User login
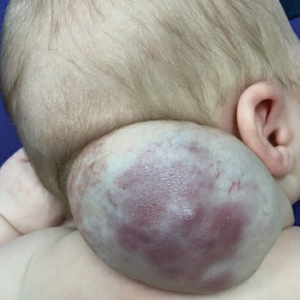
Scarring Head Wound
The Diagnosis: Brunsting-Perry Cicatricial Pemphigoid
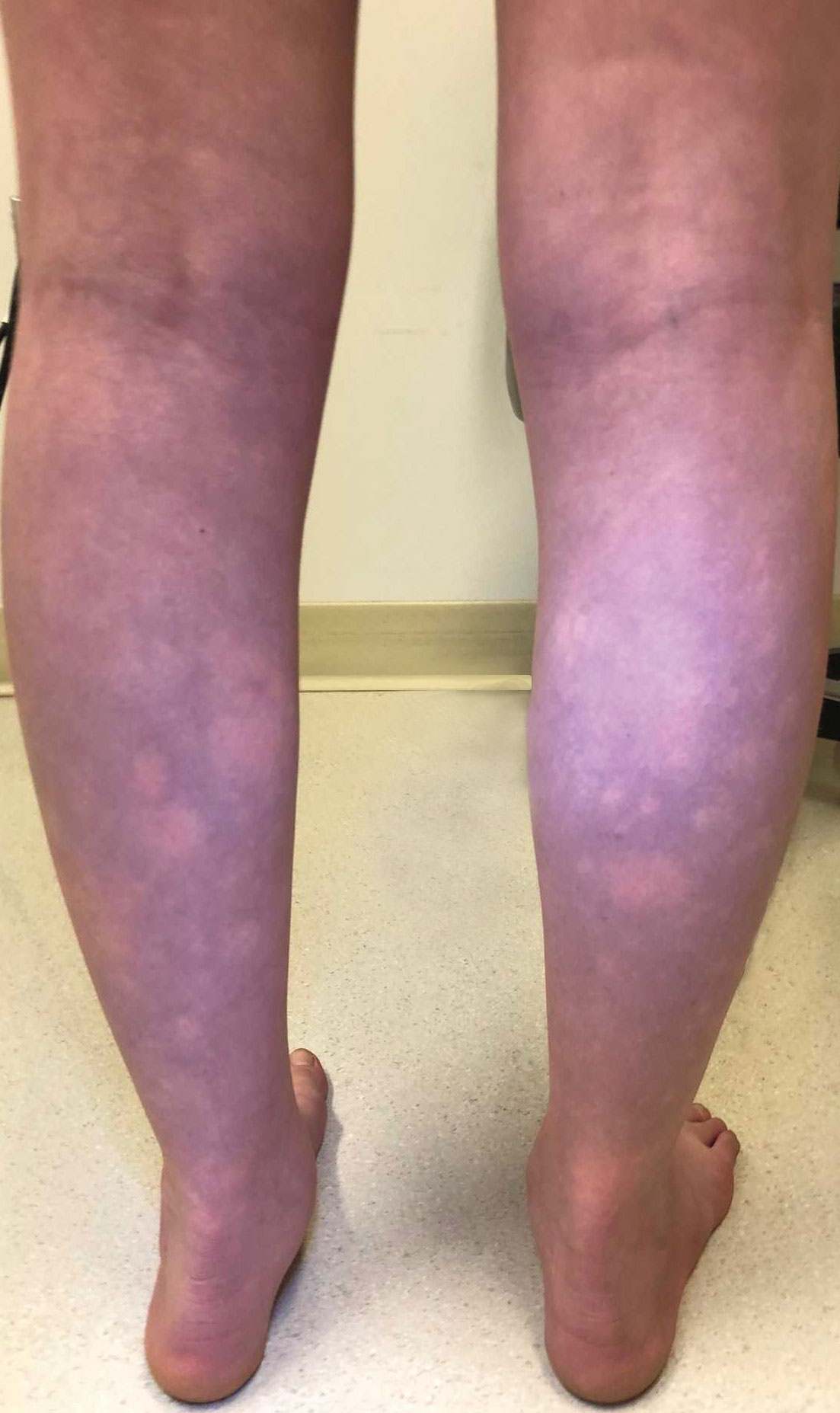
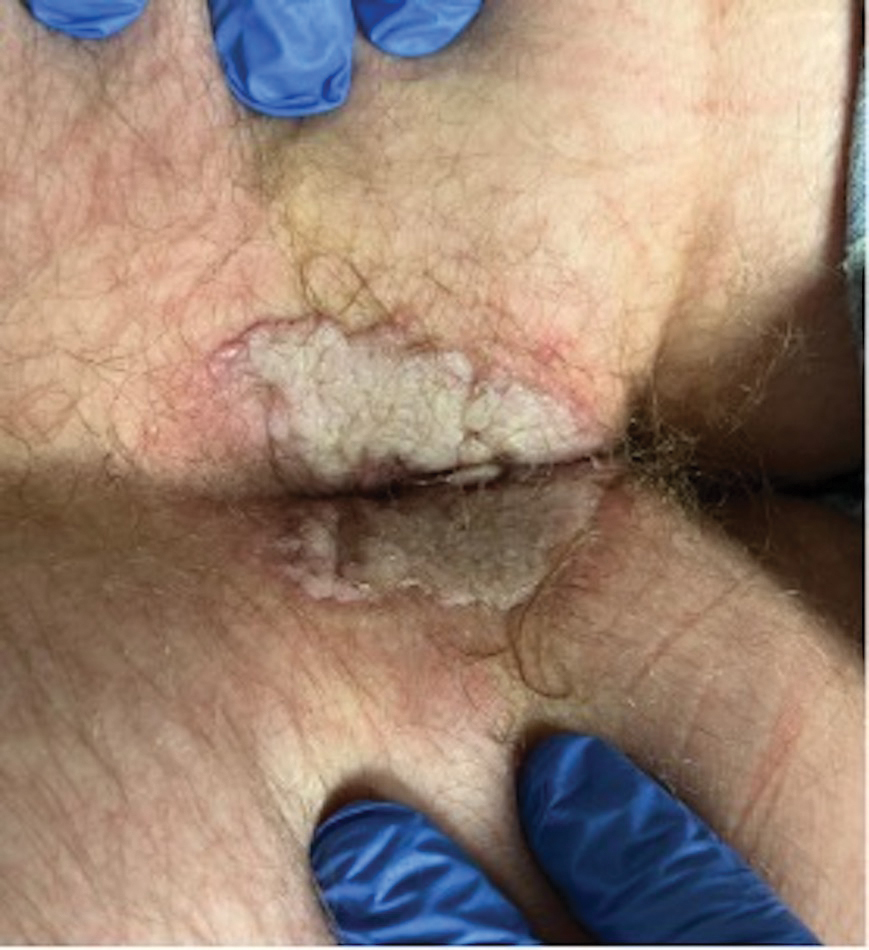
Physical examination and histopathology are paramount in diagnosing Brunsting-Perry cicatricial pemphigoid (BPCP). In our patient, histopathology showed subepidermal blistering with a mixed superficial dermal inflammatory cell infiltrate. Direct immunofluorescence was positive for linear IgG and C3 antibodies along the basement membrane. The scarring erosions on the scalp combined with the autoantibody findings on direct immunofluorescence were consistent with BPCP. He was started on dapsone 100 mg daily and demonstrated complete resolution of symptoms after 10 months, with the exception of persistent scarring hair loss (Figure).

Brunsting-Perry cicatricial pemphigoid is a rare dermatologic condition. It was first defined in 1957 when Brunsting and Perry1 examined 7 patients with cicatricial pemphigoid that predominantly affected the head and neck region, with occasional mucous membrane involvement but no mucosal scarring. Characteristically, BPCP manifests as scarring herpetiform plaques with varied blisters, erosions, crusts, and scarring.1 It primarily affects middle-aged men.2
Historically, BPCP has been considered a variant of cicatricial pemphigoid (now known as mucous membrane pemphigoid), bullous pemphigoid, or epidermolysis bullosa acquisita.3 The antigen target has not been established clearly; however, autoantibodies against laminin 332, collagen VII, and BP180 and BP230 have been proposed.2,4,5 Jacoby et al6 described BPCP on a spectrum with bullous pemphigoid and cicatricial pemphigoid, with primarily circulating autoantibodies on one end and tissue-fixed autoantibodies on the other.
The differential for BPCP also includes anti-p200 pemphigoid and anti–laminin 332 pemphigoid. Anti-p200 pemphigoid also is known as bullous pemphigoid with antibodies against the 200-kDa protein.7 It may clinically manifest similar to bullous pemphigoid and other subepidermal autoimmune blistering diseases; thus, immunopathologic differentiation can be helpful. Anti–laminin 332 pemphigoid (also known as anti–laminin gamma-1 pemphigoid) is characterized by autoantibodies targeting the laminin 332 protein in the basement membrane zone, resulting in blistering and erosions.8 Similar to BPCP and epidermolysis bullosa aquisita, anti–laminin 332 pemphigoid may affect cephalic regions and mucous membrane surfaces, resulting in scarring and cicatricial changes. Anti–laminin 332 pemphigoid also has been associated with internal malignancy.8 The use of the salt-split skin technique can be utilized to differentiate these entities based on their autoantibody-binding patterns in relation to the lamina densa.
Treatment options for mild BPCP include potent topical or intralesional steroids and dapsone, while more severe cases may require systemic therapy with rituximab, azathioprine, mycophenolate mofetil, or cyclophosphamide.4
This case highlights the importance of histopathologic examination of skin lesions with an unusual history or clinical presentation. Dermatologists should consider BPCP when presented with erosions, ulcerations, or blisters of the head and neck in middle-aged male patients.
- Brunsting LA, Perry HO. Benign pemphigoid? a report of seven cases with chronic, scarring, herpetiform plaques about the head and neck. AMA Arch Derm. 1957;75:489-501. doi:10.1001 /archderm.1957.01550160015002
- Jedlickova H, Neidermeier A, Zgažarová S, et al. Brunsting-Perry pemphigoid of the scalp with antibodies against laminin 332. Dermatology. 2011;222:193-195. doi:10.1159/000322842
- Eichhoff G. Brunsting-Perry pemphigoid as differential diagnosis of nonmelanoma skin cancer. Cureus. 2019;11:E5400. doi:10.7759/cureus.5400
- Asfour L, Chong H, Mee J, et al. Epidermolysis bullosa acquisita (Brunsting-Perry pemphigoid variant) localized to the face and diagnosed with antigen identification using skin deficient in type VII collagen. Am J Dermatopathol. 2017;39:e90-e96. doi:10.1097 /DAD.0000000000000829
- Zhou S, Zou Y, Pan M. Brunsting-Perry pemphigoid transitioning from previous bullous pemphigoid. JAAD Case Rep. 2020;6:192-194. doi:10.1016/j.jdcr.2019.12.018
- Jacoby WD Jr, Bartholome CW, Ramchand SC, et al. Cicatricial pemphigoid (Brunsting-Perry type). case report and immunofluorescence findings. Arch Dermatol. 1978;114:779-781. doi:10.1001/archderm.1978.01640170079018
- Kridin K, Ahmed AR. Anti-p200 pemphigoid: a systematic review. Front Immunol. 2019;10:2466. doi:10.3389/fimmu.2019.02466
- Shi L, Li X, Qian H. Anti-laminin 332-type mucous membrane pemphigoid. Biomolecules. 2022;12:1461. doi:10.3390/biom12101461
The Diagnosis: Brunsting-Perry Cicatricial Pemphigoid
Physical examination and histopathology are paramount in diagnosing Brunsting-Perry cicatricial pemphigoid (BPCP). In our patient, histopathology showed subepidermal blistering with a mixed superficial dermal inflammatory cell infiltrate. Direct immunofluorescence was positive for linear IgG and C3 antibodies along the basement membrane. The scarring erosions on the scalp combined with the autoantibody findings on direct immunofluorescence were consistent with BPCP. He was started on dapsone 100 mg daily and demonstrated complete resolution of symptoms after 10 months, with the exception of persistent scarring hair loss (Figure).

Brunsting-Perry cicatricial pemphigoid is a rare dermatologic condition. It was first defined in 1957 when Brunsting and Perry1 examined 7 patients with cicatricial pemphigoid that predominantly affected the head and neck region, with occasional mucous membrane involvement but no mucosal scarring. Characteristically, BPCP manifests as scarring herpetiform plaques with varied blisters, erosions, crusts, and scarring.1 It primarily affects middle-aged men.2
Historically, BPCP has been considered a variant of cicatricial pemphigoid (now known as mucous membrane pemphigoid), bullous pemphigoid, or epidermolysis bullosa acquisita.3 The antigen target has not been established clearly; however, autoantibodies against laminin 332, collagen VII, and BP180 and BP230 have been proposed.2,4,5 Jacoby et al6 described BPCP on a spectrum with bullous pemphigoid and cicatricial pemphigoid, with primarily circulating autoantibodies on one end and tissue-fixed autoantibodies on the other.
The differential for BPCP also includes anti-p200 pemphigoid and anti–laminin 332 pemphigoid. Anti-p200 pemphigoid also is known as bullous pemphigoid with antibodies against the 200-kDa protein.7 It may clinically manifest similar to bullous pemphigoid and other subepidermal autoimmune blistering diseases; thus, immunopathologic differentiation can be helpful. Anti–laminin 332 pemphigoid (also known as anti–laminin gamma-1 pemphigoid) is characterized by autoantibodies targeting the laminin 332 protein in the basement membrane zone, resulting in blistering and erosions.8 Similar to BPCP and epidermolysis bullosa aquisita, anti–laminin 332 pemphigoid may affect cephalic regions and mucous membrane surfaces, resulting in scarring and cicatricial changes. Anti–laminin 332 pemphigoid also has been associated with internal malignancy.8 The use of the salt-split skin technique can be utilized to differentiate these entities based on their autoantibody-binding patterns in relation to the lamina densa.
Treatment options for mild BPCP include potent topical or intralesional steroids and dapsone, while more severe cases may require systemic therapy with rituximab, azathioprine, mycophenolate mofetil, or cyclophosphamide.4
This case highlights the importance of histopathologic examination of skin lesions with an unusual history or clinical presentation. Dermatologists should consider BPCP when presented with erosions, ulcerations, or blisters of the head and neck in middle-aged male patients.
The Diagnosis: Brunsting-Perry Cicatricial Pemphigoid
Physical examination and histopathology are paramount in diagnosing Brunsting-Perry cicatricial pemphigoid (BPCP). In our patient, histopathology showed subepidermal blistering with a mixed superficial dermal inflammatory cell infiltrate. Direct immunofluorescence was positive for linear IgG and C3 antibodies along the basement membrane. The scarring erosions on the scalp combined with the autoantibody findings on direct immunofluorescence were consistent with BPCP. He was started on dapsone 100 mg daily and demonstrated complete resolution of symptoms after 10 months, with the exception of persistent scarring hair loss (Figure).

Brunsting-Perry cicatricial pemphigoid is a rare dermatologic condition. It was first defined in 1957 when Brunsting and Perry1 examined 7 patients with cicatricial pemphigoid that predominantly affected the head and neck region, with occasional mucous membrane involvement but no mucosal scarring. Characteristically, BPCP manifests as scarring herpetiform plaques with varied blisters, erosions, crusts, and scarring.1 It primarily affects middle-aged men.2
Historically, BPCP has been considered a variant of cicatricial pemphigoid (now known as mucous membrane pemphigoid), bullous pemphigoid, or epidermolysis bullosa acquisita.3 The antigen target has not been established clearly; however, autoantibodies against laminin 332, collagen VII, and BP180 and BP230 have been proposed.2,4,5 Jacoby et al6 described BPCP on a spectrum with bullous pemphigoid and cicatricial pemphigoid, with primarily circulating autoantibodies on one end and tissue-fixed autoantibodies on the other.
The differential for BPCP also includes anti-p200 pemphigoid and anti–laminin 332 pemphigoid. Anti-p200 pemphigoid also is known as bullous pemphigoid with antibodies against the 200-kDa protein.7 It may clinically manifest similar to bullous pemphigoid and other subepidermal autoimmune blistering diseases; thus, immunopathologic differentiation can be helpful. Anti–laminin 332 pemphigoid (also known as anti–laminin gamma-1 pemphigoid) is characterized by autoantibodies targeting the laminin 332 protein in the basement membrane zone, resulting in blistering and erosions.8 Similar to BPCP and epidermolysis bullosa aquisita, anti–laminin 332 pemphigoid may affect cephalic regions and mucous membrane surfaces, resulting in scarring and cicatricial changes. Anti–laminin 332 pemphigoid also has been associated with internal malignancy.8 The use of the salt-split skin technique can be utilized to differentiate these entities based on their autoantibody-binding patterns in relation to the lamina densa.
Treatment options for mild BPCP include potent topical or intralesional steroids and dapsone, while more severe cases may require systemic therapy with rituximab, azathioprine, mycophenolate mofetil, or cyclophosphamide.4
This case highlights the importance of histopathologic examination of skin lesions with an unusual history or clinical presentation. Dermatologists should consider BPCP when presented with erosions, ulcerations, or blisters of the head and neck in middle-aged male patients.
- Brunsting LA, Perry HO. Benign pemphigoid? a report of seven cases with chronic, scarring, herpetiform plaques about the head and neck. AMA Arch Derm. 1957;75:489-501. doi:10.1001 /archderm.1957.01550160015002
- Jedlickova H, Neidermeier A, Zgažarová S, et al. Brunsting-Perry pemphigoid of the scalp with antibodies against laminin 332. Dermatology. 2011;222:193-195. doi:10.1159/000322842
- Eichhoff G. Brunsting-Perry pemphigoid as differential diagnosis of nonmelanoma skin cancer. Cureus. 2019;11:E5400. doi:10.7759/cureus.5400
- Asfour L, Chong H, Mee J, et al. Epidermolysis bullosa acquisita (Brunsting-Perry pemphigoid variant) localized to the face and diagnosed with antigen identification using skin deficient in type VII collagen. Am J Dermatopathol. 2017;39:e90-e96. doi:10.1097 /DAD.0000000000000829
- Zhou S, Zou Y, Pan M. Brunsting-Perry pemphigoid transitioning from previous bullous pemphigoid. JAAD Case Rep. 2020;6:192-194. doi:10.1016/j.jdcr.2019.12.018
- Jacoby WD Jr, Bartholome CW, Ramchand SC, et al. Cicatricial pemphigoid (Brunsting-Perry type). case report and immunofluorescence findings. Arch Dermatol. 1978;114:779-781. doi:10.1001/archderm.1978.01640170079018
- Kridin K, Ahmed AR. Anti-p200 pemphigoid: a systematic review. Front Immunol. 2019;10:2466. doi:10.3389/fimmu.2019.02466
- Shi L, Li X, Qian H. Anti-laminin 332-type mucous membrane pemphigoid. Biomolecules. 2022;12:1461. doi:10.3390/biom12101461
- Brunsting LA, Perry HO. Benign pemphigoid? a report of seven cases with chronic, scarring, herpetiform plaques about the head and neck. AMA Arch Derm. 1957;75:489-501. doi:10.1001 /archderm.1957.01550160015002
- Jedlickova H, Neidermeier A, Zgažarová S, et al. Brunsting-Perry pemphigoid of the scalp with antibodies against laminin 332. Dermatology. 2011;222:193-195. doi:10.1159/000322842
- Eichhoff G. Brunsting-Perry pemphigoid as differential diagnosis of nonmelanoma skin cancer. Cureus. 2019;11:E5400. doi:10.7759/cureus.5400
- Asfour L, Chong H, Mee J, et al. Epidermolysis bullosa acquisita (Brunsting-Perry pemphigoid variant) localized to the face and diagnosed with antigen identification using skin deficient in type VII collagen. Am J Dermatopathol. 2017;39:e90-e96. doi:10.1097 /DAD.0000000000000829
- Zhou S, Zou Y, Pan M. Brunsting-Perry pemphigoid transitioning from previous bullous pemphigoid. JAAD Case Rep. 2020;6:192-194. doi:10.1016/j.jdcr.2019.12.018
- Jacoby WD Jr, Bartholome CW, Ramchand SC, et al. Cicatricial pemphigoid (Brunsting-Perry type). case report and immunofluorescence findings. Arch Dermatol. 1978;114:779-781. doi:10.1001/archderm.1978.01640170079018
- Kridin K, Ahmed AR. Anti-p200 pemphigoid: a systematic review. Front Immunol. 2019;10:2466. doi:10.3389/fimmu.2019.02466
- Shi L, Li X, Qian H. Anti-laminin 332-type mucous membrane pemphigoid. Biomolecules. 2022;12:1461. doi:10.3390/biom12101461
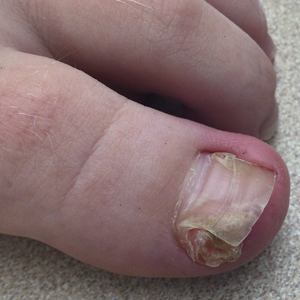
A 60-year-old man presented to a dermatology clinic with a wound on the scalp that had persisted for 11 months. The lesion started as a small erosion that eventually progressed to involve the entire parietal scalp. He had a history of type 2 diabetes mellitus, hypertension, and Graves disease. Physical examination demonstrated a large scar over the vertex scalp with central erosion, overlying crust, peripheral scalp atrophy, hypopigmentation at the periphery, and exaggerated superficial vasculature. Some oral erosions also were observed. A review of systems was negative for any constitutional symptoms. A month prior, the patient had been started on dapsone 50 mg with a prednisone taper by an outside dermatologist and noticed some improvement.

