User login
COVID-19 accelerated psychological problems for critical care clinicians
Approximately one-third of critical care workers reported some degree of depression, anxiety, or somatic symptoms in the early phase of the COVID-19 pandemic, based on survey results from 939 health care professionals.
The emotional response of professionals in a critical care setting in the early phase of the COVID-19 pandemic has not been well studied, Robyn Branca, PhD, and Paul Branca, MD, of Carson Newman University and the University of Tennessee Medical Center, both in Knoxville, wrote in an abstract presented at the virtual Critical Care Congress sponsored by the Society of Critical Care Medicine.
The prevalence of depression, anxiety, and somatization is low in the general population overall, but the researchers predicted that these conditions increased among workers in critical care settings early in the pandemic.
To assess the prevalence of psychological problems during that time, they sent an email survey on April 7, 2020, to members of the Society of Critical Care Medicine. The survey collected data on demographics, perceived caseload, and potential course of the pandemic. The survey also collected responses to assessments for depression (using the Patient Health Questionnaire–9), anxiety (using the Generalized Anxiety Disorder [GAD] Scale–7), and symptom somatization (using the PHQ-15).
Of the 939 survey respondents, 37% were male, 61.4% were female, and 1.4% gave another or no response.
Overall, 32.3% reported encountering 0-50 COVID-19 cases, 31.1% had encountered 51-200 cases, 12.5% had encountered 201-500 cases, 9.4% had encountered 501-1000 cases, and 13.7% had encountered more than 1,000 cases.
Based on the PHQ-9 depression scale, 44.9% of the respondents had minimal symptoms, 31.1% mild symptoms, 14.3% moderate symptoms, and 9.7% met criteria for severe depressive symptoms. Based on the GAD-7 anxiety scale, 35.5% had minimal symptoms, 32.9% mild, 16.8% moderate, and 14.8% had severe symptoms. Based on the PHQ-15 somatization scale, 39.6% of respondents showed minimal symptoms, whereas 38.2% showed mild symptoms, 17.3% moderate symptoms, and 4.9% had a severe degree of somatic symptoms.
The study findings were limited by the reliance on self-reports; however, the results indicate that a high percentage of critical care workers experienced significant, diagnosable levels of depression, anxiety, and somatic symptoms, the researchers said.
The standard guidance is to pursue individual intervention for anyone with scores of moderate or severe on the scales used in the survey, the researchers said.
Therefore, the findings represent “an alarming degree of mental health impact,” they emphasized. “Immediate mitigation efforts are needed to preserve the health of our ICU workforce.”
The study is important at this time because clinician fatigue and occupational stress are at endemic levels, Bernard Chang, MD, of Columbia University Irving Medical Center, New York City, said in an interview. “It is vital that we take stock of how frontline workers in critical care settings are doing overall,” said Dr. Chang.
Dr. Chang, who was not involved with the study but has conducted research on mental health in frontline health care workers during the pandemic, said he was not surprised by the findings. “This work builds on the growing body of literature in the pandemic noting high levels of stress, fatigue, and depression/anxiety symptoms across many frontline workers, from emergency department staff, first responders and others. These are all data points highlighting the urgent need for a broad safety net, not only for patients but the providers serving them.”
The takeaway message: “Clinicians are often so focused on providing care for their patients that they may overlook the need to care for their own well-being and mental health,” said Dr. Chang.
As for additional research, “we need to now take this important data and build on creating and identifying tangible solutions to improve the morale of the acute care/health care workforce to ensure career longevity, professional satisfaction, and overall well-being,” Dr. Chang emphasized. Mental health and morale affect not only health care workers, but also the patients they care for. Well–cared for health care providers can be at their best to provide the optimal care for their patients.
The study received no outside funding. The researchers and Dr. Chang disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Approximately one-third of critical care workers reported some degree of depression, anxiety, or somatic symptoms in the early phase of the COVID-19 pandemic, based on survey results from 939 health care professionals.
The emotional response of professionals in a critical care setting in the early phase of the COVID-19 pandemic has not been well studied, Robyn Branca, PhD, and Paul Branca, MD, of Carson Newman University and the University of Tennessee Medical Center, both in Knoxville, wrote in an abstract presented at the virtual Critical Care Congress sponsored by the Society of Critical Care Medicine.
The prevalence of depression, anxiety, and somatization is low in the general population overall, but the researchers predicted that these conditions increased among workers in critical care settings early in the pandemic.
To assess the prevalence of psychological problems during that time, they sent an email survey on April 7, 2020, to members of the Society of Critical Care Medicine. The survey collected data on demographics, perceived caseload, and potential course of the pandemic. The survey also collected responses to assessments for depression (using the Patient Health Questionnaire–9), anxiety (using the Generalized Anxiety Disorder [GAD] Scale–7), and symptom somatization (using the PHQ-15).
Of the 939 survey respondents, 37% were male, 61.4% were female, and 1.4% gave another or no response.
Overall, 32.3% reported encountering 0-50 COVID-19 cases, 31.1% had encountered 51-200 cases, 12.5% had encountered 201-500 cases, 9.4% had encountered 501-1000 cases, and 13.7% had encountered more than 1,000 cases.
Based on the PHQ-9 depression scale, 44.9% of the respondents had minimal symptoms, 31.1% mild symptoms, 14.3% moderate symptoms, and 9.7% met criteria for severe depressive symptoms. Based on the GAD-7 anxiety scale, 35.5% had minimal symptoms, 32.9% mild, 16.8% moderate, and 14.8% had severe symptoms. Based on the PHQ-15 somatization scale, 39.6% of respondents showed minimal symptoms, whereas 38.2% showed mild symptoms, 17.3% moderate symptoms, and 4.9% had a severe degree of somatic symptoms.
The study findings were limited by the reliance on self-reports; however, the results indicate that a high percentage of critical care workers experienced significant, diagnosable levels of depression, anxiety, and somatic symptoms, the researchers said.
The standard guidance is to pursue individual intervention for anyone with scores of moderate or severe on the scales used in the survey, the researchers said.
Therefore, the findings represent “an alarming degree of mental health impact,” they emphasized. “Immediate mitigation efforts are needed to preserve the health of our ICU workforce.”
The study is important at this time because clinician fatigue and occupational stress are at endemic levels, Bernard Chang, MD, of Columbia University Irving Medical Center, New York City, said in an interview. “It is vital that we take stock of how frontline workers in critical care settings are doing overall,” said Dr. Chang.
Dr. Chang, who was not involved with the study but has conducted research on mental health in frontline health care workers during the pandemic, said he was not surprised by the findings. “This work builds on the growing body of literature in the pandemic noting high levels of stress, fatigue, and depression/anxiety symptoms across many frontline workers, from emergency department staff, first responders and others. These are all data points highlighting the urgent need for a broad safety net, not only for patients but the providers serving them.”
The takeaway message: “Clinicians are often so focused on providing care for their patients that they may overlook the need to care for their own well-being and mental health,” said Dr. Chang.
As for additional research, “we need to now take this important data and build on creating and identifying tangible solutions to improve the morale of the acute care/health care workforce to ensure career longevity, professional satisfaction, and overall well-being,” Dr. Chang emphasized. Mental health and morale affect not only health care workers, but also the patients they care for. Well–cared for health care providers can be at their best to provide the optimal care for their patients.
The study received no outside funding. The researchers and Dr. Chang disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Approximately one-third of critical care workers reported some degree of depression, anxiety, or somatic symptoms in the early phase of the COVID-19 pandemic, based on survey results from 939 health care professionals.
The emotional response of professionals in a critical care setting in the early phase of the COVID-19 pandemic has not been well studied, Robyn Branca, PhD, and Paul Branca, MD, of Carson Newman University and the University of Tennessee Medical Center, both in Knoxville, wrote in an abstract presented at the virtual Critical Care Congress sponsored by the Society of Critical Care Medicine.
The prevalence of depression, anxiety, and somatization is low in the general population overall, but the researchers predicted that these conditions increased among workers in critical care settings early in the pandemic.
To assess the prevalence of psychological problems during that time, they sent an email survey on April 7, 2020, to members of the Society of Critical Care Medicine. The survey collected data on demographics, perceived caseload, and potential course of the pandemic. The survey also collected responses to assessments for depression (using the Patient Health Questionnaire–9), anxiety (using the Generalized Anxiety Disorder [GAD] Scale–7), and symptom somatization (using the PHQ-15).
Of the 939 survey respondents, 37% were male, 61.4% were female, and 1.4% gave another or no response.
Overall, 32.3% reported encountering 0-50 COVID-19 cases, 31.1% had encountered 51-200 cases, 12.5% had encountered 201-500 cases, 9.4% had encountered 501-1000 cases, and 13.7% had encountered more than 1,000 cases.
Based on the PHQ-9 depression scale, 44.9% of the respondents had minimal symptoms, 31.1% mild symptoms, 14.3% moderate symptoms, and 9.7% met criteria for severe depressive symptoms. Based on the GAD-7 anxiety scale, 35.5% had minimal symptoms, 32.9% mild, 16.8% moderate, and 14.8% had severe symptoms. Based on the PHQ-15 somatization scale, 39.6% of respondents showed minimal symptoms, whereas 38.2% showed mild symptoms, 17.3% moderate symptoms, and 4.9% had a severe degree of somatic symptoms.
The study findings were limited by the reliance on self-reports; however, the results indicate that a high percentage of critical care workers experienced significant, diagnosable levels of depression, anxiety, and somatic symptoms, the researchers said.
The standard guidance is to pursue individual intervention for anyone with scores of moderate or severe on the scales used in the survey, the researchers said.
Therefore, the findings represent “an alarming degree of mental health impact,” they emphasized. “Immediate mitigation efforts are needed to preserve the health of our ICU workforce.”
The study is important at this time because clinician fatigue and occupational stress are at endemic levels, Bernard Chang, MD, of Columbia University Irving Medical Center, New York City, said in an interview. “It is vital that we take stock of how frontline workers in critical care settings are doing overall,” said Dr. Chang.
Dr. Chang, who was not involved with the study but has conducted research on mental health in frontline health care workers during the pandemic, said he was not surprised by the findings. “This work builds on the growing body of literature in the pandemic noting high levels of stress, fatigue, and depression/anxiety symptoms across many frontline workers, from emergency department staff, first responders and others. These are all data points highlighting the urgent need for a broad safety net, not only for patients but the providers serving them.”
The takeaway message: “Clinicians are often so focused on providing care for their patients that they may overlook the need to care for their own well-being and mental health,” said Dr. Chang.
As for additional research, “we need to now take this important data and build on creating and identifying tangible solutions to improve the morale of the acute care/health care workforce to ensure career longevity, professional satisfaction, and overall well-being,” Dr. Chang emphasized. Mental health and morale affect not only health care workers, but also the patients they care for. Well–cared for health care providers can be at their best to provide the optimal care for their patients.
The study received no outside funding. The researchers and Dr. Chang disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SCCM 2022
ILD progression, not diagnosis, triggers palliative care
Most health care providers are comfortable recommending palliative care (PC) for their patients with interstitial lung disease (ILD), but most do so at the time of disease progression, rather than diagnosis, as indicated on survey data from 128 clinicians.
ILD is associated with a high mortality rate and profound symptoms that contribute to poor quality of life, Rebecca A. Gersen, MD, of Johns Hopkins University, Baltimore, and colleagues wrote.
“Nevertheless, there is often a lack of preparedness for death by both patients and providers, contributing to increased distress,” they said. Clinician perspectives on the use of PC for ILD patients have not been well studied, although PC is not limited to end-of-life care and is recommended for ILD patients by professional organizations, including the American Thoracic Society. “PC is successful in improving breathlessness in chronic lung disease and can increase survival.”
In a study published in the journal CHEST®, the researchers surveyed health care providers at 68 Pulmonary Fibrosis Foundation centers across the United States. The survey was sent and collected by email and a restricted social media platform. A total of 128 providers from 34 states completed the survey between October 2020 and January 2021. Of these, 61% were physicians, and 67% identified as White.
Overall, 95% of the respondents agreed or strongly agreed that addressing advance directives is important, but only 66% agreed or strongly agreed that they themselves addressed advance directives in the outpatient ILD clinic setting. A greater number (91%) agreed or strongly agreed that they had a high level of comfort in discussing prognosis, while 88% agreed or strongly agreed that they felt comfortable assessing a patient’s readiness for and acceptance of PC. Approximately two-thirds (67%) agreed or strongly agreed that they use PC services for ILD patients. There were no significant differences in responses from clinicians who had more than 10 years of experience and those who had less.
Of the providers who referred patients to PC, 54% did so at objective disease progression, and 80% did so at objective and/or symptomatic progress; 2% referred patients to PC at initial ILD diagnosis.
Lack of resources
Health care providers who reported that they rarely referred patients to palliative care were significantly more likely to cite a lack of local PC options (P < .01). Those who rarely referred patients for PC also were significantly less likely to feel comfortable discussing prognoses or advance directives in the ILD clinic (P = .03 and P = .02, respectively).
Among the 23% of responders who reported that they rarely referred patients, 66% said they did not have PC at their institution.
“In addition to understanding and addressing barriers to care, educational resources may be key to improving PC delivery to the ILD population,” the researchers wrote.
The study findings were limited by several factors, including voluntary participation, lack of a validated questionnaire, and use of self-reports, which may not reflect physicians’ actual practice, the researchers noted. Other limitations include the use of U.S. data only, which may not generalize to countries with different health care models.
However, the results were strengthened by the use of data from providers at a range of institutions across the United States and by the high overall survey response rate, the researchers said.
“While ILD providers reassuringly demonstrate knowledge and interest in PC involvement, no current system exists to facilitate and monitor response to referral,” they noted. “Future research is desperately needed to address barriers to the provision of PC in order to enhance access to a critical service in the management and care of patients with ILD.”
The study was supported by the National Heart, Lung, and Blood Institute. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most health care providers are comfortable recommending palliative care (PC) for their patients with interstitial lung disease (ILD), but most do so at the time of disease progression, rather than diagnosis, as indicated on survey data from 128 clinicians.
ILD is associated with a high mortality rate and profound symptoms that contribute to poor quality of life, Rebecca A. Gersen, MD, of Johns Hopkins University, Baltimore, and colleagues wrote.
“Nevertheless, there is often a lack of preparedness for death by both patients and providers, contributing to increased distress,” they said. Clinician perspectives on the use of PC for ILD patients have not been well studied, although PC is not limited to end-of-life care and is recommended for ILD patients by professional organizations, including the American Thoracic Society. “PC is successful in improving breathlessness in chronic lung disease and can increase survival.”
In a study published in the journal CHEST®, the researchers surveyed health care providers at 68 Pulmonary Fibrosis Foundation centers across the United States. The survey was sent and collected by email and a restricted social media platform. A total of 128 providers from 34 states completed the survey between October 2020 and January 2021. Of these, 61% were physicians, and 67% identified as White.
Overall, 95% of the respondents agreed or strongly agreed that addressing advance directives is important, but only 66% agreed or strongly agreed that they themselves addressed advance directives in the outpatient ILD clinic setting. A greater number (91%) agreed or strongly agreed that they had a high level of comfort in discussing prognosis, while 88% agreed or strongly agreed that they felt comfortable assessing a patient’s readiness for and acceptance of PC. Approximately two-thirds (67%) agreed or strongly agreed that they use PC services for ILD patients. There were no significant differences in responses from clinicians who had more than 10 years of experience and those who had less.
Of the providers who referred patients to PC, 54% did so at objective disease progression, and 80% did so at objective and/or symptomatic progress; 2% referred patients to PC at initial ILD diagnosis.
Lack of resources
Health care providers who reported that they rarely referred patients to palliative care were significantly more likely to cite a lack of local PC options (P < .01). Those who rarely referred patients for PC also were significantly less likely to feel comfortable discussing prognoses or advance directives in the ILD clinic (P = .03 and P = .02, respectively).
Among the 23% of responders who reported that they rarely referred patients, 66% said they did not have PC at their institution.
“In addition to understanding and addressing barriers to care, educational resources may be key to improving PC delivery to the ILD population,” the researchers wrote.
The study findings were limited by several factors, including voluntary participation, lack of a validated questionnaire, and use of self-reports, which may not reflect physicians’ actual practice, the researchers noted. Other limitations include the use of U.S. data only, which may not generalize to countries with different health care models.
However, the results were strengthened by the use of data from providers at a range of institutions across the United States and by the high overall survey response rate, the researchers said.
“While ILD providers reassuringly demonstrate knowledge and interest in PC involvement, no current system exists to facilitate and monitor response to referral,” they noted. “Future research is desperately needed to address barriers to the provision of PC in order to enhance access to a critical service in the management and care of patients with ILD.”
The study was supported by the National Heart, Lung, and Blood Institute. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most health care providers are comfortable recommending palliative care (PC) for their patients with interstitial lung disease (ILD), but most do so at the time of disease progression, rather than diagnosis, as indicated on survey data from 128 clinicians.
ILD is associated with a high mortality rate and profound symptoms that contribute to poor quality of life, Rebecca A. Gersen, MD, of Johns Hopkins University, Baltimore, and colleagues wrote.
“Nevertheless, there is often a lack of preparedness for death by both patients and providers, contributing to increased distress,” they said. Clinician perspectives on the use of PC for ILD patients have not been well studied, although PC is not limited to end-of-life care and is recommended for ILD patients by professional organizations, including the American Thoracic Society. “PC is successful in improving breathlessness in chronic lung disease and can increase survival.”
In a study published in the journal CHEST®, the researchers surveyed health care providers at 68 Pulmonary Fibrosis Foundation centers across the United States. The survey was sent and collected by email and a restricted social media platform. A total of 128 providers from 34 states completed the survey between October 2020 and January 2021. Of these, 61% were physicians, and 67% identified as White.
Overall, 95% of the respondents agreed or strongly agreed that addressing advance directives is important, but only 66% agreed or strongly agreed that they themselves addressed advance directives in the outpatient ILD clinic setting. A greater number (91%) agreed or strongly agreed that they had a high level of comfort in discussing prognosis, while 88% agreed or strongly agreed that they felt comfortable assessing a patient’s readiness for and acceptance of PC. Approximately two-thirds (67%) agreed or strongly agreed that they use PC services for ILD patients. There were no significant differences in responses from clinicians who had more than 10 years of experience and those who had less.
Of the providers who referred patients to PC, 54% did so at objective disease progression, and 80% did so at objective and/or symptomatic progress; 2% referred patients to PC at initial ILD diagnosis.
Lack of resources
Health care providers who reported that they rarely referred patients to palliative care were significantly more likely to cite a lack of local PC options (P < .01). Those who rarely referred patients for PC also were significantly less likely to feel comfortable discussing prognoses or advance directives in the ILD clinic (P = .03 and P = .02, respectively).
Among the 23% of responders who reported that they rarely referred patients, 66% said they did not have PC at their institution.
“In addition to understanding and addressing barriers to care, educational resources may be key to improving PC delivery to the ILD population,” the researchers wrote.
The study findings were limited by several factors, including voluntary participation, lack of a validated questionnaire, and use of self-reports, which may not reflect physicians’ actual practice, the researchers noted. Other limitations include the use of U.S. data only, which may not generalize to countries with different health care models.
However, the results were strengthened by the use of data from providers at a range of institutions across the United States and by the high overall survey response rate, the researchers said.
“While ILD providers reassuringly demonstrate knowledge and interest in PC involvement, no current system exists to facilitate and monitor response to referral,” they noted. “Future research is desperately needed to address barriers to the provision of PC in order to enhance access to a critical service in the management and care of patients with ILD.”
The study was supported by the National Heart, Lung, and Blood Institute. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL CHEST®
Can gram stains guide antibiotics for pneumonia in critical care?
Similar outcomes in patients with ventilator-associated pneumonia (VAP) suggest that antibiotics selected by Gram staining were noninferior to those based on guidelines and also significantly decreased the use of broad-spectrum antibiotics in this patient population.
The findings were published in JAMA Network Open. The multicenter, open-label, noninferiority, randomized trial, Gram Stain-Guided Antibiotics Choice for VAP (GRACE-VAP), was conducted for 2 years in intensive care units (ICUs) of a dozen tertiary referral hospitals in Japan, from April 1, 2018, through May 31, 2020.
The authors noted in their paper that the 2016 clinical practice guidelines for VAP published by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society recommend antibiotic agents active against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa as an empirical treatment. Adherence to these guidelines may lead to overuse of broad-spectrum antibiotic agents and could be associated with the accelerated emergence of antimicrobial-resistant organisms, the authors postulated.
The study sought to answer the question: Can Gram staining be used as an alternative to established guidelines to direct antibiotic use – thereby curbing the use of broad-spectrum antibiotics – without compromising patient safety and clinical outcomes?
A total of 206 patients, with a mean age of 69, took part in the study. The same number of patients were assigned to each arm. Patients aged 15 years or older with a VAP diagnosis and a modified Clinical Pulmonary Infection Score of 5 or higher were included.
Investigators reported that 79 patients (76.7%) responded to antibiotics in the Gram stain-guided group and 74 (71.8%) responded in the guideline-based group (risk difference, 0.05; 95% confidence interval, –0.07 to 0.17; P < .001, for noninferiority).
There was a decrease in antipseudomonal agent use comparing the Gram stain-guided group with the guideline-based group (30.1%; 95% CI, 21.5% to 39.9%; P < .001). There also was a decrease in anti-MRSA agents in the Gram stain-guided group, compared with the guideline-based group (38.8%; 95% CI, 29.4% to 48.9%; P < .001).
The 28-day cumulative incidence of mortality was 13.6% (n = 14) in the Gram stain-guided group versus 17.5% (n = 18) in the guideline-based group. Escalation of antibiotics according to culture results was performed in seven patients (6.8%) in the Gram stain-guided group and in one patient (1.0%) in the guideline-based group. No significant differences in study arms were observed on other measures, such as ICU-free days, ventilator-free days, and adverse events.
The authors concluded that their findings support the use of Gram staining as a strategy to manage infectious diseases and contain the development of multidrug resistant organisms (MDROs) in the setting of critical care.
“In the GRACE-VAP trial, we used the time-honored Gram stain technique as part of the daily management of infectious diseases. We believe that the trial results are acceptable and have the potential to change the strategy of antibiotic choice worldwide,” the authors wrote.
Benjamin D. Galvan MLS(ASCP), CIC, an infection preventionist with a professional background in clinical microbiology, noted that Gram staining is more accessible and significantly less costly than the rapid polymerase chain reaction testing certain institutions use to rapidly identify MDROs to help tailor therapy.
But one of the pitfalls with relying on Gram stain collection to guide antibiotic use is that it is operator dependent and subject to extrinsic factors, like prior antibiotic use, he pointed out.
“If it is not collected, set up, and read properly, the Gram stain is not going to necessarily be reliable” said Mr. Galvan, also a member of the national communications committee for the Association for Professionals in Infection Control and Epidemiology. He added that the sample in the study was not representative of institutions dealing with elevated rates of multidrug resistance.
“Even from their own results, they were looking at hospitals that have a low rate of multidrug resistance,” he said. “It was not clear if MRSA or just Staphylococcus aureus was identified in significant quantities upon review, and they recognized a lower-than-expected number of isolates of Pseudomonas aeruginosa.”
Establishing antibiotic treatment from the results of Gram-stain collection may not be sufficiently comprehensive, he said.
“Generally speaking, basing it (antibiotic therapy) solely off of a Gram stain is not looking at the whole picture,” said Mr. Galvan, noting that the 2016 IDSA guidelines call for an evaluation of the clinical status, including risk, of the individual patient, as well as locally available antibiotic resistance data.
Moreover, the evidence-based IDSA guidelines are in place to help address the issue of antimicrobial resistance trends, already recommending tailoring empiric antibiotic therapy based upon the levels of resistance in the local population, according to Galvan.
While the study suggests that this Gram-stain-driven tailoring of empiric antibiotic therapy may be noninferior to current guidelines in health care settings with low MDRO rates, its utility may not be suitable in hospitals that are already dealing with high rates of MDROs, such as Pseudomonas aeruginosa and Acinetobacter baumannii, or severe clinical cases of VAP, Mr. Galvan explained.
The researchers and Mr. Galvan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Similar outcomes in patients with ventilator-associated pneumonia (VAP) suggest that antibiotics selected by Gram staining were noninferior to those based on guidelines and also significantly decreased the use of broad-spectrum antibiotics in this patient population.
The findings were published in JAMA Network Open. The multicenter, open-label, noninferiority, randomized trial, Gram Stain-Guided Antibiotics Choice for VAP (GRACE-VAP), was conducted for 2 years in intensive care units (ICUs) of a dozen tertiary referral hospitals in Japan, from April 1, 2018, through May 31, 2020.
The authors noted in their paper that the 2016 clinical practice guidelines for VAP published by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society recommend antibiotic agents active against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa as an empirical treatment. Adherence to these guidelines may lead to overuse of broad-spectrum antibiotic agents and could be associated with the accelerated emergence of antimicrobial-resistant organisms, the authors postulated.
The study sought to answer the question: Can Gram staining be used as an alternative to established guidelines to direct antibiotic use – thereby curbing the use of broad-spectrum antibiotics – without compromising patient safety and clinical outcomes?
A total of 206 patients, with a mean age of 69, took part in the study. The same number of patients were assigned to each arm. Patients aged 15 years or older with a VAP diagnosis and a modified Clinical Pulmonary Infection Score of 5 or higher were included.
Investigators reported that 79 patients (76.7%) responded to antibiotics in the Gram stain-guided group and 74 (71.8%) responded in the guideline-based group (risk difference, 0.05; 95% confidence interval, –0.07 to 0.17; P < .001, for noninferiority).
There was a decrease in antipseudomonal agent use comparing the Gram stain-guided group with the guideline-based group (30.1%; 95% CI, 21.5% to 39.9%; P < .001). There also was a decrease in anti-MRSA agents in the Gram stain-guided group, compared with the guideline-based group (38.8%; 95% CI, 29.4% to 48.9%; P < .001).
The 28-day cumulative incidence of mortality was 13.6% (n = 14) in the Gram stain-guided group versus 17.5% (n = 18) in the guideline-based group. Escalation of antibiotics according to culture results was performed in seven patients (6.8%) in the Gram stain-guided group and in one patient (1.0%) in the guideline-based group. No significant differences in study arms were observed on other measures, such as ICU-free days, ventilator-free days, and adverse events.
The authors concluded that their findings support the use of Gram staining as a strategy to manage infectious diseases and contain the development of multidrug resistant organisms (MDROs) in the setting of critical care.
“In the GRACE-VAP trial, we used the time-honored Gram stain technique as part of the daily management of infectious diseases. We believe that the trial results are acceptable and have the potential to change the strategy of antibiotic choice worldwide,” the authors wrote.
Benjamin D. Galvan MLS(ASCP), CIC, an infection preventionist with a professional background in clinical microbiology, noted that Gram staining is more accessible and significantly less costly than the rapid polymerase chain reaction testing certain institutions use to rapidly identify MDROs to help tailor therapy.
But one of the pitfalls with relying on Gram stain collection to guide antibiotic use is that it is operator dependent and subject to extrinsic factors, like prior antibiotic use, he pointed out.
“If it is not collected, set up, and read properly, the Gram stain is not going to necessarily be reliable” said Mr. Galvan, also a member of the national communications committee for the Association for Professionals in Infection Control and Epidemiology. He added that the sample in the study was not representative of institutions dealing with elevated rates of multidrug resistance.
“Even from their own results, they were looking at hospitals that have a low rate of multidrug resistance,” he said. “It was not clear if MRSA or just Staphylococcus aureus was identified in significant quantities upon review, and they recognized a lower-than-expected number of isolates of Pseudomonas aeruginosa.”
Establishing antibiotic treatment from the results of Gram-stain collection may not be sufficiently comprehensive, he said.
“Generally speaking, basing it (antibiotic therapy) solely off of a Gram stain is not looking at the whole picture,” said Mr. Galvan, noting that the 2016 IDSA guidelines call for an evaluation of the clinical status, including risk, of the individual patient, as well as locally available antibiotic resistance data.
Moreover, the evidence-based IDSA guidelines are in place to help address the issue of antimicrobial resistance trends, already recommending tailoring empiric antibiotic therapy based upon the levels of resistance in the local population, according to Galvan.
While the study suggests that this Gram-stain-driven tailoring of empiric antibiotic therapy may be noninferior to current guidelines in health care settings with low MDRO rates, its utility may not be suitable in hospitals that are already dealing with high rates of MDROs, such as Pseudomonas aeruginosa and Acinetobacter baumannii, or severe clinical cases of VAP, Mr. Galvan explained.
The researchers and Mr. Galvan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Similar outcomes in patients with ventilator-associated pneumonia (VAP) suggest that antibiotics selected by Gram staining were noninferior to those based on guidelines and also significantly decreased the use of broad-spectrum antibiotics in this patient population.
The findings were published in JAMA Network Open. The multicenter, open-label, noninferiority, randomized trial, Gram Stain-Guided Antibiotics Choice for VAP (GRACE-VAP), was conducted for 2 years in intensive care units (ICUs) of a dozen tertiary referral hospitals in Japan, from April 1, 2018, through May 31, 2020.
The authors noted in their paper that the 2016 clinical practice guidelines for VAP published by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society recommend antibiotic agents active against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa as an empirical treatment. Adherence to these guidelines may lead to overuse of broad-spectrum antibiotic agents and could be associated with the accelerated emergence of antimicrobial-resistant organisms, the authors postulated.
The study sought to answer the question: Can Gram staining be used as an alternative to established guidelines to direct antibiotic use – thereby curbing the use of broad-spectrum antibiotics – without compromising patient safety and clinical outcomes?
A total of 206 patients, with a mean age of 69, took part in the study. The same number of patients were assigned to each arm. Patients aged 15 years or older with a VAP diagnosis and a modified Clinical Pulmonary Infection Score of 5 or higher were included.
Investigators reported that 79 patients (76.7%) responded to antibiotics in the Gram stain-guided group and 74 (71.8%) responded in the guideline-based group (risk difference, 0.05; 95% confidence interval, –0.07 to 0.17; P < .001, for noninferiority).
There was a decrease in antipseudomonal agent use comparing the Gram stain-guided group with the guideline-based group (30.1%; 95% CI, 21.5% to 39.9%; P < .001). There also was a decrease in anti-MRSA agents in the Gram stain-guided group, compared with the guideline-based group (38.8%; 95% CI, 29.4% to 48.9%; P < .001).
The 28-day cumulative incidence of mortality was 13.6% (n = 14) in the Gram stain-guided group versus 17.5% (n = 18) in the guideline-based group. Escalation of antibiotics according to culture results was performed in seven patients (6.8%) in the Gram stain-guided group and in one patient (1.0%) in the guideline-based group. No significant differences in study arms were observed on other measures, such as ICU-free days, ventilator-free days, and adverse events.
The authors concluded that their findings support the use of Gram staining as a strategy to manage infectious diseases and contain the development of multidrug resistant organisms (MDROs) in the setting of critical care.
“In the GRACE-VAP trial, we used the time-honored Gram stain technique as part of the daily management of infectious diseases. We believe that the trial results are acceptable and have the potential to change the strategy of antibiotic choice worldwide,” the authors wrote.
Benjamin D. Galvan MLS(ASCP), CIC, an infection preventionist with a professional background in clinical microbiology, noted that Gram staining is more accessible and significantly less costly than the rapid polymerase chain reaction testing certain institutions use to rapidly identify MDROs to help tailor therapy.
But one of the pitfalls with relying on Gram stain collection to guide antibiotic use is that it is operator dependent and subject to extrinsic factors, like prior antibiotic use, he pointed out.
“If it is not collected, set up, and read properly, the Gram stain is not going to necessarily be reliable” said Mr. Galvan, also a member of the national communications committee for the Association for Professionals in Infection Control and Epidemiology. He added that the sample in the study was not representative of institutions dealing with elevated rates of multidrug resistance.
“Even from their own results, they were looking at hospitals that have a low rate of multidrug resistance,” he said. “It was not clear if MRSA or just Staphylococcus aureus was identified in significant quantities upon review, and they recognized a lower-than-expected number of isolates of Pseudomonas aeruginosa.”
Establishing antibiotic treatment from the results of Gram-stain collection may not be sufficiently comprehensive, he said.
“Generally speaking, basing it (antibiotic therapy) solely off of a Gram stain is not looking at the whole picture,” said Mr. Galvan, noting that the 2016 IDSA guidelines call for an evaluation of the clinical status, including risk, of the individual patient, as well as locally available antibiotic resistance data.
Moreover, the evidence-based IDSA guidelines are in place to help address the issue of antimicrobial resistance trends, already recommending tailoring empiric antibiotic therapy based upon the levels of resistance in the local population, according to Galvan.
While the study suggests that this Gram-stain-driven tailoring of empiric antibiotic therapy may be noninferior to current guidelines in health care settings with low MDRO rates, its utility may not be suitable in hospitals that are already dealing with high rates of MDROs, such as Pseudomonas aeruginosa and Acinetobacter baumannii, or severe clinical cases of VAP, Mr. Galvan explained.
The researchers and Mr. Galvan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
What COVID-19 taught us: The challenge of maintaining contingency level care to proactively forestall crisis care
In 2014, the Task Force for Mass Critical Care (TFMCC) published a CHEST consensus statement on disaster preparedness principles in caring for the critically ill during disasters and pandemics (Christian et al. CHEST. 2014;146[4_suppl]:8s-34s). This publication attempted to guide preparedness for both single-event disasters and more prolonged events, including a feared influenza pandemic.
Despite the foundation of planning and support this guidance provided, the COVID-19 pandemic response revealed substantial gaps in our understanding and preparedness for these more prolonged and widespread events.
In New York City, as the first COVID-19 wave began in March and April of 2020, area hospitals responded with surge plans that prioritized what was felt to be most important (Griffin et al. Am J Respir Crit Care Med. 2020 Jun 1;201[11]:1337-44). Tiered, creative staffing structures were rapidly created with intensivists supervising non-ICU physicians and APPs. Procedure teams were created for intubation, proning, and central line placement. ICU space was created with adaptations to ORs and PACUs, and rooms on med-surg floors and step-down units underwent emergency renovations to allow creation of new “pop-up” ICUs. Triage protocols were altered: patients on high levels of supplemental oxygen, who would under normal circumstances have been admitted to an ICU, were triaged to floors and stepdown units. Equipment was reused, modified, and substituted creatively to optimize care for the maximum number of patients.
In the face of all of these struggles, many around the country and the world felt the efforts, though heroic, resulted in less than standard of care. Two subsequent publications validated this concern (Kadri et al. Ann Int Med. 2021,174;9:1240-51; Bravata DM et al. JAMA Open Network. 2021;4[1]:e2034266), demonstrating during severe surge, COVID-19 patients’ mortality increased significantly beyond that seen in non-surging or less-severe surging times, demonstrating a mortality effect of surge itself. Though these studies observed COVID-19 patients only, there is every reason to believe the findings applied to all critically ill patients cared for during these surges.
These experiences led the TFMCC to report updated strategies for remaining in contingency care levels and avoiding crisis care (Dichter JR et al. CHEST. 2022;161[2]:429-47). Contingency is equivalent to routine care though may require adaptations and employment of otherwise non-traditional resources. The ultimate goal of mass critical care in a public health emergency is to avoid crisis-operating conditions, crisis standards of care, and their associated challenging triage decisions regarding allocation of scarce resources.
The 10 suggestions included in the most recent TFMCC publication include staffing strategies and suggestions based on COVID-19 experiences for graded staff-to-patient ratios, and support processes to preserve the existing health care work force. Strategies also include reduction of redundant documentation, limiting overtime, and most importantly, approaches for improving teamwork and supporting psychological well-being and resilience. Examples include daily unit huddles to update care and share experiences, genuine intra-team recognition and appreciation, and embedding emotional health experts within teams to provide ongoing support.
Consistent communication between incident command and frontline clinicians was also a suggested priority, perhaps with a newly proposed position of physician clinical support supervisor. This would be a formal role within hospital incident command, a liaison between the two groups.
Surge strategies should include empowerment of bedside clinicians and leaders with both planning and real-time assessment of the clinical situation, as being at the front line of care enables the situational awareness to assess ICU strain most effectively. Further, ICU clinicians must recognize when progression deeper into contingency operations occurs and they become perilously close to crisis mode. At this point, decisions are made and scarce resources are modified beyond routine standards of care to preserve life. TFMCC designates this gray area between contingency and crisis as the Critical Clinical Prioritization level (Figure).
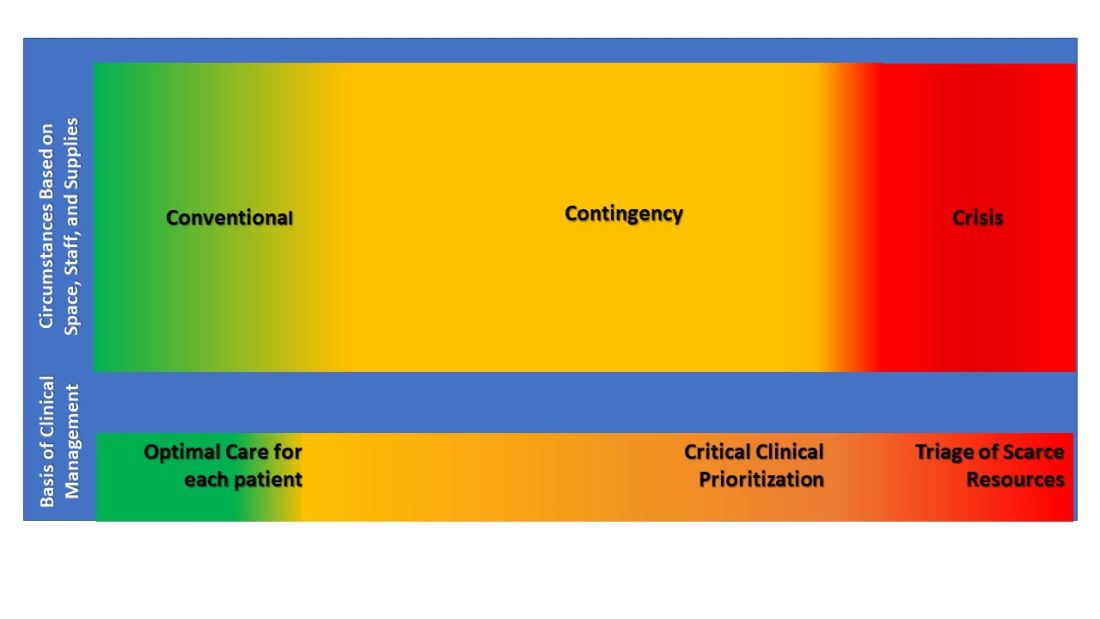
At this point, more resources must be provided, or patients must be transferred to other resourced hospitals.
Critical Clinical Prioritization is an illustration of necessity being the mother of invention, as these are adaptations clinicians devised under duress. Some particularly poignant examples are the spreading of 24 hours of continuous renal replacement therapy (CRRT) resource between two and sometimes three patients to provide life sustainment to all; and when ventilators were in short supply, determining which patients required full ICU ventilator support vs those who could manage with lower functioning ventilators, and trading them between patients when demands changed.
These adaptations can only be done by experienced clinicians proactively managing bedside critical care under duress, further underscoring the importance of our suggestion that Critical Clinical Prioritization and ICU strain be managed by bedside clinicians and leaders.
The response of early transfer of patients – load-balancing - should be considered as soon as any hospital enters contingency conditions. This strategy is commonly implemented within larger health systems, ideally before reaching Critical Clinical Prioritization. Formal, organized state or regional load-balancing coordination, now referred to as medical operations command centers (MOCCs), were highly effective and proved lifesaving for those states that implemented them (including Arizona, Washington, California, Minnesota, and others). Support for establishment of MOCC’s is crucial in prolonging contingency operations and further helps support and protect disadvantaged populations (White et al. N Engl J Med. 2021;385[24]:2211-4).
Establishment of MOCCs has met resistance due to challenges that include interhospital/intersystem competition, logistics of moving critically ill patients sometimes across significant physical distance, and the costs of assuming care of uninsured or underinsured patients. Nevertheless, the benefits to the population as a whole necessitate working through these obstacles as successful MOCCs have done, usually with government and hospital association support.
In their final suggestion of the 2022 updated strategies, TFMCC suggests that hospitals use telemedicine technology both to expand specialists’ ability to provide care and facilitate families virtually visiting their critically ill loved one when safety precludes in-person visits.
These suggestions are pivotal in planning for future public health emergencies that include mass critical care, even during events that are limited in scope and duration.
Lastly, intensivists struggled with legal and ethical concerns when mired in crisis care circumstances and decisions of allocation, and potential reallocation, of scarce resources. These issues were not well addressed during the COVID-19 pandemic, further emphasizing the importance of maintaining contingency level care and requiring further involvement from legal and medical ethics professionals for future planning.
The guiding principle of disaster preparedness is that we must do all the planning we can to ensure that we never need crisis standards of care (National Academies of Sciences, Engineering, and Medicine. 2020 Mar 28. Rapid Expert Consultation on Crisis Standards of Care for the COVID-19 Pandemic. Washington, DC: The National Academies Press.).
We must be prepared. Guidelines and suggestions laid out through decades of experience gained a real-world test in the COVID-19 pandemic. Now we must all reorganize and create new plans or augment old ones with the information we have gained. The time is now. The work must continue.
Dr. Griffin is Assistant Professor of Medicine, New York Presbyterian Hospital – Weill Cornell Medicine. Dr. Dichter is Associate Professor of Medicine, University of Minnesota.
In 2014, the Task Force for Mass Critical Care (TFMCC) published a CHEST consensus statement on disaster preparedness principles in caring for the critically ill during disasters and pandemics (Christian et al. CHEST. 2014;146[4_suppl]:8s-34s). This publication attempted to guide preparedness for both single-event disasters and more prolonged events, including a feared influenza pandemic.
Despite the foundation of planning and support this guidance provided, the COVID-19 pandemic response revealed substantial gaps in our understanding and preparedness for these more prolonged and widespread events.
In New York City, as the first COVID-19 wave began in March and April of 2020, area hospitals responded with surge plans that prioritized what was felt to be most important (Griffin et al. Am J Respir Crit Care Med. 2020 Jun 1;201[11]:1337-44). Tiered, creative staffing structures were rapidly created with intensivists supervising non-ICU physicians and APPs. Procedure teams were created for intubation, proning, and central line placement. ICU space was created with adaptations to ORs and PACUs, and rooms on med-surg floors and step-down units underwent emergency renovations to allow creation of new “pop-up” ICUs. Triage protocols were altered: patients on high levels of supplemental oxygen, who would under normal circumstances have been admitted to an ICU, were triaged to floors and stepdown units. Equipment was reused, modified, and substituted creatively to optimize care for the maximum number of patients.
In the face of all of these struggles, many around the country and the world felt the efforts, though heroic, resulted in less than standard of care. Two subsequent publications validated this concern (Kadri et al. Ann Int Med. 2021,174;9:1240-51; Bravata DM et al. JAMA Open Network. 2021;4[1]:e2034266), demonstrating during severe surge, COVID-19 patients’ mortality increased significantly beyond that seen in non-surging or less-severe surging times, demonstrating a mortality effect of surge itself. Though these studies observed COVID-19 patients only, there is every reason to believe the findings applied to all critically ill patients cared for during these surges.
These experiences led the TFMCC to report updated strategies for remaining in contingency care levels and avoiding crisis care (Dichter JR et al. CHEST. 2022;161[2]:429-47). Contingency is equivalent to routine care though may require adaptations and employment of otherwise non-traditional resources. The ultimate goal of mass critical care in a public health emergency is to avoid crisis-operating conditions, crisis standards of care, and their associated challenging triage decisions regarding allocation of scarce resources.
The 10 suggestions included in the most recent TFMCC publication include staffing strategies and suggestions based on COVID-19 experiences for graded staff-to-patient ratios, and support processes to preserve the existing health care work force. Strategies also include reduction of redundant documentation, limiting overtime, and most importantly, approaches for improving teamwork and supporting psychological well-being and resilience. Examples include daily unit huddles to update care and share experiences, genuine intra-team recognition and appreciation, and embedding emotional health experts within teams to provide ongoing support.
Consistent communication between incident command and frontline clinicians was also a suggested priority, perhaps with a newly proposed position of physician clinical support supervisor. This would be a formal role within hospital incident command, a liaison between the two groups.
Surge strategies should include empowerment of bedside clinicians and leaders with both planning and real-time assessment of the clinical situation, as being at the front line of care enables the situational awareness to assess ICU strain most effectively. Further, ICU clinicians must recognize when progression deeper into contingency operations occurs and they become perilously close to crisis mode. At this point, decisions are made and scarce resources are modified beyond routine standards of care to preserve life. TFMCC designates this gray area between contingency and crisis as the Critical Clinical Prioritization level (Figure).

