User login
Recount of FOURIER data finds higher mortality with evolocumab; trialists push back
Readjudication of mortality data from the FOURIER trial suggests a higher risk for cardiovascular death with evolocumab (Repatha) among patients with established atherosclerotic cardiovascular disease than originally reported for the first-in-class PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor.
The Restoring Invisible and Abandoned Trials (RIAT) investigators launched this review in 2018, citing “significant inconsistencies and misreporting” between information in death narratives in the trial’s clinical study report (CSR) and the 2017 New England Journal of Medicine publication of the primary trial results.
“After readjudication, deaths of cardiac origin were numerically higher in the evolocumab group than in the placebo group in the FOURIER trial, suggesting possible cardiac harm,” the researchers conclude in the new report published online in BMJ Open. “At the time the trial was terminated early, a non-significantly higher risk of cardiovascular mortality was observed with evolocumab, which was numerically greater in our adjudication.
“Our findings indicate that complete restoration of all clinical outcomes from the FOURIER trial is required,” they wrote. “Meanwhile, clinicians should be skeptical about benefits vs harms of prescribing evolocumab for patients with established atherosclerotic cardiovascular disease.”
Asked to comment on the reanalysis, FOURIER lead investigator Marc Sabatine, MD, MPH, a professor of medicine at Harvard Medical School and the Lewis Dexter distinguished chair in cardiovascular medicine at Brigham and Women’s Hospital, both in Boston, said: “It’s hard to call this science. I think it lacks all scientific rigor and is fundamentally flawed and, because their process was flawed, it has led them to erroneous conclusions.”
Reached for comment, Sanjay Kaul, MD, a cardiologist and professor of medicine at Cedars-Sinai Medical Center in Los Angeles, who was not involved with either study, said: “If I were to describe this in one sentence, I would say much ado about nothing. A tempest in a teapot.”
Evaluating hard outcomes
The Food and Drug Administration approved evolocumab in 2015 for lowering LDL cholesterol levels, but without results from any trial evaluating hard outcomes.
As previously reported in 2017, FOURIER showed that adding evolocumab to high-intensity statins slashed LDL cholesterol by 59% and was associated with a 15% reduction in the primary composite cardiovascular events endpoint, compared with placebo, but numerically more all-cause and CV mortality.
The NEJM data analysis reported the risk for cardiovascular mortality was 5% (hazard ratio, 1.05; 95% confidence interval, 0.88-1.25), whereas the new review found a still nonsignificant 20% relative risk (R95% CI, 0.95-1.51).
Cardiac deaths were also numerically higher in the evolocumab group (113 vs. 88), corresponding to a 28% higher relative risk (95% CI, 0.97-1.69). Vascular deaths were similar at 37 in both groups (RR, 1.00; 95% CI, 0.63-1.58).
For 360 of the 870 deaths, the cause of death adjudicated by the FOURIER clinical events committee differs from that identified by the local clinical investigators in the CSR death narrative, the authors said.
The RIAT investigators found 11 more deaths from myocardial infarction in the evolocumab group (36 vs. 25 in NEJM) and 3 fewer deaths in the placebo group (27 vs. 30). In addition, their review indicated that deaths as a result of cardiac failure in the evolocumab group were almost double those in the placebo group, at 31 versus 16, respectively.
An ‘obvious disconnect’
Thomas L. Perry, MD, a coauthor of the BMJ Open paper and a general internist in the department of anesthesiology, pharmacology, and therapeutics at the University of British Columbia, Vancouver, said in an interview that the team repeatedly sought information from the FOURIER investigators but never received a response.
They petitioned and received the FOURIER CSR from the European Medicines Agency and Health Canada and made a similar request with the FDA but were told in October 2019 it would take up to 7 years to release the information. Case report forms were also requested but not received from all three agencies.
Dr. Perry noted that no autopsies were performed in the trial, a claim Dr. Sabatine rejected, and that their review of the death narratives in the CSR found 91 deaths classified by the local investigator as “undetermined” but subsequently adjudicated by the FOURIER clinical events committee as “sudden cardiac” deaths without any documented evidence to support the change.
At his request, Dr. Perry said they included two case examples (figures 1 and 2) in the BMJ Open paper of the “obvious disconnect” in death endpoints. Both of these were identified by the local investigator as a myocardial infarction but later “misreported” according to Dr. Perry, as a sudden cardiac death and noncardiovascular death (trauma), respectively.
“What’s so important about this is not only that it throws into doubt the reliability of what the people at Harvard and elsewhere reported in the New England Journal of Medicine in 2017, but also raises a question about any other large study like this where you rely on supposedly ethical local investigators to run the trial well and to report accurately what happens to people,” Dr. Perry said in an interview.
Although he never prescribed evolocumab after the initial results were published, Dr. Perry said he’s even less convinced of a benefit now. “Basically, I don’t believe that they are telling us the facts. I have no reason to say there’s an element of deliberately misleading us. I think it’s sloppiness, incompetence, laziness.”
Dr. Perry also favors readjudication of the mortality data in the ODYSSEY trial, which showed an all-cause mortality benefit with the PCSK9 inhibitor alirocumab (Praluent).
The ‘full picture’
Dr. Sabatine explained that when a patient had a cardiovascular event, including a death, it triggered the collection of a full dossier containing all available source documents, such as discharge summaries, laboratory and imaging data, and autopsy reports, that were independently reviewed by two board certified physicians blinded to treatment. To suggest, as the RIAT investigators have, that no autopsies were performed is “obviously ridiculous and wrong.”
In contrast, he said the new analysis was post hoc, involved unblinded individuals, and relied on serious adverse event narratives, which include a small text box that must be filled out with the site’s initial impression of the case and sent within 24 hours of the event.
Further, when the FOURIER investigators pulled the dossiers for the two more egregious examples cited in the paper, they found that the first patient died in his sleep at home. “The investigator then just said, ‘oh, I assume it’s an MI,’ but there’s no biochemical data, there’s no ECGs, there’s nothing to make the diagnosis of MI. So that’s why that is a sudden cardiac death per the FDA definition,” Dr. Sabatine said.
When the FOURIER investigators reviewed the full dossier for the second case example, they found the patient had slipped in his kitchen at home, sustained a serious head trauma, was brought into the emergency department, and died.
“That’s why we rely on the source documents. That gives the full picture,” he said. The FDA also reviewed the death narratives.
“They comment, ironically, that they were surprised at the inconsistencies between the investigator-reported causes of death and the central events committee-adjudicated ones, making it sound like something nefarious has happened. But that’s the whole point of adjudication, right? That you have a central events committee that reviews and then classifies based on all the data,” Dr. Sabatine said.
Dr. Sabatine said he sees no reason to reevaluate the ODYSSEY mortality data and that the RIAT analysis should not change the overall interpretation of FOURIER.
“I think this is in fact a disservice to the medical community because it’s not real science,” he said. “It’s just sensationalism and sends the wrong message. But I completely stand by the results that we published, as the FDA has.”
Dr. Kaul also thought the new analysis doesn’t materially change the overall benefit–risk balance. He observed that there isn’t a major difference between the reanalysis and the original evaluation. Total mortality was similar and, for cardiovascular deaths, the original NEJM paper lists 251 for evolocumab versus 240 for placebo and the reanalysis lists 150 versus 125, respectively.
Undetermined deaths were 144 for evolocumab and 164 for placebo in the reanalysis. “The conservative approach is to count them as presumed cardiovascular deaths,” Dr. Kaul said. “So, if you do the math and add those undetermined as cardiovascular deaths, we get a total of 294 (150 + 144) versus 289 (125 + 164). That’s five excess deaths with evolocumab.”
Open access
Although the RIAT group has called for the public release of the FOURIER data, commercial and legal issues will complicate that process, Steven Grover, MD, professor of medicine and director of the comprehensive health improvement program at McGill University, Montreal, said in an interview. Amgen is back in court over patent protection, filing an appeal with the Supreme Court after losing in the lower courts in a protracted battle, Reuters reported.
“One thing that’s for sure after they’ve raised questions about the results of this study [is that] somebody needs to take a good hard look at the adjudicated results,” said Dr. Grover, who coauthored several iterations of the Canadian Cardiovascular Society dyslipidemia guidelines, including the latest in 2021.
“I think the thing that got so many of us back in 2017 when the study was first published is the mortality data stuck out like a sore thumb,” he said in an interview. “It didn’t have to be statistically significant, but it did need to move in the same direction as the nonfatal coronary events. That’s what we’ve seen happen time and again and, in this case, it was going in the opposite direction.”
Dr. Sabatine said he doesn’t know whether the data will be released but that the FOURIER trialists plan to submit a rebuttal to BMJ Open to the RIAT analysis, which has caused a stir on CardioTwitter. “Now that people live with tweets of information, it necessitates then dispelling the misinformation that comes out. So yes, we will draft a rebuttal pointing out all the flaws in this analysis.”
Dr. Kaul commented that the FDA’s response not to provide the data was “rather curious” and that Dr. Sabatine and colleagues had the opportunity to address the RIAT group’s concerns, but the paper notes they did not even bother to respond. “You can’t be holier than thou in medicine. You have to treat every question with respect and humility and can’t be dismissive. ... He could have nipped the evil in the bud, so to speak.”
The study was funded by a grant from the University of Maryland, Baltimore. The authors, Dr. Kaul, and Dr. Grover reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Readjudication of mortality data from the FOURIER trial suggests a higher risk for cardiovascular death with evolocumab (Repatha) among patients with established atherosclerotic cardiovascular disease than originally reported for the first-in-class PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor.
The Restoring Invisible and Abandoned Trials (RIAT) investigators launched this review in 2018, citing “significant inconsistencies and misreporting” between information in death narratives in the trial’s clinical study report (CSR) and the 2017 New England Journal of Medicine publication of the primary trial results.
“After readjudication, deaths of cardiac origin were numerically higher in the evolocumab group than in the placebo group in the FOURIER trial, suggesting possible cardiac harm,” the researchers conclude in the new report published online in BMJ Open. “At the time the trial was terminated early, a non-significantly higher risk of cardiovascular mortality was observed with evolocumab, which was numerically greater in our adjudication.
“Our findings indicate that complete restoration of all clinical outcomes from the FOURIER trial is required,” they wrote. “Meanwhile, clinicians should be skeptical about benefits vs harms of prescribing evolocumab for patients with established atherosclerotic cardiovascular disease.”
Asked to comment on the reanalysis, FOURIER lead investigator Marc Sabatine, MD, MPH, a professor of medicine at Harvard Medical School and the Lewis Dexter distinguished chair in cardiovascular medicine at Brigham and Women’s Hospital, both in Boston, said: “It’s hard to call this science. I think it lacks all scientific rigor and is fundamentally flawed and, because their process was flawed, it has led them to erroneous conclusions.”
Reached for comment, Sanjay Kaul, MD, a cardiologist and professor of medicine at Cedars-Sinai Medical Center in Los Angeles, who was not involved with either study, said: “If I were to describe this in one sentence, I would say much ado about nothing. A tempest in a teapot.”
Evaluating hard outcomes
The Food and Drug Administration approved evolocumab in 2015 for lowering LDL cholesterol levels, but without results from any trial evaluating hard outcomes.
As previously reported in 2017, FOURIER showed that adding evolocumab to high-intensity statins slashed LDL cholesterol by 59% and was associated with a 15% reduction in the primary composite cardiovascular events endpoint, compared with placebo, but numerically more all-cause and CV mortality.
The NEJM data analysis reported the risk for cardiovascular mortality was 5% (hazard ratio, 1.05; 95% confidence interval, 0.88-1.25), whereas the new review found a still nonsignificant 20% relative risk (R95% CI, 0.95-1.51).
Cardiac deaths were also numerically higher in the evolocumab group (113 vs. 88), corresponding to a 28% higher relative risk (95% CI, 0.97-1.69). Vascular deaths were similar at 37 in both groups (RR, 1.00; 95% CI, 0.63-1.58).
For 360 of the 870 deaths, the cause of death adjudicated by the FOURIER clinical events committee differs from that identified by the local clinical investigators in the CSR death narrative, the authors said.
The RIAT investigators found 11 more deaths from myocardial infarction in the evolocumab group (36 vs. 25 in NEJM) and 3 fewer deaths in the placebo group (27 vs. 30). In addition, their review indicated that deaths as a result of cardiac failure in the evolocumab group were almost double those in the placebo group, at 31 versus 16, respectively.
An ‘obvious disconnect’
Thomas L. Perry, MD, a coauthor of the BMJ Open paper and a general internist in the department of anesthesiology, pharmacology, and therapeutics at the University of British Columbia, Vancouver, said in an interview that the team repeatedly sought information from the FOURIER investigators but never received a response.
They petitioned and received the FOURIER CSR from the European Medicines Agency and Health Canada and made a similar request with the FDA but were told in October 2019 it would take up to 7 years to release the information. Case report forms were also requested but not received from all three agencies.
Dr. Perry noted that no autopsies were performed in the trial, a claim Dr. Sabatine rejected, and that their review of the death narratives in the CSR found 91 deaths classified by the local investigator as “undetermined” but subsequently adjudicated by the FOURIER clinical events committee as “sudden cardiac” deaths without any documented evidence to support the change.
At his request, Dr. Perry said they included two case examples (figures 1 and 2) in the BMJ Open paper of the “obvious disconnect” in death endpoints. Both of these were identified by the local investigator as a myocardial infarction but later “misreported” according to Dr. Perry, as a sudden cardiac death and noncardiovascular death (trauma), respectively.
“What’s so important about this is not only that it throws into doubt the reliability of what the people at Harvard and elsewhere reported in the New England Journal of Medicine in 2017, but also raises a question about any other large study like this where you rely on supposedly ethical local investigators to run the trial well and to report accurately what happens to people,” Dr. Perry said in an interview.
Although he never prescribed evolocumab after the initial results were published, Dr. Perry said he’s even less convinced of a benefit now. “Basically, I don’t believe that they are telling us the facts. I have no reason to say there’s an element of deliberately misleading us. I think it’s sloppiness, incompetence, laziness.”
Dr. Perry also favors readjudication of the mortality data in the ODYSSEY trial, which showed an all-cause mortality benefit with the PCSK9 inhibitor alirocumab (Praluent).
The ‘full picture’
Dr. Sabatine explained that when a patient had a cardiovascular event, including a death, it triggered the collection of a full dossier containing all available source documents, such as discharge summaries, laboratory and imaging data, and autopsy reports, that were independently reviewed by two board certified physicians blinded to treatment. To suggest, as the RIAT investigators have, that no autopsies were performed is “obviously ridiculous and wrong.”
In contrast, he said the new analysis was post hoc, involved unblinded individuals, and relied on serious adverse event narratives, which include a small text box that must be filled out with the site’s initial impression of the case and sent within 24 hours of the event.
Further, when the FOURIER investigators pulled the dossiers for the two more egregious examples cited in the paper, they found that the first patient died in his sleep at home. “The investigator then just said, ‘oh, I assume it’s an MI,’ but there’s no biochemical data, there’s no ECGs, there’s nothing to make the diagnosis of MI. So that’s why that is a sudden cardiac death per the FDA definition,” Dr. Sabatine said.
When the FOURIER investigators reviewed the full dossier for the second case example, they found the patient had slipped in his kitchen at home, sustained a serious head trauma, was brought into the emergency department, and died.
“That’s why we rely on the source documents. That gives the full picture,” he said. The FDA also reviewed the death narratives.
“They comment, ironically, that they were surprised at the inconsistencies between the investigator-reported causes of death and the central events committee-adjudicated ones, making it sound like something nefarious has happened. But that’s the whole point of adjudication, right? That you have a central events committee that reviews and then classifies based on all the data,” Dr. Sabatine said.
Dr. Sabatine said he sees no reason to reevaluate the ODYSSEY mortality data and that the RIAT analysis should not change the overall interpretation of FOURIER.
“I think this is in fact a disservice to the medical community because it’s not real science,” he said. “It’s just sensationalism and sends the wrong message. But I completely stand by the results that we published, as the FDA has.”
Dr. Kaul also thought the new analysis doesn’t materially change the overall benefit–risk balance. He observed that there isn’t a major difference between the reanalysis and the original evaluation. Total mortality was similar and, for cardiovascular deaths, the original NEJM paper lists 251 for evolocumab versus 240 for placebo and the reanalysis lists 150 versus 125, respectively.
Undetermined deaths were 144 for evolocumab and 164 for placebo in the reanalysis. “The conservative approach is to count them as presumed cardiovascular deaths,” Dr. Kaul said. “So, if you do the math and add those undetermined as cardiovascular deaths, we get a total of 294 (150 + 144) versus 289 (125 + 164). That’s five excess deaths with evolocumab.”
Open access
Although the RIAT group has called for the public release of the FOURIER data, commercial and legal issues will complicate that process, Steven Grover, MD, professor of medicine and director of the comprehensive health improvement program at McGill University, Montreal, said in an interview. Amgen is back in court over patent protection, filing an appeal with the Supreme Court after losing in the lower courts in a protracted battle, Reuters reported.
“One thing that’s for sure after they’ve raised questions about the results of this study [is that] somebody needs to take a good hard look at the adjudicated results,” said Dr. Grover, who coauthored several iterations of the Canadian Cardiovascular Society dyslipidemia guidelines, including the latest in 2021.
“I think the thing that got so many of us back in 2017 when the study was first published is the mortality data stuck out like a sore thumb,” he said in an interview. “It didn’t have to be statistically significant, but it did need to move in the same direction as the nonfatal coronary events. That’s what we’ve seen happen time and again and, in this case, it was going in the opposite direction.”
Dr. Sabatine said he doesn’t know whether the data will be released but that the FOURIER trialists plan to submit a rebuttal to BMJ Open to the RIAT analysis, which has caused a stir on CardioTwitter. “Now that people live with tweets of information, it necessitates then dispelling the misinformation that comes out. So yes, we will draft a rebuttal pointing out all the flaws in this analysis.”
