User login
Any level of physical activity tied to better later-life memory
new research suggests.
A prospective study of 1,400 participants showed that those who exercised to any extent in adulthood had significantly better cognitive scores later in life, compared with their peers who were physically inactive.
Maintaining an exercise routine throughout adulthood showed the strongest link to subsequent mental acuity.
Although these associations lessened when investigators controlled for childhood cognitive ability, socioeconomic background, and education, they remained statistically significant.
“Our findings support recommendations for greater participation in physical activity across adulthood,” lead investigator Sarah-Naomi James, PhD, research fellow at the Medical Research Council Unit for Lifelong Health and Ageing at the University College London, told this news organization.
“We provide evidence to encourage inactive adults to be active even to a small extent … at any point during adulthood,” which can improve cognition and memory later in life, Dr. James said.
The findings were published online in the Journal of Neurology, Neurosurgery & Psychiatry.
Exercise timing
Previous studies have established a link between fitness training and cognitive benefit later in life, but the researchers wanted to explore whether the timing or type of exercise influenced cognitive outcomes in later life.
The investigators asked more than 1,400 participants in the 1946 British birth cohort how much they had exercised at ages 36, 43, 60, and 69 years.
The questions changed slightly for each assessment period, but in general, participants were asked whether in the past month they had exercised or participated in such activities as badminton, swimming, fitness exercises, yoga, dancing, football, mountain climbing, jogging, or brisk walks for 30 minutes or more; and if so, how many times they participated per month.
Prior research showed that when the participants were aged 60 years, the most commonly reported activities were walking (71%), swimming (33%), floor exercises (24%), and cycling (15%).
When they turned 69, researchers tested participants’ cognitive performance using the Addenbrooke’s Cognitive Examination–III, which measures attention and orientation, verbal fluency, memory, language, and visuospatial function. In this study sample, 53% were women, and all were White.
Physical activity levels were classified as inactive, moderately active (one to four times per month), and most active (five or more times per month). In addition, they were summed across all five assessments to create a total score ranging from 0 (inactive at all ages) to 5 (active at all ages).
Overall, 11% of participants were physically inactive at all five time points; 17% were active at one time point; 20% were active at two and three time points; 17% were active at four time points; and 15% were active at all five time points.
‘Cradle to grave’ study?
Results showed that being physically active at all study time points was significantly associated with higher cognitive performance, verbal memory, and processing speed when participants were aged 69 (P < .01).
Those who exercised to any extent in adulthood – even just once a month during one of the time periods, fared better cognitively in later life, compared with physically inactive participants. (P < .01).
Study limitations cited include a lack of diversity among participants and a disproportionately high attrition rate among those who were socially disadvantaged.
“Our findings show that being active during every decade from their 30s on was associated with better cognition at around 70. Indeed, those who were active for longer had the highest cognitive function,” Dr. James said.
“However, it is also never too late to start. People in our study who only started being active in their 50s or 60s still had higher cognitive scores at age 70, compared to people of the same age who had never been active,” she added.
Dr. James intends to continue following the study sample to determine whether physical activity is linked to preserved cognitive aging “and buffers the effects of cognitive deterioration in the presence of disease markers that cause dementia, ultimately delaying dementia onset.
“We hope the cohort we study will be the first ‘cradle to grave’ study in the world, where we have followed people for their entire lives,” she said.
Encouraging finding
In a comment, Joel Hughes, PhD, professor of psychology and director of clinical training at Kent (Ohio) State University, said the study contributes to the idea that “accumulation of physical activity over one’s lifetime fits the data better than a ‘sensitive period’ – which suggests that it’s never too late to start exercising.”
Dr. Hughes, who was not involved in the research, noted that “exercise can improve cerebral blood flow and hemodynamic function, as well as greater activation of relevant brain regions such as the frontal lobes.”
While observing that the effects of exercise on cognition are likely complex from a mechanistic point of view, the finding that “exercise preserves or improves cognition later in life is encouraging,” he said.
The study received funding from the UK Medical Research Council and Alzheimer’s Research UK. The investigators and Dr. Hughes report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
A prospective study of 1,400 participants showed that those who exercised to any extent in adulthood had significantly better cognitive scores later in life, compared with their peers who were physically inactive.
Maintaining an exercise routine throughout adulthood showed the strongest link to subsequent mental acuity.
Although these associations lessened when investigators controlled for childhood cognitive ability, socioeconomic background, and education, they remained statistically significant.
“Our findings support recommendations for greater participation in physical activity across adulthood,” lead investigator Sarah-Naomi James, PhD, research fellow at the Medical Research Council Unit for Lifelong Health and Ageing at the University College London, told this news organization.
“We provide evidence to encourage inactive adults to be active even to a small extent … at any point during adulthood,” which can improve cognition and memory later in life, Dr. James said.
The findings were published online in the Journal of Neurology, Neurosurgery & Psychiatry.
Exercise timing
Previous studies have established a link between fitness training and cognitive benefit later in life, but the researchers wanted to explore whether the timing or type of exercise influenced cognitive outcomes in later life.
The investigators asked more than 1,400 participants in the 1946 British birth cohort how much they had exercised at ages 36, 43, 60, and 69 years.
The questions changed slightly for each assessment period, but in general, participants were asked whether in the past month they had exercised or participated in such activities as badminton, swimming, fitness exercises, yoga, dancing, football, mountain climbing, jogging, or brisk walks for 30 minutes or more; and if so, how many times they participated per month.
Prior research showed that when the participants were aged 60 years, the most commonly reported activities were walking (71%), swimming (33%), floor exercises (24%), and cycling (15%).
When they turned 69, researchers tested participants’ cognitive performance using the Addenbrooke’s Cognitive Examination–III, which measures attention and orientation, verbal fluency, memory, language, and visuospatial function. In this study sample, 53% were women, and all were White.
Physical activity levels were classified as inactive, moderately active (one to four times per month), and most active (five or more times per month). In addition, they were summed across all five assessments to create a total score ranging from 0 (inactive at all ages) to 5 (active at all ages).
Overall, 11% of participants were physically inactive at all five time points; 17% were active at one time point; 20% were active at two and three time points; 17% were active at four time points; and 15% were active at all five time points.
‘Cradle to grave’ study?
Results showed that being physically active at all study time points was significantly associated with higher cognitive performance, verbal memory, and processing speed when participants were aged 69 (P < .01).
Those who exercised to any extent in adulthood – even just once a month during one of the time periods, fared better cognitively in later life, compared with physically inactive participants. (P < .01).
Study limitations cited include a lack of diversity among participants and a disproportionately high attrition rate among those who were socially disadvantaged.
“Our findings show that being active during every decade from their 30s on was associated with better cognition at around 70. Indeed, those who were active for longer had the highest cognitive function,” Dr. James said.
“However, it is also never too late to start. People in our study who only started being active in their 50s or 60s still had higher cognitive scores at age 70, compared to people of the same age who had never been active,” she added.
Dr. James intends to continue following the study sample to determine whether physical activity is linked to preserved cognitive aging “and buffers the effects of cognitive deterioration in the presence of disease markers that cause dementia, ultimately delaying dementia onset.
“We hope the cohort we study will be the first ‘cradle to grave’ study in the world, where we have followed people for their entire lives,” she said.
Encouraging finding
In a comment, Joel Hughes, PhD, professor of psychology and director of clinical training at Kent (Ohio) State University, said the study contributes to the idea that “accumulation of physical activity over one’s lifetime fits the data better than a ‘sensitive period’ – which suggests that it’s never too late to start exercising.”
Dr. Hughes, who was not involved in the research, noted that “exercise can improve cerebral blood flow and hemodynamic function, as well as greater activation of relevant brain regions such as the frontal lobes.”
While observing that the effects of exercise on cognition are likely complex from a mechanistic point of view, the finding that “exercise preserves or improves cognition later in life is encouraging,” he said.
The study received funding from the UK Medical Research Council and Alzheimer’s Research UK. The investigators and Dr. Hughes report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
A prospective study of 1,400 participants showed that those who exercised to any extent in adulthood had significantly better cognitive scores later in life, compared with their peers who were physically inactive.
Maintaining an exercise routine throughout adulthood showed the strongest link to subsequent mental acuity.
Although these associations lessened when investigators controlled for childhood cognitive ability, socioeconomic background, and education, they remained statistically significant.
“Our findings support recommendations for greater participation in physical activity across adulthood,” lead investigator Sarah-Naomi James, PhD, research fellow at the Medical Research Council Unit for Lifelong Health and Ageing at the University College London, told this news organization.
“We provide evidence to encourage inactive adults to be active even to a small extent … at any point during adulthood,” which can improve cognition and memory later in life, Dr. James said.
The findings were published online in the Journal of Neurology, Neurosurgery & Psychiatry.
Exercise timing
Previous studies have established a link between fitness training and cognitive benefit later in life, but the researchers wanted to explore whether the timing or type of exercise influenced cognitive outcomes in later life.
The investigators asked more than 1,400 participants in the 1946 British birth cohort how much they had exercised at ages 36, 43, 60, and 69 years.
The questions changed slightly for each assessment period, but in general, participants were asked whether in the past month they had exercised or participated in such activities as badminton, swimming, fitness exercises, yoga, dancing, football, mountain climbing, jogging, or brisk walks for 30 minutes or more; and if so, how many times they participated per month.
Prior research showed that when the participants were aged 60 years, the most commonly reported activities were walking (71%), swimming (33%), floor exercises (24%), and cycling (15%).
When they turned 69, researchers tested participants’ cognitive performance using the Addenbrooke’s Cognitive Examination–III, which measures attention and orientation, verbal fluency, memory, language, and visuospatial function. In this study sample, 53% were women, and all were White.
Physical activity levels were classified as inactive, moderately active (one to four times per month), and most active (five or more times per month). In addition, they were summed across all five assessments to create a total score ranging from 0 (inactive at all ages) to 5 (active at all ages).
