User login
Trump administration rule erodes ACA contraceptive mandate
More employers can opt out of providing contraception coverage to their employees under final regulations from the Trump administration that narrow the Affordable Care Act’s contraceptive mandate.
The two regulations, released Nov. 7, allow an expanded group of employers and insurers to get out of covering contraception methods by objecting on either religious or moral grounds.
The first rule broadens exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of sincerely held religious beliefs. The second rule protects nonprofit organizations and small businesses that have nonreligious moral convictions that oppose services covered by the mandate. The religious and moral exemptions apply to institutions of education, issuers, and individuals, but not to governmental entities.
When first proposed in 2017, Trump administration officials said the new policies would “better balance the government’s interest in promoting coverage for contraceptive and sterilization services with the government’s interests in providing conscience protections for entities with sincerely held moral convictions.” The U.S. Department of Health & Human Services estimates that the rules, which take effect in January 2019, will affect no more than 200 employers.
The American College of Obstetricians and Gynecologists expressed concern that the final rules will restrict patient access to meaningful contraceptive methods and will erode decades of progress in increasing women’s reproductive autonomy and restrict patient access to contraception.
“Women, families and our nation all benefit from seamless, affordable access to contraception,” ACOG President Lisa M. Hollier, MD, said in a statement. “Contraception improves women’s health and well-being, reduces unintended pregnancy, enables pregnancy spacing for safer pregnancies and deliveries, and empowers women’s engagement in the workforce and economic self-sufficiency. A woman’s employer should not determine whether or not she has this access.”
Marjorie Dannenfelser, president of Susan B. Anthony List, an anti-abortion group, praised the final rules, calling them needed protections from the burdensome Obama-era ACA abortifacient drug mandate.
“President Trump and HHS Secretary Azar delivered a huge victory for conscience rights and religious liberty in America,” Ms. Dannenfelser said in a statement. “No longer will Catholic nuns who care for the elderly poor be forced by the government to provide abortion-inducing drugs in their health care plans. Not only that, moral objectors such as Susan B. Anthony List, will also no longer have to pay for life-ending drugs that are antithetical to their mission and for which we have argued there is certainly no compelling state interest.”
The ACA initially required all employers to cover birth control for employees with no copayments, except for group health plans of religious employers, which were deemed exempt. Those religious employers were primarily churches and other houses of worship. After a number of complaints and legal challenges, the Obama administration created a workaround for nonprofit religious employers to opt out of the mandate.
However, critics argued the process itself was a violation of their religious freedom. The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved. In May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
Under the approved regulations, employers or insurers can stop their coverage of contraceptive services if they have religious beliefs or moral convictions against covering birth control. Exempted entities and individuals also can choose to cover some, but not all, contraceptive services, depending on their specific religious or moral objection, according to an HHS fact sheet.
The agency emphasized that the regulations leave in place government programs that provide free or subsidized contraceptive coverage to low-income women, such as through community health centers, and that the rules do not ban any employer from covering contraceptives.
The regulations become effective 60 days after they are published in the Federal Register.
More employers can opt out of providing contraception coverage to their employees under final regulations from the Trump administration that narrow the Affordable Care Act’s contraceptive mandate.
The two regulations, released Nov. 7, allow an expanded group of employers and insurers to get out of covering contraception methods by objecting on either religious or moral grounds.
The first rule broadens exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of sincerely held religious beliefs. The second rule protects nonprofit organizations and small businesses that have nonreligious moral convictions that oppose services covered by the mandate. The religious and moral exemptions apply to institutions of education, issuers, and individuals, but not to governmental entities.
When first proposed in 2017, Trump administration officials said the new policies would “better balance the government’s interest in promoting coverage for contraceptive and sterilization services with the government’s interests in providing conscience protections for entities with sincerely held moral convictions.” The U.S. Department of Health & Human Services estimates that the rules, which take effect in January 2019, will affect no more than 200 employers.
The American College of Obstetricians and Gynecologists expressed concern that the final rules will restrict patient access to meaningful contraceptive methods and will erode decades of progress in increasing women’s reproductive autonomy and restrict patient access to contraception.
“Women, families and our nation all benefit from seamless, affordable access to contraception,” ACOG President Lisa M. Hollier, MD, said in a statement. “Contraception improves women’s health and well-being, reduces unintended pregnancy, enables pregnancy spacing for safer pregnancies and deliveries, and empowers women’s engagement in the workforce and economic self-sufficiency. A woman’s employer should not determine whether or not she has this access.”
Marjorie Dannenfelser, president of Susan B. Anthony List, an anti-abortion group, praised the final rules, calling them needed protections from the burdensome Obama-era ACA abortifacient drug mandate.
“President Trump and HHS Secretary Azar delivered a huge victory for conscience rights and religious liberty in America,” Ms. Dannenfelser said in a statement. “No longer will Catholic nuns who care for the elderly poor be forced by the government to provide abortion-inducing drugs in their health care plans. Not only that, moral objectors such as Susan B. Anthony List, will also no longer have to pay for life-ending drugs that are antithetical to their mission and for which we have argued there is certainly no compelling state interest.”
The ACA initially required all employers to cover birth control for employees with no copayments, except for group health plans of religious employers, which were deemed exempt. Those religious employers were primarily churches and other houses of worship. After a number of complaints and legal challenges, the Obama administration created a workaround for nonprofit religious employers to opt out of the mandate.
However, critics argued the process itself was a violation of their religious freedom. The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved. In May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
Under the approved regulations, employers or insurers can stop their coverage of contraceptive services if they have religious beliefs or moral convictions against covering birth control. Exempted entities and individuals also can choose to cover some, but not all, contraceptive services, depending on their specific religious or moral objection, according to an HHS fact sheet.
The agency emphasized that the regulations leave in place government programs that provide free or subsidized contraceptive coverage to low-income women, such as through community health centers, and that the rules do not ban any employer from covering contraceptives.
The regulations become effective 60 days after they are published in the Federal Register.
More employers can opt out of providing contraception coverage to their employees under final regulations from the Trump administration that narrow the Affordable Care Act’s contraceptive mandate.
The two regulations, released Nov. 7, allow an expanded group of employers and insurers to get out of covering contraception methods by objecting on either religious or moral grounds.
The first rule broadens exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of sincerely held religious beliefs. The second rule protects nonprofit organizations and small businesses that have nonreligious moral convictions that oppose services covered by the mandate. The religious and moral exemptions apply to institutions of education, issuers, and individuals, but not to governmental entities.
When first proposed in 2017, Trump administration officials said the new policies would “better balance the government’s interest in promoting coverage for contraceptive and sterilization services with the government’s interests in providing conscience protections for entities with sincerely held moral convictions.” The U.S. Department of Health & Human Services estimates that the rules, which take effect in January 2019, will affect no more than 200 employers.
The American College of Obstetricians and Gynecologists expressed concern that the final rules will restrict patient access to meaningful contraceptive methods and will erode decades of progress in increasing women’s reproductive autonomy and restrict patient access to contraception.
“Women, families and our nation all benefit from seamless, affordable access to contraception,” ACOG President Lisa M. Hollier, MD, said in a statement. “Contraception improves women’s health and well-being, reduces unintended pregnancy, enables pregnancy spacing for safer pregnancies and deliveries, and empowers women’s engagement in the workforce and economic self-sufficiency. A woman’s employer should not determine whether or not she has this access.”
Marjorie Dannenfelser, president of Susan B. Anthony List, an anti-abortion group, praised the final rules, calling them needed protections from the burdensome Obama-era ACA abortifacient drug mandate.
“President Trump and HHS Secretary Azar delivered a huge victory for conscience rights and religious liberty in America,” Ms. Dannenfelser said in a statement. “No longer will Catholic nuns who care for the elderly poor be forced by the government to provide abortion-inducing drugs in their health care plans. Not only that, moral objectors such as Susan B. Anthony List, will also no longer have to pay for life-ending drugs that are antithetical to their mission and for which we have argued there is certainly no compelling state interest.”
The ACA initially required all employers to cover birth control for employees with no copayments, except for group health plans of religious employers, which were deemed exempt. Those religious employers were primarily churches and other houses of worship. After a number of complaints and legal challenges, the Obama administration created a workaround for nonprofit religious employers to opt out of the mandate.
However, critics argued the process itself was a violation of their religious freedom. The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved. In May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
Under the approved regulations, employers or insurers can stop their coverage of contraceptive services if they have religious beliefs or moral convictions against covering birth control. Exempted entities and individuals also can choose to cover some, but not all, contraceptive services, depending on their specific religious or moral objection, according to an HHS fact sheet.
The agency emphasized that the regulations leave in place government programs that provide free or subsidized contraceptive coverage to low-income women, such as through community health centers, and that the rules do not ban any employer from covering contraceptives.
The regulations become effective 60 days after they are published in the Federal Register.
Innovations in Dermatology: Sarecycline Approved for Acne



Think barrier repair for acne patients
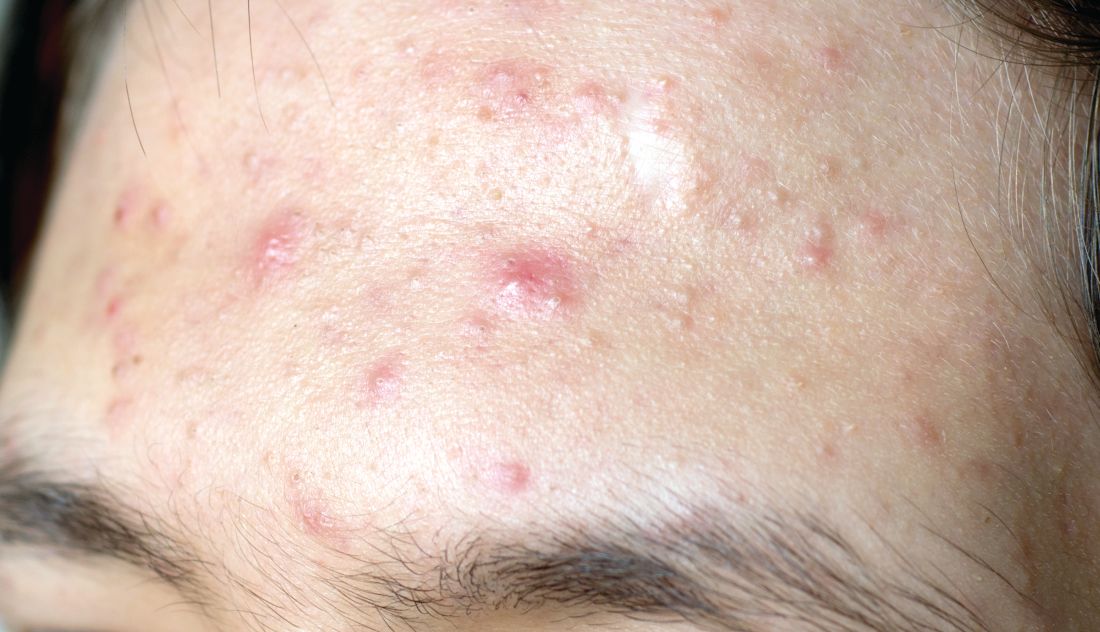
LAS VEGAS – It’s time to start thinking about barrier repair in acne patients, Hilary E. Baldwin, MD, asserts.
“We think about barrier repair with our psoriasis patients, with our dermatitis patients, and our rosacea patients, but we don’t talk about it much with our acne patients,” Dr. Baldwin said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “Now there is increasing evidence that acne pathophysiology may include a barrier defect.”
Both topical and systemic medications exacerbate the problem, because they are so drying, added Dr. Baldwin, medical director of the Acne Treatment & Research Center in Morristown, N.J. Benzoyl peroxide has been shown to increase transepidermal water loss (TEWL) and deplete tocopherol levels in the stratum corneum, while topical retinoids thin the stratum corneum, increase epidermal fragility, and increase TEWL.
The barrier defect may actually decrease medication use – patients are irritated and they stop taking their medication. “,” she said.
She described the results of an Internet-based survey of 200 acne patients aged 15-40 years who had been prescribed clindamycin/benzoyl peroxide 5% within the last 6 months (J Drugs Dermatol. 2011 Jun;10[6]:605-8). The researchers found that side effects lead to suboptimal use of the topical medication, as patients reported spot application, use only in flares, infrequent use, and discontinuation.
Further, 31% of patients complained to their doctors’ offices about dryness, 23% said their doctors did not understand the potential side effects, 21% lost confidence in their doctors, 11% said they were less likely to see their doctors again, and 41% reported using moisturizer to combat dryness and erythema.
“No acne visit is complete without a discussion of skin care,” Dr. Baldwin said. Quality moisturization is a must for acne patients and has been shown to improve TEWL, normalize ceramides, and repair the microbiome.
“I recommend moisturizers with ceramides, hyaluronic acid, and certainly moisturizers that have been shown to be noncomedogenic,” Dr. Baldwin said. “Most acne patients aren’t willing to use a cream moisturizer during the day – they just feel too greasy – so I have them use a cream in the evening when they are home and a lotion in the morning.”
While there has been one very specific study of using a moisturizer before topical medications, there is no good data for this practice, Dr. Baldwin said. “Clearly, though, using a moisturizer reduces irritation, so I prefer to have my patients put on the moisturizer at a different time, but if that doesn’t work, I have them put it on before the medication.”
Dr. Baldwin indicated that she is on the speakers bureau for LaRoche Posay, Galderma, and Valeant, and had received grant and/or contracted research support from Dermira and Valeant.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – It’s time to start thinking about barrier repair in acne patients, Hilary E. Baldwin, MD, asserts.
