User login
Is Being ‘Manly’ a Threat to a Man’s Health?
When my normally adorable cat Biscuit bit my ankle in a playful stalking exercise gone wrong, I washed it with soap and some rubbing alcohol, slapped on a Band-Aid, and went about my day.
The next morning, when it was swollen, I told myself it was probably just a hematoma and went about my day.
The next day, when the swelling had increased and red lines started creeping up my leg, I called my doctor. Long story short, I ended up hospitalized for intravenous antibiotics.
This is all to say that, yes, I’m sort of an idiot, but also to introduce the idea that maybe I minimized my very obvious lymphangitis because I am a man.
This week, we have empirical evidence that men downplay their medical symptoms — and that manlier men downplay them even more.
I’m going to talk about a study that links manliness (or, scientifically speaking, “male gender expressivity”) to medical diagnoses that are based on hard evidence and medical diagnoses that are based on self-report. You see where this is going but I want to walk you through the methods here because they are fairly interesting.
This study used data from the US National Longitudinal Study of Adolescent to Adult Health. This study enrolled 20,000 adolescents who were in grades 7-12 in the 1994-1995 school year and has been following them ever since — about 30 years so far.
The authors wanted to link early gender roles to long-term outcomes, so they cut that 20,000 number down to the 4230 males in the group who had complete follow-up.
Now comes the first interesting question. How do you quantify the “male gender expressivity” of boys in 7th-12th grade? There was no survey item that asked them how masculine or manly they felt. What the authors did was look at the surveys that were administered and identify the questions on those surveys where boys and girls gave the most disparate answers. I have some examples here. 
Some of these questions make sense when it comes to gender expressivity: “How often do you cry?” for example, has a lot of validity for the social construct that is gender. But some questions where boys and girls gave very different answers — like “How often do you exercise?” — don’t quite fit that mold. Regardless, this structure allowed the researchers to take individual kids’ responses to these questions and combine them into what amounts to a manliness score — how much their answers aligned with the typical male answer.
The score was established in adolescence — which is interesting because I’m sure some of this stuff may change over time — but notable because adolescence is where many gender roles develop.
Now we can fast-forward 30 years and see how these manliness scores link to various outcomes. The authors were interested in fairly common diseases: diabetes, hypertension, and hyperlipidemia.
Let’s start simply. Are males with higher gender expressivity in adolescence more or less likely to have these diseases in the future?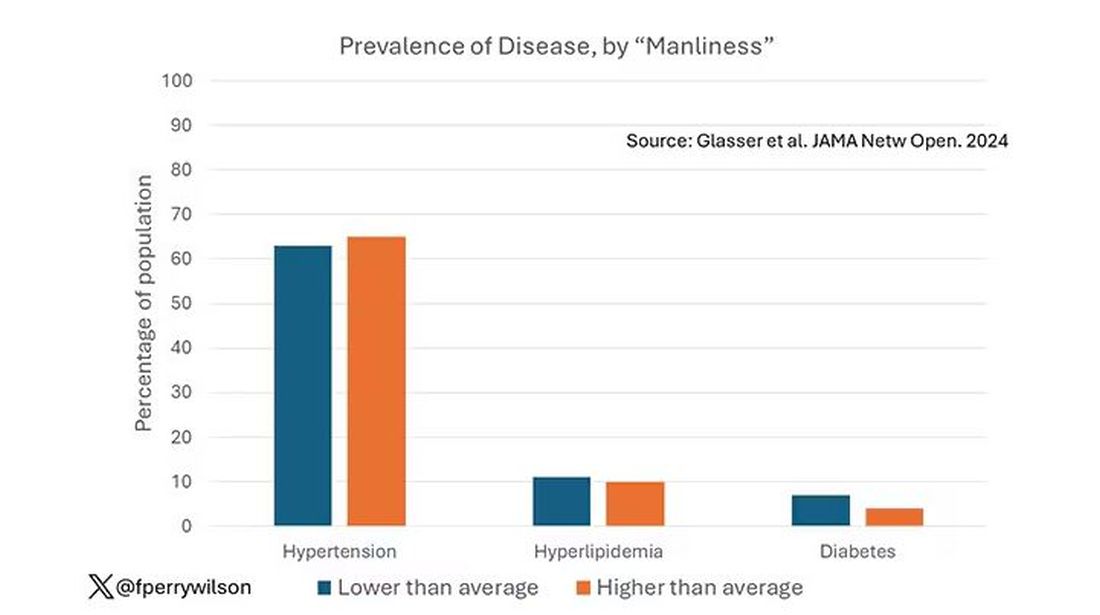
Not really. Those above the average in male gender expressivity had similar rates of hypertension and hyperlipidemia as those below the median. They were actually a bit less likely to have diabetes.
But that’s not what’s really interesting here.
I told you that there was no difference in the rate of hypertension among those with high vs low male gender expressivity. But there was a significant difference in their answer to the question “Do you have hypertension?” The same was seen for hyperlipidemia. In other words, those with higher manliness scores are less likely to admit (or perhaps know) that they have a particular disease.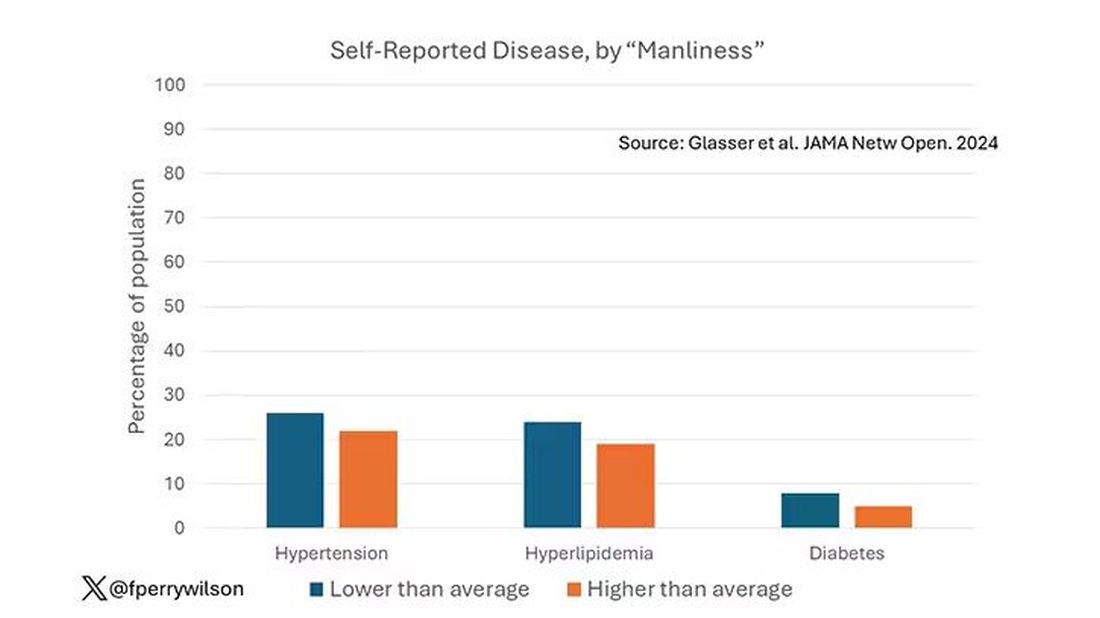
You can see the relationship across the manliness spectrum here in a series of adjusted models. The x-axis is the male gender expressivity score, and the y-axis is the percentage of people who report having the disease that we know they have based on the actual laboratory tests or vital sign measurements. As manliness increases, the self-report of a given disease decreases.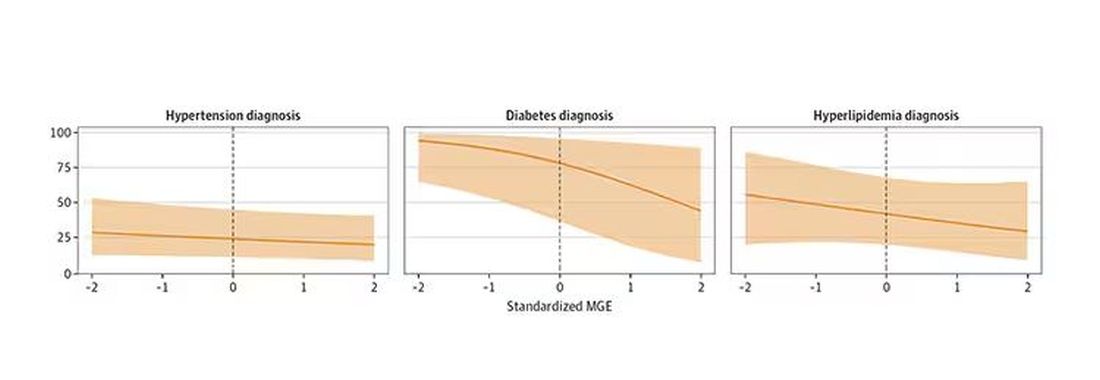
There are some important consequences of this systematic denial. Specifically, men with the diseases of interest who have higher male gender expressivity are less likely to get treatment. And, as we all know, the lack of treatment of something like hypertension puts people at risk for bad downstream outcomes.
Putting this all together, I’m not that surprised. Society trains boys from a young age to behave in certain ways: to hide emotions, to eschew vulnerability, to not complain when we are hurt. And those lessons can persist into later life. Whether the disease that strikes is hypertension or Pasteurella multocida from a slightly psychotic house cat, men are more likely to ignore it, to their detriment.
So, gents, be brave. Get your blood tests and check your blood pressure. If there’s something wrong, admit it, and fix it. After all, fixing problems — that’s a manly thing, right?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
When my normally adorable cat Biscuit bit my ankle in a playful stalking exercise gone wrong, I washed it with soap and some rubbing alcohol, slapped on a Band-Aid, and went about my day.
The next morning, when it was swollen, I told myself it was probably just a hematoma and went about my day.
The next day, when the swelling had increased and red lines started creeping up my leg, I called my doctor. Long story short, I ended up hospitalized for intravenous antibiotics.
This is all to say that, yes, I’m sort of an idiot, but also to introduce the idea that maybe I minimized my very obvious lymphangitis because I am a man.
This week, we have empirical evidence that men downplay their medical symptoms — and that manlier men downplay them even more.
I’m going to talk about a study that links manliness (or, scientifically speaking, “male gender expressivity”) to medical diagnoses that are based on hard evidence and medical diagnoses that are based on self-report. You see where this is going but I want to walk you through the methods here because they are fairly interesting.
This study used data from the US National Longitudinal Study of Adolescent to Adult Health. This study enrolled 20,000 adolescents who were in grades 7-12 in the 1994-1995 school year and has been following them ever since — about 30 years so far.
The authors wanted to link early gender roles to long-term outcomes, so they cut that 20,000 number down to the 4230 males in the group who had complete follow-up.
Now comes the first interesting question. How do you quantify the “male gender expressivity” of boys in 7th-12th grade? There was no survey item that asked them how masculine or manly they felt. What the authors did was look at the surveys that were administered and identify the questions on those surveys where boys and girls gave the most disparate answers. I have some examples here. 
Some of these questions make sense when it comes to gender expressivity: “How often do you cry?” for example, has a lot of validity for the social construct that is gender. But some questions where boys and girls gave very different answers — like “How often do you exercise?” — don’t quite fit that mold. Regardless, this structure allowed the researchers to take individual kids’ responses to these questions and combine them into what amounts to a manliness score — how much their answers aligned with the typical male answer.
The score was established in adolescence — which is interesting because I’m sure some of this stuff may change over time — but notable because adolescence is where many gender roles develop.
Now we can fast-forward 30 years and see how these manliness scores link to various outcomes. The authors were interested in fairly common diseases: diabetes, hypertension, and hyperlipidemia.
Let’s start simply. Are males with higher gender expressivity in adolescence more or less likely to have these diseases in the future?
Not really. Those above the average in male gender expressivity had similar rates of hypertension and hyperlipidemia as those below the median. They were actually a bit less likely to have diabetes.
But that’s not what’s really interesting here.
I told you that there was no difference in the rate of hypertension among those with high vs low male gender expressivity. But there was a significant difference in their answer to the question “Do you have hypertension?” The same was seen for hyperlipidemia. In other words, those with higher manliness scores are less likely to admit (or perhaps know) that they have a particular disease.
You can see the relationship across the manliness spectrum here in a series of adjusted models. The x-axis is the male gender expressivity score, and the y-axis is the percentage of people who report having the disease that we know they have based on the actual laboratory tests or vital sign measurements. As manliness increases, the self-report of a given disease decreases.
There are some important consequences of this systematic denial. Specifically, men with the diseases of interest who have higher male gender expressivity are less likely to get treatment. And, as we all know, the lack of treatment of something like hypertension puts people at risk for bad downstream outcomes.
Putting this all together, I’m not that surprised. Society trains boys from a young age to behave in certain ways: to hide emotions, to eschew vulnerability, to not complain when we are hurt. And those lessons can persist into later life. Whether the disease that strikes is hypertension or Pasteurella multocida from a slightly psychotic house cat, men are more likely to ignore it, to their detriment.
So, gents, be brave. Get your blood tests and check your blood pressure. If there’s something wrong, admit it, and fix it. After all, fixing problems — that’s a manly thing, right?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
When my normally adorable cat Biscuit bit my ankle in a playful stalking exercise gone wrong, I washed it with soap and some rubbing alcohol, slapped on a Band-Aid, and went about my day.
The next morning, when it was swollen, I told myself it was probably just a hematoma and went about my day.
The next day, when the swelling had increased and red lines started creeping up my leg, I called my doctor. Long story short, I ended up hospitalized for intravenous antibiotics.
This is all to say that, yes, I’m sort of an idiot, but also to introduce the idea that maybe I minimized my very obvious lymphangitis because I am a man.
This week, we have empirical evidence that men downplay their medical symptoms — and that manlier men downplay them even more.
I’m going to talk about a study that links manliness (or, scientifically speaking, “male gender expressivity”) to medical diagnoses that are based on hard evidence and medical diagnoses that are based on self-report. You see where this is going but I want to walk you through the methods here because they are fairly interesting.
This study used data from the US National Longitudinal Study of Adolescent to Adult Health. This study enrolled 20,000 adolescents who were in grades 7-12 in the 1994-1995 school year and has been following them ever since — about 30 years so far.
The authors wanted to link early gender roles to long-term outcomes, so they cut that 20,000 number down to the 4230 males in the group who had complete follow-up.
Now comes the first interesting question. How do you quantify the “male gender expressivity” of boys in 7th-12th grade? There was no survey item that asked them how masculine or manly they felt. What the authors did was look at the surveys that were administered and identify the questions on those surveys where boys and girls gave the most disparate answers. I have some examples here. 
Some of these questions make sense when it comes to gender expressivity: “How often do you cry?” for example, has a lot of validity for the social construct that is gender. But some questions where boys and girls gave very different answers — like “How often do you exercise?” — don’t quite fit that mold. Regardless, this structure allowed the researchers to take individual kids’ responses to these questions and combine them into what amounts to a manliness score — how much their answers aligned with the typical male answer.
The score was established in adolescence — which is interesting because I’m sure some of this stuff may change over time — but notable because adolescence is where many gender roles develop.
Now we can fast-forward 30 years and see how these manliness scores link to various outcomes. The authors were interested in fairly common diseases: diabetes, hypertension, and hyperlipidemia.
Let’s start simply. Are males with higher gender expressivity in adolescence more or less likely to have these diseases in the future?
Not really. Those above the average in male gender expressivity had similar rates of hypertension and hyperlipidemia as those below the median. They were actually a bit less likely to have diabetes.
But that’s not what’s really interesting here.
I told you that there was no difference in the rate of hypertension among those with high vs low male gender expressivity. But there was a significant difference in their answer to the question “Do you have hypertension?” The same was seen for hyperlipidemia. In other words, those with higher manliness scores are less likely to admit (or perhaps know) that they have a particular disease.
You can see the relationship across the manliness spectrum here in a series of adjusted models. The x-axis is the male gender expressivity score, and the y-axis is the percentage of people who report having the disease that we know they have based on the actual laboratory tests or vital sign measurements. As manliness increases, the self-report of a given disease decreases.
There are some important consequences of this systematic denial. Specifically, men with the diseases of interest who have higher male gender expressivity are less likely to get treatment. And, as we all know, the lack of treatment of something like hypertension puts people at risk for bad downstream outcomes.
Putting this all together, I’m not that surprised. Society trains boys from a young age to behave in certain ways: to hide emotions, to eschew vulnerability, to not complain when we are hurt. And those lessons can persist into later life. Whether the disease that strikes is hypertension or Pasteurella multocida from a slightly psychotic house cat, men are more likely to ignore it, to their detriment.
So, gents, be brave. Get your blood tests and check your blood pressure. If there’s something wrong, admit it, and fix it. After all, fixing problems — that’s a manly thing, right?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Can Better Diet Improve Survival in Black Women With Ovarian Cancer?
TOPLINE:
No significant survival association was found among the full study sample, which included women with multiple types of epithelial ovarian cancer (EOC).
METHODOLOGY:
- Researchers conducted a prospective cohort study among 483 self-identified Black women aged 20-79 years newly diagnosed with histologically confirmed EOC between December 2010 and December 2015.