Overall, 11% of participants were physically inactive at all five time points; 17% were active at one time point; 20% were active at two and three time points; 17% were active at four time points; and 15% were active at all five time points.
‘Cradle to grave’ study?
Results showed that being physically active at all study time points was significantly associated with higher cognitive performance, verbal memory, and processing speed when participants were aged 69 (P < .01).
Those who exercised to any extent in adulthood – even just once a month during one of the time periods, fared better cognitively in later life, compared with physically inactive participants. (P < .01).
Study limitations cited include a lack of diversity among participants and a disproportionately high attrition rate among those who were socially disadvantaged.
“Our findings show that being active during every decade from their 30s on was associated with better cognition at around 70. Indeed, those who were active for longer had the highest cognitive function,” Dr. James said.
“However, it is also never too late to start. People in our study who only started being active in their 50s or 60s still had higher cognitive scores at age 70, compared to people of the same age who had never been active,” she added.
Dr. James intends to continue following the study sample to determine whether physical activity is linked to preserved cognitive aging “and buffers the effects of cognitive deterioration in the presence of disease markers that cause dementia, ultimately delaying dementia onset.
“We hope the cohort we study will be the first ‘cradle to grave’ study in the world, where we have followed people for their entire lives,” she said.
Encouraging finding
In a comment, Joel Hughes, PhD, professor of psychology and director of clinical training at Kent (Ohio) State University, said the study contributes to the idea that “accumulation of physical activity over one’s lifetime fits the data better than a ‘sensitive period’ – which suggests that it’s never too late to start exercising.”
Dr. Hughes, who was not involved in the research, noted that “exercise can improve cerebral blood flow and hemodynamic function, as well as greater activation of relevant brain regions such as the frontal lobes.”
While observing that the effects of exercise on cognition are likely complex from a mechanistic point of view, the finding that “exercise preserves or improves cognition later in life is encouraging,” he said.
The study received funding from the UK Medical Research Council and Alzheimer’s Research UK. The investigators and Dr. Hughes report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF NEUROLOGY, NEUROSURGERY & PSYCHIATRY
Alzheimer’s disease: What is ‘clinically meaningful’?
A recent report in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association suggested that, at least for now, we need to lower the bar in Alzheimer’s disease drug trials.
Their point is that there’s no consensus on “clinically meaningful benefit.” Does it mean a complete cure for Alzheimer’s disease, with reversal of deficits? Or stopping disease progression where it is? Or just slowing things down enough that it means something to patients, family members, and caregivers?
The last one is, realistically, where we are now.
The problem with this is that many nonmedical people equate “treatment” with “cure,” which isn’t close to the truth for many diseases. In Alzheimer’s disease, it’s even trickier to figure out. There’s a disparity between imaging (which suggests something that should be quite effective) and clinical results (which aren’t nearly as impressive as the PET scans).
So when I prescribe any of the Alzheimer’s medications, I make it pretty clear to patients, and more importantly the patient’s family, what they can and can’t expect. This isn’t easy, because most will come back a month later, tell me their loved one is no better, and want to try something else. So I have to explain it again. These people aren’t stupid. They’re hopeful, and also facing an impossible question. “Better” is a lot easier to judge than “slowed progression.”
“Better” is a great word for migraines. Or seizures. Or Parkinson’s disease. These are condition where patients and families can tell us whether they’ve seen an improvement.
But with the current treatments for Alzheimer’s disease we’re asking patients and families “do you think you’ve gotten any worse than you would have if you hadn’t taken the drug at all?”
That’s an impossible question to answer, unless you’re following people with objective cognitive data over time and comparing them against a placebo group, which is how these drugs got here in the first place – we know they do that.
But to a family watching their loved ones go downhill, such reassurances aren’t what they want to hear.
Regrettably, it’s where things stand. While I want to strive for absolute success in these things, today it’s simply not possible. Maybe it never will be, though I hope it is.
But, for now, I agree that we need to reframe what we’re going to consider clinically meaningful. Sometimes you have to settle for a flight of stairs instead of an elevator, but still hope that you’ll get to the top. It just takes longer, and it’s better than not going anywhere at all.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A recent report in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association suggested that, at least for now, we need to lower the bar in Alzheimer’s disease drug trials.
Their point is that there’s no consensus on “clinically meaningful benefit.” Does it mean a complete cure for Alzheimer’s disease, with reversal of deficits? Or stopping disease progression where it is? Or just slowing things down enough that it means something to patients, family members, and caregivers?
The last one is, realistically, where we are now.
The problem with this is that many nonmedical people equate “treatment” with “cure,” which isn’t close to the truth for many diseases. In Alzheimer’s disease, it’s even trickier to figure out. There’s a disparity between imaging (which suggests something that should be quite effective) and clinical results (which aren’t nearly as impressive as the PET scans).
So when I prescribe any of the Alzheimer’s medications, I make it pretty clear to patients, and more importantly the patient’s family, what they can and can’t expect. This isn’t easy, because most will come back a month later, tell me their loved one is no better, and want to try something else. So I have to explain it again. These people aren’t stupid. They’re hopeful, and also facing an impossible question. “Better” is a lot easier to judge than “slowed progression.”
“Better” is a great word for migraines. Or seizures. Or Parkinson’s disease. These are condition where patients and families can tell us whether they’ve seen an improvement.
But with the current treatments for Alzheimer’s disease we’re asking patients and families “do you think you’ve gotten any worse than you would have if you hadn’t taken the drug at all?”
That’s an impossible question to answer, unless you’re following people with objective cognitive data over time and comparing them against a placebo group, which is how these drugs got here in the first place – we know they do that.
But to a family watching their loved ones go downhill, such reassurances aren’t what they want to hear.
Regrettably, it’s where things stand. While I want to strive for absolute success in these things, today it’s simply not possible. Maybe it never will be, though I hope it is.
But, for now, I agree that we need to reframe what we’re going to consider clinically meaningful. Sometimes you have to settle for a flight of stairs instead of an elevator, but still hope that you’ll get to the top. It just takes longer, and it’s better than not going anywhere at all.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A recent report in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association suggested that, at least for now, we need to lower the bar in Alzheimer’s disease drug trials.
Their point is that there’s no consensus on “clinically meaningful benefit.” Does it mean a complete cure for Alzheimer’s disease, with reversal of deficits? Or stopping disease progression where it is? Or just slowing things down enough that it means something to patients, family members, and caregivers?
The last one is, realistically, where we are now.
The problem with this is that many nonmedical people equate “treatment” with “cure,” which isn’t close to the truth for many diseases. In Alzheimer’s disease, it’s even trickier to figure out. There’s a disparity between imaging (which suggests something that should be quite effective) and clinical results (which aren’t nearly as impressive as the PET scans).
So when I prescribe any of the Alzheimer’s medications, I make it pretty clear to patients, and more importantly the patient’s family, what they can and can’t expect. This isn’t easy, because most will come back a month later, tell me their loved one is no better, and want to try something else. So I have to explain it again. These people aren’t stupid. They’re hopeful, and also facing an impossible question. “Better” is a lot easier to judge than “slowed progression.”
“Better” is a great word for migraines. Or seizures. Or Parkinson’s disease. These are condition where patients and families can tell us whether they’ve seen an improvement.
But with the current treatments for Alzheimer’s disease we’re asking patients and families “do you think you’ve gotten any worse than you would have if you hadn’t taken the drug at all?”
That’s an impossible question to answer, unless you’re following people with objective cognitive data over time and comparing them against a placebo group, which is how these drugs got here in the first place – we know they do that.
But to a family watching their loved ones go downhill, such reassurances aren’t what they want to hear.
Regrettably, it’s where things stand. While I want to strive for absolute success in these things, today it’s simply not possible. Maybe it never will be, though I hope it is.
But, for now, I agree that we need to reframe what we’re going to consider clinically meaningful. Sometimes you have to settle for a flight of stairs instead of an elevator, but still hope that you’ll get to the top. It just takes longer, and it’s better than not going anywhere at all.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Higher dementia risk in women explained?
a study suggests.
Prior research has found a higher lifetime dementia risk in women, and one explanation cited has been that women tend to live longer than men.
However, this new analysis of data from nearly 30,000 people in 18 countries found almost no evidence of sex differences in most known risk factors for dementia, including age.
The risk of dementia among women was significantly higher in poorer countries, pointing to economic disadvantages as a possible explanation.
“In general, we found that the greater dementia risk found in women compared to men was more pronounced in poorer countries, which points to the need for greater efforts to narrow the gaps in health disparities between women and men in these countries,” lead investigator Jessica Gong, MSc, a doctoral student at the George Institute for Global Health, Newtown, Australia, told this news organization. “It is likely that socioeconomic factors are potentially more important than biological factors when assessing dementia risk.”
The findings were published online in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association.
Global data
Most previous studies that examined sex differences in dementia risk were conducted in high-income countries, Ms. Gong noted, leaving a gap in the literature on risk in low- and middle-income countries.
To address this issue, researchers conducted an individual participant meta-analysis of 21 studies from the Cohort Studies of Memory in an International Consortium. Data analysis included information on 29,850 people from 18 countries on six continents. None of the participants had dementia at baseline, and the average age was 71.6 years.
Over a median of 4.6 years, incident dementia was reported in 2,089 people, 66% of whom were women.
Overall, women had higher dementia risk (hazard ratio, 1.12; 95% confidence interval, 1.02-1.23) than men, but the rates were highest in low- to middle-income economies (HR, 1.73; P = .03).
Dementia risk in women was higher than in men in 14 countries. Risk was highest in Nigeria, where dementia risk was more than double in women (aHR, 2.11; 95% CI, 1.46-3.04), and lowest in Brazil, where risk was 46% lower in women than in men (aHR, 0.54; 95% CI, 0.29-1.00).
In the United States, dementia risk was 7% higher in women than men (aHR, 1.07; 0.73-1.57).