“We think about barrier repair with our psoriasis patients, with our dermatitis patients, and our rosacea patients, but we don’t talk about it much with our acne patients,” Dr. Baldwin said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “Now there is increasing evidence that acne pathophysiology may include a barrier defect.”
Both topical and systemic medications exacerbate the problem, because they are so drying, added Dr. Baldwin, medical director of the Acne Treatment & Research Center in Morristown, N.J. Benzoyl peroxide has been shown to increase transepidermal water loss (TEWL) and deplete tocopherol levels in the stratum corneum, while topical retinoids thin the stratum corneum, increase epidermal fragility, and increase TEWL.
The barrier defect may actually decrease medication use – patients are irritated and they stop taking their medication. “,” she said.
She described the results of an Internet-based survey of 200 acne patients aged 15-40 years who had been prescribed clindamycin/benzoyl peroxide 5% within the last 6 months (J Drugs Dermatol. 2011 Jun;10[6]:605-8). The researchers found that side effects lead to suboptimal use of the topical medication, as patients reported spot application, use only in flares, infrequent use, and discontinuation.
Further, 31% of patients complained to their doctors’ offices about dryness, 23% said their doctors did not understand the potential side effects, 21% lost confidence in their doctors, 11% said they were less likely to see their doctors again, and 41% reported using moisturizer to combat dryness and erythema.
“No acne visit is complete without a discussion of skin care,” Dr. Baldwin said. Quality moisturization is a must for acne patients and has been shown to improve TEWL, normalize ceramides, and repair the microbiome.
“I recommend moisturizers with ceramides, hyaluronic acid, and certainly moisturizers that have been shown to be noncomedogenic,” Dr. Baldwin said. “Most acne patients aren’t willing to use a cream moisturizer during the day – they just feel too greasy – so I have them use a cream in the evening when they are home and a lotion in the morning.”
While there has been one very specific study of using a moisturizer before topical medications, there is no good data for this practice, Dr. Baldwin said. “Clearly, though, using a moisturizer reduces irritation, so I prefer to have my patients put on the moisturizer at a different time, but if that doesn’t work, I have them put it on before the medication.”
Dr. Baldwin indicated that she is on the speakers bureau for LaRoche Posay, Galderma, and Valeant, and had received grant and/or contracted research support from Dermira and Valeant.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – It’s time to start thinking about barrier repair in acne patients, Hilary E. Baldwin, MD, asserts.
“We think about barrier repair with our psoriasis patients, with our dermatitis patients, and our rosacea patients, but we don’t talk about it much with our acne patients,” Dr. Baldwin said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “Now there is increasing evidence that acne pathophysiology may include a barrier defect.”
Both topical and systemic medications exacerbate the problem, because they are so drying, added Dr. Baldwin, medical director of the Acne Treatment & Research Center in Morristown, N.J. Benzoyl peroxide has been shown to increase transepidermal water loss (TEWL) and deplete tocopherol levels in the stratum corneum, while topical retinoids thin the stratum corneum, increase epidermal fragility, and increase TEWL.
The barrier defect may actually decrease medication use – patients are irritated and they stop taking their medication. “,” she said.
She described the results of an Internet-based survey of 200 acne patients aged 15-40 years who had been prescribed clindamycin/benzoyl peroxide 5% within the last 6 months (J Drugs Dermatol. 2011 Jun;10[6]:605-8). The researchers found that side effects lead to suboptimal use of the topical medication, as patients reported spot application, use only in flares, infrequent use, and discontinuation.
Further, 31% of patients complained to their doctors’ offices about dryness, 23% said their doctors did not understand the potential side effects, 21% lost confidence in their doctors, 11% said they were less likely to see their doctors again, and 41% reported using moisturizer to combat dryness and erythema.
“No acne visit is complete without a discussion of skin care,” Dr. Baldwin said. Quality moisturization is a must for acne patients and has been shown to improve TEWL, normalize ceramides, and repair the microbiome.
“I recommend moisturizers with ceramides, hyaluronic acid, and certainly moisturizers that have been shown to be noncomedogenic,” Dr. Baldwin said. “Most acne patients aren’t willing to use a cream moisturizer during the day – they just feel too greasy – so I have them use a cream in the evening when they are home and a lotion in the morning.”
While there has been one very specific study of using a moisturizer before topical medications, there is no good data for this practice, Dr. Baldwin said. “Clearly, though, using a moisturizer reduces irritation, so I prefer to have my patients put on the moisturizer at a different time, but if that doesn’t work, I have them put it on before the medication.”
Dr. Baldwin indicated that she is on the speakers bureau for LaRoche Posay, Galderma, and Valeant, and had received grant and/or contracted research support from Dermira and Valeant.
SDEF and this news organization are owned by the same parent company.
REPORTING FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Taurine
Taurine, also known as 2-aminoethanesulfonic acid, is a naturally occurring beta-amino acid (which has a sulphonic acid group instead of carboxylic acid, differentiating it from other amino acids) yielded by methionine and cysteine metabolism in the liver.1,2 An important free beta-amino acid in mammals, it is often the free amino acid present in the greatest concentrations in several cell types in humans.1,2 Dietary intake of taurine also plays an important role in maintaining the body’s taurine levels because of mammals’ limited ability to synthesize it.1
Notably in terms of dermatologic treatment options, the combination product taurine bromamine is known to impart antioxidant, anti-inflammatory, and antibacterial activities.3 And taurine itself is associated with antioxidant, anti-inflammatory, antifibrotic, and immunomodulatory characteristics,1,4 and is noted for conferring antiaging benefits.5
Acne and other inflammatory conditions
The use of .6,7
In response to the problem of evolving antibiotic resistance, Marcinkiewicz reported in 2009 on the then-new therapeutic option of topical taurine bromamine for the treatment of inflammatory skin disorders such as acne. The author pointed out that Propionibacterium acnes is particularly sensitive to taurine bromamine, with the substance now known to suppress H2O2 production by activated neutrophils, likely contributing to moderating the severity and lowering the number of inflammatory acne lesions. In a 6-week double-blind pilot clinical study, Marcinkiewicz and his team compared the efficacy of 0.5% taurine bromamine cream with 1% clindamycin gel in 40 patients with mild to moderate acne. Treatments, which were randomly assigned, occurred twice daily through the study. Amelioration of acne symptoms was comparable in the two groups, with more than 90% of patients improving clinically and experiencing similar decreases in acne lesions (65% in the taurine bromamine group and 68% in the clindamycin group). Marcinkiewicz concluded that these results indicate the viability of taurine bromamine as an option for inflammatory acne therapy, particularly for patients who have shown antibiotic resistance.3
Wide-ranging protection potential
In 2003, Janeke et al. conducted analyses that showed that taurine accumulation defended cultured human keratinocytes from osmotically- and UV-induced apoptosis, suggesting the importance of taurine as an epidermal osmolyte necessary for maintaining keratinocyte hydration in a dry environment.2
Three years later, Collin et al. demonstrated the dynamic protective effects of taurine on the human hair follicle in an in vitro study in which taurine promoted hair survival and protected against TGF-beta1-induced damage.1
Taurine has also been found to stabilize and protect the catalytic activity of the hemoprotein cytochrome P450 3A4, which is a key enzyme responsible for metabolizing various endogenous as well as foreign substances, including drugs.8
Penetration enhancement
In 2016, Mueller et al. studied the effects of urea and taurine as hydrophilic penetration enhancers on stratum corneum lipid models as both substances are known to exert such effects. With inconclusive results as to the roots of such activity, they speculated that both entities enhance penetration through the introduction of copious water into the corneocytes, resulting from the robust water-binding capacity of urea and the consequent osmotic pressure related to taurine.9
Possible skin whitening and anti-aging roles and other promising lab results
Based on their previous work demonstrating that azelaic acid, a saturated dicarboxylic acid found naturally in wheat, rye, and barley, suppressed melanogenesis, Yu and Kim investigated the antimelanogenic activity of azelaic acid and taurine in B16F10 mouse melanoma cells in 2010. They found that the combination of the two substances exhibited a greater inhibitory effect in melanocytes than azelaic acid alone, with melanin production and tyrosinase activity suppressed without inducing cytotoxicity. The investigators concluded the combination of azelaic acid and taurine may be an effective approach for treating hyperpigmentation.10
In 2015, Ito et al. investigated the possible anti-aging role of taurine using a taurine transporter knockout mouse model. They noted that aging-related disorders affecting the skin, heart, skeletal muscle, and liver and resulting in a shorter lifespan have been correlated with tissue taurine depletion. The researchers proposed that proper protein folding allows endogenous taurine to perform as an antiaging molecule.5
Also in 2015, Kim et al. investigated potential mechanisms of the antiproliferative activity of taurine on murine B16F10 melanoma cells via the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and neutral red assays and microscopic analysis. They found that taurine prevented cell proliferation and engendered apoptosis in B16F10 cells, concluding that taurine may have a role to play as a chemotherapeutic agent for skin cancer.11
In 2014, Ashkani-Esfahani et al. studied the impact of taurine on cutaneous leishmaniasis wounds in a mouse model. Investigators induced 18 mice with wounds using L. major promastigotes, and divided them into a taurine injection group, taurine gel group, and no treatment group, performing treatments every 24 hours over 21 days. The taurine treatment groups exhibited significantly greater numerical fibroblast density, collagen bundle volume density, and vessel length densities compared with the nontreatment group. The taurine injection group displayed higher fibroblast numerical density than did the taurine gel group. The researchers concluded that taurine has the capacity to enhance wound healing and tissue regeneration but showed no direct anti-leishmaniasis effect.4
Conclusion
Taurine has been found over the last few decades to impart salutary effects for human health. This beta-amino acid that occurs naturally in humans and other mammals also appears to hold promising potential in the dermatologic realm, particularly for its anti-inflammatory and antioxidant effects. More research is needed to ascertain just how pivotal this compound can be for skin health.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Int J Cosmet Sci. 2006 Aug;28(4):289-98.
2. J Invest Dermatol. 2003 Aug;121(2):354-61.
3. Pol Arch Med Wewn. 2009 Oct;119(10):673-6.
4. Adv Biomed Res. 2014 Oct 7;3:204.
5. Adv Exp Med Biol. 2015;803:481-7.
6. Am J Clin Dermatol. 2012 Dec 1;13(6):357-64.
7. Eur J Dermatol. 2008 Jul-Aug;18(4):433-9.
8. Biochemistry (Mosc). 2015 Mar;80(3):366-73.
9. Biochim Biophys Acta. 2016 Sep;1858(9):2006-18.
10. J Biomed Sci. 2010 Aug 24;17 Suppl 1:S45.
11. Adv Exp Med Biol. 2015;803:167-77.
Taurine, also known as 2-aminoethanesulfonic acid, is a naturally occurring beta-amino acid (which has a sulphonic acid group instead of carboxylic acid, differentiating it from other amino acids) yielded by methionine and cysteine metabolism in the liver.1,2 An important free beta-amino acid in mammals, it is often the free amino acid present in the greatest concentrations in several cell types in humans.1,2 Dietary intake of taurine also plays an important role in maintaining the body’s taurine levels because of mammals’ limited ability to synthesize it.1
Notably in terms of dermatologic treatment options, the combination product taurine bromamine is known to impart antioxidant, anti-inflammatory, and antibacterial activities.3 And taurine itself is associated with antioxidant, anti-inflammatory, antifibrotic, and immunomodulatory characteristics,1,4 and is noted for conferring antiaging benefits.5
Acne and other inflammatory conditions
The use of .6,7
In response to the problem of evolving antibiotic resistance, Marcinkiewicz reported in 2009 on the then-new therapeutic option of topical taurine bromamine for the treatment of inflammatory skin disorders such as acne. The author pointed out that Propionibacterium acnes is particularly sensitive to taurine bromamine, with the substance now known to suppress H2O2 production by activated neutrophils, likely contributing to moderating the severity and lowering the number of inflammatory acne lesions. In a 6-week double-blind pilot clinical study, Marcinkiewicz and his team compared the efficacy of 0.5% taurine bromamine cream with 1% clindamycin gel in 40 patients with mild to moderate acne. Treatments, which were randomly assigned, occurred twice daily through the study. Amelioration of acne symptoms was comparable in the two groups, with more than 90% of patients improving clinically and experiencing similar decreases in acne lesions (65% in the taurine bromamine group and 68% in the clindamycin group). Marcinkiewicz concluded that these results indicate the viability of taurine bromamine as an option for inflammatory acne therapy, particularly for patients who have shown antibiotic resistance.3
Wide-ranging protection potential
In 2003, Janeke et al. conducted analyses that showed that taurine accumulation defended cultured human keratinocytes from osmotically- and UV-induced apoptosis, suggesting the importance of taurine as an epidermal osmolyte necessary for maintaining keratinocyte hydration in a dry environment.2
Three years later, Collin et al. demonstrated the dynamic protective effects of taurine on the human hair follicle in an in vitro study in which taurine promoted hair survival and protected against TGF-beta1-induced damage.1
Taurine has also been found to stabilize and protect the catalytic activity of the hemoprotein cytochrome P450 3A4, which is a key enzyme responsible for metabolizing various endogenous as well as foreign substances, including drugs.8
Penetration enhancement
In 2016, Mueller et al. studied the effects of urea and taurine as hydrophilic penetration enhancers on stratum corneum lipid models as both substances are known to exert such effects. With inconclusive results as to the roots of such activity, they speculated that both entities enhance penetration through the introduction of copious water into the corneocytes, resulting from the robust water-binding capacity of urea and the consequent osmotic pressure related to taurine.9
Possible skin whitening and anti-aging roles and other promising lab results
Based on their previous work demonstrating that azelaic acid, a saturated dicarboxylic acid found naturally in wheat, rye, and barley, suppressed melanogenesis, Yu and Kim investigated the antimelanogenic activity of azelaic acid and taurine in B16F10 mouse melanoma cells in 2010. They found that the combination of the two substances exhibited a greater inhibitory effect in melanocytes than azelaic acid alone, with melanin production and tyrosinase activity suppressed without inducing cytotoxicity. The investigators concluded the combination of azelaic acid and taurine may be an effective approach for treating hyperpigmentation.10
In 2015, Ito et al. investigated the possible anti-aging role of taurine using a taurine transporter knockout mouse model. They noted that aging-related disorders affecting the skin, heart, skeletal muscle, and liver and resulting in a shorter lifespan have been correlated with tissue taurine depletion. The researchers proposed that proper protein folding allows endogenous taurine to perform as an antiaging molecule.5
Also in 2015, Kim et al. investigated potential mechanisms of the antiproliferative activity of taurine on murine B16F10 melanoma cells via the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and neutral red assays and microscopic analysis. They found that taurine prevented cell proliferation and engendered apoptosis in B16F10 cells, concluding that taurine may have a role to play as a chemotherapeutic agent for skin cancer.11
In 2014, Ashkani-Esfahani et al. studied the impact of taurine on cutaneous leishmaniasis wounds in a mouse model. Investigators induced 18 mice with wounds using L. major promastigotes, and divided them into a taurine injection group, taurine gel group, and no treatment group, performing treatments every 24 hours over 21 days. The taurine treatment groups exhibited significantly greater numerical fibroblast density, collagen bundle volume density, and vessel length densities compared with the nontreatment group. The taurine injection group displayed higher fibroblast numerical density than did the taurine gel group. The researchers concluded that taurine has the capacity to enhance wound healing and tissue regeneration but showed no direct anti-leishmaniasis effect.4
Conclusion
Taurine has been found over the last few decades to impart salutary effects for human health. This beta-amino acid that occurs naturally in humans and other mammals also appears to hold promising potential in the dermatologic realm, particularly for its anti-inflammatory and antioxidant effects. More research is needed to ascertain just how pivotal this compound can be for skin health.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Int J Cosmet Sci. 2006 Aug;28(4):289-98.