Dr. Kaul commented that the FDA’s response not to provide the data was “rather curious” and that Dr. Sabatine and colleagues had the opportunity to address the RIAT group’s concerns, but the paper notes they did not even bother to respond. “You can’t be holier than thou in medicine. You have to treat every question with respect and humility and can’t be dismissive. ... He could have nipped the evil in the bud, so to speak.”
The study was funded by a grant from the University of Maryland, Baltimore. The authors, Dr. Kaul, and Dr. Grover reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Readjudication of mortality data from the FOURIER trial suggests a higher risk for cardiovascular death with evolocumab (Repatha) among patients with established atherosclerotic cardiovascular disease than originally reported for the first-in-class PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor.
The Restoring Invisible and Abandoned Trials (RIAT) investigators launched this review in 2018, citing “significant inconsistencies and misreporting” between information in death narratives in the trial’s clinical study report (CSR) and the 2017 New England Journal of Medicine publication of the primary trial results.
“After readjudication, deaths of cardiac origin were numerically higher in the evolocumab group than in the placebo group in the FOURIER trial, suggesting possible cardiac harm,” the researchers conclude in the new report published online in BMJ Open. “At the time the trial was terminated early, a non-significantly higher risk of cardiovascular mortality was observed with evolocumab, which was numerically greater in our adjudication.
“Our findings indicate that complete restoration of all clinical outcomes from the FOURIER trial is required,” they wrote. “Meanwhile, clinicians should be skeptical about benefits vs harms of prescribing evolocumab for patients with established atherosclerotic cardiovascular disease.”
Asked to comment on the reanalysis, FOURIER lead investigator Marc Sabatine, MD, MPH, a professor of medicine at Harvard Medical School and the Lewis Dexter distinguished chair in cardiovascular medicine at Brigham and Women’s Hospital, both in Boston, said: “It’s hard to call this science. I think it lacks all scientific rigor and is fundamentally flawed and, because their process was flawed, it has led them to erroneous conclusions.”
Reached for comment, Sanjay Kaul, MD, a cardiologist and professor of medicine at Cedars-Sinai Medical Center in Los Angeles, who was not involved with either study, said: “If I were to describe this in one sentence, I would say much ado about nothing. A tempest in a teapot.”
Evaluating hard outcomes
The Food and Drug Administration approved evolocumab in 2015 for lowering LDL cholesterol levels, but without results from any trial evaluating hard outcomes.
As previously reported in 2017, FOURIER showed that adding evolocumab to high-intensity statins slashed LDL cholesterol by 59% and was associated with a 15% reduction in the primary composite cardiovascular events endpoint, compared with placebo, but numerically more all-cause and CV mortality.
The NEJM data analysis reported the risk for cardiovascular mortality was 5% (hazard ratio, 1.05; 95% confidence interval, 0.88-1.25), whereas the new review found a still nonsignificant 20% relative risk (R95% CI, 0.95-1.51).
Cardiac deaths were also numerically higher in the evolocumab group (113 vs. 88), corresponding to a 28% higher relative risk (95% CI, 0.97-1.69). Vascular deaths were similar at 37 in both groups (RR, 1.00; 95% CI, 0.63-1.58).
For 360 of the 870 deaths, the cause of death adjudicated by the FOURIER clinical events committee differs from that identified by the local clinical investigators in the CSR death narrative, the authors said.
The RIAT investigators found 11 more deaths from myocardial infarction in the evolocumab group (36 vs. 25 in NEJM) and 3 fewer deaths in the placebo group (27 vs. 30). In addition, their review indicated that deaths as a result of cardiac failure in the evolocumab group were almost double those in the placebo group, at 31 versus 16, respectively.
An ‘obvious disconnect’
Thomas L. Perry, MD, a coauthor of the BMJ Open paper and a general internist in the department of anesthesiology, pharmacology, and therapeutics at the University of British Columbia, Vancouver, said in an interview that the team repeatedly sought information from the FOURIER investigators but never received a response.
They petitioned and received the FOURIER CSR from the European Medicines Agency and Health Canada and made a similar request with the FDA but were told in October 2019 it would take up to 7 years to release the information. Case report forms were also requested but not received from all three agencies.
Dr. Perry noted that no autopsies were performed in the trial, a claim Dr. Sabatine rejected, and that their review of the death narratives in the CSR found 91 deaths classified by the local investigator as “undetermined” but subsequently adjudicated by the FOURIER clinical events committee as “sudden cardiac” deaths without any documented evidence to support the change.
At his request, Dr. Perry said they included two case examples (figures 1 and 2) in the BMJ Open paper of the “obvious disconnect” in death endpoints. Both of these were identified by the local investigator as a myocardial infarction but later “misreported” according to Dr. Perry, as a sudden cardiac death and noncardiovascular death (trauma), respectively.
“What’s so important about this is not only that it throws into doubt the reliability of what the people at Harvard and elsewhere reported in the New England Journal of Medicine in 2017, but also raises a question about any other large study like this where you rely on supposedly ethical local investigators to run the trial well and to report accurately what happens to people,” Dr. Perry said in an interview.
Although he never prescribed evolocumab after the initial results were published, Dr. Perry said he’s even less convinced of a benefit now. “Basically, I don’t believe that they are telling us the facts. I have no reason to say there’s an element of deliberately misleading us. I think it’s sloppiness, incompetence, laziness.”
Dr. Perry also favors readjudication of the mortality data in the ODYSSEY trial, which showed an all-cause mortality benefit with the PCSK9 inhibitor alirocumab (Praluent).
The ‘full picture’
Dr. Sabatine explained that when a patient had a cardiovascular event, including a death, it triggered the collection of a full dossier containing all available source documents, such as discharge summaries, laboratory and imaging data, and autopsy reports, that were independently reviewed by two board certified physicians blinded to treatment. To suggest, as the RIAT investigators have, that no autopsies were performed is “obviously ridiculous and wrong.”
In contrast, he said the new analysis was post hoc, involved unblinded individuals, and relied on serious adverse event narratives, which include a small text box that must be filled out with the site’s initial impression of the case and sent within 24 hours of the event.
Further, when the FOURIER investigators pulled the dossiers for the two more egregious examples cited in the paper, they found that the first patient died in his sleep at home. “The investigator then just said, ‘oh, I assume it’s an MI,’ but there’s no biochemical data, there’s no ECGs, there’s nothing to make the diagnosis of MI. So that’s why that is a sudden cardiac death per the FDA definition,” Dr. Sabatine said.
When the FOURIER investigators reviewed the full dossier for the second case example, they found the patient had slipped in his kitchen at home, sustained a serious head trauma, was brought into the emergency department, and died.
“That’s why we rely on the source documents. That gives the full picture,” he said. The FDA also reviewed the death narratives.
“They comment, ironically, that they were surprised at the inconsistencies between the investigator-reported causes of death and the central events committee-adjudicated ones, making it sound like something nefarious has happened. But that’s the whole point of adjudication, right? That you have a central events committee that reviews and then classifies based on all the data,” Dr. Sabatine said.
Dr. Sabatine said he sees no reason to reevaluate the ODYSSEY mortality data and that the RIAT analysis should not change the overall interpretation of FOURIER.
“I think this is in fact a disservice to the medical community because it’s not real science,” he said. “It’s just sensationalism and sends the wrong message. But I completely stand by the results that we published, as the FDA has.”
Dr. Kaul also thought the new analysis doesn’t materially change the overall benefit–risk balance. He observed that there isn’t a major difference between the reanalysis and the original evaluation. Total mortality was similar and, for cardiovascular deaths, the original NEJM paper lists 251 for evolocumab versus 240 for placebo and the reanalysis lists 150 versus 125, respectively.
Undetermined deaths were 144 for evolocumab and 164 for placebo in the reanalysis. “The conservative approach is to count them as presumed cardiovascular deaths,” Dr. Kaul said. “So, if you do the math and add those undetermined as cardiovascular deaths, we get a total of 294 (150 + 144) versus 289 (125 + 164). That’s five excess deaths with evolocumab.”
Open access
Although the RIAT group has called for the public release of the FOURIER data, commercial and legal issues will complicate that process, Steven Grover, MD, professor of medicine and director of the comprehensive health improvement program at McGill University, Montreal, said in an interview. Amgen is back in court over patent protection, filing an appeal with the Supreme Court after losing in the lower courts in a protracted battle, Reuters reported.
“One thing that’s for sure after they’ve raised questions about the results of this study [is that] somebody needs to take a good hard look at the adjudicated results,” said Dr. Grover, who coauthored several iterations of the Canadian Cardiovascular Society dyslipidemia guidelines, including the latest in 2021.
“I think the thing that got so many of us back in 2017 when the study was first published is the mortality data stuck out like a sore thumb,” he said in an interview. “It didn’t have to be statistically significant, but it did need to move in the same direction as the nonfatal coronary events. That’s what we’ve seen happen time and again and, in this case, it was going in the opposite direction.”
Dr. Sabatine said he doesn’t know whether the data will be released but that the FOURIER trialists plan to submit a rebuttal to BMJ Open to the RIAT analysis, which has caused a stir on CardioTwitter. “Now that people live with tweets of information, it necessitates then dispelling the misinformation that comes out. So yes, we will draft a rebuttal pointing out all the flaws in this analysis.”
Dr. Kaul commented that the FDA’s response not to provide the data was “rather curious” and that Dr. Sabatine and colleagues had the opportunity to address the RIAT group’s concerns, but the paper notes they did not even bother to respond. “You can’t be holier than thou in medicine. You have to treat every question with respect and humility and can’t be dismissive. ... He could have nipped the evil in the bud, so to speak.”
The study was funded by a grant from the University of Maryland, Baltimore. The authors, Dr. Kaul, and Dr. Grover reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN
Five thoughts on the Damar Hamlin collapse
The obvious first statement is that it’s neither wise nor appropriate to speculate on the specifics of Damar Hamlin’s cardiac event during a football game on Jan. 2 (including the possibility of commotio cordis) or his ongoing care. The public nature of his collapse induces intense curiosity but people with illness deserve privacy. Privacy in health care is in short supply. I disagree strongly with those who say his doctors ought to be giving public updates. That’s up to the family.
But there are important general concepts to consider about this incident. These include ...
Cardiac arrest can happen to anyone
People with structural heart disease or other chronic illnesses have a higher risk of arrhythmia, but the notion that athletes are immune from cardiac arrest is wrong. This sentence almost seems too obvious to write, but to this day, I hear clinicians express surprise that an athletic person has heart disease.
Survival turns on rapid and effective intervention
In the old days of electrophysiology, we used to test implantable cardioverter-defibrillators during an implant procedure by inducing ventricular fibrillation (VF) and watching the device convert it. Thankfully, trials have shown that this is no longer necessary for most implants.
When you induce VF In the EP lab, you learn quickly that a) it causes loss of consciousness in a matter of seconds, b) rapid defibrillation restores consciousness, often without the patients knowing or remembering they passed out, and c) the failure of the shock to terminate VF results in deterioration in a matter of 1-2 minutes. Even 1 minute in VF feels so long.
Need is an appropriate word in VF treatment
Clinicians often use the verb need. As in, this patient needs this pill or this procedure. It’s rarely appropriate.
But in the case of treating VF, patients truly need rapid defibrillation. Survival of out-of-hospital cardiac arrest is low because there just aren’t enough automated external defibrillators (AEDs) or people trained to use them. A study of patients who had out-of-hospital cardiac arrest in Denmark found that 30-day survival almost doubled (28.8% vs. 16.4%), when the nearest AED was accessible.
Bystanders must act
The public messages are simple: If a person loses consciousness in front of you, and is not breathing normally, assume it is a cardiac arrest, call 911 to get professional help, and start hands-only chest compressions. Don’t spend time checking for a pulse or trying to wake the person. If this is not a cardiac arrest, they will soon tell you to stop compressing their chest. Seconds matter.
Chest compressions are important but what is really needed is defibrillation. A crucial step in CPR is to send someone to get an AED and get the pads attached. If this is a shockable rhythm, deliver the shock. Hamlin’s collapse emphasizes the importance of the AED; without it, his survival to the hospital would have been unlikely.
Widespread preparticipation screening of young athletes remains a bad idea
Whenever cardiac arrest occurs in an athlete, in such a public way, people think about prevention. Surely it is better to prevent such an event than react to it, goes the thinking. The argument against this idea has four prongs:
The incidence of cardiac disease in a young athlete is extremely low, which sets up a situation where most “positive” tests are false positive. A false positive screening ECG or echocardiogram can create harm in multiple ways. One is the risk from downstream procedures, but worse is the inappropriate disqualification from sport. Healthwise, few harms could be greater than creating long-term fear of exercise in someone.
There is also the problem of false-negative screening tests. An ECG may be normal in the setting of hypertrophic cardiomyopathy. And a normal echocardiogram does not exclude arrhythmogenic right ventricular cardiomyopathy or other genetic causes of cardiac arrest. In a 2018 study from a major sports cardiology center in London, 6 of the 8 sudden cardiac deaths in their series were in athletes who had no detectable abnormalities on screening.
Even when disease is found, it’s not clear that prohibiting participation in sports prevents sudden death. Many previous class III recommendations against participation in sport now carry class II – may be considered – designations.
Finally, screening for any disease loses value as treatments improve. Public education regarding rapid intervention with CPR and AED use is the best treatment option. A great example is the case of Christian Erikson, a Danish soccer player who suffered cardiac arrest during a match at the European Championships in 2021 and was rapidly defibrillated on the field. Therapy was so effective that he was conscious and able to wave to fans on his way out of the stadium. He has now returned to elite competition.
Proponents of screening might oppose my take by saying that National Football League players are intensely screened. But this is different from widespread screening of high school and college athletes. It might sound harsh to say, but professional teams have dualities of interests in the health of their athletes given the million-dollar contracts.
What’s more, professional teams can afford to hire expert cardiologists to perform the testing. This would likely reduce the rate of false-positive findings, compared with screening in the community setting. I often have young people referred to me because of asymptomatic bradycardia found during athletic screening – an obviously normal finding.
Conclusions
As long as there are sports, there will be athletes who suffer cardiac arrest.
We can both hope for Hamlin’s full recovery and learn lessons to help reduce the rate of death from out-of-hospital cardiac arrest. This mostly involves education on how to help fellow humans and a public health commitment to access to AEDs.
John Mandrola, MD, practices cardiac electrophysiology in Louisville, Ky. and is a writer and podcaster for Medscape. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
The obvious first statement is that it’s neither wise nor appropriate to speculate on the specifics of Damar Hamlin’s cardiac event during a football game on Jan. 2 (including the possibility of commotio cordis) or his ongoing care. The public nature of his collapse induces intense curiosity but people with illness deserve privacy. Privacy in health care is in short supply. I disagree strongly with those who say his doctors ought to be giving public updates. That’s up to the family.
But there are important general concepts to consider about this incident. These include ...
Cardiac arrest can happen to anyone
People with structural heart disease or other chronic illnesses have a higher risk of arrhythmia, but the notion that athletes are immune from cardiac arrest is wrong. This sentence almost seems too obvious to write, but to this day, I hear clinicians express surprise that an athletic person has heart disease.
Survival turns on rapid and effective intervention
In the old days of electrophysiology, we used to test implantable cardioverter-defibrillators during an implant procedure by inducing ventricular fibrillation (VF) and watching the device convert it. Thankfully, trials have shown that this is no longer necessary for most implants.
When you induce VF In the EP lab, you learn quickly that a) it causes loss of consciousness in a matter of seconds, b) rapid defibrillation restores consciousness, often without the patients knowing or remembering they passed out, and c) the failure of the shock to terminate VF results in deterioration in a matter of 1-2 minutes. Even 1 minute in VF feels so long.
Need is an appropriate word in VF treatment
Clinicians often use the verb need. As in, this patient needs this pill or this procedure. It’s rarely appropriate.
But in the case of treating VF, patients truly need rapid defibrillation. Survival of out-of-hospital cardiac arrest is low because there just aren’t enough automated external defibrillators (AEDs) or people trained to use them. A study of patients who had out-of-hospital cardiac arrest in Denmark found that 30-day survival almost doubled (28.8% vs. 16.4%), when the nearest AED was accessible.
Bystanders must act
The public messages are simple: If a person loses consciousness in front of you, and is not breathing normally, assume it is a cardiac arrest, call 911 to get professional help, and start hands-only chest compressions. Don’t spend time checking for a pulse or trying to wake the person. If this is not a cardiac arrest, they will soon tell you to stop compressing their chest. Seconds matter.
Chest compressions are important but what is really needed is defibrillation. A crucial step in CPR is to send someone to get an AED and get the pads attached. If this is a shockable rhythm, deliver the shock. Hamlin’s collapse emphasizes the importance of the AED; without it, his survival to the hospital would have been unlikely.
Widespread preparticipation screening of young athletes remains a bad idea
Whenever cardiac arrest occurs in an athlete, in such a public way, people think about prevention. Surely it is better to prevent such an event than react to it, goes the thinking. The argument against this idea has four prongs:
The incidence of cardiac disease in a young athlete is extremely low, which sets up a situation where most “positive” tests are false positive. A false positive screening ECG or echocardiogram can create harm in multiple ways. One is the risk from downstream procedures, but worse is the inappropriate disqualification from sport. Healthwise, few harms could be greater than creating long-term fear of exercise in someone.
There is also the problem of false-negative screening tests. An ECG may be normal in the setting of hypertrophic cardiomyopathy. And a normal echocardiogram does not exclude arrhythmogenic right ventricular cardiomyopathy or other genetic causes of cardiac arrest. In a 2018 study from a major sports cardiology center in London, 6 of the 8 sudden cardiac deaths in their series were in athletes who had no detectable abnormalities on screening.
Even when disease is found, it’s not clear that prohibiting participation in sports prevents sudden death. Many previous class III recommendations against participation in sport now carry class II – may be considered – designations.
Finally, screening for any disease loses value as treatments improve. Public education regarding rapid intervention with CPR and AED use is the best treatment option. A great example is the case of Christian Erikson, a Danish soccer player who suffered cardiac arrest during a match at the European Championships in 2021 and was rapidly defibrillated on the field. Therapy was so effective that he was conscious and able to wave to fans on his way out of the stadium. He has now returned to elite competition.
