedence, too, for vaccine boosters in cancer. As Dr. Parikh noted in his editorial, guidelines recommend revaccination after stem cell transplant for meningococcus, tetanus, and varicella, and other infections.
In France, COVID booster shots are already standard care for patients on dialysis and those on anti-CD20 agents, as well as for solid organ transplant recipients, for whom the literature supporting the benefit of COVID boosters is much more evolved than in cancer.
Israel has also authorized vaccine boosters for immunocompromised patients, including those with cancer, according to news reports.
It is also almost certain that the FDA will grant a formal approval for the COVID vaccines, at which point doctors will be free to administer boosters as they see fit.
“People are going to have to think really hard about what to do with them” if guidance hasn’t changed by then, Dr. Haidar said.
As the story unfolds, Dr. Haidar and others said in an interview that the take-home message for oncologists remains largely what it has been – namely to get patients vaccinated but also to consider masks and social distancing afterward for those at risk of a poor response.
Dr. Shah, Dr. Haidar, and Dr. Parikh have disclosed no relevant financial relationships. Dr. Parikh is a regular contributor to Medscape Oncology.
A version of this article first appeared on Medscape.com.
Patients with cancer have shown varying responses to COVID-19 vaccination, with good responses in patients with solid tumors (even while on systemic therapy) and poor responses in patients with blood cancers, particularly those on immunosuppressive therapies.
The data are evolving to show factors associated with a poor response but are not strong enough yet to recommend booster shots, say researchers.
The work is defining who will likely need a COVID vaccine booster when they become available. “It’s definitely not all cancer patients,” said Dimpy Shah, MD, PhD, a cancer epidemiologist at the Mays Cancer Center, University of Texas, San Antonio.
Public anxiously awaiting boosters
Boosters aren’t recommended in the United States at the moment, in large part because the Emergency Use Authorization under which the vaccines are being administered allows for only two shots of the Pfizer and Moderna vaccines and one shot of the Johnson & Johnson vaccine.
Even so, regulators and policymakers are “keenly aware that physicians and patients alike are anxious to get going and start doing boosters,” Dr. Shah said. There’s concern that antibody response might wane over time, perhaps even more quickly in patients with cancer.
Pfizer is already in talks with the U.S. Food and Drug Administration to authorize a third dose of its vaccine in the United States. Guidelines could very well change in coming months, said Ghady Haidar, MD, a specialist in infectious diseases and cancer at the University of Pittsburgh.
However, it’s still early in the game, and it’s not clear yet if boosters are necessary in cancer, Dr. Haidar said in an interview.
For one thing, it’s unknown if poor antibody response really means that patients aren’t protected, he explained. The vaccines elicit T-cell responses that could protect patients regardless of antibody levels. It’s also unclear if antibody titer levels are clinically relevant, and there hasn’t been much indication yet that less-than-robust vaccine responses translate to worse COVID outcomes in patients with cancer.
Those and other questions are areas of active investigation by Dr. Shah, Dr. Haidar, and others. Dozens of clinical trials are investigating vaccine response in patients with cancer, including the use of boosters.
Meanwhile, some cancer patients aren’t waiting around for more study results. “I get many, many emails a day” about booster shots, Dr. Haidar said. “We recommend against” them for now but some people bend the rules and get an extra shot anyway. “I get it. People are apprehensive.”
Three COVID deaths despite full vaccination
The vaccine clinical trials had fewer patients with cancer, so researchers are moving fast to backfill the data. Although there is some variation in what’s being reported, an overall picture is slowly emerging.
Dr. Shah and her team reported on responses to the mRNA COVID vaccines from Pfizer and Moderna and found a 94% seroconversion rate in 131 patients with cancer 3-4 weeks after their second dose of vaccine. They also found good responses among patients on cytotoxic chemotherapy within 6 months of their first vaccine dose, although their antibody titer levels were significantly lower than seen in other patients with cancer.
Investigators from Montefiore Medical Center in New York City also recently reported a 94% seroconversion rate among 200 patients with cancer, including 98% seroconversion in patients with solid tumors. Rates were lower in patients with blood cancers but were still 85% overall, with 70% conversion among patients on anti-CD20 therapies and 73% among stem cell transplant patients.
Dr. Haidar’s group reported a seroconversion rate of 82.4% among patients with solid tumors but only 54.7% among those with blood cancer. Risk factors for poor response included treatment with antimetabolites and anti-CD20 therapies, and, in the solid tumor group, radiation therapy, likely because of its overall toxicity and impact on lymphocyte function.
Israeli investigators reported in May a 90% seroconversion rate after two doses of the Pfizer vaccine among 102 patients with solid tumors on active treatment, which compared favorably to the 100% conversion rate in healthy controls, but they noted that antibody titers were considerably lower in patients with cancer.
The only variable associated with lower titer levels was combined use of chemotherapy and immunotherapy, they noted. There were also three women on dose-dense chemotherapy for breast cancer who did not produce any antibodies.
In a study limited to patients with blood cancers, a Lithuanian team recently reported that among 885 patients, those on Bruton tyrosine kinase inhibitors, ruxolitinib (Jakafi), venetoclax (Venclexta), or anti-CD20 therapies mounted almost no antibody response to the Pfizer vaccine.
The Lithuanian group also reported nine breakthrough COVID infections among their fully vaccinated blood cancer patients, including three deaths.
A team from the Icahn School of Medicine at Mount Sinai, New York reported that more than 15% of 260 patients with multiple myeloma also had no response to the Pfizer or Moderna vaccine; they were on BCMA-targeted therapy or anti-CD38 monoclonal antibody therapy at the time of vaccination, but a few had undergone CAR-T cell therapy more than 3 months beforehand.
Heated debate about antibody testing
Despite these reports of some patients with cancer having poorer responses, there’s some uncertainty over the benefit of giving a third (booster) shot.
There’s the question about the clinical relevance of antibody titer levels, and very little work has been done to date on cellular T-cell immunity from the vaccines.
“Right now, we are using titer levels like they actually mean something when they might not,” said Ravi Parikh, MD, a genitourinary and thoracic oncologist at the University of Pennsylvania, Philadelphia, who co-wrote an editorial that accompanies the Israeli report.
That’s one of the reasons why the FDA and others do not currently recommend antibody tests for COVID vaccine decisions outside of a clinical trial, but not everyone agrees with that position.
There’s been “a lot of heated debate in the medical community” over the issue, Dr. Haidar said.
The Icahn team, for instance, said that their results “underscore the need for routine serological monitoring of [multiple myeloma] patients following COVID-19 vaccination” to see if they might still need to mask-up and socially distance.
There is precedence, too, for vaccine boosters in cancer. As Dr. Parikh noted in his editorial, guidelines recommend revaccination after stem cell transplant for meningococcus, tetanus, and varicella, and other infections.
In France, COVID booster shots are already standard care for patients on dialysis and those on anti-CD20 agents, as well as for solid organ transplant recipients, for whom the literature supporting the benefit of COVID boosters is much more evolved than in cancer.
Israel has also authorized vaccine boosters for immunocompromised patients, including those with cancer, according to news reports.
It is also almost certain that the FDA will grant a formal approval for the COVID vaccines, at which point doctors will be free to administer boosters as they see fit.
“People are going to have to think really hard about what to do with them” if guidance hasn’t changed by then, Dr. Haidar said.
As the story unfolds, Dr. Haidar and others said in an interview that the take-home message for oncologists remains largely what it has been – namely to get patients vaccinated but also to consider masks and social distancing afterward for those at risk of a poor response.
Dr. Shah, Dr. Haidar, and Dr. Parikh have disclosed no relevant financial relationships. Dr. Parikh is a regular contributor to Medscape Oncology.
A version of this article first appeared on Medscape.com.
Testosterone replacement shows CV benefit in hypogonadal men
Data from a long-term study suggest that testosterone replacement therapy (TRT) for men with hypogonadism may reduce the risk for major adverse cardiovascular events. Previous studies have yielded conflicting results on whether there is a benefit.
The latest results come from a study of 805 men with hypogonadism from Germany and Qatar who were followed for nearly a decade. For those who received parenteral testosterone 1,000 mg every 12 weeks, there were improvements in classical cardiovascular risk factors, such as obesity, lipid level, and inflammatory markers, whereas among those who chose not to take testosterone (control patients), all of these factors worsened.
In addition, there were only 16 deaths among patients in the TRT group, and none of the deaths were from myocardial infarction or stroke. In contrast, there were 74 deaths among the control patients, as well as 70 cases of MI and 59 strokes.
The men in the study were all at relatively high risk for cardiovascular adverse events. In the TRT group, the mean Framingham Risk score was 15.5; in the control group, it was 15.8. This translates into mean 10-year risks of 22.7% and 23.5%, respectively.
“Given that all these men would normally have been expected to suffer a heart attack or stroke in the next 5-10 years with no other intervention, it was a real surprise to see no cardiovascular events at all in the group on testosterone therapy. It’s clear that this treatment can significantly reduce the risks in this particular group,” commented lead investigator Omar Aboumarzouk, MD, from Hamad Medical in Doha, Qatar.
He presented the new data at the 2021 annual congress of the European Association of Urology.
Dr. Aboumarzouk emphasized, however, that, “while men need testosterone for certain psychological and biological functions, only those with low levels who display other symptoms are likely to benefit from testosterone therapy.”
