User login
Systemic Treatment for Advanced Hepatocellular Carcinoma
From the University of Alabama at Birmingham, Division of Hematology Oncology, Birmingham, AL, and the University of South Alabama, Division of Hematology Oncology, Mobile, AL. Dr. Paluri and Dr. Hatic contributed equally to this article.
Abstract
- Objective: To review systemic treatment options for patients with locally advanced unresectable hepatocellular carcinoma (HCC).
- Methods: Review of the literature.
- Results: The paradigm of what constitutes first-line treatment for advanced HCC is shifting. Until recently, many patients with advanced HCC were treated with repeated locoregional therapies, such as transartertial embolization (TACE). However, retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
- Conclusion: Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
Keywords: liver cancer; molecular therapy; immunotherapy.
Hepatocellular carcinoma (HCC) represents 90% of primary liver malignancies. It is the fifth most common malignancy in males and the ninth most common in females worldwide.1 In contrast to other major cancers (colon, breast, prostate), the incidence of and mortality from HCC has increased over the past decade, following a brief decline between 1999 and 2004.2 The epidemiology and incidence of HCC is closely linked to chronic liver disease and conditions predisposing to liver cirrhosis. Worldwide, hepatitis B virus infection is the leading cause of liver cirrhosis and, hence, HCC. In the United States, 50% of HCC cases are linked to hepatitis C virus (HCV) infection. Diabetes mellitus and alcoholic and nonalcoholic steatohepatitis are the other major etiologies of HCC. Indeed, the metabolic syndrome, independent of other factors, is associated with a 2-fold increase in the risk of HCC.3
Although most cases of HCC are predated by liver cirrhosis, in about 20% of patients HCC occurs without liver cirrhosis.4 Similar to other malignancies, surgery in the form of resection (for isolated lesions in the context of good liver function) or liver transplant (for low-volume disease with mildly impaired liver function) provides the best chance of a cure. Locoregional therapies involving hepatic artery–directed therapy are offered for patients with more advanced disease that is limited to the liver, while systemic therapy is offered for advanced unresectable disease that involves portal vein invasion, lymph nodes, and distant metastasis. The
Molecular Pathogenesis
Similar to other malignancies, a multistep process of carcinogenesis, with accumulation of genomic alterations at the molecular and cellular levels, is recognized in HCC. In about 80% of cases, repeated and chronic injury, inflammation, and repair lead to a distortion of normal liver architecture and development of cirrhotic nodules. Exome sequencing of HCC tissues has identified risk factor–specific mutational signatures, including those related to the tumor microenvironment, and defined the extensive landscape of altered genes and pathways in HCC (eg, angiogenic and MET pathways).7 In the Schulze et al study, the frequency of alterations that could be targeted by available Food and Drug Administration (FDA)–approved drugs comprised either amplifications or mutations of FLTs (6%), FGF3 or 4 or 19 (4%), PDGFRs (3%), VEGFA (1%), HGF (3%), MTOR (2%), EGFR (1%), FGFRs (1%), and MET (1%).7 Epigenetic modification of growth-factor expression, particularly insulin-like growth factor 2 and transforming growth factor alpha, and structural alterations that lead to loss of heterozygosity are early events that cause hepatocyte proliferation and progression of dysplastic nodules.8,9 Advances in whole-exome sequencing have identified TERT promoter mutations, leading to activation of telomerase, as an early event in HCC pathogenesis. Other commonly altered genes include CTNNB1 (B-Catenin) and TP53. As a group, alterations in the MAP kinase pathway genes occur in about 40% of HCC cases.
Actionable oncogenic driver alterations are not as common as tumor suppressor pathway alterations in HCC, making targeted drug development challenging.10,11 The FGFR (fibroblast growth factor receptor) pathway, which plays a critical role in carcinogenesis-related cell growth, survival, neo-angiogenesis, and acquired resistance to other cancer treatments, is being explored as a treatment target.12 The molecular characterization of HCC may help with identifying new biomarkers and present opportunities for developing therapeutic targets.
CASE PRESENTATION
A 61-year-old man with a history of chronic hepatitis C and hypertension presents to his primary care physician due to right upper quadrant pain. Laboratory evaluation shows transaminases elevated 2 times the upper limit of normal. This leads to an ultrasound and follow-up magnetic resonance imaging. Imaging shows diffuse cirrhotic changes, with a 6-cm, well-circumscribed lesion within the left lobe of the liver that shows rapid arterial enhancement with venous washout. These vascular characteristics are consistent with HCC. In addition, 2 satellite lesions in the left lobe and sonographic evidence of invasion into the portal vein are present. Periportal lymph nodes are pathologically enlarged.
The physical examination is unremarkable, except for mild tenderness over the right upper quadrant of the abdomen. Serum bilirubin, albumin, platelets, and international normalized ratio are normal, and alpha fetoprotein (AFP) is elevated at 1769 ng/mL. The patient’s family history is unremarkable for any major illness or cancer. Computed tomography scan of the chest and pelvis shows no evidence of other lesions. His liver disease is classified as Child–Pugh A. Due to locally advanced presentation, the tumor is deemed to be nontransplantable and unresectable, and is staged as BCLC-C. The patient continues to work and his performance status is ECOG (
What systemic treatment would you recommend for this patient with locally advanced unresectable HCC with nodal metastasis?
First-Line Therapeutic Options
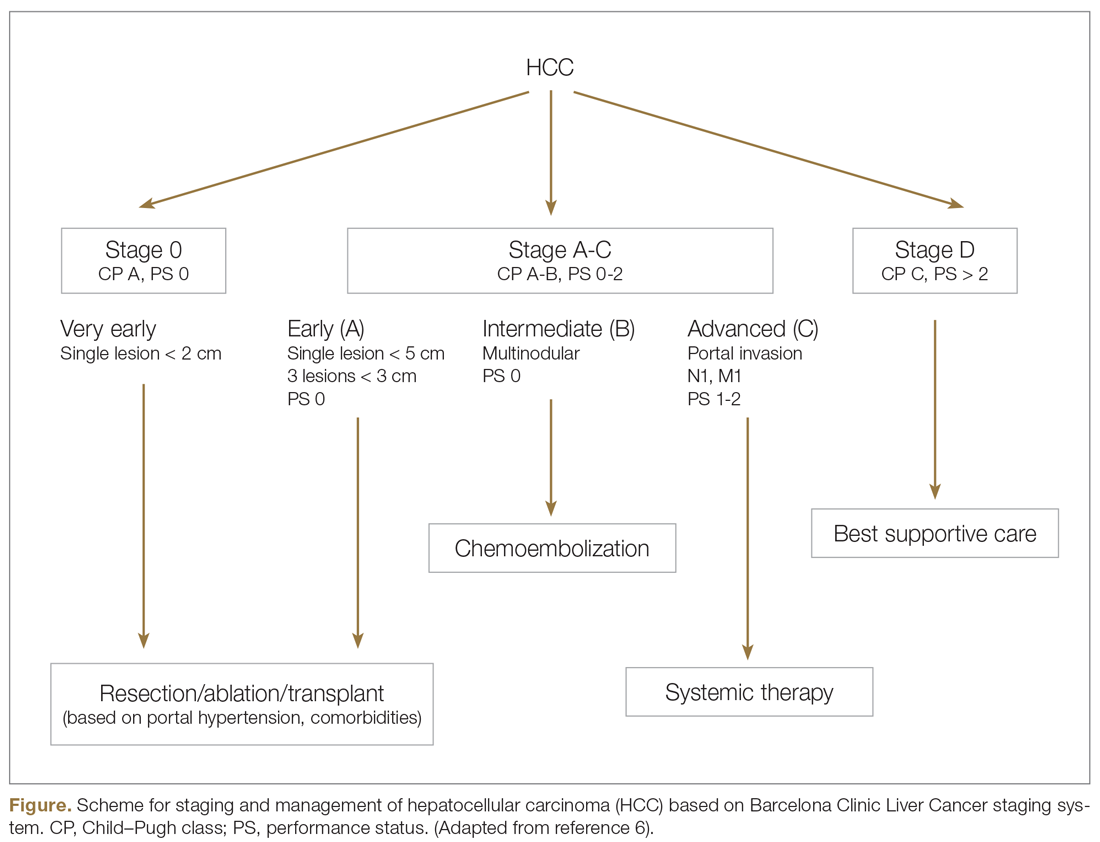
Systemic treatment of HCC is challenging because of the underlying liver cirrhosis and hepatic dysfunction present in most patients. Overall prognosis is therefore dependent on the disease biology and burden and on the degree of hepatic dysfunction. These factors must be considered in patients with advanced disease who present for systemic therapy. As such, patients with BCLC class D HCC with poor performance status and impaired liver function are better off with best supportive care and hospice services (Figure). Table 1 and Table 2 outline the landmark trials that led to the approval of agents for advanced HCC treatment.
Sorafenib
In the patient with BCLC class C HCC who has preserved liver function (traditionally based on a Child–Pugh score of ≤ 6 and a decent functional status [ECOG performance status 1-2]), sorafenib is the first FDA-approved first-line treatment. Sorafenib is a small-molecule tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR) kinase signaling, in addition to many other tyrosine kinase pathways (including the platelet-derived growth factor and Raf-Ras pathways). Evidence for the clinical benefit of sorafenib comes from the SHARP trial.13 This was a multinational, but primarily European, randomized phase 3 study that compared sorafenib to best supportive care for advanced HCC in patients with a Child–Pugh score ≤ 6A and a robust performance status (ECOG 0 and 1). Overall survival (OS) with placebo and sorafenib was 7.9 months and 10.7 months, respectively. There was no difference in time to radiologic progression, and the progression-free survival (PFS) at 4 months was 62% with sorafenib and 42% with placebo. Patients with HCV-associated HCC appeared to derive a more substantial benefit from sorafenib.14 In a smaller randomized study of sorafenib in Asian patients with predominantly hepatitis B–associated HCC, OS in the sorafenib and best supportive care arms was lower than that reported in the SHARP study (6.5 months vs 4.2 months), although OS still was longer in the sorafenib group.15
Significant adverse events reported with sorafenib include fatigue (30%), hand and foot syndrome (30%), diarrhea (15%), and mucositis (10%). Major proportions of patients in the clinical setting have not tolerated the standard dose of 400 mg twice daily. Dose-adjusted administration of sorafenib has been advocated in patients with more impaired liver function (Child–Pugh class 7B) and bilirubin of 1.5 to 3 times the upper limit of normal, although it is unclear whether these patients are deriving any benefit from sorafenib.16 At this time, in a patient with preserved liver function, starting with 400 mg twice daily, followed by dose reduction based on toxicity, remains standard.
Lenvatinib
After multiple attempts to develop newer first-line treatments for HCC,
Second-Line Therapeutic Options
Following the sorafenib approval, clinical studies of several other agents did not meet their primary endpoint and failed to show improvement in clinical outcomes compared to sorafenib. However, over the past years the treatment landscape for advanced HCC has been changed with the approval of several agents in the second line. The overall response rate (ORR) has become the new theme for management of advanced disease. With multiple therapeutic options available, optimal sequencing now plays a critical role, especially for transitioning from locoregional to systemic therapy. Five drugs are now indicated for second-line treatment of patients who progressed on or were intolerant to sorafenib: regorafenib, cabozantinib, ramucirumab, nivolumab, and pembrolizumab.
Regorafenib
Regorafenib was evaluated in the advanced HCC setting in a single-arm, phase 2 trial involving 36 patients with Child–Pugh class A liver disease who had progressed on prior sorafenib.18 Patients received regorafenib 160 mg orally once daily for 3 weeks on/1 week off cycles. Disease control was achieved in 72% of patients, with stable disease in 25 patients (69%). Based on these results, regorafenib was further evaluated in the multicenter, phase 3, 2:1 randomized, double-blind, placebo-controlled RESORCE study, which enrolled 573 patients.19 Due to the overlapping safety profiles of sorafenib and regorafenib, the inclusion criteria required patients to have tolerated a sorafenib dose of at least 400 mg daily for 20 of the past 28 days of treatment prior to enrollment. The primary endpoint of the study, OS, was met (median OS of 10.6 months in regorafenib arm versus 7.8 months in placebo arm; hazard ratio [HR], 0.63; P < 0.0001).
Cabozantinib
CELESTIAL was a phase 3, double-blind study that assessed the efficacy of cabozantinib versus placebo in patients with advanced HCC who had received prior sorafenib.22 In this study, 707 patients with Child–Pugh class A liver disease who progressed on at least 1 prior systemic therapy were randomized in a 2:1 ratio to treatment with cabozantinib at 60 mg daily or placebo. Patients treated with cabozantinib had a longer OS (10.2 months vs 8.0 months), resulting in a 24% reduction in the risk of death (HR, 0.76), and a longer median PFS (5.2 months versus 1.9 months). The disease control rate was higher with cabozantinib (64% vs 33%) as well. The incidence of high‐grade adverse events in the cabozantinib group was twice that of the placebo group. Common adverse events reported with cabozantinib included HFSR (17%), hypertension (16%), increased aspartate aminotransferase (12%), fatigue (10%), and diarrhea (10%).
Ramucirumab
REACH was a phase 3 study exploring the efficacy of ramucirumab that did not meet its primary endpoint; however, the subgroup analysis in AFP-high patients showed an OS improvement with ramucirumab.23 This led to the phase 3 REACH-2 trial, a multicenter, randomized, double-blind biomarker study in patients with advanced HCC who either progressed on or were intolerant to sorafenib and had an AFP level ≥ 400 ng/mL.24 Patients were randomized to ramucirumab 8 mg/kg every 2 weeks or placebo. The study met its primary endpoint, showing improved OS (8.5 months vs 7.3 months; P = 0.0059). The most common treatment-related adverse events in the ramucirumab group were ascites (5%), hypertension (12%), asthenia (5%), malignant neoplasm progression (6%), increased aspartate aminotransferase concentration (5%), and thrombocytopenia.
Immunotherapy
HCC is considered an inflammation-induced cancer, which renders immunotherapeutic strategies more appealing. The PD-L1/PD-1 pathway is the critical immune checkpoint mechanism and is an important target for treatment. HCC uses a complex, overlapping set of mechanisms to evade cancer-specific immunity and to suppress the immune system. Initial efforts to develop immunotherapies for HCC focused on anti-PD-1 and anti-PD-L1 antibodies. CheckMate 040 evaluated nivolumab in 262 sorafenib-naïve and -treated patients with advanced HCC (98% with Child–Pugh scores of 5 or 6), with a median follow-up of 12.9 months.25 In sorafenib-naïve patients (n = 80), the ORR was 23%, and the disease control rate was 63%. In sorafenib-treated patients (n = 182), the ORR was 18%. Response was not associated with PD-L1 expression. Durable objective responses, a manageable safety profile, and promising efficacy led the FDA to grant accelerated approval of nivolumab for the treatment of patients with HCC who have been previously treated with sorafenib. Based on this, the phase 3 randomized CheckMate-459 trial evaluated the efficacy of nivolumab versus sorafenib in the first-line. Median OS and ORR were better with nivolumab (16.4 months vs 14.7 months; HR 0.85; P = 0.752; and 15% [with 5 complete responses] vs 7%), as was the safety profile (22% vs 49% reporting grade 3 and 4 adverse events). 26
The KEYNOTE-224 study27 evaluated pembrolizumab in 104 patients with previously treated advanced HCC. This study showed an ORR of 17%, with 1 complete response and 17 partial responses. One-third of the patients had progressive disease, while 46 had stable disease. Among those who responded, 56% maintained a durable response for more than 1 year. Subsequently, in KEYNOTE 240, pembrolizumab showed an improvement in OS (13.9 months vs 10.6 months; HR, 0.78; P = 0.0238) and PFS (3.0 months versus 2.8 months; HR, 0.78; P = 0.0186) compared with placebo.28 The ORR for pembrolizumab was 16.9% (95% confidence interval [CI], 12.7%-21.8%) versus 2.2% (95% CI, 0.5%-6.4%; P = 0.00001) for placebo. Mean duration of response was 13.8 months.
In the IMbrave150 trial, atezolizumab/bevacizumab combination, compared to sorafenib, had better OS (not estimable vs 13.2 months; P = 0.0006), PFS (6.8 months vs 4.5 months, P < 0.0001), and ORR (33% vs 13%, P < 0.0001), but grade 3-4 events were similar.29 This combination has potential for first-line approval. The COSMIC–312 study is comparing the combination of cabozantinib and atezolizumab to sorafenib monotherapy and cabozantinib monotherapy in advanced HCC.
Resistance to immunotherapy can be extrinsic, associated with activation mechanisms of T-cells, or intrinsic, related to immune recognition, gene expression, and cell-signaling pathways.30 Tumor-immune heterogeneity and antigen presentation contribute to complex resistance mechanisms.31,32 Although clinical outcomes have improved with immune checkpoint inhibitors, the response rate is low and responses are inconsistent, likely due to an immunosuppressive tumor microenvironment.33 Therefore, several novel combinations of checkpoint inhibitors and targeted drugs are being evaluated to bypass some of the resistance mechanisms (Table 3).
Chemotherapy
Multiple combinations of cytotoxic regimens have been evaluated, but efficacy has been modest, suggesting the limited role for traditional chemotherapy in the systemic management of advanced HCC. Response rates to chemotherapy are low and responses are not durable. Gemcitabine- and doxorubicin-based treatment and FOLFOX (5-fluorouracil, leucovorin, oxaliplatin) are some regimens that have been studied, with a median OS of less than 1 year for these regimens.34-36 FOLFOX had a higher response rate (8.15% vs 2.67%; P = 0.02) and longer median OS (6.40 months versus 4.97 months; HR, 0.80; 95% CI, 0.63-1.02; P = 0.07) than doxorubicin.34 With the gemcitabine/oxaliplatin combination, ORR was 18%, with stable disease in 58% of patients, and median PFS and OS were 6.3 months and 11.5 months, respectively.35 In a study that compared doxorubicin and PIAF (cisplatin/interferon a-2b/doxorubicin/5-fluorouracil), median OS was 6.83 months and 8.67 months, respectively (P = 0.83). The hazard ratio for death from any cause in the PIAF group compared with the doxorubicin group was 0.97 (95% CI, 0.71-1.32). PIAF had a higher ORR (20.9%; 95% CI, 12.5%-29.2%) than doxorubicin (10.5%; 95% CI, 3.9%-16.9%).
The phase 3 ALLIANCE study evaluated the combination of sorafenib and doxorubicin in treatment-naïve HCC patients with Child–Pugh class A liver disease, and did not demonstrate superiority with the addition of cytotoxic chemotherapy.37 Indeed, the combination of chemotherapy with sorafenib appears harmful in terms of lower OS (9.3 months vs 10.6 months; HR, 1.06; 95% CI, 0.8-1.4) and worse toxicity. Patients treated with the combination experienced more hematologic (37.8% vs 8.1%) and nonhematologic adverse events (63.6% vs 61.5%).
Locoregional Therapy
The role of locoregional therapy in advanced HCC remains the subject of intense debate. Patients with BCLC stage C HCC with metastatic disease and those with lymph node involvement are candidates for systemic therapy. The optimal candidate for locoregional therapy is the patient with localized intermediate-stage disease, particularly hepatic artery–delivered therapeutic interventions. However, the presence of a solitary large tumor or portal vein involvement constitutes gray areas regarding which therapy to deliver directly to the tumor via the hepatic artery, and increasingly stereotactic body radiation therapy is being offered.
Transarterial Chemoembolization
Transarterial chemoembolization (TACE), with or without chemotherapy, is the most widely adopted locoregional therapy in the management of HCC. TACE exploits the differential vascular supply to the HCC and normal liver parenchyma. Normal liver receives only one-fourth of its blood supply from the hepatic artery (three-fourths from the portal vein), whereas HCC is mainly supplied by the hepatic artery. A survival benefit for TACE compared to best supportive care is widely acknowledged for intermediate-stage HCC, and transarterial embolization (TAE) with gelatin sponge or microspheres is noninferior to TACE.38,39 Overall safety profile and efficacy inform therapy selection in advanced HCC, although the evidence for TACE in advanced HCC is less robust. Although single-institution experiences suggest survival numbers similar to and even superior to sorafenib,40,41 there is a scarcity of large randomized clinical trial data to back this up. Based on this, patients with advanced HCC should only be offered liver-directed therapy within a clinical trial or on a case-by-case basis under multidisciplinary tumor board consensus.
A serious adverse effect of TACE is post-embolization syndrome, which occurs in about 30% of patients and may be associated with poor prognosis.42 The syndrome consists of right upper quadrant abdominal pain, malaise, and worsening nausea/vomiting following the embolization procedure. Laboratory abnormalities and other complications may persist for up to 30 days after the procedure. This is a concern, because post-embolization syndrome may affect the ability to deliver systemic therapy.
Transarterial Radioembolization
In the past few years, there has been an uptick in the utilization of transarterial radioembolization (TARE), which instead of delivering glass beads, as done in TAE, or chemotherapy-infused beads, as done in TACE, delivers the radioisotope Y-90 to the tumor via the hepatic artery. TARE is able to administer larger doses of radiation to the tumor while avoiding normal liver tissue, as compared to external-beam radiation. There has been no head-to-head comparison of these different intra-arterial therapy approaches, but TARE with Y-90 has been shown to be safe in patients with portal vein thrombosis. A recent multicenter retrospective study of TARE demonstrated a median OS of 8.8 to 10.8 months in patients with BCLC C HCC,43 and in a large randomized study of Y-90 compared to sorafenib in advanced and previously treated intermediate HCC, there was no difference in median OS between the treatment modalities (8 months for selective internal radiotherapy, 9 months for sorafenib; P = 0.18). Treatment with Y-90 was better tolerated.44 A major impediment to the adoption of TARE is the time it takes to order, plan, and deliver Y-90 to patients. Radio-embolization-induced liver disease, similar to post-embolization syndrome, is characterized by jaundice and ascites, which may occur 4 to 8 weeks postprocedure and is more common in patients with HCC who do not have cirrhosis. Compared to TACE, TARE may offer a better adverse effect profile, with improvement in quality of life.
Combination of Systemic and Locoregional Therapy
Even in carefully selected patients with intermediate- and advanced-stage HCC, locoregional therapy is not curative. Tumor embolization may promote more angiogenesis, and hence tumor progression, by causing hypoxia and upregulation of hypoxia-inducible factor.45 This upregulation of angiogenesis as a resistance mechanism to tumor embolization provides a rationale for combining systemic therapy (typically based on abrogating angiogenesis) with TACE/TAE. Most of the experience has been with sorafenib in intermediate-stage disease, and the results have been disappointing. The administration of sorafenib after at least a partial response with TACE did not provide additional benefit in terms of time to progression.46 Similarly, in the SPACE trial, concurrent therapy with TACE-doxorubicin-eluting beads and sorafenib compared to TACE-doxorubicin-eluting beads and placebo yielded similar time to progression numbers for both treatment modalities.47 While the data have been disappointing in intermediate-stage disease, as described earlier, registry data suggest that patients with advanced-stage disease may benefit from this approach.48
In the phase 2 TACTICS trial, 156 patients with unresectable HCC were randomized to receive TACE alone or sorafenib plus TACE, with a novel endpoint, time to untreatable progression (TTUP) and/or progression to TACE refractoriness.49 Treatment with sorafenib following TACE was continued until TTUP, decline in liver function to Child–Pugh class C, or the development of vascular invasion or extrahepatic spread. Development of new lesions while on sorafenib was not considered as progressive disease as long as the lesions were amenable to TACE. In this study, PFS was longer with sorafenib-TACE compared to TACE alone (26.7 months vs 20.6 months; P = 0.02). However, the TTUP endpoint needs further validation, and we are still awaiting the survival outcomes of this study. At this time, there are insufficient data to recommend the combination of liver-directed locoregional therapy and sorafenib or other systemic therapy options outside of a clinical trial setting.
Current Treatment Approach for Advanced HCC (BCLC-C)
Although progress is being made in the development of effective therapies, advanced HCC is generally incurable. These patients experience significant symptom burden throughout the course of the disease. Therefore, the optimal treatment plan must focus on improving or maintaining quality of life, in addition to overall efficacy. It is important to actively involve patients in treatment decisions for an individualized treatment plan, and to discuss the best strategy for incorporating current advances in targeted and immunotherapies. The paradigm of what constitutes first-line treatment for advanced HCC is shifting due to the recent systemic therapy approvals. Prior to the availability of these therapies, many patients with advanced HCC were treated with repeated locoregional therapies. For instance, TACE was often used to treat unresectable HCC multiple times beyond progression. There was no consensus on the definition of TACE failure, and hence it was used in broader, unselected populations. Retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial, and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
CASE CONCLUSION
An important part of evaluating a new patient with HCC is to determine whether they are a candidate for curative therapies, such as transplant or surgical resection. These are no longer an option for patients with intermediate disease. For patients with advanced disease characteristics, such as vascular invasion or systemic metastasis, the evidence supports using systemic therapy with sorafenib or lenvatinib. Lenvatinib, with a better tolerance profile and response rate, is the treatment of choice for the patient described in the case scenario. Lenvatinib is also indicated for first-line treatment of advanced HCC, and is useful in very aggressive tumors, such as those with an AFP level exceeding 200 ng/mL.
