User login
Pulse oximeters lead to less oxygen supplementation for people of color
The new research suggests that skin color–related differences in pulse oximeter readings are in fact impacting clinical decision-making, lead author Eric R. Gottlieb, MD, of Brigham and Women’s Hospital and Massachusetts Institute of Technology, both in Boston, and colleagues wrote. This suggests that technology needs to updated to improve health equity, they continued, in their paper published in JAMA Internal Medicine.
“It has been known for decades that these readings are affected by various surface pigmentations, including nail polish and skin melanin, which may affect light absorption and scattering,” the investigators wrote. “This increases the risk of hidden hypoxemia [among patients with darker skin], in which patients have falsely elevated SpO2 readings, usually defined as 92% or greater, with a blood hemoglobin oxygen saturation less than 88%.”
Although published reports on this phenomenon date back to the 1980s, clinical significance has been largely discounted, they said, citing a 2008 paper on the topic, which stated that “oximetry need not have exact accuracy” to determine if a patient needs oxygen supplementation.
‘We’re not providing equal care’
Questioning the validity of this statement, Dr. Gottlieb and colleagues conducted a retrospective cohort study involving 3,069 patients admitted to intensive care at the Beth Israel Deaconess Medical Center in Boston between 2008 and 2019, thereby excluding patients treated during the COVID-19 pandemic. The population consisted of four races/ethnicities: White (87%), Black (7%), Hispanic (4%), and Asian (3%).
Aligning with previous studies, multivariable linear regression analyses showed that Asian, Black, and Hispanic patients had significantly higher SpO2 readings than White patients in relation to hemoglobin oxygen saturation values, suggesting falsely elevated readings.
Further modeling showed that these same patient groups also received lower oxygen delivery rates, which were not explained directly by race/ethnicity, but instead were mediated by the discrepancy between SpO2 and hemoglobin oxygen saturation values. In other words, physicians were responding consistently to pulse oximetry readings, rather than exhibiting a direct racial/ethnic bias in their clinical decision-making.
“We’re not providing equal care,” Dr. Gottlieb said in an interview. “It’s not that the patients are sicker, or have other socioeconomic explanations for why this happens to them. It’s us. It’s our technology. And that’s something that really has to be fixed.”
The investigators offered a cautionary view of corrective algorithms, as these “have exacerbated disparities and are subject to ethical concerns;” for example, with glomerular filtration rate estimations in Black patients.
Dr. Gottlieb also cautioned against action by individual physicians, who may now be inclined to change how they interpret pulse oximeter readings based on a patient’s race or ethnicity.
“I don’t think that we can expect physicians, every time they see a patient, to be second guessing whether the number basically reflects the truth,” he said.
Instead, Dr. Gottlieb suggested that the burden of change rests upon the shoulders of institutions, including hospitals and device manufacturers, both of which “really need to take the responsibility” for making sure that pulse oximeters are “equitable and have similar performance across races.”
While Dr. Gottlieb said that skin color likely plays the greatest role in measurement discrepancies, he encouraged stakeholders “to think broadly about this, and not just assume that it’s entirely skin color,” noting a small amount of evidence indicating that blood chemistry may also play a role. Still, he predicted that colorimetry – the direct measurement of skin color – will probably be incorporated into pulse oximeters of the future.
Black patients 3X more likely to have hidden hypoxia than White patients
Michael Sjoding, MD, of the University of Michigan, Ann Arbor, was one of the first to raise awareness of skin color–related issues with pulse oximeters during the throes of the COVID-19 pandemic. His study, which involved more than 10,000 patients, showed that Black patients were threefold more likely to have hidden hypoxia than White patients.
The present study shows that such discrepancies are indeed clinically significant, Dr. Sjoding said in an interview. And these data are needed, he added, to bring about change.
“What is being asked is potentially a big deal,” Dr. Sjoding said. “Pulse oximeters are everywhere, and it would be a big undertaking to redesign pulse oximeters and purchase new pulse oximeters. You need a compelling body of evidence to do that. I think it’s there now, clearly. So I’m hopeful that we’re going to finally move forward, towards having devices that we are confident work accurately in everyone.”
Why it has taken so long to gather this evidence, however, is a thornier topic, considering race-related discrepancies in pulse oximeter readings were first documented more than 3 decades ago.
“We sort of rediscovered something that had been known and had been described in the past,” Dr. Sjoding said. He explained how he and many of his colleagues had completed pulmonary fellowships, yet none of them knew of these potential issues with pulse oximeters until they began to observe differences in their own patients during the pandemic.
“I’ll give previous generations of researchers the benefit of the doubt,” Dr. Sjoding said, pointing out that techniques in data gathering and analysis have advanced considerably over the years. “The types of studies that were done before were very different than what we did.”
Yet Dr. Sjoding entertained the possibility that other factors may have been at play.
“I think definitely there’s a social commentary on prioritization of research,” he said.
The study was supported by grants from the National Institutes of Health. The investigators and Dr. Sjoding reported no conflicts of interest.
The new research suggests that skin color–related differences in pulse oximeter readings are in fact impacting clinical decision-making, lead author Eric R. Gottlieb, MD, of Brigham and Women’s Hospital and Massachusetts Institute of Technology, both in Boston, and colleagues wrote. This suggests that technology needs to updated to improve health equity, they continued, in their paper published in JAMA Internal Medicine.
“It has been known for decades that these readings are affected by various surface pigmentations, including nail polish and skin melanin, which may affect light absorption and scattering,” the investigators wrote. “This increases the risk of hidden hypoxemia [among patients with darker skin], in which patients have falsely elevated SpO2 readings, usually defined as 92% or greater, with a blood hemoglobin oxygen saturation less than 88%.”
Although published reports on this phenomenon date back to the 1980s, clinical significance has been largely discounted, they said, citing a 2008 paper on the topic, which stated that “oximetry need not have exact accuracy” to determine if a patient needs oxygen supplementation.
‘We’re not providing equal care’
Questioning the validity of this statement, Dr. Gottlieb and colleagues conducted a retrospective cohort study involving 3,069 patients admitted to intensive care at the Beth Israel Deaconess Medical Center in Boston between 2008 and 2019, thereby excluding patients treated during the COVID-19 pandemic. The population consisted of four races/ethnicities: White (87%), Black (7%), Hispanic (4%), and Asian (3%).
Aligning with previous studies, multivariable linear regression analyses showed that Asian, Black, and Hispanic patients had significantly higher SpO2 readings than White patients in relation to hemoglobin oxygen saturation values, suggesting falsely elevated readings.
Further modeling showed that these same patient groups also received lower oxygen delivery rates, which were not explained directly by race/ethnicity, but instead were mediated by the discrepancy between SpO2 and hemoglobin oxygen saturation values. In other words, physicians were responding consistently to pulse oximetry readings, rather than exhibiting a direct racial/ethnic bias in their clinical decision-making.
“We’re not providing equal care,” Dr. Gottlieb said in an interview. “It’s not that the patients are sicker, or have other socioeconomic explanations for why this happens to them. It’s us. It’s our technology. And that’s something that really has to be fixed.”
The investigators offered a cautionary view of corrective algorithms, as these “have exacerbated disparities and are subject to ethical concerns;” for example, with glomerular filtration rate estimations in Black patients.
Dr. Gottlieb also cautioned against action by individual physicians, who may now be inclined to change how they interpret pulse oximeter readings based on a patient’s race or ethnicity.
“I don’t think that we can expect physicians, every time they see a patient, to be second guessing whether the number basically reflects the truth,” he said.
Instead, Dr. Gottlieb suggested that the burden of change rests upon the shoulders of institutions, including hospitals and device manufacturers, both of which “really need to take the responsibility” for making sure that pulse oximeters are “equitable and have similar performance across races.”
While Dr. Gottlieb said that skin color likely plays the greatest role in measurement discrepancies, he encouraged stakeholders “to think broadly about this, and not just assume that it’s entirely skin color,” noting a small amount of evidence indicating that blood chemistry may also play a role. Still, he predicted that colorimetry – the direct measurement of skin color – will probably be incorporated into pulse oximeters of the future.
Black patients 3X more likely to have hidden hypoxia than White patients
Michael Sjoding, MD, of the University of Michigan, Ann Arbor, was one of the first to raise awareness of skin color–related issues with pulse oximeters during the throes of the COVID-19 pandemic. His study, which involved more than 10,000 patients, showed that Black patients were threefold more likely to have hidden hypoxia than White patients.
The present study shows that such discrepancies are indeed clinically significant, Dr. Sjoding said in an interview. And these data are needed, he added, to bring about change.
“What is being asked is potentially a big deal,” Dr. Sjoding said. “Pulse oximeters are everywhere, and it would be a big undertaking to redesign pulse oximeters and purchase new pulse oximeters. You need a compelling body of evidence to do that. I think it’s there now, clearly. So I’m hopeful that we’re going to finally move forward, towards having devices that we are confident work accurately in everyone.”
Why it has taken so long to gather this evidence, however, is a thornier topic, considering race-related discrepancies in pulse oximeter readings were first documented more than 3 decades ago.
“We sort of rediscovered something that had been known and had been described in the past,” Dr. Sjoding said. He explained how he and many of his colleagues had completed pulmonary fellowships, yet none of them knew of these potential issues with pulse oximeters until they began to observe differences in their own patients during the pandemic.
“I’ll give previous generations of researchers the benefit of the doubt,” Dr. Sjoding said, pointing out that techniques in data gathering and analysis have advanced considerably over the years. “The types of studies that were done before were very different than what we did.”
Yet Dr. Sjoding entertained the possibility that other factors may have been at play.
“I think definitely there’s a social commentary on prioritization of research,” he said.
The study was supported by grants from the National Institutes of Health. The investigators and Dr. Sjoding reported no conflicts of interest.
The new research suggests that skin color–related differences in pulse oximeter readings are in fact impacting clinical decision-making, lead author Eric R. Gottlieb, MD, of Brigham and Women’s Hospital and Massachusetts Institute of Technology, both in Boston, and colleagues wrote. This suggests that technology needs to updated to improve health equity, they continued, in their paper published in JAMA Internal Medicine.
“It has been known for decades that these readings are affected by various surface pigmentations, including nail polish and skin melanin, which may affect light absorption and scattering,” the investigators wrote. “This increases the risk of hidden hypoxemia [among patients with darker skin], in which patients have falsely elevated SpO2 readings, usually defined as 92% or greater, with a blood hemoglobin oxygen saturation less than 88%.”
Although published reports on this phenomenon date back to the 1980s, clinical significance has been largely discounted, they said, citing a 2008 paper on the topic, which stated that “oximetry need not have exact accuracy” to determine if a patient needs oxygen supplementation.
‘We’re not providing equal care’
Questioning the validity of this statement, Dr. Gottlieb and colleagues conducted a retrospective cohort study involving 3,069 patients admitted to intensive care at the Beth Israel Deaconess Medical Center in Boston between 2008 and 2019, thereby excluding patients treated during the COVID-19 pandemic. The population consisted of four races/ethnicities: White (87%), Black (7%), Hispanic (4%), and Asian (3%).
Aligning with previous studies, multivariable linear regression analyses showed that Asian, Black, and Hispanic patients had significantly higher SpO2 readings than White patients in relation to hemoglobin oxygen saturation values, suggesting falsely elevated readings.
Further modeling showed that these same patient groups also received lower oxygen delivery rates, which were not explained directly by race/ethnicity, but instead were mediated by the discrepancy between SpO2 and hemoglobin oxygen saturation values. In other words, physicians were responding consistently to pulse oximetry readings, rather than exhibiting a direct racial/ethnic bias in their clinical decision-making.
“We’re not providing equal care,” Dr. Gottlieb said in an interview. “It’s not that the patients are sicker, or have other socioeconomic explanations for why this happens to them. It’s us. It’s our technology. And that’s something that really has to be fixed.”
The investigators offered a cautionary view of corrective algorithms, as these “have exacerbated disparities and are subject to ethical concerns;” for example, with glomerular filtration rate estimations in Black patients.
Dr. Gottlieb also cautioned against action by individual physicians, who may now be inclined to change how they interpret pulse oximeter readings based on a patient’s race or ethnicity.
“I don’t think that we can expect physicians, every time they see a patient, to be second guessing whether the number basically reflects the truth,” he said.
Instead, Dr. Gottlieb suggested that the burden of change rests upon the shoulders of institutions, including hospitals and device manufacturers, both of which “really need to take the responsibility” for making sure that pulse oximeters are “equitable and have similar performance across races.”
While Dr. Gottlieb said that skin color likely plays the greatest role in measurement discrepancies, he encouraged stakeholders “to think broadly about this, and not just assume that it’s entirely skin color,” noting a small amount of evidence indicating that blood chemistry may also play a role. Still, he predicted that colorimetry – the direct measurement of skin color – will probably be incorporated into pulse oximeters of the future.
Black patients 3X more likely to have hidden hypoxia than White patients
Michael Sjoding, MD, of the University of Michigan, Ann Arbor, was one of the first to raise awareness of skin color–related issues with pulse oximeters during the throes of the COVID-19 pandemic. His study, which involved more than 10,000 patients, showed that Black patients were threefold more likely to have hidden hypoxia than White patients.
The present study shows that such discrepancies are indeed clinically significant, Dr. Sjoding said in an interview. And these data are needed, he added, to bring about change.
“What is being asked is potentially a big deal,” Dr. Sjoding said. “Pulse oximeters are everywhere, and it would be a big undertaking to redesign pulse oximeters and purchase new pulse oximeters. You need a compelling body of evidence to do that. I think it’s there now, clearly. So I’m hopeful that we’re going to finally move forward, towards having devices that we are confident work accurately in everyone.”
Why it has taken so long to gather this evidence, however, is a thornier topic, considering race-related discrepancies in pulse oximeter readings were first documented more than 3 decades ago.
“We sort of rediscovered something that had been known and had been described in the past,” Dr. Sjoding said. He explained how he and many of his colleagues had completed pulmonary fellowships, yet none of them knew of these potential issues with pulse oximeters until they began to observe differences in their own patients during the pandemic.
“I’ll give previous generations of researchers the benefit of the doubt,” Dr. Sjoding said, pointing out that techniques in data gathering and analysis have advanced considerably over the years. “The types of studies that were done before were very different than what we did.”
Yet Dr. Sjoding entertained the possibility that other factors may have been at play.
“I think definitely there’s a social commentary on prioritization of research,” he said.
The study was supported by grants from the National Institutes of Health. The investigators and Dr. Sjoding reported no conflicts of interest.
FROM JAMA INTERNAL MEDICINE
Experimental cancer drug promising for hospitalized COVID patients
, a new study shows.
The medication, called sabizabulin and given as a pill, reduced by half the risk of death among participants. It could be more effective than other drugs for those severely sick with COVID-19, The New York Times reports.
The manufacturer, Veru, is seeking emergency use authorization from the Food and Drug Administration. Hospitalized COVID-19 patients currently have only a few pharmaceutical options.
Sabizabulin blocks cells from building molecular cables that carry material from one part of a cell to another. It was created to fight cancer, because tumor cells need those cables (called microtubules) to grow quickly.
Researchers tried it against COVID-19 2 years ago, because viral replication also requires microtubules to bring pieces of new viruses together.
To participate in the small trial, patients had to be receiving oxygen or on a ventilator and at a high risk of dying from COVID-19, “with risk factors such as hypertension, advanced age or obesity,” the Times reported.
A total of 134 patients received the medicine; 70 got a placebo. Among those receiving sabizabulin, 20.2% died within 2 months; 45.1% of those who took the placebo died.
One infectious disease expert told the Times that the high mortality rate of those on the placebo could mean the study was too small to offer conclusive results.
“The 45% mortality rate in the control group jumps out at me as rather high,” said David Boulware, MD, of the University of Minnesota.
A version of this article first appeared on WebMD.com.
, a new study shows.
The medication, called sabizabulin and given as a pill, reduced by half the risk of death among participants. It could be more effective than other drugs for those severely sick with COVID-19, The New York Times reports.
