User login
Pandemic-related school closures tied to mental health inequities
Back-to-school jitters are heightened this year, as children head back to the risk of COVID transmission in class, but one upside to the return of in-person school may be better mental health for students.
New research shows that virtual schooling, which dominated in many districts last year, was associated with worse mental health outcomes for students – especially older ones – and youth from Black, Hispanic, or lower-income families were hit hardest because they experienced the most closures.
“Schools with lower funding may have had more difficulty meeting guidelines for safe reopening, including updates to ventilation systems and finding the physical space to create safe distancing between children,” explained lead author Matt Hawrilenko, PhD, of the department of psychiatry and behavioral sciences at the University of Washington, Seattle, in an interview.
“In the context of complex school reopening decisions that balance competing risks and benefits, these findings suggest that allocating funding to support safe in-person instruction may reduce mental health inequities associated with race/ethnicity and income,” he and his coauthors noted in the study, published in JAMA Network Open. “Ensuring that all students have access to additional educational and mental health resources must be an important public health priority, met with appropriate funding and work force augmentation, during and beyond the COVID-19 pandemic.”
The study used a cross-sectional population-based survey of 2,324 parents of school-age children in the United States. It was administered in English and Spanish via web and telephone between Dec. 2 and Dec. 21, 2020, and used the parent-report version of the Strengths and Difficulties Questionnaire (SDQ) to assess mental health difficulties of one child per family in four domains: emotional problems, peer problems, conduct, and hyperactivity. Parents were also asked about what kind of schooling their child had received in the last year (remote, in-person, or hybrid) and about demographic information such as child age, gender, household income, parent race and ethnicity, and parent education.
The results showed that, during the 2020 school year, 58.0% of children attended school remotely, 24% attended fully in person, and 18.0% attended in a hybrid format. “Fully remote schooling was strongly patterned along lines of parent race and ethnicity as well as income,” the authors noted. “Parents of 336 children attending school in person (65.8%) but of 597 children attending school fully remotely (44.5%) were White, whereas all other racial/ethnic groups had larger proportions of children attending school fully remotely (P < .001).”
In terms of mental health, the findings showed that older children who attended school remotely had more difficulties, compared with those who attended in-person – but among younger children, remote learning was comparable or slightly better for mental health.
Specifically, “a child aged 17 years attending school remotely would be expected to have a total difficulty score 2.4 points higher than a child of the same age attending school in person, corresponding to a small effect size in favor of in-person schooling,” the authors wrote. “Conversely, a child aged 4 years attending school remotely would be expected to have a total difficulty score 0.5 points lower than a child of the same age attending school in person, corresponding to a very small effect size in favor of remote schooling.”
Age of child proves critical
“Our best estimate is that remote schooling was associated with no difference in mental health difficulties at age 6, and with slightly more difficulties with each year of age after that, with differences most clearly apparent for high school–aged kids,” explained Dr. Hawrilenko, adding the finding suggests that school reopenings should prioritize older children.
However, “what kids are doing at home matters,” he added. “In the youngest age group, the biggest work kids are doing in school and childcare settings is social and emotional development. … Finding opportunities for regular, safe social interactions with peers – perhaps during outdoor playdates – can help them build those skills.”
He emphasized with the anticipated starts and stutters of the new school year there is an important role that doctors can play.
“First, they can help families assess their own risk profile, and whether it makes sense for their children to attend school in person or remotely [to the extent that is an option]. Second, they can help families think through how school closures might impact their child specifically. For those kids who wind up with long chunks of remote schooling, scheduling in regular interactions with other kids in safe ways could make a big difference. Another driver of child anxiety might be learning loss, and this is a good place to reinforce that not every mental health problem needs a mental health solution.
“A lot of kids might be rightfully anxious about having fallen behind over the pandemic. These kids are preparing to transition to college or to the workforce and may be feeling increasingly behind while approaching these moments of transition. Pointing families toward the resources to help them navigate these issues could go a long way to helping quell child anxiety.”
Research helps fill vacuum
Elizabeth A. Stuart, PhD, who was not involved in the study, said in an interview that this research is particularly valuable because there have been very few data on this topic, especially on a large-scale national sample.
“Sadly, many of the results are not surprising,” said Dr. Stuart, a statistician and professor of mental health at Johns Hopkins University, Baltimore. “Data have shown significant mental health challenges for adults during the pandemic, and it is not surprising that children and youth would experience that as well, especially for those whose daily routines and structures changed dramatically and who were not able to be interacting in-person with teachers, staff, and classmates. This is an important reminder that schools provide not just academic instruction for students, but that the social interactions and other services (such as behavioral health supports, meals, and connections with other social services) students might receive in school are crucial.
“It has been heartening to see a stronger commitment to getting students safely back into school this fall across the country, and that the Centers for Disease Control and Prevention highlighted the benefits of in-person schooling in their COVID-19–related guidance for schools.”
Dr. Stuart added that, as students return to classes, it will be important for schools to tackle ongoing mental health challenges.
“Some students may be struggling in obvious ways; for others it may be harder to identify. It will also be important to continue to monitor children’s and youth mental health … as returning to in-person school may bring its own challenges. ”
Dr. Hawrilenko agreed.
“From a policy perspective, I am quite frankly terrified about how these inequities – in particular, learning loss – might play out long after school closures are a distant memory,” he said. “It is critical to provide schools the resources not just to minimize risk when reopening, but additional funding for workforce augmentation – both for mental health staffing and for additional educational support – to help students navigate the months and years over which they transition back into the classroom.”
Dr. Hawrilenko and Dr. Stuart had no disclosures.
Back-to-school jitters are heightened this year, as children head back to the risk of COVID transmission in class, but one upside to the return of in-person school may be better mental health for students.
New research shows that virtual schooling, which dominated in many districts last year, was associated with worse mental health outcomes for students – especially older ones – and youth from Black, Hispanic, or lower-income families were hit hardest because they experienced the most closures.
“Schools with lower funding may have had more difficulty meeting guidelines for safe reopening, including updates to ventilation systems and finding the physical space to create safe distancing between children,” explained lead author Matt Hawrilenko, PhD, of the department of psychiatry and behavioral sciences at the University of Washington, Seattle, in an interview.
“In the context of complex school reopening decisions that balance competing risks and benefits, these findings suggest that allocating funding to support safe in-person instruction may reduce mental health inequities associated with race/ethnicity and income,” he and his coauthors noted in the study, published in JAMA Network Open. “Ensuring that all students have access to additional educational and mental health resources must be an important public health priority, met with appropriate funding and work force augmentation, during and beyond the COVID-19 pandemic.”
The study used a cross-sectional population-based survey of 2,324 parents of school-age children in the United States. It was administered in English and Spanish via web and telephone between Dec. 2 and Dec. 21, 2020, and used the parent-report version of the Strengths and Difficulties Questionnaire (SDQ) to assess mental health difficulties of one child per family in four domains: emotional problems, peer problems, conduct, and hyperactivity. Parents were also asked about what kind of schooling their child had received in the last year (remote, in-person, or hybrid) and about demographic information such as child age, gender, household income, parent race and ethnicity, and parent education.
The results showed that, during the 2020 school year, 58.0% of children attended school remotely, 24% attended fully in person, and 18.0% attended in a hybrid format. “Fully remote schooling was strongly patterned along lines of parent race and ethnicity as well as income,” the authors noted. “Parents of 336 children attending school in person (65.8%) but of 597 children attending school fully remotely (44.5%) were White, whereas all other racial/ethnic groups had larger proportions of children attending school fully remotely (P < .001).”
In terms of mental health, the findings showed that older children who attended school remotely had more difficulties, compared with those who attended in-person – but among younger children, remote learning was comparable or slightly better for mental health.
Specifically, “a child aged 17 years attending school remotely would be expected to have a total difficulty score 2.4 points higher than a child of the same age attending school in person, corresponding to a small effect size in favor of in-person schooling,” the authors wrote. “Conversely, a child aged 4 years attending school remotely would be expected to have a total difficulty score 0.5 points lower than a child of the same age attending school in person, corresponding to a very small effect size in favor of remote schooling.”
Age of child proves critical
“Our best estimate is that remote schooling was associated with no difference in mental health difficulties at age 6, and with slightly more difficulties with each year of age after that, with differences most clearly apparent for high school–aged kids,” explained Dr. Hawrilenko, adding the finding suggests that school reopenings should prioritize older children.
However, “what kids are doing at home matters,” he added. “In the youngest age group, the biggest work kids are doing in school and childcare settings is social and emotional development. … Finding opportunities for regular, safe social interactions with peers – perhaps during outdoor playdates – can help them build those skills.”
He emphasized with the anticipated starts and stutters of the new school year there is an important role that doctors can play.
“First, they can help families assess their own risk profile, and whether it makes sense for their children to attend school in person or remotely [to the extent that is an option]. Second, they can help families think through how school closures might impact their child specifically. For those kids who wind up with long chunks of remote schooling, scheduling in regular interactions with other kids in safe ways could make a big difference. Another driver of child anxiety might be learning loss, and this is a good place to reinforce that not every mental health problem needs a mental health solution.
“A lot of kids might be rightfully anxious about having fallen behind over the pandemic. These kids are preparing to transition to college or to the workforce and may be feeling increasingly behind while approaching these moments of transition. Pointing families toward the resources to help them navigate these issues could go a long way to helping quell child anxiety.”
Research helps fill vacuum
Elizabeth A. Stuart, PhD, who was not involved in the study, said in an interview that this research is particularly valuable because there have been very few data on this topic, especially on a large-scale national sample.
“Sadly, many of the results are not surprising,” said Dr. Stuart, a statistician and professor of mental health at Johns Hopkins University, Baltimore. “Data have shown significant mental health challenges for adults during the pandemic, and it is not surprising that children and youth would experience that as well, especially for those whose daily routines and structures changed dramatically and who were not able to be interacting in-person with teachers, staff, and classmates. This is an important reminder that schools provide not just academic instruction for students, but that the social interactions and other services (such as behavioral health supports, meals, and connections with other social services) students might receive in school are crucial.
“It has been heartening to see a stronger commitment to getting students safely back into school this fall across the country, and that the Centers for Disease Control and Prevention highlighted the benefits of in-person schooling in their COVID-19–related guidance for schools.”
Dr. Stuart added that, as students return to classes, it will be important for schools to tackle ongoing mental health challenges.
“Some students may be struggling in obvious ways; for others it may be harder to identify. It will also be important to continue to monitor children’s and youth mental health … as returning to in-person school may bring its own challenges. ”
Dr. Hawrilenko agreed.
“From a policy perspective, I am quite frankly terrified about how these inequities – in particular, learning loss – might play out long after school closures are a distant memory,” he said. “It is critical to provide schools the resources not just to minimize risk when reopening, but additional funding for workforce augmentation – both for mental health staffing and for additional educational support – to help students navigate the months and years over which they transition back into the classroom.”
Dr. Hawrilenko and Dr. Stuart had no disclosures.
Back-to-school jitters are heightened this year, as children head back to the risk of COVID transmission in class, but one upside to the return of in-person school may be better mental health for students.
New research shows that virtual schooling, which dominated in many districts last year, was associated with worse mental health outcomes for students – especially older ones – and youth from Black, Hispanic, or lower-income families were hit hardest because they experienced the most closures.
“Schools with lower funding may have had more difficulty meeting guidelines for safe reopening, including updates to ventilation systems and finding the physical space to create safe distancing between children,” explained lead author Matt Hawrilenko, PhD, of the department of psychiatry and behavioral sciences at the University of Washington, Seattle, in an interview.
“In the context of complex school reopening decisions that balance competing risks and benefits, these findings suggest that allocating funding to support safe in-person instruction may reduce mental health inequities associated with race/ethnicity and income,” he and his coauthors noted in the study, published in JAMA Network Open. “Ensuring that all students have access to additional educational and mental health resources must be an important public health priority, met with appropriate funding and work force augmentation, during and beyond the COVID-19 pandemic.”
The study used a cross-sectional population-based survey of 2,324 parents of school-age children in the United States. It was administered in English and Spanish via web and telephone between Dec. 2 and Dec. 21, 2020, and used the parent-report version of the Strengths and Difficulties Questionnaire (SDQ) to assess mental health difficulties of one child per family in four domains: emotional problems, peer problems, conduct, and hyperactivity. Parents were also asked about what kind of schooling their child had received in the last year (remote, in-person, or hybrid) and about demographic information such as child age, gender, household income, parent race and ethnicity, and parent education.
The results showed that, during the 2020 school year, 58.0% of children attended school remotely, 24% attended fully in person, and 18.0% attended in a hybrid format. “Fully remote schooling was strongly patterned along lines of parent race and ethnicity as well as income,” the authors noted. “Parents of 336 children attending school in person (65.8%) but of 597 children attending school fully remotely (44.5%) were White, whereas all other racial/ethnic groups had larger proportions of children attending school fully remotely (P < .001).”
In terms of mental health, the findings showed that older children who attended school remotely had more difficulties, compared with those who attended in-person – but among younger children, remote learning was comparable or slightly better for mental health.
Specifically, “a child aged 17 years attending school remotely would be expected to have a total difficulty score 2.4 points higher than a child of the same age attending school in person, corresponding to a small effect size in favor of in-person schooling,” the authors wrote. “Conversely, a child aged 4 years attending school remotely would be expected to have a total difficulty score 0.5 points lower than a child of the same age attending school in person, corresponding to a very small effect size in favor of remote schooling.”
Age of child proves critical
“Our best estimate is that remote schooling was associated with no difference in mental health difficulties at age 6, and with slightly more difficulties with each year of age after that, with differences most clearly apparent for high school–aged kids,” explained Dr. Hawrilenko, adding the finding suggests that school reopenings should prioritize older children.
However, “what kids are doing at home matters,” he added. “In the youngest age group, the biggest work kids are doing in school and childcare settings is social and emotional development. … Finding opportunities for regular, safe social interactions with peers – perhaps during outdoor playdates – can help them build those skills.”
He emphasized with the anticipated starts and stutters of the new school year there is an important role that doctors can play.
“First, they can help families assess their own risk profile, and whether it makes sense for their children to attend school in person or remotely [to the extent that is an option]. Second, they can help families think through how school closures might impact their child specifically. For those kids who wind up with long chunks of remote schooling, scheduling in regular interactions with other kids in safe ways could make a big difference. Another driver of child anxiety might be learning loss, and this is a good place to reinforce that not every mental health problem needs a mental health solution.
“A lot of kids might be rightfully anxious about having fallen behind over the pandemic. These kids are preparing to transition to college or to the workforce and may be feeling increasingly behind while approaching these moments of transition. Pointing families toward the resources to help them navigate these issues could go a long way to helping quell child anxiety.”
Research helps fill vacuum
Elizabeth A. Stuart, PhD, who was not involved in the study, said in an interview that this research is particularly valuable because there have been very few data on this topic, especially on a large-scale national sample.
“Sadly, many of the results are not surprising,” said Dr. Stuart, a statistician and professor of mental health at Johns Hopkins University, Baltimore. “Data have shown significant mental health challenges for adults during the pandemic, and it is not surprising that children and youth would experience that as well, especially for those whose daily routines and structures changed dramatically and who were not able to be interacting in-person with teachers, staff, and classmates. This is an important reminder that schools provide not just academic instruction for students, but that the social interactions and other services (such as behavioral health supports, meals, and connections with other social services) students might receive in school are crucial.
“It has been heartening to see a stronger commitment to getting students safely back into school this fall across the country, and that the Centers for Disease Control and Prevention highlighted the benefits of in-person schooling in their COVID-19–related guidance for schools.”
Dr. Stuart added that, as students return to classes, it will be important for schools to tackle ongoing mental health challenges.
“Some students may be struggling in obvious ways; for others it may be harder to identify. It will also be important to continue to monitor children’s and youth mental health … as returning to in-person school may bring its own challenges. ”
Dr. Hawrilenko agreed.
“From a policy perspective, I am quite frankly terrified about how these inequities – in particular, learning loss – might play out long after school closures are a distant memory,” he said. “It is critical to provide schools the resources not just to minimize risk when reopening, but additional funding for workforce augmentation – both for mental health staffing and for additional educational support – to help students navigate the months and years over which they transition back into the classroom.”
Dr. Hawrilenko and Dr. Stuart had no disclosures.
FROM JAMA NETWORK OPEN
COVID-19 linked to rise in suicide-related ED visits among youth
After a steep decline in the first months of the COVID-19 pandemic, youth with suicidal thoughts and behaviors visiting an emergency department (ED) resurged with an overrepresentation among girls and children without a prior psychiatric history, according to a cross-sectional study conducted at Kaiser Permanente Northern California.
The latest findings, published in JAMA Psychiatry, are highly consistent with Centers for Disease Control and Prevention data published a few months earlier in the Morbidity and Mortality Weekly Report that looked at these trends among young people aged 12-25 before and during the pandemic. In addition, the new data suggest that prevention efforts might be particularly helpful for these young people and their families.
“As suicide-related encounters have made up more ED volume during the pandemic, increasing ED-based interventions, staff trained in addressing emergency mental health needs, and aftercare resources may also be valuable in addressing the needs of this population,” wrote Kathryn K. Erickson-Ridout, MD, PhD, first author of the more recent study and an adjunct investigator with the Kaiser Permanente Division of Research, Oakland, Calif.
“While these results suggest many of our youth are coping well through the pandemic, we need to be vigilant as health care workers,” she said in an interview.
Dr. Ridout and associates evaluated suicide-related ED visits among children aged 5-17 years presenting to EDs in the Kaiser Permanente Northern California system. Four periods in 2020 were compared with the same four periods in 2019.
In the first period, March through May 2020, the incidence rate ratio (IRR) of suicide-related ED visits among children and adolescents fell more than 40% (IRR, 0.53; P < .001) relative to the same months in 2019. This is consistent with a broader decline in other types of ED visits during the beginning of the COVID-19 pandemic.
In the periods of June through August and September to Dec. 15, suicide-related ED visits increased, reaching prepandemic levels overall but with differences between genders. In girls, an increase in the first period reached significance (IRR, 1.19; P = .04) and then climbed even higher in the second (hazard ratio, 1.22; P < .001).
Youth present with no prior psychiatric history
Among boys, the increase relative to 2019 in the first period was modest and nonsignificant (IRR, 1.05; P = .26) In the second period, suicide-related ED visits fell and the difference relative to the same period in 2019 reached statistical significance (IRR, 0.81; P = .02).
Other characteristics of suicide-related ED visits during the pandemic were also different from those observed in 2019. One was an increase in visits from youth with no prior history of mental health or suicide-related outpatient visits.
“This finding may suggest that a youth’s first presentation to the ED for suicidal thoughts and behaviors differed during the pandemic, compared to prior to the pandemic,” Dr. Erickson-Ridout explained. “Mental health comorbidities first diagnosed at the ED encounter may suggest an increased complexity at first presentation.”
These data are remarkably similar to ED visits for suicide attempts that were reported by the CDC several months earlier. The CDC data were drawn from the National Syndromic Surveillance Program, which captures data from 71% of the EDs in the United States.
Measured from March 29 to April 25, 2020, ED visits for a suicide attempt declined by more than 25% in both girls and boys, but by early May, those visits were already starting to return to prepandemic levels, particularly among girls, according to the CDC report. When evaluated from July 26 through Aug. 22, 2020, the mean weekly ED visits for suspected suicide attempts had increased 26.2% among girls but only 10.8% among boys, compared with the same period in 2019.
‘More severe distress’ found among girls
In the most recent period evaluated, starting Feb. 21, 2021, and extending to March 20, , compared with the same period in 2019. For boys, the increase was 3.7%.
Higher rates of suicide attempts among girls have been reported by others independent of the COVID-19 pandemic, but the CDC investigators led by Ellen Yard, PhD, a researcher in the CDC’s National Center for Environmental Health, Chamblee, Ga., reported that others have also found greater suicide ideation and attempts by girls during the pandemic.
Neither study was designed to isolate the reason or reasons for an increase in suicide-related ED visits overall or among girls specifically, but Dr. Yard and her coinvestigators speculated that social distancing and isolation during the COVID-19 pandemic might be affecting girls more.
The faster increase in suicide attempts among girls compared with boys “speaks to the importance of upstream prevention to prevent this group from becoming suicidal in the first place as well as improving suicide care both during and after ED visits,” said Dr. Yard, who was interviewed about this research.
For clinicians, she recommended “scaling up adoption of evidence-based best practice.” She cited two such resources from the National Action Alliance for Suicide Prevention (NAASP). One resource is the Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care; the other is called Recommended Standard Care. Both are available online for free from the NAASP.
Dr. Erickson-Ridout and Dr. Yard reported no potential conflicts of interest.
After a steep decline in the first months of the COVID-19 pandemic, youth with suicidal thoughts and behaviors visiting an emergency department (ED) resurged with an overrepresentation among girls and children without a prior psychiatric history, according to a cross-sectional study conducted at Kaiser Permanente Northern California.
The latest findings, published in JAMA Psychiatry, are highly consistent with Centers for Disease Control and Prevention data published a few months earlier in the Morbidity and Mortality Weekly Report that looked at these trends among young people aged 12-25 before and during the pandemic. In addition, the new data suggest that prevention efforts might be particularly helpful for these young people and their families.
“As suicide-related encounters have made up more ED volume during the pandemic, increasing ED-based interventions, staff trained in addressing emergency mental health needs, and aftercare resources may also be valuable in addressing the needs of this population,” wrote Kathryn K. Erickson-Ridout, MD, PhD, first author of the more recent study and an adjunct investigator with the Kaiser Permanente Division of Research, Oakland, Calif.
“While these results suggest many of our youth are coping well through the pandemic, we need to be vigilant as health care workers,” she said in an interview.
Dr. Ridout and associates evaluated suicide-related ED visits among children aged 5-17 years presenting to EDs in the Kaiser Permanente Northern California system. Four periods in 2020 were compared with the same four periods in 2019.
In the first period, March through May 2020, the incidence rate ratio (IRR) of suicide-related ED visits among children and adolescents fell more than 40% (IRR, 0.53; P < .001) relative to the same months in 2019. This is consistent with a broader decline in other types of ED visits during the beginning of the COVID-19 pandemic.
In the periods of June through August and September to Dec. 15, suicide-related ED visits increased, reaching prepandemic levels overall but with differences between genders. In girls, an increase in the first period reached significance (IRR, 1.19; P = .04) and then climbed even higher in the second (hazard ratio, 1.22; P < .001).
Youth present with no prior psychiatric history
Among boys, the increase relative to 2019 in the first period was modest and nonsignificant (IRR, 1.05; P = .26) In the second period, suicide-related ED visits fell and the difference relative to the same period in 2019 reached statistical significance (IRR, 0.81; P = .02).
Other characteristics of suicide-related ED visits during the pandemic were also different from those observed in 2019. One was an increase in visits from youth with no prior history of mental health or suicide-related outpatient visits.
“This finding may suggest that a youth’s first presentation to the ED for suicidal thoughts and behaviors differed during the pandemic, compared to prior to the pandemic,” Dr. Erickson-Ridout explained. “Mental health comorbidities first diagnosed at the ED encounter may suggest an increased complexity at first presentation.”
These data are remarkably similar to ED visits for suicide attempts that were reported by the CDC several months earlier. The CDC data were drawn from the National Syndromic Surveillance Program, which captures data from 71% of the EDs in the United States.
Measured from March 29 to April 25, 2020, ED visits for a suicide attempt declined by more than 25% in both girls and boys, but by early May, those visits were already starting to return to prepandemic levels, particularly among girls, according to the CDC report. When evaluated from July 26 through Aug. 22, 2020, the mean weekly ED visits for suspected suicide attempts had increased 26.2% among girls but only 10.8% among boys, compared with the same period in 2019.