Maarten Albersen, MD, a urologist at the University of Leuven (Belgium), who was not involved in the study, noted that, although the study showed a reduction in major adverse cardiovascular events and mortality among the men who received TRT, the risk scores were in the intermediate range, and the men in the TRT group were slightly younger and were at slightly lower risk at baseline.
“The study was long enough to see differences in the rate of cardiovascular events. However, the numbers involved and the fact that the trial was not randomized mean it’s still difficult to draw any hard conclusions,” he said.
Registry study
The data came from a cumulative registry study begun in 2004 to assess the long-term efficacy and safety of TRT every 3 months in men with hypogonadism. The study, conducted in Bremen, Dresden, and Muenster in Germany, as well as in Doha, Qatar, is ongoing.
At total of 805 men were enrolled; 412 received TRT, and 393 declined testosterone replacement and served as control patients.
The investigators reported 10-year data. Statistical models controlled for age, body mass index, smoking, alcohol, total and HDL cholesterol level, systolic blood pressure, and type 2 diabetes.
The median age at baseline was lower among those in the TRT arm, at 57.7 years versus 63.7 years for control patients (P < .001).
All classical cardiovascular risk factors, including obesity, glycemic control, lipid pattern, and C-reactive protein, improved in the TRT group and worsened in the control group.
Dr. Albersen noted that “a new trial is now underway, aiming to recruit 6,000 participants, and this should provide definitive answers on the cardiovascular risks or even benefits of hormone therapy in men with low testosterone.”
No funding source for the study was reported. Dr. Aboumarzouk and Dr. Albersen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Data from a long-term study suggest that testosterone replacement therapy (TRT) for men with hypogonadism may reduce the risk for major adverse cardiovascular events. Previous studies have yielded conflicting results on whether there is a benefit.
The latest results come from a study of 805 men with hypogonadism from Germany and Qatar who were followed for nearly a decade. For those who received parenteral testosterone 1,000 mg every 12 weeks, there were improvements in classical cardiovascular risk factors, such as obesity, lipid level, and inflammatory markers, whereas among those who chose not to take testosterone (control patients), all of these factors worsened.
In addition, there were only 16 deaths among patients in the TRT group, and none of the deaths were from myocardial infarction or stroke. In contrast, there were 74 deaths among the control patients, as well as 70 cases of MI and 59 strokes.
The men in the study were all at relatively high risk for cardiovascular adverse events. In the TRT group, the mean Framingham Risk score was 15.5; in the control group, it was 15.8. This translates into mean 10-year risks of 22.7% and 23.5%, respectively.
“Given that all these men would normally have been expected to suffer a heart attack or stroke in the next 5-10 years with no other intervention, it was a real surprise to see no cardiovascular events at all in the group on testosterone therapy. It’s clear that this treatment can significantly reduce the risks in this particular group,” commented lead investigator Omar Aboumarzouk, MD, from Hamad Medical in Doha, Qatar.
He presented the new data at the 2021 annual congress of the European Association of Urology.
Dr. Aboumarzouk emphasized, however, that, “while men need testosterone for certain psychological and biological functions, only those with low levels who display other symptoms are likely to benefit from testosterone therapy.”
Maarten Albersen, MD, a urologist at the University of Leuven (Belgium), who was not involved in the study, noted that, although the study showed a reduction in major adverse cardiovascular events and mortality among the men who received TRT, the risk scores were in the intermediate range, and the men in the TRT group were slightly younger and were at slightly lower risk at baseline.
“The study was long enough to see differences in the rate of cardiovascular events. However, the numbers involved and the fact that the trial was not randomized mean it’s still difficult to draw any hard conclusions,” he said.
Registry study
The data came from a cumulative registry study begun in 2004 to assess the long-term efficacy and safety of TRT every 3 months in men with hypogonadism. The study, conducted in Bremen, Dresden, and Muenster in Germany, as well as in Doha, Qatar, is ongoing.
At total of 805 men were enrolled; 412 received TRT, and 393 declined testosterone replacement and served as control patients.
The investigators reported 10-year data. Statistical models controlled for age, body mass index, smoking, alcohol, total and HDL cholesterol level, systolic blood pressure, and type 2 diabetes.
The median age at baseline was lower among those in the TRT arm, at 57.7 years versus 63.7 years for control patients (P < .001).
All classical cardiovascular risk factors, including obesity, glycemic control, lipid pattern, and C-reactive protein, improved in the TRT group and worsened in the control group.
Dr. Albersen noted that “a new trial is now underway, aiming to recruit 6,000 participants, and this should provide definitive answers on the cardiovascular risks or even benefits of hormone therapy in men with low testosterone.”
No funding source for the study was reported. Dr. Aboumarzouk and Dr. Albersen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Data from a long-term study suggest that testosterone replacement therapy (TRT) for men with hypogonadism may reduce the risk for major adverse cardiovascular events. Previous studies have yielded conflicting results on whether there is a benefit.
The latest results come from a study of 805 men with hypogonadism from Germany and Qatar who were followed for nearly a decade. For those who received parenteral testosterone 1,000 mg every 12 weeks, there were improvements in classical cardiovascular risk factors, such as obesity, lipid level, and inflammatory markers, whereas among those who chose not to take testosterone (control patients), all of these factors worsened.
In addition, there were only 16 deaths among patients in the TRT group, and none of the deaths were from myocardial infarction or stroke. In contrast, there were 74 deaths among the control patients, as well as 70 cases of MI and 59 strokes.
The men in the study were all at relatively high risk for cardiovascular adverse events. In the TRT group, the mean Framingham Risk score was 15.5; in the control group, it was 15.8. This translates into mean 10-year risks of 22.7% and 23.5%, respectively.
“Given that all these men would normally have been expected to suffer a heart attack or stroke in the next 5-10 years with no other intervention, it was a real surprise to see no cardiovascular events at all in the group on testosterone therapy. It’s clear that this treatment can significantly reduce the risks in this particular group,” commented lead investigator Omar Aboumarzouk, MD, from Hamad Medical in Doha, Qatar.
He presented the new data at the 2021 annual congress of the European Association of Urology.
Dr. Aboumarzouk emphasized, however, that, “while men need testosterone for certain psychological and biological functions, only those with low levels who display other symptoms are likely to benefit from testosterone therapy.”
Maarten Albersen, MD, a urologist at the University of Leuven (Belgium), who was not involved in the study, noted that, although the study showed a reduction in major adverse cardiovascular events and mortality among the men who received TRT, the risk scores were in the intermediate range, and the men in the TRT group were slightly younger and were at slightly lower risk at baseline.
“The study was long enough to see differences in the rate of cardiovascular events. However, the numbers involved and the fact that the trial was not randomized mean it’s still difficult to draw any hard conclusions,” he said.
Registry study
The data came from a cumulative registry study begun in 2004 to assess the long-term efficacy and safety of TRT every 3 months in men with hypogonadism. The study, conducted in Bremen, Dresden, and Muenster in Germany, as well as in Doha, Qatar, is ongoing.
At total of 805 men were enrolled; 412 received TRT, and 393 declined testosterone replacement and served as control patients.
The investigators reported 10-year data. Statistical models controlled for age, body mass index, smoking, alcohol, total and HDL cholesterol level, systolic blood pressure, and type 2 diabetes.
The median age at baseline was lower among those in the TRT arm, at 57.7 years versus 63.7 years for control patients (P < .001).
All classical cardiovascular risk factors, including obesity, glycemic control, lipid pattern, and C-reactive protein, improved in the TRT group and worsened in the control group.
Dr. Albersen noted that “a new trial is now underway, aiming to recruit 6,000 participants, and this should provide definitive answers on the cardiovascular risks or even benefits of hormone therapy in men with low testosterone.”
No funding source for the study was reported. Dr. Aboumarzouk and Dr. Albersen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Assessment of a Medication Deprescribing Tool on Polypharmacy and Cost Avoidance
According to the Centers for Disease Control and Prevention National Center for Health Statistics (NCHS), the use of prescription drugs has increased in the past half century. Although prescription drugs have played an important role in preventing, controlling, and delaying onset or progression of disease, their growth in use also has posed many risks.1 One ramification of this growth is the occurrence of polypharmacy, which does not have a universal, clear definition. In general, it can be described as the concurrent use of multiple medications by a single patient to treat one or more medical ailments. Five or more medications taken simultaneously is the most common definition to date, but this is just one of many acceptable definitions and that varies from one health care facility to another.1,2
Regardless of the cutoffs established to indicate polypharmacy, its incidence can result in poor and potentially harmful health outcomes. Polypharmacy increases the risk of experiencing adverse drug events (ADEs), drug-drug interactions (DDIs), geriatric-related syndromes, falls, hospitalization, and mortality. Issues with adherence may begin to unfold secondary to increased pill burden. Both the patient and the health care system may encounter financial strain, as polypharmacy can lead to unnecessary and essentially preventable costs of care. When evaluating the likelihood of polypharmacy based on age group, NCHS found that 47.5% of patients taking ≥ 5 medications were aged ≥ 65 years.1-5 This indicates that polypharmacy is of great concern in the geriatric population, which also represents a large proportion of individuals accessing Veterans Health Administration (VHA) care.
