User login
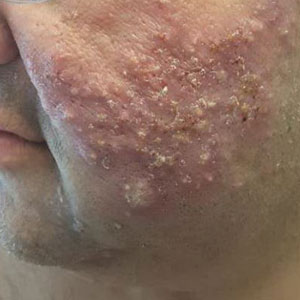
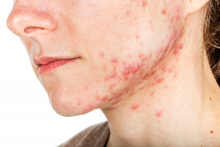
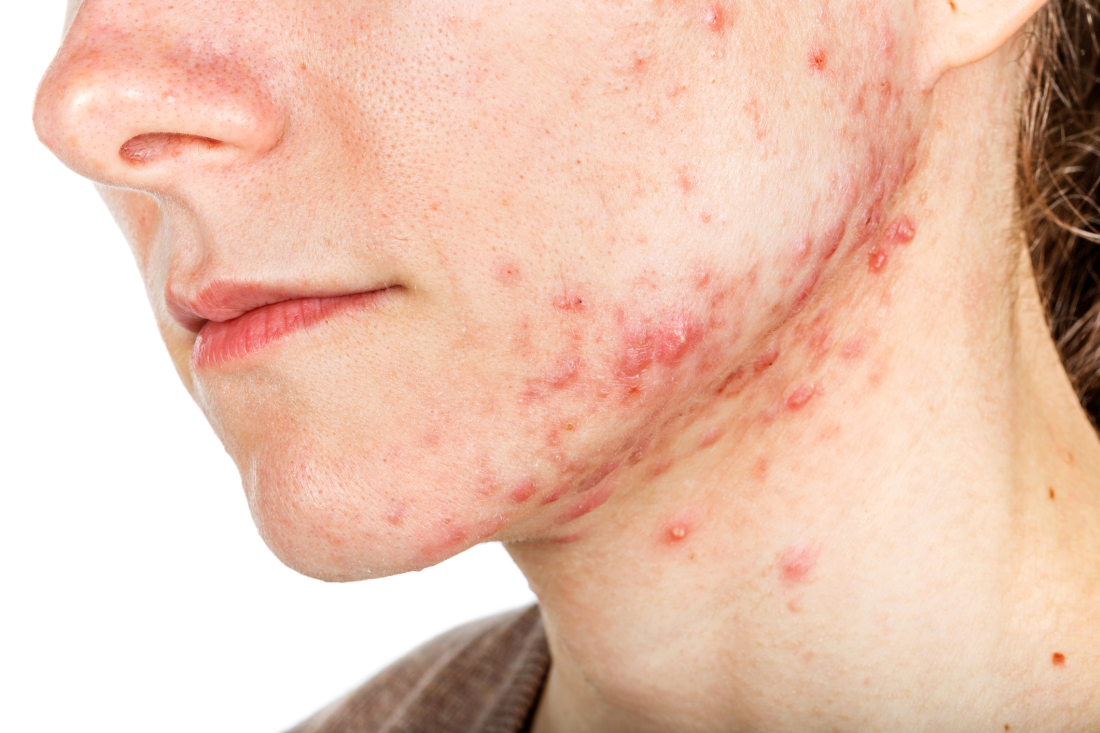
Combining treatment options for scar revision often a useful approach
DENVER –
“It’s important to manage expectations,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual meeting of the American Society for Dermatologic Surgery. “I tell them I can improve their scar and make it look less noticeable, but I can’t make it look like normal skin. It’s going to require multiple treatments. It’s not a one-time thing; it’s going to take several months to see the full benefit. And, it’s an investment of time and money.”
Nonablative, ablative, and fractional resurfacing stimulates dermal fibroblasts to replace lost collagen and elastin. Traditional lasers offer impressive clinical results for scars but are associated with significant preprocedural discomfort, prolonged recovery, and a significant risk of side effects, Dr. Ortiz said, while nonablative lasers are more tolerable with shorter recovery times.
Multiple sessions are required, and results are often less clinically impressive. “It’s often difficult for patients to have a lot of downtime with each treatment so often I prefer to use the nonablative laser, especially for acne scarring,” she said.
Mounting evidence suggests that the sooner scars are treated after they are formed, the better. That may not be feasible for patients with a long history of acne scars, but for surgical scars, Dr. Ortiz prefers to start treatment on the day of suture removal. “Whenever I do that, I always get better results,” she said.
Outcomes may also improve by combining different treatment options, but the type of scar drives the type of modality to consider. There are red scars from postinflammatory erythema, hyperpigmented scars, hypopigmented scars, atrophic scars, hypertrophic scars, spread scars, pin cushion scars, and keloid scars, “which are the most difficult to treat,” she said. “When I’m using a combination approach, I start with the redness component of the scar, because you don’t want to exacerbate nonspecific erythema, or it’ll be difficult to see where the redness is. So, I always use vascular laser first, then a pigment-specific laser, followed by resurfacing, and augmentation with filler if needed.”
Red scars generally fade with time, but that can take several months to more than a year. “If you use a laser, that can speed up the recovery,” said Dr. Ortiz, who is the vice president of the American Society for Laser Medicine and Surgery. “A vascular laser will work, such as KTP, or intense pulsed light. Studies favor a low fluence and a short pulse duration. Pulsed dye laser (PDL) penetrates deeper than KTP, so theoretically you get a bit of collagen remodeling because it can increase TGF-beta [transforming growth factor–beta], so theoretically, PDL is a little bit better than KTP for red scars, but both will work.”
In a comparative study, researchers used purpuric and nonpurpuric parameters to treat surgical scars but found no significant differences between the two treatment settings. “I tend to stick to short pulse duration and low fluence settings,” said Dr. Ortiz, who was not affiliated with the study.
A separate, single-blinded, split scar study, which compared the efficacy of KTP to 595 nm PDL in reduction of erythema in surgical scars, found no significant difference between the two approaches. A review of available therapeutic lasers for acne scarring found that the thermal energy delivered by KTP extends only to the papillary dermis, making it useful for postinflammatory erythema without significant effects on collagen remodeling.
Hyperpigmentation
Use of concomitant bleaching cream can also help as a preventive strategy for hyperpigmentation. But one study of 100 patients found that pretreatment with a bleaching regimen prior to undergoing CO2 laser resurfacing made no significant difference in hyperpigmentation compared with those who received no pretreatment regimen.
When Dr. Ortiz is concerned about hyperpigmentation after laser treatment, she prescribes post-treatment tranexamic acid 325 mg twice daily for 6 months or longer. “I don’t do any kind of workup or labs, but I do not prescribe it if a patient has increased risk of clotting,” she said. Those at increased risk include smokers, those on birth control pills, those on hormonal supplementation, those with a current malignancy, and those with a history of a cerebrovascular accident or deep vein thrombosis.
Hypopigmented, atrophic scars
In Dr. Ortiz’s clinical experience, hypopigmented scars respond well to treatment with the 1550 nonablative laser. “The idea is that you’re removing some of the scarred collagen and it allows the melanocytes to migrate in and repigment,” she said. Following laser treatment, consider applying topical bimatoprost 0.03% twice daily for at least 3 months to optimize results, she added.
For atrophic scars, options include subcision, laser treatment, radiofrequency microneedling, fillers, or biostimulators. “I caution against using permanent fillers because there is a higher risk of granuloma formation,” Dr. Ortiz said. “I tend to use hyaluronic acid fillers, which have a low G prime. I inject superficially.”
She shared a technique she learned from Mathew Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston. It entails spreading the skin with one’s fingers for a scar, especially an acne scar. “If it improves when you spread the skin, then you know it’s amenable to laser treatment,” Dr. Ortiz said. “But if it doesn’t improve when you spread the skin, it probably needs a little subcision. Insert an 18- or 20-gauge tribeveled hypodermic needle or an 18-gauge Nokor under the scar to sever the fibrous components that anchor the scar. This can take more than one treatment. I’ll often do this immediately before resurfacing.”
For hypertrophic scars, consider laser-assisted drug delivery, which creates vertical channels that assist the delivery of topically applied drugs into the skin. “You never want to use something that isn’t meant to be injected into the skin because you can get a granulomatous reaction,” she warned. “I often use topical triamcinolone acetonide, 5-FU, or poly-l-lactic acid.”
Dr. Ortiz noted that botulinum toxin type A may be helpful for scars, despite the paucity of evidence regarding specific mechanisms of action. “There is some thought that it can modulate TGF-beta,” she said. “It also may modulate collagen deposition. Currently we’re looking into Botox alone for keloid scars. The initial results look just okay.”
Dr. Ortiz disclosed that she has received consulting fees from Alastin, Cutera, and Sciton, and honoraria from BTL and Procter & Gamble. She is also a member of the advisory board for Aerolase, Allergan, Bausch Health, Endo, Galderma, Rodan + Fields, and Sciton, and has received equipment from BTL, Sciton, and SmartGraft.
DENVER –
“It’s important to manage expectations,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual meeting of the American Society for Dermatologic Surgery. “I tell them I can improve their scar and make it look less noticeable, but I can’t make it look like normal skin. It’s going to require multiple treatments. It’s not a one-time thing; it’s going to take several months to see the full benefit. And, it’s an investment of time and money.”
Nonablative, ablative, and fractional resurfacing stimulates dermal fibroblasts to replace lost collagen and elastin. Traditional lasers offer impressive clinical results for scars but are associated with significant preprocedural discomfort, prolonged recovery, and a significant risk of side effects, Dr. Ortiz said, while nonablative lasers are more tolerable with shorter recovery times.
Multiple sessions are required, and results are often less clinically impressive. “It’s often difficult for patients to have a lot of downtime with each treatment so often I prefer to use the nonablative laser, especially for acne scarring,” she said.
Mounting evidence suggests that the sooner scars are treated after they are formed, the better. That may not be feasible for patients with a long history of acne scars, but for surgical scars, Dr. Ortiz prefers to start treatment on the day of suture removal. “Whenever I do that, I always get better results,” she said.
Outcomes may also improve by combining different treatment options, but the type of scar drives the type of modality to consider. There are red scars from postinflammatory erythema, hyperpigmented scars, hypopigmented scars, atrophic scars, hypertrophic scars, spread scars, pin cushion scars, and keloid scars, “which are the most difficult to treat,” she said. “When I’m using a combination approach, I start with the redness component of the scar, because you don’t want to exacerbate nonspecific erythema, or it’ll be difficult to see where the redness is. So, I always use vascular laser first, then a pigment-specific laser, followed by resurfacing, and augmentation with filler if needed.”
Red scars generally fade with time, but that can take several months to more than a year. “If you use a laser, that can speed up the recovery,” said Dr. Ortiz, who is the vice president of the American Society for Laser Medicine and Surgery. “A vascular laser will work, such as KTP, or intense pulsed light. Studies favor a low fluence and a short pulse duration. Pulsed dye laser (PDL) penetrates deeper than KTP, so theoretically you get a bit of collagen remodeling because it can increase TGF-beta [transforming growth factor–beta], so theoretically, PDL is a little bit better than KTP for red scars, but both will work.”
In a comparative study, researchers used purpuric and nonpurpuric parameters to treat surgical scars but found no significant differences between the two treatment settings. “I tend to stick to short pulse duration and low fluence settings,” said Dr. Ortiz, who was not affiliated with the study.
A separate, single-blinded, split scar study, which compared the efficacy of KTP to 595 nm PDL in reduction of erythema in surgical scars, found no significant difference between the two approaches. A review of available therapeutic lasers for acne scarring found that the thermal energy delivered by KTP extends only to the papillary dermis, making it useful for postinflammatory erythema without significant effects on collagen remodeling.
Hyperpigmentation
Use of concomitant bleaching cream can also help as a preventive strategy for hyperpigmentation. But one study of 100 patients found that pretreatment with a bleaching regimen prior to undergoing CO2 laser resurfacing made no significant difference in hyperpigmentation compared with those who received no pretreatment regimen.
When Dr. Ortiz is concerned about hyperpigmentation after laser treatment, she prescribes post-treatment tranexamic acid 325 mg twice daily for 6 months or longer. “I don’t do any kind of workup or labs, but I do not prescribe it if a patient has increased risk of clotting,” she said. Those at increased risk include smokers, those on birth control pills, those on hormonal supplementation, those with a current malignancy, and those with a history of a cerebrovascular accident or deep vein thrombosis.
Hypopigmented, atrophic scars
In Dr. Ortiz’s clinical experience, hypopigmented scars respond well to treatment with the 1550 nonablative laser. “The idea is that you’re removing some of the scarred collagen and it allows the melanocytes to migrate in and repigment,” she said. Following laser treatment, consider applying topical bimatoprost 0.03% twice daily for at least 3 months to optimize results, she added.
For atrophic scars, options include subcision, laser treatment, radiofrequency microneedling, fillers, or biostimulators. “I caution against using permanent fillers because there is a higher risk of granuloma formation,” Dr. Ortiz said. “I tend to use hyaluronic acid fillers, which have a low G prime. I inject superficially.”
She shared a technique she learned from Mathew Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston. It entails spreading the skin with one’s fingers for a scar, especially an acne scar. “If it improves when you spread the skin, then you know it’s amenable to laser treatment,” Dr. Ortiz said. “But if it doesn’t improve when you spread the skin, it probably needs a little subcision. Insert an 18- or 20-gauge tribeveled hypodermic needle or an 18-gauge Nokor under the scar to sever the fibrous components that anchor the scar. This can take more than one treatment. I’ll often do this immediately before resurfacing.”
For hypertrophic scars, consider laser-assisted drug delivery, which creates vertical channels that assist the delivery of topically applied drugs into the skin. “You never want to use something that isn’t meant to be injected into the skin because you can get a granulomatous reaction,” she warned. “I often use topical triamcinolone acetonide, 5-FU, or poly-l-lactic acid.”
Dr. Ortiz noted that botulinum toxin type A may be helpful for scars, despite the paucity of evidence regarding specific mechanisms of action. “There is some thought that it can modulate TGF-beta,” she said. “It also may modulate collagen deposition. Currently we’re looking into Botox alone for keloid scars. The initial results look just okay.”
Dr. Ortiz disclosed that she has received consulting fees from Alastin, Cutera, and Sciton, and honoraria from BTL and Procter & Gamble. She is also a member of the advisory board for Aerolase, Allergan, Bausch Health, Endo, Galderma, Rodan + Fields, and Sciton, and has received equipment from BTL, Sciton, and SmartGraft.
DENVER –
“It’s important to manage expectations,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual meeting of the American Society for Dermatologic Surgery. “I tell them I can improve their scar and make it look less noticeable, but I can’t make it look like normal skin. It’s going to require multiple treatments. It’s not a one-time thing; it’s going to take several months to see the full benefit. And, it’s an investment of time and money.”
Nonablative, ablative, and fractional resurfacing stimulates dermal fibroblasts to replace lost collagen and elastin. Traditional lasers offer impressive clinical results for scars but are associated with significant preprocedural discomfort, prolonged recovery, and a significant risk of side effects, Dr. Ortiz said, while nonablative lasers are more tolerable with shorter recovery times.
Multiple sessions are required, and results are often less clinically impressive. “It’s often difficult for patients to have a lot of downtime with each treatment so often I prefer to use the nonablative laser, especially for acne scarring,” she said.
Mounting evidence suggests that the sooner scars are treated after they are formed, the better. That may not be feasible for patients with a long history of acne scars, but for surgical scars, Dr. Ortiz prefers to start treatment on the day of suture removal. “Whenever I do that, I always get better results,” she said.
Outcomes may also improve by combining different treatment options, but the type of scar drives the type of modality to consider. There are red scars from postinflammatory erythema, hyperpigmented scars, hypopigmented scars, atrophic scars, hypertrophic scars, spread scars, pin cushion scars, and keloid scars, “which are the most difficult to treat,” she said. “When I’m using a combination approach, I start with the redness component of the scar, because you don’t want to exacerbate nonspecific erythema, or it’ll be difficult to see where the redness is. So, I always use vascular laser first, then a pigment-specific laser, followed by resurfacing, and augmentation with filler if needed.”
Red scars generally fade with time, but that can take several months to more than a year. “If you use a laser, that can speed up the recovery,” said Dr. Ortiz, who is the vice president of the American Society for Laser Medicine and Surgery. “A vascular laser will work, such as KTP, or intense pulsed light. Studies favor a low fluence and a short pulse duration. Pulsed dye laser (PDL) penetrates deeper than KTP, so theoretically you get a bit of collagen remodeling because it can increase TGF-beta [transforming growth factor–beta], so theoretically, PDL is a little bit better than KTP for red scars, but both will work.”
In a comparative study, researchers used purpuric and nonpurpuric parameters to treat surgical scars but found no significant differences between the two treatment settings. “I tend to stick to short pulse duration and low fluence settings,” said Dr. Ortiz, who was not affiliated with the study.
A separate, single-blinded, split scar study, which compared the efficacy of KTP to 595 nm PDL in reduction of erythema in surgical scars, found no significant difference between the two approaches. A review of available therapeutic lasers for acne scarring found that the thermal energy delivered by KTP extends only to the papillary dermis, making it useful for postinflammatory erythema without significant effects on collagen remodeling.
Hyperpigmentation
Use of concomitant bleaching cream can also help as a preventive strategy for hyperpigmentation. But one study of 100 patients found that pretreatment with a bleaching regimen prior to undergoing CO2 laser resurfacing made no significant difference in hyperpigmentation compared with those who received no pretreatment regimen.
When Dr. Ortiz is concerned about hyperpigmentation after laser treatment, she prescribes post-treatment tranexamic acid 325 mg twice daily for 6 months or longer. “I don’t do any kind of workup or labs, but I do not prescribe it if a patient has increased risk of clotting,” she said. Those at increased risk include smokers, those on birth control pills, those on hormonal supplementation, those with a current malignancy, and those with a history of a cerebrovascular accident or deep vein thrombosis.
Hypopigmented, atrophic scars
In Dr. Ortiz’s clinical experience, hypopigmented scars respond well to treatment with the 1550 nonablative laser. “The idea is that you’re removing some of the scarred collagen and it allows the melanocytes to migrate in and repigment,” she said. Following laser treatment, consider applying topical bimatoprost 0.03% twice daily for at least 3 months to optimize results, she added.
For atrophic scars, options include subcision, laser treatment, radiofrequency microneedling, fillers, or biostimulators. “I caution against using permanent fillers because there is a higher risk of granuloma formation,” Dr. Ortiz said. “I tend to use hyaluronic acid fillers, which have a low G prime. I inject superficially.”