The manufacturer, Veru, is seeking emergency use authorization from the Food and Drug Administration. Hospitalized COVID-19 patients currently have only a few pharmaceutical options.
Sabizabulin blocks cells from building molecular cables that carry material from one part of a cell to another. It was created to fight cancer, because tumor cells need those cables (called microtubules) to grow quickly.
Researchers tried it against COVID-19 2 years ago, because viral replication also requires microtubules to bring pieces of new viruses together.
To participate in the small trial, patients had to be receiving oxygen or on a ventilator and at a high risk of dying from COVID-19, “with risk factors such as hypertension, advanced age or obesity,” the Times reported.
A total of 134 patients received the medicine; 70 got a placebo. Among those receiving sabizabulin, 20.2% died within 2 months; 45.1% of those who took the placebo died.
One infectious disease expert told the Times that the high mortality rate of those on the placebo could mean the study was too small to offer conclusive results.
“The 45% mortality rate in the control group jumps out at me as rather high,” said David Boulware, MD, of the University of Minnesota.
A version of this article first appeared on WebMD.com.
, a new study shows.
The medication, called sabizabulin and given as a pill, reduced by half the risk of death among participants. It could be more effective than other drugs for those severely sick with COVID-19, The New York Times reports.
The manufacturer, Veru, is seeking emergency use authorization from the Food and Drug Administration. Hospitalized COVID-19 patients currently have only a few pharmaceutical options.
Sabizabulin blocks cells from building molecular cables that carry material from one part of a cell to another. It was created to fight cancer, because tumor cells need those cables (called microtubules) to grow quickly.
Researchers tried it against COVID-19 2 years ago, because viral replication also requires microtubules to bring pieces of new viruses together.
To participate in the small trial, patients had to be receiving oxygen or on a ventilator and at a high risk of dying from COVID-19, “with risk factors such as hypertension, advanced age or obesity,” the Times reported.
A total of 134 patients received the medicine; 70 got a placebo. Among those receiving sabizabulin, 20.2% died within 2 months; 45.1% of those who took the placebo died.
One infectious disease expert told the Times that the high mortality rate of those on the placebo could mean the study was too small to offer conclusive results.
“The 45% mortality rate in the control group jumps out at me as rather high,” said David Boulware, MD, of the University of Minnesota.
A version of this article first appeared on WebMD.com.
Acute exacerbations common and often fatal in RA-ILD
Acute exacerbations (AEs) are both common in rheumatoid arthritis–associated interstitial lung disease (RA-ILD) and are a frequent cause of imminent mortality, a retrospective Japanese study suggests.
The same is also true for patients with idiopathic pulmonary fibrosis (IPF) for whom an AE is the most frequent cause of death as well, the same comparative study indicates.
“Several studies have reported that acute exacerbation, which occurs during the clinical course of idiopathic pulmonary fibrosis (IPF), also occurs in rheumatoid arthritis–associated interstitial lung disease (RA-ILD),” lead author Junji Otsuka, MD, PhD, of the National Hospital Organization Omuta National Hospital, Fukuoka, Japan, and colleagues observed.
“[We found that] AE was not uncommon in RA-ILD or IPF ... but the prognosis after AE of RA-ILD was significantly better than that of IPF [even though] the most frequent cause of death in RA-ILD and IPF was AE,” they stated.
The study was published online in Respiratory Medicine.
Patient features
The study involved 149 RA-ILD patients with a median age of 72 years at RA onset. The median time from ILD diagnosis to onset of AE was 48.5 months, while the median survival time after the onset of AE was 196 days (range 1-3,463 days), as the authors detailed. “All patients were treated with corticosteroids,” the authors noted, and almost all of them were treated with steroid pulse therapy.
Noninvasive positive pressure ventilation (NPPV) was used to maintain oxygenation in 18.5% of patients with severe respiratory failure, while invasive positive pressure ventilation (IPPV) was used in almost 26% of patients with the same degree of respiratory failure. Features of patients who developed an AE were then compared with those who did not.
Interestingly, no significant differences in clinical parameters were seen between those who developed an AE and those who did not. Nor were there any significant differences between the 2 groups in the length of time from the ILD diagnosis to the development of an AE. Some 18% of RA-ILD patients developed an AE, as did over 27% of patients with IPF, investigators report.
, compared with only 60 days for those with IPF (P = .038). In a multivariable analysis, hypoalbuminemia at an odds ratio of .090 (95% confidence interval, 0.011-0.733; P = .012) as well as percent carbon monoxide diffusion capacity at an OR of .810 (95% CI, 0.814-0.964; P < .01) were both independent risk factors for the development of an AE, the investigators pointed out.
The best cut-off level for predicting the risk of an AE was 3.0 g/dL (95% CI, 0.011-0.733; P = .012) for serum albumin and 53% (95% CI, 0.814-0.964; P < .01) for carbon monoxide diffusion capacity. As Dr. Otsuka noted in an email to this news organization, low serum albumin likely correlates with a generally poor condition, while low carbon monoxide diffusion capacity is likely due to lung fibrosis.
“But if low albumin and low carbon monoxide diffusion capacity are due to the progression of ILD, both values may be difficult to improve,” he added.
Survivors versus nonsurvivors
Of those patients with RA-ILD who developed an AE, approximately half recovered. Among the IPF patients who developed an AE during the study period, approximately 39% recovered from the event, while 70% did not. Comparing RA-ILD patients who survived versus whose who did not, again, no significant demographic or clinical differences were seen between the 2 groups. On the other hand, the number of patients treated with immunosuppressants for their AE was significantly higher among patients who did not survive the AE, compared with those who did (P =.022), investigators note.
Similarly, the number of patients who required NPPV was also significantly higher among those who did not survive, compared with those who did. In fact, “none of the surviving patients used NPPV (P <0.01),” the authors stress. The number of patients who required IPPV was also significantly higher among nonsurvivors than among survivors (P =.017), and of the small number of patients who were treated with IPPV, all but one died without recovery.
As the authors suggested, these findings suggest that RA-ILD patients who recover from an AE with the help of corticosteroids alone have a relatively decent prognosis. In contrast, those who require immunosuppressive drugs in addition to steroids or mechanical ventilation for AE management can be expected to have a poor prognosis.
The same can also be said for IPF patients and even with the help of mechanical ventilation “with IPF patients, the survival rate is low anyway, so the indication for mechanical ventilation should be carefully judged,” Dr. Otsuka stressed.
Cause of death
The authors also compared the cause of death between patients with RA-ILD and those with IPF. “In RA-ILD patients, the most frequent cause of death was AE,” they report, at close to 35% of all patients with RA-ILD. This was also true for IPF patients among whom AE was the cause of death for over 44%. “These results indicate that, as in IPF, AE develops in the clinical course of RA-ILD with considerable frequency,” investigators note.
“During the clinical course of RA-ILD, as with IPD, it is necessary to pay attention to AE,” they stress. Dr. Otsuka added: “It may be difficult to change the prognosis of these patients.”
“However, knowing which patients are more likely to develop AE may help predict the prognosis, and it may be improved if antifibrotic agents are used for these patients,” he said. Elizabeth Volkmann, MD, director, UCLA scleroderma program, University of California, Los Angeles, felt that understanding the risk factors for AEs in this patient population may help physicians identify a subgroup of patients with RA-ILD who require closer monitoring and follow-up.
“These patients may also require more aggressive treatment for RA-ILD to prevent AEs,” she said in an email to this news organization. Given that the study was retrospective in nature, Dr. Volkmann cautioned that there were likely multiple confounding factors that could have affected survival in this patient population and not to take away from the study that survival was solely affected by immunosuppressant use, for example.
“It is possible that patients [treated with] immunosuppressants had other features of their disease that independently heightened their risk of mortality,” Dr. Volkmann said. Similarly, physicians should not assume that the high mortality rate seen in RA-ILD patients who were treated with mechanical ventilation had anything to do with mechanical ventilation itself, as patients requiring ventilation are likely to have worse outcomes, as she stressed.
As for hypoalbuminemia, Dr. Volkmann pointed out that hypoalbuminemia is often a sign of malnutrition in these patients. “Studies have demonstrated that malnutrition is an independent predictor of mortality in patients with ILD,” she emphasized.
“Optimizing patients’ nutritional status could potentially help lower the risk of AEs,” Dr. Volkmann suggested.
Limitations of the study include the fact that it was a single-center design study and included only a limited number of patients.
No specific funding source was noted. The authors have no conflicts of interest to declare.
Acute exacerbations (AEs) are both common in rheumatoid arthritis–associated interstitial lung disease (RA-ILD) and are a frequent cause of imminent mortality, a retrospective Japanese study suggests.
The same is also true for patients with idiopathic pulmonary fibrosis (IPF) for whom an AE is the most frequent cause of death as well, the same comparative study indicates.
“Several studies have reported that acute exacerbation, which occurs during the clinical course of idiopathic pulmonary fibrosis (IPF), also occurs in rheumatoid arthritis–associated interstitial lung disease (RA-ILD),” lead author Junji Otsuka, MD, PhD, of the National Hospital Organization Omuta National Hospital, Fukuoka, Japan, and colleagues observed.
“[We found that] AE was not uncommon in RA-ILD or IPF ... but the prognosis after AE of RA-ILD was significantly better than that of IPF [even though] the most frequent cause of death in RA-ILD and IPF was AE,” they stated.
The study was published online in Respiratory Medicine.
Patient features
The study involved 149 RA-ILD patients with a median age of 72 years at RA onset. The median time from ILD diagnosis to onset of AE was 48.5 months, while the median survival time after the onset of AE was 196 days (range 1-3,463 days), as the authors detailed. “All patients were treated with corticosteroids,” the authors noted, and almost all of them were treated with steroid pulse therapy.
Noninvasive positive pressure ventilation (NPPV) was used to maintain oxygenation in 18.5% of patients with severe respiratory failure, while invasive positive pressure ventilation (IPPV) was used in almost 26% of patients with the same degree of respiratory failure. Features of patients who developed an AE were then compared with those who did not.
Interestingly, no significant differences in clinical parameters were seen between those who developed an AE and those who did not. Nor were there any significant differences between the 2 groups in the length of time from the ILD diagnosis to the development of an AE. Some 18% of RA-ILD patients developed an AE, as did over 27% of patients with IPF, investigators report.
, compared with only 60 days for those with IPF (P = .038). In a multivariable analysis, hypoalbuminemia at an odds ratio of .090 (95% confidence interval, 0.011-0.733; P = .012) as well as percent carbon monoxide diffusion capacity at an OR of .810 (95% CI, 0.814-0.964; P < .01) were both independent risk factors for the development of an AE, the investigators pointed out.
The best cut-off level for predicting the risk of an AE was 3.0 g/dL (95% CI, 0.011-0.733; P = .012) for serum albumin and 53% (95% CI, 0.814-0.964; P < .01) for carbon monoxide diffusion capacity. As Dr. Otsuka noted in an email to this news organization, low serum albumin likely correlates with a generally poor condition, while low carbon monoxide diffusion capacity is likely due to lung fibrosis.
“But if low albumin and low carbon monoxide diffusion capacity are due to the progression of ILD, both values may be difficult to improve,” he added.
Survivors versus nonsurvivors
Of those patients with RA-ILD who developed an AE, approximately half recovered. Among the IPF patients who developed an AE during the study period, approximately 39% recovered from the event, while 70% did not. Comparing RA-ILD patients who survived versus whose who did not, again, no significant demographic or clinical differences were seen between the 2 groups. On the other hand, the number of patients treated with immunosuppressants for their AE was significantly higher among patients who did not survive the AE, compared with those who did (P =.022), investigators note.
Similarly, the number of patients who required NPPV was also significantly higher among those who did not survive, compared with those who did. In fact, “none of the surviving patients used NPPV (P <0.01),” the authors stress. The number of patients who required IPPV was also significantly higher among nonsurvivors than among survivors (P =.017), and of the small number of patients who were treated with IPPV, all but one died without recovery.
As the authors suggested, these findings suggest that RA-ILD patients who recover from an AE with the help of corticosteroids alone have a relatively decent prognosis. In contrast, those who require immunosuppressive drugs in addition to steroids or mechanical ventilation for AE management can be expected to have a poor prognosis.
The same can also be said for IPF patients and even with the help of mechanical ventilation “with IPF patients, the survival rate is low anyway, so the indication for mechanical ventilation should be carefully judged,” Dr. Otsuka stressed.
Cause of death
The authors also compared the cause of death between patients with RA-ILD and those with IPF. “In RA-ILD patients, the most frequent cause of death was AE,” they report, at close to 35% of all patients with RA-ILD. This was also true for IPF patients among whom AE was the cause of death for over 44%. “These results indicate that, as in IPF, AE develops in the clinical course of RA-ILD with considerable frequency,” investigators note.
“During the clinical course of RA-ILD, as with IPD, it is necessary to pay attention to AE,” they stress. Dr. Otsuka added: “It may be difficult to change the prognosis of these patients.”
“However, knowing which patients are more likely to develop AE may help predict the prognosis, and it may be improved if antifibrotic agents are used for these patients,” he said. Elizabeth Volkmann, MD, director, UCLA scleroderma program, University of California, Los Angeles, felt that understanding the risk factors for AEs in this patient population may help physicians identify a subgroup of patients with RA-ILD who require closer monitoring and follow-up.
“These patients may also require more aggressive treatment for RA-ILD to prevent AEs,” she said in an email to this news organization. Given that the study was retrospective in nature, Dr. Volkmann cautioned that there were likely multiple confounding factors that could have affected survival in this patient population and not to take away from the study that survival was solely affected by immunosuppressant use, for example.
“It is possible that patients [treated with] immunosuppressants had other features of their disease that independently heightened their risk of mortality,” Dr. Volkmann said. Similarly, physicians should not assume that the high mortality rate seen in RA-ILD patients who were treated with mechanical ventilation had anything to do with mechanical ventilation itself, as patients requiring ventilation are likely to have worse outcomes, as she stressed.
As for hypoalbuminemia, Dr. Volkmann pointed out that hypoalbuminemia is often a sign of malnutrition in these patients. “Studies have demonstrated that malnutrition is an independent predictor of mortality in patients with ILD,” she emphasized.
“Optimizing patients’ nutritional status could potentially help lower the risk of AEs,” Dr. Volkmann suggested.
Limitations of the study include the fact that it was a single-center design study and included only a limited number of patients.
No specific funding source was noted. The authors have no conflicts of interest to declare.
Acute exacerbations (AEs) are both common in rheumatoid arthritis–associated interstitial lung disease (RA-ILD) and are a frequent cause of imminent mortality, a retrospective Japanese study suggests.
The same is also true for patients with idiopathic pulmonary fibrosis (IPF) for whom an AE is the most frequent cause of death as well, the same comparative study indicates.
“Several studies have reported that acute exacerbation, which occurs during the clinical course of idiopathic pulmonary fibrosis (IPF), also occurs in rheumatoid arthritis–associated interstitial lung disease (RA-ILD),” lead author Junji Otsuka, MD, PhD, of the National Hospital Organization Omuta National Hospital, Fukuoka, Japan, and colleagues observed.
“[We found that] AE was not uncommon in RA-ILD or IPF ... but the prognosis after AE of RA-ILD was significantly better than that of IPF [even though] the most frequent cause of death in RA-ILD and IPF was AE,” they stated.
The study was published online in Respiratory Medicine.
Patient features
The study involved 149 RA-ILD patients with a median age of 72 years at RA onset. The median time from ILD diagnosis to onset of AE was 48.5 months, while the median survival time after the onset of AE was 196 days (range 1-3,463 days), as the authors detailed. “All patients were treated with corticosteroids,” the authors noted, and almost all of them were treated with steroid pulse therapy.
Noninvasive positive pressure ventilation (NPPV) was used to maintain oxygenation in 18.5% of patients with severe respiratory failure, while invasive positive pressure ventilation (IPPV) was used in almost 26% of patients with the same degree of respiratory failure. Features of patients who developed an AE were then compared with those who did not.