‘More severe distress’ found among girls
In the most recent period evaluated, starting Feb. 21, 2021, and extending to March 20, , compared with the same period in 2019. For boys, the increase was 3.7%.
Higher rates of suicide attempts among girls have been reported by others independent of the COVID-19 pandemic, but the CDC investigators led by Ellen Yard, PhD, a researcher in the CDC’s National Center for Environmental Health, Chamblee, Ga., reported that others have also found greater suicide ideation and attempts by girls during the pandemic.
Neither study was designed to isolate the reason or reasons for an increase in suicide-related ED visits overall or among girls specifically, but Dr. Yard and her coinvestigators speculated that social distancing and isolation during the COVID-19 pandemic might be affecting girls more.
The faster increase in suicide attempts among girls compared with boys “speaks to the importance of upstream prevention to prevent this group from becoming suicidal in the first place as well as improving suicide care both during and after ED visits,” said Dr. Yard, who was interviewed about this research.
For clinicians, she recommended “scaling up adoption of evidence-based best practice.” She cited two such resources from the National Action Alliance for Suicide Prevention (NAASP). One resource is the Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care; the other is called Recommended Standard Care. Both are available online for free from the NAASP.
Dr. Erickson-Ridout and Dr. Yard reported no potential conflicts of interest.
After a steep decline in the first months of the COVID-19 pandemic, youth with suicidal thoughts and behaviors visiting an emergency department (ED) resurged with an overrepresentation among girls and children without a prior psychiatric history, according to a cross-sectional study conducted at Kaiser Permanente Northern California.
The latest findings, published in JAMA Psychiatry, are highly consistent with Centers for Disease Control and Prevention data published a few months earlier in the Morbidity and Mortality Weekly Report that looked at these trends among young people aged 12-25 before and during the pandemic. In addition, the new data suggest that prevention efforts might be particularly helpful for these young people and their families.
“As suicide-related encounters have made up more ED volume during the pandemic, increasing ED-based interventions, staff trained in addressing emergency mental health needs, and aftercare resources may also be valuable in addressing the needs of this population,” wrote Kathryn K. Erickson-Ridout, MD, PhD, first author of the more recent study and an adjunct investigator with the Kaiser Permanente Division of Research, Oakland, Calif.
“While these results suggest many of our youth are coping well through the pandemic, we need to be vigilant as health care workers,” she said in an interview.
Dr. Ridout and associates evaluated suicide-related ED visits among children aged 5-17 years presenting to EDs in the Kaiser Permanente Northern California system. Four periods in 2020 were compared with the same four periods in 2019.
In the first period, March through May 2020, the incidence rate ratio (IRR) of suicide-related ED visits among children and adolescents fell more than 40% (IRR, 0.53; P < .001) relative to the same months in 2019. This is consistent with a broader decline in other types of ED visits during the beginning of the COVID-19 pandemic.
In the periods of June through August and September to Dec. 15, suicide-related ED visits increased, reaching prepandemic levels overall but with differences between genders. In girls, an increase in the first period reached significance (IRR, 1.19; P = .04) and then climbed even higher in the second (hazard ratio, 1.22; P < .001).
Youth present with no prior psychiatric history
Among boys, the increase relative to 2019 in the first period was modest and nonsignificant (IRR, 1.05; P = .26) In the second period, suicide-related ED visits fell and the difference relative to the same period in 2019 reached statistical significance (IRR, 0.81; P = .02).
Other characteristics of suicide-related ED visits during the pandemic were also different from those observed in 2019. One was an increase in visits from youth with no prior history of mental health or suicide-related outpatient visits.
“This finding may suggest that a youth’s first presentation to the ED for suicidal thoughts and behaviors differed during the pandemic, compared to prior to the pandemic,” Dr. Erickson-Ridout explained. “Mental health comorbidities first diagnosed at the ED encounter may suggest an increased complexity at first presentation.”
These data are remarkably similar to ED visits for suicide attempts that were reported by the CDC several months earlier. The CDC data were drawn from the National Syndromic Surveillance Program, which captures data from 71% of the EDs in the United States.
Measured from March 29 to April 25, 2020, ED visits for a suicide attempt declined by more than 25% in both girls and boys, but by early May, those visits were already starting to return to prepandemic levels, particularly among girls, according to the CDC report. When evaluated from July 26 through Aug. 22, 2020, the mean weekly ED visits for suspected suicide attempts had increased 26.2% among girls but only 10.8% among boys, compared with the same period in 2019.
‘More severe distress’ found among girls
In the most recent period evaluated, starting Feb. 21, 2021, and extending to March 20, , compared with the same period in 2019. For boys, the increase was 3.7%.
Higher rates of suicide attempts among girls have been reported by others independent of the COVID-19 pandemic, but the CDC investigators led by Ellen Yard, PhD, a researcher in the CDC’s National Center for Environmental Health, Chamblee, Ga., reported that others have also found greater suicide ideation and attempts by girls during the pandemic.
Neither study was designed to isolate the reason or reasons for an increase in suicide-related ED visits overall or among girls specifically, but Dr. Yard and her coinvestigators speculated that social distancing and isolation during the COVID-19 pandemic might be affecting girls more.
The faster increase in suicide attempts among girls compared with boys “speaks to the importance of upstream prevention to prevent this group from becoming suicidal in the first place as well as improving suicide care both during and after ED visits,” said Dr. Yard, who was interviewed about this research.
For clinicians, she recommended “scaling up adoption of evidence-based best practice.” She cited two such resources from the National Action Alliance for Suicide Prevention (NAASP). One resource is the Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care; the other is called Recommended Standard Care. Both are available online for free from the NAASP.
Dr. Erickson-Ridout and Dr. Yard reported no potential conflicts of interest.
FROM JAMA PSYCHIATRY
FDA inaction on hair loss drug’s suicide, depression, erectile dysfunction risk sparks lawsuit
Consumer advocacy group 4 years ago.
The September 2017 petition requested that the FDA take the popular hair-loss drug (1 mg finasteride, Propecia) off the market because of evidence of serious risk of patient injury, including depression and suicidal ideation.
As an alternative, PFSF requested that the FDA require the drug’s manufacturers revise the safety information on the labeling and add boxed warnings to disclose the potential for side effects, another of which is erectile dysfunction.
Public Citizen points to a recent analysis of the VigiBase global database, which tracks adverse effects from global pharmacovigilance agencies, lists 356 reports of suicidality and 2,926 reports of psychological adverse events in finasteride users. Yet, 4 years after submitting the petition, the FDA has neither granted nor denied it.
The lawsuit claims that FDA has acted unlawfully in failing to act on PFSF’s petition, and further cites “88 cases of completed suicide associated with finasteride use” per data from the VigiBase database.
“On the same day that PFSF submitted the petition, FDA’s docket management division acknowledged receipt and assigned the petition a docket number,” Michael Kirkpatrick, the Public Citizen attorney serving as lead counsel for PFSF, told this news organization.
Yet, to date, “there has been no substantive response to the petition. The lawsuit filed today seeks to force FDA to issue a decision on PFSF’s petition,” Mr. Kirkpatrick said.
“The FDA needs to act in a timely way to protect the public from the risks associated with use of Propecia. The FDA’s failure to act exposes consumers to potentially life-threatening harm,” he added in a statement.
The complaint filed today by Public Citizen in the U.S. District Court for the District of Columbia is available online.
This news organization reached out to the FDA for comment but did not receive a response by press time.
A version of this article first appeared on Medscape.com.
Consumer advocacy group 4 years ago.
The September 2017 petition requested that the FDA take the popular hair-loss drug (1 mg finasteride, Propecia) off the market because of evidence of serious risk of patient injury, including depression and suicidal ideation.
As an alternative, PFSF requested that the FDA require the drug’s manufacturers revise the safety information on the labeling and add boxed warnings to disclose the potential for side effects, another of which is erectile dysfunction.
Public Citizen points to a recent analysis of the VigiBase global database, which tracks adverse effects from global pharmacovigilance agencies, lists 356 reports of suicidality and 2,926 reports of psychological adverse events in finasteride users. Yet, 4 years after submitting the petition, the FDA has neither granted nor denied it.
The lawsuit claims that FDA has acted unlawfully in failing to act on PFSF’s petition, and further cites “88 cases of completed suicide associated with finasteride use” per data from the VigiBase database.
“On the same day that PFSF submitted the petition, FDA’s docket management division acknowledged receipt and assigned the petition a docket number,” Michael Kirkpatrick, the Public Citizen attorney serving as lead counsel for PFSF, told this news organization.
Yet, to date, “there has been no substantive response to the petition. The lawsuit filed today seeks to force FDA to issue a decision on PFSF’s petition,” Mr. Kirkpatrick said.
“The FDA needs to act in a timely way to protect the public from the risks associated with use of Propecia. The FDA’s failure to act exposes consumers to potentially life-threatening harm,” he added in a statement.
The complaint filed today by Public Citizen in the U.S. District Court for the District of Columbia is available online.
This news organization reached out to the FDA for comment but did not receive a response by press time.
A version of this article first appeared on Medscape.com.
Consumer advocacy group 4 years ago.
The September 2017 petition requested that the FDA take the popular hair-loss drug (1 mg finasteride, Propecia) off the market because of evidence of serious risk of patient injury, including depression and suicidal ideation.
As an alternative, PFSF requested that the FDA require the drug’s manufacturers revise the safety information on the labeling and add boxed warnings to disclose the potential for side effects, another of which is erectile dysfunction.
Public Citizen points to a recent analysis of the VigiBase global database, which tracks adverse effects from global pharmacovigilance agencies, lists 356 reports of suicidality and 2,926 reports of psychological adverse events in finasteride users. Yet, 4 years after submitting the petition, the FDA has neither granted nor denied it.
The lawsuit claims that FDA has acted unlawfully in failing to act on PFSF’s petition, and further cites “88 cases of completed suicide associated with finasteride use” per data from the VigiBase database.
“On the same day that PFSF submitted the petition, FDA’s docket management division acknowledged receipt and assigned the petition a docket number,” Michael Kirkpatrick, the Public Citizen attorney serving as lead counsel for PFSF, told this news organization.
Yet, to date, “there has been no substantive response to the petition. The lawsuit filed today seeks to force FDA to issue a decision on PFSF’s petition,” Mr. Kirkpatrick said.
“The FDA needs to act in a timely way to protect the public from the risks associated with use of Propecia. The FDA’s failure to act exposes consumers to potentially life-threatening harm,” he added in a statement.
The complaint filed today by Public Citizen in the U.S. District Court for the District of Columbia is available online.
This news organization reached out to the FDA for comment but did not receive a response by press time.
A version of this article first appeared on Medscape.com.
Atopic dermatitis doubles risk of mental health issues in children
, according to a newly published cohort study of more than 11,000 children between the ages of 3 and 18 years.
Along with previous studies that have also linked AD to depression and other mental health issues in children, these data highlight the need for “clinical awareness of the psychosocial needs of children and adolescents with AD,” reported a multicenter team of investigators from the University of California, San Francisco, the University of Pennsylvania, and the London School of Hygiene and Tropical Medicine.
Unlike some previous studies, in this study, published online in JAMA Dermatology on Sept. 1, children were evaluated longitudinally, rather than at a single point in time, with a mean follow-up of 10 years. For those with active AD, compared with children without AD, the odds ratio for depression overall in any child with AD relative to those without AD was not significant after adjustment for variables such socioeconomic factors.
However, among children with severe AD, the risk was more than twofold greater even after adjustment (adjusted OR, 2.38; 95% confidence interval, 1.21- 4.72), reported the investigators, led by senior author Katrina Abuabara, MD, associate professor of dermatology and epidemiology at UCSF.
Internalizing symptoms seen with mild to severe AD
Internalizing behavior, which is closely linked to depression and describes a spectrum of inward-focusing activities, such as social withdrawal, was significantly more common in children with any degree of AD relative to those without AD: After adjustment, the risk climbed from a 29% increased risk in those with mild AD (aOR, 1.29; 95% CI, 1.06-1.57) to a more than 80% increased risk in children with moderate AD (aOR, 1.84; 95% CI, 1.40-2.41) and in children with severe AD (aOR, 1.90; 95% CI, 1.14-3.16).
In the study, depression was measured with the Short Moods and Feelings Questionnaire (SMFQ). Parental response to the Emotional Symptoms subscale of the Strength and Difficulties Questionnaire (SDQ) was used to measure internalizing behaviors.
The data were drawn from the Avon Longitudinal Study for Parents and Children (ALSPAC), a cohort that enrolled pregnant women in a defined area in southwest England and then followed children born from these pregnancies. Of the 14,062 children enrolled in ALSPAC, data from 11,181 children were available for this study.
In a previous meta-analysis of studies that have documented a link between AD and adverse effects on mood and mental health, an impact was identified in both children and adults. In children, AD was associated with a 27% increase in risk of depression (OR, 1.27; 95% CI, 1.12 -1.45). In adults, the risk was more than doubled (OR, 2.19; 95% CI, 1.87-2.57). The same meta-analysis found that the risk of suicidal ideation among adolescents and adults with AD was increased more than fourfold (OR, 4.32; 95% CI, 1.93-9.66).
In the ALSPAC data, the investigators were unable to find compelling evidence that sleep disturbances or concomitant asthma contributed to the increased risk of depression, which is a mechanism proposed by past investigators.
In an interview, Dr. Abuabara said that these and other data provide the basis for encouraging clinical awareness of the psychological needs of children with AD, but she suggested there is a gap in understanding what this means clinically. “We need more data on how dermatologists can effectively screen and manage these patients before we try to set expectations for clinical practice,” she said.
In addition, these data along with previously published studies suggest that change in mental health outcomes should be included in the evaluation of new therapies, according to Dr. Abuabara. She noted that there are several tools for evaluating mental health in children that might be appropriate, each with their own advantages and disadvantages.
“Ideally, recommendations would be issued through a group consensus process with patients, clinicians, researchers, and industry representatives working together as has been done for other outcomes through the Harmonizing Measures for Eczema (HOME) group,” Dr. Abuabara said.
Mental health assessments recommended
Others who have looked at the relationship between AD and depression have also recommended adding mental health outcomes to an assessment of efficacy for AD therapies.
Jonathan I. Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, is one such investigator. He is already monitoring depression systematically with the Hospital Anxiety and Depression Scale (HADS).
“HADS has been validated in AD and provides very important information about the emotional burden of AD,” explained Dr. Silverberg, whose most recent article on this topic appeared earlier this year. In that study, the relationship between AD and depression was found to be more pronounced in White children from families with lower incomes.
“Just a few hours ago, one of my patients thanked me for asking about their mental health and recognizing the holistic effects of AD,” Dr. Silverberg said.
The recent study based on ALSPAC data add to the evidence that AD, particularly severe AD, produces deleterious effects on mental health in children, and Dr. Silverberg believes clinicians should be acting on this evidence.
“I strongly encourage clinicians to routinely assess mental health. It will elevate the quality of care they provide, and their patients will appreciate them more for it,” he said.
Dr. Abuabara and another author report receiving research funding from Pfizer to their universities for unrelated work; there were no other disclosures. Dr. Silverberg reports financial relationships with more than 15 pharmaceutical companies.
Commentary by Lawrence F. Eichenfield, MD
More severe atopic dermatitis (AD) carries with it significant mental health concerns in children, as well as adults. Multiple studies have shown significantly higher rates of depression, anxiety, and “internalizing behaviors” (discussed as social withdrawal and other inward-focused activities) as well as attention-deficit/hyperactivity disorder. The study by Dr. Abuabara and colleagues is important as it followed children over time (an average of 10 years) and adjusted the data for socioeconomic factors, showing a rate of depression in children with severe AD twice that of those without. It appears that we are in the midst of a mental health crisis in children and teens, with markedly higher rates of pediatric and adolescent depression and anxiety, certainly influenced by COVID-19 societal changes. As the literature has developed on depression and AD, we have appreciated the importance of addressing this as part of our assessment of the disease effect on the individual and family, and it is one factor we consider in selections of systemic vs. topical therapies.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
, according to a newly published cohort study of more than 11,000 children between the ages of 3 and 18 years.
Along with previous studies that have also linked AD to depression and other mental health issues in children, these data highlight the need for “clinical awareness of the psychosocial needs of children and adolescents with AD,” reported a multicenter team of investigators from the University of California, San Francisco, the University of Pennsylvania, and the London School of Hygiene and Tropical Medicine.
Unlike some previous studies, in this study, published online in JAMA Dermatology on Sept. 1, children were evaluated longitudinally, rather than at a single point in time, with a mean follow-up of 10 years. For those with active AD, compared with children without AD, the odds ratio for depression overall in any child with AD relative to those without AD was not significant after adjustment for variables such socioeconomic factors.
However, among children with severe AD, the risk was more than twofold greater even after adjustment (adjusted OR, 2.38; 95% confidence interval, 1.21- 4.72), reported the investigators, led by senior author Katrina Abuabara, MD, associate professor of dermatology and epidemiology at UCSF.
Internalizing symptoms seen with mild to severe AD
Internalizing behavior, which is closely linked to depression and describes a spectrum of inward-focusing activities, such as social withdrawal, was significantly more common in children with any degree of AD relative to those without AD: After adjustment, the risk climbed from a 29% increased risk in those with mild AD (aOR, 1.29; 95% CI, 1.06-1.57) to a more than 80% increased risk in children with moderate AD (aOR, 1.84; 95% CI, 1.40-2.41) and in children with severe AD (aOR, 1.90; 95% CI, 1.14-3.16).
In the study, depression was measured with the Short Moods and Feelings Questionnaire (SMFQ). Parental response to the Emotional Symptoms subscale of the Strength and Difficulties Questionnaire (SDQ) was used to measure internalizing behaviors.
The data were drawn from the Avon Longitudinal Study for Parents and Children (ALSPAC), a cohort that enrolled pregnant women in a defined area in southwest England and then followed children born from these pregnancies. Of the 14,062 children enrolled in ALSPAC, data from 11,181 children were available for this study.
In a previous meta-analysis of studies that have documented a link between AD and adverse effects on mood and mental health, an impact was identified in both children and adults. In children, AD was associated with a 27% increase in risk of depression (OR, 1.27; 95% CI, 1.12 -1.45). In adults, the risk was more than doubled (OR, 2.19; 95% CI, 1.87-2.57). The same meta-analysis found that the risk of suicidal ideation among adolescents and adults with AD was increased more than fourfold (OR, 4.32; 95% CI, 1.93-9.66).
In the ALSPAC data, the investigators were unable to find compelling evidence that sleep disturbances or concomitant asthma contributed to the increased risk of depression, which is a mechanism proposed by past investigators.
In an interview, Dr. Abuabara said that these and other data provide the basis for encouraging clinical awareness of the psychological needs of children with AD, but she suggested there is a gap in understanding what this means clinically. “We need more data on how dermatologists can effectively screen and manage these patients before we try to set expectations for clinical practice,” she said.
In addition, these data along with previously published studies suggest that change in mental health outcomes should be included in the evaluation of new therapies, according to Dr. Abuabara. She noted that there are several tools for evaluating mental health in children that might be appropriate, each with their own advantages and disadvantages.
“Ideally, recommendations would be issued through a group consensus process with patients, clinicians, researchers, and industry representatives working together as has been done for other outcomes through the Harmonizing Measures for Eczema (HOME) group,” Dr. Abuabara said.
Mental health assessments recommended
Others who have looked at the relationship between AD and depression have also recommended adding mental health outcomes to an assessment of efficacy for AD therapies.
Jonathan I. Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, is one such investigator. He is already monitoring depression systematically with the Hospital Anxiety and Depression Scale (HADS).
“HADS has been validated in AD and provides very important information about the emotional burden of AD,” explained Dr. Silverberg, whose most recent article on this topic appeared earlier this year. In that study, the relationship between AD and depression was found to be more pronounced in White children from families with lower incomes.
“Just a few hours ago, one of my patients thanked me for asking about their mental health and recognizing the holistic effects of AD,” Dr. Silverberg said.
The recent study based on ALSPAC data add to the evidence that AD, particularly severe AD, produces deleterious effects on mental health in children, and Dr. Silverberg believes clinicians should be acting on this evidence.
“I strongly encourage clinicians to routinely assess mental health. It will elevate the quality of care they provide, and their patients will appreciate them more for it,” he said.
Dr. Abuabara and another author report receiving research funding from Pfizer to their universities for unrelated work; there were no other disclosures. Dr. Silverberg reports financial relationships with more than 15 pharmaceutical companies.
Commentary by Lawrence F. Eichenfield, MD
More severe atopic dermatitis (AD) carries with it significant mental health concerns in children, as well as adults. Multiple studies have shown significantly higher rates of depression, anxiety, and “internalizing behaviors” (discussed as social withdrawal and other inward-focused activities) as well as attention-deficit/hyperactivity disorder. The study by Dr. Abuabara and colleagues is important as it followed children over time (an average of 10 years) and adjusted the data for socioeconomic factors, showing a rate of depression in children with severe AD twice that of those without. It appears that we are in the midst of a mental health crisis in children and teens, with markedly higher rates of pediatric and adolescent depression and anxiety, certainly influenced by COVID-19 societal changes. As the literature has developed on depression and AD, we have appreciated the importance of addressing this as part of our assessment of the disease effect on the individual and family, and it is one factor we consider in selections of systemic vs. topical therapies.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
, according to a newly published cohort study of more than 11,000 children between the ages of 3 and 18 years.
Along with previous studies that have also linked AD to depression and other mental health issues in children, these data highlight the need for “clinical awareness of the psychosocial needs of children and adolescents with AD,” reported a multicenter team of investigators from the University of California, San Francisco, the University of Pennsylvania, and the London School of Hygiene and Tropical Medicine.
Unlike some previous studies, in this study, published online in JAMA Dermatology on Sept. 1, children were evaluated longitudinally, rather than at a single point in time, with a mean follow-up of 10 years. For those with active AD, compared with children without AD, the odds ratio for depression overall in any child with AD relative to those without AD was not significant after adjustment for variables such socioeconomic factors.
However, among children with severe AD, the risk was more than twofold greater even after adjustment (adjusted OR, 2.38; 95% confidence interval, 1.21- 4.72), reported the investigators, led by senior author Katrina Abuabara, MD, associate professor of dermatology and epidemiology at UCSF.
Internalizing symptoms seen with mild to severe AD
Internalizing behavior, which is closely linked to depression and describes a spectrum of inward-focusing activities, such as social withdrawal, was significantly more common in children with any degree of AD relative to those without AD: After adjustment, the risk climbed from a 29% increased risk in those with mild AD (aOR, 1.29; 95% CI, 1.06-1.57) to a more than 80% increased risk in children with moderate AD (aOR, 1.84; 95% CI, 1.40-2.41) and in children with severe AD (aOR, 1.90; 95% CI, 1.14-3.16).
In the study, depression was measured with the Short Moods and Feelings Questionnaire (SMFQ). Parental response to the Emotional Symptoms subscale of the Strength and Difficulties Questionnaire (SDQ) was used to measure internalizing behaviors.
The data were drawn from the Avon Longitudinal Study for Parents and Children (ALSPAC), a cohort that enrolled pregnant women in a defined area in southwest England and then followed children born from these pregnancies. Of the 14,062 children enrolled in ALSPAC, data from 11,181 children were available for this study.
In a previous meta-analysis of studies that have documented a link between AD and adverse effects on mood and mental health, an impact was identified in both children and adults. In children, AD was associated with a 27% increase in risk of depression (OR, 1.27; 95% CI, 1.12 -1.45). In adults, the risk was more than doubled (OR, 2.19; 95% CI, 1.87-2.57). The same meta-analysis found that the risk of suicidal ideation among adolescents and adults with AD was increased more than fourfold (OR, 4.32; 95% CI, 1.93-9.66).
In the ALSPAC data, the investigators were unable to find compelling evidence that sleep disturbances or concomitant asthma contributed to the increased risk of depression, which is a mechanism proposed by past investigators.