She shared a technique she learned from Mathew Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston. It entails spreading the skin with one’s fingers for a scar, especially an acne scar. “If it improves when you spread the skin, then you know it’s amenable to laser treatment,” Dr. Ortiz said. “But if it doesn’t improve when you spread the skin, it probably needs a little subcision. Insert an 18- or 20-gauge tribeveled hypodermic needle or an 18-gauge Nokor under the scar to sever the fibrous components that anchor the scar. This can take more than one treatment. I’ll often do this immediately before resurfacing.”
For hypertrophic scars, consider laser-assisted drug delivery, which creates vertical channels that assist the delivery of topically applied drugs into the skin. “You never want to use something that isn’t meant to be injected into the skin because you can get a granulomatous reaction,” she warned. “I often use topical triamcinolone acetonide, 5-FU, or poly-l-lactic acid.”
Dr. Ortiz noted that botulinum toxin type A may be helpful for scars, despite the paucity of evidence regarding specific mechanisms of action. “There is some thought that it can modulate TGF-beta,” she said. “It also may modulate collagen deposition. Currently we’re looking into Botox alone for keloid scars. The initial results look just okay.”
Dr. Ortiz disclosed that she has received consulting fees from Alastin, Cutera, and Sciton, and honoraria from BTL and Procter & Gamble. She is also a member of the advisory board for Aerolase, Allergan, Bausch Health, Endo, Galderma, Rodan + Fields, and Sciton, and has received equipment from BTL, Sciton, and SmartGraft.
AT ASDS 2022
Study addresses whether cosmetic treatments make patients happier
DENVER – compared with the general population, according to a study of 42 individuals. However, these treatments did not improve their baseline happiness or life satisfaction scores at follow-up.
Those are key findings from the study that lead author Rishi Chopra, MD, MS, presented during an oral abstract session at the annual meeting of the American Society for Dermatologic Surgery.
“These are interesting and surprising results,” said Dr. Chopra, a dermatologist and laser and cosmetic dermatologic surgery fellow at Harvard Medical School and Massachusetts General Hospital in Boston. “Patients are seeking consultations with us with the hope that the treatments we offer may potentially help them feel happier, but are we really delivering on that?”
In a pivotal 2018 study that examined patient motivations for undergoing cosmetic dermatology procedures, investigators found that 67.2% did so to “feel happier and more confident or improve total quality of life”. Moreover, 38.5% cited the desire to “feel happier, better overall, or improve total quality of life” as the key reason for pursuing cosmetic procedures.
Prior published evidence validates this benefit of procedures, as neuromodulators have repeatedly demonstrated to improve mood and depression, including a 2020 randomized, single-blind crossover study that examined the impact of neuromodulators on mood and appearance during the COVID-19 pandemic. It found that patients who received treatment with neuromodulators prior to the pandemic, stopped during the pandemic, and restarted again, reported increased happiness, self-satisfaction with appearance, and overall treatment satisfaction.
“However, studies evaluating the effect of filler on happiness have failed to demonstrate an impact,” Dr. Chopra said. “Thus, the jury is still out.”
Study evaluated 42 patients
In what he said is the first study of its kind, he and his colleagues evaluated the impact of minimally invasive cosmetic procedures on the happiness of 42 treatment non-naive patients (those who regularly undergo cosmetic procedures) with a mean age of 47 years who were surveyed in November and December of 2021 during the COVID-19 Omicron subvariant outbreak at the cosmetic dermatology practices of Sabrina G. Fabi, MD, in San Diego, and Nicole Kanaris, MBBCh, in Johannesburg, South Africa.
“On average, these patients were undergoing six treatments per year during four visits per year, so these were frequent flyers,” Dr. Chopra said. “We set out to assess: Are patients who seek cosmetic procedures happy at baseline? And, do cosmetic procedures make us happier or more satisfied with life?”
Prior to treatment, patients completed the Subjective Happiness Scale (SHS) and Satisfaction With Life Scale (SWLS). Three weeks later, patients completed the SHS, SWLS, the Global Aesthetic Improvement Scale (GAIS) and a 5-point satisfaction score. The researchers used paired and unpaired t-tests, independent sample t-tests, and Spearman rank correlations to conduct statistical analyses.
The baseline SHS score of study participants was an average of 5.87, which Dr. Chopra said is higher than the worldwide population range between 4.57 and 5.33, and 5.05 in the U.S. population. “The patients in our study were very happy to begin with,” an important point to consider, he said. Following their treatments, respondents felt “improved” or “much improved” on the GAIS (a mean score of 3.64) and “somewhat satisfied” or “very satisfied” based on the SWLS (a mean score of 4.4). “So overall, they viewed their treatments as a success,” Dr. Chopra said.
In terms of happiness, however, the researchers observed no significant differences between pre- and posttreatment scores on the SHS (a mean of 5.87 vs. 6.61, respectively; P = .634) nor on the SWLS (a mean of 29.62 vs. 29.1; P = .709). On stratified analysis, no significant differences in the SHS, SWLS, and the GAIS were observed when the researchers accounted for the aggressiveness of the procedure, the number of treatments, the number of sites treated, the type of treatment, and whether the respondents were happier or sadder at baseline. “Surprisingly, this had no effect whatsoever on happiness,” he said. “Not only that, these factors didn’t improve a patient’s perception of the efficacy or satisfaction with a treatment either.”
According to Dr. Chopra, this is the first study to evaluate the impact of a broad spectrum of minimally invasive cosmetic procedures, including injectables and lasers, on the happiness and life satisfaction of treatment non-naive patients.
“Surprisingly, we found these patients were no happier after treatment,” he told this news organization. “However, before rushing to declare that cosmetic procedures don’t make us happier, it is critical to evaluate these results in the context of our study population. We believe there to be a distinction between treatment naive and non-naive patients. All the patients in our study were treatment non-naive, routinely and frequently undergoing cosmetic procedures. Moreover, our treatment non-naive patients were very happy at baseline prior to treatment.”
He and his colleagues hypothesize that there is a “ceiling effect” to the happiness one can attain via these procedures. “Our treatment non-naive patients had already reached this ceiling-peak happiness of their treatment journey, and at this point were only pursuing procedures to maintain their results and happiness,” he said. “Thus, we were unable to measure any effect this late in the ‘maintenance-phase’ of their journey via our study. On the other hand, treatment naive patients (those who have never undergone a cosmetic procedure) were not included. We hypothesize that evaluating patients at the start of their journey after their first round of treatments will demonstrate an impact on happiness, prior to reaching the ceiling and subsequent ‘maintenance phase.’ ”
Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study results, said that it was not clear which specific cosmetic treatments the study participants received. “I would like to see if different injectable or device treatments would give different happiness scale results,” Dr. Green said.
“In addition, only two locations were surveyed, so the results could have location bias. I think it would be a great idea to replicate this survey of experienced cosmetic treatment patients with many locations and to include survey responses based on the procedure that was done. That said, it is interesting that overall, investigator satisfaction did not correlate with patient happiness from the treatments.”
Dr. Chopra reported having no financial disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
DENVER – compared with the general population, according to a study of 42 individuals. However, these treatments did not improve their baseline happiness or life satisfaction scores at follow-up.
Those are key findings from the study that lead author Rishi Chopra, MD, MS, presented during an oral abstract session at the annual meeting of the American Society for Dermatologic Surgery.
“These are interesting and surprising results,” said Dr. Chopra, a dermatologist and laser and cosmetic dermatologic surgery fellow at Harvard Medical School and Massachusetts General Hospital in Boston. “Patients are seeking consultations with us with the hope that the treatments we offer may potentially help them feel happier, but are we really delivering on that?”
In a pivotal 2018 study that examined patient motivations for undergoing cosmetic dermatology procedures, investigators found that 67.2% did so to “feel happier and more confident or improve total quality of life”. Moreover, 38.5% cited the desire to “feel happier, better overall, or improve total quality of life” as the key reason for pursuing cosmetic procedures.
Prior published evidence validates this benefit of procedures, as neuromodulators have repeatedly demonstrated to improve mood and depression, including a 2020 randomized, single-blind crossover study that examined the impact of neuromodulators on mood and appearance during the COVID-19 pandemic. It found that patients who received treatment with neuromodulators prior to the pandemic, stopped during the pandemic, and restarted again, reported increased happiness, self-satisfaction with appearance, and overall treatment satisfaction.
“However, studies evaluating the effect of filler on happiness have failed to demonstrate an impact,” Dr. Chopra said. “Thus, the jury is still out.”
Study evaluated 42 patients
In what he said is the first study of its kind, he and his colleagues evaluated the impact of minimally invasive cosmetic procedures on the happiness of 42 treatment non-naive patients (those who regularly undergo cosmetic procedures) with a mean age of 47 years who were surveyed in November and December of 2021 during the COVID-19 Omicron subvariant outbreak at the cosmetic dermatology practices of Sabrina G. Fabi, MD, in San Diego, and Nicole Kanaris, MBBCh, in Johannesburg, South Africa.
“On average, these patients were undergoing six treatments per year during four visits per year, so these were frequent flyers,” Dr. Chopra said. “We set out to assess: Are patients who seek cosmetic procedures happy at baseline? And, do cosmetic procedures make us happier or more satisfied with life?”
Prior to treatment, patients completed the Subjective Happiness Scale (SHS) and Satisfaction With Life Scale (SWLS). Three weeks later, patients completed the SHS, SWLS, the Global Aesthetic Improvement Scale (GAIS) and a 5-point satisfaction score. The researchers used paired and unpaired t-tests, independent sample t-tests, and Spearman rank correlations to conduct statistical analyses.
The baseline SHS score of study participants was an average of 5.87, which Dr. Chopra said is higher than the worldwide population range between 4.57 and 5.33, and 5.05 in the U.S. population. “The patients in our study were very happy to begin with,” an important point to consider, he said. Following their treatments, respondents felt “improved” or “much improved” on the GAIS (a mean score of 3.64) and “somewhat satisfied” or “very satisfied” based on the SWLS (a mean score of 4.4). “So overall, they viewed their treatments as a success,” Dr. Chopra said.
In terms of happiness, however, the researchers observed no significant differences between pre- and posttreatment scores on the SHS (a mean of 5.87 vs. 6.61, respectively; P = .634) nor on the SWLS (a mean of 29.62 vs. 29.1; P = .709). On stratified analysis, no significant differences in the SHS, SWLS, and the GAIS were observed when the researchers accounted for the aggressiveness of the procedure, the number of treatments, the number of sites treated, the type of treatment, and whether the respondents were happier or sadder at baseline. “Surprisingly, this had no effect whatsoever on happiness,” he said. “Not only that, these factors didn’t improve a patient’s perception of the efficacy or satisfaction with a treatment either.”
According to Dr. Chopra, this is the first study to evaluate the impact of a broad spectrum of minimally invasive cosmetic procedures, including injectables and lasers, on the happiness and life satisfaction of treatment non-naive patients.
“Surprisingly, we found these patients were no happier after treatment,” he told this news organization. “However, before rushing to declare that cosmetic procedures don’t make us happier, it is critical to evaluate these results in the context of our study population. We believe there to be a distinction between treatment naive and non-naive patients. All the patients in our study were treatment non-naive, routinely and frequently undergoing cosmetic procedures. Moreover, our treatment non-naive patients were very happy at baseline prior to treatment.”
He and his colleagues hypothesize that there is a “ceiling effect” to the happiness one can attain via these procedures. “Our treatment non-naive patients had already reached this ceiling-peak happiness of their treatment journey, and at this point were only pursuing procedures to maintain their results and happiness,” he said. “Thus, we were unable to measure any effect this late in the ‘maintenance-phase’ of their journey via our study. On the other hand, treatment naive patients (those who have never undergone a cosmetic procedure) were not included. We hypothesize that evaluating patients at the start of their journey after their first round of treatments will demonstrate an impact on happiness, prior to reaching the ceiling and subsequent ‘maintenance phase.’ ”
Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study results, said that it was not clear which specific cosmetic treatments the study participants received. “I would like to see if different injectable or device treatments would give different happiness scale results,” Dr. Green said.
“In addition, only two locations were surveyed, so the results could have location bias. I think it would be a great idea to replicate this survey of experienced cosmetic treatment patients with many locations and to include survey responses based on the procedure that was done. That said, it is interesting that overall, investigator satisfaction did not correlate with patient happiness from the treatments.”
Dr. Chopra reported having no financial disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
DENVER – compared with the general population, according to a study of 42 individuals. However, these treatments did not improve their baseline happiness or life satisfaction scores at follow-up.
Those are key findings from the study that lead author Rishi Chopra, MD, MS, presented during an oral abstract session at the annual meeting of the American Society for Dermatologic Surgery.
“These are interesting and surprising results,” said Dr. Chopra, a dermatologist and laser and cosmetic dermatologic surgery fellow at Harvard Medical School and Massachusetts General Hospital in Boston. “Patients are seeking consultations with us with the hope that the treatments we offer may potentially help them feel happier, but are we really delivering on that?”
In a pivotal 2018 study that examined patient motivations for undergoing cosmetic dermatology procedures, investigators found that 67.2% did so to “feel happier and more confident or improve total quality of life”. Moreover, 38.5% cited the desire to “feel happier, better overall, or improve total quality of life” as the key reason for pursuing cosmetic procedures.
Prior published evidence validates this benefit of procedures, as neuromodulators have repeatedly demonstrated to improve mood and depression, including a 2020 randomized, single-blind crossover study that examined the impact of neuromodulators on mood and appearance during the COVID-19 pandemic. It found that patients who received treatment with neuromodulators prior to the pandemic, stopped during the pandemic, and restarted again, reported increased happiness, self-satisfaction with appearance, and overall treatment satisfaction.
“However, studies evaluating the effect of filler on happiness have failed to demonstrate an impact,” Dr. Chopra said. “Thus, the jury is still out.”
Study evaluated 42 patients
In what he said is the first study of its kind, he and his colleagues evaluated the impact of minimally invasive cosmetic procedures on the happiness of 42 treatment non-naive patients (those who regularly undergo cosmetic procedures) with a mean age of 47 years who were surveyed in November and December of 2021 during the COVID-19 Omicron subvariant outbreak at the cosmetic dermatology practices of Sabrina G. Fabi, MD, in San Diego, and Nicole Kanaris, MBBCh, in Johannesburg, South Africa.
“On average, these patients were undergoing six treatments per year during four visits per year, so these were frequent flyers,” Dr. Chopra said. “We set out to assess: Are patients who seek cosmetic procedures happy at baseline? And, do cosmetic procedures make us happier or more satisfied with life?”
Prior to treatment, patients completed the Subjective Happiness Scale (SHS) and Satisfaction With Life Scale (SWLS). Three weeks later, patients completed the SHS, SWLS, the Global Aesthetic Improvement Scale (GAIS) and a 5-point satisfaction score. The researchers used paired and unpaired t-tests, independent sample t-tests, and Spearman rank correlations to conduct statistical analyses.
The baseline SHS score of study participants was an average of 5.87, which Dr. Chopra said is higher than the worldwide population range between 4.57 and 5.33, and 5.05 in the U.S. population. “The patients in our study were very happy to begin with,” an important point to consider, he said. Following their treatments, respondents felt “improved” or “much improved” on the GAIS (a mean score of 3.64) and “somewhat satisfied” or “very satisfied” based on the SWLS (a mean score of 4.4). “So overall, they viewed their treatments as a success,” Dr. Chopra said.
In terms of happiness, however, the researchers observed no significant differences between pre- and posttreatment scores on the SHS (a mean of 5.87 vs. 6.61, respectively; P = .634) nor on the SWLS (a mean of 29.62 vs. 29.1; P = .709). On stratified analysis, no significant differences in the SHS, SWLS, and the GAIS were observed when the researchers accounted for the aggressiveness of the procedure, the number of treatments, the number of sites treated, the type of treatment, and whether the respondents were happier or sadder at baseline. “Surprisingly, this had no effect whatsoever on happiness,” he said. “Not only that, these factors didn’t improve a patient’s perception of the efficacy or satisfaction with a treatment either.”
According to Dr. Chopra, this is the first study to evaluate the impact of a broad spectrum of minimally invasive cosmetic procedures, including injectables and lasers, on the happiness and life satisfaction of treatment non-naive patients.
“Surprisingly, we found these patients were no happier after treatment,” he told this news organization. “However, before rushing to declare that cosmetic procedures don’t make us happier, it is critical to evaluate these results in the context of our study population. We believe there to be a distinction between treatment naive and non-naive patients. All the patients in our study were treatment non-naive, routinely and frequently undergoing cosmetic procedures. Moreover, our treatment non-naive patients were very happy at baseline prior to treatment.”
He and his colleagues hypothesize that there is a “ceiling effect” to the happiness one can attain via these procedures. “Our treatment non-naive patients had already reached this ceiling-peak happiness of their treatment journey, and at this point were only pursuing procedures to maintain their results and happiness,” he said. “Thus, we were unable to measure any effect this late in the ‘maintenance-phase’ of their journey via our study. On the other hand, treatment naive patients (those who have never undergone a cosmetic procedure) were not included. We hypothesize that evaluating patients at the start of their journey after their first round of treatments will demonstrate an impact on happiness, prior to reaching the ceiling and subsequent ‘maintenance phase.’ ”
Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study results, said that it was not clear which specific cosmetic treatments the study participants received. “I would like to see if different injectable or device treatments would give different happiness scale results,” Dr. Green said.
“In addition, only two locations were surveyed, so the results could have location bias. I think it would be a great idea to replicate this survey of experienced cosmetic treatment patients with many locations and to include survey responses based on the procedure that was done. That said, it is interesting that overall, investigator satisfaction did not correlate with patient happiness from the treatments.”
Dr. Chopra reported having no financial disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
AT ASDS 2022
First-in-class device for facial wrinkles, tightening hits the market
DENVER – .
“It’s early yet, but I have treated dozens of patients with this device, and they have been happy with the results,” Mathew M. Avram, MD, JD, said at the annual meeting of the American Society for Dermatologic Surgery. “This is a new technique that offers the ability to remove a significant amount of damaged, lax skin without concern for scarring,” he said.
A brainchild of dermatologists and plastic surgeons at Massachusetts General Hospital, Boston, the first-in-class device is cleared by the Food and Drug Administration for the treatment of moderate and severe wrinkles in the mid and lower face in adults aged 22 years or older with Fitzpatrick skin types I-IV. It features a proprietary needle design that makes a series of high throughput microexcisions in epidermal and dermal tissue, with minimal downtime and without using thermal energy.
