User login
COVID-19 hospitalization 80% more likely for smokers
Observational data was analyzed alongside hospital coronavirus test data and UK Biobank genetic information for the first time, and the findings are published in Thorax.
The data cover 421,469 people overall. Of these, 3.2% took a polymerase chain reaction swab test, 0.4% of these tested positive, 0.2% of them required hospitalization for COVID-19, and 0.1% of them died because of COVID-19.
When it came to smoking status, 59% had never smoked, 37% were ex-smokers, and 3% were current smokers.
Current smokers were 80% more likely to be admitted to hospital, and significantly more likely to die from COVID-19, than nonsmokers.
Time to quit
Heavy smokers who smoked more than 20 cigarettes a day were 6.11 times more likely to die from COVID-19 than people who had never smoked.
Analysis also showed those with a genetic predisposition to being smokers had a 45% higher infection risk, and 60% higher hospitalization risk.
The authors wrote: “Overall, the congruence of observational analyses indicating associations with recent smoking behaviors and [Mendelian randomization] analyses indicating associations with lifelong predisposition to smoking and smoking heaviness support a causal effect of smoking on COVID-19 severity.”
In a linked podcast, lead researcher Dr. Ashley Clift, said: “Our results strongly suggest that smoking is related to your risk of getting severe COVID, and just as smoking affects your risk of heart disease, different cancers, and all those other conditions we know smoking is linked to, it appears that it’s the same for COVID. So now might be as good a time as any to quit cigarettes and quit smoking.”
These results contrast with previous studies that have suggested a protective effect of smoking against COVID-19. In a linked editorial, Anthony Laverty, PhD, and Christopher Millet, PhD, Imperial College London, wrote: “The idea that tobacco smoking may protect against COVID-19 was always an improbable one.”
A version of this article first appeared on Medscape.com.
Observational data was analyzed alongside hospital coronavirus test data and UK Biobank genetic information for the first time, and the findings are published in Thorax.
The data cover 421,469 people overall. Of these, 3.2% took a polymerase chain reaction swab test, 0.4% of these tested positive, 0.2% of them required hospitalization for COVID-19, and 0.1% of them died because of COVID-19.
When it came to smoking status, 59% had never smoked, 37% were ex-smokers, and 3% were current smokers.
Current smokers were 80% more likely to be admitted to hospital, and significantly more likely to die from COVID-19, than nonsmokers.
Time to quit
Heavy smokers who smoked more than 20 cigarettes a day were 6.11 times more likely to die from COVID-19 than people who had never smoked.
Analysis also showed those with a genetic predisposition to being smokers had a 45% higher infection risk, and 60% higher hospitalization risk.
The authors wrote: “Overall, the congruence of observational analyses indicating associations with recent smoking behaviors and [Mendelian randomization] analyses indicating associations with lifelong predisposition to smoking and smoking heaviness support a causal effect of smoking on COVID-19 severity.”
In a linked podcast, lead researcher Dr. Ashley Clift, said: “Our results strongly suggest that smoking is related to your risk of getting severe COVID, and just as smoking affects your risk of heart disease, different cancers, and all those other conditions we know smoking is linked to, it appears that it’s the same for COVID. So now might be as good a time as any to quit cigarettes and quit smoking.”
These results contrast with previous studies that have suggested a protective effect of smoking against COVID-19. In a linked editorial, Anthony Laverty, PhD, and Christopher Millet, PhD, Imperial College London, wrote: “The idea that tobacco smoking may protect against COVID-19 was always an improbable one.”
A version of this article first appeared on Medscape.com.
Observational data was analyzed alongside hospital coronavirus test data and UK Biobank genetic information for the first time, and the findings are published in Thorax.
The data cover 421,469 people overall. Of these, 3.2% took a polymerase chain reaction swab test, 0.4% of these tested positive, 0.2% of them required hospitalization for COVID-19, and 0.1% of them died because of COVID-19.
When it came to smoking status, 59% had never smoked, 37% were ex-smokers, and 3% were current smokers.
Current smokers were 80% more likely to be admitted to hospital, and significantly more likely to die from COVID-19, than nonsmokers.
Time to quit
Heavy smokers who smoked more than 20 cigarettes a day were 6.11 times more likely to die from COVID-19 than people who had never smoked.
Analysis also showed those with a genetic predisposition to being smokers had a 45% higher infection risk, and 60% higher hospitalization risk.
The authors wrote: “Overall, the congruence of observational analyses indicating associations with recent smoking behaviors and [Mendelian randomization] analyses indicating associations with lifelong predisposition to smoking and smoking heaviness support a causal effect of smoking on COVID-19 severity.”
In a linked podcast, lead researcher Dr. Ashley Clift, said: “Our results strongly suggest that smoking is related to your risk of getting severe COVID, and just as smoking affects your risk of heart disease, different cancers, and all those other conditions we know smoking is linked to, it appears that it’s the same for COVID. So now might be as good a time as any to quit cigarettes and quit smoking.”
These results contrast with previous studies that have suggested a protective effect of smoking against COVID-19. In a linked editorial, Anthony Laverty, PhD, and Christopher Millet, PhD, Imperial College London, wrote: “The idea that tobacco smoking may protect against COVID-19 was always an improbable one.”
A version of this article first appeared on Medscape.com.
‘Alarming’ increase in fake pills laced with fentanyl, methamphetamine, DEA warns
The U.S. Drug Enforcement Administration has issued a public safety alert over an “alarming” increase in fake prescription pills laced with the synthetic opioid fentanyl or the stimulant methamphetamine.
“The United States is facing an unprecedented crisis of overdose deaths fueled by illegally manufactured fentanyl and methamphetamine,” DEA Administrator Anne Milgram said in the alert.
“Counterfeit pills that contain these dangerous and extremely addictive drugs are more lethal and more accessible than ever before. DEA is focusing resources on taking down the violent drug traffickers causing the greatest harm and posing the greatest threat to the safety and health of Americans,” Ms. Milgram said.
Criminal drug networks are mass-producing fake fentanyl- and methamphetamine-laced pills and deceptively marketing them as legitimate prescription pills, the DEA warns.
such as oxycodone (Oxycontin, Percocet), hydrocodone (Vicodin), and alprazolam (Xanax); or stimulants like amphetamines (Adderall).
The agency has seized fake pills in every U.S. state. More than 9.5 million fake pills have been seized so far this year – more than the last 2 years combined.
The number of seized counterfeit pills with fentanyl has jumped nearly 430% since 2019. DEA lab tests reveal that two out of every five pills with fentanyl contain a potentially lethal dose.
These deadly pills are widely accessible and often sold on social media and e-commerce platforms – making them available to anyone with a smartphone, including minors, the DEA warns.
More than 93,000 people died of a drug overdose in the United States last year, according to federal statistics, and fentanyl is the primary driver of this alarming increase in overdose deaths, the DEA says.
The agency has launched a “One Pill Can Kill” public awareness campaign to educate the public of the dangers of counterfeit pills purchased outside of a licensed pharmacy. These pills are “illegal, dangerous, and potentially lethal,” the DEA warns.
This alert does not apply to legitimate pharmaceutical medications prescribed by doctors and dispensed by licensed pharmacists, the DEA says.
“The legitimate prescription supply chain is not impacted. Anyone filling a prescription at a licensed pharmacy can be confident that the medications they receive are safe when taken as directed by a medical professional,” the agency says.
A version of this article first appeared on Medscape.com.
The U.S. Drug Enforcement Administration has issued a public safety alert over an “alarming” increase in fake prescription pills laced with the synthetic opioid fentanyl or the stimulant methamphetamine.
“The United States is facing an unprecedented crisis of overdose deaths fueled by illegally manufactured fentanyl and methamphetamine,” DEA Administrator Anne Milgram said in the alert.
“Counterfeit pills that contain these dangerous and extremely addictive drugs are more lethal and more accessible than ever before. DEA is focusing resources on taking down the violent drug traffickers causing the greatest harm and posing the greatest threat to the safety and health of Americans,” Ms. Milgram said.
Criminal drug networks are mass-producing fake fentanyl- and methamphetamine-laced pills and deceptively marketing them as legitimate prescription pills, the DEA warns.
such as oxycodone (Oxycontin, Percocet), hydrocodone (Vicodin), and alprazolam (Xanax); or stimulants like amphetamines (Adderall).
The agency has seized fake pills in every U.S. state. More than 9.5 million fake pills have been seized so far this year – more than the last 2 years combined.
The number of seized counterfeit pills with fentanyl has jumped nearly 430% since 2019. DEA lab tests reveal that two out of every five pills with fentanyl contain a potentially lethal dose.
These deadly pills are widely accessible and often sold on social media and e-commerce platforms – making them available to anyone with a smartphone, including minors, the DEA warns.
More than 93,000 people died of a drug overdose in the United States last year, according to federal statistics, and fentanyl is the primary driver of this alarming increase in overdose deaths, the DEA says.
The agency has launched a “One Pill Can Kill” public awareness campaign to educate the public of the dangers of counterfeit pills purchased outside of a licensed pharmacy. These pills are “illegal, dangerous, and potentially lethal,” the DEA warns.
This alert does not apply to legitimate pharmaceutical medications prescribed by doctors and dispensed by licensed pharmacists, the DEA says.
“The legitimate prescription supply chain is not impacted. Anyone filling a prescription at a licensed pharmacy can be confident that the medications they receive are safe when taken as directed by a medical professional,” the agency says.
A version of this article first appeared on Medscape.com.
The U.S. Drug Enforcement Administration has issued a public safety alert over an “alarming” increase in fake prescription pills laced with the synthetic opioid fentanyl or the stimulant methamphetamine.
“The United States is facing an unprecedented crisis of overdose deaths fueled by illegally manufactured fentanyl and methamphetamine,” DEA Administrator Anne Milgram said in the alert.
“Counterfeit pills that contain these dangerous and extremely addictive drugs are more lethal and more accessible than ever before. DEA is focusing resources on taking down the violent drug traffickers causing the greatest harm and posing the greatest threat to the safety and health of Americans,” Ms. Milgram said.
Criminal drug networks are mass-producing fake fentanyl- and methamphetamine-laced pills and deceptively marketing them as legitimate prescription pills, the DEA warns.
such as oxycodone (Oxycontin, Percocet), hydrocodone (Vicodin), and alprazolam (Xanax); or stimulants like amphetamines (Adderall).
The agency has seized fake pills in every U.S. state. More than 9.5 million fake pills have been seized so far this year – more than the last 2 years combined.
The number of seized counterfeit pills with fentanyl has jumped nearly 430% since 2019. DEA lab tests reveal that two out of every five pills with fentanyl contain a potentially lethal dose.
These deadly pills are widely accessible and often sold on social media and e-commerce platforms – making them available to anyone with a smartphone, including minors, the DEA warns.
More than 93,000 people died of a drug overdose in the United States last year, according to federal statistics, and fentanyl is the primary driver of this alarming increase in overdose deaths, the DEA says.
The agency has launched a “One Pill Can Kill” public awareness campaign to educate the public of the dangers of counterfeit pills purchased outside of a licensed pharmacy. These pills are “illegal, dangerous, and potentially lethal,” the DEA warns.
This alert does not apply to legitimate pharmaceutical medications prescribed by doctors and dispensed by licensed pharmacists, the DEA says.
“The legitimate prescription supply chain is not impacted. Anyone filling a prescription at a licensed pharmacy can be confident that the medications they receive are safe when taken as directed by a medical professional,” the agency says.
A version of this article first appeared on Medscape.com.
Dopamine and reward: The story of social media
How often do you find yourself on social media? The first thing I do when I wake up is check my email and text messages, as well as my Facebook, Snapchat, and Instagram notifications.
Some 150,000 messages are shared on Facebook each minute; 293 million daily active users worldwide were recorded on Snapchat during the second quarter of 2021; 127.2 million monthly active users in the United States are projected to be on Instagram by 2023.
Social media has gained the hearts and wonder of many around the world. It’s absolutely incredible how ingrained it has become in our lives as a medium for creativity, outlet for communication, and platform for information. In fact, these online network tools have now become essential during COVID-19 to ensure productive workflow, keep in touch with our loved ones, and, overall, maintain social capital. Social media has truly emerged as a powerful form of living beyond our physical selves.
Yet, increased (and addictive) social media use is associated with negative health outcomes, especially among adolescents. For example, in a study reporting parent and adolescent accounts of social media use, it was reported that social media use was associated with hyperactivity/impulsivity, depression, anxiety, loneliness, and a fear of missing out. Furthermore, a meta-analysis investigating the relationship between social media use and depressive symptoms among adolescents found a small but significant and positive relationship between the two. However, additional research is required to elucidate this association.
Notwithstanding, the addictive nature of social media has previously been called out as analogous to the addictive nature of gambling. Let’s think about it. Whether you’re on Instagram, TikTok, or a similar platform, you can’t help but scroll from one video to the next. It’s one 5- to 10-second video after the next, and before you know it, you’ve spent the past hour going through random videos – but you can’t stop. Why is that so?
Social media actually “rewires” our brain such that we expect instant gratification. In other words, when we get a notification, message, like, or share, we expect fast and short-term pleasure/reward because the brain will produce a “hit of dopamine.” However, it is important to note that the reward system is not delimited to the dopaminergic pathway and, in fact, should be understood as a complex network system (i.e., governed by changes in brain morphology through addiction and excessive behavior). Given the quick pace of the social media world, the reward pathways in our brain change and there’s an increasing demand for attention, perpetuating an addictive mindset.
When we refresh our page, we expect instant gratification. But what happens when we don’t get a like, or a message, or some sort of “reward”? Recounts of social media use by adolescents have likened online attention to popularity. Accordingly, a lack of constant attention on social media has created a vicious cycle of anxiety, loneliness, and depression because of a failure to receive “virtual” reward. Taken together, social media may be harmful because it distorts our self-image, and while social media platforms help connect us, they can also ironically make us feel isolated, lower our self-confidence, and diminish our overall sense of well-being.
As the platforms for communication and information have evolved so rapidly over the past decade, there is a need to establish boundaries between what is beneficial and what is potentially detrimental to our mental health. While social media companies should play a role in mitigating addictive social network behavior, it would also seem counterintuitive to the general business model. In that case, who takes charge? This multifaceted problem requires a multidisciplinary approach.
Leanna M.W. Lui is an MSc candidate at the University of Toronto.
A version of this article first appeared on Medscape.com.
How often do you find yourself on social media? The first thing I do when I wake up is check my email and text messages, as well as my Facebook, Snapchat, and Instagram notifications.
Some 150,000 messages are shared on Facebook each minute; 293 million daily active users worldwide were recorded on Snapchat during the second quarter of 2021; 127.2 million monthly active users in the United States are projected to be on Instagram by 2023.
Social media has gained the hearts and wonder of many around the world. It’s absolutely incredible how ingrained it has become in our lives as a medium for creativity, outlet for communication, and platform for information. In fact, these online network tools have now become essential during COVID-19 to ensure productive workflow, keep in touch with our loved ones, and, overall, maintain social capital. Social media has truly emerged as a powerful form of living beyond our physical selves.
Yet, increased (and addictive) social media use is associated with negative health outcomes, especially among adolescents. For example, in a study reporting parent and adolescent accounts of social media use, it was reported that social media use was associated with hyperactivity/impulsivity, depression, anxiety, loneliness, and a fear of missing out. Furthermore, a meta-analysis investigating the relationship between social media use and depressive symptoms among adolescents found a small but significant and positive relationship between the two. However, additional research is required to elucidate this association.
Notwithstanding, the addictive nature of social media has previously been called out as analogous to the addictive nature of gambling. Let’s think about it. Whether you’re on Instagram, TikTok, or a similar platform, you can’t help but scroll from one video to the next. It’s one 5- to 10-second video after the next, and before you know it, you’ve spent the past hour going through random videos – but you can’t stop. Why is that so?