In an interview, Dr. Abuabara said that these and other data provide the basis for encouraging clinical awareness of the psychological needs of children with AD, but she suggested there is a gap in understanding what this means clinically. “We need more data on how dermatologists can effectively screen and manage these patients before we try to set expectations for clinical practice,” she said.
In addition, these data along with previously published studies suggest that change in mental health outcomes should be included in the evaluation of new therapies, according to Dr. Abuabara. She noted that there are several tools for evaluating mental health in children that might be appropriate, each with their own advantages and disadvantages.
“Ideally, recommendations would be issued through a group consensus process with patients, clinicians, researchers, and industry representatives working together as has been done for other outcomes through the Harmonizing Measures for Eczema (HOME) group,” Dr. Abuabara said.
Mental health assessments recommended
Others who have looked at the relationship between AD and depression have also recommended adding mental health outcomes to an assessment of efficacy for AD therapies.
Jonathan I. Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, is one such investigator. He is already monitoring depression systematically with the Hospital Anxiety and Depression Scale (HADS).
“HADS has been validated in AD and provides very important information about the emotional burden of AD,” explained Dr. Silverberg, whose most recent article on this topic appeared earlier this year. In that study, the relationship between AD and depression was found to be more pronounced in White children from families with lower incomes.
“Just a few hours ago, one of my patients thanked me for asking about their mental health and recognizing the holistic effects of AD,” Dr. Silverberg said.
The recent study based on ALSPAC data add to the evidence that AD, particularly severe AD, produces deleterious effects on mental health in children, and Dr. Silverberg believes clinicians should be acting on this evidence.
“I strongly encourage clinicians to routinely assess mental health. It will elevate the quality of care they provide, and their patients will appreciate them more for it,” he said.
Dr. Abuabara and another author report receiving research funding from Pfizer to their universities for unrelated work; there were no other disclosures. Dr. Silverberg reports financial relationships with more than 15 pharmaceutical companies.
Commentary by Lawrence F. Eichenfield, MD
More severe atopic dermatitis (AD) carries with it significant mental health concerns in children, as well as adults. Multiple studies have shown significantly higher rates of depression, anxiety, and “internalizing behaviors” (discussed as social withdrawal and other inward-focused activities) as well as attention-deficit/hyperactivity disorder. The study by Dr. Abuabara and colleagues is important as it followed children over time (an average of 10 years) and adjusted the data for socioeconomic factors, showing a rate of depression in children with severe AD twice that of those without. It appears that we are in the midst of a mental health crisis in children and teens, with markedly higher rates of pediatric and adolescent depression and anxiety, certainly influenced by COVID-19 societal changes. As the literature has developed on depression and AD, we have appreciated the importance of addressing this as part of our assessment of the disease effect on the individual and family, and it is one factor we consider in selections of systemic vs. topical therapies.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
FROM JAMA DERMATOLOGY
The trauma and healing of 9/11 echo in COVID-19
The scope and magnitude of the Sept. 11, 2001, attacks on the World Trade Center and the Pentagon were unprecedented in U.S. history. It was arguably the most serious trauma to beset Americans on U.S. soil. The 20th anniversary of 9/11 will take place during another crisis, not only in American history but also in world history – the COVID-19 pandemic.

“As different as these two events are, there are obvious points of comparison,” Jonathan DePierro, PhD, assistant professor of psychiatry, Icahn School of Medicine at Mount Sinai, New York, said in an interview. “Both were unprecedented life-threatening situations, presenting threats to individuals’ lives and profoundly traumatizing not only society as a whole but also first responders.”
Dr. DePierro, who is also the clinical director of the Center for Stress, Resilience, and Personal Growth at Mount Sinai, thinks there are many lessons to be learned from the mental health response to 9/11 that can inform our understanding of and response to the mental health needs of today’s first responders in the COVID-19 crisis, particularly health care professionals.
“Every one of our hospitals became a ‘ground zero’ early during the pandemic, and we see the numbers rising again and hospitals again overwhelmed, so he said.
Placing trauma within a new framework
According to Priscilla Dass-Brailsford, EdD, MPH, professor of psychology, department of psychiatry, Georgetown University, Washington, Sept. 11, 2001, “placed trauma within a new framework.”
“Prior to 9/11, crisis protocols and how to manage stress in the aftermath of violent events were uncommon,” Dr. Dass-Brailsford, a clinical psychologist with expertise in trauma who also chairs a clinical psychology program for the Chicago School of Professional Psychology, said in an interview.
As a first responder, she was involved in early interventions for survivors of 9/11. On Sept. 11, 2001, she had just resigned her position as coordinator of the community crisis response team – the first of its kind in the United States – through the Victims of Violence Program in Cambridge, in Cambridge, Mass.
The program responded to communities in which there were high rates of drive-by shootings and similar acts of violence. Because of her crisis experience, Dr. Dass-Brailsford was asked to conduct debriefings in Boston in the area where the 9/11 terrorists had stayed prior to boarding the planes that were used in the terrorist attacks. She subsequently went to New York City to conduct similar psychological debriefings with affected communities.
“What we’ve learned is that we had no crisis protocol on how to manage the stress in the aftermath of such a violent event, no standard operating procedures. There were very few people trained in crisis and trauma response at that time. Partially spurred by 9/11, trauma training programs became more prolific,” she said. Dr. Dass-Brailsford developed a trauma certification program at Lesley University in Cambridge, Mass., where she began to teach after 9/11. “I saw the importance of having clinicians trained to respond in a crisis, because responding to a crisis is very different from conducting regular mental health interventions.”
Short- and long-term interventions
Dr. DePierro said that Mount Sinai has a 20-year history of responding to the physical and mental health needs of 9/11 responders.
“We saw a number of first responders experiencing clinical depression, anxiety, a lot of worry, symptoms of posttraumatic stress disorder, and an increase in alcohol and/or substance use,” he recounted. In some, these responses were immediate; in others, the onset of symptoms was more gradual. Some responders had acute reactions that lasted for several months to a year, whereas for others, the reactions were prolonged, and they remained “chronically distressed long after the immediate exposure to the event,” he said.
Recent studies have shown that, during the COVID-19 pandemic, health care professionals and many essential workers have experienced similar symptoms, Dr. DePierro noted.
Mental health care professionals who provided interventions for workers involved in recovery and cleanup at the World Trade Center have highlighted the need for long-term monitoring of people on the front lines during the COVID-19 pandemic – especially health care workers, other essential personnel (for example, delivery, postal, and grocery store workers) and surviving family members. “Health monitoring and treatment efforts for 9/11 survivors and responders were put into place soon after the attacks and continue to this day,” using funding provided through the James Zadroga Act, Dr. DePierro said.
“Without similarly unified health registry and treatment services, many individuals – especially from underserved groups – will likely experience chronic mental health consequences and will be unable to access high-quality health care services,” he stated.
‘Psychological first aid’
“Although many people who go through a crisis – whether as a result of terrorism, such as 9/11, or a medical crisis, such as the current pandemic, or a natural disaster, such as Hurricane Katrina – experience PTSD, it’s important to note that not everyone who goes through a crisis and is traumatized will go on to develop PTSD,” Dr. Dass-Brailsford emphasized.
“To me, 9/11 placed psychological first aid on the map. Even if you are not a clinician, you can be trained to provide psychological first aid by becoming familiar with people’s reactions to trauma and how you can support them through it,” she continued.
For example, if a coworker is agitated or “seems to be having a meltdown, you can be there by offering support and getting them the appropriate help.” Research has suggested that having social support before and after a traumatic event can be helpful in determining vulnerability to the development of PTSD and in modulating the impact of the trauma.
Psychological first aid is helpful as an interim measure. “If you see a coworker holding their head in their hands all day and staring at the screen, identifying whether the person might be having a dissociative episode is critical. Providing some support is important, but if more intensive professional support is needed, determining that and making a referral becomes key,” Dr. Dass-Brailsford stated.
Dr. DePierro added: “One of the most important messages that I want health care workers to know from my years of working with 9/11 survivors is that feeling distressed after a traumatic event is very common, but with effective care, one doesn’t necessarily need to be in treatment for years.”
Danielle Ofri, MD, PhD, clinical professor, department of medicine, New York University, agreed. “It is important to continue keeping tabs on each other and remaining sensitive to the collateral struggles of our colleagues. Some have children who are struggling in school, others have parents who have lost a job. Continuing to check in on others and offer support is critical going forward,” she said in an interview.
Cohesiveness and volunteerism
One of the most powerful antidotes to long-term traumatization is a sense of community cohesiveness. This was the case following 9/11, and it is the case during the COVID-19 pandemic, according to Dr. Ofri, an internist at Bellevue Hospital in New York.
“There was an enormous mobilization. Bellevue is a city hospital with a level 1 trauma center, and we expected to be swamped, so the whole hospital shifted into gear,” said Dr. Ofri. “What would have been terrifying seemed tolerable because we felt that we were in it together. We discharged the inpatients to make beds available. Within hours, we had converted clinics into emergency departments and ICUs. We worked seamlessly, and the crisis brought us together ... but then, of course, no patients showed up.”
She described her relationship with her colleagues as “feeling almost like a family, especially during the pandemic, when so many others were in lockdown and feeling isolated and useless.”
She and her colleagues saw each other daily. Although the content of their tasks and responsibilities changed and people were redeployed to other areas, “our workday didn’t really change. It would have been overwhelming if we hadn’t had our daily meetings to regroup and assess where we were. Each day, everything we had learned or implemented the day before – treatment protocols, testing protocols, our understanding of how the virus was communicated – would change and need to be reevaluated. Those morning meetings were critical to staying centered. It felt as though we were building a plane and flying it at the same time, which felt both scary and heady. Luckily, it took place within the fraternity of a committed and caring group.”
Dr. Ofri recounted that, after 9/11, as well as during the pandemic, “professionals kept jumping in from the sidelines to volunteer. Within hours of the collapse of the towers, the ED had filled with staff. People came out of retirement and out from vacation and out of the woodwork. It was very heartening.”
Even more inspiring, “all the departmental barriers seemed to break down. People were willing to step out of their ordinary roles and check their egos at the door. Seasoned physicians were willing to function as medical interns.”
This generosity of time and spirit “helped keep us going,” she said.
Dr. DePierro agreed. “One of the things I’ve seen on medical floors is that COVID actually brought some units together, increasing their cohesion and mutual support and increasing the bonds between people.” These intensified bonds “increased the resilience of everyone involved.”
Commitment to the community
Dr. Ofri recalls families gathering at the hospital after 9/11, watching posters of missing people going up all over the hospital as well as on mailboxes and lampposts. Because the center for missing people was located right next door to Bellevue, there were long lines of families coming in to register. The chief medical office was there, and a huge tent was built to accommodate the families. The tent took up the entire block. “We felt a lot of ownership, because families were coming here,” she said.
The street remained closed even as the days, weeks, and years stretched on, and the tent remained. It was used as a reflection area for families. During the pandemic, that area was used for refrigerated trucks that served as temporary morgues.
“Both logistically and emotionally, we had a feeling during the pandemic of, ‘We’ve been here before, we’ll do it again and be there for the community,’ ” Dr. Ofri said.
She noted that the sense of commitment to the community carried her and fellow clinicians through the toughest parts of 9/11 and of the COVID-19 pandemic.
“People look to the medical system as a lodestar. ‘Where’s my family member? What should I do? Should I be tested? Vaccinated?’ We were there to be a steady presence for the community physically, psychologically, emotionally, and medically, which helped center us as well,” Dr. Ofri said. “If we didn’t have that, we might have all given in to existential panic.”
She added: “Although we had to work twice as hard, often amid great personal risk, we had the good fortune of having a sense of purpose, something to contribute, plus the community of colleagues we cared about and trusted with our lives.”
Crisis and personal growth
Dr. DePierro said that participants who went through 9/11 have been coming to Mount Sinai’s World Trade Center Health Program for care for nearly two decades. “Many are doing quite well, despite the emotional trauma and the dust and toxin exposure, which has given us a window into what makes people resilient.”
Social and community support are key factors in resilience. Another is recognizing opportunities for personal or professional growth during the crisis, according to Dr. DePierro.
During the pandemic, hospital staff were redeployed to departments where they didn’t typically work. They worked with new colleagues and used skills in patient care that they hadn’t needed for years or even decades. “Although this was stressful and distressing, quite a number said they came through with more medical knowledge than before and that they had forged relationships in the trenches that have been lasting and have become important to them,” he reported.
He noted that, during both crises, for first responders and health care practitioners, religious or spiritual faith was a source of resilience. “During the peak of the pandemic, chaplains provided an exorbitant amount of staff support as clinicians turned to the chaplain to help make sense of what they were going through and connect to something greater than themselves.” Similarly, during 9/11, police and fire department chaplains “played a huge role in supporting the first responders,” Dr. DePierro said.
He said that Mount Sinai holds resilience workshops “where we focus on these topics and teach health care workers how to build resilience in their lives, heal day-to-day stressors, and even grow from the experience.”
Dr. Ofri, who is the founder and editor-in-chief of the Bellevue Literary Review, added that the arts played an important role in bolstering resilience and providing a creative outlet for clinicians after 9/11 and again during the pandemic.
The publication is celebrating its twentieth anniversary – its first issue went to press in September 2001. The cover contained an acknowledgment of 9/11.
Dr. Ofri said that a gala event had been planned for Oct. 7, 2001, to celebrate the inaugural issue of the publication. She assumed no one would show up, given that the United States had invaded Afghanistan only hours earlier. To her surprise, over a hundred people attended, “which made me realize the role of the arts during trauma. People were seeking to come together and hear poetry, fiction, and creative nonfiction.”
Dr. Ofri has been “impressed by the amount of incredible creative writing of all sorts that has been submitted [to the publication] during the pandemic, an unexpected flowering of the arts.”
Unique challenges, unique opportunities
All three experts pointed to several noteworthy differences between the experiences of first responders following 9/11 and those of today’s health care professionals during the pandemic.
“What happened on Sept. 11 was one discrete event, and although it obviously led to years of recovering body parts and cleaning up Ground Zero, and on a national level it led to a war, it nevertheless was a single event,” Dr. DePierro observed. By contrast, the COVID-19 pandemic is ongoing, and for health care practitioners, “it’s by no means over. Again and again, they are being thrown back into battle, dealing with fatigue, weariness, and loss of life.”
Moreover, “it is my understanding that immediately following 9/11, there was a general coming together in our country, but it’s obvious that today, there’s a great deal of fractiousness, contention, disagreement, and disunity in our country when it comes to COVID-19,” Dr. DePierro continued.
“This takes a great toll, particularly on health care workers who are dealing with COVID-19 on a daily basis and experience a disconnect between what they see on their floors and ICUs of the hospital, experiencing loss of life they’ve likely never encountered in their careers, and what people are saying when they downplay the seriousness of COVID-19,” he said.
Dr. Ofri agreed. “The fragmentation of our country and the failure of leadership at the highest level to provide even the basics, such as PPE [personal protective equipment] for health care professionals, left us baffled, profoundly hurt, and angry.”
A positive difference between the COVID-19 pandemic and the aftermath of 9/11 is the development of sophisticated technology that allows interventions for traumatized individuals – both health care professionals and the general public – through telehealth, Dr. DePierro pointed out.
“I would say that these resources and technologies are a silver lining and should continue to be expanded on,” he said. “Now, busy health care workers can access all manner of supportive services, including teletherapy, right from home or between shifts.”
Another “silver lining” is that the pandemic has shone a spotlight on an issue that predated the pandemic – the mental health of health care professionals. Opening a discussion about this has reduced stigma and hopefully has paved the way for improved treatments and for providing resources.
Dr. Dass-Brailsford added that “it is important, going forward, for all of us to be trauma informed, to know how trauma and trauma-related stress unfolds in both other people and yourself, and to know what coping skills can be used to avoid crises from developing – a task that extends across all types of disasters.”
A version of this article first appeared on Medscape.com.
The scope and magnitude of the Sept. 11, 2001, attacks on the World Trade Center and the Pentagon were unprecedented in U.S. history. It was arguably the most serious trauma to beset Americans on U.S. soil. The 20th anniversary of 9/11 will take place during another crisis, not only in American history but also in world history – the COVID-19 pandemic.

“As different as these two events are, there are obvious points of comparison,” Jonathan DePierro, PhD, assistant professor of psychiatry, Icahn School of Medicine at Mount Sinai, New York, said in an interview. “Both were unprecedented life-threatening situations, presenting threats to individuals’ lives and profoundly traumatizing not only society as a whole but also first responders.”
Dr. DePierro, who is also the clinical director of the Center for Stress, Resilience, and Personal Growth at Mount Sinai, thinks there are many lessons to be learned from the mental health response to 9/11 that can inform our understanding of and response to the mental health needs of today’s first responders in the COVID-19 crisis, particularly health care professionals.
“Every one of our hospitals became a ‘ground zero’ early during the pandemic, and we see the numbers rising again and hospitals again overwhelmed, so he said.
Placing trauma within a new framework
According to Priscilla Dass-Brailsford, EdD, MPH, professor of psychology, department of psychiatry, Georgetown University, Washington, Sept. 11, 2001, “placed trauma within a new framework.”
“Prior to 9/11, crisis protocols and how to manage stress in the aftermath of violent events were uncommon,” Dr. Dass-Brailsford, a clinical psychologist with expertise in trauma who also chairs a clinical psychology program for the Chicago School of Professional Psychology, said in an interview.
As a first responder, she was involved in early interventions for survivors of 9/11. On Sept. 11, 2001, she had just resigned her position as coordinator of the community crisis response team – the first of its kind in the United States – through the Victims of Violence Program in Cambridge, in Cambridge, Mass.
The program responded to communities in which there were high rates of drive-by shootings and similar acts of violence. Because of her crisis experience, Dr. Dass-Brailsford was asked to conduct debriefings in Boston in the area where the 9/11 terrorists had stayed prior to boarding the planes that were used in the terrorist attacks. She subsequently went to New York City to conduct similar psychological debriefings with affected communities.
“What we’ve learned is that we had no crisis protocol on how to manage the stress in the aftermath of such a violent event, no standard operating procedures. There were very few people trained in crisis and trauma response at that time. Partially spurred by 9/11, trauma training programs became more prolific,” she said. Dr. Dass-Brailsford developed a trauma certification program at Lesley University in Cambridge, Mass., where she began to teach after 9/11. “I saw the importance of having clinicians trained to respond in a crisis, because responding to a crisis is very different from conducting regular mental health interventions.”
Short- and long-term interventions
Dr. DePierro said that Mount Sinai has a 20-year history of responding to the physical and mental health needs of 9/11 responders.
“We saw a number of first responders experiencing clinical depression, anxiety, a lot of worry, symptoms of posttraumatic stress disorder, and an increase in alcohol and/or substance use,” he recounted. In some, these responses were immediate; in others, the onset of symptoms was more gradual. Some responders had acute reactions that lasted for several months to a year, whereas for others, the reactions were prolonged, and they remained “chronically distressed long after the immediate exposure to the event,” he said.
Recent studies have shown that, during the COVID-19 pandemic, health care professionals and many essential workers have experienced similar symptoms, Dr. DePierro noted.
Mental health care professionals who provided interventions for workers involved in recovery and cleanup at the World Trade Center have highlighted the need for long-term monitoring of people on the front lines during the COVID-19 pandemic – especially health care workers, other essential personnel (for example, delivery, postal, and grocery store workers) and surviving family members. “Health monitoring and treatment efforts for 9/11 survivors and responders were put into place soon after the attacks and continue to this day,” using funding provided through the James Zadroga Act, Dr. DePierro said.
“Without similarly unified health registry and treatment services, many individuals – especially from underserved groups – will likely experience chronic mental health consequences and will be unable to access high-quality health care services,” he stated.
‘Psychological first aid’
“Although many people who go through a crisis – whether as a result of terrorism, such as 9/11, or a medical crisis, such as the current pandemic, or a natural disaster, such as Hurricane Katrina – experience PTSD, it’s important to note that not everyone who goes through a crisis and is traumatized will go on to develop PTSD,” Dr. Dass-Brailsford emphasized.
“To me, 9/11 placed psychological first aid on the map. Even if you are not a clinician, you can be trained to provide psychological first aid by becoming familiar with people’s reactions to trauma and how you can support them through it,” she continued.
For example, if a coworker is agitated or “seems to be having a meltdown, you can be there by offering support and getting them the appropriate help.” Research has suggested that having social support before and after a traumatic event can be helpful in determining vulnerability to the development of PTSD and in modulating the impact of the trauma.
Psychological first aid is helpful as an interim measure. “If you see a coworker holding their head in their hands all day and staring at the screen, identifying whether the person might be having a dissociative episode is critical. Providing some support is important, but if more intensive professional support is needed, determining that and making a referral becomes key,” Dr. Dass-Brailsford stated.
Dr. DePierro added: “One of the most important messages that I want health care workers to know from my years of working with 9/11 survivors is that feeling distressed after a traumatic event is very common, but with effective care, one doesn’t necessarily need to be in treatment for years.”
Danielle Ofri, MD, PhD, clinical professor, department of medicine, New York University, agreed. “It is important to continue keeping tabs on each other and remaining sensitive to the collateral struggles of our colleagues. Some have children who are struggling in school, others have parents who have lost a job. Continuing to check in on others and offer support is critical going forward,” she said in an interview.
Cohesiveness and volunteerism
One of the most powerful antidotes to long-term traumatization is a sense of community cohesiveness. This was the case following 9/11, and it is the case during the COVID-19 pandemic, according to Dr. Ofri, an internist at Bellevue Hospital in New York.
“There was an enormous mobilization. Bellevue is a city hospital with a level 1 trauma center, and we expected to be swamped, so the whole hospital shifted into gear,” said Dr. Ofri. “What would have been terrifying seemed tolerable because we felt that we were in it together. We discharged the inpatients to make beds available. Within hours, we had converted clinics into emergency departments and ICUs. We worked seamlessly, and the crisis brought us together ... but then, of course, no patients showed up.”
She described her relationship with her colleagues as “feeling almost like a family, especially during the pandemic, when so many others were in lockdown and feeling isolated and useless.”
She and her colleagues saw each other daily. Although the content of their tasks and responsibilities changed and people were redeployed to other areas, “our workday didn’t really change. It would have been overwhelming if we hadn’t had our daily meetings to regroup and assess where we were. Each day, everything we had learned or implemented the day before – treatment protocols, testing protocols, our understanding of how the virus was communicated – would change and need to be reevaluated. Those morning meetings were critical to staying centered. It felt as though we were building a plane and flying it at the same time, which felt both scary and heady. Luckily, it took place within the fraternity of a committed and caring group.”
Dr. Ofri recounted that, after 9/11, as well as during the pandemic, “professionals kept jumping in from the sidelines to volunteer. Within hours of the collapse of the towers, the ED had filled with staff. People came out of retirement and out from vacation and out of the woodwork. It was very heartening.”
Even more inspiring, “all the departmental barriers seemed to break down. People were willing to step out of their ordinary roles and check their egos at the door. Seasoned physicians were willing to function as medical interns.”
This generosity of time and spirit “helped keep us going,” she said.
Dr. DePierro agreed. “One of the things I’ve seen on medical floors is that COVID actually brought some units together, increasing their cohesion and mutual support and increasing the bonds between people.” These intensified bonds “increased the resilience of everyone involved.”
Commitment to the community
Dr. Ofri recalls families gathering at the hospital after 9/11, watching posters of missing people going up all over the hospital as well as on mailboxes and lampposts. Because the center for missing people was located right next door to Bellevue, there were long lines of families coming in to register. The chief medical office was there, and a huge tent was built to accommodate the families. The tent took up the entire block. “We felt a lot of ownership, because families were coming here,” she said.