- The study aimed to examine associations between dietary patterns and survival among Black women diagnosed with EOC using data from the African American Cancer Epidemiology Study.
- Dietary patterns were assessed using the Healthy Eating Index–2020 (HEI-2020) and Alternative Healthy Eating Index–2010 (AHEI-2010), based on dietary intake in the year prior to diagnosis collected via the validated Block 2005 Food Frequency Questionnaire (FFQ). Participant characteristics were summarized across quartiles of HEI-2020 and AHEI-2010 scores.
- The researchers obtained and summarized clinical characteristics, including tumor characteristics, first-line treatment regimen, debulking status, residual disease, and cancer antigen 125 levels, from medical records.
- The main outcome measure was overall survival, with hazard ratios (HRs) and 95% CIs estimated from multivariable Cox models for the association between adherence to dietary recommendations and overall mortality. Follow-up was conducted until October 2022, with data analyzed from March 2023 to June 2024.
TAKEAWAY:
- No significant association was found between dietary patterns and overall mortality among women with EOC.
- Among women with HGSOC, the most lethal histotype of EOC, better adherence to the HEI-2020 was associated with decreased mortality in later quartiles vs the first quartile (HR, 0.63; 95% CI, 0.44-0.92).
- Similar results were observed with the AHEI-2010 among women with HGSOC for the second (HR, 0.62; 95% CI, 0.43-0.89) and fourth (HR, 0.67; 95% CI, 0.45-0.98) quartiles vs the first quartile.
- Women with moderate and high prediagnosis dietary quality had significantly lower mortality rates from HGSOC than those with the lowest prediagnosis dietary quality.
IN PRACTICE:
“Our findings suggest that prediagnosis dietary patterns (ie, the combination of foods and nutrients) are more important than individual components for ovarian cancer survival as shown by comparing results of dietary patterns with individual components,” the authors of the study wrote.
SOURCE:
This study was led by Tsion A. Armidie, MPH, Rollins School of Public Health, Emory University in Atlanta, Georgia. It was published online on October 18 in JAMA Network Open.
LIMITATIONS:
This study’s limitations included the potential for residual confounding, despite accounting for a wide array of covariates. The median time between diagnosis and FFQ completion was 5.8 months, which may have introduced measurement errors in dietary recall. Additionally, the study did not collect postdiagnostic dietary information, which could have provided further insights into the association between diet and survival.
DISCLOSURES:
This study was supported by grants from the National Cancer Institute. One coauthor reported receiving personal fees from Pfizer outside the submitted work. One coauthor reported receiving grants from the US Department of Defense during the conduct of the study and Bristol-Myers Squibb and Karyopharm outside the submitted work. One coauthor reported receiving personal fees from Ashcraft and Gerel outside the submitted work. One coauthor reported receiving personal fees from Epidemiologic Research & Methods outside the submitted work. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
No significant survival association was found among the full study sample, which included women with multiple types of epithelial ovarian cancer (EOC).
METHODOLOGY:
- Researchers conducted a prospective cohort study among 483 self-identified Black women aged 20-79 years newly diagnosed with histologically confirmed EOC between December 2010 and December 2015.
- The study aimed to examine associations between dietary patterns and survival among Black women diagnosed with EOC using data from the African American Cancer Epidemiology Study.
- Dietary patterns were assessed using the Healthy Eating Index–2020 (HEI-2020) and Alternative Healthy Eating Index–2010 (AHEI-2010), based on dietary intake in the year prior to diagnosis collected via the validated Block 2005 Food Frequency Questionnaire (FFQ). Participant characteristics were summarized across quartiles of HEI-2020 and AHEI-2010 scores.
- The researchers obtained and summarized clinical characteristics, including tumor characteristics, first-line treatment regimen, debulking status, residual disease, and cancer antigen 125 levels, from medical records.
- The main outcome measure was overall survival, with hazard ratios (HRs) and 95% CIs estimated from multivariable Cox models for the association between adherence to dietary recommendations and overall mortality. Follow-up was conducted until October 2022, with data analyzed from March 2023 to June 2024.
TAKEAWAY:
- No significant association was found between dietary patterns and overall mortality among women with EOC.
- Among women with HGSOC, the most lethal histotype of EOC, better adherence to the HEI-2020 was associated with decreased mortality in later quartiles vs the first quartile (HR, 0.63; 95% CI, 0.44-0.92).
- Similar results were observed with the AHEI-2010 among women with HGSOC for the second (HR, 0.62; 95% CI, 0.43-0.89) and fourth (HR, 0.67; 95% CI, 0.45-0.98) quartiles vs the first quartile.
- Women with moderate and high prediagnosis dietary quality had significantly lower mortality rates from HGSOC than those with the lowest prediagnosis dietary quality.
IN PRACTICE:
“Our findings suggest that prediagnosis dietary patterns (ie, the combination of foods and nutrients) are more important than individual components for ovarian cancer survival as shown by comparing results of dietary patterns with individual components,” the authors of the study wrote.
SOURCE:
This study was led by Tsion A. Armidie, MPH, Rollins School of Public Health, Emory University in Atlanta, Georgia. It was published online on October 18 in JAMA Network Open.
LIMITATIONS:
This study’s limitations included the potential for residual confounding, despite accounting for a wide array of covariates. The median time between diagnosis and FFQ completion was 5.8 months, which may have introduced measurement errors in dietary recall. Additionally, the study did not collect postdiagnostic dietary information, which could have provided further insights into the association between diet and survival.
DISCLOSURES:
This study was supported by grants from the National Cancer Institute. One coauthor reported receiving personal fees from Pfizer outside the submitted work. One coauthor reported receiving grants from the US Department of Defense during the conduct of the study and Bristol-Myers Squibb and Karyopharm outside the submitted work. One coauthor reported receiving personal fees from Ashcraft and Gerel outside the submitted work. One coauthor reported receiving personal fees from Epidemiologic Research & Methods outside the submitted work. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
No significant survival association was found among the full study sample, which included women with multiple types of epithelial ovarian cancer (EOC).
METHODOLOGY:
- Researchers conducted a prospective cohort study among 483 self-identified Black women aged 20-79 years newly diagnosed with histologically confirmed EOC between December 2010 and December 2015.
- The study aimed to examine associations between dietary patterns and survival among Black women diagnosed with EOC using data from the African American Cancer Epidemiology Study.
- Dietary patterns were assessed using the Healthy Eating Index–2020 (HEI-2020) and Alternative Healthy Eating Index–2010 (AHEI-2010), based on dietary intake in the year prior to diagnosis collected via the validated Block 2005 Food Frequency Questionnaire (FFQ). Participant characteristics were summarized across quartiles of HEI-2020 and AHEI-2010 scores.
- The researchers obtained and summarized clinical characteristics, including tumor characteristics, first-line treatment regimen, debulking status, residual disease, and cancer antigen 125 levels, from medical records.
- The main outcome measure was overall survival, with hazard ratios (HRs) and 95% CIs estimated from multivariable Cox models for the association between adherence to dietary recommendations and overall mortality. Follow-up was conducted until October 2022, with data analyzed from March 2023 to June 2024.
TAKEAWAY:
- No significant association was found between dietary patterns and overall mortality among women with EOC.
- Among women with HGSOC, the most lethal histotype of EOC, better adherence to the HEI-2020 was associated with decreased mortality in later quartiles vs the first quartile (HR, 0.63; 95% CI, 0.44-0.92).
- Similar results were observed with the AHEI-2010 among women with HGSOC for the second (HR, 0.62; 95% CI, 0.43-0.89) and fourth (HR, 0.67; 95% CI, 0.45-0.98) quartiles vs the first quartile.
- Women with moderate and high prediagnosis dietary quality had significantly lower mortality rates from HGSOC than those with the lowest prediagnosis dietary quality.
IN PRACTICE:
“Our findings suggest that prediagnosis dietary patterns (ie, the combination of foods and nutrients) are more important than individual components for ovarian cancer survival as shown by comparing results of dietary patterns with individual components,” the authors of the study wrote.
SOURCE:
This study was led by Tsion A. Armidie, MPH, Rollins School of Public Health, Emory University in Atlanta, Georgia. It was published online on October 18 in JAMA Network Open.
LIMITATIONS:
This study’s limitations included the potential for residual confounding, despite accounting for a wide array of covariates. The median time between diagnosis and FFQ completion was 5.8 months, which may have introduced measurement errors in dietary recall. Additionally, the study did not collect postdiagnostic dietary information, which could have provided further insights into the association between diet and survival.
DISCLOSURES:
This study was supported by grants from the National Cancer Institute. One coauthor reported receiving personal fees from Pfizer outside the submitted work. One coauthor reported receiving grants from the US Department of Defense during the conduct of the study and Bristol-Myers Squibb and Karyopharm outside the submitted work. One coauthor reported receiving personal fees from Ashcraft and Gerel outside the submitted work. One coauthor reported receiving personal fees from Epidemiologic Research & Methods outside the submitted work. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Community Outreach Benefits Dermatology Residents and Their Patients
The sun often is rising in the rearview mirror as I travel with the University of New Mexico dermatology team from Albuquerque to our satellite clinic in Gallup, New Mexico. This twice-monthly trip—with a group usually comprising an attending physician, residents, and medical students—provides an invaluable opportunity for me to take part in delivering care to a majority Native American population and connects our institution and its trainees to the state’s rural and indigenous cultures and communities.
Community outreach is an important initiative for many dermatology residency training programs. Engaging with the community outside the clinic setting allows residents to hone their clinical skills, interact with and meet new people, and help to improve access to health care, especially for members of underserved populations.
Limited access to health care remains a pressing issue in the United States, especially for underserved and rural communities. There currently is no standardized way to measure access to care, but multiple contributing factors have been identified, including but not limited to patient wait times and throughput, provider turnover, ratio of dermatologists to patient population, insurance type, and patient outcomes.1 Fortunately, there are many ways for dermatology residents to get involved and improve access to dermatologic services in their communities, including skin cancer screenings, free clinics, and teledermatology.
Skin Cancer Screenings
More than 40% of community outreach initiatives offered by dermatology residency programs are related to skin cancer screening and prevention.2 The American Academy of Dermatology’s free skin cancer check program (https://www.aad.org/member/career/volunteer/spot) offers a way to participate in or even host a skin cancer screening in your community. Since 1985, this program has identified nearly 300,000 suspicious lesions and more than 30,000 suspected melanomas. Resources for setting up a skin cancer screening in your community are available on the program’s website. Residents may take this opportunity to teach medical students how to perform full-body skin examinations and/or practice making independent decisions as the supervisor for medical trainees. Skin cancer screening events not only expand access to care in underserved communities but also help residents feel more connected to the local community, especially if they have moved to a new location for their residency training.
Free Clinics
Engaging in educational opportunities offered through residency programs is another way to participate in community outreach. In particular, many programs are affiliated with a School of Medicine within their institution that allows residents to spearhead volunteer opportunities such as working at a free clinic. In fact, more than 30% of initiatives offered at dermatology residency programs are free general dermatology clinics.2 Residents are in the unique position of being both learners themselves as well as educators to trainees.3 As part of our role, we can provide crucial specialty care to the community by working in concert with medical students and while also familiarizing ourselves with treating populations that we may not reach in our daily clinical work. For example, by participating in free clinics, we can provide care to vulnerable populations who typically may have financial or time barriers that prevent them from seeking care at the institution-associated clinic, including individuals experiencing homelessness, patients who are uninsured, and individuals who cannot take time off work to pursue medical care. Our presence in the community helps to reduce barriers to specialty care, particularly in the field of dermatology where the access shortage in the context of rising skin cancer rates prompts public health concerns.4
Teledermatology
Teledermatology became a way to extend our reach in the community more than ever before during the COVID-19 pandemic. Advances in audio, visual, and data telecommunication have been particularly helpful in dermatology, a specialty that relies heavily on visual cues for diagnosis. Synchronous, asynchronous, and hybrid teledermatology services implemented during the pandemic have gained favor among patients and dermatologists and are still applied in current practice.5,6
For example, in the state of New Mexico (where there is a severe shortage of board-certified dermatologists to care for the state’s population), teledermatology has allowed rural providers of all specialties to consult University of New Mexico dermatologists by sending clinical photographs along with patient information and history via secure messaging. Instead of having the patient travel hundreds of miles to see the nearest dermatologist for their skin condition or endure long wait times to get in to see a specialist, primary providers now can initiate treatment or work-up for their patient’s skin issue in a timely manner with the use of teledermatology to consult specialists.
Teledermatology has demonstrated cost-effectiveness, accuracy, and efficiency in conveniently expanding access to care. It offers patients and dermatologists flexibility in receiving and delivering health care, respectively.7 As residents, learning how to navigate this technologic frontier in health care delivery is imperative, as it will remain a prevalent tool in the future care of our communities, particularly in underserved areas.
Final Thoughts
Through community outreach initiatives, dermatology residents have an opportunity not only to enrich our education but also to connect with and become closer to our patients. Skin cancer screenings, free clinics, and teledermatology have provided ways to reach more communities and remain important aspects of dermatology residency.
- Patel B, Blalock TW. Defining “access to care” for dermatology at academic medical institutions. J Am Acad Dermatol. 2023;89:627-628. doi:10.1016/j.jaad.2023.03.014
- Fritsche M, Maglakelidze N, Zaenglein A, et al. Community outreach initiatives in dermatology: cross-sectional study. Arch Dermatol Res. 2023;315:2693-2695. doi:10.1007/s00403-023-02629-y
- Chiu LW. Teaching tips for dermatology residents. Cutis. 2024;113:E17-E19. doi:10.12788/cutis.1046
- Duniphin DD. Limited access to dermatology specialty care: barriers and teledermatology. Dermatol Pract Concept. 2023;13:E2023031. doi:10.5826/dpc.1301a31
- Ibrahim AE, Magdy M, Khalaf EM, et al. Teledermatology in the time of COVID-19. Int J Clin Pract. 2021;75:e15000. doi:10.1111/ijcp.15000
- Farr MA, Duvic M, Joshi TP. Teledermatology during COVID-19: an updated review. Am J Clin Dermatol. 2021;22:467-475. doi:10.1007/s40257-021-00601-y
- Lipner SR. Optimizing patient care with teledermatology: improving access, efficiency, and satisfaction. Cutis. 2024;114:63-64. doi:10.12788/cutis.1073
The sun often is rising in the rearview mirror as I travel with the University of New Mexico dermatology team from Albuquerque to our satellite clinic in Gallup, New Mexico. This twice-monthly trip—with a group usually comprising an attending physician, residents, and medical students—provides an invaluable opportunity for me to take part in delivering care to a majority Native American population and connects our institution and its trainees to the state’s rural and indigenous cultures and communities.
Community outreach is an important initiative for many dermatology residency training programs. Engaging with the community outside the clinic setting allows residents to hone their clinical skills, interact with and meet new people, and help to improve access to health care, especially for members of underserved populations.
Limited access to health care remains a pressing issue in the United States, especially for underserved and rural communities. There currently is no standardized way to measure access to care, but multiple contributing factors have been identified, including but not limited to patient wait times and throughput, provider turnover, ratio of dermatologists to patient population, insurance type, and patient outcomes.1 Fortunately, there are many ways for dermatology residents to get involved and improve access to dermatologic services in their communities, including skin cancer screenings, free clinics, and teledermatology.
Skin Cancer Screenings
More than 40% of community outreach initiatives offered by dermatology residency programs are related to skin cancer screening and prevention.2 The American Academy of Dermatology’s free skin cancer check program (https://www.aad.org/member/career/volunteer/spot) offers a way to participate in or even host a skin cancer screening in your community. Since 1985, this program has identified nearly 300,000 suspicious lesions and more than 30,000 suspected melanomas. Resources for setting up a skin cancer screening in your community are available on the program’s website. Residents may take this opportunity to teach medical students how to perform full-body skin examinations and/or practice making independent decisions as the supervisor for medical trainees. Skin cancer screening events not only expand access to care in underserved communities but also help residents feel more connected to the local community, especially if they have moved to a new location for their residency training.
Free Clinics
Engaging in educational opportunities offered through residency programs is another way to participate in community outreach. In particular, many programs are affiliated with a School of Medicine within their institution that allows residents to spearhead volunteer opportunities such as working at a free clinic. In fact, more than 30% of initiatives offered at dermatology residency programs are free general dermatology clinics.2 Residents are in the unique position of being both learners themselves as well as educators to trainees.3 As part of our role, we can provide crucial specialty care to the community by working in concert with medical students and while also familiarizing ourselves with treating populations that we may not reach in our daily clinical work. For example, by participating in free clinics, we can provide care to vulnerable populations who typically may have financial or time barriers that prevent them from seeking care at the institution-associated clinic, including individuals experiencing homelessness, patients who are uninsured, and individuals who cannot take time off work to pursue medical care. Our presence in the community helps to reduce barriers to specialty care, particularly in the field of dermatology where the access shortage in the context of rising skin cancer rates prompts public health concerns.4
Teledermatology
Teledermatology became a way to extend our reach in the community more than ever before during the COVID-19 pandemic. Advances in audio, visual, and data telecommunication have been particularly helpful in dermatology, a specialty that relies heavily on visual cues for diagnosis. Synchronous, asynchronous, and hybrid teledermatology services implemented during the pandemic have gained favor among patients and dermatologists and are still applied in current practice.5,6
For example, in the state of New Mexico (where there is a severe shortage of board-certified dermatologists to care for the state’s population), teledermatology has allowed rural providers of all specialties to consult University of New Mexico dermatologists by sending clinical photographs along with patient information and history via secure messaging. Instead of having the patient travel hundreds of miles to see the nearest dermatologist for their skin condition or endure long wait times to get in to see a specialist, primary providers now can initiate treatment or work-up for their patient’s skin issue in a timely manner with the use of teledermatology to consult specialists.