Proponents of screening might oppose my take by saying that National Football League players are intensely screened. But this is different from widespread screening of high school and college athletes. It might sound harsh to say, but professional teams have dualities of interests in the health of their athletes given the million-dollar contracts.
What’s more, professional teams can afford to hire expert cardiologists to perform the testing. This would likely reduce the rate of false-positive findings, compared with screening in the community setting. I often have young people referred to me because of asymptomatic bradycardia found during athletic screening – an obviously normal finding.
Conclusions
As long as there are sports, there will be athletes who suffer cardiac arrest.
We can both hope for Hamlin’s full recovery and learn lessons to help reduce the rate of death from out-of-hospital cardiac arrest. This mostly involves education on how to help fellow humans and a public health commitment to access to AEDs.
John Mandrola, MD, practices cardiac electrophysiology in Louisville, Ky. and is a writer and podcaster for Medscape. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
The obvious first statement is that it’s neither wise nor appropriate to speculate on the specifics of Damar Hamlin’s cardiac event during a football game on Jan. 2 (including the possibility of commotio cordis) or his ongoing care. The public nature of his collapse induces intense curiosity but people with illness deserve privacy. Privacy in health care is in short supply. I disagree strongly with those who say his doctors ought to be giving public updates. That’s up to the family.
But there are important general concepts to consider about this incident. These include ...
Cardiac arrest can happen to anyone
People with structural heart disease or other chronic illnesses have a higher risk of arrhythmia, but the notion that athletes are immune from cardiac arrest is wrong. This sentence almost seems too obvious to write, but to this day, I hear clinicians express surprise that an athletic person has heart disease.
Survival turns on rapid and effective intervention
In the old days of electrophysiology, we used to test implantable cardioverter-defibrillators during an implant procedure by inducing ventricular fibrillation (VF) and watching the device convert it. Thankfully, trials have shown that this is no longer necessary for most implants.
When you induce VF In the EP lab, you learn quickly that a) it causes loss of consciousness in a matter of seconds, b) rapid defibrillation restores consciousness, often without the patients knowing or remembering they passed out, and c) the failure of the shock to terminate VF results in deterioration in a matter of 1-2 minutes. Even 1 minute in VF feels so long.
Need is an appropriate word in VF treatment
Clinicians often use the verb need. As in, this patient needs this pill or this procedure. It’s rarely appropriate.
But in the case of treating VF, patients truly need rapid defibrillation. Survival of out-of-hospital cardiac arrest is low because there just aren’t enough automated external defibrillators (AEDs) or people trained to use them. A study of patients who had out-of-hospital cardiac arrest in Denmark found that 30-day survival almost doubled (28.8% vs. 16.4%), when the nearest AED was accessible.
Bystanders must act
The public messages are simple: If a person loses consciousness in front of you, and is not breathing normally, assume it is a cardiac arrest, call 911 to get professional help, and start hands-only chest compressions. Don’t spend time checking for a pulse or trying to wake the person. If this is not a cardiac arrest, they will soon tell you to stop compressing their chest. Seconds matter.
Chest compressions are important but what is really needed is defibrillation. A crucial step in CPR is to send someone to get an AED and get the pads attached. If this is a shockable rhythm, deliver the shock. Hamlin’s collapse emphasizes the importance of the AED; without it, his survival to the hospital would have been unlikely.
Widespread preparticipation screening of young athletes remains a bad idea
Whenever cardiac arrest occurs in an athlete, in such a public way, people think about prevention. Surely it is better to prevent such an event than react to it, goes the thinking. The argument against this idea has four prongs:
The incidence of cardiac disease in a young athlete is extremely low, which sets up a situation where most “positive” tests are false positive. A false positive screening ECG or echocardiogram can create harm in multiple ways. One is the risk from downstream procedures, but worse is the inappropriate disqualification from sport. Healthwise, few harms could be greater than creating long-term fear of exercise in someone.
There is also the problem of false-negative screening tests. An ECG may be normal in the setting of hypertrophic cardiomyopathy. And a normal echocardiogram does not exclude arrhythmogenic right ventricular cardiomyopathy or other genetic causes of cardiac arrest. In a 2018 study from a major sports cardiology center in London, 6 of the 8 sudden cardiac deaths in their series were in athletes who had no detectable abnormalities on screening.
Even when disease is found, it’s not clear that prohibiting participation in sports prevents sudden death. Many previous class III recommendations against participation in sport now carry class II – may be considered – designations.
Finally, screening for any disease loses value as treatments improve. Public education regarding rapid intervention with CPR and AED use is the best treatment option. A great example is the case of Christian Erikson, a Danish soccer player who suffered cardiac arrest during a match at the European Championships in 2021 and was rapidly defibrillated on the field. Therapy was so effective that he was conscious and able to wave to fans on his way out of the stadium. He has now returned to elite competition.
Proponents of screening might oppose my take by saying that National Football League players are intensely screened. But this is different from widespread screening of high school and college athletes. It might sound harsh to say, but professional teams have dualities of interests in the health of their athletes given the million-dollar contracts.
What’s more, professional teams can afford to hire expert cardiologists to perform the testing. This would likely reduce the rate of false-positive findings, compared with screening in the community setting. I often have young people referred to me because of asymptomatic bradycardia found during athletic screening – an obviously normal finding.
Conclusions
As long as there are sports, there will be athletes who suffer cardiac arrest.
We can both hope for Hamlin’s full recovery and learn lessons to help reduce the rate of death from out-of-hospital cardiac arrest. This mostly involves education on how to help fellow humans and a public health commitment to access to AEDs.
John Mandrola, MD, practices cardiac electrophysiology in Louisville, Ky. and is a writer and podcaster for Medscape. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
One in four cardiologists worldwide report mental health issues
ranging from anxiety or anger issues to major depression or other psychiatric disorders.
Such conditions varied in prevalence by cardiology subspecialty and years in the field, were more common in women than in men, and were closely linked to enduring hostile work environments and other strains of professional life.
The survey, conducted only months before the COVID-19 pandemic and with its share of limitations, still paints a picture that’s not pretty.
For example, mental health concerns were reported by about 42% of respondents who cited a hostile work environment, defined as workplace experience of discrimination based on age, sex, religion, race or ethnicity, or emotional or sexual harassment. Conversely, the prevalence of these concerns reached only 17% among those without such workplace conditions.
The study shows substantial overlap between cardiologists reporting hostility at work and those with mental health concerns, “and that was a significant finding,” Garima Sharma, MD, Johns Hopkins University, Baltimore, said in an interview.
Still, only 31% of male and 42% of female cardiologists (P < .001) reporting mental health concerns also said they had sought professional help either within or outside their own institutions.
That means “there is a lot of silent suffering” in the field, said Dr. Sharma, who is lead author on the study, published in the Journal of the American College of Cardiology.
Bringing back the conversation
The survey findings, she added, point to at least two potential ways the cardiology community can strive to diminish what may be a major underlying cause of the mental health concerns and their consequences.
“If you work towards reducing hostility at work and making mental health a priority for your workforce, then those experiencing these types of egregious conditions based on age, gender, race, ethnicity, or sexual orientation are less likely to be harmed.”
Mental health concerns among cardiologists are seldom openly discussed, so the current study can be “a way to bring them back into the conversation,” Dr. Sharma said. Clinician mental health “is extremely important because it directly impacts patient care and productivity.”
The survey’s reported mental health conditions “are an issue across the board in medicine, and amongst our medical students as well,” senior author Laxmi S. Mehta, MD, professor of internal medicine at Ohio State University, Columbus, said in an interview. The current study provides new details about their prevalence and predictors in cardiology and, she hopes, may improve the field’s awareness of and efforts to address the problem.
“We need to support those who have underlying mental health conditions, as well as improve the work environment to reduce contributory factors to mental illnesses. And we also need to work on reducing the stigma associated with seeking treatment and on reducing the barriers to receiving treatment,” said Dr. Mehta, who chairs the Workgroup on Clinician Well-Being of the ACC, which conducted the survey in 2019.
A global perspective
Cardiologists in Africa, the Americas, Asia, Europe, the Middle East, and Oceania – 5,890 in all – responded to mental health questions on the survey, which was novel for its global reach and insights across continents and cultures.
Respondents in South America and Central America reported the highest prevalences of mental health concerns, outliers at about 39% and 33%, respectively. Rates for most other geographic regions ranged narrowly from about 20% to 26%, the lowest reported in Asia and the Middle East.
Dr. Sharma acknowledged that the countries probably varied widely in social and cultural factors likely to influence survey responses, such as interpretation of the questionnaire’s mental health terminology or the degree to which the disorders are stigmatized.
“I think it’s hard to say how people may or may not respond culturally to a certain word or metric,” she said. But on the survey results, “whether you’re practicing in rural America, in rural India, or in the United Arab Emirates, Oceania, or Eastern Europe, there is a level of consistency, across the board, in what people are recognizing as mental health conditions.”
Junior vs. senior physicians
The global perspective “is a nice positive of the study, and the high rates in Central America and South America I think were something the field was not aware of and are an important contribution,” Srijan Sen, MD, PhD, said in an interview.
The psychological toll of hostile work environments is an issue throughout medicine, “but it seems greater in certain specialties, and cardiology may be one where it’s more of a problem,” observed Dr. Sen, who studies physician mental health at the University of Michigan, Ann Arbor, and wasn’t associated with the survey.
Mental health concerns in the survey were significantly more common among women than men (33.7% vs 26.3%), and for younger cardiologists, compared with older cardiologists (32.2% for those < 40 vs. 22.1% and 16.8% for those 55-69 and 70 or older, respectively).
Those findings seem to make sense, Dr. Sen observed. “Generally, cardiology and medicine broadly are hierarchical, so being more junior can be stressful.” And if there’s more hostility in the workplace, “it might fall on junior people.”
In other studies, moreover, “a high level of work-family conflict has been a real driver of depression and burnout, and that likely is affecting younger physicians, particularly young women physicians,” who may have smaller children and a greater burden of childcare than their seniors.
He pointed to the survey’s low response rate as an important limitation of the study. Of the 71,022 cardiologists invited to participate, only 5,890 (8.3%) responded and answered the queries on mental health.
With a response rate that low, a survey “can be biased in ways that we can’t predict,” Dr. Sen noted. Also, anyone concerned about the toxicity of their own workplace might be “more likely to respond to the survey than if they worked in a more pleasant place. That would provide a skewed sense of the overall experience of cardiologists.”
Those issues might not be a concern with the current survey, however, “because the results are consistent with other studies with higher response rates.”
‘Sobering report’
An accompanying editorial said Dr. Sharm and colleagues have provided “a sobering report on the global prevalence and potential contributors to mental health concerns” in the surveyed population.
Based on its lessons, Andrew J. Sauer, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., proposed several potential “interventions” the field could enact.
It could “selectively promote leaders who strive to mitigate implicit bias, discrimination, and harassment while advancing diversity, equity, and inclusion within the broad ranks of cardiologists.”
Also, he continued, “we must eliminate the stigmatization of mental illness among physicians. We need to handle mental health concerns with compassion and without blaming, like how we strive to treat our veterans who suffer from posttraumatic stress disorder.”
Lastly, Dr. Sauer wrote, “mentorship programs should be formalized to assist the cardiologist in transition zones from early to mid-career, with particular attention to women and those experiencing a simultaneously increased load of family burdens that compound existing workplace contributors to burnout and psychological distress.”
Years in practice
Of the cardiologists who responded to the survey’s mental health questions, 28% reported they have experienced mental health issues that could include alcohol/drug use disorder, suicidal tendencies, psychological distress (including anxiety, irritability, or anger), “other psychiatric disorders” (such as panic disorder, posttraumatic stress, or eating disorders) or major psychiatric disorders such as major depression, bipolar disorder, or schizophrenia.
Cardiologists with 5-10 years of practice post-training were more likely than cardiologists practicing for at least 20 years to have mental health concerns (31.9% vs. 22.6%, P < .001).
Mental health concerns were cited by 42% of respondents who cited “any type of discrimination” based on age, sex, race or ethnicity, or sexual orientation, the report noted.
Among those reporting any mental health concern, 2.7% considered suicide within the past year and 2.9% considered suicide more than 12 months previously. Women were more likely than men to consider suicide within the past year (3.8% vs. 2.3%) but were also more likely to seek help (42.3% vs. 31.1%; P < .001 for both differences), the authors wrote.
In multivariate analysis, predictors of mental health concerns included emotional harassment, 2.81 (odds ratio, 2.81; 95% confidence interval, 2.46-3.20), any discrimination (OR, 1.85; 95% CI, 1.61-2.12), being divorced (OR, 1.73; 95% CI, 1.26-2.36, age less than 55 years (OR, 1.43; 95% CI, 1.24-1.66), and being mid-career versus late (OR, 1.36; 95% CI, 1.14-1.62).
Because the survey was conducted from September to October 2019, before the pandemic’s traumatic effects unfolded on health care nearly everywhere, “I think there needs to be a follow-up at some point when everything has leveled out,” Dr. Sharma said. The current study is “a baseline, and not a healthy baseline,” for the field’s state of mental health that has likely grown worse during the pandemic.
But even without such a follow-up, the current study “is actionable enough that it forces us to do something about it right now.”
Dr. Sharma, Dr. Mehta, their coauthors, Dr. Sen, and Dr. Sauer reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
ranging from anxiety or anger issues to major depression or other psychiatric disorders.
Such conditions varied in prevalence by cardiology subspecialty and years in the field, were more common in women than in men, and were closely linked to enduring hostile work environments and other strains of professional life.
The survey, conducted only months before the COVID-19 pandemic and with its share of limitations, still paints a picture that’s not pretty.
For example, mental health concerns were reported by about 42% of respondents who cited a hostile work environment, defined as workplace experience of discrimination based on age, sex, religion, race or ethnicity, or emotional or sexual harassment. Conversely, the prevalence of these concerns reached only 17% among those without such workplace conditions.
The study shows substantial overlap between cardiologists reporting hostility at work and those with mental health concerns, “and that was a significant finding,” Garima Sharma, MD, Johns Hopkins University, Baltimore, said in an interview.
Still, only 31% of male and 42% of female cardiologists (P < .001) reporting mental health concerns also said they had sought professional help either within or outside their own institutions.
That means “there is a lot of silent suffering” in the field, said Dr. Sharma, who is lead author on the study, published in the Journal of the American College of Cardiology.
Bringing back the conversation
The survey findings, she added, point to at least two potential ways the cardiology community can strive to diminish what may be a major underlying cause of the mental health concerns and their consequences.
“If you work towards reducing hostility at work and making mental health a priority for your workforce, then those experiencing these types of egregious conditions based on age, gender, race, ethnicity, or sexual orientation are less likely to be harmed.”
Mental health concerns among cardiologists are seldom openly discussed, so the current study can be “a way to bring them back into the conversation,” Dr. Sharma said. Clinician mental health “is extremely important because it directly impacts patient care and productivity.”
The survey’s reported mental health conditions “are an issue across the board in medicine, and amongst our medical students as well,” senior author Laxmi S. Mehta, MD, professor of internal medicine at Ohio State University, Columbus, said in an interview. The current study provides new details about their prevalence and predictors in cardiology and, she hopes, may improve the field’s awareness of and efforts to address the problem.
“We need to support those who have underlying mental health conditions, as well as improve the work environment to reduce contributory factors to mental illnesses. And we also need to work on reducing the stigma associated with seeking treatment and on reducing the barriers to receiving treatment,” said Dr. Mehta, who chairs the Workgroup on Clinician Well-Being of the ACC, which conducted the survey in 2019.
A global perspective
Cardiologists in Africa, the Americas, Asia, Europe, the Middle East, and Oceania – 5,890 in all – responded to mental health questions on the survey, which was novel for its global reach and insights across continents and cultures.
Respondents in South America and Central America reported the highest prevalences of mental health concerns, outliers at about 39% and 33%, respectively. Rates for most other geographic regions ranged narrowly from about 20% to 26%, the lowest reported in Asia and the Middle East.
Dr. Sharma acknowledged that the countries probably varied widely in social and cultural factors likely to influence survey responses, such as interpretation of the questionnaire’s mental health terminology or the degree to which the disorders are stigmatized.
“I think it’s hard to say how people may or may not respond culturally to a certain word or metric,” she said. But on the survey results, “whether you’re practicing in rural America, in rural India, or in the United Arab Emirates, Oceania, or Eastern Europe, there is a level of consistency, across the board, in what people are recognizing as mental health conditions.”
Junior vs. senior physicians
The global perspective “is a nice positive of the study, and the high rates in Central America and South America I think were something the field was not aware of and are an important contribution,” Srijan Sen, MD, PhD, said in an interview.
The psychological toll of hostile work environments is an issue throughout medicine, “but it seems greater in certain specialties, and cardiology may be one where it’s more of a problem,” observed Dr. Sen, who studies physician mental health at the University of Michigan, Ann Arbor, and wasn’t associated with the survey.
Mental health concerns in the survey were significantly more common among women than men (33.7% vs 26.3%), and for younger cardiologists, compared with older cardiologists (32.2% for those < 40 vs. 22.1% and 16.8% for those 55-69 and 70 or older, respectively).
Those findings seem to make sense, Dr. Sen observed. “Generally, cardiology and medicine broadly are hierarchical, so being more junior can be stressful.” And if there’s more hostility in the workplace, “it might fall on junior people.”
In other studies, moreover, “a high level of work-family conflict has been a real driver of depression and burnout, and that likely is affecting younger physicians, particularly young women physicians,” who may have smaller children and a greater burden of childcare than their seniors.
He pointed to the survey’s low response rate as an important limitation of the study. Of the 71,022 cardiologists invited to participate, only 5,890 (8.3%) responded and answered the queries on mental health.
With a response rate that low, a survey “can be biased in ways that we can’t predict,” Dr. Sen noted. Also, anyone concerned about the toxicity of their own workplace might be “more likely to respond to the survey than if they worked in a more pleasant place. That would provide a skewed sense of the overall experience of cardiologists.”