Painful Plaque on the Forearm
The Diagnosis: Mycobacterium marinum Infection
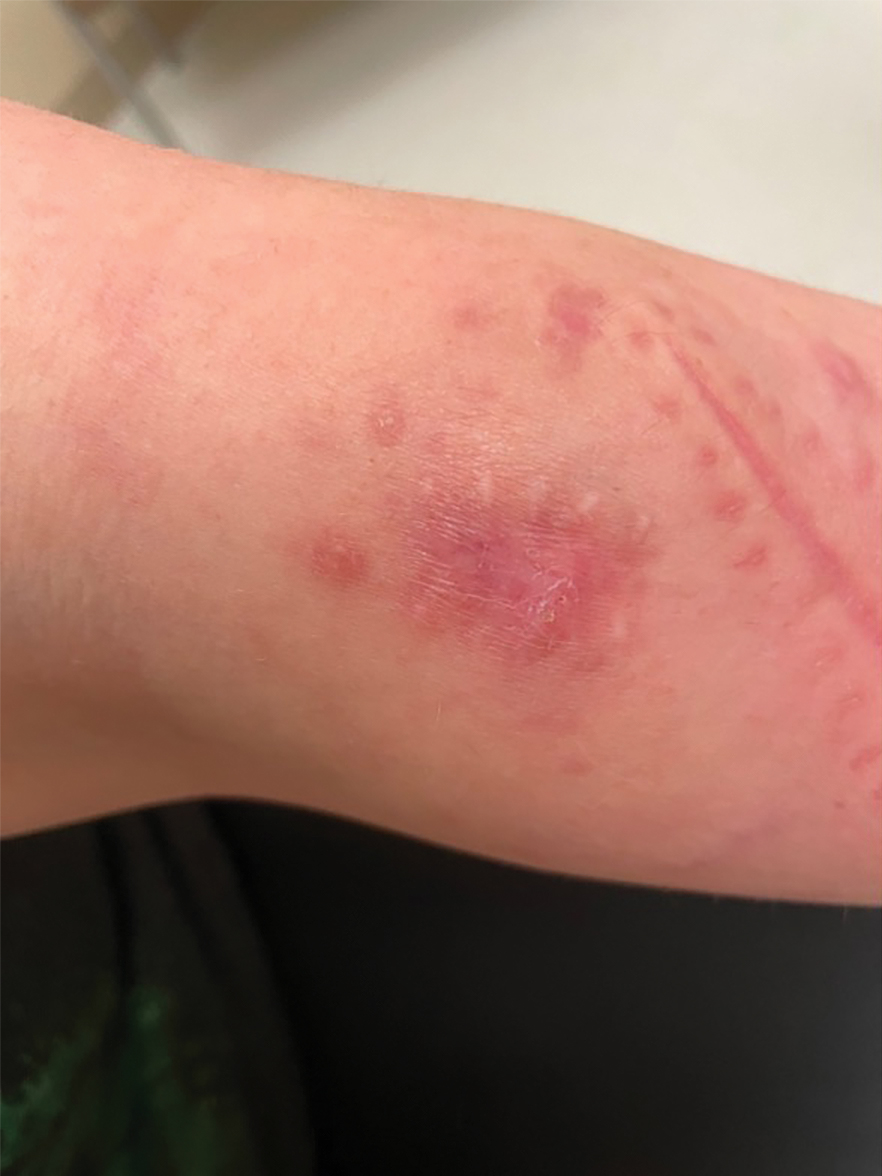
A repeat excisional biopsy showed suppurative granulomatous dermatitis with negative stains for infectious organisms; however, tissue culture grew Mycobacterium marinum. The patient had a history of exposure to fish tanks, which are a potential habitat for nontuberculous mycobacteria. These bacteria can enter the body through a minor laceration or cut in the skin, which was likely due to her occupation and pet care activities.1 Her fish tank exposure combined with the cutaneous findings of a long-standing indurated plaque with proximal nodular lymphangitis made M marinum infection the most likely diagnosis.2
Due to the limited specificity and sensitivity of patient symptoms, histologic staining, and direct microscopy, the gold standard for diagnosing acid-fast bacilli is tissue culture. 3 Tissue polymerase chain reaction testing is most useful in identifying the species of mycobacteria when histologic stains identify acid-fast bacilli but repeated tissue cultures are negative.4 With M marinum, a high clinical suspicion is needed to acquire a positive tissue culture because it needs to be grown for several weeks and at a temperature of 30 °C.5 Therefore, the physician should inform the laboratory if there is any suspicion for M marinum to increase the likelihood of obtaining a positive culture.
The differential diagnosis for M marinum infection includes other skin diseases that can cause nodular lymphangitis (also known as sporotrichoid spread) such as sporotrichosis, leishmaniasis, and certain bacterial and fungal infections. Although cat scratch disease, which is caused by Bartonella henselae, can appear similar to M marinum on histopathology, it clinically manifests with a single papulovesicular lesion at the site of inoculation that then forms a central eschar and resolves within a few weeks. Cat scratch disease typically causes painful lymphadenopathy, but it does not cause nodular lymphangitis or sporotrichoid spread.6 Sporotrichosis can have a similar clinical and histologic manifestation to M marinum infection, but the patient history typically includes exposure to Sporothrix schenckii through gardening or other contact with thorns, plants, or soil.2 Cutaneous sarcoidosis can have a similar clinical appearance to M marinum infection, but nodular lymphangitis does not occur and histopathology would demonstrate noncaseating epithelioid cell granulomas.7 Lastly, although vegetative pyoderma gangrenosum can have some of the same histologic findings as M marinum, it typically also demonstrates sinus tract formation, which was not present in our case. Additionally, vegetative pyoderma gangrenosum manifests with a verrucous and pustular plaque that would not have lymphocutaneous spread.8
Treatment of cutaneous M marinum infection is guided by antibiotic susceptibility testing. One regimen is clarithromycin (500 mg twice daily9) plus ethambutol. 10 Treatment often entails a multidrug combination due to the high rates of antibiotic resistance. Other antibiotics that potentially can be used include rifampin, trimethoprim-sulfamethoxazole, minocycline, and quinolones. The treatment duration typically is more than 3 months, and therapy is continued for 4 to 6 weeks after the skin lesions resolve.11 Excision of the lesion is reserved for patients with M marinum infection that fails to respond to antibiotic therapy.5
- Wayne LG, Sramek HA. Agents of newly recognized or infrequently encountered mycobacterial diseases. Clin Microbiol Rev. 1992;5:1-25. doi:10.1128/CMR.5.1.1
- Tobin EH, Jih WW. Sporotrichoid lymphocutaneous infections: etiology, diagnosis and therapy. Am Fam Physician. 2001;63:326-332.
- van Ingen J. Diagnosis of nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:103-109. doi:10.1055/s-0033-1333569
- Williamson H, Phillips R, Sarfo S, et al. Genetic diversity of PCR-positive, culture-negative and culture-positive Mycobacterium ulcerans isolated from Buruli ulcer patients in Ghana. PLoS One. 2014;9:E88007. doi:10.1371/journal.pone.0088007
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Baranowski K, Huang B. Cat scratch disease. StatPearls [Internet]. Updated June 12, 2023. Accessed July 15, 2024. https://www.ncbi.nlm .nih.gov/books/NBK482139/
- Sanchez M, Haimovic A, Prystowsky S. Sarcoidosis. Dermatol Clin. 2015;33:389-416. doi:10.1016/j.det.2015.03.006
- Borg Grech S, Vella Baldacchino A, Corso R, et al. Superficial granulomatous pyoderma successfully treated with intravenous immunoglobulin. Eur J Case Rep Intern Med. 2021;8:002656. doi:10.12890/2021_002656
- Krooks J, Weatherall A, Markowitz S. Complete resolution of Mycobacterium marinum infection with clarithromycin and ethambutol: a case report and a review of the literature. J Clin Aesthet Dermatol. 2018;11:48-51.
- Medel-Plaza M., Esteban J. Current treatment options for Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2023;24:1113-1123. doi:10.1080/14656566.2023.2211258
- Tirado-Sánchez A, Bonifaz A. Nodular lymphangitis (sporotrichoid lymphocutaneous infections): clues to differential diagnosis. J Fungi (Basel). 2018;4:56. doi:10.3390/jof4020056
The Diagnosis: Mycobacterium marinum Infection
A repeat excisional biopsy showed suppurative granulomatous dermatitis with negative stains for infectious organisms; however, tissue culture grew Mycobacterium marinum. The patient had a history of exposure to fish tanks, which are a potential habitat for nontuberculous mycobacteria. These bacteria can enter the body through a minor laceration or cut in the skin, which was likely due to her occupation and pet care activities.1 Her fish tank exposure combined with the cutaneous findings of a long-standing indurated plaque with proximal nodular lymphangitis made M marinum infection the most likely diagnosis.2
Due to the limited specificity and sensitivity of patient symptoms, histologic staining, and direct microscopy, the gold standard for diagnosing acid-fast bacilli is tissue culture. 3 Tissue polymerase chain reaction testing is most useful in identifying the species of mycobacteria when histologic stains identify acid-fast bacilli but repeated tissue cultures are negative.4 With M marinum, a high clinical suspicion is needed to acquire a positive tissue culture because it needs to be grown for several weeks and at a temperature of 30 °C.5 Therefore, the physician should inform the laboratory if there is any suspicion for M marinum to increase the likelihood of obtaining a positive culture.
The differential diagnosis for M marinum infection includes other skin diseases that can cause nodular lymphangitis (also known as sporotrichoid spread) such as sporotrichosis, leishmaniasis, and certain bacterial and fungal infections. Although cat scratch disease, which is caused by Bartonella henselae, can appear similar to M marinum on histopathology, it clinically manifests with a single papulovesicular lesion at the site of inoculation that then forms a central eschar and resolves within a few weeks. Cat scratch disease typically causes painful lymphadenopathy, but it does not cause nodular lymphangitis or sporotrichoid spread.6 Sporotrichosis can have a similar clinical and histologic manifestation to M marinum infection, but the patient history typically includes exposure to Sporothrix schenckii through gardening or other contact with thorns, plants, or soil.2 Cutaneous sarcoidosis can have a similar clinical appearance to M marinum infection, but nodular lymphangitis does not occur and histopathology would demonstrate noncaseating epithelioid cell granulomas.7 Lastly, although vegetative pyoderma gangrenosum can have some of the same histologic findings as M marinum, it typically also demonstrates sinus tract formation, which was not present in our case. Additionally, vegetative pyoderma gangrenosum manifests with a verrucous and pustular plaque that would not have lymphocutaneous spread.8
Treatment of cutaneous M marinum infection is guided by antibiotic susceptibility testing. One regimen is clarithromycin (500 mg twice daily9) plus ethambutol. 10 Treatment often entails a multidrug combination due to the high rates of antibiotic resistance. Other antibiotics that potentially can be used include rifampin, trimethoprim-sulfamethoxazole, minocycline, and quinolones. The treatment duration typically is more than 3 months, and therapy is continued for 4 to 6 weeks after the skin lesions resolve.11 Excision of the lesion is reserved for patients with M marinum infection that fails to respond to antibiotic therapy.5
The Diagnosis: Mycobacterium marinum Infection
A repeat excisional biopsy showed suppurative granulomatous dermatitis with negative stains for infectious organisms; however, tissue culture grew Mycobacterium marinum. The patient had a history of exposure to fish tanks, which are a potential habitat for nontuberculous mycobacteria. These bacteria can enter the body through a minor laceration or cut in the skin, which was likely due to her occupation and pet care activities.1 Her fish tank exposure combined with the cutaneous findings of a long-standing indurated plaque with proximal nodular lymphangitis made M marinum infection the most likely diagnosis.2
Due to the limited specificity and sensitivity of patient symptoms, histologic staining, and direct microscopy, the gold standard for diagnosing acid-fast bacilli is tissue culture. 3 Tissue polymerase chain reaction testing is most useful in identifying the species of mycobacteria when histologic stains identify acid-fast bacilli but repeated tissue cultures are negative.4 With M marinum, a high clinical suspicion is needed to acquire a positive tissue culture because it needs to be grown for several weeks and at a temperature of 30 °C.5 Therefore, the physician should inform the laboratory if there is any suspicion for M marinum to increase the likelihood of obtaining a positive culture.
The differential diagnosis for M marinum infection includes other skin diseases that can cause nodular lymphangitis (also known as sporotrichoid spread) such as sporotrichosis, leishmaniasis, and certain bacterial and fungal infections. Although cat scratch disease, which is caused by Bartonella henselae, can appear similar to M marinum on histopathology, it clinically manifests with a single papulovesicular lesion at the site of inoculation that then forms a central eschar and resolves within a few weeks. Cat scratch disease typically causes painful lymphadenopathy, but it does not cause nodular lymphangitis or sporotrichoid spread.6 Sporotrichosis can have a similar clinical and histologic manifestation to M marinum infection, but the patient history typically includes exposure to Sporothrix schenckii through gardening or other contact with thorns, plants, or soil.2 Cutaneous sarcoidosis can have a similar clinical appearance to M marinum infection, but nodular lymphangitis does not occur and histopathology would demonstrate noncaseating epithelioid cell granulomas.7 Lastly, although vegetative pyoderma gangrenosum can have some of the same histologic findings as M marinum, it typically also demonstrates sinus tract formation, which was not present in our case. Additionally, vegetative pyoderma gangrenosum manifests with a verrucous and pustular plaque that would not have lymphocutaneous spread.8
Treatment of cutaneous M marinum infection is guided by antibiotic susceptibility testing. One regimen is clarithromycin (500 mg twice daily9) plus ethambutol. 10 Treatment often entails a multidrug combination due to the high rates of antibiotic resistance. Other antibiotics that potentially can be used include rifampin, trimethoprim-sulfamethoxazole, minocycline, and quinolones. The treatment duration typically is more than 3 months, and therapy is continued for 4 to 6 weeks after the skin lesions resolve.11 Excision of the lesion is reserved for patients with M marinum infection that fails to respond to antibiotic therapy.5
- Wayne LG, Sramek HA. Agents of newly recognized or infrequently encountered mycobacterial diseases. Clin Microbiol Rev. 1992;5:1-25. doi:10.1128/CMR.5.1.1
- Tobin EH, Jih WW. Sporotrichoid lymphocutaneous infections: etiology, diagnosis and therapy. Am Fam Physician. 2001;63:326-332.
- van Ingen J. Diagnosis of nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:103-109. doi:10.1055/s-0033-1333569
- Williamson H, Phillips R, Sarfo S, et al. Genetic diversity of PCR-positive, culture-negative and culture-positive Mycobacterium ulcerans isolated from Buruli ulcer patients in Ghana. PLoS One. 2014;9:E88007. doi:10.1371/journal.pone.0088007
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Baranowski K, Huang B. Cat scratch disease. StatPearls [Internet]. Updated June 12, 2023. Accessed July 15, 2024. https://www.ncbi.nlm .nih.gov/books/NBK482139/
- Sanchez M, Haimovic A, Prystowsky S. Sarcoidosis. Dermatol Clin. 2015;33:389-416. doi:10.1016/j.det.2015.03.006
- Borg Grech S, Vella Baldacchino A, Corso R, et al. Superficial granulomatous pyoderma successfully treated with intravenous immunoglobulin. Eur J Case Rep Intern Med. 2021;8:002656. doi:10.12890/2021_002656
- Krooks J, Weatherall A, Markowitz S. Complete resolution of Mycobacterium marinum infection with clarithromycin and ethambutol: a case report and a review of the literature. J Clin Aesthet Dermatol. 2018;11:48-51.
- Medel-Plaza M., Esteban J. Current treatment options for Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2023;24:1113-1123. doi:10.1080/14656566.2023.2211258
- Tirado-Sánchez A, Bonifaz A. Nodular lymphangitis (sporotrichoid lymphocutaneous infections): clues to differential diagnosis. J Fungi (Basel). 2018;4:56. doi:10.3390/jof4020056
- Wayne LG, Sramek HA. Agents of newly recognized or infrequently encountered mycobacterial diseases. Clin Microbiol Rev. 1992;5:1-25. doi:10.1128/CMR.5.1.1
- Tobin EH, Jih WW. Sporotrichoid lymphocutaneous infections: etiology, diagnosis and therapy. Am Fam Physician. 2001;63:326-332.
- van Ingen J. Diagnosis of nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:103-109. doi:10.1055/s-0033-1333569
- Williamson H, Phillips R, Sarfo S, et al. Genetic diversity of PCR-positive, culture-negative and culture-positive Mycobacterium ulcerans isolated from Buruli ulcer patients in Ghana. PLoS One. 2014;9:E88007. doi:10.1371/journal.pone.0088007
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Baranowski K, Huang B. Cat scratch disease. StatPearls [Internet]. Updated June 12, 2023. Accessed July 15, 2024. https://www.ncbi.nlm .nih.gov/books/NBK482139/
- Sanchez M, Haimovic A, Prystowsky S. Sarcoidosis. Dermatol Clin. 2015;33:389-416. doi:10.1016/j.det.2015.03.006
- Borg Grech S, Vella Baldacchino A, Corso R, et al. Superficial granulomatous pyoderma successfully treated with intravenous immunoglobulin. Eur J Case Rep Intern Med. 2021;8:002656. doi:10.12890/2021_002656
- Krooks J, Weatherall A, Markowitz S. Complete resolution of Mycobacterium marinum infection with clarithromycin and ethambutol: a case report and a review of the literature. J Clin Aesthet Dermatol. 2018;11:48-51.
- Medel-Plaza M., Esteban J. Current treatment options for Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2023;24:1113-1123. doi:10.1080/14656566.2023.2211258
- Tirado-Sánchez A, Bonifaz A. Nodular lymphangitis (sporotrichoid lymphocutaneous infections): clues to differential diagnosis. J Fungi (Basel). 2018;4:56. doi:10.3390/jof4020056
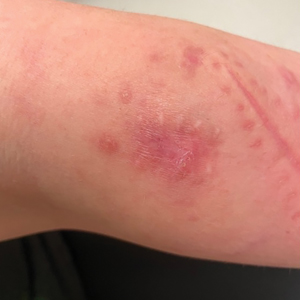
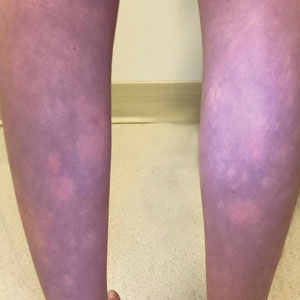
A 30-year-old woman presented to the dermatology clinic with lesions on the right forearm of 2 years’ duration. Her medical history was unremarkable. She reported working as a chef and caring for multiple pets in her home, including 3 cats, 6 fish tanks, 3 dogs, and 3 lizards. Physical examination revealed a painful, indurated, red-violaceous plaque on the right forearm with satellite pink nodules that had been slowly migrating proximally up the forearm. An outside excisional biopsy performed 1 year prior had shown suppurative granulomatous dermatitis with negative stains for infectious organisms and negative tissue cultures. At that time, the patient was diagnosed with ruptured folliculitis; however, a subsequent lack of clinical improvement prompted her to seek a second opinion at our clinic.
Painful Anal Lesions in a Patient With HIV
The Diagnosis: Condyloma Latum
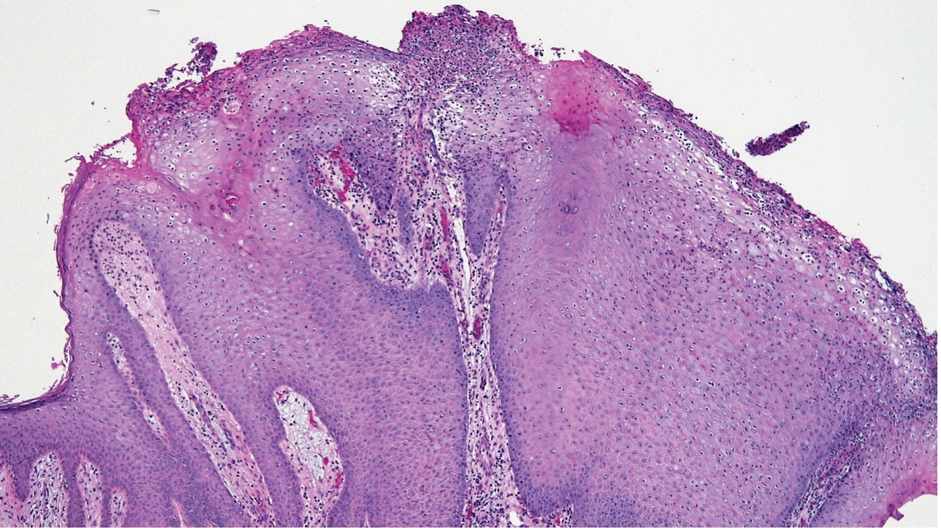
Laboratory test results were notable for a rapid plasma reagin titer of 1:512, a positive Treponema pallidum particle agglutination test, negative rectal nucleic acid amplification tests for gonorrhea and chlamydia, and a negative herpes simplex virus polymerase chain reaction. A VDRL test of cerebrospinal fluid from a lumbar puncture was negative. Histopathology of the punch biopsy sample revealed marked verrucous epidermal hyperplasia without keratinocytic atypia and with mixed inflammation (Figure 1), while immunohistochemical staining showed numerus T pallidum organisms (Figure 2). A diagnosis of condyloma latum was made based on the laboratory, lumbar puncture, and punch biopsy results. Due to a penicillin allergy, the patient was treated with oral doxycycline for 14 days. On follow-up at day 12 of therapy, he reported cessation of rectal pain, and resolution of anal lesions was noted on physical examination.
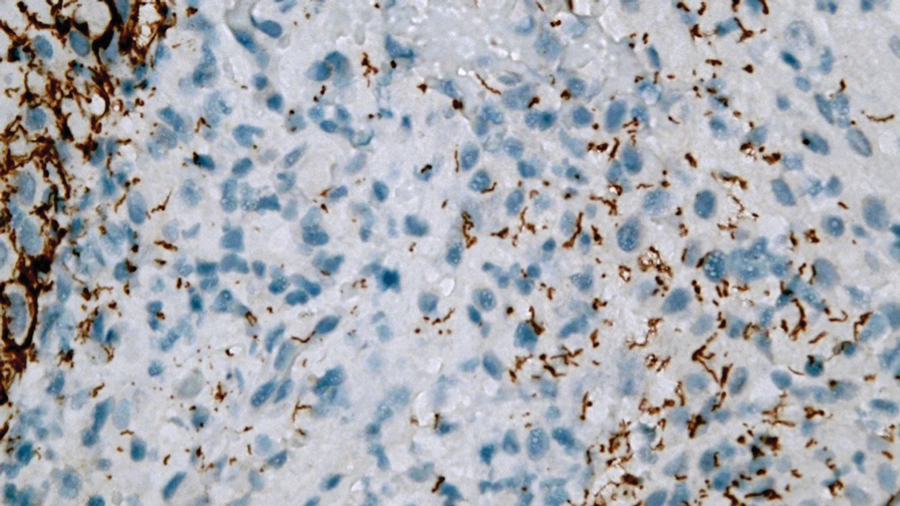
Condylomata lata are highly infectious cutaneous lesions that can manifest during secondary syphilis.1 They typically are described as white or gray, raised, flatappearing plaques and occur in moist areas or skin folds including the anus, scrotum, and vulva. However, these lesions also have been reported in the axillae, umbilicus, nasolabial folds, and other anatomic areas.1,2 The lesions can be painful and often manifest in multiples, especially in patients living with HIV.3
Condylomata lata can have a verrucous appearance and may mimic other anogenital lesions, such as condylomata acuminata, genital herpes, and malignant tumors, leading to an initial misdiagnosis.1,2 Condylomata lata should always be included in the differential when evaluating anogenital lesions. Other conditions in the differential diagnosis include psoriasis, typically manifesting as erythematous plaques with silver scale, and molluscum contagiosum, appearing as small umbilicated papules on physical examination.
Condylomata lata have been reported to occur in 6% to 23% of patients with secondary syphilis.1 Although secondary syphilis more typically manifests with a diffuse maculopapular rash, condylomata lata may be the sole dermatologic manifestation.4
Histopathology of condylomata lata consists of epithelial hyperplasia as well as lymphocytic and plasma cell infiltrates. It is diagnosed by serologic testing as well as immunohistochemical staining or dark-field microscopy.
First-line treatment of secondary syphilis is a single dose of benzathine penicillin G administered intramuscularly.5 However, a 14-day course of oral doxycycline can be used in patients with a penicillin allergy. When compliance and follow-up cannot be guaranteed, penicillin desensitization and treatment with benzathine penicillin G is recommended. Clinical evaluation and repeat serologic testing should be performed at 6 and 12 months follow-up, or more frequently if clinically indicated.5
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21. doi:10.1016/j.jdcr.2021.01.025
- Liu Z, Wang L, Zhang G, et al. Warty mucosal lesions: oral condyloma lata of secondary syphilis. Indian J Dermatol Venereol Leprol. 2017;83:277. doi:10.4103/0378-6323.191129
- Rompalo AM, Joesoef MR, O’Donnell JA, et al; Syphilis and HIV Study Group. Clinical manifestations of early syphilis by HIV status and gender: results of the syphilis and HIV study. Sex Transm Dis.2001;28:158-165.
- Kumar P, Das A, Mondal A. Secondary syphilis: an unusual presentation. Indian J Sex Transm Dis AIDS. 2017;38:98-99. doi:10.4103/0253-7184.194318
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
The Diagnosis: Condyloma Latum
Laboratory test results were notable for a rapid plasma reagin titer of 1:512, a positive Treponema pallidum particle agglutination test, negative rectal nucleic acid amplification tests for gonorrhea and chlamydia, and a negative herpes simplex virus polymerase chain reaction. A VDRL test of cerebrospinal fluid from a lumbar puncture was negative. Histopathology of the punch biopsy sample revealed marked verrucous epidermal hyperplasia without keratinocytic atypia and with mixed inflammation (Figure 1), while immunohistochemical staining showed numerus T pallidum organisms (Figure 2). A diagnosis of condyloma latum was made based on the laboratory, lumbar puncture, and punch biopsy results. Due to a penicillin allergy, the patient was treated with oral doxycycline for 14 days. On follow-up at day 12 of therapy, he reported cessation of rectal pain, and resolution of anal lesions was noted on physical examination.


Condylomata lata are highly infectious cutaneous lesions that can manifest during secondary syphilis.1 They typically are described as white or gray, raised, flatappearing plaques and occur in moist areas or skin folds including the anus, scrotum, and vulva. However, these lesions also have been reported in the axillae, umbilicus, nasolabial folds, and other anatomic areas.1,2 The lesions can be painful and often manifest in multiples, especially in patients living with HIV.3
Condylomata lata can have a verrucous appearance and may mimic other anogenital lesions, such as condylomata acuminata, genital herpes, and malignant tumors, leading to an initial misdiagnosis.1,2 Condylomata lata should always be included in the differential when evaluating anogenital lesions. Other conditions in the differential diagnosis include psoriasis, typically manifesting as erythematous plaques with silver scale, and molluscum contagiosum, appearing as small umbilicated papules on physical examination.
Condylomata lata have been reported to occur in 6% to 23% of patients with secondary syphilis.1 Although secondary syphilis more typically manifests with a diffuse maculopapular rash, condylomata lata may be the sole dermatologic manifestation.4
Histopathology of condylomata lata consists of epithelial hyperplasia as well as lymphocytic and plasma cell infiltrates. It is diagnosed by serologic testing as well as immunohistochemical staining or dark-field microscopy.
First-line treatment of secondary syphilis is a single dose of benzathine penicillin G administered intramuscularly.5 However, a 14-day course of oral doxycycline can be used in patients with a penicillin allergy. When compliance and follow-up cannot be guaranteed, penicillin desensitization and treatment with benzathine penicillin G is recommended. Clinical evaluation and repeat serologic testing should be performed at 6 and 12 months follow-up, or more frequently if clinically indicated.5
The Diagnosis: Condyloma Latum
Laboratory test results were notable for a rapid plasma reagin titer of 1:512, a positive Treponema pallidum particle agglutination test, negative rectal nucleic acid amplification tests for gonorrhea and chlamydia, and a negative herpes simplex virus polymerase chain reaction. A VDRL test of cerebrospinal fluid from a lumbar puncture was negative. Histopathology of the punch biopsy sample revealed marked verrucous epidermal hyperplasia without keratinocytic atypia and with mixed inflammation (Figure 1), while immunohistochemical staining showed numerus T pallidum organisms (Figure 2). A diagnosis of condyloma latum was made based on the laboratory, lumbar puncture, and punch biopsy results. Due to a penicillin allergy, the patient was treated with oral doxycycline for 14 days. On follow-up at day 12 of therapy, he reported cessation of rectal pain, and resolution of anal lesions was noted on physical examination.


