User login
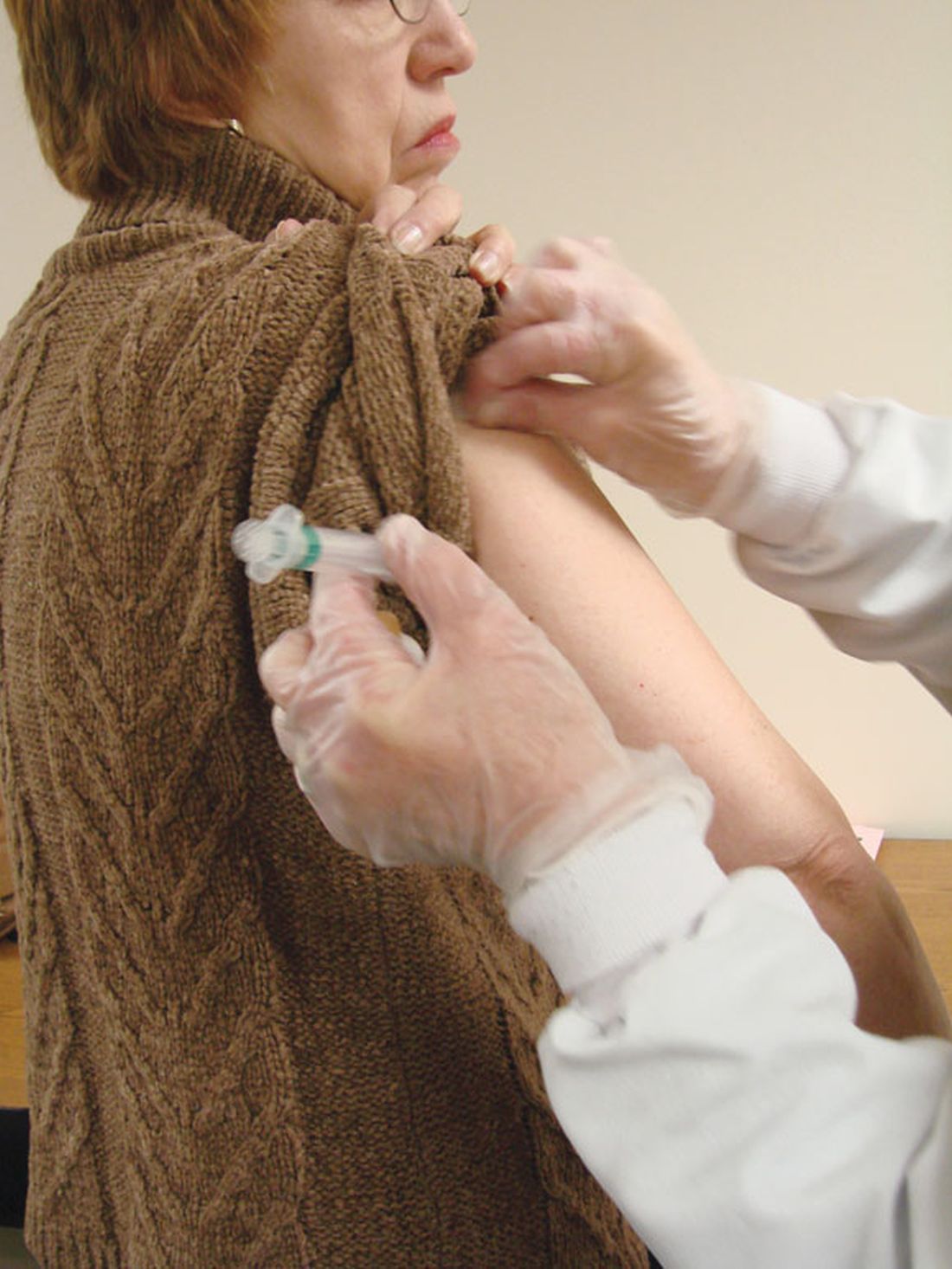
NUDGE-FLU: Electronic ‘nudges’ boost flu shot uptake in seniors
Two types of electronically delivered letter strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination and a repeat reminder letter – increased flu shot uptake, compared with usual care alone, in a national study of seniors in Denmark.
And in a prespecified subanalysis focusing on older adults with cardiovascular disease, these two strategies were also effective in boosting vaccine uptake in those with or without CVD.
The findings are from the Nationwide Utilization of Danish Government Electronic Letter System for Increasing Influenza Vaccine Uptake (NUDGE-FLU) trial, which compared usual care alone with one of nine different electronic letter “behavioral nudge” strategies during the 2022-2023 flu season in people aged 65 years and older.
Niklas Dyrby Johansen, MD, Hospital–Herlev and Gentofte and Copenhagen University, presented the main study findings in a late-breaking clinical trial session at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, and the article was simultaneously published in The Lancet
The subanalysis in patients with CVD was published online March 5 in Circulation.
“Despite modest effect sizes, the results may have important implications when translated to a population level,” Dr. Dyrby Johansen concluded during his presentation. Still, the authors write, “the low-touch (no person-to-person interaction), inexpensive, and highly scalable nature of these electronic letters might have important population-level public health implications.”
They note that, among approximately 63 million Medicare beneficiaries in the United States, a 0.89–percentage point absolute increase in vaccination rate achieved through the most successful electronic letter in NUDGE-FLU, the one highlighting cardiovascular gain, would be expected to lead to 500,000 additional vaccinations and potentially prevent 7,849 illnesses, 4,395 medical visits, 714 hospitalizations, and 66 deaths each year.
Electronic letter systems similar to the one used in this trial are already in place in several European countries, including Sweden, Norway, and Ireland, the researchers note.
In countries such as the United States, where implementing a nationwide government electronic letter system might not be feasible, nudges could be done via email, text message, or other systems, but whether this would be as effective remains to be seen.
Commenting on the findings, David Cho, MD, UCLA Health and chair of the ACC Health Care Innovation Council, commended the researchers on engaging patients with more than a million separate nudges sent out during one flu season, and randomly assigning participants to 10 different types of nudges, calling it “impressive.”
“I think the concept that the nudge is to plant an idea that leads to an action is pretty much the basis of a lot of these health care interventions, which seems like a small way to have a big impact at outcome,” Dr. Cho noted. “The behavioral science aspects of the nudges are also fascinating to me personally, and I think to a lot of the cardiologists in the audience – about how you actually get people to act. I think it’s been a lifelong question for people in general, how do you get people to follow through on an action?”
“So I found the fact that secondary gain from a cardiovascular health standpoint, but also the repeated nudges were sort of simple ways that you could have people take ownership and get their flu vaccination,” he said.
“This is ACC, this is a cardiovascular conference, but the influence of vaccine is not just a primary care problem, it is also directly affecting cardiovascular disease,” Dr. Cho concluded.
‘Small but important effect’
In an accompanying editorial (Lancet. 2023 Mar 5. doi: 10.1016/S0140-6736(23)00453-1), Melissa Stockwell, MD, Columbia University, New York, writes, “The study by Johansen and colleagues highlights the small but still important effect of scalable, digital interventions across an entire at-risk population.”
A difference of 0.89% in the entire study population of over 960,000 adults age 65 years or older would be more than 8,500 additional adults protected, she notes. “That increase is important for a scalable intervention that has a low cost per letter.”
Moreover, “that the cardiovascular gain–framed messages worked best in those who had not been vaccinated in the previous season further highlights the potential impact on a more vaccine-hesitant population,” Dr. Stockwell notes.
However, with the mandatory government electronic notification system in Denmark, “notifications are sent via regular email and SMS message, and recipients log in through a portal or smartphone app to view the letter.” Similar studies in the United States that included this extra step of needing to sign in online have not been effective in older populations.
Another limitation is that the intervention may have a different effect in populations for which there is a digital divide between people with or without Internet access of sufficient data on their mobile phones.
First-of-its kind, nationwide pragmatic trial
The NUDGE-FLU protocol was previously published in the American Heart Journal. NUDGE-FLU is a first-of-its kind nationwide, pragmatic, registry-based, cluster-randomized implementation trial of electronically delivered nudges to increase influenza vaccination uptake, the researchers note.
They identified 964,870 individuals who were 65 years or older (or would turn 65 by Jan. 15, 2023) who lived in one of 691,820 households in Denmark.
This excluded individuals who lived in a nursing home or were exempt from the government’s mandatory electronic letter system that is used for official communications.
Households were randomly assigned 9:1:1:1:1:1:1:1:1:1 to receive usual care alone or to one of nine electronic letter strategies based on different behavioral science approaches to encourage influenza vaccination uptake:
- Standard electronic letter
- Standard electronic letter sent at randomization and again 14 days later (repeated letter)
- Depersonalized letter without the recipient’s name
- Gain-framing nudge (“Vaccinations help end pandemics, like COVID-19 and the flu. Protect yourself and your loved ones.”)
- Loss-framing nudge (“When too few people get vaccinated, pandemics from diseases like COVID-19 and the flu can spread and place you and your loved ones at risk.”)
- Collective-goal nudge (“78% of Danes 65 and above were vaccinated against influenza last year. Help us achieve an even higher goal this year.”)
- Active choice or implementation-intention prompt (“We encourage you to record your appointment time here.”)
- Cardiovascular gain–framing nudge (“In addition to its protection against influenza infection, influenza vaccination also seems to protect against cardiovascular disease such as heart attacks and heart failure.”)
- Expert-authority statement (“I recommend everyone over the age of 65 years to get vaccinated against influenza – Tyra Grove Krause, Executive Vice President, Statens Serum Institut.”)
The electronic letters were sent out Sept. 16, 2022, and the primary endpoint was vaccine receipt on or before Jan. 1, 2023.
All individuals received an informative vaccination encouragement letter from the Danish Health Authority (usual care) delivered via the same electronic letter system during Sept. 17 through Sept. 21, 2022.
The individuals had a mean age of 73.8 years, 51.5% were women, and 27.4% had chronic cardiovascular disease.
The analyses were done in one randomly selected individual per household.
Influenza vaccination rates were significantly higher in the cardiovascular gain–framing nudge group vs. usual care (81.00% vs. 80.12%; difference, 0.89 percentage points; P < .0001) and in the repeat-letter group vs. usual care (80.85% vs 80.12%; difference, 0.73 percentage points; P = .0006).
These two strategies also improved vaccination rates across major subgroups.
The cardiovascular gain–framed letter was particularly effective among participants who had not been vaccinated for influenza in the previous season.
The seven other letter strategies did not increase flu shot uptake.
Subanalysis in CVD
In the prespecified subanalysis of the NUDGE-FLU trial of patients aged 65 and older that focused on patients with CVD, Daniel Modin, MB, and colleagues report that 83.1% of patients with CVD vs. 79.2% of patients without CVD received influenza vaccination within the requested time (P < .0001).
The two nudging strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination or a repeat letter – that were effective in boosting flu shot rates in the main analysis were also effective in all major CVD subgroups (ischemic heart disease, pulmonary heart disease, heart failure, atrial fibrillation, cerebrovascular disease, atherosclerotic CVD, embolic or thrombotic disease, and congenital heart disease).
Despite strong guideline endorsement, “influenza vaccination rates remain suboptimal in patients with high-risk cardiovascular disease,” Dr. Morin and colleagues write, possibly because of “insufficient knowledge among patients and providers of potential clinical benefits, concerns about vaccine safety, and other forms of vaccine hesitancy.”
Their findings suggest that “select digital behaviorally informed nudges delivered in advance of vaccine availability might be utilized to increase influenza vaccinate uptake in individuals with cardiovascular disease.”
NUDGE-HF was funded by Sanofi. Dr. Johansen and Dr. Modin have no disclosures. The disclosures of the other authors are listed with the articles. Dr. Stockwell has no disclosures.
A version of this article first appeared on Medscape.com.
Two types of electronically delivered letter strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination and a repeat reminder letter – increased flu shot uptake, compared with usual care alone, in a national study of seniors in Denmark.
And in a prespecified subanalysis focusing on older adults with cardiovascular disease, these two strategies were also effective in boosting vaccine uptake in those with or without CVD.
The findings are from the Nationwide Utilization of Danish Government Electronic Letter System for Increasing Influenza Vaccine Uptake (NUDGE-FLU) trial, which compared usual care alone with one of nine different electronic letter “behavioral nudge” strategies during the 2022-2023 flu season in people aged 65 years and older.
Niklas Dyrby Johansen, MD, Hospital–Herlev and Gentofte and Copenhagen University, presented the main study findings in a late-breaking clinical trial session at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, and the article was simultaneously published in The Lancet
The subanalysis in patients with CVD was published online March 5 in Circulation.
“Despite modest effect sizes, the results may have important implications when translated to a population level,” Dr. Dyrby Johansen concluded during his presentation. Still, the authors write, “the low-touch (no person-to-person interaction), inexpensive, and highly scalable nature of these electronic letters might have important population-level public health implications.”
They note that, among approximately 63 million Medicare beneficiaries in the United States, a 0.89–percentage point absolute increase in vaccination rate achieved through the most successful electronic letter in NUDGE-FLU, the one highlighting cardiovascular gain, would be expected to lead to 500,000 additional vaccinations and potentially prevent 7,849 illnesses, 4,395 medical visits, 714 hospitalizations, and 66 deaths each year.
Electronic letter systems similar to the one used in this trial are already in place in several European countries, including Sweden, Norway, and Ireland, the researchers note.
In countries such as the United States, where implementing a nationwide government electronic letter system might not be feasible, nudges could be done via email, text message, or other systems, but whether this would be as effective remains to be seen.
Commenting on the findings, David Cho, MD, UCLA Health and chair of the ACC Health Care Innovation Council, commended the researchers on engaging patients with more than a million separate nudges sent out during one flu season, and randomly assigning participants to 10 different types of nudges, calling it “impressive.”
“I think the concept that the nudge is to plant an idea that leads to an action is pretty much the basis of a lot of these health care interventions, which seems like a small way to have a big impact at outcome,” Dr. Cho noted. “The behavioral science aspects of the nudges are also fascinating to me personally, and I think to a lot of the cardiologists in the audience – about how you actually get people to act. I think it’s been a lifelong question for people in general, how do you get people to follow through on an action?”
“So I found the fact that secondary gain from a cardiovascular health standpoint, but also the repeated nudges were sort of simple ways that you could have people take ownership and get their flu vaccination,” he said.
“This is ACC, this is a cardiovascular conference, but the influence of vaccine is not just a primary care problem, it is also directly affecting cardiovascular disease,” Dr. Cho concluded.
‘Small but important effect’
In an accompanying editorial (Lancet. 2023 Mar 5. doi: 10.1016/S0140-6736(23)00453-1), Melissa Stockwell, MD, Columbia University, New York, writes, “The study by Johansen and colleagues highlights the small but still important effect of scalable, digital interventions across an entire at-risk population.”
A difference of 0.89% in the entire study population of over 960,000 adults age 65 years or older would be more than 8,500 additional adults protected, she notes. “That increase is important for a scalable intervention that has a low cost per letter.”
Moreover, “that the cardiovascular gain–framed messages worked best in those who had not been vaccinated in the previous season further highlights the potential impact on a more vaccine-hesitant population,” Dr. Stockwell notes.
However, with the mandatory government electronic notification system in Denmark, “notifications are sent via regular email and SMS message, and recipients log in through a portal or smartphone app to view the letter.” Similar studies in the United States that included this extra step of needing to sign in online have not been effective in older populations.
Another limitation is that the intervention may have a different effect in populations for which there is a digital divide between people with or without Internet access of sufficient data on their mobile phones.
First-of-its kind, nationwide pragmatic trial
The NUDGE-FLU protocol was previously published in the American Heart Journal. NUDGE-FLU is a first-of-its kind nationwide, pragmatic, registry-based, cluster-randomized implementation trial of electronically delivered nudges to increase influenza vaccination uptake, the researchers note.
They identified 964,870 individuals who were 65 years or older (or would turn 65 by Jan. 15, 2023) who lived in one of 691,820 households in Denmark.
This excluded individuals who lived in a nursing home or were exempt from the government’s mandatory electronic letter system that is used for official communications.
Households were randomly assigned 9:1:1:1:1:1:1:1:1:1 to receive usual care alone or to one of nine electronic letter strategies based on different behavioral science approaches to encourage influenza vaccination uptake:
- Standard electronic letter
- Standard electronic letter sent at randomization and again 14 days later (repeated letter)
- Depersonalized letter without the recipient’s name
- Gain-framing nudge (“Vaccinations help end pandemics, like COVID-19 and the flu. Protect yourself and your loved ones.”)
- Loss-framing nudge (“When too few people get vaccinated, pandemics from diseases like COVID-19 and the flu can spread and place you and your loved ones at risk.”)
- Collective-goal nudge (“78% of Danes 65 and above were vaccinated against influenza last year. Help us achieve an even higher goal this year.”)
- Active choice or implementation-intention prompt (“We encourage you to record your appointment time here.”)
- Cardiovascular gain–framing nudge (“In addition to its protection against influenza infection, influenza vaccination also seems to protect against cardiovascular disease such as heart attacks and heart failure.”)
- Expert-authority statement (“I recommend everyone over the age of 65 years to get vaccinated against influenza – Tyra Grove Krause, Executive Vice President, Statens Serum Institut.”)
The electronic letters were sent out Sept. 16, 2022, and the primary endpoint was vaccine receipt on or before Jan. 1, 2023.
All individuals received an informative vaccination encouragement letter from the Danish Health Authority (usual care) delivered via the same electronic letter system during Sept. 17 through Sept. 21, 2022.
The individuals had a mean age of 73.8 years, 51.5% were women, and 27.4% had chronic cardiovascular disease.
The analyses were done in one randomly selected individual per household.
Influenza vaccination rates were significantly higher in the cardiovascular gain–framing nudge group vs. usual care (81.00% vs. 80.12%; difference, 0.89 percentage points; P < .0001) and in the repeat-letter group vs. usual care (80.85% vs 80.12%; difference, 0.73 percentage points; P = .0006).
These two strategies also improved vaccination rates across major subgroups.
The cardiovascular gain–framed letter was particularly effective among participants who had not been vaccinated for influenza in the previous season.
The seven other letter strategies did not increase flu shot uptake.