Those issues might not be a concern with the current survey, however, “because the results are consistent with other studies with higher response rates.”
‘Sobering report’
An accompanying editorial said Dr. Sharm and colleagues have provided “a sobering report on the global prevalence and potential contributors to mental health concerns” in the surveyed population.
Based on its lessons, Andrew J. Sauer, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., proposed several potential “interventions” the field could enact.
It could “selectively promote leaders who strive to mitigate implicit bias, discrimination, and harassment while advancing diversity, equity, and inclusion within the broad ranks of cardiologists.”
Also, he continued, “we must eliminate the stigmatization of mental illness among physicians. We need to handle mental health concerns with compassion and without blaming, like how we strive to treat our veterans who suffer from posttraumatic stress disorder.”
Lastly, Dr. Sauer wrote, “mentorship programs should be formalized to assist the cardiologist in transition zones from early to mid-career, with particular attention to women and those experiencing a simultaneously increased load of family burdens that compound existing workplace contributors to burnout and psychological distress.”
Years in practice
Of the cardiologists who responded to the survey’s mental health questions, 28% reported they have experienced mental health issues that could include alcohol/drug use disorder, suicidal tendencies, psychological distress (including anxiety, irritability, or anger), “other psychiatric disorders” (such as panic disorder, posttraumatic stress, or eating disorders) or major psychiatric disorders such as major depression, bipolar disorder, or schizophrenia.
Cardiologists with 5-10 years of practice post-training were more likely than cardiologists practicing for at least 20 years to have mental health concerns (31.9% vs. 22.6%, P < .001).
Mental health concerns were cited by 42% of respondents who cited “any type of discrimination” based on age, sex, race or ethnicity, or sexual orientation, the report noted.
Among those reporting any mental health concern, 2.7% considered suicide within the past year and 2.9% considered suicide more than 12 months previously. Women were more likely than men to consider suicide within the past year (3.8% vs. 2.3%) but were also more likely to seek help (42.3% vs. 31.1%; P < .001 for both differences), the authors wrote.
In multivariate analysis, predictors of mental health concerns included emotional harassment, 2.81 (odds ratio, 2.81; 95% confidence interval, 2.46-3.20), any discrimination (OR, 1.85; 95% CI, 1.61-2.12), being divorced (OR, 1.73; 95% CI, 1.26-2.36, age less than 55 years (OR, 1.43; 95% CI, 1.24-1.66), and being mid-career versus late (OR, 1.36; 95% CI, 1.14-1.62).
Because the survey was conducted from September to October 2019, before the pandemic’s traumatic effects unfolded on health care nearly everywhere, “I think there needs to be a follow-up at some point when everything has leveled out,” Dr. Sharma said. The current study is “a baseline, and not a healthy baseline,” for the field’s state of mental health that has likely grown worse during the pandemic.
But even without such a follow-up, the current study “is actionable enough that it forces us to do something about it right now.”
Dr. Sharma, Dr. Mehta, their coauthors, Dr. Sen, and Dr. Sauer reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
ranging from anxiety or anger issues to major depression or other psychiatric disorders.
Such conditions varied in prevalence by cardiology subspecialty and years in the field, were more common in women than in men, and were closely linked to enduring hostile work environments and other strains of professional life.
The survey, conducted only months before the COVID-19 pandemic and with its share of limitations, still paints a picture that’s not pretty.
For example, mental health concerns were reported by about 42% of respondents who cited a hostile work environment, defined as workplace experience of discrimination based on age, sex, religion, race or ethnicity, or emotional or sexual harassment. Conversely, the prevalence of these concerns reached only 17% among those without such workplace conditions.
The study shows substantial overlap between cardiologists reporting hostility at work and those with mental health concerns, “and that was a significant finding,” Garima Sharma, MD, Johns Hopkins University, Baltimore, said in an interview.
Still, only 31% of male and 42% of female cardiologists (P < .001) reporting mental health concerns also said they had sought professional help either within or outside their own institutions.
That means “there is a lot of silent suffering” in the field, said Dr. Sharma, who is lead author on the study, published in the Journal of the American College of Cardiology.
Bringing back the conversation
The survey findings, she added, point to at least two potential ways the cardiology community can strive to diminish what may be a major underlying cause of the mental health concerns and their consequences.
“If you work towards reducing hostility at work and making mental health a priority for your workforce, then those experiencing these types of egregious conditions based on age, gender, race, ethnicity, or sexual orientation are less likely to be harmed.”
Mental health concerns among cardiologists are seldom openly discussed, so the current study can be “a way to bring them back into the conversation,” Dr. Sharma said. Clinician mental health “is extremely important because it directly impacts patient care and productivity.”
The survey’s reported mental health conditions “are an issue across the board in medicine, and amongst our medical students as well,” senior author Laxmi S. Mehta, MD, professor of internal medicine at Ohio State University, Columbus, said in an interview. The current study provides new details about their prevalence and predictors in cardiology and, she hopes, may improve the field’s awareness of and efforts to address the problem.
“We need to support those who have underlying mental health conditions, as well as improve the work environment to reduce contributory factors to mental illnesses. And we also need to work on reducing the stigma associated with seeking treatment and on reducing the barriers to receiving treatment,” said Dr. Mehta, who chairs the Workgroup on Clinician Well-Being of the ACC, which conducted the survey in 2019.
A global perspective
Cardiologists in Africa, the Americas, Asia, Europe, the Middle East, and Oceania – 5,890 in all – responded to mental health questions on the survey, which was novel for its global reach and insights across continents and cultures.
Respondents in South America and Central America reported the highest prevalences of mental health concerns, outliers at about 39% and 33%, respectively. Rates for most other geographic regions ranged narrowly from about 20% to 26%, the lowest reported in Asia and the Middle East.
Dr. Sharma acknowledged that the countries probably varied widely in social and cultural factors likely to influence survey responses, such as interpretation of the questionnaire’s mental health terminology or the degree to which the disorders are stigmatized.
“I think it’s hard to say how people may or may not respond culturally to a certain word or metric,” she said. But on the survey results, “whether you’re practicing in rural America, in rural India, or in the United Arab Emirates, Oceania, or Eastern Europe, there is a level of consistency, across the board, in what people are recognizing as mental health conditions.”
Junior vs. senior physicians
The global perspective “is a nice positive of the study, and the high rates in Central America and South America I think were something the field was not aware of and are an important contribution,” Srijan Sen, MD, PhD, said in an interview.
The psychological toll of hostile work environments is an issue throughout medicine, “but it seems greater in certain specialties, and cardiology may be one where it’s more of a problem,” observed Dr. Sen, who studies physician mental health at the University of Michigan, Ann Arbor, and wasn’t associated with the survey.
Mental health concerns in the survey were significantly more common among women than men (33.7% vs 26.3%), and for younger cardiologists, compared with older cardiologists (32.2% for those < 40 vs. 22.1% and 16.8% for those 55-69 and 70 or older, respectively).
Those findings seem to make sense, Dr. Sen observed. “Generally, cardiology and medicine broadly are hierarchical, so being more junior can be stressful.” And if there’s more hostility in the workplace, “it might fall on junior people.”
In other studies, moreover, “a high level of work-family conflict has been a real driver of depression and burnout, and that likely is affecting younger physicians, particularly young women physicians,” who may have smaller children and a greater burden of childcare than their seniors.
He pointed to the survey’s low response rate as an important limitation of the study. Of the 71,022 cardiologists invited to participate, only 5,890 (8.3%) responded and answered the queries on mental health.
With a response rate that low, a survey “can be biased in ways that we can’t predict,” Dr. Sen noted. Also, anyone concerned about the toxicity of their own workplace might be “more likely to respond to the survey than if they worked in a more pleasant place. That would provide a skewed sense of the overall experience of cardiologists.”
Those issues might not be a concern with the current survey, however, “because the results are consistent with other studies with higher response rates.”
‘Sobering report’
An accompanying editorial said Dr. Sharm and colleagues have provided “a sobering report on the global prevalence and potential contributors to mental health concerns” in the surveyed population.
Based on its lessons, Andrew J. Sauer, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., proposed several potential “interventions” the field could enact.
It could “selectively promote leaders who strive to mitigate implicit bias, discrimination, and harassment while advancing diversity, equity, and inclusion within the broad ranks of cardiologists.”
Also, he continued, “we must eliminate the stigmatization of mental illness among physicians. We need to handle mental health concerns with compassion and without blaming, like how we strive to treat our veterans who suffer from posttraumatic stress disorder.”
Lastly, Dr. Sauer wrote, “mentorship programs should be formalized to assist the cardiologist in transition zones from early to mid-career, with particular attention to women and those experiencing a simultaneously increased load of family burdens that compound existing workplace contributors to burnout and psychological distress.”
Years in practice
Of the cardiologists who responded to the survey’s mental health questions, 28% reported they have experienced mental health issues that could include alcohol/drug use disorder, suicidal tendencies, psychological distress (including anxiety, irritability, or anger), “other psychiatric disorders” (such as panic disorder, posttraumatic stress, or eating disorders) or major psychiatric disorders such as major depression, bipolar disorder, or schizophrenia.
Cardiologists with 5-10 years of practice post-training were more likely than cardiologists practicing for at least 20 years to have mental health concerns (31.9% vs. 22.6%, P < .001).
Mental health concerns were cited by 42% of respondents who cited “any type of discrimination” based on age, sex, race or ethnicity, or sexual orientation, the report noted.
Among those reporting any mental health concern, 2.7% considered suicide within the past year and 2.9% considered suicide more than 12 months previously. Women were more likely than men to consider suicide within the past year (3.8% vs. 2.3%) but were also more likely to seek help (42.3% vs. 31.1%; P < .001 for both differences), the authors wrote.
In multivariate analysis, predictors of mental health concerns included emotional harassment, 2.81 (odds ratio, 2.81; 95% confidence interval, 2.46-3.20), any discrimination (OR, 1.85; 95% CI, 1.61-2.12), being divorced (OR, 1.73; 95% CI, 1.26-2.36, age less than 55 years (OR, 1.43; 95% CI, 1.24-1.66), and being mid-career versus late (OR, 1.36; 95% CI, 1.14-1.62).
Because the survey was conducted from September to October 2019, before the pandemic’s traumatic effects unfolded on health care nearly everywhere, “I think there needs to be a follow-up at some point when everything has leveled out,” Dr. Sharma said. The current study is “a baseline, and not a healthy baseline,” for the field’s state of mental health that has likely grown worse during the pandemic.
But even without such a follow-up, the current study “is actionable enough that it forces us to do something about it right now.”
Dr. Sharma, Dr. Mehta, their coauthors, Dr. Sen, and Dr. Sauer reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
STEMI times to treatment usually miss established goals
Therapy initiated within national treatment-time goals set a decade ago for patients with ST-segment elevation myocardial infarction (STEMI) remains associated with improved survival in recent years. But for many such patients, time from first symptoms to initiation of reperfusion therapy still fails to meet those goals, suggests a cross-sectional registry analysis.
For example, patients initially transported to centers with percutaneous coronary intervention (PCI) capability had a median treatment time of 148 minutes, in the analysis spanning the second quarter (Q2) of 2018 to the third quarter (Q3) of 2021. But the goal for centers called for treatment initiation within 90 minutes for at least 75% of such STEMI patients.
Moreover, overall STEMI treatment times and in-hospital mortality rose in tandem significantly from Q2 2018 through the first quarter (Q1) of 2021, which included the first year of the COVID-19 pandemic. Median time to treatment went from 86 minutes to 91 minutes during that period. Meanwhile, in-hospital mortality went from 5.6% to 8.7%, report the study authors led by James G. Jollis, MD, Duke University, Durham, N.C.
Their report, based on 114,871 STEMI patients at 601 US hospitals contributing to the Get With The Guidelines – Coronary Artery Disease registry, was published online in JAMA.
Of those patients, 25,085 had been transferred from non-PCI hospitals, 32,483 were walk-ins, and 57,303 arrived via emergency medical services (EMS). Their median times from symptom onset to PCI were 240, 195, and 148 minutes, respectively.
In-hospital mortality was significantly reduced in an adjusted analysis for patients treated within target times, compared with those whose treatment missed the time goals, regardless of whether they were transported by EMS, walked into a hospital with on-site PCI, or were transferred from a non-PCI center (Table 1).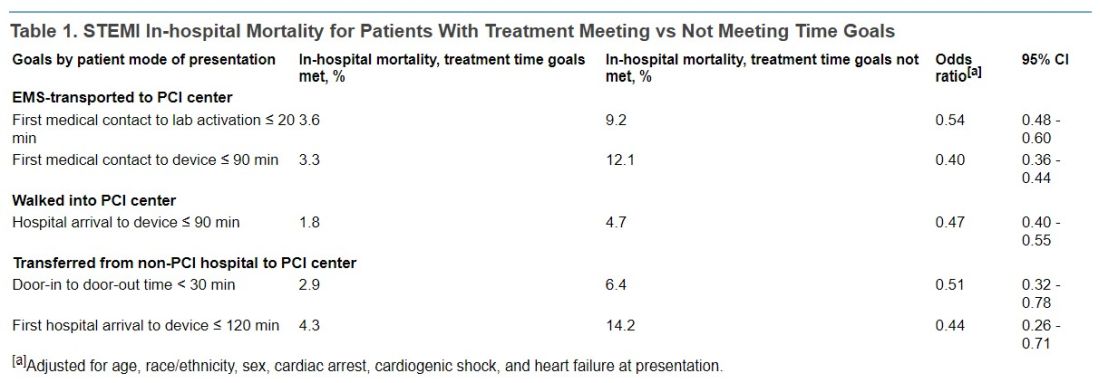
Regardless of mode of patient presentation, treatment time goals were not met most of the time, the group reports. Patients who required interhospital transfer experienced the longest system delays; only 17% were treated within 120 minutes.
Among patients who received primary PCI, 20% had a registry-defined hospital-specified reason for delay, including cardiac arrest and/or need for intubation in 6.8%, “difficulty crossing the culprit lesion” in 3.8%, and “other reasons” in 5.8%, the group reports.
“In 2020, a new reason for delay was added to the registry, ‘need for additional personal protective equipment for suspected/confirmed infectious disease.’ This reason was most commonly used in the second quarter of 2020 (6%) and then declined over time to 1% in the final 2 quarters,” they write.
“Thus, active SARS-CoV-2 infection appeared to have a smaller direct role in longer treatment times or worse outcomes.” Rather, they continue, “the pandemic potentially had a significant indirect role as hospitals were overwhelmed with patients, EMS and hospitals were challenged in maintaining paramedic and nurse staffing and intensive care bed availability, and patients experienced delayed care due to barriers to access or perceived fear of becoming entangled in an overwhelmed medical system.”
Still an important quality metric
STEMI treatment times remain an important quality metric to which hospitals should continue to pay attention because shorter times improve patient care, Deepak Bhatt, MD, MPH, told this news organization.
“Having said that, as with all metrics, one needs to be thoughtful and realize that a difference of a couple of minutes is probably not a crucial thing,” said Dr. Bhatt, Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not involved with the current study.
Interhospital transfers indeed involve longer delays, he observed, suggesting that regional integrated health systems should develop methods for optimizing STEMI care – even, for example, if they involve bypassing non-PCI centers or stopping patients briefly for stabilization followed by rapid transport to a PCI-capable facility.
“That, of course, requires cooperation among hospitals. Sometimes that requires hospitals putting aside economic considerations and just focusing on doing the right thing for that individual patient,” Dr. Bhatt said.
Transfer delays are common for patients presenting with STEMI at hospitals without PCI capability, he noted. “Having clear protocols in place that expedite that type of transfer, I think, could go a long way in reducing the time to treatment in patients that are presenting to the hospital without cath labs. That’s an important message that these data provide.”
The onset of COVID-19 led to widespread delays in STEMI time to treatment early in the pandemic. There were concerns about exposing cath lab personnel to SARS-CoV-2 and potential adverse consequences of sick personnel being unable to provide patient care in the subsequent weeks and months, Dr. Bhatt observed.
However, “All of that seems to have quieted down, and I don’t think COVID is impacting time to treatment right now.”
‘Suboptimal compliance’ with standards
The current findings of “suboptimal compliance with national targets underscore why reassessing quality metrics, in light of changing practice patterns and other secular trends, is critical,” write Andrew S. Oseran, MD, MBA, and Robert W. Yeh, MD, both of Harvard Medical School, in an accompanying editorial.
“While the importance of coordinated and expeditious care for this high-risk patient population is undeniable, the specific actions that hospitals can – or should – take to further improve overall STEMI outcomes are less clear,” they say.
“As physicians contemplate the optimal path forward in managing the care of STEMI patients, they must recognize the clinical and operational nuance that exists in caring for this diverse population and acknowledge the trade-offs associated with uniform quality metrics,” write the editorialists.
“Global reductions in time to treatment for STEMI patients has been one of health care’s great success stories. As we move forward, it may be time to consider whether efforts to achieve additional improvement in target treatment times will result in substantive benefits, or whether we have reached the point of diminishing returns.”
A version of this article first appeared on Medscape.com.
Therapy initiated within national treatment-time goals set a decade ago for patients with ST-segment elevation myocardial infarction (STEMI) remains associated with improved survival in recent years. But for many such patients, time from first symptoms to initiation of reperfusion therapy still fails to meet those goals, suggests a cross-sectional registry analysis.
For example, patients initially transported to centers with percutaneous coronary intervention (PCI) capability had a median treatment time of 148 minutes, in the analysis spanning the second quarter (Q2) of 2018 to the third quarter (Q3) of 2021. But the goal for centers called for treatment initiation within 90 minutes for at least 75% of such STEMI patients.
Moreover, overall STEMI treatment times and in-hospital mortality rose in tandem significantly from Q2 2018 through the first quarter (Q1) of 2021, which included the first year of the COVID-19 pandemic. Median time to treatment went from 86 minutes to 91 minutes during that period. Meanwhile, in-hospital mortality went from 5.6% to 8.7%, report the study authors led by James G. Jollis, MD, Duke University, Durham, N.C.