Teledermatology has demonstrated cost-effectiveness, accuracy, and efficiency in conveniently expanding access to care. It offers patients and dermatologists flexibility in receiving and delivering health care, respectively.7 As residents, learning how to navigate this technologic frontier in health care delivery is imperative, as it will remain a prevalent tool in the future care of our communities, particularly in underserved areas.
Final Thoughts
Through community outreach initiatives, dermatology residents have an opportunity not only to enrich our education but also to connect with and become closer to our patients. Skin cancer screenings, free clinics, and teledermatology have provided ways to reach more communities and remain important aspects of dermatology residency.
The sun often is rising in the rearview mirror as I travel with the University of New Mexico dermatology team from Albuquerque to our satellite clinic in Gallup, New Mexico. This twice-monthly trip—with a group usually comprising an attending physician, residents, and medical students—provides an invaluable opportunity for me to take part in delivering care to a majority Native American population and connects our institution and its trainees to the state’s rural and indigenous cultures and communities.
Community outreach is an important initiative for many dermatology residency training programs. Engaging with the community outside the clinic setting allows residents to hone their clinical skills, interact with and meet new people, and help to improve access to health care, especially for members of underserved populations.
Limited access to health care remains a pressing issue in the United States, especially for underserved and rural communities. There currently is no standardized way to measure access to care, but multiple contributing factors have been identified, including but not limited to patient wait times and throughput, provider turnover, ratio of dermatologists to patient population, insurance type, and patient outcomes.1 Fortunately, there are many ways for dermatology residents to get involved and improve access to dermatologic services in their communities, including skin cancer screenings, free clinics, and teledermatology.
Skin Cancer Screenings
More than 40% of community outreach initiatives offered by dermatology residency programs are related to skin cancer screening and prevention.2 The American Academy of Dermatology’s free skin cancer check program (https://www.aad.org/member/career/volunteer/spot) offers a way to participate in or even host a skin cancer screening in your community. Since 1985, this program has identified nearly 300,000 suspicious lesions and more than 30,000 suspected melanomas. Resources for setting up a skin cancer screening in your community are available on the program’s website. Residents may take this opportunity to teach medical students how to perform full-body skin examinations and/or practice making independent decisions as the supervisor for medical trainees. Skin cancer screening events not only expand access to care in underserved communities but also help residents feel more connected to the local community, especially if they have moved to a new location for their residency training.
Free Clinics
Engaging in educational opportunities offered through residency programs is another way to participate in community outreach. In particular, many programs are affiliated with a School of Medicine within their institution that allows residents to spearhead volunteer opportunities such as working at a free clinic. In fact, more than 30% of initiatives offered at dermatology residency programs are free general dermatology clinics.2 Residents are in the unique position of being both learners themselves as well as educators to trainees.3 As part of our role, we can provide crucial specialty care to the community by working in concert with medical students and while also familiarizing ourselves with treating populations that we may not reach in our daily clinical work. For example, by participating in free clinics, we can provide care to vulnerable populations who typically may have financial or time barriers that prevent them from seeking care at the institution-associated clinic, including individuals experiencing homelessness, patients who are uninsured, and individuals who cannot take time off work to pursue medical care. Our presence in the community helps to reduce barriers to specialty care, particularly in the field of dermatology where the access shortage in the context of rising skin cancer rates prompts public health concerns.4
Teledermatology
Teledermatology became a way to extend our reach in the community more than ever before during the COVID-19 pandemic. Advances in audio, visual, and data telecommunication have been particularly helpful in dermatology, a specialty that relies heavily on visual cues for diagnosis. Synchronous, asynchronous, and hybrid teledermatology services implemented during the pandemic have gained favor among patients and dermatologists and are still applied in current practice.5,6
For example, in the state of New Mexico (where there is a severe shortage of board-certified dermatologists to care for the state’s population), teledermatology has allowed rural providers of all specialties to consult University of New Mexico dermatologists by sending clinical photographs along with patient information and history via secure messaging. Instead of having the patient travel hundreds of miles to see the nearest dermatologist for their skin condition or endure long wait times to get in to see a specialist, primary providers now can initiate treatment or work-up for their patient’s skin issue in a timely manner with the use of teledermatology to consult specialists.
Teledermatology has demonstrated cost-effectiveness, accuracy, and efficiency in conveniently expanding access to care. It offers patients and dermatologists flexibility in receiving and delivering health care, respectively.7 As residents, learning how to navigate this technologic frontier in health care delivery is imperative, as it will remain a prevalent tool in the future care of our communities, particularly in underserved areas.
Final Thoughts
Through community outreach initiatives, dermatology residents have an opportunity not only to enrich our education but also to connect with and become closer to our patients. Skin cancer screenings, free clinics, and teledermatology have provided ways to reach more communities and remain important aspects of dermatology residency.
- Patel B, Blalock TW. Defining “access to care” for dermatology at academic medical institutions. J Am Acad Dermatol. 2023;89:627-628. doi:10.1016/j.jaad.2023.03.014
- Fritsche M, Maglakelidze N, Zaenglein A, et al. Community outreach initiatives in dermatology: cross-sectional study. Arch Dermatol Res. 2023;315:2693-2695. doi:10.1007/s00403-023-02629-y
- Chiu LW. Teaching tips for dermatology residents. Cutis. 2024;113:E17-E19. doi:10.12788/cutis.1046
- Duniphin DD. Limited access to dermatology specialty care: barriers and teledermatology. Dermatol Pract Concept. 2023;13:E2023031. doi:10.5826/dpc.1301a31
- Ibrahim AE, Magdy M, Khalaf EM, et al. Teledermatology in the time of COVID-19. Int J Clin Pract. 2021;75:e15000. doi:10.1111/ijcp.15000
- Farr MA, Duvic M, Joshi TP. Teledermatology during COVID-19: an updated review. Am J Clin Dermatol. 2021;22:467-475. doi:10.1007/s40257-021-00601-y
- Lipner SR. Optimizing patient care with teledermatology: improving access, efficiency, and satisfaction. Cutis. 2024;114:63-64. doi:10.12788/cutis.1073
- Patel B, Blalock TW. Defining “access to care” for dermatology at academic medical institutions. J Am Acad Dermatol. 2023;89:627-628. doi:10.1016/j.jaad.2023.03.014
- Fritsche M, Maglakelidze N, Zaenglein A, et al. Community outreach initiatives in dermatology: cross-sectional study. Arch Dermatol Res. 2023;315:2693-2695. doi:10.1007/s00403-023-02629-y
- Chiu LW. Teaching tips for dermatology residents. Cutis. 2024;113:E17-E19. doi:10.12788/cutis.1046
- Duniphin DD. Limited access to dermatology specialty care: barriers and teledermatology. Dermatol Pract Concept. 2023;13:E2023031. doi:10.5826/dpc.1301a31
- Ibrahim AE, Magdy M, Khalaf EM, et al. Teledermatology in the time of COVID-19. Int J Clin Pract. 2021;75:e15000. doi:10.1111/ijcp.15000
- Farr MA, Duvic M, Joshi TP. Teledermatology during COVID-19: an updated review. Am J Clin Dermatol. 2021;22:467-475. doi:10.1007/s40257-021-00601-y
- Lipner SR. Optimizing patient care with teledermatology: improving access, efficiency, and satisfaction. Cutis. 2024;114:63-64. doi:10.12788/cutis.1073
Resident Pearls
- Outreach initiatives can help residents feel more connected to their community and expand access to care.
- Skin cancer screenings, free clinics, and teledermatology are a few ways residents may get involved in their local communities.
Pediatric Melanoma Outcomes by Race and Socioeconomic Factors
To the Editor:
Skin cancers are extremely common worldwide. Malignant melanomas comprise approximately 1 in 5 of these cancers. Exposure to UV radiation is postulated to be responsible for a global rise in melanoma cases over the past 50 years.1 Pediatric melanoma is a particularly rare condition that affects approximately 6 in every 1 million children.2 Melanoma incidence in children ranges by age, increasing by approximately 10-fold from age 1 to 4 years to age 15 to 19 years. Tumor ulceration is a feature more commonly seen among children younger than 10 years and is associated with worse outcomes. Tumor thickness and ulceration strongly predict sentinel lymph node metastases among children, which also is associated with a poor prognosis.3
A recent study evaluating stage IV melanoma survival rates in adolescents and young adults (AYAs) vs older adults found that survival is much worse among AYAs. Thicker tumors and public health insurance also were associated with worse survival rates for AYAs, while early detection was associated with better survival rates.4
Health disparities and their role in the prognosis of pediatric melanoma is another important factor. One study analyzed this relationship at the state level using Texas Cancer Registry data (1995-2009).5 Patients’ socioeconomic status (SES) and driving distance to the nearest pediatric cancer care center were included in the analysis. Hispanic children were found to be 3 times more likely to present with advanced disease than non-Hispanic White children. Although SES and distance to the nearest treatment center were not found to affect the melanoma stage at presentation, Hispanic ethnicity or being in the lowest SES quartile were correlated with a higher mortality risk.5
When considering specific subtypes of melanoma, acral lentiginous melanoma (ALM) is known to develop in patients with skin of color. A 2023 study by Holman et al6 reported that the percentage of melanomas that were ALMs ranged from 0.8% in non-Hispanic White individuals to 19.1% in Hispanic Black, American Indian/Alaska Native, and Asian/Pacific Islander individuals. However, ALM is rare in children. In a pooled cohort study with patient information retrieved from the nationwide Dutch Pathology Registry, only 1 child and 1 adolescent were found to have ALM across a total of 514 patients.7 We sought to analyze pediatric melanoma outcomes based on race and other barriers to appropriate care.
We conducted a search of the Surveillance, Epidemiology, and End Results (SEER) database from January 1995 to December 2016 for patients aged 21 years and younger with a primary melanoma diagnosis. The primary outcome was the 5-year survival rate. County-level SES variables were used to calculate a prosperity index. Kaplan-Meier analysis and Cox proportional hazards model were used to compare 5-year survival rates among the different racial/ethnic groups.
A sample of 2742 patients was identified during the study period and followed for 5 years. Eighty-two percent were White, 6% Hispanic, 2% Asian, 1% Black, and 5% classified as other/unknown race (data were missing for 4%). The cohort was predominantly female (61%). White patients were more likely to present with localized disease than any other race/ethnicity (83% vs 65% in Hispanic, 60% in Asian/Pacific Islander, and 45% in Black patients [P<.05]).
Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis. On multivariate analysis, this finding remained significant for Hispanic patients when compared with White patients (hazard ratio, 2.37 [P<.05]). Increasing age, male sex, advanced stage at diagnosis, and failure to receive surgery were associated with increased odds of mortality.
Patients with regionalized and disseminated disease had increased odds of mortality (6.16 and 64.45, respectively; P<.05) compared with patients with localized disease. Socioeconomic status and urbanization were not found to influence 5-year survival rates.
Pediatric melanoma often presents a clinical challenge with special considerations. Pediatric-specific predisposing risk factors for melanoma and an atypical clinical presentation are some of the major concerns that necessitate a tailored approach to this malignancy, especially among different age groups, skin types, and racial and socioeconomic groups.5
Standard ABCDE criteria often are inadequate for accurate detection of pediatric melanomas. Initial lesions often manifest as raised, red, amelanotic lesions mimicking pyogenic granulomas. Lesions tend to be very small (<6 mm in diameter) and can be uniform in color, thereby making the melanoma more difficult to detect compared to the characteristic findings in adults.5 Bleeding or ulceration often can be a warning sign during physical examination.
With regard to incidence, pediatric melanoma is relatively rare. Since the 1970s, the incidence of pediatric melanoma has been increasing; however, a recent analysis of the SEER database showed a decreasing trend from 2000 to 2010.4
Our analysis of the SEER data showed an increased risk for pediatric melanoma in older adolescents. In addition, the incidence of pediatric melanoma was higher in females of all racial groups except Asian/Pacific Islander individuals. However, SES was not found to significantly influence the 5-year survival rate in pediatric melanoma.
White pediatric patients were more likely to present with localized disease compared with other races. Pediatric melanoma patients with regional disease had a 6-fold increase in mortality rate vs those with localized disease; those with disseminated disease had a 65-fold higher risk. Consistent with this, Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis.
These findings suggest a relationship between race, melanoma spread, and disease severity. Patient education programs need to be directed specifically to minority groups to improve their knowledge on evolving skin lesions and sun protection practices. Physicians also need to have heightened suspicion and better knowledge of the unique traits of pediatric melanoma.5
Given the considerable influence these disparities can have on melanoma outcomes, further research is needed to characterize outcomes based on race and determine obstacles to appropriate care. Improved public outreach initiatives that accommodate specific cultural barriers (eg, language, traditional patterns of behavior) also are required to improve current circumstances.
- Arnold M, Singh D, Laversanne M, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 2022;158:495-503.
- McCormack L, Hawryluk EB. Pediatric melanoma update. G Ital Dermatol Venereol. 2018;153:707-715.
- Saiyed FK, Hamilton EC, Austin MT. Pediatric melanoma: incidence, treatment, and prognosis. Pediatric Health Med Ther. 2017;8:39-45.
- Wojcik KY, Hawkins M, Anderson-Mellies A, et al. Melanoma survival by age group: population-based disparities for adolescent and young adult patients by stage, tumor thickness, and insurance type. J Am Acad Dermatol. 2023;88:831-840.
- Hamilton EC, Nguyen HT, Chang YC, et al. Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr. 2016;175:182-187.
- Holman DM, King JB, White A, et al. Acral lentiginous melanoma incidence by sex, race, ethnicity, and stage in the United States, 2010-2019. Prev Med. 2023;175:107692. doi:10.1016/j.ypmed.2023.107692
- El Sharouni MA, Rawson RV, Potter AJ, et al. Melanomas in children and adolescents: clinicopathologic features and survival outcomes. J Am Acad Dermatol. 2023;88:609-616. doi:10.1016/j.jaad.2022.08.067
To the Editor:
Skin cancers are extremely common worldwide. Malignant melanomas comprise approximately 1 in 5 of these cancers. Exposure to UV radiation is postulated to be responsible for a global rise in melanoma cases over the past 50 years.1 Pediatric melanoma is a particularly rare condition that affects approximately 6 in every 1 million children.2 Melanoma incidence in children ranges by age, increasing by approximately 10-fold from age 1 to 4 years to age 15 to 19 years. Tumor ulceration is a feature more commonly seen among children younger than 10 years and is associated with worse outcomes. Tumor thickness and ulceration strongly predict sentinel lymph node metastases among children, which also is associated with a poor prognosis.3
A recent study evaluating stage IV melanoma survival rates in adolescents and young adults (AYAs) vs older adults found that survival is much worse among AYAs. Thicker tumors and public health insurance also were associated with worse survival rates for AYAs, while early detection was associated with better survival rates.4
Health disparities and their role in the prognosis of pediatric melanoma is another important factor. One study analyzed this relationship at the state level using Texas Cancer Registry data (1995-2009).5 Patients’ socioeconomic status (SES) and driving distance to the nearest pediatric cancer care center were included in the analysis. Hispanic children were found to be 3 times more likely to present with advanced disease than non-Hispanic White children. Although SES and distance to the nearest treatment center were not found to affect the melanoma stage at presentation, Hispanic ethnicity or being in the lowest SES quartile were correlated with a higher mortality risk.5
When considering specific subtypes of melanoma, acral lentiginous melanoma (ALM) is known to develop in patients with skin of color. A 2023 study by Holman et al6 reported that the percentage of melanomas that were ALMs ranged from 0.8% in non-Hispanic White individuals to 19.1% in Hispanic Black, American Indian/Alaska Native, and Asian/Pacific Islander individuals. However, ALM is rare in children. In a pooled cohort study with patient information retrieved from the nationwide Dutch Pathology Registry, only 1 child and 1 adolescent were found to have ALM across a total of 514 patients.7 We sought to analyze pediatric melanoma outcomes based on race and other barriers to appropriate care.
We conducted a search of the Surveillance, Epidemiology, and End Results (SEER) database from January 1995 to December 2016 for patients aged 21 years and younger with a primary melanoma diagnosis. The primary outcome was the 5-year survival rate. County-level SES variables were used to calculate a prosperity index. Kaplan-Meier analysis and Cox proportional hazards model were used to compare 5-year survival rates among the different racial/ethnic groups.