Deprescibing
Deprescribing is the act of withdrawing or discontinuing potentially inappropriate medications (PIM), or medications used by older patients harboring ADEs that generally outweigh the clinical benefits of the drug. Deprescribing is an effective tool for managing or reducing polypharmacy. A variety of tools have been created whose sole purpose is to simplify deprescribing. Some tools explicitly identify PIM and are widely familiar in medical practice. Examples are the Beers Criteria developed in 1991 or Screening Tool to Alert Right Treatment/Screening Tool of Older Persons Prescriptions (START/STOPP) criteria created in 2003. Other tools that are less commonplace but equally as resourceful are MedStopper and Deprescribing.org. The former was launched in 2015 and is a Canadian online system that provides risk assessments for medications with guidance for tapering or stopping medications if continuation of the drug presents higher risk than benefit.5-7 The latter is a full-fledged website developed by a physician, a pharmacist, and their research teams that serves as an exchange hub for deprescribing information.
In 2016, the VIONE (Vital, Important, Optional, Not indicated/treatment complete, and Every medication has an indication) deprescribing tool was developed by Saraswathy Battar, MD, at Central Arkansas Veterans Healthcare System (CAVHS) in Little Rock, as a system that could go beyond medication reconciliation (Table 1). Health care providers (HCPs) and pharmacists evaluate each medication that a patient has been prescribed and places each medication in a VIONE category. Prescribers may then take the opportunity to deprescribe or discontinue medications if deemed appropriate based on their clinical assessments and shared decision making.8 Traditionally, medication reconciliation involves the process of obtaining a complete and accurate list of medications as reported by a patient or caregiver to a HCP. VIONE encourages HCPs and pharmacists not only to ensure medication lists are accurate, but also that each medication reported is appropriate for continued use. In other words, VIONE is meant to help implement deprescribing at opportune times. More than 14,000 medications have been deprescribed using the VIONE method, resulting in more than $2,000,000 of annualized cost avoidance after just 1 year of implementation at CAVHS.9
VIONE consists of 2 major components in the Computerized Patient Record System (CPRS): a template and a dropdown discontinuation menu. The template captured patient allergies, pertinent laboratory data, the patient’s active problem list and applicable diagnoses, and active medication list. Patient aligned care team (PACT) pharmacists used the information captured in the template to conduct medication reconciliations and polypharmacy reviews. Each medication is categorized in VIONE using data collected during reviews. A menu delineates reasons for discontinuation: optional, dose decrease, no diagnosis, not indicated/treatment complete, discontinue alternate medication prescribed, and patient reported no longer taking. The discontinuation menu allowed PACT pharmacists and physicians to choose 1 VIONE option per medication to clarify the reason for discontinuation. VIONE-based discontinuations are recorded in CPRS and identified as deprescribed.
At the time of this project, > 30 US Department of Veterans Affairs (VA) facilities had adopted VIONE. Use of VIONE at VA Southern Nevada Healthcare System (VASNHS) in North Las Vegas has been incorporated in the everyday practices of home-based primary care pharmacists and physicians but has yet to be implemented in other areas of the facility. The purpose of this project was to determine the impact of the VIONE tool on polypharmacy and cost avoidance at VASNHS when used by primary care physicians (PCPs) and PACT primary care clinics.
Methods
Veterans receiving care at VASNHS aged ≥ 65 years with ≥ 10 active medications noted in CPRS were included in this project. PACT pharmacists and physicians were educated on the proper use of the VIONE tool prior to its implementation. Education included a 15-minute slide presentation followed by dissemination of a 1-page VIONE tool handout during a PACT all-staff clinic meeting.
Data were collected for 3 months before and after the intervention. Data were made available for assessment by the Automated Data Processing Application Coordinator (ADPAC) at VASNHS. The ADPAC created and generated an Excel spreadsheet report, which listed all medications deprescribed using the VIONE method. The primary endpoint was the total number of medications discontinued using the VIONE template and/or discontinuation menu. For the purpose of this project, appropriate discontinuation was considered any prescription deprescribed, excluding medical supplies, by pharmacists and PCPs who received VIONE education.
The secondary endpoint was the estimated annualized cost avoidance for the facility (Figure). The calculation does not include medications discontinued due to the prescription of an alternative medication or dose decreases since these VIONE selections imply that a new prescription or order was placed and the original prescription was not deprescribed. Annualized cost avoidance was determined with use of the VIONE dashboard, a database that retrospectively gathers information regarding patients at risk of polypharmacy, polypharmacy-related ADEs, and cost. Manual adjustments were made to various parameters on the Veterans Integrated Service Network 15 VIONE dashboard by the author in order to obtain data specific to this project. These parameters allowed selection of service sections, specific staff members or the option to include or exclude chronic or nonchronic medications. The annualized cost avoidance figure was then compared to raw data pulled by a VIONE dashboard correspondent to ensure the manual calculation was accurate. Finally, the 5 most common classes of medications deprescribed were identified for information purposes and to provide a better postulation on the types of medications being discontinued using the VIONE method.
Results
A total of 2,442 veterans met inclusion criteria, and the VIONE method was applied to 598 between late October 2018 and January 2019. The 13 PACT pharmacists contacted at least 10 veterans each, thus at least 130 were randomly selected for telephone calls to perform polypharmacy reviews using the VIONE note template. The discontinuation menu was used if a medication qualified to be deprescribed. After 3 months, 1986 prescriptions were deprescribed using VIONE; however, 1060 prescriptions were considered appropriately deprescribed (Table 2). The 13 PACT pharmacists deprescribed 361 medications, and the 29 PACT physicians deprescribed 699 medications. These prescriptions were then separated into medication categories to determine the most common discontinued classes. Vitamins and supplements were the medication class most frequently deprescribed (19.4%), followed by pain medications (15.5%), antimicrobial agents (9.6%), antihypertensive medications (9.2%), and diabetes medications (6.4%) (Table 3). The top 5 medication categories accounted for 60% of all medications appropriately deprescribed.
The estimated annualized cost avoidance for all medications deprescribed in the 3-month project period was $84,030.46. To provide the most appropriate and accurate calculation, medication classes excluded from this figure were acute or short-term prescriptions and antimicrobial agents. Medications prescribed short-term typically are not suitable to continue for an extended period, and antimicrobial agents were excluded since they are normally associated with higher costs, and may overestimate the cost avoidance calculation for the facility.
Discussion
The outcomes for the primary and secondary endpoints of this project illustrate that using VIONE in PACT primary care clinics had a notable impact on polypharmacy and cost avoidance over a short period. This outcome can be attributed to 2 significant effects of using the deprescribing tool. VIONE’s simplicity in application allowed clinicians to incorporate daily use of the tool with minimal effort. Education was all that was required to fully enable clinicians to work together successfully and exercise collaborative practice to promote deprescribing. VIONE also elicited a cascade of favorable effects that improve patient safety and health outcomes. The tool aided in identification of PIM, which helped reduce polypharmacy and medication burden. The risk for DDIs and ADEs may decrease; therefore, the incidence of falls, need for emergency department visits or inpatient care related to polypharmacy may decline. Less complex medication regimens may alleviate issues with adherence and avoid the various consequences of polypharmacy in theory. Simplified regimens can potentially improve disease management and quality of life for patients. Further studies are needed to substantiate deprescribing and its true effect on patient adherence and better health outcomes at this time.10
Reducing polypharmacy can lead to cost savings. Based on the results of this 3-month study, we expect that VASNHS would save more than $84,000 by reducing polypharmacy among its patients. Those savings can be funneled back into the health care system, and allotted to necessary patient care, prescriptions, and health care facility needs.
Limitations
There are some important limitations to this study. Definitions of polypharmacy may vary from one health care facility to another. The cutoffs for polypharmacy may differ, causing the prevalence of polypharmacy and potential costs savings to vary. Use of VIONE may be inconsistent among users if not previously educated or properly trained. For instance, VIONE selections are listed in the same menu as the standard CPRS discontinuation options, which may lead to discontinuation of medical supplies or laboratory orders instead of prescriptions.
The method of data analysis and project design used in this study may have been subject to error. For example, the list of PCPs may have been inaccurate or outdated, which would result in an over- or underrepresentation of those who contributed to data collection. Furthermore, there is some volatility in calculating the total cost avoidance. For example, medications for chronic conditions that were only taken on an as needed basis may have overestimated savings. Either under- or overestimations could occur when parameters are adjusted on the VIONE discontinuation dashboard without appropriate guidance. With the ability to manually adjust the dashboard parameters, dissimilarities in calculations may follow.
Conclusions
The VIONE tool may be useful in improving patient safety through deprescribing and discontinuing PIM. Decreasing the number of medications being taken concomitantly by a patient and continuing only those that are imperative in their medical treatment is the first step to reducing the incidence of polypharmacy. Consequently, chances of ADEs or DDIs are lessened, especially among older individuals who are considered high risk for experiencing the detrimental effects that may ensue. These effects include geriatric-related syndromes, increased risk of fall, hospital visits or admissions, or death. Use of VIONE easily promotes collaboration among clinicians to evaluate medications eligible for discontinuation more regularly. If this deprescribing tool is continuously used, costs avoided can likely be maximized within VA health care systems.
The results of this project should serve as an incentive to push for better prescribing practices and increase deprescribing efforts. It should provoke the need for change in regimens and the subsequent discontinuation of prescriptions that are not considered vital to continue. Finally, the result of this project should substantiate the positive impact a deprescribing tool can possess to avert the issues commonly associated with polypharmacy.
