User login
B-cell level may affect COVID booster efficacy in MS
Patients with multiple sclerosis (MS) treated with the B-cell-depleting medication rituximab who have not yet been vaccinated against COVID-19 should get the initial vaccination as soon as possible but wait to get a booster shot until B-cell levels increase, new research suggests.
In a prospective cohort study, 90% of patients taking rituximab whose B-cell level was at least 40 cells/mcL had a sufficient antibody response to the Pfizer vaccine, whereas among those with lower levels, the antibody response was significantly lower.
Results also showed a wide variation in the length of time needed for adequate B-cell restoration. Some patients needed a year or longer for levels to become adequate.
The findings led the hospital where the study was conducted to suspend rituximab therapy until patients could be vaccinated. The findings also prompted researchers to call for new guidelines on vaccine scheduling that are based on B-cell levels and not on the current criteria of length of time since last treatment.
“It’s meaningless to just go by some recommendation covering time since the last treatment,” study investigator Joachim Burman, MD, PhD, a consultant neurologist at Uppsala University Hospital and an associate professor at Uppsala University, both in Sweden, told this news organization.
“It’s misleading and potentially harmful for patients,” Dr. Burman said.
The findings were published online in JAMA Network Open.
Finding the cutoff
Drugs such as rituximab target CD20, a protein found on the surface of B cells, resulting in B-cell depletion.
Rituximab is the most common MS therapy used in Sweden. The drug is approved in the United States to treat rheumatoid arthritis and some forms of cancer, but it is not approved for treatment of MS.
Prior research showed that antibody response to COVID-19 vaccines was lower in patients receiving B-cell therapy than in the general population. That was not altogether surprising, given the fact that studies have found a similarly weakened antibody response to other vaccines.
But before now, there was no known B-cell threshold sufficient to mount an acceptable antibody response following COVID vaccination.
Researchers enrolled 67 patients in the study. Of those patients, 60 had received rituximab treatment, and seven had not.
Approximately 6 months after the last rituximab dose, the B-cell count was lower than 10/mcL for 40% of patients. In that group, rituximab treatment duration was the only factor significantly associated with slower B-cell mobilization (median duration, 4.0 years, vs. 2.1; P = .002).
Close monitoring needed
Six weeks after vaccination with tozinameran, the mRNA vaccine manufactured by Pfizer, 28% of patients failed to generate a sufficient antibody response. Among those patients, the median B-cell count was 22/mcL, versus 51/mcL for the remainder of the cohort (P < .001).
A cutoff value of 40/mcL rendered adequate levels of anti-spike immunoglobulin G antibodies in 90% of patients and a strong response in anti-RBD antibodies in 72%.
Study participants did register an adequate T-cell response to the vaccine, suggesting at least some level of protection.
Because MS patients are at increased risk for serious illness from SARS-CoV-2 infection, the investigators recommend that patients with MS receive their initial COVID vaccines as soon as possible – but that they should hold off on receiving a booster until their B-cell counts reach 40/mcL.
Regarding when a clinician should re-vaccinate, “the results from our study strongly suggest that you should not do that right away or just follow some generic guideline,” Dr. Burman said.
“You should closely monitor the B-cell values, and re-vaccinate once those B- cells hit the level of 40 cells/mcL” he added.
Dr. Burman said he would expect that their findings would hold with the other mRNA vaccine and with any other B-cell therapy.
Too soon for B-cell measures?
Commenting for this news organization, Robert J. Fox, MD, staff neurologist at the Mellen Center for MS and vice-chair for research at the Neurological Institute at Cleveland Clinic, Ohio, said the B-cell threshold identified in the study is much higher than what is typically seen in patients who undergo treatment with ocrelizumab, an anti-CD20 B-cell therapy approved in the United States for treating MS.
“Decisions about treatment interval need to balance efficacy in treating MS with safety, including response to vaccines,” said Dr. Fox, who was not involved with the research.
“Given the unknown efficacy of these extended intervals, I don’t think we’re at the point of making management recommendations based upon B-cell counts,” he added.
And yet, Uppsala University Hospital, where the study was conducted, and other centers in Sweden decided to do just that. They suspended administering rituximab to patients with MS until the patients were vaccinated. For patients newly diagnosed with MS, therapy was initiated using another disease-modifying treatment, and for those who were due for a rituximab infusion, that treatment was delayed.
Only one patient experienced a mild MS relapse during the rituximab suspension, and that case went into remission within a week, Dr. Burman reported.
“Ever since the Bar-Or report showing that the humeral response to vaccines is markedly diminished in MS patients treated with anti-CD20 therapies, clinicians have been struggling to balance those safety concerns related to anti-CD20 monoclonal antibody treatments and the clinical benefit of this treatment class,” Dr. Fox said.
“Given the uncharted waters of the COVID pandemic, clinicians made judgments and decisions as best they could, given the paucity of data,” he noted.
“At this point, we don’t know which decisions were right or wrong, but I certainly don’t think we should judge clinicians for making decisions the best they could.”
The study was funded by the Engkvist Foundation, the Marianne and Marcus Wallenberg Foundation, and the Swedish Society for Medical Research. Dr. Burman reported no relevant financial relationships. Dr. Fox has received consulting fees from Genentech/Roche, Biogen, and other companies that promote MS therapies.
A version of this article first appeared on Medscape.com.
Patients with multiple sclerosis (MS) treated with the B-cell-depleting medication rituximab who have not yet been vaccinated against COVID-19 should get the initial vaccination as soon as possible but wait to get a booster shot until B-cell levels increase, new research suggests.
In a prospective cohort study, 90% of patients taking rituximab whose B-cell level was at least 40 cells/mcL had a sufficient antibody response to the Pfizer vaccine, whereas among those with lower levels, the antibody response was significantly lower.
Results also showed a wide variation in the length of time needed for adequate B-cell restoration. Some patients needed a year or longer for levels to become adequate.
The findings led the hospital where the study was conducted to suspend rituximab therapy until patients could be vaccinated. The findings also prompted researchers to call for new guidelines on vaccine scheduling that are based on B-cell levels and not on the current criteria of length of time since last treatment.
“It’s meaningless to just go by some recommendation covering time since the last treatment,” study investigator Joachim Burman, MD, PhD, a consultant neurologist at Uppsala University Hospital and an associate professor at Uppsala University, both in Sweden, told this news organization.
“It’s misleading and potentially harmful for patients,” Dr. Burman said.
The findings were published online in JAMA Network Open.
Finding the cutoff
Drugs such as rituximab target CD20, a protein found on the surface of B cells, resulting in B-cell depletion.
Rituximab is the most common MS therapy used in Sweden. The drug is approved in the United States to treat rheumatoid arthritis and some forms of cancer, but it is not approved for treatment of MS.
Prior research showed that antibody response to COVID-19 vaccines was lower in patients receiving B-cell therapy than in the general population. That was not altogether surprising, given the fact that studies have found a similarly weakened antibody response to other vaccines.
But before now, there was no known B-cell threshold sufficient to mount an acceptable antibody response following COVID vaccination.
Researchers enrolled 67 patients in the study. Of those patients, 60 had received rituximab treatment, and seven had not.
Approximately 6 months after the last rituximab dose, the B-cell count was lower than 10/mcL for 40% of patients. In that group, rituximab treatment duration was the only factor significantly associated with slower B-cell mobilization (median duration, 4.0 years, vs. 2.1; P = .002).
Close monitoring needed
Six weeks after vaccination with tozinameran, the mRNA vaccine manufactured by Pfizer, 28% of patients failed to generate a sufficient antibody response. Among those patients, the median B-cell count was 22/mcL, versus 51/mcL for the remainder of the cohort (P < .001).
A cutoff value of 40/mcL rendered adequate levels of anti-spike immunoglobulin G antibodies in 90% of patients and a strong response in anti-RBD antibodies in 72%.
Study participants did register an adequate T-cell response to the vaccine, suggesting at least some level of protection.
Because MS patients are at increased risk for serious illness from SARS-CoV-2 infection, the investigators recommend that patients with MS receive their initial COVID vaccines as soon as possible – but that they should hold off on receiving a booster until their B-cell counts reach 40/mcL.
Regarding when a clinician should re-vaccinate, “the results from our study strongly suggest that you should not do that right away or just follow some generic guideline,” Dr. Burman said.
“You should closely monitor the B-cell values, and re-vaccinate once those B- cells hit the level of 40 cells/mcL” he added.
Dr. Burman said he would expect that their findings would hold with the other mRNA vaccine and with any other B-cell therapy.
Too soon for B-cell measures?
Commenting for this news organization, Robert J. Fox, MD, staff neurologist at the Mellen Center for MS and vice-chair for research at the Neurological Institute at Cleveland Clinic, Ohio, said the B-cell threshold identified in the study is much higher than what is typically seen in patients who undergo treatment with ocrelizumab, an anti-CD20 B-cell therapy approved in the United States for treating MS.
“Decisions about treatment interval need to balance efficacy in treating MS with safety, including response to vaccines,” said Dr. Fox, who was not involved with the research.
“Given the unknown efficacy of these extended intervals, I don’t think we’re at the point of making management recommendations based upon B-cell counts,” he added.
And yet, Uppsala University Hospital, where the study was conducted, and other centers in Sweden decided to do just that. They suspended administering rituximab to patients with MS until the patients were vaccinated. For patients newly diagnosed with MS, therapy was initiated using another disease-modifying treatment, and for those who were due for a rituximab infusion, that treatment was delayed.
Only one patient experienced a mild MS relapse during the rituximab suspension, and that case went into remission within a week, Dr. Burman reported.