Interestingly, no significant differences in clinical parameters were seen between those who developed an AE and those who did not. Nor were there any significant differences between the 2 groups in the length of time from the ILD diagnosis to the development of an AE. Some 18% of RA-ILD patients developed an AE, as did over 27% of patients with IPF, investigators report.
, compared with only 60 days for those with IPF (P = .038). In a multivariable analysis, hypoalbuminemia at an odds ratio of .090 (95% confidence interval, 0.011-0.733; P = .012) as well as percent carbon monoxide diffusion capacity at an OR of .810 (95% CI, 0.814-0.964; P < .01) were both independent risk factors for the development of an AE, the investigators pointed out.
The best cut-off level for predicting the risk of an AE was 3.0 g/dL (95% CI, 0.011-0.733; P = .012) for serum albumin and 53% (95% CI, 0.814-0.964; P < .01) for carbon monoxide diffusion capacity. As Dr. Otsuka noted in an email to this news organization, low serum albumin likely correlates with a generally poor condition, while low carbon monoxide diffusion capacity is likely due to lung fibrosis.
“But if low albumin and low carbon monoxide diffusion capacity are due to the progression of ILD, both values may be difficult to improve,” he added.
Survivors versus nonsurvivors
Of those patients with RA-ILD who developed an AE, approximately half recovered. Among the IPF patients who developed an AE during the study period, approximately 39% recovered from the event, while 70% did not. Comparing RA-ILD patients who survived versus whose who did not, again, no significant demographic or clinical differences were seen between the 2 groups. On the other hand, the number of patients treated with immunosuppressants for their AE was significantly higher among patients who did not survive the AE, compared with those who did (P =.022), investigators note.
Similarly, the number of patients who required NPPV was also significantly higher among those who did not survive, compared with those who did. In fact, “none of the surviving patients used NPPV (P <0.01),” the authors stress. The number of patients who required IPPV was also significantly higher among nonsurvivors than among survivors (P =.017), and of the small number of patients who were treated with IPPV, all but one died without recovery.
As the authors suggested, these findings suggest that RA-ILD patients who recover from an AE with the help of corticosteroids alone have a relatively decent prognosis. In contrast, those who require immunosuppressive drugs in addition to steroids or mechanical ventilation for AE management can be expected to have a poor prognosis.
The same can also be said for IPF patients and even with the help of mechanical ventilation “with IPF patients, the survival rate is low anyway, so the indication for mechanical ventilation should be carefully judged,” Dr. Otsuka stressed.
Cause of death
The authors also compared the cause of death between patients with RA-ILD and those with IPF. “In RA-ILD patients, the most frequent cause of death was AE,” they report, at close to 35% of all patients with RA-ILD. This was also true for IPF patients among whom AE was the cause of death for over 44%. “These results indicate that, as in IPF, AE develops in the clinical course of RA-ILD with considerable frequency,” investigators note.
“During the clinical course of RA-ILD, as with IPD, it is necessary to pay attention to AE,” they stress. Dr. Otsuka added: “It may be difficult to change the prognosis of these patients.”
“However, knowing which patients are more likely to develop AE may help predict the prognosis, and it may be improved if antifibrotic agents are used for these patients,” he said. Elizabeth Volkmann, MD, director, UCLA scleroderma program, University of California, Los Angeles, felt that understanding the risk factors for AEs in this patient population may help physicians identify a subgroup of patients with RA-ILD who require closer monitoring and follow-up.
“These patients may also require more aggressive treatment for RA-ILD to prevent AEs,” she said in an email to this news organization. Given that the study was retrospective in nature, Dr. Volkmann cautioned that there were likely multiple confounding factors that could have affected survival in this patient population and not to take away from the study that survival was solely affected by immunosuppressant use, for example.
“It is possible that patients [treated with] immunosuppressants had other features of their disease that independently heightened their risk of mortality,” Dr. Volkmann said. Similarly, physicians should not assume that the high mortality rate seen in RA-ILD patients who were treated with mechanical ventilation had anything to do with mechanical ventilation itself, as patients requiring ventilation are likely to have worse outcomes, as she stressed.
As for hypoalbuminemia, Dr. Volkmann pointed out that hypoalbuminemia is often a sign of malnutrition in these patients. “Studies have demonstrated that malnutrition is an independent predictor of mortality in patients with ILD,” she emphasized.
“Optimizing patients’ nutritional status could potentially help lower the risk of AEs,” Dr. Volkmann suggested.
Limitations of the study include the fact that it was a single-center design study and included only a limited number of patients.
No specific funding source was noted. The authors have no conflicts of interest to declare.
FDA Class I recall: Batteries for CARESCAPE 2860 Ventilator
A total of 1,533 complaints allege that the batteries are draining much faster than expected, prompting manufacturer GE Healthcare to initiate the recall. There have been no injuries, and no deaths associated with the use of this device, according to an FDA corrected announcement.
Health care personnel and those patients who receive breathing support with these ventilators should be cautious about using CARESCAPE battery products moving forward, the agency said.
This type of ventilator is primarily powered by plugging into a wall outlet, but it has the capability to operate on backup batteries. These batteries are not solely for emergency situations such as power outages, but are also for routine situations such as transporting a patient within the hospital. GE Healthcare supplies these backup batteries with the ventilator, and sells replacements when they run out.
However, if the ventilator loses power because of battery malfunction, the patient may lose access to oxygen, leading to hypoxia, which can lead to brain injury and death. Therefore, if these batteries drain quicker than anticipated, it may put the patient at risk.
To prevent this danger, GE Healthcare recommends customers perform a battery performance test after they see this notice and every 3 months following. Consumers should take extra precaution and make sure their batteries are charged following a long period of inactivity. If the device is inactive for a while, the company says users should keep it plugged in to avoid draining the battery. Batteries should be replaced at a minimum of every 3 years.
When these devices are still plugged into the wall, they’re safe to use, according to the FDA. But when using the backup power source, clinicians should make sure to have alternative routes for breathing support on hand, such as with a bag-valve mask system.
There are 4,222 of these possibly defective batteries currently on the market. They were distributed from April 2, 2019, through April 18 of this year, when GE Healthcare stopped distributing these products and began the recall process. Any issues with these products should be reported to the FDA’s MedWatch database or by sending a medical device notification acknowledgment response to GE at the email address listed at the bottom of the recall announcement.
A version of this article first appeared on Medscape.com.
This article was updated 7/6/22.
A total of 1,533 complaints allege that the batteries are draining much faster than expected, prompting manufacturer GE Healthcare to initiate the recall. There have been no injuries, and no deaths associated with the use of this device, according to an FDA corrected announcement.
Health care personnel and those patients who receive breathing support with these ventilators should be cautious about using CARESCAPE battery products moving forward, the agency said.
This type of ventilator is primarily powered by plugging into a wall outlet, but it has the capability to operate on backup batteries. These batteries are not solely for emergency situations such as power outages, but are also for routine situations such as transporting a patient within the hospital. GE Healthcare supplies these backup batteries with the ventilator, and sells replacements when they run out.
However, if the ventilator loses power because of battery malfunction, the patient may lose access to oxygen, leading to hypoxia, which can lead to brain injury and death. Therefore, if these batteries drain quicker than anticipated, it may put the patient at risk.
To prevent this danger, GE Healthcare recommends customers perform a battery performance test after they see this notice and every 3 months following. Consumers should take extra precaution and make sure their batteries are charged following a long period of inactivity. If the device is inactive for a while, the company says users should keep it plugged in to avoid draining the battery. Batteries should be replaced at a minimum of every 3 years.
When these devices are still plugged into the wall, they’re safe to use, according to the FDA. But when using the backup power source, clinicians should make sure to have alternative routes for breathing support on hand, such as with a bag-valve mask system.
There are 4,222 of these possibly defective batteries currently on the market. They were distributed from April 2, 2019, through April 18 of this year, when GE Healthcare stopped distributing these products and began the recall process. Any issues with these products should be reported to the FDA’s MedWatch database or by sending a medical device notification acknowledgment response to GE at the email address listed at the bottom of the recall announcement.
A version of this article first appeared on Medscape.com.
This article was updated 7/6/22.
A total of 1,533 complaints allege that the batteries are draining much faster than expected, prompting manufacturer GE Healthcare to initiate the recall. There have been no injuries, and no deaths associated with the use of this device, according to an FDA corrected announcement.
Health care personnel and those patients who receive breathing support with these ventilators should be cautious about using CARESCAPE battery products moving forward, the agency said.
This type of ventilator is primarily powered by plugging into a wall outlet, but it has the capability to operate on backup batteries. These batteries are not solely for emergency situations such as power outages, but are also for routine situations such as transporting a patient within the hospital. GE Healthcare supplies these backup batteries with the ventilator, and sells replacements when they run out.
However, if the ventilator loses power because of battery malfunction, the patient may lose access to oxygen, leading to hypoxia, which can lead to brain injury and death. Therefore, if these batteries drain quicker than anticipated, it may put the patient at risk.
To prevent this danger, GE Healthcare recommends customers perform a battery performance test after they see this notice and every 3 months following. Consumers should take extra precaution and make sure their batteries are charged following a long period of inactivity. If the device is inactive for a while, the company says users should keep it plugged in to avoid draining the battery. Batteries should be replaced at a minimum of every 3 years.
When these devices are still plugged into the wall, they’re safe to use, according to the FDA. But when using the backup power source, clinicians should make sure to have alternative routes for breathing support on hand, such as with a bag-valve mask system.
There are 4,222 of these possibly defective batteries currently on the market. They were distributed from April 2, 2019, through April 18 of this year, when GE Healthcare stopped distributing these products and began the recall process. Any issues with these products should be reported to the FDA’s MedWatch database or by sending a medical device notification acknowledgment response to GE at the email address listed at the bottom of the recall announcement.
A version of this article first appeared on Medscape.com.
This article was updated 7/6/22.
Childhood melatonin poisonings skyrocket in the past 10 years
The number of children in the United States who unintentionally ingested melatonin supplements over the past 10 years has skyrocketed to the point where, as of 2021, melatonin ingestions by children accounted for almost 5% of all poisonings reported to poison control centers in the United States, data from the National Poison Data System (NPDS) indicate.
This compared with only 0.6% of melatonin ingestions reported to poison control centers in 2012, the authors added.
“Basically the number of pediatric melatonin ingestions increased 530% from 8,337 in 2012 to 52,563 in 2021 so it’s a 6.3-fold increase from the beginning of the study until the end,” Michael Toce, MD, one of the study authors and attending, pediatric emergency medicine/medical toxicology, Boston Children’s Hospital, said in an interview.
“And I think the biggest driver of this increase is simply that sales of melatonin have increased astronomically so there is just more melatonin at home and studies have shown there is a correlation between the amount of an individual medication in the home and the risk of pediatric exposure – so simply put: The more of a single substance in a home, the greater the chance that a child is going to get into it,” he underscored.
The study was published in the Morbidity and Mortality Weekly Report .
Melatonin ingestions
All cases of single substance melatonin ingestions involving children and adolescents between Jan. 1, 2012, and Dec. 31, 2021, were included in the analysis. During the 10-year study interval, 260,435 pediatric melatonin ingestions were reported to the NPDS. Over 94% of the reported ingestions were unintentional and 99% occurred in the home.
Over 88% of them were managed on-site; most involved young male children aged 5 years and under, and almost 83% of children who ingested melatonin supplements remained asymptomatic. On the other hand, 27,795 patients sought care at a health care facility and close to 15% of them were hospitalized. Among all melatonin ingestions, 1.6% resulted in more serious outcomes; more serious outcomes being defined as a moderate or major effects or death. Five children required mechanical ventilation in order to treat their symptoms and 2 patients died.
The largest number of patients who were hospitalized were adolescents who took melatonin intentionally but the largest increase in the rate of exposure was in young, unintentional patients, as Dr. Toce observed. Interestingly, the largest yearly increase in pediatric melatonin ingestions – almost 38% – coincided with the onset of the COVID-19 pandemic.
“This might be related to increased accessibility of melatonin during the pandemic, as children spent more time at home because of stay-at-home orders and school closures,” the authors speculate. Moreover, sleep disturbances were common during the pandemic, leading to a greater likelihood that parents were buying melatonin and thus exposing children to more melatonin at home.
Taken appropriately and at normal does, melatonin in itself is quite safe, as Dr. Toce stressed. However, “for any substance, the dose makes the poison, so taken in any significant quantity, anything is going to be dangerous.” Moreover, it’s important to appreciate that melatonin, at least in the United States, is regulated as a dietary supplement, not as a pharmaceutical.
“Thus, it doesn’t get the same rigorous testing that something like acetaminophen does by the FDA and that means two things,” Dr. Toce noted. First, if the product says that each gummy contains 3 mg of melatonin, no independent body is verifying whether or not that statement is true so there could be 3 mg of melatonin in each gummy or there could be 10 mg,.
Secondly, because there is no impartial oversight for dietary supplements, there may in fact be no melatonin at all in the product or something else may be added to it that might be harmful. “Just because something is sold over-the-counter does not necessarily mean that it’s safe,” Dr. Toce stressed. To keep children safe from pharmaceuticals and supplements, he recommended several generic poison prevention tips. This advice could be passed on to patients who are parents.
- Keep all pharmaceuticals and supplements preferably locked away so there is less risk of children and adolescents taking products either unintentionally or intentionally
- If parents have no place to lock their products up, put them out of reach, high-up so children cannot easily access them
- Keep the product in the original child-resistant packaging as opposed to taking the pills out of the packaging and putting it in a plastic bag bag. “Certainly we’ve seen that when medications are moved into a non–child-resistant container, ingestions go up,” Dr. Toce warned
- Don’t refer to any medicine or supplement a child might take as “candy.” “A lot of children have difficulty taking medications so some families will say: ‘It’s time for your candy,’ ” Dr. Toce explained. Then, if a child does discover the “candy” on a table where they have access to it, they will not recognize it as medication and they’re likely to pop it into their mouth, thinking it is candy.
Lastly, and most importantly, parents who are considering trying a melatonin supplement to help a child sleep better should first establish a stable sleep routine for their child. “They also need to limit caffeinated beverages before bed as well as screen time,” Dr. Toce added.
And they should talk with their primary care provider as to whether or not initiation of a melatonin supplement is appropriate for their child – “and not just jump right into giving them melatonin without first discussing whether it is appropriate to do so,” Dr. Toce stressed.
Remarkable rise
In a comment on his own experience with melatonin poisoning over recent years, toxicology expert Kevin Osterhoudt, MD, of the University of Pennsylvania, Philadelphia and the Children’s Hospital of Philadelphia, noted that it has been their experience that there has been a remarkable rise in poison center reports of children ingesting melatonin in the recent past. For example, the Poison Control Center at CHOP received nearly 4,000 calls involving melatonin ingestion by children 5 years old or younger in the 5 years between 2017 and 2021 with increasing numbers every year.
“The [current study] supports that our regional observation that this has been a national trend,” Dr. Osterhoudt said. Dr. Osterhoudt agreed with Dr. Toce that good sleep is healthy, and it is very important to develop good sleep habits and a regular bedtime routine in order to do so. “In some situations, melatonin may be useful as a short-term sleep aid and that’s a good discussion to have with your child’s health care provider.”
If parents do decide to give their child a melatonin supplement, they need to keep in mind that melatonin may alter how the body handles other drugs such as those used to treat epilepsy or blood clotting. They also need to know experts are still uncertain about how melatonin affects the body over the long term and whether it is safe for mothers to take during pregnancy.
Dr. Osterhoudt offered his own recommendations for safe melatonin use in the home:
- Discuss planned melatonin use with your health care provider.
- Buy only high-quality supplements by looking for the “USP Verified” mark.
- Insist that manufacturers sell products in child-resistant bottles.
- Periodically inspect the medications in your home and dispose of medications that are no longer being used.