1. Centers for Disease Control and Prevention, National Center for Health Statistics. Health, United States, 2013: with special feature on prescription drugs. Published May 2014. Accessed May 13, 2021. https://www.cdc.gov/nchs/data/hus/hus13.pdf
2. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. Published 2017 Oct 10. doi:10.1186/s12877-017-0621-2
3. Parulekar MS, Rogers CK. Polypharmacy and mobility. In: Cifu DX, Lew HL, Oh-Park M., eds Geriatric Rehabilitation. Elsevier; 2018. doi:10.1016/B978-0-323-54454-2.12001-1
4. Rieckert A, Trampisch US, Klaaßen-Mielke R, et al. Polypharmacy in older patients with chronic diseases: a cross-sectional analysis of factors associated with excessive polypharmacy. BMC Fam Pract. 2018;19(1):113. Published 2018 Jul 18. doi:10.1186/s12875-018-0795-5
5. Thompson CA. New medication review method cuts veterans’ Rx load, saves millions. Am J Health Syst Pharm. 2018;75(8):502-503. doi:10.2146/news180023
6. Reeve E. Deprescribing tools: a review of the types of tools available to aid deprescribing in clinical practice. J Pharm Pract Res. 2020;50(1):98-107. doi:10.1002/jppr.1626
7. Fried TR, Niehoff KM, Street RL, et al. Effect of the Tool to Reduce Inappropriate Medications on Medication Communication and Deprescribing. J Am Geriatr Soc. 2017;65(10):2265-2271. doi:10.1111/jgs.15042
8. Battar S, Dickerson KR, Sedgwick C, et al. Understanding principles of high reliability organizations through the eyes of VIONE, a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
9. Battar S, Cmelik T, Dickerson K, Scott, M. Experience better health with VIONE a safe medication deprescribing tool [Nonpublic source, not verified]
10. Ulley J, Harrop D, Ali A, et al. Desprescribing interventions and their impact on medication adherence in community-dwelling older adults with polypharmacy: a systematic review. BMC Geriatr. 2019;19(15):1-13.
According to the Centers for Disease Control and Prevention National Center for Health Statistics (NCHS), the use of prescription drugs has increased in the past half century. Although prescription drugs have played an important role in preventing, controlling, and delaying onset or progression of disease, their growth in use also has posed many risks.1 One ramification of this growth is the occurrence of polypharmacy, which does not have a universal, clear definition. In general, it can be described as the concurrent use of multiple medications by a single patient to treat one or more medical ailments. Five or more medications taken simultaneously is the most common definition to date, but this is just one of many acceptable definitions and that varies from one health care facility to another.1,2
Regardless of the cutoffs established to indicate polypharmacy, its incidence can result in poor and potentially harmful health outcomes. Polypharmacy increases the risk of experiencing adverse drug events (ADEs), drug-drug interactions (DDIs), geriatric-related syndromes, falls, hospitalization, and mortality. Issues with adherence may begin to unfold secondary to increased pill burden. Both the patient and the health care system may encounter financial strain, as polypharmacy can lead to unnecessary and essentially preventable costs of care. When evaluating the likelihood of polypharmacy based on age group, NCHS found that 47.5% of patients taking ≥ 5 medications were aged ≥ 65 years.1-5 This indicates that polypharmacy is of great concern in the geriatric population, which also represents a large proportion of individuals accessing Veterans Health Administration (VHA) care.
Deprescibing
Deprescribing is the act of withdrawing or discontinuing potentially inappropriate medications (PIM), or medications used by older patients harboring ADEs that generally outweigh the clinical benefits of the drug. Deprescribing is an effective tool for managing or reducing polypharmacy. A variety of tools have been created whose sole purpose is to simplify deprescribing. Some tools explicitly identify PIM and are widely familiar in medical practice. Examples are the Beers Criteria developed in 1991 or Screening Tool to Alert Right Treatment/Screening Tool of Older Persons Prescriptions (START/STOPP) criteria created in 2003. Other tools that are less commonplace but equally as resourceful are MedStopper and Deprescribing.org. The former was launched in 2015 and is a Canadian online system that provides risk assessments for medications with guidance for tapering or stopping medications if continuation of the drug presents higher risk than benefit.5-7 The latter is a full-fledged website developed by a physician, a pharmacist, and their research teams that serves as an exchange hub for deprescribing information.
In 2016, the VIONE (Vital, Important, Optional, Not indicated/treatment complete, and Every medication has an indication) deprescribing tool was developed by Saraswathy Battar, MD, at Central Arkansas Veterans Healthcare System (CAVHS) in Little Rock, as a system that could go beyond medication reconciliation (Table 1). Health care providers (HCPs) and pharmacists evaluate each medication that a patient has been prescribed and places each medication in a VIONE category. Prescribers may then take the opportunity to deprescribe or discontinue medications if deemed appropriate based on their clinical assessments and shared decision making.8 Traditionally, medication reconciliation involves the process of obtaining a complete and accurate list of medications as reported by a patient or caregiver to a HCP. VIONE encourages HCPs and pharmacists not only to ensure medication lists are accurate, but also that each medication reported is appropriate for continued use. In other words, VIONE is meant to help implement deprescribing at opportune times. More than 14,000 medications have been deprescribed using the VIONE method, resulting in more than $2,000,000 of annualized cost avoidance after just 1 year of implementation at CAVHS.9
VIONE consists of 2 major components in the Computerized Patient Record System (CPRS): a template and a dropdown discontinuation menu. The template captured patient allergies, pertinent laboratory data, the patient’s active problem list and applicable diagnoses, and active medication list. Patient aligned care team (PACT) pharmacists used the information captured in the template to conduct medication reconciliations and polypharmacy reviews. Each medication is categorized in VIONE using data collected during reviews. A menu delineates reasons for discontinuation: optional, dose decrease, no diagnosis, not indicated/treatment complete, discontinue alternate medication prescribed, and patient reported no longer taking. The discontinuation menu allowed PACT pharmacists and physicians to choose 1 VIONE option per medication to clarify the reason for discontinuation. VIONE-based discontinuations are recorded in CPRS and identified as deprescribed.
At the time of this project, > 30 US Department of Veterans Affairs (VA) facilities had adopted VIONE. Use of VIONE at VA Southern Nevada Healthcare System (VASNHS) in North Las Vegas has been incorporated in the everyday practices of home-based primary care pharmacists and physicians but has yet to be implemented in other areas of the facility. The purpose of this project was to determine the impact of the VIONE tool on polypharmacy and cost avoidance at VASNHS when used by primary care physicians (PCPs) and PACT primary care clinics.
Methods
Veterans receiving care at VASNHS aged ≥ 65 years with ≥ 10 active medications noted in CPRS were included in this project. PACT pharmacists and physicians were educated on the proper use of the VIONE tool prior to its implementation. Education included a 15-minute slide presentation followed by dissemination of a 1-page VIONE tool handout during a PACT all-staff clinic meeting.
Data were collected for 3 months before and after the intervention. Data were made available for assessment by the Automated Data Processing Application Coordinator (ADPAC) at VASNHS. The ADPAC created and generated an Excel spreadsheet report, which listed all medications deprescribed using the VIONE method. The primary endpoint was the total number of medications discontinued using the VIONE template and/or discontinuation menu. For the purpose of this project, appropriate discontinuation was considered any prescription deprescribed, excluding medical supplies, by pharmacists and PCPs who received VIONE education.
The secondary endpoint was the estimated annualized cost avoidance for the facility (Figure). The calculation does not include medications discontinued due to the prescription of an alternative medication or dose decreases since these VIONE selections imply that a new prescription or order was placed and the original prescription was not deprescribed. Annualized cost avoidance was determined with use of the VIONE dashboard, a database that retrospectively gathers information regarding patients at risk of polypharmacy, polypharmacy-related ADEs, and cost. Manual adjustments were made to various parameters on the Veterans Integrated Service Network 15 VIONE dashboard by the author in order to obtain data specific to this project. These parameters allowed selection of service sections, specific staff members or the option to include or exclude chronic or nonchronic medications. The annualized cost avoidance figure was then compared to raw data pulled by a VIONE dashboard correspondent to ensure the manual calculation was accurate. Finally, the 5 most common classes of medications deprescribed were identified for information purposes and to provide a better postulation on the types of medications being discontinued using the VIONE method.
Results
A total of 2,442 veterans met inclusion criteria, and the VIONE method was applied to 598 between late October 2018 and January 2019. The 13 PACT pharmacists contacted at least 10 veterans each, thus at least 130 were randomly selected for telephone calls to perform polypharmacy reviews using the VIONE note template. The discontinuation menu was used if a medication qualified to be deprescribed. After 3 months, 1986 prescriptions were deprescribed using VIONE; however, 1060 prescriptions were considered appropriately deprescribed (Table 2). The 13 PACT pharmacists deprescribed 361 medications, and the 29 PACT physicians deprescribed 699 medications. These prescriptions were then separated into medication categories to determine the most common discontinued classes. Vitamins and supplements were the medication class most frequently deprescribed (19.4%), followed by pain medications (15.5%), antimicrobial agents (9.6%), antihypertensive medications (9.2%), and diabetes medications (6.4%) (Table 3). The top 5 medication categories accounted for 60% of all medications appropriately deprescribed.
The estimated annualized cost avoidance for all medications deprescribed in the 3-month project period was $84,030.46. To provide the most appropriate and accurate calculation, medication classes excluded from this figure were acute or short-term prescriptions and antimicrobial agents. Medications prescribed short-term typically are not suitable to continue for an extended period, and antimicrobial agents were excluded since they are normally associated with higher costs, and may overestimate the cost avoidance calculation for the facility.