“Ever since the Bar-Or report showing that the humeral response to vaccines is markedly diminished in MS patients treated with anti-CD20 therapies, clinicians have been struggling to balance those safety concerns related to anti-CD20 monoclonal antibody treatments and the clinical benefit of this treatment class,” Dr. Fox said.
“Given the uncharted waters of the COVID pandemic, clinicians made judgments and decisions as best they could, given the paucity of data,” he noted.
“At this point, we don’t know which decisions were right or wrong, but I certainly don’t think we should judge clinicians for making decisions the best they could.”
The study was funded by the Engkvist Foundation, the Marianne and Marcus Wallenberg Foundation, and the Swedish Society for Medical Research. Dr. Burman reported no relevant financial relationships. Dr. Fox has received consulting fees from Genentech/Roche, Biogen, and other companies that promote MS therapies.
A version of this article first appeared on Medscape.com.
Patients with multiple sclerosis (MS) treated with the B-cell-depleting medication rituximab who have not yet been vaccinated against COVID-19 should get the initial vaccination as soon as possible but wait to get a booster shot until B-cell levels increase, new research suggests.
In a prospective cohort study, 90% of patients taking rituximab whose B-cell level was at least 40 cells/mcL had a sufficient antibody response to the Pfizer vaccine, whereas among those with lower levels, the antibody response was significantly lower.
Results also showed a wide variation in the length of time needed for adequate B-cell restoration. Some patients needed a year or longer for levels to become adequate.
The findings led the hospital where the study was conducted to suspend rituximab therapy until patients could be vaccinated. The findings also prompted researchers to call for new guidelines on vaccine scheduling that are based on B-cell levels and not on the current criteria of length of time since last treatment.
“It’s meaningless to just go by some recommendation covering time since the last treatment,” study investigator Joachim Burman, MD, PhD, a consultant neurologist at Uppsala University Hospital and an associate professor at Uppsala University, both in Sweden, told this news organization.
“It’s misleading and potentially harmful for patients,” Dr. Burman said.
The findings were published online in JAMA Network Open.
Finding the cutoff
Drugs such as rituximab target CD20, a protein found on the surface of B cells, resulting in B-cell depletion.
Rituximab is the most common MS therapy used in Sweden. The drug is approved in the United States to treat rheumatoid arthritis and some forms of cancer, but it is not approved for treatment of MS.
Prior research showed that antibody response to COVID-19 vaccines was lower in patients receiving B-cell therapy than in the general population. That was not altogether surprising, given the fact that studies have found a similarly weakened antibody response to other vaccines.
But before now, there was no known B-cell threshold sufficient to mount an acceptable antibody response following COVID vaccination.
Researchers enrolled 67 patients in the study. Of those patients, 60 had received rituximab treatment, and seven had not.
Approximately 6 months after the last rituximab dose, the B-cell count was lower than 10/mcL for 40% of patients. In that group, rituximab treatment duration was the only factor significantly associated with slower B-cell mobilization (median duration, 4.0 years, vs. 2.1; P = .002).
Close monitoring needed
Six weeks after vaccination with tozinameran, the mRNA vaccine manufactured by Pfizer, 28% of patients failed to generate a sufficient antibody response. Among those patients, the median B-cell count was 22/mcL, versus 51/mcL for the remainder of the cohort (P < .001).
A cutoff value of 40/mcL rendered adequate levels of anti-spike immunoglobulin G antibodies in 90% of patients and a strong response in anti-RBD antibodies in 72%.
Study participants did register an adequate T-cell response to the vaccine, suggesting at least some level of protection.
Because MS patients are at increased risk for serious illness from SARS-CoV-2 infection, the investigators recommend that patients with MS receive their initial COVID vaccines as soon as possible – but that they should hold off on receiving a booster until their B-cell counts reach 40/mcL.
Regarding when a clinician should re-vaccinate, “the results from our study strongly suggest that you should not do that right away or just follow some generic guideline,” Dr. Burman said.
“You should closely monitor the B-cell values, and re-vaccinate once those B- cells hit the level of 40 cells/mcL” he added.
Dr. Burman said he would expect that their findings would hold with the other mRNA vaccine and with any other B-cell therapy.
Too soon for B-cell measures?
Commenting for this news organization, Robert J. Fox, MD, staff neurologist at the Mellen Center for MS and vice-chair for research at the Neurological Institute at Cleveland Clinic, Ohio, said the B-cell threshold identified in the study is much higher than what is typically seen in patients who undergo treatment with ocrelizumab, an anti-CD20 B-cell therapy approved in the United States for treating MS.
“Decisions about treatment interval need to balance efficacy in treating MS with safety, including response to vaccines,” said Dr. Fox, who was not involved with the research.
“Given the unknown efficacy of these extended intervals, I don’t think we’re at the point of making management recommendations based upon B-cell counts,” he added.
And yet, Uppsala University Hospital, where the study was conducted, and other centers in Sweden decided to do just that. They suspended administering rituximab to patients with MS until the patients were vaccinated. For patients newly diagnosed with MS, therapy was initiated using another disease-modifying treatment, and for those who were due for a rituximab infusion, that treatment was delayed.
Only one patient experienced a mild MS relapse during the rituximab suspension, and that case went into remission within a week, Dr. Burman reported.
“Ever since the Bar-Or report showing that the humeral response to vaccines is markedly diminished in MS patients treated with anti-CD20 therapies, clinicians have been struggling to balance those safety concerns related to anti-CD20 monoclonal antibody treatments and the clinical benefit of this treatment class,” Dr. Fox said.
“Given the uncharted waters of the COVID pandemic, clinicians made judgments and decisions as best they could, given the paucity of data,” he noted.
“At this point, we don’t know which decisions were right or wrong, but I certainly don’t think we should judge clinicians for making decisions the best they could.”
The study was funded by the Engkvist Foundation, the Marianne and Marcus Wallenberg Foundation, and the Swedish Society for Medical Research. Dr. Burman reported no relevant financial relationships. Dr. Fox has received consulting fees from Genentech/Roche, Biogen, and other companies that promote MS therapies.
A version of this article first appeared on Medscape.com.
Pfizer asks FDA to authorize COVID vaccine for children younger than 5
The FDA has accepted Pfizer’s application for a COVID-19 vaccine for children under age 5, which clears the way for approval and distribution in June.
Pfizer announced June 1 that it completed the application for a three-dose vaccine for kids between 6 months and 5 years old, and the FDA said it received the emergency use application.
Children in this age group – the last to be eligible for COVID-19 vaccines – could begin getting shots as early as June 21, according to White House COVID-19 response coordinator Ashish Jha, MD.
Meanwhile, COVID-19 cases are still high – an average of 100,000 cases a day – but death numbers are about 90% lower than they were when President Joe Biden first took office, Dr. Jha said.
The FDA’s advisory group, the Vaccines and Related Biological Products Advisory Committee, is scheduled to meet June 14 and June 15 to discuss data submitted by both Pfizer and Moderna.
If the FDA gives them the green light, the CDC will then weigh in.
“We know that many, many parents are eager to vaccinate their youngest kids, and it’s important to do this right,” Dr. Jha said at a White House press briefing on June 2. “We expect that vaccinations will begin in earnest as early as June 21 and really roll on throughout that week.”
States can place their orders as early as June 3, Dr. Jha said, and there will initially be 10 million doses available. If the FDA gives emergency use authorization for the vaccines, the government will begin shipping doses to thousands of sites across the country.
“The good news is we have plenty of supply of Pfizer and Moderna vaccines,” Dr. Jha said. “We’ve asked states to distribute to their highest priority sites, serving the highest risk and hardest to reach areas.”
Pfizer’s clinical trials found that three doses of the vaccine for children 6 months to under 5 years were safe and effective and proved to be 80% effective against Omicron.
The FDA announced its meeting information with a conversation about the Moderna vaccine for ages 6-17 scheduled for June 14 and a conversation about the Pfizer and Moderna vaccines for young children scheduled for June 15.
Moderna applied for FDA authorization of its two-dose vaccine for children under age 6 on April 28. The company said the vaccine was 51% effective against infections with symptoms for children ages 6 months to 2 years and 37% effective for ages 2-5.
Pfizer’s 3-microgram dose is one-tenth of its adult dose. Moderna’s 25-microgram dose is one-quarter of its adult dose.
A version of this article first appeared on Medscape.com.
The FDA has accepted Pfizer’s application for a COVID-19 vaccine for children under age 5, which clears the way for approval and distribution in June.
Pfizer announced June 1 that it completed the application for a three-dose vaccine for kids between 6 months and 5 years old, and the FDA said it received the emergency use application.
Children in this age group – the last to be eligible for COVID-19 vaccines – could begin getting shots as early as June 21, according to White House COVID-19 response coordinator Ashish Jha, MD.
Meanwhile, COVID-19 cases are still high – an average of 100,000 cases a day – but death numbers are about 90% lower than they were when President Joe Biden first took office, Dr. Jha said.
The FDA’s advisory group, the Vaccines and Related Biological Products Advisory Committee, is scheduled to meet June 14 and June 15 to discuss data submitted by both Pfizer and Moderna.
If the FDA gives them the green light, the CDC will then weigh in.
“We know that many, many parents are eager to vaccinate their youngest kids, and it’s important to do this right,” Dr. Jha said at a White House press briefing on June 2. “We expect that vaccinations will begin in earnest as early as June 21 and really roll on throughout that week.”
States can place their orders as early as June 3, Dr. Jha said, and there will initially be 10 million doses available. If the FDA gives emergency use authorization for the vaccines, the government will begin shipping doses to thousands of sites across the country.
“The good news is we have plenty of supply of Pfizer and Moderna vaccines,” Dr. Jha said. “We’ve asked states to distribute to their highest priority sites, serving the highest risk and hardest to reach areas.”