Their report, based on 114,871 STEMI patients at 601 US hospitals contributing to the Get With The Guidelines – Coronary Artery Disease registry, was published online in JAMA.
Of those patients, 25,085 had been transferred from non-PCI hospitals, 32,483 were walk-ins, and 57,303 arrived via emergency medical services (EMS). Their median times from symptom onset to PCI were 240, 195, and 148 minutes, respectively.
In-hospital mortality was significantly reduced in an adjusted analysis for patients treated within target times, compared with those whose treatment missed the time goals, regardless of whether they were transported by EMS, walked into a hospital with on-site PCI, or were transferred from a non-PCI center (Table 1).
Regardless of mode of patient presentation, treatment time goals were not met most of the time, the group reports. Patients who required interhospital transfer experienced the longest system delays; only 17% were treated within 120 minutes.
Among patients who received primary PCI, 20% had a registry-defined hospital-specified reason for delay, including cardiac arrest and/or need for intubation in 6.8%, “difficulty crossing the culprit lesion” in 3.8%, and “other reasons” in 5.8%, the group reports.
“In 2020, a new reason for delay was added to the registry, ‘need for additional personal protective equipment for suspected/confirmed infectious disease.’ This reason was most commonly used in the second quarter of 2020 (6%) and then declined over time to 1% in the final 2 quarters,” they write.
“Thus, active SARS-CoV-2 infection appeared to have a smaller direct role in longer treatment times or worse outcomes.” Rather, they continue, “the pandemic potentially had a significant indirect role as hospitals were overwhelmed with patients, EMS and hospitals were challenged in maintaining paramedic and nurse staffing and intensive care bed availability, and patients experienced delayed care due to barriers to access or perceived fear of becoming entangled in an overwhelmed medical system.”
Still an important quality metric
STEMI treatment times remain an important quality metric to which hospitals should continue to pay attention because shorter times improve patient care, Deepak Bhatt, MD, MPH, told this news organization.
“Having said that, as with all metrics, one needs to be thoughtful and realize that a difference of a couple of minutes is probably not a crucial thing,” said Dr. Bhatt, Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not involved with the current study.
Interhospital transfers indeed involve longer delays, he observed, suggesting that regional integrated health systems should develop methods for optimizing STEMI care – even, for example, if they involve bypassing non-PCI centers or stopping patients briefly for stabilization followed by rapid transport to a PCI-capable facility.
“That, of course, requires cooperation among hospitals. Sometimes that requires hospitals putting aside economic considerations and just focusing on doing the right thing for that individual patient,” Dr. Bhatt said.
Transfer delays are common for patients presenting with STEMI at hospitals without PCI capability, he noted. “Having clear protocols in place that expedite that type of transfer, I think, could go a long way in reducing the time to treatment in patients that are presenting to the hospital without cath labs. That’s an important message that these data provide.”
The onset of COVID-19 led to widespread delays in STEMI time to treatment early in the pandemic. There were concerns about exposing cath lab personnel to SARS-CoV-2 and potential adverse consequences of sick personnel being unable to provide patient care in the subsequent weeks and months, Dr. Bhatt observed.
However, “All of that seems to have quieted down, and I don’t think COVID is impacting time to treatment right now.”
‘Suboptimal compliance’ with standards
The current findings of “suboptimal compliance with national targets underscore why reassessing quality metrics, in light of changing practice patterns and other secular trends, is critical,” write Andrew S. Oseran, MD, MBA, and Robert W. Yeh, MD, both of Harvard Medical School, in an accompanying editorial.
“While the importance of coordinated and expeditious care for this high-risk patient population is undeniable, the specific actions that hospitals can – or should – take to further improve overall STEMI outcomes are less clear,” they say.
“As physicians contemplate the optimal path forward in managing the care of STEMI patients, they must recognize the clinical and operational nuance that exists in caring for this diverse population and acknowledge the trade-offs associated with uniform quality metrics,” write the editorialists.
“Global reductions in time to treatment for STEMI patients has been one of health care’s great success stories. As we move forward, it may be time to consider whether efforts to achieve additional improvement in target treatment times will result in substantive benefits, or whether we have reached the point of diminishing returns.”
A version of this article first appeared on Medscape.com.
Therapy initiated within national treatment-time goals set a decade ago for patients with ST-segment elevation myocardial infarction (STEMI) remains associated with improved survival in recent years. But for many such patients, time from first symptoms to initiation of reperfusion therapy still fails to meet those goals, suggests a cross-sectional registry analysis.
For example, patients initially transported to centers with percutaneous coronary intervention (PCI) capability had a median treatment time of 148 minutes, in the analysis spanning the second quarter (Q2) of 2018 to the third quarter (Q3) of 2021. But the goal for centers called for treatment initiation within 90 minutes for at least 75% of such STEMI patients.
Moreover, overall STEMI treatment times and in-hospital mortality rose in tandem significantly from Q2 2018 through the first quarter (Q1) of 2021, which included the first year of the COVID-19 pandemic. Median time to treatment went from 86 minutes to 91 minutes during that period. Meanwhile, in-hospital mortality went from 5.6% to 8.7%, report the study authors led by James G. Jollis, MD, Duke University, Durham, N.C.
Their report, based on 114,871 STEMI patients at 601 US hospitals contributing to the Get With The Guidelines – Coronary Artery Disease registry, was published online in JAMA.
Of those patients, 25,085 had been transferred from non-PCI hospitals, 32,483 were walk-ins, and 57,303 arrived via emergency medical services (EMS). Their median times from symptom onset to PCI were 240, 195, and 148 minutes, respectively.
In-hospital mortality was significantly reduced in an adjusted analysis for patients treated within target times, compared with those whose treatment missed the time goals, regardless of whether they were transported by EMS, walked into a hospital with on-site PCI, or were transferred from a non-PCI center (Table 1).
Regardless of mode of patient presentation, treatment time goals were not met most of the time, the group reports. Patients who required interhospital transfer experienced the longest system delays; only 17% were treated within 120 minutes.
Among patients who received primary PCI, 20% had a registry-defined hospital-specified reason for delay, including cardiac arrest and/or need for intubation in 6.8%, “difficulty crossing the culprit lesion” in 3.8%, and “other reasons” in 5.8%, the group reports.
“In 2020, a new reason for delay was added to the registry, ‘need for additional personal protective equipment for suspected/confirmed infectious disease.’ This reason was most commonly used in the second quarter of 2020 (6%) and then declined over time to 1% in the final 2 quarters,” they write.
“Thus, active SARS-CoV-2 infection appeared to have a smaller direct role in longer treatment times or worse outcomes.” Rather, they continue, “the pandemic potentially had a significant indirect role as hospitals were overwhelmed with patients, EMS and hospitals were challenged in maintaining paramedic and nurse staffing and intensive care bed availability, and patients experienced delayed care due to barriers to access or perceived fear of becoming entangled in an overwhelmed medical system.”
Still an important quality metric
STEMI treatment times remain an important quality metric to which hospitals should continue to pay attention because shorter times improve patient care, Deepak Bhatt, MD, MPH, told this news organization.
“Having said that, as with all metrics, one needs to be thoughtful and realize that a difference of a couple of minutes is probably not a crucial thing,” said Dr. Bhatt, Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not involved with the current study.
Interhospital transfers indeed involve longer delays, he observed, suggesting that regional integrated health systems should develop methods for optimizing STEMI care – even, for example, if they involve bypassing non-PCI centers or stopping patients briefly for stabilization followed by rapid transport to a PCI-capable facility.
“That, of course, requires cooperation among hospitals. Sometimes that requires hospitals putting aside economic considerations and just focusing on doing the right thing for that individual patient,” Dr. Bhatt said.
Transfer delays are common for patients presenting with STEMI at hospitals without PCI capability, he noted. “Having clear protocols in place that expedite that type of transfer, I think, could go a long way in reducing the time to treatment in patients that are presenting to the hospital without cath labs. That’s an important message that these data provide.”
The onset of COVID-19 led to widespread delays in STEMI time to treatment early in the pandemic. There were concerns about exposing cath lab personnel to SARS-CoV-2 and potential adverse consequences of sick personnel being unable to provide patient care in the subsequent weeks and months, Dr. Bhatt observed.
However, “All of that seems to have quieted down, and I don’t think COVID is impacting time to treatment right now.”
‘Suboptimal compliance’ with standards
The current findings of “suboptimal compliance with national targets underscore why reassessing quality metrics, in light of changing practice patterns and other secular trends, is critical,” write Andrew S. Oseran, MD, MBA, and Robert W. Yeh, MD, both of Harvard Medical School, in an accompanying editorial.
“While the importance of coordinated and expeditious care for this high-risk patient population is undeniable, the specific actions that hospitals can – or should – take to further improve overall STEMI outcomes are less clear,” they say.
“As physicians contemplate the optimal path forward in managing the care of STEMI patients, they must recognize the clinical and operational nuance that exists in caring for this diverse population and acknowledge the trade-offs associated with uniform quality metrics,” write the editorialists.
“Global reductions in time to treatment for STEMI patients has been one of health care’s great success stories. As we move forward, it may be time to consider whether efforts to achieve additional improvement in target treatment times will result in substantive benefits, or whether we have reached the point of diminishing returns.”
A version of this article first appeared on Medscape.com.
FROM JAMA
Mediterranean diet linked with fewer pregnancy complications
Women in the United States who followed a Mediterranean-style diet – heavy on fresh foods, fish, and olive oil – around the time of conception had lower risk of developing a pregnancy complication, results of a large new study suggest.
The study included 7,798 women who had not given birth before. The group was geographically, racially, and ethnically diverse.
Researchers led by Nour Makarem, PhD, MS, with the department of epidemiology, Columbia University, New York, published their results in JAMA Network Open.
“Generally, higher intakes of vegetables, fruits, legumes, fish, and whole grains and lower intakes of red and processed meat were associated with lower risk of APOs [adverse pregnancy outcomes],” the authors wrote.
21% lower risk of complications
The investigators found that women in the study – who were part of the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be, which enrolled 10,038 women between Oct. 1, 2010, and Sept. 30, 2013, and scored high on adherence to a Mediterranean diet – had a 21% lower risk of developing any adverse pregnancy outcome (APO) than those who had low adherence. And the better the adherence, the lower the risk of adverse outcomes, especially preeclampsia or eclampsia and gestational diabetes, the researchers wrote.
The research team also studied how following the diet correlated with gestational high blood pressure, preterm birth, delivery of a small-for-gestational-age infant, and stillbirth.
Women were scored on consumption of nine components: vegetables (excluding potatoes), fruits, nuts, whole grains, legumes, fish, monounsaturated to saturated fat ratio, red and processed meats, and alcohol.
No differences by race, ethnicity, or BMI
There were no differences in adverse pregnancy outcomes by race, ethnicity, or the woman’s body mass index before pregnancy, but associations were stronger in the women who were 35 years or older, according to the paper.
The authors pointed out that the women in the study had access to prenatal care at a large academic medical center during their first 3 months of pregnancy so the study may actually underestimate the importance of the diet in the pregnancy outcomes.
Christina Han, MD, division director of maternal-fetal medicine at University of California, Los Angeles, who was not part of the study, said that the results make sense as the researchers looked at the time of conception, which is a time that reflects the way a person chooses to live their life.
“We know that your health state as you enter pregnancy can significantly affect your outcomes for that pregnancy,” she said. “We’ve known for decades now that a Mediterranean diet is good for just about everybody.”
Unequal access to foods on diet
Dr. Han said that, while it’s great the researchers were able to confirm the benefit of the Mediterranean diet, it highlights inequity as lower income people are not as likely to be able to afford fresh fruits and vegetables and fish.
“This is a call to arms for our food distribution system to even out the big divide in what patients have access to,” Dr. Han said.
She noted that most of the women in this study were married, non-Hispanic White, and had higher levels of education which may make it hard to generalize these results to the general population.
A limitation of the study is that the women were asked to report what they ate themselves, which can be less accurate than when researchers record what is eaten in a controlled setting.
The researchers suggested a next step: “Long-term intervention studies are needed to assess whether promoting a Mediterranean-style diet around the time of conception and throughout pregnancy can prevent APOs.”
Dr. Makarem reported receiving grants from the National Institutes of Health and the American Heart Association outside the submitted work. One coauthor reported receiving grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor reported receiving personal fees for serving on the board of directors for iRhythm and from fees paid through Cedars-Sinai Medical Center from Abbott Diagnostics and Sanofi outside the submitted work, and one coauthor reported serving as a clinical end point committee member for GlaxoSmithKline outside the submitted work. No other disclosures were reported. Dr. Han reported no relevant financial relationships.
Women in the United States who followed a Mediterranean-style diet – heavy on fresh foods, fish, and olive oil – around the time of conception had lower risk of developing a pregnancy complication, results of a large new study suggest.
The study included 7,798 women who had not given birth before. The group was geographically, racially, and ethnically diverse.
Researchers led by Nour Makarem, PhD, MS, with the department of epidemiology, Columbia University, New York, published their results in JAMA Network Open.
“Generally, higher intakes of vegetables, fruits, legumes, fish, and whole grains and lower intakes of red and processed meat were associated with lower risk of APOs [adverse pregnancy outcomes],” the authors wrote.
21% lower risk of complications
The investigators found that women in the study – who were part of the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be, which enrolled 10,038 women between Oct. 1, 2010, and Sept. 30, 2013, and scored high on adherence to a Mediterranean diet – had a 21% lower risk of developing any adverse pregnancy outcome (APO) than those who had low adherence. And the better the adherence, the lower the risk of adverse outcomes, especially preeclampsia or eclampsia and gestational diabetes, the researchers wrote.
The research team also studied how following the diet correlated with gestational high blood pressure, preterm birth, delivery of a small-for-gestational-age infant, and stillbirth.
Women were scored on consumption of nine components: vegetables (excluding potatoes), fruits, nuts, whole grains, legumes, fish, monounsaturated to saturated fat ratio, red and processed meats, and alcohol.
No differences by race, ethnicity, or BMI
There were no differences in adverse pregnancy outcomes by race, ethnicity, or the woman’s body mass index before pregnancy, but associations were stronger in the women who were 35 years or older, according to the paper.
The authors pointed out that the women in the study had access to prenatal care at a large academic medical center during their first 3 months of pregnancy so the study may actually underestimate the importance of the diet in the pregnancy outcomes.
Christina Han, MD, division director of maternal-fetal medicine at University of California, Los Angeles, who was not part of the study, said that the results make sense as the researchers looked at the time of conception, which is a time that reflects the way a person chooses to live their life.
“We know that your health state as you enter pregnancy can significantly affect your outcomes for that pregnancy,” she said. “We’ve known for decades now that a Mediterranean diet is good for just about everybody.”
Unequal access to foods on diet
Dr. Han said that, while it’s great the researchers were able to confirm the benefit of the Mediterranean diet, it highlights inequity as lower income people are not as likely to be able to afford fresh fruits and vegetables and fish.
“This is a call to arms for our food distribution system to even out the big divide in what patients have access to,” Dr. Han said.
She noted that most of the women in this study were married, non-Hispanic White, and had higher levels of education which may make it hard to generalize these results to the general population.
A limitation of the study is that the women were asked to report what they ate themselves, which can be less accurate than when researchers record what is eaten in a controlled setting.
The researchers suggested a next step: “Long-term intervention studies are needed to assess whether promoting a Mediterranean-style diet around the time of conception and throughout pregnancy can prevent APOs.”
Dr. Makarem reported receiving grants from the National Institutes of Health and the American Heart Association outside the submitted work. One coauthor reported receiving grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor reported receiving personal fees for serving on the board of directors for iRhythm and from fees paid through Cedars-Sinai Medical Center from Abbott Diagnostics and Sanofi outside the submitted work, and one coauthor reported serving as a clinical end point committee member for GlaxoSmithKline outside the submitted work. No other disclosures were reported. Dr. Han reported no relevant financial relationships.
Women in the United States who followed a Mediterranean-style diet – heavy on fresh foods, fish, and olive oil – around the time of conception had lower risk of developing a pregnancy complication, results of a large new study suggest.
The study included 7,798 women who had not given birth before. The group was geographically, racially, and ethnically diverse.
Researchers led by Nour Makarem, PhD, MS, with the department of epidemiology, Columbia University, New York, published their results in JAMA Network Open.
“Generally, higher intakes of vegetables, fruits, legumes, fish, and whole grains and lower intakes of red and processed meat were associated with lower risk of APOs [adverse pregnancy outcomes],” the authors wrote.
21% lower risk of complications
The investigators found that women in the study – who were part of the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be, which enrolled 10,038 women between Oct. 1, 2010, and Sept. 30, 2013, and scored high on adherence to a Mediterranean diet – had a 21% lower risk of developing any adverse pregnancy outcome (APO) than those who had low adherence. And the better the adherence, the lower the risk of adverse outcomes, especially preeclampsia or eclampsia and gestational diabetes, the researchers wrote.
The research team also studied how following the diet correlated with gestational high blood pressure, preterm birth, delivery of a small-for-gestational-age infant, and stillbirth.
Women were scored on consumption of nine components: vegetables (excluding potatoes), fruits, nuts, whole grains, legumes, fish, monounsaturated to saturated fat ratio, red and processed meats, and alcohol.
No differences by race, ethnicity, or BMI
There were no differences in adverse pregnancy outcomes by race, ethnicity, or the woman’s body mass index before pregnancy, but associations were stronger in the women who were 35 years or older, according to the paper.
The authors pointed out that the women in the study had access to prenatal care at a large academic medical center during their first 3 months of pregnancy so the study may actually underestimate the importance of the diet in the pregnancy outcomes.
Christina Han, MD, division director of maternal-fetal medicine at University of California, Los Angeles, who was not part of the study, said that the results make sense as the researchers looked at the time of conception, which is a time that reflects the way a person chooses to live their life.
“We know that your health state as you enter pregnancy can significantly affect your outcomes for that pregnancy,” she said. “We’ve known for decades now that a Mediterranean diet is good for just about everybody.”