Subanalysis in CVD
In the prespecified subanalysis of the NUDGE-FLU trial of patients aged 65 and older that focused on patients with CVD, Daniel Modin, MB, and colleagues report that 83.1% of patients with CVD vs. 79.2% of patients without CVD received influenza vaccination within the requested time (P < .0001).
The two nudging strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination or a repeat letter – that were effective in boosting flu shot rates in the main analysis were also effective in all major CVD subgroups (ischemic heart disease, pulmonary heart disease, heart failure, atrial fibrillation, cerebrovascular disease, atherosclerotic CVD, embolic or thrombotic disease, and congenital heart disease).
Despite strong guideline endorsement, “influenza vaccination rates remain suboptimal in patients with high-risk cardiovascular disease,” Dr. Morin and colleagues write, possibly because of “insufficient knowledge among patients and providers of potential clinical benefits, concerns about vaccine safety, and other forms of vaccine hesitancy.”
Their findings suggest that “select digital behaviorally informed nudges delivered in advance of vaccine availability might be utilized to increase influenza vaccinate uptake in individuals with cardiovascular disease.”
NUDGE-HF was funded by Sanofi. Dr. Johansen and Dr. Modin have no disclosures. The disclosures of the other authors are listed with the articles. Dr. Stockwell has no disclosures.
A version of this article first appeared on Medscape.com.
Two types of electronically delivered letter strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination and a repeat reminder letter – increased flu shot uptake, compared with usual care alone, in a national study of seniors in Denmark.
And in a prespecified subanalysis focusing on older adults with cardiovascular disease, these two strategies were also effective in boosting vaccine uptake in those with or without CVD.
The findings are from the Nationwide Utilization of Danish Government Electronic Letter System for Increasing Influenza Vaccine Uptake (NUDGE-FLU) trial, which compared usual care alone with one of nine different electronic letter “behavioral nudge” strategies during the 2022-2023 flu season in people aged 65 years and older.
Niklas Dyrby Johansen, MD, Hospital–Herlev and Gentofte and Copenhagen University, presented the main study findings in a late-breaking clinical trial session at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, and the article was simultaneously published in The Lancet
The subanalysis in patients with CVD was published online March 5 in Circulation.
“Despite modest effect sizes, the results may have important implications when translated to a population level,” Dr. Dyrby Johansen concluded during his presentation. Still, the authors write, “the low-touch (no person-to-person interaction), inexpensive, and highly scalable nature of these electronic letters might have important population-level public health implications.”
They note that, among approximately 63 million Medicare beneficiaries in the United States, a 0.89–percentage point absolute increase in vaccination rate achieved through the most successful electronic letter in NUDGE-FLU, the one highlighting cardiovascular gain, would be expected to lead to 500,000 additional vaccinations and potentially prevent 7,849 illnesses, 4,395 medical visits, 714 hospitalizations, and 66 deaths each year.
Electronic letter systems similar to the one used in this trial are already in place in several European countries, including Sweden, Norway, and Ireland, the researchers note.
In countries such as the United States, where implementing a nationwide government electronic letter system might not be feasible, nudges could be done via email, text message, or other systems, but whether this would be as effective remains to be seen.
Commenting on the findings, David Cho, MD, UCLA Health and chair of the ACC Health Care Innovation Council, commended the researchers on engaging patients with more than a million separate nudges sent out during one flu season, and randomly assigning participants to 10 different types of nudges, calling it “impressive.”
“I think the concept that the nudge is to plant an idea that leads to an action is pretty much the basis of a lot of these health care interventions, which seems like a small way to have a big impact at outcome,” Dr. Cho noted. “The behavioral science aspects of the nudges are also fascinating to me personally, and I think to a lot of the cardiologists in the audience – about how you actually get people to act. I think it’s been a lifelong question for people in general, how do you get people to follow through on an action?”
“So I found the fact that secondary gain from a cardiovascular health standpoint, but also the repeated nudges were sort of simple ways that you could have people take ownership and get their flu vaccination,” he said.
“This is ACC, this is a cardiovascular conference, but the influence of vaccine is not just a primary care problem, it is also directly affecting cardiovascular disease,” Dr. Cho concluded.
‘Small but important effect’
In an accompanying editorial (Lancet. 2023 Mar 5. doi: 10.1016/S0140-6736(23)00453-1), Melissa Stockwell, MD, Columbia University, New York, writes, “The study by Johansen and colleagues highlights the small but still important effect of scalable, digital interventions across an entire at-risk population.”
A difference of 0.89% in the entire study population of over 960,000 adults age 65 years or older would be more than 8,500 additional adults protected, she notes. “That increase is important for a scalable intervention that has a low cost per letter.”
Moreover, “that the cardiovascular gain–framed messages worked best in those who had not been vaccinated in the previous season further highlights the potential impact on a more vaccine-hesitant population,” Dr. Stockwell notes.
However, with the mandatory government electronic notification system in Denmark, “notifications are sent via regular email and SMS message, and recipients log in through a portal or smartphone app to view the letter.” Similar studies in the United States that included this extra step of needing to sign in online have not been effective in older populations.
Another limitation is that the intervention may have a different effect in populations for which there is a digital divide between people with or without Internet access of sufficient data on their mobile phones.
First-of-its kind, nationwide pragmatic trial
The NUDGE-FLU protocol was previously published in the American Heart Journal. NUDGE-FLU is a first-of-its kind nationwide, pragmatic, registry-based, cluster-randomized implementation trial of electronically delivered nudges to increase influenza vaccination uptake, the researchers note.
They identified 964,870 individuals who were 65 years or older (or would turn 65 by Jan. 15, 2023) who lived in one of 691,820 households in Denmark.
This excluded individuals who lived in a nursing home or were exempt from the government’s mandatory electronic letter system that is used for official communications.
Households were randomly assigned 9:1:1:1:1:1:1:1:1:1 to receive usual care alone or to one of nine electronic letter strategies based on different behavioral science approaches to encourage influenza vaccination uptake:
- Standard electronic letter
- Standard electronic letter sent at randomization and again 14 days later (repeated letter)
- Depersonalized letter without the recipient’s name
- Gain-framing nudge (“Vaccinations help end pandemics, like COVID-19 and the flu. Protect yourself and your loved ones.”)
- Loss-framing nudge (“When too few people get vaccinated, pandemics from diseases like COVID-19 and the flu can spread and place you and your loved ones at risk.”)
- Collective-goal nudge (“78% of Danes 65 and above were vaccinated against influenza last year. Help us achieve an even higher goal this year.”)
- Active choice or implementation-intention prompt (“We encourage you to record your appointment time here.”)
- Cardiovascular gain–framing nudge (“In addition to its protection against influenza infection, influenza vaccination also seems to protect against cardiovascular disease such as heart attacks and heart failure.”)
- Expert-authority statement (“I recommend everyone over the age of 65 years to get vaccinated against influenza – Tyra Grove Krause, Executive Vice President, Statens Serum Institut.”)
The electronic letters were sent out Sept. 16, 2022, and the primary endpoint was vaccine receipt on or before Jan. 1, 2023.
All individuals received an informative vaccination encouragement letter from the Danish Health Authority (usual care) delivered via the same electronic letter system during Sept. 17 through Sept. 21, 2022.
The individuals had a mean age of 73.8 years, 51.5% were women, and 27.4% had chronic cardiovascular disease.
The analyses were done in one randomly selected individual per household.
Influenza vaccination rates were significantly higher in the cardiovascular gain–framing nudge group vs. usual care (81.00% vs. 80.12%; difference, 0.89 percentage points; P < .0001) and in the repeat-letter group vs. usual care (80.85% vs 80.12%; difference, 0.73 percentage points; P = .0006).
These two strategies also improved vaccination rates across major subgroups.
The cardiovascular gain–framed letter was particularly effective among participants who had not been vaccinated for influenza in the previous season.
The seven other letter strategies did not increase flu shot uptake.
Subanalysis in CVD
In the prespecified subanalysis of the NUDGE-FLU trial of patients aged 65 and older that focused on patients with CVD, Daniel Modin, MB, and colleagues report that 83.1% of patients with CVD vs. 79.2% of patients without CVD received influenza vaccination within the requested time (P < .0001).
The two nudging strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination or a repeat letter – that were effective in boosting flu shot rates in the main analysis were also effective in all major CVD subgroups (ischemic heart disease, pulmonary heart disease, heart failure, atrial fibrillation, cerebrovascular disease, atherosclerotic CVD, embolic or thrombotic disease, and congenital heart disease).
Despite strong guideline endorsement, “influenza vaccination rates remain suboptimal in patients with high-risk cardiovascular disease,” Dr. Morin and colleagues write, possibly because of “insufficient knowledge among patients and providers of potential clinical benefits, concerns about vaccine safety, and other forms of vaccine hesitancy.”
Their findings suggest that “select digital behaviorally informed nudges delivered in advance of vaccine availability might be utilized to increase influenza vaccinate uptake in individuals with cardiovascular disease.”
NUDGE-HF was funded by Sanofi. Dr. Johansen and Dr. Modin have no disclosures. The disclosures of the other authors are listed with the articles. Dr. Stockwell has no disclosures.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
Old drug verapamil may have new use in type 1 diabetes
In children and adolescents with new-onset type 1 diabetes, the calcium channel blocker verapamil slowed the destruction of insulin-producing pancreatic beta cells for up to a year, new data show.
Use of daily verapamil within a month of diagnosis resulted in a 30% increase in C-peptide secretion (a measure of preserved beta-cell function), compared with placebo at 52 weeks, without serious adverse events.
To put it another way, verapamil delayed the expected decline in C-peptide production from 3 months after diagnosis of type 1 diabetes to 6 months after diagnosis.
“We think this is a really, really exciting finding that’s hopefully going to impact the care for children with type 1 diabetes in the new-onset period,” lead author Gregory P. Forlenza, MD, said during his presentation of the data on Feb. 24 at the annual Advanced Technologies & Treatments for Diabetes (ATTD) meeting in Berlin.
“In view of the favorable safety profile, particularly compared with immune-suppressive agents, once-a-day oral administration, and low cost, initiation of verapamil should be a consideration for newly diagnosed patients with type 1 diabetes,” added Dr. Forlenza, a pediatric endocrinologist at the Barbara Davis Center for Diabetes, Anschutz Medical Campus, University of Colorado, Aurora.
The data were also simultaneously published in JAMA, as part of the CLVer (Hybrid Closed Loop Therapy and Verapamil for Beta Cell Preservation in New Onset Type 1 Diabetes) trial.
The randomized, double-blind, six-center trial involved 113 participants, aged 7-17 years, with newly diagnosed type 1 diabetes. They were randomized to the most advanced commercially available automated insulin delivery systems available or standard care to test the effects of intensive glucose control on C-peptide levels for 52 weeks during the COVID-19 pandemic (July 2020 to September 2022). Eighty-eight patients who weighed 30 kg (66 lb) or more were further randomized (1:1) to daily extended-release verapamil or placebo for the same duration.
The positive findings for verapamil, published in one paper, contrasted with the negative ones for the automated insulin delivery (AID) system. The latter did not prevent the expected decline in C-peptide, putting to rest a long-held hypothesis that reducing glucotoxicity might preserve beta-cell function in newly diagnosed individuals with type 1 diabetes, noted Dr. Forlenza.
Could combination therapy work?
In recent years, immune-modulating agents have increasingly been shown to preserve beta-cell function in both new-onset and preclinical type 1 diabetes. One such agent, teplizumab (Tzield, Provention Bio), was approved by the U.S. Food and Drug Administration in November 2022 to delay type 1 diabetes onset in those at high risk.
Calcium channel blockers such as verapamil – used for years to treat hypertension and cardiac arrhythmias – may accomplish the same goal as teplizumab but in a different way, by reducing the protein overexpression that induces beta-cell apoptosis and death.
Dr. Forlenza showed a slide comparing the preservation of C-peptide, which was much lower with verapamil, at 30%, than with teplizumab, at 75%.
Asked to comment, session moderator Torben Biester, MD, a pediatric diabetologist at Auf der Bult-Zentrum Diabetes-Center for Children and Adolescents, Hanover, Germany, said: “[Verapamil] is a very cheap [daily] pill. [Teplizumab] is a very high-priced ... immune therapy in the United States ... an infusion twice for 10 days, so it’s a lot more burden for the patients and a lot more risk of side effects.”
“The future might be combination therapy,” added Dr. Biester.
And in an editorial published in JAMA and accompanying the two CLVer papers, Jennifer Couper, MD, of the University of Adelaide, agrees: “A well-tolerated, inexpensive, oral treatment such as verapamil with modest benefits on C-peptide production is relevant to practice.”
The new work “supports investigation of verapamil in combination with other effective agents during the earlier stages of type 1 diabetes before insulin dependence develops,” she noted.
Verapamil results ‘brilliant’ but more work needed
In the verapamil part of the CLVer trial, by 52 weeks, verapamil doses in the youth who received it ranged from 120-360 mg/day based on weight and tolerance.
The primary outcome, C-peptide area under the curve, stayed stable, from 0.66 pmol/mL at baseline to 0.65 pmol/mL at 52 weeks in the verapamil group, compared with a drop from 0.60 pmol/mL down to 0.44 pmol/mL with placebo, a significant difference of 0.14 pmol/mL (P = .04), representing a 30% higher C-peptide level in the verapamil group.
“For us, this is a phenomenally exciting result,” Dr. Forlenza commented during his presentation.
At 52 weeks, A1c was 6.6% in the verapamil group versus 6.9% with placebo, which was not significantly different. Daily insulin dose was 0.65 versus 0.74 units/kg per day, respectively, also not significantly different.
One severe hypoglycemic event occurred in each group, and one diabetic ketoacidosis event occurred in the placebo group. In the verapamil group, three participants experienced “nonserious” electrocardiogram abnormalities and one had hypertension.
Dr. Biester said he isn’t “that concerned” about the small number of mild ECG abnormalities seen in the study with verapamil, as this is a known side effect. But overall, he said, “I would think that for a recommendation for routine use it’s too early after one study, even though the results are brilliant.”
He noted that he is involved in a similar ongoing study of verapamil in adults with new-onset type 1 diabetes, called Ver-A-T1D.
No C-peptide effect of tight glycemic control: ‘A tough pill’
In the AID part of the study, the 113 participants were randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact (a median of 35 times) by study staff, or standard management using a continuous glucose monitor (CGM) with an insulin pump or multiple daily injections.
At 52 weeks, A1c was 6.5% for the intensive group versus 7.1% with standard care, a significant difference. Time in blood glucose range of 70-180 mg/dL was significantly longer with intensive management, at 78%, compared with standard care, at 64%.
Nonetheless, the change in C-peptide area under the curve did not differ between the two groups, decreasing from 0.57 pmol/mL at baseline to 0.45 pmol/mL at 52 weeks with the AID system, compared with a decrease from 0.60 pmol/L down to 0.50 pmol/L with standard care (P = .89).
Dr. Forlenza commented that the hypothesis that tight glycemic control would delay the decline in C-peptide secretion “is something I think a lot of endocrinologists assumed to be true and something I’ve heard lots of colleagues over the years talk about.”
Consequently, he said these findings are “a tough pill for us to swallow ... but it’s important for us in the field to understand.”
“Even with frequent contacts that are well above the level we’d be able to do in standard clinical care, and even with use of the most advanced AID systems we have ... we saw absolutely no difference in stimulated C-peptide levels at any of the timepoints throughout the first year or at 52 weeks.”
“So, in our opinion, this,” combined with a prior study from 2022, “should put this hypothesis to rest,” he said.
“Excellent glycemic control has a benefit in and of itself, but it was not a successful intervention for beta-cell preservation.”
Dr. Forlenza has reported serving as a consultant, speaker, or advisory board member for Medtronic, Dexcom, Abbott, Tandem Diabetes Care, Insulet, Lilly, and Beta Bionics, and his institution has also received funding on his behalf for research grants from these companies. Dr. Biester has reported receiving speaker’s fees from DexCom, Medtronic, Novo Nordisk, F. Hoffmann–La Roche, Sanofi, and Ypsomed Holding; serving on advisory boards for Ascensia Diabetes Care Holdings, AstraZeneca, DexCom, and Medtronic; and receiving personal fees from SYNLAB; and is a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes. Dr. Couper has reported no relevant financial relationships.
The rationale for the companion CLVer analysis of the effect of reducing glucose toxicity via tight glycemic control on C-peptide progression dates back to an inpatient study published in 1989 involving 26 adolescents using an early artificial pancreas prototype called a Biostator, in which beta-cell preservation was achieved. However, two more recent studies of this approach, including one published in late 2022, did not show a difference. The CLVer analysis involved 113 participants randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact by study staff, or standard management using a CGM with a pump or multiple daily injections.
A version of this article originally appeared on Medscape.com.
In children and adolescents with new-onset type 1 diabetes, the calcium channel blocker verapamil slowed the destruction of insulin-producing pancreatic beta cells for up to a year, new data show.
Use of daily verapamil within a month of diagnosis resulted in a 30% increase in C-peptide secretion (a measure of preserved beta-cell function), compared with placebo at 52 weeks, without serious adverse events.
To put it another way, verapamil delayed the expected decline in C-peptide production from 3 months after diagnosis of type 1 diabetes to 6 months after diagnosis.
“We think this is a really, really exciting finding that’s hopefully going to impact the care for children with type 1 diabetes in the new-onset period,” lead author Gregory P. Forlenza, MD, said during his presentation of the data on Feb. 24 at the annual Advanced Technologies & Treatments for Diabetes (ATTD) meeting in Berlin.
“In view of the favorable safety profile, particularly compared with immune-suppressive agents, once-a-day oral administration, and low cost, initiation of verapamil should be a consideration for newly diagnosed patients with type 1 diabetes,” added Dr. Forlenza, a pediatric endocrinologist at the Barbara Davis Center for Diabetes, Anschutz Medical Campus, University of Colorado, Aurora.
The data were also simultaneously published in JAMA, as part of the CLVer (Hybrid Closed Loop Therapy and Verapamil for Beta Cell Preservation in New Onset Type 1 Diabetes) trial.
