User login
Alcohol abstinence reduces A-fib burden in drinkers
ILLUSTRATIVE CASE
A 61-year-old man with hypertension and paroxysmal AF presents to your office shortly after experiencing his third episode of AF in the past 6 months. He describes these episodes, which last for several days, as “just awful,” noting that when he experiences AF, he has fatigue, palpitations, and shortness of breath and “can’t stop paying attention to my heart.” The patient, who has a body mass index of 32, consumes more than 15 alcoholic drinks per week. What can you recommend to him that will decrease his likelihood of experiencing more episodes of AF?
AF is the most common sustained cardiac arrhythmia. It is associated with significant morbidity and mortality. Known risk factors include obesity, physical inactivity, sleep apnea, diabetes, and hypertension.2
According to the Centers for Disease Control and Prevention, an estimated 12.1 million people in the United States will have AF by 2030. In 2018, AF was mentioned on more than 183,000 death certificates and was the underlying cause of more than 26,000 of those deaths.3 AF is the primary diagnosis in 450,000 hospitalizations annually,4 and the death rate from AF as the primary or contributing cause of death has been rising for more than 2 decades.3
More than 50% of Americans report alcohol consumption within the past month.5 Although alcohol use is associated with new and recurrent AF, only limited prospective data show a clear and causal association between abstaining from alcohol and decreasing AF recurrence.
STUDY SUMMARY
Reduction in AF recurrence and total AF burden following alcohol abstinence
This multicenter, prospective, open-label, randomized controlled trial (N = 140) from 6 sites in Australia evaluated the impact of alcohol abstinence on both the recurrence of AF and the amount of time in AF. Study participants were ages 18 to 85 years, consumed 10 or more standard alcohol-containing drinks per week, had paroxysmal or persistent AF, and were in sinus rhythm at the time of enrollment, regardless of antiarrhythmic therapy. Exclusion criteria included alcohol dependence or abuse, severe left ventricular systolic dysfunction (ejection fraction < 35%), clinically significant noncardiac illness, and/or coexisting psychiatric disorder.1
After a 4-week run-in period, patients were randomized to either an abstinence or a control group in a 1:1 fashion. Patients enrolled in the abstinence group were encouraged to abstain from alcohol consumption for 6 months and were provided with written and oral instructions to assist with abstaining. Control group patients continued their same level of alcohol consumption. Comprehensive rhythm monitoring occurred for all patients after randomization.
Alcohol consumption was reported by both groups using a weekly alcohol diary, supplemented with a visual guide showing pictures of standard alcohol drinks. For the abstinence group, random urine testing for ethyl glucuronide (an alcohol metabolite) was possible if no alcohol intake was reported. Primary outcomes during the 6-month study included recurrence of AF and total AF burden (percentage of time in AF).
Continue to: Secondary outcomes included hospitalizations...
Secondary outcomes included hospitalizations for AF, AF symptom severity, and change in weight. Blood pressure, quality-of-life, and depression scores were missing for > 35% of patients.1
Patients were randomized evenly to the control and abstinence groups. The typical patient was an overweight male in his early 60s with paroxysmal AF, who was taking an antiarrhythmic agent. Patients in the abstinence group decreased their alcohol consumption from 16.8 to 2.1 drinks per week (87.5% reduction; mean difference = –14.7; 95% CI, –12.7 to –16.7). Patients in the control group reduced their intake from 16.4 to 13.2 drinks per week (19.5% reduction; mean difference = –3.2; 95% CI, –1.9 to –4.4).1
AF recurred in 53% vs 73% of the abstinence and control groups, respectively, with a longer period before recurrence in the abstinence group than in the control group (hazard ratio = 0.55; 95% CI, 0.36-0.84; P = .005; number needed to treat = 5). The AF burden was also lower in the abstinence group (0.5%; interquartile range [IQR] = 0.0-3.0) than in the control group (1.2%; IQR = 0.0-10.3; P = .01). The abstinence group had a lower percentage of AF hospitalizations compared with the control group (9% vs 20%), and fewer patients reporting moderate or severe AF symptoms (10% vs 32%). In addition, the abstinence group lost 3.7 kg more weight than did the control group at 6 months.1
WHAT’S NEW
Objective new evidence for effective patient counseling
Alcohol consumption and its association with the onset and recurrence of AF has been documented previously.6 This study was the first to prospectively examine if abstaining from alcohol reduces paroxysmal AF episodes in moderate drinkers.
The study identified clinically meaningful findings among those who abstained from alcohol, including decreased AF recurrence rates, increased time to recurrence, and lower overall AF burden. This provides objective evidence that can be used for motivational interviewing in patients with paroxysmal AF who may be receptive to reducing or abstaining from alcohol consumption.
Continue to: CAVEATS
CAVEATS
The narrow study population may not be widely applicable
The study population was predominantly male, in their seventh decade of life (mean age, 61), and living in Australia. Rates of AF and symptomatology differ by gender and age, making this information challenging to apply to women or older populations. The study excluded patients with alcohol dependence or abuse, left ventricular systolic dysfunction (ejection fraction < 35%), coexisting psychiatric disorders, and clinically significant noncardiac illnesses, limiting the study’s generalizability to these patient populations. Overall, AF recurrence was low in both groups despite the intervention, and the study did not evaluate the efficacy of the counseling method for abstinence.
Since publication of this article, a prospective cohort study of approximately 3800 Swiss patients with AF evaluated the effect of alcohol consumption on the rate of stroke and embolic events. That study did not find statistically significant correlations between patients who drank no alcohol per day, > 0 to < 1, 1 to < 2, or ≥ 2 drinks per day and their rate of stroke.7 However, this study did not specifically evaluate the rate of AF recurrence or time spent in AF among the cohort, which is clinically meaningful for patient morbidity.1
CHALLENGES TO IMPLEMENTATION
Patient willingness to cut alcohol consumption may be limited
The largest challenge to implementation of this intervention is most likely the willingness of patients to cut their alcohol consumption. In this study population, 697 patients were screened for enrollment and met inclusion criteria; however, 491 patients (70.4%) were not willing to consider abstinence from alcohol, and after the run-in phase, another 17 declined randomization. Many primary care physicians would likely agree that while it is easy to encourage patients to drink less, patient adherence to these recommendations, particularly abstaining, is likely to be limited.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382:20-28. doi: 10.1056/NEJMoa1817591
2. Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141:e750-e772. doi: 10.1161/CIR.0000000000000748
3. Atrial fibrillation. Centers for Disease Control and Prevention. Last reviewed September 27, 2021. Accessed February 9, 2022. www.cdc.gov/heartdisease/atrial_fibrillation.htm
4. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:e56-e528. doi: 10.1161/CIR.0000000000000659
5. Alcohol facts and statistics. National Institute on Alcohol Abuse and Alcoholism. Updated June 2021. Accessed February 9, 2022. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics
6. Kodama S, Saito K, Tanaka S, et al. Alcohol consumption and risk of atrial fibrillation: a meta-analysis. J Am Coll Cardiol. 2011;57:427-436. doi: 10.1016/j.jacc.2010.08.641
7. Reddiess P, Aeschbacher S, Meyre P, et al. Alcohol consumption and risk of cardiovascular outcomes and bleeding in patients with established atrial fibrillation. CMAJ. 2021;193:E117-E123. doi: 10.1503/cmaj.200778
ILLUSTRATIVE CASE
A 61-year-old man with hypertension and paroxysmal AF presents to your office shortly after experiencing his third episode of AF in the past 6 months. He describes these episodes, which last for several days, as “just awful,” noting that when he experiences AF, he has fatigue, palpitations, and shortness of breath and “can’t stop paying attention to my heart.” The patient, who has a body mass index of 32, consumes more than 15 alcoholic drinks per week. What can you recommend to him that will decrease his likelihood of experiencing more episodes of AF?
AF is the most common sustained cardiac arrhythmia. It is associated with significant morbidity and mortality. Known risk factors include obesity, physical inactivity, sleep apnea, diabetes, and hypertension.2
According to the Centers for Disease Control and Prevention, an estimated 12.1 million people in the United States will have AF by 2030. In 2018, AF was mentioned on more than 183,000 death certificates and was the underlying cause of more than 26,000 of those deaths.3 AF is the primary diagnosis in 450,000 hospitalizations annually,4 and the death rate from AF as the primary or contributing cause of death has been rising for more than 2 decades.3
More than 50% of Americans report alcohol consumption within the past month.5 Although alcohol use is associated with new and recurrent AF, only limited prospective data show a clear and causal association between abstaining from alcohol and decreasing AF recurrence.
STUDY SUMMARY
Reduction in AF recurrence and total AF burden following alcohol abstinence
This multicenter, prospective, open-label, randomized controlled trial (N = 140) from 6 sites in Australia evaluated the impact of alcohol abstinence on both the recurrence of AF and the amount of time in AF. Study participants were ages 18 to 85 years, consumed 10 or more standard alcohol-containing drinks per week, had paroxysmal or persistent AF, and were in sinus rhythm at the time of enrollment, regardless of antiarrhythmic therapy. Exclusion criteria included alcohol dependence or abuse, severe left ventricular systolic dysfunction (ejection fraction < 35%), clinically significant noncardiac illness, and/or coexisting psychiatric disorder.1
After a 4-week run-in period, patients were randomized to either an abstinence or a control group in a 1:1 fashion. Patients enrolled in the abstinence group were encouraged to abstain from alcohol consumption for 6 months and were provided with written and oral instructions to assist with abstaining. Control group patients continued their same level of alcohol consumption. Comprehensive rhythm monitoring occurred for all patients after randomization.
Alcohol consumption was reported by both groups using a weekly alcohol diary, supplemented with a visual guide showing pictures of standard alcohol drinks. For the abstinence group, random urine testing for ethyl glucuronide (an alcohol metabolite) was possible if no alcohol intake was reported. Primary outcomes during the 6-month study included recurrence of AF and total AF burden (percentage of time in AF).
Continue to: Secondary outcomes included hospitalizations...
Secondary outcomes included hospitalizations for AF, AF symptom severity, and change in weight. Blood pressure, quality-of-life, and depression scores were missing for > 35% of patients.1
Patients were randomized evenly to the control and abstinence groups. The typical patient was an overweight male in his early 60s with paroxysmal AF, who was taking an antiarrhythmic agent. Patients in the abstinence group decreased their alcohol consumption from 16.8 to 2.1 drinks per week (87.5% reduction; mean difference = –14.7; 95% CI, –12.7 to –16.7). Patients in the control group reduced their intake from 16.4 to 13.2 drinks per week (19.5% reduction; mean difference = –3.2; 95% CI, –1.9 to –4.4).1
AF recurred in 53% vs 73% of the abstinence and control groups, respectively, with a longer period before recurrence in the abstinence group than in the control group (hazard ratio = 0.55; 95% CI, 0.36-0.84; P = .005; number needed to treat = 5). The AF burden was also lower in the abstinence group (0.5%; interquartile range [IQR] = 0.0-3.0) than in the control group (1.2%; IQR = 0.0-10.3; P = .01). The abstinence group had a lower percentage of AF hospitalizations compared with the control group (9% vs 20%), and fewer patients reporting moderate or severe AF symptoms (10% vs 32%). In addition, the abstinence group lost 3.7 kg more weight than did the control group at 6 months.1
WHAT’S NEW
Objective new evidence for effective patient counseling
Alcohol consumption and its association with the onset and recurrence of AF has been documented previously.6 This study was the first to prospectively examine if abstaining from alcohol reduces paroxysmal AF episodes in moderate drinkers.
The study identified clinically meaningful findings among those who abstained from alcohol, including decreased AF recurrence rates, increased time to recurrence, and lower overall AF burden. This provides objective evidence that can be used for motivational interviewing in patients with paroxysmal AF who may be receptive to reducing or abstaining from alcohol consumption.
Continue to: CAVEATS
CAVEATS
The narrow study population may not be widely applicable
The study population was predominantly male, in their seventh decade of life (mean age, 61), and living in Australia. Rates of AF and symptomatology differ by gender and age, making this information challenging to apply to women or older populations. The study excluded patients with alcohol dependence or abuse, left ventricular systolic dysfunction (ejection fraction < 35%), coexisting psychiatric disorders, and clinically significant noncardiac illnesses, limiting the study’s generalizability to these patient populations. Overall, AF recurrence was low in both groups despite the intervention, and the study did not evaluate the efficacy of the counseling method for abstinence.
Since publication of this article, a prospective cohort study of approximately 3800 Swiss patients with AF evaluated the effect of alcohol consumption on the rate of stroke and embolic events. That study did not find statistically significant correlations between patients who drank no alcohol per day, > 0 to < 1, 1 to < 2, or ≥ 2 drinks per day and their rate of stroke.7 However, this study did not specifically evaluate the rate of AF recurrence or time spent in AF among the cohort, which is clinically meaningful for patient morbidity.1
CHALLENGES TO IMPLEMENTATION
Patient willingness to cut alcohol consumption may be limited
The largest challenge to implementation of this intervention is most likely the willingness of patients to cut their alcohol consumption. In this study population, 697 patients were screened for enrollment and met inclusion criteria; however, 491 patients (70.4%) were not willing to consider abstinence from alcohol, and after the run-in phase, another 17 declined randomization. Many primary care physicians would likely agree that while it is easy to encourage patients to drink less, patient adherence to these recommendations, particularly abstaining, is likely to be limited.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 61-year-old man with hypertension and paroxysmal AF presents to your office shortly after experiencing his third episode of AF in the past 6 months. He describes these episodes, which last for several days, as “just awful,” noting that when he experiences AF, he has fatigue, palpitations, and shortness of breath and “can’t stop paying attention to my heart.” The patient, who has a body mass index of 32, consumes more than 15 alcoholic drinks per week. What can you recommend to him that will decrease his likelihood of experiencing more episodes of AF?
AF is the most common sustained cardiac arrhythmia. It is associated with significant morbidity and mortality. Known risk factors include obesity, physical inactivity, sleep apnea, diabetes, and hypertension.2
According to the Centers for Disease Control and Prevention, an estimated 12.1 million people in the United States will have AF by 2030. In 2018, AF was mentioned on more than 183,000 death certificates and was the underlying cause of more than 26,000 of those deaths.3 AF is the primary diagnosis in 450,000 hospitalizations annually,4 and the death rate from AF as the primary or contributing cause of death has been rising for more than 2 decades.3
More than 50% of Americans report alcohol consumption within the past month.5 Although alcohol use is associated with new and recurrent AF, only limited prospective data show a clear and causal association between abstaining from alcohol and decreasing AF recurrence.
STUDY SUMMARY
Reduction in AF recurrence and total AF burden following alcohol abstinence
This multicenter, prospective, open-label, randomized controlled trial (N = 140) from 6 sites in Australia evaluated the impact of alcohol abstinence on both the recurrence of AF and the amount of time in AF. Study participants were ages 18 to 85 years, consumed 10 or more standard alcohol-containing drinks per week, had paroxysmal or persistent AF, and were in sinus rhythm at the time of enrollment, regardless of antiarrhythmic therapy. Exclusion criteria included alcohol dependence or abuse, severe left ventricular systolic dysfunction (ejection fraction < 35%), clinically significant noncardiac illness, and/or coexisting psychiatric disorder.1
After a 4-week run-in period, patients were randomized to either an abstinence or a control group in a 1:1 fashion. Patients enrolled in the abstinence group were encouraged to abstain from alcohol consumption for 6 months and were provided with written and oral instructions to assist with abstaining. Control group patients continued their same level of alcohol consumption. Comprehensive rhythm monitoring occurred for all patients after randomization.
Alcohol consumption was reported by both groups using a weekly alcohol diary, supplemented with a visual guide showing pictures of standard alcohol drinks. For the abstinence group, random urine testing for ethyl glucuronide (an alcohol metabolite) was possible if no alcohol intake was reported. Primary outcomes during the 6-month study included recurrence of AF and total AF burden (percentage of time in AF).
Continue to: Secondary outcomes included hospitalizations...
Secondary outcomes included hospitalizations for AF, AF symptom severity, and change in weight. Blood pressure, quality-of-life, and depression scores were missing for > 35% of patients.1
Patients were randomized evenly to the control and abstinence groups. The typical patient was an overweight male in his early 60s with paroxysmal AF, who was taking an antiarrhythmic agent. Patients in the abstinence group decreased their alcohol consumption from 16.8 to 2.1 drinks per week (87.5% reduction; mean difference = –14.7; 95% CI, –12.7 to –16.7). Patients in the control group reduced their intake from 16.4 to 13.2 drinks per week (19.5% reduction; mean difference = –3.2; 95% CI, –1.9 to –4.4).1
AF recurred in 53% vs 73% of the abstinence and control groups, respectively, with a longer period before recurrence in the abstinence group than in the control group (hazard ratio = 0.55; 95% CI, 0.36-0.84; P = .005; number needed to treat = 5). The AF burden was also lower in the abstinence group (0.5%; interquartile range [IQR] = 0.0-3.0) than in the control group (1.2%; IQR = 0.0-10.3; P = .01). The abstinence group had a lower percentage of AF hospitalizations compared with the control group (9% vs 20%), and fewer patients reporting moderate or severe AF symptoms (10% vs 32%). In addition, the abstinence group lost 3.7 kg more weight than did the control group at 6 months.1
WHAT’S NEW
Objective new evidence for effective patient counseling
Alcohol consumption and its association with the onset and recurrence of AF has been documented previously.6 This study was the first to prospectively examine if abstaining from alcohol reduces paroxysmal AF episodes in moderate drinkers.
The study identified clinically meaningful findings among those who abstained from alcohol, including decreased AF recurrence rates, increased time to recurrence, and lower overall AF burden. This provides objective evidence that can be used for motivational interviewing in patients with paroxysmal AF who may be receptive to reducing or abstaining from alcohol consumption.
Continue to: CAVEATS
CAVEATS
The narrow study population may not be widely applicable
The study population was predominantly male, in their seventh decade of life (mean age, 61), and living in Australia. Rates of AF and symptomatology differ by gender and age, making this information challenging to apply to women or older populations. The study excluded patients with alcohol dependence or abuse, left ventricular systolic dysfunction (ejection fraction < 35%), coexisting psychiatric disorders, and clinically significant noncardiac illnesses, limiting the study’s generalizability to these patient populations. Overall, AF recurrence was low in both groups despite the intervention, and the study did not evaluate the efficacy of the counseling method for abstinence.
Since publication of this article, a prospective cohort study of approximately 3800 Swiss patients with AF evaluated the effect of alcohol consumption on the rate of stroke and embolic events. That study did not find statistically significant correlations between patients who drank no alcohol per day, > 0 to < 1, 1 to < 2, or ≥ 2 drinks per day and their rate of stroke.7 However, this study did not specifically evaluate the rate of AF recurrence or time spent in AF among the cohort, which is clinically meaningful for patient morbidity.1
CHALLENGES TO IMPLEMENTATION
Patient willingness to cut alcohol consumption may be limited
The largest challenge to implementation of this intervention is most likely the willingness of patients to cut their alcohol consumption. In this study population, 697 patients were screened for enrollment and met inclusion criteria; however, 491 patients (70.4%) were not willing to consider abstinence from alcohol, and after the run-in phase, another 17 declined randomization. Many primary care physicians would likely agree that while it is easy to encourage patients to drink less, patient adherence to these recommendations, particularly abstaining, is likely to be limited.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382:20-28. doi: 10.1056/NEJMoa1817591
2. Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141:e750-e772. doi: 10.1161/CIR.0000000000000748
3. Atrial fibrillation. Centers for Disease Control and Prevention. Last reviewed September 27, 2021. Accessed February 9, 2022. www.cdc.gov/heartdisease/atrial_fibrillation.htm
4. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:e56-e528. doi: 10.1161/CIR.0000000000000659
5. Alcohol facts and statistics. National Institute on Alcohol Abuse and Alcoholism. Updated June 2021. Accessed February 9, 2022. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics
6. Kodama S, Saito K, Tanaka S, et al. Alcohol consumption and risk of atrial fibrillation: a meta-analysis. J Am Coll Cardiol. 2011;57:427-436. doi: 10.1016/j.jacc.2010.08.641
7. Reddiess P, Aeschbacher S, Meyre P, et al. Alcohol consumption and risk of cardiovascular outcomes and bleeding in patients with established atrial fibrillation. CMAJ. 2021;193:E117-E123. doi: 10.1503/cmaj.200778
1. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382:20-28. doi: 10.1056/NEJMoa1817591
2. Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141:e750-e772. doi: 10.1161/CIR.0000000000000748
3. Atrial fibrillation. Centers for Disease Control and Prevention. Last reviewed September 27, 2021. Accessed February 9, 2022. www.cdc.gov/heartdisease/atrial_fibrillation.htm
4. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:e56-e528. doi: 10.1161/CIR.0000000000000659
5. Alcohol facts and statistics. National Institute on Alcohol Abuse and Alcoholism. Updated June 2021. Accessed February 9, 2022. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics
6. Kodama S, Saito K, Tanaka S, et al. Alcohol consumption and risk of atrial fibrillation: a meta-analysis. J Am Coll Cardiol. 2011;57:427-436. doi: 10.1016/j.jacc.2010.08.641
7. Reddiess P, Aeschbacher S, Meyre P, et al. Alcohol consumption and risk of cardiovascular outcomes and bleeding in patients with established atrial fibrillation. CMAJ. 2021;193:E117-E123. doi: 10.1503/cmaj.200778
PRACTICE CHANGER
Counsel patients with paroxysmal or persistent atrial fibrillation (AF) who drink moderately (≥ 10 drinks per week) that they can reduce their time in AF, as well as their overall recurrence of AF, by decreasing their alcohol consumption by half or more.
STRENGTH OF RECOMMENDATION
B: Based on a well-performed randomized controlled trial1
Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382:20-28.
Examining Interventions and Adverse Events After Nonfatal Opioid Overdoses in Veterans
The number of opioid-related overdose deaths in the United States is estimated to have increased 6-fold over the past 2 decades.1 In 2017, more than two-thirds of drug overdose deaths involved opioids, yielding a mortality rate of 14.9 per 100,000.2 Not only does the opioid epidemic currently pose a significant public health crisis characterized by high morbidity and mortality, but it is also projected to worsen in coming years. According to Chen and colleagues, opioid overdose deaths are estimated to increase by 147% from 2015 to 2025.3 That projects almost 82,000 US deaths annually and > 700,000 deaths in this period—even before accounting for surges in opioid overdoses and opioid-related mortality coinciding with the COVID-19 pandemic.3,4
As health systems and communities globally struggle with unprecedented losses and stressors introduced by the pandemic, emerging data warrants escalating concerns with regard to increased vulnerability to relapse and overdose among those with mental health and substance use disorders (SUDs). In a recent report, the American Medical Association estimates that opioid-related deaths have increased in more than 40 states with the COVID-19 pandemic.4
Veterans are twice as likely to experience a fatal opioid overdose compared with their civilian counterparts.5 While several risk mitigation strategies have been employed in recent years to improve opioid prescribing and safety within the US Department of Veterans Affairs (VA), veterans continue to overdose on opioids, both prescribed and obtained illicitly.6 Variables shown to be strongly associated with opioid overdose risk include presence of mental health disorders, SUDs, medical conditions involving impaired drug metabolism or excretion, respiratory disorders, higher doses of opioids, concomitant use of sedative medications, and history of overdose.6-8 Many veterans struggle with chronic pain and those prescribed high doses of opioids were more likely to have comorbid pain diagnoses, mental health disorders, and SUDs.9 Dashboards and predictive models, such as the Stratification Tool for Opioid Risk Mitigation (STORM) and the Risk Index for Overdose or Serious Opioid-induced Respiratory Depression (RIOSORD), incorporate such factors to stratify overdose risk among veterans, in an effort to prioritize high-risk individuals for review and provision of care.6,10,11 Despite recent recognition that overdose prevention likely requires a holistic approach that addresses the biopsychosocial factors contributing to opioid-related morbidity and mortality, it is unclear whether veterans are receiving adequate and appropriate treatment for contributing conditions.
There are currently no existing studies that describe health service utilization (HSU), medication interventions, and rates of opioid-related adverse events (ORAEs) among veterans after survival of a nonfatal opioid overdose (NFO). Clinical characteristics of veterans treated for opioid overdose at a VA emergency department (ED) have previously been described by Clement and Stock.12 Despite improvements that have been made in VA opioid prescribing and safety, knowledge gaps remain with regard to best practices for opioid overdose prevention. The aim of this study was to characterize HSU and medication interventions in veterans following NFO, as well as the frequency of ORAEs after overdose. The findings of this study may aid in the identification of areas for targeted improvement in the prevention and reduction of opioid overdoses and adverse opioid-related sequelae.
Methods
This retrospective descriptive study was conducted at VA San Diego Healthcare System (VASDHCS) in California. Subjects included were veterans administered naloxone in the ED for suspected opioid overdose between July 1, 2013 and April 1, 2017. The study population was identified through data retrieved from automated drug dispensing systems, which was then confirmed through manual chart review of notes associated with the index ED visit. Inclusion criteria included documented increased respiration or responsiveness following naloxone administration. Subjects were excluded if they demonstrated lack of response to naloxone, overdosed secondary to inpatient administration of opioids, received palliative or hospice care during the study period, or were lost to follow-up.
Data were collected via retrospective chart review and included date of index ED visit, demographics, active prescriptions, urine drug screen (UDS) results, benzodiazepine (BZD) use corroborated by positive UDS or mention of BZD in index visit chart notes, whether overdose was determined to be a suicide attempt, and naloxone kit dispensing. Patient data was collected for 2 years following overdose, including: ORAEs; ED visits; hospitalizations; repeat overdoses; fatal overdose; whether subjects were still alive; follow-up visits for pain management, mental health, and addiction treatment services; and visits to the psychiatric emergency clinic. Clinical characteristics, such as mental health disorder diagnoses, SUDs, and relevant medical conditions also were collected. Statistical analysis was performed using Microsoft Excel and included only descriptive statistics.
Results
Ninety-three patients received naloxone in the VASDHCS ED. Thirty-five met inclusion criteria and were included in the primary analysis. All subjects received IV naloxone with a mean 0.8 mg IV boluses (range, 0.1-4.4 mg).
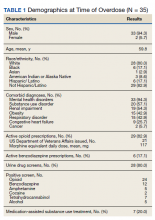
Most patients were male with a mean age of 59.8 years (Table 1). Almost all overdoses were nonintentional except for 3 suicide attempts that were reviewed by the Suicide Prevention Committee. Three patients had previously been treated for opioid overdose at the VA with a documented positive clinical response to naloxone administration.
At the time of overdose, 29 patients (82.9%) had an active opioid prescription. Of these, the majority were issued through the VA with a mean 117 mg morphine equivalent daily dose (MEDD). Interestingly, only 24 of the 28 patients with a UDS collected at time of overdose tested positive for opioids, which may be attributable to the use of synthetic opioids, which are not reliably detected by traditional UDS. Concomitant BZD use was involved in 13 of the 35 index overdoses (37.1%), although only 6 patients (17.1%) had an active BZD prescription at time of overdose. Seven patients (20.0%) were prescribed medication-assisted treatment (MAT) for opioid use disorder (OUD), with all 7 using methadone. According to VA records, only 1 patient had previously been dispensed a naloxone kit at any point prior to overdosing. Mental health and SUD diagnoses frequently co-occurred, with 20 patients (57.1%) having at least 1 mental health condition and at least 1 SUD.
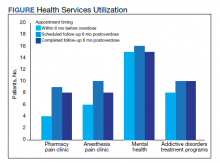
Rates of follow-up varied by clinician type in the 6 months after NFO (Figure). Of those with mental health disorders, 15 patients (45.5%) received mental health services before and after overdose, while 8 (40.0%) and 10 (50.0%) of those with SUDs received addiction treatment services before and after overdose, respectively. Seven patients presented to the psychiatric emergency clinic within 6 months prior to overdose and 5 patients within the 6 months following overdose.
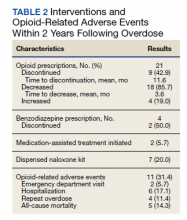
Of patients with VA opioid prescriptions, within 2 years of NFO, 9 (42.9%) had their opioids discontinued, and 18 (85.7%) had MEDD reductions ranging from 10 mg to 150 mg (12.5-71.4% reduction) with a mean of 63 mg. Two of the 4 patients with active BZD prescriptions at the time of the overdose event had their prescriptions continued. Seven patients (20.0%) were dispensed naloxone kits following overdose (Table 2).
Rates of ORAEs ranged from 0% to 17% with no documented overdose fatalities. Examples of AEs observed in this study included ED visits or hospitalizations involving opioid withdrawal, opioid-related personality changes, and opioid overdose. Five patients died during the study period, yielding an all-cause mortality rate of 14.3% with a mean time to death of 10.8 months. The causes of death were largely unknown except for 1 patient, whose death was reportedly investigated as an accidental medication overdose without additional information.
Repeat overdose verified by hospital records occurred in 4 patients (11.4%) within 2 years. Patients who experienced a subsequent overdose were prescribed higher doses of opioids with a mean MEDD among VA prescriptions of 130 mg vs 114 mg for those without repeat overdose. In this group, 3 patients (75.0%) also had concomitant BZD use, which was proportionally higher than the 10 patients (32.3%) without a subsequent overdose. Of note, 2 of the 4 patients with a repeat overdose had their opioid doses increased above the MEDD prescribed at the time of index overdose. None of the 4 subjects who experienced a repeat overdose were initiated on MAT within 2 years according to VA records.
Discussions
This retrospective study is representative of many veterans receiving VA care, despite the small sample size. Clinical characteristics observed in the study population were generally consistent with those published by Clement and Stock, including high rates of medical and psychiatric comorbidities.12 Subjects in both studies were prescribed comparable dosages of opioids; among those prescribed opioids but not BZDs through the VA, the mean MEDD was 117 mg in our study compared with 126 mg in the Clement and Stock study. Since implementation of the Opioid Safety Initiative (OSI) in 2013, opioid prescribing practices have improved nationwide across VA facilities, including successful reduction in the numbers of patients prescribed high-dose opioids and concurrent BZDs.13
Despite the tools and resources available to clinicians, discontinuing opioid therapy remains a difficult process. Concerns related to mental health and/or substance-use related decompensations often exist in the setting of rapid dose reductions or abrupt discontinuation of opioids.6 Although less than half of patients in the present study with an active opioid prescription at time of index overdose had their opioids discontinued within 2 years, it is reassuring to note the much higher rate of those with subsequent decreases in their prescribed doses, as well as the 50% reduction in BZD coprescribing. Ultimately, these findings remain consistent with the VA goals of mitigating harm, improving opioid prescribing, and ensuring the safe use of opioid medications when clinically appropriate.
Moreover, recent evidence suggests that interventions focused solely on opioid prescribing practices are becoming increasingly limited in their impact on reducing opioid-related deaths and will likely be insufficient for addressing the opioid epidemic as it continues to evolve. According to Chen and colleagues, opioid overdose deaths are projected to increase over the next several years, while further reduction in the incidence of prescription opioid misuse is estimated to decrease overdose deaths by only 3% to 5.3%. In the context of recent surges in synthetic opioid use, it is projected that 80% of overdose deaths between 2016 and 2025 will be attributable to illicit opioids.3 Such predictions underscore the urgent need to adopt alternative approaches to risk-reducing measures and policy change.
The increased risk of mortality associated with opioid misuse and overdose is well established in the current literature. However, less is known regarding the rate of ORAEs after survival of an NFO. Olfson and colleagues sought to address this knowledge gap by characterizing mortality risks in 76,325 US adults within 1 year following NFO.14 Among their studied population, all-cause mortality occurred at a rate of 778.3 per 10,000 person-years, which was 24 times greater than that of the general population. This emphasizes the need for the optimization of mental health services, addiction treatment, and medical care for these individuals at higher risk.
Limitations
Certain factors and limitations should be considered when interpreting the results of this study. Given that the study included only veterans, factors such as the demographic and clinical characteristics more commonly observed among these patients should be taken into account and may in turn limit the generalizability of these findings to nonveteran populations. Another major limitation is the small sample size; the study period and by extension, the number of patients able to be included in the present study were restricted by the availability of retrievable data from automated drug dispensing systems. Patients without documented response to naloxone were excluded from the study due to low clinical suspicion for opioid overdose, although the possibility that the dose administered was too low to produce a robust clinical response cannot be definitively ruled out. The lack of reliable methods to capture events and overdoses treated outside of the VA may have resulted in underestimations of the true occurrence of ORAEs following NFO. Information regarding naloxone administration outside VA facilities, such as in transport to the hospital, self-reported, or bystander administration, was similarly limited by lack of reliable methods for retrieving such data and absence of documentation in VA records. Although all interventions and outcomes reported in the present study occurred within 2 years following NFO, further conclusions pertaining to the relative timing of specific interventions and ORAEs cannot be made. Lastly, this study did not investigate the direct impact of opioid risk mitigation initiatives implemented by the VA in the years coinciding with the study period.
Future Directions
Despite these limitations, an important strength of this study is its ability to identify potential areas for targeted improvement and to guide further efforts relating to the prevention of opioid overdose and opioid-related mortality among veterans. Identification of individuals at high risk for opioid overdose and misuse is an imperative first step that allows for the implementation of downstream risk-mitigating interventions. Within the VA, several tools have been developed in recent years to provide clinicians with additional resources and support in this regard.6,15
No more than half of those diagnosed with mental health disorders and SUDs in the present study received outpatient follow-up care for these conditions within 6 months following NFO, which may suggest high rates of inadequate treatment. Given the strong association between mental health disorders, SUDs, and increased risk of overdose, increasing engagement with mental health and addiction treatment services may be paramount to preventing subsequent ORAEs, including repeat overdose.6-9,11
Naloxone kit dispensing represents another area for targeted improvement. Interventions may include clinician education and systematic changes, such as implementing protocols that boost the likelihood of high-risk individuals being provided with naloxone at the earliest opportunity. Bystander-administered naloxone programs can also be considered for increasing naloxone access and reducing opioid-related mortality.16
Finally, despite evidence supporting the benefit of MAT in OUD treatment and reducing all-cause and opioid-related mortality after NFO, the low rates of MAT observed in this study are consistent with previous reports that these medications remain underutilized.17 Screening for OUD, in conjunction with increasing access to and utilization of OUD treatment modalities, is an established and integral component of overdose prevention efforts. For VA clinicians, the Psychotropic Drug Safety Initiative (PDSI) dashboard can be used to identify patients diagnosed with OUD who are not yet on MAT.18 Initiatives to expand MAT access through the ED have the potential to provide life-saving interventions and bridge care in the interim until patients are able to become established with a long-term health care practitioner.19
Conclusions
This is the first study to describe HSU, medication interventions, and ORAEs among veterans who survive NFO. Studies have shown that veterans with a history of NFO are at increased risk of subsequent AEs and premature death.6,7,10,14 As such, NFOs represent crucial opportunities to identify high-risk individuals and ensure provision of adequate care. Recent data supports the development of a holistic, multimodal approach focused on adequate treatment of conditions that contribute to opioid-related risks, including mental health disorders, SUDs, pain diagnoses, and medical comorbidities.3,14 Interventions designed to improve access, engagement, and retention in such care therefore play a pivotal role in overdose prevention and reducing mortality.
Although existing risk mitigation initiatives have improved opioid prescribing and safety within the VA, the findings of this study suggest that there remains room for improvement, and the need for well-coordinated efforts to reduce risks associated with both prescribed and illicit opioid use cannot be overstated. Rates of overdose deaths not only remain high but are projected to continue increasing in coming years, despite advances in clinical practice aimed at reducing harms associated with opioid use. The present findings aim to help identify processes with the potential to reduce rates of overdose, death, and adverse sequelae in high-risk populations. However, future studies are warranted to expand on these findings and contribute to ongoing efforts in reducing opioid-related harms and overdose deaths. This study may provide critical insight to inform further investigations to guide such interventions and highlight tools that health care facilities even outside the VA can consider implementing.
Acknowledgments
The authors would like to thank Jonathan Lacro, PharmD, BCPP, for his guidance with this important clinical topic and navigating IRB submissions.
1. Centers for Disease Control and Prevention. Data overview: the drug overdose epidemic: behind the numbers. Updated March 25, 2021. Accessed February 9, 2022. www.cdc.gov/drugoverdose/data/index.html
2. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and Opioid-Involved Overdose Deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. Published 2018 Jan 4. doi:10.15585/mmwr.mm675152e1 3. Chen Q, Larochelle MR, Weaver DT, et al. Prevention of prescription opioid misuse and projected overdose deaths in the United States. JAMA Netw Open. 2019;2(2):e187621. Published 2019 Feb 1. doi:10.1001/jamanetworkopen.2018.7621
4. American Medical Association. Issue brief: nation’s drug-related overdose and death epidemic continues to worsen. Updated November 12, 2021. Accessed February 11, 2022. https://www.ama-assn.org/system/files/issue-brief-increases-in-opioid-related-overdose.pdf
5. Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care. 2011;49(4):393-396. doi:10.1097/MLR.0b013e318202aa27
6. Lewis ET, Trafton J, Oliva E. Data-based case reviews of patients with opioid related risk factors as a tool to prevent overdose and suicide. Accessed February 9, 2022. www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/2488-notes.pdf
7. Zedler B, Xie L, Wang L, et al. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med. 2014;15(11):1911-1929. doi:10.1111/pme.12480
8. Webster LR. Risk Factors for Opioid-Use Disorder and Overdose. Anesth Analg. 2017;125(5):1741-1748. doi:10.1213/ANE.0000000000002496
9. Morasco BJ, Duckart JP, Carr TP, Deyo RA, Dobscha SK. Clinical characteristics of veterans prescribed high doses of opioid medications for chronic non-cancer pain. Pain. 2010;151(3):625-632. doi:10.1016/j.pain.2010.08.002
10. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
11. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans’ Health Administration patients. Pain Med. 2015;16(8):1566-1579. doi:10.1111/pme.12777
12. Clement C, Stock C. Who Overdoses at a VA Emergency Department? Fed Pract. 2016;33(11):14-20.
13. Lin LA, Bohnert ASB, Kerns RD, Clay MA, Ganoczy D, Ilgen MA. Impact of the Opioid Safety Initiative on opioid-related prescribing in veterans. Pain. 2017;158(5):833-839. doi:10.1097/j.pain.0000000000000837
14. Olfson M, Crystal S, Wall M, Wang S, Liu SM, Blanco C. Causes of death after nonfatal opioid overdose [published correction appears in JAMA Psychiatry. 2018 Aug 1;75(8):867]. JAMA Psychiatry. 2018;75(8):820-827. doi:10.1001/jamapsychiatry.2018.1471
15. US Department of Veterans Affairs, Veterans Health Administration. VHA pain management – opioid safety – clinical tools. Updated November 14, 2019. Accessed February 9, 2022. https://www.va.gov/PAINMANAGEMENT/Opioid_Safety/Clinical_Tools.asp
16. Doe-Simkins M, Walley AY, Epstein A, Moyer P. Saved by the nose: bystander-administered intranasal naloxone hydrochloride for opioid overdose. Am J Public Health. 2009;99(5):788-791. doi:10.2105/AJPH.2008.146647
17. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. doi:10.7326/M17-3107
18. Wiechers I. Program focuses on safe psychiatric medication. Published April 21, 2016. Accessed February 9, 2022. https://blogs.va.gov/VAntage/27099/program-focuses-safe-psychiatric-medication/
19. Newman S; California Health Care Foundation. How to pay for it – MAT in the emergency department: FAQ. Published March 2019. Accessed February 9, 2022. https://www.chcf.org/wp-content/uploads/2019/03/HowToPayForMATinED.pdf
The number of opioid-related overdose deaths in the United States is estimated to have increased 6-fold over the past 2 decades.1 In 2017, more than two-thirds of drug overdose deaths involved opioids, yielding a mortality rate of 14.9 per 100,000.2 Not only does the opioid epidemic currently pose a significant public health crisis characterized by high morbidity and mortality, but it is also projected to worsen in coming years. According to Chen and colleagues, opioid overdose deaths are estimated to increase by 147% from 2015 to 2025.3 That projects almost 82,000 US deaths annually and > 700,000 deaths in this period—even before accounting for surges in opioid overdoses and opioid-related mortality coinciding with the COVID-19 pandemic.3,4
As health systems and communities globally struggle with unprecedented losses and stressors introduced by the pandemic, emerging data warrants escalating concerns with regard to increased vulnerability to relapse and overdose among those with mental health and substance use disorders (SUDs). In a recent report, the American Medical Association estimates that opioid-related deaths have increased in more than 40 states with the COVID-19 pandemic.4
Veterans are twice as likely to experience a fatal opioid overdose compared with their civilian counterparts.5 While several risk mitigation strategies have been employed in recent years to improve opioid prescribing and safety within the US Department of Veterans Affairs (VA), veterans continue to overdose on opioids, both prescribed and obtained illicitly.6 Variables shown to be strongly associated with opioid overdose risk include presence of mental health disorders, SUDs, medical conditions involving impaired drug metabolism or excretion, respiratory disorders, higher doses of opioids, concomitant use of sedative medications, and history of overdose.6-8 Many veterans struggle with chronic pain and those prescribed high doses of opioids were more likely to have comorbid pain diagnoses, mental health disorders, and SUDs.9 Dashboards and predictive models, such as the Stratification Tool for Opioid Risk Mitigation (STORM) and the Risk Index for Overdose or Serious Opioid-induced Respiratory Depression (RIOSORD), incorporate such factors to stratify overdose risk among veterans, in an effort to prioritize high-risk individuals for review and provision of care.6,10,11 Despite recent recognition that overdose prevention likely requires a holistic approach that addresses the biopsychosocial factors contributing to opioid-related morbidity and mortality, it is unclear whether veterans are receiving adequate and appropriate treatment for contributing conditions.
There are currently no existing studies that describe health service utilization (HSU), medication interventions, and rates of opioid-related adverse events (ORAEs) among veterans after survival of a nonfatal opioid overdose (NFO). Clinical characteristics of veterans treated for opioid overdose at a VA emergency department (ED) have previously been described by Clement and Stock.12 Despite improvements that have been made in VA opioid prescribing and safety, knowledge gaps remain with regard to best practices for opioid overdose prevention. The aim of this study was to characterize HSU and medication interventions in veterans following NFO, as well as the frequency of ORAEs after overdose. The findings of this study may aid in the identification of areas for targeted improvement in the prevention and reduction of opioid overdoses and adverse opioid-related sequelae.
Methods
This retrospective descriptive study was conducted at VA San Diego Healthcare System (VASDHCS) in California. Subjects included were veterans administered naloxone in the ED for suspected opioid overdose between July 1, 2013 and April 1, 2017. The study population was identified through data retrieved from automated drug dispensing systems, which was then confirmed through manual chart review of notes associated with the index ED visit. Inclusion criteria included documented increased respiration or responsiveness following naloxone administration. Subjects were excluded if they demonstrated lack of response to naloxone, overdosed secondary to inpatient administration of opioids, received palliative or hospice care during the study period, or were lost to follow-up.
Data were collected via retrospective chart review and included date of index ED visit, demographics, active prescriptions, urine drug screen (UDS) results, benzodiazepine (BZD) use corroborated by positive UDS or mention of BZD in index visit chart notes, whether overdose was determined to be a suicide attempt, and naloxone kit dispensing. Patient data was collected for 2 years following overdose, including: ORAEs; ED visits; hospitalizations; repeat overdoses; fatal overdose; whether subjects were still alive; follow-up visits for pain management, mental health, and addiction treatment services; and visits to the psychiatric emergency clinic. Clinical characteristics, such as mental health disorder diagnoses, SUDs, and relevant medical conditions also were collected. Statistical analysis was performed using Microsoft Excel and included only descriptive statistics.
Results
Ninety-three patients received naloxone in the VASDHCS ED. Thirty-five met inclusion criteria and were included in the primary analysis. All subjects received IV naloxone with a mean 0.8 mg IV boluses (range, 0.1-4.4 mg).
Most patients were male with a mean age of 59.8 years (Table 1). Almost all overdoses were nonintentional except for 3 suicide attempts that were reviewed by the Suicide Prevention Committee. Three patients had previously been treated for opioid overdose at the VA with a documented positive clinical response to naloxone administration.
At the time of overdose, 29 patients (82.9%) had an active opioid prescription. Of these, the majority were issued through the VA with a mean 117 mg morphine equivalent daily dose (MEDD). Interestingly, only 24 of the 28 patients with a UDS collected at time of overdose tested positive for opioids, which may be attributable to the use of synthetic opioids, which are not reliably detected by traditional UDS. Concomitant BZD use was involved in 13 of the 35 index overdoses (37.1%), although only 6 patients (17.1%) had an active BZD prescription at time of overdose. Seven patients (20.0%) were prescribed medication-assisted treatment (MAT) for opioid use disorder (OUD), with all 7 using methadone. According to VA records, only 1 patient had previously been dispensed a naloxone kit at any point prior to overdosing. Mental health and SUD diagnoses frequently co-occurred, with 20 patients (57.1%) having at least 1 mental health condition and at least 1 SUD.
Rates of follow-up varied by clinician type in the 6 months after NFO (Figure). Of those with mental health disorders, 15 patients (45.5%) received mental health services before and after overdose, while 8 (40.0%) and 10 (50.0%) of those with SUDs received addiction treatment services before and after overdose, respectively. Seven patients presented to the psychiatric emergency clinic within 6 months prior to overdose and 5 patients within the 6 months following overdose.
Of patients with VA opioid prescriptions, within 2 years of NFO, 9 (42.9%) had their opioids discontinued, and 18 (85.7%) had MEDD reductions ranging from 10 mg to 150 mg (12.5-71.4% reduction) with a mean of 63 mg. Two of the 4 patients with active BZD prescriptions at the time of the overdose event had their prescriptions continued. Seven patients (20.0%) were dispensed naloxone kits following overdose (Table 2).
Rates of ORAEs ranged from 0% to 17% with no documented overdose fatalities. Examples of AEs observed in this study included ED visits or hospitalizations involving opioid withdrawal, opioid-related personality changes, and opioid overdose. Five patients died during the study period, yielding an all-cause mortality rate of 14.3% with a mean time to death of 10.8 months. The causes of death were largely unknown except for 1 patient, whose death was reportedly investigated as an accidental medication overdose without additional information.
Repeat overdose verified by hospital records occurred in 4 patients (11.4%) within 2 years. Patients who experienced a subsequent overdose were prescribed higher doses of opioids with a mean MEDD among VA prescriptions of 130 mg vs 114 mg for those without repeat overdose. In this group, 3 patients (75.0%) also had concomitant BZD use, which was proportionally higher than the 10 patients (32.3%) without a subsequent overdose. Of note, 2 of the 4 patients with a repeat overdose had their opioid doses increased above the MEDD prescribed at the time of index overdose. None of the 4 subjects who experienced a repeat overdose were initiated on MAT within 2 years according to VA records.
Discussions
This retrospective study is representative of many veterans receiving VA care, despite the small sample size. Clinical characteristics observed in the study population were generally consistent with those published by Clement and Stock, including high rates of medical and psychiatric comorbidities.12 Subjects in both studies were prescribed comparable dosages of opioids; among those prescribed opioids but not BZDs through the VA, the mean MEDD was 117 mg in our study compared with 126 mg in the Clement and Stock study. Since implementation of the Opioid Safety Initiative (OSI) in 2013, opioid prescribing practices have improved nationwide across VA facilities, including successful reduction in the numbers of patients prescribed high-dose opioids and concurrent BZDs.13
Despite the tools and resources available to clinicians, discontinuing opioid therapy remains a difficult process. Concerns related to mental health and/or substance-use related decompensations often exist in the setting of rapid dose reductions or abrupt discontinuation of opioids.6 Although less than half of patients in the present study with an active opioid prescription at time of index overdose had their opioids discontinued within 2 years, it is reassuring to note the much higher rate of those with subsequent decreases in their prescribed doses, as well as the 50% reduction in BZD coprescribing. Ultimately, these findings remain consistent with the VA goals of mitigating harm, improving opioid prescribing, and ensuring the safe use of opioid medications when clinically appropriate.
Moreover, recent evidence suggests that interventions focused solely on opioid prescribing practices are becoming increasingly limited in their impact on reducing opioid-related deaths and will likely be insufficient for addressing the opioid epidemic as it continues to evolve. According to Chen and colleagues, opioid overdose deaths are projected to increase over the next several years, while further reduction in the incidence of prescription opioid misuse is estimated to decrease overdose deaths by only 3% to 5.3%. In the context of recent surges in synthetic opioid use, it is projected that 80% of overdose deaths between 2016 and 2025 will be attributable to illicit opioids.3 Such predictions underscore the urgent need to adopt alternative approaches to risk-reducing measures and policy change.
The increased risk of mortality associated with opioid misuse and overdose is well established in the current literature. However, less is known regarding the rate of ORAEs after survival of an NFO. Olfson and colleagues sought to address this knowledge gap by characterizing mortality risks in 76,325 US adults within 1 year following NFO.14 Among their studied population, all-cause mortality occurred at a rate of 778.3 per 10,000 person-years, which was 24 times greater than that of the general population. This emphasizes the need for the optimization of mental health services, addiction treatment, and medical care for these individuals at higher risk.
Limitations
Certain factors and limitations should be considered when interpreting the results of this study. Given that the study included only veterans, factors such as the demographic and clinical characteristics more commonly observed among these patients should be taken into account and may in turn limit the generalizability of these findings to nonveteran populations. Another major limitation is the small sample size; the study period and by extension, the number of patients able to be included in the present study were restricted by the availability of retrievable data from automated drug dispensing systems. Patients without documented response to naloxone were excluded from the study due to low clinical suspicion for opioid overdose, although the possibility that the dose administered was too low to produce a robust clinical response cannot be definitively ruled out. The lack of reliable methods to capture events and overdoses treated outside of the VA may have resulted in underestimations of the true occurrence of ORAEs following NFO. Information regarding naloxone administration outside VA facilities, such as in transport to the hospital, self-reported, or bystander administration, was similarly limited by lack of reliable methods for retrieving such data and absence of documentation in VA records. Although all interventions and outcomes reported in the present study occurred within 2 years following NFO, further conclusions pertaining to the relative timing of specific interventions and ORAEs cannot be made. Lastly, this study did not investigate the direct impact of opioid risk mitigation initiatives implemented by the VA in the years coinciding with the study period.
Future Directions
Despite these limitations, an important strength of this study is its ability to identify potential areas for targeted improvement and to guide further efforts relating to the prevention of opioid overdose and opioid-related mortality among veterans. Identification of individuals at high risk for opioid overdose and misuse is an imperative first step that allows for the implementation of downstream risk-mitigating interventions. Within the VA, several tools have been developed in recent years to provide clinicians with additional resources and support in this regard.6,15
No more than half of those diagnosed with mental health disorders and SUDs in the present study received outpatient follow-up care for these conditions within 6 months following NFO, which may suggest high rates of inadequate treatment. Given the strong association between mental health disorders, SUDs, and increased risk of overdose, increasing engagement with mental health and addiction treatment services may be paramount to preventing subsequent ORAEs, including repeat overdose.6-9,11
Naloxone kit dispensing represents another area for targeted improvement. Interventions may include clinician education and systematic changes, such as implementing protocols that boost the likelihood of high-risk individuals being provided with naloxone at the earliest opportunity. Bystander-administered naloxone programs can also be considered for increasing naloxone access and reducing opioid-related mortality.16
Finally, despite evidence supporting the benefit of MAT in OUD treatment and reducing all-cause and opioid-related mortality after NFO, the low rates of MAT observed in this study are consistent with previous reports that these medications remain underutilized.17 Screening for OUD, in conjunction with increasing access to and utilization of OUD treatment modalities, is an established and integral component of overdose prevention efforts. For VA clinicians, the Psychotropic Drug Safety Initiative (PDSI) dashboard can be used to identify patients diagnosed with OUD who are not yet on MAT.18 Initiatives to expand MAT access through the ED have the potential to provide life-saving interventions and bridge care in the interim until patients are able to become established with a long-term health care practitioner.19
Conclusions
This is the first study to describe HSU, medication interventions, and ORAEs among veterans who survive NFO. Studies have shown that veterans with a history of NFO are at increased risk of subsequent AEs and premature death.6,7,10,14 As such, NFOs represent crucial opportunities to identify high-risk individuals and ensure provision of adequate care. Recent data supports the development of a holistic, multimodal approach focused on adequate treatment of conditions that contribute to opioid-related risks, including mental health disorders, SUDs, pain diagnoses, and medical comorbidities.3,14 Interventions designed to improve access, engagement, and retention in such care therefore play a pivotal role in overdose prevention and reducing mortality.
Although existing risk mitigation initiatives have improved opioid prescribing and safety within the VA, the findings of this study suggest that there remains room for improvement, and the need for well-coordinated efforts to reduce risks associated with both prescribed and illicit opioid use cannot be overstated. Rates of overdose deaths not only remain high but are projected to continue increasing in coming years, despite advances in clinical practice aimed at reducing harms associated with opioid use. The present findings aim to help identify processes with the potential to reduce rates of overdose, death, and adverse sequelae in high-risk populations. However, future studies are warranted to expand on these findings and contribute to ongoing efforts in reducing opioid-related harms and overdose deaths. This study may provide critical insight to inform further investigations to guide such interventions and highlight tools that health care facilities even outside the VA can consider implementing.
Acknowledgments
The authors would like to thank Jonathan Lacro, PharmD, BCPP, for his guidance with this important clinical topic and navigating IRB submissions.
The number of opioid-related overdose deaths in the United States is estimated to have increased 6-fold over the past 2 decades.1 In 2017, more than two-thirds of drug overdose deaths involved opioids, yielding a mortality rate of 14.9 per 100,000.2 Not only does the opioid epidemic currently pose a significant public health crisis characterized by high morbidity and mortality, but it is also projected to worsen in coming years. According to Chen and colleagues, opioid overdose deaths are estimated to increase by 147% from 2015 to 2025.3 That projects almost 82,000 US deaths annually and > 700,000 deaths in this period—even before accounting for surges in opioid overdoses and opioid-related mortality coinciding with the COVID-19 pandemic.3,4
As health systems and communities globally struggle with unprecedented losses and stressors introduced by the pandemic, emerging data warrants escalating concerns with regard to increased vulnerability to relapse and overdose among those with mental health and substance use disorders (SUDs). In a recent report, the American Medical Association estimates that opioid-related deaths have increased in more than 40 states with the COVID-19 pandemic.4
Veterans are twice as likely to experience a fatal opioid overdose compared with their civilian counterparts.5 While several risk mitigation strategies have been employed in recent years to improve opioid prescribing and safety within the US Department of Veterans Affairs (VA), veterans continue to overdose on opioids, both prescribed and obtained illicitly.6 Variables shown to be strongly associated with opioid overdose risk include presence of mental health disorders, SUDs, medical conditions involving impaired drug metabolism or excretion, respiratory disorders, higher doses of opioids, concomitant use of sedative medications, and history of overdose.6-8 Many veterans struggle with chronic pain and those prescribed high doses of opioids were more likely to have comorbid pain diagnoses, mental health disorders, and SUDs.9 Dashboards and predictive models, such as the Stratification Tool for Opioid Risk Mitigation (STORM) and the Risk Index for Overdose or Serious Opioid-induced Respiratory Depression (RIOSORD), incorporate such factors to stratify overdose risk among veterans, in an effort to prioritize high-risk individuals for review and provision of care.6,10,11 Despite recent recognition that overdose prevention likely requires a holistic approach that addresses the biopsychosocial factors contributing to opioid-related morbidity and mortality, it is unclear whether veterans are receiving adequate and appropriate treatment for contributing conditions.
There are currently no existing studies that describe health service utilization (HSU), medication interventions, and rates of opioid-related adverse events (ORAEs) among veterans after survival of a nonfatal opioid overdose (NFO). Clinical characteristics of veterans treated for opioid overdose at a VA emergency department (ED) have previously been described by Clement and Stock.12 Despite improvements that have been made in VA opioid prescribing and safety, knowledge gaps remain with regard to best practices for opioid overdose prevention. The aim of this study was to characterize HSU and medication interventions in veterans following NFO, as well as the frequency of ORAEs after overdose. The findings of this study may aid in the identification of areas for targeted improvement in the prevention and reduction of opioid overdoses and adverse opioid-related sequelae.
Methods
This retrospective descriptive study was conducted at VA San Diego Healthcare System (VASDHCS) in California. Subjects included were veterans administered naloxone in the ED for suspected opioid overdose between July 1, 2013 and April 1, 2017. The study population was identified through data retrieved from automated drug dispensing systems, which was then confirmed through manual chart review of notes associated with the index ED visit. Inclusion criteria included documented increased respiration or responsiveness following naloxone administration. Subjects were excluded if they demonstrated lack of response to naloxone, overdosed secondary to inpatient administration of opioids, received palliative or hospice care during the study period, or were lost to follow-up.
Data were collected via retrospective chart review and included date of index ED visit, demographics, active prescriptions, urine drug screen (UDS) results, benzodiazepine (BZD) use corroborated by positive UDS or mention of BZD in index visit chart notes, whether overdose was determined to be a suicide attempt, and naloxone kit dispensing. Patient data was collected for 2 years following overdose, including: ORAEs; ED visits; hospitalizations; repeat overdoses; fatal overdose; whether subjects were still alive; follow-up visits for pain management, mental health, and addiction treatment services; and visits to the psychiatric emergency clinic. Clinical characteristics, such as mental health disorder diagnoses, SUDs, and relevant medical conditions also were collected. Statistical analysis was performed using Microsoft Excel and included only descriptive statistics.
Results
Ninety-three patients received naloxone in the VASDHCS ED. Thirty-five met inclusion criteria and were included in the primary analysis. All subjects received IV naloxone with a mean 0.8 mg IV boluses (range, 0.1-4.4 mg).
Most patients were male with a mean age of 59.8 years (Table 1). Almost all overdoses were nonintentional except for 3 suicide attempts that were reviewed by the Suicide Prevention Committee. Three patients had previously been treated for opioid overdose at the VA with a documented positive clinical response to naloxone administration.
At the time of overdose, 29 patients (82.9%) had an active opioid prescription. Of these, the majority were issued through the VA with a mean 117 mg morphine equivalent daily dose (MEDD). Interestingly, only 24 of the 28 patients with a UDS collected at time of overdose tested positive for opioids, which may be attributable to the use of synthetic opioids, which are not reliably detected by traditional UDS. Concomitant BZD use was involved in 13 of the 35 index overdoses (37.1%), although only 6 patients (17.1%) had an active BZD prescription at time of overdose. Seven patients (20.0%) were prescribed medication-assisted treatment (MAT) for opioid use disorder (OUD), with all 7 using methadone. According to VA records, only 1 patient had previously been dispensed a naloxone kit at any point prior to overdosing. Mental health and SUD diagnoses frequently co-occurred, with 20 patients (57.1%) having at least 1 mental health condition and at least 1 SUD.
Rates of follow-up varied by clinician type in the 6 months after NFO (Figure). Of those with mental health disorders, 15 patients (45.5%) received mental health services before and after overdose, while 8 (40.0%) and 10 (50.0%) of those with SUDs received addiction treatment services before and after overdose, respectively. Seven patients presented to the psychiatric emergency clinic within 6 months prior to overdose and 5 patients within the 6 months following overdose.
Of patients with VA opioid prescriptions, within 2 years of NFO, 9 (42.9%) had their opioids discontinued, and 18 (85.7%) had MEDD reductions ranging from 10 mg to 150 mg (12.5-71.4% reduction) with a mean of 63 mg. Two of the 4 patients with active BZD prescriptions at the time of the overdose event had their prescriptions continued. Seven patients (20.0%) were dispensed naloxone kits following overdose (Table 2).
Rates of ORAEs ranged from 0% to 17% with no documented overdose fatalities. Examples of AEs observed in this study included ED visits or hospitalizations involving opioid withdrawal, opioid-related personality changes, and opioid overdose. Five patients died during the study period, yielding an all-cause mortality rate of 14.3% with a mean time to death of 10.8 months. The causes of death were largely unknown except for 1 patient, whose death was reportedly investigated as an accidental medication overdose without additional information.
Repeat overdose verified by hospital records occurred in 4 patients (11.4%) within 2 years. Patients who experienced a subsequent overdose were prescribed higher doses of opioids with a mean MEDD among VA prescriptions of 130 mg vs 114 mg for those without repeat overdose. In this group, 3 patients (75.0%) also had concomitant BZD use, which was proportionally higher than the 10 patients (32.3%) without a subsequent overdose. Of note, 2 of the 4 patients with a repeat overdose had their opioid doses increased above the MEDD prescribed at the time of index overdose. None of the 4 subjects who experienced a repeat overdose were initiated on MAT within 2 years according to VA records.
Discussions
This retrospective study is representative of many veterans receiving VA care, despite the small sample size. Clinical characteristics observed in the study population were generally consistent with those published by Clement and Stock, including high rates of medical and psychiatric comorbidities.12 Subjects in both studies were prescribed comparable dosages of opioids; among those prescribed opioids but not BZDs through the VA, the mean MEDD was 117 mg in our study compared with 126 mg in the Clement and Stock study. Since implementation of the Opioid Safety Initiative (OSI) in 2013, opioid prescribing practices have improved nationwide across VA facilities, including successful reduction in the numbers of patients prescribed high-dose opioids and concurrent BZDs.13
Despite the tools and resources available to clinicians, discontinuing opioid therapy remains a difficult process. Concerns related to mental health and/or substance-use related decompensations often exist in the setting of rapid dose reductions or abrupt discontinuation of opioids.6 Although less than half of patients in the present study with an active opioid prescription at time of index overdose had their opioids discontinued within 2 years, it is reassuring to note the much higher rate of those with subsequent decreases in their prescribed doses, as well as the 50% reduction in BZD coprescribing. Ultimately, these findings remain consistent with the VA goals of mitigating harm, improving opioid prescribing, and ensuring the safe use of opioid medications when clinically appropriate.
Moreover, recent evidence suggests that interventions focused solely on opioid prescribing practices are becoming increasingly limited in their impact on reducing opioid-related deaths and will likely be insufficient for addressing the opioid epidemic as it continues to evolve. According to Chen and colleagues, opioid overdose deaths are projected to increase over the next several years, while further reduction in the incidence of prescription opioid misuse is estimated to decrease overdose deaths by only 3% to 5.3%. In the context of recent surges in synthetic opioid use, it is projected that 80% of overdose deaths between 2016 and 2025 will be attributable to illicit opioids.3 Such predictions underscore the urgent need to adopt alternative approaches to risk-reducing measures and policy change.
The increased risk of mortality associated with opioid misuse and overdose is well established in the current literature. However, less is known regarding the rate of ORAEs after survival of an NFO. Olfson and colleagues sought to address this knowledge gap by characterizing mortality risks in 76,325 US adults within 1 year following NFO.14 Among their studied population, all-cause mortality occurred at a rate of 778.3 per 10,000 person-years, which was 24 times greater than that of the general population. This emphasizes the need for the optimization of mental health services, addiction treatment, and medical care for these individuals at higher risk.
Limitations
Certain factors and limitations should be considered when interpreting the results of this study. Given that the study included only veterans, factors such as the demographic and clinical characteristics more commonly observed among these patients should be taken into account and may in turn limit the generalizability of these findings to nonveteran populations. Another major limitation is the small sample size; the study period and by extension, the number of patients able to be included in the present study were restricted by the availability of retrievable data from automated drug dispensing systems. Patients without documented response to naloxone were excluded from the study due to low clinical suspicion for opioid overdose, although the possibility that the dose administered was too low to produce a robust clinical response cannot be definitively ruled out. The lack of reliable methods to capture events and overdoses treated outside of the VA may have resulted in underestimations of the true occurrence of ORAEs following NFO. Information regarding naloxone administration outside VA facilities, such as in transport to the hospital, self-reported, or bystander administration, was similarly limited by lack of reliable methods for retrieving such data and absence of documentation in VA records. Although all interventions and outcomes reported in the present study occurred within 2 years following NFO, further conclusions pertaining to the relative timing of specific interventions and ORAEs cannot be made. Lastly, this study did not investigate the direct impact of opioid risk mitigation initiatives implemented by the VA in the years coinciding with the study period.
Future Directions
Despite these limitations, an important strength of this study is its ability to identify potential areas for targeted improvement and to guide further efforts relating to the prevention of opioid overdose and opioid-related mortality among veterans. Identification of individuals at high risk for opioid overdose and misuse is an imperative first step that allows for the implementation of downstream risk-mitigating interventions. Within the VA, several tools have been developed in recent years to provide clinicians with additional resources and support in this regard.6,15
No more than half of those diagnosed with mental health disorders and SUDs in the present study received outpatient follow-up care for these conditions within 6 months following NFO, which may suggest high rates of inadequate treatment. Given the strong association between mental health disorders, SUDs, and increased risk of overdose, increasing engagement with mental health and addiction treatment services may be paramount to preventing subsequent ORAEs, including repeat overdose.6-9,11
Naloxone kit dispensing represents another area for targeted improvement. Interventions may include clinician education and systematic changes, such as implementing protocols that boost the likelihood of high-risk individuals being provided with naloxone at the earliest opportunity. Bystander-administered naloxone programs can also be considered for increasing naloxone access and reducing opioid-related mortality.16
Finally, despite evidence supporting the benefit of MAT in OUD treatment and reducing all-cause and opioid-related mortality after NFO, the low rates of MAT observed in this study are consistent with previous reports that these medications remain underutilized.17 Screening for OUD, in conjunction with increasing access to and utilization of OUD treatment modalities, is an established and integral component of overdose prevention efforts. For VA clinicians, the Psychotropic Drug Safety Initiative (PDSI) dashboard can be used to identify patients diagnosed with OUD who are not yet on MAT.18 Initiatives to expand MAT access through the ED have the potential to provide life-saving interventions and bridge care in the interim until patients are able to become established with a long-term health care practitioner.19
Conclusions
This is the first study to describe HSU, medication interventions, and ORAEs among veterans who survive NFO. Studies have shown that veterans with a history of NFO are at increased risk of subsequent AEs and premature death.6,7,10,14 As such, NFOs represent crucial opportunities to identify high-risk individuals and ensure provision of adequate care. Recent data supports the development of a holistic, multimodal approach focused on adequate treatment of conditions that contribute to opioid-related risks, including mental health disorders, SUDs, pain diagnoses, and medical comorbidities.3,14 Interventions designed to improve access, engagement, and retention in such care therefore play a pivotal role in overdose prevention and reducing mortality.
Although existing risk mitigation initiatives have improved opioid prescribing and safety within the VA, the findings of this study suggest that there remains room for improvement, and the need for well-coordinated efforts to reduce risks associated with both prescribed and illicit opioid use cannot be overstated. Rates of overdose deaths not only remain high but are projected to continue increasing in coming years, despite advances in clinical practice aimed at reducing harms associated with opioid use. The present findings aim to help identify processes with the potential to reduce rates of overdose, death, and adverse sequelae in high-risk populations. However, future studies are warranted to expand on these findings and contribute to ongoing efforts in reducing opioid-related harms and overdose deaths. This study may provide critical insight to inform further investigations to guide such interventions and highlight tools that health care facilities even outside the VA can consider implementing.
Acknowledgments
The authors would like to thank Jonathan Lacro, PharmD, BCPP, for his guidance with this important clinical topic and navigating IRB submissions.
1. Centers for Disease Control and Prevention. Data overview: the drug overdose epidemic: behind the numbers. Updated March 25, 2021. Accessed February 9, 2022. www.cdc.gov/drugoverdose/data/index.html
2. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and Opioid-Involved Overdose Deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. Published 2018 Jan 4. doi:10.15585/mmwr.mm675152e1 3. Chen Q, Larochelle MR, Weaver DT, et al. Prevention of prescription opioid misuse and projected overdose deaths in the United States. JAMA Netw Open. 2019;2(2):e187621. Published 2019 Feb 1. doi:10.1001/jamanetworkopen.2018.7621
4. American Medical Association. Issue brief: nation’s drug-related overdose and death epidemic continues to worsen. Updated November 12, 2021. Accessed February 11, 2022. https://www.ama-assn.org/system/files/issue-brief-increases-in-opioid-related-overdose.pdf
5. Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care. 2011;49(4):393-396. doi:10.1097/MLR.0b013e318202aa27
6. Lewis ET, Trafton J, Oliva E. Data-based case reviews of patients with opioid related risk factors as a tool to prevent overdose and suicide. Accessed February 9, 2022. www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/2488-notes.pdf
7. Zedler B, Xie L, Wang L, et al. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med. 2014;15(11):1911-1929. doi:10.1111/pme.12480
8. Webster LR. Risk Factors for Opioid-Use Disorder and Overdose. Anesth Analg. 2017;125(5):1741-1748. doi:10.1213/ANE.0000000000002496
9. Morasco BJ, Duckart JP, Carr TP, Deyo RA, Dobscha SK. Clinical characteristics of veterans prescribed high doses of opioid medications for chronic non-cancer pain. Pain. 2010;151(3):625-632. doi:10.1016/j.pain.2010.08.002
10. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
11. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans’ Health Administration patients. Pain Med. 2015;16(8):1566-1579. doi:10.1111/pme.12777
12. Clement C, Stock C. Who Overdoses at a VA Emergency Department? Fed Pract. 2016;33(11):14-20.
13. Lin LA, Bohnert ASB, Kerns RD, Clay MA, Ganoczy D, Ilgen MA. Impact of the Opioid Safety Initiative on opioid-related prescribing in veterans. Pain. 2017;158(5):833-839. doi:10.1097/j.pain.0000000000000837
14. Olfson M, Crystal S, Wall M, Wang S, Liu SM, Blanco C. Causes of death after nonfatal opioid overdose [published correction appears in JAMA Psychiatry. 2018 Aug 1;75(8):867]. JAMA Psychiatry. 2018;75(8):820-827. doi:10.1001/jamapsychiatry.2018.1471
15. US Department of Veterans Affairs, Veterans Health Administration. VHA pain management – opioid safety – clinical tools. Updated November 14, 2019. Accessed February 9, 2022. https://www.va.gov/PAINMANAGEMENT/Opioid_Safety/Clinical_Tools.asp
16. Doe-Simkins M, Walley AY, Epstein A, Moyer P. Saved by the nose: bystander-administered intranasal naloxone hydrochloride for opioid overdose. Am J Public Health. 2009;99(5):788-791. doi:10.2105/AJPH.2008.146647
17. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. doi:10.7326/M17-3107
18. Wiechers I. Program focuses on safe psychiatric medication. Published April 21, 2016. Accessed February 9, 2022. https://blogs.va.gov/VAntage/27099/program-focuses-safe-psychiatric-medication/
19. Newman S; California Health Care Foundation. How to pay for it – MAT in the emergency department: FAQ. Published March 2019. Accessed February 9, 2022. https://www.chcf.org/wp-content/uploads/2019/03/HowToPayForMATinED.pdf
1. Centers for Disease Control and Prevention. Data overview: the drug overdose epidemic: behind the numbers. Updated March 25, 2021. Accessed February 9, 2022. www.cdc.gov/drugoverdose/data/index.html
2. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and Opioid-Involved Overdose Deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. Published 2018 Jan 4. doi:10.15585/mmwr.mm675152e1 3. Chen Q, Larochelle MR, Weaver DT, et al. Prevention of prescription opioid misuse and projected overdose deaths in the United States. JAMA Netw Open. 2019;2(2):e187621. Published 2019 Feb 1. doi:10.1001/jamanetworkopen.2018.7621
4. American Medical Association. Issue brief: nation’s drug-related overdose and death epidemic continues to worsen. Updated November 12, 2021. Accessed February 11, 2022. https://www.ama-assn.org/system/files/issue-brief-increases-in-opioid-related-overdose.pdf
5. Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care. 2011;49(4):393-396. doi:10.1097/MLR.0b013e318202aa27
6. Lewis ET, Trafton J, Oliva E. Data-based case reviews of patients with opioid related risk factors as a tool to prevent overdose and suicide. Accessed February 9, 2022. www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/2488-notes.pdf
7. Zedler B, Xie L, Wang L, et al. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med. 2014;15(11):1911-1929. doi:10.1111/pme.12480
8. Webster LR. Risk Factors for Opioid-Use Disorder and Overdose. Anesth Analg. 2017;125(5):1741-1748. doi:10.1213/ANE.0000000000002496
9. Morasco BJ, Duckart JP, Carr TP, Deyo RA, Dobscha SK. Clinical characteristics of veterans prescribed high doses of opioid medications for chronic non-cancer pain. Pain. 2010;151(3):625-632. doi:10.1016/j.pain.2010.08.002
10. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
11. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans’ Health Administration patients. Pain Med. 2015;16(8):1566-1579. doi:10.1111/pme.12777
12. Clement C, Stock C. Who Overdoses at a VA Emergency Department? Fed Pract. 2016;33(11):14-20.
13. Lin LA, Bohnert ASB, Kerns RD, Clay MA, Ganoczy D, Ilgen MA. Impact of the Opioid Safety Initiative on opioid-related prescribing in veterans. Pain. 2017;158(5):833-839. doi:10.1097/j.pain.0000000000000837
14. Olfson M, Crystal S, Wall M, Wang S, Liu SM, Blanco C. Causes of death after nonfatal opioid overdose [published correction appears in JAMA Psychiatry. 2018 Aug 1;75(8):867]. JAMA Psychiatry. 2018;75(8):820-827. doi:10.1001/jamapsychiatry.2018.1471
15. US Department of Veterans Affairs, Veterans Health Administration. VHA pain management – opioid safety – clinical tools. Updated November 14, 2019. Accessed February 9, 2022. https://www.va.gov/PAINMANAGEMENT/Opioid_Safety/Clinical_Tools.asp
16. Doe-Simkins M, Walley AY, Epstein A, Moyer P. Saved by the nose: bystander-administered intranasal naloxone hydrochloride for opioid overdose. Am J Public Health. 2009;99(5):788-791. doi:10.2105/AJPH.2008.146647
17. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. doi:10.7326/M17-3107
18. Wiechers I. Program focuses on safe psychiatric medication. Published April 21, 2016. Accessed February 9, 2022. https://blogs.va.gov/VAntage/27099/program-focuses-safe-psychiatric-medication/
19. Newman S; California Health Care Foundation. How to pay for it – MAT in the emergency department: FAQ. Published March 2019. Accessed February 9, 2022. https://www.chcf.org/wp-content/uploads/2019/03/HowToPayForMATinED.pdf
Mindfulness intervention curbs opioid misuse, chronic pain
In a randomized clinical trial, 250 adults with both opioid misuse and chronic pain received either the intervention, called mindfulness-oriented recovery enhancement (MORE), or supportive psychotherapy.
Results showed the first group was twice as likely to reduce opioid misuse after 9 months than the latter group.
The intervention was developed by Eric Garland, PhD, director of the Center on Mindfulness and Integrative Health Intervention Development (C-MIIND), University of Utah, Salt Lake City. “As the largest and longest-term clinical trial of MORE ever conducted, this study definitively establishes the efficacy of MORE as a treatment for chronic pain and opioid misuse,” he told this news organization.
The findings were published online Feb. 28 in JAMA Internal Medicine.
Self-regulation
Study participants included 250 adults (64% women; mean age, 51.8 years) with co-occurring opioid misuse and chronic pain who were randomly allocated to receive MORE or supportive psychotherapy, which served as a control group.
Both interventions were delivered by trained clinical social workers in six primary care clinics in Utah to groups of 6-12 participants across 8 weekly 2-hour sessions.
The MORE intervention, detailed on Dr. Garland’s website, provides sequenced training in mindfulness, reappraisal, and savoring skills.
Mindfulness consisted of meditation on breathing and body sensations to strengthen self-regulation of compulsive opioid use and to mitigate pain and opioid craving by reinterpreting these experiences as innocuous sensory information.
Reappraisal consisted of reframing maladaptive thoughts to decrease negative emotions and engender meaning in life.
Savoring consisted of training in focusing awareness on pleasurable events and sensations to amplify positive emotions and reward.
Fewer depressive symptoms
Through 9 months of follow-up, the MORE group had about a twofold greater likelihood than the supportive psychotherapy group for reduction in opioid misuse (odds ratio [OR], 2.06; 95% confidence interval, 1.17-3.61; P = .01)
“MORE reduced opioid misuse by 45% 9 months after the end of treatment, more than doubling the effect of standard supportive psychotherapy and exceeding the effect size of other therapies for opioid misuse among people with chronic pain,” Dr. Garland said.
Members of the MORE group experienced greater reduction in pain severity and pain-related functional interference compared with members of the control group.
“MORE’s effect size on chronic pain symptoms was greater than that observed for CBT, the current gold standard psychological treatment for chronic pain,” Dr. Garland noted.
Compared with supportive psychotherapy, MORE decreased emotional distress, depressive symptoms, and real-time reports of opioid craving in daily life.
“Although nearly 70% of participants met criteria for depression at the beginning of the trial, on average, patients in MORE no longer exhibited symptoms consistent with major depressive disorder by the end of the study,” Dr. Garland said.
The current study builds on prior studies of MORE showing similar results, as reported previously by this news organization.
MORE can be successfully delivered in routine primary care, Dr. Garland noted. “In this trial, we delivered MORE in conference rooms, break rooms, and lunch rooms at community primary care clinics,” he added.
‘Powerful program’
To date, Dr. Garland has trained more than 450 physicians, nurses, social workers, and psychologists in health care systems across the country to implement MORE as an insurance-reimbursable group visit for patients in need.
One of them is Nancy Sudak, MD, chief well-being officer and director of integrative health, Essentia Health, Duluth, Minn.
“MORE is a very powerful program that teaches patients how to turn down the volume of their pain. I’ve been quite impressed by the power of MORE,” Dr. Sudak told this news organization
She noted that “buy-in” from patients is key – and the more a clinician knows a patient, the easier the buy-in.
“I recruited most of the patients in my groups from my own practice, so I already knew the patients quite well and there wasn’t really a need to sell it,” Dr. Sudak said.
“We have tried to operationalize it through our system and find that, as long as our recruitment techniques are robust enough, it’s not that hard to find patients to fill the groups, especially because chronic pain is just so common,” she added.
Dr. Sudak has found that patients who participate in MORE “bond and learn with each other and support each other. Patients love it, providers love it, and it’s a way to address isolation and loneliness” that can come with certain conditions.
“There are really only upsides to the group visit model and I think we’ll be seeing quite a bit more of it in the future,” she added.
Evidence-based data
Anna Parisi, PhD, is also delivering MORE to patients. She told this news organization, she was “really drawn” to the MORE program because oftentimes patients who require the most sophisticated therapies receive the ones with the least evidence.
This is often “what folks in the community are getting when they’re struggling with substance use,” added Dr. Parisi, a postdoctoral research associate working with Dr. Garland at the University of Utah. Dr. Parisi was not a coauthor on the current study.
“With MORE, all of the strategies and techniques are tied to mechanistic studies of their efficacy, so you know that what you’re delivering has a rationale behind it,” she said.
Like Dr. Sudak, Dr. Parisi said her patients, for the most part, have been receptive to the program. Although at first some were skeptical about mindfulness – with one patient using the term “tree-hugging” – they found immediate benefit even after the first session.
“That really helps them stay motivated to finish the program,” Dr. Parisi said.
This work was supported by a grant from the National Institute on Drug Abuse. Dr. Garland serves as director of the Center on Mindfulness and Integrative Health Intervention Development, which provides MORE, mindfulness-based therapy, and CBT in the context of research trials for no cost to research participants. He receives honoraria and payment for delivering seminars, lectures, and teaching engagements related to training clinicians in MORE and mindfulness and receives royalties from BehaVR and from the sales of books related to MORE outside the submitted work. Dr. Sudak and Dr. Parisi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized clinical trial, 250 adults with both opioid misuse and chronic pain received either the intervention, called mindfulness-oriented recovery enhancement (MORE), or supportive psychotherapy.
Results showed the first group was twice as likely to reduce opioid misuse after 9 months than the latter group.
The intervention was developed by Eric Garland, PhD, director of the Center on Mindfulness and Integrative Health Intervention Development (C-MIIND), University of Utah, Salt Lake City. “As the largest and longest-term clinical trial of MORE ever conducted, this study definitively establishes the efficacy of MORE as a treatment for chronic pain and opioid misuse,” he told this news organization.
The findings were published online Feb. 28 in JAMA Internal Medicine.
Self-regulation
Study participants included 250 adults (64% women; mean age, 51.8 years) with co-occurring opioid misuse and chronic pain who were randomly allocated to receive MORE or supportive psychotherapy, which served as a control group.
Both interventions were delivered by trained clinical social workers in six primary care clinics in Utah to groups of 6-12 participants across 8 weekly 2-hour sessions.
The MORE intervention, detailed on Dr. Garland’s website, provides sequenced training in mindfulness, reappraisal, and savoring skills.
Mindfulness consisted of meditation on breathing and body sensations to strengthen self-regulation of compulsive opioid use and to mitigate pain and opioid craving by reinterpreting these experiences as innocuous sensory information.
Reappraisal consisted of reframing maladaptive thoughts to decrease negative emotions and engender meaning in life.
Savoring consisted of training in focusing awareness on pleasurable events and sensations to amplify positive emotions and reward.
Fewer depressive symptoms
Through 9 months of follow-up, the MORE group had about a twofold greater likelihood than the supportive psychotherapy group for reduction in opioid misuse (odds ratio [OR], 2.06; 95% confidence interval, 1.17-3.61; P = .01)
“MORE reduced opioid misuse by 45% 9 months after the end of treatment, more than doubling the effect of standard supportive psychotherapy and exceeding the effect size of other therapies for opioid misuse among people with chronic pain,” Dr. Garland said.
Members of the MORE group experienced greater reduction in pain severity and pain-related functional interference compared with members of the control group.
“MORE’s effect size on chronic pain symptoms was greater than that observed for CBT, the current gold standard psychological treatment for chronic pain,” Dr. Garland noted.
Compared with supportive psychotherapy, MORE decreased emotional distress, depressive symptoms, and real-time reports of opioid craving in daily life.
“Although nearly 70% of participants met criteria for depression at the beginning of the trial, on average, patients in MORE no longer exhibited symptoms consistent with major depressive disorder by the end of the study,” Dr. Garland said.
The current study builds on prior studies of MORE showing similar results, as reported previously by this news organization.
MORE can be successfully delivered in routine primary care, Dr. Garland noted. “In this trial, we delivered MORE in conference rooms, break rooms, and lunch rooms at community primary care clinics,” he added.
‘Powerful program’
To date, Dr. Garland has trained more than 450 physicians, nurses, social workers, and psychologists in health care systems across the country to implement MORE as an insurance-reimbursable group visit for patients in need.
One of them is Nancy Sudak, MD, chief well-being officer and director of integrative health, Essentia Health, Duluth, Minn.
“MORE is a very powerful program that teaches patients how to turn down the volume of their pain. I’ve been quite impressed by the power of MORE,” Dr. Sudak told this news organization
She noted that “buy-in” from patients is key – and the more a clinician knows a patient, the easier the buy-in.
“I recruited most of the patients in my groups from my own practice, so I already knew the patients quite well and there wasn’t really a need to sell it,” Dr. Sudak said.
“We have tried to operationalize it through our system and find that, as long as our recruitment techniques are robust enough, it’s not that hard to find patients to fill the groups, especially because chronic pain is just so common,” she added.
Dr. Sudak has found that patients who participate in MORE “bond and learn with each other and support each other. Patients love it, providers love it, and it’s a way to address isolation and loneliness” that can come with certain conditions.
“There are really only upsides to the group visit model and I think we’ll be seeing quite a bit more of it in the future,” she added.
Evidence-based data
Anna Parisi, PhD, is also delivering MORE to patients. She told this news organization, she was “really drawn” to the MORE program because oftentimes patients who require the most sophisticated therapies receive the ones with the least evidence.
This is often “what folks in the community are getting when they’re struggling with substance use,” added Dr. Parisi, a postdoctoral research associate working with Dr. Garland at the University of Utah. Dr. Parisi was not a coauthor on the current study.
“With MORE, all of the strategies and techniques are tied to mechanistic studies of their efficacy, so you know that what you’re delivering has a rationale behind it,” she said.
Like Dr. Sudak, Dr. Parisi said her patients, for the most part, have been receptive to the program. Although at first some were skeptical about mindfulness – with one patient using the term “tree-hugging” – they found immediate benefit even after the first session.
“That really helps them stay motivated to finish the program,” Dr. Parisi said.
This work was supported by a grant from the National Institute on Drug Abuse. Dr. Garland serves as director of the Center on Mindfulness and Integrative Health Intervention Development, which provides MORE, mindfulness-based therapy, and CBT in the context of research trials for no cost to research participants. He receives honoraria and payment for delivering seminars, lectures, and teaching engagements related to training clinicians in MORE and mindfulness and receives royalties from BehaVR and from the sales of books related to MORE outside the submitted work. Dr. Sudak and Dr. Parisi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized clinical trial, 250 adults with both opioid misuse and chronic pain received either the intervention, called mindfulness-oriented recovery enhancement (MORE), or supportive psychotherapy.
Results showed the first group was twice as likely to reduce opioid misuse after 9 months than the latter group.
The intervention was developed by Eric Garland, PhD, director of the Center on Mindfulness and Integrative Health Intervention Development (C-MIIND), University of Utah, Salt Lake City. “As the largest and longest-term clinical trial of MORE ever conducted, this study definitively establishes the efficacy of MORE as a treatment for chronic pain and opioid misuse,” he told this news organization.
The findings were published online Feb. 28 in JAMA Internal Medicine.
Self-regulation
Study participants included 250 adults (64% women; mean age, 51.8 years) with co-occurring opioid misuse and chronic pain who were randomly allocated to receive MORE or supportive psychotherapy, which served as a control group.
Both interventions were delivered by trained clinical social workers in six primary care clinics in Utah to groups of 6-12 participants across 8 weekly 2-hour sessions.
The MORE intervention, detailed on Dr. Garland’s website, provides sequenced training in mindfulness, reappraisal, and savoring skills.
Mindfulness consisted of meditation on breathing and body sensations to strengthen self-regulation of compulsive opioid use and to mitigate pain and opioid craving by reinterpreting these experiences as innocuous sensory information.
Reappraisal consisted of reframing maladaptive thoughts to decrease negative emotions and engender meaning in life.
Savoring consisted of training in focusing awareness on pleasurable events and sensations to amplify positive emotions and reward.
Fewer depressive symptoms
Through 9 months of follow-up, the MORE group had about a twofold greater likelihood than the supportive psychotherapy group for reduction in opioid misuse (odds ratio [OR], 2.06; 95% confidence interval, 1.17-3.61; P = .01)
“MORE reduced opioid misuse by 45% 9 months after the end of treatment, more than doubling the effect of standard supportive psychotherapy and exceeding the effect size of other therapies for opioid misuse among people with chronic pain,” Dr. Garland said.
Members of the MORE group experienced greater reduction in pain severity and pain-related functional interference compared with members of the control group.
“MORE’s effect size on chronic pain symptoms was greater than that observed for CBT, the current gold standard psychological treatment for chronic pain,” Dr. Garland noted.
Compared with supportive psychotherapy, MORE decreased emotional distress, depressive symptoms, and real-time reports of opioid craving in daily life.
“Although nearly 70% of participants met criteria for depression at the beginning of the trial, on average, patients in MORE no longer exhibited symptoms consistent with major depressive disorder by the end of the study,” Dr. Garland said.
The current study builds on prior studies of MORE showing similar results, as reported previously by this news organization.
MORE can be successfully delivered in routine primary care, Dr. Garland noted. “In this trial, we delivered MORE in conference rooms, break rooms, and lunch rooms at community primary care clinics,” he added.
‘Powerful program’
To date, Dr. Garland has trained more than 450 physicians, nurses, social workers, and psychologists in health care systems across the country to implement MORE as an insurance-reimbursable group visit for patients in need.
One of them is Nancy Sudak, MD, chief well-being officer and director of integrative health, Essentia Health, Duluth, Minn.
“MORE is a very powerful program that teaches patients how to turn down the volume of their pain. I’ve been quite impressed by the power of MORE,” Dr. Sudak told this news organization
She noted that “buy-in” from patients is key – and the more a clinician knows a patient, the easier the buy-in.
“I recruited most of the patients in my groups from my own practice, so I already knew the patients quite well and there wasn’t really a need to sell it,” Dr. Sudak said.
“We have tried to operationalize it through our system and find that, as long as our recruitment techniques are robust enough, it’s not that hard to find patients to fill the groups, especially because chronic pain is just so common,” she added.
Dr. Sudak has found that patients who participate in MORE “bond and learn with each other and support each other. Patients love it, providers love it, and it’s a way to address isolation and loneliness” that can come with certain conditions.
“There are really only upsides to the group visit model and I think we’ll be seeing quite a bit more of it in the future,” she added.
Evidence-based data
Anna Parisi, PhD, is also delivering MORE to patients. She told this news organization, she was “really drawn” to the MORE program because oftentimes patients who require the most sophisticated therapies receive the ones with the least evidence.
This is often “what folks in the community are getting when they’re struggling with substance use,” added Dr. Parisi, a postdoctoral research associate working with Dr. Garland at the University of Utah. Dr. Parisi was not a coauthor on the current study.
“With MORE, all of the strategies and techniques are tied to mechanistic studies of their efficacy, so you know that what you’re delivering has a rationale behind it,” she said.
Like Dr. Sudak, Dr. Parisi said her patients, for the most part, have been receptive to the program. Although at first some were skeptical about mindfulness – with one patient using the term “tree-hugging” – they found immediate benefit even after the first session.
“That really helps them stay motivated to finish the program,” Dr. Parisi said.
This work was supported by a grant from the National Institute on Drug Abuse. Dr. Garland serves as director of the Center on Mindfulness and Integrative Health Intervention Development, which provides MORE, mindfulness-based therapy, and CBT in the context of research trials for no cost to research participants. He receives honoraria and payment for delivering seminars, lectures, and teaching engagements related to training clinicians in MORE and mindfulness and receives royalties from BehaVR and from the sales of books related to MORE outside the submitted work. Dr. Sudak and Dr. Parisi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tips for managing youth with substance use disorders
LAS VEGAS – Timothy E. Wilens, MD, advised during an annual psychopharmacology update held by the Nevada Psychiatric Association.
“We see high rates of STDs, and we have about 10% of our kids who use opioids who already have hepatitis C,” said Dr. Wilens, who is chief of the division of child & adolescent psychiatry at Massachusetts General Hospital, Boston. “These are kids who may be 16, 17, or 18.”
While the CRAFTT Screening Test has been widely used to screen for substance-related risks and problems in adolescents, another more recent option is the Screening to Brief Intervention (S2BI). Both tools collect information about both alcohol and drug use, are supported by strong research, are available for free, and are easy to use, Dr. Wilens said.
After you generate a differential diagnosis for psychiatric/medical symptoms, clinicians should order urine, saliva, or hair toxicology screens. “We don’t recommend that toxicology screens be done by parents; we do the toxicology screens,” he said. “Be careful about certain things like limitations of detection in the case of high-potency benzodiazepines and duration of detection in the case of marijuana use. The other thing is some of our screens can be used qualitatively or quantitatively. Why is that helpful? If you’re following someone who’s on marijuana and they’re cutting back, you can see if use [really] goes down over time.”
In Dr. Wilen’s clinical experience, efforts to stabilize adolescents with substance use disorders are most effective when patients join support groups comprised of other people from similar sociodemographic backgrounds. “There are different self-help philosophies, but when you’re referring, I always tell people: ‘Have the kid look in a mirror.’ So, if you have an LGBTQ patient from the inner city, that person should not be going to an Alcoholics Anonymous meeting of middle-aged persons in the suburbs. That’s not going to work for them. You want them to be with very similar sociodemographic groups if possible.”
Support groups for parents are also helpful. “There are two levels here: Peer groups of parents that help each other with support and find referrals, and there are parent coaching groups, where you have patients work with professionals,” said Dr. Wilens, who is also codirector of the MGH Center for Addiction Medicine. He advises parents to avoid “tough love” as the first step in efforts to help their child. “Tough love is, you throw the kid out of the house because they won’t stop using,” he said. “Where do you think the kid lives if they’re not at home? Where do you think they’re going to go? Maybe to the home of a friend or a family member for 1 or 2 nights but otherwise they’re living on the streets. How do you think they’re going to make a living if they’re living on the streets? They either sell drugs, or they get involved in prostitution. I have worked with more kids who are furious at their parents because they threw them out of the house. I understand where the patients are coming from, but maybe have a graduated exit instead, where the kid has to sleep outside in a camper for 2 nights, or in an isolated room in the house, or to grandma’s house, which smells like mothballs. Have a graduated approach.”
Psychotherapy is the mainstay of treatment and begins with motivational interviewing. To foster a collaborative connection, Dr. Wilens advises clinicians to discuss issues that are problematic instead of focusing on the substance use right off the bat. “Rather than go right to saying, ‘let’s talk about you smoking too much marijuana,’ instead say, ‘what is it you think may be causing the fights with your parents?’ Or, maybe their peer group isn’t accepting them like they used to.”
In his experience, adolescents respond well to goal setting. For example, for patients who say they’re smoking marijuana every day, Dr. Wilens may ask if they can cut back use to three days per week. “I’ll say: ‘I’m going to write this down in the chart,’ ” he said. “They start to work on it. If they come back and they didn’t reach that goal I say: ‘If you can’t cut back it’s okay; I just need to know it.’ ” He also recommends “sobriety sampling” which asks the patient to make a minimal commitment to stop using, for say, 30 days. “Don’t forget to monitor substance use during follow-up meetings.”
According to Dr. Wilens, child psychiatrists can help prevent substance abuse by encouraging discussion within families by the time kids are in fifth grade and encouraging parents to monitor children’s activities, friends, and personal space. “Privacy is a relative term,” he said. “It’s good you’re in their space. Make their beds; go into their bedroom.” He also advises parents to not smoke marijuana behind their kids’ backs. “I love it when parents tell me: ‘They don’t know I smoke marijuana.’ My counter to that is ‘not only do they know, they’re smoking your marijuana.’ ”
He concluded his remarks by encouraging child psychiatrists to advocate for sensible public laws related to marijuana and other substances. “Zero tolerance laws don’t work, because 85% of kids experiment [with drugs],” said Dr. Wilens, who is also professor of psychiatry at Harvard Medical School, Boston. “It works great until it’s your kid or a neighbor’s kid who’s a good kid but gets thrown out of school.”
Dr. Wilens reported that he has received grant support from the National Institutes of Health and the Food and Drug Administration. He has also served as a consultant to Vallon and has a licensing/collaborative agreement with Ironshore and 3D Therapy.
LAS VEGAS – Timothy E. Wilens, MD, advised during an annual psychopharmacology update held by the Nevada Psychiatric Association.
“We see high rates of STDs, and we have about 10% of our kids who use opioids who already have hepatitis C,” said Dr. Wilens, who is chief of the division of child & adolescent psychiatry at Massachusetts General Hospital, Boston. “These are kids who may be 16, 17, or 18.”
While the CRAFTT Screening Test has been widely used to screen for substance-related risks and problems in adolescents, another more recent option is the Screening to Brief Intervention (S2BI). Both tools collect information about both alcohol and drug use, are supported by strong research, are available for free, and are easy to use, Dr. Wilens said.
After you generate a differential diagnosis for psychiatric/medical symptoms, clinicians should order urine, saliva, or hair toxicology screens. “We don’t recommend that toxicology screens be done by parents; we do the toxicology screens,” he said. “Be careful about certain things like limitations of detection in the case of high-potency benzodiazepines and duration of detection in the case of marijuana use. The other thing is some of our screens can be used qualitatively or quantitatively. Why is that helpful? If you’re following someone who’s on marijuana and they’re cutting back, you can see if use [really] goes down over time.”
In Dr. Wilen’s clinical experience, efforts to stabilize adolescents with substance use disorders are most effective when patients join support groups comprised of other people from similar sociodemographic backgrounds. “There are different self-help philosophies, but when you’re referring, I always tell people: ‘Have the kid look in a mirror.’ So, if you have an LGBTQ patient from the inner city, that person should not be going to an Alcoholics Anonymous meeting of middle-aged persons in the suburbs. That’s not going to work for them. You want them to be with very similar sociodemographic groups if possible.”
Support groups for parents are also helpful. “There are two levels here: Peer groups of parents that help each other with support and find referrals, and there are parent coaching groups, where you have patients work with professionals,” said Dr. Wilens, who is also codirector of the MGH Center for Addiction Medicine. He advises parents to avoid “tough love” as the first step in efforts to help their child. “Tough love is, you throw the kid out of the house because they won’t stop using,” he said. “Where do you think the kid lives if they’re not at home? Where do you think they’re going to go? Maybe to the home of a friend or a family member for 1 or 2 nights but otherwise they’re living on the streets. How do you think they’re going to make a living if they’re living on the streets? They either sell drugs, or they get involved in prostitution. I have worked with more kids who are furious at their parents because they threw them out of the house. I understand where the patients are coming from, but maybe have a graduated exit instead, where the kid has to sleep outside in a camper for 2 nights, or in an isolated room in the house, or to grandma’s house, which smells like mothballs. Have a graduated approach.”
Psychotherapy is the mainstay of treatment and begins with motivational interviewing. To foster a collaborative connection, Dr. Wilens advises clinicians to discuss issues that are problematic instead of focusing on the substance use right off the bat. “Rather than go right to saying, ‘let’s talk about you smoking too much marijuana,’ instead say, ‘what is it you think may be causing the fights with your parents?’ Or, maybe their peer group isn’t accepting them like they used to.”
In his experience, adolescents respond well to goal setting. For example, for patients who say they’re smoking marijuana every day, Dr. Wilens may ask if they can cut back use to three days per week. “I’ll say: ‘I’m going to write this down in the chart,’ ” he said. “They start to work on it. If they come back and they didn’t reach that goal I say: ‘If you can’t cut back it’s okay; I just need to know it.’ ” He also recommends “sobriety sampling” which asks the patient to make a minimal commitment to stop using, for say, 30 days. “Don’t forget to monitor substance use during follow-up meetings.”
According to Dr. Wilens, child psychiatrists can help prevent substance abuse by encouraging discussion within families by the time kids are in fifth grade and encouraging parents to monitor children’s activities, friends, and personal space. “Privacy is a relative term,” he said. “It’s good you’re in their space. Make their beds; go into their bedroom.” He also advises parents to not smoke marijuana behind their kids’ backs. “I love it when parents tell me: ‘They don’t know I smoke marijuana.’ My counter to that is ‘not only do they know, they’re smoking your marijuana.’ ”
He concluded his remarks by encouraging child psychiatrists to advocate for sensible public laws related to marijuana and other substances. “Zero tolerance laws don’t work, because 85% of kids experiment [with drugs],” said Dr. Wilens, who is also professor of psychiatry at Harvard Medical School, Boston. “It works great until it’s your kid or a neighbor’s kid who’s a good kid but gets thrown out of school.”
Dr. Wilens reported that he has received grant support from the National Institutes of Health and the Food and Drug Administration. He has also served as a consultant to Vallon and has a licensing/collaborative agreement with Ironshore and 3D Therapy.
LAS VEGAS – Timothy E. Wilens, MD, advised during an annual psychopharmacology update held by the Nevada Psychiatric Association.
“We see high rates of STDs, and we have about 10% of our kids who use opioids who already have hepatitis C,” said Dr. Wilens, who is chief of the division of child & adolescent psychiatry at Massachusetts General Hospital, Boston. “These are kids who may be 16, 17, or 18.”
While the CRAFTT Screening Test has been widely used to screen for substance-related risks and problems in adolescents, another more recent option is the Screening to Brief Intervention (S2BI). Both tools collect information about both alcohol and drug use, are supported by strong research, are available for free, and are easy to use, Dr. Wilens said.
After you generate a differential diagnosis for psychiatric/medical symptoms, clinicians should order urine, saliva, or hair toxicology screens. “We don’t recommend that toxicology screens be done by parents; we do the toxicology screens,” he said. “Be careful about certain things like limitations of detection in the case of high-potency benzodiazepines and duration of detection in the case of marijuana use. The other thing is some of our screens can be used qualitatively or quantitatively. Why is that helpful? If you’re following someone who’s on marijuana and they’re cutting back, you can see if use [really] goes down over time.”
In Dr. Wilen’s clinical experience, efforts to stabilize adolescents with substance use disorders are most effective when patients join support groups comprised of other people from similar sociodemographic backgrounds. “There are different self-help philosophies, but when you’re referring, I always tell people: ‘Have the kid look in a mirror.’ So, if you have an LGBTQ patient from the inner city, that person should not be going to an Alcoholics Anonymous meeting of middle-aged persons in the suburbs. That’s not going to work for them. You want them to be with very similar sociodemographic groups if possible.”
Support groups for parents are also helpful. “There are two levels here: Peer groups of parents that help each other with support and find referrals, and there are parent coaching groups, where you have patients work with professionals,” said Dr. Wilens, who is also codirector of the MGH Center for Addiction Medicine. He advises parents to avoid “tough love” as the first step in efforts to help their child. “Tough love is, you throw the kid out of the house because they won’t stop using,” he said. “Where do you think the kid lives if they’re not at home? Where do you think they’re going to go? Maybe to the home of a friend or a family member for 1 or 2 nights but otherwise they’re living on the streets. How do you think they’re going to make a living if they’re living on the streets? They either sell drugs, or they get involved in prostitution. I have worked with more kids who are furious at their parents because they threw them out of the house. I understand where the patients are coming from, but maybe have a graduated exit instead, where the kid has to sleep outside in a camper for 2 nights, or in an isolated room in the house, or to grandma’s house, which smells like mothballs. Have a graduated approach.”
Psychotherapy is the mainstay of treatment and begins with motivational interviewing. To foster a collaborative connection, Dr. Wilens advises clinicians to discuss issues that are problematic instead of focusing on the substance use right off the bat. “Rather than go right to saying, ‘let’s talk about you smoking too much marijuana,’ instead say, ‘what is it you think may be causing the fights with your parents?’ Or, maybe their peer group isn’t accepting them like they used to.”
In his experience, adolescents respond well to goal setting. For example, for patients who say they’re smoking marijuana every day, Dr. Wilens may ask if they can cut back use to three days per week. “I’ll say: ‘I’m going to write this down in the chart,’ ” he said. “They start to work on it. If they come back and they didn’t reach that goal I say: ‘If you can’t cut back it’s okay; I just need to know it.’ ” He also recommends “sobriety sampling” which asks the patient to make a minimal commitment to stop using, for say, 30 days. “Don’t forget to monitor substance use during follow-up meetings.”
According to Dr. Wilens, child psychiatrists can help prevent substance abuse by encouraging discussion within families by the time kids are in fifth grade and encouraging parents to monitor children’s activities, friends, and personal space. “Privacy is a relative term,” he said. “It’s good you’re in their space. Make their beds; go into their bedroom.” He also advises parents to not smoke marijuana behind their kids’ backs. “I love it when parents tell me: ‘They don’t know I smoke marijuana.’ My counter to that is ‘not only do they know, they’re smoking your marijuana.’ ”
He concluded his remarks by encouraging child psychiatrists to advocate for sensible public laws related to marijuana and other substances. “Zero tolerance laws don’t work, because 85% of kids experiment [with drugs],” said Dr. Wilens, who is also professor of psychiatry at Harvard Medical School, Boston. “It works great until it’s your kid or a neighbor’s kid who’s a good kid but gets thrown out of school.”
Dr. Wilens reported that he has received grant support from the National Institutes of Health and the Food and Drug Administration. He has also served as a consultant to Vallon and has a licensing/collaborative agreement with Ironshore and 3D Therapy.
AT NPA 2022
Did you know these things about nicotine? Your patients don’t
When asked, young people report that their reasons for starting smoking include rebellion, a new thing to try, and a peer social activity, among others. While you recognize these as developmentally expected drives, it is frustrating and scary that youth don’t realize how their brains are especially sensitive to permanent changes from nicotine.
Smoking even five packs of cigarettes is enough to cause addiction in youth; an influence as powerful as for cocaine or heroin. One pod of a vaping device delivers as much nicotine as one to five packs of cigarettes, depending on the strength and brand. There are no standards for this content and youth often are unaware of any nicotine and chemicals in vapes. Over 90% of adult smokers started before age 18, some as young as 6, mainly because quitting is so difficult. Cigarettes and vaping are not the only sources of nicotine used by youth; others are oral tobacco (chewing tobacco and dip), cigars, pipes, snus (between cheek and gum), hookahs, electronic devices, bidis (tobacco in a tendu leaf), kreteks (tobacco with cloves), and dissolvable tobacco products. Many youth use both cigarettes and noncigarette tobacco.
Given these predispositions, short-term COVID-19 and asthma exacerbation, and the long-lasting detriment of smoking on neurological, cardiac, pulmonary, and emotional health, actually the “leading preventable cause of death,” our job as pediatric providers is to do our best to prevent smoking/vaping or help our patients quit. But adolescent development is notoriously characterized by short-term thinking and feeling immune from long-term health consequences. So what approach has the best results? Focus on aspects of smoking important to the youth now, such as sports performance, bad breath, social stigma, insomnia, cost, lack of benefit for weight loss, and hazardous waste produced. Add to that loss of independence and being manipulated by Big Business by getting them (and targeted minorities) hooked may be salient in our discussion.
Even a brief 3-minute discussion using the AAC (Ask/Assess, Advise, Connect) format has shown effectiveness in getting teens and adults to quit smoking. Our assessment needs to include asking the extent of current use and symptoms of dependence to inform the treatment plan. We need to use their trust in us to advise that quitting is the best thing they can do for their health.
If the youth’s readiness stage is “thinking about stopping” nicotine, our motivational interview–style discussion of pros and cons could include asking “How important is it to you to stop?” and “What are some things that would help you?” If they are open to trying to stop, advise them to set a quit date within 2 weeks and suggest reducing gradually before then (and schedule follow-up). The plan needs to include dealing with the inevitable urges by finding ways to avoid current triggers to smoke (e.g., certain school bathrooms, people drinking or smoking, or stress over homework, conflict at home, etc.). Encourage exercise and meditation to distract and deal with the anxiety; asking family to quit; having a snack handy (such as sugarless gum or sunflower seeds) for when oral cravings develop; and setting rewards for early days of smoke-free success. We need to inform youth that using e-cigs actually reduces rates of success in quitting.
We need to warn youth of the withdrawal symptoms and their usual course when quitting: cravings each lasting 15-20 minutes (starting at 1/2-4 hours); restlessness, sadness, hopelessness (10 hours); irritability, trouble concentrating, insomnia, hunger and weight gain (5-10 pounds over 2 weeks, starting 24 hrs); headaches, dizziness, fatigue (starting 2 days); and anxiety (starting 3 days). There tends to be less brain fog, and less hunger after 2-4 weeks, but depression, anxiety, irritability, cough, constipation, and even suicidal thoughts may last weeks to months. Sounds nasty, right? No wonder quitting is so hard.
Support is crucial to quitting and staying off nicotine. You can provide this but, in addition to friends and family, we should connect youth to free ongoing phone counselors (1-800-QUIT-NOW or 877-44U-QUIT for Spanish), text services (text QUIT to 47848), apps (quit START), or community support.
While behavioral treatments are best for youth with minimal to mild dependence, risk of relapse is minimized with fewer withdrawal symptoms, thus the role for nicotine replacement therapy (NRT) for those with moderate to strong dependence and to help anyone ad lib with cravings. NRT is recommended by the American Academy of Pediatrics (AAP) to supplement counseling, although NRT is not Food and Drug Administration approved and requires a prescription for those under 18.
How can we determine the degree of dependence? Smoking more than 15 cigarettes per day (or vape equivalent) and inhaling even “seldom” counts as “moderate” dependence and more than 26 with difficulty refraining in several situations as “substantial” in the Fagerstrom Tolerance test. Early morning smoking is asked about, important to which NRT to use (gum or lozenge for faster onset). The Hooked on Nicotine Checklist assesses “loss of autonomy” over smoking by any “yes” item and is incorporated in the CRAFFT screen. The recommended dose of NRT and length of weaning is greater in substantial addiction versus moderate. Besides gum, lozenges, patch, inhaler, and nasal spray, you can prescribe bupropion (Wellbutrin or Zyban) or varenicline (Chantix), making note of the black box suicide warning. Combining NRTs is similarly effective compared with varenicline.
Relapse after quitting is more common than not. As for any chronic condition, in relapse we need to query adherence, and consider increasing NRT dose or wean duration, even years. Discussion should have a positive focus on “what was learned” from past attempts in making a new plan that incorporates Relevance, Risks, Rewards, Roadblocks, and Repetition.
Many youth smokers start because their parents smoke. While addressing adults may seem out of scope, we often treat parents when managing scabies, pinworms, meningococcal disease, and even depression for the benefit of the child. The AAP recommends prescribing NRT for parents, when needed.
Nicotine dependence is a chronic relapsing condition with comorbidities of substance use and psychiatric disorders that requires similar monitoring and support as for other chronic conditions we manage and is more likely to shorten lifespan than many.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Reference
Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke, Pediatrics 2015;136(5):1008-17. doi: 10.1542/peds.2015-31088.
When asked, young people report that their reasons for starting smoking include rebellion, a new thing to try, and a peer social activity, among others. While you recognize these as developmentally expected drives, it is frustrating and scary that youth don’t realize how their brains are especially sensitive to permanent changes from nicotine.
Smoking even five packs of cigarettes is enough to cause addiction in youth; an influence as powerful as for cocaine or heroin. One pod of a vaping device delivers as much nicotine as one to five packs of cigarettes, depending on the strength and brand. There are no standards for this content and youth often are unaware of any nicotine and chemicals in vapes. Over 90% of adult smokers started before age 18, some as young as 6, mainly because quitting is so difficult. Cigarettes and vaping are not the only sources of nicotine used by youth; others are oral tobacco (chewing tobacco and dip), cigars, pipes, snus (between cheek and gum), hookahs, electronic devices, bidis (tobacco in a tendu leaf), kreteks (tobacco with cloves), and dissolvable tobacco products. Many youth use both cigarettes and noncigarette tobacco.
Given these predispositions, short-term COVID-19 and asthma exacerbation, and the long-lasting detriment of smoking on neurological, cardiac, pulmonary, and emotional health, actually the “leading preventable cause of death,” our job as pediatric providers is to do our best to prevent smoking/vaping or help our patients quit. But adolescent development is notoriously characterized by short-term thinking and feeling immune from long-term health consequences. So what approach has the best results? Focus on aspects of smoking important to the youth now, such as sports performance, bad breath, social stigma, insomnia, cost, lack of benefit for weight loss, and hazardous waste produced. Add to that loss of independence and being manipulated by Big Business by getting them (and targeted minorities) hooked may be salient in our discussion.
Even a brief 3-minute discussion using the AAC (Ask/Assess, Advise, Connect) format has shown effectiveness in getting teens and adults to quit smoking. Our assessment needs to include asking the extent of current use and symptoms of dependence to inform the treatment plan. We need to use their trust in us to advise that quitting is the best thing they can do for their health.
If the youth’s readiness stage is “thinking about stopping” nicotine, our motivational interview–style discussion of pros and cons could include asking “How important is it to you to stop?” and “What are some things that would help you?” If they are open to trying to stop, advise them to set a quit date within 2 weeks and suggest reducing gradually before then (and schedule follow-up). The plan needs to include dealing with the inevitable urges by finding ways to avoid current triggers to smoke (e.g., certain school bathrooms, people drinking or smoking, or stress over homework, conflict at home, etc.). Encourage exercise and meditation to distract and deal with the anxiety; asking family to quit; having a snack handy (such as sugarless gum or sunflower seeds) for when oral cravings develop; and setting rewards for early days of smoke-free success. We need to inform youth that using e-cigs actually reduces rates of success in quitting.
We need to warn youth of the withdrawal symptoms and their usual course when quitting: cravings each lasting 15-20 minutes (starting at 1/2-4 hours); restlessness, sadness, hopelessness (10 hours); irritability, trouble concentrating, insomnia, hunger and weight gain (5-10 pounds over 2 weeks, starting 24 hrs); headaches, dizziness, fatigue (starting 2 days); and anxiety (starting 3 days). There tends to be less brain fog, and less hunger after 2-4 weeks, but depression, anxiety, irritability, cough, constipation, and even suicidal thoughts may last weeks to months. Sounds nasty, right? No wonder quitting is so hard.
Support is crucial to quitting and staying off nicotine. You can provide this but, in addition to friends and family, we should connect youth to free ongoing phone counselors (1-800-QUIT-NOW or 877-44U-QUIT for Spanish), text services (text QUIT to 47848), apps (quit START), or community support.
While behavioral treatments are best for youth with minimal to mild dependence, risk of relapse is minimized with fewer withdrawal symptoms, thus the role for nicotine replacement therapy (NRT) for those with moderate to strong dependence and to help anyone ad lib with cravings. NRT is recommended by the American Academy of Pediatrics (AAP) to supplement counseling, although NRT is not Food and Drug Administration approved and requires a prescription for those under 18.
How can we determine the degree of dependence? Smoking more than 15 cigarettes per day (or vape equivalent) and inhaling even “seldom” counts as “moderate” dependence and more than 26 with difficulty refraining in several situations as “substantial” in the Fagerstrom Tolerance test. Early morning smoking is asked about, important to which NRT to use (gum or lozenge for faster onset). The Hooked on Nicotine Checklist assesses “loss of autonomy” over smoking by any “yes” item and is incorporated in the CRAFFT screen. The recommended dose of NRT and length of weaning is greater in substantial addiction versus moderate. Besides gum, lozenges, patch, inhaler, and nasal spray, you can prescribe bupropion (Wellbutrin or Zyban) or varenicline (Chantix), making note of the black box suicide warning. Combining NRTs is similarly effective compared with varenicline.
Relapse after quitting is more common than not. As for any chronic condition, in relapse we need to query adherence, and consider increasing NRT dose or wean duration, even years. Discussion should have a positive focus on “what was learned” from past attempts in making a new plan that incorporates Relevance, Risks, Rewards, Roadblocks, and Repetition.
Many youth smokers start because their parents smoke. While addressing adults may seem out of scope, we often treat parents when managing scabies, pinworms, meningococcal disease, and even depression for the benefit of the child. The AAP recommends prescribing NRT for parents, when needed.
Nicotine dependence is a chronic relapsing condition with comorbidities of substance use and psychiatric disorders that requires similar monitoring and support as for other chronic conditions we manage and is more likely to shorten lifespan than many.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Reference
Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke, Pediatrics 2015;136(5):1008-17. doi: 10.1542/peds.2015-31088.
When asked, young people report that their reasons for starting smoking include rebellion, a new thing to try, and a peer social activity, among others. While you recognize these as developmentally expected drives, it is frustrating and scary that youth don’t realize how their brains are especially sensitive to permanent changes from nicotine.
Smoking even five packs of cigarettes is enough to cause addiction in youth; an influence as powerful as for cocaine or heroin. One pod of a vaping device delivers as much nicotine as one to five packs of cigarettes, depending on the strength and brand. There are no standards for this content and youth often are unaware of any nicotine and chemicals in vapes. Over 90% of adult smokers started before age 18, some as young as 6, mainly because quitting is so difficult. Cigarettes and vaping are not the only sources of nicotine used by youth; others are oral tobacco (chewing tobacco and dip), cigars, pipes, snus (between cheek and gum), hookahs, electronic devices, bidis (tobacco in a tendu leaf), kreteks (tobacco with cloves), and dissolvable tobacco products. Many youth use both cigarettes and noncigarette tobacco.
Given these predispositions, short-term COVID-19 and asthma exacerbation, and the long-lasting detriment of smoking on neurological, cardiac, pulmonary, and emotional health, actually the “leading preventable cause of death,” our job as pediatric providers is to do our best to prevent smoking/vaping or help our patients quit. But adolescent development is notoriously characterized by short-term thinking and feeling immune from long-term health consequences. So what approach has the best results? Focus on aspects of smoking important to the youth now, such as sports performance, bad breath, social stigma, insomnia, cost, lack of benefit for weight loss, and hazardous waste produced. Add to that loss of independence and being manipulated by Big Business by getting them (and targeted minorities) hooked may be salient in our discussion.
Even a brief 3-minute discussion using the AAC (Ask/Assess, Advise, Connect) format has shown effectiveness in getting teens and adults to quit smoking. Our assessment needs to include asking the extent of current use and symptoms of dependence to inform the treatment plan. We need to use their trust in us to advise that quitting is the best thing they can do for their health.
If the youth’s readiness stage is “thinking about stopping” nicotine, our motivational interview–style discussion of pros and cons could include asking “How important is it to you to stop?” and “What are some things that would help you?” If they are open to trying to stop, advise them to set a quit date within 2 weeks and suggest reducing gradually before then (and schedule follow-up). The plan needs to include dealing with the inevitable urges by finding ways to avoid current triggers to smoke (e.g., certain school bathrooms, people drinking or smoking, or stress over homework, conflict at home, etc.). Encourage exercise and meditation to distract and deal with the anxiety; asking family to quit; having a snack handy (such as sugarless gum or sunflower seeds) for when oral cravings develop; and setting rewards for early days of smoke-free success. We need to inform youth that using e-cigs actually reduces rates of success in quitting.
We need to warn youth of the withdrawal symptoms and their usual course when quitting: cravings each lasting 15-20 minutes (starting at 1/2-4 hours); restlessness, sadness, hopelessness (10 hours); irritability, trouble concentrating, insomnia, hunger and weight gain (5-10 pounds over 2 weeks, starting 24 hrs); headaches, dizziness, fatigue (starting 2 days); and anxiety (starting 3 days). There tends to be less brain fog, and less hunger after 2-4 weeks, but depression, anxiety, irritability, cough, constipation, and even suicidal thoughts may last weeks to months. Sounds nasty, right? No wonder quitting is so hard.
Support is crucial to quitting and staying off nicotine. You can provide this but, in addition to friends and family, we should connect youth to free ongoing phone counselors (1-800-QUIT-NOW or 877-44U-QUIT for Spanish), text services (text QUIT to 47848), apps (quit START), or community support.
While behavioral treatments are best for youth with minimal to mild dependence, risk of relapse is minimized with fewer withdrawal symptoms, thus the role for nicotine replacement therapy (NRT) for those with moderate to strong dependence and to help anyone ad lib with cravings. NRT is recommended by the American Academy of Pediatrics (AAP) to supplement counseling, although NRT is not Food and Drug Administration approved and requires a prescription for those under 18.
How can we determine the degree of dependence? Smoking more than 15 cigarettes per day (or vape equivalent) and inhaling even “seldom” counts as “moderate” dependence and more than 26 with difficulty refraining in several situations as “substantial” in the Fagerstrom Tolerance test. Early morning smoking is asked about, important to which NRT to use (gum or lozenge for faster onset). The Hooked on Nicotine Checklist assesses “loss of autonomy” over smoking by any “yes” item and is incorporated in the CRAFFT screen. The recommended dose of NRT and length of weaning is greater in substantial addiction versus moderate. Besides gum, lozenges, patch, inhaler, and nasal spray, you can prescribe bupropion (Wellbutrin or Zyban) or varenicline (Chantix), making note of the black box suicide warning. Combining NRTs is similarly effective compared with varenicline.
Relapse after quitting is more common than not. As for any chronic condition, in relapse we need to query adherence, and consider increasing NRT dose or wean duration, even years. Discussion should have a positive focus on “what was learned” from past attempts in making a new plan that incorporates Relevance, Risks, Rewards, Roadblocks, and Repetition.
Many youth smokers start because their parents smoke. While addressing adults may seem out of scope, we often treat parents when managing scabies, pinworms, meningococcal disease, and even depression for the benefit of the child. The AAP recommends prescribing NRT for parents, when needed.
Nicotine dependence is a chronic relapsing condition with comorbidities of substance use and psychiatric disorders that requires similar monitoring and support as for other chronic conditions we manage and is more likely to shorten lifespan than many.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Reference
Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke, Pediatrics 2015;136(5):1008-17. doi: 10.1542/peds.2015-31088.
Opioid deaths in North America predicted to soar
“Over the past quarter-century, the opioid epidemic has taken nearly 600,000 lives and triggered a cascade of public health catastrophes such as disability, family breakdown, unemployment, and child neglect in North America,” commission chair Keith Humphreys, PhD, said in a news release.
“If no action is taken, by the end of this decade, we are predicting the number of deaths to be twice as high as it has been over the last 20 years,” said Dr. Humphreys, professor of psychiatry and behavioral sciences at Stanford (Calif.) University.
The report was published online Feb. 2, 2022, in The Lancet.
Blame it on COVID-19?
The COVID-19 pandemic has both overshadowed and exacerbated the opioid crisis in North America, the commission pointed out in their report.
Their analysis suggests that 2020 was the worst year on record for overdose deaths in the United States and Canada in terms of both the total number of deaths and percentage annual increase.
In the United States, opioid overdose deaths increased by 37%, from 51,133 in 2019 to 70,168 in 2020, bringing the total number of deaths since 1999 to 583,000.
In Canada, opioid overdose deaths jumped by 72%, from 3,668 in 2019 to 6,306 in 2020, with a further 3,515 deaths reported in the first 6 months of 2021.
Although the 2020 spikes might be partly caused by the effects of the COVID-19 pandemic, a rising trajectory of deaths was evident in both the United States and Canada before the pandemic hit, the Stanford-Lancet Commission said.
Profit motives, lack of regulation
The commission blames the opioid epidemic on a lack of adequate regulation and oversight coupled with profit motives of the pharmaceutical and health care industry.
“To ensure safeguards are in place to curb the opioid addiction epidemic and prevent future ones involving other addictive drugs, we must end the pharmaceutical and health care industry’s undue influence on the government and its unregulated push for opioid use,” commission member Howard Koh, MD, MPH, said in the news release.
“This includes insulating the medical community from pharmaceutical company influence and closing the constantly revolving door between regulators and industry,” said Dr. Koh, with the Harvard School of Public Health, Boston.
In addition to regulation and policy reform, the commission said prevention efforts that focus on treating addiction as a chronic condition are key.
The United States in particular lacks accessible, high-quality, nonstigmatizing, and integrated health and social care services for people experiencing opioid use disorder, the Commission notes.
Addiction-related services must become a permanent feature of health and social care systems in the United States and Canada, in line with established chronic disease management models that are financed and organized as a core public health commitment, the commission said.
“Addiction is an enduring part of population health and should not be treated as a moral failing that needs punishment but as a chronic health condition that requires ongoing treatment and long-term support,” commission member Yasmin Hurd, PhD, director of the Addiction Institute at Icahn School of Medicine at Mount Sinai, New York, said in the release.
Investing in young people to reduce the risk of addiction will also be important going forward.
“Preventing drug addiction should be part of a comprehensive public health strategy that starts in childhood and lays the foundation for long-term declines in addiction,” said commission member Chelsea Shover, PhD, with the University of California, Los Angeles.
‘Audacious but achievable goal’
The commission calls for a nuanced approach to pain management that prioritizes innovation both in society’s response to drug addiction through policy reform and by supporting the development of new, nonaddictive pain management options.
“Opioids should not be viewed as good or bad, but instead as a class of medications essential to the management of pain. However, opioids also come with serious risks, some of which can be difficult to recognize,” commission member David Juurlink, MD, PhD, said in the release.
“Clinicians should begin learning about responsible pain management prescribing in medical school and continue to learn about it as part of their commitment to continued medical education throughout their careers,” said Dr. Juurlink, with Sunnybrook Health Sciences Centre in Toronto.
Humphreys said ending the opioid epidemic in North America and preventing its global spread is “an audacious but achievable goal” that will require a “dramatic shift in policy and culture where innovation, collaboration, and regulation are encouraged.
“We can save and improve lives by summoning the resources and political will necessary to eliminate the sources of addiction and boldly implement policies that will maximize efforts to treat it,” Dr. Humphreys added.
The study was funded by Stanford University.
A version of this article first appeared on Medscape.com.
“Over the past quarter-century, the opioid epidemic has taken nearly 600,000 lives and triggered a cascade of public health catastrophes such as disability, family breakdown, unemployment, and child neglect in North America,” commission chair Keith Humphreys, PhD, said in a news release.
“If no action is taken, by the end of this decade, we are predicting the number of deaths to be twice as high as it has been over the last 20 years,” said Dr. Humphreys, professor of psychiatry and behavioral sciences at Stanford (Calif.) University.
The report was published online Feb. 2, 2022, in The Lancet.
Blame it on COVID-19?
The COVID-19 pandemic has both overshadowed and exacerbated the opioid crisis in North America, the commission pointed out in their report.
Their analysis suggests that 2020 was the worst year on record for overdose deaths in the United States and Canada in terms of both the total number of deaths and percentage annual increase.
In the United States, opioid overdose deaths increased by 37%, from 51,133 in 2019 to 70,168 in 2020, bringing the total number of deaths since 1999 to 583,000.
In Canada, opioid overdose deaths jumped by 72%, from 3,668 in 2019 to 6,306 in 2020, with a further 3,515 deaths reported in the first 6 months of 2021.
Although the 2020 spikes might be partly caused by the effects of the COVID-19 pandemic, a rising trajectory of deaths was evident in both the United States and Canada before the pandemic hit, the Stanford-Lancet Commission said.
Profit motives, lack of regulation
The commission blames the opioid epidemic on a lack of adequate regulation and oversight coupled with profit motives of the pharmaceutical and health care industry.
“To ensure safeguards are in place to curb the opioid addiction epidemic and prevent future ones involving other addictive drugs, we must end the pharmaceutical and health care industry’s undue influence on the government and its unregulated push for opioid use,” commission member Howard Koh, MD, MPH, said in the news release.
“This includes insulating the medical community from pharmaceutical company influence and closing the constantly revolving door between regulators and industry,” said Dr. Koh, with the Harvard School of Public Health, Boston.
In addition to regulation and policy reform, the commission said prevention efforts that focus on treating addiction as a chronic condition are key.
The United States in particular lacks accessible, high-quality, nonstigmatizing, and integrated health and social care services for people experiencing opioid use disorder, the Commission notes.
Addiction-related services must become a permanent feature of health and social care systems in the United States and Canada, in line with established chronic disease management models that are financed and organized as a core public health commitment, the commission said.
“Addiction is an enduring part of population health and should not be treated as a moral failing that needs punishment but as a chronic health condition that requires ongoing treatment and long-term support,” commission member Yasmin Hurd, PhD, director of the Addiction Institute at Icahn School of Medicine at Mount Sinai, New York, said in the release.
Investing in young people to reduce the risk of addiction will also be important going forward.
“Preventing drug addiction should be part of a comprehensive public health strategy that starts in childhood and lays the foundation for long-term declines in addiction,” said commission member Chelsea Shover, PhD, with the University of California, Los Angeles.
‘Audacious but achievable goal’
The commission calls for a nuanced approach to pain management that prioritizes innovation both in society’s response to drug addiction through policy reform and by supporting the development of new, nonaddictive pain management options.
“Opioids should not be viewed as good or bad, but instead as a class of medications essential to the management of pain. However, opioids also come with serious risks, some of which can be difficult to recognize,” commission member David Juurlink, MD, PhD, said in the release.
“Clinicians should begin learning about responsible pain management prescribing in medical school and continue to learn about it as part of their commitment to continued medical education throughout their careers,” said Dr. Juurlink, with Sunnybrook Health Sciences Centre in Toronto.
Humphreys said ending the opioid epidemic in North America and preventing its global spread is “an audacious but achievable goal” that will require a “dramatic shift in policy and culture where innovation, collaboration, and regulation are encouraged.
“We can save and improve lives by summoning the resources and political will necessary to eliminate the sources of addiction and boldly implement policies that will maximize efforts to treat it,” Dr. Humphreys added.
The study was funded by Stanford University.
A version of this article first appeared on Medscape.com.
“Over the past quarter-century, the opioid epidemic has taken nearly 600,000 lives and triggered a cascade of public health catastrophes such as disability, family breakdown, unemployment, and child neglect in North America,” commission chair Keith Humphreys, PhD, said in a news release.
“If no action is taken, by the end of this decade, we are predicting the number of deaths to be twice as high as it has been over the last 20 years,” said Dr. Humphreys, professor of psychiatry and behavioral sciences at Stanford (Calif.) University.
The report was published online Feb. 2, 2022, in The Lancet.
Blame it on COVID-19?
The COVID-19 pandemic has both overshadowed and exacerbated the opioid crisis in North America, the commission pointed out in their report.
Their analysis suggests that 2020 was the worst year on record for overdose deaths in the United States and Canada in terms of both the total number of deaths and percentage annual increase.
In the United States, opioid overdose deaths increased by 37%, from 51,133 in 2019 to 70,168 in 2020, bringing the total number of deaths since 1999 to 583,000.
In Canada, opioid overdose deaths jumped by 72%, from 3,668 in 2019 to 6,306 in 2020, with a further 3,515 deaths reported in the first 6 months of 2021.
Although the 2020 spikes might be partly caused by the effects of the COVID-19 pandemic, a rising trajectory of deaths was evident in both the United States and Canada before the pandemic hit, the Stanford-Lancet Commission said.
Profit motives, lack of regulation
The commission blames the opioid epidemic on a lack of adequate regulation and oversight coupled with profit motives of the pharmaceutical and health care industry.
“To ensure safeguards are in place to curb the opioid addiction epidemic and prevent future ones involving other addictive drugs, we must end the pharmaceutical and health care industry’s undue influence on the government and its unregulated push for opioid use,” commission member Howard Koh, MD, MPH, said in the news release.
“This includes insulating the medical community from pharmaceutical company influence and closing the constantly revolving door between regulators and industry,” said Dr. Koh, with the Harvard School of Public Health, Boston.
In addition to regulation and policy reform, the commission said prevention efforts that focus on treating addiction as a chronic condition are key.
The United States in particular lacks accessible, high-quality, nonstigmatizing, and integrated health and social care services for people experiencing opioid use disorder, the Commission notes.
Addiction-related services must become a permanent feature of health and social care systems in the United States and Canada, in line with established chronic disease management models that are financed and organized as a core public health commitment, the commission said.
“Addiction is an enduring part of population health and should not be treated as a moral failing that needs punishment but as a chronic health condition that requires ongoing treatment and long-term support,” commission member Yasmin Hurd, PhD, director of the Addiction Institute at Icahn School of Medicine at Mount Sinai, New York, said in the release.
Investing in young people to reduce the risk of addiction will also be important going forward.
“Preventing drug addiction should be part of a comprehensive public health strategy that starts in childhood and lays the foundation for long-term declines in addiction,” said commission member Chelsea Shover, PhD, with the University of California, Los Angeles.
‘Audacious but achievable goal’
The commission calls for a nuanced approach to pain management that prioritizes innovation both in society’s response to drug addiction through policy reform and by supporting the development of new, nonaddictive pain management options.
“Opioids should not be viewed as good or bad, but instead as a class of medications essential to the management of pain. However, opioids also come with serious risks, some of which can be difficult to recognize,” commission member David Juurlink, MD, PhD, said in the release.
“Clinicians should begin learning about responsible pain management prescribing in medical school and continue to learn about it as part of their commitment to continued medical education throughout their careers,” said Dr. Juurlink, with Sunnybrook Health Sciences Centre in Toronto.
Humphreys said ending the opioid epidemic in North America and preventing its global spread is “an audacious but achievable goal” that will require a “dramatic shift in policy and culture where innovation, collaboration, and regulation are encouraged.
“We can save and improve lives by summoning the resources and political will necessary to eliminate the sources of addiction and boldly implement policies that will maximize efforts to treat it,” Dr. Humphreys added.
The study was funded by Stanford University.
A version of this article first appeared on Medscape.com.
FROM THE LANCET
E-cigarettes don’t help smokers quit, suggests new research
From 2013 to 2017, e-cigarette sales in the United States nearly doubled, driven by a rapid uptake of use by adolescents, wrote Riufeng Chen, MD, of the University of California, San Diego, and colleagues, in their paper published in Tobacco Control. However, the subsequent effect of increased e-cigarette use on smoking cessation have not been examined, they said.
In their study, Dr. Chen and colleagues analyzed data from 3,578 previous-year smokers with a recent quit attempt and 1,323 recent former smokers who were part of the PATH cohort in 2017. The participants reported using e-cigarettes or other products to quit cigarette smoking. The primary outcomes were at least 12 months of cigarette abstinence, and tobacco abstinence in 2019. In 2017, 32.8% of established smokers reported trying to quit. Of these, 12.6% used e-cigarettes to help them quit. Cigarette abstinence for at least 12 months for these individuals was 9.9%, which was lower than for those who used either nicotine replacement therapy or a pharmaceutical aid only (15.2%), and about half of the 18.6% abstinence in those who used no products to help them quit.
“In our study, e-cigarettes resulted in seven fewer successful quitters than those who used pharmaceutical aids,” emphasized corresponding author, John P. Pierce, PhD, of the University of California, San Diego.
Among smokers attempting to quit, the adjusted risk difference for cigarette abstinence for a least 12 months with e-cigarettes vs. pharmaceutical aids was –7.3%, and –7.7% for e-cigarettes vs. other smoking cessation methods.
*“Among recent former smokers who had switched to daily use of e-cigarettes in 2017, 43.2% had successfully quit cigarette smoking by 2019, which was similar to those who used e-cigarettes on a nondaily basis (34.6%) or to those who switched to another tobacco product, whether daily (43.6%) or nondaily (44.7%),” the researchers wrote.
The rapid growth in e-cigarette use between 2014 and 2017 has been attributed in part to aggressive marketing of high-nicotine e-cigarettes, they said. “The high-nicotine JUUL e-cigarette has been noted as the closest match to cigarettes in both nicotine delivery and user satisfaction, which should make it one of the best candidates as a product to which smokers could switch in order to maintain their nicotine habit,” they said in their discussion of the findings.
More research needed
The researchers acknowledged the need to review more recent data.
“When we looked ahead to 2019, recent former smokers had started using high-nicotine e-cigarettes. The effectiveness of high-nicotine e-cigarettes at preventing relapse will require another follow-up PATH survey,” they said.
Among recent former smokers, 2.2% reported switching to a high-nicotine e-cigarette. Although individuals who switched to e-cigarettes showed a higher rate of relapse to cigarettes than those who did not switch to other tobacco or e-cigarette products, this difference was not significant.
The study findings were limited by several factors including the observational design and inability to control for all potential confounding factors, the researchers noted. However, the results were strengthened by the use of a large and representative study population, and the inclusion of biological samples to validate self-reported smoking, they said.
Several findings surprised study author
Dr. Pierce said he was surprised by several aspects of the study findings.
“First of all, contrary to what we expected, there was a 25% decline in using e-cigarettes to quit, compared to the previous year (not the 40% increase that was expected from the increase in e-cigarette sales) and almost no smokers were using high-nicotine JUUL products to help them quit,” he said. “In this study, e-cigarettes were much less helpful (7 less successful quitters per 100) than pharmaceutical cessation aids in helping people quit,” he added.
“The fact that the proportion of smokers using e-cigarettes for cessation dropped from 17% to 12% was unexpected, and it suggests that the belief that they are a cessation aid is declining,” he said.
The implication for clinical practice is that e-cigarettes are not a useful tool for smoking cessation, Dr. Pierce said. “We are not finding any evidence in this very large nationally representative study that smokers who switch to getting their nicotine from e-cigarettes are less likely to relapse back to cigarette smoking,” he said.
“We don’t know about the high-nicotine versions,” he added.
New review advises against e-cigarettes for cessation
A recent review article published in JAMA supported the use of pharmacotherapy and behavioral support for smokers wanting to quit. In the review, Nancy A. Rigotti, MD, of Massachusetts General Hospital, Boston, and colleagues summarized the evidence for managing tobacco smoking in clinical practice.
“The health risk from cigarette smoking is primarily due to chemicals produced by the burning of tobacco and not to nicotine,” they noted. However, the physical dependence on nicotine makes quitting a challenge, but it is one worth pursuing, the authors said.
The authors of this review identified 30 reviews, 12 randomized clinical trials, and 7 recent guidelines and evidence reviews. Their key message: Pharmacotherapy and behavioral support are effective when used alone, but even more effective when combined. Pharmacotherapy helps reduce the symptoms of nicotine withdrawal, while behavioral intervention tackles the challenge of changing learned behaviors associated with smoking, the researchers said.
Although combining medications, such as varenicline and nicotine replacement therapy or bupropion might improve successful quit rates, these combinations have not been well studied, they noted.
With regard to e-cigarettes, the researchers cited a 2021 Cochrane review of 16,759 individuals who used e-cigarettes for smoking cessation, which found no evidence of harm, but insufficient evidence to asses the balance of risks vs. benefits.
In addition to the lack of randomized trials, “the FDA regulates e-cigarettes as tobacco products, not as medical products and has not evaluated any e-cigarette for medical use as a cessation aid,” the authors of the new review noted.
The review was limited by several factors, including the lack of quality assessment for the selected studies and the exclusion of pharmacotherapy not licensed in the United States.
Commenting on the JAMA paper, Dr. Pierce said, “This review looks like a number of Cochrane Reports that have been published recently. Of course, it only considers randomized trials and not population evidence.”
“If public health had limited itself to this form of evidence, then we still would not know that smoking caused cancer,” he noted. “Randomized trials are very important for testing new drugs; they use selected populations and provide considerable support that is not available in the real world. Sometimes they do not generalize to the population.”
Findings may guide patient conversations
The Tobacco Control study was important, because few studies on e-cigarettes have been conducted, said Linda Girgis, MD, a family physician in private practice in South River, N.J., in an interview.
“As clinicians, we do not have a lot of data available in order to make clinical decisions that are evidence based. Also, getting patients to quit smoking is often very difficult, and having more tools available is a great benefit; however, we need to have the evidence that these tools are effective,” she said.
Dr. Girgis also said she was not surprised by the findings.
“Patients still have the same concerns from e-cigarettes regarding nicotine exposure, but just to a lesser degree; and we still don’t know the long-term effects of e-cigarette use, she said. Based on these studies, recommending e-cigarettes for smokers looking to quit may not be the best method, she noted.
“While it may seem reasonable that exposing lungs to lower doses of nicotine will reduce harm, we need to see actual evidence of this. Also, we also need to study the additives that are frequently used in e-cigs, such as artificial flavorings, to see what harms they may pose, she emphasized.
With regard to the JAMA review, Dr. Girgis said she agreed with the recommendations for pharmacotherapy and behavior therapy as first-line treatments for smoking cessation. “There is evidence regarding the efficacy and safety of these methods, and they have been used for decades,” she said.
Dr. Girgis added that there is a role for e-cigarettes in smoking cessation strategies as a method of harm reduction, but pointed out the problem of many people thinking these products are safe and not understanding the hazards they pose.
“They think they can replace smoking with e-cigarettes and be safe from the health risks associated with smoking. I think if the plan were to switch to e-cigarettes for a short period and then quit, there would be a role,” Dr. Girgis said. “However, replacing one risk for another may reduce harm, but doesn’t eliminate it.”
“To continue to use e-cigarettes indefinitely should not be the goal,” she added.
The Tobacco Control study was funded by the National Institutes of Health and the Tobacco-Related Disease Research Program of the University of California. The researchers had no financial conflicts to disclose.
The JAMA study was funded in part by a grant from the National Institute for Health Research, via Cochrane Infrastructure funds to the Cochrane Tobacco Addiction Group. Lead author Dr. Rigotti disclosed funding from the National Heart, Lung, and Blood Institute and Achieve Life Sciences and personal fees from UpToDate and Achieve Life Sciences. Dr. Girgis had no financial conflicts to disclose.
*This article was updated on 2/28/2022.
From 2013 to 2017, e-cigarette sales in the United States nearly doubled, driven by a rapid uptake of use by adolescents, wrote Riufeng Chen, MD, of the University of California, San Diego, and colleagues, in their paper published in Tobacco Control. However, the subsequent effect of increased e-cigarette use on smoking cessation have not been examined, they said.
In their study, Dr. Chen and colleagues analyzed data from 3,578 previous-year smokers with a recent quit attempt and 1,323 recent former smokers who were part of the PATH cohort in 2017. The participants reported using e-cigarettes or other products to quit cigarette smoking. The primary outcomes were at least 12 months of cigarette abstinence, and tobacco abstinence in 2019. In 2017, 32.8% of established smokers reported trying to quit. Of these, 12.6% used e-cigarettes to help them quit. Cigarette abstinence for at least 12 months for these individuals was 9.9%, which was lower than for those who used either nicotine replacement therapy or a pharmaceutical aid only (15.2%), and about half of the 18.6% abstinence in those who used no products to help them quit.
“In our study, e-cigarettes resulted in seven fewer successful quitters than those who used pharmaceutical aids,” emphasized corresponding author, John P. Pierce, PhD, of the University of California, San Diego.
Among smokers attempting to quit, the adjusted risk difference for cigarette abstinence for a least 12 months with e-cigarettes vs. pharmaceutical aids was –7.3%, and –7.7% for e-cigarettes vs. other smoking cessation methods.
*“Among recent former smokers who had switched to daily use of e-cigarettes in 2017, 43.2% had successfully quit cigarette smoking by 2019, which was similar to those who used e-cigarettes on a nondaily basis (34.6%) or to those who switched to another tobacco product, whether daily (43.6%) or nondaily (44.7%),” the researchers wrote.
The rapid growth in e-cigarette use between 2014 and 2017 has been attributed in part to aggressive marketing of high-nicotine e-cigarettes, they said. “The high-nicotine JUUL e-cigarette has been noted as the closest match to cigarettes in both nicotine delivery and user satisfaction, which should make it one of the best candidates as a product to which smokers could switch in order to maintain their nicotine habit,” they said in their discussion of the findings.
More research needed
The researchers acknowledged the need to review more recent data.
“When we looked ahead to 2019, recent former smokers had started using high-nicotine e-cigarettes. The effectiveness of high-nicotine e-cigarettes at preventing relapse will require another follow-up PATH survey,” they said.
Among recent former smokers, 2.2% reported switching to a high-nicotine e-cigarette. Although individuals who switched to e-cigarettes showed a higher rate of relapse to cigarettes than those who did not switch to other tobacco or e-cigarette products, this difference was not significant.
The study findings were limited by several factors including the observational design and inability to control for all potential confounding factors, the researchers noted. However, the results were strengthened by the use of a large and representative study population, and the inclusion of biological samples to validate self-reported smoking, they said.
Several findings surprised study author
Dr. Pierce said he was surprised by several aspects of the study findings.
“First of all, contrary to what we expected, there was a 25% decline in using e-cigarettes to quit, compared to the previous year (not the 40% increase that was expected from the increase in e-cigarette sales) and almost no smokers were using high-nicotine JUUL products to help them quit,” he said. “In this study, e-cigarettes were much less helpful (7 less successful quitters per 100) than pharmaceutical cessation aids in helping people quit,” he added.
“The fact that the proportion of smokers using e-cigarettes for cessation dropped from 17% to 12% was unexpected, and it suggests that the belief that they are a cessation aid is declining,” he said.
The implication for clinical practice is that e-cigarettes are not a useful tool for smoking cessation, Dr. Pierce said. “We are not finding any evidence in this very large nationally representative study that smokers who switch to getting their nicotine from e-cigarettes are less likely to relapse back to cigarette smoking,” he said.
“We don’t know about the high-nicotine versions,” he added.
New review advises against e-cigarettes for cessation
A recent review article published in JAMA supported the use of pharmacotherapy and behavioral support for smokers wanting to quit. In the review, Nancy A. Rigotti, MD, of Massachusetts General Hospital, Boston, and colleagues summarized the evidence for managing tobacco smoking in clinical practice.
“The health risk from cigarette smoking is primarily due to chemicals produced by the burning of tobacco and not to nicotine,” they noted. However, the physical dependence on nicotine makes quitting a challenge, but it is one worth pursuing, the authors said.
The authors of this review identified 30 reviews, 12 randomized clinical trials, and 7 recent guidelines and evidence reviews. Their key message: Pharmacotherapy and behavioral support are effective when used alone, but even more effective when combined. Pharmacotherapy helps reduce the symptoms of nicotine withdrawal, while behavioral intervention tackles the challenge of changing learned behaviors associated with smoking, the researchers said.
Although combining medications, such as varenicline and nicotine replacement therapy or bupropion might improve successful quit rates, these combinations have not been well studied, they noted.
With regard to e-cigarettes, the researchers cited a 2021 Cochrane review of 16,759 individuals who used e-cigarettes for smoking cessation, which found no evidence of harm, but insufficient evidence to asses the balance of risks vs. benefits.
In addition to the lack of randomized trials, “the FDA regulates e-cigarettes as tobacco products, not as medical products and has not evaluated any e-cigarette for medical use as a cessation aid,” the authors of the new review noted.
The review was limited by several factors, including the lack of quality assessment for the selected studies and the exclusion of pharmacotherapy not licensed in the United States.
Commenting on the JAMA paper, Dr. Pierce said, “This review looks like a number of Cochrane Reports that have been published recently. Of course, it only considers randomized trials and not population evidence.”
“If public health had limited itself to this form of evidence, then we still would not know that smoking caused cancer,” he noted. “Randomized trials are very important for testing new drugs; they use selected populations and provide considerable support that is not available in the real world. Sometimes they do not generalize to the population.”
Findings may guide patient conversations
The Tobacco Control study was important, because few studies on e-cigarettes have been conducted, said Linda Girgis, MD, a family physician in private practice in South River, N.J., in an interview.
“As clinicians, we do not have a lot of data available in order to make clinical decisions that are evidence based. Also, getting patients to quit smoking is often very difficult, and having more tools available is a great benefit; however, we need to have the evidence that these tools are effective,” she said.
Dr. Girgis also said she was not surprised by the findings.
“Patients still have the same concerns from e-cigarettes regarding nicotine exposure, but just to a lesser degree; and we still don’t know the long-term effects of e-cigarette use, she said. Based on these studies, recommending e-cigarettes for smokers looking to quit may not be the best method, she noted.
“While it may seem reasonable that exposing lungs to lower doses of nicotine will reduce harm, we need to see actual evidence of this. Also, we also need to study the additives that are frequently used in e-cigs, such as artificial flavorings, to see what harms they may pose, she emphasized.
With regard to the JAMA review, Dr. Girgis said she agreed with the recommendations for pharmacotherapy and behavior therapy as first-line treatments for smoking cessation. “There is evidence regarding the efficacy and safety of these methods, and they have been used for decades,” she said.
Dr. Girgis added that there is a role for e-cigarettes in smoking cessation strategies as a method of harm reduction, but pointed out the problem of many people thinking these products are safe and not understanding the hazards they pose.
“They think they can replace smoking with e-cigarettes and be safe from the health risks associated with smoking. I think if the plan were to switch to e-cigarettes for a short period and then quit, there would be a role,” Dr. Girgis said. “However, replacing one risk for another may reduce harm, but doesn’t eliminate it.”
“To continue to use e-cigarettes indefinitely should not be the goal,” she added.
The Tobacco Control study was funded by the National Institutes of Health and the Tobacco-Related Disease Research Program of the University of California. The researchers had no financial conflicts to disclose.
The JAMA study was funded in part by a grant from the National Institute for Health Research, via Cochrane Infrastructure funds to the Cochrane Tobacco Addiction Group. Lead author Dr. Rigotti disclosed funding from the National Heart, Lung, and Blood Institute and Achieve Life Sciences and personal fees from UpToDate and Achieve Life Sciences. Dr. Girgis had no financial conflicts to disclose.
*This article was updated on 2/28/2022.
From 2013 to 2017, e-cigarette sales in the United States nearly doubled, driven by a rapid uptake of use by adolescents, wrote Riufeng Chen, MD, of the University of California, San Diego, and colleagues, in their paper published in Tobacco Control. However, the subsequent effect of increased e-cigarette use on smoking cessation have not been examined, they said.
In their study, Dr. Chen and colleagues analyzed data from 3,578 previous-year smokers with a recent quit attempt and 1,323 recent former smokers who were part of the PATH cohort in 2017. The participants reported using e-cigarettes or other products to quit cigarette smoking. The primary outcomes were at least 12 months of cigarette abstinence, and tobacco abstinence in 2019. In 2017, 32.8% of established smokers reported trying to quit. Of these, 12.6% used e-cigarettes to help them quit. Cigarette abstinence for at least 12 months for these individuals was 9.9%, which was lower than for those who used either nicotine replacement therapy or a pharmaceutical aid only (15.2%), and about half of the 18.6% abstinence in those who used no products to help them quit.
“In our study, e-cigarettes resulted in seven fewer successful quitters than those who used pharmaceutical aids,” emphasized corresponding author, John P. Pierce, PhD, of the University of California, San Diego.
Among smokers attempting to quit, the adjusted risk difference for cigarette abstinence for a least 12 months with e-cigarettes vs. pharmaceutical aids was –7.3%, and –7.7% for e-cigarettes vs. other smoking cessation methods.
*“Among recent former smokers who had switched to daily use of e-cigarettes in 2017, 43.2% had successfully quit cigarette smoking by 2019, which was similar to those who used e-cigarettes on a nondaily basis (34.6%) or to those who switched to another tobacco product, whether daily (43.6%) or nondaily (44.7%),” the researchers wrote.
The rapid growth in e-cigarette use between 2014 and 2017 has been attributed in part to aggressive marketing of high-nicotine e-cigarettes, they said. “The high-nicotine JUUL e-cigarette has been noted as the closest match to cigarettes in both nicotine delivery and user satisfaction, which should make it one of the best candidates as a product to which smokers could switch in order to maintain their nicotine habit,” they said in their discussion of the findings.
More research needed
The researchers acknowledged the need to review more recent data.
“When we looked ahead to 2019, recent former smokers had started using high-nicotine e-cigarettes. The effectiveness of high-nicotine e-cigarettes at preventing relapse will require another follow-up PATH survey,” they said.
Among recent former smokers, 2.2% reported switching to a high-nicotine e-cigarette. Although individuals who switched to e-cigarettes showed a higher rate of relapse to cigarettes than those who did not switch to other tobacco or e-cigarette products, this difference was not significant.
The study findings were limited by several factors including the observational design and inability to control for all potential confounding factors, the researchers noted. However, the results were strengthened by the use of a large and representative study population, and the inclusion of biological samples to validate self-reported smoking, they said.
Several findings surprised study author
Dr. Pierce said he was surprised by several aspects of the study findings.
“First of all, contrary to what we expected, there was a 25% decline in using e-cigarettes to quit, compared to the previous year (not the 40% increase that was expected from the increase in e-cigarette sales) and almost no smokers were using high-nicotine JUUL products to help them quit,” he said. “In this study, e-cigarettes were much less helpful (7 less successful quitters per 100) than pharmaceutical cessation aids in helping people quit,” he added.
“The fact that the proportion of smokers using e-cigarettes for cessation dropped from 17% to 12% was unexpected, and it suggests that the belief that they are a cessation aid is declining,” he said.
The implication for clinical practice is that e-cigarettes are not a useful tool for smoking cessation, Dr. Pierce said. “We are not finding any evidence in this very large nationally representative study that smokers who switch to getting their nicotine from e-cigarettes are less likely to relapse back to cigarette smoking,” he said.
“We don’t know about the high-nicotine versions,” he added.
New review advises against e-cigarettes for cessation
A recent review article published in JAMA supported the use of pharmacotherapy and behavioral support for smokers wanting to quit. In the review, Nancy A. Rigotti, MD, of Massachusetts General Hospital, Boston, and colleagues summarized the evidence for managing tobacco smoking in clinical practice.
“The health risk from cigarette smoking is primarily due to chemicals produced by the burning of tobacco and not to nicotine,” they noted. However, the physical dependence on nicotine makes quitting a challenge, but it is one worth pursuing, the authors said.
The authors of this review identified 30 reviews, 12 randomized clinical trials, and 7 recent guidelines and evidence reviews. Their key message: Pharmacotherapy and behavioral support are effective when used alone, but even more effective when combined. Pharmacotherapy helps reduce the symptoms of nicotine withdrawal, while behavioral intervention tackles the challenge of changing learned behaviors associated with smoking, the researchers said.
Although combining medications, such as varenicline and nicotine replacement therapy or bupropion might improve successful quit rates, these combinations have not been well studied, they noted.
With regard to e-cigarettes, the researchers cited a 2021 Cochrane review of 16,759 individuals who used e-cigarettes for smoking cessation, which found no evidence of harm, but insufficient evidence to asses the balance of risks vs. benefits.
In addition to the lack of randomized trials, “the FDA regulates e-cigarettes as tobacco products, not as medical products and has not evaluated any e-cigarette for medical use as a cessation aid,” the authors of the new review noted.
The review was limited by several factors, including the lack of quality assessment for the selected studies and the exclusion of pharmacotherapy not licensed in the United States.
Commenting on the JAMA paper, Dr. Pierce said, “This review looks like a number of Cochrane Reports that have been published recently. Of course, it only considers randomized trials and not population evidence.”
“If public health had limited itself to this form of evidence, then we still would not know that smoking caused cancer,” he noted. “Randomized trials are very important for testing new drugs; they use selected populations and provide considerable support that is not available in the real world. Sometimes they do not generalize to the population.”
Findings may guide patient conversations
The Tobacco Control study was important, because few studies on e-cigarettes have been conducted, said Linda Girgis, MD, a family physician in private practice in South River, N.J., in an interview.
“As clinicians, we do not have a lot of data available in order to make clinical decisions that are evidence based. Also, getting patients to quit smoking is often very difficult, and having more tools available is a great benefit; however, we need to have the evidence that these tools are effective,” she said.
Dr. Girgis also said she was not surprised by the findings.
“Patients still have the same concerns from e-cigarettes regarding nicotine exposure, but just to a lesser degree; and we still don’t know the long-term effects of e-cigarette use, she said. Based on these studies, recommending e-cigarettes for smokers looking to quit may not be the best method, she noted.
“While it may seem reasonable that exposing lungs to lower doses of nicotine will reduce harm, we need to see actual evidence of this. Also, we also need to study the additives that are frequently used in e-cigs, such as artificial flavorings, to see what harms they may pose, she emphasized.
With regard to the JAMA review, Dr. Girgis said she agreed with the recommendations for pharmacotherapy and behavior therapy as first-line treatments for smoking cessation. “There is evidence regarding the efficacy and safety of these methods, and they have been used for decades,” she said.
Dr. Girgis added that there is a role for e-cigarettes in smoking cessation strategies as a method of harm reduction, but pointed out the problem of many people thinking these products are safe and not understanding the hazards they pose.
“They think they can replace smoking with e-cigarettes and be safe from the health risks associated with smoking. I think if the plan were to switch to e-cigarettes for a short period and then quit, there would be a role,” Dr. Girgis said. “However, replacing one risk for another may reduce harm, but doesn’t eliminate it.”
“To continue to use e-cigarettes indefinitely should not be the goal,” she added.
The Tobacco Control study was funded by the National Institutes of Health and the Tobacco-Related Disease Research Program of the University of California. The researchers had no financial conflicts to disclose.
The JAMA study was funded in part by a grant from the National Institute for Health Research, via Cochrane Infrastructure funds to the Cochrane Tobacco Addiction Group. Lead author Dr. Rigotti disclosed funding from the National Heart, Lung, and Blood Institute and Achieve Life Sciences and personal fees from UpToDate and Achieve Life Sciences. Dr. Girgis had no financial conflicts to disclose.
*This article was updated on 2/28/2022.
FROM TOBACCO CONTROL
Heavy cannabis use tied to less diabetes in women
Women who used marijuana (cannabis) at least four times in the previous month (heavy users) were less likely to have type 2 diabetes than women who were light users or nonusers, in a nationally representative U.S. observational study.
In contrast, there were no differences in the prevalence of type 2 diabetes in men who were light or heavy cannabis users versus nonusers.
These findings are based on data from the 2013-2018 National Health and Nutrition Examination Survey (NHANES), whereby participants self-reported their cannabis use.
The study by Ayobami S. Ogunsola, MD, MPH, a graduate student at Texas A&M University, College Station, and colleagues was recently published in Cannabis and Cannabinoid Research.
What do the findings mean?
Although overall findings linking cannabis use and diabetes have been inconsistent, the gender differences in the current study are consistent with animal studies and some clinical studies, senior author Ibraheem M. Karaye, MD, MPH, said in an interview.
However, these gender differences need to be confirmed, and “we strongly recommend that more biological or biochemical studies be conducted that could actually tell us the mechanisms,” said Dr. Karaye, an assistant professor in the department of population health, Hofstra University, Hempstead, N.Y.
“It’s indisputable that medical marijuana has some medical benefits,” he added. “Women [who use cannabis] have been shown to lose more weight than men, for example.”
“If women [cannabis users] are less likely to develop diabetes or more likely to express improvement of symptoms of diabetes,” he noted, “this means that hyperglycemic medications that are being prescribed should be watched scrupulously. Otherwise, there is a risk that [women] may overrespond.”
That is, Dr. Karaye continued, women “may be at risk of developing hypoglycemia because the cannabis is acting synergistically with the regular drug that is being used to treat the diabetes.”
U.S. clinicians, especially in states with legalized medical marijuana, need to be aware of the potential synergy.
“One would have to consider the patient as a whole,” he stressed. “For example, a woman that uses medical marijuana may actually respond differently to hyperglycemic medication.”
Conflicting reports explained by sex differences?
Evidence on whether cannabis use is linked with type 2 diabetes is limited and conflicting, the researchers wrote. They hypothesized that these conflicting findings might be explained by sex differences.
To “help inform current diabetes prevention and mitigation efforts,” they investigated sex differences in cannabis use and prevalence of type 2 diabetes in 15,602 men and women in the 2013-2014, 2015-2016, and 2017-2018 NHANES surveys.
Participants were classified as having type 2 diabetes if they had a physician’s diagnosis; a 2-hour plasma glucose of at least 200 mg/dL (in a glucose tolerance test); fasting blood glucose of at least 126 mg/dL; or A1c of at least 6.5%.
About half of respondents were women (52%) and close to half (44%) were age 18-39.
More than a third (38%) had a body mass index (BMI) of at least 30 kg/m2, indicating obesity.
Roughly 1 in 10 had a diagnosis of type 2 diabetes (13.5%) or A1c of at least 6.5% (9.8%).
Close to a fifth smoked cigarettes (16%). Similarly, 14.5% used cannabis at least four times a week, 3.3% used it less often, and the rest did not use it. Half of participants were not physically active (49%).
Just over half had at least a college education (55%).
Heavy cannabis users were more likely to be younger than age 40 (57% of men, 57% of women), college graduates (54% of men, 63% of women), cigarette smokers (79% of men, 83% of women), and physically inactive (39% of men, 49% of women).
Among women, heavy cannabis users were 49% less likely to have type 2 diabetes than nonusers, after adjusting for age, sex, race/ethnicity, educational level, physical activity, tobacco use, alcohol use, marital status, difficulty walking, employment status, income, and BMI (adjusted odds ratio, 0.51; 95% confidence interval, 0.31-0.84).
There were no significant differences between light cannabis users versus nonusers and diabetes prevalence in women, or between light or heavy cannabis users versus nonusers and diabetes prevalence in men.
Limitations, yet biologically plausible
The researchers acknowledged several study limitations.
They do not know how long participants had used marijuana. The men and women may have underreported their cannabis use, especially in states where medical marijuana was not legal, and the NHANES data did not specify whether the cannabis was recreational or medicinal.
The study may have been underpowered to detect a smaller difference in men who used versus did not use marijuana.
And importantly, this was an observational study (a snapshot at one point in time), so it cannot say whether the heavy cannabis use in women caused a decreased likelihood of diabetes.
Nevertheless, the inverse association between cannabis use and presence of type 2 diabetes is biologically plausible, Dr. Ogunsola and colleagues wrote.
The two major cannabis compounds, cannabidiol and delta-9-tetrahydrocannabinol, stimulate CBD1 and CBD2 receptors in the central and peripheral nervous systems, respectively. And “activation of the CBD1 receptor increases insulin secretion, glucagon, and somatostatin, and activates metabolic processes in fat and skeletal muscles – mechanisms that improve glucose disposal,” they explained.
The researchers speculated that the sex differences they found for this association may be caused by differences in sex hormones, or the endocannabinoid system, or fat deposits.
Therefore, “additional studies are needed to investigate the sex-based heterogeneity reported in this study and to elucidate potential mechanisms for the observation,” they concluded.
The study did not receive any funding and the researchers have no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Women who used marijuana (cannabis) at least four times in the previous month (heavy users) were less likely to have type 2 diabetes than women who were light users or nonusers, in a nationally representative U.S. observational study.
In contrast, there were no differences in the prevalence of type 2 diabetes in men who were light or heavy cannabis users versus nonusers.
These findings are based on data from the 2013-2018 National Health and Nutrition Examination Survey (NHANES), whereby participants self-reported their cannabis use.
The study by Ayobami S. Ogunsola, MD, MPH, a graduate student at Texas A&M University, College Station, and colleagues was recently published in Cannabis and Cannabinoid Research.
What do the findings mean?
Although overall findings linking cannabis use and diabetes have been inconsistent, the gender differences in the current study are consistent with animal studies and some clinical studies, senior author Ibraheem M. Karaye, MD, MPH, said in an interview.
However, these gender differences need to be confirmed, and “we strongly recommend that more biological or biochemical studies be conducted that could actually tell us the mechanisms,” said Dr. Karaye, an assistant professor in the department of population health, Hofstra University, Hempstead, N.Y.
“It’s indisputable that medical marijuana has some medical benefits,” he added. “Women [who use cannabis] have been shown to lose more weight than men, for example.”
“If women [cannabis users] are less likely to develop diabetes or more likely to express improvement of symptoms of diabetes,” he noted, “this means that hyperglycemic medications that are being prescribed should be watched scrupulously. Otherwise, there is a risk that [women] may overrespond.”
That is, Dr. Karaye continued, women “may be at risk of developing hypoglycemia because the cannabis is acting synergistically with the regular drug that is being used to treat the diabetes.”
U.S. clinicians, especially in states with legalized medical marijuana, need to be aware of the potential synergy.
“One would have to consider the patient as a whole,” he stressed. “For example, a woman that uses medical marijuana may actually respond differently to hyperglycemic medication.”
Conflicting reports explained by sex differences?
Evidence on whether cannabis use is linked with type 2 diabetes is limited and conflicting, the researchers wrote. They hypothesized that these conflicting findings might be explained by sex differences.
To “help inform current diabetes prevention and mitigation efforts,” they investigated sex differences in cannabis use and prevalence of type 2 diabetes in 15,602 men and women in the 2013-2014, 2015-2016, and 2017-2018 NHANES surveys.
Participants were classified as having type 2 diabetes if they had a physician’s diagnosis; a 2-hour plasma glucose of at least 200 mg/dL (in a glucose tolerance test); fasting blood glucose of at least 126 mg/dL; or A1c of at least 6.5%.
About half of respondents were women (52%) and close to half (44%) were age 18-39.
More than a third (38%) had a body mass index (BMI) of at least 30 kg/m2, indicating obesity.
Roughly 1 in 10 had a diagnosis of type 2 diabetes (13.5%) or A1c of at least 6.5% (9.8%).
Close to a fifth smoked cigarettes (16%). Similarly, 14.5% used cannabis at least four times a week, 3.3% used it less often, and the rest did not use it. Half of participants were not physically active (49%).
Just over half had at least a college education (55%).
Heavy cannabis users were more likely to be younger than age 40 (57% of men, 57% of women), college graduates (54% of men, 63% of women), cigarette smokers (79% of men, 83% of women), and physically inactive (39% of men, 49% of women).
Among women, heavy cannabis users were 49% less likely to have type 2 diabetes than nonusers, after adjusting for age, sex, race/ethnicity, educational level, physical activity, tobacco use, alcohol use, marital status, difficulty walking, employment status, income, and BMI (adjusted odds ratio, 0.51; 95% confidence interval, 0.31-0.84).
There were no significant differences between light cannabis users versus nonusers and diabetes prevalence in women, or between light or heavy cannabis users versus nonusers and diabetes prevalence in men.
Limitations, yet biologically plausible
The researchers acknowledged several study limitations.
They do not know how long participants had used marijuana. The men and women may have underreported their cannabis use, especially in states where medical marijuana was not legal, and the NHANES data did not specify whether the cannabis was recreational or medicinal.
The study may have been underpowered to detect a smaller difference in men who used versus did not use marijuana.
And importantly, this was an observational study (a snapshot at one point in time), so it cannot say whether the heavy cannabis use in women caused a decreased likelihood of diabetes.
Nevertheless, the inverse association between cannabis use and presence of type 2 diabetes is biologically plausible, Dr. Ogunsola and colleagues wrote.
The two major cannabis compounds, cannabidiol and delta-9-tetrahydrocannabinol, stimulate CBD1 and CBD2 receptors in the central and peripheral nervous systems, respectively. And “activation of the CBD1 receptor increases insulin secretion, glucagon, and somatostatin, and activates metabolic processes in fat and skeletal muscles – mechanisms that improve glucose disposal,” they explained.
The researchers speculated that the sex differences they found for this association may be caused by differences in sex hormones, or the endocannabinoid system, or fat deposits.
Therefore, “additional studies are needed to investigate the sex-based heterogeneity reported in this study and to elucidate potential mechanisms for the observation,” they concluded.
The study did not receive any funding and the researchers have no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Women who used marijuana (cannabis) at least four times in the previous month (heavy users) were less likely to have type 2 diabetes than women who were light users or nonusers, in a nationally representative U.S. observational study.
In contrast, there were no differences in the prevalence of type 2 diabetes in men who were light or heavy cannabis users versus nonusers.
These findings are based on data from the 2013-2018 National Health and Nutrition Examination Survey (NHANES), whereby participants self-reported their cannabis use.
The study by Ayobami S. Ogunsola, MD, MPH, a graduate student at Texas A&M University, College Station, and colleagues was recently published in Cannabis and Cannabinoid Research.
What do the findings mean?
Although overall findings linking cannabis use and diabetes have been inconsistent, the gender differences in the current study are consistent with animal studies and some clinical studies, senior author Ibraheem M. Karaye, MD, MPH, said in an interview.
However, these gender differences need to be confirmed, and “we strongly recommend that more biological or biochemical studies be conducted that could actually tell us the mechanisms,” said Dr. Karaye, an assistant professor in the department of population health, Hofstra University, Hempstead, N.Y.
“It’s indisputable that medical marijuana has some medical benefits,” he added. “Women [who use cannabis] have been shown to lose more weight than men, for example.”
“If women [cannabis users] are less likely to develop diabetes or more likely to express improvement of symptoms of diabetes,” he noted, “this means that hyperglycemic medications that are being prescribed should be watched scrupulously. Otherwise, there is a risk that [women] may overrespond.”
That is, Dr. Karaye continued, women “may be at risk of developing hypoglycemia because the cannabis is acting synergistically with the regular drug that is being used to treat the diabetes.”
U.S. clinicians, especially in states with legalized medical marijuana, need to be aware of the potential synergy.
“One would have to consider the patient as a whole,” he stressed. “For example, a woman that uses medical marijuana may actually respond differently to hyperglycemic medication.”
Conflicting reports explained by sex differences?
Evidence on whether cannabis use is linked with type 2 diabetes is limited and conflicting, the researchers wrote. They hypothesized that these conflicting findings might be explained by sex differences.
To “help inform current diabetes prevention and mitigation efforts,” they investigated sex differences in cannabis use and prevalence of type 2 diabetes in 15,602 men and women in the 2013-2014, 2015-2016, and 2017-2018 NHANES surveys.
Participants were classified as having type 2 diabetes if they had a physician’s diagnosis; a 2-hour plasma glucose of at least 200 mg/dL (in a glucose tolerance test); fasting blood glucose of at least 126 mg/dL; or A1c of at least 6.5%.
About half of respondents were women (52%) and close to half (44%) were age 18-39.
More than a third (38%) had a body mass index (BMI) of at least 30 kg/m2, indicating obesity.
Roughly 1 in 10 had a diagnosis of type 2 diabetes (13.5%) or A1c of at least 6.5% (9.8%).
Close to a fifth smoked cigarettes (16%). Similarly, 14.5% used cannabis at least four times a week, 3.3% used it less often, and the rest did not use it. Half of participants were not physically active (49%).
Just over half had at least a college education (55%).
Heavy cannabis users were more likely to be younger than age 40 (57% of men, 57% of women), college graduates (54% of men, 63% of women), cigarette smokers (79% of men, 83% of women), and physically inactive (39% of men, 49% of women).
Among women, heavy cannabis users were 49% less likely to have type 2 diabetes than nonusers, after adjusting for age, sex, race/ethnicity, educational level, physical activity, tobacco use, alcohol use, marital status, difficulty walking, employment status, income, and BMI (adjusted odds ratio, 0.51; 95% confidence interval, 0.31-0.84).
There were no significant differences between light cannabis users versus nonusers and diabetes prevalence in women, or between light or heavy cannabis users versus nonusers and diabetes prevalence in men.
Limitations, yet biologically plausible
The researchers acknowledged several study limitations.
They do not know how long participants had used marijuana. The men and women may have underreported their cannabis use, especially in states where medical marijuana was not legal, and the NHANES data did not specify whether the cannabis was recreational or medicinal.
The study may have been underpowered to detect a smaller difference in men who used versus did not use marijuana.
And importantly, this was an observational study (a snapshot at one point in time), so it cannot say whether the heavy cannabis use in women caused a decreased likelihood of diabetes.
Nevertheless, the inverse association between cannabis use and presence of type 2 diabetes is biologically plausible, Dr. Ogunsola and colleagues wrote.
The two major cannabis compounds, cannabidiol and delta-9-tetrahydrocannabinol, stimulate CBD1 and CBD2 receptors in the central and peripheral nervous systems, respectively. And “activation of the CBD1 receptor increases insulin secretion, glucagon, and somatostatin, and activates metabolic processes in fat and skeletal muscles – mechanisms that improve glucose disposal,” they explained.
The researchers speculated that the sex differences they found for this association may be caused by differences in sex hormones, or the endocannabinoid system, or fat deposits.
Therefore, “additional studies are needed to investigate the sex-based heterogeneity reported in this study and to elucidate potential mechanisms for the observation,” they concluded.
The study did not receive any funding and the researchers have no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM CANNABIS AND CANNABINOID RESEARCH
Chronic marijuana use linked to recurrent stroke
, new observational research suggests. “Our analysis shows young marijuana users with a history of stroke or transient ischemic attack remain at significantly high risk for future strokes,” said lead study author Akhil Jain, MD, a resident physician at Mercy Fitzgerald Hospital in Darby, Pennsylvania.
“It’s essential to raise awareness among young adults about the impact of chronic habitual use of marijuana, especially if they have established cardiovascular risk factors or previous stroke.”
The study will be presented during the International Stroke Conference, presented by the American Stroke Association, a division of the American Heart Association.
An increasing number of jurisdictions are allowing marijuana use. To date, 18 states and the District of Columbia have legalized recreational cannabis use, the investigators noted.
Research suggests cannabis use disorder – defined as the chronic habitual use of cannabis – is more prevalent in the young adult population. But Dr. Jain said the population of marijuana users is “a changing dynamic.”
Cannabis use has been linked to an increased risk for first-time stroke or transient ischemic attack (TIA). Traditional stroke risk factors include hypertension, diabetes, and diseases related to blood vessels or blood circulation, including atherosclerosis.
Young adults might have additional stroke risk factors, such as behavioral habits like substance abuse, low physical activity, and smoking, oral contraceptives use among females, and brain infections, especially in the immunocompromised, said Dr. Jain.
Research from the American Heart Association shows stroke rates are increasing among adults 18 to 45 years of age. Each year, young adults account for up to 15% of strokes in the United States.
Prevalence and risk for recurrent stroke in patients with previous stroke or TIA in cannabis users have not been clearly established, the researchers pointed out.
A higher rate of recurrent stroke
For this new study, Dr. Jain and colleagues used data from the National Inpatient Sample from October 2015 to December 2017. They identified hospitalizations among young adults 18 to 45 years of age with a previous history of stroke or TIA.
They then grouped these patients into those with cannabis use disorder (4,690) and those without cannabis use disorder (156,700). The median age in both cohorts was 37 years.
The analysis did not include those who were considered in remission from cannabis use disorder.
Results showed that 6.9% of those with cannabis use disorder were hospitalized for a recurrent stroke, compared with 5.4% of those without cannabis use disorder (P < .001).
After adjustment for demographic factors (age, sex, race, household income), and pre-existing conditions, patients with cannabis use disorder were 48% more likely to be hospitalized for recurrent stroke than those without cannabis use disorder (odds ratio, 1.48; 95% confidence interval, 1.28-1.71; P < .001).
Compared with the group without cannabis use disorder, the cannabis use disorder group had more men (55.2% vs. 40.2%), more African American people (44.6% vs. 37.2%), and more use of tobacco (73.9% vs. 39.6%) and alcohol (16.5% vs. 3.6%). They also had a greater percentage of chronic obstructive pulmonary disease, depression, and psychoses.
But a smaller percentage of those with cannabis use disorder had hypertension (51.3% vs. 55.6%; P = .001) and diabetes (16.3% vs. 22.7%; P < .001), which is an “interesting” finding, said Dr. Jain.
“We observed that even with a lower rate of cardiovascular risk factors, after controlling for all the risk factors, we still found the cannabis users had a higher rate of recurrent stroke.”
He noted this was a retrospective study without a control group. “If both groups had comparable hypertension, then this risk might actually be more evident,” said Dr. Jain. “We need a prospective study with comparable groups.”
Living in low-income neighborhoods and in northeast and southern regions of the United States was also more common in the cannabis use disorder group.
Hypothesis-generating research
The study did not investigate the possible mechanisms by which marijuana use might increase stroke risk, but Dr. Jain speculated that these could include factors such as impaired blood vessel function, changes in blood supply, an increased tendency of blood clotting, impaired energy production in brain cells, and an imbalance between molecules that harm healthy tissue and the antioxidant defenses that neutralize them.
As cannabis use may pose a different risk for a new stroke, as opposed a previous stroke, Dr. Jain said it would be interesting to study the amount of “residual function deficit” experienced with the first stroke.
The new study represents “foundational research” upon which other research teams can build, said Dr. Jain. “Our study is hypothesis-generating research for a future prospective randomized controlled trial.”
A limitation of the study is that it did not consider the effect of various doses, duration, and forms of cannabis abuse, or use of medicinal cannabis or other drugs.
Robert L. Page II, PharmD, professor, departments of clinical pharmacy and physical medicine/rehabilitation, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, provided a comment on this new research.
A cannabis use disorder diagnosis provides “specific criteria” with regard to chronicity of use and reflects “more of a physical and psychological dependence upon cannabis,” said Dr. Page, who chaired the writing group for the AHA 2020 cannabis and cardiovascular disease scientific statement.
He explained what sets people with cannabis use disorder apart from “run-of-the-mill” recreational cannabis users is that “these are individuals who use a cannabis product, whether it’s smoking it, vaping it, or consuming it via an edible, and are using it on a regular basis, in a chronic fashion.”
The study received no outside funding. The authors report no relevant disclosures.
A version of this article first appeared on Medscape.com.
, new observational research suggests. “Our analysis shows young marijuana users with a history of stroke or transient ischemic attack remain at significantly high risk for future strokes,” said lead study author Akhil Jain, MD, a resident physician at Mercy Fitzgerald Hospital in Darby, Pennsylvania.
“It’s essential to raise awareness among young adults about the impact of chronic habitual use of marijuana, especially if they have established cardiovascular risk factors or previous stroke.”
The study will be presented during the International Stroke Conference, presented by the American Stroke Association, a division of the American Heart Association.
An increasing number of jurisdictions are allowing marijuana use. To date, 18 states and the District of Columbia have legalized recreational cannabis use, the investigators noted.
Research suggests cannabis use disorder – defined as the chronic habitual use of cannabis – is more prevalent in the young adult population. But Dr. Jain said the population of marijuana users is “a changing dynamic.”
Cannabis use has been linked to an increased risk for first-time stroke or transient ischemic attack (TIA). Traditional stroke risk factors include hypertension, diabetes, and diseases related to blood vessels or blood circulation, including atherosclerosis.
Young adults might have additional stroke risk factors, such as behavioral habits like substance abuse, low physical activity, and smoking, oral contraceptives use among females, and brain infections, especially in the immunocompromised, said Dr. Jain.
Research from the American Heart Association shows stroke rates are increasing among adults 18 to 45 years of age. Each year, young adults account for up to 15% of strokes in the United States.
Prevalence and risk for recurrent stroke in patients with previous stroke or TIA in cannabis users have not been clearly established, the researchers pointed out.
A higher rate of recurrent stroke
For this new study, Dr. Jain and colleagues used data from the National Inpatient Sample from October 2015 to December 2017. They identified hospitalizations among young adults 18 to 45 years of age with a previous history of stroke or TIA.
They then grouped these patients into those with cannabis use disorder (4,690) and those without cannabis use disorder (156,700). The median age in both cohorts was 37 years.
The analysis did not include those who were considered in remission from cannabis use disorder.
Results showed that 6.9% of those with cannabis use disorder were hospitalized for a recurrent stroke, compared with 5.4% of those without cannabis use disorder (P < .001).
After adjustment for demographic factors (age, sex, race, household income), and pre-existing conditions, patients with cannabis use disorder were 48% more likely to be hospitalized for recurrent stroke than those without cannabis use disorder (odds ratio, 1.48; 95% confidence interval, 1.28-1.71; P < .001).
Compared with the group without cannabis use disorder, the cannabis use disorder group had more men (55.2% vs. 40.2%), more African American people (44.6% vs. 37.2%), and more use of tobacco (73.9% vs. 39.6%) and alcohol (16.5% vs. 3.6%). They also had a greater percentage of chronic obstructive pulmonary disease, depression, and psychoses.
But a smaller percentage of those with cannabis use disorder had hypertension (51.3% vs. 55.6%; P = .001) and diabetes (16.3% vs. 22.7%; P < .001), which is an “interesting” finding, said Dr. Jain.
“We observed that even with a lower rate of cardiovascular risk factors, after controlling for all the risk factors, we still found the cannabis users had a higher rate of recurrent stroke.”
He noted this was a retrospective study without a control group. “If both groups had comparable hypertension, then this risk might actually be more evident,” said Dr. Jain. “We need a prospective study with comparable groups.”
Living in low-income neighborhoods and in northeast and southern regions of the United States was also more common in the cannabis use disorder group.
Hypothesis-generating research
The study did not investigate the possible mechanisms by which marijuana use might increase stroke risk, but Dr. Jain speculated that these could include factors such as impaired blood vessel function, changes in blood supply, an increased tendency of blood clotting, impaired energy production in brain cells, and an imbalance between molecules that harm healthy tissue and the antioxidant defenses that neutralize them.
As cannabis use may pose a different risk for a new stroke, as opposed a previous stroke, Dr. Jain said it would be interesting to study the amount of “residual function deficit” experienced with the first stroke.
The new study represents “foundational research” upon which other research teams can build, said Dr. Jain. “Our study is hypothesis-generating research for a future prospective randomized controlled trial.”
A limitation of the study is that it did not consider the effect of various doses, duration, and forms of cannabis abuse, or use of medicinal cannabis or other drugs.
Robert L. Page II, PharmD, professor, departments of clinical pharmacy and physical medicine/rehabilitation, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, provided a comment on this new research.
A cannabis use disorder diagnosis provides “specific criteria” with regard to chronicity of use and reflects “more of a physical and psychological dependence upon cannabis,” said Dr. Page, who chaired the writing group for the AHA 2020 cannabis and cardiovascular disease scientific statement.
He explained what sets people with cannabis use disorder apart from “run-of-the-mill” recreational cannabis users is that “these are individuals who use a cannabis product, whether it’s smoking it, vaping it, or consuming it via an edible, and are using it on a regular basis, in a chronic fashion.”
The study received no outside funding. The authors report no relevant disclosures.
A version of this article first appeared on Medscape.com.
, new observational research suggests. “Our analysis shows young marijuana users with a history of stroke or transient ischemic attack remain at significantly high risk for future strokes,” said lead study author Akhil Jain, MD, a resident physician at Mercy Fitzgerald Hospital in Darby, Pennsylvania.
“It’s essential to raise awareness among young adults about the impact of chronic habitual use of marijuana, especially if they have established cardiovascular risk factors or previous stroke.”
The study will be presented during the International Stroke Conference, presented by the American Stroke Association, a division of the American Heart Association.
An increasing number of jurisdictions are allowing marijuana use. To date, 18 states and the District of Columbia have legalized recreational cannabis use, the investigators noted.
Research suggests cannabis use disorder – defined as the chronic habitual use of cannabis – is more prevalent in the young adult population. But Dr. Jain said the population of marijuana users is “a changing dynamic.”
Cannabis use has been linked to an increased risk for first-time stroke or transient ischemic attack (TIA). Traditional stroke risk factors include hypertension, diabetes, and diseases related to blood vessels or blood circulation, including atherosclerosis.
Young adults might have additional stroke risk factors, such as behavioral habits like substance abuse, low physical activity, and smoking, oral contraceptives use among females, and brain infections, especially in the immunocompromised, said Dr. Jain.
Research from the American Heart Association shows stroke rates are increasing among adults 18 to 45 years of age. Each year, young adults account for up to 15% of strokes in the United States.
Prevalence and risk for recurrent stroke in patients with previous stroke or TIA in cannabis users have not been clearly established, the researchers pointed out.
A higher rate of recurrent stroke
For this new study, Dr. Jain and colleagues used data from the National Inpatient Sample from October 2015 to December 2017. They identified hospitalizations among young adults 18 to 45 years of age with a previous history of stroke or TIA.
They then grouped these patients into those with cannabis use disorder (4,690) and those without cannabis use disorder (156,700). The median age in both cohorts was 37 years.
The analysis did not include those who were considered in remission from cannabis use disorder.
Results showed that 6.9% of those with cannabis use disorder were hospitalized for a recurrent stroke, compared with 5.4% of those without cannabis use disorder (P < .001).
After adjustment for demographic factors (age, sex, race, household income), and pre-existing conditions, patients with cannabis use disorder were 48% more likely to be hospitalized for recurrent stroke than those without cannabis use disorder (odds ratio, 1.48; 95% confidence interval, 1.28-1.71; P < .001).
Compared with the group without cannabis use disorder, the cannabis use disorder group had more men (55.2% vs. 40.2%), more African American people (44.6% vs. 37.2%), and more use of tobacco (73.9% vs. 39.6%) and alcohol (16.5% vs. 3.6%). They also had a greater percentage of chronic obstructive pulmonary disease, depression, and psychoses.
But a smaller percentage of those with cannabis use disorder had hypertension (51.3% vs. 55.6%; P = .001) and diabetes (16.3% vs. 22.7%; P < .001), which is an “interesting” finding, said Dr. Jain.
“We observed that even with a lower rate of cardiovascular risk factors, after controlling for all the risk factors, we still found the cannabis users had a higher rate of recurrent stroke.”
He noted this was a retrospective study without a control group. “If both groups had comparable hypertension, then this risk might actually be more evident,” said Dr. Jain. “We need a prospective study with comparable groups.”
Living in low-income neighborhoods and in northeast and southern regions of the United States was also more common in the cannabis use disorder group.
Hypothesis-generating research
The study did not investigate the possible mechanisms by which marijuana use might increase stroke risk, but Dr. Jain speculated that these could include factors such as impaired blood vessel function, changes in blood supply, an increased tendency of blood clotting, impaired energy production in brain cells, and an imbalance between molecules that harm healthy tissue and the antioxidant defenses that neutralize them.
As cannabis use may pose a different risk for a new stroke, as opposed a previous stroke, Dr. Jain said it would be interesting to study the amount of “residual function deficit” experienced with the first stroke.
The new study represents “foundational research” upon which other research teams can build, said Dr. Jain. “Our study is hypothesis-generating research for a future prospective randomized controlled trial.”
A limitation of the study is that it did not consider the effect of various doses, duration, and forms of cannabis abuse, or use of medicinal cannabis or other drugs.
Robert L. Page II, PharmD, professor, departments of clinical pharmacy and physical medicine/rehabilitation, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, provided a comment on this new research.
A cannabis use disorder diagnosis provides “specific criteria” with regard to chronicity of use and reflects “more of a physical and psychological dependence upon cannabis,” said Dr. Page, who chaired the writing group for the AHA 2020 cannabis and cardiovascular disease scientific statement.
He explained what sets people with cannabis use disorder apart from “run-of-the-mill” recreational cannabis users is that “these are individuals who use a cannabis product, whether it’s smoking it, vaping it, or consuming it via an edible, and are using it on a regular basis, in a chronic fashion.”
The study received no outside funding. The authors report no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ISC 2022
Study questions reliability of maternal drug testing
A new study finding that samples from maternal urine and the meconium of their newborn babies frequently produce different results is raising more questions about drug testing of pregnant women.
The study found concerningly high rates of disagreement (or “discordance”) in biochemical testing between maternal urine in women with a documented history of or active drug use and the meconium in their newborns. In some cases, such discordance might be triggering the inappropriate intervention of childcare protective services, including the separation of infants from their mothers, according to the researchers, who presented their findings Feb. 4 at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“There’s a very big debate right now in the obstetrics and perinatology communities about the utility of biochemical testing and the identification of high-risk women,” lead author Cassandra Heiselman, DO, MPH, clinical assistant professor in the department of obstetrics, gynecology and reproductive medicine at Stony Brook (N.Y.) University, said in an interview. “We know that each biochemical test has limitations, which can include basically the inability to detect all substances, especially synthetic opioids like fentanyl, [and] the possibility for false results.”
Inaccuracies in testing can potentially result in inappropriate separation of mother and baby. “Careful scrutiny of results is needed,” Dr. Heiselman said.
The Stony Brook team conducted a retrospective cohort study that identified women presenting for delivery from January 2017 to March 2021 with indications for drug testing, including a known history of or current substance use disorder/misuse, and late or no prenatal care. A standardized panel was used for testing maternal urine and newborn meconium.
Urine tests of 327 women resulted in 187 (57%) positive and 98 (30%) negative results, along with 42 (13%) samples with incomplete data, the researchers reported. In contrast, drug testing of newborn meconium was positive in 273 (83%) cases, negative in 42 (13%), and was not performed in 12 (4%) – for a rate of concordance of 41%.
Concordance of urine/meconium occurred more frequently in male newborns (65%), compared with females (35%). “It is unclear biologically why there is such a difference based on the sex of the infants’ test and is an area that needs further investigation,” Dr. Heiselman said.
Comparing urine and meconium tests for 11 substances resulted in 195/483 (40%) concordance, the researchers said; 18% were discordant with positive maternal urine, and 41% were discordant with newborn positive meconium.
Oxycodone and fentanyl were significantly discordant with positive maternal urine. Cannabis use was the most common factor associated with a positive test of meconium, according to the researchers.
“Some studies have shown cannabis use in the second trimester can show up in meconium testing even if the mother has stopped that behavior,” Dr. Heiselman said. “Then there is also cross-reactivity with other substances that can lead to higher false positive results, especially in the urine toxicology.”
The reasons for the discordant results are not clear and vary by substance, Dr. Heiselman said.
“Cannabis and methadone were the significant factors leading to discordance with positive newborn meconium, which may reflect prior use earlier in pregnancy without recent use before delivery,” she said in an interview. “Urine and meconium reflect potentially different timing in perinatal exposure and the potential differences in windows of detection for different substances. Therefore, we would expect some discordance in our comparisons, just not the extent that we saw.”
Some test results might also have been false positives. Many commonly used medications, from cough syrups to proton pump inhibitors, have the potential to generate positive results for illicit drugs, Dr. Heiselman said.
“The issue of discordance is a complex one, where there are limitations of the tests being performed, possible cross-reactivity with false positives, and the difference in what test reflects as far as timing of prenatal exposure. Furthermore, a negative test does not rule out sporadic use, nor does a positive result diagnose substance use disorder or its severity,” she said.
Lack of standards
Dr. Heiselman said states and the federal government lack standards to biochemically evaluate women at risk for drug abuse and their newborns.
“My institution uses a risk-based protocol. Basically, we test cases where we have a known history of substance use disorder or active use, a history in the last 3 years of any kind of substance use, initiation of late prenatal care after 20 weeks, or no prenatal care at all,” she said. “And then the pediatricians on the other side will test neonates if the mother has any of that history or if the neonates themselves have unexplained complications or drug withdrawal symptoms.”
High rates of discordance can result in the inappropriate intervention by childcare protective service agencies when the mother may not have a substance use disorder, she noted.
Perinatologist Kecia Gaither, MD, MPH, associate professor of clinical obstetrics and gynecology at Weill Cornell Medicine, New York, called the findings “no surprise,” but added that negative findings in neonates “do not exclude the possibility of substance abuse by the mother. It is important to recognize the limitations inherent with screening tests for illicit substances in neonates from substance-abusing mothers.”
Dr. Heiselman added that understanding what maternal and infant drug tests truly reflect “can help us as clinicians in deciding when we test, whether it’s medically necessary, instead of just thinking biochemical tests are the best screening tool, because we know that we are screening. We must engage these women in empathetic and nonjudgmental discussions, which often will elucidate a substance use disorder history more so than just biochemical testing, negative or positive.”
The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study finding that samples from maternal urine and the meconium of their newborn babies frequently produce different results is raising more questions about drug testing of pregnant women.
The study found concerningly high rates of disagreement (or “discordance”) in biochemical testing between maternal urine in women with a documented history of or active drug use and the meconium in their newborns. In some cases, such discordance might be triggering the inappropriate intervention of childcare protective services, including the separation of infants from their mothers, according to the researchers, who presented their findings Feb. 4 at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“There’s a very big debate right now in the obstetrics and perinatology communities about the utility of biochemical testing and the identification of high-risk women,” lead author Cassandra Heiselman, DO, MPH, clinical assistant professor in the department of obstetrics, gynecology and reproductive medicine at Stony Brook (N.Y.) University, said in an interview. “We know that each biochemical test has limitations, which can include basically the inability to detect all substances, especially synthetic opioids like fentanyl, [and] the possibility for false results.”
Inaccuracies in testing can potentially result in inappropriate separation of mother and baby. “Careful scrutiny of results is needed,” Dr. Heiselman said.
The Stony Brook team conducted a retrospective cohort study that identified women presenting for delivery from January 2017 to March 2021 with indications for drug testing, including a known history of or current substance use disorder/misuse, and late or no prenatal care. A standardized panel was used for testing maternal urine and newborn meconium.
Urine tests of 327 women resulted in 187 (57%) positive and 98 (30%) negative results, along with 42 (13%) samples with incomplete data, the researchers reported. In contrast, drug testing of newborn meconium was positive in 273 (83%) cases, negative in 42 (13%), and was not performed in 12 (4%) – for a rate of concordance of 41%.
Concordance of urine/meconium occurred more frequently in male newborns (65%), compared with females (35%). “It is unclear biologically why there is such a difference based on the sex of the infants’ test and is an area that needs further investigation,” Dr. Heiselman said.
Comparing urine and meconium tests for 11 substances resulted in 195/483 (40%) concordance, the researchers said; 18% were discordant with positive maternal urine, and 41% were discordant with newborn positive meconium.
Oxycodone and fentanyl were significantly discordant with positive maternal urine. Cannabis use was the most common factor associated with a positive test of meconium, according to the researchers.
“Some studies have shown cannabis use in the second trimester can show up in meconium testing even if the mother has stopped that behavior,” Dr. Heiselman said. “Then there is also cross-reactivity with other substances that can lead to higher false positive results, especially in the urine toxicology.”
The reasons for the discordant results are not clear and vary by substance, Dr. Heiselman said.
“Cannabis and methadone were the significant factors leading to discordance with positive newborn meconium, which may reflect prior use earlier in pregnancy without recent use before delivery,” she said in an interview. “Urine and meconium reflect potentially different timing in perinatal exposure and the potential differences in windows of detection for different substances. Therefore, we would expect some discordance in our comparisons, just not the extent that we saw.”
Some test results might also have been false positives. Many commonly used medications, from cough syrups to proton pump inhibitors, have the potential to generate positive results for illicit drugs, Dr. Heiselman said.
“The issue of discordance is a complex one, where there are limitations of the tests being performed, possible cross-reactivity with false positives, and the difference in what test reflects as far as timing of prenatal exposure. Furthermore, a negative test does not rule out sporadic use, nor does a positive result diagnose substance use disorder or its severity,” she said.
Lack of standards
Dr. Heiselman said states and the federal government lack standards to biochemically evaluate women at risk for drug abuse and their newborns.
“My institution uses a risk-based protocol. Basically, we test cases where we have a known history of substance use disorder or active use, a history in the last 3 years of any kind of substance use, initiation of late prenatal care after 20 weeks, or no prenatal care at all,” she said. “And then the pediatricians on the other side will test neonates if the mother has any of that history or if the neonates themselves have unexplained complications or drug withdrawal symptoms.”
High rates of discordance can result in the inappropriate intervention by childcare protective service agencies when the mother may not have a substance use disorder, she noted.
Perinatologist Kecia Gaither, MD, MPH, associate professor of clinical obstetrics and gynecology at Weill Cornell Medicine, New York, called the findings “no surprise,” but added that negative findings in neonates “do not exclude the possibility of substance abuse by the mother. It is important to recognize the limitations inherent with screening tests for illicit substances in neonates from substance-abusing mothers.”
Dr. Heiselman added that understanding what maternal and infant drug tests truly reflect “can help us as clinicians in deciding when we test, whether it’s medically necessary, instead of just thinking biochemical tests are the best screening tool, because we know that we are screening. We must engage these women in empathetic and nonjudgmental discussions, which often will elucidate a substance use disorder history more so than just biochemical testing, negative or positive.”
The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study finding that samples from maternal urine and the meconium of their newborn babies frequently produce different results is raising more questions about drug testing of pregnant women.
The study found concerningly high rates of disagreement (or “discordance”) in biochemical testing between maternal urine in women with a documented history of or active drug use and the meconium in their newborns. In some cases, such discordance might be triggering the inappropriate intervention of childcare protective services, including the separation of infants from their mothers, according to the researchers, who presented their findings Feb. 4 at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“There’s a very big debate right now in the obstetrics and perinatology communities about the utility of biochemical testing and the identification of high-risk women,” lead author Cassandra Heiselman, DO, MPH, clinical assistant professor in the department of obstetrics, gynecology and reproductive medicine at Stony Brook (N.Y.) University, said in an interview. “We know that each biochemical test has limitations, which can include basically the inability to detect all substances, especially synthetic opioids like fentanyl, [and] the possibility for false results.”
Inaccuracies in testing can potentially result in inappropriate separation of mother and baby. “Careful scrutiny of results is needed,” Dr. Heiselman said.
The Stony Brook team conducted a retrospective cohort study that identified women presenting for delivery from January 2017 to March 2021 with indications for drug testing, including a known history of or current substance use disorder/misuse, and late or no prenatal care. A standardized panel was used for testing maternal urine and newborn meconium.
Urine tests of 327 women resulted in 187 (57%) positive and 98 (30%) negative results, along with 42 (13%) samples with incomplete data, the researchers reported. In contrast, drug testing of newborn meconium was positive in 273 (83%) cases, negative in 42 (13%), and was not performed in 12 (4%) – for a rate of concordance of 41%.
Concordance of urine/meconium occurred more frequently in male newborns (65%), compared with females (35%). “It is unclear biologically why there is such a difference based on the sex of the infants’ test and is an area that needs further investigation,” Dr. Heiselman said.
Comparing urine and meconium tests for 11 substances resulted in 195/483 (40%) concordance, the researchers said; 18% were discordant with positive maternal urine, and 41% were discordant with newborn positive meconium.
Oxycodone and fentanyl were significantly discordant with positive maternal urine. Cannabis use was the most common factor associated with a positive test of meconium, according to the researchers.
“Some studies have shown cannabis use in the second trimester can show up in meconium testing even if the mother has stopped that behavior,” Dr. Heiselman said. “Then there is also cross-reactivity with other substances that can lead to higher false positive results, especially in the urine toxicology.”
The reasons for the discordant results are not clear and vary by substance, Dr. Heiselman said.
“Cannabis and methadone were the significant factors leading to discordance with positive newborn meconium, which may reflect prior use earlier in pregnancy without recent use before delivery,” she said in an interview. “Urine and meconium reflect potentially different timing in perinatal exposure and the potential differences in windows of detection for different substances. Therefore, we would expect some discordance in our comparisons, just not the extent that we saw.”
Some test results might also have been false positives. Many commonly used medications, from cough syrups to proton pump inhibitors, have the potential to generate positive results for illicit drugs, Dr. Heiselman said.
“The issue of discordance is a complex one, where there are limitations of the tests being performed, possible cross-reactivity with false positives, and the difference in what test reflects as far as timing of prenatal exposure. Furthermore, a negative test does not rule out sporadic use, nor does a positive result diagnose substance use disorder or its severity,” she said.
Lack of standards
Dr. Heiselman said states and the federal government lack standards to biochemically evaluate women at risk for drug abuse and their newborns.
“My institution uses a risk-based protocol. Basically, we test cases where we have a known history of substance use disorder or active use, a history in the last 3 years of any kind of substance use, initiation of late prenatal care after 20 weeks, or no prenatal care at all,” she said. “And then the pediatricians on the other side will test neonates if the mother has any of that history or if the neonates themselves have unexplained complications or drug withdrawal symptoms.”
High rates of discordance can result in the inappropriate intervention by childcare protective service agencies when the mother may not have a substance use disorder, she noted.
Perinatologist Kecia Gaither, MD, MPH, associate professor of clinical obstetrics and gynecology at Weill Cornell Medicine, New York, called the findings “no surprise,” but added that negative findings in neonates “do not exclude the possibility of substance abuse by the mother. It is important to recognize the limitations inherent with screening tests for illicit substances in neonates from substance-abusing mothers.”
Dr. Heiselman added that understanding what maternal and infant drug tests truly reflect “can help us as clinicians in deciding when we test, whether it’s medically necessary, instead of just thinking biochemical tests are the best screening tool, because we know that we are screening. We must engage these women in empathetic and nonjudgmental discussions, which often will elucidate a substance use disorder history more so than just biochemical testing, negative or positive.”
The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE PREGNANCY MEETING