Unequal access to foods on diet
Dr. Han said that, while it’s great the researchers were able to confirm the benefit of the Mediterranean diet, it highlights inequity as lower income people are not as likely to be able to afford fresh fruits and vegetables and fish.
“This is a call to arms for our food distribution system to even out the big divide in what patients have access to,” Dr. Han said.
She noted that most of the women in this study were married, non-Hispanic White, and had higher levels of education which may make it hard to generalize these results to the general population.
A limitation of the study is that the women were asked to report what they ate themselves, which can be less accurate than when researchers record what is eaten in a controlled setting.
The researchers suggested a next step: “Long-term intervention studies are needed to assess whether promoting a Mediterranean-style diet around the time of conception and throughout pregnancy can prevent APOs.”
Dr. Makarem reported receiving grants from the National Institutes of Health and the American Heart Association outside the submitted work. One coauthor reported receiving grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor reported receiving personal fees for serving on the board of directors for iRhythm and from fees paid through Cedars-Sinai Medical Center from Abbott Diagnostics and Sanofi outside the submitted work, and one coauthor reported serving as a clinical end point committee member for GlaxoSmithKline outside the submitted work. No other disclosures were reported. Dr. Han reported no relevant financial relationships.
FROM JAMA NETWORK OPEN
Heart benefits begin at well under 10,000 daily steps
– and the benefits accrue at well below the widely promoted threshold of 10,000 steps per day, new research shows.
Among adults aged 60 and older, those who took roughly 6,000 to 9,000 steps per day had a 40% to 50% lower risk of CVD, compared with peers logging just 2,000 steps per day.
“We hope this study will contribute evidence to future public health and clinical guidance on how many steps we need for health,” Amanda Paluch, PhD, with University of Massachusetts Amherst, told this news organization.
Getting in more steps per day can lower an individual’s risk for heart disease – but it’s not an “all or nothing” situation, Dr. Paluch said.
“The heart health benefits begin at lower than 10,000 steps per day. So, for the many adults that may find 10,000 steps a bit out of reach, it is important to promote that even small increases in steps can be beneficial for health,” Dr. Paluch said.
The study was published online in Circulation.
Attainable step goals
As part of the Steps for Health Collaborative, Dr. Paluch and colleagues examined the dose-response relationship between steps per day and CVD in a meta-analysis of eight prospective studies involving 20,152 adults (mean age 63, 52% women).
Steps were measured in each study using one of five different commercially available step-measuring devices. Adults aged 60 years and older took a median of 4,323 steps per day (interquartile range, 2,760-6,924), while younger adults walked a bit more (median 6,911 daily steps; IQR, 4,783-9,794).
During follow-up lasting an average of 6.2 years, a total of 1,523 CVD events were reported.
In the final adjusted model, for older adults, compared with those in quartile 1 who got the fewest steps per day (median 1,811), the risk of CVD was 20% lower in those in quartile 2, who got a median of 3,823 steps per day (hazard ratio, 0.80; 95% confidence interval, 0.69-0.93).
CVD risk was 38% lower in older adults in quartile 3 who got a median of 5,520 steps per day (HR, 0.62; 95% CI, 0.52-0.74) and 49% lower in those in quartile 4 who walked the most (a median of 9,259 steps per day; HR, 0.51; 95% CI, 0.41-0.63).
Restricting the analysis to individuals without known CVD at baseline showed similar results.
Among six studies that excluded adults with a history of CVD at baseline, compared with the lowest quartile, the HR for incident CVD events was 0.74 (95% CI, 0.60-0.91) in the second quartile, 0.60 (95% CI, 0.47-0.77) in the third quartile, and 0.55 (95% CI, 0.40-0.76) in the fourth quartile.
Despite the inverse association of steps with CVD in older adults, there was no association in younger adults. The researchers caution, however, that CVD is a disease of aging, and the follow-up period in these studies may not have been long enough to capture CVD incidence in younger adults.
Stepping rate (pace or cadence) was not associated with CVD risk beyond that of total steps per day. However, only four of the eight studies reported data on stepping rate, so this finding should be viewed as preliminary, Dr. Paluch and colleagues say.
Start small and go from there
Dr. Paluch said the take-home message from this study and numerous others is simple.
“Move more and sit less! Being physically active, by getting in your steps, is an important part of keeping your heart healthy,” she said in an interview.
For adults who are currently inactive, Dr. Paluch suggests finding small ways to get in a few more steps per day. “It does not need to be drastic changes. Consider a brief 5- to 10-minute walking break at lunch, taking the stairs, or playing a game of hide and seek with the grandchildren,” Dr. Paluch advised.
“For adults starting at 3,000 steps a day, set a goal of 4,000, and then 5,000. Each improvement can lead to better heart health,” Dr. Paluch said. “And for those who are already active, keep it up, as there are benefits with higher volumes of steps per day as well.”
Support for this research was provided by the Intergovernmental Personnel Act Agreement through the Centers for Disease Control and Prevention. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– and the benefits accrue at well below the widely promoted threshold of 10,000 steps per day, new research shows.
Among adults aged 60 and older, those who took roughly 6,000 to 9,000 steps per day had a 40% to 50% lower risk of CVD, compared with peers logging just 2,000 steps per day.
“We hope this study will contribute evidence to future public health and clinical guidance on how many steps we need for health,” Amanda Paluch, PhD, with University of Massachusetts Amherst, told this news organization.
Getting in more steps per day can lower an individual’s risk for heart disease – but it’s not an “all or nothing” situation, Dr. Paluch said.
“The heart health benefits begin at lower than 10,000 steps per day. So, for the many adults that may find 10,000 steps a bit out of reach, it is important to promote that even small increases in steps can be beneficial for health,” Dr. Paluch said.
The study was published online in Circulation.
Attainable step goals
As part of the Steps for Health Collaborative, Dr. Paluch and colleagues examined the dose-response relationship between steps per day and CVD in a meta-analysis of eight prospective studies involving 20,152 adults (mean age 63, 52% women).
Steps were measured in each study using one of five different commercially available step-measuring devices. Adults aged 60 years and older took a median of 4,323 steps per day (interquartile range, 2,760-6,924), while younger adults walked a bit more (median 6,911 daily steps; IQR, 4,783-9,794).
During follow-up lasting an average of 6.2 years, a total of 1,523 CVD events were reported.
In the final adjusted model, for older adults, compared with those in quartile 1 who got the fewest steps per day (median 1,811), the risk of CVD was 20% lower in those in quartile 2, who got a median of 3,823 steps per day (hazard ratio, 0.80; 95% confidence interval, 0.69-0.93).
CVD risk was 38% lower in older adults in quartile 3 who got a median of 5,520 steps per day (HR, 0.62; 95% CI, 0.52-0.74) and 49% lower in those in quartile 4 who walked the most (a median of 9,259 steps per day; HR, 0.51; 95% CI, 0.41-0.63).
Restricting the analysis to individuals without known CVD at baseline showed similar results.
Among six studies that excluded adults with a history of CVD at baseline, compared with the lowest quartile, the HR for incident CVD events was 0.74 (95% CI, 0.60-0.91) in the second quartile, 0.60 (95% CI, 0.47-0.77) in the third quartile, and 0.55 (95% CI, 0.40-0.76) in the fourth quartile.
Despite the inverse association of steps with CVD in older adults, there was no association in younger adults. The researchers caution, however, that CVD is a disease of aging, and the follow-up period in these studies may not have been long enough to capture CVD incidence in younger adults.
Stepping rate (pace or cadence) was not associated with CVD risk beyond that of total steps per day. However, only four of the eight studies reported data on stepping rate, so this finding should be viewed as preliminary, Dr. Paluch and colleagues say.
Start small and go from there
Dr. Paluch said the take-home message from this study and numerous others is simple.
“Move more and sit less! Being physically active, by getting in your steps, is an important part of keeping your heart healthy,” she said in an interview.
For adults who are currently inactive, Dr. Paluch suggests finding small ways to get in a few more steps per day. “It does not need to be drastic changes. Consider a brief 5- to 10-minute walking break at lunch, taking the stairs, or playing a game of hide and seek with the grandchildren,” Dr. Paluch advised.
“For adults starting at 3,000 steps a day, set a goal of 4,000, and then 5,000. Each improvement can lead to better heart health,” Dr. Paluch said. “And for those who are already active, keep it up, as there are benefits with higher volumes of steps per day as well.”
Support for this research was provided by the Intergovernmental Personnel Act Agreement through the Centers for Disease Control and Prevention. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– and the benefits accrue at well below the widely promoted threshold of 10,000 steps per day, new research shows.
Among adults aged 60 and older, those who took roughly 6,000 to 9,000 steps per day had a 40% to 50% lower risk of CVD, compared with peers logging just 2,000 steps per day.
“We hope this study will contribute evidence to future public health and clinical guidance on how many steps we need for health,” Amanda Paluch, PhD, with University of Massachusetts Amherst, told this news organization.
Getting in more steps per day can lower an individual’s risk for heart disease – but it’s not an “all or nothing” situation, Dr. Paluch said.
“The heart health benefits begin at lower than 10,000 steps per day. So, for the many adults that may find 10,000 steps a bit out of reach, it is important to promote that even small increases in steps can be beneficial for health,” Dr. Paluch said.
The study was published online in Circulation.
Attainable step goals
As part of the Steps for Health Collaborative, Dr. Paluch and colleagues examined the dose-response relationship between steps per day and CVD in a meta-analysis of eight prospective studies involving 20,152 adults (mean age 63, 52% women).
Steps were measured in each study using one of five different commercially available step-measuring devices. Adults aged 60 years and older took a median of 4,323 steps per day (interquartile range, 2,760-6,924), while younger adults walked a bit more (median 6,911 daily steps; IQR, 4,783-9,794).
During follow-up lasting an average of 6.2 years, a total of 1,523 CVD events were reported.
In the final adjusted model, for older adults, compared with those in quartile 1 who got the fewest steps per day (median 1,811), the risk of CVD was 20% lower in those in quartile 2, who got a median of 3,823 steps per day (hazard ratio, 0.80; 95% confidence interval, 0.69-0.93).
CVD risk was 38% lower in older adults in quartile 3 who got a median of 5,520 steps per day (HR, 0.62; 95% CI, 0.52-0.74) and 49% lower in those in quartile 4 who walked the most (a median of 9,259 steps per day; HR, 0.51; 95% CI, 0.41-0.63).
Restricting the analysis to individuals without known CVD at baseline showed similar results.
Among six studies that excluded adults with a history of CVD at baseline, compared with the lowest quartile, the HR for incident CVD events was 0.74 (95% CI, 0.60-0.91) in the second quartile, 0.60 (95% CI, 0.47-0.77) in the third quartile, and 0.55 (95% CI, 0.40-0.76) in the fourth quartile.
Despite the inverse association of steps with CVD in older adults, there was no association in younger adults. The researchers caution, however, that CVD is a disease of aging, and the follow-up period in these studies may not have been long enough to capture CVD incidence in younger adults.
Stepping rate (pace or cadence) was not associated with CVD risk beyond that of total steps per day. However, only four of the eight studies reported data on stepping rate, so this finding should be viewed as preliminary, Dr. Paluch and colleagues say.
Start small and go from there
Dr. Paluch said the take-home message from this study and numerous others is simple.
“Move more and sit less! Being physically active, by getting in your steps, is an important part of keeping your heart healthy,” she said in an interview.
For adults who are currently inactive, Dr. Paluch suggests finding small ways to get in a few more steps per day. “It does not need to be drastic changes. Consider a brief 5- to 10-minute walking break at lunch, taking the stairs, or playing a game of hide and seek with the grandchildren,” Dr. Paluch advised.
“For adults starting at 3,000 steps a day, set a goal of 4,000, and then 5,000. Each improvement can lead to better heart health,” Dr. Paluch said. “And for those who are already active, keep it up, as there are benefits with higher volumes of steps per day as well.”
Support for this research was provided by the Intergovernmental Personnel Act Agreement through the Centers for Disease Control and Prevention. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Women with cycle disorders across their life span may be at increased risk of cardiovascular disease
This finding is demonstrated in a new analysis of the Nurses’ Health Study II.
“To date, several studies have reported increased risks of cardiovascular risk factors or cardiovascular disease in connection with cycle disorders,” Yi-Xin Wang, MD, PhD, a research fellow in nutrition, and associates from the Harvard School of Public Health, Boston, wrote in an article published in JAMA Network Open.
Ute Seeland, MD, speaker of the Gender Medicine in Cardiology Working Group of the German Cardiology Society, said in an interview“We know that women who have indicated in their medical history that they have irregular menstrual cycles, invariably in connection with polycystic ovary syndrome (PCOS), more commonly develop diabetes and other metabolic disorders, as well as cardiovascular diseases.”
Cycle disorders’ role
However, the role that irregular or especially long cycles play at different points of a woman’s reproductive life span was unclear. Therefore, the research group investigated the associations in the Nurses’ Health Study II between cycle irregularity and cycle length in women of different age groups who later experienced cardiovascular events.
At the end of this study in 1989, the participants also provided information regarding the length and irregularity of their menstrual cycle from ages 14 to 17 years and again from ages 18 to 22 years. The information was updated in 1993 when the participants were aged 29-46 years. The data from 2019 to 2022 were analyzed.
“This kind of long-term cohort study is extremely rare and therefore something special,” said Dr. Seeland, who conducts research at the Institute for Social Medicine, Epidemiology, and Health Economics at the Charité – University Hospital Berlin.
The investigators used the following cycle classifications: very regular (no more than 3 or 4 days before or after the expected date), regular (within 5-7 days), usually irregular, always irregular, or no periods.
The cycle lengths were divided into the following categories: less than 21 days, 21-25 days, 26-31 days, 32-39 days, 40-50 days, more than 50 days, and too irregular to estimate the length.
The onset of cardiovascular diseases was determined using information from the participants and was confirmed by reviewing the medical files. Relevant to the study were lethal and nonlethal coronary heart diseases (such as myocardial infarction or coronary artery revascularization), as well as strokes.
Significant in adulthood
The data from 80,630 study participants were included in the analysis. At study inclusion, the average age of the participants was 37.7 years, and the average body mass index (BMI) was 25.1. “Since it was predominantly White nurses who took part in the study, the data are not transferable to other, more diverse populations,” said Dr. Seeland.
Over 24 years, 1,816 women (2.4%) had a cardiovascular event. “We observed an increased rate of cardiovascular events in women with an irregular cycle and longer cycle, both in early an in mid-adulthood,” wrote Dr. Wang and associates. “Similar trends were also observed for cycle disorders when younger, but this association was weaker than in adulthood.”
Compared with women with very regular cycles, women with irregular cycles or without periods who were aged 14-17 years, 18-22 years, or 29-49 years exhibited a 15%, 36%, and 40% higher risk of a cardiovascular event, respectively.
Similarly, women aged 18-22 years or 29-46 years with long cycles of 40 days or more had a 44% or 30% higher risk of cardiovascular disease, respectively, compared with women with cycle lengths of 26-31 days.
“The coronary heart diseases were decisive for the increase, and less so, the strokes,” wrote the researchers.
Classic risk factors?
Dr. Seeland praised the fact that the study authors tried to determine the role that classic cardiovascular risk factors played. “Compared with women with a regular cycle, women with an irregular cycle had a higher BMI, more frequently increased cholesterol levels, and an elevated blood pressure,” she said. Women with a long cycle displayed a similar pattern.
It can be assumed from this that over a woman’s life span, BMI affects the risk of cardiovascular disease. Therefore, Dr. Wang and coauthors adjusted the results on the basis of BMI, which varies over time.
Regarding other classic risk factors that may have played a role, “hypercholesterolemia, chronic high blood pressure, and type 2 diabetes were only responsible in 5.4%-13.5% of the associations,” wrote the researchers.
“Our results suggest that certain characteristics of the menstrual cycle across a woman’s reproductive lifespan may constitute additional risk markers for cardiovascular disease,” according to the authors.
The highest rates of cardiovascular disease were among women with permanently irregular or long cycles in early to mid adulthood, as well as women who had regular cycles when younger but had irregular cycles in mid adulthood. “This indicates that the change from one cycle phenotype to another could be a surrogate marker for metabolic changes, which in turn contribute to the formation of cardiovascular diseases,” wrote the authors.
The study was observational and so conclusions cannot be drawn regarding causal relationships. But Dr. Wang and associates indicate that the most common cause of an irregular menstrual cycle may be PCOS. “Roughly 90% of women with cycle disorders or oligomenorrhea have signs of PCOS. And it was shown that women with PCOS have an increased risk of cardiovascular disease.”
They concluded that “the associations observed between irregular and long cycles in early to mid-adulthood and cardiovascular diseases are likely attributable to underlying PCOS.”
For Dr. Seeland, however, this conclusion is “too monocausal. At no point in time did there seem to be any direct information regarding the frequency of PCOS during the data collection by the respondents.”
For now, we can only speculate about the mechanisms. “The association between a very irregular and long cycle and the increased risk of cardiovascular diseases has now only been described. More research should be done on the causes,” said Dr. Seeland.
This article was translated from the Medscape German edition. A version of this article first appeared on Medscape.com.
This finding is demonstrated in a new analysis of the Nurses’ Health Study II.
“To date, several studies have reported increased risks of cardiovascular risk factors or cardiovascular disease in connection with cycle disorders,” Yi-Xin Wang, MD, PhD, a research fellow in nutrition, and associates from the Harvard School of Public Health, Boston, wrote in an article published in JAMA Network Open.
Ute Seeland, MD, speaker of the Gender Medicine in Cardiology Working Group of the German Cardiology Society, said in an interview“We know that women who have indicated in their medical history that they have irregular menstrual cycles, invariably in connection with polycystic ovary syndrome (PCOS), more commonly develop diabetes and other metabolic disorders, as well as cardiovascular diseases.”
Cycle disorders’ role
However, the role that irregular or especially long cycles play at different points of a woman’s reproductive life span was unclear. Therefore, the research group investigated the associations in the Nurses’ Health Study II between cycle irregularity and cycle length in women of different age groups who later experienced cardiovascular events.