A sample of 2742 patients was identified during the study period and followed for 5 years. Eighty-two percent were White, 6% Hispanic, 2% Asian, 1% Black, and 5% classified as other/unknown race (data were missing for 4%). The cohort was predominantly female (61%). White patients were more likely to present with localized disease than any other race/ethnicity (83% vs 65% in Hispanic, 60% in Asian/Pacific Islander, and 45% in Black patients [P<.05]).
Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis. On multivariate analysis, this finding remained significant for Hispanic patients when compared with White patients (hazard ratio, 2.37 [P<.05]). Increasing age, male sex, advanced stage at diagnosis, and failure to receive surgery were associated with increased odds of mortality.
Patients with regionalized and disseminated disease had increased odds of mortality (6.16 and 64.45, respectively; P<.05) compared with patients with localized disease. Socioeconomic status and urbanization were not found to influence 5-year survival rates.
Pediatric melanoma often presents a clinical challenge with special considerations. Pediatric-specific predisposing risk factors for melanoma and an atypical clinical presentation are some of the major concerns that necessitate a tailored approach to this malignancy, especially among different age groups, skin types, and racial and socioeconomic groups.5
Standard ABCDE criteria often are inadequate for accurate detection of pediatric melanomas. Initial lesions often manifest as raised, red, amelanotic lesions mimicking pyogenic granulomas. Lesions tend to be very small (<6 mm in diameter) and can be uniform in color, thereby making the melanoma more difficult to detect compared to the characteristic findings in adults.5 Bleeding or ulceration often can be a warning sign during physical examination.
With regard to incidence, pediatric melanoma is relatively rare. Since the 1970s, the incidence of pediatric melanoma has been increasing; however, a recent analysis of the SEER database showed a decreasing trend from 2000 to 2010.4
Our analysis of the SEER data showed an increased risk for pediatric melanoma in older adolescents. In addition, the incidence of pediatric melanoma was higher in females of all racial groups except Asian/Pacific Islander individuals. However, SES was not found to significantly influence the 5-year survival rate in pediatric melanoma.
White pediatric patients were more likely to present with localized disease compared with other races. Pediatric melanoma patients with regional disease had a 6-fold increase in mortality rate vs those with localized disease; those with disseminated disease had a 65-fold higher risk. Consistent with this, Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis.
These findings suggest a relationship between race, melanoma spread, and disease severity. Patient education programs need to be directed specifically to minority groups to improve their knowledge on evolving skin lesions and sun protection practices. Physicians also need to have heightened suspicion and better knowledge of the unique traits of pediatric melanoma.5
Given the considerable influence these disparities can have on melanoma outcomes, further research is needed to characterize outcomes based on race and determine obstacles to appropriate care. Improved public outreach initiatives that accommodate specific cultural barriers (eg, language, traditional patterns of behavior) also are required to improve current circumstances.
To the Editor:
Skin cancers are extremely common worldwide. Malignant melanomas comprise approximately 1 in 5 of these cancers. Exposure to UV radiation is postulated to be responsible for a global rise in melanoma cases over the past 50 years.1 Pediatric melanoma is a particularly rare condition that affects approximately 6 in every 1 million children.2 Melanoma incidence in children ranges by age, increasing by approximately 10-fold from age 1 to 4 years to age 15 to 19 years. Tumor ulceration is a feature more commonly seen among children younger than 10 years and is associated with worse outcomes. Tumor thickness and ulceration strongly predict sentinel lymph node metastases among children, which also is associated with a poor prognosis.3
A recent study evaluating stage IV melanoma survival rates in adolescents and young adults (AYAs) vs older adults found that survival is much worse among AYAs. Thicker tumors and public health insurance also were associated with worse survival rates for AYAs, while early detection was associated with better survival rates.4
Health disparities and their role in the prognosis of pediatric melanoma is another important factor. One study analyzed this relationship at the state level using Texas Cancer Registry data (1995-2009).5 Patients’ socioeconomic status (SES) and driving distance to the nearest pediatric cancer care center were included in the analysis. Hispanic children were found to be 3 times more likely to present with advanced disease than non-Hispanic White children. Although SES and distance to the nearest treatment center were not found to affect the melanoma stage at presentation, Hispanic ethnicity or being in the lowest SES quartile were correlated with a higher mortality risk.5
When considering specific subtypes of melanoma, acral lentiginous melanoma (ALM) is known to develop in patients with skin of color. A 2023 study by Holman et al6 reported that the percentage of melanomas that were ALMs ranged from 0.8% in non-Hispanic White individuals to 19.1% in Hispanic Black, American Indian/Alaska Native, and Asian/Pacific Islander individuals. However, ALM is rare in children. In a pooled cohort study with patient information retrieved from the nationwide Dutch Pathology Registry, only 1 child and 1 adolescent were found to have ALM across a total of 514 patients.7 We sought to analyze pediatric melanoma outcomes based on race and other barriers to appropriate care.
We conducted a search of the Surveillance, Epidemiology, and End Results (SEER) database from January 1995 to December 2016 for patients aged 21 years and younger with a primary melanoma diagnosis. The primary outcome was the 5-year survival rate. County-level SES variables were used to calculate a prosperity index. Kaplan-Meier analysis and Cox proportional hazards model were used to compare 5-year survival rates among the different racial/ethnic groups.
A sample of 2742 patients was identified during the study period and followed for 5 years. Eighty-two percent were White, 6% Hispanic, 2% Asian, 1% Black, and 5% classified as other/unknown race (data were missing for 4%). The cohort was predominantly female (61%). White patients were more likely to present with localized disease than any other race/ethnicity (83% vs 65% in Hispanic, 60% in Asian/Pacific Islander, and 45% in Black patients [P<.05]).
Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis. On multivariate analysis, this finding remained significant for Hispanic patients when compared with White patients (hazard ratio, 2.37 [P<.05]). Increasing age, male sex, advanced stage at diagnosis, and failure to receive surgery were associated with increased odds of mortality.
Patients with regionalized and disseminated disease had increased odds of mortality (6.16 and 64.45, respectively; P<.05) compared with patients with localized disease. Socioeconomic status and urbanization were not found to influence 5-year survival rates.
Pediatric melanoma often presents a clinical challenge with special considerations. Pediatric-specific predisposing risk factors for melanoma and an atypical clinical presentation are some of the major concerns that necessitate a tailored approach to this malignancy, especially among different age groups, skin types, and racial and socioeconomic groups.5
Standard ABCDE criteria often are inadequate for accurate detection of pediatric melanomas. Initial lesions often manifest as raised, red, amelanotic lesions mimicking pyogenic granulomas. Lesions tend to be very small (<6 mm in diameter) and can be uniform in color, thereby making the melanoma more difficult to detect compared to the characteristic findings in adults.5 Bleeding or ulceration often can be a warning sign during physical examination.
With regard to incidence, pediatric melanoma is relatively rare. Since the 1970s, the incidence of pediatric melanoma has been increasing; however, a recent analysis of the SEER database showed a decreasing trend from 2000 to 2010.4
Our analysis of the SEER data showed an increased risk for pediatric melanoma in older adolescents. In addition, the incidence of pediatric melanoma was higher in females of all racial groups except Asian/Pacific Islander individuals. However, SES was not found to significantly influence the 5-year survival rate in pediatric melanoma.
White pediatric patients were more likely to present with localized disease compared with other races. Pediatric melanoma patients with regional disease had a 6-fold increase in mortality rate vs those with localized disease; those with disseminated disease had a 65-fold higher risk. Consistent with this, Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis.
These findings suggest a relationship between race, melanoma spread, and disease severity. Patient education programs need to be directed specifically to minority groups to improve their knowledge on evolving skin lesions and sun protection practices. Physicians also need to have heightened suspicion and better knowledge of the unique traits of pediatric melanoma.5
Given the considerable influence these disparities can have on melanoma outcomes, further research is needed to characterize outcomes based on race and determine obstacles to appropriate care. Improved public outreach initiatives that accommodate specific cultural barriers (eg, language, traditional patterns of behavior) also are required to improve current circumstances.
- Arnold M, Singh D, Laversanne M, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 2022;158:495-503.
- McCormack L, Hawryluk EB. Pediatric melanoma update. G Ital Dermatol Venereol. 2018;153:707-715.
- Saiyed FK, Hamilton EC, Austin MT. Pediatric melanoma: incidence, treatment, and prognosis. Pediatric Health Med Ther. 2017;8:39-45.
- Wojcik KY, Hawkins M, Anderson-Mellies A, et al. Melanoma survival by age group: population-based disparities for adolescent and young adult patients by stage, tumor thickness, and insurance type. J Am Acad Dermatol. 2023;88:831-840.
- Hamilton EC, Nguyen HT, Chang YC, et al. Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr. 2016;175:182-187.
- Holman DM, King JB, White A, et al. Acral lentiginous melanoma incidence by sex, race, ethnicity, and stage in the United States, 2010-2019. Prev Med. 2023;175:107692. doi:10.1016/j.ypmed.2023.107692
- El Sharouni MA, Rawson RV, Potter AJ, et al. Melanomas in children and adolescents: clinicopathologic features and survival outcomes. J Am Acad Dermatol. 2023;88:609-616. doi:10.1016/j.jaad.2022.08.067
- Arnold M, Singh D, Laversanne M, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 2022;158:495-503.
- McCormack L, Hawryluk EB. Pediatric melanoma update. G Ital Dermatol Venereol. 2018;153:707-715.
- Saiyed FK, Hamilton EC, Austin MT. Pediatric melanoma: incidence, treatment, and prognosis. Pediatric Health Med Ther. 2017;8:39-45.
- Wojcik KY, Hawkins M, Anderson-Mellies A, et al. Melanoma survival by age group: population-based disparities for adolescent and young adult patients by stage, tumor thickness, and insurance type. J Am Acad Dermatol. 2023;88:831-840.
- Hamilton EC, Nguyen HT, Chang YC, et al. Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr. 2016;175:182-187.
- Holman DM, King JB, White A, et al. Acral lentiginous melanoma incidence by sex, race, ethnicity, and stage in the United States, 2010-2019. Prev Med. 2023;175:107692. doi:10.1016/j.ypmed.2023.107692
- El Sharouni MA, Rawson RV, Potter AJ, et al. Melanomas in children and adolescents: clinicopathologic features and survival outcomes. J Am Acad Dermatol. 2023;88:609-616. doi:10.1016/j.jaad.2022.08.067
Practice Points
- Pediatric melanoma is a unique clinical entity with a different clinical presentation than in adults.
- Thicker tumors and disseminated disease are associated with a worse prognosis, and these factors are more commonly seen in Black and Hispanic patients.
AACR Cancer Progress Report: Big Strides and Big Gaps
The AACR’s 216-page report — an annual endeavor now in its 14th year — focused on the “tremendous” strides made in cancer care, prevention, and early detection and highlighted areas where more research and attention are warranted.
One key area is funding. For the first time since 2016, federal funding for the National Institutes of Health (NIH) and National Cancer Institute (NCI) decreased in the past year. The cuts followed nearly a decade of funding increases that saw the NIH budget expand by nearly $15 billion, and that allowed for a “rapid pace and broad scope” of advances in cancer, AACR’s chief executive officer Margaret Foti, MD, PhD, said during a press briefing.
These recent cuts “threaten to curtail the medical progress seen in recent years and stymie future advancements,” said Dr. Foti, who called on Congress to commit to funding cancer research at significant and consistent levels to “maintain the momentum of progress against cancer.”
Inside the Report: Big Progress
Overall, advances in prevention, early detection, and treatment have helped catch more cancers earlier and save lives.
According to the AACR report, the age-adjusted overall cancer death rate in the United States fell by 33% between 1991 and 2021, meaning about 4.1 million cancer deaths were averted. The overall cancer death rate for children and adolescents has declined by 24% in the past 2 decades. The 5-year relative survival rate for children diagnosed with cancer in the US has improved from 58% for those diagnosed in the mid-1970s to 85% for those diagnosed between 2013 and 2019.
The past fiscal year has seen many new approvals for cancer drugs, diagnostics, and screening tests. From July 1, 2023, to June 30, 2024, the Food and Drug Administration (FDA) approved 15 new anticancer therapeutics, as well as 15 new indications for previously approved agents, one new imaging agent, several artificial intelligence (AI) tools to improve early cancer detection and diagnosis, and two minimally invasive tests for assessing inherited cancer risk or early cancer detection, according to the report.
“Cancer diagnostics are becoming more sophisticated,” AACR president Patricia M. LoRusso, DO, PhD, said during the briefing. “New technologies, such as spatial transcriptomics, are helping us study tumors at a cellular level, and helping to unveil things that we did not initially even begin to understand or think of. AI-based approaches are beginning to transform cancer detection, diagnosis, clinical decision-making, and treatment response monitoring.”
The report also highlights the significant progress in many childhood and adolescent/young adult cancers, Dr. LoRusso noted. These include FDA approvals for two new molecularly targeted therapeutics: tovorafenib for children with certain types of brain tumor and repotrectinib for children with a wide array of cancer types that have a specific genetic alteration known as NTRK gene fusion. It also includes an expanded approval for eflornithine to reduce the risk for relapse in children with high-risk neuroblastoma.
“Decades — decades — of basic research discoveries, have led to these clinical breakthroughs,” she stressed. “These gains against cancer are because of the rapid progress in our ability to decode the cancer genome, which has opened new and innovative avenues for drug development.”
The Gaps
Even with progress in cancer prevention, early detection, and treatment, cancer remains a significant issue.
“In 2024, it is estimated that more than 2 million new cases of cancer will be diagnosed in the United States. More than 611,000 people will die from the disease,” according to the report.
The 2024 report shows that incidence rates for some cancers are increasing in the United States, including vaccine-preventable cancers such as human papillomavirus (HPV)–associated oral cancers and, in young adults, cervical cancers. A recent analysis also found that overall cervical cancer incidence among women aged 30-34 years increased by 2.5% a year between 2012 and 2019.
Furthermore, despite clear evidence demonstrating that the HPV vaccine reduces cervical cancer incidence, uptake has remained poor, with only 38.6% of US children and adolescents aged 9-17 years receiving at least one dose of the vaccine in 2022.
Early-onset cancers are also increasing. Rates of breast, colorectal, and other cancers are on the rise in adults younger than 50 years, the report noted.
The report also pointed to data that 40% of all cancer cases in the United States can be attributed to preventable factors, such as smoking, excess body weight, and alcohol. However, our understanding of these risk factors has improved. Excessive levels of alcohol consumption have, for instance, been shown to increase the risk for six different types of cancer: certain types of head and neck cancer, esophageal squamous cell carcinoma, and breast, colorectal, liver, and stomach cancers.
Financial toxicity remains prevalent as well.
The report explains that financial hardship following a cancer diagnosis is widespread, and the effects can last for years. In fact, more than 40% of patients can spend their entire life savings within the first 2 years of cancer treatment. Among adult survivors of childhood cancers, 20.7% had trouble paying their medical bills, 29.9% said they had been sent to debt collection for unpaid bills, 14.1% had forgone medical care, and 26.8% could not afford nutritious meals.
For young cancer survivors, the lifetime costs associated with a diagnosis of cancer are substantial, reaching an average of $259,324 per person.
On a global level, it is estimated that from 2020 to 2050, the cumulative economic burden of cancer will be $25.2 trillion.
The Path Forward
Despite these challenges, Dr. LoRusso said, “it is unquestionable that we are in a time of unparalleled opportunities in cancer research.
“I am excited about what the future holds for cancer research, and especially for patient care,” she said.
However, funding commitments are needed to avoid impeding this momentum and losing a “talented and creative young workforce” that has brought new ideas and new technologies to the table.
Continued robust funding will help “to markedly improve cancer care, increase cancer survivorship, spur economic growth, and maintain the United States’ position as the global leader in science and medical research,” she added.
The AACR report specifically calls on Congress to:
- Appropriate at least $51.3 billion in fiscal year 2025 for the base budget of the NIH and at least $7.934 billion for the NCI.
- Provide $3.6 billion in dedicated funding for Cancer Moonshot activities through fiscal year 2026 in addition to other funding, consistent with the President’s fiscal year 2025 budget.
- Appropriate at least $472.4 million in fiscal year 2025 for the CDC’s Division of Cancer Prevention to support comprehensive cancer control, central cancer registries, and screening and awareness programs for specific cancers.
- Allocate $55 million in funding for the Oncology Center of Excellence at FDA in fiscal year 2025 to provide regulators with the staff and tools necessary to conduct expedited review of cancer-related medical products.
By working together with Congress and other stakeholders, “we will be able to accelerate the pace of progress and make major strides toward the lifesaving goal of preventing and curing all cancers at the earliest possible time,” Dr. Foti said. “I believe if we do that ... one day we will win this war on cancer.”
A version of this article first appeared on Medscape.com.
The AACR’s 216-page report — an annual endeavor now in its 14th year — focused on the “tremendous” strides made in cancer care, prevention, and early detection and highlighted areas where more research and attention are warranted.
One key area is funding. For the first time since 2016, federal funding for the National Institutes of Health (NIH) and National Cancer Institute (NCI) decreased in the past year. The cuts followed nearly a decade of funding increases that saw the NIH budget expand by nearly $15 billion, and that allowed for a “rapid pace and broad scope” of advances in cancer, AACR’s chief executive officer Margaret Foti, MD, PhD, said during a press briefing.