At this point, more resources must be provided, or patients must be transferred to other resourced hospitals.
Critical Clinical Prioritization is an illustration of necessity being the mother of invention, as these are adaptations clinicians devised under duress. Some particularly poignant examples are the spreading of 24 hours of continuous renal replacement therapy (CRRT) resource between two and sometimes three patients to provide life sustainment to all; and when ventilators were in short supply, determining which patients required full ICU ventilator support vs those who could manage with lower functioning ventilators, and trading them between patients when demands changed.
These adaptations can only be done by experienced clinicians proactively managing bedside critical care under duress, further underscoring the importance of our suggestion that Critical Clinical Prioritization and ICU strain be managed by bedside clinicians and leaders.
The response of early transfer of patients – load-balancing - should be considered as soon as any hospital enters contingency conditions. This strategy is commonly implemented within larger health systems, ideally before reaching Critical Clinical Prioritization. Formal, organized state or regional load-balancing coordination, now referred to as medical operations command centers (MOCCs), were highly effective and proved lifesaving for those states that implemented them (including Arizona, Washington, California, Minnesota, and others). Support for establishment of MOCC’s is crucial in prolonging contingency operations and further helps support and protect disadvantaged populations (White et al. N Engl J Med. 2021;385[24]:2211-4).
Establishment of MOCCs has met resistance due to challenges that include interhospital/intersystem competition, logistics of moving critically ill patients sometimes across significant physical distance, and the costs of assuming care of uninsured or underinsured patients. Nevertheless, the benefits to the population as a whole necessitate working through these obstacles as successful MOCCs have done, usually with government and hospital association support.
In their final suggestion of the 2022 updated strategies, TFMCC suggests that hospitals use telemedicine technology both to expand specialists’ ability to provide care and facilitate families virtually visiting their critically ill loved one when safety precludes in-person visits.
These suggestions are pivotal in planning for future public health emergencies that include mass critical care, even during events that are limited in scope and duration.
Lastly, intensivists struggled with legal and ethical concerns when mired in crisis care circumstances and decisions of allocation, and potential reallocation, of scarce resources. These issues were not well addressed during the COVID-19 pandemic, further emphasizing the importance of maintaining contingency level care and requiring further involvement from legal and medical ethics professionals for future planning.
The guiding principle of disaster preparedness is that we must do all the planning we can to ensure that we never need crisis standards of care (National Academies of Sciences, Engineering, and Medicine. 2020 Mar 28. Rapid Expert Consultation on Crisis Standards of Care for the COVID-19 Pandemic. Washington, DC: The National Academies Press.).
We must be prepared. Guidelines and suggestions laid out through decades of experience gained a real-world test in the COVID-19 pandemic. Now we must all reorganize and create new plans or augment old ones with the information we have gained. The time is now. The work must continue.
Dr. Griffin is Assistant Professor of Medicine, New York Presbyterian Hospital – Weill Cornell Medicine. Dr. Dichter is Associate Professor of Medicine, University of Minnesota.
In 2014, the Task Force for Mass Critical Care (TFMCC) published a CHEST consensus statement on disaster preparedness principles in caring for the critically ill during disasters and pandemics (Christian et al. CHEST. 2014;146[4_suppl]:8s-34s). This publication attempted to guide preparedness for both single-event disasters and more prolonged events, including a feared influenza pandemic.
Despite the foundation of planning and support this guidance provided, the COVID-19 pandemic response revealed substantial gaps in our understanding and preparedness for these more prolonged and widespread events.
In New York City, as the first COVID-19 wave began in March and April of 2020, area hospitals responded with surge plans that prioritized what was felt to be most important (Griffin et al. Am J Respir Crit Care Med. 2020 Jun 1;201[11]:1337-44). Tiered, creative staffing structures were rapidly created with intensivists supervising non-ICU physicians and APPs. Procedure teams were created for intubation, proning, and central line placement. ICU space was created with adaptations to ORs and PACUs, and rooms on med-surg floors and step-down units underwent emergency renovations to allow creation of new “pop-up” ICUs. Triage protocols were altered: patients on high levels of supplemental oxygen, who would under normal circumstances have been admitted to an ICU, were triaged to floors and stepdown units. Equipment was reused, modified, and substituted creatively to optimize care for the maximum number of patients.
In the face of all of these struggles, many around the country and the world felt the efforts, though heroic, resulted in less than standard of care. Two subsequent publications validated this concern (Kadri et al. Ann Int Med. 2021,174;9:1240-51; Bravata DM et al. JAMA Open Network. 2021;4[1]:e2034266), demonstrating during severe surge, COVID-19 patients’ mortality increased significantly beyond that seen in non-surging or less-severe surging times, demonstrating a mortality effect of surge itself. Though these studies observed COVID-19 patients only, there is every reason to believe the findings applied to all critically ill patients cared for during these surges.
These experiences led the TFMCC to report updated strategies for remaining in contingency care levels and avoiding crisis care (Dichter JR et al. CHEST. 2022;161[2]:429-47). Contingency is equivalent to routine care though may require adaptations and employment of otherwise non-traditional resources. The ultimate goal of mass critical care in a public health emergency is to avoid crisis-operating conditions, crisis standards of care, and their associated challenging triage decisions regarding allocation of scarce resources.
The 10 suggestions included in the most recent TFMCC publication include staffing strategies and suggestions based on COVID-19 experiences for graded staff-to-patient ratios, and support processes to preserve the existing health care work force. Strategies also include reduction of redundant documentation, limiting overtime, and most importantly, approaches for improving teamwork and supporting psychological well-being and resilience. Examples include daily unit huddles to update care and share experiences, genuine intra-team recognition and appreciation, and embedding emotional health experts within teams to provide ongoing support.
Consistent communication between incident command and frontline clinicians was also a suggested priority, perhaps with a newly proposed position of physician clinical support supervisor. This would be a formal role within hospital incident command, a liaison between the two groups.
Surge strategies should include empowerment of bedside clinicians and leaders with both planning and real-time assessment of the clinical situation, as being at the front line of care enables the situational awareness to assess ICU strain most effectively. Further, ICU clinicians must recognize when progression deeper into contingency operations occurs and they become perilously close to crisis mode. At this point, decisions are made and scarce resources are modified beyond routine standards of care to preserve life. TFMCC designates this gray area between contingency and crisis as the Critical Clinical Prioritization level (Figure).