The street remained closed even as the days, weeks, and years stretched on, and the tent remained. It was used as a reflection area for families. During the pandemic, that area was used for refrigerated trucks that served as temporary morgues.
“Both logistically and emotionally, we had a feeling during the pandemic of, ‘We’ve been here before, we’ll do it again and be there for the community,’ ” Dr. Ofri said.
She noted that the sense of commitment to the community carried her and fellow clinicians through the toughest parts of 9/11 and of the COVID-19 pandemic.
“People look to the medical system as a lodestar. ‘Where’s my family member? What should I do? Should I be tested? Vaccinated?’ We were there to be a steady presence for the community physically, psychologically, emotionally, and medically, which helped center us as well,” Dr. Ofri said. “If we didn’t have that, we might have all given in to existential panic.”
She added: “Although we had to work twice as hard, often amid great personal risk, we had the good fortune of having a sense of purpose, something to contribute, plus the community of colleagues we cared about and trusted with our lives.”
Crisis and personal growth
Dr. DePierro said that participants who went through 9/11 have been coming to Mount Sinai’s World Trade Center Health Program for care for nearly two decades. “Many are doing quite well, despite the emotional trauma and the dust and toxin exposure, which has given us a window into what makes people resilient.”
Social and community support are key factors in resilience. Another is recognizing opportunities for personal or professional growth during the crisis, according to Dr. DePierro.
During the pandemic, hospital staff were redeployed to departments where they didn’t typically work. They worked with new colleagues and used skills in patient care that they hadn’t needed for years or even decades. “Although this was stressful and distressing, quite a number said they came through with more medical knowledge than before and that they had forged relationships in the trenches that have been lasting and have become important to them,” he reported.
He noted that, during both crises, for first responders and health care practitioners, religious or spiritual faith was a source of resilience. “During the peak of the pandemic, chaplains provided an exorbitant amount of staff support as clinicians turned to the chaplain to help make sense of what they were going through and connect to something greater than themselves.” Similarly, during 9/11, police and fire department chaplains “played a huge role in supporting the first responders,” Dr. DePierro said.
He said that Mount Sinai holds resilience workshops “where we focus on these topics and teach health care workers how to build resilience in their lives, heal day-to-day stressors, and even grow from the experience.”
Dr. Ofri, who is the founder and editor-in-chief of the Bellevue Literary Review, added that the arts played an important role in bolstering resilience and providing a creative outlet for clinicians after 9/11 and again during the pandemic.
The publication is celebrating its twentieth anniversary – its first issue went to press in September 2001. The cover contained an acknowledgment of 9/11.
Dr. Ofri said that a gala event had been planned for Oct. 7, 2001, to celebrate the inaugural issue of the publication. She assumed no one would show up, given that the United States had invaded Afghanistan only hours earlier. To her surprise, over a hundred people attended, “which made me realize the role of the arts during trauma. People were seeking to come together and hear poetry, fiction, and creative nonfiction.”
Dr. Ofri has been “impressed by the amount of incredible creative writing of all sorts that has been submitted [to the publication] during the pandemic, an unexpected flowering of the arts.”
Unique challenges, unique opportunities
All three experts pointed to several noteworthy differences between the experiences of first responders following 9/11 and those of today’s health care professionals during the pandemic.
“What happened on Sept. 11 was one discrete event, and although it obviously led to years of recovering body parts and cleaning up Ground Zero, and on a national level it led to a war, it nevertheless was a single event,” Dr. DePierro observed. By contrast, the COVID-19 pandemic is ongoing, and for health care practitioners, “it’s by no means over. Again and again, they are being thrown back into battle, dealing with fatigue, weariness, and loss of life.”
Moreover, “it is my understanding that immediately following 9/11, there was a general coming together in our country, but it’s obvious that today, there’s a great deal of fractiousness, contention, disagreement, and disunity in our country when it comes to COVID-19,” Dr. DePierro continued.
“This takes a great toll, particularly on health care workers who are dealing with COVID-19 on a daily basis and experience a disconnect between what they see on their floors and ICUs of the hospital, experiencing loss of life they’ve likely never encountered in their careers, and what people are saying when they downplay the seriousness of COVID-19,” he said.
Dr. Ofri agreed. “The fragmentation of our country and the failure of leadership at the highest level to provide even the basics, such as PPE [personal protective equipment] for health care professionals, left us baffled, profoundly hurt, and angry.”
A positive difference between the COVID-19 pandemic and the aftermath of 9/11 is the development of sophisticated technology that allows interventions for traumatized individuals – both health care professionals and the general public – through telehealth, Dr. DePierro pointed out.
“I would say that these resources and technologies are a silver lining and should continue to be expanded on,” he said. “Now, busy health care workers can access all manner of supportive services, including teletherapy, right from home or between shifts.”
Another “silver lining” is that the pandemic has shone a spotlight on an issue that predated the pandemic – the mental health of health care professionals. Opening a discussion about this has reduced stigma and hopefully has paved the way for improved treatments and for providing resources.
Dr. Dass-Brailsford added that “it is important, going forward, for all of us to be trauma informed, to know how trauma and trauma-related stress unfolds in both other people and yourself, and to know what coping skills can be used to avoid crises from developing – a task that extends across all types of disasters.”
A version of this article first appeared on Medscape.com.
The scope and magnitude of the Sept. 11, 2001, attacks on the World Trade Center and the Pentagon were unprecedented in U.S. history. It was arguably the most serious trauma to beset Americans on U.S. soil. The 20th anniversary of 9/11 will take place during another crisis, not only in American history but also in world history – the COVID-19 pandemic.

“As different as these two events are, there are obvious points of comparison,” Jonathan DePierro, PhD, assistant professor of psychiatry, Icahn School of Medicine at Mount Sinai, New York, said in an interview. “Both were unprecedented life-threatening situations, presenting threats to individuals’ lives and profoundly traumatizing not only society as a whole but also first responders.”
Dr. DePierro, who is also the clinical director of the Center for Stress, Resilience, and Personal Growth at Mount Sinai, thinks there are many lessons to be learned from the mental health response to 9/11 that can inform our understanding of and response to the mental health needs of today’s first responders in the COVID-19 crisis, particularly health care professionals.
“Every one of our hospitals became a ‘ground zero’ early during the pandemic, and we see the numbers rising again and hospitals again overwhelmed, so he said.
Placing trauma within a new framework
According to Priscilla Dass-Brailsford, EdD, MPH, professor of psychology, department of psychiatry, Georgetown University, Washington, Sept. 11, 2001, “placed trauma within a new framework.”
“Prior to 9/11, crisis protocols and how to manage stress in the aftermath of violent events were uncommon,” Dr. Dass-Brailsford, a clinical psychologist with expertise in trauma who also chairs a clinical psychology program for the Chicago School of Professional Psychology, said in an interview.
As a first responder, she was involved in early interventions for survivors of 9/11. On Sept. 11, 2001, she had just resigned her position as coordinator of the community crisis response team – the first of its kind in the United States – through the Victims of Violence Program in Cambridge, in Cambridge, Mass.
The program responded to communities in which there were high rates of drive-by shootings and similar acts of violence. Because of her crisis experience, Dr. Dass-Brailsford was asked to conduct debriefings in Boston in the area where the 9/11 terrorists had stayed prior to boarding the planes that were used in the terrorist attacks. She subsequently went to New York City to conduct similar psychological debriefings with affected communities.
“What we’ve learned is that we had no crisis protocol on how to manage the stress in the aftermath of such a violent event, no standard operating procedures. There were very few people trained in crisis and trauma response at that time. Partially spurred by 9/11, trauma training programs became more prolific,” she said. Dr. Dass-Brailsford developed a trauma certification program at Lesley University in Cambridge, Mass., where she began to teach after 9/11. “I saw the importance of having clinicians trained to respond in a crisis, because responding to a crisis is very different from conducting regular mental health interventions.”
Short- and long-term interventions
Dr. DePierro said that Mount Sinai has a 20-year history of responding to the physical and mental health needs of 9/11 responders.
“We saw a number of first responders experiencing clinical depression, anxiety, a lot of worry, symptoms of posttraumatic stress disorder, and an increase in alcohol and/or substance use,” he recounted. In some, these responses were immediate; in others, the onset of symptoms was more gradual. Some responders had acute reactions that lasted for several months to a year, whereas for others, the reactions were prolonged, and they remained “chronically distressed long after the immediate exposure to the event,” he said.
Recent studies have shown that, during the COVID-19 pandemic, health care professionals and many essential workers have experienced similar symptoms, Dr. DePierro noted.
Mental health care professionals who provided interventions for workers involved in recovery and cleanup at the World Trade Center have highlighted the need for long-term monitoring of people on the front lines during the COVID-19 pandemic – especially health care workers, other essential personnel (for example, delivery, postal, and grocery store workers) and surviving family members. “Health monitoring and treatment efforts for 9/11 survivors and responders were put into place soon after the attacks and continue to this day,” using funding provided through the James Zadroga Act, Dr. DePierro said.
“Without similarly unified health registry and treatment services, many individuals – especially from underserved groups – will likely experience chronic mental health consequences and will be unable to access high-quality health care services,” he stated.
‘Psychological first aid’
“Although many people who go through a crisis – whether as a result of terrorism, such as 9/11, or a medical crisis, such as the current pandemic, or a natural disaster, such as Hurricane Katrina – experience PTSD, it’s important to note that not everyone who goes through a crisis and is traumatized will go on to develop PTSD,” Dr. Dass-Brailsford emphasized.
“To me, 9/11 placed psychological first aid on the map. Even if you are not a clinician, you can be trained to provide psychological first aid by becoming familiar with people’s reactions to trauma and how you can support them through it,” she continued.
For example, if a coworker is agitated or “seems to be having a meltdown, you can be there by offering support and getting them the appropriate help.” Research has suggested that having social support before and after a traumatic event can be helpful in determining vulnerability to the development of PTSD and in modulating the impact of the trauma.
Psychological first aid is helpful as an interim measure. “If you see a coworker holding their head in their hands all day and staring at the screen, identifying whether the person might be having a dissociative episode is critical. Providing some support is important, but if more intensive professional support is needed, determining that and making a referral becomes key,” Dr. Dass-Brailsford stated.
Dr. DePierro added: “One of the most important messages that I want health care workers to know from my years of working with 9/11 survivors is that feeling distressed after a traumatic event is very common, but with effective care, one doesn’t necessarily need to be in treatment for years.”
Danielle Ofri, MD, PhD, clinical professor, department of medicine, New York University, agreed. “It is important to continue keeping tabs on each other and remaining sensitive to the collateral struggles of our colleagues. Some have children who are struggling in school, others have parents who have lost a job. Continuing to check in on others and offer support is critical going forward,” she said in an interview.
Cohesiveness and volunteerism
One of the most powerful antidotes to long-term traumatization is a sense of community cohesiveness. This was the case following 9/11, and it is the case during the COVID-19 pandemic, according to Dr. Ofri, an internist at Bellevue Hospital in New York.
“There was an enormous mobilization. Bellevue is a city hospital with a level 1 trauma center, and we expected to be swamped, so the whole hospital shifted into gear,” said Dr. Ofri. “What would have been terrifying seemed tolerable because we felt that we were in it together. We discharged the inpatients to make beds available. Within hours, we had converted clinics into emergency departments and ICUs. We worked seamlessly, and the crisis brought us together ... but then, of course, no patients showed up.”
She described her relationship with her colleagues as “feeling almost like a family, especially during the pandemic, when so many others were in lockdown and feeling isolated and useless.”
She and her colleagues saw each other daily. Although the content of their tasks and responsibilities changed and people were redeployed to other areas, “our workday didn’t really change. It would have been overwhelming if we hadn’t had our daily meetings to regroup and assess where we were. Each day, everything we had learned or implemented the day before – treatment protocols, testing protocols, our understanding of how the virus was communicated – would change and need to be reevaluated. Those morning meetings were critical to staying centered. It felt as though we were building a plane and flying it at the same time, which felt both scary and heady. Luckily, it took place within the fraternity of a committed and caring group.”
Dr. Ofri recounted that, after 9/11, as well as during the pandemic, “professionals kept jumping in from the sidelines to volunteer. Within hours of the collapse of the towers, the ED had filled with staff. People came out of retirement and out from vacation and out of the woodwork. It was very heartening.”
Even more inspiring, “all the departmental barriers seemed to break down. People were willing to step out of their ordinary roles and check their egos at the door. Seasoned physicians were willing to function as medical interns.”
This generosity of time and spirit “helped keep us going,” she said.
Dr. DePierro agreed. “One of the things I’ve seen on medical floors is that COVID actually brought some units together, increasing their cohesion and mutual support and increasing the bonds between people.” These intensified bonds “increased the resilience of everyone involved.”
Commitment to the community
Dr. Ofri recalls families gathering at the hospital after 9/11, watching posters of missing people going up all over the hospital as well as on mailboxes and lampposts. Because the center for missing people was located right next door to Bellevue, there were long lines of families coming in to register. The chief medical office was there, and a huge tent was built to accommodate the families. The tent took up the entire block. “We felt a lot of ownership, because families were coming here,” she said.
The street remained closed even as the days, weeks, and years stretched on, and the tent remained. It was used as a reflection area for families. During the pandemic, that area was used for refrigerated trucks that served as temporary morgues.
“Both logistically and emotionally, we had a feeling during the pandemic of, ‘We’ve been here before, we’ll do it again and be there for the community,’ ” Dr. Ofri said.
She noted that the sense of commitment to the community carried her and fellow clinicians through the toughest parts of 9/11 and of the COVID-19 pandemic.
“People look to the medical system as a lodestar. ‘Where’s my family member? What should I do? Should I be tested? Vaccinated?’ We were there to be a steady presence for the community physically, psychologically, emotionally, and medically, which helped center us as well,” Dr. Ofri said. “If we didn’t have that, we might have all given in to existential panic.”
She added: “Although we had to work twice as hard, often amid great personal risk, we had the good fortune of having a sense of purpose, something to contribute, plus the community of colleagues we cared about and trusted with our lives.”
Crisis and personal growth
Dr. DePierro said that participants who went through 9/11 have been coming to Mount Sinai’s World Trade Center Health Program for care for nearly two decades. “Many are doing quite well, despite the emotional trauma and the dust and toxin exposure, which has given us a window into what makes people resilient.”
Social and community support are key factors in resilience. Another is recognizing opportunities for personal or professional growth during the crisis, according to Dr. DePierro.
During the pandemic, hospital staff were redeployed to departments where they didn’t typically work. They worked with new colleagues and used skills in patient care that they hadn’t needed for years or even decades. “Although this was stressful and distressing, quite a number said they came through with more medical knowledge than before and that they had forged relationships in the trenches that have been lasting and have become important to them,” he reported.
He noted that, during both crises, for first responders and health care practitioners, religious or spiritual faith was a source of resilience. “During the peak of the pandemic, chaplains provided an exorbitant amount of staff support as clinicians turned to the chaplain to help make sense of what they were going through and connect to something greater than themselves.” Similarly, during 9/11, police and fire department chaplains “played a huge role in supporting the first responders,” Dr. DePierro said.
He said that Mount Sinai holds resilience workshops “where we focus on these topics and teach health care workers how to build resilience in their lives, heal day-to-day stressors, and even grow from the experience.”
Dr. Ofri, who is the founder and editor-in-chief of the Bellevue Literary Review, added that the arts played an important role in bolstering resilience and providing a creative outlet for clinicians after 9/11 and again during the pandemic.
The publication is celebrating its twentieth anniversary – its first issue went to press in September 2001. The cover contained an acknowledgment of 9/11.
Dr. Ofri said that a gala event had been planned for Oct. 7, 2001, to celebrate the inaugural issue of the publication. She assumed no one would show up, given that the United States had invaded Afghanistan only hours earlier. To her surprise, over a hundred people attended, “which made me realize the role of the arts during trauma. People were seeking to come together and hear poetry, fiction, and creative nonfiction.”
Dr. Ofri has been “impressed by the amount of incredible creative writing of all sorts that has been submitted [to the publication] during the pandemic, an unexpected flowering of the arts.”
Unique challenges, unique opportunities
All three experts pointed to several noteworthy differences between the experiences of first responders following 9/11 and those of today’s health care professionals during the pandemic.
“What happened on Sept. 11 was one discrete event, and although it obviously led to years of recovering body parts and cleaning up Ground Zero, and on a national level it led to a war, it nevertheless was a single event,” Dr. DePierro observed. By contrast, the COVID-19 pandemic is ongoing, and for health care practitioners, “it’s by no means over. Again and again, they are being thrown back into battle, dealing with fatigue, weariness, and loss of life.”
Moreover, “it is my understanding that immediately following 9/11, there was a general coming together in our country, but it’s obvious that today, there’s a great deal of fractiousness, contention, disagreement, and disunity in our country when it comes to COVID-19,” Dr. DePierro continued.
“This takes a great toll, particularly on health care workers who are dealing with COVID-19 on a daily basis and experience a disconnect between what they see on their floors and ICUs of the hospital, experiencing loss of life they’ve likely never encountered in their careers, and what people are saying when they downplay the seriousness of COVID-19,” he said.
Dr. Ofri agreed. “The fragmentation of our country and the failure of leadership at the highest level to provide even the basics, such as PPE [personal protective equipment] for health care professionals, left us baffled, profoundly hurt, and angry.”
A positive difference between the COVID-19 pandemic and the aftermath of 9/11 is the development of sophisticated technology that allows interventions for traumatized individuals – both health care professionals and the general public – through telehealth, Dr. DePierro pointed out.
“I would say that these resources and technologies are a silver lining and should continue to be expanded on,” he said. “Now, busy health care workers can access all manner of supportive services, including teletherapy, right from home or between shifts.”
Another “silver lining” is that the pandemic has shone a spotlight on an issue that predated the pandemic – the mental health of health care professionals. Opening a discussion about this has reduced stigma and hopefully has paved the way for improved treatments and for providing resources.
Dr. Dass-Brailsford added that “it is important, going forward, for all of us to be trauma informed, to know how trauma and trauma-related stress unfolds in both other people and yourself, and to know what coping skills can be used to avoid crises from developing – a task that extends across all types of disasters.”
A version of this article first appeared on Medscape.com.
‘Innovative’ equine therapy helps overcome PTSD symptoms
Equine therapy, which involves interactions with horses in a controlled environment, reduces fear and other symptoms of posttraumatic stress disorder, new research suggests.

Results from a study of about 60 military veterans who underwent weekly sessions of horse-assisted therapy showed “marked reductions” in clinician-rated and self-reported symptoms of PTSD and depression up to 3 months post treatment.
“What we’re doing here with horses is helping people overcome something very specific to PTSD,” coinvestigator Yuval Neria, PhD, professor of clinical medical psychology and director of the PTSD treatment and research program, Columbia University Medical Center, New York, said in an interview.
“It offers the opportunity to overcome fear, to facilitate self-efficacy, to facilitate trust in yourself, to understand your feelings, and perhaps to change them over time, he said.
In addition, veterans loved the experience, Dr. Neria reported. He noted that many patients with PTSD have trouble with traditional treatments and are eager to try something “creative and new.”
The findings were published online Aug. 31, 2021, in the Journal of Clinical Psychiatry.
Building bonds
PTSD affects an estimated 10%-30% of U.S. military personnel. These rates are higher than in the general population because veterans may experience increased trauma through combat, injury, and sexual assault, the investigators noted.
Previous research has suggested that horse-human interactions can build bonds that foster behavioral changes. These powerful animals provide instantaneous feedback, allowing patients to develop emotional awareness.
“Horses are very sensitive to whatever we communicate with them, whether it’s fear or anger or stress,” said Dr. Neria.
Equine-assisted therapy is increasingly being used for various mental and physical conditions. Launching an open-label study to examine this type of treatment for PTSD “was an opportunity to look at something very, very different,” Dr. Neria said.
“This is not psychotherapy, it’s not medication, and it’s not neural stimulation,” he added.
The study included 63 veterans with PTSD (mean age, 50 years; 37% women). Of these, 47 were receiving psychotherapy alone, pharmacotherapy alone, or both. In addition, 48 had at least one comorbid disorder. All were divided into 16 groups of three to five participants each.
The program consisted of eight 90-minute weekly sessions conducted at a large equestrian center. Sessions were coled by a mental health professional and an equine specialist who guided participants in horse communication and behavior.
Early sessions focused on acquainting patients with the horses, grooming exercises, and learning “leading,” which involved directing a horse with a rope or wand. During subsequent sessions, patients became more comfortable with managing the horses in individual and teamwork exercises.
The horses were specifically chosen for their temperament and had no history of aggression. A horse wrangler attended sessions to further ensure safety.
Few dropouts
The study included four assessment points: pretreatment, midpoint, post treatment, and 3-month follow-up.
All 63 participants completed baseline assessments. Only five patients (7.9%) discontinued the program.
“We didn’t see dropouts at the rate we usually see in evidence-based therapies for PTSD, which is remarkable and suggests that people really loved it,” said Dr. Neria.
The primary outcome was the Clinician-Administered PTSD Scale–5 (CAPS-5), a structured interview that evaluates intrusive memories, social avoidance, and other symptoms based on DSM-5 criteria.
In the intent-to-treat analysis, mean CAPS-5 scores decreased from 38.6 at baseline to 26.9 post treatment. In addition, 29 (46.0%) and 23 (36.5%) participants scored below the PTSD diagnostic threshold of 25 at posttreatment and follow-up, respectively.
Notably, 50.8% of the study population had a clinically significant change, defined as 30% or greater decrease in CAPS-5 score, at post treatment; 54.0% had a significant change at follow-up.
Mean scores on the self-reported 20-item PTSD Checklist for DSM-5 questionnaire decreased from 50.7 at baseline to 34.6 at study termination.
Depression symptoms, measured by the clinician-rated Hamilton Depression Rating Scale and the self-reported Beck Depression Inventory–II, also improved.
Structural, functional change
The results did not differ by age, gender, or type of trauma. Dr. Neria noted that many women in the study had suffered sexual abuse or assault, suggesting that the intervention might be appropriate for PTSD outside the military.
“I’m very keen on moving this along into a civilian population,” he said.
The study did not examine potential mechanisms of action. where the treatment took place, the investigators noted.
However, Dr. Neria thinks there is another potential explanation – real changes in the brain.
Neuroimaging of a subsample of 20 participants before and after the intervention showed a significant increase in caudate functional connectivity and a reduction in gray matter density of the thalamus and the caudate.
“We see a big change both structurally and functionally,” with the results pointing to an impact on the reward network of the brain, said Dr. Neria.
“This suggests that pleasure was perhaps the main mechanism of action,” which corresponds with patient reports of really enjoying the experience, he added.
Dr. Neria noted that equine therapy is different from bonding with a loyal dog. Interacting with a large and powerful animal may give veterans a sense of accomplishment and self-worth, which can be tremendously therapeutic.
Next step in therapy?
Commenting on the research, retired Col. Elspeth Cameron Ritchie, MD, chair of psychiatry, MedStar Washington Hospital Center, Washington, called equine therapy “innovative” in PTSD.
“I see this as the next step in finding acceptable therapies that people like to do,” she said.
Some patients have an aversion to talk therapy because it makes them relive their trauma; and many dislike the side effects of medications, which can include erectile dysfunction, said Dr. Ritchie, who was not involved with the research.
“So something like this that they can enjoy, have a sense of mastery, can bond with an animal, I think is wonderful,” she said.
Dr. Ritchie noted that working with animals offers “a kind of biofeedback” that may calm anxieties, help maintain control, and “is very nonjudgmental.”
However, she pointed out that equine therapy is not new. For example, horses have been used previously to treat patients with a variety of disabilities, including autism.
Dr. Ritchie thought it was “very wise” that study participants just learned to control the horses and didn’t actually ride them, because that could be a frightening experience.
Nonetheless, she noted equine therapy “is not going to be accessible for everybody.”