“It doesn’t do anything equivalent to a facelift, but the concept is a facelift by thousands of micro-punch excisions,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital. “Rather than pulling up the skin and lifting it and cutting the excess skin like we do with a facelift, we are creating thousands of smaller-scale tissue removals with immediate closures to do the same thing. The micro-cores are about the size of a 22-gauge needle and there is no scarring due to the small size of these tissue extractions.”
The device features needle cartridges capable of excising up to 24,000 cores per treatment. According to data from Cytrellis, the manufacturer, the equivalent of about 2 inches of skin can be removed during the procedure, which typically takes fewer than 30 minutes to perform. “There is no heat whatsoever,” Dr. Avram said. “In my experience, it especially helps with jawline definition, the lower medial cheek excess skin, and accordion lines in that area.”
In a pivotal trial of the device, 51 patients with mid to lower face wrinkles (moderately deep or deep wrinkles with well-defined edges) were treated 2-3 times with 7%-8% skin removal and up to a 5-mm needle coring depth). The investigators found that 40% of study participants achieved an improvement of 2 grades on the Lemperle Wrinkle Severity Scale and that the rate of overall satisfaction (slightly, somewhat, and extremely satisfied) was 86%.
In addition, 90% showed improvement of treated sites on the Global Aesthetic Improvement Scale, and 70% were comfortable enough to go out in public or return to work 3 days after treatment. Common side effects that can occur immediately post treatment include redness, swelling, and pinpoint bleeding, which typically clear in a few days.
Dr. Avram, immediate past president of the ASDS, has posted videos to his Instagram feed that show him treating patients with the Ellacor device and he admits that the procedure looks painful. “There are all these tear emojis and people cursing me out,” he said, referring to responses from his Instagram followers.
Proper local anesthesia prior to treatment is key. “I perform nerve blocks and infiltrate the skin,” he said. “You have to cover the whole treatment area. If you don’t, then it’s going to hurt. The average pain score is 1.9 out of 10. The highest pain score I’ve gotten from a patient is a 3 out of 10.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton, and has ownership and/or shareholder interest in Cytrellis.
DENVER – .
“It’s early yet, but I have treated dozens of patients with this device, and they have been happy with the results,” Mathew M. Avram, MD, JD, said at the annual meeting of the American Society for Dermatologic Surgery. “This is a new technique that offers the ability to remove a significant amount of damaged, lax skin without concern for scarring,” he said.
A brainchild of dermatologists and plastic surgeons at Massachusetts General Hospital, Boston, the first-in-class device is cleared by the Food and Drug Administration for the treatment of moderate and severe wrinkles in the mid and lower face in adults aged 22 years or older with Fitzpatrick skin types I-IV. It features a proprietary needle design that makes a series of high throughput microexcisions in epidermal and dermal tissue, with minimal downtime and without using thermal energy.
“It doesn’t do anything equivalent to a facelift, but the concept is a facelift by thousands of micro-punch excisions,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital. “Rather than pulling up the skin and lifting it and cutting the excess skin like we do with a facelift, we are creating thousands of smaller-scale tissue removals with immediate closures to do the same thing. The micro-cores are about the size of a 22-gauge needle and there is no scarring due to the small size of these tissue extractions.”
The device features needle cartridges capable of excising up to 24,000 cores per treatment. According to data from Cytrellis, the manufacturer, the equivalent of about 2 inches of skin can be removed during the procedure, which typically takes fewer than 30 minutes to perform. “There is no heat whatsoever,” Dr. Avram said. “In my experience, it especially helps with jawline definition, the lower medial cheek excess skin, and accordion lines in that area.”
In a pivotal trial of the device, 51 patients with mid to lower face wrinkles (moderately deep or deep wrinkles with well-defined edges) were treated 2-3 times with 7%-8% skin removal and up to a 5-mm needle coring depth). The investigators found that 40% of study participants achieved an improvement of 2 grades on the Lemperle Wrinkle Severity Scale and that the rate of overall satisfaction (slightly, somewhat, and extremely satisfied) was 86%.
In addition, 90% showed improvement of treated sites on the Global Aesthetic Improvement Scale, and 70% were comfortable enough to go out in public or return to work 3 days after treatment. Common side effects that can occur immediately post treatment include redness, swelling, and pinpoint bleeding, which typically clear in a few days.
Dr. Avram, immediate past president of the ASDS, has posted videos to his Instagram feed that show him treating patients with the Ellacor device and he admits that the procedure looks painful. “There are all these tear emojis and people cursing me out,” he said, referring to responses from his Instagram followers.
Proper local anesthesia prior to treatment is key. “I perform nerve blocks and infiltrate the skin,” he said. “You have to cover the whole treatment area. If you don’t, then it’s going to hurt. The average pain score is 1.9 out of 10. The highest pain score I’ve gotten from a patient is a 3 out of 10.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton, and has ownership and/or shareholder interest in Cytrellis.
DENVER – .
“It’s early yet, but I have treated dozens of patients with this device, and they have been happy with the results,” Mathew M. Avram, MD, JD, said at the annual meeting of the American Society for Dermatologic Surgery. “This is a new technique that offers the ability to remove a significant amount of damaged, lax skin without concern for scarring,” he said.
A brainchild of dermatologists and plastic surgeons at Massachusetts General Hospital, Boston, the first-in-class device is cleared by the Food and Drug Administration for the treatment of moderate and severe wrinkles in the mid and lower face in adults aged 22 years or older with Fitzpatrick skin types I-IV. It features a proprietary needle design that makes a series of high throughput microexcisions in epidermal and dermal tissue, with minimal downtime and without using thermal energy.
“It doesn’t do anything equivalent to a facelift, but the concept is a facelift by thousands of micro-punch excisions,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital. “Rather than pulling up the skin and lifting it and cutting the excess skin like we do with a facelift, we are creating thousands of smaller-scale tissue removals with immediate closures to do the same thing. The micro-cores are about the size of a 22-gauge needle and there is no scarring due to the small size of these tissue extractions.”
The device features needle cartridges capable of excising up to 24,000 cores per treatment. According to data from Cytrellis, the manufacturer, the equivalent of about 2 inches of skin can be removed during the procedure, which typically takes fewer than 30 minutes to perform. “There is no heat whatsoever,” Dr. Avram said. “In my experience, it especially helps with jawline definition, the lower medial cheek excess skin, and accordion lines in that area.”
In a pivotal trial of the device, 51 patients with mid to lower face wrinkles (moderately deep or deep wrinkles with well-defined edges) were treated 2-3 times with 7%-8% skin removal and up to a 5-mm needle coring depth). The investigators found that 40% of study participants achieved an improvement of 2 grades on the Lemperle Wrinkle Severity Scale and that the rate of overall satisfaction (slightly, somewhat, and extremely satisfied) was 86%.
In addition, 90% showed improvement of treated sites on the Global Aesthetic Improvement Scale, and 70% were comfortable enough to go out in public or return to work 3 days after treatment. Common side effects that can occur immediately post treatment include redness, swelling, and pinpoint bleeding, which typically clear in a few days.
Dr. Avram, immediate past president of the ASDS, has posted videos to his Instagram feed that show him treating patients with the Ellacor device and he admits that the procedure looks painful. “There are all these tear emojis and people cursing me out,” he said, referring to responses from his Instagram followers.
Proper local anesthesia prior to treatment is key. “I perform nerve blocks and infiltrate the skin,” he said. “You have to cover the whole treatment area. If you don’t, then it’s going to hurt. The average pain score is 1.9 out of 10. The highest pain score I’ve gotten from a patient is a 3 out of 10.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton, and has ownership and/or shareholder interest in Cytrellis.
AT ASDS 2022
Dermatologists fear effects of Dobbs decision for patients on isotretinoin, methotrexate
More than 3 months after the Dobbs decision by the U.S. Supreme Court overturned Roe v. Wade and revoked the constitutional right to an abortion, Some have beefed up their already stringent instructions and lengthy conversations about avoiding pregnancy while on the medication.
The major fear is that a patient who is taking contraceptive precautions, in accordance with the isotretinoin risk-management program, iPLEDGE, but still becomes pregnant while on isotretinoin may find out about the pregnancy too late to undergo an abortion in her own state and may not be able to travel to another state – or the patient may live in a state where abortions are entirely prohibited and is unable to travel to another state.
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane; its former brand name was Accutane.
As of Oct. 7, a total of 14 states have banned most abortions, while 4 others have bans at 6, 15, 18, or 20 weeks. Attempts to restrict abortion on several other states are underway.
“To date, we don’t know of any specific effects of the Dobbs decision on isotretinoin prescribing, but with abortion access banned in many states, we anticipate that this could be a very real issue for individuals who accidentally become pregnant while taking isotretinoin,” said Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and chair of the American Academy of Dermatology Association’s iPLEDGE Workgroup.
The iPLEDGE REMS (Risk Evaluation and Mitigation Strategy) is the Food and Drug Administration–required safety program that is in place to manage the risk of isotretinoin teratogenicity and minimize fetal exposure. The work group meets with the FDA and isotretinoin manufacturers to keep the program safe and operating smoothly. The iPLEDGE workgroup has not yet issued any specific statements on the implications of the Dobbs decision on prescribing isotretinoin.
But work on the issue is ongoing by the American Academy of Dermatology. In a statement issued in September, Mark D. Kaufmann, MD, president of the AAD, said that the academy “is continuing to work with its Patient Guidance for State Regulations Regarding Reproductive Health Task Force to help dermatologists best navigate state laws about how care should be implemented for patients who are or might become pregnant, and have been exposed to teratogenic medications.”
The task force, working with the academy, is “in the process of developing resources to help members better assist patients and have a productive and caring dialogue with them,” according to the statement. No specific timeline was given for when those resources might be available.
Methotrexate prescriptions
Also of concern are prescriptions for methotrexate, which is prescribed for psoriasis, atopic dermatitis, and other skin diseases. Soon after the Dobbs decision was announced on June 24, pharmacies began to require pharmacists in states that banned abortions to verify that a prescription for methotrexate was not intended for an abortion, since methotrexate is used in combination with misoprostol for termination of an early pregnancy.
The action was taken, spokespersons for several major pharmacies said, to comply with state laws. According to Kara Page, a CVS spokesperson: “Pharmacists are caught in the middle on this issue.” Laws in some states, she told this news organization, “restrict the dispensing of medications for the purpose of inducing an abortion. These laws, some of which include criminal penalties, have forced us to require pharmacists in these states to validate that the intended indication is not to terminate a pregnancy before they can fill a prescription for methotrexate.”
“New laws in various states require additional steps for dispensing certain prescriptions and apply to all pharmacies, including Walgreens,” Fraser Engerman, a spokesperson for Walgreens, told this news organization. “In these states, our pharmacists work closely with prescribers as needed, to fill lawful, clinically appropriate prescriptions. We provide ongoing training and information to help our pharmacists understand the latest requirements in their area, and with these supports, the expectation is they are empowered to fill these prescriptions.”
The iPLEDGE program has numerous requirements before a patient can begin isotretinoin treatment. Patients capable of becoming pregnant must agree to use two effective forms of birth control during the entire treatment period, which typically lasts 4 or 5 months, as well as 1 month before and 1 month after treatment, or commit to total abstinence during that time.
Perspective: A Georgia dermatologist
Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, who sees patients regularly, practices in Georgia, where abortion is now banned at about 6 weeks of pregnancy. Dr. Yeung worries that some dermatologists in Georgia and elsewhere may not even want to take the risk of prescribing isotretinoin, although the results in treating resistant acne are well documented.
That isn’t his only concern. “Some may not want to prescribe it to a patient who reports they are abstinent and instead require them to go on two forms [of contraception].” Or some women who are not sexually active with anyone who can get them pregnant may also be asked to go on contraception, he said. Abstinence is an alternative option in iPLEDGE.
In the past, he said, well before the Dobbs decision, some doctors have argued that iPLEDGE should not include abstinence as an option. That 2020 report was challenged by others who pointed out that removing the abstinence option would pose ethical issues and may disproportionately affect minorities and others.
Before the Dobbs decision, Dr. Yeung noted, dermatologists prescribing isotretinoin focused on pregnancy prevention but knew that if pregnancy accidentally occurred, abortion was available as an option. “The reality after the decision is, it may or may not be available to all our patients.”
Of the 14 states banning most abortions, 10 are clustered within the South and Southeast. A woman living in Arkansas, which bans most abortions, for example, is surrounded by 6 other states that do the same.
Perspective: An Arizona dermatologist
Christina Kranc, MD, is a general dermatologist in Phoenix and Scottsdale. Arizona now bans most abortions. However, this has not changed her practice much when prescribing isotretinoin, she told this news organization, because when selecting appropriate candidates for the medication, she is strict on the contraceptive requirement, and only very rarely agrees to a patient relying on abstinence.
And if a patient capable of becoming pregnant was only having sex with another patient capable of becoming pregnant? Dr. Kranc said she would still require contraception unless it was impossible for pregnancy to occur.
Among the many scenarios a dermatologist might have to consider are a lesbian cisgender woman who is having, or has only had, sexual activity with another cisgender women.
Perspective: A Connecticut dermatologist
The concern is not only about isotretinoin but all teratogenic drugs, according to Jane M. Grant-Kels, MD, vice chair of dermatology and professor of dermatology, pathology, and pediatrics at the University of Connecticut, Farmington. She often prescribes methotrexate, which is also teratogenic.
Her advice for colleagues: “Whether you believe in abortion or not is irrelevant; it’s something you discuss with your patients.” She, too, fears that doctors in states banning abortions will stop prescribing these medications, “and that is very sad.”
For those practicing in states limiting or banning abortions, Dr. Grant-Kels said, “They need to have an even longer discussion with their patients about how serious this is.” Those doctors need to talk about not only two or three types of birth control, but also discuss with the patient about the potential need for travel, should pregnancy occur and abortion be the chosen option.
Although the newer biologics are an option for psoriasis, they are expensive. And, she said, many insurers require a step-therapy approach, and “want you to start with cheaper medications,” such as methotrexate. As a result, “in some states you won’t have access to the targeted therapies unless a patient fails something like methotrexate.”
Dr. Grant-Kels worries in particular about low-income women who may not have the means to travel to get an abortion.
Need for EC education
In a recent survey of 57 pediatric dermatologists who prescribe isotretinoin, only a third said they felt confident in their understanding of emergency contraception.
The authors of the study noted that the most common reasons for pregnancies during isotretinoin therapy reported to the FDA from 2011 to 2017 “included ineffective or inconsistent use” of contraceptives and “unsuccessful abstinence,” and recommended that physicians who prescribe isotretinoin update and increase their understanding of emergency contraception.
Dr. Yeung, Dr. Kranc, Dr. Grant-Kels, and Dr. Frieden reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than 3 months after the Dobbs decision by the U.S. Supreme Court overturned Roe v. Wade and revoked the constitutional right to an abortion, Some have beefed up their already stringent instructions and lengthy conversations about avoiding pregnancy while on the medication.
The major fear is that a patient who is taking contraceptive precautions, in accordance with the isotretinoin risk-management program, iPLEDGE, but still becomes pregnant while on isotretinoin may find out about the pregnancy too late to undergo an abortion in her own state and may not be able to travel to another state – or the patient may live in a state where abortions are entirely prohibited and is unable to travel to another state.
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane; its former brand name was Accutane.
As of Oct. 7, a total of 14 states have banned most abortions, while 4 others have bans at 6, 15, 18, or 20 weeks. Attempts to restrict abortion on several other states are underway.
“To date, we don’t know of any specific effects of the Dobbs decision on isotretinoin prescribing, but with abortion access banned in many states, we anticipate that this could be a very real issue for individuals who accidentally become pregnant while taking isotretinoin,” said Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and chair of the American Academy of Dermatology Association’s iPLEDGE Workgroup.
The iPLEDGE REMS (Risk Evaluation and Mitigation Strategy) is the Food and Drug Administration–required safety program that is in place to manage the risk of isotretinoin teratogenicity and minimize fetal exposure. The work group meets with the FDA and isotretinoin manufacturers to keep the program safe and operating smoothly. The iPLEDGE workgroup has not yet issued any specific statements on the implications of the Dobbs decision on prescribing isotretinoin.
But work on the issue is ongoing by the American Academy of Dermatology. In a statement issued in September, Mark D. Kaufmann, MD, president of the AAD, said that the academy “is continuing to work with its Patient Guidance for State Regulations Regarding Reproductive Health Task Force to help dermatologists best navigate state laws about how care should be implemented for patients who are or might become pregnant, and have been exposed to teratogenic medications.”
The task force, working with the academy, is “in the process of developing resources to help members better assist patients and have a productive and caring dialogue with them,” according to the statement. No specific timeline was given for when those resources might be available.
Methotrexate prescriptions
Also of concern are prescriptions for methotrexate, which is prescribed for psoriasis, atopic dermatitis, and other skin diseases. Soon after the Dobbs decision was announced on June 24, pharmacies began to require pharmacists in states that banned abortions to verify that a prescription for methotrexate was not intended for an abortion, since methotrexate is used in combination with misoprostol for termination of an early pregnancy.
The action was taken, spokespersons for several major pharmacies said, to comply with state laws. According to Kara Page, a CVS spokesperson: “Pharmacists are caught in the middle on this issue.” Laws in some states, she told this news organization, “restrict the dispensing of medications for the purpose of inducing an abortion. These laws, some of which include criminal penalties, have forced us to require pharmacists in these states to validate that the intended indication is not to terminate a pregnancy before they can fill a prescription for methotrexate.”
“New laws in various states require additional steps for dispensing certain prescriptions and apply to all pharmacies, including Walgreens,” Fraser Engerman, a spokesperson for Walgreens, told this news organization. “In these states, our pharmacists work closely with prescribers as needed, to fill lawful, clinically appropriate prescriptions. We provide ongoing training and information to help our pharmacists understand the latest requirements in their area, and with these supports, the expectation is they are empowered to fill these prescriptions.”
The iPLEDGE program has numerous requirements before a patient can begin isotretinoin treatment. Patients capable of becoming pregnant must agree to use two effective forms of birth control during the entire treatment period, which typically lasts 4 or 5 months, as well as 1 month before and 1 month after treatment, or commit to total abstinence during that time.
Perspective: A Georgia dermatologist
Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, who sees patients regularly, practices in Georgia, where abortion is now banned at about 6 weeks of pregnancy. Dr. Yeung worries that some dermatologists in Georgia and elsewhere may not even want to take the risk of prescribing isotretinoin, although the results in treating resistant acne are well documented.
That isn’t his only concern. “Some may not want to prescribe it to a patient who reports they are abstinent and instead require them to go on two forms [of contraception].” Or some women who are not sexually active with anyone who can get them pregnant may also be asked to go on contraception, he said. Abstinence is an alternative option in iPLEDGE.