Future Directions
The emerging role of novel systemic therapeutics, including immunotherapy, has drastically changed the treatment landscape for hepatocellular cancers, with 6 new drugs for treating advanced hepatocellular cancers approved recently. While these systemic drugs have improved survival in advanced HCC in the past decade, patient selection and treatment sequencing remain a challenge, due to a lack of biomarkers capable of predicting antitumor responses. In addition, there is an unmet need for treatment options for patients with Child–Pugh class B7 and C liver disease and poor performance status.
The goal of future management should be to achieve personalized care aimed at improved safety and efficacy by targeting multiple cancer pathways in the HCC cascade with combination treatments. Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
2. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491.
3. Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463-471.
4. Schutte K, Schulz C, Poranzke J, et al. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117.
5. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338.
6. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314.
7. Schulze K, Imbeaud S, Letouzé E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
8. Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339-346.
9. Dhanasekaran R, Bandoh S, Roberts LR. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000Res. 2016;5.
10. Schulze K, Imbeaud S, Letouze E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
11. Cancer Genome Atlas Research Network. Electronic address: [email protected]; Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327-1134.
12. Chae YK, Ranganath K, Hammerman PS, et al: Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget. 2016;8:16052-16074.
13. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390.
14. Jackson R, Psarelli EE, Berhane S, et al. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized phase III trials. J Clin Oncol. 2017;35:622-628.
15. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34.
16. Da Fonseca LG, Barroso-Sousa R, Bento AD, et al. Safety and efficacy of sorafenib in patients with Child-Pugh B advanced hepatocellular carcinoma. Mol Clin Oncol. 2015;3:793-796.
17. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173.
18. Bruix J, Tak W-Y, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: Multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49:3412-3419.
19. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66.
20. Bruix J, Merle P, Granito A, et al. Hand-foot skin reaction (HFSR) and overall survival (OS) in the phase 3 RESORCE trial of regorafenib for treatment of hepatocellular carcinoma (HCC) progressing on sorafenib. J Clin Oncol. 2018;36:412-412.
21. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353-358.
22. Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2018;36:207-207.
23. Zhu AX, Park JO, Ryoo B-Y, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncology. 2015;16:859-870.
24. Zhu AX, Kang Y-K, Yen C-J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased αfetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncology. 2019;20:282-296.
25. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502.
26. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2020;30:v874-v875.
27. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36:942-952.
28. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193-202.
29. Cheng A-L, Qin S, Ikeda M, et al. IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30 (suppl_9):ix183-ix202.
30. Jiang Y, Han Q-J, Zhang J. Hepatocellular carcinoma: Mechanisms of progression and immunotherapy. World J Gastroenterol. 2019;25:3151-3167.
31. Xu F, Jin T, Zhu Y, et al. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37:110.
32. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501.
33. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681-700.
34. Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501-3508.
35. Louafi S, Boige V, Ducreux M, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC). Cancer. 2007;109:1384-1390.
36. Tang A, Chan AT, Zee B, et al. A randomized phase iii study of doxorubicin versus cisplatin/interferon α-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532-1538.
37. Abou-Alfa GK, Niedzwieski D, Knox JJ, et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J Clin Oncol. 2016;34:192.
38. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739.
39. Brown KT, Do RK, Gonen M, et al. randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol. 2016;34:2046-2053.
40. Kirstein MM, Voigtlander T, Schweitzer N, et al. Transarterial chemoembolization versus sorafenib in patients with hepatocellular carcinoma and extrahepatic disease. United European Gastroenterol J. 2018;6:238-246.
41. Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590-599.
42. Mason MC, Massarweh NN, Salami A, et al. Post-embolization syndrome as an early predictor of overall survival after transarterial chemoembolization for hepatocellular carcinoma. HPB (Oxford). 2015;17:1137-1144.
43. Sangro B, Maini CL, Ettorre GM, et al. Radioembolisation in patients with hepatocellular carcinoma that have previously received liver-directed therapies. Eur J Nucl Med Mol Imaging. 2018;45:1721-1730.
44. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624-1636.
45. Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921.
46. Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117-2127.
47. Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098.
48. Geschwind JF, Chapiro J. Sorafenib in combination with transarterial chemoembolization for the treatment of hepatocellular carcinoma. Clin Adv Hematol Oncol. 2016;14:585-587.
49. Kudo M, Ueshima K, Ikeda M, et al. Randomized, open label, multicenter, phase II trial comparing transarterial chemoembolization (TACE) plus sorafenib with TACE alone in patients with hepatocellular carcinoma (HCC): TACTICS trial. J Clin Oncol. 2018;36:206.
From the University of Alabama at Birmingham, Division of Hematology Oncology, Birmingham, AL, and the University of South Alabama, Division of Hematology Oncology, Mobile, AL. Dr. Paluri and Dr. Hatic contributed equally to this article.
Abstract
- Objective: To review systemic treatment options for patients with locally advanced unresectable hepatocellular carcinoma (HCC).
- Methods: Review of the literature.
- Results: The paradigm of what constitutes first-line treatment for advanced HCC is shifting. Until recently, many patients with advanced HCC were treated with repeated locoregional therapies, such as transartertial embolization (TACE). However, retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
- Conclusion: Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
Keywords: liver cancer; molecular therapy; immunotherapy.
Hepatocellular carcinoma (HCC) represents 90% of primary liver malignancies. It is the fifth most common malignancy in males and the ninth most common in females worldwide.1 In contrast to other major cancers (colon, breast, prostate), the incidence of and mortality from HCC has increased over the past decade, following a brief decline between 1999 and 2004.2 The epidemiology and incidence of HCC is closely linked to chronic liver disease and conditions predisposing to liver cirrhosis. Worldwide, hepatitis B virus infection is the leading cause of liver cirrhosis and, hence, HCC. In the United States, 50% of HCC cases are linked to hepatitis C virus (HCV) infection. Diabetes mellitus and alcoholic and nonalcoholic steatohepatitis are the other major etiologies of HCC. Indeed, the metabolic syndrome, independent of other factors, is associated with a 2-fold increase in the risk of HCC.3
Although most cases of HCC are predated by liver cirrhosis, in about 20% of patients HCC occurs without liver cirrhosis.4 Similar to other malignancies, surgery in the form of resection (for isolated lesions in the context of good liver function) or liver transplant (for low-volume disease with mildly impaired liver function) provides the best chance of a cure. Locoregional therapies involving hepatic artery–directed therapy are offered for patients with more advanced disease that is limited to the liver, while systemic therapy is offered for advanced unresectable disease that involves portal vein invasion, lymph nodes, and distant metastasis. The
Molecular Pathogenesis
Similar to other malignancies, a multistep process of carcinogenesis, with accumulation of genomic alterations at the molecular and cellular levels, is recognized in HCC. In about 80% of cases, repeated and chronic injury, inflammation, and repair lead to a distortion of normal liver architecture and development of cirrhotic nodules. Exome sequencing of HCC tissues has identified risk factor–specific mutational signatures, including those related to the tumor microenvironment, and defined the extensive landscape of altered genes and pathways in HCC (eg, angiogenic and MET pathways).7 In the Schulze et al study, the frequency of alterations that could be targeted by available Food and Drug Administration (FDA)–approved drugs comprised either amplifications or mutations of FLTs (6%), FGF3 or 4 or 19 (4%), PDGFRs (3%), VEGFA (1%), HGF (3%), MTOR (2%), EGFR (1%), FGFRs (1%), and MET (1%).7 Epigenetic modification of growth-factor expression, particularly insulin-like growth factor 2 and transforming growth factor alpha, and structural alterations that lead to loss of heterozygosity are early events that cause hepatocyte proliferation and progression of dysplastic nodules.8,9 Advances in whole-exome sequencing have identified TERT promoter mutations, leading to activation of telomerase, as an early event in HCC pathogenesis. Other commonly altered genes include CTNNB1 (B-Catenin) and TP53. As a group, alterations in the MAP kinase pathway genes occur in about 40% of HCC cases.
Actionable oncogenic driver alterations are not as common as tumor suppressor pathway alterations in HCC, making targeted drug development challenging.10,11 The FGFR (fibroblast growth factor receptor) pathway, which plays a critical role in carcinogenesis-related cell growth, survival, neo-angiogenesis, and acquired resistance to other cancer treatments, is being explored as a treatment target.12 The molecular characterization of HCC may help with identifying new biomarkers and present opportunities for developing therapeutic targets.
CASE PRESENTATION
A 61-year-old man with a history of chronic hepatitis C and hypertension presents to his primary care physician due to right upper quadrant pain. Laboratory evaluation shows transaminases elevated 2 times the upper limit of normal. This leads to an ultrasound and follow-up magnetic resonance imaging. Imaging shows diffuse cirrhotic changes, with a 6-cm, well-circumscribed lesion within the left lobe of the liver that shows rapid arterial enhancement with venous washout. These vascular characteristics are consistent with HCC. In addition, 2 satellite lesions in the left lobe and sonographic evidence of invasion into the portal vein are present. Periportal lymph nodes are pathologically enlarged.
The physical examination is unremarkable, except for mild tenderness over the right upper quadrant of the abdomen. Serum bilirubin, albumin, platelets, and international normalized ratio are normal, and alpha fetoprotein (AFP) is elevated at 1769 ng/mL. The patient’s family history is unremarkable for any major illness or cancer. Computed tomography scan of the chest and pelvis shows no evidence of other lesions. His liver disease is classified as Child–Pugh A. Due to locally advanced presentation, the tumor is deemed to be nontransplantable and unresectable, and is staged as BCLC-C. The patient continues to work and his performance status is ECOG (
What systemic treatment would you recommend for this patient with locally advanced unresectable HCC with nodal metastasis?
First-Line Therapeutic Options
Systemic treatment of HCC is challenging because of the underlying liver cirrhosis and hepatic dysfunction present in most patients. Overall prognosis is therefore dependent on the disease biology and burden and on the degree of hepatic dysfunction. These factors must be considered in patients with advanced disease who present for systemic therapy. As such, patients with BCLC class D HCC with poor performance status and impaired liver function are better off with best supportive care and hospice services (Figure). Table 1 and Table 2 outline the landmark trials that led to the approval of agents for advanced HCC treatment.

Sorafenib
In the patient with BCLC class C HCC who has preserved liver function (traditionally based on a Child–Pugh score of ≤ 6 and a decent functional status [ECOG performance status 1-2]), sorafenib is the first FDA-approved first-line treatment. Sorafenib is a small-molecule tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR) kinase signaling, in addition to many other tyrosine kinase pathways (including the platelet-derived growth factor and Raf-Ras pathways). Evidence for the clinical benefit of sorafenib comes from the SHARP trial.13 This was a multinational, but primarily European, randomized phase 3 study that compared sorafenib to best supportive care for advanced HCC in patients with a Child–Pugh score ≤ 6A and a robust performance status (ECOG 0 and 1). Overall survival (OS) with placebo and sorafenib was 7.9 months and 10.7 months, respectively. There was no difference in time to radiologic progression, and the progression-free survival (PFS) at 4 months was 62% with sorafenib and 42% with placebo. Patients with HCV-associated HCC appeared to derive a more substantial benefit from sorafenib.14 In a smaller randomized study of sorafenib in Asian patients with predominantly hepatitis B–associated HCC, OS in the sorafenib and best supportive care arms was lower than that reported in the SHARP study (6.5 months vs 4.2 months), although OS still was longer in the sorafenib group.15

Significant adverse events reported with sorafenib include fatigue (30%), hand and foot syndrome (30%), diarrhea (15%), and mucositis (10%). Major proportions of patients in the clinical setting have not tolerated the standard dose of 400 mg twice daily. Dose-adjusted administration of sorafenib has been advocated in patients with more impaired liver function (Child–Pugh class 7B) and bilirubin of 1.5 to 3 times the upper limit of normal, although it is unclear whether these patients are deriving any benefit from sorafenib.16 At this time, in a patient with preserved liver function, starting with 400 mg twice daily, followed by dose reduction based on toxicity, remains standard.

Lenvatinib
After multiple attempts to develop newer first-line treatments for HCC,
Second-Line Therapeutic Options
Following the sorafenib approval, clinical studies of several other agents did not meet their primary endpoint and failed to show improvement in clinical outcomes compared to sorafenib. However, over the past years the treatment landscape for advanced HCC has been changed with the approval of several agents in the second line. The overall response rate (ORR) has become the new theme for management of advanced disease. With multiple therapeutic options available, optimal sequencing now plays a critical role, especially for transitioning from locoregional to systemic therapy. Five drugs are now indicated for second-line treatment of patients who progressed on or were intolerant to sorafenib: regorafenib, cabozantinib, ramucirumab, nivolumab, and pembrolizumab.
Regorafenib
Regorafenib was evaluated in the advanced HCC setting in a single-arm, phase 2 trial involving 36 patients with Child–Pugh class A liver disease who had progressed on prior sorafenib.18 Patients received regorafenib 160 mg orally once daily for 3 weeks on/1 week off cycles. Disease control was achieved in 72% of patients, with stable disease in 25 patients (69%). Based on these results, regorafenib was further evaluated in the multicenter, phase 3, 2:1 randomized, double-blind, placebo-controlled RESORCE study, which enrolled 573 patients.19 Due to the overlapping safety profiles of sorafenib and regorafenib, the inclusion criteria required patients to have tolerated a sorafenib dose of at least 400 mg daily for 20 of the past 28 days of treatment prior to enrollment. The primary endpoint of the study, OS, was met (median OS of 10.6 months in regorafenib arm versus 7.8 months in placebo arm; hazard ratio [HR], 0.63; P < 0.0001).
Cabozantinib
CELESTIAL was a phase 3, double-blind study that assessed the efficacy of cabozantinib versus placebo in patients with advanced HCC who had received prior sorafenib.22 In this study, 707 patients with Child–Pugh class A liver disease who progressed on at least 1 prior systemic therapy were randomized in a 2:1 ratio to treatment with cabozantinib at 60 mg daily or placebo. Patients treated with cabozantinib had a longer OS (10.2 months vs 8.0 months), resulting in a 24% reduction in the risk of death (HR, 0.76), and a longer median PFS (5.2 months versus 1.9 months). The disease control rate was higher with cabozantinib (64% vs 33%) as well. The incidence of high‐grade adverse events in the cabozantinib group was twice that of the placebo group. Common adverse events reported with cabozantinib included HFSR (17%), hypertension (16%), increased aspartate aminotransferase (12%), fatigue (10%), and diarrhea (10%).
Ramucirumab
REACH was a phase 3 study exploring the efficacy of ramucirumab that did not meet its primary endpoint; however, the subgroup analysis in AFP-high patients showed an OS improvement with ramucirumab.23 This led to the phase 3 REACH-2 trial, a multicenter, randomized, double-blind biomarker study in patients with advanced HCC who either progressed on or were intolerant to sorafenib and had an AFP level ≥ 400 ng/mL.24 Patients were randomized to ramucirumab 8 mg/kg every 2 weeks or placebo. The study met its primary endpoint, showing improved OS (8.5 months vs 7.3 months; P = 0.0059). The most common treatment-related adverse events in the ramucirumab group were ascites (5%), hypertension (12%), asthenia (5%), malignant neoplasm progression (6%), increased aspartate aminotransferase concentration (5%), and thrombocytopenia.
Immunotherapy
HCC is considered an inflammation-induced cancer, which renders immunotherapeutic strategies more appealing. The PD-L1/PD-1 pathway is the critical immune checkpoint mechanism and is an important target for treatment. HCC uses a complex, overlapping set of mechanisms to evade cancer-specific immunity and to suppress the immune system. Initial efforts to develop immunotherapies for HCC focused on anti-PD-1 and anti-PD-L1 antibodies. CheckMate 040 evaluated nivolumab in 262 sorafenib-naïve and -treated patients with advanced HCC (98% with Child–Pugh scores of 5 or 6), with a median follow-up of 12.9 months.25 In sorafenib-naïve patients (n = 80), the ORR was 23%, and the disease control rate was 63%. In sorafenib-treated patients (n = 182), the ORR was 18%. Response was not associated with PD-L1 expression. Durable objective responses, a manageable safety profile, and promising efficacy led the FDA to grant accelerated approval of nivolumab for the treatment of patients with HCC who have been previously treated with sorafenib. Based on this, the phase 3 randomized CheckMate-459 trial evaluated the efficacy of nivolumab versus sorafenib in the first-line. Median OS and ORR were better with nivolumab (16.4 months vs 14.7 months; HR 0.85; P = 0.752; and 15% [with 5 complete responses] vs 7%), as was the safety profile (22% vs 49% reporting grade 3 and 4 adverse events). 26
The KEYNOTE-224 study27 evaluated pembrolizumab in 104 patients with previously treated advanced HCC. This study showed an ORR of 17%, with 1 complete response and 17 partial responses. One-third of the patients had progressive disease, while 46 had stable disease. Among those who responded, 56% maintained a durable response for more than 1 year. Subsequently, in KEYNOTE 240, pembrolizumab showed an improvement in OS (13.9 months vs 10.6 months; HR, 0.78; P = 0.0238) and PFS (3.0 months versus 2.8 months; HR, 0.78; P = 0.0186) compared with placebo.28 The ORR for pembrolizumab was 16.9% (95% confidence interval [CI], 12.7%-21.8%) versus 2.2% (95% CI, 0.5%-6.4%; P = 0.00001) for placebo. Mean duration of response was 13.8 months.
In the IMbrave150 trial, atezolizumab/bevacizumab combination, compared to sorafenib, had better OS (not estimable vs 13.2 months; P = 0.0006), PFS (6.8 months vs 4.5 months, P < 0.0001), and ORR (33% vs 13%, P < 0.0001), but grade 3-4 events were similar.29 This combination has potential for first-line approval. The COSMIC–312 study is comparing the combination of cabozantinib and atezolizumab to sorafenib monotherapy and cabozantinib monotherapy in advanced HCC.
Resistance to immunotherapy can be extrinsic, associated with activation mechanisms of T-cells, or intrinsic, related to immune recognition, gene expression, and cell-signaling pathways.30 Tumor-immune heterogeneity and antigen presentation contribute to complex resistance mechanisms.31,32 Although clinical outcomes have improved with immune checkpoint inhibitors, the response rate is low and responses are inconsistent, likely due to an immunosuppressive tumor microenvironment.33 Therefore, several novel combinations of checkpoint inhibitors and targeted drugs are being evaluated to bypass some of the resistance mechanisms (Table 3).

Chemotherapy
Multiple combinations of cytotoxic regimens have been evaluated, but efficacy has been modest, suggesting the limited role for traditional chemotherapy in the systemic management of advanced HCC. Response rates to chemotherapy are low and responses are not durable. Gemcitabine- and doxorubicin-based treatment and FOLFOX (5-fluorouracil, leucovorin, oxaliplatin) are some regimens that have been studied, with a median OS of less than 1 year for these regimens.34-36 FOLFOX had a higher response rate (8.15% vs 2.67%; P = 0.02) and longer median OS (6.40 months versus 4.97 months; HR, 0.80; 95% CI, 0.63-1.02; P = 0.07) than doxorubicin.34 With the gemcitabine/oxaliplatin combination, ORR was 18%, with stable disease in 58% of patients, and median PFS and OS were 6.3 months and 11.5 months, respectively.35 In a study that compared doxorubicin and PIAF (cisplatin/interferon a-2b/doxorubicin/5-fluorouracil), median OS was 6.83 months and 8.67 months, respectively (P = 0.83). The hazard ratio for death from any cause in the PIAF group compared with the doxorubicin group was 0.97 (95% CI, 0.71-1.32). PIAF had a higher ORR (20.9%; 95% CI, 12.5%-29.2%) than doxorubicin (10.5%; 95% CI, 3.9%-16.9%).
The phase 3 ALLIANCE study evaluated the combination of sorafenib and doxorubicin in treatment-naïve HCC patients with Child–Pugh class A liver disease, and did not demonstrate superiority with the addition of cytotoxic chemotherapy.37 Indeed, the combination of chemotherapy with sorafenib appears harmful in terms of lower OS (9.3 months vs 10.6 months; HR, 1.06; 95% CI, 0.8-1.4) and worse toxicity. Patients treated with the combination experienced more hematologic (37.8% vs 8.1%) and nonhematologic adverse events (63.6% vs 61.5%).
Locoregional Therapy
The role of locoregional therapy in advanced HCC remains the subject of intense debate. Patients with BCLC stage C HCC with metastatic disease and those with lymph node involvement are candidates for systemic therapy. The optimal candidate for locoregional therapy is the patient with localized intermediate-stage disease, particularly hepatic artery–delivered therapeutic interventions. However, the presence of a solitary large tumor or portal vein involvement constitutes gray areas regarding which therapy to deliver directly to the tumor via the hepatic artery, and increasingly stereotactic body radiation therapy is being offered.
Transarterial Chemoembolization
Transarterial chemoembolization (TACE), with or without chemotherapy, is the most widely adopted locoregional therapy in the management of HCC. TACE exploits the differential vascular supply to the HCC and normal liver parenchyma. Normal liver receives only one-fourth of its blood supply from the hepatic artery (three-fourths from the portal vein), whereas HCC is mainly supplied by the hepatic artery. A survival benefit for TACE compared to best supportive care is widely acknowledged for intermediate-stage HCC, and transarterial embolization (TAE) with gelatin sponge or microspheres is noninferior to TACE.38,39 Overall safety profile and efficacy inform therapy selection in advanced HCC, although the evidence for TACE in advanced HCC is less robust. Although single-institution experiences suggest survival numbers similar to and even superior to sorafenib,40,41 there is a scarcity of large randomized clinical trial data to back this up. Based on this, patients with advanced HCC should only be offered liver-directed therapy within a clinical trial or on a case-by-case basis under multidisciplinary tumor board consensus.
A serious adverse effect of TACE is post-embolization syndrome, which occurs in about 30% of patients and may be associated with poor prognosis.42 The syndrome consists of right upper quadrant abdominal pain, malaise, and worsening nausea/vomiting following the embolization procedure. Laboratory abnormalities and other complications may persist for up to 30 days after the procedure. This is a concern, because post-embolization syndrome may affect the ability to deliver systemic therapy.
Transarterial Radioembolization
In the past few years, there has been an uptick in the utilization of transarterial radioembolization (TARE), which instead of delivering glass beads, as done in TAE, or chemotherapy-infused beads, as done in TACE, delivers the radioisotope Y-90 to the tumor via the hepatic artery. TARE is able to administer larger doses of radiation to the tumor while avoiding normal liver tissue, as compared to external-beam radiation. There has been no head-to-head comparison of these different intra-arterial therapy approaches, but TARE with Y-90 has been shown to be safe in patients with portal vein thrombosis. A recent multicenter retrospective study of TARE demonstrated a median OS of 8.8 to 10.8 months in patients with BCLC C HCC,43 and in a large randomized study of Y-90 compared to sorafenib in advanced and previously treated intermediate HCC, there was no difference in median OS between the treatment modalities (8 months for selective internal radiotherapy, 9 months for sorafenib; P = 0.18). Treatment with Y-90 was better tolerated.44 A major impediment to the adoption of TARE is the time it takes to order, plan, and deliver Y-90 to patients. Radio-embolization-induced liver disease, similar to post-embolization syndrome, is characterized by jaundice and ascites, which may occur 4 to 8 weeks postprocedure and is more common in patients with HCC who do not have cirrhosis. Compared to TACE, TARE may offer a better adverse effect profile, with improvement in quality of life.
Combination of Systemic and Locoregional Therapy
Even in carefully selected patients with intermediate- and advanced-stage HCC, locoregional therapy is not curative. Tumor embolization may promote more angiogenesis, and hence tumor progression, by causing hypoxia and upregulation of hypoxia-inducible factor.45 This upregulation of angiogenesis as a resistance mechanism to tumor embolization provides a rationale for combining systemic therapy (typically based on abrogating angiogenesis) with TACE/TAE. Most of the experience has been with sorafenib in intermediate-stage disease, and the results have been disappointing. The administration of sorafenib after at least a partial response with TACE did not provide additional benefit in terms of time to progression.46 Similarly, in the SPACE trial, concurrent therapy with TACE-doxorubicin-eluting beads and sorafenib compared to TACE-doxorubicin-eluting beads and placebo yielded similar time to progression numbers for both treatment modalities.47 While the data have been disappointing in intermediate-stage disease, as described earlier, registry data suggest that patients with advanced-stage disease may benefit from this approach.48
In the phase 2 TACTICS trial, 156 patients with unresectable HCC were randomized to receive TACE alone or sorafenib plus TACE, with a novel endpoint, time to untreatable progression (TTUP) and/or progression to TACE refractoriness.49 Treatment with sorafenib following TACE was continued until TTUP, decline in liver function to Child–Pugh class C, or the development of vascular invasion or extrahepatic spread. Development of new lesions while on sorafenib was not considered as progressive disease as long as the lesions were amenable to TACE. In this study, PFS was longer with sorafenib-TACE compared to TACE alone (26.7 months vs 20.6 months; P = 0.02). However, the TTUP endpoint needs further validation, and we are still awaiting the survival outcomes of this study. At this time, there are insufficient data to recommend the combination of liver-directed locoregional therapy and sorafenib or other systemic therapy options outside of a clinical trial setting.