Condylomata lata are highly infectious cutaneous lesions that can manifest during secondary syphilis.1 They typically are described as white or gray, raised, flatappearing plaques and occur in moist areas or skin folds including the anus, scrotum, and vulva. However, these lesions also have been reported in the axillae, umbilicus, nasolabial folds, and other anatomic areas.1,2 The lesions can be painful and often manifest in multiples, especially in patients living with HIV.3
Condylomata lata can have a verrucous appearance and may mimic other anogenital lesions, such as condylomata acuminata, genital herpes, and malignant tumors, leading to an initial misdiagnosis.1,2 Condylomata lata should always be included in the differential when evaluating anogenital lesions. Other conditions in the differential diagnosis include psoriasis, typically manifesting as erythematous plaques with silver scale, and molluscum contagiosum, appearing as small umbilicated papules on physical examination.
Condylomata lata have been reported to occur in 6% to 23% of patients with secondary syphilis.1 Although secondary syphilis more typically manifests with a diffuse maculopapular rash, condylomata lata may be the sole dermatologic manifestation.4
Histopathology of condylomata lata consists of epithelial hyperplasia as well as lymphocytic and plasma cell infiltrates. It is diagnosed by serologic testing as well as immunohistochemical staining or dark-field microscopy.
First-line treatment of secondary syphilis is a single dose of benzathine penicillin G administered intramuscularly.5 However, a 14-day course of oral doxycycline can be used in patients with a penicillin allergy. When compliance and follow-up cannot be guaranteed, penicillin desensitization and treatment with benzathine penicillin G is recommended. Clinical evaluation and repeat serologic testing should be performed at 6 and 12 months follow-up, or more frequently if clinically indicated.5
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21. doi:10.1016/j.jdcr.2021.01.025
- Liu Z, Wang L, Zhang G, et al. Warty mucosal lesions: oral condyloma lata of secondary syphilis. Indian J Dermatol Venereol Leprol. 2017;83:277. doi:10.4103/0378-6323.191129
- Rompalo AM, Joesoef MR, O’Donnell JA, et al; Syphilis and HIV Study Group. Clinical manifestations of early syphilis by HIV status and gender: results of the syphilis and HIV study. Sex Transm Dis.2001;28:158-165.
- Kumar P, Das A, Mondal A. Secondary syphilis: an unusual presentation. Indian J Sex Transm Dis AIDS. 2017;38:98-99. doi:10.4103/0253-7184.194318
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21. doi:10.1016/j.jdcr.2021.01.025
- Liu Z, Wang L, Zhang G, et al. Warty mucosal lesions: oral condyloma lata of secondary syphilis. Indian J Dermatol Venereol Leprol. 2017;83:277. doi:10.4103/0378-6323.191129
- Rompalo AM, Joesoef MR, O’Donnell JA, et al; Syphilis and HIV Study Group. Clinical manifestations of early syphilis by HIV status and gender: results of the syphilis and HIV study. Sex Transm Dis.2001;28:158-165.
- Kumar P, Das A, Mondal A. Secondary syphilis: an unusual presentation. Indian J Sex Transm Dis AIDS. 2017;38:98-99. doi:10.4103/0253-7184.194318
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
A 24-year-old man presented to the emergency department with rectal pain and lesions of 3 weeks’ duration that were progressively worsening. He had a medical history of poorly controlled HIV, cerebral toxoplasmosis, and genital herpes, as well as a social history of sexual activity with other men.
He had been diagnosed with HIV 7 years prior and had been off therapy until 1 year prior to the current presentation, when he was hospitalized with encephalopathy (CD4 count, <50 cells/mm3). A diagnosis of cerebral toxoplasmosis was made, and he began a treatment regimen of sulfadiazine, pyrimethamine, and leucovorin, as well as bictegravir, emtricitabine, and tenofovir alafenamide. Since then, the patient admitted to difficulty with medication adherence.
Rapid plasma reagin, gonorrhea, and chlamydia testing were negative during a routine workup 6 months prior to the current presentation. He initially presented to an urgent care clinic for evaluation of the rectal pain and lesions and was treated empirically with topical podofilox. He presented to the emergency department 1 week later (3 weeks after symptom onset) with anal warts and apparent vesicular lesions. Empiric treatment with oral valacyclovir was prescribed.
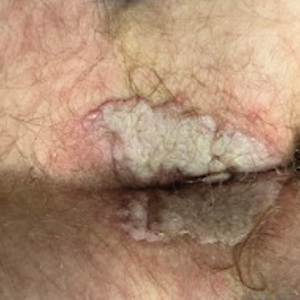
Despite these treatments, the rectal pain became severe—especially upon sitting, defecation, and physical exertion—prompting further evaluation. Physical examination revealed soft, flat-topped, moist-appearing, gray plaques with minimal surrounding erythema at the anus. Laboratory test results demonstrated a CD4 count of 161 cells/mm3 and an HIV viral load of 137 copies/mL.
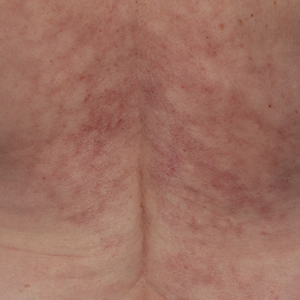
Pruritic Rash on the Neck and Back
The Diagnosis: Prurigo Pigmentosa
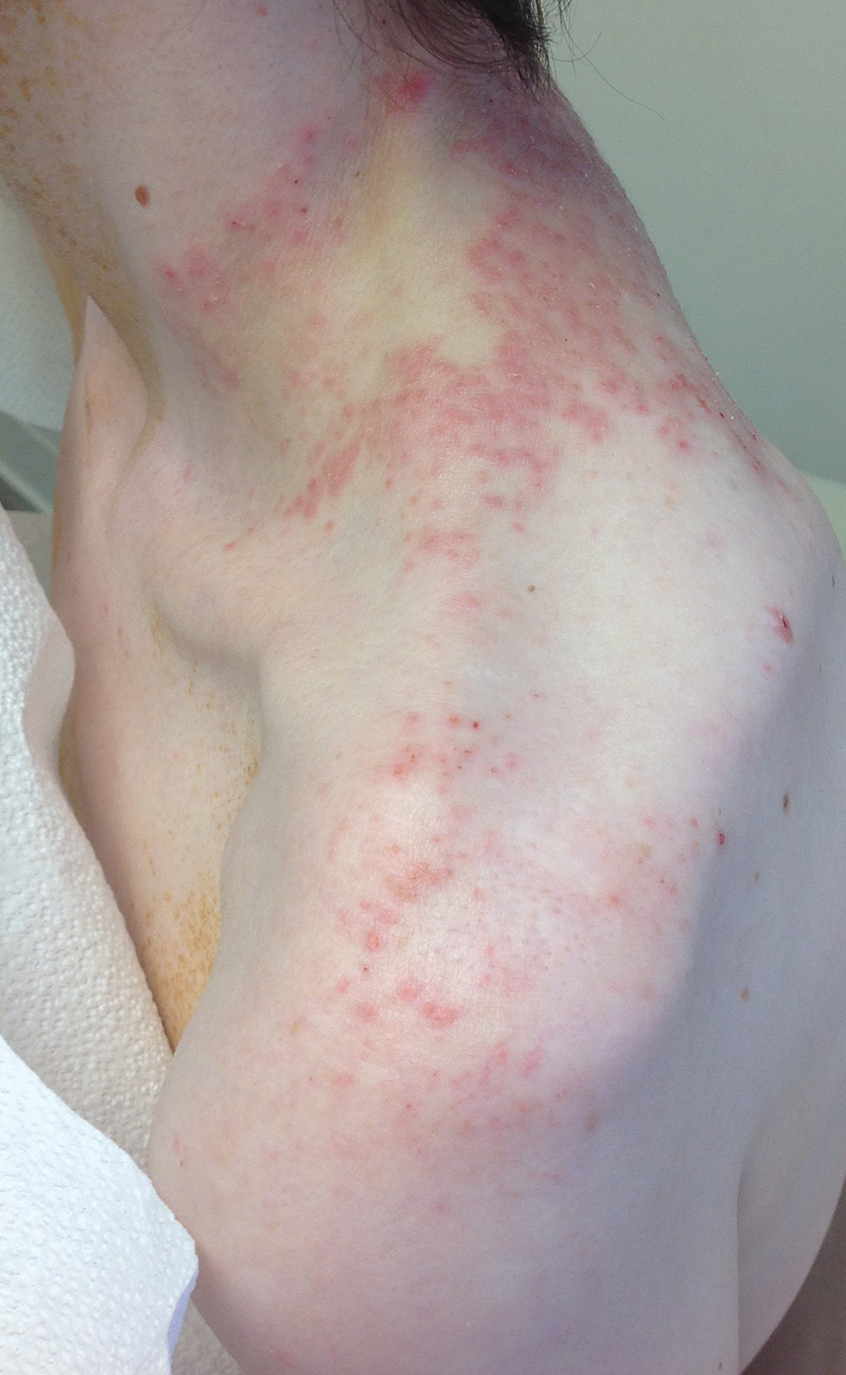
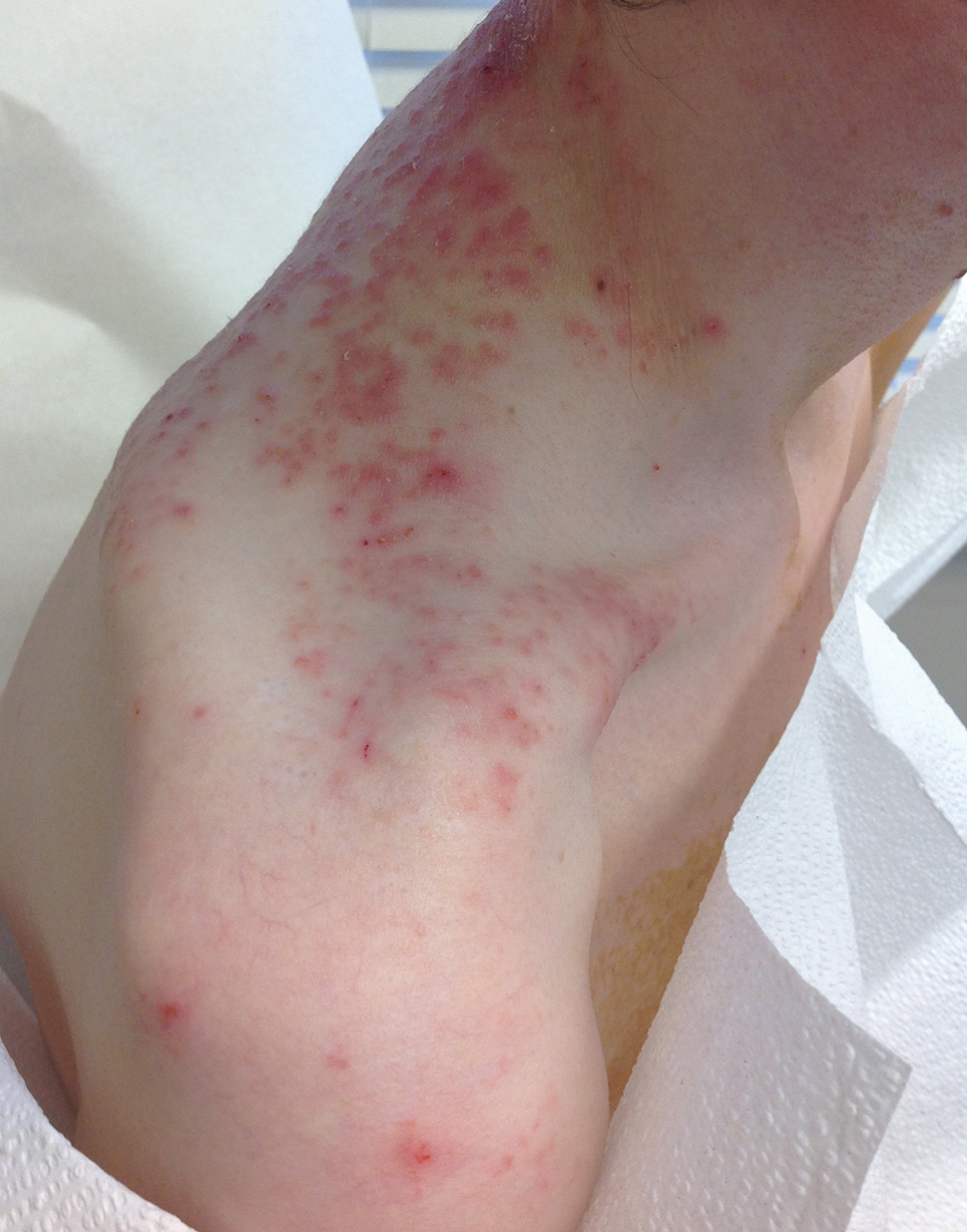
A comprehensive metabolic panel collected from our patient 1 month earlier did not reveal any abnormalities. Serum methylmalonic acid and homocysteine were both elevated at 417 nmol/L (reference range [for those aged 2–59 years], 55–335 nmol/L) and 23 μmol/L (reference range, 5–15 μmol/L), respectively. Serum folate and 25-hydroxyvitamin D were low at 3.1 ng/mL (reference range, >4.8 ng/mL) and 5 ng/mL (reference range, 30–80 ng/mL), respectively. Vitamin B12 was within reference range. Two 4-mm punch biopsies collected from the upper back showed spongiotic dermatitis.
Our patient’s histopathology results along with the rash distribution and medical history of anorexia increased suspicion for prurigo pigmentosa. A trial of oral doxycycline 100 mg twice daily for 2 weeks was prescribed. At 2-week follow-up, the patient’s mother revealed a history of ketosis in her daughter, solidifying the diagnosis. The patient was counseled on maintaining a healthy diet to prevent future breakouts. The patient’s rash resolved with diet modification and doxycycline; however, it recurred upon relapse of anorexia 4 months later.
Prurigo pigmentosa, originally identified in Japan by Nagashima et al,1 is an uncommon recurrent inflammatory disorder predominantly observed in young adults of Asian descent. Subsequently, it was reported to occur among individuals from different ethnic backgrounds, indicating potential underdiagnosis or misdiagnosis in Western countries.2 Although a direct pathogenic cause for prurigo pigmentosa has not been identified, a strong association has been linked to diet, specifically when ketosis is induced, such as in ketogenic diets and anorexia nervosa.3-5 Other possible causes include sunlight exposure, clothing friction, and sweating.1,5 The disease course is characterized by intermittent flares and spontaneous resolution, with recurrence in most cases. During the active phase, intensely pruritic, papulovesicular or urticarial papules are predominant and most often are localized to the upper body and torso, including the back, shoulders, neck, and chest.5 These flares can persist for several days but eventually subside, leaving behind a characteristic reticular pigmentation that can persist for months.5 First-line treatment often involves the use of tetracycline antibiotics, such as minocycline or doxycycline. 2,4,5 Dapsone often is used with successful resolution. 6 Dietary modifications also have been found to be effective in treating prurigo pigmentosa, particularly in patients presenting with dietary insufficiency.6,7 Increased carbohydrate intake has been shown to promote resolution. 6 Topical corticosteroids demonstrate limited efficacy in controlling flares.6,8
Histopathology has been variably described, with initial findings reported as nonspecific.1 However, it was later described as a distinct inflammatory disease of the skin with histologically distinct stages.2,9 Early stages reveal scattered dermal, dermal papillary, and perivascular neutrophilic infiltration.9 The lesions then progress and become fully developed, at which point neutrophilic infiltration becomes more prominent, accompanied by the presence of intraepidermal neutrophils and spongiosis. As the lesions resolve, the infiltration transitions to lymphocytic, and lichenoid changes can sometimes be appreciated along with epidermal hyperplasia, hyperpigmentation, and dermal melanophages.9 Although these findings aid in the diagnosis of prurigo pigmentosa, a clinicopathologic correlation is necessary to establish a definitive diagnosis.
Because prurigo pigmentosa is rare, it often is misdiagnosed as another condition with a similar presentation and nonspecific biopsy findings.6 Allergic contact dermatitis is a common type IV delayed hypersensitivity reaction that manifests similar to prurigo pigmentosa with pruritus and a well-demarcated distribution10 that is related to the pattern of allergen exposure; in the case of allergic contact dermatitis related to textiles, a well-demarcated rash will appear in the distribution area of the associated clothing (eg, shirt, pants, shorts).11 Development of allergy involves exposure and sensitization to an allergen, followed by subsequent re-exposure that results in cutaneous T-cell activation and inflammation. 10 Histopathology shows nonspecific spongiotic inflammation, and the gold standard for diagnosis is patch testing to identify the causative substance(s). Definitive treatment includes avoidance of identified allergies; however, if patients are unable to avoid the allergen or the cause is unknown, then corticosteroids, antihistamines, and/or calcineurin inhibitors are beneficial in controlling symptoms and flares.10
Pityrosporum folliculitis (also known as Malassezia folliculitis) is a fungal acneform condition that arises from overgrowth of normal skin flora Malassezia yeast,12 which may be due to occlusion of follicles or disruption of the normal flora composition. Clinically, the manifestation may resemble prurigo pigmentosa in distribution and presence of intense pruritus. However, pustular lesions and involvement of the face can aid in differentiating Pityrosporum from prurigo pigmentosa, which can be confirmed via periodic acid–Schiff staining with numerous round yeasts within affected follicles. Oral antifungal therapy typically yields rapid improvement and resolution of symptoms.12
Urticaria and prurigo pigmentosa share similar clinical characteristics, with symptoms of intense pruritus and urticarial lesions on the trunk.2,13 Urticaria is an IgEmediated type I hypersensitivity reaction characterized by wheals (ie, edematous red or pink lesions of variable size and shape that typically resolve spontaneously within 24–48 hours).13 Notably, urticaria will improve and in some cases completely resolve with antihistamines or anti-IgE antibody treatment, which may aid in distinguishing it from prurigo pigmentosa, as the latter typically exhibits limited response to such treatment.2 Histopathology also can assist in the diagnosis by ruling out other causes of similar rash; however, biopsies are not routinely done unless other inflammatory conditions are of high suspicion.13
Bullous pemphigoid is an autoimmune, subepidermal, blistering dermatosis that is most common among the elderly.14 It is characterized by the presence of IgG antibodies that target BP180 and BP230, which initiate inflammatory cascades that lead to tissue damage and blister formation. It typically manifests as pruritic blistering eruptions, primarily on the limbs and trunk, but may involve the head, neck, or palmoplantar regions.14 Although blistering eruptions are the prodrome of the disease, some cases may present with nonspecific urticarial or eczematous lesions14,15 that may resemble prurigo pigmentosa. The diagnosis is confirmed through direct immunofluorescence microscopy of biopsied lesions, which reveals IgG and/or C3 deposits along the dermoepidermal junction.14 Management of bullous pemphigoid involves timely initiation of dapsone or systemic corticosteroids, which have demonstrated high efficacy in controlling the disease and its associated symptoms.15
Our patient achieved a favorable response to diet modification and doxycycline therapy consistent with the diagnosis of prurigo pigmentosa. Unfortunately, the condition recurred following a relapse of anorexia. Management of prurigo pigmentosa necessitates not only accurate diagnosis but also addressing any underlying factors that may contribute to disease exacerbation. We anticipate the eating disorder will pose a major challenge in achieving long-term control of prurigo pigmentosa.
- Nagashima M, Ohshiro A, Shimizu N. A peculiar pruriginous dermatosis with gross reticular pigmentation. Jpn J Dermatol. 1971;81:38-39.
- Boer A, Asgari M. Prurigo pigmentosa: an underdiagnosed disease? Indian J Dermatol Venereol Leprol. 2006;72:405-409. doi:10.4103/0378-6323.29334
- Michaels JD, Hoss E, DiCaudo DJ, et al. Prurigo pigmentosa after a strict ketogenic diet. Pediatr Dermatol. 2013;32:248-251. doi:10.1111/pde.12275
- Teraki Y, Teraki E, Kawashima M, et al. Ketosis is involved in the origin of prurigo pigmentosa. J Am Acad Dermatol. 1996;34:509-511. doi:10.1016/s0190-9622(96)90460-0
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79-87. doi:10.1016/j.jdin.2021.03.003
- Wong M, Lee E, Wu Y, et al. Treatment of prurigo pigmentosa with diet modification: a medical case study. Hawaii J Med Public Health. 2018;77:114-117.
- Almaani N, Al-Tarawneh AH, Msallam H. Prurigo pigmentosa: a clinicopathological report of three Middle Eastern patients. Case Rep Dermatol Med. 2018;2018:9406797. doi:10.1155/2018/9406797
- Kim JK, Chung WK, Chang SE, et al. Prurigo pigmentosa: clinicopathological study and analysis of 50 cases in Korea. J Dermatol. 2012;39:891-897. doi:10.1111/j.1346-8138.2012.01640.x
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi:10.1016/j.jaad.2015.02.1139
- Lazarov A, Cordoba M, Plosk N, et al. Atypical and unusual clinical manifestations of contact dermatitis to clothing (textile contact dermatitis)—case presentation and review of the literature. Dermatol Online J. 2003;9. doi:10.5070/d30kd1d259
- Rubenstein RM, Malerich SA. Malassezia (Pityrosporum) folliculitis. J Clin Aesthet Dermatol. 2014;7:37-41.
- Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270-1277. doi:10.1016/j.jaci.2014.02.036
- della Torre R, Combescure C, Cortés B, et al. Clinical presentation and diagnostic delay in bullous pemphigoid: a prospective nationwide cohort. Br J Dermatol. 2012;167:1111-1117. doi:10.1111/j.1365-2133.2012.11108.x
- Alonso-Llamazares J, Rogers RS 3rd, Oursler JR, et al. Bullous pemphigoid presenting as generalized pruritus: observations in six patients. Int J Dermatol. 1998;37:508-514.
The Diagnosis: Prurigo Pigmentosa
A comprehensive metabolic panel collected from our patient 1 month earlier did not reveal any abnormalities. Serum methylmalonic acid and homocysteine were both elevated at 417 nmol/L (reference range [for those aged 2–59 years], 55–335 nmol/L) and 23 μmol/L (reference range, 5–15 μmol/L), respectively. Serum folate and 25-hydroxyvitamin D were low at 3.1 ng/mL (reference range, >4.8 ng/mL) and 5 ng/mL (reference range, 30–80 ng/mL), respectively. Vitamin B12 was within reference range. Two 4-mm punch biopsies collected from the upper back showed spongiotic dermatitis.
Our patient’s histopathology results along with the rash distribution and medical history of anorexia increased suspicion for prurigo pigmentosa. A trial of oral doxycycline 100 mg twice daily for 2 weeks was prescribed. At 2-week follow-up, the patient’s mother revealed a history of ketosis in her daughter, solidifying the diagnosis. The patient was counseled on maintaining a healthy diet to prevent future breakouts. The patient’s rash resolved with diet modification and doxycycline; however, it recurred upon relapse of anorexia 4 months later.
Prurigo pigmentosa, originally identified in Japan by Nagashima et al,1 is an uncommon recurrent inflammatory disorder predominantly observed in young adults of Asian descent. Subsequently, it was reported to occur among individuals from different ethnic backgrounds, indicating potential underdiagnosis or misdiagnosis in Western countries.2 Although a direct pathogenic cause for prurigo pigmentosa has not been identified, a strong association has been linked to diet, specifically when ketosis is induced, such as in ketogenic diets and anorexia nervosa.3-5 Other possible causes include sunlight exposure, clothing friction, and sweating.1,5 The disease course is characterized by intermittent flares and spontaneous resolution, with recurrence in most cases. During the active phase, intensely pruritic, papulovesicular or urticarial papules are predominant and most often are localized to the upper body and torso, including the back, shoulders, neck, and chest.5 These flares can persist for several days but eventually subside, leaving behind a characteristic reticular pigmentation that can persist for months.5 First-line treatment often involves the use of tetracycline antibiotics, such as minocycline or doxycycline. 2,4,5 Dapsone often is used with successful resolution. 6 Dietary modifications also have been found to be effective in treating prurigo pigmentosa, particularly in patients presenting with dietary insufficiency.6,7 Increased carbohydrate intake has been shown to promote resolution. 6 Topical corticosteroids demonstrate limited efficacy in controlling flares.6,8
Histopathology has been variably described, with initial findings reported as nonspecific.1 However, it was later described as a distinct inflammatory disease of the skin with histologically distinct stages.2,9 Early stages reveal scattered dermal, dermal papillary, and perivascular neutrophilic infiltration.9 The lesions then progress and become fully developed, at which point neutrophilic infiltration becomes more prominent, accompanied by the presence of intraepidermal neutrophils and spongiosis. As the lesions resolve, the infiltration transitions to lymphocytic, and lichenoid changes can sometimes be appreciated along with epidermal hyperplasia, hyperpigmentation, and dermal melanophages.9 Although these findings aid in the diagnosis of prurigo pigmentosa, a clinicopathologic correlation is necessary to establish a definitive diagnosis.
Because prurigo pigmentosa is rare, it often is misdiagnosed as another condition with a similar presentation and nonspecific biopsy findings.6 Allergic contact dermatitis is a common type IV delayed hypersensitivity reaction that manifests similar to prurigo pigmentosa with pruritus and a well-demarcated distribution10 that is related to the pattern of allergen exposure; in the case of allergic contact dermatitis related to textiles, a well-demarcated rash will appear in the distribution area of the associated clothing (eg, shirt, pants, shorts).11 Development of allergy involves exposure and sensitization to an allergen, followed by subsequent re-exposure that results in cutaneous T-cell activation and inflammation. 10 Histopathology shows nonspecific spongiotic inflammation, and the gold standard for diagnosis is patch testing to identify the causative substance(s). Definitive treatment includes avoidance of identified allergies; however, if patients are unable to avoid the allergen or the cause is unknown, then corticosteroids, antihistamines, and/or calcineurin inhibitors are beneficial in controlling symptoms and flares.10
Pityrosporum folliculitis (also known as Malassezia folliculitis) is a fungal acneform condition that arises from overgrowth of normal skin flora Malassezia yeast,12 which may be due to occlusion of follicles or disruption of the normal flora composition. Clinically, the manifestation may resemble prurigo pigmentosa in distribution and presence of intense pruritus. However, pustular lesions and involvement of the face can aid in differentiating Pityrosporum from prurigo pigmentosa, which can be confirmed via periodic acid–Schiff staining with numerous round yeasts within affected follicles. Oral antifungal therapy typically yields rapid improvement and resolution of symptoms.12
Urticaria and prurigo pigmentosa share similar clinical characteristics, with symptoms of intense pruritus and urticarial lesions on the trunk.2,13 Urticaria is an IgEmediated type I hypersensitivity reaction characterized by wheals (ie, edematous red or pink lesions of variable size and shape that typically resolve spontaneously within 24–48 hours).13 Notably, urticaria will improve and in some cases completely resolve with antihistamines or anti-IgE antibody treatment, which may aid in distinguishing it from prurigo pigmentosa, as the latter typically exhibits limited response to such treatment.2 Histopathology also can assist in the diagnosis by ruling out other causes of similar rash; however, biopsies are not routinely done unless other inflammatory conditions are of high suspicion.13
Bullous pemphigoid is an autoimmune, subepidermal, blistering dermatosis that is most common among the elderly.14 It is characterized by the presence of IgG antibodies that target BP180 and BP230, which initiate inflammatory cascades that lead to tissue damage and blister formation. It typically manifests as pruritic blistering eruptions, primarily on the limbs and trunk, but may involve the head, neck, or palmoplantar regions.14 Although blistering eruptions are the prodrome of the disease, some cases may present with nonspecific urticarial or eczematous lesions14,15 that may resemble prurigo pigmentosa. The diagnosis is confirmed through direct immunofluorescence microscopy of biopsied lesions, which reveals IgG and/or C3 deposits along the dermoepidermal junction.14 Management of bullous pemphigoid involves timely initiation of dapsone or systemic corticosteroids, which have demonstrated high efficacy in controlling the disease and its associated symptoms.15
Our patient achieved a favorable response to diet modification and doxycycline therapy consistent with the diagnosis of prurigo pigmentosa. Unfortunately, the condition recurred following a relapse of anorexia. Management of prurigo pigmentosa necessitates not only accurate diagnosis but also addressing any underlying factors that may contribute to disease exacerbation. We anticipate the eating disorder will pose a major challenge in achieving long-term control of prurigo pigmentosa.
The Diagnosis: Prurigo Pigmentosa
A comprehensive metabolic panel collected from our patient 1 month earlier did not reveal any abnormalities. Serum methylmalonic acid and homocysteine were both elevated at 417 nmol/L (reference range [for those aged 2–59 years], 55–335 nmol/L) and 23 μmol/L (reference range, 5–15 μmol/L), respectively. Serum folate and 25-hydroxyvitamin D were low at 3.1 ng/mL (reference range, >4.8 ng/mL) and 5 ng/mL (reference range, 30–80 ng/mL), respectively. Vitamin B12 was within reference range. Two 4-mm punch biopsies collected from the upper back showed spongiotic dermatitis.
Our patient’s histopathology results along with the rash distribution and medical history of anorexia increased suspicion for prurigo pigmentosa. A trial of oral doxycycline 100 mg twice daily for 2 weeks was prescribed. At 2-week follow-up, the patient’s mother revealed a history of ketosis in her daughter, solidifying the diagnosis. The patient was counseled on maintaining a healthy diet to prevent future breakouts. The patient’s rash resolved with diet modification and doxycycline; however, it recurred upon relapse of anorexia 4 months later.
Prurigo pigmentosa, originally identified in Japan by Nagashima et al,1 is an uncommon recurrent inflammatory disorder predominantly observed in young adults of Asian descent. Subsequently, it was reported to occur among individuals from different ethnic backgrounds, indicating potential underdiagnosis or misdiagnosis in Western countries.2 Although a direct pathogenic cause for prurigo pigmentosa has not been identified, a strong association has been linked to diet, specifically when ketosis is induced, such as in ketogenic diets and anorexia nervosa.3-5 Other possible causes include sunlight exposure, clothing friction, and sweating.1,5 The disease course is characterized by intermittent flares and spontaneous resolution, with recurrence in most cases. During the active phase, intensely pruritic, papulovesicular or urticarial papules are predominant and most often are localized to the upper body and torso, including the back, shoulders, neck, and chest.5 These flares can persist for several days but eventually subside, leaving behind a characteristic reticular pigmentation that can persist for months.5 First-line treatment often involves the use of tetracycline antibiotics, such as minocycline or doxycycline. 2,4,5 Dapsone often is used with successful resolution. 6 Dietary modifications also have been found to be effective in treating prurigo pigmentosa, particularly in patients presenting with dietary insufficiency.6,7 Increased carbohydrate intake has been shown to promote resolution. 6 Topical corticosteroids demonstrate limited efficacy in controlling flares.6,8
Histopathology has been variably described, with initial findings reported as nonspecific.1 However, it was later described as a distinct inflammatory disease of the skin with histologically distinct stages.2,9 Early stages reveal scattered dermal, dermal papillary, and perivascular neutrophilic infiltration.9 The lesions then progress and become fully developed, at which point neutrophilic infiltration becomes more prominent, accompanied by the presence of intraepidermal neutrophils and spongiosis. As the lesions resolve, the infiltration transitions to lymphocytic, and lichenoid changes can sometimes be appreciated along with epidermal hyperplasia, hyperpigmentation, and dermal melanophages.9 Although these findings aid in the diagnosis of prurigo pigmentosa, a clinicopathologic correlation is necessary to establish a definitive diagnosis.
Because prurigo pigmentosa is rare, it often is misdiagnosed as another condition with a similar presentation and nonspecific biopsy findings.6 Allergic contact dermatitis is a common type IV delayed hypersensitivity reaction that manifests similar to prurigo pigmentosa with pruritus and a well-demarcated distribution10 that is related to the pattern of allergen exposure; in the case of allergic contact dermatitis related to textiles, a well-demarcated rash will appear in the distribution area of the associated clothing (eg, shirt, pants, shorts).11 Development of allergy involves exposure and sensitization to an allergen, followed by subsequent re-exposure that results in cutaneous T-cell activation and inflammation. 10 Histopathology shows nonspecific spongiotic inflammation, and the gold standard for diagnosis is patch testing to identify the causative substance(s). Definitive treatment includes avoidance of identified allergies; however, if patients are unable to avoid the allergen or the cause is unknown, then corticosteroids, antihistamines, and/or calcineurin inhibitors are beneficial in controlling symptoms and flares.10
Pityrosporum folliculitis (also known as Malassezia folliculitis) is a fungal acneform condition that arises from overgrowth of normal skin flora Malassezia yeast,12 which may be due to occlusion of follicles or disruption of the normal flora composition. Clinically, the manifestation may resemble prurigo pigmentosa in distribution and presence of intense pruritus. However, pustular lesions and involvement of the face can aid in differentiating Pityrosporum from prurigo pigmentosa, which can be confirmed via periodic acid–Schiff staining with numerous round yeasts within affected follicles. Oral antifungal therapy typically yields rapid improvement and resolution of symptoms.12
Urticaria and prurigo pigmentosa share similar clinical characteristics, with symptoms of intense pruritus and urticarial lesions on the trunk.2,13 Urticaria is an IgEmediated type I hypersensitivity reaction characterized by wheals (ie, edematous red or pink lesions of variable size and shape that typically resolve spontaneously within 24–48 hours).13 Notably, urticaria will improve and in some cases completely resolve with antihistamines or anti-IgE antibody treatment, which may aid in distinguishing it from prurigo pigmentosa, as the latter typically exhibits limited response to such treatment.2 Histopathology also can assist in the diagnosis by ruling out other causes of similar rash; however, biopsies are not routinely done unless other inflammatory conditions are of high suspicion.13
Bullous pemphigoid is an autoimmune, subepidermal, blistering dermatosis that is most common among the elderly.14 It is characterized by the presence of IgG antibodies that target BP180 and BP230, which initiate inflammatory cascades that lead to tissue damage and blister formation. It typically manifests as pruritic blistering eruptions, primarily on the limbs and trunk, but may involve the head, neck, or palmoplantar regions.14 Although blistering eruptions are the prodrome of the disease, some cases may present with nonspecific urticarial or eczematous lesions14,15 that may resemble prurigo pigmentosa. The diagnosis is confirmed through direct immunofluorescence microscopy of biopsied lesions, which reveals IgG and/or C3 deposits along the dermoepidermal junction.14 Management of bullous pemphigoid involves timely initiation of dapsone or systemic corticosteroids, which have demonstrated high efficacy in controlling the disease and its associated symptoms.15
Our patient achieved a favorable response to diet modification and doxycycline therapy consistent with the diagnosis of prurigo pigmentosa. Unfortunately, the condition recurred following a relapse of anorexia. Management of prurigo pigmentosa necessitates not only accurate diagnosis but also addressing any underlying factors that may contribute to disease exacerbation. We anticipate the eating disorder will pose a major challenge in achieving long-term control of prurigo pigmentosa.
- Nagashima M, Ohshiro A, Shimizu N. A peculiar pruriginous dermatosis with gross reticular pigmentation. Jpn J Dermatol. 1971;81:38-39.
- Boer A, Asgari M. Prurigo pigmentosa: an underdiagnosed disease? Indian J Dermatol Venereol Leprol. 2006;72:405-409. doi:10.4103/0378-6323.29334
- Michaels JD, Hoss E, DiCaudo DJ, et al. Prurigo pigmentosa after a strict ketogenic diet. Pediatr Dermatol. 2013;32:248-251. doi:10.1111/pde.12275
- Teraki Y, Teraki E, Kawashima M, et al. Ketosis is involved in the origin of prurigo pigmentosa. J Am Acad Dermatol. 1996;34:509-511. doi:10.1016/s0190-9622(96)90460-0
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79-87. doi:10.1016/j.jdin.2021.03.003
- Wong M, Lee E, Wu Y, et al. Treatment of prurigo pigmentosa with diet modification: a medical case study. Hawaii J Med Public Health. 2018;77:114-117.
- Almaani N, Al-Tarawneh AH, Msallam H. Prurigo pigmentosa: a clinicopathological report of three Middle Eastern patients. Case Rep Dermatol Med. 2018;2018:9406797. doi:10.1155/2018/9406797
- Kim JK, Chung WK, Chang SE, et al. Prurigo pigmentosa: clinicopathological study and analysis of 50 cases in Korea. J Dermatol. 2012;39:891-897. doi:10.1111/j.1346-8138.2012.01640.x
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi:10.1016/j.jaad.2015.02.1139
- Lazarov A, Cordoba M, Plosk N, et al. Atypical and unusual clinical manifestations of contact dermatitis to clothing (textile contact dermatitis)—case presentation and review of the literature. Dermatol Online J. 2003;9. doi:10.5070/d30kd1d259
- Rubenstein RM, Malerich SA. Malassezia (Pityrosporum) folliculitis. J Clin Aesthet Dermatol. 2014;7:37-41.
- Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270-1277. doi:10.1016/j.jaci.2014.02.036
- della Torre R, Combescure C, Cortés B, et al. Clinical presentation and diagnostic delay in bullous pemphigoid: a prospective nationwide cohort. Br J Dermatol. 2012;167:1111-1117. doi:10.1111/j.1365-2133.2012.11108.x
- Alonso-Llamazares J, Rogers RS 3rd, Oursler JR, et al. Bullous pemphigoid presenting as generalized pruritus: observations in six patients. Int J Dermatol. 1998;37:508-514.
- Nagashima M, Ohshiro A, Shimizu N. A peculiar pruriginous dermatosis with gross reticular pigmentation. Jpn J Dermatol. 1971;81:38-39.
- Boer A, Asgari M. Prurigo pigmentosa: an underdiagnosed disease? Indian J Dermatol Venereol Leprol. 2006;72:405-409. doi:10.4103/0378-6323.29334
- Michaels JD, Hoss E, DiCaudo DJ, et al. Prurigo pigmentosa after a strict ketogenic diet. Pediatr Dermatol. 2013;32:248-251. doi:10.1111/pde.12275
- Teraki Y, Teraki E, Kawashima M, et al. Ketosis is involved in the origin of prurigo pigmentosa. J Am Acad Dermatol. 1996;34:509-511. doi:10.1016/s0190-9622(96)90460-0
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79-87. doi:10.1016/j.jdin.2021.03.003
- Wong M, Lee E, Wu Y, et al. Treatment of prurigo pigmentosa with diet modification: a medical case study. Hawaii J Med Public Health. 2018;77:114-117.
- Almaani N, Al-Tarawneh AH, Msallam H. Prurigo pigmentosa: a clinicopathological report of three Middle Eastern patients. Case Rep Dermatol Med. 2018;2018:9406797. doi:10.1155/2018/9406797
- Kim JK, Chung WK, Chang SE, et al. Prurigo pigmentosa: clinicopathological study and analysis of 50 cases in Korea. J Dermatol. 2012;39:891-897. doi:10.1111/j.1346-8138.2012.01640.x
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi:10.1016/j.jaad.2015.02.1139
- Lazarov A, Cordoba M, Plosk N, et al. Atypical and unusual clinical manifestations of contact dermatitis to clothing (textile contact dermatitis)—case presentation and review of the literature. Dermatol Online J. 2003;9. doi:10.5070/d30kd1d259
- Rubenstein RM, Malerich SA. Malassezia (Pityrosporum) folliculitis. J Clin Aesthet Dermatol. 2014;7:37-41.
- Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270-1277. doi:10.1016/j.jaci.2014.02.036
- della Torre R, Combescure C, Cortés B, et al. Clinical presentation and diagnostic delay in bullous pemphigoid: a prospective nationwide cohort. Br J Dermatol. 2012;167:1111-1117. doi:10.1111/j.1365-2133.2012.11108.x
- Alonso-Llamazares J, Rogers RS 3rd, Oursler JR, et al. Bullous pemphigoid presenting as generalized pruritus: observations in six patients. Int J Dermatol. 1998;37:508-514.
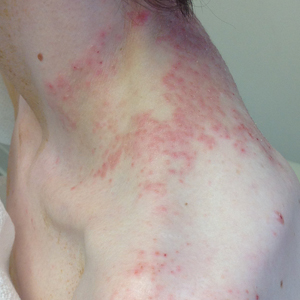
A 43-year-old woman presented with a pruritic rash across the neck and back of 6 months’ duration that progressively worsened. She had a medical history of anorexia nervosa, herpes zoster with a recent flare, and peripheral neuropathy. Physical examination showed numerous red scaly papules across the upper back and shoulders that coalesced in a reticular pattern. No similar papules were seen elsewhere on the body.
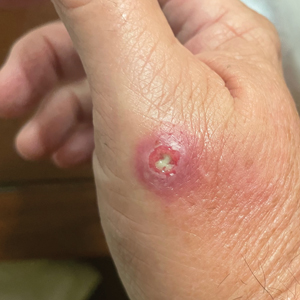
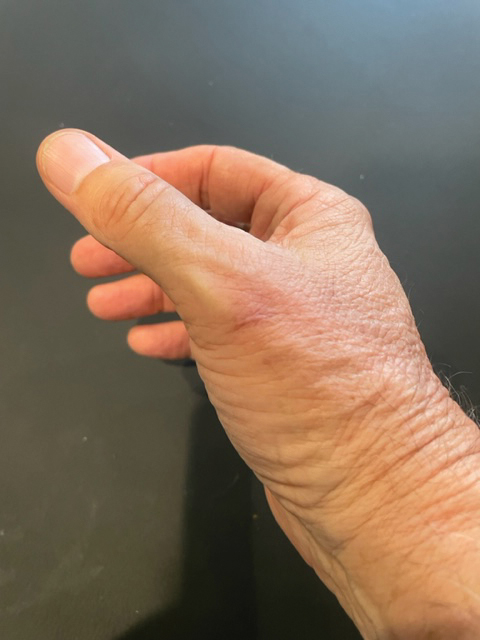
Draining Nodule of the Hand
The Diagnosis: Cutaneous Nocardiosis
The wound culture was positive for Nocardia farcinica. The patient received a 5-day course of intravenous sulfamethoxazole-trimethoprim in the hospital and was transitioned to oral sulfamethoxazoletrimethoprim (800 mg/160 mg taken as 1 tablet twice daily) for 6 months. Complete resolution of the infection was noted at 6-month follow-up (Figure).
Nocardia is a gram-positive, aerobic bacterium that typically is found in soil, water, and decaying organic matter.1 There are more than 50 species; N farcinica, Nocardia nova, and Nocardia asteroides are the leading causes of infection in humans and animals. Nocardia asteroides is the most common cause of infection in humans.1,2 Nocardiosis is an uncommon opportunistic infection that usually targets the skin, lungs, and central nervous system.3 Although it mainly affects individuals who are immunocompromised, up to 30% of infections can be seen in immunocompetent hosts who can contract cutaneous nocardiosis after experiencing traumatic injury to the skin.1
Nocardiosis is difficult to diagnose due to its diverse clinical presentations. For example, cutaneous nocardiosis can manifest similar to mycetoma, sporotrichosis, spider bites, nontuberculous mycobacteria such as Mycobacterium marinum, or methicillin-resistant Staphylococcus aureus infections, thus making cutaneous nocardiosis one of the great imitators.1 A culture is required for definitive diagnosis, as Nocardia grows well on nonselective media such as blood or Löwenstein-Jensen agar. It grows as waxy, pigmented, cerebriform colonies 3 to 5 days following incubation.3 The bacterium can be difficult to culture, and it is important to notify the microbiology laboratory if there is a high index of clinical suspicion for infection.
A history of exposure to gardening or handling animals can increase the risk for an individual contracting Nocardia.3 Although nocardiosis can be found across the world, it is native to tropical and subtropical climates such as those found in India, Africa, Latin America, and Southeast Asia.1 Infections mostly are observed in individuals aged 20 to 40 years and tend to affect men more than women. Lesions typically are seen on the lower extremities, but localized infections also can be found on the torso, neck, and upper extremities.1
Cutaneous nocardiosis is a granulomatous infection encompassing both cutaneous and subcutaneous tissue, which ultimately can lead to injury of bone and viscera.1 Primary cutaneous nocardiosis can manifest as tumors or nodules that have a sporotrichoid pattern, in which they ascend along the lymphatics. Histopathology of infected tissue frequently shows a subcutaneous dermal infiltrate of neutrophils accompanied with abscess formation, and everlasting lesions may show signs of chronic inflammation and nonspecific granulomas.3
Treatment of nocardiosis should be guided by in vitro susceptibility tests. Sulfamethoxazole-trimethoprim 800 mg/160 mg taken as 1 tablet twice daily is the first-line option. The treatment duration is contingent on the extent, severity, and complications of infection but typically is 3 to 6 months.1
- Yu Q, Song J, Liu Y, et al. Progressive primary cutaneous nocardiosis in an immunocompetent patient. Cutis. 2023;111:E22-E25.
- Gaines RJ, Randall CJ, Ruland RT. Lymphocutaneous nocardiosis from commercially treated lumber: a case report. Cutis. 2006;78:249-251.
- Riswold KJ, Tjarks BJ, Kerkvliet AM. Cutaneous nocardiosis in an immunocompromised patient. Cutis. 2019;104:226-229.
The Diagnosis: Cutaneous Nocardiosis
The wound culture was positive for Nocardia farcinica. The patient received a 5-day course of intravenous sulfamethoxazole-trimethoprim in the hospital and was transitioned to oral sulfamethoxazoletrimethoprim (800 mg/160 mg taken as 1 tablet twice daily) for 6 months. Complete resolution of the infection was noted at 6-month follow-up (Figure).
Nocardia is a gram-positive, aerobic bacterium that typically is found in soil, water, and decaying organic matter.1 There are more than 50 species; N farcinica, Nocardia nova, and Nocardia asteroides are the leading causes of infection in humans and animals. Nocardia asteroides is the most common cause of infection in humans.1,2 Nocardiosis is an uncommon opportunistic infection that usually targets the skin, lungs, and central nervous system.3 Although it mainly affects individuals who are immunocompromised, up to 30% of infections can be seen in immunocompetent hosts who can contract cutaneous nocardiosis after experiencing traumatic injury to the skin.1
Nocardiosis is difficult to diagnose due to its diverse clinical presentations. For example, cutaneous nocardiosis can manifest similar to mycetoma, sporotrichosis, spider bites, nontuberculous mycobacteria such as Mycobacterium marinum, or methicillin-resistant Staphylococcus aureus infections, thus making cutaneous nocardiosis one of the great imitators.1 A culture is required for definitive diagnosis, as Nocardia grows well on nonselective media such as blood or Löwenstein-Jensen agar. It grows as waxy, pigmented, cerebriform colonies 3 to 5 days following incubation.3 The bacterium can be difficult to culture, and it is important to notify the microbiology laboratory if there is a high index of clinical suspicion for infection.
A history of exposure to gardening or handling animals can increase the risk for an individual contracting Nocardia.3 Although nocardiosis can be found across the world, it is native to tropical and subtropical climates such as those found in India, Africa, Latin America, and Southeast Asia.1 Infections mostly are observed in individuals aged 20 to 40 years and tend to affect men more than women. Lesions typically are seen on the lower extremities, but localized infections also can be found on the torso, neck, and upper extremities.1

