User login
Is vaping a gateway to cigarettes for kids?
Vaping may not be a gateway to long-term cigarette use for adolescents, a new study published in JAMA Network Open suggests.
Many studies have found that youth who vape are more likely to take up cigarette smoking, but whether that new habit lasts for a month or a lifetime has been unclear.
The percentage of adolescents who move on to smoking after starting to vape remains low, and those who do start smoking are unlikely to continue doing so for a long time, the new research shows.
“If they simply experiment with smoking but do not continue, their risks of smoking-related adverse health outcomes are low,” said Ruoyan Sun, PhD, assistant professor with the department of health policy and organization at the University of Alabama at Birmingham and the study’s lead author. “But if they do become regular or established smokers, then the risks can be substantial.”
Dr. Sun and her colleagues analyzed data from several waves of the longitudinal Population Assessment of Tobacco and Health study. Participants included 8,671 children and adolescents aged 12-17 years. Among teens who had ever vaped, 6% began smoking cigarettes and continued to smoke in the subsequent 3 years, the researchers found (95% confidence interval, 4.5%-8.0%), compared with 1.1% among teens who never vaped (95% CI, 0.8%-1.3%).
“The real concern is whether vaping is inducing significant numbers of young people to become confirmed smokers,” said Dr. Sun. “The answer is that it does not.”
Previous studies using PATH data have suggested that adolescents who use e-cigarettes are up to 3.5 times more likely than nonusers to start smoking tobacco cigarettes and that they may continue to use both products.
But in the new study, despite the low overall number of cigarette smokers, those in the group who used e-cigarettes were 81% more likely to continue smoking tobacco cigarettes after 3 years, compared with those who did not use e-cigarettes, researchers found (95% CI, 1.03-3.18).
Rachel Boykan, MD, clinical professor of pediatrics and attending physician at Stony Brook (N.Y.) Children’s Hospital, said that despite the findings, the overall messaging to patients remains the same: Vaping is linked to smoking.
“There is still a risk of initiation smoking among e-cigarette users – that is the take-home message,” Dr. Boykan, who was not affiliated with the study, said. “No risk of smoking initiation is acceptable. And of course, as we are learning, there are significant health risks with e-cigarette use alone.”
Among the entire group of teens, approximately 4% of the adolescents began smoking cigarettes; only 2.5% continued to smoke in the subsequent 3 years, the researchers found.
“Based on our odds ratio result, e-cigarette users are more likely to report continued cigarette smoking,” said Dr. Sun. “However, the risk differences were not significant.”
The low numbers of teens who continued to smoke also suggests that adolescents are more likely to quit than become long-term smokers.
Nicotine dependence may adversely affect the ability of adolescents to learn, remember, and maintain attention. Early research has suggested that long-term e-cigarette smokers may be at increased risk of developing some of the same conditions as tobacco smokers, such as chronic lung disease.
Brian Jenssen, MD, a pediatrician at Children’s Hospital of Philadelphia and assistant professor in the Perelman School of Medicine at the University of Pennsylvania, Philadelphia, said that the analysis is limited in part because it does not include changes in smoking and vaping trends since the pandemic started, “which seems to have increased the risk of smoking and vaping use.”
Data from the 2022 National Youth Tobacco survey found that although the rate of middle school and high school students who begin to use e-cigarettes has steadily decreased during the past two decades, those who vape report using the devices more frequently.
Subsequent use of cigarettes is also only one measure of risk from vapes.
“The goal isn’t just about cigarettes,” said Dr. Jenssen, who was not affiliated with the new study. “The goal is about helping children live tobacco- and nicotine-free lives, and there seems to be an increasing intensity of use, which is causing its own health risks.”
The current study findings do not change how clinicians should counsel their patients, and they should continue to advise teens to abstain from vaping, he added.
Dr. Sun said it’s common for youth to experiment with multiple tobacco products.
“Clinicians should continue to monitor youth tobacco-use behaviors but with their concern being focused on youthful patients who sustain smoking instead of just trying cigarettes,” she said.
Some of the study authors received support from the National Cancer Institute of the National Institutes of Health and the U.S. Food and Drug Administration’s Center for Tobacco Products.
A version of this article first appeared on Medscape.com.
Vaping may not be a gateway to long-term cigarette use for adolescents, a new study published in JAMA Network Open suggests.
Many studies have found that youth who vape are more likely to take up cigarette smoking, but whether that new habit lasts for a month or a lifetime has been unclear.
The percentage of adolescents who move on to smoking after starting to vape remains low, and those who do start smoking are unlikely to continue doing so for a long time, the new research shows.
“If they simply experiment with smoking but do not continue, their risks of smoking-related adverse health outcomes are low,” said Ruoyan Sun, PhD, assistant professor with the department of health policy and organization at the University of Alabama at Birmingham and the study’s lead author. “But if they do become regular or established smokers, then the risks can be substantial.”
Dr. Sun and her colleagues analyzed data from several waves of the longitudinal Population Assessment of Tobacco and Health study. Participants included 8,671 children and adolescents aged 12-17 years. Among teens who had ever vaped, 6% began smoking cigarettes and continued to smoke in the subsequent 3 years, the researchers found (95% confidence interval, 4.5%-8.0%), compared with 1.1% among teens who never vaped (95% CI, 0.8%-1.3%).
“The real concern is whether vaping is inducing significant numbers of young people to become confirmed smokers,” said Dr. Sun. “The answer is that it does not.”
Previous studies using PATH data have suggested that adolescents who use e-cigarettes are up to 3.5 times more likely than nonusers to start smoking tobacco cigarettes and that they may continue to use both products.
But in the new study, despite the low overall number of cigarette smokers, those in the group who used e-cigarettes were 81% more likely to continue smoking tobacco cigarettes after 3 years, compared with those who did not use e-cigarettes, researchers found (95% CI, 1.03-3.18).
Rachel Boykan, MD, clinical professor of pediatrics and attending physician at Stony Brook (N.Y.) Children’s Hospital, said that despite the findings, the overall messaging to patients remains the same: Vaping is linked to smoking.
“There is still a risk of initiation smoking among e-cigarette users – that is the take-home message,” Dr. Boykan, who was not affiliated with the study, said. “No risk of smoking initiation is acceptable. And of course, as we are learning, there are significant health risks with e-cigarette use alone.”
Among the entire group of teens, approximately 4% of the adolescents began smoking cigarettes; only 2.5% continued to smoke in the subsequent 3 years, the researchers found.
“Based on our odds ratio result, e-cigarette users are more likely to report continued cigarette smoking,” said Dr. Sun. “However, the risk differences were not significant.”
The low numbers of teens who continued to smoke also suggests that adolescents are more likely to quit than become long-term smokers.
Nicotine dependence may adversely affect the ability of adolescents to learn, remember, and maintain attention. Early research has suggested that long-term e-cigarette smokers may be at increased risk of developing some of the same conditions as tobacco smokers, such as chronic lung disease.
Brian Jenssen, MD, a pediatrician at Children’s Hospital of Philadelphia and assistant professor in the Perelman School of Medicine at the University of Pennsylvania, Philadelphia, said that the analysis is limited in part because it does not include changes in smoking and vaping trends since the pandemic started, “which seems to have increased the risk of smoking and vaping use.”
Data from the 2022 National Youth Tobacco survey found that although the rate of middle school and high school students who begin to use e-cigarettes has steadily decreased during the past two decades, those who vape report using the devices more frequently.
Subsequent use of cigarettes is also only one measure of risk from vapes.
“The goal isn’t just about cigarettes,” said Dr. Jenssen, who was not affiliated with the new study. “The goal is about helping children live tobacco- and nicotine-free lives, and there seems to be an increasing intensity of use, which is causing its own health risks.”
The current study findings do not change how clinicians should counsel their patients, and they should continue to advise teens to abstain from vaping, he added.
Dr. Sun said it’s common for youth to experiment with multiple tobacco products.
“Clinicians should continue to monitor youth tobacco-use behaviors but with their concern being focused on youthful patients who sustain smoking instead of just trying cigarettes,” she said.
Some of the study authors received support from the National Cancer Institute of the National Institutes of Health and the U.S. Food and Drug Administration’s Center for Tobacco Products.
A version of this article first appeared on Medscape.com.
Vaping may not be a gateway to long-term cigarette use for adolescents, a new study published in JAMA Network Open suggests.
Many studies have found that youth who vape are more likely to take up cigarette smoking, but whether that new habit lasts for a month or a lifetime has been unclear.
The percentage of adolescents who move on to smoking after starting to vape remains low, and those who do start smoking are unlikely to continue doing so for a long time, the new research shows.
“If they simply experiment with smoking but do not continue, their risks of smoking-related adverse health outcomes are low,” said Ruoyan Sun, PhD, assistant professor with the department of health policy and organization at the University of Alabama at Birmingham and the study’s lead author. “But if they do become regular or established smokers, then the risks can be substantial.”
Dr. Sun and her colleagues analyzed data from several waves of the longitudinal Population Assessment of Tobacco and Health study. Participants included 8,671 children and adolescents aged 12-17 years. Among teens who had ever vaped, 6% began smoking cigarettes and continued to smoke in the subsequent 3 years, the researchers found (95% confidence interval, 4.5%-8.0%), compared with 1.1% among teens who never vaped (95% CI, 0.8%-1.3%).
“The real concern is whether vaping is inducing significant numbers of young people to become confirmed smokers,” said Dr. Sun. “The answer is that it does not.”
Previous studies using PATH data have suggested that adolescents who use e-cigarettes are up to 3.5 times more likely than nonusers to start smoking tobacco cigarettes and that they may continue to use both products.
But in the new study, despite the low overall number of cigarette smokers, those in the group who used e-cigarettes were 81% more likely to continue smoking tobacco cigarettes after 3 years, compared with those who did not use e-cigarettes, researchers found (95% CI, 1.03-3.18).
Rachel Boykan, MD, clinical professor of pediatrics and attending physician at Stony Brook (N.Y.) Children’s Hospital, said that despite the findings, the overall messaging to patients remains the same: Vaping is linked to smoking.
“There is still a risk of initiation smoking among e-cigarette users – that is the take-home message,” Dr. Boykan, who was not affiliated with the study, said. “No risk of smoking initiation is acceptable. And of course, as we are learning, there are significant health risks with e-cigarette use alone.”
Among the entire group of teens, approximately 4% of the adolescents began smoking cigarettes; only 2.5% continued to smoke in the subsequent 3 years, the researchers found.
“Based on our odds ratio result, e-cigarette users are more likely to report continued cigarette smoking,” said Dr. Sun. “However, the risk differences were not significant.”
The low numbers of teens who continued to smoke also suggests that adolescents are more likely to quit than become long-term smokers.
Nicotine dependence may adversely affect the ability of adolescents to learn, remember, and maintain attention. Early research has suggested that long-term e-cigarette smokers may be at increased risk of developing some of the same conditions as tobacco smokers, such as chronic lung disease.
Brian Jenssen, MD, a pediatrician at Children’s Hospital of Philadelphia and assistant professor in the Perelman School of Medicine at the University of Pennsylvania, Philadelphia, said that the analysis is limited in part because it does not include changes in smoking and vaping trends since the pandemic started, “which seems to have increased the risk of smoking and vaping use.”
Data from the 2022 National Youth Tobacco survey found that although the rate of middle school and high school students who begin to use e-cigarettes has steadily decreased during the past two decades, those who vape report using the devices more frequently.
Subsequent use of cigarettes is also only one measure of risk from vapes.
“The goal isn’t just about cigarettes,” said Dr. Jenssen, who was not affiliated with the new study. “The goal is about helping children live tobacco- and nicotine-free lives, and there seems to be an increasing intensity of use, which is causing its own health risks.”
The current study findings do not change how clinicians should counsel their patients, and they should continue to advise teens to abstain from vaping, he added.
Dr. Sun said it’s common for youth to experiment with multiple tobacco products.
“Clinicians should continue to monitor youth tobacco-use behaviors but with their concern being focused on youthful patients who sustain smoking instead of just trying cigarettes,” she said.
Some of the study authors received support from the National Cancer Institute of the National Institutes of Health and the U.S. Food and Drug Administration’s Center for Tobacco Products.
A version of this article first appeared on Medscape.com.
When a patient with chronic alcohol use abruptly stops drinking
CASE A difficult withdrawal
Three days after he stops drinking alcohol, Mr. G, age 49, presents to a detoxification center with his wife, who drove him there because she was concerned about his condition. She says her husband had been drinking alcohol every night for as long as she can remember. Despite numerous admissions to rehabilitation centers, Mr. G usually would resume drinking soon after he was discharged. Three days ago, Mr. G’s wife had told him she “could not take it anymore,” so he got rid of all his alcohol and stopped drinking. Mr. G’s wife felt he was doing fine the first day, but his condition increasingly worsened the second and third days. The triage nurse who attempts to interview Mr. G finds him tremulous, vomiting, and sweating. She notices that he seems preoccupied with pulling at his shirt, appearing to pick at things that are not there.
HISTORY Untreated depression, other comorbidities
Mr. G’s wife says he has never been psychiatrically hospitalized or exhibited suicidal behavior. Mr. G previously received care from a psychiatrist, who diagnosed him with major depressive disorder (MDD) and prescribed an antidepressant, though his wife cannot recall which specific medication. She shares it has been “a long time” since Mr. G has taken the antidepressant and the last time he received treatment for his MDD was 5 years ago. Mr. G’s wife says her husband had once abstained from alcohol use for >6 months following one of his stints at a rehabilitation center. She is not able to share many other details about Mr. G’s previous stays at rehabilitation centers, but says he always had “a rough time.”
She says Mr. G had been drinking an average of 10 drinks each night, usually within 4 hours. He has no history of nicotine or illicit substance use and has held a corporate job for the last 18 years. Several years ago, a physician had diagnosed Mr. G with hypertension and high cholesterol, but he did not follow up for treatment. Mr. G’s wife also recalls a physician told her husband he had a fatty liver. His family history includes heart disease and cancer.
[polldaddy:12041618]
The author’s observations
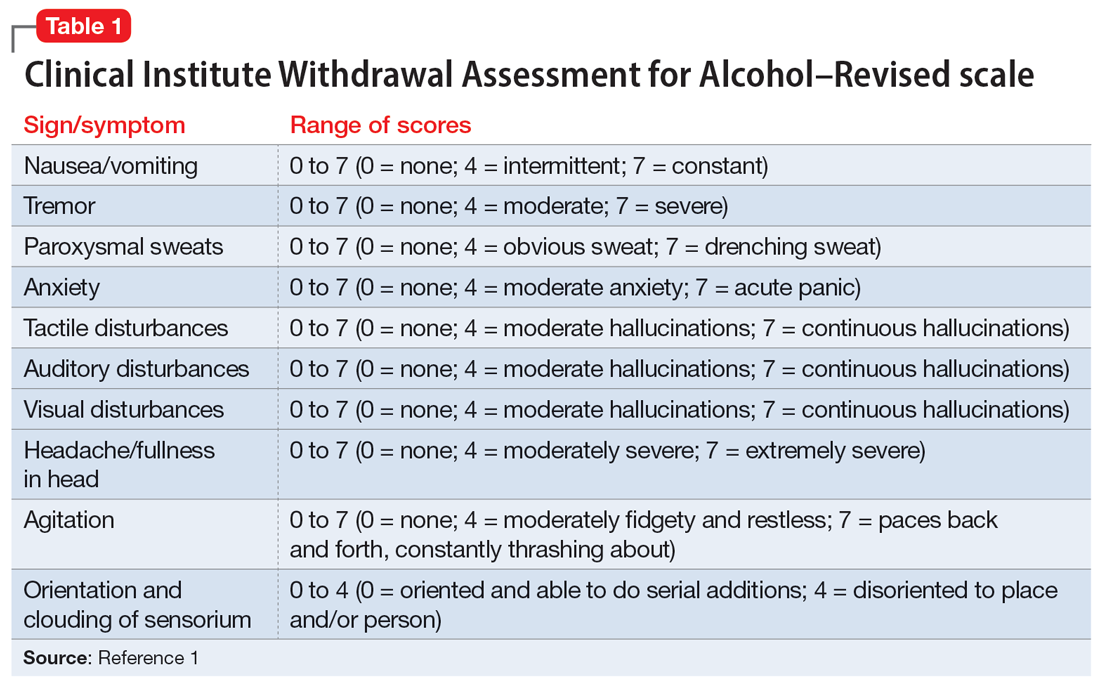
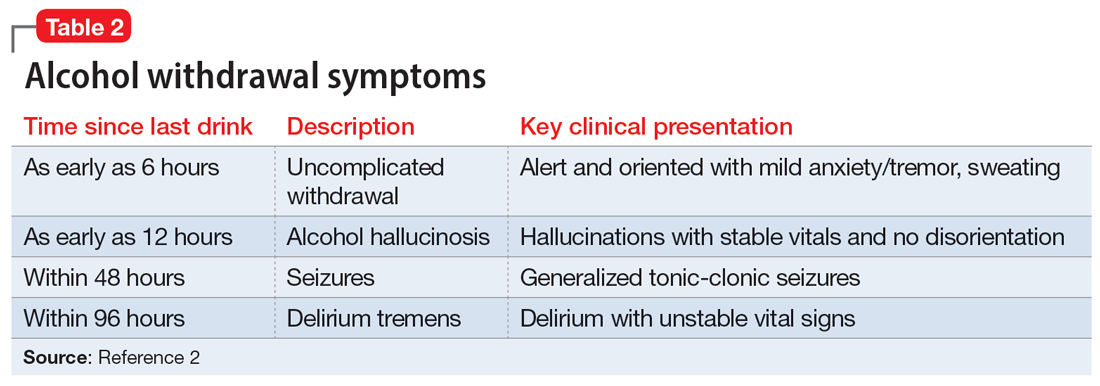
The treatment team observed several elements of alcohol withdrawal and classified Mr. G as a priority patient. If the team had completed the Clinical Institute Withdrawal Assessment for Alcohol–Revised scale (CIWA-Ar) (Table 11), Mr. G would score ≥10. While the protocol for initiating treatment for patients experiencing alcohol withdrawal varies by institution, patients with moderate to severe scores on the CIWA-Ar when experiencing withdrawal typically are managed with pharmacotherapy to address their symptoms.1 Given the timeline of his last drink as reported by his wife, Mr. G is on the brink of experiencing a cascade of symptoms concerning for delirium tremens (DTs).2 Table 22 provides a timeline and symptoms related to alcohol withdrawal. To prevent further exacerbation of symptoms, which could lead to DTs, Mr. G’s treatment team will likely initiate a benzodiazepine, using either scheduled or symptom-driven dosing.3
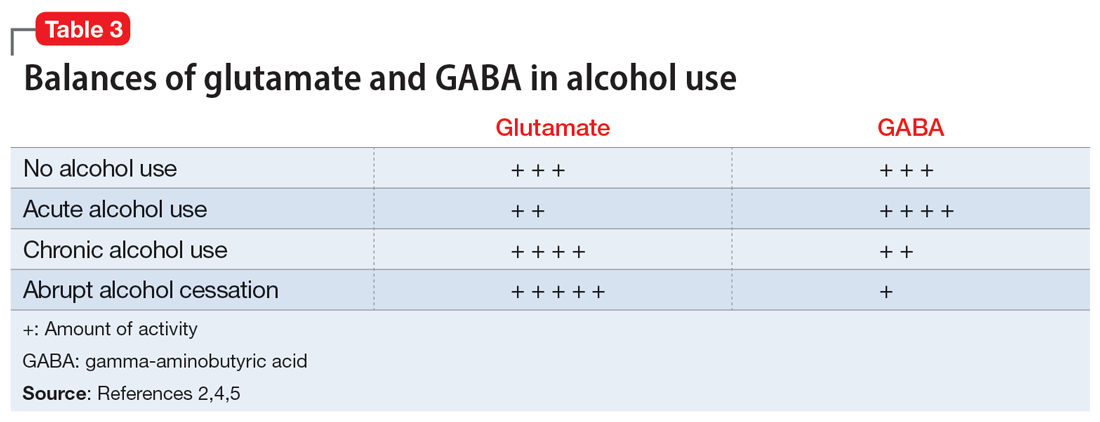
Two neurotransmitters that play a role in DTs are glutamate (excitatory) and GABA (inhibitory). In a normal state, the competing actions of these neurotransmitters balance each other. Acute alcohol intake causes a shift in the excitatory and inhibitory levels, with more inhibition taking place, thus causing disequilibrium. If chronic alcohol use continues, the amount of GABA inhibition reduction is related to downregulation of receptors.2,4 Excitation increases by way of upregulation of the N-methyl-
If alcohol is suddenly removed following chronic use, there is unchecked glutamate excitation related to a blunted GABA state. This added increase in the excitation of glutamate leads to withdrawal symptoms.2,4 Table 32,4,5 depicts the neurotransmitter equilibrium of GABA and glutamate relative to alcohol use.
EVALUATION Bleeding gums and bruising
The treatment team admits Mr. G to the triage bay and contacts the addiction psychiatrist. The physician orders laboratory tests to assess nutritional deficits and electrolyte abnormalities. Mr. G is also placed on routine assessments with symptom-triggered therapy. An assessment reveals bleeding gums and bruises, which are believed to be a result of thrombocytopenia (low blood platelet count).
[polldaddy:12041627]
Continue to: The author's observations
The author’s observations
Though regular clinical assessment of PEth varies, it is considered to have high sensitivity and specificity to detect alcohol use.6 When ethanol is present, the phospholipase D enzyme acts upon phosphatidylcholine, forming a direct biomarker, PEth, on the surface of the red blood cell.6,7 PEth’s half-life ranges from 4.5 to 12 days,6 and it can be detected in blood for 3 to 4 weeks after alcohol ingestion.6,7 A PEth value <20 ng/mL indicates light or no alcohol consumption; 20 to 199 ng/mL indicates significant consumption; and >200 ng/mL indicates heavy consumption.7 Since Mr. G has a history of chronic alcohol use, his PEth level is expected to be >200 ng/mL.
AST/ALT and MCV are indirect biomarkers, meaning the tests are not alcohol-specific and the role of alcohol is instead observed by the damage to the body with excessive use over time.7 The expected AST:ALT ratio is 2:1. This is related to 3 mechanisms. The first is a decrease in ALT usually relative to B6 deficiency in individuals with alcohol use disorder (AUD). Another mechanism is related to alcohol’s propensity to affect mitochondria, which is a source for AST. Additionally, AST is also found in higher proportions in the kidneys, heart, and muscles.8
An MCV <100 fL would be within the normal range (80 to 100 fL) for red blood cells. While the reasons for an enlarged red blood cell (or macrocyte) are extensive, alcohol can be a factor once other causes are excluded. Additional laboratory tests and a peripheral blood smear test can help in this investigation. Alcohol disrupts the complete maturation of red blood cells.9,10 If the cause of the macrocyte is alcohol-related and alcohol use is terminated, those enlarged cells can resolve in an average of 3 months.9
Vitamin B1 levels >200 nmol/L would be within normal range (74 to 222 nmol/L). Mr. G’s chronic alcohol use would likely cause him to be vitamin B1–deficient. The deficiency is usually related to diet, malabsorption, and the cells’ impaired ability to utilize vitamin B1. A consequence of vitamin B1 deficiency is Wernicke-Korsakoff syndrome.11
Due to his chronic alcohol use, Mr. G’s magnesium stores most likely would be below normal range (1.7 to 2.2 mg/dL). Acting as a diuretic, alcohol depletes magnesium and other electrolytes. The intracellular shift that occurs to balance the deficit causes the body to use its normal stores of magnesium, which leads to further magnesium depletion. Other common causes include nutritional deficiency and decreased gastrointestinal absorption.12 The bleeding the physician suspected was a result of drinking likely occurred through direct and indirect mechanisms that affect platelets.9,13 Platelets can show improvement 1 week after drinking cessation. Some evidence suggests the risk of seizure or DTs increases significantly with a platelet count <119,000 µL per unit of blood.13
Continue to: TREATMENT Pharmacotherapy for alcohol use disorder
TREATMENT Pharmacotherapy for alcohol use disorder
As Mr. G’s condition starts to stabilize, he discusses treatment options for AUD with his physician. At the end of the discussion, Mr. G expresses an interest in starting a medication. The doctor reviews his laboratory results and available treatment options.
[polldaddy:12041630]
The author’s observations
Of the 3 FDA-approved medications for treating AUD (disulfiram, acamprosate, and naltrexone), naltrexone has been shown to decrease heavy drinking days5,14 and comes in oral and injectable forms. Reducing drinking is achieved by reducing the rewarding effects of alcohol5,14 and alcohol cravings.5 Disulfiram often has poor adherence, and like acamprosate it may be more helpful for maintenance of abstinence. Neither topiramate nor gabapentin are FDA-approved for AUD but may be used for their affects on GABA.5 Gabapentin may also help patients experiencing alcohol withdrawal syndrome.5,15 Mr. G did not have any concomitant medications or comorbid medical conditions, but these factors as well as any renal or hepatic dysfunction must be considered before initiating any medications.
OUTCOME Improved well-being
Mr. G’s treatment team initiates oral naltrexone 50 mg/d, which he tolerates well without complications. He stops drinking entirely and expresses an interest in transitioning to an injectable form of naltrexone in the future. In addition to taking medication, Mr. G wants to participate in psychotherapy. Mr. G thanks his team for the care he received in the hospital, telling them, “You all saved my life.” As he discusses his past issues with alcohol, Mr. G asks his physician how he could get involved to make changes to reduce excessive alcohol consumption in his community (Box5,15-21).
Box
Alcohol use disorder is undertreated5,15-17 and excessive alcohol use accounts for 1 in 5 deaths in individuals within Mr. G’s age range.18 An April 2011 report from the Community Preventive Services Task Force19 did not recommend privatization of retail alcohol sales as an intervention to reduce excessive alcohol consumption, because it would instead lead to an increase in alcohol consumption per capita, a known gateway to excessive alcohol consumption.20
The Task Force was established in 1996 by the US Department of Health and Human Services. Its objective is to identify scientifically proven interventions to save lives, increase lifespans, and improve quality of life. Recommendations are based on systematic reviews to inform lawmakers, health departments, and other organizations and agencies.21 The Task Force’s recommendations were divided into interventions that have strong evidence, sufficient evidence, or insufficient evidence. If Mr. G wanted to have the greatest impact in his efforts to reduce excessive alcohol consumption in his community, the strongest evidence supporting change focuses on electronic screening and brief intervention, maintaining limits on days of alcohol sale, increasing taxes on alcohol, and establishing dram shop liability (laws that hold retail establishments that sell alcohol liable for the injuries or harms caused by their intoxicated or underage customers).19
Bottom Line
Patients experiencing alcohol withdrawal can present with several layers of complexity. Failure to achieve acute stabilization may be life-threatening. After providing critical care, promptly start alcohol use disorder treatment for patients who expresses a desire to change.
Related Resources
- American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. https://www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association Publishing; 2018.
Drug Brand Names
Acamprosate • Campral
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone (injection) • Vivitrol
Naltrexone (oral) • ReVia
Topiramate • Topamax
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
3. Holleck JL, Merchant N, Gunderson CG. Symptom-triggered therapy for alcohol withdrawal syndrome: a systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2019;34(6):1018-1024.
4. Clapp P, Bhave SV, Hoffman PL. How adaptation of the brain to alcohol leads to dependence: a pharmacological perspective. Alcohol Res Health. 2008;31(4):310-339.
5. Burnette EM, Nieto SJ, Grodin EN, et al. Novel agents for the pharmacological treatment of alcohol use disorder. Drugs. 2022;82(3):251-274.
6. Selim R, Zhou Y, Rupp LB, et al. Availability of PEth testing is associated with reduced eligibility for liver transplant among patients with alcohol-related liver disease. Clin Transplant. 2022;36(5):e14595.
7. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63(6):1634-1640.
8. Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev. 2013;34(3):117-130.
9. Ballard HS. The hematological complications of alcoholism. Alcohol Health Res World. 1997;21(1):42-52.
10. Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009;79(3):203-208.
11. Martin PR, Singleton CK, Hiller-Sturmhöfel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27(2):134-142.
12. Palmer BF, Clegg DJ. Electrolyte disturbances in patients with chronic alcohol-use disorder. N Engl J Med. 2017;377(14):1368-1377.
13. Silczuk A, Habrat B. Alcohol-induced thrombocytopenia: current review. Alcohol. 2020;86:9-16. doi:10.1016/j.alcohol.2020.02.166
14. Pettinati HM, Rabinowitz AR. New pharmacotherapies for treating the neurobiology of alcohol and drug addiction. Psychiatry (Edgmont). 2006;3(5):14-16.
15. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736.
16. Chockalingam L, Burnham EL, Jolley SE. Medication prescribing for alcohol use disorders during alcohol-related encounters in a Colorado regional healthcare system. Alcoholism Clin Exp Res. 2022;46(6):1094-1102.
17. Mintz CM, Hartz SM, Fisher SL, et al. A cascade of care for alcohol use disorder: using 2015-2019 National Survey on Drug Use and Health data to identify gaps in past 12-month care. Alcohol Clin Exp Res. 2021;45(6):1276-1286.
18. Esser MB, Leung G, Sherk A, et al. Estimated deaths attributable to excessive alcohol use among US adults aged 20 to 64 years, 2015 to 2019. JAMA Netw Open. 2022;5(11):e2239485. doi:10.1001/jamanet workopen.2022.39485
19. The Community Guide. CPSTF Findings for Excessive Alcohol Consumption. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/task-force-findings-excessive-alcohol-consumption.html
20. The Community Guide. Alcohol Excessive Consumption: Privatization of Retail Alcohol Sales. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/findings/alcohol-excessive-consumption-privatization-retail-alcohol-sales.html
21. The Community Guide. What is the CPSTF? Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/what-is-the-cpstf.html
CASE A difficult withdrawal
Three days after he stops drinking alcohol, Mr. G, age 49, presents to a detoxification center with his wife, who drove him there because she was concerned about his condition. She says her husband had been drinking alcohol every night for as long as she can remember. Despite numerous admissions to rehabilitation centers, Mr. G usually would resume drinking soon after he was discharged. Three days ago, Mr. G’s wife had told him she “could not take it anymore,” so he got rid of all his alcohol and stopped drinking. Mr. G’s wife felt he was doing fine the first day, but his condition increasingly worsened the second and third days. The triage nurse who attempts to interview Mr. G finds him tremulous, vomiting, and sweating. She notices that he seems preoccupied with pulling at his shirt, appearing to pick at things that are not there.
HISTORY Untreated depression, other comorbidities
Mr. G’s wife says he has never been psychiatrically hospitalized or exhibited suicidal behavior. Mr. G previously received care from a psychiatrist, who diagnosed him with major depressive disorder (MDD) and prescribed an antidepressant, though his wife cannot recall which specific medication. She shares it has been “a long time” since Mr. G has taken the antidepressant and the last time he received treatment for his MDD was 5 years ago. Mr. G’s wife says her husband had once abstained from alcohol use for >6 months following one of his stints at a rehabilitation center. She is not able to share many other details about Mr. G’s previous stays at rehabilitation centers, but says he always had “a rough time.”
She says Mr. G had been drinking an average of 10 drinks each night, usually within 4 hours. He has no history of nicotine or illicit substance use and has held a corporate job for the last 18 years. Several years ago, a physician had diagnosed Mr. G with hypertension and high cholesterol, but he did not follow up for treatment. Mr. G’s wife also recalls a physician told her husband he had a fatty liver. His family history includes heart disease and cancer.
[polldaddy:12041618]
The author’s observations
The treatment team observed several elements of alcohol withdrawal and classified Mr. G as a priority patient. If the team had completed the Clinical Institute Withdrawal Assessment for Alcohol–Revised scale (CIWA-Ar) (Table 11), Mr. G would score ≥10. While the protocol for initiating treatment for patients experiencing alcohol withdrawal varies by institution, patients with moderate to severe scores on the CIWA-Ar when experiencing withdrawal typically are managed with pharmacotherapy to address their symptoms.1 Given the timeline of his last drink as reported by his wife, Mr. G is on the brink of experiencing a cascade of symptoms concerning for delirium tremens (DTs).2 Table 22 provides a timeline and symptoms related to alcohol withdrawal. To prevent further exacerbation of symptoms, which could lead to DTs, Mr. G’s treatment team will likely initiate a benzodiazepine, using either scheduled or symptom-driven dosing.3

Two neurotransmitters that play a role in DTs are glutamate (excitatory) and GABA (inhibitory). In a normal state, the competing actions of these neurotransmitters balance each other. Acute alcohol intake causes a shift in the excitatory and inhibitory levels, with more inhibition taking place, thus causing disequilibrium. If chronic alcohol use continues, the amount of GABA inhibition reduction is related to downregulation of receptors.2,4 Excitation increases by way of upregulation of the N-methyl-

If alcohol is suddenly removed following chronic use, there is unchecked glutamate excitation related to a blunted GABA state. This added increase in the excitation of glutamate leads to withdrawal symptoms.2,4 Table 32,4,5 depicts the neurotransmitter equilibrium of GABA and glutamate relative to alcohol use.

EVALUATION Bleeding gums and bruising
The treatment team admits Mr. G to the triage bay and contacts the addiction psychiatrist. The physician orders laboratory tests to assess nutritional deficits and electrolyte abnormalities. Mr. G is also placed on routine assessments with symptom-triggered therapy. An assessment reveals bleeding gums and bruises, which are believed to be a result of thrombocytopenia (low blood platelet count).
[polldaddy:12041627]
Continue to: The author's observations
The author’s observations
Though regular clinical assessment of PEth varies, it is considered to have high sensitivity and specificity to detect alcohol use.6 When ethanol is present, the phospholipase D enzyme acts upon phosphatidylcholine, forming a direct biomarker, PEth, on the surface of the red blood cell.6,7 PEth’s half-life ranges from 4.5 to 12 days,6 and it can be detected in blood for 3 to 4 weeks after alcohol ingestion.6,7 A PEth value <20 ng/mL indicates light or no alcohol consumption; 20 to 199 ng/mL indicates significant consumption; and >200 ng/mL indicates heavy consumption.7 Since Mr. G has a history of chronic alcohol use, his PEth level is expected to be >200 ng/mL.
AST/ALT and MCV are indirect biomarkers, meaning the tests are not alcohol-specific and the role of alcohol is instead observed by the damage to the body with excessive use over time.7 The expected AST:ALT ratio is 2:1. This is related to 3 mechanisms. The first is a decrease in ALT usually relative to B6 deficiency in individuals with alcohol use disorder (AUD). Another mechanism is related to alcohol’s propensity to affect mitochondria, which is a source for AST. Additionally, AST is also found in higher proportions in the kidneys, heart, and muscles.8
An MCV <100 fL would be within the normal range (80 to 100 fL) for red blood cells. While the reasons for an enlarged red blood cell (or macrocyte) are extensive, alcohol can be a factor once other causes are excluded. Additional laboratory tests and a peripheral blood smear test can help in this investigation. Alcohol disrupts the complete maturation of red blood cells.9,10 If the cause of the macrocyte is alcohol-related and alcohol use is terminated, those enlarged cells can resolve in an average of 3 months.9
Vitamin B1 levels >200 nmol/L would be within normal range (74 to 222 nmol/L). Mr. G’s chronic alcohol use would likely cause him to be vitamin B1–deficient. The deficiency is usually related to diet, malabsorption, and the cells’ impaired ability to utilize vitamin B1. A consequence of vitamin B1 deficiency is Wernicke-Korsakoff syndrome.11
Due to his chronic alcohol use, Mr. G’s magnesium stores most likely would be below normal range (1.7 to 2.2 mg/dL). Acting as a diuretic, alcohol depletes magnesium and other electrolytes. The intracellular shift that occurs to balance the deficit causes the body to use its normal stores of magnesium, which leads to further magnesium depletion. Other common causes include nutritional deficiency and decreased gastrointestinal absorption.12 The bleeding the physician suspected was a result of drinking likely occurred through direct and indirect mechanisms that affect platelets.9,13 Platelets can show improvement 1 week after drinking cessation. Some evidence suggests the risk of seizure or DTs increases significantly with a platelet count <119,000 µL per unit of blood.13
Continue to: TREATMENT Pharmacotherapy for alcohol use disorder
TREATMENT Pharmacotherapy for alcohol use disorder
As Mr. G’s condition starts to stabilize, he discusses treatment options for AUD with his physician. At the end of the discussion, Mr. G expresses an interest in starting a medication. The doctor reviews his laboratory results and available treatment options.
[polldaddy:12041630]
The author’s observations
Of the 3 FDA-approved medications for treating AUD (disulfiram, acamprosate, and naltrexone), naltrexone has been shown to decrease heavy drinking days5,14 and comes in oral and injectable forms. Reducing drinking is achieved by reducing the rewarding effects of alcohol5,14 and alcohol cravings.5 Disulfiram often has poor adherence, and like acamprosate it may be more helpful for maintenance of abstinence. Neither topiramate nor gabapentin are FDA-approved for AUD but may be used for their affects on GABA.5 Gabapentin may also help patients experiencing alcohol withdrawal syndrome.5,15 Mr. G did not have any concomitant medications or comorbid medical conditions, but these factors as well as any renal or hepatic dysfunction must be considered before initiating any medications.
OUTCOME Improved well-being
Mr. G’s treatment team initiates oral naltrexone 50 mg/d, which he tolerates well without complications. He stops drinking entirely and expresses an interest in transitioning to an injectable form of naltrexone in the future. In addition to taking medication, Mr. G wants to participate in psychotherapy. Mr. G thanks his team for the care he received in the hospital, telling them, “You all saved my life.” As he discusses his past issues with alcohol, Mr. G asks his physician how he could get involved to make changes to reduce excessive alcohol consumption in his community (Box5,15-21).
Box
Alcohol use disorder is undertreated5,15-17 and excessive alcohol use accounts for 1 in 5 deaths in individuals within Mr. G’s age range.18 An April 2011 report from the Community Preventive Services Task Force19 did not recommend privatization of retail alcohol sales as an intervention to reduce excessive alcohol consumption, because it would instead lead to an increase in alcohol consumption per capita, a known gateway to excessive alcohol consumption.20
The Task Force was established in 1996 by the US Department of Health and Human Services. Its objective is to identify scientifically proven interventions to save lives, increase lifespans, and improve quality of life. Recommendations are based on systematic reviews to inform lawmakers, health departments, and other organizations and agencies.21 The Task Force’s recommendations were divided into interventions that have strong evidence, sufficient evidence, or insufficient evidence. If Mr. G wanted to have the greatest impact in his efforts to reduce excessive alcohol consumption in his community, the strongest evidence supporting change focuses on electronic screening and brief intervention, maintaining limits on days of alcohol sale, increasing taxes on alcohol, and establishing dram shop liability (laws that hold retail establishments that sell alcohol liable for the injuries or harms caused by their intoxicated or underage customers).19
Bottom Line
Patients experiencing alcohol withdrawal can present with several layers of complexity. Failure to achieve acute stabilization may be life-threatening. After providing critical care, promptly start alcohol use disorder treatment for patients who expresses a desire to change.
Related Resources
- American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. https://www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association Publishing; 2018.
Drug Brand Names
Acamprosate • Campral
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone (injection) • Vivitrol
Naltrexone (oral) • ReVia
Topiramate • Topamax
CASE A difficult withdrawal
Three days after he stops drinking alcohol, Mr. G, age 49, presents to a detoxification center with his wife, who drove him there because she was concerned about his condition. She says her husband had been drinking alcohol every night for as long as she can remember. Despite numerous admissions to rehabilitation centers, Mr. G usually would resume drinking soon after he was discharged. Three days ago, Mr. G’s wife had told him she “could not take it anymore,” so he got rid of all his alcohol and stopped drinking. Mr. G’s wife felt he was doing fine the first day, but his condition increasingly worsened the second and third days. The triage nurse who attempts to interview Mr. G finds him tremulous, vomiting, and sweating. She notices that he seems preoccupied with pulling at his shirt, appearing to pick at things that are not there.
HISTORY Untreated depression, other comorbidities
Mr. G’s wife says he has never been psychiatrically hospitalized or exhibited suicidal behavior. Mr. G previously received care from a psychiatrist, who diagnosed him with major depressive disorder (MDD) and prescribed an antidepressant, though his wife cannot recall which specific medication. She shares it has been “a long time” since Mr. G has taken the antidepressant and the last time he received treatment for his MDD was 5 years ago. Mr. G’s wife says her husband had once abstained from alcohol use for >6 months following one of his stints at a rehabilitation center. She is not able to share many other details about Mr. G’s previous stays at rehabilitation centers, but says he always had “a rough time.”
She says Mr. G had been drinking an average of 10 drinks each night, usually within 4 hours. He has no history of nicotine or illicit substance use and has held a corporate job for the last 18 years. Several years ago, a physician had diagnosed Mr. G with hypertension and high cholesterol, but he did not follow up for treatment. Mr. G’s wife also recalls a physician told her husband he had a fatty liver. His family history includes heart disease and cancer.
[polldaddy:12041618]
The author’s observations
The treatment team observed several elements of alcohol withdrawal and classified Mr. G as a priority patient. If the team had completed the Clinical Institute Withdrawal Assessment for Alcohol–Revised scale (CIWA-Ar) (Table 11), Mr. G would score ≥10. While the protocol for initiating treatment for patients experiencing alcohol withdrawal varies by institution, patients with moderate to severe scores on the CIWA-Ar when experiencing withdrawal typically are managed with pharmacotherapy to address their symptoms.1 Given the timeline of his last drink as reported by his wife, Mr. G is on the brink of experiencing a cascade of symptoms concerning for delirium tremens (DTs).2 Table 22 provides a timeline and symptoms related to alcohol withdrawal. To prevent further exacerbation of symptoms, which could lead to DTs, Mr. G’s treatment team will likely initiate a benzodiazepine, using either scheduled or symptom-driven dosing.3

Two neurotransmitters that play a role in DTs are glutamate (excitatory) and GABA (inhibitory). In a normal state, the competing actions of these neurotransmitters balance each other. Acute alcohol intake causes a shift in the excitatory and inhibitory levels, with more inhibition taking place, thus causing disequilibrium. If chronic alcohol use continues, the amount of GABA inhibition reduction is related to downregulation of receptors.2,4 Excitation increases by way of upregulation of the N-methyl-

If alcohol is suddenly removed following chronic use, there is unchecked glutamate excitation related to a blunted GABA state. This added increase in the excitation of glutamate leads to withdrawal symptoms.2,4 Table 32,4,5 depicts the neurotransmitter equilibrium of GABA and glutamate relative to alcohol use.

EVALUATION Bleeding gums and bruising
The treatment team admits Mr. G to the triage bay and contacts the addiction psychiatrist. The physician orders laboratory tests to assess nutritional deficits and electrolyte abnormalities. Mr. G is also placed on routine assessments with symptom-triggered therapy. An assessment reveals bleeding gums and bruises, which are believed to be a result of thrombocytopenia (low blood platelet count).
[polldaddy:12041627]
Continue to: The author's observations
The author’s observations
Though regular clinical assessment of PEth varies, it is considered to have high sensitivity and specificity to detect alcohol use.6 When ethanol is present, the phospholipase D enzyme acts upon phosphatidylcholine, forming a direct biomarker, PEth, on the surface of the red blood cell.6,7 PEth’s half-life ranges from 4.5 to 12 days,6 and it can be detected in blood for 3 to 4 weeks after alcohol ingestion.6,7 A PEth value <20 ng/mL indicates light or no alcohol consumption; 20 to 199 ng/mL indicates significant consumption; and >200 ng/mL indicates heavy consumption.7 Since Mr. G has a history of chronic alcohol use, his PEth level is expected to be >200 ng/mL.
AST/ALT and MCV are indirect biomarkers, meaning the tests are not alcohol-specific and the role of alcohol is instead observed by the damage to the body with excessive use over time.7 The expected AST:ALT ratio is 2:1. This is related to 3 mechanisms. The first is a decrease in ALT usually relative to B6 deficiency in individuals with alcohol use disorder (AUD). Another mechanism is related to alcohol’s propensity to affect mitochondria, which is a source for AST. Additionally, AST is also found in higher proportions in the kidneys, heart, and muscles.8
An MCV <100 fL would be within the normal range (80 to 100 fL) for red blood cells. While the reasons for an enlarged red blood cell (or macrocyte) are extensive, alcohol can be a factor once other causes are excluded. Additional laboratory tests and a peripheral blood smear test can help in this investigation. Alcohol disrupts the complete maturation of red blood cells.9,10 If the cause of the macrocyte is alcohol-related and alcohol use is terminated, those enlarged cells can resolve in an average of 3 months.9
Vitamin B1 levels >200 nmol/L would be within normal range (74 to 222 nmol/L). Mr. G’s chronic alcohol use would likely cause him to be vitamin B1–deficient. The deficiency is usually related to diet, malabsorption, and the cells’ impaired ability to utilize vitamin B1. A consequence of vitamin B1 deficiency is Wernicke-Korsakoff syndrome.11
Due to his chronic alcohol use, Mr. G’s magnesium stores most likely would be below normal range (1.7 to 2.2 mg/dL). Acting as a diuretic, alcohol depletes magnesium and other electrolytes. The intracellular shift that occurs to balance the deficit causes the body to use its normal stores of magnesium, which leads to further magnesium depletion. Other common causes include nutritional deficiency and decreased gastrointestinal absorption.12 The bleeding the physician suspected was a result of drinking likely occurred through direct and indirect mechanisms that affect platelets.9,13 Platelets can show improvement 1 week after drinking cessation. Some evidence suggests the risk of seizure or DTs increases significantly with a platelet count <119,000 µL per unit of blood.13
Continue to: TREATMENT Pharmacotherapy for alcohol use disorder
TREATMENT Pharmacotherapy for alcohol use disorder
As Mr. G’s condition starts to stabilize, he discusses treatment options for AUD with his physician. At the end of the discussion, Mr. G expresses an interest in starting a medication. The doctor reviews his laboratory results and available treatment options.
[polldaddy:12041630]
The author’s observations
Of the 3 FDA-approved medications for treating AUD (disulfiram, acamprosate, and naltrexone), naltrexone has been shown to decrease heavy drinking days5,14 and comes in oral and injectable forms. Reducing drinking is achieved by reducing the rewarding effects of alcohol5,14 and alcohol cravings.5 Disulfiram often has poor adherence, and like acamprosate it may be more helpful for maintenance of abstinence. Neither topiramate nor gabapentin are FDA-approved for AUD but may be used for their affects on GABA.5 Gabapentin may also help patients experiencing alcohol withdrawal syndrome.5,15 Mr. G did not have any concomitant medications or comorbid medical conditions, but these factors as well as any renal or hepatic dysfunction must be considered before initiating any medications.
OUTCOME Improved well-being
Mr. G’s treatment team initiates oral naltrexone 50 mg/d, which he tolerates well without complications. He stops drinking entirely and expresses an interest in transitioning to an injectable form of naltrexone in the future. In addition to taking medication, Mr. G wants to participate in psychotherapy. Mr. G thanks his team for the care he received in the hospital, telling them, “You all saved my life.” As he discusses his past issues with alcohol, Mr. G asks his physician how he could get involved to make changes to reduce excessive alcohol consumption in his community (Box5,15-21).
Box
Alcohol use disorder is undertreated5,15-17 and excessive alcohol use accounts for 1 in 5 deaths in individuals within Mr. G’s age range.18 An April 2011 report from the Community Preventive Services Task Force19 did not recommend privatization of retail alcohol sales as an intervention to reduce excessive alcohol consumption, because it would instead lead to an increase in alcohol consumption per capita, a known gateway to excessive alcohol consumption.20
The Task Force was established in 1996 by the US Department of Health and Human Services. Its objective is to identify scientifically proven interventions to save lives, increase lifespans, and improve quality of life. Recommendations are based on systematic reviews to inform lawmakers, health departments, and other organizations and agencies.21 The Task Force’s recommendations were divided into interventions that have strong evidence, sufficient evidence, or insufficient evidence. If Mr. G wanted to have the greatest impact in his efforts to reduce excessive alcohol consumption in his community, the strongest evidence supporting change focuses on electronic screening and brief intervention, maintaining limits on days of alcohol sale, increasing taxes on alcohol, and establishing dram shop liability (laws that hold retail establishments that sell alcohol liable for the injuries or harms caused by their intoxicated or underage customers).19
Bottom Line
Patients experiencing alcohol withdrawal can present with several layers of complexity. Failure to achieve acute stabilization may be life-threatening. After providing critical care, promptly start alcohol use disorder treatment for patients who expresses a desire to change.
Related Resources
- American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. https://www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association Publishing; 2018.
Drug Brand Names
Acamprosate • Campral
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone (injection) • Vivitrol
Naltrexone (oral) • ReVia
Topiramate • Topamax
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
3. Holleck JL, Merchant N, Gunderson CG. Symptom-triggered therapy for alcohol withdrawal syndrome: a systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2019;34(6):1018-1024.
4. Clapp P, Bhave SV, Hoffman PL. How adaptation of the brain to alcohol leads to dependence: a pharmacological perspective. Alcohol Res Health. 2008;31(4):310-339.
5. Burnette EM, Nieto SJ, Grodin EN, et al. Novel agents for the pharmacological treatment of alcohol use disorder. Drugs. 2022;82(3):251-274.
6. Selim R, Zhou Y, Rupp LB, et al. Availability of PEth testing is associated with reduced eligibility for liver transplant among patients with alcohol-related liver disease. Clin Transplant. 2022;36(5):e14595.
7. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63(6):1634-1640.
8. Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev. 2013;34(3):117-130.
9. Ballard HS. The hematological complications of alcoholism. Alcohol Health Res World. 1997;21(1):42-52.
10. Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009;79(3):203-208.
11. Martin PR, Singleton CK, Hiller-Sturmhöfel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27(2):134-142.
12. Palmer BF, Clegg DJ. Electrolyte disturbances in patients with chronic alcohol-use disorder. N Engl J Med. 2017;377(14):1368-1377.
13. Silczuk A, Habrat B. Alcohol-induced thrombocytopenia: current review. Alcohol. 2020;86:9-16. doi:10.1016/j.alcohol.2020.02.166
14. Pettinati HM, Rabinowitz AR. New pharmacotherapies for treating the neurobiology of alcohol and drug addiction. Psychiatry (Edgmont). 2006;3(5):14-16.
15. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736.
16. Chockalingam L, Burnham EL, Jolley SE. Medication prescribing for alcohol use disorders during alcohol-related encounters in a Colorado regional healthcare system. Alcoholism Clin Exp Res. 2022;46(6):1094-1102.
17. Mintz CM, Hartz SM, Fisher SL, et al. A cascade of care for alcohol use disorder: using 2015-2019 National Survey on Drug Use and Health data to identify gaps in past 12-month care. Alcohol Clin Exp Res. 2021;45(6):1276-1286.
18. Esser MB, Leung G, Sherk A, et al. Estimated deaths attributable to excessive alcohol use among US adults aged 20 to 64 years, 2015 to 2019. JAMA Netw Open. 2022;5(11):e2239485. doi:10.1001/jamanet workopen.2022.39485
19. The Community Guide. CPSTF Findings for Excessive Alcohol Consumption. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/task-force-findings-excessive-alcohol-consumption.html
20. The Community Guide. Alcohol Excessive Consumption: Privatization of Retail Alcohol Sales. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/findings/alcohol-excessive-consumption-privatization-retail-alcohol-sales.html
21. The Community Guide. What is the CPSTF? Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/what-is-the-cpstf.html
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
3. Holleck JL, Merchant N, Gunderson CG. Symptom-triggered therapy for alcohol withdrawal syndrome: a systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2019;34(6):1018-1024.
4. Clapp P, Bhave SV, Hoffman PL. How adaptation of the brain to alcohol leads to dependence: a pharmacological perspective. Alcohol Res Health. 2008;31(4):310-339.
5. Burnette EM, Nieto SJ, Grodin EN, et al. Novel agents for the pharmacological treatment of alcohol use disorder. Drugs. 2022;82(3):251-274.
6. Selim R, Zhou Y, Rupp LB, et al. Availability of PEth testing is associated with reduced eligibility for liver transplant among patients with alcohol-related liver disease. Clin Transplant. 2022;36(5):e14595.
7. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63(6):1634-1640.
8. Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev. 2013;34(3):117-130.
9. Ballard HS. The hematological complications of alcoholism. Alcohol Health Res World. 1997;21(1):42-52.
10. Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009;79(3):203-208.
11. Martin PR, Singleton CK, Hiller-Sturmhöfel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27(2):134-142.
12. Palmer BF, Clegg DJ. Electrolyte disturbances in patients with chronic alcohol-use disorder. N Engl J Med. 2017;377(14):1368-1377.
13. Silczuk A, Habrat B. Alcohol-induced thrombocytopenia: current review. Alcohol. 2020;86:9-16. doi:10.1016/j.alcohol.2020.02.166
14. Pettinati HM, Rabinowitz AR. New pharmacotherapies for treating the neurobiology of alcohol and drug addiction. Psychiatry (Edgmont). 2006;3(5):14-16.
15. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736.
16. Chockalingam L, Burnham EL, Jolley SE. Medication prescribing for alcohol use disorders during alcohol-related encounters in a Colorado regional healthcare system. Alcoholism Clin Exp Res. 2022;46(6):1094-1102.
17. Mintz CM, Hartz SM, Fisher SL, et al. A cascade of care for alcohol use disorder: using 2015-2019 National Survey on Drug Use and Health data to identify gaps in past 12-month care. Alcohol Clin Exp Res. 2021;45(6):1276-1286.
18. Esser MB, Leung G, Sherk A, et al. Estimated deaths attributable to excessive alcohol use among US adults aged 20 to 64 years, 2015 to 2019. JAMA Netw Open. 2022;5(11):e2239485. doi:10.1001/jamanet workopen.2022.39485
19. The Community Guide. CPSTF Findings for Excessive Alcohol Consumption. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/task-force-findings-excessive-alcohol-consumption.html
20. The Community Guide. Alcohol Excessive Consumption: Privatization of Retail Alcohol Sales. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/findings/alcohol-excessive-consumption-privatization-retail-alcohol-sales.html
21. The Community Guide. What is the CPSTF? Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/what-is-the-cpstf.html
Telehealth services tied to a major reduction in opioid overdose deaths
, a new study of Medicare beneficiaries shows.
Telehealth services for opioid use disorder (OUD) were used far more often during the pandemic than before COVID-19, and those who used them were 33% less likely to die of a drug overdose.
Investigators also found a significant increase in MOUD use during the pandemic. Fatal drug overdoses were 59% less likely among individuals who received MOUD from an opioid treatment program and 38% less likely among those treated with buprenorphine in an office-based setting.
The results come as policymakers are preparing for the end of the public health emergency that prompted the expansion of OUD-related telehealth and MOUD prescribing and are deciding whether to make those expansions permanent.
“The expansion of telehealth during the COVID-19 pandemic appears to have had positive effects on patients receiving MOUD, improved retention among patients who received MOUD, and lowered risks for both nonfatal and fatal overdose,” lead investigator Christopher M. Jones, PharmD, DrPH, director of the National Center for Injury Prevention and Control at the Centers for Disease Control and Prevention, Atlanta, Georgia, told this news organization. “Our results suggest that telehealth is a valuable tool in the toolbox for expanding access to and improving retention on MOUD.”
The findings were published online in JAMA Psychiatry.
Increase in treatment
The study included 105,162 Medicare beneficiaries who began OUD treatment between March and August in 2019 (prepandemic cohort; 67.6%
aged 45-74 years), and 70,479 who began treatment between March and August of 2020 (pandemic cohort; 66.3% aged 45-74 years).
Participants had not received OUD treatment in the 6 months leading up to study enrollment and were followed for 6 months after treatment began.
Significantly more study participants received OUD-related telehealth services during the pandemic than prior to 2019 (19.6% vs. 0.6%; P < .001). Receipt of MOUD was also significantly higher in the pandemic cohort (12.6% vs. 10.8%; P < .001).
The rate of drug overdose deaths was higher in the pandemic cohort (5.1 deaths vs. 3.7 deaths per 1,000 beneficiaries; P < .001). But the percentage of deaths from drug overdoses did not differ between groups (4.8% in the prepandemic cohort vs. 5.1% in the pandemic cohort; P = .49).
In the pandemic cohort, fatal drug overdoses were 33% less likely among those who received OUD-related telehealth services (adjusted odds ratio, 0.67; 95% confidence interval, 0.48-0.92); 59% less likely among those who received MOUD from opioid treatment programs (aOR, 0.41; 95% CI, 0.25-0.68), and 38% less likely among those who received buprenorphine in office-based settings (aOR, 0.62; 95% CI, 0.43-0.91).
Risk of fatal overdose was significantly lower among women and those aged 65 years and older. There were no significant differences in risk based on urban or rural residency or on ethnicity.
“Against the backdrop of a highly potent illicit drug supply driven by illicit fentanyl and fentanyl analogues and historically large increases in overdose deaths during the COVID-19 pandemic, MOUD was still highly effective at reducing risk for fatal overdose,” Dr. Jones said.
While the use of buprenorphine in office-based settings was associated with a decreased risk of overdose death, use of extended-release naltrexone was not.
“Prior research has demonstrated the effectiveness of extended-release naltrexone in the treatment of opioid use disorder,” Dr. Jones said. “However, research has also shown that patients have challenges getting started, or inducted, on extended-release naltrexone.”
An earlier study by Dr. Jones and colleagues showed that rates of retention were lower with extended-release naltrexone, compared with buprenorphine in office-based settings or MOUD from opioid treatment programs.
The new study included only a small number of individuals who were receiving extended-release naltrexone, which may have influenced the findings. In addition, challenges with induction and retention may be driving the results, Dr. Jones noted.
“Efforts to improve induction and retention with extended-release naltrexone are important areas for future research and clinical practice,” he added.
An important engagement tool
A number of questions about telehealth care for OUD remain, including whether increased access to care accounts for the reduction in drug overdose risk that the investigators found or whether other factors are at play.
“There is still more we need to understand about telehealth, such as the quality of care provided and the particular aspects of care provided by telehealth and how this influences health outcomes,” Dr. Jones said.
The results also suggest treatments for OUD are still not finding their way to patients who might benefit, he added.
“Despite the positive findings and the prior research showing that MOUD is highly effective, we found that only one in five patients received telehealth services and only one in eight received any MOUD. This really underscores the need to expand these services across clinical settings,” he added.
These and earlier findings demonstrate the potential benefits of continuing pandemic-era expansion of OUD-related telehealth services and MOUD access, Dr. Jones said.
In preparation for the end of the public health emergency on May 1, the Drug Enforcement Agency recently released a proposal that would allow providers to prescribe a 30-day supply of buprenorphine, but for patients to receive additional prescriptions, a face-to-face meeting would be required. The proposal has drawn criticism from addiction medicine specialists.
The current study didn’t explore if or how the proposal might affect patients with OUD or whether it could blunt the positive effects of the findings.
“Prior research shows that keeping individuals engaged in treatment, including on medications, is a critical part of reducing the negative health and social impacts of opioid use disorder. Our results suggest that telehealth can be an important tool in helping patients engage in and stay connected in care,” said Dr. Jones.
The study was funded by the Centers for Disease Control and Prevention, the Centers for Medicare & Medicaid Services, and the National Institutes of Health. Dr. Johnson reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, a new study of Medicare beneficiaries shows.
Telehealth services for opioid use disorder (OUD) were used far more often during the pandemic than before COVID-19, and those who used them were 33% less likely to die of a drug overdose.
Investigators also found a significant increase in MOUD use during the pandemic. Fatal drug overdoses were 59% less likely among individuals who received MOUD from an opioid treatment program and 38% less likely among those treated with buprenorphine in an office-based setting.
The results come as policymakers are preparing for the end of the public health emergency that prompted the expansion of OUD-related telehealth and MOUD prescribing and are deciding whether to make those expansions permanent.
“The expansion of telehealth during the COVID-19 pandemic appears to have had positive effects on patients receiving MOUD, improved retention among patients who received MOUD, and lowered risks for both nonfatal and fatal overdose,” lead investigator Christopher M. Jones, PharmD, DrPH, director of the National Center for Injury Prevention and Control at the Centers for Disease Control and Prevention, Atlanta, Georgia, told this news organization. “Our results suggest that telehealth is a valuable tool in the toolbox for expanding access to and improving retention on MOUD.”
The findings were published online in JAMA Psychiatry.
Increase in treatment
The study included 105,162 Medicare beneficiaries who began OUD treatment between March and August in 2019 (prepandemic cohort; 67.6%
aged 45-74 years), and 70,479 who began treatment between March and August of 2020 (pandemic cohort; 66.3% aged 45-74 years).
Participants had not received OUD treatment in the 6 months leading up to study enrollment and were followed for 6 months after treatment began.
Significantly more study participants received OUD-related telehealth services during the pandemic than prior to 2019 (19.6% vs. 0.6%; P < .001). Receipt of MOUD was also significantly higher in the pandemic cohort (12.6% vs. 10.8%; P < .001).
The rate of drug overdose deaths was higher in the pandemic cohort (5.1 deaths vs. 3.7 deaths per 1,000 beneficiaries; P < .001). But the percentage of deaths from drug overdoses did not differ between groups (4.8% in the prepandemic cohort vs. 5.1% in the pandemic cohort; P = .49).
In the pandemic cohort, fatal drug overdoses were 33% less likely among those who received OUD-related telehealth services (adjusted odds ratio, 0.67; 95% confidence interval, 0.48-0.92); 59% less likely among those who received MOUD from opioid treatment programs (aOR, 0.41; 95% CI, 0.25-0.68), and 38% less likely among those who received buprenorphine in office-based settings (aOR, 0.62; 95% CI, 0.43-0.91).
Risk of fatal overdose was significantly lower among women and those aged 65 years and older. There were no significant differences in risk based on urban or rural residency or on ethnicity.
“Against the backdrop of a highly potent illicit drug supply driven by illicit fentanyl and fentanyl analogues and historically large increases in overdose deaths during the COVID-19 pandemic, MOUD was still highly effective at reducing risk for fatal overdose,” Dr. Jones said.
While the use of buprenorphine in office-based settings was associated with a decreased risk of overdose death, use of extended-release naltrexone was not.
“Prior research has demonstrated the effectiveness of extended-release naltrexone in the treatment of opioid use disorder,” Dr. Jones said. “However, research has also shown that patients have challenges getting started, or inducted, on extended-release naltrexone.”
An earlier study by Dr. Jones and colleagues showed that rates of retention were lower with extended-release naltrexone, compared with buprenorphine in office-based settings or MOUD from opioid treatment programs.
The new study included only a small number of individuals who were receiving extended-release naltrexone, which may have influenced the findings. In addition, challenges with induction and retention may be driving the results, Dr. Jones noted.
“Efforts to improve induction and retention with extended-release naltrexone are important areas for future research and clinical practice,” he added.
An important engagement tool
A number of questions about telehealth care for OUD remain, including whether increased access to care accounts for the reduction in drug overdose risk that the investigators found or whether other factors are at play.
“There is still more we need to understand about telehealth, such as the quality of care provided and the particular aspects of care provided by telehealth and how this influences health outcomes,” Dr. Jones said.
The results also suggest treatments for OUD are still not finding their way to patients who might benefit, he added.
“Despite the positive findings and the prior research showing that MOUD is highly effective, we found that only one in five patients received telehealth services and only one in eight received any MOUD. This really underscores the need to expand these services across clinical settings,” he added.
These and earlier findings demonstrate the potential benefits of continuing pandemic-era expansion of OUD-related telehealth services and MOUD access, Dr. Jones said.
In preparation for the end of the public health emergency on May 1, the Drug Enforcement Agency recently released a proposal that would allow providers to prescribe a 30-day supply of buprenorphine, but for patients to receive additional prescriptions, a face-to-face meeting would be required. The proposal has drawn criticism from addiction medicine specialists.
The current study didn’t explore if or how the proposal might affect patients with OUD or whether it could blunt the positive effects of the findings.
“Prior research shows that keeping individuals engaged in treatment, including on medications, is a critical part of reducing the negative health and social impacts of opioid use disorder. Our results suggest that telehealth can be an important tool in helping patients engage in and stay connected in care,” said Dr. Jones.
The study was funded by the Centers for Disease Control and Prevention, the Centers for Medicare & Medicaid Services, and the National Institutes of Health. Dr. Johnson reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, a new study of Medicare beneficiaries shows.
Telehealth services for opioid use disorder (OUD) were used far more often during the pandemic than before COVID-19, and those who used them were 33% less likely to die of a drug overdose.
Investigators also found a significant increase in MOUD use during the pandemic. Fatal drug overdoses were 59% less likely among individuals who received MOUD from an opioid treatment program and 38% less likely among those treated with buprenorphine in an office-based setting.
The results come as policymakers are preparing for the end of the public health emergency that prompted the expansion of OUD-related telehealth and MOUD prescribing and are deciding whether to make those expansions permanent.
“The expansion of telehealth during the COVID-19 pandemic appears to have had positive effects on patients receiving MOUD, improved retention among patients who received MOUD, and lowered risks for both nonfatal and fatal overdose,” lead investigator Christopher M. Jones, PharmD, DrPH, director of the National Center for Injury Prevention and Control at the Centers for Disease Control and Prevention, Atlanta, Georgia, told this news organization. “Our results suggest that telehealth is a valuable tool in the toolbox for expanding access to and improving retention on MOUD.”
The findings were published online in JAMA Psychiatry.
Increase in treatment
The study included 105,162 Medicare beneficiaries who began OUD treatment between March and August in 2019 (prepandemic cohort; 67.6%
aged 45-74 years), and 70,479 who began treatment between March and August of 2020 (pandemic cohort; 66.3% aged 45-74 years).
Participants had not received OUD treatment in the 6 months leading up to study enrollment and were followed for 6 months after treatment began.
Significantly more study participants received OUD-related telehealth services during the pandemic than prior to 2019 (19.6% vs. 0.6%; P < .001). Receipt of MOUD was also significantly higher in the pandemic cohort (12.6% vs. 10.8%; P < .001).
The rate of drug overdose deaths was higher in the pandemic cohort (5.1 deaths vs. 3.7 deaths per 1,000 beneficiaries; P < .001). But the percentage of deaths from drug overdoses did not differ between groups (4.8% in the prepandemic cohort vs. 5.1% in the pandemic cohort; P = .49).
In the pandemic cohort, fatal drug overdoses were 33% less likely among those who received OUD-related telehealth services (adjusted odds ratio, 0.67; 95% confidence interval, 0.48-0.92); 59% less likely among those who received MOUD from opioid treatment programs (aOR, 0.41; 95% CI, 0.25-0.68), and 38% less likely among those who received buprenorphine in office-based settings (aOR, 0.62; 95% CI, 0.43-0.91).
Risk of fatal overdose was significantly lower among women and those aged 65 years and older. There were no significant differences in risk based on urban or rural residency or on ethnicity.
“Against the backdrop of a highly potent illicit drug supply driven by illicit fentanyl and fentanyl analogues and historically large increases in overdose deaths during the COVID-19 pandemic, MOUD was still highly effective at reducing risk for fatal overdose,” Dr. Jones said.
While the use of buprenorphine in office-based settings was associated with a decreased risk of overdose death, use of extended-release naltrexone was not.
“Prior research has demonstrated the effectiveness of extended-release naltrexone in the treatment of opioid use disorder,” Dr. Jones said. “However, research has also shown that patients have challenges getting started, or inducted, on extended-release naltrexone.”
An earlier study by Dr. Jones and colleagues showed that rates of retention were lower with extended-release naltrexone, compared with buprenorphine in office-based settings or MOUD from opioid treatment programs.
The new study included only a small number of individuals who were receiving extended-release naltrexone, which may have influenced the findings. In addition, challenges with induction and retention may be driving the results, Dr. Jones noted.
“Efforts to improve induction and retention with extended-release naltrexone are important areas for future research and clinical practice,” he added.
An important engagement tool
A number of questions about telehealth care for OUD remain, including whether increased access to care accounts for the reduction in drug overdose risk that the investigators found or whether other factors are at play.
“There is still more we need to understand about telehealth, such as the quality of care provided and the particular aspects of care provided by telehealth and how this influences health outcomes,” Dr. Jones said.
The results also suggest treatments for OUD are still not finding their way to patients who might benefit, he added.
“Despite the positive findings and the prior research showing that MOUD is highly effective, we found that only one in five patients received telehealth services and only one in eight received any MOUD. This really underscores the need to expand these services across clinical settings,” he added.
These and earlier findings demonstrate the potential benefits of continuing pandemic-era expansion of OUD-related telehealth services and MOUD access, Dr. Jones said.
In preparation for the end of the public health emergency on May 1, the Drug Enforcement Agency recently released a proposal that would allow providers to prescribe a 30-day supply of buprenorphine, but for patients to receive additional prescriptions, a face-to-face meeting would be required. The proposal has drawn criticism from addiction medicine specialists.
The current study didn’t explore if or how the proposal might affect patients with OUD or whether it could blunt the positive effects of the findings.
“Prior research shows that keeping individuals engaged in treatment, including on medications, is a critical part of reducing the negative health and social impacts of opioid use disorder. Our results suggest that telehealth can be an important tool in helping patients engage in and stay connected in care,” said Dr. Jones.
The study was funded by the Centers for Disease Control and Prevention, the Centers for Medicare & Medicaid Services, and the National Institutes of Health. Dr. Johnson reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Tranq-contaminated fentanyl now in 48 states, DEA warns
The DEA warning comes on the heels of a Food and Drug Administration announcement that it would begin more closely monitoring imports of the raw materials and bulk shipments of xylazine, also known as “tranq” and “zombie drug.”
Xylazine was first approved by the FDA in 1972 as a sedative and analgesic for use only in animals, but is increasingly being detected in illicit street drugs, and is often mixed with fentanyl, cocaine, and methamphetamine.
The FDA warned in November that naloxone (Narcan) would not reverse xylazine-related overdoses because the tranquilizer is not an opioid. It does suppress respiration and repeated exposures may lead to dependence and withdrawal, said the agency. Users are also experiencing severe necrosis at injection sites.
“Xylazine is making the deadliest drug threat our country has ever faced, fentanyl, even deadlier,” said DEA Administrator Anne Milgram in a statement. “The DEA Laboratory System is reporting that in 2022 approximately 23% of fentanyl powder and 7% of fentanyl pills seized by the DEA contained xylazine.”
Xylazine use has spread quickly, from its start in the Philadelphia area to the Northeast, the South, and most recently the West.
Citing data from the Centers for Disease Control and Prevention, the DEA said that 66% of the 107,735 overdose deaths for the year ending August 2022 involved synthetic opioids such as fentanyl. The DEA said that the Sinaloa Cartel and Jalisco Cartel in Mexico, using chemicals sourced from China, are primarily responsible for trafficking fentanyl in the United States.
A version of this article originally appeared on Medscape.com.
The DEA warning comes on the heels of a Food and Drug Administration announcement that it would begin more closely monitoring imports of the raw materials and bulk shipments of xylazine, also known as “tranq” and “zombie drug.”
Xylazine was first approved by the FDA in 1972 as a sedative and analgesic for use only in animals, but is increasingly being detected in illicit street drugs, and is often mixed with fentanyl, cocaine, and methamphetamine.
The FDA warned in November that naloxone (Narcan) would not reverse xylazine-related overdoses because the tranquilizer is not an opioid. It does suppress respiration and repeated exposures may lead to dependence and withdrawal, said the agency. Users are also experiencing severe necrosis at injection sites.
“Xylazine is making the deadliest drug threat our country has ever faced, fentanyl, even deadlier,” said DEA Administrator Anne Milgram in a statement. “The DEA Laboratory System is reporting that in 2022 approximately 23% of fentanyl powder and 7% of fentanyl pills seized by the DEA contained xylazine.”
Xylazine use has spread quickly, from its start in the Philadelphia area to the Northeast, the South, and most recently the West.
Citing data from the Centers for Disease Control and Prevention, the DEA said that 66% of the 107,735 overdose deaths for the year ending August 2022 involved synthetic opioids such as fentanyl. The DEA said that the Sinaloa Cartel and Jalisco Cartel in Mexico, using chemicals sourced from China, are primarily responsible for trafficking fentanyl in the United States.
A version of this article originally appeared on Medscape.com.
The DEA warning comes on the heels of a Food and Drug Administration announcement that it would begin more closely monitoring imports of the raw materials and bulk shipments of xylazine, also known as “tranq” and “zombie drug.”
Xylazine was first approved by the FDA in 1972 as a sedative and analgesic for use only in animals, but is increasingly being detected in illicit street drugs, and is often mixed with fentanyl, cocaine, and methamphetamine.
The FDA warned in November that naloxone (Narcan) would not reverse xylazine-related overdoses because the tranquilizer is not an opioid. It does suppress respiration and repeated exposures may lead to dependence and withdrawal, said the agency. Users are also experiencing severe necrosis at injection sites.
“Xylazine is making the deadliest drug threat our country has ever faced, fentanyl, even deadlier,” said DEA Administrator Anne Milgram in a statement. “The DEA Laboratory System is reporting that in 2022 approximately 23% of fentanyl powder and 7% of fentanyl pills seized by the DEA contained xylazine.”
Xylazine use has spread quickly, from its start in the Philadelphia area to the Northeast, the South, and most recently the West.
Citing data from the Centers for Disease Control and Prevention, the DEA said that 66% of the 107,735 overdose deaths for the year ending August 2022 involved synthetic opioids such as fentanyl. The DEA said that the Sinaloa Cartel and Jalisco Cartel in Mexico, using chemicals sourced from China, are primarily responsible for trafficking fentanyl in the United States.
A version of this article originally appeared on Medscape.com.
FDA approves OTC naloxone, but will cost be a barrier?
Greater access to the drug should mean more lives saved. However, it’s unclear how much the nasal spray will cost and whether pharmacies will stock the product openly on shelves.
Currently, major pharmacy chains such as CVS and Walgreens make naloxone available without prescription, but consumers have to ask a pharmacist to dispense the drug.
“The major question is what is it going to cost,” Brian Hurley, MD, MBA, president-elect of the American Society of Addiction Medicine, said in an interview. “In order for people to access it they have to be able to afford it.”
“We won’t accomplish much if people can’t afford to buy Narcan,” said Chuck Ingoglia, president and CEO of the National Council for Mental Wellbeing, in a statement. Still, he applauded the FDA.
“No single approach will end overdose deaths but making Narcan easy to obtain and widely available likely will save countless lives annually,” he said.
“The timeline for availability and price of this OTC product is determined by the manufacturer,” the FDA said in a statement.
Commissioner Robert M. Califf, MD, called for the drug’s manufacturer to “make accessibility to the product a priority by making it available as soon as possible and at an affordable price.”
Emergent BioSolutions did not comment on cost. It said in a statement that the spray “will be available on U.S. shelves and at online retailers by the late summer,” after it has adapted Narcan for direct-to-consumer use, including more consumer-oriented packaging.
Naloxone’s cost varies, depending on geographic location and whether it is generic. According to GoodRX, a box containing two doses of generic naloxone costs $31-$100, depending on location and coupon availability.
A two-dose box of Narcan costs $135-$140. Emergent reported a 14% decline in naloxone sales in 2022 – to $373.7 million – blaming it in part on the introduction of generic formulations.
Dr. Hurley said he expects those who purchase Narcan at a drug store will primarily already be shopping there. It may or may not be those who most often experience overdose, such as people leaving incarceration or experiencing homelessness.
Having Narcan available over-the-counter “is an important supplement but it doesn’t replace the existing array of naloxone distribution programs,” Dr. Hurley said.
The FDA has encouraged naloxone manufacturers to seek OTC approval for the medication since at least 2019, when it designed a model label for a theoretical OTC product.
In November, the agency said it had determined that some naloxone products had the potential to be safe and effective for OTC use and again urged drugmakers to seek such an approval.
Emergent BioSolutions was the first to pursue OTC approval, but another manufacturer – the nonprofit Harm Reduction Therapeutics – is awaiting approval of its application to sell its spray directly to consumers.
Scott Gottlieb, MD, who was the FDA commissioner from 2017 to 2019, said in a tweet that more work needed to be done.
“This regulatory move should be followed by a strong push by elected officials to support wider deployment of Narcan, getting more doses into the hands of at risk households and frontline workers,” he tweeted.
Mr. Ingoglia said that “Narcan represents a second chance. By giving people a second chance, we also give them an opportunity to enter treatment if they so choose. You can’t recover if you’re dead, and we shouldn’t turn our backs on those who may choose a pathway to recovery that includes treatment.”
A version of this article first appeared on Medscape.com.
Greater access to the drug should mean more lives saved. However, it’s unclear how much the nasal spray will cost and whether pharmacies will stock the product openly on shelves.
Currently, major pharmacy chains such as CVS and Walgreens make naloxone available without prescription, but consumers have to ask a pharmacist to dispense the drug.
“The major question is what is it going to cost,” Brian Hurley, MD, MBA, president-elect of the American Society of Addiction Medicine, said in an interview. “In order for people to access it they have to be able to afford it.”
“We won’t accomplish much if people can’t afford to buy Narcan,” said Chuck Ingoglia, president and CEO of the National Council for Mental Wellbeing, in a statement. Still, he applauded the FDA.
“No single approach will end overdose deaths but making Narcan easy to obtain and widely available likely will save countless lives annually,” he said.
“The timeline for availability and price of this OTC product is determined by the manufacturer,” the FDA said in a statement.
Commissioner Robert M. Califf, MD, called for the drug’s manufacturer to “make accessibility to the product a priority by making it available as soon as possible and at an affordable price.”
Emergent BioSolutions did not comment on cost. It said in a statement that the spray “will be available on U.S. shelves and at online retailers by the late summer,” after it has adapted Narcan for direct-to-consumer use, including more consumer-oriented packaging.
Naloxone’s cost varies, depending on geographic location and whether it is generic. According to GoodRX, a box containing two doses of generic naloxone costs $31-$100, depending on location and coupon availability.
A two-dose box of Narcan costs $135-$140. Emergent reported a 14% decline in naloxone sales in 2022 – to $373.7 million – blaming it in part on the introduction of generic formulations.
Dr. Hurley said he expects those who purchase Narcan at a drug store will primarily already be shopping there. It may or may not be those who most often experience overdose, such as people leaving incarceration or experiencing homelessness.
Having Narcan available over-the-counter “is an important supplement but it doesn’t replace the existing array of naloxone distribution programs,” Dr. Hurley said.
The FDA has encouraged naloxone manufacturers to seek OTC approval for the medication since at least 2019, when it designed a model label for a theoretical OTC product.
In November, the agency said it had determined that some naloxone products had the potential to be safe and effective for OTC use and again urged drugmakers to seek such an approval.
Emergent BioSolutions was the first to pursue OTC approval, but another manufacturer – the nonprofit Harm Reduction Therapeutics – is awaiting approval of its application to sell its spray directly to consumers.
Scott Gottlieb, MD, who was the FDA commissioner from 2017 to 2019, said in a tweet that more work needed to be done.
“This regulatory move should be followed by a strong push by elected officials to support wider deployment of Narcan, getting more doses into the hands of at risk households and frontline workers,” he tweeted.
Mr. Ingoglia said that “Narcan represents a second chance. By giving people a second chance, we also give them an opportunity to enter treatment if they so choose. You can’t recover if you’re dead, and we shouldn’t turn our backs on those who may choose a pathway to recovery that includes treatment.”
A version of this article first appeared on Medscape.com.
Greater access to the drug should mean more lives saved. However, it’s unclear how much the nasal spray will cost and whether pharmacies will stock the product openly on shelves.
Currently, major pharmacy chains such as CVS and Walgreens make naloxone available without prescription, but consumers have to ask a pharmacist to dispense the drug.
“The major question is what is it going to cost,” Brian Hurley, MD, MBA, president-elect of the American Society of Addiction Medicine, said in an interview. “In order for people to access it they have to be able to afford it.”
“We won’t accomplish much if people can’t afford to buy Narcan,” said Chuck Ingoglia, president and CEO of the National Council for Mental Wellbeing, in a statement. Still, he applauded the FDA.
“No single approach will end overdose deaths but making Narcan easy to obtain and widely available likely will save countless lives annually,” he said.
“The timeline for availability and price of this OTC product is determined by the manufacturer,” the FDA said in a statement.
Commissioner Robert M. Califf, MD, called for the drug’s manufacturer to “make accessibility to the product a priority by making it available as soon as possible and at an affordable price.”
Emergent BioSolutions did not comment on cost. It said in a statement that the spray “will be available on U.S. shelves and at online retailers by the late summer,” after it has adapted Narcan for direct-to-consumer use, including more consumer-oriented packaging.
Naloxone’s cost varies, depending on geographic location and whether it is generic. According to GoodRX, a box containing two doses of generic naloxone costs $31-$100, depending on location and coupon availability.
A two-dose box of Narcan costs $135-$140. Emergent reported a 14% decline in naloxone sales in 2022 – to $373.7 million – blaming it in part on the introduction of generic formulations.
Dr. Hurley said he expects those who purchase Narcan at a drug store will primarily already be shopping there. It may or may not be those who most often experience overdose, such as people leaving incarceration or experiencing homelessness.
Having Narcan available over-the-counter “is an important supplement but it doesn’t replace the existing array of naloxone distribution programs,” Dr. Hurley said.
The FDA has encouraged naloxone manufacturers to seek OTC approval for the medication since at least 2019, when it designed a model label for a theoretical OTC product.
In November, the agency said it had determined that some naloxone products had the potential to be safe and effective for OTC use and again urged drugmakers to seek such an approval.
Emergent BioSolutions was the first to pursue OTC approval, but another manufacturer – the nonprofit Harm Reduction Therapeutics – is awaiting approval of its application to sell its spray directly to consumers.
Scott Gottlieb, MD, who was the FDA commissioner from 2017 to 2019, said in a tweet that more work needed to be done.
“This regulatory move should be followed by a strong push by elected officials to support wider deployment of Narcan, getting more doses into the hands of at risk households and frontline workers,” he tweeted.
Mr. Ingoglia said that “Narcan represents a second chance. By giving people a second chance, we also give them an opportunity to enter treatment if they so choose. You can’t recover if you’re dead, and we shouldn’t turn our backs on those who may choose a pathway to recovery that includes treatment.”
A version of this article first appeared on Medscape.com.
Watch for buprenorphine ‘spiking’ in urine drug tests
Urine drug testing can be valuable for monitoring patients undergoing treatment with buprenorphine for opioid use disorder (OUD). However, some patients alter their test results by adding buprenorphine directly to their urine sample to imply adherence, a new study shows.
“I anticipate a much-needed increase” in the number of people gaining access to buprenorphine therapy, given elimination of the X waiver, first author Jarratt D. Pytell, MD, with University of Colorado at Denver, Aurora, said in a statement.
“New prescribers of buprenorphine will need to learn how to conduct the increasingly complex initiation of treatment and then gauge whether it is successful or not,” added Dr. Pytell, a general internist and addiction medicine specialist.
“Spiking suggests that treatment is not working – especially in patients continuing illicit drug use. Detecting spiking allows clinicians to adjust or intensify the treatment plan,” Dr. Pytell said in an interview.
The study was published online in JAMA Psychiatry.
A sign of elevated patient risk
In a cross-sectional study using Millennium Health’s proprietary urine drug test (UDT) database, researchers analyzed 507,735 urine specimens from 58,476 OUD patients collected between January 2017 and April 2022.
A total of 9546 (1.9%) specimens from 4,550 patients (7.6%) were suggestive of spiking.
UDT specimens suggestive of spiking had two times the odds of being positive for other opioids (fentanyl or heroin), compared with opioid negative samples.
UDT specimens obtained from primary care clinics, from patients aged 35-44 years, and from patients living in the South Atlantic region of the United States were also more likely to be suggestive of buprenorphine spiking.
“Our study demonstrated that a buprenorphine to norbuprenorphine ratio of less than 0.02 indicates the possibility of spiking,” Dr. Pytell said in an interview.
“Nevertheless, it is important to note that this cutoff is not a definitive standard and further controlled studies are necessary to determine its predictive value for spiking. But recognizing possible spiking is very important since it demonstrates a point of elevated risk for the patient and the treatment approach should be reconsidered,” Dr. Pytell said.
“At Millennium Health, we have been tracking the enormity of the drug use crisis. This study suggests that spiking is an important patient safety issue, and it is not uncommon,” study coauthor Eric Dawson, PharmD, vice president of clinical affairs, Millennium Health, said in a statement.
“Detection of spiking requires definitive drug testing. Immunoassay-based, point-of-care tests cannot detect spiking because they are generally incapable of quantitative analysis and differentiating buprenorphine from norbuprenorphine,” Dr. Dawson said.
Best practices?
“We need to develop best practices specific for this situation where a patient has added buprenorphine to the urine drug test specimen,” said Dr. Pytell.
“As with all unexpected findings, it is crucial for clinicians to approach this finding in a nonjudgmental manner and work with the patient to understand what might have motivated them to alter their urine specimen,” he added.
Dr. Pytell said a common reaction for clinicians might be to discontinue treatment. However, this is actually a time to try and engage with the patient.
“Clinicians should work collaboratively with patients to identify potential reasons for spiking and determine what changes may need to be made to better support the patient’s recovery,” Dr. Pytell said.
“This could include more frequent monitoring or referral to a higher level of care. In addition, clinicians should be aware that patients who engage in spiking may be experiencing other challenges that impact their ability to adhere to treatment, such as inadequate housing, mental health issues, or financial strain. Addressing these underlying issues may help patients overcome barriers to treatment adherence and reduce the likelihood of future spiking,” Dr. Pytell said.
This study was supported by Millennium Health. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Urine drug testing can be valuable for monitoring patients undergoing treatment with buprenorphine for opioid use disorder (OUD). However, some patients alter their test results by adding buprenorphine directly to their urine sample to imply adherence, a new study shows.
“I anticipate a much-needed increase” in the number of people gaining access to buprenorphine therapy, given elimination of the X waiver, first author Jarratt D. Pytell, MD, with University of Colorado at Denver, Aurora, said in a statement.
“New prescribers of buprenorphine will need to learn how to conduct the increasingly complex initiation of treatment and then gauge whether it is successful or not,” added Dr. Pytell, a general internist and addiction medicine specialist.
“Spiking suggests that treatment is not working – especially in patients continuing illicit drug use. Detecting spiking allows clinicians to adjust or intensify the treatment plan,” Dr. Pytell said in an interview.
The study was published online in JAMA Psychiatry.
A sign of elevated patient risk
In a cross-sectional study using Millennium Health’s proprietary urine drug test (UDT) database, researchers analyzed 507,735 urine specimens from 58,476 OUD patients collected between January 2017 and April 2022.
A total of 9546 (1.9%) specimens from 4,550 patients (7.6%) were suggestive of spiking.
UDT specimens suggestive of spiking had two times the odds of being positive for other opioids (fentanyl or heroin), compared with opioid negative samples.
UDT specimens obtained from primary care clinics, from patients aged 35-44 years, and from patients living in the South Atlantic region of the United States were also more likely to be suggestive of buprenorphine spiking.
“Our study demonstrated that a buprenorphine to norbuprenorphine ratio of less than 0.02 indicates the possibility of spiking,” Dr. Pytell said in an interview.
“Nevertheless, it is important to note that this cutoff is not a definitive standard and further controlled studies are necessary to determine its predictive value for spiking. But recognizing possible spiking is very important since it demonstrates a point of elevated risk for the patient and the treatment approach should be reconsidered,” Dr. Pytell said.
“At Millennium Health, we have been tracking the enormity of the drug use crisis. This study suggests that spiking is an important patient safety issue, and it is not uncommon,” study coauthor Eric Dawson, PharmD, vice president of clinical affairs, Millennium Health, said in a statement.
“Detection of spiking requires definitive drug testing. Immunoassay-based, point-of-care tests cannot detect spiking because they are generally incapable of quantitative analysis and differentiating buprenorphine from norbuprenorphine,” Dr. Dawson said.
Best practices?
“We need to develop best practices specific for this situation where a patient has added buprenorphine to the urine drug test specimen,” said Dr. Pytell.
“As with all unexpected findings, it is crucial for clinicians to approach this finding in a nonjudgmental manner and work with the patient to understand what might have motivated them to alter their urine specimen,” he added.
Dr. Pytell said a common reaction for clinicians might be to discontinue treatment. However, this is actually a time to try and engage with the patient.
“Clinicians should work collaboratively with patients to identify potential reasons for spiking and determine what changes may need to be made to better support the patient’s recovery,” Dr. Pytell said.
“This could include more frequent monitoring or referral to a higher level of care. In addition, clinicians should be aware that patients who engage in spiking may be experiencing other challenges that impact their ability to adhere to treatment, such as inadequate housing, mental health issues, or financial strain. Addressing these underlying issues may help patients overcome barriers to treatment adherence and reduce the likelihood of future spiking,” Dr. Pytell said.
This study was supported by Millennium Health. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Urine drug testing can be valuable for monitoring patients undergoing treatment with buprenorphine for opioid use disorder (OUD). However, some patients alter their test results by adding buprenorphine directly to their urine sample to imply adherence, a new study shows.
“I anticipate a much-needed increase” in the number of people gaining access to buprenorphine therapy, given elimination of the X waiver, first author Jarratt D. Pytell, MD, with University of Colorado at Denver, Aurora, said in a statement.
“New prescribers of buprenorphine will need to learn how to conduct the increasingly complex initiation of treatment and then gauge whether it is successful or not,” added Dr. Pytell, a general internist and addiction medicine specialist.
“Spiking suggests that treatment is not working – especially in patients continuing illicit drug use. Detecting spiking allows clinicians to adjust or intensify the treatment plan,” Dr. Pytell said in an interview.
The study was published online in JAMA Psychiatry.
A sign of elevated patient risk
In a cross-sectional study using Millennium Health’s proprietary urine drug test (UDT) database, researchers analyzed 507,735 urine specimens from 58,476 OUD patients collected between January 2017 and April 2022.
A total of 9546 (1.9%) specimens from 4,550 patients (7.6%) were suggestive of spiking.
UDT specimens suggestive of spiking had two times the odds of being positive for other opioids (fentanyl or heroin), compared with opioid negative samples.
UDT specimens obtained from primary care clinics, from patients aged 35-44 years, and from patients living in the South Atlantic region of the United States were also more likely to be suggestive of buprenorphine spiking.
“Our study demonstrated that a buprenorphine to norbuprenorphine ratio of less than 0.02 indicates the possibility of spiking,” Dr. Pytell said in an interview.
“Nevertheless, it is important to note that this cutoff is not a definitive standard and further controlled studies are necessary to determine its predictive value for spiking. But recognizing possible spiking is very important since it demonstrates a point of elevated risk for the patient and the treatment approach should be reconsidered,” Dr. Pytell said.
“At Millennium Health, we have been tracking the enormity of the drug use crisis. This study suggests that spiking is an important patient safety issue, and it is not uncommon,” study coauthor Eric Dawson, PharmD, vice president of clinical affairs, Millennium Health, said in a statement.
“Detection of spiking requires definitive drug testing. Immunoassay-based, point-of-care tests cannot detect spiking because they are generally incapable of quantitative analysis and differentiating buprenorphine from norbuprenorphine,” Dr. Dawson said.
Best practices?
“We need to develop best practices specific for this situation where a patient has added buprenorphine to the urine drug test specimen,” said Dr. Pytell.
“As with all unexpected findings, it is crucial for clinicians to approach this finding in a nonjudgmental manner and work with the patient to understand what might have motivated them to alter their urine specimen,” he added.
Dr. Pytell said a common reaction for clinicians might be to discontinue treatment. However, this is actually a time to try and engage with the patient.
“Clinicians should work collaboratively with patients to identify potential reasons for spiking and determine what changes may need to be made to better support the patient’s recovery,” Dr. Pytell said.
“This could include more frequent monitoring or referral to a higher level of care. In addition, clinicians should be aware that patients who engage in spiking may be experiencing other challenges that impact their ability to adhere to treatment, such as inadequate housing, mental health issues, or financial strain. Addressing these underlying issues may help patients overcome barriers to treatment adherence and reduce the likelihood of future spiking,” Dr. Pytell said.
This study was supported by Millennium Health. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Substance abuse disorders may share a common genetic signature
suggest new findings that researchers say could eventually lead to universal therapies to treat multiple and comorbid addictions.
“Genetics play a key role in determining health throughout our lives, but they are not destiny. Our hope with genomic studies is to further illuminate factors that may protect or predispose a person to substance use disorders – knowledge that can be used to expand preventative services and empower individuals to make informed decisions about drug use,” Nora Volkow, MD, director of the National Institute on Drug Abuse, said in news release.
“A better understanding of genetics also brings us one step closer to developing personalized interventions that are tailored to an individual’s unique biology, environment, and lived experience in order to provide the most benefits,” Dr. Volkow added.
The research was published online in Nature Mental Health.
Global research
Led by a team at the Washington University in St. Louis, the study included more than 150 collaborating investigators from around the world.
The risk of developing SUDs is influenced by a complex interplay between genetics and environmental factors. In a genomewide association study, the investigators looked for variations in the genome that were closely associated with SUDs in more than 1 million people of European ancestry and 92,630 people of African ancestry.
Among the European ancestry sample, they discovered 19 single-nucleotide polymorphisms that were significantly associated with general addiction risk and 47 genetic variants linked to specific SUDs – 9 for alcohol, 32 for tobacco, 5 for cannabis, and 1 for opioids.
The strongest gene signals consistent across the various SUDs mapped to areas in the genome involved in dopamine-signaling regulation, which reinforces the role of the dopamine system in addiction.
The genomic pattern also predicted higher risk of mental and physical illness, including psychiatric disorders, suicidal behavior, respiratory disease, heart disease, and chronic pain conditions. In children aged 9 or 10 years, presumably without any SUD, these genes correlated with parental substance use and externalizing behavior.
“Substance use disorders and mental disorders often co-occur, and we know that the most effective treatments help people address both issues at the same time. The shared genetic mechanisms between substance use and mental disorders revealed in this study underscore the importance of thinking about these disorders in tandem,” Joshua A. Gordon, MD, PhD, director of the National Institute of Mental Health, said in a news release.
Repurpose existing drugs for SUDs?
Separately, the genomic analysis of individuals of African ancestry showed only one genetic variation associated with general addiction risk and one substance-specific variation for risk of alcohol use disorder. The smaller sample size may be one reason for the more limited findings in this population.
“There is a tremendous need for treatments that target addiction generally, given patterns of the use of multiple substances, lifetime substance use, and severity seen in the clinic,” lead researcher Alexander Hatoum, PhD, at Washington University in St. Louis, said in a news release.
“Our study opens the door to identifying medications that may be leveraged to treat addiction broadly, which may be especially useful for treating more severe forms, including addiction to multiple substances,” Dr. Hatoum added.
As part of the study, the researchers compiled a list of approved and investigational pharmaceutical drugs that could potentially be repurposed to treat SUDs.
The list includes more than 100 drugs to investigate in future clinical trials, including those that can influence regulation of dopamine signaling.
This research was supported by NIDA, the National Institute on Alcohol Abuse and Alcoholism, NIMH, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
suggest new findings that researchers say could eventually lead to universal therapies to treat multiple and comorbid addictions.
“Genetics play a key role in determining health throughout our lives, but they are not destiny. Our hope with genomic studies is to further illuminate factors that may protect or predispose a person to substance use disorders – knowledge that can be used to expand preventative services and empower individuals to make informed decisions about drug use,” Nora Volkow, MD, director of the National Institute on Drug Abuse, said in news release.
“A better understanding of genetics also brings us one step closer to developing personalized interventions that are tailored to an individual’s unique biology, environment, and lived experience in order to provide the most benefits,” Dr. Volkow added.
The research was published online in Nature Mental Health.
Global research
Led by a team at the Washington University in St. Louis, the study included more than 150 collaborating investigators from around the world.
The risk of developing SUDs is influenced by a complex interplay between genetics and environmental factors. In a genomewide association study, the investigators looked for variations in the genome that were closely associated with SUDs in more than 1 million people of European ancestry and 92,630 people of African ancestry.
Among the European ancestry sample, they discovered 19 single-nucleotide polymorphisms that were significantly associated with general addiction risk and 47 genetic variants linked to specific SUDs – 9 for alcohol, 32 for tobacco, 5 for cannabis, and 1 for opioids.
The strongest gene signals consistent across the various SUDs mapped to areas in the genome involved in dopamine-signaling regulation, which reinforces the role of the dopamine system in addiction.
The genomic pattern also predicted higher risk of mental and physical illness, including psychiatric disorders, suicidal behavior, respiratory disease, heart disease, and chronic pain conditions. In children aged 9 or 10 years, presumably without any SUD, these genes correlated with parental substance use and externalizing behavior.
“Substance use disorders and mental disorders often co-occur, and we know that the most effective treatments help people address both issues at the same time. The shared genetic mechanisms between substance use and mental disorders revealed in this study underscore the importance of thinking about these disorders in tandem,” Joshua A. Gordon, MD, PhD, director of the National Institute of Mental Health, said in a news release.
Repurpose existing drugs for SUDs?
Separately, the genomic analysis of individuals of African ancestry showed only one genetic variation associated with general addiction risk and one substance-specific variation for risk of alcohol use disorder. The smaller sample size may be one reason for the more limited findings in this population.
“There is a tremendous need for treatments that target addiction generally, given patterns of the use of multiple substances, lifetime substance use, and severity seen in the clinic,” lead researcher Alexander Hatoum, PhD, at Washington University in St. Louis, said in a news release.
“Our study opens the door to identifying medications that may be leveraged to treat addiction broadly, which may be especially useful for treating more severe forms, including addiction to multiple substances,” Dr. Hatoum added.
As part of the study, the researchers compiled a list of approved and investigational pharmaceutical drugs that could potentially be repurposed to treat SUDs.
The list includes more than 100 drugs to investigate in future clinical trials, including those that can influence regulation of dopamine signaling.
This research was supported by NIDA, the National Institute on Alcohol Abuse and Alcoholism, NIMH, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
suggest new findings that researchers say could eventually lead to universal therapies to treat multiple and comorbid addictions.
“Genetics play a key role in determining health throughout our lives, but they are not destiny. Our hope with genomic studies is to further illuminate factors that may protect or predispose a person to substance use disorders – knowledge that can be used to expand preventative services and empower individuals to make informed decisions about drug use,” Nora Volkow, MD, director of the National Institute on Drug Abuse, said in news release.
“A better understanding of genetics also brings us one step closer to developing personalized interventions that are tailored to an individual’s unique biology, environment, and lived experience in order to provide the most benefits,” Dr. Volkow added.
The research was published online in Nature Mental Health.
Global research
Led by a team at the Washington University in St. Louis, the study included more than 150 collaborating investigators from around the world.
The risk of developing SUDs is influenced by a complex interplay between genetics and environmental factors. In a genomewide association study, the investigators looked for variations in the genome that were closely associated with SUDs in more than 1 million people of European ancestry and 92,630 people of African ancestry.
Among the European ancestry sample, they discovered 19 single-nucleotide polymorphisms that were significantly associated with general addiction risk and 47 genetic variants linked to specific SUDs – 9 for alcohol, 32 for tobacco, 5 for cannabis, and 1 for opioids.
The strongest gene signals consistent across the various SUDs mapped to areas in the genome involved in dopamine-signaling regulation, which reinforces the role of the dopamine system in addiction.
The genomic pattern also predicted higher risk of mental and physical illness, including psychiatric disorders, suicidal behavior, respiratory disease, heart disease, and chronic pain conditions. In children aged 9 or 10 years, presumably without any SUD, these genes correlated with parental substance use and externalizing behavior.
“Substance use disorders and mental disorders often co-occur, and we know that the most effective treatments help people address both issues at the same time. The shared genetic mechanisms between substance use and mental disorders revealed in this study underscore the importance of thinking about these disorders in tandem,” Joshua A. Gordon, MD, PhD, director of the National Institute of Mental Health, said in a news release.
Repurpose existing drugs for SUDs?
Separately, the genomic analysis of individuals of African ancestry showed only one genetic variation associated with general addiction risk and one substance-specific variation for risk of alcohol use disorder. The smaller sample size may be one reason for the more limited findings in this population.
“There is a tremendous need for treatments that target addiction generally, given patterns of the use of multiple substances, lifetime substance use, and severity seen in the clinic,” lead researcher Alexander Hatoum, PhD, at Washington University in St. Louis, said in a news release.
“Our study opens the door to identifying medications that may be leveraged to treat addiction broadly, which may be especially useful for treating more severe forms, including addiction to multiple substances,” Dr. Hatoum added.
As part of the study, the researchers compiled a list of approved and investigational pharmaceutical drugs that could potentially be repurposed to treat SUDs.
The list includes more than 100 drugs to investigate in future clinical trials, including those that can influence regulation of dopamine signaling.
This research was supported by NIDA, the National Institute on Alcohol Abuse and Alcoholism, NIMH, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
FROM NATURE MENTAL HEALTH
Opioid overdose is an important cause of postpartum death
(OUD), according to research published in Obstetrics and Gynecology.
Opioid overdose deaths account for up to 10% of pregnancy-associated deaths in the United States, and 75% of the deliveries of women with OUD are covered by Medicaid, according to lead author Elizabeth Suarez, PhD, MPH, with the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital and Harvard Medical School in Boston, and colleagues.
Nearly 5 million deliveries studied
Researchers studied claims data from Medicaid and the National Death Index database in the United States from 2006 to 2013 for 4,972,061 deliveries. They also identified a subgroup of women with a documented history of OUD in the 3 months before delivery.
They found the incidence of postpartum opioid overdose deaths was 5.4 per 100,000 deliveries (95% confidence interval, 4.5-6.4) among all in the study and 118 per 100,000 (95% CI, 84-163) among individuals with OUD.
Incidence of all-cause postpartum death was six times higher in women with OUD than in all the women studied. Common causes of death of those with OUD were other drug- and alcohol-related deaths (47/100,000); suicide (26/100,000); and other injuries, including accidents and falls (33/100,000).
Risk factors strongly linked with postpartum opioid overdose death included mental health and other substance use disorders.
Medication significantly lowers death risk
The authors also documented the benefit of buprenorphine or methadone for OUD.
For women with OUD who used medication to treat OUD post partum, odds of opioid overdose death were 60% lower (odds ratio, 0.4; 95% CI 0.1-0.9).
As important as use of medication, Marcela Smid, MD, MS, writes in an accompanying editorial, is noting that 80% of the women in this study who died of opioid overdoses had contact with a health care provider before death.
“Both of these results indicate that we have the means and opportunity to prevent these deaths,” writes Dr. Smid, with the division of maternal fetal medicine, University of Utah Health in Salt Lake City.
Dismal numbers on ob.gyns. trained to prescribe medications
She points out some barriers, however. Most clinicians, she notes, lack time and training to prescribe buprenorphine, and in 2019, fewer than 2% of ob.gyns. who accept Medicaid were able to prescribe it.
Her charge to ob.gyns.: “We need to help identify individuals who are at high risk of OUD or opioid overdose by screening.” A validated screening tool should be used at prenatal and postpartum appointments.
On a bigger scale, she urges Medicaid to be expanded for a full year post partum through the American Rescue Act’s State Plan Amendment, something only 28 states and Washington, D.C., have done so far.
Dr. Smid points out some good news, however: President Joe Biden signed the Consolidated Appropriations Act 2023, which eliminated the “X” waiver.
Now all clinicians who have a Drug Enforcement Administration registration that includes Schedule III authority can prescribe buprenorphine for OUD if applicable state law allows it.
But that calls for medical schools and residency programs to prioritize addiction medicine as a core competency, Dr. Smid says.
Getting naloxone to patients, families
One of the potential interventions the study authors suggest is providing naloxone prescriptions and training to pregnant and postpartum women who have a substance use history and to their partners and significant others.
However, Mishka Terplan, MD, MPH, told this publication, “It’s one thing to write a prescription; it’s another thing for the person to actually get the medication.” He is medical director of the Friends Research Institute in Baltimore, an ob.gyn. who specializes in addiction medicine.
“What can we do?” We can think about how to get naloxone into people’s hands at discharge from the hospital after they give birth, instead of prescribing. That would mean that health systems need to prioritize this, he said. “We give people discharge medications all the time.”
Still, naloxone can’t be seen as the answer, he said.
He compares it to defibrillators in public places, which are for rescues, not reversing a population problem.
“Some people think that naloxone reversals are doing something about OUD. It’s doing about as much about OUD as defibrillators do for cardiovascular disease,” he said.
The best help, he says, will be continuation of treatment.
“Addiction is a chronic condition,” he says, “but often we only provide episodic care. We see that particularly in pregnancy. Once the pregnancy is finished, there’s not categorical continuation of insurance.”
Even if you do have insurance, it’s hard to find a clinic that’s family friendly, he notes. “You might not feel comfortable taking your newborn and standing in line in the morning to get your daily methodone dose. We have to make those environments more welcoming.”
Problem probably understated
He also says that though the study was well done given the data available, he’s frustrated that researchers still have to depend on billing data and can’t capture factors such as child care availability, living wages, and continuation of health insurance. Additionally, not everyone is coded correctly for OUD.
“It’s all Medicaid, so it’s only people who continued with care,” he pointed out. That means these numbers may actually underrepresent the problem.
Still, he says it’s important to realize the magnitude of deaths this study does highlight in this population.
In people with OUD in the postpartum period, the deaths are more than 1 in 1,000.
“That should be alarming,” Dr. Terplan said. “That’s a very big number from a public health perspective.”
Coauthor Kathryn J. Gray received payment from Aetion Inc., Roche, and BillionToOne. Funds were paid to the University of Utah for Dr. Smid from Alydia Inc. for being the site principal investigator for a study of the JADA device, and from Gilead for Dr. Smid’s study of hepatitis C in pregnancy; she was also a consultant for Organon and Rhia Ventures. Dr. Terplan reports no relevant financial relationships.
(OUD), according to research published in Obstetrics and Gynecology.
Opioid overdose deaths account for up to 10% of pregnancy-associated deaths in the United States, and 75% of the deliveries of women with OUD are covered by Medicaid, according to lead author Elizabeth Suarez, PhD, MPH, with the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital and Harvard Medical School in Boston, and colleagues.
Nearly 5 million deliveries studied
Researchers studied claims data from Medicaid and the National Death Index database in the United States from 2006 to 2013 for 4,972,061 deliveries. They also identified a subgroup of women with a documented history of OUD in the 3 months before delivery.
They found the incidence of postpartum opioid overdose deaths was 5.4 per 100,000 deliveries (95% confidence interval, 4.5-6.4) among all in the study and 118 per 100,000 (95% CI, 84-163) among individuals with OUD.
Incidence of all-cause postpartum death was six times higher in women with OUD than in all the women studied. Common causes of death of those with OUD were other drug- and alcohol-related deaths (47/100,000); suicide (26/100,000); and other injuries, including accidents and falls (33/100,000).
Risk factors strongly linked with postpartum opioid overdose death included mental health and other substance use disorders.
Medication significantly lowers death risk
The authors also documented the benefit of buprenorphine or methadone for OUD.
For women with OUD who used medication to treat OUD post partum, odds of opioid overdose death were 60% lower (odds ratio, 0.4; 95% CI 0.1-0.9).
As important as use of medication, Marcela Smid, MD, MS, writes in an accompanying editorial, is noting that 80% of the women in this study who died of opioid overdoses had contact with a health care provider before death.
“Both of these results indicate that we have the means and opportunity to prevent these deaths,” writes Dr. Smid, with the division of maternal fetal medicine, University of Utah Health in Salt Lake City.
Dismal numbers on ob.gyns. trained to prescribe medications
She points out some barriers, however. Most clinicians, she notes, lack time and training to prescribe buprenorphine, and in 2019, fewer than 2% of ob.gyns. who accept Medicaid were able to prescribe it.
Her charge to ob.gyns.: “We need to help identify individuals who are at high risk of OUD or opioid overdose by screening.” A validated screening tool should be used at prenatal and postpartum appointments.
On a bigger scale, she urges Medicaid to be expanded for a full year post partum through the American Rescue Act’s State Plan Amendment, something only 28 states and Washington, D.C., have done so far.
Dr. Smid points out some good news, however: President Joe Biden signed the Consolidated Appropriations Act 2023, which eliminated the “X” waiver.
Now all clinicians who have a Drug Enforcement Administration registration that includes Schedule III authority can prescribe buprenorphine for OUD if applicable state law allows it.
But that calls for medical schools and residency programs to prioritize addiction medicine as a core competency, Dr. Smid says.
Getting naloxone to patients, families
One of the potential interventions the study authors suggest is providing naloxone prescriptions and training to pregnant and postpartum women who have a substance use history and to their partners and significant others.
However, Mishka Terplan, MD, MPH, told this publication, “It’s one thing to write a prescription; it’s another thing for the person to actually get the medication.” He is medical director of the Friends Research Institute in Baltimore, an ob.gyn. who specializes in addiction medicine.
“What can we do?” We can think about how to get naloxone into people’s hands at discharge from the hospital after they give birth, instead of prescribing. That would mean that health systems need to prioritize this, he said. “We give people discharge medications all the time.”
Still, naloxone can’t be seen as the answer, he said.
He compares it to defibrillators in public places, which are for rescues, not reversing a population problem.
“Some people think that naloxone reversals are doing something about OUD. It’s doing about as much about OUD as defibrillators do for cardiovascular disease,” he said.
The best help, he says, will be continuation of treatment.
“Addiction is a chronic condition,” he says, “but often we only provide episodic care. We see that particularly in pregnancy. Once the pregnancy is finished, there’s not categorical continuation of insurance.”
Even if you do have insurance, it’s hard to find a clinic that’s family friendly, he notes. “You might not feel comfortable taking your newborn and standing in line in the morning to get your daily methodone dose. We have to make those environments more welcoming.”
Problem probably understated
He also says that though the study was well done given the data available, he’s frustrated that researchers still have to depend on billing data and can’t capture factors such as child care availability, living wages, and continuation of health insurance. Additionally, not everyone is coded correctly for OUD.
“It’s all Medicaid, so it’s only people who continued with care,” he pointed out. That means these numbers may actually underrepresent the problem.
Still, he says it’s important to realize the magnitude of deaths this study does highlight in this population.
In people with OUD in the postpartum period, the deaths are more than 1 in 1,000.
“That should be alarming,” Dr. Terplan said. “That’s a very big number from a public health perspective.”
Coauthor Kathryn J. Gray received payment from Aetion Inc., Roche, and BillionToOne. Funds were paid to the University of Utah for Dr. Smid from Alydia Inc. for being the site principal investigator for a study of the JADA device, and from Gilead for Dr. Smid’s study of hepatitis C in pregnancy; she was also a consultant for Organon and Rhia Ventures. Dr. Terplan reports no relevant financial relationships.
(OUD), according to research published in Obstetrics and Gynecology.
Opioid overdose deaths account for up to 10% of pregnancy-associated deaths in the United States, and 75% of the deliveries of women with OUD are covered by Medicaid, according to lead author Elizabeth Suarez, PhD, MPH, with the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital and Harvard Medical School in Boston, and colleagues.
Nearly 5 million deliveries studied
Researchers studied claims data from Medicaid and the National Death Index database in the United States from 2006 to 2013 for 4,972,061 deliveries. They also identified a subgroup of women with a documented history of OUD in the 3 months before delivery.
They found the incidence of postpartum opioid overdose deaths was 5.4 per 100,000 deliveries (95% confidence interval, 4.5-6.4) among all in the study and 118 per 100,000 (95% CI, 84-163) among individuals with OUD.
Incidence of all-cause postpartum death was six times higher in women with OUD than in all the women studied. Common causes of death of those with OUD were other drug- and alcohol-related deaths (47/100,000); suicide (26/100,000); and other injuries, including accidents and falls (33/100,000).
Risk factors strongly linked with postpartum opioid overdose death included mental health and other substance use disorders.
Medication significantly lowers death risk
The authors also documented the benefit of buprenorphine or methadone for OUD.
For women with OUD who used medication to treat OUD post partum, odds of opioid overdose death were 60% lower (odds ratio, 0.4; 95% CI 0.1-0.9).
As important as use of medication, Marcela Smid, MD, MS, writes in an accompanying editorial, is noting that 80% of the women in this study who died of opioid overdoses had contact with a health care provider before death.
“Both of these results indicate that we have the means and opportunity to prevent these deaths,” writes Dr. Smid, with the division of maternal fetal medicine, University of Utah Health in Salt Lake City.
Dismal numbers on ob.gyns. trained to prescribe medications
She points out some barriers, however. Most clinicians, she notes, lack time and training to prescribe buprenorphine, and in 2019, fewer than 2% of ob.gyns. who accept Medicaid were able to prescribe it.
Her charge to ob.gyns.: “We need to help identify individuals who are at high risk of OUD or opioid overdose by screening.” A validated screening tool should be used at prenatal and postpartum appointments.
On a bigger scale, she urges Medicaid to be expanded for a full year post partum through the American Rescue Act’s State Plan Amendment, something only 28 states and Washington, D.C., have done so far.
Dr. Smid points out some good news, however: President Joe Biden signed the Consolidated Appropriations Act 2023, which eliminated the “X” waiver.
Now all clinicians who have a Drug Enforcement Administration registration that includes Schedule III authority can prescribe buprenorphine for OUD if applicable state law allows it.
But that calls for medical schools and residency programs to prioritize addiction medicine as a core competency, Dr. Smid says.
Getting naloxone to patients, families
One of the potential interventions the study authors suggest is providing naloxone prescriptions and training to pregnant and postpartum women who have a substance use history and to their partners and significant others.
However, Mishka Terplan, MD, MPH, told this publication, “It’s one thing to write a prescription; it’s another thing for the person to actually get the medication.” He is medical director of the Friends Research Institute in Baltimore, an ob.gyn. who specializes in addiction medicine.
“What can we do?” We can think about how to get naloxone into people’s hands at discharge from the hospital after they give birth, instead of prescribing. That would mean that health systems need to prioritize this, he said. “We give people discharge medications all the time.”
Still, naloxone can’t be seen as the answer, he said.
He compares it to defibrillators in public places, which are for rescues, not reversing a population problem.
“Some people think that naloxone reversals are doing something about OUD. It’s doing about as much about OUD as defibrillators do for cardiovascular disease,” he said.
The best help, he says, will be continuation of treatment.
“Addiction is a chronic condition,” he says, “but often we only provide episodic care. We see that particularly in pregnancy. Once the pregnancy is finished, there’s not categorical continuation of insurance.”
Even if you do have insurance, it’s hard to find a clinic that’s family friendly, he notes. “You might not feel comfortable taking your newborn and standing in line in the morning to get your daily methodone dose. We have to make those environments more welcoming.”
Problem probably understated
He also says that though the study was well done given the data available, he’s frustrated that researchers still have to depend on billing data and can’t capture factors such as child care availability, living wages, and continuation of health insurance. Additionally, not everyone is coded correctly for OUD.
“It’s all Medicaid, so it’s only people who continued with care,” he pointed out. That means these numbers may actually underrepresent the problem.
Still, he says it’s important to realize the magnitude of deaths this study does highlight in this population.
In people with OUD in the postpartum period, the deaths are more than 1 in 1,000.
“That should be alarming,” Dr. Terplan said. “That’s a very big number from a public health perspective.”
Coauthor Kathryn J. Gray received payment from Aetion Inc., Roche, and BillionToOne. Funds were paid to the University of Utah for Dr. Smid from Alydia Inc. for being the site principal investigator for a study of the JADA device, and from Gilead for Dr. Smid’s study of hepatitis C in pregnancy; she was also a consultant for Organon and Rhia Ventures. Dr. Terplan reports no relevant financial relationships.
FROM OBSTETRICS AND GYNECOLOGY
FDA moves to stop the spread of illicit ‘tranq’ in the U.S.
The agency issued an import alert, which gives it the power to detain raw ingredients or bulk finished product if the shipments are suspected to be in violation of the law. Xylazine was first approved by the FDA in 1972 as a sedative and analgesic for use only in animals.
It is increasingly being detected and is usually mixed with fentanyl, cocaine, methamphetamine, and other illicit drugs. A January 2023 study by Nashville-based testing company Aegis Sciences found xylazine in 413 of about 60,000 urine samples and in 25 of 39 states that submitted tests. The vast majority of xylazine-positive samples also tested positive for fentanyl.
The FDA said it would continue to ensure the availability of xylazine for veterinary use, and the American Veterinary Medicine Association said in a statement that it “supports such efforts to combat illicit drug use.”
FDA Commissioner Robert M. Califf, MD, said in a statement that the agency “remains concerned about the increasing prevalence of xylazine mixed with illicit drugs, and this action is one part of broader efforts the agency is undertaking to address this issue.”
In November, the agency warned health care providers that because xylazine is not an opioid, the overdose reversal agent naloxone would not be effective. Xylazine acts as a central alpha-2-adrenergic receptor agonist in the brainstem, causing a rapid decrease in the release of norepinephrine and dopamine in the central nervous system. Its use can lead to central nervous system and respiratory depression, said the FDA.
Clinicians have scrambled to treat severe necrotic skin ulcerations that develop at injection sites.
Xylazine is relatively cheap and easy to access, said the Drug Enforcement Administration and Department of Justice in a November joint report. The drug is “readily available for purchase on other Internet sites in liquid and powder form, often with no association to the veterinary profession nor requirements to prove legitimate need,” said the Justice Department. A buyer can purchase xylazine powder online from Chinese suppliers for $6-$20 per kilogram, according to the report.
In 2021, xylazine-positive overdoses were highest in the South, which experienced a 1,127% increase from 2020, the Justice Department reported. The same year, there were 1,281 overdoses involving the substance in the Northeast and 351 in the Midwest.
There were just 34 overdoses involving xylazine in the West in 2021, but its use appears to be growing. The San Francisco Department of Public Health said it had detected low levels of xylazine in four people who died of overdoses in December and January.
“Identifying xylazine in San Francisco is concerning,” said the department in a statement, adding that it had not yet seen evidence of skin wounds in injection drug users in the city.
In late February, the Los Angeles County Department of Public Health issued a warning to first responders and health care professionals that xylazine had been detected in the area’s illicit drug supply.
The department said it will “work closely with other partners to understand the extent of the possible xylazine contamination in the illicit drug supply to increase awareness and education to the public.”
The FDA commissioner said the agency will coordinate with public health officials to more closely track xylazine.
“We will continue to use all tools at our disposal and partner with the Drug Enforcement Administration and other federal, state, local agencies, and stakeholders as appropriate to stem these illicit activities and protect public health,” said Dr. Califf.
A version of this article first appeared on Medscape.com.
The agency issued an import alert, which gives it the power to detain raw ingredients or bulk finished product if the shipments are suspected to be in violation of the law. Xylazine was first approved by the FDA in 1972 as a sedative and analgesic for use only in animals.
It is increasingly being detected and is usually mixed with fentanyl, cocaine, methamphetamine, and other illicit drugs. A January 2023 study by Nashville-based testing company Aegis Sciences found xylazine in 413 of about 60,000 urine samples and in 25 of 39 states that submitted tests. The vast majority of xylazine-positive samples also tested positive for fentanyl.
The FDA said it would continue to ensure the availability of xylazine for veterinary use, and the American Veterinary Medicine Association said in a statement that it “supports such efforts to combat illicit drug use.”
FDA Commissioner Robert M. Califf, MD, said in a statement that the agency “remains concerned about the increasing prevalence of xylazine mixed with illicit drugs, and this action is one part of broader efforts the agency is undertaking to address this issue.”
In November, the agency warned health care providers that because xylazine is not an opioid, the overdose reversal agent naloxone would not be effective. Xylazine acts as a central alpha-2-adrenergic receptor agonist in the brainstem, causing a rapid decrease in the release of norepinephrine and dopamine in the central nervous system. Its use can lead to central nervous system and respiratory depression, said the FDA.
Clinicians have scrambled to treat severe necrotic skin ulcerations that develop at injection sites.
Xylazine is relatively cheap and easy to access, said the Drug Enforcement Administration and Department of Justice in a November joint report. The drug is “readily available for purchase on other Internet sites in liquid and powder form, often with no association to the veterinary profession nor requirements to prove legitimate need,” said the Justice Department. A buyer can purchase xylazine powder online from Chinese suppliers for $6-$20 per kilogram, according to the report.
In 2021, xylazine-positive overdoses were highest in the South, which experienced a 1,127% increase from 2020, the Justice Department reported. The same year, there were 1,281 overdoses involving the substance in the Northeast and 351 in the Midwest.
There were just 34 overdoses involving xylazine in the West in 2021, but its use appears to be growing. The San Francisco Department of Public Health said it had detected low levels of xylazine in four people who died of overdoses in December and January.
“Identifying xylazine in San Francisco is concerning,” said the department in a statement, adding that it had not yet seen evidence of skin wounds in injection drug users in the city.
In late February, the Los Angeles County Department of Public Health issued a warning to first responders and health care professionals that xylazine had been detected in the area’s illicit drug supply.
The department said it will “work closely with other partners to understand the extent of the possible xylazine contamination in the illicit drug supply to increase awareness and education to the public.”
The FDA commissioner said the agency will coordinate with public health officials to more closely track xylazine.
“We will continue to use all tools at our disposal and partner with the Drug Enforcement Administration and other federal, state, local agencies, and stakeholders as appropriate to stem these illicit activities and protect public health,” said Dr. Califf.
A version of this article first appeared on Medscape.com.
The agency issued an import alert, which gives it the power to detain raw ingredients or bulk finished product if the shipments are suspected to be in violation of the law. Xylazine was first approved by the FDA in 1972 as a sedative and analgesic for use only in animals.
It is increasingly being detected and is usually mixed with fentanyl, cocaine, methamphetamine, and other illicit drugs. A January 2023 study by Nashville-based testing company Aegis Sciences found xylazine in 413 of about 60,000 urine samples and in 25 of 39 states that submitted tests. The vast majority of xylazine-positive samples also tested positive for fentanyl.
The FDA said it would continue to ensure the availability of xylazine for veterinary use, and the American Veterinary Medicine Association said in a statement that it “supports such efforts to combat illicit drug use.”
FDA Commissioner Robert M. Califf, MD, said in a statement that the agency “remains concerned about the increasing prevalence of xylazine mixed with illicit drugs, and this action is one part of broader efforts the agency is undertaking to address this issue.”
In November, the agency warned health care providers that because xylazine is not an opioid, the overdose reversal agent naloxone would not be effective. Xylazine acts as a central alpha-2-adrenergic receptor agonist in the brainstem, causing a rapid decrease in the release of norepinephrine and dopamine in the central nervous system. Its use can lead to central nervous system and respiratory depression, said the FDA.
Clinicians have scrambled to treat severe necrotic skin ulcerations that develop at injection sites.
Xylazine is relatively cheap and easy to access, said the Drug Enforcement Administration and Department of Justice in a November joint report. The drug is “readily available for purchase on other Internet sites in liquid and powder form, often with no association to the veterinary profession nor requirements to prove legitimate need,” said the Justice Department. A buyer can purchase xylazine powder online from Chinese suppliers for $6-$20 per kilogram, according to the report.
In 2021, xylazine-positive overdoses were highest in the South, which experienced a 1,127% increase from 2020, the Justice Department reported. The same year, there were 1,281 overdoses involving the substance in the Northeast and 351 in the Midwest.
There were just 34 overdoses involving xylazine in the West in 2021, but its use appears to be growing. The San Francisco Department of Public Health said it had detected low levels of xylazine in four people who died of overdoses in December and January.
“Identifying xylazine in San Francisco is concerning,” said the department in a statement, adding that it had not yet seen evidence of skin wounds in injection drug users in the city.
In late February, the Los Angeles County Department of Public Health issued a warning to first responders and health care professionals that xylazine had been detected in the area’s illicit drug supply.
The department said it will “work closely with other partners to understand the extent of the possible xylazine contamination in the illicit drug supply to increase awareness and education to the public.”
The FDA commissioner said the agency will coordinate with public health officials to more closely track xylazine.
“We will continue to use all tools at our disposal and partner with the Drug Enforcement Administration and other federal, state, local agencies, and stakeholders as appropriate to stem these illicit activities and protect public health,” said Dr. Califf.
A version of this article first appeared on Medscape.com.
DEA proposals on telehealth for controlled substances draw fire
The proposed rules – one for Schedule III-V substances, and the other for buprenorphine – are due to go into effect on May 11, when the COVID-19 public health emergency (PHE), and temporary flexibilities, end.
Essentially, both proposals would allow providers to prescribe a 30-day supply of a controlled substance or buprenorphine, but then require a face-to-face meeting for patients to receive additional prescriptions.
The DEA says that the rules are aimed at preventing abuse and diversion of the substances, but clinicians claim they are creating unnecessary hurdles that will probably lead to some patients dropping out of treatment.
“We were happy to see that there is ongoing flexibility to be able to initiate buprenorphine through telehealth, but we were disappointed to see that the DEA set an arbitrary time frame, in this case, a 30-day time frame after which the patient would have to be seen in person before ongoing care with buprenorphine for opioid use disorder could be provided,” Brian Hurley, MD, MBA, the president-elect of the American Society of Addiction Medicine told this news organization.
Dr. Hurley agreed that it is best practice to see patients in person for ongoing care, but he noted they have many reasons why they might not be able to make it into an office every month.
“What this rule would do if instituted as written is prevent me from continuing care for patients unless I can get them in in person,” he said. “And while I’d make every effort as a clinician, it’s not always feasible to do so.”
The addiction specialist noted that only about 20% of Americans with opioid use disorder have access to medications for the disorder. “I would posit that untreated opioid use disorder is a bigger threat to public safety currently than the risk of diversion,” he said.
The DEA is also proposing to allow state laws to supersede its regulations, which concerns Dr. Hurley and other clinicians because some states are more restrictive. “Our position is that state laws that restrict access to medications for opioid use disorder through telehealth means are inconsistent with our policy recommendation. I certainly hope that the DEA hears our concerns and amends the proposal,” said Dr. Hurley.
A potential ‘telehealth cliff’
Shabana Khan, MD, chair of the American Psychiatric Association’s telepsychiatry committee, said that “because of potential overlap with state rules that may be more stringent than these new regulations, APA is concerned that the proposed rules will create a telehealth cliff for those in most need of critical psychiatric and opioid use disorder treatment, particularly in communities where this specialty care is limited or nonexistent.”
Dr. Khan noted that “clarification is necessary on how patients who started treatment during the PHE can continue treatment with a prescribing provider, if at all, through an in-person evaluation with a DEA-registered provider referral.”
Telehealth companies were also disappointed in the DEA proposals.
“The continuity of care for countless Americans will be severed, potentially leaving these patients to fall through the cracks of our health care system without access to needed medications,” said Kyle Zebley, the American Telemedicine Association’s senior vice president of public policy, in a statement.
“Requiring every patient who has initiated treatment via telemedicine during the pandemic to now visit a provider in person clearly falls on the side of being overly restrictive,” Mr. Zebley added.
The DEA is proposing to allow patients who have been receiving telehealth over the past 3 years to continue to do so for 180 days after the PHE ends.
But the American Telemedicine Association and others said that they still want to see a change in the proposal as written. “Our hope is that the DEA works with us to avoid unnecessary and inappropriate restrictions on the prescription of essential medications for these vulnerable and underserved populations,” Mr. Zebley said in the statement.
DEA Administrator Anne Milgram said in a statement that the agency believes that “the telemedicine regulations would continue to expand access to buprenorphine for patients with opioid use disorder,” and that the DEA “is committed to the expansion of telemedicine with guardrails that prevent the online overprescribing of controlled medications that can cause harm.”
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said in a statement that “This proposed rule builds on President Biden’s historic move to eliminate the X-waiver that prevented many prescribers from treating patients with buprenorphine.” He added, “Thanks to these changes, millions of Americans will be able to access the lifesaving care they need.”
The DEA estimated that there were 15.7 million prescriptions for buprenorphine in 2021 and that about 67,000 were for initial prescriptions.
Ketamine confusion
The rule on controlled substances has also caused some consternation, especially given that it does not differentiate between racemic ketamine and esketamine, said Lisa Marie Harding, MD, vice president of the board of the American Society of Ketamine Physicians, Psychotherapists & Practitioners.
Esketamine (Spravato) is approved by the Food and Drug Administration and, under a Risk Evaluation and Mitigation Strategy, can only be administered in FDA-monitored treatment facilities. Racemic ketamine is being prescribed – often for home use – with almost no regulatory oversight.
Dr. Harding, who is an approved Spravato provider and also administers intravenous ketamine in her practice, does not believe that ketamine should be used at home without supervision.
“I had a patient who had a very powerful dissociative experience in my office earlier this week,” Dr. Harding said in an interview. One of her staff asked what would happen if the patient had experienced that at home. “We don’t know. Nor do we want this to happen,” said Dr. Harding.
However, the DEA proposal would continue to allow for home use, at least initially. “If it’s open to interpretation, those people that prescribe ketamine for home use can use that leeway to then continue to do it,” she said. “That is not safe.”
Dr. Harding approves of the proposed DEA requirement for face-to-face visits. “It’s good patient care,” she said. But she wants the administration to adjust the rules to make it harder to offer home ketamine therapy.
“Lots of people are using racemic ketamine off-label for treating depression with success but doing it in treatment settings that are appropriate,” said Dr. Harding.
Dr. Hurley and Dr. Harding report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The proposed rules – one for Schedule III-V substances, and the other for buprenorphine – are due to go into effect on May 11, when the COVID-19 public health emergency (PHE), and temporary flexibilities, end.
Essentially, both proposals would allow providers to prescribe a 30-day supply of a controlled substance or buprenorphine, but then require a face-to-face meeting for patients to receive additional prescriptions.
The DEA says that the rules are aimed at preventing abuse and diversion of the substances, but clinicians claim they are creating unnecessary hurdles that will probably lead to some patients dropping out of treatment.
“We were happy to see that there is ongoing flexibility to be able to initiate buprenorphine through telehealth, but we were disappointed to see that the DEA set an arbitrary time frame, in this case, a 30-day time frame after which the patient would have to be seen in person before ongoing care with buprenorphine for opioid use disorder could be provided,” Brian Hurley, MD, MBA, the president-elect of the American Society of Addiction Medicine told this news organization.
Dr. Hurley agreed that it is best practice to see patients in person for ongoing care, but he noted they have many reasons why they might not be able to make it into an office every month.
“What this rule would do if instituted as written is prevent me from continuing care for patients unless I can get them in in person,” he said. “And while I’d make every effort as a clinician, it’s not always feasible to do so.”
The addiction specialist noted that only about 20% of Americans with opioid use disorder have access to medications for the disorder. “I would posit that untreated opioid use disorder is a bigger threat to public safety currently than the risk of diversion,” he said.
The DEA is also proposing to allow state laws to supersede its regulations, which concerns Dr. Hurley and other clinicians because some states are more restrictive. “Our position is that state laws that restrict access to medications for opioid use disorder through telehealth means are inconsistent with our policy recommendation. I certainly hope that the DEA hears our concerns and amends the proposal,” said Dr. Hurley.
A potential ‘telehealth cliff’
Shabana Khan, MD, chair of the American Psychiatric Association’s telepsychiatry committee, said that “because of potential overlap with state rules that may be more stringent than these new regulations, APA is concerned that the proposed rules will create a telehealth cliff for those in most need of critical psychiatric and opioid use disorder treatment, particularly in communities where this specialty care is limited or nonexistent.”
Dr. Khan noted that “clarification is necessary on how patients who started treatment during the PHE can continue treatment with a prescribing provider, if at all, through an in-person evaluation with a DEA-registered provider referral.”
Telehealth companies were also disappointed in the DEA proposals.
“The continuity of care for countless Americans will be severed, potentially leaving these patients to fall through the cracks of our health care system without access to needed medications,” said Kyle Zebley, the American Telemedicine Association’s senior vice president of public policy, in a statement.
“Requiring every patient who has initiated treatment via telemedicine during the pandemic to now visit a provider in person clearly falls on the side of being overly restrictive,” Mr. Zebley added.
The DEA is proposing to allow patients who have been receiving telehealth over the past 3 years to continue to do so for 180 days after the PHE ends.
But the American Telemedicine Association and others said that they still want to see a change in the proposal as written. “Our hope is that the DEA works with us to avoid unnecessary and inappropriate restrictions on the prescription of essential medications for these vulnerable and underserved populations,” Mr. Zebley said in the statement.
DEA Administrator Anne Milgram said in a statement that the agency believes that “the telemedicine regulations would continue to expand access to buprenorphine for patients with opioid use disorder,” and that the DEA “is committed to the expansion of telemedicine with guardrails that prevent the online overprescribing of controlled medications that can cause harm.”
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said in a statement that “This proposed rule builds on President Biden’s historic move to eliminate the X-waiver that prevented many prescribers from treating patients with buprenorphine.” He added, “Thanks to these changes, millions of Americans will be able to access the lifesaving care they need.”
The DEA estimated that there were 15.7 million prescriptions for buprenorphine in 2021 and that about 67,000 were for initial prescriptions.
Ketamine confusion
The rule on controlled substances has also caused some consternation, especially given that it does not differentiate between racemic ketamine and esketamine, said Lisa Marie Harding, MD, vice president of the board of the American Society of Ketamine Physicians, Psychotherapists & Practitioners.
Esketamine (Spravato) is approved by the Food and Drug Administration and, under a Risk Evaluation and Mitigation Strategy, can only be administered in FDA-monitored treatment facilities. Racemic ketamine is being prescribed – often for home use – with almost no regulatory oversight.
Dr. Harding, who is an approved Spravato provider and also administers intravenous ketamine in her practice, does not believe that ketamine should be used at home without supervision.
“I had a patient who had a very powerful dissociative experience in my office earlier this week,” Dr. Harding said in an interview. One of her staff asked what would happen if the patient had experienced that at home. “We don’t know. Nor do we want this to happen,” said Dr. Harding.
However, the DEA proposal would continue to allow for home use, at least initially. “If it’s open to interpretation, those people that prescribe ketamine for home use can use that leeway to then continue to do it,” she said. “That is not safe.”
Dr. Harding approves of the proposed DEA requirement for face-to-face visits. “It’s good patient care,” she said. But she wants the administration to adjust the rules to make it harder to offer home ketamine therapy.
“Lots of people are using racemic ketamine off-label for treating depression with success but doing it in treatment settings that are appropriate,” said Dr. Harding.
Dr. Hurley and Dr. Harding report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The proposed rules – one for Schedule III-V substances, and the other for buprenorphine – are due to go into effect on May 11, when the COVID-19 public health emergency (PHE), and temporary flexibilities, end.
Essentially, both proposals would allow providers to prescribe a 30-day supply of a controlled substance or buprenorphine, but then require a face-to-face meeting for patients to receive additional prescriptions.
The DEA says that the rules are aimed at preventing abuse and diversion of the substances, but clinicians claim they are creating unnecessary hurdles that will probably lead to some patients dropping out of treatment.
“We were happy to see that there is ongoing flexibility to be able to initiate buprenorphine through telehealth, but we were disappointed to see that the DEA set an arbitrary time frame, in this case, a 30-day time frame after which the patient would have to be seen in person before ongoing care with buprenorphine for opioid use disorder could be provided,” Brian Hurley, MD, MBA, the president-elect of the American Society of Addiction Medicine told this news organization.
Dr. Hurley agreed that it is best practice to see patients in person for ongoing care, but he noted they have many reasons why they might not be able to make it into an office every month.
“What this rule would do if instituted as written is prevent me from continuing care for patients unless I can get them in in person,” he said. “And while I’d make every effort as a clinician, it’s not always feasible to do so.”
The addiction specialist noted that only about 20% of Americans with opioid use disorder have access to medications for the disorder. “I would posit that untreated opioid use disorder is a bigger threat to public safety currently than the risk of diversion,” he said.
The DEA is also proposing to allow state laws to supersede its regulations, which concerns Dr. Hurley and other clinicians because some states are more restrictive. “Our position is that state laws that restrict access to medications for opioid use disorder through telehealth means are inconsistent with our policy recommendation. I certainly hope that the DEA hears our concerns and amends the proposal,” said Dr. Hurley.
A potential ‘telehealth cliff’
Shabana Khan, MD, chair of the American Psychiatric Association’s telepsychiatry committee, said that “because of potential overlap with state rules that may be more stringent than these new regulations, APA is concerned that the proposed rules will create a telehealth cliff for those in most need of critical psychiatric and opioid use disorder treatment, particularly in communities where this specialty care is limited or nonexistent.”
Dr. Khan noted that “clarification is necessary on how patients who started treatment during the PHE can continue treatment with a prescribing provider, if at all, through an in-person evaluation with a DEA-registered provider referral.”
Telehealth companies were also disappointed in the DEA proposals.
“The continuity of care for countless Americans will be severed, potentially leaving these patients to fall through the cracks of our health care system without access to needed medications,” said Kyle Zebley, the American Telemedicine Association’s senior vice president of public policy, in a statement.
“Requiring every patient who has initiated treatment via telemedicine during the pandemic to now visit a provider in person clearly falls on the side of being overly restrictive,” Mr. Zebley added.
The DEA is proposing to allow patients who have been receiving telehealth over the past 3 years to continue to do so for 180 days after the PHE ends.
But the American Telemedicine Association and others said that they still want to see a change in the proposal as written. “Our hope is that the DEA works with us to avoid unnecessary and inappropriate restrictions on the prescription of essential medications for these vulnerable and underserved populations,” Mr. Zebley said in the statement.
DEA Administrator Anne Milgram said in a statement that the agency believes that “the telemedicine regulations would continue to expand access to buprenorphine for patients with opioid use disorder,” and that the DEA “is committed to the expansion of telemedicine with guardrails that prevent the online overprescribing of controlled medications that can cause harm.”
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said in a statement that “This proposed rule builds on President Biden’s historic move to eliminate the X-waiver that prevented many prescribers from treating patients with buprenorphine.” He added, “Thanks to these changes, millions of Americans will be able to access the lifesaving care they need.”
The DEA estimated that there were 15.7 million prescriptions for buprenorphine in 2021 and that about 67,000 were for initial prescriptions.
Ketamine confusion
The rule on controlled substances has also caused some consternation, especially given that it does not differentiate between racemic ketamine and esketamine, said Lisa Marie Harding, MD, vice president of the board of the American Society of Ketamine Physicians, Psychotherapists & Practitioners.
Esketamine (Spravato) is approved by the Food and Drug Administration and, under a Risk Evaluation and Mitigation Strategy, can only be administered in FDA-monitored treatment facilities. Racemic ketamine is being prescribed – often for home use – with almost no regulatory oversight.
Dr. Harding, who is an approved Spravato provider and also administers intravenous ketamine in her practice, does not believe that ketamine should be used at home without supervision.
“I had a patient who had a very powerful dissociative experience in my office earlier this week,” Dr. Harding said in an interview. One of her staff asked what would happen if the patient had experienced that at home. “We don’t know. Nor do we want this to happen,” said Dr. Harding.
However, the DEA proposal would continue to allow for home use, at least initially. “If it’s open to interpretation, those people that prescribe ketamine for home use can use that leeway to then continue to do it,” she said. “That is not safe.”
Dr. Harding approves of the proposed DEA requirement for face-to-face visits. “It’s good patient care,” she said. But she wants the administration to adjust the rules to make it harder to offer home ketamine therapy.
“Lots of people are using racemic ketamine off-label for treating depression with success but doing it in treatment settings that are appropriate,” said Dr. Harding.
Dr. Hurley and Dr. Harding report no relevant financial relationships.
A version of this article first appeared on Medscape.com.







