User login
Clinical Utility of Methicillin-Resistant Staphylococcus aureus Polymerase Chain Reaction Nasal Swab Testing in Lower Respiratory Tract Infections
From the Hospital of Central Connecticut, New Britain, CT (Dr. Caulfield and Dr. Shepard); Hartford Hospital, Hartford, CT (Dr. Linder and Dr. Dempsey); and the Hartford HealthCare Research Program, Hartford, CT (Dr. O’Sullivan).
Abstract
- Objective: To assess the utility of methicillin-resistant Staphylococcus aureus (MRSA) polymerase chain reaction (PCR) nasal swab testing in patients with lower respiratory tract infections (LRTI).
- Design and setting: Multicenter, retrospective, electronic chart review conducted within the Hartford HealthCare system.
- Participants: Patients who were treated for LRTIs at the Hospital of Central Connecticut or Hartford Hospital between July 1, 2018, and June 30, 2019.
- Measurements: The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing versus those who did not. In a subgroup analysis, we compared anti-MRSA DOT among patients with appropriate versus inappropriate utilization of the MRSA PCR test.
- Results: Of the 319 patients treated for LRTIs, 155 (48.6%) had a MRSA PCR ordered, and appropriate utilization occurred in 94 (60.6%) of these patients. Anti-MRSA DOT in the MRSA PCR group (n = 155) was shorter than in the group that did not undergo MRSA PCR testing (n = 164), but this difference did not reach statistical significance (1.68 days [interquartile range {IQR}, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458). In the subgroup analysis, anti-MRSA DOT was significantly shorter in the MRSA PCR group with appropriate utilization compared to the inappropriate utilization group (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76], P < 0.001)
- Conclusion: Appropriate utilization of MRSA PCR nasal swab testing can reduce DOT in patients with LRTI. Further education is necessary to expand the appropriate use of the MRSA PCR test across our health system.
Keywords: MRSA; LRTI; pneumonia; antimicrobial stewardship; antibiotic resistance.
More than 300,000 patients were hospitalized with methicillin-resistant Staphylococcus aureus (MRSA) infections in the United States in 2017, and at least 10,000 of these cases resulted in mortality.1 While MRSA infections overall are decreasing, it is crucial to continue to employ antimicrobial stewardship tactics to keep these infections at bay. Recently, strains of S. aureus have become resistant to vancomycin, making this bacterium even more difficult to treat.2
A novel tactic in antimicrobial stewardship involves the use of MRSA polymerase chain reaction (PCR) nasal swab testing to rule out the presence of MRSA in patients with lower respiratory tract infections (LRTI). If used appropriately, this approach may decrease the number of days patients are treated with anti-MRSA antimicrobials. Waiting for cultures to speciate can take up to 72 hours,3 meaning that patients may be exposed to 3 days of unnecessary broad-spectrum antibiotics. Results of MRSA PCR assay of nasal swab specimens can be available in 1 to 2 hours,4 allowing for more rapid de-escalation of therapy. Numerous studies have shown that this test has a negative predictive value (NPV) greater than 95%, indicating that a negative nasal swab result may be useful to guide de-escalation of antibiotic therapy.5-8 The purpose of this study was to assess the utility of MRSA PCR nasal swab testing in patients with LRTI throughout the Hartford HealthCare system.
Methods
Design
This study was a multicenter, retrospective, electronic chart review. It was approved by the Hartford HealthCare Institutional Review Board (HHC-2019-0169).
Selection of Participants
Patients were identified through electronic medical record reports based on ICD-10 codes. Records were categorized into 2 groups: patients who received a MRSA PCR nasal swab testing and patients who did not. Patients who received the MRSA PCR were further categorized by appropriate or inappropriate utilization. Appropriate utilization of the MRSA PCR was defined as MRSA PCR ordered within 48 hours of a new vancomycin or linezolid order, and anti-MRSA therapy discontinued within 24 hours of a negative result. Inappropriate utilization of the MRSA PCR was defined as MRSA PCR ordered more than 48 hours after a new vancomycin or linezolid order, or continuation of anti-MRSA therapy despite a negative MRSA PCR and no other evidence of a MRSA infection.
Patients were included if they met all of the following criteria: age 18 years or older, with no upper age limit; treated for a LRTI, identified by ICD-10 codes (J13-22, J44, J85); treated with empiric antibiotics active against MRSA, specifically vancomycin or linezolid; and treated at the Hospital of Central Connecticut (HOCC) or Hartford Hospital (HH) between July 1, 2018, and June 30, 2019, inclusive. Patients were excluded if they met 1 or more of the following criteria: age less than 18 years old; admitted for 48 hours or fewer or discharged from the emergency department; not treated at either facility; treated before July 1, 2018, or after June 30, 2019; treated for ventilator-associated pneumonia; received anti-MRSA therapy within 30 days prior to admission; or treated for a concurrent bacterial infection requiring anti-MRSA therapy.
Outcome Measures
The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing compared to patients who did not undergo MRSA PCR testing. A subgroup analysis was completed to compare anti-MRSA DOT within patients in the MRSA PCR group. Patients in the subgroup were categorized by appropriate or inappropriate utilization, and anti-MRSA DOT were compared between these groups. Secondary outcomes that were evaluated included length of stay (LOS), 30-day readmission rate, and incidence of acute kidney injury (AKI). Thirty-day readmission was defined as admission to HOCC, HH, or any institution within Hartford HealthCare within 30 days of discharge. AKI was defined as an increase in serum creatinine by ≥ 0.3 mg/dL in 48 hours, increase ≥ 1.5 times baseline, or a urine volume < 0.5 mL/kg/hr for 6 hours.
Statistical Analyses
The study was powered for the primary outcome, anti-MRSA DOT, with a clinically meaningful difference of 1 day. Group sample sizes of 240 in the MRSA PCR group and 160 in the no MRSA PCR group would have afforded 92% power to detect that difference, if the null hypothesis was that both group means were 4 days and the alternative hypothesis was that the mean of the MRSA PCR group was 3 days, with estimated group standard deviations of 80% of each mean. This estimate used an alpha level of 0.05 with a 2-sided t-test. Among those who received a MRSA PCR test, a clinically meaningful difference between appropriate and inappropriate utilization was 5%.
Descriptive statistics were provided for all variables as a function of the individual hospital and for the combined data set. Continuous data were summarized with means and standard deviations (SD), or with median and interquartile ranges (IQR), depending on distribution. Categorical variables were reported as frequencies, using percentages. All data were evaluated for normality of distribution. Inferential statistics comprised a Student’s t-test to compare normally distributed, continuous data between groups. Nonparametric distributions were compared using a Mann-Whitney U test. Categorical comparisons were made using a Fisher’s exact test for 2×2 tables and a Pearson chi-square test for comparisons involving more than 2 groups.
Since anti-MRSA DOT (primary outcome) and LOS (secondary outcome) are often non-normally distributed, they have been transformed (eg, log or square root, again depending on distribution). Whichever native variable or transformation variable was appropriate was used as the outcome measure in a linear regression model to account for the influence of factors (covariates) that show significant univariate differences. Given the relatively small sample size, a maximum of 10 variables were included in the model. All factors were iterated in a forward regression model (most influential first) until no significant changes were observed.
All calculations were performed with SPSS v. 21 (IBM; Armonk, NY) using an a priori alpha level of 0.05, such that all results yielding P < 0.05 were deemed statistically significant.
Results
Of the 561 patient records reviewed, 319 patients were included and 242 patients were excluded. Reasons for exclusion included 65 patients admitted for a duration of 48 hours or less or discharged from the emergency department; 61 patients having another infection requiring anti-MRSA therapy; 60 patients not having a diagnosis of a LRTI or not receiving anti-MRSA therapy; 52 patients having received anti-MRSA therapy within 30 days prior to admission; and 4 patients treated outside of the specified date range.
Of the 319 patients included, 155 (48.6%) were in the MRSA PCR group and 164 (51.4%) were in the group that did not undergo MRSA PCR (Table 1). Of the 155 patients with a MRSA PCR ordered, the test was utilized appropriately in 94 (60.6%) patients and inappropriately in 61 (39.4%) patients (Table 2). In the MRSA PCR group, 135 patients had a negative result on PCR assay, with 133 of those patients having negative respiratory cultures, resulting in a NPV of 98.5%. Differences in baseline characteristics between the MRSA PCR and no MRSA PCR groups were observed. The patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, as indicated by statistically significant differences in intensive care unit (ICU) admissions (P = 0.001), positive chest radiographs (P = 0.034), sepsis at time of anti-MRSA initiation (P = 0.013), pulmonary consults placed (P = 0.003), and carbapenem usage (P = 0.047).
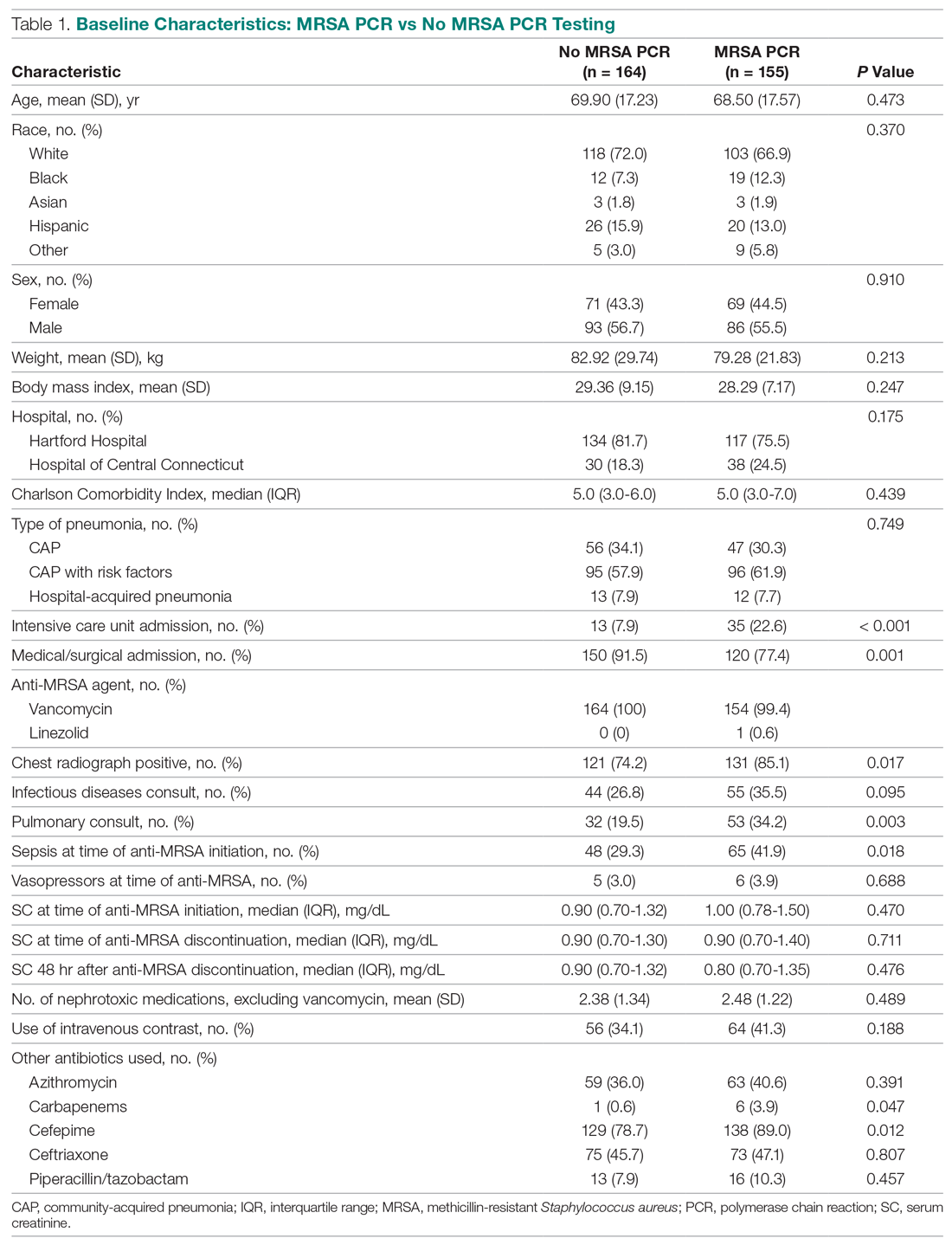
In the subgroup analysis comparing appropriate and inappropriate utilization within the MRSA PCR group, the inappropriate utilization group had significantly higher numbers of infectious diseases consults placed, patients with hospital-acquired pneumonia, and patients with community-acquired pneumonia with risk factors.
Outcomes
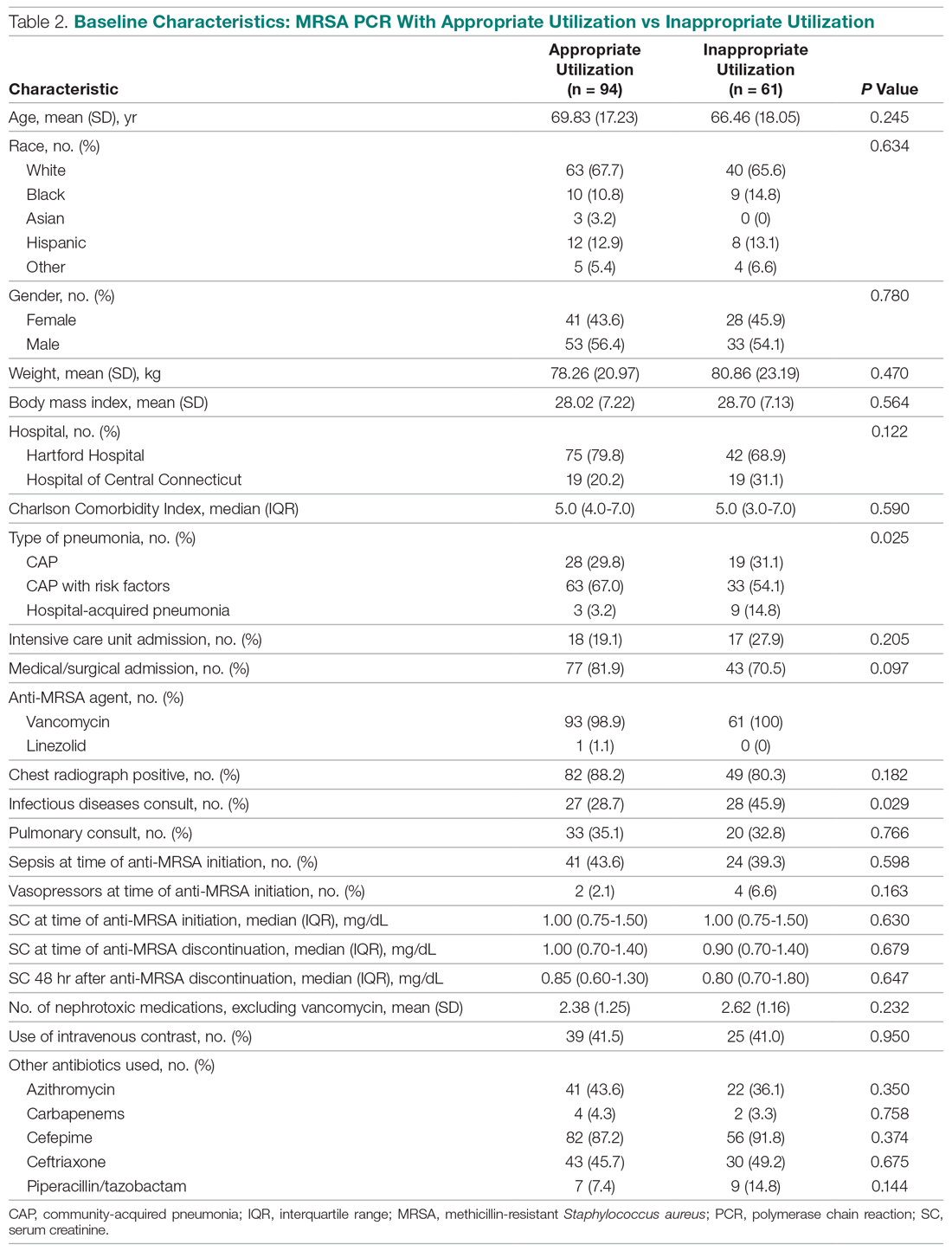
Median anti-MRSA DOT in the MRSA PCR group was shorter than DOT in the no MRSA PCR group, but this difference did not reach statistical significance (1.68 [IQR, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458; Table 3). LOS in the MRSA PCR group was longer than in the no MRSA PCR group (6.0 [IQR, 4.0-10.0] vs 5.0 [IQR, 3.0-7.0] days, P = 0.001). There was no difference in 30-day readmissions that were related to the previous visit or incidence of AKI between groups.
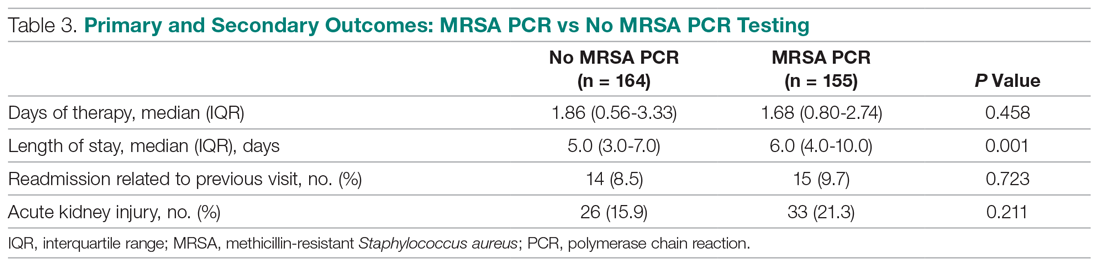
In the subgroup analysis, anti-MRSA DOT in the MRSA PCR group with appropriate utilization was shorter than DOT in the MRSA PCR group with inappropriate utilization (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76] days, P < 0.001; Table 4). LOS in the MRSA PCR group with appropriate utilization was shorter than LOS in the inappropriate utilization group (5.0 [IQR, 4.0-7.0] vs 7.0 [IQR, 5.0-12.0] days, P < 0.001). Thirty-day readmissions that were related to the previous visit were significantly higher in patients in the MRSA PCR group with appropriate utilization (13 vs 2, P = 0.030). There was no difference in incidence of AKI between the groups.
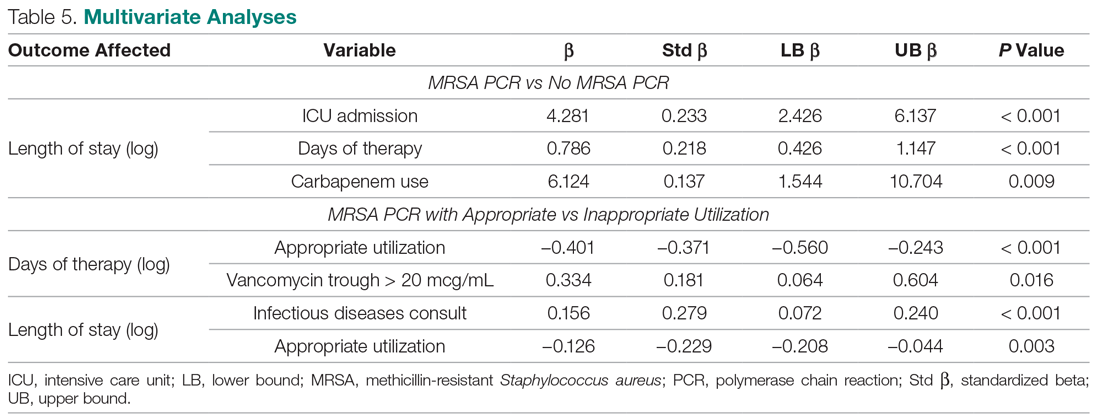
A multivariate analysis was completed to determine whether the sicker MRSA PCR population was confounding outcomes, particularly the secondary outcome of LOS, which was noted to be longer in the MRSA PCR group (Table 5). When comparing LOS in the MRSA PCR and the no MRSA PCR patients, the multivariate analysis showed that admission to the ICU and carbapenem use were associated with a longer LOS (P < 0.001 and P = 0.009, respectively). The incidence of admission to the ICU and carbapenem use were higher in the MRSA PCR group (P = 0.001 and P = 0.047). Therefore, longer LOS in the MRSA PCR patients could be a result of the higher prevalence of ICU admissions and infections requiring carbapenem therapy rather than the result of the MRSA PCR itself.
Discussion
A MRSA PCR nasal swab protocol can be used to minimize a patient’s exposure to unnecessary broad-spectrum antibiotics, thereby preventing antimicrobial resistance. Thus, it is important to assess how our health system is utilizing this antimicrobial stewardship tactic. With the MRSA PCR’s high NPV, providers can be confident that MRSA pneumonia is unlikely in the absence of MRSA colonization. Our study established a NPV of 98.5%, which is similar to other studies, all of which have shown NPVs greater than 95%.5-8 Despite the high NPV, this study demonstrated that only 51.4% of patients with LRTI had orders for a MRSA PCR. Of the 155 patients with a MRSA PCR, the test was utilized appropriately only 60.6% of the time. A majority of the inappropriately utilized tests were due to MRSA PCR orders placed more than 48 hours after anti-MRSA therapy initiation. To our knowledge, no other studies have assessed the clinical utility of MRSA PCR nasal swabs as an antimicrobial stewardship tool in a diverse health system; therefore, these results are useful to guide future practices at our institution. There is a clear need for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing for patients with LRTI being treated with anti-MRSA therapy. Additionally, clinician education regarding the initial timing of the MRSA PCR order and the proper utilization of the results of the MRSA PCR likely will benefit patient outcomes at our institution.
When evaluating anti-MRSA DOT, this study demonstrated a reduction of only 0.18 days (about 4 hours) of anti-MRSA therapy in the patients who received MRSA PCR testing compared to the patients without a MRSA PCR ordered. Our anti-MRSA DOT reduction was lower than what has been reported in similar studies. For example, Baby et al found that the use of the MRSA PCR was associated with 46.6 fewer hours of unnecessary antimicrobial treatment. Willis et al evaluated a pharmacist-driven protocol that resulted in a reduction of 1.8 days of anti-MRSA therapy, despite a protocol compliance rate of only 55%.9,10 In our study, the patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, which may be the reason for the incongruences in our results compared to the current literature. Characteristics such as ICU admissions, positive chest radiographs, sepsis cases, pulmonary consults, and carbapenem usage—all of which are indicative of a sicker population—were more prevalent in the MRSA PCR group. This sicker population could have underestimated the reduction of DOT in the MRSA PCR group compared to the no MRSA PCR group.
After isolating the MRSA PCR patients in the subgroup analysis, anti-MRSA DOT was 1.5 days shorter when the test was appropriately utilized, which is more comparable to what has been reported in the literature.9,10 Only 60.6% of the MRSA PCR patients had their anti-MRSA therapy appropriately managed based on the MRSA PCR. Interestingly, a majority of patients in the inappropriate utilization group had MRSA PCR tests ordered more than 48 hours after beginning anti-MRSA therapy. More prompt and efficient ordering of the MRSA PCR may have resulted in more opportunities for earlier de-escalation of therapy. Due to these factors, the patients in the inappropriate utilization group could have further contributed to the underestimated difference in anti-MRSA DOT between the MRSA PCR and no MRSA PCR patients in the primary outcome. Additionally, there were no notable differences between the appropriate and inappropriate utilization groups, unlike in the MRSA PCR and no MRSA PCR groups, which is why we were able to draw more robust conclusions in the subgroup analysis. Therefore, the subgroup analysis confirmed that if the results of the MRSA PCR are used appropriately to guide anti-MRSA therapy, patients can potentially avoid 36 hours of broad-spectrum antibiotics.
Data on how the utilization of the MRSA PCR nasal swab can affect LOS are limited; however, one study did report a 2.8-day reduction in LOS after implementation of a pharmacist-driven MRSA PCR nasal swab protocol.11 Our study demonstrated that LOS was significantly longer in the MRSA PCR group than in the no MRSA PCR group. This result was likely affected by the aforementioned sicker MRSA PCR population. Our multivariate analysis further confirmed that ICU admissions were associated with a longer LOS, and, given that the MRSA PCR group had a significantly higher ICU population, this likely confounded these results. If our 2 groups had had more evenly distributed characteristics, it is possible that we could have found a shorter LOS in the MRSA PCR group, similar to what is reported in the literature. In the subgroup analysis, LOS was 2 days shorter in the appropriate utilization group compared to the inappropriate utilization group. This further affirms that the results of the MRSA PCR must be used appropriately in order for patient outcomes, like LOS, to benefit.
The effects of the MRSA PCR nasal swab on 30-day readmission rates and incidence of AKI are not well-documented in the literature. One study did report 30-day readmission rates as an outcome, but did not cite any difference after the implementation of a protocol that utilized MRSA PCR nasal swab testing.12 The outcome of AKI is slightly better represented in the literature, but the results are conflicting. Some studies report no difference after the implementation of a MRSA PCR-based protocol,11 and others report a significant decrease in AKI with the use of the MRSA PCR.9 Our study detected no difference in 30-day readmission rates related to the previous admission or in AKI between the MRSA PCR and no MRSA PCR populations. In the subgroup analysis, 30-day readmission rates were significantly higher in the MRSA PCR group with appropriate utilization than in the group with inappropriate utilization; however, our study was not powered to detect a difference in this secondary outcome.
This study had some limitations that may have affected our results. First, this study was a retrospective chart review. Additionally, the baseline characteristics were not well balanced across the different groups. There were sicker patients in the MRSA PCR group, which may have led to an underestimate of the reduction in DOT and LOS in these patients. Finally, we did not include enough patient records to reach power in the MRSA PCR group due to a higher than expected number of patients meeting exclusion criteria. Had we attained sufficient power, there may have been more profound reductions in DOT and LOS.
Conclusion
MRSA infections are a common cause for hospitalization, and there is a growing need for antimicrobial stewardship efforts to limit unnecessary antibiotic usage in order to prevent resistance. As illustrated in our study, appropriate utilization of the MRSA PCR can reduce DOT up to 1.5 days. However, our results suggest that there is room for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing in patients with LRTI receiving anti-MRSA therapy. Further emphasis on the appropriate utilization of the MRSA PCR within our health care system is essential.
Corresponding author: Casey Dempsey, PharmD, BCIDP, 80 Seymour St., Hartford, CT 06106; [email protected].
Financial disclosures: None.
1. Antimicrobial resistance threats. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest-threats.html. Accessed September 9, 2020.
2. Biggest threats and data. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest_threats.html#mrsa. Accessed September 9, 2020.
3. Smith MN, Erdman MJ, Ferreira JA, et al. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care. 2017;38:168-171.
4. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86:307-310.
5. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58:859-864.
6. Johnson JA, Wright ME, Sheperd LA, et al. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19: 34-36.
7. Dureau AF, Duclos G, Antonini F, et al. Rapid diagnostic test and use of antibiotic against methicillin-resistant Staphylococcus aureus in adult intensive care unit. Eur J Clin Microbiol Infect Dis. 2017;36:267-272.
8. Tilahun B, Faust AC, McCorstin P, Ortegon A. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care. 2015;24:8-12.
9. Baby N, Faust AC, Smith T, et al. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61:e02432-16.
10. Willis C, Allen B, Tucker C, et al. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am J Health-Syst Pharm. 2017;74:1765-1773.
11. Dadzie P, Dietrich T, Ashurst J. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus polymerase chain reaction nasal swab protocol on the de-escalation of empiric vancomycin in patients with pneumonia in a rural healthcare setting. Cureus. 2019;11:e6378
12. Dunaway S, Orwig KW, Arbogast ZQ, et al. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int J Clin Pharm. 2018;40;526-532.
From the Hospital of Central Connecticut, New Britain, CT (Dr. Caulfield and Dr. Shepard); Hartford Hospital, Hartford, CT (Dr. Linder and Dr. Dempsey); and the Hartford HealthCare Research Program, Hartford, CT (Dr. O’Sullivan).
Abstract
- Objective: To assess the utility of methicillin-resistant Staphylococcus aureus (MRSA) polymerase chain reaction (PCR) nasal swab testing in patients with lower respiratory tract infections (LRTI).
- Design and setting: Multicenter, retrospective, electronic chart review conducted within the Hartford HealthCare system.
- Participants: Patients who were treated for LRTIs at the Hospital of Central Connecticut or Hartford Hospital between July 1, 2018, and June 30, 2019.
- Measurements: The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing versus those who did not. In a subgroup analysis, we compared anti-MRSA DOT among patients with appropriate versus inappropriate utilization of the MRSA PCR test.
- Results: Of the 319 patients treated for LRTIs, 155 (48.6%) had a MRSA PCR ordered, and appropriate utilization occurred in 94 (60.6%) of these patients. Anti-MRSA DOT in the MRSA PCR group (n = 155) was shorter than in the group that did not undergo MRSA PCR testing (n = 164), but this difference did not reach statistical significance (1.68 days [interquartile range {IQR}, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458). In the subgroup analysis, anti-MRSA DOT was significantly shorter in the MRSA PCR group with appropriate utilization compared to the inappropriate utilization group (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76], P < 0.001)
- Conclusion: Appropriate utilization of MRSA PCR nasal swab testing can reduce DOT in patients with LRTI. Further education is necessary to expand the appropriate use of the MRSA PCR test across our health system.
Keywords: MRSA; LRTI; pneumonia; antimicrobial stewardship; antibiotic resistance.
More than 300,000 patients were hospitalized with methicillin-resistant Staphylococcus aureus (MRSA) infections in the United States in 2017, and at least 10,000 of these cases resulted in mortality.1 While MRSA infections overall are decreasing, it is crucial to continue to employ antimicrobial stewardship tactics to keep these infections at bay. Recently, strains of S. aureus have become resistant to vancomycin, making this bacterium even more difficult to treat.2
A novel tactic in antimicrobial stewardship involves the use of MRSA polymerase chain reaction (PCR) nasal swab testing to rule out the presence of MRSA in patients with lower respiratory tract infections (LRTI). If used appropriately, this approach may decrease the number of days patients are treated with anti-MRSA antimicrobials. Waiting for cultures to speciate can take up to 72 hours,3 meaning that patients may be exposed to 3 days of unnecessary broad-spectrum antibiotics. Results of MRSA PCR assay of nasal swab specimens can be available in 1 to 2 hours,4 allowing for more rapid de-escalation of therapy. Numerous studies have shown that this test has a negative predictive value (NPV) greater than 95%, indicating that a negative nasal swab result may be useful to guide de-escalation of antibiotic therapy.5-8 The purpose of this study was to assess the utility of MRSA PCR nasal swab testing in patients with LRTI throughout the Hartford HealthCare system.
Methods
Design
This study was a multicenter, retrospective, electronic chart review. It was approved by the Hartford HealthCare Institutional Review Board (HHC-2019-0169).
Selection of Participants
Patients were identified through electronic medical record reports based on ICD-10 codes. Records were categorized into 2 groups: patients who received a MRSA PCR nasal swab testing and patients who did not. Patients who received the MRSA PCR were further categorized by appropriate or inappropriate utilization. Appropriate utilization of the MRSA PCR was defined as MRSA PCR ordered within 48 hours of a new vancomycin or linezolid order, and anti-MRSA therapy discontinued within 24 hours of a negative result. Inappropriate utilization of the MRSA PCR was defined as MRSA PCR ordered more than 48 hours after a new vancomycin or linezolid order, or continuation of anti-MRSA therapy despite a negative MRSA PCR and no other evidence of a MRSA infection.