2. J Invest Dermatol. 2003 Aug;121(2):354-61.
3. Pol Arch Med Wewn. 2009 Oct;119(10):673-6.
4. Adv Biomed Res. 2014 Oct 7;3:204.
5. Adv Exp Med Biol. 2015;803:481-7.
6. Am J Clin Dermatol. 2012 Dec 1;13(6):357-64.
7. Eur J Dermatol. 2008 Jul-Aug;18(4):433-9.
8. Biochemistry (Mosc). 2015 Mar;80(3):366-73.
9. Biochim Biophys Acta. 2016 Sep;1858(9):2006-18.
10. J Biomed Sci. 2010 Aug 24;17 Suppl 1:S45.
11. Adv Exp Med Biol. 2015;803:167-77.
Taurine, also known as 2-aminoethanesulfonic acid, is a naturally occurring beta-amino acid (which has a sulphonic acid group instead of carboxylic acid, differentiating it from other amino acids) yielded by methionine and cysteine metabolism in the liver.1,2 An important free beta-amino acid in mammals, it is often the free amino acid present in the greatest concentrations in several cell types in humans.1,2 Dietary intake of taurine also plays an important role in maintaining the body’s taurine levels because of mammals’ limited ability to synthesize it.1
Notably in terms of dermatologic treatment options, the combination product taurine bromamine is known to impart antioxidant, anti-inflammatory, and antibacterial activities.3 And taurine itself is associated with antioxidant, anti-inflammatory, antifibrotic, and immunomodulatory characteristics,1,4 and is noted for conferring antiaging benefits.5
Acne and other inflammatory conditions
The use of .6,7
In response to the problem of evolving antibiotic resistance, Marcinkiewicz reported in 2009 on the then-new therapeutic option of topical taurine bromamine for the treatment of inflammatory skin disorders such as acne. The author pointed out that Propionibacterium acnes is particularly sensitive to taurine bromamine, with the substance now known to suppress H2O2 production by activated neutrophils, likely contributing to moderating the severity and lowering the number of inflammatory acne lesions. In a 6-week double-blind pilot clinical study, Marcinkiewicz and his team compared the efficacy of 0.5% taurine bromamine cream with 1% clindamycin gel in 40 patients with mild to moderate acne. Treatments, which were randomly assigned, occurred twice daily through the study. Amelioration of acne symptoms was comparable in the two groups, with more than 90% of patients improving clinically and experiencing similar decreases in acne lesions (65% in the taurine bromamine group and 68% in the clindamycin group). Marcinkiewicz concluded that these results indicate the viability of taurine bromamine as an option for inflammatory acne therapy, particularly for patients who have shown antibiotic resistance.3
Wide-ranging protection potential
In 2003, Janeke et al. conducted analyses that showed that taurine accumulation defended cultured human keratinocytes from osmotically- and UV-induced apoptosis, suggesting the importance of taurine as an epidermal osmolyte necessary for maintaining keratinocyte hydration in a dry environment.2
Three years later, Collin et al. demonstrated the dynamic protective effects of taurine on the human hair follicle in an in vitro study in which taurine promoted hair survival and protected against TGF-beta1-induced damage.1
Taurine has also been found to stabilize and protect the catalytic activity of the hemoprotein cytochrome P450 3A4, which is a key enzyme responsible for metabolizing various endogenous as well as foreign substances, including drugs.8
Penetration enhancement
In 2016, Mueller et al. studied the effects of urea and taurine as hydrophilic penetration enhancers on stratum corneum lipid models as both substances are known to exert such effects. With inconclusive results as to the roots of such activity, they speculated that both entities enhance penetration through the introduction of copious water into the corneocytes, resulting from the robust water-binding capacity of urea and the consequent osmotic pressure related to taurine.9
Possible skin whitening and anti-aging roles and other promising lab results
Based on their previous work demonstrating that azelaic acid, a saturated dicarboxylic acid found naturally in wheat, rye, and barley, suppressed melanogenesis, Yu and Kim investigated the antimelanogenic activity of azelaic acid and taurine in B16F10 mouse melanoma cells in 2010. They found that the combination of the two substances exhibited a greater inhibitory effect in melanocytes than azelaic acid alone, with melanin production and tyrosinase activity suppressed without inducing cytotoxicity. The investigators concluded the combination of azelaic acid and taurine may be an effective approach for treating hyperpigmentation.10
In 2015, Ito et al. investigated the possible anti-aging role of taurine using a taurine transporter knockout mouse model. They noted that aging-related disorders affecting the skin, heart, skeletal muscle, and liver and resulting in a shorter lifespan have been correlated with tissue taurine depletion. The researchers proposed that proper protein folding allows endogenous taurine to perform as an antiaging molecule.5
Also in 2015, Kim et al. investigated potential mechanisms of the antiproliferative activity of taurine on murine B16F10 melanoma cells via the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and neutral red assays and microscopic analysis. They found that taurine prevented cell proliferation and engendered apoptosis in B16F10 cells, concluding that taurine may have a role to play as a chemotherapeutic agent for skin cancer.11
In 2014, Ashkani-Esfahani et al. studied the impact of taurine on cutaneous leishmaniasis wounds in a mouse model. Investigators induced 18 mice with wounds using L. major promastigotes, and divided them into a taurine injection group, taurine gel group, and no treatment group, performing treatments every 24 hours over 21 days. The taurine treatment groups exhibited significantly greater numerical fibroblast density, collagen bundle volume density, and vessel length densities compared with the nontreatment group. The taurine injection group displayed higher fibroblast numerical density than did the taurine gel group. The researchers concluded that taurine has the capacity to enhance wound healing and tissue regeneration but showed no direct anti-leishmaniasis effect.4
Conclusion
Taurine has been found over the last few decades to impart salutary effects for human health. This beta-amino acid that occurs naturally in humans and other mammals also appears to hold promising potential in the dermatologic realm, particularly for its anti-inflammatory and antioxidant effects. More research is needed to ascertain just how pivotal this compound can be for skin health.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Int J Cosmet Sci. 2006 Aug;28(4):289-98.
2. J Invest Dermatol. 2003 Aug;121(2):354-61.
3. Pol Arch Med Wewn. 2009 Oct;119(10):673-6.
4. Adv Biomed Res. 2014 Oct 7;3:204.
5. Adv Exp Med Biol. 2015;803:481-7.
6. Am J Clin Dermatol. 2012 Dec 1;13(6):357-64.
7. Eur J Dermatol. 2008 Jul-Aug;18(4):433-9.
8. Biochemistry (Mosc). 2015 Mar;80(3):366-73.
9. Biochim Biophys Acta. 2016 Sep;1858(9):2006-18.
10. J Biomed Sci. 2010 Aug 24;17 Suppl 1:S45.
11. Adv Exp Med Biol. 2015;803:167-77.
Topical treatment with retinoid/benzoyl peroxide combination reduced acne scars
PARIS – Treatment with the fixed combination in a multicenter, randomized trial, Brigitte Dreno, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“To my knowledge, this is the first time that we have seen a topical therapy showing a reduction in atrophic acne scars,” said Dr. Dreno, professor and chair of the department of dermatology at Nantes (France) University Hospital.
She reported on 67 adolescents and adults with mainly moderate facial acne randomized to treat half their face with adapalene 0.3%/benzoyl peroxide 2.5% gel (Epiduo Forte) and the other half with the product’s vehicle daily for 6 months. Investigators were blinded as to which side was which. At baseline, patients averaged 40 acne lesions and 12 scars per half face.
The primary efficacy endpoint was the atrophic acne scar count per half face at week 24. At that point, the mean total was 9.5 scars on the active treatment side, compared with 13.3 on the control side. This translated to a statistically significant and clinically meaningful 15.5% decrease in scars with active treatment versus a 14.4% increase with vehicle. The between-side difference achieved statistical significance at week 1 and remained so at all follow-up visits through week 24.
By Scar Global Assessment at week 24, 32.9% of half faces treated with the combination product were rated clear or almost clear, compared with 16.4% with vehicle.
At 24 weeks, 24.1% of participants reported having moderately or very visible holes or indents on the active treatment side of their face, compared with 51.8% on the control side. The number of inflammatory acne lesions fell by 86.7% with the active treatment and 57.9% with vehicle over the course of 24 weeks. Again, the difference became statistically significant starting at week 1. By the Investigator’s Global Assessment at week 24, 64.2% of adapalene/benzoyl peroxide gel–treated faces were rated clear or almost clear, as were 19.4% with vehicle. In addition, 32% of patients reported a marked improvement in skin texture on their active treatment side at 24 weeks, as did 14% on the control side.
The salutary effect on acne scars documented with a topical therapy in this study represents a real advance in clinical care.
“Facial acne scars are a very important and difficult problem for our patients and also for dermatologists,” Dr. Dreno observed, adding that the evidence base for procedural interventions for acne scars, such as dermabrasion and laser resurfacing, is not top quality.
Not surprisingly with a topical retinoid, skin irritation was the most common treatment-emergent adverse event, reported by 14.9% of patients on their active treatment side and 6% with vehicle. This side effect was typically mild and resolved within the first 2-3 weeks.
The improvement in preexisting acne scars documented in this trial was probably caused by drug-induced remodeling of the dermal matrix, according to Dr. Dreno.
The study was funded by Galderma. Dr. Dreno reported receiving research grants from and/or serving as a consultant to Galderma, Bioderma, Pierre Fabre, and La Roche–Posay.
PARIS – Treatment with the fixed combination in a multicenter, randomized trial, Brigitte Dreno, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“To my knowledge, this is the first time that we have seen a topical therapy showing a reduction in atrophic acne scars,” said Dr. Dreno, professor and chair of the department of dermatology at Nantes (France) University Hospital.
She reported on 67 adolescents and adults with mainly moderate facial acne randomized to treat half their face with adapalene 0.3%/benzoyl peroxide 2.5% gel (Epiduo Forte) and the other half with the product’s vehicle daily for 6 months. Investigators were blinded as to which side was which. At baseline, patients averaged 40 acne lesions and 12 scars per half face.
The primary efficacy endpoint was the atrophic acne scar count per half face at week 24. At that point, the mean total was 9.5 scars on the active treatment side, compared with 13.3 on the control side. This translated to a statistically significant and clinically meaningful 15.5% decrease in scars with active treatment versus a 14.4% increase with vehicle. The between-side difference achieved statistical significance at week 1 and remained so at all follow-up visits through week 24.
By Scar Global Assessment at week 24, 32.9% of half faces treated with the combination product were rated clear or almost clear, compared with 16.4% with vehicle.
At 24 weeks, 24.1% of participants reported having moderately or very visible holes or indents on the active treatment side of their face, compared with 51.8% on the control side. The number of inflammatory acne lesions fell by 86.7% with the active treatment and 57.9% with vehicle over the course of 24 weeks. Again, the difference became statistically significant starting at week 1. By the Investigator’s Global Assessment at week 24, 64.2% of adapalene/benzoyl peroxide gel–treated faces were rated clear or almost clear, as were 19.4% with vehicle. In addition, 32% of patients reported a marked improvement in skin texture on their active treatment side at 24 weeks, as did 14% on the control side.
The salutary effect on acne scars documented with a topical therapy in this study represents a real advance in clinical care.
“Facial acne scars are a very important and difficult problem for our patients and also for dermatologists,” Dr. Dreno observed, adding that the evidence base for procedural interventions for acne scars, such as dermabrasion and laser resurfacing, is not top quality.