At this point, more resources must be provided, or patients must be transferred to other resourced hospitals.
Critical Clinical Prioritization is an illustration of necessity being the mother of invention, as these are adaptations clinicians devised under duress. Some particularly poignant examples are the spreading of 24 hours of continuous renal replacement therapy (CRRT) resource between two and sometimes three patients to provide life sustainment to all; and when ventilators were in short supply, determining which patients required full ICU ventilator support vs those who could manage with lower functioning ventilators, and trading them between patients when demands changed.
These adaptations can only be done by experienced clinicians proactively managing bedside critical care under duress, further underscoring the importance of our suggestion that Critical Clinical Prioritization and ICU strain be managed by bedside clinicians and leaders.
The response of early transfer of patients – load-balancing - should be considered as soon as any hospital enters contingency conditions. This strategy is commonly implemented within larger health systems, ideally before reaching Critical Clinical Prioritization. Formal, organized state or regional load-balancing coordination, now referred to as medical operations command centers (MOCCs), were highly effective and proved lifesaving for those states that implemented them (including Arizona, Washington, California, Minnesota, and others). Support for establishment of MOCC’s is crucial in prolonging contingency operations and further helps support and protect disadvantaged populations (White et al. N Engl J Med. 2021;385[24]:2211-4).
Establishment of MOCCs has met resistance due to challenges that include interhospital/intersystem competition, logistics of moving critically ill patients sometimes across significant physical distance, and the costs of assuming care of uninsured or underinsured patients. Nevertheless, the benefits to the population as a whole necessitate working through these obstacles as successful MOCCs have done, usually with government and hospital association support.
In their final suggestion of the 2022 updated strategies, TFMCC suggests that hospitals use telemedicine technology both to expand specialists’ ability to provide care and facilitate families virtually visiting their critically ill loved one when safety precludes in-person visits.
These suggestions are pivotal in planning for future public health emergencies that include mass critical care, even during events that are limited in scope and duration.
Lastly, intensivists struggled with legal and ethical concerns when mired in crisis care circumstances and decisions of allocation, and potential reallocation, of scarce resources. These issues were not well addressed during the COVID-19 pandemic, further emphasizing the importance of maintaining contingency level care and requiring further involvement from legal and medical ethics professionals for future planning.
The guiding principle of disaster preparedness is that we must do all the planning we can to ensure that we never need crisis standards of care (National Academies of Sciences, Engineering, and Medicine. 2020 Mar 28. Rapid Expert Consultation on Crisis Standards of Care for the COVID-19 Pandemic. Washington, DC: The National Academies Press.).
We must be prepared. Guidelines and suggestions laid out through decades of experience gained a real-world test in the COVID-19 pandemic. Now we must all reorganize and create new plans or augment old ones with the information we have gained. The time is now. The work must continue.
Dr. Griffin is Assistant Professor of Medicine, New York Presbyterian Hospital – Weill Cornell Medicine. Dr. Dichter is Associate Professor of Medicine, University of Minnesota.
Hospitalists and PCPs crave greater communication
Hospitalists and PCPs want more dialogue while patients are in the hospital in order to coordinate and personalize care, according to data collected at Beth Israel Deaconess Medical Center, Boston. The results were presented at the annual meeting of the Society of General Internal Medicine.
“I think a major takeaway is that both hospitalists and primary care doctors agree that it’s important for primary care doctors to be involved in a patient’s hospitalization. They both identified a value that PCPs can bring to the table,” coresearcher Kristen Flint, MD, a primary care resident, told this news organization.
A majority in both camps reported that communication with the other party occurred in less than 25% of cases, whereas ideally it would happen half of the time. Dr. Flint noted that communication tools differ among hospitals, limiting the applicability of the findings.
The research team surveyed 39 hospitalists and 28 PCPs employed by the medical center during the first half of 2021. They also interviewed six hospitalists as they admitted and discharged patients.
The hospitalist movement, which took hold in response to cost and efficiency demands of managed care, led to the start of inpatient specialists, thereby reducing the need for PCPs to commute between their offices and the hospital to care for patients in both settings.
Primary care involvement is important during hospitalization
In the Beth Israel Deaconess survey, four out of five hospitalists and three-quarters of PCPs agreed that primary care involvement is still important during hospitalization, most critically during discharge and admission. Hospitalists reported that PCPs provide valuable data about a patient’s medical status, social supports, mental health, and goals for care. They also said having such data helps to boost patient trust and improve the quality of inpatient care.
“Most projects around communication between inpatient and outpatient doctors have really focused on the time of discharge,” when clinicians identify what care a patient will need after they leave the hospital, Dr. Flint said. “But we found that both sides felt increased communication at time of admission would also be beneficial.”
The biggest barrier for PCPs, cited by 82% of respondents, was lack of time. Hospitalists’ top impediment was being unable to find contact information for the other party, which was cited by 79% of these survey participants.
Hospitalists operate ‘in a very stressful environment’
The Beth Israel Deaconess research “documents what has largely been suspected,” said primary care general internist Allan Goroll, MD.
Dr. Goroll, a professor of medicine at Harvard Medical School, Boston, said in an interview that hospitalists operate “in a very stressful environment.”
“They [hospitalists] appreciate accurate information about a patient’s recent medical history, test results, and responses to treatment as well as a briefing on patient values and preferences, family dynamics, and priorities for the admission. It makes for a safer, more personalized, and more efficient hospital admission,” said Dr. Goroll, who was not involved in the research.
In a 2015 article in the New England Journal of Medicine, Dr. Goroll and Daniel Hunt, MD, director of hospital medicine at Emory University, Atlanta, proposed a collaborative model in which PCPs visit hospitalized patients and serve as consultants to inpatient staff. Dr. Goroll said Massachusetts General Hospital in Boston, where he practices, initiated a study of that approach, but it was interrupted by the pandemic.
“As limited time is the most often cited barrier to communication, future interventions such as asynchronous forms of communication between the two groups should be considered,” the researchers wrote in the NEJM perspective.
To narrow the gap, Beth Israel Deaconess will study converting an admission notification letter sent to PCPs into a two-way communication tool in which PCPs can insert patient information, Dr. Flint said.
Dr. Flint and Dr. Goroll have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hospitalists and PCPs want more dialogue while patients are in the hospital in order to coordinate and personalize care, according to data collected at Beth Israel Deaconess Medical Center, Boston. The results were presented at the annual meeting of the Society of General Internal Medicine.
“I think a major takeaway is that both hospitalists and primary care doctors agree that it’s important for primary care doctors to be involved in a patient’s hospitalization. They both identified a value that PCPs can bring to the table,” coresearcher Kristen Flint, MD, a primary care resident, told this news organization.
A majority in both camps reported that communication with the other party occurred in less than 25% of cases, whereas ideally it would happen half of the time. Dr. Flint noted that communication tools differ among hospitals, limiting the applicability of the findings.
The research team surveyed 39 hospitalists and 28 PCPs employed by the medical center during the first half of 2021. They also interviewed six hospitalists as they admitted and discharged patients.
The hospitalist movement, which took hold in response to cost and efficiency demands of managed care, led to the start of inpatient specialists, thereby reducing the need for PCPs to commute between their offices and the hospital to care for patients in both settings.
Primary care involvement is important during hospitalization
In the Beth Israel Deaconess survey, four out of five hospitalists and three-quarters of PCPs agreed that primary care involvement is still important during hospitalization, most critically during discharge and admission. Hospitalists reported that PCPs provide valuable data about a patient’s medical status, social supports, mental health, and goals for care. They also said having such data helps to boost patient trust and improve the quality of inpatient care.
“Most projects around communication between inpatient and outpatient doctors have really focused on the time of discharge,” when clinicians identify what care a patient will need after they leave the hospital, Dr. Flint said. “But we found that both sides felt increased communication at time of admission would also be beneficial.”
The biggest barrier for PCPs, cited by 82% of respondents, was lack of time. Hospitalists’ top impediment was being unable to find contact information for the other party, which was cited by 79% of these survey participants.
Hospitalists operate ‘in a very stressful environment’
The Beth Israel Deaconess research “documents what has largely been suspected,” said primary care general internist Allan Goroll, MD.
Dr. Goroll, a professor of medicine at Harvard Medical School, Boston, said in an interview that hospitalists operate “in a very stressful environment.”
“They [hospitalists] appreciate accurate information about a patient’s recent medical history, test results, and responses to treatment as well as a briefing on patient values and preferences, family dynamics, and priorities for the admission. It makes for a safer, more personalized, and more efficient hospital admission,” said Dr. Goroll, who was not involved in the research.
In a 2015 article in the New England Journal of Medicine, Dr. Goroll and Daniel Hunt, MD, director of hospital medicine at Emory University, Atlanta, proposed a collaborative model in which PCPs visit hospitalized patients and serve as consultants to inpatient staff. Dr. Goroll said Massachusetts General Hospital in Boston, where he practices, initiated a study of that approach, but it was interrupted by the pandemic.
“As limited time is the most often cited barrier to communication, future interventions such as asynchronous forms of communication between the two groups should be considered,” the researchers wrote in the NEJM perspective.
To narrow the gap, Beth Israel Deaconess will study converting an admission notification letter sent to PCPs into a two-way communication tool in which PCPs can insert patient information, Dr. Flint said.
Dr. Flint and Dr. Goroll have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hospitalists and PCPs want more dialogue while patients are in the hospital in order to coordinate and personalize care, according to data collected at Beth Israel Deaconess Medical Center, Boston. The results were presented at the annual meeting of the Society of General Internal Medicine.
“I think a major takeaway is that both hospitalists and primary care doctors agree that it’s important for primary care doctors to be involved in a patient’s hospitalization. They both identified a value that PCPs can bring to the table,” coresearcher Kristen Flint, MD, a primary care resident, told this news organization.
A majority in both camps reported that communication with the other party occurred in less than 25% of cases, whereas ideally it would happen half of the time. Dr. Flint noted that communication tools differ among hospitals, limiting the applicability of the findings.
The research team surveyed 39 hospitalists and 28 PCPs employed by the medical center during the first half of 2021. They also interviewed six hospitalists as they admitted and discharged patients.
The hospitalist movement, which took hold in response to cost and efficiency demands of managed care, led to the start of inpatient specialists, thereby reducing the need for PCPs to commute between their offices and the hospital to care for patients in both settings.
Primary care involvement is important during hospitalization
In the Beth Israel Deaconess survey, four out of five hospitalists and three-quarters of PCPs agreed that primary care involvement is still important during hospitalization, most critically during discharge and admission. Hospitalists reported that PCPs provide valuable data about a patient’s medical status, social supports, mental health, and goals for care. They also said having such data helps to boost patient trust and improve the quality of inpatient care.
“Most projects around communication between inpatient and outpatient doctors have really focused on the time of discharge,” when clinicians identify what care a patient will need after they leave the hospital, Dr. Flint said. “But we found that both sides felt increased communication at time of admission would also be beneficial.”
The biggest barrier for PCPs, cited by 82% of respondents, was lack of time. Hospitalists’ top impediment was being unable to find contact information for the other party, which was cited by 79% of these survey participants.
Hospitalists operate ‘in a very stressful environment’
The Beth Israel Deaconess research “documents what has largely been suspected,” said primary care general internist Allan Goroll, MD.
Dr. Goroll, a professor of medicine at Harvard Medical School, Boston, said in an interview that hospitalists operate “in a very stressful environment.”
“They [hospitalists] appreciate accurate information about a patient’s recent medical history, test results, and responses to treatment as well as a briefing on patient values and preferences, family dynamics, and priorities for the admission. It makes for a safer, more personalized, and more efficient hospital admission,” said Dr. Goroll, who was not involved in the research.
In a 2015 article in the New England Journal of Medicine, Dr. Goroll and Daniel Hunt, MD, director of hospital medicine at Emory University, Atlanta, proposed a collaborative model in which PCPs visit hospitalized patients and serve as consultants to inpatient staff. Dr. Goroll said Massachusetts General Hospital in Boston, where he practices, initiated a study of that approach, but it was interrupted by the pandemic.
“As limited time is the most often cited barrier to communication, future interventions such as asynchronous forms of communication between the two groups should be considered,” the researchers wrote in the NEJM perspective.
To narrow the gap, Beth Israel Deaconess will study converting an admission notification letter sent to PCPs into a two-way communication tool in which PCPs can insert patient information, Dr. Flint said.
Dr. Flint and Dr. Goroll have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SGIM 2022
Pneumococcal pneumonia outcomes worse than those of Legionnaires disease
Outcomes for patients with bacteremic Streptococcus pneumoniae were significantly worse than those for patients with Legionnaires disease (LD), based on data from 106 individuals.
Reported cases of LD in the United States have increased in recent decades, but they are likely under-reported, wrote Sima Salahie, MD, of Wayne State University School of Medicine, Detroit, and Central Michigan University College of Medicine, Grosse Pointe Woods, and colleagues.
Clinical presentations may be similar for both conditions, but different antimicrobial therapies are needed; therefore, identifying distinguishing factors can promote better management of hospitalized patients, they reported.
In a retrospective case companion study published in the American Journal of the Medical Sciences, the researchers reviewed data from 51 adults with LD and 55 with bacteremic S. pneumoniae pneumonia (SP) who were hospitalized at a single center between 2013 and 2018. Diagnoses were confirmed by laboratory and radiology results. In addition, data were collected on clinical features including body mass index, systolic and diastolic blood pressure, pulse, respiratory rate, and temperature.
Overall, patients with SP were significantly more likely than those with LD to require mechanical ventilation (P = .04), intensive care unit stay (P = .004), and to die (P = .002). Patients with SP also had higher rates of septic shock compared to LD patients, although this difference fell short of statistical significance (49.1% vs. 30.4%; P = .06).
In a multivariate analysis, male sex, diarrhea, higher body mass index, hyponatremia, and lower Charleston Weighted Index of Comorbidity (CWIC) score were significant independent predictors of LD, with odds ratios of 21.6, 4.5, 1.13, 5.6, and 0.61, respectively.
The incidence of LD peaked in summer, while the incidence of SP peaked in the winter, the researchers noted. “Seasonality is a variable that has not always been included in previous scoring systems but should be considered in future modeling,” they said.
“Noteworthy is that LD represented almost as many cases as documented bacteremic pneumococcal pneumonia,” the researchers wrote in their discussion. “This occurred at a time when there was no outbreak of L. pneumophila in our community, and as these were all community acquired, there was no evidence of a nosocomial outbreak in our institution,” they said.
The study findings were limited by several factors, including the possible underestimation of SP because of the requirement for positive blood cultures and the lack of other methods of diagnosing SP, the researchers noted.
“However, the data suggest variables to distinguish LD from SP,” they said. “Establishing reliable clinical and laboratory parameters embedded in a simple diagnostic score that can accurately identify patients with LD may be helpful in aiding physicians’ early diagnosis in distinguishing LD from SP but will need to be defined.”
The study received no outside funding. The researchers disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
Outcomes for patients with bacteremic Streptococcus pneumoniae were significantly worse than those for patients with Legionnaires disease (LD), based on data from 106 individuals.
Reported cases of LD in the United States have increased in recent decades, but they are likely under-reported, wrote Sima Salahie, MD, of Wayne State University School of Medicine, Detroit, and Central Michigan University College of Medicine, Grosse Pointe Woods, and colleagues.
Clinical presentations may be similar for both conditions, but different antimicrobial therapies are needed; therefore, identifying distinguishing factors can promote better management of hospitalized patients, they reported.
In a retrospective case companion study published in the American Journal of the Medical Sciences, the researchers reviewed data from 51 adults with LD and 55 with bacteremic S. pneumoniae pneumonia (SP) who were hospitalized at a single center between 2013 and 2018. Diagnoses were confirmed by laboratory and radiology results. In addition, data were collected on clinical features including body mass index, systolic and diastolic blood pressure, pulse, respiratory rate, and temperature.
Overall, patients with SP were significantly more likely than those with LD to require mechanical ventilation (P = .04), intensive care unit stay (P = .004), and to die (P = .002). Patients with SP also had higher rates of septic shock compared to LD patients, although this difference fell short of statistical significance (49.1% vs. 30.4%; P = .06).
In a multivariate analysis, male sex, diarrhea, higher body mass index, hyponatremia, and lower Charleston Weighted Index of Comorbidity (CWIC) score were significant independent predictors of LD, with odds ratios of 21.6, 4.5, 1.13, 5.6, and 0.61, respectively.
The incidence of LD peaked in summer, while the incidence of SP peaked in the winter, the researchers noted. “Seasonality is a variable that has not always been included in previous scoring systems but should be considered in future modeling,” they said.
“Noteworthy is that LD represented almost as many cases as documented bacteremic pneumococcal pneumonia,” the researchers wrote in their discussion. “This occurred at a time when there was no outbreak of L. pneumophila in our community, and as these were all community acquired, there was no evidence of a nosocomial outbreak in our institution,” they said.
The study findings were limited by several factors, including the possible underestimation of SP because of the requirement for positive blood cultures and the lack of other methods of diagnosing SP, the researchers noted.
“However, the data suggest variables to distinguish LD from SP,” they said. “Establishing reliable clinical and laboratory parameters embedded in a simple diagnostic score that can accurately identify patients with LD may be helpful in aiding physicians’ early diagnosis in distinguishing LD from SP but will need to be defined.”
The study received no outside funding. The researchers disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
Outcomes for patients with bacteremic Streptococcus pneumoniae were significantly worse than those for patients with Legionnaires disease (LD), based on data from 106 individuals.
Reported cases of LD in the United States have increased in recent decades, but they are likely under-reported, wrote Sima Salahie, MD, of Wayne State University School of Medicine, Detroit, and Central Michigan University College of Medicine, Grosse Pointe Woods, and colleagues.
Clinical presentations may be similar for both conditions, but different antimicrobial therapies are needed; therefore, identifying distinguishing factors can promote better management of hospitalized patients, they reported.
In a retrospective case companion study published in the American Journal of the Medical Sciences, the researchers reviewed data from 51 adults with LD and 55 with bacteremic S. pneumoniae pneumonia (SP) who were hospitalized at a single center between 2013 and 2018. Diagnoses were confirmed by laboratory and radiology results. In addition, data were collected on clinical features including body mass index, systolic and diastolic blood pressure, pulse, respiratory rate, and temperature.
Overall, patients with SP were significantly more likely than those with LD to require mechanical ventilation (P = .04), intensive care unit stay (P = .004), and to die (P = .002). Patients with SP also had higher rates of septic shock compared to LD patients, although this difference fell short of statistical significance (49.1% vs. 30.4%; P = .06).
In a multivariate analysis, male sex, diarrhea, higher body mass index, hyponatremia, and lower Charleston Weighted Index of Comorbidity (CWIC) score were significant independent predictors of LD, with odds ratios of 21.6, 4.5, 1.13, 5.6, and 0.61, respectively.
The incidence of LD peaked in summer, while the incidence of SP peaked in the winter, the researchers noted. “Seasonality is a variable that has not always been included in previous scoring systems but should be considered in future modeling,” they said.
“Noteworthy is that LD represented almost as many cases as documented bacteremic pneumococcal pneumonia,” the researchers wrote in their discussion. “This occurred at a time when there was no outbreak of L. pneumophila in our community, and as these were all community acquired, there was no evidence of a nosocomial outbreak in our institution,” they said.
The study findings were limited by several factors, including the possible underestimation of SP because of the requirement for positive blood cultures and the lack of other methods of diagnosing SP, the researchers noted.
“However, the data suggest variables to distinguish LD from SP,” they said. “Establishing reliable clinical and laboratory parameters embedded in a simple diagnostic score that can accurately identify patients with LD may be helpful in aiding physicians’ early diagnosis in distinguishing LD from SP but will need to be defined.”
The study received no outside funding. The researchers disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
‘Vast majority’ of COVID patients wake up after mechanical ventilation
COVID-19 patients who are successfully weaned off a ventilator may take days, or even weeks, to regain consciousness, especially those who experienced episodes of hypoxemia while intubated, a new study shows.
“As we started to see the first patients waking up after successful COVID-19 ICU treatments, we also encountered many patients who remained comatose for days and weeks and then regained consciousness to become fully oriented,” co-senior investigator Nicholas Schiff, MD, with NewYork-Presbyterian/Weill Cornell Medical Center, says in a news release.
The findings have immediate implications regarding life-sustaining therapies for unresponsive COVID-19 patients, the investigators note.
“In critical care medicine, one of our main tasks is to advise families about planning in the event a patient does not regain consciousness,” said co-senior author Jan Claassen, MD, with New York-Presbyterian/Columbia University Irving Medical Center.
“Our findings suggest that for patients with severe COVID, the decision to withdraw life support shouldn’t be based solely on prolonged periods of unconsciousness, as these patients may eventually recover,” Dr. Claassen adds.
The study was published online March 7 in Annals of Neurology.
Slow road back
The researchers examined 795 intubated patients with severe COVID-19 at three medical centers in New York during the first wave of the pandemic (March-July 2020). All patients had impaired consciousness (Glasgow Coma Scale [GCS] motor score less than 6) on day 7 of intubation.
A total of 571 patients (72%) survived and regained consciousness.
The median time to recovery of consciousness was 30 days. One-quarter of the patients recovered consciousness 10 days or longer after they stopped receiving ventilator support and 10% took 23 days or longer to recover.
Time to recovery of consciousness was associated with hypoxemia. The hazard ratio was 0.56 (95% confidence interval, 0.46-0.68) with arterial partial pressure of oxygen (PaO2) less than or equal to 55 mm Hg and 0.88 (95% CI, 0.85-0.91) with a PaO2 less than or equal to 70 mm Hg.
Each additional day of hypoxemia decreased the odds of recovery of consciousness after accounting for confounding factors including sedation.
These findings were confirmed among patients without any imaging evidence of structural brain injury and in a non-overlapping cohort of 427 patients from the second wave of the pandemic (October-April 2021).
“These findings provide us with more accurate information to guide families who are deciding whether to continue life-sustaining therapy in unconscious COVID-19 patients,” co-senior author Brian Edlow, MD, with Massachusetts General Hospital and Harvard Medical School in Boston, says in the news release.
“Encouragingly,” adds Dr. Claassen, “our study shows that the vast majority of unconscious COVID patients recover consciousness, but it is important to consider that we did not look at the quality of recovery. That’s something that should be the focus of long-term follow-up studies.”
The study was supported by the James S. McDonnell Foundation (JSMF). Dr. Schiff, Dr. Claassen, and Dr. Edlow have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 patients who are successfully weaned off a ventilator may take days, or even weeks, to regain consciousness, especially those who experienced episodes of hypoxemia while intubated, a new study shows.
“As we started to see the first patients waking up after successful COVID-19 ICU treatments, we also encountered many patients who remained comatose for days and weeks and then regained consciousness to become fully oriented,” co-senior investigator Nicholas Schiff, MD, with NewYork-Presbyterian/Weill Cornell Medical Center, says in a news release.
The findings have immediate implications regarding life-sustaining therapies for unresponsive COVID-19 patients, the investigators note.
“In critical care medicine, one of our main tasks is to advise families about planning in the event a patient does not regain consciousness,” said co-senior author Jan Claassen, MD, with New York-Presbyterian/Columbia University Irving Medical Center.
“Our findings suggest that for patients with severe COVID, the decision to withdraw life support shouldn’t be based solely on prolonged periods of unconsciousness, as these patients may eventually recover,” Dr. Claassen adds.
The study was published online March 7 in Annals of Neurology.
Slow road back
The researchers examined 795 intubated patients with severe COVID-19 at three medical centers in New York during the first wave of the pandemic (March-July 2020). All patients had impaired consciousness (Glasgow Coma Scale [GCS] motor score less than 6) on day 7 of intubation.
A total of 571 patients (72%) survived and regained consciousness.
The median time to recovery of consciousness was 30 days. One-quarter of the patients recovered consciousness 10 days or longer after they stopped receiving ventilator support and 10% took 23 days or longer to recover.
Time to recovery of consciousness was associated with hypoxemia. The hazard ratio was 0.56 (95% confidence interval, 0.46-0.68) with arterial partial pressure of oxygen (PaO2) less than or equal to 55 mm Hg and 0.88 (95% CI, 0.85-0.91) with a PaO2 less than or equal to 70 mm Hg.
Each additional day of hypoxemia decreased the odds of recovery of consciousness after accounting for confounding factors including sedation.
These findings were confirmed among patients without any imaging evidence of structural brain injury and in a non-overlapping cohort of 427 patients from the second wave of the pandemic (October-April 2021).
“These findings provide us with more accurate information to guide families who are deciding whether to continue life-sustaining therapy in unconscious COVID-19 patients,” co-senior author Brian Edlow, MD, with Massachusetts General Hospital and Harvard Medical School in Boston, says in the news release.
“Encouragingly,” adds Dr. Claassen, “our study shows that the vast majority of unconscious COVID patients recover consciousness, but it is important to consider that we did not look at the quality of recovery. That’s something that should be the focus of long-term follow-up studies.”
The study was supported by the James S. McDonnell Foundation (JSMF). Dr. Schiff, Dr. Claassen, and Dr. Edlow have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 patients who are successfully weaned off a ventilator may take days, or even weeks, to regain consciousness, especially those who experienced episodes of hypoxemia while intubated, a new study shows.
“As we started to see the first patients waking up after successful COVID-19 ICU treatments, we also encountered many patients who remained comatose for days and weeks and then regained consciousness to become fully oriented,” co-senior investigator Nicholas Schiff, MD, with NewYork-Presbyterian/Weill Cornell Medical Center, says in a news release.
The findings have immediate implications regarding life-sustaining therapies for unresponsive COVID-19 patients, the investigators note.
“In critical care medicine, one of our main tasks is to advise families about planning in the event a patient does not regain consciousness,” said co-senior author Jan Claassen, MD, with New York-Presbyterian/Columbia University Irving Medical Center.
“Our findings suggest that for patients with severe COVID, the decision to withdraw life support shouldn’t be based solely on prolonged periods of unconsciousness, as these patients may eventually recover,” Dr. Claassen adds.
The study was published online March 7 in Annals of Neurology.
Slow road back
The researchers examined 795 intubated patients with severe COVID-19 at three medical centers in New York during the first wave of the pandemic (March-July 2020). All patients had impaired consciousness (Glasgow Coma Scale [GCS] motor score less than 6) on day 7 of intubation.
A total of 571 patients (72%) survived and regained consciousness.
The median time to recovery of consciousness was 30 days. One-quarter of the patients recovered consciousness 10 days or longer after they stopped receiving ventilator support and 10% took 23 days or longer to recover.
Time to recovery of consciousness was associated with hypoxemia. The hazard ratio was 0.56 (95% confidence interval, 0.46-0.68) with arterial partial pressure of oxygen (PaO2) less than or equal to 55 mm Hg and 0.88 (95% CI, 0.85-0.91) with a PaO2 less than or equal to 70 mm Hg.
Each additional day of hypoxemia decreased the odds of recovery of consciousness after accounting for confounding factors including sedation.
These findings were confirmed among patients without any imaging evidence of structural brain injury and in a non-overlapping cohort of 427 patients from the second wave of the pandemic (October-April 2021).
“These findings provide us with more accurate information to guide families who are deciding whether to continue life-sustaining therapy in unconscious COVID-19 patients,” co-senior author Brian Edlow, MD, with Massachusetts General Hospital and Harvard Medical School in Boston, says in the news release.
“Encouragingly,” adds Dr. Claassen, “our study shows that the vast majority of unconscious COVID patients recover consciousness, but it is important to consider that we did not look at the quality of recovery. That’s something that should be the focus of long-term follow-up studies.”
The study was supported by the James S. McDonnell Foundation (JSMF). Dr. Schiff, Dr. Claassen, and Dr. Edlow have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF NEUROLOGY
Acute STEMI During the COVID-19 Pandemic at a Regional Hospital: Incidence, Clinical Characteristics, and Outcomes
From the Department of Medicine, Medical College of Georgia at the Augusta University-University of Georgia Medical Partnership, Athens, GA (Syed H. Ali, Syed Hyder, and Dr. Murrow), and the Department of Cardiology, Piedmont Heart Institute, Piedmont Athens Regional, Athens, GA (Dr. Murrow and Mrs. Davis).
Abstract
Objectives: The aim of this study was to describe the characteristics and in-hospital outcomes of patients with acute ST-segment elevation myocardial infarction (STEMI) during the early COVID-19 pandemic at Piedmont Athens Regional (PAR), a 330-bed tertiary referral center in Northeast Georgia.
Methods: A retrospective study was conducted at PAR to evaluate patients with acute STEMI admitted over an 8-week period during the initial COVID-19 outbreak. This study group was compared to patients admitted during the corresponding period in 2019. The primary endpoint of this study was defined as a composite of sustained ventricular arrhythmia, congestive heart failure (CHF) with pulmonary congestion, and/or in-hospital mortality.
Results: This study cohort was composed of 64 patients with acute STEMI; 30 patients (46.9%) were hospitalized during the COVID-19 pandemic. Patients with STEMI in both the COVID-19 and control groups had similar comorbidities, Killip classification score, and clinical presentations. The median (interquartile range) time from symptom onset to reperfusion (total ischemic time) increased from 99.5 minutes (84.8-132) in 2019 to 149 minutes (96.3-231.8; P = .032) in 2020. Hospitalization during the COVID-19 period was associated with an increased risk for combined in-hospital outcome (odds ratio, 3.96; P = .046).
Conclusion: Patients with STEMI admitted during the first wave of the COVID-19 outbreak experienced longer total ischemic time and increased risk for combined in-hospital outcomes compared to patients admitted during the corresponding period in 2019.
Keywords: myocardial infarction, acute coronary syndrome, hospitalization, outcomes.

