User login
Fournier gangrene cases surge in patients using SGLT2 inhibitors
since the US Food and Drug Administration (FDA) issued a 2018 warning about this rare but serious infection, researchers say.
Health care providers prescribing SGLT2 inhibitors to patients with diabetes should have a high index of suspicion for the signs and symptoms of Fournier gangrene, given its substantial morbidity and mortality, according to Susan J. Bersoff-Matcha, MD, and her colleagues at the FDA.
“Although the risk for [Fournier gangrene] is low, serious infection should be considered and weighed against the benefits of SGLT2 inhibitor therapy,” said Dr. Bersoff-Matcha and co-authors in their recent report published in the Annals of Internal Medicine (2019 May 6. doi: 10.7326/M19-0085).
In the previous warning, FDA officials said 12 cases of Fournier gangrene in patients taking an SGLT2 inhibitor had been reported to the agency or in medical literature from March 2013, when the first such inhibitor was approved, and May 2018.
In this latest report, a total of 55 Fournier gangrene cases had been reported in patients receiving SGLT2 inhibitors from March 2, 2013 through January 31, 2019.
The influx of reports may have been prompted by growing awareness of the safety issue, investigators said, but could also reflect the increasing prevalence of diabetes combined with SGLT2 inhibitor use. The researchers also noted that diabetes is a comorbidity in 32% to 66% of cases of Fournier gangrene.
But the likliehood that diabetes mellitus alone causes Fournier gangrene seems unlikley, given that Dr. Bersoff-Matcha and co-authors only found 19 Fournier gangrene cases associated with other classes of antiglycemic agents reported to the FDA or in the literature over a 35-year time frame.
“If Fournier gangrene were associated only with diabetes mellitus and not SGLT2 inhibitors, we would expect far more cases reported with the other antiglycemic agents, considering the 35-year timeframe and the large number of agents,” they said in their report.
Cases were reported for all FDA-approved SGLT2 inhibitors besides ertugliflozin, an agent approved for use in the U.S. in December 2017. The lack of cases reported for this drug could be related to its limited time on the market, the investigators said.
Fournier gangrene, marked by rapidly progressing necrotizing infection of the genitalia, perineum, and perianal region, requires antibiotics and immediate surgery, according to Dr. Bersoff-Matcha and colleagues.
“Serious complications and death are likely if Fournier gangrene is not recognized immediately and surgical intervention is not carried out within the first few hours of diagnosis,” they said in the report.
Of the 55 cases reported in patients receiving SGLT2 inhibitors, 39 were men and 16 were women, with an average of 9 months from the start of treatment to the event, investigators said.
At least 25 patients required multiple surgeries, including one patient who had 17 trips to the operating room, they said. A total of 8 patients had a fecal diversion procedure, and 4 patients had skin grafting.
Six patients had multiple encounters with a provider before being diagnosed, suggesting that the provider may have not recognized the infection due to its nonspecific symptoms, which include fatigue, fever, and malaise.
“Pain that seems out of proportion to findings on physical examination is a strong clinical indicator of necrotizing fasciitis and may be the most important diagnostic clue,” Dr. Bersoff-Matcha and co-authors said in their report.
The incidence of Fournier gangrene in patients taking SGLT2 inhibitors can’t be established by these cases reported to the FDA, which are spontaneously provided by health care providers and patients, investigators said.
“We suspect that our numbers underestimate the true burden,” they said in their report.
Dr. Bersoff-Matcha and co-authors disclosed no conflicts of interest related to their report.
SOURCE: Bersoff-Matcha SJ, et al. Ann Intern Med. 2019 May 6. Doi: doi:10.7326/M19-0085.
This article was updated May 9, 2019.
since the US Food and Drug Administration (FDA) issued a 2018 warning about this rare but serious infection, researchers say.
Health care providers prescribing SGLT2 inhibitors to patients with diabetes should have a high index of suspicion for the signs and symptoms of Fournier gangrene, given its substantial morbidity and mortality, according to Susan J. Bersoff-Matcha, MD, and her colleagues at the FDA.
“Although the risk for [Fournier gangrene] is low, serious infection should be considered and weighed against the benefits of SGLT2 inhibitor therapy,” said Dr. Bersoff-Matcha and co-authors in their recent report published in the Annals of Internal Medicine (2019 May 6. doi: 10.7326/M19-0085).
In the previous warning, FDA officials said 12 cases of Fournier gangrene in patients taking an SGLT2 inhibitor had been reported to the agency or in medical literature from March 2013, when the first such inhibitor was approved, and May 2018.
In this latest report, a total of 55 Fournier gangrene cases had been reported in patients receiving SGLT2 inhibitors from March 2, 2013 through January 31, 2019.
The influx of reports may have been prompted by growing awareness of the safety issue, investigators said, but could also reflect the increasing prevalence of diabetes combined with SGLT2 inhibitor use. The researchers also noted that diabetes is a comorbidity in 32% to 66% of cases of Fournier gangrene.
But the likliehood that diabetes mellitus alone causes Fournier gangrene seems unlikley, given that Dr. Bersoff-Matcha and co-authors only found 19 Fournier gangrene cases associated with other classes of antiglycemic agents reported to the FDA or in the literature over a 35-year time frame.
“If Fournier gangrene were associated only with diabetes mellitus and not SGLT2 inhibitors, we would expect far more cases reported with the other antiglycemic agents, considering the 35-year timeframe and the large number of agents,” they said in their report.
Cases were reported for all FDA-approved SGLT2 inhibitors besides ertugliflozin, an agent approved for use in the U.S. in December 2017. The lack of cases reported for this drug could be related to its limited time on the market, the investigators said.
Fournier gangrene, marked by rapidly progressing necrotizing infection of the genitalia, perineum, and perianal region, requires antibiotics and immediate surgery, according to Dr. Bersoff-Matcha and colleagues.
“Serious complications and death are likely if Fournier gangrene is not recognized immediately and surgical intervention is not carried out within the first few hours of diagnosis,” they said in the report.
Of the 55 cases reported in patients receiving SGLT2 inhibitors, 39 were men and 16 were women, with an average of 9 months from the start of treatment to the event, investigators said.
At least 25 patients required multiple surgeries, including one patient who had 17 trips to the operating room, they said. A total of 8 patients had a fecal diversion procedure, and 4 patients had skin grafting.
Six patients had multiple encounters with a provider before being diagnosed, suggesting that the provider may have not recognized the infection due to its nonspecific symptoms, which include fatigue, fever, and malaise.
“Pain that seems out of proportion to findings on physical examination is a strong clinical indicator of necrotizing fasciitis and may be the most important diagnostic clue,” Dr. Bersoff-Matcha and co-authors said in their report.
The incidence of Fournier gangrene in patients taking SGLT2 inhibitors can’t be established by these cases reported to the FDA, which are spontaneously provided by health care providers and patients, investigators said.
“We suspect that our numbers underestimate the true burden,” they said in their report.
Dr. Bersoff-Matcha and co-authors disclosed no conflicts of interest related to their report.
SOURCE: Bersoff-Matcha SJ, et al. Ann Intern Med. 2019 May 6. Doi: doi:10.7326/M19-0085.
This article was updated May 9, 2019.
since the US Food and Drug Administration (FDA) issued a 2018 warning about this rare but serious infection, researchers say.
Health care providers prescribing SGLT2 inhibitors to patients with diabetes should have a high index of suspicion for the signs and symptoms of Fournier gangrene, given its substantial morbidity and mortality, according to Susan J. Bersoff-Matcha, MD, and her colleagues at the FDA.
“Although the risk for [Fournier gangrene] is low, serious infection should be considered and weighed against the benefits of SGLT2 inhibitor therapy,” said Dr. Bersoff-Matcha and co-authors in their recent report published in the Annals of Internal Medicine (2019 May 6. doi: 10.7326/M19-0085).
In the previous warning, FDA officials said 12 cases of Fournier gangrene in patients taking an SGLT2 inhibitor had been reported to the agency or in medical literature from March 2013, when the first such inhibitor was approved, and May 2018.
In this latest report, a total of 55 Fournier gangrene cases had been reported in patients receiving SGLT2 inhibitors from March 2, 2013 through January 31, 2019.
The influx of reports may have been prompted by growing awareness of the safety issue, investigators said, but could also reflect the increasing prevalence of diabetes combined with SGLT2 inhibitor use. The researchers also noted that diabetes is a comorbidity in 32% to 66% of cases of Fournier gangrene.
But the likliehood that diabetes mellitus alone causes Fournier gangrene seems unlikley, given that Dr. Bersoff-Matcha and co-authors only found 19 Fournier gangrene cases associated with other classes of antiglycemic agents reported to the FDA or in the literature over a 35-year time frame.
“If Fournier gangrene were associated only with diabetes mellitus and not SGLT2 inhibitors, we would expect far more cases reported with the other antiglycemic agents, considering the 35-year timeframe and the large number of agents,” they said in their report.
Cases were reported for all FDA-approved SGLT2 inhibitors besides ertugliflozin, an agent approved for use in the U.S. in December 2017. The lack of cases reported for this drug could be related to its limited time on the market, the investigators said.
Fournier gangrene, marked by rapidly progressing necrotizing infection of the genitalia, perineum, and perianal region, requires antibiotics and immediate surgery, according to Dr. Bersoff-Matcha and colleagues.
“Serious complications and death are likely if Fournier gangrene is not recognized immediately and surgical intervention is not carried out within the first few hours of diagnosis,” they said in the report.
Of the 55 cases reported in patients receiving SGLT2 inhibitors, 39 were men and 16 were women, with an average of 9 months from the start of treatment to the event, investigators said.
At least 25 patients required multiple surgeries, including one patient who had 17 trips to the operating room, they said. A total of 8 patients had a fecal diversion procedure, and 4 patients had skin grafting.
Six patients had multiple encounters with a provider before being diagnosed, suggesting that the provider may have not recognized the infection due to its nonspecific symptoms, which include fatigue, fever, and malaise.
“Pain that seems out of proportion to findings on physical examination is a strong clinical indicator of necrotizing fasciitis and may be the most important diagnostic clue,” Dr. Bersoff-Matcha and co-authors said in their report.
The incidence of Fournier gangrene in patients taking SGLT2 inhibitors can’t be established by these cases reported to the FDA, which are spontaneously provided by health care providers and patients, investigators said.
“We suspect that our numbers underestimate the true burden,” they said in their report.
Dr. Bersoff-Matcha and co-authors disclosed no conflicts of interest related to their report.
SOURCE: Bersoff-Matcha SJ, et al. Ann Intern Med. 2019 May 6. Doi: doi:10.7326/M19-0085.
This article was updated May 9, 2019.
FROM THE ANNALS OF INTERNAL MEDICINE
Key clinical point: The number of Fournier gangrene cases reported in patients receiving sodium-glucose cotransporter-2 (SGLT2) inhibitors has increased in the time since an FDA warning was issued about this rare but potentially serious infection.
Major finding: The previous FDA warning noted 12 reported cases from March 1, 2013 through March 1, 2018. This latest report included a total of 55 cases reported through January 31, 2019.
Study details: A review of spontaneous postmarketing cases of Fournier gangrene reported to the FDA or in the medical literature.
Disclosures: Authors disclosed no conflicts of interest related to the study.
Source: Bersoff-Matcha SJ, et al. Ann Intern Med. 2019 May 6.
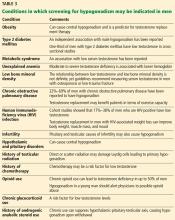
An obese 48-year-old man with progressive fatigue and decreased libido
A 48-year-old man presents to his primary care physician because of progressively decreasing energy and gradual decline in both libido and erectile function for the past 18 months. He has noticed decreased morning erections as well. He rates his libido at 3 to 4 on a scale of 10 for the past 6 months. He also reports poor motivation, depressed mood, impaired concentration, and sleep disturbances. He reports no hair loss, headache, or dizziness, and no decrease in shaving frequency. Review of his systems is otherwise unremarkable.
He has had dyslipidemia for 3 years and is not known to have hypertension or diabetes. His medications include atorvastatin, vitamin E, and multivitamins.
He is married with 3 children and does not wish to have more. He works as a software engineer and leads a sedentary lifestyle. He is a nonsmoker and occasionally drinks alcohol on the weekends.
On physical examination, he is alert and oriented and appears well. His height is 5 feet 10 inches (178 cm), weight 230 lb (104 kg), and body mass index (BMI) 32.8 kg/m2. His blood pressure is 115/83 mm Hg and pulse rate is 82 beats per minute and regular. Findings on cardiovascular and pulmonary examination are normal. He has large fatty breasts but without palpable glandular tissue.
Genitourinary examination reveals normal hair distribution, a normal-sized penis, and slightly soft testes with testicular volume of 18–20 mL bilaterally.
His primary care physician suspects that he has low testosterone and orders some basic laboratory tests; the results are normal except for a low total testosterone level (Table 1).
FURTHER TESTING
1. Which of the following tests should his physician order next?
- Repeat total testosterone measurement
- Free testosterone measurement by commercial assay
- Calculated free testosterone
- Bioavailable testosterone measurement
- Serum inhibin B measurement
This patient presents with several nonspecific symptoms. But collectively they suggest testosterone deficiency (hypogonadism).
Together, erectile dysfunction, low libido, and decreased morning erections strongly suggest hypogonadism.2 Loss of body hair and decreased shaving frequency are specific symptoms of hypogonadism; however, they require years to develop.3 Gynecomastia can also occur due to loss of the inhibitory action of testosterone on breast growth and a relative increase in estradiol. This occurs more in primary hypogonadism, due to the increase in luteinizing hormone (LH), which stimulates the remaining Leydig cells to secrete estradiol rather than testosterone.4
To diagnose hypogonadism in men and to start treatment for it, current guidelines recommend that the patient should have clinical features as well as laboratory evidence of low testosterone.5,6
Measuring testosterone: Total, free, bound, and bioavailable
Testosterone, a steroid hormone, circulates in the serum either as free testosterone or bound to several plasma proteins, mainly sex-hormone binding globulin (SHBG) and albumin.
Total testosterone includes both the free and bound fractions, whereas bioavailable testosterone includes both free and the portion bound to albumin, which has low affinity and can dissociate and be used at the tissue level.11
Low levels of total testosterone do not necessarily reflect a hypogonadal state, as a man with altered SHBG levels or binding capabilities can have low total but normal free testosterone levels and no manifestations.12 Several conditions can alter the levels of SHBG, including obesity, diabetes, aging, thyroid dysfunction, and others.5,13
Because our patient is obese, his total testosterone level is not a reliable indicator of hypogonadism, and repeating its measurement will not add diagnostic value.
Therefore, an alternative measurement should be used to accurately reflect the testosterone levels. From a physiologic point of view, bioavailable testosterone is the active form of testosterone and is the most accurate to be measured in a patient with hypogonadism. Nevertheless, because of technical difficulties in its measurement and lack of evidence correlating bioavailable testosterone with the clinical picture of hypogonadism, it is recommended that the level of free testosterone be used.5
The gold standard for direct measurement of serum free testosterone is equilibrium dialysis, but this is expensive and time-consuming.14 Commercial assays for free testosterone exist but have been deemed unreliable.14,15 It is recommended that free testosterone be measured by equilibrium dialysis or calculated using equations based on total testosterone, SHBG, and albumin levels.5 These equations are reliable and give results very close to the values obtained by equilibrium dialysis.15 Therefore, in our patient, it would be suitable to calculate the free testosterone level next.
Serum levels of free testosterone vary according to several factors. Diurnal variation of testosterone has been established: levels are highest in the morning and decline throughout the day.16 Food decreases testosterone levels.17 In addition, there is considerable day-to-day variation.18 Therefore, at least 2 readings of fasting morning testosterone on 2 separate days are recommended for the diagnosis of hypogonadism.5
Inhibin B is a hormone produced by Sertoli cells in the testes in response to follicle-stimulating hormone (FSH) stimulation. In turn, it acts as negative feedback, together with testosterone, to inhibit FSH release from the pituitary. Inhibin B has been shown to reflect spermatogenesis in the testes and therefore fertility.19 Inhibin B levels were found to be low in patients with central hypogonadism, due to less FSH release; however, they did not correlate with testosterone levels.20
CASE RESUMED: CHARACTERIZING HIS HYPOGONADISM
The patient’s physician orders morning fasting total testosterone, SHBG, and albumin testing and calculates the free testosterone level, which yields a value of 3 ng/dL (reference range 4.5–17). This is confirmed by a repeat measurement, which yields a value of 2.9 ng/dL. Laboratory test results combined with his clinical presentation are consistent with hypogonadism.
2. What is the most appropriate next step?
- Measurement of serum LH and FSH
- Measurement of serum prolactin
- Scrotal ultrasonography
- Gonadotropin-releasing hormone (GnRH) stimulation test
- Semen analysis
After hypogonadism is diagnosed, it is important to distinguish if it is primary or central. This is achieved by measuring serum LH and FSH.5 All biotin supplements should be stopped at least 72 hours before measuring LH and FSH, as biotin can interfere with the assays, yielding false values.21
Secretion of FSH and LH from the anterior pituitary is under the influence of pulsatile release of GnRH from the hypothalamus. LH acts on Leydig cells in the testes to produce testosterone, whereas FSH acts on Sertoli cells, together with testosterone, to bring about spermatogenesis in the seminiferous tubules. Testosterone acts centrally as negative feedback to decrease the release of LH and FSH.
Primary hypogonadism occurs due to testicular failure, ie, the testes themselves fail to produce testosterone, leading to hypogonadism. The decrease in testosterone levels, together with inhibin B if Sertoli cells are damaged, lead to loss of negative feedback on the hypothalamus and pituitary, and therefore increased levels of LH and FSH. This is termed hypergonadotropic hypogonadism. Testicular failure may also result in impaired spermatogenesis and infertility due to destruction of testicular structures, in which case fertility cannot be restored.
Central hypogonadism occurs when the pituitary fails to produce LH and FSH (secondary hypogonadism) or when the hypothalamus fails to produce GnRH and subsequently the lack of secretion of LH and FSH from the pituitary (tertiary hypogonadism). The lack of LH will result in no stimulation of Leydig cells to produce testosterone, and therefore its deficiency. Serum hormone levels in central hypogonadism will reveal low testosterone, with either low or inappropriately normal gonadotropins (LH and FSH). This is termed hypogonadotropic hypogonadism. The lack of FSH, together with testosterone deficiency will also result in decreased spermatogenesis and therefore infertility. Testicular structures are preserved, however, and fertility can be restored with appropriate therapy, as discussed below.
Prolactin should be measured only if the patient has central hypogonadism. Its measurement is not warranted at this point in the patient’s workup. The implications of prolactin and its relationship to hypogonadism will be discussed later.
Although, this stepwise approach is not convenient for many patients, some physicians follow it because it is cost-effective, especially in those who are not insured. However, other physicians order FSH, LH, and sometimes prolactin with the confirmatory low testosterone measurement. Laboratories can also be instructed to wait to measure the pituitary hormones and to do so only if low testosterone is confirmed.
Varicocele, a possible cause of male infertility, can also impair Leydig cell function and cause low testosterone. In fact, surgical repair of varicocele has been demonstrated to increase serum testosterone.22 Scrotal ultrasonography is used to diagnose varicocele, but this also should be ordered at a later stage in the workup if primary hypogonadism is diagnosed.
The GnRH stimulation test is important for the diagnosis and evaluation of precocious or delayed puberty in children. In boys with delayed puberty, a poorer response to GnRH stimulation indicates central hypogonadism rather than constitutional delay.23 It has no role in the evaluation of postpubertal or adult-onset hypogonadism.
Semen analysis is important to evaluate fertility if the patient is interested in further procreation.5 Low testosterone levels may result in impaired spermatogenesis and therefore infertility. On the other hand, treatment with exogenous testosterone will also result in infertility, by feedback inhibition of LH and FSH and therefore inhibition of spermatogenesis. If the patient wishes to preserve fertility, treatment options other than testosterone should be considered; examples include clomiphene citrate, human menopausal gonadotropin, and human chorionic gonadotropin.23,24
Our patient has no desire to expand his family; therefore, a semen analysis and attempts to preserve spermatogenesis are not indicated.
CASE RESUMED: SEARCHING FOR CAUSES
His physician orders testing of serum LH and FSH, yielding the following values:
- LH 1.6 mIU/mL (reference range 1.8–12)
- FSH 1.9 mIU/mL (reference range 1.5–12.5).
The diagnosis of central hypogonadism is established.
3. Which investigation is the least appropriate in the further evaluation of this patient?
- Serum prolactin measurement
- Serum ferritin measurement
- Pituitary magnetic resonance imaging (MRI)
- Chromosomal karyotyping
The diagnosis of central hypogonadism warrants evaluation for possible causes. These are summarized in Table 4.
Serum free thyroxine and morning cortisol
Since this patient’s LH and FSH values are abnormal, it is important to evaluate the status of other anterior pituitary hormones. In patients with pituitary abnormalities, serum free T4 is a more reliable test for assessing thyroid function than thyroid-stimulating hormone (TSH), because of loss of the negative feedback of thyroid hormones on the diseased pituitary. In contrast, serum TSH is considered the best single thyroid test to assess primary thyroid dysfunction.
Other measurements include prolactin and morning cortisol (reflecting adrenocorticotropic hormone status).
Prolactin measurement
Prolactin measurement is important to evaluate for hyperprolactinemia, as this will lead to hypogonadism by inhibition of GnRH secretion.25 Different pathologic, pharmacologic, and physiologic conditions can result in hyperprolactinemia, including prolactinomas, other pituitary and hypothalamic lesions, primary hypothyroidism, and medications such as antipsychotics.25 Dopamine agonists are the mainstay treatment for hyperprolactinemia.
Ferritin measurement
Ferritin measurement is indicated to diagnose iron overload conditions such as hemochromatosis, which can result in primary hypogonadism via testicular damage or in secondary hypogonadism via pituitary damage.26
Pituitary MRI with contrast
Pituitary MRI with contrast is used to diagnose structural lesions of the pituitary or hypothalamus. This diagnostic modality is indicated for patients with pituitary dysfunction, including central hypogonadism, manifestations of a mass effect (headache, visual field defects), persistent hyperprolactinemia, and panhypopituitarism, among others. To improve the diagnostic yield of pituitary MRI, the Endocrine Society guidelines recommend it for men with serum total testosterone levels below 150 ng/dL.5 However, some clinicians have a lower threshold for ordering pituitary MRI for patients with central hypogonadism. Physician judgment and expertise should be exercised and the decision made on an individual basis.
Chromosomal karyotyping
Chromosomal karyotyping is not indicated in our patient. It is reserved for those with primary hypogonadism to diagnose Klinefelter syndrome, which has a karyotype of 47,XXY.
CASE RESUMED: MOSH SYNDROME
Our patient’s prolactin, free T4, morning cortisol, and ferritin levels are measured, yielding normal values. No abnormalities are seen on pituitary MRI. A clinical reevaluation is conducted, revealing no history of head trauma or head and neck radiation. The lack of an obvious cause in our patient’s clinical presentation and workup, together with his obesity (BMI 32.8 kg/m2) supports the diagnosis of obesity as the cause of his hypogonadism.
Obesity can be a cause of secondary hypogonadism, which has led to the term “MOSH” (male obesity-associated secondary hypogonadism) syndrome. In fact, a cross-sectional study has demonstrated that 40% of nondiabetic obese (BMI ≥ 30 kg/m2) men over age 45 have low serum free testosterone levels, compared with 26% for lean (BMI < 25 kg/m2) men.27 Moreover, obesity has been found to be a strong predictor of testosterone replacement therapy.28 Other studies have also found an inverse relationship between BMI and testosterone levels.29
Several mechanisms interact in the pathogenesis of MOSH syndrome. Adipose tissue possesses aromatase activity, which converts androgens into estrogens.30 Peripheral estrogen production can in turn exert feedback inhibition on pituitary gonadotropin secretion.31 In obese men, increased adipose tissue leads to increased aromatase activity and more estrogen, so more feedback inhibition on the pituitary and subsequently secondary hypogonadism.
Leptin, a hormone produced by adipocytes, is also increased in obesity, and was found to be inversely correlated with serum testosterone.32 Studies have demonstrated that leptin has an inhibitory effect on the enzymatic pathway that synthesizes testosterone in Leydig cells.33
Proinflammatory cytokines have also been implicated, as central obesity is associated with an increase in these cytokines, which in turn act negatively on the hypothalamus and impair GnRH release leading to lower testosterone.34,35
Treating obesity-related hypogonadism
In a pilot study,36 lifestyle attempts to reduce obesity were shown to improve hormonal levels. Bariatric surgery has also been demonstrated to be successful.37
Clomiphene citrate, a selective estrogen receptor modulator, increases endogenous testosterone secretion by inhibiting the negative feedback of estrogen on the hypothalamus and pituitary and thus increasing LH and FSH. It also preserves endogenous testosterone production, since it does not suppress the hypothalamic-pituitary-testicular axis.38 This made clomiphene citrate a potential treatment for men with central hypogonadism including those with MOSH.39
Nevertheless, there are no randomized trials to prove its safety and efficacy in the management of central hypogonadism.5 Regarding its use in men wishing to preserve fertility, most studies did not show improvement. However, a meta-analysis demonstrated statistically significant increased pregnancy rates in partners of men with idiopathic infertility if the men used 50 mg of clomiphene citrate daily.40
Testosterone deficiency can be a marker of metabolic syndrome, which needs to be managed more urgently than hypogonadism. A cross-sectional study found not only an association between metabolic syndrome and low serum testosterone, but also with each individual component of metabolic syndrome on its own, all of which need to be addressed.10
CASE CONTINUED: BEGINNING TREATMENT
The physician counsels the patient regarding the implications, potential adverse outcomes, and available treatments for his obesity, including lifestyle modification and bariatric surgery. The patient declines surgery and wishes to adopt a weight-reducing diet and exercise program, for which he is referred to a dietitian.