Not surprisingly with a topical retinoid, skin irritation was the most common treatment-emergent adverse event, reported by 14.9% of patients on their active treatment side and 6% with vehicle. This side effect was typically mild and resolved within the first 2-3 weeks.
The improvement in preexisting acne scars documented in this trial was probably caused by drug-induced remodeling of the dermal matrix, according to Dr. Dreno.
The study was funded by Galderma. Dr. Dreno reported receiving research grants from and/or serving as a consultant to Galderma, Bioderma, Pierre Fabre, and La Roche–Posay.
PARIS – Treatment with the fixed combination in a multicenter, randomized trial, Brigitte Dreno, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“To my knowledge, this is the first time that we have seen a topical therapy showing a reduction in atrophic acne scars,” said Dr. Dreno, professor and chair of the department of dermatology at Nantes (France) University Hospital.
She reported on 67 adolescents and adults with mainly moderate facial acne randomized to treat half their face with adapalene 0.3%/benzoyl peroxide 2.5% gel (Epiduo Forte) and the other half with the product’s vehicle daily for 6 months. Investigators were blinded as to which side was which. At baseline, patients averaged 40 acne lesions and 12 scars per half face.
The primary efficacy endpoint was the atrophic acne scar count per half face at week 24. At that point, the mean total was 9.5 scars on the active treatment side, compared with 13.3 on the control side. This translated to a statistically significant and clinically meaningful 15.5% decrease in scars with active treatment versus a 14.4% increase with vehicle. The between-side difference achieved statistical significance at week 1 and remained so at all follow-up visits through week 24.
By Scar Global Assessment at week 24, 32.9% of half faces treated with the combination product were rated clear or almost clear, compared with 16.4% with vehicle.
At 24 weeks, 24.1% of participants reported having moderately or very visible holes or indents on the active treatment side of their face, compared with 51.8% on the control side. The number of inflammatory acne lesions fell by 86.7% with the active treatment and 57.9% with vehicle over the course of 24 weeks. Again, the difference became statistically significant starting at week 1. By the Investigator’s Global Assessment at week 24, 64.2% of adapalene/benzoyl peroxide gel–treated faces were rated clear or almost clear, as were 19.4% with vehicle. In addition, 32% of patients reported a marked improvement in skin texture on their active treatment side at 24 weeks, as did 14% on the control side.
The salutary effect on acne scars documented with a topical therapy in this study represents a real advance in clinical care.
“Facial acne scars are a very important and difficult problem for our patients and also for dermatologists,” Dr. Dreno observed, adding that the evidence base for procedural interventions for acne scars, such as dermabrasion and laser resurfacing, is not top quality.
Not surprisingly with a topical retinoid, skin irritation was the most common treatment-emergent adverse event, reported by 14.9% of patients on their active treatment side and 6% with vehicle. This side effect was typically mild and resolved within the first 2-3 weeks.
The improvement in preexisting acne scars documented in this trial was probably caused by drug-induced remodeling of the dermal matrix, according to Dr. Dreno.
The study was funded by Galderma. Dr. Dreno reported receiving research grants from and/or serving as a consultant to Galderma, Bioderma, Pierre Fabre, and La Roche–Posay.
REPORTING FROM THE EADV CONGRESS
Key clinical point: A fixed combination adapalene 0.3%/benzoyl peroxide 2.5% gel reduced the number of preexisting facial atrophic acne scars and prevented new scar formation.
Major finding: Facial atrophic acne scar count dropped by 15.5% with 6 months of treatment with adapalene 0.3%/benzoyl peroxide 2.5% gel while increasing by 14.4% with vehicle.
Study details: This was a 6-month, prospective, multicenter, randomized, vehicle-controlled, split-face study involving 67 acne patients.
Disclosures: The study was funded by Galderma. The presenter reported receiving research grants from and/or serving as a consultant to Galderma, Bioderma, Pierre Fabre, and La Roche–Posay.
Innovations in Dermatology: Antibiotic Stewardship in Acne Therapy



Q and A with Dr. Julie Harper: Treating acne and rosacea
MONTEREY, CALIF. – Julie C. Harper, MD, likes to warn her patients with acne about an unexpected possible side effect of treatment with isotretinoin. “You may become a dermatologist.”
After all, that’s exactly how Dr. Harper herself was inspired to pursue a career in dermatology. As a teenager, she had acne and was treated with isotretinoin three times. The experience was so influential that she went into dermatology with a specific goal of treating acne.
“I love all of it, and in my practice I treat everything,” said Dr. Harper, “but I have a special interest in helping people with acne be as clear as they can be.” Indeed, she helped found the American Acne and Rosacea Society, which she now serves as president.
Dr. Harper, who practices in Birmingham, Ala., spoke about her approach to acne and rosacea in an interview following one of her presentations at the annual Coastal Dermatology Symposium.
DERMATOLOGY NEWS: What drew you to focus on rosacea in addition to acne?
Dr. Harper: But they’re very distinct diagnoses, and their pathogenesis is completely different. My interest in treating rosacea was secondary to acne, but I love to treat them both.
DN: Are they both equally challenging to treat?
Dr. Harper: In some ways, rosacea is more challenging to treat.
With acne, we have a pretty good algorithm for how we treat it. We can end with isotretinoin, which for many people is a cure. But we really don’t have that last step in rosacea.
DN: What are you doing differently with rosacea than you might not have done a few years ago?
Dr. Harper: More combination therapy. The trend is more toward a comprehensive combination approach to treat everything we see in rosacea: Hit this as hard as you can. Hit everything you see. Part of that is because we have some newer drugs like the alpha-adrenergic agonists that work differently than anything we’ve had before.
We have a couple of good combination studies. One study examined ivermectin plus brimonidine (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16). Those two worked better together if you did not delay the brimonidine for 4 weeks and only used it with the ivermectin for part of the study.
There are also the newer studies that look at doxycycline plus ivermectin and compare it with ivermectin plus placebo. The combination works better, and it works faster (Adv Ther. 2016;33[9]:1481-1501; unpublished clinical trial data on file with Galderma, NCT03075891).
On top of those treatments, we may need to add laser for background redness, or an oral beta-blocker for flushing if the patient still complains of the symptoms.
DN: What’s most challenging to treat in rosacea?
Dr. Harper: The redness and phymatous changes are the hardest. Once you get phymatous changes, you have to do a physical modality.
Most of us think that if we treat rosacea aggressively up front, maybe we can prevent the phymatous changes. Prevention is key, just like prevention of acne scarring is easier than getting rid of scars once you have it.
Other than phyma, it’s the redness. Even the Food and Drug Administration–approved products we have for redness don’t work for flushing. Patients stand up to give up a presentation and “Oh no, here comes a red face.” That’s the hardest part to manage.
DN: How do beta-blockers fare at treating flushing?
Dr. Harper: They can help, but I don’t know that they can knock it out completely.
And we should remember that there are no FDA-approved beta-blockers to treat this. Most of the data we have are small case reports or case series. We don’t have a lot of data.
DN: Is there anything that’s used too much in rosacea?
Dr. Harper: Probably metronidazole. I understand why it’s used. It’s not a bad drug. But we have better drugs now.
I think we use metronidazole whenever things aren’t covered by insurance. And we use it to do too many things. Don’t try to make metronidazole do everything.
Metronidazole is FDA-approved for papules and pustules. It wasn’t ever intended to help with flushing and background erythema, and you’ll need to use something else with it.
DN: What’s coming down the line for rosacea?
Dr. Harper: We’ve got a couple new antibiotics: a new topical antibiotic and another oral antibiotic.
DN: Let’s talk about acne. Do you think isotretinoin is underused?
Dr. Harper: We should be using more of it. Why do we hold this drug hostage from our patients? In many people, it will cure their acne if they take it for just 5-6 months.
Are we worried about inflammatory bowel disease? The most recent studies say that’s not really an association. Are we worried about depression? We’ve had a meta-analysis that suggests if you take all that data, depression – if anything – gets better in people who take isotretinoin (Am J Gastroenterol. 2014 Apr;109[4]:563-9; J Am Acad Dermatol. 2017 Jun;76[6]:1068-76.e9).
We need to take [the risk with pregnancy] seriously. But we need to be putting more people on the drug and giving them the opportunity to be clear.
DN: What should be used less in acne?
Dr. Harper: We should use less antibiotics and more of everything else – more hormonal treatments, more isotretinoin, more topical retinoids.
That doesn’t mean no antibiotics. But instead of doing three repetitive courses of antibiotics, do one. If acne recurs, go to isotretinoin. Go to an alternative.
DN: What about spironolactone in acne?
Dr. Harper: It’s a blood pressure medicine, but it’s got an antiandrogenic qualities. It blocks the androgen receptor so it’s like getting the benefits of the birth control pill without the estrogen. It can be very beneficial for acne in women.
Its use increased from 2004 to 2013, and people are getting the hang of it. But when you compare it with the number of antibiotics prescribed, antibiotics are written a whole lot more (J Am Acad Dermatol. 2017 Sep;77[3],456-63.e4).
DN: Is there anything that is especially helpful in treating men?
Dr. Harper: Part of the way that birth control and spironolactone work is by decreasing sebum, and we don’t have anything like that for men. But potentially, there may be a topical antiandrogen product that decreases sebum.
DN: How do you deal with patients who are in a lot of distress because of acne or rosacea?
Dr. Harper: You listen to them and tell them you hear what they’re saying. “I understand that you want to be clear, and I’ll help you do that.”
Listen to why they’re not doing well and why they’re frustrated with what they’ve used. They might say, “I don’t use what you gave me because I don’t like the way it feels.” Or, “the drug that you prescribed is too expensive.”
If they’re really doing everything you said, and they’re not doing well, in both of those conditions it may be time for isotretinoin.
In acne, I’ve never seen it fail. It doesn’t work as predictably in rosacea, but it does pretty well if you do low-dose, intermittent isotretinoin.
DN: Do you ever try treatments that are unexpected for acne and rosacea?
Dr. Harper: If you use the right combination of what we have, and try to target pathogenesis, I don’t think we have to go off the reservation very often. We can get good results with what we have available.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications. Dr. Harper disclosed speaker/advisor relationships with Allergan, Bayer, BioPharmX, Galderma, La Roche–Posay, and Ortho Pharmaceutical, and has served as an investigator for Bayer.
MONTEREY, CALIF. – Julie C. Harper, MD, likes to warn her patients with acne about an unexpected possible side effect of treatment with isotretinoin. “You may become a dermatologist.”
After all, that’s exactly how Dr. Harper herself was inspired to pursue a career in dermatology. As a teenager, she had acne and was treated with isotretinoin three times. The experience was so influential that she went into dermatology with a specific goal of treating acne.
“I love all of it, and in my practice I treat everything,” said Dr. Harper, “but I have a special interest in helping people with acne be as clear as they can be.” Indeed, she helped found the American Acne and Rosacea Society, which she now serves as president.
Dr. Harper, who practices in Birmingham, Ala., spoke about her approach to acne and rosacea in an interview following one of her presentations at the annual Coastal Dermatology Symposium.
DERMATOLOGY NEWS: What drew you to focus on rosacea in addition to acne?
Dr. Harper: But they’re very distinct diagnoses, and their pathogenesis is completely different. My interest in treating rosacea was secondary to acne, but I love to treat them both.
DN: Are they both equally challenging to treat?
Dr. Harper: In some ways, rosacea is more challenging to treat.
With acne, we have a pretty good algorithm for how we treat it. We can end with isotretinoin, which for many people is a cure. But we really don’t have that last step in rosacea.
DN: What are you doing differently with rosacea than you might not have done a few years ago?
Dr. Harper: More combination therapy. The trend is more toward a comprehensive combination approach to treat everything we see in rosacea: Hit this as hard as you can. Hit everything you see. Part of that is because we have some newer drugs like the alpha-adrenergic agonists that work differently than anything we’ve had before.
We have a couple of good combination studies. One study examined ivermectin plus brimonidine (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16). Those two worked better together if you did not delay the brimonidine for 4 weeks and only used it with the ivermectin for part of the study.
There are also the newer studies that look at doxycycline plus ivermectin and compare it with ivermectin plus placebo. The combination works better, and it works faster (Adv Ther. 2016;33[9]:1481-1501; unpublished clinical trial data on file with Galderma, NCT03075891).
On top of those treatments, we may need to add laser for background redness, or an oral beta-blocker for flushing if the patient still complains of the symptoms.
DN: What’s most challenging to treat in rosacea?
Dr. Harper: The redness and phymatous changes are the hardest. Once you get phymatous changes, you have to do a physical modality.
Most of us think that if we treat rosacea aggressively up front, maybe we can prevent the phymatous changes. Prevention is key, just like prevention of acne scarring is easier than getting rid of scars once you have it.
Other than phyma, it’s the redness. Even the Food and Drug Administration–approved products we have for redness don’t work for flushing. Patients stand up to give up a presentation and “Oh no, here comes a red face.” That’s the hardest part to manage.
DN: How do beta-blockers fare at treating flushing?
Dr. Harper: They can help, but I don’t know that they can knock it out completely.
And we should remember that there are no FDA-approved beta-blockers to treat this. Most of the data we have are small case reports or case series. We don’t have a lot of data.
DN: Is there anything that’s used too much in rosacea?
Dr. Harper: Probably metronidazole. I understand why it’s used. It’s not a bad drug. But we have better drugs now.
I think we use metronidazole whenever things aren’t covered by insurance. And we use it to do too many things. Don’t try to make metronidazole do everything.