Pfizer’s clinical trials found that three doses of the vaccine for children 6 months to under 5 years were safe and effective and proved to be 80% effective against Omicron.
The FDA announced its meeting information with a conversation about the Moderna vaccine for ages 6-17 scheduled for June 14 and a conversation about the Pfizer and Moderna vaccines for young children scheduled for June 15.
Moderna applied for FDA authorization of its two-dose vaccine for children under age 6 on April 28. The company said the vaccine was 51% effective against infections with symptoms for children ages 6 months to 2 years and 37% effective for ages 2-5.
Pfizer’s 3-microgram dose is one-tenth of its adult dose. Moderna’s 25-microgram dose is one-quarter of its adult dose.
A version of this article first appeared on Medscape.com.
The FDA has accepted Pfizer’s application for a COVID-19 vaccine for children under age 5, which clears the way for approval and distribution in June.
Pfizer announced June 1 that it completed the application for a three-dose vaccine for kids between 6 months and 5 years old, and the FDA said it received the emergency use application.
Children in this age group – the last to be eligible for COVID-19 vaccines – could begin getting shots as early as June 21, according to White House COVID-19 response coordinator Ashish Jha, MD.
Meanwhile, COVID-19 cases are still high – an average of 100,000 cases a day – but death numbers are about 90% lower than they were when President Joe Biden first took office, Dr. Jha said.
The FDA’s advisory group, the Vaccines and Related Biological Products Advisory Committee, is scheduled to meet June 14 and June 15 to discuss data submitted by both Pfizer and Moderna.
If the FDA gives them the green light, the CDC will then weigh in.
“We know that many, many parents are eager to vaccinate their youngest kids, and it’s important to do this right,” Dr. Jha said at a White House press briefing on June 2. “We expect that vaccinations will begin in earnest as early as June 21 and really roll on throughout that week.”
States can place their orders as early as June 3, Dr. Jha said, and there will initially be 10 million doses available. If the FDA gives emergency use authorization for the vaccines, the government will begin shipping doses to thousands of sites across the country.
“The good news is we have plenty of supply of Pfizer and Moderna vaccines,” Dr. Jha said. “We’ve asked states to distribute to their highest priority sites, serving the highest risk and hardest to reach areas.”
Pfizer’s clinical trials found that three doses of the vaccine for children 6 months to under 5 years were safe and effective and proved to be 80% effective against Omicron.
The FDA announced its meeting information with a conversation about the Moderna vaccine for ages 6-17 scheduled for June 14 and a conversation about the Pfizer and Moderna vaccines for young children scheduled for June 15.
Moderna applied for FDA authorization of its two-dose vaccine for children under age 6 on April 28. The company said the vaccine was 51% effective against infections with symptoms for children ages 6 months to 2 years and 37% effective for ages 2-5.
Pfizer’s 3-microgram dose is one-tenth of its adult dose. Moderna’s 25-microgram dose is one-quarter of its adult dose.
A version of this article first appeared on Medscape.com.
COVID-19 vaccines equally effective in patients on dialysis
Two doses of either the Pfizer-BioNTech COVID-19 vaccine or the Oxford AstraZeneca alternative provide equal and significant protection against severe disease in patients on hemodialysis who have contracted SARS-CoV-2 infection, results of a multicenter observational study indicate.
Following two doses of either vaccine, the risk of hospital admission was 75% lower among vaccinated patients while the risk of death was 88% lower, compared with those who remained unvaccinated.
No difference was seen between the two vaccine types in terms of outcome severity, and there was no loss of protection in patients over the age of 65 or with increasing time since vaccination, the authors add. The need for oxygen and ventilation was also halved among those who had received two shots, compared with those who had not.
“The coronavirus disease 2019 (COVID-19) pandemic has had a devastating effect on the CKD (chronic kidney disease) community, particularly for individuals receiving maintenance dialysis,” Matthew Oliver, MD, University of Toronto, and Peter Blake, MD, Western University, London, Ont., write in an editorial published with the study.
“Overall, [this and other studies] show that COVID-19 vaccination in the maintenance dialysis population provides moderate protection against acquiring SARS-CoV-2 infection but is highly protective against severe outcomes,” they conclude.
The study was published in the June issue of the Clinical Journal of the American Society of Nephrology.
Severe outcomes observed less in patients who tested positive
The cohort included 1,323 patients on hemodialysis who tested positive on PCR testing to SARS-CoV-2 during a surveillance interval between December 2020 and September 2021, report, Damien Ashby, MD, Hammersmith Hospital, London, and colleagues report.
Among those who tested positive, 79% had not been vaccinated, 7% tested positive after their first dose of either vaccine, and 14% tested positive at least 10 days beyond their second dose.
The course of illness was mild in 61% of patients in that they did not require hospital admission, investigators note. Oxygen support was required by 29% of those who tested positive, and 13% died before 28 days, they added. Among those who died within 28 days of testing positive, 90% of the deaths were deemed to be caused by the virus itself.
“Compared with unvaccinated patients, severe COVID-19 outcomes were observed less than half as often in patients testing positive for SARS-Co-V-2 at least 10 days after the second dose,” Dr. Ashby and colleagues emphasize.
“And the protection from severe illness associated with vaccination was most obvious in patients over 65 years, in whom severe COVID-19 outcomes were reduced at least as much after vaccination as in their younger peers,” they add. Following vaccination with the Pfizer-BioNTech vaccine, antibody levels in patients on dialysis were comparable with those of healthy controls.
In contrast, this was not the case for the Oxford AstraZeneca vaccine where neutralizing titers in patients who received the vaccine were less effective against most variants. Despite its ability to produce comparable immunogenicity, the Oxford AstraZeneca vaccine was clearly associated with clinical protection against severe illness, the authors stress.
They also note that their results are relevant to vaccine uptake in the dialysis population where vaccine hesitancy remains a problem. “This study may, therefore, be useful in reducing vaccine hesitancy, which has resulted in low uptake in some countries (for example, Australia, where almost a quarter of patients on dialysis declined),” Dr. Ashby and colleagues point out.
Although significant vulnerability in the dialysis population remains, “this population has much to gain from vaccination, regardless of age or vaccine type,” the authors underscore.
CKD community quick to prioritize vaccine
As the editorialists point out, leaders in the CKD community were quick – and successful – in prioritizing vaccination in the dialysis population right from the beginning of the pandemic. For example, in Ontario, 90% of the maintenance dialysis population had received two doses of a COVID-19 vaccine by September 2021 and 78% had received three doses by January 2022.
Moreover, in Ontario, “our group found that two doses of mRNA vaccine reduced the risk of infection by 69%,” Dr. Oliver and Dr. Blake point out. U.S. researchers also found that the Pfizer mRNA vaccine reduced infection risk from COVID-19 by 79% while the Moderna mRNA vaccine reduced that risk by 73%. Vaccine effectiveness (VE) in the real-world setting indicates that COVID-19 vaccines provide moderate protection against being infected with the SARS-Co-V-2 virus, as the editorialists note.
However, “the VE for preventing severe outcomes is clinically more important for patients on dialysis because their risk of [morbid] events is high,” Dr. Oliver and Dr. Blake write. Indeed, their own study estimated that two doses of an mRNA vaccine reduced severe outcomes by 83%, “a greater benefit than for infection prevention,” they stress.
The editorialists caution that the SARS-CoV-2 virus continues to mutate and serology studies do show that vaccine-induced immunity does wane over time. Thus, while the COVID-19 pandemic is ever-changing, “we should conduct [VE] studies rigorously and expeditiously to bolster the case for prioritizing vaccination in the dialysis population,” Dr. Oliver and Dr. Blake recommend.
Need to increase vaccine acceptance
Commenting on the study, Uwe K.H. Korst from Bensheim, Germany, notes that COVID-19 is a daily reminder of how fragile life is for people with CKD. “Daily, the virus continues its horrific and unprecedented course through immunocompromised and immunosuppressed patients with kidney disease,” he writes.
Thus, Mr. Korst continues to call for additional education for health care professionals, patients, and the public to increase vaccine acceptance as well as more research to better understand the virus and its long-term consequences.
“Finally, patients need to express their needs, and physicians need to listen to patients’ voices,” Mr. Korst advises.
Dr. Oliver is a contracted medical lead of Ontario Renal Network and owner of Oliver Medical Management for which he holds patents and has received royalties. He has also reported receiving honoraria for speaking from Baxter Healthcare and participating in advisory boards for Amgen and Janssen. Dr. Blake has reported receiving honoraria from Baxter Global for speaking engagements and serves on the editorial board for the American Journal of Nephrology. Dr. Ashby and Dr. Korst have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Two doses of either the Pfizer-BioNTech COVID-19 vaccine or the Oxford AstraZeneca alternative provide equal and significant protection against severe disease in patients on hemodialysis who have contracted SARS-CoV-2 infection, results of a multicenter observational study indicate.
Following two doses of either vaccine, the risk of hospital admission was 75% lower among vaccinated patients while the risk of death was 88% lower, compared with those who remained unvaccinated.
No difference was seen between the two vaccine types in terms of outcome severity, and there was no loss of protection in patients over the age of 65 or with increasing time since vaccination, the authors add. The need for oxygen and ventilation was also halved among those who had received two shots, compared with those who had not.
“The coronavirus disease 2019 (COVID-19) pandemic has had a devastating effect on the CKD (chronic kidney disease) community, particularly for individuals receiving maintenance dialysis,” Matthew Oliver, MD, University of Toronto, and Peter Blake, MD, Western University, London, Ont., write in an editorial published with the study.