The emergence of the SARS-Cov-2 virus in December 2019 caused a worldwide shift in resource allocation and the restructuring of health care systems within the span of a few months. With the rapid spread of infection, the World Health Organization officially declared a pandemic in March 2020. The pandemic led to the deferral and cancellation of in-person patient visits, routine diagnostic studies, and nonessential surgeries and procedures. This response occurred secondary to a joint effort to reduce transmission via stay-at-home mandates and appropriate social distancing.1
Alongside the reduction in elective procedures and health care visits, significant reductions in hospitalization rates due to decreases in acute ST-segment elevation myocardial infarction (STEMI) and catheterization laboratory utilization have been reported in many studies from around the world.2-7 Comprehensive data demonstrating the impact of the COVID-19 pandemic on acute STEMI patient characteristics, clinical presentation, and in-hospital outcomes are lacking. Although patients with previously diagnosed cardiovascular disease are more likely to encounter worse outcomes in the setting of COVID-19, there may also be an indirect impact of the pandemic on high-risk patients, including those without the infection.8 Several theories have been hypothesized to explain this phenomenon. One theory postulates that the fear of contracting the virus during hospitalization is great enough to prevent patients from seeking care.2 Another theory suggests that the increased utilization of telemedicine prevents exacerbation of chronic conditions and the need for hospitalization.9 Contrary to this trend, previous studies have shown an increased incidence of acute STEMI following stressful events such as natural disasters.10
The aim of this study was to describe trends pertaining to clinical characteristics and in-hospital outcomes of patients with acute STEMI during the early COVID-19 pandemic at Piedmont Athens Regional (PAR), a 330-bed tertiary referral center in Northeast Georgia.
Methods
A retrospective cohort study was conducted at PAR to evaluate patients with STEMI admitted to the cardiovascular intensive care unit over an 8-week period (March 5 to May 5, 2020) during the COVID-19 outbreak. COVID-19 was declared a national emergency on March 13, 2020, in the United States. The institutional review board at PAR approved the study; the need for individual consent was waived under the condition that participant data would undergo de-identification and be strictly safeguarded.
Data Collection
Because there are seasonal variations in cardiovascular admissions, patient data from a control period (March 9 to May 9, 2019) were obtained to compare with data from the 2020 period. The number of patients with the diagnosis of acute STEMI during the COVID-19 period was recorded. Demographic data, clinical characteristics, and primary angiographic findings were gathered for all patients. Time from symptom onset to hospital admission and time from hospital admission to reperfusion (defined as door-to-balloon time) were documented for each patient. Killip classification was used to assess patients’ clinical status on admission. Length of stay was determined as days from hospital admission to discharge or death (if occurring during the same hospitalization).
Adverse in-hospital complications were also recorded. These were selected based on inclusion of the following categories of acute STEMI complications: ischemic, mechanical, arrhythmic, embolic, and inflammatory. The following complications occurred in our patient cohort: sustained ventricular arrhythmia, congestive heart failure (CHF) defined as congestion requiring intravenous diuretics, re-infarction, mechanical complications (free-wall rupture, ventricular septal defect, or mitral regurgitation), second- or third-degree atrioventricular block, atrial fibrillation, stroke, mechanical ventilation, major bleeding, pericarditis, cardiogenic shock, cardiac arrest, and in-hospital mortality. The primary outcome of this study was defined as a composite of sustained ventricular arrhythmia, CHF with congestion requiring intravenous diuretics, and/or in-hospital mortality. Ventricular arrythmia and CHF were included in the composite outcome because they are defined as the 2 most common causes of sudden cardiac death following acute STEMI.11,12
Statistical Analysis
Normally distributed continuous variables and categorical variables were compared using the paired t-test. A 2-sided P value <.05 was considered to be statistically significant. Mean admission rates for acute STEMI hospitalizations were determined by dividing the number of admissions by the number of days in each time period. The daily rate of COVID-19 cases per 100,000 individuals was obtained from the Centers for Disease Control and Prevention COVID-19 database. All data analyses were performed using Microsoft Excel.
Results
The study cohort consisted of 64 patients, of whom 30 (46.9%) were hospitalized between March 5 and May 5, 2020, and 34 (53.1%) who were admitted during the analogous time period in 2019. This reflected a 6% decrease in STEMI admissions at PAR in the COVID-19 cohort.
Acute STEMI Hospitalization Rates and COVID-19 Incidence
The mean daily acute STEMI admission rate was 0.50 during the study period compared to 0.57 during the control period. During the study period in 2020 in the state of Georgia, the daily rate of newly confirmed COVID-19 cases ranged from 0.194 per 100,000 on March 5 to 8.778 per 100,000 on May 5. Results of COVID-19 testing were available for 9 STEMI patients, and of these 0 tests were positive.
Baseline Characteristics
Baseline characteristics of the acute STEMI cohorts are presented in Table 1. Approximately 75% were male; median (interquartile range [IQR]) age was 60 (51-72) years. There were no significant differences in age and gender between the study periods. Three-quarters of patients had a history of hypertension, and 87.5% had a history of dyslipidemia. There was no significant difference in baseline comorbidity profiles between the 2 study periods; therefore, our sample populations shared similar characteristics.

Clinical Presentation
Significant differences were observed regarding the time intervals of STEMI patients in the COVID-19 period and the control period (Table 2). Median time from symptom onset to hospital admission (patient delay) was extended from 57.5 minutes (IQR, 40.3-106) in 2019 to 93 minutes (IQR, 48.8-132) in 2020; however, this difference was not statistically significant (P = .697). Median time from hospital admission to reperfusion (system delay) was prolonged from 45 minutes (IQR, 28-61) in 2019 to 78 minutes (IQR, 50-110) in 2020 (P < .001). Overall time from symptom onset to reperfusion (total ischemic time) increased from 99.5 minutes (IQR, 84.8-132) in 2019 to 149 minutes (IQR, 96.3-231.8) in 2020 (P = .032).

Regarding mode of transportation, 23.5% of patients in 2019 were walk-in admissions to the emergency department. During the COVID-19 period, walk-in admissions decreased to 6.7% (P = .065). There were no significant differences between emergency medical service, transfer, or in-patient admissions for STEMI cases between the 2 study periods.
Killip classification scores were calculated for all patients on admission; 90.6% of patients were classified as Killip Class 1. There was no significant difference between hemodynamic presentations during the COVID-19 period compared to the control period.
Angiographic Data
Overall, 53 (82.8%) patients admitted with acute STEMI underwent coronary angiography during their hospital stay. The proportion of patients who underwent primary reperfusion was greater in the control period than in the COVID-19 period (85.3% vs 80%; P = .582). Angiographic characteristics and findings were similar between the 2 study groups (Table 2).
In-Hospital Outcomes
In-hospital outcome data were available for all patients. As shown in Table 3, hospitalization during the COVID-19 period was independently associated with an increased risk for combined in-hospital outcome (odds ratio, 3.96; P = .046). The rate of in-hospital mortality was greater in the COVID-19 period (P = .013). We found no significant difference when comparing secondary outcomes from admissions during the COVID-19 period and the control period in 2019. For the 5 patients who died during the study period, the primary diagnosis at death was acute STEMI complicated by CHF (3 patients) or cardiogenic shock (2 patients).

Discussion
This single-center retrospective study at PAR looks at the impact of COVID-19 on hospitalizations for acute STEMI during the initial peak of the pandemic. The key findings of this study show a significant increase in ischemic time parameters (symptom onset to reperfusion, hospital admission to reperfusion), in-hospital mortality, and combined in-hospital outcomes.
There was a 49.5-minute increase in total ischemic time noted in this study (P = .032). Though there was a numerical increase in time of symptom onset to hospital admission by 23.5 minutes, this difference was not statistically significant (P = .697). However, this study observed a statistically significant 33-minute increase in ischemic time from hospital admission to reperfusion (P < .001). Multiple studies globally have found a similar increase in total ischemic times, including those conducted in China and Europe.13-15 Every level of potential delay must be considered, including pre-hospital, triage and emergency department, and/or reperfusion team. Pre-hospital sources of delays that have been suggested include “stay-at-home” orders and apprehension to seek medical care due to concern about contracting the virus or overwhelming the health care facilities. There was a clinically significant 4-fold decrease in the number of walk-in acute STEMI cases in the study period. In 2019, there were 8 walk-in cases compared to 2 cases in 2020 (P = .065). However, this change was not statistically significant. In-hospital/systemic sources of delays have been mentioned in other studies; they include increased time taken to rule out COVID-19 (nasopharyngeal swab/chest x-ray) and increased time due to the need for intensive gowning and gloving procedures by staff. It was difficult to objectively determine the sources of system delay by the reperfusion team due to a lack of quantitative data.
In the current study, we found a significant increase in in-hospital mortality during the COVID-19 period compared to a parallel time frame in 2019. This finding is contrary to a multicenter study from Spain that reported no difference in in-hospital outcomes or mortality rates among all acute coronary syndrome cases.16 The worsening outcomes and prognosis may simply be a result of increased ischemic time; however, the virus that causes COVID-19 itself may play a role as well. Studies have found that SARS-Cov-2 infection places patients at greater risk for cardiovascular conditions such as hypercoagulability, myocarditis, and arrhythmias.17 In our study, however, there were no acute STEMI patients who tested positive for COVID-19. Therefore, we cannot discuss the impact of increased thrombus burden in patients with COVID-19. Piedmont Healthcare published a STEMI treatment protocol in May 2020 that advised increased use of tissue plasminogen activator (tPA) in COVID-19-positive cases; during the study period, however, there were no occasions when tPA use was deemed appropriate based on clinical judgment.
Our findings align with previous studies that describe an increase in combined in-hospital adverse outcomes during the COVID-19 era. Previous studies detected a higher rate of complications in the COVID-19 cohort, but in the current study, the adverse in-hospital course is unrelated to underlying infection.18,19 This study reports a higher incidence of major in-hospital outcomes, including a 65% increase in the rate of combined in-hospital outcomes, which is similar to a multicenter study conducted in Israel.19 There was a 2.3-fold numerical increase in sustained ventricular arrhythmias and a 2.5-fold numerical increase in the incidence of cardiac arrest in the study period. This phenomenon was observed despite a similar rate of reperfusion procedures in both groups.
Acute STEMI is a highly fatal condition with an incidence of 8.5 in 10,000 annually in the United States. While studies across the world have shown a 25% to 40% reduction in the rate of hospitalized acute coronary syndrome cases during the COVID-19 pandemic, the decrease from 34 to 30 STEMI admissions at PAR is not statistically significant.20 Possible reasons for the reduction globally include increased out-of-hospital mortality and decreased incidence of acute STEMI across the general population as a result of improved access to telemedicine or decreased levels of life stressors.20
In summary, there was an increase in ischemic time to reperfusion, in-hospital mortality, and combined in-hospital outcomes for acute STEMI patients at PAR during the COVID period.
Limitations
This study has several limitations. This is a single-center study, so the sample size is small and may not be generalizable to a larger population. This is a retrospective observational study, so causation cannot be inferred. This study analyzed ischemic time parameters as average rates over time rather than in an interrupted time series. Post-reperfusion outcomes were limited to hospital stay. Post-hospital follow-up would provide a better picture of the effects of STEMI intervention. There is no account of patients who died out-of-hospital secondary to acute STEMI. COVID-19 testing was not introduced until midway in our study period. Therefore, we cannot rule out the possibility of the SARS-Cov-2 virus inciting acute STEMI and subsequently leading to worse outcomes and poor prognosis.
Conclusions
This study provides an analysis of the incidence, characteristics, and clinical outcomes of patients presenting with acute STEMI during the early period of the COVID-19 pandemic. In-hospital mortality and ischemic time to reperfusion increased while combined in-hospital outcomes worsened.
Acknowledgment: The authors thank Piedmont Athens Regional IRB for approving this project and allowing access to patient data.
Corresponding author: Syed H. Ali; Department of Medicine, Medical College of Georgia at the Augusta University-University of Georgia Medical Partnership, 30606, Athens, GA; [email protected]
Disclosures: None reported.
doi:10.12788/jcom.0085
1. Bhatt AS, Moscone A, McElrath EE, et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280-288. doi:10.1016/j.jacc.2020.05.038
2. Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJR. Decline of acute coronary syndrome admissions in Austria since the outbreak of Covid-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41:1852-1853. doi:10.1093/eurheartj/ehaa314
3. De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the Covid-19 era. Eur Heart J. 2020;41(22):2083-2088.
4. Wilson SJ, Connolly MJ, Elghamry Z, et al. Effect of the COVID-19 pandemic on ST-segment-elevation myocardial infarction presentations and in-hospital outcomes. Circ Cardiovasc Interv. 2020; 13(7):e009438. doi:10.1161/CIRCINTERVENTIONS.120.009438
5. Mafham MM, Spata E, Goldacre R, et al. Covid-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396 (10248):381-389. doi:10.1016/S0140-6736(20)31356-8
6. Bhatt AS, Moscone A, McElrath EE, et al. Fewer Hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280-288. doi:10.1016/j.jacc.2020.05.038
7. Tam CF, Cheung KS, Lam S, et al. Impact of Coronavirus disease 2019 (Covid-19) outbreak on ST-segment elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13(4):e006631. doi:10.1161/CIRCOUTCOMES.120.006631
8. Clerkin KJ, Fried JA, Raikhelkar J, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141:1648-1655. doi:10.1161/CIRCULATIONAHA.120.046941
9. Ebinger JE, Shah PK. Declining admissions for acute cardiovascular illness: The Covid-19 paradox. J Am Coll Cardiol. 2020;76(3):289-291. doi:10.1016/j.jacc.2020.05.039
10 Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med. 1996;334(7):413-419. doi:10.1056/NEJM199602153340701
11. Hiramori K. Major causes of death from acute myocardial infarction in a coronary care unit. Jpn Circ J. 1987;51(9):1041-1047. doi:10.1253/jcj.51.1041
12. Bui AH, Waks JW. Risk stratification of sudden cardiac death after acute myocardial infarction. J Innov Card Rhythm Manag. 2018;9(2):3035-3049. doi:10.19102/icrm.2018.090201
13. Xiang D, Xiang X, Zhang W, et al. Management and outcomes of patients with STEMI during the COVID-19 pandemic in China. J Am Coll Cardiol. 2020;76(11):1318-1324. doi:10.1016/j.jacc.2020.06.039
14. Hakim R, Motreff P, Rangé G. COVID-19 and STEMI. [Article in French]. Ann Cardiol Angeiol (Paris). 2020;69(6):355-359. doi:10.1016/j.ancard.2020.09.034
15. Soylu K, Coksevim M, Yanık A, Bugra Cerik I, Aksan G. Effect of Covid-19 pandemic process on STEMI patients timeline. Int J Clin Pract. 2021;75(5):e14005. doi:10.1111/ijcp.14005
16. Salinas P, Travieso A, Vergara-Uzcategui C, et al. Clinical profile and 30-day mortality of invasively managed patients with suspected acute coronary syndrome during the COVID-19 outbreak. Int Heart J. 2021;62(2):274-281. doi:10.1536/ihj.20-574
17. Hu Y, Sun J, Dai Z, et al. Prevalence and severity of corona virus disease 2019 (Covid-19): a systematic review and meta-analysis. J Clin Virol. 2020;127:104371. doi:10.1016/j.jcv.2020.104371
18. Rodriguez-Leor O, Cid Alvarez AB, Perez de Prado A, et al. In-hospital outcomes of COVID-19 ST-elevation myocardial infarction patients. EuroIntervention. 2021;16(17):1426-1433. doi:10.4244/EIJ-D-20-00935
19. Fardman A, Zahger D, Orvin K, et al. Acute myocardial infarction in the Covid-19 era: incidence, clinical characteristics and in-hospital outcomes—A multicenter registry. PLoS ONE. 2021;16(6): e0253524. doi:10.1371/journal.pone.0253524
20. Pessoa-Amorim G, Camm CF, Gajendragadkar P, et al. Admission of patients with STEMI since the outbreak of the COVID-19 pandemic: a survey by the European Society of Cardiology. Eur Heart J Qual Care Clin Outcomes. 2020;6(3):210-216. doi:10.1093/ehjqcco/qcaa046
From the Department of Medicine, Medical College of Georgia at the Augusta University-University of Georgia Medical Partnership, Athens, GA (Syed H. Ali, Syed Hyder, and Dr. Murrow), and the Department of Cardiology, Piedmont Heart Institute, Piedmont Athens Regional, Athens, GA (Dr. Murrow and Mrs. Davis).
Abstract
Objectives: The aim of this study was to describe the characteristics and in-hospital outcomes of patients with acute ST-segment elevation myocardial infarction (STEMI) during the early COVID-19 pandemic at Piedmont Athens Regional (PAR), a 330-bed tertiary referral center in Northeast Georgia.
Methods: A retrospective study was conducted at PAR to evaluate patients with acute STEMI admitted over an 8-week period during the initial COVID-19 outbreak. This study group was compared to patients admitted during the corresponding period in 2019. The primary endpoint of this study was defined as a composite of sustained ventricular arrhythmia, congestive heart failure (CHF) with pulmonary congestion, and/or in-hospital mortality.
Results: This study cohort was composed of 64 patients with acute STEMI; 30 patients (46.9%) were hospitalized during the COVID-19 pandemic. Patients with STEMI in both the COVID-19 and control groups had similar comorbidities, Killip classification score, and clinical presentations. The median (interquartile range) time from symptom onset to reperfusion (total ischemic time) increased from 99.5 minutes (84.8-132) in 2019 to 149 minutes (96.3-231.8; P = .032) in 2020. Hospitalization during the COVID-19 period was associated with an increased risk for combined in-hospital outcome (odds ratio, 3.96; P = .046).
Conclusion: Patients with STEMI admitted during the first wave of the COVID-19 outbreak experienced longer total ischemic time and increased risk for combined in-hospital outcomes compared to patients admitted during the corresponding period in 2019.
Keywords: myocardial infarction, acute coronary syndrome, hospitalization, outcomes.