Social media actually “rewires” our brain such that we expect instant gratification. In other words, when we get a notification, message, like, or share, we expect fast and short-term pleasure/reward because the brain will produce a “hit of dopamine.” However, it is important to note that the reward system is not delimited to the dopaminergic pathway and, in fact, should be understood as a complex network system (i.e., governed by changes in brain morphology through addiction and excessive behavior). Given the quick pace of the social media world, the reward pathways in our brain change and there’s an increasing demand for attention, perpetuating an addictive mindset.
When we refresh our page, we expect instant gratification. But what happens when we don’t get a like, or a message, or some sort of “reward”? Recounts of social media use by adolescents have likened online attention to popularity. Accordingly, a lack of constant attention on social media has created a vicious cycle of anxiety, loneliness, and depression because of a failure to receive “virtual” reward. Taken together, social media may be harmful because it distorts our self-image, and while social media platforms help connect us, they can also ironically make us feel isolated, lower our self-confidence, and diminish our overall sense of well-being.
As the platforms for communication and information have evolved so rapidly over the past decade, there is a need to establish boundaries between what is beneficial and what is potentially detrimental to our mental health. While social media companies should play a role in mitigating addictive social network behavior, it would also seem counterintuitive to the general business model. In that case, who takes charge? This multifaceted problem requires a multidisciplinary approach.
Leanna M.W. Lui is an MSc candidate at the University of Toronto.
A version of this article first appeared on Medscape.com.
How often do you find yourself on social media? The first thing I do when I wake up is check my email and text messages, as well as my Facebook, Snapchat, and Instagram notifications.
Some 150,000 messages are shared on Facebook each minute; 293 million daily active users worldwide were recorded on Snapchat during the second quarter of 2021; 127.2 million monthly active users in the United States are projected to be on Instagram by 2023.
Social media has gained the hearts and wonder of many around the world. It’s absolutely incredible how ingrained it has become in our lives as a medium for creativity, outlet for communication, and platform for information. In fact, these online network tools have now become essential during COVID-19 to ensure productive workflow, keep in touch with our loved ones, and, overall, maintain social capital. Social media has truly emerged as a powerful form of living beyond our physical selves.
Yet, increased (and addictive) social media use is associated with negative health outcomes, especially among adolescents. For example, in a study reporting parent and adolescent accounts of social media use, it was reported that social media use was associated with hyperactivity/impulsivity, depression, anxiety, loneliness, and a fear of missing out. Furthermore, a meta-analysis investigating the relationship between social media use and depressive symptoms among adolescents found a small but significant and positive relationship between the two. However, additional research is required to elucidate this association.
Notwithstanding, the addictive nature of social media has previously been called out as analogous to the addictive nature of gambling. Let’s think about it. Whether you’re on Instagram, TikTok, or a similar platform, you can’t help but scroll from one video to the next. It’s one 5- to 10-second video after the next, and before you know it, you’ve spent the past hour going through random videos – but you can’t stop. Why is that so?
Social media actually “rewires” our brain such that we expect instant gratification. In other words, when we get a notification, message, like, or share, we expect fast and short-term pleasure/reward because the brain will produce a “hit of dopamine.” However, it is important to note that the reward system is not delimited to the dopaminergic pathway and, in fact, should be understood as a complex network system (i.e., governed by changes in brain morphology through addiction and excessive behavior). Given the quick pace of the social media world, the reward pathways in our brain change and there’s an increasing demand for attention, perpetuating an addictive mindset.
When we refresh our page, we expect instant gratification. But what happens when we don’t get a like, or a message, or some sort of “reward”? Recounts of social media use by adolescents have likened online attention to popularity. Accordingly, a lack of constant attention on social media has created a vicious cycle of anxiety, loneliness, and depression because of a failure to receive “virtual” reward. Taken together, social media may be harmful because it distorts our self-image, and while social media platforms help connect us, they can also ironically make us feel isolated, lower our self-confidence, and diminish our overall sense of well-being.
As the platforms for communication and information have evolved so rapidly over the past decade, there is a need to establish boundaries between what is beneficial and what is potentially detrimental to our mental health. While social media companies should play a role in mitigating addictive social network behavior, it would also seem counterintuitive to the general business model. In that case, who takes charge? This multifaceted problem requires a multidisciplinary approach.
Leanna M.W. Lui is an MSc candidate at the University of Toronto.
A version of this article first appeared on Medscape.com.
Buprenorphine offers a way to rise from the ashes of addiction
One of the most rewarding aspects of being a physician is having a direct impact on alleviating patient suffering. On the other hand, one of the more difficult elements is a confrontational patient with unreasonable expectations or inappropriate demands. I have experienced both ends of the spectrum while engaging with patients who have opioid use disorder (OUD).
An untreated patient with OUD might provide an untruthful history, attempt to falsify exam findings, or even become threatening or abusive in an attempt to secure opiate pain medication. Managing a patient with OUD by providing buprenorphine treatment, however, is a completely different experience.
There is no controversy about the effectiveness of buprenorphine treatment for OUD. Patients seeking it are not looking for inappropriate care but rather a treatment that is established as an unequivocal standard with proven results for better treatment outcomes1-3 and reduced mortality.4 Personally, I’ve found offering buprenorphine treatment to be one of the most rewarding aspects of practicing medicine. It is a real joy to witness people turn their lives around with meaningful outcomes such as gainful employment, eradication of hepatitis C, reconciliation of broken relationships, resolution of legal troubles, and long-term sobriety. Being a part of lives that are practically resurrected from the ashes of addiction by prescribing medicine is indeed an exceptional experience.
On April 28, 2021, the Department of Health and Human Services provided notice for immediate action allowing for any DEA-licensed provider to obtain an X-waiver to treat 30 active patients without educational prerequisite or certification of behavioral health referral capacity.5 The X-waiver requirements were reduced, as outlined by SAMSHA,6 to a simple online notice of intent7 that can be completed in less than 5 minutes.
I encourage my colleagues to obtain the X-waiver by the simplified process, start prescribing buprenorphine, and be a part of the solution to the opioid epidemic. Of course, there will be struggles and lessons learned, but these can most certainly be eclipsed by a focus on the rewarding experience of restoring wholeness to the lives of many patients.
Aaron Newcomb, DO
Carbondale, IL
1. Norton BL, Beitin A, Glenn M, et al. Retention in buprenorphine treatment is associated with improved HCV care outcomes. J Subst Abuse Treat. 2017;75:38-42. doi: 10.1016/j.jsat.2017.01.015
2. Evans EA, Zhu Y, Yoo C, et al. Criminal justice outcomes over 5 years after randomization to buprenorphine-naloxone or methadone treatment for opioid use disorder. Addiction. 2019;114:1396-1404. doi: 10.1111/add.14620
3. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane. Published February 6, 2014. Accessed August 10, 2021. www.cochrane.org/CD002207/ADDICTN_buprenorphine-maintenance-versus-placebo-or-methadone-maintenance-for-opioid-dependence
4. Methadone and buprenorphine reduce risk of death after opioid overdose. National Institutes of Health. Published June 19, 2018. Accessed August 10, 2021. www.nih.gov/news-events/news-releases/methadone-buprenorphine-reduce-risk-death-after-opioid-overdose
5. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. Department of Health and Human Services; 2021. Accessed August 10, 2021. www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
6. US Department of Health & Human Services. Become a buprenorphine waivered practitioner. SAMHSA. Updated May 14, 2021. Accessed August 10, 2021. www.samhsa.gov/medication-assisted-treatment/become-buprenorphine-waivered-practitioner
7. Buprenorphine waiver notification. SAMHSA. Accessed August 10, 2021. https://buprenorphine.samhsa.gov/forms/select-practitioner-type.php
One of the most rewarding aspects of being a physician is having a direct impact on alleviating patient suffering. On the other hand, one of the more difficult elements is a confrontational patient with unreasonable expectations or inappropriate demands. I have experienced both ends of the spectrum while engaging with patients who have opioid use disorder (OUD).
An untreated patient with OUD might provide an untruthful history, attempt to falsify exam findings, or even become threatening or abusive in an attempt to secure opiate pain medication. Managing a patient with OUD by providing buprenorphine treatment, however, is a completely different experience.
There is no controversy about the effectiveness of buprenorphine treatment for OUD. Patients seeking it are not looking for inappropriate care but rather a treatment that is established as an unequivocal standard with proven results for better treatment outcomes1-3 and reduced mortality.4 Personally, I’ve found offering buprenorphine treatment to be one of the most rewarding aspects of practicing medicine. It is a real joy to witness people turn their lives around with meaningful outcomes such as gainful employment, eradication of hepatitis C, reconciliation of broken relationships, resolution of legal troubles, and long-term sobriety. Being a part of lives that are practically resurrected from the ashes of addiction by prescribing medicine is indeed an exceptional experience.
On April 28, 2021, the Department of Health and Human Services provided notice for immediate action allowing for any DEA-licensed provider to obtain an X-waiver to treat 30 active patients without educational prerequisite or certification of behavioral health referral capacity.5 The X-waiver requirements were reduced, as outlined by SAMSHA,6 to a simple online notice of intent7 that can be completed in less than 5 minutes.
I encourage my colleagues to obtain the X-waiver by the simplified process, start prescribing buprenorphine, and be a part of the solution to the opioid epidemic. Of course, there will be struggles and lessons learned, but these can most certainly be eclipsed by a focus on the rewarding experience of restoring wholeness to the lives of many patients.
Aaron Newcomb, DO
Carbondale, IL
One of the most rewarding aspects of being a physician is having a direct impact on alleviating patient suffering. On the other hand, one of the more difficult elements is a confrontational patient with unreasonable expectations or inappropriate demands. I have experienced both ends of the spectrum while engaging with patients who have opioid use disorder (OUD).
An untreated patient with OUD might provide an untruthful history, attempt to falsify exam findings, or even become threatening or abusive in an attempt to secure opiate pain medication. Managing a patient with OUD by providing buprenorphine treatment, however, is a completely different experience.
There is no controversy about the effectiveness of buprenorphine treatment for OUD. Patients seeking it are not looking for inappropriate care but rather a treatment that is established as an unequivocal standard with proven results for better treatment outcomes1-3 and reduced mortality.4 Personally, I’ve found offering buprenorphine treatment to be one of the most rewarding aspects of practicing medicine. It is a real joy to witness people turn their lives around with meaningful outcomes such as gainful employment, eradication of hepatitis C, reconciliation of broken relationships, resolution of legal troubles, and long-term sobriety. Being a part of lives that are practically resurrected from the ashes of addiction by prescribing medicine is indeed an exceptional experience.
On April 28, 2021, the Department of Health and Human Services provided notice for immediate action allowing for any DEA-licensed provider to obtain an X-waiver to treat 30 active patients without educational prerequisite or certification of behavioral health referral capacity.5 The X-waiver requirements were reduced, as outlined by SAMSHA,6 to a simple online notice of intent7 that can be completed in less than 5 minutes.
I encourage my colleagues to obtain the X-waiver by the simplified process, start prescribing buprenorphine, and be a part of the solution to the opioid epidemic. Of course, there will be struggles and lessons learned, but these can most certainly be eclipsed by a focus on the rewarding experience of restoring wholeness to the lives of many patients.
Aaron Newcomb, DO
Carbondale, IL
1. Norton BL, Beitin A, Glenn M, et al. Retention in buprenorphine treatment is associated with improved HCV care outcomes. J Subst Abuse Treat. 2017;75:38-42. doi: 10.1016/j.jsat.2017.01.015
2. Evans EA, Zhu Y, Yoo C, et al. Criminal justice outcomes over 5 years after randomization to buprenorphine-naloxone or methadone treatment for opioid use disorder. Addiction. 2019;114:1396-1404. doi: 10.1111/add.14620
3. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane. Published February 6, 2014. Accessed August 10, 2021. www.cochrane.org/CD002207/ADDICTN_buprenorphine-maintenance-versus-placebo-or-methadone-maintenance-for-opioid-dependence
4. Methadone and buprenorphine reduce risk of death after opioid overdose. National Institutes of Health. Published June 19, 2018. Accessed August 10, 2021. www.nih.gov/news-events/news-releases/methadone-buprenorphine-reduce-risk-death-after-opioid-overdose
5. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. Department of Health and Human Services; 2021. Accessed August 10, 2021. www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
6. US Department of Health & Human Services. Become a buprenorphine waivered practitioner. SAMHSA. Updated May 14, 2021. Accessed August 10, 2021. www.samhsa.gov/medication-assisted-treatment/become-buprenorphine-waivered-practitioner
7. Buprenorphine waiver notification. SAMHSA. Accessed August 10, 2021. https://buprenorphine.samhsa.gov/forms/select-practitioner-type.php
1. Norton BL, Beitin A, Glenn M, et al. Retention in buprenorphine treatment is associated with improved HCV care outcomes. J Subst Abuse Treat. 2017;75:38-42. doi: 10.1016/j.jsat.2017.01.015
2. Evans EA, Zhu Y, Yoo C, et al. Criminal justice outcomes over 5 years after randomization to buprenorphine-naloxone or methadone treatment for opioid use disorder. Addiction. 2019;114:1396-1404. doi: 10.1111/add.14620
3. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane. Published February 6, 2014. Accessed August 10, 2021. www.cochrane.org/CD002207/ADDICTN_buprenorphine-maintenance-versus-placebo-or-methadone-maintenance-for-opioid-dependence
4. Methadone and buprenorphine reduce risk of death after opioid overdose. National Institutes of Health. Published June 19, 2018. Accessed August 10, 2021. www.nih.gov/news-events/news-releases/methadone-buprenorphine-reduce-risk-death-after-opioid-overdose
5. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. Department of Health and Human Services; 2021. Accessed August 10, 2021. www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
6. US Department of Health & Human Services. Become a buprenorphine waivered practitioner. SAMHSA. Updated May 14, 2021. Accessed August 10, 2021. www.samhsa.gov/medication-assisted-treatment/become-buprenorphine-waivered-practitioner
7. Buprenorphine waiver notification. SAMHSA. Accessed August 10, 2021. https://buprenorphine.samhsa.gov/forms/select-practitioner-type.php
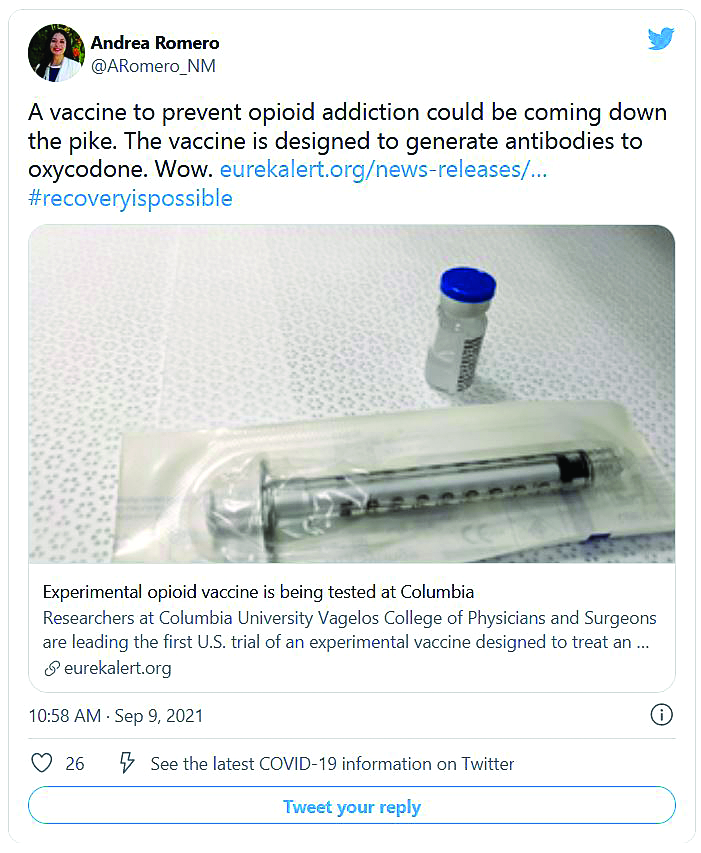
As opioid deaths climb, human trials begin for vaccine
Opioid-related drug overdose deaths in the United States exploded to an estimated record high of 69,031 people in 2020, topping the 49,860 deaths logged in 2019, according to a new report from the Centers for Disease Control and Prevention. Most of the deaths involved synthetic opioids such as fentanyl.
President Joe Biden has pledged more than $10 billion to expand access to prevention, treatment, and recovery services. The money is important as people receiving treatment for opioid use disorder have a high risk for relapse, and that means a high risk for opioid overdose.
Now, researchers are studying a possible bridge to successful recovery: A vaccine that could blunt the drugs’ ability to cause harm.
The first such vaccines are now entering clinical trials, raising hopes of adding another tool to the antiaddiction armamentarium. But even if the vaccines prove safe and effective, their success could generate some new problems to solve.
An advantage of vaccines is that their effects can last for several months, said trial investigator Sandra Comer, PhD, professor of neurobiology and psychiatry at Columbia University Irving Medical Center, New York. Dropout rates for existing medical therapies for opioid use disorder are as high as 50% at 6 months, and a vaccine could protect people from overdose and give them time to re-enter treatment.
“It serves as a bit of a safety net,” she said.
The first vaccine to enter a trial targets oxycodone. Volunteers are being recruited who have a diagnosis of opioid use disorder but are not being medically treated and are still using opioids. A third of them will receive a placebo vaccine, a third will receive a low-dose injection of vaccine, and the other third will receive a high-dose vaccine.
A shot against oxycodone
Researchers are primarily tracking the safety of the shot, but they’re also looking at whether vaccination prevents the euphoria that opioids usually produce. They expect to enroll 24 people initially but expand to 45 if results look promising.
In response to the shot, the body produces antibodies, proteins that tag oxycodone and keep it from reaching the brain. If the drug can’t reach brain cells, it can’t produce euphoria. And more important for lifesaving effects, it can’t block the brain’s signals to the body to breathe. The vaccine has already performed well in animal studies.
Previous trials of vaccines for cocaine and nicotine failed. Those vaccines made it to the last clinical trial stage, but didn’t prove effective overall. So this time, investigators plan to track antibody levels in participants, examining blood samples for signs of a good immune response to the vaccine.
But even though earlier cocaine and nicotine vaccines didn’t work for everybody, there were some people they seemed to help. This is why investigators involved in opioid vaccine trials want to track immune responses, said Marco Pravetoni, PhD, associate professor of pharmacology and medicine at the University of Minnesota, Minneapolis, whose team will be assessing the blood samples. Ultimately, a doctor might even be able to use this information to tailor vaccine selection to a specific person.
Dr. Pravetoni also said that oxycodone is one of three vaccine targets – the other two are heroin and fentanyl – that researchers hope to combine into a single shot. Recipients might need to have one shot a month for the first 3 to 4 months and then receive annual boosters.
Stopping the pain
The vaccines also raise some issues that need attention, said Cody Wenthur, PharmD, PhD, assistant professor of pharmacy at the University of Wisconsin–Madison, who is not involved in the vaccine trials.
“If you’re vaccinated against oxycodone, you might not have access to adequate pain control if you get into a car accident, for example,” he said.
Clinicians could use other opioids for pain management, but limiting the opioids that the vaccine targets is a “double-edged sword,” said Dr. Wenthur, because vaccinated people could just switch their opioid of choice to one that a vaccine does not inhibit.
Although these issues need to be addressed, vaccines, if successful, will have an important role. Dr. Wenthur noted a survey of pharmacists and pharmacy students that he and his group conducted showing that respondents “overwhelmingly” viewed a potential vaccine as helpful.
said Dr. Pravetoni. He mentioned the 2002 incident when terrorists took over a theater in Moscow and Russian special forces are thought to have used an aerosolized form of fentanyl to incapacitate everyone in the room. More than 100 of the hostages died, and the episode raised the specter of opioids being used in chemical attacks.
Dr. Pravetoni said vaccination could offer protection for first responders, law enforcement or other people whose professions place them at risk for inhalation, either accidentally or through such attacks.
These or other real-world applications for people at risk for exposure are several years away. Dr. Pravetoni said it took 10 years to get to this phase and estimates that, in about 5 years, a vaccine that targets multiple opioid drugs might enter the first clinical trial.
A version of this article first appeared on WebMD.com.
Opioid-related drug overdose deaths in the United States exploded to an estimated record high of 69,031 people in 2020, topping the 49,860 deaths logged in 2019, according to a new report from the Centers for Disease Control and Prevention. Most of the deaths involved synthetic opioids such as fentanyl.
President Joe Biden has pledged more than $10 billion to expand access to prevention, treatment, and recovery services. The money is important as people receiving treatment for opioid use disorder have a high risk for relapse, and that means a high risk for opioid overdose.
Now, researchers are studying a possible bridge to successful recovery: A vaccine that could blunt the drugs’ ability to cause harm.
The first such vaccines are now entering clinical trials, raising hopes of adding another tool to the antiaddiction armamentarium. But even if the vaccines prove safe and effective, their success could generate some new problems to solve.
An advantage of vaccines is that their effects can last for several months, said trial investigator Sandra Comer, PhD, professor of neurobiology and psychiatry at Columbia University Irving Medical Center, New York. Dropout rates for existing medical therapies for opioid use disorder are as high as 50% at 6 months, and a vaccine could protect people from overdose and give them time to re-enter treatment.
“It serves as a bit of a safety net,” she said.
The first vaccine to enter a trial targets oxycodone. Volunteers are being recruited who have a diagnosis of opioid use disorder but are not being medically treated and are still using opioids. A third of them will receive a placebo vaccine, a third will receive a low-dose injection of vaccine, and the other third will receive a high-dose vaccine.
A shot against oxycodone
Researchers are primarily tracking the safety of the shot, but they’re also looking at whether vaccination prevents the euphoria that opioids usually produce. They expect to enroll 24 people initially but expand to 45 if results look promising.
In response to the shot, the body produces antibodies, proteins that tag oxycodone and keep it from reaching the brain. If the drug can’t reach brain cells, it can’t produce euphoria. And more important for lifesaving effects, it can’t block the brain’s signals to the body to breathe. The vaccine has already performed well in animal studies.
Previous trials of vaccines for cocaine and nicotine failed. Those vaccines made it to the last clinical trial stage, but didn’t prove effective overall. So this time, investigators plan to track antibody levels in participants, examining blood samples for signs of a good immune response to the vaccine.
But even though earlier cocaine and nicotine vaccines didn’t work for everybody, there were some people they seemed to help. This is why investigators involved in opioid vaccine trials want to track immune responses, said Marco Pravetoni, PhD, associate professor of pharmacology and medicine at the University of Minnesota, Minneapolis, whose team will be assessing the blood samples. Ultimately, a doctor might even be able to use this information to tailor vaccine selection to a specific person.
Dr. Pravetoni also said that oxycodone is one of three vaccine targets – the other two are heroin and fentanyl – that researchers hope to combine into a single shot. Recipients might need to have one shot a month for the first 3 to 4 months and then receive annual boosters.
Stopping the pain
The vaccines also raise some issues that need attention, said Cody Wenthur, PharmD, PhD, assistant professor of pharmacy at the University of Wisconsin–Madison, who is not involved in the vaccine trials.
“If you’re vaccinated against oxycodone, you might not have access to adequate pain control if you get into a car accident, for example,” he said.
Clinicians could use other opioids for pain management, but limiting the opioids that the vaccine targets is a “double-edged sword,” said Dr. Wenthur, because vaccinated people could just switch their opioid of choice to one that a vaccine does not inhibit.
Although these issues need to be addressed, vaccines, if successful, will have an important role. Dr. Wenthur noted a survey of pharmacists and pharmacy students that he and his group conducted showing that respondents “overwhelmingly” viewed a potential vaccine as helpful.
said Dr. Pravetoni. He mentioned the 2002 incident when terrorists took over a theater in Moscow and Russian special forces are thought to have used an aerosolized form of fentanyl to incapacitate everyone in the room. More than 100 of the hostages died, and the episode raised the specter of opioids being used in chemical attacks.
Dr. Pravetoni said vaccination could offer protection for first responders, law enforcement or other people whose professions place them at risk for inhalation, either accidentally or through such attacks.
These or other real-world applications for people at risk for exposure are several years away. Dr. Pravetoni said it took 10 years to get to this phase and estimates that, in about 5 years, a vaccine that targets multiple opioid drugs might enter the first clinical trial.
A version of this article first appeared on WebMD.com.
Opioid-related drug overdose deaths in the United States exploded to an estimated record high of 69,031 people in 2020, topping the 49,860 deaths logged in 2019, according to a new report from the Centers for Disease Control and Prevention. Most of the deaths involved synthetic opioids such as fentanyl.
President Joe Biden has pledged more than $10 billion to expand access to prevention, treatment, and recovery services. The money is important as people receiving treatment for opioid use disorder have a high risk for relapse, and that means a high risk for opioid overdose.
Now, researchers are studying a possible bridge to successful recovery: A vaccine that could blunt the drugs’ ability to cause harm.
The first such vaccines are now entering clinical trials, raising hopes of adding another tool to the antiaddiction armamentarium. But even if the vaccines prove safe and effective, their success could generate some new problems to solve.
An advantage of vaccines is that their effects can last for several months, said trial investigator Sandra Comer, PhD, professor of neurobiology and psychiatry at Columbia University Irving Medical Center, New York. Dropout rates for existing medical therapies for opioid use disorder are as high as 50% at 6 months, and a vaccine could protect people from overdose and give them time to re-enter treatment.
“It serves as a bit of a safety net,” she said.
The first vaccine to enter a trial targets oxycodone. Volunteers are being recruited who have a diagnosis of opioid use disorder but are not being medically treated and are still using opioids. A third of them will receive a placebo vaccine, a third will receive a low-dose injection of vaccine, and the other third will receive a high-dose vaccine.
A shot against oxycodone
Researchers are primarily tracking the safety of the shot, but they’re also looking at whether vaccination prevents the euphoria that opioids usually produce. They expect to enroll 24 people initially but expand to 45 if results look promising.
In response to the shot, the body produces antibodies, proteins that tag oxycodone and keep it from reaching the brain. If the drug can’t reach brain cells, it can’t produce euphoria. And more important for lifesaving effects, it can’t block the brain’s signals to the body to breathe. The vaccine has already performed well in animal studies.
Previous trials of vaccines for cocaine and nicotine failed. Those vaccines made it to the last clinical trial stage, but didn’t prove effective overall. So this time, investigators plan to track antibody levels in participants, examining blood samples for signs of a good immune response to the vaccine.
But even though earlier cocaine and nicotine vaccines didn’t work for everybody, there were some people they seemed to help. This is why investigators involved in opioid vaccine trials want to track immune responses, said Marco Pravetoni, PhD, associate professor of pharmacology and medicine at the University of Minnesota, Minneapolis, whose team will be assessing the blood samples. Ultimately, a doctor might even be able to use this information to tailor vaccine selection to a specific person.
Dr. Pravetoni also said that oxycodone is one of three vaccine targets – the other two are heroin and fentanyl – that researchers hope to combine into a single shot. Recipients might need to have one shot a month for the first 3 to 4 months and then receive annual boosters.
Stopping the pain
The vaccines also raise some issues that need attention, said Cody Wenthur, PharmD, PhD, assistant professor of pharmacy at the University of Wisconsin–Madison, who is not involved in the vaccine trials.
“If you’re vaccinated against oxycodone, you might not have access to adequate pain control if you get into a car accident, for example,” he said.
Clinicians could use other opioids for pain management, but limiting the opioids that the vaccine targets is a “double-edged sword,” said Dr. Wenthur, because vaccinated people could just switch their opioid of choice to one that a vaccine does not inhibit.
Although these issues need to be addressed, vaccines, if successful, will have an important role. Dr. Wenthur noted a survey of pharmacists and pharmacy students that he and his group conducted showing that respondents “overwhelmingly” viewed a potential vaccine as helpful.
said Dr. Pravetoni. He mentioned the 2002 incident when terrorists took over a theater in Moscow and Russian special forces are thought to have used an aerosolized form of fentanyl to incapacitate everyone in the room. More than 100 of the hostages died, and the episode raised the specter of opioids being used in chemical attacks.
Dr. Pravetoni said vaccination could offer protection for first responders, law enforcement or other people whose professions place them at risk for inhalation, either accidentally or through such attacks.
These or other real-world applications for people at risk for exposure are several years away. Dr. Pravetoni said it took 10 years to get to this phase and estimates that, in about 5 years, a vaccine that targets multiple opioid drugs might enter the first clinical trial.
A version of this article first appeared on WebMD.com.
Opioid overdoses tied to lasting cognitive impairment
Opioid overdoses usually aren’t fatal, but a new review of numerous studies, mostly case reports and case series, suggests that they can have long-lasting effects on cognition, possibly because of hypoxia resulting from respiratory depression.
Erin L. Winstanley, PhD, MA, and associates noted in the review that opioids cause about 80% of worldwide deaths from illicit drug use, and the Centers for Disease Control and Prevention’s provisional August 2021 number of more than 88,000 opioid-caused deaths in the United States is the highest ever recorded – a 27% increase over what was reported last December. That number suggests that the opioid epidemic continues to rage, but the study results also show that the neurological consequences of nonfatal overdoses are an important public health problem.
And that’s something that may be overlooked, according to Mark S. Gold, MD, who was not involved with the study and was asked to comment on the review, which was published in the Journal of Addiction Science.
“Assuming that an overdose has no effect on the brain, mood, and behavior is not supported by experience or the literature. He is a University of Florida, Gainesville, Emeritus Eminent Scholar, adjunct professor of psychiatry at Washington University in St. Louis, and a member of the clinical council of Washington University’s Public Health Institute.
A common pattern among patients with opioid use disorder (OUD) is that they undergo treatment with medication-assisted therapy (MAT), only to drop out of treatment and then repeat the treatment at a later date. That suggests that physicians should take a harder look at the limitations of MAT and other treatments, Dr. Gold said.
Although the review found some associations between neurocognitive deficits and opioid overdose, the authors point out that it is difficult to make direct comparisons because of biases and differences in methodology among the included studies. They were not able to reach conclusions about the prevalence of brain injuries following nonfatal opioid overdoses. Few included studies controlled for confounding factors that might contribute to or explain neurocognitive impairments, reported Dr. Winstanley, associate professor in the department of behavioral medicine and psychiatry at the University of West Virginia, Morgantown, and associates.
Still, distinct patterns emerged from the analysis of almost 3,500 subjects in 79 studies in 21 countries. Twenty-nine studies reported diagnoses of leukoencephalopathy, which affects white matter. Spongiform leukoencephalopathy is known to occur secondarily after exposure to a variety of toxic agents, including carbon monoxide poisoning and drugs of abuse. The damage can lead to erosion of higher cerebral function. The condition can occur from 2 to 180 days after a hypoxic brain injury, potentially complicating efforts to attribute it specifically to an opioid overdose. Amnestic syndrome was also reported in some studies. One study found that about 39% of people seeking buprenorphine treatment suffered from neurocognitive impairment.
Dr. Gold called the study’s findings novel and of public health importance. “Each overdose takes a toll on the body, and especially the brain,” he said.
Better documentation needed
The variability in symptoms, as well as their timing, present challenges to initial treatment, which often occur before a patient reaches the hospital. This is a vital window because the length of time of inadequate respiration because of opioid overdose is likely to predict the extent of brain injury. The duration of inadequate respiration may not be captured in electronic medical records, and emergency departments don’t typically collect toxicology information, which may lead health care providers to attribute neurocognitive impairments to ongoing drug use rather than an acute anoxic or hypoxic episode. Further neurocognitive damage may have a delayed onset, and better documentation of these events could help physicians determine whether those symptoms stem from the acute event.
Dr. Winstanley and associates called for more research, including prospective case-control studies to identify brain changes following opioid-related overdose.
The authors also suggested that physicians might want to consider screening patients who experience prolonged anoxia or hypoxia for neurocognitive impairments and brain injuries. Dr. Gold agreed.
“Clinicians working with OUD patients should take these data to heart and take a comprehensive history of previous overdoses, loss of consciousness, head trauma, and following up on the history with neuropsychological and other tests of brain function,” Dr. Gold said. “After an assessment, rehabilitation and treatment might then be more personalized and effective.”
Dr. Gold had no relevant financial disclosures.
Opioid overdoses usually aren’t fatal, but a new review of numerous studies, mostly case reports and case series, suggests that they can have long-lasting effects on cognition, possibly because of hypoxia resulting from respiratory depression.
Erin L. Winstanley, PhD, MA, and associates noted in the review that opioids cause about 80% of worldwide deaths from illicit drug use, and the Centers for Disease Control and Prevention’s provisional August 2021 number of more than 88,000 opioid-caused deaths in the United States is the highest ever recorded – a 27% increase over what was reported last December. That number suggests that the opioid epidemic continues to rage, but the study results also show that the neurological consequences of nonfatal overdoses are an important public health problem.
And that’s something that may be overlooked, according to Mark S. Gold, MD, who was not involved with the study and was asked to comment on the review, which was published in the Journal of Addiction Science.
“Assuming that an overdose has no effect on the brain, mood, and behavior is not supported by experience or the literature. He is a University of Florida, Gainesville, Emeritus Eminent Scholar, adjunct professor of psychiatry at Washington University in St. Louis, and a member of the clinical council of Washington University’s Public Health Institute.
A common pattern among patients with opioid use disorder (OUD) is that they undergo treatment with medication-assisted therapy (MAT), only to drop out of treatment and then repeat the treatment at a later date. That suggests that physicians should take a harder look at the limitations of MAT and other treatments, Dr. Gold said.
Although the review found some associations between neurocognitive deficits and opioid overdose, the authors point out that it is difficult to make direct comparisons because of biases and differences in methodology among the included studies. They were not able to reach conclusions about the prevalence of brain injuries following nonfatal opioid overdoses. Few included studies controlled for confounding factors that might contribute to or explain neurocognitive impairments, reported Dr. Winstanley, associate professor in the department of behavioral medicine and psychiatry at the University of West Virginia, Morgantown, and associates.
Still, distinct patterns emerged from the analysis of almost 3,500 subjects in 79 studies in 21 countries. Twenty-nine studies reported diagnoses of leukoencephalopathy, which affects white matter. Spongiform leukoencephalopathy is known to occur secondarily after exposure to a variety of toxic agents, including carbon monoxide poisoning and drugs of abuse. The damage can lead to erosion of higher cerebral function. The condition can occur from 2 to 180 days after a hypoxic brain injury, potentially complicating efforts to attribute it specifically to an opioid overdose. Amnestic syndrome was also reported in some studies. One study found that about 39% of people seeking buprenorphine treatment suffered from neurocognitive impairment.
Dr. Gold called the study’s findings novel and of public health importance. “Each overdose takes a toll on the body, and especially the brain,” he said.
Better documentation needed
The variability in symptoms, as well as their timing, present challenges to initial treatment, which often occur before a patient reaches the hospital. This is a vital window because the length of time of inadequate respiration because of opioid overdose is likely to predict the extent of brain injury. The duration of inadequate respiration may not be captured in electronic medical records, and emergency departments don’t typically collect toxicology information, which may lead health care providers to attribute neurocognitive impairments to ongoing drug use rather than an acute anoxic or hypoxic episode. Further neurocognitive damage may have a delayed onset, and better documentation of these events could help physicians determine whether those symptoms stem from the acute event.
Dr. Winstanley and associates called for more research, including prospective case-control studies to identify brain changes following opioid-related overdose.
The authors also suggested that physicians might want to consider screening patients who experience prolonged anoxia or hypoxia for neurocognitive impairments and brain injuries. Dr. Gold agreed.
“Clinicians working with OUD patients should take these data to heart and take a comprehensive history of previous overdoses, loss of consciousness, head trauma, and following up on the history with neuropsychological and other tests of brain function,” Dr. Gold said. “After an assessment, rehabilitation and treatment might then be more personalized and effective.”
Dr. Gold had no relevant financial disclosures.
Opioid overdoses usually aren’t fatal, but a new review of numerous studies, mostly case reports and case series, suggests that they can have long-lasting effects on cognition, possibly because of hypoxia resulting from respiratory depression.
Erin L. Winstanley, PhD, MA, and associates noted in the review that opioids cause about 80% of worldwide deaths from illicit drug use, and the Centers for Disease Control and Prevention’s provisional August 2021 number of more than 88,000 opioid-caused deaths in the United States is the highest ever recorded – a 27% increase over what was reported last December. That number suggests that the opioid epidemic continues to rage, but the study results also show that the neurological consequences of nonfatal overdoses are an important public health problem.
And that’s something that may be overlooked, according to Mark S. Gold, MD, who was not involved with the study and was asked to comment on the review, which was published in the Journal of Addiction Science.
“Assuming that an overdose has no effect on the brain, mood, and behavior is not supported by experience or the literature. He is a University of Florida, Gainesville, Emeritus Eminent Scholar, adjunct professor of psychiatry at Washington University in St. Louis, and a member of the clinical council of Washington University’s Public Health Institute.
A common pattern among patients with opioid use disorder (OUD) is that they undergo treatment with medication-assisted therapy (MAT), only to drop out of treatment and then repeat the treatment at a later date. That suggests that physicians should take a harder look at the limitations of MAT and other treatments, Dr. Gold said.
Although the review found some associations between neurocognitive deficits and opioid overdose, the authors point out that it is difficult to make direct comparisons because of biases and differences in methodology among the included studies. They were not able to reach conclusions about the prevalence of brain injuries following nonfatal opioid overdoses. Few included studies controlled for confounding factors that might contribute to or explain neurocognitive impairments, reported Dr. Winstanley, associate professor in the department of behavioral medicine and psychiatry at the University of West Virginia, Morgantown, and associates.
Still, distinct patterns emerged from the analysis of almost 3,500 subjects in 79 studies in 21 countries. Twenty-nine studies reported diagnoses of leukoencephalopathy, which affects white matter. Spongiform leukoencephalopathy is known to occur secondarily after exposure to a variety of toxic agents, including carbon monoxide poisoning and drugs of abuse. The damage can lead to erosion of higher cerebral function. The condition can occur from 2 to 180 days after a hypoxic brain injury, potentially complicating efforts to attribute it specifically to an opioid overdose. Amnestic syndrome was also reported in some studies. One study found that about 39% of people seeking buprenorphine treatment suffered from neurocognitive impairment.
Dr. Gold called the study’s findings novel and of public health importance. “Each overdose takes a toll on the body, and especially the brain,” he said.
Better documentation needed
The variability in symptoms, as well as their timing, present challenges to initial treatment, which often occur before a patient reaches the hospital. This is a vital window because the length of time of inadequate respiration because of opioid overdose is likely to predict the extent of brain injury. The duration of inadequate respiration may not be captured in electronic medical records, and emergency departments don’t typically collect toxicology information, which may lead health care providers to attribute neurocognitive impairments to ongoing drug use rather than an acute anoxic or hypoxic episode. Further neurocognitive damage may have a delayed onset, and better documentation of these events could help physicians determine whether those symptoms stem from the acute event.
Dr. Winstanley and associates called for more research, including prospective case-control studies to identify brain changes following opioid-related overdose.
The authors also suggested that physicians might want to consider screening patients who experience prolonged anoxia or hypoxia for neurocognitive impairments and brain injuries. Dr. Gold agreed.
“Clinicians working with OUD patients should take these data to heart and take a comprehensive history of previous overdoses, loss of consciousness, head trauma, and following up on the history with neuropsychological and other tests of brain function,” Dr. Gold said. “After an assessment, rehabilitation and treatment might then be more personalized and effective.”
Dr. Gold had no relevant financial disclosures.
FROM THE JOURNAL OF ADDICTION SCIENCE
Growing proportion of cardiac arrests in U.S. considered opioid related
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
FROM ESC 2021
The trauma and healing of 9/11 echo in COVID-19
The scope and magnitude of the Sept. 11, 2001, attacks on the World Trade Center and the Pentagon were unprecedented in U.S. history. It was arguably the most serious trauma to beset Americans on U.S. soil. The 20th anniversary of 9/11 will take place during another crisis, not only in American history but also in world history – the COVID-19 pandemic.

“As different as these two events are, there are obvious points of comparison,” Jonathan DePierro, PhD, assistant professor of psychiatry, Icahn School of Medicine at Mount Sinai, New York, said in an interview. “Both were unprecedented life-threatening situations, presenting threats to individuals’ lives and profoundly traumatizing not only society as a whole but also first responders.”
Dr. DePierro, who is also the clinical director of the Center for Stress, Resilience, and Personal Growth at Mount Sinai, thinks there are many lessons to be learned from the mental health response to 9/11 that can inform our understanding of and response to the mental health needs of today’s first responders in the COVID-19 crisis, particularly health care professionals.
“Every one of our hospitals became a ‘ground zero’ early during the pandemic, and we see the numbers rising again and hospitals again overwhelmed, so he said.
Placing trauma within a new framework
According to Priscilla Dass-Brailsford, EdD, MPH, professor of psychology, department of psychiatry, Georgetown University, Washington, Sept. 11, 2001, “placed trauma within a new framework.”
“Prior to 9/11, crisis protocols and how to manage stress in the aftermath of violent events were uncommon,” Dr. Dass-Brailsford, a clinical psychologist with expertise in trauma who also chairs a clinical psychology program for the Chicago School of Professional Psychology, said in an interview.
As a first responder, she was involved in early interventions for survivors of 9/11. On Sept. 11, 2001, she had just resigned her position as coordinator of the community crisis response team – the first of its kind in the United States – through the Victims of Violence Program in Cambridge, in Cambridge, Mass.
The program responded to communities in which there were high rates of drive-by shootings and similar acts of violence. Because of her crisis experience, Dr. Dass-Brailsford was asked to conduct debriefings in Boston in the area where the 9/11 terrorists had stayed prior to boarding the planes that were used in the terrorist attacks. She subsequently went to New York City to conduct similar psychological debriefings with affected communities.
“What we’ve learned is that we had no crisis protocol on how to manage the stress in the aftermath of such a violent event, no standard operating procedures. There were very few people trained in crisis and trauma response at that time. Partially spurred by 9/11, trauma training programs became more prolific,” she said. Dr. Dass-Brailsford developed a trauma certification program at Lesley University in Cambridge, Mass., where she began to teach after 9/11. “I saw the importance of having clinicians trained to respond in a crisis, because responding to a crisis is very different from conducting regular mental health interventions.”
Short- and long-term interventions
Dr. DePierro said that Mount Sinai has a 20-year history of responding to the physical and mental health needs of 9/11 responders.
“We saw a number of first responders experiencing clinical depression, anxiety, a lot of worry, symptoms of posttraumatic stress disorder, and an increase in alcohol and/or substance use,” he recounted. In some, these responses were immediate; in others, the onset of symptoms was more gradual. Some responders had acute reactions that lasted for several months to a year, whereas for others, the reactions were prolonged, and they remained “chronically distressed long after the immediate exposure to the event,” he said.
Recent studies have shown that, during the COVID-19 pandemic, health care professionals and many essential workers have experienced similar symptoms, Dr. DePierro noted.
Mental health care professionals who provided interventions for workers involved in recovery and cleanup at the World Trade Center have highlighted the need for long-term monitoring of people on the front lines during the COVID-19 pandemic – especially health care workers, other essential personnel (for example, delivery, postal, and grocery store workers) and surviving family members. “Health monitoring and treatment efforts for 9/11 survivors and responders were put into place soon after the attacks and continue to this day,” using funding provided through the James Zadroga Act, Dr. DePierro said.
“Without similarly unified health registry and treatment services, many individuals – especially from underserved groups – will likely experience chronic mental health consequences and will be unable to access high-quality health care services,” he stated.
‘Psychological first aid’
“Although many people who go through a crisis – whether as a result of terrorism, such as 9/11, or a medical crisis, such as the current pandemic, or a natural disaster, such as Hurricane Katrina – experience PTSD, it’s important to note that not everyone who goes through a crisis and is traumatized will go on to develop PTSD,” Dr. Dass-Brailsford emphasized.
“To me, 9/11 placed psychological first aid on the map. Even if you are not a clinician, you can be trained to provide psychological first aid by becoming familiar with people’s reactions to trauma and how you can support them through it,” she continued.
For example, if a coworker is agitated or “seems to be having a meltdown, you can be there by offering support and getting them the appropriate help.” Research has suggested that having social support before and after a traumatic event can be helpful in determining vulnerability to the development of PTSD and in modulating the impact of the trauma.
Psychological first aid is helpful as an interim measure. “If you see a coworker holding their head in their hands all day and staring at the screen, identifying whether the person might be having a dissociative episode is critical. Providing some support is important, but if more intensive professional support is needed, determining that and making a referral becomes key,” Dr. Dass-Brailsford stated.
Dr. DePierro added: “One of the most important messages that I want health care workers to know from my years of working with 9/11 survivors is that feeling distressed after a traumatic event is very common, but with effective care, one doesn’t necessarily need to be in treatment for years.”
Danielle Ofri, MD, PhD, clinical professor, department of medicine, New York University, agreed. “It is important to continue keeping tabs on each other and remaining sensitive to the collateral struggles of our colleagues. Some have children who are struggling in school, others have parents who have lost a job. Continuing to check in on others and offer support is critical going forward,” she said in an interview.
Cohesiveness and volunteerism
One of the most powerful antidotes to long-term traumatization is a sense of community cohesiveness. This was the case following 9/11, and it is the case during the COVID-19 pandemic, according to Dr. Ofri, an internist at Bellevue Hospital in New York.
“There was an enormous mobilization. Bellevue is a city hospital with a level 1 trauma center, and we expected to be swamped, so the whole hospital shifted into gear,” said Dr. Ofri. “What would have been terrifying seemed tolerable because we felt that we were in it together. We discharged the inpatients to make beds available. Within hours, we had converted clinics into emergency departments and ICUs. We worked seamlessly, and the crisis brought us together ... but then, of course, no patients showed up.”
She described her relationship with her colleagues as “feeling almost like a family, especially during the pandemic, when so many others were in lockdown and feeling isolated and useless.”
She and her colleagues saw each other daily. Although the content of their tasks and responsibilities changed and people were redeployed to other areas, “our workday didn’t really change. It would have been overwhelming if we hadn’t had our daily meetings to regroup and assess where we were. Each day, everything we had learned or implemented the day before – treatment protocols, testing protocols, our understanding of how the virus was communicated – would change and need to be reevaluated. Those morning meetings were critical to staying centered. It felt as though we were building a plane and flying it at the same time, which felt both scary and heady. Luckily, it took place within the fraternity of a committed and caring group.”
Dr. Ofri recounted that, after 9/11, as well as during the pandemic, “professionals kept jumping in from the sidelines to volunteer. Within hours of the collapse of the towers, the ED had filled with staff. People came out of retirement and out from vacation and out of the woodwork. It was very heartening.”
Even more inspiring, “all the departmental barriers seemed to break down. People were willing to step out of their ordinary roles and check their egos at the door. Seasoned physicians were willing to function as medical interns.”
This generosity of time and spirit “helped keep us going,” she said.
Dr. DePierro agreed. “One of the things I’ve seen on medical floors is that COVID actually brought some units together, increasing their cohesion and mutual support and increasing the bonds between people.” These intensified bonds “increased the resilience of everyone involved.”
Commitment to the community
Dr. Ofri recalls families gathering at the hospital after 9/11, watching posters of missing people going up all over the hospital as well as on mailboxes and lampposts. Because the center for missing people was located right next door to Bellevue, there were long lines of families coming in to register. The chief medical office was there, and a huge tent was built to accommodate the families. The tent took up the entire block. “We felt a lot of ownership, because families were coming here,” she said.
The street remained closed even as the days, weeks, and years stretched on, and the tent remained. It was used as a reflection area for families. During the pandemic, that area was used for refrigerated trucks that served as temporary morgues.
“Both logistically and emotionally, we had a feeling during the pandemic of, ‘We’ve been here before, we’ll do it again and be there for the community,’ ” Dr. Ofri said.
She noted that the sense of commitment to the community carried her and fellow clinicians through the toughest parts of 9/11 and of the COVID-19 pandemic.
“People look to the medical system as a lodestar. ‘Where’s my family member? What should I do? Should I be tested? Vaccinated?’ We were there to be a steady presence for the community physically, psychologically, emotionally, and medically, which helped center us as well,” Dr. Ofri said. “If we didn’t have that, we might have all given in to existential panic.”
She added: “Although we had to work twice as hard, often amid great personal risk, we had the good fortune of having a sense of purpose, something to contribute, plus the community of colleagues we cared about and trusted with our lives.”
Crisis and personal growth
Dr. DePierro said that participants who went through 9/11 have been coming to Mount Sinai’s World Trade Center Health Program for care for nearly two decades. “Many are doing quite well, despite the emotional trauma and the dust and toxin exposure, which has given us a window into what makes people resilient.”
Social and community support are key factors in resilience. Another is recognizing opportunities for personal or professional growth during the crisis, according to Dr. DePierro.
During the pandemic, hospital staff were redeployed to departments where they didn’t typically work. They worked with new colleagues and used skills in patient care that they hadn’t needed for years or even decades. “Although this was stressful and distressing, quite a number said they came through with more medical knowledge than before and that they had forged relationships in the trenches that have been lasting and have become important to them,” he reported.
He noted that, during both crises, for first responders and health care practitioners, religious or spiritual faith was a source of resilience. “During the peak of the pandemic, chaplains provided an exorbitant amount of staff support as clinicians turned to the chaplain to help make sense of what they were going through and connect to something greater than themselves.” Similarly, during 9/11, police and fire department chaplains “played a huge role in supporting the first responders,” Dr. DePierro said.
He said that Mount Sinai holds resilience workshops “where we focus on these topics and teach health care workers how to build resilience in their lives, heal day-to-day stressors, and even grow from the experience.”
Dr. Ofri, who is the founder and editor-in-chief of the Bellevue Literary Review, added that the arts played an important role in bolstering resilience and providing a creative outlet for clinicians after 9/11 and again during the pandemic.
The publication is celebrating its twentieth anniversary – its first issue went to press in September 2001. The cover contained an acknowledgment of 9/11.
Dr. Ofri said that a gala event had been planned for Oct. 7, 2001, to celebrate the inaugural issue of the publication. She assumed no one would show up, given that the United States had invaded Afghanistan only hours earlier. To her surprise, over a hundred people attended, “which made me realize the role of the arts during trauma. People were seeking to come together and hear poetry, fiction, and creative nonfiction.”
Dr. Ofri has been “impressed by the amount of incredible creative writing of all sorts that has been submitted [to the publication] during the pandemic, an unexpected flowering of the arts.”
Unique challenges, unique opportunities
All three experts pointed to several noteworthy differences between the experiences of first responders following 9/11 and those of today’s health care professionals during the pandemic.
“What happened on Sept. 11 was one discrete event, and although it obviously led to years of recovering body parts and cleaning up Ground Zero, and on a national level it led to a war, it nevertheless was a single event,” Dr. DePierro observed. By contrast, the COVID-19 pandemic is ongoing, and for health care practitioners, “it’s by no means over. Again and again, they are being thrown back into battle, dealing with fatigue, weariness, and loss of life.”
Moreover, “it is my understanding that immediately following 9/11, there was a general coming together in our country, but it’s obvious that today, there’s a great deal of fractiousness, contention, disagreement, and disunity in our country when it comes to COVID-19,” Dr. DePierro continued.
“This takes a great toll, particularly on health care workers who are dealing with COVID-19 on a daily basis and experience a disconnect between what they see on their floors and ICUs of the hospital, experiencing loss of life they’ve likely never encountered in their careers, and what people are saying when they downplay the seriousness of COVID-19,” he said.
Dr. Ofri agreed. “The fragmentation of our country and the failure of leadership at the highest level to provide even the basics, such as PPE [personal protective equipment] for health care professionals, left us baffled, profoundly hurt, and angry.”
A positive difference between the COVID-19 pandemic and the aftermath of 9/11 is the development of sophisticated technology that allows interventions for traumatized individuals – both health care professionals and the general public – through telehealth, Dr. DePierro pointed out.
“I would say that these resources and technologies are a silver lining and should continue to be expanded on,” he said. “Now, busy health care workers can access all manner of supportive services, including teletherapy, right from home or between shifts.”
Another “silver lining” is that the pandemic has shone a spotlight on an issue that predated the pandemic – the mental health of health care professionals. Opening a discussion about this has reduced stigma and hopefully has paved the way for improved treatments and for providing resources.
Dr. Dass-Brailsford added that “it is important, going forward, for all of us to be trauma informed, to know how trauma and trauma-related stress unfolds in both other people and yourself, and to know what coping skills can be used to avoid crises from developing – a task that extends across all types of disasters.”
A version of this article first appeared on Medscape.com.
The scope and magnitude of the Sept. 11, 2001, attacks on the World Trade Center and the Pentagon were unprecedented in U.S. history. It was arguably the most serious trauma to beset Americans on U.S. soil. The 20th anniversary of 9/11 will take place during another crisis, not only in American history but also in world history – the COVID-19 pandemic.

“As different as these two events are, there are obvious points of comparison,” Jonathan DePierro, PhD, assistant professor of psychiatry, Icahn School of Medicine at Mount Sinai, New York, said in an interview. “Both were unprecedented life-threatening situations, presenting threats to individuals’ lives and profoundly traumatizing not only society as a whole but also first responders.”
Dr. DePierro, who is also the clinical director of the Center for Stress, Resilience, and Personal Growth at Mount Sinai, thinks there are many lessons to be learned from the mental health response to 9/11 that can inform our understanding of and response to the mental health needs of today’s first responders in the COVID-19 crisis, particularly health care professionals.
“Every one of our hospitals became a ‘ground zero’ early during the pandemic, and we see the numbers rising again and hospitals again overwhelmed, so he said.
Placing trauma within a new framework
According to Priscilla Dass-Brailsford, EdD, MPH, professor of psychology, department of psychiatry, Georgetown University, Washington, Sept. 11, 2001, “placed trauma within a new framework.”
“Prior to 9/11, crisis protocols and how to manage stress in the aftermath of violent events were uncommon,” Dr. Dass-Brailsford, a clinical psychologist with expertise in trauma who also chairs a clinical psychology program for the Chicago School of Professional Psychology, said in an interview.
As a first responder, she was involved in early interventions for survivors of 9/11. On Sept. 11, 2001, she had just resigned her position as coordinator of the community crisis response team – the first of its kind in the United States – through the Victims of Violence Program in Cambridge, in Cambridge, Mass.
The program responded to communities in which there were high rates of drive-by shootings and similar acts of violence. Because of her crisis experience, Dr. Dass-Brailsford was asked to conduct debriefings in Boston in the area where the 9/11 terrorists had stayed prior to boarding the planes that were used in the terrorist attacks. She subsequently went to New York City to conduct similar psychological debriefings with affected communities.
“What we’ve learned is that we had no crisis protocol on how to manage the stress in the aftermath of such a violent event, no standard operating procedures. There were very few people trained in crisis and trauma response at that time. Partially spurred by 9/11, trauma training programs became more prolific,” she said. Dr. Dass-Brailsford developed a trauma certification program at Lesley University in Cambridge, Mass., where she began to teach after 9/11. “I saw the importance of having clinicians trained to respond in a crisis, because responding to a crisis is very different from conducting regular mental health interventions.”
Short- and long-term interventions
Dr. DePierro said that Mount Sinai has a 20-year history of responding to the physical and mental health needs of 9/11 responders.
“We saw a number of first responders experiencing clinical depression, anxiety, a lot of worry, symptoms of posttraumatic stress disorder, and an increase in alcohol and/or substance use,” he recounted. In some, these responses were immediate; in others, the onset of symptoms was more gradual. Some responders had acute reactions that lasted for several months to a year, whereas for others, the reactions were prolonged, and they remained “chronically distressed long after the immediate exposure to the event,” he said.
Recent studies have shown that, during the COVID-19 pandemic, health care professionals and many essential workers have experienced similar symptoms, Dr. DePierro noted.
Mental health care professionals who provided interventions for workers involved in recovery and cleanup at the World Trade Center have highlighted the need for long-term monitoring of people on the front lines during the COVID-19 pandemic – especially health care workers, other essential personnel (for example, delivery, postal, and grocery store workers) and surviving family members. “Health monitoring and treatment efforts for 9/11 survivors and responders were put into place soon after the attacks and continue to this day,” using funding provided through the James Zadroga Act, Dr. DePierro said.
“Without similarly unified health registry and treatment services, many individuals – especially from underserved groups – will likely experience chronic mental health consequences and will be unable to access high-quality health care services,” he stated.
‘Psychological first aid’
“Although many people who go through a crisis – whether as a result of terrorism, such as 9/11, or a medical crisis, such as the current pandemic, or a natural disaster, such as Hurricane Katrina – experience PTSD, it’s important to note that not everyone who goes through a crisis and is traumatized will go on to develop PTSD,” Dr. Dass-Brailsford emphasized.
“To me, 9/11 placed psychological first aid on the map. Even if you are not a clinician, you can be trained to provide psychological first aid by becoming familiar with people’s reactions to trauma and how you can support them through it,” she continued.
For example, if a coworker is agitated or “seems to be having a meltdown, you can be there by offering support and getting them the appropriate help.” Research has suggested that having social support before and after a traumatic event can be helpful in determining vulnerability to the development of PTSD and in modulating the impact of the trauma.
Psychological first aid is helpful as an interim measure. “If you see a coworker holding their head in their hands all day and staring at the screen, identifying whether the person might be having a dissociative episode is critical. Providing some support is important, but if more intensive professional support is needed, determining that and making a referral becomes key,” Dr. Dass-Brailsford stated.
Dr. DePierro added: “One of the most important messages that I want health care workers to know from my years of working with 9/11 survivors is that feeling distressed after a traumatic event is very common, but with effective care, one doesn’t necessarily need to be in treatment for years.”
Danielle Ofri, MD, PhD, clinical professor, department of medicine, New York University, agreed. “It is important to continue keeping tabs on each other and remaining sensitive to the collateral struggles of our colleagues. Some have children who are struggling in school, others have parents who have lost a job. Continuing to check in on others and offer support is critical going forward,” she said in an interview.
Cohesiveness and volunteerism
One of the most powerful antidotes to long-term traumatization is a sense of community cohesiveness. This was the case following 9/11, and it is the case during the COVID-19 pandemic, according to Dr. Ofri, an internist at Bellevue Hospital in New York.
“There was an enormous mobilization. Bellevue is a city hospital with a level 1 trauma center, and we expected to be swamped, so the whole hospital shifted into gear,” said Dr. Ofri. “What would have been terrifying seemed tolerable because we felt that we were in it together. We discharged the inpatients to make beds available. Within hours, we had converted clinics into emergency departments and ICUs. We worked seamlessly, and the crisis brought us together ... but then, of course, no patients showed up.”
She described her relationship with her colleagues as “feeling almost like a family, especially during the pandemic, when so many others were in lockdown and feeling isolated and useless.”
She and her colleagues saw each other daily. Although the content of their tasks and responsibilities changed and people were redeployed to other areas, “our workday didn’t really change. It would have been overwhelming if we hadn’t had our daily meetings to regroup and assess where we were. Each day, everything we had learned or implemented the day before – treatment protocols, testing protocols, our understanding of how the virus was communicated – would change and need to be reevaluated. Those morning meetings were critical to staying centered. It felt as though we were building a plane and flying it at the same time, which felt both scary and heady. Luckily, it took place within the fraternity of a committed and caring group.”
Dr. Ofri recounted that, after 9/11, as well as during the pandemic, “professionals kept jumping in from the sidelines to volunteer. Within hours of the collapse of the towers, the ED had filled with staff. People came out of retirement and out from vacation and out of the woodwork. It was very heartening.”
Even more inspiring, “all the departmental barriers seemed to break down. People were willing to step out of their ordinary roles and check their egos at the door. Seasoned physicians were willing to function as medical interns.”
This generosity of time and spirit “helped keep us going,” she said.
Dr. DePierro agreed. “One of the things I’ve seen on medical floors is that COVID actually brought some units together, increasing their cohesion and mutual support and increasing the bonds between people.” These intensified bonds “increased the resilience of everyone involved.”
Commitment to the community
Dr. Ofri recalls families gathering at the hospital after 9/11, watching posters of missing people going up all over the hospital as well as on mailboxes and lampposts. Because the center for missing people was located right next door to Bellevue, there were long lines of families coming in to register. The chief medical office was there, and a huge tent was built to accommodate the families. The tent took up the entire block. “We felt a lot of ownership, because families were coming here,” she said.
The street remained closed even as the days, weeks, and years stretched on, and the tent remained. It was used as a reflection area for families. During the pandemic, that area was used for refrigerated trucks that served as temporary morgues.
“Both logistically and emotionally, we had a feeling during the pandemic of, ‘We’ve been here before, we’ll do it again and be there for the community,’ ” Dr. Ofri said.
She noted that the sense of commitment to the community carried her and fellow clinicians through the toughest parts of 9/11 and of the COVID-19 pandemic.
“People look to the medical system as a lodestar. ‘Where’s my family member? What should I do? Should I be tested? Vaccinated?’ We were there to be a steady presence for the community physically, psychologically, emotionally, and medically, which helped center us as well,” Dr. Ofri said. “If we didn’t have that, we might have all given in to existential panic.”
She added: “Although we had to work twice as hard, often amid great personal risk, we had the good fortune of having a sense of purpose, something to contribute, plus the community of colleagues we cared about and trusted with our lives.”
Crisis and personal growth
Dr. DePierro said that participants who went through 9/11 have been coming to Mount Sinai’s World Trade Center Health Program for care for nearly two decades. “Many are doing quite well, despite the emotional trauma and the dust and toxin exposure, which has given us a window into what makes people resilient.”
Social and community support are key factors in resilience. Another is recognizing opportunities for personal or professional growth during the crisis, according to Dr. DePierro.
During the pandemic, hospital staff were redeployed to departments where they didn’t typically work. They worked with new colleagues and used skills in patient care that they hadn’t needed for years or even decades. “Although this was stressful and distressing, quite a number said they came through with more medical knowledge than before and that they had forged relationships in the trenches that have been lasting and have become important to them,” he reported.
He noted that, during both crises, for first responders and health care practitioners, religious or spiritual faith was a source of resilience. “During the peak of the pandemic, chaplains provided an exorbitant amount of staff support as clinicians turned to the chaplain to help make sense of what they were going through and connect to something greater than themselves.” Similarly, during 9/11, police and fire department chaplains “played a huge role in supporting the first responders,” Dr. DePierro said.