In the past, he said, well before the Dobbs decision, some doctors have argued that iPLEDGE should not include abstinence as an option. That 2020 report was challenged by others who pointed out that removing the abstinence option would pose ethical issues and may disproportionately affect minorities and others.
Before the Dobbs decision, Dr. Yeung noted, dermatologists prescribing isotretinoin focused on pregnancy prevention but knew that if pregnancy accidentally occurred, abortion was available as an option. “The reality after the decision is, it may or may not be available to all our patients.”
Of the 14 states banning most abortions, 10 are clustered within the South and Southeast. A woman living in Arkansas, which bans most abortions, for example, is surrounded by 6 other states that do the same.
Perspective: An Arizona dermatologist
Christina Kranc, MD, is a general dermatologist in Phoenix and Scottsdale. Arizona now bans most abortions. However, this has not changed her practice much when prescribing isotretinoin, she told this news organization, because when selecting appropriate candidates for the medication, she is strict on the contraceptive requirement, and only very rarely agrees to a patient relying on abstinence.
And if a patient capable of becoming pregnant was only having sex with another patient capable of becoming pregnant? Dr. Kranc said she would still require contraception unless it was impossible for pregnancy to occur.
Among the many scenarios a dermatologist might have to consider are a lesbian cisgender woman who is having, or has only had, sexual activity with another cisgender women.
Perspective: A Connecticut dermatologist
The concern is not only about isotretinoin but all teratogenic drugs, according to Jane M. Grant-Kels, MD, vice chair of dermatology and professor of dermatology, pathology, and pediatrics at the University of Connecticut, Farmington. She often prescribes methotrexate, which is also teratogenic.
Her advice for colleagues: “Whether you believe in abortion or not is irrelevant; it’s something you discuss with your patients.” She, too, fears that doctors in states banning abortions will stop prescribing these medications, “and that is very sad.”
For those practicing in states limiting or banning abortions, Dr. Grant-Kels said, “They need to have an even longer discussion with their patients about how serious this is.” Those doctors need to talk about not only two or three types of birth control, but also discuss with the patient about the potential need for travel, should pregnancy occur and abortion be the chosen option.
Although the newer biologics are an option for psoriasis, they are expensive. And, she said, many insurers require a step-therapy approach, and “want you to start with cheaper medications,” such as methotrexate. As a result, “in some states you won’t have access to the targeted therapies unless a patient fails something like methotrexate.”
Dr. Grant-Kels worries in particular about low-income women who may not have the means to travel to get an abortion.
Need for EC education
In a recent survey of 57 pediatric dermatologists who prescribe isotretinoin, only a third said they felt confident in their understanding of emergency contraception.
The authors of the study noted that the most common reasons for pregnancies during isotretinoin therapy reported to the FDA from 2011 to 2017 “included ineffective or inconsistent use” of contraceptives and “unsuccessful abstinence,” and recommended that physicians who prescribe isotretinoin update and increase their understanding of emergency contraception.
Dr. Yeung, Dr. Kranc, Dr. Grant-Kels, and Dr. Frieden reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than 3 months after the Dobbs decision by the U.S. Supreme Court overturned Roe v. Wade and revoked the constitutional right to an abortion, Some have beefed up their already stringent instructions and lengthy conversations about avoiding pregnancy while on the medication.
The major fear is that a patient who is taking contraceptive precautions, in accordance with the isotretinoin risk-management program, iPLEDGE, but still becomes pregnant while on isotretinoin may find out about the pregnancy too late to undergo an abortion in her own state and may not be able to travel to another state – or the patient may live in a state where abortions are entirely prohibited and is unable to travel to another state.
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane; its former brand name was Accutane.
As of Oct. 7, a total of 14 states have banned most abortions, while 4 others have bans at 6, 15, 18, or 20 weeks. Attempts to restrict abortion on several other states are underway.
“To date, we don’t know of any specific effects of the Dobbs decision on isotretinoin prescribing, but with abortion access banned in many states, we anticipate that this could be a very real issue for individuals who accidentally become pregnant while taking isotretinoin,” said Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and chair of the American Academy of Dermatology Association’s iPLEDGE Workgroup.
The iPLEDGE REMS (Risk Evaluation and Mitigation Strategy) is the Food and Drug Administration–required safety program that is in place to manage the risk of isotretinoin teratogenicity and minimize fetal exposure. The work group meets with the FDA and isotretinoin manufacturers to keep the program safe and operating smoothly. The iPLEDGE workgroup has not yet issued any specific statements on the implications of the Dobbs decision on prescribing isotretinoin.
But work on the issue is ongoing by the American Academy of Dermatology. In a statement issued in September, Mark D. Kaufmann, MD, president of the AAD, said that the academy “is continuing to work with its Patient Guidance for State Regulations Regarding Reproductive Health Task Force to help dermatologists best navigate state laws about how care should be implemented for patients who are or might become pregnant, and have been exposed to teratogenic medications.”
The task force, working with the academy, is “in the process of developing resources to help members better assist patients and have a productive and caring dialogue with them,” according to the statement. No specific timeline was given for when those resources might be available.
Methotrexate prescriptions
Also of concern are prescriptions for methotrexate, which is prescribed for psoriasis, atopic dermatitis, and other skin diseases. Soon after the Dobbs decision was announced on June 24, pharmacies began to require pharmacists in states that banned abortions to verify that a prescription for methotrexate was not intended for an abortion, since methotrexate is used in combination with misoprostol for termination of an early pregnancy.
The action was taken, spokespersons for several major pharmacies said, to comply with state laws. According to Kara Page, a CVS spokesperson: “Pharmacists are caught in the middle on this issue.” Laws in some states, she told this news organization, “restrict the dispensing of medications for the purpose of inducing an abortion. These laws, some of which include criminal penalties, have forced us to require pharmacists in these states to validate that the intended indication is not to terminate a pregnancy before they can fill a prescription for methotrexate.”
“New laws in various states require additional steps for dispensing certain prescriptions and apply to all pharmacies, including Walgreens,” Fraser Engerman, a spokesperson for Walgreens, told this news organization. “In these states, our pharmacists work closely with prescribers as needed, to fill lawful, clinically appropriate prescriptions. We provide ongoing training and information to help our pharmacists understand the latest requirements in their area, and with these supports, the expectation is they are empowered to fill these prescriptions.”
The iPLEDGE program has numerous requirements before a patient can begin isotretinoin treatment. Patients capable of becoming pregnant must agree to use two effective forms of birth control during the entire treatment period, which typically lasts 4 or 5 months, as well as 1 month before and 1 month after treatment, or commit to total abstinence during that time.
Perspective: A Georgia dermatologist
Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, who sees patients regularly, practices in Georgia, where abortion is now banned at about 6 weeks of pregnancy. Dr. Yeung worries that some dermatologists in Georgia and elsewhere may not even want to take the risk of prescribing isotretinoin, although the results in treating resistant acne are well documented.
That isn’t his only concern. “Some may not want to prescribe it to a patient who reports they are abstinent and instead require them to go on two forms [of contraception].” Or some women who are not sexually active with anyone who can get them pregnant may also be asked to go on contraception, he said. Abstinence is an alternative option in iPLEDGE.
In the past, he said, well before the Dobbs decision, some doctors have argued that iPLEDGE should not include abstinence as an option. That 2020 report was challenged by others who pointed out that removing the abstinence option would pose ethical issues and may disproportionately affect minorities and others.
Before the Dobbs decision, Dr. Yeung noted, dermatologists prescribing isotretinoin focused on pregnancy prevention but knew that if pregnancy accidentally occurred, abortion was available as an option. “The reality after the decision is, it may or may not be available to all our patients.”
Of the 14 states banning most abortions, 10 are clustered within the South and Southeast. A woman living in Arkansas, which bans most abortions, for example, is surrounded by 6 other states that do the same.
Perspective: An Arizona dermatologist
Christina Kranc, MD, is a general dermatologist in Phoenix and Scottsdale. Arizona now bans most abortions. However, this has not changed her practice much when prescribing isotretinoin, she told this news organization, because when selecting appropriate candidates for the medication, she is strict on the contraceptive requirement, and only very rarely agrees to a patient relying on abstinence.
And if a patient capable of becoming pregnant was only having sex with another patient capable of becoming pregnant? Dr. Kranc said she would still require contraception unless it was impossible for pregnancy to occur.
Among the many scenarios a dermatologist might have to consider are a lesbian cisgender woman who is having, or has only had, sexual activity with another cisgender women.
Perspective: A Connecticut dermatologist
The concern is not only about isotretinoin but all teratogenic drugs, according to Jane M. Grant-Kels, MD, vice chair of dermatology and professor of dermatology, pathology, and pediatrics at the University of Connecticut, Farmington. She often prescribes methotrexate, which is also teratogenic.
Her advice for colleagues: “Whether you believe in abortion or not is irrelevant; it’s something you discuss with your patients.” She, too, fears that doctors in states banning abortions will stop prescribing these medications, “and that is very sad.”
For those practicing in states limiting or banning abortions, Dr. Grant-Kels said, “They need to have an even longer discussion with their patients about how serious this is.” Those doctors need to talk about not only two or three types of birth control, but also discuss with the patient about the potential need for travel, should pregnancy occur and abortion be the chosen option.
Although the newer biologics are an option for psoriasis, they are expensive. And, she said, many insurers require a step-therapy approach, and “want you to start with cheaper medications,” such as methotrexate. As a result, “in some states you won’t have access to the targeted therapies unless a patient fails something like methotrexate.”
Dr. Grant-Kels worries in particular about low-income women who may not have the means to travel to get an abortion.
Need for EC education
In a recent survey of 57 pediatric dermatologists who prescribe isotretinoin, only a third said they felt confident in their understanding of emergency contraception.
The authors of the study noted that the most common reasons for pregnancies during isotretinoin therapy reported to the FDA from 2011 to 2017 “included ineffective or inconsistent use” of contraceptives and “unsuccessful abstinence,” and recommended that physicians who prescribe isotretinoin update and increase their understanding of emergency contraception.
Dr. Yeung, Dr. Kranc, Dr. Grant-Kels, and Dr. Frieden reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Liquid injectable silicone safe for acne scarring in dark-skinned patients, study finds
DENVER – Highly , results from a recent study showed.
“Acne is pervasive, and acne scarring disproportionately affects darker skin types,” lead study author Nicole Salame, MD, told this news organization in advance of the annual meeting of the American Society for Dermatologic Surgery, where she presented the results of the study. “Treatment of acne scarring in darker skin is also particularly challenging since resurfacing can be problematic. Numerous treatment options exist but vary in effectiveness, sustainability, and side-effect profile, especially for patients with darker skin.”
Highly purified liquid injectable silicone (also known as LIS) is approved by the Food and Drug Administration for treating intraocular tamponade of retinal detachment, and has been used off label for skin augmentation. A 2005 study of LIS for five patients with acne scarring, with up to 30 years of follow-up, showed efficacy and preservation of product without complications for depressed, broad-based acne scars .
“Use of LIS as a permanent treatment for acne scarring in darker skin types has yet to be evaluated,” said Dr. Salame, a 4th-year dermatology resident at Emory University, Atlanta. “Our study is the first to retrospectively evaluate the safety and efficacy of highly purified LIS for the treatment of acne scars in all skin types.”
Dr. Salame and coauthor Harold J. Brody, MD, evaluated the charts of 96 patients with a mean age of 51 years who received highly purified LIS for the treatment of acne scars at Dr. Brody’s Atlanta-based private dermatology practice between July 2010 and March 2021. Of the 96 patients, 31 had darker skin types (20 were Fitzpatrick skin type IV and 11 were Fitzpatrick skin type V). Dr. Brody performed all treatments: a total of 206 in the 96 patients.
The average time of follow-up was 6.31 years; 19 patients had a follow-up of 1-3 years, 25 had a follow-up of 3-5 years, and 52 had a follow-up of greater than 5 years. The researchers did not observe any complications along the course of the patients’ treatments, and no patients reported complications or dissatisfaction with treatment.
“Among the most impressive findings of our study was the permanence of effectiveness of LIS for acne scarring in patients who had treatment over a decade before,” Dr. Salame said. “Our longest follow up was 12 years. These patients continued to show improvement in their acne scarring years after treatment with LIS, even as they lost collagen and volume in their face with advancing age.”
In addition, she said, none of the patients experienced complications of granulomatous reactions, migration, or extrusion of product, which were previously documented with the use of macrodroplet injectable silicone techniques. “This is likely due to the consistent use of the microdroplet injection technique in our study – less than 0.01 cc per injection at minimum 6- to 8-week intervals or more,” Dr. Salame said.
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment on the study, said that the findings “show safety and durability of highly purified microdroplet liquid silicone to treat acne scars. The numbers of patients reviewed are small and selective (one highly skilled dermatologist), but with the right material (highly purified liquid silicone) and in a qualified and experienced physician’s hand, this treatment seems like a great option.”
Dr. Salame acknowledged certain limitations of the study, including its single-center, retrospective design. “Future prospective studies with larger patient populations of all skin types recruited from multiple centers may be needed,” she said.
The researchers reported having no relevant conflicts of interest or funding sources to disclose. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
DENVER – Highly , results from a recent study showed.
“Acne is pervasive, and acne scarring disproportionately affects darker skin types,” lead study author Nicole Salame, MD, told this news organization in advance of the annual meeting of the American Society for Dermatologic Surgery, where she presented the results of the study. “Treatment of acne scarring in darker skin is also particularly challenging since resurfacing can be problematic. Numerous treatment options exist but vary in effectiveness, sustainability, and side-effect profile, especially for patients with darker skin.”
Highly purified liquid injectable silicone (also known as LIS) is approved by the Food and Drug Administration for treating intraocular tamponade of retinal detachment, and has been used off label for skin augmentation. A 2005 study of LIS for five patients with acne scarring, with up to 30 years of follow-up, showed efficacy and preservation of product without complications for depressed, broad-based acne scars .
“Use of LIS as a permanent treatment for acne scarring in darker skin types has yet to be evaluated,” said Dr. Salame, a 4th-year dermatology resident at Emory University, Atlanta. “Our study is the first to retrospectively evaluate the safety and efficacy of highly purified LIS for the treatment of acne scars in all skin types.”
Dr. Salame and coauthor Harold J. Brody, MD, evaluated the charts of 96 patients with a mean age of 51 years who received highly purified LIS for the treatment of acne scars at Dr. Brody’s Atlanta-based private dermatology practice between July 2010 and March 2021. Of the 96 patients, 31 had darker skin types (20 were Fitzpatrick skin type IV and 11 were Fitzpatrick skin type V). Dr. Brody performed all treatments: a total of 206 in the 96 patients.
The average time of follow-up was 6.31 years; 19 patients had a follow-up of 1-3 years, 25 had a follow-up of 3-5 years, and 52 had a follow-up of greater than 5 years. The researchers did not observe any complications along the course of the patients’ treatments, and no patients reported complications or dissatisfaction with treatment.
“Among the most impressive findings of our study was the permanence of effectiveness of LIS for acne scarring in patients who had treatment over a decade before,” Dr. Salame said. “Our longest follow up was 12 years. These patients continued to show improvement in their acne scarring years after treatment with LIS, even as they lost collagen and volume in their face with advancing age.”
In addition, she said, none of the patients experienced complications of granulomatous reactions, migration, or extrusion of product, which were previously documented with the use of macrodroplet injectable silicone techniques. “This is likely due to the consistent use of the microdroplet injection technique in our study – less than 0.01 cc per injection at minimum 6- to 8-week intervals or more,” Dr. Salame said.
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment on the study, said that the findings “show safety and durability of highly purified microdroplet liquid silicone to treat acne scars. The numbers of patients reviewed are small and selective (one highly skilled dermatologist), but with the right material (highly purified liquid silicone) and in a qualified and experienced physician’s hand, this treatment seems like a great option.”
Dr. Salame acknowledged certain limitations of the study, including its single-center, retrospective design. “Future prospective studies with larger patient populations of all skin types recruited from multiple centers may be needed,” she said.
The researchers reported having no relevant conflicts of interest or funding sources to disclose. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
DENVER – Highly , results from a recent study showed.
“Acne is pervasive, and acne scarring disproportionately affects darker skin types,” lead study author Nicole Salame, MD, told this news organization in advance of the annual meeting of the American Society for Dermatologic Surgery, where she presented the results of the study. “Treatment of acne scarring in darker skin is also particularly challenging since resurfacing can be problematic. Numerous treatment options exist but vary in effectiveness, sustainability, and side-effect profile, especially for patients with darker skin.”
Highly purified liquid injectable silicone (also known as LIS) is approved by the Food and Drug Administration for treating intraocular tamponade of retinal detachment, and has been used off label for skin augmentation. A 2005 study of LIS for five patients with acne scarring, with up to 30 years of follow-up, showed efficacy and preservation of product without complications for depressed, broad-based acne scars .
“Use of LIS as a permanent treatment for acne scarring in darker skin types has yet to be evaluated,” said Dr. Salame, a 4th-year dermatology resident at Emory University, Atlanta. “Our study is the first to retrospectively evaluate the safety and efficacy of highly purified LIS for the treatment of acne scars in all skin types.”
Dr. Salame and coauthor Harold J. Brody, MD, evaluated the charts of 96 patients with a mean age of 51 years who received highly purified LIS for the treatment of acne scars at Dr. Brody’s Atlanta-based private dermatology practice between July 2010 and March 2021. Of the 96 patients, 31 had darker skin types (20 were Fitzpatrick skin type IV and 11 were Fitzpatrick skin type V). Dr. Brody performed all treatments: a total of 206 in the 96 patients.
The average time of follow-up was 6.31 years; 19 patients had a follow-up of 1-3 years, 25 had a follow-up of 3-5 years, and 52 had a follow-up of greater than 5 years. The researchers did not observe any complications along the course of the patients’ treatments, and no patients reported complications or dissatisfaction with treatment.
“Among the most impressive findings of our study was the permanence of effectiveness of LIS for acne scarring in patients who had treatment over a decade before,” Dr. Salame said. “Our longest follow up was 12 years. These patients continued to show improvement in their acne scarring years after treatment with LIS, even as they lost collagen and volume in their face with advancing age.”
In addition, she said, none of the patients experienced complications of granulomatous reactions, migration, or extrusion of product, which were previously documented with the use of macrodroplet injectable silicone techniques. “This is likely due to the consistent use of the microdroplet injection technique in our study – less than 0.01 cc per injection at minimum 6- to 8-week intervals or more,” Dr. Salame said.
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment on the study, said that the findings “show safety and durability of highly purified microdroplet liquid silicone to treat acne scars. The numbers of patients reviewed are small and selective (one highly skilled dermatologist), but with the right material (highly purified liquid silicone) and in a qualified and experienced physician’s hand, this treatment seems like a great option.”
Dr. Salame acknowledged certain limitations of the study, including its single-center, retrospective design. “Future prospective studies with larger patient populations of all skin types recruited from multiple centers may be needed,” she said.
The researchers reported having no relevant conflicts of interest or funding sources to disclose. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
AT ASDS 2022
Vedolizumab-Induced Acne Fulminans: An Uncommon and Severe Adverse Effect
To the Editor:
Vedolizumab is an innovative monoclonal antibody targeted against the α4β7 integrin that is approved for treatment of moderate to severe ulcerative colitis and Crohn disease refractory to standard treatment.1 Vedolizumab is thought to be gut specific, blocking integrins specific to T lymphocytes destined for the gastrointestinal tract and their interaction with endothelial cells, thereby modulating the adaptive immune system in the gut without systemic immunosuppression.2 It generally is well tolerated, and acne rarely has been reported as an adverse event.3,4 We present a case of acne fulminans without systemic symptoms (AF-WOSS) as a severe side effect of vedolizumab that responded very well to systemic steroids and oral isotretinoin in addition to the discontinuation of treatment.
A 46-year-old obese man presented to our dermatology clinic with a chief complaint of rapidly progressive tender skin lesions. The patient had a long-standing history of severe fistulating and stricturing Crohn disease status post–bowel resection with ileostomy and had recently started treatment with vedolizumab after failing treatment with infliximab, adalimumab, certolizumab pegol, ustekinumab, and methotrexate. Several weeks after beginning infusions of vedolizumab, the patient began to develop many erythematous papules and pustules on the face, chest (Figure 1), and buttocks that rapidly progressed into painful and coalescing nodules and cysts over the next several months. He was prescribed benzoyl peroxide wash 10% as well as several weeks of oral doxycycline 100 mg twice daily with no improvement. The patient denied any other new medications or triggers, fever, chills, bone pain, headache, fatigue, or myalgia. The skin involvement continued to worsen with successive vedolizumab infusions over a period of 8 weeks, which ultimately resulted in cessation of vedolizumab.
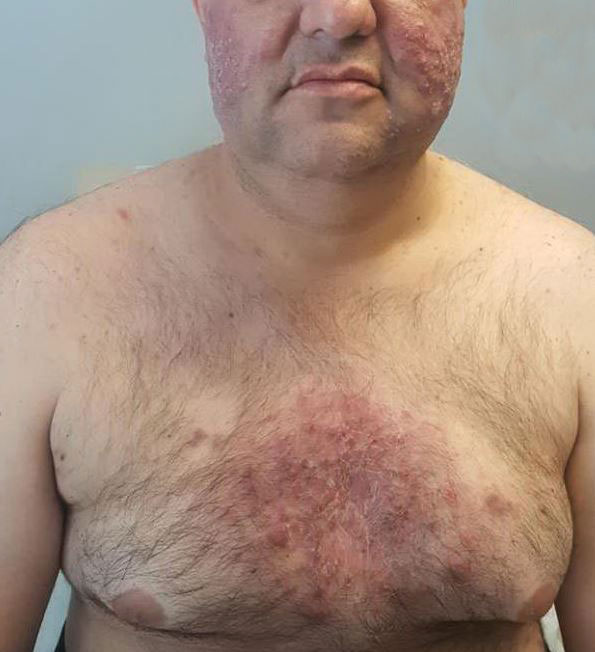
Physical examination revealed large, tender, pink, erythematous, and indurated plaques that were heavily studded with pink papules, pustules, and nodules on the cheeks (Figure 2), central chest, and buttocks. A punch biopsy of a pustule on the cheek showed ruptured suppurative folliculitis. The patient subsequently was diagnosed with AF-WOSS.
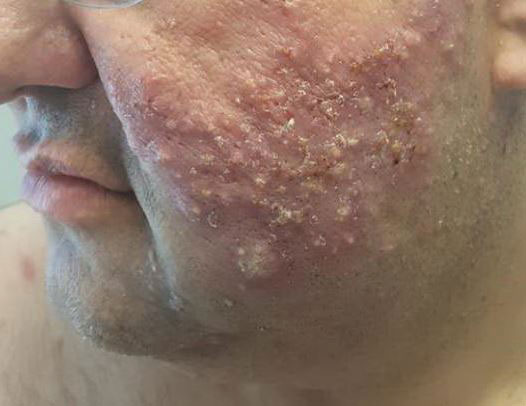
The patient then completed a 7-day course of sulfamethoxazole-trimethoprim followed by a 10-day course of amoxicillin-clavulanic acid, neither of which led to improvement of the lesions. He then was started on an oral prednisone taper (1 mg/kg starting dose) that ultimately totaled 14 weeks in length due to his frequent flares any time prednisone was decreased below 40 mg daily. After 3 weeks on the oral prednisone, the patient was started on 0.3 mg/kg of concomitant oral isotretinoin every other day, which slowly was increased as tolerated until he reached a goal dose of roughly 150 mg/kg, which resolved the acneform papules and pustules and allowed for successful tapering off the prednisone.
Many studies have been published regarding the safety and side-effect profile of vedolizumab, but most do not report acne as an adverse event.3-5 A German cohort study by Baumgart et al3 reported acne as a side effect in 15 of 212 (7.1%) patients but did not classify the severity. Another case report noted nodulocystic acne in a patient receiving vedolizumab for treatment of inflammatory bowel disease; however, this patient responded well to the use of a tetracycline antibiotic and was able to continue therapy with vedolizumab.5 Our patient demonstrated a severe and uncommon case of acne classified as AF-WOSS following initiation of therapy with vedolizumab, which required treatment with systemic steroids plus oral isotretinoin and resulted in cessation of vedolizumab.
As new therapies emerge, it is important to document new or severe adverse effects so providers can choose an appropriate therapy and adequately counsel patients regarding the side effects. Although vedolizumab was thought to have gut-specific action, there is new evidence to suggest that the principal ligand of the α4β7 integrin, mucosal addressin cell adhesion molecule-1, is not only expressed on gut endothelial cells but also on fibroblasts and melanomas, which may provide insight into the observed extraintestinal side effects of vedolizumab.6
- Smith MA, Mohammad RA. Vedolizumab: an α4β7 integrin inhibitor for inflammatory bowel diseases. Ann Pharmacother. 2014;48:1629-1635.
- Singh H, Grewal N, Arora E, et al. Vedolizumab: a novel anti-integrin drug for treatment of inflammatory bowel disease. J Nat Sci Bio Med. 2016;7:4-9.
- Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice: a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102.
- Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3-15.
- Gilhooley E, Doherty G, Lally A. Vedolizumab-induced acne in inflammatory bowel disease. Int J Dermatol. 2018;57:752-753.
- Leung E, Kanwar RK, Kanwar JR, et al. Mucosal vascular addressin cell adhesion molecule-1 is expressed outside the endothelial lineage on fibroblasts and melanoma cells. Immunol Cell Biol. 2003;81:320-327.
To the Editor:
Vedolizumab is an innovative monoclonal antibody targeted against the α4β7 integrin that is approved for treatment of moderate to severe ulcerative colitis and Crohn disease refractory to standard treatment.1 Vedolizumab is thought to be gut specific, blocking integrins specific to T lymphocytes destined for the gastrointestinal tract and their interaction with endothelial cells, thereby modulating the adaptive immune system in the gut without systemic immunosuppression.2 It generally is well tolerated, and acne rarely has been reported as an adverse event.3,4 We present a case of acne fulminans without systemic symptoms (AF-WOSS) as a severe side effect of vedolizumab that responded very well to systemic steroids and oral isotretinoin in addition to the discontinuation of treatment.
A 46-year-old obese man presented to our dermatology clinic with a chief complaint of rapidly progressive tender skin lesions. The patient had a long-standing history of severe fistulating and stricturing Crohn disease status post–bowel resection with ileostomy and had recently started treatment with vedolizumab after failing treatment with infliximab, adalimumab, certolizumab pegol, ustekinumab, and methotrexate. Several weeks after beginning infusions of vedolizumab, the patient began to develop many erythematous papules and pustules on the face, chest (Figure 1), and buttocks that rapidly progressed into painful and coalescing nodules and cysts over the next several months. He was prescribed benzoyl peroxide wash 10% as well as several weeks of oral doxycycline 100 mg twice daily with no improvement. The patient denied any other new medications or triggers, fever, chills, bone pain, headache, fatigue, or myalgia. The skin involvement continued to worsen with successive vedolizumab infusions over a period of 8 weeks, which ultimately resulted in cessation of vedolizumab.

Physical examination revealed large, tender, pink, erythematous, and indurated plaques that were heavily studded with pink papules, pustules, and nodules on the cheeks (Figure 2), central chest, and buttocks. A punch biopsy of a pustule on the cheek showed ruptured suppurative folliculitis. The patient subsequently was diagnosed with AF-WOSS.

The patient then completed a 7-day course of sulfamethoxazole-trimethoprim followed by a 10-day course of amoxicillin-clavulanic acid, neither of which led to improvement of the lesions. He then was started on an oral prednisone taper (1 mg/kg starting dose) that ultimately totaled 14 weeks in length due to his frequent flares any time prednisone was decreased below 40 mg daily. After 3 weeks on the oral prednisone, the patient was started on 0.3 mg/kg of concomitant oral isotretinoin every other day, which slowly was increased as tolerated until he reached a goal dose of roughly 150 mg/kg, which resolved the acneform papules and pustules and allowed for successful tapering off the prednisone.
Many studies have been published regarding the safety and side-effect profile of vedolizumab, but most do not report acne as an adverse event.3-5 A German cohort study by Baumgart et al3 reported acne as a side effect in 15 of 212 (7.1%) patients but did not classify the severity. Another case report noted nodulocystic acne in a patient receiving vedolizumab for treatment of inflammatory bowel disease; however, this patient responded well to the use of a tetracycline antibiotic and was able to continue therapy with vedolizumab.5 Our patient demonstrated a severe and uncommon case of acne classified as AF-WOSS following initiation of therapy with vedolizumab, which required treatment with systemic steroids plus oral isotretinoin and resulted in cessation of vedolizumab.
As new therapies emerge, it is important to document new or severe adverse effects so providers can choose an appropriate therapy and adequately counsel patients regarding the side effects. Although vedolizumab was thought to have gut-specific action, there is new evidence to suggest that the principal ligand of the α4β7 integrin, mucosal addressin cell adhesion molecule-1, is not only expressed on gut endothelial cells but also on fibroblasts and melanomas, which may provide insight into the observed extraintestinal side effects of vedolizumab.6
To the Editor:
Vedolizumab is an innovative monoclonal antibody targeted against the α4β7 integrin that is approved for treatment of moderate to severe ulcerative colitis and Crohn disease refractory to standard treatment.1 Vedolizumab is thought to be gut specific, blocking integrins specific to T lymphocytes destined for the gastrointestinal tract and their interaction with endothelial cells, thereby modulating the adaptive immune system in the gut without systemic immunosuppression.2 It generally is well tolerated, and acne rarely has been reported as an adverse event.3,4 We present a case of acne fulminans without systemic symptoms (AF-WOSS) as a severe side effect of vedolizumab that responded very well to systemic steroids and oral isotretinoin in addition to the discontinuation of treatment.
A 46-year-old obese man presented to our dermatology clinic with a chief complaint of rapidly progressive tender skin lesions. The patient had a long-standing history of severe fistulating and stricturing Crohn disease status post–bowel resection with ileostomy and had recently started treatment with vedolizumab after failing treatment with infliximab, adalimumab, certolizumab pegol, ustekinumab, and methotrexate. Several weeks after beginning infusions of vedolizumab, the patient began to develop many erythematous papules and pustules on the face, chest (Figure 1), and buttocks that rapidly progressed into painful and coalescing nodules and cysts over the next several months. He was prescribed benzoyl peroxide wash 10% as well as several weeks of oral doxycycline 100 mg twice daily with no improvement. The patient denied any other new medications or triggers, fever, chills, bone pain, headache, fatigue, or myalgia. The skin involvement continued to worsen with successive vedolizumab infusions over a period of 8 weeks, which ultimately resulted in cessation of vedolizumab.

Physical examination revealed large, tender, pink, erythematous, and indurated plaques that were heavily studded with pink papules, pustules, and nodules on the cheeks (Figure 2), central chest, and buttocks. A punch biopsy of a pustule on the cheek showed ruptured suppurative folliculitis. The patient subsequently was diagnosed with AF-WOSS.

The patient then completed a 7-day course of sulfamethoxazole-trimethoprim followed by a 10-day course of amoxicillin-clavulanic acid, neither of which led to improvement of the lesions. He then was started on an oral prednisone taper (1 mg/kg starting dose) that ultimately totaled 14 weeks in length due to his frequent flares any time prednisone was decreased below 40 mg daily. After 3 weeks on the oral prednisone, the patient was started on 0.3 mg/kg of concomitant oral isotretinoin every other day, which slowly was increased as tolerated until he reached a goal dose of roughly 150 mg/kg, which resolved the acneform papules and pustules and allowed for successful tapering off the prednisone.
Many studies have been published regarding the safety and side-effect profile of vedolizumab, but most do not report acne as an adverse event.3-5 A German cohort study by Baumgart et al3 reported acne as a side effect in 15 of 212 (7.1%) patients but did not classify the severity. Another case report noted nodulocystic acne in a patient receiving vedolizumab for treatment of inflammatory bowel disease; however, this patient responded well to the use of a tetracycline antibiotic and was able to continue therapy with vedolizumab.5 Our patient demonstrated a severe and uncommon case of acne classified as AF-WOSS following initiation of therapy with vedolizumab, which required treatment with systemic steroids plus oral isotretinoin and resulted in cessation of vedolizumab.
As new therapies emerge, it is important to document new or severe adverse effects so providers can choose an appropriate therapy and adequately counsel patients regarding the side effects. Although vedolizumab was thought to have gut-specific action, there is new evidence to suggest that the principal ligand of the α4β7 integrin, mucosal addressin cell adhesion molecule-1, is not only expressed on gut endothelial cells but also on fibroblasts and melanomas, which may provide insight into the observed extraintestinal side effects of vedolizumab.6
- Smith MA, Mohammad RA. Vedolizumab: an α4β7 integrin inhibitor for inflammatory bowel diseases. Ann Pharmacother. 2014;48:1629-1635.
- Singh H, Grewal N, Arora E, et al. Vedolizumab: a novel anti-integrin drug for treatment of inflammatory bowel disease. J Nat Sci Bio Med. 2016;7:4-9.
- Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice: a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102.
- Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3-15.
- Gilhooley E, Doherty G, Lally A. Vedolizumab-induced acne in inflammatory bowel disease. Int J Dermatol. 2018;57:752-753.
- Leung E, Kanwar RK, Kanwar JR, et al. Mucosal vascular addressin cell adhesion molecule-1 is expressed outside the endothelial lineage on fibroblasts and melanoma cells. Immunol Cell Biol. 2003;81:320-327.
- Smith MA, Mohammad RA. Vedolizumab: an α4β7 integrin inhibitor for inflammatory bowel diseases. Ann Pharmacother. 2014;48:1629-1635.
- Singh H, Grewal N, Arora E, et al. Vedolizumab: a novel anti-integrin drug for treatment of inflammatory bowel disease. J Nat Sci Bio Med. 2016;7:4-9.
- Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice: a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102.
- Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3-15.
- Gilhooley E, Doherty G, Lally A. Vedolizumab-induced acne in inflammatory bowel disease. Int J Dermatol. 2018;57:752-753.
- Leung E, Kanwar RK, Kanwar JR, et al. Mucosal vascular addressin cell adhesion molecule-1 is expressed outside the endothelial lineage on fibroblasts and melanoma cells. Immunol Cell Biol. 2003;81:320-327.
Practice Points
- Vedolizumab, a monoclonal antibody for the treatment of refractory inflammatory bowel disease, was found to cause acne fulminans without systemic symptoms.
- Vedolizumab previously was believed to be a gut-limited immune modulator.
- Off-target cutaneous effects may indicate wider expression of the target integrin of vedolizumab and should be recognized as the drug becomes more widely used.
Consider the mnemonic ‘CLEAR’ when counseling acne patients
to use when treating this group of patients.
During a presentation at Medscape Live’s annual Coastal Dermatology Symposium, Dr. Harper, who practices at Dermatology and Skin Care of Birmingham, Ala., elaborated on the mnemonic, as follows:
C: Communicate expectations. “I look right at the acne patient and say, ‘I know you don’t just want to be better; I know you want to be clear,’ ” she said at the meeting. “ ‘That’s my goal for you, too. That may take us more than one visit and more than one treatment, but I am on your team, and that’s what we’re shooting for.’ If you don’t communicate that, they’re going to think that their acne is not that important to you.”
L: Listen for clues to customize the patient’s treatment. “We’re quick to say, ‘my patients don’t do what I recommend,’ or ‘they didn’t do what the last doctor recommended,’ ” Dr. Harper said. “Sometimes that is true, but there may be a reason why. Maybe the medication was too expensive. Maybe it was bleaching their fabrics. Maybe the regimen was too complex. Listen for opportunities to make adjustments to get their acne closer to clear.”
E: Treat early to improve quality of life and to decrease the risk of scarring. “I have a laser in my practice that is good at treating acne scarring,” she said. “Do I ever look at my patient and say, ‘don’t worry about those scars; I can make them go away?’ No. I look at them and say, ‘we can maybe make this 40% better,’ something like that. We have to prevent acne scars, because we’re not good at treating them.”
A: Treat aggressively with more combination therapies, more hormonal therapies, more isotretinoin, and perhaps more prior authorizations. She characterized the effort to obtain a prior authorization as “our megaphone back to insurance companies that says, ‘we think it is worth taking the time to do this prior authorization because the acne patient will benefit.’ ”
R: Don’t resist isotretinoin. Dr. Harper, who began practicing dermatology more than 20 years ago, said that over time, she has gradually prescribed more isotretinoin for her patients with acne. “It’s not a first-line [treatment], but I’m not afraid of it. If I can’t get somebody clear on other oral or topical treatments, we are going to try isotretinoin.”
The goal of acne treatment, she added, is to affect four key aspects of pathogenesis: follicular epithelial hyperproliferation, inflammation, Cutibacterium acnes (C. acnes), and sebum. “That’s what we’re always shooting for,” she said.