- Program the phone number of your regional poison control center into your phone; poison center experts are available 24/7 to answer questions and concerns about ingestions of melatonin (in the United States the number is 1-800-222-1222).
The study authors and neither Dr. Toce nor Dr. Osterhoudt had any relevant conflicts of interest to declare.
The number of children in the United States who unintentionally ingested melatonin supplements over the past 10 years has skyrocketed to the point where, as of 2021, melatonin ingestions by children accounted for almost 5% of all poisonings reported to poison control centers in the United States, data from the National Poison Data System (NPDS) indicate.
This compared with only 0.6% of melatonin ingestions reported to poison control centers in 2012, the authors added.
“Basically the number of pediatric melatonin ingestions increased 530% from 8,337 in 2012 to 52,563 in 2021 so it’s a 6.3-fold increase from the beginning of the study until the end,” Michael Toce, MD, one of the study authors and attending, pediatric emergency medicine/medical toxicology, Boston Children’s Hospital, said in an interview.
“And I think the biggest driver of this increase is simply that sales of melatonin have increased astronomically so there is just more melatonin at home and studies have shown there is a correlation between the amount of an individual medication in the home and the risk of pediatric exposure – so simply put: The more of a single substance in a home, the greater the chance that a child is going to get into it,” he underscored.
The study was published in the Morbidity and Mortality Weekly Report .
Melatonin ingestions
All cases of single substance melatonin ingestions involving children and adolescents between Jan. 1, 2012, and Dec. 31, 2021, were included in the analysis. During the 10-year study interval, 260,435 pediatric melatonin ingestions were reported to the NPDS. Over 94% of the reported ingestions were unintentional and 99% occurred in the home.
Over 88% of them were managed on-site; most involved young male children aged 5 years and under, and almost 83% of children who ingested melatonin supplements remained asymptomatic. On the other hand, 27,795 patients sought care at a health care facility and close to 15% of them were hospitalized. Among all melatonin ingestions, 1.6% resulted in more serious outcomes; more serious outcomes being defined as a moderate or major effects or death. Five children required mechanical ventilation in order to treat their symptoms and 2 patients died.
The largest number of patients who were hospitalized were adolescents who took melatonin intentionally but the largest increase in the rate of exposure was in young, unintentional patients, as Dr. Toce observed. Interestingly, the largest yearly increase in pediatric melatonin ingestions – almost 38% – coincided with the onset of the COVID-19 pandemic.
“This might be related to increased accessibility of melatonin during the pandemic, as children spent more time at home because of stay-at-home orders and school closures,” the authors speculate. Moreover, sleep disturbances were common during the pandemic, leading to a greater likelihood that parents were buying melatonin and thus exposing children to more melatonin at home.
Taken appropriately and at normal does, melatonin in itself is quite safe, as Dr. Toce stressed. However, “for any substance, the dose makes the poison, so taken in any significant quantity, anything is going to be dangerous.” Moreover, it’s important to appreciate that melatonin, at least in the United States, is regulated as a dietary supplement, not as a pharmaceutical.
“Thus, it doesn’t get the same rigorous testing that something like acetaminophen does by the FDA and that means two things,” Dr. Toce noted. First, if the product says that each gummy contains 3 mg of melatonin, no independent body is verifying whether or not that statement is true so there could be 3 mg of melatonin in each gummy or there could be 10 mg,.
Secondly, because there is no impartial oversight for dietary supplements, there may in fact be no melatonin at all in the product or something else may be added to it that might be harmful. “Just because something is sold over-the-counter does not necessarily mean that it’s safe,” Dr. Toce stressed. To keep children safe from pharmaceuticals and supplements, he recommended several generic poison prevention tips. This advice could be passed on to patients who are parents.
- Keep all pharmaceuticals and supplements preferably locked away so there is less risk of children and adolescents taking products either unintentionally or intentionally
- If parents have no place to lock their products up, put them out of reach, high-up so children cannot easily access them
- Keep the product in the original child-resistant packaging as opposed to taking the pills out of the packaging and putting it in a plastic bag bag. “Certainly we’ve seen that when medications are moved into a non–child-resistant container, ingestions go up,” Dr. Toce warned
- Don’t refer to any medicine or supplement a child might take as “candy.” “A lot of children have difficulty taking medications so some families will say: ‘It’s time for your candy,’ ” Dr. Toce explained. Then, if a child does discover the “candy” on a table where they have access to it, they will not recognize it as medication and they’re likely to pop it into their mouth, thinking it is candy.
Lastly, and most importantly, parents who are considering trying a melatonin supplement to help a child sleep better should first establish a stable sleep routine for their child. “They also need to limit caffeinated beverages before bed as well as screen time,” Dr. Toce added.
And they should talk with their primary care provider as to whether or not initiation of a melatonin supplement is appropriate for their child – “and not just jump right into giving them melatonin without first discussing whether it is appropriate to do so,” Dr. Toce stressed.
Remarkable rise
In a comment on his own experience with melatonin poisoning over recent years, toxicology expert Kevin Osterhoudt, MD, of the University of Pennsylvania, Philadelphia and the Children’s Hospital of Philadelphia, noted that it has been their experience that there has been a remarkable rise in poison center reports of children ingesting melatonin in the recent past. For example, the Poison Control Center at CHOP received nearly 4,000 calls involving melatonin ingestion by children 5 years old or younger in the 5 years between 2017 and 2021 with increasing numbers every year.
“The [current study] supports that our regional observation that this has been a national trend,” Dr. Osterhoudt said. Dr. Osterhoudt agreed with Dr. Toce that good sleep is healthy, and it is very important to develop good sleep habits and a regular bedtime routine in order to do so. “In some situations, melatonin may be useful as a short-term sleep aid and that’s a good discussion to have with your child’s health care provider.”
If parents do decide to give their child a melatonin supplement, they need to keep in mind that melatonin may alter how the body handles other drugs such as those used to treat epilepsy or blood clotting. They also need to know experts are still uncertain about how melatonin affects the body over the long term and whether it is safe for mothers to take during pregnancy.
Dr. Osterhoudt offered his own recommendations for safe melatonin use in the home:
- Discuss planned melatonin use with your health care provider.
- Buy only high-quality supplements by looking for the “USP Verified” mark.
- Insist that manufacturers sell products in child-resistant bottles.
- Periodically inspect the medications in your home and dispose of medications that are no longer being used.
- Program the phone number of your regional poison control center into your phone; poison center experts are available 24/7 to answer questions and concerns about ingestions of melatonin (in the United States the number is 1-800-222-1222).
The study authors and neither Dr. Toce nor Dr. Osterhoudt had any relevant conflicts of interest to declare.
The number of children in the United States who unintentionally ingested melatonin supplements over the past 10 years has skyrocketed to the point where, as of 2021, melatonin ingestions by children accounted for almost 5% of all poisonings reported to poison control centers in the United States, data from the National Poison Data System (NPDS) indicate.
This compared with only 0.6% of melatonin ingestions reported to poison control centers in 2012, the authors added.
“Basically the number of pediatric melatonin ingestions increased 530% from 8,337 in 2012 to 52,563 in 2021 so it’s a 6.3-fold increase from the beginning of the study until the end,” Michael Toce, MD, one of the study authors and attending, pediatric emergency medicine/medical toxicology, Boston Children’s Hospital, said in an interview.
“And I think the biggest driver of this increase is simply that sales of melatonin have increased astronomically so there is just more melatonin at home and studies have shown there is a correlation between the amount of an individual medication in the home and the risk of pediatric exposure – so simply put: The more of a single substance in a home, the greater the chance that a child is going to get into it,” he underscored.
The study was published in the Morbidity and Mortality Weekly Report .
Melatonin ingestions
All cases of single substance melatonin ingestions involving children and adolescents between Jan. 1, 2012, and Dec. 31, 2021, were included in the analysis. During the 10-year study interval, 260,435 pediatric melatonin ingestions were reported to the NPDS. Over 94% of the reported ingestions were unintentional and 99% occurred in the home.
Over 88% of them were managed on-site; most involved young male children aged 5 years and under, and almost 83% of children who ingested melatonin supplements remained asymptomatic. On the other hand, 27,795 patients sought care at a health care facility and close to 15% of them were hospitalized. Among all melatonin ingestions, 1.6% resulted in more serious outcomes; more serious outcomes being defined as a moderate or major effects or death. Five children required mechanical ventilation in order to treat their symptoms and 2 patients died.
The largest number of patients who were hospitalized were adolescents who took melatonin intentionally but the largest increase in the rate of exposure was in young, unintentional patients, as Dr. Toce observed. Interestingly, the largest yearly increase in pediatric melatonin ingestions – almost 38% – coincided with the onset of the COVID-19 pandemic.
“This might be related to increased accessibility of melatonin during the pandemic, as children spent more time at home because of stay-at-home orders and school closures,” the authors speculate. Moreover, sleep disturbances were common during the pandemic, leading to a greater likelihood that parents were buying melatonin and thus exposing children to more melatonin at home.
Taken appropriately and at normal does, melatonin in itself is quite safe, as Dr. Toce stressed. However, “for any substance, the dose makes the poison, so taken in any significant quantity, anything is going to be dangerous.” Moreover, it’s important to appreciate that melatonin, at least in the United States, is regulated as a dietary supplement, not as a pharmaceutical.
“Thus, it doesn’t get the same rigorous testing that something like acetaminophen does by the FDA and that means two things,” Dr. Toce noted. First, if the product says that each gummy contains 3 mg of melatonin, no independent body is verifying whether or not that statement is true so there could be 3 mg of melatonin in each gummy or there could be 10 mg,.
Secondly, because there is no impartial oversight for dietary supplements, there may in fact be no melatonin at all in the product or something else may be added to it that might be harmful. “Just because something is sold over-the-counter does not necessarily mean that it’s safe,” Dr. Toce stressed. To keep children safe from pharmaceuticals and supplements, he recommended several generic poison prevention tips. This advice could be passed on to patients who are parents.
- Keep all pharmaceuticals and supplements preferably locked away so there is less risk of children and adolescents taking products either unintentionally or intentionally
- If parents have no place to lock their products up, put them out of reach, high-up so children cannot easily access them
- Keep the product in the original child-resistant packaging as opposed to taking the pills out of the packaging and putting it in a plastic bag bag. “Certainly we’ve seen that when medications are moved into a non–child-resistant container, ingestions go up,” Dr. Toce warned
- Don’t refer to any medicine or supplement a child might take as “candy.” “A lot of children have difficulty taking medications so some families will say: ‘It’s time for your candy,’ ” Dr. Toce explained. Then, if a child does discover the “candy” on a table where they have access to it, they will not recognize it as medication and they’re likely to pop it into their mouth, thinking it is candy.
Lastly, and most importantly, parents who are considering trying a melatonin supplement to help a child sleep better should first establish a stable sleep routine for their child. “They also need to limit caffeinated beverages before bed as well as screen time,” Dr. Toce added.
And they should talk with their primary care provider as to whether or not initiation of a melatonin supplement is appropriate for their child – “and not just jump right into giving them melatonin without first discussing whether it is appropriate to do so,” Dr. Toce stressed.
Remarkable rise
In a comment on his own experience with melatonin poisoning over recent years, toxicology expert Kevin Osterhoudt, MD, of the University of Pennsylvania, Philadelphia and the Children’s Hospital of Philadelphia, noted that it has been their experience that there has been a remarkable rise in poison center reports of children ingesting melatonin in the recent past. For example, the Poison Control Center at CHOP received nearly 4,000 calls involving melatonin ingestion by children 5 years old or younger in the 5 years between 2017 and 2021 with increasing numbers every year.
“The [current study] supports that our regional observation that this has been a national trend,” Dr. Osterhoudt said. Dr. Osterhoudt agreed with Dr. Toce that good sleep is healthy, and it is very important to develop good sleep habits and a regular bedtime routine in order to do so. “In some situations, melatonin may be useful as a short-term sleep aid and that’s a good discussion to have with your child’s health care provider.”
If parents do decide to give their child a melatonin supplement, they need to keep in mind that melatonin may alter how the body handles other drugs such as those used to treat epilepsy or blood clotting. They also need to know experts are still uncertain about how melatonin affects the body over the long term and whether it is safe for mothers to take during pregnancy.
Dr. Osterhoudt offered his own recommendations for safe melatonin use in the home:
- Discuss planned melatonin use with your health care provider.
- Buy only high-quality supplements by looking for the “USP Verified” mark.
- Insist that manufacturers sell products in child-resistant bottles.
- Periodically inspect the medications in your home and dispose of medications that are no longer being used.
- Program the phone number of your regional poison control center into your phone; poison center experts are available 24/7 to answer questions and concerns about ingestions of melatonin (in the United States the number is 1-800-222-1222).
The study authors and neither Dr. Toce nor Dr. Osterhoudt had any relevant conflicts of interest to declare.
FROM THE MMWR
What are the signs of post–acute infection syndromes?
The long-term health consequences of COVID-19 have refocused our attention on post–acute infection syndromes (PAIS), starting a discussion on the need for a complete understanding of multisystemic pathophysiology, clinical indicators, and the epidemiology of these syndromes, representing a significant blind spot in the field of medicine. A better understanding of these persistent symptom profiles, not only for post-acute sequelae of SARS-CoV-2 infection (PASC), better known as long COVID, but also for other diseases with unexplainable post-acute sequelae, would allow doctors to fine tune the diagnostic criteria. Having a clear definition and better understanding of post–acute infection symptoms is a necessary step toward developing an evidence-based, multidisciplinary management approach.
PAIS, PASC, or long COVID
The observation of unexplained chronic sequelae after SARS-CoV-2 is known as PASC or long COVID.
Long COVID has been reported as a syndrome in survivors of serious and critical disease, but the effects also persist over time for subjects who experienced a mild infection that did not require admission to hospital. This means that PASC, especially when occurring after a mild or moderate COVID-19 infection, shares many of the same characteristics as chronic diseases triggered by other pathogenic organisms, many of which have not been sufficiently clarified.
PAIS are characterized by a set of core symptoms centering on the following:
- Exertion intolerance
- Disproportionate levels of fatigue
- Neurocognitive and sensory impairment
- Flu-like symptoms
- Unrefreshing sleep
- Myalgia/arthralgia
A plethora of nonspecific symptoms are often present to various degrees.
These similarities suggest a unifying pathophysiology that needs to be elucidated to properly understand and manage postinfectious chronic disability.
Overview of PAIS
A detailed revision on what is currently known about PAIS was published in Nature Medicine. It provided various useful pieces of information to assist with the poor recognition of these conditions in clinical practice, a result of which is that patients might experience delayed or a complete lack of clinical care.
The following consolidated postinfection sequelae are mentioned:
- Q fever fatigue syndrome, which follows infection by the intracellular bacterium Coxiella burnetii
- Post-dengue fatigue syndrome, which can follow infection by the mosquito-borne dengue virus
- Fatiguing and rheumatic symptoms in a subset of individuals infected with chikungunya virus, a mosquito-borne virus that causes fever and joint pain in the acute phase
- Post-polio syndrome, which can emerge as many as 15-40 years after an initial poliomyelitis attack (similarly, some other neurotropic microbes, such as West Nile virus, might lead to persistent effects)
- Prolonged, debilitating, chronic symptoms have long been reported in a subset of patients after common and typically nonserious infections. For example, after mononucleosis, a condition generally caused by Epstein-Barr virus (EBV), and after an outbreak of Giardia lamblia, an intestinal parasite that usually causes acute intestinal illness. In fact, several studies identified the association of this outbreak of giardiasis with chronic fatigue, irritable bowel syndrome (IBS), and fibromyalgia persisting for many years.
- Views expressed in the literature regarding the frequency and the validity of posttreatment Lyme disease syndrome are divided. Although substantial evidence points to persistence of arthralgia, fatigue, and subjective neurocognitive impairments in a minority of patients with Lyme disease after the recommended antibiotic treatment, some of the early studies have failed to characterize the initial Lyme disease episode with sufficient rigor.