He said that Mount Sinai holds resilience workshops “where we focus on these topics and teach health care workers how to build resilience in their lives, heal day-to-day stressors, and even grow from the experience.”
Dr. Ofri, who is the founder and editor-in-chief of the Bellevue Literary Review, added that the arts played an important role in bolstering resilience and providing a creative outlet for clinicians after 9/11 and again during the pandemic.
The publication is celebrating its twentieth anniversary – its first issue went to press in September 2001. The cover contained an acknowledgment of 9/11.
Dr. Ofri said that a gala event had been planned for Oct. 7, 2001, to celebrate the inaugural issue of the publication. She assumed no one would show up, given that the United States had invaded Afghanistan only hours earlier. To her surprise, over a hundred people attended, “which made me realize the role of the arts during trauma. People were seeking to come together and hear poetry, fiction, and creative nonfiction.”
Dr. Ofri has been “impressed by the amount of incredible creative writing of all sorts that has been submitted [to the publication] during the pandemic, an unexpected flowering of the arts.”
Unique challenges, unique opportunities
All three experts pointed to several noteworthy differences between the experiences of first responders following 9/11 and those of today’s health care professionals during the pandemic.
“What happened on Sept. 11 was one discrete event, and although it obviously led to years of recovering body parts and cleaning up Ground Zero, and on a national level it led to a war, it nevertheless was a single event,” Dr. DePierro observed. By contrast, the COVID-19 pandemic is ongoing, and for health care practitioners, “it’s by no means over. Again and again, they are being thrown back into battle, dealing with fatigue, weariness, and loss of life.”
Moreover, “it is my understanding that immediately following 9/11, there was a general coming together in our country, but it’s obvious that today, there’s a great deal of fractiousness, contention, disagreement, and disunity in our country when it comes to COVID-19,” Dr. DePierro continued.
“This takes a great toll, particularly on health care workers who are dealing with COVID-19 on a daily basis and experience a disconnect between what they see on their floors and ICUs of the hospital, experiencing loss of life they’ve likely never encountered in their careers, and what people are saying when they downplay the seriousness of COVID-19,” he said.
Dr. Ofri agreed. “The fragmentation of our country and the failure of leadership at the highest level to provide even the basics, such as PPE [personal protective equipment] for health care professionals, left us baffled, profoundly hurt, and angry.”
A positive difference between the COVID-19 pandemic and the aftermath of 9/11 is the development of sophisticated technology that allows interventions for traumatized individuals – both health care professionals and the general public – through telehealth, Dr. DePierro pointed out.
“I would say that these resources and technologies are a silver lining and should continue to be expanded on,” he said. “Now, busy health care workers can access all manner of supportive services, including teletherapy, right from home or between shifts.”
Another “silver lining” is that the pandemic has shone a spotlight on an issue that predated the pandemic – the mental health of health care professionals. Opening a discussion about this has reduced stigma and hopefully has paved the way for improved treatments and for providing resources.
Dr. Dass-Brailsford added that “it is important, going forward, for all of us to be trauma informed, to know how trauma and trauma-related stress unfolds in both other people and yourself, and to know what coping skills can be used to avoid crises from developing – a task that extends across all types of disasters.”
A version of this article first appeared on Medscape.com.
The scope and magnitude of the Sept. 11, 2001, attacks on the World Trade Center and the Pentagon were unprecedented in U.S. history. It was arguably the most serious trauma to beset Americans on U.S. soil. The 20th anniversary of 9/11 will take place during another crisis, not only in American history but also in world history – the COVID-19 pandemic.

“As different as these two events are, there are obvious points of comparison,” Jonathan DePierro, PhD, assistant professor of psychiatry, Icahn School of Medicine at Mount Sinai, New York, said in an interview. “Both were unprecedented life-threatening situations, presenting threats to individuals’ lives and profoundly traumatizing not only society as a whole but also first responders.”
Dr. DePierro, who is also the clinical director of the Center for Stress, Resilience, and Personal Growth at Mount Sinai, thinks there are many lessons to be learned from the mental health response to 9/11 that can inform our understanding of and response to the mental health needs of today’s first responders in the COVID-19 crisis, particularly health care professionals.
“Every one of our hospitals became a ‘ground zero’ early during the pandemic, and we see the numbers rising again and hospitals again overwhelmed, so he said.
Placing trauma within a new framework
According to Priscilla Dass-Brailsford, EdD, MPH, professor of psychology, department of psychiatry, Georgetown University, Washington, Sept. 11, 2001, “placed trauma within a new framework.”
“Prior to 9/11, crisis protocols and how to manage stress in the aftermath of violent events were uncommon,” Dr. Dass-Brailsford, a clinical psychologist with expertise in trauma who also chairs a clinical psychology program for the Chicago School of Professional Psychology, said in an interview.
As a first responder, she was involved in early interventions for survivors of 9/11. On Sept. 11, 2001, she had just resigned her position as coordinator of the community crisis response team – the first of its kind in the United States – through the Victims of Violence Program in Cambridge, in Cambridge, Mass.
The program responded to communities in which there were high rates of drive-by shootings and similar acts of violence. Because of her crisis experience, Dr. Dass-Brailsford was asked to conduct debriefings in Boston in the area where the 9/11 terrorists had stayed prior to boarding the planes that were used in the terrorist attacks. She subsequently went to New York City to conduct similar psychological debriefings with affected communities.
“What we’ve learned is that we had no crisis protocol on how to manage the stress in the aftermath of such a violent event, no standard operating procedures. There were very few people trained in crisis and trauma response at that time. Partially spurred by 9/11, trauma training programs became more prolific,” she said. Dr. Dass-Brailsford developed a trauma certification program at Lesley University in Cambridge, Mass., where she began to teach after 9/11. “I saw the importance of having clinicians trained to respond in a crisis, because responding to a crisis is very different from conducting regular mental health interventions.”
Short- and long-term interventions
Dr. DePierro said that Mount Sinai has a 20-year history of responding to the physical and mental health needs of 9/11 responders.
“We saw a number of first responders experiencing clinical depression, anxiety, a lot of worry, symptoms of posttraumatic stress disorder, and an increase in alcohol and/or substance use,” he recounted. In some, these responses were immediate; in others, the onset of symptoms was more gradual. Some responders had acute reactions that lasted for several months to a year, whereas for others, the reactions were prolonged, and they remained “chronically distressed long after the immediate exposure to the event,” he said.
Recent studies have shown that, during the COVID-19 pandemic, health care professionals and many essential workers have experienced similar symptoms, Dr. DePierro noted.
Mental health care professionals who provided interventions for workers involved in recovery and cleanup at the World Trade Center have highlighted the need for long-term monitoring of people on the front lines during the COVID-19 pandemic – especially health care workers, other essential personnel (for example, delivery, postal, and grocery store workers) and surviving family members. “Health monitoring and treatment efforts for 9/11 survivors and responders were put into place soon after the attacks and continue to this day,” using funding provided through the James Zadroga Act, Dr. DePierro said.
“Without similarly unified health registry and treatment services, many individuals – especially from underserved groups – will likely experience chronic mental health consequences and will be unable to access high-quality health care services,” he stated.
‘Psychological first aid’
“Although many people who go through a crisis – whether as a result of terrorism, such as 9/11, or a medical crisis, such as the current pandemic, or a natural disaster, such as Hurricane Katrina – experience PTSD, it’s important to note that not everyone who goes through a crisis and is traumatized will go on to develop PTSD,” Dr. Dass-Brailsford emphasized.
“To me, 9/11 placed psychological first aid on the map. Even if you are not a clinician, you can be trained to provide psychological first aid by becoming familiar with people’s reactions to trauma and how you can support them through it,” she continued.
For example, if a coworker is agitated or “seems to be having a meltdown, you can be there by offering support and getting them the appropriate help.” Research has suggested that having social support before and after a traumatic event can be helpful in determining vulnerability to the development of PTSD and in modulating the impact of the trauma.
Psychological first aid is helpful as an interim measure. “If you see a coworker holding their head in their hands all day and staring at the screen, identifying whether the person might be having a dissociative episode is critical. Providing some support is important, but if more intensive professional support is needed, determining that and making a referral becomes key,” Dr. Dass-Brailsford stated.
Dr. DePierro added: “One of the most important messages that I want health care workers to know from my years of working with 9/11 survivors is that feeling distressed after a traumatic event is very common, but with effective care, one doesn’t necessarily need to be in treatment for years.”
Danielle Ofri, MD, PhD, clinical professor, department of medicine, New York University, agreed. “It is important to continue keeping tabs on each other and remaining sensitive to the collateral struggles of our colleagues. Some have children who are struggling in school, others have parents who have lost a job. Continuing to check in on others and offer support is critical going forward,” she said in an interview.
Cohesiveness and volunteerism
One of the most powerful antidotes to long-term traumatization is a sense of community cohesiveness. This was the case following 9/11, and it is the case during the COVID-19 pandemic, according to Dr. Ofri, an internist at Bellevue Hospital in New York.
“There was an enormous mobilization. Bellevue is a city hospital with a level 1 trauma center, and we expected to be swamped, so the whole hospital shifted into gear,” said Dr. Ofri. “What would have been terrifying seemed tolerable because we felt that we were in it together. We discharged the inpatients to make beds available. Within hours, we had converted clinics into emergency departments and ICUs. We worked seamlessly, and the crisis brought us together ... but then, of course, no patients showed up.”
She described her relationship with her colleagues as “feeling almost like a family, especially during the pandemic, when so many others were in lockdown and feeling isolated and useless.”
She and her colleagues saw each other daily. Although the content of their tasks and responsibilities changed and people were redeployed to other areas, “our workday didn’t really change. It would have been overwhelming if we hadn’t had our daily meetings to regroup and assess where we were. Each day, everything we had learned or implemented the day before – treatment protocols, testing protocols, our understanding of how the virus was communicated – would change and need to be reevaluated. Those morning meetings were critical to staying centered. It felt as though we were building a plane and flying it at the same time, which felt both scary and heady. Luckily, it took place within the fraternity of a committed and caring group.”
Dr. Ofri recounted that, after 9/11, as well as during the pandemic, “professionals kept jumping in from the sidelines to volunteer. Within hours of the collapse of the towers, the ED had filled with staff. People came out of retirement and out from vacation and out of the woodwork. It was very heartening.”
Even more inspiring, “all the departmental barriers seemed to break down. People were willing to step out of their ordinary roles and check their egos at the door. Seasoned physicians were willing to function as medical interns.”
This generosity of time and spirit “helped keep us going,” she said.
Dr. DePierro agreed. “One of the things I’ve seen on medical floors is that COVID actually brought some units together, increasing their cohesion and mutual support and increasing the bonds between people.” These intensified bonds “increased the resilience of everyone involved.”
Commitment to the community
Dr. Ofri recalls families gathering at the hospital after 9/11, watching posters of missing people going up all over the hospital as well as on mailboxes and lampposts. Because the center for missing people was located right next door to Bellevue, there were long lines of families coming in to register. The chief medical office was there, and a huge tent was built to accommodate the families. The tent took up the entire block. “We felt a lot of ownership, because families were coming here,” she said.
The street remained closed even as the days, weeks, and years stretched on, and the tent remained. It was used as a reflection area for families. During the pandemic, that area was used for refrigerated trucks that served as temporary morgues.
“Both logistically and emotionally, we had a feeling during the pandemic of, ‘We’ve been here before, we’ll do it again and be there for the community,’ ” Dr. Ofri said.
She noted that the sense of commitment to the community carried her and fellow clinicians through the toughest parts of 9/11 and of the COVID-19 pandemic.
“People look to the medical system as a lodestar. ‘Where’s my family member? What should I do? Should I be tested? Vaccinated?’ We were there to be a steady presence for the community physically, psychologically, emotionally, and medically, which helped center us as well,” Dr. Ofri said. “If we didn’t have that, we might have all given in to existential panic.”
She added: “Although we had to work twice as hard, often amid great personal risk, we had the good fortune of having a sense of purpose, something to contribute, plus the community of colleagues we cared about and trusted with our lives.”
Crisis and personal growth
Dr. DePierro said that participants who went through 9/11 have been coming to Mount Sinai’s World Trade Center Health Program for care for nearly two decades. “Many are doing quite well, despite the emotional trauma and the dust and toxin exposure, which has given us a window into what makes people resilient.”
Social and community support are key factors in resilience. Another is recognizing opportunities for personal or professional growth during the crisis, according to Dr. DePierro.
During the pandemic, hospital staff were redeployed to departments where they didn’t typically work. They worked with new colleagues and used skills in patient care that they hadn’t needed for years or even decades. “Although this was stressful and distressing, quite a number said they came through with more medical knowledge than before and that they had forged relationships in the trenches that have been lasting and have become important to them,” he reported.
He noted that, during both crises, for first responders and health care practitioners, religious or spiritual faith was a source of resilience. “During the peak of the pandemic, chaplains provided an exorbitant amount of staff support as clinicians turned to the chaplain to help make sense of what they were going through and connect to something greater than themselves.” Similarly, during 9/11, police and fire department chaplains “played a huge role in supporting the first responders,” Dr. DePierro said.
He said that Mount Sinai holds resilience workshops “where we focus on these topics and teach health care workers how to build resilience in their lives, heal day-to-day stressors, and even grow from the experience.”
Dr. Ofri, who is the founder and editor-in-chief of the Bellevue Literary Review, added that the arts played an important role in bolstering resilience and providing a creative outlet for clinicians after 9/11 and again during the pandemic.
The publication is celebrating its twentieth anniversary – its first issue went to press in September 2001. The cover contained an acknowledgment of 9/11.
Dr. Ofri said that a gala event had been planned for Oct. 7, 2001, to celebrate the inaugural issue of the publication. She assumed no one would show up, given that the United States had invaded Afghanistan only hours earlier. To her surprise, over a hundred people attended, “which made me realize the role of the arts during trauma. People were seeking to come together and hear poetry, fiction, and creative nonfiction.”
Dr. Ofri has been “impressed by the amount of incredible creative writing of all sorts that has been submitted [to the publication] during the pandemic, an unexpected flowering of the arts.”
Unique challenges, unique opportunities
All three experts pointed to several noteworthy differences between the experiences of first responders following 9/11 and those of today’s health care professionals during the pandemic.
“What happened on Sept. 11 was one discrete event, and although it obviously led to years of recovering body parts and cleaning up Ground Zero, and on a national level it led to a war, it nevertheless was a single event,” Dr. DePierro observed. By contrast, the COVID-19 pandemic is ongoing, and for health care practitioners, “it’s by no means over. Again and again, they are being thrown back into battle, dealing with fatigue, weariness, and loss of life.”
Moreover, “it is my understanding that immediately following 9/11, there was a general coming together in our country, but it’s obvious that today, there’s a great deal of fractiousness, contention, disagreement, and disunity in our country when it comes to COVID-19,” Dr. DePierro continued.
“This takes a great toll, particularly on health care workers who are dealing with COVID-19 on a daily basis and experience a disconnect between what they see on their floors and ICUs of the hospital, experiencing loss of life they’ve likely never encountered in their careers, and what people are saying when they downplay the seriousness of COVID-19,” he said.
Dr. Ofri agreed. “The fragmentation of our country and the failure of leadership at the highest level to provide even the basics, such as PPE [personal protective equipment] for health care professionals, left us baffled, profoundly hurt, and angry.”
A positive difference between the COVID-19 pandemic and the aftermath of 9/11 is the development of sophisticated technology that allows interventions for traumatized individuals – both health care professionals and the general public – through telehealth, Dr. DePierro pointed out.
“I would say that these resources and technologies are a silver lining and should continue to be expanded on,” he said. “Now, busy health care workers can access all manner of supportive services, including teletherapy, right from home or between shifts.”