Patients were included if they met all of the following criteria: age 18 years or older, with no upper age limit; treated for a LRTI, identified by ICD-10 codes (J13-22, J44, J85); treated with empiric antibiotics active against MRSA, specifically vancomycin or linezolid; and treated at the Hospital of Central Connecticut (HOCC) or Hartford Hospital (HH) between July 1, 2018, and June 30, 2019, inclusive. Patients were excluded if they met 1 or more of the following criteria: age less than 18 years old; admitted for 48 hours or fewer or discharged from the emergency department; not treated at either facility; treated before July 1, 2018, or after June 30, 2019; treated for ventilator-associated pneumonia; received anti-MRSA therapy within 30 days prior to admission; or treated for a concurrent bacterial infection requiring anti-MRSA therapy.
Outcome Measures
The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing compared to patients who did not undergo MRSA PCR testing. A subgroup analysis was completed to compare anti-MRSA DOT within patients in the MRSA PCR group. Patients in the subgroup were categorized by appropriate or inappropriate utilization, and anti-MRSA DOT were compared between these groups. Secondary outcomes that were evaluated included length of stay (LOS), 30-day readmission rate, and incidence of acute kidney injury (AKI). Thirty-day readmission was defined as admission to HOCC, HH, or any institution within Hartford HealthCare within 30 days of discharge. AKI was defined as an increase in serum creatinine by ≥ 0.3 mg/dL in 48 hours, increase ≥ 1.5 times baseline, or a urine volume < 0.5 mL/kg/hr for 6 hours.
Statistical Analyses
The study was powered for the primary outcome, anti-MRSA DOT, with a clinically meaningful difference of 1 day. Group sample sizes of 240 in the MRSA PCR group and 160 in the no MRSA PCR group would have afforded 92% power to detect that difference, if the null hypothesis was that both group means were 4 days and the alternative hypothesis was that the mean of the MRSA PCR group was 3 days, with estimated group standard deviations of 80% of each mean. This estimate used an alpha level of 0.05 with a 2-sided t-test. Among those who received a MRSA PCR test, a clinically meaningful difference between appropriate and inappropriate utilization was 5%.
Descriptive statistics were provided for all variables as a function of the individual hospital and for the combined data set. Continuous data were summarized with means and standard deviations (SD), or with median and interquartile ranges (IQR), depending on distribution. Categorical variables were reported as frequencies, using percentages. All data were evaluated for normality of distribution. Inferential statistics comprised a Student’s t-test to compare normally distributed, continuous data between groups. Nonparametric distributions were compared using a Mann-Whitney U test. Categorical comparisons were made using a Fisher’s exact test for 2×2 tables and a Pearson chi-square test for comparisons involving more than 2 groups.
Since anti-MRSA DOT (primary outcome) and LOS (secondary outcome) are often non-normally distributed, they have been transformed (eg, log or square root, again depending on distribution). Whichever native variable or transformation variable was appropriate was used as the outcome measure in a linear regression model to account for the influence of factors (covariates) that show significant univariate differences. Given the relatively small sample size, a maximum of 10 variables were included in the model. All factors were iterated in a forward regression model (most influential first) until no significant changes were observed.
All calculations were performed with SPSS v. 21 (IBM; Armonk, NY) using an a priori alpha level of 0.05, such that all results yielding P < 0.05 were deemed statistically significant.
Results
Of the 561 patient records reviewed, 319 patients were included and 242 patients were excluded. Reasons for exclusion included 65 patients admitted for a duration of 48 hours or less or discharged from the emergency department; 61 patients having another infection requiring anti-MRSA therapy; 60 patients not having a diagnosis of a LRTI or not receiving anti-MRSA therapy; 52 patients having received anti-MRSA therapy within 30 days prior to admission; and 4 patients treated outside of the specified date range.
Of the 319 patients included, 155 (48.6%) were in the MRSA PCR group and 164 (51.4%) were in the group that did not undergo MRSA PCR (Table 1). Of the 155 patients with a MRSA PCR ordered, the test was utilized appropriately in 94 (60.6%) patients and inappropriately in 61 (39.4%) patients (Table 2). In the MRSA PCR group, 135 patients had a negative result on PCR assay, with 133 of those patients having negative respiratory cultures, resulting in a NPV of 98.5%. Differences in baseline characteristics between the MRSA PCR and no MRSA PCR groups were observed. The patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, as indicated by statistically significant differences in intensive care unit (ICU) admissions (P = 0.001), positive chest radiographs (P = 0.034), sepsis at time of anti-MRSA initiation (P = 0.013), pulmonary consults placed (P = 0.003), and carbapenem usage (P = 0.047).

In the subgroup analysis comparing appropriate and inappropriate utilization within the MRSA PCR group, the inappropriate utilization group had significantly higher numbers of infectious diseases consults placed, patients with hospital-acquired pneumonia, and patients with community-acquired pneumonia with risk factors.

Outcomes
Median anti-MRSA DOT in the MRSA PCR group was shorter than DOT in the no MRSA PCR group, but this difference did not reach statistical significance (1.68 [IQR, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458; Table 3). LOS in the MRSA PCR group was longer than in the no MRSA PCR group (6.0 [IQR, 4.0-10.0] vs 5.0 [IQR, 3.0-7.0] days, P = 0.001). There was no difference in 30-day readmissions that were related to the previous visit or incidence of AKI between groups.

In the subgroup analysis, anti-MRSA DOT in the MRSA PCR group with appropriate utilization was shorter than DOT in the MRSA PCR group with inappropriate utilization (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76] days, P < 0.001; Table 4). LOS in the MRSA PCR group with appropriate utilization was shorter than LOS in the inappropriate utilization group (5.0 [IQR, 4.0-7.0] vs 7.0 [IQR, 5.0-12.0] days, P < 0.001). Thirty-day readmissions that were related to the previous visit were significantly higher in patients in the MRSA PCR group with appropriate utilization (13 vs 2, P = 0.030). There was no difference in incidence of AKI between the groups.

A multivariate analysis was completed to determine whether the sicker MRSA PCR population was confounding outcomes, particularly the secondary outcome of LOS, which was noted to be longer in the MRSA PCR group (Table 5). When comparing LOS in the MRSA PCR and the no MRSA PCR patients, the multivariate analysis showed that admission to the ICU and carbapenem use were associated with a longer LOS (P < 0.001 and P = 0.009, respectively). The incidence of admission to the ICU and carbapenem use were higher in the MRSA PCR group (P = 0.001 and P = 0.047). Therefore, longer LOS in the MRSA PCR patients could be a result of the higher prevalence of ICU admissions and infections requiring carbapenem therapy rather than the result of the MRSA PCR itself.

Discussion
A MRSA PCR nasal swab protocol can be used to minimize a patient’s exposure to unnecessary broad-spectrum antibiotics, thereby preventing antimicrobial resistance. Thus, it is important to assess how our health system is utilizing this antimicrobial stewardship tactic. With the MRSA PCR’s high NPV, providers can be confident that MRSA pneumonia is unlikely in the absence of MRSA colonization. Our study established a NPV of 98.5%, which is similar to other studies, all of which have shown NPVs greater than 95%.5-8 Despite the high NPV, this study demonstrated that only 51.4% of patients with LRTI had orders for a MRSA PCR. Of the 155 patients with a MRSA PCR, the test was utilized appropriately only 60.6% of the time. A majority of the inappropriately utilized tests were due to MRSA PCR orders placed more than 48 hours after anti-MRSA therapy initiation. To our knowledge, no other studies have assessed the clinical utility of MRSA PCR nasal swabs as an antimicrobial stewardship tool in a diverse health system; therefore, these results are useful to guide future practices at our institution. There is a clear need for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing for patients with LRTI being treated with anti-MRSA therapy. Additionally, clinician education regarding the initial timing of the MRSA PCR order and the proper utilization of the results of the MRSA PCR likely will benefit patient outcomes at our institution.
When evaluating anti-MRSA DOT, this study demonstrated a reduction of only 0.18 days (about 4 hours) of anti-MRSA therapy in the patients who received MRSA PCR testing compared to the patients without a MRSA PCR ordered. Our anti-MRSA DOT reduction was lower than what has been reported in similar studies. For example, Baby et al found that the use of the MRSA PCR was associated with 46.6 fewer hours of unnecessary antimicrobial treatment. Willis et al evaluated a pharmacist-driven protocol that resulted in a reduction of 1.8 days of anti-MRSA therapy, despite a protocol compliance rate of only 55%.9,10 In our study, the patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, which may be the reason for the incongruences in our results compared to the current literature. Characteristics such as ICU admissions, positive chest radiographs, sepsis cases, pulmonary consults, and carbapenem usage—all of which are indicative of a sicker population—were more prevalent in the MRSA PCR group. This sicker population could have underestimated the reduction of DOT in the MRSA PCR group compared to the no MRSA PCR group.
After isolating the MRSA PCR patients in the subgroup analysis, anti-MRSA DOT was 1.5 days shorter when the test was appropriately utilized, which is more comparable to what has been reported in the literature.9,10 Only 60.6% of the MRSA PCR patients had their anti-MRSA therapy appropriately managed based on the MRSA PCR. Interestingly, a majority of patients in the inappropriate utilization group had MRSA PCR tests ordered more than 48 hours after beginning anti-MRSA therapy. More prompt and efficient ordering of the MRSA PCR may have resulted in more opportunities for earlier de-escalation of therapy. Due to these factors, the patients in the inappropriate utilization group could have further contributed to the underestimated difference in anti-MRSA DOT between the MRSA PCR and no MRSA PCR patients in the primary outcome. Additionally, there were no notable differences between the appropriate and inappropriate utilization groups, unlike in the MRSA PCR and no MRSA PCR groups, which is why we were able to draw more robust conclusions in the subgroup analysis. Therefore, the subgroup analysis confirmed that if the results of the MRSA PCR are used appropriately to guide anti-MRSA therapy, patients can potentially avoid 36 hours of broad-spectrum antibiotics.
Data on how the utilization of the MRSA PCR nasal swab can affect LOS are limited; however, one study did report a 2.8-day reduction in LOS after implementation of a pharmacist-driven MRSA PCR nasal swab protocol.11 Our study demonstrated that LOS was significantly longer in the MRSA PCR group than in the no MRSA PCR group. This result was likely affected by the aforementioned sicker MRSA PCR population. Our multivariate analysis further confirmed that ICU admissions were associated with a longer LOS, and, given that the MRSA PCR group had a significantly higher ICU population, this likely confounded these results. If our 2 groups had had more evenly distributed characteristics, it is possible that we could have found a shorter LOS in the MRSA PCR group, similar to what is reported in the literature. In the subgroup analysis, LOS was 2 days shorter in the appropriate utilization group compared to the inappropriate utilization group. This further affirms that the results of the MRSA PCR must be used appropriately in order for patient outcomes, like LOS, to benefit.
The effects of the MRSA PCR nasal swab on 30-day readmission rates and incidence of AKI are not well-documented in the literature. One study did report 30-day readmission rates as an outcome, but did not cite any difference after the implementation of a protocol that utilized MRSA PCR nasal swab testing.12 The outcome of AKI is slightly better represented in the literature, but the results are conflicting. Some studies report no difference after the implementation of a MRSA PCR-based protocol,11 and others report a significant decrease in AKI with the use of the MRSA PCR.9 Our study detected no difference in 30-day readmission rates related to the previous admission or in AKI between the MRSA PCR and no MRSA PCR populations. In the subgroup analysis, 30-day readmission rates were significantly higher in the MRSA PCR group with appropriate utilization than in the group with inappropriate utilization; however, our study was not powered to detect a difference in this secondary outcome.
This study had some limitations that may have affected our results. First, this study was a retrospective chart review. Additionally, the baseline characteristics were not well balanced across the different groups. There were sicker patients in the MRSA PCR group, which may have led to an underestimate of the reduction in DOT and LOS in these patients. Finally, we did not include enough patient records to reach power in the MRSA PCR group due to a higher than expected number of patients meeting exclusion criteria. Had we attained sufficient power, there may have been more profound reductions in DOT and LOS.
Conclusion
MRSA infections are a common cause for hospitalization, and there is a growing need for antimicrobial stewardship efforts to limit unnecessary antibiotic usage in order to prevent resistance. As illustrated in our study, appropriate utilization of the MRSA PCR can reduce DOT up to 1.5 days. However, our results suggest that there is room for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing in patients with LRTI receiving anti-MRSA therapy. Further emphasis on the appropriate utilization of the MRSA PCR within our health care system is essential.
Corresponding author: Casey Dempsey, PharmD, BCIDP, 80 Seymour St., Hartford, CT 06106; [email protected].
Financial disclosures: None.
From the Hospital of Central Connecticut, New Britain, CT (Dr. Caulfield and Dr. Shepard); Hartford Hospital, Hartford, CT (Dr. Linder and Dr. Dempsey); and the Hartford HealthCare Research Program, Hartford, CT (Dr. O’Sullivan).
Abstract
- Objective: To assess the utility of methicillin-resistant Staphylococcus aureus (MRSA) polymerase chain reaction (PCR) nasal swab testing in patients with lower respiratory tract infections (LRTI).
- Design and setting: Multicenter, retrospective, electronic chart review conducted within the Hartford HealthCare system.
- Participants: Patients who were treated for LRTIs at the Hospital of Central Connecticut or Hartford Hospital between July 1, 2018, and June 30, 2019.
- Measurements: The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing versus those who did not. In a subgroup analysis, we compared anti-MRSA DOT among patients with appropriate versus inappropriate utilization of the MRSA PCR test.
- Results: Of the 319 patients treated for LRTIs, 155 (48.6%) had a MRSA PCR ordered, and appropriate utilization occurred in 94 (60.6%) of these patients. Anti-MRSA DOT in the MRSA PCR group (n = 155) was shorter than in the group that did not undergo MRSA PCR testing (n = 164), but this difference did not reach statistical significance (1.68 days [interquartile range {IQR}, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458). In the subgroup analysis, anti-MRSA DOT was significantly shorter in the MRSA PCR group with appropriate utilization compared to the inappropriate utilization group (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76], P < 0.001)
- Conclusion: Appropriate utilization of MRSA PCR nasal swab testing can reduce DOT in patients with LRTI. Further education is necessary to expand the appropriate use of the MRSA PCR test across our health system.
Keywords: MRSA; LRTI; pneumonia; antimicrobial stewardship; antibiotic resistance.
More than 300,000 patients were hospitalized with methicillin-resistant Staphylococcus aureus (MRSA) infections in the United States in 2017, and at least 10,000 of these cases resulted in mortality.1 While MRSA infections overall are decreasing, it is crucial to continue to employ antimicrobial stewardship tactics to keep these infections at bay. Recently, strains of S. aureus have become resistant to vancomycin, making this bacterium even more difficult to treat.2
A novel tactic in antimicrobial stewardship involves the use of MRSA polymerase chain reaction (PCR) nasal swab testing to rule out the presence of MRSA in patients with lower respiratory tract infections (LRTI). If used appropriately, this approach may decrease the number of days patients are treated with anti-MRSA antimicrobials. Waiting for cultures to speciate can take up to 72 hours,3 meaning that patients may be exposed to 3 days of unnecessary broad-spectrum antibiotics. Results of MRSA PCR assay of nasal swab specimens can be available in 1 to 2 hours,4 allowing for more rapid de-escalation of therapy. Numerous studies have shown that this test has a negative predictive value (NPV) greater than 95%, indicating that a negative nasal swab result may be useful to guide de-escalation of antibiotic therapy.5-8 The purpose of this study was to assess the utility of MRSA PCR nasal swab testing in patients with LRTI throughout the Hartford HealthCare system.
Methods
Design
This study was a multicenter, retrospective, electronic chart review. It was approved by the Hartford HealthCare Institutional Review Board (HHC-2019-0169).
Selection of Participants
Patients were identified through electronic medical record reports based on ICD-10 codes. Records were categorized into 2 groups: patients who received a MRSA PCR nasal swab testing and patients who did not. Patients who received the MRSA PCR were further categorized by appropriate or inappropriate utilization. Appropriate utilization of the MRSA PCR was defined as MRSA PCR ordered within 48 hours of a new vancomycin or linezolid order, and anti-MRSA therapy discontinued within 24 hours of a negative result. Inappropriate utilization of the MRSA PCR was defined as MRSA PCR ordered more than 48 hours after a new vancomycin or linezolid order, or continuation of anti-MRSA therapy despite a negative MRSA PCR and no other evidence of a MRSA infection.
Patients were included if they met all of the following criteria: age 18 years or older, with no upper age limit; treated for a LRTI, identified by ICD-10 codes (J13-22, J44, J85); treated with empiric antibiotics active against MRSA, specifically vancomycin or linezolid; and treated at the Hospital of Central Connecticut (HOCC) or Hartford Hospital (HH) between July 1, 2018, and June 30, 2019, inclusive. Patients were excluded if they met 1 or more of the following criteria: age less than 18 years old; admitted for 48 hours or fewer or discharged from the emergency department; not treated at either facility; treated before July 1, 2018, or after June 30, 2019; treated for ventilator-associated pneumonia; received anti-MRSA therapy within 30 days prior to admission; or treated for a concurrent bacterial infection requiring anti-MRSA therapy.
Outcome Measures
The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing compared to patients who did not undergo MRSA PCR testing. A subgroup analysis was completed to compare anti-MRSA DOT within patients in the MRSA PCR group. Patients in the subgroup were categorized by appropriate or inappropriate utilization, and anti-MRSA DOT were compared between these groups. Secondary outcomes that were evaluated included length of stay (LOS), 30-day readmission rate, and incidence of acute kidney injury (AKI). Thirty-day readmission was defined as admission to HOCC, HH, or any institution within Hartford HealthCare within 30 days of discharge. AKI was defined as an increase in serum creatinine by ≥ 0.3 mg/dL in 48 hours, increase ≥ 1.5 times baseline, or a urine volume < 0.5 mL/kg/hr for 6 hours.
Statistical Analyses
The study was powered for the primary outcome, anti-MRSA DOT, with a clinically meaningful difference of 1 day. Group sample sizes of 240 in the MRSA PCR group and 160 in the no MRSA PCR group would have afforded 92% power to detect that difference, if the null hypothesis was that both group means were 4 days and the alternative hypothesis was that the mean of the MRSA PCR group was 3 days, with estimated group standard deviations of 80% of each mean. This estimate used an alpha level of 0.05 with a 2-sided t-test. Among those who received a MRSA PCR test, a clinically meaningful difference between appropriate and inappropriate utilization was 5%.
Descriptive statistics were provided for all variables as a function of the individual hospital and for the combined data set. Continuous data were summarized with means and standard deviations (SD), or with median and interquartile ranges (IQR), depending on distribution. Categorical variables were reported as frequencies, using percentages. All data were evaluated for normality of distribution. Inferential statistics comprised a Student’s t-test to compare normally distributed, continuous data between groups. Nonparametric distributions were compared using a Mann-Whitney U test. Categorical comparisons were made using a Fisher’s exact test for 2×2 tables and a Pearson chi-square test for comparisons involving more than 2 groups.
Since anti-MRSA DOT (primary outcome) and LOS (secondary outcome) are often non-normally distributed, they have been transformed (eg, log or square root, again depending on distribution). Whichever native variable or transformation variable was appropriate was used as the outcome measure in a linear regression model to account for the influence of factors (covariates) that show significant univariate differences. Given the relatively small sample size, a maximum of 10 variables were included in the model. All factors were iterated in a forward regression model (most influential first) until no significant changes were observed.
All calculations were performed with SPSS v. 21 (IBM; Armonk, NY) using an a priori alpha level of 0.05, such that all results yielding P < 0.05 were deemed statistically significant.
Results
Of the 561 patient records reviewed, 319 patients were included and 242 patients were excluded. Reasons for exclusion included 65 patients admitted for a duration of 48 hours or less or discharged from the emergency department; 61 patients having another infection requiring anti-MRSA therapy; 60 patients not having a diagnosis of a LRTI or not receiving anti-MRSA therapy; 52 patients having received anti-MRSA therapy within 30 days prior to admission; and 4 patients treated outside of the specified date range.
Of the 319 patients included, 155 (48.6%) were in the MRSA PCR group and 164 (51.4%) were in the group that did not undergo MRSA PCR (Table 1). Of the 155 patients with a MRSA PCR ordered, the test was utilized appropriately in 94 (60.6%) patients and inappropriately in 61 (39.4%) patients (Table 2). In the MRSA PCR group, 135 patients had a negative result on PCR assay, with 133 of those patients having negative respiratory cultures, resulting in a NPV of 98.5%. Differences in baseline characteristics between the MRSA PCR and no MRSA PCR groups were observed. The patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, as indicated by statistically significant differences in intensive care unit (ICU) admissions (P = 0.001), positive chest radiographs (P = 0.034), sepsis at time of anti-MRSA initiation (P = 0.013), pulmonary consults placed (P = 0.003), and carbapenem usage (P = 0.047).

In the subgroup analysis comparing appropriate and inappropriate utilization within the MRSA PCR group, the inappropriate utilization group had significantly higher numbers of infectious diseases consults placed, patients with hospital-acquired pneumonia, and patients with community-acquired pneumonia with risk factors.

Outcomes
Median anti-MRSA DOT in the MRSA PCR group was shorter than DOT in the no MRSA PCR group, but this difference did not reach statistical significance (1.68 [IQR, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458; Table 3). LOS in the MRSA PCR group was longer than in the no MRSA PCR group (6.0 [IQR, 4.0-10.0] vs 5.0 [IQR, 3.0-7.0] days, P = 0.001). There was no difference in 30-day readmissions that were related to the previous visit or incidence of AKI between groups.

In the subgroup analysis, anti-MRSA DOT in the MRSA PCR group with appropriate utilization was shorter than DOT in the MRSA PCR group with inappropriate utilization (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76] days, P < 0.001; Table 4). LOS in the MRSA PCR group with appropriate utilization was shorter than LOS in the inappropriate utilization group (5.0 [IQR, 4.0-7.0] vs 7.0 [IQR, 5.0-12.0] days, P < 0.001). Thirty-day readmissions that were related to the previous visit were significantly higher in patients in the MRSA PCR group with appropriate utilization (13 vs 2, P = 0.030). There was no difference in incidence of AKI between the groups.

A multivariate analysis was completed to determine whether the sicker MRSA PCR population was confounding outcomes, particularly the secondary outcome of LOS, which was noted to be longer in the MRSA PCR group (Table 5). When comparing LOS in the MRSA PCR and the no MRSA PCR patients, the multivariate analysis showed that admission to the ICU and carbapenem use were associated with a longer LOS (P < 0.001 and P = 0.009, respectively). The incidence of admission to the ICU and carbapenem use were higher in the MRSA PCR group (P = 0.001 and P = 0.047). Therefore, longer LOS in the MRSA PCR patients could be a result of the higher prevalence of ICU admissions and infections requiring carbapenem therapy rather than the result of the MRSA PCR itself.

Discussion
A MRSA PCR nasal swab protocol can be used to minimize a patient’s exposure to unnecessary broad-spectrum antibiotics, thereby preventing antimicrobial resistance. Thus, it is important to assess how our health system is utilizing this antimicrobial stewardship tactic. With the MRSA PCR’s high NPV, providers can be confident that MRSA pneumonia is unlikely in the absence of MRSA colonization. Our study established a NPV of 98.5%, which is similar to other studies, all of which have shown NPVs greater than 95%.5-8 Despite the high NPV, this study demonstrated that only 51.4% of patients with LRTI had orders for a MRSA PCR. Of the 155 patients with a MRSA PCR, the test was utilized appropriately only 60.6% of the time. A majority of the inappropriately utilized tests were due to MRSA PCR orders placed more than 48 hours after anti-MRSA therapy initiation. To our knowledge, no other studies have assessed the clinical utility of MRSA PCR nasal swabs as an antimicrobial stewardship tool in a diverse health system; therefore, these results are useful to guide future practices at our institution. There is a clear need for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing for patients with LRTI being treated with anti-MRSA therapy. Additionally, clinician education regarding the initial timing of the MRSA PCR order and the proper utilization of the results of the MRSA PCR likely will benefit patient outcomes at our institution.
When evaluating anti-MRSA DOT, this study demonstrated a reduction of only 0.18 days (about 4 hours) of anti-MRSA therapy in the patients who received MRSA PCR testing compared to the patients without a MRSA PCR ordered. Our anti-MRSA DOT reduction was lower than what has been reported in similar studies. For example, Baby et al found that the use of the MRSA PCR was associated with 46.6 fewer hours of unnecessary antimicrobial treatment. Willis et al evaluated a pharmacist-driven protocol that resulted in a reduction of 1.8 days of anti-MRSA therapy, despite a protocol compliance rate of only 55%.9,10 In our study, the patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, which may be the reason for the incongruences in our results compared to the current literature. Characteristics such as ICU admissions, positive chest radiographs, sepsis cases, pulmonary consults, and carbapenem usage—all of which are indicative of a sicker population—were more prevalent in the MRSA PCR group. This sicker population could have underestimated the reduction of DOT in the MRSA PCR group compared to the no MRSA PCR group.
After isolating the MRSA PCR patients in the subgroup analysis, anti-MRSA DOT was 1.5 days shorter when the test was appropriately utilized, which is more comparable to what has been reported in the literature.9,10 Only 60.6% of the MRSA PCR patients had their anti-MRSA therapy appropriately managed based on the MRSA PCR. Interestingly, a majority of patients in the inappropriate utilization group had MRSA PCR tests ordered more than 48 hours after beginning anti-MRSA therapy. More prompt and efficient ordering of the MRSA PCR may have resulted in more opportunities for earlier de-escalation of therapy. Due to these factors, the patients in the inappropriate utilization group could have further contributed to the underestimated difference in anti-MRSA DOT between the MRSA PCR and no MRSA PCR patients in the primary outcome. Additionally, there were no notable differences between the appropriate and inappropriate utilization groups, unlike in the MRSA PCR and no MRSA PCR groups, which is why we were able to draw more robust conclusions in the subgroup analysis. Therefore, the subgroup analysis confirmed that if the results of the MRSA PCR are used appropriately to guide anti-MRSA therapy, patients can potentially avoid 36 hours of broad-spectrum antibiotics.
Data on how the utilization of the MRSA PCR nasal swab can affect LOS are limited; however, one study did report a 2.8-day reduction in LOS after implementation of a pharmacist-driven MRSA PCR nasal swab protocol.11 Our study demonstrated that LOS was significantly longer in the MRSA PCR group than in the no MRSA PCR group. This result was likely affected by the aforementioned sicker MRSA PCR population. Our multivariate analysis further confirmed that ICU admissions were associated with a longer LOS, and, given that the MRSA PCR group had a significantly higher ICU population, this likely confounded these results. If our 2 groups had had more evenly distributed characteristics, it is possible that we could have found a shorter LOS in the MRSA PCR group, similar to what is reported in the literature. In the subgroup analysis, LOS was 2 days shorter in the appropriate utilization group compared to the inappropriate utilization group. This further affirms that the results of the MRSA PCR must be used appropriately in order for patient outcomes, like LOS, to benefit.
The effects of the MRSA PCR nasal swab on 30-day readmission rates and incidence of AKI are not well-documented in the literature. One study did report 30-day readmission rates as an outcome, but did not cite any difference after the implementation of a protocol that utilized MRSA PCR nasal swab testing.12 The outcome of AKI is slightly better represented in the literature, but the results are conflicting. Some studies report no difference after the implementation of a MRSA PCR-based protocol,11 and others report a significant decrease in AKI with the use of the MRSA PCR.9 Our study detected no difference in 30-day readmission rates related to the previous admission or in AKI between the MRSA PCR and no MRSA PCR populations. In the subgroup analysis, 30-day readmission rates were significantly higher in the MRSA PCR group with appropriate utilization than in the group with inappropriate utilization; however, our study was not powered to detect a difference in this secondary outcome.
This study had some limitations that may have affected our results. First, this study was a retrospective chart review. Additionally, the baseline characteristics were not well balanced across the different groups. There were sicker patients in the MRSA PCR group, which may have led to an underestimate of the reduction in DOT and LOS in these patients. Finally, we did not include enough patient records to reach power in the MRSA PCR group due to a higher than expected number of patients meeting exclusion criteria. Had we attained sufficient power, there may have been more profound reductions in DOT and LOS.
Conclusion
MRSA infections are a common cause for hospitalization, and there is a growing need for antimicrobial stewardship efforts to limit unnecessary antibiotic usage in order to prevent resistance. As illustrated in our study, appropriate utilization of the MRSA PCR can reduce DOT up to 1.5 days. However, our results suggest that there is room for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing in patients with LRTI receiving anti-MRSA therapy. Further emphasis on the appropriate utilization of the MRSA PCR within our health care system is essential.
Corresponding author: Casey Dempsey, PharmD, BCIDP, 80 Seymour St., Hartford, CT 06106; [email protected].
Financial disclosures: None.
1. Antimicrobial resistance threats. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest-threats.html. Accessed September 9, 2020.
2. Biggest threats and data. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest_threats.html#mrsa. Accessed September 9, 2020.
3. Smith MN, Erdman MJ, Ferreira JA, et al. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care. 2017;38:168-171.
4. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86:307-310.
5. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58:859-864.
6. Johnson JA, Wright ME, Sheperd LA, et al. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19: 34-36.
7. Dureau AF, Duclos G, Antonini F, et al. Rapid diagnostic test and use of antibiotic against methicillin-resistant Staphylococcus aureus in adult intensive care unit. Eur J Clin Microbiol Infect Dis. 2017;36:267-272.
8. Tilahun B, Faust AC, McCorstin P, Ortegon A. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care. 2015;24:8-12.
9. Baby N, Faust AC, Smith T, et al. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61:e02432-16.
10. Willis C, Allen B, Tucker C, et al. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am J Health-Syst Pharm. 2017;74:1765-1773.
11. Dadzie P, Dietrich T, Ashurst J. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus polymerase chain reaction nasal swab protocol on the de-escalation of empiric vancomycin in patients with pneumonia in a rural healthcare setting. Cureus. 2019;11:e6378
12. Dunaway S, Orwig KW, Arbogast ZQ, et al. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int J Clin Pharm. 2018;40;526-532.
1. Antimicrobial resistance threats. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest-threats.html. Accessed September 9, 2020.
2. Biggest threats and data. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest_threats.html#mrsa. Accessed September 9, 2020.
3. Smith MN, Erdman MJ, Ferreira JA, et al. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care. 2017;38:168-171.
4. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86:307-310.
5. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58:859-864.
6. Johnson JA, Wright ME, Sheperd LA, et al. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19: 34-36.
7. Dureau AF, Duclos G, Antonini F, et al. Rapid diagnostic test and use of antibiotic against methicillin-resistant Staphylococcus aureus in adult intensive care unit. Eur J Clin Microbiol Infect Dis. 2017;36:267-272.
8. Tilahun B, Faust AC, McCorstin P, Ortegon A. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care. 2015;24:8-12.
9. Baby N, Faust AC, Smith T, et al. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61:e02432-16.
10. Willis C, Allen B, Tucker C, et al. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am J Health-Syst Pharm. 2017;74:1765-1773.
11. Dadzie P, Dietrich T, Ashurst J. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus polymerase chain reaction nasal swab protocol on the de-escalation of empiric vancomycin in patients with pneumonia in a rural healthcare setting. Cureus. 2019;11:e6378
12. Dunaway S, Orwig KW, Arbogast ZQ, et al. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int J Clin Pharm. 2018;40;526-532.
Systemic Corticosteroids in Critically Ill Patients With COVID-19
Study Overview
Objective. To assess the association between administration of systemic corticosteroids, compared with usual care or placebo, and 28-day all-cause mortality in critically ill patients with coronavirus disease 2019 (COVID-19).
Design. Prospective meta-analysis with data from 7 randomized clinical trials conducted in 12 countries.
Setting and participants. This prospective meta-analysis included randomized clinical trials conducted between February 26, 2020, and June 9, 2020, that examined the clinical efficacy of administration of corticosteroids in hospitalized COVID-19 patients who were critically ill. Trials were systematically identified from ClinicalTrials.gov, the Chinese Clinical Trial Registry, and the EU Clinical Trials Register, using the search terms COVID-19, corticosteroids, and steroids. Additional trials were identified by experts from the WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Senior investigators of these identified trials were asked to participate in weekly calls to develop a protocol for the prospective meta-analysis.1 Subsequently, trials that had randomly assigned critically ill patients to receive corticosteroids versus usual care or placebo were invited to participate in this meta-analysis. Data were pooled from patients recruited to the participating trials through June 9, 2020, and aggregated in overall and in predefined subgroups.