Metronidazole is FDA-approved for papules and pustules. It wasn’t ever intended to help with flushing and background erythema, and you’ll need to use something else with it.
DN: What’s coming down the line for rosacea?
Dr. Harper: We’ve got a couple new antibiotics: a new topical antibiotic and another oral antibiotic.
DN: Let’s talk about acne. Do you think isotretinoin is underused?
Dr. Harper: We should be using more of it. Why do we hold this drug hostage from our patients? In many people, it will cure their acne if they take it for just 5-6 months.
Are we worried about inflammatory bowel disease? The most recent studies say that’s not really an association. Are we worried about depression? We’ve had a meta-analysis that suggests if you take all that data, depression – if anything – gets better in people who take isotretinoin (Am J Gastroenterol. 2014 Apr;109[4]:563-9; J Am Acad Dermatol. 2017 Jun;76[6]:1068-76.e9).
We need to take [the risk with pregnancy] seriously. But we need to be putting more people on the drug and giving them the opportunity to be clear.
DN: What should be used less in acne?
Dr. Harper: We should use less antibiotics and more of everything else – more hormonal treatments, more isotretinoin, more topical retinoids.
That doesn’t mean no antibiotics. But instead of doing three repetitive courses of antibiotics, do one. If acne recurs, go to isotretinoin. Go to an alternative.
DN: What about spironolactone in acne?
Dr. Harper: It’s a blood pressure medicine, but it’s got an antiandrogenic qualities. It blocks the androgen receptor so it’s like getting the benefits of the birth control pill without the estrogen. It can be very beneficial for acne in women.
Its use increased from 2004 to 2013, and people are getting the hang of it. But when you compare it with the number of antibiotics prescribed, antibiotics are written a whole lot more (J Am Acad Dermatol. 2017 Sep;77[3],456-63.e4).
DN: Is there anything that is especially helpful in treating men?
Dr. Harper: Part of the way that birth control and spironolactone work is by decreasing sebum, and we don’t have anything like that for men. But potentially, there may be a topical antiandrogen product that decreases sebum.
DN: How do you deal with patients who are in a lot of distress because of acne or rosacea?
Dr. Harper: You listen to them and tell them you hear what they’re saying. “I understand that you want to be clear, and I’ll help you do that.”
Listen to why they’re not doing well and why they’re frustrated with what they’ve used. They might say, “I don’t use what you gave me because I don’t like the way it feels.” Or, “the drug that you prescribed is too expensive.”
If they’re really doing everything you said, and they’re not doing well, in both of those conditions it may be time for isotretinoin.
In acne, I’ve never seen it fail. It doesn’t work as predictably in rosacea, but it does pretty well if you do low-dose, intermittent isotretinoin.
DN: Do you ever try treatments that are unexpected for acne and rosacea?
Dr. Harper: If you use the right combination of what we have, and try to target pathogenesis, I don’t think we have to go off the reservation very often. We can get good results with what we have available.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications. Dr. Harper disclosed speaker/advisor relationships with Allergan, Bayer, BioPharmX, Galderma, La Roche–Posay, and Ortho Pharmaceutical, and has served as an investigator for Bayer.
MONTEREY, CALIF. – Julie C. Harper, MD, likes to warn her patients with acne about an unexpected possible side effect of treatment with isotretinoin. “You may become a dermatologist.”
After all, that’s exactly how Dr. Harper herself was inspired to pursue a career in dermatology. As a teenager, she had acne and was treated with isotretinoin three times. The experience was so influential that she went into dermatology with a specific goal of treating acne.
“I love all of it, and in my practice I treat everything,” said Dr. Harper, “but I have a special interest in helping people with acne be as clear as they can be.” Indeed, she helped found the American Acne and Rosacea Society, which she now serves as president.
Dr. Harper, who practices in Birmingham, Ala., spoke about her approach to acne and rosacea in an interview following one of her presentations at the annual Coastal Dermatology Symposium.
DERMATOLOGY NEWS: What drew you to focus on rosacea in addition to acne?
Dr. Harper: But they’re very distinct diagnoses, and their pathogenesis is completely different. My interest in treating rosacea was secondary to acne, but I love to treat them both.
DN: Are they both equally challenging to treat?
Dr. Harper: In some ways, rosacea is more challenging to treat.
With acne, we have a pretty good algorithm for how we treat it. We can end with isotretinoin, which for many people is a cure. But we really don’t have that last step in rosacea.
DN: What are you doing differently with rosacea than you might not have done a few years ago?
Dr. Harper: More combination therapy. The trend is more toward a comprehensive combination approach to treat everything we see in rosacea: Hit this as hard as you can. Hit everything you see. Part of that is because we have some newer drugs like the alpha-adrenergic agonists that work differently than anything we’ve had before.
We have a couple of good combination studies. One study examined ivermectin plus brimonidine (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16). Those two worked better together if you did not delay the brimonidine for 4 weeks and only used it with the ivermectin for part of the study.
There are also the newer studies that look at doxycycline plus ivermectin and compare it with ivermectin plus placebo. The combination works better, and it works faster (Adv Ther. 2016;33[9]:1481-1501; unpublished clinical trial data on file with Galderma, NCT03075891).
On top of those treatments, we may need to add laser for background redness, or an oral beta-blocker for flushing if the patient still complains of the symptoms.
DN: What’s most challenging to treat in rosacea?
Dr. Harper: The redness and phymatous changes are the hardest. Once you get phymatous changes, you have to do a physical modality.
Most of us think that if we treat rosacea aggressively up front, maybe we can prevent the phymatous changes. Prevention is key, just like prevention of acne scarring is easier than getting rid of scars once you have it.
Other than phyma, it’s the redness. Even the Food and Drug Administration–approved products we have for redness don’t work for flushing. Patients stand up to give up a presentation and “Oh no, here comes a red face.” That’s the hardest part to manage.
DN: How do beta-blockers fare at treating flushing?
Dr. Harper: They can help, but I don’t know that they can knock it out completely.
And we should remember that there are no FDA-approved beta-blockers to treat this. Most of the data we have are small case reports or case series. We don’t have a lot of data.
DN: Is there anything that’s used too much in rosacea?
Dr. Harper: Probably metronidazole. I understand why it’s used. It’s not a bad drug. But we have better drugs now.
I think we use metronidazole whenever things aren’t covered by insurance. And we use it to do too many things. Don’t try to make metronidazole do everything.
Metronidazole is FDA-approved for papules and pustules. It wasn’t ever intended to help with flushing and background erythema, and you’ll need to use something else with it.
DN: What’s coming down the line for rosacea?
Dr. Harper: We’ve got a couple new antibiotics: a new topical antibiotic and another oral antibiotic.
DN: Let’s talk about acne. Do you think isotretinoin is underused?
Dr. Harper: We should be using more of it. Why do we hold this drug hostage from our patients? In many people, it will cure their acne if they take it for just 5-6 months.
Are we worried about inflammatory bowel disease? The most recent studies say that’s not really an association. Are we worried about depression? We’ve had a meta-analysis that suggests if you take all that data, depression – if anything – gets better in people who take isotretinoin (Am J Gastroenterol. 2014 Apr;109[4]:563-9; J Am Acad Dermatol. 2017 Jun;76[6]:1068-76.e9).
We need to take [the risk with pregnancy] seriously. But we need to be putting more people on the drug and giving them the opportunity to be clear.
DN: What should be used less in acne?
Dr. Harper: We should use less antibiotics and more of everything else – more hormonal treatments, more isotretinoin, more topical retinoids.
That doesn’t mean no antibiotics. But instead of doing three repetitive courses of antibiotics, do one. If acne recurs, go to isotretinoin. Go to an alternative.
DN: What about spironolactone in acne?
Dr. Harper: It’s a blood pressure medicine, but it’s got an antiandrogenic qualities. It blocks the androgen receptor so it’s like getting the benefits of the birth control pill without the estrogen. It can be very beneficial for acne in women.
Its use increased from 2004 to 2013, and people are getting the hang of it. But when you compare it with the number of antibiotics prescribed, antibiotics are written a whole lot more (J Am Acad Dermatol. 2017 Sep;77[3],456-63.e4).
DN: Is there anything that is especially helpful in treating men?
Dr. Harper: Part of the way that birth control and spironolactone work is by decreasing sebum, and we don’t have anything like that for men. But potentially, there may be a topical antiandrogen product that decreases sebum.
DN: How do you deal with patients who are in a lot of distress because of acne or rosacea?
Dr. Harper: You listen to them and tell them you hear what they’re saying. “I understand that you want to be clear, and I’ll help you do that.”
Listen to why they’re not doing well and why they’re frustrated with what they’ve used. They might say, “I don’t use what you gave me because I don’t like the way it feels.” Or, “the drug that you prescribed is too expensive.”
If they’re really doing everything you said, and they’re not doing well, in both of those conditions it may be time for isotretinoin.
In acne, I’ve never seen it fail. It doesn’t work as predictably in rosacea, but it does pretty well if you do low-dose, intermittent isotretinoin.
DN: Do you ever try treatments that are unexpected for acne and rosacea?
Dr. Harper: If you use the right combination of what we have, and try to target pathogenesis, I don’t think we have to go off the reservation very often. We can get good results with what we have available.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications. Dr. Harper disclosed speaker/advisor relationships with Allergan, Bayer, BioPharmX, Galderma, La Roche–Posay, and Ortho Pharmaceutical, and has served as an investigator for Bayer.
EXPERT ANALYSIS FROM COASTAL DERMATOLOGY SYMPOSIUM
Acne more common in adults with hidradenitis suppurativa
, according to Sara Wertenteil and her colleagues in the department of dermatology at Hofstra University in Hempstead, N.Y.
Using data collected by IBM Watson Health, the study authors examined a total of 48,050 adults with HS and 16.9 million adults in the general U.S. population. In this study population, 15.2% of adults with HS had acne, compared with only 2.9% of adults in the general population (P less than .0001), the investigators wrote in the Journal of the American Academy of Dermatology.
After adjusting for age, sex, obesity, smoking status, and polycystic ovarian syndrome (PCOS) status, the odds ratio of adults with HS having acne was 4.51 over those without HS (95% confidence interval, 4.40-4.63). In all subgroups measured (male; female; adults aged 18-44 years, 45-64 years, and 65 years and older; white; nonwhite; obese; nonobese; smoker; nonsmoker; positive for PCOS; non-PCOS) adults with HS were significantly more likely to have acne. The strongest association was in patients who were aged 65 years and older (odds ratio, 10.14; 95% CI, 8.97-11.46).
“Patients with HS have an increased prevalence of [acne vulgaris]. Clinicians treating HS patients should be aware of this burden and its potential implications including a further impact on quality of life. Management strategies should include consideration of both conditions, either with treatments that have overlapping efficacy, or with concomitant therapies,” the authors concluded.
The study was sponsored in part by AbbVie. One coauthor reported having served as an advisor for AbbVie, Pfizer, Janssen, and Asana Biosciences.
SOURCE: Wertentiel S et al. J Am Acad Dermatol. 2018 Oct 1. doi: 10.1016/j.jaad.2018.09.040.
, according to Sara Wertenteil and her colleagues in the department of dermatology at Hofstra University in Hempstead, N.Y.
Using data collected by IBM Watson Health, the study authors examined a total of 48,050 adults with HS and 16.9 million adults in the general U.S. population. In this study population, 15.2% of adults with HS had acne, compared with only 2.9% of adults in the general population (P less than .0001), the investigators wrote in the Journal of the American Academy of Dermatology.
After adjusting for age, sex, obesity, smoking status, and polycystic ovarian syndrome (PCOS) status, the odds ratio of adults with HS having acne was 4.51 over those without HS (95% confidence interval, 4.40-4.63). In all subgroups measured (male; female; adults aged 18-44 years, 45-64 years, and 65 years and older; white; nonwhite; obese; nonobese; smoker; nonsmoker; positive for PCOS; non-PCOS) adults with HS were significantly more likely to have acne. The strongest association was in patients who were aged 65 years and older (odds ratio, 10.14; 95% CI, 8.97-11.46).
“Patients with HS have an increased prevalence of [acne vulgaris]. Clinicians treating HS patients should be aware of this burden and its potential implications including a further impact on quality of life. Management strategies should include consideration of both conditions, either with treatments that have overlapping efficacy, or with concomitant therapies,” the authors concluded.
The study was sponsored in part by AbbVie. One coauthor reported having served as an advisor for AbbVie, Pfizer, Janssen, and Asana Biosciences.
SOURCE: Wertentiel S et al. J Am Acad Dermatol. 2018 Oct 1. doi: 10.1016/j.jaad.2018.09.040.
, according to Sara Wertenteil and her colleagues in the department of dermatology at Hofstra University in Hempstead, N.Y.
Using data collected by IBM Watson Health, the study authors examined a total of 48,050 adults with HS and 16.9 million adults in the general U.S. population. In this study population, 15.2% of adults with HS had acne, compared with only 2.9% of adults in the general population (P less than .0001), the investigators wrote in the Journal of the American Academy of Dermatology.
After adjusting for age, sex, obesity, smoking status, and polycystic ovarian syndrome (PCOS) status, the odds ratio of adults with HS having acne was 4.51 over those without HS (95% confidence interval, 4.40-4.63). In all subgroups measured (male; female; adults aged 18-44 years, 45-64 years, and 65 years and older; white; nonwhite; obese; nonobese; smoker; nonsmoker; positive for PCOS; non-PCOS) adults with HS were significantly more likely to have acne. The strongest association was in patients who were aged 65 years and older (odds ratio, 10.14; 95% CI, 8.97-11.46).