At the end of this study in 1989, the participants also provided information regarding the length and irregularity of their menstrual cycle from ages 14 to 17 years and again from ages 18 to 22 years. The information was updated in 1993 when the participants were aged 29-46 years. The data from 2019 to 2022 were analyzed.
“This kind of long-term cohort study is extremely rare and therefore something special,” said Dr. Seeland, who conducts research at the Institute for Social Medicine, Epidemiology, and Health Economics at the Charité – University Hospital Berlin.
The investigators used the following cycle classifications: very regular (no more than 3 or 4 days before or after the expected date), regular (within 5-7 days), usually irregular, always irregular, or no periods.
The cycle lengths were divided into the following categories: less than 21 days, 21-25 days, 26-31 days, 32-39 days, 40-50 days, more than 50 days, and too irregular to estimate the length.
The onset of cardiovascular diseases was determined using information from the participants and was confirmed by reviewing the medical files. Relevant to the study were lethal and nonlethal coronary heart diseases (such as myocardial infarction or coronary artery revascularization), as well as strokes.
Significant in adulthood
The data from 80,630 study participants were included in the analysis. At study inclusion, the average age of the participants was 37.7 years, and the average body mass index (BMI) was 25.1. “Since it was predominantly White nurses who took part in the study, the data are not transferable to other, more diverse populations,” said Dr. Seeland.
Over 24 years, 1,816 women (2.4%) had a cardiovascular event. “We observed an increased rate of cardiovascular events in women with an irregular cycle and longer cycle, both in early an in mid-adulthood,” wrote Dr. Wang and associates. “Similar trends were also observed for cycle disorders when younger, but this association was weaker than in adulthood.”
Compared with women with very regular cycles, women with irregular cycles or without periods who were aged 14-17 years, 18-22 years, or 29-49 years exhibited a 15%, 36%, and 40% higher risk of a cardiovascular event, respectively.
Similarly, women aged 18-22 years or 29-46 years with long cycles of 40 days or more had a 44% or 30% higher risk of cardiovascular disease, respectively, compared with women with cycle lengths of 26-31 days.
“The coronary heart diseases were decisive for the increase, and less so, the strokes,” wrote the researchers.
Classic risk factors?
Dr. Seeland praised the fact that the study authors tried to determine the role that classic cardiovascular risk factors played. “Compared with women with a regular cycle, women with an irregular cycle had a higher BMI, more frequently increased cholesterol levels, and an elevated blood pressure,” she said. Women with a long cycle displayed a similar pattern.
It can be assumed from this that over a woman’s life span, BMI affects the risk of cardiovascular disease. Therefore, Dr. Wang and coauthors adjusted the results on the basis of BMI, which varies over time.
Regarding other classic risk factors that may have played a role, “hypercholesterolemia, chronic high blood pressure, and type 2 diabetes were only responsible in 5.4%-13.5% of the associations,” wrote the researchers.
“Our results suggest that certain characteristics of the menstrual cycle across a woman’s reproductive lifespan may constitute additional risk markers for cardiovascular disease,” according to the authors.
The highest rates of cardiovascular disease were among women with permanently irregular or long cycles in early to mid adulthood, as well as women who had regular cycles when younger but had irregular cycles in mid adulthood. “This indicates that the change from one cycle phenotype to another could be a surrogate marker for metabolic changes, which in turn contribute to the formation of cardiovascular diseases,” wrote the authors.
The study was observational and so conclusions cannot be drawn regarding causal relationships. But Dr. Wang and associates indicate that the most common cause of an irregular menstrual cycle may be PCOS. “Roughly 90% of women with cycle disorders or oligomenorrhea have signs of PCOS. And it was shown that women with PCOS have an increased risk of cardiovascular disease.”
They concluded that “the associations observed between irregular and long cycles in early to mid-adulthood and cardiovascular diseases are likely attributable to underlying PCOS.”
For Dr. Seeland, however, this conclusion is “too monocausal. At no point in time did there seem to be any direct information regarding the frequency of PCOS during the data collection by the respondents.”
For now, we can only speculate about the mechanisms. “The association between a very irregular and long cycle and the increased risk of cardiovascular diseases has now only been described. More research should be done on the causes,” said Dr. Seeland.
This article was translated from the Medscape German edition. A version of this article first appeared on Medscape.com.
This finding is demonstrated in a new analysis of the Nurses’ Health Study II.
“To date, several studies have reported increased risks of cardiovascular risk factors or cardiovascular disease in connection with cycle disorders,” Yi-Xin Wang, MD, PhD, a research fellow in nutrition, and associates from the Harvard School of Public Health, Boston, wrote in an article published in JAMA Network Open.
Ute Seeland, MD, speaker of the Gender Medicine in Cardiology Working Group of the German Cardiology Society, said in an interview“We know that women who have indicated in their medical history that they have irregular menstrual cycles, invariably in connection with polycystic ovary syndrome (PCOS), more commonly develop diabetes and other metabolic disorders, as well as cardiovascular diseases.”
Cycle disorders’ role
However, the role that irregular or especially long cycles play at different points of a woman’s reproductive life span was unclear. Therefore, the research group investigated the associations in the Nurses’ Health Study II between cycle irregularity and cycle length in women of different age groups who later experienced cardiovascular events.
At the end of this study in 1989, the participants also provided information regarding the length and irregularity of their menstrual cycle from ages 14 to 17 years and again from ages 18 to 22 years. The information was updated in 1993 when the participants were aged 29-46 years. The data from 2019 to 2022 were analyzed.
“This kind of long-term cohort study is extremely rare and therefore something special,” said Dr. Seeland, who conducts research at the Institute for Social Medicine, Epidemiology, and Health Economics at the Charité – University Hospital Berlin.
The investigators used the following cycle classifications: very regular (no more than 3 or 4 days before or after the expected date), regular (within 5-7 days), usually irregular, always irregular, or no periods.
The cycle lengths were divided into the following categories: less than 21 days, 21-25 days, 26-31 days, 32-39 days, 40-50 days, more than 50 days, and too irregular to estimate the length.
The onset of cardiovascular diseases was determined using information from the participants and was confirmed by reviewing the medical files. Relevant to the study were lethal and nonlethal coronary heart diseases (such as myocardial infarction or coronary artery revascularization), as well as strokes.
Significant in adulthood
The data from 80,630 study participants were included in the analysis. At study inclusion, the average age of the participants was 37.7 years, and the average body mass index (BMI) was 25.1. “Since it was predominantly White nurses who took part in the study, the data are not transferable to other, more diverse populations,” said Dr. Seeland.
Over 24 years, 1,816 women (2.4%) had a cardiovascular event. “We observed an increased rate of cardiovascular events in women with an irregular cycle and longer cycle, both in early an in mid-adulthood,” wrote Dr. Wang and associates. “Similar trends were also observed for cycle disorders when younger, but this association was weaker than in adulthood.”
Compared with women with very regular cycles, women with irregular cycles or without periods who were aged 14-17 years, 18-22 years, or 29-49 years exhibited a 15%, 36%, and 40% higher risk of a cardiovascular event, respectively.
Similarly, women aged 18-22 years or 29-46 years with long cycles of 40 days or more had a 44% or 30% higher risk of cardiovascular disease, respectively, compared with women with cycle lengths of 26-31 days.
“The coronary heart diseases were decisive for the increase, and less so, the strokes,” wrote the researchers.
Classic risk factors?
Dr. Seeland praised the fact that the study authors tried to determine the role that classic cardiovascular risk factors played. “Compared with women with a regular cycle, women with an irregular cycle had a higher BMI, more frequently increased cholesterol levels, and an elevated blood pressure,” she said. Women with a long cycle displayed a similar pattern.
It can be assumed from this that over a woman’s life span, BMI affects the risk of cardiovascular disease. Therefore, Dr. Wang and coauthors adjusted the results on the basis of BMI, which varies over time.
Regarding other classic risk factors that may have played a role, “hypercholesterolemia, chronic high blood pressure, and type 2 diabetes were only responsible in 5.4%-13.5% of the associations,” wrote the researchers.
“Our results suggest that certain characteristics of the menstrual cycle across a woman’s reproductive lifespan may constitute additional risk markers for cardiovascular disease,” according to the authors.
The highest rates of cardiovascular disease were among women with permanently irregular or long cycles in early to mid adulthood, as well as women who had regular cycles when younger but had irregular cycles in mid adulthood. “This indicates that the change from one cycle phenotype to another could be a surrogate marker for metabolic changes, which in turn contribute to the formation of cardiovascular diseases,” wrote the authors.
The study was observational and so conclusions cannot be drawn regarding causal relationships. But Dr. Wang and associates indicate that the most common cause of an irregular menstrual cycle may be PCOS. “Roughly 90% of women with cycle disorders or oligomenorrhea have signs of PCOS. And it was shown that women with PCOS have an increased risk of cardiovascular disease.”
They concluded that “the associations observed between irregular and long cycles in early to mid-adulthood and cardiovascular diseases are likely attributable to underlying PCOS.”
For Dr. Seeland, however, this conclusion is “too monocausal. At no point in time did there seem to be any direct information regarding the frequency of PCOS during the data collection by the respondents.”
For now, we can only speculate about the mechanisms. “The association between a very irregular and long cycle and the increased risk of cardiovascular diseases has now only been described. More research should be done on the causes,” said Dr. Seeland.
This article was translated from the Medscape German edition. A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Top cardiology societies call for revamp of clinical trials
Leading cardiology societies have issued a “call for action” on a global scale to reinvent randomized clinical trials fit for the 21st century.
“Randomized trials are an essential tool for reliably assessing the effects of treatments, but they have become too costly and too burdensome,” first author Louise Bowman, University of Oxford, England, told this news organization. “We urgently need to modernize our approach to clinical trials in order to continue to improve patient care.”
The joint opinion is from the European Society of Cardiology, the American Heart Association, the American College of Cardiology, and the World Heart Federation. It was simultaneously published online in the European Heart Journal, Circulation, Journal of the American College of Cardiology, and Global Heart.
The authors note that the availability of large-scale “real-world” data is increasingly being touted as a way to bypass the challenges of conducting randomized trials. Yet, observational analyses of real-world data “are not a suitable alternative to randomization,” Prof. Bowman said.
Cardiology has historically led the way in transforming clinical practice with groundbreaking “mega-trials,” such as the International Study of Infarct Survival (ISIS), Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto (GISSI), and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO).
But over the past 25 years, there has been a huge increase in the rules and related bureaucracy governing clinical trials, which hinders the ability to conduct trials swiftly and affordably, the authors point out.
The COVID-19 pandemic has shown that important clinical trials can be performed quickly and efficiently in busy hospitals, they note.
“The RECOVERY trial in COVID-19 has been an excellent example of this, with results that are estimated to have saved around 1 million lives worldwide within just 1 year,” Prof. Bowman told this news organization.
A Good Clinical Trials Collaborative made up of key stakeholders recently developed new guidelines designed to promote better, more efficient randomized controlled trials.
“If widely adopted and used alongside valuable 21st century electronic health records, we could transform the clinical trials landscape and do many more high-quality trials very cost-effectively,” Prof. Bowman said.
“Widespread adoption and implementation of the revised guidelines will require collaboration with a wide range of national and international organizations, including patient, professional, academic, and industry groups, funders and government organizations, and ethics, health policy, and regulatory bodies,” Prof. Bowman acknowledged.
“This is work that the Good Clinical Trials Collaborative is leading. It is hoped that this endorsement by the joint cardiovascular societies will increase awareness and provide valuable support to his important work,” she added.
No commercial funding was received. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Leading cardiology societies have issued a “call for action” on a global scale to reinvent randomized clinical trials fit for the 21st century.
“Randomized trials are an essential tool for reliably assessing the effects of treatments, but they have become too costly and too burdensome,” first author Louise Bowman, University of Oxford, England, told this news organization. “We urgently need to modernize our approach to clinical trials in order to continue to improve patient care.”
The joint opinion is from the European Society of Cardiology, the American Heart Association, the American College of Cardiology, and the World Heart Federation. It was simultaneously published online in the European Heart Journal, Circulation, Journal of the American College of Cardiology, and Global Heart.
The authors note that the availability of large-scale “real-world” data is increasingly being touted as a way to bypass the challenges of conducting randomized trials. Yet, observational analyses of real-world data “are not a suitable alternative to randomization,” Prof. Bowman said.
Cardiology has historically led the way in transforming clinical practice with groundbreaking “mega-trials,” such as the International Study of Infarct Survival (ISIS), Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto (GISSI), and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO).
But over the past 25 years, there has been a huge increase in the rules and related bureaucracy governing clinical trials, which hinders the ability to conduct trials swiftly and affordably, the authors point out.
The COVID-19 pandemic has shown that important clinical trials can be performed quickly and efficiently in busy hospitals, they note.
“The RECOVERY trial in COVID-19 has been an excellent example of this, with results that are estimated to have saved around 1 million lives worldwide within just 1 year,” Prof. Bowman told this news organization.
A Good Clinical Trials Collaborative made up of key stakeholders recently developed new guidelines designed to promote better, more efficient randomized controlled trials.
“If widely adopted and used alongside valuable 21st century electronic health records, we could transform the clinical trials landscape and do many more high-quality trials very cost-effectively,” Prof. Bowman said.
“Widespread adoption and implementation of the revised guidelines will require collaboration with a wide range of national and international organizations, including patient, professional, academic, and industry groups, funders and government organizations, and ethics, health policy, and regulatory bodies,” Prof. Bowman acknowledged.
“This is work that the Good Clinical Trials Collaborative is leading. It is hoped that this endorsement by the joint cardiovascular societies will increase awareness and provide valuable support to his important work,” she added.
No commercial funding was received. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Leading cardiology societies have issued a “call for action” on a global scale to reinvent randomized clinical trials fit for the 21st century.
“Randomized trials are an essential tool for reliably assessing the effects of treatments, but they have become too costly and too burdensome,” first author Louise Bowman, University of Oxford, England, told this news organization. “We urgently need to modernize our approach to clinical trials in order to continue to improve patient care.”
The joint opinion is from the European Society of Cardiology, the American Heart Association, the American College of Cardiology, and the World Heart Federation. It was simultaneously published online in the European Heart Journal, Circulation, Journal of the American College of Cardiology, and Global Heart.
The authors note that the availability of large-scale “real-world” data is increasingly being touted as a way to bypass the challenges of conducting randomized trials. Yet, observational analyses of real-world data “are not a suitable alternative to randomization,” Prof. Bowman said.
Cardiology has historically led the way in transforming clinical practice with groundbreaking “mega-trials,” such as the International Study of Infarct Survival (ISIS), Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto (GISSI), and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO).
But over the past 25 years, there has been a huge increase in the rules and related bureaucracy governing clinical trials, which hinders the ability to conduct trials swiftly and affordably, the authors point out.
The COVID-19 pandemic has shown that important clinical trials can be performed quickly and efficiently in busy hospitals, they note.
“The RECOVERY trial in COVID-19 has been an excellent example of this, with results that are estimated to have saved around 1 million lives worldwide within just 1 year,” Prof. Bowman told this news organization.
A Good Clinical Trials Collaborative made up of key stakeholders recently developed new guidelines designed to promote better, more efficient randomized controlled trials.
“If widely adopted and used alongside valuable 21st century electronic health records, we could transform the clinical trials landscape and do many more high-quality trials very cost-effectively,” Prof. Bowman said.
“Widespread adoption and implementation of the revised guidelines will require collaboration with a wide range of national and international organizations, including patient, professional, academic, and industry groups, funders and government organizations, and ethics, health policy, and regulatory bodies,” Prof. Bowman acknowledged.
“This is work that the Good Clinical Trials Collaborative is leading. It is hoped that this endorsement by the joint cardiovascular societies will increase awareness and provide valuable support to his important work,” she added.
No commercial funding was received. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Lupin recalls quinapril tablets because of potential carcinogen
Lupin Pharmaceuticals is recalling four lots of quinapril tablets because of unacceptable levels of the nitrosamine impurity, N-nitroso-quinapril, a potential carcinogen.
Nitrosamines “may increase the risk of cancer if people are exposed to them above acceptable levels over long periods of time,” the company says in a recall notice posted on the Food and Drug Administration website.
Lupin says it “has received no reports of illness that appear to relate to this issue.”
Quinapril is an ACE inhibitor used to treat hypertension. Lupin stopped marketing quinapril tablets in September 2022.
The recalled product – quinapril tablets USP 20 mg and 40 mg – are packaged in 90-count bottles and were distributed nationwide to U.S. wholesalers, drug chains, mail order pharmacies, and supermarkets between March 15, 2021, and Sept. 1, 2022.
Lupin is notifying customers to immediately stop distribution of the recalled product and is arranging for the affected product lots to be returned to the company.
Questions regarding this recall should be directed to Inmar Rx Solutions at (877) 538-8445 Monday to Friday between 9:00 a.m. to 5:00 p.m. EST.
Patients and physicians are also advised to report any adverse events or side effects related to the affected products to MedWatch, the FDA’s Safety Information and Adverse Event Reporting program.
Pfizer recalled several lots of quinapril owing to the presence of the same impurity in March 2022and again in April.
A version of this article first appeared on Medscape.com.
Lupin Pharmaceuticals is recalling four lots of quinapril tablets because of unacceptable levels of the nitrosamine impurity, N-nitroso-quinapril, a potential carcinogen.
Nitrosamines “may increase the risk of cancer if people are exposed to them above acceptable levels over long periods of time,” the company says in a recall notice posted on the Food and Drug Administration website.
Lupin says it “has received no reports of illness that appear to relate to this issue.”
Quinapril is an ACE inhibitor used to treat hypertension. Lupin stopped marketing quinapril tablets in September 2022.
The recalled product – quinapril tablets USP 20 mg and 40 mg – are packaged in 90-count bottles and were distributed nationwide to U.S. wholesalers, drug chains, mail order pharmacies, and supermarkets between March 15, 2021, and Sept. 1, 2022.
Lupin is notifying customers to immediately stop distribution of the recalled product and is arranging for the affected product lots to be returned to the company.