The emergence of the SARS-Cov-2 virus in December 2019 caused a worldwide shift in resource allocation and the restructuring of health care systems within the span of a few months. With the rapid spread of infection, the World Health Organization officially declared a pandemic in March 2020. The pandemic led to the deferral and cancellation of in-person patient visits, routine diagnostic studies, and nonessential surgeries and procedures. This response occurred secondary to a joint effort to reduce transmission via stay-at-home mandates and appropriate social distancing.1
Alongside the reduction in elective procedures and health care visits, significant reductions in hospitalization rates due to decreases in acute ST-segment elevation myocardial infarction (STEMI) and catheterization laboratory utilization have been reported in many studies from around the world.2-7 Comprehensive data demonstrating the impact of the COVID-19 pandemic on acute STEMI patient characteristics, clinical presentation, and in-hospital outcomes are lacking. Although patients with previously diagnosed cardiovascular disease are more likely to encounter worse outcomes in the setting of COVID-19, there may also be an indirect impact of the pandemic on high-risk patients, including those without the infection.8 Several theories have been hypothesized to explain this phenomenon. One theory postulates that the fear of contracting the virus during hospitalization is great enough to prevent patients from seeking care.2 Another theory suggests that the increased utilization of telemedicine prevents exacerbation of chronic conditions and the need for hospitalization.9 Contrary to this trend, previous studies have shown an increased incidence of acute STEMI following stressful events such as natural disasters.10
The aim of this study was to describe trends pertaining to clinical characteristics and in-hospital outcomes of patients with acute STEMI during the early COVID-19 pandemic at Piedmont Athens Regional (PAR), a 330-bed tertiary referral center in Northeast Georgia.
Methods
A retrospective cohort study was conducted at PAR to evaluate patients with STEMI admitted to the cardiovascular intensive care unit over an 8-week period (March 5 to May 5, 2020) during the COVID-19 outbreak. COVID-19 was declared a national emergency on March 13, 2020, in the United States. The institutional review board at PAR approved the study; the need for individual consent was waived under the condition that participant data would undergo de-identification and be strictly safeguarded.
Data Collection
Because there are seasonal variations in cardiovascular admissions, patient data from a control period (March 9 to May 9, 2019) were obtained to compare with data from the 2020 period. The number of patients with the diagnosis of acute STEMI during the COVID-19 period was recorded. Demographic data, clinical characteristics, and primary angiographic findings were gathered for all patients. Time from symptom onset to hospital admission and time from hospital admission to reperfusion (defined as door-to-balloon time) were documented for each patient. Killip classification was used to assess patients’ clinical status on admission. Length of stay was determined as days from hospital admission to discharge or death (if occurring during the same hospitalization).
Adverse in-hospital complications were also recorded. These were selected based on inclusion of the following categories of acute STEMI complications: ischemic, mechanical, arrhythmic, embolic, and inflammatory. The following complications occurred in our patient cohort: sustained ventricular arrhythmia, congestive heart failure (CHF) defined as congestion requiring intravenous diuretics, re-infarction, mechanical complications (free-wall rupture, ventricular septal defect, or mitral regurgitation), second- or third-degree atrioventricular block, atrial fibrillation, stroke, mechanical ventilation, major bleeding, pericarditis, cardiogenic shock, cardiac arrest, and in-hospital mortality. The primary outcome of this study was defined as a composite of sustained ventricular arrhythmia, CHF with congestion requiring intravenous diuretics, and/or in-hospital mortality. Ventricular arrythmia and CHF were included in the composite outcome because they are defined as the 2 most common causes of sudden cardiac death following acute STEMI.11,12
Statistical Analysis
Normally distributed continuous variables and categorical variables were compared using the paired t-test. A 2-sided P value <.05 was considered to be statistically significant. Mean admission rates for acute STEMI hospitalizations were determined by dividing the number of admissions by the number of days in each time period. The daily rate of COVID-19 cases per 100,000 individuals was obtained from the Centers for Disease Control and Prevention COVID-19 database. All data analyses were performed using Microsoft Excel.
Results
The study cohort consisted of 64 patients, of whom 30 (46.9%) were hospitalized between March 5 and May 5, 2020, and 34 (53.1%) who were admitted during the analogous time period in 2019. This reflected a 6% decrease in STEMI admissions at PAR in the COVID-19 cohort.
Acute STEMI Hospitalization Rates and COVID-19 Incidence
The mean daily acute STEMI admission rate was 0.50 during the study period compared to 0.57 during the control period. During the study period in 2020 in the state of Georgia, the daily rate of newly confirmed COVID-19 cases ranged from 0.194 per 100,000 on March 5 to 8.778 per 100,000 on May 5. Results of COVID-19 testing were available for 9 STEMI patients, and of these 0 tests were positive.
Baseline Characteristics
Baseline characteristics of the acute STEMI cohorts are presented in Table 1. Approximately 75% were male; median (interquartile range [IQR]) age was 60 (51-72) years. There were no significant differences in age and gender between the study periods. Three-quarters of patients had a history of hypertension, and 87.5% had a history of dyslipidemia. There was no significant difference in baseline comorbidity profiles between the 2 study periods; therefore, our sample populations shared similar characteristics.

Clinical Presentation
Significant differences were observed regarding the time intervals of STEMI patients in the COVID-19 period and the control period (Table 2). Median time from symptom onset to hospital admission (patient delay) was extended from 57.5 minutes (IQR, 40.3-106) in 2019 to 93 minutes (IQR, 48.8-132) in 2020; however, this difference was not statistically significant (P = .697). Median time from hospital admission to reperfusion (system delay) was prolonged from 45 minutes (IQR, 28-61) in 2019 to 78 minutes (IQR, 50-110) in 2020 (P < .001). Overall time from symptom onset to reperfusion (total ischemic time) increased from 99.5 minutes (IQR, 84.8-132) in 2019 to 149 minutes (IQR, 96.3-231.8) in 2020 (P = .032).

Regarding mode of transportation, 23.5% of patients in 2019 were walk-in admissions to the emergency department. During the COVID-19 period, walk-in admissions decreased to 6.7% (P = .065). There were no significant differences between emergency medical service, transfer, or in-patient admissions for STEMI cases between the 2 study periods.
Killip classification scores were calculated for all patients on admission; 90.6% of patients were classified as Killip Class 1. There was no significant difference between hemodynamic presentations during the COVID-19 period compared to the control period.
Angiographic Data
Overall, 53 (82.8%) patients admitted with acute STEMI underwent coronary angiography during their hospital stay. The proportion of patients who underwent primary reperfusion was greater in the control period than in the COVID-19 period (85.3% vs 80%; P = .582). Angiographic characteristics and findings were similar between the 2 study groups (Table 2).
In-Hospital Outcomes
In-hospital outcome data were available for all patients. As shown in Table 3, hospitalization during the COVID-19 period was independently associated with an increased risk for combined in-hospital outcome (odds ratio, 3.96; P = .046). The rate of in-hospital mortality was greater in the COVID-19 period (P = .013). We found no significant difference when comparing secondary outcomes from admissions during the COVID-19 period and the control period in 2019. For the 5 patients who died during the study period, the primary diagnosis at death was acute STEMI complicated by CHF (3 patients) or cardiogenic shock (2 patients).

Discussion
This single-center retrospective study at PAR looks at the impact of COVID-19 on hospitalizations for acute STEMI during the initial peak of the pandemic. The key findings of this study show a significant increase in ischemic time parameters (symptom onset to reperfusion, hospital admission to reperfusion), in-hospital mortality, and combined in-hospital outcomes.
There was a 49.5-minute increase in total ischemic time noted in this study (P = .032). Though there was a numerical increase in time of symptom onset to hospital admission by 23.5 minutes, this difference was not statistically significant (P = .697). However, this study observed a statistically significant 33-minute increase in ischemic time from hospital admission to reperfusion (P < .001). Multiple studies globally have found a similar increase in total ischemic times, including those conducted in China and Europe.13-15 Every level of potential delay must be considered, including pre-hospital, triage and emergency department, and/or reperfusion team. Pre-hospital sources of delays that have been suggested include “stay-at-home” orders and apprehension to seek medical care due to concern about contracting the virus or overwhelming the health care facilities. There was a clinically significant 4-fold decrease in the number of walk-in acute STEMI cases in the study period. In 2019, there were 8 walk-in cases compared to 2 cases in 2020 (P = .065). However, this change was not statistically significant. In-hospital/systemic sources of delays have been mentioned in other studies; they include increased time taken to rule out COVID-19 (nasopharyngeal swab/chest x-ray) and increased time due to the need for intensive gowning and gloving procedures by staff. It was difficult to objectively determine the sources of system delay by the reperfusion team due to a lack of quantitative data.
In the current study, we found a significant increase in in-hospital mortality during the COVID-19 period compared to a parallel time frame in 2019. This finding is contrary to a multicenter study from Spain that reported no difference in in-hospital outcomes or mortality rates among all acute coronary syndrome cases.16 The worsening outcomes and prognosis may simply be a result of increased ischemic time; however, the virus that causes COVID-19 itself may play a role as well. Studies have found that SARS-Cov-2 infection places patients at greater risk for cardiovascular conditions such as hypercoagulability, myocarditis, and arrhythmias.17 In our study, however, there were no acute STEMI patients who tested positive for COVID-19. Therefore, we cannot discuss the impact of increased thrombus burden in patients with COVID-19. Piedmont Healthcare published a STEMI treatment protocol in May 2020 that advised increased use of tissue plasminogen activator (tPA) in COVID-19-positive cases; during the study period, however, there were no occasions when tPA use was deemed appropriate based on clinical judgment.
Our findings align with previous studies that describe an increase in combined in-hospital adverse outcomes during the COVID-19 era. Previous studies detected a higher rate of complications in the COVID-19 cohort, but in the current study, the adverse in-hospital course is unrelated to underlying infection.18,19 This study reports a higher incidence of major in-hospital outcomes, including a 65% increase in the rate of combined in-hospital outcomes, which is similar to a multicenter study conducted in Israel.19 There was a 2.3-fold numerical increase in sustained ventricular arrhythmias and a 2.5-fold numerical increase in the incidence of cardiac arrest in the study period. This phenomenon was observed despite a similar rate of reperfusion procedures in both groups.
Acute STEMI is a highly fatal condition with an incidence of 8.5 in 10,000 annually in the United States. While studies across the world have shown a 25% to 40% reduction in the rate of hospitalized acute coronary syndrome cases during the COVID-19 pandemic, the decrease from 34 to 30 STEMI admissions at PAR is not statistically significant.20 Possible reasons for the reduction globally include increased out-of-hospital mortality and decreased incidence of acute STEMI across the general population as a result of improved access to telemedicine or decreased levels of life stressors.20
In summary, there was an increase in ischemic time to reperfusion, in-hospital mortality, and combined in-hospital outcomes for acute STEMI patients at PAR during the COVID period.
Limitations
This study has several limitations. This is a single-center study, so the sample size is small and may not be generalizable to a larger population. This is a retrospective observational study, so causation cannot be inferred. This study analyzed ischemic time parameters as average rates over time rather than in an interrupted time series. Post-reperfusion outcomes were limited to hospital stay. Post-hospital follow-up would provide a better picture of the effects of STEMI intervention. There is no account of patients who died out-of-hospital secondary to acute STEMI. COVID-19 testing was not introduced until midway in our study period. Therefore, we cannot rule out the possibility of the SARS-Cov-2 virus inciting acute STEMI and subsequently leading to worse outcomes and poor prognosis.
Conclusions
This study provides an analysis of the incidence, characteristics, and clinical outcomes of patients presenting with acute STEMI during the early period of the COVID-19 pandemic. In-hospital mortality and ischemic time to reperfusion increased while combined in-hospital outcomes worsened.
Acknowledgment: The authors thank Piedmont Athens Regional IRB for approving this project and allowing access to patient data.
Corresponding author: Syed H. Ali; Department of Medicine, Medical College of Georgia at the Augusta University-University of Georgia Medical Partnership, 30606, Athens, GA; [email protected]
Disclosures: None reported.
doi:10.12788/jcom.0085
From the Department of Medicine, Medical College of Georgia at the Augusta University-University of Georgia Medical Partnership, Athens, GA (Syed H. Ali, Syed Hyder, and Dr. Murrow), and the Department of Cardiology, Piedmont Heart Institute, Piedmont Athens Regional, Athens, GA (Dr. Murrow and Mrs. Davis).
Abstract
Objectives: The aim of this study was to describe the characteristics and in-hospital outcomes of patients with acute ST-segment elevation myocardial infarction (STEMI) during the early COVID-19 pandemic at Piedmont Athens Regional (PAR), a 330-bed tertiary referral center in Northeast Georgia.
Methods: A retrospective study was conducted at PAR to evaluate patients with acute STEMI admitted over an 8-week period during the initial COVID-19 outbreak. This study group was compared to patients admitted during the corresponding period in 2019. The primary endpoint of this study was defined as a composite of sustained ventricular arrhythmia, congestive heart failure (CHF) with pulmonary congestion, and/or in-hospital mortality.
Results: This study cohort was composed of 64 patients with acute STEMI; 30 patients (46.9%) were hospitalized during the COVID-19 pandemic. Patients with STEMI in both the COVID-19 and control groups had similar comorbidities, Killip classification score, and clinical presentations. The median (interquartile range) time from symptom onset to reperfusion (total ischemic time) increased from 99.5 minutes (84.8-132) in 2019 to 149 minutes (96.3-231.8; P = .032) in 2020. Hospitalization during the COVID-19 period was associated with an increased risk for combined in-hospital outcome (odds ratio, 3.96; P = .046).
Conclusion: Patients with STEMI admitted during the first wave of the COVID-19 outbreak experienced longer total ischemic time and increased risk for combined in-hospital outcomes compared to patients admitted during the corresponding period in 2019.
Keywords: myocardial infarction, acute coronary syndrome, hospitalization, outcomes.

The emergence of the SARS-Cov-2 virus in December 2019 caused a worldwide shift in resource allocation and the restructuring of health care systems within the span of a few months. With the rapid spread of infection, the World Health Organization officially declared a pandemic in March 2020. The pandemic led to the deferral and cancellation of in-person patient visits, routine diagnostic studies, and nonessential surgeries and procedures. This response occurred secondary to a joint effort to reduce transmission via stay-at-home mandates and appropriate social distancing.1
Alongside the reduction in elective procedures and health care visits, significant reductions in hospitalization rates due to decreases in acute ST-segment elevation myocardial infarction (STEMI) and catheterization laboratory utilization have been reported in many studies from around the world.2-7 Comprehensive data demonstrating the impact of the COVID-19 pandemic on acute STEMI patient characteristics, clinical presentation, and in-hospital outcomes are lacking. Although patients with previously diagnosed cardiovascular disease are more likely to encounter worse outcomes in the setting of COVID-19, there may also be an indirect impact of the pandemic on high-risk patients, including those without the infection.8 Several theories have been hypothesized to explain this phenomenon. One theory postulates that the fear of contracting the virus during hospitalization is great enough to prevent patients from seeking care.2 Another theory suggests that the increased utilization of telemedicine prevents exacerbation of chronic conditions and the need for hospitalization.9 Contrary to this trend, previous studies have shown an increased incidence of acute STEMI following stressful events such as natural disasters.10
The aim of this study was to describe trends pertaining to clinical characteristics and in-hospital outcomes of patients with acute STEMI during the early COVID-19 pandemic at Piedmont Athens Regional (PAR), a 330-bed tertiary referral center in Northeast Georgia.
Methods
A retrospective cohort study was conducted at PAR to evaluate patients with STEMI admitted to the cardiovascular intensive care unit over an 8-week period (March 5 to May 5, 2020) during the COVID-19 outbreak. COVID-19 was declared a national emergency on March 13, 2020, in the United States. The institutional review board at PAR approved the study; the need for individual consent was waived under the condition that participant data would undergo de-identification and be strictly safeguarded.
Data Collection
Because there are seasonal variations in cardiovascular admissions, patient data from a control period (March 9 to May 9, 2019) were obtained to compare with data from the 2020 period. The number of patients with the diagnosis of acute STEMI during the COVID-19 period was recorded. Demographic data, clinical characteristics, and primary angiographic findings were gathered for all patients. Time from symptom onset to hospital admission and time from hospital admission to reperfusion (defined as door-to-balloon time) were documented for each patient. Killip classification was used to assess patients’ clinical status on admission. Length of stay was determined as days from hospital admission to discharge or death (if occurring during the same hospitalization).
Adverse in-hospital complications were also recorded. These were selected based on inclusion of the following categories of acute STEMI complications: ischemic, mechanical, arrhythmic, embolic, and inflammatory. The following complications occurred in our patient cohort: sustained ventricular arrhythmia, congestive heart failure (CHF) defined as congestion requiring intravenous diuretics, re-infarction, mechanical complications (free-wall rupture, ventricular septal defect, or mitral regurgitation), second- or third-degree atrioventricular block, atrial fibrillation, stroke, mechanical ventilation, major bleeding, pericarditis, cardiogenic shock, cardiac arrest, and in-hospital mortality. The primary outcome of this study was defined as a composite of sustained ventricular arrhythmia, CHF with congestion requiring intravenous diuretics, and/or in-hospital mortality. Ventricular arrythmia and CHF were included in the composite outcome because they are defined as the 2 most common causes of sudden cardiac death following acute STEMI.11,12
Statistical Analysis
Normally distributed continuous variables and categorical variables were compared using the paired t-test. A 2-sided P value <.05 was considered to be statistically significant. Mean admission rates for acute STEMI hospitalizations were determined by dividing the number of admissions by the number of days in each time period. The daily rate of COVID-19 cases per 100,000 individuals was obtained from the Centers for Disease Control and Prevention COVID-19 database. All data analyses were performed using Microsoft Excel.
Results
The study cohort consisted of 64 patients, of whom 30 (46.9%) were hospitalized between March 5 and May 5, 2020, and 34 (53.1%) who were admitted during the analogous time period in 2019. This reflected a 6% decrease in STEMI admissions at PAR in the COVID-19 cohort.
Acute STEMI Hospitalization Rates and COVID-19 Incidence
The mean daily acute STEMI admission rate was 0.50 during the study period compared to 0.57 during the control period. During the study period in 2020 in the state of Georgia, the daily rate of newly confirmed COVID-19 cases ranged from 0.194 per 100,000 on March 5 to 8.778 per 100,000 on May 5. Results of COVID-19 testing were available for 9 STEMI patients, and of these 0 tests were positive.
Baseline Characteristics
Baseline characteristics of the acute STEMI cohorts are presented in Table 1. Approximately 75% were male; median (interquartile range [IQR]) age was 60 (51-72) years. There were no significant differences in age and gender between the study periods. Three-quarters of patients had a history of hypertension, and 87.5% had a history of dyslipidemia. There was no significant difference in baseline comorbidity profiles between the 2 study periods; therefore, our sample populations shared similar characteristics.

Clinical Presentation
Significant differences were observed regarding the time intervals of STEMI patients in the COVID-19 period and the control period (Table 2). Median time from symptom onset to hospital admission (patient delay) was extended from 57.5 minutes (IQR, 40.3-106) in 2019 to 93 minutes (IQR, 48.8-132) in 2020; however, this difference was not statistically significant (P = .697). Median time from hospital admission to reperfusion (system delay) was prolonged from 45 minutes (IQR, 28-61) in 2019 to 78 minutes (IQR, 50-110) in 2020 (P < .001). Overall time from symptom onset to reperfusion (total ischemic time) increased from 99.5 minutes (IQR, 84.8-132) in 2019 to 149 minutes (IQR, 96.3-231.8) in 2020 (P = .032).

Regarding mode of transportation, 23.5% of patients in 2019 were walk-in admissions to the emergency department. During the COVID-19 period, walk-in admissions decreased to 6.7% (P = .065). There were no significant differences between emergency medical service, transfer, or in-patient admissions for STEMI cases between the 2 study periods.
Killip classification scores were calculated for all patients on admission; 90.6% of patients were classified as Killip Class 1. There was no significant difference between hemodynamic presentations during the COVID-19 period compared to the control period.
Angiographic Data
Overall, 53 (82.8%) patients admitted with acute STEMI underwent coronary angiography during their hospital stay. The proportion of patients who underwent primary reperfusion was greater in the control period than in the COVID-19 period (85.3% vs 80%; P = .582). Angiographic characteristics and findings were similar between the 2 study groups (Table 2).
In-Hospital Outcomes
In-hospital outcome data were available for all patients. As shown in Table 3, hospitalization during the COVID-19 period was independently associated with an increased risk for combined in-hospital outcome (odds ratio, 3.96; P = .046). The rate of in-hospital mortality was greater in the COVID-19 period (P = .013). We found no significant difference when comparing secondary outcomes from admissions during the COVID-19 period and the control period in 2019. For the 5 patients who died during the study period, the primary diagnosis at death was acute STEMI complicated by CHF (3 patients) or cardiogenic shock (2 patients).