These recent cuts “threaten to curtail the medical progress seen in recent years and stymie future advancements,” said Dr. Foti, who called on Congress to commit to funding cancer research at significant and consistent levels to “maintain the momentum of progress against cancer.”
Inside the Report: Big Progress
Overall, advances in prevention, early detection, and treatment have helped catch more cancers earlier and save lives.
According to the AACR report, the age-adjusted overall cancer death rate in the United States fell by 33% between 1991 and 2021, meaning about 4.1 million cancer deaths were averted. The overall cancer death rate for children and adolescents has declined by 24% in the past 2 decades. The 5-year relative survival rate for children diagnosed with cancer in the US has improved from 58% for those diagnosed in the mid-1970s to 85% for those diagnosed between 2013 and 2019.
The past fiscal year has seen many new approvals for cancer drugs, diagnostics, and screening tests. From July 1, 2023, to June 30, 2024, the Food and Drug Administration (FDA) approved 15 new anticancer therapeutics, as well as 15 new indications for previously approved agents, one new imaging agent, several artificial intelligence (AI) tools to improve early cancer detection and diagnosis, and two minimally invasive tests for assessing inherited cancer risk or early cancer detection, according to the report.
“Cancer diagnostics are becoming more sophisticated,” AACR president Patricia M. LoRusso, DO, PhD, said during the briefing. “New technologies, such as spatial transcriptomics, are helping us study tumors at a cellular level, and helping to unveil things that we did not initially even begin to understand or think of. AI-based approaches are beginning to transform cancer detection, diagnosis, clinical decision-making, and treatment response monitoring.”
The report also highlights the significant progress in many childhood and adolescent/young adult cancers, Dr. LoRusso noted. These include FDA approvals for two new molecularly targeted therapeutics: tovorafenib for children with certain types of brain tumor and repotrectinib for children with a wide array of cancer types that have a specific genetic alteration known as NTRK gene fusion. It also includes an expanded approval for eflornithine to reduce the risk for relapse in children with high-risk neuroblastoma.
“Decades — decades — of basic research discoveries, have led to these clinical breakthroughs,” she stressed. “These gains against cancer are because of the rapid progress in our ability to decode the cancer genome, which has opened new and innovative avenues for drug development.”
The Gaps
Even with progress in cancer prevention, early detection, and treatment, cancer remains a significant issue.
“In 2024, it is estimated that more than 2 million new cases of cancer will be diagnosed in the United States. More than 611,000 people will die from the disease,” according to the report.
The 2024 report shows that incidence rates for some cancers are increasing in the United States, including vaccine-preventable cancers such as human papillomavirus (HPV)–associated oral cancers and, in young adults, cervical cancers. A recent analysis also found that overall cervical cancer incidence among women aged 30-34 years increased by 2.5% a year between 2012 and 2019.
Furthermore, despite clear evidence demonstrating that the HPV vaccine reduces cervical cancer incidence, uptake has remained poor, with only 38.6% of US children and adolescents aged 9-17 years receiving at least one dose of the vaccine in 2022.
Early-onset cancers are also increasing. Rates of breast, colorectal, and other cancers are on the rise in adults younger than 50 years, the report noted.
The report also pointed to data that 40% of all cancer cases in the United States can be attributed to preventable factors, such as smoking, excess body weight, and alcohol. However, our understanding of these risk factors has improved. Excessive levels of alcohol consumption have, for instance, been shown to increase the risk for six different types of cancer: certain types of head and neck cancer, esophageal squamous cell carcinoma, and breast, colorectal, liver, and stomach cancers.
Financial toxicity remains prevalent as well.
The report explains that financial hardship following a cancer diagnosis is widespread, and the effects can last for years. In fact, more than 40% of patients can spend their entire life savings within the first 2 years of cancer treatment. Among adult survivors of childhood cancers, 20.7% had trouble paying their medical bills, 29.9% said they had been sent to debt collection for unpaid bills, 14.1% had forgone medical care, and 26.8% could not afford nutritious meals.
For young cancer survivors, the lifetime costs associated with a diagnosis of cancer are substantial, reaching an average of $259,324 per person.
On a global level, it is estimated that from 2020 to 2050, the cumulative economic burden of cancer will be $25.2 trillion.
The Path Forward
Despite these challenges, Dr. LoRusso said, “it is unquestionable that we are in a time of unparalleled opportunities in cancer research.
“I am excited about what the future holds for cancer research, and especially for patient care,” she said.
However, funding commitments are needed to avoid impeding this momentum and losing a “talented and creative young workforce” that has brought new ideas and new technologies to the table.
Continued robust funding will help “to markedly improve cancer care, increase cancer survivorship, spur economic growth, and maintain the United States’ position as the global leader in science and medical research,” she added.
The AACR report specifically calls on Congress to:
- Appropriate at least $51.3 billion in fiscal year 2025 for the base budget of the NIH and at least $7.934 billion for the NCI.
- Provide $3.6 billion in dedicated funding for Cancer Moonshot activities through fiscal year 2026 in addition to other funding, consistent with the President’s fiscal year 2025 budget.
- Appropriate at least $472.4 million in fiscal year 2025 for the CDC’s Division of Cancer Prevention to support comprehensive cancer control, central cancer registries, and screening and awareness programs for specific cancers.
- Allocate $55 million in funding for the Oncology Center of Excellence at FDA in fiscal year 2025 to provide regulators with the staff and tools necessary to conduct expedited review of cancer-related medical products.
By working together with Congress and other stakeholders, “we will be able to accelerate the pace of progress and make major strides toward the lifesaving goal of preventing and curing all cancers at the earliest possible time,” Dr. Foti said. “I believe if we do that ... one day we will win this war on cancer.”
A version of this article first appeared on Medscape.com.
The AACR’s 216-page report — an annual endeavor now in its 14th year — focused on the “tremendous” strides made in cancer care, prevention, and early detection and highlighted areas where more research and attention are warranted.
One key area is funding. For the first time since 2016, federal funding for the National Institutes of Health (NIH) and National Cancer Institute (NCI) decreased in the past year. The cuts followed nearly a decade of funding increases that saw the NIH budget expand by nearly $15 billion, and that allowed for a “rapid pace and broad scope” of advances in cancer, AACR’s chief executive officer Margaret Foti, MD, PhD, said during a press briefing.
These recent cuts “threaten to curtail the medical progress seen in recent years and stymie future advancements,” said Dr. Foti, who called on Congress to commit to funding cancer research at significant and consistent levels to “maintain the momentum of progress against cancer.”
Inside the Report: Big Progress
Overall, advances in prevention, early detection, and treatment have helped catch more cancers earlier and save lives.
According to the AACR report, the age-adjusted overall cancer death rate in the United States fell by 33% between 1991 and 2021, meaning about 4.1 million cancer deaths were averted. The overall cancer death rate for children and adolescents has declined by 24% in the past 2 decades. The 5-year relative survival rate for children diagnosed with cancer in the US has improved from 58% for those diagnosed in the mid-1970s to 85% for those diagnosed between 2013 and 2019.
The past fiscal year has seen many new approvals for cancer drugs, diagnostics, and screening tests. From July 1, 2023, to June 30, 2024, the Food and Drug Administration (FDA) approved 15 new anticancer therapeutics, as well as 15 new indications for previously approved agents, one new imaging agent, several artificial intelligence (AI) tools to improve early cancer detection and diagnosis, and two minimally invasive tests for assessing inherited cancer risk or early cancer detection, according to the report.
“Cancer diagnostics are becoming more sophisticated,” AACR president Patricia M. LoRusso, DO, PhD, said during the briefing. “New technologies, such as spatial transcriptomics, are helping us study tumors at a cellular level, and helping to unveil things that we did not initially even begin to understand or think of. AI-based approaches are beginning to transform cancer detection, diagnosis, clinical decision-making, and treatment response monitoring.”
The report also highlights the significant progress in many childhood and adolescent/young adult cancers, Dr. LoRusso noted. These include FDA approvals for two new molecularly targeted therapeutics: tovorafenib for children with certain types of brain tumor and repotrectinib for children with a wide array of cancer types that have a specific genetic alteration known as NTRK gene fusion. It also includes an expanded approval for eflornithine to reduce the risk for relapse in children with high-risk neuroblastoma.
“Decades — decades — of basic research discoveries, have led to these clinical breakthroughs,” she stressed. “These gains against cancer are because of the rapid progress in our ability to decode the cancer genome, which has opened new and innovative avenues for drug development.”
The Gaps
Even with progress in cancer prevention, early detection, and treatment, cancer remains a significant issue.
“In 2024, it is estimated that more than 2 million new cases of cancer will be diagnosed in the United States. More than 611,000 people will die from the disease,” according to the report.
The 2024 report shows that incidence rates for some cancers are increasing in the United States, including vaccine-preventable cancers such as human papillomavirus (HPV)–associated oral cancers and, in young adults, cervical cancers. A recent analysis also found that overall cervical cancer incidence among women aged 30-34 years increased by 2.5% a year between 2012 and 2019.
Furthermore, despite clear evidence demonstrating that the HPV vaccine reduces cervical cancer incidence, uptake has remained poor, with only 38.6% of US children and adolescents aged 9-17 years receiving at least one dose of the vaccine in 2022.
Early-onset cancers are also increasing. Rates of breast, colorectal, and other cancers are on the rise in adults younger than 50 years, the report noted.
The report also pointed to data that 40% of all cancer cases in the United States can be attributed to preventable factors, such as smoking, excess body weight, and alcohol. However, our understanding of these risk factors has improved. Excessive levels of alcohol consumption have, for instance, been shown to increase the risk for six different types of cancer: certain types of head and neck cancer, esophageal squamous cell carcinoma, and breast, colorectal, liver, and stomach cancers.
Financial toxicity remains prevalent as well.
The report explains that financial hardship following a cancer diagnosis is widespread, and the effects can last for years. In fact, more than 40% of patients can spend their entire life savings within the first 2 years of cancer treatment. Among adult survivors of childhood cancers, 20.7% had trouble paying their medical bills, 29.9% said they had been sent to debt collection for unpaid bills, 14.1% had forgone medical care, and 26.8% could not afford nutritious meals.
For young cancer survivors, the lifetime costs associated with a diagnosis of cancer are substantial, reaching an average of $259,324 per person.
On a global level, it is estimated that from 2020 to 2050, the cumulative economic burden of cancer will be $25.2 trillion.
The Path Forward
Despite these challenges, Dr. LoRusso said, “it is unquestionable that we are in a time of unparalleled opportunities in cancer research.
“I am excited about what the future holds for cancer research, and especially for patient care,” she said.
However, funding commitments are needed to avoid impeding this momentum and losing a “talented and creative young workforce” that has brought new ideas and new technologies to the table.
Continued robust funding will help “to markedly improve cancer care, increase cancer survivorship, spur economic growth, and maintain the United States’ position as the global leader in science and medical research,” she added.
The AACR report specifically calls on Congress to:
- Appropriate at least $51.3 billion in fiscal year 2025 for the base budget of the NIH and at least $7.934 billion for the NCI.
- Provide $3.6 billion in dedicated funding for Cancer Moonshot activities through fiscal year 2026 in addition to other funding, consistent with the President’s fiscal year 2025 budget.
- Appropriate at least $472.4 million in fiscal year 2025 for the CDC’s Division of Cancer Prevention to support comprehensive cancer control, central cancer registries, and screening and awareness programs for specific cancers.
- Allocate $55 million in funding for the Oncology Center of Excellence at FDA in fiscal year 2025 to provide regulators with the staff and tools necessary to conduct expedited review of cancer-related medical products.
By working together with Congress and other stakeholders, “we will be able to accelerate the pace of progress and make major strides toward the lifesaving goal of preventing and curing all cancers at the earliest possible time,” Dr. Foti said. “I believe if we do that ... one day we will win this war on cancer.”
A version of this article first appeared on Medscape.com.
The Uneven Surge in Diabetes in the United States
TOPLINE:
METHODOLOGY:
- Over 37 million people in the United States have diabetes, and its prevalence is only expected to increase in the coming years, making identifying high-risk demographic groups particularly crucial.
- To assess recent national trends and disparities in diabetes prevalence among US adults, researchers conducted an observational study using data from the Behavioral Risk Factor Surveillance System and included 5,312,827 observations from 2012 to 2022.
- Diabetes was defined on the basis of a previous self-reported diagnosis using standardized questionnaires.
- The sociodemographic factors of age, sex, race, education, physical activity, income, and body mass index were used to establish the risk indicators for diabetes diagnosis.
- Age-standardized diabetes prevalence and the association between risk factors and diabetes were assessed both overall and across various sociodemographic groups.
TAKEAWAY:
- The overall prevalence of diabetes increased by 18.6% (P < .001) from 2012 to 2022, with the highest prevalence observed among non-Hispanic Black individuals (15.8%) and people aged ≥ 65 years (23.86%).
- The likelihood of being diagnosed with diabetes was 1.15 times higher in men than in women, 5.16 times higher in adults aged 45-64 years than in those aged 18-24 years, and 3.64 times higher in those with obesity than in those with normal weight.
- The risk for being diagnosed with diabetes was 1.60 times higher among Hispanic individuals, 1.67 times higher among non-Hispanic Asian individuals, and 2.10 times higher among non-Hispanic Black individuals than among non-Hispanic White individuals.
- Individuals with a college education and higher income level were 24% and 41% less likely, respectively, to be diagnosed with diabetes.
IN PRACTICE:
“Improving access to quality care, implementing diabetes prevention programs focusing on high-risk groups, and addressing social determinants through multilevel interventions may help curb the diabetes epidemic in the United States,” the authors wrote.
SOURCE:
The study, led by Sulakshan Neupane, MS, Department of Agricultural and Applied Economics, University of Georgia, Athens, Georgia, was published online in Diabetes, Obesity, and Metabolism.
LIMITATIONS:
The self-reported diagnoses and lack of clinical data may have introduced bias. Diabetes prevalence could not be analyzed in South-East Asian and South Asian populations owing to limitations in the data collection process.
DISCLOSURES:
The study was not supported by any funding, and no potential author disclosures or conflicts were identified.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Over 37 million people in the United States have diabetes, and its prevalence is only expected to increase in the coming years, making identifying high-risk demographic groups particularly crucial.
- To assess recent national trends and disparities in diabetes prevalence among US adults, researchers conducted an observational study using data from the Behavioral Risk Factor Surveillance System and included 5,312,827 observations from 2012 to 2022.
- Diabetes was defined on the basis of a previous self-reported diagnosis using standardized questionnaires.
- The sociodemographic factors of age, sex, race, education, physical activity, income, and body mass index were used to establish the risk indicators for diabetes diagnosis.
- Age-standardized diabetes prevalence and the association between risk factors and diabetes were assessed both overall and across various sociodemographic groups.
TAKEAWAY:
- The overall prevalence of diabetes increased by 18.6% (P < .001) from 2012 to 2022, with the highest prevalence observed among non-Hispanic Black individuals (15.8%) and people aged ≥ 65 years (23.86%).
- The likelihood of being diagnosed with diabetes was 1.15 times higher in men than in women, 5.16 times higher in adults aged 45-64 years than in those aged 18-24 years, and 3.64 times higher in those with obesity than in those with normal weight.
- The risk for being diagnosed with diabetes was 1.60 times higher among Hispanic individuals, 1.67 times higher among non-Hispanic Asian individuals, and 2.10 times higher among non-Hispanic Black individuals than among non-Hispanic White individuals.
- Individuals with a college education and higher income level were 24% and 41% less likely, respectively, to be diagnosed with diabetes.
IN PRACTICE:
“Improving access to quality care, implementing diabetes prevention programs focusing on high-risk groups, and addressing social determinants through multilevel interventions may help curb the diabetes epidemic in the United States,” the authors wrote.
SOURCE:
The study, led by Sulakshan Neupane, MS, Department of Agricultural and Applied Economics, University of Georgia, Athens, Georgia, was published online in Diabetes, Obesity, and Metabolism.
LIMITATIONS:
The self-reported diagnoses and lack of clinical data may have introduced bias. Diabetes prevalence could not be analyzed in South-East Asian and South Asian populations owing to limitations in the data collection process.
DISCLOSURES:
The study was not supported by any funding, and no potential author disclosures or conflicts were identified.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Over 37 million people in the United States have diabetes, and its prevalence is only expected to increase in the coming years, making identifying high-risk demographic groups particularly crucial.
- To assess recent national trends and disparities in diabetes prevalence among US adults, researchers conducted an observational study using data from the Behavioral Risk Factor Surveillance System and included 5,312,827 observations from 2012 to 2022.
- Diabetes was defined on the basis of a previous self-reported diagnosis using standardized questionnaires.
- The sociodemographic factors of age, sex, race, education, physical activity, income, and body mass index were used to establish the risk indicators for diabetes diagnosis.
- Age-standardized diabetes prevalence and the association between risk factors and diabetes were assessed both overall and across various sociodemographic groups.
TAKEAWAY:
- The overall prevalence of diabetes increased by 18.6% (P < .001) from 2012 to 2022, with the highest prevalence observed among non-Hispanic Black individuals (15.8%) and people aged ≥ 65 years (23.86%).