“Overall, [this and other studies] show that COVID-19 vaccination in the maintenance dialysis population provides moderate protection against acquiring SARS-CoV-2 infection but is highly protective against severe outcomes,” they conclude.
The study was published in the June issue of the Clinical Journal of the American Society of Nephrology.
Severe outcomes observed less in patients who tested positive
The cohort included 1,323 patients on hemodialysis who tested positive on PCR testing to SARS-CoV-2 during a surveillance interval between December 2020 and September 2021, report, Damien Ashby, MD, Hammersmith Hospital, London, and colleagues report.
Among those who tested positive, 79% had not been vaccinated, 7% tested positive after their first dose of either vaccine, and 14% tested positive at least 10 days beyond their second dose.
The course of illness was mild in 61% of patients in that they did not require hospital admission, investigators note. Oxygen support was required by 29% of those who tested positive, and 13% died before 28 days, they added. Among those who died within 28 days of testing positive, 90% of the deaths were deemed to be caused by the virus itself.
“Compared with unvaccinated patients, severe COVID-19 outcomes were observed less than half as often in patients testing positive for SARS-Co-V-2 at least 10 days after the second dose,” Dr. Ashby and colleagues emphasize.
“And the protection from severe illness associated with vaccination was most obvious in patients over 65 years, in whom severe COVID-19 outcomes were reduced at least as much after vaccination as in their younger peers,” they add. Following vaccination with the Pfizer-BioNTech vaccine, antibody levels in patients on dialysis were comparable with those of healthy controls.
In contrast, this was not the case for the Oxford AstraZeneca vaccine where neutralizing titers in patients who received the vaccine were less effective against most variants. Despite its ability to produce comparable immunogenicity, the Oxford AstraZeneca vaccine was clearly associated with clinical protection against severe illness, the authors stress.
They also note that their results are relevant to vaccine uptake in the dialysis population where vaccine hesitancy remains a problem. “This study may, therefore, be useful in reducing vaccine hesitancy, which has resulted in low uptake in some countries (for example, Australia, where almost a quarter of patients on dialysis declined),” Dr. Ashby and colleagues point out.
Although significant vulnerability in the dialysis population remains, “this population has much to gain from vaccination, regardless of age or vaccine type,” the authors underscore.
CKD community quick to prioritize vaccine
As the editorialists point out, leaders in the CKD community were quick – and successful – in prioritizing vaccination in the dialysis population right from the beginning of the pandemic. For example, in Ontario, 90% of the maintenance dialysis population had received two doses of a COVID-19 vaccine by September 2021 and 78% had received three doses by January 2022.
Moreover, in Ontario, “our group found that two doses of mRNA vaccine reduced the risk of infection by 69%,” Dr. Oliver and Dr. Blake point out. U.S. researchers also found that the Pfizer mRNA vaccine reduced infection risk from COVID-19 by 79% while the Moderna mRNA vaccine reduced that risk by 73%. Vaccine effectiveness (VE) in the real-world setting indicates that COVID-19 vaccines provide moderate protection against being infected with the SARS-Co-V-2 virus, as the editorialists note.
However, “the VE for preventing severe outcomes is clinically more important for patients on dialysis because their risk of [morbid] events is high,” Dr. Oliver and Dr. Blake write. Indeed, their own study estimated that two doses of an mRNA vaccine reduced severe outcomes by 83%, “a greater benefit than for infection prevention,” they stress.
The editorialists caution that the SARS-CoV-2 virus continues to mutate and serology studies do show that vaccine-induced immunity does wane over time. Thus, while the COVID-19 pandemic is ever-changing, “we should conduct [VE] studies rigorously and expeditiously to bolster the case for prioritizing vaccination in the dialysis population,” Dr. Oliver and Dr. Blake recommend.
Need to increase vaccine acceptance
Commenting on the study, Uwe K.H. Korst from Bensheim, Germany, notes that COVID-19 is a daily reminder of how fragile life is for people with CKD. “Daily, the virus continues its horrific and unprecedented course through immunocompromised and immunosuppressed patients with kidney disease,” he writes.
Thus, Mr. Korst continues to call for additional education for health care professionals, patients, and the public to increase vaccine acceptance as well as more research to better understand the virus and its long-term consequences.
“Finally, patients need to express their needs, and physicians need to listen to patients’ voices,” Mr. Korst advises.
Dr. Oliver is a contracted medical lead of Ontario Renal Network and owner of Oliver Medical Management for which he holds patents and has received royalties. He has also reported receiving honoraria for speaking from Baxter Healthcare and participating in advisory boards for Amgen and Janssen. Dr. Blake has reported receiving honoraria from Baxter Global for speaking engagements and serves on the editorial board for the American Journal of Nephrology. Dr. Ashby and Dr. Korst have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Two doses of either the Pfizer-BioNTech COVID-19 vaccine or the Oxford AstraZeneca alternative provide equal and significant protection against severe disease in patients on hemodialysis who have contracted SARS-CoV-2 infection, results of a multicenter observational study indicate.
Following two doses of either vaccine, the risk of hospital admission was 75% lower among vaccinated patients while the risk of death was 88% lower, compared with those who remained unvaccinated.
No difference was seen between the two vaccine types in terms of outcome severity, and there was no loss of protection in patients over the age of 65 or with increasing time since vaccination, the authors add. The need for oxygen and ventilation was also halved among those who had received two shots, compared with those who had not.
“The coronavirus disease 2019 (COVID-19) pandemic has had a devastating effect on the CKD (chronic kidney disease) community, particularly for individuals receiving maintenance dialysis,” Matthew Oliver, MD, University of Toronto, and Peter Blake, MD, Western University, London, Ont., write in an editorial published with the study.
“Overall, [this and other studies] show that COVID-19 vaccination in the maintenance dialysis population provides moderate protection against acquiring SARS-CoV-2 infection but is highly protective against severe outcomes,” they conclude.
The study was published in the June issue of the Clinical Journal of the American Society of Nephrology.
Severe outcomes observed less in patients who tested positive
The cohort included 1,323 patients on hemodialysis who tested positive on PCR testing to SARS-CoV-2 during a surveillance interval between December 2020 and September 2021, report, Damien Ashby, MD, Hammersmith Hospital, London, and colleagues report.
Among those who tested positive, 79% had not been vaccinated, 7% tested positive after their first dose of either vaccine, and 14% tested positive at least 10 days beyond their second dose.
The course of illness was mild in 61% of patients in that they did not require hospital admission, investigators note. Oxygen support was required by 29% of those who tested positive, and 13% died before 28 days, they added. Among those who died within 28 days of testing positive, 90% of the deaths were deemed to be caused by the virus itself.
“Compared with unvaccinated patients, severe COVID-19 outcomes were observed less than half as often in patients testing positive for SARS-Co-V-2 at least 10 days after the second dose,” Dr. Ashby and colleagues emphasize.
“And the protection from severe illness associated with vaccination was most obvious in patients over 65 years, in whom severe COVID-19 outcomes were reduced at least as much after vaccination as in their younger peers,” they add. Following vaccination with the Pfizer-BioNTech vaccine, antibody levels in patients on dialysis were comparable with those of healthy controls.
In contrast, this was not the case for the Oxford AstraZeneca vaccine where neutralizing titers in patients who received the vaccine were less effective against most variants. Despite its ability to produce comparable immunogenicity, the Oxford AstraZeneca vaccine was clearly associated with clinical protection against severe illness, the authors stress.
They also note that their results are relevant to vaccine uptake in the dialysis population where vaccine hesitancy remains a problem. “This study may, therefore, be useful in reducing vaccine hesitancy, which has resulted in low uptake in some countries (for example, Australia, where almost a quarter of patients on dialysis declined),” Dr. Ashby and colleagues point out.
Although significant vulnerability in the dialysis population remains, “this population has much to gain from vaccination, regardless of age or vaccine type,” the authors underscore.
CKD community quick to prioritize vaccine
As the editorialists point out, leaders in the CKD community were quick – and successful – in prioritizing vaccination in the dialysis population right from the beginning of the pandemic. For example, in Ontario, 90% of the maintenance dialysis population had received two doses of a COVID-19 vaccine by September 2021 and 78% had received three doses by January 2022.
Moreover, in Ontario, “our group found that two doses of mRNA vaccine reduced the risk of infection by 69%,” Dr. Oliver and Dr. Blake point out. U.S. researchers also found that the Pfizer mRNA vaccine reduced infection risk from COVID-19 by 79% while the Moderna mRNA vaccine reduced that risk by 73%. Vaccine effectiveness (VE) in the real-world setting indicates that COVID-19 vaccines provide moderate protection against being infected with the SARS-Co-V-2 virus, as the editorialists note.
However, “the VE for preventing severe outcomes is clinically more important for patients on dialysis because their risk of [morbid] events is high,” Dr. Oliver and Dr. Blake write. Indeed, their own study estimated that two doses of an mRNA vaccine reduced severe outcomes by 83%, “a greater benefit than for infection prevention,” they stress.
The editorialists caution that the SARS-CoV-2 virus continues to mutate and serology studies do show that vaccine-induced immunity does wane over time. Thus, while the COVID-19 pandemic is ever-changing, “we should conduct [VE] studies rigorously and expeditiously to bolster the case for prioritizing vaccination in the dialysis population,” Dr. Oliver and Dr. Blake recommend.
Need to increase vaccine acceptance
Commenting on the study, Uwe K.H. Korst from Bensheim, Germany, notes that COVID-19 is a daily reminder of how fragile life is for people with CKD. “Daily, the virus continues its horrific and unprecedented course through immunocompromised and immunosuppressed patients with kidney disease,” he writes.
Thus, Mr. Korst continues to call for additional education for health care professionals, patients, and the public to increase vaccine acceptance as well as more research to better understand the virus and its long-term consequences.