Discussion
This single-center retrospective study at PAR looks at the impact of COVID-19 on hospitalizations for acute STEMI during the initial peak of the pandemic. The key findings of this study show a significant increase in ischemic time parameters (symptom onset to reperfusion, hospital admission to reperfusion), in-hospital mortality, and combined in-hospital outcomes.
There was a 49.5-minute increase in total ischemic time noted in this study (P = .032). Though there was a numerical increase in time of symptom onset to hospital admission by 23.5 minutes, this difference was not statistically significant (P = .697). However, this study observed a statistically significant 33-minute increase in ischemic time from hospital admission to reperfusion (P < .001). Multiple studies globally have found a similar increase in total ischemic times, including those conducted in China and Europe.13-15 Every level of potential delay must be considered, including pre-hospital, triage and emergency department, and/or reperfusion team. Pre-hospital sources of delays that have been suggested include “stay-at-home” orders and apprehension to seek medical care due to concern about contracting the virus or overwhelming the health care facilities. There was a clinically significant 4-fold decrease in the number of walk-in acute STEMI cases in the study period. In 2019, there were 8 walk-in cases compared to 2 cases in 2020 (P = .065). However, this change was not statistically significant. In-hospital/systemic sources of delays have been mentioned in other studies; they include increased time taken to rule out COVID-19 (nasopharyngeal swab/chest x-ray) and increased time due to the need for intensive gowning and gloving procedures by staff. It was difficult to objectively determine the sources of system delay by the reperfusion team due to a lack of quantitative data.
In the current study, we found a significant increase in in-hospital mortality during the COVID-19 period compared to a parallel time frame in 2019. This finding is contrary to a multicenter study from Spain that reported no difference in in-hospital outcomes or mortality rates among all acute coronary syndrome cases.16 The worsening outcomes and prognosis may simply be a result of increased ischemic time; however, the virus that causes COVID-19 itself may play a role as well. Studies have found that SARS-Cov-2 infection places patients at greater risk for cardiovascular conditions such as hypercoagulability, myocarditis, and arrhythmias.17 In our study, however, there were no acute STEMI patients who tested positive for COVID-19. Therefore, we cannot discuss the impact of increased thrombus burden in patients with COVID-19. Piedmont Healthcare published a STEMI treatment protocol in May 2020 that advised increased use of tissue plasminogen activator (tPA) in COVID-19-positive cases; during the study period, however, there were no occasions when tPA use was deemed appropriate based on clinical judgment.
Our findings align with previous studies that describe an increase in combined in-hospital adverse outcomes during the COVID-19 era. Previous studies detected a higher rate of complications in the COVID-19 cohort, but in the current study, the adverse in-hospital course is unrelated to underlying infection.18,19 This study reports a higher incidence of major in-hospital outcomes, including a 65% increase in the rate of combined in-hospital outcomes, which is similar to a multicenter study conducted in Israel.19 There was a 2.3-fold numerical increase in sustained ventricular arrhythmias and a 2.5-fold numerical increase in the incidence of cardiac arrest in the study period. This phenomenon was observed despite a similar rate of reperfusion procedures in both groups.
Acute STEMI is a highly fatal condition with an incidence of 8.5 in 10,000 annually in the United States. While studies across the world have shown a 25% to 40% reduction in the rate of hospitalized acute coronary syndrome cases during the COVID-19 pandemic, the decrease from 34 to 30 STEMI admissions at PAR is not statistically significant.20 Possible reasons for the reduction globally include increased out-of-hospital mortality and decreased incidence of acute STEMI across the general population as a result of improved access to telemedicine or decreased levels of life stressors.20
In summary, there was an increase in ischemic time to reperfusion, in-hospital mortality, and combined in-hospital outcomes for acute STEMI patients at PAR during the COVID period.
Limitations
This study has several limitations. This is a single-center study, so the sample size is small and may not be generalizable to a larger population. This is a retrospective observational study, so causation cannot be inferred. This study analyzed ischemic time parameters as average rates over time rather than in an interrupted time series. Post-reperfusion outcomes were limited to hospital stay. Post-hospital follow-up would provide a better picture of the effects of STEMI intervention. There is no account of patients who died out-of-hospital secondary to acute STEMI. COVID-19 testing was not introduced until midway in our study period. Therefore, we cannot rule out the possibility of the SARS-Cov-2 virus inciting acute STEMI and subsequently leading to worse outcomes and poor prognosis.
Conclusions
This study provides an analysis of the incidence, characteristics, and clinical outcomes of patients presenting with acute STEMI during the early period of the COVID-19 pandemic. In-hospital mortality and ischemic time to reperfusion increased while combined in-hospital outcomes worsened.
Acknowledgment: The authors thank Piedmont Athens Regional IRB for approving this project and allowing access to patient data.
Corresponding author: Syed H. Ali; Department of Medicine, Medical College of Georgia at the Augusta University-University of Georgia Medical Partnership, 30606, Athens, GA; [email protected]
Disclosures: None reported.
doi:10.12788/jcom.0085
1. Bhatt AS, Moscone A, McElrath EE, et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280-288. doi:10.1016/j.jacc.2020.05.038
2. Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJR. Decline of acute coronary syndrome admissions in Austria since the outbreak of Covid-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41:1852-1853. doi:10.1093/eurheartj/ehaa314
3. De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the Covid-19 era. Eur Heart J. 2020;41(22):2083-2088.
4. Wilson SJ, Connolly MJ, Elghamry Z, et al. Effect of the COVID-19 pandemic on ST-segment-elevation myocardial infarction presentations and in-hospital outcomes. Circ Cardiovasc Interv. 2020; 13(7):e009438. doi:10.1161/CIRCINTERVENTIONS.120.009438
5. Mafham MM, Spata E, Goldacre R, et al. Covid-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396 (10248):381-389. doi:10.1016/S0140-6736(20)31356-8
6. Bhatt AS, Moscone A, McElrath EE, et al. Fewer Hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280-288. doi:10.1016/j.jacc.2020.05.038
7. Tam CF, Cheung KS, Lam S, et al. Impact of Coronavirus disease 2019 (Covid-19) outbreak on ST-segment elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13(4):e006631. doi:10.1161/CIRCOUTCOMES.120.006631
8. Clerkin KJ, Fried JA, Raikhelkar J, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141:1648-1655. doi:10.1161/CIRCULATIONAHA.120.046941
9. Ebinger JE, Shah PK. Declining admissions for acute cardiovascular illness: The Covid-19 paradox. J Am Coll Cardiol. 2020;76(3):289-291. doi:10.1016/j.jacc.2020.05.039
10 Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med. 1996;334(7):413-419. doi:10.1056/NEJM199602153340701
11. Hiramori K. Major causes of death from acute myocardial infarction in a coronary care unit. Jpn Circ J. 1987;51(9):1041-1047. doi:10.1253/jcj.51.1041
12. Bui AH, Waks JW. Risk stratification of sudden cardiac death after acute myocardial infarction. J Innov Card Rhythm Manag. 2018;9(2):3035-3049. doi:10.19102/icrm.2018.090201
13. Xiang D, Xiang X, Zhang W, et al. Management and outcomes of patients with STEMI during the COVID-19 pandemic in China. J Am Coll Cardiol. 2020;76(11):1318-1324. doi:10.1016/j.jacc.2020.06.039
14. Hakim R, Motreff P, Rangé G. COVID-19 and STEMI. [Article in French]. Ann Cardiol Angeiol (Paris). 2020;69(6):355-359. doi:10.1016/j.ancard.2020.09.034
15. Soylu K, Coksevim M, Yanık A, Bugra Cerik I, Aksan G. Effect of Covid-19 pandemic process on STEMI patients timeline. Int J Clin Pract. 2021;75(5):e14005. doi:10.1111/ijcp.14005
16. Salinas P, Travieso A, Vergara-Uzcategui C, et al. Clinical profile and 30-day mortality of invasively managed patients with suspected acute coronary syndrome during the COVID-19 outbreak. Int Heart J. 2021;62(2):274-281. doi:10.1536/ihj.20-574
17. Hu Y, Sun J, Dai Z, et al. Prevalence and severity of corona virus disease 2019 (Covid-19): a systematic review and meta-analysis. J Clin Virol. 2020;127:104371. doi:10.1016/j.jcv.2020.104371
18. Rodriguez-Leor O, Cid Alvarez AB, Perez de Prado A, et al. In-hospital outcomes of COVID-19 ST-elevation myocardial infarction patients. EuroIntervention. 2021;16(17):1426-1433. doi:10.4244/EIJ-D-20-00935
19. Fardman A, Zahger D, Orvin K, et al. Acute myocardial infarction in the Covid-19 era: incidence, clinical characteristics and in-hospital outcomes—A multicenter registry. PLoS ONE. 2021;16(6): e0253524. doi:10.1371/journal.pone.0253524
20. Pessoa-Amorim G, Camm CF, Gajendragadkar P, et al. Admission of patients with STEMI since the outbreak of the COVID-19 pandemic: a survey by the European Society of Cardiology. Eur Heart J Qual Care Clin Outcomes. 2020;6(3):210-216. doi:10.1093/ehjqcco/qcaa046
1. Bhatt AS, Moscone A, McElrath EE, et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280-288. doi:10.1016/j.jacc.2020.05.038
2. Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJR. Decline of acute coronary syndrome admissions in Austria since the outbreak of Covid-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41:1852-1853. doi:10.1093/eurheartj/ehaa314
3. De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the Covid-19 era. Eur Heart J. 2020;41(22):2083-2088.
4. Wilson SJ, Connolly MJ, Elghamry Z, et al. Effect of the COVID-19 pandemic on ST-segment-elevation myocardial infarction presentations and in-hospital outcomes. Circ Cardiovasc Interv. 2020; 13(7):e009438. doi:10.1161/CIRCINTERVENTIONS.120.009438
5. Mafham MM, Spata E, Goldacre R, et al. Covid-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396 (10248):381-389. doi:10.1016/S0140-6736(20)31356-8
6. Bhatt AS, Moscone A, McElrath EE, et al. Fewer Hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280-288. doi:10.1016/j.jacc.2020.05.038
7. Tam CF, Cheung KS, Lam S, et al. Impact of Coronavirus disease 2019 (Covid-19) outbreak on ST-segment elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13(4):e006631. doi:10.1161/CIRCOUTCOMES.120.006631
8. Clerkin KJ, Fried JA, Raikhelkar J, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141:1648-1655. doi:10.1161/CIRCULATIONAHA.120.046941
9. Ebinger JE, Shah PK. Declining admissions for acute cardiovascular illness: The Covid-19 paradox. J Am Coll Cardiol. 2020;76(3):289-291. doi:10.1016/j.jacc.2020.05.039
10 Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med. 1996;334(7):413-419. doi:10.1056/NEJM199602153340701
11. Hiramori K. Major causes of death from acute myocardial infarction in a coronary care unit. Jpn Circ J. 1987;51(9):1041-1047. doi:10.1253/jcj.51.1041
12. Bui AH, Waks JW. Risk stratification of sudden cardiac death after acute myocardial infarction. J Innov Card Rhythm Manag. 2018;9(2):3035-3049. doi:10.19102/icrm.2018.090201
13. Xiang D, Xiang X, Zhang W, et al. Management and outcomes of patients with STEMI during the COVID-19 pandemic in China. J Am Coll Cardiol. 2020;76(11):1318-1324. doi:10.1016/j.jacc.2020.06.039
14. Hakim R, Motreff P, Rangé G. COVID-19 and STEMI. [Article in French]. Ann Cardiol Angeiol (Paris). 2020;69(6):355-359. doi:10.1016/j.ancard.2020.09.034
15. Soylu K, Coksevim M, Yanık A, Bugra Cerik I, Aksan G. Effect of Covid-19 pandemic process on STEMI patients timeline. Int J Clin Pract. 2021;75(5):e14005. doi:10.1111/ijcp.14005
16. Salinas P, Travieso A, Vergara-Uzcategui C, et al. Clinical profile and 30-day mortality of invasively managed patients with suspected acute coronary syndrome during the COVID-19 outbreak. Int Heart J. 2021;62(2):274-281. doi:10.1536/ihj.20-574
17. Hu Y, Sun J, Dai Z, et al. Prevalence and severity of corona virus disease 2019 (Covid-19): a systematic review and meta-analysis. J Clin Virol. 2020;127:104371. doi:10.1016/j.jcv.2020.104371
18. Rodriguez-Leor O, Cid Alvarez AB, Perez de Prado A, et al. In-hospital outcomes of COVID-19 ST-elevation myocardial infarction patients. EuroIntervention. 2021;16(17):1426-1433. doi:10.4244/EIJ-D-20-00935
19. Fardman A, Zahger D, Orvin K, et al. Acute myocardial infarction in the Covid-19 era: incidence, clinical characteristics and in-hospital outcomes—A multicenter registry. PLoS ONE. 2021;16(6): e0253524. doi:10.1371/journal.pone.0253524
20. Pessoa-Amorim G, Camm CF, Gajendragadkar P, et al. Admission of patients with STEMI since the outbreak of the COVID-19 pandemic: a survey by the European Society of Cardiology. Eur Heart J Qual Care Clin Outcomes. 2020;6(3):210-216. doi:10.1093/ehjqcco/qcaa046
Oxygen Therapies and Clinical Outcomes for Patients Hospitalized With COVID-19: First Surge vs Second Surge
From Lahey Hospital and Medical Center, Burlington, MA (Drs. Liesching and Lei), and Tufts University School of Medicine, Boston, MA (Dr. Liesching)
ABSTRACT
Objective: To compare the utilization of oxygen therapies and clinical outcomes of patients admitted for COVID-19 during the second surge of the pandemic to that of patients admitted during the first surge.
Design: Observational study using a registry database.
Setting: Three hospitals (791 inpatient beds and 76 intensive care unit [ICU] beds) within the Beth Israel Lahey Health system in Massachusetts.
Participants: We included 3183 patients with COVID-19 admitted to hospitals.
Measurements: Baseline data included demographics and comorbidities. Treatments included low-flow supplemental oxygen (2-6 L/min), high-flow oxygen via nasal cannula, and invasive mechanical ventilation. Outcomes included ICU admission, length of stay, ventilator days, and mortality.
Results: A total of 3183 patients were included: 1586 during the first surge and 1597 during the second surge. Compared to the first surge, patients admitted during the second surge had a similar rate of receiving low-flow supplemental oxygen (65.8% vs 64.1%, P = .3), a higher rate of receiving high-flow nasal cannula (15.4% vs 10.8%, P = .0001), and a lower ventilation rate (5.6% vs 9.7%, P < .0001). The outcomes during the second surge were better than those during the first surge: lower ICU admission rate (8.1% vs 12.7%, P < .0001), shorter length of hospital stay (5 vs 6 days, P < .0001), fewer ventilator days (10 vs 16, P = .01), and lower mortality (8.3% vs 19.2%, P < .0001). Among ventilated patients, those who received high-flow nasal cannula had lower mortality.
Conclusion: Compared to the first surge of the COVID-19 pandemic, patients admitted during the second surge had similar likelihood of receiving low-flow supplemental oxygen, were more likely to receive high-flow nasal cannula, were less likely to be ventilated, and had better outcomes.
Keywords: supplemental oxygen, high-flow nasal cannula, ventilator.
The respiratory system receives the major impact of SARS-CoV-2 virus, and hypoxemia has been the predominant diagnosis for patients hospitalized with COVID-19.1,2 During the initial stage of the pandemic, oxygen therapies and mechanical ventilation were the only choices for these patients.3-6 Standard-of-care treatment for patients with COVID-19 during the initial surge included oxygen therapies and mechanical ventilation for hypoxemia and medications for comorbidities and COVID-19–associated sequelae, such as multi-organ dysfunction and failure. A report from New York during the first surge (May 2020) showed that among 5700 hospitalized patients with COVID-19, 27.8% received supplemental oxygen and 12.2% received invasive mechanical ventilation.7 High-flow nasal cannula (HFNC) oxygen delivery has been utilized widely throughout the pandemic due to its superiority over other noninvasive respiratory support techniques.8-12 Mechanical ventilation is always necessary for critically ill patients with acute respiratory distress syndrome. However, ventilator scarcity has become a bottleneck in caring for severely ill patients with COVID-19 during the pandemic.13
The clinical outcomes of hospitalized COVID-19 patients include a high intubation rate, long length of hospital and intensive care unit (ICU) stay, and high mortality.14,15 As the pandemic evolved, new medications, including remdesivir, hydroxychloroquine, lopinavir, or interferon β-1a, were used in addition to the standard of care, but these did not result in significantly different mortality from standard of care.16 Steroids are becoming foundational to the treatment of severe COVID-19 pneumonia, but evidence from high-quality randomized controlled clinical trials is lacking.17
During the first surge from March to May 2020, Massachusetts had the third highest number of COVID-19 cases among states in the United States.18 In early 2021, COVID-19 cases were climbing close to the peak of the second surge in Massachusetts. In this study, we compared utilization of low-flow supplemental oxygen, HFNC, and mechanical ventilation and clinical outcomes of patients admitted to 3 hospitals in Massachusetts during the second surge of the pandemic to that of patients admitted during the first surge.
Methods
Setting
Beth Israel Lahey Health is a system of academic and teaching hospitals with primary care and specialty care providers. We included 3 centers within the Beth Israel Lahey Health system in Massachusetts: Lahey Hospital and Medical Center, with 335 inpatient hospital beds and 52 critical care beds; Beverly Hospital, with 227 beds and 14 critical care beds; and Winchester Hospital, with 229 beds and 10 ICU beds.
Participants
We included patients admitted to the 3 hospitals with COVID-19 as a primary or secondary diagnosis during the first surge of the pandemic (March 1, 2020 to June 15, 2020) and the second surge (November 15, 2020 to January 27, 2021). The timeframe of the first surge was defined as the window between the start date and the end date of data collection. During the time window of the first surge, 1586 patients were included. The start time of the second surge was defined as the date when the data collection was restarted; the end date was set when the number of patients (1597) accumulated was close to the number of patients in the first surge (1586), so that the two groups had similar sample size.
Study Design
A data registry of COVID-19 patients was created by our institution, and the data were prospectively collected starting in March 2020. We retrospectively extracted data on the following from the registry database for this observational study: demographics and baseline comorbidities; the use of low-flow supplemental oxygen, HFNC, and invasive mechanical ventilator; and ICU admission, length of hospital stay, length of ICU stay, and hospital discharge disposition. Start and end times for each oxygen therapy were not entered in the registry. Data about other oxygen therapies, such as noninvasive positive-pressure ventilation, were not collected in the registry database, and therefore were not included in the analysis.
Statistical Analysis
Continuous variables (eg, age) were tested for data distribution normality using the Shapiro-Wilk test. Normally distributed data were tested using unpaired t-tests and displayed as mean (SD). The skewed data were tested using the Wilcoxon rank sum test and displayed as median (interquartile range [IQR]). The categorical variables were compared using chi-square test. Comparisons with P ≤ .05 were considered significantly different. Statistical analysis for this study was generated using Statistical Analysis Software (SAS), version 9.4 for Windows (SAS Institute Inc.).
Results
Baseline Characteristics
We included 3183 patients: 1586 admitted during the first surge and 1597 admitted during the second surge. Baseline characteristics of patients with COVID-19 admitted during the first and second surges are shown in Table 1. Patients admitted during the second surge were older (73 years vs 71 years, P = .01) and had higher rates of hypertension (64.8% vs 59.6%, P = .003) and asthma (12.9% vs 10.7%, P = .049) but a lower rate of interstitial lung disease (3.3% vs 7.7%, P < .001). Sequential organ failure assessment scores at admission and the rates of other comorbidities were not significantly different between the 2 surges.

Oxygen Therapies
The number of patients who were hospitalized and received low-flow supplemental oxygen, and/or HFNC, and/or ventilator in the first surge and the second surge is shown in the Figure. Of all patients included, 2067 (64.9%) received low-flow supplemental oxygen; of these, 374 (18.1%) subsequently received HFNC, and 85 (22.7%) of these subsequently received mechanical ventilation. Of all 3183 patients, 417 (13.1%) received HFNC; 43 of these patients received HFNC without receiving low-flow supplemental oxygen, and 98 (23.5%) subsequently received mechanical ventilation. Out of all 3183 patients, 244 (7.7%) received mechanical ventilation; 98 (40.2%) of these received HFNC while the remaining 146 (59.8%) did not. At the beginning of the first surge, the ratio of patients who received invasive mechanical ventilation to patients who received HFNC was close to 1:1 (10/10); the ratio decreased to 6:10 in May and June 2020. At the beginning of the second surge, the ratio was 8:10 and then decreased to 3:10 in December 2020 and January 2021.

As shown in Table 2, the proportion of patients who received low-flow supplemental oxygen during the second surge was similar to that during the first surge (65.8% vs 64.1%, P = .3). Patients admitted during the second surge were more likely to receive HFNC than patients admitted during the first surge (15.4% vs 10.8%, P = .0001). Patients admitted during the second surge were less likely to be ventilated than the patients admitted during the first surge (5.6% vs 9.7%, P < .0001).

Clinical Outcomes
As shown in Table 3, second surge outcomes were much better than first surge outcomes: the ICU admission rate was lower (8.1% vs 12.7%, P < .0001); patients were more likely to be discharged to home (60.2% vs 47.4%, P < .0001), had a shorter length of hospital stay (5 vs 6 days, P < .0001), and had fewer ventilator days (10 vs 16, P = .01); and mortality was lower (8.3% vs 19.2%, P < .0001). There was a trend that length of ICU stay was shorter during the second surge than during the first surge (7 days vs 9 days, P = .09).

As noted (Figure), the ratio of patients who received invasive mechanical ventilation to patients who received HFNC was decreasing during both the first surge and the second surge. To further analyze the relation between ventilator and HFNC, we performed a subgroup analysis for 244 ventilated patients during both surges to compare outcomes between patients who received HFNC and those who did not receive HFNC (Table 4). Ninety-eight (40%) patients received HFNC. Ventilated patients who received HFNC had lower mortality than those patients who did not receive HFNC (31.6% vs 48%, P = .01), but had a longer length of hospital stay (29 days vs 14 days, P < .0001), longer length of ICU stay (17 days vs 9 days, P < .0001), and a higher number of ventilator days (16 vs 11, P = .001).