Main outcome measures. The primary outcome was all-cause mortality up to 30 days after randomization. Because 5 of the included trials reported mortality at 28 days after randomization, the primary outcome was reported as 28-day all-cause mortality. The secondary outcome was serious adverse events (SAEs). The authors also gathered data on the demographic and clinical characteristics of patients, the number of patients lost to follow-up, and outcomes according to intervention group, overall, and in subgroups (ie, patients receiving invasive mechanical ventilation or vasoactive medication; age ≤ 60 years or > 60 years [the median across trials]; sex [male or female]; and the duration patients were symptomatic [≤ 7 days or > 7 days]). For each trial, the risk of bias was assessed independently by 4 investigators using the Cochrane Risk of Bias Assessment Tool for the overall effects of corticosteroids on mortality and SAEs and the effect of assignment and allocated interventions. Inconsistency between trial results was evaluated using the I2 statistic. The trials were classified according to the corticosteroids used in the intervention group and the dose administered using a priori-defined cutoffs (15 mg/day of dexamethasone, 400 mg/day of hydrocortisone, and 1 mg/kg/day of methylprednisolone). The primary analysis utilized was an inverse variance-weighted fixed-effect meta-analysis of odds ratios (ORs) for overall mortality. Random-effects meta-analyses with Paule-Mandel estimate of heterogeneity were also performed.
Main results. Seven trials (DEXA-COVID 19, CoDEX, RECOVERY, CAPE COVID, COVID STEROID, REMAP-CAP, and Steroids-SARI) were included in the final meta-analysis. The enrolled patients were from Australia, Brazil, Canada, China, Denmark, France, Ireland, the Netherlands, New Zealand, Spain, the United Kingdom, and the United States. The date of final follow-up was July 6, 2020. The corticosteroids groups included dexamethasone at low (6 mg/day orally or intravenously [IV]) and high (20 mg/day IV) doses; low-dose hydrocortisone (200 mg/day IV or 50 mg every 6 hr IV); and high-dose methylprednisolone (40 mg every 12 hr IV). In total, 1703 patients were randomized, with 678 assigned to the corticosteroids group and 1025 to the usual-care or placebo group. The median age of patients was 60 years (interquartile range, 52-68 years), and 29% were women. The larger number of patients in the usual-care/placebo group was a result of the 1:2 randomization (corticosteroids versus usual care or placebo) in the RECOVERY trial, which contributed 59.1% of patients included in this prospective meta-analysis. The majority of patients were receiving invasive mechanical ventilation at randomization (1559 patients). The administration of adjunctive treatments, such as azithromycin or antiviral agents, varied among the trials. The risk of bias was determined as low for 6 of the 7 mortality results.
A total of 222 of 678 patients in the corticosteroids group died, and 425 of 1025 patients in the usual care or placebo group died. The summary OR was 0.66 (95% confidence interval [CI], 0.53-0.82; P < 0.001) based on a fixed-effect meta-analysis, and 0.70 (95% CI, 0.48-1.01; P = 0.053) based on the random-effects meta-analysis, for 28-day all-cause mortality comparing all corticosteroids with usual care or placebo. There was little inconsistency between trial results (I2 = 15.6%; P = 0.31). The fixed-effect summary OR for the association with 28-day all-cause mortality was 0.64 (95% CI, 0.50-0.82; P < 0.001) for dexamethasone compared with usual care or placebo (3 trials, 1282 patients, and 527 deaths); the OR was 0.69 (95% CI, 0.43-1.12; P = 0.13) for hydrocortisone (3 trials, 374 patients, and 94 deaths); and the OR was 0.91 (95% CI, 0.29-2.87; P = 0.87) for methylprednisolone (1 trial, 47 patients, and 26 deaths). Moreover, in trials that administered low-dose corticosteroids, the overall fixed-effect OR for 28-day all-cause mortality was 0.61 (95% CI, 0.48-0.78; P < 0.001). In the subgroup analysis, the overall fixed-effect OR was 0.69 (95% CI, 0.55-0.86) in patients who were receiving invasive mechanical ventilation at randomization, and the OR was 0.41 (95% CI, 0.19-0.88) in patients who were not receiving invasive mechanical ventilation at randomization.
Six trials (all except the RECOVERY trial) reported SAEs, with 64 events occurring among 354 patients assigned to the corticosteroids group and 80 SAEs occurring among 342 patients assigned to the usual-care or placebo group. There was no suggestion that the risk of SAEs was higher in patients who were administered corticosteroids.
Conclusion. The administration of systemic corticosteroids was associated with a lower 28-day all-cause mortality in critically ill patients with COVID-19 compared to those who received usual care or placebo.
Commentary
Corticosteroids are anti-inflammatory and vasoconstrictive medications that have long been used in intensive care units for the treatment of acute respiratory distress syndrome and septic shock. However, the therapeutic role of corticosteroids for treating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was uncertain at the outset of the COVID-19 pandemic due to concerns that this class of medications may cause an impaired immune response in the setting of a life-threatening SARS-CoV-2 infection. Evidence supporting this notion included prior studies showing that corticosteroid therapy was associated with delayed viral clearance of Middle East respiratory syndrome or a higher viral load of SARS-CoV.2,3 The uncertainty surrounding the therapeutic use of corticosteroids in treating COVID-19 led to a simultaneous global effort to conduct randomized controlled trials to urgently examine this important clinical question. The open-label Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial, conducted in the UK, was the first large-scale randomized clinical trial that reported the clinical benefit of corticosteroids in treating patients hospitalized with COVID-19. Specifically, it showed that low-dose dexamethasone (6 mg/day) administered orally or IV for up to 10 days resulted in a 2.8% absolute reduction in 28-day mortality, with the greatest benefit, an absolute risk reduction of 12.1%, conferred to patients who were receiving invasive mechanical ventilation at the time of randomization.4 In response to these findings, the National Institutes of Health COVID-19 Treatment Guidelines Panel recommended the use of dexamethasone in patients with COVID-19 who are on mechanical ventilation or who require supplemental oxygen, and recommended against the use of dexamethasone for those not requiring supplemental oxygen.5
The meta-analysis discussed in this commentary, conducted by the WHO REACT Working Group, has replicated initial findings from the RECOVERY trial. This prospective meta-analysis pooled data from 7 randomized controlled trials of corticosteroid therapy in 1703 critically ill patients hospitalized with COVID-19. Similar to findings from the RECOVERY trial, corticosteroids were associated with lower all-cause mortality at 28 days after randomization, and this benefit was observed both in critically ill patients who were receiving mechanical ventilation or supplemental oxygen without mechanical ventilation. Interestingly, while the OR estimates were imprecise, the reduction in mortality rates was similar between patients who were administered dexamethasone and hydrocortisone, which may suggest a general drug class effect. In addition, the mortality benefit of corticosteroids appeared similar for those aged ≤ 60 years and those aged > 60 years, between female and male patients, and those who were symptomatic for ≤ 7 days or > 7 days before randomization. Moreover, the administration of corticosteroids did not appear to increase the risk of SAEs. While more data are needed, results from the RECOVERY trial and this prospective meta-analysis indicate that corticosteroids should be an essential pharmacologic treatment for COVID-19, and suggest its potential role as a standard of care for critically ill patients with COVID-19.
This study has several limitations. First, not all trials systematically identified participated in the meta-analysis. Second, long-term outcomes after hospital discharge were not captured, and thus the effect of corticosteroids on long-term mortality and other adverse outcomes, such as hospital readmission, remain unknown. Third, because children were excluded from study participation, the effect of corticosteroids on pediatric COVID-19 patients is unknown. Fourth, the RECOVERY trial contributed more than 50% of patients in the current analysis, although there was little inconsistency in the effects of corticosteroids on mortality between individual trials. Last, the meta-analysis was unable to establish the optimal dose or duration of corticosteroid intervention in critically ill COVID-19 patients, or determine its efficacy in patients with mild-to-moderate COVID-19, all of which are key clinical questions that will need to be addressed with further clinical investigations.
The development of effective treatments for COVID-19 is critical to mitigating the devastating consequences of SARS-CoV-2 infection. Several recent COVID-19 clinical trials have shown promise in this endeavor. For instance, the Adaptive COVID-19 Treatment Trial (ACCT-1) found that intravenous remdesivir, as compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.6 Moreover, there is some evidence to suggest that convalescent plasma and aerosol inhalation of IFN-κ may have beneficial effects in treating COVID-19.7,8 Thus, clinical trials designed to investigate combination therapy approaches including corticosteroids, remdesivir, convalescent plasma, and others are urgently needed to help identify interventions that most effectively treat COVID-19.
Applications for Clinical Practice
The use of corticosteroids in critically ill patients with COVID-19 reduces overall mortality. This treatment is inexpensive and available in most care settings, including low-resource regions, and provides hope for better outcomes in the COVID-19 pandemic.
Katerina Oikonomou, MD, PhD
General Hospital of Larissa, Larissa, Greece
Fred Ko, MD, MS
1. Sterne JAC, Diaz J, Villar J, et al. Corticosteroid therapy for critically ill patients with COVID-19: A structured summary of a study protocol for a prospective meta-analysis of randomized trials. Trials. 2020;21:734.
2. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304-309.
3. Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for citically Ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757-767.
4. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report [published online ahead of print, 2020 Jul 17]. N Engl J Med. 2020;NEJMoa2021436.
5. NIH COVID-19 Treatment Guidelines. National Institutes of Health. www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/. Accessed September 11, 2020.
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19--preliminary report [published online ahead of print, 2020 May 22]. N Engl J Med. 2020;NEJMoa2007764.
7. Casadevall A, Joyner MJ, Pirofski LA. A randomized trial of convalescent plasma for covid-19-potentially hopeful signals. JAMA. 2020;324:455-457.
8. Fu W, Liu Y, Xia L, et al. A clinical pilot study on the safety and efficacy of aerosol inhalation treatment of IFN-κ plus TFF2 in patients with moderate COVID-19. EClinicalMedicine. 2020;25:100478.
Study Overview
Objective. To assess the association between administration of systemic corticosteroids, compared with usual care or placebo, and 28-day all-cause mortality in critically ill patients with coronavirus disease 2019 (COVID-19).
Design. Prospective meta-analysis with data from 7 randomized clinical trials conducted in 12 countries.
Setting and participants. This prospective meta-analysis included randomized clinical trials conducted between February 26, 2020, and June 9, 2020, that examined the clinical efficacy of administration of corticosteroids in hospitalized COVID-19 patients who were critically ill. Trials were systematically identified from ClinicalTrials.gov, the Chinese Clinical Trial Registry, and the EU Clinical Trials Register, using the search terms COVID-19, corticosteroids, and steroids. Additional trials were identified by experts from the WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Senior investigators of these identified trials were asked to participate in weekly calls to develop a protocol for the prospective meta-analysis.1 Subsequently, trials that had randomly assigned critically ill patients to receive corticosteroids versus usual care or placebo were invited to participate in this meta-analysis. Data were pooled from patients recruited to the participating trials through June 9, 2020, and aggregated in overall and in predefined subgroups.
Main outcome measures. The primary outcome was all-cause mortality up to 30 days after randomization. Because 5 of the included trials reported mortality at 28 days after randomization, the primary outcome was reported as 28-day all-cause mortality. The secondary outcome was serious adverse events (SAEs). The authors also gathered data on the demographic and clinical characteristics of patients, the number of patients lost to follow-up, and outcomes according to intervention group, overall, and in subgroups (ie, patients receiving invasive mechanical ventilation or vasoactive medication; age ≤ 60 years or > 60 years [the median across trials]; sex [male or female]; and the duration patients were symptomatic [≤ 7 days or > 7 days]). For each trial, the risk of bias was assessed independently by 4 investigators using the Cochrane Risk of Bias Assessment Tool for the overall effects of corticosteroids on mortality and SAEs and the effect of assignment and allocated interventions. Inconsistency between trial results was evaluated using the I2 statistic. The trials were classified according to the corticosteroids used in the intervention group and the dose administered using a priori-defined cutoffs (15 mg/day of dexamethasone, 400 mg/day of hydrocortisone, and 1 mg/kg/day of methylprednisolone). The primary analysis utilized was an inverse variance-weighted fixed-effect meta-analysis of odds ratios (ORs) for overall mortality. Random-effects meta-analyses with Paule-Mandel estimate of heterogeneity were also performed.
Main results. Seven trials (DEXA-COVID 19, CoDEX, RECOVERY, CAPE COVID, COVID STEROID, REMAP-CAP, and Steroids-SARI) were included in the final meta-analysis. The enrolled patients were from Australia, Brazil, Canada, China, Denmark, France, Ireland, the Netherlands, New Zealand, Spain, the United Kingdom, and the United States. The date of final follow-up was July 6, 2020. The corticosteroids groups included dexamethasone at low (6 mg/day orally or intravenously [IV]) and high (20 mg/day IV) doses; low-dose hydrocortisone (200 mg/day IV or 50 mg every 6 hr IV); and high-dose methylprednisolone (40 mg every 12 hr IV). In total, 1703 patients were randomized, with 678 assigned to the corticosteroids group and 1025 to the usual-care or placebo group. The median age of patients was 60 years (interquartile range, 52-68 years), and 29% were women. The larger number of patients in the usual-care/placebo group was a result of the 1:2 randomization (corticosteroids versus usual care or placebo) in the RECOVERY trial, which contributed 59.1% of patients included in this prospective meta-analysis. The majority of patients were receiving invasive mechanical ventilation at randomization (1559 patients). The administration of adjunctive treatments, such as azithromycin or antiviral agents, varied among the trials. The risk of bias was determined as low for 6 of the 7 mortality results.
A total of 222 of 678 patients in the corticosteroids group died, and 425 of 1025 patients in the usual care or placebo group died. The summary OR was 0.66 (95% confidence interval [CI], 0.53-0.82; P < 0.001) based on a fixed-effect meta-analysis, and 0.70 (95% CI, 0.48-1.01; P = 0.053) based on the random-effects meta-analysis, for 28-day all-cause mortality comparing all corticosteroids with usual care or placebo. There was little inconsistency between trial results (I2 = 15.6%; P = 0.31). The fixed-effect summary OR for the association with 28-day all-cause mortality was 0.64 (95% CI, 0.50-0.82; P < 0.001) for dexamethasone compared with usual care or placebo (3 trials, 1282 patients, and 527 deaths); the OR was 0.69 (95% CI, 0.43-1.12; P = 0.13) for hydrocortisone (3 trials, 374 patients, and 94 deaths); and the OR was 0.91 (95% CI, 0.29-2.87; P = 0.87) for methylprednisolone (1 trial, 47 patients, and 26 deaths). Moreover, in trials that administered low-dose corticosteroids, the overall fixed-effect OR for 28-day all-cause mortality was 0.61 (95% CI, 0.48-0.78; P < 0.001). In the subgroup analysis, the overall fixed-effect OR was 0.69 (95% CI, 0.55-0.86) in patients who were receiving invasive mechanical ventilation at randomization, and the OR was 0.41 (95% CI, 0.19-0.88) in patients who were not receiving invasive mechanical ventilation at randomization.
Six trials (all except the RECOVERY trial) reported SAEs, with 64 events occurring among 354 patients assigned to the corticosteroids group and 80 SAEs occurring among 342 patients assigned to the usual-care or placebo group. There was no suggestion that the risk of SAEs was higher in patients who were administered corticosteroids.
Conclusion. The administration of systemic corticosteroids was associated with a lower 28-day all-cause mortality in critically ill patients with COVID-19 compared to those who received usual care or placebo.
Commentary
Corticosteroids are anti-inflammatory and vasoconstrictive medications that have long been used in intensive care units for the treatment of acute respiratory distress syndrome and septic shock. However, the therapeutic role of corticosteroids for treating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was uncertain at the outset of the COVID-19 pandemic due to concerns that this class of medications may cause an impaired immune response in the setting of a life-threatening SARS-CoV-2 infection. Evidence supporting this notion included prior studies showing that corticosteroid therapy was associated with delayed viral clearance of Middle East respiratory syndrome or a higher viral load of SARS-CoV.2,3 The uncertainty surrounding the therapeutic use of corticosteroids in treating COVID-19 led to a simultaneous global effort to conduct randomized controlled trials to urgently examine this important clinical question. The open-label Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial, conducted in the UK, was the first large-scale randomized clinical trial that reported the clinical benefit of corticosteroids in treating patients hospitalized with COVID-19. Specifically, it showed that low-dose dexamethasone (6 mg/day) administered orally or IV for up to 10 days resulted in a 2.8% absolute reduction in 28-day mortality, with the greatest benefit, an absolute risk reduction of 12.1%, conferred to patients who were receiving invasive mechanical ventilation at the time of randomization.4 In response to these findings, the National Institutes of Health COVID-19 Treatment Guidelines Panel recommended the use of dexamethasone in patients with COVID-19 who are on mechanical ventilation or who require supplemental oxygen, and recommended against the use of dexamethasone for those not requiring supplemental oxygen.5
The meta-analysis discussed in this commentary, conducted by the WHO REACT Working Group, has replicated initial findings from the RECOVERY trial. This prospective meta-analysis pooled data from 7 randomized controlled trials of corticosteroid therapy in 1703 critically ill patients hospitalized with COVID-19. Similar to findings from the RECOVERY trial, corticosteroids were associated with lower all-cause mortality at 28 days after randomization, and this benefit was observed both in critically ill patients who were receiving mechanical ventilation or supplemental oxygen without mechanical ventilation. Interestingly, while the OR estimates were imprecise, the reduction in mortality rates was similar between patients who were administered dexamethasone and hydrocortisone, which may suggest a general drug class effect. In addition, the mortality benefit of corticosteroids appeared similar for those aged ≤ 60 years and those aged > 60 years, between female and male patients, and those who were symptomatic for ≤ 7 days or > 7 days before randomization. Moreover, the administration of corticosteroids did not appear to increase the risk of SAEs. While more data are needed, results from the RECOVERY trial and this prospective meta-analysis indicate that corticosteroids should be an essential pharmacologic treatment for COVID-19, and suggest its potential role as a standard of care for critically ill patients with COVID-19.
This study has several limitations. First, not all trials systematically identified participated in the meta-analysis. Second, long-term outcomes after hospital discharge were not captured, and thus the effect of corticosteroids on long-term mortality and other adverse outcomes, such as hospital readmission, remain unknown. Third, because children were excluded from study participation, the effect of corticosteroids on pediatric COVID-19 patients is unknown. Fourth, the RECOVERY trial contributed more than 50% of patients in the current analysis, although there was little inconsistency in the effects of corticosteroids on mortality between individual trials. Last, the meta-analysis was unable to establish the optimal dose or duration of corticosteroid intervention in critically ill COVID-19 patients, or determine its efficacy in patients with mild-to-moderate COVID-19, all of which are key clinical questions that will need to be addressed with further clinical investigations.
The development of effective treatments for COVID-19 is critical to mitigating the devastating consequences of SARS-CoV-2 infection. Several recent COVID-19 clinical trials have shown promise in this endeavor. For instance, the Adaptive COVID-19 Treatment Trial (ACCT-1) found that intravenous remdesivir, as compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.6 Moreover, there is some evidence to suggest that convalescent plasma and aerosol inhalation of IFN-κ may have beneficial effects in treating COVID-19.7,8 Thus, clinical trials designed to investigate combination therapy approaches including corticosteroids, remdesivir, convalescent plasma, and others are urgently needed to help identify interventions that most effectively treat COVID-19.
Applications for Clinical Practice
The use of corticosteroids in critically ill patients with COVID-19 reduces overall mortality. This treatment is inexpensive and available in most care settings, including low-resource regions, and provides hope for better outcomes in the COVID-19 pandemic.
Katerina Oikonomou, MD, PhD
General Hospital of Larissa, Larissa, Greece
Fred Ko, MD, MS
Study Overview
Objective. To assess the association between administration of systemic corticosteroids, compared with usual care or placebo, and 28-day all-cause mortality in critically ill patients with coronavirus disease 2019 (COVID-19).
Design. Prospective meta-analysis with data from 7 randomized clinical trials conducted in 12 countries.
Setting and participants. This prospective meta-analysis included randomized clinical trials conducted between February 26, 2020, and June 9, 2020, that examined the clinical efficacy of administration of corticosteroids in hospitalized COVID-19 patients who were critically ill. Trials were systematically identified from ClinicalTrials.gov, the Chinese Clinical Trial Registry, and the EU Clinical Trials Register, using the search terms COVID-19, corticosteroids, and steroids. Additional trials were identified by experts from the WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Senior investigators of these identified trials were asked to participate in weekly calls to develop a protocol for the prospective meta-analysis.1 Subsequently, trials that had randomly assigned critically ill patients to receive corticosteroids versus usual care or placebo were invited to participate in this meta-analysis. Data were pooled from patients recruited to the participating trials through June 9, 2020, and aggregated in overall and in predefined subgroups.
Main outcome measures. The primary outcome was all-cause mortality up to 30 days after randomization. Because 5 of the included trials reported mortality at 28 days after randomization, the primary outcome was reported as 28-day all-cause mortality. The secondary outcome was serious adverse events (SAEs). The authors also gathered data on the demographic and clinical characteristics of patients, the number of patients lost to follow-up, and outcomes according to intervention group, overall, and in subgroups (ie, patients receiving invasive mechanical ventilation or vasoactive medication; age ≤ 60 years or > 60 years [the median across trials]; sex [male or female]; and the duration patients were symptomatic [≤ 7 days or > 7 days]). For each trial, the risk of bias was assessed independently by 4 investigators using the Cochrane Risk of Bias Assessment Tool for the overall effects of corticosteroids on mortality and SAEs and the effect of assignment and allocated interventions. Inconsistency between trial results was evaluated using the I2 statistic. The trials were classified according to the corticosteroids used in the intervention group and the dose administered using a priori-defined cutoffs (15 mg/day of dexamethasone, 400 mg/day of hydrocortisone, and 1 mg/kg/day of methylprednisolone). The primary analysis utilized was an inverse variance-weighted fixed-effect meta-analysis of odds ratios (ORs) for overall mortality. Random-effects meta-analyses with Paule-Mandel estimate of heterogeneity were also performed.
Main results. Seven trials (DEXA-COVID 19, CoDEX, RECOVERY, CAPE COVID, COVID STEROID, REMAP-CAP, and Steroids-SARI) were included in the final meta-analysis. The enrolled patients were from Australia, Brazil, Canada, China, Denmark, France, Ireland, the Netherlands, New Zealand, Spain, the United Kingdom, and the United States. The date of final follow-up was July 6, 2020. The corticosteroids groups included dexamethasone at low (6 mg/day orally or intravenously [IV]) and high (20 mg/day IV) doses; low-dose hydrocortisone (200 mg/day IV or 50 mg every 6 hr IV); and high-dose methylprednisolone (40 mg every 12 hr IV). In total, 1703 patients were randomized, with 678 assigned to the corticosteroids group and 1025 to the usual-care or placebo group. The median age of patients was 60 years (interquartile range, 52-68 years), and 29% were women. The larger number of patients in the usual-care/placebo group was a result of the 1:2 randomization (corticosteroids versus usual care or placebo) in the RECOVERY trial, which contributed 59.1% of patients included in this prospective meta-analysis. The majority of patients were receiving invasive mechanical ventilation at randomization (1559 patients). The administration of adjunctive treatments, such as azithromycin or antiviral agents, varied among the trials. The risk of bias was determined as low for 6 of the 7 mortality results.
A total of 222 of 678 patients in the corticosteroids group died, and 425 of 1025 patients in the usual care or placebo group died. The summary OR was 0.66 (95% confidence interval [CI], 0.53-0.82; P < 0.001) based on a fixed-effect meta-analysis, and 0.70 (95% CI, 0.48-1.01; P = 0.053) based on the random-effects meta-analysis, for 28-day all-cause mortality comparing all corticosteroids with usual care or placebo. There was little inconsistency between trial results (I2 = 15.6%; P = 0.31). The fixed-effect summary OR for the association with 28-day all-cause mortality was 0.64 (95% CI, 0.50-0.82; P < 0.001) for dexamethasone compared with usual care or placebo (3 trials, 1282 patients, and 527 deaths); the OR was 0.69 (95% CI, 0.43-1.12; P = 0.13) for hydrocortisone (3 trials, 374 patients, and 94 deaths); and the OR was 0.91 (95% CI, 0.29-2.87; P = 0.87) for methylprednisolone (1 trial, 47 patients, and 26 deaths). Moreover, in trials that administered low-dose corticosteroids, the overall fixed-effect OR for 28-day all-cause mortality was 0.61 (95% CI, 0.48-0.78; P < 0.001). In the subgroup analysis, the overall fixed-effect OR was 0.69 (95% CI, 0.55-0.86) in patients who were receiving invasive mechanical ventilation at randomization, and the OR was 0.41 (95% CI, 0.19-0.88) in patients who were not receiving invasive mechanical ventilation at randomization.
Six trials (all except the RECOVERY trial) reported SAEs, with 64 events occurring among 354 patients assigned to the corticosteroids group and 80 SAEs occurring among 342 patients assigned to the usual-care or placebo group. There was no suggestion that the risk of SAEs was higher in patients who were administered corticosteroids.
Conclusion. The administration of systemic corticosteroids was associated with a lower 28-day all-cause mortality in critically ill patients with COVID-19 compared to those who received usual care or placebo.
Commentary
Corticosteroids are anti-inflammatory and vasoconstrictive medications that have long been used in intensive care units for the treatment of acute respiratory distress syndrome and septic shock. However, the therapeutic role of corticosteroids for treating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was uncertain at the outset of the COVID-19 pandemic due to concerns that this class of medications may cause an impaired immune response in the setting of a life-threatening SARS-CoV-2 infection. Evidence supporting this notion included prior studies showing that corticosteroid therapy was associated with delayed viral clearance of Middle East respiratory syndrome or a higher viral load of SARS-CoV.2,3 The uncertainty surrounding the therapeutic use of corticosteroids in treating COVID-19 led to a simultaneous global effort to conduct randomized controlled trials to urgently examine this important clinical question. The open-label Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial, conducted in the UK, was the first large-scale randomized clinical trial that reported the clinical benefit of corticosteroids in treating patients hospitalized with COVID-19. Specifically, it showed that low-dose dexamethasone (6 mg/day) administered orally or IV for up to 10 days resulted in a 2.8% absolute reduction in 28-day mortality, with the greatest benefit, an absolute risk reduction of 12.1%, conferred to patients who were receiving invasive mechanical ventilation at the time of randomization.4 In response to these findings, the National Institutes of Health COVID-19 Treatment Guidelines Panel recommended the use of dexamethasone in patients with COVID-19 who are on mechanical ventilation or who require supplemental oxygen, and recommended against the use of dexamethasone for those not requiring supplemental oxygen.5
The meta-analysis discussed in this commentary, conducted by the WHO REACT Working Group, has replicated initial findings from the RECOVERY trial. This prospective meta-analysis pooled data from 7 randomized controlled trials of corticosteroid therapy in 1703 critically ill patients hospitalized with COVID-19. Similar to findings from the RECOVERY trial, corticosteroids were associated with lower all-cause mortality at 28 days after randomization, and this benefit was observed both in critically ill patients who were receiving mechanical ventilation or supplemental oxygen without mechanical ventilation. Interestingly, while the OR estimates were imprecise, the reduction in mortality rates was similar between patients who were administered dexamethasone and hydrocortisone, which may suggest a general drug class effect. In addition, the mortality benefit of corticosteroids appeared similar for those aged ≤ 60 years and those aged > 60 years, between female and male patients, and those who were symptomatic for ≤ 7 days or > 7 days before randomization. Moreover, the administration of corticosteroids did not appear to increase the risk of SAEs. While more data are needed, results from the RECOVERY trial and this prospective meta-analysis indicate that corticosteroids should be an essential pharmacologic treatment for COVID-19, and suggest its potential role as a standard of care for critically ill patients with COVID-19.
This study has several limitations. First, not all trials systematically identified participated in the meta-analysis. Second, long-term outcomes after hospital discharge were not captured, and thus the effect of corticosteroids on long-term mortality and other adverse outcomes, such as hospital readmission, remain unknown. Third, because children were excluded from study participation, the effect of corticosteroids on pediatric COVID-19 patients is unknown. Fourth, the RECOVERY trial contributed more than 50% of patients in the current analysis, although there was little inconsistency in the effects of corticosteroids on mortality between individual trials. Last, the meta-analysis was unable to establish the optimal dose or duration of corticosteroid intervention in critically ill COVID-19 patients, or determine its efficacy in patients with mild-to-moderate COVID-19, all of which are key clinical questions that will need to be addressed with further clinical investigations.
The development of effective treatments for COVID-19 is critical to mitigating the devastating consequences of SARS-CoV-2 infection. Several recent COVID-19 clinical trials have shown promise in this endeavor. For instance, the Adaptive COVID-19 Treatment Trial (ACCT-1) found that intravenous remdesivir, as compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.6 Moreover, there is some evidence to suggest that convalescent plasma and aerosol inhalation of IFN-κ may have beneficial effects in treating COVID-19.7,8 Thus, clinical trials designed to investigate combination therapy approaches including corticosteroids, remdesivir, convalescent plasma, and others are urgently needed to help identify interventions that most effectively treat COVID-19.
Applications for Clinical Practice
The use of corticosteroids in critically ill patients with COVID-19 reduces overall mortality. This treatment is inexpensive and available in most care settings, including low-resource regions, and provides hope for better outcomes in the COVID-19 pandemic.
Katerina Oikonomou, MD, PhD
General Hospital of Larissa, Larissa, Greece
Fred Ko, MD, MS
1. Sterne JAC, Diaz J, Villar J, et al. Corticosteroid therapy for critically ill patients with COVID-19: A structured summary of a study protocol for a prospective meta-analysis of randomized trials. Trials. 2020;21:734.
2. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304-309.
3. Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for citically Ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757-767.
4. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report [published online ahead of print, 2020 Jul 17]. N Engl J Med. 2020;NEJMoa2021436.
5. NIH COVID-19 Treatment Guidelines. National Institutes of Health. www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/. Accessed September 11, 2020.
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19--preliminary report [published online ahead of print, 2020 May 22]. N Engl J Med. 2020;NEJMoa2007764.
7. Casadevall A, Joyner MJ, Pirofski LA. A randomized trial of convalescent plasma for covid-19-potentially hopeful signals. JAMA. 2020;324:455-457.
8. Fu W, Liu Y, Xia L, et al. A clinical pilot study on the safety and efficacy of aerosol inhalation treatment of IFN-κ plus TFF2 in patients with moderate COVID-19. EClinicalMedicine. 2020;25:100478.
1. Sterne JAC, Diaz J, Villar J, et al. Corticosteroid therapy for critically ill patients with COVID-19: A structured summary of a study protocol for a prospective meta-analysis of randomized trials. Trials. 2020;21:734.
2. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304-309.
3. Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for citically Ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757-767.
4. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report [published online ahead of print, 2020 Jul 17]. N Engl J Med. 2020;NEJMoa2021436.
5. NIH COVID-19 Treatment Guidelines. National Institutes of Health. www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/. Accessed September 11, 2020.
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19--preliminary report [published online ahead of print, 2020 May 22]. N Engl J Med. 2020;NEJMoa2007764.
7. Casadevall A, Joyner MJ, Pirofski LA. A randomized trial of convalescent plasma for covid-19-potentially hopeful signals. JAMA. 2020;324:455-457.
8. Fu W, Liu Y, Xia L, et al. A clinical pilot study on the safety and efficacy of aerosol inhalation treatment of IFN-κ plus TFF2 in patients with moderate COVID-19. EClinicalMedicine. 2020;25:100478.
Dapagliflozin Improves Cardiovascular Outcomes in Patients With Heart Failure and Reduced Ejection Fraction
Study Overview
Objective. To evaluate the effects of dapagliflozin in patients with heart failure with reduced ejection fraction in the presence or absence of type 2 diabetes.
Design. Multicenter, international, double-blind, prospective, randomized, controlled trial.
Setting and participants. Adult patients with symptomatic heart failure with an ejection fraction of 40% or less and elevated heart failure biomarkers who were already on appropriate guideline-directed therapies were eligible for the study.
Intervention. A total of 4744 patients were randomly assigned to receive dapagliflozin (10 mg once daily) or placebo, in addition to recommended therapy. Randomization was stratified by the presence or absence of type 2 diabetes.
Main outcome measures. The primary outcome was the composite of a first episode of worsening heart failure (hospitalization or urgent intravenous therapy) or cardiovascular death.
Main results. Median follow-up was 18.2 months; during this time, the primary outcome occurred in 16.3% (386 of 2373) of patients in the dapagliflozin group and in 21.2% (502 of 2371) of patients in the placebo group (hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.65-0.85; P < 0.001). In the dapagliflozin group, 237 patients (10.0%) experienced a first worsening heart failure event, as compared with 326 patients (13.7%) in the placebo group (HR, 0.70; 95% CI, 0.59-0.83). The dapagliflozin group hadlower rates of death from cardiovascular causes (9.6% vs 11.5%; HR, 0.82; 95% CI, 0.69-0.98) and from any causes (11.6% vs 13.9%; HR, 0.83; 95% CI, 0.71-0.97), compared to the placebo group. Findings in patients with diabetes were similar to those in patients without diabetes.
Conclusion. Among patients with heart failure and a reduced ejection fraction, the risk of worsening heart failure or death from cardiovascular causes was lower among those who received dapagliflozin than among those who received placebo, regardless of the presence or absence of diabetes.
Commentary
Inhibitors of sodium-glucose cotransporter 2 (SGLT-2) are a novel class of diabetic medication that decrease renal glucose reabsorption, thereby increasing urinary glucose excretion. In several large clinical trials of these medications for patients with diabetes, which were designed to meet the regulatory requirements for cardiovascular safety in novel diabetic agents, investigators unexpectedly found that SGLT-2 inhibitors were associated with a reduction in cardiovascular events, driven by a reduction in heart failure hospitalizations. The results of EMPA-REG OUTCOME, the first of these trials, showed significantly lower risks of both death from any cause and hospitalization for heart failure in patients treated with empagliflozin.1 This improvement in cardiovascular outcomes was subsequently confirmed as a class effect of SGLT-2 inhibitors in the CANVAS Program (canagliflozin) and DECLARE TIMI 58 (dapagliflozin) trials.2,3
While these trials were designed for patients with type 2 diabetes who had either established cardiovascular disease or multiple risk factors for it, most patients did not have heart failure at baseline. Accordingly, despite a signal toward benefit of SGLT-2 inhibitors in patients with heart failure, the trials were not powered to test the hypothesis that SGLT-2 inhibitors benefit patients with heart failure, regardless of diabetes status. Therefore, McMurray et al designed the DAPA-HF trial to investigate whether SGLT-2 inhibitors can improve cardiovascular outcomes in patients with heart failure with reduced ejection fraction, with or without diabetes. The trial included 4744 patients with heart failure with reduced ejection fraction, who were randomly assigned to dapagliflozin 10 mg once daily or placebo, atop guideline-directed heart failure therapy, with randomization stratified by presence or absence of type 2 diabetes. Investigators found that the composite primary outcome, a first episode of worsening heart failure or cardiovascular death, occurred less frequently in patients in the dapagliflozin group compared to the placebo group (16.3% vs 21.2%; HR, 0.74; 95% CI, 0.65-0.85; P < 0.001). Individual components of the primary outcome and death from any cause were all significantly lower, and heart failure–related quality of life was significantly improved in the dapagliflozin group compared to placebo.
DAPA-HF was the first randomized study to investigate the effect of SGLT-2 inhibitors on patients with heart failure regardless of the presence of diabetes. In addition to the reduction in the above-mentioned primary and secondary endpoints, the study yielded other important findings worth noting. First, the consistent benefit of dapagliflozin on cardiovascular outcomes in patients with and without diabetes suggests that the cardioprotective effect of dapagliflozin is independent of its glucose-lowering effect. Prior studies have proposed alternative mechanisms, such as diuretic function and related hemodynamic actions, effects on myocardial metabolism, ion transporters, fibrosis, adipokines, vascular function, and the preservation of renal function. Future studies are needed to fully understand the likely pleiotropic effects of this class of medication on patients with heart failure. Second, there was no difference in the safety endpoints between the groups, including renal adverse events and major hypoglycemia, implying dapagliflozin is as safe as placebo.
There are a few limitations of this trial. First, as the authors point out, the study included mostly white males—less than 5% of participants were African Americans—and the finding may not be generalizable to all patient populations. Second, although all patients were already treated with guideline-directed heart failure therapy, only 10% of patients were on sacubitril–valsartan, which is more effective than renin–angiotensin system blockade alone at reducing the incidence of hospitalization for heart failure and death from cardiovascular causes. Also, mineralocorticoid receptor blockers were used in only 70% of the population. Finally, since the doses were not provided, whether patients were on the maximal tolerated dose of heart failure therapy prior to enrollment is unclear.
Based on the results of the DAPA-HF trial, the Food and Drug Administration approved dapagliflozin for the treatment of heart failure with reduced ejection fraction on May 5, 2020. This is the first diabetic drug approved for the treatment of heart failure.
Applications for Clinical Practice
SGLT-2 inhibitors represent a fourth class of medication that patients with heart failure with reduced ejection fraction should be initiated on, in addition to beta blocker, ACE inhibitor/angiotensin receptor blocker/neprilysin inhibitor, and mineralocorticoid receptor blocker. SGLT-2 inhibitors may be especially applicable in patients with heart failure with reduced ejection fraction and relative hypotension, as these agents are not associated with a significant blood-pressure-lowering effect, which can often limit our ability to initiate or uptitrate the other main 3 classes of guideline-directed medical therapy.
—Rie Hirai, MD, Fukui Kosei Hospital, Fukui, Japan
—Taishi Hirai, MD, University of Missouri Medical Center, Columbia, MO
—Timothy Fendler, MD, St. Luke’s Mid America Heart Institute, Kansas City, MO
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
Study Overview
Objective. To evaluate the effects of dapagliflozin in patients with heart failure with reduced ejection fraction in the presence or absence of type 2 diabetes.
Design. Multicenter, international, double-blind, prospective, randomized, controlled trial.
Setting and participants. Adult patients with symptomatic heart failure with an ejection fraction of 40% or less and elevated heart failure biomarkers who were already on appropriate guideline-directed therapies were eligible for the study.
Intervention. A total of 4744 patients were randomly assigned to receive dapagliflozin (10 mg once daily) or placebo, in addition to recommended therapy. Randomization was stratified by the presence or absence of type 2 diabetes.
Main outcome measures. The primary outcome was the composite of a first episode of worsening heart failure (hospitalization or urgent intravenous therapy) or cardiovascular death.
Main results. Median follow-up was 18.2 months; during this time, the primary outcome occurred in 16.3% (386 of 2373) of patients in the dapagliflozin group and in 21.2% (502 of 2371) of patients in the placebo group (hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.65-0.85; P < 0.001). In the dapagliflozin group, 237 patients (10.0%) experienced a first worsening heart failure event, as compared with 326 patients (13.7%) in the placebo group (HR, 0.70; 95% CI, 0.59-0.83). The dapagliflozin group hadlower rates of death from cardiovascular causes (9.6% vs 11.5%; HR, 0.82; 95% CI, 0.69-0.98) and from any causes (11.6% vs 13.9%; HR, 0.83; 95% CI, 0.71-0.97), compared to the placebo group. Findings in patients with diabetes were similar to those in patients without diabetes.
Conclusion. Among patients with heart failure and a reduced ejection fraction, the risk of worsening heart failure or death from cardiovascular causes was lower among those who received dapagliflozin than among those who received placebo, regardless of the presence or absence of diabetes.
Commentary
Inhibitors of sodium-glucose cotransporter 2 (SGLT-2) are a novel class of diabetic medication that decrease renal glucose reabsorption, thereby increasing urinary glucose excretion. In several large clinical trials of these medications for patients with diabetes, which were designed to meet the regulatory requirements for cardiovascular safety in novel diabetic agents, investigators unexpectedly found that SGLT-2 inhibitors were associated with a reduction in cardiovascular events, driven by a reduction in heart failure hospitalizations. The results of EMPA-REG OUTCOME, the first of these trials, showed significantly lower risks of both death from any cause and hospitalization for heart failure in patients treated with empagliflozin.1 This improvement in cardiovascular outcomes was subsequently confirmed as a class effect of SGLT-2 inhibitors in the CANVAS Program (canagliflozin) and DECLARE TIMI 58 (dapagliflozin) trials.2,3
While these trials were designed for patients with type 2 diabetes who had either established cardiovascular disease or multiple risk factors for it, most patients did not have heart failure at baseline. Accordingly, despite a signal toward benefit of SGLT-2 inhibitors in patients with heart failure, the trials were not powered to test the hypothesis that SGLT-2 inhibitors benefit patients with heart failure, regardless of diabetes status. Therefore, McMurray et al designed the DAPA-HF trial to investigate whether SGLT-2 inhibitors can improve cardiovascular outcomes in patients with heart failure with reduced ejection fraction, with or without diabetes. The trial included 4744 patients with heart failure with reduced ejection fraction, who were randomly assigned to dapagliflozin 10 mg once daily or placebo, atop guideline-directed heart failure therapy, with randomization stratified by presence or absence of type 2 diabetes. Investigators found that the composite primary outcome, a first episode of worsening heart failure or cardiovascular death, occurred less frequently in patients in the dapagliflozin group compared to the placebo group (16.3% vs 21.2%; HR, 0.74; 95% CI, 0.65-0.85; P < 0.001). Individual components of the primary outcome and death from any cause were all significantly lower, and heart failure–related quality of life was significantly improved in the dapagliflozin group compared to placebo.
DAPA-HF was the first randomized study to investigate the effect of SGLT-2 inhibitors on patients with heart failure regardless of the presence of diabetes. In addition to the reduction in the above-mentioned primary and secondary endpoints, the study yielded other important findings worth noting. First, the consistent benefit of dapagliflozin on cardiovascular outcomes in patients with and without diabetes suggests that the cardioprotective effect of dapagliflozin is independent of its glucose-lowering effect. Prior studies have proposed alternative mechanisms, such as diuretic function and related hemodynamic actions, effects on myocardial metabolism, ion transporters, fibrosis, adipokines, vascular function, and the preservation of renal function. Future studies are needed to fully understand the likely pleiotropic effects of this class of medication on patients with heart failure. Second, there was no difference in the safety endpoints between the groups, including renal adverse events and major hypoglycemia, implying dapagliflozin is as safe as placebo.
There are a few limitations of this trial. First, as the authors point out, the study included mostly white males—less than 5% of participants were African Americans—and the finding may not be generalizable to all patient populations. Second, although all patients were already treated with guideline-directed heart failure therapy, only 10% of patients were on sacubitril–valsartan, which is more effective than renin–angiotensin system blockade alone at reducing the incidence of hospitalization for heart failure and death from cardiovascular causes. Also, mineralocorticoid receptor blockers were used in only 70% of the population. Finally, since the doses were not provided, whether patients were on the maximal tolerated dose of heart failure therapy prior to enrollment is unclear.
Based on the results of the DAPA-HF trial, the Food and Drug Administration approved dapagliflozin for the treatment of heart failure with reduced ejection fraction on May 5, 2020. This is the first diabetic drug approved for the treatment of heart failure.
Applications for Clinical Practice
SGLT-2 inhibitors represent a fourth class of medication that patients with heart failure with reduced ejection fraction should be initiated on, in addition to beta blocker, ACE inhibitor/angiotensin receptor blocker/neprilysin inhibitor, and mineralocorticoid receptor blocker. SGLT-2 inhibitors may be especially applicable in patients with heart failure with reduced ejection fraction and relative hypotension, as these agents are not associated with a significant blood-pressure-lowering effect, which can often limit our ability to initiate or uptitrate the other main 3 classes of guideline-directed medical therapy.
—Rie Hirai, MD, Fukui Kosei Hospital, Fukui, Japan
—Taishi Hirai, MD, University of Missouri Medical Center, Columbia, MO
—Timothy Fendler, MD, St. Luke’s Mid America Heart Institute, Kansas City, MO
Study Overview
Objective. To evaluate the effects of dapagliflozin in patients with heart failure with reduced ejection fraction in the presence or absence of type 2 diabetes.
Design. Multicenter, international, double-blind, prospective, randomized, controlled trial.
Setting and participants. Adult patients with symptomatic heart failure with an ejection fraction of 40% or less and elevated heart failure biomarkers who were already on appropriate guideline-directed therapies were eligible for the study.
Intervention. A total of 4744 patients were randomly assigned to receive dapagliflozin (10 mg once daily) or placebo, in addition to recommended therapy. Randomization was stratified by the presence or absence of type 2 diabetes.
Main outcome measures. The primary outcome was the composite of a first episode of worsening heart failure (hospitalization or urgent intravenous therapy) or cardiovascular death.
Main results. Median follow-up was 18.2 months; during this time, the primary outcome occurred in 16.3% (386 of 2373) of patients in the dapagliflozin group and in 21.2% (502 of 2371) of patients in the placebo group (hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.65-0.85; P < 0.001). In the dapagliflozin group, 237 patients (10.0%) experienced a first worsening heart failure event, as compared with 326 patients (13.7%) in the placebo group (HR, 0.70; 95% CI, 0.59-0.83). The dapagliflozin group hadlower rates of death from cardiovascular causes (9.6% vs 11.5%; HR, 0.82; 95% CI, 0.69-0.98) and from any causes (11.6% vs 13.9%; HR, 0.83; 95% CI, 0.71-0.97), compared to the placebo group. Findings in patients with diabetes were similar to those in patients without diabetes.
Conclusion. Among patients with heart failure and a reduced ejection fraction, the risk of worsening heart failure or death from cardiovascular causes was lower among those who received dapagliflozin than among those who received placebo, regardless of the presence or absence of diabetes.
Commentary
Inhibitors of sodium-glucose cotransporter 2 (SGLT-2) are a novel class of diabetic medication that decrease renal glucose reabsorption, thereby increasing urinary glucose excretion. In several large clinical trials of these medications for patients with diabetes, which were designed to meet the regulatory requirements for cardiovascular safety in novel diabetic agents, investigators unexpectedly found that SGLT-2 inhibitors were associated with a reduction in cardiovascular events, driven by a reduction in heart failure hospitalizations. The results of EMPA-REG OUTCOME, the first of these trials, showed significantly lower risks of both death from any cause and hospitalization for heart failure in patients treated with empagliflozin.1 This improvement in cardiovascular outcomes was subsequently confirmed as a class effect of SGLT-2 inhibitors in the CANVAS Program (canagliflozin) and DECLARE TIMI 58 (dapagliflozin) trials.2,3
While these trials were designed for patients with type 2 diabetes who had either established cardiovascular disease or multiple risk factors for it, most patients did not have heart failure at baseline. Accordingly, despite a signal toward benefit of SGLT-2 inhibitors in patients with heart failure, the trials were not powered to test the hypothesis that SGLT-2 inhibitors benefit patients with heart failure, regardless of diabetes status. Therefore, McMurray et al designed the DAPA-HF trial to investigate whether SGLT-2 inhibitors can improve cardiovascular outcomes in patients with heart failure with reduced ejection fraction, with or without diabetes. The trial included 4744 patients with heart failure with reduced ejection fraction, who were randomly assigned to dapagliflozin 10 mg once daily or placebo, atop guideline-directed heart failure therapy, with randomization stratified by presence or absence of type 2 diabetes. Investigators found that the composite primary outcome, a first episode of worsening heart failure or cardiovascular death, occurred less frequently in patients in the dapagliflozin group compared to the placebo group (16.3% vs 21.2%; HR, 0.74; 95% CI, 0.65-0.85; P < 0.001). Individual components of the primary outcome and death from any cause were all significantly lower, and heart failure–related quality of life was significantly improved in the dapagliflozin group compared to placebo.
DAPA-HF was the first randomized study to investigate the effect of SGLT-2 inhibitors on patients with heart failure regardless of the presence of diabetes. In addition to the reduction in the above-mentioned primary and secondary endpoints, the study yielded other important findings worth noting. First, the consistent benefit of dapagliflozin on cardiovascular outcomes in patients with and without diabetes suggests that the cardioprotective effect of dapagliflozin is independent of its glucose-lowering effect. Prior studies have proposed alternative mechanisms, such as diuretic function and related hemodynamic actions, effects on myocardial metabolism, ion transporters, fibrosis, adipokines, vascular function, and the preservation of renal function. Future studies are needed to fully understand the likely pleiotropic effects of this class of medication on patients with heart failure. Second, there was no difference in the safety endpoints between the groups, including renal adverse events and major hypoglycemia, implying dapagliflozin is as safe as placebo.
There are a few limitations of this trial. First, as the authors point out, the study included mostly white males—less than 5% of participants were African Americans—and the finding may not be generalizable to all patient populations. Second, although all patients were already treated with guideline-directed heart failure therapy, only 10% of patients were on sacubitril–valsartan, which is more effective than renin–angiotensin system blockade alone at reducing the incidence of hospitalization for heart failure and death from cardiovascular causes. Also, mineralocorticoid receptor blockers were used in only 70% of the population. Finally, since the doses were not provided, whether patients were on the maximal tolerated dose of heart failure therapy prior to enrollment is unclear.
Based on the results of the DAPA-HF trial, the Food and Drug Administration approved dapagliflozin for the treatment of heart failure with reduced ejection fraction on May 5, 2020. This is the first diabetic drug approved for the treatment of heart failure.
Applications for Clinical Practice
SGLT-2 inhibitors represent a fourth class of medication that patients with heart failure with reduced ejection fraction should be initiated on, in addition to beta blocker, ACE inhibitor/angiotensin receptor blocker/neprilysin inhibitor, and mineralocorticoid receptor blocker. SGLT-2 inhibitors may be especially applicable in patients with heart failure with reduced ejection fraction and relative hypotension, as these agents are not associated with a significant blood-pressure-lowering effect, which can often limit our ability to initiate or uptitrate the other main 3 classes of guideline-directed medical therapy.
—Rie Hirai, MD, Fukui Kosei Hospital, Fukui, Japan
—Taishi Hirai, MD, University of Missouri Medical Center, Columbia, MO
—Timothy Fendler, MD, St. Luke’s Mid America Heart Institute, Kansas City, MO
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
Oral Relugolix Yields Superior Testosterone Suppression and Decreased Cardiovascular Events Compared With GnRH Agonist
Study Overview
Objective. To evaluate the safety and efficacy of the highly selective oral gonadotropin-releasing hormone (GnRH) antagonist relugolix in men with advanced prostate cancer.
Design. Global, multicenter, randomized, open-label, phase 3 trial.
Intervention. Patients were randomized in a 2:1 ratio to receive either relugolix 120 mg once daily after receiving a single loading dose of 360 mg, or 22.5 mg of leuprolide acetate every 3 months. Patients in Japan and Taiwan received 11.25 mg of leuprolide. The randomization was stratified by age (> 75 years or ≤ 75 years), metastatic disease status, and geographic region (Asia, Europe, North and South America). The intervention period was 48 weeks.
Setting and participants. 1327 patients were screened, and 934 patients underwent randomization: 622 patients to the relugolix group and 308 to the leuprolide group. Patients had histologically or cytologically confirmed adenocarcinoma of the prostate. All patients had to have 1 of the following: evidence of biochemical or clinical relapse after primary curative therapy, newly diagnosed hormone-sensitive metastatic disease, or advance localized disease unlikely to be cured by local primary intervention. The patients with disease progression or rising prostate-specific antigen (PSA) had the option to receive enzalutamide or docetaxel after the confirmation of progression. Patients were excluded if they had a major cardiovascular event within 6 months of enrollment.
Main outcome measures. The primary endpoint was sustained castration rate, defined as the cumulative probability of testosterone suppression to ≤ 50 ng/dL while on study treatment from week 5 through week 48. Secondary endpoints included noninferiority of relugolix to leuprolide in regard to sustained castration rate. Superiority testing was performed if the noninferiority margin of –10 percentage points was met. Additional secondary endpoints were probability of testosterone suppression to ≤ 50 ng/dL on day 4 and day 15 and the percentage of patients with a > 50% decrease in PSA at day 15 and follicle-stimulating hormone (FSH) levels at the end of week 24.
Main results. The baseline characteristics were well balanced between the treatment groups. Approximately 30% of the patients in each group had metastatic disease. Approximately 50% of patients enrolled had biochemical recurrence following primary treatment for prostate cancer. The mean PSA was 104.2 ng/mL in the relugolix group and 68.6 ng/mL in the leuprolide group. The majority of patients had at least 1 cardiovascular risk factor (ie, tobacco use, obesity, diabetes, hypertension, or a history of a major adverse cardiac event [MACE]). Adherence to oral therapy was reported as 99% in both groups. The median follow-up time was 52 weeks; 90% of patients in the relugolix arm and 89% in the leuprolide arm completed 48 weeks of treatment.
Sustained testosterone suppression to ≤ 50 ng/dL from day 29 through week 48 was seen in 96.7% of patients in the relugolix group and 88.8% in the leuprolide group, which was determined to be noninferior. Additionally, relugolix was also found to be superior to leuprolide in regard to sustained testosterone suppression (P < 0.001). These results were consistent across all subgroups. Relugolix was also found to be superior to leuprolide for all secondary endpoints, including cumulative probability of castration on day 4 (56% vs 0%) and day 15 (98.7% vs 12%) and testosterone suppression to ≤ 20 ng/dL on day 15 (78.4% vs 1%). Confirmed PSA response on day 15 was seen in 79.4% of patients in the relugolix arm and in 19.8% in the leuprolide arm (P < 0.001). FSH suppression was greater in the relugolix arm compared with the leuprolide arm by the end of week 24. An increase of testosterone levels from baseline was noted in the leuprolide patients at day 4, with the level decreasing to castrate level by day 29. In contrast, relugolix patients maintained castrate testosterone levels from day 4 throughout the intervention period. Testosterone recovery at 90 days was seen in 54% of patients in the relugolix group compared with 3% in the leuprolide group (P = 0.002).
The most frequent adverse event seen in both groups was hot flashes (54.3% in the relugolix group and 51.6% in the leuprolide group). The second most common adverse event report was fatigue, which occurred in 21.5% of patients in the relugolix arm and 18.5% in the leuprolide arm. Diarrhea was reported more frequently with relugolix than with leuprolide (12.2% vs 6.8%); however, diarrhea did not lead to discontinuation of therapy in any patient. Fatal events were reported more frequently in the leuprolide group (2.9%) compared with the relugolix group (1.1%). MACE were defined as nonfatal myocardial infarction, stroke, and death from any cause. After completing the intervention period of 48 weeks, the relugolix group had a 2.9% incidence of major cardiovascular events, compared with 6.2% in the leuprolide group. In patients having a medical history of cardiovascular events, the adverse event rate during the trial period was 3.6% in the relugolix group and 17.8% in leuprolide group. This translated into a 54% lower risk of MACE in the relugolix arm compared with the leuprolide arm.
Conclusion. The use of relugolix in advanced prostate cancer led to rapid, sustained suppression and faster recovery of testosterone level compared with leuprolide. Relugolix appeared safer to use for men with a medical history of cardiovascular events and showed a 54% lower risk of MACE than leuprolide.
Commentary
Relugolix is a highly selective oral GnRH antagonist that rapidly inhibits pituitary release of luteinizing hormone and FSH. The current phase 3 HERO trial highlights the efficacy of relugolix in regard to testosterone suppression, adding to potential therapeutic options for these men. Relugolix yielded superior sustained testosterone suppression to less than 50 ng/dL throughout the 48-week study period, meeting its primary endpoint. Additionally, relugolix showed superiority in all secondary endpoints across all subgroups of patients. To date, the only GnRH antagonist on the market is degarelix, which is given as a monthly subcutaneous injection.1 Injection-site reactions remain an issue with this formulation.
Cardiovascular disease is the leading cause of death in the United States, and it is known that men with prostate cancer have a higher incidence of cardiovascular disease.2 While data regarding adverse cardiac outcomes with androgen deprivation therapy have been mixed, it is thought that this therapy increases the risk for MACE. There is mounting evidence that GnRH antagonists may have a less detrimental effect on cardiovascular outcomes compared with GnRH agonists. For example, a pooled analysis of 6 phase 3 trials showed a lower incidence of cardiovascular events in men with preexisting cardiovascular disease using the GnRH antagonist degarelix compared with GnRH agonists after 12 months of treatment.3 Furthermore, a more recent phase 2 randomized trial showed that 20% of patients treated with a GnRH agonist developed cardiovascular events, compared to 3% in the GnRH antagonist group. The absolute risk reduction of cardiovascular events at 12 months was 18%.4 The results of the current trial support such findings, showing a 54% reduction in MACE after 48 weeks of therapy when compared with leuprolide (2.9% in relugolix arm vs 6.2% in leuprolide arm). More importantly perhaps, in the subgroup of men with preexisting cardiovascular disease, the benefit was even greater, with a MACE incidence of 3.6% with relugolix compared with 17.8% with leuprolide.
Studies have also shown that second-generation antiandrogens such as enzalutamide are associated with an increased risk of death from cardiovascular causes. For example, data from the recently updated PROSPER trial, which evaluated the use of enzalutamide in men with nonmetastatic, castration-resistant prostate cancer, showed an increased risk of adverse events, including falls, fatigue, hypertension, and death from cardiovascular events.5 Furthermore, adding second-generation antiandrogens to GnRH-agonist therapy is associated with a high risk of cardiovascular events in men with preexisting cardiovascular disease.3 These results were noted in all of the trials of second-generation antiandrogens, including enzalutamide, apalutamide, and darolutamide, in combination with GnRH agonists.6-8 Taken together, one might consider whether the use of a GnRH antagonist would result in improved cardiovascular outcomes in high-risk patients.
In light of the efficacy of relugolix in regard to testosterone suppression highlighted in the current trial, it is likely that its efficacy in regard to cancer outcomes will be similar; however, to date there is no level 1 evidence to support this. Nevertheless, there is a clear association of adverse cardiovascular outcomes in men treated with GnRH agonists, and the notable 54% risk reduction seen in the current trial certainly would support considering the use of a GnRH antagonist for the subgroup of patients with preexisting cardiovascular disease or those at high risk for MACE. Further work is needed to define the role of GnRH antagonists in conjunction with second-generation antiandrogens to help mitigate cardiovascular toxicities.
Clinical Implications
The use of GnRH antagonists should be considered in men with advanced prostate cancer who have underlying cardiovascular disease to help mitigate the risk of MACE. Currently, degarelix is the only commercially available agent; however, pending regulatory approval, oral relugolix may be considered an appropriate oral option in such patients, with data supporting superior testosterone suppressive effects. Further follow-up will be needed.
–Saud Alsubait, MD, Michigan State University, East Lansing, MI
–Daniel Isaac, MD, MS
1. Barkin J, Burton S, Lambert C. Optimizing subcutaneous injection of the gonadotropin-releasing hormone receptor antagonist degarelix. Can J Urol. 2016;23:8179-8183.
2. Higano CS. Cardiovascular disease and androgen axis-targeted drugs for prostate cancer. N Engl J Med. 2020;382:2257-2259.
3. Albertsen PC, Klotz L, Tombal B, et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65:565-573.
4. Margel D, Peer A, Ber Y, et al. Cardiovascular morbidity in a randomized trial comparing GnRH agonist and GnRH antagonist among patients with advanced prostate cancer and preexisting cardiovascular disease. J Urol. 2019;202:1199-1208.
5. Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382:2197-2206.
6. Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418.
7. Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380:1235-1246.
Study Overview
Objective. To evaluate the safety and efficacy of the highly selective oral gonadotropin-releasing hormone (GnRH) antagonist relugolix in men with advanced prostate cancer.
Design. Global, multicenter, randomized, open-label, phase 3 trial.
Intervention. Patients were randomized in a 2:1 ratio to receive either relugolix 120 mg once daily after receiving a single loading dose of 360 mg, or 22.5 mg of leuprolide acetate every 3 months. Patients in Japan and Taiwan received 11.25 mg of leuprolide. The randomization was stratified by age (> 75 years or ≤ 75 years), metastatic disease status, and geographic region (Asia, Europe, North and South America). The intervention period was 48 weeks.
Setting and participants. 1327 patients were screened, and 934 patients underwent randomization: 622 patients to the relugolix group and 308 to the leuprolide group. Patients had histologically or cytologically confirmed adenocarcinoma of the prostate. All patients had to have 1 of the following: evidence of biochemical or clinical relapse after primary curative therapy, newly diagnosed hormone-sensitive metastatic disease, or advance localized disease unlikely to be cured by local primary intervention. The patients with disease progression or rising prostate-specific antigen (PSA) had the option to receive enzalutamide or docetaxel after the confirmation of progression. Patients were excluded if they had a major cardiovascular event within 6 months of enrollment.
Main outcome measures. The primary endpoint was sustained castration rate, defined as the cumulative probability of testosterone suppression to ≤ 50 ng/dL while on study treatment from week 5 through week 48. Secondary endpoints included noninferiority of relugolix to leuprolide in regard to sustained castration rate. Superiority testing was performed if the noninferiority margin of –10 percentage points was met. Additional secondary endpoints were probability of testosterone suppression to ≤ 50 ng/dL on day 4 and day 15 and the percentage of patients with a > 50% decrease in PSA at day 15 and follicle-stimulating hormone (FSH) levels at the end of week 24.
Main results. The baseline characteristics were well balanced between the treatment groups. Approximately 30% of the patients in each group had metastatic disease. Approximately 50% of patients enrolled had biochemical recurrence following primary treatment for prostate cancer. The mean PSA was 104.2 ng/mL in the relugolix group and 68.6 ng/mL in the leuprolide group. The majority of patients had at least 1 cardiovascular risk factor (ie, tobacco use, obesity, diabetes, hypertension, or a history of a major adverse cardiac event [MACE]). Adherence to oral therapy was reported as 99% in both groups. The median follow-up time was 52 weeks; 90% of patients in the relugolix arm and 89% in the leuprolide arm completed 48 weeks of treatment.
Sustained testosterone suppression to ≤ 50 ng/dL from day 29 through week 48 was seen in 96.7% of patients in the relugolix group and 88.8% in the leuprolide group, which was determined to be noninferior. Additionally, relugolix was also found to be superior to leuprolide in regard to sustained testosterone suppression (P < 0.001). These results were consistent across all subgroups. Relugolix was also found to be superior to leuprolide for all secondary endpoints, including cumulative probability of castration on day 4 (56% vs 0%) and day 15 (98.7% vs 12%) and testosterone suppression to ≤ 20 ng/dL on day 15 (78.4% vs 1%). Confirmed PSA response on day 15 was seen in 79.4% of patients in the relugolix arm and in 19.8% in the leuprolide arm (P < 0.001). FSH suppression was greater in the relugolix arm compared with the leuprolide arm by the end of week 24. An increase of testosterone levels from baseline was noted in the leuprolide patients at day 4, with the level decreasing to castrate level by day 29. In contrast, relugolix patients maintained castrate testosterone levels from day 4 throughout the intervention period. Testosterone recovery at 90 days was seen in 54% of patients in the relugolix group compared with 3% in the leuprolide group (P = 0.002).
The most frequent adverse event seen in both groups was hot flashes (54.3% in the relugolix group and 51.6% in the leuprolide group). The second most common adverse event report was fatigue, which occurred in 21.5% of patients in the relugolix arm and 18.5% in the leuprolide arm. Diarrhea was reported more frequently with relugolix than with leuprolide (12.2% vs 6.8%); however, diarrhea did not lead to discontinuation of therapy in any patient. Fatal events were reported more frequently in the leuprolide group (2.9%) compared with the relugolix group (1.1%). MACE were defined as nonfatal myocardial infarction, stroke, and death from any cause. After completing the intervention period of 48 weeks, the relugolix group had a 2.9% incidence of major cardiovascular events, compared with 6.2% in the leuprolide group. In patients having a medical history of cardiovascular events, the adverse event rate during the trial period was 3.6% in the relugolix group and 17.8% in leuprolide group. This translated into a 54% lower risk of MACE in the relugolix arm compared with the leuprolide arm.
Conclusion. The use of relugolix in advanced prostate cancer led to rapid, sustained suppression and faster recovery of testosterone level compared with leuprolide. Relugolix appeared safer to use for men with a medical history of cardiovascular events and showed a 54% lower risk of MACE than leuprolide.
Commentary
Relugolix is a highly selective oral GnRH antagonist that rapidly inhibits pituitary release of luteinizing hormone and FSH. The current phase 3 HERO trial highlights the efficacy of relugolix in regard to testosterone suppression, adding to potential therapeutic options for these men. Relugolix yielded superior sustained testosterone suppression to less than 50 ng/dL throughout the 48-week study period, meeting its primary endpoint. Additionally, relugolix showed superiority in all secondary endpoints across all subgroups of patients. To date, the only GnRH antagonist on the market is degarelix, which is given as a monthly subcutaneous injection.1 Injection-site reactions remain an issue with this formulation.
Cardiovascular disease is the leading cause of death in the United States, and it is known that men with prostate cancer have a higher incidence of cardiovascular disease.2 While data regarding adverse cardiac outcomes with androgen deprivation therapy have been mixed, it is thought that this therapy increases the risk for MACE. There is mounting evidence that GnRH antagonists may have a less detrimental effect on cardiovascular outcomes compared with GnRH agonists. For example, a pooled analysis of 6 phase 3 trials showed a lower incidence of cardiovascular events in men with preexisting cardiovascular disease using the GnRH antagonist degarelix compared with GnRH agonists after 12 months of treatment.3 Furthermore, a more recent phase 2 randomized trial showed that 20% of patients treated with a GnRH agonist developed cardiovascular events, compared to 3% in the GnRH antagonist group. The absolute risk reduction of cardiovascular events at 12 months was 18%.4 The results of the current trial support such findings, showing a 54% reduction in MACE after 48 weeks of therapy when compared with leuprolide (2.9% in relugolix arm vs 6.2% in leuprolide arm). More importantly perhaps, in the subgroup of men with preexisting cardiovascular disease, the benefit was even greater, with a MACE incidence of 3.6% with relugolix compared with 17.8% with leuprolide.
Studies have also shown that second-generation antiandrogens such as enzalutamide are associated with an increased risk of death from cardiovascular causes. For example, data from the recently updated PROSPER trial, which evaluated the use of enzalutamide in men with nonmetastatic, castration-resistant prostate cancer, showed an increased risk of adverse events, including falls, fatigue, hypertension, and death from cardiovascular events.5 Furthermore, adding second-generation antiandrogens to GnRH-agonist therapy is associated with a high risk of cardiovascular events in men with preexisting cardiovascular disease.3 These results were noted in all of the trials of second-generation antiandrogens, including enzalutamide, apalutamide, and darolutamide, in combination with GnRH agonists.6-8 Taken together, one might consider whether the use of a GnRH antagonist would result in improved cardiovascular outcomes in high-risk patients.
In light of the efficacy of relugolix in regard to testosterone suppression highlighted in the current trial, it is likely that its efficacy in regard to cancer outcomes will be similar; however, to date there is no level 1 evidence to support this. Nevertheless, there is a clear association of adverse cardiovascular outcomes in men treated with GnRH agonists, and the notable 54% risk reduction seen in the current trial certainly would support considering the use of a GnRH antagonist for the subgroup of patients with preexisting cardiovascular disease or those at high risk for MACE. Further work is needed to define the role of GnRH antagonists in conjunction with second-generation antiandrogens to help mitigate cardiovascular toxicities.
Clinical Implications
The use of GnRH antagonists should be considered in men with advanced prostate cancer who have underlying cardiovascular disease to help mitigate the risk of MACE. Currently, degarelix is the only commercially available agent; however, pending regulatory approval, oral relugolix may be considered an appropriate oral option in such patients, with data supporting superior testosterone suppressive effects. Further follow-up will be needed.
–Saud Alsubait, MD, Michigan State University, East Lansing, MI
–Daniel Isaac, MD, MS
Study Overview
Objective. To evaluate the safety and efficacy of the highly selective oral gonadotropin-releasing hormone (GnRH) antagonist relugolix in men with advanced prostate cancer.
Design. Global, multicenter, randomized, open-label, phase 3 trial.
Intervention. Patients were randomized in a 2:1 ratio to receive either relugolix 120 mg once daily after receiving a single loading dose of 360 mg, or 22.5 mg of leuprolide acetate every 3 months. Patients in Japan and Taiwan received 11.25 mg of leuprolide. The randomization was stratified by age (> 75 years or ≤ 75 years), metastatic disease status, and geographic region (Asia, Europe, North and South America). The intervention period was 48 weeks.
Setting and participants. 1327 patients were screened, and 934 patients underwent randomization: 622 patients to the relugolix group and 308 to the leuprolide group. Patients had histologically or cytologically confirmed adenocarcinoma of the prostate. All patients had to have 1 of the following: evidence of biochemical or clinical relapse after primary curative therapy, newly diagnosed hormone-sensitive metastatic disease, or advance localized disease unlikely to be cured by local primary intervention. The patients with disease progression or rising prostate-specific antigen (PSA) had the option to receive enzalutamide or docetaxel after the confirmation of progression. Patients were excluded if they had a major cardiovascular event within 6 months of enrollment.
Main outcome measures. The primary endpoint was sustained castration rate, defined as the cumulative probability of testosterone suppression to ≤ 50 ng/dL while on study treatment from week 5 through week 48. Secondary endpoints included noninferiority of relugolix to leuprolide in regard to sustained castration rate. Superiority testing was performed if the noninferiority margin of –10 percentage points was met. Additional secondary endpoints were probability of testosterone suppression to ≤ 50 ng/dL on day 4 and day 15 and the percentage of patients with a > 50% decrease in PSA at day 15 and follicle-stimulating hormone (FSH) levels at the end of week 24.
Main results. The baseline characteristics were well balanced between the treatment groups. Approximately 30% of the patients in each group had metastatic disease. Approximately 50% of patients enrolled had biochemical recurrence following primary treatment for prostate cancer. The mean PSA was 104.2 ng/mL in the relugolix group and 68.6 ng/mL in the leuprolide group. The majority of patients had at least 1 cardiovascular risk factor (ie, tobacco use, obesity, diabetes, hypertension, or a history of a major adverse cardiac event [MACE]). Adherence to oral therapy was reported as 99% in both groups. The median follow-up time was 52 weeks; 90% of patients in the relugolix arm and 89% in the leuprolide arm completed 48 weeks of treatment.
Sustained testosterone suppression to ≤ 50 ng/dL from day 29 through week 48 was seen in 96.7% of patients in the relugolix group and 88.8% in the leuprolide group, which was determined to be noninferior. Additionally, relugolix was also found to be superior to leuprolide in regard to sustained testosterone suppression (P < 0.001). These results were consistent across all subgroups. Relugolix was also found to be superior to leuprolide for all secondary endpoints, including cumulative probability of castration on day 4 (56% vs 0%) and day 15 (98.7% vs 12%) and testosterone suppression to ≤ 20 ng/dL on day 15 (78.4% vs 1%). Confirmed PSA response on day 15 was seen in 79.4% of patients in the relugolix arm and in 19.8% in the leuprolide arm (P < 0.001). FSH suppression was greater in the relugolix arm compared with the leuprolide arm by the end of week 24. An increase of testosterone levels from baseline was noted in the leuprolide patients at day 4, with the level decreasing to castrate level by day 29. In contrast, relugolix patients maintained castrate testosterone levels from day 4 throughout the intervention period. Testosterone recovery at 90 days was seen in 54% of patients in the relugolix group compared with 3% in the leuprolide group (P = 0.002).
The most frequent adverse event seen in both groups was hot flashes (54.3% in the relugolix group and 51.6% in the leuprolide group). The second most common adverse event report was fatigue, which occurred in 21.5% of patients in the relugolix arm and 18.5% in the leuprolide arm. Diarrhea was reported more frequently with relugolix than with leuprolide (12.2% vs 6.8%); however, diarrhea did not lead to discontinuation of therapy in any patient. Fatal events were reported more frequently in the leuprolide group (2.9%) compared with the relugolix group (1.1%). MACE were defined as nonfatal myocardial infarction, stroke, and death from any cause. After completing the intervention period of 48 weeks, the relugolix group had a 2.9% incidence of major cardiovascular events, compared with 6.2% in the leuprolide group. In patients having a medical history of cardiovascular events, the adverse event rate during the trial period was 3.6% in the relugolix group and 17.8% in leuprolide group. This translated into a 54% lower risk of MACE in the relugolix arm compared with the leuprolide arm.
Conclusion. The use of relugolix in advanced prostate cancer led to rapid, sustained suppression and faster recovery of testosterone level compared with leuprolide. Relugolix appeared safer to use for men with a medical history of cardiovascular events and showed a 54% lower risk of MACE than leuprolide.
Commentary
Relugolix is a highly selective oral GnRH antagonist that rapidly inhibits pituitary release of luteinizing hormone and FSH. The current phase 3 HERO trial highlights the efficacy of relugolix in regard to testosterone suppression, adding to potential therapeutic options for these men. Relugolix yielded superior sustained testosterone suppression to less than 50 ng/dL throughout the 48-week study period, meeting its primary endpoint. Additionally, relugolix showed superiority in all secondary endpoints across all subgroups of patients. To date, the only GnRH antagonist on the market is degarelix, which is given as a monthly subcutaneous injection.1 Injection-site reactions remain an issue with this formulation.
Cardiovascular disease is the leading cause of death in the United States, and it is known that men with prostate cancer have a higher incidence of cardiovascular disease.2 While data regarding adverse cardiac outcomes with androgen deprivation therapy have been mixed, it is thought that this therapy increases the risk for MACE. There is mounting evidence that GnRH antagonists may have a less detrimental effect on cardiovascular outcomes compared with GnRH agonists. For example, a pooled analysis of 6 phase 3 trials showed a lower incidence of cardiovascular events in men with preexisting cardiovascular disease using the GnRH antagonist degarelix compared with GnRH agonists after 12 months of treatment.3 Furthermore, a more recent phase 2 randomized trial showed that 20% of patients treated with a GnRH agonist developed cardiovascular events, compared to 3% in the GnRH antagonist group. The absolute risk reduction of cardiovascular events at 12 months was 18%.4 The results of the current trial support such findings, showing a 54% reduction in MACE after 48 weeks of therapy when compared with leuprolide (2.9% in relugolix arm vs 6.2% in leuprolide arm). More importantly perhaps, in the subgroup of men with preexisting cardiovascular disease, the benefit was even greater, with a MACE incidence of 3.6% with relugolix compared with 17.8% with leuprolide.
Studies have also shown that second-generation antiandrogens such as enzalutamide are associated with an increased risk of death from cardiovascular causes. For example, data from the recently updated PROSPER trial, which evaluated the use of enzalutamide in men with nonmetastatic, castration-resistant prostate cancer, showed an increased risk of adverse events, including falls, fatigue, hypertension, and death from cardiovascular events.5 Furthermore, adding second-generation antiandrogens to GnRH-agonist therapy is associated with a high risk of cardiovascular events in men with preexisting cardiovascular disease.3 These results were noted in all of the trials of second-generation antiandrogens, including enzalutamide, apalutamide, and darolutamide, in combination with GnRH agonists.6-8 Taken together, one might consider whether the use of a GnRH antagonist would result in improved cardiovascular outcomes in high-risk patients.
In light of the efficacy of relugolix in regard to testosterone suppression highlighted in the current trial, it is likely that its efficacy in regard to cancer outcomes will be similar; however, to date there is no level 1 evidence to support this. Nevertheless, there is a clear association of adverse cardiovascular outcomes in men treated with GnRH agonists, and the notable 54% risk reduction seen in the current trial certainly would support considering the use of a GnRH antagonist for the subgroup of patients with preexisting cardiovascular disease or those at high risk for MACE. Further work is needed to define the role of GnRH antagonists in conjunction with second-generation antiandrogens to help mitigate cardiovascular toxicities.
Clinical Implications
The use of GnRH antagonists should be considered in men with advanced prostate cancer who have underlying cardiovascular disease to help mitigate the risk of MACE. Currently, degarelix is the only commercially available agent; however, pending regulatory approval, oral relugolix may be considered an appropriate oral option in such patients, with data supporting superior testosterone suppressive effects. Further follow-up will be needed.
–Saud Alsubait, MD, Michigan State University, East Lansing, MI
–Daniel Isaac, MD, MS
1. Barkin J, Burton S, Lambert C. Optimizing subcutaneous injection of the gonadotropin-releasing hormone receptor antagonist degarelix. Can J Urol. 2016;23:8179-8183.
2. Higano CS. Cardiovascular disease and androgen axis-targeted drugs for prostate cancer. N Engl J Med. 2020;382:2257-2259.
3. Albertsen PC, Klotz L, Tombal B, et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65:565-573.
4. Margel D, Peer A, Ber Y, et al. Cardiovascular morbidity in a randomized trial comparing GnRH agonist and GnRH antagonist among patients with advanced prostate cancer and preexisting cardiovascular disease. J Urol. 2019;202:1199-1208.
5. Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382:2197-2206.
6. Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418.
7. Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380:1235-1246.
1. Barkin J, Burton S, Lambert C. Optimizing subcutaneous injection of the gonadotropin-releasing hormone receptor antagonist degarelix. Can J Urol. 2016;23:8179-8183.
2. Higano CS. Cardiovascular disease and androgen axis-targeted drugs for prostate cancer. N Engl J Med. 2020;382:2257-2259.
3. Albertsen PC, Klotz L, Tombal B, et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65:565-573.
4. Margel D, Peer A, Ber Y, et al. Cardiovascular morbidity in a randomized trial comparing GnRH agonist and GnRH antagonist among patients with advanced prostate cancer and preexisting cardiovascular disease. J Urol. 2019;202:1199-1208.
5. Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382:2197-2206.
6. Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418.
7. Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380:1235-1246.
Remdesivir Reduces Time to Recovery in Adults Hospitalized With COVID-19: A Meaningful Step in Therapeutic Discovery
Study Overview
Objective. To assess the clinical efficacy and safety of remdesivir in hospitalized adults with laboratory-confirmed COVID-19 and with evidence of lower respiratory tract involvement.
Design. Double-blinded, randomized, placebo-controlled, multicenter trial.
Setting and participants. Enrollment for the study took place between February 21, 2020, and April 19, 2020, at 60 trial sites and 13 subsites in the United States, Denmark, the United Kingdom, Greece, Germany, Korea, Mexico, Spain, Japan, and Singapore. Study participants included patients aged ≥ 18 years who were hospitalized and had laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, as determined by a positive reverse transcription polymerase chain reaction assay on a respiratory specimen. Participants had evidence of lower respiratory tract infection at the time of enrollment; this was defined as radiographic infiltrates by imaging study, peripheral oxygen saturation (SpO2) ≤ 94% on room air, or requiring supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). Exclusion criteria for study participation included abnormal liver enzymes (alanine aminotransferase, aspartate aminotransferase) more than 5 times the upper limit of normal range; impaired renal function or need for hemodialysis or hemofiltration; pregnancy or breastfeeding; or anticipated hospital discharge or transfer to another hospital within 72 hours of enrollment.
Intervention. Participants were randomized in a 1:1 ratio to the remdesivir group or the placebo group and were administered either intravenous infusions of remdesivir (200-mg loading dose on day 1, followed by a 100-mg maintenance dose daily on days 2 through 10, or until hospital discharge or death) or placebo for up to 10 days. Blinding was maintained by masking infusions with an opaque bag and tubing. Randomization was stratified by study site and disease severity at enrollment. Supportive care was delivered to all participants according to the standard of care at each trial site hospital. Clinical status, determined using an 8-category ordinal scale and the National Early Warning Score, was assessed daily for each participant while hospitalized (day 1 through day 29).
Blood samples for safety laboratory tests were collected, and oropharyngeal or nasopharyngeal swab testing was performed for viral RNA detection and quantification on days 1, 3, 5, 8, and 11. All serious adverse events (AEs) and grade 3/4 AEs that represented an increase in severity from day 1 and any grade 2 or higher suspected drug-related hypersensitivity reactions associated with the study drug or placebo administration were recorded.
Main outcome measures. The primary endpoint measure of this study was time to recovery, defined as the first day during the 28 days after enrollment on which a participant satisfied category 1 (ie, not hospitalized, no limitations of activities), 2 (ie, not hospitalized, limitation of activities, home oxygen requirement, or both), or 3 (ie, hospitalized, not requiring supplemental oxygen and no longer requiring ongoing medical care; hospitalization was extended for infection-control reason) on the 8-category ordinal scale. Secondary outcomes included all-cause mortality at 14 and 28 days after enrollment and grade 3/4 AEs and serious AEs that occurred during trial participation. Analysis of the primary outcome was performed using a log-rank test of the time to recovery comparing remdesivir with placebo group, stratified by disease severity.
The study’s primary outcome was initially defined as a difference in clinical status as ascertained by the 8-category ordinal scale between groups of participants who were administered remdesivir versus placebo on day 15. Because of new knowledge gained external to the study about a more protracted COVID-19 clinical course than previously recognized, a change in primary outcome to time to recovery was proposed by trial statisticians, who were unaware of treatment assignments (72 participants had been enrolled) or outcome data (no interim data) on March 22, 2020, with subsequent amendment approval on April 2, 2020. On April 27, 2020, the Data and Safety Monitoring Board (DSMB) reviewed the interim study analysis (with data cutoff date of April 22, 2020) and recommended the report and mortality data to be provided to trial team members from the National Institute of Allergy and Infectious Diseases; these findings were subsequently made public.
Main results. A total of 1107 patients were assessed for eligibility, of whom 1063 underwent randomization, with 541 assigned to remdesivir and 522 to placebo. Results were unblinded early at the recommendation of DSMB due to findings from the interim analysis that showed reduced time to recovery in the group that received remdesivir. As of April 28, 2020, a total of 391 participants in the remdesivir group and 340 participants in the placebo group had completed the trial (day 29), recovered, or died. The mean age of participants was 58.9 ± 15.0 years, the majority were men (64.3%) and were White (53.2%), and the most common prespecified coexisting conditions were hypertension (49.6%), obesity (37.0%), and type 2 diabetes mellitus (29.7%). The vast majority of participants (88.7%) had severe COVID-19 disease at enrollment, defined as requiring invasive or noninvasive mechanical ventilation, requiring supplemental oxygen, SpO2 ≤ 94% on room air, or tachypnea (respiratory rate ≥ 24 breaths per minute).
Based on available data from 1059 participants (538 from the remdesivir group and 521 from the placebo group), those in the remdesivir group had a shorter median recovery time of 11 days (95% confidence interval [CI], 9-12) as compared to 15 days (95% CI, 13-19) in the placebo group, with a rate ratio for recovery of 1.32 (95% CI, 1.12-1.55; P < 0.001). Moreover, the odds of improvement on day 15 in the 8-category ordinal scale score were higher in the remdesivir group, compared to the placebo group (proportional odds model; odds ratio, 1.50; 95% CI, 1.18-1.91; P = 0.001; 844 participants).
Mortality rate by 14 days was numerically lower in the remdesivir group (7.1%) compared to the placebo group (11.9%), but the difference was not statistically significant (Kaplan-Meier, hazard ratio for death, 0.70; 95% CI, 0.47-1.04). Serious AEs were reported in 114 of the 541 (21.1%) participants in the remdesivir group and 141 of the 522 (27.0%) participants in the placebo group. Moreover, grade 3/4 AEs occurred in 156 (28.8%) participants in the remdesivir group and in 172 (33.0%) in the placebo group.
Conclusion. The study found that remdesivir, compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.
Commentary
Since the initial reporting of a cluster of cases of pneumonia in Wuhan, China, on December 31, 2019, SARS-CoV-2 has been identified as the cause of this new disease (COVID-19), and to-date SARS-CoV-2 infection has affected more than 15.2 million people globally, with more than 3.9 million cases in the United States alone.1 Despite an unprecedented global research effort, as well as public-private research partnerships, both in terms of scale and scope, an effective pharmacologic therapy for COVID-19 has so far eluded the scientific and medical community. Early trials of hydroxychloroquine and lopinavir-ritonavir did not demonstrate a clinical benefit in patients with COVID-19.2,3 Moreover, the first randomized controlled trial of remdesivir in COVID-19, a nucleoside analogue prodrug and a broad-spectrum antiviral agent previously shown to have inhibitory effects on pathogenic coronaviruses, was an underpowered study, and thus inconclusive.4 Thus, given the persistence of the COVID-19 pandemic and a current lack of effective vaccines or curative treatments, the study reported by Beigel and colleagues is timely and provides much needed knowledge in developing potential therapies for COVID-19.
The present report described the preliminary results of the first stage of the Adaptive Covid-19 Treatment Trial (ACCT-1), which aimed to evaluate the clinical efficacy and safety of intravenous remdesivir, as compared to placebo, in hospitalized adults with laboratory-confirmed COVID-19. The study itself was well-designed and conducted. The successful enrollment of more than 1000 participants randomized in a 1:1 ratio within a 2-month recruitment window, involving 60 international trial sites, shortly after the emergence of a new global pandemic was remarkable. This study provided the first evidence that remdesivir, an antiviral, can shorten time to recovery by approximately 31% compared to placebo in COVID-19 patients with lower respiratory tract involvement.
Interestingly, this beneficial effect of remdesivir on time to recovery was primarily observed in participants within the severe disease stratum (those requiring supplemental oxygen) at baseline (12 days in remdesivir group versus 18 days in placebo group), but not in those with mild-moderate disease at the time of study enrollment (5 days in either remdesivir or placebo group). Moreover, the beneficial effects of remdesivir on reducing time to recovery was not observed in participants who required mechanical ventilation or ECMO at enrollment. Thus, these preliminary results suggest that COVID-19 disease severity and timing, particularly in patients who require supplemental oxygen but prior to disease progression towards requiring mechanical ventilation, may present a window of opportunity to initiate remdesivir treatment in order to improve outcomes. Further analysis utilizing data from the entire cohort, including outcomes data from the full 28-day follow-up period, may better delineate the subgroup of hospitalized COVID-19 patients who may benefit most from remdesivir. Last, safety data from the present study, along with that reported by Wang and colleagues,4 provides evidence that intravenous remdesivir administration is likely safe in adults during the treatment period.
The preliminary results from the ACCT-1 provide early evidence that remdesivir shortens time to recovery in adult patients hospitalized for COVID-19 with pulmonary involvement. In light of these results, the US Food and Drug Administration issued an emergency use authorization for remdesivir on May 1, 2020, for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease.5 In addition, remdesivir has also recently been approved as a therapy for COVID-19 in Japan, Taiwan, India, Singapore, and the United Arab Emirates, and has received conditional approval for use by the European Commission.6
Although these are encouraging developments in the race to identify effective therapeutics for COVID-19, a number of unanswered questions regarding the administration of remdesivir in the treatment of this disease remain. For instance, in an open-label, randomized, multicenter trial of patients with severe COVID-19 not requiring mechanical ventilation, treatment with a 5-day course versus a 10-day course of intravenous remdesivir did not result in a significant difference in efficacy.7 Thus, more studies are needed to better determine the shortest effective duration of remdesivir therapy in COVID-19 patients with different disease severity. Also, the mortality rate in COVID-19 patients who were treated with remdesivir remained high in the current study. Therefore, there is ample opportunity to evaluate treatment strategies, including multidrug interventions with remdesivir, to reduce mortality and improve clinical outcomes in patients hospitalized with COVID-19.
Applications for Clinical Practice
Remdesivir shortens time to recovery in adult patients hospitalized with COVID-19 who require supplemental oxygen therapy. While much needs to be learned in order to optimize treatment of COVID-19, preliminary findings from the current study provide an important first step towards these discoveries.
–Fred Ko, MD, MS
1. Johns Hopkins University Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Accessed July 16, 2020.
2. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial [published online April 14, 2020]. medRxiv 2020; doi:10.1101/2020.04.10.20060558.
3. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787-1799.
4. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578.
5. Coronavirus (COVID-19) update: FDA issues Emergency Use Authorization for potential COVID-19 treatment. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed July 16, 2020.
6. Gilead’s COVID-19 antiviral remdesivir gets conditional EU clearance. www.reuters.com/article/us-health-coronavirus-eu-remdesivir/gileads-covid-19-antiviral-remdesivir-gets-conditional-eu-clearance-idUSKBN2441GK. Accessed July 6, 2020.
7. Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med. 2020 May 27.doi: 10.1056/NEJMoa2015301. Online ahead of print.
Study Overview
Objective. To assess the clinical efficacy and safety of remdesivir in hospitalized adults with laboratory-confirmed COVID-19 and with evidence of lower respiratory tract involvement.
Design. Double-blinded, randomized, placebo-controlled, multicenter trial.
Setting and participants. Enrollment for the study took place between February 21, 2020, and April 19, 2020, at 60 trial sites and 13 subsites in the United States, Denmark, the United Kingdom, Greece, Germany, Korea, Mexico, Spain, Japan, and Singapore. Study participants included patients aged ≥ 18 years who were hospitalized and had laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, as determined by a positive reverse transcription polymerase chain reaction assay on a respiratory specimen. Participants had evidence of lower respiratory tract infection at the time of enrollment; this was defined as radiographic infiltrates by imaging study, peripheral oxygen saturation (SpO2) ≤ 94% on room air, or requiring supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). Exclusion criteria for study participation included abnormal liver enzymes (alanine aminotransferase, aspartate aminotransferase) more than 5 times the upper limit of normal range; impaired renal function or need for hemodialysis or hemofiltration; pregnancy or breastfeeding; or anticipated hospital discharge or transfer to another hospital within 72 hours of enrollment.
Intervention. Participants were randomized in a 1:1 ratio to the remdesivir group or the placebo group and were administered either intravenous infusions of remdesivir (200-mg loading dose on day 1, followed by a 100-mg maintenance dose daily on days 2 through 10, or until hospital discharge or death) or placebo for up to 10 days. Blinding was maintained by masking infusions with an opaque bag and tubing. Randomization was stratified by study site and disease severity at enrollment. Supportive care was delivered to all participants according to the standard of care at each trial site hospital. Clinical status, determined using an 8-category ordinal scale and the National Early Warning Score, was assessed daily for each participant while hospitalized (day 1 through day 29).
Blood samples for safety laboratory tests were collected, and oropharyngeal or nasopharyngeal swab testing was performed for viral RNA detection and quantification on days 1, 3, 5, 8, and 11. All serious adverse events (AEs) and grade 3/4 AEs that represented an increase in severity from day 1 and any grade 2 or higher suspected drug-related hypersensitivity reactions associated with the study drug or placebo administration were recorded.
Main outcome measures. The primary endpoint measure of this study was time to recovery, defined as the first day during the 28 days after enrollment on which a participant satisfied category 1 (ie, not hospitalized, no limitations of activities), 2 (ie, not hospitalized, limitation of activities, home oxygen requirement, or both), or 3 (ie, hospitalized, not requiring supplemental oxygen and no longer requiring ongoing medical care; hospitalization was extended for infection-control reason) on the 8-category ordinal scale. Secondary outcomes included all-cause mortality at 14 and 28 days after enrollment and grade 3/4 AEs and serious AEs that occurred during trial participation. Analysis of the primary outcome was performed using a log-rank test of the time to recovery comparing remdesivir with placebo group, stratified by disease severity.
The study’s primary outcome was initially defined as a difference in clinical status as ascertained by the 8-category ordinal scale between groups of participants who were administered remdesivir versus placebo on day 15. Because of new knowledge gained external to the study about a more protracted COVID-19 clinical course than previously recognized, a change in primary outcome to time to recovery was proposed by trial statisticians, who were unaware of treatment assignments (72 participants had been enrolled) or outcome data (no interim data) on March 22, 2020, with subsequent amendment approval on April 2, 2020. On April 27, 2020, the Data and Safety Monitoring Board (DSMB) reviewed the interim study analysis (with data cutoff date of April 22, 2020) and recommended the report and mortality data to be provided to trial team members from the National Institute of Allergy and Infectious Diseases; these findings were subsequently made public.
Main results. A total of 1107 patients were assessed for eligibility, of whom 1063 underwent randomization, with 541 assigned to remdesivir and 522 to placebo. Results were unblinded early at the recommendation of DSMB due to findings from the interim analysis that showed reduced time to recovery in the group that received remdesivir. As of April 28, 2020, a total of 391 participants in the remdesivir group and 340 participants in the placebo group had completed the trial (day 29), recovered, or died. The mean age of participants was 58.9 ± 15.0 years, the majority were men (64.3%) and were White (53.2%), and the most common prespecified coexisting conditions were hypertension (49.6%), obesity (37.0%), and type 2 diabetes mellitus (29.7%). The vast majority of participants (88.7%) had severe COVID-19 disease at enrollment, defined as requiring invasive or noninvasive mechanical ventilation, requiring supplemental oxygen, SpO2 ≤ 94% on room air, or tachypnea (respiratory rate ≥ 24 breaths per minute).
Based on available data from 1059 participants (538 from the remdesivir group and 521 from the placebo group), those in the remdesivir group had a shorter median recovery time of 11 days (95% confidence interval [CI], 9-12) as compared to 15 days (95% CI, 13-19) in the placebo group, with a rate ratio for recovery of 1.32 (95% CI, 1.12-1.55; P < 0.001). Moreover, the odds of improvement on day 15 in the 8-category ordinal scale score were higher in the remdesivir group, compared to the placebo group (proportional odds model; odds ratio, 1.50; 95% CI, 1.18-1.91; P = 0.001; 844 participants).
Mortality rate by 14 days was numerically lower in the remdesivir group (7.1%) compared to the placebo group (11.9%), but the difference was not statistically significant (Kaplan-Meier, hazard ratio for death, 0.70; 95% CI, 0.47-1.04). Serious AEs were reported in 114 of the 541 (21.1%) participants in the remdesivir group and 141 of the 522 (27.0%) participants in the placebo group. Moreover, grade 3/4 AEs occurred in 156 (28.8%) participants in the remdesivir group and in 172 (33.0%) in the placebo group.
Conclusion. The study found that remdesivir, compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.
Commentary
Since the initial reporting of a cluster of cases of pneumonia in Wuhan, China, on December 31, 2019, SARS-CoV-2 has been identified as the cause of this new disease (COVID-19), and to-date SARS-CoV-2 infection has affected more than 15.2 million people globally, with more than 3.9 million cases in the United States alone.1 Despite an unprecedented global research effort, as well as public-private research partnerships, both in terms of scale and scope, an effective pharmacologic therapy for COVID-19 has so far eluded the scientific and medical community. Early trials of hydroxychloroquine and lopinavir-ritonavir did not demonstrate a clinical benefit in patients with COVID-19.2,3 Moreover, the first randomized controlled trial of remdesivir in COVID-19, a nucleoside analogue prodrug and a broad-spectrum antiviral agent previously shown to have inhibitory effects on pathogenic coronaviruses, was an underpowered study, and thus inconclusive.4 Thus, given the persistence of the COVID-19 pandemic and a current lack of effective vaccines or curative treatments, the study reported by Beigel and colleagues is timely and provides much needed knowledge in developing potential therapies for COVID-19.
The present report described the preliminary results of the first stage of the Adaptive Covid-19 Treatment Trial (ACCT-1), which aimed to evaluate the clinical efficacy and safety of intravenous remdesivir, as compared to placebo, in hospitalized adults with laboratory-confirmed COVID-19. The study itself was well-designed and conducted. The successful enrollment of more than 1000 participants randomized in a 1:1 ratio within a 2-month recruitment window, involving 60 international trial sites, shortly after the emergence of a new global pandemic was remarkable. This study provided the first evidence that remdesivir, an antiviral, can shorten time to recovery by approximately 31% compared to placebo in COVID-19 patients with lower respiratory tract involvement.
Interestingly, this beneficial effect of remdesivir on time to recovery was primarily observed in participants within the severe disease stratum (those requiring supplemental oxygen) at baseline (12 days in remdesivir group versus 18 days in placebo group), but not in those with mild-moderate disease at the time of study enrollment (5 days in either remdesivir or placebo group). Moreover, the beneficial effects of remdesivir on reducing time to recovery was not observed in participants who required mechanical ventilation or ECMO at enrollment. Thus, these preliminary results suggest that COVID-19 disease severity and timing, particularly in patients who require supplemental oxygen but prior to disease progression towards requiring mechanical ventilation, may present a window of opportunity to initiate remdesivir treatment in order to improve outcomes. Further analysis utilizing data from the entire cohort, including outcomes data from the full 28-day follow-up period, may better delineate the subgroup of hospitalized COVID-19 patients who may benefit most from remdesivir. Last, safety data from the present study, along with that reported by Wang and colleagues,4 provides evidence that intravenous remdesivir administration is likely safe in adults during the treatment period.
The preliminary results from the ACCT-1 provide early evidence that remdesivir shortens time to recovery in adult patients hospitalized for COVID-19 with pulmonary involvement. In light of these results, the US Food and Drug Administration issued an emergency use authorization for remdesivir on May 1, 2020, for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease.5 In addition, remdesivir has also recently been approved as a therapy for COVID-19 in Japan, Taiwan, India, Singapore, and the United Arab Emirates, and has received conditional approval for use by the European Commission.6
Although these are encouraging developments in the race to identify effective therapeutics for COVID-19, a number of unanswered questions regarding the administration of remdesivir in the treatment of this disease remain. For instance, in an open-label, randomized, multicenter trial of patients with severe COVID-19 not requiring mechanical ventilation, treatment with a 5-day course versus a 10-day course of intravenous remdesivir did not result in a significant difference in efficacy.7 Thus, more studies are needed to better determine the shortest effective duration of remdesivir therapy in COVID-19 patients with different disease severity. Also, the mortality rate in COVID-19 patients who were treated with remdesivir remained high in the current study. Therefore, there is ample opportunity to evaluate treatment strategies, including multidrug interventions with remdesivir, to reduce mortality and improve clinical outcomes in patients hospitalized with COVID-19.
Applications for Clinical Practice
Remdesivir shortens time to recovery in adult patients hospitalized with COVID-19 who require supplemental oxygen therapy. While much needs to be learned in order to optimize treatment of COVID-19, preliminary findings from the current study provide an important first step towards these discoveries.
–Fred Ko, MD, MS
Study Overview
Objective. To assess the clinical efficacy and safety of remdesivir in hospitalized adults with laboratory-confirmed COVID-19 and with evidence of lower respiratory tract involvement.
Design. Double-blinded, randomized, placebo-controlled, multicenter trial.
Setting and participants. Enrollment for the study took place between February 21, 2020, and April 19, 2020, at 60 trial sites and 13 subsites in the United States, Denmark, the United Kingdom, Greece, Germany, Korea, Mexico, Spain, Japan, and Singapore. Study participants included patients aged ≥ 18 years who were hospitalized and had laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, as determined by a positive reverse transcription polymerase chain reaction assay on a respiratory specimen. Participants had evidence of lower respiratory tract infection at the time of enrollment; this was defined as radiographic infiltrates by imaging study, peripheral oxygen saturation (SpO2) ≤ 94% on room air, or requiring supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). Exclusion criteria for study participation included abnormal liver enzymes (alanine aminotransferase, aspartate aminotransferase) more than 5 times the upper limit of normal range; impaired renal function or need for hemodialysis or hemofiltration; pregnancy or breastfeeding; or anticipated hospital discharge or transfer to another hospital within 72 hours of enrollment.
Intervention. Participants were randomized in a 1:1 ratio to the remdesivir group or the placebo group and were administered either intravenous infusions of remdesivir (200-mg loading dose on day 1, followed by a 100-mg maintenance dose daily on days 2 through 10, or until hospital discharge or death) or placebo for up to 10 days. Blinding was maintained by masking infusions with an opaque bag and tubing. Randomization was stratified by study site and disease severity at enrollment. Supportive care was delivered to all participants according to the standard of care at each trial site hospital. Clinical status, determined using an 8-category ordinal scale and the National Early Warning Score, was assessed daily for each participant while hospitalized (day 1 through day 29).
Blood samples for safety laboratory tests were collected, and oropharyngeal or nasopharyngeal swab testing was performed for viral RNA detection and quantification on days 1, 3, 5, 8, and 11. All serious adverse events (AEs) and grade 3/4 AEs that represented an increase in severity from day 1 and any grade 2 or higher suspected drug-related hypersensitivity reactions associated with the study drug or placebo administration were recorded.
Main outcome measures. The primary endpoint measure of this study was time to recovery, defined as the first day during the 28 days after enrollment on which a participant satisfied category 1 (ie, not hospitalized, no limitations of activities), 2 (ie, not hospitalized, limitation of activities, home oxygen requirement, or both), or 3 (ie, hospitalized, not requiring supplemental oxygen and no longer requiring ongoing medical care; hospitalization was extended for infection-control reason) on the 8-category ordinal scale. Secondary outcomes included all-cause mortality at 14 and 28 days after enrollment and grade 3/4 AEs and serious AEs that occurred during trial participation. Analysis of the primary outcome was performed using a log-rank test of the time to recovery comparing remdesivir with placebo group, stratified by disease severity.
The study’s primary outcome was initially defined as a difference in clinical status as ascertained by the 8-category ordinal scale between groups of participants who were administered remdesivir versus placebo on day 15. Because of new knowledge gained external to the study about a more protracted COVID-19 clinical course than previously recognized, a change in primary outcome to time to recovery was proposed by trial statisticians, who were unaware of treatment assignments (72 participants had been enrolled) or outcome data (no interim data) on March 22, 2020, with subsequent amendment approval on April 2, 2020. On April 27, 2020, the Data and Safety Monitoring Board (DSMB) reviewed the interim study analysis (with data cutoff date of April 22, 2020) and recommended the report and mortality data to be provided to trial team members from the National Institute of Allergy and Infectious Diseases; these findings were subsequently made public.
Main results. A total of 1107 patients were assessed for eligibility, of whom 1063 underwent randomization, with 541 assigned to remdesivir and 522 to placebo. Results were unblinded early at the recommendation of DSMB due to findings from the interim analysis that showed reduced time to recovery in the group that received remdesivir. As of April 28, 2020, a total of 391 participants in the remdesivir group and 340 participants in the placebo group had completed the trial (day 29), recovered, or died. The mean age of participants was 58.9 ± 15.0 years, the majority were men (64.3%) and were White (53.2%), and the most common prespecified coexisting conditions were hypertension (49.6%), obesity (37.0%), and type 2 diabetes mellitus (29.7%). The vast majority of participants (88.7%) had severe COVID-19 disease at enrollment, defined as requiring invasive or noninvasive mechanical ventilation, requiring supplemental oxygen, SpO2 ≤ 94% on room air, or tachypnea (respiratory rate ≥ 24 breaths per minute).
Based on available data from 1059 participants (538 from the remdesivir group and 521 from the placebo group), those in the remdesivir group had a shorter median recovery time of 11 days (95% confidence interval [CI], 9-12) as compared to 15 days (95% CI, 13-19) in the placebo group, with a rate ratio for recovery of 1.32 (95% CI, 1.12-1.55; P < 0.001). Moreover, the odds of improvement on day 15 in the 8-category ordinal scale score were higher in the remdesivir group, compared to the placebo group (proportional odds model; odds ratio, 1.50; 95% CI, 1.18-1.91; P = 0.001; 844 participants).
Mortality rate by 14 days was numerically lower in the remdesivir group (7.1%) compared to the placebo group (11.9%), but the difference was not statistically significant (Kaplan-Meier, hazard ratio for death, 0.70; 95% CI, 0.47-1.04). Serious AEs were reported in 114 of the 541 (21.1%) participants in the remdesivir group and 141 of the 522 (27.0%) participants in the placebo group. Moreover, grade 3/4 AEs occurred in 156 (28.8%) participants in the remdesivir group and in 172 (33.0%) in the placebo group.
Conclusion. The study found that remdesivir, compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.
Commentary
Since the initial reporting of a cluster of cases of pneumonia in Wuhan, China, on December 31, 2019, SARS-CoV-2 has been identified as the cause of this new disease (COVID-19), and to-date SARS-CoV-2 infection has affected more than 15.2 million people globally, with more than 3.9 million cases in the United States alone.1 Despite an unprecedented global research effort, as well as public-private research partnerships, both in terms of scale and scope, an effective pharmacologic therapy for COVID-19 has so far eluded the scientific and medical community. Early trials of hydroxychloroquine and lopinavir-ritonavir did not demonstrate a clinical benefit in patients with COVID-19.2,3 Moreover, the first randomized controlled trial of remdesivir in COVID-19, a nucleoside analogue prodrug and a broad-spectrum antiviral agent previously shown to have inhibitory effects on pathogenic coronaviruses, was an underpowered study, and thus inconclusive.4 Thus, given the persistence of the COVID-19 pandemic and a current lack of effective vaccines or curative treatments, the study reported by Beigel and colleagues is timely and provides much needed knowledge in developing potential therapies for COVID-19.
The present report described the preliminary results of the first stage of the Adaptive Covid-19 Treatment Trial (ACCT-1), which aimed to evaluate the clinical efficacy and safety of intravenous remdesivir, as compared to placebo, in hospitalized adults with laboratory-confirmed COVID-19. The study itself was well-designed and conducted. The successful enrollment of more than 1000 participants randomized in a 1:1 ratio within a 2-month recruitment window, involving 60 international trial sites, shortly after the emergence of a new global pandemic was remarkable. This study provided the first evidence that remdesivir, an antiviral, can shorten time to recovery by approximately 31% compared to placebo in COVID-19 patients with lower respiratory tract involvement.
Interestingly, this beneficial effect of remdesivir on time to recovery was primarily observed in participants within the severe disease stratum (those requiring supplemental oxygen) at baseline (12 days in remdesivir group versus 18 days in placebo group), but not in those with mild-moderate disease at the time of study enrollment (5 days in either remdesivir or placebo group). Moreover, the beneficial effects of remdesivir on reducing time to recovery was not observed in participants who required mechanical ventilation or ECMO at enrollment. Thus, these preliminary results suggest that COVID-19 disease severity and timing, particularly in patients who require supplemental oxygen but prior to disease progression towards requiring mechanical ventilation, may present a window of opportunity to initiate remdesivir treatment in order to improve outcomes. Further analysis utilizing data from the entire cohort, including outcomes data from the full 28-day follow-up period, may better delineate the subgroup of hospitalized COVID-19 patients who may benefit most from remdesivir. Last, safety data from the present study, along with that reported by Wang and colleagues,4 provides evidence that intravenous remdesivir administration is likely safe in adults during the treatment period.
The preliminary results from the ACCT-1 provide early evidence that remdesivir shortens time to recovery in adult patients hospitalized for COVID-19 with pulmonary involvement. In light of these results, the US Food and Drug Administration issued an emergency use authorization for remdesivir on May 1, 2020, for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease.5 In addition, remdesivir has also recently been approved as a therapy for COVID-19 in Japan, Taiwan, India, Singapore, and the United Arab Emirates, and has received conditional approval for use by the European Commission.6
Although these are encouraging developments in the race to identify effective therapeutics for COVID-19, a number of unanswered questions regarding the administration of remdesivir in the treatment of this disease remain. For instance, in an open-label, randomized, multicenter trial of patients with severe COVID-19 not requiring mechanical ventilation, treatment with a 5-day course versus a 10-day course of intravenous remdesivir did not result in a significant difference in efficacy.7 Thus, more studies are needed to better determine the shortest effective duration of remdesivir therapy in COVID-19 patients with different disease severity. Also, the mortality rate in COVID-19 patients who were treated with remdesivir remained high in the current study. Therefore, there is ample opportunity to evaluate treatment strategies, including multidrug interventions with remdesivir, to reduce mortality and improve clinical outcomes in patients hospitalized with COVID-19.
Applications for Clinical Practice
Remdesivir shortens time to recovery in adult patients hospitalized with COVID-19 who require supplemental oxygen therapy. While much needs to be learned in order to optimize treatment of COVID-19, preliminary findings from the current study provide an important first step towards these discoveries.
–Fred Ko, MD, MS
1. Johns Hopkins University Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Accessed July 16, 2020.
2. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial [published online April 14, 2020]. medRxiv 2020; doi:10.1101/2020.04.10.20060558.
3. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787-1799.
4. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578.
5. Coronavirus (COVID-19) update: FDA issues Emergency Use Authorization for potential COVID-19 treatment. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed July 16, 2020.
6. Gilead’s COVID-19 antiviral remdesivir gets conditional EU clearance. www.reuters.com/article/us-health-coronavirus-eu-remdesivir/gileads-covid-19-antiviral-remdesivir-gets-conditional-eu-clearance-idUSKBN2441GK. Accessed July 6, 2020.
7. Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med. 2020 May 27.doi: 10.1056/NEJMoa2015301. Online ahead of print.
1. Johns Hopkins University Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Accessed July 16, 2020.
2. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial [published online April 14, 2020]. medRxiv 2020; doi:10.1101/2020.04.10.20060558.
3. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787-1799.
4. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578.
5. Coronavirus (COVID-19) update: FDA issues Emergency Use Authorization for potential COVID-19 treatment. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed July 16, 2020.
6. Gilead’s COVID-19 antiviral remdesivir gets conditional EU clearance. www.reuters.com/article/us-health-coronavirus-eu-remdesivir/gileads-covid-19-antiviral-remdesivir-gets-conditional-eu-clearance-idUSKBN2441GK. Accessed July 6, 2020.
7. Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med. 2020 May 27.doi: 10.1056/NEJMoa2015301. Online ahead of print.
Procalcitonin-Guided Antibiotic Discontinuation: An Antimicrobial Stewardship Initiative to Assist Providers
From Western Michigan University, Homer Stryker MD School of Medicine, Kalamazoo, MI (Dr. Vaillant and Dr. Kavanaugh), Ferris State University, Grand Rapids, MI (Dr. Mersfelder), and Bronson Methodist Hospital, Kalamazoo, MI (Dr. Maynard).
Abstract
- Background: Procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin. The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied, and use of this biomarker has been shown to decrease antibiotic usage in clinical trials. We sought to evaluate the impact of a pharmacist-driven initiative regarding discontinuation of antibiotics utilizing procalcitonin levels at a community teaching hospital.
- Methods: We retrospectively gathered baseline data on adult patients admitted to a community teaching hospital who were 18 years of age and older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during admission. We then prospectively identified an intervention group of similar patients using a web-based, real-time clinical surveillance system. When a low procalcitonin level was identified in the intervention group, the participating clinical pharmacists screened for antibiotic use and the indication(s), determined whether the antibiotic could be discontinued based on the low procalcitonin level and the absence of another indication for antibiotics, and, when appropriate, contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. The total antibiotic treatment duration was compared between the baseline and intervention groups.
- Results: A total of 172 patients were included in this study (86 in each group). The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and the intervention (3.34 ± 2.8 days) groups (P = 0.1083). Other patient demographics did not influence antibiotic duration.
- Conclusion: Our study did not demonstrate a difference in total antibiotic treatment duration with the utilization of procalcitonin and an oral communication intervention made by a clinical pharmacist at a community-based teaching hospital. Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis and treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program to reduce antibiotic use and effectively use laboratory values.
Keywords: antibiotic use; bacterial infection; biomarkers; procalcitonin.
Procalcitonin is the precursor of the hormone calcitonin, which is normally produced in the parafollicular cells of the thyroid gland under physiological conditions.1 However, procalcitonin is also released in response to a proinflammatory stimulus, especially that of bacterial origin.1 The source of the procalcitonin surge seen during proinflammatory states is not the parafollicular cells of the thyroid, but rather the neuroendocrine cells of the lung and intestine.1 Stimulants of procalcitonin in these scenarios include bacterial endotoxin, tumor necrosis factor, and interleukin-6.1,2 Due to these observations, procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin.3
The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied.4,5 Various randomized controlled trials have shown that antibiotic stewardship guided by procalcitonin levels resulted in lower rates of antibiotic initiation and shorter duration of antibiotic use.4-6 Similar results were obtained in prospective studies evaluating its role in patients with chronic obstructive pulmonary disease and sepsis.7,8 Based on these data, protocol-driven procalcitonin-guided antibiotic stewardship appears beneficial.
Many of these studies employed rigorous protocols. Studies of procalcitonin use in a so-called real-world setting, in which the provider can order and use procalcitonin levels without the use of protocols, are limited. The objective of our study was to evaluate the impact of a pharmacist-driven initiative on discontinuing antibiotics, if indicated, utilizing single procalcitonin measurement results of < 0.25 mcg/L at a community teaching hospital.
Methods
Our study utilized a 2-phase approach. The first phase was a retrospective chart review to establish baseline data regarding adult inpatients with a low procalcitonin level; these patients were randomly selected over a 1-year period (2017). Patients were included if they were 18 years of age or older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during their admission. Patients admitted to the intensive care unit were excluded. In the second phase, we prospectively identified similar patients admitted between January and March 2018 using a web-based, real-time clinical surveillance system. When patients with low procalcitonin levels were identified, 2 participating clinical pharmacists screened for antibiotic use and indication. If it was determined that the antibiotic could be discontinued as a result of the low procalcitonin level and no additional indication for antibiotics was present, the pharmacist contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. Data collected before and after the intervention included total antibiotic treatment duration, white blood cell count, maximum temperature, age, and procalcitonin level.
A sample size of 86 was calculated to provide an alpha of 0.05 and a power of 0.8. A nonparametric Wilcoxon 2-sample test was used to test for a difference in duration of antibiotic treatment between the baseline and intervention groups. A nonparametric test was used due to right-skewed data. All patients were included in the group analysis, regardless of antibiotic use, as the procalcitonin level may have been used in the decision to initiate antibiotics, and this is more representative of a real-world application of the test. This allowed for detection of a significant decrease of 2 days in antibiotic duration post intervention, with a 10% margin to compensate for potential missing data. Data from 86 patients obtained prior to the pharmacist intervention acted as a control comparison group. Statistical analysis was performed using SAS 9.4.
Results
A total of 172 patients were included in this study: 86 patients prior to the intervention, and 86 after implementation. Baseline demographics, laboratory values, vitals, and principal diagnoses for both groups are shown in Table 1 and Table 2. The most common indications for procalcitonin measurement were pneumonia (45.9%), chronic obstructive pulmonary disease (15.7%), and sepsis (14.5%). The remaining diagnoses were encephalopathy, fever and leukocytosis, skin and soft tissue infection, urinary tract infection or pyelonephritis, bone and joint infection, meningitis, intra-abdominal infection, and asthma exacerbation.
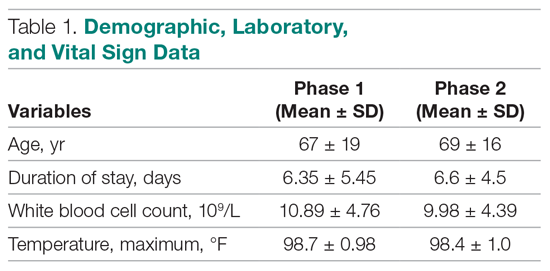
Antibiotic therapy was initiated in 68% of the patients overall, 59% in the baseline group and 76% in the intervention group. The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and intervention (3.34 ± 2.8 days) groups (P = 0.1083). Furthermore, antibiotic treatment duration did not vary significantly with patient age, white blood cell count, maximum temperature, or procalcitonin level in either group. Although there was no difference in total antibiotic treatment duration, a post-hoc analysis revealed a 0.6-day decrease in the interval between the date of procalcitonin measurement and the stop date of antibiotics in the intervention group. The average time from admission to obtaining a procalcitonin level was 3 days in the baseline group and 2 days in the intervention group.
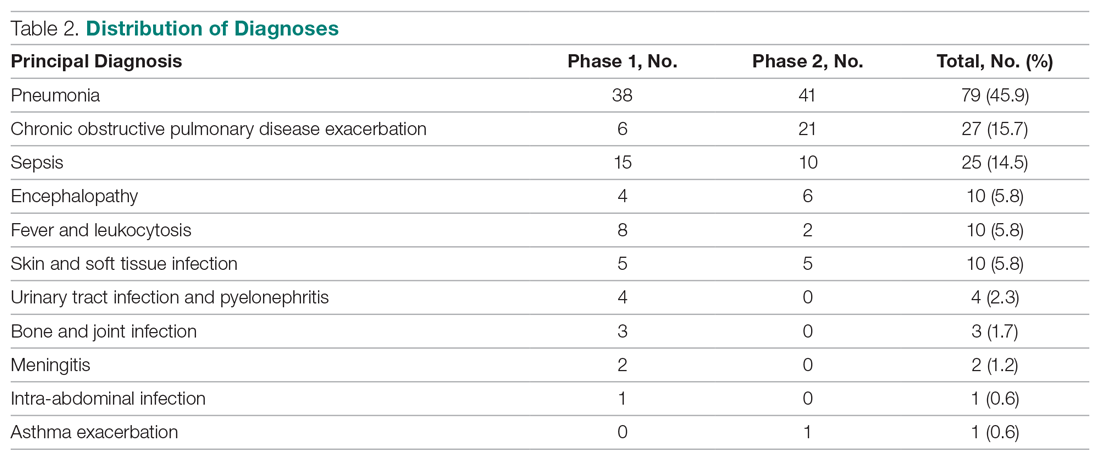
Discussion
Our study did not demonstrate a difference in total antibiotic treatment duration with procalcitonin measurement and an oral communication intervention made by a clinical pharmacist at a community teaching hospital with a well-established antimicrobial stewardship program. This may be due to several factors. First, the providers did not receive ongoing education regarding the appropriate use or interpretation of procalcitonin. The procalcitonin result in the electronic health record references the risk for progression to severe sepsis and/or septic shock, but does not indicate how to use procalcitonin as an aid in antibiotic decision-making. However, a recent study in patients with lower respiratory tract infections treated by providers who had been educated on the use of procalcitonin failed to find a reduction in total antibiotic use.9 Second, our study included hospital-wide use of procalcitonin, and was not limited to infections for which procalcitonin use has the strongest evidence (eg, upper respiratory tract infections, pneumonia, sepsis). Thus, providers may have been less likely to use protocolized guidelines. Last, we did not limit the data on antibiotic duration to patients with a procalcitonin level obtained within a defined time frame from antibiotic initiation or time of admission, and some patients had procalcitonin levels measured several days into their hospital stay. While this is likely to have skewed the data in favor of longer antibiotic treatment courses, it also represents a more realistic way in which this laboratory test is being used. Our post-hoc finding of earlier discontinuation of antibiotics after procalcitonin measurement suggests that our intervention may have influenced the decision to discontinue antibiotics. Such an effect may be augmented if procalcitonin is measured earlier in a hospital admission.
Previous studies have also failed to show that the use of procalcitonin decreased duration of antibiotics.9,10 In the aforementioned study regarding real-world outcomes in patients with lower respiratory tract infections, antibiotic duration was not reduced, despite provider education.9 A large observational study that evaluated real-world outcomes in intensive care unit patients did not find decreased antibiotic use or improved outcomes with procalcitonin use.10 With these large studies evaluating the 2 most common infectious diseases for which procalcitonin has previously been found to have clinical benefit, it is important for institutions to re-evaluate how procalcitonin is being utilized by providers. Furthermore, institutions should explore ways to optimize procalcitonin use and decrease unnecessary health care costs. Notably, the current community-acquired pneumonia guidelines recommend against routine use of procalcitonin.11
Conclusion
Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis or treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program that includes an algorithmic protocol to promote appropriate laboratory testing and reduce total antibiotic use. In addition to improved communication with providers, other interventions need to be investigated to effectively use this biomarker or limit its use.
Acknowledgment: The authors thank the Western Michigan University Department of Epidemiology and Biostatistics for their assistance in preparing this article.
Corresponding author: James Vaillant, MD, Western Michigan University, Homer Stryker MD School of Medicine, 1000 Oakland Drive, Kalamazoo, MI, 49008; [email protected].
Financial disclosures: None.
1. Maruna P, Nedelníková K, Gürlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;(49 suppl 1):S57-S61.
2. Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159:253-264.
3. Vijayan AL, Vanimaya RS, Saikant R, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care. 2017;5:51.
4. Hey J, Thompson-Leduc P, Kirson NY, et al. Procalcitonin guidance in patients with lower respiratory tract infections: A systematic review and meta-analysis. Clin Chem Lab Med. 2018;56:1200-1209.
5. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
6. Huang HB, Peng JM, Weng L, et al. Procalcitonin-guided antibiotic therapy in intensive care unit patients: a systematic review and meta-analysis. Ann Intensive Care. 2017;7:114.
7. Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
8. Prkno A, Wacker C, Brunkhorst FM, Schlattmann P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock—a systematic review and meta-analysis. Crit Care. 2013;17:R291.
9. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infections. N Engl J Med. 2018;379:236-249.
10. Chu DC, Mehta AB, Walkey AJ. Practice patterns and outcomes associated with procalcitonin use in critically ill patients with sepsis. Clin Infect Dis. 2017;64:1509-1515.
11. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
From Western Michigan University, Homer Stryker MD School of Medicine, Kalamazoo, MI (Dr. Vaillant and Dr. Kavanaugh), Ferris State University, Grand Rapids, MI (Dr. Mersfelder), and Bronson Methodist Hospital, Kalamazoo, MI (Dr. Maynard).
Abstract
- Background: Procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin. The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied, and use of this biomarker has been shown to decrease antibiotic usage in clinical trials. We sought to evaluate the impact of a pharmacist-driven initiative regarding discontinuation of antibiotics utilizing procalcitonin levels at a community teaching hospital.
- Methods: We retrospectively gathered baseline data on adult patients admitted to a community teaching hospital who were 18 years of age and older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during admission. We then prospectively identified an intervention group of similar patients using a web-based, real-time clinical surveillance system. When a low procalcitonin level was identified in the intervention group, the participating clinical pharmacists screened for antibiotic use and the indication(s), determined whether the antibiotic could be discontinued based on the low procalcitonin level and the absence of another indication for antibiotics, and, when appropriate, contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. The total antibiotic treatment duration was compared between the baseline and intervention groups.
- Results: A total of 172 patients were included in this study (86 in each group). The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and the intervention (3.34 ± 2.8 days) groups (P = 0.1083). Other patient demographics did not influence antibiotic duration.
- Conclusion: Our study did not demonstrate a difference in total antibiotic treatment duration with the utilization of procalcitonin and an oral communication intervention made by a clinical pharmacist at a community-based teaching hospital. Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis and treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program to reduce antibiotic use and effectively use laboratory values.
Keywords: antibiotic use; bacterial infection; biomarkers; procalcitonin.
Procalcitonin is the precursor of the hormone calcitonin, which is normally produced in the parafollicular cells of the thyroid gland under physiological conditions.1 However, procalcitonin is also released in response to a proinflammatory stimulus, especially that of bacterial origin.1 The source of the procalcitonin surge seen during proinflammatory states is not the parafollicular cells of the thyroid, but rather the neuroendocrine cells of the lung and intestine.1 Stimulants of procalcitonin in these scenarios include bacterial endotoxin, tumor necrosis factor, and interleukin-6.1,2 Due to these observations, procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin.3
The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied.4,5 Various randomized controlled trials have shown that antibiotic stewardship guided by procalcitonin levels resulted in lower rates of antibiotic initiation and shorter duration of antibiotic use.4-6 Similar results were obtained in prospective studies evaluating its role in patients with chronic obstructive pulmonary disease and sepsis.7,8 Based on these data, protocol-driven procalcitonin-guided antibiotic stewardship appears beneficial.
Many of these studies employed rigorous protocols. Studies of procalcitonin use in a so-called real-world setting, in which the provider can order and use procalcitonin levels without the use of protocols, are limited. The objective of our study was to evaluate the impact of a pharmacist-driven initiative on discontinuing antibiotics, if indicated, utilizing single procalcitonin measurement results of < 0.25 mcg/L at a community teaching hospital.
Methods
Our study utilized a 2-phase approach. The first phase was a retrospective chart review to establish baseline data regarding adult inpatients with a low procalcitonin level; these patients were randomly selected over a 1-year period (2017). Patients were included if they were 18 years of age or older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during their admission. Patients admitted to the intensive care unit were excluded. In the second phase, we prospectively identified similar patients admitted between January and March 2018 using a web-based, real-time clinical surveillance system. When patients with low procalcitonin levels were identified, 2 participating clinical pharmacists screened for antibiotic use and indication. If it was determined that the antibiotic could be discontinued as a result of the low procalcitonin level and no additional indication for antibiotics was present, the pharmacist contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. Data collected before and after the intervention included total antibiotic treatment duration, white blood cell count, maximum temperature, age, and procalcitonin level.
A sample size of 86 was calculated to provide an alpha of 0.05 and a power of 0.8. A nonparametric Wilcoxon 2-sample test was used to test for a difference in duration of antibiotic treatment between the baseline and intervention groups. A nonparametric test was used due to right-skewed data. All patients were included in the group analysis, regardless of antibiotic use, as the procalcitonin level may have been used in the decision to initiate antibiotics, and this is more representative of a real-world application of the test. This allowed for detection of a significant decrease of 2 days in antibiotic duration post intervention, with a 10% margin to compensate for potential missing data. Data from 86 patients obtained prior to the pharmacist intervention acted as a control comparison group. Statistical analysis was performed using SAS 9.4.
Results
A total of 172 patients were included in this study: 86 patients prior to the intervention, and 86 after implementation. Baseline demographics, laboratory values, vitals, and principal diagnoses for both groups are shown in Table 1 and Table 2. The most common indications for procalcitonin measurement were pneumonia (45.9%), chronic obstructive pulmonary disease (15.7%), and sepsis (14.5%). The remaining diagnoses were encephalopathy, fever and leukocytosis, skin and soft tissue infection, urinary tract infection or pyelonephritis, bone and joint infection, meningitis, intra-abdominal infection, and asthma exacerbation.

Antibiotic therapy was initiated in 68% of the patients overall, 59% in the baseline group and 76% in the intervention group. The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and intervention (3.34 ± 2.8 days) groups (P = 0.1083). Furthermore, antibiotic treatment duration did not vary significantly with patient age, white blood cell count, maximum temperature, or procalcitonin level in either group. Although there was no difference in total antibiotic treatment duration, a post-hoc analysis revealed a 0.6-day decrease in the interval between the date of procalcitonin measurement and the stop date of antibiotics in the intervention group. The average time from admission to obtaining a procalcitonin level was 3 days in the baseline group and 2 days in the intervention group.

Discussion
Our study did not demonstrate a difference in total antibiotic treatment duration with procalcitonin measurement and an oral communication intervention made by a clinical pharmacist at a community teaching hospital with a well-established antimicrobial stewardship program. This may be due to several factors. First, the providers did not receive ongoing education regarding the appropriate use or interpretation of procalcitonin. The procalcitonin result in the electronic health record references the risk for progression to severe sepsis and/or septic shock, but does not indicate how to use procalcitonin as an aid in antibiotic decision-making. However, a recent study in patients with lower respiratory tract infections treated by providers who had been educated on the use of procalcitonin failed to find a reduction in total antibiotic use.9 Second, our study included hospital-wide use of procalcitonin, and was not limited to infections for which procalcitonin use has the strongest evidence (eg, upper respiratory tract infections, pneumonia, sepsis). Thus, providers may have been less likely to use protocolized guidelines. Last, we did not limit the data on antibiotic duration to patients with a procalcitonin level obtained within a defined time frame from antibiotic initiation or time of admission, and some patients had procalcitonin levels measured several days into their hospital stay. While this is likely to have skewed the data in favor of longer antibiotic treatment courses, it also represents a more realistic way in which this laboratory test is being used. Our post-hoc finding of earlier discontinuation of antibiotics after procalcitonin measurement suggests that our intervention may have influenced the decision to discontinue antibiotics. Such an effect may be augmented if procalcitonin is measured earlier in a hospital admission.
Previous studies have also failed to show that the use of procalcitonin decreased duration of antibiotics.9,10 In the aforementioned study regarding real-world outcomes in patients with lower respiratory tract infections, antibiotic duration was not reduced, despite provider education.9 A large observational study that evaluated real-world outcomes in intensive care unit patients did not find decreased antibiotic use or improved outcomes with procalcitonin use.10 With these large studies evaluating the 2 most common infectious diseases for which procalcitonin has previously been found to have clinical benefit, it is important for institutions to re-evaluate how procalcitonin is being utilized by providers. Furthermore, institutions should explore ways to optimize procalcitonin use and decrease unnecessary health care costs. Notably, the current community-acquired pneumonia guidelines recommend against routine use of procalcitonin.11
Conclusion
Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis or treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program that includes an algorithmic protocol to promote appropriate laboratory testing and reduce total antibiotic use. In addition to improved communication with providers, other interventions need to be investigated to effectively use this biomarker or limit its use.
Acknowledgment: The authors thank the Western Michigan University Department of Epidemiology and Biostatistics for their assistance in preparing this article.
Corresponding author: James Vaillant, MD, Western Michigan University, Homer Stryker MD School of Medicine, 1000 Oakland Drive, Kalamazoo, MI, 49008; [email protected].
Financial disclosures: None.
From Western Michigan University, Homer Stryker MD School of Medicine, Kalamazoo, MI (Dr. Vaillant and Dr. Kavanaugh), Ferris State University, Grand Rapids, MI (Dr. Mersfelder), and Bronson Methodist Hospital, Kalamazoo, MI (Dr. Maynard).
Abstract
- Background: Procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin. The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied, and use of this biomarker has been shown to decrease antibiotic usage in clinical trials. We sought to evaluate the impact of a pharmacist-driven initiative regarding discontinuation of antibiotics utilizing procalcitonin levels at a community teaching hospital.
- Methods: We retrospectively gathered baseline data on adult patients admitted to a community teaching hospital who were 18 years of age and older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during admission. We then prospectively identified an intervention group of similar patients using a web-based, real-time clinical surveillance system. When a low procalcitonin level was identified in the intervention group, the participating clinical pharmacists screened for antibiotic use and the indication(s), determined whether the antibiotic could be discontinued based on the low procalcitonin level and the absence of another indication for antibiotics, and, when appropriate, contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. The total antibiotic treatment duration was compared between the baseline and intervention groups.
- Results: A total of 172 patients were included in this study (86 in each group). The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and the intervention (3.34 ± 2.8 days) groups (P = 0.1083). Other patient demographics did not influence antibiotic duration.
- Conclusion: Our study did not demonstrate a difference in total antibiotic treatment duration with the utilization of procalcitonin and an oral communication intervention made by a clinical pharmacist at a community-based teaching hospital. Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis and treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program to reduce antibiotic use and effectively use laboratory values.
Keywords: antibiotic use; bacterial infection; biomarkers; procalcitonin.
Procalcitonin is the precursor of the hormone calcitonin, which is normally produced in the parafollicular cells of the thyroid gland under physiological conditions.1 However, procalcitonin is also released in response to a proinflammatory stimulus, especially that of bacterial origin.1 The source of the procalcitonin surge seen during proinflammatory states is not the parafollicular cells of the thyroid, but rather the neuroendocrine cells of the lung and intestine.1 Stimulants of procalcitonin in these scenarios include bacterial endotoxin, tumor necrosis factor, and interleukin-6.1,2 Due to these observations, procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin.3
The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied.4,5 Various randomized controlled trials have shown that antibiotic stewardship guided by procalcitonin levels resulted in lower rates of antibiotic initiation and shorter duration of antibiotic use.4-6 Similar results were obtained in prospective studies evaluating its role in patients with chronic obstructive pulmonary disease and sepsis.7,8 Based on these data, protocol-driven procalcitonin-guided antibiotic stewardship appears beneficial.
Many of these studies employed rigorous protocols. Studies of procalcitonin use in a so-called real-world setting, in which the provider can order and use procalcitonin levels without the use of protocols, are limited. The objective of our study was to evaluate the impact of a pharmacist-driven initiative on discontinuing antibiotics, if indicated, utilizing single procalcitonin measurement results of < 0.25 mcg/L at a community teaching hospital.
Methods
Our study utilized a 2-phase approach. The first phase was a retrospective chart review to establish baseline data regarding adult inpatients with a low procalcitonin level; these patients were randomly selected over a 1-year period (2017). Patients were included if they were 18 years of age or older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during their admission. Patients admitted to the intensive care unit were excluded. In the second phase, we prospectively identified similar patients admitted between January and March 2018 using a web-based, real-time clinical surveillance system. When patients with low procalcitonin levels were identified, 2 participating clinical pharmacists screened for antibiotic use and indication. If it was determined that the antibiotic could be discontinued as a result of the low procalcitonin level and no additional indication for antibiotics was present, the pharmacist contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. Data collected before and after the intervention included total antibiotic treatment duration, white blood cell count, maximum temperature, age, and procalcitonin level.
A sample size of 86 was calculated to provide an alpha of 0.05 and a power of 0.8. A nonparametric Wilcoxon 2-sample test was used to test for a difference in duration of antibiotic treatment between the baseline and intervention groups. A nonparametric test was used due to right-skewed data. All patients were included in the group analysis, regardless of antibiotic use, as the procalcitonin level may have been used in the decision to initiate antibiotics, and this is more representative of a real-world application of the test. This allowed for detection of a significant decrease of 2 days in antibiotic duration post intervention, with a 10% margin to compensate for potential missing data. Data from 86 patients obtained prior to the pharmacist intervention acted as a control comparison group. Statistical analysis was performed using SAS 9.4.
Results
A total of 172 patients were included in this study: 86 patients prior to the intervention, and 86 after implementation. Baseline demographics, laboratory values, vitals, and principal diagnoses for both groups are shown in Table 1 and Table 2. The most common indications for procalcitonin measurement were pneumonia (45.9%), chronic obstructive pulmonary disease (15.7%), and sepsis (14.5%). The remaining diagnoses were encephalopathy, fever and leukocytosis, skin and soft tissue infection, urinary tract infection or pyelonephritis, bone and joint infection, meningitis, intra-abdominal infection, and asthma exacerbation.

Antibiotic therapy was initiated in 68% of the patients overall, 59% in the baseline group and 76% in the intervention group. The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and intervention (3.34 ± 2.8 days) groups (P = 0.1083). Furthermore, antibiotic treatment duration did not vary significantly with patient age, white blood cell count, maximum temperature, or procalcitonin level in either group. Although there was no difference in total antibiotic treatment duration, a post-hoc analysis revealed a 0.6-day decrease in the interval between the date of procalcitonin measurement and the stop date of antibiotics in the intervention group. The average time from admission to obtaining a procalcitonin level was 3 days in the baseline group and 2 days in the intervention group.

Discussion
Our study did not demonstrate a difference in total antibiotic treatment duration with procalcitonin measurement and an oral communication intervention made by a clinical pharmacist at a community teaching hospital with a well-established antimicrobial stewardship program. This may be due to several factors. First, the providers did not receive ongoing education regarding the appropriate use or interpretation of procalcitonin. The procalcitonin result in the electronic health record references the risk for progression to severe sepsis and/or septic shock, but does not indicate how to use procalcitonin as an aid in antibiotic decision-making. However, a recent study in patients with lower respiratory tract infections treated by providers who had been educated on the use of procalcitonin failed to find a reduction in total antibiotic use.9 Second, our study included hospital-wide use of procalcitonin, and was not limited to infections for which procalcitonin use has the strongest evidence (eg, upper respiratory tract infections, pneumonia, sepsis). Thus, providers may have been less likely to use protocolized guidelines. Last, we did not limit the data on antibiotic duration to patients with a procalcitonin level obtained within a defined time frame from antibiotic initiation or time of admission, and some patients had procalcitonin levels measured several days into their hospital stay. While this is likely to have skewed the data in favor of longer antibiotic treatment courses, it also represents a more realistic way in which this laboratory test is being used. Our post-hoc finding of earlier discontinuation of antibiotics after procalcitonin measurement suggests that our intervention may have influenced the decision to discontinue antibiotics. Such an effect may be augmented if procalcitonin is measured earlier in a hospital admission.
Previous studies have also failed to show that the use of procalcitonin decreased duration of antibiotics.9,10 In the aforementioned study regarding real-world outcomes in patients with lower respiratory tract infections, antibiotic duration was not reduced, despite provider education.9 A large observational study that evaluated real-world outcomes in intensive care unit patients did not find decreased antibiotic use or improved outcomes with procalcitonin use.10 With these large studies evaluating the 2 most common infectious diseases for which procalcitonin has previously been found to have clinical benefit, it is important for institutions to re-evaluate how procalcitonin is being utilized by providers. Furthermore, institutions should explore ways to optimize procalcitonin use and decrease unnecessary health care costs. Notably, the current community-acquired pneumonia guidelines recommend against routine use of procalcitonin.11
Conclusion
Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis or treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program that includes an algorithmic protocol to promote appropriate laboratory testing and reduce total antibiotic use. In addition to improved communication with providers, other interventions need to be investigated to effectively use this biomarker or limit its use.
Acknowledgment: The authors thank the Western Michigan University Department of Epidemiology and Biostatistics for their assistance in preparing this article.
Corresponding author: James Vaillant, MD, Western Michigan University, Homer Stryker MD School of Medicine, 1000 Oakland Drive, Kalamazoo, MI, 49008; [email protected].
Financial disclosures: None.
1. Maruna P, Nedelníková K, Gürlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;(49 suppl 1):S57-S61.
2. Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159:253-264.
3. Vijayan AL, Vanimaya RS, Saikant R, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care. 2017;5:51.
4. Hey J, Thompson-Leduc P, Kirson NY, et al. Procalcitonin guidance in patients with lower respiratory tract infections: A systematic review and meta-analysis. Clin Chem Lab Med. 2018;56:1200-1209.
5. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
6. Huang HB, Peng JM, Weng L, et al. Procalcitonin-guided antibiotic therapy in intensive care unit patients: a systematic review and meta-analysis. Ann Intensive Care. 2017;7:114.
7. Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
8. Prkno A, Wacker C, Brunkhorst FM, Schlattmann P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock—a systematic review and meta-analysis. Crit Care. 2013;17:R291.
9. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infections. N Engl J Med. 2018;379:236-249.
10. Chu DC, Mehta AB, Walkey AJ. Practice patterns and outcomes associated with procalcitonin use in critically ill patients with sepsis. Clin Infect Dis. 2017;64:1509-1515.
11. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
1. Maruna P, Nedelníková K, Gürlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;(49 suppl 1):S57-S61.
2. Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159:253-264.
3. Vijayan AL, Vanimaya RS, Saikant R, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care. 2017;5:51.
4. Hey J, Thompson-Leduc P, Kirson NY, et al. Procalcitonin guidance in patients with lower respiratory tract infections: A systematic review and meta-analysis. Clin Chem Lab Med. 2018;56:1200-1209.
5. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
6. Huang HB, Peng JM, Weng L, et al. Procalcitonin-guided antibiotic therapy in intensive care unit patients: a systematic review and meta-analysis. Ann Intensive Care. 2017;7:114.
7. Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
8. Prkno A, Wacker C, Brunkhorst FM, Schlattmann P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock—a systematic review and meta-analysis. Crit Care. 2013;17:R291.
9. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infections. N Engl J Med. 2018;379:236-249.
10. Chu DC, Mehta AB, Walkey AJ. Practice patterns and outcomes associated with procalcitonin use in critically ill patients with sepsis. Clin Infect Dis. 2017;64:1509-1515.
11. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
Remdesivir in Hospitalized Adults With Severe COVID-19: Lessons Learned From the First Randomized Trial
Study Overview
Objective. To assess the efficacy, safety, and clinical benefit of remdesivir in hospitalized adults with confirmed pneumonia due to severe SARS-CoV-2 infection.
Design. Randomized, investigator-initiated, placebo-controlled, double-blind, multicenter trial.
Setting and participants. The trial took place between February 6, 2020 and March 12, 2020, at 10 hospitals in Wuhan, China. Study participants included adult patients (aged ≥ 18 years) admitted to hospital who tested positive for SARS-CoV-2 by reverse transcription polymerase chain reaction assay and had the following clinical characteristics: radiographic evidence of pneumonia; hypoxia with oxygen saturation ≤ 94% on room air or a ratio of arterial oxygen partial pressure to fractional inspired oxygen ≤ 300 mm Hg; and symptom onset to enrollment ≤ 12 days. Some of the exclusion criteria for participation in the study were pregnancy or breast feeding, liver cirrhosis, abnormal liver enzymes ≥ 5 times the upper limit of normal, severe renal impairment or receipt of renal replacement therapy, plan for transfer to a non-study hospital, and enrollment in a trial for COVID-19 within the previous month.
Intervention. Participants were randomized in a 2:1 ratio to the remdesivir group or the placebo group and were administered either intravenous infusions of remdesivir (200 mg on day 1 followed by 100 mg daily on days 2-10) or the same volume of placebo for 10 days. Clinical and safety data assessed included laboratory testing, electrocardiogram, and medication adverse effects. Testing of oropharyngeal and nasopharyngeal swab samples, anal swab samples, sputum, and stool was performed for viral RNA detection and quantification on days 1, 3, 5, 7, 10, 14, 21, and 28.
Main outcome measures. The primary endpoint of this study was time to clinical improvement within 28 days after randomization. Clinical improvement was defined as a 2-point reduction in participants’ admission status on a 6-point ordinal scale (1 = discharged or clinical recovery, 6 = death) or live discharge from hospital, whichever came first. Secondary outcomes included all-cause mortality at day 28 and duration of hospital admission, oxygen support, and invasive mechanical ventilation. Virological measures and safety outcomes ascertained included treatment-emergent adverse events, serious adverse events, and premature discontinuation of remdesivir.
The sample size estimate for the original study design was a total of 453 patients (302 in the remdesivir group and 151 in the placebo group). This sample size would provide 80% power, assuming a hazard ratio (HR) of 1.4 comparing remdesivir to placebo, and corresponding to a change in time to clinical improvement of 6 days. The analysis of primary outcome was performed on an intention-to-treat basis. Time to clinical improvement within 28 days was assessed with Kaplan-Meier plots.
Main results. A total of 255 patients were screened, of whom 237 were enrolled and randomized to remdesivir (158) or placebo (79) group. Of the participants in the remdesivir group, 155 started study treatment and 150 completed treatment per protocol. For the participants in the placebo group, 78 started study treatment and 76 completed treatment per-protocol. Study enrollment was terminated after March 12, 2020, before attaining the prespecified sample size, because no additional patients met study eligibility criteria due to various public health measures implemented in Wuhan. The median age of participants was 65 years (IQR, 56-71), the majority were men (56% in remdesivir group vs 65% in placebo group), and the most common comorbidities included hypertension, diabetes, and coronary artery disease. Median time from symptom onset to study enrollment was 10 days (IQR, 9-12). The time to clinical improvement between treatments (21 days for remdesivir group vs 23 days for placebo group) was not significantly different (HR, 1.23; 95% confidence interval [CI], 0.87-1.75). In addition, in participants who received treatment within 10 days of symptom onset, those who were administered remdesivir had a nonsignificant (HR, 1.52; 95% CI, 0.95-2.43) but faster time (18 days) to clinical improvement, compared to those administered placebo (23 days). Moreover, treatment with remdesivir versus placebo did not lead to differences in secondary outcomes (eg, 28-day mortality and duration of hospital stay, oxygen support, and invasive mechanical ventilation), changes in viral load over time, or adverse events between the groups.
Conclusion. This study found that, compared with placebo, intravenous remdesivir did not significantly improve the time to clinical improvement, mortality, or time to clearance of SARS-CoV-2 in hospitalized adults with severe COVID-19. A numeric reduction in time to clinical improvement with early remdesivir treatment (ie, within 10 days of symptom onset) that approached statistical significance was observed in this underpowered study.
Commentary
Within a few short months since its emergence. SARS-CoV-2 infection has caused a global pandemic, posing a dire threat to public health due to its adverse effects on morbidity (eg, respiratory failure, thromboembolic diseases, multiorgan failure) and mortality. To date, no pharmacologic treatment has been shown to effectively improve clinical outcomes in patients with COVID-19. Multiple ongoing clinical trials are being conducted globally to determine potential therapeutic treatments for severe COVID-19. The first clinical trials of hydroxychloroquine and lopinavir-ritonavir, agents traditionally used for other indications, such as malaria and HIV, did not show a clear benefit in COVID-19.1,2 Remdesivir, a nucleoside analogue prodrug, is a broad-spectrum antiviral agent that was previously used for treatment of Ebola and has been shown to have inhibitory effects on pathogenic coronaviruses. The study reported by Wang and colleagues was the first randomized controlled trial (RCT) aimed at evaluating whether remdesivir improves outcomes in patients with severe COVID-19. Thus, the worsening COVID-19 pandemic, coupled with the absence of a curative treatment, underscore the urgency of this trial.
The study was grounded on observational data from several recent case reports and case series centering on the potential efficacy of remdesivir in treating COVID-19.3 The study itself was designed well (ie, randomized, placebo-controlled, double-blind, multicenter) and carefully implemented (ie, high protocol adherence to treatments, no loss to follow-up). The principal limitation of this study was its inability to reach the estimated statistical power of study. Due to successful epidemic control in Wuhan, which led to marked reductions in hospital admission of patients with COVID-19, and implementation of stringent termination criteria per the study protocol, only 237 participants were enrolled, instead of the 453, as specified by the sample estimate. This corresponded to a reduction of statistical power from 80% to 58%. Due to this limitation, the study was underpowered, rendering its findings inconclusive.
Despite this limitation, the study found that those treated with remdesivir within 10 days of symptom onset had a numerically faster time (although not statistically significant) to clinical improvement. This leads to an interesting question: whether remdesivir administration early in COVID-19 course could improve clinical outcomes, a question that warrants further investigation by an adequately powered trial. Also, data from this study provided evidence that intravenous remdesivir administration is likely safe in adults during the treatment period, although the long-term drug effects, as well as the safety profile in pediatric patients, remain unknown at this time.
While the study reported by Wang and colleagues was underpowered and is thus inconclusive, several other ongoing RCTs are evaluating the potential clinical benefit of remdesivir treatment in patients hospitalized with COVID-19. On the date of online publication of this report in The Lancet, the National Institutes of Health (NIH) published a news release summarizing preliminary findings from the Adaptive COVID-19 Treatment Trial (ACTT), which showed positive effects of remdesivir on clinical recovery from advanced COVID-19.4 The ACTT, the first RCT launched in the United States to evaluate experimental treatment for COVID-19, included 1063 hospitalized participants with advanced COVID-19 and lung involvement. Participants who were administered remdesivir had a 31% faster time to recovery compared to those in the placebo group (median time to recovery, 11 days vs 15 days, respectively; P < 0.001), and had near statistically significant improved survival (mortality rate, 8.0% vs 11.6%, respectively; P = 0.059). In response to these findings, the US Food and Drug Administration (FDA) issued an emergency use authorization for remdesivir on May 1, 2020, for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease.5 While the findings noted from the NIH news release are very encouraging and provide the first evidence of a potentially beneficial antiviral treatment for severe COVID-19 in humans, the scientific community awaits the peer-reviewed publication of the ACTT to better assess the safety and effectiveness of remdesivir therapy and determine the trial’s implications in the management of COVID-19.
Applications for Clinical Practice
The discovery of an effective pharmacologic intervention for COVID-19 is of utmost urgency. While the present study was unable to answer the question of whether remdesivir is effective in improving clinical outcomes in patients with severe COVID-19, other ongoing or completed (ie, ACTT) studies will likely address this knowledge gap in the coming months. The FDA’s emergency use authorization for remdesivir provides a glimpse into this possibility.
–Katerina Oikonomou, MD, Brookdale Department of Geriatrics & Palliative Medicine, Icahn School of Medicine at Mount Sinai, New York, NY
–Fred Ko, MD
1. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial [published online April 14, 2020]. medRxiv.org. doi:10.1101/2020.04.10.20060558.
2. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787-1799.
3. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID-19 [published online April 10, 2020]. N Engl J Med. doi:10.1056/NEJMoa2007016.
4. NIH clinical trial shows remdesivir accelerates recovery from advanced COVID-19. www.niaid.nih.gov/news-events/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19. Accessed May 9, 2020
5. Coronavirus (COVID-19) update: FDA issues Emergency Use Authorization for potential COVID-19 treatment. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed May 9, 2020.
Study Overview
Objective. To assess the efficacy, safety, and clinical benefit of remdesivir in hospitalized adults with confirmed pneumonia due to severe SARS-CoV-2 infection.
Design. Randomized, investigator-initiated, placebo-controlled, double-blind, multicenter trial.
Setting and participants. The trial took place between February 6, 2020 and March 12, 2020, at 10 hospitals in Wuhan, China. Study participants included adult patients (aged ≥ 18 years) admitted to hospital who tested positive for SARS-CoV-2 by reverse transcription polymerase chain reaction assay and had the following clinical characteristics: radiographic evidence of pneumonia; hypoxia with oxygen saturation ≤ 94% on room air or a ratio of arterial oxygen partial pressure to fractional inspired oxygen ≤ 300 mm Hg; and symptom onset to enrollment ≤ 12 days. Some of the exclusion criteria for participation in the study were pregnancy or breast feeding, liver cirrhosis, abnormal liver enzymes ≥ 5 times the upper limit of normal, severe renal impairment or receipt of renal replacement therapy, plan for transfer to a non-study hospital, and enrollment in a trial for COVID-19 within the previous month.
Intervention. Participants were randomized in a 2:1 ratio to the remdesivir group or the placebo group and were administered either intravenous infusions of remdesivir (200 mg on day 1 followed by 100 mg daily on days 2-10) or the same volume of placebo for 10 days. Clinical and safety data assessed included laboratory testing, electrocardiogram, and medication adverse effects. Testing of oropharyngeal and nasopharyngeal swab samples, anal swab samples, sputum, and stool was performed for viral RNA detection and quantification on days 1, 3, 5, 7, 10, 14, 21, and 28.
Main outcome measures. The primary endpoint of this study was time to clinical improvement within 28 days after randomization. Clinical improvement was defined as a 2-point reduction in participants’ admission status on a 6-point ordinal scale (1 = discharged or clinical recovery, 6 = death) or live discharge from hospital, whichever came first. Secondary outcomes included all-cause mortality at day 28 and duration of hospital admission, oxygen support, and invasive mechanical ventilation. Virological measures and safety outcomes ascertained included treatment-emergent adverse events, serious adverse events, and premature discontinuation of remdesivir.
The sample size estimate for the original study design was a total of 453 patients (302 in the remdesivir group and 151 in the placebo group). This sample size would provide 80% power, assuming a hazard ratio (HR) of 1.4 comparing remdesivir to placebo, and corresponding to a change in time to clinical improvement of 6 days. The analysis of primary outcome was performed on an intention-to-treat basis. Time to clinical improvement within 28 days was assessed with Kaplan-Meier plots.
Main results. A total of 255 patients were screened, of whom 237 were enrolled and randomized to remdesivir (158) or placebo (79) group. Of the participants in the remdesivir group, 155 started study treatment and 150 completed treatment per protocol. For the participants in the placebo group, 78 started study treatment and 76 completed treatment per-protocol. Study enrollment was terminated after March 12, 2020, before attaining the prespecified sample size, because no additional patients met study eligibility criteria due to various public health measures implemented in Wuhan. The median age of participants was 65 years (IQR, 56-71), the majority were men (56% in remdesivir group vs 65% in placebo group), and the most common comorbidities included hypertension, diabetes, and coronary artery disease. Median time from symptom onset to study enrollment was 10 days (IQR, 9-12). The time to clinical improvement between treatments (21 days for remdesivir group vs 23 days for placebo group) was not significantly different (HR, 1.23; 95% confidence interval [CI], 0.87-1.75). In addition, in participants who received treatment within 10 days of symptom onset, those who were administered remdesivir had a nonsignificant (HR, 1.52; 95% CI, 0.95-2.43) but faster time (18 days) to clinical improvement, compared to those administered placebo (23 days). Moreover, treatment with remdesivir versus placebo did not lead to differences in secondary outcomes (eg, 28-day mortality and duration of hospital stay, oxygen support, and invasive mechanical ventilation), changes in viral load over time, or adverse events between the groups.
Conclusion. This study found that, compared with placebo, intravenous remdesivir did not significantly improve the time to clinical improvement, mortality, or time to clearance of SARS-CoV-2 in hospitalized adults with severe COVID-19. A numeric reduction in time to clinical improvement with early remdesivir treatment (ie, within 10 days of symptom onset) that approached statistical significance was observed in this underpowered study.
Commentary
Within a few short months since its emergence. SARS-CoV-2 infection has caused a global pandemic, posing a dire threat to public health due to its adverse effects on morbidity (eg, respiratory failure, thromboembolic diseases, multiorgan failure) and mortality. To date, no pharmacologic treatment has been shown to effectively improve clinical outcomes in patients with COVID-19. Multiple ongoing clinical trials are being conducted globally to determine potential therapeutic treatments for severe COVID-19. The first clinical trials of hydroxychloroquine and lopinavir-ritonavir, agents traditionally used for other indications, such as malaria and HIV, did not show a clear benefit in COVID-19.1,2 Remdesivir, a nucleoside analogue prodrug, is a broad-spectrum antiviral agent that was previously used for treatment of Ebola and has been shown to have inhibitory effects on pathogenic coronaviruses. The study reported by Wang and colleagues was the first randomized controlled trial (RCT) aimed at evaluating whether remdesivir improves outcomes in patients with severe COVID-19. Thus, the worsening COVID-19 pandemic, coupled with the absence of a curative treatment, underscore the urgency of this trial.
The study was grounded on observational data from several recent case reports and case series centering on the potential efficacy of remdesivir in treating COVID-19.3 The study itself was designed well (ie, randomized, placebo-controlled, double-blind, multicenter) and carefully implemented (ie, high protocol adherence to treatments, no loss to follow-up). The principal limitation of this study was its inability to reach the estimated statistical power of study. Due to successful epidemic control in Wuhan, which led to marked reductions in hospital admission of patients with COVID-19, and implementation of stringent termination criteria per the study protocol, only 237 participants were enrolled, instead of the 453, as specified by the sample estimate. This corresponded to a reduction of statistical power from 80% to 58%. Due to this limitation, the study was underpowered, rendering its findings inconclusive.
Despite this limitation, the study found that those treated with remdesivir within 10 days of symptom onset had a numerically faster time (although not statistically significant) to clinical improvement. This leads to an interesting question: whether remdesivir administration early in COVID-19 course could improve clinical outcomes, a question that warrants further investigation by an adequately powered trial. Also, data from this study provided evidence that intravenous remdesivir administration is likely safe in adults during the treatment period, although the long-term drug effects, as well as the safety profile in pediatric patients, remain unknown at this time.
While the study reported by Wang and colleagues was underpowered and is thus inconclusive, several other ongoing RCTs are evaluating the potential clinical benefit of remdesivir treatment in patients hospitalized with COVID-19. On the date of online publication of this report in The Lancet, the National Institutes of Health (NIH) published a news release summarizing preliminary findings from the Adaptive COVID-19 Treatment Trial (ACTT), which showed positive effects of remdesivir on clinical recovery from advanced COVID-19.4 The ACTT, the first RCT launched in the United States to evaluate experimental treatment for COVID-19, included 1063 hospitalized participants with advanced COVID-19 and lung involvement. Participants who were administered remdesivir had a 31% faster time to recovery compared to those in the placebo group (median time to recovery, 11 days vs 15 days, respectively; P < 0.001), and had near statistically significant improved survival (mortality rate, 8.0% vs 11.6%, respectively; P = 0.059). In response to these findings, the US Food and Drug Administration (FDA) issued an emergency use authorization for remdesivir on May 1, 2020, for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease.5 While the findings noted from the NIH news release are very encouraging and provide the first evidence of a potentially beneficial antiviral treatment for severe COVID-19 in humans, the scientific community awaits the peer-reviewed publication of the ACTT to better assess the safety and effectiveness of remdesivir therapy and determine the trial’s implications in the management of COVID-19.
Applications for Clinical Practice
The discovery of an effective pharmacologic intervention for COVID-19 is of utmost urgency. While the present study was unable to answer the question of whether remdesivir is effective in improving clinical outcomes in patients with severe COVID-19, other ongoing or completed (ie, ACTT) studies will likely address this knowledge gap in the coming months. The FDA’s emergency use authorization for remdesivir provides a glimpse into this possibility.
–Katerina Oikonomou, MD, Brookdale Department of Geriatrics & Palliative Medicine, Icahn School of Medicine at Mount Sinai, New York, NY
–Fred Ko, MD
Study Overview
Objective. To assess the efficacy, safety, and clinical benefit of remdesivir in hospitalized adults with confirmed pneumonia due to severe SARS-CoV-2 infection.
Design. Randomized, investigator-initiated, placebo-controlled, double-blind, multicenter trial.
Setting and participants. The trial took place between February 6, 2020 and March 12, 2020, at 10 hospitals in Wuhan, China. Study participants included adult patients (aged ≥ 18 years) admitted to hospital who tested positive for SARS-CoV-2 by reverse transcription polymerase chain reaction assay and had the following clinical characteristics: radiographic evidence of pneumonia; hypoxia with oxygen saturation ≤ 94% on room air or a ratio of arterial oxygen partial pressure to fractional inspired oxygen ≤ 300 mm Hg; and symptom onset to enrollment ≤ 12 days. Some of the exclusion criteria for participation in the study were pregnancy or breast feeding, liver cirrhosis, abnormal liver enzymes ≥ 5 times the upper limit of normal, severe renal impairment or receipt of renal replacement therapy, plan for transfer to a non-study hospital, and enrollment in a trial for COVID-19 within the previous month.
Intervention. Participants were randomized in a 2:1 ratio to the remdesivir group or the placebo group and were administered either intravenous infusions of remdesivir (200 mg on day 1 followed by 100 mg daily on days 2-10) or the same volume of placebo for 10 days. Clinical and safety data assessed included laboratory testing, electrocardiogram, and medication adverse effects. Testing of oropharyngeal and nasopharyngeal swab samples, anal swab samples, sputum, and stool was performed for viral RNA detection and quantification on days 1, 3, 5, 7, 10, 14, 21, and 28.
Main outcome measures. The primary endpoint of this study was time to clinical improvement within 28 days after randomization. Clinical improvement was defined as a 2-point reduction in participants’ admission status on a 6-point ordinal scale (1 = discharged or clinical recovery, 6 = death) or live discharge from hospital, whichever came first. Secondary outcomes included all-cause mortality at day 28 and duration of hospital admission, oxygen support, and invasive mechanical ventilation. Virological measures and safety outcomes ascertained included treatment-emergent adverse events, serious adverse events, and premature discontinuation of remdesivir.
The sample size estimate for the original study design was a total of 453 patients (302 in the remdesivir group and 151 in the placebo group). This sample size would provide 80% power, assuming a hazard ratio (HR) of 1.4 comparing remdesivir to placebo, and corresponding to a change in time to clinical improvement of 6 days. The analysis of primary outcome was performed on an intention-to-treat basis. Time to clinical improvement within 28 days was assessed with Kaplan-Meier plots.
Main results. A total of 255 patients were screened, of whom 237 were enrolled and randomized to remdesivir (158) or placebo (79) group. Of the participants in the remdesivir group, 155 started study treatment and 150 completed treatment per protocol. For the participants in the placebo group, 78 started study treatment and 76 completed treatment per-protocol. Study enrollment was terminated after March 12, 2020, before attaining the prespecified sample size, because no additional patients met study eligibility criteria due to various public health measures implemented in Wuhan. The median age of participants was 65 years (IQR, 56-71), the majority were men (56% in remdesivir group vs 65% in placebo group), and the most common comorbidities included hypertension, diabetes, and coronary artery disease. Median time from symptom onset to study enrollment was 10 days (IQR, 9-12). The time to clinical improvement between treatments (21 days for remdesivir group vs 23 days for placebo group) was not significantly different (HR, 1.23; 95% confidence interval [CI], 0.87-1.75). In addition, in participants who received treatment within 10 days of symptom onset, those who were administered remdesivir had a nonsignificant (HR, 1.52; 95% CI, 0.95-2.43) but faster time (18 days) to clinical improvement, compared to those administered placebo (23 days). Moreover, treatment with remdesivir versus placebo did not lead to differences in secondary outcomes (eg, 28-day mortality and duration of hospital stay, oxygen support, and invasive mechanical ventilation), changes in viral load over time, or adverse events between the groups.
Conclusion. This study found that, compared with placebo, intravenous remdesivir did not significantly improve the time to clinical improvement, mortality, or time to clearance of SARS-CoV-2 in hospitalized adults with severe COVID-19. A numeric reduction in time to clinical improvement with early remdesivir treatment (ie, within 10 days of symptom onset) that approached statistical significance was observed in this underpowered study.
Commentary
Within a few short months since its emergence. SARS-CoV-2 infection has caused a global pandemic, posing a dire threat to public health due to its adverse effects on morbidity (eg, respiratory failure, thromboembolic diseases, multiorgan failure) and mortality. To date, no pharmacologic treatment has been shown to effectively improve clinical outcomes in patients with COVID-19. Multiple ongoing clinical trials are being conducted globally to determine potential therapeutic treatments for severe COVID-19. The first clinical trials of hydroxychloroquine and lopinavir-ritonavir, agents traditionally used for other indications, such as malaria and HIV, did not show a clear benefit in COVID-19.1,2 Remdesivir, a nucleoside analogue prodrug, is a broad-spectrum antiviral agent that was previously used for treatment of Ebola and has been shown to have inhibitory effects on pathogenic coronaviruses. The study reported by Wang and colleagues was the first randomized controlled trial (RCT) aimed at evaluating whether remdesivir improves outcomes in patients with severe COVID-19. Thus, the worsening COVID-19 pandemic, coupled with the absence of a curative treatment, underscore the urgency of this trial.
The study was grounded on observational data from several recent case reports and case series centering on the potential efficacy of remdesivir in treating COVID-19.3 The study itself was designed well (ie, randomized, placebo-controlled, double-blind, multicenter) and carefully implemented (ie, high protocol adherence to treatments, no loss to follow-up). The principal limitation of this study was its inability to reach the estimated statistical power of study. Due to successful epidemic control in Wuhan, which led to marked reductions in hospital admission of patients with COVID-19, and implementation of stringent termination criteria per the study protocol, only 237 participants were enrolled, instead of the 453, as specified by the sample estimate. This corresponded to a reduction of statistical power from 80% to 58%. Due to this limitation, the study was underpowered, rendering its findings inconclusive.
Despite this limitation, the study found that those treated with remdesivir within 10 days of symptom onset had a numerically faster time (although not statistically significant) to clinical improvement. This leads to an interesting question: whether remdesivir administration early in COVID-19 course could improve clinical outcomes, a question that warrants further investigation by an adequately powered trial. Also, data from this study provided evidence that intravenous remdesivir administration is likely safe in adults during the treatment period, although the long-term drug effects, as well as the safety profile in pediatric patients, remain unknown at this time.
While the study reported by Wang and colleagues was underpowered and is thus inconclusive, several other ongoing RCTs are evaluating the potential clinical benefit of remdesivir treatment in patients hospitalized with COVID-19. On the date of online publication of this report in The Lancet, the National Institutes of Health (NIH) published a news release summarizing preliminary findings from the Adaptive COVID-19 Treatment Trial (ACTT), which showed positive effects of remdesivir on clinical recovery from advanced COVID-19.4 The ACTT, the first RCT launched in the United States to evaluate experimental treatment for COVID-19, included 1063 hospitalized participants with advanced COVID-19 and lung involvement. Participants who were administered remdesivir had a 31% faster time to recovery compared to those in the placebo group (median time to recovery, 11 days vs 15 days, respectively; P < 0.001), and had near statistically significant improved survival (mortality rate, 8.0% vs 11.6%, respectively; P = 0.059). In response to these findings, the US Food and Drug Administration (FDA) issued an emergency use authorization for remdesivir on May 1, 2020, for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease.5 While the findings noted from the NIH news release are very encouraging and provide the first evidence of a potentially beneficial antiviral treatment for severe COVID-19 in humans, the scientific community awaits the peer-reviewed publication of the ACTT to better assess the safety and effectiveness of remdesivir therapy and determine the trial’s implications in the management of COVID-19.
Applications for Clinical Practice
The discovery of an effective pharmacologic intervention for COVID-19 is of utmost urgency. While the present study was unable to answer the question of whether remdesivir is effective in improving clinical outcomes in patients with severe COVID-19, other ongoing or completed (ie, ACTT) studies will likely address this knowledge gap in the coming months. The FDA’s emergency use authorization for remdesivir provides a glimpse into this possibility.
–Katerina Oikonomou, MD, Brookdale Department of Geriatrics & Palliative Medicine, Icahn School of Medicine at Mount Sinai, New York, NY
–Fred Ko, MD
1. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial [published online April 14, 2020]. medRxiv.org. doi:10.1101/2020.04.10.20060558.
2. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787-1799.
3. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID-19 [published online April 10, 2020]. N Engl J Med. doi:10.1056/NEJMoa2007016.
4. NIH clinical trial shows remdesivir accelerates recovery from advanced COVID-19. www.niaid.nih.gov/news-events/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19. Accessed May 9, 2020
5. Coronavirus (COVID-19) update: FDA issues Emergency Use Authorization for potential COVID-19 treatment. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed May 9, 2020.
1. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial [published online April 14, 2020]. medRxiv.org. doi:10.1101/2020.04.10.20060558.
2. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787-1799.
3. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID-19 [published online April 10, 2020]. N Engl J Med. doi:10.1056/NEJMoa2007016.
4. NIH clinical trial shows remdesivir accelerates recovery from advanced COVID-19. www.niaid.nih.gov/news-events/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19. Accessed May 9, 2020
5. Coronavirus (COVID-19) update: FDA issues Emergency Use Authorization for potential COVID-19 treatment. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed May 9, 2020.
Biologics better solo than with methotrexate in psoriatic arthritis
Ustekinumab or a tumor necrosis factor inhibitor (TNFi) are better used alone than with methotrexate in the treatment of psoriatic arthritis suggest the results of PsABio (A Study on Assessment of STELARA and Tumor Necrosis Factor Alpha Inhibitor Therapies in Participants With Psoriatic Arthritis), a large, ongoing, prospective observational study.
The percentages of patients achieving multiple psoriatic arthritis disease activity outcome measures at 6 months were higher if biologic monotherapy was used rather than a biologic in combination with methotrexate.
For example, minimal disease activity (MDA) was achieved by 27.5% of patients taking ustekinumab as monotherapy and by 32.1% of those taking a TNFi alone. When methotrexate was used in combination, the respective percentages of patients achieving MDA were 23.7% and 27.8%.
A similar pattern was seen for very-low disease activity (VLDA), with 9.8% of patients in the ustekinumab monotherapy arm and 12% of those in the TNFi monotherapy arm achieving this target, compared with 5.7% and 5.4% when these drugs were combined with methotrexate.
MDA is defined as meeting five or more cutoffs for seven domains of disease activity, and VLDA for all seven: 0-1 tender joints, 0-1 swollen joints, Psoriasis Area Severity Index 1 or less or body surface area involved 3% or less, 0-1 tender entheseal points, Health Assessment Questionnaire score of 0.5 or less, patient global disease activity visual analog scale score of 20 or lower, and patient pain visual analog scale score of 15 or lower.
Other outcome measures used that showed no advantage of adding methotrexate to these biologics were the Clinical Disease Activity in Psoriatic Arthritis low disease activity and remission scores, the patient acceptable symptoms rate of the 12-item Psoriatic Arthritis Impact of Disease Questionnaire, and improvement in skin involvement.
“Patients were no more likely to achieve lower disease activity or a remission target having received methotrexate than they did just on the biologic drug on its own,” Stefan Siebert, MBBCh, PhD, one of the PsABio investigators, said in an interview.
Dr. Siebert, who is clinical senior lecturer in inflammation and rheumatology at the University of Glasgow (Scotland), was scheduled to present the findings at the British Society for Rheumatology annual conference. The meeting was canceled because of the ongoing COVID-19 crisis. Abstracts and ePosters from the meeting have since been released in a supplement to Rheumatology and via the BSR’s conference app.
First data for ustekinumab
“There certainly doesn’t appear to be any added benefit from using methotrexate on a group level in patients getting ustekinumab and TNF inhibitors,” Dr. Siebert said. “We’ve looked at everything,” he emphasized, and “none of the single domains or composite measures were improved by the addition of methotrexate. I think we knew that for TNF inhibitors, but the key thing is we’ve never known that for ustekinumab, and this is the first study to show that.”
Indeed, the findings match up with those from the SEAM-PsA (Etanercept and Methotrexate in Subjects with Psoriatic Arthritis) study in which patients who were treated with the TNFi etanercept as monotherapy did much better than those given the TNFi in combination with methotrexate or methotrexate alone. While not a randomized trial, PsABio now shows that the same is true for ustekinumab.
Obviously, there are some clear differences between a clinical trial and an observational study such as PsABio. For one thing, there was no randomization and patients taking methotrexate were presumably doing so for good reason, Dr. Siebert said. Secondly, there was no methotrexate-only arm.
PsABio recruited patients who were starting treatment with either ustekinumab or a new TNFi as first-, second-, or third-line biologic disease-modifying antirheumatic therapy (DMARD). “These are all people starting on a biologic, so they’ve already got severe disease and have failed methotrexate on some level. So everything we’ve done is biologic without methotrexate or biologic with methotrexate,” Dr. Siebert explained. Patients may not have been taking methotrexate for a variety of reasons, such as inefficacy or side effects, so PsABio “doesn’t tell us anything about methotrexate on its own.”
Time to rethink ingrained methotrexate use
The rationale for using methotrexate in combination with biologics in psoriatic arthritis comes from its long-standing use in rheumatoid arthritis. Much of what is advocated in guidelines comes from experience in RA, Dr. Siebert said.
“In rheumatoid arthritis, we know that the TNF inhibitors work much better if you use methotrexate, that’s a given,” he noted. “We’ve been trained that you have to have methotrexate to have a biologic. However, PsABio, together with other studies, show that you don’t have to, and you should have a good reason to add methotrexate.”
Individual patients may still benefit from methotrexate use, but the decision to treat all patients the same is not supported by the current evidence. “It’s good that it shows that, actually, once you get someone on a decent biologic, it’s working: It’s doing what it ‘says on the tin’ for a lot of patients. I really think that is the key message, here, that you don’t have to; this reassures clinicians and actually makes them think ‘should this patient be on methotrexate?’ ” Dr. Siebert said.
The PsABio study was funded by Janssen. Dr. Siebert has acted as a consultant to and received research funding from Janssen, UCB, Pfizer, Boehringer Ingelheim, Novartis, and Celgene. He has also acted as a consultant for AbbVie and received research support from Bristol-Myers Squibb.
SOURCE: Siebert S et al. Rheumatology. 2020;59(Suppl 2). doi: 10.1093/rheumatology/keaa110.023, Abstract O24.
Ustekinumab or a tumor necrosis factor inhibitor (TNFi) are better used alone than with methotrexate in the treatment of psoriatic arthritis suggest the results of PsABio (A Study on Assessment of STELARA and Tumor Necrosis Factor Alpha Inhibitor Therapies in Participants With Psoriatic Arthritis), a large, ongoing, prospective observational study.
The percentages of patients achieving multiple psoriatic arthritis disease activity outcome measures at 6 months were higher if biologic monotherapy was used rather than a biologic in combination with methotrexate.
For example, minimal disease activity (MDA) was achieved by 27.5% of patients taking ustekinumab as monotherapy and by 32.1% of those taking a TNFi alone. When methotrexate was used in combination, the respective percentages of patients achieving MDA were 23.7% and 27.8%.
A similar pattern was seen for very-low disease activity (VLDA), with 9.8% of patients in the ustekinumab monotherapy arm and 12% of those in the TNFi monotherapy arm achieving this target, compared with 5.7% and 5.4% when these drugs were combined with methotrexate.
MDA is defined as meeting five or more cutoffs for seven domains of disease activity, and VLDA for all seven: 0-1 tender joints, 0-1 swollen joints, Psoriasis Area Severity Index 1 or less or body surface area involved 3% or less, 0-1 tender entheseal points, Health Assessment Questionnaire score of 0.5 or less, patient global disease activity visual analog scale score of 20 or lower, and patient pain visual analog scale score of 15 or lower.
Other outcome measures used that showed no advantage of adding methotrexate to these biologics were the Clinical Disease Activity in Psoriatic Arthritis low disease activity and remission scores, the patient acceptable symptoms rate of the 12-item Psoriatic Arthritis Impact of Disease Questionnaire, and improvement in skin involvement.
“Patients were no more likely to achieve lower disease activity or a remission target having received methotrexate than they did just on the biologic drug on its own,” Stefan Siebert, MBBCh, PhD, one of the PsABio investigators, said in an interview.
Dr. Siebert, who is clinical senior lecturer in inflammation and rheumatology at the University of Glasgow (Scotland), was scheduled to present the findings at the British Society for Rheumatology annual conference. The meeting was canceled because of the ongoing COVID-19 crisis. Abstracts and ePosters from the meeting have since been released in a supplement to Rheumatology and via the BSR’s conference app.
First data for ustekinumab
“There certainly doesn’t appear to be any added benefit from using methotrexate on a group level in patients getting ustekinumab and TNF inhibitors,” Dr. Siebert said. “We’ve looked at everything,” he emphasized, and “none of the single domains or composite measures were improved by the addition of methotrexate. I think we knew that for TNF inhibitors, but the key thing is we’ve never known that for ustekinumab, and this is the first study to show that.”
Indeed, the findings match up with those from the SEAM-PsA (Etanercept and Methotrexate in Subjects with Psoriatic Arthritis) study in which patients who were treated with the TNFi etanercept as monotherapy did much better than those given the TNFi in combination with methotrexate or methotrexate alone. While not a randomized trial, PsABio now shows that the same is true for ustekinumab.
Obviously, there are some clear differences between a clinical trial and an observational study such as PsABio. For one thing, there was no randomization and patients taking methotrexate were presumably doing so for good reason, Dr. Siebert said. Secondly, there was no methotrexate-only arm.
PsABio recruited patients who were starting treatment with either ustekinumab or a new TNFi as first-, second-, or third-line biologic disease-modifying antirheumatic therapy (DMARD). “These are all people starting on a biologic, so they’ve already got severe disease and have failed methotrexate on some level. So everything we’ve done is biologic without methotrexate or biologic with methotrexate,” Dr. Siebert explained. Patients may not have been taking methotrexate for a variety of reasons, such as inefficacy or side effects, so PsABio “doesn’t tell us anything about methotrexate on its own.”
Time to rethink ingrained methotrexate use
The rationale for using methotrexate in combination with biologics in psoriatic arthritis comes from its long-standing use in rheumatoid arthritis. Much of what is advocated in guidelines comes from experience in RA, Dr. Siebert said.
“In rheumatoid arthritis, we know that the TNF inhibitors work much better if you use methotrexate, that’s a given,” he noted. “We’ve been trained that you have to have methotrexate to have a biologic. However, PsABio, together with other studies, show that you don’t have to, and you should have a good reason to add methotrexate.”
Individual patients may still benefit from methotrexate use, but the decision to treat all patients the same is not supported by the current evidence. “It’s good that it shows that, actually, once you get someone on a decent biologic, it’s working: It’s doing what it ‘says on the tin’ for a lot of patients. I really think that is the key message, here, that you don’t have to; this reassures clinicians and actually makes them think ‘should this patient be on methotrexate?’ ” Dr. Siebert said.
The PsABio study was funded by Janssen. Dr. Siebert has acted as a consultant to and received research funding from Janssen, UCB, Pfizer, Boehringer Ingelheim, Novartis, and Celgene. He has also acted as a consultant for AbbVie and received research support from Bristol-Myers Squibb.
SOURCE: Siebert S et al. Rheumatology. 2020;59(Suppl 2). doi: 10.1093/rheumatology/keaa110.023, Abstract O24.
Ustekinumab or a tumor necrosis factor inhibitor (TNFi) are better used alone than with methotrexate in the treatment of psoriatic arthritis suggest the results of PsABio (A Study on Assessment of STELARA and Tumor Necrosis Factor Alpha Inhibitor Therapies in Participants With Psoriatic Arthritis), a large, ongoing, prospective observational study.
The percentages of patients achieving multiple psoriatic arthritis disease activity outcome measures at 6 months were higher if biologic monotherapy was used rather than a biologic in combination with methotrexate.
For example, minimal disease activity (MDA) was achieved by 27.5% of patients taking ustekinumab as monotherapy and by 32.1% of those taking a TNFi alone. When methotrexate was used in combination, the respective percentages of patients achieving MDA were 23.7% and 27.8%.
A similar pattern was seen for very-low disease activity (VLDA), with 9.8% of patients in the ustekinumab monotherapy arm and 12% of those in the TNFi monotherapy arm achieving this target, compared with 5.7% and 5.4% when these drugs were combined with methotrexate.
MDA is defined as meeting five or more cutoffs for seven domains of disease activity, and VLDA for all seven: 0-1 tender joints, 0-1 swollen joints, Psoriasis Area Severity Index 1 or less or body surface area involved 3% or less, 0-1 tender entheseal points, Health Assessment Questionnaire score of 0.5 or less, patient global disease activity visual analog scale score of 20 or lower, and patient pain visual analog scale score of 15 or lower.
Other outcome measures used that showed no advantage of adding methotrexate to these biologics were the Clinical Disease Activity in Psoriatic Arthritis low disease activity and remission scores, the patient acceptable symptoms rate of the 12-item Psoriatic Arthritis Impact of Disease Questionnaire, and improvement in skin involvement.
“Patients were no more likely to achieve lower disease activity or a remission target having received methotrexate than they did just on the biologic drug on its own,” Stefan Siebert, MBBCh, PhD, one of the PsABio investigators, said in an interview.
Dr. Siebert, who is clinical senior lecturer in inflammation and rheumatology at the University of Glasgow (Scotland), was scheduled to present the findings at the British Society for Rheumatology annual conference. The meeting was canceled because of the ongoing COVID-19 crisis. Abstracts and ePosters from the meeting have since been released in a supplement to Rheumatology and via the BSR’s conference app.
First data for ustekinumab
“There certainly doesn’t appear to be any added benefit from using methotrexate on a group level in patients getting ustekinumab and TNF inhibitors,” Dr. Siebert said. “We’ve looked at everything,” he emphasized, and “none of the single domains or composite measures were improved by the addition of methotrexate. I think we knew that for TNF inhibitors, but the key thing is we’ve never known that for ustekinumab, and this is the first study to show that.”
Indeed, the findings match up with those from the SEAM-PsA (Etanercept and Methotrexate in Subjects with Psoriatic Arthritis) study in which patients who were treated with the TNFi etanercept as monotherapy did much better than those given the TNFi in combination with methotrexate or methotrexate alone. While not a randomized trial, PsABio now shows that the same is true for ustekinumab.
Obviously, there are some clear differences between a clinical trial and an observational study such as PsABio. For one thing, there was no randomization and patients taking methotrexate were presumably doing so for good reason, Dr. Siebert said. Secondly, there was no methotrexate-only arm.
PsABio recruited patients who were starting treatment with either ustekinumab or a new TNFi as first-, second-, or third-line biologic disease-modifying antirheumatic therapy (DMARD). “These are all people starting on a biologic, so they’ve already got severe disease and have failed methotrexate on some level. So everything we’ve done is biologic without methotrexate or biologic with methotrexate,” Dr. Siebert explained. Patients may not have been taking methotrexate for a variety of reasons, such as inefficacy or side effects, so PsABio “doesn’t tell us anything about methotrexate on its own.”
Time to rethink ingrained methotrexate use
The rationale for using methotrexate in combination with biologics in psoriatic arthritis comes from its long-standing use in rheumatoid arthritis. Much of what is advocated in guidelines comes from experience in RA, Dr. Siebert said.
“In rheumatoid arthritis, we know that the TNF inhibitors work much better if you use methotrexate, that’s a given,” he noted. “We’ve been trained that you have to have methotrexate to have a biologic. However, PsABio, together with other studies, show that you don’t have to, and you should have a good reason to add methotrexate.”
Individual patients may still benefit from methotrexate use, but the decision to treat all patients the same is not supported by the current evidence. “It’s good that it shows that, actually, once you get someone on a decent biologic, it’s working: It’s doing what it ‘says on the tin’ for a lot of patients. I really think that is the key message, here, that you don’t have to; this reassures clinicians and actually makes them think ‘should this patient be on methotrexate?’ ” Dr. Siebert said.
The PsABio study was funded by Janssen. Dr. Siebert has acted as a consultant to and received research funding from Janssen, UCB, Pfizer, Boehringer Ingelheim, Novartis, and Celgene. He has also acted as a consultant for AbbVie and received research support from Bristol-Myers Squibb.
SOURCE: Siebert S et al. Rheumatology. 2020;59(Suppl 2). doi: 10.1093/rheumatology/keaa110.023, Abstract O24.
FROM BSR 2020
FDA approves ixekizumab for pediatric plaque psoriasis
The according to an announcement from Lilly.
Patients need to be candidates for systemic therapy or phototherapy and have no known hypersensitivity to the biologic.
The safety, tolerability, and efficacy of the interleukin-17a antagonist were demonstrated in a phase 3 study that included 171 patients aged 6-17 years with moderate to severe plaque psoriasis. At 12 weeks, 89% those on ixekizumab achieved a 75% improvement on Psoriasis Area and Severity Index score, compared with 25% of those on placebo, and 81% achieved a static Physician’s Global Assessment of clear or almost clear, compared with 11% of those on placebo, according to the Lilly statement.
The safety profile seen with ixekizumab (Taltz) among the pediatric patients with plaque psoriasis is consistent with what has been observed among adult patients, although there were higher rates of conjunctivitis, influenza, and urticaria among the pediatric patients, the statement noted. The biologic may increase the risk of infection, and patients should be evaluated for tuberculosis, hypersensitivity, and inflammatory bowel disease. It is also recommended that routine immunizations be completed before initiating treatment.
Ixekizumab was initially approved for treating adults with moderate to severe plaque psoriasis in 2016, followed by approvals for treatment of adults with active psoriatic arthritis in 2017, and for adults with ankylosing spondylitis in August 2019.
The biologic therapies – etanercept, a tumor necrosis factor blocker, and ustekinumab (Stelara), an IL-12/23 antagonist – were previously approved by the FDA for pediatric psoriasis, in children ages 4 years and older and 12 years and older, respectively.
Updated prescribing information for ixekizumab can be found on the Lilly website.
[email protected]
The according to an announcement from Lilly.
Patients need to be candidates for systemic therapy or phototherapy and have no known hypersensitivity to the biologic.
The safety, tolerability, and efficacy of the interleukin-17a antagonist were demonstrated in a phase 3 study that included 171 patients aged 6-17 years with moderate to severe plaque psoriasis. At 12 weeks, 89% those on ixekizumab achieved a 75% improvement on Psoriasis Area and Severity Index score, compared with 25% of those on placebo, and 81% achieved a static Physician’s Global Assessment of clear or almost clear, compared with 11% of those on placebo, according to the Lilly statement.
The safety profile seen with ixekizumab (Taltz) among the pediatric patients with plaque psoriasis is consistent with what has been observed among adult patients, although there were higher rates of conjunctivitis, influenza, and urticaria among the pediatric patients, the statement noted. The biologic may increase the risk of infection, and patients should be evaluated for tuberculosis, hypersensitivity, and inflammatory bowel disease. It is also recommended that routine immunizations be completed before initiating treatment.
Ixekizumab was initially approved for treating adults with moderate to severe plaque psoriasis in 2016, followed by approvals for treatment of adults with active psoriatic arthritis in 2017, and for adults with ankylosing spondylitis in August 2019.
The biologic therapies – etanercept, a tumor necrosis factor blocker, and ustekinumab (Stelara), an IL-12/23 antagonist – were previously approved by the FDA for pediatric psoriasis, in children ages 4 years and older and 12 years and older, respectively.
Updated prescribing information for ixekizumab can be found on the Lilly website.
[email protected]
The according to an announcement from Lilly.
Patients need to be candidates for systemic therapy or phototherapy and have no known hypersensitivity to the biologic.
The safety, tolerability, and efficacy of the interleukin-17a antagonist were demonstrated in a phase 3 study that included 171 patients aged 6-17 years with moderate to severe plaque psoriasis. At 12 weeks, 89% those on ixekizumab achieved a 75% improvement on Psoriasis Area and Severity Index score, compared with 25% of those on placebo, and 81% achieved a static Physician’s Global Assessment of clear or almost clear, compared with 11% of those on placebo, according to the Lilly statement.
The safety profile seen with ixekizumab (Taltz) among the pediatric patients with plaque psoriasis is consistent with what has been observed among adult patients, although there were higher rates of conjunctivitis, influenza, and urticaria among the pediatric patients, the statement noted. The biologic may increase the risk of infection, and patients should be evaluated for tuberculosis, hypersensitivity, and inflammatory bowel disease. It is also recommended that routine immunizations be completed before initiating treatment.
Ixekizumab was initially approved for treating adults with moderate to severe plaque psoriasis in 2016, followed by approvals for treatment of adults with active psoriatic arthritis in 2017, and for adults with ankylosing spondylitis in August 2019.
The biologic therapies – etanercept, a tumor necrosis factor blocker, and ustekinumab (Stelara), an IL-12/23 antagonist – were previously approved by the FDA for pediatric psoriasis, in children ages 4 years and older and 12 years and older, respectively.
Updated prescribing information for ixekizumab can be found on the Lilly website.
[email protected]
Dapagliflozin trial in CKD halted because of high efficacy
AstraZeneca has announced that the phase 3 DAPA-CKD trial for dapagliflozin (Farxiga) in patients with chronic kidney disease has been halted early because of overwhelming efficacy of the drug, at the recommendation of an independent data monitoring committee.
DAPA-CKD is an international, multicenter, randomized, double-blinded trial in 4,245 patients with stage 2-4 chronic kidney disease. Patients received either 10 mg of the dapagliflozin once-daily or a placebo. The primary composite endpoint is worsening of renal function, defined as a composite of an estimated glomerular filtration rate decline of at least 50%, onset of end-stage kidney disease, and death from cardiovascular or renal cause.
The decision to stop the trial came after a routine assessment of efficacy and safety that showed dapagliflozin’s benefits significantly earlier than expected. AstraZeneca will initiate closure of the study, and results will be published and submitted for presentation at a forthcoming medical meeting.
Dapagliflozin is a sodium-glucose transporter 2 inhibitor currently indicated for the treatment type 2 diabetes patients with inadequately controlled type 2 diabetes and for reduction of the risk of hospitalization for heart failure. In August 2019, the drug was granted Fast Track status by the Food and Drug Administration for the treatment of chronic kidney disease. In January 2020, the agency also granted Fast Track status for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients, regardless of diabetes status, with heart failure with reduced ejection fraction.
“Chronic kidney disease patients have limited treatment options, particularly those without type-2 diabetes. We are very pleased the data monitoring committee concluded that patients experienced overwhelming benefit. Farxiga has the potential to change the management of chronic kidney disease for patients around the world,” Mene Pangalos, executive vice president of BioPharmaceuticals R&D, said in the press release.
AstraZeneca has announced that the phase 3 DAPA-CKD trial for dapagliflozin (Farxiga) in patients with chronic kidney disease has been halted early because of overwhelming efficacy of the drug, at the recommendation of an independent data monitoring committee.
DAPA-CKD is an international, multicenter, randomized, double-blinded trial in 4,245 patients with stage 2-4 chronic kidney disease. Patients received either 10 mg of the dapagliflozin once-daily or a placebo. The primary composite endpoint is worsening of renal function, defined as a composite of an estimated glomerular filtration rate decline of at least 50%, onset of end-stage kidney disease, and death from cardiovascular or renal cause.
The decision to stop the trial came after a routine assessment of efficacy and safety that showed dapagliflozin’s benefits significantly earlier than expected. AstraZeneca will initiate closure of the study, and results will be published and submitted for presentation at a forthcoming medical meeting.
Dapagliflozin is a sodium-glucose transporter 2 inhibitor currently indicated for the treatment type 2 diabetes patients with inadequately controlled type 2 diabetes and for reduction of the risk of hospitalization for heart failure. In August 2019, the drug was granted Fast Track status by the Food and Drug Administration for the treatment of chronic kidney disease. In January 2020, the agency also granted Fast Track status for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients, regardless of diabetes status, with heart failure with reduced ejection fraction.
“Chronic kidney disease patients have limited treatment options, particularly those without type-2 diabetes. We are very pleased the data monitoring committee concluded that patients experienced overwhelming benefit. Farxiga has the potential to change the management of chronic kidney disease for patients around the world,” Mene Pangalos, executive vice president of BioPharmaceuticals R&D, said in the press release.
AstraZeneca has announced that the phase 3 DAPA-CKD trial for dapagliflozin (Farxiga) in patients with chronic kidney disease has been halted early because of overwhelming efficacy of the drug, at the recommendation of an independent data monitoring committee.
DAPA-CKD is an international, multicenter, randomized, double-blinded trial in 4,245 patients with stage 2-4 chronic kidney disease. Patients received either 10 mg of the dapagliflozin once-daily or a placebo. The primary composite endpoint is worsening of renal function, defined as a composite of an estimated glomerular filtration rate decline of at least 50%, onset of end-stage kidney disease, and death from cardiovascular or renal cause.
The decision to stop the trial came after a routine assessment of efficacy and safety that showed dapagliflozin’s benefits significantly earlier than expected. AstraZeneca will initiate closure of the study, and results will be published and submitted for presentation at a forthcoming medical meeting.
Dapagliflozin is a sodium-glucose transporter 2 inhibitor currently indicated for the treatment type 2 diabetes patients with inadequately controlled type 2 diabetes and for reduction of the risk of hospitalization for heart failure. In August 2019, the drug was granted Fast Track status by the Food and Drug Administration for the treatment of chronic kidney disease. In January 2020, the agency also granted Fast Track status for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients, regardless of diabetes status, with heart failure with reduced ejection fraction.
“Chronic kidney disease patients have limited treatment options, particularly those without type-2 diabetes. We are very pleased the data monitoring committee concluded that patients experienced overwhelming benefit. Farxiga has the potential to change the management of chronic kidney disease for patients around the world,” Mene Pangalos, executive vice president of BioPharmaceuticals R&D, said in the press release.