Dr. Harper is a past president of the American Acne & Rosacea Society. She disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speaker’s bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
to use when treating this group of patients.
During a presentation at Medscape Live’s annual Coastal Dermatology Symposium, Dr. Harper, who practices at Dermatology and Skin Care of Birmingham, Ala., elaborated on the mnemonic, as follows:
C: Communicate expectations. “I look right at the acne patient and say, ‘I know you don’t just want to be better; I know you want to be clear,’ ” she said at the meeting. “ ‘That’s my goal for you, too. That may take us more than one visit and more than one treatment, but I am on your team, and that’s what we’re shooting for.’ If you don’t communicate that, they’re going to think that their acne is not that important to you.”
L: Listen for clues to customize the patient’s treatment. “We’re quick to say, ‘my patients don’t do what I recommend,’ or ‘they didn’t do what the last doctor recommended,’ ” Dr. Harper said. “Sometimes that is true, but there may be a reason why. Maybe the medication was too expensive. Maybe it was bleaching their fabrics. Maybe the regimen was too complex. Listen for opportunities to make adjustments to get their acne closer to clear.”
E: Treat early to improve quality of life and to decrease the risk of scarring. “I have a laser in my practice that is good at treating acne scarring,” she said. “Do I ever look at my patient and say, ‘don’t worry about those scars; I can make them go away?’ No. I look at them and say, ‘we can maybe make this 40% better,’ something like that. We have to prevent acne scars, because we’re not good at treating them.”
A: Treat aggressively with more combination therapies, more hormonal therapies, more isotretinoin, and perhaps more prior authorizations. She characterized the effort to obtain a prior authorization as “our megaphone back to insurance companies that says, ‘we think it is worth taking the time to do this prior authorization because the acne patient will benefit.’ ”
R: Don’t resist isotretinoin. Dr. Harper, who began practicing dermatology more than 20 years ago, said that over time, she has gradually prescribed more isotretinoin for her patients with acne. “It’s not a first-line [treatment], but I’m not afraid of it. If I can’t get somebody clear on other oral or topical treatments, we are going to try isotretinoin.”
The goal of acne treatment, she added, is to affect four key aspects of pathogenesis: follicular epithelial hyperproliferation, inflammation, Cutibacterium acnes (C. acnes), and sebum. “That’s what we’re always shooting for,” she said.
Dr. Harper is a past president of the American Acne & Rosacea Society. She disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speaker’s bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
to use when treating this group of patients.
During a presentation at Medscape Live’s annual Coastal Dermatology Symposium, Dr. Harper, who practices at Dermatology and Skin Care of Birmingham, Ala., elaborated on the mnemonic, as follows:
C: Communicate expectations. “I look right at the acne patient and say, ‘I know you don’t just want to be better; I know you want to be clear,’ ” she said at the meeting. “ ‘That’s my goal for you, too. That may take us more than one visit and more than one treatment, but I am on your team, and that’s what we’re shooting for.’ If you don’t communicate that, they’re going to think that their acne is not that important to you.”
L: Listen for clues to customize the patient’s treatment. “We’re quick to say, ‘my patients don’t do what I recommend,’ or ‘they didn’t do what the last doctor recommended,’ ” Dr. Harper said. “Sometimes that is true, but there may be a reason why. Maybe the medication was too expensive. Maybe it was bleaching their fabrics. Maybe the regimen was too complex. Listen for opportunities to make adjustments to get their acne closer to clear.”
E: Treat early to improve quality of life and to decrease the risk of scarring. “I have a laser in my practice that is good at treating acne scarring,” she said. “Do I ever look at my patient and say, ‘don’t worry about those scars; I can make them go away?’ No. I look at them and say, ‘we can maybe make this 40% better,’ something like that. We have to prevent acne scars, because we’re not good at treating them.”
A: Treat aggressively with more combination therapies, more hormonal therapies, more isotretinoin, and perhaps more prior authorizations. She characterized the effort to obtain a prior authorization as “our megaphone back to insurance companies that says, ‘we think it is worth taking the time to do this prior authorization because the acne patient will benefit.’ ”
R: Don’t resist isotretinoin. Dr. Harper, who began practicing dermatology more than 20 years ago, said that over time, she has gradually prescribed more isotretinoin for her patients with acne. “It’s not a first-line [treatment], but I’m not afraid of it. If I can’t get somebody clear on other oral or topical treatments, we are going to try isotretinoin.”
The goal of acne treatment, she added, is to affect four key aspects of pathogenesis: follicular epithelial hyperproliferation, inflammation, Cutibacterium acnes (C. acnes), and sebum. “That’s what we’re always shooting for,” she said.
Dr. Harper is a past president of the American Acne & Rosacea Society. She disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speaker’s bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
FROM MEDSCAPE LIVE COASTAL DERM
Expert calls for thoughtful approach to curbing costs in dermatology
PORTLAND, ORE. – About 10 years ago when Arash Mostaghimi, MD, MPA, MPH, became an attending physician at Brigham and Women’s Hospital, Boston, he noticed that some of his dermatology colleagues checked the potassium levels religiously in their female patients taking spironolactone, while others never did.
“It led to this question: Dr. Mostaghimi, director of the dermatology inpatient service at Brigham and Women’s, said at the annual meeting of the Pacific Dermatologic Association.
To find out, he and his colleagues reviewed 1,802 serum potassium measurements in a study of healthy young women with no known health conditions who were taking spironolactone, published in 2015. They discovered that 13 of those tests suggested mild hyperkalemia, defined as a level greater than 5.0 mEq/L. Of these, six were rechecked and were normal; no action was taken in the other seven patients.
“This led us to conclude that we spent $78,000 at our institution on testing that did not appear to yield clinically significant information for these patients, and that routine potassium monitoring is unnecessary for most women taking spironolactone for acne,” he said. Their findings have been validated “in many cohorts of data,” he added.
The study serves as an example of efforts dermatologists can take to curb unnecessary costs within the field to be “appropriate stewards of resources,” he continued. “We have to think about the ratio of benefit over cost. It’s not just about the cost, it’s about what you’re getting for the amount of money that you’re spending. The idea of this is not restricting or not giving people medications or access to things that they need. The idea is to do it in a thoughtful way that works across the population.”
Value thresholds
Determining the value thresholds of a particular medicine or procedure is also essential to good dermatology practice. To illustrate, Dr. Mostaghimi cited a prospective cohort study that compared treatment patterns and clinical outcomes in 1,536 consecutive patients with nonmelanoma skin cancer (NMSC) with and without limited life expectancy. More than two-thirds of the NMSCs (69%) were treated surgically. After adjusting for tumor and patient characteristics, the researchers found that 43% of patients with low life expectancy died within 5 years, but not from NMSC.
“Does that mean we shouldn’t do surgery for NMSC patients with low life expectancy?” he asked. “Should we do it less? Should we let the patients decide? It’s complicated. As a society, we have to decide what’s worth doing and what’s not worth doing,” he said. “What about old diseases with new treatments, like alopecia areata? Is alopecia areata a cosmetic condition? Dermatologists and patients wouldn’t classify it that way, but many insurers do. How do you negotiate that?”
In 2013, the American Academy of Dermatology identified 10 evidence-based recommendations that can support conversations between patients and dermatologists about treatments, tests, and procedures that may not be necessary. One of the recommendations was not to prescribe oral antifungal therapy for suspected nail fungus without confirmation of fungal infection.
“If a clinician thinks a patient has onychomycosis, he or she is usually right,” Dr. Mostaghimi said. “But what’s the added cost/benefit of performing a KOH followed by PAS testing if negative or performing a PAS test directly versus just treating the patient?”
In 2006, he and his colleagues published the results of a decision analysis to address these questions. They determined that the costs of testing to avoid one case of clinically apparent liver injury with terbinafine treatment was $18.2-$43.7 million for the KOH screening pathway and $37.6 to $90.2 million for the PAS testing pathway.
“Is that worth it?” he asked. “Would we get more value for spending the money elsewhere? In this case, the answer is most likely yes.”
Isotretinoin lab testing
Translating research into recommendations and standards of care is one way to help curb costs in dermatology. As an example, he cited lab monitoring for patients treated with isotretinoin for acne.
“There have been a number of papers over the years that have suggested that the number of labs we do is excessive, that the value that they provide is low, and that abnormal results do not impact our decision-making,” Dr. Mostaghimi said. “Do some patients on isotretinoin get mildly elevated [liver function tests] and hypertriglyceridemia? Yes, that happens. Does it matter? Nothing has demonstrated that it matters. Does it matter that an 18-year-old has high triglycerides for 6 months? Rarely, if ever.”
To promote a new approach, he and a panel of acne experts from five continents performed a Delphi consensus study. Based on their consensus, they proposed a simple approach: For “generally healthy patients without underlying abnormalities or preexisting conditions warranting further investigation,” check ALT and triglycerides prior to initiating isotretinoin. Then start isotretinoin.
“At the peak dose, recheck ALT and triglycerides – this might be at month 2,” Dr. Mostaghimi said. “Other people wait a little bit longer. No labs are required once treatment is complete. Of course, adjust this approach based on your assessment of the patient in front of you. None of these recommendations should replace your clinical judgment and intuition.”
He proposed a new paradigm where dermatologists can ask themselves three questions for every patient they see: Why is this intervention or test being done? Why is it being done in this patient? And why do it at that time? “If we think this way, we can identify some inconsistencies in our own thinking and opportunities for improvement,” he said.
Dr. Mostaghimi reported that he is a consultant to Pfizer, Concert, Lilly, and Bioniz. He is also an advisor to Him & Hers Cosmetics and Digital Diagnostics and is an associate editor for JAMA Dermatology.
PORTLAND, ORE. – About 10 years ago when Arash Mostaghimi, MD, MPA, MPH, became an attending physician at Brigham and Women’s Hospital, Boston, he noticed that some of his dermatology colleagues checked the potassium levels religiously in their female patients taking spironolactone, while others never did.
“It led to this question: Dr. Mostaghimi, director of the dermatology inpatient service at Brigham and Women’s, said at the annual meeting of the Pacific Dermatologic Association.
To find out, he and his colleagues reviewed 1,802 serum potassium measurements in a study of healthy young women with no known health conditions who were taking spironolactone, published in 2015. They discovered that 13 of those tests suggested mild hyperkalemia, defined as a level greater than 5.0 mEq/L. Of these, six were rechecked and were normal; no action was taken in the other seven patients.
“This led us to conclude that we spent $78,000 at our institution on testing that did not appear to yield clinically significant information for these patients, and that routine potassium monitoring is unnecessary for most women taking spironolactone for acne,” he said. Their findings have been validated “in many cohorts of data,” he added.
The study serves as an example of efforts dermatologists can take to curb unnecessary costs within the field to be “appropriate stewards of resources,” he continued. “We have to think about the ratio of benefit over cost. It’s not just about the cost, it’s about what you’re getting for the amount of money that you’re spending. The idea of this is not restricting or not giving people medications or access to things that they need. The idea is to do it in a thoughtful way that works across the population.”
Value thresholds
Determining the value thresholds of a particular medicine or procedure is also essential to good dermatology practice. To illustrate, Dr. Mostaghimi cited a prospective cohort study that compared treatment patterns and clinical outcomes in 1,536 consecutive patients with nonmelanoma skin cancer (NMSC) with and without limited life expectancy. More than two-thirds of the NMSCs (69%) were treated surgically. After adjusting for tumor and patient characteristics, the researchers found that 43% of patients with low life expectancy died within 5 years, but not from NMSC.
“Does that mean we shouldn’t do surgery for NMSC patients with low life expectancy?” he asked. “Should we do it less? Should we let the patients decide? It’s complicated. As a society, we have to decide what’s worth doing and what’s not worth doing,” he said. “What about old diseases with new treatments, like alopecia areata? Is alopecia areata a cosmetic condition? Dermatologists and patients wouldn’t classify it that way, but many insurers do. How do you negotiate that?”
In 2013, the American Academy of Dermatology identified 10 evidence-based recommendations that can support conversations between patients and dermatologists about treatments, tests, and procedures that may not be necessary. One of the recommendations was not to prescribe oral antifungal therapy for suspected nail fungus without confirmation of fungal infection.
“If a clinician thinks a patient has onychomycosis, he or she is usually right,” Dr. Mostaghimi said. “But what’s the added cost/benefit of performing a KOH followed by PAS testing if negative or performing a PAS test directly versus just treating the patient?”
In 2006, he and his colleagues published the results of a decision analysis to address these questions. They determined that the costs of testing to avoid one case of clinically apparent liver injury with terbinafine treatment was $18.2-$43.7 million for the KOH screening pathway and $37.6 to $90.2 million for the PAS testing pathway.
“Is that worth it?” he asked. “Would we get more value for spending the money elsewhere? In this case, the answer is most likely yes.”
Isotretinoin lab testing
Translating research into recommendations and standards of care is one way to help curb costs in dermatology. As an example, he cited lab monitoring for patients treated with isotretinoin for acne.
“There have been a number of papers over the years that have suggested that the number of labs we do is excessive, that the value that they provide is low, and that abnormal results do not impact our decision-making,” Dr. Mostaghimi said. “Do some patients on isotretinoin get mildly elevated [liver function tests] and hypertriglyceridemia? Yes, that happens. Does it matter? Nothing has demonstrated that it matters. Does it matter that an 18-year-old has high triglycerides for 6 months? Rarely, if ever.”
To promote a new approach, he and a panel of acne experts from five continents performed a Delphi consensus study. Based on their consensus, they proposed a simple approach: For “generally healthy patients without underlying abnormalities or preexisting conditions warranting further investigation,” check ALT and triglycerides prior to initiating isotretinoin. Then start isotretinoin.
“At the peak dose, recheck ALT and triglycerides – this might be at month 2,” Dr. Mostaghimi said. “Other people wait a little bit longer. No labs are required once treatment is complete. Of course, adjust this approach based on your assessment of the patient in front of you. None of these recommendations should replace your clinical judgment and intuition.”
He proposed a new paradigm where dermatologists can ask themselves three questions for every patient they see: Why is this intervention or test being done? Why is it being done in this patient? And why do it at that time? “If we think this way, we can identify some inconsistencies in our own thinking and opportunities for improvement,” he said.
Dr. Mostaghimi reported that he is a consultant to Pfizer, Concert, Lilly, and Bioniz. He is also an advisor to Him & Hers Cosmetics and Digital Diagnostics and is an associate editor for JAMA Dermatology.
PORTLAND, ORE. – About 10 years ago when Arash Mostaghimi, MD, MPA, MPH, became an attending physician at Brigham and Women’s Hospital, Boston, he noticed that some of his dermatology colleagues checked the potassium levels religiously in their female patients taking spironolactone, while others never did.
“It led to this question: Dr. Mostaghimi, director of the dermatology inpatient service at Brigham and Women’s, said at the annual meeting of the Pacific Dermatologic Association.
To find out, he and his colleagues reviewed 1,802 serum potassium measurements in a study of healthy young women with no known health conditions who were taking spironolactone, published in 2015. They discovered that 13 of those tests suggested mild hyperkalemia, defined as a level greater than 5.0 mEq/L. Of these, six were rechecked and were normal; no action was taken in the other seven patients.
“This led us to conclude that we spent $78,000 at our institution on testing that did not appear to yield clinically significant information for these patients, and that routine potassium monitoring is unnecessary for most women taking spironolactone for acne,” he said. Their findings have been validated “in many cohorts of data,” he added.
The study serves as an example of efforts dermatologists can take to curb unnecessary costs within the field to be “appropriate stewards of resources,” he continued. “We have to think about the ratio of benefit over cost. It’s not just about the cost, it’s about what you’re getting for the amount of money that you’re spending. The idea of this is not restricting or not giving people medications or access to things that they need. The idea is to do it in a thoughtful way that works across the population.”
Value thresholds
Determining the value thresholds of a particular medicine or procedure is also essential to good dermatology practice. To illustrate, Dr. Mostaghimi cited a prospective cohort study that compared treatment patterns and clinical outcomes in 1,536 consecutive patients with nonmelanoma skin cancer (NMSC) with and without limited life expectancy. More than two-thirds of the NMSCs (69%) were treated surgically. After adjusting for tumor and patient characteristics, the researchers found that 43% of patients with low life expectancy died within 5 years, but not from NMSC.
“Does that mean we shouldn’t do surgery for NMSC patients with low life expectancy?” he asked. “Should we do it less? Should we let the patients decide? It’s complicated. As a society, we have to decide what’s worth doing and what’s not worth doing,” he said. “What about old diseases with new treatments, like alopecia areata? Is alopecia areata a cosmetic condition? Dermatologists and patients wouldn’t classify it that way, but many insurers do. How do you negotiate that?”
In 2013, the American Academy of Dermatology identified 10 evidence-based recommendations that can support conversations between patients and dermatologists about treatments, tests, and procedures that may not be necessary. One of the recommendations was not to prescribe oral antifungal therapy for suspected nail fungus without confirmation of fungal infection.
“If a clinician thinks a patient has onychomycosis, he or she is usually right,” Dr. Mostaghimi said. “But what’s the added cost/benefit of performing a KOH followed by PAS testing if negative or performing a PAS test directly versus just treating the patient?”
In 2006, he and his colleagues published the results of a decision analysis to address these questions. They determined that the costs of testing to avoid one case of clinically apparent liver injury with terbinafine treatment was $18.2-$43.7 million for the KOH screening pathway and $37.6 to $90.2 million for the PAS testing pathway.
“Is that worth it?” he asked. “Would we get more value for spending the money elsewhere? In this case, the answer is most likely yes.”
Isotretinoin lab testing
Translating research into recommendations and standards of care is one way to help curb costs in dermatology. As an example, he cited lab monitoring for patients treated with isotretinoin for acne.
“There have been a number of papers over the years that have suggested that the number of labs we do is excessive, that the value that they provide is low, and that abnormal results do not impact our decision-making,” Dr. Mostaghimi said. “Do some patients on isotretinoin get mildly elevated [liver function tests] and hypertriglyceridemia? Yes, that happens. Does it matter? Nothing has demonstrated that it matters. Does it matter that an 18-year-old has high triglycerides for 6 months? Rarely, if ever.”
To promote a new approach, he and a panel of acne experts from five continents performed a Delphi consensus study. Based on their consensus, they proposed a simple approach: For “generally healthy patients without underlying abnormalities or preexisting conditions warranting further investigation,” check ALT and triglycerides prior to initiating isotretinoin. Then start isotretinoin.