Discussion
The outcomes for the primary and secondary endpoints of this project illustrate that using VIONE in PACT primary care clinics had a notable impact on polypharmacy and cost avoidance over a short period. This outcome can be attributed to 2 significant effects of using the deprescribing tool. VIONE’s simplicity in application allowed clinicians to incorporate daily use of the tool with minimal effort. Education was all that was required to fully enable clinicians to work together successfully and exercise collaborative practice to promote deprescribing. VIONE also elicited a cascade of favorable effects that improve patient safety and health outcomes. The tool aided in identification of PIM, which helped reduce polypharmacy and medication burden. The risk for DDIs and ADEs may decrease; therefore, the incidence of falls, need for emergency department visits or inpatient care related to polypharmacy may decline. Less complex medication regimens may alleviate issues with adherence and avoid the various consequences of polypharmacy in theory. Simplified regimens can potentially improve disease management and quality of life for patients. Further studies are needed to substantiate deprescribing and its true effect on patient adherence and better health outcomes at this time.10
Reducing polypharmacy can lead to cost savings. Based on the results of this 3-month study, we expect that VASNHS would save more than $84,000 by reducing polypharmacy among its patients. Those savings can be funneled back into the health care system, and allotted to necessary patient care, prescriptions, and health care facility needs.
Limitations
There are some important limitations to this study. Definitions of polypharmacy may vary from one health care facility to another. The cutoffs for polypharmacy may differ, causing the prevalence of polypharmacy and potential costs savings to vary. Use of VIONE may be inconsistent among users if not previously educated or properly trained. For instance, VIONE selections are listed in the same menu as the standard CPRS discontinuation options, which may lead to discontinuation of medical supplies or laboratory orders instead of prescriptions.
The method of data analysis and project design used in this study may have been subject to error. For example, the list of PCPs may have been inaccurate or outdated, which would result in an over- or underrepresentation of those who contributed to data collection. Furthermore, there is some volatility in calculating the total cost avoidance. For example, medications for chronic conditions that were only taken on an as needed basis may have overestimated savings. Either under- or overestimations could occur when parameters are adjusted on the VIONE discontinuation dashboard without appropriate guidance. With the ability to manually adjust the dashboard parameters, dissimilarities in calculations may follow.
Conclusions
The VIONE tool may be useful in improving patient safety through deprescribing and discontinuing PIM. Decreasing the number of medications being taken concomitantly by a patient and continuing only those that are imperative in their medical treatment is the first step to reducing the incidence of polypharmacy. Consequently, chances of ADEs or DDIs are lessened, especially among older individuals who are considered high risk for experiencing the detrimental effects that may ensue. These effects include geriatric-related syndromes, increased risk of fall, hospital visits or admissions, or death. Use of VIONE easily promotes collaboration among clinicians to evaluate medications eligible for discontinuation more regularly. If this deprescribing tool is continuously used, costs avoided can likely be maximized within VA health care systems.
The results of this project should serve as an incentive to push for better prescribing practices and increase deprescribing efforts. It should provoke the need for change in regimens and the subsequent discontinuation of prescriptions that are not considered vital to continue. Finally, the result of this project should substantiate the positive impact a deprescribing tool can possess to avert the issues commonly associated with polypharmacy.
According to the Centers for Disease Control and Prevention National Center for Health Statistics (NCHS), the use of prescription drugs has increased in the past half century. Although prescription drugs have played an important role in preventing, controlling, and delaying onset or progression of disease, their growth in use also has posed many risks.1 One ramification of this growth is the occurrence of polypharmacy, which does not have a universal, clear definition. In general, it can be described as the concurrent use of multiple medications by a single patient to treat one or more medical ailments. Five or more medications taken simultaneously is the most common definition to date, but this is just one of many acceptable definitions and that varies from one health care facility to another.1,2
Regardless of the cutoffs established to indicate polypharmacy, its incidence can result in poor and potentially harmful health outcomes. Polypharmacy increases the risk of experiencing adverse drug events (ADEs), drug-drug interactions (DDIs), geriatric-related syndromes, falls, hospitalization, and mortality. Issues with adherence may begin to unfold secondary to increased pill burden. Both the patient and the health care system may encounter financial strain, as polypharmacy can lead to unnecessary and essentially preventable costs of care. When evaluating the likelihood of polypharmacy based on age group, NCHS found that 47.5% of patients taking ≥ 5 medications were aged ≥ 65 years.1-5 This indicates that polypharmacy is of great concern in the geriatric population, which also represents a large proportion of individuals accessing Veterans Health Administration (VHA) care.
Deprescibing
Deprescribing is the act of withdrawing or discontinuing potentially inappropriate medications (PIM), or medications used by older patients harboring ADEs that generally outweigh the clinical benefits of the drug. Deprescribing is an effective tool for managing or reducing polypharmacy. A variety of tools have been created whose sole purpose is to simplify deprescribing. Some tools explicitly identify PIM and are widely familiar in medical practice. Examples are the Beers Criteria developed in 1991 or Screening Tool to Alert Right Treatment/Screening Tool of Older Persons Prescriptions (START/STOPP) criteria created in 2003. Other tools that are less commonplace but equally as resourceful are MedStopper and Deprescribing.org. The former was launched in 2015 and is a Canadian online system that provides risk assessments for medications with guidance for tapering or stopping medications if continuation of the drug presents higher risk than benefit.5-7 The latter is a full-fledged website developed by a physician, a pharmacist, and their research teams that serves as an exchange hub for deprescribing information.
In 2016, the VIONE (Vital, Important, Optional, Not indicated/treatment complete, and Every medication has an indication) deprescribing tool was developed by Saraswathy Battar, MD, at Central Arkansas Veterans Healthcare System (CAVHS) in Little Rock, as a system that could go beyond medication reconciliation (Table 1). Health care providers (HCPs) and pharmacists evaluate each medication that a patient has been prescribed and places each medication in a VIONE category. Prescribers may then take the opportunity to deprescribe or discontinue medications if deemed appropriate based on their clinical assessments and shared decision making.8 Traditionally, medication reconciliation involves the process of obtaining a complete and accurate list of medications as reported by a patient or caregiver to a HCP. VIONE encourages HCPs and pharmacists not only to ensure medication lists are accurate, but also that each medication reported is appropriate for continued use. In other words, VIONE is meant to help implement deprescribing at opportune times. More than 14,000 medications have been deprescribed using the VIONE method, resulting in more than $2,000,000 of annualized cost avoidance after just 1 year of implementation at CAVHS.9
VIONE consists of 2 major components in the Computerized Patient Record System (CPRS): a template and a dropdown discontinuation menu. The template captured patient allergies, pertinent laboratory data, the patient’s active problem list and applicable diagnoses, and active medication list. Patient aligned care team (PACT) pharmacists used the information captured in the template to conduct medication reconciliations and polypharmacy reviews. Each medication is categorized in VIONE using data collected during reviews. A menu delineates reasons for discontinuation: optional, dose decrease, no diagnosis, not indicated/treatment complete, discontinue alternate medication prescribed, and patient reported no longer taking. The discontinuation menu allowed PACT pharmacists and physicians to choose 1 VIONE option per medication to clarify the reason for discontinuation. VIONE-based discontinuations are recorded in CPRS and identified as deprescribed.
At the time of this project, > 30 US Department of Veterans Affairs (VA) facilities had adopted VIONE. Use of VIONE at VA Southern Nevada Healthcare System (VASNHS) in North Las Vegas has been incorporated in the everyday practices of home-based primary care pharmacists and physicians but has yet to be implemented in other areas of the facility. The purpose of this project was to determine the impact of the VIONE tool on polypharmacy and cost avoidance at VASNHS when used by primary care physicians (PCPs) and PACT primary care clinics.
Methods
Veterans receiving care at VASNHS aged ≥ 65 years with ≥ 10 active medications noted in CPRS were included in this project. PACT pharmacists and physicians were educated on the proper use of the VIONE tool prior to its implementation. Education included a 15-minute slide presentation followed by dissemination of a 1-page VIONE tool handout during a PACT all-staff clinic meeting.
Data were collected for 3 months before and after the intervention. Data were made available for assessment by the Automated Data Processing Application Coordinator (ADPAC) at VASNHS. The ADPAC created and generated an Excel spreadsheet report, which listed all medications deprescribed using the VIONE method. The primary endpoint was the total number of medications discontinued using the VIONE template and/or discontinuation menu. For the purpose of this project, appropriate discontinuation was considered any prescription deprescribed, excluding medical supplies, by pharmacists and PCPs who received VIONE education.
The secondary endpoint was the estimated annualized cost avoidance for the facility (Figure). The calculation does not include medications discontinued due to the prescription of an alternative medication or dose decreases since these VIONE selections imply that a new prescription or order was placed and the original prescription was not deprescribed. Annualized cost avoidance was determined with use of the VIONE dashboard, a database that retrospectively gathers information regarding patients at risk of polypharmacy, polypharmacy-related ADEs, and cost. Manual adjustments were made to various parameters on the Veterans Integrated Service Network 15 VIONE dashboard by the author in order to obtain data specific to this project. These parameters allowed selection of service sections, specific staff members or the option to include or exclude chronic or nonchronic medications. The annualized cost avoidance figure was then compared to raw data pulled by a VIONE dashboard correspondent to ensure the manual calculation was accurate. Finally, the 5 most common classes of medications deprescribed were identified for information purposes and to provide a better postulation on the types of medications being discontinued using the VIONE method.