Symptoms and signs
The symptoms and signs which, based on the evidence available, are seen more frequently in health care checks may be characterized as the following:
- Exertion intolerance, fatigue
- Flu-like and ‘sickness behavior’ symptoms: fever, feverishness, muscle pain, feeling sick, malaise, sweating, irritability
- Neurological/neurocognitive symptoms: brain fog, impaired concentration or memory, trouble finding words
- Rheumatologic symptoms: chronic or recurrent joint pain
- Trigger-specific symptoms: for example, eye problems post Ebola, IBS post Giardia, anosmia and ageusia post COVID-19, motor disturbances post polio and post West Nile virus
Myalgic encephalomyelitis/chronic fatigue syndrome
Patients with this disorder experience worsening of symptoms following physical, cognitive, or emotional exertion above their (very low) tolerated limit. Other prominent features frequently observed in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) are neurocognitive impairments (colloquially referred to as brain fog), unrefreshing sleep, pain, sensory disturbances, gastrointestinal issues, and various forms of dysautonomia. Up to 75% of ME/CFS cases report an infection-like episode preceding the onset of their illness. Postinfectious and postviral fatigue syndromes were originally postulated as subsets of chronic fatigue syndrome. However, there appears to be no clear consensus at present about whether these terms should be considered synonymous to the ME/CFS label or any of its subsets, or include a wider range of postinfectious fatigue conditions.
Practical diagnostic criteria
From a revision of the available criteria, it emerges that the diagnostic criteria for a PAIS should include not only the presence of symptoms, but ideally also the intensity, course, and constellation of symptoms within an individual, as the individual symptoms and symptom trajectories of PAIS vary over time, rendering a mere comparison of symptom presence at a single time point misleading. Furthermore, when a diagnosis of ME/CFS is made, attention should be given to the choice of diagnostic criteria, with preference given to the more conservative criteria, so as not to run the risk of overestimating the syndrome.
Asthenia is the cornerstone symptom for most epidemiological studies on PAIS, but it would be reductive to concentrate only on this rather than the other characteristics, such as the exacerbation of symptoms following exertion, together with other characteristic symptoms and signs that may allow for better identification of the overall, observable clinical picture in these postinfection syndromes, which have significant impacts on a patient’s quality of life.
This article was translated from Univadis Italy. A version of this article appeared on Medscape.com.
The long-term health consequences of COVID-19 have refocused our attention on post–acute infection syndromes (PAIS), starting a discussion on the need for a complete understanding of multisystemic pathophysiology, clinical indicators, and the epidemiology of these syndromes, representing a significant blind spot in the field of medicine. A better understanding of these persistent symptom profiles, not only for post-acute sequelae of SARS-CoV-2 infection (PASC), better known as long COVID, but also for other diseases with unexplainable post-acute sequelae, would allow doctors to fine tune the diagnostic criteria. Having a clear definition and better understanding of post–acute infection symptoms is a necessary step toward developing an evidence-based, multidisciplinary management approach.
PAIS, PASC, or long COVID
The observation of unexplained chronic sequelae after SARS-CoV-2 is known as PASC or long COVID.
Long COVID has been reported as a syndrome in survivors of serious and critical disease, but the effects also persist over time for subjects who experienced a mild infection that did not require admission to hospital. This means that PASC, especially when occurring after a mild or moderate COVID-19 infection, shares many of the same characteristics as chronic diseases triggered by other pathogenic organisms, many of which have not been sufficiently clarified.
PAIS are characterized by a set of core symptoms centering on the following:
- Exertion intolerance
- Disproportionate levels of fatigue
- Neurocognitive and sensory impairment
- Flu-like symptoms
- Unrefreshing sleep
- Myalgia/arthralgia
A plethora of nonspecific symptoms are often present to various degrees.
These similarities suggest a unifying pathophysiology that needs to be elucidated to properly understand and manage postinfectious chronic disability.
Overview of PAIS
A detailed revision on what is currently known about PAIS was published in Nature Medicine. It provided various useful pieces of information to assist with the poor recognition of these conditions in clinical practice, a result of which is that patients might experience delayed or a complete lack of clinical care.
The following consolidated postinfection sequelae are mentioned:
- Q fever fatigue syndrome, which follows infection by the intracellular bacterium Coxiella burnetii
- Post-dengue fatigue syndrome, which can follow infection by the mosquito-borne dengue virus
- Fatiguing and rheumatic symptoms in a subset of individuals infected with chikungunya virus, a mosquito-borne virus that causes fever and joint pain in the acute phase
- Post-polio syndrome, which can emerge as many as 15-40 years after an initial poliomyelitis attack (similarly, some other neurotropic microbes, such as West Nile virus, might lead to persistent effects)
- Prolonged, debilitating, chronic symptoms have long been reported in a subset of patients after common and typically nonserious infections. For example, after mononucleosis, a condition generally caused by Epstein-Barr virus (EBV), and after an outbreak of Giardia lamblia, an intestinal parasite that usually causes acute intestinal illness. In fact, several studies identified the association of this outbreak of giardiasis with chronic fatigue, irritable bowel syndrome (IBS), and fibromyalgia persisting for many years.
- Views expressed in the literature regarding the frequency and the validity of posttreatment Lyme disease syndrome are divided. Although substantial evidence points to persistence of arthralgia, fatigue, and subjective neurocognitive impairments in a minority of patients with Lyme disease after the recommended antibiotic treatment, some of the early studies have failed to characterize the initial Lyme disease episode with sufficient rigor.
Symptoms and signs
The symptoms and signs which, based on the evidence available, are seen more frequently in health care checks may be characterized as the following:
- Exertion intolerance, fatigue
- Flu-like and ‘sickness behavior’ symptoms: fever, feverishness, muscle pain, feeling sick, malaise, sweating, irritability
- Neurological/neurocognitive symptoms: brain fog, impaired concentration or memory, trouble finding words
- Rheumatologic symptoms: chronic or recurrent joint pain
- Trigger-specific symptoms: for example, eye problems post Ebola, IBS post Giardia, anosmia and ageusia post COVID-19, motor disturbances post polio and post West Nile virus
Myalgic encephalomyelitis/chronic fatigue syndrome
Patients with this disorder experience worsening of symptoms following physical, cognitive, or emotional exertion above their (very low) tolerated limit. Other prominent features frequently observed in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) are neurocognitive impairments (colloquially referred to as brain fog), unrefreshing sleep, pain, sensory disturbances, gastrointestinal issues, and various forms of dysautonomia. Up to 75% of ME/CFS cases report an infection-like episode preceding the onset of their illness. Postinfectious and postviral fatigue syndromes were originally postulated as subsets of chronic fatigue syndrome. However, there appears to be no clear consensus at present about whether these terms should be considered synonymous to the ME/CFS label or any of its subsets, or include a wider range of postinfectious fatigue conditions.
Practical diagnostic criteria
From a revision of the available criteria, it emerges that the diagnostic criteria for a PAIS should include not only the presence of symptoms, but ideally also the intensity, course, and constellation of symptoms within an individual, as the individual symptoms and symptom trajectories of PAIS vary over time, rendering a mere comparison of symptom presence at a single time point misleading. Furthermore, when a diagnosis of ME/CFS is made, attention should be given to the choice of diagnostic criteria, with preference given to the more conservative criteria, so as not to run the risk of overestimating the syndrome.
Asthenia is the cornerstone symptom for most epidemiological studies on PAIS, but it would be reductive to concentrate only on this rather than the other characteristics, such as the exacerbation of symptoms following exertion, together with other characteristic symptoms and signs that may allow for better identification of the overall, observable clinical picture in these postinfection syndromes, which have significant impacts on a patient’s quality of life.
This article was translated from Univadis Italy. A version of this article appeared on Medscape.com.
The long-term health consequences of COVID-19 have refocused our attention on post–acute infection syndromes (PAIS), starting a discussion on the need for a complete understanding of multisystemic pathophysiology, clinical indicators, and the epidemiology of these syndromes, representing a significant blind spot in the field of medicine. A better understanding of these persistent symptom profiles, not only for post-acute sequelae of SARS-CoV-2 infection (PASC), better known as long COVID, but also for other diseases with unexplainable post-acute sequelae, would allow doctors to fine tune the diagnostic criteria. Having a clear definition and better understanding of post–acute infection symptoms is a necessary step toward developing an evidence-based, multidisciplinary management approach.
PAIS, PASC, or long COVID
The observation of unexplained chronic sequelae after SARS-CoV-2 is known as PASC or long COVID.
Long COVID has been reported as a syndrome in survivors of serious and critical disease, but the effects also persist over time for subjects who experienced a mild infection that did not require admission to hospital. This means that PASC, especially when occurring after a mild or moderate COVID-19 infection, shares many of the same characteristics as chronic diseases triggered by other pathogenic organisms, many of which have not been sufficiently clarified.
PAIS are characterized by a set of core symptoms centering on the following:
- Exertion intolerance
- Disproportionate levels of fatigue
- Neurocognitive and sensory impairment
- Flu-like symptoms
- Unrefreshing sleep
- Myalgia/arthralgia
A plethora of nonspecific symptoms are often present to various degrees.
These similarities suggest a unifying pathophysiology that needs to be elucidated to properly understand and manage postinfectious chronic disability.
Overview of PAIS
A detailed revision on what is currently known about PAIS was published in Nature Medicine. It provided various useful pieces of information to assist with the poor recognition of these conditions in clinical practice, a result of which is that patients might experience delayed or a complete lack of clinical care.
The following consolidated postinfection sequelae are mentioned:
- Q fever fatigue syndrome, which follows infection by the intracellular bacterium Coxiella burnetii
- Post-dengue fatigue syndrome, which can follow infection by the mosquito-borne dengue virus
- Fatiguing and rheumatic symptoms in a subset of individuals infected with chikungunya virus, a mosquito-borne virus that causes fever and joint pain in the acute phase
- Post-polio syndrome, which can emerge as many as 15-40 years after an initial poliomyelitis attack (similarly, some other neurotropic microbes, such as West Nile virus, might lead to persistent effects)
- Prolonged, debilitating, chronic symptoms have long been reported in a subset of patients after common and typically nonserious infections. For example, after mononucleosis, a condition generally caused by Epstein-Barr virus (EBV), and after an outbreak of Giardia lamblia, an intestinal parasite that usually causes acute intestinal illness. In fact, several studies identified the association of this outbreak of giardiasis with chronic fatigue, irritable bowel syndrome (IBS), and fibromyalgia persisting for many years.
- Views expressed in the literature regarding the frequency and the validity of posttreatment Lyme disease syndrome are divided. Although substantial evidence points to persistence of arthralgia, fatigue, and subjective neurocognitive impairments in a minority of patients with Lyme disease after the recommended antibiotic treatment, some of the early studies have failed to characterize the initial Lyme disease episode with sufficient rigor.
Symptoms and signs
The symptoms and signs which, based on the evidence available, are seen more frequently in health care checks may be characterized as the following:
- Exertion intolerance, fatigue
- Flu-like and ‘sickness behavior’ symptoms: fever, feverishness, muscle pain, feeling sick, malaise, sweating, irritability
- Neurological/neurocognitive symptoms: brain fog, impaired concentration or memory, trouble finding words
- Rheumatologic symptoms: chronic or recurrent joint pain
- Trigger-specific symptoms: for example, eye problems post Ebola, IBS post Giardia, anosmia and ageusia post COVID-19, motor disturbances post polio and post West Nile virus
Myalgic encephalomyelitis/chronic fatigue syndrome
Patients with this disorder experience worsening of symptoms following physical, cognitive, or emotional exertion above their (very low) tolerated limit. Other prominent features frequently observed in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) are neurocognitive impairments (colloquially referred to as brain fog), unrefreshing sleep, pain, sensory disturbances, gastrointestinal issues, and various forms of dysautonomia. Up to 75% of ME/CFS cases report an infection-like episode preceding the onset of their illness. Postinfectious and postviral fatigue syndromes were originally postulated as subsets of chronic fatigue syndrome. However, there appears to be no clear consensus at present about whether these terms should be considered synonymous to the ME/CFS label or any of its subsets, or include a wider range of postinfectious fatigue conditions.
Practical diagnostic criteria
From a revision of the available criteria, it emerges that the diagnostic criteria for a PAIS should include not only the presence of symptoms, but ideally also the intensity, course, and constellation of symptoms within an individual, as the individual symptoms and symptom trajectories of PAIS vary over time, rendering a mere comparison of symptom presence at a single time point misleading. Furthermore, when a diagnosis of ME/CFS is made, attention should be given to the choice of diagnostic criteria, with preference given to the more conservative criteria, so as not to run the risk of overestimating the syndrome.
Asthenia is the cornerstone symptom for most epidemiological studies on PAIS, but it would be reductive to concentrate only on this rather than the other characteristics, such as the exacerbation of symptoms following exertion, together with other characteristic symptoms and signs that may allow for better identification of the overall, observable clinical picture in these postinfection syndromes, which have significant impacts on a patient’s quality of life.
This article was translated from Univadis Italy. A version of this article appeared on Medscape.com.
Pneumothorax, pneumomediastinum, and subcutaneous emphysema: The many faces of COVID-19 ARDS
I recall early in the pandemic being called to the bedside to examine an acutely decompensating patient with COVID-19. This was a 33-year-old, previously healthy woman, admitted to the medical ICU with hypoxemic respiratory failure requiring mechanical ventilation and undergoing treatment for severe acute respiratory distress syndrome (ARDS). I quickly realized she was seconds away from an arrest. As I examined her, one thing caught my eye. Her airway pressures had skyrocketed over the past few minutes. Could it be? I thought to myself as I reached for the ultrasound that confirmed my suspicions, tension pneumothorax. One emergent needle decompression and chest tube later and she survives, only to die a week later from overwhelming hypoxemia.
As we reflect on these past 26 months, we recall that caring for the critically ill patient with COVID-19 has posed numerous challenges. One challenge was the overwhelming incidence of the so-called “barotrauma-related complications.” However, we also recall seeing many patients develop such complications while receiving supplemental noninvasive forms of respiratory support. Perhaps, this is in agreement with prior literature that specifically discusses the presence of air outside the tracheobronchial tree and how it does not always correlate with high airway pressure and high tidal volumes, refuting the argument that these complications always fall under the umbrella of barotrauma. We will discuss these complications and attempt to shed light on the potential variables associated with their development.
The development of pneumothorax is a well-recognized complication associated with ventilator-dependent ARDS thought to be a form of barotrauma, with some reports indicating an incidence of 48.8% (Gattinoni L et al. JAMA. 1994;271[2]):1772-9) and a significantly increased mortality rate compared with postprocedural pneumothorax in the ICU (Chen K et al. Chest. 2002;122[2]:678-83). The incidence of such complication in COVID-19-related ARDS is significantly higher than in ARDS from other causes (Belletti A et al. Crit Care Med. 2022;50[3]:491-500), with a mortality rate approaching 100% (Chong WH et al. Heart Lung. 2021;50[5]:599-608).
So why are patients with COVID-19 developing these complications at a higher rate? When we examine the literature, we note that Leisman and colleagues (Am J Respir Crit Care Med. 2022;205[5]:507-19) describe higher baseline markers of alveolar damage, including RAGE (receptor for advanced glycation end-products) in mechanically ventilated patients with COVID-19 vs patients requiring mechanical ventilation for other causes. This poses a question that perhaps one of the main reasons patients with COVID-19 ARDS are at an increased risk for developing certain complications, such as pneumothorax, is inherent to the unique type of alveolar injury sustained with the infection. The authors also note that alveolar markers of injury had moderate to poor discrimination for invasive ventilation early in the disease and diminished over time in both ventilated patients receiving lung protective ventilation strategy and those spontaneously breathing. Likewise, this important finding suggests that the development of pneumothorax in patients with COVID-19 may not be entirely related to barotrauma.
Another phenomenon worth investigating is the development of pneumomediastinum and subcutaneous emphysema, with a reported seven-fold increased risk of development in patients with COVID-19. Lemmers and colleagues (ERJ Open Res. 2020;6[4]:00385-2020) found no statistically significant difference in PEEP, plateau pressure, ratio of tidal volume to ideal body weight, or compliance between patients who developed this complication and those who did not, again, signifying that perhaps there is more to the story here.
Belletti and colleagues (J Cardiothorac Vasc Anesth. 2021;35[12]:3642-51) published an article examining the predictors of pneumothorax and pneumomediastinum in patients with COVID-19. The authors found that the time from symptom onset to intubation and the total bilirubin level were the only two significant predictors for the development of these complications. They explain that longer time from symptom onset to intubation likely increased the risk for self-induced lung injury, inflammation, and fibrosis, contributing to the development of such complications. It is important to note that the authors did not find a significant difference in the ventilation parameters between patients who developed pneumothorax/pneumomediastinum and those who did not.
In our institute, we examined a total of 102 patients admitted to the ICU with COVID-19 ARDS over a 3-month period from March 2020 to May 2020. We identified a total of 36 patients who developed pneumothorax, pneumomediastinum, and/or subcutaneous emphysema. We compared these subjects to age- and gender-matched control subjects. Higher age was associated with an increased risk of development of these complications, whereas the presence of diabetes mellitus, hypertension, and chronic kidney disease at baseline was associated with lower risk. This translated into lower mSOFA scores in our subjects as opposed to the control subjects mainly due to higher creatinine levels at baseline in the control group, skewing our data and indicating that some predictive criteria may not reflect the underlying disease severity and risk for development of such complications. In analyzing our ventilator data and comparing the subjects to the control group, we found no differences in mode of ventilation, set tidal volumes, or PEEP levels between the two. The subjects had significantly higher peak airway pressures, lower compliance, and longer ventilator days. Intubation was needed significantly earlier in the subjects compared with the control group with a median of 2 days vs 6 days from admission. Our data are in concordance with prior published reports and are set to be presented in abstract form this May.
COVID-19 remains a challenging disease with the potential for morbid outcomes. As we phase out of the pandemic and move into an epidemic, future research direction will likely focus on some of the more unusually common complications, such as the ones presented here.
Dr. Abdullah is with the Henry Ford Health System, Detroit, Michigan.
I recall early in the pandemic being called to the bedside to examine an acutely decompensating patient with COVID-19. This was a 33-year-old, previously healthy woman, admitted to the medical ICU with hypoxemic respiratory failure requiring mechanical ventilation and undergoing treatment for severe acute respiratory distress syndrome (ARDS). I quickly realized she was seconds away from an arrest. As I examined her, one thing caught my eye. Her airway pressures had skyrocketed over the past few minutes. Could it be? I thought to myself as I reached for the ultrasound that confirmed my suspicions, tension pneumothorax. One emergent needle decompression and chest tube later and she survives, only to die a week later from overwhelming hypoxemia.
As we reflect on these past 26 months, we recall that caring for the critically ill patient with COVID-19 has posed numerous challenges. One challenge was the overwhelming incidence of the so-called “barotrauma-related complications.” However, we also recall seeing many patients develop such complications while receiving supplemental noninvasive forms of respiratory support. Perhaps, this is in agreement with prior literature that specifically discusses the presence of air outside the tracheobronchial tree and how it does not always correlate with high airway pressure and high tidal volumes, refuting the argument that these complications always fall under the umbrella of barotrauma. We will discuss these complications and attempt to shed light on the potential variables associated with their development.
The development of pneumothorax is a well-recognized complication associated with ventilator-dependent ARDS thought to be a form of barotrauma, with some reports indicating an incidence of 48.8% (Gattinoni L et al. JAMA. 1994;271[2]):1772-9) and a significantly increased mortality rate compared with postprocedural pneumothorax in the ICU (Chen K et al. Chest. 2002;122[2]:678-83). The incidence of such complication in COVID-19-related ARDS is significantly higher than in ARDS from other causes (Belletti A et al. Crit Care Med. 2022;50[3]:491-500), with a mortality rate approaching 100% (Chong WH et al. Heart Lung. 2021;50[5]:599-608).
So why are patients with COVID-19 developing these complications at a higher rate? When we examine the literature, we note that Leisman and colleagues (Am J Respir Crit Care Med. 2022;205[5]:507-19) describe higher baseline markers of alveolar damage, including RAGE (receptor for advanced glycation end-products) in mechanically ventilated patients with COVID-19 vs patients requiring mechanical ventilation for other causes. This poses a question that perhaps one of the main reasons patients with COVID-19 ARDS are at an increased risk for developing certain complications, such as pneumothorax, is inherent to the unique type of alveolar injury sustained with the infection. The authors also note that alveolar markers of injury had moderate to poor discrimination for invasive ventilation early in the disease and diminished over time in both ventilated patients receiving lung protective ventilation strategy and those spontaneously breathing. Likewise, this important finding suggests that the development of pneumothorax in patients with COVID-19 may not be entirely related to barotrauma.
Another phenomenon worth investigating is the development of pneumomediastinum and subcutaneous emphysema, with a reported seven-fold increased risk of development in patients with COVID-19. Lemmers and colleagues (ERJ Open Res. 2020;6[4]:00385-2020) found no statistically significant difference in PEEP, plateau pressure, ratio of tidal volume to ideal body weight, or compliance between patients who developed this complication and those who did not, again, signifying that perhaps there is more to the story here.
Belletti and colleagues (J Cardiothorac Vasc Anesth. 2021;35[12]:3642-51) published an article examining the predictors of pneumothorax and pneumomediastinum in patients with COVID-19. The authors found that the time from symptom onset to intubation and the total bilirubin level were the only two significant predictors for the development of these complications. They explain that longer time from symptom onset to intubation likely increased the risk for self-induced lung injury, inflammation, and fibrosis, contributing to the development of such complications. It is important to note that the authors did not find a significant difference in the ventilation parameters between patients who developed pneumothorax/pneumomediastinum and those who did not.
In our institute, we examined a total of 102 patients admitted to the ICU with COVID-19 ARDS over a 3-month period from March 2020 to May 2020. We identified a total of 36 patients who developed pneumothorax, pneumomediastinum, and/or subcutaneous emphysema. We compared these subjects to age- and gender-matched control subjects. Higher age was associated with an increased risk of development of these complications, whereas the presence of diabetes mellitus, hypertension, and chronic kidney disease at baseline was associated with lower risk. This translated into lower mSOFA scores in our subjects as opposed to the control subjects mainly due to higher creatinine levels at baseline in the control group, skewing our data and indicating that some predictive criteria may not reflect the underlying disease severity and risk for development of such complications. In analyzing our ventilator data and comparing the subjects to the control group, we found no differences in mode of ventilation, set tidal volumes, or PEEP levels between the two. The subjects had significantly higher peak airway pressures, lower compliance, and longer ventilator days. Intubation was needed significantly earlier in the subjects compared with the control group with a median of 2 days vs 6 days from admission. Our data are in concordance with prior published reports and are set to be presented in abstract form this May.
COVID-19 remains a challenging disease with the potential for morbid outcomes. As we phase out of the pandemic and move into an epidemic, future research direction will likely focus on some of the more unusually common complications, such as the ones presented here.
Dr. Abdullah is with the Henry Ford Health System, Detroit, Michigan.
I recall early in the pandemic being called to the bedside to examine an acutely decompensating patient with COVID-19. This was a 33-year-old, previously healthy woman, admitted to the medical ICU with hypoxemic respiratory failure requiring mechanical ventilation and undergoing treatment for severe acute respiratory distress syndrome (ARDS). I quickly realized she was seconds away from an arrest. As I examined her, one thing caught my eye. Her airway pressures had skyrocketed over the past few minutes. Could it be? I thought to myself as I reached for the ultrasound that confirmed my suspicions, tension pneumothorax. One emergent needle decompression and chest tube later and she survives, only to die a week later from overwhelming hypoxemia.
As we reflect on these past 26 months, we recall that caring for the critically ill patient with COVID-19 has posed numerous challenges. One challenge was the overwhelming incidence of the so-called “barotrauma-related complications.” However, we also recall seeing many patients develop such complications while receiving supplemental noninvasive forms of respiratory support. Perhaps, this is in agreement with prior literature that specifically discusses the presence of air outside the tracheobronchial tree and how it does not always correlate with high airway pressure and high tidal volumes, refuting the argument that these complications always fall under the umbrella of barotrauma. We will discuss these complications and attempt to shed light on the potential variables associated with their development.
The development of pneumothorax is a well-recognized complication associated with ventilator-dependent ARDS thought to be a form of barotrauma, with some reports indicating an incidence of 48.8% (Gattinoni L et al. JAMA. 1994;271[2]):1772-9) and a significantly increased mortality rate compared with postprocedural pneumothorax in the ICU (Chen K et al. Chest. 2002;122[2]:678-83). The incidence of such complication in COVID-19-related ARDS is significantly higher than in ARDS from other causes (Belletti A et al. Crit Care Med. 2022;50[3]:491-500), with a mortality rate approaching 100% (Chong WH et al. Heart Lung. 2021;50[5]:599-608).
So why are patients with COVID-19 developing these complications at a higher rate? When we examine the literature, we note that Leisman and colleagues (Am J Respir Crit Care Med. 2022;205[5]:507-19) describe higher baseline markers of alveolar damage, including RAGE (receptor for advanced glycation end-products) in mechanically ventilated patients with COVID-19 vs patients requiring mechanical ventilation for other causes. This poses a question that perhaps one of the main reasons patients with COVID-19 ARDS are at an increased risk for developing certain complications, such as pneumothorax, is inherent to the unique type of alveolar injury sustained with the infection. The authors also note that alveolar markers of injury had moderate to poor discrimination for invasive ventilation early in the disease and diminished over time in both ventilated patients receiving lung protective ventilation strategy and those spontaneously breathing. Likewise, this important finding suggests that the development of pneumothorax in patients with COVID-19 may not be entirely related to barotrauma.
Another phenomenon worth investigating is the development of pneumomediastinum and subcutaneous emphysema, with a reported seven-fold increased risk of development in patients with COVID-19. Lemmers and colleagues (ERJ Open Res. 2020;6[4]:00385-2020) found no statistically significant difference in PEEP, plateau pressure, ratio of tidal volume to ideal body weight, or compliance between patients who developed this complication and those who did not, again, signifying that perhaps there is more to the story here.
Belletti and colleagues (J Cardiothorac Vasc Anesth. 2021;35[12]:3642-51) published an article examining the predictors of pneumothorax and pneumomediastinum in patients with COVID-19. The authors found that the time from symptom onset to intubation and the total bilirubin level were the only two significant predictors for the development of these complications. They explain that longer time from symptom onset to intubation likely increased the risk for self-induced lung injury, inflammation, and fibrosis, contributing to the development of such complications. It is important to note that the authors did not find a significant difference in the ventilation parameters between patients who developed pneumothorax/pneumomediastinum and those who did not.
In our institute, we examined a total of 102 patients admitted to the ICU with COVID-19 ARDS over a 3-month period from March 2020 to May 2020. We identified a total of 36 patients who developed pneumothorax, pneumomediastinum, and/or subcutaneous emphysema. We compared these subjects to age- and gender-matched control subjects. Higher age was associated with an increased risk of development of these complications, whereas the presence of diabetes mellitus, hypertension, and chronic kidney disease at baseline was associated with lower risk. This translated into lower mSOFA scores in our subjects as opposed to the control subjects mainly due to higher creatinine levels at baseline in the control group, skewing our data and indicating that some predictive criteria may not reflect the underlying disease severity and risk for development of such complications. In analyzing our ventilator data and comparing the subjects to the control group, we found no differences in mode of ventilation, set tidal volumes, or PEEP levels between the two. The subjects had significantly higher peak airway pressures, lower compliance, and longer ventilator days. Intubation was needed significantly earlier in the subjects compared with the control group with a median of 2 days vs 6 days from admission. Our data are in concordance with prior published reports and are set to be presented in abstract form this May.
COVID-19 remains a challenging disease with the potential for morbid outcomes. As we phase out of the pandemic and move into an epidemic, future research direction will likely focus on some of the more unusually common complications, such as the ones presented here.
Dr. Abdullah is with the Henry Ford Health System, Detroit, Michigan.
Surgeons, who see it up close, offer ways to stop gun violence
Their strategies can work regardless of where you stand on the Second Amendment of the Constitution, said Patricia Turner, MD. “Our proposals are embraced by both gun owners and non–gun owners alike, and we are unique in that regard.”
These “implementable solutions” could prevent the next massacre, Dr. Turner, executive director of the American College of Surgeons, said during a news briefing the group sponsored on June 2.
“Our future – indeed all of our futures – depend on our ability to find durable, actionable steps that we can implement tomorrow to save lives,” she said.
Firsthand perspective
“Sadly I’m here today as a trauma surgeon who has cared for two of the largest mass shootings in modern U.S. history,” said Ronald Stewart, MD, chair of the department of surgery at University Hospital in San Antonio, Texas.
Dr. Stewart treated victims of the 2017 Sutherland Springs First Baptist Church shooting – where 27 people died, including the shooter – and the recent Uvalde school shooting, both in Texas.
“The injuries inflicted by high-velocity weapons used at both of these attacks are horrific. A high-capacity, magazine-fed automatic rifle such as the AR-15 causes extremely destructive tissue wounds,” he said.
One of the group’s proposals is to increase the regulation of high-velocity weapons, including AR-15s.
“These wounds are horribly lethal at close range, and sadly, most victims do not survive long enough to make it to a trauma center,” Dr. Stewart said.
On a positive note, “all of our current [Uvalde] patients are improving, which really brings us joy in this dark time,” he said. “But all of them have a long road to deal with recovery with both the physical and emotional impact of their injuries.”
Jeffrey Kerby, MD, agreed.
“Trauma surgeons see the short-term physical effects of these injuries and watch patients struggle with the long-term impact of these wounds,” said Dr. Kerby, director of trauma and acute care surgery at the University of Alabama at Birmingham.
Surgeons feel ‘profound impact’ of shootings
“Firearm violence has a profound impact on surgeons, and we are the undisputed subject matter experts in treating the tragic results,” said Patrick Bailey, MD, medical director for advocacy at the American College of Surgeons.
“This impacts surgeons as well,” said Dr. Kerby, chair of the Committee on Trauma for the surgeons’ group. “We are human, and we can’t help but share in the grief, the pain, and the suffering that our patients endure.
“As a pediatric surgeon ... I have too often witnessed the impact of firearm violence, and obviously, the devastation extends beyond the victims to their families,” he said. “To put it succinctly, in our culture, parents are not supposed to be put in a position of burying their children.”
A public health crisis
“It’s important to recognize that we’ve been talking about a public health approach,” said Eileen Bulger, MD, acting chief of the trauma division at the University of Washington in Seattle. That strategy is important for engaging both firearm owners and communities that have a higher risk for firearm violence, she said.
A committee of the American College of Surgeons developed specific recommendations in 2018, which are still valid today. The group brought together surgeons from across the U.S. including “passionate firearm owners and experts in firearm safety,” Dr. Bulger said.
The committee, for example, agreed on 10 specific recommendations “that we believe are bipartisan and could have an immediate impact in saving lives.”
“I’m a lifelong gun owner,” Dr. Bailey said, emphasizing that the team’s process included participation and perspective from other surgeons “who, like me, are also gun owners, but gun owners who also seek to reduce the impact of firearm violence in our country.”
The recommendations address these areas:
- Gun ownership
- Firearm registration
- Licensure
- Education and training
- Ownership responsibilities
- Mandatory reporting and risk reduction
- Safety innovation and technology
- Research
- The culture of violence
- Social isolation and mental health
For example, “we currently have certain classes of weapons with significant offensive capability,” Dr. Bulger said, “that are appropriately restricted and regulated under the National Firearms Act as Class 3 weapons.”
This group includes fully automatic machine guns, explosive devices, and short-barrel shotguns.
“We recommend a formal reassessment of the firearms designated within each of these national firearms classifications,” Dr. Bulger said.
For example, high-capacity, magazine-fed semiautomatic rifles, such as the AR-15, should be considered for reclassification as NFA Class 3 firearms, or they should get a new designation with tighter regulation.
The ACS endorses formal firearm safety training for all new gun owners. Also, owners who do not provide reasonably safe firearm storage should be held responsible for events related to the discharge of their firearms, Dr. Bulger said. And people who are deemed an imminent threat to themselves or others through firearm ownership should be temporarily or permanently restricted, with due process.
Research and reporting reforms
The ACS is also calling for research on firearm injuries and firearm injury prevention to be federally funded, Dr. Bulger said. The research should be done in a nonpartisan manner, she said.
“We have concerns that the manner and tone in which information is released to the public may lead to copycat mass killers,” she said. “The ACS recommends that law enforcement officials and the press take steps to eliminate the notoriety of the shooter, for example.”
Dr. Bulger also addressed the mental health angle. “We encourage recognition of mental health warning signs and social isolation by teachers, counselors, peers, and parents.” When identified, immediate referral to professionals is needed.
In addition to these recommendations, another team from the American College of Surgeons has published an overview of ways to address the inequities that contribute to violence. “We advocate for federal funding to support the development of hospital-based and community programs for violence intervention and prevention,” Dr. Bulger said.
Dr. Bailey said that as a gun owner himself, he thinks other gun owners would support these recommendations.
“I do not believe that the steps recommended ... pose undue burden on the rights of individual gun owners,” he said.
The time is now
Most firearm injuries are not from mass shooting events, Dr. Kerby said.
“My own trauma center has seen a 40% increase in the number of firearm injuries just in the last 2 years,” he added, “and these numbers continue to grow.”
A version of this article first appeared on WebMD.com.
Their strategies can work regardless of where you stand on the Second Amendment of the Constitution, said Patricia Turner, MD. “Our proposals are embraced by both gun owners and non–gun owners alike, and we are unique in that regard.”
These “implementable solutions” could prevent the next massacre, Dr. Turner, executive director of the American College of Surgeons, said during a news briefing the group sponsored on June 2.
“Our future – indeed all of our futures – depend on our ability to find durable, actionable steps that we can implement tomorrow to save lives,” she said.
Firsthand perspective
“Sadly I’m here today as a trauma surgeon who has cared for two of the largest mass shootings in modern U.S. history,” said Ronald Stewart, MD, chair of the department of surgery at University Hospital in San Antonio, Texas.
Dr. Stewart treated victims of the 2017 Sutherland Springs First Baptist Church shooting – where 27 people died, including the shooter – and the recent Uvalde school shooting, both in Texas.
“The injuries inflicted by high-velocity weapons used at both of these attacks are horrific. A high-capacity, magazine-fed automatic rifle such as the AR-15 causes extremely destructive tissue wounds,” he said.
One of the group’s proposals is to increase the regulation of high-velocity weapons, including AR-15s.
“These wounds are horribly lethal at close range, and sadly, most victims do not survive long enough to make it to a trauma center,” Dr. Stewart said.
On a positive note, “all of our current [Uvalde] patients are improving, which really brings us joy in this dark time,” he said. “But all of them have a long road to deal with recovery with both the physical and emotional impact of their injuries.”
Jeffrey Kerby, MD, agreed.
“Trauma surgeons see the short-term physical effects of these injuries and watch patients struggle with the long-term impact of these wounds,” said Dr. Kerby, director of trauma and acute care surgery at the University of Alabama at Birmingham.
Surgeons feel ‘profound impact’ of shootings
“Firearm violence has a profound impact on surgeons, and we are the undisputed subject matter experts in treating the tragic results,” said Patrick Bailey, MD, medical director for advocacy at the American College of Surgeons.
“This impacts surgeons as well,” said Dr. Kerby, chair of the Committee on Trauma for the surgeons’ group. “We are human, and we can’t help but share in the grief, the pain, and the suffering that our patients endure.
“As a pediatric surgeon ... I have too often witnessed the impact of firearm violence, and obviously, the devastation extends beyond the victims to their families,” he said. “To put it succinctly, in our culture, parents are not supposed to be put in a position of burying their children.”
A public health crisis
“It’s important to recognize that we’ve been talking about a public health approach,” said Eileen Bulger, MD, acting chief of the trauma division at the University of Washington in Seattle. That strategy is important for engaging both firearm owners and communities that have a higher risk for firearm violence, she said.
A committee of the American College of Surgeons developed specific recommendations in 2018, which are still valid today. The group brought together surgeons from across the U.S. including “passionate firearm owners and experts in firearm safety,” Dr. Bulger said.
The committee, for example, agreed on 10 specific recommendations “that we believe are bipartisan and could have an immediate impact in saving lives.”
“I’m a lifelong gun owner,” Dr. Bailey said, emphasizing that the team’s process included participation and perspective from other surgeons “who, like me, are also gun owners, but gun owners who also seek to reduce the impact of firearm violence in our country.”
The recommendations address these areas:
- Gun ownership
- Firearm registration
- Licensure
- Education and training
- Ownership responsibilities
- Mandatory reporting and risk reduction
- Safety innovation and technology
- Research
- The culture of violence
- Social isolation and mental health
For example, “we currently have certain classes of weapons with significant offensive capability,” Dr. Bulger said, “that are appropriately restricted and regulated under the National Firearms Act as Class 3 weapons.”
This group includes fully automatic machine guns, explosive devices, and short-barrel shotguns.
“We recommend a formal reassessment of the firearms designated within each of these national firearms classifications,” Dr. Bulger said.
For example, high-capacity, magazine-fed semiautomatic rifles, such as the AR-15, should be considered for reclassification as NFA Class 3 firearms, or they should get a new designation with tighter regulation.
The ACS endorses formal firearm safety training for all new gun owners. Also, owners who do not provide reasonably safe firearm storage should be held responsible for events related to the discharge of their firearms, Dr. Bulger said. And people who are deemed an imminent threat to themselves or others through firearm ownership should be temporarily or permanently restricted, with due process.
Research and reporting reforms
The ACS is also calling for research on firearm injuries and firearm injury prevention to be federally funded, Dr. Bulger said. The research should be done in a nonpartisan manner, she said.
“We have concerns that the manner and tone in which information is released to the public may lead to copycat mass killers,” she said. “The ACS recommends that law enforcement officials and the press take steps to eliminate the notoriety of the shooter, for example.”
Dr. Bulger also addressed the mental health angle. “We encourage recognition of mental health warning signs and social isolation by teachers, counselors, peers, and parents.” When identified, immediate referral to professionals is needed.
In addition to these recommendations, another team from the American College of Surgeons has published an overview of ways to address the inequities that contribute to violence. “We advocate for federal funding to support the development of hospital-based and community programs for violence intervention and prevention,” Dr. Bulger said.
Dr. Bailey said that as a gun owner himself, he thinks other gun owners would support these recommendations.
“I do not believe that the steps recommended ... pose undue burden on the rights of individual gun owners,” he said.
The time is now
Most firearm injuries are not from mass shooting events, Dr. Kerby said.
“My own trauma center has seen a 40% increase in the number of firearm injuries just in the last 2 years,” he added, “and these numbers continue to grow.”
A version of this article first appeared on WebMD.com.
Their strategies can work regardless of where you stand on the Second Amendment of the Constitution, said Patricia Turner, MD. “Our proposals are embraced by both gun owners and non–gun owners alike, and we are unique in that regard.”
These “implementable solutions” could prevent the next massacre, Dr. Turner, executive director of the American College of Surgeons, said during a news briefing the group sponsored on June 2.
“Our future – indeed all of our futures – depend on our ability to find durable, actionable steps that we can implement tomorrow to save lives,” she said.
Firsthand perspective
“Sadly I’m here today as a trauma surgeon who has cared for two of the largest mass shootings in modern U.S. history,” said Ronald Stewart, MD, chair of the department of surgery at University Hospital in San Antonio, Texas.
Dr. Stewart treated victims of the 2017 Sutherland Springs First Baptist Church shooting – where 27 people died, including the shooter – and the recent Uvalde school shooting, both in Texas.
“The injuries inflicted by high-velocity weapons used at both of these attacks are horrific. A high-capacity, magazine-fed automatic rifle such as the AR-15 causes extremely destructive tissue wounds,” he said.
One of the group’s proposals is to increase the regulation of high-velocity weapons, including AR-15s.
“These wounds are horribly lethal at close range, and sadly, most victims do not survive long enough to make it to a trauma center,” Dr. Stewart said.
On a positive note, “all of our current [Uvalde] patients are improving, which really brings us joy in this dark time,” he said. “But all of them have a long road to deal with recovery with both the physical and emotional impact of their injuries.”
Jeffrey Kerby, MD, agreed.
“Trauma surgeons see the short-term physical effects of these injuries and watch patients struggle with the long-term impact of these wounds,” said Dr. Kerby, director of trauma and acute care surgery at the University of Alabama at Birmingham.
Surgeons feel ‘profound impact’ of shootings
“Firearm violence has a profound impact on surgeons, and we are the undisputed subject matter experts in treating the tragic results,” said Patrick Bailey, MD, medical director for advocacy at the American College of Surgeons.
“This impacts surgeons as well,” said Dr. Kerby, chair of the Committee on Trauma for the surgeons’ group. “We are human, and we can’t help but share in the grief, the pain, and the suffering that our patients endure.
“As a pediatric surgeon ... I have too often witnessed the impact of firearm violence, and obviously, the devastation extends beyond the victims to their families,” he said. “To put it succinctly, in our culture, parents are not supposed to be put in a position of burying their children.”
A public health crisis
“It’s important to recognize that we’ve been talking about a public health approach,” said Eileen Bulger, MD, acting chief of the trauma division at the University of Washington in Seattle. That strategy is important for engaging both firearm owners and communities that have a higher risk for firearm violence, she said.
A committee of the American College of Surgeons developed specific recommendations in 2018, which are still valid today. The group brought together surgeons from across the U.S. including “passionate firearm owners and experts in firearm safety,” Dr. Bulger said.
The committee, for example, agreed on 10 specific recommendations “that we believe are bipartisan and could have an immediate impact in saving lives.”
“I’m a lifelong gun owner,” Dr. Bailey said, emphasizing that the team’s process included participation and perspective from other surgeons “who, like me, are also gun owners, but gun owners who also seek to reduce the impact of firearm violence in our country.”
The recommendations address these areas:
- Gun ownership
- Firearm registration
- Licensure
- Education and training
- Ownership responsibilities
- Mandatory reporting and risk reduction
- Safety innovation and technology
- Research
- The culture of violence
- Social isolation and mental health
For example, “we currently have certain classes of weapons with significant offensive capability,” Dr. Bulger said, “that are appropriately restricted and regulated under the National Firearms Act as Class 3 weapons.”
This group includes fully automatic machine guns, explosive devices, and short-barrel shotguns.
“We recommend a formal reassessment of the firearms designated within each of these national firearms classifications,” Dr. Bulger said.
For example, high-capacity, magazine-fed semiautomatic rifles, such as the AR-15, should be considered for reclassification as NFA Class 3 firearms, or they should get a new designation with tighter regulation.
The ACS endorses formal firearm safety training for all new gun owners. Also, owners who do not provide reasonably safe firearm storage should be held responsible for events related to the discharge of their firearms, Dr. Bulger said. And people who are deemed an imminent threat to themselves or others through firearm ownership should be temporarily or permanently restricted, with due process.
Research and reporting reforms
The ACS is also calling for research on firearm injuries and firearm injury prevention to be federally funded, Dr. Bulger said. The research should be done in a nonpartisan manner, she said.
“We have concerns that the manner and tone in which information is released to the public may lead to copycat mass killers,” she said. “The ACS recommends that law enforcement officials and the press take steps to eliminate the notoriety of the shooter, for example.”
Dr. Bulger also addressed the mental health angle. “We encourage recognition of mental health warning signs and social isolation by teachers, counselors, peers, and parents.” When identified, immediate referral to professionals is needed.
In addition to these recommendations, another team from the American College of Surgeons has published an overview of ways to address the inequities that contribute to violence. “We advocate for federal funding to support the development of hospital-based and community programs for violence intervention and prevention,” Dr. Bulger said.
Dr. Bailey said that as a gun owner himself, he thinks other gun owners would support these recommendations.
“I do not believe that the steps recommended ... pose undue burden on the rights of individual gun owners,” he said.
The time is now
Most firearm injuries are not from mass shooting events, Dr. Kerby said.
“My own trauma center has seen a 40% increase in the number of firearm injuries just in the last 2 years,” he added, “and these numbers continue to grow.”
A version of this article first appeared on WebMD.com.
Intravenous Immunoglobulin in Treating Nonventilated COVID-19 Patients With Moderate-to-Severe Hypoxia: A Pharmacoeconomic Analysis
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board ([email protected]), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.
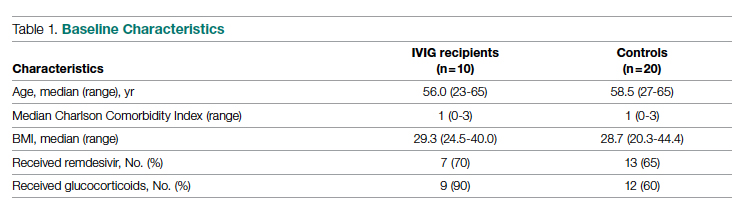
Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).
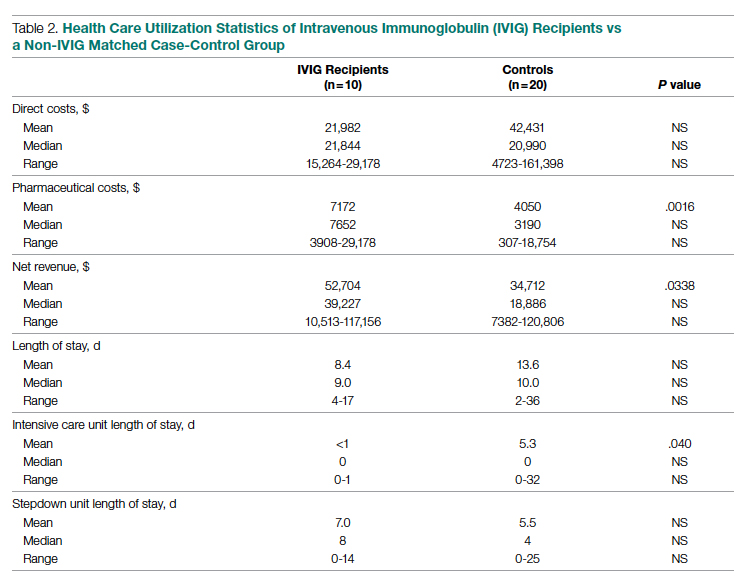
LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).

Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; [email protected]
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board ([email protected]), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.

Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).

LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).

Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; [email protected]
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board ([email protected]), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.

Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).

LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).

Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; [email protected]
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
ED staff speak out about workplace violence, ask for mitigation
WASHINGTON – Speaker after speaker, veteran emergency department physicians and nurses approached the podium for a May 4 press conference on the U.S. Capitol lawn across from the East Senate steps to describe violent incidents – being bitten, punched, slapped, kicked, choked, spat on, threatened – that they have both observed and have been subject to while working in EDs.
The press conference was cosponsored by the American College of Emergency Physicians and the Emergency Nurses Association, which have partnered since 2019 on the No Silence on ED Violence campaign.
The numbers confirm their experience. A 2018 poll of 3,500 ED physicians nationwide, which was conducted by Marketing General and was reported at ACEP’s annual meeting, found that nearly half of respondents had been assaulted at work; 27% of them were injured from the assault. Nurses, who spend more time with patients, may face even higher rates.
Incidence was reported to be increasing in 2018, and that was before the social and psychological upheavals imposed by the COVID pandemic caused assaults on staff in the hospital to go up an estimated 200%-300%.