- The likelihood of being diagnosed with diabetes was 1.15 times higher in men than in women, 5.16 times higher in adults aged 45-64 years than in those aged 18-24 years, and 3.64 times higher in those with obesity than in those with normal weight.
- The risk for being diagnosed with diabetes was 1.60 times higher among Hispanic individuals, 1.67 times higher among non-Hispanic Asian individuals, and 2.10 times higher among non-Hispanic Black individuals than among non-Hispanic White individuals.
- Individuals with a college education and higher income level were 24% and 41% less likely, respectively, to be diagnosed with diabetes.
IN PRACTICE:
“Improving access to quality care, implementing diabetes prevention programs focusing on high-risk groups, and addressing social determinants through multilevel interventions may help curb the diabetes epidemic in the United States,” the authors wrote.
SOURCE:
The study, led by Sulakshan Neupane, MS, Department of Agricultural and Applied Economics, University of Georgia, Athens, Georgia, was published online in Diabetes, Obesity, and Metabolism.
LIMITATIONS:
The self-reported diagnoses and lack of clinical data may have introduced bias. Diabetes prevalence could not be analyzed in South-East Asian and South Asian populations owing to limitations in the data collection process.
DISCLOSURES:
The study was not supported by any funding, and no potential author disclosures or conflicts were identified.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Black Women Have a Higher Risk for Death in BC Subtypes
TOPLINE:
The greatest disparity in BC-specific survival was observed in those with hormone receptor-positive (HR+), human epidermal growth factor 2–negative (HER2−) tumors, with Black women having a 50% higher risk for death.
METHODOLOGY:
- US Black women have a 40% higher risk for death from BC than White women, and many cancer specialists believe that disparities are worse among more treatable subtypes, such as HR+ tumors.
- Researchers conducted a systematic review and meta-analysis of 18 US studies published during 2009-2022 that included 228,885 women (34,262 Black women; 182,466 White women) and examined racial differences in BC survival by subtype.
- The analysis included hormone receptor and HER2/neu status to define subtypes: HR+ HER2+, HR+ HER2−, HR− HER2+, and HR− HER2−.
- Random-effects models were used to generate pooled relative risks and 95% CI for BC-specific survival and overall survival.
- The primary outcome was BC-specific survival, with overall survival as a secondary analysis.
TAKEAWAY:
- Black women had a higher risk for BC death across all tumor subtypes than White women, with the greatest disparity observed in HR+ HER2− tumors (hazard ratio [HR], 1.50; 95% CI, 1.30-1.72).
- The risk for BC death was also higher for Black women with HR+ HER2+ tumors (HR, 1.34; 95% CI, 1.10-1.64); HR− HER2+ tumors (HR, 1.20; 95% CI, 1.00-1.43); and HR− HER2− tumors (HR, 1.17; 95% CI, 1.10-1.25).
- Overall survival was poorer for Black women across all subtypes, although estimates for HR− HER2+ tumors did not reach statistical significance.
- In analysis of two subtypes with significant heterogeneity among studies, adjustments for socioeconomic status and number of Black participants explained about half and all the variance for HR+ HER2− and HR− HER2+ tumors, respectively.
IN PRACTICE:
“These results suggest there are both subtype-specific and subtype-independent mechanisms that contribute to disparities in breast cancer survival between Black and White women, which require multilevel interventions to address and achieve health equity,” wrote the authors.
SOURCE:
The study was led by Juliana M. Torres, Dana-Farber/Harvard Cancer Center, CURE Program, Boston. It was published online in the Journal of Clinical Oncology.
LIMITATIONS:
The study’s limitations included potential heterogeneity between studies as indicated by significant heterogeneity in some analyses. The use of different subtype definitions and potential overlap in data sets may have also affected the results. Many included studies did not capture the extent to which treatments were completed or detection and treatment of recurrences. Additionally, the study’s findings may not fully capture socioeconomic inequality and other unmeasured factors contributing to disparities. The racial and ethnic disparities analysis focused only on Black and White women.
DISCLOSURES:
Individual authors disclosed financial relationships with Pfizer, Healthix, Merck, AstraZeneca, LabCorp, and Takeda. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
The greatest disparity in BC-specific survival was observed in those with hormone receptor-positive (HR+), human epidermal growth factor 2–negative (HER2−) tumors, with Black women having a 50% higher risk for death.
METHODOLOGY:
- US Black women have a 40% higher risk for death from BC than White women, and many cancer specialists believe that disparities are worse among more treatable subtypes, such as HR+ tumors.
- Researchers conducted a systematic review and meta-analysis of 18 US studies published during 2009-2022 that included 228,885 women (34,262 Black women; 182,466 White women) and examined racial differences in BC survival by subtype.
- The analysis included hormone receptor and HER2/neu status to define subtypes: HR+ HER2+, HR+ HER2−, HR− HER2+, and HR− HER2−.
- Random-effects models were used to generate pooled relative risks and 95% CI for BC-specific survival and overall survival.
- The primary outcome was BC-specific survival, with overall survival as a secondary analysis.
TAKEAWAY:
- Black women had a higher risk for BC death across all tumor subtypes than White women, with the greatest disparity observed in HR+ HER2− tumors (hazard ratio [HR], 1.50; 95% CI, 1.30-1.72).
- The risk for BC death was also higher for Black women with HR+ HER2+ tumors (HR, 1.34; 95% CI, 1.10-1.64); HR− HER2+ tumors (HR, 1.20; 95% CI, 1.00-1.43); and HR− HER2− tumors (HR, 1.17; 95% CI, 1.10-1.25).
- Overall survival was poorer for Black women across all subtypes, although estimates for HR− HER2+ tumors did not reach statistical significance.
- In analysis of two subtypes with significant heterogeneity among studies, adjustments for socioeconomic status and number of Black participants explained about half and all the variance for HR+ HER2− and HR− HER2+ tumors, respectively.
IN PRACTICE:
“These results suggest there are both subtype-specific and subtype-independent mechanisms that contribute to disparities in breast cancer survival between Black and White women, which require multilevel interventions to address and achieve health equity,” wrote the authors.
SOURCE:
The study was led by Juliana M. Torres, Dana-Farber/Harvard Cancer Center, CURE Program, Boston. It was published online in the Journal of Clinical Oncology.
LIMITATIONS:
The study’s limitations included potential heterogeneity between studies as indicated by significant heterogeneity in some analyses. The use of different subtype definitions and potential overlap in data sets may have also affected the results. Many included studies did not capture the extent to which treatments were completed or detection and treatment of recurrences. Additionally, the study’s findings may not fully capture socioeconomic inequality and other unmeasured factors contributing to disparities. The racial and ethnic disparities analysis focused only on Black and White women.
DISCLOSURES:
Individual authors disclosed financial relationships with Pfizer, Healthix, Merck, AstraZeneca, LabCorp, and Takeda. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
The greatest disparity in BC-specific survival was observed in those with hormone receptor-positive (HR+), human epidermal growth factor 2–negative (HER2−) tumors, with Black women having a 50% higher risk for death.
METHODOLOGY:
- US Black women have a 40% higher risk for death from BC than White women, and many cancer specialists believe that disparities are worse among more treatable subtypes, such as HR+ tumors.
- Researchers conducted a systematic review and meta-analysis of 18 US studies published during 2009-2022 that included 228,885 women (34,262 Black women; 182,466 White women) and examined racial differences in BC survival by subtype.
- The analysis included hormone receptor and HER2/neu status to define subtypes: HR+ HER2+, HR+ HER2−, HR− HER2+, and HR− HER2−.
- Random-effects models were used to generate pooled relative risks and 95% CI for BC-specific survival and overall survival.
- The primary outcome was BC-specific survival, with overall survival as a secondary analysis.
TAKEAWAY:
- Black women had a higher risk for BC death across all tumor subtypes than White women, with the greatest disparity observed in HR+ HER2− tumors (hazard ratio [HR], 1.50; 95% CI, 1.30-1.72).
- The risk for BC death was also higher for Black women with HR+ HER2+ tumors (HR, 1.34; 95% CI, 1.10-1.64); HR− HER2+ tumors (HR, 1.20; 95% CI, 1.00-1.43); and HR− HER2− tumors (HR, 1.17; 95% CI, 1.10-1.25).
- Overall survival was poorer for Black women across all subtypes, although estimates for HR− HER2+ tumors did not reach statistical significance.
- In analysis of two subtypes with significant heterogeneity among studies, adjustments for socioeconomic status and number of Black participants explained about half and all the variance for HR+ HER2− and HR− HER2+ tumors, respectively.
IN PRACTICE:
“These results suggest there are both subtype-specific and subtype-independent mechanisms that contribute to disparities in breast cancer survival between Black and White women, which require multilevel interventions to address and achieve health equity,” wrote the authors.
SOURCE:
The study was led by Juliana M. Torres, Dana-Farber/Harvard Cancer Center, CURE Program, Boston. It was published online in the Journal of Clinical Oncology.
LIMITATIONS:
The study’s limitations included potential heterogeneity between studies as indicated by significant heterogeneity in some analyses. The use of different subtype definitions and potential overlap in data sets may have also affected the results. Many included studies did not capture the extent to which treatments were completed or detection and treatment of recurrences. Additionally, the study’s findings may not fully capture socioeconomic inequality and other unmeasured factors contributing to disparities. The racial and ethnic disparities analysis focused only on Black and White women.
DISCLOSURES:
Individual authors disclosed financial relationships with Pfizer, Healthix, Merck, AstraZeneca, LabCorp, and Takeda. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
Seborrheic Dermatitis in Black Patients: New Therapies Offer Hope
NEW YORK — not only in this group but also overall, now that there is an approved therapy with an array of alternatives and adjunctive medications, according to Shawn Kwatra, MD.
The list of therapies effective against SD, often employed in combination, is lengthy, but topical 0.3% roflumilast foam (Zoryve), approved by the Food and Drug Administration (FDA) late last year for treating SD, has a high rate of efficacy and should now be considered a first-line treatment option, according to Dr. Kwatra, professor and chair of the Department of Dermatology, University of Maryland School of Medicine, Baltimore.
New Approved Therapy Draws Attention to SD
Emphasizing that topical roflumilast does not necessarily replace the use of over-the-counter shampoos and emollients or a list of prescription drugs used off-label to control this condition, he said it is also important for another reason.
“It shines a light on this disease,” said Dr. Kwatra, speaking at the 2024 Skin of Color Update. While his comments were focused primarily on individuals with darker skin, his major take home messages were broadly relevant across skin types.
He acknowledged that for years he “had not given seborrheic dermatitis the respect that it deserves” even though this condition comes after only acne and eczema as chief complaints among Black individuals seeing a dermatologist. The estimated global incidence is 5%, according to Dr. Kwatra, but he considers this estimate of an often “forgotten disease” too low.
One reason is that many individuals self-treat with over-the-counter solutions and never bring the complaint to a clinician. Dr. Kwatra said that he now looks for it routinely and points it out to patients who have come to him for another reason.
In patients with darker skin, the signs of SD can differ. While scalp involvement is generally easy to identify across skin types, the inflammation and erythema, sebum production, scaling and itch, and Malassezia that accompanies and drives SD might be missed in a patient with darker skin without specifically looking for these signs.
Skin and Gut Microbiome Involvement Suspected
The underlying causes of SD are understood as an inflammatory process involving keratinocyte disruption and proliferation that ultimately impairs skin barrier function, causes water loss, and produces scale stemming from stratum corneum, but Dr. Kwatra said that there is increasing evidence of a major role for both the skin and gut microbiome.
In regard to the skin microbiome, Malassezia has long been recognized as linked to SD and is a target of treatment, but evidence that the gut microbiome might be participating is relatively new. One clue comes from the fact that oral antifungal therapies, such as itraconazole, are known to reduce risk for SD relapse, an effect that might be a function of their ability to modulate the gut microbiome, according to Dr. Kwatra.
Topical roflumilast, a phosphodiesterase-4 inhibitor, was effective for SD in a vehicle-controlled phase 3 trial published in 2023. He characterized the adverse event profile as “pretty clean,” but he emphasized that a role for many other strategies remains. This is particularly true for challenging forms of SD. For example, topical tacrolimus provided meaningful protection against relapse over a period of more than 6 months in a 2021 trial that enrolled patients with severe facial SD.
The topical Janus kinase inhibitor ruxolitinib, 1.5%, (approved for atopic dermatitis and vitiligo) has also been reported to be effective for refractory facial SD. It is being evaluated in a phase 2 study of SD, according to Dr. Kwatra. A topical PDE4 inhibitor is also being evaluated for SD in a phase 2 study, he said.
Given the heterogeneity of the presentation of SD and the value of combining different mechanisms of action, Dr. Kwatra does not think any drug by itself will be a cure for SD. However, the chances of success with current drug combinations are high.
It is for this reason that Dr. Kwatra encourages clinicians to look for this disease routinely, including among patients who have a different presenting complaint. “Patients do not always bring it up, so bring it up,” he said.
This is good advice, according to Andrew F. Alexis, MD, MPH, professor of clinical dermatology and Vice-chair for Diversity and Inclusion of the Department of Dermatology, Weill Cornell Medicine, New York City. He agreed that the recent introduction of a therapy approved by the FDA is an impetus to look for SD and to talk with patients about treatment options.
In addition, while he also considers roflumilast foam to be a first-line drug, he agreed that combination therapies might be needed to increase the likely of rapid control of scalp and skin involvement. “SD is probably underestimated as a clinical problem, and we do have good treatments to offer for the patients who are affected,” he said at the meeting.
Dr. Kwatra reported no relevant disclosures. Dr. Alexis reported financial relationships with more than 25 pharmaceutical companies.
A version of this article appeared on Medscape.com.
NEW YORK — not only in this group but also overall, now that there is an approved therapy with an array of alternatives and adjunctive medications, according to Shawn Kwatra, MD.
The list of therapies effective against SD, often employed in combination, is lengthy, but topical 0.3% roflumilast foam (Zoryve), approved by the Food and Drug Administration (FDA) late last year for treating SD, has a high rate of efficacy and should now be considered a first-line treatment option, according to Dr. Kwatra, professor and chair of the Department of Dermatology, University of Maryland School of Medicine, Baltimore.
New Approved Therapy Draws Attention to SD
Emphasizing that topical roflumilast does not necessarily replace the use of over-the-counter shampoos and emollients or a list of prescription drugs used off-label to control this condition, he said it is also important for another reason.
“It shines a light on this disease,” said Dr. Kwatra, speaking at the 2024 Skin of Color Update. While his comments were focused primarily on individuals with darker skin, his major take home messages were broadly relevant across skin types.
He acknowledged that for years he “had not given seborrheic dermatitis the respect that it deserves” even though this condition comes after only acne and eczema as chief complaints among Black individuals seeing a dermatologist. The estimated global incidence is 5%, according to Dr. Kwatra, but he considers this estimate of an often “forgotten disease” too low.
One reason is that many individuals self-treat with over-the-counter solutions and never bring the complaint to a clinician. Dr. Kwatra said that he now looks for it routinely and points it out to patients who have come to him for another reason.
In patients with darker skin, the signs of SD can differ. While scalp involvement is generally easy to identify across skin types, the inflammation and erythema, sebum production, scaling and itch, and Malassezia that accompanies and drives SD might be missed in a patient with darker skin without specifically looking for these signs.
Skin and Gut Microbiome Involvement Suspected
The underlying causes of SD are understood as an inflammatory process involving keratinocyte disruption and proliferation that ultimately impairs skin barrier function, causes water loss, and produces scale stemming from stratum corneum, but Dr. Kwatra said that there is increasing evidence of a major role for both the skin and gut microbiome.
In regard to the skin microbiome, Malassezia has long been recognized as linked to SD and is a target of treatment, but evidence that the gut microbiome might be participating is relatively new. One clue comes from the fact that oral antifungal therapies, such as itraconazole, are known to reduce risk for SD relapse, an effect that might be a function of their ability to modulate the gut microbiome, according to Dr. Kwatra.
Topical roflumilast, a phosphodiesterase-4 inhibitor, was effective for SD in a vehicle-controlled phase 3 trial published in 2023. He characterized the adverse event profile as “pretty clean,” but he emphasized that a role for many other strategies remains. This is particularly true for challenging forms of SD. For example, topical tacrolimus provided meaningful protection against relapse over a period of more than 6 months in a 2021 trial that enrolled patients with severe facial SD.
The topical Janus kinase inhibitor ruxolitinib, 1.5%, (approved for atopic dermatitis and vitiligo) has also been reported to be effective for refractory facial SD. It is being evaluated in a phase 2 study of SD, according to Dr. Kwatra. A topical PDE4 inhibitor is also being evaluated for SD in a phase 2 study, he said.
Given the heterogeneity of the presentation of SD and the value of combining different mechanisms of action, Dr. Kwatra does not think any drug by itself will be a cure for SD. However, the chances of success with current drug combinations are high.
It is for this reason that Dr. Kwatra encourages clinicians to look for this disease routinely, including among patients who have a different presenting complaint. “Patients do not always bring it up, so bring it up,” he said.