Cutaneous nocardiosis is a granulomatous infection encompassing both cutaneous and subcutaneous tissue, which ultimately can lead to injury of bone and viscera.1 Primary cutaneous nocardiosis can manifest as tumors or nodules that have a sporotrichoid pattern, in which they ascend along the lymphatics. Histopathology of infected tissue frequently shows a subcutaneous dermal infiltrate of neutrophils accompanied with abscess formation, and everlasting lesions may show signs of chronic inflammation and nonspecific granulomas.3
Treatment of nocardiosis should be guided by in vitro susceptibility tests. Sulfamethoxazole-trimethoprim 800 mg/160 mg taken as 1 tablet twice daily is the first-line option. The treatment duration is contingent on the extent, severity, and complications of infection but typically is 3 to 6 months.1
The Diagnosis: Cutaneous Nocardiosis
The wound culture was positive for Nocardia farcinica. The patient received a 5-day course of intravenous sulfamethoxazole-trimethoprim in the hospital and was transitioned to oral sulfamethoxazoletrimethoprim (800 mg/160 mg taken as 1 tablet twice daily) for 6 months. Complete resolution of the infection was noted at 6-month follow-up (Figure).
Nocardia is a gram-positive, aerobic bacterium that typically is found in soil, water, and decaying organic matter.1 There are more than 50 species; N farcinica, Nocardia nova, and Nocardia asteroides are the leading causes of infection in humans and animals. Nocardia asteroides is the most common cause of infection in humans.1,2 Nocardiosis is an uncommon opportunistic infection that usually targets the skin, lungs, and central nervous system.3 Although it mainly affects individuals who are immunocompromised, up to 30% of infections can be seen in immunocompetent hosts who can contract cutaneous nocardiosis after experiencing traumatic injury to the skin.1
Nocardiosis is difficult to diagnose due to its diverse clinical presentations. For example, cutaneous nocardiosis can manifest similar to mycetoma, sporotrichosis, spider bites, nontuberculous mycobacteria such as Mycobacterium marinum, or methicillin-resistant Staphylococcus aureus infections, thus making cutaneous nocardiosis one of the great imitators.1 A culture is required for definitive diagnosis, as Nocardia grows well on nonselective media such as blood or Löwenstein-Jensen agar. It grows as waxy, pigmented, cerebriform colonies 3 to 5 days following incubation.3 The bacterium can be difficult to culture, and it is important to notify the microbiology laboratory if there is a high index of clinical suspicion for infection.
A history of exposure to gardening or handling animals can increase the risk for an individual contracting Nocardia.3 Although nocardiosis can be found across the world, it is native to tropical and subtropical climates such as those found in India, Africa, Latin America, and Southeast Asia.1 Infections mostly are observed in individuals aged 20 to 40 years and tend to affect men more than women. Lesions typically are seen on the lower extremities, but localized infections also can be found on the torso, neck, and upper extremities.1

Cutaneous nocardiosis is a granulomatous infection encompassing both cutaneous and subcutaneous tissue, which ultimately can lead to injury of bone and viscera.1 Primary cutaneous nocardiosis can manifest as tumors or nodules that have a sporotrichoid pattern, in which they ascend along the lymphatics. Histopathology of infected tissue frequently shows a subcutaneous dermal infiltrate of neutrophils accompanied with abscess formation, and everlasting lesions may show signs of chronic inflammation and nonspecific granulomas.3
Treatment of nocardiosis should be guided by in vitro susceptibility tests. Sulfamethoxazole-trimethoprim 800 mg/160 mg taken as 1 tablet twice daily is the first-line option. The treatment duration is contingent on the extent, severity, and complications of infection but typically is 3 to 6 months.1
- Yu Q, Song J, Liu Y, et al. Progressive primary cutaneous nocardiosis in an immunocompetent patient. Cutis. 2023;111:E22-E25.
- Gaines RJ, Randall CJ, Ruland RT. Lymphocutaneous nocardiosis from commercially treated lumber: a case report. Cutis. 2006;78:249-251.
- Riswold KJ, Tjarks BJ, Kerkvliet AM. Cutaneous nocardiosis in an immunocompromised patient. Cutis. 2019;104:226-229.
- Yu Q, Song J, Liu Y, et al. Progressive primary cutaneous nocardiosis in an immunocompetent patient. Cutis. 2023;111:E22-E25.
- Gaines RJ, Randall CJ, Ruland RT. Lymphocutaneous nocardiosis from commercially treated lumber: a case report. Cutis. 2006;78:249-251.
- Riswold KJ, Tjarks BJ, Kerkvliet AM. Cutaneous nocardiosis in an immunocompromised patient. Cutis. 2019;104:226-229.
A 67-year-old man presented to the emergency department with a draining nodule on the right hand of 4 days’ duration. He reported that the swelling and redness started 1 hour after handling a succulent plant. The following day, the nodule increased in size and exudated yellow pus. He presented with swelling of the thumb and hand, which resulted in a decreased range of motion. He had a history of prediabetes and denied any recent travel, allergies, or animal exposures. Physical examination revealed a draining nodule on the dorsal aspect of the right hand that measured approximately 15×15 mm with surrounding erythema and tenderness. There also was progression of ascending erythema up to the axilla. The patient was admitted to the hospital.
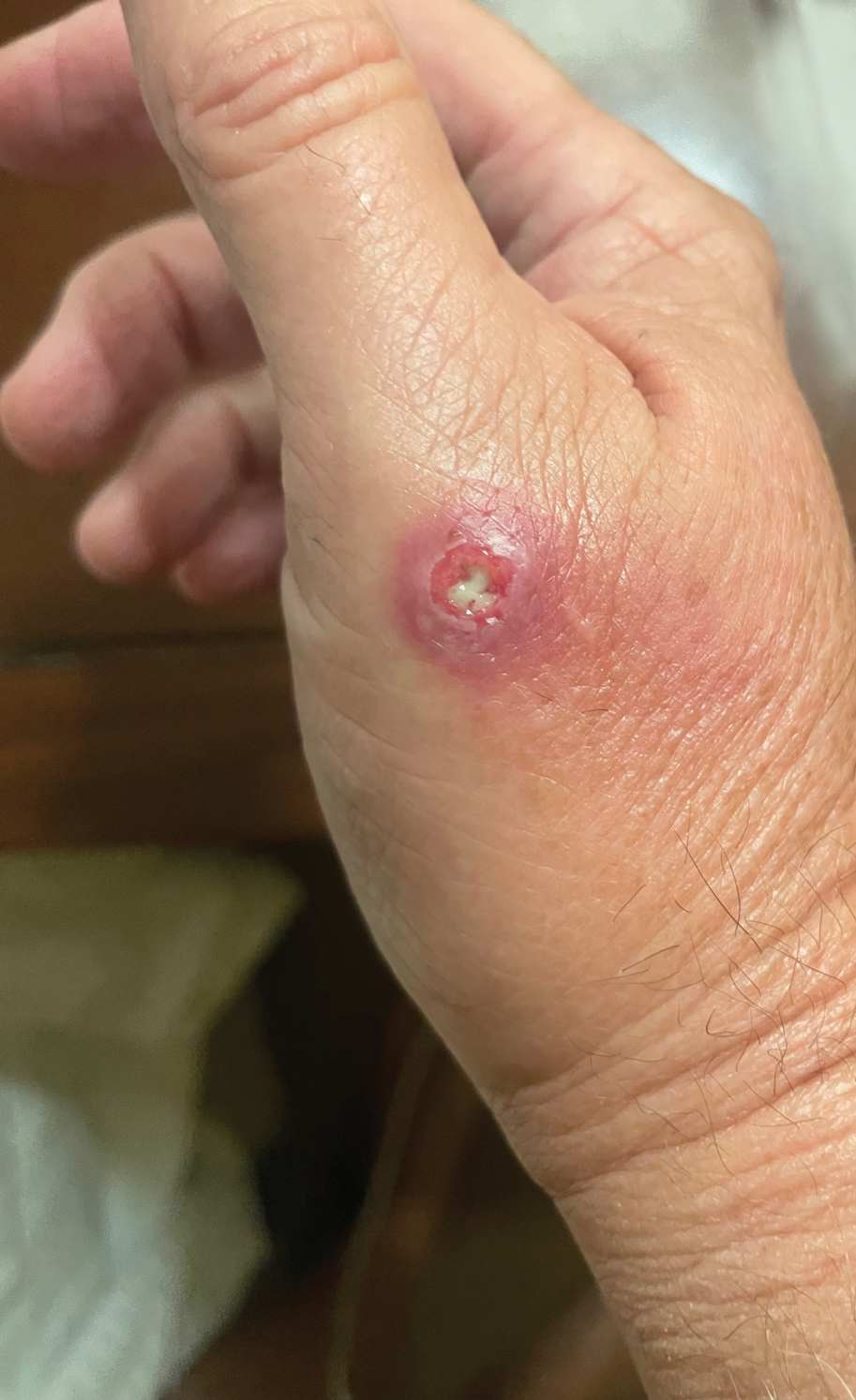
Vascular Mass on the Posterior Neck in a Newborn
The Diagnosis: Congenital Hemangioma
Surgical resection of the mass was performed at 4 months of age without complication (Figure 1). Histopathology revealed a lobular endothelial cell proliferation within a densely fibrotic stroma, multiple thin-walled vessels, and negative immunoreactivity to glucose transporter type 1 (GLUT-1)(Figures 2 and 3). Combined with the patient’s clinical history and findings on imaging (Figure 4), the most accurate diagnosis was a congenital hemangioma (CH). The mass was determined to be a noninvoluting congenital hemangioma (NICH).
A variety of vascular anomalies manifest in newborns and can be differentiated by the patient’s clinical history—particularly whether the lesion is present at birth or develops after birth. Imaging and histopathology of the lesion(s) may be utilized when clinical examination alone is not sufficient to make a diagnosis. Histopathology and immunohistochemistry further aid in differentiating the type of vascular lesion.

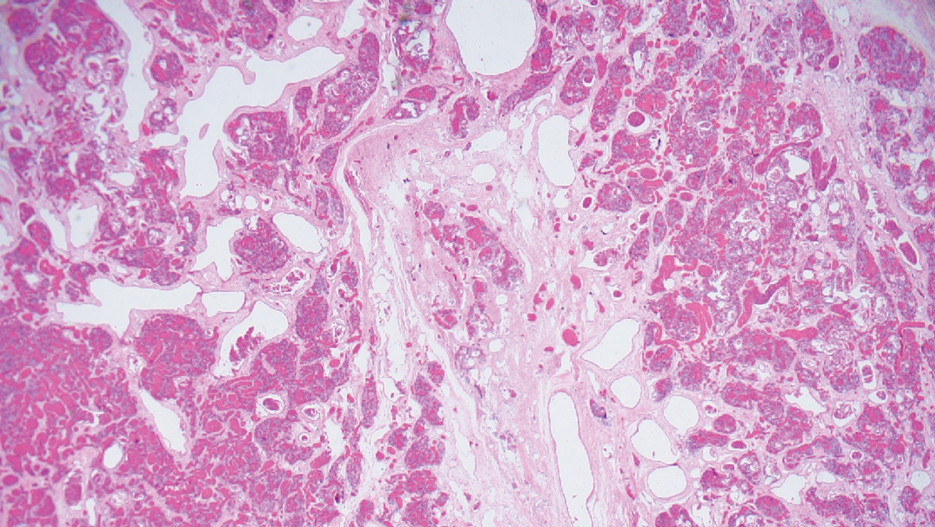
Overall, vascular anomalies are classified broadly into 2 categories based on their pathogenesis: tumors and malformations. Vascular tumors are composed of proliferating endothelial cells that have the potential to resolve spontaneously over time. Examples include CH, infantile hemangioma (IH), kaposiform hemangioendothelioma (KHE), and tufted angioma (TA). In contrast, vascular malformations (ie, arteriovenous malformations) are composed of dysplastic vessels with normal endothelial cell turnover and do not resolve without intervention.1
Congenital hemangiomas are rare vascular tumors that are fully developed at birth. These tumors proliferate in utero, enabling prenatal detection via ultrasonography as early as 12 weeks’ gestation for large heterogeneous vascular masses.2-4 Congenital hemangiomas are described as solitary, well-circumscribed, raised, violaceous lesions most commonly located in the head and neck region.4-6 Histopathologically, they are characterized by lobules of proliferating capillaries surrounded by fibrous stroma and dysplastic vascular channels.6,7
Congenital hemangiomas are categorized based on their postnatal involution patterns.2 Fetally involuting CH both develops and begins regression in utero and often is completely regressed at birth.8 Rapidly involuting CH begins regression in the first few weeks of life and usually is completely involuted by 14 months of age.6,9-11 Conversely, NICH does not regress, often requiring surgical excision due to functional and cosmetic issues.12,13 Partially involuting CH is intermediary, beginning as rapidly involuting but not involuting completely and persisting as lesions that resemble NICH.14-16 Although generally benign and asymptomatic, these tumors can cause transient thrombocytopenia and coagulopathy at birth, as seen in our patient.17,18
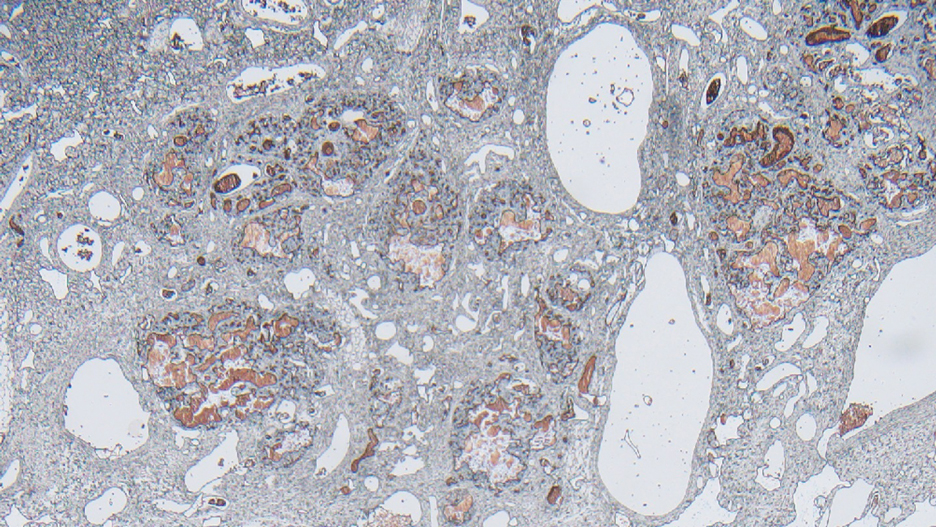
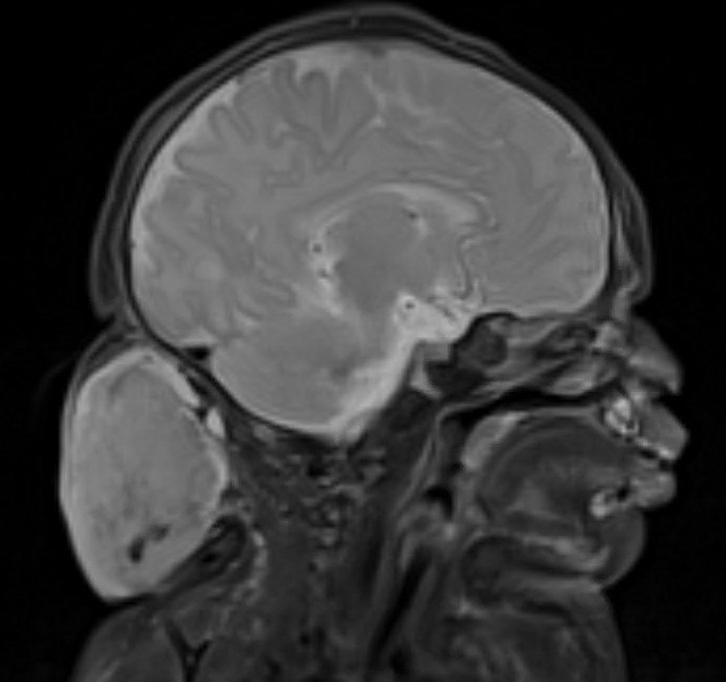
Infantile hemangioma is the most common vascular tumor of infancy.19-21 Although a precursor lesion may be present at birth, generally this tumor becomes apparent after the first few weeks of life as a solitary vascular plaque or nodule with a predilection for the head and neck.22-25 Once it arises, IH quickly enters a period of rapid growth, followed by a period of slower continued growth, with most reaching maximum size by 3 months.22 Thereafter, IH enters a slow period of involution (range, 3–9 years)26; more recent data suggest near resolution by 5 years of age.27 Infantile hemangioma is categorized based on its depth in the skin and subcutaneous tissues and can be classified as superficial, mixed, or deep.22,24,28,29 Superficial IH appears as a red plaque and may exhibit lobulation, while deep IH can be identified as flesh-colored or blue subcutaneous masses. Mixed IH may manifest with both superficial and deep features depending on the extent of its involvement in the dermal and subcutaneous layers. The pattern of involvement may be focal, segmental, or indeterminate.24 In contrast, CH typically is a solitary vascular mass with prominent telangiectases, nodules, and radiating veins.6 Histologically, IH is composed of proliferative plump endothelial cells that form capillaries, and the lesion stains positively for GLUT-1, whereas CH does not.30
Kaposiform hemangioendothelioma is classified as a locally aggressive vascular tumor that manifests either prenatally or in early infancy.31 It is described as a solitary, ill-defined, firm, purple plaque most commonly located on the extremities and retroperitoneum.32-34 Histopathologically, these lesions are characterized by dilated lymphatic channels and irregular sheets or lobules of spindle-shaped endothelial cells infiltrating the dermis and subcutaneous fat.33,35 In contrast to CH, KHE lesions show immunoreactivity to the markers podoplanin, lymphatic vessel endothelial receptor 1, and prospero homeobox 1 protein.36,37 Notably, 70% of these tumors are complicated by the presence of Kasabach-Merritt phenomenon, a potentially life-threatening emergency that occurs when platelets are trapped within a vascular tumor, leading to the consumption of clotting factors, intralesional bleeding, and rapid enlargement of the tumor.32 The Kasabach-Merritt phenomenon manifests clinically as microangiopathic hemolytic anemia, severe thrombocytopenia, and disseminated intravascular coagulation. 38 Although CH lesions also can be associated with thrombocytopenia and coagulopathy, they generally are mild and self-limited.18
Tufted angioma is a vascular tumor that arises within the first 5 years of life as firm violaceous papules or plaques, often with associated hyperhidrosis or hypertrichosis.39,40 Although TA grows slowly for a period of time, it eventually stabilizes and persists, rarely regressing completely.41 These tumors share many similarities with KHE, and it has been suggested that they may be part of the same spectrum. 42 As with KHE, TA lesions show immunoreactivity to the markers podoplanin, lymphatic vessel endothelial receptor 1, and prospero homeobox 1 protein, which are negative in CH.36,37 Although TA also can be complicated by Kasabach-Merritt phenomenon, the incidence is much lower (up to 38%).43,44 As such, TAs tend to be recognized as more superficial benign lesions. However, they still can cause notable cosmetic and functional impairment and should be monitored closely, especially in the presence of associated symptoms or complications.
Arteriovenous malformation is a vascular lesion that results from errors during the embryonic development of vascular channels.45 Although present at birth, it may not become clinically apparent until later in life. Arteriovenous malformations enlarge postnatally, and their growth is proportional to the developmental growth of the affected individual rather than the result of endothelial proliferation.46 In infants, AVM may manifest as a faint vascular stain that can evolve over time into a pink patch associated with a palpable thrill during adolescence. 4 On Doppler flow imaging, AVMs are identified as fast-flow anomalies arising from an abnormal communication between high-pressure arterial systems and low-pressure venous systems without the presence of a capillary bed.47 One of the differentiating factors between AVM and CH is that AVMs do not regress spontaneously and tend to have high recurrence rates, even with intervention. 48 In contrast, CH can be categorized based on its postnatal involution pattern. Another distinguishing factor is that AVMs tend to be larger and more invasive than CHs.46 Therefore, early diagnosis and intervention are crucial to prevent complications such as bleeding, seizures, or neurologic deficits associated with AVMs.1
- Enjolras O, Wassef M, Chapot R. Introduction: ISSVA Classification. In: Enjolras O, Wassef M, Chapot R, eds. Color Atlas of Vascular Tumors and Vascular Malformations. Cambridge University Press; 2007:3-11.
- Fadell MF, Jones BV, Adams DM. Prenatal diagnosis and postnatal follow-up of rapidly involuting congenital hemangioma (RICH). Pediatr Radiol. 2011;41:1057-1060.
- Feygin T, Khalek N, Moldenhauer JS. Fetal brain, head, and neck tumors: prenatal imaging and management. Prenat Diagn. 2020;40:1203-1219.
- Foley LS, Kulungowski AM. Vascular anomalies in pediatrics. Adv Pediatr. 2015;62:227-255.
- Bruder E, Alaggio R, Kozakewich HPW, et al. Vascular and perivascular lesions of skin and soft tissues in children and adolescents. Pediatr Dev Pathol. 2012;15:26-61.
- Berenguer B, Mulliken JB, Enjolras O, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol. 2003;6:495-510.
- North PE, Waner M, James CA, et al. Congenital nonprogressive hemangioma: a distinct clinicopathologic entity unlike infantile hemangioma. Arch Dermatol. 2001;137:1607-1620.
- Maguiness S, Uihlein LC, Liang MG, et al. Rapidly involuting congenital hemangioma with fetal involution. Pediatr Dermatol. 2015;32:321-326.
- Keating LJ, Soares GM, Muratore CS. Rapidly involuting congenital hemangioma. Med Health R I. 2012;95:149-152.
- Schafer F, Tapia M, Pinto C. Rapidly involuting congenital haemangioma. Arch Dis Child Fetal Neonatal Ed. 2014;99:F422.
- Boon LM, Enjolras O, Mulliken JB. Congenital hemangioma: evidence of accelerated involution. J Pediatr. 1996;128:329-335.
- Liang MG, Frieden IJ. Infantile and congenital hemangiomas. Semin Pediatr Surg. 2014;23:162-167.
- Enjolras O, Mulliken JB, Boon LM, et al. Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg. 2001;107:1647-1654.
- Nasseri E, Piram M, McCuaig CC, et al. Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. J Am Acad Dermatol. 2014;70:75-79.
- Wassef M, Blei F, Adams D, et al. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136:E203-E214.
- Boull C, Maguiness SM. Congenital hemangiomas. Semin Cutan Med Surg. 2016;35:124-127.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Baselga E, Cordisco MR, Garzon M, et al. Rapidly involuting congenital haemangioma associated with transient thrombocytopenia and coagulopathy: a case series. Br J Dermatol. 2008;158:1363-1370.
- Kanada KN, Merin MR, Munden A, et al. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr. 2012;161:240-245.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360-367.
- Hidano A, Nakajima S. Earliest features of the strawberry mark in the newborn. Br J Dermatol. 1972;87:138-144.
- Martinez-Perez D, Fein NA, Boon LM, et al. Not all hemangiomas look like strawberries: uncommon presentations of the most common tumor of infancy. Pediatr Dermatol. 1995;12:1-6.
- Payne MM, Moyer F, Marcks KM, et al. The precursor to the hemangioma. Plast Reconstr Surg. 1966;38:64-67.
- Bowers RE, Graham EA, Tomlinson KM. The natural history of the strawberry nevus. Arch Dermatol. 1960;82:667-680.
- Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624.
- Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med. 1999;341:173-181.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567-1576.
- North PE, Waner M, Mizeracki A, et al. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31:11-22.
- Gruman A, Liang MG, Mulliken JB, et al. Kaposiform hemangioendothelioma without Kasabach-Merritt phenomenon. J Am Acad Dermatol. 2005;52:616-622.
- Croteau SE, Liang MG, Kozakewich HP, et al. Kaposiform hemangioendothelioma: atypical features and risks of Kasabach- Merritt phenomenon in 107 referrals. J Pediatr. 2013;162:142-147.
- Zukerberg LR, Nickoloff BJ, Weiss SW. Kaposiform hemangioendothelioma of infancy and childhood. an aggressive neoplasm associated with Kasabach-Merritt syndrome and lymphangiomatosis. Am J Surg Pathol. 1993;17:321-328.
- Mac-Moune Lai F, To KF, Choi PC, et al. Kaposiform hemangioendothelioma: five patients with cutaneous lesion and long follow-up. Mod Pathol. 2001;14:1087-1092.
- O’Rafferty C, O’Regan GM, Irvine AD, et al. Recent advances in the pathobiology and management of Kasabach-Merritt phenomenon. Br J Haematol. 2015;171:38-51.
- Le Huu AR, Jokinen CH, Rubin BP, et al. Expression of prox1, lymphatic endothelial nuclear transcription factor, in kaposiform hemangioendothelioma and tufted angioma. Am J Surg Pathol. 2010;34:1563-1573.
- Debelenko LV, Perez-Atayde AR, Mulliken JB, et al. D2-40 immuno-histochemical analysis of pediatric vascular tumors reveals positivity in kaposiform hemangioendothelioma. Mod Pathol. 2005;18:1454-1460.
- Haisley-Royster C, Enjolras O, Frieden IJ, et al. Kasabach-Merritt phenomenon: a retrospective study of treatment with vincristine. J Pediatr Hematol Oncol. 2002;24:459-462.
- Wilmer A, Kaatz M, Bocker T, et al. Tufted angioma. Eur J Dermatol. 1999;9:51-53.
- Herron MD, Coffin CM, Vanderhooft SL. Tufted angiomas: variability of the clinical morphology. Pediatr Dermatol. 2002;19:394-401.
- North PE. Pediatric vascular tumors and malformations. Surg Pathol Clin. 2010,3:455-494.
- Chu CY, Hsiao CH, Chiu HC. Transformation between kaposiform hemangioendothelioma and tufted angioma. Dermatology. 2003;206:334-337.
- Osio A, Fraitag S, Hadj-Rabia S, et al. Clinical spectrum of tufted angiomas in childhood: a report of 13 cases and a review of the literature. Arch Dermatol. 2010;146:758-763.
- Johnson EF, Davis DM, Tollefson MM, et al. Vascular tumors in infants: case report and review of clinical, histopathologic, and immunohistochemical characteristics of infantile hemangioma, pyogenic granuloma, noninvoluting congenital hemangioma, tufted angioma, and kaposiform hemangioendothelioma. Am J Dermatopathol. 2018;40:231-239.
- Christison-Lagay ER, Fishman SJ. Vascular anomalies. Surg Clin North Am. 2006;86:393-425.
- Liu AS, Mulliken JB, Zurakowski D, et al. Extracranial arteriovenous malformations: natural progression and recurrence after treatment. Plast Reconstr Surg. 2010;125:1185-1194.
- Young AE, Mulliken JB. Arteriovenous malformations. In: Mulliken JB, Young AE, eds. Vascular Birthmarks: Haemangiomas and Malformations. WB Saunders; 1988:228-245.
- Duggan EM, Fishman SJ. Vascular anomalies. In: Holcomb GW III, Murphy JP, St Peter SD, eds. Holcomb and Ashcraft’s Pediatric Surgery. 7th edition. Elsevier; 2019:1147-1170.
The Diagnosis: Congenital Hemangioma
Surgical resection of the mass was performed at 4 months of age without complication (Figure 1). Histopathology revealed a lobular endothelial cell proliferation within a densely fibrotic stroma, multiple thin-walled vessels, and negative immunoreactivity to glucose transporter type 1 (GLUT-1)(Figures 2 and 3). Combined with the patient’s clinical history and findings on imaging (Figure 4), the most accurate diagnosis was a congenital hemangioma (CH). The mass was determined to be a noninvoluting congenital hemangioma (NICH).
A variety of vascular anomalies manifest in newborns and can be differentiated by the patient’s clinical history—particularly whether the lesion is present at birth or develops after birth. Imaging and histopathology of the lesion(s) may be utilized when clinical examination alone is not sufficient to make a diagnosis. Histopathology and immunohistochemistry further aid in differentiating the type of vascular lesion.


Overall, vascular anomalies are classified broadly into 2 categories based on their pathogenesis: tumors and malformations. Vascular tumors are composed of proliferating endothelial cells that have the potential to resolve spontaneously over time. Examples include CH, infantile hemangioma (IH), kaposiform hemangioendothelioma (KHE), and tufted angioma (TA). In contrast, vascular malformations (ie, arteriovenous malformations) are composed of dysplastic vessels with normal endothelial cell turnover and do not resolve without intervention.1
Congenital hemangiomas are rare vascular tumors that are fully developed at birth. These tumors proliferate in utero, enabling prenatal detection via ultrasonography as early as 12 weeks’ gestation for large heterogeneous vascular masses.2-4 Congenital hemangiomas are described as solitary, well-circumscribed, raised, violaceous lesions most commonly located in the head and neck region.4-6 Histopathologically, they are characterized by lobules of proliferating capillaries surrounded by fibrous stroma and dysplastic vascular channels.6,7
Congenital hemangiomas are categorized based on their postnatal involution patterns.2 Fetally involuting CH both develops and begins regression in utero and often is completely regressed at birth.8 Rapidly involuting CH begins regression in the first few weeks of life and usually is completely involuted by 14 months of age.6,9-11 Conversely, NICH does not regress, often requiring surgical excision due to functional and cosmetic issues.12,13 Partially involuting CH is intermediary, beginning as rapidly involuting but not involuting completely and persisting as lesions that resemble NICH.14-16 Although generally benign and asymptomatic, these tumors can cause transient thrombocytopenia and coagulopathy at birth, as seen in our patient.17,18


Infantile hemangioma is the most common vascular tumor of infancy.19-21 Although a precursor lesion may be present at birth, generally this tumor becomes apparent after the first few weeks of life as a solitary vascular plaque or nodule with a predilection for the head and neck.22-25 Once it arises, IH quickly enters a period of rapid growth, followed by a period of slower continued growth, with most reaching maximum size by 3 months.22 Thereafter, IH enters a slow period of involution (range, 3–9 years)26; more recent data suggest near resolution by 5 years of age.27 Infantile hemangioma is categorized based on its depth in the skin and subcutaneous tissues and can be classified as superficial, mixed, or deep.22,24,28,29 Superficial IH appears as a red plaque and may exhibit lobulation, while deep IH can be identified as flesh-colored or blue subcutaneous masses. Mixed IH may manifest with both superficial and deep features depending on the extent of its involvement in the dermal and subcutaneous layers. The pattern of involvement may be focal, segmental, or indeterminate.24 In contrast, CH typically is a solitary vascular mass with prominent telangiectases, nodules, and radiating veins.6 Histologically, IH is composed of proliferative plump endothelial cells that form capillaries, and the lesion stains positively for GLUT-1, whereas CH does not.30
Kaposiform hemangioendothelioma is classified as a locally aggressive vascular tumor that manifests either prenatally or in early infancy.31 It is described as a solitary, ill-defined, firm, purple plaque most commonly located on the extremities and retroperitoneum.32-34 Histopathologically, these lesions are characterized by dilated lymphatic channels and irregular sheets or lobules of spindle-shaped endothelial cells infiltrating the dermis and subcutaneous fat.33,35 In contrast to CH, KHE lesions show immunoreactivity to the markers podoplanin, lymphatic vessel endothelial receptor 1, and prospero homeobox 1 protein.36,37 Notably, 70% of these tumors are complicated by the presence of Kasabach-Merritt phenomenon, a potentially life-threatening emergency that occurs when platelets are trapped within a vascular tumor, leading to the consumption of clotting factors, intralesional bleeding, and rapid enlargement of the tumor.32 The Kasabach-Merritt phenomenon manifests clinically as microangiopathic hemolytic anemia, severe thrombocytopenia, and disseminated intravascular coagulation. 38 Although CH lesions also can be associated with thrombocytopenia and coagulopathy, they generally are mild and self-limited.18
Tufted angioma is a vascular tumor that arises within the first 5 years of life as firm violaceous papules or plaques, often with associated hyperhidrosis or hypertrichosis.39,40 Although TA grows slowly for a period of time, it eventually stabilizes and persists, rarely regressing completely.41 These tumors share many similarities with KHE, and it has been suggested that they may be part of the same spectrum. 42 As with KHE, TA lesions show immunoreactivity to the markers podoplanin, lymphatic vessel endothelial receptor 1, and prospero homeobox 1 protein, which are negative in CH.36,37 Although TA also can be complicated by Kasabach-Merritt phenomenon, the incidence is much lower (up to 38%).43,44 As such, TAs tend to be recognized as more superficial benign lesions. However, they still can cause notable cosmetic and functional impairment and should be monitored closely, especially in the presence of associated symptoms or complications.
Arteriovenous malformation is a vascular lesion that results from errors during the embryonic development of vascular channels.45 Although present at birth, it may not become clinically apparent until later in life. Arteriovenous malformations enlarge postnatally, and their growth is proportional to the developmental growth of the affected individual rather than the result of endothelial proliferation.46 In infants, AVM may manifest as a faint vascular stain that can evolve over time into a pink patch associated with a palpable thrill during adolescence. 4 On Doppler flow imaging, AVMs are identified as fast-flow anomalies arising from an abnormal communication between high-pressure arterial systems and low-pressure venous systems without the presence of a capillary bed.47 One of the differentiating factors between AVM and CH is that AVMs do not regress spontaneously and tend to have high recurrence rates, even with intervention. 48 In contrast, CH can be categorized based on its postnatal involution pattern. Another distinguishing factor is that AVMs tend to be larger and more invasive than CHs.46 Therefore, early diagnosis and intervention are crucial to prevent complications such as bleeding, seizures, or neurologic deficits associated with AVMs.1
The Diagnosis: Congenital Hemangioma
Surgical resection of the mass was performed at 4 months of age without complication (Figure 1). Histopathology revealed a lobular endothelial cell proliferation within a densely fibrotic stroma, multiple thin-walled vessels, and negative immunoreactivity to glucose transporter type 1 (GLUT-1)(Figures 2 and 3). Combined with the patient’s clinical history and findings on imaging (Figure 4), the most accurate diagnosis was a congenital hemangioma (CH). The mass was determined to be a noninvoluting congenital hemangioma (NICH).
A variety of vascular anomalies manifest in newborns and can be differentiated by the patient’s clinical history—particularly whether the lesion is present at birth or develops after birth. Imaging and histopathology of the lesion(s) may be utilized when clinical examination alone is not sufficient to make a diagnosis. Histopathology and immunohistochemistry further aid in differentiating the type of vascular lesion.


Overall, vascular anomalies are classified broadly into 2 categories based on their pathogenesis: tumors and malformations. Vascular tumors are composed of proliferating endothelial cells that have the potential to resolve spontaneously over time. Examples include CH, infantile hemangioma (IH), kaposiform hemangioendothelioma (KHE), and tufted angioma (TA). In contrast, vascular malformations (ie, arteriovenous malformations) are composed of dysplastic vessels with normal endothelial cell turnover and do not resolve without intervention.1
Congenital hemangiomas are rare vascular tumors that are fully developed at birth. These tumors proliferate in utero, enabling prenatal detection via ultrasonography as early as 12 weeks’ gestation for large heterogeneous vascular masses.2-4 Congenital hemangiomas are described as solitary, well-circumscribed, raised, violaceous lesions most commonly located in the head and neck region.4-6 Histopathologically, they are characterized by lobules of proliferating capillaries surrounded by fibrous stroma and dysplastic vascular channels.6,7
Congenital hemangiomas are categorized based on their postnatal involution patterns.2 Fetally involuting CH both develops and begins regression in utero and often is completely regressed at birth.8 Rapidly involuting CH begins regression in the first few weeks of life and usually is completely involuted by 14 months of age.6,9-11 Conversely, NICH does not regress, often requiring surgical excision due to functional and cosmetic issues.12,13 Partially involuting CH is intermediary, beginning as rapidly involuting but not involuting completely and persisting as lesions that resemble NICH.14-16 Although generally benign and asymptomatic, these tumors can cause transient thrombocytopenia and coagulopathy at birth, as seen in our patient.17,18


Infantile hemangioma is the most common vascular tumor of infancy.19-21 Although a precursor lesion may be present at birth, generally this tumor becomes apparent after the first few weeks of life as a solitary vascular plaque or nodule with a predilection for the head and neck.22-25 Once it arises, IH quickly enters a period of rapid growth, followed by a period of slower continued growth, with most reaching maximum size by 3 months.22 Thereafter, IH enters a slow period of involution (range, 3–9 years)26; more recent data suggest near resolution by 5 years of age.27 Infantile hemangioma is categorized based on its depth in the skin and subcutaneous tissues and can be classified as superficial, mixed, or deep.22,24,28,29 Superficial IH appears as a red plaque and may exhibit lobulation, while deep IH can be identified as flesh-colored or blue subcutaneous masses. Mixed IH may manifest with both superficial and deep features depending on the extent of its involvement in the dermal and subcutaneous layers. The pattern of involvement may be focal, segmental, or indeterminate.24 In contrast, CH typically is a solitary vascular mass with prominent telangiectases, nodules, and radiating veins.6 Histologically, IH is composed of proliferative plump endothelial cells that form capillaries, and the lesion stains positively for GLUT-1, whereas CH does not.30
Kaposiform hemangioendothelioma is classified as a locally aggressive vascular tumor that manifests either prenatally or in early infancy.31 It is described as a solitary, ill-defined, firm, purple plaque most commonly located on the extremities and retroperitoneum.32-34 Histopathologically, these lesions are characterized by dilated lymphatic channels and irregular sheets or lobules of spindle-shaped endothelial cells infiltrating the dermis and subcutaneous fat.33,35 In contrast to CH, KHE lesions show immunoreactivity to the markers podoplanin, lymphatic vessel endothelial receptor 1, and prospero homeobox 1 protein.36,37 Notably, 70% of these tumors are complicated by the presence of Kasabach-Merritt phenomenon, a potentially life-threatening emergency that occurs when platelets are trapped within a vascular tumor, leading to the consumption of clotting factors, intralesional bleeding, and rapid enlargement of the tumor.32 The Kasabach-Merritt phenomenon manifests clinically as microangiopathic hemolytic anemia, severe thrombocytopenia, and disseminated intravascular coagulation. 38 Although CH lesions also can be associated with thrombocytopenia and coagulopathy, they generally are mild and self-limited.18
Tufted angioma is a vascular tumor that arises within the first 5 years of life as firm violaceous papules or plaques, often with associated hyperhidrosis or hypertrichosis.39,40 Although TA grows slowly for a period of time, it eventually stabilizes and persists, rarely regressing completely.41 These tumors share many similarities with KHE, and it has been suggested that they may be part of the same spectrum. 42 As with KHE, TA lesions show immunoreactivity to the markers podoplanin, lymphatic vessel endothelial receptor 1, and prospero homeobox 1 protein, which are negative in CH.36,37 Although TA also can be complicated by Kasabach-Merritt phenomenon, the incidence is much lower (up to 38%).43,44 As such, TAs tend to be recognized as more superficial benign lesions. However, they still can cause notable cosmetic and functional impairment and should be monitored closely, especially in the presence of associated symptoms or complications.
Arteriovenous malformation is a vascular lesion that results from errors during the embryonic development of vascular channels.45 Although present at birth, it may not become clinically apparent until later in life. Arteriovenous malformations enlarge postnatally, and their growth is proportional to the developmental growth of the affected individual rather than the result of endothelial proliferation.46 In infants, AVM may manifest as a faint vascular stain that can evolve over time into a pink patch associated with a palpable thrill during adolescence. 4 On Doppler flow imaging, AVMs are identified as fast-flow anomalies arising from an abnormal communication between high-pressure arterial systems and low-pressure venous systems without the presence of a capillary bed.47 One of the differentiating factors between AVM and CH is that AVMs do not regress spontaneously and tend to have high recurrence rates, even with intervention. 48 In contrast, CH can be categorized based on its postnatal involution pattern. Another distinguishing factor is that AVMs tend to be larger and more invasive than CHs.46 Therefore, early diagnosis and intervention are crucial to prevent complications such as bleeding, seizures, or neurologic deficits associated with AVMs.1
- Enjolras O, Wassef M, Chapot R. Introduction: ISSVA Classification. In: Enjolras O, Wassef M, Chapot R, eds. Color Atlas of Vascular Tumors and Vascular Malformations. Cambridge University Press; 2007:3-11.
- Fadell MF, Jones BV, Adams DM. Prenatal diagnosis and postnatal follow-up of rapidly involuting congenital hemangioma (RICH). Pediatr Radiol. 2011;41:1057-1060.
- Feygin T, Khalek N, Moldenhauer JS. Fetal brain, head, and neck tumors: prenatal imaging and management. Prenat Diagn. 2020;40:1203-1219.
- Foley LS, Kulungowski AM. Vascular anomalies in pediatrics. Adv Pediatr. 2015;62:227-255.
- Bruder E, Alaggio R, Kozakewich HPW, et al. Vascular and perivascular lesions of skin and soft tissues in children and adolescents. Pediatr Dev Pathol. 2012;15:26-61.
- Berenguer B, Mulliken JB, Enjolras O, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol. 2003;6:495-510.
- North PE, Waner M, James CA, et al. Congenital nonprogressive hemangioma: a distinct clinicopathologic entity unlike infantile hemangioma. Arch Dermatol. 2001;137:1607-1620.
- Maguiness S, Uihlein LC, Liang MG, et al. Rapidly involuting congenital hemangioma with fetal involution. Pediatr Dermatol. 2015;32:321-326.
- Keating LJ, Soares GM, Muratore CS. Rapidly involuting congenital hemangioma. Med Health R I. 2012;95:149-152.
- Schafer F, Tapia M, Pinto C. Rapidly involuting congenital haemangioma. Arch Dis Child Fetal Neonatal Ed. 2014;99:F422.
- Boon LM, Enjolras O, Mulliken JB. Congenital hemangioma: evidence of accelerated involution. J Pediatr. 1996;128:329-335.
- Liang MG, Frieden IJ. Infantile and congenital hemangiomas. Semin Pediatr Surg. 2014;23:162-167.
- Enjolras O, Mulliken JB, Boon LM, et al. Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg. 2001;107:1647-1654.
- Nasseri E, Piram M, McCuaig CC, et al. Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. J Am Acad Dermatol. 2014;70:75-79.
- Wassef M, Blei F, Adams D, et al. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136:E203-E214.
- Boull C, Maguiness SM. Congenital hemangiomas. Semin Cutan Med Surg. 2016;35:124-127.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Baselga E, Cordisco MR, Garzon M, et al. Rapidly involuting congenital haemangioma associated with transient thrombocytopenia and coagulopathy: a case series. Br J Dermatol. 2008;158:1363-1370.
- Kanada KN, Merin MR, Munden A, et al. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr. 2012;161:240-245.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360-367.
- Hidano A, Nakajima S. Earliest features of the strawberry mark in the newborn. Br J Dermatol. 1972;87:138-144.
- Martinez-Perez D, Fein NA, Boon LM, et al. Not all hemangiomas look like strawberries: uncommon presentations of the most common tumor of infancy. Pediatr Dermatol. 1995;12:1-6.
- Payne MM, Moyer F, Marcks KM, et al. The precursor to the hemangioma. Plast Reconstr Surg. 1966;38:64-67.
- Bowers RE, Graham EA, Tomlinson KM. The natural history of the strawberry nevus. Arch Dermatol. 1960;82:667-680.
- Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624.
- Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med. 1999;341:173-181.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567-1576.
- North PE, Waner M, Mizeracki A, et al. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31:11-22.
- Gruman A, Liang MG, Mulliken JB, et al. Kaposiform hemangioendothelioma without Kasabach-Merritt phenomenon. J Am Acad Dermatol. 2005;52:616-622.
- Croteau SE, Liang MG, Kozakewich HP, et al. Kaposiform hemangioendothelioma: atypical features and risks of Kasabach- Merritt phenomenon in 107 referrals. J Pediatr. 2013;162:142-147.
- Zukerberg LR, Nickoloff BJ, Weiss SW. Kaposiform hemangioendothelioma of infancy and childhood. an aggressive neoplasm associated with Kasabach-Merritt syndrome and lymphangiomatosis. Am J Surg Pathol. 1993;17:321-328.
- Mac-Moune Lai F, To KF, Choi PC, et al. Kaposiform hemangioendothelioma: five patients with cutaneous lesion and long follow-up. Mod Pathol. 2001;14:1087-1092.
- O’Rafferty C, O’Regan GM, Irvine AD, et al. Recent advances in the pathobiology and management of Kasabach-Merritt phenomenon. Br J Haematol. 2015;171:38-51.
- Le Huu AR, Jokinen CH, Rubin BP, et al. Expression of prox1, lymphatic endothelial nuclear transcription factor, in kaposiform hemangioendothelioma and tufted angioma. Am J Surg Pathol. 2010;34:1563-1573.
- Debelenko LV, Perez-Atayde AR, Mulliken JB, et al. D2-40 immuno-histochemical analysis of pediatric vascular tumors reveals positivity in kaposiform hemangioendothelioma. Mod Pathol. 2005;18:1454-1460.
- Haisley-Royster C, Enjolras O, Frieden IJ, et al. Kasabach-Merritt phenomenon: a retrospective study of treatment with vincristine. J Pediatr Hematol Oncol. 2002;24:459-462.
- Wilmer A, Kaatz M, Bocker T, et al. Tufted angioma. Eur J Dermatol. 1999;9:51-53.
- Herron MD, Coffin CM, Vanderhooft SL. Tufted angiomas: variability of the clinical morphology. Pediatr Dermatol. 2002;19:394-401.
- North PE. Pediatric vascular tumors and malformations. Surg Pathol Clin. 2010,3:455-494.
- Chu CY, Hsiao CH, Chiu HC. Transformation between kaposiform hemangioendothelioma and tufted angioma. Dermatology. 2003;206:334-337.
- Osio A, Fraitag S, Hadj-Rabia S, et al. Clinical spectrum of tufted angiomas in childhood: a report of 13 cases and a review of the literature. Arch Dermatol. 2010;146:758-763.
- Johnson EF, Davis DM, Tollefson MM, et al. Vascular tumors in infants: case report and review of clinical, histopathologic, and immunohistochemical characteristics of infantile hemangioma, pyogenic granuloma, noninvoluting congenital hemangioma, tufted angioma, and kaposiform hemangioendothelioma. Am J Dermatopathol. 2018;40:231-239.
- Christison-Lagay ER, Fishman SJ. Vascular anomalies. Surg Clin North Am. 2006;86:393-425.
- Liu AS, Mulliken JB, Zurakowski D, et al. Extracranial arteriovenous malformations: natural progression and recurrence after treatment. Plast Reconstr Surg. 2010;125:1185-1194.
- Young AE, Mulliken JB. Arteriovenous malformations. In: Mulliken JB, Young AE, eds. Vascular Birthmarks: Haemangiomas and Malformations. WB Saunders; 1988:228-245.
- Duggan EM, Fishman SJ. Vascular anomalies. In: Holcomb GW III, Murphy JP, St Peter SD, eds. Holcomb and Ashcraft’s Pediatric Surgery. 7th edition. Elsevier; 2019:1147-1170.
- Enjolras O, Wassef M, Chapot R. Introduction: ISSVA Classification. In: Enjolras O, Wassef M, Chapot R, eds. Color Atlas of Vascular Tumors and Vascular Malformations. Cambridge University Press; 2007:3-11.
- Fadell MF, Jones BV, Adams DM. Prenatal diagnosis and postnatal follow-up of rapidly involuting congenital hemangioma (RICH). Pediatr Radiol. 2011;41:1057-1060.
- Feygin T, Khalek N, Moldenhauer JS. Fetal brain, head, and neck tumors: prenatal imaging and management. Prenat Diagn. 2020;40:1203-1219.
- Foley LS, Kulungowski AM. Vascular anomalies in pediatrics. Adv Pediatr. 2015;62:227-255.
- Bruder E, Alaggio R, Kozakewich HPW, et al. Vascular and perivascular lesions of skin and soft tissues in children and adolescents. Pediatr Dev Pathol. 2012;15:26-61.
- Berenguer B, Mulliken JB, Enjolras O, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol. 2003;6:495-510.
- North PE, Waner M, James CA, et al. Congenital nonprogressive hemangioma: a distinct clinicopathologic entity unlike infantile hemangioma. Arch Dermatol. 2001;137:1607-1620.
- Maguiness S, Uihlein LC, Liang MG, et al. Rapidly involuting congenital hemangioma with fetal involution. Pediatr Dermatol. 2015;32:321-326.
- Keating LJ, Soares GM, Muratore CS. Rapidly involuting congenital hemangioma. Med Health R I. 2012;95:149-152.
- Schafer F, Tapia M, Pinto C. Rapidly involuting congenital haemangioma. Arch Dis Child Fetal Neonatal Ed. 2014;99:F422.
- Boon LM, Enjolras O, Mulliken JB. Congenital hemangioma: evidence of accelerated involution. J Pediatr. 1996;128:329-335.
- Liang MG, Frieden IJ. Infantile and congenital hemangiomas. Semin Pediatr Surg. 2014;23:162-167.
- Enjolras O, Mulliken JB, Boon LM, et al. Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg. 2001;107:1647-1654.
- Nasseri E, Piram M, McCuaig CC, et al. Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. J Am Acad Dermatol. 2014;70:75-79.
- Wassef M, Blei F, Adams D, et al. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136:E203-E214.
- Boull C, Maguiness SM. Congenital hemangiomas. Semin Cutan Med Surg. 2016;35:124-127.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Baselga E, Cordisco MR, Garzon M, et al. Rapidly involuting congenital haemangioma associated with transient thrombocytopenia and coagulopathy: a case series. Br J Dermatol. 2008;158:1363-1370.
- Kanada KN, Merin MR, Munden A, et al. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr. 2012;161:240-245.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360-367.
- Hidano A, Nakajima S. Earliest features of the strawberry mark in the newborn. Br J Dermatol. 1972;87:138-144.
- Martinez-Perez D, Fein NA, Boon LM, et al. Not all hemangiomas look like strawberries: uncommon presentations of the most common tumor of infancy. Pediatr Dermatol. 1995;12:1-6.
- Payne MM, Moyer F, Marcks KM, et al. The precursor to the hemangioma. Plast Reconstr Surg. 1966;38:64-67.
- Bowers RE, Graham EA, Tomlinson KM. The natural history of the strawberry nevus. Arch Dermatol. 1960;82:667-680.
- Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624.
- Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med. 1999;341:173-181.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567-1576.
- North PE, Waner M, Mizeracki A, et al. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31:11-22.
- Gruman A, Liang MG, Mulliken JB, et al. Kaposiform hemangioendothelioma without Kasabach-Merritt phenomenon. J Am Acad Dermatol. 2005;52:616-622.
- Croteau SE, Liang MG, Kozakewich HP, et al. Kaposiform hemangioendothelioma: atypical features and risks of Kasabach- Merritt phenomenon in 107 referrals. J Pediatr. 2013;162:142-147.
- Zukerberg LR, Nickoloff BJ, Weiss SW. Kaposiform hemangioendothelioma of infancy and childhood. an aggressive neoplasm associated with Kasabach-Merritt syndrome and lymphangiomatosis. Am J Surg Pathol. 1993;17:321-328.
- Mac-Moune Lai F, To KF, Choi PC, et al. Kaposiform hemangioendothelioma: five patients with cutaneous lesion and long follow-up. Mod Pathol. 2001;14:1087-1092.
- O’Rafferty C, O’Regan GM, Irvine AD, et al. Recent advances in the pathobiology and management of Kasabach-Merritt phenomenon. Br J Haematol. 2015;171:38-51.
- Le Huu AR, Jokinen CH, Rubin BP, et al. Expression of prox1, lymphatic endothelial nuclear transcription factor, in kaposiform hemangioendothelioma and tufted angioma. Am J Surg Pathol. 2010;34:1563-1573.
- Debelenko LV, Perez-Atayde AR, Mulliken JB, et al. D2-40 immuno-histochemical analysis of pediatric vascular tumors reveals positivity in kaposiform hemangioendothelioma. Mod Pathol. 2005;18:1454-1460.
- Haisley-Royster C, Enjolras O, Frieden IJ, et al. Kasabach-Merritt phenomenon: a retrospective study of treatment with vincristine. J Pediatr Hematol Oncol. 2002;24:459-462.
- Wilmer A, Kaatz M, Bocker T, et al. Tufted angioma. Eur J Dermatol. 1999;9:51-53.
- Herron MD, Coffin CM, Vanderhooft SL. Tufted angiomas: variability of the clinical morphology. Pediatr Dermatol. 2002;19:394-401.
- North PE. Pediatric vascular tumors and malformations. Surg Pathol Clin. 2010,3:455-494.
- Chu CY, Hsiao CH, Chiu HC. Transformation between kaposiform hemangioendothelioma and tufted angioma. Dermatology. 2003;206:334-337.
- Osio A, Fraitag S, Hadj-Rabia S, et al. Clinical spectrum of tufted angiomas in childhood: a report of 13 cases and a review of the literature. Arch Dermatol. 2010;146:758-763.
- Johnson EF, Davis DM, Tollefson MM, et al. Vascular tumors in infants: case report and review of clinical, histopathologic, and immunohistochemical characteristics of infantile hemangioma, pyogenic granuloma, noninvoluting congenital hemangioma, tufted angioma, and kaposiform hemangioendothelioma. Am J Dermatopathol. 2018;40:231-239.
- Christison-Lagay ER, Fishman SJ. Vascular anomalies. Surg Clin North Am. 2006;86:393-425.
- Liu AS, Mulliken JB, Zurakowski D, et al. Extracranial arteriovenous malformations: natural progression and recurrence after treatment. Plast Reconstr Surg. 2010;125:1185-1194.
- Young AE, Mulliken JB. Arteriovenous malformations. In: Mulliken JB, Young AE, eds. Vascular Birthmarks: Haemangiomas and Malformations. WB Saunders; 1988:228-245.
- Duggan EM, Fishman SJ. Vascular anomalies. In: Holcomb GW III, Murphy JP, St Peter SD, eds. Holcomb and Ashcraft’s Pediatric Surgery. 7th edition. Elsevier; 2019:1147-1170.
A newborn male was delivered via cesarean section at 38 weeks 5 days’ gestation with a large vascular mass on the posterior neck. The mass previously had been identified on a 23-week prenatal ultrasound. Physical examination by dermatology at birth revealed a well-defined violaceous mass measuring 6×5 cm with prominent radiating veins, coarse telangiectases, and a pale rim. Magnetic resonance imaging demonstrated a well-circumscribed mass with avid arterial phase enhancement. The patient experienced transient thrombocytopenia that resolved following administration of methylprednisolone. No evidence of rapid involution was noted after 3 months of observation.
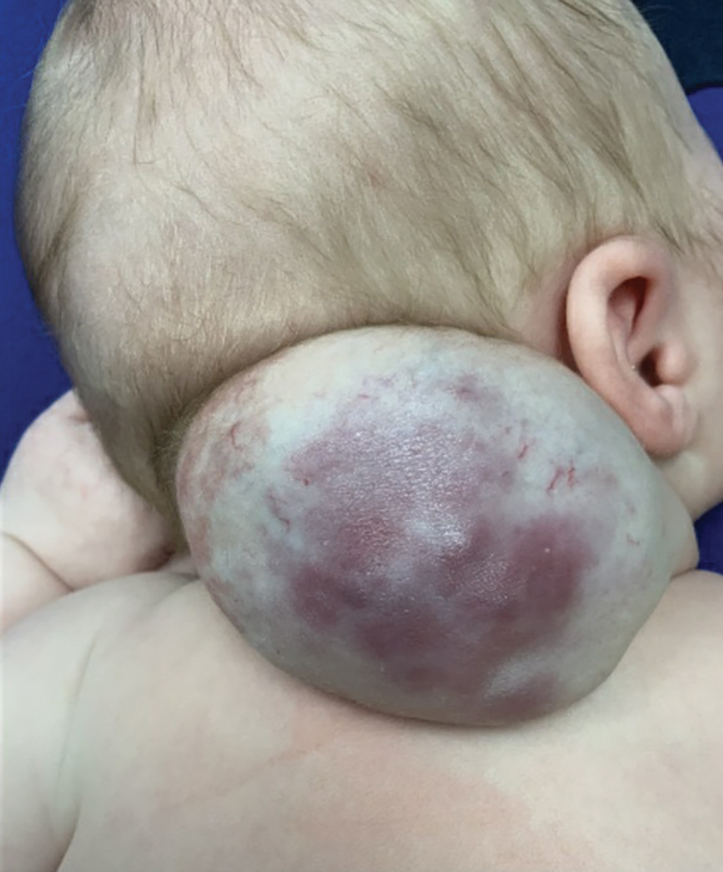
Flesh-Colored Pinpoint Papules With Fine White Spicules on the Upper Body
The Diagnosis: Trichodysplasia Spinulosa
A diagnosis of trichodysplasia spinulosa (TS) was rendered based on the clinical presentation— diffuse folliculocentric keratotic papules with spicules and leonine facies—coinciding with cyclosporine initiation. Biopsy was deferred given the classic presentation. The patient applied cidofovir cream 1% daily to lesions on the face. She was prescribed leflunomide 10 mg daily, which was later increased to 20 mg daily, for polyarthritis associated with systemic lupus erythematosus (SLE). Her transplant physician increased her cyclosporine dosage from 50 mg twice daily to 75 mg each morning and 50 mg each evening due to rising creatinine and donor-specific antibodies from the renal transplant. The patient’s TS eruption mildly improved 3 months after the cyclosporine dose was increased. To treat persistent lesions, oral valganciclovir was started at 450 mg once daily and later reduced to every other day due to leukopenia. After 3 months of taking valganciclovir 450 mg every other day, the patient’s TS rash resolved.
Trichodysplasia spinulosa is a rare condition caused by TS-associated polyomavirus1 that may arise in immunosuppressed patients, especially in solid organ transplant recipients.2 It is characterized by spiculated and folliculocentric papules, mainly on the face,1 and often is diagnosed clinically, but if the presentation is not classic, a skin biopsy can help to confirm the diagnosis. Because of its rarity, treatment options do not have well-established efficacy1 but include reducing immunosuppression and using the antivirals cidofovir1 or valganciclovir3 to treat the polyomavirus. Topical retinoids,3 photodynamic therapy, 4 and leflunomide5 also may be effective.
Although the typical approach to treating TS is to reduce immunosuppression, this was not an option for our patient, as she required increased immunosuppression for the treatment of active SLE. Leflunomide can be used for SLE, and in some reports it can be effective for BK viremia in kidney transplant recipients5 as well as for TS in solid organ transplant recipients.6 Our patient showed improvement of the TS, BK viremia, renal function, and SLE while taking leflunomide and valganciclovir.
The differential diagnosis includes keratosis pilaris, lichen nitidus, scleromyxedema, and trichostasis spinulosa. Keratosis pilaris is a benign skin disorder consisting of patches of keratotic papules with varying degrees of erythema and inflammation that are formed by dead keratinocytes plugging the hair follicles and often are seen on the extremities, face, and trunk.7 Our patient’s papules were flesh colored with no notable background erythema. Additionally, the presence of leonine facies was atypical for keratosis pilaris. Acids, steroids, and kinase inhibitors are the most frequently used treatments for keratosis pilaris.8
Lichen nitidus is a skin condition characterized by multiple shiny, dome-shaped, flesh-colored papules usually found on the flexor surfaces of the arms, anterior trunk, and genitalia. It is mostly asymptomatic, but patients may experience pruritus. Most cases occur in children and young adults, with no obvious racial or gender predilection. The diagnosis often is clinical, but biopsy shows downward enlargement of the epidermal rete ridges surrounding a focal inflammatory infiltrate, known as a ball-in-claw configuration.9-11 Lichen nitidus spontaneously resolves within a few years without treatment. Our patient did have flesh-colored papules on the arms and chest; however, major involvement of the face is not typical in lichen nitidus. Additionally, fine white spicules would not be seen in lichen nitidus. For severe generalized lichen nitidus, treatment options include topical corticosteroids, topical calcineurin inhibitors, oral antihistamines, or UV light to decrease inflammation.9-11
Scleromyxedema is a rare condition involving the deposition of mucinous material in the papillary dermis to cause the formation of infiltrative skin lesions.12 It is thought that immunoglobulins and cytokines secreted by inflammatory cells lead to the synthesis of glycosaminoglycans, which then causes deposition of mucin in the dermis.13 The classic cutaneous features of scleromyxedema include waxy indurated papules and plaques with skin thickening throughout the entire body.12 Our patient’s papules were not notably indurated and involved less than 50% of the total body surface area. An important diagnostic feature of scleromyxedema is monoclonal gammopathy, which our patient did not have. Intravenous immunoglobulin is the first-line treatment of scleromyxedema, and second-line treatments include systemic corticosteroids and thalidomide.14 Our patient also did not require treatment with intravenous immunoglobulin, as her rash improved with antiviral medication, which would not address the underlying inflammatory processes associated with scleromyxedema.
Trichostasis spinulosa is a rare hair follicle disorder consisting of dark, spiny, hyperkeratotic follicular papules that can be found on the extremities and face, especially the nose. The etiology is unknown, but risk factors include congenital dysplasia of hair follicles; exposure to UV light, dust, oil, or heat; chronic renal failure; Malassezia yeast; and Propionibacterium acnes. Adult women with darker skin types are most commonly affected by trichostasis spinulosa.15,16 Our patient fit the epidemiologic demographic of trichostasis spinulosa, including a history of chronic renal failure. Her rash covered the face, nose, and arms; however, the papules were flesh colored, whereas trichostasis spinulosa would appear as black papules. Furthermore, yeast and bacterial infections have been identified as potential agents associated with trichostasis spinulosa; therefore, antiviral agents would be ineffective. Viable treatments for trichostasis spinulosa include emollients, topical keratolytic agents, retinoic acids, and lasers to remove abnormal hair follicles.15,16
- Curman P, Näsman A, Brauner H. Trichodysplasia spinulosa: a comprehensive disease and its treatment. J Eur Acad Dermatol Venereol. 2021;35:1067-1076.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2021;148:726-733.
- Shah PR, Esaa FS, Gupta P, et al. Trichodysplasia spinulosa successfully treated with adapalene 0.1% gel and oral valganciclovir in a renal transplant recipient. JAAD Case Rep. 2020;6:23-25.
- Liew YCC, Kee TYS, Kwek JL, et al. Photodynamic therapy for the treatment of trichodysplasia spinulosa in an Asian renal transplant recipient: a case report and review of the literature. JAAD Case Rep. 2021;7:74-83.
- Pierrotti LC, Urbano PRP, da Silva Nali LH, et al. Viremia and viuria of trichodysplasia spinulosa-associated polyomavirus before the development of clinical disease in a kidney transplant recipient. Transpl Infect Dis. 2019;21:E13133.
- Kassar R, Chang J, Chan AW, et al. Leflunomide for the treatment of trichodysplasia spinulosa in a liver transplant recipient. Transpl Infect Dis. 2017;19:E12702.
- Eckburg A, Kazemi T, Maguiness S. Keratosis pilaris rubra successfully treated with topical sirolimus: report of a case and review of the literature. Pediatr Dermatol. 2022;39:429-431.
- Reddy S, Brahmbhatt H. A narrative review on the role of acids, steroids, and kinase inhibitors in the treatment of keratosis pilaris. Cureus. 2021;13:E18917.
- Jordan AS, Green MC, Sulit DJ. Lichen nitidus. J Am Osteopath Assoc. 2019;119:704.
- Arizaga AT, Gaughan MD, Bang RH. Generalized lichen nitidus. Clin Exp Dermatol. 2002;27:115-117.
- Chu J, Lam JM. Lichen nitidus. CMAJ. 2014;186:E688.
- Haber R, Bachour J, El Gemayel M. Scleromyxedema treatment: a systematic review and update. Int J Dermatol. 2020;59:1191-1201.
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosis (LM) (discrete papular type). Dermatol Online J. 2017;23:8.
- Hoffman JHO, Enk AH. Scleromyxedema. J Dtsch Dermatol Ges. 2020;18:1449-1467.
- Kositkuljorn C, Suchonwanit P. Trichostasis spinulosa: a case report with an unusual presentation. Case Rep Dermatol. 2020;12:178-185.
- Ramteke MN, Bhide AA. Trichostasis spinulosa at an unusual site. Int J Trichology. 2016;8:78-80.
The Diagnosis: Trichodysplasia Spinulosa
A diagnosis of trichodysplasia spinulosa (TS) was rendered based on the clinical presentation— diffuse folliculocentric keratotic papules with spicules and leonine facies—coinciding with cyclosporine initiation. Biopsy was deferred given the classic presentation. The patient applied cidofovir cream 1% daily to lesions on the face. She was prescribed leflunomide 10 mg daily, which was later increased to 20 mg daily, for polyarthritis associated with systemic lupus erythematosus (SLE). Her transplant physician increased her cyclosporine dosage from 50 mg twice daily to 75 mg each morning and 50 mg each evening due to rising creatinine and donor-specific antibodies from the renal transplant. The patient’s TS eruption mildly improved 3 months after the cyclosporine dose was increased. To treat persistent lesions, oral valganciclovir was started at 450 mg once daily and later reduced to every other day due to leukopenia. After 3 months of taking valganciclovir 450 mg every other day, the patient’s TS rash resolved.
Trichodysplasia spinulosa is a rare condition caused by TS-associated polyomavirus1 that may arise in immunosuppressed patients, especially in solid organ transplant recipients.2 It is characterized by spiculated and folliculocentric papules, mainly on the face,1 and often is diagnosed clinically, but if the presentation is not classic, a skin biopsy can help to confirm the diagnosis. Because of its rarity, treatment options do not have well-established efficacy1 but include reducing immunosuppression and using the antivirals cidofovir1 or valganciclovir3 to treat the polyomavirus. Topical retinoids,3 photodynamic therapy, 4 and leflunomide5 also may be effective.
Although the typical approach to treating TS is to reduce immunosuppression, this was not an option for our patient, as she required increased immunosuppression for the treatment of active SLE. Leflunomide can be used for SLE, and in some reports it can be effective for BK viremia in kidney transplant recipients5 as well as for TS in solid organ transplant recipients.6 Our patient showed improvement of the TS, BK viremia, renal function, and SLE while taking leflunomide and valganciclovir.
The differential diagnosis includes keratosis pilaris, lichen nitidus, scleromyxedema, and trichostasis spinulosa. Keratosis pilaris is a benign skin disorder consisting of patches of keratotic papules with varying degrees of erythema and inflammation that are formed by dead keratinocytes plugging the hair follicles and often are seen on the extremities, face, and trunk.7 Our patient’s papules were flesh colored with no notable background erythema. Additionally, the presence of leonine facies was atypical for keratosis pilaris. Acids, steroids, and kinase inhibitors are the most frequently used treatments for keratosis pilaris.8
Lichen nitidus is a skin condition characterized by multiple shiny, dome-shaped, flesh-colored papules usually found on the flexor surfaces of the arms, anterior trunk, and genitalia. It is mostly asymptomatic, but patients may experience pruritus. Most cases occur in children and young adults, with no obvious racial or gender predilection. The diagnosis often is clinical, but biopsy shows downward enlargement of the epidermal rete ridges surrounding a focal inflammatory infiltrate, known as a ball-in-claw configuration.9-11 Lichen nitidus spontaneously resolves within a few years without treatment. Our patient did have flesh-colored papules on the arms and chest; however, major involvement of the face is not typical in lichen nitidus. Additionally, fine white spicules would not be seen in lichen nitidus. For severe generalized lichen nitidus, treatment options include topical corticosteroids, topical calcineurin inhibitors, oral antihistamines, or UV light to decrease inflammation.9-11
Scleromyxedema is a rare condition involving the deposition of mucinous material in the papillary dermis to cause the formation of infiltrative skin lesions.12 It is thought that immunoglobulins and cytokines secreted by inflammatory cells lead to the synthesis of glycosaminoglycans, which then causes deposition of mucin in the dermis.13 The classic cutaneous features of scleromyxedema include waxy indurated papules and plaques with skin thickening throughout the entire body.12 Our patient’s papules were not notably indurated and involved less than 50% of the total body surface area. An important diagnostic feature of scleromyxedema is monoclonal gammopathy, which our patient did not have. Intravenous immunoglobulin is the first-line treatment of scleromyxedema, and second-line treatments include systemic corticosteroids and thalidomide.14 Our patient also did not require treatment with intravenous immunoglobulin, as her rash improved with antiviral medication, which would not address the underlying inflammatory processes associated with scleromyxedema.
Trichostasis spinulosa is a rare hair follicle disorder consisting of dark, spiny, hyperkeratotic follicular papules that can be found on the extremities and face, especially the nose. The etiology is unknown, but risk factors include congenital dysplasia of hair follicles; exposure to UV light, dust, oil, or heat; chronic renal failure; Malassezia yeast; and Propionibacterium acnes. Adult women with darker skin types are most commonly affected by trichostasis spinulosa.15,16 Our patient fit the epidemiologic demographic of trichostasis spinulosa, including a history of chronic renal failure. Her rash covered the face, nose, and arms; however, the papules were flesh colored, whereas trichostasis spinulosa would appear as black papules. Furthermore, yeast and bacterial infections have been identified as potential agents associated with trichostasis spinulosa; therefore, antiviral agents would be ineffective. Viable treatments for trichostasis spinulosa include emollients, topical keratolytic agents, retinoic acids, and lasers to remove abnormal hair follicles.15,16
The Diagnosis: Trichodysplasia Spinulosa
A diagnosis of trichodysplasia spinulosa (TS) was rendered based on the clinical presentation— diffuse folliculocentric keratotic papules with spicules and leonine facies—coinciding with cyclosporine initiation. Biopsy was deferred given the classic presentation. The patient applied cidofovir cream 1% daily to lesions on the face. She was prescribed leflunomide 10 mg daily, which was later increased to 20 mg daily, for polyarthritis associated with systemic lupus erythematosus (SLE). Her transplant physician increased her cyclosporine dosage from 50 mg twice daily to 75 mg each morning and 50 mg each evening due to rising creatinine and donor-specific antibodies from the renal transplant. The patient’s TS eruption mildly improved 3 months after the cyclosporine dose was increased. To treat persistent lesions, oral valganciclovir was started at 450 mg once daily and later reduced to every other day due to leukopenia. After 3 months of taking valganciclovir 450 mg every other day, the patient’s TS rash resolved.
Trichodysplasia spinulosa is a rare condition caused by TS-associated polyomavirus1 that may arise in immunosuppressed patients, especially in solid organ transplant recipients.2 It is characterized by spiculated and folliculocentric papules, mainly on the face,1 and often is diagnosed clinically, but if the presentation is not classic, a skin biopsy can help to confirm the diagnosis. Because of its rarity, treatment options do not have well-established efficacy1 but include reducing immunosuppression and using the antivirals cidofovir1 or valganciclovir3 to treat the polyomavirus. Topical retinoids,3 photodynamic therapy, 4 and leflunomide5 also may be effective.
Although the typical approach to treating TS is to reduce immunosuppression, this was not an option for our patient, as she required increased immunosuppression for the treatment of active SLE. Leflunomide can be used for SLE, and in some reports it can be effective for BK viremia in kidney transplant recipients5 as well as for TS in solid organ transplant recipients.6 Our patient showed improvement of the TS, BK viremia, renal function, and SLE while taking leflunomide and valganciclovir.
The differential diagnosis includes keratosis pilaris, lichen nitidus, scleromyxedema, and trichostasis spinulosa. Keratosis pilaris is a benign skin disorder consisting of patches of keratotic papules with varying degrees of erythema and inflammation that are formed by dead keratinocytes plugging the hair follicles and often are seen on the extremities, face, and trunk.7 Our patient’s papules were flesh colored with no notable background erythema. Additionally, the presence of leonine facies was atypical for keratosis pilaris. Acids, steroids, and kinase inhibitors are the most frequently used treatments for keratosis pilaris.8
Lichen nitidus is a skin condition characterized by multiple shiny, dome-shaped, flesh-colored papules usually found on the flexor surfaces of the arms, anterior trunk, and genitalia. It is mostly asymptomatic, but patients may experience pruritus. Most cases occur in children and young adults, with no obvious racial or gender predilection. The diagnosis often is clinical, but biopsy shows downward enlargement of the epidermal rete ridges surrounding a focal inflammatory infiltrate, known as a ball-in-claw configuration.9-11 Lichen nitidus spontaneously resolves within a few years without treatment. Our patient did have flesh-colored papules on the arms and chest; however, major involvement of the face is not typical in lichen nitidus. Additionally, fine white spicules would not be seen in lichen nitidus. For severe generalized lichen nitidus, treatment options include topical corticosteroids, topical calcineurin inhibitors, oral antihistamines, or UV light to decrease inflammation.9-11
Scleromyxedema is a rare condition involving the deposition of mucinous material in the papillary dermis to cause the formation of infiltrative skin lesions.12 It is thought that immunoglobulins and cytokines secreted by inflammatory cells lead to the synthesis of glycosaminoglycans, which then causes deposition of mucin in the dermis.13 The classic cutaneous features of scleromyxedema include waxy indurated papules and plaques with skin thickening throughout the entire body.12 Our patient’s papules were not notably indurated and involved less than 50% of the total body surface area. An important diagnostic feature of scleromyxedema is monoclonal gammopathy, which our patient did not have. Intravenous immunoglobulin is the first-line treatment of scleromyxedema, and second-line treatments include systemic corticosteroids and thalidomide.14 Our patient also did not require treatment with intravenous immunoglobulin, as her rash improved with antiviral medication, which would not address the underlying inflammatory processes associated with scleromyxedema.
Trichostasis spinulosa is a rare hair follicle disorder consisting of dark, spiny, hyperkeratotic follicular papules that can be found on the extremities and face, especially the nose. The etiology is unknown, but risk factors include congenital dysplasia of hair follicles; exposure to UV light, dust, oil, or heat; chronic renal failure; Malassezia yeast; and Propionibacterium acnes. Adult women with darker skin types are most commonly affected by trichostasis spinulosa.15,16 Our patient fit the epidemiologic demographic of trichostasis spinulosa, including a history of chronic renal failure. Her rash covered the face, nose, and arms; however, the papules were flesh colored, whereas trichostasis spinulosa would appear as black papules. Furthermore, yeast and bacterial infections have been identified as potential agents associated with trichostasis spinulosa; therefore, antiviral agents would be ineffective. Viable treatments for trichostasis spinulosa include emollients, topical keratolytic agents, retinoic acids, and lasers to remove abnormal hair follicles.15,16
- Curman P, Näsman A, Brauner H. Trichodysplasia spinulosa: a comprehensive disease and its treatment. J Eur Acad Dermatol Venereol. 2021;35:1067-1076.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2021;148:726-733.
- Shah PR, Esaa FS, Gupta P, et al. Trichodysplasia spinulosa successfully treated with adapalene 0.1% gel and oral valganciclovir in a renal transplant recipient. JAAD Case Rep. 2020;6:23-25.
- Liew YCC, Kee TYS, Kwek JL, et al. Photodynamic therapy for the treatment of trichodysplasia spinulosa in an Asian renal transplant recipient: a case report and review of the literature. JAAD Case Rep. 2021;7:74-83.
- Pierrotti LC, Urbano PRP, da Silva Nali LH, et al. Viremia and viuria of trichodysplasia spinulosa-associated polyomavirus before the development of clinical disease in a kidney transplant recipient. Transpl Infect Dis. 2019;21:E13133.
- Kassar R, Chang J, Chan AW, et al. Leflunomide for the treatment of trichodysplasia spinulosa in a liver transplant recipient. Transpl Infect Dis. 2017;19:E12702.
- Eckburg A, Kazemi T, Maguiness S. Keratosis pilaris rubra successfully treated with topical sirolimus: report of a case and review of the literature. Pediatr Dermatol. 2022;39:429-431.
- Reddy S, Brahmbhatt H. A narrative review on the role of acids, steroids, and kinase inhibitors in the treatment of keratosis pilaris. Cureus. 2021;13:E18917.
- Jordan AS, Green MC, Sulit DJ. Lichen nitidus. J Am Osteopath Assoc. 2019;119:704.
- Arizaga AT, Gaughan MD, Bang RH. Generalized lichen nitidus. Clin Exp Dermatol. 2002;27:115-117.
- Chu J, Lam JM. Lichen nitidus. CMAJ. 2014;186:E688.
- Haber R, Bachour J, El Gemayel M. Scleromyxedema treatment: a systematic review and update. Int J Dermatol. 2020;59:1191-1201.
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosis (LM) (discrete papular type). Dermatol Online J. 2017;23:8.
- Hoffman JHO, Enk AH. Scleromyxedema. J Dtsch Dermatol Ges. 2020;18:1449-1467.
- Kositkuljorn C, Suchonwanit P. Trichostasis spinulosa: a case report with an unusual presentation. Case Rep Dermatol. 2020;12:178-185.
- Ramteke MN, Bhide AA. Trichostasis spinulosa at an unusual site. Int J Trichology. 2016;8:78-80.
- Curman P, Näsman A, Brauner H. Trichodysplasia spinulosa: a comprehensive disease and its treatment. J Eur Acad Dermatol Venereol. 2021;35:1067-1076.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2021;148:726-733.
- Shah PR, Esaa FS, Gupta P, et al. Trichodysplasia spinulosa successfully treated with adapalene 0.1% gel and oral valganciclovir in a renal transplant recipient. JAAD Case Rep. 2020;6:23-25.
- Liew YCC, Kee TYS, Kwek JL, et al. Photodynamic therapy for the treatment of trichodysplasia spinulosa in an Asian renal transplant recipient: a case report and review of the literature. JAAD Case Rep. 2021;7:74-83.
- Pierrotti LC, Urbano PRP, da Silva Nali LH, et al. Viremia and viuria of trichodysplasia spinulosa-associated polyomavirus before the development of clinical disease in a kidney transplant recipient. Transpl Infect Dis. 2019;21:E13133.
- Kassar R, Chang J, Chan AW, et al. Leflunomide for the treatment of trichodysplasia spinulosa in a liver transplant recipient. Transpl Infect Dis. 2017;19:E12702.
- Eckburg A, Kazemi T, Maguiness S. Keratosis pilaris rubra successfully treated with topical sirolimus: report of a case and review of the literature. Pediatr Dermatol. 2022;39:429-431.
- Reddy S, Brahmbhatt H. A narrative review on the role of acids, steroids, and kinase inhibitors in the treatment of keratosis pilaris. Cureus. 2021;13:E18917.
- Jordan AS, Green MC, Sulit DJ. Lichen nitidus. J Am Osteopath Assoc. 2019;119:704.
- Arizaga AT, Gaughan MD, Bang RH. Generalized lichen nitidus. Clin Exp Dermatol. 2002;27:115-117.
- Chu J, Lam JM. Lichen nitidus. CMAJ. 2014;186:E688.
- Haber R, Bachour J, El Gemayel M. Scleromyxedema treatment: a systematic review and update. Int J Dermatol. 2020;59:1191-1201.
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosis (LM) (discrete papular type). Dermatol Online J. 2017;23:8.
- Hoffman JHO, Enk AH. Scleromyxedema. J Dtsch Dermatol Ges. 2020;18:1449-1467.
- Kositkuljorn C, Suchonwanit P. Trichostasis spinulosa: a case report with an unusual presentation. Case Rep Dermatol. 2020;12:178-185.
- Ramteke MN, Bhide AA. Trichostasis spinulosa at an unusual site. Int J Trichology. 2016;8:78-80.
A 54-year-old Black woman presented with a rash that developed 6 months after a renal transplant due to a history of systemic lupus erythematosus with lupus nephritis. She was started on mycophenolate mofetil and tacrolimus after the transplant but was switched to cyclosporine because of BK viremia. The rash developed 1 week after cyclosporine was initiated and consisted of pruritic papules that started on the face and spread to the trunk and arms. Physical examination revealed innumerable follicular-based, keratotic, flesh-colored, pinpoint papules with fine white spicules on the face (top), neck, chest, arms, and back. Leonine facies was seen along the glabella with madarosis of the lateral eyebrows (top) and ears (bottom).
Reticulated Brownish Erythema on the Lower Back
The Diagnosis: Erythema Ab Igne
Based on the patient's long-standing history of back pain treated with heating pads as well as the normal laboratory findings and skin examination, a diagnosis of erythema ab igne (EAI) was made.
Erythema ab igne presents as reticulated brownish erythema or hyperpigmentation on sites exposed to prolonged use of heat sources such as heating pads, laptops, and space heaters. Erythema ab igne most commonly affects the lower back, thighs, or legs1-6; however, EAI can appear on atypical sites such as the forehead and eyebrows due to newer technology (eg, virtual reality headsets).7 The level of heat required for EAI to occur is below the threshold for thermal burns (<45 °C [113 °F]).1 Erythema ab igne can occur at any age, and woman are more commonly affected than men.8 The pathophysiology currently is unknown; however, recurrent and prolonged heat exposure may damage superficial vessels. As a result, hemosiderin accumulates in the skin, and hyperpigmentation subsequently occurs.9
The diagnosis of EAI is clinical, and early stages of the rash present as blanching reticulated erythema in areas associated with heat exposure. If the offending source of heat is not removed, EAI can progress to nonblanching, fixed, hyperpigmented plaques with skin atrophy, bullae, or hyperkeratosis. Patients often are asymptomatic; however, mild burning may occur.2 Histopathology reveals cellular atypia, epidermal atrophy, dilation of dermal blood vessels, a minute inflammatory infiltrate, and keratinocyte apoptosis.10 Skin biopsy may be necessary in cases of suspected malignancy due to chronic heat exposure. Lesions that ulcerate or evolve should raise suspicion for malignancy.11 Squamous cell carcinoma is the most common malignancy associated with EAI; other malignancies that may manifest include basal cell carcinoma, Merkel cell carcinoma, or cutaneous marginal zone lymphoma.2,12-14
Erythema ab igne often is mistaken for livedo reticularis, which appears more erythematous without hyperpigmentation or epidermal changes and may be associated with a pathologic state.15 The differential diagnosis in our patient, who was in her 40s with a history of fatigue and joint pain, included livedo reticularis associated with lupus; however, the history of heating pad use, normal laboratory findings, and presence of epidermal changes suggested EAI. Lupus typically affects the hand and knee joints.16 Additionally, livedo reticularis more commonly appears on the legs.15
Other differentials for EAI include livedo racemosa, cutaneous T-cell lymphoma, and cutis marmorata. Livedo racemosa presents with broken rings of erythema in young to middle-aged women and primarily affects the trunk and proximal limbs. It is associated with an underlying condition such as polyarteritis nodosa and less commonly with lupus erythematosus with antiphospholipid or Sneddon syndrome.15,17 Cutaneous T-cell lymphoma typically manifests with poikilodermatous patches larger than the palm, especially in covered areas of skin.18 Cutis marmorata is transient and temperature dependent.9
The key intervention for EAI is removal of the offending heat source.2 Patients should be counseled that the erythema and hyperpigmentation may take months to years to resolve. Topical hydroquinone or tretinoin may be used in cases of persistent hyperpigmentation.19 Patients who continue to use heating pads for long-standing pain should be advised to limit their use to short intervals without occlusion. If malignancy is a concern, a biopsy should be performed.20
- Wipf AJ, Brown MR. Malignant transformation of erythema ab igne. JAAD Case Rep. 2022;26:85-87. doi:10.1016/j.jdcr.2022.06.018
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Patel DP. The evolving nomenclature of erythema ab igne-redness from fire. JAMA Dermatol. 2017;153:685. doi:10.1001/jamadermatol.2017.2021
- Arnold AW, Itin PH. Laptop computer-induced erythema ab igne in a child and review of the literature. Pediatrics. 2010;126:E1227-E1230. doi:10.1542/peds.2010-1390
- Riahi RR, Cohen PR. Laptop-induced erythema ab igne: report and review of literature. Dermatol Online J. 2012;18:5.
- Haleem Z, Philip J, Muhammad S. Erythema ab igne: a rare presentation of toasted skin syndrome with the use of a space heater. Cureus. 2021;13:e13401. doi:10.7759/cureus.13401
- Moreau T, Benzaquen M, Gueissaz F. Erythema ab igne after using a virtual reality headset: a new phenomenon to know. J Eur Acad Dermatol Venereol. 2022;36:E932-E933. doi:10.1111/jdv.18371
- Ozturk M, An I. Clinical features and etiology of patients with erythema ab igne: a retrospective multicenter study. J Cosmet Dermatol. 2020;19:1774-1779. doi:10.1111/jocd.13210
- Gmuca S, Yu J, Weiss PF, et al. Erythema ab igne in an adolescent with chronic pain: an alarming cutaneous eruption from heat exposure. Pediatr Emerg Care. 2020;36:E236-E238. doi:10.1097 /PEC.0000000000001460
- Wells A, Desai A, Rudnick EW, et al. Erythema ab igne with features resembling keratosis lichenoides chronica. J Cutan Pathol. 2021;48:151-153. doi:10.1111/cup.13885
- Milchak M, Smucker J, Chung CG, et al. Erythema ab igne due to heating pad use: a case report and review of clinical presentation, prevention, and complications. Case Rep Med. 2016;2016:1862480. doi:10.1155/2016/1862480
- Daneshvar E, Seraji S, Kamyab-Hesari K, et al. Basal cell carcinoma associated with erythema ab igne. Dermatol Online J. 2020;26:13030 /qt3kz985b4.
- Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124:110-113.
- Wharton J, Roffwarg D, Miller J, et al. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62:1080-1081. doi:10.1016/j.jaad.2009.08.005
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103 /2229-5178.164493
- Grossman JM. Lupus arthritis. Best Pract Res Clin Rheumatol. 2009;23:495-506. doi:10.1016/j.berh.2009.04.003
- Aria AB, Chen L, Silapunt S. Erythema ab igne from heating pad use: a report of three clinical cases and a differential diagnosis. Cureus. 2018;10:E2635. doi:10.7759/cureus.2635
- Wilcox RA. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2017;92:1085-1102. doi:10.1002/ajh.24876
- Pennitz A, Kinberger M, Avila Valle G, et al. Self-applied topical interventions for melasma: a systematic review and meta-analysis of data from randomized, investigator-blinded clinical trials. Br J Dermatol. 2022;187:309-317.
- Sahl WJ, Taira JW. Erythema ab igne: treatment with 5-fluorouracil cream. J Am Acad Dermatol. 1992;27:109-110.
The Diagnosis: Erythema Ab Igne
Based on the patient's long-standing history of back pain treated with heating pads as well as the normal laboratory findings and skin examination, a diagnosis of erythema ab igne (EAI) was made.
Erythema ab igne presents as reticulated brownish erythema or hyperpigmentation on sites exposed to prolonged use of heat sources such as heating pads, laptops, and space heaters. Erythema ab igne most commonly affects the lower back, thighs, or legs1-6; however, EAI can appear on atypical sites such as the forehead and eyebrows due to newer technology (eg, virtual reality headsets).7 The level of heat required for EAI to occur is below the threshold for thermal burns (<45 °C [113 °F]).1 Erythema ab igne can occur at any age, and woman are more commonly affected than men.8 The pathophysiology currently is unknown; however, recurrent and prolonged heat exposure may damage superficial vessels. As a result, hemosiderin accumulates in the skin, and hyperpigmentation subsequently occurs.9
The diagnosis of EAI is clinical, and early stages of the rash present as blanching reticulated erythema in areas associated with heat exposure. If the offending source of heat is not removed, EAI can progress to nonblanching, fixed, hyperpigmented plaques with skin atrophy, bullae, or hyperkeratosis. Patients often are asymptomatic; however, mild burning may occur.2 Histopathology reveals cellular atypia, epidermal atrophy, dilation of dermal blood vessels, a minute inflammatory infiltrate, and keratinocyte apoptosis.10 Skin biopsy may be necessary in cases of suspected malignancy due to chronic heat exposure. Lesions that ulcerate or evolve should raise suspicion for malignancy.11 Squamous cell carcinoma is the most common malignancy associated with EAI; other malignancies that may manifest include basal cell carcinoma, Merkel cell carcinoma, or cutaneous marginal zone lymphoma.2,12-14
Erythema ab igne often is mistaken for livedo reticularis, which appears more erythematous without hyperpigmentation or epidermal changes and may be associated with a pathologic state.15 The differential diagnosis in our patient, who was in her 40s with a history of fatigue and joint pain, included livedo reticularis associated with lupus; however, the history of heating pad use, normal laboratory findings, and presence of epidermal changes suggested EAI. Lupus typically affects the hand and knee joints.16 Additionally, livedo reticularis more commonly appears on the legs.15
Other differentials for EAI include livedo racemosa, cutaneous T-cell lymphoma, and cutis marmorata. Livedo racemosa presents with broken rings of erythema in young to middle-aged women and primarily affects the trunk and proximal limbs. It is associated with an underlying condition such as polyarteritis nodosa and less commonly with lupus erythematosus with antiphospholipid or Sneddon syndrome.15,17 Cutaneous T-cell lymphoma typically manifests with poikilodermatous patches larger than the palm, especially in covered areas of skin.18 Cutis marmorata is transient and temperature dependent.9
The key intervention for EAI is removal of the offending heat source.2 Patients should be counseled that the erythema and hyperpigmentation may take months to years to resolve. Topical hydroquinone or tretinoin may be used in cases of persistent hyperpigmentation.19 Patients who continue to use heating pads for long-standing pain should be advised to limit their use to short intervals without occlusion. If malignancy is a concern, a biopsy should be performed.20
The Diagnosis: Erythema Ab Igne
Based on the patient's long-standing history of back pain treated with heating pads as well as the normal laboratory findings and skin examination, a diagnosis of erythema ab igne (EAI) was made.
Erythema ab igne presents as reticulated brownish erythema or hyperpigmentation on sites exposed to prolonged use of heat sources such as heating pads, laptops, and space heaters. Erythema ab igne most commonly affects the lower back, thighs, or legs1-6; however, EAI can appear on atypical sites such as the forehead and eyebrows due to newer technology (eg, virtual reality headsets).7 The level of heat required for EAI to occur is below the threshold for thermal burns (<45 °C [113 °F]).1 Erythema ab igne can occur at any age, and woman are more commonly affected than men.8 The pathophysiology currently is unknown; however, recurrent and prolonged heat exposure may damage superficial vessels. As a result, hemosiderin accumulates in the skin, and hyperpigmentation subsequently occurs.9
The diagnosis of EAI is clinical, and early stages of the rash present as blanching reticulated erythema in areas associated with heat exposure. If the offending source of heat is not removed, EAI can progress to nonblanching, fixed, hyperpigmented plaques with skin atrophy, bullae, or hyperkeratosis. Patients often are asymptomatic; however, mild burning may occur.2 Histopathology reveals cellular atypia, epidermal atrophy, dilation of dermal blood vessels, a minute inflammatory infiltrate, and keratinocyte apoptosis.10 Skin biopsy may be necessary in cases of suspected malignancy due to chronic heat exposure. Lesions that ulcerate or evolve should raise suspicion for malignancy.11 Squamous cell carcinoma is the most common malignancy associated with EAI; other malignancies that may manifest include basal cell carcinoma, Merkel cell carcinoma, or cutaneous marginal zone lymphoma.2,12-14
Erythema ab igne often is mistaken for livedo reticularis, which appears more erythematous without hyperpigmentation or epidermal changes and may be associated with a pathologic state.15 The differential diagnosis in our patient, who was in her 40s with a history of fatigue and joint pain, included livedo reticularis associated with lupus; however, the history of heating pad use, normal laboratory findings, and presence of epidermal changes suggested EAI. Lupus typically affects the hand and knee joints.16 Additionally, livedo reticularis more commonly appears on the legs.15
Other differentials for EAI include livedo racemosa, cutaneous T-cell lymphoma, and cutis marmorata. Livedo racemosa presents with broken rings of erythema in young to middle-aged women and primarily affects the trunk and proximal limbs. It is associated with an underlying condition such as polyarteritis nodosa and less commonly with lupus erythematosus with antiphospholipid or Sneddon syndrome.15,17 Cutaneous T-cell lymphoma typically manifests with poikilodermatous patches larger than the palm, especially in covered areas of skin.18 Cutis marmorata is transient and temperature dependent.9
The key intervention for EAI is removal of the offending heat source.2 Patients should be counseled that the erythema and hyperpigmentation may take months to years to resolve. Topical hydroquinone or tretinoin may be used in cases of persistent hyperpigmentation.19 Patients who continue to use heating pads for long-standing pain should be advised to limit their use to short intervals without occlusion. If malignancy is a concern, a biopsy should be performed.20
- Wipf AJ, Brown MR. Malignant transformation of erythema ab igne. JAAD Case Rep. 2022;26:85-87. doi:10.1016/j.jdcr.2022.06.018
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Patel DP. The evolving nomenclature of erythema ab igne-redness from fire. JAMA Dermatol. 2017;153:685. doi:10.1001/jamadermatol.2017.2021
- Arnold AW, Itin PH. Laptop computer-induced erythema ab igne in a child and review of the literature. Pediatrics. 2010;126:E1227-E1230. doi:10.1542/peds.2010-1390
- Riahi RR, Cohen PR. Laptop-induced erythema ab igne: report and review of literature. Dermatol Online J. 2012;18:5.
- Haleem Z, Philip J, Muhammad S. Erythema ab igne: a rare presentation of toasted skin syndrome with the use of a space heater. Cureus. 2021;13:e13401. doi:10.7759/cureus.13401
- Moreau T, Benzaquen M, Gueissaz F. Erythema ab igne after using a virtual reality headset: a new phenomenon to know. J Eur Acad Dermatol Venereol. 2022;36:E932-E933. doi:10.1111/jdv.18371
- Ozturk M, An I. Clinical features and etiology of patients with erythema ab igne: a retrospective multicenter study. J Cosmet Dermatol. 2020;19:1774-1779. doi:10.1111/jocd.13210
- Gmuca S, Yu J, Weiss PF, et al. Erythema ab igne in an adolescent with chronic pain: an alarming cutaneous eruption from heat exposure. Pediatr Emerg Care. 2020;36:E236-E238. doi:10.1097 /PEC.0000000000001460
- Wells A, Desai A, Rudnick EW, et al. Erythema ab igne with features resembling keratosis lichenoides chronica. J Cutan Pathol. 2021;48:151-153. doi:10.1111/cup.13885
- Milchak M, Smucker J, Chung CG, et al. Erythema ab igne due to heating pad use: a case report and review of clinical presentation, prevention, and complications. Case Rep Med. 2016;2016:1862480. doi:10.1155/2016/1862480
- Daneshvar E, Seraji S, Kamyab-Hesari K, et al. Basal cell carcinoma associated with erythema ab igne. Dermatol Online J. 2020;26:13030 /qt3kz985b4.
- Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124:110-113.
- Wharton J, Roffwarg D, Miller J, et al. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62:1080-1081. doi:10.1016/j.jaad.2009.08.005
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103 /2229-5178.164493
- Grossman JM. Lupus arthritis. Best Pract Res Clin Rheumatol. 2009;23:495-506. doi:10.1016/j.berh.2009.04.003
- Aria AB, Chen L, Silapunt S. Erythema ab igne from heating pad use: a report of three clinical cases and a differential diagnosis. Cureus. 2018;10:E2635. doi:10.7759/cureus.2635
- Wilcox RA. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2017;92:1085-1102. doi:10.1002/ajh.24876
- Pennitz A, Kinberger M, Avila Valle G, et al. Self-applied topical interventions for melasma: a systematic review and meta-analysis of data from randomized, investigator-blinded clinical trials. Br J Dermatol. 2022;187:309-317.
- Sahl WJ, Taira JW. Erythema ab igne: treatment with 5-fluorouracil cream. J Am Acad Dermatol. 1992;27:109-110.
- Wipf AJ, Brown MR. Malignant transformation of erythema ab igne. JAAD Case Rep. 2022;26:85-87. doi:10.1016/j.jdcr.2022.06.018
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Patel DP. The evolving nomenclature of erythema ab igne-redness from fire. JAMA Dermatol. 2017;153:685. doi:10.1001/jamadermatol.2017.2021
- Arnold AW, Itin PH. Laptop computer-induced erythema ab igne in a child and review of the literature. Pediatrics. 2010;126:E1227-E1230. doi:10.1542/peds.2010-1390
- Riahi RR, Cohen PR. Laptop-induced erythema ab igne: report and review of literature. Dermatol Online J. 2012;18:5.
- Haleem Z, Philip J, Muhammad S. Erythema ab igne: a rare presentation of toasted skin syndrome with the use of a space heater. Cureus. 2021;13:e13401. doi:10.7759/cureus.13401
- Moreau T, Benzaquen M, Gueissaz F. Erythema ab igne after using a virtual reality headset: a new phenomenon to know. J Eur Acad Dermatol Venereol. 2022;36:E932-E933. doi:10.1111/jdv.18371
- Ozturk M, An I. Clinical features and etiology of patients with erythema ab igne: a retrospective multicenter study. J Cosmet Dermatol. 2020;19:1774-1779. doi:10.1111/jocd.13210
- Gmuca S, Yu J, Weiss PF, et al. Erythema ab igne in an adolescent with chronic pain: an alarming cutaneous eruption from heat exposure. Pediatr Emerg Care. 2020;36:E236-E238. doi:10.1097 /PEC.0000000000001460
- Wells A, Desai A, Rudnick EW, et al. Erythema ab igne with features resembling keratosis lichenoides chronica. J Cutan Pathol. 2021;48:151-153. doi:10.1111/cup.13885
- Milchak M, Smucker J, Chung CG, et al. Erythema ab igne due to heating pad use: a case report and review of clinical presentation, prevention, and complications. Case Rep Med. 2016;2016:1862480. doi:10.1155/2016/1862480
- Daneshvar E, Seraji S, Kamyab-Hesari K, et al. Basal cell carcinoma associated with erythema ab igne. Dermatol Online J. 2020;26:13030 /qt3kz985b4.
- Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124:110-113.
- Wharton J, Roffwarg D, Miller J, et al. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62:1080-1081. doi:10.1016/j.jaad.2009.08.005
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103 /2229-5178.164493
- Grossman JM. Lupus arthritis. Best Pract Res Clin Rheumatol. 2009;23:495-506. doi:10.1016/j.berh.2009.04.003
- Aria AB, Chen L, Silapunt S. Erythema ab igne from heating pad use: a report of three clinical cases and a differential diagnosis. Cureus. 2018;10:E2635. doi:10.7759/cureus.2635
- Wilcox RA. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2017;92:1085-1102. doi:10.1002/ajh.24876
- Pennitz A, Kinberger M, Avila Valle G, et al. Self-applied topical interventions for melasma: a systematic review and meta-analysis of data from randomized, investigator-blinded clinical trials. Br J Dermatol. 2022;187:309-317.
- Sahl WJ, Taira JW. Erythema ab igne: treatment with 5-fluorouracil cream. J Am Acad Dermatol. 1992;27:109-110.
A 42-year-old woman presented with an asymptomatic, erythematous, lacelike rash on the lower back of 8 months’ duration that was first noticed by her husband. The patient had a long-standing history of chronic fatigue and lower back pain treated with acetaminophen, diclofenac gel, and heating pads. Physical examination revealed reticulated brownish erythema confined to the lower back. Laboratory findings were unremarkable.
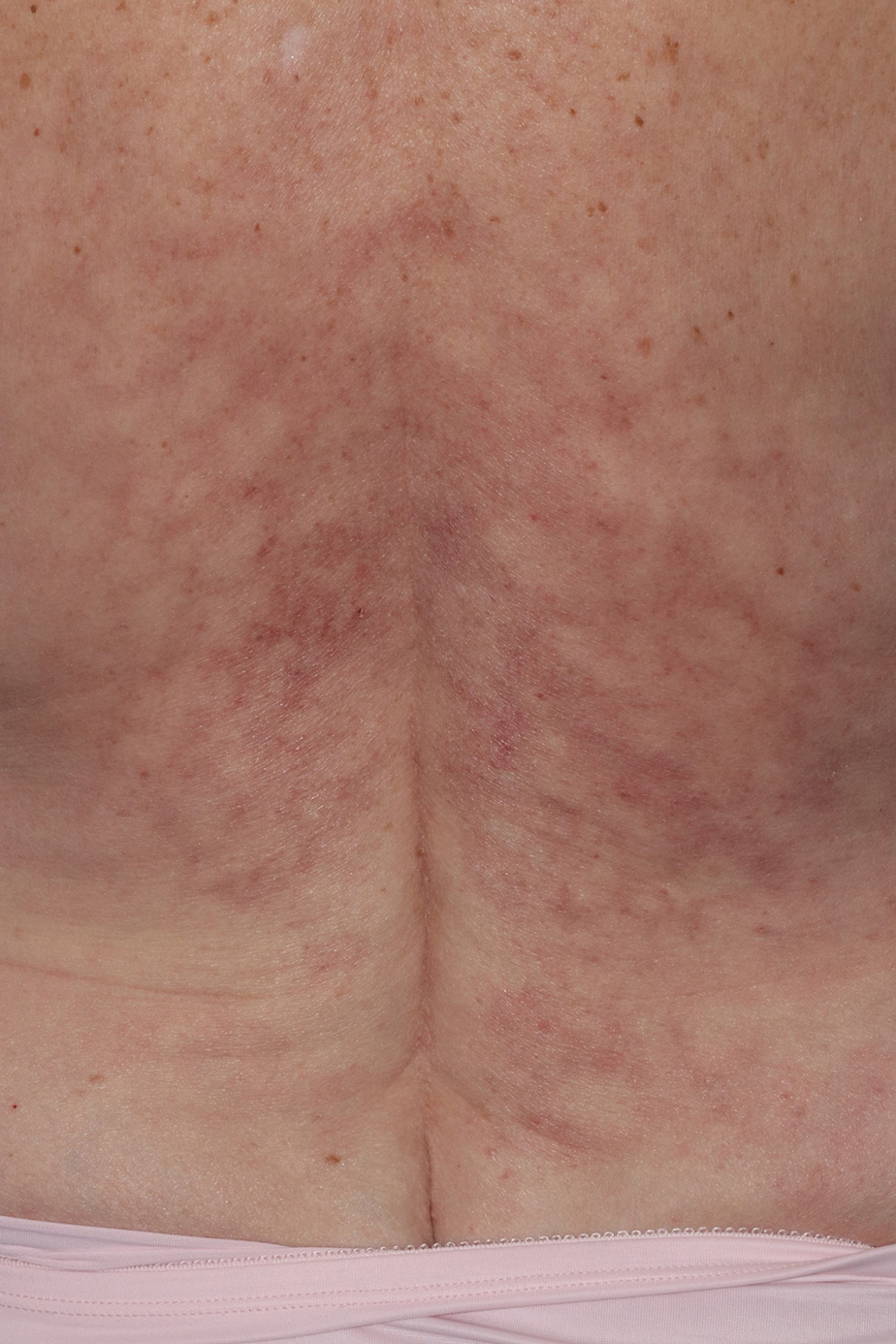
Subungual Nodule in a Pediatric Patient
The Diagnosis: Subungual Exostosis
Subungual exostosis should be considered as a possible cause of an exophytic subungual nodule in a young active female. In our patient, the involvement of the great toe was a clue, as the hallux is the most common location of subungual exostosis. The patient’s age and sex also were supportive, as subungual exostosis is most common in female children and adolescents— particularly those who are active, as trauma is thought to play a possible role in development of this benign tumor.1-3 Radiography is the preferred modality for diagnosis; in our case, it showed a trabecular bony overgrowth (Figure 1), which confirmed the diagnosis. Subungual exostosis is a rare, benign, osteocartilaginous tumor of trabecular bone. The etiology is unknown but is hypothesized to be related to trauma, infection, or activation of a cartilaginous cyst.1,3 The subungual nodule may be asymptomatic or painful. Disruption and elevation of the nail plate is common.4 The differential diagnosis includes amelanotic melanoma, fibroma, fibrokeratoma, osteochondroma, pyogenic granuloma, squamous cell carcinoma, glomus tumor, and verruca vulgaris, among others.5
Physical examination demonstrates a firm, fixed, subungual nodule, often with an accompanying nail deformity. Further workup is required to confirm the benign nature of the lesion and exclude nail tumors such as melanoma or squamous cell carcinoma. Radiography is the gold standard for diagnosis, demonstrating a trabecular bony overgrowth.6 Performing a radiograph as the initial diagnostic test spares the patient from unnecessary procedures such as biopsy or expensive imaging techniques such as magnetic resonance imaging. Early lesions may not demonstrate sufficient bone formation shown on radiography. In these situations, a combination of dermoscopy and histopathologic examination may aid in diagnosis (Figure 2).4 Vascular ectasia, hyperkeratosis, onycholysis, and ulceration are the most common findings on dermoscopy (in ascending order).7 Histopathology typically demonstrates a base or stalk of normal-appearing trabecular bone with a fibrocartilage cap.8 However, initial clinical workup via radiography allows for the least-invasive and highest-yield intervention. Clinical suspicion for this condition is important, as it can be diagnosed with noninvasive inexpensive imaging rather than biopsy or more specialized imaging modalities. Appropriate recognition can save young patients from unnecessary and expensive procedures. Treatment typically involves surgical excision; to prevent regrowth, removal of the lesion at the base of the bone is recommended.2
Although amelanotic melanoma also can manifest as a subungual nail tumor, it would be unusual in a young child and would not be expected to show characteristic changes on radiography. A glomus tumor would be painful, is more common on the fingers than on the toes, and typically has a bluish hue.9 Verruca vulgaris can occur subungually but is more common around the nailfold and often has the characteristic dermoscopic finding of thrombosed capillaries. It also would not be expected to show characteristic radiographic findings. Osteochondroma can occur in young patients and can appear clinically similar to subungual exostosis; however, it typically is painful.10
- Pascoal D, Balaco I, Alves C, et al. Subungual exostosis—treatment results with preservation of the nail bed. J Pediatr Orthop B. 2020;29:382-386.
- Yousefian F, Davis B, Browning JC. Pediatric subungual exostosis. Cutis. 2021;108:256-257.
- Chiheb S, Slimani Y, Karam R, et al. Subungual exostosis: a case series of 48 patients. Skin Appendage Disord. 2021;7:475-479.
- Zhang W, Gu L, Fan H, et al. Subungual exostosis with an unusual dermoscopic feature. JAAD Case Rep. 2020;6:725-726.
- Demirdag HG, Tugrul Ayanoglu B, Akay BN. Dermoscopic features of subungual exostosis. Australas J Dermatol. 2019;60:E138-E141.
- Tritto M, Mirkin G, Hao X. Subungual exostosis on the right hallux. J Am Podiatr Med Assoc. 2021;111.
- Piccolo V, Argenziano G, Alessandrini AM, et al. Dermoscopy of subungual exostosis: a retrospective study of 10 patients. Dermatology. 2017;233:80-85.
- Lee SK, Jung MS, Lee YH, et al. Two distinctive subungual pathologies: subungual exostosis and subungual osteochondroma. Foot Ankle Int. 2007;28:595-601. doi:10.3113/FAI.2007.0595
- Samaniego E, Crespo A, Sanz A. Key diagnostic features and treatment of subungual glomus tumor. Actas Dermosifiliogr. 2009;100:875-882.
- Glick S. Subungual osteochondroma of the third toe. Consult.360. 2013;12.
The Diagnosis: Subungual Exostosis
Subungual exostosis should be considered as a possible cause of an exophytic subungual nodule in a young active female. In our patient, the involvement of the great toe was a clue, as the hallux is the most common location of subungual exostosis. The patient’s age and sex also were supportive, as subungual exostosis is most common in female children and adolescents— particularly those who are active, as trauma is thought to play a possible role in development of this benign tumor.1-3 Radiography is the preferred modality for diagnosis; in our case, it showed a trabecular bony overgrowth (Figure 1), which confirmed the diagnosis. Subungual exostosis is a rare, benign, osteocartilaginous tumor of trabecular bone. The etiology is unknown but is hypothesized to be related to trauma, infection, or activation of a cartilaginous cyst.1,3 The subungual nodule may be asymptomatic or painful. Disruption and elevation of the nail plate is common.4 The differential diagnosis includes amelanotic melanoma, fibroma, fibrokeratoma, osteochondroma, pyogenic granuloma, squamous cell carcinoma, glomus tumor, and verruca vulgaris, among others.5

Physical examination demonstrates a firm, fixed, subungual nodule, often with an accompanying nail deformity. Further workup is required to confirm the benign nature of the lesion and exclude nail tumors such as melanoma or squamous cell carcinoma. Radiography is the gold standard for diagnosis, demonstrating a trabecular bony overgrowth.6 Performing a radiograph as the initial diagnostic test spares the patient from unnecessary procedures such as biopsy or expensive imaging techniques such as magnetic resonance imaging. Early lesions may not demonstrate sufficient bone formation shown on radiography. In these situations, a combination of dermoscopy and histopathologic examination may aid in diagnosis (Figure 2).4 Vascular ectasia, hyperkeratosis, onycholysis, and ulceration are the most common findings on dermoscopy (in ascending order).7 Histopathology typically demonstrates a base or stalk of normal-appearing trabecular bone with a fibrocartilage cap.8 However, initial clinical workup via radiography allows for the least-invasive and highest-yield intervention. Clinical suspicion for this condition is important, as it can be diagnosed with noninvasive inexpensive imaging rather than biopsy or more specialized imaging modalities. Appropriate recognition can save young patients from unnecessary and expensive procedures. Treatment typically involves surgical excision; to prevent regrowth, removal of the lesion at the base of the bone is recommended.2

Although amelanotic melanoma also can manifest as a subungual nail tumor, it would be unusual in a young child and would not be expected to show characteristic changes on radiography. A glomus tumor would be painful, is more common on the fingers than on the toes, and typically has a bluish hue.9 Verruca vulgaris can occur subungually but is more common around the nailfold and often has the characteristic dermoscopic finding of thrombosed capillaries. It also would not be expected to show characteristic radiographic findings. Osteochondroma can occur in young patients and can appear clinically similar to subungual exostosis; however, it typically is painful.10
The Diagnosis: Subungual Exostosis
Subungual exostosis should be considered as a possible cause of an exophytic subungual nodule in a young active female. In our patient, the involvement of the great toe was a clue, as the hallux is the most common location of subungual exostosis. The patient’s age and sex also were supportive, as subungual exostosis is most common in female children and adolescents— particularly those who are active, as trauma is thought to play a possible role in development of this benign tumor.1-3 Radiography is the preferred modality for diagnosis; in our case, it showed a trabecular bony overgrowth (Figure 1), which confirmed the diagnosis. Subungual exostosis is a rare, benign, osteocartilaginous tumor of trabecular bone. The etiology is unknown but is hypothesized to be related to trauma, infection, or activation of a cartilaginous cyst.1,3 The subungual nodule may be asymptomatic or painful. Disruption and elevation of the nail plate is common.4 The differential diagnosis includes amelanotic melanoma, fibroma, fibrokeratoma, osteochondroma, pyogenic granuloma, squamous cell carcinoma, glomus tumor, and verruca vulgaris, among others.5

Physical examination demonstrates a firm, fixed, subungual nodule, often with an accompanying nail deformity. Further workup is required to confirm the benign nature of the lesion and exclude nail tumors such as melanoma or squamous cell carcinoma. Radiography is the gold standard for diagnosis, demonstrating a trabecular bony overgrowth.6 Performing a radiograph as the initial diagnostic test spares the patient from unnecessary procedures such as biopsy or expensive imaging techniques such as magnetic resonance imaging. Early lesions may not demonstrate sufficient bone formation shown on radiography. In these situations, a combination of dermoscopy and histopathologic examination may aid in diagnosis (Figure 2).4 Vascular ectasia, hyperkeratosis, onycholysis, and ulceration are the most common findings on dermoscopy (in ascending order).7 Histopathology typically demonstrates a base or stalk of normal-appearing trabecular bone with a fibrocartilage cap.8 However, initial clinical workup via radiography allows for the least-invasive and highest-yield intervention. Clinical suspicion for this condition is important, as it can be diagnosed with noninvasive inexpensive imaging rather than biopsy or more specialized imaging modalities. Appropriate recognition can save young patients from unnecessary and expensive procedures. Treatment typically involves surgical excision; to prevent regrowth, removal of the lesion at the base of the bone is recommended.2

Although amelanotic melanoma also can manifest as a subungual nail tumor, it would be unusual in a young child and would not be expected to show characteristic changes on radiography. A glomus tumor would be painful, is more common on the fingers than on the toes, and typically has a bluish hue.9 Verruca vulgaris can occur subungually but is more common around the nailfold and often has the characteristic dermoscopic finding of thrombosed capillaries. It also would not be expected to show characteristic radiographic findings. Osteochondroma can occur in young patients and can appear clinically similar to subungual exostosis; however, it typically is painful.10
- Pascoal D, Balaco I, Alves C, et al. Subungual exostosis—treatment results with preservation of the nail bed. J Pediatr Orthop B. 2020;29:382-386.
- Yousefian F, Davis B, Browning JC. Pediatric subungual exostosis. Cutis. 2021;108:256-257.
- Chiheb S, Slimani Y, Karam R, et al. Subungual exostosis: a case series of 48 patients. Skin Appendage Disord. 2021;7:475-479.
- Zhang W, Gu L, Fan H, et al. Subungual exostosis with an unusual dermoscopic feature. JAAD Case Rep. 2020;6:725-726.
- Demirdag HG, Tugrul Ayanoglu B, Akay BN. Dermoscopic features of subungual exostosis. Australas J Dermatol. 2019;60:E138-E141.
- Tritto M, Mirkin G, Hao X. Subungual exostosis on the right hallux. J Am Podiatr Med Assoc. 2021;111.
- Piccolo V, Argenziano G, Alessandrini AM, et al. Dermoscopy of subungual exostosis: a retrospective study of 10 patients. Dermatology. 2017;233:80-85.
- Lee SK, Jung MS, Lee YH, et al. Two distinctive subungual pathologies: subungual exostosis and subungual osteochondroma. Foot Ankle Int. 2007;28:595-601. doi:10.3113/FAI.2007.0595
- Samaniego E, Crespo A, Sanz A. Key diagnostic features and treatment of subungual glomus tumor. Actas Dermosifiliogr. 2009;100:875-882.
- Glick S. Subungual osteochondroma of the third toe. Consult.360. 2013;12.
- Pascoal D, Balaco I, Alves C, et al. Subungual exostosis—treatment results with preservation of the nail bed. J Pediatr Orthop B. 2020;29:382-386.
- Yousefian F, Davis B, Browning JC. Pediatric subungual exostosis. Cutis. 2021;108:256-257.
- Chiheb S, Slimani Y, Karam R, et al. Subungual exostosis: a case series of 48 patients. Skin Appendage Disord. 2021;7:475-479.
- Zhang W, Gu L, Fan H, et al. Subungual exostosis with an unusual dermoscopic feature. JAAD Case Rep. 2020;6:725-726.
- Demirdag HG, Tugrul Ayanoglu B, Akay BN. Dermoscopic features of subungual exostosis. Australas J Dermatol. 2019;60:E138-E141.
- Tritto M, Mirkin G, Hao X. Subungual exostosis on the right hallux. J Am Podiatr Med Assoc. 2021;111.
- Piccolo V, Argenziano G, Alessandrini AM, et al. Dermoscopy of subungual exostosis: a retrospective study of 10 patients. Dermatology. 2017;233:80-85.
- Lee SK, Jung MS, Lee YH, et al. Two distinctive subungual pathologies: subungual exostosis and subungual osteochondroma. Foot Ankle Int. 2007;28:595-601. doi:10.3113/FAI.2007.0595
- Samaniego E, Crespo A, Sanz A. Key diagnostic features and treatment of subungual glomus tumor. Actas Dermosifiliogr. 2009;100:875-882.
- Glick S. Subungual osteochondroma of the third toe. Consult.360. 2013;12.
A 13-year-old girl presented to her pediatrician with a small pink bump under the left great toenail of 8 months’ duration that was slowly growing. Months later, she developed an ingrown nail on the same toe, which was treated with partial nail avulsion by the pediatrician. Given continued nail dystrophy and a visible bump under the nail, the patient was referred to dermatology. Physical examination revealed a subungual, flesh-colored, sessile nodule causing distortion of the nail plate on the left great toe with associated intermittent redness and swelling. She denied wearing new shoes or experiencing any pain, pruritus, or purulent drainage or bleeding from the lesion. She reported being physically active and playing tennis.
Transient Symmetric Blanching Macules on a Background of Reticulate Erythema
The Diagnosis: BASCULE Syndrome
The patient had previously been thought to have livedo reticularis by primary care. Repeat antinuclear antibody (ANA) testing was positive (1:1280 homogeneous [reflexive titers all negative]). However, upon dermatologic evaluation, the manifestation of the rash in addition to onset occurring with postural changes challenged the livedo reticularis diagnosis. Extensive research and consultation with dermatologic colleagues led to the diagnosis of the rare entity BASCULE syndrome. BASCULE (Bier anemic spots, cyanosis, and urticarialike eruption) syndrome was described by Bessis et al1 in 2016. It is a rare condition but may be underreported.2 It is a benign pediatric disorder in the vascular acrosyndrome family that is characterized by underlying vasomotor dysfunction in distal regions of the body. Raynaud phenomenon is a widely known member of this family. As seen in our patient, it typically presents on the distal legs and feet with numerous irregular hypopigmented macules on a cyanotic background. Red-orange papules may appear on the hypopigmented macules and often are pruritic. Lesions on the distal upper extremities are less common, and a case involving the trunk has been reported.3 Onset generally begins within a couple of minutes of standing or mechanical compression of the lower legs, with full reversal of symptoms occurring within minutes of laying down or walking. Commonly reported associated symptoms include tenderness, pruritus, edema, and pain; however, the cutaneous lesions may be asymptomatic. The condition tends to affect adolescents, as seen in our patient; however, there have been reports in infants as young as 3 months to adults aged 19 years.2
The pathophysiology behind BASCULE syndrome remains unclear but is believed to be centered around the role of physiologic venous stasis that occurs when standing. The hypoxia secondary to stasis is thought to induce amplified vasoconstriction of arterioles. These responses are further exaggerated due to absence of venoarteriolar reflexes in dermal ascending arterioles, leading to Bier spots.2 The role of mast cells and eosinophils remains unclear. It is a clinical diagnosis without clear histologic findings; therefore, biopsy was not pursued in our patient.
Although BASCULE syndrome is a benign entity, it is imperative that it be recognized to avoid a time consuming, expensive, and anxiety-producing diagnostic workup, as occurred in our patient. Although not a manifestation of systemic disease, BASCULE syndrome may be associated with orthostatic hypotension in up to 20% of cases.2,4 Therefore, these patients should undergo orthostatic testing, including the tilt table test. In our patient, these manifestations were not appreciated.
There are no current guidelines for effective treatment of BASCULE syndrome. Given the possible role of mast cells in the condition, H1 antihistamines are proposed as first-line treatment. Desloratadine (10 mg/d for 7 days) has been found to be associated with improvement of pruritus. However, a recent literature review found little evidence to support the use of H1 antihistamines for resolution of other symptoms.2
The differential diagnosis includes livedo reticularis, Bier spots, Sneddon syndrome, and urticarial vasculitis. Livedo reticularis presents as distinct, netlike, blue-erythematousviolaceous discoloration, which differs from the distinct orange-red macules in BASCULE syndrome.5 In addition to distinct variances in dermatologic presentation, livedo reticularis typically is associated with cold exposure as a causative agent, with cold avoidance as the treatment for this benign and often transient condition.6 This phenomenon was not appreciated in our patient. Livedo reticularis commonly occurs with antiphospholipid syndrome.5 This association in combination with our patient's positive ANA findings and her mother's history of miscarriages resulted in the misdiagnosis as livedo reticularis.
Bier spots manifest as white macules with surrounding erythema and typically present in young adults. When first described in the literature, it was debated if BASCULE syndrome was simply another manifestation of Bier spots or postural orthostatic intolerance,4 as there was a large consensus that postural orthostatic intolerance was associated with BASCULE syndrome, with the majority of patients not meeting criteria for the condition. Heymann4 addressed the differences in BASCULE manifestations vs typical Bier spots. The author extended the syndrome to include cyanosis, an urticarialike eruption of red-orange macules with central papules located centrally, pruritus, tenderness, and partial or diffuse edema, in addition to Bier spots.4
Sneddon syndrome is a rare progressive disorder that affects small- to medium-sized blood vessels resulting in multiple episodes of ischemia in the brain. Skin manifestations of these repeated strokes are similar to livedo reticularis, typically manifesting as livedo racemosa—irregular reticular patterns of skin mottling with reddish-blue hues.6 However, Sneddon syndrome is more generalized and widespread and differs from BASCULE syndrome in shape and histologic findings. Our patient presented with findings on the legs, which is more characteristic of livedo reticularis vs livedo racemosa. Our patient experienced resolution upon laying down and sitting, and Sneddon syndrome persists beyond postural changes. Furthermore, patients with Sneddon syndrome present with neurologic symptoms such as prodromal headaches.6
Urticarial vasculitis was ruled out in our patient because of the duration of symptoms as well as the spatial changes. Urticarial vasculitis is a rare skin condition characterized by chronic recurring urticarial lesions that may persist for more than a day. This condition typically presents in middle-aged women and rarely in children. Urticarial vasculitis is thought to be immune-complex mediated, but its cause is largely unknown. It is a common manifestation of underlying conditions such as systemic lupus erythematosus.6 Our patient had a positive ANA and possible autoimmune history from her mother; however, urticarial vasculitis does not present transiently on the legs or in the rash pattern appreciated in our patient.
- Bessis D, Jeziorski E, Rigau V, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome: a new entity? Br J Dermatol. 2016;175:218-220. doi:10.1111/bjd.14589
- Baurens N, Briand C, Giovannini-Chami L, et al. Case report, practices survey and literature review of an under-recognized pediatric vascular disorder: the BASCULE syndrome. Front Pediatr. 2022;10:849914. doi:10.3389/fped.2022.849914
- Jiménez-Gallo D, Collantes-Rodríguez C, Ossorio-García L, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome on trunk and upper limbs. Pediatr Dermatol. 2018;35:E313-E315. doi:10.1111/pde.13558
- Heymann WR. BASCULE syndrome: is something brewing with Bier spots? Dermatology World Insights and Inquiries. September 7, 2022. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2022/bascule-syndrome
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Gu SL, Jorizzo JL. Urticarial vasculitis. Int J Womens Dermatol. 2021;7:290-297. doi:10.1016/j.ijwd.2021.01.021
The Diagnosis: BASCULE Syndrome
The patient had previously been thought to have livedo reticularis by primary care. Repeat antinuclear antibody (ANA) testing was positive (1:1280 homogeneous [reflexive titers all negative]). However, upon dermatologic evaluation, the manifestation of the rash in addition to onset occurring with postural changes challenged the livedo reticularis diagnosis. Extensive research and consultation with dermatologic colleagues led to the diagnosis of the rare entity BASCULE syndrome. BASCULE (Bier anemic spots, cyanosis, and urticarialike eruption) syndrome was described by Bessis et al1 in 2016. It is a rare condition but may be underreported.2 It is a benign pediatric disorder in the vascular acrosyndrome family that is characterized by underlying vasomotor dysfunction in distal regions of the body. Raynaud phenomenon is a widely known member of this family. As seen in our patient, it typically presents on the distal legs and feet with numerous irregular hypopigmented macules on a cyanotic background. Red-orange papules may appear on the hypopigmented macules and often are pruritic. Lesions on the distal upper extremities are less common, and a case involving the trunk has been reported.3 Onset generally begins within a couple of minutes of standing or mechanical compression of the lower legs, with full reversal of symptoms occurring within minutes of laying down or walking. Commonly reported associated symptoms include tenderness, pruritus, edema, and pain; however, the cutaneous lesions may be asymptomatic. The condition tends to affect adolescents, as seen in our patient; however, there have been reports in infants as young as 3 months to adults aged 19 years.2
The pathophysiology behind BASCULE syndrome remains unclear but is believed to be centered around the role of physiologic venous stasis that occurs when standing. The hypoxia secondary to stasis is thought to induce amplified vasoconstriction of arterioles. These responses are further exaggerated due to absence of venoarteriolar reflexes in dermal ascending arterioles, leading to Bier spots.2 The role of mast cells and eosinophils remains unclear. It is a clinical diagnosis without clear histologic findings; therefore, biopsy was not pursued in our patient.
Although BASCULE syndrome is a benign entity, it is imperative that it be recognized to avoid a time consuming, expensive, and anxiety-producing diagnostic workup, as occurred in our patient. Although not a manifestation of systemic disease, BASCULE syndrome may be associated with orthostatic hypotension in up to 20% of cases.2,4 Therefore, these patients should undergo orthostatic testing, including the tilt table test. In our patient, these manifestations were not appreciated.
There are no current guidelines for effective treatment of BASCULE syndrome. Given the possible role of mast cells in the condition, H1 antihistamines are proposed as first-line treatment. Desloratadine (10 mg/d for 7 days) has been found to be associated with improvement of pruritus. However, a recent literature review found little evidence to support the use of H1 antihistamines for resolution of other symptoms.2
The differential diagnosis includes livedo reticularis, Bier spots, Sneddon syndrome, and urticarial vasculitis. Livedo reticularis presents as distinct, netlike, blue-erythematousviolaceous discoloration, which differs from the distinct orange-red macules in BASCULE syndrome.5 In addition to distinct variances in dermatologic presentation, livedo reticularis typically is associated with cold exposure as a causative agent, with cold avoidance as the treatment for this benign and often transient condition.6 This phenomenon was not appreciated in our patient. Livedo reticularis commonly occurs with antiphospholipid syndrome.5 This association in combination with our patient's positive ANA findings and her mother's history of miscarriages resulted in the misdiagnosis as livedo reticularis.
Bier spots manifest as white macules with surrounding erythema and typically present in young adults. When first described in the literature, it was debated if BASCULE syndrome was simply another manifestation of Bier spots or postural orthostatic intolerance,4 as there was a large consensus that postural orthostatic intolerance was associated with BASCULE syndrome, with the majority of patients not meeting criteria for the condition. Heymann4 addressed the differences in BASCULE manifestations vs typical Bier spots. The author extended the syndrome to include cyanosis, an urticarialike eruption of red-orange macules with central papules located centrally, pruritus, tenderness, and partial or diffuse edema, in addition to Bier spots.4
Sneddon syndrome is a rare progressive disorder that affects small- to medium-sized blood vessels resulting in multiple episodes of ischemia in the brain. Skin manifestations of these repeated strokes are similar to livedo reticularis, typically manifesting as livedo racemosa—irregular reticular patterns of skin mottling with reddish-blue hues.6 However, Sneddon syndrome is more generalized and widespread and differs from BASCULE syndrome in shape and histologic findings. Our patient presented with findings on the legs, which is more characteristic of livedo reticularis vs livedo racemosa. Our patient experienced resolution upon laying down and sitting, and Sneddon syndrome persists beyond postural changes. Furthermore, patients with Sneddon syndrome present with neurologic symptoms such as prodromal headaches.6
Urticarial vasculitis was ruled out in our patient because of the duration of symptoms as well as the spatial changes. Urticarial vasculitis is a rare skin condition characterized by chronic recurring urticarial lesions that may persist for more than a day. This condition typically presents in middle-aged women and rarely in children. Urticarial vasculitis is thought to be immune-complex mediated, but its cause is largely unknown. It is a common manifestation of underlying conditions such as systemic lupus erythematosus.6 Our patient had a positive ANA and possible autoimmune history from her mother; however, urticarial vasculitis does not present transiently on the legs or in the rash pattern appreciated in our patient.
The Diagnosis: BASCULE Syndrome
The patient had previously been thought to have livedo reticularis by primary care. Repeat antinuclear antibody (ANA) testing was positive (1:1280 homogeneous [reflexive titers all negative]). However, upon dermatologic evaluation, the manifestation of the rash in addition to onset occurring with postural changes challenged the livedo reticularis diagnosis. Extensive research and consultation with dermatologic colleagues led to the diagnosis of the rare entity BASCULE syndrome. BASCULE (Bier anemic spots, cyanosis, and urticarialike eruption) syndrome was described by Bessis et al1 in 2016. It is a rare condition but may be underreported.2 It is a benign pediatric disorder in the vascular acrosyndrome family that is characterized by underlying vasomotor dysfunction in distal regions of the body. Raynaud phenomenon is a widely known member of this family. As seen in our patient, it typically presents on the distal legs and feet with numerous irregular hypopigmented macules on a cyanotic background. Red-orange papules may appear on the hypopigmented macules and often are pruritic. Lesions on the distal upper extremities are less common, and a case involving the trunk has been reported.3 Onset generally begins within a couple of minutes of standing or mechanical compression of the lower legs, with full reversal of symptoms occurring within minutes of laying down or walking. Commonly reported associated symptoms include tenderness, pruritus, edema, and pain; however, the cutaneous lesions may be asymptomatic. The condition tends to affect adolescents, as seen in our patient; however, there have been reports in infants as young as 3 months to adults aged 19 years.2
The pathophysiology behind BASCULE syndrome remains unclear but is believed to be centered around the role of physiologic venous stasis that occurs when standing. The hypoxia secondary to stasis is thought to induce amplified vasoconstriction of arterioles. These responses are further exaggerated due to absence of venoarteriolar reflexes in dermal ascending arterioles, leading to Bier spots.2 The role of mast cells and eosinophils remains unclear. It is a clinical diagnosis without clear histologic findings; therefore, biopsy was not pursued in our patient.
Although BASCULE syndrome is a benign entity, it is imperative that it be recognized to avoid a time consuming, expensive, and anxiety-producing diagnostic workup, as occurred in our patient. Although not a manifestation of systemic disease, BASCULE syndrome may be associated with orthostatic hypotension in up to 20% of cases.2,4 Therefore, these patients should undergo orthostatic testing, including the tilt table test. In our patient, these manifestations were not appreciated.
There are no current guidelines for effective treatment of BASCULE syndrome. Given the possible role of mast cells in the condition, H1 antihistamines are proposed as first-line treatment. Desloratadine (10 mg/d for 7 days) has been found to be associated with improvement of pruritus. However, a recent literature review found little evidence to support the use of H1 antihistamines for resolution of other symptoms.2
The differential diagnosis includes livedo reticularis, Bier spots, Sneddon syndrome, and urticarial vasculitis. Livedo reticularis presents as distinct, netlike, blue-erythematousviolaceous discoloration, which differs from the distinct orange-red macules in BASCULE syndrome.5 In addition to distinct variances in dermatologic presentation, livedo reticularis typically is associated with cold exposure as a causative agent, with cold avoidance as the treatment for this benign and often transient condition.6 This phenomenon was not appreciated in our patient. Livedo reticularis commonly occurs with antiphospholipid syndrome.5 This association in combination with our patient's positive ANA findings and her mother's history of miscarriages resulted in the misdiagnosis as livedo reticularis.
Bier spots manifest as white macules with surrounding erythema and typically present in young adults. When first described in the literature, it was debated if BASCULE syndrome was simply another manifestation of Bier spots or postural orthostatic intolerance,4 as there was a large consensus that postural orthostatic intolerance was associated with BASCULE syndrome, with the majority of patients not meeting criteria for the condition. Heymann4 addressed the differences in BASCULE manifestations vs typical Bier spots. The author extended the syndrome to include cyanosis, an urticarialike eruption of red-orange macules with central papules located centrally, pruritus, tenderness, and partial or diffuse edema, in addition to Bier spots.4
Sneddon syndrome is a rare progressive disorder that affects small- to medium-sized blood vessels resulting in multiple episodes of ischemia in the brain. Skin manifestations of these repeated strokes are similar to livedo reticularis, typically manifesting as livedo racemosa—irregular reticular patterns of skin mottling with reddish-blue hues.6 However, Sneddon syndrome is more generalized and widespread and differs from BASCULE syndrome in shape and histologic findings. Our patient presented with findings on the legs, which is more characteristic of livedo reticularis vs livedo racemosa. Our patient experienced resolution upon laying down and sitting, and Sneddon syndrome persists beyond postural changes. Furthermore, patients with Sneddon syndrome present with neurologic symptoms such as prodromal headaches.6
Urticarial vasculitis was ruled out in our patient because of the duration of symptoms as well as the spatial changes. Urticarial vasculitis is a rare skin condition characterized by chronic recurring urticarial lesions that may persist for more than a day. This condition typically presents in middle-aged women and rarely in children. Urticarial vasculitis is thought to be immune-complex mediated, but its cause is largely unknown. It is a common manifestation of underlying conditions such as systemic lupus erythematosus.6 Our patient had a positive ANA and possible autoimmune history from her mother; however, urticarial vasculitis does not present transiently on the legs or in the rash pattern appreciated in our patient.
- Bessis D, Jeziorski E, Rigau V, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome: a new entity? Br J Dermatol. 2016;175:218-220. doi:10.1111/bjd.14589
- Baurens N, Briand C, Giovannini-Chami L, et al. Case report, practices survey and literature review of an under-recognized pediatric vascular disorder: the BASCULE syndrome. Front Pediatr. 2022;10:849914. doi:10.3389/fped.2022.849914
- Jiménez-Gallo D, Collantes-Rodríguez C, Ossorio-García L, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome on trunk and upper limbs. Pediatr Dermatol. 2018;35:E313-E315. doi:10.1111/pde.13558
- Heymann WR. BASCULE syndrome: is something brewing with Bier spots? Dermatology World Insights and Inquiries. September 7, 2022. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2022/bascule-syndrome
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Gu SL, Jorizzo JL. Urticarial vasculitis. Int J Womens Dermatol. 2021;7:290-297. doi:10.1016/j.ijwd.2021.01.021
- Bessis D, Jeziorski E, Rigau V, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome: a new entity? Br J Dermatol. 2016;175:218-220. doi:10.1111/bjd.14589
- Baurens N, Briand C, Giovannini-Chami L, et al. Case report, practices survey and literature review of an under-recognized pediatric vascular disorder: the BASCULE syndrome. Front Pediatr. 2022;10:849914. doi:10.3389/fped.2022.849914
- Jiménez-Gallo D, Collantes-Rodríguez C, Ossorio-García L, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome on trunk and upper limbs. Pediatr Dermatol. 2018;35:E313-E315. doi:10.1111/pde.13558
- Heymann WR. BASCULE syndrome: is something brewing with Bier spots? Dermatology World Insights and Inquiries. September 7, 2022. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2022/bascule-syndrome
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Gu SL, Jorizzo JL. Urticarial vasculitis. Int J Womens Dermatol. 2021;7:290-297. doi:10.1016/j.ijwd.2021.01.021
An 11-year-old girl was referred to the dermatology clinic for evaluation of a rash on the legs and feet of 1 year’s duration. The rash appeared every time she was standing for longer than 10 to 15 minutes and resolved when sitting or laying down. After the initial onset, the rash did not spread to other body areas but became more prominent in appearance. The patient endorsed intense pruritus associated with the rash. A review of systems was negative for fever, headaches, history of blood clots, and joint pain. She did not have any known medical conditions or take any medications. The patient’s mother reported that the patient experienced episodes of leg numbness while sitting in vehicles from 6 to 10 years of age. There was no family history of rheumatologic, hematologic, or cardiac conditions. The patient’s mother had experienced 2 miscarriages but denied any other obstetric complications. The patient had 1 sibling who was unaffected. Physical examination revealed reticulate erythema on the calves with scattered regions of blanching and evanescent pink macules as well as dermatographism.
One month prior to presenting to dermatology, the patient was evaluated by rheumatology, endocrinology, and hematology. Laboratory workup completed at age 3 years included antinuclear antibody, anticardiolipin antibody, and antithrombin III activity; factor V Leiden; cryoglobulins; quantitation (human chorionic gonadotropin); proteins S and C activity; antineutrophil cytoplasmic antibody screen; thyroid studies; prothrombin time; and partial thromboplastin time. All laboratory results were within reference range.