In addition, Dr. Ritchie was surprised that the investigators didn’t mention more of the quite extensive research that has been conducted on dog therapy in patients with PTSD.
Dr. Neria and Ritchie disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Equine therapy, which involves interactions with horses in a controlled environment, reduces fear and other symptoms of posttraumatic stress disorder, new research suggests.

Results from a study of about 60 military veterans who underwent weekly sessions of horse-assisted therapy showed “marked reductions” in clinician-rated and self-reported symptoms of PTSD and depression up to 3 months post treatment.
“What we’re doing here with horses is helping people overcome something very specific to PTSD,” coinvestigator Yuval Neria, PhD, professor of clinical medical psychology and director of the PTSD treatment and research program, Columbia University Medical Center, New York, said in an interview.
“It offers the opportunity to overcome fear, to facilitate self-efficacy, to facilitate trust in yourself, to understand your feelings, and perhaps to change them over time, he said.
In addition, veterans loved the experience, Dr. Neria reported. He noted that many patients with PTSD have trouble with traditional treatments and are eager to try something “creative and new.”
The findings were published online Aug. 31, 2021, in the Journal of Clinical Psychiatry.
Building bonds
PTSD affects an estimated 10%-30% of U.S. military personnel. These rates are higher than in the general population because veterans may experience increased trauma through combat, injury, and sexual assault, the investigators noted.
Previous research has suggested that horse-human interactions can build bonds that foster behavioral changes. These powerful animals provide instantaneous feedback, allowing patients to develop emotional awareness.
“Horses are very sensitive to whatever we communicate with them, whether it’s fear or anger or stress,” said Dr. Neria.
Equine-assisted therapy is increasingly being used for various mental and physical conditions. Launching an open-label study to examine this type of treatment for PTSD “was an opportunity to look at something very, very different,” Dr. Neria said.
“This is not psychotherapy, it’s not medication, and it’s not neural stimulation,” he added.
The study included 63 veterans with PTSD (mean age, 50 years; 37% women). Of these, 47 were receiving psychotherapy alone, pharmacotherapy alone, or both. In addition, 48 had at least one comorbid disorder. All were divided into 16 groups of three to five participants each.
The program consisted of eight 90-minute weekly sessions conducted at a large equestrian center. Sessions were coled by a mental health professional and an equine specialist who guided participants in horse communication and behavior.
Early sessions focused on acquainting patients with the horses, grooming exercises, and learning “leading,” which involved directing a horse with a rope or wand. During subsequent sessions, patients became more comfortable with managing the horses in individual and teamwork exercises.
The horses were specifically chosen for their temperament and had no history of aggression. A horse wrangler attended sessions to further ensure safety.
Few dropouts
The study included four assessment points: pretreatment, midpoint, post treatment, and 3-month follow-up.
All 63 participants completed baseline assessments. Only five patients (7.9%) discontinued the program.
“We didn’t see dropouts at the rate we usually see in evidence-based therapies for PTSD, which is remarkable and suggests that people really loved it,” said Dr. Neria.
The primary outcome was the Clinician-Administered PTSD Scale–5 (CAPS-5), a structured interview that evaluates intrusive memories, social avoidance, and other symptoms based on DSM-5 criteria.
In the intent-to-treat analysis, mean CAPS-5 scores decreased from 38.6 at baseline to 26.9 post treatment. In addition, 29 (46.0%) and 23 (36.5%) participants scored below the PTSD diagnostic threshold of 25 at posttreatment and follow-up, respectively.
Notably, 50.8% of the study population had a clinically significant change, defined as 30% or greater decrease in CAPS-5 score, at post treatment; 54.0% had a significant change at follow-up.
Mean scores on the self-reported 20-item PTSD Checklist for DSM-5 questionnaire decreased from 50.7 at baseline to 34.6 at study termination.
Depression symptoms, measured by the clinician-rated Hamilton Depression Rating Scale and the self-reported Beck Depression Inventory–II, also improved.
Structural, functional change
The results did not differ by age, gender, or type of trauma. Dr. Neria noted that many women in the study had suffered sexual abuse or assault, suggesting that the intervention might be appropriate for PTSD outside the military.
“I’m very keen on moving this along into a civilian population,” he said.
The study did not examine potential mechanisms of action. where the treatment took place, the investigators noted.
However, Dr. Neria thinks there is another potential explanation – real changes in the brain.
Neuroimaging of a subsample of 20 participants before and after the intervention showed a significant increase in caudate functional connectivity and a reduction in gray matter density of the thalamus and the caudate.
“We see a big change both structurally and functionally,” with the results pointing to an impact on the reward network of the brain, said Dr. Neria.
“This suggests that pleasure was perhaps the main mechanism of action,” which corresponds with patient reports of really enjoying the experience, he added.
Dr. Neria noted that equine therapy is different from bonding with a loyal dog. Interacting with a large and powerful animal may give veterans a sense of accomplishment and self-worth, which can be tremendously therapeutic.
Next step in therapy?
Commenting on the research, retired Col. Elspeth Cameron Ritchie, MD, chair of psychiatry, MedStar Washington Hospital Center, Washington, called equine therapy “innovative” in PTSD.
“I see this as the next step in finding acceptable therapies that people like to do,” she said.
Some patients have an aversion to talk therapy because it makes them relive their trauma; and many dislike the side effects of medications, which can include erectile dysfunction, said Dr. Ritchie, who was not involved with the research.
“So something like this that they can enjoy, have a sense of mastery, can bond with an animal, I think is wonderful,” she said.
Dr. Ritchie noted that working with animals offers “a kind of biofeedback” that may calm anxieties, help maintain control, and “is very nonjudgmental.”
However, she pointed out that equine therapy is not new. For example, horses have been used previously to treat patients with a variety of disabilities, including autism.
Dr. Ritchie thought it was “very wise” that study participants just learned to control the horses and didn’t actually ride them, because that could be a frightening experience.
Nonetheless, she noted equine therapy “is not going to be accessible for everybody.”
In addition, Dr. Ritchie was surprised that the investigators didn’t mention more of the quite extensive research that has been conducted on dog therapy in patients with PTSD.
Dr. Neria and Ritchie disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Equine therapy, which involves interactions with horses in a controlled environment, reduces fear and other symptoms of posttraumatic stress disorder, new research suggests.

Results from a study of about 60 military veterans who underwent weekly sessions of horse-assisted therapy showed “marked reductions” in clinician-rated and self-reported symptoms of PTSD and depression up to 3 months post treatment.
“What we’re doing here with horses is helping people overcome something very specific to PTSD,” coinvestigator Yuval Neria, PhD, professor of clinical medical psychology and director of the PTSD treatment and research program, Columbia University Medical Center, New York, said in an interview.
“It offers the opportunity to overcome fear, to facilitate self-efficacy, to facilitate trust in yourself, to understand your feelings, and perhaps to change them over time, he said.
In addition, veterans loved the experience, Dr. Neria reported. He noted that many patients with PTSD have trouble with traditional treatments and are eager to try something “creative and new.”
The findings were published online Aug. 31, 2021, in the Journal of Clinical Psychiatry.
Building bonds
PTSD affects an estimated 10%-30% of U.S. military personnel. These rates are higher than in the general population because veterans may experience increased trauma through combat, injury, and sexual assault, the investigators noted.
Previous research has suggested that horse-human interactions can build bonds that foster behavioral changes. These powerful animals provide instantaneous feedback, allowing patients to develop emotional awareness.
“Horses are very sensitive to whatever we communicate with them, whether it’s fear or anger or stress,” said Dr. Neria.
Equine-assisted therapy is increasingly being used for various mental and physical conditions. Launching an open-label study to examine this type of treatment for PTSD “was an opportunity to look at something very, very different,” Dr. Neria said.
“This is not psychotherapy, it’s not medication, and it’s not neural stimulation,” he added.
The study included 63 veterans with PTSD (mean age, 50 years; 37% women). Of these, 47 were receiving psychotherapy alone, pharmacotherapy alone, or both. In addition, 48 had at least one comorbid disorder. All were divided into 16 groups of three to five participants each.
The program consisted of eight 90-minute weekly sessions conducted at a large equestrian center. Sessions were coled by a mental health professional and an equine specialist who guided participants in horse communication and behavior.
Early sessions focused on acquainting patients with the horses, grooming exercises, and learning “leading,” which involved directing a horse with a rope or wand. During subsequent sessions, patients became more comfortable with managing the horses in individual and teamwork exercises.
The horses were specifically chosen for their temperament and had no history of aggression. A horse wrangler attended sessions to further ensure safety.
Few dropouts
The study included four assessment points: pretreatment, midpoint, post treatment, and 3-month follow-up.
All 63 participants completed baseline assessments. Only five patients (7.9%) discontinued the program.
“We didn’t see dropouts at the rate we usually see in evidence-based therapies for PTSD, which is remarkable and suggests that people really loved it,” said Dr. Neria.
The primary outcome was the Clinician-Administered PTSD Scale–5 (CAPS-5), a structured interview that evaluates intrusive memories, social avoidance, and other symptoms based on DSM-5 criteria.
In the intent-to-treat analysis, mean CAPS-5 scores decreased from 38.6 at baseline to 26.9 post treatment. In addition, 29 (46.0%) and 23 (36.5%) participants scored below the PTSD diagnostic threshold of 25 at posttreatment and follow-up, respectively.
Notably, 50.8% of the study population had a clinically significant change, defined as 30% or greater decrease in CAPS-5 score, at post treatment; 54.0% had a significant change at follow-up.
Mean scores on the self-reported 20-item PTSD Checklist for DSM-5 questionnaire decreased from 50.7 at baseline to 34.6 at study termination.
Depression symptoms, measured by the clinician-rated Hamilton Depression Rating Scale and the self-reported Beck Depression Inventory–II, also improved.
Structural, functional change
The results did not differ by age, gender, or type of trauma. Dr. Neria noted that many women in the study had suffered sexual abuse or assault, suggesting that the intervention might be appropriate for PTSD outside the military.
“I’m very keen on moving this along into a civilian population,” he said.
The study did not examine potential mechanisms of action. where the treatment took place, the investigators noted.
However, Dr. Neria thinks there is another potential explanation – real changes in the brain.
Neuroimaging of a subsample of 20 participants before and after the intervention showed a significant increase in caudate functional connectivity and a reduction in gray matter density of the thalamus and the caudate.
“We see a big change both structurally and functionally,” with the results pointing to an impact on the reward network of the brain, said Dr. Neria.
“This suggests that pleasure was perhaps the main mechanism of action,” which corresponds with patient reports of really enjoying the experience, he added.
Dr. Neria noted that equine therapy is different from bonding with a loyal dog. Interacting with a large and powerful animal may give veterans a sense of accomplishment and self-worth, which can be tremendously therapeutic.
Next step in therapy?
Commenting on the research, retired Col. Elspeth Cameron Ritchie, MD, chair of psychiatry, MedStar Washington Hospital Center, Washington, called equine therapy “innovative” in PTSD.
“I see this as the next step in finding acceptable therapies that people like to do,” she said.
Some patients have an aversion to talk therapy because it makes them relive their trauma; and many dislike the side effects of medications, which can include erectile dysfunction, said Dr. Ritchie, who was not involved with the research.
“So something like this that they can enjoy, have a sense of mastery, can bond with an animal, I think is wonderful,” she said.
Dr. Ritchie noted that working with animals offers “a kind of biofeedback” that may calm anxieties, help maintain control, and “is very nonjudgmental.”
However, she pointed out that equine therapy is not new. For example, horses have been used previously to treat patients with a variety of disabilities, including autism.
Dr. Ritchie thought it was “very wise” that study participants just learned to control the horses and didn’t actually ride them, because that could be a frightening experience.
Nonetheless, she noted equine therapy “is not going to be accessible for everybody.”
In addition, Dr. Ritchie was surprised that the investigators didn’t mention more of the quite extensive research that has been conducted on dog therapy in patients with PTSD.
Dr. Neria and Ritchie disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Toward ‘superhuman cognition’: The future of brain-computer interfaces
The brain is inarguably the most complex and mysterious organ in the human body.
As the epicenter of intelligence, mastermind of movement, and song for our senses, the brain is more than a 3-lb organ encased in shell and fluid. Rather, it is the crown jewel that defines the self and, broadly, humanity.
For decades now, researchers have been exploring the potential for connecting our own astounding biological “computer” with actual physical mainframes. These so-called “brain-computer interfaces” (BCIs) are showing promise in treating an array of conditions, including paralysis, deafness, stroke, and even psychiatric disorders.
Among the big players in this area of research is billionaire entrepreneur Elon Musk, who in 2016 founded Neuralink. The company’s short-term mission is to develop a brain-to-machine interface to help people with neurologic conditions (for example, Parkinson’s disease). The long-term mission is to steer humanity into the era of “superhuman cognition.”
But first, some neuroscience 101.
Neurons are specialized cells that transmit and receive information. The basic structure of a neuron includes the dendrite, soma, and axon. The dendrite is the signal receiver. The soma is the cell body that is connected to the dendrites and serves as a structure to pass signals. The axon, also known as the nerve fiber, transmits the signal away from the soma.
Neurons communicate with each other at the synapse (for example, axon-dendrite connection). Neurons send information to each other through action potentials. An action potential may be defined as an electric impulse that transmits down the axon, causing the release of neurotransmitters, which may consequently either inhibit or excite the next neuron (leading to the initiation of another action potential).
So how will the company and other BCI companies tap into this evolutionarily ancient system to develop an implant that will obtain and decode information output from the brain?
The Neuralink implant is composed of three parts: The Link, neural threads, and the charger.
A robotic system, controlled by a neurosurgeon, will place an implant into the brain. The Link is the central component. It processes and transmits neural signals. The micron-scale neural threads are connected to the Link and other areas of the brain. The threads also contain electrodes, which are responsible for detecting neural signals. The charger ensures the battery is charged via wireless connection.
The invasive nature of this implant allows for precise readouts of electric outputs from the brain – unlike noninvasive devices, which are less sensitive and specific. Additionally, owing to its small size, engineers and neurosurgeons can implant the device in very specific brain regions as well as customize electrode distribution.
The Neuralink implant would be paired with an application via Bluetooth connection. The goal is to enable someone with the implant to control their device or computer by simply thinking. The application offers several exercises to help guide and train individuals on how to use the implant for its intended purpose. , as well as partake in creative activities such as photography.
Existing text and speech synthesis technology are already underway. For example, Synchron, a BCI platform company, is investigating the use of Stentrode for people with severe paralysis. This neuroprosthesis was designed to help people associate thought with movement through Bluetooth technology (for example, texting, emailing, shopping, online banking). Preliminary results from a study in which the device was used for patients with amyotrophic lateral sclerosis showed improvements in functional independence via direct thinking.
Software intended to enable high-performance handwriting utilizing BCI technology is being developed by Francis R. Willett, PhD, at Stanford (Calif.) University. The technology has also shown promise.
“We’ve learned that the brain retains its ability to prescribe fine movements a full decade after the body has lost its ability to execute those movements,” says Dr. Willett, who recently reported on results from a BCI study of handwriting conversion in an individual with full-body paralysis. Through a recurrent neural networking decoding approach, the BrainGate study participant was able to type 90 characters per minute – with an impressive 94.1% raw accuracy – using thoughts alone.
Although not a fully implantable brain device, this percutaneous implant has also been studied of its capacity to restore arm function among individuals who suffered from chronic stroke. Preliminary results from the Cortimo trials, led by Mijail D. Serruya, MD, an assistant professor at Thomas Jefferson University, Philadelphia, have been positive. Researchers implanted microelectrode arrays to decode brain signals and power motor function in a participant who had experienced a stroke 2 years earlier. The participant was able to use a powered arm brace on their paralyzed arm.
Neuralink recently released a video demonstrating the use of the interface in a monkey named Pager as it played a game with a joystick. Company researchers inserted a 1024-Electrode neural recording and data transmission device called the N1 Link into the left and right motor cortices. Using the implant, neural activity was sent to a decoder algorithm. Throughout the process, the decoder algorithm was refined and calibrated. After a few minutes, Pager was able to control the cursor on the screen using his mind instead of the joystick.
Mr. Musk hopes to develop Neuralink further to change not only the way we treat neurological disorders but also the way we interact with ourselves and our environment. It’s a lofty goal to be sure, but one that doesn’t seem outside the realm of possibility in the near future.
Known unknowns: The ethical dilemmas
One major conundrum facing the future of BCI technology is that researchers don’t fully understand the science regarding how brain signaling, artificial intelligence (AI) software, and prostheses interact. Although offloading computations improves the predictive nature of AI algorithms, there are concerns of identity and personal agency.
How do we know that an action is truly the result of one’s own thinking or, rather, the outcome of AI software? In this context, the autocorrect function while typing can be incredibly useful when we’re in a pinch for time, when we’re using one hand to type, or because of ease. However, it’s also easy to create and send out unintended or inappropriate messages.
These algorithms are designed to learn from our behavior and anticipate our next move. However, a question arises as to whether we are the authors of our own thoughts or whether we are simply the device that delivers messages under the control of external forces.
“People may question whether new personality changes they experience are truly representative of themselves or whether they are now a product of the implant (e.g., ‘Is that really me?’; ‘Have I grown as a person, or is it the technology?’). This then raises questions about agency and who we are as people,” says Kerry Bowman, PhD, a clinical bioethicist and assistant professor at the Temerty Faculty of Medicine of the University of Toronto.
It’s important to have safeguards in place to ensure the privacy of our thoughts. In an age where data is currency, it’s crucial to establish boundaries to preserve our autonomy and prevent exploitation (for example, by private companies or hackers). Although Neuralink and BCIs generally are certainly pushing the boundaries of neural engineering in profound ways, it’s important to note the biological and ethical implications of this technology.
As Dr. Bowman points out, “throughout the entire human story, under the worst of human circumstances, such as captivity and torture, the one safe ground and place for all people has been the privacy of one’s own mind. No one could ever interfere, take away, or be aware of those thoughts. However, this technology challenges one’s own privacy – that this technology (and, by extension, a company) could be aware of those thoughts.”
A version of this article first appeared on Medscape.com.
The brain is inarguably the most complex and mysterious organ in the human body.
As the epicenter of intelligence, mastermind of movement, and song for our senses, the brain is more than a 3-lb organ encased in shell and fluid. Rather, it is the crown jewel that defines the self and, broadly, humanity.
For decades now, researchers have been exploring the potential for connecting our own astounding biological “computer” with actual physical mainframes. These so-called “brain-computer interfaces” (BCIs) are showing promise in treating an array of conditions, including paralysis, deafness, stroke, and even psychiatric disorders.
Among the big players in this area of research is billionaire entrepreneur Elon Musk, who in 2016 founded Neuralink. The company’s short-term mission is to develop a brain-to-machine interface to help people with neurologic conditions (for example, Parkinson’s disease). The long-term mission is to steer humanity into the era of “superhuman cognition.”
But first, some neuroscience 101.
Neurons are specialized cells that transmit and receive information. The basic structure of a neuron includes the dendrite, soma, and axon. The dendrite is the signal receiver. The soma is the cell body that is connected to the dendrites and serves as a structure to pass signals. The axon, also known as the nerve fiber, transmits the signal away from the soma.
Neurons communicate with each other at the synapse (for example, axon-dendrite connection). Neurons send information to each other through action potentials. An action potential may be defined as an electric impulse that transmits down the axon, causing the release of neurotransmitters, which may consequently either inhibit or excite the next neuron (leading to the initiation of another action potential).
So how will the company and other BCI companies tap into this evolutionarily ancient system to develop an implant that will obtain and decode information output from the brain?
The Neuralink implant is composed of three parts: The Link, neural threads, and the charger.
A robotic system, controlled by a neurosurgeon, will place an implant into the brain. The Link is the central component. It processes and transmits neural signals. The micron-scale neural threads are connected to the Link and other areas of the brain. The threads also contain electrodes, which are responsible for detecting neural signals. The charger ensures the battery is charged via wireless connection.
The invasive nature of this implant allows for precise readouts of electric outputs from the brain – unlike noninvasive devices, which are less sensitive and specific. Additionally, owing to its small size, engineers and neurosurgeons can implant the device in very specific brain regions as well as customize electrode distribution.
The Neuralink implant would be paired with an application via Bluetooth connection. The goal is to enable someone with the implant to control their device or computer by simply thinking. The application offers several exercises to help guide and train individuals on how to use the implant for its intended purpose. , as well as partake in creative activities such as photography.
Existing text and speech synthesis technology are already underway. For example, Synchron, a BCI platform company, is investigating the use of Stentrode for people with severe paralysis. This neuroprosthesis was designed to help people associate thought with movement through Bluetooth technology (for example, texting, emailing, shopping, online banking). Preliminary results from a study in which the device was used for patients with amyotrophic lateral sclerosis showed improvements in functional independence via direct thinking.
Software intended to enable high-performance handwriting utilizing BCI technology is being developed by Francis R. Willett, PhD, at Stanford (Calif.) University. The technology has also shown promise.
“We’ve learned that the brain retains its ability to prescribe fine movements a full decade after the body has lost its ability to execute those movements,” says Dr. Willett, who recently reported on results from a BCI study of handwriting conversion in an individual with full-body paralysis. Through a recurrent neural networking decoding approach, the BrainGate study participant was able to type 90 characters per minute – with an impressive 94.1% raw accuracy – using thoughts alone.
Although not a fully implantable brain device, this percutaneous implant has also been studied of its capacity to restore arm function among individuals who suffered from chronic stroke. Preliminary results from the Cortimo trials, led by Mijail D. Serruya, MD, an assistant professor at Thomas Jefferson University, Philadelphia, have been positive. Researchers implanted microelectrode arrays to decode brain signals and power motor function in a participant who had experienced a stroke 2 years earlier. The participant was able to use a powered arm brace on their paralyzed arm.
Neuralink recently released a video demonstrating the use of the interface in a monkey named Pager as it played a game with a joystick. Company researchers inserted a 1024-Electrode neural recording and data transmission device called the N1 Link into the left and right motor cortices. Using the implant, neural activity was sent to a decoder algorithm. Throughout the process, the decoder algorithm was refined and calibrated. After a few minutes, Pager was able to control the cursor on the screen using his mind instead of the joystick.
Mr. Musk hopes to develop Neuralink further to change not only the way we treat neurological disorders but also the way we interact with ourselves and our environment. It’s a lofty goal to be sure, but one that doesn’t seem outside the realm of possibility in the near future.
Known unknowns: The ethical dilemmas
One major conundrum facing the future of BCI technology is that researchers don’t fully understand the science regarding how brain signaling, artificial intelligence (AI) software, and prostheses interact. Although offloading computations improves the predictive nature of AI algorithms, there are concerns of identity and personal agency.
How do we know that an action is truly the result of one’s own thinking or, rather, the outcome of AI software? In this context, the autocorrect function while typing can be incredibly useful when we’re in a pinch for time, when we’re using one hand to type, or because of ease. However, it’s also easy to create and send out unintended or inappropriate messages.
These algorithms are designed to learn from our behavior and anticipate our next move. However, a question arises as to whether we are the authors of our own thoughts or whether we are simply the device that delivers messages under the control of external forces.
“People may question whether new personality changes they experience are truly representative of themselves or whether they are now a product of the implant (e.g., ‘Is that really me?’; ‘Have I grown as a person, or is it the technology?’). This then raises questions about agency and who we are as people,” says Kerry Bowman, PhD, a clinical bioethicist and assistant professor at the Temerty Faculty of Medicine of the University of Toronto.
It’s important to have safeguards in place to ensure the privacy of our thoughts. In an age where data is currency, it’s crucial to establish boundaries to preserve our autonomy and prevent exploitation (for example, by private companies or hackers). Although Neuralink and BCIs generally are certainly pushing the boundaries of neural engineering in profound ways, it’s important to note the biological and ethical implications of this technology.
As Dr. Bowman points out, “throughout the entire human story, under the worst of human circumstances, such as captivity and torture, the one safe ground and place for all people has been the privacy of one’s own mind. No one could ever interfere, take away, or be aware of those thoughts. However, this technology challenges one’s own privacy – that this technology (and, by extension, a company) could be aware of those thoughts.”
A version of this article first appeared on Medscape.com.
The brain is inarguably the most complex and mysterious organ in the human body.
As the epicenter of intelligence, mastermind of movement, and song for our senses, the brain is more than a 3-lb organ encased in shell and fluid. Rather, it is the crown jewel that defines the self and, broadly, humanity.
For decades now, researchers have been exploring the potential for connecting our own astounding biological “computer” with actual physical mainframes. These so-called “brain-computer interfaces” (BCIs) are showing promise in treating an array of conditions, including paralysis, deafness, stroke, and even psychiatric disorders.
Among the big players in this area of research is billionaire entrepreneur Elon Musk, who in 2016 founded Neuralink. The company’s short-term mission is to develop a brain-to-machine interface to help people with neurologic conditions (for example, Parkinson’s disease). The long-term mission is to steer humanity into the era of “superhuman cognition.”
But first, some neuroscience 101.
Neurons are specialized cells that transmit and receive information. The basic structure of a neuron includes the dendrite, soma, and axon. The dendrite is the signal receiver. The soma is the cell body that is connected to the dendrites and serves as a structure to pass signals. The axon, also known as the nerve fiber, transmits the signal away from the soma.
Neurons communicate with each other at the synapse (for example, axon-dendrite connection). Neurons send information to each other through action potentials. An action potential may be defined as an electric impulse that transmits down the axon, causing the release of neurotransmitters, which may consequently either inhibit or excite the next neuron (leading to the initiation of another action potential).
So how will the company and other BCI companies tap into this evolutionarily ancient system to develop an implant that will obtain and decode information output from the brain?
The Neuralink implant is composed of three parts: The Link, neural threads, and the charger.
A robotic system, controlled by a neurosurgeon, will place an implant into the brain. The Link is the central component. It processes and transmits neural signals. The micron-scale neural threads are connected to the Link and other areas of the brain. The threads also contain electrodes, which are responsible for detecting neural signals. The charger ensures the battery is charged via wireless connection.
The invasive nature of this implant allows for precise readouts of electric outputs from the brain – unlike noninvasive devices, which are less sensitive and specific. Additionally, owing to its small size, engineers and neurosurgeons can implant the device in very specific brain regions as well as customize electrode distribution.
The Neuralink implant would be paired with an application via Bluetooth connection. The goal is to enable someone with the implant to control their device or computer by simply thinking. The application offers several exercises to help guide and train individuals on how to use the implant for its intended purpose. , as well as partake in creative activities such as photography.
Existing text and speech synthesis technology are already underway. For example, Synchron, a BCI platform company, is investigating the use of Stentrode for people with severe paralysis. This neuroprosthesis was designed to help people associate thought with movement through Bluetooth technology (for example, texting, emailing, shopping, online banking). Preliminary results from a study in which the device was used for patients with amyotrophic lateral sclerosis showed improvements in functional independence via direct thinking.
Software intended to enable high-performance handwriting utilizing BCI technology is being developed by Francis R. Willett, PhD, at Stanford (Calif.) University. The technology has also shown promise.
“We’ve learned that the brain retains its ability to prescribe fine movements a full decade after the body has lost its ability to execute those movements,” says Dr. Willett, who recently reported on results from a BCI study of handwriting conversion in an individual with full-body paralysis. Through a recurrent neural networking decoding approach, the BrainGate study participant was able to type 90 characters per minute – with an impressive 94.1% raw accuracy – using thoughts alone.
Although not a fully implantable brain device, this percutaneous implant has also been studied of its capacity to restore arm function among individuals who suffered from chronic stroke. Preliminary results from the Cortimo trials, led by Mijail D. Serruya, MD, an assistant professor at Thomas Jefferson University, Philadelphia, have been positive. Researchers implanted microelectrode arrays to decode brain signals and power motor function in a participant who had experienced a stroke 2 years earlier. The participant was able to use a powered arm brace on their paralyzed arm.
Neuralink recently released a video demonstrating the use of the interface in a monkey named Pager as it played a game with a joystick. Company researchers inserted a 1024-Electrode neural recording and data transmission device called the N1 Link into the left and right motor cortices. Using the implant, neural activity was sent to a decoder algorithm. Throughout the process, the decoder algorithm was refined and calibrated. After a few minutes, Pager was able to control the cursor on the screen using his mind instead of the joystick.
Mr. Musk hopes to develop Neuralink further to change not only the way we treat neurological disorders but also the way we interact with ourselves and our environment. It’s a lofty goal to be sure, but one that doesn’t seem outside the realm of possibility in the near future.
Known unknowns: The ethical dilemmas
One major conundrum facing the future of BCI technology is that researchers don’t fully understand the science regarding how brain signaling, artificial intelligence (AI) software, and prostheses interact. Although offloading computations improves the predictive nature of AI algorithms, there are concerns of identity and personal agency.
How do we know that an action is truly the result of one’s own thinking or, rather, the outcome of AI software? In this context, the autocorrect function while typing can be incredibly useful when we’re in a pinch for time, when we’re using one hand to type, or because of ease. However, it’s also easy to create and send out unintended or inappropriate messages.
These algorithms are designed to learn from our behavior and anticipate our next move. However, a question arises as to whether we are the authors of our own thoughts or whether we are simply the device that delivers messages under the control of external forces.
“People may question whether new personality changes they experience are truly representative of themselves or whether they are now a product of the implant (e.g., ‘Is that really me?’; ‘Have I grown as a person, or is it the technology?’). This then raises questions about agency and who we are as people,” says Kerry Bowman, PhD, a clinical bioethicist and assistant professor at the Temerty Faculty of Medicine of the University of Toronto.
It’s important to have safeguards in place to ensure the privacy of our thoughts. In an age where data is currency, it’s crucial to establish boundaries to preserve our autonomy and prevent exploitation (for example, by private companies or hackers). Although Neuralink and BCIs generally are certainly pushing the boundaries of neural engineering in profound ways, it’s important to note the biological and ethical implications of this technology.
As Dr. Bowman points out, “throughout the entire human story, under the worst of human circumstances, such as captivity and torture, the one safe ground and place for all people has been the privacy of one’s own mind. No one could ever interfere, take away, or be aware of those thoughts. However, this technology challenges one’s own privacy – that this technology (and, by extension, a company) could be aware of those thoughts.”
A version of this article first appeared on Medscape.com.
MS plus depression can increase risk of death, vascular disease
, a new study has found. “The effects of depression and MS on all-cause mortality are synergistic,” wrote lead author Raffaele Palladino, MD, PhD, research associate, faculty of medicine, Imperial College London.
The study was published in Neurology.
To assess the association between depression, vascular disease, and death in patients with MS, the researchers launched a population-based retrospective cohort study that reviewed English medical records from January 1987 to December 2018 and matched people with and without MS. Ultimately, 12,251 people with MS were matched with 72,572 controls. At baseline, 21% of the MS group (n = 2,535) and 9% of the controls (n = 6,278) had depression. Women were the majority in both cohorts and were more likely than men to be depressed.
People with both MS and depression had an all-cause mortality rate of 10.3 cases per 100,000 person-years (95% confidence interval, 9.17-11.57), compared with 10.6 for people with MS without depression (95% CI, 9.99-11.21), 3.6 for people with depression but not MS (95% CI, 3.18-4.05), and 2.5 for people with neither condition (95% CI, 2.42-2.64). Compared with controls without depression, the 10-year hazard of all-cause mortality was increasingly greater in controls with depression (hazard ratio, 1.75; 95% CI, 1.59-1.91), people with MS but not depression (HR, 3.88; 95% CI, 3.66-4.10), and people with MS and depression (HR, 5.43; 95% CI, 4.88-5.96). Overall, 14% of the observed effect on mortality was attributable to the interaction between MS status and depression.
As for vascular diseases, people with MS had an increased risk regardless of their depression status. That said, people with MS and depression (HR, 3.30; 95% CI, 2.37-4.23) had a notably higher risk than people with MS and no depression (HR, 1.48; 95% CI, 1.23-1.74). Women with MS and depression also had a greater risk of vascular disease than women with MS and no depression, while men with MS did not have significantly different risks of acute coronary syndrome or composite macrovascular disease than those in the control group who did not suffer from depression.
Does treating depression decrease the likelihood of vascular disease?
“The take-home message for me is the importance of treating depression in this population, in which we see it with great regularity,” Joseph Berger, MD, professor of neurology and associate chief of the multiple sclerosis division at the University of Pennsylvania, Philadelphia, said in an interview. “The question that I have is: If you treat depression in an individual with MS or an individual who is simply depressed and thus at risk for the subsequent development of vascular disease, does it decrease the likelihood of their subsequent development of vascular disease in comparison to had you not?
“I presume it does,” he added, noting that “the theories underlying why depression would increase one’s risk of subsequent vascular disease are enumerated by the authors, including such things as increased inflammation. Now, the inflammation may be contributing to the depression, or the depression may be contributing to the inflammation; it may be one of those chicken-and-egg scenarios. But if you decrease the depression, do you thereby decrease the inflammation, which has a pernicious effect on endothelial cells and increases one’s vascular risk?
“Alternatively, lifestyle in depressed patients is also altered,” he said. “They’re far less likely to engage in exercise, healthy habits, and healthy diets, and more likely perhaps to smoke. These all need to be addressed, but this study certainly gives you a greater impetus as a MS neurologist to address the issue of depression, realizing that there is also this comorbidity of vascular disease.”
Evaluating the biological interaction between MS and depression
Based on this and other studies, the joint effect of MS and depression on all-cause mortality may qualify as a biological interaction, Amber Salter, PhD, of the University of Texas Southwestern Medical Center, Dallas, wrote in an accompanying editorial.
“Biological interactions consider whether the joint effect of two factors follow an additive pattern, or the joint effect of two factors is greater than the sum of the individual effects for each factor alone,” she wrote. And though the interaction was not found to be present for vascular disease and cardiovascular mortality, it was for all-cause mortality.
“When warranted, the evaluation of biological interactions in future studies should be considered to provide insight on target subpopulations for interventions or test for potential mechanistic forms of interaction,” she added.
Dr. Salter highlighted the study’s strengths, including a large sample size and six controls matched to each MS patient. She also stated that the researchers’ inability to control for risk factors like body mass index and physical activity means the 14% increase in mortality “may not be a large absolute increase in mortality when other covariates cannot be considered.” In addition, their lack of data on suicide – and its association with depression – offers up the possibility that increases in mortality could be tied to a “potentially modifiable risk” as opposed to a biologically increased one.
In acknowledging their study’s limitations, the authors stated that body mass index, though an important vascular risk factor, has a “modest” association with mortality, and that the average annual suicide rate in the MS population – though higher than in the non-MS population – is still “relatively low.”
Two of the authors disclosed receiving support, including grants and research funding, from various institutions and organizations in the United Kingdom, the United States, and Canada, as well as several pharmaceutical companies. Dr. Salter reported no relevant disclosures.
, a new study has found. “The effects of depression and MS on all-cause mortality are synergistic,” wrote lead author Raffaele Palladino, MD, PhD, research associate, faculty of medicine, Imperial College London.
The study was published in Neurology.
To assess the association between depression, vascular disease, and death in patients with MS, the researchers launched a population-based retrospective cohort study that reviewed English medical records from January 1987 to December 2018 and matched people with and without MS. Ultimately, 12,251 people with MS were matched with 72,572 controls. At baseline, 21% of the MS group (n = 2,535) and 9% of the controls (n = 6,278) had depression. Women were the majority in both cohorts and were more likely than men to be depressed.
People with both MS and depression had an all-cause mortality rate of 10.3 cases per 100,000 person-years (95% confidence interval, 9.17-11.57), compared with 10.6 for people with MS without depression (95% CI, 9.99-11.21), 3.6 for people with depression but not MS (95% CI, 3.18-4.05), and 2.5 for people with neither condition (95% CI, 2.42-2.64). Compared with controls without depression, the 10-year hazard of all-cause mortality was increasingly greater in controls with depression (hazard ratio, 1.75; 95% CI, 1.59-1.91), people with MS but not depression (HR, 3.88; 95% CI, 3.66-4.10), and people with MS and depression (HR, 5.43; 95% CI, 4.88-5.96). Overall, 14% of the observed effect on mortality was attributable to the interaction between MS status and depression.
As for vascular diseases, people with MS had an increased risk regardless of their depression status. That said, people with MS and depression (HR, 3.30; 95% CI, 2.37-4.23) had a notably higher risk than people with MS and no depression (HR, 1.48; 95% CI, 1.23-1.74). Women with MS and depression also had a greater risk of vascular disease than women with MS and no depression, while men with MS did not have significantly different risks of acute coronary syndrome or composite macrovascular disease than those in the control group who did not suffer from depression.
Does treating depression decrease the likelihood of vascular disease?
“The take-home message for me is the importance of treating depression in this population, in which we see it with great regularity,” Joseph Berger, MD, professor of neurology and associate chief of the multiple sclerosis division at the University of Pennsylvania, Philadelphia, said in an interview. “The question that I have is: If you treat depression in an individual with MS or an individual who is simply depressed and thus at risk for the subsequent development of vascular disease, does it decrease the likelihood of their subsequent development of vascular disease in comparison to had you not?
“I presume it does,” he added, noting that “the theories underlying why depression would increase one’s risk of subsequent vascular disease are enumerated by the authors, including such things as increased inflammation. Now, the inflammation may be contributing to the depression, or the depression may be contributing to the inflammation; it may be one of those chicken-and-egg scenarios. But if you decrease the depression, do you thereby decrease the inflammation, which has a pernicious effect on endothelial cells and increases one’s vascular risk?
“Alternatively, lifestyle in depressed patients is also altered,” he said. “They’re far less likely to engage in exercise, healthy habits, and healthy diets, and more likely perhaps to smoke. These all need to be addressed, but this study certainly gives you a greater impetus as a MS neurologist to address the issue of depression, realizing that there is also this comorbidity of vascular disease.”
Evaluating the biological interaction between MS and depression
Based on this and other studies, the joint effect of MS and depression on all-cause mortality may qualify as a biological interaction, Amber Salter, PhD, of the University of Texas Southwestern Medical Center, Dallas, wrote in an accompanying editorial.
“Biological interactions consider whether the joint effect of two factors follow an additive pattern, or the joint effect of two factors is greater than the sum of the individual effects for each factor alone,” she wrote. And though the interaction was not found to be present for vascular disease and cardiovascular mortality, it was for all-cause mortality.
“When warranted, the evaluation of biological interactions in future studies should be considered to provide insight on target subpopulations for interventions or test for potential mechanistic forms of interaction,” she added.
Dr. Salter highlighted the study’s strengths, including a large sample size and six controls matched to each MS patient. She also stated that the researchers’ inability to control for risk factors like body mass index and physical activity means the 14% increase in mortality “may not be a large absolute increase in mortality when other covariates cannot be considered.” In addition, their lack of data on suicide – and its association with depression – offers up the possibility that increases in mortality could be tied to a “potentially modifiable risk” as opposed to a biologically increased one.
In acknowledging their study’s limitations, the authors stated that body mass index, though an important vascular risk factor, has a “modest” association with mortality, and that the average annual suicide rate in the MS population – though higher than in the non-MS population – is still “relatively low.”
Two of the authors disclosed receiving support, including grants and research funding, from various institutions and organizations in the United Kingdom, the United States, and Canada, as well as several pharmaceutical companies. Dr. Salter reported no relevant disclosures.
, a new study has found. “The effects of depression and MS on all-cause mortality are synergistic,” wrote lead author Raffaele Palladino, MD, PhD, research associate, faculty of medicine, Imperial College London.
The study was published in Neurology.
To assess the association between depression, vascular disease, and death in patients with MS, the researchers launched a population-based retrospective cohort study that reviewed English medical records from January 1987 to December 2018 and matched people with and without MS. Ultimately, 12,251 people with MS were matched with 72,572 controls. At baseline, 21% of the MS group (n = 2,535) and 9% of the controls (n = 6,278) had depression. Women were the majority in both cohorts and were more likely than men to be depressed.
People with both MS and depression had an all-cause mortality rate of 10.3 cases per 100,000 person-years (95% confidence interval, 9.17-11.57), compared with 10.6 for people with MS without depression (95% CI, 9.99-11.21), 3.6 for people with depression but not MS (95% CI, 3.18-4.05), and 2.5 for people with neither condition (95% CI, 2.42-2.64). Compared with controls without depression, the 10-year hazard of all-cause mortality was increasingly greater in controls with depression (hazard ratio, 1.75; 95% CI, 1.59-1.91), people with MS but not depression (HR, 3.88; 95% CI, 3.66-4.10), and people with MS and depression (HR, 5.43; 95% CI, 4.88-5.96). Overall, 14% of the observed effect on mortality was attributable to the interaction between MS status and depression.
As for vascular diseases, people with MS had an increased risk regardless of their depression status. That said, people with MS and depression (HR, 3.30; 95% CI, 2.37-4.23) had a notably higher risk than people with MS and no depression (HR, 1.48; 95% CI, 1.23-1.74). Women with MS and depression also had a greater risk of vascular disease than women with MS and no depression, while men with MS did not have significantly different risks of acute coronary syndrome or composite macrovascular disease than those in the control group who did not suffer from depression.
Does treating depression decrease the likelihood of vascular disease?
“The take-home message for me is the importance of treating depression in this population, in which we see it with great regularity,” Joseph Berger, MD, professor of neurology and associate chief of the multiple sclerosis division at the University of Pennsylvania, Philadelphia, said in an interview. “The question that I have is: If you treat depression in an individual with MS or an individual who is simply depressed and thus at risk for the subsequent development of vascular disease, does it decrease the likelihood of their subsequent development of vascular disease in comparison to had you not?
“I presume it does,” he added, noting that “the theories underlying why depression would increase one’s risk of subsequent vascular disease are enumerated by the authors, including such things as increased inflammation. Now, the inflammation may be contributing to the depression, or the depression may be contributing to the inflammation; it may be one of those chicken-and-egg scenarios. But if you decrease the depression, do you thereby decrease the inflammation, which has a pernicious effect on endothelial cells and increases one’s vascular risk?
“Alternatively, lifestyle in depressed patients is also altered,” he said. “They’re far less likely to engage in exercise, healthy habits, and healthy diets, and more likely perhaps to smoke. These all need to be addressed, but this study certainly gives you a greater impetus as a MS neurologist to address the issue of depression, realizing that there is also this comorbidity of vascular disease.”
Evaluating the biological interaction between MS and depression
Based on this and other studies, the joint effect of MS and depression on all-cause mortality may qualify as a biological interaction, Amber Salter, PhD, of the University of Texas Southwestern Medical Center, Dallas, wrote in an accompanying editorial.
“Biological interactions consider whether the joint effect of two factors follow an additive pattern, or the joint effect of two factors is greater than the sum of the individual effects for each factor alone,” she wrote. And though the interaction was not found to be present for vascular disease and cardiovascular mortality, it was for all-cause mortality.
“When warranted, the evaluation of biological interactions in future studies should be considered to provide insight on target subpopulations for interventions or test for potential mechanistic forms of interaction,” she added.
Dr. Salter highlighted the study’s strengths, including a large sample size and six controls matched to each MS patient. She also stated that the researchers’ inability to control for risk factors like body mass index and physical activity means the 14% increase in mortality “may not be a large absolute increase in mortality when other covariates cannot be considered.” In addition, their lack of data on suicide – and its association with depression – offers up the possibility that increases in mortality could be tied to a “potentially modifiable risk” as opposed to a biologically increased one.
In acknowledging their study’s limitations, the authors stated that body mass index, though an important vascular risk factor, has a “modest” association with mortality, and that the average annual suicide rate in the MS population – though higher than in the non-MS population – is still “relatively low.”
Two of the authors disclosed receiving support, including grants and research funding, from various institutions and organizations in the United Kingdom, the United States, and Canada, as well as several pharmaceutical companies. Dr. Salter reported no relevant disclosures.
FROM NEUROLOGY
‘Deeper dive’ into opioid overdose deaths during COVID pandemic
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.
Nontraditional therapies for treatment-resistant depression
Presently, FDA-approved first-line treatments and standard adjunctive strategies (eg, lithium, thyroid supplementation, stimulants, second-generation antipsychotics) for major depressive disorder (MDD) often produce less-than-desired outcomes while carrying a potentially substantial safety and tolerability burden. The lack of clinically useful and individual-based biomarkers (eg, genetic, neurophysiological, imaging) is a major obstacle to enhancing treatment efficacy and/or decreasing associated adverse effects (AEs). While the discovery of such tools is being aggressively pursued and ultimately will facilitate a more precision-based choice of therapy, empirical strategies remain our primary approach.
In controlled trials, several nontraditional treatments used primarily as adjuncts to standard antidepressants have shown promise. These include “repurposed” (off-label) medications, herbal/nutraceuticals, anti-inflammatory/immune system therapies, device-based treatments, and other alternative approaches.
Importantly, some nontraditional treatments also demonstrate AEs (Table1-16). With a careful consideration of the risk/benefit balance, this article reviews some of the better-studied treatment options for patients with treatment-resistant depression (TRD). In Part 1, we will examine off-label medications. In Part 2, we will review other nontraditional approaches to TRD, including herbal/nutraceuticals, anti-inflammatory/immune system therapies, device-based treatments, and other alternative approaches.
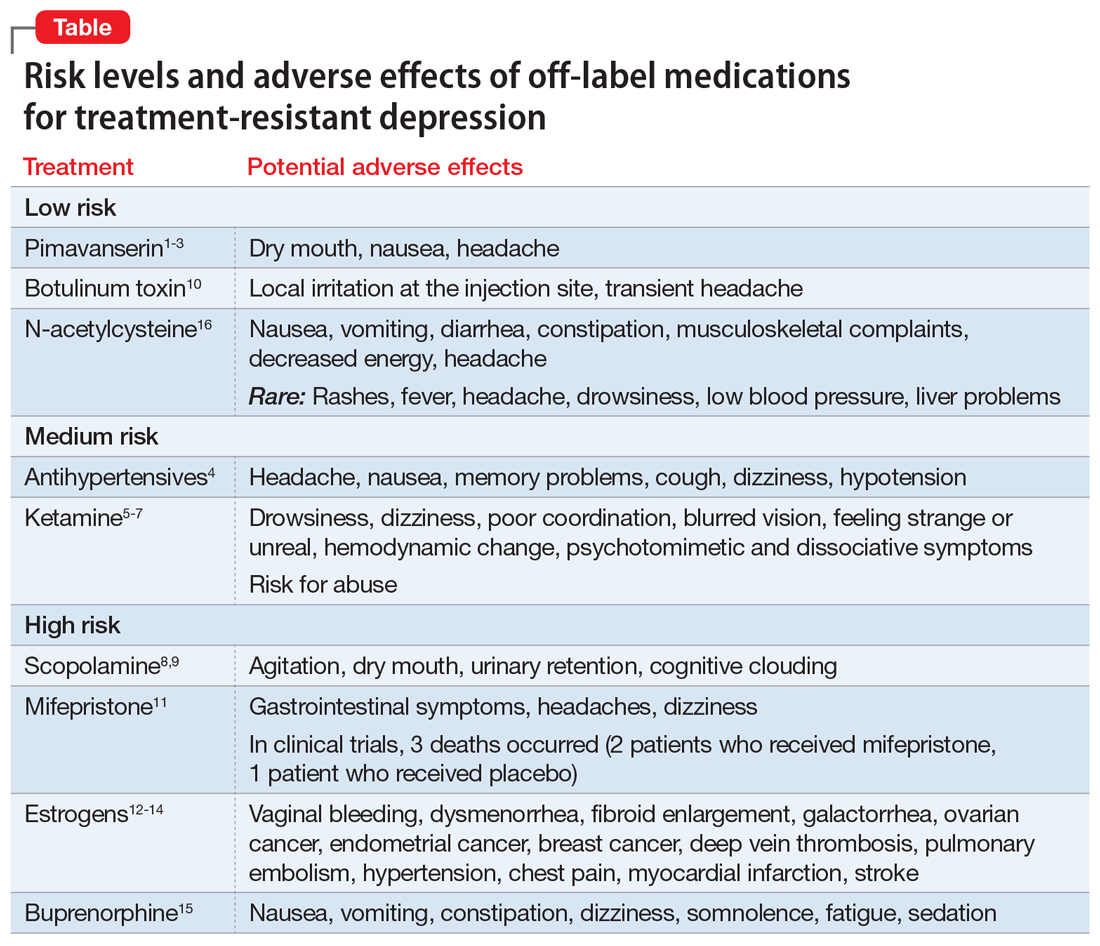
We believe this review will help clinicians who need to formulate a different approach after their patient with depression is not helped by traditional first-, second-, and third-line treatments. The potential options discussed in Part 1 of this article are categorized based on their putative mechanism of action (MOA) for depression.
Serotonergic and noradrenergic strategies
Pimavanserin is FDA-approved for treatment of Parkinson’s psychosis. Its potential MOA as an adjunctive strategy for MDD may involve 5-HT2A antagonist and inverse agonist receptor activity, as well as lesser effects at the 5-HT2Creceptor.
A 2-stage, 5-week randomized controlled trial (RCT) (CLARITY; N = 207) found adjunctive pimavanserin (34 mg/d) produced a robust antidepressant effect vs placebo in patients whose depression did not respond to selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs).1 Furthermore, a secondary analysis of the data suggested that pimavanserin also improved sleepiness (P < .0003) and daily functioning (P < .014) at Week 5.2
Unfortunately, two 6-week, Phase III RCTs (CLARITY-2 and -3; N = 298) did not find a statistically significant difference between active treatment and placebo. This was based on change in the primary outcome measure (Hamilton Depression Rating Scale-17 score) when adjunctive pimavanserin (34 mg/d) was added to an SSRI or SNRI in patients with TRD.3 There was, however, a significant difference favoring active treatment over placebo based on the Clinical Global Impression–Severity score.
Continue to: In these trials...
In these trials, pimavanserin was generally well-tolerated. The most common AEs were dry mouth, nausea, and headache. Pimavanserin has minimal activity at norepinephrine, dopamine, histamine, or acetylcholine receptors, thus avoiding AEs associated with these receptor interactions.
Given the mixed efficacy results of existing trials, further studies are needed to clarify this agent’s overall risk/benefit in the context of TRD.
Antihypertensive medications
Emerging data suggest that some beta-adrenergic blockers, angiotensin-inhibiting agents, and calcium antagonists are associated with a decreased incidence of depression. A large 2020 study (N = 3,747,190) used population-based Danish registries (2005 to 2015) to evaluate if any of the 41 most commonly prescribed antihypertensive medications were associated with the diagnosis of depressive disorder or use of antidepressants.4 These researchers found that enalapril, ramipril, amlodipine, propranolol, atenolol, bisoprolol, carvedilol (P < .001), and verapamil (P < .004) were strongly associated with a decreased risk of depression.4
Adverse effects across these different classes of antihypertensives are well characterized, can be substantial, and commonly are related to their impact on cardiovascular function (eg, hypotension). Clinically, these agents may be potential adjuncts for patients with TRD who need antihypertensive therapy. Their use and the choice of specific agent should only be determined in consultation with the patient’s primary care physician (PCP) or appropriate specialist.
Glutamatergic strategies
Ketamine is a dissociative anesthetic and analgesic. Its MOA for treating depression appears to occur primarily through antagonist activity at the N-methyl-
Continue to: Many published studies...
Many published studies and reviews have described ketamine’s role for treating MDD. Several studies have reported that low-dose (0.5 mg/kg) IV ketamine infusions can rapidly attenuate severe episodes of MDD as well as associated suicidality. For example, a meta-analysis of 9 RCTs (N = 368) comparing ketamine to placebo for acute treatment of unipolar and bipolar depression reported superior therapeutic effects with active treatment at 24 hours, 72 hours, and 7 days.6 The response and remission rates for ketamine were 52% and 21% at 24 hours; 48% and 24% at 72 hours; and 40% and 26% at 7 days, respectively.6
The most commonly reported AEs during the 4 hours after ketamine infusion included7:
- drowsiness, dizziness, poor coordination
- blurred vision, feeling strange or unreal
- hemodynamic changes (approximately 33%)
- small but significant (P < .05) increases in psychotomimetic and dissociative symptoms.
Because some individuals use ketamine recreationally, this agent also carries the risk of abuse.
Research is ongoing on strategies for long-term maintenance ketamine treatment, and the results of both short- and long-term trials will require careful scrutiny to better assess this agent’s safety and tolerability. Clinicians should first consider esketamine—the S-enantiomer of ketamine—because an intranasal formulation of this agent is FDA-approved for treating patients with TRD or MDD with suicidality when administered in a Risk Evaluation and Mitigation Strategy–certified setting.
Cholinergic strategies
Scopolamine is a potent muscarinic receptor antagonist used to prevent nausea and vomiting caused by motion sickness or medications used during surgery. Its use for MDD is based on the theory that muscarinic receptors may be hypersensitive in mood disorders.
Continue to: Several double-blind RCTs...
Several double-blind RCTs of patients with unipolar or bipolar depression that used 3 pulsed IV infusions (4.0 mcg/kg) over 15 minutes found a rapid, robust antidepressant effect with scopolamine vs placebo.8,9 The oral formulation might also be effective, but would not have a rapid onset.
Common adverse effects of scopolamine include agitation, dry mouth, urinary retention, and cognitive clouding. Given scopolamine’s substantial AE profile, it should be considered only for patients with TRD who could also benefit from the oral formulation for the medical indications noted above, should generally be avoided in older patients, and should be prescribed in consultation with the patient’s PCP.
Botulinum toxin. This neurotoxin inhibits acetylcholine release. It is used to treat disorders characterized by abnormal muscular contraction, such as strabismus, blepharospasm, and chronic pain syndromes. Its MOA for depression may involve its paralytic effects after injection into the glabella forehead muscle (based on the facial feedback hypothesis), as well as modulation of neurotransmitters implicated in the pathophysiology of depression.
In several small trials, injectable botulinum toxin type A (BTA) (29 units) demonstrated antidepressant effects. A recent review that considered 6 trials (N = 235; 4 of the 6 studies were RCTs, 3 of which were rated as high quality) concluded that BTA may be a promising treatment for MDD.10 Limitations of this review included lack of a priori hypotheses, small sample sizes, gender bias, and difficulty in blinding.
In clinical trials, the most common AEs included local irritation at the injection site and transient headache. This agent’s relatively mild AE profile and possible overlap when used for some of the medical indications noted above opens its potential use as an adjunct in patients with comorbid TRD.
Continue to: Endocrine strategies
Endocrine strategies
Mifepristone (RU486). This anti-glucocorticoid receptor antagonist is used as an abortifacient. Based on the theory that hyperactivity of the hypothalamic-pituitary-adrenal axis is implicated in the pathophysiology of MDD with psychotic features (psychotic depression), this agent has been studied as a treatment for this indication.
An analysis of 5 double-blind RCTs (N = 1,460) found that 7 days of mifepristone, 1,200 mg/d, was superior to placebo (P < .004) in reducing psychotic symptoms of depression.11 Plasma concentrations ≥1,600 ng/mL may be required to maximize benefit.11
Overall, this agent demonstrated a good safety profile in clinical trials, with treatment-emergent AEs reported in 556 (66.7%) patients who received mifepristone vs 386 (61.6%) patients who received placebo.11 Common AEs included gastrointestinal (GI) symptoms, headache, and dizziness. However, 3 deaths occurred: 2 patients who received mifepristone and 1 patient who received placebo. Given this potential for a fatal outcome, clinicians should first consider prescribing an adjunctive antipsychotic agent or electroconvulsive therapy.
Estrogens. These hormones are important for sexual and reproductive development and are used to treat various sexual/reproductive disorders, primarily in women. Their role in treating depression is based on the observation that perimenopause is accompanied by an increased risk of new and recurrent depression coincident with declining ovarian function.
Evidence supports the antidepressant efficacy of transdermal estradiol plus progesterone for perimenopausal depression, but not for postmenopausal depression.12-14 However, estrogens carry significant risks that must be carefully considered in relationship to their potential benefits. These risks include:
- vaginal bleeding, dysmenorrhea
- fibroid enlargement
- galactorrhea
- ovarian cancer, endometrial cancer, breast cancer
- deep vein thrombosis, pulmonary embolism
- hypertension, chest pain, myocardial infarction, stroke.
Continue to: The use of estrogens...
The use of estrogens as an adjunctive therapy for women with treatment-resistant perimenopausal depression should only be undertaken when standard strategies have failed, and in consultation with an endocrine specialist who can monitor for potentially serious AEs.
Opioid medications
Buprenorphine is used to treat opioid use disorder (OUD) as well as acute and chronic pain. The opioid system is involved in the regulation of mood and may be an appropriate target for novel antidepressants. The use of buprenorphine in combination with samidorphan (a preferential mu-opioid receptor antagonist) has shown initial promise for TRD while minimizing abuse potential.
Although earlier results were mixed, a pooled analysis of 2 recent large RCTs (N = 760) of patients with MDD who had not responded to antidepressants reported greater reduction in Montgomery-Åsberg Depression Rating Scale scores from baseline for active treatment (buprenorphine/samidorphan; 2 mg/2 mg) vs placebo at multiple timepoints, including end of treatment (-1.8; P < .010).15
The most common AEs included nausea, constipation, dizziness, vomiting, somnolence, fatigue, and sedation. There was minimal evidence of abuse, dependence, or opioid withdrawal. Due to the opioid crisis in the United States, the resulting relaxation of regulations regarding prescribing buprenorphine, and the high rates of depression among patients with OUD, buprenorphine/samidorphan, which is an investigational agent that is not FDA-approved, may be particularly helpful for patients with OUD who also experience comorbid TRD.
Antioxidant agents
N-acetylcysteine (NAC) is an amino acid that can treat acetaminophen toxicity and moderate hepatic damage by increasing glutathione levels. Glutathione is also the primary antioxidant in the CNS. NAC may protect against oxidative stress, chelate heavy metals, reduce inflammation, protect against mitochondrial dysfunction, inhibit apoptosis, and enhance neurogenesis, all potential pathophysiological processes that may contribute to depression.16
Continue to: A systematic review...
A systematic review and meta-analysis of 5 RCTs (N = 574) considered patients with various depression diagnoses who were randomized to adjunctive NAC, 1,000 mg twice a day, or placebo. Over 12 to 24 weeks, there was a significantly greater improvement in mood symptoms and functionality with NAC vs placebo.16
Overall, NAC was well-tolerated. The most common AEs were GI symptoms, musculoskeletal complaints, decreased energy, and headache. While NAC has been touted as a potential adjunct therapy for several psychiatric disorders, including TRD, the evidence for benefit remains limited. Given its favorable AE profile, however, and over-the-counter availability, it remains an option for select patients. It is important to ask patients if they are already taking NAC.
Options beyond off-label medications
There are a multitude of options available for addressing TRD. Many FDA-approved medications are repurposed and prescribed off-label for other indications when the risk/benefit balance is favorable. In Part 1 of this article, we reviewed several off-label medications that have supportive controlled data for treating TRD. In Part 2, we will review other nontraditional therapies for TRD, including herbal/nutraceuticals, anti-inflammatory/immune system therapies, device-based treatments, and other alternative approaches.
Bottom Line
Off-label medications that may offer benefit for patients with treatment-resistant depression (TRD) include pimavanserin, antihypertensive agents, ketamine, scopolamine, botulinum toxin, mifepristone, estrogens, buprenorphine, and N-acetylcysteine. Although some evidence supports use of these agents as adjuncts for TRD, an individualized risk/benefit analysis is required.
Related Resource
- Joshi KG, Frierson RL. Off-label prescribing: How to limit your liability. Current Psychiatry. 2020;19(9):12,39.
Drug Brand Names
Amlodipine • Katerzia, Norvasc
Atenolol • Tenormin
Bisoprolol • Zebeta
Buprenorphine • Sublocade, Subutex
Carvedilol • Coreg
Enalapril • Vasotec
Esketamine • Spravato
Estradiol transdermal • Estraderm
Ketamine • Ketalar
Mifepristone • Mifeprex
Pimavanserin • Nuplazid
Progesterone • Prometrium
Propranolol • Inderal
Ramipril • Altace
Verapamil • Calan, Verelan
1. Fava M, Dirks B, Freeman M, et al. A phase 2, randomized, double-blind, placebo-controlled study of adjunctive pimavanserin in patients with major depressive disorder and an inadequate response to therapy (CLARITY). J Clin Psychiatry. 2019;80(6):19m12928.
2. Jha MK, Fava M, Freeman MP, et al. Effect of adjunctive pimavanserin on sleep/wakefulness in patients with major depressive disorder: secondary analysis from CLARITY. J Clin Psychiatry. 2020;82(1):20m13425.
3. ACADIA Pharmaceuticals announces top-line results from the Phase 3 CLARITY study evaluating pimavanserin for the adjunctive treatment of major depressive disorder. News release. Acadia Pharmaceuticals Inc. Published July 20, 2020. https://ir.acadia-pharm.com/news-releases/news-release-details/acadia-pharmaceuticals-announces-top-line-results-phase-3-0
4. Kessing LV, Rytgaard HC, Ekstrom CT, et al. Antihypertensive drugs and risk of depression: a nationwide population-based study. Hypertension. 2020;76(4):1263-1279.
5. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
6. Han Y, Chen J, Zou D, et al. Efficacy of ketamine in the rapid treatment of major depressive disorder: a meta-analysis of randomized, double-blind, placebo-controlled studies. Neuropsychiatr Dis Treat. 2016;12:2859-2867.
7. Wan LB, Levitch CF, Perez AM, et al. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry. 2015;76(3):247-252.
8. Hasselmann, H. Scopolamine and depression: a role for muscarinic antagonism? CNS Neurol Disord Drug Targets. 2014;13(4):673-683.
9. Drevets WC, Zarate CA Jr, Furey ML. Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol Psychiatry. 2013;73(12):1156-1163.
10. Stearns TP, Shad MU, Guzman GC. Glabellar botulinum toxin injections in major depressive disorder: a critical review. Prim Care Companion CNS Disord. 2018;20(5): 18r02298.
11. Block TS, Kushner H, Kalin N, et al. Combined analysis of mifepristone for psychotic depression: plasma levels associated with clinical response. Biol Psychiatry. 2018;84(1):46-54.
12. Rubinow DR, Johnson SL, Schmidt PJ, et al. Efficacy of estradiol in perimenopausal depression: so much promise and so few answers. Depress Anxiety. 2015;32(8):539-549.
13. Schmidt PJ, Ben Dor R, Martinez PE, et al. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. 2015;72(7):714-726.
14. Gordon JL, Rubinow DR, Eisenlohr-Moul TA, et al. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):149-157.
15. Fava M, Thase ME, Trivedi MH, et al. Opioid system modulation with buprenorphine/samidorphan combination for major depressive disorder: two randomized controlled studies. Mol Psychiatry. 2020;25(7):1580-1591.
16. Fernandes BS, Dean OM, Dodd S, et al. N-Acetylcysteine in depressive symptoms and functionality: a systematic review and meta-analysis. J Clin Psychiatry. 2016;77(4):e457-466.
Presently, FDA-approved first-line treatments and standard adjunctive strategies (eg, lithium, thyroid supplementation, stimulants, second-generation antipsychotics) for major depressive disorder (MDD) often produce less-than-desired outcomes while carrying a potentially substantial safety and tolerability burden. The lack of clinically useful and individual-based biomarkers (eg, genetic, neurophysiological, imaging) is a major obstacle to enhancing treatment efficacy and/or decreasing associated adverse effects (AEs). While the discovery of such tools is being aggressively pursued and ultimately will facilitate a more precision-based choice of therapy, empirical strategies remain our primary approach.
In controlled trials, several nontraditional treatments used primarily as adjuncts to standard antidepressants have shown promise. These include “repurposed” (off-label) medications, herbal/nutraceuticals, anti-inflammatory/immune system therapies, device-based treatments, and other alternative approaches.
Importantly, some nontraditional treatments also demonstrate AEs (Table1-16). With a careful consideration of the risk/benefit balance, this article reviews some of the better-studied treatment options for patients with treatment-resistant depression (TRD). In Part 1, we will examine off-label medications. In Part 2, we will review other nontraditional approaches to TRD, including herbal/nutraceuticals, anti-inflammatory/immune system therapies, device-based treatments, and other alternative approaches.

We believe this review will help clinicians who need to formulate a different approach after their patient with depression is not helped by traditional first-, second-, and third-line treatments. The potential options discussed in Part 1 of this article are categorized based on their putative mechanism of action (MOA) for depression.
Serotonergic and noradrenergic strategies
Pimavanserin is FDA-approved for treatment of Parkinson’s psychosis. Its potential MOA as an adjunctive strategy for MDD may involve 5-HT2A antagonist and inverse agonist receptor activity, as well as lesser effects at the 5-HT2Creceptor.
A 2-stage, 5-week randomized controlled trial (RCT) (CLARITY; N = 207) found adjunctive pimavanserin (34 mg/d) produced a robust antidepressant effect vs placebo in patients whose depression did not respond to selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs).1 Furthermore, a secondary analysis of the data suggested that pimavanserin also improved sleepiness (P < .0003) and daily functioning (P < .014) at Week 5.2
Unfortunately, two 6-week, Phase III RCTs (CLARITY-2 and -3; N = 298) did not find a statistically significant difference between active treatment and placebo. This was based on change in the primary outcome measure (Hamilton Depression Rating Scale-17 score) when adjunctive pimavanserin (34 mg/d) was added to an SSRI or SNRI in patients with TRD.3 There was, however, a significant difference favoring active treatment over placebo based on the Clinical Global Impression–Severity score.
Continue to: In these trials...
In these trials, pimavanserin was generally well-tolerated. The most common AEs were dry mouth, nausea, and headache. Pimavanserin has minimal activity at norepinephrine, dopamine, histamine, or acetylcholine receptors, thus avoiding AEs associated with these receptor interactions.
Given the mixed efficacy results of existing trials, further studies are needed to clarify this agent’s overall risk/benefit in the context of TRD.
Antihypertensive medications
Emerging data suggest that some beta-adrenergic blockers, angiotensin-inhibiting agents, and calcium antagonists are associated with a decreased incidence of depression. A large 2020 study (N = 3,747,190) used population-based Danish registries (2005 to 2015) to evaluate if any of the 41 most commonly prescribed antihypertensive medications were associated with the diagnosis of depressive disorder or use of antidepressants.4 These researchers found that enalapril, ramipril, amlodipine, propranolol, atenolol, bisoprolol, carvedilol (P < .001), and verapamil (P < .004) were strongly associated with a decreased risk of depression.4
Adverse effects across these different classes of antihypertensives are well characterized, can be substantial, and commonly are related to their impact on cardiovascular function (eg, hypotension). Clinically, these agents may be potential adjuncts for patients with TRD who need antihypertensive therapy. Their use and the choice of specific agent should only be determined in consultation with the patient’s primary care physician (PCP) or appropriate specialist.
Glutamatergic strategies
Ketamine is a dissociative anesthetic and analgesic. Its MOA for treating depression appears to occur primarily through antagonist activity at the N-methyl-
Continue to: Many published studies...
Many published studies and reviews have described ketamine’s role for treating MDD. Several studies have reported that low-dose (0.5 mg/kg) IV ketamine infusions can rapidly attenuate severe episodes of MDD as well as associated suicidality. For example, a meta-analysis of 9 RCTs (N = 368) comparing ketamine to placebo for acute treatment of unipolar and bipolar depression reported superior therapeutic effects with active treatment at 24 hours, 72 hours, and 7 days.6 The response and remission rates for ketamine were 52% and 21% at 24 hours; 48% and 24% at 72 hours; and 40% and 26% at 7 days, respectively.6
The most commonly reported AEs during the 4 hours after ketamine infusion included7:
- drowsiness, dizziness, poor coordination
- blurred vision, feeling strange or unreal
- hemodynamic changes (approximately 33%)
- small but significant (P < .05) increases in psychotomimetic and dissociative symptoms.
Because some individuals use ketamine recreationally, this agent also carries the risk of abuse.
Research is ongoing on strategies for long-term maintenance ketamine treatment, and the results of both short- and long-term trials will require careful scrutiny to better assess this agent’s safety and tolerability. Clinicians should first consider esketamine—the S-enantiomer of ketamine—because an intranasal formulation of this agent is FDA-approved for treating patients with TRD or MDD with suicidality when administered in a Risk Evaluation and Mitigation Strategy–certified setting.
Cholinergic strategies
Scopolamine is a potent muscarinic receptor antagonist used to prevent nausea and vomiting caused by motion sickness or medications used during surgery. Its use for MDD is based on the theory that muscarinic receptors may be hypersensitive in mood disorders.
Continue to: Several double-blind RCTs...
Several double-blind RCTs of patients with unipolar or bipolar depression that used 3 pulsed IV infusions (4.0 mcg/kg) over 15 minutes found a rapid, robust antidepressant effect with scopolamine vs placebo.8,9 The oral formulation might also be effective, but would not have a rapid onset.
Common adverse effects of scopolamine include agitation, dry mouth, urinary retention, and cognitive clouding. Given scopolamine’s substantial AE profile, it should be considered only for patients with TRD who could also benefit from the oral formulation for the medical indications noted above, should generally be avoided in older patients, and should be prescribed in consultation with the patient’s PCP.
Botulinum toxin. This neurotoxin inhibits acetylcholine release. It is used to treat disorders characterized by abnormal muscular contraction, such as strabismus, blepharospasm, and chronic pain syndromes. Its MOA for depression may involve its paralytic effects after injection into the glabella forehead muscle (based on the facial feedback hypothesis), as well as modulation of neurotransmitters implicated in the pathophysiology of depression.
In several small trials, injectable botulinum toxin type A (BTA) (29 units) demonstrated antidepressant effects. A recent review that considered 6 trials (N = 235; 4 of the 6 studies were RCTs, 3 of which were rated as high quality) concluded that BTA may be a promising treatment for MDD.10 Limitations of this review included lack of a priori hypotheses, small sample sizes, gender bias, and difficulty in blinding.
In clinical trials, the most common AEs included local irritation at the injection site and transient headache. This agent’s relatively mild AE profile and possible overlap when used for some of the medical indications noted above opens its potential use as an adjunct in patients with comorbid TRD.
Continue to: Endocrine strategies
Endocrine strategies
Mifepristone (RU486). This anti-glucocorticoid receptor antagonist is used as an abortifacient. Based on the theory that hyperactivity of the hypothalamic-pituitary-adrenal axis is implicated in the pathophysiology of MDD with psychotic features (psychotic depression), this agent has been studied as a treatment for this indication.
An analysis of 5 double-blind RCTs (N = 1,460) found that 7 days of mifepristone, 1,200 mg/d, was superior to placebo (P < .004) in reducing psychotic symptoms of depression.11 Plasma concentrations ≥1,600 ng/mL may be required to maximize benefit.11
Overall, this agent demonstrated a good safety profile in clinical trials, with treatment-emergent AEs reported in 556 (66.7%) patients who received mifepristone vs 386 (61.6%) patients who received placebo.11 Common AEs included gastrointestinal (GI) symptoms, headache, and dizziness. However, 3 deaths occurred: 2 patients who received mifepristone and 1 patient who received placebo. Given this potential for a fatal outcome, clinicians should first consider prescribing an adjunctive antipsychotic agent or electroconvulsive therapy.