Results
A total of 2,442 veterans met inclusion criteria, and the VIONE method was applied to 598 between late October 2018 and January 2019. The 13 PACT pharmacists contacted at least 10 veterans each, thus at least 130 were randomly selected for telephone calls to perform polypharmacy reviews using the VIONE note template. The discontinuation menu was used if a medication qualified to be deprescribed. After 3 months, 1986 prescriptions were deprescribed using VIONE; however, 1060 prescriptions were considered appropriately deprescribed (Table 2). The 13 PACT pharmacists deprescribed 361 medications, and the 29 PACT physicians deprescribed 699 medications. These prescriptions were then separated into medication categories to determine the most common discontinued classes. Vitamins and supplements were the medication class most frequently deprescribed (19.4%), followed by pain medications (15.5%), antimicrobial agents (9.6%), antihypertensive medications (9.2%), and diabetes medications (6.4%) (Table 3). The top 5 medication categories accounted for 60% of all medications appropriately deprescribed.
The estimated annualized cost avoidance for all medications deprescribed in the 3-month project period was $84,030.46. To provide the most appropriate and accurate calculation, medication classes excluded from this figure were acute or short-term prescriptions and antimicrobial agents. Medications prescribed short-term typically are not suitable to continue for an extended period, and antimicrobial agents were excluded since they are normally associated with higher costs, and may overestimate the cost avoidance calculation for the facility.
Discussion
The outcomes for the primary and secondary endpoints of this project illustrate that using VIONE in PACT primary care clinics had a notable impact on polypharmacy and cost avoidance over a short period. This outcome can be attributed to 2 significant effects of using the deprescribing tool. VIONE’s simplicity in application allowed clinicians to incorporate daily use of the tool with minimal effort. Education was all that was required to fully enable clinicians to work together successfully and exercise collaborative practice to promote deprescribing. VIONE also elicited a cascade of favorable effects that improve patient safety and health outcomes. The tool aided in identification of PIM, which helped reduce polypharmacy and medication burden. The risk for DDIs and ADEs may decrease; therefore, the incidence of falls, need for emergency department visits or inpatient care related to polypharmacy may decline. Less complex medication regimens may alleviate issues with adherence and avoid the various consequences of polypharmacy in theory. Simplified regimens can potentially improve disease management and quality of life for patients. Further studies are needed to substantiate deprescribing and its true effect on patient adherence and better health outcomes at this time.10
Reducing polypharmacy can lead to cost savings. Based on the results of this 3-month study, we expect that VASNHS would save more than $84,000 by reducing polypharmacy among its patients. Those savings can be funneled back into the health care system, and allotted to necessary patient care, prescriptions, and health care facility needs.
Limitations
There are some important limitations to this study. Definitions of polypharmacy may vary from one health care facility to another. The cutoffs for polypharmacy may differ, causing the prevalence of polypharmacy and potential costs savings to vary. Use of VIONE may be inconsistent among users if not previously educated or properly trained. For instance, VIONE selections are listed in the same menu as the standard CPRS discontinuation options, which may lead to discontinuation of medical supplies or laboratory orders instead of prescriptions.
The method of data analysis and project design used in this study may have been subject to error. For example, the list of PCPs may have been inaccurate or outdated, which would result in an over- or underrepresentation of those who contributed to data collection. Furthermore, there is some volatility in calculating the total cost avoidance. For example, medications for chronic conditions that were only taken on an as needed basis may have overestimated savings. Either under- or overestimations could occur when parameters are adjusted on the VIONE discontinuation dashboard without appropriate guidance. With the ability to manually adjust the dashboard parameters, dissimilarities in calculations may follow.
Conclusions
The VIONE tool may be useful in improving patient safety through deprescribing and discontinuing PIM. Decreasing the number of medications being taken concomitantly by a patient and continuing only those that are imperative in their medical treatment is the first step to reducing the incidence of polypharmacy. Consequently, chances of ADEs or DDIs are lessened, especially among older individuals who are considered high risk for experiencing the detrimental effects that may ensue. These effects include geriatric-related syndromes, increased risk of fall, hospital visits or admissions, or death. Use of VIONE easily promotes collaboration among clinicians to evaluate medications eligible for discontinuation more regularly. If this deprescribing tool is continuously used, costs avoided can likely be maximized within VA health care systems.
The results of this project should serve as an incentive to push for better prescribing practices and increase deprescribing efforts. It should provoke the need for change in regimens and the subsequent discontinuation of prescriptions that are not considered vital to continue. Finally, the result of this project should substantiate the positive impact a deprescribing tool can possess to avert the issues commonly associated with polypharmacy.
1. Centers for Disease Control and Prevention, National Center for Health Statistics. Health, United States, 2013: with special feature on prescription drugs. Published May 2014. Accessed May 13, 2021. https://www.cdc.gov/nchs/data/hus/hus13.pdf
2. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. Published 2017 Oct 10. doi:10.1186/s12877-017-0621-2
3. Parulekar MS, Rogers CK. Polypharmacy and mobility. In: Cifu DX, Lew HL, Oh-Park M., eds Geriatric Rehabilitation. Elsevier; 2018. doi:10.1016/B978-0-323-54454-2.12001-1
4. Rieckert A, Trampisch US, Klaaßen-Mielke R, et al. Polypharmacy in older patients with chronic diseases: a cross-sectional analysis of factors associated with excessive polypharmacy. BMC Fam Pract. 2018;19(1):113. Published 2018 Jul 18. doi:10.1186/s12875-018-0795-5
5. Thompson CA. New medication review method cuts veterans’ Rx load, saves millions. Am J Health Syst Pharm. 2018;75(8):502-503. doi:10.2146/news180023
6. Reeve E. Deprescribing tools: a review of the types of tools available to aid deprescribing in clinical practice. J Pharm Pract Res. 2020;50(1):98-107. doi:10.1002/jppr.1626
7. Fried TR, Niehoff KM, Street RL, et al. Effect of the Tool to Reduce Inappropriate Medications on Medication Communication and Deprescribing. J Am Geriatr Soc. 2017;65(10):2265-2271. doi:10.1111/jgs.15042
8. Battar S, Dickerson KR, Sedgwick C, et al. Understanding principles of high reliability organizations through the eyes of VIONE, a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
9. Battar S, Cmelik T, Dickerson K, Scott, M. Experience better health with VIONE a safe medication deprescribing tool [Nonpublic source, not verified]
10. Ulley J, Harrop D, Ali A, et al. Desprescribing interventions and their impact on medication adherence in community-dwelling older adults with polypharmacy: a systematic review. BMC Geriatr. 2019;19(15):1-13.
1. Centers for Disease Control and Prevention, National Center for Health Statistics. Health, United States, 2013: with special feature on prescription drugs. Published May 2014. Accessed May 13, 2021. https://www.cdc.gov/nchs/data/hus/hus13.pdf
2. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. Published 2017 Oct 10. doi:10.1186/s12877-017-0621-2
3. Parulekar MS, Rogers CK. Polypharmacy and mobility. In: Cifu DX, Lew HL, Oh-Park M., eds Geriatric Rehabilitation. Elsevier; 2018. doi:10.1016/B978-0-323-54454-2.12001-1
4. Rieckert A, Trampisch US, Klaaßen-Mielke R, et al. Polypharmacy in older patients with chronic diseases: a cross-sectional analysis of factors associated with excessive polypharmacy. BMC Fam Pract. 2018;19(1):113. Published 2018 Jul 18. doi:10.1186/s12875-018-0795-5
5. Thompson CA. New medication review method cuts veterans’ Rx load, saves millions. Am J Health Syst Pharm. 2018;75(8):502-503. doi:10.2146/news180023
6. Reeve E. Deprescribing tools: a review of the types of tools available to aid deprescribing in clinical practice. J Pharm Pract Res. 2020;50(1):98-107. doi:10.1002/jppr.1626
7. Fried TR, Niehoff KM, Street RL, et al. Effect of the Tool to Reduce Inappropriate Medications on Medication Communication and Deprescribing. J Am Geriatr Soc. 2017;65(10):2265-2271. doi:10.1111/jgs.15042
8. Battar S, Dickerson KR, Sedgwick C, et al. Understanding principles of high reliability organizations through the eyes of VIONE, a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
9. Battar S, Cmelik T, Dickerson K, Scott, M. Experience better health with VIONE a safe medication deprescribing tool [Nonpublic source, not verified]
10. Ulley J, Harrop D, Ali A, et al. Desprescribing interventions and their impact on medication adherence in community-dwelling older adults with polypharmacy: a systematic review. BMC Geriatr. 2019;19(15):1-13.
FDA updates label for controversial Alzheimer’s drug aducanumab (Aduhelm)
– the group studied in the clinical trials.
The FDA approved aducanumab in early June amid significant controversy and disregarding the recommendation by its own advisory panel not to approve the drug. The original prescribing information implied that the drug – which is administered intravenously and costs around $56,000 a year – could be used for treatment of any patient with Alzheimer’s disease.
The updated label now states that aducanumab should be initiated only in patients with mild cognitive impairment (MCI) or mild dementia stage of disease – the population in which treatment was initiated in the clinical trials leading to approval of the anti-amyloid drug.
The FDA granted accelerated approval of the drug based on data from clinical trials showing a reduction in amyloid beta plaques observed in patients with MCI or mild dementia stage of disease.
“Continued approval for the indication may be contingent upon verification of clinical benefit in confirmatory trial(s),” the label states. It emphasizes that there are no safety or effectiveness data on starting aducanumab treatment at earlier or later stages of the disease than were studied.