“Finally, patients need to express their needs, and physicians need to listen to patients’ voices,” Mr. Korst advises.
Dr. Oliver is a contracted medical lead of Ontario Renal Network and owner of Oliver Medical Management for which he holds patents and has received royalties. He has also reported receiving honoraria for speaking from Baxter Healthcare and participating in advisory boards for Amgen and Janssen. Dr. Blake has reported receiving honoraria from Baxter Global for speaking engagements and serves on the editorial board for the American Journal of Nephrology. Dr. Ashby and Dr. Korst have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The latest on COVID-19 and the heart in children
The 2022 Pediatric Academic Societies meeting included an excellent session on the acute and delayed effects of COVID-19 on children’s hearts. Data on the risk for cardiac injury during acute COVID-19, return-to-play guidelines after COVID-19–related heart injury, and post–vaccine-associated myocarditis were reviewed.
COVID-induced cardiac injury
The risk for COVID-induced cardiac injury is directly associated with age. Recent Centers for Disease Control and Prevention data revealed a “myocarditis or pericarditis” rate in the range of 12-17 cases per 100,000 SARS-CoV-2 infections among male children aged 5-11 years (lower rates for females); the rate jumps to 50-65 cases per 100,000 infections among male children aged 12-17 years. So cardiac injury caused by acute COVID-19 appears rare, but the risk is clearly associated with male sex and adolescent age.
Return to play after COVID-19
Clinicians may be pressed by patients and parents for advice on return to play after illness with COVID-19. In July 2020, the American College of Cardiology published an algorithm that has been adjusted over time, most recently in 2022 by the American Academy of Pediatrics. These algorithms stratify recommendations by degree of illness. One rule of thumb: Patients with severe COVID-19 (ICU care or multisystem inflammatory syndrome in children [MIS-C]) have only one box on the algorithm, and that is to rest for 3-6 months and only return to usual activity after cardiac clearance. Moderate disease (defined as ≥ 4 days of fever > 100.4 °F; ≥ 1 week of myalgia, chills, lethargy, or any non-ICU hospital stay; and no evidence of MIS-C) require undergoing an ECG to look for cardiac dysfunction, followed by at least 10 days of rest if the ECG is negative or referral for cardiac evaluation if either ECG or exam by a pediatric cardiologist is abnormal.
Clinicians can perhaps be more permissible with patients who are younger or who have had less severe disease. For example, if a patient aged younger than 12 years is asymptomatic with routine activity at the time of evaluation, an ECG is not indicated. For patients aged 12-15 years who are asymptomatic at the time of evaluation but participate in a high-intensity sport, clinicians might consider obtaining an ECG. As few as 3 days of rest might be enough for select patients who are asymptomatic at presentation. For other patients, clinicians should work with parents to introduce activity gradually and make it clear to parents that any activity intolerance requires quick reevaluation. On existing athlete registries, no deaths that are attributable to post–COVID-19 cardiac effects have been confirmed in children; however, all data presented during the session were from prior to the Omicron variant surge in early 2022, so more information may be forthcoming.
Considerations for MIS-C
Among children experiencing MIS-C, 35% had ECG changes, 40% exhibited left ventricular systolic or diastolic dysfunction, and 30% had mitral regurgitation, meaning that a large percentage of patients with MIS-C show some degree of cardiac dysfunction. Unfortunately, we are still in the data-gathering phase for long-term outcomes. Functional parameters tend to improve within a week, and most patients will return to normal cardiac function by 3-4 months.
Return to play after MIS-C is quite different from that for acute COVID-19. Patients with MIS-C should be treated much like other patients with myocarditis with an expected return to play in 3-6 months and only after cardiac follow-up. Another good-to-remember recommendation is to delay COVID-19 vaccination for at least 90 days after an episode of MIS-C.
Vaccine-related myocarditis
Once again, older age appears to be a risk factor because most patients with postvaccine myocarditis have been in their mid-teens to early 20s, with events more likely after the second vaccine dose and also more likely in male children (4:1 ratio to female children). No deaths have occurred from postvaccination myocarditis in patients younger than 30 years. Still, many individuals have exhibited residual MRI enhancement in the cardiac tissue for some time after experiencing postvaccination myocarditis; it’s currently unclear whether that has clinical implications. By comparison, CDC data demonstrates convincingly that the risk for cardiac effects is much greater after acute COVID-19 than after COVID-19 vaccination, with risk ratios often higher than 20, depending on age and condition (for example, myocarditis vs. pericarditis). Data are still insufficient to determine whether clinicians should recommend or avoid COVID-19 vaccination in children with congenital heart disease.
In summary, administering COVID-19 vaccines requires a great deal of shared decision-making with parents, and the clinician’s role is to educate parents about all potential risks related to both the vaccine and COVID-19 illness. Research has consistently shown that acute COVID-19 myocarditis and myocarditis associated with MIS-C are much more likely to occur in unvaccinated youth and more likely than postvaccination myocarditis, regardless of age.
William T. Basco, Jr., MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
The 2022 Pediatric Academic Societies meeting included an excellent session on the acute and delayed effects of COVID-19 on children’s hearts. Data on the risk for cardiac injury during acute COVID-19, return-to-play guidelines after COVID-19–related heart injury, and post–vaccine-associated myocarditis were reviewed.
COVID-induced cardiac injury
The risk for COVID-induced cardiac injury is directly associated with age. Recent Centers for Disease Control and Prevention data revealed a “myocarditis or pericarditis” rate in the range of 12-17 cases per 100,000 SARS-CoV-2 infections among male children aged 5-11 years (lower rates for females); the rate jumps to 50-65 cases per 100,000 infections among male children aged 12-17 years. So cardiac injury caused by acute COVID-19 appears rare, but the risk is clearly associated with male sex and adolescent age.
Return to play after COVID-19
Clinicians may be pressed by patients and parents for advice on return to play after illness with COVID-19. In July 2020, the American College of Cardiology published an algorithm that has been adjusted over time, most recently in 2022 by the American Academy of Pediatrics. These algorithms stratify recommendations by degree of illness. One rule of thumb: Patients with severe COVID-19 (ICU care or multisystem inflammatory syndrome in children [MIS-C]) have only one box on the algorithm, and that is to rest for 3-6 months and only return to usual activity after cardiac clearance. Moderate disease (defined as ≥ 4 days of fever > 100.4 °F; ≥ 1 week of myalgia, chills, lethargy, or any non-ICU hospital stay; and no evidence of MIS-C) require undergoing an ECG to look for cardiac dysfunction, followed by at least 10 days of rest if the ECG is negative or referral for cardiac evaluation if either ECG or exam by a pediatric cardiologist is abnormal.
Clinicians can perhaps be more permissible with patients who are younger or who have had less severe disease. For example, if a patient aged younger than 12 years is asymptomatic with routine activity at the time of evaluation, an ECG is not indicated. For patients aged 12-15 years who are asymptomatic at the time of evaluation but participate in a high-intensity sport, clinicians might consider obtaining an ECG. As few as 3 days of rest might be enough for select patients who are asymptomatic at presentation. For other patients, clinicians should work with parents to introduce activity gradually and make it clear to parents that any activity intolerance requires quick reevaluation. On existing athlete registries, no deaths that are attributable to post–COVID-19 cardiac effects have been confirmed in children; however, all data presented during the session were from prior to the Omicron variant surge in early 2022, so more information may be forthcoming.
Considerations for MIS-C
Among children experiencing MIS-C, 35% had ECG changes, 40% exhibited left ventricular systolic or diastolic dysfunction, and 30% had mitral regurgitation, meaning that a large percentage of patients with MIS-C show some degree of cardiac dysfunction. Unfortunately, we are still in the data-gathering phase for long-term outcomes. Functional parameters tend to improve within a week, and most patients will return to normal cardiac function by 3-4 months.
Return to play after MIS-C is quite different from that for acute COVID-19. Patients with MIS-C should be treated much like other patients with myocarditis with an expected return to play in 3-6 months and only after cardiac follow-up. Another good-to-remember recommendation is to delay COVID-19 vaccination for at least 90 days after an episode of MIS-C.
Vaccine-related myocarditis
Once again, older age appears to be a risk factor because most patients with postvaccine myocarditis have been in their mid-teens to early 20s, with events more likely after the second vaccine dose and also more likely in male children (4:1 ratio to female children). No deaths have occurred from postvaccination myocarditis in patients younger than 30 years. Still, many individuals have exhibited residual MRI enhancement in the cardiac tissue for some time after experiencing postvaccination myocarditis; it’s currently unclear whether that has clinical implications. By comparison, CDC data demonstrates convincingly that the risk for cardiac effects is much greater after acute COVID-19 than after COVID-19 vaccination, with risk ratios often higher than 20, depending on age and condition (for example, myocarditis vs. pericarditis). Data are still insufficient to determine whether clinicians should recommend or avoid COVID-19 vaccination in children with congenital heart disease.
In summary, administering COVID-19 vaccines requires a great deal of shared decision-making with parents, and the clinician’s role is to educate parents about all potential risks related to both the vaccine and COVID-19 illness. Research has consistently shown that acute COVID-19 myocarditis and myocarditis associated with MIS-C are much more likely to occur in unvaccinated youth and more likely than postvaccination myocarditis, regardless of age.
William T. Basco, Jr., MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
The 2022 Pediatric Academic Societies meeting included an excellent session on the acute and delayed effects of COVID-19 on children’s hearts. Data on the risk for cardiac injury during acute COVID-19, return-to-play guidelines after COVID-19–related heart injury, and post–vaccine-associated myocarditis were reviewed.
COVID-induced cardiac injury
The risk for COVID-induced cardiac injury is directly associated with age. Recent Centers for Disease Control and Prevention data revealed a “myocarditis or pericarditis” rate in the range of 12-17 cases per 100,000 SARS-CoV-2 infections among male children aged 5-11 years (lower rates for females); the rate jumps to 50-65 cases per 100,000 infections among male children aged 12-17 years. So cardiac injury caused by acute COVID-19 appears rare, but the risk is clearly associated with male sex and adolescent age.
Return to play after COVID-19
Clinicians may be pressed by patients and parents for advice on return to play after illness with COVID-19. In July 2020, the American College of Cardiology published an algorithm that has been adjusted over time, most recently in 2022 by the American Academy of Pediatrics. These algorithms stratify recommendations by degree of illness. One rule of thumb: Patients with severe COVID-19 (ICU care or multisystem inflammatory syndrome in children [MIS-C]) have only one box on the algorithm, and that is to rest for 3-6 months and only return to usual activity after cardiac clearance. Moderate disease (defined as ≥ 4 days of fever > 100.4 °F; ≥ 1 week of myalgia, chills, lethargy, or any non-ICU hospital stay; and no evidence of MIS-C) require undergoing an ECG to look for cardiac dysfunction, followed by at least 10 days of rest if the ECG is negative or referral for cardiac evaluation if either ECG or exam by a pediatric cardiologist is abnormal.
Clinicians can perhaps be more permissible with patients who are younger or who have had less severe disease. For example, if a patient aged younger than 12 years is asymptomatic with routine activity at the time of evaluation, an ECG is not indicated. For patients aged 12-15 years who are asymptomatic at the time of evaluation but participate in a high-intensity sport, clinicians might consider obtaining an ECG. As few as 3 days of rest might be enough for select patients who are asymptomatic at presentation. For other patients, clinicians should work with parents to introduce activity gradually and make it clear to parents that any activity intolerance requires quick reevaluation. On existing athlete registries, no deaths that are attributable to post–COVID-19 cardiac effects have been confirmed in children; however, all data presented during the session were from prior to the Omicron variant surge in early 2022, so more information may be forthcoming.
Considerations for MIS-C
Among children experiencing MIS-C, 35% had ECG changes, 40% exhibited left ventricular systolic or diastolic dysfunction, and 30% had mitral regurgitation, meaning that a large percentage of patients with MIS-C show some degree of cardiac dysfunction. Unfortunately, we are still in the data-gathering phase for long-term outcomes. Functional parameters tend to improve within a week, and most patients will return to normal cardiac function by 3-4 months.
Return to play after MIS-C is quite different from that for acute COVID-19. Patients with MIS-C should be treated much like other patients with myocarditis with an expected return to play in 3-6 months and only after cardiac follow-up. Another good-to-remember recommendation is to delay COVID-19 vaccination for at least 90 days after an episode of MIS-C.
Vaccine-related myocarditis
Once again, older age appears to be a risk factor because most patients with postvaccine myocarditis have been in their mid-teens to early 20s, with events more likely after the second vaccine dose and also more likely in male children (4:1 ratio to female children). No deaths have occurred from postvaccination myocarditis in patients younger than 30 years. Still, many individuals have exhibited residual MRI enhancement in the cardiac tissue for some time after experiencing postvaccination myocarditis; it’s currently unclear whether that has clinical implications. By comparison, CDC data demonstrates convincingly that the risk for cardiac effects is much greater after acute COVID-19 than after COVID-19 vaccination, with risk ratios often higher than 20, depending on age and condition (for example, myocarditis vs. pericarditis). Data are still insufficient to determine whether clinicians should recommend or avoid COVID-19 vaccination in children with congenital heart disease.
In summary, administering COVID-19 vaccines requires a great deal of shared decision-making with parents, and the clinician’s role is to educate parents about all potential risks related to both the vaccine and COVID-19 illness. Research has consistently shown that acute COVID-19 myocarditis and myocarditis associated with MIS-C are much more likely to occur in unvaccinated youth and more likely than postvaccination myocarditis, regardless of age.
William T. Basco, Jr., MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
Meet the JCOM Author with Dr. Barkoudah: IVIG in Treating Nonventilated COVID-19 Patients With Moderate-to-Severe Hypoxia
Children & COVID: Rise in new cases slows
New cases of COVID-19 in children climbed for the seventh consecutive week, but the latest increase was the smallest of the seven, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Since the weekly total bottomed out at just under 26,000 in early April, the new-case count has risen by 28.0%, 11.8%, 43.5%, 17.4%, 50%, 14.6%, and 5.0%, based on data from the AAP/CHA weekly COVID-19 report.
The cumulative number of pediatric cases is almost 13.4 million since the pandemic began, and those infected children represent 18.9% of all cases, the AAP and CHA said based on data from 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
That 18.9% is noteworthy because it marks the first decline in that particular measure since the AAP and CHA started keeping track in April of 2020. Children’s share of the overall COVID burden had been holding at 19.0% for 14 straight weeks, the AAP/CHA data show.
Regionally, new cases were up in the South and the West, where recent rising trends continued, and down in the Midwest and Northeast, where the recent rising trends were reversed for the first time. At the state/territory level, Puerto Rico had the largest percent increase over the last 2 weeks, followed by Maryland and Delaware, the organizations noted in their joint report.
Hospital admissions in children aged 0-17 have changed little in the last week, with the Centers for Disease Control and Prevention reporting rates of 0.25 per 100,000 population on May 23 and 0.25 per 100,000 on May 29, the latest date available. There was, however, a move up to 0.26 per 100,000 from May 24 to May 28, and the CDC acknowledges a possible reporting delay over the most recent 7-day period.
Emergency department visits have dipped slightly in recent days, with children aged 0-11 years at a 7-day average of 2.0% of ED visits with diagnosed COVID on May 28, down from a 5-day stretch at 2.2% from May 19 to May 23. Children aged 12-15 years were at 1.8% on May 28, compared with 2.0% on May 23-24, and 15- to 17-year-olds were at 2.0% on May 28, down from the 2.1% reached over the previous 2 days, the CDC reported on its COVID Data Tracker.
New cases of COVID-19 in children climbed for the seventh consecutive week, but the latest increase was the smallest of the seven, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Since the weekly total bottomed out at just under 26,000 in early April, the new-case count has risen by 28.0%, 11.8%, 43.5%, 17.4%, 50%, 14.6%, and 5.0%, based on data from the AAP/CHA weekly COVID-19 report.
The cumulative number of pediatric cases is almost 13.4 million since the pandemic began, and those infected children represent 18.9% of all cases, the AAP and CHA said based on data from 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
That 18.9% is noteworthy because it marks the first decline in that particular measure since the AAP and CHA started keeping track in April of 2020. Children’s share of the overall COVID burden had been holding at 19.0% for 14 straight weeks, the AAP/CHA data show.
Regionally, new cases were up in the South and the West, where recent rising trends continued, and down in the Midwest and Northeast, where the recent rising trends were reversed for the first time. At the state/territory level, Puerto Rico had the largest percent increase over the last 2 weeks, followed by Maryland and Delaware, the organizations noted in their joint report.
Hospital admissions in children aged 0-17 have changed little in the last week, with the Centers for Disease Control and Prevention reporting rates of 0.25 per 100,000 population on May 23 and 0.25 per 100,000 on May 29, the latest date available. There was, however, a move up to 0.26 per 100,000 from May 24 to May 28, and the CDC acknowledges a possible reporting delay over the most recent 7-day period.
Emergency department visits have dipped slightly in recent days, with children aged 0-11 years at a 7-day average of 2.0% of ED visits with diagnosed COVID on May 28, down from a 5-day stretch at 2.2% from May 19 to May 23. Children aged 12-15 years were at 1.8% on May 28, compared with 2.0% on May 23-24, and 15- to 17-year-olds were at 2.0% on May 28, down from the 2.1% reached over the previous 2 days, the CDC reported on its COVID Data Tracker.
New cases of COVID-19 in children climbed for the seventh consecutive week, but the latest increase was the smallest of the seven, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Since the weekly total bottomed out at just under 26,000 in early April, the new-case count has risen by 28.0%, 11.8%, 43.5%, 17.4%, 50%, 14.6%, and 5.0%, based on data from the AAP/CHA weekly COVID-19 report.
The cumulative number of pediatric cases is almost 13.4 million since the pandemic began, and those infected children represent 18.9% of all cases, the AAP and CHA said based on data from 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
That 18.9% is noteworthy because it marks the first decline in that particular measure since the AAP and CHA started keeping track in April of 2020. Children’s share of the overall COVID burden had been holding at 19.0% for 14 straight weeks, the AAP/CHA data show.
Regionally, new cases were up in the South and the West, where recent rising trends continued, and down in the Midwest and Northeast, where the recent rising trends were reversed for the first time. At the state/territory level, Puerto Rico had the largest percent increase over the last 2 weeks, followed by Maryland and Delaware, the organizations noted in their joint report.
Hospital admissions in children aged 0-17 have changed little in the last week, with the Centers for Disease Control and Prevention reporting rates of 0.25 per 100,000 population on May 23 and 0.25 per 100,000 on May 29, the latest date available. There was, however, a move up to 0.26 per 100,000 from May 24 to May 28, and the CDC acknowledges a possible reporting delay over the most recent 7-day period.
Emergency department visits have dipped slightly in recent days, with children aged 0-11 years at a 7-day average of 2.0% of ED visits with diagnosed COVID on May 28, down from a 5-day stretch at 2.2% from May 19 to May 23. Children aged 12-15 years were at 1.8% on May 28, compared with 2.0% on May 23-24, and 15- to 17-year-olds were at 2.0% on May 28, down from the 2.1% reached over the previous 2 days, the CDC reported on its COVID Data Tracker.