Current Treatment Approach for Advanced HCC (BCLC-C)
Although progress is being made in the development of effective therapies, advanced HCC is generally incurable. These patients experience significant symptom burden throughout the course of the disease. Therefore, the optimal treatment plan must focus on improving or maintaining quality of life, in addition to overall efficacy. It is important to actively involve patients in treatment decisions for an individualized treatment plan, and to discuss the best strategy for incorporating current advances in targeted and immunotherapies. The paradigm of what constitutes first-line treatment for advanced HCC is shifting due to the recent systemic therapy approvals. Prior to the availability of these therapies, many patients with advanced HCC were treated with repeated locoregional therapies. For instance, TACE was often used to treat unresectable HCC multiple times beyond progression. There was no consensus on the definition of TACE failure, and hence it was used in broader, unselected populations. Retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial, and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
CASE CONCLUSION
An important part of evaluating a new patient with HCC is to determine whether they are a candidate for curative therapies, such as transplant or surgical resection. These are no longer an option for patients with intermediate disease. For patients with advanced disease characteristics, such as vascular invasion or systemic metastasis, the evidence supports using systemic therapy with sorafenib or lenvatinib. Lenvatinib, with a better tolerance profile and response rate, is the treatment of choice for the patient described in the case scenario. Lenvatinib is also indicated for first-line treatment of advanced HCC, and is useful in very aggressive tumors, such as those with an AFP level exceeding 200 ng/mL.
Future Directions
The emerging role of novel systemic therapeutics, including immunotherapy, has drastically changed the treatment landscape for hepatocellular cancers, with 6 new drugs for treating advanced hepatocellular cancers approved recently. While these systemic drugs have improved survival in advanced HCC in the past decade, patient selection and treatment sequencing remain a challenge, due to a lack of biomarkers capable of predicting antitumor responses. In addition, there is an unmet need for treatment options for patients with Child–Pugh class B7 and C liver disease and poor performance status.
The goal of future management should be to achieve personalized care aimed at improved safety and efficacy by targeting multiple cancer pathways in the HCC cascade with combination treatments. Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
From the University of Alabama at Birmingham, Division of Hematology Oncology, Birmingham, AL, and the University of South Alabama, Division of Hematology Oncology, Mobile, AL. Dr. Paluri and Dr. Hatic contributed equally to this article.
Abstract
- Objective: To review systemic treatment options for patients with locally advanced unresectable hepatocellular carcinoma (HCC).
- Methods: Review of the literature.
- Results: The paradigm of what constitutes first-line treatment for advanced HCC is shifting. Until recently, many patients with advanced HCC were treated with repeated locoregional therapies, such as transartertial embolization (TACE). However, retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
- Conclusion: Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
Keywords: liver cancer; molecular therapy; immunotherapy.
Hepatocellular carcinoma (HCC) represents 90% of primary liver malignancies. It is the fifth most common malignancy in males and the ninth most common in females worldwide.1 In contrast to other major cancers (colon, breast, prostate), the incidence of and mortality from HCC has increased over the past decade, following a brief decline between 1999 and 2004.2 The epidemiology and incidence of HCC is closely linked to chronic liver disease and conditions predisposing to liver cirrhosis. Worldwide, hepatitis B virus infection is the leading cause of liver cirrhosis and, hence, HCC. In the United States, 50% of HCC cases are linked to hepatitis C virus (HCV) infection. Diabetes mellitus and alcoholic and nonalcoholic steatohepatitis are the other major etiologies of HCC. Indeed, the metabolic syndrome, independent of other factors, is associated with a 2-fold increase in the risk of HCC.3
Although most cases of HCC are predated by liver cirrhosis, in about 20% of patients HCC occurs without liver cirrhosis.4 Similar to other malignancies, surgery in the form of resection (for isolated lesions in the context of good liver function) or liver transplant (for low-volume disease with mildly impaired liver function) provides the best chance of a cure. Locoregional therapies involving hepatic artery–directed therapy are offered for patients with more advanced disease that is limited to the liver, while systemic therapy is offered for advanced unresectable disease that involves portal vein invasion, lymph nodes, and distant metastasis. The
Molecular Pathogenesis
Similar to other malignancies, a multistep process of carcinogenesis, with accumulation of genomic alterations at the molecular and cellular levels, is recognized in HCC. In about 80% of cases, repeated and chronic injury, inflammation, and repair lead to a distortion of normal liver architecture and development of cirrhotic nodules. Exome sequencing of HCC tissues has identified risk factor–specific mutational signatures, including those related to the tumor microenvironment, and defined the extensive landscape of altered genes and pathways in HCC (eg, angiogenic and MET pathways).7 In the Schulze et al study, the frequency of alterations that could be targeted by available Food and Drug Administration (FDA)–approved drugs comprised either amplifications or mutations of FLTs (6%), FGF3 or 4 or 19 (4%), PDGFRs (3%), VEGFA (1%), HGF (3%), MTOR (2%), EGFR (1%), FGFRs (1%), and MET (1%).7 Epigenetic modification of growth-factor expression, particularly insulin-like growth factor 2 and transforming growth factor alpha, and structural alterations that lead to loss of heterozygosity are early events that cause hepatocyte proliferation and progression of dysplastic nodules.8,9 Advances in whole-exome sequencing have identified TERT promoter mutations, leading to activation of telomerase, as an early event in HCC pathogenesis. Other commonly altered genes include CTNNB1 (B-Catenin) and TP53. As a group, alterations in the MAP kinase pathway genes occur in about 40% of HCC cases.
Actionable oncogenic driver alterations are not as common as tumor suppressor pathway alterations in HCC, making targeted drug development challenging.10,11 The FGFR (fibroblast growth factor receptor) pathway, which plays a critical role in carcinogenesis-related cell growth, survival, neo-angiogenesis, and acquired resistance to other cancer treatments, is being explored as a treatment target.12 The molecular characterization of HCC may help with identifying new biomarkers and present opportunities for developing therapeutic targets.
CASE PRESENTATION
A 61-year-old man with a history of chronic hepatitis C and hypertension presents to his primary care physician due to right upper quadrant pain. Laboratory evaluation shows transaminases elevated 2 times the upper limit of normal. This leads to an ultrasound and follow-up magnetic resonance imaging. Imaging shows diffuse cirrhotic changes, with a 6-cm, well-circumscribed lesion within the left lobe of the liver that shows rapid arterial enhancement with venous washout. These vascular characteristics are consistent with HCC. In addition, 2 satellite lesions in the left lobe and sonographic evidence of invasion into the portal vein are present. Periportal lymph nodes are pathologically enlarged.
The physical examination is unremarkable, except for mild tenderness over the right upper quadrant of the abdomen. Serum bilirubin, albumin, platelets, and international normalized ratio are normal, and alpha fetoprotein (AFP) is elevated at 1769 ng/mL. The patient’s family history is unremarkable for any major illness or cancer. Computed tomography scan of the chest and pelvis shows no evidence of other lesions. His liver disease is classified as Child–Pugh A. Due to locally advanced presentation, the tumor is deemed to be nontransplantable and unresectable, and is staged as BCLC-C. The patient continues to work and his performance status is ECOG (
What systemic treatment would you recommend for this patient with locally advanced unresectable HCC with nodal metastasis?
First-Line Therapeutic Options
Systemic treatment of HCC is challenging because of the underlying liver cirrhosis and hepatic dysfunction present in most patients. Overall prognosis is therefore dependent on the disease biology and burden and on the degree of hepatic dysfunction. These factors must be considered in patients with advanced disease who present for systemic therapy. As such, patients with BCLC class D HCC with poor performance status and impaired liver function are better off with best supportive care and hospice services (Figure). Table 1 and Table 2 outline the landmark trials that led to the approval of agents for advanced HCC treatment.

Sorafenib
In the patient with BCLC class C HCC who has preserved liver function (traditionally based on a Child–Pugh score of ≤ 6 and a decent functional status [ECOG performance status 1-2]), sorafenib is the first FDA-approved first-line treatment. Sorafenib is a small-molecule tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR) kinase signaling, in addition to many other tyrosine kinase pathways (including the platelet-derived growth factor and Raf-Ras pathways). Evidence for the clinical benefit of sorafenib comes from the SHARP trial.13 This was a multinational, but primarily European, randomized phase 3 study that compared sorafenib to best supportive care for advanced HCC in patients with a Child–Pugh score ≤ 6A and a robust performance status (ECOG 0 and 1). Overall survival (OS) with placebo and sorafenib was 7.9 months and 10.7 months, respectively. There was no difference in time to radiologic progression, and the progression-free survival (PFS) at 4 months was 62% with sorafenib and 42% with placebo. Patients with HCV-associated HCC appeared to derive a more substantial benefit from sorafenib.14 In a smaller randomized study of sorafenib in Asian patients with predominantly hepatitis B–associated HCC, OS in the sorafenib and best supportive care arms was lower than that reported in the SHARP study (6.5 months vs 4.2 months), although OS still was longer in the sorafenib group.15

Significant adverse events reported with sorafenib include fatigue (30%), hand and foot syndrome (30%), diarrhea (15%), and mucositis (10%). Major proportions of patients in the clinical setting have not tolerated the standard dose of 400 mg twice daily. Dose-adjusted administration of sorafenib has been advocated in patients with more impaired liver function (Child–Pugh class 7B) and bilirubin of 1.5 to 3 times the upper limit of normal, although it is unclear whether these patients are deriving any benefit from sorafenib.16 At this time, in a patient with preserved liver function, starting with 400 mg twice daily, followed by dose reduction based on toxicity, remains standard.

Lenvatinib
After multiple attempts to develop newer first-line treatments for HCC,
Second-Line Therapeutic Options
Following the sorafenib approval, clinical studies of several other agents did not meet their primary endpoint and failed to show improvement in clinical outcomes compared to sorafenib. However, over the past years the treatment landscape for advanced HCC has been changed with the approval of several agents in the second line. The overall response rate (ORR) has become the new theme for management of advanced disease. With multiple therapeutic options available, optimal sequencing now plays a critical role, especially for transitioning from locoregional to systemic therapy. Five drugs are now indicated for second-line treatment of patients who progressed on or were intolerant to sorafenib: regorafenib, cabozantinib, ramucirumab, nivolumab, and pembrolizumab.
Regorafenib
Regorafenib was evaluated in the advanced HCC setting in a single-arm, phase 2 trial involving 36 patients with Child–Pugh class A liver disease who had progressed on prior sorafenib.18 Patients received regorafenib 160 mg orally once daily for 3 weeks on/1 week off cycles. Disease control was achieved in 72% of patients, with stable disease in 25 patients (69%). Based on these results, regorafenib was further evaluated in the multicenter, phase 3, 2:1 randomized, double-blind, placebo-controlled RESORCE study, which enrolled 573 patients.19 Due to the overlapping safety profiles of sorafenib and regorafenib, the inclusion criteria required patients to have tolerated a sorafenib dose of at least 400 mg daily for 20 of the past 28 days of treatment prior to enrollment. The primary endpoint of the study, OS, was met (median OS of 10.6 months in regorafenib arm versus 7.8 months in placebo arm; hazard ratio [HR], 0.63; P < 0.0001).
Cabozantinib
CELESTIAL was a phase 3, double-blind study that assessed the efficacy of cabozantinib versus placebo in patients with advanced HCC who had received prior sorafenib.22 In this study, 707 patients with Child–Pugh class A liver disease who progressed on at least 1 prior systemic therapy were randomized in a 2:1 ratio to treatment with cabozantinib at 60 mg daily or placebo. Patients treated with cabozantinib had a longer OS (10.2 months vs 8.0 months), resulting in a 24% reduction in the risk of death (HR, 0.76), and a longer median PFS (5.2 months versus 1.9 months). The disease control rate was higher with cabozantinib (64% vs 33%) as well. The incidence of high‐grade adverse events in the cabozantinib group was twice that of the placebo group. Common adverse events reported with cabozantinib included HFSR (17%), hypertension (16%), increased aspartate aminotransferase (12%), fatigue (10%), and diarrhea (10%).
Ramucirumab
REACH was a phase 3 study exploring the efficacy of ramucirumab that did not meet its primary endpoint; however, the subgroup analysis in AFP-high patients showed an OS improvement with ramucirumab.23 This led to the phase 3 REACH-2 trial, a multicenter, randomized, double-blind biomarker study in patients with advanced HCC who either progressed on or were intolerant to sorafenib and had an AFP level ≥ 400 ng/mL.24 Patients were randomized to ramucirumab 8 mg/kg every 2 weeks or placebo. The study met its primary endpoint, showing improved OS (8.5 months vs 7.3 months; P = 0.0059). The most common treatment-related adverse events in the ramucirumab group were ascites (5%), hypertension (12%), asthenia (5%), malignant neoplasm progression (6%), increased aspartate aminotransferase concentration (5%), and thrombocytopenia.
Immunotherapy
HCC is considered an inflammation-induced cancer, which renders immunotherapeutic strategies more appealing. The PD-L1/PD-1 pathway is the critical immune checkpoint mechanism and is an important target for treatment. HCC uses a complex, overlapping set of mechanisms to evade cancer-specific immunity and to suppress the immune system. Initial efforts to develop immunotherapies for HCC focused on anti-PD-1 and anti-PD-L1 antibodies. CheckMate 040 evaluated nivolumab in 262 sorafenib-naïve and -treated patients with advanced HCC (98% with Child–Pugh scores of 5 or 6), with a median follow-up of 12.9 months.25 In sorafenib-naïve patients (n = 80), the ORR was 23%, and the disease control rate was 63%. In sorafenib-treated patients (n = 182), the ORR was 18%. Response was not associated with PD-L1 expression. Durable objective responses, a manageable safety profile, and promising efficacy led the FDA to grant accelerated approval of nivolumab for the treatment of patients with HCC who have been previously treated with sorafenib. Based on this, the phase 3 randomized CheckMate-459 trial evaluated the efficacy of nivolumab versus sorafenib in the first-line. Median OS and ORR were better with nivolumab (16.4 months vs 14.7 months; HR 0.85; P = 0.752; and 15% [with 5 complete responses] vs 7%), as was the safety profile (22% vs 49% reporting grade 3 and 4 adverse events). 26
The KEYNOTE-224 study27 evaluated pembrolizumab in 104 patients with previously treated advanced HCC. This study showed an ORR of 17%, with 1 complete response and 17 partial responses. One-third of the patients had progressive disease, while 46 had stable disease. Among those who responded, 56% maintained a durable response for more than 1 year. Subsequently, in KEYNOTE 240, pembrolizumab showed an improvement in OS (13.9 months vs 10.6 months; HR, 0.78; P = 0.0238) and PFS (3.0 months versus 2.8 months; HR, 0.78; P = 0.0186) compared with placebo.28 The ORR for pembrolizumab was 16.9% (95% confidence interval [CI], 12.7%-21.8%) versus 2.2% (95% CI, 0.5%-6.4%; P = 0.00001) for placebo. Mean duration of response was 13.8 months.
In the IMbrave150 trial, atezolizumab/bevacizumab combination, compared to sorafenib, had better OS (not estimable vs 13.2 months; P = 0.0006), PFS (6.8 months vs 4.5 months, P < 0.0001), and ORR (33% vs 13%, P < 0.0001), but grade 3-4 events were similar.29 This combination has potential for first-line approval. The COSMIC–312 study is comparing the combination of cabozantinib and atezolizumab to sorafenib monotherapy and cabozantinib monotherapy in advanced HCC.
Resistance to immunotherapy can be extrinsic, associated with activation mechanisms of T-cells, or intrinsic, related to immune recognition, gene expression, and cell-signaling pathways.30 Tumor-immune heterogeneity and antigen presentation contribute to complex resistance mechanisms.31,32 Although clinical outcomes have improved with immune checkpoint inhibitors, the response rate is low and responses are inconsistent, likely due to an immunosuppressive tumor microenvironment.33 Therefore, several novel combinations of checkpoint inhibitors and targeted drugs are being evaluated to bypass some of the resistance mechanisms (Table 3).

Chemotherapy
Multiple combinations of cytotoxic regimens have been evaluated, but efficacy has been modest, suggesting the limited role for traditional chemotherapy in the systemic management of advanced HCC. Response rates to chemotherapy are low and responses are not durable. Gemcitabine- and doxorubicin-based treatment and FOLFOX (5-fluorouracil, leucovorin, oxaliplatin) are some regimens that have been studied, with a median OS of less than 1 year for these regimens.34-36 FOLFOX had a higher response rate (8.15% vs 2.67%; P = 0.02) and longer median OS (6.40 months versus 4.97 months; HR, 0.80; 95% CI, 0.63-1.02; P = 0.07) than doxorubicin.34 With the gemcitabine/oxaliplatin combination, ORR was 18%, with stable disease in 58% of patients, and median PFS and OS were 6.3 months and 11.5 months, respectively.35 In a study that compared doxorubicin and PIAF (cisplatin/interferon a-2b/doxorubicin/5-fluorouracil), median OS was 6.83 months and 8.67 months, respectively (P = 0.83). The hazard ratio for death from any cause in the PIAF group compared with the doxorubicin group was 0.97 (95% CI, 0.71-1.32). PIAF had a higher ORR (20.9%; 95% CI, 12.5%-29.2%) than doxorubicin (10.5%; 95% CI, 3.9%-16.9%).
The phase 3 ALLIANCE study evaluated the combination of sorafenib and doxorubicin in treatment-naïve HCC patients with Child–Pugh class A liver disease, and did not demonstrate superiority with the addition of cytotoxic chemotherapy.37 Indeed, the combination of chemotherapy with sorafenib appears harmful in terms of lower OS (9.3 months vs 10.6 months; HR, 1.06; 95% CI, 0.8-1.4) and worse toxicity. Patients treated with the combination experienced more hematologic (37.8% vs 8.1%) and nonhematologic adverse events (63.6% vs 61.5%).
Locoregional Therapy
The role of locoregional therapy in advanced HCC remains the subject of intense debate. Patients with BCLC stage C HCC with metastatic disease and those with lymph node involvement are candidates for systemic therapy. The optimal candidate for locoregional therapy is the patient with localized intermediate-stage disease, particularly hepatic artery–delivered therapeutic interventions. However, the presence of a solitary large tumor or portal vein involvement constitutes gray areas regarding which therapy to deliver directly to the tumor via the hepatic artery, and increasingly stereotactic body radiation therapy is being offered.
Transarterial Chemoembolization
Transarterial chemoembolization (TACE), with or without chemotherapy, is the most widely adopted locoregional therapy in the management of HCC. TACE exploits the differential vascular supply to the HCC and normal liver parenchyma. Normal liver receives only one-fourth of its blood supply from the hepatic artery (three-fourths from the portal vein), whereas HCC is mainly supplied by the hepatic artery. A survival benefit for TACE compared to best supportive care is widely acknowledged for intermediate-stage HCC, and transarterial embolization (TAE) with gelatin sponge or microspheres is noninferior to TACE.38,39 Overall safety profile and efficacy inform therapy selection in advanced HCC, although the evidence for TACE in advanced HCC is less robust. Although single-institution experiences suggest survival numbers similar to and even superior to sorafenib,40,41 there is a scarcity of large randomized clinical trial data to back this up. Based on this, patients with advanced HCC should only be offered liver-directed therapy within a clinical trial or on a case-by-case basis under multidisciplinary tumor board consensus.
A serious adverse effect of TACE is post-embolization syndrome, which occurs in about 30% of patients and may be associated with poor prognosis.42 The syndrome consists of right upper quadrant abdominal pain, malaise, and worsening nausea/vomiting following the embolization procedure. Laboratory abnormalities and other complications may persist for up to 30 days after the procedure. This is a concern, because post-embolization syndrome may affect the ability to deliver systemic therapy.
Transarterial Radioembolization
In the past few years, there has been an uptick in the utilization of transarterial radioembolization (TARE), which instead of delivering glass beads, as done in TAE, or chemotherapy-infused beads, as done in TACE, delivers the radioisotope Y-90 to the tumor via the hepatic artery. TARE is able to administer larger doses of radiation to the tumor while avoiding normal liver tissue, as compared to external-beam radiation. There has been no head-to-head comparison of these different intra-arterial therapy approaches, but TARE with Y-90 has been shown to be safe in patients with portal vein thrombosis. A recent multicenter retrospective study of TARE demonstrated a median OS of 8.8 to 10.8 months in patients with BCLC C HCC,43 and in a large randomized study of Y-90 compared to sorafenib in advanced and previously treated intermediate HCC, there was no difference in median OS between the treatment modalities (8 months for selective internal radiotherapy, 9 months for sorafenib; P = 0.18). Treatment with Y-90 was better tolerated.44 A major impediment to the adoption of TARE is the time it takes to order, plan, and deliver Y-90 to patients. Radio-embolization-induced liver disease, similar to post-embolization syndrome, is characterized by jaundice and ascites, which may occur 4 to 8 weeks postprocedure and is more common in patients with HCC who do not have cirrhosis. Compared to TACE, TARE may offer a better adverse effect profile, with improvement in quality of life.
Combination of Systemic and Locoregional Therapy
Even in carefully selected patients with intermediate- and advanced-stage HCC, locoregional therapy is not curative. Tumor embolization may promote more angiogenesis, and hence tumor progression, by causing hypoxia and upregulation of hypoxia-inducible factor.45 This upregulation of angiogenesis as a resistance mechanism to tumor embolization provides a rationale for combining systemic therapy (typically based on abrogating angiogenesis) with TACE/TAE. Most of the experience has been with sorafenib in intermediate-stage disease, and the results have been disappointing. The administration of sorafenib after at least a partial response with TACE did not provide additional benefit in terms of time to progression.46 Similarly, in the SPACE trial, concurrent therapy with TACE-doxorubicin-eluting beads and sorafenib compared to TACE-doxorubicin-eluting beads and placebo yielded similar time to progression numbers for both treatment modalities.47 While the data have been disappointing in intermediate-stage disease, as described earlier, registry data suggest that patients with advanced-stage disease may benefit from this approach.48
In the phase 2 TACTICS trial, 156 patients with unresectable HCC were randomized to receive TACE alone or sorafenib plus TACE, with a novel endpoint, time to untreatable progression (TTUP) and/or progression to TACE refractoriness.49 Treatment with sorafenib following TACE was continued until TTUP, decline in liver function to Child–Pugh class C, or the development of vascular invasion or extrahepatic spread. Development of new lesions while on sorafenib was not considered as progressive disease as long as the lesions were amenable to TACE. In this study, PFS was longer with sorafenib-TACE compared to TACE alone (26.7 months vs 20.6 months; P = 0.02). However, the TTUP endpoint needs further validation, and we are still awaiting the survival outcomes of this study. At this time, there are insufficient data to recommend the combination of liver-directed locoregional therapy and sorafenib or other systemic therapy options outside of a clinical trial setting.
Current Treatment Approach for Advanced HCC (BCLC-C)
Although progress is being made in the development of effective therapies, advanced HCC is generally incurable. These patients experience significant symptom burden throughout the course of the disease. Therefore, the optimal treatment plan must focus on improving or maintaining quality of life, in addition to overall efficacy. It is important to actively involve patients in treatment decisions for an individualized treatment plan, and to discuss the best strategy for incorporating current advances in targeted and immunotherapies. The paradigm of what constitutes first-line treatment for advanced HCC is shifting due to the recent systemic therapy approvals. Prior to the availability of these therapies, many patients with advanced HCC were treated with repeated locoregional therapies. For instance, TACE was often used to treat unresectable HCC multiple times beyond progression. There was no consensus on the definition of TACE failure, and hence it was used in broader, unselected populations. Retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial, and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
CASE CONCLUSION
An important part of evaluating a new patient with HCC is to determine whether they are a candidate for curative therapies, such as transplant or surgical resection. These are no longer an option for patients with intermediate disease. For patients with advanced disease characteristics, such as vascular invasion or systemic metastasis, the evidence supports using systemic therapy with sorafenib or lenvatinib. Lenvatinib, with a better tolerance profile and response rate, is the treatment of choice for the patient described in the case scenario. Lenvatinib is also indicated for first-line treatment of advanced HCC, and is useful in very aggressive tumors, such as those with an AFP level exceeding 200 ng/mL.
Future Directions
The emerging role of novel systemic therapeutics, including immunotherapy, has drastically changed the treatment landscape for hepatocellular cancers, with 6 new drugs for treating advanced hepatocellular cancers approved recently. While these systemic drugs have improved survival in advanced HCC in the past decade, patient selection and treatment sequencing remain a challenge, due to a lack of biomarkers capable of predicting antitumor responses. In addition, there is an unmet need for treatment options for patients with Child–Pugh class B7 and C liver disease and poor performance status.
The goal of future management should be to achieve personalized care aimed at improved safety and efficacy by targeting multiple cancer pathways in the HCC cascade with combination treatments. Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
2. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491.
3. Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463-471.
4. Schutte K, Schulz C, Poranzke J, et al. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117.
5. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338.
6. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314.
7. Schulze K, Imbeaud S, Letouzé E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
8. Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339-346.
9. Dhanasekaran R, Bandoh S, Roberts LR. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000Res. 2016;5.
10. Schulze K, Imbeaud S, Letouze E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
11. Cancer Genome Atlas Research Network. Electronic address: [email protected]; Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327-1134.
12. Chae YK, Ranganath K, Hammerman PS, et al: Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget. 2016;8:16052-16074.
13. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390.
14. Jackson R, Psarelli EE, Berhane S, et al. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized phase III trials. J Clin Oncol. 2017;35:622-628.
15. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34.
16. Da Fonseca LG, Barroso-Sousa R, Bento AD, et al. Safety and efficacy of sorafenib in patients with Child-Pugh B advanced hepatocellular carcinoma. Mol Clin Oncol. 2015;3:793-796.
17. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173.
18. Bruix J, Tak W-Y, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: Multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49:3412-3419.
19. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66.
20. Bruix J, Merle P, Granito A, et al. Hand-foot skin reaction (HFSR) and overall survival (OS) in the phase 3 RESORCE trial of regorafenib for treatment of hepatocellular carcinoma (HCC) progressing on sorafenib. J Clin Oncol. 2018;36:412-412.
21. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353-358.
22. Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2018;36:207-207.
23. Zhu AX, Park JO, Ryoo B-Y, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncology. 2015;16:859-870.
24. Zhu AX, Kang Y-K, Yen C-J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased αfetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncology. 2019;20:282-296.
25. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502.
26. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2020;30:v874-v875.
27. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36:942-952.
28. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193-202.
29. Cheng A-L, Qin S, Ikeda M, et al. IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30 (suppl_9):ix183-ix202.
30. Jiang Y, Han Q-J, Zhang J. Hepatocellular carcinoma: Mechanisms of progression and immunotherapy. World J Gastroenterol. 2019;25:3151-3167.
31. Xu F, Jin T, Zhu Y, et al. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37:110.
32. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501.
33. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681-700.
34. Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501-3508.
35. Louafi S, Boige V, Ducreux M, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC). Cancer. 2007;109:1384-1390.
36. Tang A, Chan AT, Zee B, et al. A randomized phase iii study of doxorubicin versus cisplatin/interferon α-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532-1538.
37. Abou-Alfa GK, Niedzwieski D, Knox JJ, et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J Clin Oncol. 2016;34:192.
38. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739.
39. Brown KT, Do RK, Gonen M, et al. randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol. 2016;34:2046-2053.
40. Kirstein MM, Voigtlander T, Schweitzer N, et al. Transarterial chemoembolization versus sorafenib in patients with hepatocellular carcinoma and extrahepatic disease. United European Gastroenterol J. 2018;6:238-246.
41. Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590-599.
42. Mason MC, Massarweh NN, Salami A, et al. Post-embolization syndrome as an early predictor of overall survival after transarterial chemoembolization for hepatocellular carcinoma. HPB (Oxford). 2015;17:1137-1144.
43. Sangro B, Maini CL, Ettorre GM, et al. Radioembolisation in patients with hepatocellular carcinoma that have previously received liver-directed therapies. Eur J Nucl Med Mol Imaging. 2018;45:1721-1730.
44. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624-1636.
45. Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921.
46. Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117-2127.
47. Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098.
48. Geschwind JF, Chapiro J. Sorafenib in combination with transarterial chemoembolization for the treatment of hepatocellular carcinoma. Clin Adv Hematol Oncol. 2016;14:585-587.
49. Kudo M, Ueshima K, Ikeda M, et al. Randomized, open label, multicenter, phase II trial comparing transarterial chemoembolization (TACE) plus sorafenib with TACE alone in patients with hepatocellular carcinoma (HCC): TACTICS trial. J Clin Oncol. 2018;36:206.
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
2. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491.
3. Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463-471.
4. Schutte K, Schulz C, Poranzke J, et al. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117.
5. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338.
6. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314.
7. Schulze K, Imbeaud S, Letouzé E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
8. Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339-346.
9. Dhanasekaran R, Bandoh S, Roberts LR. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000Res. 2016;5.
10. Schulze K, Imbeaud S, Letouze E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
11. Cancer Genome Atlas Research Network. Electronic address: [email protected]; Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327-1134.
12. Chae YK, Ranganath K, Hammerman PS, et al: Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget. 2016;8:16052-16074.
13. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390.
14. Jackson R, Psarelli EE, Berhane S, et al. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized phase III trials. J Clin Oncol. 2017;35:622-628.
15. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34.
16. Da Fonseca LG, Barroso-Sousa R, Bento AD, et al. Safety and efficacy of sorafenib in patients with Child-Pugh B advanced hepatocellular carcinoma. Mol Clin Oncol. 2015;3:793-796.
17. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173.
18. Bruix J, Tak W-Y, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: Multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49:3412-3419.
19. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66.
20. Bruix J, Merle P, Granito A, et al. Hand-foot skin reaction (HFSR) and overall survival (OS) in the phase 3 RESORCE trial of regorafenib for treatment of hepatocellular carcinoma (HCC) progressing on sorafenib. J Clin Oncol. 2018;36:412-412.
21. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353-358.
22. Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2018;36:207-207.
23. Zhu AX, Park JO, Ryoo B-Y, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncology. 2015;16:859-870.
24. Zhu AX, Kang Y-K, Yen C-J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased αfetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncology. 2019;20:282-296.
25. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502.
26. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2020;30:v874-v875.
27. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36:942-952.
28. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193-202.
29. Cheng A-L, Qin S, Ikeda M, et al. IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30 (suppl_9):ix183-ix202.
30. Jiang Y, Han Q-J, Zhang J. Hepatocellular carcinoma: Mechanisms of progression and immunotherapy. World J Gastroenterol. 2019;25:3151-3167.
31. Xu F, Jin T, Zhu Y, et al. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37:110.
32. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501.
33. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681-700.
34. Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501-3508.
35. Louafi S, Boige V, Ducreux M, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC). Cancer. 2007;109:1384-1390.
36. Tang A, Chan AT, Zee B, et al. A randomized phase iii study of doxorubicin versus cisplatin/interferon α-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532-1538.
37. Abou-Alfa GK, Niedzwieski D, Knox JJ, et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J Clin Oncol. 2016;34:192.
38. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739.
39. Brown KT, Do RK, Gonen M, et al. randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol. 2016;34:2046-2053.
40. Kirstein MM, Voigtlander T, Schweitzer N, et al. Transarterial chemoembolization versus sorafenib in patients with hepatocellular carcinoma and extrahepatic disease. United European Gastroenterol J. 2018;6:238-246.
41. Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590-599.
42. Mason MC, Massarweh NN, Salami A, et al. Post-embolization syndrome as an early predictor of overall survival after transarterial chemoembolization for hepatocellular carcinoma. HPB (Oxford). 2015;17:1137-1144.
43. Sangro B, Maini CL, Ettorre GM, et al. Radioembolisation in patients with hepatocellular carcinoma that have previously received liver-directed therapies. Eur J Nucl Med Mol Imaging. 2018;45:1721-1730.
44. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624-1636.
45. Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921.
46. Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117-2127.
47. Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098.
48. Geschwind JF, Chapiro J. Sorafenib in combination with transarterial chemoembolization for the treatment of hepatocellular carcinoma. Clin Adv Hematol Oncol. 2016;14:585-587.
49. Kudo M, Ueshima K, Ikeda M, et al. Randomized, open label, multicenter, phase II trial comparing transarterial chemoembolization (TACE) plus sorafenib with TACE alone in patients with hepatocellular carcinoma (HCC): TACTICS trial. J Clin Oncol. 2018;36:206.
Geriatric Assessment and Collaborative Medication Review for Older Adults With Polypharmacy
Study Overview
Objective. To examine the effect of clinical geriatric assessments and collaborative medication review by geriatricians and family physicians on quality of life and other patient outcomes in home-dwelling older adults with polypharmacy.
Design. The study was a single-blind, cluster randomized clinical trial enrolling home-dwelling adults aged 70 years and older who were taking 7 or more medications. Family physicians in Norway were recruited to participate in the trial with their patients. Randomization was at the family physician level to avoid contamination between intervention and control groups.
Setting and participants. The study was conducted in Akershus and Oslo, Norway. Family physicians were recruited to participate in the trial with their patients. A total of 84 family physicians were recruited, of which 70 were included in the trial and randomized to intervention versus control; 14 were excluded because they had no eligible patients. The cluster size of each family physician was limited to 5 patients per physician to avoid large variation in cluster sizes. Patients were eligible for enrollment if they were home-dwelling, aged 70 years or older, and were taking 7 or more systemic medications regularly and had medications administered by the home nursing service. Patients were excluded if they were expected to die or be institutionalized within 6 months, or if they were discouraged from participation by their family physician. A total of 174 patients were recruited, with 87 patients in each group (34 family physicians were in the control group and 36 in the intervention group).
Intervention. The intervention included a geriatric assessment performed by a physician trained in geriatric medicine and supervised by a senior consultant. The geriatric assessment consisted of review of medical history; systematic screening for current problems; clinical examination; supplementary tests, if indicated; and review of each medication being used. The review of medication included the indication for each medication, dosage, adverse effects, and interactions. The geriatric assessment consultation took 1 hour to complete, on average. After the geriatric assessment, the family physician and the geriatrician met to discuss each medication and to establish a collaborative plan for adjustments and follow-up; this meeting was approximately 15 minutes in duration. Lastly, clinical follow-up with the older adult was conducted by the geriatrician or the family physician, as agreed upon in the plan, with most follow-up conducted by the family physician. Participants randomized to the control group received usual care without any intervention.
Main outcome measures. Outcomes were assessed at 16-week and 24-week follow-up. The main study outcome measure was health-related quality of life (HRQoL), as measured by the 15D instrument, at 16 weeks. The quality-of-life measure included the following aspects, each rated on an ordinal scale of 5 levels: mobility, vision, hearing, breathing, sleeping, eating, speech, elimination, usual activities, mental function, discomfort or symptoms, depression, distress, vitality, and sexual activity. The index scale including all aspects is in the range of 0 to 1, with a higher score indicating better quality of life. A predetermined change of 0.015 or more is considered clinically important, and a positive change of 0.035 indicates much better HRQoL. Other outcomes included: appropriateness of medications measured by the Medication Appropriateness Index and the Assessment of Underutilization; physical function (short Physical Performance battery); gait speed; grip strength; cognitive functioning; physical and cognitive disability (Functional Independence Measure); caregiver burden (Relative Stress Scale); physical measures, including orthostatic blood pressure, falls, and weight; hospital admissions; use of home nursing service; incidence of institutionalization; and mortality.
Main results. The study included 174 patients with an average age of 83.3 years (SD, 7.3); 67.8% were women. Of those who were randomized to the intervention and control groups, 158 (90.8%) completed the trial. The average number of regularly used medications was 10.1 (SD, 2.7) in the intervention group and 9.5 (SD, 2.6) in the control group. At week 16 of follow-up, patients in the intervention group had an improved HRQoL score measured by the 15D instrument; the difference between the intervention group and control groups was 0.045 (95% confidence interval [CI], 0.004 -0.086; P = 0.03). Medication appropriateness was better in the intervention group, as compared with the control group at both 16 weeks and 24 weeks. Nearly all (99%) patients in the intervention group experienced medication changes, which included withdrawal of medications, dosage adjustment, or new drug regimens. There was a trend towards a higher rate of hospitalization during follow-up in the intervention group (adjusted risk ratio, 2.03; 95% CI, 0.98-4.24; P = 0.06). Other secondary outcomes were not substantially different between the intervention and control groups.
Conclusion. The study demonstrated that a clinical geriatric assessment and collaborative medication review by geriatrician and family physician led to improved HRQoL and improved medication use.
Commentary
The use of multiple medications in older adults is common, with almost 20% of older adults over age 65 taking 10 or more medications.1 Polypharmacy in older adults is associated with lower adherence rates and increases the potential for interactions between medications.2 Age-related changes, such as changes in absorption, metabolism, and excretion, affect pharmacokinetics of medications and potentiate adverse drug reactions, requiring adjustments in use and dosing to optimize safety and outcomes. Recognizing the potential effects of medications in older adults, evidence-based guidelines, such as the Beers criteria3 and START/STOPP criteria,4 have been developed to identify potentially inappropriate medications in older adults and to improve prescribing. Randomized trials using the START/STOPP criteria have demonstrated improved medication appropriateness, reduced polypharmacy, and reduced adverse drug reactions.5 Although this study did not use a criteria-based approach for improving medication use, it demonstrated that in a population of older adults with polypharmacy, medication review with geriatricians can lead to improved HRQoL while improving medication appropriateness. The collaborative approach between the family physician and geriatrician, rather than a consultative approach with recommendations from a geriatrician, may have contributed to increased uptake of medication changes. Such an approach may be a reasonable strategy to improve medication use in older adults.
A limitation of the study is that the improvement in HRQoL could have been the result of medication changes, but could also have been due to other changes in the plan of care that resulted from the geriatric assessment. As noted by the authors, the increase in hospital admissions, though not statistically significant, could have resulted from the medication modifications; however, it was also noted that the geriatric assessments could have identified severe illnesses that required hospitalization, as the timeline from geriatric assessment to hospitalization suggested was the case. Thus, the increase in hospitalization resulting from timely identification of severe illness was more likely a benefit than an adverse effect; however, further studies should be done to elucidate this.
Applications for Clinical Practice
Older adults with multiple chronic conditions and complex medication regimens are at risk for poor health outcomes, and a purposeful medication review to improve medication use, leading to the removal of unnecessary and potentially harmful medications, adjustment of dosages, and initiation of appropriate medications, may yield health benefits, such as improved HRQoL. The present study utilized an approach that could be scalable, which is important given the limited number of clinicians with geriatrics expertise. For health systems with geriatrics clinical expertise, it may be reasonable to consider adopting a similar collaborative approach in order to improve care for older adults most at risk. Further reports on how patients and family physicians perceive this intervention will enhance our understanding of whether it could be implemented widely.
–William W. Hung, MD, MPH
1. Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There’s got to be a happy medium”. JAMA. 2010;304:1592-1601.
2. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
3. American Geriatrics Society 2015 Updated Beers criteria for potentially inappropriate medication use in older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
4. Hill-Taylor B, Sketris I, Hayden J, et al. Application of the STOPP/START criteria: a systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther. 2013;38:360-372.
5. O’Mahony D. STOPP/START criteria for potentially inappropriate medications/ potential prescribing omissions in older people: origin and progress. Expert Rev Clin Pharmacol. 2020;13:15-22.
Study Overview
Objective. To examine the effect of clinical geriatric assessments and collaborative medication review by geriatricians and family physicians on quality of life and other patient outcomes in home-dwelling older adults with polypharmacy.
Design. The study was a single-blind, cluster randomized clinical trial enrolling home-dwelling adults aged 70 years and older who were taking 7 or more medications. Family physicians in Norway were recruited to participate in the trial with their patients. Randomization was at the family physician level to avoid contamination between intervention and control groups.
Setting and participants. The study was conducted in Akershus and Oslo, Norway. Family physicians were recruited to participate in the trial with their patients. A total of 84 family physicians were recruited, of which 70 were included in the trial and randomized to intervention versus control; 14 were excluded because they had no eligible patients. The cluster size of each family physician was limited to 5 patients per physician to avoid large variation in cluster sizes. Patients were eligible for enrollment if they were home-dwelling, aged 70 years or older, and were taking 7 or more systemic medications regularly and had medications administered by the home nursing service. Patients were excluded if they were expected to die or be institutionalized within 6 months, or if they were discouraged from participation by their family physician. A total of 174 patients were recruited, with 87 patients in each group (34 family physicians were in the control group and 36 in the intervention group).
Intervention. The intervention included a geriatric assessment performed by a physician trained in geriatric medicine and supervised by a senior consultant. The geriatric assessment consisted of review of medical history; systematic screening for current problems; clinical examination; supplementary tests, if indicated; and review of each medication being used. The review of medication included the indication for each medication, dosage, adverse effects, and interactions. The geriatric assessment consultation took 1 hour to complete, on average. After the geriatric assessment, the family physician and the geriatrician met to discuss each medication and to establish a collaborative plan for adjustments and follow-up; this meeting was approximately 15 minutes in duration. Lastly, clinical follow-up with the older adult was conducted by the geriatrician or the family physician, as agreed upon in the plan, with most follow-up conducted by the family physician. Participants randomized to the control group received usual care without any intervention.
Main outcome measures. Outcomes were assessed at 16-week and 24-week follow-up. The main study outcome measure was health-related quality of life (HRQoL), as measured by the 15D instrument, at 16 weeks. The quality-of-life measure included the following aspects, each rated on an ordinal scale of 5 levels: mobility, vision, hearing, breathing, sleeping, eating, speech, elimination, usual activities, mental function, discomfort or symptoms, depression, distress, vitality, and sexual activity. The index scale including all aspects is in the range of 0 to 1, with a higher score indicating better quality of life. A predetermined change of 0.015 or more is considered clinically important, and a positive change of 0.035 indicates much better HRQoL. Other outcomes included: appropriateness of medications measured by the Medication Appropriateness Index and the Assessment of Underutilization; physical function (short Physical Performance battery); gait speed; grip strength; cognitive functioning; physical and cognitive disability (Functional Independence Measure); caregiver burden (Relative Stress Scale); physical measures, including orthostatic blood pressure, falls, and weight; hospital admissions; use of home nursing service; incidence of institutionalization; and mortality.
Main results. The study included 174 patients with an average age of 83.3 years (SD, 7.3); 67.8% were women. Of those who were randomized to the intervention and control groups, 158 (90.8%) completed the trial. The average number of regularly used medications was 10.1 (SD, 2.7) in the intervention group and 9.5 (SD, 2.6) in the control group. At week 16 of follow-up, patients in the intervention group had an improved HRQoL score measured by the 15D instrument; the difference between the intervention group and control groups was 0.045 (95% confidence interval [CI], 0.004 -0.086; P = 0.03). Medication appropriateness was better in the intervention group, as compared with the control group at both 16 weeks and 24 weeks. Nearly all (99%) patients in the intervention group experienced medication changes, which included withdrawal of medications, dosage adjustment, or new drug regimens. There was a trend towards a higher rate of hospitalization during follow-up in the intervention group (adjusted risk ratio, 2.03; 95% CI, 0.98-4.24; P = 0.06). Other secondary outcomes were not substantially different between the intervention and control groups.
Conclusion. The study demonstrated that a clinical geriatric assessment and collaborative medication review by geriatrician and family physician led to improved HRQoL and improved medication use.
Commentary
The use of multiple medications in older adults is common, with almost 20% of older adults over age 65 taking 10 or more medications.1 Polypharmacy in older adults is associated with lower adherence rates and increases the potential for interactions between medications.2 Age-related changes, such as changes in absorption, metabolism, and excretion, affect pharmacokinetics of medications and potentiate adverse drug reactions, requiring adjustments in use and dosing to optimize safety and outcomes. Recognizing the potential effects of medications in older adults, evidence-based guidelines, such as the Beers criteria3 and START/STOPP criteria,4 have been developed to identify potentially inappropriate medications in older adults and to improve prescribing. Randomized trials using the START/STOPP criteria have demonstrated improved medication appropriateness, reduced polypharmacy, and reduced adverse drug reactions.5 Although this study did not use a criteria-based approach for improving medication use, it demonstrated that in a population of older adults with polypharmacy, medication review with geriatricians can lead to improved HRQoL while improving medication appropriateness. The collaborative approach between the family physician and geriatrician, rather than a consultative approach with recommendations from a geriatrician, may have contributed to increased uptake of medication changes. Such an approach may be a reasonable strategy to improve medication use in older adults.
A limitation of the study is that the improvement in HRQoL could have been the result of medication changes, but could also have been due to other changes in the plan of care that resulted from the geriatric assessment. As noted by the authors, the increase in hospital admissions, though not statistically significant, could have resulted from the medication modifications; however, it was also noted that the geriatric assessments could have identified severe illnesses that required hospitalization, as the timeline from geriatric assessment to hospitalization suggested was the case. Thus, the increase in hospitalization resulting from timely identification of severe illness was more likely a benefit than an adverse effect; however, further studies should be done to elucidate this.
Applications for Clinical Practice
Older adults with multiple chronic conditions and complex medication regimens are at risk for poor health outcomes, and a purposeful medication review to improve medication use, leading to the removal of unnecessary and potentially harmful medications, adjustment of dosages, and initiation of appropriate medications, may yield health benefits, such as improved HRQoL. The present study utilized an approach that could be scalable, which is important given the limited number of clinicians with geriatrics expertise. For health systems with geriatrics clinical expertise, it may be reasonable to consider adopting a similar collaborative approach in order to improve care for older adults most at risk. Further reports on how patients and family physicians perceive this intervention will enhance our understanding of whether it could be implemented widely.
–William W. Hung, MD, MPH
Study Overview
Objective. To examine the effect of clinical geriatric assessments and collaborative medication review by geriatricians and family physicians on quality of life and other patient outcomes in home-dwelling older adults with polypharmacy.
Design. The study was a single-blind, cluster randomized clinical trial enrolling home-dwelling adults aged 70 years and older who were taking 7 or more medications. Family physicians in Norway were recruited to participate in the trial with their patients. Randomization was at the family physician level to avoid contamination between intervention and control groups.
Setting and participants. The study was conducted in Akershus and Oslo, Norway. Family physicians were recruited to participate in the trial with their patients. A total of 84 family physicians were recruited, of which 70 were included in the trial and randomized to intervention versus control; 14 were excluded because they had no eligible patients. The cluster size of each family physician was limited to 5 patients per physician to avoid large variation in cluster sizes. Patients were eligible for enrollment if they were home-dwelling, aged 70 years or older, and were taking 7 or more systemic medications regularly and had medications administered by the home nursing service. Patients were excluded if they were expected to die or be institutionalized within 6 months, or if they were discouraged from participation by their family physician. A total of 174 patients were recruited, with 87 patients in each group (34 family physicians were in the control group and 36 in the intervention group).
Intervention. The intervention included a geriatric assessment performed by a physician trained in geriatric medicine and supervised by a senior consultant. The geriatric assessment consisted of review of medical history; systematic screening for current problems; clinical examination; supplementary tests, if indicated; and review of each medication being used. The review of medication included the indication for each medication, dosage, adverse effects, and interactions. The geriatric assessment consultation took 1 hour to complete, on average. After the geriatric assessment, the family physician and the geriatrician met to discuss each medication and to establish a collaborative plan for adjustments and follow-up; this meeting was approximately 15 minutes in duration. Lastly, clinical follow-up with the older adult was conducted by the geriatrician or the family physician, as agreed upon in the plan, with most follow-up conducted by the family physician. Participants randomized to the control group received usual care without any intervention.
Main outcome measures. Outcomes were assessed at 16-week and 24-week follow-up. The main study outcome measure was health-related quality of life (HRQoL), as measured by the 15D instrument, at 16 weeks. The quality-of-life measure included the following aspects, each rated on an ordinal scale of 5 levels: mobility, vision, hearing, breathing, sleeping, eating, speech, elimination, usual activities, mental function, discomfort or symptoms, depression, distress, vitality, and sexual activity. The index scale including all aspects is in the range of 0 to 1, with a higher score indicating better quality of life. A predetermined change of 0.015 or more is considered clinically important, and a positive change of 0.035 indicates much better HRQoL. Other outcomes included: appropriateness of medications measured by the Medication Appropriateness Index and the Assessment of Underutilization; physical function (short Physical Performance battery); gait speed; grip strength; cognitive functioning; physical and cognitive disability (Functional Independence Measure); caregiver burden (Relative Stress Scale); physical measures, including orthostatic blood pressure, falls, and weight; hospital admissions; use of home nursing service; incidence of institutionalization; and mortality.
Main results. The study included 174 patients with an average age of 83.3 years (SD, 7.3); 67.8% were women. Of those who were randomized to the intervention and control groups, 158 (90.8%) completed the trial. The average number of regularly used medications was 10.1 (SD, 2.7) in the intervention group and 9.5 (SD, 2.6) in the control group. At week 16 of follow-up, patients in the intervention group had an improved HRQoL score measured by the 15D instrument; the difference between the intervention group and control groups was 0.045 (95% confidence interval [CI], 0.004 -0.086; P = 0.03). Medication appropriateness was better in the intervention group, as compared with the control group at both 16 weeks and 24 weeks. Nearly all (99%) patients in the intervention group experienced medication changes, which included withdrawal of medications, dosage adjustment, or new drug regimens. There was a trend towards a higher rate of hospitalization during follow-up in the intervention group (adjusted risk ratio, 2.03; 95% CI, 0.98-4.24; P = 0.06). Other secondary outcomes were not substantially different between the intervention and control groups.
Conclusion. The study demonstrated that a clinical geriatric assessment and collaborative medication review by geriatrician and family physician led to improved HRQoL and improved medication use.
Commentary
The use of multiple medications in older adults is common, with almost 20% of older adults over age 65 taking 10 or more medications.1 Polypharmacy in older adults is associated with lower adherence rates and increases the potential for interactions between medications.2 Age-related changes, such as changes in absorption, metabolism, and excretion, affect pharmacokinetics of medications and potentiate adverse drug reactions, requiring adjustments in use and dosing to optimize safety and outcomes. Recognizing the potential effects of medications in older adults, evidence-based guidelines, such as the Beers criteria3 and START/STOPP criteria,4 have been developed to identify potentially inappropriate medications in older adults and to improve prescribing. Randomized trials using the START/STOPP criteria have demonstrated improved medication appropriateness, reduced polypharmacy, and reduced adverse drug reactions.5 Although this study did not use a criteria-based approach for improving medication use, it demonstrated that in a population of older adults with polypharmacy, medication review with geriatricians can lead to improved HRQoL while improving medication appropriateness. The collaborative approach between the family physician and geriatrician, rather than a consultative approach with recommendations from a geriatrician, may have contributed to increased uptake of medication changes. Such an approach may be a reasonable strategy to improve medication use in older adults.
A limitation of the study is that the improvement in HRQoL could have been the result of medication changes, but could also have been due to other changes in the plan of care that resulted from the geriatric assessment. As noted by the authors, the increase in hospital admissions, though not statistically significant, could have resulted from the medication modifications; however, it was also noted that the geriatric assessments could have identified severe illnesses that required hospitalization, as the timeline from geriatric assessment to hospitalization suggested was the case. Thus, the increase in hospitalization resulting from timely identification of severe illness was more likely a benefit than an adverse effect; however, further studies should be done to elucidate this.
Applications for Clinical Practice
Older adults with multiple chronic conditions and complex medication regimens are at risk for poor health outcomes, and a purposeful medication review to improve medication use, leading to the removal of unnecessary and potentially harmful medications, adjustment of dosages, and initiation of appropriate medications, may yield health benefits, such as improved HRQoL. The present study utilized an approach that could be scalable, which is important given the limited number of clinicians with geriatrics expertise. For health systems with geriatrics clinical expertise, it may be reasonable to consider adopting a similar collaborative approach in order to improve care for older adults most at risk. Further reports on how patients and family physicians perceive this intervention will enhance our understanding of whether it could be implemented widely.
–William W. Hung, MD, MPH
1. Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There’s got to be a happy medium”. JAMA. 2010;304:1592-1601.
2. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
3. American Geriatrics Society 2015 Updated Beers criteria for potentially inappropriate medication use in older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
4. Hill-Taylor B, Sketris I, Hayden J, et al. Application of the STOPP/START criteria: a systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther. 2013;38:360-372.
5. O’Mahony D. STOPP/START criteria for potentially inappropriate medications/ potential prescribing omissions in older people: origin and progress. Expert Rev Clin Pharmacol. 2020;13:15-22.
1. Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There’s got to be a happy medium”. JAMA. 2010;304:1592-1601.
2. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
3. American Geriatrics Society 2015 Updated Beers criteria for potentially inappropriate medication use in older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
4. Hill-Taylor B, Sketris I, Hayden J, et al. Application of the STOPP/START criteria: a systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther. 2013;38:360-372.
5. O’Mahony D. STOPP/START criteria for potentially inappropriate medications/ potential prescribing omissions in older people: origin and progress. Expert Rev Clin Pharmacol. 2020;13:15-22.
Pembrolizumab Plus Neoadjuvant Chemotherapy Improves Pathologic Complete Response Rates in Triple-Negative Breast Cancer
Study Overview
Objective. To evaluate the efficacy and safety of pembrolizumab in combination with neoadjuvant chemotherapy followed by adjuvant pembrolizumab in early-stage triple-negative breast cancer.
Design. International, multicenter, randomized, double-blind, phase 3 trial.
Intervention. Patients were randomly assigned in a 2:1 fashion to receive either pembrolizumab or placebo. Patients received 4 cycles of neoadjuvant pembrolizumab or placebo once every 3 weeks, in addition to weekly paclitaxel 80 mg/m2 plus carboplatin AUC5 once every 3 weeks. This was followed by 4 cycles of pembrolizumab or placebo plus doxorubicin 60 mg/m2 or epirubicin 90 mg/m2 plus cyclophosphamide 600 mg/m2 once every 3 weeks. Patients then underwent definitive surgery 3 to 6 weeks after completion of neoadjuvant therapy. In the adjuvant setting, patients received pembrolizumab or placebo once every 3 weeks for up to 9 cycles. Adjuvant capecitabine was not allowed.
Setting and participants. A total of 1174 patients underwent randomization: 784 patients in the pembrolizumab/chemotherapy group and 390 patients in the placebo/chemotherapy group. Eligible patients had newly diagnosed, centrally confirmed triple-negative breast cancer (nonmetastatic: T1c, N1-2 or T2-4, N0-2). Patients were eligible regardless of PD-L1 status, and those with inflammatory breast cancer and multifocal primaries were eligible.
Main outcome measures. The primary endpoints of this study were pathologic complete response (pCR) rate (defined as ypT0/ypTis, ypN0) at the time of surgery and event-free survival (EFS) in the intention-to-treat population. Secondary endpoints included pCR in all patients, pCR among patients with PD-L1–positive tumors, EFS among patients with PD-L1–positive tumors, and overall survival among all patients and those with PD-L1–positive tumors. PD-L1 expression was assessed using the PD-L1 IHC 22C3 pharmDx assay (Agilent, Santa Clara, CA). Expression was characterized according to the combined positive score, with a score of 1% or greater being considered positive.
Results. The baseline characteristics were well balanced between the treatment arms. At the time of the second interim analysis, the median duration of follow-up was 15.5 months. The pCR rate among the first 602 patients who were randomized was 64.8% in the pembrolizumab/chemotherapy group and 51.2% in the placebo group (P < 0.001; 95% confidence interval, 5.4-21.8). The pCR rate in the PD-L1–positive population was 68.9% in the pembrolizumab/chemotherapy group, as compared to 54.9% in the placebo group. In the PD-L1–negative population, the pCR rate was 45.3% in the pembrolizumab/chemotherapy group, as compared to 30.3% in the placebo group. At the time of analysis, 104 events had occurred, and the estimated percentage of patients at 18 months who were alive without disease progression was 91% in the pembrolizumab group and 85% in the placebo group. The median was not reached in either group.
Grade 3 or higher adverse events in the neoadjuvant phase were seen in 76.8% and 72.2% of patients in the pembrolizumab and placebo arms, respectively. Serious treatment-related adverse events occurred in 32% of patients in the pembrolizumab group compared to 19% in the placebo group. Febrile neutropenia and anemia were the most common. Discontinuation of the trial drug due to adverse events occurred in 23% of patients in the pembrolizumab arm and in 12% in the placebo arm. The majority of treatment-related adverse events occurred in the neoadjuvant phase. In the adjuvant phase, treatment-related adverse events occurred in 48% and 43% of patients in the pembrolizumab and placebo groups, respectively.
Conclusion. The combination of neoadjuvant chemotherapy and pembrolizumab in patients with newly diagnosed, early-stage, triple-negative breast cancer yielded a higher percentage of patients achieving a pCR as compared with chemotherapy plus placebo.
Commentary
The current study adds to the growing body of literature outlining the efficacy of immune checkpoint inhibition in triple-negative breast cancer. The previously published IMpassion130 trial showed that the addition of the PD-L1 antibody atezolizumab to nab-paclitaxel improved progression-free survival in patients with PD-L1–positive (1% or greater), metastatic triple-negative breast cancer.1 Similarly, in the phase 2 I-SPY2 trial, the addition of pembrolizumab to standard neoadjuvant chemotherapy led to a near tripling of the pCR rates in triple-negative breast cancer.2 While the current study demonstrated improved pCR rates with pembrolizumab, no difference in EFS has yet been demonstrated; however, longer-term follow-up will be required. There certainly are numerous studies documenting an association between pCR and improved disease-free survival and possibly overall survival. Cortazar and colleagues performed a pooled analysis of 12 international trials, which demonstrated an association between pCR and improved EFS (hazard ratio [HR], 0.24) and overall survival (HR, 0.16) in patients with triple-negative breast cancer.3 The results of the current study will require longer-term follow-up to confirm such an association.
The current study appears to have demonstrated a benefit with the addition of pembrolizumab across treatment subgroups, particularly in the PD-L1–positive and PD-L1–negative populations. While this differs from the findings of the IMpassion130 trial, it is quite difficult to draw definitive conclusions because the 2 trials studied different antibodies, and thus used a different assay to define PD-L1 positivity. Notable differences exist in determination of PD-L1 status across assays, and it is important for providers to use the appropriate assay for each antibody. These differences highlight the need for more informative biomarkers to predict a benefit from immune checkpoint inhibition.
It is also noteworthy that the control arm in the current trial was a platinum-based regimen. Platinum-based neoadjuvant regimens previously have been shown to induce higher pCR rates in triple-negative breast cancer; however, the incorporation of carboplatin as standard of care remains a topic of debate.4 Nevertheless, a similar trial evaluating the efficacy of atezolizumab combined with platinum-based neoadjuvant chemotherapy in triple-negative breast cancer, NSABP B-59 (NCT03281954), is underway, with the control arm also incorporating carboplatin. The results of this study will also help validate the role of checkpoint inhibitors in the neoadjuvant setting in triple-negative breast cancer. Of note, this trial did not allow for the use of adjuvant capecitabine, which has been previously shown in the CREATE-X trial to prolong survival in this population.5 How the use of adjuvant capecitabine would impact these results is completely unknown.6 The incidence of grade 3 or higher toxicities in the current trial appeared to be similar in both groups. There did appear to be a higher incidence of infusion reactions and skin reactions in the pembrolizumab groups. Immune-related adverse events were consistent with prior pembrolizumab data.
Applications for Clinical Practice
KEYNOTE-522 adds to the growing evidence suggesting that incorporation of immune checkpoint inhibitors into neoadjuvant therapy in patients with triple-negative breast cancer can improve pCR rates; however, its use as a standard of care will require longer-term follow-up to ensure the noted findings translate into improvement in EFS and, ultimately, overall survival.
– Daniel Isaac, DO, MS
1. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108-2121.
2. Nanda R, Liu MC, Yau C, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): results from I-SPY 2. J Clin Oncol. 2017;35: Suppl:506. Abstract 506.
3. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164-172.
4. Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant one-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33:13-21.
5. Masuda N, Lee S-J, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147-2159.
6. von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15:747-756.
Study Overview
Objective. To evaluate the efficacy and safety of pembrolizumab in combination with neoadjuvant chemotherapy followed by adjuvant pembrolizumab in early-stage triple-negative breast cancer.
Design. International, multicenter, randomized, double-blind, phase 3 trial.
Intervention. Patients were randomly assigned in a 2:1 fashion to receive either pembrolizumab or placebo. Patients received 4 cycles of neoadjuvant pembrolizumab or placebo once every 3 weeks, in addition to weekly paclitaxel 80 mg/m2 plus carboplatin AUC5 once every 3 weeks. This was followed by 4 cycles of pembrolizumab or placebo plus doxorubicin 60 mg/m2 or epirubicin 90 mg/m2 plus cyclophosphamide 600 mg/m2 once every 3 weeks. Patients then underwent definitive surgery 3 to 6 weeks after completion of neoadjuvant therapy. In the adjuvant setting, patients received pembrolizumab or placebo once every 3 weeks for up to 9 cycles. Adjuvant capecitabine was not allowed.
Setting and participants. A total of 1174 patients underwent randomization: 784 patients in the pembrolizumab/chemotherapy group and 390 patients in the placebo/chemotherapy group. Eligible patients had newly diagnosed, centrally confirmed triple-negative breast cancer (nonmetastatic: T1c, N1-2 or T2-4, N0-2). Patients were eligible regardless of PD-L1 status, and those with inflammatory breast cancer and multifocal primaries were eligible.
Main outcome measures. The primary endpoints of this study were pathologic complete response (pCR) rate (defined as ypT0/ypTis, ypN0) at the time of surgery and event-free survival (EFS) in the intention-to-treat population. Secondary endpoints included pCR in all patients, pCR among patients with PD-L1–positive tumors, EFS among patients with PD-L1–positive tumors, and overall survival among all patients and those with PD-L1–positive tumors. PD-L1 expression was assessed using the PD-L1 IHC 22C3 pharmDx assay (Agilent, Santa Clara, CA). Expression was characterized according to the combined positive score, with a score of 1% or greater being considered positive.
Results. The baseline characteristics were well balanced between the treatment arms. At the time of the second interim analysis, the median duration of follow-up was 15.5 months. The pCR rate among the first 602 patients who were randomized was 64.8% in the pembrolizumab/chemotherapy group and 51.2% in the placebo group (P < 0.001; 95% confidence interval, 5.4-21.8). The pCR rate in the PD-L1–positive population was 68.9% in the pembrolizumab/chemotherapy group, as compared to 54.9% in the placebo group. In the PD-L1–negative population, the pCR rate was 45.3% in the pembrolizumab/chemotherapy group, as compared to 30.3% in the placebo group. At the time of analysis, 104 events had occurred, and the estimated percentage of patients at 18 months who were alive without disease progression was 91% in the pembrolizumab group and 85% in the placebo group. The median was not reached in either group.
Grade 3 or higher adverse events in the neoadjuvant phase were seen in 76.8% and 72.2% of patients in the pembrolizumab and placebo arms, respectively. Serious treatment-related adverse events occurred in 32% of patients in the pembrolizumab group compared to 19% in the placebo group. Febrile neutropenia and anemia were the most common. Discontinuation of the trial drug due to adverse events occurred in 23% of patients in the pembrolizumab arm and in 12% in the placebo arm. The majority of treatment-related adverse events occurred in the neoadjuvant phase. In the adjuvant phase, treatment-related adverse events occurred in 48% and 43% of patients in the pembrolizumab and placebo groups, respectively.
Conclusion. The combination of neoadjuvant chemotherapy and pembrolizumab in patients with newly diagnosed, early-stage, triple-negative breast cancer yielded a higher percentage of patients achieving a pCR as compared with chemotherapy plus placebo.
Commentary
The current study adds to the growing body of literature outlining the efficacy of immune checkpoint inhibition in triple-negative breast cancer. The previously published IMpassion130 trial showed that the addition of the PD-L1 antibody atezolizumab to nab-paclitaxel improved progression-free survival in patients with PD-L1–positive (1% or greater), metastatic triple-negative breast cancer.1 Similarly, in the phase 2 I-SPY2 trial, the addition of pembrolizumab to standard neoadjuvant chemotherapy led to a near tripling of the pCR rates in triple-negative breast cancer.2 While the current study demonstrated improved pCR rates with pembrolizumab, no difference in EFS has yet been demonstrated; however, longer-term follow-up will be required. There certainly are numerous studies documenting an association between pCR and improved disease-free survival and possibly overall survival. Cortazar and colleagues performed a pooled analysis of 12 international trials, which demonstrated an association between pCR and improved EFS (hazard ratio [HR], 0.24) and overall survival (HR, 0.16) in patients with triple-negative breast cancer.3 The results of the current study will require longer-term follow-up to confirm such an association.
The current study appears to have demonstrated a benefit with the addition of pembrolizumab across treatment subgroups, particularly in the PD-L1–positive and PD-L1–negative populations. While this differs from the findings of the IMpassion130 trial, it is quite difficult to draw definitive conclusions because the 2 trials studied different antibodies, and thus used a different assay to define PD-L1 positivity. Notable differences exist in determination of PD-L1 status across assays, and it is important for providers to use the appropriate assay for each antibody. These differences highlight the need for more informative biomarkers to predict a benefit from immune checkpoint inhibition.
It is also noteworthy that the control arm in the current trial was a platinum-based regimen. Platinum-based neoadjuvant regimens previously have been shown to induce higher pCR rates in triple-negative breast cancer; however, the incorporation of carboplatin as standard of care remains a topic of debate.4 Nevertheless, a similar trial evaluating the efficacy of atezolizumab combined with platinum-based neoadjuvant chemotherapy in triple-negative breast cancer, NSABP B-59 (NCT03281954), is underway, with the control arm also incorporating carboplatin. The results of this study will also help validate the role of checkpoint inhibitors in the neoadjuvant setting in triple-negative breast cancer. Of note, this trial did not allow for the use of adjuvant capecitabine, which has been previously shown in the CREATE-X trial to prolong survival in this population.5 How the use of adjuvant capecitabine would impact these results is completely unknown.6 The incidence of grade 3 or higher toxicities in the current trial appeared to be similar in both groups. There did appear to be a higher incidence of infusion reactions and skin reactions in the pembrolizumab groups. Immune-related adverse events were consistent with prior pembrolizumab data.
Applications for Clinical Practice
KEYNOTE-522 adds to the growing evidence suggesting that incorporation of immune checkpoint inhibitors into neoadjuvant therapy in patients with triple-negative breast cancer can improve pCR rates; however, its use as a standard of care will require longer-term follow-up to ensure the noted findings translate into improvement in EFS and, ultimately, overall survival.
– Daniel Isaac, DO, MS
Study Overview
Objective. To evaluate the efficacy and safety of pembrolizumab in combination with neoadjuvant chemotherapy followed by adjuvant pembrolizumab in early-stage triple-negative breast cancer.
Design. International, multicenter, randomized, double-blind, phase 3 trial.
Intervention. Patients were randomly assigned in a 2:1 fashion to receive either pembrolizumab or placebo. Patients received 4 cycles of neoadjuvant pembrolizumab or placebo once every 3 weeks, in addition to weekly paclitaxel 80 mg/m2 plus carboplatin AUC5 once every 3 weeks. This was followed by 4 cycles of pembrolizumab or placebo plus doxorubicin 60 mg/m2 or epirubicin 90 mg/m2 plus cyclophosphamide 600 mg/m2 once every 3 weeks. Patients then underwent definitive surgery 3 to 6 weeks after completion of neoadjuvant therapy. In the adjuvant setting, patients received pembrolizumab or placebo once every 3 weeks for up to 9 cycles. Adjuvant capecitabine was not allowed.
Setting and participants. A total of 1174 patients underwent randomization: 784 patients in the pembrolizumab/chemotherapy group and 390 patients in the placebo/chemotherapy group. Eligible patients had newly diagnosed, centrally confirmed triple-negative breast cancer (nonmetastatic: T1c, N1-2 or T2-4, N0-2). Patients were eligible regardless of PD-L1 status, and those with inflammatory breast cancer and multifocal primaries were eligible.
Main outcome measures. The primary endpoints of this study were pathologic complete response (pCR) rate (defined as ypT0/ypTis, ypN0) at the time of surgery and event-free survival (EFS) in the intention-to-treat population. Secondary endpoints included pCR in all patients, pCR among patients with PD-L1–positive tumors, EFS among patients with PD-L1–positive tumors, and overall survival among all patients and those with PD-L1–positive tumors. PD-L1 expression was assessed using the PD-L1 IHC 22C3 pharmDx assay (Agilent, Santa Clara, CA). Expression was characterized according to the combined positive score, with a score of 1% or greater being considered positive.
Results. The baseline characteristics were well balanced between the treatment arms. At the time of the second interim analysis, the median duration of follow-up was 15.5 months. The pCR rate among the first 602 patients who were randomized was 64.8% in the pembrolizumab/chemotherapy group and 51.2% in the placebo group (P < 0.001; 95% confidence interval, 5.4-21.8). The pCR rate in the PD-L1–positive population was 68.9% in the pembrolizumab/chemotherapy group, as compared to 54.9% in the placebo group. In the PD-L1–negative population, the pCR rate was 45.3% in the pembrolizumab/chemotherapy group, as compared to 30.3% in the placebo group. At the time of analysis, 104 events had occurred, and the estimated percentage of patients at 18 months who were alive without disease progression was 91% in the pembrolizumab group and 85% in the placebo group. The median was not reached in either group.
Grade 3 or higher adverse events in the neoadjuvant phase were seen in 76.8% and 72.2% of patients in the pembrolizumab and placebo arms, respectively. Serious treatment-related adverse events occurred in 32% of patients in the pembrolizumab group compared to 19% in the placebo group. Febrile neutropenia and anemia were the most common. Discontinuation of the trial drug due to adverse events occurred in 23% of patients in the pembrolizumab arm and in 12% in the placebo arm. The majority of treatment-related adverse events occurred in the neoadjuvant phase. In the adjuvant phase, treatment-related adverse events occurred in 48% and 43% of patients in the pembrolizumab and placebo groups, respectively.
Conclusion. The combination of neoadjuvant chemotherapy and pembrolizumab in patients with newly diagnosed, early-stage, triple-negative breast cancer yielded a higher percentage of patients achieving a pCR as compared with chemotherapy plus placebo.
Commentary
The current study adds to the growing body of literature outlining the efficacy of immune checkpoint inhibition in triple-negative breast cancer. The previously published IMpassion130 trial showed that the addition of the PD-L1 antibody atezolizumab to nab-paclitaxel improved progression-free survival in patients with PD-L1–positive (1% or greater), metastatic triple-negative breast cancer.1 Similarly, in the phase 2 I-SPY2 trial, the addition of pembrolizumab to standard neoadjuvant chemotherapy led to a near tripling of the pCR rates in triple-negative breast cancer.2 While the current study demonstrated improved pCR rates with pembrolizumab, no difference in EFS has yet been demonstrated; however, longer-term follow-up will be required. There certainly are numerous studies documenting an association between pCR and improved disease-free survival and possibly overall survival. Cortazar and colleagues performed a pooled analysis of 12 international trials, which demonstrated an association between pCR and improved EFS (hazard ratio [HR], 0.24) and overall survival (HR, 0.16) in patients with triple-negative breast cancer.3 The results of the current study will require longer-term follow-up to confirm such an association.
The current study appears to have demonstrated a benefit with the addition of pembrolizumab across treatment subgroups, particularly in the PD-L1–positive and PD-L1–negative populations. While this differs from the findings of the IMpassion130 trial, it is quite difficult to draw definitive conclusions because the 2 trials studied different antibodies, and thus used a different assay to define PD-L1 positivity. Notable differences exist in determination of PD-L1 status across assays, and it is important for providers to use the appropriate assay for each antibody. These differences highlight the need for more informative biomarkers to predict a benefit from immune checkpoint inhibition.
It is also noteworthy that the control arm in the current trial was a platinum-based regimen. Platinum-based neoadjuvant regimens previously have been shown to induce higher pCR rates in triple-negative breast cancer; however, the incorporation of carboplatin as standard of care remains a topic of debate.4 Nevertheless, a similar trial evaluating the efficacy of atezolizumab combined with platinum-based neoadjuvant chemotherapy in triple-negative breast cancer, NSABP B-59 (NCT03281954), is underway, with the control arm also incorporating carboplatin. The results of this study will also help validate the role of checkpoint inhibitors in the neoadjuvant setting in triple-negative breast cancer. Of note, this trial did not allow for the use of adjuvant capecitabine, which has been previously shown in the CREATE-X trial to prolong survival in this population.5 How the use of adjuvant capecitabine would impact these results is completely unknown.6 The incidence of grade 3 or higher toxicities in the current trial appeared to be similar in both groups. There did appear to be a higher incidence of infusion reactions and skin reactions in the pembrolizumab groups. Immune-related adverse events were consistent with prior pembrolizumab data.
Applications for Clinical Practice
KEYNOTE-522 adds to the growing evidence suggesting that incorporation of immune checkpoint inhibitors into neoadjuvant therapy in patients with triple-negative breast cancer can improve pCR rates; however, its use as a standard of care will require longer-term follow-up to ensure the noted findings translate into improvement in EFS and, ultimately, overall survival.
– Daniel Isaac, DO, MS
1. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108-2121.