In addition, in view of the patient’s clinically and biochemically proven hypogonadism, his physician offers testosterone replacement therapy. He orders a serum prostate-specific antigen (PSA) level, which is 1.3 ng/dL (reference range < 4 ng/dL). The patient is prescribed 5 g of 1% testosterone gel daily.
TESTOSTERONE REPLACEMENT THERAPY
4. Which is the most common adverse effect of testosterone replacement therapy?
- Cardiovascular events
- Erythrocytosis
- Prostate cancer
- Infertility
- Obstructive sleep apnea
Clinicians should be very cautious in initiating testosterone replacement therapy in any patient with an unstable medical condition.
There are several formulations of testosterone replacement therapy, including intramuscular injections, transdermal gels or patches, buccal tablets, an intranasal gel, and oral tablets. Of note, there are 2 different forms of oral testosterone preparations: testosterone undecanoate and 17-alpha alkylated testosterone. The former is unavailable in the United States and the latter is not recommended for use due to its proven hepatic toxicity.41
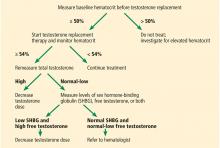
Testosterone and erythrocytosis
Meta-analyses have concluded that the most frequent adverse event of testosterone replacement therapy is a significant rise in hematocrit.42 This rise was found to be dose-dependent and was more marked in older men.43 Although all preparations can cause erythrocytosis, parenteral forms have been observed to raise it the most, particularly short-term injectables.44,45
The mechanism behind this increase is attributed to increased erythropoietin levels and improved usage of iron for red blood cell synthesis.46 In fact, testosterone replacement therapy has been shown to improve hemoglobin levels in patients with anemia.47 On the other hand, increasing hematocrit levels may lead to thrombotic and vasoocclusive events.44
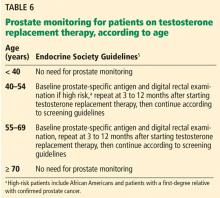
Testosterone and prostate cancer
The relationship between testosterone treatment and prostate cancer has long been studied. Historically, testosterone replacement therapy was believed to increase the risk of prostate cancer; however, recent studies and meta-analyses have shown that this is not the case.42,48 Nevertheless, clinical guidelines still recommend prostate monitoring for men on testosterone replacement therapy.5,6
Testosterone and cardiovascular risk
The evidence regarding this issue has been contradictory and inconsistent. Meta-analyses have demonstrated that low testosterone is associated with higher risk of major adverse cardiovascular events.50 These studies argue for the use of testosterone replacement therapy in hypogonadal men to decrease the risk. However, other studies and meta-analyses have found that testosterone replacement therapy is associated with increased cardiovascular risk and have concluded that major adverse cardiac events are in fact a risk of testosterone replacement therapy.51
Current recommendations advocate against the use of testosterone replacement therapy in men with uncontrolled heart failure or with cardiovascular events in the past 3 to 6 months.5,6 Cardiovascular risk factors should be addressed and corrected, and patients should be educated on cardiovascular symptoms and the need to report them if they occur.
Testosterone and infertility
As described earlier, testosterone replacement therapy increases negative feedback on the pituitary and decreases LH and FSH production, leading to less spermatogenesis. Other treatment options should be sought for hypogonadal men wishing to preserve fertility.
Other adverse effects
Other adverse effects of testosterone replacement therapy include acne, oily skin, obstructive sleep apnea, gynecomastia, and balding.
Given all the adverse events that can be associated with testosterone replacement therapy, the risks and benefits of treating hypogonadism in each patient should be taken into consideration, and an individualized approach is required.
CASE RESUMED: FOLLOW-UP
The patient presents 3 months later for follow-up. He reports significant improvement in his presenting symptoms including energy, libido, and erectile function. He also reports some improvement in his mood and concentration. He has lost 12 lb (5.4 kg) and is still trying to improve his diet and exercise program. He is compliant with his testosterone gel therapy.
His serum calculated free testosterone level is 7.8 ng/dL (4.5–17), and his hematocrit is 46%. The patient is instructed to continue his treatment and to return after 9 months for further follow-up.
TAKE-HOME POINTS
- Men with hypogonadism usually present with nonspecific manifestations, so clinicians should keep a high index of suspicion.
- Both clinical and biochemical evidence of hypogonadism should be present to diagnose and start treatment for it.
- Low levels of serum total testosterone do not necessarily reflect hypogonadism.
- The hormonal profile of central hypogonadism reveals low serum testosterone with low or inappropriately normal serum LH and FSH levels.
Obesity can cause central hypogonadism and should be suspected after pituitary and other systemic causes are excluded.
- Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 2007; 92(11):4241–4247. doi:10.1210/jc.2007-1245
- Wu FCW, Tajar A, Beynon JM, et al; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010; 363(2):123–135. doi:10.1056/NEJMoa0911101
- Arver S, Lehtihet M. Current guidelines for the diagnosis of testosterone deficiency. Front Horm Res 2009; 37:5–20. doi:10.1159/000175839
- Narula HS, Carlson HE. Gynaecomastia—pathophysiology, diagnosis and treatment. Nat Rev Endocrinol 2014; 10(11):684–698. doi:10.1038/nrendo.2014.139
- Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2018; 103(5):1715–1744. doi:10.1210/jc.2018-00229
- Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol 2018; 200(2):423–432. doi:10.1016/j.juro.2018.03.115
- Balasubramanian V, Naing S. Hypogonadism in chronic obstructive pulmonary disease: incidence and effects. Curr Opin Pulm Med 2012; 18(2):112–117. doi:10.1097/MCP.0b013e32834feb37
- Atlantis E, Fahey P, Cochrane B, Wittert G, Smith S. Endogenous testosterone level and testosterone supplementation therapy in chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. BMJ Open 2013; 3(8)pii:e003127. doi:10.1136/bmjopen-2013-003127
- Bawor M, Bami H, Dennis BB, et al. Testosterone suppression in opioid users: a systematic review and meta-analysis. Drug Alcohol Depend 2015; 149:1–9. doi:10.1016/j.drugalcdep.2015.01.038
- Tan WS, Ng CJ, Khoo EM, Low WY, Tan HM. The triad of erectile dysfunction, testosterone deficiency syndrome and metabolic syndrome: findings from a multi-ethnic Asian men study (The Subang Men's Health Study). Aging Male 2011; 14(4):231–236. doi:10.3109/13685538.2011.597463
- Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A reappraisal of testosterone’s binding in circulation: physiological and clinical implications. Endocr Rev 2017; 38(4):302–324. doi:10.1210/er.2017-00025
- Antonio L, Wu FC, O’Neill TW, et al; European Male Ageing Study Study Group. Low free testosterone is associated with hypogonadal signs and symptoms in men with normal total testosterone. J Clin Endocrinol Metab 2016; 101(7):2647–2657. doi:10.1210/jc.2015-4106
- Liu F, Shen X, Wang R, et al. Association of central obesity with sex hormone binding globulin: a cross-sectional study of 1166 Chinese men. Open Med (Wars) 2018; 13:196–202. doi:10.1515/med-2018-0030
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999; 84(10):3666–3672. doi:10.1210/jcem.84.10.6079
- Halmenschlager G, Rhoden EL, Riedner CE. Calculated free testosterone and radioimmunoassay free testosterone as a predictor of subnormal levels of total testosterone. Int Urol Nephrol 2012; 44(3):673–681. doi:10.1007/s11255-011-0066-z
- Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab 2009; 94(3):907–913. doi:10.1210/jc.2008-1902
- Lehtihet M, Arver S, Bartuseviciene I, Pousette Å. S-testosterone decrease after a mixed meal in healthy men independent of SHBG and gonadotrophin levels. Andrologia 2012; 44(6):405–410. doi:10.1111/j.1439-0272.2012.01296.x
- Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf) 2007; 67(6):853–862. doi:10.1111/j.1365-2265.2007.02976.x
- Manzoor SM, Sattar A, Hashim R, et al. Serum inhibin B as a diagnostic marker of male infertility. J Ayub Med Coll Abbottabad 2012; 24(3–4):113–116. pmid:24669628
- Kolb BA, Stanczyk FZ, Sokol RZ. Serum inhibin B levels in males with gonadal dysfunction. Fertil Steril 2000; 74(2):234–238. pmid:10927037
- Trambas CM, Sikaris KA, Lu ZX. More on biotin treatment mimicking Graves’ disease. N Engl J Med 2016; 375(17):1698. doi:10.1056/NEJMc1611875
- Li F, Yue H, Yamaguchi K, et al. Effect of surgical repair on testosterone production in infertile men with varicocele: a meta-analysis. Int J Urol 2012; 19(2):149–154. doi:10.1111/j.1442-2042.2011.02890.x
- Crosnoe-Shipley LE, Elkelany OO, Rahnema CD, Kim ED. Treatment of hypogonadotropic male hypogonadism: case-based scenarios. World J Nephrol 2015; 4(2):245–253. doi:10.5527/wjn.v4.i2.245
- Majzoub A, Sabanegh E Jr. Testosterone replacement in the infertile man. Transl Androl Urol 2016; 5(6):859–865. doi:10.21037/tau.2016.08.03
- Majumdar A, Mangal NS. Hyperprolactinemia. J Hum Reprod Sci 2013; 6(3):168–175. doi:10.4103/0974-1208.121400
- El Osta R, Grandpre N, Monnin N, Hubert J, Koscinski I. Hypogonadotropic hypogonadism in men with hereditary hemochromatosis. Basic Clin Androl 2017; 27:13. doi:10.1186/s12610-017-0057-8
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010; 33(6):1186–1192. doi:10.2337/dc09-1649
- Jasuja GK, Bhasin S, Reisman JI, et al. Who gets testosterone? Patient characteristics associated with testosterone prescribing in the Veteran Affairs system: a cross-sectional study. J Gen Intern Med 2017; 32(3):304–311. doi:10.1007/s11606-016-3940-7
- Kaplan SA, Lee JY, O’Neill EA, Meehan AG, Kusek JW. Prevalence of low testosterone and its relationship to body mass index in older men with lower urinary tract symptoms associated with benign prostatic hyperplasia. Aging Male 2013; 16(4):169–172. doi:10.3109/13685538.2013.844786
- Lee HK, Lee JK, Cho B. The role of androgen in the adipose tissue of males. World J Mens Health 2013; 31(2):136–140. doi:10.5534/wjmh.2013.31.2.136
- Raven G, De Jong FH, Kaufman JM, De Ronde W. In men, peripheral estradiol levels directly reflect the action of estrogens at the hypothalamo-pituitary level to inhibit gonadotropin secretion. J Clin Endocrinol Metab 2006; 91(9):3324–3328. doi:10.1210/jc.2006-0462
- Hofny ER, Ali ME, Abdel-Hafez HZ, et al. Semen parameters and hormonal profile in obese fertile and infertile males. Fertil Steril 2010; 94(2):581–584. doi:10.1016/j.fertnstert.2009.03.085
- Isidori AM, Caprio M, Strollo F, et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab 1999; 84(10):3673–3680. doi:10.1210/jcem.84.10.6082
- El-Wakkad A, Hassan NM, Sibaii H, El-Zayat SR. Proinflammatory, anti-inflammatory cytokines and adiponkines in students with central obesity. Cytokine 2013; 61(2):682–687. doi:10.1016/j.cyto.2012.11.010
- Maggio M, Basaria S, Ceda GP, et al. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest 2005; 28(suppl proceedings 11):116–119. pmid:16760639
- de Lorenzo A, Noce A, Moriconi E, et al. MOSH syndrome (male obesity secondary hypogonadism): clinical assessment and possible therapeutic approaches. Nutrients 2018; 10(4)pii:E474. doi:10.3390/nu10040474
- Escobar-Morreale HF, Santacruz E, Luque-Ramírez M, Botella Carretero JI. Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum Reprod Update 2017; 23(4):390–408. doi:10.1093/humupd/dmx012
- Lo EM, Rodriguez KM, Pastuszak AW, Khera M. Alternatives to testosterone therapy: a review. Sex Med Rev 2018; 6(1):106–113. doi:10.1016/j.sxmr.2017.09.004
- Soares AH, Horie NC, Chiang LAP, et al. Effects of clomiphene citrate on male obesity-associated hypogonadism: a randomized, double-blind, placebo-controlled study. Int J Obes (Lond) 2018; 42(5):953–963. doi:10.1038/s41366-018-0105-2
- Chua ME, Escusa KG, Luna S, Tapia LC, Dofitas B, Morales M. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology 2013; 1(5):749–757. doi:10.1111/j.2047-2927.2013.00107.x
- Westaby D, Ogle SJ, Paradinas FJ, Randell JB, Murray-Lyon IM. Liver damage from long-term methyltestosterone. Lancet 1977; 2(8032):262–263. pmid:69876
- Fernández-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2010; 95(6):2560–2575. doi:10.1210/jc.2009-2575
- Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab 2008; 93(3):914–919. doi:10.1210/jc.2007-1692
- Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis following testosterone therapy. Sex Med Rev 2018; 6(1):77–85. doi:10.1016/j.sxmr.2017.04.001
- Jones SD Jr, Dukovac T, Sangkum P, Yafi FA, Hellstrom WJ. Erythrocytosis and polycythemia secondary to testosterone replacement therapy in the aging male. Sex Med Rev 2015; 3(2):101–112. doi:10.1002/smrj.43
- Bachman E, Travison TG, Basaria S, et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci 2014; 69(6):725–735. doi:10.1093/gerona/glt154
- Roy CN, Snyder PJ, Stephens-Shields AJ, et al. Association of testosterone levels with anemia in older men: a controlled clinical trial. JAMA Intern Med 2017; 177(4):480–490. doi:10.1001/jamainternmed.2016.9540
- Klap J, Schmid M, Loughlin KR. The relationship between total testosterone levels and prostate cancer: a review of the continuing controversy. J Urol 2015; 193(2):403–413. doi:10.1016/j.juro.2014.07.123
- Gilbert SM, Cavallo CB, Kahane H, Lowe FC. Evidence suggesting PSA cutpoint of 2.5 ng/mL for prompting prostate biopsy: review of 36,316 biopsies. Urology 2005; 65(3):549–553. doi:10.1016/j.urology.2004.10.064
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011; 96(10):3007–3019. doi:10.1210/jc.2011-1137
- Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med 2013; 11:108. doi:10.1186/1741-7015-11-108
A 48-year-old man presents to his primary care physician because of progressively decreasing energy and gradual decline in both libido and erectile function for the past 18 months. He has noticed decreased morning erections as well. He rates his libido at 3 to 4 on a scale of 10 for the past 6 months. He also reports poor motivation, depressed mood, impaired concentration, and sleep disturbances. He reports no hair loss, headache, or dizziness, and no decrease in shaving frequency. Review of his systems is otherwise unremarkable.
He has had dyslipidemia for 3 years and is not known to have hypertension or diabetes. His medications include atorvastatin, vitamin E, and multivitamins.
He is married with 3 children and does not wish to have more. He works as a software engineer and leads a sedentary lifestyle. He is a nonsmoker and occasionally drinks alcohol on the weekends.
On physical examination, he is alert and oriented and appears well. His height is 5 feet 10 inches (178 cm), weight 230 lb (104 kg), and body mass index (BMI) 32.8 kg/m2. His blood pressure is 115/83 mm Hg and pulse rate is 82 beats per minute and regular. Findings on cardiovascular and pulmonary examination are normal. He has large fatty breasts but without palpable glandular tissue.
Genitourinary examination reveals normal hair distribution, a normal-sized penis, and slightly soft testes with testicular volume of 18–20 mL bilaterally.
His primary care physician suspects that he has low testosterone and orders some basic laboratory tests; the results are normal except for a low total testosterone level (Table 1).
FURTHER TESTING
1. Which of the following tests should his physician order next?
- Repeat total testosterone measurement
- Free testosterone measurement by commercial assay
- Calculated free testosterone
- Bioavailable testosterone measurement
- Serum inhibin B measurement
This patient presents with several nonspecific symptoms. But collectively they suggest testosterone deficiency (hypogonadism).
Together, erectile dysfunction, low libido, and decreased morning erections strongly suggest hypogonadism.2 Loss of body hair and decreased shaving frequency are specific symptoms of hypogonadism; however, they require years to develop.3 Gynecomastia can also occur due to loss of the inhibitory action of testosterone on breast growth and a relative increase in estradiol. This occurs more in primary hypogonadism, due to the increase in luteinizing hormone (LH), which stimulates the remaining Leydig cells to secrete estradiol rather than testosterone.4
To diagnose hypogonadism in men and to start treatment for it, current guidelines recommend that the patient should have clinical features as well as laboratory evidence of low testosterone.5,6
Measuring testosterone: Total, free, bound, and bioavailable
Testosterone, a steroid hormone, circulates in the serum either as free testosterone or bound to several plasma proteins, mainly sex-hormone binding globulin (SHBG) and albumin.
Total testosterone includes both the free and bound fractions, whereas bioavailable testosterone includes both free and the portion bound to albumin, which has low affinity and can dissociate and be used at the tissue level.11
Low levels of total testosterone do not necessarily reflect a hypogonadal state, as a man with altered SHBG levels or binding capabilities can have low total but normal free testosterone levels and no manifestations.12 Several conditions can alter the levels of SHBG, including obesity, diabetes, aging, thyroid dysfunction, and others.5,13
Because our patient is obese, his total testosterone level is not a reliable indicator of hypogonadism, and repeating its measurement will not add diagnostic value.
Therefore, an alternative measurement should be used to accurately reflect the testosterone levels. From a physiologic point of view, bioavailable testosterone is the active form of testosterone and is the most accurate to be measured in a patient with hypogonadism. Nevertheless, because of technical difficulties in its measurement and lack of evidence correlating bioavailable testosterone with the clinical picture of hypogonadism, it is recommended that the level of free testosterone be used.5
The gold standard for direct measurement of serum free testosterone is equilibrium dialysis, but this is expensive and time-consuming.14 Commercial assays for free testosterone exist but have been deemed unreliable.14,15 It is recommended that free testosterone be measured by equilibrium dialysis or calculated using equations based on total testosterone, SHBG, and albumin levels.5 These equations are reliable and give results very close to the values obtained by equilibrium dialysis.15 Therefore, in our patient, it would be suitable to calculate the free testosterone level next.
Serum levels of free testosterone vary according to several factors. Diurnal variation of testosterone has been established: levels are highest in the morning and decline throughout the day.16 Food decreases testosterone levels.17 In addition, there is considerable day-to-day variation.18 Therefore, at least 2 readings of fasting morning testosterone on 2 separate days are recommended for the diagnosis of hypogonadism.5
Inhibin B is a hormone produced by Sertoli cells in the testes in response to follicle-stimulating hormone (FSH) stimulation. In turn, it acts as negative feedback, together with testosterone, to inhibit FSH release from the pituitary. Inhibin B has been shown to reflect spermatogenesis in the testes and therefore fertility.19 Inhibin B levels were found to be low in patients with central hypogonadism, due to less FSH release; however, they did not correlate with testosterone levels.20
CASE RESUMED: CHARACTERIZING HIS HYPOGONADISM
The patient’s physician orders morning fasting total testosterone, SHBG, and albumin testing and calculates the free testosterone level, which yields a value of 3 ng/dL (reference range 4.5–17). This is confirmed by a repeat measurement, which yields a value of 2.9 ng/dL. Laboratory test results combined with his clinical presentation are consistent with hypogonadism.
2. What is the most appropriate next step?
- Measurement of serum LH and FSH
- Measurement of serum prolactin
- Scrotal ultrasonography
- Gonadotropin-releasing hormone (GnRH) stimulation test
- Semen analysis
After hypogonadism is diagnosed, it is important to distinguish if it is primary or central. This is achieved by measuring serum LH and FSH.5 All biotin supplements should be stopped at least 72 hours before measuring LH and FSH, as biotin can interfere with the assays, yielding false values.21
Secretion of FSH and LH from the anterior pituitary is under the influence of pulsatile release of GnRH from the hypothalamus. LH acts on Leydig cells in the testes to produce testosterone, whereas FSH acts on Sertoli cells, together with testosterone, to bring about spermatogenesis in the seminiferous tubules. Testosterone acts centrally as negative feedback to decrease the release of LH and FSH.
Primary hypogonadism occurs due to testicular failure, ie, the testes themselves fail to produce testosterone, leading to hypogonadism. The decrease in testosterone levels, together with inhibin B if Sertoli cells are damaged, lead to loss of negative feedback on the hypothalamus and pituitary, and therefore increased levels of LH and FSH. This is termed hypergonadotropic hypogonadism. Testicular failure may also result in impaired spermatogenesis and infertility due to destruction of testicular structures, in which case fertility cannot be restored.
Central hypogonadism occurs when the pituitary fails to produce LH and FSH (secondary hypogonadism) or when the hypothalamus fails to produce GnRH and subsequently the lack of secretion of LH and FSH from the pituitary (tertiary hypogonadism). The lack of LH will result in no stimulation of Leydig cells to produce testosterone, and therefore its deficiency. Serum hormone levels in central hypogonadism will reveal low testosterone, with either low or inappropriately normal gonadotropins (LH and FSH). This is termed hypogonadotropic hypogonadism. The lack of FSH, together with testosterone deficiency will also result in decreased spermatogenesis and therefore infertility. Testicular structures are preserved, however, and fertility can be restored with appropriate therapy, as discussed below.
Prolactin should be measured only if the patient has central hypogonadism. Its measurement is not warranted at this point in the patient’s workup. The implications of prolactin and its relationship to hypogonadism will be discussed later.
Although, this stepwise approach is not convenient for many patients, some physicians follow it because it is cost-effective, especially in those who are not insured. However, other physicians order FSH, LH, and sometimes prolactin with the confirmatory low testosterone measurement. Laboratories can also be instructed to wait to measure the pituitary hormones and to do so only if low testosterone is confirmed.
Varicocele, a possible cause of male infertility, can also impair Leydig cell function and cause low testosterone. In fact, surgical repair of varicocele has been demonstrated to increase serum testosterone.22 Scrotal ultrasonography is used to diagnose varicocele, but this also should be ordered at a later stage in the workup if primary hypogonadism is diagnosed.
The GnRH stimulation test is important for the diagnosis and evaluation of precocious or delayed puberty in children. In boys with delayed puberty, a poorer response to GnRH stimulation indicates central hypogonadism rather than constitutional delay.23 It has no role in the evaluation of postpubertal or adult-onset hypogonadism.
Semen analysis is important to evaluate fertility if the patient is interested in further procreation.5 Low testosterone levels may result in impaired spermatogenesis and therefore infertility. On the other hand, treatment with exogenous testosterone will also result in infertility, by feedback inhibition of LH and FSH and therefore inhibition of spermatogenesis. If the patient wishes to preserve fertility, treatment options other than testosterone should be considered; examples include clomiphene citrate, human menopausal gonadotropin, and human chorionic gonadotropin.23,24
Our patient has no desire to expand his family; therefore, a semen analysis and attempts to preserve spermatogenesis are not indicated.
CASE RESUMED: SEARCHING FOR CAUSES
His physician orders testing of serum LH and FSH, yielding the following values:
- LH 1.6 mIU/mL (reference range 1.8–12)
- FSH 1.9 mIU/mL (reference range 1.5–12.5).
The diagnosis of central hypogonadism is established.
3. Which investigation is the least appropriate in the further evaluation of this patient?
- Serum prolactin measurement
- Serum ferritin measurement
- Pituitary magnetic resonance imaging (MRI)
- Chromosomal karyotyping
The diagnosis of central hypogonadism warrants evaluation for possible causes. These are summarized in Table 4.
Serum free thyroxine and morning cortisol
Since this patient’s LH and FSH values are abnormal, it is important to evaluate the status of other anterior pituitary hormones. In patients with pituitary abnormalities, serum free T4 is a more reliable test for assessing thyroid function than thyroid-stimulating hormone (TSH), because of loss of the negative feedback of thyroid hormones on the diseased pituitary. In contrast, serum TSH is considered the best single thyroid test to assess primary thyroid dysfunction.
Other measurements include prolactin and morning cortisol (reflecting adrenocorticotropic hormone status).
Prolactin measurement
Prolactin measurement is important to evaluate for hyperprolactinemia, as this will lead to hypogonadism by inhibition of GnRH secretion.25 Different pathologic, pharmacologic, and physiologic conditions can result in hyperprolactinemia, including prolactinomas, other pituitary and hypothalamic lesions, primary hypothyroidism, and medications such as antipsychotics.25 Dopamine agonists are the mainstay treatment for hyperprolactinemia.
Ferritin measurement
Ferritin measurement is indicated to diagnose iron overload conditions such as hemochromatosis, which can result in primary hypogonadism via testicular damage or in secondary hypogonadism via pituitary damage.26
Pituitary MRI with contrast
Pituitary MRI with contrast is used to diagnose structural lesions of the pituitary or hypothalamus. This diagnostic modality is indicated for patients with pituitary dysfunction, including central hypogonadism, manifestations of a mass effect (headache, visual field defects), persistent hyperprolactinemia, and panhypopituitarism, among others. To improve the diagnostic yield of pituitary MRI, the Endocrine Society guidelines recommend it for men with serum total testosterone levels below 150 ng/dL.5 However, some clinicians have a lower threshold for ordering pituitary MRI for patients with central hypogonadism. Physician judgment and expertise should be exercised and the decision made on an individual basis.
Chromosomal karyotyping
Chromosomal karyotyping is not indicated in our patient. It is reserved for those with primary hypogonadism to diagnose Klinefelter syndrome, which has a karyotype of 47,XXY.
CASE RESUMED: MOSH SYNDROME
Our patient’s prolactin, free T4, morning cortisol, and ferritin levels are measured, yielding normal values. No abnormalities are seen on pituitary MRI. A clinical reevaluation is conducted, revealing no history of head trauma or head and neck radiation. The lack of an obvious cause in our patient’s clinical presentation and workup, together with his obesity (BMI 32.8 kg/m2) supports the diagnosis of obesity as the cause of his hypogonadism.
Obesity can be a cause of secondary hypogonadism, which has led to the term “MOSH” (male obesity-associated secondary hypogonadism) syndrome. In fact, a cross-sectional study has demonstrated that 40% of nondiabetic obese (BMI ≥ 30 kg/m2) men over age 45 have low serum free testosterone levels, compared with 26% for lean (BMI < 25 kg/m2) men.27 Moreover, obesity has been found to be a strong predictor of testosterone replacement therapy.28 Other studies have also found an inverse relationship between BMI and testosterone levels.29
Several mechanisms interact in the pathogenesis of MOSH syndrome. Adipose tissue possesses aromatase activity, which converts androgens into estrogens.30 Peripheral estrogen production can in turn exert feedback inhibition on pituitary gonadotropin secretion.31 In obese men, increased adipose tissue leads to increased aromatase activity and more estrogen, so more feedback inhibition on the pituitary and subsequently secondary hypogonadism.
Leptin, a hormone produced by adipocytes, is also increased in obesity, and was found to be inversely correlated with serum testosterone.32 Studies have demonstrated that leptin has an inhibitory effect on the enzymatic pathway that synthesizes testosterone in Leydig cells.33
Proinflammatory cytokines have also been implicated, as central obesity is associated with an increase in these cytokines, which in turn act negatively on the hypothalamus and impair GnRH release leading to lower testosterone.34,35
Treating obesity-related hypogonadism
In a pilot study,36 lifestyle attempts to reduce obesity were shown to improve hormonal levels. Bariatric surgery has also been demonstrated to be successful.37
Clomiphene citrate, a selective estrogen receptor modulator, increases endogenous testosterone secretion by inhibiting the negative feedback of estrogen on the hypothalamus and pituitary and thus increasing LH and FSH. It also preserves endogenous testosterone production, since it does not suppress the hypothalamic-pituitary-testicular axis.38 This made clomiphene citrate a potential treatment for men with central hypogonadism including those with MOSH.39
Nevertheless, there are no randomized trials to prove its safety and efficacy in the management of central hypogonadism.5 Regarding its use in men wishing to preserve fertility, most studies did not show improvement. However, a meta-analysis demonstrated statistically significant increased pregnancy rates in partners of men with idiopathic infertility if the men used 50 mg of clomiphene citrate daily.40
Testosterone deficiency can be a marker of metabolic syndrome, which needs to be managed more urgently than hypogonadism. A cross-sectional study found not only an association between metabolic syndrome and low serum testosterone, but also with each individual component of metabolic syndrome on its own, all of which need to be addressed.10
CASE CONTINUED: BEGINNING TREATMENT
The physician counsels the patient regarding the implications, potential adverse outcomes, and available treatments for his obesity, including lifestyle modification and bariatric surgery. The patient declines surgery and wishes to adopt a weight-reducing diet and exercise program, for which he is referred to a dietitian.
In addition, in view of the patient’s clinically and biochemically proven hypogonadism, his physician offers testosterone replacement therapy. He orders a serum prostate-specific antigen (PSA) level, which is 1.3 ng/dL (reference range < 4 ng/dL). The patient is prescribed 5 g of 1% testosterone gel daily.
TESTOSTERONE REPLACEMENT THERAPY
4. Which is the most common adverse effect of testosterone replacement therapy?
- Cardiovascular events
- Erythrocytosis
- Prostate cancer
- Infertility
- Obstructive sleep apnea
Clinicians should be very cautious in initiating testosterone replacement therapy in any patient with an unstable medical condition.
There are several formulations of testosterone replacement therapy, including intramuscular injections, transdermal gels or patches, buccal tablets, an intranasal gel, and oral tablets. Of note, there are 2 different forms of oral testosterone preparations: testosterone undecanoate and 17-alpha alkylated testosterone. The former is unavailable in the United States and the latter is not recommended for use due to its proven hepatic toxicity.41
Testosterone and erythrocytosis
Meta-analyses have concluded that the most frequent adverse event of testosterone replacement therapy is a significant rise in hematocrit.42 This rise was found to be dose-dependent and was more marked in older men.43 Although all preparations can cause erythrocytosis, parenteral forms have been observed to raise it the most, particularly short-term injectables.44,45
The mechanism behind this increase is attributed to increased erythropoietin levels and improved usage of iron for red blood cell synthesis.46 In fact, testosterone replacement therapy has been shown to improve hemoglobin levels in patients with anemia.47 On the other hand, increasing hematocrit levels may lead to thrombotic and vasoocclusive events.44
Testosterone and prostate cancer
The relationship between testosterone treatment and prostate cancer has long been studied. Historically, testosterone replacement therapy was believed to increase the risk of prostate cancer; however, recent studies and meta-analyses have shown that this is not the case.42,48 Nevertheless, clinical guidelines still recommend prostate monitoring for men on testosterone replacement therapy.5,6
Testosterone and cardiovascular risk
The evidence regarding this issue has been contradictory and inconsistent. Meta-analyses have demonstrated that low testosterone is associated with higher risk of major adverse cardiovascular events.50 These studies argue for the use of testosterone replacement therapy in hypogonadal men to decrease the risk. However, other studies and meta-analyses have found that testosterone replacement therapy is associated with increased cardiovascular risk and have concluded that major adverse cardiac events are in fact a risk of testosterone replacement therapy.51
Current recommendations advocate against the use of testosterone replacement therapy in men with uncontrolled heart failure or with cardiovascular events in the past 3 to 6 months.5,6 Cardiovascular risk factors should be addressed and corrected, and patients should be educated on cardiovascular symptoms and the need to report them if they occur.
Testosterone and infertility
As described earlier, testosterone replacement therapy increases negative feedback on the pituitary and decreases LH and FSH production, leading to less spermatogenesis. Other treatment options should be sought for hypogonadal men wishing to preserve fertility.
Other adverse effects
Other adverse effects of testosterone replacement therapy include acne, oily skin, obstructive sleep apnea, gynecomastia, and balding.
Given all the adverse events that can be associated with testosterone replacement therapy, the risks and benefits of treating hypogonadism in each patient should be taken into consideration, and an individualized approach is required.
CASE RESUMED: FOLLOW-UP
The patient presents 3 months later for follow-up. He reports significant improvement in his presenting symptoms including energy, libido, and erectile function. He also reports some improvement in his mood and concentration. He has lost 12 lb (5.4 kg) and is still trying to improve his diet and exercise program. He is compliant with his testosterone gel therapy.
His serum calculated free testosterone level is 7.8 ng/dL (4.5–17), and his hematocrit is 46%. The patient is instructed to continue his treatment and to return after 9 months for further follow-up.
TAKE-HOME POINTS
- Men with hypogonadism usually present with nonspecific manifestations, so clinicians should keep a high index of suspicion.
- Both clinical and biochemical evidence of hypogonadism should be present to diagnose and start treatment for it.
- Low levels of serum total testosterone do not necessarily reflect hypogonadism.
- The hormonal profile of central hypogonadism reveals low serum testosterone with low or inappropriately normal serum LH and FSH levels.
Obesity can cause central hypogonadism and should be suspected after pituitary and other systemic causes are excluded.
A 48-year-old man presents to his primary care physician because of progressively decreasing energy and gradual decline in both libido and erectile function for the past 18 months. He has noticed decreased morning erections as well. He rates his libido at 3 to 4 on a scale of 10 for the past 6 months. He also reports poor motivation, depressed mood, impaired concentration, and sleep disturbances. He reports no hair loss, headache, or dizziness, and no decrease in shaving frequency. Review of his systems is otherwise unremarkable.
He has had dyslipidemia for 3 years and is not known to have hypertension or diabetes. His medications include atorvastatin, vitamin E, and multivitamins.
He is married with 3 children and does not wish to have more. He works as a software engineer and leads a sedentary lifestyle. He is a nonsmoker and occasionally drinks alcohol on the weekends.
On physical examination, he is alert and oriented and appears well. His height is 5 feet 10 inches (178 cm), weight 230 lb (104 kg), and body mass index (BMI) 32.8 kg/m2. His blood pressure is 115/83 mm Hg and pulse rate is 82 beats per minute and regular. Findings on cardiovascular and pulmonary examination are normal. He has large fatty breasts but without palpable glandular tissue.
Genitourinary examination reveals normal hair distribution, a normal-sized penis, and slightly soft testes with testicular volume of 18–20 mL bilaterally.
His primary care physician suspects that he has low testosterone and orders some basic laboratory tests; the results are normal except for a low total testosterone level (Table 1).
FURTHER TESTING
1. Which of the following tests should his physician order next?
- Repeat total testosterone measurement
- Free testosterone measurement by commercial assay
- Calculated free testosterone
- Bioavailable testosterone measurement
- Serum inhibin B measurement
This patient presents with several nonspecific symptoms. But collectively they suggest testosterone deficiency (hypogonadism).
Together, erectile dysfunction, low libido, and decreased morning erections strongly suggest hypogonadism.2 Loss of body hair and decreased shaving frequency are specific symptoms of hypogonadism; however, they require years to develop.3 Gynecomastia can also occur due to loss of the inhibitory action of testosterone on breast growth and a relative increase in estradiol. This occurs more in primary hypogonadism, due to the increase in luteinizing hormone (LH), which stimulates the remaining Leydig cells to secrete estradiol rather than testosterone.4
To diagnose hypogonadism in men and to start treatment for it, current guidelines recommend that the patient should have clinical features as well as laboratory evidence of low testosterone.5,6
Measuring testosterone: Total, free, bound, and bioavailable
Testosterone, a steroid hormone, circulates in the serum either as free testosterone or bound to several plasma proteins, mainly sex-hormone binding globulin (SHBG) and albumin.
Total testosterone includes both the free and bound fractions, whereas bioavailable testosterone includes both free and the portion bound to albumin, which has low affinity and can dissociate and be used at the tissue level.11
Low levels of total testosterone do not necessarily reflect a hypogonadal state, as a man with altered SHBG levels or binding capabilities can have low total but normal free testosterone levels and no manifestations.12 Several conditions can alter the levels of SHBG, including obesity, diabetes, aging, thyroid dysfunction, and others.5,13
Because our patient is obese, his total testosterone level is not a reliable indicator of hypogonadism, and repeating its measurement will not add diagnostic value.
Therefore, an alternative measurement should be used to accurately reflect the testosterone levels. From a physiologic point of view, bioavailable testosterone is the active form of testosterone and is the most accurate to be measured in a patient with hypogonadism. Nevertheless, because of technical difficulties in its measurement and lack of evidence correlating bioavailable testosterone with the clinical picture of hypogonadism, it is recommended that the level of free testosterone be used.5
The gold standard for direct measurement of serum free testosterone is equilibrium dialysis, but this is expensive and time-consuming.14 Commercial assays for free testosterone exist but have been deemed unreliable.14,15 It is recommended that free testosterone be measured by equilibrium dialysis or calculated using equations based on total testosterone, SHBG, and albumin levels.5 These equations are reliable and give results very close to the values obtained by equilibrium dialysis.15 Therefore, in our patient, it would be suitable to calculate the free testosterone level next.
Serum levels of free testosterone vary according to several factors. Diurnal variation of testosterone has been established: levels are highest in the morning and decline throughout the day.16 Food decreases testosterone levels.17 In addition, there is considerable day-to-day variation.18 Therefore, at least 2 readings of fasting morning testosterone on 2 separate days are recommended for the diagnosis of hypogonadism.5
Inhibin B is a hormone produced by Sertoli cells in the testes in response to follicle-stimulating hormone (FSH) stimulation. In turn, it acts as negative feedback, together with testosterone, to inhibit FSH release from the pituitary. Inhibin B has been shown to reflect spermatogenesis in the testes and therefore fertility.19 Inhibin B levels were found to be low in patients with central hypogonadism, due to less FSH release; however, they did not correlate with testosterone levels.20
CASE RESUMED: CHARACTERIZING HIS HYPOGONADISM
The patient’s physician orders morning fasting total testosterone, SHBG, and albumin testing and calculates the free testosterone level, which yields a value of 3 ng/dL (reference range 4.5–17). This is confirmed by a repeat measurement, which yields a value of 2.9 ng/dL. Laboratory test results combined with his clinical presentation are consistent with hypogonadism.
2. What is the most appropriate next step?
- Measurement of serum LH and FSH
- Measurement of serum prolactin
- Scrotal ultrasonography
- Gonadotropin-releasing hormone (GnRH) stimulation test
- Semen analysis
After hypogonadism is diagnosed, it is important to distinguish if it is primary or central. This is achieved by measuring serum LH and FSH.5 All biotin supplements should be stopped at least 72 hours before measuring LH and FSH, as biotin can interfere with the assays, yielding false values.21
Secretion of FSH and LH from the anterior pituitary is under the influence of pulsatile release of GnRH from the hypothalamus. LH acts on Leydig cells in the testes to produce testosterone, whereas FSH acts on Sertoli cells, together with testosterone, to bring about spermatogenesis in the seminiferous tubules. Testosterone acts centrally as negative feedback to decrease the release of LH and FSH.
Primary hypogonadism occurs due to testicular failure, ie, the testes themselves fail to produce testosterone, leading to hypogonadism. The decrease in testosterone levels, together with inhibin B if Sertoli cells are damaged, lead to loss of negative feedback on the hypothalamus and pituitary, and therefore increased levels of LH and FSH. This is termed hypergonadotropic hypogonadism. Testicular failure may also result in impaired spermatogenesis and infertility due to destruction of testicular structures, in which case fertility cannot be restored.
Central hypogonadism occurs when the pituitary fails to produce LH and FSH (secondary hypogonadism) or when the hypothalamus fails to produce GnRH and subsequently the lack of secretion of LH and FSH from the pituitary (tertiary hypogonadism). The lack of LH will result in no stimulation of Leydig cells to produce testosterone, and therefore its deficiency. Serum hormone levels in central hypogonadism will reveal low testosterone, with either low or inappropriately normal gonadotropins (LH and FSH). This is termed hypogonadotropic hypogonadism. The lack of FSH, together with testosterone deficiency will also result in decreased spermatogenesis and therefore infertility. Testicular structures are preserved, however, and fertility can be restored with appropriate therapy, as discussed below.
Prolactin should be measured only if the patient has central hypogonadism. Its measurement is not warranted at this point in the patient’s workup. The implications of prolactin and its relationship to hypogonadism will be discussed later.
Although, this stepwise approach is not convenient for many patients, some physicians follow it because it is cost-effective, especially in those who are not insured. However, other physicians order FSH, LH, and sometimes prolactin with the confirmatory low testosterone measurement. Laboratories can also be instructed to wait to measure the pituitary hormones and to do so only if low testosterone is confirmed.
Varicocele, a possible cause of male infertility, can also impair Leydig cell function and cause low testosterone. In fact, surgical repair of varicocele has been demonstrated to increase serum testosterone.22 Scrotal ultrasonography is used to diagnose varicocele, but this also should be ordered at a later stage in the workup if primary hypogonadism is diagnosed.
The GnRH stimulation test is important for the diagnosis and evaluation of precocious or delayed puberty in children. In boys with delayed puberty, a poorer response to GnRH stimulation indicates central hypogonadism rather than constitutional delay.23 It has no role in the evaluation of postpubertal or adult-onset hypogonadism.
Semen analysis is important to evaluate fertility if the patient is interested in further procreation.5 Low testosterone levels may result in impaired spermatogenesis and therefore infertility. On the other hand, treatment with exogenous testosterone will also result in infertility, by feedback inhibition of LH and FSH and therefore inhibition of spermatogenesis. If the patient wishes to preserve fertility, treatment options other than testosterone should be considered; examples include clomiphene citrate, human menopausal gonadotropin, and human chorionic gonadotropin.23,24
Our patient has no desire to expand his family; therefore, a semen analysis and attempts to preserve spermatogenesis are not indicated.
CASE RESUMED: SEARCHING FOR CAUSES
His physician orders testing of serum LH and FSH, yielding the following values:
- LH 1.6 mIU/mL (reference range 1.8–12)
- FSH 1.9 mIU/mL (reference range 1.5–12.5).
The diagnosis of central hypogonadism is established.
3. Which investigation is the least appropriate in the further evaluation of this patient?
- Serum prolactin measurement
- Serum ferritin measurement
- Pituitary magnetic resonance imaging (MRI)
- Chromosomal karyotyping
The diagnosis of central hypogonadism warrants evaluation for possible causes. These are summarized in Table 4.
Serum free thyroxine and morning cortisol
Since this patient’s LH and FSH values are abnormal, it is important to evaluate the status of other anterior pituitary hormones. In patients with pituitary abnormalities, serum free T4 is a more reliable test for assessing thyroid function than thyroid-stimulating hormone (TSH), because of loss of the negative feedback of thyroid hormones on the diseased pituitary. In contrast, serum TSH is considered the best single thyroid test to assess primary thyroid dysfunction.
Other measurements include prolactin and morning cortisol (reflecting adrenocorticotropic hormone status).
Prolactin measurement
Prolactin measurement is important to evaluate for hyperprolactinemia, as this will lead to hypogonadism by inhibition of GnRH secretion.25 Different pathologic, pharmacologic, and physiologic conditions can result in hyperprolactinemia, including prolactinomas, other pituitary and hypothalamic lesions, primary hypothyroidism, and medications such as antipsychotics.25 Dopamine agonists are the mainstay treatment for hyperprolactinemia.
Ferritin measurement
Ferritin measurement is indicated to diagnose iron overload conditions such as hemochromatosis, which can result in primary hypogonadism via testicular damage or in secondary hypogonadism via pituitary damage.26
Pituitary MRI with contrast
Pituitary MRI with contrast is used to diagnose structural lesions of the pituitary or hypothalamus. This diagnostic modality is indicated for patients with pituitary dysfunction, including central hypogonadism, manifestations of a mass effect (headache, visual field defects), persistent hyperprolactinemia, and panhypopituitarism, among others. To improve the diagnostic yield of pituitary MRI, the Endocrine Society guidelines recommend it for men with serum total testosterone levels below 150 ng/dL.5 However, some clinicians have a lower threshold for ordering pituitary MRI for patients with central hypogonadism. Physician judgment and expertise should be exercised and the decision made on an individual basis.
Chromosomal karyotyping
Chromosomal karyotyping is not indicated in our patient. It is reserved for those with primary hypogonadism to diagnose Klinefelter syndrome, which has a karyotype of 47,XXY.
CASE RESUMED: MOSH SYNDROME
Our patient’s prolactin, free T4, morning cortisol, and ferritin levels are measured, yielding normal values. No abnormalities are seen on pituitary MRI. A clinical reevaluation is conducted, revealing no history of head trauma or head and neck radiation. The lack of an obvious cause in our patient’s clinical presentation and workup, together with his obesity (BMI 32.8 kg/m2) supports the diagnosis of obesity as the cause of his hypogonadism.
Obesity can be a cause of secondary hypogonadism, which has led to the term “MOSH” (male obesity-associated secondary hypogonadism) syndrome. In fact, a cross-sectional study has demonstrated that 40% of nondiabetic obese (BMI ≥ 30 kg/m2) men over age 45 have low serum free testosterone levels, compared with 26% for lean (BMI < 25 kg/m2) men.27 Moreover, obesity has been found to be a strong predictor of testosterone replacement therapy.28 Other studies have also found an inverse relationship between BMI and testosterone levels.29
Several mechanisms interact in the pathogenesis of MOSH syndrome. Adipose tissue possesses aromatase activity, which converts androgens into estrogens.30 Peripheral estrogen production can in turn exert feedback inhibition on pituitary gonadotropin secretion.31 In obese men, increased adipose tissue leads to increased aromatase activity and more estrogen, so more feedback inhibition on the pituitary and subsequently secondary hypogonadism.
Leptin, a hormone produced by adipocytes, is also increased in obesity, and was found to be inversely correlated with serum testosterone.32 Studies have demonstrated that leptin has an inhibitory effect on the enzymatic pathway that synthesizes testosterone in Leydig cells.33
Proinflammatory cytokines have also been implicated, as central obesity is associated with an increase in these cytokines, which in turn act negatively on the hypothalamus and impair GnRH release leading to lower testosterone.34,35
Treating obesity-related hypogonadism
In a pilot study,36 lifestyle attempts to reduce obesity were shown to improve hormonal levels. Bariatric surgery has also been demonstrated to be successful.37
Clomiphene citrate, a selective estrogen receptor modulator, increases endogenous testosterone secretion by inhibiting the negative feedback of estrogen on the hypothalamus and pituitary and thus increasing LH and FSH. It also preserves endogenous testosterone production, since it does not suppress the hypothalamic-pituitary-testicular axis.38 This made clomiphene citrate a potential treatment for men with central hypogonadism including those with MOSH.39
Nevertheless, there are no randomized trials to prove its safety and efficacy in the management of central hypogonadism.5 Regarding its use in men wishing to preserve fertility, most studies did not show improvement. However, a meta-analysis demonstrated statistically significant increased pregnancy rates in partners of men with idiopathic infertility if the men used 50 mg of clomiphene citrate daily.40
Testosterone deficiency can be a marker of metabolic syndrome, which needs to be managed more urgently than hypogonadism. A cross-sectional study found not only an association between metabolic syndrome and low serum testosterone, but also with each individual component of metabolic syndrome on its own, all of which need to be addressed.10
CASE CONTINUED: BEGINNING TREATMENT
The physician counsels the patient regarding the implications, potential adverse outcomes, and available treatments for his obesity, including lifestyle modification and bariatric surgery. The patient declines surgery and wishes to adopt a weight-reducing diet and exercise program, for which he is referred to a dietitian.
In addition, in view of the patient’s clinically and biochemically proven hypogonadism, his physician offers testosterone replacement therapy. He orders a serum prostate-specific antigen (PSA) level, which is 1.3 ng/dL (reference range < 4 ng/dL). The patient is prescribed 5 g of 1% testosterone gel daily.
TESTOSTERONE REPLACEMENT THERAPY
4. Which is the most common adverse effect of testosterone replacement therapy?
- Cardiovascular events
- Erythrocytosis
- Prostate cancer
- Infertility
- Obstructive sleep apnea
Clinicians should be very cautious in initiating testosterone replacement therapy in any patient with an unstable medical condition.
There are several formulations of testosterone replacement therapy, including intramuscular injections, transdermal gels or patches, buccal tablets, an intranasal gel, and oral tablets. Of note, there are 2 different forms of oral testosterone preparations: testosterone undecanoate and 17-alpha alkylated testosterone. The former is unavailable in the United States and the latter is not recommended for use due to its proven hepatic toxicity.41
Testosterone and erythrocytosis
Meta-analyses have concluded that the most frequent adverse event of testosterone replacement therapy is a significant rise in hematocrit.42 This rise was found to be dose-dependent and was more marked in older men.43 Although all preparations can cause erythrocytosis, parenteral forms have been observed to raise it the most, particularly short-term injectables.44,45
The mechanism behind this increase is attributed to increased erythropoietin levels and improved usage of iron for red blood cell synthesis.46 In fact, testosterone replacement therapy has been shown to improve hemoglobin levels in patients with anemia.47 On the other hand, increasing hematocrit levels may lead to thrombotic and vasoocclusive events.44
Testosterone and prostate cancer
The relationship between testosterone treatment and prostate cancer has long been studied. Historically, testosterone replacement therapy was believed to increase the risk of prostate cancer; however, recent studies and meta-analyses have shown that this is not the case.42,48 Nevertheless, clinical guidelines still recommend prostate monitoring for men on testosterone replacement therapy.5,6
Testosterone and cardiovascular risk
The evidence regarding this issue has been contradictory and inconsistent. Meta-analyses have demonstrated that low testosterone is associated with higher risk of major adverse cardiovascular events.50 These studies argue for the use of testosterone replacement therapy in hypogonadal men to decrease the risk. However, other studies and meta-analyses have found that testosterone replacement therapy is associated with increased cardiovascular risk and have concluded that major adverse cardiac events are in fact a risk of testosterone replacement therapy.51
Current recommendations advocate against the use of testosterone replacement therapy in men with uncontrolled heart failure or with cardiovascular events in the past 3 to 6 months.5,6 Cardiovascular risk factors should be addressed and corrected, and patients should be educated on cardiovascular symptoms and the need to report them if they occur.
Testosterone and infertility
As described earlier, testosterone replacement therapy increases negative feedback on the pituitary and decreases LH and FSH production, leading to less spermatogenesis. Other treatment options should be sought for hypogonadal men wishing to preserve fertility.
Other adverse effects
Other adverse effects of testosterone replacement therapy include acne, oily skin, obstructive sleep apnea, gynecomastia, and balding.
Given all the adverse events that can be associated with testosterone replacement therapy, the risks and benefits of treating hypogonadism in each patient should be taken into consideration, and an individualized approach is required.
CASE RESUMED: FOLLOW-UP
The patient presents 3 months later for follow-up. He reports significant improvement in his presenting symptoms including energy, libido, and erectile function. He also reports some improvement in his mood and concentration. He has lost 12 lb (5.4 kg) and is still trying to improve his diet and exercise program. He is compliant with his testosterone gel therapy.
His serum calculated free testosterone level is 7.8 ng/dL (4.5–17), and his hematocrit is 46%. The patient is instructed to continue his treatment and to return after 9 months for further follow-up.
TAKE-HOME POINTS
- Men with hypogonadism usually present with nonspecific manifestations, so clinicians should keep a high index of suspicion.
- Both clinical and biochemical evidence of hypogonadism should be present to diagnose and start treatment for it.
- Low levels of serum total testosterone do not necessarily reflect hypogonadism.
- The hormonal profile of central hypogonadism reveals low serum testosterone with low or inappropriately normal serum LH and FSH levels.
Obesity can cause central hypogonadism and should be suspected after pituitary and other systemic causes are excluded.
- Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 2007; 92(11):4241–4247. doi:10.1210/jc.2007-1245
- Wu FCW, Tajar A, Beynon JM, et al; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010; 363(2):123–135. doi:10.1056/NEJMoa0911101
- Arver S, Lehtihet M. Current guidelines for the diagnosis of testosterone deficiency. Front Horm Res 2009; 37:5–20. doi:10.1159/000175839
- Narula HS, Carlson HE. Gynaecomastia—pathophysiology, diagnosis and treatment. Nat Rev Endocrinol 2014; 10(11):684–698. doi:10.1038/nrendo.2014.139
- Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2018; 103(5):1715–1744. doi:10.1210/jc.2018-00229
- Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol 2018; 200(2):423–432. doi:10.1016/j.juro.2018.03.115
- Balasubramanian V, Naing S. Hypogonadism in chronic obstructive pulmonary disease: incidence and effects. Curr Opin Pulm Med 2012; 18(2):112–117. doi:10.1097/MCP.0b013e32834feb37
- Atlantis E, Fahey P, Cochrane B, Wittert G, Smith S. Endogenous testosterone level and testosterone supplementation therapy in chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. BMJ Open 2013; 3(8)pii:e003127. doi:10.1136/bmjopen-2013-003127
- Bawor M, Bami H, Dennis BB, et al. Testosterone suppression in opioid users: a systematic review and meta-analysis. Drug Alcohol Depend 2015; 149:1–9. doi:10.1016/j.drugalcdep.2015.01.038
- Tan WS, Ng CJ, Khoo EM, Low WY, Tan HM. The triad of erectile dysfunction, testosterone deficiency syndrome and metabolic syndrome: findings from a multi-ethnic Asian men study (The Subang Men's Health Study). Aging Male 2011; 14(4):231–236. doi:10.3109/13685538.2011.597463
- Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A reappraisal of testosterone’s binding in circulation: physiological and clinical implications. Endocr Rev 2017; 38(4):302–324. doi:10.1210/er.2017-00025
- Antonio L, Wu FC, O’Neill TW, et al; European Male Ageing Study Study Group. Low free testosterone is associated with hypogonadal signs and symptoms in men with normal total testosterone. J Clin Endocrinol Metab 2016; 101(7):2647–2657. doi:10.1210/jc.2015-4106
- Liu F, Shen X, Wang R, et al. Association of central obesity with sex hormone binding globulin: a cross-sectional study of 1166 Chinese men. Open Med (Wars) 2018; 13:196–202. doi:10.1515/med-2018-0030
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999; 84(10):3666–3672. doi:10.1210/jcem.84.10.6079
- Halmenschlager G, Rhoden EL, Riedner CE. Calculated free testosterone and radioimmunoassay free testosterone as a predictor of subnormal levels of total testosterone. Int Urol Nephrol 2012; 44(3):673–681. doi:10.1007/s11255-011-0066-z
- Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab 2009; 94(3):907–913. doi:10.1210/jc.2008-1902
- Lehtihet M, Arver S, Bartuseviciene I, Pousette Å. S-testosterone decrease after a mixed meal in healthy men independent of SHBG and gonadotrophin levels. Andrologia 2012; 44(6):405–410. doi:10.1111/j.1439-0272.2012.01296.x
- Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf) 2007; 67(6):853–862. doi:10.1111/j.1365-2265.2007.02976.x
- Manzoor SM, Sattar A, Hashim R, et al. Serum inhibin B as a diagnostic marker of male infertility. J Ayub Med Coll Abbottabad 2012; 24(3–4):113–116. pmid:24669628
- Kolb BA, Stanczyk FZ, Sokol RZ. Serum inhibin B levels in males with gonadal dysfunction. Fertil Steril 2000; 74(2):234–238. pmid:10927037
- Trambas CM, Sikaris KA, Lu ZX. More on biotin treatment mimicking Graves’ disease. N Engl J Med 2016; 375(17):1698. doi:10.1056/NEJMc1611875
- Li F, Yue H, Yamaguchi K, et al. Effect of surgical repair on testosterone production in infertile men with varicocele: a meta-analysis. Int J Urol 2012; 19(2):149–154. doi:10.1111/j.1442-2042.2011.02890.x
- Crosnoe-Shipley LE, Elkelany OO, Rahnema CD, Kim ED. Treatment of hypogonadotropic male hypogonadism: case-based scenarios. World J Nephrol 2015; 4(2):245–253. doi:10.5527/wjn.v4.i2.245
- Majzoub A, Sabanegh E Jr. Testosterone replacement in the infertile man. Transl Androl Urol 2016; 5(6):859–865. doi:10.21037/tau.2016.08.03
- Majumdar A, Mangal NS. Hyperprolactinemia. J Hum Reprod Sci 2013; 6(3):168–175. doi:10.4103/0974-1208.121400
- El Osta R, Grandpre N, Monnin N, Hubert J, Koscinski I. Hypogonadotropic hypogonadism in men with hereditary hemochromatosis. Basic Clin Androl 2017; 27:13. doi:10.1186/s12610-017-0057-8
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010; 33(6):1186–1192. doi:10.2337/dc09-1649
- Jasuja GK, Bhasin S, Reisman JI, et al. Who gets testosterone? Patient characteristics associated with testosterone prescribing in the Veteran Affairs system: a cross-sectional study. J Gen Intern Med 2017; 32(3):304–311. doi:10.1007/s11606-016-3940-7
- Kaplan SA, Lee JY, O’Neill EA, Meehan AG, Kusek JW. Prevalence of low testosterone and its relationship to body mass index in older men with lower urinary tract symptoms associated with benign prostatic hyperplasia. Aging Male 2013; 16(4):169–172. doi:10.3109/13685538.2013.844786
- Lee HK, Lee JK, Cho B. The role of androgen in the adipose tissue of males. World J Mens Health 2013; 31(2):136–140. doi:10.5534/wjmh.2013.31.2.136
- Raven G, De Jong FH, Kaufman JM, De Ronde W. In men, peripheral estradiol levels directly reflect the action of estrogens at the hypothalamo-pituitary level to inhibit gonadotropin secretion. J Clin Endocrinol Metab 2006; 91(9):3324–3328. doi:10.1210/jc.2006-0462
- Hofny ER, Ali ME, Abdel-Hafez HZ, et al. Semen parameters and hormonal profile in obese fertile and infertile males. Fertil Steril 2010; 94(2):581–584. doi:10.1016/j.fertnstert.2009.03.085
- Isidori AM, Caprio M, Strollo F, et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab 1999; 84(10):3673–3680. doi:10.1210/jcem.84.10.6082
- El-Wakkad A, Hassan NM, Sibaii H, El-Zayat SR. Proinflammatory, anti-inflammatory cytokines and adiponkines in students with central obesity. Cytokine 2013; 61(2):682–687. doi:10.1016/j.cyto.2012.11.010
- Maggio M, Basaria S, Ceda GP, et al. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest 2005; 28(suppl proceedings 11):116–119. pmid:16760639
- de Lorenzo A, Noce A, Moriconi E, et al. MOSH syndrome (male obesity secondary hypogonadism): clinical assessment and possible therapeutic approaches. Nutrients 2018; 10(4)pii:E474. doi:10.3390/nu10040474
- Escobar-Morreale HF, Santacruz E, Luque-Ramírez M, Botella Carretero JI. Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum Reprod Update 2017; 23(4):390–408. doi:10.1093/humupd/dmx012
- Lo EM, Rodriguez KM, Pastuszak AW, Khera M. Alternatives to testosterone therapy: a review. Sex Med Rev 2018; 6(1):106–113. doi:10.1016/j.sxmr.2017.09.004
- Soares AH, Horie NC, Chiang LAP, et al. Effects of clomiphene citrate on male obesity-associated hypogonadism: a randomized, double-blind, placebo-controlled study. Int J Obes (Lond) 2018; 42(5):953–963. doi:10.1038/s41366-018-0105-2
- Chua ME, Escusa KG, Luna S, Tapia LC, Dofitas B, Morales M. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology 2013; 1(5):749–757. doi:10.1111/j.2047-2927.2013.00107.x
- Westaby D, Ogle SJ, Paradinas FJ, Randell JB, Murray-Lyon IM. Liver damage from long-term methyltestosterone. Lancet 1977; 2(8032):262–263. pmid:69876
- Fernández-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2010; 95(6):2560–2575. doi:10.1210/jc.2009-2575
- Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab 2008; 93(3):914–919. doi:10.1210/jc.2007-1692
- Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis following testosterone therapy. Sex Med Rev 2018; 6(1):77–85. doi:10.1016/j.sxmr.2017.04.001
- Jones SD Jr, Dukovac T, Sangkum P, Yafi FA, Hellstrom WJ. Erythrocytosis and polycythemia secondary to testosterone replacement therapy in the aging male. Sex Med Rev 2015; 3(2):101–112. doi:10.1002/smrj.43
- Bachman E, Travison TG, Basaria S, et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci 2014; 69(6):725–735. doi:10.1093/gerona/glt154
- Roy CN, Snyder PJ, Stephens-Shields AJ, et al. Association of testosterone levels with anemia in older men: a controlled clinical trial. JAMA Intern Med 2017; 177(4):480–490. doi:10.1001/jamainternmed.2016.9540
- Klap J, Schmid M, Loughlin KR. The relationship between total testosterone levels and prostate cancer: a review of the continuing controversy. J Urol 2015; 193(2):403–413. doi:10.1016/j.juro.2014.07.123
- Gilbert SM, Cavallo CB, Kahane H, Lowe FC. Evidence suggesting PSA cutpoint of 2.5 ng/mL for prompting prostate biopsy: review of 36,316 biopsies. Urology 2005; 65(3):549–553. doi:10.1016/j.urology.2004.10.064
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011; 96(10):3007–3019. doi:10.1210/jc.2011-1137
- Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med 2013; 11:108. doi:10.1186/1741-7015-11-108
- Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 2007; 92(11):4241–4247. doi:10.1210/jc.2007-1245
- Wu FCW, Tajar A, Beynon JM, et al; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010; 363(2):123–135. doi:10.1056/NEJMoa0911101
- Arver S, Lehtihet M. Current guidelines for the diagnosis of testosterone deficiency. Front Horm Res 2009; 37:5–20. doi:10.1159/000175839
- Narula HS, Carlson HE. Gynaecomastia—pathophysiology, diagnosis and treatment. Nat Rev Endocrinol 2014; 10(11):684–698. doi:10.1038/nrendo.2014.139
- Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2018; 103(5):1715–1744. doi:10.1210/jc.2018-00229
- Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol 2018; 200(2):423–432. doi:10.1016/j.juro.2018.03.115
- Balasubramanian V, Naing S. Hypogonadism in chronic obstructive pulmonary disease: incidence and effects. Curr Opin Pulm Med 2012; 18(2):112–117. doi:10.1097/MCP.0b013e32834feb37
- Atlantis E, Fahey P, Cochrane B, Wittert G, Smith S. Endogenous testosterone level and testosterone supplementation therapy in chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. BMJ Open 2013; 3(8)pii:e003127. doi:10.1136/bmjopen-2013-003127
- Bawor M, Bami H, Dennis BB, et al. Testosterone suppression in opioid users: a systematic review and meta-analysis. Drug Alcohol Depend 2015; 149:1–9. doi:10.1016/j.drugalcdep.2015.01.038
- Tan WS, Ng CJ, Khoo EM, Low WY, Tan HM. The triad of erectile dysfunction, testosterone deficiency syndrome and metabolic syndrome: findings from a multi-ethnic Asian men study (The Subang Men's Health Study). Aging Male 2011; 14(4):231–236. doi:10.3109/13685538.2011.597463
- Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A reappraisal of testosterone’s binding in circulation: physiological and clinical implications. Endocr Rev 2017; 38(4):302–324. doi:10.1210/er.2017-00025
- Antonio L, Wu FC, O’Neill TW, et al; European Male Ageing Study Study Group. Low free testosterone is associated with hypogonadal signs and symptoms in men with normal total testosterone. J Clin Endocrinol Metab 2016; 101(7):2647–2657. doi:10.1210/jc.2015-4106
- Liu F, Shen X, Wang R, et al. Association of central obesity with sex hormone binding globulin: a cross-sectional study of 1166 Chinese men. Open Med (Wars) 2018; 13:196–202. doi:10.1515/med-2018-0030
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999; 84(10):3666–3672. doi:10.1210/jcem.84.10.6079
- Halmenschlager G, Rhoden EL, Riedner CE. Calculated free testosterone and radioimmunoassay free testosterone as a predictor of subnormal levels of total testosterone. Int Urol Nephrol 2012; 44(3):673–681. doi:10.1007/s11255-011-0066-z
- Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab 2009; 94(3):907–913. doi:10.1210/jc.2008-1902
- Lehtihet M, Arver S, Bartuseviciene I, Pousette Å. S-testosterone decrease after a mixed meal in healthy men independent of SHBG and gonadotrophin levels. Andrologia 2012; 44(6):405–410. doi:10.1111/j.1439-0272.2012.01296.x
- Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf) 2007; 67(6):853–862. doi:10.1111/j.1365-2265.2007.02976.x
- Manzoor SM, Sattar A, Hashim R, et al. Serum inhibin B as a diagnostic marker of male infertility. J Ayub Med Coll Abbottabad 2012; 24(3–4):113–116. pmid:24669628
- Kolb BA, Stanczyk FZ, Sokol RZ. Serum inhibin B levels in males with gonadal dysfunction. Fertil Steril 2000; 74(2):234–238. pmid:10927037
- Trambas CM, Sikaris KA, Lu ZX. More on biotin treatment mimicking Graves’ disease. N Engl J Med 2016; 375(17):1698. doi:10.1056/NEJMc1611875
- Li F, Yue H, Yamaguchi K, et al. Effect of surgical repair on testosterone production in infertile men with varicocele: a meta-analysis. Int J Urol 2012; 19(2):149–154. doi:10.1111/j.1442-2042.2011.02890.x
- Crosnoe-Shipley LE, Elkelany OO, Rahnema CD, Kim ED. Treatment of hypogonadotropic male hypogonadism: case-based scenarios. World J Nephrol 2015; 4(2):245–253. doi:10.5527/wjn.v4.i2.245
- Majzoub A, Sabanegh E Jr. Testosterone replacement in the infertile man. Transl Androl Urol 2016; 5(6):859–865. doi:10.21037/tau.2016.08.03
- Majumdar A, Mangal NS. Hyperprolactinemia. J Hum Reprod Sci 2013; 6(3):168–175. doi:10.4103/0974-1208.121400
- El Osta R, Grandpre N, Monnin N, Hubert J, Koscinski I. Hypogonadotropic hypogonadism in men with hereditary hemochromatosis. Basic Clin Androl 2017; 27:13. doi:10.1186/s12610-017-0057-8
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010; 33(6):1186–1192. doi:10.2337/dc09-1649
- Jasuja GK, Bhasin S, Reisman JI, et al. Who gets testosterone? Patient characteristics associated with testosterone prescribing in the Veteran Affairs system: a cross-sectional study. J Gen Intern Med 2017; 32(3):304–311. doi:10.1007/s11606-016-3940-7
- Kaplan SA, Lee JY, O’Neill EA, Meehan AG, Kusek JW. Prevalence of low testosterone and its relationship to body mass index in older men with lower urinary tract symptoms associated with benign prostatic hyperplasia. Aging Male 2013; 16(4):169–172. doi:10.3109/13685538.2013.844786
- Lee HK, Lee JK, Cho B. The role of androgen in the adipose tissue of males. World J Mens Health 2013; 31(2):136–140. doi:10.5534/wjmh.2013.31.2.136
- Raven G, De Jong FH, Kaufman JM, De Ronde W. In men, peripheral estradiol levels directly reflect the action of estrogens at the hypothalamo-pituitary level to inhibit gonadotropin secretion. J Clin Endocrinol Metab 2006; 91(9):3324–3328. doi:10.1210/jc.2006-0462
- Hofny ER, Ali ME, Abdel-Hafez HZ, et al. Semen parameters and hormonal profile in obese fertile and infertile males. Fertil Steril 2010; 94(2):581–584. doi:10.1016/j.fertnstert.2009.03.085
- Isidori AM, Caprio M, Strollo F, et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab 1999; 84(10):3673–3680. doi:10.1210/jcem.84.10.6082
- El-Wakkad A, Hassan NM, Sibaii H, El-Zayat SR. Proinflammatory, anti-inflammatory cytokines and adiponkines in students with central obesity. Cytokine 2013; 61(2):682–687. doi:10.1016/j.cyto.2012.11.010
- Maggio M, Basaria S, Ceda GP, et al. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest 2005; 28(suppl proceedings 11):116–119. pmid:16760639
- de Lorenzo A, Noce A, Moriconi E, et al. MOSH syndrome (male obesity secondary hypogonadism): clinical assessment and possible therapeutic approaches. Nutrients 2018; 10(4)pii:E474. doi:10.3390/nu10040474
- Escobar-Morreale HF, Santacruz E, Luque-Ramírez M, Botella Carretero JI. Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum Reprod Update 2017; 23(4):390–408. doi:10.1093/humupd/dmx012
- Lo EM, Rodriguez KM, Pastuszak AW, Khera M. Alternatives to testosterone therapy: a review. Sex Med Rev 2018; 6(1):106–113. doi:10.1016/j.sxmr.2017.09.004
- Soares AH, Horie NC, Chiang LAP, et al. Effects of clomiphene citrate on male obesity-associated hypogonadism: a randomized, double-blind, placebo-controlled study. Int J Obes (Lond) 2018; 42(5):953–963. doi:10.1038/s41366-018-0105-2
- Chua ME, Escusa KG, Luna S, Tapia LC, Dofitas B, Morales M. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology 2013; 1(5):749–757. doi:10.1111/j.2047-2927.2013.00107.x
- Westaby D, Ogle SJ, Paradinas FJ, Randell JB, Murray-Lyon IM. Liver damage from long-term methyltestosterone. Lancet 1977; 2(8032):262–263. pmid:69876
- Fernández-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2010; 95(6):2560–2575. doi:10.1210/jc.2009-2575
- Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab 2008; 93(3):914–919. doi:10.1210/jc.2007-1692
- Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis following testosterone therapy. Sex Med Rev 2018; 6(1):77–85. doi:10.1016/j.sxmr.2017.04.001
- Jones SD Jr, Dukovac T, Sangkum P, Yafi FA, Hellstrom WJ. Erythrocytosis and polycythemia secondary to testosterone replacement therapy in the aging male. Sex Med Rev 2015; 3(2):101–112. doi:10.1002/smrj.43
- Bachman E, Travison TG, Basaria S, et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci 2014; 69(6):725–735. doi:10.1093/gerona/glt154
- Roy CN, Snyder PJ, Stephens-Shields AJ, et al. Association of testosterone levels with anemia in older men: a controlled clinical trial. JAMA Intern Med 2017; 177(4):480–490. doi:10.1001/jamainternmed.2016.9540
- Klap J, Schmid M, Loughlin KR. The relationship between total testosterone levels and prostate cancer: a review of the continuing controversy. J Urol 2015; 193(2):403–413. doi:10.1016/j.juro.2014.07.123
- Gilbert SM, Cavallo CB, Kahane H, Lowe FC. Evidence suggesting PSA cutpoint of 2.5 ng/mL for prompting prostate biopsy: review of 36,316 biopsies. Urology 2005; 65(3):549–553. doi:10.1016/j.urology.2004.10.064
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011; 96(10):3007–3019. doi:10.1210/jc.2011-1137
- Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med 2013; 11:108. doi:10.1186/1741-7015-11-108
Infection or not infection, that is the question—Is procalcitonin the answer?
Diagnostic algorithms have been proposed to help recognize infection in chronic obstructive pulmonary disease, rhinosinusitis syndrome, acute arthritis, pharyngitis, and possible sepsis. The algorithms have included laboratory tests and potential biomarkers, but all are imperfect despite achieving various degrees of acceptance in practice.
In this issue of the Journal, Dr. Fakheri updates us on using the data on serum procalcitonin levels to guide starting and stopping antibiotics in different clinical scenarios. As I read the paper, I wondered what was different about procalcitonin that might allow it to succeed where seemingly similar biomarkers like C-reactive protein (CRP) and the erythrocyte sedimentation rate (ESR) have failed.
Procalcitonin is the approximately 15,000-kD product of the CALC1 gene and the precursor of calcitonin. Not surprisingly, then, it is increased in patients with thyroid medullary carcinoma, and it is also often elevated in nonthyroid neuroendocrine malignancies. Proteolytic cleavage of procalcitonin to active calcitonin takes place mainly or only in the thyroid, and under normal homeostatic conditions, procalcitonin is almost unmeasurable in the circulation. However, under major stress such as systemic inflammation, sepsis, or burns, the CALC1 gene is activated in parenchymal cells in many organs, and procalcitonin is synthesized and released. Notably, under these conditions, the procalcitonin does not seem to be of thyroid origin; hence, calcitonin levels do not rise markedly. The physiologic role of nonthyroidal procalcitonin is unknown.
Procalcitonin synthesis and secretion is turned on in nonthyroid tissue by multiple cytokines; the cytokines most likely relevant to its association with inflammation and infections are interleukin (IL) 1 beta, tumor necrosis factor (TNF) alpha, and IL-6. Since these same mediators drive the acute-phase response and elicit the increase in circulating CRP and fibrinogen (the major contributor to the ESR), the obvious question is why procalcitonin might be a more reliable biomarker to distinguish bacterial infection from inflammation or a viral infection than the CRP level or ESR. And although it does indeed seem to do so in several conditions, as Dr. Fakheri discusses, the explanation is not obvious. But it is intriguing to hypothesize.
Induction of procalcitonin by endotoxin-stimulated cytokines is rapid and seems to be slightly faster than that of CRP, although there may be issues of assay sensitivity. The half-life of procalcitonin is similar to that of CRP (about 24 hours). Its degradation does not seem to be altered in renal insufficiency, and its synthesis seems to rapidly shut off as the cytokine level drops. But interestingly, and perhaps relevant to its possible unique biomarker behavior, its synthesis seems to depend on factors other than the increase in inflammatory cytokines such as IL-6. Under certain circumstances, in the same patient, there is a discrepancy between the levels of procalcitonin and CRP.
In a small study of patients with pulmonary embolism and fever, IL-6 levels increased in many with an expected accompanying increase in CRP and ESR, but procalcitonin did not markedly rise,1 although all 3 markers rose as expected in patients with bacterial pneumonia.
Even more provocative is another study in 69 patients with systemic lupus erythematosus and bacterial infection (43 patients had sepsis, 11 of whom died). The CRP level rose dramatically in the infected patients, but procalcitonin did not.2
The intriguing aspect of this, assuming it holds true in other studies, is that interferon activity is high in lupus and many viral infections, and if interferon can suppress CALC1 gene activation3 but leave CRP activation unaffected, this may provide a clue as to why CRP but not procalcitonin is elevated in serious viral infections, thus allowing procalcitonin to more effectively distinguish bacterial from viral and other nonbacterial inflammatory responses.
The two studies I mention are small, some conflicting results have been published, and the results cannot yet be generalized. Plus, it has long been recognized there is sometimes discordance in a given patient between the elevation in ESR and CRP, not readily explained by the presence of a paraprotein, rheologic factors, or the different time course of decay in the ESR and CRP response. Whatever the explanation, procalcitonin’s biology is interesting, and clinical study results show promise. While tracking procalcitonin levels is not uniformly useful (eg, there is no convincing value in using procalcitonin in the diagnosis of prosthetic joint infections), there is accumulating evidence that it can guide us to using shorter but still effective courses of antibiotics in several clinical scenarios. Hopefully, more frequent use of the test will make a dent in our apparent excess use of antibiotics in patients with nonbacterial upper-respiratory infections.
- Köktürk N, Kanbay A, Bukan N, Ekim N. The value of serum procalcitonin in differential diagnosis of pulmonary embolism and community acquired pneumonia. Clin App Thromb Hemostasis 2011; 17(5):519–525. doi:10.1177/1076029610375425
- El-Serougy E, Zayed HS, Ibrahim NM, Maged LA. Procalcitonin and C-reactive protein as markers of infection in systemic lupus erythematosus: the controversy continues. Lupus 2018 Jan 1:961203318777101. doi:10.1177/0961203318777101 (e-pub ahead of print)
- Linscheid P, Seboek D, Nylen ES, et al. In vitro and in vivo calcitonin I gene expression in parenchymal cells: a novel product of human adipose tissue. Endocrinology 2003; 144(12): 5578–5584. doi:10.1210/en.2003-0854
Diagnostic algorithms have been proposed to help recognize infection in chronic obstructive pulmonary disease, rhinosinusitis syndrome, acute arthritis, pharyngitis, and possible sepsis. The algorithms have included laboratory tests and potential biomarkers, but all are imperfect despite achieving various degrees of acceptance in practice.
In this issue of the Journal, Dr. Fakheri updates us on using the data on serum procalcitonin levels to guide starting and stopping antibiotics in different clinical scenarios. As I read the paper, I wondered what was different about procalcitonin that might allow it to succeed where seemingly similar biomarkers like C-reactive protein (CRP) and the erythrocyte sedimentation rate (ESR) have failed.
Procalcitonin is the approximately 15,000-kD product of the CALC1 gene and the precursor of calcitonin. Not surprisingly, then, it is increased in patients with thyroid medullary carcinoma, and it is also often elevated in nonthyroid neuroendocrine malignancies. Proteolytic cleavage of procalcitonin to active calcitonin takes place mainly or only in the thyroid, and under normal homeostatic conditions, procalcitonin is almost unmeasurable in the circulation. However, under major stress such as systemic inflammation, sepsis, or burns, the CALC1 gene is activated in parenchymal cells in many organs, and procalcitonin is synthesized and released. Notably, under these conditions, the procalcitonin does not seem to be of thyroid origin; hence, calcitonin levels do not rise markedly. The physiologic role of nonthyroidal procalcitonin is unknown.
Procalcitonin synthesis and secretion is turned on in nonthyroid tissue by multiple cytokines; the cytokines most likely relevant to its association with inflammation and infections are interleukin (IL) 1 beta, tumor necrosis factor (TNF) alpha, and IL-6. Since these same mediators drive the acute-phase response and elicit the increase in circulating CRP and fibrinogen (the major contributor to the ESR), the obvious question is why procalcitonin might be a more reliable biomarker to distinguish bacterial infection from inflammation or a viral infection than the CRP level or ESR. And although it does indeed seem to do so in several conditions, as Dr. Fakheri discusses, the explanation is not obvious. But it is intriguing to hypothesize.
Induction of procalcitonin by endotoxin-stimulated cytokines is rapid and seems to be slightly faster than that of CRP, although there may be issues of assay sensitivity. The half-life of procalcitonin is similar to that of CRP (about 24 hours). Its degradation does not seem to be altered in renal insufficiency, and its synthesis seems to rapidly shut off as the cytokine level drops. But interestingly, and perhaps relevant to its possible unique biomarker behavior, its synthesis seems to depend on factors other than the increase in inflammatory cytokines such as IL-6. Under certain circumstances, in the same patient, there is a discrepancy between the levels of procalcitonin and CRP.
In a small study of patients with pulmonary embolism and fever, IL-6 levels increased in many with an expected accompanying increase in CRP and ESR, but procalcitonin did not markedly rise,1 although all 3 markers rose as expected in patients with bacterial pneumonia.
Even more provocative is another study in 69 patients with systemic lupus erythematosus and bacterial infection (43 patients had sepsis, 11 of whom died). The CRP level rose dramatically in the infected patients, but procalcitonin did not.2
The intriguing aspect of this, assuming it holds true in other studies, is that interferon activity is high in lupus and many viral infections, and if interferon can suppress CALC1 gene activation3 but leave CRP activation unaffected, this may provide a clue as to why CRP but not procalcitonin is elevated in serious viral infections, thus allowing procalcitonin to more effectively distinguish bacterial from viral and other nonbacterial inflammatory responses.
The two studies I mention are small, some conflicting results have been published, and the results cannot yet be generalized. Plus, it has long been recognized there is sometimes discordance in a given patient between the elevation in ESR and CRP, not readily explained by the presence of a paraprotein, rheologic factors, or the different time course of decay in the ESR and CRP response. Whatever the explanation, procalcitonin’s biology is interesting, and clinical study results show promise. While tracking procalcitonin levels is not uniformly useful (eg, there is no convincing value in using procalcitonin in the diagnosis of prosthetic joint infections), there is accumulating evidence that it can guide us to using shorter but still effective courses of antibiotics in several clinical scenarios. Hopefully, more frequent use of the test will make a dent in our apparent excess use of antibiotics in patients with nonbacterial upper-respiratory infections.
Diagnostic algorithms have been proposed to help recognize infection in chronic obstructive pulmonary disease, rhinosinusitis syndrome, acute arthritis, pharyngitis, and possible sepsis. The algorithms have included laboratory tests and potential biomarkers, but all are imperfect despite achieving various degrees of acceptance in practice.
In this issue of the Journal, Dr. Fakheri updates us on using the data on serum procalcitonin levels to guide starting and stopping antibiotics in different clinical scenarios. As I read the paper, I wondered what was different about procalcitonin that might allow it to succeed where seemingly similar biomarkers like C-reactive protein (CRP) and the erythrocyte sedimentation rate (ESR) have failed.
Procalcitonin is the approximately 15,000-kD product of the CALC1 gene and the precursor of calcitonin. Not surprisingly, then, it is increased in patients with thyroid medullary carcinoma, and it is also often elevated in nonthyroid neuroendocrine malignancies. Proteolytic cleavage of procalcitonin to active calcitonin takes place mainly or only in the thyroid, and under normal homeostatic conditions, procalcitonin is almost unmeasurable in the circulation. However, under major stress such as systemic inflammation, sepsis, or burns, the CALC1 gene is activated in parenchymal cells in many organs, and procalcitonin is synthesized and released. Notably, under these conditions, the procalcitonin does not seem to be of thyroid origin; hence, calcitonin levels do not rise markedly. The physiologic role of nonthyroidal procalcitonin is unknown.
Procalcitonin synthesis and secretion is turned on in nonthyroid tissue by multiple cytokines; the cytokines most likely relevant to its association with inflammation and infections are interleukin (IL) 1 beta, tumor necrosis factor (TNF) alpha, and IL-6. Since these same mediators drive the acute-phase response and elicit the increase in circulating CRP and fibrinogen (the major contributor to the ESR), the obvious question is why procalcitonin might be a more reliable biomarker to distinguish bacterial infection from inflammation or a viral infection than the CRP level or ESR. And although it does indeed seem to do so in several conditions, as Dr. Fakheri discusses, the explanation is not obvious. But it is intriguing to hypothesize.
Induction of procalcitonin by endotoxin-stimulated cytokines is rapid and seems to be slightly faster than that of CRP, although there may be issues of assay sensitivity. The half-life of procalcitonin is similar to that of CRP (about 24 hours). Its degradation does not seem to be altered in renal insufficiency, and its synthesis seems to rapidly shut off as the cytokine level drops. But interestingly, and perhaps relevant to its possible unique biomarker behavior, its synthesis seems to depend on factors other than the increase in inflammatory cytokines such as IL-6. Under certain circumstances, in the same patient, there is a discrepancy between the levels of procalcitonin and CRP.
In a small study of patients with pulmonary embolism and fever, IL-6 levels increased in many with an expected accompanying increase in CRP and ESR, but procalcitonin did not markedly rise,1 although all 3 markers rose as expected in patients with bacterial pneumonia.
Even more provocative is another study in 69 patients with systemic lupus erythematosus and bacterial infection (43 patients had sepsis, 11 of whom died). The CRP level rose dramatically in the infected patients, but procalcitonin did not.2
The intriguing aspect of this, assuming it holds true in other studies, is that interferon activity is high in lupus and many viral infections, and if interferon can suppress CALC1 gene activation3 but leave CRP activation unaffected, this may provide a clue as to why CRP but not procalcitonin is elevated in serious viral infections, thus allowing procalcitonin to more effectively distinguish bacterial from viral and other nonbacterial inflammatory responses.
The two studies I mention are small, some conflicting results have been published, and the results cannot yet be generalized. Plus, it has long been recognized there is sometimes discordance in a given patient between the elevation in ESR and CRP, not readily explained by the presence of a paraprotein, rheologic factors, or the different time course of decay in the ESR and CRP response. Whatever the explanation, procalcitonin’s biology is interesting, and clinical study results show promise. While tracking procalcitonin levels is not uniformly useful (eg, there is no convincing value in using procalcitonin in the diagnosis of prosthetic joint infections), there is accumulating evidence that it can guide us to using shorter but still effective courses of antibiotics in several clinical scenarios. Hopefully, more frequent use of the test will make a dent in our apparent excess use of antibiotics in patients with nonbacterial upper-respiratory infections.
- Köktürk N, Kanbay A, Bukan N, Ekim N. The value of serum procalcitonin in differential diagnosis of pulmonary embolism and community acquired pneumonia. Clin App Thromb Hemostasis 2011; 17(5):519–525. doi:10.1177/1076029610375425
- El-Serougy E, Zayed HS, Ibrahim NM, Maged LA. Procalcitonin and C-reactive protein as markers of infection in systemic lupus erythematosus: the controversy continues. Lupus 2018 Jan 1:961203318777101. doi:10.1177/0961203318777101 (e-pub ahead of print)
- Linscheid P, Seboek D, Nylen ES, et al. In vitro and in vivo calcitonin I gene expression in parenchymal cells: a novel product of human adipose tissue. Endocrinology 2003; 144(12): 5578–5584. doi:10.1210/en.2003-0854
- Köktürk N, Kanbay A, Bukan N, Ekim N. The value of serum procalcitonin in differential diagnosis of pulmonary embolism and community acquired pneumonia. Clin App Thromb Hemostasis 2011; 17(5):519–525. doi:10.1177/1076029610375425
- El-Serougy E, Zayed HS, Ibrahim NM, Maged LA. Procalcitonin and C-reactive protein as markers of infection in systemic lupus erythematosus: the controversy continues. Lupus 2018 Jan 1:961203318777101. doi:10.1177/0961203318777101 (e-pub ahead of print)
- Linscheid P, Seboek D, Nylen ES, et al. In vitro and in vivo calcitonin I gene expression in parenchymal cells: a novel product of human adipose tissue. Endocrinology 2003; 144(12): 5578–5584. doi:10.1210/en.2003-0854
Dabigatran-induced esophagitis
A 74-year-old man presented to the gastroenterology clinic with a 2-day history of retrosternal discomfort. His vital signs were normal, and laboratory testing showed a normal leukocyte count.
Esophagogastroduodenoscopy (EGD) revealed longitudinal sloughing mucosal casts in the middle and lower esophagus (Figure 1).
The patient had been taking dabigatran 110 mg twice daily for 2 years because of nonvalvular atrial fibrillation. He was also taking amlodipine 2.5 mg/day for hypertension.
DABIGATRAN-INDUCED ESOPHAGITIS
Although no study has investigated the overall prevalence of dabigatran-induced esophagitis, a retrospective database review of 91 patients taking dabigatran and undergoing upper-gastrointestinal endoscopy reported that 19 (20.9%) had endoscopic signs of dabigatran-induced esophagitis.2
Typical symptoms are the acute onset of chest pain, epigastralgia, odynophagia, and dysphagia. But patients can also have no symptoms or only mild symptoms.2,3
Despite dabigatran’s anticoagulant activity, there have been few reports of bleeding, perhaps because the lesions tend to be superficial on the surface of the esophageal mucosa.
Symptoms usually resolve within 1 week after stopping dabigatran and starting a proton pump inhibitor. To prevent mucosal injury, patients should be instructed to take dabigatran with sufficient water and to remain in an upright position for at least 30 minutes afterward.4
- Baehr PH, McDonald GB. Esophageal infections: risk factors, presentation, diagnosis, and treatment. Gastroenterology 1994; 106(2):509–532. pmid:7980741
- Toya Y, Nakamura S, Tomita K, et al. Dabigatran-induced esophagitis: the prevalence and endoscopic characteristics. J Gastroenterol Hepatol 2016; 31(3):610–614. doi:10.1111/jgh.13024
- Ueta E, Fujikawa T, Imagawa A. A case of a slightly symptomatic exfoliative oesophagitis. BMJ Case Rep 2015; pii:bcr2015211925. doi:10.1136/bcr-2015-211925
- Ootani A, Hayashi Y, Miyagi Y. Dabigatran-induced esophagitis. Clin Gastroenterol Hepatol 2014; 12(7):e55–e56. doi:10.1016/j.cgh.2013.09.010
A 74-year-old man presented to the gastroenterology clinic with a 2-day history of retrosternal discomfort. His vital signs were normal, and laboratory testing showed a normal leukocyte count.
Esophagogastroduodenoscopy (EGD) revealed longitudinal sloughing mucosal casts in the middle and lower esophagus (Figure 1).
The patient had been taking dabigatran 110 mg twice daily for 2 years because of nonvalvular atrial fibrillation. He was also taking amlodipine 2.5 mg/day for hypertension.
DABIGATRAN-INDUCED ESOPHAGITIS
Although no study has investigated the overall prevalence of dabigatran-induced esophagitis, a retrospective database review of 91 patients taking dabigatran and undergoing upper-gastrointestinal endoscopy reported that 19 (20.9%) had endoscopic signs of dabigatran-induced esophagitis.2
Typical symptoms are the acute onset of chest pain, epigastralgia, odynophagia, and dysphagia. But patients can also have no symptoms or only mild symptoms.2,3
Despite dabigatran’s anticoagulant activity, there have been few reports of bleeding, perhaps because the lesions tend to be superficial on the surface of the esophageal mucosa.
Symptoms usually resolve within 1 week after stopping dabigatran and starting a proton pump inhibitor. To prevent mucosal injury, patients should be instructed to take dabigatran with sufficient water and to remain in an upright position for at least 30 minutes afterward.4
A 74-year-old man presented to the gastroenterology clinic with a 2-day history of retrosternal discomfort. His vital signs were normal, and laboratory testing showed a normal leukocyte count.
Esophagogastroduodenoscopy (EGD) revealed longitudinal sloughing mucosal casts in the middle and lower esophagus (Figure 1).
The patient had been taking dabigatran 110 mg twice daily for 2 years because of nonvalvular atrial fibrillation. He was also taking amlodipine 2.5 mg/day for hypertension.
DABIGATRAN-INDUCED ESOPHAGITIS
Although no study has investigated the overall prevalence of dabigatran-induced esophagitis, a retrospective database review of 91 patients taking dabigatran and undergoing upper-gastrointestinal endoscopy reported that 19 (20.9%) had endoscopic signs of dabigatran-induced esophagitis.2
Typical symptoms are the acute onset of chest pain, epigastralgia, odynophagia, and dysphagia. But patients can also have no symptoms or only mild symptoms.2,3
Despite dabigatran’s anticoagulant activity, there have been few reports of bleeding, perhaps because the lesions tend to be superficial on the surface of the esophageal mucosa.
Symptoms usually resolve within 1 week after stopping dabigatran and starting a proton pump inhibitor. To prevent mucosal injury, patients should be instructed to take dabigatran with sufficient water and to remain in an upright position for at least 30 minutes afterward.4
- Baehr PH, McDonald GB. Esophageal infections: risk factors, presentation, diagnosis, and treatment. Gastroenterology 1994; 106(2):509–532. pmid:7980741
- Toya Y, Nakamura S, Tomita K, et al. Dabigatran-induced esophagitis: the prevalence and endoscopic characteristics. J Gastroenterol Hepatol 2016; 31(3):610–614. doi:10.1111/jgh.13024
- Ueta E, Fujikawa T, Imagawa A. A case of a slightly symptomatic exfoliative oesophagitis. BMJ Case Rep 2015; pii:bcr2015211925. doi:10.1136/bcr-2015-211925
- Ootani A, Hayashi Y, Miyagi Y. Dabigatran-induced esophagitis. Clin Gastroenterol Hepatol 2014; 12(7):e55–e56. doi:10.1016/j.cgh.2013.09.010
- Baehr PH, McDonald GB. Esophageal infections: risk factors, presentation, diagnosis, and treatment. Gastroenterology 1994; 106(2):509–532. pmid:7980741
- Toya Y, Nakamura S, Tomita K, et al. Dabigatran-induced esophagitis: the prevalence and endoscopic characteristics. J Gastroenterol Hepatol 2016; 31(3):610–614. doi:10.1111/jgh.13024
- Ueta E, Fujikawa T, Imagawa A. A case of a slightly symptomatic exfoliative oesophagitis. BMJ Case Rep 2015; pii:bcr2015211925. doi:10.1136/bcr-2015-211925
- Ootani A, Hayashi Y, Miyagi Y. Dabigatran-induced esophagitis. Clin Gastroenterol Hepatol 2014; 12(7):e55–e56. doi:10.1016/j.cgh.2013.09.010
Can procalcitonin guide decisions about antibiotic management?
Yes, but with caution. Multiple randomized controlled trials showed that procalcitonin testing can help guide antibiotic management in a variety of clinical scenarios including sepsis, respiratory tract infection, and exacerbation of chronic obstructive pulmonary disease (COPD), and that procalcitonin guidance led to less antibiotic use with either unchanged or better outcomes. Moreover, observational studies have shown high negative predictive values for procalcitonin testing in other clinical situations such as bacteremia and bacterial meningitis, allowing clinicians to rule out these diagnoses if the clinical probability is low or moderate.
Nonetheless, clinical judgment must be exercised to consider the possibility of false- positive and false-negative results, especially if clinical suspicion for bacterial infection is high.
A RESPONSE TO BACTERIAL TOXIN
Procalcitonin is a peptide precursor of calcitonin that is produced by C cells of the thyroid and by neuroendocrine cells of the lung and intestine in response to bacterial toxin. In contrast, procalcitonin levels are down-regulated in viral infection.
Levels of procalcitonin increase 6 to 12 hours after stimulation, and the half-life is roughly 24 hours.1 This suggests levels should decrease by one-half daily if an infection is controlled and is responding to therapy (assuming normal clearance).
The test costs about $25, with a turnaround time of 20 to 60 minutes, or longer at institutions that send the test out or run the tests in batches.
Point-of-care procalcitonin testing is emerging but not yet commercially available in the United States. Despite extensive observational studies and randomized controlled trials over the past 20 years, procalcitonin’s physiologic role remains unclear. The large body of evidence of the clinical utility of procalcitonin measurement has been summarized in several meta-analyses in different diseases.
PROCALCITONIN TESTING IN SEPSIS
Trials of procalcitonin testing have had slightly different inclusion criteria that commonly overlap with similar diagnoses. Sepsis is the broadest cohort studied.
The Procalcitonin to Reduce Antibiotic Treatments in Acutely Ill Patients (PRORATA) trial2 randomized 621 patients admitted to the intensive care unit (ICU) with suspected bacterial infections to antibiotic therapy guided by procalcitonin concentrations or to antibiotic therapy based on current guidelines. The source of infection varied, but 73% of patients had pulmonary infections.The procalcitonin algorithm was as follows:
- Starting antibiotics was discouraged if the procalcitonin concentration was less than 0.5 ng/mL, and strongly discouraged if less than 0.25 ng/mL
- Starting antibiotics was encouraged if the concentration was 0.5 ng/mL or higher, and strongly encouraged if 1 ng/mL or higher
- Stopping antibiotics was encouraged if the concentration dropped by at least 80% from the peak level or to a level greater than or equal to 0.25 ng/mL; stopping was strongly encouraged if the concentration fell below 0.25 ng/mL.
There was also guidance to change antibiotics if procalcitonin increased on therapy and was above 0.5 ng/mL.
Although the study physicians generally followed the algorithm, they were allowed to override it based on clinical judgment. The main results were that the number of days without antibiotics was higher in the procalcitonin group than in the controls (14.3 vs 11.6 days), with no other statistically significant difference between groups. These findings supported the idea that procalcitonin can guide clinicians to safely “deprescribe” antibiotics.
The Stop Antibiotics on Guidance of Procalcitonin Study (SAPS),3 published in 2016, was a larger trial with similar design, in 1,575 patients admitted to the ICU with suspected infection. Antibiotic use was less and the 28-day mortality rate was lower with procalcitonin guidance: 20% vs 25% in the intention-to-treat analysis.
ACUTE RESPIRATORY TRACT INFECTION
The Procalcitonin Guided Antibiotic Therapy and Hospitalisation in Patients With Lower Respiratory Tract Infections (ProHOSP) trial4 randomized 1,381 patients to antibiotic therapy guided by procalcitonin levels or standard guidelines. Most patients had community-acquired pneumonia, while the rest had exacerbations of COPD, acute bronchitis, or other lower respiratory tract infections.
In the study algorithm, starting or continuing antibiotics was discouraged if procalcitonin levels were 0.25 ng/mL or less, and strongly discouraged if less than 0.1 ng/mL. Starting or continuing antibiotics was encouraged if levels were greater than 0.25 ng/mL, and strongly encouraged if greater than 0.5 ng/mL.
The algorithm recommended stopping antibiotics if procalcitonin levels fell below 0.25 ng/mL or decreased by 80%, and strongly recommended stopping them if procalcitonin fell below 0.1 ng/mL or decreased by 90%.
The treating physician could override the algorithm if the patient was unstable, was in an ICU, or had Legionella infection.
Antibiotic use was less in the procalcitonin-guided arm (75.4% vs 87.7%; mean duration 5.7 days vs 8.7 days), as was the rate of adverse effects from antibiotics (19.8% vs 28.1%). Rates of recurrence or rehospitalization were also lower with procalcitonin guidance (3.7% vs 6.5%), presumably because of fewer antibiotic-related side effects or better diagnostic accuracy. Rates of death and ICU admission were similar in the 2 groups. These findings were similar to those of PRORATA and SAPS, demonstrating that guidance with procalcitonin levels decreased antibiotic utilization, with other outcomes either improved or unchanged.
Schuetz et al,5 in a 2018 meta-analysis, collected data on 6,708 patients from 26 trials in 12 countries and found that procalcitonin guidance decreased antibiotic exposure by 2.4 days and reduced the rate of antibiotic-related side effects (16% vs 22%). Although there was skepticism about the mortality benefit reported in the SAPS trial, a similar mortality benefit was found in this meta-analysis (30-day mortality rates were 9% vs 10%), suggesting that measuring procalcitonin not only reduces unnecessary antibiotic exposure, but also saves lives.
Although decreasing antibiotic exposure may not confer a survival benefit, procalcitonin guidance likely clarifies the diagnosis and thus expedites proper treatment in patients with sepsis-like syndromes that are actually due to a noninfectious pathology (eg, pulmonary embolism, myocardial infarction, adrenal insufficiency).
Negative findings in ProACT
The Procalcitonin Antibiotic Consensus Trial (ProACT)6 subsequently reported findings discordant with those above but was flawed in that adherence to the procalcitonin guideline by physicians was only 62% in the subgroup of patients with low procalcitonin results, which accounted for almost 90% of patients. Overall adherence by physicians to the procalcitonin guideline was 65%, much lower than in other trials (ProHOSP had over 90% adherence).4 Further, ProACT was done in American centers unfamiliar with procalcitonin, and it seems they did not trust low procalcitonin values as a reason to stop or avoid antibiotics.
ACUTE EXACERBATIONS OF COPD
Multiple small randomized controlled trials and subgroups of larger studies like ProHOSP have studied the use of procalcitonin in acute exacerbations of COPD. Most studies used a design similar to the algorithm in ProHOSP.
Mathioudakis et al,7 in a meta-analysis of 8 trials with a total of 1,062 patients with acute exacerbation of COPD, found that with procalcitonin guidance, prescription of antibiotics on admission decreased by almost one-half, and courses of antibiotics were approximately 4 days shorter without any statistically significant difference in rates of treatment failure, length of hospital stay, recurrence, rehospitalization, or overall mortality.
However, the quality of the studies included in the meta-analysis was deemed only low to moderate, and thus the authors concluded, “Procalcitonin-based protocols appear to be clinically effective; however, confirmatory trials with rigorous methodology are required.”7 Nonetheless, given the lack of data supporting current practices for patient selection for antibiotics in COPD exacerbations, a strategy involving procalcitonin seems to be reasonable.
BACTEREMIA
Observational studies from as far as back as 1999 have examined the association of procalcitonin levels with bacteremia. The study designs were generally similar, with procalcitonin levels checked at time of blood culture, mostly in emergency rooms, and the procalcitonin value correlated with blood culture results. The general conclusion has been that procalcitonin has diagnostic value in ruling out bacteremia but should be used in the context of pretest probability rather than in isolation.
Hattori et al8 performed one of the largest studies, in 1,331 patients, using a procalcitonin level cutoff of 0.9 ng/mL. The sensitivity was 72% and specificity was 69%, which are not impressive; however, the negative predictive value was 95%, and even higher at lower cutoff values. Further, procalcitonin was significantly better at predicting bacteremia than either the white blood cell count or C-reactive protein level, with the latter two being hardly better than random chance.
Hoeboer et al9 performed a meta-analysis of various studies with a total of 16,514 patients. Using a cutoff of 0.5 ng/mL, they reported a sensitivity of 76% and a specificity of 69% with a negative predictive value of 97% in emergency rooms, 95% on regular wards, and 98% in ICUs. The high negative predictive value of procalcitonin can allow clinicians to stratify bacteremia risk to determine which patients need blood cultures, which in turn may help clinicians order blood cultures more appropriately and avoid unnecessary costs, delays, and harms associated with false-positive results, such as additional visits, additional testing, and unnecessary use of antibiotics.
MENINGITIS
As with bacteremia, observational studies have reported fairly high negative predictive values for procalcitonin in bacterial meningitis. The correlation is not surprising, given that most cases of bacterial meningitis occur due to hematogenous dissemination.
A 2015 meta-analysis of 9 studies and 725 patients reported a pooled sensitivity of 90%, specificity 90%, positive likelihood ratio 27.3, and negative likelihood ratio 0.13.10 Cutoffs for procalcitonin levels varied, but the most common value was 0.5 ng/mL. The authors also noted that the diagnostic utility of procalcitonin was far superior to C-reactive protein in this scenario, concluding that serum procalcitonin is a highly accurate test to distinguish between bacterial and viral causes in suspected meningitis.10
OTHER CLINICAL APPLICATIONS
Postoperative infection
Small studies have assessed procalcitonin as a marker to rule out postoperative infections,11,12 but the heterogeneity of study designs and populations makes it difficult to combine the studies for meta-analysis. Nevertheless, the general trend is that there may be a role for procalcitonin, and that procalcitonin has better diagnostic yield than the white blood cell count or C-reactive protein level. The optimal cutoff depends on the surgery, since a small elevation in procalcitonin can be expected with the stress of surgery; and since the degree of elevation varies with type of surgery, the result must be interpreted with caution.
Malignancy
In malignancy-associated conditions such as neutropenic fever and tumor fever, the clinical utility of procalcitonin is somewhat diminished, as malignancy can cause elevated procalcitonin levels (especially in metastatic disease), but a low concentration still has a fair negative predictive value (approximately 90%) for bloodstream infections.13
A retrospective study suggested that the ratio of procalcitonin to C-reactive protein could improve diagnostic accuracy in patients with malignancies, presumably because an elevation of procalcitonin out of proportion to elevation in C-reactive protein favored a bacterial infection rather than nonspecific inflammation related to malignancy.14
Cardiac syndromes
In cardiac syndromes, dyspnea and abnormal chest imaging may make it difficult to exclude respiratory infections. Schuetz et al15 reviewed the potential value of procalcitonin testing in a variety of cardiac disorders, especially in acute cardiovascular conditions whose presentation resembles that of sepsis or acute respiratory tract infection. They concluded it may have a role in diagnosis and prognosis in these settings, as well as guiding drug therapy.
Localized infections
Though localized infections such as cystitis, cellulitis, and osteomyelitis often do not affect procalcitonin levels, the test may help assess illness severity and rule out associated bacteremia.
One study found that a low procalcitonin level was insufficient to rule out urinary tract infection, but procalcitonin levels predicted bacteremia better than any other variable or combination of variables; moreover, procalcitonin had a negative predictive value as high as 97% for ruling out bacteremia associated with urinary tract infection.16
ROLE IN PROGNOSIS
In addition to being a useful marker for diagnosis of bacterial infections, the procalcitonin level has significant prognostic implications, as a high or persistently elevated level correlates with a higher rate of all-cause mortality.17 The prognostic capability may enhance triage decisions.
Because the procalcitonin level lacks specificity, clinicians need to be aware of noninfectious causes of elevations such as malignancy, surgery, impaired renal function,8 and myocardial infarction.18 In these scenarios, it is important to think critically about the procalcitonin result and consider an adjusted cutoff.
A study of procalcitonin to predict a positive blood culture in patients with renal disease suggested an optimal cutoff value of 1.06 ng/mL for patients with an estimated glomerular filtration rate of 30 to 60 mL/min/1.73m2, and a value of 2.50 ng/mL for a rate less than 30 mL/min/1.73m2.8
In a chronic process like malignancy, the procalcitonin level is usually not markedly elevated. But it can also remain persistently elevated, with no improvement associated with effective antibiotic treatment and no clinical deterioration associated with treatment failure.
Use of procalcitonin and troponin
For some patients, there may be diagnostic uncertainty about interpreting procalcitonin and troponin results, as both plaque-rupture myocardial infarction and demand ischemia from sepsis can cause elevation in both values. In a study of patients with acute myocardial infarction, the procalcitonin level peaked at 3.57 ng/mL and troponin peaked at 60 ng/mL at about 24 hours after admission.18 This suggests that a troponin-to-procalcitonin ratio may help distinguish acute myocardial infarction from demand ischemia, though the optimal cutoff is unknown.
Both troponin and procalcitonin levels can help rule out acute severe illness (eg, bloodstream infection, acute myocardial infarction). But both can be falsely negative in early presentation or in less severe disease (eg, localized infection, unstable angina), as well as in noninfectious inflammation and nonobstructive myocardial injury.
Both are important prognostic markers. Furthermore, both can be chronically elevated in patients with renal disease, but both still have a characteristic rise and fall in acute disease states. But neither should be used in isolation without information from electrocardiography, other tests, and the clinical context.
CAVEATS AND CHALLENGES
Based on clinical experience and reported studies, procalcitonin testing has proven valuable in the diagnosis, prognosis, and management of a range of diseases, particularly certain infections.
However, procalcitonin testing must be applied cautiously and judiciously. There is a potential for early false-negative results, and false-positive results can occur in conditions such as kidney disease, myocardial infarction, postoperative stress response, and malignancy, though there may be ways to factor these conditions into interpretation of procalcitonin results.
Widespread procalcitonin testing may lead to excessive costs, though the cost for each test is reasonable and probably offset by benefits of diagnostic clarification and decreased use of antibiotics, if appropriately applied.
The primary roles for procalcitonin testing are to rule out infection in patients with low probability of infection and to allow safe early cessation of antibiotic therapy in patients with presumed bacterial infection. Procalcitonin testing can enable providers to stop antibiotics safely, with the general trend showing decreased antibiotic utilization without patient harm. This can result in healthcare cost savings and improved patient outcomes such as decreased length of hospital stay, decreased readmission rates, fewer adverse effects from antibiotics, and possibly improved mortality rates.
Despite the potential benefits from procalcitonin testing, results must be interpreted within the clinical context because a host of factors can affect the values. Extreme values are more useful than intermediate values, which are difficult to interpret and have poor predictive value.
Although all current biomarkers for infection are imperfect, procalcitonin appears to have better diagnostic accuracy than other markers such as the white blood cell count and C-reactive protein in multiple clinical scenarios, and its appropriate use appears to improve important outcomes such as survival.
- Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med 2011; 9:107. doi:10.1186/1741-7015-9-107
- Bouadma L, Luyt CE, Tubach F, et al; PRORATA trial group. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010; 375(9713):463–474. doi:10.1016/S0140-6736(09)61879-1
- de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 2016; 16(7):819–827. doi:10.1016/S1473-3099(16)00053-0
- Schuetz P, Christ-Crain M, Thomann R, et al; ProHOSP Study Group. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302(10):1059–1066. doi:10.1001/jama.2009.1297
- Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 2018; 18(1):95–107. doi:10.1016/S1473-3099(17)30592-3
- Huang DT, Yealy DM, Filbin MR, et al; ProACT Investigators. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med 2018; 379(3):236–249. doi:10.1056/NEJMoa1802670
- Mathioudakis AG, Chatzimavridou-Grigoriadou V, Corlateanu A, Vestbo J. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev 2017; 26(143)pii:160073. doi:10.1183/16000617.0073-2016
- Hattori T, Nishiyama H, Kato H, et al. Clinical value of procalcitonin for patients with suspected bloodstream infection. Am J Clin Pathol 2014; 141(1):43–51. doi:10.1309/AJCP4GV7ZFDTANGC
- Hoeboer SH, van der Geest PJ, Nieboer D, Groeneveld AB. The diagnostic accuracy of procalcitonin for bacteraemia: a systematic review and meta-analysis. Clin Microbiol Infect 2015; 21(5):474–481. doi:10.1016/j.cmi.2014.12.026
- Vikse J, Henry BM, Roy J, Ramakrishnan PK, Tomaszewski KA, Walocha JA. The role of serum procalcitonin in the diagnosis of bacterial meningitis in adults: a systematic review and meta-analysis. Int J Infect Dis 2015; 38:68–76. doi:10.1016/j.ijid.2015.07.011
- Aouifi A, Piriou V, Bastien O, et al. Usefulness of procalcitonin for diagnosis of infection in cardiac surgical patients. Crit Care Med 2000; 28(9):3171–3176. pmid:11008977
- Hunziker S, Hugle T, Schuchardt K, et al. The value of serum procalcitonin level for differentiation of infectious from noninfectious causes of fever after orthopaedic surgery. J Bone Joint Surg Am 2010; 92(1):138–148. doi:10.2106/JBJS.H.01600
- Shomali W, Hachem R, Chaftari AM, et al. Can procalcitonin distinguish infectious fever from tumor-related fever in non-neutropenic cancer patients? Cancer 2012; 118(23):5823–5829. doi:10.1002/cncr.27602
- Hangai S, Nannya Y, Kurokawa M. Role of procalcitonin and C-reactive protein for discrimination between tumor fever and infection in patients with hematological diseases. Leuk Lymphoma 2015; 56(4):910–914. doi:10.3109/10428194.2014.938329
- Schuetz P, Daniels LB, Kulkarni P, Anker SD, Mueller B. Procalcitonin: a new biomarker for the cardiologist. Int J Cardiol 2016; 223:390–397. doi:10.1016/j.ijcard.2016.08.204
- van Nieuwkoop C, Bonten TN, van't Wout JW, et al. Procalcitonin reflects bacteremia and bacterial load in urosepsis syndrome: a prospective observational study. Crit Care 2010; 14(6):R206. doi:10.1186/cc9328
- Liu D, Su L, Han G, Yan P, Xie L. Prognostic value of procalcitonin in adult patients with sepsis: a systematic review and meta-analysis. PLoS One 2015; 10(6):e0129450. doi:10.1371/journal.pone.0129450
- Kafkas N, Venetsanou K, Patsilinakos S, et al. Procalcitonin in acute myocardial infarction. Acute Card Care 2008; 10(1):30–36. doi:10.1080/17482940701534800
Yes, but with caution. Multiple randomized controlled trials showed that procalcitonin testing can help guide antibiotic management in a variety of clinical scenarios including sepsis, respiratory tract infection, and exacerbation of chronic obstructive pulmonary disease (COPD), and that procalcitonin guidance led to less antibiotic use with either unchanged or better outcomes. Moreover, observational studies have shown high negative predictive values for procalcitonin testing in other clinical situations such as bacteremia and bacterial meningitis, allowing clinicians to rule out these diagnoses if the clinical probability is low or moderate.
Nonetheless, clinical judgment must be exercised to consider the possibility of false- positive and false-negative results, especially if clinical suspicion for bacterial infection is high.
A RESPONSE TO BACTERIAL TOXIN
Procalcitonin is a peptide precursor of calcitonin that is produced by C cells of the thyroid and by neuroendocrine cells of the lung and intestine in response to bacterial toxin. In contrast, procalcitonin levels are down-regulated in viral infection.
Levels of procalcitonin increase 6 to 12 hours after stimulation, and the half-life is roughly 24 hours.1 This suggests levels should decrease by one-half daily if an infection is controlled and is responding to therapy (assuming normal clearance).
The test costs about $25, with a turnaround time of 20 to 60 minutes, or longer at institutions that send the test out or run the tests in batches.
Point-of-care procalcitonin testing is emerging but not yet commercially available in the United States. Despite extensive observational studies and randomized controlled trials over the past 20 years, procalcitonin’s physiologic role remains unclear. The large body of evidence of the clinical utility of procalcitonin measurement has been summarized in several meta-analyses in different diseases.
PROCALCITONIN TESTING IN SEPSIS
Trials of procalcitonin testing have had slightly different inclusion criteria that commonly overlap with similar diagnoses. Sepsis is the broadest cohort studied.
The Procalcitonin to Reduce Antibiotic Treatments in Acutely Ill Patients (PRORATA) trial2 randomized 621 patients admitted to the intensive care unit (ICU) with suspected bacterial infections to antibiotic therapy guided by procalcitonin concentrations or to antibiotic therapy based on current guidelines. The source of infection varied, but 73% of patients had pulmonary infections.The procalcitonin algorithm was as follows:
- Starting antibiotics was discouraged if the procalcitonin concentration was less than 0.5 ng/mL, and strongly discouraged if less than 0.25 ng/mL
- Starting antibiotics was encouraged if the concentration was 0.5 ng/mL or higher, and strongly encouraged if 1 ng/mL or higher
- Stopping antibiotics was encouraged if the concentration dropped by at least 80% from the peak level or to a level greater than or equal to 0.25 ng/mL; stopping was strongly encouraged if the concentration fell below 0.25 ng/mL.
There was also guidance to change antibiotics if procalcitonin increased on therapy and was above 0.5 ng/mL.
Although the study physicians generally followed the algorithm, they were allowed to override it based on clinical judgment. The main results were that the number of days without antibiotics was higher in the procalcitonin group than in the controls (14.3 vs 11.6 days), with no other statistically significant difference between groups. These findings supported the idea that procalcitonin can guide clinicians to safely “deprescribe” antibiotics.
The Stop Antibiotics on Guidance of Procalcitonin Study (SAPS),3 published in 2016, was a larger trial with similar design, in 1,575 patients admitted to the ICU with suspected infection. Antibiotic use was less and the 28-day mortality rate was lower with procalcitonin guidance: 20% vs 25% in the intention-to-treat analysis.
ACUTE RESPIRATORY TRACT INFECTION
The Procalcitonin Guided Antibiotic Therapy and Hospitalisation in Patients With Lower Respiratory Tract Infections (ProHOSP) trial4 randomized 1,381 patients to antibiotic therapy guided by procalcitonin levels or standard guidelines. Most patients had community-acquired pneumonia, while the rest had exacerbations of COPD, acute bronchitis, or other lower respiratory tract infections.
In the study algorithm, starting or continuing antibiotics was discouraged if procalcitonin levels were 0.25 ng/mL or less, and strongly discouraged if less than 0.1 ng/mL. Starting or continuing antibiotics was encouraged if levels were greater than 0.25 ng/mL, and strongly encouraged if greater than 0.5 ng/mL.
The algorithm recommended stopping antibiotics if procalcitonin levels fell below 0.25 ng/mL or decreased by 80%, and strongly recommended stopping them if procalcitonin fell below 0.1 ng/mL or decreased by 90%.
The treating physician could override the algorithm if the patient was unstable, was in an ICU, or had Legionella infection.
Antibiotic use was less in the procalcitonin-guided arm (75.4% vs 87.7%; mean duration 5.7 days vs 8.7 days), as was the rate of adverse effects from antibiotics (19.8% vs 28.1%). Rates of recurrence or rehospitalization were also lower with procalcitonin guidance (3.7% vs 6.5%), presumably because of fewer antibiotic-related side effects or better diagnostic accuracy. Rates of death and ICU admission were similar in the 2 groups. These findings were similar to those of PRORATA and SAPS, demonstrating that guidance with procalcitonin levels decreased antibiotic utilization, with other outcomes either improved or unchanged.
Schuetz et al,5 in a 2018 meta-analysis, collected data on 6,708 patients from 26 trials in 12 countries and found that procalcitonin guidance decreased antibiotic exposure by 2.4 days and reduced the rate of antibiotic-related side effects (16% vs 22%). Although there was skepticism about the mortality benefit reported in the SAPS trial, a similar mortality benefit was found in this meta-analysis (30-day mortality rates were 9% vs 10%), suggesting that measuring procalcitonin not only reduces unnecessary antibiotic exposure, but also saves lives.
Although decreasing antibiotic exposure may not confer a survival benefit, procalcitonin guidance likely clarifies the diagnosis and thus expedites proper treatment in patients with sepsis-like syndromes that are actually due to a noninfectious pathology (eg, pulmonary embolism, myocardial infarction, adrenal insufficiency).
Negative findings in ProACT
The Procalcitonin Antibiotic Consensus Trial (ProACT)6 subsequently reported findings discordant with those above but was flawed in that adherence to the procalcitonin guideline by physicians was only 62% in the subgroup of patients with low procalcitonin results, which accounted for almost 90% of patients. Overall adherence by physicians to the procalcitonin guideline was 65%, much lower than in other trials (ProHOSP had over 90% adherence).4 Further, ProACT was done in American centers unfamiliar with procalcitonin, and it seems they did not trust low procalcitonin values as a reason to stop or avoid antibiotics.
ACUTE EXACERBATIONS OF COPD
Multiple small randomized controlled trials and subgroups of larger studies like ProHOSP have studied the use of procalcitonin in acute exacerbations of COPD. Most studies used a design similar to the algorithm in ProHOSP.
Mathioudakis et al,7 in a meta-analysis of 8 trials with a total of 1,062 patients with acute exacerbation of COPD, found that with procalcitonin guidance, prescription of antibiotics on admission decreased by almost one-half, and courses of antibiotics were approximately 4 days shorter without any statistically significant difference in rates of treatment failure, length of hospital stay, recurrence, rehospitalization, or overall mortality.
However, the quality of the studies included in the meta-analysis was deemed only low to moderate, and thus the authors concluded, “Procalcitonin-based protocols appear to be clinically effective; however, confirmatory trials with rigorous methodology are required.”7 Nonetheless, given the lack of data supporting current practices for patient selection for antibiotics in COPD exacerbations, a strategy involving procalcitonin seems to be reasonable.
BACTEREMIA
Observational studies from as far as back as 1999 have examined the association of procalcitonin levels with bacteremia. The study designs were generally similar, with procalcitonin levels checked at time of blood culture, mostly in emergency rooms, and the procalcitonin value correlated with blood culture results. The general conclusion has been that procalcitonin has diagnostic value in ruling out bacteremia but should be used in the context of pretest probability rather than in isolation.
Hattori et al8 performed one of the largest studies, in 1,331 patients, using a procalcitonin level cutoff of 0.9 ng/mL. The sensitivity was 72% and specificity was 69%, which are not impressive; however, the negative predictive value was 95%, and even higher at lower cutoff values. Further, procalcitonin was significantly better at predicting bacteremia than either the white blood cell count or C-reactive protein level, with the latter two being hardly better than random chance.
Hoeboer et al9 performed a meta-analysis of various studies with a total of 16,514 patients. Using a cutoff of 0.5 ng/mL, they reported a sensitivity of 76% and a specificity of 69% with a negative predictive value of 97% in emergency rooms, 95% on regular wards, and 98% in ICUs. The high negative predictive value of procalcitonin can allow clinicians to stratify bacteremia risk to determine which patients need blood cultures, which in turn may help clinicians order blood cultures more appropriately and avoid unnecessary costs, delays, and harms associated with false-positive results, such as additional visits, additional testing, and unnecessary use of antibiotics.
MENINGITIS
As with bacteremia, observational studies have reported fairly high negative predictive values for procalcitonin in bacterial meningitis. The correlation is not surprising, given that most cases of bacterial meningitis occur due to hematogenous dissemination.
A 2015 meta-analysis of 9 studies and 725 patients reported a pooled sensitivity of 90%, specificity 90%, positive likelihood ratio 27.3, and negative likelihood ratio 0.13.10 Cutoffs for procalcitonin levels varied, but the most common value was 0.5 ng/mL. The authors also noted that the diagnostic utility of procalcitonin was far superior to C-reactive protein in this scenario, concluding that serum procalcitonin is a highly accurate test to distinguish between bacterial and viral causes in suspected meningitis.10
OTHER CLINICAL APPLICATIONS
Postoperative infection
Small studies have assessed procalcitonin as a marker to rule out postoperative infections,11,12 but the heterogeneity of study designs and populations makes it difficult to combine the studies for meta-analysis. Nevertheless, the general trend is that there may be a role for procalcitonin, and that procalcitonin has better diagnostic yield than the white blood cell count or C-reactive protein level. The optimal cutoff depends on the surgery, since a small elevation in procalcitonin can be expected with the stress of surgery; and since the degree of elevation varies with type of surgery, the result must be interpreted with caution.
Malignancy
In malignancy-associated conditions such as neutropenic fever and tumor fever, the clinical utility of procalcitonin is somewhat diminished, as malignancy can cause elevated procalcitonin levels (especially in metastatic disease), but a low concentration still has a fair negative predictive value (approximately 90%) for bloodstream infections.13
A retrospective study suggested that the ratio of procalcitonin to C-reactive protein could improve diagnostic accuracy in patients with malignancies, presumably because an elevation of procalcitonin out of proportion to elevation in C-reactive protein favored a bacterial infection rather than nonspecific inflammation related to malignancy.14
Cardiac syndromes
In cardiac syndromes, dyspnea and abnormal chest imaging may make it difficult to exclude respiratory infections. Schuetz et al15 reviewed the potential value of procalcitonin testing in a variety of cardiac disorders, especially in acute cardiovascular conditions whose presentation resembles that of sepsis or acute respiratory tract infection. They concluded it may have a role in diagnosis and prognosis in these settings, as well as guiding drug therapy.
Localized infections
Though localized infections such as cystitis, cellulitis, and osteomyelitis often do not affect procalcitonin levels, the test may help assess illness severity and rule out associated bacteremia.
One study found that a low procalcitonin level was insufficient to rule out urinary tract infection, but procalcitonin levels predicted bacteremia better than any other variable or combination of variables; moreover, procalcitonin had a negative predictive value as high as 97% for ruling out bacteremia associated with urinary tract infection.16
ROLE IN PROGNOSIS
In addition to being a useful marker for diagnosis of bacterial infections, the procalcitonin level has significant prognostic implications, as a high or persistently elevated level correlates with a higher rate of all-cause mortality.17 The prognostic capability may enhance triage decisions.
Because the procalcitonin level lacks specificity, clinicians need to be aware of noninfectious causes of elevations such as malignancy, surgery, impaired renal function,8 and myocardial infarction.18 In these scenarios, it is important to think critically about the procalcitonin result and consider an adjusted cutoff.
A study of procalcitonin to predict a positive blood culture in patients with renal disease suggested an optimal cutoff value of 1.06 ng/mL for patients with an estimated glomerular filtration rate of 30 to 60 mL/min/1.73m2, and a value of 2.50 ng/mL for a rate less than 30 mL/min/1.73m2.8
In a chronic process like malignancy, the procalcitonin level is usually not markedly elevated. But it can also remain persistently elevated, with no improvement associated with effective antibiotic treatment and no clinical deterioration associated with treatment failure.
Use of procalcitonin and troponin
For some patients, there may be diagnostic uncertainty about interpreting procalcitonin and troponin results, as both plaque-rupture myocardial infarction and demand ischemia from sepsis can cause elevation in both values. In a study of patients with acute myocardial infarction, the procalcitonin level peaked at 3.57 ng/mL and troponin peaked at 60 ng/mL at about 24 hours after admission.18 This suggests that a troponin-to-procalcitonin ratio may help distinguish acute myocardial infarction from demand ischemia, though the optimal cutoff is unknown.
Both troponin and procalcitonin levels can help rule out acute severe illness (eg, bloodstream infection, acute myocardial infarction). But both can be falsely negative in early presentation or in less severe disease (eg, localized infection, unstable angina), as well as in noninfectious inflammation and nonobstructive myocardial injury.
Both are important prognostic markers. Furthermore, both can be chronically elevated in patients with renal disease, but both still have a characteristic rise and fall in acute disease states. But neither should be used in isolation without information from electrocardiography, other tests, and the clinical context.
CAVEATS AND CHALLENGES
Based on clinical experience and reported studies, procalcitonin testing has proven valuable in the diagnosis, prognosis, and management of a range of diseases, particularly certain infections.
However, procalcitonin testing must be applied cautiously and judiciously. There is a potential for early false-negative results, and false-positive results can occur in conditions such as kidney disease, myocardial infarction, postoperative stress response, and malignancy, though there may be ways to factor these conditions into interpretation of procalcitonin results.
Widespread procalcitonin testing may lead to excessive costs, though the cost for each test is reasonable and probably offset by benefits of diagnostic clarification and decreased use of antibiotics, if appropriately applied.
The primary roles for procalcitonin testing are to rule out infection in patients with low probability of infection and to allow safe early cessation of antibiotic therapy in patients with presumed bacterial infection. Procalcitonin testing can enable providers to stop antibiotics safely, with the general trend showing decreased antibiotic utilization without patient harm. This can result in healthcare cost savings and improved patient outcomes such as decreased length of hospital stay, decreased readmission rates, fewer adverse effects from antibiotics, and possibly improved mortality rates.
Despite the potential benefits from procalcitonin testing, results must be interpreted within the clinical context because a host of factors can affect the values. Extreme values are more useful than intermediate values, which are difficult to interpret and have poor predictive value.
Although all current biomarkers for infection are imperfect, procalcitonin appears to have better diagnostic accuracy than other markers such as the white blood cell count and C-reactive protein in multiple clinical scenarios, and its appropriate use appears to improve important outcomes such as survival.
Yes, but with caution. Multiple randomized controlled trials showed that procalcitonin testing can help guide antibiotic management in a variety of clinical scenarios including sepsis, respiratory tract infection, and exacerbation of chronic obstructive pulmonary disease (COPD), and that procalcitonin guidance led to less antibiotic use with either unchanged or better outcomes. Moreover, observational studies have shown high negative predictive values for procalcitonin testing in other clinical situations such as bacteremia and bacterial meningitis, allowing clinicians to rule out these diagnoses if the clinical probability is low or moderate.
Nonetheless, clinical judgment must be exercised to consider the possibility of false- positive and false-negative results, especially if clinical suspicion for bacterial infection is high.
A RESPONSE TO BACTERIAL TOXIN
Procalcitonin is a peptide precursor of calcitonin that is produced by C cells of the thyroid and by neuroendocrine cells of the lung and intestine in response to bacterial toxin. In contrast, procalcitonin levels are down-regulated in viral infection.
Levels of procalcitonin increase 6 to 12 hours after stimulation, and the half-life is roughly 24 hours.1 This suggests levels should decrease by one-half daily if an infection is controlled and is responding to therapy (assuming normal clearance).
The test costs about $25, with a turnaround time of 20 to 60 minutes, or longer at institutions that send the test out or run the tests in batches.
Point-of-care procalcitonin testing is emerging but not yet commercially available in the United States. Despite extensive observational studies and randomized controlled trials over the past 20 years, procalcitonin’s physiologic role remains unclear. The large body of evidence of the clinical utility of procalcitonin measurement has been summarized in several meta-analyses in different diseases.
PROCALCITONIN TESTING IN SEPSIS
Trials of procalcitonin testing have had slightly different inclusion criteria that commonly overlap with similar diagnoses. Sepsis is the broadest cohort studied.
The Procalcitonin to Reduce Antibiotic Treatments in Acutely Ill Patients (PRORATA) trial2 randomized 621 patients admitted to the intensive care unit (ICU) with suspected bacterial infections to antibiotic therapy guided by procalcitonin concentrations or to antibiotic therapy based on current guidelines. The source of infection varied, but 73% of patients had pulmonary infections.The procalcitonin algorithm was as follows:
- Starting antibiotics was discouraged if the procalcitonin concentration was less than 0.5 ng/mL, and strongly discouraged if less than 0.25 ng/mL
- Starting antibiotics was encouraged if the concentration was 0.5 ng/mL or higher, and strongly encouraged if 1 ng/mL or higher
- Stopping antibiotics was encouraged if the concentration dropped by at least 80% from the peak level or to a level greater than or equal to 0.25 ng/mL; stopping was strongly encouraged if the concentration fell below 0.25 ng/mL.
There was also guidance to change antibiotics if procalcitonin increased on therapy and was above 0.5 ng/mL.
Although the study physicians generally followed the algorithm, they were allowed to override it based on clinical judgment. The main results were that the number of days without antibiotics was higher in the procalcitonin group than in the controls (14.3 vs 11.6 days), with no other statistically significant difference between groups. These findings supported the idea that procalcitonin can guide clinicians to safely “deprescribe” antibiotics.
The Stop Antibiotics on Guidance of Procalcitonin Study (SAPS),3 published in 2016, was a larger trial with similar design, in 1,575 patients admitted to the ICU with suspected infection. Antibiotic use was less and the 28-day mortality rate was lower with procalcitonin guidance: 20% vs 25% in the intention-to-treat analysis.
ACUTE RESPIRATORY TRACT INFECTION
The Procalcitonin Guided Antibiotic Therapy and Hospitalisation in Patients With Lower Respiratory Tract Infections (ProHOSP) trial4 randomized 1,381 patients to antibiotic therapy guided by procalcitonin levels or standard guidelines. Most patients had community-acquired pneumonia, while the rest had exacerbations of COPD, acute bronchitis, or other lower respiratory tract infections.
In the study algorithm, starting or continuing antibiotics was discouraged if procalcitonin levels were 0.25 ng/mL or less, and strongly discouraged if less than 0.1 ng/mL. Starting or continuing antibiotics was encouraged if levels were greater than 0.25 ng/mL, and strongly encouraged if greater than 0.5 ng/mL.
The algorithm recommended stopping antibiotics if procalcitonin levels fell below 0.25 ng/mL or decreased by 80%, and strongly recommended stopping them if procalcitonin fell below 0.1 ng/mL or decreased by 90%.
The treating physician could override the algorithm if the patient was unstable, was in an ICU, or had Legionella infection.
Antibiotic use was less in the procalcitonin-guided arm (75.4% vs 87.7%; mean duration 5.7 days vs 8.7 days), as was the rate of adverse effects from antibiotics (19.8% vs 28.1%). Rates of recurrence or rehospitalization were also lower with procalcitonin guidance (3.7% vs 6.5%), presumably because of fewer antibiotic-related side effects or better diagnostic accuracy. Rates of death and ICU admission were similar in the 2 groups. These findings were similar to those of PRORATA and SAPS, demonstrating that guidance with procalcitonin levels decreased antibiotic utilization, with other outcomes either improved or unchanged.
Schuetz et al,5 in a 2018 meta-analysis, collected data on 6,708 patients from 26 trials in 12 countries and found that procalcitonin guidance decreased antibiotic exposure by 2.4 days and reduced the rate of antibiotic-related side effects (16% vs 22%). Although there was skepticism about the mortality benefit reported in the SAPS trial, a similar mortality benefit was found in this meta-analysis (30-day mortality rates were 9% vs 10%), suggesting that measuring procalcitonin not only reduces unnecessary antibiotic exposure, but also saves lives.
Although decreasing antibiotic exposure may not confer a survival benefit, procalcitonin guidance likely clarifies the diagnosis and thus expedites proper treatment in patients with sepsis-like syndromes that are actually due to a noninfectious pathology (eg, pulmonary embolism, myocardial infarction, adrenal insufficiency).
Negative findings in ProACT
The Procalcitonin Antibiotic Consensus Trial (ProACT)6 subsequently reported findings discordant with those above but was flawed in that adherence to the procalcitonin guideline by physicians was only 62% in the subgroup of patients with low procalcitonin results, which accounted for almost 90% of patients. Overall adherence by physicians to the procalcitonin guideline was 65%, much lower than in other trials (ProHOSP had over 90% adherence).4 Further, ProACT was done in American centers unfamiliar with procalcitonin, and it seems they did not trust low procalcitonin values as a reason to stop or avoid antibiotics.
ACUTE EXACERBATIONS OF COPD
Multiple small randomized controlled trials and subgroups of larger studies like ProHOSP have studied the use of procalcitonin in acute exacerbations of COPD. Most studies used a design similar to the algorithm in ProHOSP.
Mathioudakis et al,7 in a meta-analysis of 8 trials with a total of 1,062 patients with acute exacerbation of COPD, found that with procalcitonin guidance, prescription of antibiotics on admission decreased by almost one-half, and courses of antibiotics were approximately 4 days shorter without any statistically significant difference in rates of treatment failure, length of hospital stay, recurrence, rehospitalization, or overall mortality.
However, the quality of the studies included in the meta-analysis was deemed only low to moderate, and thus the authors concluded, “Procalcitonin-based protocols appear to be clinically effective; however, confirmatory trials with rigorous methodology are required.”7 Nonetheless, given the lack of data supporting current practices for patient selection for antibiotics in COPD exacerbations, a strategy involving procalcitonin seems to be reasonable.
BACTEREMIA
Observational studies from as far as back as 1999 have examined the association of procalcitonin levels with bacteremia. The study designs were generally similar, with procalcitonin levels checked at time of blood culture, mostly in emergency rooms, and the procalcitonin value correlated with blood culture results. The general conclusion has been that procalcitonin has diagnostic value in ruling out bacteremia but should be used in the context of pretest probability rather than in isolation.
Hattori et al8 performed one of the largest studies, in 1,331 patients, using a procalcitonin level cutoff of 0.9 ng/mL. The sensitivity was 72% and specificity was 69%, which are not impressive; however, the negative predictive value was 95%, and even higher at lower cutoff values. Further, procalcitonin was significantly better at predicting bacteremia than either the white blood cell count or C-reactive protein level, with the latter two being hardly better than random chance.
Hoeboer et al9 performed a meta-analysis of various studies with a total of 16,514 patients. Using a cutoff of 0.5 ng/mL, they reported a sensitivity of 76% and a specificity of 69% with a negative predictive value of 97% in emergency rooms, 95% on regular wards, and 98% in ICUs. The high negative predictive value of procalcitonin can allow clinicians to stratify bacteremia risk to determine which patients need blood cultures, which in turn may help clinicians order blood cultures more appropriately and avoid unnecessary costs, delays, and harms associated with false-positive results, such as additional visits, additional testing, and unnecessary use of antibiotics.
MENINGITIS
As with bacteremia, observational studies have reported fairly high negative predictive values for procalcitonin in bacterial meningitis. The correlation is not surprising, given that most cases of bacterial meningitis occur due to hematogenous dissemination.
A 2015 meta-analysis of 9 studies and 725 patients reported a pooled sensitivity of 90%, specificity 90%, positive likelihood ratio 27.3, and negative likelihood ratio 0.13.10 Cutoffs for procalcitonin levels varied, but the most common value was 0.5 ng/mL. The authors also noted that the diagnostic utility of procalcitonin was far superior to C-reactive protein in this scenario, concluding that serum procalcitonin is a highly accurate test to distinguish between bacterial and viral causes in suspected meningitis.10
OTHER CLINICAL APPLICATIONS
Postoperative infection
Small studies have assessed procalcitonin as a marker to rule out postoperative infections,11,12 but the heterogeneity of study designs and populations makes it difficult to combine the studies for meta-analysis. Nevertheless, the general trend is that there may be a role for procalcitonin, and that procalcitonin has better diagnostic yield than the white blood cell count or C-reactive protein level. The optimal cutoff depends on the surgery, since a small elevation in procalcitonin can be expected with the stress of surgery; and since the degree of elevation varies with type of surgery, the result must be interpreted with caution.
Malignancy
In malignancy-associated conditions such as neutropenic fever and tumor fever, the clinical utility of procalcitonin is somewhat diminished, as malignancy can cause elevated procalcitonin levels (especially in metastatic disease), but a low concentration still has a fair negative predictive value (approximately 90%) for bloodstream infections.13
A retrospective study suggested that the ratio of procalcitonin to C-reactive protein could improve diagnostic accuracy in patients with malignancies, presumably because an elevation of procalcitonin out of proportion to elevation in C-reactive protein favored a bacterial infection rather than nonspecific inflammation related to malignancy.14
Cardiac syndromes
In cardiac syndromes, dyspnea and abnormal chest imaging may make it difficult to exclude respiratory infections. Schuetz et al15 reviewed the potential value of procalcitonin testing in a variety of cardiac disorders, especially in acute cardiovascular conditions whose presentation resembles that of sepsis or acute respiratory tract infection. They concluded it may have a role in diagnosis and prognosis in these settings, as well as guiding drug therapy.
Localized infections
Though localized infections such as cystitis, cellulitis, and osteomyelitis often do not affect procalcitonin levels, the test may help assess illness severity and rule out associated bacteremia.
One study found that a low procalcitonin level was insufficient to rule out urinary tract infection, but procalcitonin levels predicted bacteremia better than any other variable or combination of variables; moreover, procalcitonin had a negative predictive value as high as 97% for ruling out bacteremia associated with urinary tract infection.16
ROLE IN PROGNOSIS
In addition to being a useful marker for diagnosis of bacterial infections, the procalcitonin level has significant prognostic implications, as a high or persistently elevated level correlates with a higher rate of all-cause mortality.17 The prognostic capability may enhance triage decisions.
Because the procalcitonin level lacks specificity, clinicians need to be aware of noninfectious causes of elevations such as malignancy, surgery, impaired renal function,8 and myocardial infarction.18 In these scenarios, it is important to think critically about the procalcitonin result and consider an adjusted cutoff.
A study of procalcitonin to predict a positive blood culture in patients with renal disease suggested an optimal cutoff value of 1.06 ng/mL for patients with an estimated glomerular filtration rate of 30 to 60 mL/min/1.73m2, and a value of 2.50 ng/mL for a rate less than 30 mL/min/1.73m2.8
In a chronic process like malignancy, the procalcitonin level is usually not markedly elevated. But it can also remain persistently elevated, with no improvement associated with effective antibiotic treatment and no clinical deterioration associated with treatment failure.
Use of procalcitonin and troponin
For some patients, there may be diagnostic uncertainty about interpreting procalcitonin and troponin results, as both plaque-rupture myocardial infarction and demand ischemia from sepsis can cause elevation in both values. In a study of patients with acute myocardial infarction, the procalcitonin level peaked at 3.57 ng/mL and troponin peaked at 60 ng/mL at about 24 hours after admission.18 This suggests that a troponin-to-procalcitonin ratio may help distinguish acute myocardial infarction from demand ischemia, though the optimal cutoff is unknown.
Both troponin and procalcitonin levels can help rule out acute severe illness (eg, bloodstream infection, acute myocardial infarction). But both can be falsely negative in early presentation or in less severe disease (eg, localized infection, unstable angina), as well as in noninfectious inflammation and nonobstructive myocardial injury.
Both are important prognostic markers. Furthermore, both can be chronically elevated in patients with renal disease, but both still have a characteristic rise and fall in acute disease states. But neither should be used in isolation without information from electrocardiography, other tests, and the clinical context.
CAVEATS AND CHALLENGES
Based on clinical experience and reported studies, procalcitonin testing has proven valuable in the diagnosis, prognosis, and management of a range of diseases, particularly certain infections.
However, procalcitonin testing must be applied cautiously and judiciously. There is a potential for early false-negative results, and false-positive results can occur in conditions such as kidney disease, myocardial infarction, postoperative stress response, and malignancy, though there may be ways to factor these conditions into interpretation of procalcitonin results.
Widespread procalcitonin testing may lead to excessive costs, though the cost for each test is reasonable and probably offset by benefits of diagnostic clarification and decreased use of antibiotics, if appropriately applied.
The primary roles for procalcitonin testing are to rule out infection in patients with low probability of infection and to allow safe early cessation of antibiotic therapy in patients with presumed bacterial infection. Procalcitonin testing can enable providers to stop antibiotics safely, with the general trend showing decreased antibiotic utilization without patient harm. This can result in healthcare cost savings and improved patient outcomes such as decreased length of hospital stay, decreased readmission rates, fewer adverse effects from antibiotics, and possibly improved mortality rates.
Despite the potential benefits from procalcitonin testing, results must be interpreted within the clinical context because a host of factors can affect the values. Extreme values are more useful than intermediate values, which are difficult to interpret and have poor predictive value.
Although all current biomarkers for infection are imperfect, procalcitonin appears to have better diagnostic accuracy than other markers such as the white blood cell count and C-reactive protein in multiple clinical scenarios, and its appropriate use appears to improve important outcomes such as survival.
- Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med 2011; 9:107. doi:10.1186/1741-7015-9-107
- Bouadma L, Luyt CE, Tubach F, et al; PRORATA trial group. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010; 375(9713):463–474. doi:10.1016/S0140-6736(09)61879-1
- de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 2016; 16(7):819–827. doi:10.1016/S1473-3099(16)00053-0
- Schuetz P, Christ-Crain M, Thomann R, et al; ProHOSP Study Group. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302(10):1059–1066. doi:10.1001/jama.2009.1297
- Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 2018; 18(1):95–107. doi:10.1016/S1473-3099(17)30592-3
- Huang DT, Yealy DM, Filbin MR, et al; ProACT Investigators. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med 2018; 379(3):236–249. doi:10.1056/NEJMoa1802670
- Mathioudakis AG, Chatzimavridou-Grigoriadou V, Corlateanu A, Vestbo J. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev 2017; 26(143)pii:160073. doi:10.1183/16000617.0073-2016
- Hattori T, Nishiyama H, Kato H, et al. Clinical value of procalcitonin for patients with suspected bloodstream infection. Am J Clin Pathol 2014; 141(1):43–51. doi:10.1309/AJCP4GV7ZFDTANGC
- Hoeboer SH, van der Geest PJ, Nieboer D, Groeneveld AB. The diagnostic accuracy of procalcitonin for bacteraemia: a systematic review and meta-analysis. Clin Microbiol Infect 2015; 21(5):474–481. doi:10.1016/j.cmi.2014.12.026
- Vikse J, Henry BM, Roy J, Ramakrishnan PK, Tomaszewski KA, Walocha JA. The role of serum procalcitonin in the diagnosis of bacterial meningitis in adults: a systematic review and meta-analysis. Int J Infect Dis 2015; 38:68–76. doi:10.1016/j.ijid.2015.07.011
- Aouifi A, Piriou V, Bastien O, et al. Usefulness of procalcitonin for diagnosis of infection in cardiac surgical patients. Crit Care Med 2000; 28(9):3171–3176. pmid:11008977
- Hunziker S, Hugle T, Schuchardt K, et al. The value of serum procalcitonin level for differentiation of infectious from noninfectious causes of fever after orthopaedic surgery. J Bone Joint Surg Am 2010; 92(1):138–148. doi:10.2106/JBJS.H.01600
- Shomali W, Hachem R, Chaftari AM, et al. Can procalcitonin distinguish infectious fever from tumor-related fever in non-neutropenic cancer patients? Cancer 2012; 118(23):5823–5829. doi:10.1002/cncr.27602
- Hangai S, Nannya Y, Kurokawa M. Role of procalcitonin and C-reactive protein for discrimination between tumor fever and infection in patients with hematological diseases. Leuk Lymphoma 2015; 56(4):910–914. doi:10.3109/10428194.2014.938329
- Schuetz P, Daniels LB, Kulkarni P, Anker SD, Mueller B. Procalcitonin: a new biomarker for the cardiologist. Int J Cardiol 2016; 223:390–397. doi:10.1016/j.ijcard.2016.08.204
- van Nieuwkoop C, Bonten TN, van't Wout JW, et al. Procalcitonin reflects bacteremia and bacterial load in urosepsis syndrome: a prospective observational study. Crit Care 2010; 14(6):R206. doi:10.1186/cc9328
- Liu D, Su L, Han G, Yan P, Xie L. Prognostic value of procalcitonin in adult patients with sepsis: a systematic review and meta-analysis. PLoS One 2015; 10(6):e0129450. doi:10.1371/journal.pone.0129450
- Kafkas N, Venetsanou K, Patsilinakos S, et al. Procalcitonin in acute myocardial infarction. Acute Card Care 2008; 10(1):30–36. doi:10.1080/17482940701534800
- Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med 2011; 9:107. doi:10.1186/1741-7015-9-107
- Bouadma L, Luyt CE, Tubach F, et al; PRORATA trial group. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010; 375(9713):463–474. doi:10.1016/S0140-6736(09)61879-1
- de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 2016; 16(7):819–827. doi:10.1016/S1473-3099(16)00053-0
- Schuetz P, Christ-Crain M, Thomann R, et al; ProHOSP Study Group. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302(10):1059–1066. doi:10.1001/jama.2009.1297
- Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 2018; 18(1):95–107. doi:10.1016/S1473-3099(17)30592-3
- Huang DT, Yealy DM, Filbin MR, et al; ProACT Investigators. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med 2018; 379(3):236–249. doi:10.1056/NEJMoa1802670
- Mathioudakis AG, Chatzimavridou-Grigoriadou V, Corlateanu A, Vestbo J. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev 2017; 26(143)pii:160073. doi:10.1183/16000617.0073-2016
- Hattori T, Nishiyama H, Kato H, et al. Clinical value of procalcitonin for patients with suspected bloodstream infection. Am J Clin Pathol 2014; 141(1):43–51. doi:10.1309/AJCP4GV7ZFDTANGC
- Hoeboer SH, van der Geest PJ, Nieboer D, Groeneveld AB. The diagnostic accuracy of procalcitonin for bacteraemia: a systematic review and meta-analysis. Clin Microbiol Infect 2015; 21(5):474–481. doi:10.1016/j.cmi.2014.12.026
- Vikse J, Henry BM, Roy J, Ramakrishnan PK, Tomaszewski KA, Walocha JA. The role of serum procalcitonin in the diagnosis of bacterial meningitis in adults: a systematic review and meta-analysis. Int J Infect Dis 2015; 38:68–76. doi:10.1016/j.ijid.2015.07.011
- Aouifi A, Piriou V, Bastien O, et al. Usefulness of procalcitonin for diagnosis of infection in cardiac surgical patients. Crit Care Med 2000; 28(9):3171–3176. pmid:11008977
- Hunziker S, Hugle T, Schuchardt K, et al. The value of serum procalcitonin level for differentiation of infectious from noninfectious causes of fever after orthopaedic surgery. J Bone Joint Surg Am 2010; 92(1):138–148. doi:10.2106/JBJS.H.01600
- Shomali W, Hachem R, Chaftari AM, et al. Can procalcitonin distinguish infectious fever from tumor-related fever in non-neutropenic cancer patients? Cancer 2012; 118(23):5823–5829. doi:10.1002/cncr.27602
- Hangai S, Nannya Y, Kurokawa M. Role of procalcitonin and C-reactive protein for discrimination between tumor fever and infection in patients with hematological diseases. Leuk Lymphoma 2015; 56(4):910–914. doi:10.3109/10428194.2014.938329
- Schuetz P, Daniels LB, Kulkarni P, Anker SD, Mueller B. Procalcitonin: a new biomarker for the cardiologist. Int J Cardiol 2016; 223:390–397. doi:10.1016/j.ijcard.2016.08.204
- van Nieuwkoop C, Bonten TN, van't Wout JW, et al. Procalcitonin reflects bacteremia and bacterial load in urosepsis syndrome: a prospective observational study. Crit Care 2010; 14(6):R206. doi:10.1186/cc9328
- Liu D, Su L, Han G, Yan P, Xie L. Prognostic value of procalcitonin in adult patients with sepsis: a systematic review and meta-analysis. PLoS One 2015; 10(6):e0129450. doi:10.1371/journal.pone.0129450
- Kafkas N, Venetsanou K, Patsilinakos S, et al. Procalcitonin in acute myocardial infarction. Acute Card Care 2008; 10(1):30–36. doi:10.1080/17482940701534800
Mavyret approved for children with any HCV genotype
The Food and Drug Administration has approved glecaprevir/pibrentasvir tablets (Mavyret) for treating any of six identified genotypes of hepatitis C virus in children ages 12-17 years.
The agency noted in its press announcement that, Dosing information now will be provided for patients aged 12 years and older or weighing at least 99 lbs, without cirrhosis or who have compensated cirrhosis. It is not recommended for patients with moderate cirrhosis, and it is contraindicated in patients with severe cirrhosis, as well as patients taking atazanavir and rifampin.
In clinical trials of 47 patients with genotype 1, 2, 3, or 4 HCV without cirrhosis or with only mild cirrhosis, results at 12 weeks after 8 or 16 weeks’ treatment suggested patients’ infections had been cured – 100% had no virus detected in their blood. Adverse reactions observed were consistent with those previously observed in adults during clinical trials.
The most common reactions were headache and fatigue. Hepatitis B virus reactivation has been reported in coinfected adults during or after treatment with direct-acting antivirals, and in those who were not receiving HBV antiviral treatment. Full prescribing information can be found on the FDA website, and more information about this approval can be found in the agency’s announcement.
The Food and Drug Administration has approved glecaprevir/pibrentasvir tablets (Mavyret) for treating any of six identified genotypes of hepatitis C virus in children ages 12-17 years.
The agency noted in its press announcement that, Dosing information now will be provided for patients aged 12 years and older or weighing at least 99 lbs, without cirrhosis or who have compensated cirrhosis. It is not recommended for patients with moderate cirrhosis, and it is contraindicated in patients with severe cirrhosis, as well as patients taking atazanavir and rifampin.
In clinical trials of 47 patients with genotype 1, 2, 3, or 4 HCV without cirrhosis or with only mild cirrhosis, results at 12 weeks after 8 or 16 weeks’ treatment suggested patients’ infections had been cured – 100% had no virus detected in their blood. Adverse reactions observed were consistent with those previously observed in adults during clinical trials.
The most common reactions were headache and fatigue. Hepatitis B virus reactivation has been reported in coinfected adults during or after treatment with direct-acting antivirals, and in those who were not receiving HBV antiviral treatment. Full prescribing information can be found on the FDA website, and more information about this approval can be found in the agency’s announcement.
The Food and Drug Administration has approved glecaprevir/pibrentasvir tablets (Mavyret) for treating any of six identified genotypes of hepatitis C virus in children ages 12-17 years.
The agency noted in its press announcement that, Dosing information now will be provided for patients aged 12 years and older or weighing at least 99 lbs, without cirrhosis or who have compensated cirrhosis. It is not recommended for patients with moderate cirrhosis, and it is contraindicated in patients with severe cirrhosis, as well as patients taking atazanavir and rifampin.
In clinical trials of 47 patients with genotype 1, 2, 3, or 4 HCV without cirrhosis or with only mild cirrhosis, results at 12 weeks after 8 or 16 weeks’ treatment suggested patients’ infections had been cured – 100% had no virus detected in their blood. Adverse reactions observed were consistent with those previously observed in adults during clinical trials.
The most common reactions were headache and fatigue. Hepatitis B virus reactivation has been reported in coinfected adults during or after treatment with direct-acting antivirals, and in those who were not receiving HBV antiviral treatment. Full prescribing information can be found on the FDA website, and more information about this approval can be found in the agency’s announcement.
Benlysta approved for children with SLE
The B-lymphocyte stimulator–inhibitor called Benlysta already is approved for use in adults alongside standard therapy for SLE, and this approval marks the first such treatment available for children. Although there are regulatory submissions for use of this drug among children elsewhere, the United States is the first to approve its use among this age group, according to a press release from GSK. According to an FDA press announcement, the agency expedited the review and approval because belimumab could fulfill an unmet need.
The approval is based on a 1-year postapproval commitment study, which assessed efficacy, safety, and pharmacokinetics of 10 mg/kg belimumab plus standard therapy versus placebo plus standard therapy among children with SLE aged 5-11 years (n = 13) and those aged 12-17 years (n = 80). Although the study was not fully powered because of the rarity of the disease in this age group, it did find numerically higher SLE responder index response rates over 1 year among children treated with belimumab plus standard therapy (53%) than in those treated with placebo and standard therapy (44%).
Adverse reactions seen among this age group were consistent with those seen in adults, including nausea, diarrhea, pyrexia, nasopharyngitis, and bronchitis. The most common serious adverse reactions were serious infections. Belimumab has not been studied in combination with certain other drugs, such as other biologics or cyclophosphamide; therefore, combining it with such treatments is not recommended. Acute hypersensitivity reactions – including anaphylaxis and death – have been observed, even among patients who had previously tolerated belimumab.
Infusion reactions were common, so pretreat patients with an antihistamine. Also, do not administer the drug with live vaccines, the FDA noted.
More information can be found in the press announcement on the FDA website.
The B-lymphocyte stimulator–inhibitor called Benlysta already is approved for use in adults alongside standard therapy for SLE, and this approval marks the first such treatment available for children. Although there are regulatory submissions for use of this drug among children elsewhere, the United States is the first to approve its use among this age group, according to a press release from GSK. According to an FDA press announcement, the agency expedited the review and approval because belimumab could fulfill an unmet need.
The approval is based on a 1-year postapproval commitment study, which assessed efficacy, safety, and pharmacokinetics of 10 mg/kg belimumab plus standard therapy versus placebo plus standard therapy among children with SLE aged 5-11 years (n = 13) and those aged 12-17 years (n = 80). Although the study was not fully powered because of the rarity of the disease in this age group, it did find numerically higher SLE responder index response rates over 1 year among children treated with belimumab plus standard therapy (53%) than in those treated with placebo and standard therapy (44%).
Adverse reactions seen among this age group were consistent with those seen in adults, including nausea, diarrhea, pyrexia, nasopharyngitis, and bronchitis. The most common serious adverse reactions were serious infections. Belimumab has not been studied in combination with certain other drugs, such as other biologics or cyclophosphamide; therefore, combining it with such treatments is not recommended. Acute hypersensitivity reactions – including anaphylaxis and death – have been observed, even among patients who had previously tolerated belimumab.
Infusion reactions were common, so pretreat patients with an antihistamine. Also, do not administer the drug with live vaccines, the FDA noted.
More information can be found in the press announcement on the FDA website.
The B-lymphocyte stimulator–inhibitor called Benlysta already is approved for use in adults alongside standard therapy for SLE, and this approval marks the first such treatment available for children. Although there are regulatory submissions for use of this drug among children elsewhere, the United States is the first to approve its use among this age group, according to a press release from GSK. According to an FDA press announcement, the agency expedited the review and approval because belimumab could fulfill an unmet need.
The approval is based on a 1-year postapproval commitment study, which assessed efficacy, safety, and pharmacokinetics of 10 mg/kg belimumab plus standard therapy versus placebo plus standard therapy among children with SLE aged 5-11 years (n = 13) and those aged 12-17 years (n = 80). Although the study was not fully powered because of the rarity of the disease in this age group, it did find numerically higher SLE responder index response rates over 1 year among children treated with belimumab plus standard therapy (53%) than in those treated with placebo and standard therapy (44%).
Adverse reactions seen among this age group were consistent with those seen in adults, including nausea, diarrhea, pyrexia, nasopharyngitis, and bronchitis. The most common serious adverse reactions were serious infections. Belimumab has not been studied in combination with certain other drugs, such as other biologics or cyclophosphamide; therefore, combining it with such treatments is not recommended. Acute hypersensitivity reactions – including anaphylaxis and death – have been observed, even among patients who had previously tolerated belimumab.
Infusion reactions were common, so pretreat patients with an antihistamine. Also, do not administer the drug with live vaccines, the FDA noted.
More information can be found in the press announcement on the FDA website.
Long-term antibiotic use may heighten stroke, CHD risk
, according to a study in the European Heart Journal.
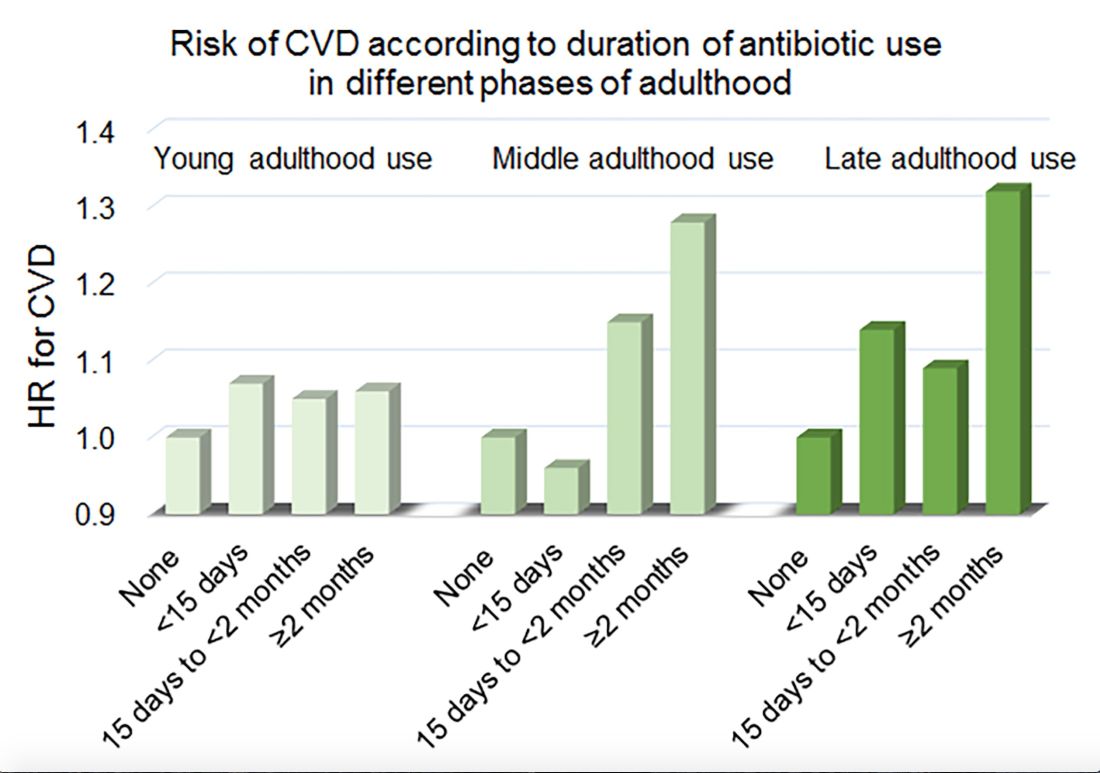
Women in the Nurses’ Health Study who used antibiotics for 2 or more months between ages 40 and 59 years or at age 60 years and older had a significantly increased risk of cardiovascular disease, compared with those who did not use antibiotics. Antibiotic use between 20 and 39 years old was not significantly related to cardiovascular disease.
Prior research has found that antibiotics may have long-lasting effects on gut microbiota and relate to cardiovascular disease risk.
“Antibiotic use is the most critical factor in altering the balance of microorganisms in the gut,” said lead investigator Lu Qi, MD, PhD, in a news release. “Previous studies have shown a link between alterations in the microbiotic environment of the gut and inflammation and narrowing of the blood vessels, stroke, and heart disease,” said Dr. Qi, who is the director of the Tulane University Obesity Research Center in New Orleans and an adjunct professor of nutrition at Harvard T.C. Chan School of Public Health in Boston.
To evaluate associations between life stage, antibiotic exposure, and subsequent cardiovascular disease, researchers analyzed data from 36,429 participants in the Nurses’ Health Study. The women were at least 60 years old and had no history of cardiovascular disease or cancer when they completed a 2004 questionnaire about antibiotic usage during young, middle, and late adulthood. The questionnaire asked participants to indicate the total time using antibiotics with eight categories ranging from none to 5 or more years.
The researchers defined incident cardiovascular disease as a composite endpoint of coronary heart disease (nonfatal myocardial infarction or fatal coronary heart disease) and stroke (nonfatal or fatal). They calculated person-years of follow-up from the questionnaire return date until date of cardiovascular disease diagnosis, death, or end of follow-up in 2012.
Women with longer duration of antibiotic use were more likely to use other medications and have unfavorable cardiovascular risk profiles, including family history of myocardial infarction and higher body mass index. Antibiotics most often were used to treat respiratory infections. During an average follow-up of 7.6 years, 1,056 participants developed cardiovascular disease.
In a multivariable model that adjusted for demographics, diet, lifestyle, reason for antibiotic use, medications, overweight status, and other factors, long-term antibiotic use – 2 months or more – in late adulthood was associated with significantly increased risk of cardiovascular disease (hazard ratio, 1.32), as was long-term antibiotic use in middle adulthood (HR, 1.28).
Although antibiotic use was self-reported, which could lead to misclassification, the participants were health professionals, which may mitigate this limitation, the authors noted. Whether these findings apply to men and other populations requires further study, they said.
Because of the study’s observational design, the results “cannot show that antibiotics cause heart disease and stroke, only that there is a link between them,” Dr. Qi said. “It’s possible that women who reported more antibiotic use might be sicker in other ways that we were unable to measure, or there may be other factors that could affect the results that we have not been able take account of.”
“Our study suggests that antibiotics should be used only when they are absolutely needed,” he concluded. “Considering the potentially cumulative adverse effects, the shorter time of antibiotic use the better.”
The study was supported by National Institutes of Health grants, the Boston Obesity Nutrition Research Center, and the United States–Israel Binational Science Foundation. One author received support from the Japan Society for the Promotion of Science. The authors had no conflicts of interest.
SOURCE: Heianza Y et al. Eur Heart J. 2019 Apr 24. doi: 10.1093/eurheartj/ehz231.
, according to a study in the European Heart Journal.

Women in the Nurses’ Health Study who used antibiotics for 2 or more months between ages 40 and 59 years or at age 60 years and older had a significantly increased risk of cardiovascular disease, compared with those who did not use antibiotics. Antibiotic use between 20 and 39 years old was not significantly related to cardiovascular disease.
Prior research has found that antibiotics may have long-lasting effects on gut microbiota and relate to cardiovascular disease risk.
“Antibiotic use is the most critical factor in altering the balance of microorganisms in the gut,” said lead investigator Lu Qi, MD, PhD, in a news release. “Previous studies have shown a link between alterations in the microbiotic environment of the gut and inflammation and narrowing of the blood vessels, stroke, and heart disease,” said Dr. Qi, who is the director of the Tulane University Obesity Research Center in New Orleans and an adjunct professor of nutrition at Harvard T.C. Chan School of Public Health in Boston.
To evaluate associations between life stage, antibiotic exposure, and subsequent cardiovascular disease, researchers analyzed data from 36,429 participants in the Nurses’ Health Study. The women were at least 60 years old and had no history of cardiovascular disease or cancer when they completed a 2004 questionnaire about antibiotic usage during young, middle, and late adulthood. The questionnaire asked participants to indicate the total time using antibiotics with eight categories ranging from none to 5 or more years.
The researchers defined incident cardiovascular disease as a composite endpoint of coronary heart disease (nonfatal myocardial infarction or fatal coronary heart disease) and stroke (nonfatal or fatal). They calculated person-years of follow-up from the questionnaire return date until date of cardiovascular disease diagnosis, death, or end of follow-up in 2012.
Women with longer duration of antibiotic use were more likely to use other medications and have unfavorable cardiovascular risk profiles, including family history of myocardial infarction and higher body mass index. Antibiotics most often were used to treat respiratory infections. During an average follow-up of 7.6 years, 1,056 participants developed cardiovascular disease.
In a multivariable model that adjusted for demographics, diet, lifestyle, reason for antibiotic use, medications, overweight status, and other factors, long-term antibiotic use – 2 months or more – in late adulthood was associated with significantly increased risk of cardiovascular disease (hazard ratio, 1.32), as was long-term antibiotic use in middle adulthood (HR, 1.28).
Although antibiotic use was self-reported, which could lead to misclassification, the participants were health professionals, which may mitigate this limitation, the authors noted. Whether these findings apply to men and other populations requires further study, they said.
Because of the study’s observational design, the results “cannot show that antibiotics cause heart disease and stroke, only that there is a link between them,” Dr. Qi said. “It’s possible that women who reported more antibiotic use might be sicker in other ways that we were unable to measure, or there may be other factors that could affect the results that we have not been able take account of.”
“Our study suggests that antibiotics should be used only when they are absolutely needed,” he concluded. “Considering the potentially cumulative adverse effects, the shorter time of antibiotic use the better.”
The study was supported by National Institutes of Health grants, the Boston Obesity Nutrition Research Center, and the United States–Israel Binational Science Foundation. One author received support from the Japan Society for the Promotion of Science. The authors had no conflicts of interest.
SOURCE: Heianza Y et al. Eur Heart J. 2019 Apr 24. doi: 10.1093/eurheartj/ehz231.
, according to a study in the European Heart Journal.

Women in the Nurses’ Health Study who used antibiotics for 2 or more months between ages 40 and 59 years or at age 60 years and older had a significantly increased risk of cardiovascular disease, compared with those who did not use antibiotics. Antibiotic use between 20 and 39 years old was not significantly related to cardiovascular disease.
Prior research has found that antibiotics may have long-lasting effects on gut microbiota and relate to cardiovascular disease risk.
“Antibiotic use is the most critical factor in altering the balance of microorganisms in the gut,” said lead investigator Lu Qi, MD, PhD, in a news release. “Previous studies have shown a link between alterations in the microbiotic environment of the gut and inflammation and narrowing of the blood vessels, stroke, and heart disease,” said Dr. Qi, who is the director of the Tulane University Obesity Research Center in New Orleans and an adjunct professor of nutrition at Harvard T.C. Chan School of Public Health in Boston.
To evaluate associations between life stage, antibiotic exposure, and subsequent cardiovascular disease, researchers analyzed data from 36,429 participants in the Nurses’ Health Study. The women were at least 60 years old and had no history of cardiovascular disease or cancer when they completed a 2004 questionnaire about antibiotic usage during young, middle, and late adulthood. The questionnaire asked participants to indicate the total time using antibiotics with eight categories ranging from none to 5 or more years.
The researchers defined incident cardiovascular disease as a composite endpoint of coronary heart disease (nonfatal myocardial infarction or fatal coronary heart disease) and stroke (nonfatal or fatal). They calculated person-years of follow-up from the questionnaire return date until date of cardiovascular disease diagnosis, death, or end of follow-up in 2012.
Women with longer duration of antibiotic use were more likely to use other medications and have unfavorable cardiovascular risk profiles, including family history of myocardial infarction and higher body mass index. Antibiotics most often were used to treat respiratory infections. During an average follow-up of 7.6 years, 1,056 participants developed cardiovascular disease.
In a multivariable model that adjusted for demographics, diet, lifestyle, reason for antibiotic use, medications, overweight status, and other factors, long-term antibiotic use – 2 months or more – in late adulthood was associated with significantly increased risk of cardiovascular disease (hazard ratio, 1.32), as was long-term antibiotic use in middle adulthood (HR, 1.28).
Although antibiotic use was self-reported, which could lead to misclassification, the participants were health professionals, which may mitigate this limitation, the authors noted. Whether these findings apply to men and other populations requires further study, they said.
Because of the study’s observational design, the results “cannot show that antibiotics cause heart disease and stroke, only that there is a link between them,” Dr. Qi said. “It’s possible that women who reported more antibiotic use might be sicker in other ways that we were unable to measure, or there may be other factors that could affect the results that we have not been able take account of.”
“Our study suggests that antibiotics should be used only when they are absolutely needed,” he concluded. “Considering the potentially cumulative adverse effects, the shorter time of antibiotic use the better.”
The study was supported by National Institutes of Health grants, the Boston Obesity Nutrition Research Center, and the United States–Israel Binational Science Foundation. One author received support from the Japan Society for the Promotion of Science. The authors had no conflicts of interest.
SOURCE: Heianza Y et al. Eur Heart J. 2019 Apr 24. doi: 10.1093/eurheartj/ehz231.
FROM THE EUROPEAN HEART JOURNAL
Key clinical point: Among middle-aged and older women, 2 or more months’ exposure to antibiotics is associated with an increased risk of coronary heart disease or stroke.
Major finding: Long-term antibiotic use in late adulthood was associated with significantly increased risk of cardiovascular disease (hazard ratio, 1.32), as was long-term antibiotic use in middle adulthood (HR, 1.28).
Study details: An analysis of data from nearly 36,500 women in the Nurses’ Health Study.
Disclosures: The study was supported by National Institutes of Health grants, the Boston Obesity Nutrition Research Center, and the United States–Israel Binational Science Foundation. One author received support from the Japan Society for the Promotion of Science. The authors had no conflicts of interest.
Source: Heianza Y et al. Eur Heart J. 2019 Apr 24. doi: 10.1093/eurheartj/ehz231.
Gaps exist in rotavirus vaccination coverage in young U.S. children
falling short of the Healthy People 2020 goal of 80% complete vaccination, according to Bethany K. Sederdahl, MPH, and her associates at Emory University, Atlanta.
In an analysis published in Pediatrics of data from 14,571 children included in the 2014 National Immunization Survey, 71% of children received full vaccination for rotavirus, 15% received partial vaccination, and 14% received no vaccination. Children whose mothers were not college graduates, lived in households with at least four children, or were uninsured at any point had an increased likelihood of being unvaccinated; African American children also faced an increased risk of being unvaccinated.
Among the unvaccinated, 72% had at least one missed opportunity according to the Advisory Committee on Immunization Practices schedule, and 83% had at least one missed opportunity according to the World Health Organization schedule. For the partially vaccinated, 54% at least one missed opportunity according to the ACIP schedule, and 96% had at least one missed opportunity according to the WHO schedule. While poorer socioeconomic conditions were associated with the risk of being unvaccinated, children who were partially vaccinated and who missed vaccination opportunities according to the ACIP-recommended schedule were more likely to have mothers with a college degree or an income of more than $75,000.
According to the investigators, if all missed opportunities for vaccination according to the ACIP schedule were addressed, coverage would improve from 71% to 81%; if all opportunities according to the WHO schedule were addressed, coverage would increase to 94%.
“Low rotavirus vaccine uptake may be attributable to both socioeconomic barriers and possibly vaccine hesitancy. Understanding the barriers to rotavirus vaccine uptake and developing effective public health measures to promote vaccine use will be essential to reducing rotavirus morbidity in the United States,” Ms. Sederdahl and her associates wrote.
The study received no external funding. One coauthor reported receiving personal fees from AbbVie, funds to conduct clinical research from Merck, and that his institution receives funds to conduct clinical research from MedImmune, Regeneron, PaxVax, Pfizer, Merck, Novavax, Sanofi Pasteur, and Micron Technology.
SOURCE: Sederdahl BK et al. Pediatrics. 2019 Apr 25. doi: 10.1542/peds.2018-2498.
falling short of the Healthy People 2020 goal of 80% complete vaccination, according to Bethany K. Sederdahl, MPH, and her associates at Emory University, Atlanta.
In an analysis published in Pediatrics of data from 14,571 children included in the 2014 National Immunization Survey, 71% of children received full vaccination for rotavirus, 15% received partial vaccination, and 14% received no vaccination. Children whose mothers were not college graduates, lived in households with at least four children, or were uninsured at any point had an increased likelihood of being unvaccinated; African American children also faced an increased risk of being unvaccinated.
Among the unvaccinated, 72% had at least one missed opportunity according to the Advisory Committee on Immunization Practices schedule, and 83% had at least one missed opportunity according to the World Health Organization schedule. For the partially vaccinated, 54% at least one missed opportunity according to the ACIP schedule, and 96% had at least one missed opportunity according to the WHO schedule. While poorer socioeconomic conditions were associated with the risk of being unvaccinated, children who were partially vaccinated and who missed vaccination opportunities according to the ACIP-recommended schedule were more likely to have mothers with a college degree or an income of more than $75,000.
According to the investigators, if all missed opportunities for vaccination according to the ACIP schedule were addressed, coverage would improve from 71% to 81%; if all opportunities according to the WHO schedule were addressed, coverage would increase to 94%.
“Low rotavirus vaccine uptake may be attributable to both socioeconomic barriers and possibly vaccine hesitancy. Understanding the barriers to rotavirus vaccine uptake and developing effective public health measures to promote vaccine use will be essential to reducing rotavirus morbidity in the United States,” Ms. Sederdahl and her associates wrote.
The study received no external funding. One coauthor reported receiving personal fees from AbbVie, funds to conduct clinical research from Merck, and that his institution receives funds to conduct clinical research from MedImmune, Regeneron, PaxVax, Pfizer, Merck, Novavax, Sanofi Pasteur, and Micron Technology.
SOURCE: Sederdahl BK et al. Pediatrics. 2019 Apr 25. doi: 10.1542/peds.2018-2498.
falling short of the Healthy People 2020 goal of 80% complete vaccination, according to Bethany K. Sederdahl, MPH, and her associates at Emory University, Atlanta.
In an analysis published in Pediatrics of data from 14,571 children included in the 2014 National Immunization Survey, 71% of children received full vaccination for rotavirus, 15% received partial vaccination, and 14% received no vaccination. Children whose mothers were not college graduates, lived in households with at least four children, or were uninsured at any point had an increased likelihood of being unvaccinated; African American children also faced an increased risk of being unvaccinated.
Among the unvaccinated, 72% had at least one missed opportunity according to the Advisory Committee on Immunization Practices schedule, and 83% had at least one missed opportunity according to the World Health Organization schedule. For the partially vaccinated, 54% at least one missed opportunity according to the ACIP schedule, and 96% had at least one missed opportunity according to the WHO schedule. While poorer socioeconomic conditions were associated with the risk of being unvaccinated, children who were partially vaccinated and who missed vaccination opportunities according to the ACIP-recommended schedule were more likely to have mothers with a college degree or an income of more than $75,000.
According to the investigators, if all missed opportunities for vaccination according to the ACIP schedule were addressed, coverage would improve from 71% to 81%; if all opportunities according to the WHO schedule were addressed, coverage would increase to 94%.
“Low rotavirus vaccine uptake may be attributable to both socioeconomic barriers and possibly vaccine hesitancy. Understanding the barriers to rotavirus vaccine uptake and developing effective public health measures to promote vaccine use will be essential to reducing rotavirus morbidity in the United States,” Ms. Sederdahl and her associates wrote.
The study received no external funding. One coauthor reported receiving personal fees from AbbVie, funds to conduct clinical research from Merck, and that his institution receives funds to conduct clinical research from MedImmune, Regeneron, PaxVax, Pfizer, Merck, Novavax, Sanofi Pasteur, and Micron Technology.
SOURCE: Sederdahl BK et al. Pediatrics. 2019 Apr 25. doi: 10.1542/peds.2018-2498.
FROM PEDIATRICS
CDC warns against misuse of opioid-prescribing guideline
Officials at the Centers for Disease Control and Prevention are warning against the misapplication of the agency’s 2016 guidelines on opioid prescribing, as well as clarifying dosage recommendations for patients starting or stopping pain medications.
In a perspective published in the New England Journal of Medicine on April 24, lead author Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control, conveyed concern that some policies and practices derived from the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain are inconsistent with the recommendations and often go beyond their scope.
Misapplication examples include inappropriately applying the guideline to patients in active cancer treatment, patients experiencing acute sickle cell crises, or patients experiencing postsurgical pain, Dr. Dowell wrote.
The guideline offers guidance to clinicians treating chronic pain in adults who are already receiving opioids long-term at high dosages, she noted. It includes advice on maximizing nonopioid treatment, reviewing risks associated with continuing high-dose opioids, and collaborating with patients who agree to taper dosage, among other guidance.
Any application of the guideline’s dosage recommendation that results in hard limits or “cutting off” opioids is also an incorrect use of the recommendations, according to Dr. Dowell.
While the guideline advises clinicians to start opioids at the lowest effective dosage and avoid increasing dosage to 90 morphine milligram equivalents per day or more, that statement does not suggest discontinuation of opioids already prescribed at high dosages, according to the CDC’s clarification.
The guidance also does not apply to patients receiving or starting medication-assisted treatment for opioid use disorder.
The commentary comes after a trio of organizations raised concerns that insurers are inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology, raised the issue in a letter to the CDC in February. In response, Dr. Dowell clarified that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
In the perspective, Dr. Dowell wrote that the CDC is evaluating the intended and unintended impact of the 2016 opioid-prescribing guideline on clinician and patient outcomes and that the agency is committed to updating the recommendations when new evidence is available.
Officials at the Centers for Disease Control and Prevention are warning against the misapplication of the agency’s 2016 guidelines on opioid prescribing, as well as clarifying dosage recommendations for patients starting or stopping pain medications.
In a perspective published in the New England Journal of Medicine on April 24, lead author Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control, conveyed concern that some policies and practices derived from the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain are inconsistent with the recommendations and often go beyond their scope.
Misapplication examples include inappropriately applying the guideline to patients in active cancer treatment, patients experiencing acute sickle cell crises, or patients experiencing postsurgical pain, Dr. Dowell wrote.
The guideline offers guidance to clinicians treating chronic pain in adults who are already receiving opioids long-term at high dosages, she noted. It includes advice on maximizing nonopioid treatment, reviewing risks associated with continuing high-dose opioids, and collaborating with patients who agree to taper dosage, among other guidance.
Any application of the guideline’s dosage recommendation that results in hard limits or “cutting off” opioids is also an incorrect use of the recommendations, according to Dr. Dowell.
While the guideline advises clinicians to start opioids at the lowest effective dosage and avoid increasing dosage to 90 morphine milligram equivalents per day or more, that statement does not suggest discontinuation of opioids already prescribed at high dosages, according to the CDC’s clarification.
The guidance also does not apply to patients receiving or starting medication-assisted treatment for opioid use disorder.
The commentary comes after a trio of organizations raised concerns that insurers are inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology, raised the issue in a letter to the CDC in February. In response, Dr. Dowell clarified that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
In the perspective, Dr. Dowell wrote that the CDC is evaluating the intended and unintended impact of the 2016 opioid-prescribing guideline on clinician and patient outcomes and that the agency is committed to updating the recommendations when new evidence is available.
Officials at the Centers for Disease Control and Prevention are warning against the misapplication of the agency’s 2016 guidelines on opioid prescribing, as well as clarifying dosage recommendations for patients starting or stopping pain medications.
In a perspective published in the New England Journal of Medicine on April 24, lead author Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control, conveyed concern that some policies and practices derived from the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain are inconsistent with the recommendations and often go beyond their scope.
Misapplication examples include inappropriately applying the guideline to patients in active cancer treatment, patients experiencing acute sickle cell crises, or patients experiencing postsurgical pain, Dr. Dowell wrote.
The guideline offers guidance to clinicians treating chronic pain in adults who are already receiving opioids long-term at high dosages, she noted. It includes advice on maximizing nonopioid treatment, reviewing risks associated with continuing high-dose opioids, and collaborating with patients who agree to taper dosage, among other guidance.
Any application of the guideline’s dosage recommendation that results in hard limits or “cutting off” opioids is also an incorrect use of the recommendations, according to Dr. Dowell.
While the guideline advises clinicians to start opioids at the lowest effective dosage and avoid increasing dosage to 90 morphine milligram equivalents per day or more, that statement does not suggest discontinuation of opioids already prescribed at high dosages, according to the CDC’s clarification.
The guidance also does not apply to patients receiving or starting medication-assisted treatment for opioid use disorder.
The commentary comes after a trio of organizations raised concerns that insurers are inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology, raised the issue in a letter to the CDC in February. In response, Dr. Dowell clarified that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
In the perspective, Dr. Dowell wrote that the CDC is evaluating the intended and unintended impact of the 2016 opioid-prescribing guideline on clinician and patient outcomes and that the agency is committed to updating the recommendations when new evidence is available.