Discussion
Our study compared the baseline patient characteristics; utilization of low-flow supplemental oxygen therapy, HFNC, and mechanical ventilation; and clinical outcomes between the first surge (n = 1586) and the second surge (n = 1597) of the COVID-19 pandemic. During both surges, about two-thirds of admitted patients received low-flow supplemental oxygen. A higher proportion of the admitted patients received HFNC during the second surge than during the first surge, while the intubation rate was lower during the second surge than during the first surge.
Reported low-flow supplemental oxygen use ranged from 28% to 63% depending on the cohort characteristics and location during the first surge.6,7,19 A report from New York during the first surge (March 1 to April 4, 2020) showed that among 5700 hospitalized patients with COVID-19, 27.8% received low-flow supplemental oxygen.7 HFNC is recommended in guidelines on management of patients with acute respiratory failure due to COVID-19.20 In our study, HFNC was utilized in a higher proportion of patients admitted for COVID-19 during the second surge (15.5% vs 10.8%, P = .0001). During the early pandemic period in Wuhan, China, 11% to 21% of admitted COVID-19 patients received HFNC.21,22 Utilization of HFNC in New York during the first surge (March to May 2020) varied from 5% to 14.3% of patients admitted with COVID-19.23,24 Our subgroup analysis of the ventilated patients showed that patients who received HFNC had lower mortality than those who did not (31.6% vs 48.0%, P = .011). Comparably, a report from Paris, France, showed that among patients admitted to ICUs for acute hypoxemic respiratory failure, those who received HFNC had lower mortality at day 60 than those who did not (21% vs 31%, P = .052).25 Our recent analysis showed that patients treated with HFNC prior to mechanical ventilation had lower mortality than those treated with only conventional oxygen (30% vs 52%, P = .05).26 In this subgroup analysis, we could not determine if HFNC treatment was administered before or after ventilation because HFNC was entered as dichotomous data (“Yes” or “No”) in the registry database. We merely showed the beneficial effect of HFNC on reducing mortality for ventilated COVID-19 patients, but did not mean to focus on how and when to apply HFNC.
We observed that the patients admitted during the second surge were less likely to be ventilated than the patients admitted during the first surge (5.6% vs 9.7%, P < .0001). During the first surge in New York, among 5700 patients admitted with COVID-19, 12.2% received invasive mechanical ventilation.7 In another report, also from New York during the first surge, 26.1% of 2015 hospitalized COVID-19 patients received mechanical ventilation.27 In our study, the ventilation rate of 9.7% during the first surge was lower.
Outcomes during the second surge were better than during the first surge, including ICU admission rate, hospital and ICU length of stay, ventilator days, and mortality. The mortality was 19.2% during the first surge vs 8.3% during the second surge (P < .0001). The mortality of 19.2% was lower than the 30.6% mortality reported for 2015 hospitalized COVID-19 patients in New York during the first surge.27 A retrospective study showed that early administration of remdesivir was associated with reduced ICU admission, ventilation use, and mortality.28 The RECOVERY clinical trial showed that dexamethasone improved mortality for COVID-19 patients who received respiratory support, but not for patients who did not receive any respiratory support.29 Perhaps some, if not all, of the improvement in ICU admission and mortality during the second surge was attributed to the new medications, such as antivirals and steroids.
The length of hospital stay for patients with moderate to severe COVID-19 varied from 4 to 53 days at different locations of the world, as shown in a meta-analysis by Rees and colleagues.30 Our results showing a length of stay of 6 days during the first surge and 5 days during the second surge fell into the shorter end of this range. In a retrospective analysis of 1643 adults with severe COVID-19 admitted to hospitals in New York City between March 9, 2020 and April 23, 2020, median hospital length of stay was 7 (IQR, 3-14) days.31 For the ventilated patients in our study, the length of stay of 14 days (did not receive HFNC) and 29 days (received HFNC) was much longer. This longer length of stay might be attributed to the patients in our study being older and having more severe comorbidities.
The main purpose of this study was to compare the oxygen therapies and outcomes between 2 surges. It is difficult to associate the clinical outcomes with the oxygen therapies because new therapies and medications were available after the first surge. It was not possible to adjust the outcomes with confounders (other therapies and medications) because the registry data did not include the new therapies and medications.
A strength of this study was that we included a large, balanced number of patients in the first surge and the second surge. We did not plan the sample size in both groups as we could not predict the number of admissions. We set the end date of data collection for analysis as the time when the number of patients admitted during the second surge was similar to the number of patients admitted during the first surge. A limitation was that the registry database was created by the institution and was not designed solely for this study. The data for oxygen therapies were limited to low-flow supplemental oxygen, HFNC, and invasive mechanical ventilation; data for noninvasive ventilation were not included.
Conclusion
At our centers, during the second surge of COVID-19 pandemic, patients hospitalized with COVID-19 infection were more likely to receive HFNC but less likely to be ventilated. Compared to the first surge, the hospitalized patients with COVID-19 infection had a lower ICU admission rate, shorter length of hospital stay, fewer ventilator days, and lower mortality. For ventilated patients, those who received HFNC had lower mortality than those who did not.
Corresponding author: Timothy N. Liesching, MD, 41 Mall Road, Burlington, MA 01805; [email protected]
Disclosures: None reported.
doi:10.12788/jcom.0086
1. Xie J, Covassin N, Fan Z, et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020;95(6):1138-1147. doi:10.1016/j.mayocp.2020.04.006
2. Asleh R, Asher E, Yagel O, et al. Predictors of hypoxemia and related adverse outcomes in patients hospitalized with COVID-19: a double-center retrospective study. J Clin Med. 2021;10(16):3581. doi:10.3390/jcm10163581
3. Choi KJ, Hong HL, Kim EJ. Association between oxygen saturation/fraction of inhaled oxygen and mortality in patients with COVID-19 associated pneumonia requiring oxygen therapy. Tuberc Respir Dis (Seoul). 2021;84(2):125-133. doi:10.4046/trd.2020.0126
4. Dixit SB. Role of noninvasive oxygen therapy strategies in COVID-19 patients: Where are we going? Indian J Crit Care Med. 2020;24(10):897-898. doi:10.5005/jp-journals-10071-23625
5. Gonzalez-Castro A, Fajardo Campoverde A, Medina A, et al. Non-invasive mechanical ventilation and high-flow oxygen therapy in the COVID-19 pandemic: the value of a draw. Med Intensiva (Engl Ed). 2021;45(5):320-321. doi:10.1016/j.medine.2021.04.001
6. Pan W, Li J, Ou Y, et al. Clinical outcome of standardized oxygen therapy nursing strategy in COVID-19. Ann Palliat Med. 2020;9(4):2171-2177. doi:10.21037/apm-20-1272
7. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
8. He G, Han Y, Fang Q, et al. Clinical experience of high-flow nasal cannula oxygen therapy in severe COVID-19 patients. Article in Chinese. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(2):232-239. doi:10.3785/j.issn.1008-9292.2020.03.13
9. Lalla U, Allwood BW, Louw EH, et al. The utility of high-flow nasal cannula oxygen therapy in the management of respiratory failure secondary to COVID-19 pneumonia. S Afr Med J. 2020;110(6):12941.
10. Zhang TT, Dai B, Wang W. Should the high-flow nasal oxygen therapy be used or avoided in COVID-19? J Transl Int Med. 2020;8(2):57-58. doi:10.2478/jtim-2020-0018
11. Agarwal A, Basmaji J, Muttalib F, et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can J Anaesth. 2020;67(9):1217-1248. doi:10.1007/s12630-020-01740-2
12. Geng S, Mei Q, Zhu C, et al. High flow nasal cannula is a good treatment option for COVID-19. Heart Lung. 2020;49(5):444-445. doi:10.1016/j.hrtlng.2020.03.018
13. Feinstein MM, Niforatos JD, Hyun I, et al. Considerations for ventilator triage during the COVID-19 pandemic. Lancet Respir Med. 2020;8(6):e53. doi:10.1016/S2213-2600(20)30192-2
14. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi:10.1001/jama.2020.2648
15. Rojas-Marte G, Hashmi AT, Khalid M, et al. Outcomes in patients with COVID-19 disease and high oxygen requirements. J Clin Med Res. 2021;13(1):26-37. doi:10.14740/jocmr4405
16. Zhang R, Mylonakis E. In inpatients with COVID-19, none of remdesivir, hydroxychloroquine, lopinavir, or interferon β-1a differed from standard care for in-hospital mortality. Ann Intern Med. 2021;174(2):JC17. doi:10.7326/ACPJ202102160-017
17. Rello J, Waterer GW, Bourdiol A, Roquilly A. COVID-19, steroids and other immunomodulators: The jigsaw is not complete. Anaesth Crit Care Pain Med. 2020;39(6):699-701. doi:10.1016/j.accpm.2020.10.011
18. Dargin J, Stempek S, Lei Y, Gray Jr. A, Liesching T. The effect of a tiered provider staffing model on patient outcomes during the coronavirus disease 2019 pandemic: A single-center observational study. Int J Crit Illn Inj Sci. 2021;11(3). doi:10.4103/ijciis.ijciis_37_21
19. Ni YN, Wang T, Liang BM, Liang ZA. The independent factors associated with oxygen therapy in COVID-19 patients under 65 years old. PLoS One. 2021;16(1):e0245690. doi:10.1371/journal.pone.0245690
20. Alhazzani W, Moller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. doi:10.1097/CCM.0000000000004363
21. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. doi:10.1001/jama.2020.1585
22. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:10.1016/S0140-6736(20)30566-3
23. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi:10.1136/bmj.m1996
24. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
25. Demoule A, Vieillard Baron A, Darmon M, et al. High-flow nasal cannula in critically ill patients with severe COVID-19. Am J Respir Crit Care Med. 2020;202(7):1039-1042. doi:10.1164/rccm.202005-2007LE
26. Hansen CK, Stempek S, Liesching T, Lei Y, Dargin J. Characteristics and outcomes of patients receiving high flow nasal cannula therapy prior to mechanical ventilation in COVID-19 respiratory failure: a prospective observational study. Int J Crit Illn Inj Sci. 2021;11(2):56-60. doi:10.4103/IJCIIS.IJCIIS_181_20
27. van Gerwen M, Alsen M, Little C, et al. Risk factors and outcomes of COVID-19 in New York City; a retrospective cohort study. J Med Virol. 2021;93(2):907-915. doi:10.1002/jmv.26337
28. Hussain Alsayed HA, Saheb Sharif-Askari F, Saheb Sharif-Askari N, Hussain AAS, Hamid Q, Halwani R. Early administration of remdesivir to COVID-19 patients associates with higher recovery rate and lower need for ICU admission: A retrospective cohort study. PLoS One. 2021;16(10):e0258643. doi:10.1371/journal.pone.0258643
29. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704. doi:10.1056/NEJMoa2021436
30. Rees EM, Nightingale ES, Jafari Y, et al. COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Med. 2020;18(1):270. doi:10.1186/s12916-020-01726-3
31. Anderson M, Bach P, Baldwin MR. Hospital length of stay for severe COVID-19: implications for Remdesivir’s value. medRxiv. 2020;2020.08.10.20171637. doi:10.1101/2020.08.10.20171637
From Lahey Hospital and Medical Center, Burlington, MA (Drs. Liesching and Lei), and Tufts University School of Medicine, Boston, MA (Dr. Liesching)
ABSTRACT
Objective: To compare the utilization of oxygen therapies and clinical outcomes of patients admitted for COVID-19 during the second surge of the pandemic to that of patients admitted during the first surge.
Design: Observational study using a registry database.
Setting: Three hospitals (791 inpatient beds and 76 intensive care unit [ICU] beds) within the Beth Israel Lahey Health system in Massachusetts.
Participants: We included 3183 patients with COVID-19 admitted to hospitals.
Measurements: Baseline data included demographics and comorbidities. Treatments included low-flow supplemental oxygen (2-6 L/min), high-flow oxygen via nasal cannula, and invasive mechanical ventilation. Outcomes included ICU admission, length of stay, ventilator days, and mortality.
Results: A total of 3183 patients were included: 1586 during the first surge and 1597 during the second surge. Compared to the first surge, patients admitted during the second surge had a similar rate of receiving low-flow supplemental oxygen (65.8% vs 64.1%, P = .3), a higher rate of receiving high-flow nasal cannula (15.4% vs 10.8%, P = .0001), and a lower ventilation rate (5.6% vs 9.7%, P < .0001). The outcomes during the second surge were better than those during the first surge: lower ICU admission rate (8.1% vs 12.7%, P < .0001), shorter length of hospital stay (5 vs 6 days, P < .0001), fewer ventilator days (10 vs 16, P = .01), and lower mortality (8.3% vs 19.2%, P < .0001). Among ventilated patients, those who received high-flow nasal cannula had lower mortality.
Conclusion: Compared to the first surge of the COVID-19 pandemic, patients admitted during the second surge had similar likelihood of receiving low-flow supplemental oxygen, were more likely to receive high-flow nasal cannula, were less likely to be ventilated, and had better outcomes.
Keywords: supplemental oxygen, high-flow nasal cannula, ventilator.
The respiratory system receives the major impact of SARS-CoV-2 virus, and hypoxemia has been the predominant diagnosis for patients hospitalized with COVID-19.1,2 During the initial stage of the pandemic, oxygen therapies and mechanical ventilation were the only choices for these patients.3-6 Standard-of-care treatment for patients with COVID-19 during the initial surge included oxygen therapies and mechanical ventilation for hypoxemia and medications for comorbidities and COVID-19–associated sequelae, such as multi-organ dysfunction and failure. A report from New York during the first surge (May 2020) showed that among 5700 hospitalized patients with COVID-19, 27.8% received supplemental oxygen and 12.2% received invasive mechanical ventilation.7 High-flow nasal cannula (HFNC) oxygen delivery has been utilized widely throughout the pandemic due to its superiority over other noninvasive respiratory support techniques.8-12 Mechanical ventilation is always necessary for critically ill patients with acute respiratory distress syndrome. However, ventilator scarcity has become a bottleneck in caring for severely ill patients with COVID-19 during the pandemic.13
The clinical outcomes of hospitalized COVID-19 patients include a high intubation rate, long length of hospital and intensive care unit (ICU) stay, and high mortality.14,15 As the pandemic evolved, new medications, including remdesivir, hydroxychloroquine, lopinavir, or interferon β-1a, were used in addition to the standard of care, but these did not result in significantly different mortality from standard of care.16 Steroids are becoming foundational to the treatment of severe COVID-19 pneumonia, but evidence from high-quality randomized controlled clinical trials is lacking.17
During the first surge from March to May 2020, Massachusetts had the third highest number of COVID-19 cases among states in the United States.18 In early 2021, COVID-19 cases were climbing close to the peak of the second surge in Massachusetts. In this study, we compared utilization of low-flow supplemental oxygen, HFNC, and mechanical ventilation and clinical outcomes of patients admitted to 3 hospitals in Massachusetts during the second surge of the pandemic to that of patients admitted during the first surge.
Methods
Setting
Beth Israel Lahey Health is a system of academic and teaching hospitals with primary care and specialty care providers. We included 3 centers within the Beth Israel Lahey Health system in Massachusetts: Lahey Hospital and Medical Center, with 335 inpatient hospital beds and 52 critical care beds; Beverly Hospital, with 227 beds and 14 critical care beds; and Winchester Hospital, with 229 beds and 10 ICU beds.
Participants
We included patients admitted to the 3 hospitals with COVID-19 as a primary or secondary diagnosis during the first surge of the pandemic (March 1, 2020 to June 15, 2020) and the second surge (November 15, 2020 to January 27, 2021). The timeframe of the first surge was defined as the window between the start date and the end date of data collection. During the time window of the first surge, 1586 patients were included. The start time of the second surge was defined as the date when the data collection was restarted; the end date was set when the number of patients (1597) accumulated was close to the number of patients in the first surge (1586), so that the two groups had similar sample size.
Study Design
A data registry of COVID-19 patients was created by our institution, and the data were prospectively collected starting in March 2020. We retrospectively extracted data on the following from the registry database for this observational study: demographics and baseline comorbidities; the use of low-flow supplemental oxygen, HFNC, and invasive mechanical ventilator; and ICU admission, length of hospital stay, length of ICU stay, and hospital discharge disposition. Start and end times for each oxygen therapy were not entered in the registry. Data about other oxygen therapies, such as noninvasive positive-pressure ventilation, were not collected in the registry database, and therefore were not included in the analysis.
Statistical Analysis
Continuous variables (eg, age) were tested for data distribution normality using the Shapiro-Wilk test. Normally distributed data were tested using unpaired t-tests and displayed as mean (SD). The skewed data were tested using the Wilcoxon rank sum test and displayed as median (interquartile range [IQR]). The categorical variables were compared using chi-square test. Comparisons with P ≤ .05 were considered significantly different. Statistical analysis for this study was generated using Statistical Analysis Software (SAS), version 9.4 for Windows (SAS Institute Inc.).
Results
Baseline Characteristics
We included 3183 patients: 1586 admitted during the first surge and 1597 admitted during the second surge. Baseline characteristics of patients with COVID-19 admitted during the first and second surges are shown in Table 1. Patients admitted during the second surge were older (73 years vs 71 years, P = .01) and had higher rates of hypertension (64.8% vs 59.6%, P = .003) and asthma (12.9% vs 10.7%, P = .049) but a lower rate of interstitial lung disease (3.3% vs 7.7%, P < .001). Sequential organ failure assessment scores at admission and the rates of other comorbidities were not significantly different between the 2 surges.

Oxygen Therapies
The number of patients who were hospitalized and received low-flow supplemental oxygen, and/or HFNC, and/or ventilator in the first surge and the second surge is shown in the Figure. Of all patients included, 2067 (64.9%) received low-flow supplemental oxygen; of these, 374 (18.1%) subsequently received HFNC, and 85 (22.7%) of these subsequently received mechanical ventilation. Of all 3183 patients, 417 (13.1%) received HFNC; 43 of these patients received HFNC without receiving low-flow supplemental oxygen, and 98 (23.5%) subsequently received mechanical ventilation. Out of all 3183 patients, 244 (7.7%) received mechanical ventilation; 98 (40.2%) of these received HFNC while the remaining 146 (59.8%) did not. At the beginning of the first surge, the ratio of patients who received invasive mechanical ventilation to patients who received HFNC was close to 1:1 (10/10); the ratio decreased to 6:10 in May and June 2020. At the beginning of the second surge, the ratio was 8:10 and then decreased to 3:10 in December 2020 and January 2021.

As shown in Table 2, the proportion of patients who received low-flow supplemental oxygen during the second surge was similar to that during the first surge (65.8% vs 64.1%, P = .3). Patients admitted during the second surge were more likely to receive HFNC than patients admitted during the first surge (15.4% vs 10.8%, P = .0001). Patients admitted during the second surge were less likely to be ventilated than the patients admitted during the first surge (5.6% vs 9.7%, P < .0001).

Clinical Outcomes
As shown in Table 3, second surge outcomes were much better than first surge outcomes: the ICU admission rate was lower (8.1% vs 12.7%, P < .0001); patients were more likely to be discharged to home (60.2% vs 47.4%, P < .0001), had a shorter length of hospital stay (5 vs 6 days, P < .0001), and had fewer ventilator days (10 vs 16, P = .01); and mortality was lower (8.3% vs 19.2%, P < .0001). There was a trend that length of ICU stay was shorter during the second surge than during the first surge (7 days vs 9 days, P = .09).

As noted (Figure), the ratio of patients who received invasive mechanical ventilation to patients who received HFNC was decreasing during both the first surge and the second surge. To further analyze the relation between ventilator and HFNC, we performed a subgroup analysis for 244 ventilated patients during both surges to compare outcomes between patients who received HFNC and those who did not receive HFNC (Table 4). Ninety-eight (40%) patients received HFNC. Ventilated patients who received HFNC had lower mortality than those patients who did not receive HFNC (31.6% vs 48%, P = .01), but had a longer length of hospital stay (29 days vs 14 days, P < .0001), longer length of ICU stay (17 days vs 9 days, P < .0001), and a higher number of ventilator days (16 vs 11, P = .001).

Discussion
Our study compared the baseline patient characteristics; utilization of low-flow supplemental oxygen therapy, HFNC, and mechanical ventilation; and clinical outcomes between the first surge (n = 1586) and the second surge (n = 1597) of the COVID-19 pandemic. During both surges, about two-thirds of admitted patients received low-flow supplemental oxygen. A higher proportion of the admitted patients received HFNC during the second surge than during the first surge, while the intubation rate was lower during the second surge than during the first surge.
Reported low-flow supplemental oxygen use ranged from 28% to 63% depending on the cohort characteristics and location during the first surge.6,7,19 A report from New York during the first surge (March 1 to April 4, 2020) showed that among 5700 hospitalized patients with COVID-19, 27.8% received low-flow supplemental oxygen.7 HFNC is recommended in guidelines on management of patients with acute respiratory failure due to COVID-19.20 In our study, HFNC was utilized in a higher proportion of patients admitted for COVID-19 during the second surge (15.5% vs 10.8%, P = .0001). During the early pandemic period in Wuhan, China, 11% to 21% of admitted COVID-19 patients received HFNC.21,22 Utilization of HFNC in New York during the first surge (March to May 2020) varied from 5% to 14.3% of patients admitted with COVID-19.23,24 Our subgroup analysis of the ventilated patients showed that patients who received HFNC had lower mortality than those who did not (31.6% vs 48.0%, P = .011). Comparably, a report from Paris, France, showed that among patients admitted to ICUs for acute hypoxemic respiratory failure, those who received HFNC had lower mortality at day 60 than those who did not (21% vs 31%, P = .052).25 Our recent analysis showed that patients treated with HFNC prior to mechanical ventilation had lower mortality than those treated with only conventional oxygen (30% vs 52%, P = .05).26 In this subgroup analysis, we could not determine if HFNC treatment was administered before or after ventilation because HFNC was entered as dichotomous data (“Yes” or “No”) in the registry database. We merely showed the beneficial effect of HFNC on reducing mortality for ventilated COVID-19 patients, but did not mean to focus on how and when to apply HFNC.
We observed that the patients admitted during the second surge were less likely to be ventilated than the patients admitted during the first surge (5.6% vs 9.7%, P < .0001). During the first surge in New York, among 5700 patients admitted with COVID-19, 12.2% received invasive mechanical ventilation.7 In another report, also from New York during the first surge, 26.1% of 2015 hospitalized COVID-19 patients received mechanical ventilation.27 In our study, the ventilation rate of 9.7% during the first surge was lower.
Outcomes during the second surge were better than during the first surge, including ICU admission rate, hospital and ICU length of stay, ventilator days, and mortality. The mortality was 19.2% during the first surge vs 8.3% during the second surge (P < .0001). The mortality of 19.2% was lower than the 30.6% mortality reported for 2015 hospitalized COVID-19 patients in New York during the first surge.27 A retrospective study showed that early administration of remdesivir was associated with reduced ICU admission, ventilation use, and mortality.28 The RECOVERY clinical trial showed that dexamethasone improved mortality for COVID-19 patients who received respiratory support, but not for patients who did not receive any respiratory support.29 Perhaps some, if not all, of the improvement in ICU admission and mortality during the second surge was attributed to the new medications, such as antivirals and steroids.
The length of hospital stay for patients with moderate to severe COVID-19 varied from 4 to 53 days at different locations of the world, as shown in a meta-analysis by Rees and colleagues.30 Our results showing a length of stay of 6 days during the first surge and 5 days during the second surge fell into the shorter end of this range. In a retrospective analysis of 1643 adults with severe COVID-19 admitted to hospitals in New York City between March 9, 2020 and April 23, 2020, median hospital length of stay was 7 (IQR, 3-14) days.31 For the ventilated patients in our study, the length of stay of 14 days (did not receive HFNC) and 29 days (received HFNC) was much longer. This longer length of stay might be attributed to the patients in our study being older and having more severe comorbidities.
The main purpose of this study was to compare the oxygen therapies and outcomes between 2 surges. It is difficult to associate the clinical outcomes with the oxygen therapies because new therapies and medications were available after the first surge. It was not possible to adjust the outcomes with confounders (other therapies and medications) because the registry data did not include the new therapies and medications.
A strength of this study was that we included a large, balanced number of patients in the first surge and the second surge. We did not plan the sample size in both groups as we could not predict the number of admissions. We set the end date of data collection for analysis as the time when the number of patients admitted during the second surge was similar to the number of patients admitted during the first surge. A limitation was that the registry database was created by the institution and was not designed solely for this study. The data for oxygen therapies were limited to low-flow supplemental oxygen, HFNC, and invasive mechanical ventilation; data for noninvasive ventilation were not included.
Conclusion
At our centers, during the second surge of COVID-19 pandemic, patients hospitalized with COVID-19 infection were more likely to receive HFNC but less likely to be ventilated. Compared to the first surge, the hospitalized patients with COVID-19 infection had a lower ICU admission rate, shorter length of hospital stay, fewer ventilator days, and lower mortality. For ventilated patients, those who received HFNC had lower mortality than those who did not.
Corresponding author: Timothy N. Liesching, MD, 41 Mall Road, Burlington, MA 01805; [email protected]
Disclosures: None reported.
doi:10.12788/jcom.0086
From Lahey Hospital and Medical Center, Burlington, MA (Drs. Liesching and Lei), and Tufts University School of Medicine, Boston, MA (Dr. Liesching)
ABSTRACT
Objective: To compare the utilization of oxygen therapies and clinical outcomes of patients admitted for COVID-19 during the second surge of the pandemic to that of patients admitted during the first surge.
Design: Observational study using a registry database.
Setting: Three hospitals (791 inpatient beds and 76 intensive care unit [ICU] beds) within the Beth Israel Lahey Health system in Massachusetts.
Participants: We included 3183 patients with COVID-19 admitted to hospitals.
Measurements: Baseline data included demographics and comorbidities. Treatments included low-flow supplemental oxygen (2-6 L/min), high-flow oxygen via nasal cannula, and invasive mechanical ventilation. Outcomes included ICU admission, length of stay, ventilator days, and mortality.
Results: A total of 3183 patients were included: 1586 during the first surge and 1597 during the second surge. Compared to the first surge, patients admitted during the second surge had a similar rate of receiving low-flow supplemental oxygen (65.8% vs 64.1%, P = .3), a higher rate of receiving high-flow nasal cannula (15.4% vs 10.8%, P = .0001), and a lower ventilation rate (5.6% vs 9.7%, P < .0001). The outcomes during the second surge were better than those during the first surge: lower ICU admission rate (8.1% vs 12.7%, P < .0001), shorter length of hospital stay (5 vs 6 days, P < .0001), fewer ventilator days (10 vs 16, P = .01), and lower mortality (8.3% vs 19.2%, P < .0001). Among ventilated patients, those who received high-flow nasal cannula had lower mortality.
Conclusion: Compared to the first surge of the COVID-19 pandemic, patients admitted during the second surge had similar likelihood of receiving low-flow supplemental oxygen, were more likely to receive high-flow nasal cannula, were less likely to be ventilated, and had better outcomes.
Keywords: supplemental oxygen, high-flow nasal cannula, ventilator.
The respiratory system receives the major impact of SARS-CoV-2 virus, and hypoxemia has been the predominant diagnosis for patients hospitalized with COVID-19.1,2 During the initial stage of the pandemic, oxygen therapies and mechanical ventilation were the only choices for these patients.3-6 Standard-of-care treatment for patients with COVID-19 during the initial surge included oxygen therapies and mechanical ventilation for hypoxemia and medications for comorbidities and COVID-19–associated sequelae, such as multi-organ dysfunction and failure. A report from New York during the first surge (May 2020) showed that among 5700 hospitalized patients with COVID-19, 27.8% received supplemental oxygen and 12.2% received invasive mechanical ventilation.7 High-flow nasal cannula (HFNC) oxygen delivery has been utilized widely throughout the pandemic due to its superiority over other noninvasive respiratory support techniques.8-12 Mechanical ventilation is always necessary for critically ill patients with acute respiratory distress syndrome. However, ventilator scarcity has become a bottleneck in caring for severely ill patients with COVID-19 during the pandemic.13
The clinical outcomes of hospitalized COVID-19 patients include a high intubation rate, long length of hospital and intensive care unit (ICU) stay, and high mortality.14,15 As the pandemic evolved, new medications, including remdesivir, hydroxychloroquine, lopinavir, or interferon β-1a, were used in addition to the standard of care, but these did not result in significantly different mortality from standard of care.16 Steroids are becoming foundational to the treatment of severe COVID-19 pneumonia, but evidence from high-quality randomized controlled clinical trials is lacking.17
During the first surge from March to May 2020, Massachusetts had the third highest number of COVID-19 cases among states in the United States.18 In early 2021, COVID-19 cases were climbing close to the peak of the second surge in Massachusetts. In this study, we compared utilization of low-flow supplemental oxygen, HFNC, and mechanical ventilation and clinical outcomes of patients admitted to 3 hospitals in Massachusetts during the second surge of the pandemic to that of patients admitted during the first surge.
Methods
Setting
Beth Israel Lahey Health is a system of academic and teaching hospitals with primary care and specialty care providers. We included 3 centers within the Beth Israel Lahey Health system in Massachusetts: Lahey Hospital and Medical Center, with 335 inpatient hospital beds and 52 critical care beds; Beverly Hospital, with 227 beds and 14 critical care beds; and Winchester Hospital, with 229 beds and 10 ICU beds.
Participants
We included patients admitted to the 3 hospitals with COVID-19 as a primary or secondary diagnosis during the first surge of the pandemic (March 1, 2020 to June 15, 2020) and the second surge (November 15, 2020 to January 27, 2021). The timeframe of the first surge was defined as the window between the start date and the end date of data collection. During the time window of the first surge, 1586 patients were included. The start time of the second surge was defined as the date when the data collection was restarted; the end date was set when the number of patients (1597) accumulated was close to the number of patients in the first surge (1586), so that the two groups had similar sample size.
Study Design
A data registry of COVID-19 patients was created by our institution, and the data were prospectively collected starting in March 2020. We retrospectively extracted data on the following from the registry database for this observational study: demographics and baseline comorbidities; the use of low-flow supplemental oxygen, HFNC, and invasive mechanical ventilator; and ICU admission, length of hospital stay, length of ICU stay, and hospital discharge disposition. Start and end times for each oxygen therapy were not entered in the registry. Data about other oxygen therapies, such as noninvasive positive-pressure ventilation, were not collected in the registry database, and therefore were not included in the analysis.
Statistical Analysis
Continuous variables (eg, age) were tested for data distribution normality using the Shapiro-Wilk test. Normally distributed data were tested using unpaired t-tests and displayed as mean (SD). The skewed data were tested using the Wilcoxon rank sum test and displayed as median (interquartile range [IQR]). The categorical variables were compared using chi-square test. Comparisons with P ≤ .05 were considered significantly different. Statistical analysis for this study was generated using Statistical Analysis Software (SAS), version 9.4 for Windows (SAS Institute Inc.).
Results
Baseline Characteristics
We included 3183 patients: 1586 admitted during the first surge and 1597 admitted during the second surge. Baseline characteristics of patients with COVID-19 admitted during the first and second surges are shown in Table 1. Patients admitted during the second surge were older (73 years vs 71 years, P = .01) and had higher rates of hypertension (64.8% vs 59.6%, P = .003) and asthma (12.9% vs 10.7%, P = .049) but a lower rate of interstitial lung disease (3.3% vs 7.7%, P < .001). Sequential organ failure assessment scores at admission and the rates of other comorbidities were not significantly different between the 2 surges.

Oxygen Therapies
The number of patients who were hospitalized and received low-flow supplemental oxygen, and/or HFNC, and/or ventilator in the first surge and the second surge is shown in the Figure. Of all patients included, 2067 (64.9%) received low-flow supplemental oxygen; of these, 374 (18.1%) subsequently received HFNC, and 85 (22.7%) of these subsequently received mechanical ventilation. Of all 3183 patients, 417 (13.1%) received HFNC; 43 of these patients received HFNC without receiving low-flow supplemental oxygen, and 98 (23.5%) subsequently received mechanical ventilation. Out of all 3183 patients, 244 (7.7%) received mechanical ventilation; 98 (40.2%) of these received HFNC while the remaining 146 (59.8%) did not. At the beginning of the first surge, the ratio of patients who received invasive mechanical ventilation to patients who received HFNC was close to 1:1 (10/10); the ratio decreased to 6:10 in May and June 2020. At the beginning of the second surge, the ratio was 8:10 and then decreased to 3:10 in December 2020 and January 2021.

As shown in Table 2, the proportion of patients who received low-flow supplemental oxygen during the second surge was similar to that during the first surge (65.8% vs 64.1%, P = .3). Patients admitted during the second surge were more likely to receive HFNC than patients admitted during the first surge (15.4% vs 10.8%, P = .0001). Patients admitted during the second surge were less likely to be ventilated than the patients admitted during the first surge (5.6% vs 9.7%, P < .0001).

Clinical Outcomes
As shown in Table 3, second surge outcomes were much better than first surge outcomes: the ICU admission rate was lower (8.1% vs 12.7%, P < .0001); patients were more likely to be discharged to home (60.2% vs 47.4%, P < .0001), had a shorter length of hospital stay (5 vs 6 days, P < .0001), and had fewer ventilator days (10 vs 16, P = .01); and mortality was lower (8.3% vs 19.2%, P < .0001). There was a trend that length of ICU stay was shorter during the second surge than during the first surge (7 days vs 9 days, P = .09).