Questions regarding this recall should be directed to Inmar Rx Solutions at (877) 538-8445 Monday to Friday between 9:00 a.m. to 5:00 p.m. EST.
Patients and physicians are also advised to report any adverse events or side effects related to the affected products to MedWatch, the FDA’s Safety Information and Adverse Event Reporting program.
Pfizer recalled several lots of quinapril owing to the presence of the same impurity in March 2022and again in April.
A version of this article first appeared on Medscape.com.
Lupin Pharmaceuticals is recalling four lots of quinapril tablets because of unacceptable levels of the nitrosamine impurity, N-nitroso-quinapril, a potential carcinogen.
Nitrosamines “may increase the risk of cancer if people are exposed to them above acceptable levels over long periods of time,” the company says in a recall notice posted on the Food and Drug Administration website.
Lupin says it “has received no reports of illness that appear to relate to this issue.”
Quinapril is an ACE inhibitor used to treat hypertension. Lupin stopped marketing quinapril tablets in September 2022.
The recalled product – quinapril tablets USP 20 mg and 40 mg – are packaged in 90-count bottles and were distributed nationwide to U.S. wholesalers, drug chains, mail order pharmacies, and supermarkets between March 15, 2021, and Sept. 1, 2022.
Lupin is notifying customers to immediately stop distribution of the recalled product and is arranging for the affected product lots to be returned to the company.
Questions regarding this recall should be directed to Inmar Rx Solutions at (877) 538-8445 Monday to Friday between 9:00 a.m. to 5:00 p.m. EST.
Patients and physicians are also advised to report any adverse events or side effects related to the affected products to MedWatch, the FDA’s Safety Information and Adverse Event Reporting program.
Pfizer recalled several lots of quinapril owing to the presence of the same impurity in March 2022and again in April.
A version of this article first appeared on Medscape.com.
A doctor saves a drowning family in a dangerous river
Is There a Doctor in the House? is a new series telling these stories.
I live on the Maumee River in Ohio, about 50 yards from the water. I had an early quit time and came home to meet my wife for lunch. Afterward, I went up to my barn across the main road to tinker around. It was a nice day out, so my wife had opened some windows. Suddenly, she heard screaming from the river. It did not sound like fun.
She ran down to the river’s edge and saw a dad and three boys struggling in the water. She phoned me screaming: “They’re drowning! They’re drowning!” I jumped in my truck and drove up our driveway through the yard right down to the river.
My wife was on the phone with 911 at that point, and I could see them about 75-100 yards out. The dad had two of the boys clinging around his neck. They were going under the water and coming up and going under again. The other boy was just floating nearby, face down, motionless.
I threw my shoes and scrubs off and started to walk towards the water. My wife screamed at me, “You’re not going in there!” I said, “I’m not going to stand here and watch this. It’s not going to happen.”
I’m not a kid anymore, but I was a high school swimmer, and to this day I work out all the time. I felt like I had to try something. So, I went in the water despite my wife yelling and I swam towards them.
What happens when you get in that deep water is that you panic. You can’t hear anyone because of the rapids, and your instinct is to swim back towards where you went in, which is against the current. Unless you’re a very strong swimmer, you’re just wasting your time, swimming in place.
But these guys weren’t trying to go anywhere. Dad was just trying to stay up and keep the boys alive. He was in about 10 feet of water. What they didn’t see or just didn’t know: About 20 yards upstream from that deep water is a little island.
When I got to them, I yelled at the dad to move towards the island, “Go backwards! Go back!” I flipped the boy over who wasn’t moving. He was the oldest of the three, around 10 or 11 years old. When I turned him over, he was blue and wasn’t breathing. I put my fingers on his neck and didn’t feel a pulse.
So, I’m treading water, holding him. I put an arm behind his back and started doing chest compressions on him. I probably did a dozen to 15 compressions – nothing. I thought, I’ve got to get some air in this kid. So, I gave him two deep breaths and then started doing compressions again. I know ACLS and CPR training would say we don’t do that anymore. But I couldn’t just sit there and give up. Shortly after that, he coughed out a large amount of water and started breathing.
The dad and the other two boys had made it to the island. So, I started moving towards it with the boy. It was a few minutes before he regained consciousness. Of course, he was unaware of what had happened. He started to scream, because here’s this strange man holding him. But he was breathing. That’s all I cared about.
When we got to the island, I saw that my neighbor downstream had launched his canoe. He’s a retired gentleman who lives next to me, a very physically fit man. He started rolling as hard as he could towards us, against the stream. I kind of gave him a thumbs up, like, “we’re safe now. We’re standing.” We loaded the kids and the dad in the canoe and made it back against the stream to the parking lot where they went in.
All this took probably 10 or 15 minutes, and by then the paramedics were there. Life Flight had been dispatched up by my barn where there’s room to land. So, they drove up there in the ambulance. The boy I revived was flown to the hospital. The others went in the ambulance.
I know all the ED docs, so I talked to somebody later who, with permission from the family, said they were all doing fine. They were getting x-rays on the boy’s lungs. And then I heard the dad and two boys were released that night. The other boy I worked on was observed overnight and discharged the following morning.
Four or 5 days later, I heard from their pediatrician, who also had permission to share. He sent me a very nice note through Epic that he had seen the boys. Besides some mental trauma, they were all healthy and doing fine.
The family lives in the area and the kids go to school 5 miles from my house. So, the following weekend they came over. It was Father’s Day, which was kind of cool. They brought me some flowers and candy and a card the boys had drawn to thank me.
I learned that the dad had brought the boys to the fishing site. They were horsing around in knee deep water. One of the boys walked off a little way and didn’t realize there was a drop off. He went in, and of course the dad went after him, and the other two followed.
I said to the parents: “Look, things like this happen for a reason. People like your son are saved and go on in this world because they’ve got special things to do. I can’t wait to see what kind of man he becomes.”
Two or 3 months later, it was football season, and I got at a message from the dad saying their son was playing football on Saturday at the school. He wondered if I could drop by. So, I kind of snuck over and watched, but I didn’t go say hi. There’s trauma there, and I didn’t want them to have to relive that.
I’m very fortunate that I exercise every day and I know how to do CPR and swim. And thank God the boy was floating when I got to him, or I never would’ve found him. The Maumee River is known as the “muddy Maumee.” You can’t see anything under the water.
Depending on the time of year, the river can be almost dry or overflowing into the parking lot with the current rushing hard. If it had been like that, I wouldn’t have considered going in. And they wouldn’t they have been there in the first place. They’d have been a mile downstream.
I took a risk. I could have gone out there and had the dad and two other kids jump on top of me. Then we all would have been in trouble. But like I told my wife, I couldn’t stand there and watch it. I’m just not that person.
I think it was also about being a dad myself and having grandkids now. Doctor or no doctor, I felt like I was in reasonably good shape and I had to go in there to help. This dad was trying his butt off, but three little kids is too many. You can’t do that by yourself. They were not going to make it.
I go to the hospital and I save lives as part of my job, and I don’t even come home and talk about it. But this is a whole different thing. Being able to save someone’s life when put in this situation is very gratifying. It’s a tremendous feeling. There’s a reason that young man is here today, and I’ll be watching for great things from him.
A version of this article first appeared on Medscape.com.
Daniel Cassavar, MD, is a cardiologist with ProMedica in Perrysburg, Ohio.
Is There a Doctor in the House? is a new series telling these stories.
I live on the Maumee River in Ohio, about 50 yards from the water. I had an early quit time and came home to meet my wife for lunch. Afterward, I went up to my barn across the main road to tinker around. It was a nice day out, so my wife had opened some windows. Suddenly, she heard screaming from the river. It did not sound like fun.
She ran down to the river’s edge and saw a dad and three boys struggling in the water. She phoned me screaming: “They’re drowning! They’re drowning!” I jumped in my truck and drove up our driveway through the yard right down to the river.
My wife was on the phone with 911 at that point, and I could see them about 75-100 yards out. The dad had two of the boys clinging around his neck. They were going under the water and coming up and going under again. The other boy was just floating nearby, face down, motionless.
I threw my shoes and scrubs off and started to walk towards the water. My wife screamed at me, “You’re not going in there!” I said, “I’m not going to stand here and watch this. It’s not going to happen.”
I’m not a kid anymore, but I was a high school swimmer, and to this day I work out all the time. I felt like I had to try something. So, I went in the water despite my wife yelling and I swam towards them.
What happens when you get in that deep water is that you panic. You can’t hear anyone because of the rapids, and your instinct is to swim back towards where you went in, which is against the current. Unless you’re a very strong swimmer, you’re just wasting your time, swimming in place.
But these guys weren’t trying to go anywhere. Dad was just trying to stay up and keep the boys alive. He was in about 10 feet of water. What they didn’t see or just didn’t know: About 20 yards upstream from that deep water is a little island.
When I got to them, I yelled at the dad to move towards the island, “Go backwards! Go back!” I flipped the boy over who wasn’t moving. He was the oldest of the three, around 10 or 11 years old. When I turned him over, he was blue and wasn’t breathing. I put my fingers on his neck and didn’t feel a pulse.
So, I’m treading water, holding him. I put an arm behind his back and started doing chest compressions on him. I probably did a dozen to 15 compressions – nothing. I thought, I’ve got to get some air in this kid. So, I gave him two deep breaths and then started doing compressions again. I know ACLS and CPR training would say we don’t do that anymore. But I couldn’t just sit there and give up. Shortly after that, he coughed out a large amount of water and started breathing.
The dad and the other two boys had made it to the island. So, I started moving towards it with the boy. It was a few minutes before he regained consciousness. Of course, he was unaware of what had happened. He started to scream, because here’s this strange man holding him. But he was breathing. That’s all I cared about.
When we got to the island, I saw that my neighbor downstream had launched his canoe. He’s a retired gentleman who lives next to me, a very physically fit man. He started rolling as hard as he could towards us, against the stream. I kind of gave him a thumbs up, like, “we’re safe now. We’re standing.” We loaded the kids and the dad in the canoe and made it back against the stream to the parking lot where they went in.
All this took probably 10 or 15 minutes, and by then the paramedics were there. Life Flight had been dispatched up by my barn where there’s room to land. So, they drove up there in the ambulance. The boy I revived was flown to the hospital. The others went in the ambulance.
I know all the ED docs, so I talked to somebody later who, with permission from the family, said they were all doing fine. They were getting x-rays on the boy’s lungs. And then I heard the dad and two boys were released that night. The other boy I worked on was observed overnight and discharged the following morning.
Four or 5 days later, I heard from their pediatrician, who also had permission to share. He sent me a very nice note through Epic that he had seen the boys. Besides some mental trauma, they were all healthy and doing fine.
The family lives in the area and the kids go to school 5 miles from my house. So, the following weekend they came over. It was Father’s Day, which was kind of cool. They brought me some flowers and candy and a card the boys had drawn to thank me.
I learned that the dad had brought the boys to the fishing site. They were horsing around in knee deep water. One of the boys walked off a little way and didn’t realize there was a drop off. He went in, and of course the dad went after him, and the other two followed.
I said to the parents: “Look, things like this happen for a reason. People like your son are saved and go on in this world because they’ve got special things to do. I can’t wait to see what kind of man he becomes.”
Two or 3 months later, it was football season, and I got at a message from the dad saying their son was playing football on Saturday at the school. He wondered if I could drop by. So, I kind of snuck over and watched, but I didn’t go say hi. There’s trauma there, and I didn’t want them to have to relive that.
I’m very fortunate that I exercise every day and I know how to do CPR and swim. And thank God the boy was floating when I got to him, or I never would’ve found him. The Maumee River is known as the “muddy Maumee.” You can’t see anything under the water.
Depending on the time of year, the river can be almost dry or overflowing into the parking lot with the current rushing hard. If it had been like that, I wouldn’t have considered going in. And they wouldn’t they have been there in the first place. They’d have been a mile downstream.
I took a risk. I could have gone out there and had the dad and two other kids jump on top of me. Then we all would have been in trouble. But like I told my wife, I couldn’t stand there and watch it. I’m just not that person.
I think it was also about being a dad myself and having grandkids now. Doctor or no doctor, I felt like I was in reasonably good shape and I had to go in there to help. This dad was trying his butt off, but three little kids is too many. You can’t do that by yourself. They were not going to make it.
I go to the hospital and I save lives as part of my job, and I don’t even come home and talk about it. But this is a whole different thing. Being able to save someone’s life when put in this situation is very gratifying. It’s a tremendous feeling. There’s a reason that young man is here today, and I’ll be watching for great things from him.
A version of this article first appeared on Medscape.com.
Daniel Cassavar, MD, is a cardiologist with ProMedica in Perrysburg, Ohio.
Is There a Doctor in the House? is a new series telling these stories.
I live on the Maumee River in Ohio, about 50 yards from the water. I had an early quit time and came home to meet my wife for lunch. Afterward, I went up to my barn across the main road to tinker around. It was a nice day out, so my wife had opened some windows. Suddenly, she heard screaming from the river. It did not sound like fun.
She ran down to the river’s edge and saw a dad and three boys struggling in the water. She phoned me screaming: “They’re drowning! They’re drowning!” I jumped in my truck and drove up our driveway through the yard right down to the river.
My wife was on the phone with 911 at that point, and I could see them about 75-100 yards out. The dad had two of the boys clinging around his neck. They were going under the water and coming up and going under again. The other boy was just floating nearby, face down, motionless.
I threw my shoes and scrubs off and started to walk towards the water. My wife screamed at me, “You’re not going in there!” I said, “I’m not going to stand here and watch this. It’s not going to happen.”
I’m not a kid anymore, but I was a high school swimmer, and to this day I work out all the time. I felt like I had to try something. So, I went in the water despite my wife yelling and I swam towards them.
What happens when you get in that deep water is that you panic. You can’t hear anyone because of the rapids, and your instinct is to swim back towards where you went in, which is against the current. Unless you’re a very strong swimmer, you’re just wasting your time, swimming in place.
But these guys weren’t trying to go anywhere. Dad was just trying to stay up and keep the boys alive. He was in about 10 feet of water. What they didn’t see or just didn’t know: About 20 yards upstream from that deep water is a little island.
When I got to them, I yelled at the dad to move towards the island, “Go backwards! Go back!” I flipped the boy over who wasn’t moving. He was the oldest of the three, around 10 or 11 years old. When I turned him over, he was blue and wasn’t breathing. I put my fingers on his neck and didn’t feel a pulse.
So, I’m treading water, holding him. I put an arm behind his back and started doing chest compressions on him. I probably did a dozen to 15 compressions – nothing. I thought, I’ve got to get some air in this kid. So, I gave him two deep breaths and then started doing compressions again. I know ACLS and CPR training would say we don’t do that anymore. But I couldn’t just sit there and give up. Shortly after that, he coughed out a large amount of water and started breathing.
The dad and the other two boys had made it to the island. So, I started moving towards it with the boy. It was a few minutes before he regained consciousness. Of course, he was unaware of what had happened. He started to scream, because here’s this strange man holding him. But he was breathing. That’s all I cared about.
When we got to the island, I saw that my neighbor downstream had launched his canoe. He’s a retired gentleman who lives next to me, a very physically fit man. He started rolling as hard as he could towards us, against the stream. I kind of gave him a thumbs up, like, “we’re safe now. We’re standing.” We loaded the kids and the dad in the canoe and made it back against the stream to the parking lot where they went in.
All this took probably 10 or 15 minutes, and by then the paramedics were there. Life Flight had been dispatched up by my barn where there’s room to land. So, they drove up there in the ambulance. The boy I revived was flown to the hospital. The others went in the ambulance.
I know all the ED docs, so I talked to somebody later who, with permission from the family, said they were all doing fine. They were getting x-rays on the boy’s lungs. And then I heard the dad and two boys were released that night. The other boy I worked on was observed overnight and discharged the following morning.
Four or 5 days later, I heard from their pediatrician, who also had permission to share. He sent me a very nice note through Epic that he had seen the boys. Besides some mental trauma, they were all healthy and doing fine.
The family lives in the area and the kids go to school 5 miles from my house. So, the following weekend they came over. It was Father’s Day, which was kind of cool. They brought me some flowers and candy and a card the boys had drawn to thank me.
I learned that the dad had brought the boys to the fishing site. They were horsing around in knee deep water. One of the boys walked off a little way and didn’t realize there was a drop off. He went in, and of course the dad went after him, and the other two followed.
I said to the parents: “Look, things like this happen for a reason. People like your son are saved and go on in this world because they’ve got special things to do. I can’t wait to see what kind of man he becomes.”
Two or 3 months later, it was football season, and I got at a message from the dad saying their son was playing football on Saturday at the school. He wondered if I could drop by. So, I kind of snuck over and watched, but I didn’t go say hi. There’s trauma there, and I didn’t want them to have to relive that.
I’m very fortunate that I exercise every day and I know how to do CPR and swim. And thank God the boy was floating when I got to him, or I never would’ve found him. The Maumee River is known as the “muddy Maumee.” You can’t see anything under the water.
Depending on the time of year, the river can be almost dry or overflowing into the parking lot with the current rushing hard. If it had been like that, I wouldn’t have considered going in. And they wouldn’t they have been there in the first place. They’d have been a mile downstream.
I took a risk. I could have gone out there and had the dad and two other kids jump on top of me. Then we all would have been in trouble. But like I told my wife, I couldn’t stand there and watch it. I’m just not that person.
I think it was also about being a dad myself and having grandkids now. Doctor or no doctor, I felt like I was in reasonably good shape and I had to go in there to help. This dad was trying his butt off, but three little kids is too many. You can’t do that by yourself. They were not going to make it.
I go to the hospital and I save lives as part of my job, and I don’t even come home and talk about it. But this is a whole different thing. Being able to save someone’s life when put in this situation is very gratifying. It’s a tremendous feeling. There’s a reason that young man is here today, and I’ll be watching for great things from him.
A version of this article first appeared on Medscape.com.
Daniel Cassavar, MD, is a cardiologist with ProMedica in Perrysburg, Ohio.