Similar risk factors
In both women and men, older age, diabetes, depression, hearing impairment, and apo E–epsilon 4 carriage were associated with a greater risk of dementia, and more years of education, higher hip circumference, current alcohol use (vs. never), and high physical activity (vs. none to minimal) were associated with a lower risk of dementia.
Among all these risk factors, sex differences were only significant for longer education and former alcohol use, with both demonstrating a stronger association in men than women.
Global dementia rates are expected to triple over the next 25 years unless steps are taken to reduce risk factors. A 2020 report found that dementia risk could be reduced by addressing 12 modifiable risk factors, including obesity, air pollution, diabetes, social isolation, and hypertension. All of these risk factors are more common in low- to middle-income countries, Ms. Gong noted.
“These findings justify ongoing efforts to support programs to improve sex and gender equity in brain health, particularly in underrepresented and underserved populations, in turn to narrow the gaps within and between country,” Ms. Gong said.
Understanding the puzzle
Commenting on the findings for Medscape Medical News, Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, said the findings add to the body of work about sex differences in dementia risk.
“This is an interesting study looking at risk factors for dementia and suggests that, while some risk factors are more pronounced in men than in women, women may be more at risk of progressing to dementia,” Dr. Snyder said. “The findings outline the importance of understanding how the underlying biology, particularly biology that differs in males and females, may be contributing to risk.”
Data on the country and geographical variations highlighted in the study also point to a potential risk influencer, she said.
“Studying geography-specific risk factors is important because it helps us understand the ‘why’ behind geographic differences in dementia risk,” Dr. Snyder said. “This type of collaboration among countries and researchers is essential for us to understand these puzzle pieces.”
Funding for the study was provided by the U.K. Medical Research Council Skills Development Fellowship, Australian National Health and Medical Research Council Investigator Grant, National Institute on Aging, among others. See the original article for full funding sources. Ms. Gong reported no relevant financial conflicts. Dr. Snyder is employed by the Alzheimer’s Association.
A version of this article originally appeared on Medscape.com.
a study suggests.
Prior research has found a higher lifetime dementia risk in women, and one explanation cited has been that women tend to live longer than men.
However, this new analysis of data from nearly 30,000 people in 18 countries found almost no evidence of sex differences in most known risk factors for dementia, including age.
The risk of dementia among women was significantly higher in poorer countries, pointing to economic disadvantages as a possible explanation.
“In general, we found that the greater dementia risk found in women compared to men was more pronounced in poorer countries, which points to the need for greater efforts to narrow the gaps in health disparities between women and men in these countries,” lead investigator Jessica Gong, MSc, a doctoral student at the George Institute for Global Health, Newtown, Australia, told this news organization. “It is likely that socioeconomic factors are potentially more important than biological factors when assessing dementia risk.”
The findings were published online in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association.
Global data
Most previous studies that examined sex differences in dementia risk were conducted in high-income countries, Ms. Gong noted, leaving a gap in the literature on risk in low- and middle-income countries.
To address this issue, researchers conducted an individual participant meta-analysis of 21 studies from the Cohort Studies of Memory in an International Consortium. Data analysis included information on 29,850 people from 18 countries on six continents. None of the participants had dementia at baseline, and the average age was 71.6 years.
Over a median of 4.6 years, incident dementia was reported in 2,089 people, 66% of whom were women.
Overall, women had higher dementia risk (hazard ratio, 1.12; 95% confidence interval, 1.02-1.23) than men, but the rates were highest in low- to middle-income economies (HR, 1.73; P = .03).
Dementia risk in women was higher than in men in 14 countries. Risk was highest in Nigeria, where dementia risk was more than double in women (aHR, 2.11; 95% CI, 1.46-3.04), and lowest in Brazil, where risk was 46% lower in women than in men (aHR, 0.54; 95% CI, 0.29-1.00).
In the United States, dementia risk was 7% higher in women than men (aHR, 1.07; 0.73-1.57).
Similar risk factors
In both women and men, older age, diabetes, depression, hearing impairment, and apo E–epsilon 4 carriage were associated with a greater risk of dementia, and more years of education, higher hip circumference, current alcohol use (vs. never), and high physical activity (vs. none to minimal) were associated with a lower risk of dementia.
Among all these risk factors, sex differences were only significant for longer education and former alcohol use, with both demonstrating a stronger association in men than women.
Global dementia rates are expected to triple over the next 25 years unless steps are taken to reduce risk factors. A 2020 report found that dementia risk could be reduced by addressing 12 modifiable risk factors, including obesity, air pollution, diabetes, social isolation, and hypertension. All of these risk factors are more common in low- to middle-income countries, Ms. Gong noted.
“These findings justify ongoing efforts to support programs to improve sex and gender equity in brain health, particularly in underrepresented and underserved populations, in turn to narrow the gaps within and between country,” Ms. Gong said.
Understanding the puzzle
Commenting on the findings for Medscape Medical News, Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, said the findings add to the body of work about sex differences in dementia risk.
“This is an interesting study looking at risk factors for dementia and suggests that, while some risk factors are more pronounced in men than in women, women may be more at risk of progressing to dementia,” Dr. Snyder said. “The findings outline the importance of understanding how the underlying biology, particularly biology that differs in males and females, may be contributing to risk.”
Data on the country and geographical variations highlighted in the study also point to a potential risk influencer, she said.
“Studying geography-specific risk factors is important because it helps us understand the ‘why’ behind geographic differences in dementia risk,” Dr. Snyder said. “This type of collaboration among countries and researchers is essential for us to understand these puzzle pieces.”
Funding for the study was provided by the U.K. Medical Research Council Skills Development Fellowship, Australian National Health and Medical Research Council Investigator Grant, National Institute on Aging, among others. See the original article for full funding sources. Ms. Gong reported no relevant financial conflicts. Dr. Snyder is employed by the Alzheimer’s Association.
A version of this article originally appeared on Medscape.com.
a study suggests.
Prior research has found a higher lifetime dementia risk in women, and one explanation cited has been that women tend to live longer than men.
However, this new analysis of data from nearly 30,000 people in 18 countries found almost no evidence of sex differences in most known risk factors for dementia, including age.
The risk of dementia among women was significantly higher in poorer countries, pointing to economic disadvantages as a possible explanation.
“In general, we found that the greater dementia risk found in women compared to men was more pronounced in poorer countries, which points to the need for greater efforts to narrow the gaps in health disparities between women and men in these countries,” lead investigator Jessica Gong, MSc, a doctoral student at the George Institute for Global Health, Newtown, Australia, told this news organization. “It is likely that socioeconomic factors are potentially more important than biological factors when assessing dementia risk.”
The findings were published online in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association.
Global data
Most previous studies that examined sex differences in dementia risk were conducted in high-income countries, Ms. Gong noted, leaving a gap in the literature on risk in low- and middle-income countries.
To address this issue, researchers conducted an individual participant meta-analysis of 21 studies from the Cohort Studies of Memory in an International Consortium. Data analysis included information on 29,850 people from 18 countries on six continents. None of the participants had dementia at baseline, and the average age was 71.6 years.
Over a median of 4.6 years, incident dementia was reported in 2,089 people, 66% of whom were women.
Overall, women had higher dementia risk (hazard ratio, 1.12; 95% confidence interval, 1.02-1.23) than men, but the rates were highest in low- to middle-income economies (HR, 1.73; P = .03).
Dementia risk in women was higher than in men in 14 countries. Risk was highest in Nigeria, where dementia risk was more than double in women (aHR, 2.11; 95% CI, 1.46-3.04), and lowest in Brazil, where risk was 46% lower in women than in men (aHR, 0.54; 95% CI, 0.29-1.00).
In the United States, dementia risk was 7% higher in women than men (aHR, 1.07; 0.73-1.57).
Similar risk factors
In both women and men, older age, diabetes, depression, hearing impairment, and apo E–epsilon 4 carriage were associated with a greater risk of dementia, and more years of education, higher hip circumference, current alcohol use (vs. never), and high physical activity (vs. none to minimal) were associated with a lower risk of dementia.
Among all these risk factors, sex differences were only significant for longer education and former alcohol use, with both demonstrating a stronger association in men than women.
Global dementia rates are expected to triple over the next 25 years unless steps are taken to reduce risk factors. A 2020 report found that dementia risk could be reduced by addressing 12 modifiable risk factors, including obesity, air pollution, diabetes, social isolation, and hypertension. All of these risk factors are more common in low- to middle-income countries, Ms. Gong noted.
“These findings justify ongoing efforts to support programs to improve sex and gender equity in brain health, particularly in underrepresented and underserved populations, in turn to narrow the gaps within and between country,” Ms. Gong said.
Understanding the puzzle
Commenting on the findings for Medscape Medical News, Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, said the findings add to the body of work about sex differences in dementia risk.
“This is an interesting study looking at risk factors for dementia and suggests that, while some risk factors are more pronounced in men than in women, women may be more at risk of progressing to dementia,” Dr. Snyder said. “The findings outline the importance of understanding how the underlying biology, particularly biology that differs in males and females, may be contributing to risk.”
Data on the country and geographical variations highlighted in the study also point to a potential risk influencer, she said.
“Studying geography-specific risk factors is important because it helps us understand the ‘why’ behind geographic differences in dementia risk,” Dr. Snyder said. “This type of collaboration among countries and researchers is essential for us to understand these puzzle pieces.”
Funding for the study was provided by the U.K. Medical Research Council Skills Development Fellowship, Australian National Health and Medical Research Council Investigator Grant, National Institute on Aging, among others. See the original article for full funding sources. Ms. Gong reported no relevant financial conflicts. Dr. Snyder is employed by the Alzheimer’s Association.
A version of this article originally appeared on Medscape.com.
FROM ALZHEIMER’S & DEMENTIA
Regular laxative use tied to increased dementia risk
Among more than 500,000 middle-aged or older adults in the UK Biobank, those who reported regular laxative use had a 51% increased risk of dementia due to any cause, compared with their counterparts who did not regularly use laxatives.