But what really grated was that more than 95% of such cases, mostly perpetrated by patients, were never prosecuted, said Jennifer Casaletto, MD, FACEP, a North Carolina emergency physician and president of the state’s ACEP chapter. “Hospital and law enforcement see violence as just part of the job in our EDs.”
It’s no secret that workplace violence is increasing, Dr. Casaletto said. Four weeks ago, she stitched up the face of a charge nurse who had been assaulted. The nurse didn’t report the incident because she didn’t believe anything would change.
“Listening to my colleagues, I know the terror they have felt in the moment – for themselves, their colleagues, their patients. I know that raw fear of being attacked, and the complex emotions that follow. I’ve been hit, bit, and punched and watched colleagues getting choked.”
Dr. Casaletto was present in the ED when an out-of-control patient clubbed a nurse with an IV pole as she tried to close the doors to other patients’ rooms. “Instinctively, I pulled my stethoscope from around my neck, hoping I wouldn’t be strangled with it.”
Tennessee emergency nurse Todd Haines, MSN, RN, AEMT, CEN, said he has stepped in to help pull patients off coworkers. “I’ve seen some staff so severely injured they could not return to the bedside. I’ve been verbally threatened. My family has been threatened by patients and their families,” he reported. “We’ve all seen it. And COVID has made some people even meaner. They just lose their minds, and ED staff take the brunt of their aggression. But then to report these incidents and hear: ‘It’s just part of your job,’ well, it’s not part of my job.”
Mr. Haines spent 10 years in law enforcement with a sheriff’s department in middle Tennessee and was on its special tactical response team before becoming an ED nurse. He said he saw many more verbal and physical assaults in 11 years in the ED than during his police career.
“I love emergency nursing at the bedside, but it got to the point where I took the first chance to leave the bedside. And I’m not alone. Other nurses are leaving in droves.” Mr. Haines now has a job directing a trauma program, and he volunteers on policy issues for the Tennessee ENA. But he worries about the toll of this violence on the ED workforce, with so many professionals already mulling over leaving the field because of job stress and burnout.
“We have to do something to keep experienced hospital emergency staff at the bedside.”
What’s the answer?
Also speaking at the press conference was Senator Tammy Baldwin (D-Wis.), who pledged to introduce the Workplace Violence Prevention for Health Care and Social Services Workers Act, which passed the House in April. This bill would direct the Occupational Health and Safety Administration to issue a standard requiring employers in health care and social services to develop and implement workplace violence prevention plans. It would cover a variety of health facilities but not doctor’s offices or home-based services.
An interim final standard would be due within a year of enactment, with a final version to follow. Covered employers would have 6 months to develop and implement their own comprehensive workplace violence prevention plans, with the meaningful participation of direct care employees, tailored for and specific to the conditions and hazards of their facility, informed by past violent incidents, and subject to the size and complexity of the setting.
The plan would also name an individual responsible for its implementation, would include staff training and education, and would require facilities to track incidents and prohibit retaliation against employees who reported incidents of workplace violence.
On Wednesday, Sen. Baldwin called for unanimous consent on the Senate floor to fast-track this bill, but that was opposed by Senator Mike Braun (R-Ind.). She will soon introduce legislation similar to HR 1195, which the House passed.
“This bill will provide long overdue protections and safety standards,” she said. It will ensure that workplaces adopt proven protection techniques, such as those in OSHA’s 2015 guideline for preventing health care workplace violence. The American Hospital Association opposed the House bill on the grounds that hospitals have already implemented policies and programs specifically tailored to address workplace violence, so the OSHA standards required by the bill are not warranted.
Another speaker at the press conference, Aisha Terry, MD, MPH, FACEP, an emergency physician for George Washington University and Veterans Affairs in Washington, D.C., and current vice president of ACEP, described an incident that occurred when she was at work. A patient punched the nurse caring for him in the face, knocking her unconscious to the floor. “I’ll never forget that sound,” Dr. Terry said. “To this day, it has impacted her career. She hasn’t known what to do.”
Many people don’t realize how bad workplace violence really is, Dr. Terry added. “You assume you can serve as the safety net of this country, taking care of patients in the context of the pandemic, and feel safe – and not have to worry about your own safety. It’s past due that we put an end to this.”
Biggest win
Mr. Haines called the workplace violence bill a game changer for ED professionals, now and into the future. “We’re not going to totally eliminate violence in the emergency department. That is part of our business. But this legislation will support us and give a safer environment for us to do the work we love,” he said.
“The biggest win for this legislation is that it will create a supportive, nonretaliatory environment. It will give us as nurses a structured way to report things.” And, when these incidents do get reported, staff will get the help they need, Mr. Haines said. “The legislation will help show the importance of implementing systems and processes in emergency settings to address the risks and hazards that makes us all vulnerable to violence.”
No relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
WASHINGTON – Speaker after speaker, veteran emergency department physicians and nurses approached the podium for a May 4 press conference on the U.S. Capitol lawn across from the East Senate steps to describe violent incidents – being bitten, punched, slapped, kicked, choked, spat on, threatened – that they have both observed and have been subject to while working in EDs.
The press conference was cosponsored by the American College of Emergency Physicians and the Emergency Nurses Association, which have partnered since 2019 on the No Silence on ED Violence campaign.
The numbers confirm their experience. A 2018 poll of 3,500 ED physicians nationwide, which was conducted by Marketing General and was reported at ACEP’s annual meeting, found that nearly half of respondents had been assaulted at work; 27% of them were injured from the assault. Nurses, who spend more time with patients, may face even higher rates.
Incidence was reported to be increasing in 2018, and that was before the social and psychological upheavals imposed by the COVID pandemic caused assaults on staff in the hospital to go up an estimated 200%-300%.
But what really grated was that more than 95% of such cases, mostly perpetrated by patients, were never prosecuted, said Jennifer Casaletto, MD, FACEP, a North Carolina emergency physician and president of the state’s ACEP chapter. “Hospital and law enforcement see violence as just part of the job in our EDs.”
It’s no secret that workplace violence is increasing, Dr. Casaletto said. Four weeks ago, she stitched up the face of a charge nurse who had been assaulted. The nurse didn’t report the incident because she didn’t believe anything would change.
“Listening to my colleagues, I know the terror they have felt in the moment – for themselves, their colleagues, their patients. I know that raw fear of being attacked, and the complex emotions that follow. I’ve been hit, bit, and punched and watched colleagues getting choked.”
Dr. Casaletto was present in the ED when an out-of-control patient clubbed a nurse with an IV pole as she tried to close the doors to other patients’ rooms. “Instinctively, I pulled my stethoscope from around my neck, hoping I wouldn’t be strangled with it.”
Tennessee emergency nurse Todd Haines, MSN, RN, AEMT, CEN, said he has stepped in to help pull patients off coworkers. “I’ve seen some staff so severely injured they could not return to the bedside. I’ve been verbally threatened. My family has been threatened by patients and their families,” he reported. “We’ve all seen it. And COVID has made some people even meaner. They just lose their minds, and ED staff take the brunt of their aggression. But then to report these incidents and hear: ‘It’s just part of your job,’ well, it’s not part of my job.”
Mr. Haines spent 10 years in law enforcement with a sheriff’s department in middle Tennessee and was on its special tactical response team before becoming an ED nurse. He said he saw many more verbal and physical assaults in 11 years in the ED than during his police career.
“I love emergency nursing at the bedside, but it got to the point where I took the first chance to leave the bedside. And I’m not alone. Other nurses are leaving in droves.” Mr. Haines now has a job directing a trauma program, and he volunteers on policy issues for the Tennessee ENA. But he worries about the toll of this violence on the ED workforce, with so many professionals already mulling over leaving the field because of job stress and burnout.
“We have to do something to keep experienced hospital emergency staff at the bedside.”
What’s the answer?
Also speaking at the press conference was Senator Tammy Baldwin (D-Wis.), who pledged to introduce the Workplace Violence Prevention for Health Care and Social Services Workers Act, which passed the House in April. This bill would direct the Occupational Health and Safety Administration to issue a standard requiring employers in health care and social services to develop and implement workplace violence prevention plans. It would cover a variety of health facilities but not doctor’s offices or home-based services.
An interim final standard would be due within a year of enactment, with a final version to follow. Covered employers would have 6 months to develop and implement their own comprehensive workplace violence prevention plans, with the meaningful participation of direct care employees, tailored for and specific to the conditions and hazards of their facility, informed by past violent incidents, and subject to the size and complexity of the setting.
The plan would also name an individual responsible for its implementation, would include staff training and education, and would require facilities to track incidents and prohibit retaliation against employees who reported incidents of workplace violence.
On Wednesday, Sen. Baldwin called for unanimous consent on the Senate floor to fast-track this bill, but that was opposed by Senator Mike Braun (R-Ind.). She will soon introduce legislation similar to HR 1195, which the House passed.
“This bill will provide long overdue protections and safety standards,” she said. It will ensure that workplaces adopt proven protection techniques, such as those in OSHA’s 2015 guideline for preventing health care workplace violence. The American Hospital Association opposed the House bill on the grounds that hospitals have already implemented policies and programs specifically tailored to address workplace violence, so the OSHA standards required by the bill are not warranted.
Another speaker at the press conference, Aisha Terry, MD, MPH, FACEP, an emergency physician for George Washington University and Veterans Affairs in Washington, D.C., and current vice president of ACEP, described an incident that occurred when she was at work. A patient punched the nurse caring for him in the face, knocking her unconscious to the floor. “I’ll never forget that sound,” Dr. Terry said. “To this day, it has impacted her career. She hasn’t known what to do.”
Many people don’t realize how bad workplace violence really is, Dr. Terry added. “You assume you can serve as the safety net of this country, taking care of patients in the context of the pandemic, and feel safe – and not have to worry about your own safety. It’s past due that we put an end to this.”
Biggest win
Mr. Haines called the workplace violence bill a game changer for ED professionals, now and into the future. “We’re not going to totally eliminate violence in the emergency department. That is part of our business. But this legislation will support us and give a safer environment for us to do the work we love,” he said.
“The biggest win for this legislation is that it will create a supportive, nonretaliatory environment. It will give us as nurses a structured way to report things.” And, when these incidents do get reported, staff will get the help they need, Mr. Haines said. “The legislation will help show the importance of implementing systems and processes in emergency settings to address the risks and hazards that makes us all vulnerable to violence.”
No relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
WASHINGTON – Speaker after speaker, veteran emergency department physicians and nurses approached the podium for a May 4 press conference on the U.S. Capitol lawn across from the East Senate steps to describe violent incidents – being bitten, punched, slapped, kicked, choked, spat on, threatened – that they have both observed and have been subject to while working in EDs.
The press conference was cosponsored by the American College of Emergency Physicians and the Emergency Nurses Association, which have partnered since 2019 on the No Silence on ED Violence campaign.
The numbers confirm their experience. A 2018 poll of 3,500 ED physicians nationwide, which was conducted by Marketing General and was reported at ACEP’s annual meeting, found that nearly half of respondents had been assaulted at work; 27% of them were injured from the assault. Nurses, who spend more time with patients, may face even higher rates.
Incidence was reported to be increasing in 2018, and that was before the social and psychological upheavals imposed by the COVID pandemic caused assaults on staff in the hospital to go up an estimated 200%-300%.
But what really grated was that more than 95% of such cases, mostly perpetrated by patients, were never prosecuted, said Jennifer Casaletto, MD, FACEP, a North Carolina emergency physician and president of the state’s ACEP chapter. “Hospital and law enforcement see violence as just part of the job in our EDs.”
It’s no secret that workplace violence is increasing, Dr. Casaletto said. Four weeks ago, she stitched up the face of a charge nurse who had been assaulted. The nurse didn’t report the incident because she didn’t believe anything would change.
“Listening to my colleagues, I know the terror they have felt in the moment – for themselves, their colleagues, their patients. I know that raw fear of being attacked, and the complex emotions that follow. I’ve been hit, bit, and punched and watched colleagues getting choked.”
Dr. Casaletto was present in the ED when an out-of-control patient clubbed a nurse with an IV pole as she tried to close the doors to other patients’ rooms. “Instinctively, I pulled my stethoscope from around my neck, hoping I wouldn’t be strangled with it.”
Tennessee emergency nurse Todd Haines, MSN, RN, AEMT, CEN, said he has stepped in to help pull patients off coworkers. “I’ve seen some staff so severely injured they could not return to the bedside. I’ve been verbally threatened. My family has been threatened by patients and their families,” he reported. “We’ve all seen it. And COVID has made some people even meaner. They just lose their minds, and ED staff take the brunt of their aggression. But then to report these incidents and hear: ‘It’s just part of your job,’ well, it’s not part of my job.”
Mr. Haines spent 10 years in law enforcement with a sheriff’s department in middle Tennessee and was on its special tactical response team before becoming an ED nurse. He said he saw many more verbal and physical assaults in 11 years in the ED than during his police career.
“I love emergency nursing at the bedside, but it got to the point where I took the first chance to leave the bedside. And I’m not alone. Other nurses are leaving in droves.” Mr. Haines now has a job directing a trauma program, and he volunteers on policy issues for the Tennessee ENA. But he worries about the toll of this violence on the ED workforce, with so many professionals already mulling over leaving the field because of job stress and burnout.
“We have to do something to keep experienced hospital emergency staff at the bedside.”
What’s the answer?
Also speaking at the press conference was Senator Tammy Baldwin (D-Wis.), who pledged to introduce the Workplace Violence Prevention for Health Care and Social Services Workers Act, which passed the House in April. This bill would direct the Occupational Health and Safety Administration to issue a standard requiring employers in health care and social services to develop and implement workplace violence prevention plans. It would cover a variety of health facilities but not doctor’s offices or home-based services.
An interim final standard would be due within a year of enactment, with a final version to follow. Covered employers would have 6 months to develop and implement their own comprehensive workplace violence prevention plans, with the meaningful participation of direct care employees, tailored for and specific to the conditions and hazards of their facility, informed by past violent incidents, and subject to the size and complexity of the setting.
The plan would also name an individual responsible for its implementation, would include staff training and education, and would require facilities to track incidents and prohibit retaliation against employees who reported incidents of workplace violence.
On Wednesday, Sen. Baldwin called for unanimous consent on the Senate floor to fast-track this bill, but that was opposed by Senator Mike Braun (R-Ind.). She will soon introduce legislation similar to HR 1195, which the House passed.
“This bill will provide long overdue protections and safety standards,” she said. It will ensure that workplaces adopt proven protection techniques, such as those in OSHA’s 2015 guideline for preventing health care workplace violence. The American Hospital Association opposed the House bill on the grounds that hospitals have already implemented policies and programs specifically tailored to address workplace violence, so the OSHA standards required by the bill are not warranted.
Another speaker at the press conference, Aisha Terry, MD, MPH, FACEP, an emergency physician for George Washington University and Veterans Affairs in Washington, D.C., and current vice president of ACEP, described an incident that occurred when she was at work. A patient punched the nurse caring for him in the face, knocking her unconscious to the floor. “I’ll never forget that sound,” Dr. Terry said. “To this day, it has impacted her career. She hasn’t known what to do.”
Many people don’t realize how bad workplace violence really is, Dr. Terry added. “You assume you can serve as the safety net of this country, taking care of patients in the context of the pandemic, and feel safe – and not have to worry about your own safety. It’s past due that we put an end to this.”
Biggest win
Mr. Haines called the workplace violence bill a game changer for ED professionals, now and into the future. “We’re not going to totally eliminate violence in the emergency department. That is part of our business. But this legislation will support us and give a safer environment for us to do the work we love,” he said.
“The biggest win for this legislation is that it will create a supportive, nonretaliatory environment. It will give us as nurses a structured way to report things.” And, when these incidents do get reported, staff will get the help they need, Mr. Haines said. “The legislation will help show the importance of implementing systems and processes in emergency settings to address the risks and hazards that makes us all vulnerable to violence.”
No relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.