This is good advice, according to Andrew F. Alexis, MD, MPH, professor of clinical dermatology and Vice-chair for Diversity and Inclusion of the Department of Dermatology, Weill Cornell Medicine, New York City. He agreed that the recent introduction of a therapy approved by the FDA is an impetus to look for SD and to talk with patients about treatment options.
In addition, while he also considers roflumilast foam to be a first-line drug, he agreed that combination therapies might be needed to increase the likely of rapid control of scalp and skin involvement. “SD is probably underestimated as a clinical problem, and we do have good treatments to offer for the patients who are affected,” he said at the meeting.
Dr. Kwatra reported no relevant disclosures. Dr. Alexis reported financial relationships with more than 25 pharmaceutical companies.
A version of this article appeared on Medscape.com.
NEW YORK — not only in this group but also overall, now that there is an approved therapy with an array of alternatives and adjunctive medications, according to Shawn Kwatra, MD.
The list of therapies effective against SD, often employed in combination, is lengthy, but topical 0.3% roflumilast foam (Zoryve), approved by the Food and Drug Administration (FDA) late last year for treating SD, has a high rate of efficacy and should now be considered a first-line treatment option, according to Dr. Kwatra, professor and chair of the Department of Dermatology, University of Maryland School of Medicine, Baltimore.
New Approved Therapy Draws Attention to SD
Emphasizing that topical roflumilast does not necessarily replace the use of over-the-counter shampoos and emollients or a list of prescription drugs used off-label to control this condition, he said it is also important for another reason.
“It shines a light on this disease,” said Dr. Kwatra, speaking at the 2024 Skin of Color Update. While his comments were focused primarily on individuals with darker skin, his major take home messages were broadly relevant across skin types.
He acknowledged that for years he “had not given seborrheic dermatitis the respect that it deserves” even though this condition comes after only acne and eczema as chief complaints among Black individuals seeing a dermatologist. The estimated global incidence is 5%, according to Dr. Kwatra, but he considers this estimate of an often “forgotten disease” too low.
One reason is that many individuals self-treat with over-the-counter solutions and never bring the complaint to a clinician. Dr. Kwatra said that he now looks for it routinely and points it out to patients who have come to him for another reason.
In patients with darker skin, the signs of SD can differ. While scalp involvement is generally easy to identify across skin types, the inflammation and erythema, sebum production, scaling and itch, and Malassezia that accompanies and drives SD might be missed in a patient with darker skin without specifically looking for these signs.
Skin and Gut Microbiome Involvement Suspected
The underlying causes of SD are understood as an inflammatory process involving keratinocyte disruption and proliferation that ultimately impairs skin barrier function, causes water loss, and produces scale stemming from stratum corneum, but Dr. Kwatra said that there is increasing evidence of a major role for both the skin and gut microbiome.
In regard to the skin microbiome, Malassezia has long been recognized as linked to SD and is a target of treatment, but evidence that the gut microbiome might be participating is relatively new. One clue comes from the fact that oral antifungal therapies, such as itraconazole, are known to reduce risk for SD relapse, an effect that might be a function of their ability to modulate the gut microbiome, according to Dr. Kwatra.
Topical roflumilast, a phosphodiesterase-4 inhibitor, was effective for SD in a vehicle-controlled phase 3 trial published in 2023. He characterized the adverse event profile as “pretty clean,” but he emphasized that a role for many other strategies remains. This is particularly true for challenging forms of SD. For example, topical tacrolimus provided meaningful protection against relapse over a period of more than 6 months in a 2021 trial that enrolled patients with severe facial SD.
The topical Janus kinase inhibitor ruxolitinib, 1.5%, (approved for atopic dermatitis and vitiligo) has also been reported to be effective for refractory facial SD. It is being evaluated in a phase 2 study of SD, according to Dr. Kwatra. A topical PDE4 inhibitor is also being evaluated for SD in a phase 2 study, he said.
Given the heterogeneity of the presentation of SD and the value of combining different mechanisms of action, Dr. Kwatra does not think any drug by itself will be a cure for SD. However, the chances of success with current drug combinations are high.
It is for this reason that Dr. Kwatra encourages clinicians to look for this disease routinely, including among patients who have a different presenting complaint. “Patients do not always bring it up, so bring it up,” he said.
This is good advice, according to Andrew F. Alexis, MD, MPH, professor of clinical dermatology and Vice-chair for Diversity and Inclusion of the Department of Dermatology, Weill Cornell Medicine, New York City. He agreed that the recent introduction of a therapy approved by the FDA is an impetus to look for SD and to talk with patients about treatment options.
In addition, while he also considers roflumilast foam to be a first-line drug, he agreed that combination therapies might be needed to increase the likely of rapid control of scalp and skin involvement. “SD is probably underestimated as a clinical problem, and we do have good treatments to offer for the patients who are affected,” he said at the meeting.
Dr. Kwatra reported no relevant disclosures. Dr. Alexis reported financial relationships with more than 25 pharmaceutical companies.
A version of this article appeared on Medscape.com.
FROM SOC 2024
Identifying Drug-Induced Rashes in Skin of Color: Heightened Awareness Can Accelerate Diagnosis
NEW YORK — Because of their heterogeneity in appearance, to speed the diagnosis.
This risk for a delayed or missed diagnosis in patients with darker skin is shared across skin rashes, but drug-induced hypersensitivity syndrome (DIHS) is a telling example, according to Joanna Harp, MD, director of the Inpatient Dermatology Consult Service, NewYork–Presbyterian Hospital, New York City.
DIHS, also known as a drug reaction with eosinophilia and systemic symptoms, is a type IV hypersensitivity reaction, Dr. Harp explained. While the fact that this disorder does not always include eosinophilia prompted the DIHS acronym, the maculopapular rash often serves as a critical clue of the underlying etiology.
In patients with darker skin, DIHS skin manifestations “can look different, can be more severe, and can have worse outcomes,” Dr. Harp said. As with other skin rashes that are primarily erythematous, the DIHS rash is often more subtle in Black-skinned patients, typically appearing gray or violaceous rather than red.
“The high amount of scale can be a clue,” said Dr. Harp, speaking at the 2024 Skin of Color Update. Scale is particularly prominent among Black patients, she said, because of the greater relative transepidermal water loss than lighter skin, increasing dryness and susceptibility to scale.
The maculopapular rash is “similar to a simple drug eruption, although it is usually more impressive,” she said. Emphasizing that DIHS is a systemic disease, she noted that the characteristic rash is typically accompanied by inflammation in multiple organs that not only includes the mucous membranes but can include major organs such as the lungs, kidneys, and heart.
In patients with DIHS and many of the even more serious types of rashes traced to drug exposures, such as Stevens-Johnson syndrome (SJS) or erythema multiforme, the delay to appearance of the rash from the time of exposure can be the most confusing element.
“It can be months for some drugs such as allopurinol,” said Dr. Harp, pointing out that Black and Asian patients are more likely to carry the HLA-B*5801 genotype, a known risk factor for allopurinol hypersensitivity.
Signs of AGEP Can Be Subtle in Black Patients
Some of the same principles for diagnosing drug-induced rash in darker skin can also be applied to acute generalized exanthematous pustulosis (AGEP), another type IV hypersensitivity reaction. Like all drug-induced rashes, the earlier AGEP is recognized and treated, the better the outcome, but in Black patients, the signs can be subtle.
“The onset is usually fast and occurs in 1-2 days after [the causative drug] exposure,” said Dr. Harp, adding that antibiotics, such as cephalosporins or penicillin, and calcium channel blockers are among the prominent causes of AGEP.
One of the hallmark signs of early-onset AGEP are tiny erythematous pustules in flexural areas, such as the neck or the armpits. The issue of detecting erythema in darker skin is also relevant to this area, but there is an additional problem, according to Dr. Harp. The pustules often dry up quickly, leaving a neutrophilic scale that further complicates the effort to see the characteristic erythema.
“If you see a lot of scale, look for erythema underneath. Think of inflammation,” Dr. Harp said, explaining that the clinical appearance evolves quickly. “If you do not see the pustules, it does not mean they were not there; you just missed them.”
In addition to the flexural areas, “AGEP loves the ears, the face, and the geographic tongue,” she said, offering several pearls to help with the diagnosis. These include side lighting to make papules easier to see, pressing on the skin to highlight the difference between erythematous skin and blanched skin, and checking less pigmented skin, such as on the hands and feet, which makes erythema easier to see.
Steroids are often the first-line treatment for drug-induced skin rashes, but Dr. Harp moves to etanercept or cyclosporine for the most serious drug reactions, such as SJS and toxic epidermal necrolysis.
Etanercept is typically her first choice because patients with systemic hypersensitivity reactions with major organ involvement are often quite ill, making cyclosporine harder to use. In her experience, etanercept has been well tolerated.
Conversely, she cautioned against the use of intravenous immunoglobulin (IVIG). Although this has been used traditionally for severe drug hypersensitivity reactions, “the data are not there,” she said. The data are stronger for a combination of high-dose steroids and IVIG, but she thinks even these data are inconsistent and not as strong as the data supporting etanercept or cyclosporine. She encouraged centers still using IVIG to consider alternatives.
After drug sensitivity reactions are controlled, follow-up care is particularly important for Black patients who face greater risks for sequelae, such as hypopigmentation, hyperpigmentation, or keloids. She recommended aggressive use of emollients and sunscreens for an extended period after lesions resolve to lessen these risks.
Differences in the manifestations of drug-induced skin rashes by race and ethnicity are important and perhaps underappreciated, agreed Shawn Kwatra, MD, professor and chairman of the Department of Dermatology, University of Maryland, Baltimore.
Asked to comment at the meeting, Dr. Kwatra said that he appreciated Dr. Harp’s effort to translate published data and her experience into an overview that increases awareness of the risk for missed or delayed diagnoses of drug-induced rashes in skin of color. He noted that the strategies to identify erythema and pustules, such as increased suspicion in skin of color and the extra steps to rule them out, such as the use of side lighting in the case of pustules for AGEP, are simple and practical.
Dr. Harp and Dr. Kwatra had no relevant disclosures.
A version of this article appeared on Medscape.com.
NEW YORK — Because of their heterogeneity in appearance, to speed the diagnosis.
This risk for a delayed or missed diagnosis in patients with darker skin is shared across skin rashes, but drug-induced hypersensitivity syndrome (DIHS) is a telling example, according to Joanna Harp, MD, director of the Inpatient Dermatology Consult Service, NewYork–Presbyterian Hospital, New York City.
DIHS, also known as a drug reaction with eosinophilia and systemic symptoms, is a type IV hypersensitivity reaction, Dr. Harp explained. While the fact that this disorder does not always include eosinophilia prompted the DIHS acronym, the maculopapular rash often serves as a critical clue of the underlying etiology.
In patients with darker skin, DIHS skin manifestations “can look different, can be more severe, and can have worse outcomes,” Dr. Harp said. As with other skin rashes that are primarily erythematous, the DIHS rash is often more subtle in Black-skinned patients, typically appearing gray or violaceous rather than red.
“The high amount of scale can be a clue,” said Dr. Harp, speaking at the 2024 Skin of Color Update. Scale is particularly prominent among Black patients, she said, because of the greater relative transepidermal water loss than lighter skin, increasing dryness and susceptibility to scale.
The maculopapular rash is “similar to a simple drug eruption, although it is usually more impressive,” she said. Emphasizing that DIHS is a systemic disease, she noted that the characteristic rash is typically accompanied by inflammation in multiple organs that not only includes the mucous membranes but can include major organs such as the lungs, kidneys, and heart.
In patients with DIHS and many of the even more serious types of rashes traced to drug exposures, such as Stevens-Johnson syndrome (SJS) or erythema multiforme, the delay to appearance of the rash from the time of exposure can be the most confusing element.
“It can be months for some drugs such as allopurinol,” said Dr. Harp, pointing out that Black and Asian patients are more likely to carry the HLA-B*5801 genotype, a known risk factor for allopurinol hypersensitivity.
Signs of AGEP Can Be Subtle in Black Patients
Some of the same principles for diagnosing drug-induced rash in darker skin can also be applied to acute generalized exanthematous pustulosis (AGEP), another type IV hypersensitivity reaction. Like all drug-induced rashes, the earlier AGEP is recognized and treated, the better the outcome, but in Black patients, the signs can be subtle.
“The onset is usually fast and occurs in 1-2 days after [the causative drug] exposure,” said Dr. Harp, adding that antibiotics, such as cephalosporins or penicillin, and calcium channel blockers are among the prominent causes of AGEP.
One of the hallmark signs of early-onset AGEP are tiny erythematous pustules in flexural areas, such as the neck or the armpits. The issue of detecting erythema in darker skin is also relevant to this area, but there is an additional problem, according to Dr. Harp. The pustules often dry up quickly, leaving a neutrophilic scale that further complicates the effort to see the characteristic erythema.
“If you see a lot of scale, look for erythema underneath. Think of inflammation,” Dr. Harp said, explaining that the clinical appearance evolves quickly. “If you do not see the pustules, it does not mean they were not there; you just missed them.”
In addition to the flexural areas, “AGEP loves the ears, the face, and the geographic tongue,” she said, offering several pearls to help with the diagnosis. These include side lighting to make papules easier to see, pressing on the skin to highlight the difference between erythematous skin and blanched skin, and checking less pigmented skin, such as on the hands and feet, which makes erythema easier to see.
Steroids are often the first-line treatment for drug-induced skin rashes, but Dr. Harp moves to etanercept or cyclosporine for the most serious drug reactions, such as SJS and toxic epidermal necrolysis.
Etanercept is typically her first choice because patients with systemic hypersensitivity reactions with major organ involvement are often quite ill, making cyclosporine harder to use. In her experience, etanercept has been well tolerated.
Conversely, she cautioned against the use of intravenous immunoglobulin (IVIG). Although this has been used traditionally for severe drug hypersensitivity reactions, “the data are not there,” she said. The data are stronger for a combination of high-dose steroids and IVIG, but she thinks even these data are inconsistent and not as strong as the data supporting etanercept or cyclosporine. She encouraged centers still using IVIG to consider alternatives.
After drug sensitivity reactions are controlled, follow-up care is particularly important for Black patients who face greater risks for sequelae, such as hypopigmentation, hyperpigmentation, or keloids. She recommended aggressive use of emollients and sunscreens for an extended period after lesions resolve to lessen these risks.
Differences in the manifestations of drug-induced skin rashes by race and ethnicity are important and perhaps underappreciated, agreed Shawn Kwatra, MD, professor and chairman of the Department of Dermatology, University of Maryland, Baltimore.
Asked to comment at the meeting, Dr. Kwatra said that he appreciated Dr. Harp’s effort to translate published data and her experience into an overview that increases awareness of the risk for missed or delayed diagnoses of drug-induced rashes in skin of color. He noted that the strategies to identify erythema and pustules, such as increased suspicion in skin of color and the extra steps to rule them out, such as the use of side lighting in the case of pustules for AGEP, are simple and practical.
Dr. Harp and Dr. Kwatra had no relevant disclosures.
A version of this article appeared on Medscape.com.
NEW YORK — Because of their heterogeneity in appearance, to speed the diagnosis.
This risk for a delayed or missed diagnosis in patients with darker skin is shared across skin rashes, but drug-induced hypersensitivity syndrome (DIHS) is a telling example, according to Joanna Harp, MD, director of the Inpatient Dermatology Consult Service, NewYork–Presbyterian Hospital, New York City.
DIHS, also known as a drug reaction with eosinophilia and systemic symptoms, is a type IV hypersensitivity reaction, Dr. Harp explained. While the fact that this disorder does not always include eosinophilia prompted the DIHS acronym, the maculopapular rash often serves as a critical clue of the underlying etiology.
In patients with darker skin, DIHS skin manifestations “can look different, can be more severe, and can have worse outcomes,” Dr. Harp said. As with other skin rashes that are primarily erythematous, the DIHS rash is often more subtle in Black-skinned patients, typically appearing gray or violaceous rather than red.
“The high amount of scale can be a clue,” said Dr. Harp, speaking at the 2024 Skin of Color Update. Scale is particularly prominent among Black patients, she said, because of the greater relative transepidermal water loss than lighter skin, increasing dryness and susceptibility to scale.
The maculopapular rash is “similar to a simple drug eruption, although it is usually more impressive,” she said. Emphasizing that DIHS is a systemic disease, she noted that the characteristic rash is typically accompanied by inflammation in multiple organs that not only includes the mucous membranes but can include major organs such as the lungs, kidneys, and heart.
In patients with DIHS and many of the even more serious types of rashes traced to drug exposures, such as Stevens-Johnson syndrome (SJS) or erythema multiforme, the delay to appearance of the rash from the time of exposure can be the most confusing element.
“It can be months for some drugs such as allopurinol,” said Dr. Harp, pointing out that Black and Asian patients are more likely to carry the HLA-B*5801 genotype, a known risk factor for allopurinol hypersensitivity.
Signs of AGEP Can Be Subtle in Black Patients
Some of the same principles for diagnosing drug-induced rash in darker skin can also be applied to acute generalized exanthematous pustulosis (AGEP), another type IV hypersensitivity reaction. Like all drug-induced rashes, the earlier AGEP is recognized and treated, the better the outcome, but in Black patients, the signs can be subtle.
“The onset is usually fast and occurs in 1-2 days after [the causative drug] exposure,” said Dr. Harp, adding that antibiotics, such as cephalosporins or penicillin, and calcium channel blockers are among the prominent causes of AGEP.
One of the hallmark signs of early-onset AGEP are tiny erythematous pustules in flexural areas, such as the neck or the armpits. The issue of detecting erythema in darker skin is also relevant to this area, but there is an additional problem, according to Dr. Harp. The pustules often dry up quickly, leaving a neutrophilic scale that further complicates the effort to see the characteristic erythema.
“If you see a lot of scale, look for erythema underneath. Think of inflammation,” Dr. Harp said, explaining that the clinical appearance evolves quickly. “If you do not see the pustules, it does not mean they were not there; you just missed them.”
In addition to the flexural areas, “AGEP loves the ears, the face, and the geographic tongue,” she said, offering several pearls to help with the diagnosis. These include side lighting to make papules easier to see, pressing on the skin to highlight the difference between erythematous skin and blanched skin, and checking less pigmented skin, such as on the hands and feet, which makes erythema easier to see.
Steroids are often the first-line treatment for drug-induced skin rashes, but Dr. Harp moves to etanercept or cyclosporine for the most serious drug reactions, such as SJS and toxic epidermal necrolysis.
Etanercept is typically her first choice because patients with systemic hypersensitivity reactions with major organ involvement are often quite ill, making cyclosporine harder to use. In her experience, etanercept has been well tolerated.
Conversely, she cautioned against the use of intravenous immunoglobulin (IVIG). Although this has been used traditionally for severe drug hypersensitivity reactions, “the data are not there,” she said. The data are stronger for a combination of high-dose steroids and IVIG, but she thinks even these data are inconsistent and not as strong as the data supporting etanercept or cyclosporine. She encouraged centers still using IVIG to consider alternatives.
After drug sensitivity reactions are controlled, follow-up care is particularly important for Black patients who face greater risks for sequelae, such as hypopigmentation, hyperpigmentation, or keloids. She recommended aggressive use of emollients and sunscreens for an extended period after lesions resolve to lessen these risks.
Differences in the manifestations of drug-induced skin rashes by race and ethnicity are important and perhaps underappreciated, agreed Shawn Kwatra, MD, professor and chairman of the Department of Dermatology, University of Maryland, Baltimore.
Asked to comment at the meeting, Dr. Kwatra said that he appreciated Dr. Harp’s effort to translate published data and her experience into an overview that increases awareness of the risk for missed or delayed diagnoses of drug-induced rashes in skin of color. He noted that the strategies to identify erythema and pustules, such as increased suspicion in skin of color and the extra steps to rule them out, such as the use of side lighting in the case of pustules for AGEP, are simple and practical.
Dr. Harp and Dr. Kwatra had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM SOC 2024
FDA Initiative Aims to Improve Diversity in Clinical Trials
NEW YORK — Underrepresentation by gender and race in major clinical trials has been a cause for complaint for decades, but the Food and Drug Administration (FDA) has drafted a regulatory solution to this issue expected to be implemented sometime in 2025.
This initiative, known as the according to Valerie M. Harvey, MD, MPH, associate clinical professor, Edward Via College of Osteopathic Medicine, Blacksburg, Virginia. These rules will be codified, she said at the 2024 Skin of Color Update.
Once the DAP is enacted, “the sponsor must specify the rationale and goals for study enrollment by age, ethnicity, sex, and race,” she said. Furthermore, the submission to the FDA must “describe the methods to meet the diversity benchmarks.”
Lack of Trial Diversity Is Common Across Medicine
Although she focused on the relevance of this initiative to dermatology, Dr. Harvey said the lack of diversity in clinical trials is pervasive throughout medicine. In one survey of randomized controlled trials, less than 60% of trials even specified the race and ethnicity of the participants. In recent psoriasis trials, only 30% met a diversity definition of ≥ 20% of patients identifying as minority (Black, Hispanic, Asian, or other non-White group), said Dr. Harvey, who practices dermatology in Newport News, Virginia.
The FDA draft guidance for the DAP was released in June 2024 and is now available for submitting comments (until September 26). The plan is expected to be published in June 2025, according to Dr. Harvey. It will pertain to all pivotal and phase 3 trials enrolling 180 days after the publication date and will be relevant to all drugs and biologics as well as certain devices.
This initiative could be a critical step toward ensuring diversity in major clinical trials after years of stagnation, Dr. Harvey said, noting that despite repeated calls for more diversity in clinical trials, the literature suggests “little progress.”
However, she said that increasing diversity in clinical trials is just one step toward gathering data about the generalizability of efficacy and safety across racial and ethnic groups. A much more complex issue involves how race and ethnicity are defined in order to understand differences, if any, for efficacy and risk.
“Race is a dynamic social construct and a poor measure for biologic variation and skin color,” Dr. Harvey said. This means that work is needed to address the more complex issue of race and ethnicity stratification that will help clinicians understand the relative benefits and risks for the drugs in these trials.
Rather than differences based on genetic or other sources of biologic differences, she said, outcomes by race alone are often suspected of reflecting disparities in access to healthcare rather than a difference in therapeutic response.
Skin Color Is Inadequate to Define Race
When stratifying patients by race or ethnicity, Dr. Harvey said that “we have to be very, very careful in considering the study purpose and what the study question is.” A study attempting to compare benefits and risks among subgroups by race or ethnicity will require descriptors beyond skin color.
The recognized limitations of measuring skin tone as a surrogate of race are one reason for widespread interest in moving away from the Fitzpatrick skin type (FST) rating that has been widely considered a standard, according to Dr. Harvey. Several alternatives have been proposed, including the Monk Skin Tone Scale, the Individual Typology Angle, and the Eumelanin Human Skin Color Scale, but she cautioned that these are less well validated and generally have the limitations of the FST.
If skin color was ever useful for grouping individuals on the basis of shared physiology, growing rates of intermarriage and immigration have made skin color increasingly irrelevant to racial identity. If the goal is to evaluate the safety and efficacy of drugs across racial groups and ethnicities, the characterization of populations will almost certainly require multiple descriptors and biomarkers, she said.
“It is very important to have many tools for characterizing patients by skin type,” Susan Taylor, MD, professor of dermatology and vice chair for diversity, equity, and inclusion for the Department of Dermatology, University of Pennsylvania, Philadelphia, said in an interview at the meeting.
The reason is “there are limitations to all of them,” she said, noting also that the questions being asked about how and if skin color and race are relevant to therapeutic options differ by the question, such as innate response or access to care.
Dr. Taylor is part of a workshop that she said is evaluating a combination of instruments for characterizing skin color and race in ways relevant to the specific question being asked.
The solutions might differ. While simple clinical assessments involving skin color might be made with methods captured on a smartphone app, Dr. Taylor acknowledged that far more complex tools might be required to document the effect of racial or ethnic differences in drug efficacy and safety in a research setting.
Outside of a research setting, any tools that might be useful for assessing race as a variable must be practical, according to Dr. Harvey. She suggested that these must be time efficient, of reasonable cost, and most importantly, reliable.
Tools meeting these criteria do not currently exist, but Dr. Harvey said the work is underway. She expects a “top-down” collaborative approach to validate alternatives to the FST. If such tools can be developed with buy-in from the FDA, they might be particularly useful for translating trial data to patient care, she added.
Dr. Harvey reported financial relationships with AbbVie, Bristol-Myers Squibb, Janssen, Johnson & Johnson, L’Oréal, and SkinCeuticals. Dr. Taylor, president-elect of the American Academy of Dermatology, reported financial relationships with more than 25 pharmaceutical and cosmetic companies.
A version of this article appeared on Medscape.com.
NEW YORK — Underrepresentation by gender and race in major clinical trials has been a cause for complaint for decades, but the Food and Drug Administration (FDA) has drafted a regulatory solution to this issue expected to be implemented sometime in 2025.
This initiative, known as the according to Valerie M. Harvey, MD, MPH, associate clinical professor, Edward Via College of Osteopathic Medicine, Blacksburg, Virginia. These rules will be codified, she said at the 2024 Skin of Color Update.
Once the DAP is enacted, “the sponsor must specify the rationale and goals for study enrollment by age, ethnicity, sex, and race,” she said. Furthermore, the submission to the FDA must “describe the methods to meet the diversity benchmarks.”
Lack of Trial Diversity Is Common Across Medicine
Although she focused on the relevance of this initiative to dermatology, Dr. Harvey said the lack of diversity in clinical trials is pervasive throughout medicine. In one survey of randomized controlled trials, less than 60% of trials even specified the race and ethnicity of the participants. In recent psoriasis trials, only 30% met a diversity definition of ≥ 20% of patients identifying as minority (Black, Hispanic, Asian, or other non-White group), said Dr. Harvey, who practices dermatology in Newport News, Virginia.
The FDA draft guidance for the DAP was released in June 2024 and is now available for submitting comments (until September 26). The plan is expected to be published in June 2025, according to Dr. Harvey. It will pertain to all pivotal and phase 3 trials enrolling 180 days after the publication date and will be relevant to all drugs and biologics as well as certain devices.
This initiative could be a critical step toward ensuring diversity in major clinical trials after years of stagnation, Dr. Harvey said, noting that despite repeated calls for more diversity in clinical trials, the literature suggests “little progress.”
However, she said that increasing diversity in clinical trials is just one step toward gathering data about the generalizability of efficacy and safety across racial and ethnic groups. A much more complex issue involves how race and ethnicity are defined in order to understand differences, if any, for efficacy and risk.
“Race is a dynamic social construct and a poor measure for biologic variation and skin color,” Dr. Harvey said. This means that work is needed to address the more complex issue of race and ethnicity stratification that will help clinicians understand the relative benefits and risks for the drugs in these trials.
Rather than differences based on genetic or other sources of biologic differences, she said, outcomes by race alone are often suspected of reflecting disparities in access to healthcare rather than a difference in therapeutic response.
Skin Color Is Inadequate to Define Race
When stratifying patients by race or ethnicity, Dr. Harvey said that “we have to be very, very careful in considering the study purpose and what the study question is.” A study attempting to compare benefits and risks among subgroups by race or ethnicity will require descriptors beyond skin color.
The recognized limitations of measuring skin tone as a surrogate of race are one reason for widespread interest in moving away from the Fitzpatrick skin type (FST) rating that has been widely considered a standard, according to Dr. Harvey. Several alternatives have been proposed, including the Monk Skin Tone Scale, the Individual Typology Angle, and the Eumelanin Human Skin Color Scale, but she cautioned that these are less well validated and generally have the limitations of the FST.
If skin color was ever useful for grouping individuals on the basis of shared physiology, growing rates of intermarriage and immigration have made skin color increasingly irrelevant to racial identity. If the goal is to evaluate the safety and efficacy of drugs across racial groups and ethnicities, the characterization of populations will almost certainly require multiple descriptors and biomarkers, she said.
“It is very important to have many tools for characterizing patients by skin type,” Susan Taylor, MD, professor of dermatology and vice chair for diversity, equity, and inclusion for the Department of Dermatology, University of Pennsylvania, Philadelphia, said in an interview at the meeting.
The reason is “there are limitations to all of them,” she said, noting also that the questions being asked about how and if skin color and race are relevant to therapeutic options differ by the question, such as innate response or access to care.
Dr. Taylor is part of a workshop that she said is evaluating a combination of instruments for characterizing skin color and race in ways relevant to the specific question being asked.
The solutions might differ. While simple clinical assessments involving skin color might be made with methods captured on a smartphone app, Dr. Taylor acknowledged that far more complex tools might be required to document the effect of racial or ethnic differences in drug efficacy and safety in a research setting.
Outside of a research setting, any tools that might be useful for assessing race as a variable must be practical, according to Dr. Harvey. She suggested that these must be time efficient, of reasonable cost, and most importantly, reliable.
Tools meeting these criteria do not currently exist, but Dr. Harvey said the work is underway. She expects a “top-down” collaborative approach to validate alternatives to the FST. If such tools can be developed with buy-in from the FDA, they might be particularly useful for translating trial data to patient care, she added.
Dr. Harvey reported financial relationships with AbbVie, Bristol-Myers Squibb, Janssen, Johnson & Johnson, L’Oréal, and SkinCeuticals. Dr. Taylor, president-elect of the American Academy of Dermatology, reported financial relationships with more than 25 pharmaceutical and cosmetic companies.
A version of this article appeared on Medscape.com.
NEW YORK — Underrepresentation by gender and race in major clinical trials has been a cause for complaint for decades, but the Food and Drug Administration (FDA) has drafted a regulatory solution to this issue expected to be implemented sometime in 2025.
This initiative, known as the according to Valerie M. Harvey, MD, MPH, associate clinical professor, Edward Via College of Osteopathic Medicine, Blacksburg, Virginia. These rules will be codified, she said at the 2024 Skin of Color Update.
Once the DAP is enacted, “the sponsor must specify the rationale and goals for study enrollment by age, ethnicity, sex, and race,” she said. Furthermore, the submission to the FDA must “describe the methods to meet the diversity benchmarks.”
Lack of Trial Diversity Is Common Across Medicine
Although she focused on the relevance of this initiative to dermatology, Dr. Harvey said the lack of diversity in clinical trials is pervasive throughout medicine. In one survey of randomized controlled trials, less than 60% of trials even specified the race and ethnicity of the participants. In recent psoriasis trials, only 30% met a diversity definition of ≥ 20% of patients identifying as minority (Black, Hispanic, Asian, or other non-White group), said Dr. Harvey, who practices dermatology in Newport News, Virginia.
The FDA draft guidance for the DAP was released in June 2024 and is now available for submitting comments (until September 26). The plan is expected to be published in June 2025, according to Dr. Harvey. It will pertain to all pivotal and phase 3 trials enrolling 180 days after the publication date and will be relevant to all drugs and biologics as well as certain devices.
This initiative could be a critical step toward ensuring diversity in major clinical trials after years of stagnation, Dr. Harvey said, noting that despite repeated calls for more diversity in clinical trials, the literature suggests “little progress.”
However, she said that increasing diversity in clinical trials is just one step toward gathering data about the generalizability of efficacy and safety across racial and ethnic groups. A much more complex issue involves how race and ethnicity are defined in order to understand differences, if any, for efficacy and risk.
“Race is a dynamic social construct and a poor measure for biologic variation and skin color,” Dr. Harvey said. This means that work is needed to address the more complex issue of race and ethnicity stratification that will help clinicians understand the relative benefits and risks for the drugs in these trials.
Rather than differences based on genetic or other sources of biologic differences, she said, outcomes by race alone are often suspected of reflecting disparities in access to healthcare rather than a difference in therapeutic response.
Skin Color Is Inadequate to Define Race
When stratifying patients by race or ethnicity, Dr. Harvey said that “we have to be very, very careful in considering the study purpose and what the study question is.” A study attempting to compare benefits and risks among subgroups by race or ethnicity will require descriptors beyond skin color.
The recognized limitations of measuring skin tone as a surrogate of race are one reason for widespread interest in moving away from the Fitzpatrick skin type (FST) rating that has been widely considered a standard, according to Dr. Harvey. Several alternatives have been proposed, including the Monk Skin Tone Scale, the Individual Typology Angle, and the Eumelanin Human Skin Color Scale, but she cautioned that these are less well validated and generally have the limitations of the FST.
If skin color was ever useful for grouping individuals on the basis of shared physiology, growing rates of intermarriage and immigration have made skin color increasingly irrelevant to racial identity. If the goal is to evaluate the safety and efficacy of drugs across racial groups and ethnicities, the characterization of populations will almost certainly require multiple descriptors and biomarkers, she said.
“It is very important to have many tools for characterizing patients by skin type,” Susan Taylor, MD, professor of dermatology and vice chair for diversity, equity, and inclusion for the Department of Dermatology, University of Pennsylvania, Philadelphia, said in an interview at the meeting.
The reason is “there are limitations to all of them,” she said, noting also that the questions being asked about how and if skin color and race are relevant to therapeutic options differ by the question, such as innate response or access to care.
Dr. Taylor is part of a workshop that she said is evaluating a combination of instruments for characterizing skin color and race in ways relevant to the specific question being asked.
The solutions might differ. While simple clinical assessments involving skin color might be made with methods captured on a smartphone app, Dr. Taylor acknowledged that far more complex tools might be required to document the effect of racial or ethnic differences in drug efficacy and safety in a research setting.
Outside of a research setting, any tools that might be useful for assessing race as a variable must be practical, according to Dr. Harvey. She suggested that these must be time efficient, of reasonable cost, and most importantly, reliable.
Tools meeting these criteria do not currently exist, but Dr. Harvey said the work is underway. She expects a “top-down” collaborative approach to validate alternatives to the FST. If such tools can be developed with buy-in from the FDA, they might be particularly useful for translating trial data to patient care, she added.
Dr. Harvey reported financial relationships with AbbVie, Bristol-Myers Squibb, Janssen, Johnson & Johnson, L’Oréal, and SkinCeuticals. Dr. Taylor, president-elect of the American Academy of Dermatology, reported financial relationships with more than 25 pharmaceutical and cosmetic companies.
A version of this article appeared on Medscape.com.
FROM SOC 2024