Individuals who used only osmotic laxatives had a 64% increased risk, compared with peers who did not use laxatives, while those using one or more types of laxatives, including bulk-forming, stool-softening, or stimulating laxatives, had a 90% increased risk.
“Constipation and laxative use are common among middle-aged and older adults,” study investigator Feng Sha, PhD, with the Chinese Academy of Sciences in Guangdong, China, said in a news release.
“However, regular laxative use may change the microbiome of the gut, possibly affecting nerve signaling from the gut to the brain or increasing the production of intestinal toxins that may affect the brain,” Dr. Sha noted.
The study was published online in Neurology.
Robust link
The findings are based on 502,229 people (54% women; mean age, 57 at baseline) from the UK biobank database. All were dementia-free at baseline.
A total of 18,235 participants (3.6%) said they used over-the-counter laxatives regularly, which was defined as using them most days of the week during the month before the study.
Over an average of 9.8 years, dementia was recorded in 218 (1.3%) of those who regularly used laxatives and in 1,969 (0.4%) of those did not.
After adjusting for factors such as age, sex, education, other illnesses, medication use, and a family history of dementia, regular use of laxatives was significantly associated with increased risk of all-cause dementia (adjusted hazard ratio, 1.51; 95% confidence interval, 1.30-1.75) and vascular dementia (aHR, 1.65; 95% CI, 1.21-2.27), with no significant association observed for Alzheimer’s disease (aHR, 1.05; 95% CI, 0.79-1.40).
The risk of dementia also increased with the number of laxative types used. All-cause dementia risk increased by 28% (aHR, 1.28; 95% CI, 1.03-1.61) for those using a single laxative type and by 90% (aHR, 1.90; 95% CI, 1.20-3.01) for those using two or more types, compared with nonuse.
Among those who reported using only one type of laxative, only those using osmotic laxatives had a statistically significant higher risk of all-cause dementia (aHR, 1.64; 95% CI, 1.20-2.24) and vascular dementia (aHR, 1.97; 95% CI, 1.04-3.75).
“These results remained robust in various subgroup and sensitivity analyses,” the investigators report.
They caution that they had no data on laxative dosage and so they were unable to explore the relationship between various laxative dosages and dementia risk.
Interpret with caution
Commenting on the findings for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, said the results are “interesting and demonstrate an association between laxative use and later life risk of dementia.”
However, “there is no proven causation, and there are some caveats,” Dr. Snyder said. “It’s unclear what may be driving this association, though other lines of research have suggested a linkage between our overall gut health, our immune system, and our brain health.”
Dr. Snyder said it’s also worth noting that the data came from the UK Biobank, which, “while a wealth of information for research purposes, is not representative of other countries. More research is needed.”
The Alzheimer’s Association is leading a 2-year clinical trial, U.S. Pointer, to examine the impact of behavioral interventions on the gut-brain axis to “better understand how our gut health may affect our brains,” Dr. Snyder told this news organization.
“While we await the results of that study, people should talk to their doctor about the risks and benefits of laxatives for their health, as well as discuss alternative methods of alleviating constipation, such as increasing dietary fiber and drinking more water,” she advised.
The study was funded by the National Natural Science Foundation of China, Shenzhen Science and Technology Program, and the Chinese Academy of Sciences. The authors and Dr. Snyder have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Among more than 500,000 middle-aged or older adults in the UK Biobank, those who reported regular laxative use had a 51% increased risk of dementia due to any cause, compared with their counterparts who did not regularly use laxatives.
Individuals who used only osmotic laxatives had a 64% increased risk, compared with peers who did not use laxatives, while those using one or more types of laxatives, including bulk-forming, stool-softening, or stimulating laxatives, had a 90% increased risk.
“Constipation and laxative use are common among middle-aged and older adults,” study investigator Feng Sha, PhD, with the Chinese Academy of Sciences in Guangdong, China, said in a news release.
“However, regular laxative use may change the microbiome of the gut, possibly affecting nerve signaling from the gut to the brain or increasing the production of intestinal toxins that may affect the brain,” Dr. Sha noted.
The study was published online in Neurology.
Robust link
The findings are based on 502,229 people (54% women; mean age, 57 at baseline) from the UK biobank database. All were dementia-free at baseline.
A total of 18,235 participants (3.6%) said they used over-the-counter laxatives regularly, which was defined as using them most days of the week during the month before the study.
Over an average of 9.8 years, dementia was recorded in 218 (1.3%) of those who regularly used laxatives and in 1,969 (0.4%) of those did not.
After adjusting for factors such as age, sex, education, other illnesses, medication use, and a family history of dementia, regular use of laxatives was significantly associated with increased risk of all-cause dementia (adjusted hazard ratio, 1.51; 95% confidence interval, 1.30-1.75) and vascular dementia (aHR, 1.65; 95% CI, 1.21-2.27), with no significant association observed for Alzheimer’s disease (aHR, 1.05; 95% CI, 0.79-1.40).
The risk of dementia also increased with the number of laxative types used. All-cause dementia risk increased by 28% (aHR, 1.28; 95% CI, 1.03-1.61) for those using a single laxative type and by 90% (aHR, 1.90; 95% CI, 1.20-3.01) for those using two or more types, compared with nonuse.
Among those who reported using only one type of laxative, only those using osmotic laxatives had a statistically significant higher risk of all-cause dementia (aHR, 1.64; 95% CI, 1.20-2.24) and vascular dementia (aHR, 1.97; 95% CI, 1.04-3.75).
“These results remained robust in various subgroup and sensitivity analyses,” the investigators report.
They caution that they had no data on laxative dosage and so they were unable to explore the relationship between various laxative dosages and dementia risk.
Interpret with caution
Commenting on the findings for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, said the results are “interesting and demonstrate an association between laxative use and later life risk of dementia.”
However, “there is no proven causation, and there are some caveats,” Dr. Snyder said. “It’s unclear what may be driving this association, though other lines of research have suggested a linkage between our overall gut health, our immune system, and our brain health.”
Dr. Snyder said it’s also worth noting that the data came from the UK Biobank, which, “while a wealth of information for research purposes, is not representative of other countries. More research is needed.”
The Alzheimer’s Association is leading a 2-year clinical trial, U.S. Pointer, to examine the impact of behavioral interventions on the gut-brain axis to “better understand how our gut health may affect our brains,” Dr. Snyder told this news organization.
“While we await the results of that study, people should talk to their doctor about the risks and benefits of laxatives for their health, as well as discuss alternative methods of alleviating constipation, such as increasing dietary fiber and drinking more water,” she advised.
The study was funded by the National Natural Science Foundation of China, Shenzhen Science and Technology Program, and the Chinese Academy of Sciences. The authors and Dr. Snyder have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Among more than 500,000 middle-aged or older adults in the UK Biobank, those who reported regular laxative use had a 51% increased risk of dementia due to any cause, compared with their counterparts who did not regularly use laxatives.
Individuals who used only osmotic laxatives had a 64% increased risk, compared with peers who did not use laxatives, while those using one or more types of laxatives, including bulk-forming, stool-softening, or stimulating laxatives, had a 90% increased risk.
“Constipation and laxative use are common among middle-aged and older adults,” study investigator Feng Sha, PhD, with the Chinese Academy of Sciences in Guangdong, China, said in a news release.
“However, regular laxative use may change the microbiome of the gut, possibly affecting nerve signaling from the gut to the brain or increasing the production of intestinal toxins that may affect the brain,” Dr. Sha noted.
The study was published online in Neurology.
Robust link
The findings are based on 502,229 people (54% women; mean age, 57 at baseline) from the UK biobank database. All were dementia-free at baseline.
A total of 18,235 participants (3.6%) said they used over-the-counter laxatives regularly, which was defined as using them most days of the week during the month before the study.
Over an average of 9.8 years, dementia was recorded in 218 (1.3%) of those who regularly used laxatives and in 1,969 (0.4%) of those did not.
After adjusting for factors such as age, sex, education, other illnesses, medication use, and a family history of dementia, regular use of laxatives was significantly associated with increased risk of all-cause dementia (adjusted hazard ratio, 1.51; 95% confidence interval, 1.30-1.75) and vascular dementia (aHR, 1.65; 95% CI, 1.21-2.27), with no significant association observed for Alzheimer’s disease (aHR, 1.05; 95% CI, 0.79-1.40).
The risk of dementia also increased with the number of laxative types used. All-cause dementia risk increased by 28% (aHR, 1.28; 95% CI, 1.03-1.61) for those using a single laxative type and by 90% (aHR, 1.90; 95% CI, 1.20-3.01) for those using two or more types, compared with nonuse.
Among those who reported using only one type of laxative, only those using osmotic laxatives had a statistically significant higher risk of all-cause dementia (aHR, 1.64; 95% CI, 1.20-2.24) and vascular dementia (aHR, 1.97; 95% CI, 1.04-3.75).
“These results remained robust in various subgroup and sensitivity analyses,” the investigators report.
They caution that they had no data on laxative dosage and so they were unable to explore the relationship between various laxative dosages and dementia risk.
Interpret with caution
Commenting on the findings for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, said the results are “interesting and demonstrate an association between laxative use and later life risk of dementia.”
However, “there is no proven causation, and there are some caveats,” Dr. Snyder said. “It’s unclear what may be driving this association, though other lines of research have suggested a linkage between our overall gut health, our immune system, and our brain health.”
Dr. Snyder said it’s also worth noting that the data came from the UK Biobank, which, “while a wealth of information for research purposes, is not representative of other countries. More research is needed.”
The Alzheimer’s Association is leading a 2-year clinical trial, U.S. Pointer, to examine the impact of behavioral interventions on the gut-brain axis to “better understand how our gut health may affect our brains,” Dr. Snyder told this news organization.