“At the peak dose, recheck ALT and triglycerides – this might be at month 2,” Dr. Mostaghimi said. “Other people wait a little bit longer. No labs are required once treatment is complete. Of course, adjust this approach based on your assessment of the patient in front of you. None of these recommendations should replace your clinical judgment and intuition.”
He proposed a new paradigm where dermatologists can ask themselves three questions for every patient they see: Why is this intervention or test being done? Why is it being done in this patient? And why do it at that time? “If we think this way, we can identify some inconsistencies in our own thinking and opportunities for improvement,” he said.
Dr. Mostaghimi reported that he is a consultant to Pfizer, Concert, Lilly, and Bioniz. He is also an advisor to Him & Hers Cosmetics and Digital Diagnostics and is an associate editor for JAMA Dermatology.
AT PDA 2022
Isotretinoin prescribers need better education on emergency contraception
, in a survey of 57 clinicians.
Pregnancies among patients on isotretinoin have declined since the iPLEDGE risk management program was introduced in 2005, but from 2011 to 2017, 210 to 310 pregnancies were reported to the Food and Drug Administration every year, wrote Catherine E. Smiley of Penn State University, Hershey, Pa., and coauthors Melissa Butt, DrPH, and Andrea L. Zaenglein, MD, of Penn State.
For patients on isotretinoin, EC “becomes critical when abstinence fails or contraception is not used properly,” but EC merits only a brief mention in iPLEDGE materials for patients and providers, they noted.
Patients on isotretinoin who choose abstinence as their form of birth control are the group at greatest risk for pregnancy, Dr. Zaenglein, professor of dermatology and pediatric dermatology, Penn State University, said in an interview. “However, the iPLEDGE program fails to educate patients adequately on emergency contraception,” she explained.
To assess pediatric dermatologists’ understanding of EC and their contraception counseling practices for isotretinoin patients, the researchers surveyed 57 pediatric dermatologists who prescribed isotretinoin as part of their practices. The findings were published in Pediatric Dermatology.Respondents included 53 practicing dermatologists, 2 residents, and 2 fellows. Approximately one-third (31.6%) had been in practice for 6-10 years, almost 23% had been in practice for 3-5 years, and almost 20% had been in practice for 21 or more years. Almost two-thirds practiced pediatric dermatology only.
Overall, 58% of the respondents strongly agreed that they provided contraception counseling to patients at their initial visit for isotretinoin, but only 7% and 3.5% reported providing EC counseling at initial and follow-up visits, respectively. More than half (58%) said they did not counsel patients on the side effects of EC.
As for provider education, 7.1% of respondents said they had received formal education on EC counseling, 25% reported receiving informal education on EC counseling, and 68% said they received no education on EC counseling.
A total of 32% of respondents said they were at least somewhat confident in how to obtain EC in their state.
EC is an effective form of contraception if used after unprotected intercourse, and discounts can reduce the price to as low as $9.69, the researchers wrote in their discussion. “Given that most providers in this study did not receive formal education on EC, and most do not provide EC counseling to their patients of reproductive potential on isotretinoin, EC education should be a core competency in dermatology residency education on isotretinoin prescribing,” the researchers noted. In addition, EC counseling in the iPLEDGE program should be improved by including more information in education materials and reminding patients that EC is an option, they said.
The study findings were limited by several factors including the small sample size and the multiple-choice format that prevented respondents to share rationales for their responses, the researchers noted.
However, the results highlight the need to improve EC education among pediatric dermatologists to better inform patients considering isotretinoin, especially those choosing abstinence as a method of birth control, they emphasized.
“This study is very important at this specific time for two reasons,” Dr. Zaenglein said in an interview. “The first is that with the recent disastrous rollout of the new iPLEDGE changes, there have been many calls to reform the REMS program. For the first time in the 22-year history of the program, the isotretinoin manufacturers, who manage the iPLEDGE program as an unidentified group (the IPMG), have been forced by the FDA to meet with the AAD iPLEDGE Task Force,” said Dr. Zaenglein, a member of the task force.
“The task force is currently advocating for common sense changes to iPLEDGE and I think enhancing education on emergency contraception is vital to the goal of the program, stated as ‘to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure,’ ” she added. For many patients who previously became pregnant on isotretinoin, Plan B, an over-the-counter, FDA-approved form of contraception, might have prevented that pregnancy if the patients received adequate education on EC, she said.
The current study is especially relevant now, said Dr. Zaenglein. “With the reversal of Roe v. Wade, access to abortion is restricted or completely banned in many states, which makes educating our patients on how to prevent pregnancy even more important.”
Dr. Zaenglein said she was “somewhat surprised” by how many respondents were not educating their isotretinoin patients on EC. “However, these results follow a known trend among dermatologists. Only 50% of dermatologists prescribe oral contraceptives for acne, despite its being an FDA-approved treatment for the most common dermatologic condition we see in adolescents and young adults,” she noted.
“In general, dermatologists, and subsequently dermatology residents, are poorly educated on issues of reproductive health and how they are relevant to dermatologic care,” she added.
Dr. Zaenglein’s take home message: “Dermatologists should educate all patients of childbearing potential taking isotretinoin on how to acquire and use emergency contraception at every visit.” As for additional research, she said that since the study was conducted with pediatric dermatologists, “it would be very interesting to see if general dermatologists had the same lack of comfort in educating patients on emergency contraception and what their standard counseling practices are.”
The study received no outside funding. Dr. Zaenglein is a member of the AAD’s iPLEDGE Work Group and serves as an editor-in-chief of Pediatric Dermatology.
, in a survey of 57 clinicians.
Pregnancies among patients on isotretinoin have declined since the iPLEDGE risk management program was introduced in 2005, but from 2011 to 2017, 210 to 310 pregnancies were reported to the Food and Drug Administration every year, wrote Catherine E. Smiley of Penn State University, Hershey, Pa., and coauthors Melissa Butt, DrPH, and Andrea L. Zaenglein, MD, of Penn State.
For patients on isotretinoin, EC “becomes critical when abstinence fails or contraception is not used properly,” but EC merits only a brief mention in iPLEDGE materials for patients and providers, they noted.
Patients on isotretinoin who choose abstinence as their form of birth control are the group at greatest risk for pregnancy, Dr. Zaenglein, professor of dermatology and pediatric dermatology, Penn State University, said in an interview. “However, the iPLEDGE program fails to educate patients adequately on emergency contraception,” she explained.
To assess pediatric dermatologists’ understanding of EC and their contraception counseling practices for isotretinoin patients, the researchers surveyed 57 pediatric dermatologists who prescribed isotretinoin as part of their practices. The findings were published in Pediatric Dermatology.Respondents included 53 practicing dermatologists, 2 residents, and 2 fellows. Approximately one-third (31.6%) had been in practice for 6-10 years, almost 23% had been in practice for 3-5 years, and almost 20% had been in practice for 21 or more years. Almost two-thirds practiced pediatric dermatology only.
Overall, 58% of the respondents strongly agreed that they provided contraception counseling to patients at their initial visit for isotretinoin, but only 7% and 3.5% reported providing EC counseling at initial and follow-up visits, respectively. More than half (58%) said they did not counsel patients on the side effects of EC.
As for provider education, 7.1% of respondents said they had received formal education on EC counseling, 25% reported receiving informal education on EC counseling, and 68% said they received no education on EC counseling.
A total of 32% of respondents said they were at least somewhat confident in how to obtain EC in their state.
EC is an effective form of contraception if used after unprotected intercourse, and discounts can reduce the price to as low as $9.69, the researchers wrote in their discussion. “Given that most providers in this study did not receive formal education on EC, and most do not provide EC counseling to their patients of reproductive potential on isotretinoin, EC education should be a core competency in dermatology residency education on isotretinoin prescribing,” the researchers noted. In addition, EC counseling in the iPLEDGE program should be improved by including more information in education materials and reminding patients that EC is an option, they said.
The study findings were limited by several factors including the small sample size and the multiple-choice format that prevented respondents to share rationales for their responses, the researchers noted.
However, the results highlight the need to improve EC education among pediatric dermatologists to better inform patients considering isotretinoin, especially those choosing abstinence as a method of birth control, they emphasized.
“This study is very important at this specific time for two reasons,” Dr. Zaenglein said in an interview. “The first is that with the recent disastrous rollout of the new iPLEDGE changes, there have been many calls to reform the REMS program. For the first time in the 22-year history of the program, the isotretinoin manufacturers, who manage the iPLEDGE program as an unidentified group (the IPMG), have been forced by the FDA to meet with the AAD iPLEDGE Task Force,” said Dr. Zaenglein, a member of the task force.
“The task force is currently advocating for common sense changes to iPLEDGE and I think enhancing education on emergency contraception is vital to the goal of the program, stated as ‘to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure,’ ” she added. For many patients who previously became pregnant on isotretinoin, Plan B, an over-the-counter, FDA-approved form of contraception, might have prevented that pregnancy if the patients received adequate education on EC, she said.
The current study is especially relevant now, said Dr. Zaenglein. “With the reversal of Roe v. Wade, access to abortion is restricted or completely banned in many states, which makes educating our patients on how to prevent pregnancy even more important.”
Dr. Zaenglein said she was “somewhat surprised” by how many respondents were not educating their isotretinoin patients on EC. “However, these results follow a known trend among dermatologists. Only 50% of dermatologists prescribe oral contraceptives for acne, despite its being an FDA-approved treatment for the most common dermatologic condition we see in adolescents and young adults,” she noted.
“In general, dermatologists, and subsequently dermatology residents, are poorly educated on issues of reproductive health and how they are relevant to dermatologic care,” she added.
Dr. Zaenglein’s take home message: “Dermatologists should educate all patients of childbearing potential taking isotretinoin on how to acquire and use emergency contraception at every visit.” As for additional research, she said that since the study was conducted with pediatric dermatologists, “it would be very interesting to see if general dermatologists had the same lack of comfort in educating patients on emergency contraception and what their standard counseling practices are.”
The study received no outside funding. Dr. Zaenglein is a member of the AAD’s iPLEDGE Work Group and serves as an editor-in-chief of Pediatric Dermatology.
, in a survey of 57 clinicians.
Pregnancies among patients on isotretinoin have declined since the iPLEDGE risk management program was introduced in 2005, but from 2011 to 2017, 210 to 310 pregnancies were reported to the Food and Drug Administration every year, wrote Catherine E. Smiley of Penn State University, Hershey, Pa., and coauthors Melissa Butt, DrPH, and Andrea L. Zaenglein, MD, of Penn State.
For patients on isotretinoin, EC “becomes critical when abstinence fails or contraception is not used properly,” but EC merits only a brief mention in iPLEDGE materials for patients and providers, they noted.
Patients on isotretinoin who choose abstinence as their form of birth control are the group at greatest risk for pregnancy, Dr. Zaenglein, professor of dermatology and pediatric dermatology, Penn State University, said in an interview. “However, the iPLEDGE program fails to educate patients adequately on emergency contraception,” she explained.
To assess pediatric dermatologists’ understanding of EC and their contraception counseling practices for isotretinoin patients, the researchers surveyed 57 pediatric dermatologists who prescribed isotretinoin as part of their practices. The findings were published in Pediatric Dermatology.Respondents included 53 practicing dermatologists, 2 residents, and 2 fellows. Approximately one-third (31.6%) had been in practice for 6-10 years, almost 23% had been in practice for 3-5 years, and almost 20% had been in practice for 21 or more years. Almost two-thirds practiced pediatric dermatology only.
Overall, 58% of the respondents strongly agreed that they provided contraception counseling to patients at their initial visit for isotretinoin, but only 7% and 3.5% reported providing EC counseling at initial and follow-up visits, respectively. More than half (58%) said they did not counsel patients on the side effects of EC.
As for provider education, 7.1% of respondents said they had received formal education on EC counseling, 25% reported receiving informal education on EC counseling, and 68% said they received no education on EC counseling.
A total of 32% of respondents said they were at least somewhat confident in how to obtain EC in their state.
EC is an effective form of contraception if used after unprotected intercourse, and discounts can reduce the price to as low as $9.69, the researchers wrote in their discussion. “Given that most providers in this study did not receive formal education on EC, and most do not provide EC counseling to their patients of reproductive potential on isotretinoin, EC education should be a core competency in dermatology residency education on isotretinoin prescribing,” the researchers noted. In addition, EC counseling in the iPLEDGE program should be improved by including more information in education materials and reminding patients that EC is an option, they said.
The study findings were limited by several factors including the small sample size and the multiple-choice format that prevented respondents to share rationales for their responses, the researchers noted.
However, the results highlight the need to improve EC education among pediatric dermatologists to better inform patients considering isotretinoin, especially those choosing abstinence as a method of birth control, they emphasized.
“This study is very important at this specific time for two reasons,” Dr. Zaenglein said in an interview. “The first is that with the recent disastrous rollout of the new iPLEDGE changes, there have been many calls to reform the REMS program. For the first time in the 22-year history of the program, the isotretinoin manufacturers, who manage the iPLEDGE program as an unidentified group (the IPMG), have been forced by the FDA to meet with the AAD iPLEDGE Task Force,” said Dr. Zaenglein, a member of the task force.
“The task force is currently advocating for common sense changes to iPLEDGE and I think enhancing education on emergency contraception is vital to the goal of the program, stated as ‘to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure,’ ” she added. For many patients who previously became pregnant on isotretinoin, Plan B, an over-the-counter, FDA-approved form of contraception, might have prevented that pregnancy if the patients received adequate education on EC, she said.
The current study is especially relevant now, said Dr. Zaenglein. “With the reversal of Roe v. Wade, access to abortion is restricted or completely banned in many states, which makes educating our patients on how to prevent pregnancy even more important.”
Dr. Zaenglein said she was “somewhat surprised” by how many respondents were not educating their isotretinoin patients on EC. “However, these results follow a known trend among dermatologists. Only 50% of dermatologists prescribe oral contraceptives for acne, despite its being an FDA-approved treatment for the most common dermatologic condition we see in adolescents and young adults,” she noted.
“In general, dermatologists, and subsequently dermatology residents, are poorly educated on issues of reproductive health and how they are relevant to dermatologic care,” she added.
Dr. Zaenglein’s take home message: “Dermatologists should educate all patients of childbearing potential taking isotretinoin on how to acquire and use emergency contraception at every visit.” As for additional research, she said that since the study was conducted with pediatric dermatologists, “it would be very interesting to see if general dermatologists had the same lack of comfort in educating patients on emergency contraception and what their standard counseling practices are.”
The study received no outside funding. Dr. Zaenglein is a member of the AAD’s iPLEDGE Work Group and serves as an editor-in-chief of Pediatric Dermatology.
FROM PEDIATRIC DERMATOLOGY
Hormonal therapy a safe, long term option for older women with recalcitrant acne
PORTLAND, ORE. – During her dermatology residency training at the University of California, Irvine, Medical Center, Jenny Murase, MD, remembers hearing a colleague say that her most angry patients of the day were adult women with recalcitrant acne who present to the clinic with questions like, “My skin has been clear my whole life! What’s going on?”
Such . In fact, 82% fail multiple courses of systemic antibiotics and 32% relapse after using isotretinoin, Dr. Murase, director of medical dermatology consultative services and patch testing at the Palo Alto Foundation Medical Group, said at the annual meeting of the Pacific Dermatologic Association.
In her clinical experience, hormonal therapy is a safe long-term option for recalcitrant acne in postmenarcheal females over the age of 14. “Although oral antibiotics are going to be superior to hormonal therapy in the first month or two, when you get to about six months, they have equivalent efficacy,” she said.
Telltale signs of acne associated with androgen excess include the development of nodulocystic papules along the jawline and small comedones over the forehead. Female patients with acne may request that labs be ordered to check their hormone levels, but that often is not necessary, according to Dr. Murase, who is also associate clinical professor of dermatology at the University of California, San Francisco. “There aren’t strict guidelines to indicate when you should perform hormonal testing, but warning signs that warrant further evaluation include hirsutism, androgenetic alopecia, virilization, infertility, oligomenorrhea or amenorrhea, and sudden onset of severe acne. The most common situation that warrants hormonal testing is polycystic ovary syndrome (PCOS).”
When there is a strong suspicion for hyperandrogenism, essential labs include free and total testosterone. Free testosterone is commonly elevated in patients with PCOS and total testosterone levels over 200 ng/dL is suggestive of an ovarian tumor. Other essential labs include 17-hyydroxyprogesterone (values greater than 200 ng/dL indicate congenital adrenal hyperplasia), and dehydroepiandrosterone sulfate (DHEA-S); levels over 8,000 mcg/dL indicate an adrenal tumor, while levels in the 4,000-8,000 mcg/dL range indicate congenital adrenal hyperplasia.
Helpful lab tests to consider include the ratio of luteinizing hormone to follicle-stimulating hormone; a 3:1 ratio or greater is suggestive for PCOS. “Ordering a prolactin level can also help, especially if patients are describing issues with headaches, which could indicate a pituitary tumor,” Dr. Murase added. Measuring sex hormone binding globulin (SHBG) levels can also be helpful. “If a patient has been on oral contraceptives for a long time, it increases their SHBG,” which, in older women, she said, “is inversely related to the development of type 2 diabetes.”
All labs for hyperandrogenism should be performed early in the morning on day 3 of the patient’s menstrual cycle. “If patients are on some kind of hormonal therapy, they need to be off of it for at least 6 weeks in order for you get a relevant test,” she said. Other relevant labs to consider include fasting glucose and lipids, cortisol, and thyroid-stimulating hormone.
Oral contraceptives
Estrogen contained in oral contraceptives (OCs) provides the most benefit to acne patients. “It reduces sebum production, decreases free testosterone and DHEA-S by stimulating SHBG synthesis in the liver, inhibits 5-alpha-reductase, which decreases peripheral testosterone conversion, and it decreases the production of ovarian and adrenal androgens,” Dr. Murase explained. “On average, you can get about 40%-70% reduction of lesion count, which is pretty good.”
Progestins with low androgenetic activity are the most helpful for acne, including norgestimate, desogestrel, and drospirenone. FDA-approved OC options include Ortho Tri-Cyclen, EstroStep, Yaz, and Beyaz. None has data showing superior efficacy.
No Pap smear or pelvic exam is required when prescribing OCs, but the risk of clotting should be discussed with patients. According to Dr. Murase, the risk of deep vein thrombosis (DVT) at baseline is about 1 per 10,000 woman-years, while the risk of DVT after 1 year on an OC is 3.4 per 10,000 years.
“This is a very mild increased risk that we’re talking about, but it is relevant in smokers, in those with hypertension, and in those who are diabetic,” she said. As for the risk of cancer associated with the use of OCs, a large collaborative study found a relative risk of 1.24 for developing breast cancer (not dose or duration related), but a risk reduction for endometrial, colorectal, and ovarian cancer.