2. Nanda R, Liu MC, Yau C, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): results from I-SPY 2. J Clin Oncol. 2017;35: Suppl:506. Abstract 506.
3. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164-172.
4. Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant one-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33:13-21.
5. Masuda N, Lee S-J, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147-2159.
6. von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15:747-756.
1. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108-2121.
2. Nanda R, Liu MC, Yau C, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): results from I-SPY 2. J Clin Oncol. 2017;35: Suppl:506. Abstract 506.
3. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164-172.
4. Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant one-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33:13-21.
5. Masuda N, Lee S-J, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147-2159.
6. von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15:747-756.
Cabazitaxel Improves Progression-Free and Overall Survival in Metastatic Prostate Cancer After Progression on Abiraterone or Enzalutamide
Study Overview
Objective. To evaluate the efficacy of cabazitaxel compared to androgen-signaling–targeted inhibitors (ASTIs) in patients with metastatic castration-resistant prostate cancer who have received docetaxel and have progressed within 12 months of treatment with either abiraterone or enzalutamide.
Design. The CARD trial was an international, randomized, open-label phase 3 trial conducted across 13 European countries.
Setting and participants. Eligible patients were 18 years of age or older; had metastatic castration-resistant prostate cancer previously treated with docetaxel; and had disease progression during 12 months of treatment with abiraterone or enzalutamide. All patients had histologically proven prostate cancer, castrate levels of serum testosterone, and disease progression, defined by at least 2 new bone lesions or rising prostate-specific antigen (PSA) level. A total of 255 patients underwent randomization between November 2015 and November 2018, with 129 assigned to receive cabazitaxel and 126 patients assigned to receive an ASTI, 58 of whom received abiraterone and 66 of whom received enzalutamide. Patients who had received an ASTI in the setting of castrate-sensitive metastatic prostate cancer were included.
Intervention. Patients were randomized in a 1:1 fashion to receive either cabazitaxel or abiraterone or enzalutamide. Patients receiving cabazitaxel 25 mg/m2 intravenously every 3 weeks also received oral prednisone daily and primary prophylactic granulocyte-colony stimulating factor. Patients assigned to receive an ASTI received abiraterone 1000 mg orally daily with prednisone 5 mg twice daily or enzalutamide 160 mg daily. Patients in the ASTI group who had progressed on abiraterone were assigned to enzalutamide, and alternatively, those on enzalutamide were assigned to abiraterone. Patients were treated until 1 of the following occurred: imaging-based disease progression, unacceptable toxicity, or advancing to an alternative therapy.
Main outcome measures. The primary endpoint was imaging-based progression-free survival, which was defined as the time from randomization until objective tumor progression, progression of bone lesions, or death. The secondary endpoints were overall survival, progression-free survival, PSA response, tumor and pain responses, a new symptomatic skeletal event, and safety.
Results. The median follow-up was 9.2 months. Imaging-based disease progression or death from any cause occurred in 95 (73.6%) participants in the cabazitaxel group, as compared to 101 (80.2%) who were assigned to receive an ASTI. The median imaging-based progression-free survival was 8.0 months in the cabazitaxel group and 3.7 months in the abiraterone/enzalutamide group. The median duration of treatment was longer in those receiving cabazitaxel (22 vs 12.5 weeks). The primary reason for treatment discontinuation was disease progression (in 43.7% of patients receiving cabazitaxel and 71% receiving an ASTI) or an adverse event (19.8% and 8.9%, respectively).
The trial’s secondary endpoints demonstrated improved outcomes in the cabazitaxel group compared to the abiraterone/enzalutamide group. There were 70 deaths (54.2%) in the cabazitaxel group and 83 (65.9%) in the ASTI group. Both the median overall survival (13.6 months in the cabazitaxel group and 11 months in the ASTI group) and the median progression-free survival (4.4 months and 2.7 months, respectively) were improved in those who received cabazitaxel. There was a 50% or greater reduction in the PSA level from baseline in 35.7% of the cabazitaxel group and 13.5% of the ASTI group.
Regarding the safety of the agents, the incidence of adverse events was similar in each group (38.9% in the cabazitaxel group and 38.7% in the ASTI group). Treatment discontinuation occurred more frequently in the cabazitaxel group (19.8%) compared to the ASTI group (8.9%). Adverse events of grade 3 or higher occurred more frequently with cabazitaxel; these were asthenia (4% vs 2.4%), diarrhea (3.2% vs 0), peripheral neuropathy (3.2% vs 0 patients), and febrile neutropenia (3.2% vs 0 patients).
Conclusion. Patients who had disease progression within 12 months on an ASTI and had previously been treated for metastatic castration-resistant prostate cancer with docetaxel had longer imaging-based progression-free survival and overall survival when treated with cabazitaxel compared to those treated with an alternative ASTI. Other clinical outcomes, including overall survival and progression-free survival, were also improved in the cabazitaxel group.
Commentary
Four ASTIs are approved for therapy in men with advanced prostate cancer. The next line of therapy following progression on an ASTI, whether to consider second-line androgen targeted inhibitors or proceed to taxane-based chemotherapy, has been unclear. The current CARD trial sought to answer this question and provides evidence that cabazitaxel is the next line of therapy for these patients. The trial’s primary endpoint, imaging-based disease progression, was reported in 73.6% of those who received cabazitaxel and in 80.2% of those who received abiraterone or enzalutamide. Patients treated with cabazitaxel had a longer imaging-based progression-free survival (8.0 months vs 3.7 months) and a longer duration of treatment (22 vs 12.5 weeks).
Because there is clinical evidence of cross-resistance between different ASTIs, the value of sequential therapy has been unclear. Emergence of androgen-receptor splice variant 7 (AR-V7) mutational status in circulating tumor cells is associated with poor outcomes with secondary androgen-signaling inhibitor therapy, and may be an indicator of resistance to subsequent androgen-signaling inhibitors.1,2 In the PROPHECY trial, the response rates to subsequent androgen targeted therapy in patients with AR-V7 mutations ranged from 30% to 40%.3 Understanding how AR-V7 mutational status may impact such outcomes will certainly help define whether a subgroup exists in whom use of second-line androgen signaling inhibitors may be considered.
The patients enrolled in the current study appear to represent a subgroup of patients with biologically aggressive disease or with inherent resistance to ASTIs. The patients included in this study progressed within 1 year of androgen targeted therapy, which is representative of a more aggressive population of patients who may be hormone insensitive and derive more benefit from chemotherapy. Initial androgen deprivation therapy was given for 13.7 and 12.6 months to the cabazitaxel and enzalutamide/abiraterone arms, respectively, prior to developing castrate-resistant prostate cancer. Patients enrolled in this study also previously received docetaxel, deselecting those who are taxane-resistant and therefore may be less likely to respond to additional taxane-based therapy. Detection of AR-V7 splice variant expression in circulating tumor cells, consideration of biomarker data, and sensitivity to taxanes may help guide decisions regarding the use of sequential androgen-targeted agents; however, there has been no clear data to guide such an approach. It is also important to consider that, because this is a European study, the approved dose given in this trial was 25 mg/m2. The PROSELICA trial previously demonstrated noninferiority of 20 mg/m2 compared with 25 mg/m2, with fewer adverse events, which is the dose now utilized in the United States.4
The adverse events of grade 3 or greater occurring in the cabazitaxel group should be discussed with patients, including fatigue, diarrhea, peripheral neuropathy, and febrile neutropenia.
The data from the CARD trial provide guidance regarding therapy sequencing in those with advanced prostate cancer after progression on first-line androgen targeted inhibitors and docetaxel; however, further work is needed to understand the universal application of this data in this cohort.
Applications in Clinical Practice
Patients with metastatic castration-resistant prostate cancer who have received docetaxel and progressed on an androgen-signaling inhibitor within 12 months should be considered for cabazitaxel over an alternative androgen-signaling inhibitor. This decision should be based on several factors, including AR-V7 mutational status, duration of androgen deprivation therapy, and hormone and taxane sensitivity in the past. Future studies are likely to incorporate genomic biomarkers rather than clinical criteria alone to make treatment decisions.
–Britni Souther, DO, and Daniel Isaac, DO, MS, Michigan State University, East Lansing, MI
1. Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028-1038.
2. Zhang T, Karsh LI, Nissenblatt MJ, et al. Androgen receptor splice variant, AR-V7, as a biomarker of resistance to androgen axis-targeted therapies in advanced prostate cancer. Clin Genitourin Cancer. 2019;18:1-10.
3. Armstrong AJ, Halabi S, Luo J, et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J Clin Oncol. 2019;37:1120-1129.
4. Eisenberger M, Hardy-Bessard AC, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA. J Clin Oncol. 2017;35:3198-3206.
Study Overview
Objective. To evaluate the efficacy of cabazitaxel compared to androgen-signaling–targeted inhibitors (ASTIs) in patients with metastatic castration-resistant prostate cancer who have received docetaxel and have progressed within 12 months of treatment with either abiraterone or enzalutamide.
Design. The CARD trial was an international, randomized, open-label phase 3 trial conducted across 13 European countries.
Setting and participants. Eligible patients were 18 years of age or older; had metastatic castration-resistant prostate cancer previously treated with docetaxel; and had disease progression during 12 months of treatment with abiraterone or enzalutamide. All patients had histologically proven prostate cancer, castrate levels of serum testosterone, and disease progression, defined by at least 2 new bone lesions or rising prostate-specific antigen (PSA) level. A total of 255 patients underwent randomization between November 2015 and November 2018, with 129 assigned to receive cabazitaxel and 126 patients assigned to receive an ASTI, 58 of whom received abiraterone and 66 of whom received enzalutamide. Patients who had received an ASTI in the setting of castrate-sensitive metastatic prostate cancer were included.
Intervention. Patients were randomized in a 1:1 fashion to receive either cabazitaxel or abiraterone or enzalutamide. Patients receiving cabazitaxel 25 mg/m2 intravenously every 3 weeks also received oral prednisone daily and primary prophylactic granulocyte-colony stimulating factor. Patients assigned to receive an ASTI received abiraterone 1000 mg orally daily with prednisone 5 mg twice daily or enzalutamide 160 mg daily. Patients in the ASTI group who had progressed on abiraterone were assigned to enzalutamide, and alternatively, those on enzalutamide were assigned to abiraterone. Patients were treated until 1 of the following occurred: imaging-based disease progression, unacceptable toxicity, or advancing to an alternative therapy.
Main outcome measures. The primary endpoint was imaging-based progression-free survival, which was defined as the time from randomization until objective tumor progression, progression of bone lesions, or death. The secondary endpoints were overall survival, progression-free survival, PSA response, tumor and pain responses, a new symptomatic skeletal event, and safety.
Results. The median follow-up was 9.2 months. Imaging-based disease progression or death from any cause occurred in 95 (73.6%) participants in the cabazitaxel group, as compared to 101 (80.2%) who were assigned to receive an ASTI. The median imaging-based progression-free survival was 8.0 months in the cabazitaxel group and 3.7 months in the abiraterone/enzalutamide group. The median duration of treatment was longer in those receiving cabazitaxel (22 vs 12.5 weeks). The primary reason for treatment discontinuation was disease progression (in 43.7% of patients receiving cabazitaxel and 71% receiving an ASTI) or an adverse event (19.8% and 8.9%, respectively).
The trial’s secondary endpoints demonstrated improved outcomes in the cabazitaxel group compared to the abiraterone/enzalutamide group. There were 70 deaths (54.2%) in the cabazitaxel group and 83 (65.9%) in the ASTI group. Both the median overall survival (13.6 months in the cabazitaxel group and 11 months in the ASTI group) and the median progression-free survival (4.4 months and 2.7 months, respectively) were improved in those who received cabazitaxel. There was a 50% or greater reduction in the PSA level from baseline in 35.7% of the cabazitaxel group and 13.5% of the ASTI group.
Regarding the safety of the agents, the incidence of adverse events was similar in each group (38.9% in the cabazitaxel group and 38.7% in the ASTI group). Treatment discontinuation occurred more frequently in the cabazitaxel group (19.8%) compared to the ASTI group (8.9%). Adverse events of grade 3 or higher occurred more frequently with cabazitaxel; these were asthenia (4% vs 2.4%), diarrhea (3.2% vs 0), peripheral neuropathy (3.2% vs 0 patients), and febrile neutropenia (3.2% vs 0 patients).
Conclusion. Patients who had disease progression within 12 months on an ASTI and had previously been treated for metastatic castration-resistant prostate cancer with docetaxel had longer imaging-based progression-free survival and overall survival when treated with cabazitaxel compared to those treated with an alternative ASTI. Other clinical outcomes, including overall survival and progression-free survival, were also improved in the cabazitaxel group.
Commentary
Four ASTIs are approved for therapy in men with advanced prostate cancer. The next line of therapy following progression on an ASTI, whether to consider second-line androgen targeted inhibitors or proceed to taxane-based chemotherapy, has been unclear. The current CARD trial sought to answer this question and provides evidence that cabazitaxel is the next line of therapy for these patients. The trial’s primary endpoint, imaging-based disease progression, was reported in 73.6% of those who received cabazitaxel and in 80.2% of those who received abiraterone or enzalutamide. Patients treated with cabazitaxel had a longer imaging-based progression-free survival (8.0 months vs 3.7 months) and a longer duration of treatment (22 vs 12.5 weeks).
Because there is clinical evidence of cross-resistance between different ASTIs, the value of sequential therapy has been unclear. Emergence of androgen-receptor splice variant 7 (AR-V7) mutational status in circulating tumor cells is associated with poor outcomes with secondary androgen-signaling inhibitor therapy, and may be an indicator of resistance to subsequent androgen-signaling inhibitors.1,2 In the PROPHECY trial, the response rates to subsequent androgen targeted therapy in patients with AR-V7 mutations ranged from 30% to 40%.3 Understanding how AR-V7 mutational status may impact such outcomes will certainly help define whether a subgroup exists in whom use of second-line androgen signaling inhibitors may be considered.
The patients enrolled in the current study appear to represent a subgroup of patients with biologically aggressive disease or with inherent resistance to ASTIs. The patients included in this study progressed within 1 year of androgen targeted therapy, which is representative of a more aggressive population of patients who may be hormone insensitive and derive more benefit from chemotherapy. Initial androgen deprivation therapy was given for 13.7 and 12.6 months to the cabazitaxel and enzalutamide/abiraterone arms, respectively, prior to developing castrate-resistant prostate cancer. Patients enrolled in this study also previously received docetaxel, deselecting those who are taxane-resistant and therefore may be less likely to respond to additional taxane-based therapy. Detection of AR-V7 splice variant expression in circulating tumor cells, consideration of biomarker data, and sensitivity to taxanes may help guide decisions regarding the use of sequential androgen-targeted agents; however, there has been no clear data to guide such an approach. It is also important to consider that, because this is a European study, the approved dose given in this trial was 25 mg/m2. The PROSELICA trial previously demonstrated noninferiority of 20 mg/m2 compared with 25 mg/m2, with fewer adverse events, which is the dose now utilized in the United States.4
The adverse events of grade 3 or greater occurring in the cabazitaxel group should be discussed with patients, including fatigue, diarrhea, peripheral neuropathy, and febrile neutropenia.
The data from the CARD trial provide guidance regarding therapy sequencing in those with advanced prostate cancer after progression on first-line androgen targeted inhibitors and docetaxel; however, further work is needed to understand the universal application of this data in this cohort.
Applications in Clinical Practice
Patients with metastatic castration-resistant prostate cancer who have received docetaxel and progressed on an androgen-signaling inhibitor within 12 months should be considered for cabazitaxel over an alternative androgen-signaling inhibitor. This decision should be based on several factors, including AR-V7 mutational status, duration of androgen deprivation therapy, and hormone and taxane sensitivity in the past. Future studies are likely to incorporate genomic biomarkers rather than clinical criteria alone to make treatment decisions.
–Britni Souther, DO, and Daniel Isaac, DO, MS, Michigan State University, East Lansing, MI
Study Overview
Objective. To evaluate the efficacy of cabazitaxel compared to androgen-signaling–targeted inhibitors (ASTIs) in patients with metastatic castration-resistant prostate cancer who have received docetaxel and have progressed within 12 months of treatment with either abiraterone or enzalutamide.
Design. The CARD trial was an international, randomized, open-label phase 3 trial conducted across 13 European countries.
Setting and participants. Eligible patients were 18 years of age or older; had metastatic castration-resistant prostate cancer previously treated with docetaxel; and had disease progression during 12 months of treatment with abiraterone or enzalutamide. All patients had histologically proven prostate cancer, castrate levels of serum testosterone, and disease progression, defined by at least 2 new bone lesions or rising prostate-specific antigen (PSA) level. A total of 255 patients underwent randomization between November 2015 and November 2018, with 129 assigned to receive cabazitaxel and 126 patients assigned to receive an ASTI, 58 of whom received abiraterone and 66 of whom received enzalutamide. Patients who had received an ASTI in the setting of castrate-sensitive metastatic prostate cancer were included.
Intervention. Patients were randomized in a 1:1 fashion to receive either cabazitaxel or abiraterone or enzalutamide. Patients receiving cabazitaxel 25 mg/m2 intravenously every 3 weeks also received oral prednisone daily and primary prophylactic granulocyte-colony stimulating factor. Patients assigned to receive an ASTI received abiraterone 1000 mg orally daily with prednisone 5 mg twice daily or enzalutamide 160 mg daily. Patients in the ASTI group who had progressed on abiraterone were assigned to enzalutamide, and alternatively, those on enzalutamide were assigned to abiraterone. Patients were treated until 1 of the following occurred: imaging-based disease progression, unacceptable toxicity, or advancing to an alternative therapy.
Main outcome measures. The primary endpoint was imaging-based progression-free survival, which was defined as the time from randomization until objective tumor progression, progression of bone lesions, or death. The secondary endpoints were overall survival, progression-free survival, PSA response, tumor and pain responses, a new symptomatic skeletal event, and safety.
Results. The median follow-up was 9.2 months. Imaging-based disease progression or death from any cause occurred in 95 (73.6%) participants in the cabazitaxel group, as compared to 101 (80.2%) who were assigned to receive an ASTI. The median imaging-based progression-free survival was 8.0 months in the cabazitaxel group and 3.7 months in the abiraterone/enzalutamide group. The median duration of treatment was longer in those receiving cabazitaxel (22 vs 12.5 weeks). The primary reason for treatment discontinuation was disease progression (in 43.7% of patients receiving cabazitaxel and 71% receiving an ASTI) or an adverse event (19.8% and 8.9%, respectively).
The trial’s secondary endpoints demonstrated improved outcomes in the cabazitaxel group compared to the abiraterone/enzalutamide group. There were 70 deaths (54.2%) in the cabazitaxel group and 83 (65.9%) in the ASTI group. Both the median overall survival (13.6 months in the cabazitaxel group and 11 months in the ASTI group) and the median progression-free survival (4.4 months and 2.7 months, respectively) were improved in those who received cabazitaxel. There was a 50% or greater reduction in the PSA level from baseline in 35.7% of the cabazitaxel group and 13.5% of the ASTI group.
Regarding the safety of the agents, the incidence of adverse events was similar in each group (38.9% in the cabazitaxel group and 38.7% in the ASTI group). Treatment discontinuation occurred more frequently in the cabazitaxel group (19.8%) compared to the ASTI group (8.9%). Adverse events of grade 3 or higher occurred more frequently with cabazitaxel; these were asthenia (4% vs 2.4%), diarrhea (3.2% vs 0), peripheral neuropathy (3.2% vs 0 patients), and febrile neutropenia (3.2% vs 0 patients).
Conclusion. Patients who had disease progression within 12 months on an ASTI and had previously been treated for metastatic castration-resistant prostate cancer with docetaxel had longer imaging-based progression-free survival and overall survival when treated with cabazitaxel compared to those treated with an alternative ASTI. Other clinical outcomes, including overall survival and progression-free survival, were also improved in the cabazitaxel group.
Commentary
Four ASTIs are approved for therapy in men with advanced prostate cancer. The next line of therapy following progression on an ASTI, whether to consider second-line androgen targeted inhibitors or proceed to taxane-based chemotherapy, has been unclear. The current CARD trial sought to answer this question and provides evidence that cabazitaxel is the next line of therapy for these patients. The trial’s primary endpoint, imaging-based disease progression, was reported in 73.6% of those who received cabazitaxel and in 80.2% of those who received abiraterone or enzalutamide. Patients treated with cabazitaxel had a longer imaging-based progression-free survival (8.0 months vs 3.7 months) and a longer duration of treatment (22 vs 12.5 weeks).
Because there is clinical evidence of cross-resistance between different ASTIs, the value of sequential therapy has been unclear. Emergence of androgen-receptor splice variant 7 (AR-V7) mutational status in circulating tumor cells is associated with poor outcomes with secondary androgen-signaling inhibitor therapy, and may be an indicator of resistance to subsequent androgen-signaling inhibitors.1,2 In the PROPHECY trial, the response rates to subsequent androgen targeted therapy in patients with AR-V7 mutations ranged from 30% to 40%.3 Understanding how AR-V7 mutational status may impact such outcomes will certainly help define whether a subgroup exists in whom use of second-line androgen signaling inhibitors may be considered.
The patients enrolled in the current study appear to represent a subgroup of patients with biologically aggressive disease or with inherent resistance to ASTIs. The patients included in this study progressed within 1 year of androgen targeted therapy, which is representative of a more aggressive population of patients who may be hormone insensitive and derive more benefit from chemotherapy. Initial androgen deprivation therapy was given for 13.7 and 12.6 months to the cabazitaxel and enzalutamide/abiraterone arms, respectively, prior to developing castrate-resistant prostate cancer. Patients enrolled in this study also previously received docetaxel, deselecting those who are taxane-resistant and therefore may be less likely to respond to additional taxane-based therapy. Detection of AR-V7 splice variant expression in circulating tumor cells, consideration of biomarker data, and sensitivity to taxanes may help guide decisions regarding the use of sequential androgen-targeted agents; however, there has been no clear data to guide such an approach. It is also important to consider that, because this is a European study, the approved dose given in this trial was 25 mg/m2. The PROSELICA trial previously demonstrated noninferiority of 20 mg/m2 compared with 25 mg/m2, with fewer adverse events, which is the dose now utilized in the United States.4
The adverse events of grade 3 or greater occurring in the cabazitaxel group should be discussed with patients, including fatigue, diarrhea, peripheral neuropathy, and febrile neutropenia.
The data from the CARD trial provide guidance regarding therapy sequencing in those with advanced prostate cancer after progression on first-line androgen targeted inhibitors and docetaxel; however, further work is needed to understand the universal application of this data in this cohort.
Applications in Clinical Practice
Patients with metastatic castration-resistant prostate cancer who have received docetaxel and progressed on an androgen-signaling inhibitor within 12 months should be considered for cabazitaxel over an alternative androgen-signaling inhibitor. This decision should be based on several factors, including AR-V7 mutational status, duration of androgen deprivation therapy, and hormone and taxane sensitivity in the past. Future studies are likely to incorporate genomic biomarkers rather than clinical criteria alone to make treatment decisions.
–Britni Souther, DO, and Daniel Isaac, DO, MS, Michigan State University, East Lansing, MI
1. Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028-1038.
2. Zhang T, Karsh LI, Nissenblatt MJ, et al. Androgen receptor splice variant, AR-V7, as a biomarker of resistance to androgen axis-targeted therapies in advanced prostate cancer. Clin Genitourin Cancer. 2019;18:1-10.
3. Armstrong AJ, Halabi S, Luo J, et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J Clin Oncol. 2019;37:1120-1129.
4. Eisenberger M, Hardy-Bessard AC, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA. J Clin Oncol. 2017;35:3198-3206.
1. Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028-1038.
2. Zhang T, Karsh LI, Nissenblatt MJ, et al. Androgen receptor splice variant, AR-V7, as a biomarker of resistance to androgen axis-targeted therapies in advanced prostate cancer. Clin Genitourin Cancer. 2019;18:1-10.
3. Armstrong AJ, Halabi S, Luo J, et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J Clin Oncol. 2019;37:1120-1129.
4. Eisenberger M, Hardy-Bessard AC, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA. J Clin Oncol. 2017;35:3198-3206.
New topicals coming for pediatric atopic dermatitis
LAHAINA, HAWAII – Novel topical medications are in the works that will address the longstanding unmet need for a Food and Drug Administration–approved noncorticosteroid topical for use in pediatric atopic dermatitis, Lawrence F. Eichenfield, MD, reported at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
These new agents will be embraced by clinicians for use in delicate skin areas, as well as in the common clinical scenario involving steroid-averse parents, predicted Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital.
First up is crisaborole (Eucrisa), which is approved for atopic dermatitis (AD) in children aged two years and older and has been under review at the Food and Drug Administration for use in infantile AD. (On March 24, several weeks after the meeting, the FDA approved crisaborole down to aged three months for treatment of mild to moderate AD). Agents earlier in the developmental pipeline include two topical Janus kinase (JAK) inhibitors, ruxolitinib and delgocitinib, as well as tapinarof.
Crisaborole: This phosphodiesterase 4 inhibitor is FDA approved down to 2 years of age. In the phase 4, open-label CrisADe CARE 1 study, crisaborole was studied in 137 children ages 3 months to under 24 months. CrisADe CARE 1, presented at the 2019 annual conference of the Pediatric Dermatology Research Alliance (PeDRA), showed close to a 60% reduction from baseline in Eczema Area and Severity Index (EASI) scores after 28 days of twice-daily therapy in the youngsters, 61% of who had moderate AD, the rest mild disease.
Tolerability and safety were reassuring in the phase 4 study. Although about 3% of subjects each experienced application site pain, discomfort, or erythema, the rate of study discontinuation was impressively low at 2.9%, Dr. Eichenfield observed.
Delgocitinib: Japanese investigators have reported positive results in a phase 2 study of delgocitinib ointment in 98 children and adolescents aged 2-15 years, with AD. After 4 weeks of twice-daily treatment, modified EASI scores improved by a mean of 54% with delgocitinib 0.25% and by 62% with 0.5%, compared with less than a 5% improvement with the vehicle control (J Allergy Clin Immunol. 2019 Dec;144[6]:1575-83). The ointment formulation is being developed specifically for the Japanese market.
Studies of an alternative formulation of the JAK inhibitor as a cream rather than ointment, intended for the U.S. and European markets, are in the early stages, conducted by Leo Pharma. Delgocitinib cream, under study in adults and children down to age 2 years with AD, is also under study for chronic hand dermatitis, a program Dr. Eichenfield is enthusiastic about.
“Hand eczema is something you’re going to hear a lot about in the next 2 years. In the U.S., we have no drug approved specifically for hand eczema. And we actually see a lot of hand eczema in pediatric and adolescent patients. I’d say 75%-80% of the ones I see also have atopic dermatitis,” he said.
Ruxolitinib: Incyte, which is developing the topical JAK inhibitor, recently announced positive results in the first of four phase 3 randomized trials, this one conducted in AD patients aged 12 years and older. The efficacy appears to be comparable to that of topical steroids. Studies in younger children are also planned. Ruxolitinib cream is in advanced clinical trials for treatment of vitiligo.
Tapinarof: This topical aryl hydrocarbon receptor agonist downregulates Th17 cytokines, an attribute desirable for treatment of psoriasis. But it also downregulates Th2 cytokines and improves the damaged skin barrier characteristic of AD via upregulation of the filaggrin and involucrin genes in keratinocytes. In a phase 2b, double-blind clinical trial conducted in 247 adults and adolescents with moderate to severe AD, 12 weeks of once-daily tapinarof 1% enabled 51% of patients to achieve a 75% or greater improvement in EASI scores, compared with 18% in controls on vehicle (J Am Acad Dermatol. 2019 Jan;80[1]:89-98.e3).
Dermavant, which is developing the drug, plans to seek an initial indication for treatment of psoriasis, where a phase 3 study is underway, before pursuing regulatory approval in AD.
Dr. Eichenfield disclosed serving as a consultant or investigator for various pharmaceutical companies, including Pfizer, and Dermavant.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
This article was updated 3/27/20.
LAHAINA, HAWAII – Novel topical medications are in the works that will address the longstanding unmet need for a Food and Drug Administration–approved noncorticosteroid topical for use in pediatric atopic dermatitis, Lawrence F. Eichenfield, MD, reported at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
These new agents will be embraced by clinicians for use in delicate skin areas, as well as in the common clinical scenario involving steroid-averse parents, predicted Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital.
First up is crisaborole (Eucrisa), which is approved for atopic dermatitis (AD) in children aged two years and older and has been under review at the Food and Drug Administration for use in infantile AD. (On March 24, several weeks after the meeting, the FDA approved crisaborole down to aged three months for treatment of mild to moderate AD). Agents earlier in the developmental pipeline include two topical Janus kinase (JAK) inhibitors, ruxolitinib and delgocitinib, as well as tapinarof.
Crisaborole: This phosphodiesterase 4 inhibitor is FDA approved down to 2 years of age. In the phase 4, open-label CrisADe CARE 1 study, crisaborole was studied in 137 children ages 3 months to under 24 months. CrisADe CARE 1, presented at the 2019 annual conference of the Pediatric Dermatology Research Alliance (PeDRA), showed close to a 60% reduction from baseline in Eczema Area and Severity Index (EASI) scores after 28 days of twice-daily therapy in the youngsters, 61% of who had moderate AD, the rest mild disease.
Tolerability and safety were reassuring in the phase 4 study. Although about 3% of subjects each experienced application site pain, discomfort, or erythema, the rate of study discontinuation was impressively low at 2.9%, Dr. Eichenfield observed.
Delgocitinib: Japanese investigators have reported positive results in a phase 2 study of delgocitinib ointment in 98 children and adolescents aged 2-15 years, with AD. After 4 weeks of twice-daily treatment, modified EASI scores improved by a mean of 54% with delgocitinib 0.25% and by 62% with 0.5%, compared with less than a 5% improvement with the vehicle control (J Allergy Clin Immunol. 2019 Dec;144[6]:1575-83). The ointment formulation is being developed specifically for the Japanese market.
Studies of an alternative formulation of the JAK inhibitor as a cream rather than ointment, intended for the U.S. and European markets, are in the early stages, conducted by Leo Pharma. Delgocitinib cream, under study in adults and children down to age 2 years with AD, is also under study for chronic hand dermatitis, a program Dr. Eichenfield is enthusiastic about.
“Hand eczema is something you’re going to hear a lot about in the next 2 years. In the U.S., we have no drug approved specifically for hand eczema. And we actually see a lot of hand eczema in pediatric and adolescent patients. I’d say 75%-80% of the ones I see also have atopic dermatitis,” he said.
Ruxolitinib: Incyte, which is developing the topical JAK inhibitor, recently announced positive results in the first of four phase 3 randomized trials, this one conducted in AD patients aged 12 years and older. The efficacy appears to be comparable to that of topical steroids. Studies in younger children are also planned. Ruxolitinib cream is in advanced clinical trials for treatment of vitiligo.
Tapinarof: This topical aryl hydrocarbon receptor agonist downregulates Th17 cytokines, an attribute desirable for treatment of psoriasis. But it also downregulates Th2 cytokines and improves the damaged skin barrier characteristic of AD via upregulation of the filaggrin and involucrin genes in keratinocytes. In a phase 2b, double-blind clinical trial conducted in 247 adults and adolescents with moderate to severe AD, 12 weeks of once-daily tapinarof 1% enabled 51% of patients to achieve a 75% or greater improvement in EASI scores, compared with 18% in controls on vehicle (J Am Acad Dermatol. 2019 Jan;80[1]:89-98.e3).
Dermavant, which is developing the drug, plans to seek an initial indication for treatment of psoriasis, where a phase 3 study is underway, before pursuing regulatory approval in AD.
Dr. Eichenfield disclosed serving as a consultant or investigator for various pharmaceutical companies, including Pfizer, and Dermavant.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
This article was updated 3/27/20.
LAHAINA, HAWAII – Novel topical medications are in the works that will address the longstanding unmet need for a Food and Drug Administration–approved noncorticosteroid topical for use in pediatric atopic dermatitis, Lawrence F. Eichenfield, MD, reported at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
These new agents will be embraced by clinicians for use in delicate skin areas, as well as in the common clinical scenario involving steroid-averse parents, predicted Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital.
First up is crisaborole (Eucrisa), which is approved for atopic dermatitis (AD) in children aged two years and older and has been under review at the Food and Drug Administration for use in infantile AD. (On March 24, several weeks after the meeting, the FDA approved crisaborole down to aged three months for treatment of mild to moderate AD). Agents earlier in the developmental pipeline include two topical Janus kinase (JAK) inhibitors, ruxolitinib and delgocitinib, as well as tapinarof.
Crisaborole: This phosphodiesterase 4 inhibitor is FDA approved down to 2 years of age. In the phase 4, open-label CrisADe CARE 1 study, crisaborole was studied in 137 children ages 3 months to under 24 months. CrisADe CARE 1, presented at the 2019 annual conference of the Pediatric Dermatology Research Alliance (PeDRA), showed close to a 60% reduction from baseline in Eczema Area and Severity Index (EASI) scores after 28 days of twice-daily therapy in the youngsters, 61% of who had moderate AD, the rest mild disease.
Tolerability and safety were reassuring in the phase 4 study. Although about 3% of subjects each experienced application site pain, discomfort, or erythema, the rate of study discontinuation was impressively low at 2.9%, Dr. Eichenfield observed.
Delgocitinib: Japanese investigators have reported positive results in a phase 2 study of delgocitinib ointment in 98 children and adolescents aged 2-15 years, with AD. After 4 weeks of twice-daily treatment, modified EASI scores improved by a mean of 54% with delgocitinib 0.25% and by 62% with 0.5%, compared with less than a 5% improvement with the vehicle control (J Allergy Clin Immunol. 2019 Dec;144[6]:1575-83). The ointment formulation is being developed specifically for the Japanese market.
Studies of an alternative formulation of the JAK inhibitor as a cream rather than ointment, intended for the U.S. and European markets, are in the early stages, conducted by Leo Pharma. Delgocitinib cream, under study in adults and children down to age 2 years with AD, is also under study for chronic hand dermatitis, a program Dr. Eichenfield is enthusiastic about.
“Hand eczema is something you’re going to hear a lot about in the next 2 years. In the U.S., we have no drug approved specifically for hand eczema. And we actually see a lot of hand eczema in pediatric and adolescent patients. I’d say 75%-80% of the ones I see also have atopic dermatitis,” he said.
Ruxolitinib: Incyte, which is developing the topical JAK inhibitor, recently announced positive results in the first of four phase 3 randomized trials, this one conducted in AD patients aged 12 years and older. The efficacy appears to be comparable to that of topical steroids. Studies in younger children are also planned. Ruxolitinib cream is in advanced clinical trials for treatment of vitiligo.
Tapinarof: This topical aryl hydrocarbon receptor agonist downregulates Th17 cytokines, an attribute desirable for treatment of psoriasis. But it also downregulates Th2 cytokines and improves the damaged skin barrier characteristic of AD via upregulation of the filaggrin and involucrin genes in keratinocytes. In a phase 2b, double-blind clinical trial conducted in 247 adults and adolescents with moderate to severe AD, 12 weeks of once-daily tapinarof 1% enabled 51% of patients to achieve a 75% or greater improvement in EASI scores, compared with 18% in controls on vehicle (J Am Acad Dermatol. 2019 Jan;80[1]:89-98.e3).
Dermavant, which is developing the drug, plans to seek an initial indication for treatment of psoriasis, where a phase 3 study is underway, before pursuing regulatory approval in AD.
Dr. Eichenfield disclosed serving as a consultant or investigator for various pharmaceutical companies, including Pfizer, and Dermavant.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
This article was updated 3/27/20.
REPORTING FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
New ASAM guideline released amid COVID-19 concerns
Home-based buprenorphine induction deemed safe for OUD
The American Society of Addiction Medicine has released an updated practice guideline for patients with opioid use disorder.
The guideline, called a focused update, advances ASAM’s 2015 National Practice Guidelines for the Treament of Opioid Use Disorder. “During the ongoing COVID-19 pandemic and the associated need for social distancing, it is especially important that clinicians and health care providers across the country take steps to ensure that individuals with OUD can continue to receive evidence-based care,” said Paul H. Earley, MD, president of ASAM, in a press release announcing the new guideline.
The guideline specifies that home-based buprenorphine induction is safe and effective for treatment of opioid use disorder and that no individual entering the criminal justice system should be subjected to opioid withdrawal.
“The research is clear, providing methadone or buprenorphine, even without psychosocial treatment, reduces the patient’s risk of death,” said Kyle Kampman, MD, chair of the group’s Guideline Writing Committee, in the release. “Ultimately, keeping patients with the disease of addiction alive and engaged to become ready for recovery is absolutely critical in the context of the deadly overdose epidemic that has struck communities across our country.”
The society released this focused update to reflect new medications and formulations, published evidence, and clinical guidance related to treatment of OUD. This update includes the addition of 13 new recommendations and major revisions to 35 existing recommendations. One concern the society has is how to help patients being treated for OUD who are limited in their ability to leave their homes. Because of these same concerns, the Substance Abuse and Mental Health Services Administration relaxed regulations on March 16 regarding patient eligibility for take-home medications, such as buprenorphine and methadone, which dovetails with the society’s guidance regarding home-based induction.
, continuing on to pharmacologic treatment even if the patient declines recommended psychosocial treatment, keeping naloxone kits available in correctional facilities, and more. Additional information about this update can be found on ASAM’s website.
Home-based buprenorphine induction deemed safe for OUD
Home-based buprenorphine induction deemed safe for OUD
The American Society of Addiction Medicine has released an updated practice guideline for patients with opioid use disorder.
The guideline, called a focused update, advances ASAM’s 2015 National Practice Guidelines for the Treament of Opioid Use Disorder. “During the ongoing COVID-19 pandemic and the associated need for social distancing, it is especially important that clinicians and health care providers across the country take steps to ensure that individuals with OUD can continue to receive evidence-based care,” said Paul H. Earley, MD, president of ASAM, in a press release announcing the new guideline.
The guideline specifies that home-based buprenorphine induction is safe and effective for treatment of opioid use disorder and that no individual entering the criminal justice system should be subjected to opioid withdrawal.
“The research is clear, providing methadone or buprenorphine, even without psychosocial treatment, reduces the patient’s risk of death,” said Kyle Kampman, MD, chair of the group’s Guideline Writing Committee, in the release. “Ultimately, keeping patients with the disease of addiction alive and engaged to become ready for recovery is absolutely critical in the context of the deadly overdose epidemic that has struck communities across our country.”
The society released this focused update to reflect new medications and formulations, published evidence, and clinical guidance related to treatment of OUD. This update includes the addition of 13 new recommendations and major revisions to 35 existing recommendations. One concern the society has is how to help patients being treated for OUD who are limited in their ability to leave their homes. Because of these same concerns, the Substance Abuse and Mental Health Services Administration relaxed regulations on March 16 regarding patient eligibility for take-home medications, such as buprenorphine and methadone, which dovetails with the society’s guidance regarding home-based induction.
, continuing on to pharmacologic treatment even if the patient declines recommended psychosocial treatment, keeping naloxone kits available in correctional facilities, and more. Additional information about this update can be found on ASAM’s website.
The American Society of Addiction Medicine has released an updated practice guideline for patients with opioid use disorder.
The guideline, called a focused update, advances ASAM’s 2015 National Practice Guidelines for the Treament of Opioid Use Disorder. “During the ongoing COVID-19 pandemic and the associated need for social distancing, it is especially important that clinicians and health care providers across the country take steps to ensure that individuals with OUD can continue to receive evidence-based care,” said Paul H. Earley, MD, president of ASAM, in a press release announcing the new guideline.
The guideline specifies that home-based buprenorphine induction is safe and effective for treatment of opioid use disorder and that no individual entering the criminal justice system should be subjected to opioid withdrawal.
“The research is clear, providing methadone or buprenorphine, even without psychosocial treatment, reduces the patient’s risk of death,” said Kyle Kampman, MD, chair of the group’s Guideline Writing Committee, in the release. “Ultimately, keeping patients with the disease of addiction alive and engaged to become ready for recovery is absolutely critical in the context of the deadly overdose epidemic that has struck communities across our country.”
The society released this focused update to reflect new medications and formulations, published evidence, and clinical guidance related to treatment of OUD. This update includes the addition of 13 new recommendations and major revisions to 35 existing recommendations. One concern the society has is how to help patients being treated for OUD who are limited in their ability to leave their homes. Because of these same concerns, the Substance Abuse and Mental Health Services Administration relaxed regulations on March 16 regarding patient eligibility for take-home medications, such as buprenorphine and methadone, which dovetails with the society’s guidance regarding home-based induction.
, continuing on to pharmacologic treatment even if the patient declines recommended psychosocial treatment, keeping naloxone kits available in correctional facilities, and more. Additional information about this update can be found on ASAM’s website.
Advances in ankylosing spondylitis hailed as rheumatology’s story of the year
MAUI, HAWAII – Arguably the most important development in the field of rheumatology during the past year was the emergence of persuasive clinical trials data predictive of a bright future for the oral Janus kinase inhibitors as major new drugs for treating ankylosing spondylitis, two experts agreed at the 2020 Rheumatology Winter Clinical Symposium.
“These drugs are the first oral DMARDs [disease-modifying antirheumatic drugs], if you will, in AS [ankylosing spondylitis] ... I think obviously they are going to move forward to regulatory approval,” commented Arthur F. Kavanaugh, MD, professor of medicine at the University of California, San Diego, and the RWCS program director.
“I think they’re going to hit the ground running,” predicted Eric M. Ruderman, MD, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University, Chicago.
Upon approval, the Janus kinase (JAK) inhibitors will quickly change the treatment paradigm in AS, the rheumatologists forecast.
“I think this is a way forward to go with strictly oral therapy without necessarily going first with parenteral therapy in these patients. It gives you an alternative, and my guess is, by the time these drugs are approved in this space, there’ll be more and more of their preferential use over biologics in rheumatoid arthritis and potentially in psoriatic arthritis, so people will be more familiar with them. A lot of patients are much happier taking a pill once a day with none of the tolerability issues that we have with shots,” Dr. Ruderman said.
“And they act fast,” noted Dr. Kavanaugh. “You can make a case for trying a JAK inhibitor for a month or 2, and then if you’re not better, then we go to the other option.”
Dr. Ruderman cautioned that the increased need for laboratory monitoring with the oral JAK inhibitors as compared with the annual monitoring with tumor necrosis factor inhibitors could be an issue, especially since AS patients tend to be on the younger side and often dislike coming in regularly for office visits and laboratory tests.
“There may be more of a headache in having to tell patients, ‘You’re not getting your JAK inhibitor refilled until you get your labs done,’ ” he said.
In December 2019, rheumatologists received a holiday present in the form of publication of the results of SELECT-AXIS 1, a multicenter, double-blind, randomized, placebo-controlled phase 2/3 trial in which 187 patients with active AS were randomized to placebo or to the JAK inhibitor upadacitinib (Rinvoq) at the same 15-mg dose approved by the FDA earlier in the year for rheumatoid arthritis. In SELECT-AXIS 1, upadacitinib not only demonstrated clinical efficacy, with a week-14 Assessment of SpondyloArthritis International Society-40 (ASAS 40) response rate of 52% – twice that for placebo – but the active treatment arm also experienced significantly reduced inflammatory disease activity as measured by Spondyloarthritis Research Consortium of Canada (SPARCC) MRI scores of the spine and sacroiliac joint. In contrast, MRI scores remained unchanged from baseline in the placebo arm.
“This suggests a biologic effect, a hook beyond clinical disease. And the time of onset was pretty impressive, much like with our experience with JAK inhibitors in RA. Within 2 weeks, there was significant separation from placebo on the ASDAS [Ankylosing Spondylitis Disease Activity Score],” Dr. Ruderman noted.
Positive clinical trials in AS have also been reported for the oral JAK inhibitors filgotinib and tofacitinib (Xeljanz).
Dr. Ruderman and Dr. Kavanaugh also touched on other major developments in AS within the past year, including the landmark FDA approval of certolizumab pegol (Cimzia) as the first-ever drug for treatment of nonradiographic axial spondyloarthritis (nr-axSpA). Also, Dr. Kavanaugh and Dr. Ruderman gave two thumbs down to the 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis.
More biologics coming for nr-AxSpA
In order to gain an indication for treatment of nr-axSpA, the FDA requires completion of a 52-week, randomized, placebo-controlled clinical trial. That’s what UCB did with the company’s TNF inhibitor, certolizumab. Similarly, Eli Lilly sprang for the mandated 52-week trial in 303 nr-axSpA patients for its interleukin-17 inhibitor ixekizumab (Taltz), with positive findings (Lancet. 2020 Jan 4;395[10217]:53-64). And Novartis has done so with its IL-17 inhibitor secukinumab (Cosentyx) in the 555-patient PREVENT trial, again with positive outcomes. So the two IL-17 inhibitors, which are already approved for AS, are seemingly a lock for approval in nr-axSpA as well.
Will the makers of other TNF inhibitors already approved for AS fork over the considerable money entailed in a 52-week randomized trial, or will they ride on certolizumab’s coattails? Dr. Ruderman said he doesn’t know the answer, but it’s certain that, if they don’t complete the trial, they can’t market their biologic for nr-axSpA in the United States, even though there might well be a drug class effect at work.
ACR/SAA/SPARTAN guidelines critiqued
The two panelists had plenty to say about the 2019 update of the guidelines, none of it favorable. Among their criticisms: The guidelines are already out of date several months after their release, they are based heavily on opinion rather than on evidence, they appear to take medication cost into consideration when they’re not supposed to, they are complicated, and they are just not practical or useful.
“I don’t know when these guidelines would potentially be used,” Dr. Kavanaugh commented in response to an audience question.
Specifically, the guidelines strongly recommend a TNF inhibitor over the IL-17 inhibitors secukinumab or ixekizumab as the first-line biologic in AS patients with active disease while on NSAIDs, guidance that is already out of date.
“You’d almost think it would have been better just to wait a few months to see the published literature, which I think is going to have a tremendous impact on practice,” Dr. Kavanaugh said.
He also said he was troubled by the strong recommendation against switching to a TNF inhibitor–biosimilar after receiving treatment with an originator TNF inhibitor. That’s something rheumatologists all across Europe are routinely doing now for economic reasons without known harm, with the caveat that patients must be switched to a biosimilar of a different TNF inhibitor than the originator.
“There’s absolutely no data to support a recommendation against switching. I think they’re going out on a limb a little bit,” he added.
Dr. Ruderman said he struggles with the guideline development methodology, which involves posing a series of key questions at the outset, with a strict charge to provide answers.
“They always have to have an answer to the question. And in the event that there’s no data to support an answer, then it becomes a matter of the expert opinion of the people on the committee. I think many rheumatologists would say, ‘Why are they any more expert than a clinical rheumatologist who’s been seeing AS patients for 20 years?’ And the answer is they’re probably not. The fact that most of these are conditional recommendations, meaning they’re not supported by strong evidence, makes them less useful,” according to Dr. Ruderman.
Both he and Dr. Kavanaugh reported receiving research funding from and/or serving as a consultant to numerous pharmaceutical companies.
MAUI, HAWAII – Arguably the most important development in the field of rheumatology during the past year was the emergence of persuasive clinical trials data predictive of a bright future for the oral Janus kinase inhibitors as major new drugs for treating ankylosing spondylitis, two experts agreed at the 2020 Rheumatology Winter Clinical Symposium.
“These drugs are the first oral DMARDs [disease-modifying antirheumatic drugs], if you will, in AS [ankylosing spondylitis] ... I think obviously they are going to move forward to regulatory approval,” commented Arthur F. Kavanaugh, MD, professor of medicine at the University of California, San Diego, and the RWCS program director.
“I think they’re going to hit the ground running,” predicted Eric M. Ruderman, MD, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University, Chicago.
Upon approval, the Janus kinase (JAK) inhibitors will quickly change the treatment paradigm in AS, the rheumatologists forecast.
“I think this is a way forward to go with strictly oral therapy without necessarily going first with parenteral therapy in these patients. It gives you an alternative, and my guess is, by the time these drugs are approved in this space, there’ll be more and more of their preferential use over biologics in rheumatoid arthritis and potentially in psoriatic arthritis, so people will be more familiar with them. A lot of patients are much happier taking a pill once a day with none of the tolerability issues that we have with shots,” Dr. Ruderman said.
“And they act fast,” noted Dr. Kavanaugh. “You can make a case for trying a JAK inhibitor for a month or 2, and then if you’re not better, then we go to the other option.”
Dr. Ruderman cautioned that the increased need for laboratory monitoring with the oral JAK inhibitors as compared with the annual monitoring with tumor necrosis factor inhibitors could be an issue, especially since AS patients tend to be on the younger side and often dislike coming in regularly for office visits and laboratory tests.
“There may be more of a headache in having to tell patients, ‘You’re not getting your JAK inhibitor refilled until you get your labs done,’ ” he said.
In December 2019, rheumatologists received a holiday present in the form of publication of the results of SELECT-AXIS 1, a multicenter, double-blind, randomized, placebo-controlled phase 2/3 trial in which 187 patients with active AS were randomized to placebo or to the JAK inhibitor upadacitinib (Rinvoq) at the same 15-mg dose approved by the FDA earlier in the year for rheumatoid arthritis. In SELECT-AXIS 1, upadacitinib not only demonstrated clinical efficacy, with a week-14 Assessment of SpondyloArthritis International Society-40 (ASAS 40) response rate of 52% – twice that for placebo – but the active treatment arm also experienced significantly reduced inflammatory disease activity as measured by Spondyloarthritis Research Consortium of Canada (SPARCC) MRI scores of the spine and sacroiliac joint. In contrast, MRI scores remained unchanged from baseline in the placebo arm.