The randomized, double-blind, six-center trial involved 113 participants, aged 7-17 years, with newly diagnosed type 1 diabetes. They were randomized to the most advanced commercially available automated insulin delivery systems available or standard care to test the effects of intensive glucose control on C-peptide levels for 52 weeks during the COVID-19 pandemic (July 2020 to September 2022). Eighty-eight patients who weighed 30 kg (66 lb) or more were further randomized (1:1) to daily extended-release verapamil or placebo for the same duration.
The positive findings for verapamil, published in one paper, contrasted with the negative ones for the automated insulin delivery (AID) system. The latter did not prevent the expected decline in C-peptide, putting to rest a long-held hypothesis that reducing glucotoxicity might preserve beta-cell function in newly diagnosed individuals with type 1 diabetes, noted Dr. Forlenza.
Could combination therapy work?
In recent years, immune-modulating agents have increasingly been shown to preserve beta-cell function in both new-onset and preclinical type 1 diabetes. One such agent, teplizumab (Tzield, Provention Bio), was approved by the U.S. Food and Drug Administration in November 2022 to delay type 1 diabetes onset in those at high risk.
Calcium channel blockers such as verapamil – used for years to treat hypertension and cardiac arrhythmias – may accomplish the same goal as teplizumab but in a different way, by reducing the protein overexpression that induces beta-cell apoptosis and death.
Dr. Forlenza showed a slide comparing the preservation of C-peptide, which was much lower with verapamil, at 30%, than with teplizumab, at 75%.
Asked to comment, session moderator Torben Biester, MD, a pediatric diabetologist at Auf der Bult-Zentrum Diabetes-Center for Children and Adolescents, Hanover, Germany, said: “[Verapamil] is a very cheap [daily] pill. [Teplizumab] is a very high-priced ... immune therapy in the United States ... an infusion twice for 10 days, so it’s a lot more burden for the patients and a lot more risk of side effects.”
“The future might be combination therapy,” added Dr. Biester.
And in an editorial published in JAMA and accompanying the two CLVer papers, Jennifer Couper, MD, of the University of Adelaide, agrees: “A well-tolerated, inexpensive, oral treatment such as verapamil with modest benefits on C-peptide production is relevant to practice.”
The new work “supports investigation of verapamil in combination with other effective agents during the earlier stages of type 1 diabetes before insulin dependence develops,” she noted.
Verapamil results ‘brilliant’ but more work needed
In the verapamil part of the CLVer trial, by 52 weeks, verapamil doses in the youth who received it ranged from 120-360 mg/day based on weight and tolerance.
The primary outcome, C-peptide area under the curve, stayed stable, from 0.66 pmol/mL at baseline to 0.65 pmol/mL at 52 weeks in the verapamil group, compared with a drop from 0.60 pmol/mL down to 0.44 pmol/mL with placebo, a significant difference of 0.14 pmol/mL (P = .04), representing a 30% higher C-peptide level in the verapamil group.
“For us, this is a phenomenally exciting result,” Dr. Forlenza commented during his presentation.
At 52 weeks, A1c was 6.6% in the verapamil group versus 6.9% with placebo, which was not significantly different. Daily insulin dose was 0.65 versus 0.74 units/kg per day, respectively, also not significantly different.
One severe hypoglycemic event occurred in each group, and one diabetic ketoacidosis event occurred in the placebo group. In the verapamil group, three participants experienced “nonserious” electrocardiogram abnormalities and one had hypertension.
Dr. Biester said he isn’t “that concerned” about the small number of mild ECG abnormalities seen in the study with verapamil, as this is a known side effect. But overall, he said, “I would think that for a recommendation for routine use it’s too early after one study, even though the results are brilliant.”
He noted that he is involved in a similar ongoing study of verapamil in adults with new-onset type 1 diabetes, called Ver-A-T1D.
No C-peptide effect of tight glycemic control: ‘A tough pill’
In the AID part of the study, the 113 participants were randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact (a median of 35 times) by study staff, or standard management using a continuous glucose monitor (CGM) with an insulin pump or multiple daily injections.
At 52 weeks, A1c was 6.5% for the intensive group versus 7.1% with standard care, a significant difference. Time in blood glucose range of 70-180 mg/dL was significantly longer with intensive management, at 78%, compared with standard care, at 64%.
Nonetheless, the change in C-peptide area under the curve did not differ between the two groups, decreasing from 0.57 pmol/mL at baseline to 0.45 pmol/mL at 52 weeks with the AID system, compared with a decrease from 0.60 pmol/L down to 0.50 pmol/L with standard care (P = .89).
Dr. Forlenza commented that the hypothesis that tight glycemic control would delay the decline in C-peptide secretion “is something I think a lot of endocrinologists assumed to be true and something I’ve heard lots of colleagues over the years talk about.”
Consequently, he said these findings are “a tough pill for us to swallow ... but it’s important for us in the field to understand.”
“Even with frequent contacts that are well above the level we’d be able to do in standard clinical care, and even with use of the most advanced AID systems we have ... we saw absolutely no difference in stimulated C-peptide levels at any of the timepoints throughout the first year or at 52 weeks.”
“So, in our opinion, this,” combined with a prior study from 2022, “should put this hypothesis to rest,” he said.
“Excellent glycemic control has a benefit in and of itself, but it was not a successful intervention for beta-cell preservation.”
Dr. Forlenza has reported serving as a consultant, speaker, or advisory board member for Medtronic, Dexcom, Abbott, Tandem Diabetes Care, Insulet, Lilly, and Beta Bionics, and his institution has also received funding on his behalf for research grants from these companies. Dr. Biester has reported receiving speaker’s fees from DexCom, Medtronic, Novo Nordisk, F. Hoffmann–La Roche, Sanofi, and Ypsomed Holding; serving on advisory boards for Ascensia Diabetes Care Holdings, AstraZeneca, DexCom, and Medtronic; and receiving personal fees from SYNLAB; and is a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes. Dr. Couper has reported no relevant financial relationships.
The rationale for the companion CLVer analysis of the effect of reducing glucose toxicity via tight glycemic control on C-peptide progression dates back to an inpatient study published in 1989 involving 26 adolescents using an early artificial pancreas prototype called a Biostator, in which beta-cell preservation was achieved. However, two more recent studies of this approach, including one published in late 2022, did not show a difference. The CLVer analysis involved 113 participants randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact by study staff, or standard management using a CGM with a pump or multiple daily injections.
A version of this article originally appeared on Medscape.com.
In children and adolescents with new-onset type 1 diabetes, the calcium channel blocker verapamil slowed the destruction of insulin-producing pancreatic beta cells for up to a year, new data show.
Use of daily verapamil within a month of diagnosis resulted in a 30% increase in C-peptide secretion (a measure of preserved beta-cell function), compared with placebo at 52 weeks, without serious adverse events.
To put it another way, verapamil delayed the expected decline in C-peptide production from 3 months after diagnosis of type 1 diabetes to 6 months after diagnosis.
“We think this is a really, really exciting finding that’s hopefully going to impact the care for children with type 1 diabetes in the new-onset period,” lead author Gregory P. Forlenza, MD, said during his presentation of the data on Feb. 24 at the annual Advanced Technologies & Treatments for Diabetes (ATTD) meeting in Berlin.
“In view of the favorable safety profile, particularly compared with immune-suppressive agents, once-a-day oral administration, and low cost, initiation of verapamil should be a consideration for newly diagnosed patients with type 1 diabetes,” added Dr. Forlenza, a pediatric endocrinologist at the Barbara Davis Center for Diabetes, Anschutz Medical Campus, University of Colorado, Aurora.
The data were also simultaneously published in JAMA, as part of the CLVer (Hybrid Closed Loop Therapy and Verapamil for Beta Cell Preservation in New Onset Type 1 Diabetes) trial.
The randomized, double-blind, six-center trial involved 113 participants, aged 7-17 years, with newly diagnosed type 1 diabetes. They were randomized to the most advanced commercially available automated insulin delivery systems available or standard care to test the effects of intensive glucose control on C-peptide levels for 52 weeks during the COVID-19 pandemic (July 2020 to September 2022). Eighty-eight patients who weighed 30 kg (66 lb) or more were further randomized (1:1) to daily extended-release verapamil or placebo for the same duration.
The positive findings for verapamil, published in one paper, contrasted with the negative ones for the automated insulin delivery (AID) system. The latter did not prevent the expected decline in C-peptide, putting to rest a long-held hypothesis that reducing glucotoxicity might preserve beta-cell function in newly diagnosed individuals with type 1 diabetes, noted Dr. Forlenza.
Could combination therapy work?
In recent years, immune-modulating agents have increasingly been shown to preserve beta-cell function in both new-onset and preclinical type 1 diabetes. One such agent, teplizumab (Tzield, Provention Bio), was approved by the U.S. Food and Drug Administration in November 2022 to delay type 1 diabetes onset in those at high risk.
Calcium channel blockers such as verapamil – used for years to treat hypertension and cardiac arrhythmias – may accomplish the same goal as teplizumab but in a different way, by reducing the protein overexpression that induces beta-cell apoptosis and death.
Dr. Forlenza showed a slide comparing the preservation of C-peptide, which was much lower with verapamil, at 30%, than with teplizumab, at 75%.
Asked to comment, session moderator Torben Biester, MD, a pediatric diabetologist at Auf der Bult-Zentrum Diabetes-Center for Children and Adolescents, Hanover, Germany, said: “[Verapamil] is a very cheap [daily] pill. [Teplizumab] is a very high-priced ... immune therapy in the United States ... an infusion twice for 10 days, so it’s a lot more burden for the patients and a lot more risk of side effects.”
“The future might be combination therapy,” added Dr. Biester.
And in an editorial published in JAMA and accompanying the two CLVer papers, Jennifer Couper, MD, of the University of Adelaide, agrees: “A well-tolerated, inexpensive, oral treatment such as verapamil with modest benefits on C-peptide production is relevant to practice.”
The new work “supports investigation of verapamil in combination with other effective agents during the earlier stages of type 1 diabetes before insulin dependence develops,” she noted.
Verapamil results ‘brilliant’ but more work needed
In the verapamil part of the CLVer trial, by 52 weeks, verapamil doses in the youth who received it ranged from 120-360 mg/day based on weight and tolerance.
The primary outcome, C-peptide area under the curve, stayed stable, from 0.66 pmol/mL at baseline to 0.65 pmol/mL at 52 weeks in the verapamil group, compared with a drop from 0.60 pmol/mL down to 0.44 pmol/mL with placebo, a significant difference of 0.14 pmol/mL (P = .04), representing a 30% higher C-peptide level in the verapamil group.
“For us, this is a phenomenally exciting result,” Dr. Forlenza commented during his presentation.
At 52 weeks, A1c was 6.6% in the verapamil group versus 6.9% with placebo, which was not significantly different. Daily insulin dose was 0.65 versus 0.74 units/kg per day, respectively, also not significantly different.
One severe hypoglycemic event occurred in each group, and one diabetic ketoacidosis event occurred in the placebo group. In the verapamil group, three participants experienced “nonserious” electrocardiogram abnormalities and one had hypertension.
Dr. Biester said he isn’t “that concerned” about the small number of mild ECG abnormalities seen in the study with verapamil, as this is a known side effect. But overall, he said, “I would think that for a recommendation for routine use it’s too early after one study, even though the results are brilliant.”
He noted that he is involved in a similar ongoing study of verapamil in adults with new-onset type 1 diabetes, called Ver-A-T1D.
No C-peptide effect of tight glycemic control: ‘A tough pill’
In the AID part of the study, the 113 participants were randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact (a median of 35 times) by study staff, or standard management using a continuous glucose monitor (CGM) with an insulin pump or multiple daily injections.
At 52 weeks, A1c was 6.5% for the intensive group versus 7.1% with standard care, a significant difference. Time in blood glucose range of 70-180 mg/dL was significantly longer with intensive management, at 78%, compared with standard care, at 64%.
Nonetheless, the change in C-peptide area under the curve did not differ between the two groups, decreasing from 0.57 pmol/mL at baseline to 0.45 pmol/mL at 52 weeks with the AID system, compared with a decrease from 0.60 pmol/L down to 0.50 pmol/L with standard care (P = .89).
Dr. Forlenza commented that the hypothesis that tight glycemic control would delay the decline in C-peptide secretion “is something I think a lot of endocrinologists assumed to be true and something I’ve heard lots of colleagues over the years talk about.”
Consequently, he said these findings are “a tough pill for us to swallow ... but it’s important for us in the field to understand.”
“Even with frequent contacts that are well above the level we’d be able to do in standard clinical care, and even with use of the most advanced AID systems we have ... we saw absolutely no difference in stimulated C-peptide levels at any of the timepoints throughout the first year or at 52 weeks.”
“So, in our opinion, this,” combined with a prior study from 2022, “should put this hypothesis to rest,” he said.
“Excellent glycemic control has a benefit in and of itself, but it was not a successful intervention for beta-cell preservation.”
Dr. Forlenza has reported serving as a consultant, speaker, or advisory board member for Medtronic, Dexcom, Abbott, Tandem Diabetes Care, Insulet, Lilly, and Beta Bionics, and his institution has also received funding on his behalf for research grants from these companies. Dr. Biester has reported receiving speaker’s fees from DexCom, Medtronic, Novo Nordisk, F. Hoffmann–La Roche, Sanofi, and Ypsomed Holding; serving on advisory boards for Ascensia Diabetes Care Holdings, AstraZeneca, DexCom, and Medtronic; and receiving personal fees from SYNLAB; and is a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes. Dr. Couper has reported no relevant financial relationships.
The rationale for the companion CLVer analysis of the effect of reducing glucose toxicity via tight glycemic control on C-peptide progression dates back to an inpatient study published in 1989 involving 26 adolescents using an early artificial pancreas prototype called a Biostator, in which beta-cell preservation was achieved. However, two more recent studies of this approach, including one published in late 2022, did not show a difference. The CLVer analysis involved 113 participants randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact by study staff, or standard management using a CGM with a pump or multiple daily injections.
A version of this article originally appeared on Medscape.com.
Two cups of coffee increase heart dangers with hypertension
according to researchers at Institute for Global Health Policy Research, Bureau of International Health Cooperation, National Center for Global Health and Medicine, Tokyo.
What to know
People with severely high blood pressure who drink two or more cups of caffeinated coffee each day could double their risk of dying from a heart attack, stroke, or any type of cardiovascular disease.
Too much coffee may raise blood pressure and lead to anxiety, heart palpitations, and difficulty sleeping.
An 8-ounce cup of coffee has 80-100 mg of caffeine, while an 8-ounce cup of green or black tea has 30-50 mg.
Drinking one cup of coffee a day or any amount of green tea was not associated with risk of death across any blood pressure categories, and drinking green tea was not associated with increased risk of death related to cardiovascular disease at any blood pressure level.
Frequent consumers of coffee were more likely to be younger, current smokers, current drinkers, to eat fewer vegetables, and to have higher total cholesterol levels and lower systolic blood pressure regardless of their blood pressure category.
This is a summary of the article “Coffee and Green Tea Consumption and Cardiovascular Disease Mortality Among People With and Without Hypertension,” published in the Journal of the American Heart Association.
A version of this article first appeared on Medscape.com.
according to researchers at Institute for Global Health Policy Research, Bureau of International Health Cooperation, National Center for Global Health and Medicine, Tokyo.
What to know
People with severely high blood pressure who drink two or more cups of caffeinated coffee each day could double their risk of dying from a heart attack, stroke, or any type of cardiovascular disease.
Too much coffee may raise blood pressure and lead to anxiety, heart palpitations, and difficulty sleeping.
An 8-ounce cup of coffee has 80-100 mg of caffeine, while an 8-ounce cup of green or black tea has 30-50 mg.
Drinking one cup of coffee a day or any amount of green tea was not associated with risk of death across any blood pressure categories, and drinking green tea was not associated with increased risk of death related to cardiovascular disease at any blood pressure level.
Frequent consumers of coffee were more likely to be younger, current smokers, current drinkers, to eat fewer vegetables, and to have higher total cholesterol levels and lower systolic blood pressure regardless of their blood pressure category.
This is a summary of the article “Coffee and Green Tea Consumption and Cardiovascular Disease Mortality Among People With and Without Hypertension,” published in the Journal of the American Heart Association.
A version of this article first appeared on Medscape.com.
according to researchers at Institute for Global Health Policy Research, Bureau of International Health Cooperation, National Center for Global Health and Medicine, Tokyo.
What to know
People with severely high blood pressure who drink two or more cups of caffeinated coffee each day could double their risk of dying from a heart attack, stroke, or any type of cardiovascular disease.
Too much coffee may raise blood pressure and lead to anxiety, heart palpitations, and difficulty sleeping.
An 8-ounce cup of coffee has 80-100 mg of caffeine, while an 8-ounce cup of green or black tea has 30-50 mg.
Drinking one cup of coffee a day or any amount of green tea was not associated with risk of death across any blood pressure categories, and drinking green tea was not associated with increased risk of death related to cardiovascular disease at any blood pressure level.
Frequent consumers of coffee were more likely to be younger, current smokers, current drinkers, to eat fewer vegetables, and to have higher total cholesterol levels and lower systolic blood pressure regardless of their blood pressure category.
This is a summary of the article “Coffee and Green Tea Consumption and Cardiovascular Disease Mortality Among People With and Without Hypertension,” published in the Journal of the American Heart Association.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF AMERICAN HEART ASSOCIATION
Silent bradycardia common on loop recorders – pacemaker needed?
Bradycardia is a lot more common than generally believed, but is often asymptomatic and not clinically relevant, and may lead to needless pacemaker therapy, suggests a post hoc analysis of a major study.
The arrhythmia’s presence overall in the randomized LOOP trial predicted an excess risk of syncope and death, and it didn’t matter how it was detected. Bradyarrhythmia revealed incidentally at long-term cardiac rhythm monitoring was no more predictive than when it was picked up in a usual-care setting.
Still, people in the trial with implantable loop recorders (ILR) had six times the chance of being diagnosed with bradyarrhythmias than those in the usual-care control group. LOOP entered older persons in the community without known arrhythmias but with risk factors like diabetes or hypertension.
About 80% of such arrhythmias at ILR monitoring were asymptomatic, compared with less than one-fourth in the usual-care group. Yet pacemaker implantation for bradyarrhythmia was 53% more likely in the ILR group, according to a report published in JAMA Cardiology.
Most participants with asymptomatic bradycardia did not receive treatment for it, yet the study – despite the mostly conservative management – still showed “overtreatment with pacemakers” in the ILR group, observed lead author Søren Zöga Diederichsen, MD, PhD, Copenhagen University Hospital–Rigshospitalet.
Bradyarrhythmia overall predicted later syncope and all-cause and cardiovascular (CV) death, but did so regardless of whether the patient was ILR monitored or received a pacemaker, Diederichsen said in an interview.
“We didn’t see any signal, not even a small signal, toward a health benefit from monitoring and detecting bradycardias, or from acting on them conservatively or implanting pacemakers,” he noted.
The study “emphasizes that you should have symptoms” to justify pacemaker therapy for bradyarrhythmias, regardless of how they were detected, Dr. Diederichsen said.
“Clearly ILRs may identify patients with bradyarrhythmias deserving of treatment” when they are associated with symptoms, an accompanying editorial agreed. In the current analysis, however, “a large proportion of bradycardic events were completely asymptomatic.” Yet bradycardia predicted syncope and CV death in both the ILR and usual care groups, it noted.
“This does raise the question as to whether bradyarrhythmia may be a risk marker for underlying nonarrhythmic conditions to which preventive strategies and treatment should be directed,” wrote editorialists Mark H. Schoenfeld, MD, Yale University, New Haven, Conn., and Kristen K. Patton, MD, University of Washington, Seattle.
“In an aging population with ever-increasing comorbidities, it may become increasingly important to rule out bradycardia as a manifestation of a more sinister underlying disease,” they noted, and to identify “patients who may be particularly vulnerable to adverse outcomes of progressive distal conduction disease.”
The previously published LOOP trial, conducted at four sites in Denmark, compared ILR screening for atrial fibrillation to usual care in 6,004 patients at least 70 years or older, most with hypertension. The main results showed little benefit from screening for atrial fibrillation in prevention of incident stroke or systemic embolism over about 5 years.
The current LOOP analysis, post hoc with all the associated limitations, followed incident bradyarrhythmia in the ILR and usual-care groups; any treatment of the arrhythmia was at physician discretion. The total cohort averaged 75 years in age and 47.3% were women.
The rate of incident bradyarrhythmia was 8.1% overall; it was 20.8% for those with ILR monitoring and 3.8% in the usual care group, for a hazard ratio of 6.21 (95% confidence interval, 5.15-7.48, P < .001).
The arrhythmia was asymptomatic in 23.8% of usual-care patients and 79.8% of those with an ILR.
Bradyarrhythmia was significantly more likely among older patients, male patients, and those with a history of syncope, the group reported.
Pacemakers were implanted for bradyarrhythmia in 2.9% of usual-care patients and 4.5% of those with ILR monitoring for an HR of 1.53 (95% CI, 1.14-2.06, P < .001).
Among usual-care patients, bradyarrhythmia (vs no bradyarrhythmia) was associated with 5.2 times the risk for incident syncope (P < .001). That risk for syncope went up 2.6 times (P = .01) in the ILR group.
The corresponding risks for CV death among controls and among ILR patients increased 4.8 times (P < .001) and by 3.1 (P < .001), respectively. The risks for death from any cause tripled (P < .001) and rose 2.5 times (P < .001) among bradycardic controls and ILR patients, respectively.
Bradyarrhythmia was not significantly related to sudden cardiac death in either group, the report noted.
Given the increasing use of heart rhythm monitoring “inside and outside the clinical setting,” it stated, “bradyarrhythmias are likely to be detected more often, sometimes as an incidental finding. Knowledge about the underlying prevalence and prognostic significance could help guide decisions.”
The study “teaches us a little bit” about the true prevalence of bradyarrhythmias in the general population, including asymptomatic cases that appear to be subclinical or “physiological,” Dr. Diederichsen said in an interview.
It also suggests that such bradycardia will be increasingly observed as use of ILR for arrhythmia screening expands in practice, he predicted. It may also be picked up more often by wearables and other rhythm-monitoring technology used by the public.
In the latter case especially, Dr. Diederichsen said, the current analysis could potentially help alleviate any concerns that bradyarrhythmia without symptoms is something that has to be specifically treated.
Dr. Diederichsen disclosed grants from several Danish research institutions, R. og Hustrus Fond, and Medtronic, as well as receiving personal fees from Vital Beats and Bristol-Myers Squibb/Pfizer. Dr. Schoenfeld reported ownership of stock from Apple. Dr. Patton reported employment as a medical officer for the Food and Drug Administration and serving as associate editor for JAMA Cardiology.
A version of this article originally appeared on Medscape.com.
Bradycardia is a lot more common than generally believed, but is often asymptomatic and not clinically relevant, and may lead to needless pacemaker therapy, suggests a post hoc analysis of a major study.
The arrhythmia’s presence overall in the randomized LOOP trial predicted an excess risk of syncope and death, and it didn’t matter how it was detected. Bradyarrhythmia revealed incidentally at long-term cardiac rhythm monitoring was no more predictive than when it was picked up in a usual-care setting.
Still, people in the trial with implantable loop recorders (ILR) had six times the chance of being diagnosed with bradyarrhythmias than those in the usual-care control group. LOOP entered older persons in the community without known arrhythmias but with risk factors like diabetes or hypertension.
About 80% of such arrhythmias at ILR monitoring were asymptomatic, compared with less than one-fourth in the usual-care group. Yet pacemaker implantation for bradyarrhythmia was 53% more likely in the ILR group, according to a report published in JAMA Cardiology.
Most participants with asymptomatic bradycardia did not receive treatment for it, yet the study – despite the mostly conservative management – still showed “overtreatment with pacemakers” in the ILR group, observed lead author Søren Zöga Diederichsen, MD, PhD, Copenhagen University Hospital–Rigshospitalet.
Bradyarrhythmia overall predicted later syncope and all-cause and cardiovascular (CV) death, but did so regardless of whether the patient was ILR monitored or received a pacemaker, Diederichsen said in an interview.
“We didn’t see any signal, not even a small signal, toward a health benefit from monitoring and detecting bradycardias, or from acting on them conservatively or implanting pacemakers,” he noted.
The study “emphasizes that you should have symptoms” to justify pacemaker therapy for bradyarrhythmias, regardless of how they were detected, Dr. Diederichsen said.
“Clearly ILRs may identify patients with bradyarrhythmias deserving of treatment” when they are associated with symptoms, an accompanying editorial agreed. In the current analysis, however, “a large proportion of bradycardic events were completely asymptomatic.” Yet bradycardia predicted syncope and CV death in both the ILR and usual care groups, it noted.
“This does raise the question as to whether bradyarrhythmia may be a risk marker for underlying nonarrhythmic conditions to which preventive strategies and treatment should be directed,” wrote editorialists Mark H. Schoenfeld, MD, Yale University, New Haven, Conn., and Kristen K. Patton, MD, University of Washington, Seattle.
“In an aging population with ever-increasing comorbidities, it may become increasingly important to rule out bradycardia as a manifestation of a more sinister underlying disease,” they noted, and to identify “patients who may be particularly vulnerable to adverse outcomes of progressive distal conduction disease.”
The previously published LOOP trial, conducted at four sites in Denmark, compared ILR screening for atrial fibrillation to usual care in 6,004 patients at least 70 years or older, most with hypertension. The main results showed little benefit from screening for atrial fibrillation in prevention of incident stroke or systemic embolism over about 5 years.
The current LOOP analysis, post hoc with all the associated limitations, followed incident bradyarrhythmia in the ILR and usual-care groups; any treatment of the arrhythmia was at physician discretion. The total cohort averaged 75 years in age and 47.3% were women.
The rate of incident bradyarrhythmia was 8.1% overall; it was 20.8% for those with ILR monitoring and 3.8% in the usual care group, for a hazard ratio of 6.21 (95% confidence interval, 5.15-7.48, P < .001).
The arrhythmia was asymptomatic in 23.8% of usual-care patients and 79.8% of those with an ILR.
Bradyarrhythmia was significantly more likely among older patients, male patients, and those with a history of syncope, the group reported.
Pacemakers were implanted for bradyarrhythmia in 2.9% of usual-care patients and 4.5% of those with ILR monitoring for an HR of 1.53 (95% CI, 1.14-2.06, P < .001).
Among usual-care patients, bradyarrhythmia (vs no bradyarrhythmia) was associated with 5.2 times the risk for incident syncope (P < .001). That risk for syncope went up 2.6 times (P = .01) in the ILR group.
The corresponding risks for CV death among controls and among ILR patients increased 4.8 times (P < .001) and by 3.1 (P < .001), respectively. The risks for death from any cause tripled (P < .001) and rose 2.5 times (P < .001) among bradycardic controls and ILR patients, respectively.
Bradyarrhythmia was not significantly related to sudden cardiac death in either group, the report noted.
Given the increasing use of heart rhythm monitoring “inside and outside the clinical setting,” it stated, “bradyarrhythmias are likely to be detected more often, sometimes as an incidental finding. Knowledge about the underlying prevalence and prognostic significance could help guide decisions.”
The study “teaches us a little bit” about the true prevalence of bradyarrhythmias in the general population, including asymptomatic cases that appear to be subclinical or “physiological,” Dr. Diederichsen said in an interview.
It also suggests that such bradycardia will be increasingly observed as use of ILR for arrhythmia screening expands in practice, he predicted. It may also be picked up more often by wearables and other rhythm-monitoring technology used by the public.
In the latter case especially, Dr. Diederichsen said, the current analysis could potentially help alleviate any concerns that bradyarrhythmia without symptoms is something that has to be specifically treated.
Dr. Diederichsen disclosed grants from several Danish research institutions, R. og Hustrus Fond, and Medtronic, as well as receiving personal fees from Vital Beats and Bristol-Myers Squibb/Pfizer. Dr. Schoenfeld reported ownership of stock from Apple. Dr. Patton reported employment as a medical officer for the Food and Drug Administration and serving as associate editor for JAMA Cardiology.
A version of this article originally appeared on Medscape.com.
Bradycardia is a lot more common than generally believed, but is often asymptomatic and not clinically relevant, and may lead to needless pacemaker therapy, suggests a post hoc analysis of a major study.
The arrhythmia’s presence overall in the randomized LOOP trial predicted an excess risk of syncope and death, and it didn’t matter how it was detected. Bradyarrhythmia revealed incidentally at long-term cardiac rhythm monitoring was no more predictive than when it was picked up in a usual-care setting.
Still, people in the trial with implantable loop recorders (ILR) had six times the chance of being diagnosed with bradyarrhythmias than those in the usual-care control group. LOOP entered older persons in the community without known arrhythmias but with risk factors like diabetes or hypertension.
About 80% of such arrhythmias at ILR monitoring were asymptomatic, compared with less than one-fourth in the usual-care group. Yet pacemaker implantation for bradyarrhythmia was 53% more likely in the ILR group, according to a report published in JAMA Cardiology.
Most participants with asymptomatic bradycardia did not receive treatment for it, yet the study – despite the mostly conservative management – still showed “overtreatment with pacemakers” in the ILR group, observed lead author Søren Zöga Diederichsen, MD, PhD, Copenhagen University Hospital–Rigshospitalet.
Bradyarrhythmia overall predicted later syncope and all-cause and cardiovascular (CV) death, but did so regardless of whether the patient was ILR monitored or received a pacemaker, Diederichsen said in an interview.
“We didn’t see any signal, not even a small signal, toward a health benefit from monitoring and detecting bradycardias, or from acting on them conservatively or implanting pacemakers,” he noted.
The study “emphasizes that you should have symptoms” to justify pacemaker therapy for bradyarrhythmias, regardless of how they were detected, Dr. Diederichsen said.
“Clearly ILRs may identify patients with bradyarrhythmias deserving of treatment” when they are associated with symptoms, an accompanying editorial agreed. In the current analysis, however, “a large proportion of bradycardic events were completely asymptomatic.” Yet bradycardia predicted syncope and CV death in both the ILR and usual care groups, it noted.
“This does raise the question as to whether bradyarrhythmia may be a risk marker for underlying nonarrhythmic conditions to which preventive strategies and treatment should be directed,” wrote editorialists Mark H. Schoenfeld, MD, Yale University, New Haven, Conn., and Kristen K. Patton, MD, University of Washington, Seattle.
“In an aging population with ever-increasing comorbidities, it may become increasingly important to rule out bradycardia as a manifestation of a more sinister underlying disease,” they noted, and to identify “patients who may be particularly vulnerable to adverse outcomes of progressive distal conduction disease.”
The previously published LOOP trial, conducted at four sites in Denmark, compared ILR screening for atrial fibrillation to usual care in 6,004 patients at least 70 years or older, most with hypertension. The main results showed little benefit from screening for atrial fibrillation in prevention of incident stroke or systemic embolism over about 5 years.
The current LOOP analysis, post hoc with all the associated limitations, followed incident bradyarrhythmia in the ILR and usual-care groups; any treatment of the arrhythmia was at physician discretion. The total cohort averaged 75 years in age and 47.3% were women.
The rate of incident bradyarrhythmia was 8.1% overall; it was 20.8% for those with ILR monitoring and 3.8% in the usual care group, for a hazard ratio of 6.21 (95% confidence interval, 5.15-7.48, P < .001).
The arrhythmia was asymptomatic in 23.8% of usual-care patients and 79.8% of those with an ILR.
Bradyarrhythmia was significantly more likely among older patients, male patients, and those with a history of syncope, the group reported.
Pacemakers were implanted for bradyarrhythmia in 2.9% of usual-care patients and 4.5% of those with ILR monitoring for an HR of 1.53 (95% CI, 1.14-2.06, P < .001).
Among usual-care patients, bradyarrhythmia (vs no bradyarrhythmia) was associated with 5.2 times the risk for incident syncope (P < .001). That risk for syncope went up 2.6 times (P = .01) in the ILR group.
The corresponding risks for CV death among controls and among ILR patients increased 4.8 times (P < .001) and by 3.1 (P < .001), respectively. The risks for death from any cause tripled (P < .001) and rose 2.5 times (P < .001) among bradycardic controls and ILR patients, respectively.
Bradyarrhythmia was not significantly related to sudden cardiac death in either group, the report noted.
Given the increasing use of heart rhythm monitoring “inside and outside the clinical setting,” it stated, “bradyarrhythmias are likely to be detected more often, sometimes as an incidental finding. Knowledge about the underlying prevalence and prognostic significance could help guide decisions.”
The study “teaches us a little bit” about the true prevalence of bradyarrhythmias in the general population, including asymptomatic cases that appear to be subclinical or “physiological,” Dr. Diederichsen said in an interview.
It also suggests that such bradycardia will be increasingly observed as use of ILR for arrhythmia screening expands in practice, he predicted. It may also be picked up more often by wearables and other rhythm-monitoring technology used by the public.
In the latter case especially, Dr. Diederichsen said, the current analysis could potentially help alleviate any concerns that bradyarrhythmia without symptoms is something that has to be specifically treated.
Dr. Diederichsen disclosed grants from several Danish research institutions, R. og Hustrus Fond, and Medtronic, as well as receiving personal fees from Vital Beats and Bristol-Myers Squibb/Pfizer. Dr. Schoenfeld reported ownership of stock from Apple. Dr. Patton reported employment as a medical officer for the Food and Drug Administration and serving as associate editor for JAMA Cardiology.
A version of this article originally appeared on Medscape.com.
FROM JAMA CARDIOLOGY
Novel celery seed–derived drug may improve stroke outcomes
a new report suggests.
Patients treated with butylphthalide had fewer severe neurologic symptoms and better function 90 days after the stroke, compared with those receiving placebo.
Butylphthalide is approved and available for use in China, where the study was conducted. However, the medication hasn’t been approved for use by the U.S. Food and Drug Administration.
“Patients who received butylphthalide had less severe neurological symptoms and a better living status at 90 days post stroke, compared to those who received the placebo,” said coauthor Baixue Jia, MD, an attending physician in interventional neuroradiology at the Beijing Tiantan Hospital of Capital Medical University and a faculty member at the China National Clinical Research Center for Neurological Diseases in Beijing. “If the results are confirmed in other trials, this may lead to more options to treat strokes caused by clots.”
The study was presented at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Studying stroke outcomes
The researchers described butylphthalide as a cerebroprotective drug that was originally extracted from seeds of Apium graveolens. In China, previous studies have shown that the drug has cerebroprotective effects in animal models of ischemia-reperfusion, they noted.
In this randomized, double-blind, placebo-controlled trial, Dr. Jia and colleagues evaluated whether treatment with butylphthalide could improve 90-day outcomes for adults with acute ischemic stroke who received intravenous recombinant tissue plasminogen activator (tPA), endovascular treatment, or both.
The participants were treated at one of 59 medical centers in China between July 2018 and February 2022. Those who had minimal stroke symptoms on their initial exam, defined as a score of 0-3 on the National Institutes of Health Stroke Scale, or had severe stroke symptoms, defined as having a score of 26 or higher on the NIHSS, were excluded from the study.
Along with an initial revascularization intervention chosen by their physician, participants were randomly selected to receive either butylphthalide or a placebo daily for 90 days. The drug was administered through daily intravenous injections for the first 14 days, after which patients received oral capsules for 76 days.
The research team defined the outcomes as “favorable” if a patient fell into one of the following categories 90 days after the stroke: an initially mild to moderate stroke (NIHSS, 4-7) and no symptoms after treatment, defined as a score of 0 on the Modified Rankin Scale (mRS), which measures disability and dependence; an initially moderate to serious stroke (NIHSS, 8-14) and no residual symptoms or mild symptoms that don’t impair the ability to perform routine activities of daily living without assistance (mRS, 0-1); or an initially serious to severe stroke (NIHSS, 15-25) and no remaining symptoms or a slight disability that impairs some activities but allows one to conduct daily living without assistance (mRS, 0-2).
Secondary outcomes included symptomatic intracranial hemorrhage, recurrent stroke, and mortality.
Among the 1,216 participants, 607 were assigned to the treatment group, and 609 were assigned to the placebo group. The average age was 66 years, and 68% were men.
Overall, participants in the butylphthalide group were 70% more likely to have a favorable 90-day outcome, compared with the placebo group. Favorable outcomes occurred in 344 patients (56.7%) in the butylphthalide group, compared with 268 patients (44%) in the placebo group (odds ratio, 1.70; 95% confidence interval, 1.35-2.14; P < .001).
In addition, butylphthalide improved function equally well for the patients who initially received tPA, those who received endovascular treatment, and those who received both tPA and endovascular treatment.
Secondary events, such as recurrent stroke and intracranial hemorrhage, weren’t significantly different between the butylphthalide and placebo groups.
Ongoing questions
Dr. Jia and colleagues noted the need to understand how butylphthalide works in the brain. Animal studies have suggested several possible mechanisms, but it remains unclear.
“The next step should be investigating the exact mechanisms of butylphthalide in humans,” Dr. Jia said.
Additional research should assess the medication in other populations, the authors noted, particularly because the study involved participants who received initial treatment with tPA, endovascular treatment, or both. The results may not be generalizable to stroke patients who receive other treatments or to populations outside of China.
“While these are interesting results, this is only one relatively small study on a fairly select population in China. Butylphthalide, a medication initially compounded from celery seed, is not ready for use in standard stroke treatment,” said Daniel Lackland, DrPH, professor of neurology and director of the division of translational neurosciences and population studies at the Medical University of South Carolina, Charleston.
Dr. Lackland, who wasn’t involved with the study, is a member of the American Stroke Association’s Stroke Council. Although butylphthalide was originally extracted from seeds, he noted, it’s not what patients would find commercially available.
“The medication used in this study is not the same as celery seed or celery seed extract supplements,” he said. “Stroke survivors should always consult with their neurologist or healthcare professional regarding diet after a stroke.”
The study was funded by the National Key Technology Research and Development Program of the Ministry of Science and Technology of the People’s Republic of China and Shijiazhuang Pharmaceutical Group dl-3-butylphthalide Pharmaceutical. Several authors are employed with Beijing Tiantan Hospital and the Beijing Institute of Brain Disorders. Dr. Lackland reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new report suggests.
Patients treated with butylphthalide had fewer severe neurologic symptoms and better function 90 days after the stroke, compared with those receiving placebo.
Butylphthalide is approved and available for use in China, where the study was conducted. However, the medication hasn’t been approved for use by the U.S. Food and Drug Administration.
“Patients who received butylphthalide had less severe neurological symptoms and a better living status at 90 days post stroke, compared to those who received the placebo,” said coauthor Baixue Jia, MD, an attending physician in interventional neuroradiology at the Beijing Tiantan Hospital of Capital Medical University and a faculty member at the China National Clinical Research Center for Neurological Diseases in Beijing. “If the results are confirmed in other trials, this may lead to more options to treat strokes caused by clots.”
The study was presented at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Studying stroke outcomes
The researchers described butylphthalide as a cerebroprotective drug that was originally extracted from seeds of Apium graveolens. In China, previous studies have shown that the drug has cerebroprotective effects in animal models of ischemia-reperfusion, they noted.
In this randomized, double-blind, placebo-controlled trial, Dr. Jia and colleagues evaluated whether treatment with butylphthalide could improve 90-day outcomes for adults with acute ischemic stroke who received intravenous recombinant tissue plasminogen activator (tPA), endovascular treatment, or both.
The participants were treated at one of 59 medical centers in China between July 2018 and February 2022. Those who had minimal stroke symptoms on their initial exam, defined as a score of 0-3 on the National Institutes of Health Stroke Scale, or had severe stroke symptoms, defined as having a score of 26 or higher on the NIHSS, were excluded from the study.
Along with an initial revascularization intervention chosen by their physician, participants were randomly selected to receive either butylphthalide or a placebo daily for 90 days. The drug was administered through daily intravenous injections for the first 14 days, after which patients received oral capsules for 76 days.
The research team defined the outcomes as “favorable” if a patient fell into one of the following categories 90 days after the stroke: an initially mild to moderate stroke (NIHSS, 4-7) and no symptoms after treatment, defined as a score of 0 on the Modified Rankin Scale (mRS), which measures disability and dependence; an initially moderate to serious stroke (NIHSS, 8-14) and no residual symptoms or mild symptoms that don’t impair the ability to perform routine activities of daily living without assistance (mRS, 0-1); or an initially serious to severe stroke (NIHSS, 15-25) and no remaining symptoms or a slight disability that impairs some activities but allows one to conduct daily living without assistance (mRS, 0-2).
Secondary outcomes included symptomatic intracranial hemorrhage, recurrent stroke, and mortality.
Among the 1,216 participants, 607 were assigned to the treatment group, and 609 were assigned to the placebo group. The average age was 66 years, and 68% were men.
Overall, participants in the butylphthalide group were 70% more likely to have a favorable 90-day outcome, compared with the placebo group. Favorable outcomes occurred in 344 patients (56.7%) in the butylphthalide group, compared with 268 patients (44%) in the placebo group (odds ratio, 1.70; 95% confidence interval, 1.35-2.14; P < .001).
In addition, butylphthalide improved function equally well for the patients who initially received tPA, those who received endovascular treatment, and those who received both tPA and endovascular treatment.
Secondary events, such as recurrent stroke and intracranial hemorrhage, weren’t significantly different between the butylphthalide and placebo groups.
Ongoing questions
Dr. Jia and colleagues noted the need to understand how butylphthalide works in the brain. Animal studies have suggested several possible mechanisms, but it remains unclear.
“The next step should be investigating the exact mechanisms of butylphthalide in humans,” Dr. Jia said.
Additional research should assess the medication in other populations, the authors noted, particularly because the study involved participants who received initial treatment with tPA, endovascular treatment, or both. The results may not be generalizable to stroke patients who receive other treatments or to populations outside of China.
“While these are interesting results, this is only one relatively small study on a fairly select population in China. Butylphthalide, a medication initially compounded from celery seed, is not ready for use in standard stroke treatment,” said Daniel Lackland, DrPH, professor of neurology and director of the division of translational neurosciences and population studies at the Medical University of South Carolina, Charleston.
Dr. Lackland, who wasn’t involved with the study, is a member of the American Stroke Association’s Stroke Council. Although butylphthalide was originally extracted from seeds, he noted, it’s not what patients would find commercially available.
“The medication used in this study is not the same as celery seed or celery seed extract supplements,” he said. “Stroke survivors should always consult with their neurologist or healthcare professional regarding diet after a stroke.”
The study was funded by the National Key Technology Research and Development Program of the Ministry of Science and Technology of the People’s Republic of China and Shijiazhuang Pharmaceutical Group dl-3-butylphthalide Pharmaceutical. Several authors are employed with Beijing Tiantan Hospital and the Beijing Institute of Brain Disorders. Dr. Lackland reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new report suggests.
Patients treated with butylphthalide had fewer severe neurologic symptoms and better function 90 days after the stroke, compared with those receiving placebo.
Butylphthalide is approved and available for use in China, where the study was conducted. However, the medication hasn’t been approved for use by the U.S. Food and Drug Administration.
“Patients who received butylphthalide had less severe neurological symptoms and a better living status at 90 days post stroke, compared to those who received the placebo,” said coauthor Baixue Jia, MD, an attending physician in interventional neuroradiology at the Beijing Tiantan Hospital of Capital Medical University and a faculty member at the China National Clinical Research Center for Neurological Diseases in Beijing. “If the results are confirmed in other trials, this may lead to more options to treat strokes caused by clots.”
The study was presented at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Studying stroke outcomes
The researchers described butylphthalide as a cerebroprotective drug that was originally extracted from seeds of Apium graveolens. In China, previous studies have shown that the drug has cerebroprotective effects in animal models of ischemia-reperfusion, they noted.
In this randomized, double-blind, placebo-controlled trial, Dr. Jia and colleagues evaluated whether treatment with butylphthalide could improve 90-day outcomes for adults with acute ischemic stroke who received intravenous recombinant tissue plasminogen activator (tPA), endovascular treatment, or both.
The participants were treated at one of 59 medical centers in China between July 2018 and February 2022. Those who had minimal stroke symptoms on their initial exam, defined as a score of 0-3 on the National Institutes of Health Stroke Scale, or had severe stroke symptoms, defined as having a score of 26 or higher on the NIHSS, were excluded from the study.
Along with an initial revascularization intervention chosen by their physician, participants were randomly selected to receive either butylphthalide or a placebo daily for 90 days. The drug was administered through daily intravenous injections for the first 14 days, after which patients received oral capsules for 76 days.
The research team defined the outcomes as “favorable” if a patient fell into one of the following categories 90 days after the stroke: an initially mild to moderate stroke (NIHSS, 4-7) and no symptoms after treatment, defined as a score of 0 on the Modified Rankin Scale (mRS), which measures disability and dependence; an initially moderate to serious stroke (NIHSS, 8-14) and no residual symptoms or mild symptoms that don’t impair the ability to perform routine activities of daily living without assistance (mRS, 0-1); or an initially serious to severe stroke (NIHSS, 15-25) and no remaining symptoms or a slight disability that impairs some activities but allows one to conduct daily living without assistance (mRS, 0-2).
Secondary outcomes included symptomatic intracranial hemorrhage, recurrent stroke, and mortality.
Among the 1,216 participants, 607 were assigned to the treatment group, and 609 were assigned to the placebo group. The average age was 66 years, and 68% were men.
Overall, participants in the butylphthalide group were 70% more likely to have a favorable 90-day outcome, compared with the placebo group. Favorable outcomes occurred in 344 patients (56.7%) in the butylphthalide group, compared with 268 patients (44%) in the placebo group (odds ratio, 1.70; 95% confidence interval, 1.35-2.14; P < .001).
In addition, butylphthalide improved function equally well for the patients who initially received tPA, those who received endovascular treatment, and those who received both tPA and endovascular treatment.
Secondary events, such as recurrent stroke and intracranial hemorrhage, weren’t significantly different between the butylphthalide and placebo groups.
Ongoing questions
Dr. Jia and colleagues noted the need to understand how butylphthalide works in the brain. Animal studies have suggested several possible mechanisms, but it remains unclear.
“The next step should be investigating the exact mechanisms of butylphthalide in humans,” Dr. Jia said.
Additional research should assess the medication in other populations, the authors noted, particularly because the study involved participants who received initial treatment with tPA, endovascular treatment, or both. The results may not be generalizable to stroke patients who receive other treatments or to populations outside of China.
“While these are interesting results, this is only one relatively small study on a fairly select population in China. Butylphthalide, a medication initially compounded from celery seed, is not ready for use in standard stroke treatment,” said Daniel Lackland, DrPH, professor of neurology and director of the division of translational neurosciences and population studies at the Medical University of South Carolina, Charleston.
Dr. Lackland, who wasn’t involved with the study, is a member of the American Stroke Association’s Stroke Council. Although butylphthalide was originally extracted from seeds, he noted, it’s not what patients would find commercially available.
“The medication used in this study is not the same as celery seed or celery seed extract supplements,” he said. “Stroke survivors should always consult with their neurologist or healthcare professional regarding diet after a stroke.”
The study was funded by the National Key Technology Research and Development Program of the Ministry of Science and Technology of the People’s Republic of China and Shijiazhuang Pharmaceutical Group dl-3-butylphthalide Pharmaceutical. Several authors are employed with Beijing Tiantan Hospital and the Beijing Institute of Brain Disorders. Dr. Lackland reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ISC 2023
Cardiac issues twice as likely with COVID plus high troponin
Hospitalized COVID-19 patients with high troponin levels are twice as likely to have cardiac abnormalities than those with normal troponin, with or without COVID-19, a multicenter U.K. study suggests.
The causes were diverse, myocarditis prevalence was lower than previously reported, and myocardial scar emerged as an independent risk factor for adverse cardiovascular outcomes at 12 months.
“We know that multiorgan involvement in hospitalized patients with COVID-19 is common ... and may result in acute myocardial injury, detected by an increase in cardiac troponin concentrations,” John P. Greenwood, PhD, of the University of Leeds (England), told this news organization. “Elevated cardiac troponin is associated with a worse prognosis.”
“Multiple mechanisms of myocardial injury have been proposed and ... mitigation or prevention strategies likely depend on the underpinning mechanisms,” he said. “The sequelae of scar may predispose to late events.”
The study, published online in Circulation, also identified a new pattern of microinfarction on cardiac magnetic resonance (CMR) imaging, highlighting the pro-thrombotic nature of SARS-CoV-2, Dr. Greenwood said.
Injury patterns different
Three hundred and forty-two patients with COVID-19 and elevated troponin levels (COVID+/troponin+) across 25 centers were enrolled between June 2020 and March 2021 in COVID-HEART, deemed an “urgent public health study” in the United Kingdom. The aim was to characterize myocardial injury and its associations and sequelae in convalescent patients after hospitalization with COVID-19.
Enrollment took place during the Wuhan and Alpha waves of COVID-19: before vaccination and when dexamethasone and anticoagulant protocols were emerging. All participants underwent CMR at a median of 21 days after discharge.
Two prospective control groups also were recruited: 64 patients with COVID-19 and normal troponin levels (COVID+/troponin−) and 113 without COVID-19 or elevated troponin matched by age and cardiovascular comorbidities (COVID−/comorbidity+).
Overall, participants’ median age was 61 years and 69% were men. Common comorbidities included hypertension (47%), obesity (43%), and diabetes (25%).
The frequency of any heart abnormality – for example, left or right ventricular impairment, scar, or pericardial disease – was twice as great (61%) in COVID+/troponin+ cases, compared with controls (36% for COVID+/troponin− patients versus 31% for COVID−/comorbidity+ patients).
Specifically, more cases than controls had ventricular impairment (17.2% vs. 3.1% and 7.1%) or scar (42% vs. 7% and 23%).
The myocardial injury pattern differed between cases and controls, with cases more likely to have infarction (13% vs. 2% and 7%) or microinfarction (9% vs. 0% and 1%).
However, there was no between-group difference in nonischemic scar (13% vs. 5% and 14%).
The prevalence of probable recent myocarditis was 6.7% in cases, compared with 1.7% in controls without COVID-19 – “much lower” than in previous studies, Dr. Greenwood noted.
During follow-up, four COVID+/troponin+ patients (1.2%) died, and 34 (10%) experienced a subsequent major adverse cardiovascular event (MACE; 10.2%), which was similar to controls (6.1%).
Myocardial scar, but not previous COVID-19 infection or troponin level, was an independent predictor of MACE (odds ratio, 2.25).
“These findings suggest that macroangiopathic and microangiopathic thrombosis may be the key pathologic process for myocardial injury in COVID-19 survivors,” the authors conclude.
Dr. Greenwood added, “We are currently analyzing the 6-month follow-up CMR scans, the quality-of-life questionnaires, and the 6-minute walk tests. These will give us great understanding of how the heart repairs after acute myocardial injury associated with COVID-19. It will also allow us to assess the impact on patient quality of life and functional capacity.”
‘Tour de force’
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and a professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said, “This is a tour de force collaboration – obtaining this many MRIs across multiple centers in the pandemic is quite remarkable. The study highlights the multiple different processes that lead to cardiac injury in COVID patients, complements autopsy studies and prior smaller MRI studies, [and] also provides the best data on the rate of myocarditis to date among the subset of COVID patients with cardiac injury.”
Overall, he said, the findings “do support closer follow-up for patients who had COVID and elevated troponins. We need to see follow-up MRI results in this cohort, as well as longer term outcomes. We also need studies on newer, more benign variants that are likely to have lower rates of cardiac injury and even fewer MRI abnormalities.”
Matthias Stuber, PhD, and Aaron L. Baggish, MD, both of Lausanne University Hospital and University of Lausanne, Switzerland, noted in a related editorial, “We are also reminded that the clinical severity of COVID-19 is most often dictated by the presence of pre-existing comorbidity, with antecedent ischemic scar now added to the long list of bad actors. Although not the primary focus of the COVID-HEART study, the question of whether cardiac troponin levels should be checked routinely and universally during the index admission for COVID-19 remains unresolved,” they noted.
“In general, we are most effective as clinicians when we use tests to confirm or rule out the specific disease processes suspected by careful basic clinical assessment rather than in a shotgun manner among undifferentiated all-comers,” they conclude.
No commercial funding or relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.
Hospitalized COVID-19 patients with high troponin levels are twice as likely to have cardiac abnormalities than those with normal troponin, with or without COVID-19, a multicenter U.K. study suggests.
The causes were diverse, myocarditis prevalence was lower than previously reported, and myocardial scar emerged as an independent risk factor for adverse cardiovascular outcomes at 12 months.
“We know that multiorgan involvement in hospitalized patients with COVID-19 is common ... and may result in acute myocardial injury, detected by an increase in cardiac troponin concentrations,” John P. Greenwood, PhD, of the University of Leeds (England), told this news organization. “Elevated cardiac troponin is associated with a worse prognosis.”
“Multiple mechanisms of myocardial injury have been proposed and ... mitigation or prevention strategies likely depend on the underpinning mechanisms,” he said. “The sequelae of scar may predispose to late events.”
The study, published online in Circulation, also identified a new pattern of microinfarction on cardiac magnetic resonance (CMR) imaging, highlighting the pro-thrombotic nature of SARS-CoV-2, Dr. Greenwood said.
Injury patterns different
Three hundred and forty-two patients with COVID-19 and elevated troponin levels (COVID+/troponin+) across 25 centers were enrolled between June 2020 and March 2021 in COVID-HEART, deemed an “urgent public health study” in the United Kingdom. The aim was to characterize myocardial injury and its associations and sequelae in convalescent patients after hospitalization with COVID-19.
Enrollment took place during the Wuhan and Alpha waves of COVID-19: before vaccination and when dexamethasone and anticoagulant protocols were emerging. All participants underwent CMR at a median of 21 days after discharge.
Two prospective control groups also were recruited: 64 patients with COVID-19 and normal troponin levels (COVID+/troponin−) and 113 without COVID-19 or elevated troponin matched by age and cardiovascular comorbidities (COVID−/comorbidity+).
Overall, participants’ median age was 61 years and 69% were men. Common comorbidities included hypertension (47%), obesity (43%), and diabetes (25%).
The frequency of any heart abnormality – for example, left or right ventricular impairment, scar, or pericardial disease – was twice as great (61%) in COVID+/troponin+ cases, compared with controls (36% for COVID+/troponin− patients versus 31% for COVID−/comorbidity+ patients).
Specifically, more cases than controls had ventricular impairment (17.2% vs. 3.1% and 7.1%) or scar (42% vs. 7% and 23%).
The myocardial injury pattern differed between cases and controls, with cases more likely to have infarction (13% vs. 2% and 7%) or microinfarction (9% vs. 0% and 1%).
However, there was no between-group difference in nonischemic scar (13% vs. 5% and 14%).
The prevalence of probable recent myocarditis was 6.7% in cases, compared with 1.7% in controls without COVID-19 – “much lower” than in previous studies, Dr. Greenwood noted.
During follow-up, four COVID+/troponin+ patients (1.2%) died, and 34 (10%) experienced a subsequent major adverse cardiovascular event (MACE; 10.2%), which was similar to controls (6.1%).
Myocardial scar, but not previous COVID-19 infection or troponin level, was an independent predictor of MACE (odds ratio, 2.25).
“These findings suggest that macroangiopathic and microangiopathic thrombosis may be the key pathologic process for myocardial injury in COVID-19 survivors,” the authors conclude.
Dr. Greenwood added, “We are currently analyzing the 6-month follow-up CMR scans, the quality-of-life questionnaires, and the 6-minute walk tests. These will give us great understanding of how the heart repairs after acute myocardial injury associated with COVID-19. It will also allow us to assess the impact on patient quality of life and functional capacity.”
‘Tour de force’
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and a professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said, “This is a tour de force collaboration – obtaining this many MRIs across multiple centers in the pandemic is quite remarkable. The study highlights the multiple different processes that lead to cardiac injury in COVID patients, complements autopsy studies and prior smaller MRI studies, [and] also provides the best data on the rate of myocarditis to date among the subset of COVID patients with cardiac injury.”
Overall, he said, the findings “do support closer follow-up for patients who had COVID and elevated troponins. We need to see follow-up MRI results in this cohort, as well as longer term outcomes. We also need studies on newer, more benign variants that are likely to have lower rates of cardiac injury and even fewer MRI abnormalities.”
Matthias Stuber, PhD, and Aaron L. Baggish, MD, both of Lausanne University Hospital and University of Lausanne, Switzerland, noted in a related editorial, “We are also reminded that the clinical severity of COVID-19 is most often dictated by the presence of pre-existing comorbidity, with antecedent ischemic scar now added to the long list of bad actors. Although not the primary focus of the COVID-HEART study, the question of whether cardiac troponin levels should be checked routinely and universally during the index admission for COVID-19 remains unresolved,” they noted.
“In general, we are most effective as clinicians when we use tests to confirm or rule out the specific disease processes suspected by careful basic clinical assessment rather than in a shotgun manner among undifferentiated all-comers,” they conclude.
No commercial funding or relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.
Hospitalized COVID-19 patients with high troponin levels are twice as likely to have cardiac abnormalities than those with normal troponin, with or without COVID-19, a multicenter U.K. study suggests.
The causes were diverse, myocarditis prevalence was lower than previously reported, and myocardial scar emerged as an independent risk factor for adverse cardiovascular outcomes at 12 months.
“We know that multiorgan involvement in hospitalized patients with COVID-19 is common ... and may result in acute myocardial injury, detected by an increase in cardiac troponin concentrations,” John P. Greenwood, PhD, of the University of Leeds (England), told this news organization. “Elevated cardiac troponin is associated with a worse prognosis.”
“Multiple mechanisms of myocardial injury have been proposed and ... mitigation or prevention strategies likely depend on the underpinning mechanisms,” he said. “The sequelae of scar may predispose to late events.”
The study, published online in Circulation, also identified a new pattern of microinfarction on cardiac magnetic resonance (CMR) imaging, highlighting the pro-thrombotic nature of SARS-CoV-2, Dr. Greenwood said.
Injury patterns different
Three hundred and forty-two patients with COVID-19 and elevated troponin levels (COVID+/troponin+) across 25 centers were enrolled between June 2020 and March 2021 in COVID-HEART, deemed an “urgent public health study” in the United Kingdom. The aim was to characterize myocardial injury and its associations and sequelae in convalescent patients after hospitalization with COVID-19.
Enrollment took place during the Wuhan and Alpha waves of COVID-19: before vaccination and when dexamethasone and anticoagulant protocols were emerging. All participants underwent CMR at a median of 21 days after discharge.
Two prospective control groups also were recruited: 64 patients with COVID-19 and normal troponin levels (COVID+/troponin−) and 113 without COVID-19 or elevated troponin matched by age and cardiovascular comorbidities (COVID−/comorbidity+).
Overall, participants’ median age was 61 years and 69% were men. Common comorbidities included hypertension (47%), obesity (43%), and diabetes (25%).
The frequency of any heart abnormality – for example, left or right ventricular impairment, scar, or pericardial disease – was twice as great (61%) in COVID+/troponin+ cases, compared with controls (36% for COVID+/troponin− patients versus 31% for COVID−/comorbidity+ patients).
Specifically, more cases than controls had ventricular impairment (17.2% vs. 3.1% and 7.1%) or scar (42% vs. 7% and 23%).
The myocardial injury pattern differed between cases and controls, with cases more likely to have infarction (13% vs. 2% and 7%) or microinfarction (9% vs. 0% and 1%).
However, there was no between-group difference in nonischemic scar (13% vs. 5% and 14%).
The prevalence of probable recent myocarditis was 6.7% in cases, compared with 1.7% in controls without COVID-19 – “much lower” than in previous studies, Dr. Greenwood noted.
During follow-up, four COVID+/troponin+ patients (1.2%) died, and 34 (10%) experienced a subsequent major adverse cardiovascular event (MACE; 10.2%), which was similar to controls (6.1%).
Myocardial scar, but not previous COVID-19 infection or troponin level, was an independent predictor of MACE (odds ratio, 2.25).
“These findings suggest that macroangiopathic and microangiopathic thrombosis may be the key pathologic process for myocardial injury in COVID-19 survivors,” the authors conclude.
Dr. Greenwood added, “We are currently analyzing the 6-month follow-up CMR scans, the quality-of-life questionnaires, and the 6-minute walk tests. These will give us great understanding of how the heart repairs after acute myocardial injury associated with COVID-19. It will also allow us to assess the impact on patient quality of life and functional capacity.”
‘Tour de force’
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and a professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said, “This is a tour de force collaboration – obtaining this many MRIs across multiple centers in the pandemic is quite remarkable. The study highlights the multiple different processes that lead to cardiac injury in COVID patients, complements autopsy studies and prior smaller MRI studies, [and] also provides the best data on the rate of myocarditis to date among the subset of COVID patients with cardiac injury.”
Overall, he said, the findings “do support closer follow-up for patients who had COVID and elevated troponins. We need to see follow-up MRI results in this cohort, as well as longer term outcomes. We also need studies on newer, more benign variants that are likely to have lower rates of cardiac injury and even fewer MRI abnormalities.”
Matthias Stuber, PhD, and Aaron L. Baggish, MD, both of Lausanne University Hospital and University of Lausanne, Switzerland, noted in a related editorial, “We are also reminded that the clinical severity of COVID-19 is most often dictated by the presence of pre-existing comorbidity, with antecedent ischemic scar now added to the long list of bad actors. Although not the primary focus of the COVID-HEART study, the question of whether cardiac troponin levels should be checked routinely and universally during the index admission for COVID-19 remains unresolved,” they noted.
“In general, we are most effective as clinicians when we use tests to confirm or rule out the specific disease processes suspected by careful basic clinical assessment rather than in a shotgun manner among undifferentiated all-comers,” they conclude.
No commercial funding or relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.
STROKE AF at 3 years: High AFib rate after atherosclerotic stroke
In the STROKE AF study, among patients who had a stroke presumably caused by atherosclerosis, the rate of atrial fibrillation (AFib) was almost 22% at 3 years, as detected by continuous monitoring.
The 3-year results from the study were presented by Lee H. Schwamm, MD, of Massachusetts General Hospital, Boston, at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Dr. Schwamm said the high rate of AFib detection in this study suggests that continuous monitoring for AFib should be considered for a larger population of stroke patients, rather than just those with cryptogenic stroke.
“We found a much higher rate of AF[ib] than we expected in this population of patients who have had an atherosclerotic stroke,” Dr. Schwamm said in an interview.
“These AF[ib] occurrences were found by a device, so they are known as ‘device-documented AF[ib].’ The patient is not generally aware of symptoms, but 67% of the AF[ib] episodes lasted for more than 1 hour, showing that this is not trivial AF[ib]. This is meaningful AF[ib],” he said.
Dr. Schwamm said the major question is whether these cases of AFib that are detected with a device warrant treatment with anticoagulation. He noted that, in this study, clinicians decided to provide anticoagulation to 70%-80% of patients in whom AFib was detected.
“If we think it deserves treatment, then we have to look for it. And if we care about finding AF[ib], we have no choice but to monitor continuously,” he said.
“If this data doesn’t convince you that AF[ib] is present in this population, I don’t think any data will. Because it is consistent, it accumulates over time and looks remarkably similar to a set of data that we have all become very comfortable with – the CRYSTAL-AF study in patients with cryptogenic stroke,” he stated.
Dr. Schwamm noted that the STROKE AF trial was not based on the cause of the index stroke; rather, it was asking whether there are risk factors that could contribute to the 25% stroke recurrence rate in this population that are not covered in current guidelines.
“I’m really trying to move away from the anchor that I was trained in, which is to figure out the cause of the last stroke to help decide how to prevent the next stroke, towards more of a probabilistic model – of what is all the information I have at my disposal and how do I act on it to prevent the next stroke? We have to start thinking differently about building models for future stroke risk and determining therapy based on that,” he commented.
Changing practice
ISC 2023 program chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., and moderator of the session at which the results were presented, discussed the STROKE AF results in a highlights presentation.
“To me as clinician, these results are even more relevant than those at 12 months,” Dr. Jovin said. “The lesson I took is that AF[ib] is even more prevalent than we thought. The burden of AF[ib] is significant in these patients, and it doesn’t seem to be limited to a particular time. These are very thought-provoking results which are going to change clinical practice. I think the threshold for long-term monitoring will be lower.”
Comoderator Lauren Sansing, MD, Yale University, New Haven, Conn., added: “This study shows that the longer we monitor, the more patients with AF[ib] we are likely to pick up. And because in two-thirds of patients with AF[ib], it lasted longer than 1 hour, I do believe this was clinically relevant AF[ib]. The question now is, do we monitor everyone? I think it puts the burden on us to search for AF[ib] in our patients.”
In his presentation, Dr. Schwamm explained that, on the basis of the CRYSTAL-AF study, insertable cardiac monitoring devices are frequently used to identify poststroke AFib in patients with cryptogenic stroke. In the device-monitored arm of that study, AFib was detected in 12.4% of patients over 12 months versus 2.0% in the control arm.
“However, we don’t know how often AF[ib] is detected in other presumed stroke types – largely those due to atherosclerosis,” he said.
He pointed out that, at present, long-term monitoring post stroke for the detection of AFib is not currently recommended for patients with ischemic stroke, owing to presumed small-vessel occlusion or large-artery atherosclerosis.
“In these patients, we are not suspecting AF[ib] because we believe the cause of the stroke was not embolic. But we wanted to investigate what the AF[ib] risk is in these patients, who often have multiple stroke risk factors,” he said.
The trial enrolled 496 patients at 33 centers in the United States. Eligible patients were aged 60 years or older or aged 50-59 years with at least one additional stroke risk factor and had an index stroke that was attributed to large-artery or small-vessel disease. Patients were randomly assigned either to continuous monitoring with the Reveal LINQ device (Medtronic) or to the control arm following site-specific standard of care for AFib detection.
Dr. Schwamm noted that usual care for these patients normally involves monitoring for just a few days while in hospital, but this picks up less than 5% of AFib occurrences.
Baseline characteristics of patients in the STROKE AF study showed that the enrolled population was at high risk for stroke, with a CHADSVASC score of 5. But the index strokes were generally small; the median National Institutes of Health Stroke Scale score was 2.
Results at 12 months, reported 2 years ago, showed a 12.5% incidence of AFib with continuous monitoring versus 1.8% with standard of care (hazard ratio, 7.7; P < .001), rates similar to that found in the CRYSTAL-AF study.
By 3 years, the rate of detected AFib had risen to 21.7% in the continuous monitoring arm versus 2.4% in the control arm (HR, 10.0; P < .001).
“At 12 months, we were seven times more likely to detect AF[ib] with continuous monitoring in these patients, and by 3 years, it was 10 times more likely that AF would be detected with continuous monitoring. I think we’ve settled the question of the best way to find AF[ib] in these patients – it is with an inserted device,” Dr. Schwamm said.
“We have also shown that this is not a transient rise in AFib after the stroke which then diminishes over the next few years. It is a continuous and progressive detection of AF[ib].”
Dr. Schwamm pointed out that 88% of the recorded AFib episodes were asymptomatic. “So relying on patients self-reporting symptoms when deciding who to monitor is unreliable and not a sensible strategy.”
The median time to the first adjudicated AFib episode at 12-month follow-up was 99 days; at the 3-year follow-up, it was 284 days.
“This shows that 30 days of monitoring with an external patch is not sufficient to exclude the presence of AF[ib]. And this really argues for a strategy of immediate insertion of cardiac monitor placement if your goal is to look for AF[ib],” Dr. Schwamm commented.
Is this clinically relevant AFib?
Dr. Schwamm acknowledged that there is a question of whether device-detected AFib should be thought about in the same way as clinically detected AFib with respect to future stroke risk.
He noted that, in this study, 67.4% of patients for whom AFib was detected by continuous monitoring (31 of 46 patients) had at least one episode of AFib that lasted more than 1 hour.
“This is not a trivial little squiggle of something on an EKG which then goes away. This is of significant duration that the cardiologist who adjudicated these rhythm strips felt confident was AF[ib].”
He added: “AF[ib] lasting more than 1 hour crosses the threshold for most practitioners I know to feel confident in treating the patient with anticoagulation. If it was symptomatic AF, this wouldn’t even be a question.”
Dr. Schwamm made the point that device-detected A AFib F has been accepted as worthy of treatment in patients after cryptogenic stroke.
“If we are honest with ourselves and if we have no hesitation in starting anticoagulation in a patient with cryptogenic stroke who has had device-detected AF 6 months later, should we decide that if the patient has had a lacunar stroke, we can ignore that same device-detected fibrillation?”
He put forward the idea that, at some level, all stroke is cryptogenic. “We never know for sure what the cause was. We have hypotheses, we have associations, but we don’t really know. So how much should we weigh that presumptive etiology in terms of how we interpret a rhythm disturbance of fibrillation?”
When looking for predictors of AFib in this study, the investigators found that patients were more likely to have an episode of AFib detected if they had one of the four following risk factors: congestive heart failure, left atrial enlargement, obesity, or QRS prolongation.
“In patients with any one of those four factors, 30% of those had device-detected AF[ib]. These are same predictors of AF[ib] that we are all accustomed to,” Dr. Schwamm said.
Shared decision-making
Dr. Schwamm said in an interview that, in his practice, for these patients, the decision as to whether to use continuous monitoring is made with the patient through shared decision-making.
“We discuss the chance that they could have AF[ib], and I suggest that it might be worth looking for it, but there are factors to be considered. There is a cost to the device, and reimbursement may depend on insurance coverage. Also, some patients may have strong feelings about having the chip implanted in their body.”
He says implanting the chip is easy. “It takes longer to check in at the front desk than to put the device in. It is injected under the skin. It just needs two stitches and a Band-Aid.” The device connects with a smartphone, and the results are interpreted by a cardiologist.
Dr. Schwamm pointed out that the optimal antithrombotic regimen for these patients in whom AFib is detected remains uncertain and should be the focus of future research.
“Do we just stick to antiplatelet therapy or advance to anticoagulation? In moving to an anticoagulant, are we providing less effective prevention for the atherosclerotic stroke risk at the expense of reducing the AF[ib]-related stroke risk? That may be a reasonable trade-off because we know the disability from AF[ib]-associated stroke is much higher.
“Or perhaps the optimal therapy is aspirin plus low-dose anticoagulant? Or left atrial appendage closure and an antiplatelet for patients at a higher risk of bleeding?” he said. “These are the really important questions we need to start asking.”
He added that he hopes a future study will address these questions, but he noted that it would have to be a large study, that it would have to first identify these patients and then randomly assign them to anticoagulation or to no treatment. “That is quite a major undertaking.”
In the highlights presentation, Dr. Jovin said he was uncertain of which of these patients in whom AFib is detected would benefit from anticoagulation. He said he would also like to see a randomized trial on this. But he added: “This would be challenging, as there is the issue of whether there would be equipoise to allow us to randomize to a placebo.”
Dr. Sansing agreed. “I think it would be a hard sell. I would have to think carefully about randomizing a patient to anticoagulation therapy or no therapy who has been found to have AF[ib].”
Dr. Schwamm noted that the current STROKE-AF study was not designed or powered to detect differences in stroke recurrence rates and that there was no difference in stroke recurrence rates between the two arms. There was also no randomization with regard to treatment; choice of medication was left to the discretion of the treating physician.
But he noted that only for 3 of the 34 patients with recurrent stroke in the continuous-monitor arm was AFib detected prior to the recurrent stroke, and only one of those three was receiving anticoagulation at the time of the recurrent stroke.
“These strokes were occurring in patients who did not have device-detected AF[ib],” Dr. Schwamm said. “This is because the population in this study were loaded with stroke risk factors and are at risk of recurrent stroke, but we don’t have the opportunity in this study to really understand the significance of the recurrent strokes.”
The STROKE AF trial was funded by Medtronic. Dr. Schwamm is a consultant to Medtronic.
A version of this article originally appeared on Medscape.com.
In the STROKE AF study, among patients who had a stroke presumably caused by atherosclerosis, the rate of atrial fibrillation (AFib) was almost 22% at 3 years, as detected by continuous monitoring.
The 3-year results from the study were presented by Lee H. Schwamm, MD, of Massachusetts General Hospital, Boston, at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Dr. Schwamm said the high rate of AFib detection in this study suggests that continuous monitoring for AFib should be considered for a larger population of stroke patients, rather than just those with cryptogenic stroke.
“We found a much higher rate of AF[ib] than we expected in this population of patients who have had an atherosclerotic stroke,” Dr. Schwamm said in an interview.
“These AF[ib] occurrences were found by a device, so they are known as ‘device-documented AF[ib].’ The patient is not generally aware of symptoms, but 67% of the AF[ib] episodes lasted for more than 1 hour, showing that this is not trivial AF[ib]. This is meaningful AF[ib],” he said.
Dr. Schwamm said the major question is whether these cases of AFib that are detected with a device warrant treatment with anticoagulation. He noted that, in this study, clinicians decided to provide anticoagulation to 70%-80% of patients in whom AFib was detected.
“If we think it deserves treatment, then we have to look for it. And if we care about finding AF[ib], we have no choice but to monitor continuously,” he said.
“If this data doesn’t convince you that AF[ib] is present in this population, I don’t think any data will. Because it is consistent, it accumulates over time and looks remarkably similar to a set of data that we have all become very comfortable with – the CRYSTAL-AF study in patients with cryptogenic stroke,” he stated.
Dr. Schwamm noted that the STROKE AF trial was not based on the cause of the index stroke; rather, it was asking whether there are risk factors that could contribute to the 25% stroke recurrence rate in this population that are not covered in current guidelines.
“I’m really trying to move away from the anchor that I was trained in, which is to figure out the cause of the last stroke to help decide how to prevent the next stroke, towards more of a probabilistic model – of what is all the information I have at my disposal and how do I act on it to prevent the next stroke? We have to start thinking differently about building models for future stroke risk and determining therapy based on that,” he commented.
Changing practice
ISC 2023 program chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., and moderator of the session at which the results were presented, discussed the STROKE AF results in a highlights presentation.
“To me as clinician, these results are even more relevant than those at 12 months,” Dr. Jovin said. “The lesson I took is that AF[ib] is even more prevalent than we thought. The burden of AF[ib] is significant in these patients, and it doesn’t seem to be limited to a particular time. These are very thought-provoking results which are going to change clinical practice. I think the threshold for long-term monitoring will be lower.”
Comoderator Lauren Sansing, MD, Yale University, New Haven, Conn., added: “This study shows that the longer we monitor, the more patients with AF[ib] we are likely to pick up. And because in two-thirds of patients with AF[ib], it lasted longer than 1 hour, I do believe this was clinically relevant AF[ib]. The question now is, do we monitor everyone? I think it puts the burden on us to search for AF[ib] in our patients.”
In his presentation, Dr. Schwamm explained that, on the basis of the CRYSTAL-AF study, insertable cardiac monitoring devices are frequently used to identify poststroke AFib in patients with cryptogenic stroke. In the device-monitored arm of that study, AFib was detected in 12.4% of patients over 12 months versus 2.0% in the control arm.
“However, we don’t know how often AF[ib] is detected in other presumed stroke types – largely those due to atherosclerosis,” he said.
He pointed out that, at present, long-term monitoring post stroke for the detection of AFib is not currently recommended for patients with ischemic stroke, owing to presumed small-vessel occlusion or large-artery atherosclerosis.
“In these patients, we are not suspecting AF[ib] because we believe the cause of the stroke was not embolic. But we wanted to investigate what the AF[ib] risk is in these patients, who often have multiple stroke risk factors,” he said.
The trial enrolled 496 patients at 33 centers in the United States. Eligible patients were aged 60 years or older or aged 50-59 years with at least one additional stroke risk factor and had an index stroke that was attributed to large-artery or small-vessel disease. Patients were randomly assigned either to continuous monitoring with the Reveal LINQ device (Medtronic) or to the control arm following site-specific standard of care for AFib detection.
Dr. Schwamm noted that usual care for these patients normally involves monitoring for just a few days while in hospital, but this picks up less than 5% of AFib occurrences.
Baseline characteristics of patients in the STROKE AF study showed that the enrolled population was at high risk for stroke, with a CHADSVASC score of 5. But the index strokes were generally small; the median National Institutes of Health Stroke Scale score was 2.
Results at 12 months, reported 2 years ago, showed a 12.5% incidence of AFib with continuous monitoring versus 1.8% with standard of care (hazard ratio, 7.7; P < .001), rates similar to that found in the CRYSTAL-AF study.
By 3 years, the rate of detected AFib had risen to 21.7% in the continuous monitoring arm versus 2.4% in the control arm (HR, 10.0; P < .001).
“At 12 months, we were seven times more likely to detect AF[ib] with continuous monitoring in these patients, and by 3 years, it was 10 times more likely that AF would be detected with continuous monitoring. I think we’ve settled the question of the best way to find AF[ib] in these patients – it is with an inserted device,” Dr. Schwamm said.
“We have also shown that this is not a transient rise in AFib after the stroke which then diminishes over the next few years. It is a continuous and progressive detection of AF[ib].”
Dr. Schwamm pointed out that 88% of the recorded AFib episodes were asymptomatic. “So relying on patients self-reporting symptoms when deciding who to monitor is unreliable and not a sensible strategy.”
The median time to the first adjudicated AFib episode at 12-month follow-up was 99 days; at the 3-year follow-up, it was 284 days.
“This shows that 30 days of monitoring with an external patch is not sufficient to exclude the presence of AF[ib]. And this really argues for a strategy of immediate insertion of cardiac monitor placement if your goal is to look for AF[ib],” Dr. Schwamm commented.
Is this clinically relevant AFib?
Dr. Schwamm acknowledged that there is a question of whether device-detected AFib should be thought about in the same way as clinically detected AFib with respect to future stroke risk.
He noted that, in this study, 67.4% of patients for whom AFib was detected by continuous monitoring (31 of 46 patients) had at least one episode of AFib that lasted more than 1 hour.
“This is not a trivial little squiggle of something on an EKG which then goes away. This is of significant duration that the cardiologist who adjudicated these rhythm strips felt confident was AF[ib].”
He added: “AF[ib] lasting more than 1 hour crosses the threshold for most practitioners I know to feel confident in treating the patient with anticoagulation. If it was symptomatic AF, this wouldn’t even be a question.”
Dr. Schwamm made the point that device-detected A AFib F has been accepted as worthy of treatment in patients after cryptogenic stroke.
“If we are honest with ourselves and if we have no hesitation in starting anticoagulation in a patient with cryptogenic stroke who has had device-detected AF 6 months later, should we decide that if the patient has had a lacunar stroke, we can ignore that same device-detected fibrillation?”
He put forward the idea that, at some level, all stroke is cryptogenic. “We never know for sure what the cause was. We have hypotheses, we have associations, but we don’t really know. So how much should we weigh that presumptive etiology in terms of how we interpret a rhythm disturbance of fibrillation?”
When looking for predictors of AFib in this study, the investigators found that patients were more likely to have an episode of AFib detected if they had one of the four following risk factors: congestive heart failure, left atrial enlargement, obesity, or QRS prolongation.
“In patients with any one of those four factors, 30% of those had device-detected AF[ib]. These are same predictors of AF[ib] that we are all accustomed to,” Dr. Schwamm said.
Shared decision-making
Dr. Schwamm said in an interview that, in his practice, for these patients, the decision as to whether to use continuous monitoring is made with the patient through shared decision-making.
“We discuss the chance that they could have AF[ib], and I suggest that it might be worth looking for it, but there are factors to be considered. There is a cost to the device, and reimbursement may depend on insurance coverage. Also, some patients may have strong feelings about having the chip implanted in their body.”
He says implanting the chip is easy. “It takes longer to check in at the front desk than to put the device in. It is injected under the skin. It just needs two stitches and a Band-Aid.” The device connects with a smartphone, and the results are interpreted by a cardiologist.
Dr. Schwamm pointed out that the optimal antithrombotic regimen for these patients in whom AFib is detected remains uncertain and should be the focus of future research.
“Do we just stick to antiplatelet therapy or advance to anticoagulation? In moving to an anticoagulant, are we providing less effective prevention for the atherosclerotic stroke risk at the expense of reducing the AF[ib]-related stroke risk? That may be a reasonable trade-off because we know the disability from AF[ib]-associated stroke is much higher.
“Or perhaps the optimal therapy is aspirin plus low-dose anticoagulant? Or left atrial appendage closure and an antiplatelet for patients at a higher risk of bleeding?” he said. “These are the really important questions we need to start asking.”
He added that he hopes a future study will address these questions, but he noted that it would have to be a large study, that it would have to first identify these patients and then randomly assign them to anticoagulation or to no treatment. “That is quite a major undertaking.”
In the highlights presentation, Dr. Jovin said he was uncertain of which of these patients in whom AFib is detected would benefit from anticoagulation. He said he would also like to see a randomized trial on this. But he added: “This would be challenging, as there is the issue of whether there would be equipoise to allow us to randomize to a placebo.”
Dr. Sansing agreed. “I think it would be a hard sell. I would have to think carefully about randomizing a patient to anticoagulation therapy or no therapy who has been found to have AF[ib].”
Dr. Schwamm noted that the current STROKE-AF study was not designed or powered to detect differences in stroke recurrence rates and that there was no difference in stroke recurrence rates between the two arms. There was also no randomization with regard to treatment; choice of medication was left to the discretion of the treating physician.
But he noted that only for 3 of the 34 patients with recurrent stroke in the continuous-monitor arm was AFib detected prior to the recurrent stroke, and only one of those three was receiving anticoagulation at the time of the recurrent stroke.
“These strokes were occurring in patients who did not have device-detected AF[ib],” Dr. Schwamm said. “This is because the population in this study were loaded with stroke risk factors and are at risk of recurrent stroke, but we don’t have the opportunity in this study to really understand the significance of the recurrent strokes.”
The STROKE AF trial was funded by Medtronic. Dr. Schwamm is a consultant to Medtronic.
A version of this article originally appeared on Medscape.com.
In the STROKE AF study, among patients who had a stroke presumably caused by atherosclerosis, the rate of atrial fibrillation (AFib) was almost 22% at 3 years, as detected by continuous monitoring.
The 3-year results from the study were presented by Lee H. Schwamm, MD, of Massachusetts General Hospital, Boston, at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Dr. Schwamm said the high rate of AFib detection in this study suggests that continuous monitoring for AFib should be considered for a larger population of stroke patients, rather than just those with cryptogenic stroke.
“We found a much higher rate of AF[ib] than we expected in this population of patients who have had an atherosclerotic stroke,” Dr. Schwamm said in an interview.
“These AF[ib] occurrences were found by a device, so they are known as ‘device-documented AF[ib].’ The patient is not generally aware of symptoms, but 67% of the AF[ib] episodes lasted for more than 1 hour, showing that this is not trivial AF[ib]. This is meaningful AF[ib],” he said.
Dr. Schwamm said the major question is whether these cases of AFib that are detected with a device warrant treatment with anticoagulation. He noted that, in this study, clinicians decided to provide anticoagulation to 70%-80% of patients in whom AFib was detected.
“If we think it deserves treatment, then we have to look for it. And if we care about finding AF[ib], we have no choice but to monitor continuously,” he said.
“If this data doesn’t convince you that AF[ib] is present in this population, I don’t think any data will. Because it is consistent, it accumulates over time and looks remarkably similar to a set of data that we have all become very comfortable with – the CRYSTAL-AF study in patients with cryptogenic stroke,” he stated.
Dr. Schwamm noted that the STROKE AF trial was not based on the cause of the index stroke; rather, it was asking whether there are risk factors that could contribute to the 25% stroke recurrence rate in this population that are not covered in current guidelines.
“I’m really trying to move away from the anchor that I was trained in, which is to figure out the cause of the last stroke to help decide how to prevent the next stroke, towards more of a probabilistic model – of what is all the information I have at my disposal and how do I act on it to prevent the next stroke? We have to start thinking differently about building models for future stroke risk and determining therapy based on that,” he commented.
Changing practice
ISC 2023 program chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., and moderator of the session at which the results were presented, discussed the STROKE AF results in a highlights presentation.
“To me as clinician, these results are even more relevant than those at 12 months,” Dr. Jovin said. “The lesson I took is that AF[ib] is even more prevalent than we thought. The burden of AF[ib] is significant in these patients, and it doesn’t seem to be limited to a particular time. These are very thought-provoking results which are going to change clinical practice. I think the threshold for long-term monitoring will be lower.”
Comoderator Lauren Sansing, MD, Yale University, New Haven, Conn., added: “This study shows that the longer we monitor, the more patients with AF[ib] we are likely to pick up. And because in two-thirds of patients with AF[ib], it lasted longer than 1 hour, I do believe this was clinically relevant AF[ib]. The question now is, do we monitor everyone? I think it puts the burden on us to search for AF[ib] in our patients.”
In his presentation, Dr. Schwamm explained that, on the basis of the CRYSTAL-AF study, insertable cardiac monitoring devices are frequently used to identify poststroke AFib in patients with cryptogenic stroke. In the device-monitored arm of that study, AFib was detected in 12.4% of patients over 12 months versus 2.0% in the control arm.
“However, we don’t know how often AF[ib] is detected in other presumed stroke types – largely those due to atherosclerosis,” he said.
He pointed out that, at present, long-term monitoring post stroke for the detection of AFib is not currently recommended for patients with ischemic stroke, owing to presumed small-vessel occlusion or large-artery atherosclerosis.
“In these patients, we are not suspecting AF[ib] because we believe the cause of the stroke was not embolic. But we wanted to investigate what the AF[ib] risk is in these patients, who often have multiple stroke risk factors,” he said.
The trial enrolled 496 patients at 33 centers in the United States. Eligible patients were aged 60 years or older or aged 50-59 years with at least one additional stroke risk factor and had an index stroke that was attributed to large-artery or small-vessel disease. Patients were randomly assigned either to continuous monitoring with the Reveal LINQ device (Medtronic) or to the control arm following site-specific standard of care for AFib detection.
Dr. Schwamm noted that usual care for these patients normally involves monitoring for just a few days while in hospital, but this picks up less than 5% of AFib occurrences.
Baseline characteristics of patients in the STROKE AF study showed that the enrolled population was at high risk for stroke, with a CHADSVASC score of 5. But the index strokes were generally small; the median National Institutes of Health Stroke Scale score was 2.
Results at 12 months, reported 2 years ago, showed a 12.5% incidence of AFib with continuous monitoring versus 1.8% with standard of care (hazard ratio, 7.7; P < .001), rates similar to that found in the CRYSTAL-AF study.
By 3 years, the rate of detected AFib had risen to 21.7% in the continuous monitoring arm versus 2.4% in the control arm (HR, 10.0; P < .001).
“At 12 months, we were seven times more likely to detect AF[ib] with continuous monitoring in these patients, and by 3 years, it was 10 times more likely that AF would be detected with continuous monitoring. I think we’ve settled the question of the best way to find AF[ib] in these patients – it is with an inserted device,” Dr. Schwamm said.
“We have also shown that this is not a transient rise in AFib after the stroke which then diminishes over the next few years. It is a continuous and progressive detection of AF[ib].”
Dr. Schwamm pointed out that 88% of the recorded AFib episodes were asymptomatic. “So relying on patients self-reporting symptoms when deciding who to monitor is unreliable and not a sensible strategy.”
The median time to the first adjudicated AFib episode at 12-month follow-up was 99 days; at the 3-year follow-up, it was 284 days.
“This shows that 30 days of monitoring with an external patch is not sufficient to exclude the presence of AF[ib]. And this really argues for a strategy of immediate insertion of cardiac monitor placement if your goal is to look for AF[ib],” Dr. Schwamm commented.
Is this clinically relevant AFib?
Dr. Schwamm acknowledged that there is a question of whether device-detected AFib should be thought about in the same way as clinically detected AFib with respect to future stroke risk.
He noted that, in this study, 67.4% of patients for whom AFib was detected by continuous monitoring (31 of 46 patients) had at least one episode of AFib that lasted more than 1 hour.
“This is not a trivial little squiggle of something on an EKG which then goes away. This is of significant duration that the cardiologist who adjudicated these rhythm strips felt confident was AF[ib].”
He added: “AF[ib] lasting more than 1 hour crosses the threshold for most practitioners I know to feel confident in treating the patient with anticoagulation. If it was symptomatic AF, this wouldn’t even be a question.”
Dr. Schwamm made the point that device-detected A AFib F has been accepted as worthy of treatment in patients after cryptogenic stroke.
“If we are honest with ourselves and if we have no hesitation in starting anticoagulation in a patient with cryptogenic stroke who has had device-detected AF 6 months later, should we decide that if the patient has had a lacunar stroke, we can ignore that same device-detected fibrillation?”
He put forward the idea that, at some level, all stroke is cryptogenic. “We never know for sure what the cause was. We have hypotheses, we have associations, but we don’t really know. So how much should we weigh that presumptive etiology in terms of how we interpret a rhythm disturbance of fibrillation?”
When looking for predictors of AFib in this study, the investigators found that patients were more likely to have an episode of AFib detected if they had one of the four following risk factors: congestive heart failure, left atrial enlargement, obesity, or QRS prolongation.
“In patients with any one of those four factors, 30% of those had device-detected AF[ib]. These are same predictors of AF[ib] that we are all accustomed to,” Dr. Schwamm said.
Shared decision-making
Dr. Schwamm said in an interview that, in his practice, for these patients, the decision as to whether to use continuous monitoring is made with the patient through shared decision-making.
“We discuss the chance that they could have AF[ib], and I suggest that it might be worth looking for it, but there are factors to be considered. There is a cost to the device, and reimbursement may depend on insurance coverage. Also, some patients may have strong feelings about having the chip implanted in their body.”
He says implanting the chip is easy. “It takes longer to check in at the front desk than to put the device in. It is injected under the skin. It just needs two stitches and a Band-Aid.” The device connects with a smartphone, and the results are interpreted by a cardiologist.
Dr. Schwamm pointed out that the optimal antithrombotic regimen for these patients in whom AFib is detected remains uncertain and should be the focus of future research.
“Do we just stick to antiplatelet therapy or advance to anticoagulation? In moving to an anticoagulant, are we providing less effective prevention for the atherosclerotic stroke risk at the expense of reducing the AF[ib]-related stroke risk? That may be a reasonable trade-off because we know the disability from AF[ib]-associated stroke is much higher.
“Or perhaps the optimal therapy is aspirin plus low-dose anticoagulant? Or left atrial appendage closure and an antiplatelet for patients at a higher risk of bleeding?” he said. “These are the really important questions we need to start asking.”
He added that he hopes a future study will address these questions, but he noted that it would have to be a large study, that it would have to first identify these patients and then randomly assign them to anticoagulation or to no treatment. “That is quite a major undertaking.”
In the highlights presentation, Dr. Jovin said he was uncertain of which of these patients in whom AFib is detected would benefit from anticoagulation. He said he would also like to see a randomized trial on this. But he added: “This would be challenging, as there is the issue of whether there would be equipoise to allow us to randomize to a placebo.”
Dr. Sansing agreed. “I think it would be a hard sell. I would have to think carefully about randomizing a patient to anticoagulation therapy or no therapy who has been found to have AF[ib].”
Dr. Schwamm noted that the current STROKE-AF study was not designed or powered to detect differences in stroke recurrence rates and that there was no difference in stroke recurrence rates between the two arms. There was also no randomization with regard to treatment; choice of medication was left to the discretion of the treating physician.
But he noted that only for 3 of the 34 patients with recurrent stroke in the continuous-monitor arm was AFib detected prior to the recurrent stroke, and only one of those three was receiving anticoagulation at the time of the recurrent stroke.
“These strokes were occurring in patients who did not have device-detected AF[ib],” Dr. Schwamm said. “This is because the population in this study were loaded with stroke risk factors and are at risk of recurrent stroke, but we don’t have the opportunity in this study to really understand the significance of the recurrent strokes.”
The STROKE AF trial was funded by Medtronic. Dr. Schwamm is a consultant to Medtronic.
A version of this article originally appeared on Medscape.com.
FROM ISC 2023
Cardiac monitoring company settles DOJ false claims allegations
Beyond Reps (dba IronRod Health and Cardiac Monitoring Services) has agreed to pay $673,200 to resolve allegations that it submitted false claims to federal health care programs relating to remote cardiac monitoring services.
The U.S. Department of Justice alleges that between Jan. 1, 2018, and April 30, 2021, IronRod, with headquarters in Phoenix, used technicians who lacked required credentials to conduct remote cardiac monitoring readings.
The government further alleges that between June 1, 2018, and Aug. 20, 2018, the company misrepresented that it performed services in New York state in order to get higher reimbursements from Medicare for remote cardiac monitoring services.
“Providers that seek payment from federal health programs are required to follow laws meant to protect beneficiaries, as well as to protect the integrity of those programs,” U.S. Attorney Trini E. Ross said in a statement.
“Our office is committed to pursuing cases against any provider that cuts corners or seeks to obtain payments for which they are not entitled,” Ms. Ross said.
A request to Beyond Reps for comment was not returned.
The civil settlement resolves claims brought under the qui tam (whistleblower) provisions of the False Claims Act by Coleen DeGroat.
Under those provisions, a private party can file an action on behalf of the United States and receive a portion of any recovery. Ms. DeGroat will receive a share of the settlement.
A version of this article first appeared on Medscape.com.
Beyond Reps (dba IronRod Health and Cardiac Monitoring Services) has agreed to pay $673,200 to resolve allegations that it submitted false claims to federal health care programs relating to remote cardiac monitoring services.
The U.S. Department of Justice alleges that between Jan. 1, 2018, and April 30, 2021, IronRod, with headquarters in Phoenix, used technicians who lacked required credentials to conduct remote cardiac monitoring readings.
The government further alleges that between June 1, 2018, and Aug. 20, 2018, the company misrepresented that it performed services in New York state in order to get higher reimbursements from Medicare for remote cardiac monitoring services.
“Providers that seek payment from federal health programs are required to follow laws meant to protect beneficiaries, as well as to protect the integrity of those programs,” U.S. Attorney Trini E. Ross said in a statement.
“Our office is committed to pursuing cases against any provider that cuts corners or seeks to obtain payments for which they are not entitled,” Ms. Ross said.
A request to Beyond Reps for comment was not returned.
The civil settlement resolves claims brought under the qui tam (whistleblower) provisions of the False Claims Act by Coleen DeGroat.
Under those provisions, a private party can file an action on behalf of the United States and receive a portion of any recovery. Ms. DeGroat will receive a share of the settlement.
A version of this article first appeared on Medscape.com.
Beyond Reps (dba IronRod Health and Cardiac Monitoring Services) has agreed to pay $673,200 to resolve allegations that it submitted false claims to federal health care programs relating to remote cardiac monitoring services.
The U.S. Department of Justice alleges that between Jan. 1, 2018, and April 30, 2021, IronRod, with headquarters in Phoenix, used technicians who lacked required credentials to conduct remote cardiac monitoring readings.
The government further alleges that between June 1, 2018, and Aug. 20, 2018, the company misrepresented that it performed services in New York state in order to get higher reimbursements from Medicare for remote cardiac monitoring services.
“Providers that seek payment from federal health programs are required to follow laws meant to protect beneficiaries, as well as to protect the integrity of those programs,” U.S. Attorney Trini E. Ross said in a statement.
“Our office is committed to pursuing cases against any provider that cuts corners or seeks to obtain payments for which they are not entitled,” Ms. Ross said.
A request to Beyond Reps for comment was not returned.
The civil settlement resolves claims brought under the qui tam (whistleblower) provisions of the False Claims Act by Coleen DeGroat.
Under those provisions, a private party can file an action on behalf of the United States and receive a portion of any recovery. Ms. DeGroat will receive a share of the settlement.
A version of this article first appeared on Medscape.com.
Three wild technologies about to change health care
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
Acute cardiac events common during COVID hospitalization
particularly among those with underlying heart disease, and are associated with more severe disease outcomes, a new study suggests.
“We expected to see acute cardiac events occurring among adults hospitalized with COVID-19 but were surprised by how frequently they occurred,” Rebecca C. Woodruff, PhD, MPH, of the U.S. Centers for Disease Control and Prevention, Atlanta, told this news organization.
Overall, she said, “about 1 in 10 adults experienced an acute cardiac event – including heart attacks and acute heart failure – while hospitalized with COVID-19, and this included people with no preexisting heart disease.”
However, she added, “about a quarter of those with underlying heart disease had an acute cardiac event. These patients tended to experience more severe disease outcomes relative to patients hospitalized with COVID-19 who did not experience an acute cardiac event.”
The findings might be relevant to hospitalizations for other viral diseases, “though we can’t say for sure,” she noted. “This study was modeled off a previous study conducted before the COVID-19 pandemic among adults hospitalized with influenza. About 11.7% of [those] adults experienced an acute cardiac event, which was a similar percentage as what we found among patients hospitalized with COVID-19.”
The study was published online in the Journal of the American College of Cardiology.
Underlying cardiac disease key
Dr. Woodruff and colleagues analyzed medical records on a probability sample of 8,460 adults hospitalized with SARS-CoV-2 infection identified from 99 U.S. counties in 14 U.S. states (about 10% of the United States population) from January to November 2021.
Among participants, 11.4% had an acute cardiac event during their hospitalization. The median age was 69 years; 56.5% were men; 48.7%, non-Hispanic White; 33.6%, non-Hispanic Black; 7.4%, Hispanic; and 7.1%, non-Hispanic Asian or Pacific Islander.
As indicated, the prevalence was higher among those with underlying cardiac disease (23.4%), compared with those without (6.2%).
Acute ischemic heart disease (5.5%) and acute heart failure (5.4%) were the most prevalent events; 0.3% of participants had acute myocarditis or pericarditis.
Risk factors varied, depending on underlying cardiac disease status. Those who experienced one or more acute cardiac events had a greater risk for intensive care unit admission (adjusted risk ratio,1.9) and in-hospital death (aRR, 1.7) versus those who did not.
In multivariable analyses, the risk of experiencing acute heart failure was significantly greater among men (aRR, 1.5) and among those with a history of congestive heart failure (aRR, 13.5), atrial fibrillation (aRR, 1.6) or hypertension (aRR,1.3).
Among patients who experienced one or more acute cardiac events, 39.2% required an intensive care unit stay for a median of 5 days. Approximately 22.4% required invasive mechanical ventilation or extracorporeal membrane oxygenation, and 21.1% died while hospitalized.
“Persons at greater risk for experiencing acute cardiac events during COVID-19–associated hospitalizations might benefit from more intensive clinical evaluation and monitoring during hospitalization,” the authors conclude.
The team currently is taking a closer look at acute myocarditis among patients hospitalized with COVID-19, Dr. Woodruff said. Preliminary results were presented at the 2022 annual scientific sessions of the American Heart Association and a paper is forthcoming.
Contemporary data needed
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said the findings mirror his team’s clinical experience in 2020 and 2021 and echo what was seen in the AHA COVID registry: that is, a 0.3% rate of myocarditis.
“The major caveat is that [the findings] may not be generalizable to contemporary COVID infection, both due to changing viral variants and higher levels of immunity in the population,” he said.
“Rates of COVID hospitalization are markedly lower with the current dominant variants, and we would expect the cardiac risk to be lower as well. I would like to see more contemporary data with current variants, particularly focused on higher risk patients with cardiovascular disease,” Dr. de Lemos added.
In a related editorial, George A. Mensa, MD, of the National Heart, Lung, and Blood Institute in Bethesda, Md., and colleagues suggest that the broader impact of the COVID-19 pandemic on human health remains “incompletely examined.”
“The impact of COVID-19 on cardiovascular mortality, in particular, appears to have varied widely, with no large increases seen in a number of the most developed countries but marked increases in hypertensive heart disease mortality seen in the United States in 2021,” they conclude. “The potential contribution of COVID-19 to these deaths, either directly or indirectly, remains to be determined.”
No commercial funding or relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
particularly among those with underlying heart disease, and are associated with more severe disease outcomes, a new study suggests.
“We expected to see acute cardiac events occurring among adults hospitalized with COVID-19 but were surprised by how frequently they occurred,” Rebecca C. Woodruff, PhD, MPH, of the U.S. Centers for Disease Control and Prevention, Atlanta, told this news organization.
Overall, she said, “about 1 in 10 adults experienced an acute cardiac event – including heart attacks and acute heart failure – while hospitalized with COVID-19, and this included people with no preexisting heart disease.”
However, she added, “about a quarter of those with underlying heart disease had an acute cardiac event. These patients tended to experience more severe disease outcomes relative to patients hospitalized with COVID-19 who did not experience an acute cardiac event.”
The findings might be relevant to hospitalizations for other viral diseases, “though we can’t say for sure,” she noted. “This study was modeled off a previous study conducted before the COVID-19 pandemic among adults hospitalized with influenza. About 11.7% of [those] adults experienced an acute cardiac event, which was a similar percentage as what we found among patients hospitalized with COVID-19.”
The study was published online in the Journal of the American College of Cardiology.
Underlying cardiac disease key
Dr. Woodruff and colleagues analyzed medical records on a probability sample of 8,460 adults hospitalized with SARS-CoV-2 infection identified from 99 U.S. counties in 14 U.S. states (about 10% of the United States population) from January to November 2021.
Among participants, 11.4% had an acute cardiac event during their hospitalization. The median age was 69 years; 56.5% were men; 48.7%, non-Hispanic White; 33.6%, non-Hispanic Black; 7.4%, Hispanic; and 7.1%, non-Hispanic Asian or Pacific Islander.
As indicated, the prevalence was higher among those with underlying cardiac disease (23.4%), compared with those without (6.2%).
Acute ischemic heart disease (5.5%) and acute heart failure (5.4%) were the most prevalent events; 0.3% of participants had acute myocarditis or pericarditis.
Risk factors varied, depending on underlying cardiac disease status. Those who experienced one or more acute cardiac events had a greater risk for intensive care unit admission (adjusted risk ratio,1.9) and in-hospital death (aRR, 1.7) versus those who did not.
In multivariable analyses, the risk of experiencing acute heart failure was significantly greater among men (aRR, 1.5) and among those with a history of congestive heart failure (aRR, 13.5), atrial fibrillation (aRR, 1.6) or hypertension (aRR,1.3).
Among patients who experienced one or more acute cardiac events, 39.2% required an intensive care unit stay for a median of 5 days. Approximately 22.4% required invasive mechanical ventilation or extracorporeal membrane oxygenation, and 21.1% died while hospitalized.
“Persons at greater risk for experiencing acute cardiac events during COVID-19–associated hospitalizations might benefit from more intensive clinical evaluation and monitoring during hospitalization,” the authors conclude.
The team currently is taking a closer look at acute myocarditis among patients hospitalized with COVID-19, Dr. Woodruff said. Preliminary results were presented at the 2022 annual scientific sessions of the American Heart Association and a paper is forthcoming.
Contemporary data needed
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said the findings mirror his team’s clinical experience in 2020 and 2021 and echo what was seen in the AHA COVID registry: that is, a 0.3% rate of myocarditis.
“The major caveat is that [the findings] may not be generalizable to contemporary COVID infection, both due to changing viral variants and higher levels of immunity in the population,” he said.
“Rates of COVID hospitalization are markedly lower with the current dominant variants, and we would expect the cardiac risk to be lower as well. I would like to see more contemporary data with current variants, particularly focused on higher risk patients with cardiovascular disease,” Dr. de Lemos added.
In a related editorial, George A. Mensa, MD, of the National Heart, Lung, and Blood Institute in Bethesda, Md., and colleagues suggest that the broader impact of the COVID-19 pandemic on human health remains “incompletely examined.”
“The impact of COVID-19 on cardiovascular mortality, in particular, appears to have varied widely, with no large increases seen in a number of the most developed countries but marked increases in hypertensive heart disease mortality seen in the United States in 2021,” they conclude. “The potential contribution of COVID-19 to these deaths, either directly or indirectly, remains to be determined.”
No commercial funding or relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
particularly among those with underlying heart disease, and are associated with more severe disease outcomes, a new study suggests.
“We expected to see acute cardiac events occurring among adults hospitalized with COVID-19 but were surprised by how frequently they occurred,” Rebecca C. Woodruff, PhD, MPH, of the U.S. Centers for Disease Control and Prevention, Atlanta, told this news organization.
Overall, she said, “about 1 in 10 adults experienced an acute cardiac event – including heart attacks and acute heart failure – while hospitalized with COVID-19, and this included people with no preexisting heart disease.”
However, she added, “about a quarter of those with underlying heart disease had an acute cardiac event. These patients tended to experience more severe disease outcomes relative to patients hospitalized with COVID-19 who did not experience an acute cardiac event.”
The findings might be relevant to hospitalizations for other viral diseases, “though we can’t say for sure,” she noted. “This study was modeled off a previous study conducted before the COVID-19 pandemic among adults hospitalized with influenza. About 11.7% of [those] adults experienced an acute cardiac event, which was a similar percentage as what we found among patients hospitalized with COVID-19.”
The study was published online in the Journal of the American College of Cardiology.
Underlying cardiac disease key
Dr. Woodruff and colleagues analyzed medical records on a probability sample of 8,460 adults hospitalized with SARS-CoV-2 infection identified from 99 U.S. counties in 14 U.S. states (about 10% of the United States population) from January to November 2021.
Among participants, 11.4% had an acute cardiac event during their hospitalization. The median age was 69 years; 56.5% were men; 48.7%, non-Hispanic White; 33.6%, non-Hispanic Black; 7.4%, Hispanic; and 7.1%, non-Hispanic Asian or Pacific Islander.
As indicated, the prevalence was higher among those with underlying cardiac disease (23.4%), compared with those without (6.2%).
Acute ischemic heart disease (5.5%) and acute heart failure (5.4%) were the most prevalent events; 0.3% of participants had acute myocarditis or pericarditis.
Risk factors varied, depending on underlying cardiac disease status. Those who experienced one or more acute cardiac events had a greater risk for intensive care unit admission (adjusted risk ratio,1.9) and in-hospital death (aRR, 1.7) versus those who did not.
In multivariable analyses, the risk of experiencing acute heart failure was significantly greater among men (aRR, 1.5) and among those with a history of congestive heart failure (aRR, 13.5), atrial fibrillation (aRR, 1.6) or hypertension (aRR,1.3).
Among patients who experienced one or more acute cardiac events, 39.2% required an intensive care unit stay for a median of 5 days. Approximately 22.4% required invasive mechanical ventilation or extracorporeal membrane oxygenation, and 21.1% died while hospitalized.
“Persons at greater risk for experiencing acute cardiac events during COVID-19–associated hospitalizations might benefit from more intensive clinical evaluation and monitoring during hospitalization,” the authors conclude.
The team currently is taking a closer look at acute myocarditis among patients hospitalized with COVID-19, Dr. Woodruff said. Preliminary results were presented at the 2022 annual scientific sessions of the American Heart Association and a paper is forthcoming.
Contemporary data needed
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said the findings mirror his team’s clinical experience in 2020 and 2021 and echo what was seen in the AHA COVID registry: that is, a 0.3% rate of myocarditis.
“The major caveat is that [the findings] may not be generalizable to contemporary COVID infection, both due to changing viral variants and higher levels of immunity in the population,” he said.
“Rates of COVID hospitalization are markedly lower with the current dominant variants, and we would expect the cardiac risk to be lower as well. I would like to see more contemporary data with current variants, particularly focused on higher risk patients with cardiovascular disease,” Dr. de Lemos added.
In a related editorial, George A. Mensa, MD, of the National Heart, Lung, and Blood Institute in Bethesda, Md., and colleagues suggest that the broader impact of the COVID-19 pandemic on human health remains “incompletely examined.”
“The impact of COVID-19 on cardiovascular mortality, in particular, appears to have varied widely, with no large increases seen in a number of the most developed countries but marked increases in hypertensive heart disease mortality seen in the United States in 2021,” they conclude. “The potential contribution of COVID-19 to these deaths, either directly or indirectly, remains to be determined.”
No commercial funding or relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY