User login
Painful Retiform Purpura in a Peritoneal Dialysis Patient
The Diagnosis: Calcific Uremic Arteriolopathy
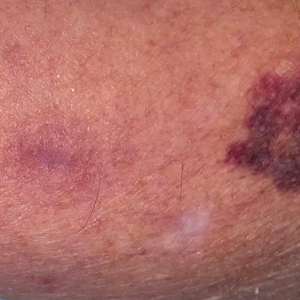
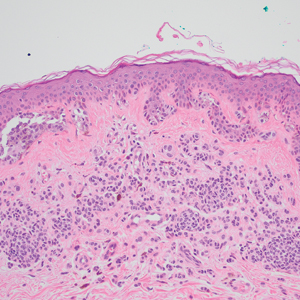
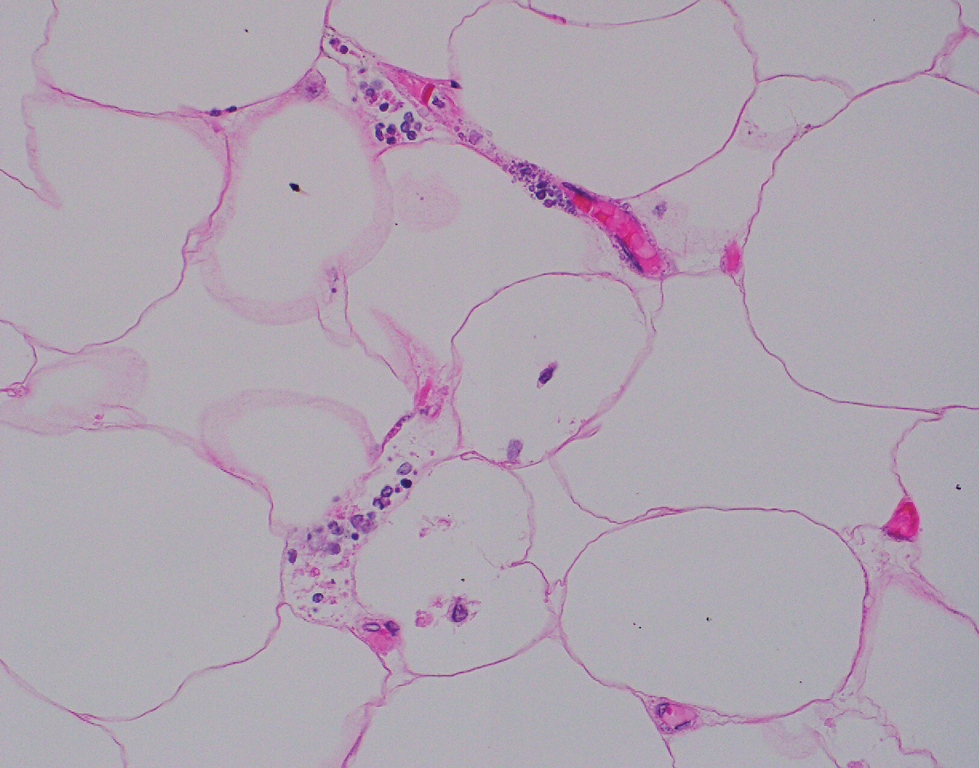
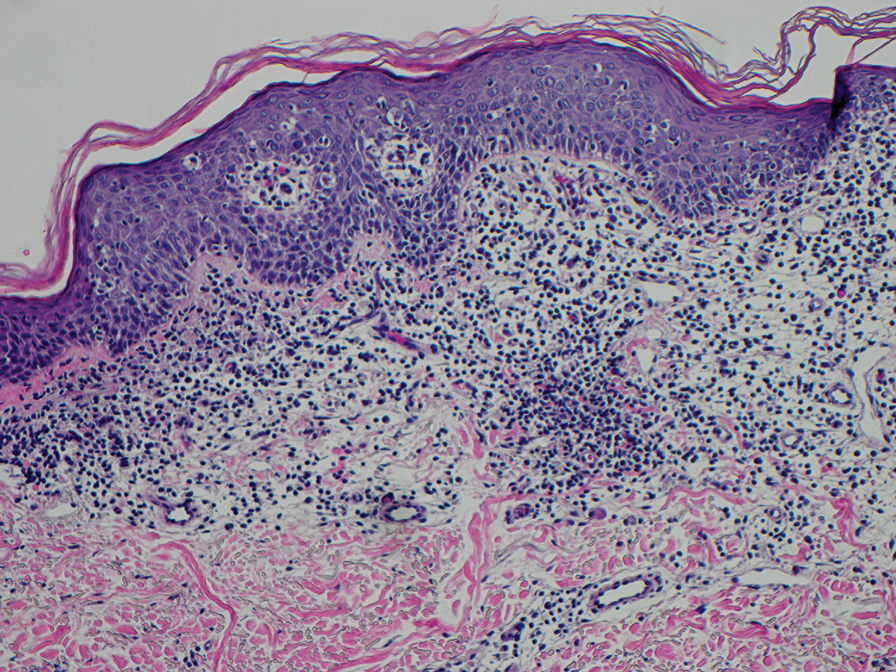
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.
Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
The Diagnosis: Calcific Uremic Arteriolopathy
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.

Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
The Diagnosis: Calcific Uremic Arteriolopathy
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.

Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
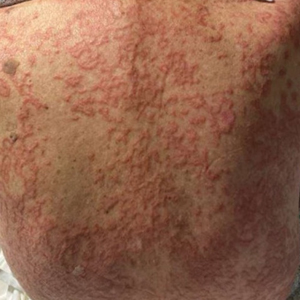
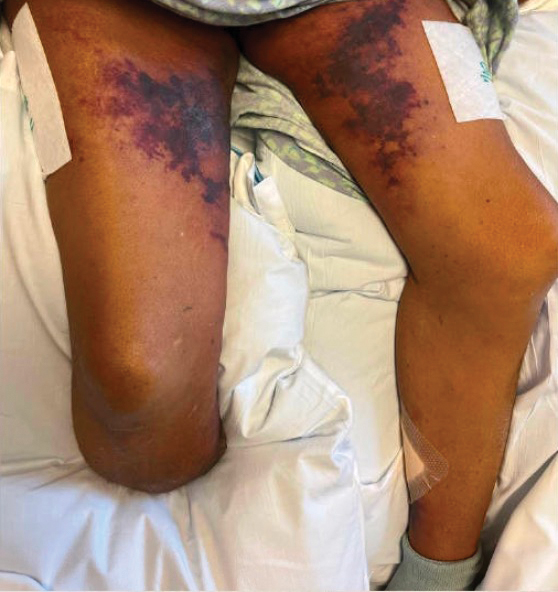
A 72-year-old woman presented to the emergency department with concerns of confusion and lethargy during a session of peritoneal dialysis, which she had been receiving for the last 2 years for end-stage renal disease. She had a history of type 2 diabetes mellitus, diabetic retinopathy, hypertension, coronary artery disease, and peripheral vascular disease preceding a recent right below-knee amputation. A review of systems was positive for a rash on the thighs of several weeks’ duration that was preceded by several days of burning pain in the same distribution. Physical examination revealed retiform purpura with irregular contours and interspersed white stellate patterns scattered across the superomedial thighs, right lower back, and left lower abdomen. An initial laboratory workup revealed an elevated creatinine level of 5.03 mg/dL (reference range, 0.6–1.1 mg/dL; baseline level, 3.0 mg/dL) and mild leukocytosis (12.5 cells/mm3 [reference range, 4.5–11.0 cells/mm3]). Dermatology was consulted, and a 4-mm punch biopsy was obtained from the left medial thigh. Nephrology, infectious disease, and wound care consultations also were placed.
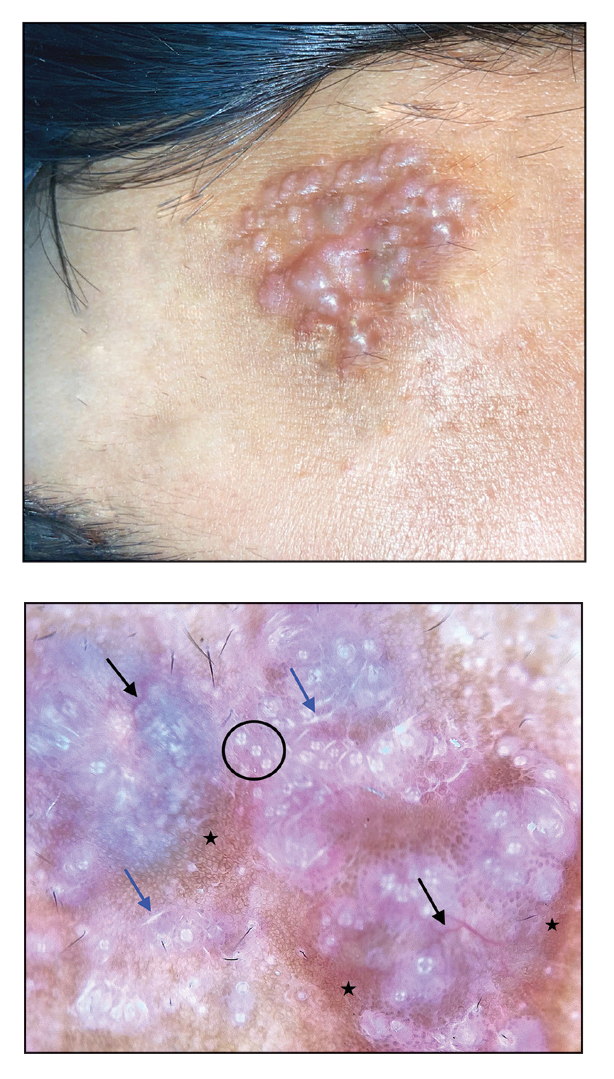
Noduloplaque on the Forehead
The Diagnosis: Giant Apocrine Hidrocystoma
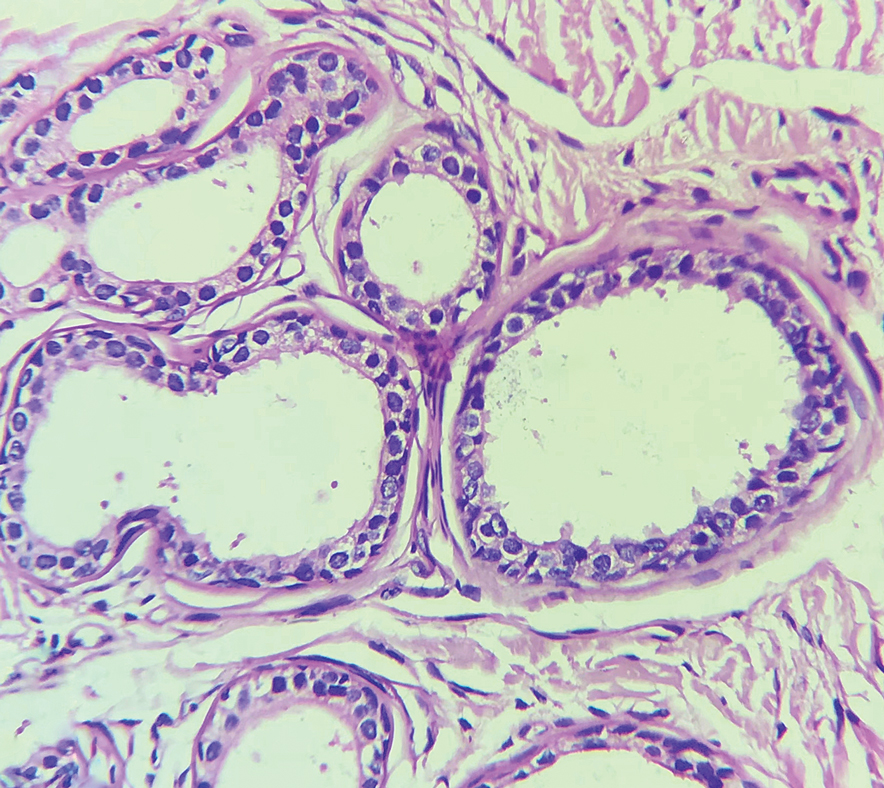
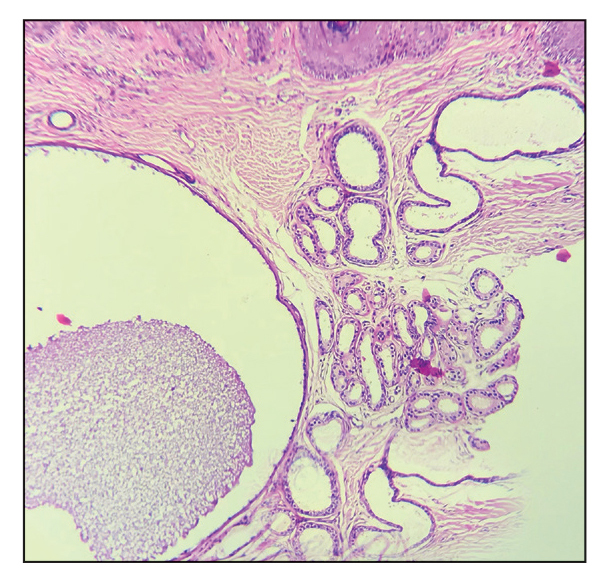
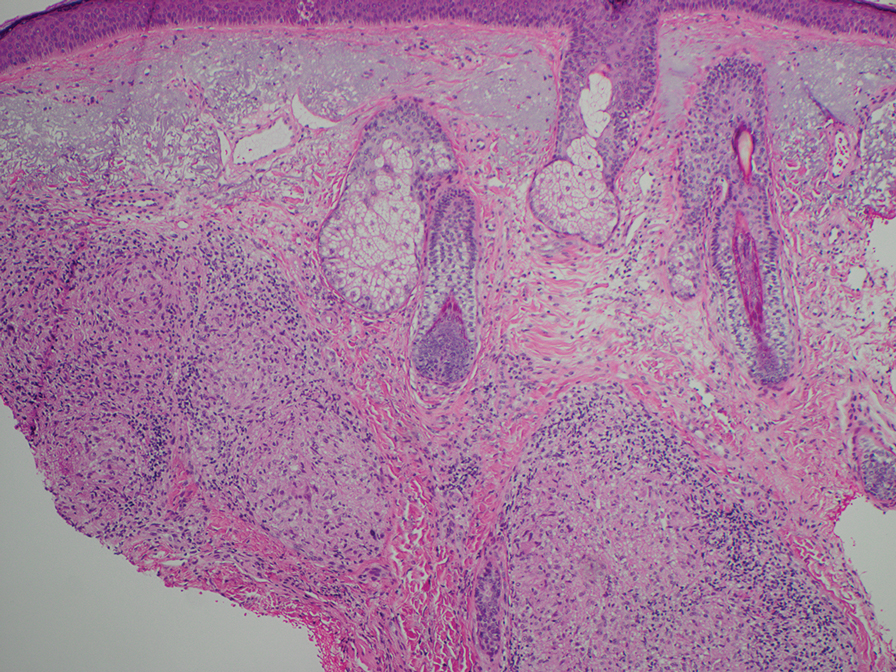
Histopathology of the noduloplaque revealed an unremarkable epidermis with multilocular cystic spaces centered in the dermis. The cysts had a double-lined epithelium with inner columnar to cuboidal cells and outer myoepithelial cells (bottom quiz image). Columnar cells showing decapitation secretion could be appreciated at places indicating apocrine secretion (Figure). A final diagnosis of apocrine hidrocystoma was made.
Hidrocystomas are rare, benign, cystic lesions derived either from apocrine or eccrine glands.1 Apocrine hidrocystoma usually manifests as asymptomatic, solitary, dome-shaped papules or nodules with a predilection for the head and neck region. Hidrocystomas can vary from flesh colored to blue, brown, or black. Pigmentation in hidrocystoma is seen in 5% to 80% of cases and is attributed to the Tyndall effect.1 The tumor usually is less than 20 mm in diameter; larger lesions are termed giant apocrine hidrocystoma.2 Apocrine hidrocystoma manifesting with multiple lesions and a size greater than 10 mm, as seen in our case, is uncommon.
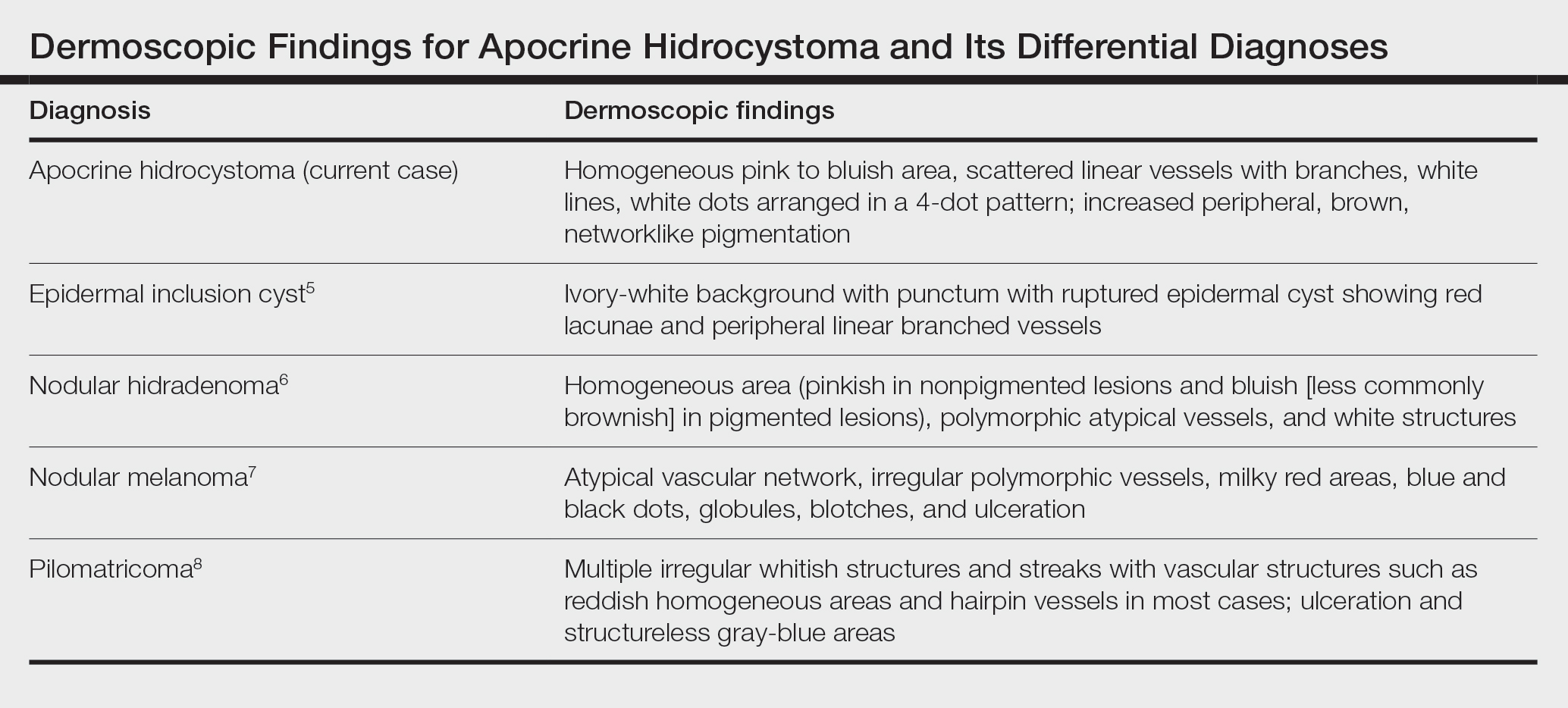
Zaballos et al3 described dermoscopy of apocrine hidrocystoma in 22 patients. Hallmark dermoscopic findings were the presence of a homogeneous flesh-colored, yellowish, blue to pinkish-blue area involving the entire lesion with arborizing vessels and whitish structures.3 Similar dermoscopic findings were present in our patient. The homogeneous area histologically correlates to the multiloculated cysts located in the dermis. The exact reason for white structures is unknown; however, their visualization in apocrine hidrocystoma could be attributed to the alternation in collagen orientation secondary to the presence of large or multiple cysts in the dermis.
The presence of shiny white dots arranged in a square resembling a four-leaf clover (also known as white rosettes) was a unique dermoscopic finding in our patient. These rosettes can be appreciated only with polarized dermoscopy, and they have been described in actinic keratosis, seborrheic keratosis, squamous cell carcinoma, and basal cell carcinoma.4 The exact morphologic correlate of white rosettes is unknown but is postulated to be secondary to material inside adnexal openings in small rosettes and concentric perifollicular fibrosis in larger rosettes.4 In our patient, we believe the white rosettes can be attributed to the accumulated secretions in the dermal glands, which also were seen via histopathology. Dermoscopy also revealed increased peripheral, brown, networklike pigmentation, which was unique and could be secondary to the patient’s darker skin phenotype.
Differential diagnoses of apocrine hidrocystoma include both melanocytic and nonmelanocytic conditions such as epidermal cyst, nodular melanoma, nodular hidradenoma, syringoma, blue nevus, pilomatricoma, eccrine poroma, nodular Kaposi sarcoma, and venous lake.1 Histopathology showing large unilocular or multilocular dermal cysts with double lining comprising outer myoepithelial cells and inner columnar or cuboidal cell with decapitation secretion is paramount in confirming the diagnosis of apocrine hidrocystoma.
Dermoscopy can act as a valuable noninvasive modality in differentiating apocrine hidrocystoma from its melanocytic and nonmelanocytic differential diagnoses (Table).5-8 In our patient, the presence of a homogeneous pink to bluish area involving the entire lesion, linear branched vessels, and whitish structures on dermoscopy pointed to the diagnosis of apocrine hidrocystoma, which was further confirmed by characteristic histopathologic findings.
The treatment of apocrine hidrocystoma includes surgical excision for solitary lesions, with electrodesiccation and curettage, chemical cautery, and CO2 laser ablation employed for multiple lesions.1 Our patient was scheduled for CO2 laser ablation, considering the multiple lesions and size of the apocrine hidrocystoma but was subsequently lost to follow-up.
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp [published online August 15, 2020]. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703. doi:10.1111/j.1365-4632.2005.02512.x
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Haspeslagh M, Noë M, De Wispelaere I, et al. Rosettes and other white shiny structures in polarized dermoscopy: histological correlate and optical explanation. J Eur Acad Dermatol Venereol. 2016;30:311-313. doi:10.1111/jdv.13080
- Suh KS, Kang DY, Park JB, et al. Usefulness of dermoscopy in the differential diagnosis of ruptured and unruptured epidermal cysts. Ann Dermatol. 2017;29:33-38. doi:10.5021/ad.2017.29.1.33
- Serrano P, Lallas A, Del Pozo LJ, et al. Dermoscopy of nodular hidradenoma, a great masquerader: a morphological study of 28 cases. Dermatology. 2016;232:78-82. doi:10.1159/000441218
- Russo T, Piccolo V, Lallas A, et al. Dermoscopy of malignant skin tumours: what’s new? Dermatology. 2017;233:64-73. doi:10.1159/000472253
- Zaballos P, Llambrich A, Puig S, et al. Dermoscopic findings of pilomatricomas. Dermatology. 2008;217:225-230. doi:10.1159 /000148248
The Diagnosis: Giant Apocrine Hidrocystoma
Histopathology of the noduloplaque revealed an unremarkable epidermis with multilocular cystic spaces centered in the dermis. The cysts had a double-lined epithelium with inner columnar to cuboidal cells and outer myoepithelial cells (bottom quiz image). Columnar cells showing decapitation secretion could be appreciated at places indicating apocrine secretion (Figure). A final diagnosis of apocrine hidrocystoma was made.

Hidrocystomas are rare, benign, cystic lesions derived either from apocrine or eccrine glands.1 Apocrine hidrocystoma usually manifests as asymptomatic, solitary, dome-shaped papules or nodules with a predilection for the head and neck region. Hidrocystomas can vary from flesh colored to blue, brown, or black. Pigmentation in hidrocystoma is seen in 5% to 80% of cases and is attributed to the Tyndall effect.1 The tumor usually is less than 20 mm in diameter; larger lesions are termed giant apocrine hidrocystoma.2 Apocrine hidrocystoma manifesting with multiple lesions and a size greater than 10 mm, as seen in our case, is uncommon.
Zaballos et al3 described dermoscopy of apocrine hidrocystoma in 22 patients. Hallmark dermoscopic findings were the presence of a homogeneous flesh-colored, yellowish, blue to pinkish-blue area involving the entire lesion with arborizing vessels and whitish structures.3 Similar dermoscopic findings were present in our patient. The homogeneous area histologically correlates to the multiloculated cysts located in the dermis. The exact reason for white structures is unknown; however, their visualization in apocrine hidrocystoma could be attributed to the alternation in collagen orientation secondary to the presence of large or multiple cysts in the dermis.
The presence of shiny white dots arranged in a square resembling a four-leaf clover (also known as white rosettes) was a unique dermoscopic finding in our patient. These rosettes can be appreciated only with polarized dermoscopy, and they have been described in actinic keratosis, seborrheic keratosis, squamous cell carcinoma, and basal cell carcinoma.4 The exact morphologic correlate of white rosettes is unknown but is postulated to be secondary to material inside adnexal openings in small rosettes and concentric perifollicular fibrosis in larger rosettes.4 In our patient, we believe the white rosettes can be attributed to the accumulated secretions in the dermal glands, which also were seen via histopathology. Dermoscopy also revealed increased peripheral, brown, networklike pigmentation, which was unique and could be secondary to the patient’s darker skin phenotype.
Differential diagnoses of apocrine hidrocystoma include both melanocytic and nonmelanocytic conditions such as epidermal cyst, nodular melanoma, nodular hidradenoma, syringoma, blue nevus, pilomatricoma, eccrine poroma, nodular Kaposi sarcoma, and venous lake.1 Histopathology showing large unilocular or multilocular dermal cysts with double lining comprising outer myoepithelial cells and inner columnar or cuboidal cell with decapitation secretion is paramount in confirming the diagnosis of apocrine hidrocystoma.
Dermoscopy can act as a valuable noninvasive modality in differentiating apocrine hidrocystoma from its melanocytic and nonmelanocytic differential diagnoses (Table).5-8 In our patient, the presence of a homogeneous pink to bluish area involving the entire lesion, linear branched vessels, and whitish structures on dermoscopy pointed to the diagnosis of apocrine hidrocystoma, which was further confirmed by characteristic histopathologic findings.

The treatment of apocrine hidrocystoma includes surgical excision for solitary lesions, with electrodesiccation and curettage, chemical cautery, and CO2 laser ablation employed for multiple lesions.1 Our patient was scheduled for CO2 laser ablation, considering the multiple lesions and size of the apocrine hidrocystoma but was subsequently lost to follow-up.
The Diagnosis: Giant Apocrine Hidrocystoma
Histopathology of the noduloplaque revealed an unremarkable epidermis with multilocular cystic spaces centered in the dermis. The cysts had a double-lined epithelium with inner columnar to cuboidal cells and outer myoepithelial cells (bottom quiz image). Columnar cells showing decapitation secretion could be appreciated at places indicating apocrine secretion (Figure). A final diagnosis of apocrine hidrocystoma was made.

Hidrocystomas are rare, benign, cystic lesions derived either from apocrine or eccrine glands.1 Apocrine hidrocystoma usually manifests as asymptomatic, solitary, dome-shaped papules or nodules with a predilection for the head and neck region. Hidrocystomas can vary from flesh colored to blue, brown, or black. Pigmentation in hidrocystoma is seen in 5% to 80% of cases and is attributed to the Tyndall effect.1 The tumor usually is less than 20 mm in diameter; larger lesions are termed giant apocrine hidrocystoma.2 Apocrine hidrocystoma manifesting with multiple lesions and a size greater than 10 mm, as seen in our case, is uncommon.
Zaballos et al3 described dermoscopy of apocrine hidrocystoma in 22 patients. Hallmark dermoscopic findings were the presence of a homogeneous flesh-colored, yellowish, blue to pinkish-blue area involving the entire lesion with arborizing vessels and whitish structures.3 Similar dermoscopic findings were present in our patient. The homogeneous area histologically correlates to the multiloculated cysts located in the dermis. The exact reason for white structures is unknown; however, their visualization in apocrine hidrocystoma could be attributed to the alternation in collagen orientation secondary to the presence of large or multiple cysts in the dermis.
The presence of shiny white dots arranged in a square resembling a four-leaf clover (also known as white rosettes) was a unique dermoscopic finding in our patient. These rosettes can be appreciated only with polarized dermoscopy, and they have been described in actinic keratosis, seborrheic keratosis, squamous cell carcinoma, and basal cell carcinoma.4 The exact morphologic correlate of white rosettes is unknown but is postulated to be secondary to material inside adnexal openings in small rosettes and concentric perifollicular fibrosis in larger rosettes.4 In our patient, we believe the white rosettes can be attributed to the accumulated secretions in the dermal glands, which also were seen via histopathology. Dermoscopy also revealed increased peripheral, brown, networklike pigmentation, which was unique and could be secondary to the patient’s darker skin phenotype.
Differential diagnoses of apocrine hidrocystoma include both melanocytic and nonmelanocytic conditions such as epidermal cyst, nodular melanoma, nodular hidradenoma, syringoma, blue nevus, pilomatricoma, eccrine poroma, nodular Kaposi sarcoma, and venous lake.1 Histopathology showing large unilocular or multilocular dermal cysts with double lining comprising outer myoepithelial cells and inner columnar or cuboidal cell with decapitation secretion is paramount in confirming the diagnosis of apocrine hidrocystoma.
Dermoscopy can act as a valuable noninvasive modality in differentiating apocrine hidrocystoma from its melanocytic and nonmelanocytic differential diagnoses (Table).5-8 In our patient, the presence of a homogeneous pink to bluish area involving the entire lesion, linear branched vessels, and whitish structures on dermoscopy pointed to the diagnosis of apocrine hidrocystoma, which was further confirmed by characteristic histopathologic findings.

The treatment of apocrine hidrocystoma includes surgical excision for solitary lesions, with electrodesiccation and curettage, chemical cautery, and CO2 laser ablation employed for multiple lesions.1 Our patient was scheduled for CO2 laser ablation, considering the multiple lesions and size of the apocrine hidrocystoma but was subsequently lost to follow-up.
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp [published online August 15, 2020]. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703. doi:10.1111/j.1365-4632.2005.02512.x
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Haspeslagh M, Noë M, De Wispelaere I, et al. Rosettes and other white shiny structures in polarized dermoscopy: histological correlate and optical explanation. J Eur Acad Dermatol Venereol. 2016;30:311-313. doi:10.1111/jdv.13080
- Suh KS, Kang DY, Park JB, et al. Usefulness of dermoscopy in the differential diagnosis of ruptured and unruptured epidermal cysts. Ann Dermatol. 2017;29:33-38. doi:10.5021/ad.2017.29.1.33
- Serrano P, Lallas A, Del Pozo LJ, et al. Dermoscopy of nodular hidradenoma, a great masquerader: a morphological study of 28 cases. Dermatology. 2016;232:78-82. doi:10.1159/000441218
- Russo T, Piccolo V, Lallas A, et al. Dermoscopy of malignant skin tumours: what’s new? Dermatology. 2017;233:64-73. doi:10.1159/000472253
- Zaballos P, Llambrich A, Puig S, et al. Dermoscopic findings of pilomatricomas. Dermatology. 2008;217:225-230. doi:10.1159 /000148248
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp [published online August 15, 2020]. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703. doi:10.1111/j.1365-4632.2005.02512.x
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Haspeslagh M, Noë M, De Wispelaere I, et al. Rosettes and other white shiny structures in polarized dermoscopy: histological correlate and optical explanation. J Eur Acad Dermatol Venereol. 2016;30:311-313. doi:10.1111/jdv.13080
- Suh KS, Kang DY, Park JB, et al. Usefulness of dermoscopy in the differential diagnosis of ruptured and unruptured epidermal cysts. Ann Dermatol. 2017;29:33-38. doi:10.5021/ad.2017.29.1.33
- Serrano P, Lallas A, Del Pozo LJ, et al. Dermoscopy of nodular hidradenoma, a great masquerader: a morphological study of 28 cases. Dermatology. 2016;232:78-82. doi:10.1159/000441218
- Russo T, Piccolo V, Lallas A, et al. Dermoscopy of malignant skin tumours: what’s new? Dermatology. 2017;233:64-73. doi:10.1159/000472253
- Zaballos P, Llambrich A, Puig S, et al. Dermoscopic findings of pilomatricomas. Dermatology. 2008;217:225-230. doi:10.1159 /000148248
A 21-year-old man presented with a raised lesion on the forehead that had started as a single papule 16 years prior and gradually increased in number and size. There were no associated symptoms and no history of seasonal variation in the size of the lesions. Physical examination revealed multiple erythematous to slightly bluish translucent papules that coalesced to form a 3×3-cm noduloplaque with cystic consistency on the right side of the forehead (top). Dermoscopic examination (middle) (polarized noncontact mode) revealed a homogeneous pink to bluish background, scattered linear vessels with branches (black arrows), multiple chrysalislike shiny white lines (blue arrows), and dots arranged in a 4-dot pattern (black circle) resembling a four-leaf clover. Increased peripheral, brown, networklike pigmentation (black stars) also was noted on dermoscopy. Histopathologic examination of the noduloplaque was performed (bottom).
Annular Erythematous Plaques on the Back
The Diagnosis: Granuloma Annulare
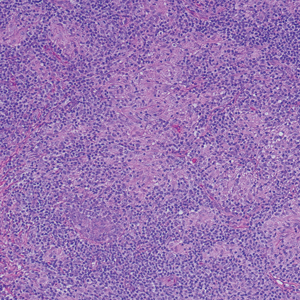
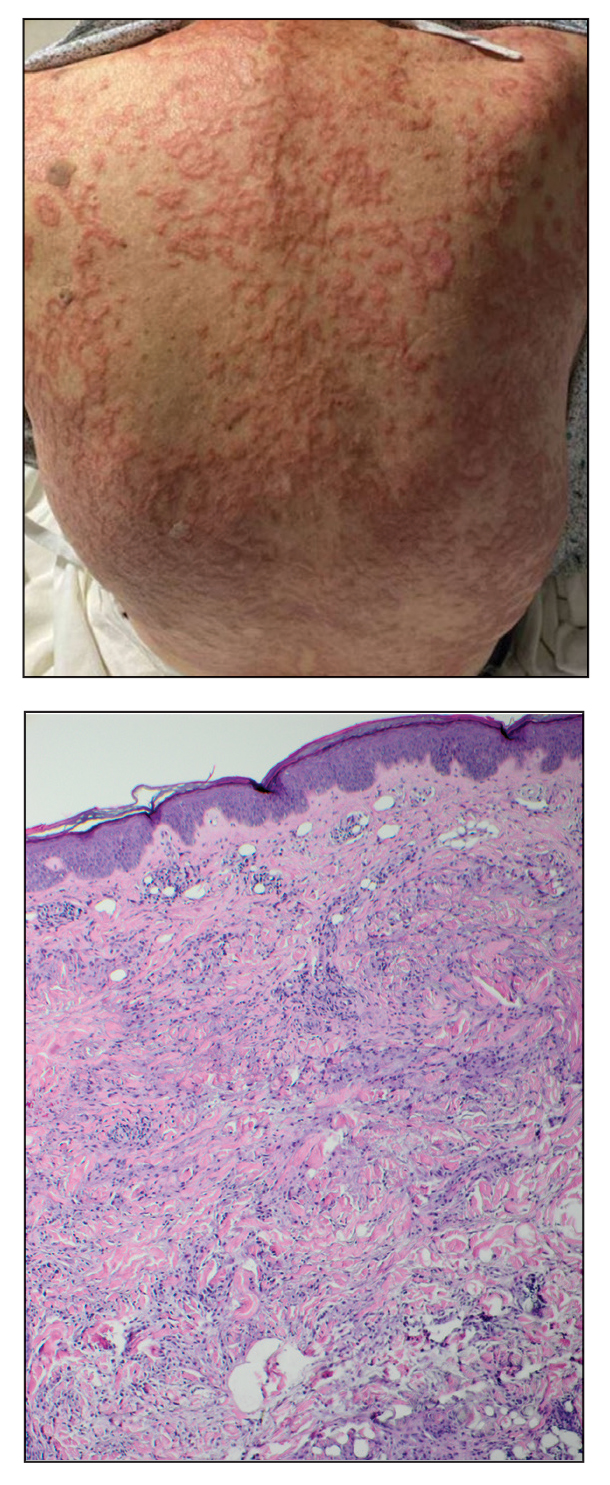
The biopsies revealed palisading granulomatous dermatitis consistent with granuloma annulare (GA). This diagnosis was supported by the clinical presentation and histopathologic findings. Although the pathogenesis of GA is unclear, it is a benign, self-limiting condition. Primarily affected sites include the trunk and forearms. Generalized GA (or GA with ≥10 lesions) may warrant workup for malignancy, as it may represent a paraneoplastic process.1 Histopathology reveals granulomas comprising a dermal lymphohistiocytic infiltrate as well as central mucin and nuclear debris. There are a few histologic subtypes of GA, including palisading and interstitial, which refer to the distribution of the histiocytic infiltrate.2,3 This case—with palisading histiocytes lining the collection of necrobiosis and mucin (bottom quiz image)—features palisading GA. Notably, GA exhibits central rather than diffuse mucin.4
Erythema gyratum repens is a paraneoplastic arcuate erythema that manifests as erythematous figurate, gyrate, or annular plaques exhibiting a trailing scale. Clinically, erythema gyratum repens spreads rapidly—as quickly as 1 cm/d—and can be extensive (as in this case). Histopathology ruled out this diagnosis in our patient. Nonspecific findings of acanthosis, parakeratosis, and superficial spongiosis can be found in erythema gyratum repens. A superficial and deep perivascular lymphohistiocytic infiltrate may be seen in figurate erythemas (Figure 1).5 Unlike GA, this infiltrate does not form granulomas, is more superficial, and does not contain mucin.
Histopathology also can help establish the diagnosis of leprosy and its specific subtype, as leprosy exists on a spectrum from tuberculoid to lepromatous, with a great deal of overlap in between.6 Lepromatous leprosy has many cutaneous clinical presentations but typically manifests as erythematous papules or nodules. It is multibacillary, and these mycobacteria form clumps known as globi that can be seen on Fite stain.7 In lepromatous leprosy, there is a characteristic dense lymphohistiocytic infiltrate (Figure 2) above which a Grenz zone can be seen.4,8 There are no well-formed granulomas in lepromatous leprosy, unlike in tuberculoid leprosy, which is paucibacillary and creates a granulomatous response surrounding nerves and adnexal structures.6
Mycosis fungoides (MF) is the most common cutaneous lymphoma. There are patch, plaque, and tumor stages of MF, each of which exhibits various histopathologic findings.9 In early patch-stage MF, lymphocytes have perinuclear clearing, and the degree of lymphocytic infiltrate is out of proportion to the spongiosis present. Epidermotropism and Pautrier microabscesses often are present in the epidermis (Figure 3). In the plaque stage, there is a denser lymphoid infiltrate in a lichenoid pattern with epidermotropism and Pautrier microabscesses. The tumor stage shows a dense dermal lymphoid infiltrate with more atypia and typically a lack of epidermotropism. Rarely, MF can exhibit a granulomatous variant in which epithelioid histiocytes collect to form granulomas along with atypical lymphocytes.10
The diagnosis of cutaneous sarcoidosis requires clinicopathologic corroboration. Histopathology demonstrates epithelioid histiocytes forming noncaseating granulomas with little to no lymphocytic infiltrate (Figure 4). There typically is no necrosis or necrobiosis as there is in GA. The diagnosis of sarcoidosis can be challenging histopathologically, and stains should be used to rule out infectious processes.4 Asteroid bodies— star-shaped eosinophilic inclusions within giant cells—may be present but are nonspecific for sarcoidosis.11 Schaumann bodies—inclusions of calcifications within giant cells—also may be present and can aid in diagnosis.12
- Kovich O, Burgin S. Generalized granuloma annulare [published online December 30, 2005]. Dermatol Online J. 2005;11:23.
- Al Ameer MA, Al-Natour SH, Alsahaf HAA, et al. Eruptive granuloma annulare in an elderly man with diabetes [published online January 14, 2022]. Cureus. 2022;14:E21242. doi:10.7759/cureus.21242
- Howard A, White CR Jr. Non-infectious granulomas. In: Bolognia JL, et al, eds. Dermatology. Mosby; 2003:1455.
- Elston DM, Ferringer T, Ko CJ, et al. Dermatopathology. 3rd ed. Elsevier; 2018.
- Gore M, Winters ME. Erythema gyratum repens: a rare paraneoplastic rash. West J Emerg Med. 2011;12:556-558. doi:10.5811/westjem.2010.11.2090
- Maymone MBC, Laughter M, Venkatesh S, et al. Leprosy: clinical aspects and diagnostic techniques. J Am Acad Dermatol. 2020;83:1-14. doi:10.1016/j.jaad.2019.12.080
- Pedley JC, Harman DJ, Waudby H, et al. Leprosy in peripheral nerves: histopathological findings in 119 untreated patients in Nepal. J Neurol Neurosurg Psychiatry. 1980;43:198-204. doi:10.1136/jnnp.43.3.198
- Booth AV, Kovich OI. Lepromatous leprosy [published online January 27, 2007]. Dermatol Online J. 2007;13:9.
- Robson A. The pathology of cutaneous T-cell lymphoma. Oncology (Williston Park). 2007;21(2 suppl 1):9-12.
- Kempf W, Ostheeren-Michaelis S, Paulli M, et al. Granulomatous mycosis fungoides and granulomatous slack skin: a multicenter study of the Cutaneous Lymphoma Histopathology Task Force Group of the European Organization for Research and Treatment of Cancer (EORTC). Arch Dermatol. 2008;144:1609-1617. doi:10.1001/archdermatol.2008.46
- Azar HA, Lunardelli C. Collagen nature of asteroid bodies of giant cells in sarcoidosis. Am J Pathol. 1969;57:81-92.
- Sreeja C, Priyadarshini A, Premika, et al. Sarcoidosis—a review article. J Oral Maxillofac Pathol. 2022;26:242-253. doi:10.4103 /jomfp.jomfp_373_21
The Diagnosis: Granuloma Annulare
The biopsies revealed palisading granulomatous dermatitis consistent with granuloma annulare (GA). This diagnosis was supported by the clinical presentation and histopathologic findings. Although the pathogenesis of GA is unclear, it is a benign, self-limiting condition. Primarily affected sites include the trunk and forearms. Generalized GA (or GA with ≥10 lesions) may warrant workup for malignancy, as it may represent a paraneoplastic process.1 Histopathology reveals granulomas comprising a dermal lymphohistiocytic infiltrate as well as central mucin and nuclear debris. There are a few histologic subtypes of GA, including palisading and interstitial, which refer to the distribution of the histiocytic infiltrate.2,3 This case—with palisading histiocytes lining the collection of necrobiosis and mucin (bottom quiz image)—features palisading GA. Notably, GA exhibits central rather than diffuse mucin.4
Erythema gyratum repens is a paraneoplastic arcuate erythema that manifests as erythematous figurate, gyrate, or annular plaques exhibiting a trailing scale. Clinically, erythema gyratum repens spreads rapidly—as quickly as 1 cm/d—and can be extensive (as in this case). Histopathology ruled out this diagnosis in our patient. Nonspecific findings of acanthosis, parakeratosis, and superficial spongiosis can be found in erythema gyratum repens. A superficial and deep perivascular lymphohistiocytic infiltrate may be seen in figurate erythemas (Figure 1).5 Unlike GA, this infiltrate does not form granulomas, is more superficial, and does not contain mucin.

Histopathology also can help establish the diagnosis of leprosy and its specific subtype, as leprosy exists on a spectrum from tuberculoid to lepromatous, with a great deal of overlap in between.6 Lepromatous leprosy has many cutaneous clinical presentations but typically manifests as erythematous papules or nodules. It is multibacillary, and these mycobacteria form clumps known as globi that can be seen on Fite stain.7 In lepromatous leprosy, there is a characteristic dense lymphohistiocytic infiltrate (Figure 2) above which a Grenz zone can be seen.4,8 There are no well-formed granulomas in lepromatous leprosy, unlike in tuberculoid leprosy, which is paucibacillary and creates a granulomatous response surrounding nerves and adnexal structures.6

Mycosis fungoides (MF) is the most common cutaneous lymphoma. There are patch, plaque, and tumor stages of MF, each of which exhibits various histopathologic findings.9 In early patch-stage MF, lymphocytes have perinuclear clearing, and the degree of lymphocytic infiltrate is out of proportion to the spongiosis present. Epidermotropism and Pautrier microabscesses often are present in the epidermis (Figure 3). In the plaque stage, there is a denser lymphoid infiltrate in a lichenoid pattern with epidermotropism and Pautrier microabscesses. The tumor stage shows a dense dermal lymphoid infiltrate with more atypia and typically a lack of epidermotropism. Rarely, MF can exhibit a granulomatous variant in which epithelioid histiocytes collect to form granulomas along with atypical lymphocytes.10

The diagnosis of cutaneous sarcoidosis requires clinicopathologic corroboration. Histopathology demonstrates epithelioid histiocytes forming noncaseating granulomas with little to no lymphocytic infiltrate (Figure 4). There typically is no necrosis or necrobiosis as there is in GA. The diagnosis of sarcoidosis can be challenging histopathologically, and stains should be used to rule out infectious processes.4 Asteroid bodies— star-shaped eosinophilic inclusions within giant cells—may be present but are nonspecific for sarcoidosis.11 Schaumann bodies—inclusions of calcifications within giant cells—also may be present and can aid in diagnosis.12

The Diagnosis: Granuloma Annulare
The biopsies revealed palisading granulomatous dermatitis consistent with granuloma annulare (GA). This diagnosis was supported by the clinical presentation and histopathologic findings. Although the pathogenesis of GA is unclear, it is a benign, self-limiting condition. Primarily affected sites include the trunk and forearms. Generalized GA (or GA with ≥10 lesions) may warrant workup for malignancy, as it may represent a paraneoplastic process.1 Histopathology reveals granulomas comprising a dermal lymphohistiocytic infiltrate as well as central mucin and nuclear debris. There are a few histologic subtypes of GA, including palisading and interstitial, which refer to the distribution of the histiocytic infiltrate.2,3 This case—with palisading histiocytes lining the collection of necrobiosis and mucin (bottom quiz image)—features palisading GA. Notably, GA exhibits central rather than diffuse mucin.4
Erythema gyratum repens is a paraneoplastic arcuate erythema that manifests as erythematous figurate, gyrate, or annular plaques exhibiting a trailing scale. Clinically, erythema gyratum repens spreads rapidly—as quickly as 1 cm/d—and can be extensive (as in this case). Histopathology ruled out this diagnosis in our patient. Nonspecific findings of acanthosis, parakeratosis, and superficial spongiosis can be found in erythema gyratum repens. A superficial and deep perivascular lymphohistiocytic infiltrate may be seen in figurate erythemas (Figure 1).5 Unlike GA, this infiltrate does not form granulomas, is more superficial, and does not contain mucin.

Histopathology also can help establish the diagnosis of leprosy and its specific subtype, as leprosy exists on a spectrum from tuberculoid to lepromatous, with a great deal of overlap in between.6 Lepromatous leprosy has many cutaneous clinical presentations but typically manifests as erythematous papules or nodules. It is multibacillary, and these mycobacteria form clumps known as globi that can be seen on Fite stain.7 In lepromatous leprosy, there is a characteristic dense lymphohistiocytic infiltrate (Figure 2) above which a Grenz zone can be seen.4,8 There are no well-formed granulomas in lepromatous leprosy, unlike in tuberculoid leprosy, which is paucibacillary and creates a granulomatous response surrounding nerves and adnexal structures.6

Mycosis fungoides (MF) is the most common cutaneous lymphoma. There are patch, plaque, and tumor stages of MF, each of which exhibits various histopathologic findings.9 In early patch-stage MF, lymphocytes have perinuclear clearing, and the degree of lymphocytic infiltrate is out of proportion to the spongiosis present. Epidermotropism and Pautrier microabscesses often are present in the epidermis (Figure 3). In the plaque stage, there is a denser lymphoid infiltrate in a lichenoid pattern with epidermotropism and Pautrier microabscesses. The tumor stage shows a dense dermal lymphoid infiltrate with more atypia and typically a lack of epidermotropism. Rarely, MF can exhibit a granulomatous variant in which epithelioid histiocytes collect to form granulomas along with atypical lymphocytes.10

The diagnosis of cutaneous sarcoidosis requires clinicopathologic corroboration. Histopathology demonstrates epithelioid histiocytes forming noncaseating granulomas with little to no lymphocytic infiltrate (Figure 4). There typically is no necrosis or necrobiosis as there is in GA. The diagnosis of sarcoidosis can be challenging histopathologically, and stains should be used to rule out infectious processes.4 Asteroid bodies— star-shaped eosinophilic inclusions within giant cells—may be present but are nonspecific for sarcoidosis.11 Schaumann bodies—inclusions of calcifications within giant cells—also may be present and can aid in diagnosis.12

- Kovich O, Burgin S. Generalized granuloma annulare [published online December 30, 2005]. Dermatol Online J. 2005;11:23.
- Al Ameer MA, Al-Natour SH, Alsahaf HAA, et al. Eruptive granuloma annulare in an elderly man with diabetes [published online January 14, 2022]. Cureus. 2022;14:E21242. doi:10.7759/cureus.21242
- Howard A, White CR Jr. Non-infectious granulomas. In: Bolognia JL, et al, eds. Dermatology. Mosby; 2003:1455.
- Elston DM, Ferringer T, Ko CJ, et al. Dermatopathology. 3rd ed. Elsevier; 2018.
- Gore M, Winters ME. Erythema gyratum repens: a rare paraneoplastic rash. West J Emerg Med. 2011;12:556-558. doi:10.5811/westjem.2010.11.2090
- Maymone MBC, Laughter M, Venkatesh S, et al. Leprosy: clinical aspects and diagnostic techniques. J Am Acad Dermatol. 2020;83:1-14. doi:10.1016/j.jaad.2019.12.080
- Pedley JC, Harman DJ, Waudby H, et al. Leprosy in peripheral nerves: histopathological findings in 119 untreated patients in Nepal. J Neurol Neurosurg Psychiatry. 1980;43:198-204. doi:10.1136/jnnp.43.3.198
- Booth AV, Kovich OI. Lepromatous leprosy [published online January 27, 2007]. Dermatol Online J. 2007;13:9.
- Robson A. The pathology of cutaneous T-cell lymphoma. Oncology (Williston Park). 2007;21(2 suppl 1):9-12.
- Kempf W, Ostheeren-Michaelis S, Paulli M, et al. Granulomatous mycosis fungoides and granulomatous slack skin: a multicenter study of the Cutaneous Lymphoma Histopathology Task Force Group of the European Organization for Research and Treatment of Cancer (EORTC). Arch Dermatol. 2008;144:1609-1617. doi:10.1001/archdermatol.2008.46
- Azar HA, Lunardelli C. Collagen nature of asteroid bodies of giant cells in sarcoidosis. Am J Pathol. 1969;57:81-92.
- Sreeja C, Priyadarshini A, Premika, et al. Sarcoidosis—a review article. J Oral Maxillofac Pathol. 2022;26:242-253. doi:10.4103 /jomfp.jomfp_373_21
- Kovich O, Burgin S. Generalized granuloma annulare [published online December 30, 2005]. Dermatol Online J. 2005;11:23.
- Al Ameer MA, Al-Natour SH, Alsahaf HAA, et al. Eruptive granuloma annulare in an elderly man with diabetes [published online January 14, 2022]. Cureus. 2022;14:E21242. doi:10.7759/cureus.21242
- Howard A, White CR Jr. Non-infectious granulomas. In: Bolognia JL, et al, eds. Dermatology. Mosby; 2003:1455.
- Elston DM, Ferringer T, Ko CJ, et al. Dermatopathology. 3rd ed. Elsevier; 2018.
- Gore M, Winters ME. Erythema gyratum repens: a rare paraneoplastic rash. West J Emerg Med. 2011;12:556-558. doi:10.5811/westjem.2010.11.2090
- Maymone MBC, Laughter M, Venkatesh S, et al. Leprosy: clinical aspects and diagnostic techniques. J Am Acad Dermatol. 2020;83:1-14. doi:10.1016/j.jaad.2019.12.080
- Pedley JC, Harman DJ, Waudby H, et al. Leprosy in peripheral nerves: histopathological findings in 119 untreated patients in Nepal. J Neurol Neurosurg Psychiatry. 1980;43:198-204. doi:10.1136/jnnp.43.3.198
- Booth AV, Kovich OI. Lepromatous leprosy [published online January 27, 2007]. Dermatol Online J. 2007;13:9.
- Robson A. The pathology of cutaneous T-cell lymphoma. Oncology (Williston Park). 2007;21(2 suppl 1):9-12.
- Kempf W, Ostheeren-Michaelis S, Paulli M, et al. Granulomatous mycosis fungoides and granulomatous slack skin: a multicenter study of the Cutaneous Lymphoma Histopathology Task Force Group of the European Organization for Research and Treatment of Cancer (EORTC). Arch Dermatol. 2008;144:1609-1617. doi:10.1001/archdermatol.2008.46
- Azar HA, Lunardelli C. Collagen nature of asteroid bodies of giant cells in sarcoidosis. Am J Pathol. 1969;57:81-92.
- Sreeja C, Priyadarshini A, Premika, et al. Sarcoidosis—a review article. J Oral Maxillofac Pathol. 2022;26:242-253. doi:10.4103 /jomfp.jomfp_373_21
An 84-year-old man presented to the clinic for evaluation of a pruritic rash on the back of 6 months’ duration that spread to the neck and chest over the past 2 months and then to the abdomen and thighs more recently. His primary care provider prescribed a 1-week course of oral steroids and steroid cream. The oral medication did not help, but the cream alleviated the pruritus. He had a medical history of coronary artery disease, hypertension, and diabetes mellitus. He also had a rash on the forearms that had waxed and waned for many years but was not associated with pruritus. He had not sought medical care for the rash and had never treated it. Physical examination revealed pink to violaceous annular plaques with central clearing and raised borders that coalesced into larger plaques on the trunk (top). Dusky, scaly, pink plaques were present on the dorsal forearms. Three punch biopsies—2 from the upper back (bottom) and 1 from the left forearm—all demonstrated consistent findings.
Punch Biopsy Extraction With Fingers
Practice Gap
Punch biopsies are utilized frequently by dermatologists to aid in the diagnosis of various skin diseases.1 When performing a punch biopsy, dermatologists are taught to use either forceps or skin hooks in addition to scissors to extract the tissue from the skin.2 However, the use of these sterile instruments for a simple biopsy adds extra costs to the procedure. Herein, a cheaper and often faster method of obtaining the specimen from the patient is described.
The Technique
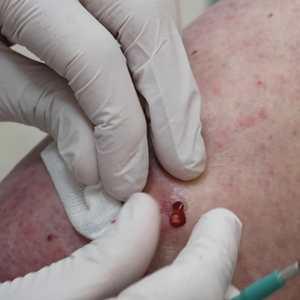
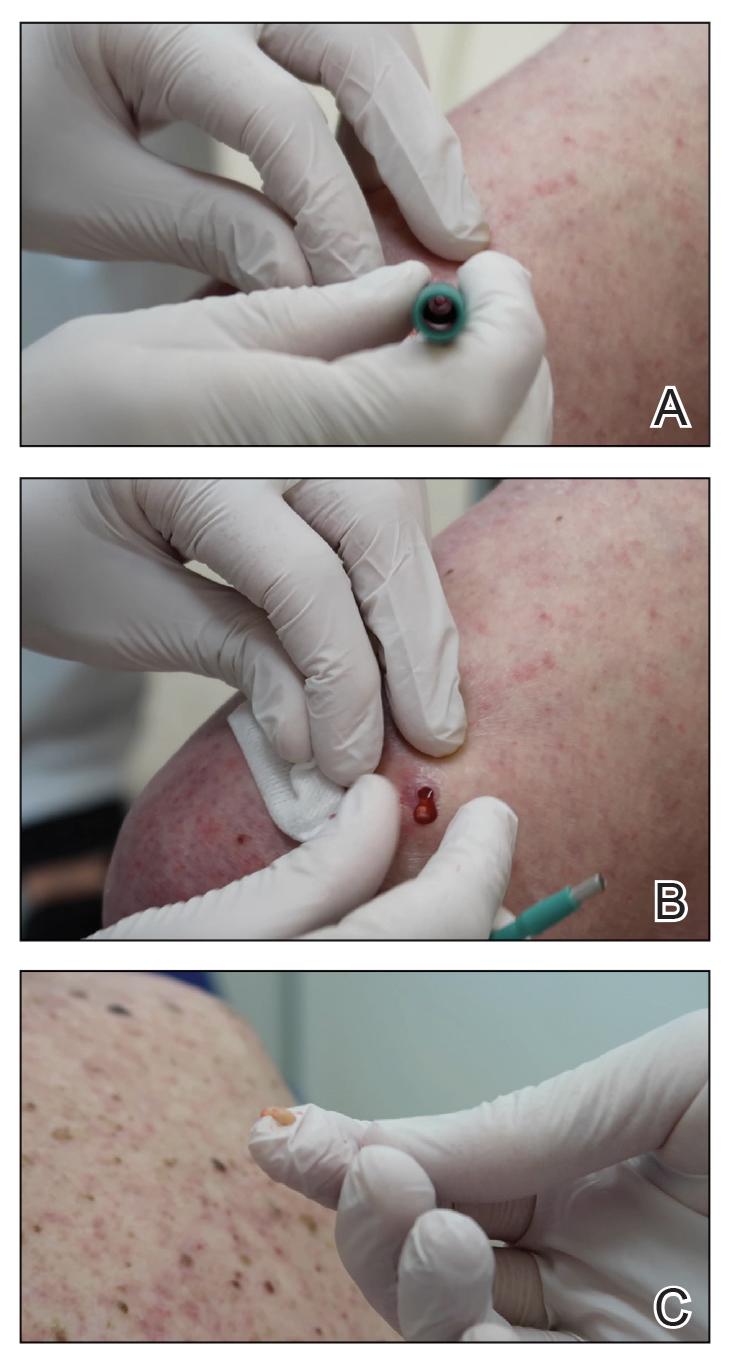
A 3- or 4-mm disposable punch biopsy tool is employed for this method. After locally anesthetizing the skin, the skin is punched to a subcutaneous depth utilizing the full length of the blade and a little extra pressure is applied downward while stretching the skin around (Figure, A). This may be helpful to dislodge the punch specimen from the surrounding skin. The specimen now can be easily removed by gently grasping it with the thumb and index finger (Figure, B and C). It then can be transferred immediately to the formalin container.
Practice Implications
This technique saves time as well as financial and environmental costs associated with the use of sterile instruments. An additional advantage to this simple method is avoiding specimen crush injuries, which are common when using forceps. This solution works in most cases but may not be suitable for certain special anatomic locations such as the scalp, nose, and ears.
- Gronbeck C, Feng H. Volume and distribution of skin biopsies performed by dermatologists and other health care providers in the Medicare population in 2019. J Am Acad Dermatol. 2022;87:675-678.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. Elsevier; 2018.
Practice Gap
Punch biopsies are utilized frequently by dermatologists to aid in the diagnosis of various skin diseases.1 When performing a punch biopsy, dermatologists are taught to use either forceps or skin hooks in addition to scissors to extract the tissue from the skin.2 However, the use of these sterile instruments for a simple biopsy adds extra costs to the procedure. Herein, a cheaper and often faster method of obtaining the specimen from the patient is described.
The Technique
A 3- or 4-mm disposable punch biopsy tool is employed for this method. After locally anesthetizing the skin, the skin is punched to a subcutaneous depth utilizing the full length of the blade and a little extra pressure is applied downward while stretching the skin around (Figure, A). This may be helpful to dislodge the punch specimen from the surrounding skin. The specimen now can be easily removed by gently grasping it with the thumb and index finger (Figure, B and C). It then can be transferred immediately to the formalin container.

Practice Implications
This technique saves time as well as financial and environmental costs associated with the use of sterile instruments. An additional advantage to this simple method is avoiding specimen crush injuries, which are common when using forceps. This solution works in most cases but may not be suitable for certain special anatomic locations such as the scalp, nose, and ears.
Practice Gap
Punch biopsies are utilized frequently by dermatologists to aid in the diagnosis of various skin diseases.1 When performing a punch biopsy, dermatologists are taught to use either forceps or skin hooks in addition to scissors to extract the tissue from the skin.2 However, the use of these sterile instruments for a simple biopsy adds extra costs to the procedure. Herein, a cheaper and often faster method of obtaining the specimen from the patient is described.
The Technique
A 3- or 4-mm disposable punch biopsy tool is employed for this method. After locally anesthetizing the skin, the skin is punched to a subcutaneous depth utilizing the full length of the blade and a little extra pressure is applied downward while stretching the skin around (Figure, A). This may be helpful to dislodge the punch specimen from the surrounding skin. The specimen now can be easily removed by gently grasping it with the thumb and index finger (Figure, B and C). It then can be transferred immediately to the formalin container.

Practice Implications
This technique saves time as well as financial and environmental costs associated with the use of sterile instruments. An additional advantage to this simple method is avoiding specimen crush injuries, which are common when using forceps. This solution works in most cases but may not be suitable for certain special anatomic locations such as the scalp, nose, and ears.
- Gronbeck C, Feng H. Volume and distribution of skin biopsies performed by dermatologists and other health care providers in the Medicare population in 2019. J Am Acad Dermatol. 2022;87:675-678.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. Elsevier; 2018.
- Gronbeck C, Feng H. Volume and distribution of skin biopsies performed by dermatologists and other health care providers in the Medicare population in 2019. J Am Acad Dermatol. 2022;87:675-678.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. Elsevier; 2018.
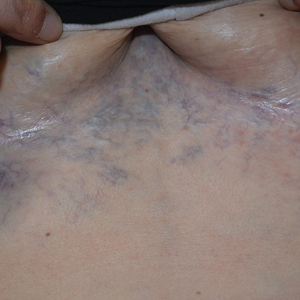
Ectatic Vessels on the Chest
The Diagnosis: Superior Vena Cava Syndrome
Computed tomography angiography of the chest confirmed a diagnosis of superior vena cava (SVC) syndrome due to external pressure of the indwelling catheter. Upon diagnosis, the left indwelling catheter was removed. Further testing to assess for a potential pulmonary embolism was negative. Resolution of the ectatic spider veins and patientreported intermittent facial swelling was achieved after catheter removal.
Superior vena cava syndrome occurs when the SVC is occluded due to extrinsic pressure or thrombosis. Although classically thought to be due to underlying bronchogenic carcinomas, all pathologies that cause compression of the SVC also can lead to vessel occlusion.1 Superior vena cava syndrome initially can be detected on physical examination. The most prominent skin finding includes diffusely dilated blood vessels on the central chest wall, which indicate the presence of collateral blood vessels.1 Imaging studies such as abdominal computed tomography can provide information on the etiology of the condition but are not required for diagnosis. Given the high correlation of SVC syndrome with underlying lung and mediastinal carcinomas, imaging was warranted in our patient. Imaging also can distinguish if the condition is due to external pressure or thrombosis.2 For SVC syndrome due to thrombosis, endovascular therapy is first-line management; however, mechanical thrombectomy may be preferred in patients with absolute contraindication to thrombolytic agents.3 In the setting of increased external pressure on the SVC, treatment includes the removal of the source of pressure.4
In a case series including 78 patients, ports and indwelling catheters accounted for 71% of benign SVC cases.5 Our patient’s SVC syndrome most likely was due to the indwelling catheter pressing on the SVC. The goal of treatment is to address the underlying cause—whether it be pressure or thrombosis. In the setting of increased external pressure, treatment includes removal of the source of pressure from the SVC.4
Other differential diagnoses to consider for newonset ectatic vessels on the chest wall include generalized essential telangiectasia, scleroderma, poikiloderma vasculare atrophicans, and caput medusae. Generalized essential telangiectasia is characterized by red or pink dilated capillary blood vessels in a branch or lacelike pattern predominantly on the lower limbs. The eruption primarily is asymptomatic, though tingling or numbness may be reported.6 The diagnosis can be made with a punch biopsy, with histopathology showing dilated vessels in the dermis.7
Scleroderma is a connective tissue fibrosis disorder with variable clinical presentations. The systemic sclerosis subset can be divided into localized systemic sclerosis and diffuse systemic sclerosis. Physical examination reveals cutaneous sclerosis in various areas of the body. Localized systemic sclerosis includes sclerosis of the fingers and face, while diffuse systemic sclerosis is notable for progression to the arms, legs, and trunk.8 In addition to sclerosis, diffuse telangiectases also can be observed. Systemic sclerosis is a clinical diagnosis based on physical examination and laboratory studies to identify antibodies such as antinuclear antibodies.
Poikiloderma vasculare atrophicans is a variant of cutaneous T-cell lymphoma. The initial presentation is characterized by plaques of hypopigmentation and hyperpigmentation with atrophy and telangiectases. The lesions may be asymptomatic or mildly pruritic and classically involve the trunk and flexural areas.9 The diagnosis is made with skin biopsy and immunohistochemical studies, with findings reflective of mycosis fungoides.
Caput medusae (palm tree sign) is a cardinal feature of portal hypertension characterized by grossly dilated and engorged periumbilical veins. To shunt blood from the portal venous system, cutaneous collateral veins between the umbilical veins and abdominal wall veins are used, resulting in the appearance of engorged veins in the anterior abdominal wall.10 The diagnosis can be made with abdominal ultrasonography showing the direction of blood flow through abdominal vessels.
- Drouin L, Pistorius MA, Lafforgue A, et al. Upper-extremity venous thrombosis: a retrospective study about 160 cases [in French]. Rev Med Interne. 2019;40:9-15.
- Richie E. Clinical pearl: diagnosing superior vena cava syndrome. Emergency Medicine News. 2017;39:22. doi:10.1097/01 .EEM.0000522220.37441.d2
- Azizi A, Shafi I, Shah N, et al. Superior vena cava syndrome. JACC Cardiovasc Interv. 2020;13:2896-2910. doi:10.1016/j.jcin.2020.08.038
- Dumantepe M, Tarhan A, Ozler A. Successful treatment of central venous catheter induced superior vena cava syndrome with ultrasound accelerated catheter-directed thrombolysis. Catheter Cardiovasc Interv. 2013;81:E269-E273.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006;85:37-42. doi:10.1097/01.md.0000198474.99876.f0
- Long D, Marshman G. Generalized essential telangiectasia. Australas J Dermatol. 2004;45:67-69. doi:10.1111/j.1440-0960.2004.00033.x
- Braverman IM. Ultrastructure and organization of the cutaneous microvasculature in normal and pathologic states. J Invest Dermatol. 1989;93(2 suppl):2S-9S.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336. doi:10.1007 /s12016-017-8625-4
- Bloom B, Marchbein S, Fischer M, et al. Poikilodermatous mycosis fungoides. Dermatol Online J. 2012;18:4.
- Sharma B, Raina S. Caput medusae. Indian J Med Res. 2015;141:494. doi:10.4103/0971-5916.159322
The Diagnosis: Superior Vena Cava Syndrome
Computed tomography angiography of the chest confirmed a diagnosis of superior vena cava (SVC) syndrome due to external pressure of the indwelling catheter. Upon diagnosis, the left indwelling catheter was removed. Further testing to assess for a potential pulmonary embolism was negative. Resolution of the ectatic spider veins and patientreported intermittent facial swelling was achieved after catheter removal.
Superior vena cava syndrome occurs when the SVC is occluded due to extrinsic pressure or thrombosis. Although classically thought to be due to underlying bronchogenic carcinomas, all pathologies that cause compression of the SVC also can lead to vessel occlusion.1 Superior vena cava syndrome initially can be detected on physical examination. The most prominent skin finding includes diffusely dilated blood vessels on the central chest wall, which indicate the presence of collateral blood vessels.1 Imaging studies such as abdominal computed tomography can provide information on the etiology of the condition but are not required for diagnosis. Given the high correlation of SVC syndrome with underlying lung and mediastinal carcinomas, imaging was warranted in our patient. Imaging also can distinguish if the condition is due to external pressure or thrombosis.2 For SVC syndrome due to thrombosis, endovascular therapy is first-line management; however, mechanical thrombectomy may be preferred in patients with absolute contraindication to thrombolytic agents.3 In the setting of increased external pressure on the SVC, treatment includes the removal of the source of pressure.4
In a case series including 78 patients, ports and indwelling catheters accounted for 71% of benign SVC cases.5 Our patient’s SVC syndrome most likely was due to the indwelling catheter pressing on the SVC. The goal of treatment is to address the underlying cause—whether it be pressure or thrombosis. In the setting of increased external pressure, treatment includes removal of the source of pressure from the SVC.4
Other differential diagnoses to consider for newonset ectatic vessels on the chest wall include generalized essential telangiectasia, scleroderma, poikiloderma vasculare atrophicans, and caput medusae. Generalized essential telangiectasia is characterized by red or pink dilated capillary blood vessels in a branch or lacelike pattern predominantly on the lower limbs. The eruption primarily is asymptomatic, though tingling or numbness may be reported.6 The diagnosis can be made with a punch biopsy, with histopathology showing dilated vessels in the dermis.7
Scleroderma is a connective tissue fibrosis disorder with variable clinical presentations. The systemic sclerosis subset can be divided into localized systemic sclerosis and diffuse systemic sclerosis. Physical examination reveals cutaneous sclerosis in various areas of the body. Localized systemic sclerosis includes sclerosis of the fingers and face, while diffuse systemic sclerosis is notable for progression to the arms, legs, and trunk.8 In addition to sclerosis, diffuse telangiectases also can be observed. Systemic sclerosis is a clinical diagnosis based on physical examination and laboratory studies to identify antibodies such as antinuclear antibodies.
Poikiloderma vasculare atrophicans is a variant of cutaneous T-cell lymphoma. The initial presentation is characterized by plaques of hypopigmentation and hyperpigmentation with atrophy and telangiectases. The lesions may be asymptomatic or mildly pruritic and classically involve the trunk and flexural areas.9 The diagnosis is made with skin biopsy and immunohistochemical studies, with findings reflective of mycosis fungoides.
Caput medusae (palm tree sign) is a cardinal feature of portal hypertension characterized by grossly dilated and engorged periumbilical veins. To shunt blood from the portal venous system, cutaneous collateral veins between the umbilical veins and abdominal wall veins are used, resulting in the appearance of engorged veins in the anterior abdominal wall.10 The diagnosis can be made with abdominal ultrasonography showing the direction of blood flow through abdominal vessels.
The Diagnosis: Superior Vena Cava Syndrome
Computed tomography angiography of the chest confirmed a diagnosis of superior vena cava (SVC) syndrome due to external pressure of the indwelling catheter. Upon diagnosis, the left indwelling catheter was removed. Further testing to assess for a potential pulmonary embolism was negative. Resolution of the ectatic spider veins and patientreported intermittent facial swelling was achieved after catheter removal.
Superior vena cava syndrome occurs when the SVC is occluded due to extrinsic pressure or thrombosis. Although classically thought to be due to underlying bronchogenic carcinomas, all pathologies that cause compression of the SVC also can lead to vessel occlusion.1 Superior vena cava syndrome initially can be detected on physical examination. The most prominent skin finding includes diffusely dilated blood vessels on the central chest wall, which indicate the presence of collateral blood vessels.1 Imaging studies such as abdominal computed tomography can provide information on the etiology of the condition but are not required for diagnosis. Given the high correlation of SVC syndrome with underlying lung and mediastinal carcinomas, imaging was warranted in our patient. Imaging also can distinguish if the condition is due to external pressure or thrombosis.2 For SVC syndrome due to thrombosis, endovascular therapy is first-line management; however, mechanical thrombectomy may be preferred in patients with absolute contraindication to thrombolytic agents.3 In the setting of increased external pressure on the SVC, treatment includes the removal of the source of pressure.4
In a case series including 78 patients, ports and indwelling catheters accounted for 71% of benign SVC cases.5 Our patient’s SVC syndrome most likely was due to the indwelling catheter pressing on the SVC. The goal of treatment is to address the underlying cause—whether it be pressure or thrombosis. In the setting of increased external pressure, treatment includes removal of the source of pressure from the SVC.4
Other differential diagnoses to consider for newonset ectatic vessels on the chest wall include generalized essential telangiectasia, scleroderma, poikiloderma vasculare atrophicans, and caput medusae. Generalized essential telangiectasia is characterized by red or pink dilated capillary blood vessels in a branch or lacelike pattern predominantly on the lower limbs. The eruption primarily is asymptomatic, though tingling or numbness may be reported.6 The diagnosis can be made with a punch biopsy, with histopathology showing dilated vessels in the dermis.7
Scleroderma is a connective tissue fibrosis disorder with variable clinical presentations. The systemic sclerosis subset can be divided into localized systemic sclerosis and diffuse systemic sclerosis. Physical examination reveals cutaneous sclerosis in various areas of the body. Localized systemic sclerosis includes sclerosis of the fingers and face, while diffuse systemic sclerosis is notable for progression to the arms, legs, and trunk.8 In addition to sclerosis, diffuse telangiectases also can be observed. Systemic sclerosis is a clinical diagnosis based on physical examination and laboratory studies to identify antibodies such as antinuclear antibodies.
Poikiloderma vasculare atrophicans is a variant of cutaneous T-cell lymphoma. The initial presentation is characterized by plaques of hypopigmentation and hyperpigmentation with atrophy and telangiectases. The lesions may be asymptomatic or mildly pruritic and classically involve the trunk and flexural areas.9 The diagnosis is made with skin biopsy and immunohistochemical studies, with findings reflective of mycosis fungoides.
Caput medusae (palm tree sign) is a cardinal feature of portal hypertension characterized by grossly dilated and engorged periumbilical veins. To shunt blood from the portal venous system, cutaneous collateral veins between the umbilical veins and abdominal wall veins are used, resulting in the appearance of engorged veins in the anterior abdominal wall.10 The diagnosis can be made with abdominal ultrasonography showing the direction of blood flow through abdominal vessels.
- Drouin L, Pistorius MA, Lafforgue A, et al. Upper-extremity venous thrombosis: a retrospective study about 160 cases [in French]. Rev Med Interne. 2019;40:9-15.
- Richie E. Clinical pearl: diagnosing superior vena cava syndrome. Emergency Medicine News. 2017;39:22. doi:10.1097/01 .EEM.0000522220.37441.d2
- Azizi A, Shafi I, Shah N, et al. Superior vena cava syndrome. JACC Cardiovasc Interv. 2020;13:2896-2910. doi:10.1016/j.jcin.2020.08.038
- Dumantepe M, Tarhan A, Ozler A. Successful treatment of central venous catheter induced superior vena cava syndrome with ultrasound accelerated catheter-directed thrombolysis. Catheter Cardiovasc Interv. 2013;81:E269-E273.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006;85:37-42. doi:10.1097/01.md.0000198474.99876.f0
- Long D, Marshman G. Generalized essential telangiectasia. Australas J Dermatol. 2004;45:67-69. doi:10.1111/j.1440-0960.2004.00033.x
- Braverman IM. Ultrastructure and organization of the cutaneous microvasculature in normal and pathologic states. J Invest Dermatol. 1989;93(2 suppl):2S-9S.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336. doi:10.1007 /s12016-017-8625-4
- Bloom B, Marchbein S, Fischer M, et al. Poikilodermatous mycosis fungoides. Dermatol Online J. 2012;18:4.
- Sharma B, Raina S. Caput medusae. Indian J Med Res. 2015;141:494. doi:10.4103/0971-5916.159322
- Drouin L, Pistorius MA, Lafforgue A, et al. Upper-extremity venous thrombosis: a retrospective study about 160 cases [in French]. Rev Med Interne. 2019;40:9-15.
- Richie E. Clinical pearl: diagnosing superior vena cava syndrome. Emergency Medicine News. 2017;39:22. doi:10.1097/01 .EEM.0000522220.37441.d2
- Azizi A, Shafi I, Shah N, et al. Superior vena cava syndrome. JACC Cardiovasc Interv. 2020;13:2896-2910. doi:10.1016/j.jcin.2020.08.038
- Dumantepe M, Tarhan A, Ozler A. Successful treatment of central venous catheter induced superior vena cava syndrome with ultrasound accelerated catheter-directed thrombolysis. Catheter Cardiovasc Interv. 2013;81:E269-E273.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006;85:37-42. doi:10.1097/01.md.0000198474.99876.f0
- Long D, Marshman G. Generalized essential telangiectasia. Australas J Dermatol. 2004;45:67-69. doi:10.1111/j.1440-0960.2004.00033.x
- Braverman IM. Ultrastructure and organization of the cutaneous microvasculature in normal and pathologic states. J Invest Dermatol. 1989;93(2 suppl):2S-9S.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336. doi:10.1007 /s12016-017-8625-4
- Bloom B, Marchbein S, Fischer M, et al. Poikilodermatous mycosis fungoides. Dermatol Online J. 2012;18:4.
- Sharma B, Raina S. Caput medusae. Indian J Med Res. 2015;141:494. doi:10.4103/0971-5916.159322
A 32-year-old woman presented to vascular surgery for evaluation of spider veins of 2 years’ duration that originated on the breasts but later spread to include the central chest, inframammary folds, and back. She reported associated pain and discomfort as well as intermittent facial swelling and tachycardia but denied pruritus and bleeding. The patient had a history of a kidney transplant 6 months prior, Langerhans cell histiocytosis, and Sjögren syndrome with a left indwelling catheter. Her current medications included systemic immunosuppressive agents. Physical examination revealed blue-purple ectatic vessels on the inframammary folds and central chest extending to the back. Erythema on the face, neck, and arms was not appreciated. No palpable cervical, supraclavicular, or axillary lymph nodes were noted.
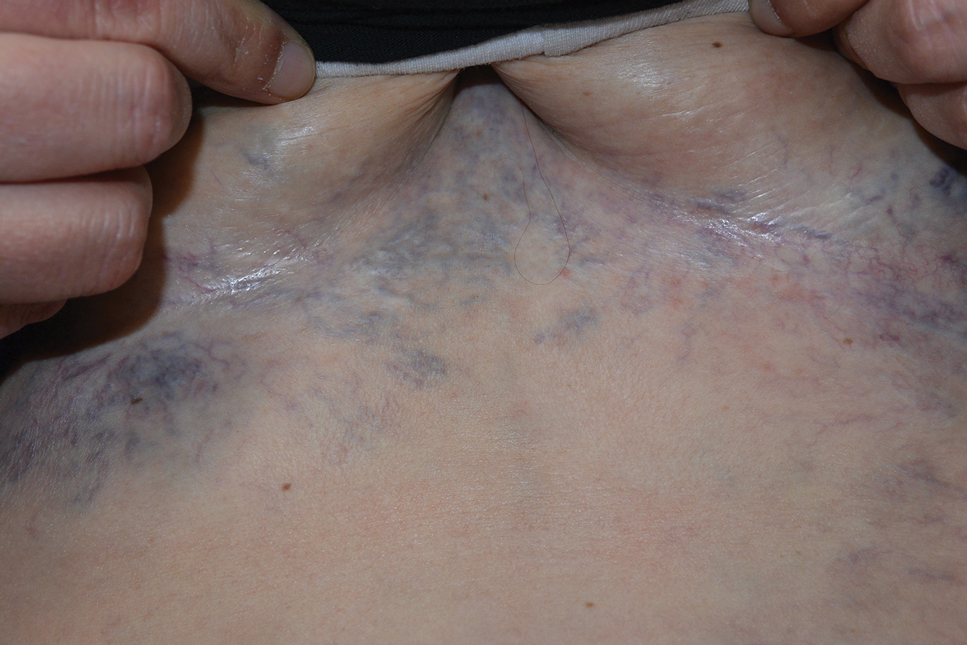
Pink Papules on the Cheek
The Diagnosis: Cutaneous Rosai-Dorfman Disease
Rosai-Dorfman disease is a rare benign non- Langerhans cell histiocytopathy that can manifest initially with lymph node involvement—classically, massive painless cervical lymphadenopathy.1 Cutaneous Rosai-Dorfman disease (CRDD) is a variant that can be associated with lymph node and internal involvement, but more than 80% of cases lack extracutaneous involvement.2,3 In cases with extracutaneous involvement, lymph node disease is most frequent.3 Cutaneous Rosai-Dorfman disease unassociated with extracutaneous disease is a benign self-limiting histiocytopathy that manifests as painless red-brown, yellow, or fleshcolored nodules, plaques, or papules that may become tender or ulcerated.4
Cutaneous Rosai-Dorfman disease represents a benign histiocytopathy of resident dendritic cell derivation.3 A characteristic immunohistochemical finding is S-100 positivity, which might suggest a Langerhans cell transdifferentiation phenotype, but other markers corroborative of a Langerhans cell phenotype—namely CD1a and langerin—will be negative. Biopsies typically show a mid to deep dermal histiocytic infiltration in a variably dense polymorphous inflammatory cell background comprised of a mixture of lymphocytes, plasma cells, and neutrophils.3 At times the extent of lymphocytic infiltration can be to a magnitude that resembles a lymphoma on histopathology. In our patient, lymphoma was excluded based on clinical presentation, as this patient lacked the typical symptoms of lymphadenopathy or B symptoms that come with B-cell lymphoma.5
The histiocytes in CRDD are characteristically large mononuclear cells exhibiting a low nuclear to cytoplasmic ratio reflective of the voluminous, nonvacuolated, watery cytoplasm. They have ill-defined cytoplasmic membranes resulting in a seemingly syncytial growth pattern. A hallmark of the histiocytes is emperipolesis characterized by intracytoplasmic localization of intact inflammatory cells including neutrophils, lymphocytes, and plasma cells.3
The differential diagnosis of CRDD includes Langerhans cell histiocytosis (LCH), indeterminate cell histiocytosis, xanthogranuloma, and reticulohistiocytoma. All of these conditions can be differentiated by their unique histopathologic and phenotypic characteristics.
Langerhans cell histiocytosis is a distinct clonal histiocytopathy that has a varied presentation ranging from cutaneous confined cases manifesting as a solitary lesion to one of disseminated cutaneous disease with the potential for multiorgan involvement. Regardless of the variant of LCH, the hallmark cell is one showing an eccentrically disposed, reniform nucleus with an open chromatin and abundant eosinophilic cytoplasm (Figure 1).6 Both LCH and CRDD stain positive for S-100. However, unlike the histiocytes in CRDD, those seen in LCH stain positive for CD1a and langerin and would not express factor XIIIA. Additionally, the neoplastic cells would not exhibit the same extent of CD68 positivity seen in CRDD.6 Treatment of LCH depends on the extent of disease, especially for the presence or absence of extracutaneous disease.7
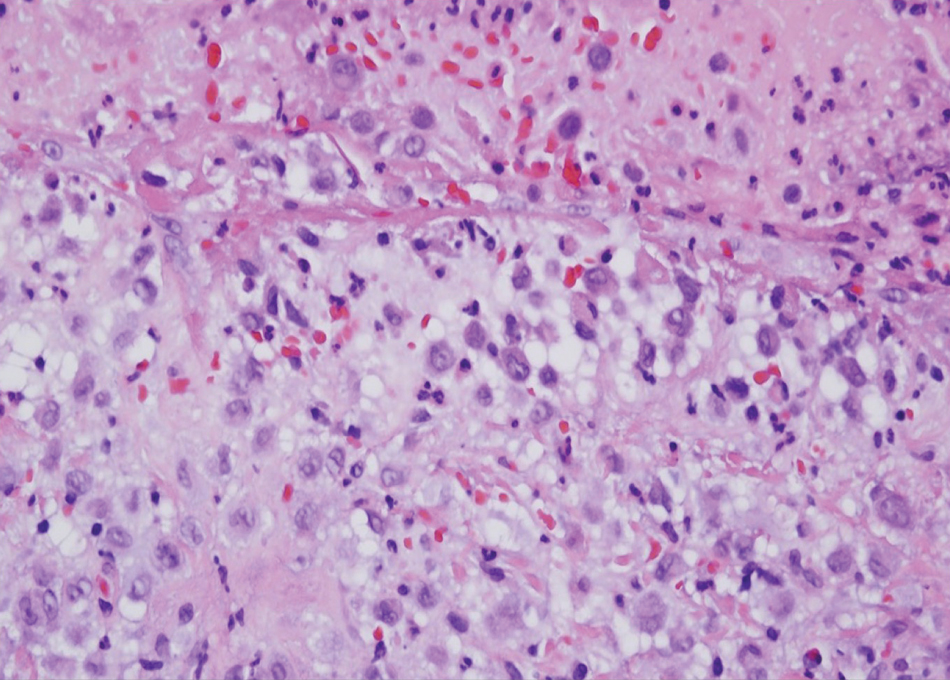
A variant of LCH is indeterminate cell histiocytosis, which can be seen in neonates or adults. It represents a neoplastic proliferation of Langerhans cells that are devoid of Birbeck granules, reflective of an immature early phase of differentiation in the skin prior to the cells acquiring the Birbeck granule (as would be seen in neonates) or a later phase of differentiation after the mature Langerhans cell has encountered antigen and is en route to the lymph node (typically seen in adults).8 The phenotypic profile is identical to conventional LCH except the cells do not express langerin. Microscopically, the infiltrates are composed of Langerhans cells that are morphologically indistinguishable from classic LCH but without epidermotropism and exhibit a dominant localization in the dermis typically unassociated with other inflammatory cell elements (Figure 2).9
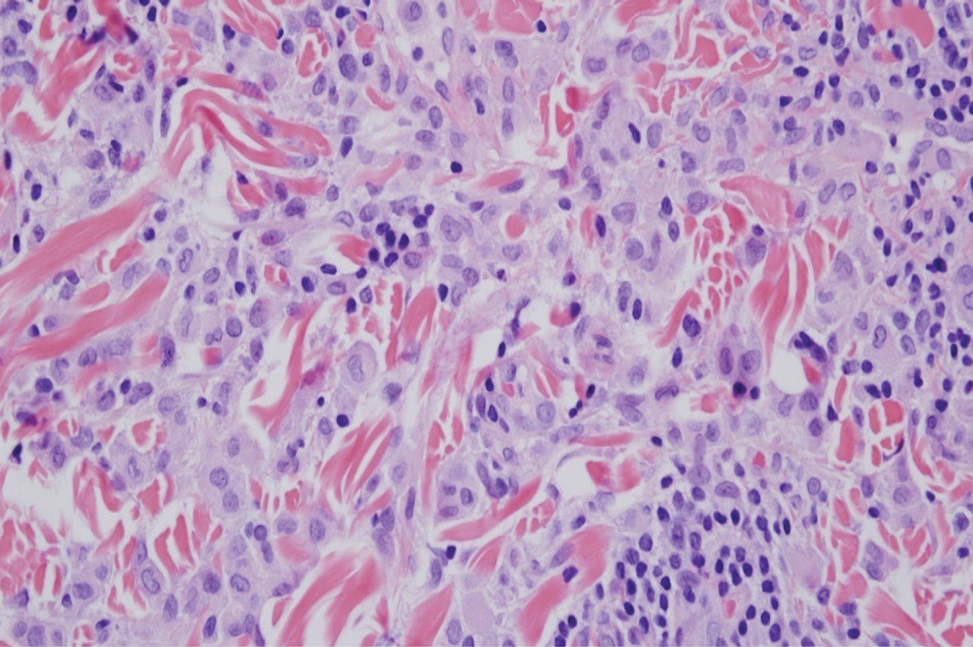
Xanthogranuloma is seen in young children (juvenile xanthogranuloma) as a solitary lesion, though a multifocal cutaneous variant and extracutaneous presentations have been described. Similar lesions can be seen in adults.10 These lesions are evolutionary in their morphology. In the inception of a juvenile xanthogranuloma, the lesions are highly cellular, and the histiocytes typically are poorly lipidized; there may be a dearth of other inflammatory cell elements. As the lesions mature, the histiocytes become lipidized, and characteristic Touton giant cells that exhibit a wreath of nuclei with peripheral lipidization may develop (Figure 3). In the later stages, there is considerable hyalinizing fibrosis, and the cells can acquire a spindled appearance. The absence of emperipolesis and the presence of notable lipidization are useful light microscopy features differentiating xanthogranuloma from CRDD.11 Treatment of xanthogranuloma can range from a conservative monitoring approach to an aggressive approach combining various antineoplastic therapies with immunosuppressive agents.12
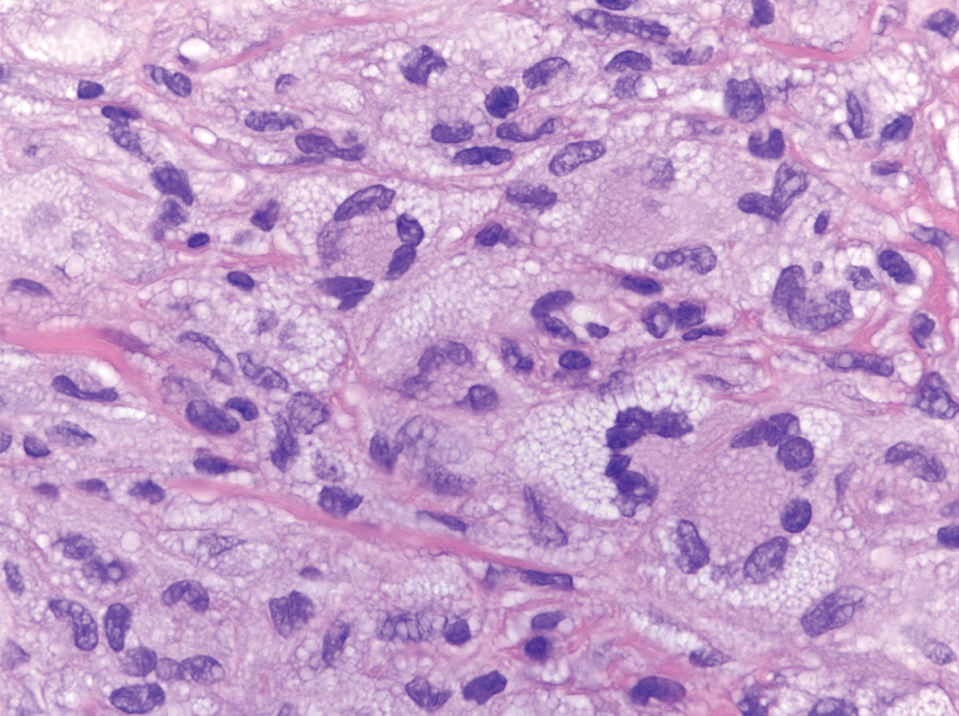
Solitary and multicentric reticulohistiocytoma is another form of resident dendritic cell histiocytopathy that can resemble Rosai-Dorfman disease. It is a dermal histiocytic infiltrate accompanied by a polymorphous inflammatory cell infiltrate (Figure 4) and can show variable fibrosis.13 One of the hallmarks is the distinct amphophilic cytoplasms, possibly attributable to nuclear DNA released into the cytoplasm from effete nuclei.13 The solitary form is unassociated with systemic disease, whereas the multicentric variant can be a paraneoplastic syndrome in the setting of solid and hematologic malignancies.14 In addition, in the multicentric variant, similar lesions can affect any organ but there can be a proclivity to involve the hand and knee joints, leading to a crippling arthritis.15 We presented a case of CRDD, a benign resident dendritic cell histiocytopathy that can manifest as a cutaneous confined process in the skin where the clinical course is characteristically benign. It potentially can be confused with LCH, indeterminate cell histiocytosis, xanthogranuloma, and reticulohistiocytoma. For a solitary lesion, intralesional triamcinolone injection and excision are options. Multifocal cutaneous disease or CRDD with notable extracutaneous disease may require systemic treatment.16 In our patient, one intralesional triamcinolone injection was performed with notable resolution.
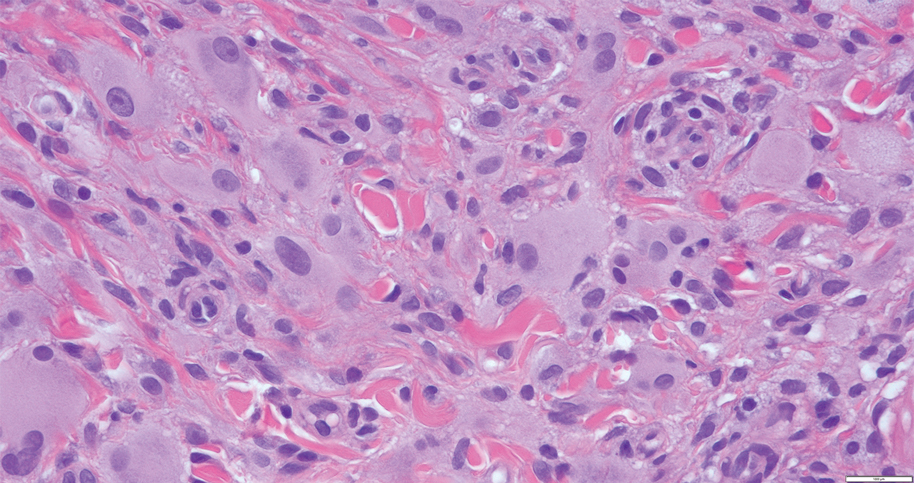
- Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: a newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63-70.
- Brenn T, Calonje E, Granter SR, et al. Cutaneous Rosai-Dorfman disease is a distinct clinical entity. Am J Dermatopathol. 2002;24:385.
- Ahmed A, Crowson N, Magro CM. A comprehensive assessment of cutaneous Rosai-Dorfman disease. Ann Diagn Pathol. 2019;40:166-173.
- Frater JL, Maddox JS, Obadiah JM, et al. Cutaneous Rosai-Dorfman disease: comprehensive review of cases reported in the medical literature since 1990 and presentation of an illustrative case. J Cutan Med Surg. 2006;10:281-290.
- Friedberg JW, Fisher RI. Diffuse large B-cell lymphoma. Hematol Oncol Clin North Am. 2008;22:941-952. Doi:10.1016/j.hoc.2008.07.002
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Board PPTE. Langerhans cell histiocytosis treatment (PDQ®). In: PDQ Cancer Information Summaries [Internet]. National Cancer Institute (US); 2009.
- Chu A, Eisinger M, Lee JS, et al. Immunoelectron microscopic identification of Langerhans cells using a new antigenic marker. J Invest Dermatol. 1982;78:177-180. doi:10.1111/1523-1747.ep12506352
- Berti E, Gianotti R, Alessi E. Unusual cutaneous histiocytosis expressing an intermediate immunophenotype between Langerhans’ cells and dermal macrophages. Arch Dermatol. 1988;124:1250-1253. doi:10.1001/archderm.1988.01670080062020
- Cypel TKS, Zuker RM. Juvenile xanthogranuloma: case report and review of the literature. Can J Plast Surg. 2008;16:175-177.
- Rodriguez J, Ackerman AB. Xanthogranuloma in adults. Arch Dermatol. 1976;112:43-44.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. In: StatPearls [Internet]. StatPearls Publishing; 2022.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492. doi:10.1016/j. clindermatol.2006.07.010
- Miettinen M, Fetsch JF. Reticulohistiocytoma (solitary epithelioid histiocytoma): a clinicopathologic and immunohistochemical study of 44 cases. Am J Surg Pathol. 2006;30:521.
- Gold RH, Metzger AL, Mirra JM, et al. Multicentric reticulohistiocytosis (lipoid dermato-arthritis). An erosive polyarthritis with distinctive clinical, roentgenographic and pathologic features. Am J Roentgenol Radium Ther Nucl Med. 1975;124:610-624. doi:10.2214/ajr.124.4.610
- Dalia S, Sagatys E, Sokol L, et al. Rosai-Dorfman disease: tumor biology, clinical features, pathology, and treatment. Cancer Control. 2014;21:322-327. doi:10.1177/107327481402100408
The Diagnosis: Cutaneous Rosai-Dorfman Disease
Rosai-Dorfman disease is a rare benign non- Langerhans cell histiocytopathy that can manifest initially with lymph node involvement—classically, massive painless cervical lymphadenopathy.1 Cutaneous Rosai-Dorfman disease (CRDD) is a variant that can be associated with lymph node and internal involvement, but more than 80% of cases lack extracutaneous involvement.2,3 In cases with extracutaneous involvement, lymph node disease is most frequent.3 Cutaneous Rosai-Dorfman disease unassociated with extracutaneous disease is a benign self-limiting histiocytopathy that manifests as painless red-brown, yellow, or fleshcolored nodules, plaques, or papules that may become tender or ulcerated.4
Cutaneous Rosai-Dorfman disease represents a benign histiocytopathy of resident dendritic cell derivation.3 A characteristic immunohistochemical finding is S-100 positivity, which might suggest a Langerhans cell transdifferentiation phenotype, but other markers corroborative of a Langerhans cell phenotype—namely CD1a and langerin—will be negative. Biopsies typically show a mid to deep dermal histiocytic infiltration in a variably dense polymorphous inflammatory cell background comprised of a mixture of lymphocytes, plasma cells, and neutrophils.3 At times the extent of lymphocytic infiltration can be to a magnitude that resembles a lymphoma on histopathology. In our patient, lymphoma was excluded based on clinical presentation, as this patient lacked the typical symptoms of lymphadenopathy or B symptoms that come with B-cell lymphoma.5
The histiocytes in CRDD are characteristically large mononuclear cells exhibiting a low nuclear to cytoplasmic ratio reflective of the voluminous, nonvacuolated, watery cytoplasm. They have ill-defined cytoplasmic membranes resulting in a seemingly syncytial growth pattern. A hallmark of the histiocytes is emperipolesis characterized by intracytoplasmic localization of intact inflammatory cells including neutrophils, lymphocytes, and plasma cells.3
The differential diagnosis of CRDD includes Langerhans cell histiocytosis (LCH), indeterminate cell histiocytosis, xanthogranuloma, and reticulohistiocytoma. All of these conditions can be differentiated by their unique histopathologic and phenotypic characteristics.
Langerhans cell histiocytosis is a distinct clonal histiocytopathy that has a varied presentation ranging from cutaneous confined cases manifesting as a solitary lesion to one of disseminated cutaneous disease with the potential for multiorgan involvement. Regardless of the variant of LCH, the hallmark cell is one showing an eccentrically disposed, reniform nucleus with an open chromatin and abundant eosinophilic cytoplasm (Figure 1).6 Both LCH and CRDD stain positive for S-100. However, unlike the histiocytes in CRDD, those seen in LCH stain positive for CD1a and langerin and would not express factor XIIIA. Additionally, the neoplastic cells would not exhibit the same extent of CD68 positivity seen in CRDD.6 Treatment of LCH depends on the extent of disease, especially for the presence or absence of extracutaneous disease.7

A variant of LCH is indeterminate cell histiocytosis, which can be seen in neonates or adults. It represents a neoplastic proliferation of Langerhans cells that are devoid of Birbeck granules, reflective of an immature early phase of differentiation in the skin prior to the cells acquiring the Birbeck granule (as would be seen in neonates) or a later phase of differentiation after the mature Langerhans cell has encountered antigen and is en route to the lymph node (typically seen in adults).8 The phenotypic profile is identical to conventional LCH except the cells do not express langerin. Microscopically, the infiltrates are composed of Langerhans cells that are morphologically indistinguishable from classic LCH but without epidermotropism and exhibit a dominant localization in the dermis typically unassociated with other inflammatory cell elements (Figure 2).9

Xanthogranuloma is seen in young children (juvenile xanthogranuloma) as a solitary lesion, though a multifocal cutaneous variant and extracutaneous presentations have been described. Similar lesions can be seen in adults.10 These lesions are evolutionary in their morphology. In the inception of a juvenile xanthogranuloma, the lesions are highly cellular, and the histiocytes typically are poorly lipidized; there may be a dearth of other inflammatory cell elements. As the lesions mature, the histiocytes become lipidized, and characteristic Touton giant cells that exhibit a wreath of nuclei with peripheral lipidization may develop (Figure 3). In the later stages, there is considerable hyalinizing fibrosis, and the cells can acquire a spindled appearance. The absence of emperipolesis and the presence of notable lipidization are useful light microscopy features differentiating xanthogranuloma from CRDD.11 Treatment of xanthogranuloma can range from a conservative monitoring approach to an aggressive approach combining various antineoplastic therapies with immunosuppressive agents.12

Solitary and multicentric reticulohistiocytoma is another form of resident dendritic cell histiocytopathy that can resemble Rosai-Dorfman disease. It is a dermal histiocytic infiltrate accompanied by a polymorphous inflammatory cell infiltrate (Figure 4) and can show variable fibrosis.13 One of the hallmarks is the distinct amphophilic cytoplasms, possibly attributable to nuclear DNA released into the cytoplasm from effete nuclei.13 The solitary form is unassociated with systemic disease, whereas the multicentric variant can be a paraneoplastic syndrome in the setting of solid and hematologic malignancies.14 In addition, in the multicentric variant, similar lesions can affect any organ but there can be a proclivity to involve the hand and knee joints, leading to a crippling arthritis.15 We presented a case of CRDD, a benign resident dendritic cell histiocytopathy that can manifest as a cutaneous confined process in the skin where the clinical course is characteristically benign. It potentially can be confused with LCH, indeterminate cell histiocytosis, xanthogranuloma, and reticulohistiocytoma. For a solitary lesion, intralesional triamcinolone injection and excision are options. Multifocal cutaneous disease or CRDD with notable extracutaneous disease may require systemic treatment.16 In our patient, one intralesional triamcinolone injection was performed with notable resolution.

The Diagnosis: Cutaneous Rosai-Dorfman Disease
Rosai-Dorfman disease is a rare benign non- Langerhans cell histiocytopathy that can manifest initially with lymph node involvement—classically, massive painless cervical lymphadenopathy.1 Cutaneous Rosai-Dorfman disease (CRDD) is a variant that can be associated with lymph node and internal involvement, but more than 80% of cases lack extracutaneous involvement.2,3 In cases with extracutaneous involvement, lymph node disease is most frequent.3 Cutaneous Rosai-Dorfman disease unassociated with extracutaneous disease is a benign self-limiting histiocytopathy that manifests as painless red-brown, yellow, or fleshcolored nodules, plaques, or papules that may become tender or ulcerated.4
Cutaneous Rosai-Dorfman disease represents a benign histiocytopathy of resident dendritic cell derivation.3 A characteristic immunohistochemical finding is S-100 positivity, which might suggest a Langerhans cell transdifferentiation phenotype, but other markers corroborative of a Langerhans cell phenotype—namely CD1a and langerin—will be negative. Biopsies typically show a mid to deep dermal histiocytic infiltration in a variably dense polymorphous inflammatory cell background comprised of a mixture of lymphocytes, plasma cells, and neutrophils.3 At times the extent of lymphocytic infiltration can be to a magnitude that resembles a lymphoma on histopathology. In our patient, lymphoma was excluded based on clinical presentation, as this patient lacked the typical symptoms of lymphadenopathy or B symptoms that come with B-cell lymphoma.5
The histiocytes in CRDD are characteristically large mononuclear cells exhibiting a low nuclear to cytoplasmic ratio reflective of the voluminous, nonvacuolated, watery cytoplasm. They have ill-defined cytoplasmic membranes resulting in a seemingly syncytial growth pattern. A hallmark of the histiocytes is emperipolesis characterized by intracytoplasmic localization of intact inflammatory cells including neutrophils, lymphocytes, and plasma cells.3
The differential diagnosis of CRDD includes Langerhans cell histiocytosis (LCH), indeterminate cell histiocytosis, xanthogranuloma, and reticulohistiocytoma. All of these conditions can be differentiated by their unique histopathologic and phenotypic characteristics.
Langerhans cell histiocytosis is a distinct clonal histiocytopathy that has a varied presentation ranging from cutaneous confined cases manifesting as a solitary lesion to one of disseminated cutaneous disease with the potential for multiorgan involvement. Regardless of the variant of LCH, the hallmark cell is one showing an eccentrically disposed, reniform nucleus with an open chromatin and abundant eosinophilic cytoplasm (Figure 1).6 Both LCH and CRDD stain positive for S-100. However, unlike the histiocytes in CRDD, those seen in LCH stain positive for CD1a and langerin and would not express factor XIIIA. Additionally, the neoplastic cells would not exhibit the same extent of CD68 positivity seen in CRDD.6 Treatment of LCH depends on the extent of disease, especially for the presence or absence of extracutaneous disease.7

A variant of LCH is indeterminate cell histiocytosis, which can be seen in neonates or adults. It represents a neoplastic proliferation of Langerhans cells that are devoid of Birbeck granules, reflective of an immature early phase of differentiation in the skin prior to the cells acquiring the Birbeck granule (as would be seen in neonates) or a later phase of differentiation after the mature Langerhans cell has encountered antigen and is en route to the lymph node (typically seen in adults).8 The phenotypic profile is identical to conventional LCH except the cells do not express langerin. Microscopically, the infiltrates are composed of Langerhans cells that are morphologically indistinguishable from classic LCH but without epidermotropism and exhibit a dominant localization in the dermis typically unassociated with other inflammatory cell elements (Figure 2).9

Xanthogranuloma is seen in young children (juvenile xanthogranuloma) as a solitary lesion, though a multifocal cutaneous variant and extracutaneous presentations have been described. Similar lesions can be seen in adults.10 These lesions are evolutionary in their morphology. In the inception of a juvenile xanthogranuloma, the lesions are highly cellular, and the histiocytes typically are poorly lipidized; there may be a dearth of other inflammatory cell elements. As the lesions mature, the histiocytes become lipidized, and characteristic Touton giant cells that exhibit a wreath of nuclei with peripheral lipidization may develop (Figure 3). In the later stages, there is considerable hyalinizing fibrosis, and the cells can acquire a spindled appearance. The absence of emperipolesis and the presence of notable lipidization are useful light microscopy features differentiating xanthogranuloma from CRDD.11 Treatment of xanthogranuloma can range from a conservative monitoring approach to an aggressive approach combining various antineoplastic therapies with immunosuppressive agents.12

Solitary and multicentric reticulohistiocytoma is another form of resident dendritic cell histiocytopathy that can resemble Rosai-Dorfman disease. It is a dermal histiocytic infiltrate accompanied by a polymorphous inflammatory cell infiltrate (Figure 4) and can show variable fibrosis.13 One of the hallmarks is the distinct amphophilic cytoplasms, possibly attributable to nuclear DNA released into the cytoplasm from effete nuclei.13 The solitary form is unassociated with systemic disease, whereas the multicentric variant can be a paraneoplastic syndrome in the setting of solid and hematologic malignancies.14 In addition, in the multicentric variant, similar lesions can affect any organ but there can be a proclivity to involve the hand and knee joints, leading to a crippling arthritis.15 We presented a case of CRDD, a benign resident dendritic cell histiocytopathy that can manifest as a cutaneous confined process in the skin where the clinical course is characteristically benign. It potentially can be confused with LCH, indeterminate cell histiocytosis, xanthogranuloma, and reticulohistiocytoma. For a solitary lesion, intralesional triamcinolone injection and excision are options. Multifocal cutaneous disease or CRDD with notable extracutaneous disease may require systemic treatment.16 In our patient, one intralesional triamcinolone injection was performed with notable resolution.

- Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: a newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63-70.
- Brenn T, Calonje E, Granter SR, et al. Cutaneous Rosai-Dorfman disease is a distinct clinical entity. Am J Dermatopathol. 2002;24:385.
- Ahmed A, Crowson N, Magro CM. A comprehensive assessment of cutaneous Rosai-Dorfman disease. Ann Diagn Pathol. 2019;40:166-173.
- Frater JL, Maddox JS, Obadiah JM, et al. Cutaneous Rosai-Dorfman disease: comprehensive review of cases reported in the medical literature since 1990 and presentation of an illustrative case. J Cutan Med Surg. 2006;10:281-290.
- Friedberg JW, Fisher RI. Diffuse large B-cell lymphoma. Hematol Oncol Clin North Am. 2008;22:941-952. Doi:10.1016/j.hoc.2008.07.002
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Board PPTE. Langerhans cell histiocytosis treatment (PDQ®). In: PDQ Cancer Information Summaries [Internet]. National Cancer Institute (US); 2009.
- Chu A, Eisinger M, Lee JS, et al. Immunoelectron microscopic identification of Langerhans cells using a new antigenic marker. J Invest Dermatol. 1982;78:177-180. doi:10.1111/1523-1747.ep12506352
- Berti E, Gianotti R, Alessi E. Unusual cutaneous histiocytosis expressing an intermediate immunophenotype between Langerhans’ cells and dermal macrophages. Arch Dermatol. 1988;124:1250-1253. doi:10.1001/archderm.1988.01670080062020
- Cypel TKS, Zuker RM. Juvenile xanthogranuloma: case report and review of the literature. Can J Plast Surg. 2008;16:175-177.
- Rodriguez J, Ackerman AB. Xanthogranuloma in adults. Arch Dermatol. 1976;112:43-44.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. In: StatPearls [Internet]. StatPearls Publishing; 2022.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492. doi:10.1016/j. clindermatol.2006.07.010
- Miettinen M, Fetsch JF. Reticulohistiocytoma (solitary epithelioid histiocytoma): a clinicopathologic and immunohistochemical study of 44 cases. Am J Surg Pathol. 2006;30:521.
- Gold RH, Metzger AL, Mirra JM, et al. Multicentric reticulohistiocytosis (lipoid dermato-arthritis). An erosive polyarthritis with distinctive clinical, roentgenographic and pathologic features. Am J Roentgenol Radium Ther Nucl Med. 1975;124:610-624. doi:10.2214/ajr.124.4.610
- Dalia S, Sagatys E, Sokol L, et al. Rosai-Dorfman disease: tumor biology, clinical features, pathology, and treatment. Cancer Control. 2014;21:322-327. doi:10.1177/107327481402100408
- Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: a newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63-70.
- Brenn T, Calonje E, Granter SR, et al. Cutaneous Rosai-Dorfman disease is a distinct clinical entity. Am J Dermatopathol. 2002;24:385.
- Ahmed A, Crowson N, Magro CM. A comprehensive assessment of cutaneous Rosai-Dorfman disease. Ann Diagn Pathol. 2019;40:166-173.
- Frater JL, Maddox JS, Obadiah JM, et al. Cutaneous Rosai-Dorfman disease: comprehensive review of cases reported in the medical literature since 1990 and presentation of an illustrative case. J Cutan Med Surg. 2006;10:281-290.
- Friedberg JW, Fisher RI. Diffuse large B-cell lymphoma. Hematol Oncol Clin North Am. 2008;22:941-952. Doi:10.1016/j.hoc.2008.07.002
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Board PPTE. Langerhans cell histiocytosis treatment (PDQ®). In: PDQ Cancer Information Summaries [Internet]. National Cancer Institute (US); 2009.
- Chu A, Eisinger M, Lee JS, et al. Immunoelectron microscopic identification of Langerhans cells using a new antigenic marker. J Invest Dermatol. 1982;78:177-180. doi:10.1111/1523-1747.ep12506352
- Berti E, Gianotti R, Alessi E. Unusual cutaneous histiocytosis expressing an intermediate immunophenotype between Langerhans’ cells and dermal macrophages. Arch Dermatol. 1988;124:1250-1253. doi:10.1001/archderm.1988.01670080062020
- Cypel TKS, Zuker RM. Juvenile xanthogranuloma: case report and review of the literature. Can J Plast Surg. 2008;16:175-177.
- Rodriguez J, Ackerman AB. Xanthogranuloma in adults. Arch Dermatol. 1976;112:43-44.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. In: StatPearls [Internet]. StatPearls Publishing; 2022.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492. doi:10.1016/j. clindermatol.2006.07.010
- Miettinen M, Fetsch JF. Reticulohistiocytoma (solitary epithelioid histiocytoma): a clinicopathologic and immunohistochemical study of 44 cases. Am J Surg Pathol. 2006;30:521.
- Gold RH, Metzger AL, Mirra JM, et al. Multicentric reticulohistiocytosis (lipoid dermato-arthritis). An erosive polyarthritis with distinctive clinical, roentgenographic and pathologic features. Am J Roentgenol Radium Ther Nucl Med. 1975;124:610-624. doi:10.2214/ajr.124.4.610
- Dalia S, Sagatys E, Sokol L, et al. Rosai-Dorfman disease: tumor biology, clinical features, pathology, and treatment. Cancer Control. 2014;21:322-327. doi:10.1177/107327481402100408
A 31-year-old woman presented with a slow-growing, tender, pruritic lesion on the right cheek of 4 to 5 months’ duration. She had been applying petroleum jelly and hydrocortisone cream 2.5% without any improvement. Physical examination revealed a 1×5-mm, pearly pink, erythematous, crusted papule with arborizing vessels surrounded by scattered pink papules with white dots within. No cervical lymphadenopathy was appreciated on physical examination, and the patient denied any other systemic symptoms. Shave and punch biopsies of the lesion were performed; stains for microorganisms were negative. The biopsy showed a dense reticular mixed inflammatory cell infiltrate comprised of a mixture of histiocytes (top), lymphocytes, neutrophils, and plasma cells that assumed a diffuse growth pattern within the dermis. The histiocytes exhibited abundant watery cytoplasms with ill-defined cytoplasmic membranes; intact leukocytes were found within the cytoplasms. The histiocytes demonstrated a unique phenotype characterized by S-100 (bottom) and CD68 positivity.
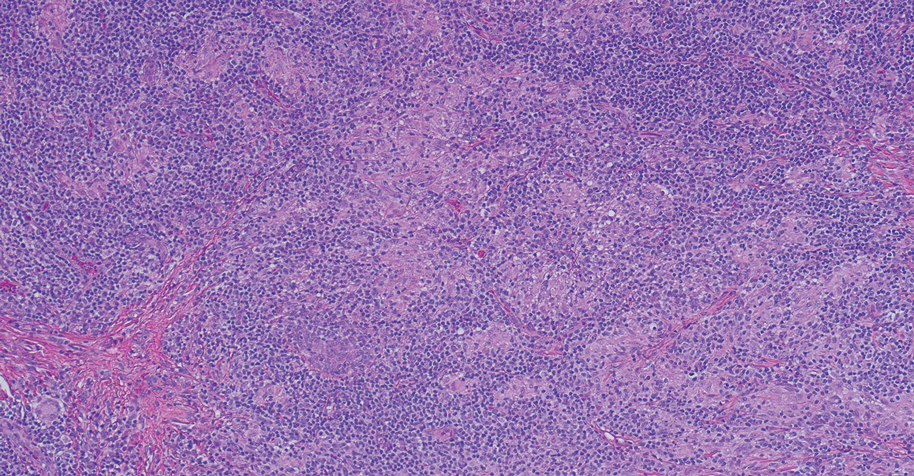
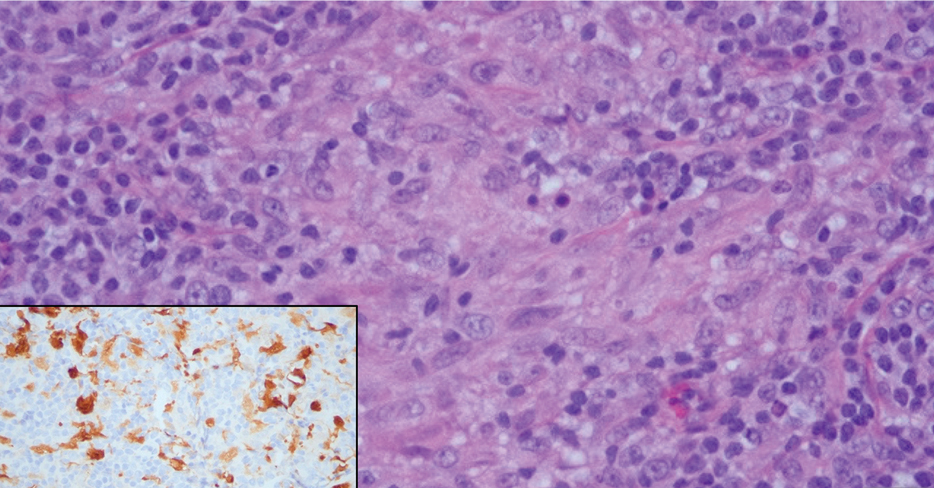
The Many Uses of the Humble Alcohol Swab
Practice Gap
In light of inflation, rising costs of procedures, and decreased reimbursements,1 there is an increased need to identify and utilize inexpensive multitasking tools that can serve the dermatologic surgeon from preoperative to postoperative care. The 70% isopropyl alcohol swab may be the dermatologist’s most cost-effective and versatile surgical tool.
The Technique
When assessing a lesion, alcohol swabs can remove scale, crust, or residue from personal care products to help reveal primary morphology. They aid in the diagnosis of porokeratosis by highlighting the cornoid lamella when used following application of gentian violet.2 The alcohol swab also can lay down a liquid interface to facilitate contact dermoscopy and improve visualization while also reducing the transmission of pathogens by the dermatoscope.3 Rubbing an area with an alcohol swab can induce vasodilation of scar tissue, which also may help localize a prior biopsy or surgical site (Figure).
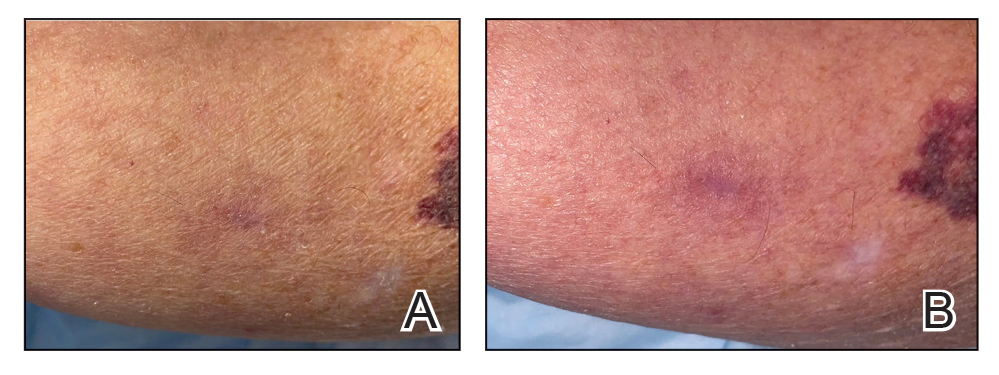
Before a surgical site is marked, an initial cleanse with an alcohol swab serves to both remove debris and provide antisepsis ahead of the procedure. Additionally, the swab may improve adherence of skin markers by clearing excess lipid from the skin surface. Assessing the amount of debris and oil removed in the process can help determine a patient’s baseline level of hygiene, which can aid postoperative wound care planning. In extreme cases, use of an alcohol swab may help diagnose dermatitis neglecta or terra firma-forme dermatosis by completely removing any pigmentation.4
After surgery, the alcohol swab can remove skin marker(s) and blood and prepare the site for the surgical dressing. There also is some evidence to suggest that cleansing the surgical site with an alcohol swab as part of routine postoperative wound care may decrease incidence of surgical-site infection.5 At follow-up, the swab can remove crust and clean the skin before suture removal. If infection is suspected, the swab can cleanse skin before a wound culture is obtained to remove skin commensals and flora on the outer surface of the wound.
Practice Implications
The 70% isopropyl alcohol swab can assist the dermatologist in numerous tasks related to everyday procedures. It is readily available in every clinic and costs only a few cents.
- Pollock JR, Chen JY, Dorius DA, et al. Decreasing physician Medicare reimbursement for dermatology services. J Am Acad Dermatol. 2022;86:1154-1156.
- Thomas CJ, Elston DM. Medical pearl: Gentian violet to highlight the cornoid lamella in disseminated superficial actinic porokeratosis.J Am Acad Dermatol. 2005;52(3 pt 1):513-514.
- Kelly SC, Purcell SM. Prevention of nosocomial infection during dermoscopy? Dermatol Surg. 2006;32:552-555.
- Blattner CM, Perry B, Snider K, et al. Clinical pearl: increasing utility of isopropyl alcohol for cutaneous dyschromia. Cutis. 2016;97:287;301.
- Vogt KN, Chadi S, Parry N, et al. Daily incision cleansing with alcohol reduces the rate of surgical site infections: a pilot study. Am Surg. 2015;81:1182-1186.
Practice Gap
In light of inflation, rising costs of procedures, and decreased reimbursements,1 there is an increased need to identify and utilize inexpensive multitasking tools that can serve the dermatologic surgeon from preoperative to postoperative care. The 70% isopropyl alcohol swab may be the dermatologist’s most cost-effective and versatile surgical tool.
The Technique
When assessing a lesion, alcohol swabs can remove scale, crust, or residue from personal care products to help reveal primary morphology. They aid in the diagnosis of porokeratosis by highlighting the cornoid lamella when used following application of gentian violet.2 The alcohol swab also can lay down a liquid interface to facilitate contact dermoscopy and improve visualization while also reducing the transmission of pathogens by the dermatoscope.3 Rubbing an area with an alcohol swab can induce vasodilation of scar tissue, which also may help localize a prior biopsy or surgical site (Figure).

Before a surgical site is marked, an initial cleanse with an alcohol swab serves to both remove debris and provide antisepsis ahead of the procedure. Additionally, the swab may improve adherence of skin markers by clearing excess lipid from the skin surface. Assessing the amount of debris and oil removed in the process can help determine a patient’s baseline level of hygiene, which can aid postoperative wound care planning. In extreme cases, use of an alcohol swab may help diagnose dermatitis neglecta or terra firma-forme dermatosis by completely removing any pigmentation.4
After surgery, the alcohol swab can remove skin marker(s) and blood and prepare the site for the surgical dressing. There also is some evidence to suggest that cleansing the surgical site with an alcohol swab as part of routine postoperative wound care may decrease incidence of surgical-site infection.5 At follow-up, the swab can remove crust and clean the skin before suture removal. If infection is suspected, the swab can cleanse skin before a wound culture is obtained to remove skin commensals and flora on the outer surface of the wound.
Practice Implications
The 70% isopropyl alcohol swab can assist the dermatologist in numerous tasks related to everyday procedures. It is readily available in every clinic and costs only a few cents.
Practice Gap
In light of inflation, rising costs of procedures, and decreased reimbursements,1 there is an increased need to identify and utilize inexpensive multitasking tools that can serve the dermatologic surgeon from preoperative to postoperative care. The 70% isopropyl alcohol swab may be the dermatologist’s most cost-effective and versatile surgical tool.
The Technique
When assessing a lesion, alcohol swabs can remove scale, crust, or residue from personal care products to help reveal primary morphology. They aid in the diagnosis of porokeratosis by highlighting the cornoid lamella when used following application of gentian violet.2 The alcohol swab also can lay down a liquid interface to facilitate contact dermoscopy and improve visualization while also reducing the transmission of pathogens by the dermatoscope.3 Rubbing an area with an alcohol swab can induce vasodilation of scar tissue, which also may help localize a prior biopsy or surgical site (Figure).

Before a surgical site is marked, an initial cleanse with an alcohol swab serves to both remove debris and provide antisepsis ahead of the procedure. Additionally, the swab may improve adherence of skin markers by clearing excess lipid from the skin surface. Assessing the amount of debris and oil removed in the process can help determine a patient’s baseline level of hygiene, which can aid postoperative wound care planning. In extreme cases, use of an alcohol swab may help diagnose dermatitis neglecta or terra firma-forme dermatosis by completely removing any pigmentation.4
After surgery, the alcohol swab can remove skin marker(s) and blood and prepare the site for the surgical dressing. There also is some evidence to suggest that cleansing the surgical site with an alcohol swab as part of routine postoperative wound care may decrease incidence of surgical-site infection.5 At follow-up, the swab can remove crust and clean the skin before suture removal. If infection is suspected, the swab can cleanse skin before a wound culture is obtained to remove skin commensals and flora on the outer surface of the wound.
Practice Implications
The 70% isopropyl alcohol swab can assist the dermatologist in numerous tasks related to everyday procedures. It is readily available in every clinic and costs only a few cents.
- Pollock JR, Chen JY, Dorius DA, et al. Decreasing physician Medicare reimbursement for dermatology services. J Am Acad Dermatol. 2022;86:1154-1156.
- Thomas CJ, Elston DM. Medical pearl: Gentian violet to highlight the cornoid lamella in disseminated superficial actinic porokeratosis.J Am Acad Dermatol. 2005;52(3 pt 1):513-514.
- Kelly SC, Purcell SM. Prevention of nosocomial infection during dermoscopy? Dermatol Surg. 2006;32:552-555.
- Blattner CM, Perry B, Snider K, et al. Clinical pearl: increasing utility of isopropyl alcohol for cutaneous dyschromia. Cutis. 2016;97:287;301.
- Vogt KN, Chadi S, Parry N, et al. Daily incision cleansing with alcohol reduces the rate of surgical site infections: a pilot study. Am Surg. 2015;81:1182-1186.
- Pollock JR, Chen JY, Dorius DA, et al. Decreasing physician Medicare reimbursement for dermatology services. J Am Acad Dermatol. 2022;86:1154-1156.
- Thomas CJ, Elston DM. Medical pearl: Gentian violet to highlight the cornoid lamella in disseminated superficial actinic porokeratosis.J Am Acad Dermatol. 2005;52(3 pt 1):513-514.
- Kelly SC, Purcell SM. Prevention of nosocomial infection during dermoscopy? Dermatol Surg. 2006;32:552-555.
- Blattner CM, Perry B, Snider K, et al. Clinical pearl: increasing utility of isopropyl alcohol for cutaneous dyschromia. Cutis. 2016;97:287;301.
- Vogt KN, Chadi S, Parry N, et al. Daily incision cleansing with alcohol reduces the rate of surgical site infections: a pilot study. Am Surg. 2015;81:1182-1186.
PRAME Expression in Melanocytic Proliferations in Special Sites
The assessment and diagnosis of melanocytic lesions can present a formidable challenge to even a seasoned pathologist, which is especially true when dealing with the subset of nevi occurring at special sites—where baseline variations inherent to particular locations on the body can preclude the use of features routinely used to diagnose malignancy elsewhere. These so-called special-site nevi previously have been described in the literature along with suggested criteria for differentiating malignant lesions from their benign counterparts.1 Locations generally considered to be special sites include the acral skin, anogenital region, breast, ear, and flexural regions.1,2
When evaluating non–special-site melanocytic lesions, general characteristics associated with a malignant diagnosis include confluence or pagetoid spread of melanocytes, nuclear pleomorphism, cytologic atypia, and irregular architecture3; however, these features can be compatible with a benign diagnosis in special-site nevi depending on their extent and the site in question. Although they can be atypical, special-site nevi tend to have the bulk of their architectural distortion and cytologic atypia in the center of the lesion as opposed to the edges.1 If a given lesion is from a special site but lacks this reassuring feature, special care should be taken to rule out malignancy.
Preferentially expressed antigen in melanoma (PRAME) is an antigen first identified in tumor-reactive T-cell populations in patients with malignant melanoma. It is the product of an oncogene that frequently is overexpressed in melanomas, lung squamous cell carcinomas, sarcomas, and acute leukemias.4 It functions as an antagonist of the retinoic acid signaling pathway, which normally serves to induce further cell differentiation, senescence, or apoptosis.5 PRAME inhibits retinoid signaling by forming a complex with both the ligand-bound retinoic acid holoreceptor and the polycomb protein EZH2, which blocks retinoid-dependent gene expression by encouraging chromatin condensation at the RARβ promoter site5; therefore, expressing PRAME allows lesional cells a substantial growth advantage.
PRAME expression has been extensively characterized in non–special-site nevi and has filled the need for a rather specific marker of melanoma.6-10 Although PRAME has been studied in acral nevi,11 the expression pattern in nevi of special sites has yet to be elucidated. Herein, we present a dataset characterizing PRAME expression in these challenging lesions.
Methods
We performed a retrospective case review at the University of Virginia (Charlottesville, Virginia) and collected a panel of 36 special-site nevi that previously were diagnosed as benign by a trained dermatopathologist from January 2020 through December 2022. Special-site nevi were identified using a natural language filter for the following terms: acral, palm, sole, ear, auricular, lip, axilla, armpit, breast, groin, labia, vulva, umbilicus, and penis. This study was approved by the University of Virginia institutional review board.
The original hematoxylin and eosin slides used for primary diagnosis were re-examined to verify the prior diagnosis of benign nevus at a special site. We performed a detailed microscopic examination of all benign nevi in our cohort to determine the frequency of various characteristics at each special site. Sections were prepared from the formalin-fixed and paraffin-embedded tissue blocks and stained with a commercial PRAME antibody (#219650 [Abcam] at a 1:50 dilution) and counterstain. A trained dermatopathologist (S.S.R.) examined the stained sections and recorded the percentage of tumor cells with nuclear PRAME staining. We reported our results using previously established criteria for scoring PRAME immunohistochemistry7: 0 for no expression, 1+ for 1% to 25% expression, 2+ for 26% to 50% expression, 3+ for 51% to 75% expression, and 4+ for diffuse or 76% to 100% expression. Only strong clonal expression within a population of cells was graded.
Data handling and statistical testing were performed using the R Project for Statistical Computing (https://www.r-project.org/). Significance testing was performed using the Fisher exact test. Plot construction was performed using ggplot2 (https://ggplot2.tidyverse.org/).
Results
Our study cohort included 36 special-site nevi, and the control cohort comprised 25 melanoma in situ (MIS) or invasive melanoma (IM) lesions occurring at special sites. Table 1 provides a breakdown of the study and control cohorts by lesion site. Table 2 details the results of our microscopic examination, describing frequency of various characteristics of special-site nevi stratified by site.
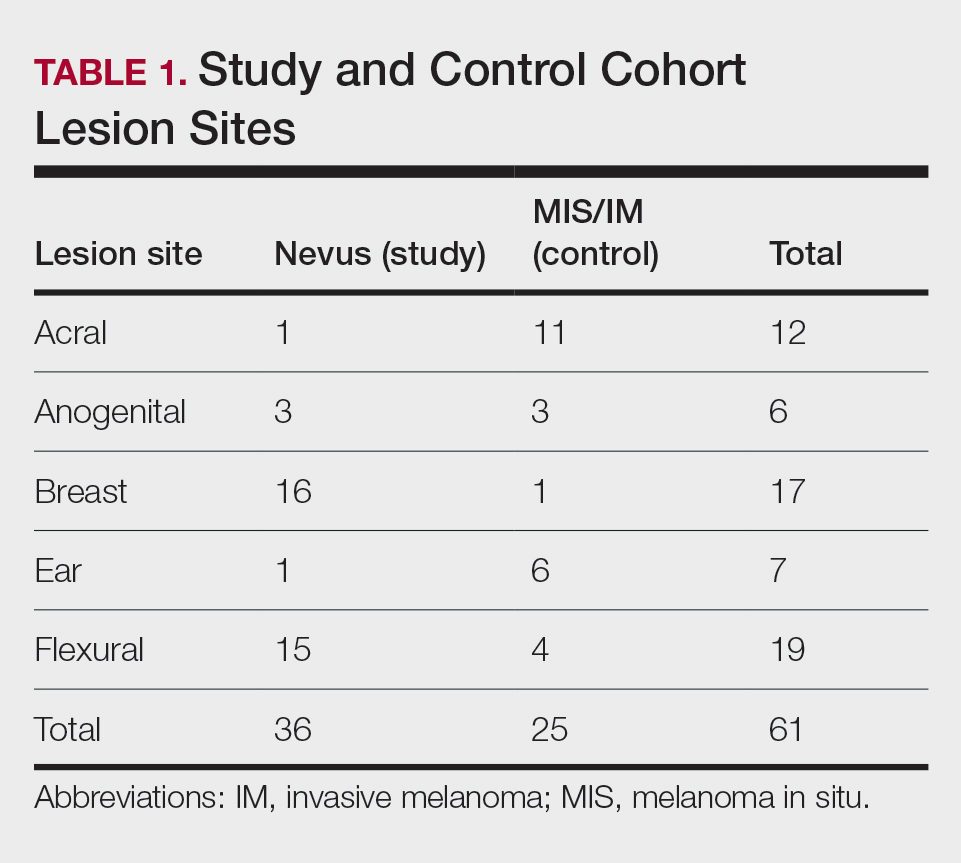
Of the 36 special-site nevi in our cohort, 20 (56%) had no staining (0) for PRAME, 11 (31%) demonstrated 1+ PRAME expression, 3 (8%) demonstrated 2+ PRAME expression, and 2 (6%) demonstrated 3+ PRAME expression. No nevi showed 4+ expression. In the control cohort, 24 of 25 (96%) MIS and IM showed 3+ or 4+ expression, with 21 (84%) demonstrating diffuse/4+ expression. One control case (4%) demonstrated 0 PRAME expression. These data are summarized in Table 3 and Figure 1. There is a significant difference in diffuse (4+) PRAME expression between special-site nevi and MIS/IM occurring at special sites (P=1.039×10-12).
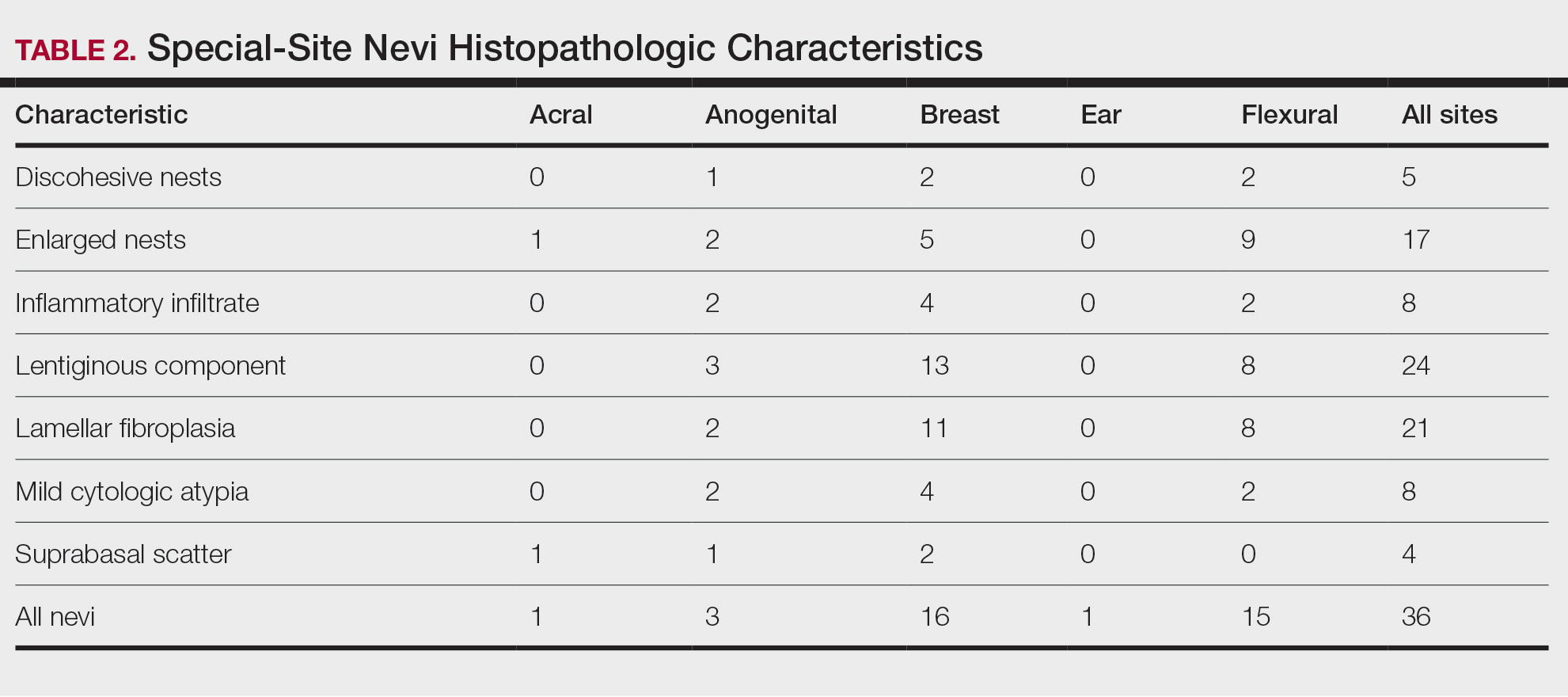
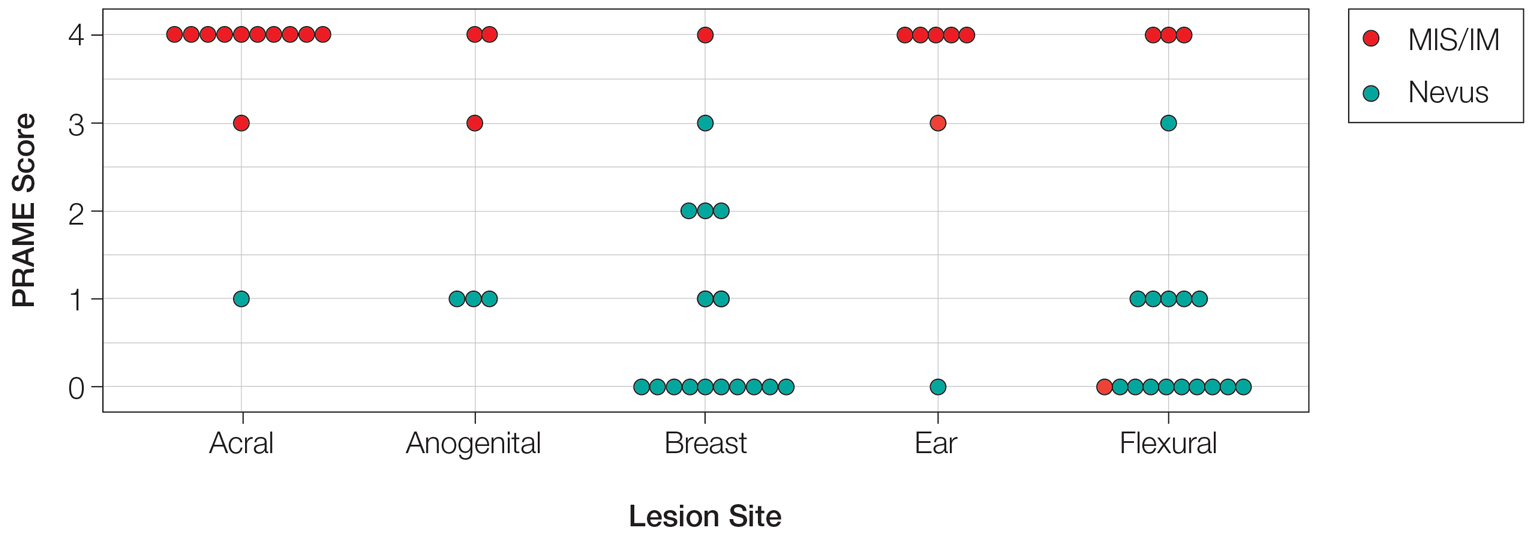
Based on our cohort, a positivity threshold of 3+ for PRAME expression for the diagnosis of melanoma in a special-site lesion would have a sensitivity of 96% and a specificity of 94%, while a positivity threshold of 4+ for PRAME expression would have a sensitivity of 84% and a specificity of 100%. Figures 2 through 4 show photomicrographs of a special-site nevus of the breast, which appropriately does not stain for PRAME; Figures 5 and 6 show an MIS at a special site that appropriately stains for PRAME.

Comment
The distinction between benign and malignant pigmented lesions at special sites presents a fair challenge for pathologists due to the larger degree of leniency for architectural distortion and cytologic atypia in benign lesions at these sites. The presence of architectural distortion or cytologic atypia at the lesion’s edge makes rendering a benign diagnosis especially difficult, and the need for a validated immunohistochemical stain is apparent. In our cohort, strong clonal PRAME expression provided a reliable immunohistochemical marker, allowing for the distinction of malignant lesions from benign nevi at special sites. Diffuse faint PRAME expression was present in several benign nevi within our cohort, and these lesions were considered negative (0) in our analysis.
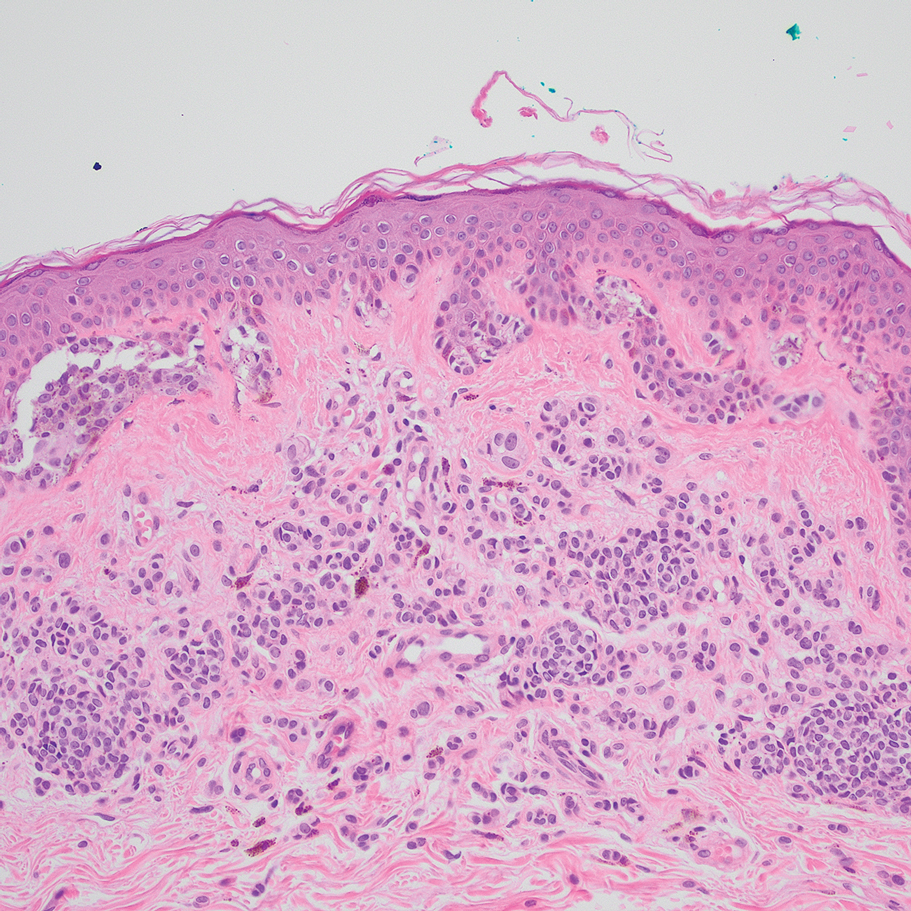
Given the described test characteristics, we support the implementation of PRAME immunohistochemistry with a positivity threshold of 4+ expression as an ancillary test supporting the diagnosis of IM or MIS in special sites, which would allow clinicians to leverage the high specificity of 4+ PRAME expression to distinguish an IM or MIS from a benign nevus occurring at a special site. We do not recommend the use of 4+ PRAME expression as a screening test for melanoma or MIS among special-site nevi due to its comparatively low sensitivity; however, no one marker is always reliable, and we recommend continued clinicopathologic correlation for all cases.
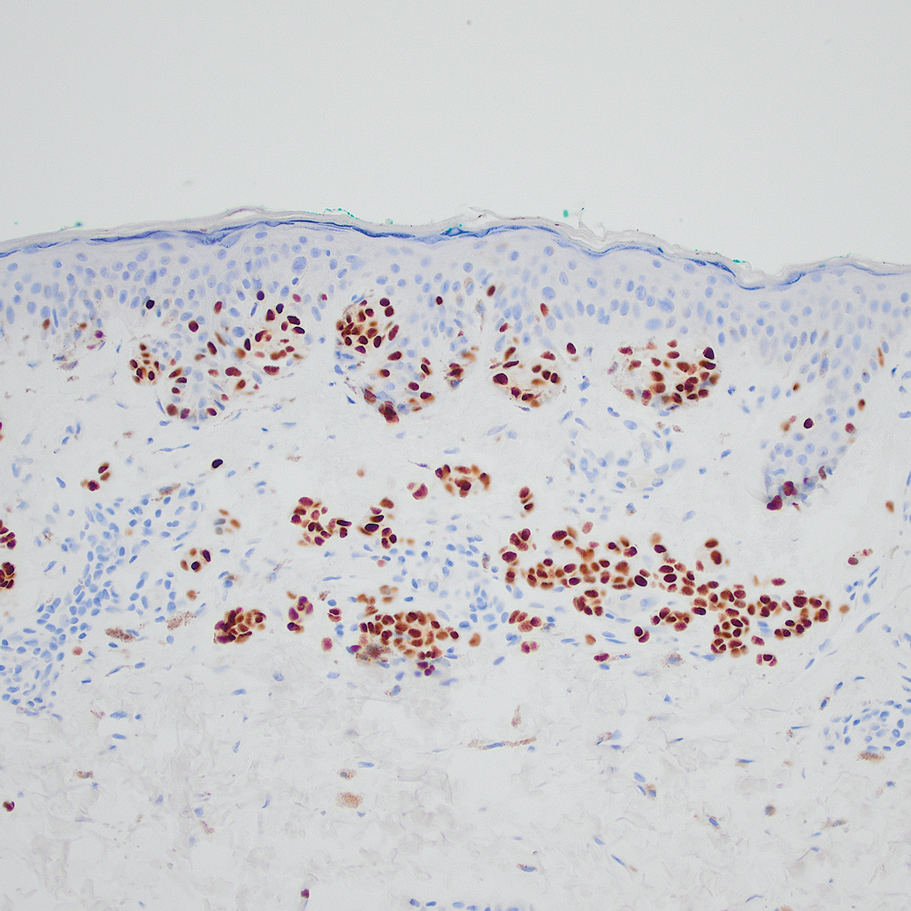
Although our case series included nevi and MIS/IM from all special sites, we were limited in the number of acrogenital and ear nevi included due to a relative paucity of biopsied benign nevi from these locations at the University of Virginia. Additionally, although the magnitude of the difference in PRAME expression between the study and control groups is sufficient to demonstrate statistical significance, the overall strength of our argument would be increased with a larger study group. We were limited by the number of cases available at our institution, which did not utilize PRAME during the initial diagnosis of the case; including these cases in the study group would have undermined the integrity of our argument because the differentiation of benign vs malignant initially was made using PRAME immunohistochemistry.
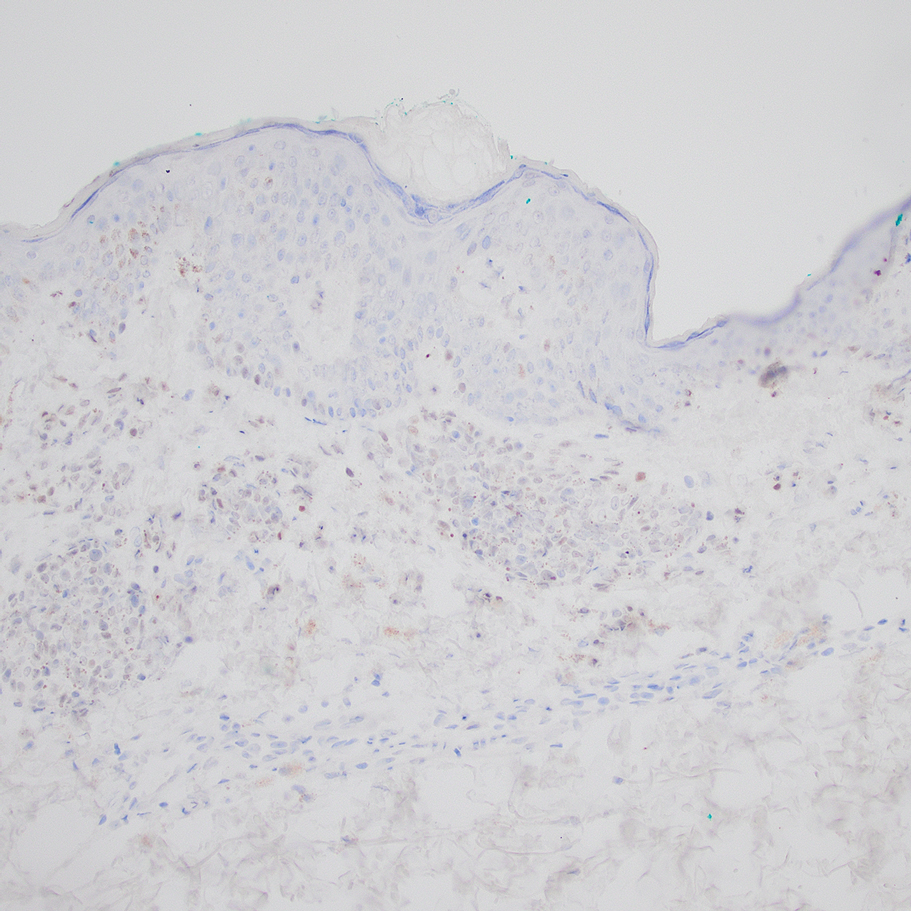
Conclusion
Due to their atypical features, special-site nevi can be challenging to assess. In this study, we showed that PRAME expression can be a reliable marker to distinguish benign from malignant lesions. Our results showed that 100% of benign special-site nevi demonstrated 3+ expression or less, with 56% (20/36) demonstrating no expression at all. The presence of diffuse PRAME expression (4+ PRAME staining) appears to be a specific indicator of a malignant lesion, but results should always be interpreted with respect to the patient’s clinical history and the lesion’s histomorphologic features. Further study of a larger sample size would allow refinement of the sensitivity and specificity of diffuse PRAME expression in the determination of malignancy for special-site lesions.
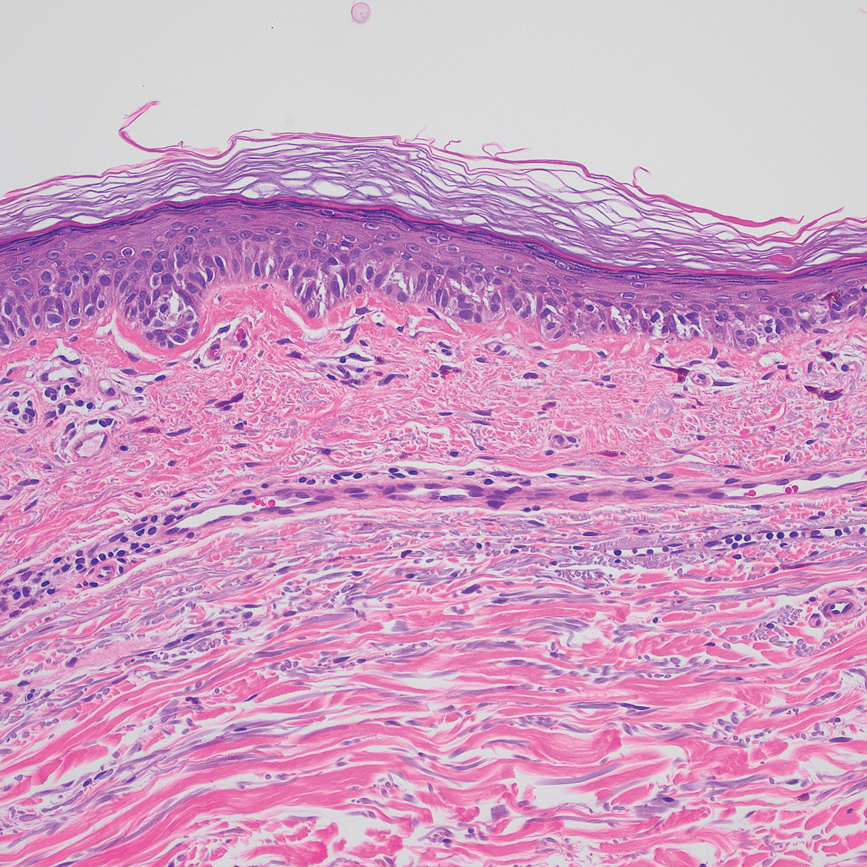
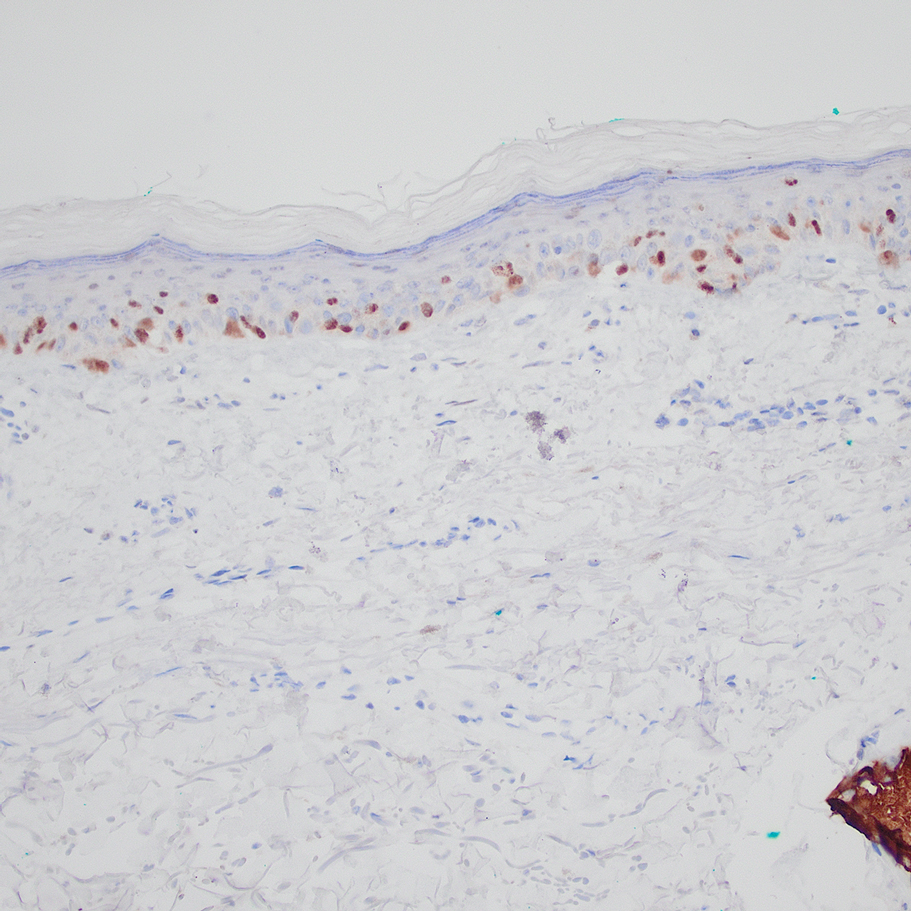
Acknowledgment—The authors thank the pathologistsat the University of Virginia Biorepository and Tissue Research Facility (Charlottesville, Virginia) for their skill and expertise in performing immunohistochemical staining for this study.
- VandenBoom T, Gerami P. Melanocytic nevi of special sites. In: Pathology of Melanocytic Tumors. Elsevier; 2019:90-100. doi:10.1016/B978-0-323-37457-6.00007-9
- Hosler GA, Moresi JM, Barrett TL. Nevi with site-related atypia: a review of melanocytic nevi with atypical histologic features based on anatomic site. J Cutan Pathol. 2008;35:889-898. doi:10.1111/j.1600-0560.2008.01041.x.
- Brenn T. Melanocytic lesions—staying out of trouble. Ann Diagn Pathol. 2018;37:91-102. doi:10.1016/j.anndiagpath.2018.09.010
- Ikeda H, Lethé B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199-208. doi:10.1016/s1074-7613(00)80426-4
- Epping MT, Wang L, Edel MJ, et al. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835-847. doi:10.1016/j.cell.2005.07.003
- Alomari AK, Tharp AW, Umphress B, et al. The utility of PRAME immunohistochemistry in the evaluation of challenging melanocytic tumors. J Cutan Pathol. 2021;48:1115-1123. doi:10.1111/cup.14000
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465. doi:10.1097/PAS.0000000000001134
- Gill P, Prieto VG, Austin MT, et al. Diagnostic utility of PRAME in distinguishing proliferative nodules from melanoma in giant congenital melanocytic nevi. J Cutan Pathol. 2021;48:1410-1415. doi:10.1111/cup.14091
- Googe PB, Flanigan KL, Miedema JR. Preferentially expressed antigen in melanoma immunostaining in a series of melanocytic neoplasms. Am J Dermatopathol. 2021;43):794-800. doi:10.1097/DAD.0000000000001885
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131. doi:10.1111/cup.13818
- McBride JD, McAfee JL, Piliang M, et al. Preferentially expressed antigen in melanoma and p16 expression in acral melanocytic neoplasms. J Cutan Pathol. 2022;49:220-230. doi:10.1111/cup.14130
The assessment and diagnosis of melanocytic lesions can present a formidable challenge to even a seasoned pathologist, which is especially true when dealing with the subset of nevi occurring at special sites—where baseline variations inherent to particular locations on the body can preclude the use of features routinely used to diagnose malignancy elsewhere. These so-called special-site nevi previously have been described in the literature along with suggested criteria for differentiating malignant lesions from their benign counterparts.1 Locations generally considered to be special sites include the acral skin, anogenital region, breast, ear, and flexural regions.1,2
When evaluating non–special-site melanocytic lesions, general characteristics associated with a malignant diagnosis include confluence or pagetoid spread of melanocytes, nuclear pleomorphism, cytologic atypia, and irregular architecture3; however, these features can be compatible with a benign diagnosis in special-site nevi depending on their extent and the site in question. Although they can be atypical, special-site nevi tend to have the bulk of their architectural distortion and cytologic atypia in the center of the lesion as opposed to the edges.1 If a given lesion is from a special site but lacks this reassuring feature, special care should be taken to rule out malignancy.
Preferentially expressed antigen in melanoma (PRAME) is an antigen first identified in tumor-reactive T-cell populations in patients with malignant melanoma. It is the product of an oncogene that frequently is overexpressed in melanomas, lung squamous cell carcinomas, sarcomas, and acute leukemias.4 It functions as an antagonist of the retinoic acid signaling pathway, which normally serves to induce further cell differentiation, senescence, or apoptosis.5 PRAME inhibits retinoid signaling by forming a complex with both the ligand-bound retinoic acid holoreceptor and the polycomb protein EZH2, which blocks retinoid-dependent gene expression by encouraging chromatin condensation at the RARβ promoter site5; therefore, expressing PRAME allows lesional cells a substantial growth advantage.
PRAME expression has been extensively characterized in non–special-site nevi and has filled the need for a rather specific marker of melanoma.6-10 Although PRAME has been studied in acral nevi,11 the expression pattern in nevi of special sites has yet to be elucidated. Herein, we present a dataset characterizing PRAME expression in these challenging lesions.
Methods
We performed a retrospective case review at the University of Virginia (Charlottesville, Virginia) and collected a panel of 36 special-site nevi that previously were diagnosed as benign by a trained dermatopathologist from January 2020 through December 2022. Special-site nevi were identified using a natural language filter for the following terms: acral, palm, sole, ear, auricular, lip, axilla, armpit, breast, groin, labia, vulva, umbilicus, and penis. This study was approved by the University of Virginia institutional review board.
The original hematoxylin and eosin slides used for primary diagnosis were re-examined to verify the prior diagnosis of benign nevus at a special site. We performed a detailed microscopic examination of all benign nevi in our cohort to determine the frequency of various characteristics at each special site. Sections were prepared from the formalin-fixed and paraffin-embedded tissue blocks and stained with a commercial PRAME antibody (#219650 [Abcam] at a 1:50 dilution) and counterstain. A trained dermatopathologist (S.S.R.) examined the stained sections and recorded the percentage of tumor cells with nuclear PRAME staining. We reported our results using previously established criteria for scoring PRAME immunohistochemistry7: 0 for no expression, 1+ for 1% to 25% expression, 2+ for 26% to 50% expression, 3+ for 51% to 75% expression, and 4+ for diffuse or 76% to 100% expression. Only strong clonal expression within a population of cells was graded.
Data handling and statistical testing were performed using the R Project for Statistical Computing (https://www.r-project.org/). Significance testing was performed using the Fisher exact test. Plot construction was performed using ggplot2 (https://ggplot2.tidyverse.org/).
Results
Our study cohort included 36 special-site nevi, and the control cohort comprised 25 melanoma in situ (MIS) or invasive melanoma (IM) lesions occurring at special sites. Table 1 provides a breakdown of the study and control cohorts by lesion site. Table 2 details the results of our microscopic examination, describing frequency of various characteristics of special-site nevi stratified by site.

Of the 36 special-site nevi in our cohort, 20 (56%) had no staining (0) for PRAME, 11 (31%) demonstrated 1+ PRAME expression, 3 (8%) demonstrated 2+ PRAME expression, and 2 (6%) demonstrated 3+ PRAME expression. No nevi showed 4+ expression. In the control cohort, 24 of 25 (96%) MIS and IM showed 3+ or 4+ expression, with 21 (84%) demonstrating diffuse/4+ expression. One control case (4%) demonstrated 0 PRAME expression. These data are summarized in Table 3 and Figure 1. There is a significant difference in diffuse (4+) PRAME expression between special-site nevi and MIS/IM occurring at special sites (P=1.039×10-12).


Based on our cohort, a positivity threshold of 3+ for PRAME expression for the diagnosis of melanoma in a special-site lesion would have a sensitivity of 96% and a specificity of 94%, while a positivity threshold of 4+ for PRAME expression would have a sensitivity of 84% and a specificity of 100%. Figures 2 through 4 show photomicrographs of a special-site nevus of the breast, which appropriately does not stain for PRAME; Figures 5 and 6 show an MIS at a special site that appropriately stains for PRAME.

Comment
The distinction between benign and malignant pigmented lesions at special sites presents a fair challenge for pathologists due to the larger degree of leniency for architectural distortion and cytologic atypia in benign lesions at these sites. The presence of architectural distortion or cytologic atypia at the lesion’s edge makes rendering a benign diagnosis especially difficult, and the need for a validated immunohistochemical stain is apparent. In our cohort, strong clonal PRAME expression provided a reliable immunohistochemical marker, allowing for the distinction of malignant lesions from benign nevi at special sites. Diffuse faint PRAME expression was present in several benign nevi within our cohort, and these lesions were considered negative (0) in our analysis.

Given the described test characteristics, we support the implementation of PRAME immunohistochemistry with a positivity threshold of 4+ expression as an ancillary test supporting the diagnosis of IM or MIS in special sites, which would allow clinicians to leverage the high specificity of 4+ PRAME expression to distinguish an IM or MIS from a benign nevus occurring at a special site. We do not recommend the use of 4+ PRAME expression as a screening test for melanoma or MIS among special-site nevi due to its comparatively low sensitivity; however, no one marker is always reliable, and we recommend continued clinicopathologic correlation for all cases.

Although our case series included nevi and MIS/IM from all special sites, we were limited in the number of acrogenital and ear nevi included due to a relative paucity of biopsied benign nevi from these locations at the University of Virginia. Additionally, although the magnitude of the difference in PRAME expression between the study and control groups is sufficient to demonstrate statistical significance, the overall strength of our argument would be increased with a larger study group. We were limited by the number of cases available at our institution, which did not utilize PRAME during the initial diagnosis of the case; including these cases in the study group would have undermined the integrity of our argument because the differentiation of benign vs malignant initially was made using PRAME immunohistochemistry.

Conclusion
Due to their atypical features, special-site nevi can be challenging to assess. In this study, we showed that PRAME expression can be a reliable marker to distinguish benign from malignant lesions. Our results showed that 100% of benign special-site nevi demonstrated 3+ expression or less, with 56% (20/36) demonstrating no expression at all. The presence of diffuse PRAME expression (4+ PRAME staining) appears to be a specific indicator of a malignant lesion, but results should always be interpreted with respect to the patient’s clinical history and the lesion’s histomorphologic features. Further study of a larger sample size would allow refinement of the sensitivity and specificity of diffuse PRAME expression in the determination of malignancy for special-site lesions.


Acknowledgment—The authors thank the pathologistsat the University of Virginia Biorepository and Tissue Research Facility (Charlottesville, Virginia) for their skill and expertise in performing immunohistochemical staining for this study.
The assessment and diagnosis of melanocytic lesions can present a formidable challenge to even a seasoned pathologist, which is especially true when dealing with the subset of nevi occurring at special sites—where baseline variations inherent to particular locations on the body can preclude the use of features routinely used to diagnose malignancy elsewhere. These so-called special-site nevi previously have been described in the literature along with suggested criteria for differentiating malignant lesions from their benign counterparts.1 Locations generally considered to be special sites include the acral skin, anogenital region, breast, ear, and flexural regions.1,2
When evaluating non–special-site melanocytic lesions, general characteristics associated with a malignant diagnosis include confluence or pagetoid spread of melanocytes, nuclear pleomorphism, cytologic atypia, and irregular architecture3; however, these features can be compatible with a benign diagnosis in special-site nevi depending on their extent and the site in question. Although they can be atypical, special-site nevi tend to have the bulk of their architectural distortion and cytologic atypia in the center of the lesion as opposed to the edges.1 If a given lesion is from a special site but lacks this reassuring feature, special care should be taken to rule out malignancy.
Preferentially expressed antigen in melanoma (PRAME) is an antigen first identified in tumor-reactive T-cell populations in patients with malignant melanoma. It is the product of an oncogene that frequently is overexpressed in melanomas, lung squamous cell carcinomas, sarcomas, and acute leukemias.4 It functions as an antagonist of the retinoic acid signaling pathway, which normally serves to induce further cell differentiation, senescence, or apoptosis.5 PRAME inhibits retinoid signaling by forming a complex with both the ligand-bound retinoic acid holoreceptor and the polycomb protein EZH2, which blocks retinoid-dependent gene expression by encouraging chromatin condensation at the RARβ promoter site5; therefore, expressing PRAME allows lesional cells a substantial growth advantage.
PRAME expression has been extensively characterized in non–special-site nevi and has filled the need for a rather specific marker of melanoma.6-10 Although PRAME has been studied in acral nevi,11 the expression pattern in nevi of special sites has yet to be elucidated. Herein, we present a dataset characterizing PRAME expression in these challenging lesions.
Methods
We performed a retrospective case review at the University of Virginia (Charlottesville, Virginia) and collected a panel of 36 special-site nevi that previously were diagnosed as benign by a trained dermatopathologist from January 2020 through December 2022. Special-site nevi were identified using a natural language filter for the following terms: acral, palm, sole, ear, auricular, lip, axilla, armpit, breast, groin, labia, vulva, umbilicus, and penis. This study was approved by the University of Virginia institutional review board.
The original hematoxylin and eosin slides used for primary diagnosis were re-examined to verify the prior diagnosis of benign nevus at a special site. We performed a detailed microscopic examination of all benign nevi in our cohort to determine the frequency of various characteristics at each special site. Sections were prepared from the formalin-fixed and paraffin-embedded tissue blocks and stained with a commercial PRAME antibody (#219650 [Abcam] at a 1:50 dilution) and counterstain. A trained dermatopathologist (S.S.R.) examined the stained sections and recorded the percentage of tumor cells with nuclear PRAME staining. We reported our results using previously established criteria for scoring PRAME immunohistochemistry7: 0 for no expression, 1+ for 1% to 25% expression, 2+ for 26% to 50% expression, 3+ for 51% to 75% expression, and 4+ for diffuse or 76% to 100% expression. Only strong clonal expression within a population of cells was graded.
Data handling and statistical testing were performed using the R Project for Statistical Computing (https://www.r-project.org/). Significance testing was performed using the Fisher exact test. Plot construction was performed using ggplot2 (https://ggplot2.tidyverse.org/).
Results
Our study cohort included 36 special-site nevi, and the control cohort comprised 25 melanoma in situ (MIS) or invasive melanoma (IM) lesions occurring at special sites. Table 1 provides a breakdown of the study and control cohorts by lesion site. Table 2 details the results of our microscopic examination, describing frequency of various characteristics of special-site nevi stratified by site.

Of the 36 special-site nevi in our cohort, 20 (56%) had no staining (0) for PRAME, 11 (31%) demonstrated 1+ PRAME expression, 3 (8%) demonstrated 2+ PRAME expression, and 2 (6%) demonstrated 3+ PRAME expression. No nevi showed 4+ expression. In the control cohort, 24 of 25 (96%) MIS and IM showed 3+ or 4+ expression, with 21 (84%) demonstrating diffuse/4+ expression. One control case (4%) demonstrated 0 PRAME expression. These data are summarized in Table 3 and Figure 1. There is a significant difference in diffuse (4+) PRAME expression between special-site nevi and MIS/IM occurring at special sites (P=1.039×10-12).


Based on our cohort, a positivity threshold of 3+ for PRAME expression for the diagnosis of melanoma in a special-site lesion would have a sensitivity of 96% and a specificity of 94%, while a positivity threshold of 4+ for PRAME expression would have a sensitivity of 84% and a specificity of 100%. Figures 2 through 4 show photomicrographs of a special-site nevus of the breast, which appropriately does not stain for PRAME; Figures 5 and 6 show an MIS at a special site that appropriately stains for PRAME.

Comment
The distinction between benign and malignant pigmented lesions at special sites presents a fair challenge for pathologists due to the larger degree of leniency for architectural distortion and cytologic atypia in benign lesions at these sites. The presence of architectural distortion or cytologic atypia at the lesion’s edge makes rendering a benign diagnosis especially difficult, and the need for a validated immunohistochemical stain is apparent. In our cohort, strong clonal PRAME expression provided a reliable immunohistochemical marker, allowing for the distinction of malignant lesions from benign nevi at special sites. Diffuse faint PRAME expression was present in several benign nevi within our cohort, and these lesions were considered negative (0) in our analysis.

Given the described test characteristics, we support the implementation of PRAME immunohistochemistry with a positivity threshold of 4+ expression as an ancillary test supporting the diagnosis of IM or MIS in special sites, which would allow clinicians to leverage the high specificity of 4+ PRAME expression to distinguish an IM or MIS from a benign nevus occurring at a special site. We do not recommend the use of 4+ PRAME expression as a screening test for melanoma or MIS among special-site nevi due to its comparatively low sensitivity; however, no one marker is always reliable, and we recommend continued clinicopathologic correlation for all cases.

Although our case series included nevi and MIS/IM from all special sites, we were limited in the number of acrogenital and ear nevi included due to a relative paucity of biopsied benign nevi from these locations at the University of Virginia. Additionally, although the magnitude of the difference in PRAME expression between the study and control groups is sufficient to demonstrate statistical significance, the overall strength of our argument would be increased with a larger study group. We were limited by the number of cases available at our institution, which did not utilize PRAME during the initial diagnosis of the case; including these cases in the study group would have undermined the integrity of our argument because the differentiation of benign vs malignant initially was made using PRAME immunohistochemistry.

Conclusion
Due to their atypical features, special-site nevi can be challenging to assess. In this study, we showed that PRAME expression can be a reliable marker to distinguish benign from malignant lesions. Our results showed that 100% of benign special-site nevi demonstrated 3+ expression or less, with 56% (20/36) demonstrating no expression at all. The presence of diffuse PRAME expression (4+ PRAME staining) appears to be a specific indicator of a malignant lesion, but results should always be interpreted with respect to the patient’s clinical history and the lesion’s histomorphologic features. Further study of a larger sample size would allow refinement of the sensitivity and specificity of diffuse PRAME expression in the determination of malignancy for special-site lesions.


Acknowledgment—The authors thank the pathologistsat the University of Virginia Biorepository and Tissue Research Facility (Charlottesville, Virginia) for their skill and expertise in performing immunohistochemical staining for this study.
- VandenBoom T, Gerami P. Melanocytic nevi of special sites. In: Pathology of Melanocytic Tumors. Elsevier; 2019:90-100. doi:10.1016/B978-0-323-37457-6.00007-9
- Hosler GA, Moresi JM, Barrett TL. Nevi with site-related atypia: a review of melanocytic nevi with atypical histologic features based on anatomic site. J Cutan Pathol. 2008;35:889-898. doi:10.1111/j.1600-0560.2008.01041.x.
- Brenn T. Melanocytic lesions—staying out of trouble. Ann Diagn Pathol. 2018;37:91-102. doi:10.1016/j.anndiagpath.2018.09.010
- Ikeda H, Lethé B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199-208. doi:10.1016/s1074-7613(00)80426-4
- Epping MT, Wang L, Edel MJ, et al. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835-847. doi:10.1016/j.cell.2005.07.003
- Alomari AK, Tharp AW, Umphress B, et al. The utility of PRAME immunohistochemistry in the evaluation of challenging melanocytic tumors. J Cutan Pathol. 2021;48:1115-1123. doi:10.1111/cup.14000
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465. doi:10.1097/PAS.0000000000001134
- Gill P, Prieto VG, Austin MT, et al. Diagnostic utility of PRAME in distinguishing proliferative nodules from melanoma in giant congenital melanocytic nevi. J Cutan Pathol. 2021;48:1410-1415. doi:10.1111/cup.14091
- Googe PB, Flanigan KL, Miedema JR. Preferentially expressed antigen in melanoma immunostaining in a series of melanocytic neoplasms. Am J Dermatopathol. 2021;43):794-800. doi:10.1097/DAD.0000000000001885
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131. doi:10.1111/cup.13818
- McBride JD, McAfee JL, Piliang M, et al. Preferentially expressed antigen in melanoma and p16 expression in acral melanocytic neoplasms. J Cutan Pathol. 2022;49:220-230. doi:10.1111/cup.14130
- VandenBoom T, Gerami P. Melanocytic nevi of special sites. In: Pathology of Melanocytic Tumors. Elsevier; 2019:90-100. doi:10.1016/B978-0-323-37457-6.00007-9
- Hosler GA, Moresi JM, Barrett TL. Nevi with site-related atypia: a review of melanocytic nevi with atypical histologic features based on anatomic site. J Cutan Pathol. 2008;35:889-898. doi:10.1111/j.1600-0560.2008.01041.x.
- Brenn T. Melanocytic lesions—staying out of trouble. Ann Diagn Pathol. 2018;37:91-102. doi:10.1016/j.anndiagpath.2018.09.010
- Ikeda H, Lethé B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199-208. doi:10.1016/s1074-7613(00)80426-4
- Epping MT, Wang L, Edel MJ, et al. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835-847. doi:10.1016/j.cell.2005.07.003
- Alomari AK, Tharp AW, Umphress B, et al. The utility of PRAME immunohistochemistry in the evaluation of challenging melanocytic tumors. J Cutan Pathol. 2021;48:1115-1123. doi:10.1111/cup.14000
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465. doi:10.1097/PAS.0000000000001134
- Gill P, Prieto VG, Austin MT, et al. Diagnostic utility of PRAME in distinguishing proliferative nodules from melanoma in giant congenital melanocytic nevi. J Cutan Pathol. 2021;48:1410-1415. doi:10.1111/cup.14091
- Googe PB, Flanigan KL, Miedema JR. Preferentially expressed antigen in melanoma immunostaining in a series of melanocytic neoplasms. Am J Dermatopathol. 2021;43):794-800. doi:10.1097/DAD.0000000000001885
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131. doi:10.1111/cup.13818
- McBride JD, McAfee JL, Piliang M, et al. Preferentially expressed antigen in melanoma and p16 expression in acral melanocytic neoplasms. J Cutan Pathol. 2022;49:220-230. doi:10.1111/cup.14130
Practice Points
- Special-site nevi are benign melanocytic proliferations at special anatomic sites. Although cytologic atypia and architectural distortion may be present, they are centrally located and should not be present at the borders of the lesion.
- Strong expression of the preferentially expressed antigen in melanoma (PRAME) via immunohistochemistry provides a reliable indicator for benignity in differentiating a special-site nevus from a malignant melanoma occurring at a special site.
Squamous Cell Carcinoma Arising in Chronic Inflammatory Dermatoses
As many as one-quarter of human cancers are related to chronic inflammation, chronic infection, or both.1 Extrinsic inflammation leads to generation of proinflammatory cytokines that in turn recruit other inflammatory cells, which is thought to generate a positive amplification loop.2 Intrinsic stimuli from proto-oncogenes and mutations in tumor suppressor genes lead to transformed cancer cells that also secrete proinflammatory cytokines, thus propagating the cycle.
Numerous factors have been observed in association with tumor growth, progression, invasion, and metastasis.3 One factor for the development of squamous cell carcinoma (SCC) may be chronic inflammatory dermatoses. To date, reviews of chronic inflammation–associated malignancy have focused on solid organ cancers. We sought to provide an up-to-date review of SCC arising within chronic dermatoses, with an emphasis on the anatomic location of dermatoses involved in the transformation of cancer cells, the lag time from onset of dermatosis to diagnosis of SCC, and the distinctive mechanisms thought to be involved in the tumorigenesis in particular dermatoses.
Discoid Lupus Erythematosus
Discoid lupus erythematosus (DLE) is a chronic cutaneous lupus erythematosus variant with a female to male predominance of 3:1,4 and DLE lesions are prone to malignant transformation. Retrospective cohort studies have attempted to characterize who is at risk for SCC and how SCCs behave depending on their location. Cohorts from China,5 India,6 and Japan7 have noted a higher rate of SCC within DLE lesions in men (female to male ratios of 1:2.2, 1:1.6, and 1:2, respectively) and shorter lag times for SCC onset within DLE lesions of the lips (13, 5, and 10 years, respectively) compared to SCC arising in DLE elsewhere (19.2, 11.2, and 26 years, respectively). Studies have noted that DLE lesions of the lips may be prone to more rapid SCC tumorigenesis compared to DLE on cutaneous sites. One study reported SCC in DLE recurrence, metastasis, and death rates of 29%, 16.1%, and 19.4%, respectively,5 which exceeds reported rates in non-DLE SCCs (20%, 0.5% to 6%, and 1%, respectively).5,8
Because SCC arising within DLE is most common on the lips (Figure 1), it has been hypothesized that the high rate of transformation of DLE lesions on the lips may be due to constant exposure to irritation and tobacco, which may accelerate carcinogenesis.5 It also has been hypothesized that atrophic discoid lesions have lost sun protection and are more prone to mutagenic UV radiation,9 as SCCs arising in DLE lesions virtually always display prominent solar elastosis6; however, SCC has been observed to arise in non–sun-exposed DLE lesions in both White and Black patients.10
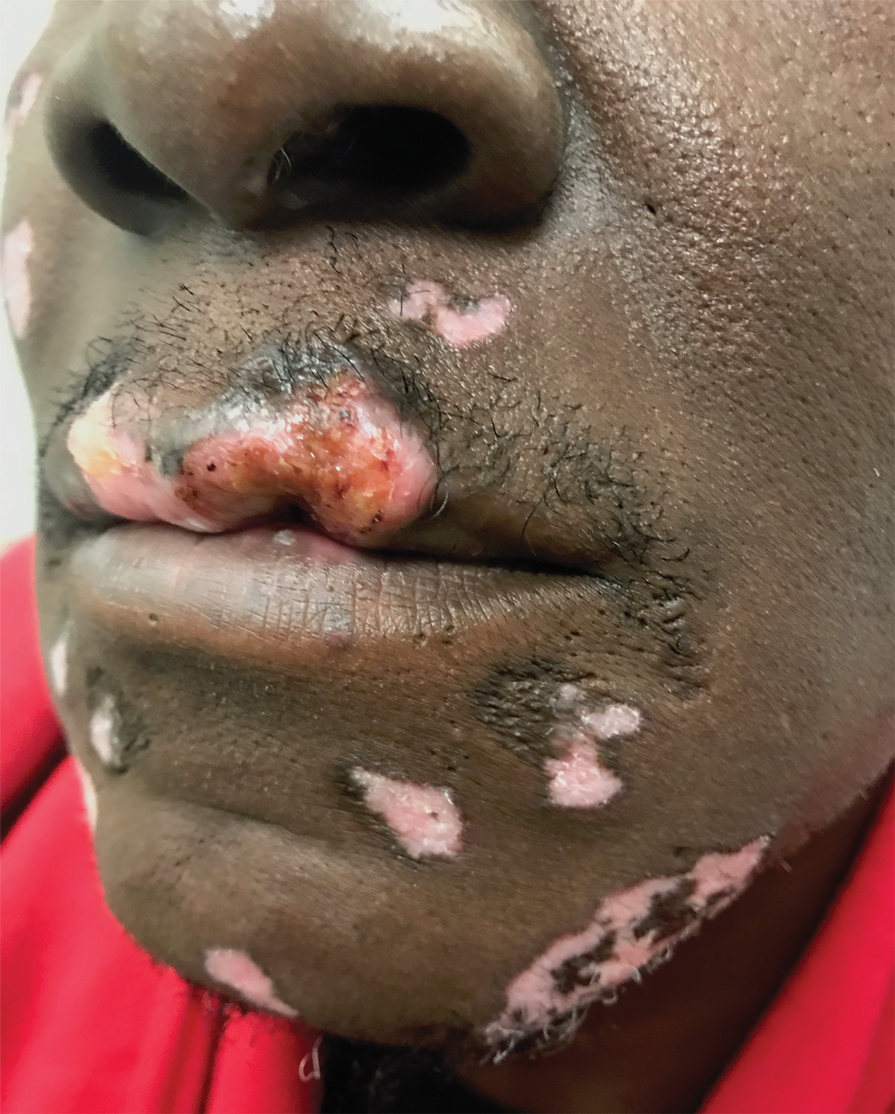
Additionally, use of immunosuppressant medications may accelerate the emergence of malignancy or more aggressive forms of malignancy; however, patients with autoimmune disease have a greater risk for malignancy at baseline,11 thus making it difficult to determine the excess risk from medications. There also may be a role for human papillomavirus (HPV) accelerating SCC development in DLE lesions, as demonstrated in a case of SCC arising in DLE lesions of the ears, with viral staining evident within the tumors.12 However, testing for HPV is not routinely performed in these cases.
Dermatologists need to be aware of the relatively rapid tumorigenesis and aggressive behavior of transformation and aggression seen with SCC arising within orolabial DLE lesions compared to cutaneous lesions, especially those on the lips.
Lichen Planus
Although patients with typical cutaneous lichen planus lesions do not have an increased risk for SCC,13 variants of lichen planus may predispose patients to SCC.
Oral Lichen Planus—Oral lichen planus (OLP) lesions are prone to malignant transformation. A systematic review of 16 studies evaluating the risk for OLP-associated SCC revealed an overall transformation rate of 1.09%, with a mean lag time of 4.3 years,14 compared to a reference rate of 0.2% for oral SCC.15 A meta-analysis of 19,676 patients with OLP and other oral lichenoid lesions revealed an oral SCC rate of 1.1%, with higher rates of transformation seen in cigarette smokers, alcoholics, and patients with hepatitis C virus infection.16 The ulcerative subtype of OLP appears to present a greater risk for malignant transformation.15 Dermatologists also should be cognizant that treatments for OLP such as topical calcineurin inhibitors may support the development of malignancy within inflammatory lesions.17
Hypertrophic Lichen Planus—The hypertrophic variant of lichen planus (HLP) also is prone to malignant transformation. A 1991 epidemiologic study from Sweden of malignancy arising in lichen planus revealed a disproportionate number of cases arising in verrucous or hypertrophic lesions, with a mean of 12.2 years from onset of the dermatosis to malignancy diagnosis.13 A subsequent 2015 retrospective study of 38 patients revealed that SCC had a propensity for the lower limb, favoring the pretibial region and the calf over the foot and the ankle with a reported lag time of 11 years.18
Although metastatic SCC arising in HLP is rare, 2 cases have been reported. A 24-year-old woman presented with an HLP plaque on the lower leg that developed during childhood and rapidly enlarged 2 months prior to presentation; she eventually died from metastatic disease.19 In another case, a 34-year-old man presented with an HLP lesion of approximately 10 years’ duration. A well-differentiated SCC was excised, and he developed lymph node metastases 5 months later.20
It is important to note that HLP on the legs often is misdiagnosed as SCC, as pseudoepitheliomatous hyperplasia and squamous metaplasia can be difficult to differentiate clinically and histologically.21,22 In the case of multiple eruptive SCCs of the lower leg, clinical correlation is essential to avoid unnecessary and ineffective surgical treatment.
Patients with HLP may exhibit Wickham striae, follicular accentuation, and mucocutaneous lichen planus at other sites, or a correlative initiation of possible culprit medications.23 Because true SCC arising within HLP is relatively rare, its malignant potential is not as clear as those arising within DLE; however, the lower limb appears to be the most common location for SCC within HLP.Nail Lichen Planus—Squamous cell carcinoma arising in nail lichen planus is rare. A report of 2 patients were diagnosed with lichen planus approximately 15 years prior to diagnosis of ungual SCC.24 Given the rarity of this presentation, it is difficult to ascertain the approximate lag time and other risk factors. Furthermore, the role of HPV in these cases was not ruled out. Oncogenic HPV strains have been reported in patients with periungual SCC.25,26
Lichen Sclerosus
Lichen sclerosus (LS) is a chronic inflammatory dermatosis that favors the anogenital area in a female to male ratio of 10:1.27 It is considered a premalignant condition for SCC tumorigenesis and may be a strong predictor of vulvar SCC (Figure 2), as 62% of vulvar SCC cases (N=78) may have adjacent LS.28
In a Dutch cohort of 3038 women with LS, 2.6% of patients developed vulvar SCC at a median of 3.3 years after LS diagnosis.29 Other studies have estimated a lag time of 4 years until SCC presentation.30 An Italian cohort of 976 women similarly observed that 2.7% of patients developed premalignancy or SCC.31 It was previously estimated that 3% to 5% of patients with LS developed SCC; however, prior studies may have included cases of vulvar intraepithelial neoplasia with low risk for invasive SCC, which might have overestimated true risk of SCC.32 Another confounding factor for elucidating SCC on a background of LS may be the presence of HPV.33 Extragenital LS does not appear to have similar potential for malignant transformation.34
In a prospective Australian cohort of 507 women with LS (mean age, 55.4 years), remission was induced with potent topical corticosteroids.35 Patients who were adherent to a topical regimen did not develop SCC during follow-up. Those who were nonadherent or partially adherent had a 4.7% risk for SCC.35 In a similar prospective study of 83 women in France, the SCC rate was 9.6% in lesions that were untreated or irregularly treated.36 These studies provide essential evidence that appropriately treating LS can prevent SCC at a later date, though longer-term data are lacking.
The rate of SCC arising in male genital LS may approach 8.4%,37 with a lag time of 17 years from onset of LS to SCC diagnosis.38 Although circumcision often is considered curative for male genital LS, patients have been observed to develop penile SCC at least 5 years after circumcision.39 Male penile SCC in a background of LS may not necessarily be HPV associated.40
Marjolin Ulcer
Chronic ulcers or scars, typically postburn scars, may undergo malignant transformation, with SCC being the most common carcinoma.41 Squamous cell carcinoma in the context of a chronic ulcer or wound is known as a Marjolin ulcer (MU). Up to 2% of burn scars have been observed to undergo malignant transformation.42 Marjolin ulcers tend to behave aggressively once they form, and it has been proposed that removal of scar tissue may be a preventive therapeutic strategy.43 Cohort studies of MU on the lower extremities have observed lag times of 26.444 and 37.945 years, with both studies also noting relatively high rates of local recurrence.
The pathogenesis of MU appears to be multifactorial. Chronic inflammation and scar formation have been implicated. Chronic inflammation and irritation of lesions at natural creases are thought to increase mitotic activity,41 and local accumulation of toxin may promote mutagenesis.46 Scar formation may create a locally immunoprivileged site, allowing for developing tumors to evade the immune system47 and become even more aggressive as the tumor accumulates.48 Scar formation also may prevent the ability of immune cells to penetrate the tumor microenvironment and access lymphatic channels.49
Hidradenitis Suppurativa
As many as 3.2% of patients with chronic hidradenitis suppurativa (HS) experience malignant transformation to SCC.50 Early HS displays subclinical lymphedema in affected sites, which can progress to chronic fibrosis, stasis, and accumulation of protein-rich fluid.51 Stasis changes have been associated with altered local inflammatory proteins, such as toll-like receptors, β-defensins, and interleukins.52
A retrospective cohort study of 12 patients revealed a lag time of 28.5 years from HS diagnosis to the manifestation of malignancy.53 After local excision, 7 patients developed recurrence, with 100% mortality. Squamous cell carcinomas were well differentiated and moderately differentiated.53 A 2017 literature review of 62 case reports calculated a mean lag time of 27 years. Despite 85% of SCCs being well differentiated and moderately differentiated, nearly half of patients died within 2 years.54 As seen in other inflammatory conditions, HPV can complicate perineal HS and promote SCC tumorigenesis.55
Squamous cell carcinomas arising within HS lesions are more prevalent in males (6.75:1 ratio),54,56 despite HS being more prevalent in females (2:1 ratio).57 Similar to DLE, SCCs arising in HS are aggressive and are seen more in males, despite both conditions being female predominant. Incidence and mortality rates for primary cutaneous SCC are higher for men vs women58; however, the discordance in aggressive behavior seen more commonly in SCC arising from HS or DLE in male patients has yet to be explained.
Necrobiosis Lipoidica Diabeticorum
Malignancy arising within necrobiosis lipoidica diabeticorum (NLD) is rare. A review of 14 published cases noted that 13 were SCC and 1 was leiomyosarcoma.59 The lag time was 21.5 years; 31% of cases (N=14) presented with regional lymph node metastasis. Although chronic ulceration is a risk factor for SCC and occurs in as many as one-third of NLD cases, its correlation with ulceration and malignant transformation has not been characterized.
Epidermolysis Bullosa
Recessive dystrophic epidermolysis bullosa (RDEB) is a noninflammatory inherited blistering disease, and patients have an inherently high risk for aggressive SCC.60 Other forms of epidermolysis bullosa can lead to SCC, but the rarer RDEB accounts for 69% of SCC cases, with a median age of 36 years at presentation.61 Although SCCs tend to be well differentiated in RDEB (73.9%),61 they also exhibit highly aggressive behavior.62 In the most severe variant—RDEB-generalized severe—the cumulative risk for SCC-related death in an Australian population was 84.4% at 34 years of age.63
As RDEB is an inherited disorder with potential for malignancy at a young age, the pathogenesis is plausibly different from the previously discussed inflammatory dermatoses. This disease is characterized by a mutation in the collagen VII gene, leading to loss of anchoring fibrils and a basement membrane zone split.64 There also can be inherent fibroblast alterations; RDEB fibroblasts create an environment for tumor growth by supporting malignant-cell adhesion and invasion.65 Mutations in p53,66 local alterations in transforming growth factor β activity,67 and downstream matrix metalloproteinase activity68 have been implicated.
Additionally, keratinocytes may retain the N-terminal noncollagenous (NC1) domain of truncated collagen VII while losing the anchoring NC2 domain in mutated collagen VII RDEB, thereby supporting anchorless keratinocyte survival and higher metastatic potential.69 Retention of this truncated NC1 domain has shown conversion of RDEB keratinocytes to tumor in a xenotransplant mouse model.70 A high level of type VII collagen itself may inherently be protumorigenic for keratinocytes.71
There does not appear to be evidence for HPV involvement in RDEB-associated SCC.72 Squamous cell carcinoma development in RDEB appears to be multifactorial,73 but validated tumor models are lacking. Other than conventional oncologic therapy, future directions in the management of RDEB may include gene-, protein- and cell-targeted therapies.73
Conclusion
Squamous cell carcinomas are known to arise within chronic cutaneous inflammatory dermatoses. Tumorigenesis peaks relatively early in new orolabial DLE, LS, and OLP cases, and can occur over many decades in cutaneous DLE, HLP, HS, NLD, and chronic wounds or scars, summarized in the Table. Frequent SCCs are observed in high-risk subtypes of epidermolysis bullosa. Dermatologists must examine areas affected by these diseases at regular intervals, being mindful of the possibility of SCC development. Furthermore, dermatologists should adopt a lower threshold to biopsy suspicious lesions, especially those that develop within relatively new orolabial DLE, chronic HS, or chronic wound cases, as SCC in these settings is particularly aggressive and displays mortality and metastasis rates that exceed those of common cutaneous SCC.
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. doi:10.1002/ijc.23173
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436-444. doi:10.1038/nature07205
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi:10.3389/fimmu.2011.00098
- Tebbe B. Clinical course and prognosis of cutaneous lupus erythematosus. Clin Dermatol. 2004;22:121-124. doi:10.1016/j.clindermatol.2003.12.018
- Tao J, Zhang X, Guo N, et al. Squamous cell carcinoma complicating discoid lupus erythematosus in Chinese patients: review of the literature, 1964-2010. J Am Acad Dermatol. 2012;66:695-696. doi:10.1016 /j.jaad.2011.09.033
- Fernandes MS, Girisha BS, Viswanathan N, et al. Discoid lupus erythematosus with squamous cell carcinoma: a case report and review of the literature in Indian patients. Lupus. 2015;24:1562-1566. doi:10.1177/0961203315599245
- Makita E, Akasaka E, Sakuraba Y, et al. Squamous cell carcinoma on the lip arising from discoid lupus erythematosus: a case report and review of Japanese patients. Eur J Dermatol. 2016;26:395-396. doi:10.1684/ejd.2016.2780
- Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23:759-765. doi:10.1200/JCO.2005.02.155
- Arvanitidou I-E, Nikitakis NG, Georgaki M, et al. Multiple primary squamous cell carcinomas of the lower lip and tongue arising in discoid lupus erythematosus: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:e22-e30. doi:10.1016/j.oooo.2017.08.012
- Alsanafi S, Werth VP. Squamous cell carcinomas arising in discoid lupus erythematosus scars: unusual occurrence in an African-American and in a sun-protected area. J Clin Rheumatol. 2011;17:35-36. doi:10.1097/RHU.0b013e3182051928
- Goobie GC, Bernatsky S, Ramsey-Goldman R, et al. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol. 2015;27:454-460. doi:10.1097/BOR.0000000000000202
- Simpson JK, Medina-Flores R, Deng J-S. Squamous cell carcinoma arising in discoid lupus erythematosus lesions of the ears infected with human papillomavirus. Cutis. 2010;86:195-198.
- Sigurgeirsson B, B. Lichen planus and malignancy. an epidemiologic study of 2071 patients and a review of the literature. Arch Dermatol. 1991;127:1684-1688. doi:10.1001/archderm.127.11.1684
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45-56. doi:10.14219/jada.2013.10
- Laniosz V, Torgerson RR, Ramos-Rodriguez AJ, et al. Incidence of squamous cell carcinoma in oral lichen planus: a 25-year population-based study. Int J Dermatol. 2019;58:296-301. doi:10.1111/ijd.14215
- Aghbari SMH, Abushouk AI, Attia A, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017;68:92-102. doi:10.1016/j.oraloncology.2017.03.012
- Morita M, Asoda S, Tsunoda K, et al. The onset risk of carcinoma in patients continuing tacrolimus topical treatment for oral lichen planus: a case report. Odontology. 2017;105:262-266. doi:10.1007/s10266-016-0255-4
- Knackstedt TJ, Collins LK, Li Z, et al. Squamous cell carcinoma arising in hypertrophic lichen planus: a review and analysis of 38 cases. Dermatol Surg. 2015;41:1411-1418. doi:10.1097/DSS.0000000000000565
- Tong LX, Weinstock MJ, Drews R, et al. Widely metastatic squamous cell carcinoma originating from malignant transformation of hypertrophic lichen planus in a 24-year-old woman: case report and review of the literature. Pediatr Dermatol. 2015;32:e98-e101. doi:10.1111/pde.12549
- Ardabili M, Gambichler T, Rotterdam S, et al. Metastatic cutaneous squamous cell carcinoma arising from a previous area of chronic hypertrophic lichen planus. Dermatol Online J. 2003;9:10.
- Bowen AR, Burt L, Boucher K, et al. Use of proliferation rate, p53 staining and perforating elastic fibers in distinguishing keratoacanthoma from hypertrophic lichen planus: a pilot study. J Cutan Pathol. 2012;39:243-250. doi:10.1111/j.1600-0560.2011.01834.x
- Totonchy MB, Leventhal JS, Ko CJ, et al. Hypertrophic lichen planus and well-differentiated squamous cell carcinoma: a diagnostic conundrum. Dermatol Surg. 2018;44:1466-1470. doi:10.1097/DSS.0000000000001465
- Levandoski KA, Nazarian RM, Asgari MM. Hypertrophic lichen planus mimicking squamous cell carcinoma: the importance of clinicopathologic correlation. JAAD Case Rep. 2017;3:151-154. doi: 10.1016/j.jdcr.2017.01.020
- Okiyama N, Satoh T, Yokozeki H, et al. Squamous cell carcinoma arising from lichen planus of nail matrix and nail bed. J Am Acad Dermatol. 2005;53:908-909. doi:10.1016/j.jaad.2005.04.052
- Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011;64:1147-1153. doi:10.1016/j.jaad.2010.02.057
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370. doi:10.1016/j.jaad.2019.03.070
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416. doi:10.1016/0190-9622(95)90060-8
- Leibowitch M, Neill S, Pelisse M, et al. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990;97:1135-1139. doi:10.1111/j.1471-0528.1990.tb02502.x
- Bleeker MCG, Visser PJ, Overbeek LIH, et al. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:1224-1230. doi:10.1158/1055-9965.EPI-16-0019
- Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol. 1998;29:932-948. doi:10.1016/s0046-8177(98)90198-8
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183. doi:10.1097/LGT.0000000000000186
- Cooper SM, Madnani N, Margesson L. Reduced risk of squamous cell carcinoma with adequate treatment of vulvar lichen sclerosus. JAMA Dermatol. 2015;151:1059-1060. doi:10.1001/jamadermatol.2015.0644
- Rakislova N, Alemany L, Clavero O, et al; VVAP Study Group. Differentiated vulvar intraepithelial neoplasia-like and lichen sclerosus-like lesions in HPV-associated squamous cell carcinomas of the vulva. Am J Surg Pathol. 2018;42:828-835. doi:10.1097/PAS.0000000000001047
- Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48:808-817. doi:10.1097/01.grf.0000179635.64663.3d
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067. doi:10.1001/jamadermatol.2015.0643
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, et al. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140:709-712. doi:10.1001/archderm.140.6.709
- Minhas S, Manseck A, Watya S, et al. Penile cancer—prevention and premalignant conditions. Urology. 2010;76(2 suppl 1):S24-S35. doi:10.1016/j.urology.2010.04.007
- Nasca MR, Innocenzi D, Micali G. Penile cancer among patients with genital lichen sclerosus. J Am Acad Dermatol. 1999;41:911-914. doi:10.1016/s0190-9622(99)70245-8
- Philippou P, Shabbir M, Ralph DJ, et al. Genital lichen sclerosus/balanitis xerotica obliterans in men with penile carcinoma: a critical analysis. BJU Int. 2013;111:970-976. doi:10.1111/j.1464-410X.2012.11773.x
- Velazquez EF, Cubilla AL. Lichen sclerosus in 68 patients with squamous cell carcinoma of the penis: frequent atypias and correlation with special carcinoma variants suggests a precancerous role. Am J Surg Pathol. 2003;27:1448-1453. doi:10.1097/00000478-200311000-00007
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64. doi:10.1016/j.jcws.2012.04.001
- Aydogdu E, Yildirim S, Akoz T. Is surgery an effective and adequate treatment in advanced Marjolin’s ulcer? Burns. 2005;31:421-431. doi:10.1016/j.burns.2005.02.008
- Xiao H, Deng K, Liu R, et al. A review of 31 cases of Marjolin’s ulcer on scalp: is it necessary to preventively remove the scar? Int Wound J. 2019;16:479-485. doi:10.1111/iwj.13058
- Chaturvedi G, Gupta AK, Das S, et al. Marjolin ulcer: an observational epidemiological study from a tertiary care centre in India. Ann Plast Surg. 2019;83:518-522. doi:10.1097/SAP.0000000000001995
- Karasoy Yesilada A, Zeynep Sevim K, D, et al. Marjolin ulcer: clinical experience with 34 patients over 15 years. J Cutan Med Surg. 2013;17:404-409. doi:10.2310/7750.2013.13016
- D, Przybek-Mita J, B, et al. Marjolin’s ulcer in chronic wounds - review of available literature. Contemp Oncol (Pozn). 2017;21:197-202. doi:10.5114/wo.2017.70109
- Visuthikosol V, Boonpucknavig V, Nitiyanant P. Squamous carcinoma in scars: clinicopathological correlations. Ann Plast Surg. 1986;16:42-48. doi:10.1097/00000637-198601000-00004
- Bostwick J 3rd, Pendergrast WJ Jr, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976;57:66-69.
- Kerr-Valentic MA, Samimi K, Rohlen BH, et al. Marjolin’s ulcer: modern analysis of an ancient problem. Plast Reconstr Surg. 2009;123:184-191. doi:10.1097/PRS.0b013e3181904d86
- Constantinou C, Widom K, Desantis J, et al. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg. 2008;74:1177-1181.
- Fabbrocini G, Ruocco E, De Vita V, et al. Squamous cell carcinoma arising in long-standing hidradenitis suppurativa: an overlooked facet of the immunocompromised district. Clin Dermatol. 2017;35:225-227. doi:10.1016/j.clindermatol.2016.10.019
- Baroni A, Buommino E, Piccolo V, et al. Alterations of skin innate immunity in lymphedematous limbs: correlations with opportunistic diseases. Clin Dermatol. 2014;32:592-598. doi:10.1016/j.clindermatol.2014.04.006
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526. doi:10.1097/DSS.0000000000001713
- Huang C, Lai Z, He M, et al. Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa: a case report and literature review. Medicine (Baltimore). 2017;96:e5857. doi:10.1097/MD.0000000000005857
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153. doi:10.1159/000269836
- Makris G-M, Poulakaki N, Papanota A-M, et al. Vulvar, perianal and perineal cancer after hidradenitis suppurativa: a systematic review and pooled analysis. Dermatol Surg. 2017;43:107-115. doi:10.1097/DSS.0000000000000944
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419. doi:10.1016/j.jaad.2012.07.027
- Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48:2046-2053. doi:10.1016/j.ejca.2012.01.003
- Uva L, Freitas J, Soares de Almeida L, et al. Squamous cell carcinoma arising in ulcerated necrobiosis lipoidica diabeticorum. Int Wound J. 2015;12:741-743. doi:10.1111/iwj.12206
- McGrath JA, Schofield OM, Mayou BJ, et al. Epidermolysis bullosa complicated by squamous cell carcinoma: report of 10 cases. J Cutan Pathol. 1992;19:116-123. doi:10.1111/j.1600-0560.1992.tb01352.x
- H, Chiaverini C, Sbidian E, et al. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis. 2016;11:117. doi:10.1186/s13023-016-0489-9.
- Fine J-D. Inherited epidermolysis bullosa: past, present, and future. Ann N Y Acad Sci. 2010;1194:213-222. doi:10.1111/j.1749-6632.2010.05463.x
- Kim M, Li M, Intong-Wheeler LRA, et al. Epidemiology and outcome of squamous cell carcinoma in epidermolysis bullosa in Australia and New Zealand. Acta Derm Venereol. 2018;98:70-76. doi:10.2340/00015555-2781
- Bruckner-Tuderman L, Mitsuhashi Y, Schnyder UW, et al. Anchoring fibrils and type VII collagen are absent from skin in severe recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1989;93:3-9. doi:10.1111/1523-1747.ep12277331
- Ng Y-Z, Pourreyron C, Salas-Alanis JC, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. 2012;72:3522-3534. doi:10.1158/0008-5472.CAN-11-2996
- Arbiser JL, Fan C-Y, Su X, et al. Involvement of p53 and p16 tumor suppressor genes in recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2004;123:788-790. doi:10.1111/j.0022-202X.2004.23418.x
- Knaup J, Gruber C, Krammer B, et al. TGFbeta-signaling in squamous cell carcinoma occurring in recessive dystrophic epidermolysis bullosa. Anal Cell Pathol (Amst). 2011;34:339-353. doi:10.3233/ACP-2011-0039
- Kivisaari AK, Kallajoki M, Mirtti T, et al. Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol. 2008;158:778-785. doi:10.1111/j.1365-2133.2008.08466.x
- Rodeck U, Fertala A, Uitto J. Anchorless keratinocyte survival: an emerging pathogenic mechanism for squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2007;16:465-467. doi:10.1111/j.1600-0625.2007.00563.x
- Ortiz-Urda S, Garcia J, Green CL, et al. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773-1776. doi:10.1126/science.1106209
- Pourreyron C, Chen M, McGrath JA, et al. High levels of type VII collagen expression in recessive dystrophic epidermolysis bullosa cutaneous squamous cell carcinoma keratinocytes increases PI3K and MAPK signalling, cell migration and invasion. Br J Dermatol. 2014;170:1256-1265. doi:10.1111/bjd.12715
- Purdie KJ, Pourreyron C, Fassihi H, et al. No evidence that human papillomavirus is responsible for the aggressive nature of recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2010;130:2853-2855. doi:10.1038/jid.2010.243
- South AP, O’Toole EA. Understanding the pathogenesis of recessive dystrophic epidermolysis bullosa squamous cell carcinoma. Dermatol Clin. 2010;28:171-178. doi:10.1016/j.det.2009.10.023
As many as one-quarter of human cancers are related to chronic inflammation, chronic infection, or both.1 Extrinsic inflammation leads to generation of proinflammatory cytokines that in turn recruit other inflammatory cells, which is thought to generate a positive amplification loop.2 Intrinsic stimuli from proto-oncogenes and mutations in tumor suppressor genes lead to transformed cancer cells that also secrete proinflammatory cytokines, thus propagating the cycle.
Numerous factors have been observed in association with tumor growth, progression, invasion, and metastasis.3 One factor for the development of squamous cell carcinoma (SCC) may be chronic inflammatory dermatoses. To date, reviews of chronic inflammation–associated malignancy have focused on solid organ cancers. We sought to provide an up-to-date review of SCC arising within chronic dermatoses, with an emphasis on the anatomic location of dermatoses involved in the transformation of cancer cells, the lag time from onset of dermatosis to diagnosis of SCC, and the distinctive mechanisms thought to be involved in the tumorigenesis in particular dermatoses.
Discoid Lupus Erythematosus
Discoid lupus erythematosus (DLE) is a chronic cutaneous lupus erythematosus variant with a female to male predominance of 3:1,4 and DLE lesions are prone to malignant transformation. Retrospective cohort studies have attempted to characterize who is at risk for SCC and how SCCs behave depending on their location. Cohorts from China,5 India,6 and Japan7 have noted a higher rate of SCC within DLE lesions in men (female to male ratios of 1:2.2, 1:1.6, and 1:2, respectively) and shorter lag times for SCC onset within DLE lesions of the lips (13, 5, and 10 years, respectively) compared to SCC arising in DLE elsewhere (19.2, 11.2, and 26 years, respectively). Studies have noted that DLE lesions of the lips may be prone to more rapid SCC tumorigenesis compared to DLE on cutaneous sites. One study reported SCC in DLE recurrence, metastasis, and death rates of 29%, 16.1%, and 19.4%, respectively,5 which exceeds reported rates in non-DLE SCCs (20%, 0.5% to 6%, and 1%, respectively).5,8
Because SCC arising within DLE is most common on the lips (Figure 1), it has been hypothesized that the high rate of transformation of DLE lesions on the lips may be due to constant exposure to irritation and tobacco, which may accelerate carcinogenesis.5 It also has been hypothesized that atrophic discoid lesions have lost sun protection and are more prone to mutagenic UV radiation,9 as SCCs arising in DLE lesions virtually always display prominent solar elastosis6; however, SCC has been observed to arise in non–sun-exposed DLE lesions in both White and Black patients.10

Additionally, use of immunosuppressant medications may accelerate the emergence of malignancy or more aggressive forms of malignancy; however, patients with autoimmune disease have a greater risk for malignancy at baseline,11 thus making it difficult to determine the excess risk from medications. There also may be a role for human papillomavirus (HPV) accelerating SCC development in DLE lesions, as demonstrated in a case of SCC arising in DLE lesions of the ears, with viral staining evident within the tumors.12 However, testing for HPV is not routinely performed in these cases.
Dermatologists need to be aware of the relatively rapid tumorigenesis and aggressive behavior of transformation and aggression seen with SCC arising within orolabial DLE lesions compared to cutaneous lesions, especially those on the lips.
Lichen Planus
Although patients with typical cutaneous lichen planus lesions do not have an increased risk for SCC,13 variants of lichen planus may predispose patients to SCC.
Oral Lichen Planus—Oral lichen planus (OLP) lesions are prone to malignant transformation. A systematic review of 16 studies evaluating the risk for OLP-associated SCC revealed an overall transformation rate of 1.09%, with a mean lag time of 4.3 years,14 compared to a reference rate of 0.2% for oral SCC.15 A meta-analysis of 19,676 patients with OLP and other oral lichenoid lesions revealed an oral SCC rate of 1.1%, with higher rates of transformation seen in cigarette smokers, alcoholics, and patients with hepatitis C virus infection.16 The ulcerative subtype of OLP appears to present a greater risk for malignant transformation.15 Dermatologists also should be cognizant that treatments for OLP such as topical calcineurin inhibitors may support the development of malignancy within inflammatory lesions.17
Hypertrophic Lichen Planus—The hypertrophic variant of lichen planus (HLP) also is prone to malignant transformation. A 1991 epidemiologic study from Sweden of malignancy arising in lichen planus revealed a disproportionate number of cases arising in verrucous or hypertrophic lesions, with a mean of 12.2 years from onset of the dermatosis to malignancy diagnosis.13 A subsequent 2015 retrospective study of 38 patients revealed that SCC had a propensity for the lower limb, favoring the pretibial region and the calf over the foot and the ankle with a reported lag time of 11 years.18
Although metastatic SCC arising in HLP is rare, 2 cases have been reported. A 24-year-old woman presented with an HLP plaque on the lower leg that developed during childhood and rapidly enlarged 2 months prior to presentation; she eventually died from metastatic disease.19 In another case, a 34-year-old man presented with an HLP lesion of approximately 10 years’ duration. A well-differentiated SCC was excised, and he developed lymph node metastases 5 months later.20
It is important to note that HLP on the legs often is misdiagnosed as SCC, as pseudoepitheliomatous hyperplasia and squamous metaplasia can be difficult to differentiate clinically and histologically.21,22 In the case of multiple eruptive SCCs of the lower leg, clinical correlation is essential to avoid unnecessary and ineffective surgical treatment.
Patients with HLP may exhibit Wickham striae, follicular accentuation, and mucocutaneous lichen planus at other sites, or a correlative initiation of possible culprit medications.23 Because true SCC arising within HLP is relatively rare, its malignant potential is not as clear as those arising within DLE; however, the lower limb appears to be the most common location for SCC within HLP.Nail Lichen Planus—Squamous cell carcinoma arising in nail lichen planus is rare. A report of 2 patients were diagnosed with lichen planus approximately 15 years prior to diagnosis of ungual SCC.24 Given the rarity of this presentation, it is difficult to ascertain the approximate lag time and other risk factors. Furthermore, the role of HPV in these cases was not ruled out. Oncogenic HPV strains have been reported in patients with periungual SCC.25,26
Lichen Sclerosus
Lichen sclerosus (LS) is a chronic inflammatory dermatosis that favors the anogenital area in a female to male ratio of 10:1.27 It is considered a premalignant condition for SCC tumorigenesis and may be a strong predictor of vulvar SCC (Figure 2), as 62% of vulvar SCC cases (N=78) may have adjacent LS.28

In a Dutch cohort of 3038 women with LS, 2.6% of patients developed vulvar SCC at a median of 3.3 years after LS diagnosis.29 Other studies have estimated a lag time of 4 years until SCC presentation.30 An Italian cohort of 976 women similarly observed that 2.7% of patients developed premalignancy or SCC.31 It was previously estimated that 3% to 5% of patients with LS developed SCC; however, prior studies may have included cases of vulvar intraepithelial neoplasia with low risk for invasive SCC, which might have overestimated true risk of SCC.32 Another confounding factor for elucidating SCC on a background of LS may be the presence of HPV.33 Extragenital LS does not appear to have similar potential for malignant transformation.34
In a prospective Australian cohort of 507 women with LS (mean age, 55.4 years), remission was induced with potent topical corticosteroids.35 Patients who were adherent to a topical regimen did not develop SCC during follow-up. Those who were nonadherent or partially adherent had a 4.7% risk for SCC.35 In a similar prospective study of 83 women in France, the SCC rate was 9.6% in lesions that were untreated or irregularly treated.36 These studies provide essential evidence that appropriately treating LS can prevent SCC at a later date, though longer-term data are lacking.
The rate of SCC arising in male genital LS may approach 8.4%,37 with a lag time of 17 years from onset of LS to SCC diagnosis.38 Although circumcision often is considered curative for male genital LS, patients have been observed to develop penile SCC at least 5 years after circumcision.39 Male penile SCC in a background of LS may not necessarily be HPV associated.40
Marjolin Ulcer
Chronic ulcers or scars, typically postburn scars, may undergo malignant transformation, with SCC being the most common carcinoma.41 Squamous cell carcinoma in the context of a chronic ulcer or wound is known as a Marjolin ulcer (MU). Up to 2% of burn scars have been observed to undergo malignant transformation.42 Marjolin ulcers tend to behave aggressively once they form, and it has been proposed that removal of scar tissue may be a preventive therapeutic strategy.43 Cohort studies of MU on the lower extremities have observed lag times of 26.444 and 37.945 years, with both studies also noting relatively high rates of local recurrence.
The pathogenesis of MU appears to be multifactorial. Chronic inflammation and scar formation have been implicated. Chronic inflammation and irritation of lesions at natural creases are thought to increase mitotic activity,41 and local accumulation of toxin may promote mutagenesis.46 Scar formation may create a locally immunoprivileged site, allowing for developing tumors to evade the immune system47 and become even more aggressive as the tumor accumulates.48 Scar formation also may prevent the ability of immune cells to penetrate the tumor microenvironment and access lymphatic channels.49
Hidradenitis Suppurativa
As many as 3.2% of patients with chronic hidradenitis suppurativa (HS) experience malignant transformation to SCC.50 Early HS displays subclinical lymphedema in affected sites, which can progress to chronic fibrosis, stasis, and accumulation of protein-rich fluid.51 Stasis changes have been associated with altered local inflammatory proteins, such as toll-like receptors, β-defensins, and interleukins.52
A retrospective cohort study of 12 patients revealed a lag time of 28.5 years from HS diagnosis to the manifestation of malignancy.53 After local excision, 7 patients developed recurrence, with 100% mortality. Squamous cell carcinomas were well differentiated and moderately differentiated.53 A 2017 literature review of 62 case reports calculated a mean lag time of 27 years. Despite 85% of SCCs being well differentiated and moderately differentiated, nearly half of patients died within 2 years.54 As seen in other inflammatory conditions, HPV can complicate perineal HS and promote SCC tumorigenesis.55
Squamous cell carcinomas arising within HS lesions are more prevalent in males (6.75:1 ratio),54,56 despite HS being more prevalent in females (2:1 ratio).57 Similar to DLE, SCCs arising in HS are aggressive and are seen more in males, despite both conditions being female predominant. Incidence and mortality rates for primary cutaneous SCC are higher for men vs women58; however, the discordance in aggressive behavior seen more commonly in SCC arising from HS or DLE in male patients has yet to be explained.
Necrobiosis Lipoidica Diabeticorum
Malignancy arising within necrobiosis lipoidica diabeticorum (NLD) is rare. A review of 14 published cases noted that 13 were SCC and 1 was leiomyosarcoma.59 The lag time was 21.5 years; 31% of cases (N=14) presented with regional lymph node metastasis. Although chronic ulceration is a risk factor for SCC and occurs in as many as one-third of NLD cases, its correlation with ulceration and malignant transformation has not been characterized.
Epidermolysis Bullosa
Recessive dystrophic epidermolysis bullosa (RDEB) is a noninflammatory inherited blistering disease, and patients have an inherently high risk for aggressive SCC.60 Other forms of epidermolysis bullosa can lead to SCC, but the rarer RDEB accounts for 69% of SCC cases, with a median age of 36 years at presentation.61 Although SCCs tend to be well differentiated in RDEB (73.9%),61 they also exhibit highly aggressive behavior.62 In the most severe variant—RDEB-generalized severe—the cumulative risk for SCC-related death in an Australian population was 84.4% at 34 years of age.63
As RDEB is an inherited disorder with potential for malignancy at a young age, the pathogenesis is plausibly different from the previously discussed inflammatory dermatoses. This disease is characterized by a mutation in the collagen VII gene, leading to loss of anchoring fibrils and a basement membrane zone split.64 There also can be inherent fibroblast alterations; RDEB fibroblasts create an environment for tumor growth by supporting malignant-cell adhesion and invasion.65 Mutations in p53,66 local alterations in transforming growth factor β activity,67 and downstream matrix metalloproteinase activity68 have been implicated.
Additionally, keratinocytes may retain the N-terminal noncollagenous (NC1) domain of truncated collagen VII while losing the anchoring NC2 domain in mutated collagen VII RDEB, thereby supporting anchorless keratinocyte survival and higher metastatic potential.69 Retention of this truncated NC1 domain has shown conversion of RDEB keratinocytes to tumor in a xenotransplant mouse model.70 A high level of type VII collagen itself may inherently be protumorigenic for keratinocytes.71
There does not appear to be evidence for HPV involvement in RDEB-associated SCC.72 Squamous cell carcinoma development in RDEB appears to be multifactorial,73 but validated tumor models are lacking. Other than conventional oncologic therapy, future directions in the management of RDEB may include gene-, protein- and cell-targeted therapies.73
Conclusion
Squamous cell carcinomas are known to arise within chronic cutaneous inflammatory dermatoses. Tumorigenesis peaks relatively early in new orolabial DLE, LS, and OLP cases, and can occur over many decades in cutaneous DLE, HLP, HS, NLD, and chronic wounds or scars, summarized in the Table. Frequent SCCs are observed in high-risk subtypes of epidermolysis bullosa. Dermatologists must examine areas affected by these diseases at regular intervals, being mindful of the possibility of SCC development. Furthermore, dermatologists should adopt a lower threshold to biopsy suspicious lesions, especially those that develop within relatively new orolabial DLE, chronic HS, or chronic wound cases, as SCC in these settings is particularly aggressive and displays mortality and metastasis rates that exceed those of common cutaneous SCC.
As many as one-quarter of human cancers are related to chronic inflammation, chronic infection, or both.1 Extrinsic inflammation leads to generation of proinflammatory cytokines that in turn recruit other inflammatory cells, which is thought to generate a positive amplification loop.2 Intrinsic stimuli from proto-oncogenes and mutations in tumor suppressor genes lead to transformed cancer cells that also secrete proinflammatory cytokines, thus propagating the cycle.
Numerous factors have been observed in association with tumor growth, progression, invasion, and metastasis.3 One factor for the development of squamous cell carcinoma (SCC) may be chronic inflammatory dermatoses. To date, reviews of chronic inflammation–associated malignancy have focused on solid organ cancers. We sought to provide an up-to-date review of SCC arising within chronic dermatoses, with an emphasis on the anatomic location of dermatoses involved in the transformation of cancer cells, the lag time from onset of dermatosis to diagnosis of SCC, and the distinctive mechanisms thought to be involved in the tumorigenesis in particular dermatoses.
Discoid Lupus Erythematosus
Discoid lupus erythematosus (DLE) is a chronic cutaneous lupus erythematosus variant with a female to male predominance of 3:1,4 and DLE lesions are prone to malignant transformation. Retrospective cohort studies have attempted to characterize who is at risk for SCC and how SCCs behave depending on their location. Cohorts from China,5 India,6 and Japan7 have noted a higher rate of SCC within DLE lesions in men (female to male ratios of 1:2.2, 1:1.6, and 1:2, respectively) and shorter lag times for SCC onset within DLE lesions of the lips (13, 5, and 10 years, respectively) compared to SCC arising in DLE elsewhere (19.2, 11.2, and 26 years, respectively). Studies have noted that DLE lesions of the lips may be prone to more rapid SCC tumorigenesis compared to DLE on cutaneous sites. One study reported SCC in DLE recurrence, metastasis, and death rates of 29%, 16.1%, and 19.4%, respectively,5 which exceeds reported rates in non-DLE SCCs (20%, 0.5% to 6%, and 1%, respectively).5,8
Because SCC arising within DLE is most common on the lips (Figure 1), it has been hypothesized that the high rate of transformation of DLE lesions on the lips may be due to constant exposure to irritation and tobacco, which may accelerate carcinogenesis.5 It also has been hypothesized that atrophic discoid lesions have lost sun protection and are more prone to mutagenic UV radiation,9 as SCCs arising in DLE lesions virtually always display prominent solar elastosis6; however, SCC has been observed to arise in non–sun-exposed DLE lesions in both White and Black patients.10

Additionally, use of immunosuppressant medications may accelerate the emergence of malignancy or more aggressive forms of malignancy; however, patients with autoimmune disease have a greater risk for malignancy at baseline,11 thus making it difficult to determine the excess risk from medications. There also may be a role for human papillomavirus (HPV) accelerating SCC development in DLE lesions, as demonstrated in a case of SCC arising in DLE lesions of the ears, with viral staining evident within the tumors.12 However, testing for HPV is not routinely performed in these cases.
Dermatologists need to be aware of the relatively rapid tumorigenesis and aggressive behavior of transformation and aggression seen with SCC arising within orolabial DLE lesions compared to cutaneous lesions, especially those on the lips.
Lichen Planus
Although patients with typical cutaneous lichen planus lesions do not have an increased risk for SCC,13 variants of lichen planus may predispose patients to SCC.
Oral Lichen Planus—Oral lichen planus (OLP) lesions are prone to malignant transformation. A systematic review of 16 studies evaluating the risk for OLP-associated SCC revealed an overall transformation rate of 1.09%, with a mean lag time of 4.3 years,14 compared to a reference rate of 0.2% for oral SCC.15 A meta-analysis of 19,676 patients with OLP and other oral lichenoid lesions revealed an oral SCC rate of 1.1%, with higher rates of transformation seen in cigarette smokers, alcoholics, and patients with hepatitis C virus infection.16 The ulcerative subtype of OLP appears to present a greater risk for malignant transformation.15 Dermatologists also should be cognizant that treatments for OLP such as topical calcineurin inhibitors may support the development of malignancy within inflammatory lesions.17
Hypertrophic Lichen Planus—The hypertrophic variant of lichen planus (HLP) also is prone to malignant transformation. A 1991 epidemiologic study from Sweden of malignancy arising in lichen planus revealed a disproportionate number of cases arising in verrucous or hypertrophic lesions, with a mean of 12.2 years from onset of the dermatosis to malignancy diagnosis.13 A subsequent 2015 retrospective study of 38 patients revealed that SCC had a propensity for the lower limb, favoring the pretibial region and the calf over the foot and the ankle with a reported lag time of 11 years.18
Although metastatic SCC arising in HLP is rare, 2 cases have been reported. A 24-year-old woman presented with an HLP plaque on the lower leg that developed during childhood and rapidly enlarged 2 months prior to presentation; she eventually died from metastatic disease.19 In another case, a 34-year-old man presented with an HLP lesion of approximately 10 years’ duration. A well-differentiated SCC was excised, and he developed lymph node metastases 5 months later.20
It is important to note that HLP on the legs often is misdiagnosed as SCC, as pseudoepitheliomatous hyperplasia and squamous metaplasia can be difficult to differentiate clinically and histologically.21,22 In the case of multiple eruptive SCCs of the lower leg, clinical correlation is essential to avoid unnecessary and ineffective surgical treatment.
Patients with HLP may exhibit Wickham striae, follicular accentuation, and mucocutaneous lichen planus at other sites, or a correlative initiation of possible culprit medications.23 Because true SCC arising within HLP is relatively rare, its malignant potential is not as clear as those arising within DLE; however, the lower limb appears to be the most common location for SCC within HLP.Nail Lichen Planus—Squamous cell carcinoma arising in nail lichen planus is rare. A report of 2 patients were diagnosed with lichen planus approximately 15 years prior to diagnosis of ungual SCC.24 Given the rarity of this presentation, it is difficult to ascertain the approximate lag time and other risk factors. Furthermore, the role of HPV in these cases was not ruled out. Oncogenic HPV strains have been reported in patients with periungual SCC.25,26
Lichen Sclerosus
Lichen sclerosus (LS) is a chronic inflammatory dermatosis that favors the anogenital area in a female to male ratio of 10:1.27 It is considered a premalignant condition for SCC tumorigenesis and may be a strong predictor of vulvar SCC (Figure 2), as 62% of vulvar SCC cases (N=78) may have adjacent LS.28

In a Dutch cohort of 3038 women with LS, 2.6% of patients developed vulvar SCC at a median of 3.3 years after LS diagnosis.29 Other studies have estimated a lag time of 4 years until SCC presentation.30 An Italian cohort of 976 women similarly observed that 2.7% of patients developed premalignancy or SCC.31 It was previously estimated that 3% to 5% of patients with LS developed SCC; however, prior studies may have included cases of vulvar intraepithelial neoplasia with low risk for invasive SCC, which might have overestimated true risk of SCC.32 Another confounding factor for elucidating SCC on a background of LS may be the presence of HPV.33 Extragenital LS does not appear to have similar potential for malignant transformation.34
In a prospective Australian cohort of 507 women with LS (mean age, 55.4 years), remission was induced with potent topical corticosteroids.35 Patients who were adherent to a topical regimen did not develop SCC during follow-up. Those who were nonadherent or partially adherent had a 4.7% risk for SCC.35 In a similar prospective study of 83 women in France, the SCC rate was 9.6% in lesions that were untreated or irregularly treated.36 These studies provide essential evidence that appropriately treating LS can prevent SCC at a later date, though longer-term data are lacking.
The rate of SCC arising in male genital LS may approach 8.4%,37 with a lag time of 17 years from onset of LS to SCC diagnosis.38 Although circumcision often is considered curative for male genital LS, patients have been observed to develop penile SCC at least 5 years after circumcision.39 Male penile SCC in a background of LS may not necessarily be HPV associated.40
Marjolin Ulcer
Chronic ulcers or scars, typically postburn scars, may undergo malignant transformation, with SCC being the most common carcinoma.41 Squamous cell carcinoma in the context of a chronic ulcer or wound is known as a Marjolin ulcer (MU). Up to 2% of burn scars have been observed to undergo malignant transformation.42 Marjolin ulcers tend to behave aggressively once they form, and it has been proposed that removal of scar tissue may be a preventive therapeutic strategy.43 Cohort studies of MU on the lower extremities have observed lag times of 26.444 and 37.945 years, with both studies also noting relatively high rates of local recurrence.
The pathogenesis of MU appears to be multifactorial. Chronic inflammation and scar formation have been implicated. Chronic inflammation and irritation of lesions at natural creases are thought to increase mitotic activity,41 and local accumulation of toxin may promote mutagenesis.46 Scar formation may create a locally immunoprivileged site, allowing for developing tumors to evade the immune system47 and become even more aggressive as the tumor accumulates.48 Scar formation also may prevent the ability of immune cells to penetrate the tumor microenvironment and access lymphatic channels.49
Hidradenitis Suppurativa
As many as 3.2% of patients with chronic hidradenitis suppurativa (HS) experience malignant transformation to SCC.50 Early HS displays subclinical lymphedema in affected sites, which can progress to chronic fibrosis, stasis, and accumulation of protein-rich fluid.51 Stasis changes have been associated with altered local inflammatory proteins, such as toll-like receptors, β-defensins, and interleukins.52
A retrospective cohort study of 12 patients revealed a lag time of 28.5 years from HS diagnosis to the manifestation of malignancy.53 After local excision, 7 patients developed recurrence, with 100% mortality. Squamous cell carcinomas were well differentiated and moderately differentiated.53 A 2017 literature review of 62 case reports calculated a mean lag time of 27 years. Despite 85% of SCCs being well differentiated and moderately differentiated, nearly half of patients died within 2 years.54 As seen in other inflammatory conditions, HPV can complicate perineal HS and promote SCC tumorigenesis.55
Squamous cell carcinomas arising within HS lesions are more prevalent in males (6.75:1 ratio),54,56 despite HS being more prevalent in females (2:1 ratio).57 Similar to DLE, SCCs arising in HS are aggressive and are seen more in males, despite both conditions being female predominant. Incidence and mortality rates for primary cutaneous SCC are higher for men vs women58; however, the discordance in aggressive behavior seen more commonly in SCC arising from HS or DLE in male patients has yet to be explained.
Necrobiosis Lipoidica Diabeticorum
Malignancy arising within necrobiosis lipoidica diabeticorum (NLD) is rare. A review of 14 published cases noted that 13 were SCC and 1 was leiomyosarcoma.59 The lag time was 21.5 years; 31% of cases (N=14) presented with regional lymph node metastasis. Although chronic ulceration is a risk factor for SCC and occurs in as many as one-third of NLD cases, its correlation with ulceration and malignant transformation has not been characterized.
Epidermolysis Bullosa
Recessive dystrophic epidermolysis bullosa (RDEB) is a noninflammatory inherited blistering disease, and patients have an inherently high risk for aggressive SCC.60 Other forms of epidermolysis bullosa can lead to SCC, but the rarer RDEB accounts for 69% of SCC cases, with a median age of 36 years at presentation.61 Although SCCs tend to be well differentiated in RDEB (73.9%),61 they also exhibit highly aggressive behavior.62 In the most severe variant—RDEB-generalized severe—the cumulative risk for SCC-related death in an Australian population was 84.4% at 34 years of age.63
As RDEB is an inherited disorder with potential for malignancy at a young age, the pathogenesis is plausibly different from the previously discussed inflammatory dermatoses. This disease is characterized by a mutation in the collagen VII gene, leading to loss of anchoring fibrils and a basement membrane zone split.64 There also can be inherent fibroblast alterations; RDEB fibroblasts create an environment for tumor growth by supporting malignant-cell adhesion and invasion.65 Mutations in p53,66 local alterations in transforming growth factor β activity,67 and downstream matrix metalloproteinase activity68 have been implicated.
Additionally, keratinocytes may retain the N-terminal noncollagenous (NC1) domain of truncated collagen VII while losing the anchoring NC2 domain in mutated collagen VII RDEB, thereby supporting anchorless keratinocyte survival and higher metastatic potential.69 Retention of this truncated NC1 domain has shown conversion of RDEB keratinocytes to tumor in a xenotransplant mouse model.70 A high level of type VII collagen itself may inherently be protumorigenic for keratinocytes.71
There does not appear to be evidence for HPV involvement in RDEB-associated SCC.72 Squamous cell carcinoma development in RDEB appears to be multifactorial,73 but validated tumor models are lacking. Other than conventional oncologic therapy, future directions in the management of RDEB may include gene-, protein- and cell-targeted therapies.73
Conclusion
Squamous cell carcinomas are known to arise within chronic cutaneous inflammatory dermatoses. Tumorigenesis peaks relatively early in new orolabial DLE, LS, and OLP cases, and can occur over many decades in cutaneous DLE, HLP, HS, NLD, and chronic wounds or scars, summarized in the Table. Frequent SCCs are observed in high-risk subtypes of epidermolysis bullosa. Dermatologists must examine areas affected by these diseases at regular intervals, being mindful of the possibility of SCC development. Furthermore, dermatologists should adopt a lower threshold to biopsy suspicious lesions, especially those that develop within relatively new orolabial DLE, chronic HS, or chronic wound cases, as SCC in these settings is particularly aggressive and displays mortality and metastasis rates that exceed those of common cutaneous SCC.
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. doi:10.1002/ijc.23173
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436-444. doi:10.1038/nature07205
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi:10.3389/fimmu.2011.00098
- Tebbe B. Clinical course and prognosis of cutaneous lupus erythematosus. Clin Dermatol. 2004;22:121-124. doi:10.1016/j.clindermatol.2003.12.018
- Tao J, Zhang X, Guo N, et al. Squamous cell carcinoma complicating discoid lupus erythematosus in Chinese patients: review of the literature, 1964-2010. J Am Acad Dermatol. 2012;66:695-696. doi:10.1016 /j.jaad.2011.09.033
- Fernandes MS, Girisha BS, Viswanathan N, et al. Discoid lupus erythematosus with squamous cell carcinoma: a case report and review of the literature in Indian patients. Lupus. 2015;24:1562-1566. doi:10.1177/0961203315599245
- Makita E, Akasaka E, Sakuraba Y, et al. Squamous cell carcinoma on the lip arising from discoid lupus erythematosus: a case report and review of Japanese patients. Eur J Dermatol. 2016;26:395-396. doi:10.1684/ejd.2016.2780
- Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23:759-765. doi:10.1200/JCO.2005.02.155
- Arvanitidou I-E, Nikitakis NG, Georgaki M, et al. Multiple primary squamous cell carcinomas of the lower lip and tongue arising in discoid lupus erythematosus: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:e22-e30. doi:10.1016/j.oooo.2017.08.012
- Alsanafi S, Werth VP. Squamous cell carcinomas arising in discoid lupus erythematosus scars: unusual occurrence in an African-American and in a sun-protected area. J Clin Rheumatol. 2011;17:35-36. doi:10.1097/RHU.0b013e3182051928
- Goobie GC, Bernatsky S, Ramsey-Goldman R, et al. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol. 2015;27:454-460. doi:10.1097/BOR.0000000000000202
- Simpson JK, Medina-Flores R, Deng J-S. Squamous cell carcinoma arising in discoid lupus erythematosus lesions of the ears infected with human papillomavirus. Cutis. 2010;86:195-198.
- Sigurgeirsson B, B. Lichen planus and malignancy. an epidemiologic study of 2071 patients and a review of the literature. Arch Dermatol. 1991;127:1684-1688. doi:10.1001/archderm.127.11.1684
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45-56. doi:10.14219/jada.2013.10
- Laniosz V, Torgerson RR, Ramos-Rodriguez AJ, et al. Incidence of squamous cell carcinoma in oral lichen planus: a 25-year population-based study. Int J Dermatol. 2019;58:296-301. doi:10.1111/ijd.14215
- Aghbari SMH, Abushouk AI, Attia A, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017;68:92-102. doi:10.1016/j.oraloncology.2017.03.012
- Morita M, Asoda S, Tsunoda K, et al. The onset risk of carcinoma in patients continuing tacrolimus topical treatment for oral lichen planus: a case report. Odontology. 2017;105:262-266. doi:10.1007/s10266-016-0255-4
- Knackstedt TJ, Collins LK, Li Z, et al. Squamous cell carcinoma arising in hypertrophic lichen planus: a review and analysis of 38 cases. Dermatol Surg. 2015;41:1411-1418. doi:10.1097/DSS.0000000000000565
- Tong LX, Weinstock MJ, Drews R, et al. Widely metastatic squamous cell carcinoma originating from malignant transformation of hypertrophic lichen planus in a 24-year-old woman: case report and review of the literature. Pediatr Dermatol. 2015;32:e98-e101. doi:10.1111/pde.12549
- Ardabili M, Gambichler T, Rotterdam S, et al. Metastatic cutaneous squamous cell carcinoma arising from a previous area of chronic hypertrophic lichen planus. Dermatol Online J. 2003;9:10.
- Bowen AR, Burt L, Boucher K, et al. Use of proliferation rate, p53 staining and perforating elastic fibers in distinguishing keratoacanthoma from hypertrophic lichen planus: a pilot study. J Cutan Pathol. 2012;39:243-250. doi:10.1111/j.1600-0560.2011.01834.x
- Totonchy MB, Leventhal JS, Ko CJ, et al. Hypertrophic lichen planus and well-differentiated squamous cell carcinoma: a diagnostic conundrum. Dermatol Surg. 2018;44:1466-1470. doi:10.1097/DSS.0000000000001465
- Levandoski KA, Nazarian RM, Asgari MM. Hypertrophic lichen planus mimicking squamous cell carcinoma: the importance of clinicopathologic correlation. JAAD Case Rep. 2017;3:151-154. doi: 10.1016/j.jdcr.2017.01.020
- Okiyama N, Satoh T, Yokozeki H, et al. Squamous cell carcinoma arising from lichen planus of nail matrix and nail bed. J Am Acad Dermatol. 2005;53:908-909. doi:10.1016/j.jaad.2005.04.052
- Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011;64:1147-1153. doi:10.1016/j.jaad.2010.02.057
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370. doi:10.1016/j.jaad.2019.03.070
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416. doi:10.1016/0190-9622(95)90060-8
- Leibowitch M, Neill S, Pelisse M, et al. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990;97:1135-1139. doi:10.1111/j.1471-0528.1990.tb02502.x
- Bleeker MCG, Visser PJ, Overbeek LIH, et al. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:1224-1230. doi:10.1158/1055-9965.EPI-16-0019
- Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol. 1998;29:932-948. doi:10.1016/s0046-8177(98)90198-8
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183. doi:10.1097/LGT.0000000000000186
- Cooper SM, Madnani N, Margesson L. Reduced risk of squamous cell carcinoma with adequate treatment of vulvar lichen sclerosus. JAMA Dermatol. 2015;151:1059-1060. doi:10.1001/jamadermatol.2015.0644
- Rakislova N, Alemany L, Clavero O, et al; VVAP Study Group. Differentiated vulvar intraepithelial neoplasia-like and lichen sclerosus-like lesions in HPV-associated squamous cell carcinomas of the vulva. Am J Surg Pathol. 2018;42:828-835. doi:10.1097/PAS.0000000000001047
- Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48:808-817. doi:10.1097/01.grf.0000179635.64663.3d
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067. doi:10.1001/jamadermatol.2015.0643
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, et al. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140:709-712. doi:10.1001/archderm.140.6.709
- Minhas S, Manseck A, Watya S, et al. Penile cancer—prevention and premalignant conditions. Urology. 2010;76(2 suppl 1):S24-S35. doi:10.1016/j.urology.2010.04.007
- Nasca MR, Innocenzi D, Micali G. Penile cancer among patients with genital lichen sclerosus. J Am Acad Dermatol. 1999;41:911-914. doi:10.1016/s0190-9622(99)70245-8
- Philippou P, Shabbir M, Ralph DJ, et al. Genital lichen sclerosus/balanitis xerotica obliterans in men with penile carcinoma: a critical analysis. BJU Int. 2013;111:970-976. doi:10.1111/j.1464-410X.2012.11773.x
- Velazquez EF, Cubilla AL. Lichen sclerosus in 68 patients with squamous cell carcinoma of the penis: frequent atypias and correlation with special carcinoma variants suggests a precancerous role. Am J Surg Pathol. 2003;27:1448-1453. doi:10.1097/00000478-200311000-00007
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64. doi:10.1016/j.jcws.2012.04.001
- Aydogdu E, Yildirim S, Akoz T. Is surgery an effective and adequate treatment in advanced Marjolin’s ulcer? Burns. 2005;31:421-431. doi:10.1016/j.burns.2005.02.008
- Xiao H, Deng K, Liu R, et al. A review of 31 cases of Marjolin’s ulcer on scalp: is it necessary to preventively remove the scar? Int Wound J. 2019;16:479-485. doi:10.1111/iwj.13058
- Chaturvedi G, Gupta AK, Das S, et al. Marjolin ulcer: an observational epidemiological study from a tertiary care centre in India. Ann Plast Surg. 2019;83:518-522. doi:10.1097/SAP.0000000000001995
- Karasoy Yesilada A, Zeynep Sevim K, D, et al. Marjolin ulcer: clinical experience with 34 patients over 15 years. J Cutan Med Surg. 2013;17:404-409. doi:10.2310/7750.2013.13016
- D, Przybek-Mita J, B, et al. Marjolin’s ulcer in chronic wounds - review of available literature. Contemp Oncol (Pozn). 2017;21:197-202. doi:10.5114/wo.2017.70109
- Visuthikosol V, Boonpucknavig V, Nitiyanant P. Squamous carcinoma in scars: clinicopathological correlations. Ann Plast Surg. 1986;16:42-48. doi:10.1097/00000637-198601000-00004
- Bostwick J 3rd, Pendergrast WJ Jr, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976;57:66-69.
- Kerr-Valentic MA, Samimi K, Rohlen BH, et al. Marjolin’s ulcer: modern analysis of an ancient problem. Plast Reconstr Surg. 2009;123:184-191. doi:10.1097/PRS.0b013e3181904d86
- Constantinou C, Widom K, Desantis J, et al. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg. 2008;74:1177-1181.
- Fabbrocini G, Ruocco E, De Vita V, et al. Squamous cell carcinoma arising in long-standing hidradenitis suppurativa: an overlooked facet of the immunocompromised district. Clin Dermatol. 2017;35:225-227. doi:10.1016/j.clindermatol.2016.10.019
- Baroni A, Buommino E, Piccolo V, et al. Alterations of skin innate immunity in lymphedematous limbs: correlations with opportunistic diseases. Clin Dermatol. 2014;32:592-598. doi:10.1016/j.clindermatol.2014.04.006
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526. doi:10.1097/DSS.0000000000001713
- Huang C, Lai Z, He M, et al. Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa: a case report and literature review. Medicine (Baltimore). 2017;96:e5857. doi:10.1097/MD.0000000000005857
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153. doi:10.1159/000269836
- Makris G-M, Poulakaki N, Papanota A-M, et al. Vulvar, perianal and perineal cancer after hidradenitis suppurativa: a systematic review and pooled analysis. Dermatol Surg. 2017;43:107-115. doi:10.1097/DSS.0000000000000944
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419. doi:10.1016/j.jaad.2012.07.027
- Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48:2046-2053. doi:10.1016/j.ejca.2012.01.003
- Uva L, Freitas J, Soares de Almeida L, et al. Squamous cell carcinoma arising in ulcerated necrobiosis lipoidica diabeticorum. Int Wound J. 2015;12:741-743. doi:10.1111/iwj.12206
- McGrath JA, Schofield OM, Mayou BJ, et al. Epidermolysis bullosa complicated by squamous cell carcinoma: report of 10 cases. J Cutan Pathol. 1992;19:116-123. doi:10.1111/j.1600-0560.1992.tb01352.x
- H, Chiaverini C, Sbidian E, et al. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis. 2016;11:117. doi:10.1186/s13023-016-0489-9.
- Fine J-D. Inherited epidermolysis bullosa: past, present, and future. Ann N Y Acad Sci. 2010;1194:213-222. doi:10.1111/j.1749-6632.2010.05463.x
- Kim M, Li M, Intong-Wheeler LRA, et al. Epidemiology and outcome of squamous cell carcinoma in epidermolysis bullosa in Australia and New Zealand. Acta Derm Venereol. 2018;98:70-76. doi:10.2340/00015555-2781
- Bruckner-Tuderman L, Mitsuhashi Y, Schnyder UW, et al. Anchoring fibrils and type VII collagen are absent from skin in severe recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1989;93:3-9. doi:10.1111/1523-1747.ep12277331
- Ng Y-Z, Pourreyron C, Salas-Alanis JC, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. 2012;72:3522-3534. doi:10.1158/0008-5472.CAN-11-2996
- Arbiser JL, Fan C-Y, Su X, et al. Involvement of p53 and p16 tumor suppressor genes in recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2004;123:788-790. doi:10.1111/j.0022-202X.2004.23418.x
- Knaup J, Gruber C, Krammer B, et al. TGFbeta-signaling in squamous cell carcinoma occurring in recessive dystrophic epidermolysis bullosa. Anal Cell Pathol (Amst). 2011;34:339-353. doi:10.3233/ACP-2011-0039
- Kivisaari AK, Kallajoki M, Mirtti T, et al. Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol. 2008;158:778-785. doi:10.1111/j.1365-2133.2008.08466.x
- Rodeck U, Fertala A, Uitto J. Anchorless keratinocyte survival: an emerging pathogenic mechanism for squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2007;16:465-467. doi:10.1111/j.1600-0625.2007.00563.x
- Ortiz-Urda S, Garcia J, Green CL, et al. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773-1776. doi:10.1126/science.1106209
- Pourreyron C, Chen M, McGrath JA, et al. High levels of type VII collagen expression in recessive dystrophic epidermolysis bullosa cutaneous squamous cell carcinoma keratinocytes increases PI3K and MAPK signalling, cell migration and invasion. Br J Dermatol. 2014;170:1256-1265. doi:10.1111/bjd.12715
- Purdie KJ, Pourreyron C, Fassihi H, et al. No evidence that human papillomavirus is responsible for the aggressive nature of recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2010;130:2853-2855. doi:10.1038/jid.2010.243
- South AP, O’Toole EA. Understanding the pathogenesis of recessive dystrophic epidermolysis bullosa squamous cell carcinoma. Dermatol Clin. 2010;28:171-178. doi:10.1016/j.det.2009.10.023
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. doi:10.1002/ijc.23173
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436-444. doi:10.1038/nature07205
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi:10.3389/fimmu.2011.00098
- Tebbe B. Clinical course and prognosis of cutaneous lupus erythematosus. Clin Dermatol. 2004;22:121-124. doi:10.1016/j.clindermatol.2003.12.018
- Tao J, Zhang X, Guo N, et al. Squamous cell carcinoma complicating discoid lupus erythematosus in Chinese patients: review of the literature, 1964-2010. J Am Acad Dermatol. 2012;66:695-696. doi:10.1016 /j.jaad.2011.09.033
- Fernandes MS, Girisha BS, Viswanathan N, et al. Discoid lupus erythematosus with squamous cell carcinoma: a case report and review of the literature in Indian patients. Lupus. 2015;24:1562-1566. doi:10.1177/0961203315599245
- Makita E, Akasaka E, Sakuraba Y, et al. Squamous cell carcinoma on the lip arising from discoid lupus erythematosus: a case report and review of Japanese patients. Eur J Dermatol. 2016;26:395-396. doi:10.1684/ejd.2016.2780
- Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23:759-765. doi:10.1200/JCO.2005.02.155
- Arvanitidou I-E, Nikitakis NG, Georgaki M, et al. Multiple primary squamous cell carcinomas of the lower lip and tongue arising in discoid lupus erythematosus: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:e22-e30. doi:10.1016/j.oooo.2017.08.012
- Alsanafi S, Werth VP. Squamous cell carcinomas arising in discoid lupus erythematosus scars: unusual occurrence in an African-American and in a sun-protected area. J Clin Rheumatol. 2011;17:35-36. doi:10.1097/RHU.0b013e3182051928
- Goobie GC, Bernatsky S, Ramsey-Goldman R, et al. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol. 2015;27:454-460. doi:10.1097/BOR.0000000000000202
- Simpson JK, Medina-Flores R, Deng J-S. Squamous cell carcinoma arising in discoid lupus erythematosus lesions of the ears infected with human papillomavirus. Cutis. 2010;86:195-198.
- Sigurgeirsson B, B. Lichen planus and malignancy. an epidemiologic study of 2071 patients and a review of the literature. Arch Dermatol. 1991;127:1684-1688. doi:10.1001/archderm.127.11.1684
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45-56. doi:10.14219/jada.2013.10
- Laniosz V, Torgerson RR, Ramos-Rodriguez AJ, et al. Incidence of squamous cell carcinoma in oral lichen planus: a 25-year population-based study. Int J Dermatol. 2019;58:296-301. doi:10.1111/ijd.14215
- Aghbari SMH, Abushouk AI, Attia A, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017;68:92-102. doi:10.1016/j.oraloncology.2017.03.012
- Morita M, Asoda S, Tsunoda K, et al. The onset risk of carcinoma in patients continuing tacrolimus topical treatment for oral lichen planus: a case report. Odontology. 2017;105:262-266. doi:10.1007/s10266-016-0255-4
- Knackstedt TJ, Collins LK, Li Z, et al. Squamous cell carcinoma arising in hypertrophic lichen planus: a review and analysis of 38 cases. Dermatol Surg. 2015;41:1411-1418. doi:10.1097/DSS.0000000000000565
- Tong LX, Weinstock MJ, Drews R, et al. Widely metastatic squamous cell carcinoma originating from malignant transformation of hypertrophic lichen planus in a 24-year-old woman: case report and review of the literature. Pediatr Dermatol. 2015;32:e98-e101. doi:10.1111/pde.12549
- Ardabili M, Gambichler T, Rotterdam S, et al. Metastatic cutaneous squamous cell carcinoma arising from a previous area of chronic hypertrophic lichen planus. Dermatol Online J. 2003;9:10.
- Bowen AR, Burt L, Boucher K, et al. Use of proliferation rate, p53 staining and perforating elastic fibers in distinguishing keratoacanthoma from hypertrophic lichen planus: a pilot study. J Cutan Pathol. 2012;39:243-250. doi:10.1111/j.1600-0560.2011.01834.x
- Totonchy MB, Leventhal JS, Ko CJ, et al. Hypertrophic lichen planus and well-differentiated squamous cell carcinoma: a diagnostic conundrum. Dermatol Surg. 2018;44:1466-1470. doi:10.1097/DSS.0000000000001465
- Levandoski KA, Nazarian RM, Asgari MM. Hypertrophic lichen planus mimicking squamous cell carcinoma: the importance of clinicopathologic correlation. JAAD Case Rep. 2017;3:151-154. doi: 10.1016/j.jdcr.2017.01.020
- Okiyama N, Satoh T, Yokozeki H, et al. Squamous cell carcinoma arising from lichen planus of nail matrix and nail bed. J Am Acad Dermatol. 2005;53:908-909. doi:10.1016/j.jaad.2005.04.052
- Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011;64:1147-1153. doi:10.1016/j.jaad.2010.02.057
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370. doi:10.1016/j.jaad.2019.03.070
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416. doi:10.1016/0190-9622(95)90060-8
- Leibowitch M, Neill S, Pelisse M, et al. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990;97:1135-1139. doi:10.1111/j.1471-0528.1990.tb02502.x
- Bleeker MCG, Visser PJ, Overbeek LIH, et al. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:1224-1230. doi:10.1158/1055-9965.EPI-16-0019
- Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol. 1998;29:932-948. doi:10.1016/s0046-8177(98)90198-8
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183. doi:10.1097/LGT.0000000000000186
- Cooper SM, Madnani N, Margesson L. Reduced risk of squamous cell carcinoma with adequate treatment of vulvar lichen sclerosus. JAMA Dermatol. 2015;151:1059-1060. doi:10.1001/jamadermatol.2015.0644
- Rakislova N, Alemany L, Clavero O, et al; VVAP Study Group. Differentiated vulvar intraepithelial neoplasia-like and lichen sclerosus-like lesions in HPV-associated squamous cell carcinomas of the vulva. Am J Surg Pathol. 2018;42:828-835. doi:10.1097/PAS.0000000000001047
- Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48:808-817. doi:10.1097/01.grf.0000179635.64663.3d
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067. doi:10.1001/jamadermatol.2015.0643
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, et al. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140:709-712. doi:10.1001/archderm.140.6.709
- Minhas S, Manseck A, Watya S, et al. Penile cancer—prevention and premalignant conditions. Urology. 2010;76(2 suppl 1):S24-S35. doi:10.1016/j.urology.2010.04.007
- Nasca MR, Innocenzi D, Micali G. Penile cancer among patients with genital lichen sclerosus. J Am Acad Dermatol. 1999;41:911-914. doi:10.1016/s0190-9622(99)70245-8
- Philippou P, Shabbir M, Ralph DJ, et al. Genital lichen sclerosus/balanitis xerotica obliterans in men with penile carcinoma: a critical analysis. BJU Int. 2013;111:970-976. doi:10.1111/j.1464-410X.2012.11773.x
- Velazquez EF, Cubilla AL. Lichen sclerosus in 68 patients with squamous cell carcinoma of the penis: frequent atypias and correlation with special carcinoma variants suggests a precancerous role. Am J Surg Pathol. 2003;27:1448-1453. doi:10.1097/00000478-200311000-00007
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64. doi:10.1016/j.jcws.2012.04.001
- Aydogdu E, Yildirim S, Akoz T. Is surgery an effective and adequate treatment in advanced Marjolin’s ulcer? Burns. 2005;31:421-431. doi:10.1016/j.burns.2005.02.008
- Xiao H, Deng K, Liu R, et al. A review of 31 cases of Marjolin’s ulcer on scalp: is it necessary to preventively remove the scar? Int Wound J. 2019;16:479-485. doi:10.1111/iwj.13058
- Chaturvedi G, Gupta AK, Das S, et al. Marjolin ulcer: an observational epidemiological study from a tertiary care centre in India. Ann Plast Surg. 2019;83:518-522. doi:10.1097/SAP.0000000000001995
- Karasoy Yesilada A, Zeynep Sevim K, D, et al. Marjolin ulcer: clinical experience with 34 patients over 15 years. J Cutan Med Surg. 2013;17:404-409. doi:10.2310/7750.2013.13016
- D, Przybek-Mita J, B, et al. Marjolin’s ulcer in chronic wounds - review of available literature. Contemp Oncol (Pozn). 2017;21:197-202. doi:10.5114/wo.2017.70109
- Visuthikosol V, Boonpucknavig V, Nitiyanant P. Squamous carcinoma in scars: clinicopathological correlations. Ann Plast Surg. 1986;16:42-48. doi:10.1097/00000637-198601000-00004
- Bostwick J 3rd, Pendergrast WJ Jr, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976;57:66-69.
- Kerr-Valentic MA, Samimi K, Rohlen BH, et al. Marjolin’s ulcer: modern analysis of an ancient problem. Plast Reconstr Surg. 2009;123:184-191. doi:10.1097/PRS.0b013e3181904d86
- Constantinou C, Widom K, Desantis J, et al. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg. 2008;74:1177-1181.
- Fabbrocini G, Ruocco E, De Vita V, et al. Squamous cell carcinoma arising in long-standing hidradenitis suppurativa: an overlooked facet of the immunocompromised district. Clin Dermatol. 2017;35:225-227. doi:10.1016/j.clindermatol.2016.10.019
- Baroni A, Buommino E, Piccolo V, et al. Alterations of skin innate immunity in lymphedematous limbs: correlations with opportunistic diseases. Clin Dermatol. 2014;32:592-598. doi:10.1016/j.clindermatol.2014.04.006
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526. doi:10.1097/DSS.0000000000001713
- Huang C, Lai Z, He M, et al. Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa: a case report and literature review. Medicine (Baltimore). 2017;96:e5857. doi:10.1097/MD.0000000000005857
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153. doi:10.1159/000269836
- Makris G-M, Poulakaki N, Papanota A-M, et al. Vulvar, perianal and perineal cancer after hidradenitis suppurativa: a systematic review and pooled analysis. Dermatol Surg. 2017;43:107-115. doi:10.1097/DSS.0000000000000944
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419. doi:10.1016/j.jaad.2012.07.027
- Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48:2046-2053. doi:10.1016/j.ejca.2012.01.003
- Uva L, Freitas J, Soares de Almeida L, et al. Squamous cell carcinoma arising in ulcerated necrobiosis lipoidica diabeticorum. Int Wound J. 2015;12:741-743. doi:10.1111/iwj.12206
- McGrath JA, Schofield OM, Mayou BJ, et al. Epidermolysis bullosa complicated by squamous cell carcinoma: report of 10 cases. J Cutan Pathol. 1992;19:116-123. doi:10.1111/j.1600-0560.1992.tb01352.x
- H, Chiaverini C, Sbidian E, et al. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis. 2016;11:117. doi:10.1186/s13023-016-0489-9.
- Fine J-D. Inherited epidermolysis bullosa: past, present, and future. Ann N Y Acad Sci. 2010;1194:213-222. doi:10.1111/j.1749-6632.2010.05463.x
- Kim M, Li M, Intong-Wheeler LRA, et al. Epidemiology and outcome of squamous cell carcinoma in epidermolysis bullosa in Australia and New Zealand. Acta Derm Venereol. 2018;98:70-76. doi:10.2340/00015555-2781
- Bruckner-Tuderman L, Mitsuhashi Y, Schnyder UW, et al. Anchoring fibrils and type VII collagen are absent from skin in severe recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1989;93:3-9. doi:10.1111/1523-1747.ep12277331
- Ng Y-Z, Pourreyron C, Salas-Alanis JC, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. 2012;72:3522-3534. doi:10.1158/0008-5472.CAN-11-2996
- Arbiser JL, Fan C-Y, Su X, et al. Involvement of p53 and p16 tumor suppressor genes in recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2004;123:788-790. doi:10.1111/j.0022-202X.2004.23418.x
- Knaup J, Gruber C, Krammer B, et al. TGFbeta-signaling in squamous cell carcinoma occurring in recessive dystrophic epidermolysis bullosa. Anal Cell Pathol (Amst). 2011;34:339-353. doi:10.3233/ACP-2011-0039
- Kivisaari AK, Kallajoki M, Mirtti T, et al. Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol. 2008;158:778-785. doi:10.1111/j.1365-2133.2008.08466.x
- Rodeck U, Fertala A, Uitto J. Anchorless keratinocyte survival: an emerging pathogenic mechanism for squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2007;16:465-467. doi:10.1111/j.1600-0625.2007.00563.x
- Ortiz-Urda S, Garcia J, Green CL, et al. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773-1776. doi:10.1126/science.1106209
- Pourreyron C, Chen M, McGrath JA, et al. High levels of type VII collagen expression in recessive dystrophic epidermolysis bullosa cutaneous squamous cell carcinoma keratinocytes increases PI3K and MAPK signalling, cell migration and invasion. Br J Dermatol. 2014;170:1256-1265. doi:10.1111/bjd.12715
- Purdie KJ, Pourreyron C, Fassihi H, et al. No evidence that human papillomavirus is responsible for the aggressive nature of recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2010;130:2853-2855. doi:10.1038/jid.2010.243
- South AP, O’Toole EA. Understanding the pathogenesis of recessive dystrophic epidermolysis bullosa squamous cell carcinoma. Dermatol Clin. 2010;28:171-178. doi:10.1016/j.det.2009.10.023
PRACTICE POINTS
- Squamous cell carcinoma can develop within chronic inflammatory dermatoses.
- Orolabial discoid lupus erythematosus (DLE), oral lichen planus, and lichen sclerosus can lead to relatively rapid tumorigenesis. Squamous cell carcinoma arising in cutaneous DLE, hidradenitis suppurativa (HS), necrobiosis lipoidica, chronic wounds, and hypertrophic lichen planus tends to appear after decades of inflammation.
- Be especially mindful of new orolabial DLE cases and chronic cases of HS and Marjolin ulcer because malignancies in these settings are particularly aggressive.
Multiple New-Onset Pyogenic Granulomas During Treatment With Paclitaxel and Ramucirumab
To the Editor:
Pyogenic granuloma (PG) is a benign vascular tumor that clinically is characterized as a small eruptive friable papule.1 Lesions typically are solitary and most commonly occur in children but also are associated with pregnancy; trauma to the skin or mucosa; and use of certain medications such as isotretinoin, capecitabine, vemurafenib, or indinavir.1 Numerous antineoplastic medications have been associated with the development of solitary PGs, including the taxane mitotic inhibitor paclitaxel (PTX) and the vascular endothelial growth factor receptor 2 (VEGFR2) monoclonal antibody ramucirumab.2 We report a case of multiple PGs in a patient undergoing treatment with PTX and ramucirumab.

A 59-year-old woman presented to the dermatology clinic with red, itchy, bleeding skin lesions on the breast, superior chest, left cheek, and forearm of 1 month’s duration. She denied any preceding trauma to the areas. Her medical history was notable for gastroesophageal junction adenocarcinoma diagnosed more than 2 years prior to presentation. Her original treatment regimen included nivolumab, which was discontinued for unknown reasons 5 months prior to presentation, and she was started on combination therapy with PTX and ramucirumab at that time. She noted the formation of small red papules 2 months after the initiation of PTX-ramucirumab combination therapy, which grew larger over the course of the next month. Physical examination revealed 5 friable hemorrhagic papules and nodules ranging in size from 3 to 10 mm on the chest, cheek, and forearm consistent with PGs (Figure 1). Several scattered cherry angiomas were noted on the scalp and torso, but the patient reported these were not new. Biopsies of the PGs demonstrated lobular aggregates of small-caliber vessels set in an edematous inflamed stroma and partially enclosed by small collarettes of adnexal epithelium, confirming the clinical diagnosis of multiple PGs (Figure 2).
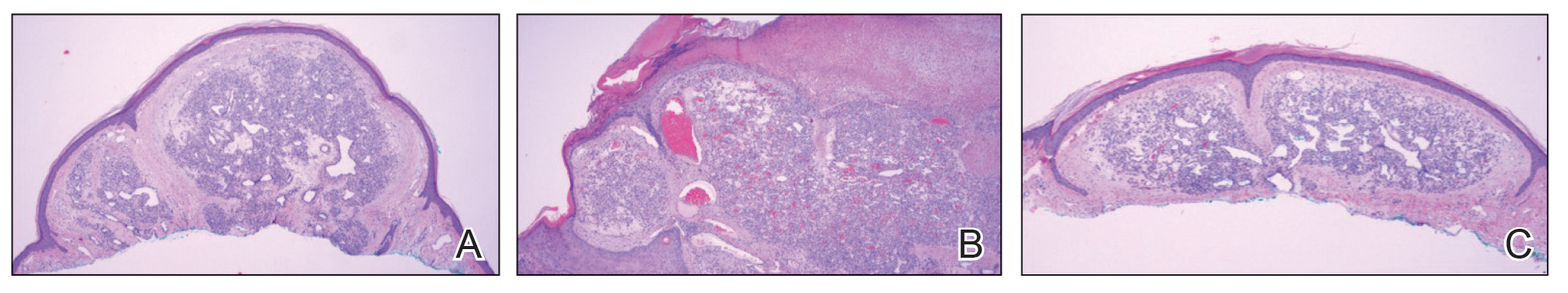
The first case of PTX-associated PG was reported in 2012.3 Based on a PubMed search of articles indexed for MEDLINE using the terms pyogenic granuloma, lobular capillary hemangioma, paclitaxel, taxane, and ramucirumab, there have been 9 cases of solitary PG development in the setting of PTX alone or in combination with ramucirumab since 2019 (Table).3-8 Pyogenic granulomas reported in patients who were treated exclusively with PTX were subungual, while the cases resulting from combined therapy were present on the scalp, face, oral mucosa, and surfaces of the hands sparing the nails. Ibe et al6 reported PG in a patient who received ramucirumab therapy without PTX but in combination with another taxane, docetaxel, which itself has been reported to cause subungual PG when used alone.9 Our case of the simultaneous development of multiple PGs in the setting of combined PTX and ramucirumab therapy added to the cutaneous distributions for which therapy-induced PGs have been observed (Table).

The development of PG, a vascular tumor, during treatment with the VEGFR2 inhibitor ramucirumab—whose mechanism of action is to inhibit angioneogenesis—is inherently paradoxical. In 2015, a rapidly expanding angioma with a mutation in the kinase domain receptor gene, KDR, that encodes VEGFR2 was identified in a patient undergoing ramucirumab therapy. The authors suggested that KDR mutation resulted in paradoxical activation of VEGFR2 in the setting of ramucirumab therapy.10 Since then, ramucirumab and PTX were suggested to have a synergistic effect in vascular proliferation,5 though an exact mechanism has not been proposed. Other authors have identified increased expression of VEGFR2 in biopsy specimens of PG during combined ramucirumab and taxane therapy.6 Although genetic studies have not been used to evaluate for the presence of KDR mutations specifically in our patient population, it is possible that patients who develop PG and other vascular tumors during combined taxane and ramucirumab therapy have a mutation that makes them more susceptible to VEGFR2 upregulation. UV exposure may have a role in the formation of PG in patients on combined ramucirumab and taxane therapy7; however, our patient’s lesions were distributed on both sun-exposed and unexposed areas. Although potential clinical implications have not yet been thoroughly investigated, following long-term outcomes for these patients may provide important information on the efficacy of the antineoplastic regimen in the subset of patients who develop cutaneous vascular tumors during antiangiogenic treatment.
Combination therapy with PTX and ramucirumab has been associated with the paradoxical development of cutaneous vascular tumors. We report a case of multiple new-onset PGs in a patient undergoing this treatment regimen.
- Elston D, Neuhaus I, James WD, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Pierson JC. Pyogenic granuloma (lobular capillary hemangioma) clinical presentation. Medscape. Updated February 21, 2020. Accessed December 26, 2023. https://emedicine.medscape.com/article/1084701-clinical#showall
- Paul LJ, Cohen PR. Paclitaxel-associated subungual pyogenic granuloma: report in a patient with breast cancer receiving paclitaxel and review of drug-induced pyogenic granulomas adjacent to and beneath the nail. J Drugs Dermatol. 2012;11:262-268.
- Alessandrini A, Starace M, Cerè G, et al. Management and outcome of taxane-induced nail side effects: experience of 79 patients from a single centre. Skin Appendage Disord. 2019;5:276-282.
- Watanabe R, Nakano E, Kawazoe A, et al. Four cases of paradoxical cephalocervical pyogenic granuloma during treatment with paclitaxel and ramucirumab. J Dermatol. 2019;46:E178-E180.
- Ibe T, Hamamoto Y, Takabatake M, et al. Development of pyogenic granuloma with strong vascular endothelial growth factor receptor-2 expression during ramucirumab treatment. BMJ Case Rep. 2019;12:E231464.
- Choi YH, Byun HJ, Lee JH, et al. Multiple cherry angiomas and pyogenic granuloma in a patient treated with ramucirumab and paclitaxel. Indian J Dermatol Venereol Leprol. 2020;86:199-202.
- Aragaki T, Tomomatsu N, Michi Y, et al. Ramucirumab-related oral pyogenic granuloma: a report of two cases [published online March 8, 2021]. Intern Med. 2021;60:2601-2605. doi:10.2169/internalmedicine.6650-20
- Devillers C, Vanhooteghem O, Henrijean A, et al. Subungual pyogenic granuloma secondary to docetaxel therapy. Clin Exp Dermatol. 2009;34:251-252.
- Lim YH, Odell ID, Ko CJ, et al. Somatic p.T771R KDR (VEGFR2) mutation arising in a sporadic angioma during ramucirumab therapy. JAMA Dermatol. 2015;151:1240-1243.
To the Editor:
Pyogenic granuloma (PG) is a benign vascular tumor that clinically is characterized as a small eruptive friable papule.1 Lesions typically are solitary and most commonly occur in children but also are associated with pregnancy; trauma to the skin or mucosa; and use of certain medications such as isotretinoin, capecitabine, vemurafenib, or indinavir.1 Numerous antineoplastic medications have been associated with the development of solitary PGs, including the taxane mitotic inhibitor paclitaxel (PTX) and the vascular endothelial growth factor receptor 2 (VEGFR2) monoclonal antibody ramucirumab.2 We report a case of multiple PGs in a patient undergoing treatment with PTX and ramucirumab.

A 59-year-old woman presented to the dermatology clinic with red, itchy, bleeding skin lesions on the breast, superior chest, left cheek, and forearm of 1 month’s duration. She denied any preceding trauma to the areas. Her medical history was notable for gastroesophageal junction adenocarcinoma diagnosed more than 2 years prior to presentation. Her original treatment regimen included nivolumab, which was discontinued for unknown reasons 5 months prior to presentation, and she was started on combination therapy with PTX and ramucirumab at that time. She noted the formation of small red papules 2 months after the initiation of PTX-ramucirumab combination therapy, which grew larger over the course of the next month. Physical examination revealed 5 friable hemorrhagic papules and nodules ranging in size from 3 to 10 mm on the chest, cheek, and forearm consistent with PGs (Figure 1). Several scattered cherry angiomas were noted on the scalp and torso, but the patient reported these were not new. Biopsies of the PGs demonstrated lobular aggregates of small-caliber vessels set in an edematous inflamed stroma and partially enclosed by small collarettes of adnexal epithelium, confirming the clinical diagnosis of multiple PGs (Figure 2).

The first case of PTX-associated PG was reported in 2012.3 Based on a PubMed search of articles indexed for MEDLINE using the terms pyogenic granuloma, lobular capillary hemangioma, paclitaxel, taxane, and ramucirumab, there have been 9 cases of solitary PG development in the setting of PTX alone or in combination with ramucirumab since 2019 (Table).3-8 Pyogenic granulomas reported in patients who were treated exclusively with PTX were subungual, while the cases resulting from combined therapy were present on the scalp, face, oral mucosa, and surfaces of the hands sparing the nails. Ibe et al6 reported PG in a patient who received ramucirumab therapy without PTX but in combination with another taxane, docetaxel, which itself has been reported to cause subungual PG when used alone.9 Our case of the simultaneous development of multiple PGs in the setting of combined PTX and ramucirumab therapy added to the cutaneous distributions for which therapy-induced PGs have been observed (Table).

The development of PG, a vascular tumor, during treatment with the VEGFR2 inhibitor ramucirumab—whose mechanism of action is to inhibit angioneogenesis—is inherently paradoxical. In 2015, a rapidly expanding angioma with a mutation in the kinase domain receptor gene, KDR, that encodes VEGFR2 was identified in a patient undergoing ramucirumab therapy. The authors suggested that KDR mutation resulted in paradoxical activation of VEGFR2 in the setting of ramucirumab therapy.10 Since then, ramucirumab and PTX were suggested to have a synergistic effect in vascular proliferation,5 though an exact mechanism has not been proposed. Other authors have identified increased expression of VEGFR2 in biopsy specimens of PG during combined ramucirumab and taxane therapy.6 Although genetic studies have not been used to evaluate for the presence of KDR mutations specifically in our patient population, it is possible that patients who develop PG and other vascular tumors during combined taxane and ramucirumab therapy have a mutation that makes them more susceptible to VEGFR2 upregulation. UV exposure may have a role in the formation of PG in patients on combined ramucirumab and taxane therapy7; however, our patient’s lesions were distributed on both sun-exposed and unexposed areas. Although potential clinical implications have not yet been thoroughly investigated, following long-term outcomes for these patients may provide important information on the efficacy of the antineoplastic regimen in the subset of patients who develop cutaneous vascular tumors during antiangiogenic treatment.
Combination therapy with PTX and ramucirumab has been associated with the paradoxical development of cutaneous vascular tumors. We report a case of multiple new-onset PGs in a patient undergoing this treatment regimen.
To the Editor:
Pyogenic granuloma (PG) is a benign vascular tumor that clinically is characterized as a small eruptive friable papule.1 Lesions typically are solitary and most commonly occur in children but also are associated with pregnancy; trauma to the skin or mucosa; and use of certain medications such as isotretinoin, capecitabine, vemurafenib, or indinavir.1 Numerous antineoplastic medications have been associated with the development of solitary PGs, including the taxane mitotic inhibitor paclitaxel (PTX) and the vascular endothelial growth factor receptor 2 (VEGFR2) monoclonal antibody ramucirumab.2 We report a case of multiple PGs in a patient undergoing treatment with PTX and ramucirumab.

A 59-year-old woman presented to the dermatology clinic with red, itchy, bleeding skin lesions on the breast, superior chest, left cheek, and forearm of 1 month’s duration. She denied any preceding trauma to the areas. Her medical history was notable for gastroesophageal junction adenocarcinoma diagnosed more than 2 years prior to presentation. Her original treatment regimen included nivolumab, which was discontinued for unknown reasons 5 months prior to presentation, and she was started on combination therapy with PTX and ramucirumab at that time. She noted the formation of small red papules 2 months after the initiation of PTX-ramucirumab combination therapy, which grew larger over the course of the next month. Physical examination revealed 5 friable hemorrhagic papules and nodules ranging in size from 3 to 10 mm on the chest, cheek, and forearm consistent with PGs (Figure 1). Several scattered cherry angiomas were noted on the scalp and torso, but the patient reported these were not new. Biopsies of the PGs demonstrated lobular aggregates of small-caliber vessels set in an edematous inflamed stroma and partially enclosed by small collarettes of adnexal epithelium, confirming the clinical diagnosis of multiple PGs (Figure 2).

The first case of PTX-associated PG was reported in 2012.3 Based on a PubMed search of articles indexed for MEDLINE using the terms pyogenic granuloma, lobular capillary hemangioma, paclitaxel, taxane, and ramucirumab, there have been 9 cases of solitary PG development in the setting of PTX alone or in combination with ramucirumab since 2019 (Table).3-8 Pyogenic granulomas reported in patients who were treated exclusively with PTX were subungual, while the cases resulting from combined therapy were present on the scalp, face, oral mucosa, and surfaces of the hands sparing the nails. Ibe et al6 reported PG in a patient who received ramucirumab therapy without PTX but in combination with another taxane, docetaxel, which itself has been reported to cause subungual PG when used alone.9 Our case of the simultaneous development of multiple PGs in the setting of combined PTX and ramucirumab therapy added to the cutaneous distributions for which therapy-induced PGs have been observed (Table).

The development of PG, a vascular tumor, during treatment with the VEGFR2 inhibitor ramucirumab—whose mechanism of action is to inhibit angioneogenesis—is inherently paradoxical. In 2015, a rapidly expanding angioma with a mutation in the kinase domain receptor gene, KDR, that encodes VEGFR2 was identified in a patient undergoing ramucirumab therapy. The authors suggested that KDR mutation resulted in paradoxical activation of VEGFR2 in the setting of ramucirumab therapy.10 Since then, ramucirumab and PTX were suggested to have a synergistic effect in vascular proliferation,5 though an exact mechanism has not been proposed. Other authors have identified increased expression of VEGFR2 in biopsy specimens of PG during combined ramucirumab and taxane therapy.6 Although genetic studies have not been used to evaluate for the presence of KDR mutations specifically in our patient population, it is possible that patients who develop PG and other vascular tumors during combined taxane and ramucirumab therapy have a mutation that makes them more susceptible to VEGFR2 upregulation. UV exposure may have a role in the formation of PG in patients on combined ramucirumab and taxane therapy7; however, our patient’s lesions were distributed on both sun-exposed and unexposed areas. Although potential clinical implications have not yet been thoroughly investigated, following long-term outcomes for these patients may provide important information on the efficacy of the antineoplastic regimen in the subset of patients who develop cutaneous vascular tumors during antiangiogenic treatment.
Combination therapy with PTX and ramucirumab has been associated with the paradoxical development of cutaneous vascular tumors. We report a case of multiple new-onset PGs in a patient undergoing this treatment regimen.
- Elston D, Neuhaus I, James WD, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Pierson JC. Pyogenic granuloma (lobular capillary hemangioma) clinical presentation. Medscape. Updated February 21, 2020. Accessed December 26, 2023. https://emedicine.medscape.com/article/1084701-clinical#showall
- Paul LJ, Cohen PR. Paclitaxel-associated subungual pyogenic granuloma: report in a patient with breast cancer receiving paclitaxel and review of drug-induced pyogenic granulomas adjacent to and beneath the nail. J Drugs Dermatol. 2012;11:262-268.
- Alessandrini A, Starace M, Cerè G, et al. Management and outcome of taxane-induced nail side effects: experience of 79 patients from a single centre. Skin Appendage Disord. 2019;5:276-282.
- Watanabe R, Nakano E, Kawazoe A, et al. Four cases of paradoxical cephalocervical pyogenic granuloma during treatment with paclitaxel and ramucirumab. J Dermatol. 2019;46:E178-E180.
- Ibe T, Hamamoto Y, Takabatake M, et al. Development of pyogenic granuloma with strong vascular endothelial growth factor receptor-2 expression during ramucirumab treatment. BMJ Case Rep. 2019;12:E231464.
- Choi YH, Byun HJ, Lee JH, et al. Multiple cherry angiomas and pyogenic granuloma in a patient treated with ramucirumab and paclitaxel. Indian J Dermatol Venereol Leprol. 2020;86:199-202.
- Aragaki T, Tomomatsu N, Michi Y, et al. Ramucirumab-related oral pyogenic granuloma: a report of two cases [published online March 8, 2021]. Intern Med. 2021;60:2601-2605. doi:10.2169/internalmedicine.6650-20
- Devillers C, Vanhooteghem O, Henrijean A, et al. Subungual pyogenic granuloma secondary to docetaxel therapy. Clin Exp Dermatol. 2009;34:251-252.
- Lim YH, Odell ID, Ko CJ, et al. Somatic p.T771R KDR (VEGFR2) mutation arising in a sporadic angioma during ramucirumab therapy. JAMA Dermatol. 2015;151:1240-1243.
- Elston D, Neuhaus I, James WD, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Pierson JC. Pyogenic granuloma (lobular capillary hemangioma) clinical presentation. Medscape. Updated February 21, 2020. Accessed December 26, 2023. https://emedicine.medscape.com/article/1084701-clinical#showall
- Paul LJ, Cohen PR. Paclitaxel-associated subungual pyogenic granuloma: report in a patient with breast cancer receiving paclitaxel and review of drug-induced pyogenic granulomas adjacent to and beneath the nail. J Drugs Dermatol. 2012;11:262-268.
- Alessandrini A, Starace M, Cerè G, et al. Management and outcome of taxane-induced nail side effects: experience of 79 patients from a single centre. Skin Appendage Disord. 2019;5:276-282.
- Watanabe R, Nakano E, Kawazoe A, et al. Four cases of paradoxical cephalocervical pyogenic granuloma during treatment with paclitaxel and ramucirumab. J Dermatol. 2019;46:E178-E180.
- Ibe T, Hamamoto Y, Takabatake M, et al. Development of pyogenic granuloma with strong vascular endothelial growth factor receptor-2 expression during ramucirumab treatment. BMJ Case Rep. 2019;12:E231464.
- Choi YH, Byun HJ, Lee JH, et al. Multiple cherry angiomas and pyogenic granuloma in a patient treated with ramucirumab and paclitaxel. Indian J Dermatol Venereol Leprol. 2020;86:199-202.
- Aragaki T, Tomomatsu N, Michi Y, et al. Ramucirumab-related oral pyogenic granuloma: a report of two cases [published online March 8, 2021]. Intern Med. 2021;60:2601-2605. doi:10.2169/internalmedicine.6650-20
- Devillers C, Vanhooteghem O, Henrijean A, et al. Subungual pyogenic granuloma secondary to docetaxel therapy. Clin Exp Dermatol. 2009;34:251-252.
- Lim YH, Odell ID, Ko CJ, et al. Somatic p.T771R KDR (VEGFR2) mutation arising in a sporadic angioma during ramucirumab therapy. JAMA Dermatol. 2015;151:1240-1243.
Practice Points
- Pyogenic granulomas (PGs) are benign vascular tumors that clinically are characterized as small, eruptive, friable papules.
- Ramucirumab is a monoclonal antibody against vascular endothelial growth factor receptor 2.
- Some patients experience paradoxical formation of vascular tumors such as PGs when treated with combination therapy with ramucirumab and a taxane such as paclitaxel.