Another “silver lining” is that the pandemic has shone a spotlight on an issue that predated the pandemic – the mental health of health care professionals. Opening a discussion about this has reduced stigma and hopefully has paved the way for improved treatments and for providing resources.
Dr. Dass-Brailsford added that “it is important, going forward, for all of us to be trauma informed, to know how trauma and trauma-related stress unfolds in both other people and yourself, and to know what coping skills can be used to avoid crises from developing – a task that extends across all types of disasters.”
A version of this article first appeared on Medscape.com.
Toward ‘superhuman cognition’: The future of brain-computer interfaces
The brain is inarguably the most complex and mysterious organ in the human body.
As the epicenter of intelligence, mastermind of movement, and song for our senses, the brain is more than a 3-lb organ encased in shell and fluid. Rather, it is the crown jewel that defines the self and, broadly, humanity.
For decades now, researchers have been exploring the potential for connecting our own astounding biological “computer” with actual physical mainframes. These so-called “brain-computer interfaces” (BCIs) are showing promise in treating an array of conditions, including paralysis, deafness, stroke, and even psychiatric disorders.
Among the big players in this area of research is billionaire entrepreneur Elon Musk, who in 2016 founded Neuralink. The company’s short-term mission is to develop a brain-to-machine interface to help people with neurologic conditions (for example, Parkinson’s disease). The long-term mission is to steer humanity into the era of “superhuman cognition.”
But first, some neuroscience 101.
Neurons are specialized cells that transmit and receive information. The basic structure of a neuron includes the dendrite, soma, and axon. The dendrite is the signal receiver. The soma is the cell body that is connected to the dendrites and serves as a structure to pass signals. The axon, also known as the nerve fiber, transmits the signal away from the soma.
Neurons communicate with each other at the synapse (for example, axon-dendrite connection). Neurons send information to each other through action potentials. An action potential may be defined as an electric impulse that transmits down the axon, causing the release of neurotransmitters, which may consequently either inhibit or excite the next neuron (leading to the initiation of another action potential).
So how will the company and other BCI companies tap into this evolutionarily ancient system to develop an implant that will obtain and decode information output from the brain?
The Neuralink implant is composed of three parts: The Link, neural threads, and the charger.
A robotic system, controlled by a neurosurgeon, will place an implant into the brain. The Link is the central component. It processes and transmits neural signals. The micron-scale neural threads are connected to the Link and other areas of the brain. The threads also contain electrodes, which are responsible for detecting neural signals. The charger ensures the battery is charged via wireless connection.
The invasive nature of this implant allows for precise readouts of electric outputs from the brain – unlike noninvasive devices, which are less sensitive and specific. Additionally, owing to its small size, engineers and neurosurgeons can implant the device in very specific brain regions as well as customize electrode distribution.
The Neuralink implant would be paired with an application via Bluetooth connection. The goal is to enable someone with the implant to control their device or computer by simply thinking. The application offers several exercises to help guide and train individuals on how to use the implant for its intended purpose. , as well as partake in creative activities such as photography.
Existing text and speech synthesis technology are already underway. For example, Synchron, a BCI platform company, is investigating the use of Stentrode for people with severe paralysis. This neuroprosthesis was designed to help people associate thought with movement through Bluetooth technology (for example, texting, emailing, shopping, online banking). Preliminary results from a study in which the device was used for patients with amyotrophic lateral sclerosis showed improvements in functional independence via direct thinking.
Software intended to enable high-performance handwriting utilizing BCI technology is being developed by Francis R. Willett, PhD, at Stanford (Calif.) University. The technology has also shown promise.
“We’ve learned that the brain retains its ability to prescribe fine movements a full decade after the body has lost its ability to execute those movements,” says Dr. Willett, who recently reported on results from a BCI study of handwriting conversion in an individual with full-body paralysis. Through a recurrent neural networking decoding approach, the BrainGate study participant was able to type 90 characters per minute – with an impressive 94.1% raw accuracy – using thoughts alone.
Although not a fully implantable brain device, this percutaneous implant has also been studied of its capacity to restore arm function among individuals who suffered from chronic stroke. Preliminary results from the Cortimo trials, led by Mijail D. Serruya, MD, an assistant professor at Thomas Jefferson University, Philadelphia, have been positive. Researchers implanted microelectrode arrays to decode brain signals and power motor function in a participant who had experienced a stroke 2 years earlier. The participant was able to use a powered arm brace on their paralyzed arm.
Neuralink recently released a video demonstrating the use of the interface in a monkey named Pager as it played a game with a joystick. Company researchers inserted a 1024-Electrode neural recording and data transmission device called the N1 Link into the left and right motor cortices. Using the implant, neural activity was sent to a decoder algorithm. Throughout the process, the decoder algorithm was refined and calibrated. After a few minutes, Pager was able to control the cursor on the screen using his mind instead of the joystick.
Mr. Musk hopes to develop Neuralink further to change not only the way we treat neurological disorders but also the way we interact with ourselves and our environment. It’s a lofty goal to be sure, but one that doesn’t seem outside the realm of possibility in the near future.
Known unknowns: The ethical dilemmas
One major conundrum facing the future of BCI technology is that researchers don’t fully understand the science regarding how brain signaling, artificial intelligence (AI) software, and prostheses interact. Although offloading computations improves the predictive nature of AI algorithms, there are concerns of identity and personal agency.
How do we know that an action is truly the result of one’s own thinking or, rather, the outcome of AI software? In this context, the autocorrect function while typing can be incredibly useful when we’re in a pinch for time, when we’re using one hand to type, or because of ease. However, it’s also easy to create and send out unintended or inappropriate messages.
These algorithms are designed to learn from our behavior and anticipate our next move. However, a question arises as to whether we are the authors of our own thoughts or whether we are simply the device that delivers messages under the control of external forces.
“People may question whether new personality changes they experience are truly representative of themselves or whether they are now a product of the implant (e.g., ‘Is that really me?’; ‘Have I grown as a person, or is it the technology?’). This then raises questions about agency and who we are as people,” says Kerry Bowman, PhD, a clinical bioethicist and assistant professor at the Temerty Faculty of Medicine of the University of Toronto.
It’s important to have safeguards in place to ensure the privacy of our thoughts. In an age where data is currency, it’s crucial to establish boundaries to preserve our autonomy and prevent exploitation (for example, by private companies or hackers). Although Neuralink and BCIs generally are certainly pushing the boundaries of neural engineering in profound ways, it’s important to note the biological and ethical implications of this technology.
As Dr. Bowman points out, “throughout the entire human story, under the worst of human circumstances, such as captivity and torture, the one safe ground and place for all people has been the privacy of one’s own mind. No one could ever interfere, take away, or be aware of those thoughts. However, this technology challenges one’s own privacy – that this technology (and, by extension, a company) could be aware of those thoughts.”
A version of this article first appeared on Medscape.com.
The brain is inarguably the most complex and mysterious organ in the human body.
As the epicenter of intelligence, mastermind of movement, and song for our senses, the brain is more than a 3-lb organ encased in shell and fluid. Rather, it is the crown jewel that defines the self and, broadly, humanity.
For decades now, researchers have been exploring the potential for connecting our own astounding biological “computer” with actual physical mainframes. These so-called “brain-computer interfaces” (BCIs) are showing promise in treating an array of conditions, including paralysis, deafness, stroke, and even psychiatric disorders.
Among the big players in this area of research is billionaire entrepreneur Elon Musk, who in 2016 founded Neuralink. The company’s short-term mission is to develop a brain-to-machine interface to help people with neurologic conditions (for example, Parkinson’s disease). The long-term mission is to steer humanity into the era of “superhuman cognition.”
But first, some neuroscience 101.
Neurons are specialized cells that transmit and receive information. The basic structure of a neuron includes the dendrite, soma, and axon. The dendrite is the signal receiver. The soma is the cell body that is connected to the dendrites and serves as a structure to pass signals. The axon, also known as the nerve fiber, transmits the signal away from the soma.
Neurons communicate with each other at the synapse (for example, axon-dendrite connection). Neurons send information to each other through action potentials. An action potential may be defined as an electric impulse that transmits down the axon, causing the release of neurotransmitters, which may consequently either inhibit or excite the next neuron (leading to the initiation of another action potential).
So how will the company and other BCI companies tap into this evolutionarily ancient system to develop an implant that will obtain and decode information output from the brain?
The Neuralink implant is composed of three parts: The Link, neural threads, and the charger.
A robotic system, controlled by a neurosurgeon, will place an implant into the brain. The Link is the central component. It processes and transmits neural signals. The micron-scale neural threads are connected to the Link and other areas of the brain. The threads also contain electrodes, which are responsible for detecting neural signals. The charger ensures the battery is charged via wireless connection.
The invasive nature of this implant allows for precise readouts of electric outputs from the brain – unlike noninvasive devices, which are less sensitive and specific. Additionally, owing to its small size, engineers and neurosurgeons can implant the device in very specific brain regions as well as customize electrode distribution.
The Neuralink implant would be paired with an application via Bluetooth connection. The goal is to enable someone with the implant to control their device or computer by simply thinking. The application offers several exercises to help guide and train individuals on how to use the implant for its intended purpose. , as well as partake in creative activities such as photography.
Existing text and speech synthesis technology are already underway. For example, Synchron, a BCI platform company, is investigating the use of Stentrode for people with severe paralysis. This neuroprosthesis was designed to help people associate thought with movement through Bluetooth technology (for example, texting, emailing, shopping, online banking). Preliminary results from a study in which the device was used for patients with amyotrophic lateral sclerosis showed improvements in functional independence via direct thinking.
Software intended to enable high-performance handwriting utilizing BCI technology is being developed by Francis R. Willett, PhD, at Stanford (Calif.) University. The technology has also shown promise.
“We’ve learned that the brain retains its ability to prescribe fine movements a full decade after the body has lost its ability to execute those movements,” says Dr. Willett, who recently reported on results from a BCI study of handwriting conversion in an individual with full-body paralysis. Through a recurrent neural networking decoding approach, the BrainGate study participant was able to type 90 characters per minute – with an impressive 94.1% raw accuracy – using thoughts alone.
Although not a fully implantable brain device, this percutaneous implant has also been studied of its capacity to restore arm function among individuals who suffered from chronic stroke. Preliminary results from the Cortimo trials, led by Mijail D. Serruya, MD, an assistant professor at Thomas Jefferson University, Philadelphia, have been positive. Researchers implanted microelectrode arrays to decode brain signals and power motor function in a participant who had experienced a stroke 2 years earlier. The participant was able to use a powered arm brace on their paralyzed arm.
Neuralink recently released a video demonstrating the use of the interface in a monkey named Pager as it played a game with a joystick. Company researchers inserted a 1024-Electrode neural recording and data transmission device called the N1 Link into the left and right motor cortices. Using the implant, neural activity was sent to a decoder algorithm. Throughout the process, the decoder algorithm was refined and calibrated. After a few minutes, Pager was able to control the cursor on the screen using his mind instead of the joystick.
Mr. Musk hopes to develop Neuralink further to change not only the way we treat neurological disorders but also the way we interact with ourselves and our environment. It’s a lofty goal to be sure, but one that doesn’t seem outside the realm of possibility in the near future.
Known unknowns: The ethical dilemmas
One major conundrum facing the future of BCI technology is that researchers don’t fully understand the science regarding how brain signaling, artificial intelligence (AI) software, and prostheses interact. Although offloading computations improves the predictive nature of AI algorithms, there are concerns of identity and personal agency.
How do we know that an action is truly the result of one’s own thinking or, rather, the outcome of AI software? In this context, the autocorrect function while typing can be incredibly useful when we’re in a pinch for time, when we’re using one hand to type, or because of ease. However, it’s also easy to create and send out unintended or inappropriate messages.
These algorithms are designed to learn from our behavior and anticipate our next move. However, a question arises as to whether we are the authors of our own thoughts or whether we are simply the device that delivers messages under the control of external forces.
“People may question whether new personality changes they experience are truly representative of themselves or whether they are now a product of the implant (e.g., ‘Is that really me?’; ‘Have I grown as a person, or is it the technology?’). This then raises questions about agency and who we are as people,” says Kerry Bowman, PhD, a clinical bioethicist and assistant professor at the Temerty Faculty of Medicine of the University of Toronto.
It’s important to have safeguards in place to ensure the privacy of our thoughts. In an age where data is currency, it’s crucial to establish boundaries to preserve our autonomy and prevent exploitation (for example, by private companies or hackers). Although Neuralink and BCIs generally are certainly pushing the boundaries of neural engineering in profound ways, it’s important to note the biological and ethical implications of this technology.
As Dr. Bowman points out, “throughout the entire human story, under the worst of human circumstances, such as captivity and torture, the one safe ground and place for all people has been the privacy of one’s own mind. No one could ever interfere, take away, or be aware of those thoughts. However, this technology challenges one’s own privacy – that this technology (and, by extension, a company) could be aware of those thoughts.”
A version of this article first appeared on Medscape.com.
The brain is inarguably the most complex and mysterious organ in the human body.
As the epicenter of intelligence, mastermind of movement, and song for our senses, the brain is more than a 3-lb organ encased in shell and fluid. Rather, it is the crown jewel that defines the self and, broadly, humanity.
For decades now, researchers have been exploring the potential for connecting our own astounding biological “computer” with actual physical mainframes. These so-called “brain-computer interfaces” (BCIs) are showing promise in treating an array of conditions, including paralysis, deafness, stroke, and even psychiatric disorders.
Among the big players in this area of research is billionaire entrepreneur Elon Musk, who in 2016 founded Neuralink. The company’s short-term mission is to develop a brain-to-machine interface to help people with neurologic conditions (for example, Parkinson’s disease). The long-term mission is to steer humanity into the era of “superhuman cognition.”
But first, some neuroscience 101.
Neurons are specialized cells that transmit and receive information. The basic structure of a neuron includes the dendrite, soma, and axon. The dendrite is the signal receiver. The soma is the cell body that is connected to the dendrites and serves as a structure to pass signals. The axon, also known as the nerve fiber, transmits the signal away from the soma.
Neurons communicate with each other at the synapse (for example, axon-dendrite connection). Neurons send information to each other through action potentials. An action potential may be defined as an electric impulse that transmits down the axon, causing the release of neurotransmitters, which may consequently either inhibit or excite the next neuron (leading to the initiation of another action potential).
So how will the company and other BCI companies tap into this evolutionarily ancient system to develop an implant that will obtain and decode information output from the brain?
The Neuralink implant is composed of three parts: The Link, neural threads, and the charger.
A robotic system, controlled by a neurosurgeon, will place an implant into the brain. The Link is the central component. It processes and transmits neural signals. The micron-scale neural threads are connected to the Link and other areas of the brain. The threads also contain electrodes, which are responsible for detecting neural signals. The charger ensures the battery is charged via wireless connection.
The invasive nature of this implant allows for precise readouts of electric outputs from the brain – unlike noninvasive devices, which are less sensitive and specific. Additionally, owing to its small size, engineers and neurosurgeons can implant the device in very specific brain regions as well as customize electrode distribution.
The Neuralink implant would be paired with an application via Bluetooth connection. The goal is to enable someone with the implant to control their device or computer by simply thinking. The application offers several exercises to help guide and train individuals on how to use the implant for its intended purpose. , as well as partake in creative activities such as photography.
Existing text and speech synthesis technology are already underway. For example, Synchron, a BCI platform company, is investigating the use of Stentrode for people with severe paralysis. This neuroprosthesis was designed to help people associate thought with movement through Bluetooth technology (for example, texting, emailing, shopping, online banking). Preliminary results from a study in which the device was used for patients with amyotrophic lateral sclerosis showed improvements in functional independence via direct thinking.
Software intended to enable high-performance handwriting utilizing BCI technology is being developed by Francis R. Willett, PhD, at Stanford (Calif.) University. The technology has also shown promise.
“We’ve learned that the brain retains its ability to prescribe fine movements a full decade after the body has lost its ability to execute those movements,” says Dr. Willett, who recently reported on results from a BCI study of handwriting conversion in an individual with full-body paralysis. Through a recurrent neural networking decoding approach, the BrainGate study participant was able to type 90 characters per minute – with an impressive 94.1% raw accuracy – using thoughts alone.
Although not a fully implantable brain device, this percutaneous implant has also been studied of its capacity to restore arm function among individuals who suffered from chronic stroke. Preliminary results from the Cortimo trials, led by Mijail D. Serruya, MD, an assistant professor at Thomas Jefferson University, Philadelphia, have been positive. Researchers implanted microelectrode arrays to decode brain signals and power motor function in a participant who had experienced a stroke 2 years earlier. The participant was able to use a powered arm brace on their paralyzed arm.
Neuralink recently released a video demonstrating the use of the interface in a monkey named Pager as it played a game with a joystick. Company researchers inserted a 1024-Electrode neural recording and data transmission device called the N1 Link into the left and right motor cortices. Using the implant, neural activity was sent to a decoder algorithm. Throughout the process, the decoder algorithm was refined and calibrated. After a few minutes, Pager was able to control the cursor on the screen using his mind instead of the joystick.
Mr. Musk hopes to develop Neuralink further to change not only the way we treat neurological disorders but also the way we interact with ourselves and our environment. It’s a lofty goal to be sure, but one that doesn’t seem outside the realm of possibility in the near future.
Known unknowns: The ethical dilemmas
One major conundrum facing the future of BCI technology is that researchers don’t fully understand the science regarding how brain signaling, artificial intelligence (AI) software, and prostheses interact. Although offloading computations improves the predictive nature of AI algorithms, there are concerns of identity and personal agency.
How do we know that an action is truly the result of one’s own thinking or, rather, the outcome of AI software? In this context, the autocorrect function while typing can be incredibly useful when we’re in a pinch for time, when we’re using one hand to type, or because of ease. However, it’s also easy to create and send out unintended or inappropriate messages.
These algorithms are designed to learn from our behavior and anticipate our next move. However, a question arises as to whether we are the authors of our own thoughts or whether we are simply the device that delivers messages under the control of external forces.
“People may question whether new personality changes they experience are truly representative of themselves or whether they are now a product of the implant (e.g., ‘Is that really me?’; ‘Have I grown as a person, or is it the technology?’). This then raises questions about agency and who we are as people,” says Kerry Bowman, PhD, a clinical bioethicist and assistant professor at the Temerty Faculty of Medicine of the University of Toronto.
It’s important to have safeguards in place to ensure the privacy of our thoughts. In an age where data is currency, it’s crucial to establish boundaries to preserve our autonomy and prevent exploitation (for example, by private companies or hackers). Although Neuralink and BCIs generally are certainly pushing the boundaries of neural engineering in profound ways, it’s important to note the biological and ethical implications of this technology.
As Dr. Bowman points out, “throughout the entire human story, under the worst of human circumstances, such as captivity and torture, the one safe ground and place for all people has been the privacy of one’s own mind. No one could ever interfere, take away, or be aware of those thoughts. However, this technology challenges one’s own privacy – that this technology (and, by extension, a company) could be aware of those thoughts.”
A version of this article first appeared on Medscape.com.
United Healthcare settles claims it violated mental health parity
The nation’s largest health care insurer has agreed to pay $16 million in restitution and penalties to consumers, the federal government, and the state of New York to settle complaints that it illegally limited outpatient psychotherapy.
The settlement resolves a joint U.S. Department of Labor and New York Attorney General investigation and lawsuit that found that United Healthcare and United Behavioral Health (UBH) had, since at least 2013, violated the federal Mental Health Parity and Addiction Equity Act of 2008.
United Healthcare and UBH are fighting similar claims in Wit v United Behavioral Health, which is currently on appeal at the U.S. Court of Appeals for the Ninth Circuit. If that complaint is decided against UBH, the insurer might have to reprocess more than 67,000 claims.
The joint New York–federal investigation determined that United Healthcare reduced reimbursement rates for out-of-network mental health services and denied payment for many services using its Algorithms for Effective Reporting and Treatment (ALERT) utilization review program.
All outpatient psychotherapy was subjected to review; by comparison, only a handful of medical and surgical services were. That violated parity laws, the New York Attorney General’s office said in a statement.
“United’s denial of these vital services was both unlawful and dangerous,” New York Attorney General Letitia James said in the statement.
“Protecting access to mental health and substance disorder treatment is a priority for the Department of Labor and something I believe in strongly as a person in long-term recovery,” U.S. Secretary of Labor Marty Walsh said in another statement.
Mr. Walsh added that the settlement “provides compensation for many people who were denied full benefits and equitable treatment.”
‘Investigations change behavior’
Commenting for this news organization, Joe Parks, MD, medical director of the National Council for Mental Wellbeing, applauded the settlement.
“These kinds of investigations change behavior, and people get better coverage,” Dr. Parks said.
He also applauded New York, saying that it “has been one of the more assertive states in expecting companies to comply with parity requirements. They should be emulated by other states.”
Dr. Parks noted that a 2019 U.S. Government Accountability Office report showed that only 20 states routinely conducted parity compliance reviews.
A spokesperson for the American Psychiatric Association said it was notable that the Department of Labor focused on three issues that it had not publicly investigated before.
These were payment parity, the use of algorithms in mental health to identify practitioners and patients who use more health care than expected of the “average” person, and requiring disclosure of how the insurer set rates, guidelines, and medical necessity standards.
The American Psychological Association’s chief of psychological practice, Jared L. Skillings, PhD, said the group was gratified that the Department of Labor and New York took complaints seriously and that it “is encouraged that United Behavioral Health and United Healthcare have agreed to change their unfair procedures regarding reimbursement and denial of psychological care.”
The association “will monitor implementation of the settlement with high hopes for fair treatment for patients with mental health needs,” Dr. Skillings said in an interview.
Across-the-board cuts for therapy
The investigation found that United Healthcare reduced the allowable reimbursement amount for all nonphysicians across the board for psychotherapy – by 25% for PhD-level psychologists and by 35% for all MA-level therapists.
The company’s ALERT system used arbitrary thresholds to trigger utilization review, which “often led to denials of coverage when providers could not justify continued treatment after 20 sessions,” said the New York attorney general.
Beneficiaries had to figure out how to pay for continuing services or abruptly end necessary treatment.
United Healthcare agreed to stop reducing reimbursement and to not employ a similar policy in New York for at least 2 years. It will stop using ALERT and stop issuing denials for psychotherapy through at least 2023.
In addition, “We are committed to ensuring all our members have access to care and to reimbursing providers consistent with the terms of the member’s health plan and state and federal rules,” United Healthcare said.
Dr. Parks noted that United Healthcare had stopped using ALERT long before the settlement and was instead employing Level of Care Utilization System for Psychiatric and Addiction Services (LOCUS) and American Society of Addiction Medicine criteria, which he called “a good step forward.”
‘Meaningful’ payout
United Healthcare will pay $14.3 million in restitution to consumers, including $9 million to 20,000 New Yorkers who received denials of or reductions in reimbursement. The company will also pay more than $2 million in penalties, with $1.3 million going to New York.
The insurer has annual revenues of some $250 billion.
“It’s not a large amount of money in United’s overall business operation,” said Dr. Parks.
However, “It certainly could be a meaningful amount of money for the individuals that didn’t get coverage that paid out of pocket,” he added.
A version of this article first appeared on Medscape.com.
The nation’s largest health care insurer has agreed to pay $16 million in restitution and penalties to consumers, the federal government, and the state of New York to settle complaints that it illegally limited outpatient psychotherapy.
The settlement resolves a joint U.S. Department of Labor and New York Attorney General investigation and lawsuit that found that United Healthcare and United Behavioral Health (UBH) had, since at least 2013, violated the federal Mental Health Parity and Addiction Equity Act of 2008.
United Healthcare and UBH are fighting similar claims in Wit v United Behavioral Health, which is currently on appeal at the U.S. Court of Appeals for the Ninth Circuit. If that complaint is decided against UBH, the insurer might have to reprocess more than 67,000 claims.
The joint New York–federal investigation determined that United Healthcare reduced reimbursement rates for out-of-network mental health services and denied payment for many services using its Algorithms for Effective Reporting and Treatment (ALERT) utilization review program.
All outpatient psychotherapy was subjected to review; by comparison, only a handful of medical and surgical services were. That violated parity laws, the New York Attorney General’s office said in a statement.
“United’s denial of these vital services was both unlawful and dangerous,” New York Attorney General Letitia James said in the statement.
“Protecting access to mental health and substance disorder treatment is a priority for the Department of Labor and something I believe in strongly as a person in long-term recovery,” U.S. Secretary of Labor Marty Walsh said in another statement.
Mr. Walsh added that the settlement “provides compensation for many people who were denied full benefits and equitable treatment.”
‘Investigations change behavior’
Commenting for this news organization, Joe Parks, MD, medical director of the National Council for Mental Wellbeing, applauded the settlement.
“These kinds of investigations change behavior, and people get better coverage,” Dr. Parks said.
He also applauded New York, saying that it “has been one of the more assertive states in expecting companies to comply with parity requirements. They should be emulated by other states.”
Dr. Parks noted that a 2019 U.S. Government Accountability Office report showed that only 20 states routinely conducted parity compliance reviews.
A spokesperson for the American Psychiatric Association said it was notable that the Department of Labor focused on three issues that it had not publicly investigated before.
These were payment parity, the use of algorithms in mental health to identify practitioners and patients who use more health care than expected of the “average” person, and requiring disclosure of how the insurer set rates, guidelines, and medical necessity standards.
The American Psychological Association’s chief of psychological practice, Jared L. Skillings, PhD, said the group was gratified that the Department of Labor and New York took complaints seriously and that it “is encouraged that United Behavioral Health and United Healthcare have agreed to change their unfair procedures regarding reimbursement and denial of psychological care.”
The association “will monitor implementation of the settlement with high hopes for fair treatment for patients with mental health needs,” Dr. Skillings said in an interview.
Across-the-board cuts for therapy
The investigation found that United Healthcare reduced the allowable reimbursement amount for all nonphysicians across the board for psychotherapy – by 25% for PhD-level psychologists and by 35% for all MA-level therapists.
The company’s ALERT system used arbitrary thresholds to trigger utilization review, which “often led to denials of coverage when providers could not justify continued treatment after 20 sessions,” said the New York attorney general.
Beneficiaries had to figure out how to pay for continuing services or abruptly end necessary treatment.
United Healthcare agreed to stop reducing reimbursement and to not employ a similar policy in New York for at least 2 years. It will stop using ALERT and stop issuing denials for psychotherapy through at least 2023.
In addition, “We are committed to ensuring all our members have access to care and to reimbursing providers consistent with the terms of the member’s health plan and state and federal rules,” United Healthcare said.
Dr. Parks noted that United Healthcare had stopped using ALERT long before the settlement and was instead employing Level of Care Utilization System for Psychiatric and Addiction Services (LOCUS) and American Society of Addiction Medicine criteria, which he called “a good step forward.”
‘Meaningful’ payout
United Healthcare will pay $14.3 million in restitution to consumers, including $9 million to 20,000 New Yorkers who received denials of or reductions in reimbursement. The company will also pay more than $2 million in penalties, with $1.3 million going to New York.
The insurer has annual revenues of some $250 billion.
“It’s not a large amount of money in United’s overall business operation,” said Dr. Parks.
However, “It certainly could be a meaningful amount of money for the individuals that didn’t get coverage that paid out of pocket,” he added.
A version of this article first appeared on Medscape.com.
The nation’s largest health care insurer has agreed to pay $16 million in restitution and penalties to consumers, the federal government, and the state of New York to settle complaints that it illegally limited outpatient psychotherapy.
The settlement resolves a joint U.S. Department of Labor and New York Attorney General investigation and lawsuit that found that United Healthcare and United Behavioral Health (UBH) had, since at least 2013, violated the federal Mental Health Parity and Addiction Equity Act of 2008.
United Healthcare and UBH are fighting similar claims in Wit v United Behavioral Health, which is currently on appeal at the U.S. Court of Appeals for the Ninth Circuit. If that complaint is decided against UBH, the insurer might have to reprocess more than 67,000 claims.
The joint New York–federal investigation determined that United Healthcare reduced reimbursement rates for out-of-network mental health services and denied payment for many services using its Algorithms for Effective Reporting and Treatment (ALERT) utilization review program.
All outpatient psychotherapy was subjected to review; by comparison, only a handful of medical and surgical services were. That violated parity laws, the New York Attorney General’s office said in a statement.
“United’s denial of these vital services was both unlawful and dangerous,” New York Attorney General Letitia James said in the statement.
“Protecting access to mental health and substance disorder treatment is a priority for the Department of Labor and something I believe in strongly as a person in long-term recovery,” U.S. Secretary of Labor Marty Walsh said in another statement.
Mr. Walsh added that the settlement “provides compensation for many people who were denied full benefits and equitable treatment.”
‘Investigations change behavior’
Commenting for this news organization, Joe Parks, MD, medical director of the National Council for Mental Wellbeing, applauded the settlement.
“These kinds of investigations change behavior, and people get better coverage,” Dr. Parks said.
He also applauded New York, saying that it “has been one of the more assertive states in expecting companies to comply with parity requirements. They should be emulated by other states.”
Dr. Parks noted that a 2019 U.S. Government Accountability Office report showed that only 20 states routinely conducted parity compliance reviews.
A spokesperson for the American Psychiatric Association said it was notable that the Department of Labor focused on three issues that it had not publicly investigated before.
These were payment parity, the use of algorithms in mental health to identify practitioners and patients who use more health care than expected of the “average” person, and requiring disclosure of how the insurer set rates, guidelines, and medical necessity standards.
The American Psychological Association’s chief of psychological practice, Jared L. Skillings, PhD, said the group was gratified that the Department of Labor and New York took complaints seriously and that it “is encouraged that United Behavioral Health and United Healthcare have agreed to change their unfair procedures regarding reimbursement and denial of psychological care.”
The association “will monitor implementation of the settlement with high hopes for fair treatment for patients with mental health needs,” Dr. Skillings said in an interview.
Across-the-board cuts for therapy
The investigation found that United Healthcare reduced the allowable reimbursement amount for all nonphysicians across the board for psychotherapy – by 25% for PhD-level psychologists and by 35% for all MA-level therapists.
The company’s ALERT system used arbitrary thresholds to trigger utilization review, which “often led to denials of coverage when providers could not justify continued treatment after 20 sessions,” said the New York attorney general.
Beneficiaries had to figure out how to pay for continuing services or abruptly end necessary treatment.
United Healthcare agreed to stop reducing reimbursement and to not employ a similar policy in New York for at least 2 years. It will stop using ALERT and stop issuing denials for psychotherapy through at least 2023.
In addition, “We are committed to ensuring all our members have access to care and to reimbursing providers consistent with the terms of the member’s health plan and state and federal rules,” United Healthcare said.
Dr. Parks noted that United Healthcare had stopped using ALERT long before the settlement and was instead employing Level of Care Utilization System for Psychiatric and Addiction Services (LOCUS) and American Society of Addiction Medicine criteria, which he called “a good step forward.”
‘Meaningful’ payout
United Healthcare will pay $14.3 million in restitution to consumers, including $9 million to 20,000 New Yorkers who received denials of or reductions in reimbursement. The company will also pay more than $2 million in penalties, with $1.3 million going to New York.
The insurer has annual revenues of some $250 billion.
“It’s not a large amount of money in United’s overall business operation,” said Dr. Parks.
However, “It certainly could be a meaningful amount of money for the individuals that didn’t get coverage that paid out of pocket,” he added.
A version of this article first appeared on Medscape.com.