The most common side effects associated with OCs are unscheduled bleeding, nausea, breast tenderness, and possible weight gain. Concomitant antibiotics can be used, with the exception of CYP3A4 inducers, such as rifampin. “That’s the main antibiotic we have to worry about that could affect the efficacy of the birth control pill,” she said. “It accounts for about three-quarters of pregnancies on antibiotics.”
Tetracyclines do not appear to increase the rate of birth defects with incidental first-trimester exposure, and data are reassuring but “tetracycline should be stopped within the first trimester as soon as the patient discovers she is pregnant,” Dr. Murase said.
Contraindications for OCs include being pregnant or breastfeeding; history of stroke, venous thromboembolism, or MI; history of smoking and being over age 35; uncontrolled hypertension; migraines with focal symptoms/aura; current or past breast cancer; hypercholesterolemia; diabetes with end-organ damage or having diabetes over age 35; liver issues such as a tumor, viral hepatitis, or cirrhosis; and a history of major surgery with prolonged immobilization.
Spironolactone
Another treatment option is spironolactone, a potassium-sparing diuretic that blocks aldosterone at a dose of 25 mg/day. At doses of 50-100 mg/day, it blocks androgen. “It can be used in combination with an oral contraceptive, with the rates of efficacy reported to range between 33% and 85%,” Dr. Murase said.
Spironolactone can also reduce hirsutism, improve androgenetic alopecia, and lower blood pressure by about 5 mm Hg systolic and 2.5 mm Hg diastolic. Dr. Murase usually checks blood pressure in patients, and “only if they’re really low I’ll talk about the potential for postural hypotension and the fact that you can get a little bit dizzy when going from a position of lying down to standing up.” Potassium levels should be checked at baseline and 4 weeks in patients older than age 46, in those with cardiac and/or renal disease, or in those on concomitant drospirenone or a third-generation progestin.
Spironolactone is classified as a pregnancy category D drug that could compromise the genital development of a male fetus. “So the onus is on us as providers to have the conversation with our patient,” she said. “If you’re putting a patient on spironolactone and they are of child-bearing age, you need to make sure that you’ve had the conversation with them about the fact that they should not get pregnant while on the medicine.”
Spironolactone also has a boxed warning citing the development of benign tumors in animal studies. That warning is based on studies in rats at doses of 10-150 mg/kg per day, “which is an extremely high dose and would never be given in humans,” said Dr. Murase, who has coauthored CME content regarding the safety of dermatologic medications in pregnancy and lactation.
In humans, there has been no evidence of the development of benign tumors associated with spironolactone therapy, and “there has been a decreased risk of prostate cancer and no association with its use and the development of breast, ovarian, bladder, kidney, gastric, or esophageal cancer,” she said.
Dr. Murase noted that during pregnancy, first-line oral antibiotics include amoxicillin for acne rosacea and cefadroxil for acne vulgaris. Macrolides are a second-line choice because of an increase in atrial/ventricular septal defects and pyloric stenosis that have been reported with first-trimester exposure.
“Erythromycin is the preferred choice over azithromycin and clarithromycin because it has the most data, [but] erythromycin estolate has been associated with increased AST levels in the second trimester,” she said. “It occurs in about 10% of cases and is reversible. Erythromycin base and erythromycin ethylsuccinate do not have this risk, and those are preferable.”
Dr. Murase disclosed that she has been a paid speaker of unbranded medical content for Regeneron and UCB. She is also a member of the advisory board for Leo Pharma, Eli Lilly, UCB, and Genzyme/Sanofi.
PORTLAND, ORE. – During her dermatology residency training at the University of California, Irvine, Medical Center, Jenny Murase, MD, remembers hearing a colleague say that her most angry patients of the day were adult women with recalcitrant acne who present to the clinic with questions like, “My skin has been clear my whole life! What’s going on?”
Such . In fact, 82% fail multiple courses of systemic antibiotics and 32% relapse after using isotretinoin, Dr. Murase, director of medical dermatology consultative services and patch testing at the Palo Alto Foundation Medical Group, said at the annual meeting of the Pacific Dermatologic Association.
In her clinical experience, hormonal therapy is a safe long-term option for recalcitrant acne in postmenarcheal females over the age of 14. “Although oral antibiotics are going to be superior to hormonal therapy in the first month or two, when you get to about six months, they have equivalent efficacy,” she said.
Telltale signs of acne associated with androgen excess include the development of nodulocystic papules along the jawline and small comedones over the forehead. Female patients with acne may request that labs be ordered to check their hormone levels, but that often is not necessary, according to Dr. Murase, who is also associate clinical professor of dermatology at the University of California, San Francisco. “There aren’t strict guidelines to indicate when you should perform hormonal testing, but warning signs that warrant further evaluation include hirsutism, androgenetic alopecia, virilization, infertility, oligomenorrhea or amenorrhea, and sudden onset of severe acne. The most common situation that warrants hormonal testing is polycystic ovary syndrome (PCOS).”
When there is a strong suspicion for hyperandrogenism, essential labs include free and total testosterone. Free testosterone is commonly elevated in patients with PCOS and total testosterone levels over 200 ng/dL is suggestive of an ovarian tumor. Other essential labs include 17-hyydroxyprogesterone (values greater than 200 ng/dL indicate congenital adrenal hyperplasia), and dehydroepiandrosterone sulfate (DHEA-S); levels over 8,000 mcg/dL indicate an adrenal tumor, while levels in the 4,000-8,000 mcg/dL range indicate congenital adrenal hyperplasia.
Helpful lab tests to consider include the ratio of luteinizing hormone to follicle-stimulating hormone; a 3:1 ratio or greater is suggestive for PCOS. “Ordering a prolactin level can also help, especially if patients are describing issues with headaches, which could indicate a pituitary tumor,” Dr. Murase added. Measuring sex hormone binding globulin (SHBG) levels can also be helpful. “If a patient has been on oral contraceptives for a long time, it increases their SHBG,” which, in older women, she said, “is inversely related to the development of type 2 diabetes.”
All labs for hyperandrogenism should be performed early in the morning on day 3 of the patient’s menstrual cycle. “If patients are on some kind of hormonal therapy, they need to be off of it for at least 6 weeks in order for you get a relevant test,” she said. Other relevant labs to consider include fasting glucose and lipids, cortisol, and thyroid-stimulating hormone.
Oral contraceptives
Estrogen contained in oral contraceptives (OCs) provides the most benefit to acne patients. “It reduces sebum production, decreases free testosterone and DHEA-S by stimulating SHBG synthesis in the liver, inhibits 5-alpha-reductase, which decreases peripheral testosterone conversion, and it decreases the production of ovarian and adrenal androgens,” Dr. Murase explained. “On average, you can get about 40%-70% reduction of lesion count, which is pretty good.”
Progestins with low androgenetic activity are the most helpful for acne, including norgestimate, desogestrel, and drospirenone. FDA-approved OC options include Ortho Tri-Cyclen, EstroStep, Yaz, and Beyaz. None has data showing superior efficacy.
No Pap smear or pelvic exam is required when prescribing OCs, but the risk of clotting should be discussed with patients. According to Dr. Murase, the risk of deep vein thrombosis (DVT) at baseline is about 1 per 10,000 woman-years, while the risk of DVT after 1 year on an OC is 3.4 per 10,000 years.
“This is a very mild increased risk that we’re talking about, but it is relevant in smokers, in those with hypertension, and in those who are diabetic,” she said. As for the risk of cancer associated with the use of OCs, a large collaborative study found a relative risk of 1.24 for developing breast cancer (not dose or duration related), but a risk reduction for endometrial, colorectal, and ovarian cancer.
The most common side effects associated with OCs are unscheduled bleeding, nausea, breast tenderness, and possible weight gain. Concomitant antibiotics can be used, with the exception of CYP3A4 inducers, such as rifampin. “That’s the main antibiotic we have to worry about that could affect the efficacy of the birth control pill,” she said. “It accounts for about three-quarters of pregnancies on antibiotics.”
Tetracyclines do not appear to increase the rate of birth defects with incidental first-trimester exposure, and data are reassuring but “tetracycline should be stopped within the first trimester as soon as the patient discovers she is pregnant,” Dr. Murase said.
Contraindications for OCs include being pregnant or breastfeeding; history of stroke, venous thromboembolism, or MI; history of smoking and being over age 35; uncontrolled hypertension; migraines with focal symptoms/aura; current or past breast cancer; hypercholesterolemia; diabetes with end-organ damage or having diabetes over age 35; liver issues such as a tumor, viral hepatitis, or cirrhosis; and a history of major surgery with prolonged immobilization.
Spironolactone
Another treatment option is spironolactone, a potassium-sparing diuretic that blocks aldosterone at a dose of 25 mg/day. At doses of 50-100 mg/day, it blocks androgen. “It can be used in combination with an oral contraceptive, with the rates of efficacy reported to range between 33% and 85%,” Dr. Murase said.
Spironolactone can also reduce hirsutism, improve androgenetic alopecia, and lower blood pressure by about 5 mm Hg systolic and 2.5 mm Hg diastolic. Dr. Murase usually checks blood pressure in patients, and “only if they’re really low I’ll talk about the potential for postural hypotension and the fact that you can get a little bit dizzy when going from a position of lying down to standing up.” Potassium levels should be checked at baseline and 4 weeks in patients older than age 46, in those with cardiac and/or renal disease, or in those on concomitant drospirenone or a third-generation progestin.
Spironolactone is classified as a pregnancy category D drug that could compromise the genital development of a male fetus. “So the onus is on us as providers to have the conversation with our patient,” she said. “If you’re putting a patient on spironolactone and they are of child-bearing age, you need to make sure that you’ve had the conversation with them about the fact that they should not get pregnant while on the medicine.”
Spironolactone also has a boxed warning citing the development of benign tumors in animal studies. That warning is based on studies in rats at doses of 10-150 mg/kg per day, “which is an extremely high dose and would never be given in humans,” said Dr. Murase, who has coauthored CME content regarding the safety of dermatologic medications in pregnancy and lactation.
In humans, there has been no evidence of the development of benign tumors associated with spironolactone therapy, and “there has been a decreased risk of prostate cancer and no association with its use and the development of breast, ovarian, bladder, kidney, gastric, or esophageal cancer,” she said.
Dr. Murase noted that during pregnancy, first-line oral antibiotics include amoxicillin for acne rosacea and cefadroxil for acne vulgaris. Macrolides are a second-line choice because of an increase in atrial/ventricular septal defects and pyloric stenosis that have been reported with first-trimester exposure.
“Erythromycin is the preferred choice over azithromycin and clarithromycin because it has the most data, [but] erythromycin estolate has been associated with increased AST levels in the second trimester,” she said. “It occurs in about 10% of cases and is reversible. Erythromycin base and erythromycin ethylsuccinate do not have this risk, and those are preferable.”
Dr. Murase disclosed that she has been a paid speaker of unbranded medical content for Regeneron and UCB. She is also a member of the advisory board for Leo Pharma, Eli Lilly, UCB, and Genzyme/Sanofi.
PORTLAND, ORE. – During her dermatology residency training at the University of California, Irvine, Medical Center, Jenny Murase, MD, remembers hearing a colleague say that her most angry patients of the day were adult women with recalcitrant acne who present to the clinic with questions like, “My skin has been clear my whole life! What’s going on?”
Such . In fact, 82% fail multiple courses of systemic antibiotics and 32% relapse after using isotretinoin, Dr. Murase, director of medical dermatology consultative services and patch testing at the Palo Alto Foundation Medical Group, said at the annual meeting of the Pacific Dermatologic Association.
In her clinical experience, hormonal therapy is a safe long-term option for recalcitrant acne in postmenarcheal females over the age of 14. “Although oral antibiotics are going to be superior to hormonal therapy in the first month or two, when you get to about six months, they have equivalent efficacy,” she said.
Telltale signs of acne associated with androgen excess include the development of nodulocystic papules along the jawline and small comedones over the forehead. Female patients with acne may request that labs be ordered to check their hormone levels, but that often is not necessary, according to Dr. Murase, who is also associate clinical professor of dermatology at the University of California, San Francisco. “There aren’t strict guidelines to indicate when you should perform hormonal testing, but warning signs that warrant further evaluation include hirsutism, androgenetic alopecia, virilization, infertility, oligomenorrhea or amenorrhea, and sudden onset of severe acne. The most common situation that warrants hormonal testing is polycystic ovary syndrome (PCOS).”
When there is a strong suspicion for hyperandrogenism, essential labs include free and total testosterone. Free testosterone is commonly elevated in patients with PCOS and total testosterone levels over 200 ng/dL is suggestive of an ovarian tumor. Other essential labs include 17-hyydroxyprogesterone (values greater than 200 ng/dL indicate congenital adrenal hyperplasia), and dehydroepiandrosterone sulfate (DHEA-S); levels over 8,000 mcg/dL indicate an adrenal tumor, while levels in the 4,000-8,000 mcg/dL range indicate congenital adrenal hyperplasia.
Helpful lab tests to consider include the ratio of luteinizing hormone to follicle-stimulating hormone; a 3:1 ratio or greater is suggestive for PCOS. “Ordering a prolactin level can also help, especially if patients are describing issues with headaches, which could indicate a pituitary tumor,” Dr. Murase added. Measuring sex hormone binding globulin (SHBG) levels can also be helpful. “If a patient has been on oral contraceptives for a long time, it increases their SHBG,” which, in older women, she said, “is inversely related to the development of type 2 diabetes.”
All labs for hyperandrogenism should be performed early in the morning on day 3 of the patient’s menstrual cycle. “If patients are on some kind of hormonal therapy, they need to be off of it for at least 6 weeks in order for you get a relevant test,” she said. Other relevant labs to consider include fasting glucose and lipids, cortisol, and thyroid-stimulating hormone.
Oral contraceptives
Estrogen contained in oral contraceptives (OCs) provides the most benefit to acne patients. “It reduces sebum production, decreases free testosterone and DHEA-S by stimulating SHBG synthesis in the liver, inhibits 5-alpha-reductase, which decreases peripheral testosterone conversion, and it decreases the production of ovarian and adrenal androgens,” Dr. Murase explained. “On average, you can get about 40%-70% reduction of lesion count, which is pretty good.”
Progestins with low androgenetic activity are the most helpful for acne, including norgestimate, desogestrel, and drospirenone. FDA-approved OC options include Ortho Tri-Cyclen, EstroStep, Yaz, and Beyaz. None has data showing superior efficacy.
No Pap smear or pelvic exam is required when prescribing OCs, but the risk of clotting should be discussed with patients. According to Dr. Murase, the risk of deep vein thrombosis (DVT) at baseline is about 1 per 10,000 woman-years, while the risk of DVT after 1 year on an OC is 3.4 per 10,000 years.
“This is a very mild increased risk that we’re talking about, but it is relevant in smokers, in those with hypertension, and in those who are diabetic,” she said. As for the risk of cancer associated with the use of OCs, a large collaborative study found a relative risk of 1.24 for developing breast cancer (not dose or duration related), but a risk reduction for endometrial, colorectal, and ovarian cancer.
The most common side effects associated with OCs are unscheduled bleeding, nausea, breast tenderness, and possible weight gain. Concomitant antibiotics can be used, with the exception of CYP3A4 inducers, such as rifampin. “That’s the main antibiotic we have to worry about that could affect the efficacy of the birth control pill,” she said. “It accounts for about three-quarters of pregnancies on antibiotics.”
Tetracyclines do not appear to increase the rate of birth defects with incidental first-trimester exposure, and data are reassuring but “tetracycline should be stopped within the first trimester as soon as the patient discovers she is pregnant,” Dr. Murase said.
Contraindications for OCs include being pregnant or breastfeeding; history of stroke, venous thromboembolism, or MI; history of smoking and being over age 35; uncontrolled hypertension; migraines with focal symptoms/aura; current or past breast cancer; hypercholesterolemia; diabetes with end-organ damage or having diabetes over age 35; liver issues such as a tumor, viral hepatitis, or cirrhosis; and a history of major surgery with prolonged immobilization.
Spironolactone
Another treatment option is spironolactone, a potassium-sparing diuretic that blocks aldosterone at a dose of 25 mg/day. At doses of 50-100 mg/day, it blocks androgen. “It can be used in combination with an oral contraceptive, with the rates of efficacy reported to range between 33% and 85%,” Dr. Murase said.
Spironolactone can also reduce hirsutism, improve androgenetic alopecia, and lower blood pressure by about 5 mm Hg systolic and 2.5 mm Hg diastolic. Dr. Murase usually checks blood pressure in patients, and “only if they’re really low I’ll talk about the potential for postural hypotension and the fact that you can get a little bit dizzy when going from a position of lying down to standing up.” Potassium levels should be checked at baseline and 4 weeks in patients older than age 46, in those with cardiac and/or renal disease, or in those on concomitant drospirenone or a third-generation progestin.
Spironolactone is classified as a pregnancy category D drug that could compromise the genital development of a male fetus. “So the onus is on us as providers to have the conversation with our patient,” she said. “If you’re putting a patient on spironolactone and they are of child-bearing age, you need to make sure that you’ve had the conversation with them about the fact that they should not get pregnant while on the medicine.”
Spironolactone also has a boxed warning citing the development of benign tumors in animal studies. That warning is based on studies in rats at doses of 10-150 mg/kg per day, “which is an extremely high dose and would never be given in humans,” said Dr. Murase, who has coauthored CME content regarding the safety of dermatologic medications in pregnancy and lactation.
In humans, there has been no evidence of the development of benign tumors associated with spironolactone therapy, and “there has been a decreased risk of prostate cancer and no association with its use and the development of breast, ovarian, bladder, kidney, gastric, or esophageal cancer,” she said.
Dr. Murase noted that during pregnancy, first-line oral antibiotics include amoxicillin for acne rosacea and cefadroxil for acne vulgaris. Macrolides are a second-line choice because of an increase in atrial/ventricular septal defects and pyloric stenosis that have been reported with first-trimester exposure.
“Erythromycin is the preferred choice over azithromycin and clarithromycin because it has the most data, [but] erythromycin estolate has been associated with increased AST levels in the second trimester,” she said. “It occurs in about 10% of cases and is reversible. Erythromycin base and erythromycin ethylsuccinate do not have this risk, and those are preferable.”
Dr. Murase disclosed that she has been a paid speaker of unbranded medical content for Regeneron and UCB. She is also a member of the advisory board for Leo Pharma, Eli Lilly, UCB, and Genzyme/Sanofi.
AT PDA 2022