“Patients with HS have an increased prevalence of [acne vulgaris]. Clinicians treating HS patients should be aware of this burden and its potential implications including a further impact on quality of life. Management strategies should include consideration of both conditions, either with treatments that have overlapping efficacy, or with concomitant therapies,” the authors concluded.
The study was sponsored in part by AbbVie. One coauthor reported having served as an advisor for AbbVie, Pfizer, Janssen, and Asana Biosciences.
SOURCE: Wertentiel S et al. J Am Acad Dermatol. 2018 Oct 1. doi: 10.1016/j.jaad.2018.09.040.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Mobile App Rankings in Dermatology
As technology continues to advance, so too does its accessibility to the general population. In 2013, 56% of Americans owned a smartphone versus 77% in 2017.1With the increase in mobile applications (apps) available, it is no surprise that the market has extended into the medical field, with dermatology being no exception.2 The majority of dermatology apps can be classified as teledermatology apps, followed by self-surveillance, disease guide, and reference apps. Additional types of dermatology apps include dermoscopy, conference, education, photograph storage and sharing, and journal apps, and others.2 In this study, we examined Apple App Store rankings to determine the types of dermatology apps that are most popular among patients and physicians.
METHODS
A popular app rankings analyzer (App Annie) was used to search for dermatology apps along with their App Store rankings.3 Although iOS is not the most popular mobile device operating system, we chose to evaluate app rankings via the App Store because iPhones are the top-selling individual phones of any kind in the United States.4
We performed our analysis on a single day (July 14, 2018) given that app rankings can change daily. We incorporated the following keywords, which were commonly used in other dermatology app studies: dermatology, psoriasis, rosacea, acne, skin cancer, melanoma, eczema, and teledermatology. The category ranking was defined as the rank of a free or paid app in the App Store’s top charts for the selected country (United States), market (Apple), and device (iPhone) within their app category (Medical). Inclusion criteria required a ranking in the top 1500 Medical apps and being categorized in the App Store as a Medical app. Exclusion criteria included apps that focused on cosmetics, private practice, direct advertisements, photograph editing, or claims to cure skin disease, as well as non–English-language apps. The App Store descriptions were assessed to determine the type of each app (eg, teledermatology, disease guide) and target audience (patient, physician, or both).
Another search was performed using the same keywords but within the Health and Fitness category to capture potentially more highly ranked apps among patients. We also conducted separate searches within the Medical category using the keywords billing, coding, and ICD (International Classification of Diseases) to evaluate rankings for billing/coding apps, as well as EMR and electronic medical records for electronic medical record (EMR) apps.
RESULTS
The initial search yielded 851 results, which was narrowed down to 29 apps after applying the exclusion criteria. Of note, prior to application of the exclusion criteria, one dermatology app that was considered to be a direct advertisement app claiming to cure acne was ranked fourth of 1500 apps in the Medical category. However, the majority of the search results were excluded because they were not popular enough to be ranked among the top 1500 apps. There were more ranked dermatology apps in the Medical category targeting patients than physicians; 18 of 29 (62%) qualifying apps targeted patients and 11 (38%) targeted physicians (Tables 1 and 2). No apps targeted both groups. The most common type of ranked app targeting patients was self-surveillance (11/18), and the most common type targeting physicians was reference (8/11). The highest ranked app targeting patients was a teledermatology app with a ranking of 184, and the highest ranked app targeting physicians was educational, ranked 353. The least common type of ranked apps targeting patients were “other” (2/18 [11%]; 1 prescription and 1 UV monitor app) and conference (1/18 [6%]). The least common type of ranked apps targeting physicians were education (2/11 [18%]) and dermoscopy (1/11 [9%]).
Our search of the Health and Fitness category yielded 6 apps, all targeting patients; 3 (50%) were self-surveillance apps, and 3 (50%) were classified as other (2 UV monitors and a conferencing app for cancer emotional support)(Table 3).

Our search of the Medical category for billing/coding and EMR apps yielded 232 and 164 apps, respectively; of them, 49 (21%) and 54 (33%) apps were ranked. These apps did not overlap with the dermatology-related search criteria; thus, we were not able to ascertain how many of these apps were used specifically by health care providers in dermatology.
COMMENT
Patient Apps
The most common apps used by patients are fitness and nutrition tracker apps categorized as Health and Fitness5,6; however, the majority of ranked dermatology apps are categorized as Medical per our findings. In a study of 557 dermatology patients, it was found that among the health-related apps they used, the most common apps after fitness/nutrition were references, followed by patient portals, self-surveillance, and emotional assistance apps.6 Our search was consistent with these findings, suggesting that the most desired dermatology apps by patients are those that allow them to be proactive with their health. It is no surprise that the top-ranked app targeting patients was a teledermatology app, followed by multiple self-surveillance apps. The highest ranked self-surveillance app in the Health and Fitness category focused on monitoring the effects of nutrition on symptoms of diseases including skin disorders, while the highest ranked (as well as the majority of) self-surveillance apps in the Medical category encompassed mole monitoring and cancer risk calculators.
Benefits of the ranked dermatology apps in the Medical and Health and Fitness categories targeting patients include more immediate access to health care and education. Despite this popularity among patients, Masud et al7 demonstrated that only 20.5% (9/44) of dermatology apps targeting patients may be reliable resources based on a rubric created by the investigators. Overall, there remains a research gap for a standardized scientific approach to evaluating app validity and reliability.
Teledermatology
Teledermatology apps are the most common dermatology apps,2 allowing for remote evaluation of patients through either live consultations or transmittance of medical information for later review by board-certified physicians.8 Features common to many teledermatology apps include accessibility on Android (Google Inc) and iOS as well as a web version. Security and Health Insurance Portability and Accountability Act compliance is especially important and is enforced through user authentications, data encryption, and automatic logout features. Data is not stored locally and is secured on a private server with backup. Referring providers and consultants often can communicate within the app. Insurance providers also may cover teledermatology services, and if not, the out-of-pocket costs often are affordable.
The highest-ranked patient app (ranked 184 in the Medical category) was a teledermatology app that did not meet the American Telemedicine Association standards for teledermatology apps.9 The popularity of this app among patients may have been attributable to multiple ease-of-use and turnaround time features. The user interface was simplistic, and the design was appealing to the eye. The entry field options were minimal to avoid confusion. The turnaround time to receive a diagnosis depended on 1 of 3 options, including a more rapid response for an increased cost. Ease of use was the highlight of this app at the cost of accuracy, as the limited amount of information that users were required to provide physicians compromised diagnostic accuracy in this app.
For comparison, we chose a nonranked (and thus less frequently used) teledermatology app that had previously undergone scientific evaluation using 13 evaluation criteria specific to teledermatology.10 The app also met the American Telemedicine Association standard for teledermatology apps.9 The app was originally a broader telemedicine app but featured a section specific to teledermatology. The user interface was simple but professional, almost resembling an EMR. The input fields included a comprehensive history that permitted a better evaluation of a lesion but might be tedious for users. This app boasted professionalism and accuracy, but from a user standpoint, it may have been too time-consuming.
Striking a balance between ensuring proper care versus appealing to patients is a difficult but important task. Based on this study, it appears that popular patient apps may in fact have less scientific rationale and therefore potentially less accuracy.
Self-surveillance
Although self-surveillance apps did not account for the highest-ranked app, they were the most frequently ranked app type in our study. Most of the ranked self-surveillance apps in the Medical category were for monitoring lesions over time to assess for changes. These apps help users take photographs that are well organized in a single, easy-to-find location. Some apps were risk calculators that assessed the risk for malignancies using a questionnaire. The majority of these self-surveillance apps were specific to skin cancer detection. Of note, one of the ranked self-surveillance apps assessed drug effectiveness by monitoring clinical appearance and symptoms. The lowest ranked self-surveillance app in the top 1500 ranked Medical apps in our search monitored cancer symptoms not specific to dermatology. Although this app had a low ranking (1380/1500), it received a high number of reviews and was well rated at 4.8 out of 5 stars; therefore, it seemed more helpful than the other higher-ranked apps targeting patients, which had higher rankings but minimal to no reviews or ratings. A comparison of the ease-of-use features of all the ranked patient-targeted self-surveillance apps in the Medical category is provided in Table 4.
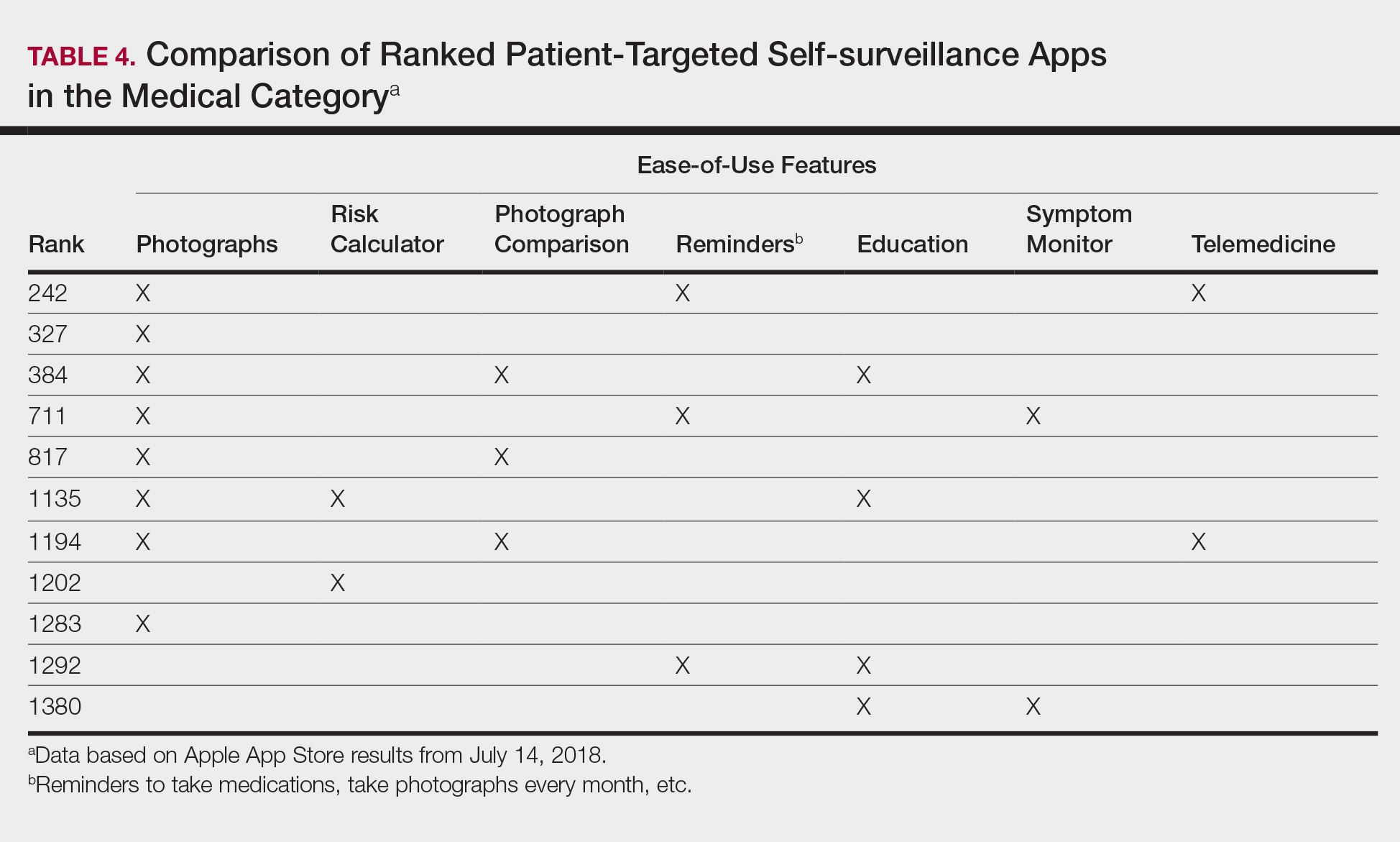
Physician Apps
After examining the results of apps targeting physicians, we realized that the data may be accurate but may not be as representative of all currently practicing dermatology providers. Given the increased usage of apps among younger age groups,11 our data may be skewed toward medical students and residents, supported by the fact that the top-ranked physician app in our study was an education app and the majority were reference apps. Future studies are needed to reexamine app ranking as this age group transitions from entry-level health care providers in the next 5 to 10 years. These findings also suggest less frequent app use among more veteran health care providers within our specific search parameters. Therefore, we decided to do subsequent searches for available billing/coding and EMR apps, which were many, but as mentioned above, none were specific to dermatology.
General Dermatology References
Most of the dermatology reference apps were formatted as e-books; however, other apps such as the Amazon Kindle app (categorized under Books) providing access to multiple e-books within one app were not included. Some apps included study aid features (eg, flash cards, quizzes), and topics spanned both dermatology and dermatopathology. Apps provide a unique way for on-the-go studying for dermatologists in training, and if the usage continues to grow, there may be a need for increased formal integration in dermatology education in the future.
Journals
Journal apps were not among those listed in the top-ranked apps we evaluated, which we suspect may be because journals were categorized differently from one journal to the next; for example, the Journal of the American Academy of Dermatology was ranked 1168 in the Magazines and Newspapers category. On the other hand, Dermatology World was ranked 1363 in the Reference category. An article’s citation affects the publishing journal’s impact factor, which is one of the most important variables in measuring a journal’s influence. In the future, there may be other variables that could aid in understanding journal impact as it relates to the journal’s accessibility.
Limitations
Our study did not look at Android apps. The top chart apps in the Android and Apple App Stores use undisclosed algorithms likely involving different characteristics such as number of downloads, frequency of updates, number of reviews, ratings, and more. Thus, the rankings across these different markets would not be comparable. Although our choice of keywords stemmed from the majority of prior studies looking at dermatology apps, our search was limited due to the use of these specific keywords. To avoid skewing data by cross-comparison of noncomparable categories, we could not compare apps in the Medical category versus those in other categories.
CONCLUSION
There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps. As app usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in our education, and as such, it will become more critical to develop formal scientific standards. Given these future trends, we may need to increase our current literature and understanding of apps in dermatology with regard to their impact on both patients and health care providers.
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center website. http://www.pewglobal.org/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/#table. Published June 19, 2018. Accessed August 28, 2018.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018;24. pii:13030/qt3hs7n9z6.
- App Annie website. https://www.appannie.com/top/. Accessed August 28, 2018.
- Number of iPhone users in the United States from 2012 to 2016 (in millions). Statista website. https://www.statista.com/statistics/232790/forecast-of-apple-users-in-the-us/. Accessed August 28, 2018.
- Burkhart C. Medical mobile apps and dermatology. Cutis. 2012;90:278-281.
- Wolf JA, Moreau JF, Patton TJ, et al. Prevalence and impact of health-related internet and smartphone use among dermatology patients. Cutis. 2015;95:323-328.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Walocko FM, Tejasvi T. Teledermatology applications in skin cancer diagnosis. Dermatol Clin. 2017;35:559-563.
- Krupinski E, Burdick A, Pak H, et al. American Telemedicine Association’s practice guidelines for teledermatology. Telemed J E Health. 2008;14:289-302.
- Ho B, Lee M, Armstrong AW. Evaluation criteria for mobile teledermatology applications and comparison of major mobile teledermatology applications. Telemed J E Health. 2013;19:678-682.
- Number of mobile app hours per smartphone and tablet app user in the United States in June 2016, by age group. Statista website. https://www.statista.com/statistics/323522/us-user-mobile-app-engagement-age/. Accessed September 18, 2018.
As technology continues to advance, so too does its accessibility to the general population. In 2013, 56% of Americans owned a smartphone versus 77% in 2017.1With the increase in mobile applications (apps) available, it is no surprise that the market has extended into the medical field, with dermatology being no exception.2 The majority of dermatology apps can be classified as teledermatology apps, followed by self-surveillance, disease guide, and reference apps. Additional types of dermatology apps include dermoscopy, conference, education, photograph storage and sharing, and journal apps, and others.2 In this study, we examined Apple App Store rankings to determine the types of dermatology apps that are most popular among patients and physicians.
METHODS
A popular app rankings analyzer (App Annie) was used to search for dermatology apps along with their App Store rankings.3 Although iOS is not the most popular mobile device operating system, we chose to evaluate app rankings via the App Store because iPhones are the top-selling individual phones of any kind in the United States.4
We performed our analysis on a single day (July 14, 2018) given that app rankings can change daily. We incorporated the following keywords, which were commonly used in other dermatology app studies: dermatology, psoriasis, rosacea, acne, skin cancer, melanoma, eczema, and teledermatology. The category ranking was defined as the rank of a free or paid app in the App Store’s top charts for the selected country (United States), market (Apple), and device (iPhone) within their app category (Medical). Inclusion criteria required a ranking in the top 1500 Medical apps and being categorized in the App Store as a Medical app. Exclusion criteria included apps that focused on cosmetics, private practice, direct advertisements, photograph editing, or claims to cure skin disease, as well as non–English-language apps. The App Store descriptions were assessed to determine the type of each app (eg, teledermatology, disease guide) and target audience (patient, physician, or both).
Another search was performed using the same keywords but within the Health and Fitness category to capture potentially more highly ranked apps among patients. We also conducted separate searches within the Medical category using the keywords billing, coding, and ICD (International Classification of Diseases) to evaluate rankings for billing/coding apps, as well as EMR and electronic medical records for electronic medical record (EMR) apps.
RESULTS
The initial search yielded 851 results, which was narrowed down to 29 apps after applying the exclusion criteria. Of note, prior to application of the exclusion criteria, one dermatology app that was considered to be a direct advertisement app claiming to cure acne was ranked fourth of 1500 apps in the Medical category. However, the majority of the search results were excluded because they were not popular enough to be ranked among the top 1500 apps. There were more ranked dermatology apps in the Medical category targeting patients than physicians; 18 of 29 (62%) qualifying apps targeted patients and 11 (38%) targeted physicians (Tables 1 and 2). No apps targeted both groups. The most common type of ranked app targeting patients was self-surveillance (11/18), and the most common type targeting physicians was reference (8/11). The highest ranked app targeting patients was a teledermatology app with a ranking of 184, and the highest ranked app targeting physicians was educational, ranked 353. The least common type of ranked apps targeting patients were “other” (2/18 [11%]; 1 prescription and 1 UV monitor app) and conference (1/18 [6%]). The least common type of ranked apps targeting physicians were education (2/11 [18%]) and dermoscopy (1/11 [9%]).


Our search of the Health and Fitness category yielded 6 apps, all targeting patients; 3 (50%) were self-surveillance apps, and 3 (50%) were classified as other (2 UV monitors and a conferencing app for cancer emotional support)(Table 3).

Our search of the Medical category for billing/coding and EMR apps yielded 232 and 164 apps, respectively; of them, 49 (21%) and 54 (33%) apps were ranked. These apps did not overlap with the dermatology-related search criteria; thus, we were not able to ascertain how many of these apps were used specifically by health care providers in dermatology.
COMMENT
Patient Apps
The most common apps used by patients are fitness and nutrition tracker apps categorized as Health and Fitness5,6; however, the majority of ranked dermatology apps are categorized as Medical per our findings. In a study of 557 dermatology patients, it was found that among the health-related apps they used, the most common apps after fitness/nutrition were references, followed by patient portals, self-surveillance, and emotional assistance apps.6 Our search was consistent with these findings, suggesting that the most desired dermatology apps by patients are those that allow them to be proactive with their health. It is no surprise that the top-ranked app targeting patients was a teledermatology app, followed by multiple self-surveillance apps. The highest ranked self-surveillance app in the Health and Fitness category focused on monitoring the effects of nutrition on symptoms of diseases including skin disorders, while the highest ranked (as well as the majority of) self-surveillance apps in the Medical category encompassed mole monitoring and cancer risk calculators.
Benefits of the ranked dermatology apps in the Medical and Health and Fitness categories targeting patients include more immediate access to health care and education. Despite this popularity among patients, Masud et al7 demonstrated that only 20.5% (9/44) of dermatology apps targeting patients may be reliable resources based on a rubric created by the investigators. Overall, there remains a research gap for a standardized scientific approach to evaluating app validity and reliability.
Teledermatology
Teledermatology apps are the most common dermatology apps,2 allowing for remote evaluation of patients through either live consultations or transmittance of medical information for later review by board-certified physicians.8 Features common to many teledermatology apps include accessibility on Android (Google Inc) and iOS as well as a web version. Security and Health Insurance Portability and Accountability Act compliance is especially important and is enforced through user authentications, data encryption, and automatic logout features. Data is not stored locally and is secured on a private server with backup. Referring providers and consultants often can communicate within the app. Insurance providers also may cover teledermatology services, and if not, the out-of-pocket costs often are affordable.
The highest-ranked patient app (ranked 184 in the Medical category) was a teledermatology app that did not meet the American Telemedicine Association standards for teledermatology apps.9 The popularity of this app among patients may have been attributable to multiple ease-of-use and turnaround time features. The user interface was simplistic, and the design was appealing to the eye. The entry field options were minimal to avoid confusion. The turnaround time to receive a diagnosis depended on 1 of 3 options, including a more rapid response for an increased cost. Ease of use was the highlight of this app at the cost of accuracy, as the limited amount of information that users were required to provide physicians compromised diagnostic accuracy in this app.
For comparison, we chose a nonranked (and thus less frequently used) teledermatology app that had previously undergone scientific evaluation using 13 evaluation criteria specific to teledermatology.10 The app also met the American Telemedicine Association standard for teledermatology apps.9 The app was originally a broader telemedicine app but featured a section specific to teledermatology. The user interface was simple but professional, almost resembling an EMR. The input fields included a comprehensive history that permitted a better evaluation of a lesion but might be tedious for users. This app boasted professionalism and accuracy, but from a user standpoint, it may have been too time-consuming.
Striking a balance between ensuring proper care versus appealing to patients is a difficult but important task. Based on this study, it appears that popular patient apps may in fact have less scientific rationale and therefore potentially less accuracy.
Self-surveillance
Although self-surveillance apps did not account for the highest-ranked app, they were the most frequently ranked app type in our study. Most of the ranked self-surveillance apps in the Medical category were for monitoring lesions over time to assess for changes. These apps help users take photographs that are well organized in a single, easy-to-find location. Some apps were risk calculators that assessed the risk for malignancies using a questionnaire. The majority of these self-surveillance apps were specific to skin cancer detection. Of note, one of the ranked self-surveillance apps assessed drug effectiveness by monitoring clinical appearance and symptoms. The lowest ranked self-surveillance app in the top 1500 ranked Medical apps in our search monitored cancer symptoms not specific to dermatology. Although this app had a low ranking (1380/1500), it received a high number of reviews and was well rated at 4.8 out of 5 stars; therefore, it seemed more helpful than the other higher-ranked apps targeting patients, which had higher rankings but minimal to no reviews or ratings. A comparison of the ease-of-use features of all the ranked patient-targeted self-surveillance apps in the Medical category is provided in Table 4.

Physician Apps
After examining the results of apps targeting physicians, we realized that the data may be accurate but may not be as representative of all currently practicing dermatology providers. Given the increased usage of apps among younger age groups,11 our data may be skewed toward medical students and residents, supported by the fact that the top-ranked physician app in our study was an education app and the majority were reference apps. Future studies are needed to reexamine app ranking as this age group transitions from entry-level health care providers in the next 5 to 10 years. These findings also suggest less frequent app use among more veteran health care providers within our specific search parameters. Therefore, we decided to do subsequent searches for available billing/coding and EMR apps, which were many, but as mentioned above, none were specific to dermatology.
General Dermatology References
Most of the dermatology reference apps were formatted as e-books; however, other apps such as the Amazon Kindle app (categorized under Books) providing access to multiple e-books within one app were not included. Some apps included study aid features (eg, flash cards, quizzes), and topics spanned both dermatology and dermatopathology. Apps provide a unique way for on-the-go studying for dermatologists in training, and if the usage continues to grow, there may be a need for increased formal integration in dermatology education in the future.
Journals
Journal apps were not among those listed in the top-ranked apps we evaluated, which we suspect may be because journals were categorized differently from one journal to the next; for example, the Journal of the American Academy of Dermatology was ranked 1168 in the Magazines and Newspapers category. On the other hand, Dermatology World was ranked 1363 in the Reference category. An article’s citation affects the publishing journal’s impact factor, which is one of the most important variables in measuring a journal’s influence. In the future, there may be other variables that could aid in understanding journal impact as it relates to the journal’s accessibility.
Limitations
Our study did not look at Android apps. The top chart apps in the Android and Apple App Stores use undisclosed algorithms likely involving different characteristics such as number of downloads, frequency of updates, number of reviews, ratings, and more. Thus, the rankings across these different markets would not be comparable. Although our choice of keywords stemmed from the majority of prior studies looking at dermatology apps, our search was limited due to the use of these specific keywords. To avoid skewing data by cross-comparison of noncomparable categories, we could not compare apps in the Medical category versus those in other categories.
CONCLUSION
There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps. As app usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in our education, and as such, it will become more critical to develop formal scientific standards. Given these future trends, we may need to increase our current literature and understanding of apps in dermatology with regard to their impact on both patients and health care providers.
As technology continues to advance, so too does its accessibility to the general population. In 2013, 56% of Americans owned a smartphone versus 77% in 2017.1With the increase in mobile applications (apps) available, it is no surprise that the market has extended into the medical field, with dermatology being no exception.2 The majority of dermatology apps can be classified as teledermatology apps, followed by self-surveillance, disease guide, and reference apps. Additional types of dermatology apps include dermoscopy, conference, education, photograph storage and sharing, and journal apps, and others.2 In this study, we examined Apple App Store rankings to determine the types of dermatology apps that are most popular among patients and physicians.
METHODS
A popular app rankings analyzer (App Annie) was used to search for dermatology apps along with their App Store rankings.3 Although iOS is not the most popular mobile device operating system, we chose to evaluate app rankings via the App Store because iPhones are the top-selling individual phones of any kind in the United States.4
We performed our analysis on a single day (July 14, 2018) given that app rankings can change daily. We incorporated the following keywords, which were commonly used in other dermatology app studies: dermatology, psoriasis, rosacea, acne, skin cancer, melanoma, eczema, and teledermatology. The category ranking was defined as the rank of a free or paid app in the App Store’s top charts for the selected country (United States), market (Apple), and device (iPhone) within their app category (Medical). Inclusion criteria required a ranking in the top 1500 Medical apps and being categorized in the App Store as a Medical app. Exclusion criteria included apps that focused on cosmetics, private practice, direct advertisements, photograph editing, or claims to cure skin disease, as well as non–English-language apps. The App Store descriptions were assessed to determine the type of each app (eg, teledermatology, disease guide) and target audience (patient, physician, or both).
Another search was performed using the same keywords but within the Health and Fitness category to capture potentially more highly ranked apps among patients. We also conducted separate searches within the Medical category using the keywords billing, coding, and ICD (International Classification of Diseases) to evaluate rankings for billing/coding apps, as well as EMR and electronic medical records for electronic medical record (EMR) apps.