“Based on our ongoing conversations with prescribing physicians, FDA, and patient advocates, we submitted this label update with the goal to further clarify the patient population that was studied across the three Aduhelm clinical trials that supported approval,” Alfred Sandrock Jr., MD, PhD, Biogen’s head of research and development, said in a statement announcing the label update.
“We are committed to continue to listen to the community’s needs as clinical practice adapts to this important, first-in-class treatment option,” said Dr. Sandrock.
A version of this article first appeared on Medscape.com.
– the group studied in the clinical trials.
The FDA approved aducanumab in early June amid significant controversy and disregarding the recommendation by its own advisory panel not to approve the drug. The original prescribing information implied that the drug – which is administered intravenously and costs around $56,000 a year – could be used for treatment of any patient with Alzheimer’s disease.
The updated label now states that aducanumab should be initiated only in patients with mild cognitive impairment (MCI) or mild dementia stage of disease – the population in which treatment was initiated in the clinical trials leading to approval of the anti-amyloid drug.
The FDA granted accelerated approval of the drug based on data from clinical trials showing a reduction in amyloid beta plaques observed in patients with MCI or mild dementia stage of disease.
“Continued approval for the indication may be contingent upon verification of clinical benefit in confirmatory trial(s),” the label states. It emphasizes that there are no safety or effectiveness data on starting aducanumab treatment at earlier or later stages of the disease than were studied.
“Based on our ongoing conversations with prescribing physicians, FDA, and patient advocates, we submitted this label update with the goal to further clarify the patient population that was studied across the three Aduhelm clinical trials that supported approval,” Alfred Sandrock Jr., MD, PhD, Biogen’s head of research and development, said in a statement announcing the label update.
“We are committed to continue to listen to the community’s needs as clinical practice adapts to this important, first-in-class treatment option,” said Dr. Sandrock.
A version of this article first appeared on Medscape.com.
– the group studied in the clinical trials.
The FDA approved aducanumab in early June amid significant controversy and disregarding the recommendation by its own advisory panel not to approve the drug. The original prescribing information implied that the drug – which is administered intravenously and costs around $56,000 a year – could be used for treatment of any patient with Alzheimer’s disease.
The updated label now states that aducanumab should be initiated only in patients with mild cognitive impairment (MCI) or mild dementia stage of disease – the population in which treatment was initiated in the clinical trials leading to approval of the anti-amyloid drug.
The FDA granted accelerated approval of the drug based on data from clinical trials showing a reduction in amyloid beta plaques observed in patients with MCI or mild dementia stage of disease.
“Continued approval for the indication may be contingent upon verification of clinical benefit in confirmatory trial(s),” the label states. It emphasizes that there are no safety or effectiveness data on starting aducanumab treatment at earlier or later stages of the disease than were studied.
“Based on our ongoing conversations with prescribing physicians, FDA, and patient advocates, we submitted this label update with the goal to further clarify the patient population that was studied across the three Aduhelm clinical trials that supported approval,” Alfred Sandrock Jr., MD, PhD, Biogen’s head of research and development, said in a statement announcing the label update.
“We are committed to continue to listen to the community’s needs as clinical practice adapts to this important, first-in-class treatment option,” said Dr. Sandrock.
A version of this article first appeared on Medscape.com.
Extra COVID-19 vaccine could help immunocompromised people
People whose immune systems are compromised by therapy or disease may benefit from additional doses of vaccines against SARS-CoV-2, researchers say.
In a study involving 101 people with solid-organ transplants, there was a significant boost in antibodies after the patients received third doses of the Pfizer vaccine, said Nassim Kamar, MD, PhD, professor of nephrology at Toulouse University Hospital, France.
None of the transplant patients had antibodies against the virus before their first dose of the vaccine, and only 4% produced antibodies after the first dose. That proportion rose to 40% after the second dose and to 68% after the third dose.
The effect is so strong that Dr. Kamar and colleagues at Toulouse University Hospital routinely administer three doses of mRNA vaccines to patients with solid-organ transplant without testing them for antibodies.
“When we observed that the second dose was not sufficient to have an immune response, the Francophone Society of Transplantation asked the National Health Authority to allow the third dose,” he told this news organization.
That agency on April 11 approved third doses of mRNA vaccines not only for people with solid-organ transplants but also for those with recent bone marrow transplants, those undergoing dialysis, and those with autoimmune diseases who were receiving strong immunosuppressive treatment, such as anti-CD20 or antimetabolites. Contrary to their procedure for people with solid-organ transplants, clinicians at Toulouse University Hospital test these patients for antibodies and administer third doses of vaccine only to those who test negative or have very low titers.
The researchers’ findings, published on June 23 as a letter to the editor of The New England Journal of Medicine, come as other researchers document more and more categories of patients whose responses to the vaccines typically fall short.
A study at the University of Pittsburgh that was published as a preprint on MedRxiv compared people with various health conditions to healthy health care workers. People with HIV who were taking antivirals against that virus responded almost as well as did the health care workers, said John Mellors, MD, chief of infectious diseases at the university. But people whose immune systems were compromised for other reasons fared less well.
“The areas of concern are hematological malignancy and solid-organ transplants, with the most nonresponsive groups being those who have had lung transplantation,” he said in an interview.
For patients with liver disease, mixed news came from the International Liver Congress (ILC) 2021 annual meeting.
In a study involving patients with liver disease who had received the Pfizer vaccine at Hadassah University Medical Center in Jerusalem, antibody titers were lower in patients who had received liver transplants or who had advanced liver fibrosis, as reported by this news organization.
A multicenter study in China that was presented at the ILC meeting and that was also published in the Journal of Hepatology, provided a more optimistic picture. Among patients with nonalcoholic fatty liver disease who were immunized against SARS-CoV-2 with the Sinopharm vaccine, 95.5% had neutralizing antibodies; the median neutralizing antibody titer was 32.
In the Toulouse University Hospital study, for the 40 patients who were seropositive after the second dose, antibody titers increased from 36 before the third dose to 2,676 a month after the third dose, a statistically significant result (P < .001).
For patients whose immune systems are compromised for reasons other than having received a transplant, clinicians at Toulouse University Hospital use a titer of 14 as the threshold below which they administer a third dose of mRNA vaccines. But Dr. Kamar acknowledged that the threshold is arbitrary and that the assays for antibodies with different vaccines in different populations can’t be compared head to head.
“We can’t tell you simply on the basis of the amount of antibody in your laboratory report how protected you are,” agreed William Schaffner, MD, professor of infectious diseases at Vanderbilt University, Nashville, Tenn., who is a spokesperson for the Infectious Diseases Society of America.
Not enough research has been done to establish that relationship, and results vary from one laboratory to another, he said.
That doesn’t mean that antibody tests don’t help, Dr. Schaffner said. On the basis of views of other experts he has consulted, Dr. Schaffner recommends that people who are immunocompromised undergo an antibody test. If the test is positive – meaning they have some antibodies to SARS-CoV-2, however low the titers – patients can take fewer precautions than before they were vaccinated.
But they should still be more cautious than people with healthy immune systems, he said. “Would I be going to large indoor gatherings without a mask? No. Would I be outside without a mask? Yes. Would I gather with three other people who are vaccinated to play a game of bridge? Yes. You have to titrate things a little and use some common sense,” he added.
If the results are negative, such patients may still be protected. Much research remains to be done on T-cell immunity, a second line of defense against the virus. And the current assays often produce false negative results. But to be on the safe side, people with this result should assume that their vaccine is not protecting them, Dr. Schaffner said.
That suggestion contradicts the Food and Drug Administration, which issued a recommendation on May 19 against using antibody tests to check the effectiveness of SARS-CoV-2 vaccination.
The studies so far suggest that vaccines are safe for people whose immune systems are compromised, Dr. Schaffner and Dr. Kamar agreed. Dr. Kamar is aware of only two case reports of transplant patients rejecting their transplants after vaccination. One of these was his own patient, and the rejection occurred after her second dose. She has not needed dialysis, although her kidney function was impaired.
But the FDA has not approved additional doses of SARS-CoV-2 vaccine to treat patients who are immunocompromised, and Dr. Kamar has not heard of any other national regulatory agencies that have.
In the United States, it may be difficult for anyone to obtain a third dose of vaccine outside of a clinical trial, Dr. Schaffner said, because vaccinators are likely to check databases and deny vaccination to anyone who has already received the recommended number.
Dr. Kamar, Dr. Mellors, and Dr. Schaffner have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People whose immune systems are compromised by therapy or disease may benefit from additional doses of vaccines against SARS-CoV-2, researchers say.
In a study involving 101 people with solid-organ transplants, there was a significant boost in antibodies after the patients received third doses of the Pfizer vaccine, said Nassim Kamar, MD, PhD, professor of nephrology at Toulouse University Hospital, France.
None of the transplant patients had antibodies against the virus before their first dose of the vaccine, and only 4% produced antibodies after the first dose. That proportion rose to 40% after the second dose and to 68% after the third dose.
The effect is so strong that Dr. Kamar and colleagues at Toulouse University Hospital routinely administer three doses of mRNA vaccines to patients with solid-organ transplant without testing them for antibodies.
“When we observed that the second dose was not sufficient to have an immune response, the Francophone Society of Transplantation asked the National Health Authority to allow the third dose,” he told this news organization.