As noted (Figure), the ratio of patients who received invasive mechanical ventilation to patients who received HFNC was decreasing during both the first surge and the second surge. To further analyze the relation between ventilator and HFNC, we performed a subgroup analysis for 244 ventilated patients during both surges to compare outcomes between patients who received HFNC and those who did not receive HFNC (Table 4). Ninety-eight (40%) patients received HFNC. Ventilated patients who received HFNC had lower mortality than those patients who did not receive HFNC (31.6% vs 48%, P = .01), but had a longer length of hospital stay (29 days vs 14 days, P < .0001), longer length of ICU stay (17 days vs 9 days, P < .0001), and a higher number of ventilator days (16 vs 11, P = .001).

Discussion
Our study compared the baseline patient characteristics; utilization of low-flow supplemental oxygen therapy, HFNC, and mechanical ventilation; and clinical outcomes between the first surge (n = 1586) and the second surge (n = 1597) of the COVID-19 pandemic. During both surges, about two-thirds of admitted patients received low-flow supplemental oxygen. A higher proportion of the admitted patients received HFNC during the second surge than during the first surge, while the intubation rate was lower during the second surge than during the first surge.
Reported low-flow supplemental oxygen use ranged from 28% to 63% depending on the cohort characteristics and location during the first surge.6,7,19 A report from New York during the first surge (March 1 to April 4, 2020) showed that among 5700 hospitalized patients with COVID-19, 27.8% received low-flow supplemental oxygen.7 HFNC is recommended in guidelines on management of patients with acute respiratory failure due to COVID-19.20 In our study, HFNC was utilized in a higher proportion of patients admitted for COVID-19 during the second surge (15.5% vs 10.8%, P = .0001). During the early pandemic period in Wuhan, China, 11% to 21% of admitted COVID-19 patients received HFNC.21,22 Utilization of HFNC in New York during the first surge (March to May 2020) varied from 5% to 14.3% of patients admitted with COVID-19.23,24 Our subgroup analysis of the ventilated patients showed that patients who received HFNC had lower mortality than those who did not (31.6% vs 48.0%, P = .011). Comparably, a report from Paris, France, showed that among patients admitted to ICUs for acute hypoxemic respiratory failure, those who received HFNC had lower mortality at day 60 than those who did not (21% vs 31%, P = .052).25 Our recent analysis showed that patients treated with HFNC prior to mechanical ventilation had lower mortality than those treated with only conventional oxygen (30% vs 52%, P = .05).26 In this subgroup analysis, we could not determine if HFNC treatment was administered before or after ventilation because HFNC was entered as dichotomous data (“Yes” or “No”) in the registry database. We merely showed the beneficial effect of HFNC on reducing mortality for ventilated COVID-19 patients, but did not mean to focus on how and when to apply HFNC.
We observed that the patients admitted during the second surge were less likely to be ventilated than the patients admitted during the first surge (5.6% vs 9.7%, P < .0001). During the first surge in New York, among 5700 patients admitted with COVID-19, 12.2% received invasive mechanical ventilation.7 In another report, also from New York during the first surge, 26.1% of 2015 hospitalized COVID-19 patients received mechanical ventilation.27 In our study, the ventilation rate of 9.7% during the first surge was lower.
Outcomes during the second surge were better than during the first surge, including ICU admission rate, hospital and ICU length of stay, ventilator days, and mortality. The mortality was 19.2% during the first surge vs 8.3% during the second surge (P < .0001). The mortality of 19.2% was lower than the 30.6% mortality reported for 2015 hospitalized COVID-19 patients in New York during the first surge.27 A retrospective study showed that early administration of remdesivir was associated with reduced ICU admission, ventilation use, and mortality.28 The RECOVERY clinical trial showed that dexamethasone improved mortality for COVID-19 patients who received respiratory support, but not for patients who did not receive any respiratory support.29 Perhaps some, if not all, of the improvement in ICU admission and mortality during the second surge was attributed to the new medications, such as antivirals and steroids.
The length of hospital stay for patients with moderate to severe COVID-19 varied from 4 to 53 days at different locations of the world, as shown in a meta-analysis by Rees and colleagues.30 Our results showing a length of stay of 6 days during the first surge and 5 days during the second surge fell into the shorter end of this range. In a retrospective analysis of 1643 adults with severe COVID-19 admitted to hospitals in New York City between March 9, 2020 and April 23, 2020, median hospital length of stay was 7 (IQR, 3-14) days.31 For the ventilated patients in our study, the length of stay of 14 days (did not receive HFNC) and 29 days (received HFNC) was much longer. This longer length of stay might be attributed to the patients in our study being older and having more severe comorbidities.
The main purpose of this study was to compare the oxygen therapies and outcomes between 2 surges. It is difficult to associate the clinical outcomes with the oxygen therapies because new therapies and medications were available after the first surge. It was not possible to adjust the outcomes with confounders (other therapies and medications) because the registry data did not include the new therapies and medications.
A strength of this study was that we included a large, balanced number of patients in the first surge and the second surge. We did not plan the sample size in both groups as we could not predict the number of admissions. We set the end date of data collection for analysis as the time when the number of patients admitted during the second surge was similar to the number of patients admitted during the first surge. A limitation was that the registry database was created by the institution and was not designed solely for this study. The data for oxygen therapies were limited to low-flow supplemental oxygen, HFNC, and invasive mechanical ventilation; data for noninvasive ventilation were not included.
Conclusion
At our centers, during the second surge of COVID-19 pandemic, patients hospitalized with COVID-19 infection were more likely to receive HFNC but less likely to be ventilated. Compared to the first surge, the hospitalized patients with COVID-19 infection had a lower ICU admission rate, shorter length of hospital stay, fewer ventilator days, and lower mortality. For ventilated patients, those who received HFNC had lower mortality than those who did not.
Corresponding author: Timothy N. Liesching, MD, 41 Mall Road, Burlington, MA 01805; [email protected]
Disclosures: None reported.
doi:10.12788/jcom.0086
1. Xie J, Covassin N, Fan Z, et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020;95(6):1138-1147. doi:10.1016/j.mayocp.2020.04.006
2. Asleh R, Asher E, Yagel O, et al. Predictors of hypoxemia and related adverse outcomes in patients hospitalized with COVID-19: a double-center retrospective study. J Clin Med. 2021;10(16):3581. doi:10.3390/jcm10163581
3. Choi KJ, Hong HL, Kim EJ. Association between oxygen saturation/fraction of inhaled oxygen and mortality in patients with COVID-19 associated pneumonia requiring oxygen therapy. Tuberc Respir Dis (Seoul). 2021;84(2):125-133. doi:10.4046/trd.2020.0126
4. Dixit SB. Role of noninvasive oxygen therapy strategies in COVID-19 patients: Where are we going? Indian J Crit Care Med. 2020;24(10):897-898. doi:10.5005/jp-journals-10071-23625
5. Gonzalez-Castro A, Fajardo Campoverde A, Medina A, et al. Non-invasive mechanical ventilation and high-flow oxygen therapy in the COVID-19 pandemic: the value of a draw. Med Intensiva (Engl Ed). 2021;45(5):320-321. doi:10.1016/j.medine.2021.04.001
6. Pan W, Li J, Ou Y, et al. Clinical outcome of standardized oxygen therapy nursing strategy in COVID-19. Ann Palliat Med. 2020;9(4):2171-2177. doi:10.21037/apm-20-1272
7. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
8. He G, Han Y, Fang Q, et al. Clinical experience of high-flow nasal cannula oxygen therapy in severe COVID-19 patients. Article in Chinese. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(2):232-239. doi:10.3785/j.issn.1008-9292.2020.03.13
9. Lalla U, Allwood BW, Louw EH, et al. The utility of high-flow nasal cannula oxygen therapy in the management of respiratory failure secondary to COVID-19 pneumonia. S Afr Med J. 2020;110(6):12941.
10. Zhang TT, Dai B, Wang W. Should the high-flow nasal oxygen therapy be used or avoided in COVID-19? J Transl Int Med. 2020;8(2):57-58. doi:10.2478/jtim-2020-0018
11. Agarwal A, Basmaji J, Muttalib F, et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can J Anaesth. 2020;67(9):1217-1248. doi:10.1007/s12630-020-01740-2
12. Geng S, Mei Q, Zhu C, et al. High flow nasal cannula is a good treatment option for COVID-19. Heart Lung. 2020;49(5):444-445. doi:10.1016/j.hrtlng.2020.03.018
13. Feinstein MM, Niforatos JD, Hyun I, et al. Considerations for ventilator triage during the COVID-19 pandemic. Lancet Respir Med. 2020;8(6):e53. doi:10.1016/S2213-2600(20)30192-2
14. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi:10.1001/jama.2020.2648
15. Rojas-Marte G, Hashmi AT, Khalid M, et al. Outcomes in patients with COVID-19 disease and high oxygen requirements. J Clin Med Res. 2021;13(1):26-37. doi:10.14740/jocmr4405
16. Zhang R, Mylonakis E. In inpatients with COVID-19, none of remdesivir, hydroxychloroquine, lopinavir, or interferon β-1a differed from standard care for in-hospital mortality. Ann Intern Med. 2021;174(2):JC17. doi:10.7326/ACPJ202102160-017
17. Rello J, Waterer GW, Bourdiol A, Roquilly A. COVID-19, steroids and other immunomodulators: The jigsaw is not complete. Anaesth Crit Care Pain Med. 2020;39(6):699-701. doi:10.1016/j.accpm.2020.10.011
18. Dargin J, Stempek S, Lei Y, Gray Jr. A, Liesching T. The effect of a tiered provider staffing model on patient outcomes during the coronavirus disease 2019 pandemic: A single-center observational study. Int J Crit Illn Inj Sci. 2021;11(3). doi:10.4103/ijciis.ijciis_37_21
19. Ni YN, Wang T, Liang BM, Liang ZA. The independent factors associated with oxygen therapy in COVID-19 patients under 65 years old. PLoS One. 2021;16(1):e0245690. doi:10.1371/journal.pone.0245690
20. Alhazzani W, Moller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. doi:10.1097/CCM.0000000000004363
21. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. doi:10.1001/jama.2020.1585
22. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:10.1016/S0140-6736(20)30566-3
23. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi:10.1136/bmj.m1996
24. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
25. Demoule A, Vieillard Baron A, Darmon M, et al. High-flow nasal cannula in critically ill patients with severe COVID-19. Am J Respir Crit Care Med. 2020;202(7):1039-1042. doi:10.1164/rccm.202005-2007LE
26. Hansen CK, Stempek S, Liesching T, Lei Y, Dargin J. Characteristics and outcomes of patients receiving high flow nasal cannula therapy prior to mechanical ventilation in COVID-19 respiratory failure: a prospective observational study. Int J Crit Illn Inj Sci. 2021;11(2):56-60. doi:10.4103/IJCIIS.IJCIIS_181_20
27. van Gerwen M, Alsen M, Little C, et al. Risk factors and outcomes of COVID-19 in New York City; a retrospective cohort study. J Med Virol. 2021;93(2):907-915. doi:10.1002/jmv.26337
28. Hussain Alsayed HA, Saheb Sharif-Askari F, Saheb Sharif-Askari N, Hussain AAS, Hamid Q, Halwani R. Early administration of remdesivir to COVID-19 patients associates with higher recovery rate and lower need for ICU admission: A retrospective cohort study. PLoS One. 2021;16(10):e0258643. doi:10.1371/journal.pone.0258643
29. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704. doi:10.1056/NEJMoa2021436
30. Rees EM, Nightingale ES, Jafari Y, et al. COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Med. 2020;18(1):270. doi:10.1186/s12916-020-01726-3
31. Anderson M, Bach P, Baldwin MR. Hospital length of stay for severe COVID-19: implications for Remdesivir’s value. medRxiv. 2020;2020.08.10.20171637. doi:10.1101/2020.08.10.20171637
1. Xie J, Covassin N, Fan Z, et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020;95(6):1138-1147. doi:10.1016/j.mayocp.2020.04.006
2. Asleh R, Asher E, Yagel O, et al. Predictors of hypoxemia and related adverse outcomes in patients hospitalized with COVID-19: a double-center retrospective study. J Clin Med. 2021;10(16):3581. doi:10.3390/jcm10163581
3. Choi KJ, Hong HL, Kim EJ. Association between oxygen saturation/fraction of inhaled oxygen and mortality in patients with COVID-19 associated pneumonia requiring oxygen therapy. Tuberc Respir Dis (Seoul). 2021;84(2):125-133. doi:10.4046/trd.2020.0126
4. Dixit SB. Role of noninvasive oxygen therapy strategies in COVID-19 patients: Where are we going? Indian J Crit Care Med. 2020;24(10):897-898. doi:10.5005/jp-journals-10071-23625
5. Gonzalez-Castro A, Fajardo Campoverde A, Medina A, et al. Non-invasive mechanical ventilation and high-flow oxygen therapy in the COVID-19 pandemic: the value of a draw. Med Intensiva (Engl Ed). 2021;45(5):320-321. doi:10.1016/j.medine.2021.04.001
6. Pan W, Li J, Ou Y, et al. Clinical outcome of standardized oxygen therapy nursing strategy in COVID-19. Ann Palliat Med. 2020;9(4):2171-2177. doi:10.21037/apm-20-1272
7. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
8. He G, Han Y, Fang Q, et al. Clinical experience of high-flow nasal cannula oxygen therapy in severe COVID-19 patients. Article in Chinese. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(2):232-239. doi:10.3785/j.issn.1008-9292.2020.03.13
9. Lalla U, Allwood BW, Louw EH, et al. The utility of high-flow nasal cannula oxygen therapy in the management of respiratory failure secondary to COVID-19 pneumonia. S Afr Med J. 2020;110(6):12941.
10. Zhang TT, Dai B, Wang W. Should the high-flow nasal oxygen therapy be used or avoided in COVID-19? J Transl Int Med. 2020;8(2):57-58. doi:10.2478/jtim-2020-0018
11. Agarwal A, Basmaji J, Muttalib F, et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can J Anaesth. 2020;67(9):1217-1248. doi:10.1007/s12630-020-01740-2
12. Geng S, Mei Q, Zhu C, et al. High flow nasal cannula is a good treatment option for COVID-19. Heart Lung. 2020;49(5):444-445. doi:10.1016/j.hrtlng.2020.03.018
13. Feinstein MM, Niforatos JD, Hyun I, et al. Considerations for ventilator triage during the COVID-19 pandemic. Lancet Respir Med. 2020;8(6):e53. doi:10.1016/S2213-2600(20)30192-2
14. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi:10.1001/jama.2020.2648
15. Rojas-Marte G, Hashmi AT, Khalid M, et al. Outcomes in patients with COVID-19 disease and high oxygen requirements. J Clin Med Res. 2021;13(1):26-37. doi:10.14740/jocmr4405
16. Zhang R, Mylonakis E. In inpatients with COVID-19, none of remdesivir, hydroxychloroquine, lopinavir, or interferon β-1a differed from standard care for in-hospital mortality. Ann Intern Med. 2021;174(2):JC17. doi:10.7326/ACPJ202102160-017
17. Rello J, Waterer GW, Bourdiol A, Roquilly A. COVID-19, steroids and other immunomodulators: The jigsaw is not complete. Anaesth Crit Care Pain Med. 2020;39(6):699-701. doi:10.1016/j.accpm.2020.10.011
18. Dargin J, Stempek S, Lei Y, Gray Jr. A, Liesching T. The effect of a tiered provider staffing model on patient outcomes during the coronavirus disease 2019 pandemic: A single-center observational study. Int J Crit Illn Inj Sci. 2021;11(3). doi:10.4103/ijciis.ijciis_37_21
19. Ni YN, Wang T, Liang BM, Liang ZA. The independent factors associated with oxygen therapy in COVID-19 patients under 65 years old. PLoS One. 2021;16(1):e0245690. doi:10.1371/journal.pone.0245690
20. Alhazzani W, Moller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. doi:10.1097/CCM.0000000000004363
21. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. doi:10.1001/jama.2020.1585
22. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:10.1016/S0140-6736(20)30566-3
23. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi:10.1136/bmj.m1996
24. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
25. Demoule A, Vieillard Baron A, Darmon M, et al. High-flow nasal cannula in critically ill patients with severe COVID-19. Am J Respir Crit Care Med. 2020;202(7):1039-1042. doi:10.1164/rccm.202005-2007LE
26. Hansen CK, Stempek S, Liesching T, Lei Y, Dargin J. Characteristics and outcomes of patients receiving high flow nasal cannula therapy prior to mechanical ventilation in COVID-19 respiratory failure: a prospective observational study. Int J Crit Illn Inj Sci. 2021;11(2):56-60. doi:10.4103/IJCIIS.IJCIIS_181_20
27. van Gerwen M, Alsen M, Little C, et al. Risk factors and outcomes of COVID-19 in New York City; a retrospective cohort study. J Med Virol. 2021;93(2):907-915. doi:10.1002/jmv.26337
28. Hussain Alsayed HA, Saheb Sharif-Askari F, Saheb Sharif-Askari N, Hussain AAS, Hamid Q, Halwani R. Early administration of remdesivir to COVID-19 patients associates with higher recovery rate and lower need for ICU admission: A retrospective cohort study. PLoS One. 2021;16(10):e0258643. doi:10.1371/journal.pone.0258643
29. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704. doi:10.1056/NEJMoa2021436
30. Rees EM, Nightingale ES, Jafari Y, et al. COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Med. 2020;18(1):270. doi:10.1186/s12916-020-01726-3
31. Anderson M, Bach P, Baldwin MR. Hospital length of stay for severe COVID-19: implications for Remdesivir’s value. medRxiv. 2020;2020.08.10.20171637. doi:10.1101/2020.08.10.20171637
COVID-19 often more severe with congenital heart defects
Adults with a congenital heart defect (CHD) are at increased risk for serious illness and death when hospitalized with COVID-19, making vaccination and other preventive measures even important in this population, say researchers with the Centers for Disease Control and Prevention.
“We found that hospitalized patients with heart defects are up to twice as likely to have critical outcomes of COVID-19 illness (admission to the intensive care unit, use of a ventilator to help with breathing, or death) compared to hospitalized COVID-19 patients without heart defects,” Karrie Downing, MPH, epidemiologist, with the CDC’s National Center on Birth Defects and Developmental Disabilities, said in an interview.
“Additionally, we learned that people with hearts defects who were older or who also had other conditions like heart failure, pulmonary hypertension, Down syndrome, diabetes, or obesity were the most likely to have critical COVID-19 illness, but children and adults with heart defects without these other conditions were still at increased risk,” Ms. Downing said.
The message for health care providers is clear: “Encourage your patients with heart defects to get vaccinated and discuss with your patients the need for other preventive measures to avoid infection that may progress to severe COVID-19 illness,” Ms. Downing added.
The study was published online March 7, 2022, in Circulation.
The researchers analyzed data on 235,638 patients hospitalized with COVID-19 between March 2020 and January 2021, including 421 (0.2%) with CHD. Most CHD patients were older than 30 years (73%) and 61% were men, with 55% non-Hispanic white, 19% Hispanic and 16% non-Hispanic Black.
Overall, 68% of CHD patients had at least one comorbidity, as did 59% of patients without CHD.
Rates of ICU admission were higher in the CHD group (54% vs. 43%), as were rates of invasive mechanical ventilation (24% vs. 15%) and in-hospital death (11% vs. 7%).
After accounting for patient characteristics, ICU admission, invasive mechanical ventilation and death were more prevalent among COVID-19 patients with rather than without CHD, with adjusted prevalence ratios of 1.4, 1.8 and 2.0, respectively.
When stratified by high-risk characteristics, prevalence estimates for ICU admission, invasive mechanical ventilation and death remained higher among patients with COVID-19 and CHD across nearly all strata, including younger age groups and those without heart failure, pulmonary hypertension, Down syndrome, diabetes, or obesity, the researchers reported.
Ms. Downing said more work is needed to identify why the clinical course of COVID-19 disease results in admission to the ICU, the need for a ventilator, or death for some hospitalized patients with CHD and not for others.
“There could be a number of social, environmental, economic, medical, and genetic factors playing a role. But staying up to date with COVID-19 vaccines and following preventive measures for COVID-19 are effective ways to reduce the risk of severe illness from COVID-19,” Ms. Downing said.
The study had no specific funding. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Adults with a congenital heart defect (CHD) are at increased risk for serious illness and death when hospitalized with COVID-19, making vaccination and other preventive measures even important in this population, say researchers with the Centers for Disease Control and Prevention.
“We found that hospitalized patients with heart defects are up to twice as likely to have critical outcomes of COVID-19 illness (admission to the intensive care unit, use of a ventilator to help with breathing, or death) compared to hospitalized COVID-19 patients without heart defects,” Karrie Downing, MPH, epidemiologist, with the CDC’s National Center on Birth Defects and Developmental Disabilities, said in an interview.
“Additionally, we learned that people with hearts defects who were older or who also had other conditions like heart failure, pulmonary hypertension, Down syndrome, diabetes, or obesity were the most likely to have critical COVID-19 illness, but children and adults with heart defects without these other conditions were still at increased risk,” Ms. Downing said.
The message for health care providers is clear: “Encourage your patients with heart defects to get vaccinated and discuss with your patients the need for other preventive measures to avoid infection that may progress to severe COVID-19 illness,” Ms. Downing added.
The study was published online March 7, 2022, in Circulation.
The researchers analyzed data on 235,638 patients hospitalized with COVID-19 between March 2020 and January 2021, including 421 (0.2%) with CHD. Most CHD patients were older than 30 years (73%) and 61% were men, with 55% non-Hispanic white, 19% Hispanic and 16% non-Hispanic Black.
Overall, 68% of CHD patients had at least one comorbidity, as did 59% of patients without CHD.
Rates of ICU admission were higher in the CHD group (54% vs. 43%), as were rates of invasive mechanical ventilation (24% vs. 15%) and in-hospital death (11% vs. 7%).
After accounting for patient characteristics, ICU admission, invasive mechanical ventilation and death were more prevalent among COVID-19 patients with rather than without CHD, with adjusted prevalence ratios of 1.4, 1.8 and 2.0, respectively.
When stratified by high-risk characteristics, prevalence estimates for ICU admission, invasive mechanical ventilation and death remained higher among patients with COVID-19 and CHD across nearly all strata, including younger age groups and those without heart failure, pulmonary hypertension, Down syndrome, diabetes, or obesity, the researchers reported.
Ms. Downing said more work is needed to identify why the clinical course of COVID-19 disease results in admission to the ICU, the need for a ventilator, or death for some hospitalized patients with CHD and not for others.
“There could be a number of social, environmental, economic, medical, and genetic factors playing a role. But staying up to date with COVID-19 vaccines and following preventive measures for COVID-19 are effective ways to reduce the risk of severe illness from COVID-19,” Ms. Downing said.
The study had no specific funding. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Adults with a congenital heart defect (CHD) are at increased risk for serious illness and death when hospitalized with COVID-19, making vaccination and other preventive measures even important in this population, say researchers with the Centers for Disease Control and Prevention.
“We found that hospitalized patients with heart defects are up to twice as likely to have critical outcomes of COVID-19 illness (admission to the intensive care unit, use of a ventilator to help with breathing, or death) compared to hospitalized COVID-19 patients without heart defects,” Karrie Downing, MPH, epidemiologist, with the CDC’s National Center on Birth Defects and Developmental Disabilities, said in an interview.
“Additionally, we learned that people with hearts defects who were older or who also had other conditions like heart failure, pulmonary hypertension, Down syndrome, diabetes, or obesity were the most likely to have critical COVID-19 illness, but children and adults with heart defects without these other conditions were still at increased risk,” Ms. Downing said.
The message for health care providers is clear: “Encourage your patients with heart defects to get vaccinated and discuss with your patients the need for other preventive measures to avoid infection that may progress to severe COVID-19 illness,” Ms. Downing added.
The study was published online March 7, 2022, in Circulation.
The researchers analyzed data on 235,638 patients hospitalized with COVID-19 between March 2020 and January 2021, including 421 (0.2%) with CHD. Most CHD patients were older than 30 years (73%) and 61% were men, with 55% non-Hispanic white, 19% Hispanic and 16% non-Hispanic Black.
Overall, 68% of CHD patients had at least one comorbidity, as did 59% of patients without CHD.
Rates of ICU admission were higher in the CHD group (54% vs. 43%), as were rates of invasive mechanical ventilation (24% vs. 15%) and in-hospital death (11% vs. 7%).
After accounting for patient characteristics, ICU admission, invasive mechanical ventilation and death were more prevalent among COVID-19 patients with rather than without CHD, with adjusted prevalence ratios of 1.4, 1.8 and 2.0, respectively.
When stratified by high-risk characteristics, prevalence estimates for ICU admission, invasive mechanical ventilation and death remained higher among patients with COVID-19 and CHD across nearly all strata, including younger age groups and those without heart failure, pulmonary hypertension, Down syndrome, diabetes, or obesity, the researchers reported.
Ms. Downing said more work is needed to identify why the clinical course of COVID-19 disease results in admission to the ICU, the need for a ventilator, or death for some hospitalized patients with CHD and not for others.
“There could be a number of social, environmental, economic, medical, and genetic factors playing a role. But staying up to date with COVID-19 vaccines and following preventive measures for COVID-19 are effective ways to reduce the risk of severe illness from COVID-19,” Ms. Downing said.
The study had no specific funding. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION


