User login
Cancer Data Trends 2023
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment
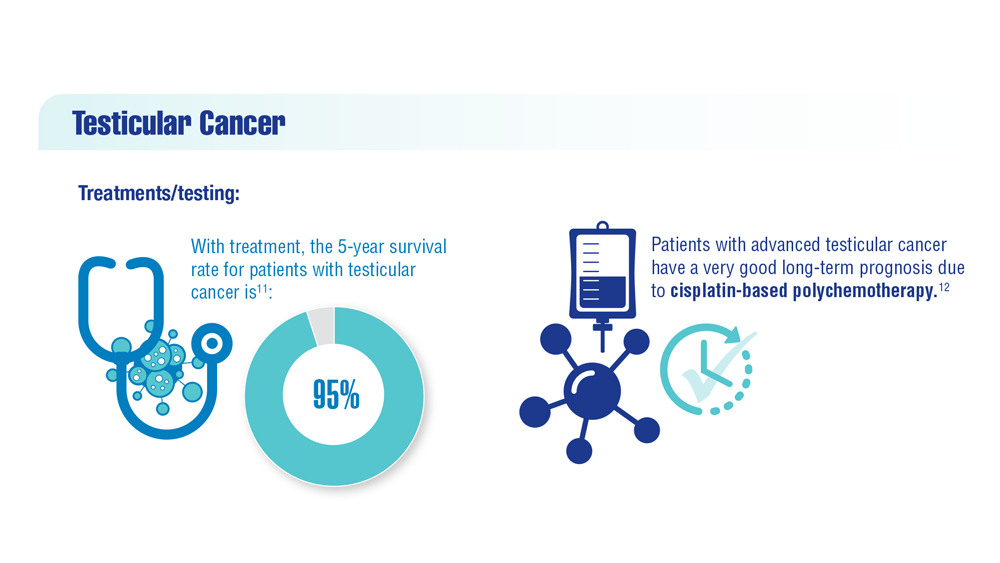
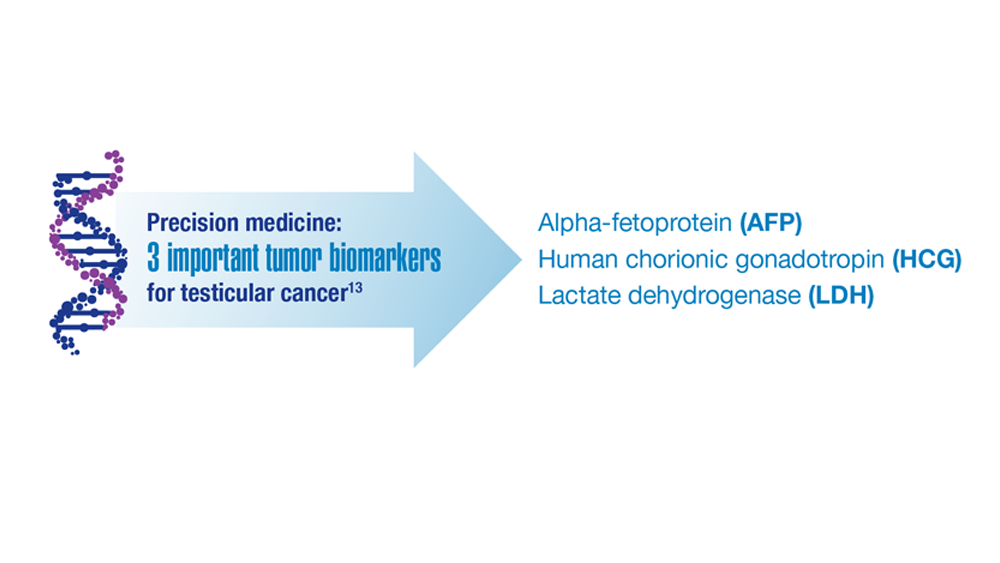
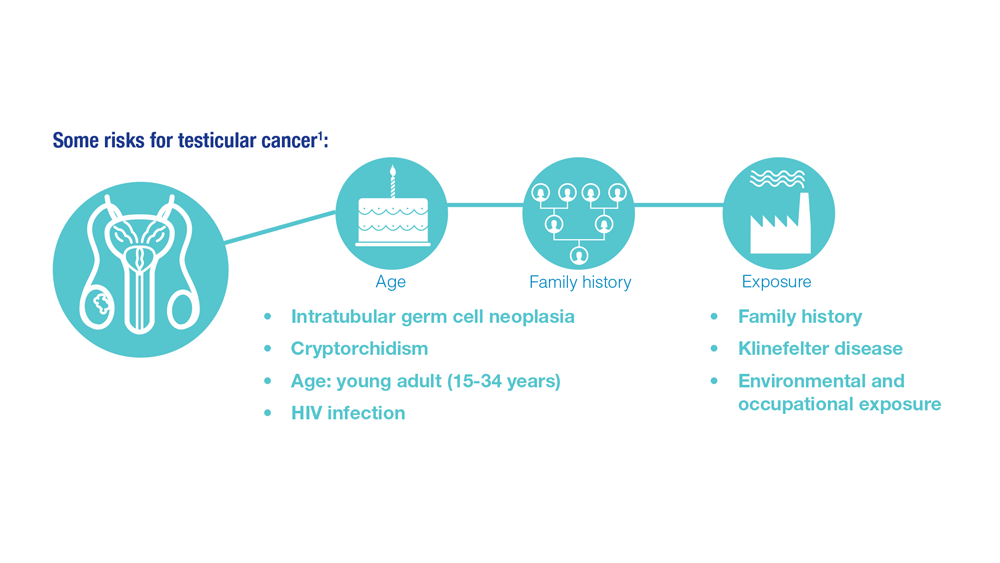
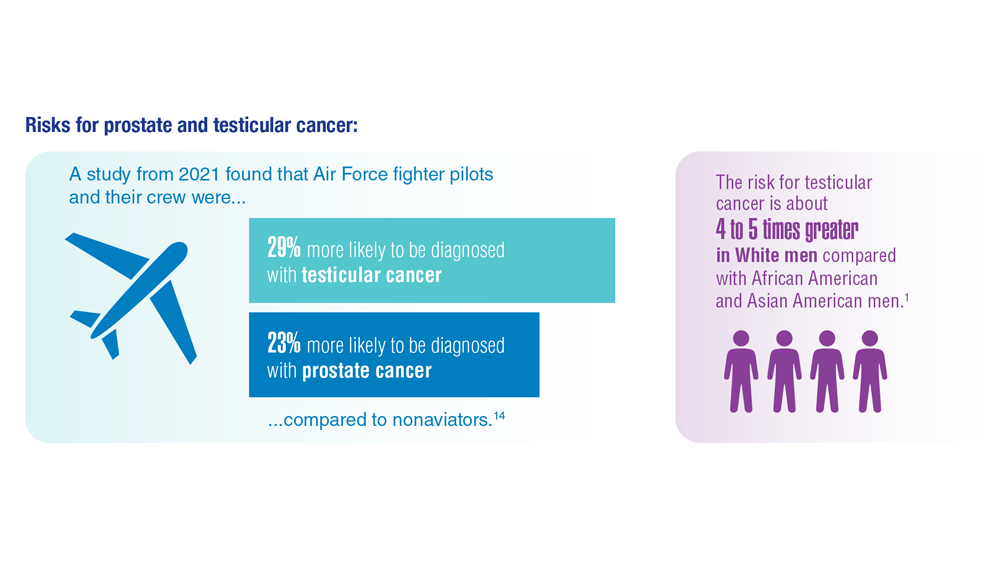
Promising New Approaches for Testicular and Prostate Cancer
- Risk factors for testicular cancer. American Cancer Society. Updated May 17, 2018. Accessed December 15, 2022. https://www.cancer.org/cancer/testicular-cancer/causes-risks-prevention/risk-factors.html
- Chovanec M, Cheng L. BMJ. 2022;379:e070499. doi:10.1136/bmj-2022-070499
- Tavares NT et al. J Pathol. 2022. doi:10.1002/path.6037
- Bryant AK et al. JAMA Oncol. 2022;e224319. doi:10.1001/jamaoncol.2022.4319
- Kabasakal L et al. Nucl Med Commun. 2017;38(2):149-155. doi:10.1097/MNM.0000000000000617
- Sartor O et al; VISION Investigators. N Engl J Med. 2021;385(12):1091-1103. doi:10.1056/NEJMoa2107322
- Rowe SP et al. Annu Rev Med. 2019;70:461-477. doi:10.1146/annurev-med-062117-073027
- Pomykala KL et al. Eur Urol Oncol. 2022;S2588-9311(22)00177-8. doi:10.1016/j.euo.2022.10.007
- Keam SJ. Mol Diagn Ther. 2022;26(4):467-475. doi:10.1007/s40291-022-00594-2
- Lovejoy LA et al. Mil Med. 2022:usac297. doi:10.1093/milmed/usac297
- Smith ZL et al. Med Clin North Am. 2018;102(2):251-264. doi:10.1016/j.mcna.2017.10.003
- Hohnloser JH et al. Eur J Med Res.1996;1(11):509-514.
- Johns Hopkins Medicine website. Testicular Cancer tumor Markers. Accessed December 2022. https://www.hopkinsmedicine.org/health/conditions-and-diseases/testicular-cancer/testicular-cancer-tumor-markers
- Webber BJ et al. J Occup Environ Med. 2022;64(1):71-78. doi:10.1097/JOM.0000000000002353
- Risk factors for testicular cancer. American Cancer Society. Updated May 17, 2018. Accessed December 15, 2022. https://www.cancer.org/cancer/testicular-cancer/causes-risks-prevention/risk-factors.html
- Chovanec M, Cheng L. BMJ. 2022;379:e070499. doi:10.1136/bmj-2022-070499
- Tavares NT et al. J Pathol. 2022. doi:10.1002/path.6037
- Bryant AK et al. JAMA Oncol. 2022;e224319. doi:10.1001/jamaoncol.2022.4319
- Kabasakal L et al. Nucl Med Commun. 2017;38(2):149-155. doi:10.1097/MNM.0000000000000617
- Sartor O et al; VISION Investigators. N Engl J Med. 2021;385(12):1091-1103. doi:10.1056/NEJMoa2107322
- Rowe SP et al. Annu Rev Med. 2019;70:461-477. doi:10.1146/annurev-med-062117-073027
- Pomykala KL et al. Eur Urol Oncol. 2022;S2588-9311(22)00177-8. doi:10.1016/j.euo.2022.10.007
- Keam SJ. Mol Diagn Ther. 2022;26(4):467-475. doi:10.1007/s40291-022-00594-2
- Lovejoy LA et al. Mil Med. 2022:usac297. doi:10.1093/milmed/usac297
- Smith ZL et al. Med Clin North Am. 2018;102(2):251-264. doi:10.1016/j.mcna.2017.10.003
- Hohnloser JH et al. Eur J Med Res.1996;1(11):509-514.
- Johns Hopkins Medicine website. Testicular Cancer tumor Markers. Accessed December 2022. https://www.hopkinsmedicine.org/health/conditions-and-diseases/testicular-cancer/testicular-cancer-tumor-markers
- Webber BJ et al. J Occup Environ Med. 2022;64(1):71-78. doi:10.1097/JOM.0000000000002353
- Risk factors for testicular cancer. American Cancer Society. Updated May 17, 2018. Accessed December 15, 2022. https://www.cancer.org/cancer/testicular-cancer/causes-risks-prevention/risk-factors.html
- Chovanec M, Cheng L. BMJ. 2022;379:e070499. doi:10.1136/bmj-2022-070499
- Tavares NT et al. J Pathol. 2022. doi:10.1002/path.6037
- Bryant AK et al. JAMA Oncol. 2022;e224319. doi:10.1001/jamaoncol.2022.4319
- Kabasakal L et al. Nucl Med Commun. 2017;38(2):149-155. doi:10.1097/MNM.0000000000000617
- Sartor O et al; VISION Investigators. N Engl J Med. 2021;385(12):1091-1103. doi:10.1056/NEJMoa2107322
- Rowe SP et al. Annu Rev Med. 2019;70:461-477. doi:10.1146/annurev-med-062117-073027
- Pomykala KL et al. Eur Urol Oncol. 2022;S2588-9311(22)00177-8. doi:10.1016/j.euo.2022.10.007
- Keam SJ. Mol Diagn Ther. 2022;26(4):467-475. doi:10.1007/s40291-022-00594-2
- Lovejoy LA et al. Mil Med. 2022:usac297. doi:10.1093/milmed/usac297
- Smith ZL et al. Med Clin North Am. 2018;102(2):251-264. doi:10.1016/j.mcna.2017.10.003
- Hohnloser JH et al. Eur J Med Res.1996;1(11):509-514.
- Johns Hopkins Medicine website. Testicular Cancer tumor Markers. Accessed December 2022. https://www.hopkinsmedicine.org/health/conditions-and-diseases/testicular-cancer/testicular-cancer-tumor-markers
- Webber BJ et al. J Occup Environ Med. 2022;64(1):71-78. doi:10.1097/JOM.0000000000002353
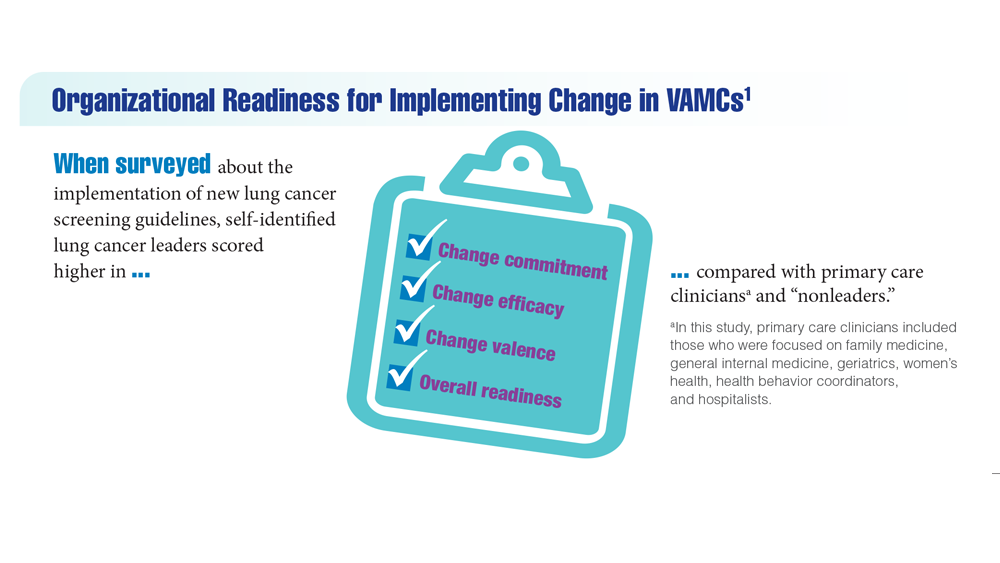
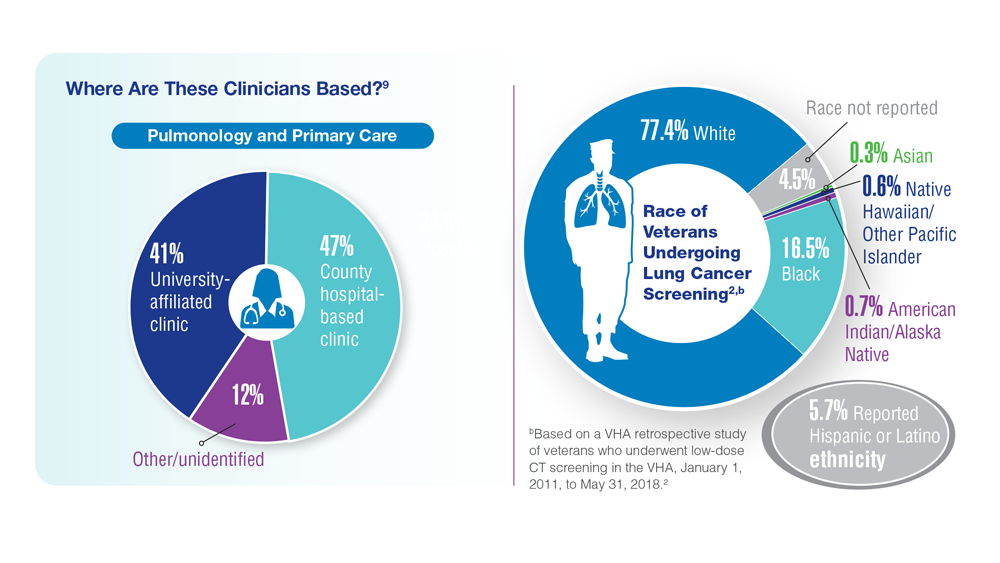
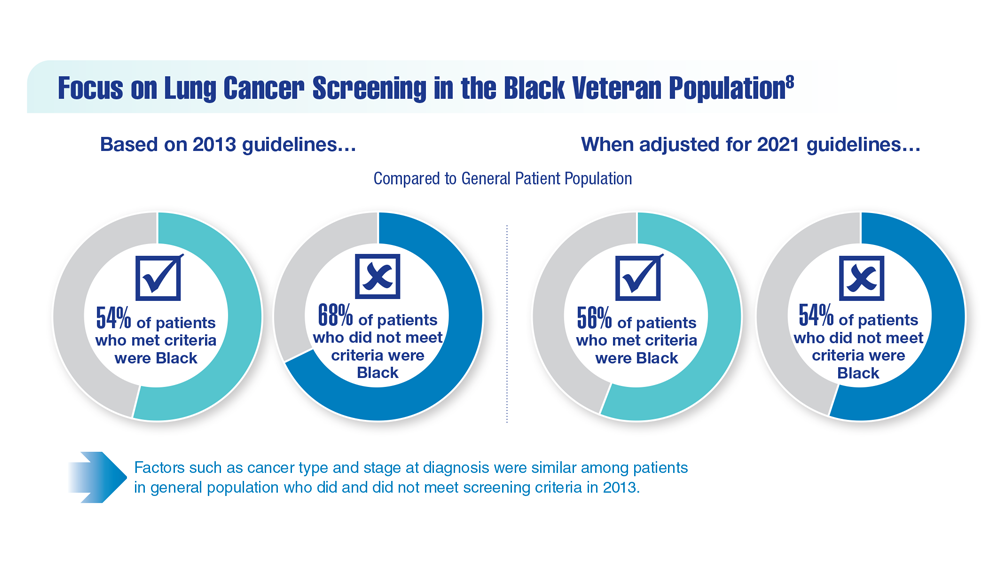
Lung Cancer Screening in Veterans
- Spalluto LB et al. J Am Coll Radiol. 2021;18(6):809-819. doi:10.1016/j.jacr.2020.12.010
- Lewis JA et al. JNCI Cancer Spectr. 2020;4(5):pkaa053. doi:10.1093/jncics/pkaa053
- Wallace C. Largest-ever lung cancer screening study reveals ways to increase screening outreach. Medical University of South Carolina. November 22, 2022. Accessed January 4, 202 https://hollingscancercenter.musc.edu/news/archive/2022/11/22/largest-ever-lung-cancer-screening-study-reveals-ways-to-increase-screening-outreach
- Screening facts & figures. Go2 For Lung Cancer. 2022. Accessed January 4, 2023. https://go2.org/risk-early-detection/screening-facts-figures/
- Dyer O. BMJ. 2021;372:n698. doi:10.1136/bmj.n698
- Boudreau JH et al. Chest. 2021;160(1):358-367. doi:10.1016/j.chest.2021.02.016
- Maurice NM, Tanner NT. Semin Oncol. 2022;S0093-7754(22)00041-0. doi:10.1053/j.seminoncol.2022.06.001
- Rusher TN et al. Fed Pract. 2022;39(suppl 2):S48-S51. doi:10.12788/fp.0269
- Núñez ER et al. JAMA Netw Open. 2021;4(7):e2116233. doi:10.1001/jamanetworkopen.2021.16233
- Lake M et al. BMC Cancer. 2020;20(1):561. doi:1186/s12885-020-06923-0
- Spalluto LB et al. J Am Coll Radiol. 2021;18(6):809-819. doi:10.1016/j.jacr.2020.12.010
- Lewis JA et al. JNCI Cancer Spectr. 2020;4(5):pkaa053. doi:10.1093/jncics/pkaa053
- Wallace C. Largest-ever lung cancer screening study reveals ways to increase screening outreach. Medical University of South Carolina. November 22, 2022. Accessed January 4, 202 https://hollingscancercenter.musc.edu/news/archive/2022/11/22/largest-ever-lung-cancer-screening-study-reveals-ways-to-increase-screening-outreach
- Screening facts & figures. Go2 For Lung Cancer. 2022. Accessed January 4, 2023. https://go2.org/risk-early-detection/screening-facts-figures/
- Dyer O. BMJ. 2021;372:n698. doi:10.1136/bmj.n698
- Boudreau JH et al. Chest. 2021;160(1):358-367. doi:10.1016/j.chest.2021.02.016
- Maurice NM, Tanner NT. Semin Oncol. 2022;S0093-7754(22)00041-0. doi:10.1053/j.seminoncol.2022.06.001
- Rusher TN et al. Fed Pract. 2022;39(suppl 2):S48-S51. doi:10.12788/fp.0269
- Núñez ER et al. JAMA Netw Open. 2021;4(7):e2116233. doi:10.1001/jamanetworkopen.2021.16233
- Lake M et al. BMC Cancer. 2020;20(1):561. doi:1186/s12885-020-06923-0
- Spalluto LB et al. J Am Coll Radiol. 2021;18(6):809-819. doi:10.1016/j.jacr.2020.12.010
- Lewis JA et al. JNCI Cancer Spectr. 2020;4(5):pkaa053. doi:10.1093/jncics/pkaa053
- Wallace C. Largest-ever lung cancer screening study reveals ways to increase screening outreach. Medical University of South Carolina. November 22, 2022. Accessed January 4, 202 https://hollingscancercenter.musc.edu/news/archive/2022/11/22/largest-ever-lung-cancer-screening-study-reveals-ways-to-increase-screening-outreach
- Screening facts & figures. Go2 For Lung Cancer. 2022. Accessed January 4, 2023. https://go2.org/risk-early-detection/screening-facts-figures/
- Dyer O. BMJ. 2021;372:n698. doi:10.1136/bmj.n698
- Boudreau JH et al. Chest. 2021;160(1):358-367. doi:10.1016/j.chest.2021.02.016
- Maurice NM, Tanner NT. Semin Oncol. 2022;S0093-7754(22)00041-0. doi:10.1053/j.seminoncol.2022.06.001
- Rusher TN et al. Fed Pract. 2022;39(suppl 2):S48-S51. doi:10.12788/fp.0269
- Núñez ER et al. JAMA Netw Open. 2021;4(7):e2116233. doi:10.1001/jamanetworkopen.2021.16233
- Lake M et al. BMC Cancer. 2020;20(1):561. doi:1186/s12885-020-06923-0
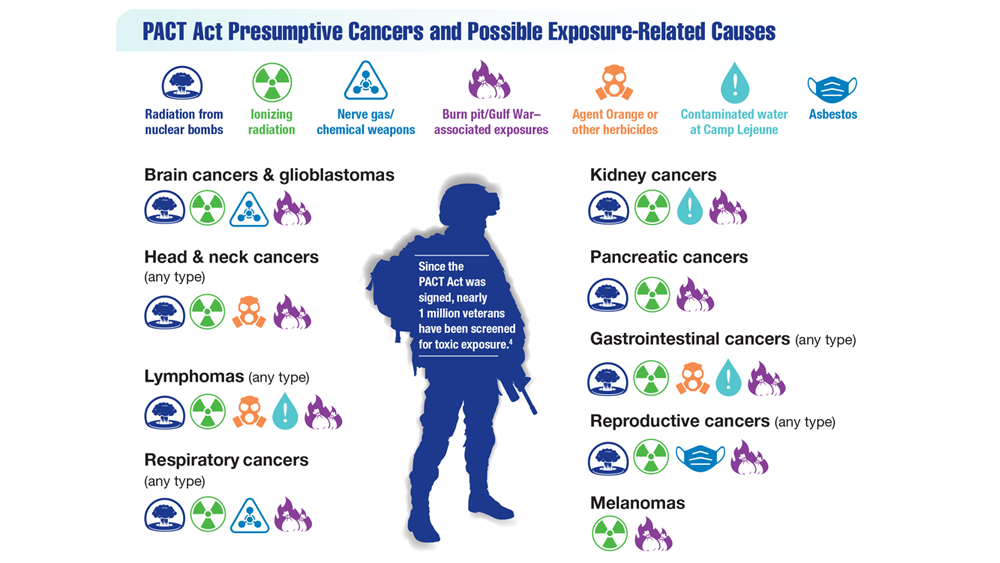
Exposure-Related Cancers: A Look at the PACT Act
- US Department of Veterans Affairs. PACT Act. Updated November 4, 2022. Accessed January 4, 2023. https://www.publichealth.va.gov/exposures/benefits/PACT_Act.asp
- The White House. FACT SHEET: President Biden signs the PACT Act and delivers on his promise to America’s veterans. August 10, 202 Accessed January 10, 2023. https://www.whitehouse.gov/briefing-room/statements-releases/2022/08/10/fact-sheet-president-biden-signs-the-pact-act-and-delivers-on-his-promise-to-americas-veterans/
- US House of Representatives. Honoring our promise to address Comprehensive Toxics Act of 2021. Title I – Expansion of health care eligibility for toxic exposed veterans. House report 117-249. February 22, 2022. Accessed January 19, 202 https://www.govinfo.gov/content/pkg/CRPT-117hrpt249/html/CRPT-117hrpt249-pt1.htm
- VA News. Cancer Moonshot week of action sees VA deploying new clinical pathways. Updated December 7, 2022. Accessed January 19, 2023. https://news.va.gov/111925/cancer-moonshot-clinical-pathways/
- US Department of Veterans Affairs. PACT Act. Updated November 4, 2022. Accessed January 4, 2023. https://www.publichealth.va.gov/exposures/benefits/PACT_Act.asp
- The White House. FACT SHEET: President Biden signs the PACT Act and delivers on his promise to America’s veterans. August 10, 202 Accessed January 10, 2023. https://www.whitehouse.gov/briefing-room/statements-releases/2022/08/10/fact-sheet-president-biden-signs-the-pact-act-and-delivers-on-his-promise-to-americas-veterans/
- US House of Representatives. Honoring our promise to address Comprehensive Toxics Act of 2021. Title I – Expansion of health care eligibility for toxic exposed veterans. House report 117-249. February 22, 2022. Accessed January 19, 202 https://www.govinfo.gov/content/pkg/CRPT-117hrpt249/html/CRPT-117hrpt249-pt1.htm
- VA News. Cancer Moonshot week of action sees VA deploying new clinical pathways. Updated December 7, 2022. Accessed January 19, 2023. https://news.va.gov/111925/cancer-moonshot-clinical-pathways/
- US Department of Veterans Affairs. PACT Act. Updated November 4, 2022. Accessed January 4, 2023. https://www.publichealth.va.gov/exposures/benefits/PACT_Act.asp
- The White House. FACT SHEET: President Biden signs the PACT Act and delivers on his promise to America’s veterans. August 10, 202 Accessed January 10, 2023. https://www.whitehouse.gov/briefing-room/statements-releases/2022/08/10/fact-sheet-president-biden-signs-the-pact-act-and-delivers-on-his-promise-to-americas-veterans/
- US House of Representatives. Honoring our promise to address Comprehensive Toxics Act of 2021. Title I – Expansion of health care eligibility for toxic exposed veterans. House report 117-249. February 22, 2022. Accessed January 19, 202 https://www.govinfo.gov/content/pkg/CRPT-117hrpt249/html/CRPT-117hrpt249-pt1.htm
- VA News. Cancer Moonshot week of action sees VA deploying new clinical pathways. Updated December 7, 2022. Accessed January 19, 2023. https://news.va.gov/111925/cancer-moonshot-clinical-pathways/
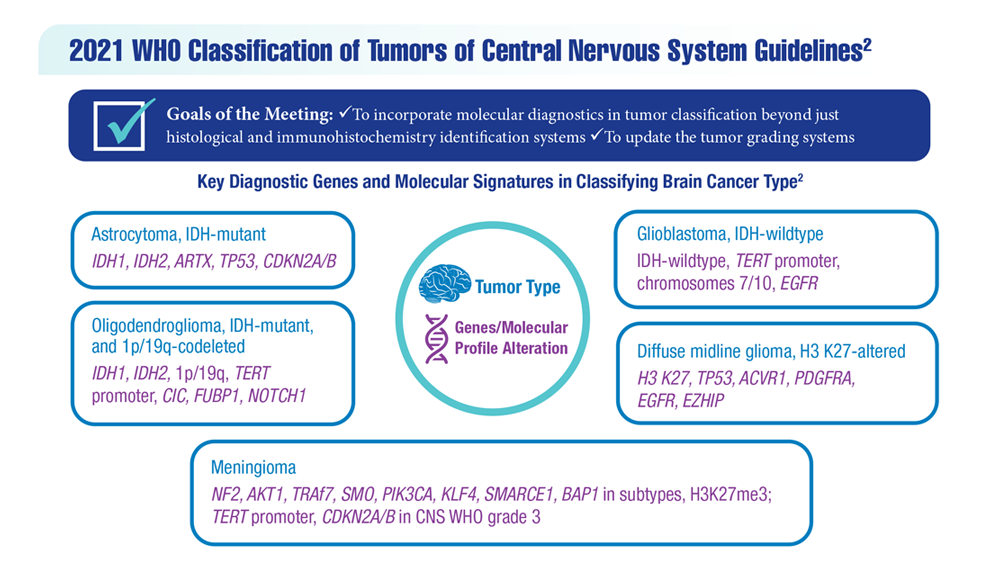
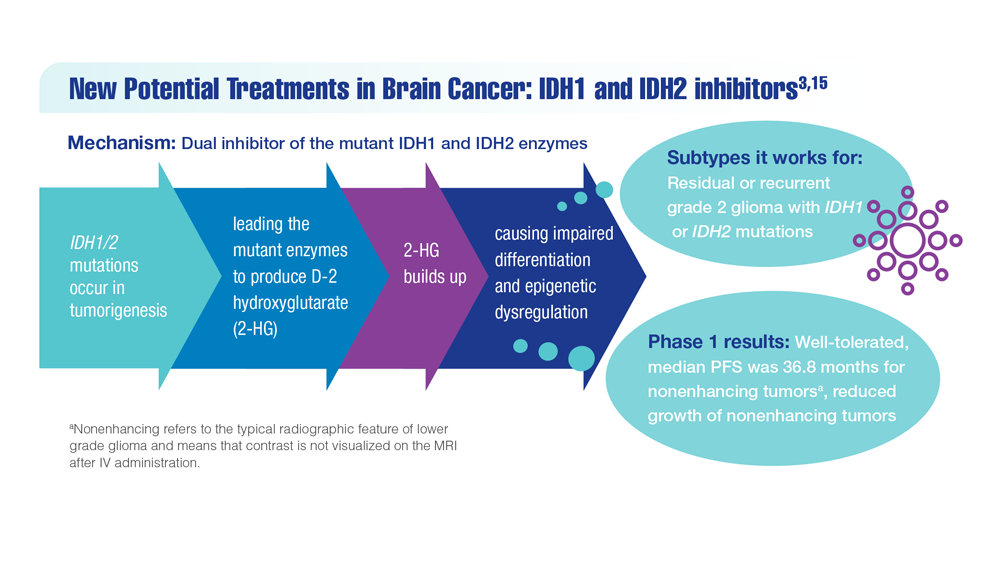
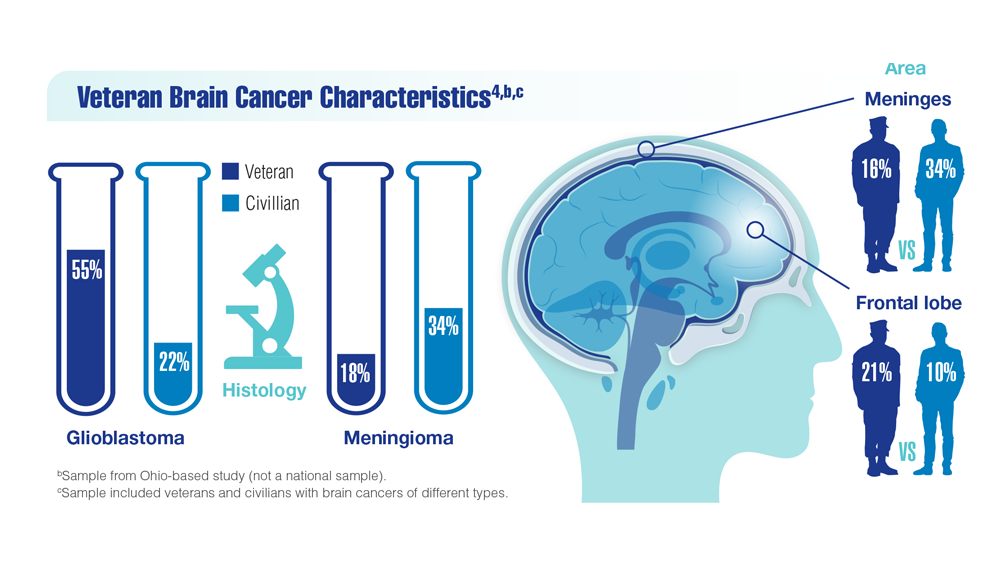
New Classifications and Emerging Treatments in Brain Cancer
- Sokolov AV et al. Pharmacol Rev. 2021;73(4):1-32. doi:10.1124/pharmrev.121.000317
- Louis DN et al. Neuro Oncol. 2021;23(8):1231-1251. doi:10.1093/neuonc/noab106
- Mellinghoff IK et al. Clin Cancer Res. 2021;27(16):4491-4499. doi:10.1158/1078-0432.CCR-21-0611
- Woo C et al. JCO Clin Cancer Inform. 2021;5:985-994. doi:10.1200/CCI.21.00052
- Study of vorasidenib (AG-881) in participants with residual or recurrent grade 2 glioma with an IDH1 or IDH2 mutation (INDIGO). ClinicalTrials.gov. Updated May 17, 2022. Accessed December 8, 2022. https://clinicaltrials.gov/ct2/show/NCT04164901
- Servier's pivotal phase 3 indigo trial investigating vorasidenib in IDH-mutant low-grade glioma meets primary endpoint of progression-free survival (PFS) and key secondary endpoint of time to next intervention (TTNI) (no date) Servier US. March 14, 2023. Accessed March 20, 2023. https://www.servier.us/serviers-pivotal-phase-3-indigo-trial-meets-primary-endpoint
- Nehra M et al. J Control Release. 2021;338:224-243. doi:10.1016/j.jconrel.2021.08.027
- Hersh AM et al. Cancers (Basel). 2022;14(19):4920. doi:10.3390/cancers14194920
- Shoaf ML, Desjardins A. Neurotherapeutics. 2022;19(6):1818-1831. doi:10.1007/s13311-022-01256-1
- Bagley SJ, O’Rourke DM. Pharmacol Ther. 2020;205:107419. doi:10.1016/j.pharmthera.2019.107419
- Batich KA et al. Clin Cancer Res. 2020;26(20):5297-5303. doi:10.1158/1078-0432.CCR-20-1082
- Lin J et al. Cancer. 2020;126(13):3053-3060. doi:10.1002/cncr.32884
- Barth SK et al. Cancer Epidemiol. 2017;50(pt A):22-29. doi:10.1016/j.canep.2017.07.012
- VA and partners hope APOLLO program will be leap forward for precision oncology. US Department of Veteran Affairs. May 1, 2019. Accessed December 8, 2022. https://www.research.va.gov/currents/0519-VA-and-partners-hope-APOLLO-program-will-be-leap-forward-for-precision-oncology.cfm
- Konteatis Z et al. ACS Med Chem Lett. 2020;11(2):101-107. doi:10.1021/acsmedchemlett.9b00509
- Sokolov AV et al. Pharmacol Rev. 2021;73(4):1-32. doi:10.1124/pharmrev.121.000317
- Louis DN et al. Neuro Oncol. 2021;23(8):1231-1251. doi:10.1093/neuonc/noab106
- Mellinghoff IK et al. Clin Cancer Res. 2021;27(16):4491-4499. doi:10.1158/1078-0432.CCR-21-0611
- Woo C et al. JCO Clin Cancer Inform. 2021;5:985-994. doi:10.1200/CCI.21.00052
- Study of vorasidenib (AG-881) in participants with residual or recurrent grade 2 glioma with an IDH1 or IDH2 mutation (INDIGO). ClinicalTrials.gov. Updated May 17, 2022. Accessed December 8, 2022. https://clinicaltrials.gov/ct2/show/NCT04164901
- Servier's pivotal phase 3 indigo trial investigating vorasidenib in IDH-mutant low-grade glioma meets primary endpoint of progression-free survival (PFS) and key secondary endpoint of time to next intervention (TTNI) (no date) Servier US. March 14, 2023. Accessed March 20, 2023. https://www.servier.us/serviers-pivotal-phase-3-indigo-trial-meets-primary-endpoint
- Nehra M et al. J Control Release. 2021;338:224-243. doi:10.1016/j.jconrel.2021.08.027
- Hersh AM et al. Cancers (Basel). 2022;14(19):4920. doi:10.3390/cancers14194920
- Shoaf ML, Desjardins A. Neurotherapeutics. 2022;19(6):1818-1831. doi:10.1007/s13311-022-01256-1
- Bagley SJ, O’Rourke DM. Pharmacol Ther. 2020;205:107419. doi:10.1016/j.pharmthera.2019.107419
- Batich KA et al. Clin Cancer Res. 2020;26(20):5297-5303. doi:10.1158/1078-0432.CCR-20-1082
- Lin J et al. Cancer. 2020;126(13):3053-3060. doi:10.1002/cncr.32884
- Barth SK et al. Cancer Epidemiol. 2017;50(pt A):22-29. doi:10.1016/j.canep.2017.07.012
- VA and partners hope APOLLO program will be leap forward for precision oncology. US Department of Veteran Affairs. May 1, 2019. Accessed December 8, 2022. https://www.research.va.gov/currents/0519-VA-and-partners-hope-APOLLO-program-will-be-leap-forward-for-precision-oncology.cfm
- Konteatis Z et al. ACS Med Chem Lett. 2020;11(2):101-107. doi:10.1021/acsmedchemlett.9b00509
- Sokolov AV et al. Pharmacol Rev. 2021;73(4):1-32. doi:10.1124/pharmrev.121.000317
- Louis DN et al. Neuro Oncol. 2021;23(8):1231-1251. doi:10.1093/neuonc/noab106
- Mellinghoff IK et al. Clin Cancer Res. 2021;27(16):4491-4499. doi:10.1158/1078-0432.CCR-21-0611
- Woo C et al. JCO Clin Cancer Inform. 2021;5:985-994. doi:10.1200/CCI.21.00052
- Study of vorasidenib (AG-881) in participants with residual or recurrent grade 2 glioma with an IDH1 or IDH2 mutation (INDIGO). ClinicalTrials.gov. Updated May 17, 2022. Accessed December 8, 2022. https://clinicaltrials.gov/ct2/show/NCT04164901
- Servier's pivotal phase 3 indigo trial investigating vorasidenib in IDH-mutant low-grade glioma meets primary endpoint of progression-free survival (PFS) and key secondary endpoint of time to next intervention (TTNI) (no date) Servier US. March 14, 2023. Accessed March 20, 2023. https://www.servier.us/serviers-pivotal-phase-3-indigo-trial-meets-primary-endpoint
- Nehra M et al. J Control Release. 2021;338:224-243. doi:10.1016/j.jconrel.2021.08.027
- Hersh AM et al. Cancers (Basel). 2022;14(19):4920. doi:10.3390/cancers14194920
- Shoaf ML, Desjardins A. Neurotherapeutics. 2022;19(6):1818-1831. doi:10.1007/s13311-022-01256-1
- Bagley SJ, O’Rourke DM. Pharmacol Ther. 2020;205:107419. doi:10.1016/j.pharmthera.2019.107419
- Batich KA et al. Clin Cancer Res. 2020;26(20):5297-5303. doi:10.1158/1078-0432.CCR-20-1082
- Lin J et al. Cancer. 2020;126(13):3053-3060. doi:10.1002/cncr.32884
- Barth SK et al. Cancer Epidemiol. 2017;50(pt A):22-29. doi:10.1016/j.canep.2017.07.012
- VA and partners hope APOLLO program will be leap forward for precision oncology. US Department of Veteran Affairs. May 1, 2019. Accessed December 8, 2022. https://www.research.va.gov/currents/0519-VA-and-partners-hope-APOLLO-program-will-be-leap-forward-for-precision-oncology.cfm
- Konteatis Z et al. ACS Med Chem Lett. 2020;11(2):101-107. doi:10.1021/acsmedchemlett.9b00509
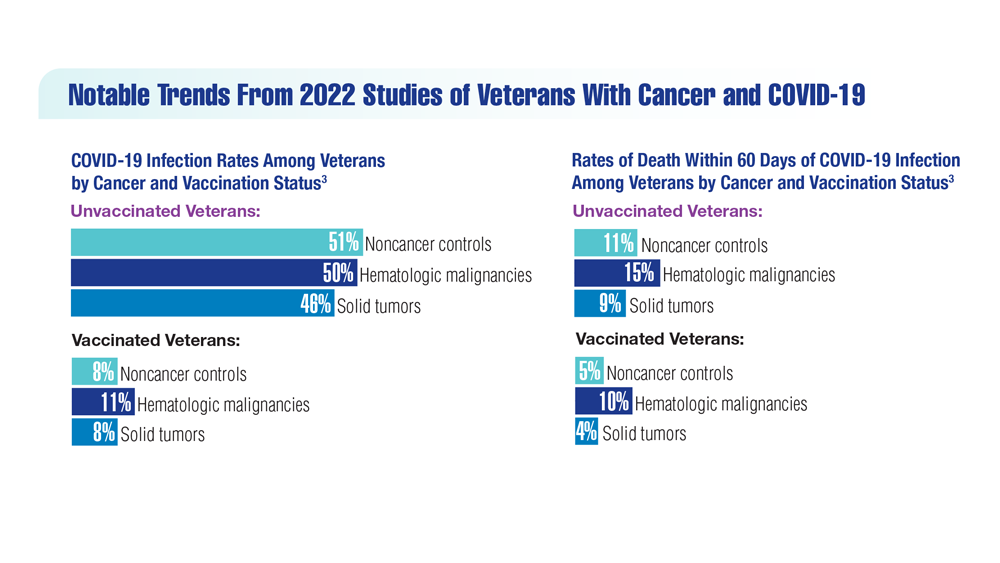
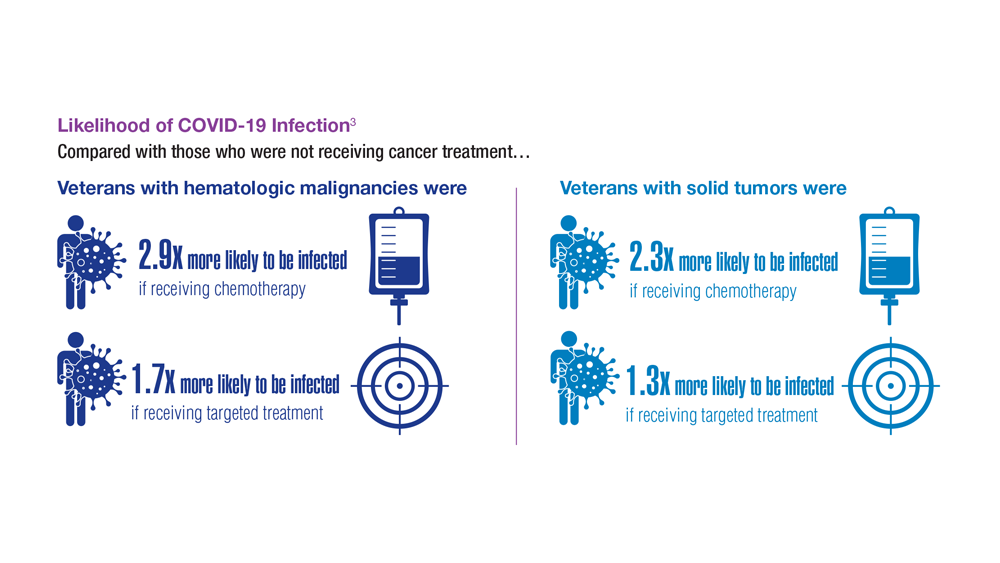
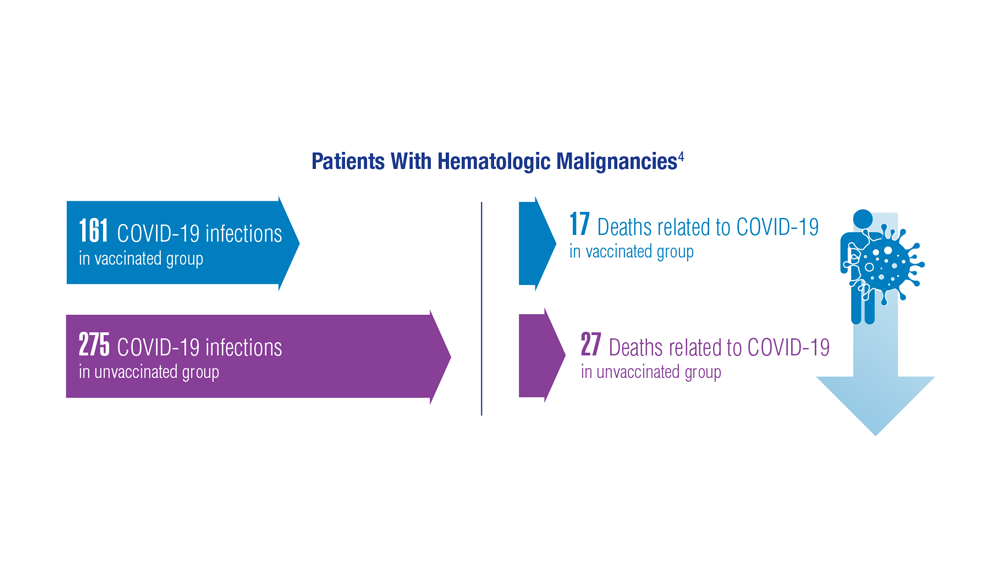
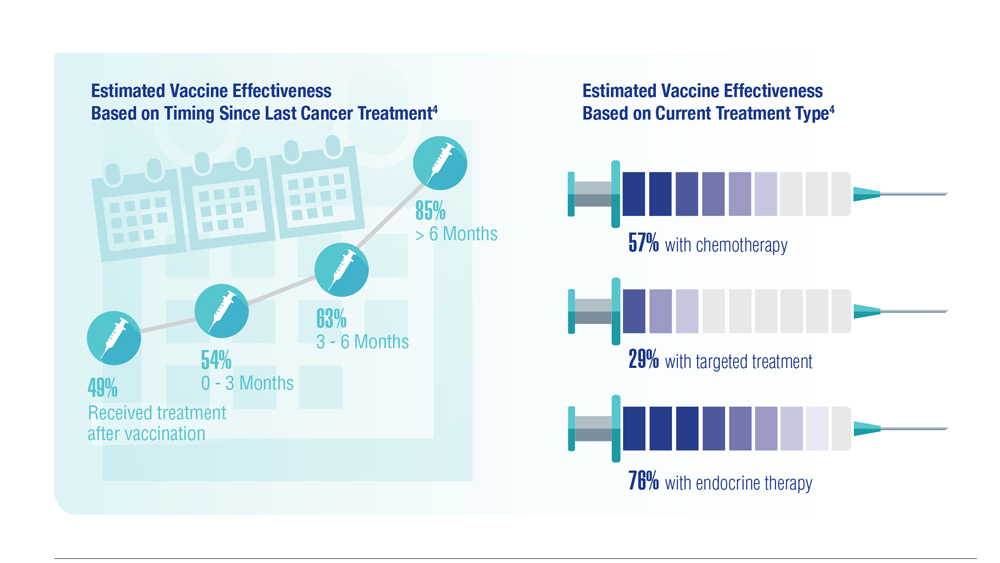
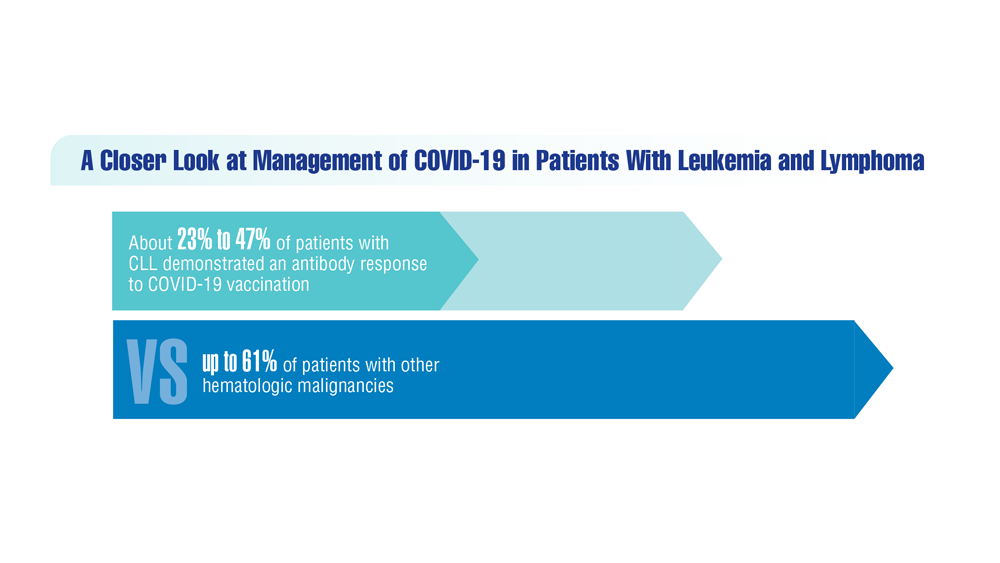
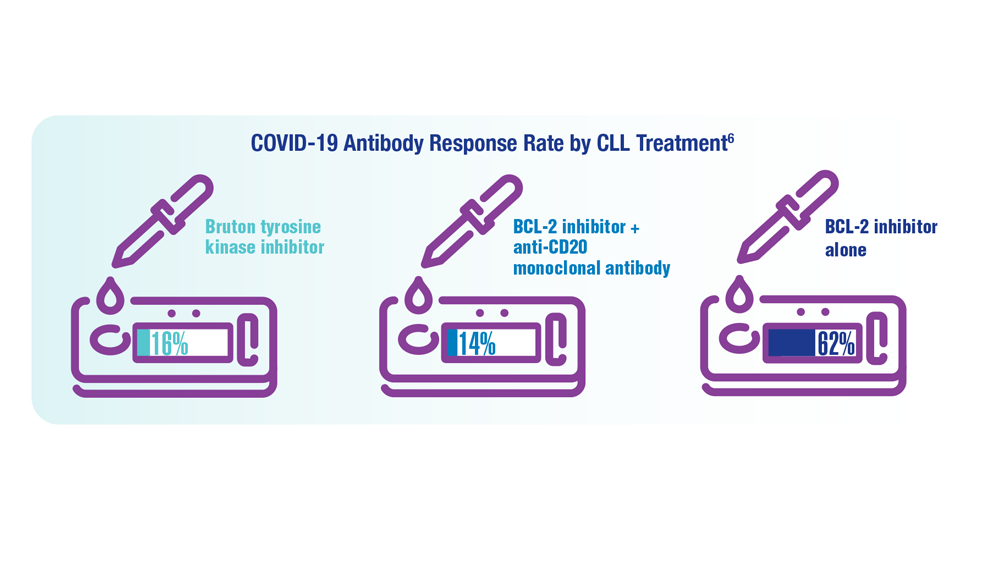
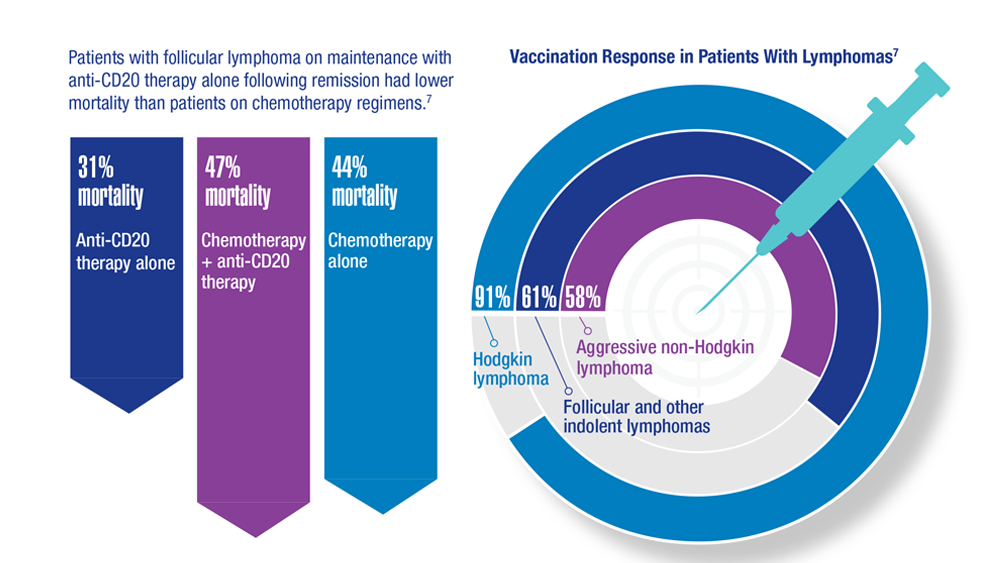
COVID-19 Outcomes in Veterans With Hematologic Malignancies
- Parker S. Lancet Oncol. 2022;23(1):2 doi:10.1016/S1470-2045(21)00713-0
- Englum BR et al. Cancer. 2022;128(5):1048-1056. doi:10.1002/cncr.34011
- Leuva H et al. Semin Oncol. 2022:49(5):363-370. doi:10.1053/j.seminoncol.2022.07.005
- Wu JTY et al. JAMA Oncol. 2022;8(2):281-286. doi:10.1001/jamaoncol.2021.5771
- Fillmore NR et al. J Natl Cancer Inst. 2021;113(6):691-698. doi:10.1093/jnci/djaa159
- Morawska M. Eur J Haematol. 2022;108(2):91-98. doi:10.1111/ejh.13722
- Passamonti F et al. Hematol Oncol. 2023;41(1):3-15. doi:10.1002/hon.3086
- Parker S. Lancet Oncol. 2022;23(1):2 doi:10.1016/S1470-2045(21)00713-0
- Englum BR et al. Cancer. 2022;128(5):1048-1056. doi:10.1002/cncr.34011
- Leuva H et al. Semin Oncol. 2022:49(5):363-370. doi:10.1053/j.seminoncol.2022.07.005
- Wu JTY et al. JAMA Oncol. 2022;8(2):281-286. doi:10.1001/jamaoncol.2021.5771
- Fillmore NR et al. J Natl Cancer Inst. 2021;113(6):691-698. doi:10.1093/jnci/djaa159
- Morawska M. Eur J Haematol. 2022;108(2):91-98. doi:10.1111/ejh.13722
- Passamonti F et al. Hematol Oncol. 2023;41(1):3-15. doi:10.1002/hon.3086
- Parker S. Lancet Oncol. 2022;23(1):2 doi:10.1016/S1470-2045(21)00713-0
- Englum BR et al. Cancer. 2022;128(5):1048-1056. doi:10.1002/cncr.34011
- Leuva H et al. Semin Oncol. 2022:49(5):363-370. doi:10.1053/j.seminoncol.2022.07.005
- Wu JTY et al. JAMA Oncol. 2022;8(2):281-286. doi:10.1001/jamaoncol.2021.5771
- Fillmore NR et al. J Natl Cancer Inst. 2021;113(6):691-698. doi:10.1093/jnci/djaa159
- Morawska M. Eur J Haematol. 2022;108(2):91-98. doi:10.1111/ejh.13722
- Passamonti F et al. Hematol Oncol. 2023;41(1):3-15. doi:10.1002/hon.3086
New cancer data spark outcry from patient advocates
The American Cancer Society on Jan. 13 revealed what it called “alarming” news about prostate cancer: After 2 decades of decline, the number of men diagnosed with the disease in the United States rose by 15% from 2014 to 2019.
“Most concerning,” according to the group’s CEO Karen Knudsen, PhD, MBA, is that the increase is being driven by diagnoses of advanced disease.
“Since 2011, the diagnosis of advanced-stage (regional- or distant-stage) prostate cancer has increased by 4%-5% annually and the proportion of men diagnosed with distant-stage disease has doubled,” said Dr. Knudsen at a press conference concerning the figures. “These findings underscore the importance of understanding and reducing this trend.”
The increase, which works out to be an additional 99,000 cases of prostate cancer, did not take the ACS by surprise; the group has been predicting a jump in diagnoses of the disease, which is the most common cancer in men after skin cancer, and the second most common cause of cancer death for that group.
The ACS announced a new action plan, “Improving Mortality from Prostate Cancer Together” – or IMPACT – to address the rise, especially in Black men, and to curb the increasing rate of advanced, difficult-to-treat cases.
“We must address these shifts in prostate cancer, especially in the Black community, since the incidence of prostate cancer in Black men is 70% higher than in White men and prostate cancer mortality rates in Black men are approximately two to four times higher than those in every other racial and ethnic group,” William Dahut, MD, PhD, chief scientific officer for the ACS, said at the press conference.
A study published in JAMA Network Open challenged that claim, finding that, after controlling for socioeconomic factors, race does not appear to be a significant predictor of mortality for prostate cancer.
Dr. Dahut said in an interview that IMPACT “is still [in the] early days for this initiative and more details will be coming out soon.”
Charles Ryan, MD, CEO of the Prostate Cancer Foundation, the world’s largest prostate cancer research charity, called IMPACT “extremely important work. Highlighting the disparities can only serve to benefit all men with prostate cancer, especially Black men.”
Bold action ... or passivity?
Overall cancer mortality has dropped 33% since 1991, averting an estimated 3.8 million deaths, according to ACS. But the story for prostate cancer is different.
The society and advocates had warned as recently as 2 years ago that prostate cancer was poised to rise again, especially advanced cases that may be too late to treat.
Leaders in the prostate cancer advocacy community praised the ACS plan for IMPACT, but some expressed frustration over what they said was ACS’ passivity in the face of long-anticipated increases in cases of the disease.
“I think prostate cancer was not high on their agenda,” said Rick Davis, founder of AnCan, which offers several support groups for patients with prostate cancer. “It’s good to see ACS get back into the prostate cancer game.”
Mr. Davis and patient advocate Darryl Mitteldorf, LCSW, founder of Malecare, another prostate support organization, said ACS dropped patient services for prostate cancer patients a decade ago and has not been a vocal supporter of screening for levels of prostate-specific antigen (PSA) to detect prostate cancer early.
“Early detection is supposed to be their goal,” Mr. Davis said.
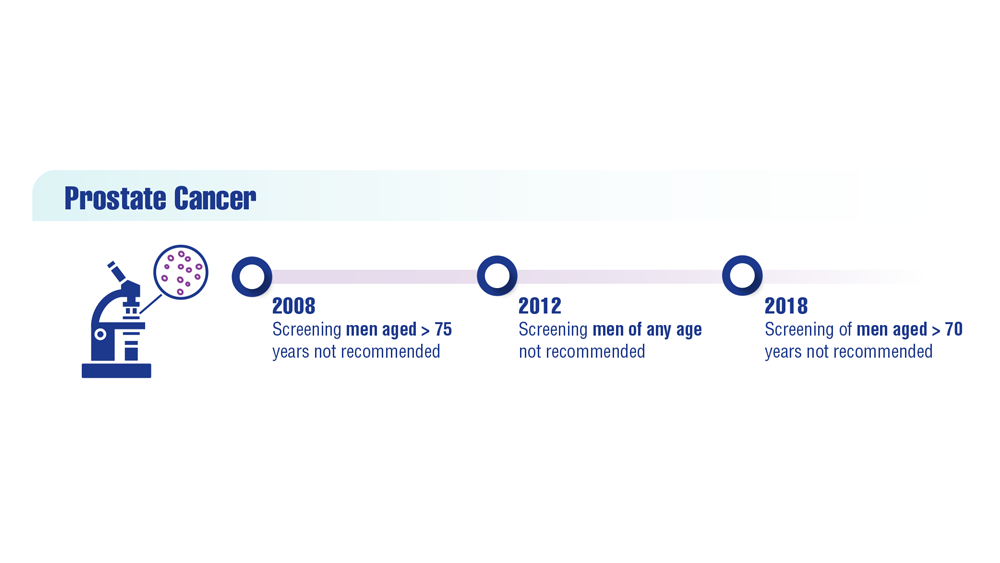
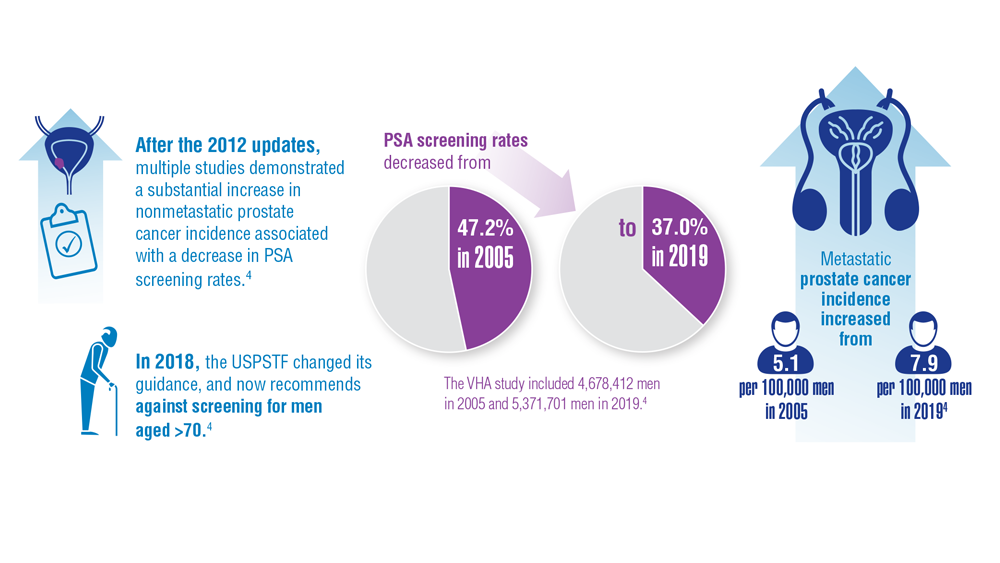
In 2012, the U.S. Preventive Services Task Force recommended against PSA screening, giving it a D-rating. The move prompted attacks on the task force from most advocates and many urologists.
Following this criticism, the task force recommended shared decision-making between patient and doctor, while giving PSA screening a C-rating. Now, the ACS recommends men in general at age 50 discuss prostate cancer screening with their doctor and that Black men do the same at age 45.
Mr. Mitteldorf said ACS “owes prostate cancer patients an explanation and analysis of its response to the USPTF’s downgrade of PSA testing and how that response might be related to death and instance rates.”
Mr. Mitteldorf added that male patients lost key support from ACS when the group dismantled its Man to Man group for prostate cancer patients and its Brother to Brother group for Blacks in particular.
Dr. Dahut said Man to Man “sunsetted” and was turned over to any local organization that chose to offer it. He said longtime staff didn’t have “a lot of information about [the demise of] Brother to Brother.”