Estrogens. These hormones are important for sexual and reproductive development and are used to treat various sexual/reproductive disorders, primarily in women. Their role in treating depression is based on the observation that perimenopause is accompanied by an increased risk of new and recurrent depression coincident with declining ovarian function.
Evidence supports the antidepressant efficacy of transdermal estradiol plus progesterone for perimenopausal depression, but not for postmenopausal depression.12-14 However, estrogens carry significant risks that must be carefully considered in relationship to their potential benefits. These risks include:
- vaginal bleeding, dysmenorrhea
- fibroid enlargement
- galactorrhea
- ovarian cancer, endometrial cancer, breast cancer
- deep vein thrombosis, pulmonary embolism
- hypertension, chest pain, myocardial infarction, stroke.
Continue to: The use of estrogens...
The use of estrogens as an adjunctive therapy for women with treatment-resistant perimenopausal depression should only be undertaken when standard strategies have failed, and in consultation with an endocrine specialist who can monitor for potentially serious AEs.
Opioid medications
Buprenorphine is used to treat opioid use disorder (OUD) as well as acute and chronic pain. The opioid system is involved in the regulation of mood and may be an appropriate target for novel antidepressants. The use of buprenorphine in combination with samidorphan (a preferential mu-opioid receptor antagonist) has shown initial promise for TRD while minimizing abuse potential.
Although earlier results were mixed, a pooled analysis of 2 recent large RCTs (N = 760) of patients with MDD who had not responded to antidepressants reported greater reduction in Montgomery-Åsberg Depression Rating Scale scores from baseline for active treatment (buprenorphine/samidorphan; 2 mg/2 mg) vs placebo at multiple timepoints, including end of treatment (-1.8; P < .010).15
The most common AEs included nausea, constipation, dizziness, vomiting, somnolence, fatigue, and sedation. There was minimal evidence of abuse, dependence, or opioid withdrawal. Due to the opioid crisis in the United States, the resulting relaxation of regulations regarding prescribing buprenorphine, and the high rates of depression among patients with OUD, buprenorphine/samidorphan, which is an investigational agent that is not FDA-approved, may be particularly helpful for patients with OUD who also experience comorbid TRD.
Antioxidant agents
N-acetylcysteine (NAC) is an amino acid that can treat acetaminophen toxicity and moderate hepatic damage by increasing glutathione levels. Glutathione is also the primary antioxidant in the CNS. NAC may protect against oxidative stress, chelate heavy metals, reduce inflammation, protect against mitochondrial dysfunction, inhibit apoptosis, and enhance neurogenesis, all potential pathophysiological processes that may contribute to depression.16
Continue to: A systematic review...
A systematic review and meta-analysis of 5 RCTs (N = 574) considered patients with various depression diagnoses who were randomized to adjunctive NAC, 1,000 mg twice a day, or placebo. Over 12 to 24 weeks, there was a significantly greater improvement in mood symptoms and functionality with NAC vs placebo.16
Overall, NAC was well-tolerated. The most common AEs were GI symptoms, musculoskeletal complaints, decreased energy, and headache. While NAC has been touted as a potential adjunct therapy for several psychiatric disorders, including TRD, the evidence for benefit remains limited. Given its favorable AE profile, however, and over-the-counter availability, it remains an option for select patients. It is important to ask patients if they are already taking NAC.
Options beyond off-label medications
There are a multitude of options available for addressing TRD. Many FDA-approved medications are repurposed and prescribed off-label for other indications when the risk/benefit balance is favorable. In Part 1 of this article, we reviewed several off-label medications that have supportive controlled data for treating TRD. In Part 2, we will review other nontraditional therapies for TRD, including herbal/nutraceuticals, anti-inflammatory/immune system therapies, device-based treatments, and other alternative approaches.
Bottom Line
Off-label medications that may offer benefit for patients with treatment-resistant depression (TRD) include pimavanserin, antihypertensive agents, ketamine, scopolamine, botulinum toxin, mifepristone, estrogens, buprenorphine, and N-acetylcysteine. Although some evidence supports use of these agents as adjuncts for TRD, an individualized risk/benefit analysis is required.
Related Resource
- Joshi KG, Frierson RL. Off-label prescribing: How to limit your liability. Current Psychiatry. 2020;19(9):12,39.
Drug Brand Names
Amlodipine • Katerzia, Norvasc
Atenolol • Tenormin
Bisoprolol • Zebeta
Buprenorphine • Sublocade, Subutex
Carvedilol • Coreg
Enalapril • Vasotec
Esketamine • Spravato
Estradiol transdermal • Estraderm
Ketamine • Ketalar
Mifepristone • Mifeprex
Pimavanserin • Nuplazid
Progesterone • Prometrium
Propranolol • Inderal
Ramipril • Altace
Verapamil • Calan, Verelan
Presently, FDA-approved first-line treatments and standard adjunctive strategies (eg, lithium, thyroid supplementation, stimulants, second-generation antipsychotics) for major depressive disorder (MDD) often produce less-than-desired outcomes while carrying a potentially substantial safety and tolerability burden. The lack of clinically useful and individual-based biomarkers (eg, genetic, neurophysiological, imaging) is a major obstacle to enhancing treatment efficacy and/or decreasing associated adverse effects (AEs). While the discovery of such tools is being aggressively pursued and ultimately will facilitate a more precision-based choice of therapy, empirical strategies remain our primary approach.
In controlled trials, several nontraditional treatments used primarily as adjuncts to standard antidepressants have shown promise. These include “repurposed” (off-label) medications, herbal/nutraceuticals, anti-inflammatory/immune system therapies, device-based treatments, and other alternative approaches.
Importantly, some nontraditional treatments also demonstrate AEs (Table1-16). With a careful consideration of the risk/benefit balance, this article reviews some of the better-studied treatment options for patients with treatment-resistant depression (TRD). In Part 1, we will examine off-label medications. In Part 2, we will review other nontraditional approaches to TRD, including herbal/nutraceuticals, anti-inflammatory/immune system therapies, device-based treatments, and other alternative approaches.

We believe this review will help clinicians who need to formulate a different approach after their patient with depression is not helped by traditional first-, second-, and third-line treatments. The potential options discussed in Part 1 of this article are categorized based on their putative mechanism of action (MOA) for depression.
Serotonergic and noradrenergic strategies
Pimavanserin is FDA-approved for treatment of Parkinson’s psychosis. Its potential MOA as an adjunctive strategy for MDD may involve 5-HT2A antagonist and inverse agonist receptor activity, as well as lesser effects at the 5-HT2Creceptor.
A 2-stage, 5-week randomized controlled trial (RCT) (CLARITY; N = 207) found adjunctive pimavanserin (34 mg/d) produced a robust antidepressant effect vs placebo in patients whose depression did not respond to selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs).1 Furthermore, a secondary analysis of the data suggested that pimavanserin also improved sleepiness (P < .0003) and daily functioning (P < .014) at Week 5.2
Unfortunately, two 6-week, Phase III RCTs (CLARITY-2 and -3; N = 298) did not find a statistically significant difference between active treatment and placebo. This was based on change in the primary outcome measure (Hamilton Depression Rating Scale-17 score) when adjunctive pimavanserin (34 mg/d) was added to an SSRI or SNRI in patients with TRD.3 There was, however, a significant difference favoring active treatment over placebo based on the Clinical Global Impression–Severity score.
Continue to: In these trials...
In these trials, pimavanserin was generally well-tolerated. The most common AEs were dry mouth, nausea, and headache. Pimavanserin has minimal activity at norepinephrine, dopamine, histamine, or acetylcholine receptors, thus avoiding AEs associated with these receptor interactions.
Given the mixed efficacy results of existing trials, further studies are needed to clarify this agent’s overall risk/benefit in the context of TRD.
Antihypertensive medications
Emerging data suggest that some beta-adrenergic blockers, angiotensin-inhibiting agents, and calcium antagonists are associated with a decreased incidence of depression. A large 2020 study (N = 3,747,190) used population-based Danish registries (2005 to 2015) to evaluate if any of the 41 most commonly prescribed antihypertensive medications were associated with the diagnosis of depressive disorder or use of antidepressants.4 These researchers found that enalapril, ramipril, amlodipine, propranolol, atenolol, bisoprolol, carvedilol (P < .001), and verapamil (P < .004) were strongly associated with a decreased risk of depression.4
Adverse effects across these different classes of antihypertensives are well characterized, can be substantial, and commonly are related to their impact on cardiovascular function (eg, hypotension). Clinically, these agents may be potential adjuncts for patients with TRD who need antihypertensive therapy. Their use and the choice of specific agent should only be determined in consultation with the patient’s primary care physician (PCP) or appropriate specialist.
Glutamatergic strategies
Ketamine is a dissociative anesthetic and analgesic. Its MOA for treating depression appears to occur primarily through antagonist activity at the N-methyl-
Continue to: Many published studies...
Many published studies and reviews have described ketamine’s role for treating MDD. Several studies have reported that low-dose (0.5 mg/kg) IV ketamine infusions can rapidly attenuate severe episodes of MDD as well as associated suicidality. For example, a meta-analysis of 9 RCTs (N = 368) comparing ketamine to placebo for acute treatment of unipolar and bipolar depression reported superior therapeutic effects with active treatment at 24 hours, 72 hours, and 7 days.6 The response and remission rates for ketamine were 52% and 21% at 24 hours; 48% and 24% at 72 hours; and 40% and 26% at 7 days, respectively.6
The most commonly reported AEs during the 4 hours after ketamine infusion included7:
- drowsiness, dizziness, poor coordination
- blurred vision, feeling strange or unreal
- hemodynamic changes (approximately 33%)
- small but significant (P < .05) increases in psychotomimetic and dissociative symptoms.
Because some individuals use ketamine recreationally, this agent also carries the risk of abuse.
Research is ongoing on strategies for long-term maintenance ketamine treatment, and the results of both short- and long-term trials will require careful scrutiny to better assess this agent’s safety and tolerability. Clinicians should first consider esketamine—the S-enantiomer of ketamine—because an intranasal formulation of this agent is FDA-approved for treating patients with TRD or MDD with suicidality when administered in a Risk Evaluation and Mitigation Strategy–certified setting.
Cholinergic strategies
Scopolamine is a potent muscarinic receptor antagonist used to prevent nausea and vomiting caused by motion sickness or medications used during surgery. Its use for MDD is based on the theory that muscarinic receptors may be hypersensitive in mood disorders.
Continue to: Several double-blind RCTs...
Several double-blind RCTs of patients with unipolar or bipolar depression that used 3 pulsed IV infusions (4.0 mcg/kg) over 15 minutes found a rapid, robust antidepressant effect with scopolamine vs placebo.8,9 The oral formulation might also be effective, but would not have a rapid onset.
Common adverse effects of scopolamine include agitation, dry mouth, urinary retention, and cognitive clouding. Given scopolamine’s substantial AE profile, it should be considered only for patients with TRD who could also benefit from the oral formulation for the medical indications noted above, should generally be avoided in older patients, and should be prescribed in consultation with the patient’s PCP.
Botulinum toxin. This neurotoxin inhibits acetylcholine release. It is used to treat disorders characterized by abnormal muscular contraction, such as strabismus, blepharospasm, and chronic pain syndromes. Its MOA for depression may involve its paralytic effects after injection into the glabella forehead muscle (based on the facial feedback hypothesis), as well as modulation of neurotransmitters implicated in the pathophysiology of depression.
In several small trials, injectable botulinum toxin type A (BTA) (29 units) demonstrated antidepressant effects. A recent review that considered 6 trials (N = 235; 4 of the 6 studies were RCTs, 3 of which were rated as high quality) concluded that BTA may be a promising treatment for MDD.10 Limitations of this review included lack of a priori hypotheses, small sample sizes, gender bias, and difficulty in blinding.
In clinical trials, the most common AEs included local irritation at the injection site and transient headache. This agent’s relatively mild AE profile and possible overlap when used for some of the medical indications noted above opens its potential use as an adjunct in patients with comorbid TRD.
Continue to: Endocrine strategies
Endocrine strategies
Mifepristone (RU486). This anti-glucocorticoid receptor antagonist is used as an abortifacient. Based on the theory that hyperactivity of the hypothalamic-pituitary-adrenal axis is implicated in the pathophysiology of MDD with psychotic features (psychotic depression), this agent has been studied as a treatment for this indication.
An analysis of 5 double-blind RCTs (N = 1,460) found that 7 days of mifepristone, 1,200 mg/d, was superior to placebo (P < .004) in reducing psychotic symptoms of depression.11 Plasma concentrations ≥1,600 ng/mL may be required to maximize benefit.11
Overall, this agent demonstrated a good safety profile in clinical trials, with treatment-emergent AEs reported in 556 (66.7%) patients who received mifepristone vs 386 (61.6%) patients who received placebo.11 Common AEs included gastrointestinal (GI) symptoms, headache, and dizziness. However, 3 deaths occurred: 2 patients who received mifepristone and 1 patient who received placebo. Given this potential for a fatal outcome, clinicians should first consider prescribing an adjunctive antipsychotic agent or electroconvulsive therapy.
Estrogens. These hormones are important for sexual and reproductive development and are used to treat various sexual/reproductive disorders, primarily in women. Their role in treating depression is based on the observation that perimenopause is accompanied by an increased risk of new and recurrent depression coincident with declining ovarian function.
Evidence supports the antidepressant efficacy of transdermal estradiol plus progesterone for perimenopausal depression, but not for postmenopausal depression.12-14 However, estrogens carry significant risks that must be carefully considered in relationship to their potential benefits. These risks include:
- vaginal bleeding, dysmenorrhea
- fibroid enlargement
- galactorrhea
- ovarian cancer, endometrial cancer, breast cancer
- deep vein thrombosis, pulmonary embolism
- hypertension, chest pain, myocardial infarction, stroke.
Continue to: The use of estrogens...
The use of estrogens as an adjunctive therapy for women with treatment-resistant perimenopausal depression should only be undertaken when standard strategies have failed, and in consultation with an endocrine specialist who can monitor for potentially serious AEs.
Opioid medications
Buprenorphine is used to treat opioid use disorder (OUD) as well as acute and chronic pain. The opioid system is involved in the regulation of mood and may be an appropriate target for novel antidepressants. The use of buprenorphine in combination with samidorphan (a preferential mu-opioid receptor antagonist) has shown initial promise for TRD while minimizing abuse potential.
Although earlier results were mixed, a pooled analysis of 2 recent large RCTs (N = 760) of patients with MDD who had not responded to antidepressants reported greater reduction in Montgomery-Åsberg Depression Rating Scale scores from baseline for active treatment (buprenorphine/samidorphan; 2 mg/2 mg) vs placebo at multiple timepoints, including end of treatment (-1.8; P < .010).15
The most common AEs included nausea, constipation, dizziness, vomiting, somnolence, fatigue, and sedation. There was minimal evidence of abuse, dependence, or opioid withdrawal. Due to the opioid crisis in the United States, the resulting relaxation of regulations regarding prescribing buprenorphine, and the high rates of depression among patients with OUD, buprenorphine/samidorphan, which is an investigational agent that is not FDA-approved, may be particularly helpful for patients with OUD who also experience comorbid TRD.
Antioxidant agents
N-acetylcysteine (NAC) is an amino acid that can treat acetaminophen toxicity and moderate hepatic damage by increasing glutathione levels. Glutathione is also the primary antioxidant in the CNS. NAC may protect against oxidative stress, chelate heavy metals, reduce inflammation, protect against mitochondrial dysfunction, inhibit apoptosis, and enhance neurogenesis, all potential pathophysiological processes that may contribute to depression.16
Continue to: A systematic review...
A systematic review and meta-analysis of 5 RCTs (N = 574) considered patients with various depression diagnoses who were randomized to adjunctive NAC, 1,000 mg twice a day, or placebo. Over 12 to 24 weeks, there was a significantly greater improvement in mood symptoms and functionality with NAC vs placebo.16
Overall, NAC was well-tolerated. The most common AEs were GI symptoms, musculoskeletal complaints, decreased energy, and headache. While NAC has been touted as a potential adjunct therapy for several psychiatric disorders, including TRD, the evidence for benefit remains limited. Given its favorable AE profile, however, and over-the-counter availability, it remains an option for select patients. It is important to ask patients if they are already taking NAC.
Options beyond off-label medications
There are a multitude of options available for addressing TRD. Many FDA-approved medications are repurposed and prescribed off-label for other indications when the risk/benefit balance is favorable. In Part 1 of this article, we reviewed several off-label medications that have supportive controlled data for treating TRD. In Part 2, we will review other nontraditional therapies for TRD, including herbal/nutraceuticals, anti-inflammatory/immune system therapies, device-based treatments, and other alternative approaches.
Bottom Line
Off-label medications that may offer benefit for patients with treatment-resistant depression (TRD) include pimavanserin, antihypertensive agents, ketamine, scopolamine, botulinum toxin, mifepristone, estrogens, buprenorphine, and N-acetylcysteine. Although some evidence supports use of these agents as adjuncts for TRD, an individualized risk/benefit analysis is required.
Related Resource
- Joshi KG, Frierson RL. Off-label prescribing: How to limit your liability. Current Psychiatry. 2020;19(9):12,39.
Drug Brand Names
Amlodipine • Katerzia, Norvasc
Atenolol • Tenormin
Bisoprolol • Zebeta
Buprenorphine • Sublocade, Subutex
Carvedilol • Coreg
Enalapril • Vasotec
Esketamine • Spravato
Estradiol transdermal • Estraderm
Ketamine • Ketalar
Mifepristone • Mifeprex
Pimavanserin • Nuplazid
Progesterone • Prometrium
Propranolol • Inderal
Ramipril • Altace
Verapamil • Calan, Verelan
1. Fava M, Dirks B, Freeman M, et al. A phase 2, randomized, double-blind, placebo-controlled study of adjunctive pimavanserin in patients with major depressive disorder and an inadequate response to therapy (CLARITY). J Clin Psychiatry. 2019;80(6):19m12928.
2. Jha MK, Fava M, Freeman MP, et al. Effect of adjunctive pimavanserin on sleep/wakefulness in patients with major depressive disorder: secondary analysis from CLARITY. J Clin Psychiatry. 2020;82(1):20m13425.
3. ACADIA Pharmaceuticals announces top-line results from the Phase 3 CLARITY study evaluating pimavanserin for the adjunctive treatment of major depressive disorder. News release. Acadia Pharmaceuticals Inc. Published July 20, 2020. https://ir.acadia-pharm.com/news-releases/news-release-details/acadia-pharmaceuticals-announces-top-line-results-phase-3-0
4. Kessing LV, Rytgaard HC, Ekstrom CT, et al. Antihypertensive drugs and risk of depression: a nationwide population-based study. Hypertension. 2020;76(4):1263-1279.
5. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
6. Han Y, Chen J, Zou D, et al. Efficacy of ketamine in the rapid treatment of major depressive disorder: a meta-analysis of randomized, double-blind, placebo-controlled studies. Neuropsychiatr Dis Treat. 2016;12:2859-2867.
7. Wan LB, Levitch CF, Perez AM, et al. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry. 2015;76(3):247-252.
8. Hasselmann, H. Scopolamine and depression: a role for muscarinic antagonism? CNS Neurol Disord Drug Targets. 2014;13(4):673-683.
9. Drevets WC, Zarate CA Jr, Furey ML. Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol Psychiatry. 2013;73(12):1156-1163.
10. Stearns TP, Shad MU, Guzman GC. Glabellar botulinum toxin injections in major depressive disorder: a critical review. Prim Care Companion CNS Disord. 2018;20(5): 18r02298.
11. Block TS, Kushner H, Kalin N, et al. Combined analysis of mifepristone for psychotic depression: plasma levels associated with clinical response. Biol Psychiatry. 2018;84(1):46-54.
12. Rubinow DR, Johnson SL, Schmidt PJ, et al. Efficacy of estradiol in perimenopausal depression: so much promise and so few answers. Depress Anxiety. 2015;32(8):539-549.
13. Schmidt PJ, Ben Dor R, Martinez PE, et al. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. 2015;72(7):714-726.
14. Gordon JL, Rubinow DR, Eisenlohr-Moul TA, et al. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):149-157.
15. Fava M, Thase ME, Trivedi MH, et al. Opioid system modulation with buprenorphine/samidorphan combination for major depressive disorder: two randomized controlled studies. Mol Psychiatry. 2020;25(7):1580-1591.
16. Fernandes BS, Dean OM, Dodd S, et al. N-Acetylcysteine in depressive symptoms and functionality: a systematic review and meta-analysis. J Clin Psychiatry. 2016;77(4):e457-466.
1. Fava M, Dirks B, Freeman M, et al. A phase 2, randomized, double-blind, placebo-controlled study of adjunctive pimavanserin in patients with major depressive disorder and an inadequate response to therapy (CLARITY). J Clin Psychiatry. 2019;80(6):19m12928.
2. Jha MK, Fava M, Freeman MP, et al. Effect of adjunctive pimavanserin on sleep/wakefulness in patients with major depressive disorder: secondary analysis from CLARITY. J Clin Psychiatry. 2020;82(1):20m13425.
3. ACADIA Pharmaceuticals announces top-line results from the Phase 3 CLARITY study evaluating pimavanserin for the adjunctive treatment of major depressive disorder. News release. Acadia Pharmaceuticals Inc. Published July 20, 2020. https://ir.acadia-pharm.com/news-releases/news-release-details/acadia-pharmaceuticals-announces-top-line-results-phase-3-0
4. Kessing LV, Rytgaard HC, Ekstrom CT, et al. Antihypertensive drugs and risk of depression: a nationwide population-based study. Hypertension. 2020;76(4):1263-1279.
5. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
6. Han Y, Chen J, Zou D, et al. Efficacy of ketamine in the rapid treatment of major depressive disorder: a meta-analysis of randomized, double-blind, placebo-controlled studies. Neuropsychiatr Dis Treat. 2016;12:2859-2867.
7. Wan LB, Levitch CF, Perez AM, et al. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry. 2015;76(3):247-252.
8. Hasselmann, H. Scopolamine and depression: a role for muscarinic antagonism? CNS Neurol Disord Drug Targets. 2014;13(4):673-683.
9. Drevets WC, Zarate CA Jr, Furey ML. Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol Psychiatry. 2013;73(12):1156-1163.
10. Stearns TP, Shad MU, Guzman GC. Glabellar botulinum toxin injections in major depressive disorder: a critical review. Prim Care Companion CNS Disord. 2018;20(5): 18r02298.
11. Block TS, Kushner H, Kalin N, et al. Combined analysis of mifepristone for psychotic depression: plasma levels associated with clinical response. Biol Psychiatry. 2018;84(1):46-54.
12. Rubinow DR, Johnson SL, Schmidt PJ, et al. Efficacy of estradiol in perimenopausal depression: so much promise and so few answers. Depress Anxiety. 2015;32(8):539-549.
13. Schmidt PJ, Ben Dor R, Martinez PE, et al. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. 2015;72(7):714-726.
14. Gordon JL, Rubinow DR, Eisenlohr-Moul TA, et al. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):149-157.
15. Fava M, Thase ME, Trivedi MH, et al. Opioid system modulation with buprenorphine/samidorphan combination for major depressive disorder: two randomized controlled studies. Mol Psychiatry. 2020;25(7):1580-1591.
16. Fernandes BS, Dean OM, Dodd S, et al. N-Acetylcysteine in depressive symptoms and functionality: a systematic review and meta-analysis. J Clin Psychiatry. 2016;77(4):e457-466.



















