User login
Exploring the Utility of Artificial Intelligence During COVID-19 in Dermatology Practice
With the need to adapt to the given challenges associated with COVID-19, artificial intelligence (AI) serves as a potential tool in providing access to medical-based diagnosis in a novel way. Artificial intelligence is defined as intelligence harnessed by machines that have the ability to perform what is called cognitive thinking and to mimic the problem-solving abilities of the human mind. Virtual AI in dermatology entails neural network–based guidance that includes developing algorithms to detect skin pathology through photographs.1 To use AI in dermatology, recognition of visual patterns must be established to give diagnoses. These neural networks have been used to classify skin diseases, including cancer, actinic keratosis, and warts.2
AI for Skin Cancer
The use of AI to classify melanoma and nonmelanoma skin cancer has been studied extensively, including the following 2 research projects.
Convolutional Neural Network
In 2017, Stanford University published a study in which a deep-learning algorithm known as a convolutional neural network was used to classify skin lesions.3 The network was trained using a dataset of 129,450 clinical images of 2032 diseases. Its performance was compared to that of 21 board-certified dermatologists on biopsy-proven clinical images with 2 classifications of cases: (1) keratinocyte carcinoma as opposed to benign seborrheic keratosis and (2) malignant melanoma as opposed to benign nevi—the first representing the most common skin cancers, and the second, the deadliest skin cancers. The study showed that the machine could accurately identify and classify skin cancers compared to the work of board-certified dermatologists. The study did not include demographic information, which limits its external validity.3
Dermoscopic Image Classification
A 2019 study by Brinker and colleagues4 showed the superiority of automated dermoscopic melanoma image classifications compared to the work of board-certified dermatologists. For the study, 804 biopsy-proven images of melanoma and nevi (1:1 ratio) were randomly presented to dermatologists for their evaluation and recommended treatment (yielding 19,296 recommendations). The dermatologists classified the lesions with a sensitivity of 67.2% and specificity of 62.2%; the trained convolutional neural network attained both higher sensitivity (82.3%) and higher specificity (77.9%).4
Smartphone Diagnosis of Melanoma
An application of AI has been to use smartphone apps for the diagnosis of melanoma. The most utilized and novel algorithm-based smartphone app that assesses skin lesions for malignancy characteristics is SkinVision. With a simple download from Apple’s App Store, this technology allows a person to check their skin spots by taking a photograph and receiving algorithmic risk-assessment feedback. This inexpensive software ($51.78 a year) also allows a patient’s physician to assess the photograph and then validate their assessment by comparing it with the algorithmic analysis that the program provides.5
A review of SkinVision conducted by Thissen and colleagues6 found that, in a hypothetical population of 1000 adults of whom 3% actually had melanoma, 4 of those 30 people would not have been flagged as at “high risk” by SkinVision. There also was a high false-positive rate with the app, with more than 200 people flagged as at high risk. The analysis pegged SkinVision as having a sensitivity of 88% and specificity of 79%.6
In summary, systematic review of diagnostic accuracy has shown that, although there is accuracy in AI analyses, it should be used only as a guide for health care advice due to variability in algorithm performance.7
Utility of AI in Telehealth
Artificial intelligence algorithms could be created to ensure telehealth image accuracy, stratify risk, and track patient progress. With teledermatology visits on the rise during the COVID-19 pandemic, AI algorithms could ensure that photographs of appropriate quality are taken. Also, patients could be organized by risk factors with such algorithms, allowing physicians to save time on triage and stratification. Algorithms also could be used to track a telehealth patient’s treatment and progress.8
Furthermore, there is a need for an algorithm that has the ability to detect, quantify, and monitor changes in dermatologic conditions using images that patients have uploaded. This capability will lead to creation of a standardized quantification scale that will allow physicians to virtually track the progression of visible skin pathologies.
Hazards of Racial Bias in AI
Artificial intelligence is limited by racial disparity bias seen in computerized medicine. For years, the majority of dermatology research, especially in skin cancer, has been conducted on fairer-skinned populations. This bias has existed at the expense of darker-skinned patients, whose skin conditions and symptoms present differently,9 and reflects directly in available data sets that can be used to develop AI algorithms. Because these data are inadequate to the task, AI might misdiagnose skin cancer in people of color or miss an existing condition entirely.10 Consequently, the higher rate of skin cancer mortality that is reported in people of color is likely to persist with the rise of AI in dermatology.11 A more representative database of imaged skin lesions needs to be utilized to create a diversely representative and applicable data set for AI algorithms.12
Benefits of Conversational Agents
Another method by which AI could be incorporated into dermatology is through what is known as a conversational agent (CA)—AI software that engages in a dialogue with users by interpreting their voice and replying to them through text, image, or voice.13 Conversational agents facilitate remote patient management, allow clinicians to focus on other functions, and aid in data collection.14 A 2014 study showed that patients were significantly more likely to disclose history and emotions when informed they were interacting with a CA than with a human clinician (P=.007).15 Such benefits could be invaluable in dermatology, where emotions and patient perceptions of skin conditions play into the treatment process.
However, some evidence showed that CAs cannot respond to patients’ statements in all circumstances.16 It also is unclear how well CAs recognize nuanced statements that might signal potential harm. This fits into the greater theme of a major problem with AI: the lack of a reliable response in all circumstances.13
Final Thoughts
The practical implementations of AI in dermatology are still being explored. Given the uncertainty surrounding the COVID-19 pandemic and the future of patient care, AI might serve as an important asset in assisting with the diagnosis and treatment of dermatologic conditions, physician productivity, and patient monitoring.
- Amisha, Malik P, Pathania M, et al. Overview of artificial intelligence in medicine. J Family Med Prim Care. 2019;8:2328-2331. doi:10.4103/jfmpc.jfmpc_440_19
- Han SS, Kim MS, Lim W, et al. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J Invest Dermatol. 2018;138:1529-1538. doi:10.1016/j.jid.2018.01.028
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Brinker TJ, Hekler A, Enk AH, et al. Deep neural networks are superior to dermatologists in melanoma image classification. Eur J Cancer. 2019;119:11-17. doi:10.1016/j.ejca.2019.05.023
- Regulated medical device for detecting skin cancer. SkinVision website. Accessed July 23, 2021. https://www.skinvision.com/hcp/
- Thissen M, Udrea A, Hacking M, et al. mHealth app for risk assessment of pigmented and nonpigmented skin lesions—a study on sensitivity and specificity in detecting malignancy. Telemed J E Health. 2017;23:948-954. doi:10.1089/tmj.2016.0259
- Freeman K, Dinnes J, Chuchu N, et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies. BMJ. 2020;368:m127. doi:10.1136/bmj.m127
- Puri P, Comfere N, Pittelkow MR, et al. COVID-19: an opportunity to build dermatology’s digital future. Dermatol Ther. 2020;33:e14149. doi:10.1111/dth.14149
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Adamson AS, Smith A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 2018;154:1247-1248. doi:10.1001/jamadermatol.2018.2348
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:S0190-9622(13)01296-6
- Alabdulkareem A. Artificial intelligence and dermatologists: friends or foes? J Dermatol Dermatolog Surg. 2019;23:57-60. doi:10.4103/jdds.jdds_19_19
- McGreevey JD 3rd, Hanson CW 3rd, Koppel R. Clinical, legal, and ethical aspects of artificial intelligence-assisted conversational agents in health care. JAMA. 2020;324:552-553. doi:10.1001/jama.2020.2724
- Piau A, Crissey R, Brechemier D, et al. A smartphone chatbot application to optimize monitoring of older patients with cancer. Int J Med Inform. 2019;128:18-23. doi:10.1016/j.ijmedinf.2019.05.013
- Lucas GM, Gratch J, King A, et al. It’s only a computer: virtual humans increase willingness to disclose. Comput Human Behav. 2014;37:94-100. https://doi.org/10.1016/j.chb.2014.04.043
- Miner AS, Milstein A, Schueller S, et al. Smartphone-based conversational agents and responses to questions about mental health, interpersonal violence, and physical health. JAMA Intern Med. 2016;176:619-625. doi:10.1001/jamainternmed.2016.0400
With the need to adapt to the given challenges associated with COVID-19, artificial intelligence (AI) serves as a potential tool in providing access to medical-based diagnosis in a novel way. Artificial intelligence is defined as intelligence harnessed by machines that have the ability to perform what is called cognitive thinking and to mimic the problem-solving abilities of the human mind. Virtual AI in dermatology entails neural network–based guidance that includes developing algorithms to detect skin pathology through photographs.1 To use AI in dermatology, recognition of visual patterns must be established to give diagnoses. These neural networks have been used to classify skin diseases, including cancer, actinic keratosis, and warts.2
AI for Skin Cancer
The use of AI to classify melanoma and nonmelanoma skin cancer has been studied extensively, including the following 2 research projects.
Convolutional Neural Network
In 2017, Stanford University published a study in which a deep-learning algorithm known as a convolutional neural network was used to classify skin lesions.3 The network was trained using a dataset of 129,450 clinical images of 2032 diseases. Its performance was compared to that of 21 board-certified dermatologists on biopsy-proven clinical images with 2 classifications of cases: (1) keratinocyte carcinoma as opposed to benign seborrheic keratosis and (2) malignant melanoma as opposed to benign nevi—the first representing the most common skin cancers, and the second, the deadliest skin cancers. The study showed that the machine could accurately identify and classify skin cancers compared to the work of board-certified dermatologists. The study did not include demographic information, which limits its external validity.3
Dermoscopic Image Classification
A 2019 study by Brinker and colleagues4 showed the superiority of automated dermoscopic melanoma image classifications compared to the work of board-certified dermatologists. For the study, 804 biopsy-proven images of melanoma and nevi (1:1 ratio) were randomly presented to dermatologists for their evaluation and recommended treatment (yielding 19,296 recommendations). The dermatologists classified the lesions with a sensitivity of 67.2% and specificity of 62.2%; the trained convolutional neural network attained both higher sensitivity (82.3%) and higher specificity (77.9%).4
Smartphone Diagnosis of Melanoma
An application of AI has been to use smartphone apps for the diagnosis of melanoma. The most utilized and novel algorithm-based smartphone app that assesses skin lesions for malignancy characteristics is SkinVision. With a simple download from Apple’s App Store, this technology allows a person to check their skin spots by taking a photograph and receiving algorithmic risk-assessment feedback. This inexpensive software ($51.78 a year) also allows a patient’s physician to assess the photograph and then validate their assessment by comparing it with the algorithmic analysis that the program provides.5
A review of SkinVision conducted by Thissen and colleagues6 found that, in a hypothetical population of 1000 adults of whom 3% actually had melanoma, 4 of those 30 people would not have been flagged as at “high risk” by SkinVision. There also was a high false-positive rate with the app, with more than 200 people flagged as at high risk. The analysis pegged SkinVision as having a sensitivity of 88% and specificity of 79%.6
In summary, systematic review of diagnostic accuracy has shown that, although there is accuracy in AI analyses, it should be used only as a guide for health care advice due to variability in algorithm performance.7
Utility of AI in Telehealth
Artificial intelligence algorithms could be created to ensure telehealth image accuracy, stratify risk, and track patient progress. With teledermatology visits on the rise during the COVID-19 pandemic, AI algorithms could ensure that photographs of appropriate quality are taken. Also, patients could be organized by risk factors with such algorithms, allowing physicians to save time on triage and stratification. Algorithms also could be used to track a telehealth patient’s treatment and progress.8
Furthermore, there is a need for an algorithm that has the ability to detect, quantify, and monitor changes in dermatologic conditions using images that patients have uploaded. This capability will lead to creation of a standardized quantification scale that will allow physicians to virtually track the progression of visible skin pathologies.
Hazards of Racial Bias in AI
Artificial intelligence is limited by racial disparity bias seen in computerized medicine. For years, the majority of dermatology research, especially in skin cancer, has been conducted on fairer-skinned populations. This bias has existed at the expense of darker-skinned patients, whose skin conditions and symptoms present differently,9 and reflects directly in available data sets that can be used to develop AI algorithms. Because these data are inadequate to the task, AI might misdiagnose skin cancer in people of color or miss an existing condition entirely.10 Consequently, the higher rate of skin cancer mortality that is reported in people of color is likely to persist with the rise of AI in dermatology.11 A more representative database of imaged skin lesions needs to be utilized to create a diversely representative and applicable data set for AI algorithms.12
Benefits of Conversational Agents
Another method by which AI could be incorporated into dermatology is through what is known as a conversational agent (CA)—AI software that engages in a dialogue with users by interpreting their voice and replying to them through text, image, or voice.13 Conversational agents facilitate remote patient management, allow clinicians to focus on other functions, and aid in data collection.14 A 2014 study showed that patients were significantly more likely to disclose history and emotions when informed they were interacting with a CA than with a human clinician (P=.007).15 Such benefits could be invaluable in dermatology, where emotions and patient perceptions of skin conditions play into the treatment process.
However, some evidence showed that CAs cannot respond to patients’ statements in all circumstances.16 It also is unclear how well CAs recognize nuanced statements that might signal potential harm. This fits into the greater theme of a major problem with AI: the lack of a reliable response in all circumstances.13
Final Thoughts
The practical implementations of AI in dermatology are still being explored. Given the uncertainty surrounding the COVID-19 pandemic and the future of patient care, AI might serve as an important asset in assisting with the diagnosis and treatment of dermatologic conditions, physician productivity, and patient monitoring.
With the need to adapt to the given challenges associated with COVID-19, artificial intelligence (AI) serves as a potential tool in providing access to medical-based diagnosis in a novel way. Artificial intelligence is defined as intelligence harnessed by machines that have the ability to perform what is called cognitive thinking and to mimic the problem-solving abilities of the human mind. Virtual AI in dermatology entails neural network–based guidance that includes developing algorithms to detect skin pathology through photographs.1 To use AI in dermatology, recognition of visual patterns must be established to give diagnoses. These neural networks have been used to classify skin diseases, including cancer, actinic keratosis, and warts.2
AI for Skin Cancer
The use of AI to classify melanoma and nonmelanoma skin cancer has been studied extensively, including the following 2 research projects.
Convolutional Neural Network
In 2017, Stanford University published a study in which a deep-learning algorithm known as a convolutional neural network was used to classify skin lesions.3 The network was trained using a dataset of 129,450 clinical images of 2032 diseases. Its performance was compared to that of 21 board-certified dermatologists on biopsy-proven clinical images with 2 classifications of cases: (1) keratinocyte carcinoma as opposed to benign seborrheic keratosis and (2) malignant melanoma as opposed to benign nevi—the first representing the most common skin cancers, and the second, the deadliest skin cancers. The study showed that the machine could accurately identify and classify skin cancers compared to the work of board-certified dermatologists. The study did not include demographic information, which limits its external validity.3
Dermoscopic Image Classification
A 2019 study by Brinker and colleagues4 showed the superiority of automated dermoscopic melanoma image classifications compared to the work of board-certified dermatologists. For the study, 804 biopsy-proven images of melanoma and nevi (1:1 ratio) were randomly presented to dermatologists for their evaluation and recommended treatment (yielding 19,296 recommendations). The dermatologists classified the lesions with a sensitivity of 67.2% and specificity of 62.2%; the trained convolutional neural network attained both higher sensitivity (82.3%) and higher specificity (77.9%).4
Smartphone Diagnosis of Melanoma
An application of AI has been to use smartphone apps for the diagnosis of melanoma. The most utilized and novel algorithm-based smartphone app that assesses skin lesions for malignancy characteristics is SkinVision. With a simple download from Apple’s App Store, this technology allows a person to check their skin spots by taking a photograph and receiving algorithmic risk-assessment feedback. This inexpensive software ($51.78 a year) also allows a patient’s physician to assess the photograph and then validate their assessment by comparing it with the algorithmic analysis that the program provides.5
A review of SkinVision conducted by Thissen and colleagues6 found that, in a hypothetical population of 1000 adults of whom 3% actually had melanoma, 4 of those 30 people would not have been flagged as at “high risk” by SkinVision. There also was a high false-positive rate with the app, with more than 200 people flagged as at high risk. The analysis pegged SkinVision as having a sensitivity of 88% and specificity of 79%.6
In summary, systematic review of diagnostic accuracy has shown that, although there is accuracy in AI analyses, it should be used only as a guide for health care advice due to variability in algorithm performance.7
Utility of AI in Telehealth
Artificial intelligence algorithms could be created to ensure telehealth image accuracy, stratify risk, and track patient progress. With teledermatology visits on the rise during the COVID-19 pandemic, AI algorithms could ensure that photographs of appropriate quality are taken. Also, patients could be organized by risk factors with such algorithms, allowing physicians to save time on triage and stratification. Algorithms also could be used to track a telehealth patient’s treatment and progress.8
Furthermore, there is a need for an algorithm that has the ability to detect, quantify, and monitor changes in dermatologic conditions using images that patients have uploaded. This capability will lead to creation of a standardized quantification scale that will allow physicians to virtually track the progression of visible skin pathologies.
Hazards of Racial Bias in AI
Artificial intelligence is limited by racial disparity bias seen in computerized medicine. For years, the majority of dermatology research, especially in skin cancer, has been conducted on fairer-skinned populations. This bias has existed at the expense of darker-skinned patients, whose skin conditions and symptoms present differently,9 and reflects directly in available data sets that can be used to develop AI algorithms. Because these data are inadequate to the task, AI might misdiagnose skin cancer in people of color or miss an existing condition entirely.10 Consequently, the higher rate of skin cancer mortality that is reported in people of color is likely to persist with the rise of AI in dermatology.11 A more representative database of imaged skin lesions needs to be utilized to create a diversely representative and applicable data set for AI algorithms.12
Benefits of Conversational Agents
Another method by which AI could be incorporated into dermatology is through what is known as a conversational agent (CA)—AI software that engages in a dialogue with users by interpreting their voice and replying to them through text, image, or voice.13 Conversational agents facilitate remote patient management, allow clinicians to focus on other functions, and aid in data collection.14 A 2014 study showed that patients were significantly more likely to disclose history and emotions when informed they were interacting with a CA than with a human clinician (P=.007).15 Such benefits could be invaluable in dermatology, where emotions and patient perceptions of skin conditions play into the treatment process.
However, some evidence showed that CAs cannot respond to patients’ statements in all circumstances.16 It also is unclear how well CAs recognize nuanced statements that might signal potential harm. This fits into the greater theme of a major problem with AI: the lack of a reliable response in all circumstances.13
Final Thoughts
The practical implementations of AI in dermatology are still being explored. Given the uncertainty surrounding the COVID-19 pandemic and the future of patient care, AI might serve as an important asset in assisting with the diagnosis and treatment of dermatologic conditions, physician productivity, and patient monitoring.
- Amisha, Malik P, Pathania M, et al. Overview of artificial intelligence in medicine. J Family Med Prim Care. 2019;8:2328-2331. doi:10.4103/jfmpc.jfmpc_440_19
- Han SS, Kim MS, Lim W, et al. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J Invest Dermatol. 2018;138:1529-1538. doi:10.1016/j.jid.2018.01.028
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Brinker TJ, Hekler A, Enk AH, et al. Deep neural networks are superior to dermatologists in melanoma image classification. Eur J Cancer. 2019;119:11-17. doi:10.1016/j.ejca.2019.05.023
- Regulated medical device for detecting skin cancer. SkinVision website. Accessed July 23, 2021. https://www.skinvision.com/hcp/
- Thissen M, Udrea A, Hacking M, et al. mHealth app for risk assessment of pigmented and nonpigmented skin lesions—a study on sensitivity and specificity in detecting malignancy. Telemed J E Health. 2017;23:948-954. doi:10.1089/tmj.2016.0259
- Freeman K, Dinnes J, Chuchu N, et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies. BMJ. 2020;368:m127. doi:10.1136/bmj.m127
- Puri P, Comfere N, Pittelkow MR, et al. COVID-19: an opportunity to build dermatology’s digital future. Dermatol Ther. 2020;33:e14149. doi:10.1111/dth.14149
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Adamson AS, Smith A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 2018;154:1247-1248. doi:10.1001/jamadermatol.2018.2348
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:S0190-9622(13)01296-6
- Alabdulkareem A. Artificial intelligence and dermatologists: friends or foes? J Dermatol Dermatolog Surg. 2019;23:57-60. doi:10.4103/jdds.jdds_19_19
- McGreevey JD 3rd, Hanson CW 3rd, Koppel R. Clinical, legal, and ethical aspects of artificial intelligence-assisted conversational agents in health care. JAMA. 2020;324:552-553. doi:10.1001/jama.2020.2724
- Piau A, Crissey R, Brechemier D, et al. A smartphone chatbot application to optimize monitoring of older patients with cancer. Int J Med Inform. 2019;128:18-23. doi:10.1016/j.ijmedinf.2019.05.013
- Lucas GM, Gratch J, King A, et al. It’s only a computer: virtual humans increase willingness to disclose. Comput Human Behav. 2014;37:94-100. https://doi.org/10.1016/j.chb.2014.04.043
- Miner AS, Milstein A, Schueller S, et al. Smartphone-based conversational agents and responses to questions about mental health, interpersonal violence, and physical health. JAMA Intern Med. 2016;176:619-625. doi:10.1001/jamainternmed.2016.0400
- Amisha, Malik P, Pathania M, et al. Overview of artificial intelligence in medicine. J Family Med Prim Care. 2019;8:2328-2331. doi:10.4103/jfmpc.jfmpc_440_19
- Han SS, Kim MS, Lim W, et al. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J Invest Dermatol. 2018;138:1529-1538. doi:10.1016/j.jid.2018.01.028
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Brinker TJ, Hekler A, Enk AH, et al. Deep neural networks are superior to dermatologists in melanoma image classification. Eur J Cancer. 2019;119:11-17. doi:10.1016/j.ejca.2019.05.023
- Regulated medical device for detecting skin cancer. SkinVision website. Accessed July 23, 2021. https://www.skinvision.com/hcp/
- Thissen M, Udrea A, Hacking M, et al. mHealth app for risk assessment of pigmented and nonpigmented skin lesions—a study on sensitivity and specificity in detecting malignancy. Telemed J E Health. 2017;23:948-954. doi:10.1089/tmj.2016.0259
- Freeman K, Dinnes J, Chuchu N, et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies. BMJ. 2020;368:m127. doi:10.1136/bmj.m127
- Puri P, Comfere N, Pittelkow MR, et al. COVID-19: an opportunity to build dermatology’s digital future. Dermatol Ther. 2020;33:e14149. doi:10.1111/dth.14149
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Adamson AS, Smith A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 2018;154:1247-1248. doi:10.1001/jamadermatol.2018.2348
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:S0190-9622(13)01296-6
- Alabdulkareem A. Artificial intelligence and dermatologists: friends or foes? J Dermatol Dermatolog Surg. 2019;23:57-60. doi:10.4103/jdds.jdds_19_19
- McGreevey JD 3rd, Hanson CW 3rd, Koppel R. Clinical, legal, and ethical aspects of artificial intelligence-assisted conversational agents in health care. JAMA. 2020;324:552-553. doi:10.1001/jama.2020.2724
- Piau A, Crissey R, Brechemier D, et al. A smartphone chatbot application to optimize monitoring of older patients with cancer. Int J Med Inform. 2019;128:18-23. doi:10.1016/j.ijmedinf.2019.05.013
- Lucas GM, Gratch J, King A, et al. It’s only a computer: virtual humans increase willingness to disclose. Comput Human Behav. 2014;37:94-100. https://doi.org/10.1016/j.chb.2014.04.043
- Miner AS, Milstein A, Schueller S, et al. Smartphone-based conversational agents and responses to questions about mental health, interpersonal violence, and physical health. JAMA Intern Med. 2016;176:619-625. doi:10.1001/jamainternmed.2016.0400
Practice Points
- Dermatologists should amass pictures of dermatologic conditions in skin of color to contribute to growing awareness and knowledge of presentation of disease in this population.
- Dermatologists should use artificial intelligence as a tool for delivering more efficient and beneficial patient care.
Trial yields evidence that laser resurfacing may prevent NMSC in aged skin
A on treated areas, according to the results of a small, randomized trial.
“Previous research suggests a new model to explain why older patients obtain nonmelanoma skin cancer in areas of ongoing sun exposure,” presenting author Jeffrey Wargo, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “Insulinlike growth factor-1 produced by dermal fibroblasts dictates how overlying skin keratinocytes respond to UVB radiation. The skin of a patient aged in their 20s produces normal levels of healthy fibroblasts, normal levels of insulinlike growth factor 1, and appropriate UVB response via activation of nucleotide excision, repair, and DNA damage checkpoint-signaling systems.”
Older patients, meanwhile, have an increase in senescent fibroblasts, decreased insulinlike growth factor-1 (IGF-1), and an inappropriate UVB response to DNA damage, continued Dr. Wargo, a dermatologist at the Ohio State University Wexner Medical Center in Columbus. Previous studies conducted by his mentor, Jeffrey B. Travers, MD, PhD, a dermatologist and pharmacologist at Wright State University, Dayton, showed that fractionated laser resurfacing (FLR) restores UVB response in older patients’ skin by resulting in new fibroblasts and increased levels of IGF 2 years post wounding.
To determine if FLR of aged skin can prevent the development of actinic keratosis (AK) and nonmelanoma skin cancer, Dr. Travers and Dr. Wargo recruited 48 patients at the Dayton VA Medical Center who were 60 years or older and had at least five AKs on each arm that were 3 mm or smaller, with nothing concerning for skin cancer at the screening visit.
Randomization of which arm was treated was based on an odd or even Social Security Number. That arm was treated with the 2,790 nm Erbium:YSSG ablative laser at 120 J/m2 with one pass at 24% coverage from the elbow to hand dorsally. Previously published data reported outcomes for 30 of these patients at 3 and 6 months following treatment. Subsequent to that report, 18 additional subjects have been recruited to the study and follow-up has been extended. Of the 48 patients, 47 were male and their average age was 74, with a range between 61 and 87 years.
At 3 months following FLR, the ratio of AKs on the treated vs. untreated arms was reduced by fourfold, with a P value less than .00001, Dr. Wargo reported. “Throughout the current 30-month follow-up period, this ratio has been maintained,” he said. “In fact, none of the ratios determined at 3, 6, 12, 18, 24, or 30 months post FLR are significantly different. Hence, as described in our first report on this work, these data indicate FLR is an effective treatment for existing AKs. However, our model predicts that FLR treatment will also prevent the occurrence of new AK lesions.”
Among 19 of the study participants who have been followed out to 30 months, untreated arms continued to accumulate increasing number of AKs. In contrast, AKs on treated arms are decreasing with time, indicating the lack of newly initiated lesions.
“A second analysis of the data posits that, if FLR were only removing existing lesions, one would predict the number of AKs that were present at 3 months on both the untreated and FLR-treated [arms] would accumulate at the same rate subsequent to 3 months point in time,” Dr. Wargo said.
He pointed out that 12 patients were removed from the study: two at 12 months, one at 18 months, eight at 24 months, and one at 30 months, as they were found to have 20 or more AKs on their untreated arm and required treatment.
Over the entire study period, “consistent with the notion that FLR was preventing new actinic neoplasia, we noted a dramatic difference in numbers of nonmelanoma skin cancer diagnosed in the untreated areas (22) versus FLR treated areas (2),” Dr. Wargo said. The majority of nonmelanoma skin cancers diagnosed were SCC (17) and 5 basal cell carcinomas on the untreated arms, whereas the 2 diagnosed on the treated arm were SCC. “These studies indicate that a dermal-wounding strategy involving FLR, which upregulates dermal IGF-1 levels, not only treats AKs but prevents nonmelanoma skin cancer,” he said.
The study was funded by the National Institutes of Health. Dr. Travers is the principal investigator. Dr. Wargo reported having no financial disclosures.
A on treated areas, according to the results of a small, randomized trial.
“Previous research suggests a new model to explain why older patients obtain nonmelanoma skin cancer in areas of ongoing sun exposure,” presenting author Jeffrey Wargo, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “Insulinlike growth factor-1 produced by dermal fibroblasts dictates how overlying skin keratinocytes respond to UVB radiation. The skin of a patient aged in their 20s produces normal levels of healthy fibroblasts, normal levels of insulinlike growth factor 1, and appropriate UVB response via activation of nucleotide excision, repair, and DNA damage checkpoint-signaling systems.”
Older patients, meanwhile, have an increase in senescent fibroblasts, decreased insulinlike growth factor-1 (IGF-1), and an inappropriate UVB response to DNA damage, continued Dr. Wargo, a dermatologist at the Ohio State University Wexner Medical Center in Columbus. Previous studies conducted by his mentor, Jeffrey B. Travers, MD, PhD, a dermatologist and pharmacologist at Wright State University, Dayton, showed that fractionated laser resurfacing (FLR) restores UVB response in older patients’ skin by resulting in new fibroblasts and increased levels of IGF 2 years post wounding.
To determine if FLR of aged skin can prevent the development of actinic keratosis (AK) and nonmelanoma skin cancer, Dr. Travers and Dr. Wargo recruited 48 patients at the Dayton VA Medical Center who were 60 years or older and had at least five AKs on each arm that were 3 mm or smaller, with nothing concerning for skin cancer at the screening visit.
Randomization of which arm was treated was based on an odd or even Social Security Number. That arm was treated with the 2,790 nm Erbium:YSSG ablative laser at 120 J/m2 with one pass at 24% coverage from the elbow to hand dorsally. Previously published data reported outcomes for 30 of these patients at 3 and 6 months following treatment. Subsequent to that report, 18 additional subjects have been recruited to the study and follow-up has been extended. Of the 48 patients, 47 were male and their average age was 74, with a range between 61 and 87 years.
At 3 months following FLR, the ratio of AKs on the treated vs. untreated arms was reduced by fourfold, with a P value less than .00001, Dr. Wargo reported. “Throughout the current 30-month follow-up period, this ratio has been maintained,” he said. “In fact, none of the ratios determined at 3, 6, 12, 18, 24, or 30 months post FLR are significantly different. Hence, as described in our first report on this work, these data indicate FLR is an effective treatment for existing AKs. However, our model predicts that FLR treatment will also prevent the occurrence of new AK lesions.”
Among 19 of the study participants who have been followed out to 30 months, untreated arms continued to accumulate increasing number of AKs. In contrast, AKs on treated arms are decreasing with time, indicating the lack of newly initiated lesions.
“A second analysis of the data posits that, if FLR were only removing existing lesions, one would predict the number of AKs that were present at 3 months on both the untreated and FLR-treated [arms] would accumulate at the same rate subsequent to 3 months point in time,” Dr. Wargo said.
He pointed out that 12 patients were removed from the study: two at 12 months, one at 18 months, eight at 24 months, and one at 30 months, as they were found to have 20 or more AKs on their untreated arm and required treatment.
Over the entire study period, “consistent with the notion that FLR was preventing new actinic neoplasia, we noted a dramatic difference in numbers of nonmelanoma skin cancer diagnosed in the untreated areas (22) versus FLR treated areas (2),” Dr. Wargo said. The majority of nonmelanoma skin cancers diagnosed were SCC (17) and 5 basal cell carcinomas on the untreated arms, whereas the 2 diagnosed on the treated arm were SCC. “These studies indicate that a dermal-wounding strategy involving FLR, which upregulates dermal IGF-1 levels, not only treats AKs but prevents nonmelanoma skin cancer,” he said.
The study was funded by the National Institutes of Health. Dr. Travers is the principal investigator. Dr. Wargo reported having no financial disclosures.
A on treated areas, according to the results of a small, randomized trial.
“Previous research suggests a new model to explain why older patients obtain nonmelanoma skin cancer in areas of ongoing sun exposure,” presenting author Jeffrey Wargo, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “Insulinlike growth factor-1 produced by dermal fibroblasts dictates how overlying skin keratinocytes respond to UVB radiation. The skin of a patient aged in their 20s produces normal levels of healthy fibroblasts, normal levels of insulinlike growth factor 1, and appropriate UVB response via activation of nucleotide excision, repair, and DNA damage checkpoint-signaling systems.”
Older patients, meanwhile, have an increase in senescent fibroblasts, decreased insulinlike growth factor-1 (IGF-1), and an inappropriate UVB response to DNA damage, continued Dr. Wargo, a dermatologist at the Ohio State University Wexner Medical Center in Columbus. Previous studies conducted by his mentor, Jeffrey B. Travers, MD, PhD, a dermatologist and pharmacologist at Wright State University, Dayton, showed that fractionated laser resurfacing (FLR) restores UVB response in older patients’ skin by resulting in new fibroblasts and increased levels of IGF 2 years post wounding.
To determine if FLR of aged skin can prevent the development of actinic keratosis (AK) and nonmelanoma skin cancer, Dr. Travers and Dr. Wargo recruited 48 patients at the Dayton VA Medical Center who were 60 years or older and had at least five AKs on each arm that were 3 mm or smaller, with nothing concerning for skin cancer at the screening visit.
Randomization of which arm was treated was based on an odd or even Social Security Number. That arm was treated with the 2,790 nm Erbium:YSSG ablative laser at 120 J/m2 with one pass at 24% coverage from the elbow to hand dorsally. Previously published data reported outcomes for 30 of these patients at 3 and 6 months following treatment. Subsequent to that report, 18 additional subjects have been recruited to the study and follow-up has been extended. Of the 48 patients, 47 were male and their average age was 74, with a range between 61 and 87 years.
At 3 months following FLR, the ratio of AKs on the treated vs. untreated arms was reduced by fourfold, with a P value less than .00001, Dr. Wargo reported. “Throughout the current 30-month follow-up period, this ratio has been maintained,” he said. “In fact, none of the ratios determined at 3, 6, 12, 18, 24, or 30 months post FLR are significantly different. Hence, as described in our first report on this work, these data indicate FLR is an effective treatment for existing AKs. However, our model predicts that FLR treatment will also prevent the occurrence of new AK lesions.”
Among 19 of the study participants who have been followed out to 30 months, untreated arms continued to accumulate increasing number of AKs. In contrast, AKs on treated arms are decreasing with time, indicating the lack of newly initiated lesions.
“A second analysis of the data posits that, if FLR were only removing existing lesions, one would predict the number of AKs that were present at 3 months on both the untreated and FLR-treated [arms] would accumulate at the same rate subsequent to 3 months point in time,” Dr. Wargo said.
He pointed out that 12 patients were removed from the study: two at 12 months, one at 18 months, eight at 24 months, and one at 30 months, as they were found to have 20 or more AKs on their untreated arm and required treatment.
Over the entire study period, “consistent with the notion that FLR was preventing new actinic neoplasia, we noted a dramatic difference in numbers of nonmelanoma skin cancer diagnosed in the untreated areas (22) versus FLR treated areas (2),” Dr. Wargo said. The majority of nonmelanoma skin cancers diagnosed were SCC (17) and 5 basal cell carcinomas on the untreated arms, whereas the 2 diagnosed on the treated arm were SCC. “These studies indicate that a dermal-wounding strategy involving FLR, which upregulates dermal IGF-1 levels, not only treats AKs but prevents nonmelanoma skin cancer,” he said.
The study was funded by the National Institutes of Health. Dr. Travers is the principal investigator. Dr. Wargo reported having no financial disclosures.
FROM ASLMS 2021
AAD unveils new guidelines for actinic keratosis management
. They also conditionally recommend the use of photodynamic therapy (PDT) and diclofenac for the treatment of AK, both individually and as part of combination therapy regimens.
Those are two of 18 recommendations made by 14 members of the multidisciplinary work group that convened to assemble the AAD’s first-ever guidelines on the management of AKs, which were published online April 2 in the Journal of the American Academy of Dermatology. The group, cochaired by Daniel B. Eisen, MD, professor of clinical dermatology at the University of California, Davis, and Todd E. Schlesinger, MD, medical director of the Dermatology and Laser Center of Charleston, S.C., conducted a systematic review to address five clinical questions on the management of AKs in adults. The questions were: What are the efficacy, effectiveness, and adverse effects of surgical and chemical peel treatments for AK; of topically applied agents for AK; of energy devices and other miscellaneous treatments for AK; and of combination therapy for the treatment of AK? And what are the special considerations to be taken into account when treating AK in immunocompromised individuals?
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
“As a participant in the work group, I was impressed by the level of care and detail and the involvement of relevant stakeholders, including a patient advocate, as well as having the draft guidelines go out to the AAD membership at large, and evaluating every comment that came in,” Maryam Asgari, MD, MPH, professor of dermatology at Harvard University, Boston, said in an interview. “The academy sought stakeholder and leadership input in revising and revamping the guidelines. The AAD also made sure the work group had minimal conflicts of interest by requiring that the majority of experts convened did not have relevant financial conflicts of interest. That might not be the case in a publication such as a systematic review, where no threshold for financial conflict of interest for coauthorship is set.”
Of the 18 recommendations the work group made for patients with AKs, only four were ranked as “strong” based on the evidence reviewed, while the rest were ranked as “conditional.”
The strong recommendations include the use of UV protection, field treatment with 5-FU, field treatment with imiquimod, and the use of cryosurgery.
The first four conditional recommendations for patients with AKs include the use of diclofenac, treatment with cryosurgery over CO2 laser ablation, aminolevulinic acid (ALA)–red-light PDT, and 1- to 4-hour 5-ALA incubation time to enhance complete clearance with red-light PDT. The work group also conditionally recommends ALA-daylight PDT as less painful than but equally effective as ALA–red-light PDT.
In the clinical experience of Catherine M. DiGiorgio, MD, who was not involved in the guidelines, daylight PDT with ALA is a viable, cost-effective option. “Patients can come into the office, apply the ALA and then they go outside for 2 hours – not in direct sunlight but in a shady area,” Dr. DiGiorgio, a dermatologist who practices at the Boston Center for Facial Rejuvenation, said in an interview. “That’s a cost-effective treatment for patients who perhaps can’t afford some of the chemotherapy creams. I don’t think we’ve adopted ALA-daylight PDT here in the U.S. very much.”
The work group noted that topical 1% tirbanibulin ointment, a novel microtubule inhibitor, was approved for treatment of AKs on the face and scalp by the Food and Drug Administration after the guidelines had been put together.
Several trials of combination therapy were included in the review of evidence, prompting several recommendations. For example, the work group conditionally recommends combined 5-FU cream and cryosurgery over cryosurgery alone, based on moderate-quality evidence and conditionally recommends combined imiquimod and cryosurgery over cryosurgery alone based on low-quality evidence. In addition, the work group conditionally recommends against the use of 3% diclofenac in addition to cryosurgery, favoring cryosurgery alone based on low-quality evidence, and conditionally recommends against the use of imiquimod typically after ALA–blue-light PDT, based on moderate-quality data.
“The additional treatment with imiquimod was thought to add both expense and burden to the patient, which negates much of the perceived convenience of using PDT as a stand-alone treatment modality and which is not mitigated by the modest increase in lesion reduction,” the authors wrote.
The guidelines emphasize the importance of shared decision-making between patients and clinicians on the choice of therapy, a point that resonates with Dr. DiGiorgio. Success of a treatment can depend on whether a patient is willing to go through with it, she said. “Some patients don’t want to do a therapeutic topical like 5-FU. They prefer to come in and have cryotherapy done. Others prefer to not come in and have the cream at home and treat themselves.”
Assembling the guidelines exposed certain gaps in research, according to the work group. Of the 18 recommendations, seven were based on low-quality evidence, and there were not enough data to make guidelines for the treatment of AKs in immunocompromised individuals.
“I can’t tell you the number of times we in the committee sat back and said, ‘we need to have a randomized trial that looks at this, or compares this to that head on,’” Dr. Asgari said. Such limitations “give researchers direction for where the areas of study need to go to help us answer some of these management conundrums.”
She added that the new guidelines “give clinicians a leg to stand on” when an insurer pushes back on a recommended treatment for AK. “It gives you a way to have dialogue with insurers if you’re prescribing some of these treatments.”
The guidelines authors write that there is “strong theoretic rationale for the treatment of AK to prevent skin cancers” but acknowledge that only a few studies in the review “report the incidence of skin cancer as an outcome measure or have sufficient follow-up to viably measure carcinoma development.” In addition, “more long-term research is needed to validate our current understanding of skin cancer progression from AKs to keratinocyte carcinoma.”
Dr. DiGiorgio thinks about this differently. “I think treatment of AKs does prevent skin cancers,” she said. “We call them precancers as we’re treating our patients because we know a certain percentage of them can develop into skin cancers over time.”
The study was funded by internal funds from the AAD. Dr. Asgari disclosed that she serves as an investigator for Pfizer. Several of the other authors reported having financial disclosures.
Dr. DiGiorgio reported having no financial disclosures.
. They also conditionally recommend the use of photodynamic therapy (PDT) and diclofenac for the treatment of AK, both individually and as part of combination therapy regimens.
Those are two of 18 recommendations made by 14 members of the multidisciplinary work group that convened to assemble the AAD’s first-ever guidelines on the management of AKs, which were published online April 2 in the Journal of the American Academy of Dermatology. The group, cochaired by Daniel B. Eisen, MD, professor of clinical dermatology at the University of California, Davis, and Todd E. Schlesinger, MD, medical director of the Dermatology and Laser Center of Charleston, S.C., conducted a systematic review to address five clinical questions on the management of AKs in adults. The questions were: What are the efficacy, effectiveness, and adverse effects of surgical and chemical peel treatments for AK; of topically applied agents for AK; of energy devices and other miscellaneous treatments for AK; and of combination therapy for the treatment of AK? And what are the special considerations to be taken into account when treating AK in immunocompromised individuals?
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
“As a participant in the work group, I was impressed by the level of care and detail and the involvement of relevant stakeholders, including a patient advocate, as well as having the draft guidelines go out to the AAD membership at large, and evaluating every comment that came in,” Maryam Asgari, MD, MPH, professor of dermatology at Harvard University, Boston, said in an interview. “The academy sought stakeholder and leadership input in revising and revamping the guidelines. The AAD also made sure the work group had minimal conflicts of interest by requiring that the majority of experts convened did not have relevant financial conflicts of interest. That might not be the case in a publication such as a systematic review, where no threshold for financial conflict of interest for coauthorship is set.”
Of the 18 recommendations the work group made for patients with AKs, only four were ranked as “strong” based on the evidence reviewed, while the rest were ranked as “conditional.”
The strong recommendations include the use of UV protection, field treatment with 5-FU, field treatment with imiquimod, and the use of cryosurgery.
The first four conditional recommendations for patients with AKs include the use of diclofenac, treatment with cryosurgery over CO2 laser ablation, aminolevulinic acid (ALA)–red-light PDT, and 1- to 4-hour 5-ALA incubation time to enhance complete clearance with red-light PDT. The work group also conditionally recommends ALA-daylight PDT as less painful than but equally effective as ALA–red-light PDT.
In the clinical experience of Catherine M. DiGiorgio, MD, who was not involved in the guidelines, daylight PDT with ALA is a viable, cost-effective option. “Patients can come into the office, apply the ALA and then they go outside for 2 hours – not in direct sunlight but in a shady area,” Dr. DiGiorgio, a dermatologist who practices at the Boston Center for Facial Rejuvenation, said in an interview. “That’s a cost-effective treatment for patients who perhaps can’t afford some of the chemotherapy creams. I don’t think we’ve adopted ALA-daylight PDT here in the U.S. very much.”
The work group noted that topical 1% tirbanibulin ointment, a novel microtubule inhibitor, was approved for treatment of AKs on the face and scalp by the Food and Drug Administration after the guidelines had been put together.
Several trials of combination therapy were included in the review of evidence, prompting several recommendations. For example, the work group conditionally recommends combined 5-FU cream and cryosurgery over cryosurgery alone, based on moderate-quality evidence and conditionally recommends combined imiquimod and cryosurgery over cryosurgery alone based on low-quality evidence. In addition, the work group conditionally recommends against the use of 3% diclofenac in addition to cryosurgery, favoring cryosurgery alone based on low-quality evidence, and conditionally recommends against the use of imiquimod typically after ALA–blue-light PDT, based on moderate-quality data.
“The additional treatment with imiquimod was thought to add both expense and burden to the patient, which negates much of the perceived convenience of using PDT as a stand-alone treatment modality and which is not mitigated by the modest increase in lesion reduction,” the authors wrote.
The guidelines emphasize the importance of shared decision-making between patients and clinicians on the choice of therapy, a point that resonates with Dr. DiGiorgio. Success of a treatment can depend on whether a patient is willing to go through with it, she said. “Some patients don’t want to do a therapeutic topical like 5-FU. They prefer to come in and have cryotherapy done. Others prefer to not come in and have the cream at home and treat themselves.”
Assembling the guidelines exposed certain gaps in research, according to the work group. Of the 18 recommendations, seven were based on low-quality evidence, and there were not enough data to make guidelines for the treatment of AKs in immunocompromised individuals.
“I can’t tell you the number of times we in the committee sat back and said, ‘we need to have a randomized trial that looks at this, or compares this to that head on,’” Dr. Asgari said. Such limitations “give researchers direction for where the areas of study need to go to help us answer some of these management conundrums.”
She added that the new guidelines “give clinicians a leg to stand on” when an insurer pushes back on a recommended treatment for AK. “It gives you a way to have dialogue with insurers if you’re prescribing some of these treatments.”
The guidelines authors write that there is “strong theoretic rationale for the treatment of AK to prevent skin cancers” but acknowledge that only a few studies in the review “report the incidence of skin cancer as an outcome measure or have sufficient follow-up to viably measure carcinoma development.” In addition, “more long-term research is needed to validate our current understanding of skin cancer progression from AKs to keratinocyte carcinoma.”
Dr. DiGiorgio thinks about this differently. “I think treatment of AKs does prevent skin cancers,” she said. “We call them precancers as we’re treating our patients because we know a certain percentage of them can develop into skin cancers over time.”
The study was funded by internal funds from the AAD. Dr. Asgari disclosed that she serves as an investigator for Pfizer. Several of the other authors reported having financial disclosures.
Dr. DiGiorgio reported having no financial disclosures.
. They also conditionally recommend the use of photodynamic therapy (PDT) and diclofenac for the treatment of AK, both individually and as part of combination therapy regimens.
Those are two of 18 recommendations made by 14 members of the multidisciplinary work group that convened to assemble the AAD’s first-ever guidelines on the management of AKs, which were published online April 2 in the Journal of the American Academy of Dermatology. The group, cochaired by Daniel B. Eisen, MD, professor of clinical dermatology at the University of California, Davis, and Todd E. Schlesinger, MD, medical director of the Dermatology and Laser Center of Charleston, S.C., conducted a systematic review to address five clinical questions on the management of AKs in adults. The questions were: What are the efficacy, effectiveness, and adverse effects of surgical and chemical peel treatments for AK; of topically applied agents for AK; of energy devices and other miscellaneous treatments for AK; and of combination therapy for the treatment of AK? And what are the special considerations to be taken into account when treating AK in immunocompromised individuals?
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
“As a participant in the work group, I was impressed by the level of care and detail and the involvement of relevant stakeholders, including a patient advocate, as well as having the draft guidelines go out to the AAD membership at large, and evaluating every comment that came in,” Maryam Asgari, MD, MPH, professor of dermatology at Harvard University, Boston, said in an interview. “The academy sought stakeholder and leadership input in revising and revamping the guidelines. The AAD also made sure the work group had minimal conflicts of interest by requiring that the majority of experts convened did not have relevant financial conflicts of interest. That might not be the case in a publication such as a systematic review, where no threshold for financial conflict of interest for coauthorship is set.”
Of the 18 recommendations the work group made for patients with AKs, only four were ranked as “strong” based on the evidence reviewed, while the rest were ranked as “conditional.”
The strong recommendations include the use of UV protection, field treatment with 5-FU, field treatment with imiquimod, and the use of cryosurgery.
The first four conditional recommendations for patients with AKs include the use of diclofenac, treatment with cryosurgery over CO2 laser ablation, aminolevulinic acid (ALA)–red-light PDT, and 1- to 4-hour 5-ALA incubation time to enhance complete clearance with red-light PDT. The work group also conditionally recommends ALA-daylight PDT as less painful than but equally effective as ALA–red-light PDT.
In the clinical experience of Catherine M. DiGiorgio, MD, who was not involved in the guidelines, daylight PDT with ALA is a viable, cost-effective option. “Patients can come into the office, apply the ALA and then they go outside for 2 hours – not in direct sunlight but in a shady area,” Dr. DiGiorgio, a dermatologist who practices at the Boston Center for Facial Rejuvenation, said in an interview. “That’s a cost-effective treatment for patients who perhaps can’t afford some of the chemotherapy creams. I don’t think we’ve adopted ALA-daylight PDT here in the U.S. very much.”
The work group noted that topical 1% tirbanibulin ointment, a novel microtubule inhibitor, was approved for treatment of AKs on the face and scalp by the Food and Drug Administration after the guidelines had been put together.
Several trials of combination therapy were included in the review of evidence, prompting several recommendations. For example, the work group conditionally recommends combined 5-FU cream and cryosurgery over cryosurgery alone, based on moderate-quality evidence and conditionally recommends combined imiquimod and cryosurgery over cryosurgery alone based on low-quality evidence. In addition, the work group conditionally recommends against the use of 3% diclofenac in addition to cryosurgery, favoring cryosurgery alone based on low-quality evidence, and conditionally recommends against the use of imiquimod typically after ALA–blue-light PDT, based on moderate-quality data.
“The additional treatment with imiquimod was thought to add both expense and burden to the patient, which negates much of the perceived convenience of using PDT as a stand-alone treatment modality and which is not mitigated by the modest increase in lesion reduction,” the authors wrote.
The guidelines emphasize the importance of shared decision-making between patients and clinicians on the choice of therapy, a point that resonates with Dr. DiGiorgio. Success of a treatment can depend on whether a patient is willing to go through with it, she said. “Some patients don’t want to do a therapeutic topical like 5-FU. They prefer to come in and have cryotherapy done. Others prefer to not come in and have the cream at home and treat themselves.”
Assembling the guidelines exposed certain gaps in research, according to the work group. Of the 18 recommendations, seven were based on low-quality evidence, and there were not enough data to make guidelines for the treatment of AKs in immunocompromised individuals.
“I can’t tell you the number of times we in the committee sat back and said, ‘we need to have a randomized trial that looks at this, or compares this to that head on,’” Dr. Asgari said. Such limitations “give researchers direction for where the areas of study need to go to help us answer some of these management conundrums.”
She added that the new guidelines “give clinicians a leg to stand on” when an insurer pushes back on a recommended treatment for AK. “It gives you a way to have dialogue with insurers if you’re prescribing some of these treatments.”
The guidelines authors write that there is “strong theoretic rationale for the treatment of AK to prevent skin cancers” but acknowledge that only a few studies in the review “report the incidence of skin cancer as an outcome measure or have sufficient follow-up to viably measure carcinoma development.” In addition, “more long-term research is needed to validate our current understanding of skin cancer progression from AKs to keratinocyte carcinoma.”
Dr. DiGiorgio thinks about this differently. “I think treatment of AKs does prevent skin cancers,” she said. “We call them precancers as we’re treating our patients because we know a certain percentage of them can develop into skin cancers over time.”
The study was funded by internal funds from the AAD. Dr. Asgari disclosed that she serves as an investigator for Pfizer. Several of the other authors reported having financial disclosures.
Dr. DiGiorgio reported having no financial disclosures.
FROM JAAD
Less pain, same gain with tirbanibulin for actinic keratosis
“with transient local reactions,” according to the results of two identically designed trials.
However, the results, assessed at day 57 and out to 1 year of follow-up, were associated with recurrence of lesions at 1 year, noted lead author Andrew Blauvelt, MD, president of the Oregon Medical Research Center, Portland, and colleagues.
“The incidence of recurrence with conventional treatment has ranged from 20% to 96%,” they noted. “Among patients who had complete clearance at day 57 in the current trials, the estimated incidence of recurrence of previously cleared lesions was 47% at 1 year.” At 1 year, they added, “the estimated incidence of any lesions (new or recurrent) within the application area was 73%” and the estimate of sustained complete clearance was 27%.
A total of 700 adults completed the two multicenter, double-blind, parallel-group, vehicle-controlled trials, conducted concurrently between September 2017 and April 2019 at 62 U.S. sites. The results were published in the New England Journal of Medicine.
To be eligible, patients, mostly White men, had to have four to eight clinically typical, visible, and discrete AK lesions on the face or scalp within a contiguous area measuring 25 cm2. They were randomly assigned to treatment with either tirbanibulin 1% ointment or vehicle ointment (containing monoglycerides, diglycerides, and propylene glycol), which they applied once daily to the entire contiguous area for 5 days.
Pooled data across the two trials showed that the primary outcome, complete clearance of all lesions at day 57, occurred in 49% of the tirbanibulin groups versus 9% of the vehicle groups, and partial clearance (the secondary outcome) occurred in 72% versus 18% respectively. For both outcomes, and in both trials, all results were statistically significant.
Of the 174 patients who received tirbanibulin and had complete clearance, 124 had one or more lesions develop within the application area during follow-up, the authors reported. Of these, 58% had recurrences, while 42% had new lesions.
While individual AK lesions are typically treated with cryosurgery, the study authors noted that treatment of multiple lesions involves topical agents, such as fluorouracil, diclofenac, imiquimod, or ingenol mebutate, and photodynamic therapy, some of which have to be administered over periods of weeks or months and “may be associated with local reactions of pain, irritation, erosions, ulcerations, and irreversible skin changes of pigmentation and scarring,” which may reduce adherence.
In contrast, the current studies showed the most common local reactions to tirbanibulin were erythema in 91% of patients and flaking or scaling in 82%, with transient adverse events including application-site pain in 10% and pruritus in 9%.
“Unlike with most topical treatments for actinic keratosis ... severe local reactions, including vesiculation or pustulation and erosion or ulceration, were infrequent with tirbanibulin ointment,” the authors noted. “This could be due to the relatively short, 5-day course of once-daily treatment.”
They concluded that “larger and longer trials are necessary to determine the effects and risks” of treatment with tirbanibulin for treating AK.
Tirbanibulin, a synthetic inhibitor of tubulin polymerization and Src kinase signaling, was approved by the Food and Drug Administration in December 2020, for the topical treatment of AK of the face or scalp.
Asked to comment on the findings, Neal Bhatia, MD, a dermatologist and researcher at Therapeutics Dermatology, San Diego, who was not involved with the study, said that “a treatment with a 5-day course and excellent tolerability will make dermatologists rethink the old practice of ‘freeze and go.’ ”
In an interview, he added, “tirbanibulin comes to the U.S. market for treating AKs at a great time, as ingenol mebutate has been withdrawn and the others are not widely supported. The mechanism of promoting apoptosis and inducing cell cycle arrest directly correlates to the local skin reaction profile of less crusting, vesiculation, and overall signs of skin necrosis as compared to [5-fluorouracil] and ingenol mebutate, which work via that pathway. As a result, there is a direct impact on the hyperproliferation of atypical keratinocytes that will treat visible and subclinical disease.”
“The ointment vehicle is also novel as previous therapies have been in either creams or gels,” he said.
The two trials were funded by tirbanibulin manufacturer Athenex. Dr. Blauvelt reported receiving consulting fees from Athenex and other pharmaceutical companies, including Almirall, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant Sciences. Other author disclosures included serving as a consultant to Athenex and other companies. Several authors are Athenex employees. Dr. Bhatia disclosed that he is an adviser and consultant for Almirall and has been an investigator for multiple other AK treatments.
“with transient local reactions,” according to the results of two identically designed trials.
However, the results, assessed at day 57 and out to 1 year of follow-up, were associated with recurrence of lesions at 1 year, noted lead author Andrew Blauvelt, MD, president of the Oregon Medical Research Center, Portland, and colleagues.
“The incidence of recurrence with conventional treatment has ranged from 20% to 96%,” they noted. “Among patients who had complete clearance at day 57 in the current trials, the estimated incidence of recurrence of previously cleared lesions was 47% at 1 year.” At 1 year, they added, “the estimated incidence of any lesions (new or recurrent) within the application area was 73%” and the estimate of sustained complete clearance was 27%.
A total of 700 adults completed the two multicenter, double-blind, parallel-group, vehicle-controlled trials, conducted concurrently between September 2017 and April 2019 at 62 U.S. sites. The results were published in the New England Journal of Medicine.
To be eligible, patients, mostly White men, had to have four to eight clinically typical, visible, and discrete AK lesions on the face or scalp within a contiguous area measuring 25 cm2. They were randomly assigned to treatment with either tirbanibulin 1% ointment or vehicle ointment (containing monoglycerides, diglycerides, and propylene glycol), which they applied once daily to the entire contiguous area for 5 days.
Pooled data across the two trials showed that the primary outcome, complete clearance of all lesions at day 57, occurred in 49% of the tirbanibulin groups versus 9% of the vehicle groups, and partial clearance (the secondary outcome) occurred in 72% versus 18% respectively. For both outcomes, and in both trials, all results were statistically significant.
Of the 174 patients who received tirbanibulin and had complete clearance, 124 had one or more lesions develop within the application area during follow-up, the authors reported. Of these, 58% had recurrences, while 42% had new lesions.
While individual AK lesions are typically treated with cryosurgery, the study authors noted that treatment of multiple lesions involves topical agents, such as fluorouracil, diclofenac, imiquimod, or ingenol mebutate, and photodynamic therapy, some of which have to be administered over periods of weeks or months and “may be associated with local reactions of pain, irritation, erosions, ulcerations, and irreversible skin changes of pigmentation and scarring,” which may reduce adherence.
In contrast, the current studies showed the most common local reactions to tirbanibulin were erythema in 91% of patients and flaking or scaling in 82%, with transient adverse events including application-site pain in 10% and pruritus in 9%.
“Unlike with most topical treatments for actinic keratosis ... severe local reactions, including vesiculation or pustulation and erosion or ulceration, were infrequent with tirbanibulin ointment,” the authors noted. “This could be due to the relatively short, 5-day course of once-daily treatment.”
They concluded that “larger and longer trials are necessary to determine the effects and risks” of treatment with tirbanibulin for treating AK.
Tirbanibulin, a synthetic inhibitor of tubulin polymerization and Src kinase signaling, was approved by the Food and Drug Administration in December 2020, for the topical treatment of AK of the face or scalp.
Asked to comment on the findings, Neal Bhatia, MD, a dermatologist and researcher at Therapeutics Dermatology, San Diego, who was not involved with the study, said that “a treatment with a 5-day course and excellent tolerability will make dermatologists rethink the old practice of ‘freeze and go.’ ”
In an interview, he added, “tirbanibulin comes to the U.S. market for treating AKs at a great time, as ingenol mebutate has been withdrawn and the others are not widely supported. The mechanism of promoting apoptosis and inducing cell cycle arrest directly correlates to the local skin reaction profile of less crusting, vesiculation, and overall signs of skin necrosis as compared to [5-fluorouracil] and ingenol mebutate, which work via that pathway. As a result, there is a direct impact on the hyperproliferation of atypical keratinocytes that will treat visible and subclinical disease.”
“The ointment vehicle is also novel as previous therapies have been in either creams or gels,” he said.
The two trials were funded by tirbanibulin manufacturer Athenex. Dr. Blauvelt reported receiving consulting fees from Athenex and other pharmaceutical companies, including Almirall, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant Sciences. Other author disclosures included serving as a consultant to Athenex and other companies. Several authors are Athenex employees. Dr. Bhatia disclosed that he is an adviser and consultant for Almirall and has been an investigator for multiple other AK treatments.
“with transient local reactions,” according to the results of two identically designed trials.
However, the results, assessed at day 57 and out to 1 year of follow-up, were associated with recurrence of lesions at 1 year, noted lead author Andrew Blauvelt, MD, president of the Oregon Medical Research Center, Portland, and colleagues.
“The incidence of recurrence with conventional treatment has ranged from 20% to 96%,” they noted. “Among patients who had complete clearance at day 57 in the current trials, the estimated incidence of recurrence of previously cleared lesions was 47% at 1 year.” At 1 year, they added, “the estimated incidence of any lesions (new or recurrent) within the application area was 73%” and the estimate of sustained complete clearance was 27%.
A total of 700 adults completed the two multicenter, double-blind, parallel-group, vehicle-controlled trials, conducted concurrently between September 2017 and April 2019 at 62 U.S. sites. The results were published in the New England Journal of Medicine.
To be eligible, patients, mostly White men, had to have four to eight clinically typical, visible, and discrete AK lesions on the face or scalp within a contiguous area measuring 25 cm2. They were randomly assigned to treatment with either tirbanibulin 1% ointment or vehicle ointment (containing monoglycerides, diglycerides, and propylene glycol), which they applied once daily to the entire contiguous area for 5 days.
Pooled data across the two trials showed that the primary outcome, complete clearance of all lesions at day 57, occurred in 49% of the tirbanibulin groups versus 9% of the vehicle groups, and partial clearance (the secondary outcome) occurred in 72% versus 18% respectively. For both outcomes, and in both trials, all results were statistically significant.
Of the 174 patients who received tirbanibulin and had complete clearance, 124 had one or more lesions develop within the application area during follow-up, the authors reported. Of these, 58% had recurrences, while 42% had new lesions.
While individual AK lesions are typically treated with cryosurgery, the study authors noted that treatment of multiple lesions involves topical agents, such as fluorouracil, diclofenac, imiquimod, or ingenol mebutate, and photodynamic therapy, some of which have to be administered over periods of weeks or months and “may be associated with local reactions of pain, irritation, erosions, ulcerations, and irreversible skin changes of pigmentation and scarring,” which may reduce adherence.
In contrast, the current studies showed the most common local reactions to tirbanibulin were erythema in 91% of patients and flaking or scaling in 82%, with transient adverse events including application-site pain in 10% and pruritus in 9%.
“Unlike with most topical treatments for actinic keratosis ... severe local reactions, including vesiculation or pustulation and erosion or ulceration, were infrequent with tirbanibulin ointment,” the authors noted. “This could be due to the relatively short, 5-day course of once-daily treatment.”
They concluded that “larger and longer trials are necessary to determine the effects and risks” of treatment with tirbanibulin for treating AK.
Tirbanibulin, a synthetic inhibitor of tubulin polymerization and Src kinase signaling, was approved by the Food and Drug Administration in December 2020, for the topical treatment of AK of the face or scalp.
Asked to comment on the findings, Neal Bhatia, MD, a dermatologist and researcher at Therapeutics Dermatology, San Diego, who was not involved with the study, said that “a treatment with a 5-day course and excellent tolerability will make dermatologists rethink the old practice of ‘freeze and go.’ ”
In an interview, he added, “tirbanibulin comes to the U.S. market for treating AKs at a great time, as ingenol mebutate has been withdrawn and the others are not widely supported. The mechanism of promoting apoptosis and inducing cell cycle arrest directly correlates to the local skin reaction profile of less crusting, vesiculation, and overall signs of skin necrosis as compared to [5-fluorouracil] and ingenol mebutate, which work via that pathway. As a result, there is a direct impact on the hyperproliferation of atypical keratinocytes that will treat visible and subclinical disease.”
“The ointment vehicle is also novel as previous therapies have been in either creams or gels,” he said.
The two trials were funded by tirbanibulin manufacturer Athenex. Dr. Blauvelt reported receiving consulting fees from Athenex and other pharmaceutical companies, including Almirall, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant Sciences. Other author disclosures included serving as a consultant to Athenex and other companies. Several authors are Athenex employees. Dr. Bhatia disclosed that he is an adviser and consultant for Almirall and has been an investigator for multiple other AK treatments.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Scaly lesion on forearm
The suspicion raised by the dermoscopy results led to a shave biopsy, which confirmed the diagnosis of squamous cell carcinoma (SCC) in situ, also known as Bowen disease.
Bowen disease typically manifests as a scaly erythematous patch, often on sun-exposed skin. If untreated, these lesions have the potential to develop into invasive SCCs. Generally, the lesions are preceded by the formation of actinic keratosis (AK). In a 2009 trial performed by the Department of Veterans Affairs, up to 65% of SCCs were found to have previously been diagnosed as AK lesions.1
The selection of treatments includes excision, electrodessication and curettage, cryotherapy, and topical options such as fluorouracil bid for 4 weeks or imiquimod qd for 9 weeks.
After the physician outlined the treatment options, this patient opted for an elliptical excision. At the patient’s next follow-up appointment, she was found to have multiple AKs on her face; they were treated with cryotherapy. Patients with a diagnosis of precancerous or cancerous skin lesions are at high risk for additional AKs and skin cancer, so they should be counseled regarding the use of sun protective measures and the importance of annual screening for new lesions.
Image courtesy of Carlos Cano, MD, and text courtesy of Carlos Cano, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the veterans affairs topical tretinoin chemoprevention trial. Cancer. 2009;115:2523-2530. doi: 10.1002/cncr.24284.

The suspicion raised by the dermoscopy results led to a shave biopsy, which confirmed the diagnosis of squamous cell carcinoma (SCC) in situ, also known as Bowen disease.
Bowen disease typically manifests as a scaly erythematous patch, often on sun-exposed skin. If untreated, these lesions have the potential to develop into invasive SCCs. Generally, the lesions are preceded by the formation of actinic keratosis (AK). In a 2009 trial performed by the Department of Veterans Affairs, up to 65% of SCCs were found to have previously been diagnosed as AK lesions.1
The selection of treatments includes excision, electrodessication and curettage, cryotherapy, and topical options such as fluorouracil bid for 4 weeks or imiquimod qd for 9 weeks.
After the physician outlined the treatment options, this patient opted for an elliptical excision. At the patient’s next follow-up appointment, she was found to have multiple AKs on her face; they were treated with cryotherapy. Patients with a diagnosis of precancerous or cancerous skin lesions are at high risk for additional AKs and skin cancer, so they should be counseled regarding the use of sun protective measures and the importance of annual screening for new lesions.
Image courtesy of Carlos Cano, MD, and text courtesy of Carlos Cano, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The suspicion raised by the dermoscopy results led to a shave biopsy, which confirmed the diagnosis of squamous cell carcinoma (SCC) in situ, also known as Bowen disease.
Bowen disease typically manifests as a scaly erythematous patch, often on sun-exposed skin. If untreated, these lesions have the potential to develop into invasive SCCs. Generally, the lesions are preceded by the formation of actinic keratosis (AK). In a 2009 trial performed by the Department of Veterans Affairs, up to 65% of SCCs were found to have previously been diagnosed as AK lesions.1
The selection of treatments includes excision, electrodessication and curettage, cryotherapy, and topical options such as fluorouracil bid for 4 weeks or imiquimod qd for 9 weeks.
After the physician outlined the treatment options, this patient opted for an elliptical excision. At the patient’s next follow-up appointment, she was found to have multiple AKs on her face; they were treated with cryotherapy. Patients with a diagnosis of precancerous or cancerous skin lesions are at high risk for additional AKs and skin cancer, so they should be counseled regarding the use of sun protective measures and the importance of annual screening for new lesions.
Image courtesy of Carlos Cano, MD, and text courtesy of Carlos Cano, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the veterans affairs topical tretinoin chemoprevention trial. Cancer. 2009;115:2523-2530. doi: 10.1002/cncr.24284.
1. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the veterans affairs topical tretinoin chemoprevention trial. Cancer. 2009;115:2523-2530. doi: 10.1002/cncr.24284.
Erythema Ab Igne and Malignant Transformation to Squamous Cell Carcinoma
Case Report
A 67-year-old Black woman presented with a long-standing history of pruritus and “scaly thick bumps” on the lower extremities. Upon further questioning, she reported a 30-year history of placing her feet by an electric space heater and daily baths in “very hot” water. A review of systems and medical history were unremarkable, and the patient was not on any medications. Initial physical examination of the lower extremities demonstrated lichenified plaques and scattered, firm, ulcerated nodules surrounded by mottled postinflammatory hyperpigmentation with sharp demarcation at the midcalf bilaterally (Figure 1).
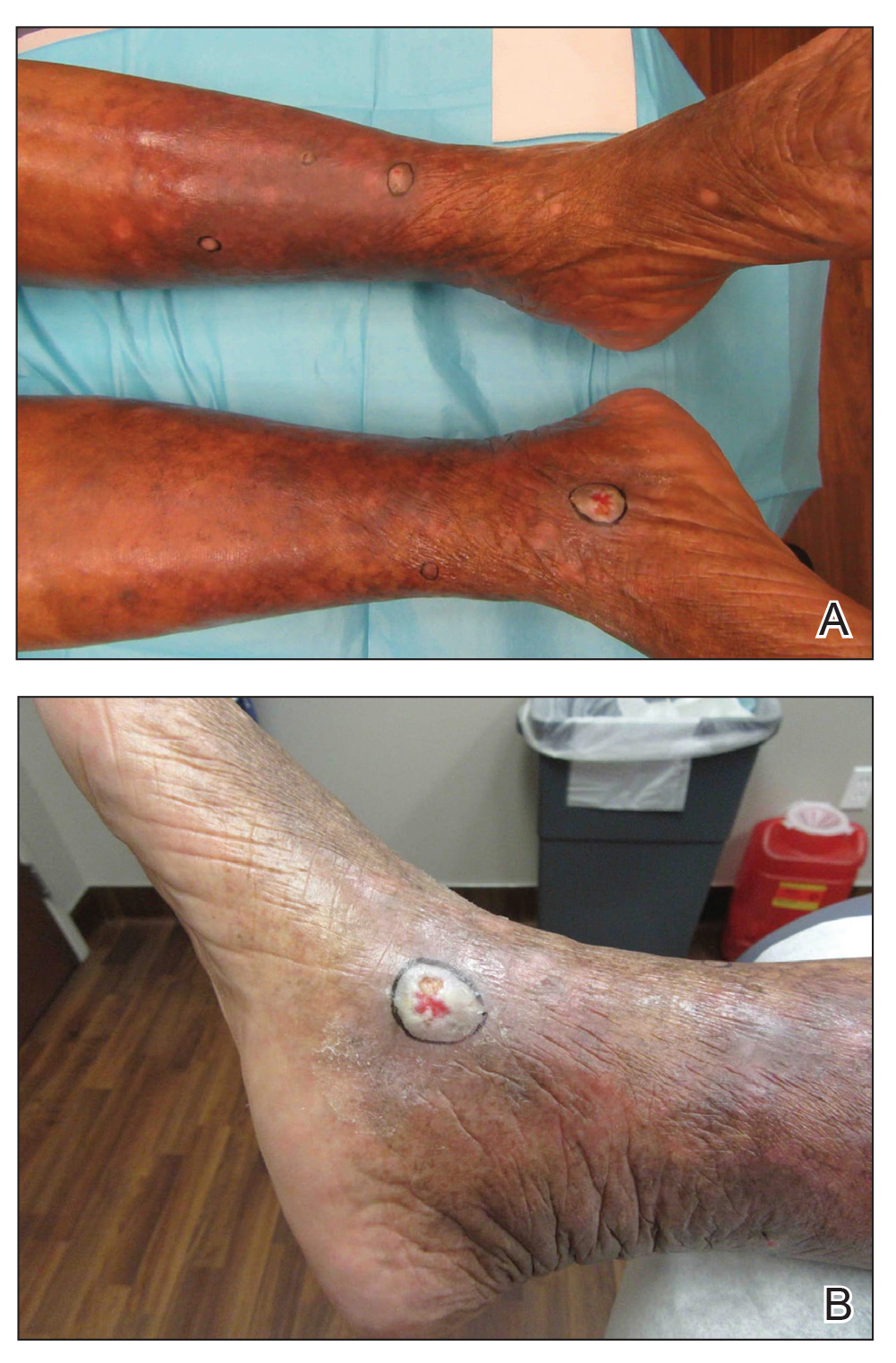
Subsequently, the patient was shown to have multiple actinic keratoses and SCCs, both in situ and invasive, within the areas of EAI (Figure 2). The patient had no actinic keratoses or other cutaneous malignant neoplasms elsewhere on the skin. Management of actinic keratoses, SCC in situ, and invasive SCC on the lower extremities included numerous excisions, treatment with liquid nitrogen, and topical 5-fluorouracil under occlusion. The patient continues to be monitored frequently.
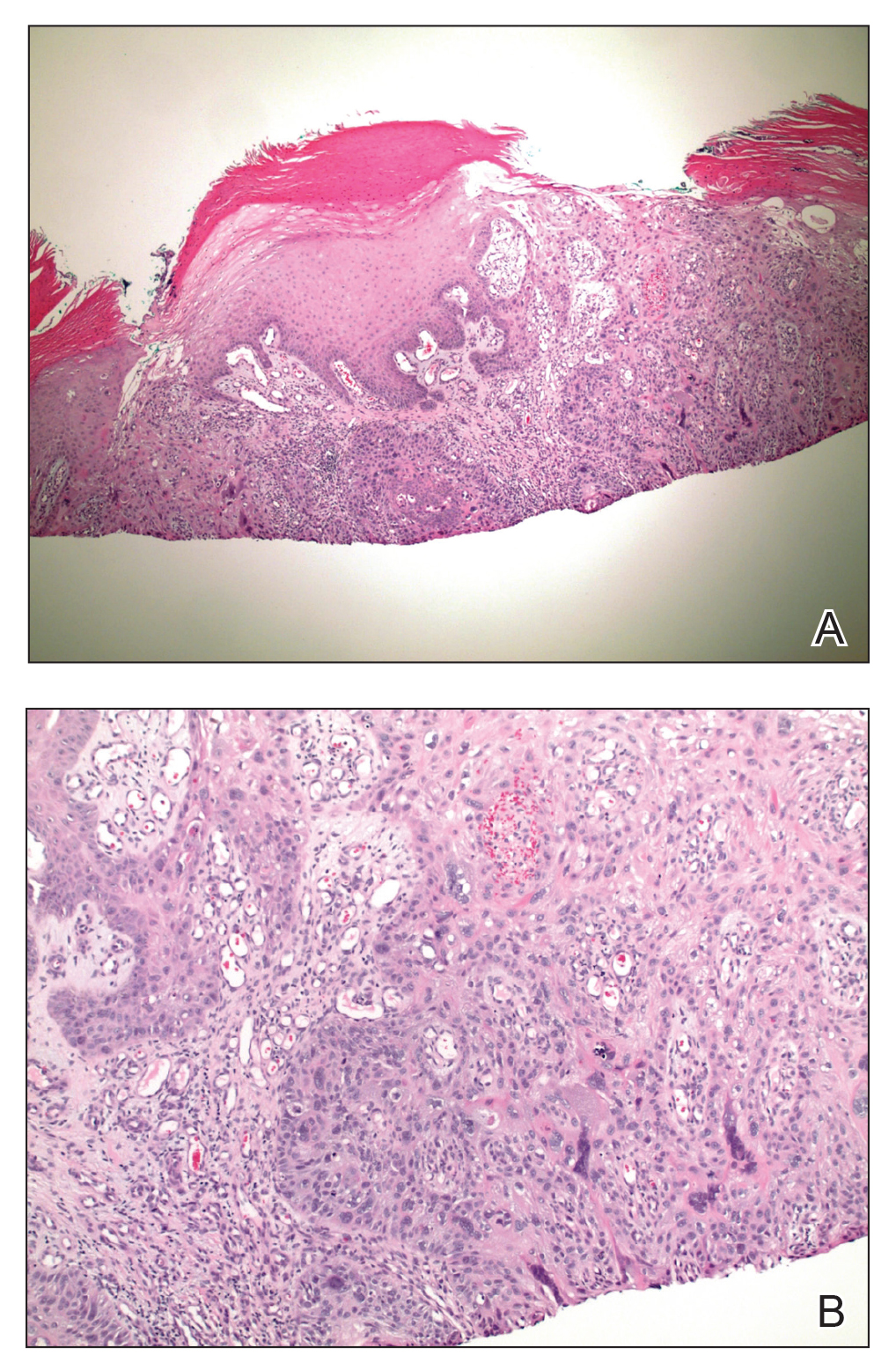
Comment
Presentation of EAI
Erythema ab igne is a cutaneous reaction resulting from prolonged exposure to an infrared heat source at temperatures insufficient to cause a burn (37 °F to 11
Histopathology of EAI
Histologically, later stages of EAI can demonstrate focal hyperkeratosis with dyskeratosis and increased dermal elastosis, similar to actinic damage, with a predisposition to develop SCC.2 Notably, early reports document various heat-induced carcinomas, including kangri-burn cancers among Kashmiris, kang thermal cancers in China, and kairo cancers in Japan.2,4,5 More recent reports identify cutaneous carcinomas arising specifically in the setting of EAI, most commonly SCC3; Merkel cell carcinoma and cutaneous marginal zone lymphoma are less commonly reported malignancies.6,7 Given the frequency of malignant transformation within sites of thermal exposure, chronic heat exposure may share a common pathophysiology with SCC and other neoplasms, including Merkel cell carcinoma and cutaneous marginal zone lymphoma.
SCC in Black Individuals
Squamous cell carcinoma is the most common skin cancer in Black individuals, with a notably higher incidence in high-risk subpopulations (immunosuppressed patients). Unlike White individuals, SCCs frequently occur in non–sun-exposed areas in Black individuals and are associated with unique risk factors, such as human papillomavirus, as demonstrated in Black transplant patients.8 A retrospective study examining the characteristics of SCC on the legs of Black individuals documented atypical hyperkeratotic neoplasms surrounded by abnormal pigmentation and mottling of surrounding skin.9 Morphologic skin changes could be the result of chronic thermal damage: Numerous patients reported a history of leg warming from an open heat source. Other patients had an actual diagnosis of EAI. The predilection for less-exposed skin suggests UV radiation (UVR) might be a less important predisposing risk factor for this racial group, and the increased mortality associated with SCC in Black individuals might represent a more aggressive nature to this subset of SCCs.9 Furthermore, infrared radiation (IRR), such as fires and coal stoves, might have the potential to stimulate skin changes similar to those associated with UVR and ultimately malignant changes.
Infrared Radiation
Compared to UVR, little is known about the biological effects of IRR (wavelength, 760 nm to 1 mm), to which human skin is constantly exposed from natural and artificial light sources. Early studies have demonstrated the carcinogenic potential of IRR, observing an augmentation of UVR-induced tumorigenesis in the presence of heat. More recently, IRR was observed to stimulate increased collagenase production from dermal fibroblasts and influence pathways (extracellular signal-related kinases 1/2 and p38 mitogen-activated protein kinases) in a similar fashion to UVB and UVA.10,11 Therefore, IRR might be capable of eliciting molecular responses comparable to those caused by UVR.
Conclusion
Although SCC in association with EAI is uncommon, historical reports of thermal cancers and scientific observations of IRR-induced biological and molecular effects support EAI as a predisposing risk factor for SCC and the important need for close monitoring by physicians. Studies are needed to further elucidate the pathologic effects of IRR, with more promotion of caution relating to thermal exposure.
- Milchak M, Smucker J, Chung CG, et al. Erythema ab igne due to heating pad use: a case report and review of clinical presentation, prevention, and complications. Case Rep Med. 2016;2016:1862480.
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28. Accessed December 10, 2020. https://escholarship.org/uc/item/47z4v01z
- Wharton JB, Sheehan DJ, Lesher JL Jr. Squamous cell carcinoma in situ arising in the setting of erythema ab igne. J Drugs Dermatol. 2008;7:488-489.
- Neve EF. Kangri-burn cancer. Br Med J. 1923;2:1255-1256.
- Laycock HT. The kang cancer of North-West China. Br Med J. 1948;1:982.
- Wharton J, Roffwarg D, Miller J, et al. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62:1080-1081.
- Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124:110-113.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- McCall CO, Chen SC. Squamous cell carcinoma of the legs in African Americans. J Am Acad Dermatol. 2002;47:524-529.
- Freeman RG, Knox JM. Influence of temperature on ultraviolet injury. Arch Dermatol. 1964;89:858-864.
- Schieke SM, Schroeder P, Krutmann J. Cutaneous effects of infrared radiation: from clinical observations to molecular response mechanisms. Photodermatol Photoimmunol Photomed. 2003;19:228-234.
Case Report
A 67-year-old Black woman presented with a long-standing history of pruritus and “scaly thick bumps” on the lower extremities. Upon further questioning, she reported a 30-year history of placing her feet by an electric space heater and daily baths in “very hot” water. A review of systems and medical history were unremarkable, and the patient was not on any medications. Initial physical examination of the lower extremities demonstrated lichenified plaques and scattered, firm, ulcerated nodules surrounded by mottled postinflammatory hyperpigmentation with sharp demarcation at the midcalf bilaterally (Figure 1).

Subsequently, the patient was shown to have multiple actinic keratoses and SCCs, both in situ and invasive, within the areas of EAI (Figure 2). The patient had no actinic keratoses or other cutaneous malignant neoplasms elsewhere on the skin. Management of actinic keratoses, SCC in situ, and invasive SCC on the lower extremities included numerous excisions, treatment with liquid nitrogen, and topical 5-fluorouracil under occlusion. The patient continues to be monitored frequently.

Comment
Presentation of EAI
Erythema ab igne is a cutaneous reaction resulting from prolonged exposure to an infrared heat source at temperatures insufficient to cause a burn (37 °F to 11
Histopathology of EAI
Histologically, later stages of EAI can demonstrate focal hyperkeratosis with dyskeratosis and increased dermal elastosis, similar to actinic damage, with a predisposition to develop SCC.2 Notably, early reports document various heat-induced carcinomas, including kangri-burn cancers among Kashmiris, kang thermal cancers in China, and kairo cancers in Japan.2,4,5 More recent reports identify cutaneous carcinomas arising specifically in the setting of EAI, most commonly SCC3; Merkel cell carcinoma and cutaneous marginal zone lymphoma are less commonly reported malignancies.6,7 Given the frequency of malignant transformation within sites of thermal exposure, chronic heat exposure may share a common pathophysiology with SCC and other neoplasms, including Merkel cell carcinoma and cutaneous marginal zone lymphoma.
SCC in Black Individuals
Squamous cell carcinoma is the most common skin cancer in Black individuals, with a notably higher incidence in high-risk subpopulations (immunosuppressed patients). Unlike White individuals, SCCs frequently occur in non–sun-exposed areas in Black individuals and are associated with unique risk factors, such as human papillomavirus, as demonstrated in Black transplant patients.8 A retrospective study examining the characteristics of SCC on the legs of Black individuals documented atypical hyperkeratotic neoplasms surrounded by abnormal pigmentation and mottling of surrounding skin.9 Morphologic skin changes could be the result of chronic thermal damage: Numerous patients reported a history of leg warming from an open heat source. Other patients had an actual diagnosis of EAI. The predilection for less-exposed skin suggests UV radiation (UVR) might be a less important predisposing risk factor for this racial group, and the increased mortality associated with SCC in Black individuals might represent a more aggressive nature to this subset of SCCs.9 Furthermore, infrared radiation (IRR), such as fires and coal stoves, might have the potential to stimulate skin changes similar to those associated with UVR and ultimately malignant changes.
Infrared Radiation
Compared to UVR, little is known about the biological effects of IRR (wavelength, 760 nm to 1 mm), to which human skin is constantly exposed from natural and artificial light sources. Early studies have demonstrated the carcinogenic potential of IRR, observing an augmentation of UVR-induced tumorigenesis in the presence of heat. More recently, IRR was observed to stimulate increased collagenase production from dermal fibroblasts and influence pathways (extracellular signal-related kinases 1/2 and p38 mitogen-activated protein kinases) in a similar fashion to UVB and UVA.10,11 Therefore, IRR might be capable of eliciting molecular responses comparable to those caused by UVR.
Conclusion
Although SCC in association with EAI is uncommon, historical reports of thermal cancers and scientific observations of IRR-induced biological and molecular effects support EAI as a predisposing risk factor for SCC and the important need for close monitoring by physicians. Studies are needed to further elucidate the pathologic effects of IRR, with more promotion of caution relating to thermal exposure.
Case Report
A 67-year-old Black woman presented with a long-standing history of pruritus and “scaly thick bumps” on the lower extremities. Upon further questioning, she reported a 30-year history of placing her feet by an electric space heater and daily baths in “very hot” water. A review of systems and medical history were unremarkable, and the patient was not on any medications. Initial physical examination of the lower extremities demonstrated lichenified plaques and scattered, firm, ulcerated nodules surrounded by mottled postinflammatory hyperpigmentation with sharp demarcation at the midcalf bilaterally (Figure 1).

Subsequently, the patient was shown to have multiple actinic keratoses and SCCs, both in situ and invasive, within the areas of EAI (Figure 2). The patient had no actinic keratoses or other cutaneous malignant neoplasms elsewhere on the skin. Management of actinic keratoses, SCC in situ, and invasive SCC on the lower extremities included numerous excisions, treatment with liquid nitrogen, and topical 5-fluorouracil under occlusion. The patient continues to be monitored frequently.

Comment
Presentation of EAI
Erythema ab igne is a cutaneous reaction resulting from prolonged exposure to an infrared heat source at temperatures insufficient to cause a burn (37 °F to 11
Histopathology of EAI
Histologically, later stages of EAI can demonstrate focal hyperkeratosis with dyskeratosis and increased dermal elastosis, similar to actinic damage, with a predisposition to develop SCC.2 Notably, early reports document various heat-induced carcinomas, including kangri-burn cancers among Kashmiris, kang thermal cancers in China, and kairo cancers in Japan.2,4,5 More recent reports identify cutaneous carcinomas arising specifically in the setting of EAI, most commonly SCC3; Merkel cell carcinoma and cutaneous marginal zone lymphoma are less commonly reported malignancies.6,7 Given the frequency of malignant transformation within sites of thermal exposure, chronic heat exposure may share a common pathophysiology with SCC and other neoplasms, including Merkel cell carcinoma and cutaneous marginal zone lymphoma.
SCC in Black Individuals
Squamous cell carcinoma is the most common skin cancer in Black individuals, with a notably higher incidence in high-risk subpopulations (immunosuppressed patients). Unlike White individuals, SCCs frequently occur in non–sun-exposed areas in Black individuals and are associated with unique risk factors, such as human papillomavirus, as demonstrated in Black transplant patients.8 A retrospective study examining the characteristics of SCC on the legs of Black individuals documented atypical hyperkeratotic neoplasms surrounded by abnormal pigmentation and mottling of surrounding skin.9 Morphologic skin changes could be the result of chronic thermal damage: Numerous patients reported a history of leg warming from an open heat source. Other patients had an actual diagnosis of EAI. The predilection for less-exposed skin suggests UV radiation (UVR) might be a less important predisposing risk factor for this racial group, and the increased mortality associated with SCC in Black individuals might represent a more aggressive nature to this subset of SCCs.9 Furthermore, infrared radiation (IRR), such as fires and coal stoves, might have the potential to stimulate skin changes similar to those associated with UVR and ultimately malignant changes.
Infrared Radiation
Compared to UVR, little is known about the biological effects of IRR (wavelength, 760 nm to 1 mm), to which human skin is constantly exposed from natural and artificial light sources. Early studies have demonstrated the carcinogenic potential of IRR, observing an augmentation of UVR-induced tumorigenesis in the presence of heat. More recently, IRR was observed to stimulate increased collagenase production from dermal fibroblasts and influence pathways (extracellular signal-related kinases 1/2 and p38 mitogen-activated protein kinases) in a similar fashion to UVB and UVA.10,11 Therefore, IRR might be capable of eliciting molecular responses comparable to those caused by UVR.
Conclusion
Although SCC in association with EAI is uncommon, historical reports of thermal cancers and scientific observations of IRR-induced biological and molecular effects support EAI as a predisposing risk factor for SCC and the important need for close monitoring by physicians. Studies are needed to further elucidate the pathologic effects of IRR, with more promotion of caution relating to thermal exposure.
- Milchak M, Smucker J, Chung CG, et al. Erythema ab igne due to heating pad use: a case report and review of clinical presentation, prevention, and complications. Case Rep Med. 2016;2016:1862480.
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28. Accessed December 10, 2020. https://escholarship.org/uc/item/47z4v01z
- Wharton JB, Sheehan DJ, Lesher JL Jr. Squamous cell carcinoma in situ arising in the setting of erythema ab igne. J Drugs Dermatol. 2008;7:488-489.
- Neve EF. Kangri-burn cancer. Br Med J. 1923;2:1255-1256.
- Laycock HT. The kang cancer of North-West China. Br Med J. 1948;1:982.
- Wharton J, Roffwarg D, Miller J, et al. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62:1080-1081.
- Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124:110-113.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- McCall CO, Chen SC. Squamous cell carcinoma of the legs in African Americans. J Am Acad Dermatol. 2002;47:524-529.
- Freeman RG, Knox JM. Influence of temperature on ultraviolet injury. Arch Dermatol. 1964;89:858-864.
- Schieke SM, Schroeder P, Krutmann J. Cutaneous effects of infrared radiation: from clinical observations to molecular response mechanisms. Photodermatol Photoimmunol Photomed. 2003;19:228-234.
- Milchak M, Smucker J, Chung CG, et al. Erythema ab igne due to heating pad use: a case report and review of clinical presentation, prevention, and complications. Case Rep Med. 2016;2016:1862480.
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28. Accessed December 10, 2020. https://escholarship.org/uc/item/47z4v01z
- Wharton JB, Sheehan DJ, Lesher JL Jr. Squamous cell carcinoma in situ arising in the setting of erythema ab igne. J Drugs Dermatol. 2008;7:488-489.
- Neve EF. Kangri-burn cancer. Br Med J. 1923;2:1255-1256.
- Laycock HT. The kang cancer of North-West China. Br Med J. 1948;1:982.
- Wharton J, Roffwarg D, Miller J, et al. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62:1080-1081.
- Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124:110-113.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- McCall CO, Chen SC. Squamous cell carcinoma of the legs in African Americans. J Am Acad Dermatol. 2002;47:524-529.
- Freeman RG, Knox JM. Influence of temperature on ultraviolet injury. Arch Dermatol. 1964;89:858-864.
- Schieke SM, Schroeder P, Krutmann J. Cutaneous effects of infrared radiation: from clinical observations to molecular response mechanisms. Photodermatol Photoimmunol Photomed. 2003;19:228-234.
Practice Points
- Erythema ab igne (EAI) is a cutaneous reaction in response to prolonged exposure to infrared heat sources at temperatures insufficient to induce a burn.
- Common infrared heat sources include open fires, coal stoves, heating pads, laptop computers, and electric space heaters.
- Although considered a chronic pigmentary disorder, EAI rarely can progress to malignant transformation, including squamous cell carcinoma. Patients with EAI should be monitored long-term for malignant transformation.
New AK treatments: Local reactions are the price for greater clearance rates
, according to an expert speaking at the annual Coastal Dermatology Symposium, held virtually.
This relationship is not new. In a review of treatments for AKs, Neal Bhatia, MD, a dermatologist and researcher at Therapeutics Dermatology, San Diego, advised that most effective agents trade a higher risk of inflammatory reactions – including erythema, flaking, and scaling – for greater therapeutic gain. In many cases, local skin reactions are an inevitable consequence of their mechanism of action.
Data from the completed phase 3 trials of tirbanibulin 1% ointment (KX01-AK-003 and KX01-AK-004), are illustrative. (Tirbanibulin 1% ointment was approved by the Food and Drug Administration in mid-December, after the Coastal Derm meeting was held.)
In the phase 3 trials, which have not yet been published, tirbanibulin, an inhibitor of Src kinase, which has an antiproliferative action, was four to five times more effective than vehicle by day 57 for overall complete clearance (P < .0001) of AKs and complete clearance of the face (P < .0001), but rates of local skin reactions were generally two to three times higher, according to Dr. Bhatia.
In the KX01-AK-004 trial, for example, 61% of patients had complete clearance of the face, versus 14% of those randomized to vehicle. The difference for overall partial clearance (76% vs. 20%; P < .0001), partial clearance of the face (80% vs. 22%; P < .0001), and partial clearance of the scalp (69% vs. 15%; P < .0001) was even greater. When compared with placebo, tirbanibulin was also associated with greater rates of erythema (90% vs. 31%), crusting (45% vs. 8%), flaking (84% vs. 35%), swelling (38% vs. 2%) and erosions or ulcers (12% vs. 1%).
Although these events might be a challenge with regard to tolerability for some patients, they might best be described as evidence that the drug is working.
“Local skin reactions are anticipated. They are not adverse events. They are not side effects,” Dr. Bhatia said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education. “Patients are going to get red, and you need to counsel patients about the 5 days when they can expected to be red. It is a sign of the civil war, if you will, that your skin is taking on with the actinic keratoses.”
Both 3- and 5-day courses of the drug were tested in the clinical trials. (The approved prescribing information recommends treatment on the face or scalp once a day for 5 consecutive days).
Other studies evaluating treatments for AKs have also associated an increased risk of local skin reactions with greater efficacy, Dr. Bhatia noted. As an example, he cited a phase 4 study comparing 0.015% ingenol mebutate gel to diclofenac sodium 3% gel in people with facial and scalp AK lesions.
At the end of the 3-month study, complete clearance was higher among those on ingenol mebutate, which was applied for 3 days, when compared with diclofenac sodium gel, which was applied daily for 3 months (34% vs. 23%; P = .006). However, patients randomized to ingenol mebutate gel had to first weather a higher rate of application-site erythema (19% vs. 12%) before achieving a greater level of clearance.
The correlation between efficacy and local reactions at the site of treatment application emphasizes the importance of educating patients about this relationship and in engaging in shared decision-making, Dr. Bhatia said.
“It is basically a tradeoff between local skin reactions, between frequency [of applications], compliance, and, of course, duration of therapy, even though both drugs served their purposes well,” said Dr. Bhatia, referring to the comparison of the ingenol mebutate and diclofenac gels.
Although not absolute, efficacy and tolerability were also generally inversely related in a recent four-treatment comparison of four commonly used field-directed therapies. In that trial, the primary endpoint was at least a 75% reduction from baseline in the number of AKs to 12 months after treatment ended.
For that outcome, 5% fluorouracil (5-FU) cream (74.7%) was significantly more effective than 5% imiquimod cream (53.9%), methyl aminolevulinate photodynamic therapy (37.7%), and 0.015% ingenol mebutate gel (28.9%). Also, 5-FU treatment was associated with the moderate or severe erythema (81.5%), severe pain (16.%), and a severe burning sensation (21.5%).
Other therapies on the horizon, some of which are already available in Europe or Canada, show a relationship between efficacy and local skin reactions. Of two that Dr. Bhatia cited, 5-FU and salicylic acid combined in a solution and 5-FU and calcipotriene combined in an ointment have demonstrated high rates of efficacy but at the cost of substantial rates of erythema and flaking.
Transient skin reactions can be made acceptable to patients who are informed of the goals of clearing AKs, which includes lowering the risk of cancer, as well as cosmetic improvement. In the phase 4 study comparing ingenol mebutate gel to diclofenac sodium gel, the end-of-study global satisfaction rates were higher (P < .001) for those randomized to the most effective therapy despite the local skin reactions.
Preparing patients for the consequences of therapy for AKs is essential, because optimal therapy involves treating uninvolved skin, according to Hassan Galadari, MD, assistant professor of dermatology, United Arab Emirates University, Dubai. A coauthor of a recent review article on actinic keratoses, Dr. Galadari said in an interview that field treatment means patients might have local skin reactions where they did not previously have lesions.
“Actinic damage may not be visible with the naked eye. That is why field treatment, which is applying medicine in adjacent areas that may appear normal, is indicated,” he said. As a result, “areas that otherwise may have appeared as normal start to react by becoming red, itchy, and even inflamed.”
He agreed with Dr. Bhatia that local skin reactions are typically the price paid for effective control of these precancerous lesions.
This publication and Global Academy for Medical Education are owned by the same parent company.
Dr. Bhatia reports financial relationships with more than 30 pharmaceutical companies with dermatologic products, including Almirall and other companies with products relevant to AK therapies.
, according to an expert speaking at the annual Coastal Dermatology Symposium, held virtually.
This relationship is not new. In a review of treatments for AKs, Neal Bhatia, MD, a dermatologist and researcher at Therapeutics Dermatology, San Diego, advised that most effective agents trade a higher risk of inflammatory reactions – including erythema, flaking, and scaling – for greater therapeutic gain. In many cases, local skin reactions are an inevitable consequence of their mechanism of action.
Data from the completed phase 3 trials of tirbanibulin 1% ointment (KX01-AK-003 and KX01-AK-004), are illustrative. (Tirbanibulin 1% ointment was approved by the Food and Drug Administration in mid-December, after the Coastal Derm meeting was held.)
In the phase 3 trials, which have not yet been published, tirbanibulin, an inhibitor of Src kinase, which has an antiproliferative action, was four to five times more effective than vehicle by day 57 for overall complete clearance (P < .0001) of AKs and complete clearance of the face (P < .0001), but rates of local skin reactions were generally two to three times higher, according to Dr. Bhatia.
In the KX01-AK-004 trial, for example, 61% of patients had complete clearance of the face, versus 14% of those randomized to vehicle. The difference for overall partial clearance (76% vs. 20%; P < .0001), partial clearance of the face (80% vs. 22%; P < .0001), and partial clearance of the scalp (69% vs. 15%; P < .0001) was even greater. When compared with placebo, tirbanibulin was also associated with greater rates of erythema (90% vs. 31%), crusting (45% vs. 8%), flaking (84% vs. 35%), swelling (38% vs. 2%) and erosions or ulcers (12% vs. 1%).
Although these events might be a challenge with regard to tolerability for some patients, they might best be described as evidence that the drug is working.
“Local skin reactions are anticipated. They are not adverse events. They are not side effects,” Dr. Bhatia said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education. “Patients are going to get red, and you need to counsel patients about the 5 days when they can expected to be red. It is a sign of the civil war, if you will, that your skin is taking on with the actinic keratoses.”
Both 3- and 5-day courses of the drug were tested in the clinical trials. (The approved prescribing information recommends treatment on the face or scalp once a day for 5 consecutive days).
Other studies evaluating treatments for AKs have also associated an increased risk of local skin reactions with greater efficacy, Dr. Bhatia noted. As an example, he cited a phase 4 study comparing 0.015% ingenol mebutate gel to diclofenac sodium 3% gel in people with facial and scalp AK lesions.
At the end of the 3-month study, complete clearance was higher among those on ingenol mebutate, which was applied for 3 days, when compared with diclofenac sodium gel, which was applied daily for 3 months (34% vs. 23%; P = .006). However, patients randomized to ingenol mebutate gel had to first weather a higher rate of application-site erythema (19% vs. 12%) before achieving a greater level of clearance.
The correlation between efficacy and local reactions at the site of treatment application emphasizes the importance of educating patients about this relationship and in engaging in shared decision-making, Dr. Bhatia said.
“It is basically a tradeoff between local skin reactions, between frequency [of applications], compliance, and, of course, duration of therapy, even though both drugs served their purposes well,” said Dr. Bhatia, referring to the comparison of the ingenol mebutate and diclofenac gels.
Although not absolute, efficacy and tolerability were also generally inversely related in a recent four-treatment comparison of four commonly used field-directed therapies. In that trial, the primary endpoint was at least a 75% reduction from baseline in the number of AKs to 12 months after treatment ended.
For that outcome, 5% fluorouracil (5-FU) cream (74.7%) was significantly more effective than 5% imiquimod cream (53.9%), methyl aminolevulinate photodynamic therapy (37.7%), and 0.015% ingenol mebutate gel (28.9%). Also, 5-FU treatment was associated with the moderate or severe erythema (81.5%), severe pain (16.%), and a severe burning sensation (21.5%).
Other therapies on the horizon, some of which are already available in Europe or Canada, show a relationship between efficacy and local skin reactions. Of two that Dr. Bhatia cited, 5-FU and salicylic acid combined in a solution and 5-FU and calcipotriene combined in an ointment have demonstrated high rates of efficacy but at the cost of substantial rates of erythema and flaking.
Transient skin reactions can be made acceptable to patients who are informed of the goals of clearing AKs, which includes lowering the risk of cancer, as well as cosmetic improvement. In the phase 4 study comparing ingenol mebutate gel to diclofenac sodium gel, the end-of-study global satisfaction rates were higher (P < .001) for those randomized to the most effective therapy despite the local skin reactions.
Preparing patients for the consequences of therapy for AKs is essential, because optimal therapy involves treating uninvolved skin, according to Hassan Galadari, MD, assistant professor of dermatology, United Arab Emirates University, Dubai. A coauthor of a recent review article on actinic keratoses, Dr. Galadari said in an interview that field treatment means patients might have local skin reactions where they did not previously have lesions.
“Actinic damage may not be visible with the naked eye. That is why field treatment, which is applying medicine in adjacent areas that may appear normal, is indicated,” he said. As a result, “areas that otherwise may have appeared as normal start to react by becoming red, itchy, and even inflamed.”
He agreed with Dr. Bhatia that local skin reactions are typically the price paid for effective control of these precancerous lesions.
This publication and Global Academy for Medical Education are owned by the same parent company.
Dr. Bhatia reports financial relationships with more than 30 pharmaceutical companies with dermatologic products, including Almirall and other companies with products relevant to AK therapies.
, according to an expert speaking at the annual Coastal Dermatology Symposium, held virtually.
This relationship is not new. In a review of treatments for AKs, Neal Bhatia, MD, a dermatologist and researcher at Therapeutics Dermatology, San Diego, advised that most effective agents trade a higher risk of inflammatory reactions – including erythema, flaking, and scaling – for greater therapeutic gain. In many cases, local skin reactions are an inevitable consequence of their mechanism of action.
Data from the completed phase 3 trials of tirbanibulin 1% ointment (KX01-AK-003 and KX01-AK-004), are illustrative. (Tirbanibulin 1% ointment was approved by the Food and Drug Administration in mid-December, after the Coastal Derm meeting was held.)
In the phase 3 trials, which have not yet been published, tirbanibulin, an inhibitor of Src kinase, which has an antiproliferative action, was four to five times more effective than vehicle by day 57 for overall complete clearance (P < .0001) of AKs and complete clearance of the face (P < .0001), but rates of local skin reactions were generally two to three times higher, according to Dr. Bhatia.
In the KX01-AK-004 trial, for example, 61% of patients had complete clearance of the face, versus 14% of those randomized to vehicle. The difference for overall partial clearance (76% vs. 20%; P < .0001), partial clearance of the face (80% vs. 22%; P < .0001), and partial clearance of the scalp (69% vs. 15%; P < .0001) was even greater. When compared with placebo, tirbanibulin was also associated with greater rates of erythema (90% vs. 31%), crusting (45% vs. 8%), flaking (84% vs. 35%), swelling (38% vs. 2%) and erosions or ulcers (12% vs. 1%).
Although these events might be a challenge with regard to tolerability for some patients, they might best be described as evidence that the drug is working.
“Local skin reactions are anticipated. They are not adverse events. They are not side effects,” Dr. Bhatia said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education. “Patients are going to get red, and you need to counsel patients about the 5 days when they can expected to be red. It is a sign of the civil war, if you will, that your skin is taking on with the actinic keratoses.”
Both 3- and 5-day courses of the drug were tested in the clinical trials. (The approved prescribing information recommends treatment on the face or scalp once a day for 5 consecutive days).
Other studies evaluating treatments for AKs have also associated an increased risk of local skin reactions with greater efficacy, Dr. Bhatia noted. As an example, he cited a phase 4 study comparing 0.015% ingenol mebutate gel to diclofenac sodium 3% gel in people with facial and scalp AK lesions.
At the end of the 3-month study, complete clearance was higher among those on ingenol mebutate, which was applied for 3 days, when compared with diclofenac sodium gel, which was applied daily for 3 months (34% vs. 23%; P = .006). However, patients randomized to ingenol mebutate gel had to first weather a higher rate of application-site erythema (19% vs. 12%) before achieving a greater level of clearance.
The correlation between efficacy and local reactions at the site of treatment application emphasizes the importance of educating patients about this relationship and in engaging in shared decision-making, Dr. Bhatia said.
“It is basically a tradeoff between local skin reactions, between frequency [of applications], compliance, and, of course, duration of therapy, even though both drugs served their purposes well,” said Dr. Bhatia, referring to the comparison of the ingenol mebutate and diclofenac gels.
Although not absolute, efficacy and tolerability were also generally inversely related in a recent four-treatment comparison of four commonly used field-directed therapies. In that trial, the primary endpoint was at least a 75% reduction from baseline in the number of AKs to 12 months after treatment ended.
For that outcome, 5% fluorouracil (5-FU) cream (74.7%) was significantly more effective than 5% imiquimod cream (53.9%), methyl aminolevulinate photodynamic therapy (37.7%), and 0.015% ingenol mebutate gel (28.9%). Also, 5-FU treatment was associated with the moderate or severe erythema (81.5%), severe pain (16.%), and a severe burning sensation (21.5%).
Other therapies on the horizon, some of which are already available in Europe or Canada, show a relationship between efficacy and local skin reactions. Of two that Dr. Bhatia cited, 5-FU and salicylic acid combined in a solution and 5-FU and calcipotriene combined in an ointment have demonstrated high rates of efficacy but at the cost of substantial rates of erythema and flaking.
Transient skin reactions can be made acceptable to patients who are informed of the goals of clearing AKs, which includes lowering the risk of cancer, as well as cosmetic improvement. In the phase 4 study comparing ingenol mebutate gel to diclofenac sodium gel, the end-of-study global satisfaction rates were higher (P < .001) for those randomized to the most effective therapy despite the local skin reactions.
Preparing patients for the consequences of therapy for AKs is essential, because optimal therapy involves treating uninvolved skin, according to Hassan Galadari, MD, assistant professor of dermatology, United Arab Emirates University, Dubai. A coauthor of a recent review article on actinic keratoses, Dr. Galadari said in an interview that field treatment means patients might have local skin reactions where they did not previously have lesions.
“Actinic damage may not be visible with the naked eye. That is why field treatment, which is applying medicine in adjacent areas that may appear normal, is indicated,” he said. As a result, “areas that otherwise may have appeared as normal start to react by becoming red, itchy, and even inflamed.”
He agreed with Dr. Bhatia that local skin reactions are typically the price paid for effective control of these precancerous lesions.
This publication and Global Academy for Medical Education are owned by the same parent company.
Dr. Bhatia reports financial relationships with more than 30 pharmaceutical companies with dermatologic products, including Almirall and other companies with products relevant to AK therapies.
FROM COASTAL DERM
CO2 Laser Ablative Fractional Resurfacing Photodynamic Therapy for Actinic Keratosis and Nonmelanoma Skin Cancer: A Randomized Split-Side Study
Actinic keratosis (AK) is the most common cutaneous lesion and is regarded as a precursor to nonmelanoma skin cancer (NMSC), particularly squamous cell carcinoma (SCC).1 Field cancerization refers to broad areas of chronically sun-exposed skin that show cumulative sun damage in the form of clinical and subclinical lesions. It is not feasible to treat large areas with multiple overt and subclinical lesions using surgical methods, and photodynamic therapy (PDT) has become a preferred method for treatment of field cancerization.2 Topical PDT uses the heme biosynthesis pathway precursors aminolevulinic acid (ALA) or methyl ALA (MAL), which localizes in the treatment area and is metabolized to protoporphyrin IX.3 After an incubation period, activation by a light source results in the formation of cytotoxic oxygen species,4 with reports of efficacy over large areas and excellent cosmetic outcomes.2
Laser ablative fractional resurfacing (AFR) also has been investigated as a treatment of AKs; CO2 laser AFR treatment resulted in a short-term reduction in the number of AK lesions and appeared to reduce the development of new lesions.5 However, case reports and small studies have indicated that pretreatment with laser AFR can increase the efficacy of PDT by creating microscopic vertical channels facilitating deeper penetration and uptake of the ALA.6 The use of erbium:YAG lasers in combination with PDT has demonstrated notable clinical and aesthetic improvements in treating basal cell carcinomas (BCCs)7 and AKs,8 with enhanced efficacy in moderate to thick AKs in particular. Hædersdal et al6 reported that CO2 laser AFR facilitated delivery of MAL into porcine skin, with AFR appearing to bypass the stratum corneum and deliver the treatment to the deep dermis.
The combination of CO2 laser AFR and PDT has shown statistically significant increases in efficacy for treatment of AKs compared to PDT alone (P<.001).9 In a small study, Alexiades10 reported a statistically significant improvement in AKs at 4 and 8 weeks posttreatment for 10 patients receiving CO2 laser AFR-PDT vs conventional PDT (P<.05). Studies of organ transplant recipients—who are at higher risk for AK and NMSC development—demonstrated favorable results for combined CO2 laser AFR and PDT vs either laser treatment11 or PDT9,12 alone, with significant reductions in the number of AKs (P=.002). Results were maintained for 3 to 4 months after treatment. Additional studies have shown that combining CO2 laser AFR and PDT may reduce the PDT incubation time or number of treatments required to achieve a response over conventional PDT.13,14
Our proof-of-concept study was designed to assess efficacy of CO2 laser AFR to enhance an approved drug delivery system in the treatment of AK and NMSC. The objective was to compare effect and durability of AFR-PDT vs standard ALA-PDT in the treatment of AK and NMSCs in a split-sided study of various body locations.
Methods
This randomized, split-sided study compared CO2 laser AFR-PDT to standard ALA-PDT for the treatment of AK and NMSC conducted at 1 site in Los Gatos, California. Patients who had a skin cancer screening and received a biopsy diagnosis of AK or NMSC were invited to attend an enrollment visit. Key inclusion criteria for enrollment were male or female patients aged 40 to 85 years with notable symmetrically comparable photodamage (at least 1 AK per square centimeter) in 1 or more skin areas—scalp, face, or distal extremities—with presence of clinically identifiable NMSCs proven by biopsy. Key exclusion criteria were patients who were pregnant; patients with epilepsy, seizures, or a photosensitive disorder; those taking photosensitizing medication (eg, doxycycline, hydrochlorothiazide); or immunocompromised patients. The study was approved by an institutional review board (Salus IRB [Austin, Texas]), and each participant underwent a complete and informed consent process.
Laterality for pretreatment with AFR followed by ALA-PDT vs ALA-PDT alone was determined at the time of treatment using a computer-based random number generator; even numbers resulted in pretreatment of the right side, and odd numbers resulted in pretreatment of the left side. Because of the difference in pretreatment methods for the 2 sides, it was not possible to perform the procedure under blinded conditions.
The treatment area was prepared by defatting the entire site with 70% isopropyl alcohol, followed by benzalkonium chloride antibacterial cleansing for the AFR pretreatment side. A 7% lidocaine/7% tetracaine ointment was applied under polyethylene wrap occlusion to the AFR pretreatment side for 20 minutes. Additionally, nerve blocks and field blocks with a mixture of 1.1% lidocaine with epinephrine/0.5% bupivacaine with epinephrine were performed wherever feasible. After 20 minutes, the lidocaine-tetracaine ointment was removed with isopropanol, and AFR treatment commenced immediately with the SmartXide DOT laser (DEKA)(1 pass of 25 W, 1200-microsecond duration at 500-µm spacing, 200-µm spot size, achieving 12% surface area ablation). Hyperkeratotic treated areas were debrided with saline and received a second pass with the laser. Aminolevulinic acid solution 20% (Levulan Kerastick; DUSA Pharmaceuticals, Inc)15 was applied to both sides of the treatment area and allowed to absorb for a 1-hour incubation period, which was followed by blue-light exposure at a power density of 10 mW/cm2 for 16 minutes and 40 seconds using the BLU-U Photodynamic Therapy Illuminator (DUSA Pharmaceuticals, Inc). Areas treated with AFR were then covered with a layer of Aquaphor ointment (Beiersdorf, Inc) and an absorptive hydrogel dressing for48 to 96 hours, with continued application of the ointment until resolution of all crusting. After treatment, patients were instructed to avoid direct sun exposure, wear a hat or visor for the first 2 weeks posttreatment when outdoors, and apply sunscreen with a sun protection factor greater than 30 once skin had healed.
Follow-up was conducted at 1 week, 1 month, 3 months, and 6 months after the PDT procedure. The primary end points were clinical clearance of NMSC lesions at 1, 3, and 6 months posttreatment and histological clearance at 6 months. Secondary end points assessed quality of life and functional improvements.
Results
Twenty-four potential participants experiencing AKs and/or NMSCs were screened for the study, with 19 meeting inclusion criteria. All participants were white, non-Hispanic, and had Fitzpatrick skin types I or II. Treated areas for all participants had field cancerization defined as at least 1 AK per square centimeter. All 19 participants enrolled in the study completed the posttreatment evaluations up to 6 months. All AFR-pretreated sites showed superior results in reduction in number, size, or hyperkeratosis of AKs at all follow-up visits, with a complete absence of new AK formation at the 6-month follow-up (Table). Conversely, sites treated with standard PDT only showed some recurrence of AKs at 6 months. Of the 3 participants who had biopsy-confirmed BCCs on the AFR-pretreated side, there were 3 persistent lesions after treatment at the 6-month visit. Two participants experienced persistence of a confirmed SCC in situ that was on the laser-pretreated side only (1 on the forehead and 1 on the hand), whereas 1 participant with an SCC on the leg at baseline had no recurrence at 6 months. A participant who received treatment on the lower lip had persistence of actinic cheilitis on both the AFR- and non–AFR-treated sides of the lip.
Scalp and facial sites healed fully in an average of 7 days, whereas upper extremities—forearm and hands—took approximately 14 days to heal completely. Lower extremity AFR-pretreated sites exhibited substantial weeping, resulting in prolonged healing of approximately 21 days for resolution of all scabbing.
Comment
In this split-sided study in patients with field cancerization, the use of CO2 laser AFR before treatment with PDT increased AK lesion clearance compared to ALA-PDT alone. Prior studies of fractional laser–assisted drug delivery on porcine skin using topical MAL showed that laser channels approximately 3-mm apart were able to distribute protoporphyrin through the entire skin.6 The ablative nature of AFR theoretically provides deeper and more effusive penetration of the ALA solution than using conventional PDT or erbium:YAG lasers with PDT.7,8 Helsing et al11 applied CO2 laser AFR MAL-PDT to AKs in organ transplant recipients and obtained complete responses in 73% of patients compared to a complete response of 31% for AFR alone. The results reported in our study are consistent with Helsing et al,11 showing a complete clinical response for 14 of 19 patients (74%), of whom 4 (21%) had no recurrence of NMSC and 10 (53%) had no recurrence of AK on the AFR-PDT–treated side.
The pretreatment process required for the laser AFR added time to the initial visit compared to conventional PDT, which is balanced by a reduced PDT incubation time (1 hour vs the approved indication of 14–18 hours for face/scalp or 3 hours for upper extremities under occlusion). The use of microneedling as an alternative pretreatment procedure before PDT also has been investigated, with the aim of decreasing the optimum ALA absorption time. The mean reduction in AKs (89.3%) was significantly greater than for PDT alone (69.5%; P<.05) in a small study by Spencer and Freeman.18 Although microneedling is less time-intensive and labor-intensive than laser AFR, the photocoagulative effect and subsequent microhemorrhages resulting from AFR should result in much deeper penetration of ALA solution than for microneedling.
The limitations of this proof-of-concept study arose from the small sample size of 19 participants and the short follow-up period of 6 months. Furthermore, the unblinded nature of the study could create selection, detection, or reporting bias. Further follow-up appointments would aid in determining the longevity of results, which may encourage future use of this technique, despite the time-consuming preparation. A larger study with follow-up greater than 1 year would be beneficial, particularly for monitoring remission from SCCs and BCCs.
Conclusion
Pretreatment with CO2 laser AFR before ALA-PDT provided superior clearance of AKs and thin NMSCs at 6 months compared to ALA-PDT alone (Figure). Additionally, the incubation period for ALA absorption can be reduced before PDT, leading to a shorter treatment time overall. The benefits of AFR pretreatment on AK clearance demonstrated in this study warrant further investigation in a larger trial with a longer follow-up period to monitor maintenance of response.
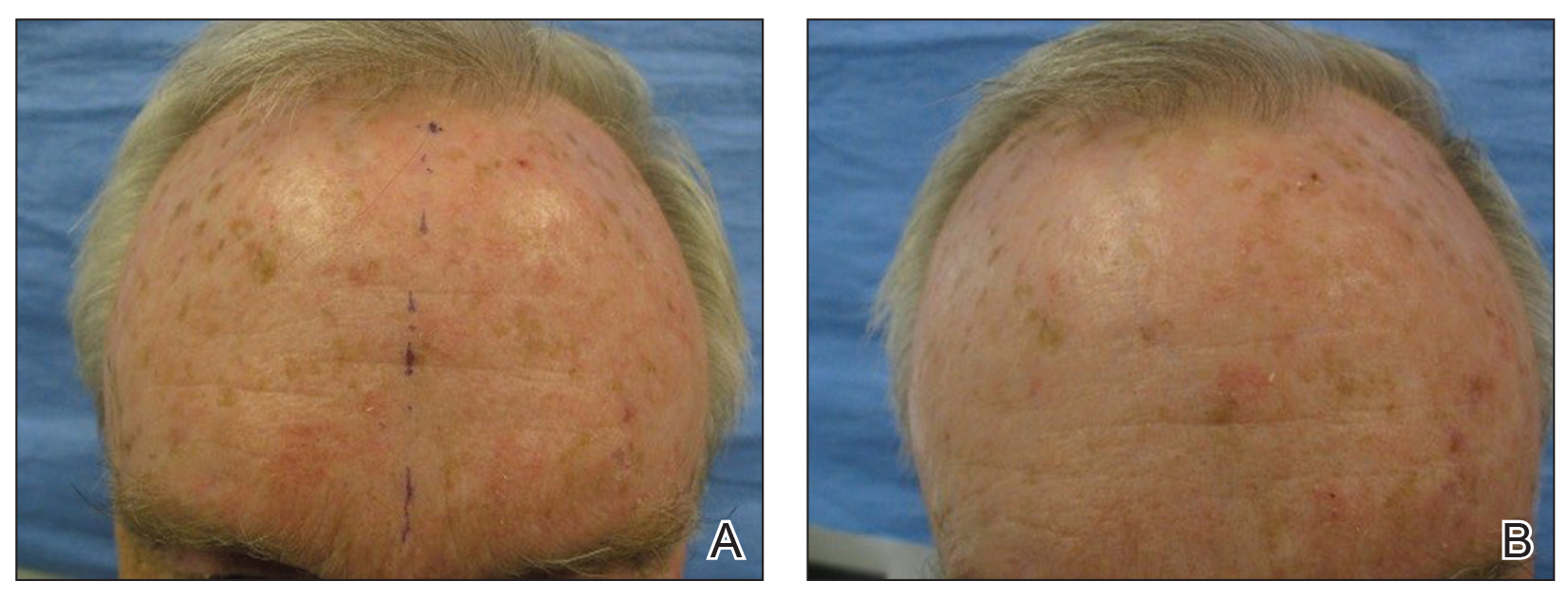
Acknowledgments
The authors thank the patients who participated in this study. Editorial assistance was provided by Louise Gildea, PhD, of JK Associates Inc, part of the Fishawack Group of Companies (Fishawack, United Kingdom), funded by Sun Pharmaceutical Industries, Inc.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245-1266.
- Casas A, Fukuda H, Di Venosa G, et al. Photosensitization and mechanism of cytotoxicity induced by the use of ALA derivatives in photodynamic therapy. Br J Cancer. 2001;85:279-284.
- Klotz LO, Fritsch C, Briviba K, et al. Activation of JNK and p38 but not ERK MAP kinases in human skin cells by 5-aminolevulinate-photodynamic therapy. Cancer Res. 1998;58:4297-4300.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Hædersdal M, Sakamoto FH, Farinelli WA, et al. Fractional CO(2) laser-assisted drug delivery. Lasers Surg Med. 2010;42:113-122.
- Šmucler R, Vlk M. Combination of Er:YAG laser and photodynamic therapy in the treatment of nodular basal cell carcinoma. Lasers Surg Med. 2008;40:153-158.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Alexiades M. Randomized, controlled trial of fractional carbon dioxide laser resurfacing followed by ultrashort incubation aminolevulinic acid blue light photodynamic therapy for actinic keratosis. Dermatol Surg. 2017;43:1053-1064.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Jang YH, Lee DJ, Shin J, et al. Photodynamic therapy with ablative carbon dioxide fractional laser in treatment of actinic keratosis. Ann Dermatol. 2013;25:417-422.
- Song HS, Jung SE, Jang YH, et al. Fractional carbon dioxide laser-assisted photodynamic therapy for patients with actinic keratosis. Photodermatol Photoimmunol Photomed. 2015;31:296-301.
- ALA Kerastick (aminolevulinic acid HCl) for topical solution, 20% [package insert]. Wilmington, MA: DUSA Pharmaceuticals; 2010.
- Data on file. Wilmington, MA: DUSA Pharmaceuticals; 2020.
- Campbell TM, Goldman MP. Adverse events of fractionated carbon dioxide laser: review of 373 treatments. Dermatol Surg. 2010;36:1645-1650.
- Spencer JM, Freeman SA. Microneedling prior to Levulan PDT for the treatment of actinic keratoses: a split-face, blinded trial. J Drugs Dermatol. 2016;15:1072-1074.
Actinic keratosis (AK) is the most common cutaneous lesion and is regarded as a precursor to nonmelanoma skin cancer (NMSC), particularly squamous cell carcinoma (SCC).1 Field cancerization refers to broad areas of chronically sun-exposed skin that show cumulative sun damage in the form of clinical and subclinical lesions. It is not feasible to treat large areas with multiple overt and subclinical lesions using surgical methods, and photodynamic therapy (PDT) has become a preferred method for treatment of field cancerization.2 Topical PDT uses the heme biosynthesis pathway precursors aminolevulinic acid (ALA) or methyl ALA (MAL), which localizes in the treatment area and is metabolized to protoporphyrin IX.3 After an incubation period, activation by a light source results in the formation of cytotoxic oxygen species,4 with reports of efficacy over large areas and excellent cosmetic outcomes.2
Laser ablative fractional resurfacing (AFR) also has been investigated as a treatment of AKs; CO2 laser AFR treatment resulted in a short-term reduction in the number of AK lesions and appeared to reduce the development of new lesions.5 However, case reports and small studies have indicated that pretreatment with laser AFR can increase the efficacy of PDT by creating microscopic vertical channels facilitating deeper penetration and uptake of the ALA.6 The use of erbium:YAG lasers in combination with PDT has demonstrated notable clinical and aesthetic improvements in treating basal cell carcinomas (BCCs)7 and AKs,8 with enhanced efficacy in moderate to thick AKs in particular. Hædersdal et al6 reported that CO2 laser AFR facilitated delivery of MAL into porcine skin, with AFR appearing to bypass the stratum corneum and deliver the treatment to the deep dermis.
The combination of CO2 laser AFR and PDT has shown statistically significant increases in efficacy for treatment of AKs compared to PDT alone (P<.001).9 In a small study, Alexiades10 reported a statistically significant improvement in AKs at 4 and 8 weeks posttreatment for 10 patients receiving CO2 laser AFR-PDT vs conventional PDT (P<.05). Studies of organ transplant recipients—who are at higher risk for AK and NMSC development—demonstrated favorable results for combined CO2 laser AFR and PDT vs either laser treatment11 or PDT9,12 alone, with significant reductions in the number of AKs (P=.002). Results were maintained for 3 to 4 months after treatment. Additional studies have shown that combining CO2 laser AFR and PDT may reduce the PDT incubation time or number of treatments required to achieve a response over conventional PDT.13,14
Our proof-of-concept study was designed to assess efficacy of CO2 laser AFR to enhance an approved drug delivery system in the treatment of AK and NMSC. The objective was to compare effect and durability of AFR-PDT vs standard ALA-PDT in the treatment of AK and NMSCs in a split-sided study of various body locations.
Methods
This randomized, split-sided study compared CO2 laser AFR-PDT to standard ALA-PDT for the treatment of AK and NMSC conducted at 1 site in Los Gatos, California. Patients who had a skin cancer screening and received a biopsy diagnosis of AK or NMSC were invited to attend an enrollment visit. Key inclusion criteria for enrollment were male or female patients aged 40 to 85 years with notable symmetrically comparable photodamage (at least 1 AK per square centimeter) in 1 or more skin areas—scalp, face, or distal extremities—with presence of clinically identifiable NMSCs proven by biopsy. Key exclusion criteria were patients who were pregnant; patients with epilepsy, seizures, or a photosensitive disorder; those taking photosensitizing medication (eg, doxycycline, hydrochlorothiazide); or immunocompromised patients. The study was approved by an institutional review board (Salus IRB [Austin, Texas]), and each participant underwent a complete and informed consent process.
Laterality for pretreatment with AFR followed by ALA-PDT vs ALA-PDT alone was determined at the time of treatment using a computer-based random number generator; even numbers resulted in pretreatment of the right side, and odd numbers resulted in pretreatment of the left side. Because of the difference in pretreatment methods for the 2 sides, it was not possible to perform the procedure under blinded conditions.
The treatment area was prepared by defatting the entire site with 70% isopropyl alcohol, followed by benzalkonium chloride antibacterial cleansing for the AFR pretreatment side. A 7% lidocaine/7% tetracaine ointment was applied under polyethylene wrap occlusion to the AFR pretreatment side for 20 minutes. Additionally, nerve blocks and field blocks with a mixture of 1.1% lidocaine with epinephrine/0.5% bupivacaine with epinephrine were performed wherever feasible. After 20 minutes, the lidocaine-tetracaine ointment was removed with isopropanol, and AFR treatment commenced immediately with the SmartXide DOT laser (DEKA)(1 pass of 25 W, 1200-microsecond duration at 500-µm spacing, 200-µm spot size, achieving 12% surface area ablation). Hyperkeratotic treated areas were debrided with saline and received a second pass with the laser. Aminolevulinic acid solution 20% (Levulan Kerastick; DUSA Pharmaceuticals, Inc)15 was applied to both sides of the treatment area and allowed to absorb for a 1-hour incubation period, which was followed by blue-light exposure at a power density of 10 mW/cm2 for 16 minutes and 40 seconds using the BLU-U Photodynamic Therapy Illuminator (DUSA Pharmaceuticals, Inc). Areas treated with AFR were then covered with a layer of Aquaphor ointment (Beiersdorf, Inc) and an absorptive hydrogel dressing for48 to 96 hours, with continued application of the ointment until resolution of all crusting. After treatment, patients were instructed to avoid direct sun exposure, wear a hat or visor for the first 2 weeks posttreatment when outdoors, and apply sunscreen with a sun protection factor greater than 30 once skin had healed.
Follow-up was conducted at 1 week, 1 month, 3 months, and 6 months after the PDT procedure. The primary end points were clinical clearance of NMSC lesions at 1, 3, and 6 months posttreatment and histological clearance at 6 months. Secondary end points assessed quality of life and functional improvements.
Results
Twenty-four potential participants experiencing AKs and/or NMSCs were screened for the study, with 19 meeting inclusion criteria. All participants were white, non-Hispanic, and had Fitzpatrick skin types I or II. Treated areas for all participants had field cancerization defined as at least 1 AK per square centimeter. All 19 participants enrolled in the study completed the posttreatment evaluations up to 6 months. All AFR-pretreated sites showed superior results in reduction in number, size, or hyperkeratosis of AKs at all follow-up visits, with a complete absence of new AK formation at the 6-month follow-up (Table). Conversely, sites treated with standard PDT only showed some recurrence of AKs at 6 months. Of the 3 participants who had biopsy-confirmed BCCs on the AFR-pretreated side, there were 3 persistent lesions after treatment at the 6-month visit. Two participants experienced persistence of a confirmed SCC in situ that was on the laser-pretreated side only (1 on the forehead and 1 on the hand), whereas 1 participant with an SCC on the leg at baseline had no recurrence at 6 months. A participant who received treatment on the lower lip had persistence of actinic cheilitis on both the AFR- and non–AFR-treated sides of the lip.

Scalp and facial sites healed fully in an average of 7 days, whereas upper extremities—forearm and hands—took approximately 14 days to heal completely. Lower extremity AFR-pretreated sites exhibited substantial weeping, resulting in prolonged healing of approximately 21 days for resolution of all scabbing.
Comment
In this split-sided study in patients with field cancerization, the use of CO2 laser AFR before treatment with PDT increased AK lesion clearance compared to ALA-PDT alone. Prior studies of fractional laser–assisted drug delivery on porcine skin using topical MAL showed that laser channels approximately 3-mm apart were able to distribute protoporphyrin through the entire skin.6 The ablative nature of AFR theoretically provides deeper and more effusive penetration of the ALA solution than using conventional PDT or erbium:YAG lasers with PDT.7,8 Helsing et al11 applied CO2 laser AFR MAL-PDT to AKs in organ transplant recipients and obtained complete responses in 73% of patients compared to a complete response of 31% for AFR alone. The results reported in our study are consistent with Helsing et al,11 showing a complete clinical response for 14 of 19 patients (74%), of whom 4 (21%) had no recurrence of NMSC and 10 (53%) had no recurrence of AK on the AFR-PDT–treated side.
The pretreatment process required for the laser AFR added time to the initial visit compared to conventional PDT, which is balanced by a reduced PDT incubation time (1 hour vs the approved indication of 14–18 hours for face/scalp or 3 hours for upper extremities under occlusion). The use of microneedling as an alternative pretreatment procedure before PDT also has been investigated, with the aim of decreasing the optimum ALA absorption time. The mean reduction in AKs (89.3%) was significantly greater than for PDT alone (69.5%; P<.05) in a small study by Spencer and Freeman.18 Although microneedling is less time-intensive and labor-intensive than laser AFR, the photocoagulative effect and subsequent microhemorrhages resulting from AFR should result in much deeper penetration of ALA solution than for microneedling.
The limitations of this proof-of-concept study arose from the small sample size of 19 participants and the short follow-up period of 6 months. Furthermore, the unblinded nature of the study could create selection, detection, or reporting bias. Further follow-up appointments would aid in determining the longevity of results, which may encourage future use of this technique, despite the time-consuming preparation. A larger study with follow-up greater than 1 year would be beneficial, particularly for monitoring remission from SCCs and BCCs.
Conclusion
Pretreatment with CO2 laser AFR before ALA-PDT provided superior clearance of AKs and thin NMSCs at 6 months compared to ALA-PDT alone (Figure). Additionally, the incubation period for ALA absorption can be reduced before PDT, leading to a shorter treatment time overall. The benefits of AFR pretreatment on AK clearance demonstrated in this study warrant further investigation in a larger trial with a longer follow-up period to monitor maintenance of response.

Acknowledgments
The authors thank the patients who participated in this study. Editorial assistance was provided by Louise Gildea, PhD, of JK Associates Inc, part of the Fishawack Group of Companies (Fishawack, United Kingdom), funded by Sun Pharmaceutical Industries, Inc.
Actinic keratosis (AK) is the most common cutaneous lesion and is regarded as a precursor to nonmelanoma skin cancer (NMSC), particularly squamous cell carcinoma (SCC).1 Field cancerization refers to broad areas of chronically sun-exposed skin that show cumulative sun damage in the form of clinical and subclinical lesions. It is not feasible to treat large areas with multiple overt and subclinical lesions using surgical methods, and photodynamic therapy (PDT) has become a preferred method for treatment of field cancerization.2 Topical PDT uses the heme biosynthesis pathway precursors aminolevulinic acid (ALA) or methyl ALA (MAL), which localizes in the treatment area and is metabolized to protoporphyrin IX.3 After an incubation period, activation by a light source results in the formation of cytotoxic oxygen species,4 with reports of efficacy over large areas and excellent cosmetic outcomes.2
Laser ablative fractional resurfacing (AFR) also has been investigated as a treatment of AKs; CO2 laser AFR treatment resulted in a short-term reduction in the number of AK lesions and appeared to reduce the development of new lesions.5 However, case reports and small studies have indicated that pretreatment with laser AFR can increase the efficacy of PDT by creating microscopic vertical channels facilitating deeper penetration and uptake of the ALA.6 The use of erbium:YAG lasers in combination with PDT has demonstrated notable clinical and aesthetic improvements in treating basal cell carcinomas (BCCs)7 and AKs,8 with enhanced efficacy in moderate to thick AKs in particular. Hædersdal et al6 reported that CO2 laser AFR facilitated delivery of MAL into porcine skin, with AFR appearing to bypass the stratum corneum and deliver the treatment to the deep dermis.
The combination of CO2 laser AFR and PDT has shown statistically significant increases in efficacy for treatment of AKs compared to PDT alone (P<.001).9 In a small study, Alexiades10 reported a statistically significant improvement in AKs at 4 and 8 weeks posttreatment for 10 patients receiving CO2 laser AFR-PDT vs conventional PDT (P<.05). Studies of organ transplant recipients—who are at higher risk for AK and NMSC development—demonstrated favorable results for combined CO2 laser AFR and PDT vs either laser treatment11 or PDT9,12 alone, with significant reductions in the number of AKs (P=.002). Results were maintained for 3 to 4 months after treatment. Additional studies have shown that combining CO2 laser AFR and PDT may reduce the PDT incubation time or number of treatments required to achieve a response over conventional PDT.13,14
Our proof-of-concept study was designed to assess efficacy of CO2 laser AFR to enhance an approved drug delivery system in the treatment of AK and NMSC. The objective was to compare effect and durability of AFR-PDT vs standard ALA-PDT in the treatment of AK and NMSCs in a split-sided study of various body locations.
Methods
This randomized, split-sided study compared CO2 laser AFR-PDT to standard ALA-PDT for the treatment of AK and NMSC conducted at 1 site in Los Gatos, California. Patients who had a skin cancer screening and received a biopsy diagnosis of AK or NMSC were invited to attend an enrollment visit. Key inclusion criteria for enrollment were male or female patients aged 40 to 85 years with notable symmetrically comparable photodamage (at least 1 AK per square centimeter) in 1 or more skin areas—scalp, face, or distal extremities—with presence of clinically identifiable NMSCs proven by biopsy. Key exclusion criteria were patients who were pregnant; patients with epilepsy, seizures, or a photosensitive disorder; those taking photosensitizing medication (eg, doxycycline, hydrochlorothiazide); or immunocompromised patients. The study was approved by an institutional review board (Salus IRB [Austin, Texas]), and each participant underwent a complete and informed consent process.
Laterality for pretreatment with AFR followed by ALA-PDT vs ALA-PDT alone was determined at the time of treatment using a computer-based random number generator; even numbers resulted in pretreatment of the right side, and odd numbers resulted in pretreatment of the left side. Because of the difference in pretreatment methods for the 2 sides, it was not possible to perform the procedure under blinded conditions.
The treatment area was prepared by defatting the entire site with 70% isopropyl alcohol, followed by benzalkonium chloride antibacterial cleansing for the AFR pretreatment side. A 7% lidocaine/7% tetracaine ointment was applied under polyethylene wrap occlusion to the AFR pretreatment side for 20 minutes. Additionally, nerve blocks and field blocks with a mixture of 1.1% lidocaine with epinephrine/0.5% bupivacaine with epinephrine were performed wherever feasible. After 20 minutes, the lidocaine-tetracaine ointment was removed with isopropanol, and AFR treatment commenced immediately with the SmartXide DOT laser (DEKA)(1 pass of 25 W, 1200-microsecond duration at 500-µm spacing, 200-µm spot size, achieving 12% surface area ablation). Hyperkeratotic treated areas were debrided with saline and received a second pass with the laser. Aminolevulinic acid solution 20% (Levulan Kerastick; DUSA Pharmaceuticals, Inc)15 was applied to both sides of the treatment area and allowed to absorb for a 1-hour incubation period, which was followed by blue-light exposure at a power density of 10 mW/cm2 for 16 minutes and 40 seconds using the BLU-U Photodynamic Therapy Illuminator (DUSA Pharmaceuticals, Inc). Areas treated with AFR were then covered with a layer of Aquaphor ointment (Beiersdorf, Inc) and an absorptive hydrogel dressing for48 to 96 hours, with continued application of the ointment until resolution of all crusting. After treatment, patients were instructed to avoid direct sun exposure, wear a hat or visor for the first 2 weeks posttreatment when outdoors, and apply sunscreen with a sun protection factor greater than 30 once skin had healed.
Follow-up was conducted at 1 week, 1 month, 3 months, and 6 months after the PDT procedure. The primary end points were clinical clearance of NMSC lesions at 1, 3, and 6 months posttreatment and histological clearance at 6 months. Secondary end points assessed quality of life and functional improvements.
Results
Twenty-four potential participants experiencing AKs and/or NMSCs were screened for the study, with 19 meeting inclusion criteria. All participants were white, non-Hispanic, and had Fitzpatrick skin types I or II. Treated areas for all participants had field cancerization defined as at least 1 AK per square centimeter. All 19 participants enrolled in the study completed the posttreatment evaluations up to 6 months. All AFR-pretreated sites showed superior results in reduction in number, size, or hyperkeratosis of AKs at all follow-up visits, with a complete absence of new AK formation at the 6-month follow-up (Table). Conversely, sites treated with standard PDT only showed some recurrence of AKs at 6 months. Of the 3 participants who had biopsy-confirmed BCCs on the AFR-pretreated side, there were 3 persistent lesions after treatment at the 6-month visit. Two participants experienced persistence of a confirmed SCC in situ that was on the laser-pretreated side only (1 on the forehead and 1 on the hand), whereas 1 participant with an SCC on the leg at baseline had no recurrence at 6 months. A participant who received treatment on the lower lip had persistence of actinic cheilitis on both the AFR- and non–AFR-treated sides of the lip.

Scalp and facial sites healed fully in an average of 7 days, whereas upper extremities—forearm and hands—took approximately 14 days to heal completely. Lower extremity AFR-pretreated sites exhibited substantial weeping, resulting in prolonged healing of approximately 21 days for resolution of all scabbing.
Comment
In this split-sided study in patients with field cancerization, the use of CO2 laser AFR before treatment with PDT increased AK lesion clearance compared to ALA-PDT alone. Prior studies of fractional laser–assisted drug delivery on porcine skin using topical MAL showed that laser channels approximately 3-mm apart were able to distribute protoporphyrin through the entire skin.6 The ablative nature of AFR theoretically provides deeper and more effusive penetration of the ALA solution than using conventional PDT or erbium:YAG lasers with PDT.7,8 Helsing et al11 applied CO2 laser AFR MAL-PDT to AKs in organ transplant recipients and obtained complete responses in 73% of patients compared to a complete response of 31% for AFR alone. The results reported in our study are consistent with Helsing et al,11 showing a complete clinical response for 14 of 19 patients (74%), of whom 4 (21%) had no recurrence of NMSC and 10 (53%) had no recurrence of AK on the AFR-PDT–treated side.
The pretreatment process required for the laser AFR added time to the initial visit compared to conventional PDT, which is balanced by a reduced PDT incubation time (1 hour vs the approved indication of 14–18 hours for face/scalp or 3 hours for upper extremities under occlusion). The use of microneedling as an alternative pretreatment procedure before PDT also has been investigated, with the aim of decreasing the optimum ALA absorption time. The mean reduction in AKs (89.3%) was significantly greater than for PDT alone (69.5%; P<.05) in a small study by Spencer and Freeman.18 Although microneedling is less time-intensive and labor-intensive than laser AFR, the photocoagulative effect and subsequent microhemorrhages resulting from AFR should result in much deeper penetration of ALA solution than for microneedling.
The limitations of this proof-of-concept study arose from the small sample size of 19 participants and the short follow-up period of 6 months. Furthermore, the unblinded nature of the study could create selection, detection, or reporting bias. Further follow-up appointments would aid in determining the longevity of results, which may encourage future use of this technique, despite the time-consuming preparation. A larger study with follow-up greater than 1 year would be beneficial, particularly for monitoring remission from SCCs and BCCs.
Conclusion
Pretreatment with CO2 laser AFR before ALA-PDT provided superior clearance of AKs and thin NMSCs at 6 months compared to ALA-PDT alone (Figure). Additionally, the incubation period for ALA absorption can be reduced before PDT, leading to a shorter treatment time overall. The benefits of AFR pretreatment on AK clearance demonstrated in this study warrant further investigation in a larger trial with a longer follow-up period to monitor maintenance of response.

Acknowledgments
The authors thank the patients who participated in this study. Editorial assistance was provided by Louise Gildea, PhD, of JK Associates Inc, part of the Fishawack Group of Companies (Fishawack, United Kingdom), funded by Sun Pharmaceutical Industries, Inc.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245-1266.
- Casas A, Fukuda H, Di Venosa G, et al. Photosensitization and mechanism of cytotoxicity induced by the use of ALA derivatives in photodynamic therapy. Br J Cancer. 2001;85:279-284.
- Klotz LO, Fritsch C, Briviba K, et al. Activation of JNK and p38 but not ERK MAP kinases in human skin cells by 5-aminolevulinate-photodynamic therapy. Cancer Res. 1998;58:4297-4300.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Hædersdal M, Sakamoto FH, Farinelli WA, et al. Fractional CO(2) laser-assisted drug delivery. Lasers Surg Med. 2010;42:113-122.
- Šmucler R, Vlk M. Combination of Er:YAG laser and photodynamic therapy in the treatment of nodular basal cell carcinoma. Lasers Surg Med. 2008;40:153-158.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Alexiades M. Randomized, controlled trial of fractional carbon dioxide laser resurfacing followed by ultrashort incubation aminolevulinic acid blue light photodynamic therapy for actinic keratosis. Dermatol Surg. 2017;43:1053-1064.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Jang YH, Lee DJ, Shin J, et al. Photodynamic therapy with ablative carbon dioxide fractional laser in treatment of actinic keratosis. Ann Dermatol. 2013;25:417-422.
- Song HS, Jung SE, Jang YH, et al. Fractional carbon dioxide laser-assisted photodynamic therapy for patients with actinic keratosis. Photodermatol Photoimmunol Photomed. 2015;31:296-301.
- ALA Kerastick (aminolevulinic acid HCl) for topical solution, 20% [package insert]. Wilmington, MA: DUSA Pharmaceuticals; 2010.
- Data on file. Wilmington, MA: DUSA Pharmaceuticals; 2020.
- Campbell TM, Goldman MP. Adverse events of fractionated carbon dioxide laser: review of 373 treatments. Dermatol Surg. 2010;36:1645-1650.
- Spencer JM, Freeman SA. Microneedling prior to Levulan PDT for the treatment of actinic keratoses: a split-face, blinded trial. J Drugs Dermatol. 2016;15:1072-1074.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245-1266.
- Casas A, Fukuda H, Di Venosa G, et al. Photosensitization and mechanism of cytotoxicity induced by the use of ALA derivatives in photodynamic therapy. Br J Cancer. 2001;85:279-284.
- Klotz LO, Fritsch C, Briviba K, et al. Activation of JNK and p38 but not ERK MAP kinases in human skin cells by 5-aminolevulinate-photodynamic therapy. Cancer Res. 1998;58:4297-4300.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Hædersdal M, Sakamoto FH, Farinelli WA, et al. Fractional CO(2) laser-assisted drug delivery. Lasers Surg Med. 2010;42:113-122.
- Šmucler R, Vlk M. Combination of Er:YAG laser and photodynamic therapy in the treatment of nodular basal cell carcinoma. Lasers Surg Med. 2008;40:153-158.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Alexiades M. Randomized, controlled trial of fractional carbon dioxide laser resurfacing followed by ultrashort incubation aminolevulinic acid blue light photodynamic therapy for actinic keratosis. Dermatol Surg. 2017;43:1053-1064.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Jang YH, Lee DJ, Shin J, et al. Photodynamic therapy with ablative carbon dioxide fractional laser in treatment of actinic keratosis. Ann Dermatol. 2013;25:417-422.
- Song HS, Jung SE, Jang YH, et al. Fractional carbon dioxide laser-assisted photodynamic therapy for patients with actinic keratosis. Photodermatol Photoimmunol Photomed. 2015;31:296-301.
- ALA Kerastick (aminolevulinic acid HCl) for topical solution, 20% [package insert]. Wilmington, MA: DUSA Pharmaceuticals; 2010.
- Data on file. Wilmington, MA: DUSA Pharmaceuticals; 2020.
- Campbell TM, Goldman MP. Adverse events of fractionated carbon dioxide laser: review of 373 treatments. Dermatol Surg. 2010;36:1645-1650.
- Spencer JM, Freeman SA. Microneedling prior to Levulan PDT for the treatment of actinic keratoses: a split-face, blinded trial. J Drugs Dermatol. 2016;15:1072-1074.
Practice Points
- Pretreatment with CO2 laser ablative fractional resurfacing (AFR) before photodynamic therapy (PDT) provided efficient clearance of actinic keratosis (AK).
- Superior clearance of lesions was seen at 6 months for AK and thin nonmelanoma skin cancers (NMSCs) on pretreated sites compared to PDT alone, with no novel adverse events reported.
- A reduced incubation period for aminolevulinic acid (ALA) absorption before PDT was used, leading to a shorter overall treatment time.
EU panel review supports decision to pull Picato from market
Picato was cleared for marketing in the European Union in November 2012. The European Commission requested a safety review of the drug in September 2019 after data suggested a higher number of skin cancer cases, including cases of squamous cell carcinoma, in patients using it, as reported by Medscape Medical News.
In January 2020, use of Picato was suspended as a precaution while the PRAC review was underway. One month later, marketing authorization was withdrawn at the request of Leo Laboratories Ltd, which marketed the medicine.
The PRAC has now concluded its review of all available data on the risk for skin cancer in patients using Picato, including results of a study that compared Picato with imiquimod.
The review found “a higher occurrence of skin cancers, especially squamous cell carcinoma, in areas of skin treated with Picato than in areas treated with imiquimod,” the EMA said Friday in a news release.
“The committee also considered that Picato’s effectiveness is not maintained over time and noted that other treatment options are available for actinic keratosis,” the EMA said.
The agency recommends that patients who have used Picato watch for unusual skin changes or growths, which may occur weeks to months after use, and seek medical advice if any occur.
Picato continues to be available in the United States, although the US Food and Drug Administration is also looking into its safety and risks.
This article first appeared on Medscape.com.
Picato was cleared for marketing in the European Union in November 2012. The European Commission requested a safety review of the drug in September 2019 after data suggested a higher number of skin cancer cases, including cases of squamous cell carcinoma, in patients using it, as reported by Medscape Medical News.
In January 2020, use of Picato was suspended as a precaution while the PRAC review was underway. One month later, marketing authorization was withdrawn at the request of Leo Laboratories Ltd, which marketed the medicine.
The PRAC has now concluded its review of all available data on the risk for skin cancer in patients using Picato, including results of a study that compared Picato with imiquimod.
The review found “a higher occurrence of skin cancers, especially squamous cell carcinoma, in areas of skin treated with Picato than in areas treated with imiquimod,” the EMA said Friday in a news release.
“The committee also considered that Picato’s effectiveness is not maintained over time and noted that other treatment options are available for actinic keratosis,” the EMA said.
The agency recommends that patients who have used Picato watch for unusual skin changes or growths, which may occur weeks to months after use, and seek medical advice if any occur.
Picato continues to be available in the United States, although the US Food and Drug Administration is also looking into its safety and risks.
This article first appeared on Medscape.com.
Picato was cleared for marketing in the European Union in November 2012. The European Commission requested a safety review of the drug in September 2019 after data suggested a higher number of skin cancer cases, including cases of squamous cell carcinoma, in patients using it, as reported by Medscape Medical News.
In January 2020, use of Picato was suspended as a precaution while the PRAC review was underway. One month later, marketing authorization was withdrawn at the request of Leo Laboratories Ltd, which marketed the medicine.
The PRAC has now concluded its review of all available data on the risk for skin cancer in patients using Picato, including results of a study that compared Picato with imiquimod.
The review found “a higher occurrence of skin cancers, especially squamous cell carcinoma, in areas of skin treated with Picato than in areas treated with imiquimod,” the EMA said Friday in a news release.
“The committee also considered that Picato’s effectiveness is not maintained over time and noted that other treatment options are available for actinic keratosis,” the EMA said.
The agency recommends that patients who have used Picato watch for unusual skin changes or growths, which may occur weeks to months after use, and seek medical advice if any occur.
Picato continues to be available in the United States, although the US Food and Drug Administration is also looking into its safety and risks.
This article first appeared on Medscape.com.
Inflammatory Changes in Actinic Keratoses Associated With Afatinib Therapy
To the Editor:
Afatinib is a small molecule covalently binding and inhibiting the epidermal growth factor receptor (EGFR) as well as HER2 and HER4 receptor tyrosine kinases.1 The EGFR family is part of a complex signal transduction network that is central to several critical cellular processes.2 The human EGFR family is dysregulated in many solid tumors, making it an attractive target for anticancer therapy.2 In 2013, the US Food and Drug Administration approved afatinib as a first-line treatment of patients with metastatic non–small cell lung cancer whose tumors have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations.3
Treatment with afatinib and other EGFR inhibitors is frequently associated with cutaneous adverse effects that occur in up to 90% of patients. These cutaneous reactions are typical for this drug family and distinct from the skin adverse effects related to other types of anticancer chemotherapy.4 The most frequent skin manifestations following afatinib treatment consist of an acneform pustular eruption in up to 90% of patients.5,6 Other dermatologic reactions include nonspecific maculopapular rashes (90%), stomatitis (71%), paronychia with some nail changes (58%), xerosis (31%), pruritus (21%), and hand-foot syndrome (7%)5,6; however, grade 3 dermatologic reactions occurred in only 0.15% of patients.
Inflammatory changes in both preexisting and undetected actinic keratoses (AKs) and even progression to squamous cell carcinoma (SCC) have been previously described as uncommon dermatologic adverse effects of 2 EGFR inhibitors, sorafenib and erlotinib.7-9 Seven of 131 patients with metastatic renal cell carcinoma treated with single-agent sorafenib developed cutaneous SCC and 3 more had AKs.9 One patient demonstrated self-limited inflammatory flare-up of AKs during erlotinib treatment.8 We report acute inflammation of AKs from afatinib treatment.
A 78-year-old woman with fair skin who was previously treated for several AKs in sun-exposed areas presented with inflammatory changes that appeared at the site of AKs on photoexposed areas 110 days after initiating afatinib therapy (40 mg/d). Physical examination revealed multiple erythematous scaly plaques on the face, neck, chest, and forearms (Figure 1).
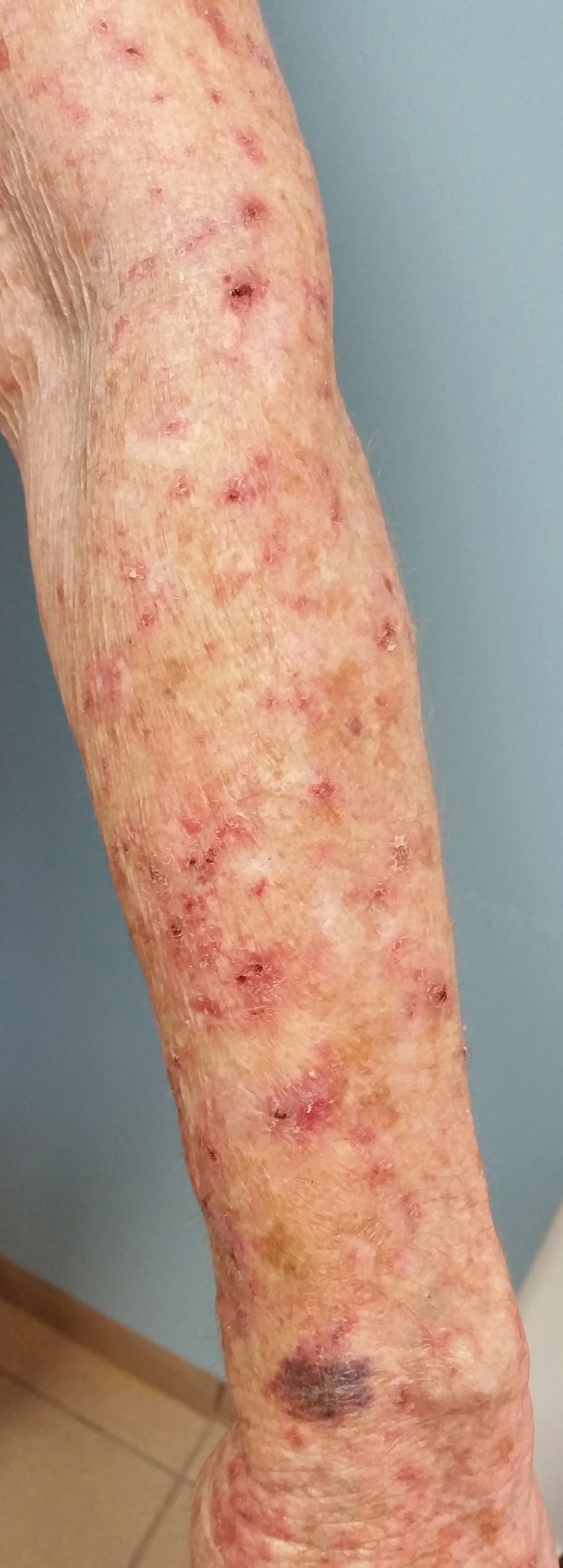
In the previous 2 decades, lesions that were surgically removed and histopathologically examined included Bowen disease (2 lesions), 2 basal cell carcinomas, 2 blue nevi, and a seborrheic keratosis. Several AKs also were surgically removed and confirmed histopathologically.
Eighteen months prior to the current presentation, the patient was diagnosed with locally advanced, inoperable, stage IIIA adenocarcinoma of the lung with deletion in exon 19 of the EGFR gene. She received definitive concomitant chemoradiation with the carboplatin-vinorelbine regimen and 60-Gy radiation. Four months later, a positron emission tomography (PET)–fludeoxyglucose scan revealed a single bone lesion in the L5 vertebra leading to irradiation to the lumbar spine. Subsequently, new metastases to the neck, right lung, T5 vertebra, and left acetabulum were detected by PET–computed tomography. One year later, afatinib 40 mg/d was initiated. A PET scan after 2 months of treatment showed excellent response.
At the current presentation, a punch biopsy obtained from an inflammatory lesion on the left dorsal forearm revealed findings consistent with an eroded and inflamed AK; the biopsy showed marked dysplasia of the keratinocytes that was predominately located in the basal layer of the epidermis. The lesion was accompanied by a dense mixed inflammatory cell infiltrate that was centered in the papillary dermis and extended to the epidermis (Figure 2). Because of this grade 3 skin toxicity, the afatinib dosage was reduced to 20 mg/d, and betamethasone cream 0.1% and emollients were applied locally for 2 weeks. A reduction in the number of AKs and clinical regression of the inflammatory changes was observed 2 weeks later (Figure 3).
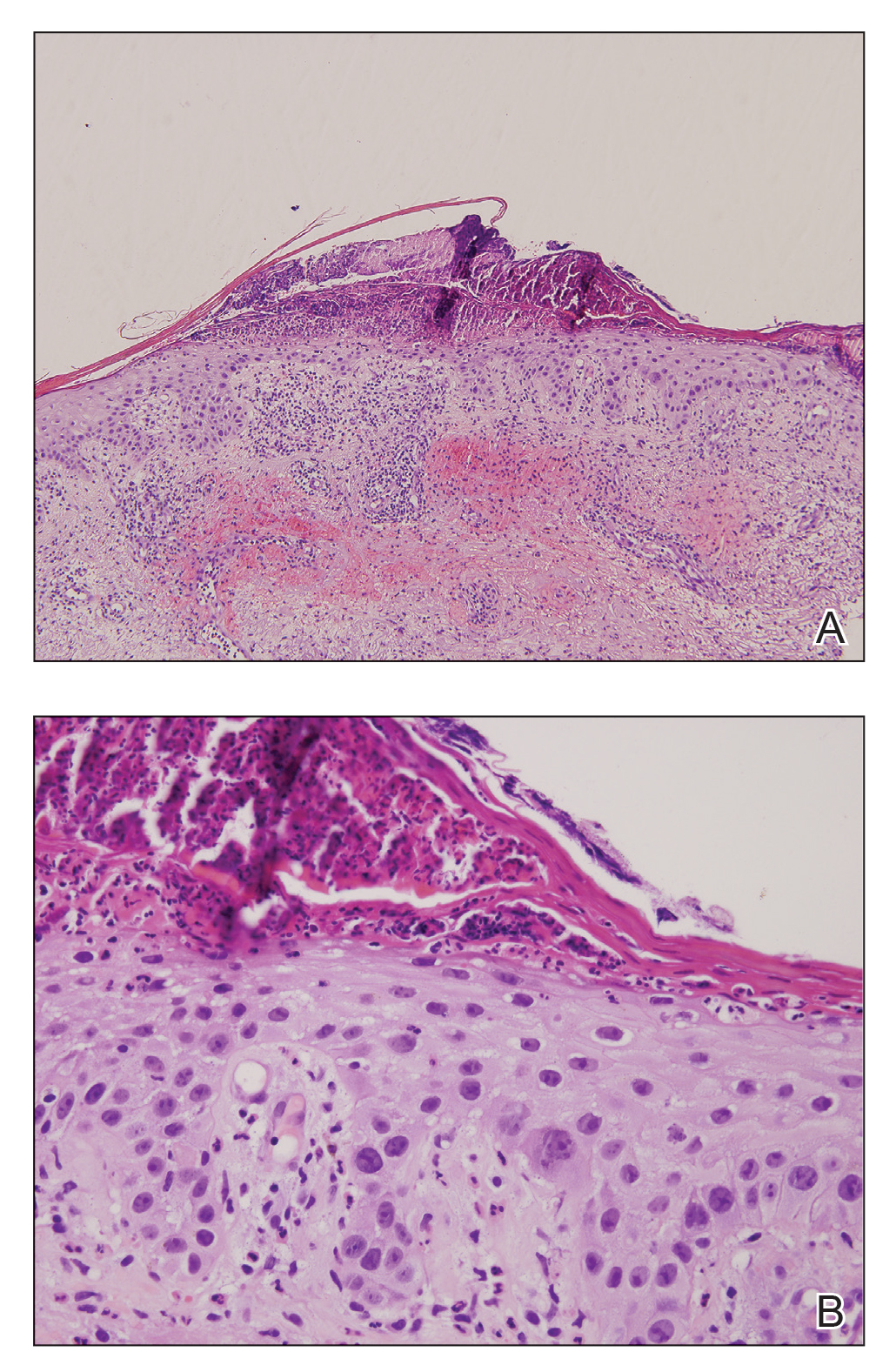
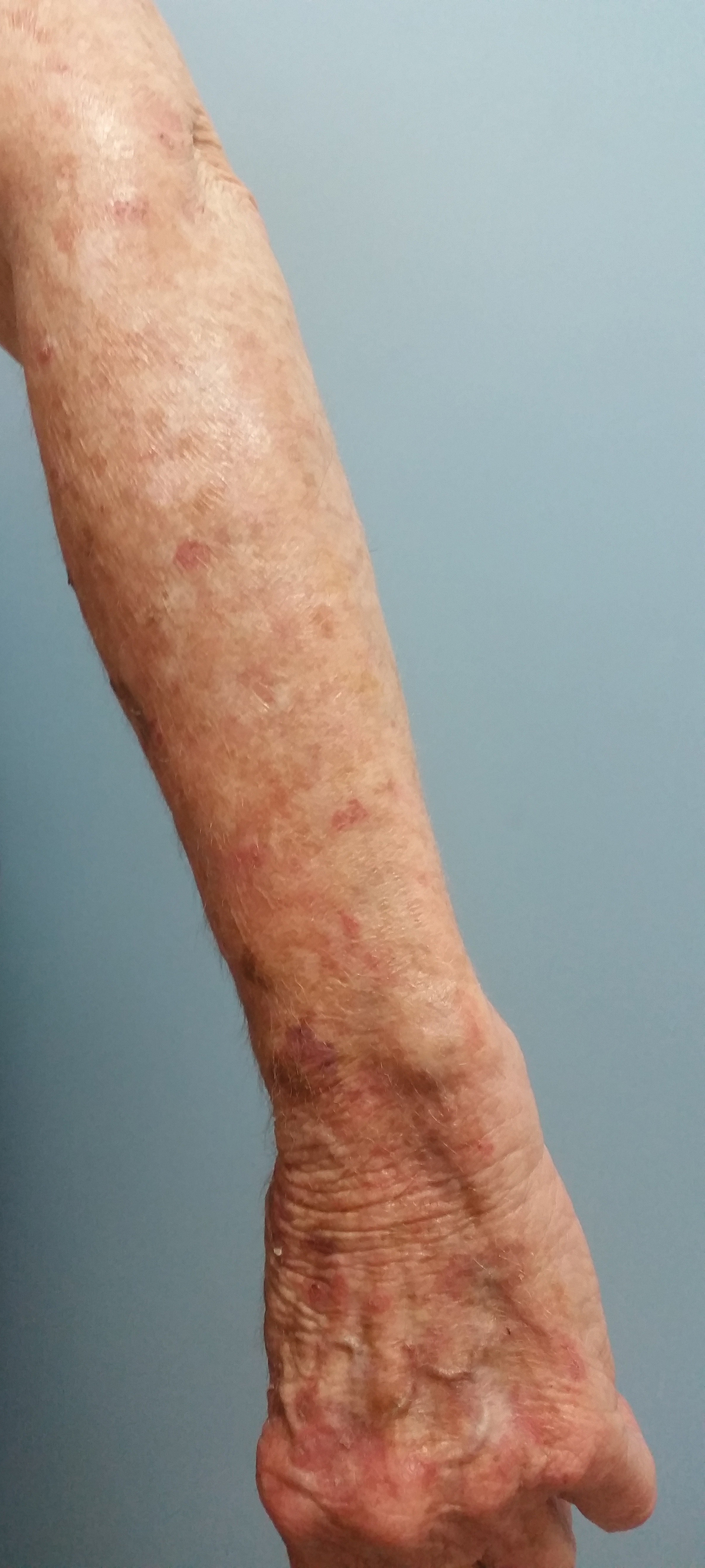
Chronically sun-exposed skin is prone to develop AKs that are at risk to progress to SCC.10-12 These lesions are increasingly diagnosed in older patients when internal cancers also are prevalent.13 Inflammatory flare-up of AKs is typically present during the regression phase14,15 but also during progression to SCC.16
There are many strategies for treating AKs. Physical procedures for destroying the lesions are commonly used. Some topical drugs, including imiquimod, 5-fluorouracil, and diclofenac sodium, also have proven efficacy.17
Conventional chemotherapeutic agents that have been described to be associated with the inflammation of AKs include docetaxel; doxorubicin; capecitabine; pentostatin; and the combination of dactinomycin, vincristine, dacarbazine and doxorubicin, cytarabine, and 6-thioguanine.7,18 The mechanism leading to this effect is unknown, though abnormal DNA synthesis and a type of radiation recall phenomenon have been postulated.7
We described inflammatory changes in AKs associated with afatinib treatment. The precise mechanism by which afatinib induces inflammation in AK has not been elucidated; however, it is known that EGFR normally downregulates chemokine expression in keratinocytes. Conversely, EGFR signaling blockade produces opposite effects, with increased CCL2, CCL5, and CXCL10, as well as reduced CXCL8 expression, leading to enhanced skin inflammation.19 Afatinib is a targeted agent that modulates the Ras/Raf/MEK/ERK signaling circuit, which is a key intracellular signal transduction pathway.20 This pathway and its downstream effectors have been implicated in cutaneous squamous cell carcinogenesis that might be accompanied by inflammatory changes.21,22 The remarkable clinical improvement of the AKs in our patient following the inflammatory flare-up supports the notion that the anticancer effect on intraepidermal neoplasms might be mediated by inflammation.23
- Katakami N, Atagi S, Goto K, et al. LUX-lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 2013;31:3335-3342.
- Liao BC, Lin CC, Yang JCH. First-line management of EGFR-mutated advanced lung adenocarcinoma: recent developments. Drugs. 2013;73:357-369.
- Jain P, Khanal R, Sharma A, et al. Afatinib and lung cancer. Expert Rev Anticancer Ther. 2014;14:1391-1406.
- Wyatt AJ, Leonard GD, Sachs DL. Cutaneous reactions to chemotherapy and their management. Am J Clin Dermatol. 2006;7:45-63.
- Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16:1425-1433.
- Agero ALC, Dusza SW, Benvenuto-Andrade C, et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657-670.
- Lacouture ME, Desai A, Soltani K, et al. Inflammation of actinic keratoses subsequent to therapy with sorafenib, a multitargeted tyrosine-kinase inhibitor. Clin Exp Dermatol. 2006;31:783-785.
- Hermanns JF, Piérard GE, Quatresooz P. Erlotinib-responsive actinic keratoses. Oncol Rep. 2007;18:581-584.
- Dubauskas Z, Kunishige J, Prieto VG, et al. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23.
- Czarnecki D, Meehan CJ, Bruce F, et al. The majority of cutaneous squamous cell carcinomas arise in actinic keratoses. J Cutan Med Surg. 2002;6:207-209.
- Ehrig T, Cockerell C, Piacquadio D, et al. Actinic keratoses and the incidence of occult squamous cell carcinoma: a clinical-histopathologic correlation. Dermatolog Surg. 2006;32:1261-1265.
- Quaedvlieg PJF, Tirsi E, Thissen MRTM, et al. Actinic keratosis: how to differentiate the good from the bad ones? Eur J Dermatol. 2006;16:335-339.
- Atkins D, Bang RH, Sternberg MR, et al. Reliable methods to evaluate the burden of actinic keratoses. J Invest Dermatol. 2006;126:591-594.
- Ooi T, Barnetson RS, Zhuang L, et al. Imiquimod-induced regression of actinic keratosis is associated with infiltration by T lymphocytes and dendritic cells: a randomized controlled trial. Br J Dermatol. 2006;154:72-78.
- Quatresooz P, Piérard GE. Imiquimod-responsive basal cell carcinomas and factor XIIIa enriched dendrocytes. Clin Exp Dermatol. 2003;28(suppl 1):27-29.
- Berhane T, Halliday GM, Cooke B, et al. Inflammation is associated with progression of actinic keratoses to squamous cell carcinomas in humans. Br J Dermatol. 2002;146:810-815.
- Ceilley RI, Jorizzo JL. Current issues in the management of actinic keratosis. J Am Acad Dermatol. 2013;68(1 suppl 1):S28-S38.
- Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol. 1999;40:367-398.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- Zebisch A, Czernilofsky AP, Keri G, et al. Signaling through RAS-RAF-MEK ERK: from basics to bedside. Curr Med Chem. 2007;14:601-623.
- Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26:1657-1667.
- Malliri A, Collard JG. Role of Rho-family proteins in cell adhesion and cancer. Curr Opin Cell Biol. 2003;15:583-589.
- Kumar S, Kumar R, Medhi B, et al. Novel strategies for effective actinic keratosis treatment: a review. Curr Cancer Ther Rev. 2015;11:119-1132.
To the Editor:
Afatinib is a small molecule covalently binding and inhibiting the epidermal growth factor receptor (EGFR) as well as HER2 and HER4 receptor tyrosine kinases.1 The EGFR family is part of a complex signal transduction network that is central to several critical cellular processes.2 The human EGFR family is dysregulated in many solid tumors, making it an attractive target for anticancer therapy.2 In 2013, the US Food and Drug Administration approved afatinib as a first-line treatment of patients with metastatic non–small cell lung cancer whose tumors have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations.3
Treatment with afatinib and other EGFR inhibitors is frequently associated with cutaneous adverse effects that occur in up to 90% of patients. These cutaneous reactions are typical for this drug family and distinct from the skin adverse effects related to other types of anticancer chemotherapy.4 The most frequent skin manifestations following afatinib treatment consist of an acneform pustular eruption in up to 90% of patients.5,6 Other dermatologic reactions include nonspecific maculopapular rashes (90%), stomatitis (71%), paronychia with some nail changes (58%), xerosis (31%), pruritus (21%), and hand-foot syndrome (7%)5,6; however, grade 3 dermatologic reactions occurred in only 0.15% of patients.
Inflammatory changes in both preexisting and undetected actinic keratoses (AKs) and even progression to squamous cell carcinoma (SCC) have been previously described as uncommon dermatologic adverse effects of 2 EGFR inhibitors, sorafenib and erlotinib.7-9 Seven of 131 patients with metastatic renal cell carcinoma treated with single-agent sorafenib developed cutaneous SCC and 3 more had AKs.9 One patient demonstrated self-limited inflammatory flare-up of AKs during erlotinib treatment.8 We report acute inflammation of AKs from afatinib treatment.
A 78-year-old woman with fair skin who was previously treated for several AKs in sun-exposed areas presented with inflammatory changes that appeared at the site of AKs on photoexposed areas 110 days after initiating afatinib therapy (40 mg/d). Physical examination revealed multiple erythematous scaly plaques on the face, neck, chest, and forearms (Figure 1).

In the previous 2 decades, lesions that were surgically removed and histopathologically examined included Bowen disease (2 lesions), 2 basal cell carcinomas, 2 blue nevi, and a seborrheic keratosis. Several AKs also were surgically removed and confirmed histopathologically.
Eighteen months prior to the current presentation, the patient was diagnosed with locally advanced, inoperable, stage IIIA adenocarcinoma of the lung with deletion in exon 19 of the EGFR gene. She received definitive concomitant chemoradiation with the carboplatin-vinorelbine regimen and 60-Gy radiation. Four months later, a positron emission tomography (PET)–fludeoxyglucose scan revealed a single bone lesion in the L5 vertebra leading to irradiation to the lumbar spine. Subsequently, new metastases to the neck, right lung, T5 vertebra, and left acetabulum were detected by PET–computed tomography. One year later, afatinib 40 mg/d was initiated. A PET scan after 2 months of treatment showed excellent response.
At the current presentation, a punch biopsy obtained from an inflammatory lesion on the left dorsal forearm revealed findings consistent with an eroded and inflamed AK; the biopsy showed marked dysplasia of the keratinocytes that was predominately located in the basal layer of the epidermis. The lesion was accompanied by a dense mixed inflammatory cell infiltrate that was centered in the papillary dermis and extended to the epidermis (Figure 2). Because of this grade 3 skin toxicity, the afatinib dosage was reduced to 20 mg/d, and betamethasone cream 0.1% and emollients were applied locally for 2 weeks. A reduction in the number of AKs and clinical regression of the inflammatory changes was observed 2 weeks later (Figure 3).


Chronically sun-exposed skin is prone to develop AKs that are at risk to progress to SCC.10-12 These lesions are increasingly diagnosed in older patients when internal cancers also are prevalent.13 Inflammatory flare-up of AKs is typically present during the regression phase14,15 but also during progression to SCC.16
There are many strategies for treating AKs. Physical procedures for destroying the lesions are commonly used. Some topical drugs, including imiquimod, 5-fluorouracil, and diclofenac sodium, also have proven efficacy.17
Conventional chemotherapeutic agents that have been described to be associated with the inflammation of AKs include docetaxel; doxorubicin; capecitabine; pentostatin; and the combination of dactinomycin, vincristine, dacarbazine and doxorubicin, cytarabine, and 6-thioguanine.7,18 The mechanism leading to this effect is unknown, though abnormal DNA synthesis and a type of radiation recall phenomenon have been postulated.7
We described inflammatory changes in AKs associated with afatinib treatment. The precise mechanism by which afatinib induces inflammation in AK has not been elucidated; however, it is known that EGFR normally downregulates chemokine expression in keratinocytes. Conversely, EGFR signaling blockade produces opposite effects, with increased CCL2, CCL5, and CXCL10, as well as reduced CXCL8 expression, leading to enhanced skin inflammation.19 Afatinib is a targeted agent that modulates the Ras/Raf/MEK/ERK signaling circuit, which is a key intracellular signal transduction pathway.20 This pathway and its downstream effectors have been implicated in cutaneous squamous cell carcinogenesis that might be accompanied by inflammatory changes.21,22 The remarkable clinical improvement of the AKs in our patient following the inflammatory flare-up supports the notion that the anticancer effect on intraepidermal neoplasms might be mediated by inflammation.23
To the Editor:
Afatinib is a small molecule covalently binding and inhibiting the epidermal growth factor receptor (EGFR) as well as HER2 and HER4 receptor tyrosine kinases.1 The EGFR family is part of a complex signal transduction network that is central to several critical cellular processes.2 The human EGFR family is dysregulated in many solid tumors, making it an attractive target for anticancer therapy.2 In 2013, the US Food and Drug Administration approved afatinib as a first-line treatment of patients with metastatic non–small cell lung cancer whose tumors have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations.3
Treatment with afatinib and other EGFR inhibitors is frequently associated with cutaneous adverse effects that occur in up to 90% of patients. These cutaneous reactions are typical for this drug family and distinct from the skin adverse effects related to other types of anticancer chemotherapy.4 The most frequent skin manifestations following afatinib treatment consist of an acneform pustular eruption in up to 90% of patients.5,6 Other dermatologic reactions include nonspecific maculopapular rashes (90%), stomatitis (71%), paronychia with some nail changes (58%), xerosis (31%), pruritus (21%), and hand-foot syndrome (7%)5,6; however, grade 3 dermatologic reactions occurred in only 0.15% of patients.
Inflammatory changes in both preexisting and undetected actinic keratoses (AKs) and even progression to squamous cell carcinoma (SCC) have been previously described as uncommon dermatologic adverse effects of 2 EGFR inhibitors, sorafenib and erlotinib.7-9 Seven of 131 patients with metastatic renal cell carcinoma treated with single-agent sorafenib developed cutaneous SCC and 3 more had AKs.9 One patient demonstrated self-limited inflammatory flare-up of AKs during erlotinib treatment.8 We report acute inflammation of AKs from afatinib treatment.
A 78-year-old woman with fair skin who was previously treated for several AKs in sun-exposed areas presented with inflammatory changes that appeared at the site of AKs on photoexposed areas 110 days after initiating afatinib therapy (40 mg/d). Physical examination revealed multiple erythematous scaly plaques on the face, neck, chest, and forearms (Figure 1).

In the previous 2 decades, lesions that were surgically removed and histopathologically examined included Bowen disease (2 lesions), 2 basal cell carcinomas, 2 blue nevi, and a seborrheic keratosis. Several AKs also were surgically removed and confirmed histopathologically.
Eighteen months prior to the current presentation, the patient was diagnosed with locally advanced, inoperable, stage IIIA adenocarcinoma of the lung with deletion in exon 19 of the EGFR gene. She received definitive concomitant chemoradiation with the carboplatin-vinorelbine regimen and 60-Gy radiation. Four months later, a positron emission tomography (PET)–fludeoxyglucose scan revealed a single bone lesion in the L5 vertebra leading to irradiation to the lumbar spine. Subsequently, new metastases to the neck, right lung, T5 vertebra, and left acetabulum were detected by PET–computed tomography. One year later, afatinib 40 mg/d was initiated. A PET scan after 2 months of treatment showed excellent response.
At the current presentation, a punch biopsy obtained from an inflammatory lesion on the left dorsal forearm revealed findings consistent with an eroded and inflamed AK; the biopsy showed marked dysplasia of the keratinocytes that was predominately located in the basal layer of the epidermis. The lesion was accompanied by a dense mixed inflammatory cell infiltrate that was centered in the papillary dermis and extended to the epidermis (Figure 2). Because of this grade 3 skin toxicity, the afatinib dosage was reduced to 20 mg/d, and betamethasone cream 0.1% and emollients were applied locally for 2 weeks. A reduction in the number of AKs and clinical regression of the inflammatory changes was observed 2 weeks later (Figure 3).


Chronically sun-exposed skin is prone to develop AKs that are at risk to progress to SCC.10-12 These lesions are increasingly diagnosed in older patients when internal cancers also are prevalent.13 Inflammatory flare-up of AKs is typically present during the regression phase14,15 but also during progression to SCC.16
There are many strategies for treating AKs. Physical procedures for destroying the lesions are commonly used. Some topical drugs, including imiquimod, 5-fluorouracil, and diclofenac sodium, also have proven efficacy.17
Conventional chemotherapeutic agents that have been described to be associated with the inflammation of AKs include docetaxel; doxorubicin; capecitabine; pentostatin; and the combination of dactinomycin, vincristine, dacarbazine and doxorubicin, cytarabine, and 6-thioguanine.7,18 The mechanism leading to this effect is unknown, though abnormal DNA synthesis and a type of radiation recall phenomenon have been postulated.7
We described inflammatory changes in AKs associated with afatinib treatment. The precise mechanism by which afatinib induces inflammation in AK has not been elucidated; however, it is known that EGFR normally downregulates chemokine expression in keratinocytes. Conversely, EGFR signaling blockade produces opposite effects, with increased CCL2, CCL5, and CXCL10, as well as reduced CXCL8 expression, leading to enhanced skin inflammation.19 Afatinib is a targeted agent that modulates the Ras/Raf/MEK/ERK signaling circuit, which is a key intracellular signal transduction pathway.20 This pathway and its downstream effectors have been implicated in cutaneous squamous cell carcinogenesis that might be accompanied by inflammatory changes.21,22 The remarkable clinical improvement of the AKs in our patient following the inflammatory flare-up supports the notion that the anticancer effect on intraepidermal neoplasms might be mediated by inflammation.23
- Katakami N, Atagi S, Goto K, et al. LUX-lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 2013;31:3335-3342.
- Liao BC, Lin CC, Yang JCH. First-line management of EGFR-mutated advanced lung adenocarcinoma: recent developments. Drugs. 2013;73:357-369.
- Jain P, Khanal R, Sharma A, et al. Afatinib and lung cancer. Expert Rev Anticancer Ther. 2014;14:1391-1406.
- Wyatt AJ, Leonard GD, Sachs DL. Cutaneous reactions to chemotherapy and their management. Am J Clin Dermatol. 2006;7:45-63.
- Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16:1425-1433.
- Agero ALC, Dusza SW, Benvenuto-Andrade C, et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657-670.
- Lacouture ME, Desai A, Soltani K, et al. Inflammation of actinic keratoses subsequent to therapy with sorafenib, a multitargeted tyrosine-kinase inhibitor. Clin Exp Dermatol. 2006;31:783-785.
- Hermanns JF, Piérard GE, Quatresooz P. Erlotinib-responsive actinic keratoses. Oncol Rep. 2007;18:581-584.
- Dubauskas Z, Kunishige J, Prieto VG, et al. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23.
- Czarnecki D, Meehan CJ, Bruce F, et al. The majority of cutaneous squamous cell carcinomas arise in actinic keratoses. J Cutan Med Surg. 2002;6:207-209.
- Ehrig T, Cockerell C, Piacquadio D, et al. Actinic keratoses and the incidence of occult squamous cell carcinoma: a clinical-histopathologic correlation. Dermatolog Surg. 2006;32:1261-1265.
- Quaedvlieg PJF, Tirsi E, Thissen MRTM, et al. Actinic keratosis: how to differentiate the good from the bad ones? Eur J Dermatol. 2006;16:335-339.
- Atkins D, Bang RH, Sternberg MR, et al. Reliable methods to evaluate the burden of actinic keratoses. J Invest Dermatol. 2006;126:591-594.
- Ooi T, Barnetson RS, Zhuang L, et al. Imiquimod-induced regression of actinic keratosis is associated with infiltration by T lymphocytes and dendritic cells: a randomized controlled trial. Br J Dermatol. 2006;154:72-78.
- Quatresooz P, Piérard GE. Imiquimod-responsive basal cell carcinomas and factor XIIIa enriched dendrocytes. Clin Exp Dermatol. 2003;28(suppl 1):27-29.
- Berhane T, Halliday GM, Cooke B, et al. Inflammation is associated with progression of actinic keratoses to squamous cell carcinomas in humans. Br J Dermatol. 2002;146:810-815.
- Ceilley RI, Jorizzo JL. Current issues in the management of actinic keratosis. J Am Acad Dermatol. 2013;68(1 suppl 1):S28-S38.
- Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol. 1999;40:367-398.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- Zebisch A, Czernilofsky AP, Keri G, et al. Signaling through RAS-RAF-MEK ERK: from basics to bedside. Curr Med Chem. 2007;14:601-623.
- Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26:1657-1667.
- Malliri A, Collard JG. Role of Rho-family proteins in cell adhesion and cancer. Curr Opin Cell Biol. 2003;15:583-589.
- Kumar S, Kumar R, Medhi B, et al. Novel strategies for effective actinic keratosis treatment: a review. Curr Cancer Ther Rev. 2015;11:119-1132.
- Katakami N, Atagi S, Goto K, et al. LUX-lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 2013;31:3335-3342.
- Liao BC, Lin CC, Yang JCH. First-line management of EGFR-mutated advanced lung adenocarcinoma: recent developments. Drugs. 2013;73:357-369.
- Jain P, Khanal R, Sharma A, et al. Afatinib and lung cancer. Expert Rev Anticancer Ther. 2014;14:1391-1406.
- Wyatt AJ, Leonard GD, Sachs DL. Cutaneous reactions to chemotherapy and their management. Am J Clin Dermatol. 2006;7:45-63.
- Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16:1425-1433.
- Agero ALC, Dusza SW, Benvenuto-Andrade C, et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657-670.
- Lacouture ME, Desai A, Soltani K, et al. Inflammation of actinic keratoses subsequent to therapy with sorafenib, a multitargeted tyrosine-kinase inhibitor. Clin Exp Dermatol. 2006;31:783-785.
- Hermanns JF, Piérard GE, Quatresooz P. Erlotinib-responsive actinic keratoses. Oncol Rep. 2007;18:581-584.
- Dubauskas Z, Kunishige J, Prieto VG, et al. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23.
- Czarnecki D, Meehan CJ, Bruce F, et al. The majority of cutaneous squamous cell carcinomas arise in actinic keratoses. J Cutan Med Surg. 2002;6:207-209.
- Ehrig T, Cockerell C, Piacquadio D, et al. Actinic keratoses and the incidence of occult squamous cell carcinoma: a clinical-histopathologic correlation. Dermatolog Surg. 2006;32:1261-1265.
- Quaedvlieg PJF, Tirsi E, Thissen MRTM, et al. Actinic keratosis: how to differentiate the good from the bad ones? Eur J Dermatol. 2006;16:335-339.
- Atkins D, Bang RH, Sternberg MR, et al. Reliable methods to evaluate the burden of actinic keratoses. J Invest Dermatol. 2006;126:591-594.
- Ooi T, Barnetson RS, Zhuang L, et al. Imiquimod-induced regression of actinic keratosis is associated with infiltration by T lymphocytes and dendritic cells: a randomized controlled trial. Br J Dermatol. 2006;154:72-78.
- Quatresooz P, Piérard GE. Imiquimod-responsive basal cell carcinomas and factor XIIIa enriched dendrocytes. Clin Exp Dermatol. 2003;28(suppl 1):27-29.
- Berhane T, Halliday GM, Cooke B, et al. Inflammation is associated with progression of actinic keratoses to squamous cell carcinomas in humans. Br J Dermatol. 2002;146:810-815.
- Ceilley RI, Jorizzo JL. Current issues in the management of actinic keratosis. J Am Acad Dermatol. 2013;68(1 suppl 1):S28-S38.
- Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol. 1999;40:367-398.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- Zebisch A, Czernilofsky AP, Keri G, et al. Signaling through RAS-RAF-MEK ERK: from basics to bedside. Curr Med Chem. 2007;14:601-623.
- Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26:1657-1667.
- Malliri A, Collard JG. Role of Rho-family proteins in cell adhesion and cancer. Curr Opin Cell Biol. 2003;15:583-589.
- Kumar S, Kumar R, Medhi B, et al. Novel strategies for effective actinic keratosis treatment: a review. Curr Cancer Ther Rev. 2015;11:119-1132.
Practice Points
- One of the underreported adverse events of afatinibis is the induction of inflammatory changes in actinic keratoses (AKs).
- Our cases showed that inflammatory changes eventually led to shrinkage and resolution of the underlying AK.