For Mr. Davis, those smaller cuts add up to a much larger insult.
“Today, in 2023, ACS continues to poke a finger in the eyes of prostate cancer patients,” he said. “Since 2010, they have not given us any respect. ACS dumped its support.”
He pointed to the group’s funding priorities, noting that outlays for prostate cancer have consistently lagged behind those for breast cancer.
The ACS spent $25.3 million on breast cancer research and $6.7 million for prostate cancer in 2018, and in 2023 will designate $126.5 for breast cancer research and $43.9 million for prostate cancer.
ACS has earmarked $62 million this year for lung cancer programs and $61 million for colorectal cancer.
“Parity between breast cancer and prostate cancer would be a good start in sizing the IMPACT program,” Mr. Davis said. “After all, breast cancer and prostate cancer are hardly different in numbers today.”
Dr. Dahut denied any gender bias in research funding. He said the group makes funding decisions “based on finding the most impactful science regardless of tumor type. Our mission includes funding every cancer, every day; thus, we generally do not go into our funding cycle with any set-asides for a particular cancer.”
Mr. Davis also said the ACS data suggest the growing number of prostate cancer cases is even worse than the group has said. Although the society cites a 3% annual increase in prostate cancer diagnoses from 2014 to 2019, since 2019 the annual increase is a much more dramatic 16%. Meanwhile, the number of new cases of the disease is projected to rise from 175,000 per year in 2019 to 288,000 this year.
Dr. Dahut said the society used the 2014-2019 time frame for technical reasons, separating confirmed cases in the earlier period from estimated cases in recent years.
“We discourage comparing projected cases over time because these cases are model-based and subject to fluctuations,” Dr. Dahut said.
A version of this article originally appeared on Medscape.com.
The American Cancer Society on Jan. 13 revealed what it called “alarming” news about prostate cancer: After 2 decades of decline, the number of men diagnosed with the disease in the United States rose by 15% from 2014 to 2019.
“Most concerning,” according to the group’s CEO Karen Knudsen, PhD, MBA, is that the increase is being driven by diagnoses of advanced disease.
“Since 2011, the diagnosis of advanced-stage (regional- or distant-stage) prostate cancer has increased by 4%-5% annually and the proportion of men diagnosed with distant-stage disease has doubled,” said Dr. Knudsen at a press conference concerning the figures. “These findings underscore the importance of understanding and reducing this trend.”
The increase, which works out to be an additional 99,000 cases of prostate cancer, did not take the ACS by surprise; the group has been predicting a jump in diagnoses of the disease, which is the most common cancer in men after skin cancer, and the second most common cause of cancer death for that group.
The ACS announced a new action plan, “Improving Mortality from Prostate Cancer Together” – or IMPACT – to address the rise, especially in Black men, and to curb the increasing rate of advanced, difficult-to-treat cases.
“We must address these shifts in prostate cancer, especially in the Black community, since the incidence of prostate cancer in Black men is 70% higher than in White men and prostate cancer mortality rates in Black men are approximately two to four times higher than those in every other racial and ethnic group,” William Dahut, MD, PhD, chief scientific officer for the ACS, said at the press conference.
A study published in JAMA Network Open challenged that claim, finding that, after controlling for socioeconomic factors, race does not appear to be a significant predictor of mortality for prostate cancer.
Dr. Dahut said in an interview that IMPACT “is still [in the] early days for this initiative and more details will be coming out soon.”
Charles Ryan, MD, CEO of the Prostate Cancer Foundation, the world’s largest prostate cancer research charity, called IMPACT “extremely important work. Highlighting the disparities can only serve to benefit all men with prostate cancer, especially Black men.”
Bold action ... or passivity?
Overall cancer mortality has dropped 33% since 1991, averting an estimated 3.8 million deaths, according to ACS. But the story for prostate cancer is different.
The society and advocates had warned as recently as 2 years ago that prostate cancer was poised to rise again, especially advanced cases that may be too late to treat.
Leaders in the prostate cancer advocacy community praised the ACS plan for IMPACT, but some expressed frustration over what they said was ACS’ passivity in the face of long-anticipated increases in cases of the disease.
“I think prostate cancer was not high on their agenda,” said Rick Davis, founder of AnCan, which offers several support groups for patients with prostate cancer. “It’s good to see ACS get back into the prostate cancer game.”
Mr. Davis and patient advocate Darryl Mitteldorf, LCSW, founder of Malecare, another prostate support organization, said ACS dropped patient services for prostate cancer patients a decade ago and has not been a vocal supporter of screening for levels of prostate-specific antigen (PSA) to detect prostate cancer early.
“Early detection is supposed to be their goal,” Mr. Davis said.
In 2012, the U.S. Preventive Services Task Force recommended against PSA screening, giving it a D-rating. The move prompted attacks on the task force from most advocates and many urologists.
Following this criticism, the task force recommended shared decision-making between patient and doctor, while giving PSA screening a C-rating. Now, the ACS recommends men in general at age 50 discuss prostate cancer screening with their doctor and that Black men do the same at age 45.
Mr. Mitteldorf said ACS “owes prostate cancer patients an explanation and analysis of its response to the USPTF’s downgrade of PSA testing and how that response might be related to death and instance rates.”
Mr. Mitteldorf added that male patients lost key support from ACS when the group dismantled its Man to Man group for prostate cancer patients and its Brother to Brother group for Blacks in particular.
Dr. Dahut said Man to Man “sunsetted” and was turned over to any local organization that chose to offer it. He said longtime staff didn’t have “a lot of information about [the demise of] Brother to Brother.”
For Mr. Davis, those smaller cuts add up to a much larger insult.
“Today, in 2023, ACS continues to poke a finger in the eyes of prostate cancer patients,” he said. “Since 2010, they have not given us any respect. ACS dumped its support.”
He pointed to the group’s funding priorities, noting that outlays for prostate cancer have consistently lagged behind those for breast cancer.
The ACS spent $25.3 million on breast cancer research and $6.7 million for prostate cancer in 2018, and in 2023 will designate $126.5 for breast cancer research and $43.9 million for prostate cancer.
ACS has earmarked $62 million this year for lung cancer programs and $61 million for colorectal cancer.
“Parity between breast cancer and prostate cancer would be a good start in sizing the IMPACT program,” Mr. Davis said. “After all, breast cancer and prostate cancer are hardly different in numbers today.”
Dr. Dahut denied any gender bias in research funding. He said the group makes funding decisions “based on finding the most impactful science regardless of tumor type. Our mission includes funding every cancer, every day; thus, we generally do not go into our funding cycle with any set-asides for a particular cancer.”
Mr. Davis also said the ACS data suggest the growing number of prostate cancer cases is even worse than the group has said. Although the society cites a 3% annual increase in prostate cancer diagnoses from 2014 to 2019, since 2019 the annual increase is a much more dramatic 16%. Meanwhile, the number of new cases of the disease is projected to rise from 175,000 per year in 2019 to 288,000 this year.
Dr. Dahut said the society used the 2014-2019 time frame for technical reasons, separating confirmed cases in the earlier period from estimated cases in recent years.
“We discourage comparing projected cases over time because these cases are model-based and subject to fluctuations,” Dr. Dahut said.
A version of this article originally appeared on Medscape.com.
The American Cancer Society on Jan. 13 revealed what it called “alarming” news about prostate cancer: After 2 decades of decline, the number of men diagnosed with the disease in the United States rose by 15% from 2014 to 2019.
“Most concerning,” according to the group’s CEO Karen Knudsen, PhD, MBA, is that the increase is being driven by diagnoses of advanced disease.
“Since 2011, the diagnosis of advanced-stage (regional- or distant-stage) prostate cancer has increased by 4%-5% annually and the proportion of men diagnosed with distant-stage disease has doubled,” said Dr. Knudsen at a press conference concerning the figures. “These findings underscore the importance of understanding and reducing this trend.”
The increase, which works out to be an additional 99,000 cases of prostate cancer, did not take the ACS by surprise; the group has been predicting a jump in diagnoses of the disease, which is the most common cancer in men after skin cancer, and the second most common cause of cancer death for that group.
The ACS announced a new action plan, “Improving Mortality from Prostate Cancer Together” – or IMPACT – to address the rise, especially in Black men, and to curb the increasing rate of advanced, difficult-to-treat cases.
“We must address these shifts in prostate cancer, especially in the Black community, since the incidence of prostate cancer in Black men is 70% higher than in White men and prostate cancer mortality rates in Black men are approximately two to four times higher than those in every other racial and ethnic group,” William Dahut, MD, PhD, chief scientific officer for the ACS, said at the press conference.
A study published in JAMA Network Open challenged that claim, finding that, after controlling for socioeconomic factors, race does not appear to be a significant predictor of mortality for prostate cancer.
Dr. Dahut said in an interview that IMPACT “is still [in the] early days for this initiative and more details will be coming out soon.”
Charles Ryan, MD, CEO of the Prostate Cancer Foundation, the world’s largest prostate cancer research charity, called IMPACT “extremely important work. Highlighting the disparities can only serve to benefit all men with prostate cancer, especially Black men.”
Bold action ... or passivity?
Overall cancer mortality has dropped 33% since 1991, averting an estimated 3.8 million deaths, according to ACS. But the story for prostate cancer is different.
The society and advocates had warned as recently as 2 years ago that prostate cancer was poised to rise again, especially advanced cases that may be too late to treat.
Leaders in the prostate cancer advocacy community praised the ACS plan for IMPACT, but some expressed frustration over what they said was ACS’ passivity in the face of long-anticipated increases in cases of the disease.
“I think prostate cancer was not high on their agenda,” said Rick Davis, founder of AnCan, which offers several support groups for patients with prostate cancer. “It’s good to see ACS get back into the prostate cancer game.”
Mr. Davis and patient advocate Darryl Mitteldorf, LCSW, founder of Malecare, another prostate support organization, said ACS dropped patient services for prostate cancer patients a decade ago and has not been a vocal supporter of screening for levels of prostate-specific antigen (PSA) to detect prostate cancer early.
“Early detection is supposed to be their goal,” Mr. Davis said.
In 2012, the U.S. Preventive Services Task Force recommended against PSA screening, giving it a D-rating. The move prompted attacks on the task force from most advocates and many urologists.
Following this criticism, the task force recommended shared decision-making between patient and doctor, while giving PSA screening a C-rating. Now, the ACS recommends men in general at age 50 discuss prostate cancer screening with their doctor and that Black men do the same at age 45.
Mr. Mitteldorf said ACS “owes prostate cancer patients an explanation and analysis of its response to the USPTF’s downgrade of PSA testing and how that response might be related to death and instance rates.”
Mr. Mitteldorf added that male patients lost key support from ACS when the group dismantled its Man to Man group for prostate cancer patients and its Brother to Brother group for Blacks in particular.
Dr. Dahut said Man to Man “sunsetted” and was turned over to any local organization that chose to offer it. He said longtime staff didn’t have “a lot of information about [the demise of] Brother to Brother.”
For Mr. Davis, those smaller cuts add up to a much larger insult.
“Today, in 2023, ACS continues to poke a finger in the eyes of prostate cancer patients,” he said. “Since 2010, they have not given us any respect. ACS dumped its support.”
He pointed to the group’s funding priorities, noting that outlays for prostate cancer have consistently lagged behind those for breast cancer.
The ACS spent $25.3 million on breast cancer research and $6.7 million for prostate cancer in 2018, and in 2023 will designate $126.5 for breast cancer research and $43.9 million for prostate cancer.
ACS has earmarked $62 million this year for lung cancer programs and $61 million for colorectal cancer.
“Parity between breast cancer and prostate cancer would be a good start in sizing the IMPACT program,” Mr. Davis said. “After all, breast cancer and prostate cancer are hardly different in numbers today.”
Dr. Dahut denied any gender bias in research funding. He said the group makes funding decisions “based on finding the most impactful science regardless of tumor type. Our mission includes funding every cancer, every day; thus, we generally do not go into our funding cycle with any set-asides for a particular cancer.”
Mr. Davis also said the ACS data suggest the growing number of prostate cancer cases is even worse than the group has said. Although the society cites a 3% annual increase in prostate cancer diagnoses from 2014 to 2019, since 2019 the annual increase is a much more dramatic 16%. Meanwhile, the number of new cases of the disease is projected to rise from 175,000 per year in 2019 to 288,000 this year.
Dr. Dahut said the society used the 2014-2019 time frame for technical reasons, separating confirmed cases in the earlier period from estimated cases in recent years.
“We discourage comparing projected cases over time because these cases are model-based and subject to fluctuations,” Dr. Dahut said.
A version of this article originally appeared on Medscape.com.
Lifestyle changes may reduce colorectal cancer risk
Changes regarding smoking, drinking, body weight, and physical activity may alter the risk for colorectal cancer (CRC), the results of a study on a large European cohort suggest.
“This is a clear message that practicing clinicians and gastroenterologists could give to their patients and to CRC screening participants to improve CRC prevention,” write Edoardo Botteri, PhD, Cancer Registry of Norway, Oslo, and colleagues in an article published in the American Journal of Gastroenterology.
Previous studies have shown a correlation between cancer in general and unhealthy lifestyle factors. They have also shown an association between weight gain and an increased risk for CRC and a reduced risk with smoking cessation. But Dr. Botteri and colleagues could not find any published research on the association of other lifestyle factors and the risk for CRC specifically, they write.
To help fill this gap, they followed 295,865 people who participated in the European Prospective Investigation into Cancer (EPIC) for a median of 7.8 years. The participants were mostly aged from 35 to 70 years and lived in Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom.
The researchers calculated a healthy lifestyle index (HLI) score on the basis of smoking status, alcohol consumption, body mass index, and physical activity. The median time between baseline and the follow-up questionnaire was 5.7 years.
They awarded points as indicated in the following table.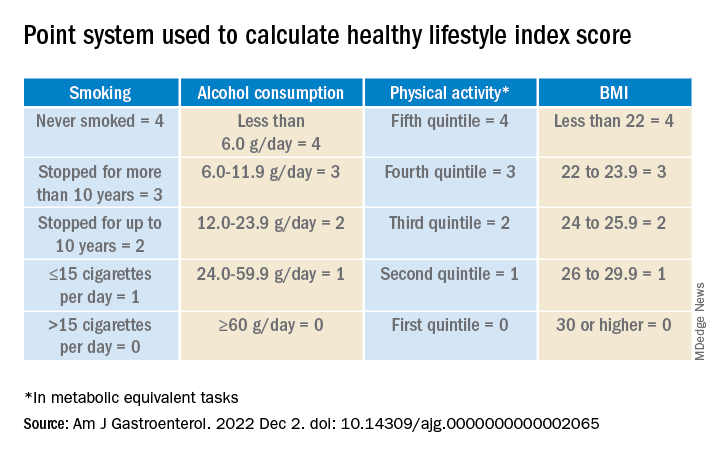
Participants’ scores ranged from 0 to 16. At baseline, the mean HLI score was 10.04. It dipped slightly to 9.95 at follow-up.
Men had more favorable changes than women, and the associations between the HLI score and CRC risk were only statistically significant among men.
Overall, a 1-unit increase in the HLI score was associated with a 3% lower risk for CRC.
When the HLI scores were grouped into tertiles, improvements from an “unfavorable lifestyle” (0-9) to a “favorable lifestyle” (12-16) were associated with a 23% lower risk for CRC (compared with no change). Likewise, a decline from a “favorable lifestyle” to an “unfavorable lifestyle” was associated with a 34% higher risk.
Changes in the BMI score from baseline showed a trend toward an association with CRC risk.
Decreases in alcohol consumption were significantly associated with a reduction in CRC risk among participants aged 55 years or younger at baseline.
Increases in physical activity were significantly associated with a lower risk for proximal colon cancer, especially in younger participants.
On the other hand, reductions in smoking were associated with an increase in CRC risk. This correlation might be the result of “inverse causation,” the researchers note; that is, people may have quit smoking because they experienced early symptoms of CRC. Smoking had only a marginal influence on the HLI calculations in this study because only a small proportion of participants changed their smoking rates.
Information on diet was collected only at baseline, so changes in this factor could not be measured. The researchers adjusted their analysis for diet at baseline, but they acknowledge that their inability to incorporate diet into the HLI score was a limitation of the study.
Similarly, they used education as a marker of socioeconomic status but acknowledge that this is only a proxy.
“The HLI score may therefore not accurately capture the complex relationship between lifestyle habits and risk for CRC,” they write.
Still, if the results of this observational study are confirmed by other research, the findings could provide evidence to design intervention studies to prevent CRC, they conclude.
The study was supported by the grant LIBERTY from the French Institut National du Cancer. Financial supporters of the national cohorts and the coordination of EPIC are listed in the published study. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Changes regarding smoking, drinking, body weight, and physical activity may alter the risk for colorectal cancer (CRC), the results of a study on a large European cohort suggest.
“This is a clear message that practicing clinicians and gastroenterologists could give to their patients and to CRC screening participants to improve CRC prevention,” write Edoardo Botteri, PhD, Cancer Registry of Norway, Oslo, and colleagues in an article published in the American Journal of Gastroenterology.
Previous studies have shown a correlation between cancer in general and unhealthy lifestyle factors. They have also shown an association between weight gain and an increased risk for CRC and a reduced risk with smoking cessation. But Dr. Botteri and colleagues could not find any published research on the association of other lifestyle factors and the risk for CRC specifically, they write.
To help fill this gap, they followed 295,865 people who participated in the European Prospective Investigation into Cancer (EPIC) for a median of 7.8 years. The participants were mostly aged from 35 to 70 years and lived in Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom.
The researchers calculated a healthy lifestyle index (HLI) score on the basis of smoking status, alcohol consumption, body mass index, and physical activity. The median time between baseline and the follow-up questionnaire was 5.7 years.
They awarded points as indicated in the following table.
Participants’ scores ranged from 0 to 16. At baseline, the mean HLI score was 10.04. It dipped slightly to 9.95 at follow-up.
Men had more favorable changes than women, and the associations between the HLI score and CRC risk were only statistically significant among men.
Overall, a 1-unit increase in the HLI score was associated with a 3% lower risk for CRC.
When the HLI scores were grouped into tertiles, improvements from an “unfavorable lifestyle” (0-9) to a “favorable lifestyle” (12-16) were associated with a 23% lower risk for CRC (compared with no change). Likewise, a decline from a “favorable lifestyle” to an “unfavorable lifestyle” was associated with a 34% higher risk.
Changes in the BMI score from baseline showed a trend toward an association with CRC risk.
Decreases in alcohol consumption were significantly associated with a reduction in CRC risk among participants aged 55 years or younger at baseline.
Increases in physical activity were significantly associated with a lower risk for proximal colon cancer, especially in younger participants.
On the other hand, reductions in smoking were associated with an increase in CRC risk. This correlation might be the result of “inverse causation,” the researchers note; that is, people may have quit smoking because they experienced early symptoms of CRC. Smoking had only a marginal influence on the HLI calculations in this study because only a small proportion of participants changed their smoking rates.
Information on diet was collected only at baseline, so changes in this factor could not be measured. The researchers adjusted their analysis for diet at baseline, but they acknowledge that their inability to incorporate diet into the HLI score was a limitation of the study.
Similarly, they used education as a marker of socioeconomic status but acknowledge that this is only a proxy.
“The HLI score may therefore not accurately capture the complex relationship between lifestyle habits and risk for CRC,” they write.
Still, if the results of this observational study are confirmed by other research, the findings could provide evidence to design intervention studies to prevent CRC, they conclude.
The study was supported by the grant LIBERTY from the French Institut National du Cancer. Financial supporters of the national cohorts and the coordination of EPIC are listed in the published study. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Changes regarding smoking, drinking, body weight, and physical activity may alter the risk for colorectal cancer (CRC), the results of a study on a large European cohort suggest.
“This is a clear message that practicing clinicians and gastroenterologists could give to their patients and to CRC screening participants to improve CRC prevention,” write Edoardo Botteri, PhD, Cancer Registry of Norway, Oslo, and colleagues in an article published in the American Journal of Gastroenterology.
Previous studies have shown a correlation between cancer in general and unhealthy lifestyle factors. They have also shown an association between weight gain and an increased risk for CRC and a reduced risk with smoking cessation. But Dr. Botteri and colleagues could not find any published research on the association of other lifestyle factors and the risk for CRC specifically, they write.
To help fill this gap, they followed 295,865 people who participated in the European Prospective Investigation into Cancer (EPIC) for a median of 7.8 years. The participants were mostly aged from 35 to 70 years and lived in Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom.
The researchers calculated a healthy lifestyle index (HLI) score on the basis of smoking status, alcohol consumption, body mass index, and physical activity. The median time between baseline and the follow-up questionnaire was 5.7 years.
They awarded points as indicated in the following table.
Participants’ scores ranged from 0 to 16. At baseline, the mean HLI score was 10.04. It dipped slightly to 9.95 at follow-up.
Men had more favorable changes than women, and the associations between the HLI score and CRC risk were only statistically significant among men.
Overall, a 1-unit increase in the HLI score was associated with a 3% lower risk for CRC.
When the HLI scores were grouped into tertiles, improvements from an “unfavorable lifestyle” (0-9) to a “favorable lifestyle” (12-16) were associated with a 23% lower risk for CRC (compared with no change). Likewise, a decline from a “favorable lifestyle” to an “unfavorable lifestyle” was associated with a 34% higher risk.
Changes in the BMI score from baseline showed a trend toward an association with CRC risk.
Decreases in alcohol consumption were significantly associated with a reduction in CRC risk among participants aged 55 years or younger at baseline.
Increases in physical activity were significantly associated with a lower risk for proximal colon cancer, especially in younger participants.
On the other hand, reductions in smoking were associated with an increase in CRC risk. This correlation might be the result of “inverse causation,” the researchers note; that is, people may have quit smoking because they experienced early symptoms of CRC. Smoking had only a marginal influence on the HLI calculations in this study because only a small proportion of participants changed their smoking rates.
Information on diet was collected only at baseline, so changes in this factor could not be measured. The researchers adjusted their analysis for diet at baseline, but they acknowledge that their inability to incorporate diet into the HLI score was a limitation of the study.
Similarly, they used education as a marker of socioeconomic status but acknowledge that this is only a proxy.
“The HLI score may therefore not accurately capture the complex relationship between lifestyle habits and risk for CRC,” they write.
Still, if the results of this observational study are confirmed by other research, the findings could provide evidence to design intervention studies to prevent CRC, they conclude.
The study was supported by the grant LIBERTY from the French Institut National du Cancer. Financial supporters of the national cohorts and the coordination of EPIC are listed in the published study. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
A Novel Text Message Protocol to Improve Bowel Preparation for Outpatient Colonoscopies in Veterans
Colorectal cancer is the third leading cause of cancer-related death in both men and women.1 Colonoscopy is the current gold standard for screening due to the ability to remove precancerous lesions but remains highly dependent on the quality of bowel preparation.2 Poor bowel preparation has been associated with impaired adenoma detection as well as increased health care utilization due to the need for a repeat colonoscopy.3
Multiple patient factors are associated with increased risk of poor bowel preparation, including age > 60 years, male sex, diabetes mellitus, and presence of a mental health diagnosis, factors that are prevalent among the veteran population.3-5 Text messages have been shown to improve the quality of bowel preparation by increasing patients' understanding and adherence with the preparation process. Improved adherence with bowel preparation directions is associated with a cleaner colon prior to colonoscopy, leading to a thorough examination. Studies using text messaging instructions prior to colonoscopies have also shown measurable improvement in adenoma detection rate, patient preparation-associated discomfort, and completion of colonoscopy.6-10
In 2016, the Veterans Health Administration (VHA) introduced Annie, one of the first automated text messaging services, named after Army Lieutenant Annie Fox, the first woman to receive the Purple Heart for combat. The Annie platform allows for notifications, instructions, and simple data collection. The development of this platform allows VHA practitioners to engage and educate veterans in a similar way to other health care systems using text messaging protocols. Annie text messages have been piloted for the use of hepatitis C treatment, demonstrating promise of improved medication adherence and patient satisfaction.11 We aimed to develop and pilot the Annie bowel preparation protocol to improve the quality of colonoscopy bowel preparation for outpatients at the Minneapolis Veterans Affairs Medical Center (MVAMC) in Minnesota. A secondary goal included measuring patient satisfaction with the text messaging instructions for outpatient colonoscopy preparation.
Methods
We conducted a single center, prospective, endoscopist-blinded, study with two 3-month long Plan-Do-Study-Act (PDSA) cycles to improve the text messaging bowel preparation protocol at MVAMC between January 2019 and April 2020. The MVAMC Institutional Review Board determined the quality improvement project was exempt. Veterans who had outpatient colonoscopies scheduled were included. Veterans undergoing inpatient colonoscopies or outpatients who could not be reached to obtain informed consent, lacked text message capability, declined participation, or required extended colonoscopy preparation were excluded. Per MVAMC procedures, extended colonoscopy preparation was provided to patients receiving general or monitored anesthesia care, with a history of poor bowel preparation, or with risk factors for poor preparation as determined by the ordering health care professional (HCP). Standard bowel preparation involves ingestion of 4 L of polyethylene glycol 3350 with electrolytes; extended bowel preparation requires ingestion of an additional 2 L to total 6 L and uses a different set of instructions. Additionally, the patient population requiring extended bowel preparation also includes patients with spinal cord injuries, who often are admitted for assistance with extended preparation. Patients who consented to receiving text messages were placed in the Annie intervention group, and all others were placed in the control group.
The control group received standardized patient education, including a mailed copy of bowel preparation instructions and a phone call from a gastroenterology service nurse about 1 to 2 weeks before the procedure. Current MVAMC standard of care involves a phone call from a nurse to confirm that patients have received the polyethylene glycol preparation solution, the mailed instructions, have an escort and transportation, and to answer any questions. Both the usual care and intervention group received the phone call. During this call, the Annie text messaging bowel preparation protocol was introduced; if the veteran chose to participate, consent and enrollment were completed.
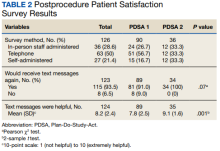
On the day of the colonoscopy, veterans in the intervention group were surveyed in the waiting room about their experience receiving the text messages and soliciting feedback for improvement or surveyed via telephone call within 3 days of their procedure. Patient satisfaction was quantified with a scale from 1 (low) to 10 (high), including questions about how helpful the texts were in relation to total number, timing, and content of messages as well as whether veterans would like to receive the text messages again for future procedures.
We reviewed individual charts and collected Boston Bowel Preparation Scale (BBPS) scores to determine adequate preparation. BBPS assigns a score of 0 to 3 for the right, transverse, and left colon applied upon withdrawal after flushing and suctioning have been completed.12 Adequate preparation is considered a total score of ≥ 6 with no segment scoring < 2. This method of preparation assessment is preferred due to its ability to account for difference in preparation quality among colonic segments, well-defined scoring characteristics, and several studies validating its use showing inter- and intraobserver reliability.12 Follow-up studies have shown validity of the BBPS when compared with relevant outcomes such as polyp detection rate and recommended timing for repeat procedure.13 Variables associated with poor bowel preparation (ie, gender, prior abdominal surgery, impaired mobility, high body mass index, diabetes mellitus, stroke, dementia, any neurologic diagnosis, cirrhosis, smoking, polypharmacy [> 8 active medications], and narcotic or tricyclic antidepressant medication use) were also collected through chart review.3-5 We note that immobility was defined by International Classification of Diseases (ICD)-9 and ICD-10 codes and prescriptions for assistive devices (ie, canes, wheelchairs, 4-wheeled walkers).
Veterans assent to be enrolled in Annie. After enrollment, veterans must text back a specific word response to an initial text message to receive the protocolized messages from the Annie program. A contact phone number to the gastrointestinal nurse line was provided for questions during business hours. The start date for the text message protocol is 6 days prior to the procedure date. If a patient rescheduled their colonoscopy, the Annie database was updated manually.
Statistical Analysis
We used both Pearson χ2 test and 2-sample t test analyses to compare demographic information and patient satisfaction scores between the control and intervention groups. We compared continuous BBPS scores between Annie intervention vs control group using parametric and nonparametric independent t tests using the Mann-Whitney U test. We repeated this analysis controlling for both mental health diagnoses and age using linear regression. We were unable to survey 61 of the 187 veterans who received Annie text messages.
RESULTS
During PDSA cycles 1 and 2, 640 veterans were scheduled for outpatient colonoscopy: 453 veterans were in the control group; 187 veterans were in the intervention group, of which 126 were surveyed. A significant percentage of veterans declined participation because they felt like they did not need reinforced education; others were not eligible for Annie due to requirement for extended bowel preparation, cancelled colonoscopy, inability to physically read text messages, or lack of cell phone.
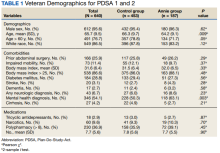
The mean (SD) age was 65 (8) years; 184 (28.8%) had a diabetes mellitus diagnosis, and the mean (SD) body mass index was 31.6 (6.4). The Annie group was slightly more likely to have mental health diagnoses and lower age compared with the control group (Table 1).
Patient Feedback
We collected feedback from veterans after each PDSA cycle to identify areas for improvement by both in-person and telephone surveys. Based on feedback from PDSA cycle 1, we decreased the total number of text messages to create a more succinct set of instructions. The most frequently requested change involved timing the text messages to align with the exact morning a specific instruction should take place.
Patient satisfaction with the Annie text messaging service was high.
DISCUSSION
To our knowledge, this is the first report of using Annie at a VAMC for colonoscopy bowel preparation improvement. We found a statistically significant improvement in the average BBPS in those receiving Annie text messages compared with the routine care control group. We also found high levels of patient satisfaction with most patients requesting to receive them again for future procedures.
The clinical significance of a BBPS of 7.8 vs 8.2 is unclear, although any score > 6 is considered to be adequate. However, subjectively speaking, the higher the BBPS the cleaner the colon, and theoretically the easier it is to see small or flat polyps. Future steps could include calculating adenoma detection rates for those enrolled in the Annie program vs the control group.
We have received inquiries regarding potential program implementation at other facilities. Success and sustainability of the program will require long-term commitment and ideally protected time for staff. It is helpful to remember that for each person who chooses to enroll in the intervention, the program currently requires that a brief consent note is placed in the patient’s chart. Thus, depending on the facilities’ resources, it is ideal for one staff member to be the designated lead to help oversee, troubleshoot, and train additional personnel. Surveys can be intermittently used to obtain feedback for improvement but are not required for sustainability. Automated text messaging is a promising addition to medicine for clinical education and communication. Future studies should examine the clinical significance (ie, adenoma detection rates) of text messaging bowel preparation protocols.
Limitations
Our study has several limitations. First, this was a single center study, thus generalizability is limited. MVAMC represents a predominantly White, male, and rural population. Second, data are likely an underestimation of the true impact of intervention, because results do not account for patients who were turned away on day of procedure (typically still reporting brown stools at time of check-in for procedure) due to poor preparation or aborted procedures secondary to poor preparation. Only about one-third of the 640 veterans opted to receive Annie text messages.
Studies have shown veterans are willing to use technology for health care; however, access to technology and lack of training remain barriers to use.14 This has been most robustly studied at the VA in veterans experiencing mental illness and homelessness. Targeted strategies to improve veteran adoption of technology within their health care include supplying veterans with cell phones and paid data plans and providing training on specific technology-based resources.15-17 Future improvement for the Annie platform should include improved integration with CPRS. Integration will facilitate automatic import of key information such as mobile phone number or colonoscopy procedure date. Unfortunately, this is not currently an automated process, and the manual workload of staff limits sustainability. Since our study ended, the Annie database now allows an “event date” to be programmed in to center the text message series around. This will be entered at the time of Annie enrollment and eliminate manual activation of the protocol. The issue of updating information for rescheduled procedures remains.
Conclusions
There is increasing evidence that automated text messaging is a promising addition to medicine for clinical education and communication. It continues to gain traction as a readily available and acceptable option, and many patients are willing to incorporate the technology platform into their care plan. We found high patient satisfaction with our protocol, and Annie patients had cleaner bowel preparations compared with control patients. Our study supports the use of text message reminders as an effective intervention for improving patient adherence with bowel preparation instructions. We suspect that creation of a text messaging protocol designed for patients requiring outpatient extended bowel preparation will yield great benefit. As technology continues to improve, future implementation of Annie text messaging will become increasingly seamless within the field of gastroenterology and beyond.
1. Centers for Disease Control and Prevention. Colorectal cancer statistics. Updated June 6, 2022. Accessed September 8, 2022. https://www.cdc.gov/cancer/colorectal/statistics
2. Lieberman D, Ladabaum U, Cruz-Correa M, et al. Screening for colorectal cancer and evolving issues for physicians and patients: a review. JAMA. 2016;316(20):2135-2145. doi:10.1001/jama.2016.17418
3. Nguyen DL, Wieland M. Risk factors predictive of poor quality preparation during average risk colonoscopy screening: the importance of health literacy. J Gastrointestin Liver Dis. 2010;19(4):369-372.
4. Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(8):819-826. doi:10.1097/MEG.0000000000001175
5. Harrington KM, Nguyen XT, Song RJ, et al. Gender differences in demographic and health characteristics of the Million Veteran Program cohort. Womens Health Issues. 2019;29(suppl 1):S56-S66. doi:10.1016/j.whi.2019.04.012
6. Zhang QX, Li J, Zhang Q, et al. Effect of education by messaging software on the quality of bowel preparation for colonoscopy. Chin Med J (Engl). 2018;131(14):1750-1752. doi:10.4103/0366-6999.235881
7. Walter B, Klare P, Strehle K, et al. Improving the quality and acceptance of colonoscopy preparation by reinforced patient education with short message service: results from a randomized, multicenter study (PERICLES-II). Gastrointest Endosc. 2019;89(3):506-513.e4. doi:10.1016/j.gie.2018.08.014
8. Nadim MM, Doshi S, Coniglio M, et al. Automated text message navigation to improve preparation quality and show rate for colonoscopy. Am J Gastroenterol. 2018;113:S64-S66.
9. Walter B, Frank R, Ludwig L, et al. Smartphone application to reinforce education increases high-quality preparation for colorectal cancer screening colonoscopies in a randomized trial. Clin Gastroenterol Hepatol. 2021;19(2):331-338.e5. doi:10.1016/j.cgh.2020.03.051
10. Guo B, Zuo X, Li Z, et al. Improving the quality of bowel preparation through an app for inpatients undergoing colonoscopy: a randomized controlled trial. J Adv Nurs. 2020;76(4):1037-1045. doi:10.1111/jan.14295
11. Yakovchenko V, Hogan TP, Houston TK, et al. Automated text messaging with patients in department of veterans affairs specialty clinics: cluster randomized trial. J Med Internet Res. 2019;21(8):e14750. doi:10.2196/14750
12. Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69(3 Pt 2):620-625. doi:10.1016/j.gie.2008.05.057
13. Calderwood AH, Jacobson BC. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc. 2010;72(4):686-692. doi:10.1016/j.gie.2010.06.068
14. Duan-Porter W, Van Houtven CH, Mahanna EP, et al. Internet use and technology-related attitudes of veterans and informal caregivers of veterans. Telemed J E Health. 2018;24(7):471-480. doi:10.1089/tmj.2017.0015
15. Boston University School of Public Health. how mobile technology can increase veteran healthcare and wellbeing. November 10, 2021. Accessed November 1, 2022. https://www.ideahub.org/research-data/how-mobile-technology-increases-veteran-healthcare-and-wellbeing/
16. Klee A, Stacy M, Rosenheck R, Harkness L, Tsai J. Interest in technology-based therapies hampered by access: A survey of veterans with serious mental illnesses. Psychiatr Rehabil J. 2016;39(2):173-179. doi:10.1037/prj0000180
17. Berrouiguet S, Baca-García E, Brandt S, Walter M, Courtet P. Fundamentals for future mobile-health (mHealth): a systematic review of mobile phone and web-based text messaging in mental health. J Med Internet Res. 2016;18(6):e135. Published 2016 Jun 10. doi:10.2196/jmir.5066
Colorectal cancer is the third leading cause of cancer-related death in both men and women.1 Colonoscopy is the current gold standard for screening due to the ability to remove precancerous lesions but remains highly dependent on the quality of bowel preparation.2 Poor bowel preparation has been associated with impaired adenoma detection as well as increased health care utilization due to the need for a repeat colonoscopy.3
Multiple patient factors are associated with increased risk of poor bowel preparation, including age > 60 years, male sex, diabetes mellitus, and presence of a mental health diagnosis, factors that are prevalent among the veteran population.3-5 Text messages have been shown to improve the quality of bowel preparation by increasing patients' understanding and adherence with the preparation process. Improved adherence with bowel preparation directions is associated with a cleaner colon prior to colonoscopy, leading to a thorough examination. Studies using text messaging instructions prior to colonoscopies have also shown measurable improvement in adenoma detection rate, patient preparation-associated discomfort, and completion of colonoscopy.6-10
In 2016, the Veterans Health Administration (VHA) introduced Annie, one of the first automated text messaging services, named after Army Lieutenant Annie Fox, the first woman to receive the Purple Heart for combat. The Annie platform allows for notifications, instructions, and simple data collection. The development of this platform allows VHA practitioners to engage and educate veterans in a similar way to other health care systems using text messaging protocols. Annie text messages have been piloted for the use of hepatitis C treatment, demonstrating promise of improved medication adherence and patient satisfaction.11 We aimed to develop and pilot the Annie bowel preparation protocol to improve the quality of colonoscopy bowel preparation for outpatients at the Minneapolis Veterans Affairs Medical Center (MVAMC) in Minnesota. A secondary goal included measuring patient satisfaction with the text messaging instructions for outpatient colonoscopy preparation.
Methods
We conducted a single center, prospective, endoscopist-blinded, study with two 3-month long Plan-Do-Study-Act (PDSA) cycles to improve the text messaging bowel preparation protocol at MVAMC between January 2019 and April 2020. The MVAMC Institutional Review Board determined the quality improvement project was exempt. Veterans who had outpatient colonoscopies scheduled were included. Veterans undergoing inpatient colonoscopies or outpatients who could not be reached to obtain informed consent, lacked text message capability, declined participation, or required extended colonoscopy preparation were excluded. Per MVAMC procedures, extended colonoscopy preparation was provided to patients receiving general or monitored anesthesia care, with a history of poor bowel preparation, or with risk factors for poor preparation as determined by the ordering health care professional (HCP). Standard bowel preparation involves ingestion of 4 L of polyethylene glycol 3350 with electrolytes; extended bowel preparation requires ingestion of an additional 2 L to total 6 L and uses a different set of instructions. Additionally, the patient population requiring extended bowel preparation also includes patients with spinal cord injuries, who often are admitted for assistance with extended preparation. Patients who consented to receiving text messages were placed in the Annie intervention group, and all others were placed in the control group.
The control group received standardized patient education, including a mailed copy of bowel preparation instructions and a phone call from a gastroenterology service nurse about 1 to 2 weeks before the procedure. Current MVAMC standard of care involves a phone call from a nurse to confirm that patients have received the polyethylene glycol preparation solution, the mailed instructions, have an escort and transportation, and to answer any questions. Both the usual care and intervention group received the phone call. During this call, the Annie text messaging bowel preparation protocol was introduced; if the veteran chose to participate, consent and enrollment were completed.
On the day of the colonoscopy, veterans in the intervention group were surveyed in the waiting room about their experience receiving the text messages and soliciting feedback for improvement or surveyed via telephone call within 3 days of their procedure. Patient satisfaction was quantified with a scale from 1 (low) to 10 (high), including questions about how helpful the texts were in relation to total number, timing, and content of messages as well as whether veterans would like to receive the text messages again for future procedures.
We reviewed individual charts and collected Boston Bowel Preparation Scale (BBPS) scores to determine adequate preparation. BBPS assigns a score of 0 to 3 for the right, transverse, and left colon applied upon withdrawal after flushing and suctioning have been completed.12 Adequate preparation is considered a total score of ≥ 6 with no segment scoring < 2. This method of preparation assessment is preferred due to its ability to account for difference in preparation quality among colonic segments, well-defined scoring characteristics, and several studies validating its use showing inter- and intraobserver reliability.12 Follow-up studies have shown validity of the BBPS when compared with relevant outcomes such as polyp detection rate and recommended timing for repeat procedure.13 Variables associated with poor bowel preparation (ie, gender, prior abdominal surgery, impaired mobility, high body mass index, diabetes mellitus, stroke, dementia, any neurologic diagnosis, cirrhosis, smoking, polypharmacy [> 8 active medications], and narcotic or tricyclic antidepressant medication use) were also collected through chart review.3-5 We note that immobility was defined by International Classification of Diseases (ICD)-9 and ICD-10 codes and prescriptions for assistive devices (ie, canes, wheelchairs, 4-wheeled walkers).
Veterans assent to be enrolled in Annie. After enrollment, veterans must text back a specific word response to an initial text message to receive the protocolized messages from the Annie program. A contact phone number to the gastrointestinal nurse line was provided for questions during business hours. The start date for the text message protocol is 6 days prior to the procedure date. If a patient rescheduled their colonoscopy, the Annie database was updated manually.
Statistical Analysis
We used both Pearson χ2 test and 2-sample t test analyses to compare demographic information and patient satisfaction scores between the control and intervention groups. We compared continuous BBPS scores between Annie intervention vs control group using parametric and nonparametric independent t tests using the Mann-Whitney U test. We repeated this analysis controlling for both mental health diagnoses and age using linear regression. We were unable to survey 61 of the 187 veterans who received Annie text messages.
RESULTS
During PDSA cycles 1 and 2, 640 veterans were scheduled for outpatient colonoscopy: 453 veterans were in the control group; 187 veterans were in the intervention group, of which 126 were surveyed. A significant percentage of veterans declined participation because they felt like they did not need reinforced education; others were not eligible for Annie due to requirement for extended bowel preparation, cancelled colonoscopy, inability to physically read text messages, or lack of cell phone.
The mean (SD) age was 65 (8) years; 184 (28.8%) had a diabetes mellitus diagnosis, and the mean (SD) body mass index was 31.6 (6.4). The Annie group was slightly more likely to have mental health diagnoses and lower age compared with the control group (Table 1).
Patient Feedback
We collected feedback from veterans after each PDSA cycle to identify areas for improvement by both in-person and telephone surveys. Based on feedback from PDSA cycle 1, we decreased the total number of text messages to create a more succinct set of instructions. The most frequently requested change involved timing the text messages to align with the exact morning a specific instruction should take place.
Patient satisfaction with the Annie text messaging service was high.
DISCUSSION
To our knowledge, this is the first report of using Annie at a VAMC for colonoscopy bowel preparation improvement. We found a statistically significant improvement in the average BBPS in those receiving Annie text messages compared with the routine care control group. We also found high levels of patient satisfaction with most patients requesting to receive them again for future procedures.
The clinical significance of a BBPS of 7.8 vs 8.2 is unclear, although any score > 6 is considered to be adequate. However, subjectively speaking, the higher the BBPS the cleaner the colon, and theoretically the easier it is to see small or flat polyps. Future steps could include calculating adenoma detection rates for those enrolled in the Annie program vs the control group.
We have received inquiries regarding potential program implementation at other facilities. Success and sustainability of the program will require long-term commitment and ideally protected time for staff. It is helpful to remember that for each person who chooses to enroll in the intervention, the program currently requires that a brief consent note is placed in the patient’s chart. Thus, depending on the facilities’ resources, it is ideal for one staff member to be the designated lead to help oversee, troubleshoot, and train additional personnel. Surveys can be intermittently used to obtain feedback for improvement but are not required for sustainability. Automated text messaging is a promising addition to medicine for clinical education and communication. Future studies should examine the clinical significance (ie, adenoma detection rates) of text messaging bowel preparation protocols.
Limitations
Our study has several limitations. First, this was a single center study, thus generalizability is limited. MVAMC represents a predominantly White, male, and rural population. Second, data are likely an underestimation of the true impact of intervention, because results do not account for patients who were turned away on day of procedure (typically still reporting brown stools at time of check-in for procedure) due to poor preparation or aborted procedures secondary to poor preparation. Only about one-third of the 640 veterans opted to receive Annie text messages.
Studies have shown veterans are willing to use technology for health care; however, access to technology and lack of training remain barriers to use.14 This has been most robustly studied at the VA in veterans experiencing mental illness and homelessness. Targeted strategies to improve veteran adoption of technology within their health care include supplying veterans with cell phones and paid data plans and providing training on specific technology-based resources.15-17 Future improvement for the Annie platform should include improved integration with CPRS. Integration will facilitate automatic import of key information such as mobile phone number or colonoscopy procedure date. Unfortunately, this is not currently an automated process, and the manual workload of staff limits sustainability. Since our study ended, the Annie database now allows an “event date” to be programmed in to center the text message series around. This will be entered at the time of Annie enrollment and eliminate manual activation of the protocol. The issue of updating information for rescheduled procedures remains.
Conclusions
There is increasing evidence that automated text messaging is a promising addition to medicine for clinical education and communication. It continues to gain traction as a readily available and acceptable option, and many patients are willing to incorporate the technology platform into their care plan. We found high patient satisfaction with our protocol, and Annie patients had cleaner bowel preparations compared with control patients. Our study supports the use of text message reminders as an effective intervention for improving patient adherence with bowel preparation instructions. We suspect that creation of a text messaging protocol designed for patients requiring outpatient extended bowel preparation will yield great benefit. As technology continues to improve, future implementation of Annie text messaging will become increasingly seamless within the field of gastroenterology and beyond.
Colorectal cancer is the third leading cause of cancer-related death in both men and women.1 Colonoscopy is the current gold standard for screening due to the ability to remove precancerous lesions but remains highly dependent on the quality of bowel preparation.2 Poor bowel preparation has been associated with impaired adenoma detection as well as increased health care utilization due to the need for a repeat colonoscopy.3
Multiple patient factors are associated with increased risk of poor bowel preparation, including age > 60 years, male sex, diabetes mellitus, and presence of a mental health diagnosis, factors that are prevalent among the veteran population.3-5 Text messages have been shown to improve the quality of bowel preparation by increasing patients' understanding and adherence with the preparation process. Improved adherence with bowel preparation directions is associated with a cleaner colon prior to colonoscopy, leading to a thorough examination. Studies using text messaging instructions prior to colonoscopies have also shown measurable improvement in adenoma detection rate, patient preparation-associated discomfort, and completion of colonoscopy.6-10
In 2016, the Veterans Health Administration (VHA) introduced Annie, one of the first automated text messaging services, named after Army Lieutenant Annie Fox, the first woman to receive the Purple Heart for combat. The Annie platform allows for notifications, instructions, and simple data collection. The development of this platform allows VHA practitioners to engage and educate veterans in a similar way to other health care systems using text messaging protocols. Annie text messages have been piloted for the use of hepatitis C treatment, demonstrating promise of improved medication adherence and patient satisfaction.11 We aimed to develop and pilot the Annie bowel preparation protocol to improve the quality of colonoscopy bowel preparation for outpatients at the Minneapolis Veterans Affairs Medical Center (MVAMC) in Minnesota. A secondary goal included measuring patient satisfaction with the text messaging instructions for outpatient colonoscopy preparation.
Methods
We conducted a single center, prospective, endoscopist-blinded, study with two 3-month long Plan-Do-Study-Act (PDSA) cycles to improve the text messaging bowel preparation protocol at MVAMC between January 2019 and April 2020. The MVAMC Institutional Review Board determined the quality improvement project was exempt. Veterans who had outpatient colonoscopies scheduled were included. Veterans undergoing inpatient colonoscopies or outpatients who could not be reached to obtain informed consent, lacked text message capability, declined participation, or required extended colonoscopy preparation were excluded. Per MVAMC procedures, extended colonoscopy preparation was provided to patients receiving general or monitored anesthesia care, with a history of poor bowel preparation, or with risk factors for poor preparation as determined by the ordering health care professional (HCP). Standard bowel preparation involves ingestion of 4 L of polyethylene glycol 3350 with electrolytes; extended bowel preparation requires ingestion of an additional 2 L to total 6 L and uses a different set of instructions. Additionally, the patient population requiring extended bowel preparation also includes patients with spinal cord injuries, who often are admitted for assistance with extended preparation. Patients who consented to receiving text messages were placed in the Annie intervention group, and all others were placed in the control group.
The control group received standardized patient education, including a mailed copy of bowel preparation instructions and a phone call from a gastroenterology service nurse about 1 to 2 weeks before the procedure. Current MVAMC standard of care involves a phone call from a nurse to confirm that patients have received the polyethylene glycol preparation solution, the mailed instructions, have an escort and transportation, and to answer any questions. Both the usual care and intervention group received the phone call. During this call, the Annie text messaging bowel preparation protocol was introduced; if the veteran chose to participate, consent and enrollment were completed.
On the day of the colonoscopy, veterans in the intervention group were surveyed in the waiting room about their experience receiving the text messages and soliciting feedback for improvement or surveyed via telephone call within 3 days of their procedure. Patient satisfaction was quantified with a scale from 1 (low) to 10 (high), including questions about how helpful the texts were in relation to total number, timing, and content of messages as well as whether veterans would like to receive the text messages again for future procedures.
We reviewed individual charts and collected Boston Bowel Preparation Scale (BBPS) scores to determine adequate preparation. BBPS assigns a score of 0 to 3 for the right, transverse, and left colon applied upon withdrawal after flushing and suctioning have been completed.12 Adequate preparation is considered a total score of ≥ 6 with no segment scoring < 2. This method of preparation assessment is preferred due to its ability to account for difference in preparation quality among colonic segments, well-defined scoring characteristics, and several studies validating its use showing inter- and intraobserver reliability.12 Follow-up studies have shown validity of the BBPS when compared with relevant outcomes such as polyp detection rate and recommended timing for repeat procedure.13 Variables associated with poor bowel preparation (ie, gender, prior abdominal surgery, impaired mobility, high body mass index, diabetes mellitus, stroke, dementia, any neurologic diagnosis, cirrhosis, smoking, polypharmacy [> 8 active medications], and narcotic or tricyclic antidepressant medication use) were also collected through chart review.3-5 We note that immobility was defined by International Classification of Diseases (ICD)-9 and ICD-10 codes and prescriptions for assistive devices (ie, canes, wheelchairs, 4-wheeled walkers).
Veterans assent to be enrolled in Annie. After enrollment, veterans must text back a specific word response to an initial text message to receive the protocolized messages from the Annie program. A contact phone number to the gastrointestinal nurse line was provided for questions during business hours. The start date for the text message protocol is 6 days prior to the procedure date. If a patient rescheduled their colonoscopy, the Annie database was updated manually.
Statistical Analysis
We used both Pearson χ2 test and 2-sample t test analyses to compare demographic information and patient satisfaction scores between the control and intervention groups. We compared continuous BBPS scores between Annie intervention vs control group using parametric and nonparametric independent t tests using the Mann-Whitney U test. We repeated this analysis controlling for both mental health diagnoses and age using linear regression. We were unable to survey 61 of the 187 veterans who received Annie text messages.
RESULTS
During PDSA cycles 1 and 2, 640 veterans were scheduled for outpatient colonoscopy: 453 veterans were in the control group; 187 veterans were in the intervention group, of which 126 were surveyed. A significant percentage of veterans declined participation because they felt like they did not need reinforced education; others were not eligible for Annie due to requirement for extended bowel preparation, cancelled colonoscopy, inability to physically read text messages, or lack of cell phone.
The mean (SD) age was 65 (8) years; 184 (28.8%) had a diabetes mellitus diagnosis, and the mean (SD) body mass index was 31.6 (6.4). The Annie group was slightly more likely to have mental health diagnoses and lower age compared with the control group (Table 1).
Patient Feedback
We collected feedback from veterans after each PDSA cycle to identify areas for improvement by both in-person and telephone surveys. Based on feedback from PDSA cycle 1, we decreased the total number of text messages to create a more succinct set of instructions. The most frequently requested change involved timing the text messages to align with the exact morning a specific instruction should take place.
Patient satisfaction with the Annie text messaging service was high.
DISCUSSION
To our knowledge, this is the first report of using Annie at a VAMC for colonoscopy bowel preparation improvement. We found a statistically significant improvement in the average BBPS in those receiving Annie text messages compared with the routine care control group. We also found high levels of patient satisfaction with most patients requesting to receive them again for future procedures.
The clinical significance of a BBPS of 7.8 vs 8.2 is unclear, although any score > 6 is considered to be adequate. However, subjectively speaking, the higher the BBPS the cleaner the colon, and theoretically the easier it is to see small or flat polyps. Future steps could include calculating adenoma detection rates for those enrolled in the Annie program vs the control group.
We have received inquiries regarding potential program implementation at other facilities. Success and sustainability of the program will require long-term commitment and ideally protected time for staff. It is helpful to remember that for each person who chooses to enroll in the intervention, the program currently requires that a brief consent note is placed in the patient’s chart. Thus, depending on the facilities’ resources, it is ideal for one staff member to be the designated lead to help oversee, troubleshoot, and train additional personnel. Surveys can be intermittently used to obtain feedback for improvement but are not required for sustainability. Automated text messaging is a promising addition to medicine for clinical education and communication. Future studies should examine the clinical significance (ie, adenoma detection rates) of text messaging bowel preparation protocols.
Limitations
Our study has several limitations. First, this was a single center study, thus generalizability is limited. MVAMC represents a predominantly White, male, and rural population. Second, data are likely an underestimation of the true impact of intervention, because results do not account for patients who were turned away on day of procedure (typically still reporting brown stools at time of check-in for procedure) due to poor preparation or aborted procedures secondary to poor preparation. Only about one-third of the 640 veterans opted to receive Annie text messages.
Studies have shown veterans are willing to use technology for health care; however, access to technology and lack of training remain barriers to use.14 This has been most robustly studied at the VA in veterans experiencing mental illness and homelessness. Targeted strategies to improve veteran adoption of technology within their health care include supplying veterans with cell phones and paid data plans and providing training on specific technology-based resources.15-17 Future improvement for the Annie platform should include improved integration with CPRS. Integration will facilitate automatic import of key information such as mobile phone number or colonoscopy procedure date. Unfortunately, this is not currently an automated process, and the manual workload of staff limits sustainability. Since our study ended, the Annie database now allows an “event date” to be programmed in to center the text message series around. This will be entered at the time of Annie enrollment and eliminate manual activation of the protocol. The issue of updating information for rescheduled procedures remains.
Conclusions
There is increasing evidence that automated text messaging is a promising addition to medicine for clinical education and communication. It continues to gain traction as a readily available and acceptable option, and many patients are willing to incorporate the technology platform into their care plan. We found high patient satisfaction with our protocol, and Annie patients had cleaner bowel preparations compared with control patients. Our study supports the use of text message reminders as an effective intervention for improving patient adherence with bowel preparation instructions. We suspect that creation of a text messaging protocol designed for patients requiring outpatient extended bowel preparation will yield great benefit. As technology continues to improve, future implementation of Annie text messaging will become increasingly seamless within the field of gastroenterology and beyond.
1. Centers for Disease Control and Prevention. Colorectal cancer statistics. Updated June 6, 2022. Accessed September 8, 2022. https://www.cdc.gov/cancer/colorectal/statistics
2. Lieberman D, Ladabaum U, Cruz-Correa M, et al. Screening for colorectal cancer and evolving issues for physicians and patients: a review. JAMA. 2016;316(20):2135-2145. doi:10.1001/jama.2016.17418
3. Nguyen DL, Wieland M. Risk factors predictive of poor quality preparation during average risk colonoscopy screening: the importance of health literacy. J Gastrointestin Liver Dis. 2010;19(4):369-372.
4. Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(8):819-826. doi:10.1097/MEG.0000000000001175
5. Harrington KM, Nguyen XT, Song RJ, et al. Gender differences in demographic and health characteristics of the Million Veteran Program cohort. Womens Health Issues. 2019;29(suppl 1):S56-S66. doi:10.1016/j.whi.2019.04.012
6. Zhang QX, Li J, Zhang Q, et al. Effect of education by messaging software on the quality of bowel preparation for colonoscopy. Chin Med J (Engl). 2018;131(14):1750-1752. doi:10.4103/0366-6999.235881
7. Walter B, Klare P, Strehle K, et al. Improving the quality and acceptance of colonoscopy preparation by reinforced patient education with short message service: results from a randomized, multicenter study (PERICLES-II). Gastrointest Endosc. 2019;89(3):506-513.e4. doi:10.1016/j.gie.2018.08.014
8. Nadim MM, Doshi S, Coniglio M, et al. Automated text message navigation to improve preparation quality and show rate for colonoscopy. Am J Gastroenterol. 2018;113:S64-S66.
9. Walter B, Frank R, Ludwig L, et al. Smartphone application to reinforce education increases high-quality preparation for colorectal cancer screening colonoscopies in a randomized trial. Clin Gastroenterol Hepatol. 2021;19(2):331-338.e5. doi:10.1016/j.cgh.2020.03.051
10. Guo B, Zuo X, Li Z, et al. Improving the quality of bowel preparation through an app for inpatients undergoing colonoscopy: a randomized controlled trial. J Adv Nurs. 2020;76(4):1037-1045. doi:10.1111/jan.14295
11. Yakovchenko V, Hogan TP, Houston TK, et al. Automated text messaging with patients in department of veterans affairs specialty clinics: cluster randomized trial. J Med Internet Res. 2019;21(8):e14750. doi:10.2196/14750
12. Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69(3 Pt 2):620-625. doi:10.1016/j.gie.2008.05.057
13. Calderwood AH, Jacobson BC. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc. 2010;72(4):686-692. doi:10.1016/j.gie.2010.06.068
14. Duan-Porter W, Van Houtven CH, Mahanna EP, et al. Internet use and technology-related attitudes of veterans and informal caregivers of veterans. Telemed J E Health. 2018;24(7):471-480. doi:10.1089/tmj.2017.0015
15. Boston University School of Public Health. how mobile technology can increase veteran healthcare and wellbeing. November 10, 2021. Accessed November 1, 2022. https://www.ideahub.org/research-data/how-mobile-technology-increases-veteran-healthcare-and-wellbeing/
16. Klee A, Stacy M, Rosenheck R, Harkness L, Tsai J. Interest in technology-based therapies hampered by access: A survey of veterans with serious mental illnesses. Psychiatr Rehabil J. 2016;39(2):173-179. doi:10.1037/prj0000180
17. Berrouiguet S, Baca-García E, Brandt S, Walter M, Courtet P. Fundamentals for future mobile-health (mHealth): a systematic review of mobile phone and web-based text messaging in mental health. J Med Internet Res. 2016;18(6):e135. Published 2016 Jun 10. doi:10.2196/jmir.5066
1. Centers for Disease Control and Prevention. Colorectal cancer statistics. Updated June 6, 2022. Accessed September 8, 2022. https://www.cdc.gov/cancer/colorectal/statistics
2. Lieberman D, Ladabaum U, Cruz-Correa M, et al. Screening for colorectal cancer and evolving issues for physicians and patients: a review. JAMA. 2016;316(20):2135-2145. doi:10.1001/jama.2016.17418
3. Nguyen DL, Wieland M. Risk factors predictive of poor quality preparation during average risk colonoscopy screening: the importance of health literacy. J Gastrointestin Liver Dis. 2010;19(4):369-372.
4. Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(8):819-826. doi:10.1097/MEG.0000000000001175
5. Harrington KM, Nguyen XT, Song RJ, et al. Gender differences in demographic and health characteristics of the Million Veteran Program cohort. Womens Health Issues. 2019;29(suppl 1):S56-S66. doi:10.1016/j.whi.2019.04.012
6. Zhang QX, Li J, Zhang Q, et al. Effect of education by messaging software on the quality of bowel preparation for colonoscopy. Chin Med J (Engl). 2018;131(14):1750-1752. doi:10.4103/0366-6999.235881
7. Walter B, Klare P, Strehle K, et al. Improving the quality and acceptance of colonoscopy preparation by reinforced patient education with short message service: results from a randomized, multicenter study (PERICLES-II). Gastrointest Endosc. 2019;89(3):506-513.e4. doi:10.1016/j.gie.2018.08.014
8. Nadim MM, Doshi S, Coniglio M, et al. Automated text message navigation to improve preparation quality and show rate for colonoscopy. Am J Gastroenterol. 2018;113:S64-S66.
9. Walter B, Frank R, Ludwig L, et al. Smartphone application to reinforce education increases high-quality preparation for colorectal cancer screening colonoscopies in a randomized trial. Clin Gastroenterol Hepatol. 2021;19(2):331-338.e5. doi:10.1016/j.cgh.2020.03.051
10. Guo B, Zuo X, Li Z, et al. Improving the quality of bowel preparation through an app for inpatients undergoing colonoscopy: a randomized controlled trial. J Adv Nurs. 2020;76(4):1037-1045. doi:10.1111/jan.14295
11. Yakovchenko V, Hogan TP, Houston TK, et al. Automated text messaging with patients in department of veterans affairs specialty clinics: cluster randomized trial. J Med Internet Res. 2019;21(8):e14750. doi:10.2196/14750
12. Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69(3 Pt 2):620-625. doi:10.1016/j.gie.2008.05.057
13. Calderwood AH, Jacobson BC. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc. 2010;72(4):686-692. doi:10.1016/j.gie.2010.06.068
14. Duan-Porter W, Van Houtven CH, Mahanna EP, et al. Internet use and technology-related attitudes of veterans and informal caregivers of veterans. Telemed J E Health. 2018;24(7):471-480. doi:10.1089/tmj.2017.0015
15. Boston University School of Public Health. how mobile technology can increase veteran healthcare and wellbeing. November 10, 2021. Accessed November 1, 2022. https://www.ideahub.org/research-data/how-mobile-technology-increases-veteran-healthcare-and-wellbeing/
16. Klee A, Stacy M, Rosenheck R, Harkness L, Tsai J. Interest in technology-based therapies hampered by access: A survey of veterans with serious mental illnesses. Psychiatr Rehabil J. 2016;39(2):173-179. doi:10.1037/prj0000180
17. Berrouiguet S, Baca-García E, Brandt S, Walter M, Courtet P. Fundamentals for future mobile-health (mHealth): a systematic review of mobile phone and web-based text messaging in mental health. J Med Internet Res. 2016;18(6):e135. Published 2016 Jun 10. doi:10.2196/jmir.5066
Contralateral Constrictor Dose Predicts Swallowing Function After Radiation for Head and Neck Cancer
Radiation therapy can cause long-term dysphagia that seriously affects quality of life for survivors of head and neck (H&N) cancer.1-3 Numerous studies have linked pharyngeal constrictor dose to long-term dysphagia, but conclusions about the dose distribution that can be safely tolerated have been inconsistent. For example, a group from the Netherlands found that the mean dose to the superior pharyngeal constrictor muscle and the supraglottic larynx were each predictive of dysphagia.4 A subsequent Vanderbilt study refuted these findings, reporting that these structures were not predictive but that dose to the inferior pharyngeal constrictor muscle was.5 Other studies have connected late dysphagia with dose to the middle pharyngeal constrictor muscle, total larynx, oral cavity, contralateral submandibular gland, contralateral parotid gland, or a combination of these structures.6-14 NRG Oncology trials commonly evaluate dose to the “uninvolved pharynx,” which is the total pharyngeal constrictor muscle volume minus the planning target volume for the lowest dose target volume. NRG H&N trials 3, 4, 5, 6, 8, and 9 all use uninvolved pharynx mean dose ≤ 45 Gy as a constraint to judge radiation plan quality.
Differences in methodology or patient population may explain the inconsistency of prior studies on dosimetric predictors of dysphagia, but it is possible that these studies did not evaluate the optimal metric for dysphagia. This study evaluates a novel organ at risk, the contralateral pharyngeal constrictor muscle, to determine whether dose to this structure is predictive of late swallowing function. The study also compares a constraint based on this structure to the NRG uninvolved pharynx constraint mentioned earlier.
Methods
This study is a retrospective review of patients treated at the Richard L. Roudebush Veterans Affairs (VA) Medical Center in Indianapolis, Indiana. Patients were identified by searching the VA Cancer Registry for patients treated for H&N squamous cell carcinoma between September 1, 2016, and August 30, 2019. Eligible sites included cancers of the nasopharynx, oropharynx, hypopharynx, larynx and oral cavity, as well as H&N cancer of an unknown primary site. Only patients treated with primary radiation with concurrent systemic therapy were included. Patients were excluded if they had prior surgery or radiation to the H&N.
The pharyngeal constrictor muscles were contoured per the techniques described by Bhide and colleagues.11 The contralateral constrictor was defined as the half of the constrictor volume contralateral to the primary site. For midline tumors, the side of the neck with a lower volume of lymph node metastases was judged to be the contralateral side.
One-year dysphagia was defined as having a gastronomy tube (G-tube) in place or an abnormal modified barium swallow (MBS) ≥ 12 months after the completion of radiation. At the study institution, MBS is not routinely done after therapy but is ordered if a patient or clinician has concerns about swallowing function. MBS was considered abnormal if there was laryngeal penetration that reached the level of the glottis or was not ejected from the larynx.
Results
The VA Cancer Registry identified 113 patients treated for H&N cancer during the study period. Of these, 55 patients met the inclusion criteria. No patients were lost to follow-up. The median follow-up was 29 months. The median age was 67 years (range, 41-83) (Table 1).
All patients were treated with intensity-modulated radiotherapy (IMRT). Patients treated with a sequential boost had an initial dose of 54 Gy and/or 50 Gy, followed by a boost to a total of 70 Gy at 2 Gy per fraction. Patients treated with a simultaneous integrated boost (SIB) technique received 69.96 Gy in 33 fractions, with elective volumes treated to 54.45 Gy in 33 fractions. Both patients with nasopharyngeal cancer were treated with SIB plans and had an intermediate dose volume of 59.4 Gy.
Systemic therapy was weekly cisplatin in 41 patients (75%) and cetuximab in 14 (25%). Twenty percent of patients receiving cisplatin switched to an alternative agent during treatment, most commonly carboplatin.
Forty-nine patients (89%) had a G-tube placed before starting radiation. G-tubes were in place for an interval of 0 to 47 months (mean, 8.6); 12 (22%) had a G-tube > 12 months. After completion of radiation, 18 patients (33%) had an abnormal MBS. These were done 1 to 50 months (mean, 14.8) after completion of radiation. Abnormal MBS occurred ≥ 12 months after radiation in 9 patients, 5 of whom had their G-tube in place for less than a year.
Forty-six patients (84%) survived more than 1 year and could be evaluated for late swallowing function. One-year dysphagia was seen in 17 (37%) of these patients. Recurrence was seen in 20 patients (36%), with locoregional recurrence in 12 (60%) of these cases. Recurrence occurred at a range of 0 to 15 months (mean, 5.6). Neither recurrence (P = .69) nor locoregional recurrence (P = .11) was associated with increased 1-year dysphagia.
In patients who could be evaluated for long-term swallowing function, contralateral constrictor V60 ranged from 0% to 100% (median, 51%). V60 was < 40% in 18 patients (39%). With V60 < 40%, there was a 6% rate of 1-year dysphagia compared with 57% for V60 ≥ 40% (P < .001).
Patients with contralateral constrictor V60 < 40 and V60 ≥ 40 both had a mean age of 65 years. χ2 analysis did not show a difference in T stage or systemic treatment but did show that patients with V60 < 40% were more likely to have N1 disease (P = .01), and less likely to have N2 disease (P = .01) compared with patients with V60 ≥ 40%. The difference in 1-year dysphagia between N0 to N1 patients (27%) and N2 to N3 patients (46%) was not statistically significant (P = .19).
In patients who could be evaluated for long-term swallowing function, the uninvolved pharynx volume median of the total constrictor volume was 32% (range, < 1%-62%). The uninvolved pharynx mean dose ranged from 28 to 68 Gy (median, 45). When the uninvolved pharynx mean dose was < 45 Gy, 1-year dysphagia was 22% compared with 52% with a dose ≥ 45 Gy (P = .03).
Air cavity editing was performed in 27 patients (49%). One-year survival was 93% with air cavity editing, and 75% without, which was not statistically significant. Locoregional recurrence occurred in 3 patients (11%) with air cavity editing, and 9 (32%) without, which was not statistically significant. In patients surviving at least 1 year, contralateral constrictor V60 averaged 33% with editing and 62% without editing (P < .001). One-year dysphagia was 12% with air cavity editing and 67% without editing (P < .001).
An SIB technique was done in 26 patients (47%). One-year survival was 85% (n = 22) with SIB and 83% (n = 24) with sequential boost, which was not statistically significant. Locoregional recurrence occurred in 19% with SIB, and 32% with sequential boost, which was not statistically significant. For SIB patients alive at 1 year, the median contralateral V60 was 28%, compared with 66% for patients treated with sequential technique. Seventeen patients (77%) with SIB had V60 < 40%. Nineteen (86%) of SIB plans also had air cavity editing. One patient (5%) with SIB had dysphagia at 1 year, compared with 16 (67%) sequential patients (P < .001).
Discussion
This is the first study to link contralateral constrictor dose to long-term dysphagia in patients treated with radiation for H&N cancer. Editing the boost volume off air cavities was associated with lower contralateral constrictor V60 and with less long-term dysphagia. This may indicate that optimizing plans to meet a contralateral constrictor constraint can reduce rates of long-term dysphagia.
The most useful clinical predictors are those that identify a patient at low risk for toxicity. These constraints are useful because they reassure physicians that treatments will have a favorable risk/benefit ratio while identifying plans that may need modification before starting treatment.
The contralateral constrictor outperformed the uninvolved pharynx in identifying patients at low risk for long-term dysphagia. This difference could not be overcome by decreasing the threshold of the pharynx constraint, as 17% of patients with dysphagia had a mean dose of < 40 Gy to the uninvolved pharynx, which was not statistically significant.
An advantage of contralateral constrictor is that it is independent of planning target volume (PTV) size. The uninvolved pharynx structure depends on the PTV contour, so it may obscure a connection between PTV size and dysphagia.
In the context of a clinical trial, only measuring dose to the uninvolved pharynx may allow more plans to meet constraints, but even in NRG trials, physicians have some control over target volumes. For example, NRG HN009, a national trial for patients with H&N cancer, recommends editing the CTV_7000 (clinical target volume treated to 70 Gy) off air cavities but does not define how much the volume should be cropped or specify protocol violations if the volume is not cropped.15 Furthermore, constraints used in clinical trials are often adopted for use outside the trial, where physicians have extensive control over target volumes.
The broad range of uninvolved pharynx volume relative to total constrictor volume confounds predictions using this variable. For example, according to the NRG constraint, a patient with an uninvolved pharynx mean dose of 44 Gy will have a low risk of dysphagia even if this structure is only 1% of the total constrictor. The contralateral constrictor is always about 50% of the total constrictor volume, which means that predictions using this structure will not be confounded by the same variation in volume size.
Figure 2 shows a representative patient who met the NRG uninvolved pharynx constraint but developed long-term dysphagia.
Pharyngoesophageal stricture is a common cause of dysphagia after IMRT for H&N cancer.16 Radiation has been shown to decrease pharyngeal function in patients with H&N cancer.17 Sparing one side of the pharynx may allow for better pharyngeal compliance throughout the length of the pharynx, possibly decreasing the rate of pharyngoesophageal stricture. Additionally, constraining the contralateral constrictor may preserve strength on this side, allowing it to compensate for weakness on the side of the primary cancer. An exercise sometimes used for dysphagia involves head rotation toward the affected side during swallowing. This technique has been shown to cause food to move to the unaffected side.18 Sparing the contralateral constrictor may help such techniques work better in patients with H&N cancer.
Few studies have commented specifically on dose to swallowing structures contralateral to the primary tumor. Two studies have proposed contralateral submandibular gland constraints for dysphagia (not xerostomia), but neither measured the dose to the contralateral constrictor muscle.9,10 Although the contralateral submandibular dose may correlate with dose to the constrictor on that side, the submandibular gland may have a less direct impact on swallowing than the constrictor muscle, and its limited dimensions may make constraints based on the gland less robust for cancers outside the oropharynx.
Another study reported improved quality of life in patients who were not treated with elective contralateral retropharyngeal radiation.19 Although it is likely that doses to the contralateral constrictor were lower in patients who did not receive elective radiation to this area, this study did not measure or constrain doses to the contralateral constrictors.
Limitations
This study is limited by its single institution, retrospective design, small sample size, and by all patients being male. The high correlation between air cavity editing and the use of SIB makes it impossible to assess the impact of each technique individually. Patients with contralateral constrictor V60 < 40% were less likely to have N2 disease, but N2 to N3 disease did not predict higher 1-year dysphagia, so the difference in N-category cannot fully explain the difference in 1-year dysphagia. It is possible that unreported factors, such as CTV, may contribute significantly to swallowing function. Nevertheless, within the study population, contralateral constrictor dose was able to identify a group with a low rate of long-term dysphagia.
Conclusions
Contralateral constrictor dose is a promising predictor of late dysphagia for patients with H&N cancer treated with radiation with concurrent systemic therapy. Contralateral constrictor V60 < 40% was able to identify a group of patients with a low rate of 1-year dysphagia in this single-center retrospective study. The correlation between air cavity editing and contralateral constrictor V60 suggests that contralateral constrictor dose may depend partly on technique. Further studies are needed to see if the contralateral constrictor dose can be used to predict long-term dysphagia prospectively and in other patient populations.
1. Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770-3776. doi:10.1200/JCO.2007.14.6647
2. Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61(3):772-778. doi:10.1016/j.ijrobp.2004.06.017
3. Ramaekers BLT, Joore MA, Grutters JPC, et al. The impact of late treatment-toxicity on generic health-related quality of life in head and neck cancer patients after radiotherapy. Oral Oncol. 2011;47(8):768-774. doi:10.1016/j.oraloncology.2011.05.012
4. Christianen MEMC, Schilstra C, Beetz I, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol. 2012;105(1):107-114. doi:10.1016/j.radonc.2011.08.009
5. Vlachich G, Spratt DE, Diaz R, et al. Dose to inferior pharyngeal conctrictor predicts prolonged gastrostomy tube dependence with concurrent intensity-modulated radiation therapy and chemotherapy for locally-advanced head and neck cancer. Radiother Oncol. 2014;110(3):435-440. doi:10.1016/j.radonc.2013.12.007
6. Mogadas S, Busch CJ, Pflug Cet al. Influence of radiation dose to pharyngeal constrictor muscles on late dysphagia and quality of life in patients with locally advanced oropharyngeal carcinoma. Strahlenther Onkol. 2020;196(6):522-529. doi:10.1007/s00066-019-01572-0
7. Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts of swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1110-1118. doi:10.1016/j.ijrobp.2008.02.048
8. Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(5):1356-1365. doi:10.1016/j.ijrobp.2009.10.002
9. Gensheimer MF, Nyflot M, Laramore GE, Laio JL, Parvathaneni U. Contribution of submandibular gland and swallowing structure sparing to post-radiation therapy peg dependence in oropharynx cancer patients treated with split-neck IMRT technique. Radiat Oncol. 2015;11(1):1-7. doi:10.1186/s13014-016-0726-3
10. Hedström J, Tuomi L, Finizia C, Olsson C. Identifying organs at risk for radiation-induced late dysphagia in head and neck cancer patients. Clin Transl Radiat Oncol. 2019;19:87-95. doi:10.1016/j.ctro.2019.08.005
11. Bhide SA, Gulliford S, Kazi R, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol. 2009;93(3):539-544. doi:10.1016/j.radonc.2009.09.017
12. Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76(2):403-409. doi:10.1016/j.ijrobp.2009.02.017
13. Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85(1):64-73. doi:10.1016/j.radonc.2007.07.009
14. Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425-1439. doi:10.1016/j.ijrobp.2004.05.050
15. Harari PM; NRG Oncology. Comparing high-dose cisplatin every three weeks to low-dose cisplatin weekly when combined with radiation for patients with advanced head and neck cancer. ClinicalTrials.gov identifier: NCT05050162. Updated November 25, 2022. Accessed December 7, 2022. https://clinicaltrials.gov/ct2/show/NCT05050162
16. Wang JJ, Goldsmith TA, Holman AS, Cianchetti M, Chan AW. Pharyngoesophageal stricture after treatment for head and neck cancer. Head Neck. 2011;34(7):967-973. doi:10.1002/hed.21842
17. Kendall KA, McKenzie SW, Leonard RJ, Jones CU. Timing of swallowing events after single-modality treatment of head and neck carcinoma with radiotherapy. Ann Otol Rhinol Laryngol. 2000;109(8, pt 1):767-775. doi:10.1177/000348940010900812
18. Ohmae Y, Ogura M, Kitahara S. Effects of head rotation on pharyngeal function during normal swallow. Ann Otol Rhinol Laryngol. 1998;107(4):344-348. doi:10.1177/000348949810700414
19. Spencer CR, Gay HA, Haughey BH, et al. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120(24):3994-4002. doi:10.1002/cncr.28938
Radiation therapy can cause long-term dysphagia that seriously affects quality of life for survivors of head and neck (H&N) cancer.1-3 Numerous studies have linked pharyngeal constrictor dose to long-term dysphagia, but conclusions about the dose distribution that can be safely tolerated have been inconsistent. For example, a group from the Netherlands found that the mean dose to the superior pharyngeal constrictor muscle and the supraglottic larynx were each predictive of dysphagia.4 A subsequent Vanderbilt study refuted these findings, reporting that these structures were not predictive but that dose to the inferior pharyngeal constrictor muscle was.5 Other studies have connected late dysphagia with dose to the middle pharyngeal constrictor muscle, total larynx, oral cavity, contralateral submandibular gland, contralateral parotid gland, or a combination of these structures.6-14 NRG Oncology trials commonly evaluate dose to the “uninvolved pharynx,” which is the total pharyngeal constrictor muscle volume minus the planning target volume for the lowest dose target volume. NRG H&N trials 3, 4, 5, 6, 8, and 9 all use uninvolved pharynx mean dose ≤ 45 Gy as a constraint to judge radiation plan quality.
Differences in methodology or patient population may explain the inconsistency of prior studies on dosimetric predictors of dysphagia, but it is possible that these studies did not evaluate the optimal metric for dysphagia. This study evaluates a novel organ at risk, the contralateral pharyngeal constrictor muscle, to determine whether dose to this structure is predictive of late swallowing function. The study also compares a constraint based on this structure to the NRG uninvolved pharynx constraint mentioned earlier.
Methods
This study is a retrospective review of patients treated at the Richard L. Roudebush Veterans Affairs (VA) Medical Center in Indianapolis, Indiana. Patients were identified by searching the VA Cancer Registry for patients treated for H&N squamous cell carcinoma between September 1, 2016, and August 30, 2019. Eligible sites included cancers of the nasopharynx, oropharynx, hypopharynx, larynx and oral cavity, as well as H&N cancer of an unknown primary site. Only patients treated with primary radiation with concurrent systemic therapy were included. Patients were excluded if they had prior surgery or radiation to the H&N.
The pharyngeal constrictor muscles were contoured per the techniques described by Bhide and colleagues.11 The contralateral constrictor was defined as the half of the constrictor volume contralateral to the primary site. For midline tumors, the side of the neck with a lower volume of lymph node metastases was judged to be the contralateral side.
One-year dysphagia was defined as having a gastronomy tube (G-tube) in place or an abnormal modified barium swallow (MBS) ≥ 12 months after the completion of radiation. At the study institution, MBS is not routinely done after therapy but is ordered if a patient or clinician has concerns about swallowing function. MBS was considered abnormal if there was laryngeal penetration that reached the level of the glottis or was not ejected from the larynx.
Results
The VA Cancer Registry identified 113 patients treated for H&N cancer during the study period. Of these, 55 patients met the inclusion criteria. No patients were lost to follow-up. The median follow-up was 29 months. The median age was 67 years (range, 41-83) (Table 1).
All patients were treated with intensity-modulated radiotherapy (IMRT). Patients treated with a sequential boost had an initial dose of 54 Gy and/or 50 Gy, followed by a boost to a total of 70 Gy at 2 Gy per fraction. Patients treated with a simultaneous integrated boost (SIB) technique received 69.96 Gy in 33 fractions, with elective volumes treated to 54.45 Gy in 33 fractions. Both patients with nasopharyngeal cancer were treated with SIB plans and had an intermediate dose volume of 59.4 Gy.
Systemic therapy was weekly cisplatin in 41 patients (75%) and cetuximab in 14 (25%). Twenty percent of patients receiving cisplatin switched to an alternative agent during treatment, most commonly carboplatin.
Forty-nine patients (89%) had a G-tube placed before starting radiation. G-tubes were in place for an interval of 0 to 47 months (mean, 8.6); 12 (22%) had a G-tube > 12 months. After completion of radiation, 18 patients (33%) had an abnormal MBS. These were done 1 to 50 months (mean, 14.8) after completion of radiation. Abnormal MBS occurred ≥ 12 months after radiation in 9 patients, 5 of whom had their G-tube in place for less than a year.
Forty-six patients (84%) survived more than 1 year and could be evaluated for late swallowing function. One-year dysphagia was seen in 17 (37%) of these patients. Recurrence was seen in 20 patients (36%), with locoregional recurrence in 12 (60%) of these cases. Recurrence occurred at a range of 0 to 15 months (mean, 5.6). Neither recurrence (P = .69) nor locoregional recurrence (P = .11) was associated with increased 1-year dysphagia.
In patients who could be evaluated for long-term swallowing function, contralateral constrictor V60 ranged from 0% to 100% (median, 51%). V60 was < 40% in 18 patients (39%). With V60 < 40%, there was a 6% rate of 1-year dysphagia compared with 57% for V60 ≥ 40% (P < .001).
Patients with contralateral constrictor V60 < 40 and V60 ≥ 40 both had a mean age of 65 years. χ2 analysis did not show a difference in T stage or systemic treatment but did show that patients with V60 < 40% were more likely to have N1 disease (P = .01), and less likely to have N2 disease (P = .01) compared with patients with V60 ≥ 40%. The difference in 1-year dysphagia between N0 to N1 patients (27%) and N2 to N3 patients (46%) was not statistically significant (P = .19).
In patients who could be evaluated for long-term swallowing function, the uninvolved pharynx volume median of the total constrictor volume was 32% (range, < 1%-62%). The uninvolved pharynx mean dose ranged from 28 to 68 Gy (median, 45). When the uninvolved pharynx mean dose was < 45 Gy, 1-year dysphagia was 22% compared with 52% with a dose ≥ 45 Gy (P = .03).
Air cavity editing was performed in 27 patients (49%). One-year survival was 93% with air cavity editing, and 75% without, which was not statistically significant. Locoregional recurrence occurred in 3 patients (11%) with air cavity editing, and 9 (32%) without, which was not statistically significant. In patients surviving at least 1 year, contralateral constrictor V60 averaged 33% with editing and 62% without editing (P < .001). One-year dysphagia was 12% with air cavity editing and 67% without editing (P < .001).
An SIB technique was done in 26 patients (47%). One-year survival was 85% (n = 22) with SIB and 83% (n = 24) with sequential boost, which was not statistically significant. Locoregional recurrence occurred in 19% with SIB, and 32% with sequential boost, which was not statistically significant. For SIB patients alive at 1 year, the median contralateral V60 was 28%, compared with 66% for patients treated with sequential technique. Seventeen patients (77%) with SIB had V60 < 40%. Nineteen (86%) of SIB plans also had air cavity editing. One patient (5%) with SIB had dysphagia at 1 year, compared with 16 (67%) sequential patients (P < .001).
Discussion
This is the first study to link contralateral constrictor dose to long-term dysphagia in patients treated with radiation for H&N cancer. Editing the boost volume off air cavities was associated with lower contralateral constrictor V60 and with less long-term dysphagia. This may indicate that optimizing plans to meet a contralateral constrictor constraint can reduce rates of long-term dysphagia.
The most useful clinical predictors are those that identify a patient at low risk for toxicity. These constraints are useful because they reassure physicians that treatments will have a favorable risk/benefit ratio while identifying plans that may need modification before starting treatment.
The contralateral constrictor outperformed the uninvolved pharynx in identifying patients at low risk for long-term dysphagia. This difference could not be overcome by decreasing the threshold of the pharynx constraint, as 17% of patients with dysphagia had a mean dose of < 40 Gy to the uninvolved pharynx, which was not statistically significant.
An advantage of contralateral constrictor is that it is independent of planning target volume (PTV) size. The uninvolved pharynx structure depends on the PTV contour, so it may obscure a connection between PTV size and dysphagia.
In the context of a clinical trial, only measuring dose to the uninvolved pharynx may allow more plans to meet constraints, but even in NRG trials, physicians have some control over target volumes. For example, NRG HN009, a national trial for patients with H&N cancer, recommends editing the CTV_7000 (clinical target volume treated to 70 Gy) off air cavities but does not define how much the volume should be cropped or specify protocol violations if the volume is not cropped.15 Furthermore, constraints used in clinical trials are often adopted for use outside the trial, where physicians have extensive control over target volumes.
The broad range of uninvolved pharynx volume relative to total constrictor volume confounds predictions using this variable. For example, according to the NRG constraint, a patient with an uninvolved pharynx mean dose of 44 Gy will have a low risk of dysphagia even if this structure is only 1% of the total constrictor. The contralateral constrictor is always about 50% of the total constrictor volume, which means that predictions using this structure will not be confounded by the same variation in volume size.
Figure 2 shows a representative patient who met the NRG uninvolved pharynx constraint but developed long-term dysphagia.
Pharyngoesophageal stricture is a common cause of dysphagia after IMRT for H&N cancer.16 Radiation has been shown to decrease pharyngeal function in patients with H&N cancer.17 Sparing one side of the pharynx may allow for better pharyngeal compliance throughout the length of the pharynx, possibly decreasing the rate of pharyngoesophageal stricture. Additionally, constraining the contralateral constrictor may preserve strength on this side, allowing it to compensate for weakness on the side of the primary cancer. An exercise sometimes used for dysphagia involves head rotation toward the affected side during swallowing. This technique has been shown to cause food to move to the unaffected side.18 Sparing the contralateral constrictor may help such techniques work better in patients with H&N cancer.
Few studies have commented specifically on dose to swallowing structures contralateral to the primary tumor. Two studies have proposed contralateral submandibular gland constraints for dysphagia (not xerostomia), but neither measured the dose to the contralateral constrictor muscle.9,10 Although the contralateral submandibular dose may correlate with dose to the constrictor on that side, the submandibular gland may have a less direct impact on swallowing than the constrictor muscle, and its limited dimensions may make constraints based on the gland less robust for cancers outside the oropharynx.
Another study reported improved quality of life in patients who were not treated with elective contralateral retropharyngeal radiation.19 Although it is likely that doses to the contralateral constrictor were lower in patients who did not receive elective radiation to this area, this study did not measure or constrain doses to the contralateral constrictors.
Limitations
This study is limited by its single institution, retrospective design, small sample size, and by all patients being male. The high correlation between air cavity editing and the use of SIB makes it impossible to assess the impact of each technique individually. Patients with contralateral constrictor V60 < 40% were less likely to have N2 disease, but N2 to N3 disease did not predict higher 1-year dysphagia, so the difference in N-category cannot fully explain the difference in 1-year dysphagia. It is possible that unreported factors, such as CTV, may contribute significantly to swallowing function. Nevertheless, within the study population, contralateral constrictor dose was able to identify a group with a low rate of long-term dysphagia.
Conclusions
Contralateral constrictor dose is a promising predictor of late dysphagia for patients with H&N cancer treated with radiation with concurrent systemic therapy. Contralateral constrictor V60 < 40% was able to identify a group of patients with a low rate of 1-year dysphagia in this single-center retrospective study. The correlation between air cavity editing and contralateral constrictor V60 suggests that contralateral constrictor dose may depend partly on technique. Further studies are needed to see if the contralateral constrictor dose can be used to predict long-term dysphagia prospectively and in other patient populations.
Radiation therapy can cause long-term dysphagia that seriously affects quality of life for survivors of head and neck (H&N) cancer.1-3 Numerous studies have linked pharyngeal constrictor dose to long-term dysphagia, but conclusions about the dose distribution that can be safely tolerated have been inconsistent. For example, a group from the Netherlands found that the mean dose to the superior pharyngeal constrictor muscle and the supraglottic larynx were each predictive of dysphagia.4 A subsequent Vanderbilt study refuted these findings, reporting that these structures were not predictive but that dose to the inferior pharyngeal constrictor muscle was.5 Other studies have connected late dysphagia with dose to the middle pharyngeal constrictor muscle, total larynx, oral cavity, contralateral submandibular gland, contralateral parotid gland, or a combination of these structures.6-14 NRG Oncology trials commonly evaluate dose to the “uninvolved pharynx,” which is the total pharyngeal constrictor muscle volume minus the planning target volume for the lowest dose target volume. NRG H&N trials 3, 4, 5, 6, 8, and 9 all use uninvolved pharynx mean dose ≤ 45 Gy as a constraint to judge radiation plan quality.
Differences in methodology or patient population may explain the inconsistency of prior studies on dosimetric predictors of dysphagia, but it is possible that these studies did not evaluate the optimal metric for dysphagia. This study evaluates a novel organ at risk, the contralateral pharyngeal constrictor muscle, to determine whether dose to this structure is predictive of late swallowing function. The study also compares a constraint based on this structure to the NRG uninvolved pharynx constraint mentioned earlier.
Methods
This study is a retrospective review of patients treated at the Richard L. Roudebush Veterans Affairs (VA) Medical Center in Indianapolis, Indiana. Patients were identified by searching the VA Cancer Registry for patients treated for H&N squamous cell carcinoma between September 1, 2016, and August 30, 2019. Eligible sites included cancers of the nasopharynx, oropharynx, hypopharynx, larynx and oral cavity, as well as H&N cancer of an unknown primary site. Only patients treated with primary radiation with concurrent systemic therapy were included. Patients were excluded if they had prior surgery or radiation to the H&N.
The pharyngeal constrictor muscles were contoured per the techniques described by Bhide and colleagues.11 The contralateral constrictor was defined as the half of the constrictor volume contralateral to the primary site. For midline tumors, the side of the neck with a lower volume of lymph node metastases was judged to be the contralateral side.
One-year dysphagia was defined as having a gastronomy tube (G-tube) in place or an abnormal modified barium swallow (MBS) ≥ 12 months after the completion of radiation. At the study institution, MBS is not routinely done after therapy but is ordered if a patient or clinician has concerns about swallowing function. MBS was considered abnormal if there was laryngeal penetration that reached the level of the glottis or was not ejected from the larynx.
Results
The VA Cancer Registry identified 113 patients treated for H&N cancer during the study period. Of these, 55 patients met the inclusion criteria. No patients were lost to follow-up. The median follow-up was 29 months. The median age was 67 years (range, 41-83) (Table 1).
All patients were treated with intensity-modulated radiotherapy (IMRT). Patients treated with a sequential boost had an initial dose of 54 Gy and/or 50 Gy, followed by a boost to a total of 70 Gy at 2 Gy per fraction. Patients treated with a simultaneous integrated boost (SIB) technique received 69.96 Gy in 33 fractions, with elective volumes treated to 54.45 Gy in 33 fractions. Both patients with nasopharyngeal cancer were treated with SIB plans and had an intermediate dose volume of 59.4 Gy.
Systemic therapy was weekly cisplatin in 41 patients (75%) and cetuximab in 14 (25%). Twenty percent of patients receiving cisplatin switched to an alternative agent during treatment, most commonly carboplatin.
Forty-nine patients (89%) had a G-tube placed before starting radiation. G-tubes were in place for an interval of 0 to 47 months (mean, 8.6); 12 (22%) had a G-tube > 12 months. After completion of radiation, 18 patients (33%) had an abnormal MBS. These were done 1 to 50 months (mean, 14.8) after completion of radiation. Abnormal MBS occurred ≥ 12 months after radiation in 9 patients, 5 of whom had their G-tube in place for less than a year.
Forty-six patients (84%) survived more than 1 year and could be evaluated for late swallowing function. One-year dysphagia was seen in 17 (37%) of these patients. Recurrence was seen in 20 patients (36%), with locoregional recurrence in 12 (60%) of these cases. Recurrence occurred at a range of 0 to 15 months (mean, 5.6). Neither recurrence (P = .69) nor locoregional recurrence (P = .11) was associated with increased 1-year dysphagia.
In patients who could be evaluated for long-term swallowing function, contralateral constrictor V60 ranged from 0% to 100% (median, 51%). V60 was < 40% in 18 patients (39%). With V60 < 40%, there was a 6% rate of 1-year dysphagia compared with 57% for V60 ≥ 40% (P < .001).
Patients with contralateral constrictor V60 < 40 and V60 ≥ 40 both had a mean age of 65 years. χ2 analysis did not show a difference in T stage or systemic treatment but did show that patients with V60 < 40% were more likely to have N1 disease (P = .01), and less likely to have N2 disease (P = .01) compared with patients with V60 ≥ 40%. The difference in 1-year dysphagia between N0 to N1 patients (27%) and N2 to N3 patients (46%) was not statistically significant (P = .19).
In patients who could be evaluated for long-term swallowing function, the uninvolved pharynx volume median of the total constrictor volume was 32% (range, < 1%-62%). The uninvolved pharynx mean dose ranged from 28 to 68 Gy (median, 45). When the uninvolved pharynx mean dose was < 45 Gy, 1-year dysphagia was 22% compared with 52% with a dose ≥ 45 Gy (P = .03).
Air cavity editing was performed in 27 patients (49%). One-year survival was 93% with air cavity editing, and 75% without, which was not statistically significant. Locoregional recurrence occurred in 3 patients (11%) with air cavity editing, and 9 (32%) without, which was not statistically significant. In patients surviving at least 1 year, contralateral constrictor V60 averaged 33% with editing and 62% without editing (P < .001). One-year dysphagia was 12% with air cavity editing and 67% without editing (P < .001).
An SIB technique was done in 26 patients (47%). One-year survival was 85% (n = 22) with SIB and 83% (n = 24) with sequential boost, which was not statistically significant. Locoregional recurrence occurred in 19% with SIB, and 32% with sequential boost, which was not statistically significant. For SIB patients alive at 1 year, the median contralateral V60 was 28%, compared with 66% for patients treated with sequential technique. Seventeen patients (77%) with SIB had V60 < 40%. Nineteen (86%) of SIB plans also had air cavity editing. One patient (5%) with SIB had dysphagia at 1 year, compared with 16 (67%) sequential patients (P < .001).
Discussion
This is the first study to link contralateral constrictor dose to long-term dysphagia in patients treated with radiation for H&N cancer. Editing the boost volume off air cavities was associated with lower contralateral constrictor V60 and with less long-term dysphagia. This may indicate that optimizing plans to meet a contralateral constrictor constraint can reduce rates of long-term dysphagia.
The most useful clinical predictors are those that identify a patient at low risk for toxicity. These constraints are useful because they reassure physicians that treatments will have a favorable risk/benefit ratio while identifying plans that may need modification before starting treatment.
The contralateral constrictor outperformed the uninvolved pharynx in identifying patients at low risk for long-term dysphagia. This difference could not be overcome by decreasing the threshold of the pharynx constraint, as 17% of patients with dysphagia had a mean dose of < 40 Gy to the uninvolved pharynx, which was not statistically significant.
An advantage of contralateral constrictor is that it is independent of planning target volume (PTV) size. The uninvolved pharynx structure depends on the PTV contour, so it may obscure a connection between PTV size and dysphagia.
In the context of a clinical trial, only measuring dose to the uninvolved pharynx may allow more plans to meet constraints, but even in NRG trials, physicians have some control over target volumes. For example, NRG HN009, a national trial for patients with H&N cancer, recommends editing the CTV_7000 (clinical target volume treated to 70 Gy) off air cavities but does not define how much the volume should be cropped or specify protocol violations if the volume is not cropped.15 Furthermore, constraints used in clinical trials are often adopted for use outside the trial, where physicians have extensive control over target volumes.
The broad range of uninvolved pharynx volume relative to total constrictor volume confounds predictions using this variable. For example, according to the NRG constraint, a patient with an uninvolved pharynx mean dose of 44 Gy will have a low risk of dysphagia even if this structure is only 1% of the total constrictor. The contralateral constrictor is always about 50% of the total constrictor volume, which means that predictions using this structure will not be confounded by the same variation in volume size.
Figure 2 shows a representative patient who met the NRG uninvolved pharynx constraint but developed long-term dysphagia.
Pharyngoesophageal stricture is a common cause of dysphagia after IMRT for H&N cancer.16 Radiation has been shown to decrease pharyngeal function in patients with H&N cancer.17 Sparing one side of the pharynx may allow for better pharyngeal compliance throughout the length of the pharynx, possibly decreasing the rate of pharyngoesophageal stricture. Additionally, constraining the contralateral constrictor may preserve strength on this side, allowing it to compensate for weakness on the side of the primary cancer. An exercise sometimes used for dysphagia involves head rotation toward the affected side during swallowing. This technique has been shown to cause food to move to the unaffected side.18 Sparing the contralateral constrictor may help such techniques work better in patients with H&N cancer.
Few studies have commented specifically on dose to swallowing structures contralateral to the primary tumor. Two studies have proposed contralateral submandibular gland constraints for dysphagia (not xerostomia), but neither measured the dose to the contralateral constrictor muscle.9,10 Although the contralateral submandibular dose may correlate with dose to the constrictor on that side, the submandibular gland may have a less direct impact on swallowing than the constrictor muscle, and its limited dimensions may make constraints based on the gland less robust for cancers outside the oropharynx.
Another study reported improved quality of life in patients who were not treated with elective contralateral retropharyngeal radiation.19 Although it is likely that doses to the contralateral constrictor were lower in patients who did not receive elective radiation to this area, this study did not measure or constrain doses to the contralateral constrictors.
Limitations
This study is limited by its single institution, retrospective design, small sample size, and by all patients being male. The high correlation between air cavity editing and the use of SIB makes it impossible to assess the impact of each technique individually. Patients with contralateral constrictor V60 < 40% were less likely to have N2 disease, but N2 to N3 disease did not predict higher 1-year dysphagia, so the difference in N-category cannot fully explain the difference in 1-year dysphagia. It is possible that unreported factors, such as CTV, may contribute significantly to swallowing function. Nevertheless, within the study population, contralateral constrictor dose was able to identify a group with a low rate of long-term dysphagia.
Conclusions
Contralateral constrictor dose is a promising predictor of late dysphagia for patients with H&N cancer treated with radiation with concurrent systemic therapy. Contralateral constrictor V60 < 40% was able to identify a group of patients with a low rate of 1-year dysphagia in this single-center retrospective study. The correlation between air cavity editing and contralateral constrictor V60 suggests that contralateral constrictor dose may depend partly on technique. Further studies are needed to see if the contralateral constrictor dose can be used to predict long-term dysphagia prospectively and in other patient populations.
1. Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770-3776. doi:10.1200/JCO.2007.14.6647
2. Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61(3):772-778. doi:10.1016/j.ijrobp.2004.06.017
3. Ramaekers BLT, Joore MA, Grutters JPC, et al. The impact of late treatment-toxicity on generic health-related quality of life in head and neck cancer patients after radiotherapy. Oral Oncol. 2011;47(8):768-774. doi:10.1016/j.oraloncology.2011.05.012
4. Christianen MEMC, Schilstra C, Beetz I, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol. 2012;105(1):107-114. doi:10.1016/j.radonc.2011.08.009
5. Vlachich G, Spratt DE, Diaz R, et al. Dose to inferior pharyngeal conctrictor predicts prolonged gastrostomy tube dependence with concurrent intensity-modulated radiation therapy and chemotherapy for locally-advanced head and neck cancer. Radiother Oncol. 2014;110(3):435-440. doi:10.1016/j.radonc.2013.12.007
6. Mogadas S, Busch CJ, Pflug Cet al. Influence of radiation dose to pharyngeal constrictor muscles on late dysphagia and quality of life in patients with locally advanced oropharyngeal carcinoma. Strahlenther Onkol. 2020;196(6):522-529. doi:10.1007/s00066-019-01572-0
7. Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts of swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1110-1118. doi:10.1016/j.ijrobp.2008.02.048
8. Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(5):1356-1365. doi:10.1016/j.ijrobp.2009.10.002
9. Gensheimer MF, Nyflot M, Laramore GE, Laio JL, Parvathaneni U. Contribution of submandibular gland and swallowing structure sparing to post-radiation therapy peg dependence in oropharynx cancer patients treated with split-neck IMRT technique. Radiat Oncol. 2015;11(1):1-7. doi:10.1186/s13014-016-0726-3
10. Hedström J, Tuomi L, Finizia C, Olsson C. Identifying organs at risk for radiation-induced late dysphagia in head and neck cancer patients. Clin Transl Radiat Oncol. 2019;19:87-95. doi:10.1016/j.ctro.2019.08.005
11. Bhide SA, Gulliford S, Kazi R, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol. 2009;93(3):539-544. doi:10.1016/j.radonc.2009.09.017
12. Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76(2):403-409. doi:10.1016/j.ijrobp.2009.02.017
13. Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85(1):64-73. doi:10.1016/j.radonc.2007.07.009
14. Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425-1439. doi:10.1016/j.ijrobp.2004.05.050
15. Harari PM; NRG Oncology. Comparing high-dose cisplatin every three weeks to low-dose cisplatin weekly when combined with radiation for patients with advanced head and neck cancer. ClinicalTrials.gov identifier: NCT05050162. Updated November 25, 2022. Accessed December 7, 2022. https://clinicaltrials.gov/ct2/show/NCT05050162
16. Wang JJ, Goldsmith TA, Holman AS, Cianchetti M, Chan AW. Pharyngoesophageal stricture after treatment for head and neck cancer. Head Neck. 2011;34(7):967-973. doi:10.1002/hed.21842
17. Kendall KA, McKenzie SW, Leonard RJ, Jones CU. Timing of swallowing events after single-modality treatment of head and neck carcinoma with radiotherapy. Ann Otol Rhinol Laryngol. 2000;109(8, pt 1):767-775. doi:10.1177/000348940010900812
18. Ohmae Y, Ogura M, Kitahara S. Effects of head rotation on pharyngeal function during normal swallow. Ann Otol Rhinol Laryngol. 1998;107(4):344-348. doi:10.1177/000348949810700414
19. Spencer CR, Gay HA, Haughey BH, et al. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120(24):3994-4002. doi:10.1002/cncr.28938
1. Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770-3776. doi:10.1200/JCO.2007.14.6647
2. Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61(3):772-778. doi:10.1016/j.ijrobp.2004.06.017
3. Ramaekers BLT, Joore MA, Grutters JPC, et al. The impact of late treatment-toxicity on generic health-related quality of life in head and neck cancer patients after radiotherapy. Oral Oncol. 2011;47(8):768-774. doi:10.1016/j.oraloncology.2011.05.012
4. Christianen MEMC, Schilstra C, Beetz I, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol. 2012;105(1):107-114. doi:10.1016/j.radonc.2011.08.009
5. Vlachich G, Spratt DE, Diaz R, et al. Dose to inferior pharyngeal conctrictor predicts prolonged gastrostomy tube dependence with concurrent intensity-modulated radiation therapy and chemotherapy for locally-advanced head and neck cancer. Radiother Oncol. 2014;110(3):435-440. doi:10.1016/j.radonc.2013.12.007
6. Mogadas S, Busch CJ, Pflug Cet al. Influence of radiation dose to pharyngeal constrictor muscles on late dysphagia and quality of life in patients with locally advanced oropharyngeal carcinoma. Strahlenther Onkol. 2020;196(6):522-529. doi:10.1007/s00066-019-01572-0
7. Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts of swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1110-1118. doi:10.1016/j.ijrobp.2008.02.048
8. Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(5):1356-1365. doi:10.1016/j.ijrobp.2009.10.002
9. Gensheimer MF, Nyflot M, Laramore GE, Laio JL, Parvathaneni U. Contribution of submandibular gland and swallowing structure sparing to post-radiation therapy peg dependence in oropharynx cancer patients treated with split-neck IMRT technique. Radiat Oncol. 2015;11(1):1-7. doi:10.1186/s13014-016-0726-3
10. Hedström J, Tuomi L, Finizia C, Olsson C. Identifying organs at risk for radiation-induced late dysphagia in head and neck cancer patients. Clin Transl Radiat Oncol. 2019;19:87-95. doi:10.1016/j.ctro.2019.08.005
11. Bhide SA, Gulliford S, Kazi R, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol. 2009;93(3):539-544. doi:10.1016/j.radonc.2009.09.017
12. Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76(2):403-409. doi:10.1016/j.ijrobp.2009.02.017
13. Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85(1):64-73. doi:10.1016/j.radonc.2007.07.009
14. Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425-1439. doi:10.1016/j.ijrobp.2004.05.050
15. Harari PM; NRG Oncology. Comparing high-dose cisplatin every three weeks to low-dose cisplatin weekly when combined with radiation for patients with advanced head and neck cancer. ClinicalTrials.gov identifier: NCT05050162. Updated November 25, 2022. Accessed December 7, 2022. https://clinicaltrials.gov/ct2/show/NCT05050162
16. Wang JJ, Goldsmith TA, Holman AS, Cianchetti M, Chan AW. Pharyngoesophageal stricture after treatment for head and neck cancer. Head Neck. 2011;34(7):967-973. doi:10.1002/hed.21842
17. Kendall KA, McKenzie SW, Leonard RJ, Jones CU. Timing of swallowing events after single-modality treatment of head and neck carcinoma with radiotherapy. Ann Otol Rhinol Laryngol. 2000;109(8, pt 1):767-775. doi:10.1177/000348940010900812
18. Ohmae Y, Ogura M, Kitahara S. Effects of head rotation on pharyngeal function during normal swallow. Ann Otol Rhinol Laryngol. 1998;107(4):344-348. doi:10.1177/000348949810700414
19. Spencer CR, Gay HA, Haughey BH, et al. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120(24):3994-4002. doi:10.1002/cncr.28938