Intravenous Immunoglobulin in Treating Nonventilated COVID-19 Patients With Moderate-to-Severe Hypoxia: A Pharmacoeconomic Analysis
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
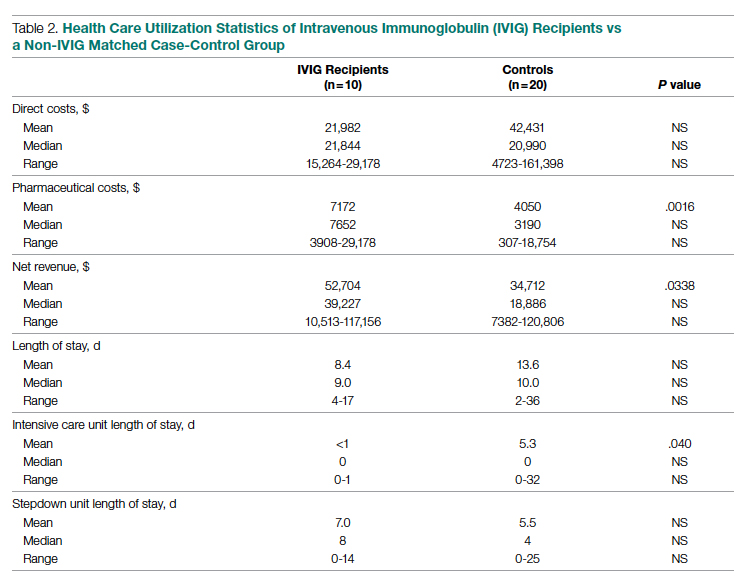
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
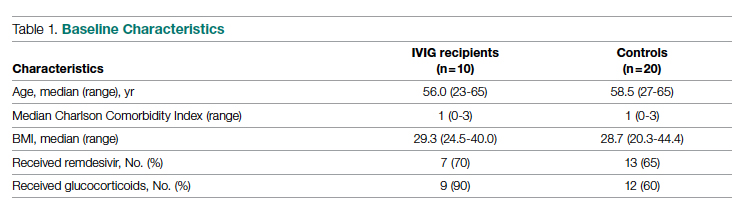
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board ([email protected]), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.
Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).
LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).
Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; [email protected]
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board ([email protected]), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.
Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).
LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).
Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; [email protected]
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board ([email protected]), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.
Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).
LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).
Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; [email protected]
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
Researchers find a pathway to prevent COVID infection
What’s more, they have succeeded in closing the lock to block the virus and prevent it from interacting with the cell, thereby preventing infection.
UCLouvain emphasized that this discovery, which was published in Nature Communications, is sparking hope that an aerosol antiviral therapy can be developed that would eradicate the virus in the case of an infection or a high-risk contact.
For 2 years, the team under David Alsteens, PhD, a researcher at the UCLouvain Institute of Biomolecular Science and Technology, has been working hard to understand the precise molecular mechanisms the virus uses to infect a cell. They investigated the interaction between sialic acids, a kind of sugar residue present on the surface of cells, and the SARS-CoV-2 spike (S) protein to clarify its role in the infection process.
It was already known that the function of the sugar residues that coat the cells is to promote cell recognition, thus enabling, in particular, viruses to identify their targets more easily, but also to provide them with a point of attachment and to facilitate infection of the cells.
The researchers have now revealed a variant of these sugars that interacts more strongly with the S protein than other sugars do.
In other words, the university explained, they found the set of keys that allows the virus to open the cell door. So, the researchers decided to catch the virus in its own trap, by preventing it from attaching to its host cell. To do this, they blocked the S protein’s points of attachment, thus suppressing any interaction with the cell surface, as if a padlock had been placed on the lock on the cell’s entry door.
Th researchers added that the advantage of this discovery is that it acts on the virus, irrespective of mutations.
The team of researchers will now conduct tests on mice to apply this blocking of virus binding sites and observe whether it works on the body. The results should make it possible to develop an antiviral therapy administered by aerosol in the case of infection or at-risk contact.
This discovery is also of interest for the future to counter other viruses with similar attachment factors.
This article was translated from MediQuality; a version appeared on Medscape.com.
What’s more, they have succeeded in closing the lock to block the virus and prevent it from interacting with the cell, thereby preventing infection.
UCLouvain emphasized that this discovery, which was published in Nature Communications, is sparking hope that an aerosol antiviral therapy can be developed that would eradicate the virus in the case of an infection or a high-risk contact.
For 2 years, the team under David Alsteens, PhD, a researcher at the UCLouvain Institute of Biomolecular Science and Technology, has been working hard to understand the precise molecular mechanisms the virus uses to infect a cell. They investigated the interaction between sialic acids, a kind of sugar residue present on the surface of cells, and the SARS-CoV-2 spike (S) protein to clarify its role in the infection process.
It was already known that the function of the sugar residues that coat the cells is to promote cell recognition, thus enabling, in particular, viruses to identify their targets more easily, but also to provide them with a point of attachment and to facilitate infection of the cells.
The researchers have now revealed a variant of these sugars that interacts more strongly with the S protein than other sugars do.
In other words, the university explained, they found the set of keys that allows the virus to open the cell door. So, the researchers decided to catch the virus in its own trap, by preventing it from attaching to its host cell. To do this, they blocked the S protein’s points of attachment, thus suppressing any interaction with the cell surface, as if a padlock had been placed on the lock on the cell’s entry door.
Th researchers added that the advantage of this discovery is that it acts on the virus, irrespective of mutations.
The team of researchers will now conduct tests on mice to apply this blocking of virus binding sites and observe whether it works on the body. The results should make it possible to develop an antiviral therapy administered by aerosol in the case of infection or at-risk contact.
This discovery is also of interest for the future to counter other viruses with similar attachment factors.
This article was translated from MediQuality; a version appeared on Medscape.com.
What’s more, they have succeeded in closing the lock to block the virus and prevent it from interacting with the cell, thereby preventing infection.
UCLouvain emphasized that this discovery, which was published in Nature Communications, is sparking hope that an aerosol antiviral therapy can be developed that would eradicate the virus in the case of an infection or a high-risk contact.
For 2 years, the team under David Alsteens, PhD, a researcher at the UCLouvain Institute of Biomolecular Science and Technology, has been working hard to understand the precise molecular mechanisms the virus uses to infect a cell. They investigated the interaction between sialic acids, a kind of sugar residue present on the surface of cells, and the SARS-CoV-2 spike (S) protein to clarify its role in the infection process.
It was already known that the function of the sugar residues that coat the cells is to promote cell recognition, thus enabling, in particular, viruses to identify their targets more easily, but also to provide them with a point of attachment and to facilitate infection of the cells.
The researchers have now revealed a variant of these sugars that interacts more strongly with the S protein than other sugars do.
In other words, the university explained, they found the set of keys that allows the virus to open the cell door. So, the researchers decided to catch the virus in its own trap, by preventing it from attaching to its host cell. To do this, they blocked the S protein’s points of attachment, thus suppressing any interaction with the cell surface, as if a padlock had been placed on the lock on the cell’s entry door.
Th researchers added that the advantage of this discovery is that it acts on the virus, irrespective of mutations.
The team of researchers will now conduct tests on mice to apply this blocking of virus binding sites and observe whether it works on the body. The results should make it possible to develop an antiviral therapy administered by aerosol in the case of infection or at-risk contact.
This discovery is also of interest for the future to counter other viruses with similar attachment factors.
This article was translated from MediQuality; a version appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
Births jump for first time since 2014
More than 3 million live births occurred in the United States in 2021, the largest increase in the nation’s birth rate since 2014, according to the U.S. Centers for Disease Control and Prevention.
Provisional data showed a 1% uptick in births, to 3.66 million, after 6 years of dropping by approximately 2% per year. The gains were concentrated among birthing people ages 25 and older. Teenage births, on the other hand, are at their lowest level since the 1990s, according to the CDC. The agency reported a record 6% decrease in births for teenagers aged 15 to 19 years between 2020 and 2021. Women ages 20 to 25 years also had a record decrease in births of 4% during that period.
Brady E. Hamilton, PhD, of the CDC’s National Center for Health Statistics, and the lead author of the new report, said the rise in births points to childbearing that was postponed during the pandemic. Data from 2021 showed a 4% drop in the nation’s birth rate between 2019 and 2020.
“The option to forgo birth is not always viable for older women, but you saw a lot of that during the pandemic,” Dr. Hamilton said. “Events happened related to job security and the economy that caused people to wait to have a child.”
Dr. Hamilton said more data are needed to determine the full impact of increased overall birth rates on individuals. The final report, which will be released in July, will delve deeper into the influence increased birth rates had on demographics and preterm births, which Dr. Hamilton and his team found have increased by 4%.
“For those beginning to have children, we see these trends, but it will be interesting to see what happens to younger women in the future,” Dr. Hamilton said. “Once we have the final data for 2021, we will be able to see a more detailed pattern emerge and draw conclusions from that.”
Dr. Hamilton has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than 3 million live births occurred in the United States in 2021, the largest increase in the nation’s birth rate since 2014, according to the U.S. Centers for Disease Control and Prevention.
Provisional data showed a 1% uptick in births, to 3.66 million, after 6 years of dropping by approximately 2% per year. The gains were concentrated among birthing people ages 25 and older. Teenage births, on the other hand, are at their lowest level since the 1990s, according to the CDC. The agency reported a record 6% decrease in births for teenagers aged 15 to 19 years between 2020 and 2021. Women ages 20 to 25 years also had a record decrease in births of 4% during that period.
Brady E. Hamilton, PhD, of the CDC’s National Center for Health Statistics, and the lead author of the new report, said the rise in births points to childbearing that was postponed during the pandemic. Data from 2021 showed a 4% drop in the nation’s birth rate between 2019 and 2020.
“The option to forgo birth is not always viable for older women, but you saw a lot of that during the pandemic,” Dr. Hamilton said. “Events happened related to job security and the economy that caused people to wait to have a child.”
Dr. Hamilton said more data are needed to determine the full impact of increased overall birth rates on individuals. The final report, which will be released in July, will delve deeper into the influence increased birth rates had on demographics and preterm births, which Dr. Hamilton and his team found have increased by 4%.
“For those beginning to have children, we see these trends, but it will be interesting to see what happens to younger women in the future,” Dr. Hamilton said. “Once we have the final data for 2021, we will be able to see a more detailed pattern emerge and draw conclusions from that.”
Dr. Hamilton has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than 3 million live births occurred in the United States in 2021, the largest increase in the nation’s birth rate since 2014, according to the U.S. Centers for Disease Control and Prevention.
Provisional data showed a 1% uptick in births, to 3.66 million, after 6 years of dropping by approximately 2% per year. The gains were concentrated among birthing people ages 25 and older. Teenage births, on the other hand, are at their lowest level since the 1990s, according to the CDC. The agency reported a record 6% decrease in births for teenagers aged 15 to 19 years between 2020 and 2021. Women ages 20 to 25 years also had a record decrease in births of 4% during that period.
Brady E. Hamilton, PhD, of the CDC’s National Center for Health Statistics, and the lead author of the new report, said the rise in births points to childbearing that was postponed during the pandemic. Data from 2021 showed a 4% drop in the nation’s birth rate between 2019 and 2020.
“The option to forgo birth is not always viable for older women, but you saw a lot of that during the pandemic,” Dr. Hamilton said. “Events happened related to job security and the economy that caused people to wait to have a child.”
Dr. Hamilton said more data are needed to determine the full impact of increased overall birth rates on individuals. The final report, which will be released in July, will delve deeper into the influence increased birth rates had on demographics and preterm births, which Dr. Hamilton and his team found have increased by 4%.
“For those beginning to have children, we see these trends, but it will be interesting to see what happens to younger women in the future,” Dr. Hamilton said. “Once we have the final data for 2021, we will be able to see a more detailed pattern emerge and draw conclusions from that.”
Dr. Hamilton has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and COVID: Weekly cases keep rising past 100,000
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
New cases were up by 14.6% over the previous week to just over 107,000 reported during May 13-16, marking the sixth straight increase since April 1-7, when the count was almost 26,000. Over that period, weekly cases rose 313%, based on data in the latest weekly COVID report from the AAP and CHA.
Rates reported by the Centers for Disease Control and Prevention show the same trend. Weekly cases per 100,000 population, which were down to 34.9 in children aged 0-4 years and 43.1 for those aged 5-11 on March 26, were up to 49.5 and 52.2, respectively, by April 16. The pace picked up right after that, and as of May 14, the rates of new cases were 125.4 per 100,000 in children aged 0-4 years and 143.1 in those aged 5-11, the CDC said.
Hospital admissions continue to rise as well. The rate of new admissions in children aged 0-17 was up to 0.25 per 100,000 population on May 18, nearly double the 0.13 per 100,000 recorded as late as April 13. The latest 7-day average count for new admissions, 163 per day from May 15-21, is down from the previous week’s 175 per day, but the CDC also acknowledges potential reporting delays in the most recent 7-day period.
Both of those weekly averages, however, are far below the peak rate for the pandemic, 914 per day, which occurred Jan. 10-16, 2022, during the Omicron surge. Since the CDC began keeping count at the beginning of August 2020, more than 125,000 children aged 0-17 years have been admitted with confirmed COVID-19, which is about 2.7% of all admissions over that period, the CDC’s data show.
Booster gets the green light
The week brought some positive news on the prevention side, though, as the CDC officially approved a COVID vaccine booster dose for children aged 5-11 years.
Even that good news came with a caveat, however. The vote by the CDC’s Advisory Committee on Immunization Practices was 11:1 in favor, with the negative vote cast by Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., who said that “boosters are great once we’ve gotten everyone their first round. That needs to be our priority in this.”
Nationally, in fact, just 35.7% of children aged 5-11 years have received at least one dose of the vaccine and only 29.0% are fully vaccinated. Those figures are nearly doubled among 12- to 17-year-olds: 69.3% have received at least one dose and 59.4% are fully vaccinated, the CDC said on its COVID Data Tracker.
Some states, meanwhile, are well below those national rates. In Wyoming, only 40% of children aged 12-17 have received an initial vaccine dose, and eight other states are below 50%. Among children aged 5-12, there are still five states below 20% in that measure, while the states on the other end of the spectrum – Vermont and Massachusetts – are above 60%, the AAP said in its separate vaccination report.
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
New cases were up by 14.6% over the previous week to just over 107,000 reported during May 13-16, marking the sixth straight increase since April 1-7, when the count was almost 26,000. Over that period, weekly cases rose 313%, based on data in the latest weekly COVID report from the AAP and CHA.
Rates reported by the Centers for Disease Control and Prevention show the same trend. Weekly cases per 100,000 population, which were down to 34.9 in children aged 0-4 years and 43.1 for those aged 5-11 on March 26, were up to 49.5 and 52.2, respectively, by April 16. The pace picked up right after that, and as of May 14, the rates of new cases were 125.4 per 100,000 in children aged 0-4 years and 143.1 in those aged 5-11, the CDC said.
Hospital admissions continue to rise as well. The rate of new admissions in children aged 0-17 was up to 0.25 per 100,000 population on May 18, nearly double the 0.13 per 100,000 recorded as late as April 13. The latest 7-day average count for new admissions, 163 per day from May 15-21, is down from the previous week’s 175 per day, but the CDC also acknowledges potential reporting delays in the most recent 7-day period.
Both of those weekly averages, however, are far below the peak rate for the pandemic, 914 per day, which occurred Jan. 10-16, 2022, during the Omicron surge. Since the CDC began keeping count at the beginning of August 2020, more than 125,000 children aged 0-17 years have been admitted with confirmed COVID-19, which is about 2.7% of all admissions over that period, the CDC’s data show.
Booster gets the green light
The week brought some positive news on the prevention side, though, as the CDC officially approved a COVID vaccine booster dose for children aged 5-11 years.
Even that good news came with a caveat, however. The vote by the CDC’s Advisory Committee on Immunization Practices was 11:1 in favor, with the negative vote cast by Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., who said that “boosters are great once we’ve gotten everyone their first round. That needs to be our priority in this.”
Nationally, in fact, just 35.7% of children aged 5-11 years have received at least one dose of the vaccine and only 29.0% are fully vaccinated. Those figures are nearly doubled among 12- to 17-year-olds: 69.3% have received at least one dose and 59.4% are fully vaccinated, the CDC said on its COVID Data Tracker.
Some states, meanwhile, are well below those national rates. In Wyoming, only 40% of children aged 12-17 have received an initial vaccine dose, and eight other states are below 50%. Among children aged 5-12, there are still five states below 20% in that measure, while the states on the other end of the spectrum – Vermont and Massachusetts – are above 60%, the AAP said in its separate vaccination report.
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
New cases were up by 14.6% over the previous week to just over 107,000 reported during May 13-16, marking the sixth straight increase since April 1-7, when the count was almost 26,000. Over that period, weekly cases rose 313%, based on data in the latest weekly COVID report from the AAP and CHA.
Rates reported by the Centers for Disease Control and Prevention show the same trend. Weekly cases per 100,000 population, which were down to 34.9 in children aged 0-4 years and 43.1 for those aged 5-11 on March 26, were up to 49.5 and 52.2, respectively, by April 16. The pace picked up right after that, and as of May 14, the rates of new cases were 125.4 per 100,000 in children aged 0-4 years and 143.1 in those aged 5-11, the CDC said.
Hospital admissions continue to rise as well. The rate of new admissions in children aged 0-17 was up to 0.25 per 100,000 population on May 18, nearly double the 0.13 per 100,000 recorded as late as April 13. The latest 7-day average count for new admissions, 163 per day from May 15-21, is down from the previous week’s 175 per day, but the CDC also acknowledges potential reporting delays in the most recent 7-day period.
Both of those weekly averages, however, are far below the peak rate for the pandemic, 914 per day, which occurred Jan. 10-16, 2022, during the Omicron surge. Since the CDC began keeping count at the beginning of August 2020, more than 125,000 children aged 0-17 years have been admitted with confirmed COVID-19, which is about 2.7% of all admissions over that period, the CDC’s data show.
Booster gets the green light
The week brought some positive news on the prevention side, though, as the CDC officially approved a COVID vaccine booster dose for children aged 5-11 years.
Even that good news came with a caveat, however. The vote by the CDC’s Advisory Committee on Immunization Practices was 11:1 in favor, with the negative vote cast by Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., who said that “boosters are great once we’ve gotten everyone their first round. That needs to be our priority in this.”
Nationally, in fact, just 35.7% of children aged 5-11 years have received at least one dose of the vaccine and only 29.0% are fully vaccinated. Those figures are nearly doubled among 12- to 17-year-olds: 69.3% have received at least one dose and 59.4% are fully vaccinated, the CDC said on its COVID Data Tracker.
Some states, meanwhile, are well below those national rates. In Wyoming, only 40% of children aged 12-17 have received an initial vaccine dose, and eight other states are below 50%. Among children aged 5-12, there are still five states below 20% in that measure, while the states on the other end of the spectrum – Vermont and Massachusetts – are above 60%, the AAP said in its separate vaccination report.