“While we await the results of that study, people should talk to their doctor about the risks and benefits of laxatives for their health, as well as discuss alternative methods of alleviating constipation, such as increasing dietary fiber and drinking more water,” she advised.
The study was funded by the National Natural Science Foundation of China, Shenzhen Science and Technology Program, and the Chinese Academy of Sciences. The authors and Dr. Snyder have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM NEUROLOGY
Decrease in cognitive functioning
The history and findings in this case are suggestive of late-onset Alzheimer's disease (AD).
AD is a neurodegenerative disease associated with progressive impairment of behavioral and cognitive functions, including memory, comprehension, language, attention, reasoning, and judgment. At least two thirds of cases of dementia in people ≥ 65 years of age are due to AD, making it the most common type of dementia. At present, there is no cure for AD, which is associated with a long preclinical stage and a progressive disease course. In the United States, AD is the sixth leading cause of death.
Individuals with AD develop amyloid plaques in the hippocampus and in other areas of the cerebral cortex. The symptoms of AD vary depending on the stage of the disease; however, in most patients with late-onset AD (≥ 65 years of age), the most common presenting symptom is episodic short-term memory loss, with relative sparing of long-term memory. Subsequently, patients may experience impairments in problem-solving, judgment, executive functioning, motivation, and organization. It is not uncommon for individuals with AD to lack insight into the impairments they are experiences, or even to deny deficits.
Neuropsychiatric symptoms, such as apathy, social withdrawal, disinhibition, agitation, psychosis, and wandering are common in the mid- to late stages of the disease. Patients may also experience difficulty performing learned motor tasks (dyspraxia), olfactory dysfunction, and sleep disturbances; develop extrapyramidal motor signs (eg, dystonia, akathisia, and parkinsonian symptoms) followed by difficulties with primitive reflexes and incontinence, and may ultimately become totally dependent on caregivers.
A thorough history and physical examination are essential for the diagnosis of AD. Because some patients may lack insight into their disease, it is vital to elicit a history from the patient's family and caregivers as well. Onset and early symptoms are important to note to aid in differentiating AD from other types of dementia. In most patients with late-onset AD, comprehensive clinical assessment can provide reasonable diagnostic certainty. This should include a detailed neurologic examination to rule out other conditions; most patients with AD will have a normal neurologic exam.
A mental status examination to evaluate concentration, attention, recent and remote memory, language, visuospatial functioning, praxis, and executive functioning should also be conducted. Brief standard examinations, such the Mini-Mental State Examination, can be used for initial screening purposes, although they are less sensitive and specific than more comprehensive tests. Follow-up visits for patients diagnosed with AD should therefore include a full mental status examination to gauge disease progression as well as the development of neuropsychiatric symptoms.
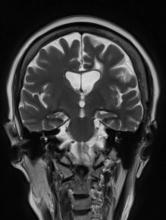
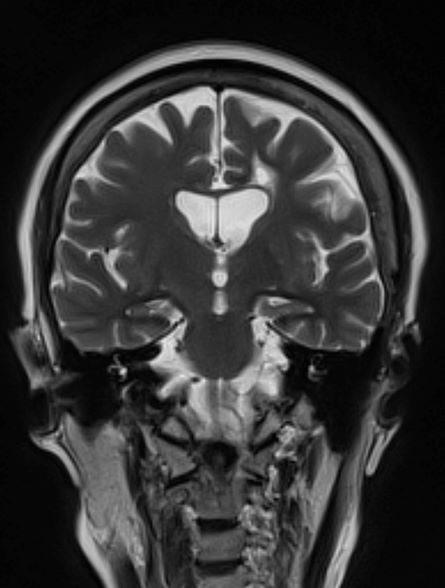
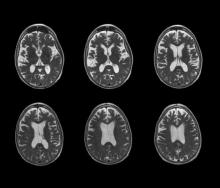
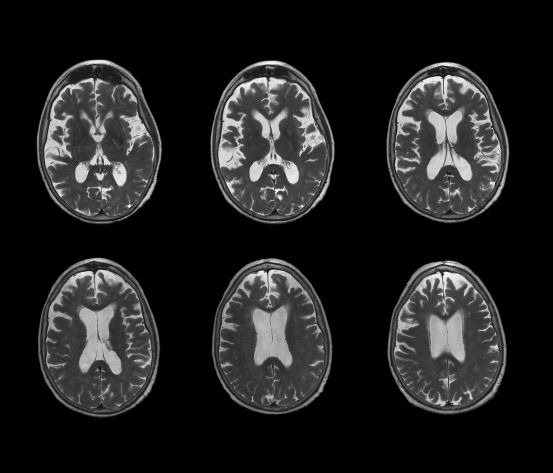
Brain imaging can be beneficial both for diagnosing AD and monitoring the disease's clinical course. MRI or CT of the brain can help eliminate alternate causes of dementia, such as stroke or tumors, from consideration. Dilated lateral ventricles and widened cortical sulci, particularly in the temporal area, are typical findings in AD.
The standard medical treatment for AD includes cholinesterase inhibitors (ChEIs) and a partial N-methyl-D-aspartate (NMDA) antagonist. Both US and European guidelines list ChEIs (donepezil, rivastigmine, galantamine, tacrine) as first-line pharmacotherapies for mild to moderate AD; however, these agents only show modest efficacy on cognitive deficits and nonsignificant efficacy on functional capacity in mild to moderate AD. Memantine, a partial NMDA antagonist, shows very limited efficacy on cognitive symptoms, with no improvement in functional domains. Newly approved anti-amyloid therapies include aducanumab, a first-in-class amyloid beta–directed antibody that was approved in 2021, and lecanemab, another amyloid beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Psychotropic agents may help to mitigate the secondary symptoms of AD, such as depression, agitation, aggression, hallucinations, delusions, and sleep disorders. Behavioral interventions (eg, patient-centered approaches and caregiver training) may be beneficial for managing the cognitive and behavioral manifestations of AD and are often combined with pharmacologic interventions (eg, anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, antidepressants or mood stabilizers for mood disorders). Regular physical activity and exercise also be beneficial for brain health and delaying disease progression.
Numerous novel agents are under investigation for AD, including anti-tau therapy, anti-neuroinflammatory therapy, neuroprotective agents (such as NMDA receptor modulators), and brain stimulation.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of late-onset Alzheimer's disease (AD).
AD is a neurodegenerative disease associated with progressive impairment of behavioral and cognitive functions, including memory, comprehension, language, attention, reasoning, and judgment. At least two thirds of cases of dementia in people ≥ 65 years of age are due to AD, making it the most common type of dementia. At present, there is no cure for AD, which is associated with a long preclinical stage and a progressive disease course. In the United States, AD is the sixth leading cause of death.
Individuals with AD develop amyloid plaques in the hippocampus and in other areas of the cerebral cortex. The symptoms of AD vary depending on the stage of the disease; however, in most patients with late-onset AD (≥ 65 years of age), the most common presenting symptom is episodic short-term memory loss, with relative sparing of long-term memory. Subsequently, patients may experience impairments in problem-solving, judgment, executive functioning, motivation, and organization. It is not uncommon for individuals with AD to lack insight into the impairments they are experiences, or even to deny deficits.
Neuropsychiatric symptoms, such as apathy, social withdrawal, disinhibition, agitation, psychosis, and wandering are common in the mid- to late stages of the disease. Patients may also experience difficulty performing learned motor tasks (dyspraxia), olfactory dysfunction, and sleep disturbances; develop extrapyramidal motor signs (eg, dystonia, akathisia, and parkinsonian symptoms) followed by difficulties with primitive reflexes and incontinence, and may ultimately become totally dependent on caregivers.
A thorough history and physical examination are essential for the diagnosis of AD. Because some patients may lack insight into their disease, it is vital to elicit a history from the patient's family and caregivers as well. Onset and early symptoms are important to note to aid in differentiating AD from other types of dementia. In most patients with late-onset AD, comprehensive clinical assessment can provide reasonable diagnostic certainty. This should include a detailed neurologic examination to rule out other conditions; most patients with AD will have a normal neurologic exam.
A mental status examination to evaluate concentration, attention, recent and remote memory, language, visuospatial functioning, praxis, and executive functioning should also be conducted. Brief standard examinations, such the Mini-Mental State Examination, can be used for initial screening purposes, although they are less sensitive and specific than more comprehensive tests. Follow-up visits for patients diagnosed with AD should therefore include a full mental status examination to gauge disease progression as well as the development of neuropsychiatric symptoms.
Brain imaging can be beneficial both for diagnosing AD and monitoring the disease's clinical course. MRI or CT of the brain can help eliminate alternate causes of dementia, such as stroke or tumors, from consideration. Dilated lateral ventricles and widened cortical sulci, particularly in the temporal area, are typical findings in AD.
The standard medical treatment for AD includes cholinesterase inhibitors (ChEIs) and a partial N-methyl-D-aspartate (NMDA) antagonist. Both US and European guidelines list ChEIs (donepezil, rivastigmine, galantamine, tacrine) as first-line pharmacotherapies for mild to moderate AD; however, these agents only show modest efficacy on cognitive deficits and nonsignificant efficacy on functional capacity in mild to moderate AD. Memantine, a partial NMDA antagonist, shows very limited efficacy on cognitive symptoms, with no improvement in functional domains. Newly approved anti-amyloid therapies include aducanumab, a first-in-class amyloid beta–directed antibody that was approved in 2021, and lecanemab, another amyloid beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Psychotropic agents may help to mitigate the secondary symptoms of AD, such as depression, agitation, aggression, hallucinations, delusions, and sleep disorders. Behavioral interventions (eg, patient-centered approaches and caregiver training) may be beneficial for managing the cognitive and behavioral manifestations of AD and are often combined with pharmacologic interventions (eg, anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, antidepressants or mood stabilizers for mood disorders). Regular physical activity and exercise also be beneficial for brain health and delaying disease progression.
Numerous novel agents are under investigation for AD, including anti-tau therapy, anti-neuroinflammatory therapy, neuroprotective agents (such as NMDA receptor modulators), and brain stimulation.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of late-onset Alzheimer's disease (AD).
AD is a neurodegenerative disease associated with progressive impairment of behavioral and cognitive functions, including memory, comprehension, language, attention, reasoning, and judgment. At least two thirds of cases of dementia in people ≥ 65 years of age are due to AD, making it the most common type of dementia. At present, there is no cure for AD, which is associated with a long preclinical stage and a progressive disease course. In the United States, AD is the sixth leading cause of death.
Individuals with AD develop amyloid plaques in the hippocampus and in other areas of the cerebral cortex. The symptoms of AD vary depending on the stage of the disease; however, in most patients with late-onset AD (≥ 65 years of age), the most common presenting symptom is episodic short-term memory loss, with relative sparing of long-term memory. Subsequently, patients may experience impairments in problem-solving, judgment, executive functioning, motivation, and organization. It is not uncommon for individuals with AD to lack insight into the impairments they are experiences, or even to deny deficits.
Neuropsychiatric symptoms, such as apathy, social withdrawal, disinhibition, agitation, psychosis, and wandering are common in the mid- to late stages of the disease. Patients may also experience difficulty performing learned motor tasks (dyspraxia), olfactory dysfunction, and sleep disturbances; develop extrapyramidal motor signs (eg, dystonia, akathisia, and parkinsonian symptoms) followed by difficulties with primitive reflexes and incontinence, and may ultimately become totally dependent on caregivers.
A thorough history and physical examination are essential for the diagnosis of AD. Because some patients may lack insight into their disease, it is vital to elicit a history from the patient's family and caregivers as well. Onset and early symptoms are important to note to aid in differentiating AD from other types of dementia. In most patients with late-onset AD, comprehensive clinical assessment can provide reasonable diagnostic certainty. This should include a detailed neurologic examination to rule out other conditions; most patients with AD will have a normal neurologic exam.
A mental status examination to evaluate concentration, attention, recent and remote memory, language, visuospatial functioning, praxis, and executive functioning should also be conducted. Brief standard examinations, such the Mini-Mental State Examination, can be used for initial screening purposes, although they are less sensitive and specific than more comprehensive tests. Follow-up visits for patients diagnosed with AD should therefore include a full mental status examination to gauge disease progression as well as the development of neuropsychiatric symptoms.
Brain imaging can be beneficial both for diagnosing AD and monitoring the disease's clinical course. MRI or CT of the brain can help eliminate alternate causes of dementia, such as stroke or tumors, from consideration. Dilated lateral ventricles and widened cortical sulci, particularly in the temporal area, are typical findings in AD.
The standard medical treatment for AD includes cholinesterase inhibitors (ChEIs) and a partial N-methyl-D-aspartate (NMDA) antagonist. Both US and European guidelines list ChEIs (donepezil, rivastigmine, galantamine, tacrine) as first-line pharmacotherapies for mild to moderate AD; however, these agents only show modest efficacy on cognitive deficits and nonsignificant efficacy on functional capacity in mild to moderate AD. Memantine, a partial NMDA antagonist, shows very limited efficacy on cognitive symptoms, with no improvement in functional domains. Newly approved anti-amyloid therapies include aducanumab, a first-in-class amyloid beta–directed antibody that was approved in 2021, and lecanemab, another amyloid beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Psychotropic agents may help to mitigate the secondary symptoms of AD, such as depression, agitation, aggression, hallucinations, delusions, and sleep disorders. Behavioral interventions (eg, patient-centered approaches and caregiver training) may be beneficial for managing the cognitive and behavioral manifestations of AD and are often combined with pharmacologic interventions (eg, anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, antidepressants or mood stabilizers for mood disorders). Regular physical activity and exercise also be beneficial for brain health and delaying disease progression.
Numerous novel agents are under investigation for AD, including anti-tau therapy, anti-neuroinflammatory therapy, neuroprotective agents (such as NMDA receptor modulators), and brain stimulation.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 79-year-old man presents to his primary care provider (PCP) for an annual examination. The patient is accompanied by his oldest daughter, with whom he has lived since the death of his spouse approximately 9 months earlier. During the examination, the patient's daughter expresses concern about her father's cognitive functioning. Specifically, she has observed him becoming increasingly forgetful since he moved in with her. She states he has repeatedly forgotten the names of her dogs and has forgotten food in the microwave or on the stove on several occasions. Recently, after leaving a restaurant, her father was unable to remember where he had parked his car, and she suspects he has gotten lost while driving to and from familiar places several times. When questioned, the patient denies impairment and states occasional memory loss is "just part of the aging process."
Neither the patient nor his daughter reports any difficulties with his ability to groom and dress himself. His medical history is notable for high cholesterol, which is managed with a statin. The patient is a former smoker (24 pack-years) and occasionally drinks alcohol. His current height and weight are 5 ft 11 in and 177 lb, respectively.
The patient appears well nourished and oriented to time and place, although he appears to have moderate difficulty hearing and questions sometimes need to be repeated to him. His blood pressure, pulse oximetry, and heart rate are within normal ranges. Laboratory tests are all within normal ranges. The patient scores 16 on the Mini-Mental State Examination. His PCP orders MRI, which reveals atrophy on both hippocampi.
Difficulty remembering words
The history and findings in this case are suggestive of early-onset Alzheimer's disease (AD) with aphasia.
AD is a neurodegenerative disorder characterized by cognitive and behavioral impairment that significantly interferes with a patient's social and occupational functioning. There is currently no cure for AD, which has a long preclinical period and a progressive course. Individuals with AD develop amyloid plaques in the hippocampus, a structure deep in the brain that helps to encode memories, and in other areas of the cerebral cortex that are involved in thinking and making decisions.
Patients with AD typically present with insidiously progressive memory loss; over the course of several years, other areas of cognition are impaired. Subsequent to memory loss, patients may also experience language disorders (eg, anomic aphasia or anomia) and impairment in visuospatial skills and executive functions. In many patients, slowly progressive behavioral changes are also observed.
AD is most prevalent in individuals older than 65 years; however, early‐onset AD (in individuals aged 60 years or older) can also occur. Early-onset AD shares the same essential neuropathological characteristics (ie, amyloid plaques and neurofibrillary tangles) as late-onset (65 years or older) AD, but it differs in several ways. For example, memory loss is an extremely common presenting symptom in late-onset AD, whereas nonamnestic presentation (ie, language, visuospatial, or executive impairment) is very rare, occurring in only about 5% of cases. Conversely, nonamnestic presentations may occur in 30%-40% of patients with early-onset AD. Frequent nonamnestic cognitive manifestations in patients with early-onset AD are those seen in mild to moderate AD, including visual agnosia (55.1%), aphasia (57.9%), and behavioral changes (61.7%). In addition, several studies have suggested that early-onset AD may have a more aggressive course than late-onset AD does, including faster cognitive and functional decline.
Presently, only symptomatic therapies are available for AD. The standard medical treatment for AD includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Newly approved antiamyloid therapies are also available for patients with mild cognitive impairment or mild dementia. These include aducanumab, a first-in-class amyloid beta–directed antibody that was approved in 2021, and lecanemab, another amyloid beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Psychotropic agents may be used to treat the secondary symptoms of AD (eg, depression, agitation, aggression, hallucinations, delusions, sleep disorders), which can be problematic. Behavioral interventions ranging from patient-centered approaches to caregiver training may also be used to help manage cognitive and behavioral manifestations of AD, often in combination with pharmacologic interventions, such as anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, and antidepressants or mood stabilizers for mood disorders and specific manifestations (eg, episodes of anger or rage). Routine physical activity and exercise may affect AD progression and may possibly exert a protective effect on brain health.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of early-onset Alzheimer's disease (AD) with aphasia.
AD is a neurodegenerative disorder characterized by cognitive and behavioral impairment that significantly interferes with a patient's social and occupational functioning. There is currently no cure for AD, which has a long preclinical period and a progressive course. Individuals with AD develop amyloid plaques in the hippocampus, a structure deep in the brain that helps to encode memories, and in other areas of the cerebral cortex that are involved in thinking and making decisions.
Patients with AD typically present with insidiously progressive memory loss; over the course of several years, other areas of cognition are impaired. Subsequent to memory loss, patients may also experience language disorders (eg, anomic aphasia or anomia) and impairment in visuospatial skills and executive functions. In many patients, slowly progressive behavioral changes are also observed.
AD is most prevalent in individuals older than 65 years; however, early‐onset AD (in individuals aged 60 years or older) can also occur. Early-onset AD shares the same essential neuropathological characteristics (ie, amyloid plaques and neurofibrillary tangles) as late-onset (65 years or older) AD, but it differs in several ways. For example, memory loss is an extremely common presenting symptom in late-onset AD, whereas nonamnestic presentation (ie, language, visuospatial, or executive impairment) is very rare, occurring in only about 5% of cases. Conversely, nonamnestic presentations may occur in 30%-40% of patients with early-onset AD. Frequent nonamnestic cognitive manifestations in patients with early-onset AD are those seen in mild to moderate AD, including visual agnosia (55.1%), aphasia (57.9%), and behavioral changes (61.7%). In addition, several studies have suggested that early-onset AD may have a more aggressive course than late-onset AD does, including faster cognitive and functional decline.
Presently, only symptomatic therapies are available for AD. The standard medical treatment for AD includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Newly approved antiamyloid therapies are also available for patients with mild cognitive impairment or mild dementia. These include aducanumab, a first-in-class amyloid beta–directed antibody that was approved in 2021, and lecanemab, another amyloid beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Psychotropic agents may be used to treat the secondary symptoms of AD (eg, depression, agitation, aggression, hallucinations, delusions, sleep disorders), which can be problematic. Behavioral interventions ranging from patient-centered approaches to caregiver training may also be used to help manage cognitive and behavioral manifestations of AD, often in combination with pharmacologic interventions, such as anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, and antidepressants or mood stabilizers for mood disorders and specific manifestations (eg, episodes of anger or rage). Routine physical activity and exercise may affect AD progression and may possibly exert a protective effect on brain health.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of early-onset Alzheimer's disease (AD) with aphasia.
AD is a neurodegenerative disorder characterized by cognitive and behavioral impairment that significantly interferes with a patient's social and occupational functioning. There is currently no cure for AD, which has a long preclinical period and a progressive course. Individuals with AD develop amyloid plaques in the hippocampus, a structure deep in the brain that helps to encode memories, and in other areas of the cerebral cortex that are involved in thinking and making decisions.
Patients with AD typically present with insidiously progressive memory loss; over the course of several years, other areas of cognition are impaired. Subsequent to memory loss, patients may also experience language disorders (eg, anomic aphasia or anomia) and impairment in visuospatial skills and executive functions. In many patients, slowly progressive behavioral changes are also observed.
AD is most prevalent in individuals older than 65 years; however, early‐onset AD (in individuals aged 60 years or older) can also occur. Early-onset AD shares the same essential neuropathological characteristics (ie, amyloid plaques and neurofibrillary tangles) as late-onset (65 years or older) AD, but it differs in several ways. For example, memory loss is an extremely common presenting symptom in late-onset AD, whereas nonamnestic presentation (ie, language, visuospatial, or executive impairment) is very rare, occurring in only about 5% of cases. Conversely, nonamnestic presentations may occur in 30%-40% of patients with early-onset AD. Frequent nonamnestic cognitive manifestations in patients with early-onset AD are those seen in mild to moderate AD, including visual agnosia (55.1%), aphasia (57.9%), and behavioral changes (61.7%). In addition, several studies have suggested that early-onset AD may have a more aggressive course than late-onset AD does, including faster cognitive and functional decline.
Presently, only symptomatic therapies are available for AD. The standard medical treatment for AD includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Newly approved antiamyloid therapies are also available for patients with mild cognitive impairment or mild dementia. These include aducanumab, a first-in-class amyloid beta–directed antibody that was approved in 2021, and lecanemab, another amyloid beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Psychotropic agents may be used to treat the secondary symptoms of AD (eg, depression, agitation, aggression, hallucinations, delusions, sleep disorders), which can be problematic. Behavioral interventions ranging from patient-centered approaches to caregiver training may also be used to help manage cognitive and behavioral manifestations of AD, often in combination with pharmacologic interventions, such as anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, and antidepressants or mood stabilizers for mood disorders and specific manifestations (eg, episodes of anger or rage). Routine physical activity and exercise may affect AD progression and may possibly exert a protective effect on brain health.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 53-year-old woman, who is a high school mathematics teacher, presents with reports of progressively increasing cognitive impairments. Specifically, she notes increasing difficulty with remembering words as well as challenges with her executive functioning. She was recently reprimanded by her principal for missing several mandatory staff meetings and deadlines for submitting student grades. The patient states her symptoms began approximately 2 years ago. She initially attributed them to hormonal changes because of menopause but is becoming concerned about the impact they are having on her ability to function. She recently began experiencing difficulties with spatial perception, which resulted in her falling down the stairs of her home and spraining an ankle. The patient lives alone and has no children. Her medical history is unremarkable except for a motor vehicle accident 5 years earlier that resulted in her sustaining a concussion and a fractured wrist. She does not currently take any medications. There is no history of tobacco use or excessive alcohol consumption. Her current height and weight are 5 ft 3 in and 147 lb, respectively.
No abnormalities are noted on physical exam; the patient's blood pressure, pulse oximetry, and heart rate are within normal ranges. Laboratory tests are all within normal ranges, including thyroid-stimulating hormone and vitamin B12 levels. The patient scores 16 on the Montreal Cognitive Assessment test. Her clinician orders an MRI, which reveals deep indentations around the front and sides of the brain.
Adult brains contain millions of ‘silent synapses’
according to neuroscientists from the Massachusetts Institute of Technology.
What to know:
- An estimated 30% of all synapses in the brain’s cortex are silent and become active to allow the adult brain to continually form new memories and leave existing conventional synapses unmodified.
- Silent synapses are looking for new connections, and when important new information is presented, connections between the relevant neurons are strengthened to allow the brain to remember new things.
- Using the silent synapses for the new memories does not overwrite the important memories stored in more mature synapses, which are harder to change.
- The brain’s neurons display a wide range of plasticity mechanisms that account for how brains can efficiently learn new things and retain them in long-term memory.
- Flexibility of synapses is critical for acquiring new information, and stability is required to retain important information, enabling one to more easily adjust and change behaviors and habits or incorporate new information.
This is a summary of the article, “Filopodia Are a Structural Substrate for Silent Synapses in Adult Neocortex,” published in Nature Nov. 30, 2022. The full article can be found at nature.com .
A version of this article first appeared on Medscape.com.
according to neuroscientists from the Massachusetts Institute of Technology.
What to know:
- An estimated 30% of all synapses in the brain’s cortex are silent and become active to allow the adult brain to continually form new memories and leave existing conventional synapses unmodified.
- Silent synapses are looking for new connections, and when important new information is presented, connections between the relevant neurons are strengthened to allow the brain to remember new things.
- Using the silent synapses for the new memories does not overwrite the important memories stored in more mature synapses, which are harder to change.
- The brain’s neurons display a wide range of plasticity mechanisms that account for how brains can efficiently learn new things and retain them in long-term memory.
- Flexibility of synapses is critical for acquiring new information, and stability is required to retain important information, enabling one to more easily adjust and change behaviors and habits or incorporate new information.
This is a summary of the article, “Filopodia Are a Structural Substrate for Silent Synapses in Adult Neocortex,” published in Nature Nov. 30, 2022. The full article can be found at nature.com .
A version of this article first appeared on Medscape.com.
according to neuroscientists from the Massachusetts Institute of Technology.
What to know:
- An estimated 30% of all synapses in the brain’s cortex are silent and become active to allow the adult brain to continually form new memories and leave existing conventional synapses unmodified.
- Silent synapses are looking for new connections, and when important new information is presented, connections between the relevant neurons are strengthened to allow the brain to remember new things.
- Using the silent synapses for the new memories does not overwrite the important memories stored in more mature synapses, which are harder to change.
- The brain’s neurons display a wide range of plasticity mechanisms that account for how brains can efficiently learn new things and retain them in long-term memory.
- Flexibility of synapses is critical for acquiring new information, and stability is required to retain important information, enabling one to more easily adjust and change behaviors and habits or incorporate new information.
This is a summary of the article, “Filopodia Are a Structural Substrate for Silent Synapses in Adult Neocortex,” published in Nature Nov. 30, 2022. The full article can be found at nature.com .
A version of this article first appeared on Medscape.com.
Alzheimer’s Disease Pathophysiology
Alzheimer’s Disease Overview
Diabetes drug tied to lower dementia risk
new research suggests.
Overall, in a large cohort study from South Korea, patients who took pioglitazone were 16% less likely to develop dementia over an average of 10 years than peers who did not take the drug.
However, the dementia risk reduction was 54% among those with ischemic heart disease and 43% among those with a history of stroke.
“Our study was to see the association between pioglitazone use and incidence of dementia, not how (with what mechanisms) this drug can suppress dementia pathology,” coinvestigator Eosu Kim, MD, PhD, Yonsei University, Seoul, South Korea, said in an interview.
However, “as we found this drug is more effective in diabetic patients who have blood circulation problems in the heart or brain than in those without such problems, we speculate that pioglitazone’s antidementia action may be related to improving blood vessel’s health,” Dr. Kim said.
This finding suggests that pioglitazone could be used as a personalized treatment approach for dementia prevention in this subgroup of patients with diabetes, the researchers noted.
The results were published online in Neurology.
Dose-response relationship
Risk for dementia is doubled in adults with T2DM, the investigators wrote. Prior studies have suggested that pioglitazone may protect against dementia, as well as a first or recurrent stroke, in patients with T2DM.
This led Dr. Kim and colleagues to examine the effects of pioglitazone on dementia risk overall and in relation to stroke and ischemic heart disease.
Using the national Korean health database, the researchers identified 91,218 adults aged 50 and older with new-onset T2DM who did not have dementia. A total of 3,467 were treated with pioglitazone.
Pioglitazone exposure was defined as a total cumulative daily dose of 90 or more calculated from all dispensations during 4 years after T2DM diagnosis, with outcomes assessed after this period.
Over an average of 10 years, 8.3% of pioglitazone users developed dementia, compared with 10.0% of nonusers.
There was a statistically significant 16% lower risk for developing all-cause dementia among pioglitazone users than among nonusers (adjusted hazard ratio, 0.84; 95% confidence interval, 0.75-0.95).
A dose-response relationship was evident; pioglitazone users who received the highest cumulative daily dose were at lower risk for dementia (aHR, 0.72; 95% CI, 0.55-0.94).
Several limitations
The reduced risk for dementia was more pronounced among patients who used pioglitazone for 4 years in comparison with patients who did not use the drug (aHR, 0.63; 95% CI, 0.44-0.90).
The apparent protective effect of pioglitazone with regard to dementia was greater among those with a history of ischemic heart disease (aHR, 0.46; 95% CI, 0.24-0.90) or stroke (aHR, 0.57; 95% CI, 0.38-0.86) before diabetes diagnosis.
The incidence of stroke was also reduced with pioglitazone use (aHR, 0.81; 95% CI, 0.66-1.0).
“These results provide valuable information on who could potentially benefit from pioglitazone use for prevention of dementia,” Dr. Kim said in a news release.
However, “the risk and benefit balance of long-term use of this drug to prevent dementia should be prospectively assessed,” he said in an interview.
The researchers cautioned that the study was observational; hence, the reported associations cannot address causal relationships. Also, because of the use of claims data, drug compliance could not be guaranteed, and exposure may have been overestimated.
There is also the potential for selection bias, and no information on apolipoprotein E was available, they noted.
More data needed
In an accompanying editorial, Colleen J. Maxwell, PhD, University of Waterloo (Ont.), and colleagues wrote that the results “not only support previous studies showing the potential cognitive benefit of pioglitazone but also extend our understanding of this benefit through the mediating effect of reducing ischemic stroke.”
However, because of their associated risks, which include fractures, weight gain, heart failure, and bladder cancer, thiazolidinediones are not currently favored in diabetes management guidelines – and their use has significantly declined since the mid to late 2000s, the editorialists noted.
They agreed that it will be important to reassess the risk-benefit profile of pioglitazone in T2DM as additional findings emerge.
They also noted that sodium-glucose cotransporter-2 inhibitors, which have significant cardiovascular and renal benefits and minimal side effects, may also lower the risk for dementia.
“As both pioglitazone and SGLT-2 inhibitors are second-line options for physicians, the current decision would easily be in favor of SGLT-2 inhibitors given their safety profile,” Dr. Maxwell and colleagues wrote.
For now, pioglitazone “should not be used to prevent dementia in patients with T2DM,” they concluded.
The study was supported by grants from the National Research Foundation of Korea funded by the Korean government and the Ministry of Health and Welfare. The investigators and editorialists report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests.
Overall, in a large cohort study from South Korea, patients who took pioglitazone were 16% less likely to develop dementia over an average of 10 years than peers who did not take the drug.
However, the dementia risk reduction was 54% among those with ischemic heart disease and 43% among those with a history of stroke.
“Our study was to see the association between pioglitazone use and incidence of dementia, not how (with what mechanisms) this drug can suppress dementia pathology,” coinvestigator Eosu Kim, MD, PhD, Yonsei University, Seoul, South Korea, said in an interview.
However, “as we found this drug is more effective in diabetic patients who have blood circulation problems in the heart or brain than in those without such problems, we speculate that pioglitazone’s antidementia action may be related to improving blood vessel’s health,” Dr. Kim said.
This finding suggests that pioglitazone could be used as a personalized treatment approach for dementia prevention in this subgroup of patients with diabetes, the researchers noted.
The results were published online in Neurology.
Dose-response relationship
Risk for dementia is doubled in adults with T2DM, the investigators wrote. Prior studies have suggested that pioglitazone may protect against dementia, as well as a first or recurrent stroke, in patients with T2DM.
This led Dr. Kim and colleagues to examine the effects of pioglitazone on dementia risk overall and in relation to stroke and ischemic heart disease.
Using the national Korean health database, the researchers identified 91,218 adults aged 50 and older with new-onset T2DM who did not have dementia. A total of 3,467 were treated with pioglitazone.
Pioglitazone exposure was defined as a total cumulative daily dose of 90 or more calculated from all dispensations during 4 years after T2DM diagnosis, with outcomes assessed after this period.
Over an average of 10 years, 8.3% of pioglitazone users developed dementia, compared with 10.0% of nonusers.
There was a statistically significant 16% lower risk for developing all-cause dementia among pioglitazone users than among nonusers (adjusted hazard ratio, 0.84; 95% confidence interval, 0.75-0.95).
A dose-response relationship was evident; pioglitazone users who received the highest cumulative daily dose were at lower risk for dementia (aHR, 0.72; 95% CI, 0.55-0.94).
Several limitations
The reduced risk for dementia was more pronounced among patients who used pioglitazone for 4 years in comparison with patients who did not use the drug (aHR, 0.63; 95% CI, 0.44-0.90).
The apparent protective effect of pioglitazone with regard to dementia was greater among those with a history of ischemic heart disease (aHR, 0.46; 95% CI, 0.24-0.90) or stroke (aHR, 0.57; 95% CI, 0.38-0.86) before diabetes diagnosis.
The incidence of stroke was also reduced with pioglitazone use (aHR, 0.81; 95% CI, 0.66-1.0).
“These results provide valuable information on who could potentially benefit from pioglitazone use for prevention of dementia,” Dr. Kim said in a news release.
However, “the risk and benefit balance of long-term use of this drug to prevent dementia should be prospectively assessed,” he said in an interview.
The researchers cautioned that the study was observational; hence, the reported associations cannot address causal relationships. Also, because of the use of claims data, drug compliance could not be guaranteed, and exposure may have been overestimated.
There is also the potential for selection bias, and no information on apolipoprotein E was available, they noted.
More data needed
In an accompanying editorial, Colleen J. Maxwell, PhD, University of Waterloo (Ont.), and colleagues wrote that the results “not only support previous studies showing the potential cognitive benefit of pioglitazone but also extend our understanding of this benefit through the mediating effect of reducing ischemic stroke.”
However, because of their associated risks, which include fractures, weight gain, heart failure, and bladder cancer, thiazolidinediones are not currently favored in diabetes management guidelines – and their use has significantly declined since the mid to late 2000s, the editorialists noted.
They agreed that it will be important to reassess the risk-benefit profile of pioglitazone in T2DM as additional findings emerge.
They also noted that sodium-glucose cotransporter-2 inhibitors, which have significant cardiovascular and renal benefits and minimal side effects, may also lower the risk for dementia.
“As both pioglitazone and SGLT-2 inhibitors are second-line options for physicians, the current decision would easily be in favor of SGLT-2 inhibitors given their safety profile,” Dr. Maxwell and colleagues wrote.
For now, pioglitazone “should not be used to prevent dementia in patients with T2DM,” they concluded.
The study was supported by grants from the National Research Foundation of Korea funded by the Korean government and the Ministry of Health and Welfare. The investigators and editorialists report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests.
Overall, in a large cohort study from South Korea, patients who took pioglitazone were 16% less likely to develop dementia over an average of 10 years than peers who did not take the drug.
However, the dementia risk reduction was 54% among those with ischemic heart disease and 43% among those with a history of stroke.
“Our study was to see the association between pioglitazone use and incidence of dementia, not how (with what mechanisms) this drug can suppress dementia pathology,” coinvestigator Eosu Kim, MD, PhD, Yonsei University, Seoul, South Korea, said in an interview.
However, “as we found this drug is more effective in diabetic patients who have blood circulation problems in the heart or brain than in those without such problems, we speculate that pioglitazone’s antidementia action may be related to improving blood vessel’s health,” Dr. Kim said.
This finding suggests that pioglitazone could be used as a personalized treatment approach for dementia prevention in this subgroup of patients with diabetes, the researchers noted.
The results were published online in Neurology.
Dose-response relationship
Risk for dementia is doubled in adults with T2DM, the investigators wrote. Prior studies have suggested that pioglitazone may protect against dementia, as well as a first or recurrent stroke, in patients with T2DM.
This led Dr. Kim and colleagues to examine the effects of pioglitazone on dementia risk overall and in relation to stroke and ischemic heart disease.
Using the national Korean health database, the researchers identified 91,218 adults aged 50 and older with new-onset T2DM who did not have dementia. A total of 3,467 were treated with pioglitazone.
Pioglitazone exposure was defined as a total cumulative daily dose of 90 or more calculated from all dispensations during 4 years after T2DM diagnosis, with outcomes assessed after this period.
Over an average of 10 years, 8.3% of pioglitazone users developed dementia, compared with 10.0% of nonusers.
There was a statistically significant 16% lower risk for developing all-cause dementia among pioglitazone users than among nonusers (adjusted hazard ratio, 0.84; 95% confidence interval, 0.75-0.95).
A dose-response relationship was evident; pioglitazone users who received the highest cumulative daily dose were at lower risk for dementia (aHR, 0.72; 95% CI, 0.55-0.94).
Several limitations
The reduced risk for dementia was more pronounced among patients who used pioglitazone for 4 years in comparison with patients who did not use the drug (aHR, 0.63; 95% CI, 0.44-0.90).
The apparent protective effect of pioglitazone with regard to dementia was greater among those with a history of ischemic heart disease (aHR, 0.46; 95% CI, 0.24-0.90) or stroke (aHR, 0.57; 95% CI, 0.38-0.86) before diabetes diagnosis.
The incidence of stroke was also reduced with pioglitazone use (aHR, 0.81; 95% CI, 0.66-1.0).
“These results provide valuable information on who could potentially benefit from pioglitazone use for prevention of dementia,” Dr. Kim said in a news release.
However, “the risk and benefit balance of long-term use of this drug to prevent dementia should be prospectively assessed,” he said in an interview.
The researchers cautioned that the study was observational; hence, the reported associations cannot address causal relationships. Also, because of the use of claims data, drug compliance could not be guaranteed, and exposure may have been overestimated.
There is also the potential for selection bias, and no information on apolipoprotein E was available, they noted.
More data needed
In an accompanying editorial, Colleen J. Maxwell, PhD, University of Waterloo (Ont.), and colleagues wrote that the results “not only support previous studies showing the potential cognitive benefit of pioglitazone but also extend our understanding of this benefit through the mediating effect of reducing ischemic stroke.”
However, because of their associated risks, which include fractures, weight gain, heart failure, and bladder cancer, thiazolidinediones are not currently favored in diabetes management guidelines – and their use has significantly declined since the mid to late 2000s, the editorialists noted.
They agreed that it will be important to reassess the risk-benefit profile of pioglitazone in T2DM as additional findings emerge.
They also noted that sodium-glucose cotransporter-2 inhibitors, which have significant cardiovascular and renal benefits and minimal side effects, may also lower the risk for dementia.
“As both pioglitazone and SGLT-2 inhibitors are second-line options for physicians, the current decision would easily be in favor of SGLT-2 inhibitors given their safety profile,” Dr. Maxwell and colleagues wrote.
For now, pioglitazone “should not be used to prevent dementia in patients with T2DM,” they concluded.
The study was supported by grants from the National Research Foundation of Korea funded by the Korean government and the Ministry of Health and Welfare. The investigators and editorialists report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM NEUROLOGY