That agency on April 11 approved third doses of mRNA vaccines not only for people with solid-organ transplants but also for those with recent bone marrow transplants, those undergoing dialysis, and those with autoimmune diseases who were receiving strong immunosuppressive treatment, such as anti-CD20 or antimetabolites. Contrary to their procedure for people with solid-organ transplants, clinicians at Toulouse University Hospital test these patients for antibodies and administer third doses of vaccine only to those who test negative or have very low titers.
The researchers’ findings, published on June 23 as a letter to the editor of The New England Journal of Medicine, come as other researchers document more and more categories of patients whose responses to the vaccines typically fall short.
A study at the University of Pittsburgh that was published as a preprint on MedRxiv compared people with various health conditions to healthy health care workers. People with HIV who were taking antivirals against that virus responded almost as well as did the health care workers, said John Mellors, MD, chief of infectious diseases at the university. But people whose immune systems were compromised for other reasons fared less well.
“The areas of concern are hematological malignancy and solid-organ transplants, with the most nonresponsive groups being those who have had lung transplantation,” he said in an interview.
For patients with liver disease, mixed news came from the International Liver Congress (ILC) 2021 annual meeting.
In a study involving patients with liver disease who had received the Pfizer vaccine at Hadassah University Medical Center in Jerusalem, antibody titers were lower in patients who had received liver transplants or who had advanced liver fibrosis, as reported by this news organization.
A multicenter study in China that was presented at the ILC meeting and that was also published in the Journal of Hepatology, provided a more optimistic picture. Among patients with nonalcoholic fatty liver disease who were immunized against SARS-CoV-2 with the Sinopharm vaccine, 95.5% had neutralizing antibodies; the median neutralizing antibody titer was 32.
In the Toulouse University Hospital study, for the 40 patients who were seropositive after the second dose, antibody titers increased from 36 before the third dose to 2,676 a month after the third dose, a statistically significant result (P < .001).
For patients whose immune systems are compromised for reasons other than having received a transplant, clinicians at Toulouse University Hospital use a titer of 14 as the threshold below which they administer a third dose of mRNA vaccines. But Dr. Kamar acknowledged that the threshold is arbitrary and that the assays for antibodies with different vaccines in different populations can’t be compared head to head.
“We can’t tell you simply on the basis of the amount of antibody in your laboratory report how protected you are,” agreed William Schaffner, MD, professor of infectious diseases at Vanderbilt University, Nashville, Tenn., who is a spokesperson for the Infectious Diseases Society of America.
Not enough research has been done to establish that relationship, and results vary from one laboratory to another, he said.
That doesn’t mean that antibody tests don’t help, Dr. Schaffner said. On the basis of views of other experts he has consulted, Dr. Schaffner recommends that people who are immunocompromised undergo an antibody test. If the test is positive – meaning they have some antibodies to SARS-CoV-2, however low the titers – patients can take fewer precautions than before they were vaccinated.
But they should still be more cautious than people with healthy immune systems, he said. “Would I be going to large indoor gatherings without a mask? No. Would I be outside without a mask? Yes. Would I gather with three other people who are vaccinated to play a game of bridge? Yes. You have to titrate things a little and use some common sense,” he added.
If the results are negative, such patients may still be protected. Much research remains to be done on T-cell immunity, a second line of defense against the virus. And the current assays often produce false negative results. But to be on the safe side, people with this result should assume that their vaccine is not protecting them, Dr. Schaffner said.
That suggestion contradicts the Food and Drug Administration, which issued a recommendation on May 19 against using antibody tests to check the effectiveness of SARS-CoV-2 vaccination.
The studies so far suggest that vaccines are safe for people whose immune systems are compromised, Dr. Schaffner and Dr. Kamar agreed. Dr. Kamar is aware of only two case reports of transplant patients rejecting their transplants after vaccination. One of these was his own patient, and the rejection occurred after her second dose. She has not needed dialysis, although her kidney function was impaired.
But the FDA has not approved additional doses of SARS-CoV-2 vaccine to treat patients who are immunocompromised, and Dr. Kamar has not heard of any other national regulatory agencies that have.
In the United States, it may be difficult for anyone to obtain a third dose of vaccine outside of a clinical trial, Dr. Schaffner said, because vaccinators are likely to check databases and deny vaccination to anyone who has already received the recommended number.
Dr. Kamar, Dr. Mellors, and Dr. Schaffner have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People whose immune systems are compromised by therapy or disease may benefit from additional doses of vaccines against SARS-CoV-2, researchers say.
In a study involving 101 people with solid-organ transplants, there was a significant boost in antibodies after the patients received third doses of the Pfizer vaccine, said Nassim Kamar, MD, PhD, professor of nephrology at Toulouse University Hospital, France.
None of the transplant patients had antibodies against the virus before their first dose of the vaccine, and only 4% produced antibodies after the first dose. That proportion rose to 40% after the second dose and to 68% after the third dose.
The effect is so strong that Dr. Kamar and colleagues at Toulouse University Hospital routinely administer three doses of mRNA vaccines to patients with solid-organ transplant without testing them for antibodies.
“When we observed that the second dose was not sufficient to have an immune response, the Francophone Society of Transplantation asked the National Health Authority to allow the third dose,” he told this news organization.
That agency on April 11 approved third doses of mRNA vaccines not only for people with solid-organ transplants but also for those with recent bone marrow transplants, those undergoing dialysis, and those with autoimmune diseases who were receiving strong immunosuppressive treatment, such as anti-CD20 or antimetabolites. Contrary to their procedure for people with solid-organ transplants, clinicians at Toulouse University Hospital test these patients for antibodies and administer third doses of vaccine only to those who test negative or have very low titers.
The researchers’ findings, published on June 23 as a letter to the editor of The New England Journal of Medicine, come as other researchers document more and more categories of patients whose responses to the vaccines typically fall short.
A study at the University of Pittsburgh that was published as a preprint on MedRxiv compared people with various health conditions to healthy health care workers. People with HIV who were taking antivirals against that virus responded almost as well as did the health care workers, said John Mellors, MD, chief of infectious diseases at the university. But people whose immune systems were compromised for other reasons fared less well.
“The areas of concern are hematological malignancy and solid-organ transplants, with the most nonresponsive groups being those who have had lung transplantation,” he said in an interview.
For patients with liver disease, mixed news came from the International Liver Congress (ILC) 2021 annual meeting.
In a study involving patients with liver disease who had received the Pfizer vaccine at Hadassah University Medical Center in Jerusalem, antibody titers were lower in patients who had received liver transplants or who had advanced liver fibrosis, as reported by this news organization.
A multicenter study in China that was presented at the ILC meeting and that was also published in the Journal of Hepatology, provided a more optimistic picture. Among patients with nonalcoholic fatty liver disease who were immunized against SARS-CoV-2 with the Sinopharm vaccine, 95.5% had neutralizing antibodies; the median neutralizing antibody titer was 32.
In the Toulouse University Hospital study, for the 40 patients who were seropositive after the second dose, antibody titers increased from 36 before the third dose to 2,676 a month after the third dose, a statistically significant result (P < .001).
For patients whose immune systems are compromised for reasons other than having received a transplant, clinicians at Toulouse University Hospital use a titer of 14 as the threshold below which they administer a third dose of mRNA vaccines. But Dr. Kamar acknowledged that the threshold is arbitrary and that the assays for antibodies with different vaccines in different populations can’t be compared head to head.
“We can’t tell you simply on the basis of the amount of antibody in your laboratory report how protected you are,” agreed William Schaffner, MD, professor of infectious diseases at Vanderbilt University, Nashville, Tenn., who is a spokesperson for the Infectious Diseases Society of America.
Not enough research has been done to establish that relationship, and results vary from one laboratory to another, he said.
That doesn’t mean that antibody tests don’t help, Dr. Schaffner said. On the basis of views of other experts he has consulted, Dr. Schaffner recommends that people who are immunocompromised undergo an antibody test. If the test is positive – meaning they have some antibodies to SARS-CoV-2, however low the titers – patients can take fewer precautions than before they were vaccinated.
But they should still be more cautious than people with healthy immune systems, he said. “Would I be going to large indoor gatherings without a mask? No. Would I be outside without a mask? Yes. Would I gather with three other people who are vaccinated to play a game of bridge? Yes. You have to titrate things a little and use some common sense,” he added.
If the results are negative, such patients may still be protected. Much research remains to be done on T-cell immunity, a second line of defense against the virus. And the current assays often produce false negative results. But to be on the safe side, people with this result should assume that their vaccine is not protecting them, Dr. Schaffner said.
That suggestion contradicts the Food and Drug Administration, which issued a recommendation on May 19 against using antibody tests to check the effectiveness of SARS-CoV-2 vaccination.
The studies so far suggest that vaccines are safe for people whose immune systems are compromised, Dr. Schaffner and Dr. Kamar agreed. Dr. Kamar is aware of only two case reports of transplant patients rejecting their transplants after vaccination. One of these was his own patient, and the rejection occurred after her second dose. She has not needed dialysis, although her kidney function was impaired.
But the FDA has not approved additional doses of SARS-CoV-2 vaccine to treat patients who are immunocompromised, and Dr. Kamar has not heard of any other national regulatory agencies that have.
In the United States, it may be difficult for anyone to obtain a third dose of vaccine outside of a clinical trial, Dr. Schaffner said, because vaccinators are likely to check databases and deny vaccination to anyone who has already received the recommended number.
Dr. Kamar, Dr. Mellors, and Dr. Schaffner have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.