RESULTS
The initial search yielded 851 results, which was narrowed down to 29 apps after applying the exclusion criteria. Of note, prior to application of the exclusion criteria, one dermatology app that was considered to be a direct advertisement app claiming to cure acne was ranked fourth of 1500 apps in the Medical category. However, the majority of the search results were excluded because they were not popular enough to be ranked among the top 1500 apps. There were more ranked dermatology apps in the Medical category targeting patients than physicians; 18 of 29 (62%) qualifying apps targeted patients and 11 (38%) targeted physicians (Tables 1 and 2). No apps targeted both groups. The most common type of ranked app targeting patients was self-surveillance (11/18), and the most common type targeting physicians was reference (8/11). The highest ranked app targeting patients was a teledermatology app with a ranking of 184, and the highest ranked app targeting physicians was educational, ranked 353. The least common type of ranked apps targeting patients were “other” (2/18 [11%]; 1 prescription and 1 UV monitor app) and conference (1/18 [6%]). The least common type of ranked apps targeting physicians were education (2/11 [18%]) and dermoscopy (1/11 [9%]).


Our search of the Health and Fitness category yielded 6 apps, all targeting patients; 3 (50%) were self-surveillance apps, and 3 (50%) were classified as other (2 UV monitors and a conferencing app for cancer emotional support)(Table 3).

Our search of the Medical category for billing/coding and EMR apps yielded 232 and 164 apps, respectively; of them, 49 (21%) and 54 (33%) apps were ranked. These apps did not overlap with the dermatology-related search criteria; thus, we were not able to ascertain how many of these apps were used specifically by health care providers in dermatology.
COMMENT
Patient Apps
The most common apps used by patients are fitness and nutrition tracker apps categorized as Health and Fitness5,6; however, the majority of ranked dermatology apps are categorized as Medical per our findings. In a study of 557 dermatology patients, it was found that among the health-related apps they used, the most common apps after fitness/nutrition were references, followed by patient portals, self-surveillance, and emotional assistance apps.6 Our search was consistent with these findings, suggesting that the most desired dermatology apps by patients are those that allow them to be proactive with their health. It is no surprise that the top-ranked app targeting patients was a teledermatology app, followed by multiple self-surveillance apps. The highest ranked self-surveillance app in the Health and Fitness category focused on monitoring the effects of nutrition on symptoms of diseases including skin disorders, while the highest ranked (as well as the majority of) self-surveillance apps in the Medical category encompassed mole monitoring and cancer risk calculators.
Benefits of the ranked dermatology apps in the Medical and Health and Fitness categories targeting patients include more immediate access to health care and education. Despite this popularity among patients, Masud et al7 demonstrated that only 20.5% (9/44) of dermatology apps targeting patients may be reliable resources based on a rubric created by the investigators. Overall, there remains a research gap for a standardized scientific approach to evaluating app validity and reliability.
Teledermatology
Teledermatology apps are the most common dermatology apps,2 allowing for remote evaluation of patients through either live consultations or transmittance of medical information for later review by board-certified physicians.8 Features common to many teledermatology apps include accessibility on Android (Google Inc) and iOS as well as a web version. Security and Health Insurance Portability and Accountability Act compliance is especially important and is enforced through user authentications, data encryption, and automatic logout features. Data is not stored locally and is secured on a private server with backup. Referring providers and consultants often can communicate within the app. Insurance providers also may cover teledermatology services, and if not, the out-of-pocket costs often are affordable.
The highest-ranked patient app (ranked 184 in the Medical category) was a teledermatology app that did not meet the American Telemedicine Association standards for teledermatology apps.9 The popularity of this app among patients may have been attributable to multiple ease-of-use and turnaround time features. The user interface was simplistic, and the design was appealing to the eye. The entry field options were minimal to avoid confusion. The turnaround time to receive a diagnosis depended on 1 of 3 options, including a more rapid response for an increased cost. Ease of use was the highlight of this app at the cost of accuracy, as the limited amount of information that users were required to provide physicians compromised diagnostic accuracy in this app.
For comparison, we chose a nonranked (and thus less frequently used) teledermatology app that had previously undergone scientific evaluation using 13 evaluation criteria specific to teledermatology.10 The app also met the American Telemedicine Association standard for teledermatology apps.9 The app was originally a broader telemedicine app but featured a section specific to teledermatology. The user interface was simple but professional, almost resembling an EMR. The input fields included a comprehensive history that permitted a better evaluation of a lesion but might be tedious for users. This app boasted professionalism and accuracy, but from a user standpoint, it may have been too time-consuming.
Striking a balance between ensuring proper care versus appealing to patients is a difficult but important task. Based on this study, it appears that popular patient apps may in fact have less scientific rationale and therefore potentially less accuracy.
Self-surveillance
Although self-surveillance apps did not account for the highest-ranked app, they were the most frequently ranked app type in our study. Most of the ranked self-surveillance apps in the Medical category were for monitoring lesions over time to assess for changes. These apps help users take photographs that are well organized in a single, easy-to-find location. Some apps were risk calculators that assessed the risk for malignancies using a questionnaire. The majority of these self-surveillance apps were specific to skin cancer detection. Of note, one of the ranked self-surveillance apps assessed drug effectiveness by monitoring clinical appearance and symptoms. The lowest ranked self-surveillance app in the top 1500 ranked Medical apps in our search monitored cancer symptoms not specific to dermatology. Although this app had a low ranking (1380/1500), it received a high number of reviews and was well rated at 4.8 out of 5 stars; therefore, it seemed more helpful than the other higher-ranked apps targeting patients, which had higher rankings but minimal to no reviews or ratings. A comparison of the ease-of-use features of all the ranked patient-targeted self-surveillance apps in the Medical category is provided in Table 4.

Physician Apps
After examining the results of apps targeting physicians, we realized that the data may be accurate but may not be as representative of all currently practicing dermatology providers. Given the increased usage of apps among younger age groups,11 our data may be skewed toward medical students and residents, supported by the fact that the top-ranked physician app in our study was an education app and the majority were reference apps. Future studies are needed to reexamine app ranking as this age group transitions from entry-level health care providers in the next 5 to 10 years. These findings also suggest less frequent app use among more veteran health care providers within our specific search parameters. Therefore, we decided to do subsequent searches for available billing/coding and EMR apps, which were many, but as mentioned above, none were specific to dermatology.
General Dermatology References
Most of the dermatology reference apps were formatted as e-books; however, other apps such as the Amazon Kindle app (categorized under Books) providing access to multiple e-books within one app were not included. Some apps included study aid features (eg, flash cards, quizzes), and topics spanned both dermatology and dermatopathology. Apps provide a unique way for on-the-go studying for dermatologists in training, and if the usage continues to grow, there may be a need for increased formal integration in dermatology education in the future.
Journals
Journal apps were not among those listed in the top-ranked apps we evaluated, which we suspect may be because journals were categorized differently from one journal to the next; for example, the Journal of the American Academy of Dermatology was ranked 1168 in the Magazines and Newspapers category. On the other hand, Dermatology World was ranked 1363 in the Reference category. An article’s citation affects the publishing journal’s impact factor, which is one of the most important variables in measuring a journal’s influence. In the future, there may be other variables that could aid in understanding journal impact as it relates to the journal’s accessibility.
Limitations
Our study did not look at Android apps. The top chart apps in the Android and Apple App Stores use undisclosed algorithms likely involving different characteristics such as number of downloads, frequency of updates, number of reviews, ratings, and more. Thus, the rankings across these different markets would not be comparable. Although our choice of keywords stemmed from the majority of prior studies looking at dermatology apps, our search was limited due to the use of these specific keywords. To avoid skewing data by cross-comparison of noncomparable categories, we could not compare apps in the Medical category versus those in other categories.
CONCLUSION
There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps. As app usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in our education, and as such, it will become more critical to develop formal scientific standards. Given these future trends, we may need to increase our current literature and understanding of apps in dermatology with regard to their impact on both patients and health care providers.
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center website. http://www.pewglobal.org/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/#table. Published June 19, 2018. Accessed August 28, 2018.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018;24. pii:13030/qt3hs7n9z6.
- App Annie website. https://www.appannie.com/top/. Accessed August 28, 2018.
- Number of iPhone users in the United States from 2012 to 2016 (in millions). Statista website. https://www.statista.com/statistics/232790/forecast-of-apple-users-in-the-us/. Accessed August 28, 2018.
- Burkhart C. Medical mobile apps and dermatology. Cutis. 2012;90:278-281.
- Wolf JA, Moreau JF, Patton TJ, et al. Prevalence and impact of health-related internet and smartphone use among dermatology patients. Cutis. 2015;95:323-328.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Walocko FM, Tejasvi T. Teledermatology applications in skin cancer diagnosis. Dermatol Clin. 2017;35:559-563.
- Krupinski E, Burdick A, Pak H, et al. American Telemedicine Association’s practice guidelines for teledermatology. Telemed J E Health. 2008;14:289-302.
- Ho B, Lee M, Armstrong AW. Evaluation criteria for mobile teledermatology applications and comparison of major mobile teledermatology applications. Telemed J E Health. 2013;19:678-682.
- Number of mobile app hours per smartphone and tablet app user in the United States in June 2016, by age group. Statista website. https://www.statista.com/statistics/323522/us-user-mobile-app-engagement-age/. Accessed September 18, 2018.
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center website. http://www.pewglobal.org/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/#table. Published June 19, 2018. Accessed August 28, 2018.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018;24. pii:13030/qt3hs7n9z6.
- App Annie website. https://www.appannie.com/top/. Accessed August 28, 2018.
- Number of iPhone users in the United States from 2012 to 2016 (in millions). Statista website. https://www.statista.com/statistics/232790/forecast-of-apple-users-in-the-us/. Accessed August 28, 2018.
- Burkhart C. Medical mobile apps and dermatology. Cutis. 2012;90:278-281.
- Wolf JA, Moreau JF, Patton TJ, et al. Prevalence and impact of health-related internet and smartphone use among dermatology patients. Cutis. 2015;95:323-328.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Walocko FM, Tejasvi T. Teledermatology applications in skin cancer diagnosis. Dermatol Clin. 2017;35:559-563.
- Krupinski E, Burdick A, Pak H, et al. American Telemedicine Association’s practice guidelines for teledermatology. Telemed J E Health. 2008;14:289-302.
- Ho B, Lee M, Armstrong AW. Evaluation criteria for mobile teledermatology applications and comparison of major mobile teledermatology applications. Telemed J E Health. 2013;19:678-682.
- Number of mobile app hours per smartphone and tablet app user in the United States in June 2016, by age group. Statista website. https://www.statista.com/statistics/323522/us-user-mobile-app-engagement-age/. Accessed September 18, 2018.
Practice Points
- As mobile application (app) usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in dermatology education. As such, it will become more critical to develop formal scientific standards.
- The most desired dermatology apps for patients were apps that allowed them to be proactive with their health.
- There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps.
FDA approves Seysara for treatment of moderate to severe acne
vulgaris for people aged 9 years and older, according to a press release from Paratek, the drug’s manufacturer.
FDA approval is based on results from two phase 3 clinical trials (NCT02320149 and NCT02322866), in which patients received either sarecycline at 1.5 mg/kg per day or placebo for 12 weeks. Patients who received sarecycline were significantly more likely to reach the primary endpoint of improved acne severity based on absolute change in facial lesion counts and percentage of participants with Investigator Global Assessment success.
Common adverse events reported in the sarecycline group were nausea (3.2%), nasopharyngitis (2.8%), and headache (2.8%). The discontinuation rate due to adverse events among sarecycline-treated patients in both studies combined was 1.4%.
Sarecycline is an oral, narrow spectrum tetracycline-derived antibiotic with anti-inflammatory properties, and is approved for once-daily use, according to Paratek. Seysara will be marketed in the United States by Almirall SA.
vulgaris for people aged 9 years and older, according to a press release from Paratek, the drug’s manufacturer.
FDA approval is based on results from two phase 3 clinical trials (NCT02320149 and NCT02322866), in which patients received either sarecycline at 1.5 mg/kg per day or placebo for 12 weeks. Patients who received sarecycline were significantly more likely to reach the primary endpoint of improved acne severity based on absolute change in facial lesion counts and percentage of participants with Investigator Global Assessment success.
Common adverse events reported in the sarecycline group were nausea (3.2%), nasopharyngitis (2.8%), and headache (2.8%). The discontinuation rate due to adverse events among sarecycline-treated patients in both studies combined was 1.4%.
Sarecycline is an oral, narrow spectrum tetracycline-derived antibiotic with anti-inflammatory properties, and is approved for once-daily use, according to Paratek. Seysara will be marketed in the United States by Almirall SA.
vulgaris for people aged 9 years and older, according to a press release from Paratek, the drug’s manufacturer.
FDA approval is based on results from two phase 3 clinical trials (NCT02320149 and NCT02322866), in which patients received either sarecycline at 1.5 mg/kg per day or placebo for 12 weeks. Patients who received sarecycline were significantly more likely to reach the primary endpoint of improved acne severity based on absolute change in facial lesion counts and percentage of participants with Investigator Global Assessment success.
Common adverse events reported in the sarecycline group were nausea (3.2%), nasopharyngitis (2.8%), and headache (2.8%). The discontinuation rate due to adverse events among sarecycline-treated patients in both studies combined was 1.4%.
Sarecycline is an oral, narrow spectrum tetracycline-derived antibiotic with anti-inflammatory properties, and is approved for once-daily use, according to Paratek. Seysara will be marketed in the United States by Almirall SA.