“This suggests a biologic effect, a hook beyond clinical disease. And the time of onset was pretty impressive, much like with our experience with JAK inhibitors in RA. Within 2 weeks, there was significant separation from placebo on the ASDAS [Ankylosing Spondylitis Disease Activity Score],” Dr. Ruderman noted.
Positive clinical trials in AS have also been reported for the oral JAK inhibitors filgotinib and tofacitinib (Xeljanz).
Dr. Ruderman and Dr. Kavanaugh also touched on other major developments in AS within the past year, including the landmark FDA approval of certolizumab pegol (Cimzia) as the first-ever drug for treatment of nonradiographic axial spondyloarthritis (nr-axSpA). Also, Dr. Kavanaugh and Dr. Ruderman gave two thumbs down to the 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis.
More biologics coming for nr-AxSpA
In order to gain an indication for treatment of nr-axSpA, the FDA requires completion of a 52-week, randomized, placebo-controlled clinical trial. That’s what UCB did with the company’s TNF inhibitor, certolizumab. Similarly, Eli Lilly sprang for the mandated 52-week trial in 303 nr-axSpA patients for its interleukin-17 inhibitor ixekizumab (Taltz), with positive findings (Lancet. 2020 Jan 4;395[10217]:53-64). And Novartis has done so with its IL-17 inhibitor secukinumab (Cosentyx) in the 555-patient PREVENT trial, again with positive outcomes. So the two IL-17 inhibitors, which are already approved for AS, are seemingly a lock for approval in nr-axSpA as well.
Will the makers of other TNF inhibitors already approved for AS fork over the considerable money entailed in a 52-week randomized trial, or will they ride on certolizumab’s coattails? Dr. Ruderman said he doesn’t know the answer, but it’s certain that, if they don’t complete the trial, they can’t market their biologic for nr-axSpA in the United States, even though there might well be a drug class effect at work.
ACR/SAA/SPARTAN guidelines critiqued
The two panelists had plenty to say about the 2019 update of the guidelines, none of it favorable. Among their criticisms: The guidelines are already out of date several months after their release, they are based heavily on opinion rather than on evidence, they appear to take medication cost into consideration when they’re not supposed to, they are complicated, and they are just not practical or useful.
“I don’t know when these guidelines would potentially be used,” Dr. Kavanaugh commented in response to an audience question.
Specifically, the guidelines strongly recommend a TNF inhibitor over the IL-17 inhibitors secukinumab or ixekizumab as the first-line biologic in AS patients with active disease while on NSAIDs, guidance that is already out of date.
“You’d almost think it would have been better just to wait a few months to see the published literature, which I think is going to have a tremendous impact on practice,” Dr. Kavanaugh said.
He also said he was troubled by the strong recommendation against switching to a TNF inhibitor–biosimilar after receiving treatment with an originator TNF inhibitor. That’s something rheumatologists all across Europe are routinely doing now for economic reasons without known harm, with the caveat that patients must be switched to a biosimilar of a different TNF inhibitor than the originator.
“There’s absolutely no data to support a recommendation against switching. I think they’re going out on a limb a little bit,” he added.
Dr. Ruderman said he struggles with the guideline development methodology, which involves posing a series of key questions at the outset, with a strict charge to provide answers.
“They always have to have an answer to the question. And in the event that there’s no data to support an answer, then it becomes a matter of the expert opinion of the people on the committee. I think many rheumatologists would say, ‘Why are they any more expert than a clinical rheumatologist who’s been seeing AS patients for 20 years?’ And the answer is they’re probably not. The fact that most of these are conditional recommendations, meaning they’re not supported by strong evidence, makes them less useful,” according to Dr. Ruderman.
Both he and Dr. Kavanaugh reported receiving research funding from and/or serving as a consultant to numerous pharmaceutical companies.
MAUI, HAWAII – Arguably the most important development in the field of rheumatology during the past year was the emergence of persuasive clinical trials data predictive of a bright future for the oral Janus kinase inhibitors as major new drugs for treating ankylosing spondylitis, two experts agreed at the 2020 Rheumatology Winter Clinical Symposium.
“These drugs are the first oral DMARDs [disease-modifying antirheumatic drugs], if you will, in AS [ankylosing spondylitis] ... I think obviously they are going to move forward to regulatory approval,” commented Arthur F. Kavanaugh, MD, professor of medicine at the University of California, San Diego, and the RWCS program director.
“I think they’re going to hit the ground running,” predicted Eric M. Ruderman, MD, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University, Chicago.
Upon approval, the Janus kinase (JAK) inhibitors will quickly change the treatment paradigm in AS, the rheumatologists forecast.
“I think this is a way forward to go with strictly oral therapy without necessarily going first with parenteral therapy in these patients. It gives you an alternative, and my guess is, by the time these drugs are approved in this space, there’ll be more and more of their preferential use over biologics in rheumatoid arthritis and potentially in psoriatic arthritis, so people will be more familiar with them. A lot of patients are much happier taking a pill once a day with none of the tolerability issues that we have with shots,” Dr. Ruderman said.
“And they act fast,” noted Dr. Kavanaugh. “You can make a case for trying a JAK inhibitor for a month or 2, and then if you’re not better, then we go to the other option.”
Dr. Ruderman cautioned that the increased need for laboratory monitoring with the oral JAK inhibitors as compared with the annual monitoring with tumor necrosis factor inhibitors could be an issue, especially since AS patients tend to be on the younger side and often dislike coming in regularly for office visits and laboratory tests.
“There may be more of a headache in having to tell patients, ‘You’re not getting your JAK inhibitor refilled until you get your labs done,’ ” he said.
In December 2019, rheumatologists received a holiday present in the form of publication of the results of SELECT-AXIS 1, a multicenter, double-blind, randomized, placebo-controlled phase 2/3 trial in which 187 patients with active AS were randomized to placebo or to the JAK inhibitor upadacitinib (Rinvoq) at the same 15-mg dose approved by the FDA earlier in the year for rheumatoid arthritis. In SELECT-AXIS 1, upadacitinib not only demonstrated clinical efficacy, with a week-14 Assessment of SpondyloArthritis International Society-40 (ASAS 40) response rate of 52% – twice that for placebo – but the active treatment arm also experienced significantly reduced inflammatory disease activity as measured by Spondyloarthritis Research Consortium of Canada (SPARCC) MRI scores of the spine and sacroiliac joint. In contrast, MRI scores remained unchanged from baseline in the placebo arm.
“This suggests a biologic effect, a hook beyond clinical disease. And the time of onset was pretty impressive, much like with our experience with JAK inhibitors in RA. Within 2 weeks, there was significant separation from placebo on the ASDAS [Ankylosing Spondylitis Disease Activity Score],” Dr. Ruderman noted.
Positive clinical trials in AS have also been reported for the oral JAK inhibitors filgotinib and tofacitinib (Xeljanz).
Dr. Ruderman and Dr. Kavanaugh also touched on other major developments in AS within the past year, including the landmark FDA approval of certolizumab pegol (Cimzia) as the first-ever drug for treatment of nonradiographic axial spondyloarthritis (nr-axSpA). Also, Dr. Kavanaugh and Dr. Ruderman gave two thumbs down to the 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis.
More biologics coming for nr-AxSpA
In order to gain an indication for treatment of nr-axSpA, the FDA requires completion of a 52-week, randomized, placebo-controlled clinical trial. That’s what UCB did with the company’s TNF inhibitor, certolizumab. Similarly, Eli Lilly sprang for the mandated 52-week trial in 303 nr-axSpA patients for its interleukin-17 inhibitor ixekizumab (Taltz), with positive findings (Lancet. 2020 Jan 4;395[10217]:53-64). And Novartis has done so with its IL-17 inhibitor secukinumab (Cosentyx) in the 555-patient PREVENT trial, again with positive outcomes. So the two IL-17 inhibitors, which are already approved for AS, are seemingly a lock for approval in nr-axSpA as well.
Will the makers of other TNF inhibitors already approved for AS fork over the considerable money entailed in a 52-week randomized trial, or will they ride on certolizumab’s coattails? Dr. Ruderman said he doesn’t know the answer, but it’s certain that, if they don’t complete the trial, they can’t market their biologic for nr-axSpA in the United States, even though there might well be a drug class effect at work.
ACR/SAA/SPARTAN guidelines critiqued
The two panelists had plenty to say about the 2019 update of the guidelines, none of it favorable. Among their criticisms: The guidelines are already out of date several months after their release, they are based heavily on opinion rather than on evidence, they appear to take medication cost into consideration when they’re not supposed to, they are complicated, and they are just not practical or useful.
“I don’t know when these guidelines would potentially be used,” Dr. Kavanaugh commented in response to an audience question.
Specifically, the guidelines strongly recommend a TNF inhibitor over the IL-17 inhibitors secukinumab or ixekizumab as the first-line biologic in AS patients with active disease while on NSAIDs, guidance that is already out of date.
“You’d almost think it would have been better just to wait a few months to see the published literature, which I think is going to have a tremendous impact on practice,” Dr. Kavanaugh said.
He also said he was troubled by the strong recommendation against switching to a TNF inhibitor–biosimilar after receiving treatment with an originator TNF inhibitor. That’s something rheumatologists all across Europe are routinely doing now for economic reasons without known harm, with the caveat that patients must be switched to a biosimilar of a different TNF inhibitor than the originator.
“There’s absolutely no data to support a recommendation against switching. I think they’re going out on a limb a little bit,” he added.
Dr. Ruderman said he struggles with the guideline development methodology, which involves posing a series of key questions at the outset, with a strict charge to provide answers.
“They always have to have an answer to the question. And in the event that there’s no data to support an answer, then it becomes a matter of the expert opinion of the people on the committee. I think many rheumatologists would say, ‘Why are they any more expert than a clinical rheumatologist who’s been seeing AS patients for 20 years?’ And the answer is they’re probably not. The fact that most of these are conditional recommendations, meaning they’re not supported by strong evidence, makes them less useful,” according to Dr. Ruderman.
Both he and Dr. Kavanaugh reported receiving research funding from and/or serving as a consultant to numerous pharmaceutical companies.
REPORTING FROM RWCS 2020
Pembro ups survival in NSCLC: ‘Really extraordinary’ results
More than a third (35%) of patients with relapsed non–small cell lung cancer (NSCLC) treated with pembrolizumab (Keytruda, Merck) were still alive at 3 years, according to long-term results from a pivotal clinical trial.
The results also showed that, among the 10% of patients who completed all 35 cycles of pembrolizumab, the 3-year overall survival was approximately 99%, with progression-free survival (PFS) at around 70%.
“It is too soon to say that pembrolizumab is a potential cure...and we know that it doesn’t work for all patients, but the agent remains very, very promising,” said lead investigator Roy Herbst, MD, PhD, Department of Medical Oncology, Yale Comprehensive Cancer Center, New Haven, Connecticut.
These new results come from the KEYNOTE-010 trial, conducted in more than 1000 patients with NSCLC who had progressed on chemotherapy, randomized to receive immunotherapy with pembrolizumab or chemotherapy with docetaxel.
The results were published online on February 20 in the Journal of Clinical Oncology and were previously presented at the 2018 annual meeting of the European Society of Medical Oncology.
Overall survival at 3 years was 35% in patients with PD-L1 expression ≥ 50% in the tumor, and 23% in those with PD-L1 ≥ 1%.
This compares with 3-year overall survival of 11-13% with docetaxel.
These results are “really extraordinary,” Herbst commented to Medscape Medical News.
The 3-year overall survival rate of 35% in patients with PD-L1 ≥ 50% “is huge,” he said. “It really shows the durability of the response.”
Herbst commented that the “almost 100%” survival at 3 years among patients who completed 35 cycles of pembrolizumab shows that this treatment period (of about 2 years) is “probably about the right time to treat.”
“Currently, the agent is being used in all potential settings, before any other treatment, after other treatment, and with other treatments,” he said.
“Our hope is to find the very best way to use pembrolizumab to treat individual lung cancer patients, assessing how much PD-L1 a tumor expresses, what stage the patient is in, as well as other variables and biomarkers we are working on. This is the story of tailored therapy,” Herbst said.
Approached for comment, Solange Peters, MD, PhD, Oncology Department, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, said that the results are “very good” and “confirm the paradigms we have been seeing in melanoma,” with good long-term control, which is “very reassuring.”
However, she told Medscape Medical News that the trial raises an important question: «How long do you need to expose your patient with lung cancer to immunotherapy in order to get this long-term control?»
She said the “good news” is that, for the 10% of patients who completed 2 years of treatment per protocol, almost all of them are still alive at 3 years, “which is not observed with chemotherapy.”
The question for Peters is “more about the definition of long-term control,” as it was seen that almost one in three patients nevertheless had some form of progression.
This suggests that you have a group of people “who are nicely controlled, you stop the drug, and 1 year later a third of them have progressed.”
Peters said: “So how long do you need to treat these patients? I would say I still don’t know.”
“If I were one of these patients probably I would still want to continue [on the drug]. Of course, some might have progressed even while remaining on the drug, but the proportion who would have progressed is probably smaller than this one.”
Responses on Re-introduction of Therapy
The study also allowed patients who had completed 35 cycles of pembrolizumab to be restarted on the drug if they experienced progression.
The team found that, among 14 patients, 43% had a partial response and 36% had stable disease.
Herbst highlighted this finding and told Medscape Medical News that this «could be very important to physicians because they might want to think about using the drug again» in patients who have progressed on it.
He believes that the progression was not because of any resistance per se but rather a slowing down of the adaptive immune response.
“It’s just that it needs a boost,” he said, while noting that tissue specimens will nevertheless be required to demonstrate the theory.
Peters agreed that these results are “very promising,” but questioned their overall significance, as it is “a very small number of patients” from a subset whose disease was controlled while on treatment and then progressed after stopping.
She also pointed out that, in another study in patients with lung cancer (CheckMate-153), some patients were rechallenged with immunotherapy after having stopped treatment at 1 year “with very poor results.”
Peters said studies in melanoma have shown “rechallenge can be useful in a significant proportion of patients, but still you have not demonstrated that stopping and rechallenging is the same as not stopping.”
Study Details
KEYNOTE-010 involved patients with NSCLC from 202 centers in 24 countries with stage IIIB/IV disease expressing PD-L1 who had experienced disease progression after at least two cycles of platinum-based chemotherapy.
They were randomized 1:1:1 to open-label pembrolizumab 2 mg/kg, pembrolizumab 10 mg/kg, or docetaxel 75 mg/m2 every 3 weeks.
Pembrolizumab was continued for 35 treatment cycles over 2 years and docetaxel was continued for the maximum duration allowed by local regulators.
Patients who stopped pembrolizumab after a complete response or completing all 35 cycles, and who subsequently experienced disease progression, could receive up to 17 additional cycles over 1 year if they had not received another anticancer therapy in the meantime.
Among the 1,034 patients originally recruited between August 2013 and February 2015, 691 were assigned to pembrolizumab at 3 mg/kg or 10 mg/kg and 343 to docetaxel.
For the intention-to-treat analysis in 1033 patients, the mean duration of follow-up was 42.6 months, with a median treatment duration of 3.5 months in the pembrolizumab group and 2.0 months in the docetaxel group.
Compared with docetaxel, pembrolizumab was associated with a significant reduction in the risk of death, at a hazard ratio of 0.53 in patients with PD-L1 ≥ 50% and 0.69 in those with PD-L1 ≥ 1% (both P < .0001).
In patients with PD-L1 ≥ 50%, median overall survival was 16.9 months in those given pembrolizumab and 8.2 months with docetaxel. Among those with PD-L1 ≥ 1%, median overall survival was 11.8 months with pembrolizumab versus 8.4 months with docetaxel.
Overall survival on Kaplan-Meier analysis was 34.5% with pembrolizumab and 12.7% with docetaxel in the PD-L1 ≥ 50% group, and 22.9% versus 11.0% in the PD-L1 ≥ 1% group.
PFS significantly improved with pembrolizumab versus docetaxel, at a hazard ratio of 0.57 (P < .00001) among patients with PD-L1 ≥ 50% and 0.83 (P < .005) in those with PD-L1 ≥ 1%.
In terms of safety, 17.7% of patients who completed 2 years of pembrolizumab had grade 3-5 treatment-related adverse events, compared with 16.6% among all pembrolizumab-treated patients and 36.6% of those given docetaxel.
The team reports that 79 patients completed 35 cycles of pembrolizumab, with a median follow-up of 43.4 months.
Compared with the overall patient group, these patients were less likely to be aged ≥ 65 years and to have received two or more prior treatment lines, although they were more likely to be current or former smokers and to have squamous tumor histology.
Patients who completed 35 cycles had an objective response rate of 94.9%, and 91.0% were still alive at the data cutoff. Overall survival rates were 98.7% at 12 months and 86.3% at 24 months.
Of 71 patients eligible for analysis, 23 experienced progression after completing pembrolizumab, at PFS rates at 12 and 24 months of 72.5% and 57.7%, respectively.
A total of 14 patients were given a second course of pembrolizumab, of whom six had a partial response and five had stable disease. At the data cutoff, five patients had completed 17 additional cycles and 11 were alive.
Pembro Approved at Fixed Dose
One notable aspect of the study is that patients in the pembrolizumab arm were given two different doses of the drug based on body weight, whereas the drug is approved in the United States at a fixed dose of 200 mg.
Herbst told Medscape Medical News he considers the 200-mg dose to be appropriate.
“I didn’t think that the 3-mg versus 10-mg dose per kg that we used in our study made much difference in an average-sized person,” he said, adding that the 200-mg dose “is something a little bit more than 3 mg/kg.”
“So I think that this is clearly the right dos, and I don’t think more would make any difference,” he said.
The study was funded by Merck, the manufacturer of pembrolizumab. Herbst has reported having a consulting or advisory role for many pharmaceutical companies. Other coauthors have also reported relationships with industry, and some of the authors are Merck employees. Peters has reported receiving education grants, providing consultation, attending advisory boards, and/or providing lectures for many pharmaceutical companies.
This article first appeared on Medscape.com.
More than a third (35%) of patients with relapsed non–small cell lung cancer (NSCLC) treated with pembrolizumab (Keytruda, Merck) were still alive at 3 years, according to long-term results from a pivotal clinical trial.
The results also showed that, among the 10% of patients who completed all 35 cycles of pembrolizumab, the 3-year overall survival was approximately 99%, with progression-free survival (PFS) at around 70%.
“It is too soon to say that pembrolizumab is a potential cure...and we know that it doesn’t work for all patients, but the agent remains very, very promising,” said lead investigator Roy Herbst, MD, PhD, Department of Medical Oncology, Yale Comprehensive Cancer Center, New Haven, Connecticut.
These new results come from the KEYNOTE-010 trial, conducted in more than 1000 patients with NSCLC who had progressed on chemotherapy, randomized to receive immunotherapy with pembrolizumab or chemotherapy with docetaxel.
The results were published online on February 20 in the Journal of Clinical Oncology and were previously presented at the 2018 annual meeting of the European Society of Medical Oncology.
Overall survival at 3 years was 35% in patients with PD-L1 expression ≥ 50% in the tumor, and 23% in those with PD-L1 ≥ 1%.
This compares with 3-year overall survival of 11-13% with docetaxel.
These results are “really extraordinary,” Herbst commented to Medscape Medical News.
The 3-year overall survival rate of 35% in patients with PD-L1 ≥ 50% “is huge,” he said. “It really shows the durability of the response.”
Herbst commented that the “almost 100%” survival at 3 years among patients who completed 35 cycles of pembrolizumab shows that this treatment period (of about 2 years) is “probably about the right time to treat.”
“Currently, the agent is being used in all potential settings, before any other treatment, after other treatment, and with other treatments,” he said.
“Our hope is to find the very best way to use pembrolizumab to treat individual lung cancer patients, assessing how much PD-L1 a tumor expresses, what stage the patient is in, as well as other variables and biomarkers we are working on. This is the story of tailored therapy,” Herbst said.
Approached for comment, Solange Peters, MD, PhD, Oncology Department, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, said that the results are “very good” and “confirm the paradigms we have been seeing in melanoma,” with good long-term control, which is “very reassuring.”
However, she told Medscape Medical News that the trial raises an important question: «How long do you need to expose your patient with lung cancer to immunotherapy in order to get this long-term control?»
She said the “good news” is that, for the 10% of patients who completed 2 years of treatment per protocol, almost all of them are still alive at 3 years, “which is not observed with chemotherapy.”
The question for Peters is “more about the definition of long-term control,” as it was seen that almost one in three patients nevertheless had some form of progression.
This suggests that you have a group of people “who are nicely controlled, you stop the drug, and 1 year later a third of them have progressed.”
Peters said: “So how long do you need to treat these patients? I would say I still don’t know.”
“If I were one of these patients probably I would still want to continue [on the drug]. Of course, some might have progressed even while remaining on the drug, but the proportion who would have progressed is probably smaller than this one.”
Responses on Re-introduction of Therapy
The study also allowed patients who had completed 35 cycles of pembrolizumab to be restarted on the drug if they experienced progression.
The team found that, among 14 patients, 43% had a partial response and 36% had stable disease.
Herbst highlighted this finding and told Medscape Medical News that this «could be very important to physicians because they might want to think about using the drug again» in patients who have progressed on it.
He believes that the progression was not because of any resistance per se but rather a slowing down of the adaptive immune response.
“It’s just that it needs a boost,” he said, while noting that tissue specimens will nevertheless be required to demonstrate the theory.
Peters agreed that these results are “very promising,” but questioned their overall significance, as it is “a very small number of patients” from a subset whose disease was controlled while on treatment and then progressed after stopping.
She also pointed out that, in another study in patients with lung cancer (CheckMate-153), some patients were rechallenged with immunotherapy after having stopped treatment at 1 year “with very poor results.”
Peters said studies in melanoma have shown “rechallenge can be useful in a significant proportion of patients, but still you have not demonstrated that stopping and rechallenging is the same as not stopping.”
Study Details
KEYNOTE-010 involved patients with NSCLC from 202 centers in 24 countries with stage IIIB/IV disease expressing PD-L1 who had experienced disease progression after at least two cycles of platinum-based chemotherapy.
They were randomized 1:1:1 to open-label pembrolizumab 2 mg/kg, pembrolizumab 10 mg/kg, or docetaxel 75 mg/m2 every 3 weeks.
Pembrolizumab was continued for 35 treatment cycles over 2 years and docetaxel was continued for the maximum duration allowed by local regulators.
Patients who stopped pembrolizumab after a complete response or completing all 35 cycles, and who subsequently experienced disease progression, could receive up to 17 additional cycles over 1 year if they had not received another anticancer therapy in the meantime.
Among the 1,034 patients originally recruited between August 2013 and February 2015, 691 were assigned to pembrolizumab at 3 mg/kg or 10 mg/kg and 343 to docetaxel.
For the intention-to-treat analysis in 1033 patients, the mean duration of follow-up was 42.6 months, with a median treatment duration of 3.5 months in the pembrolizumab group and 2.0 months in the docetaxel group.
Compared with docetaxel, pembrolizumab was associated with a significant reduction in the risk of death, at a hazard ratio of 0.53 in patients with PD-L1 ≥ 50% and 0.69 in those with PD-L1 ≥ 1% (both P < .0001).
In patients with PD-L1 ≥ 50%, median overall survival was 16.9 months in those given pembrolizumab and 8.2 months with docetaxel. Among those with PD-L1 ≥ 1%, median overall survival was 11.8 months with pembrolizumab versus 8.4 months with docetaxel.
Overall survival on Kaplan-Meier analysis was 34.5% with pembrolizumab and 12.7% with docetaxel in the PD-L1 ≥ 50% group, and 22.9% versus 11.0% in the PD-L1 ≥ 1% group.
PFS significantly improved with pembrolizumab versus docetaxel, at a hazard ratio of 0.57 (P < .00001) among patients with PD-L1 ≥ 50% and 0.83 (P < .005) in those with PD-L1 ≥ 1%.
In terms of safety, 17.7% of patients who completed 2 years of pembrolizumab had grade 3-5 treatment-related adverse events, compared with 16.6% among all pembrolizumab-treated patients and 36.6% of those given docetaxel.
The team reports that 79 patients completed 35 cycles of pembrolizumab, with a median follow-up of 43.4 months.
Compared with the overall patient group, these patients were less likely to be aged ≥ 65 years and to have received two or more prior treatment lines, although they were more likely to be current or former smokers and to have squamous tumor histology.
Patients who completed 35 cycles had an objective response rate of 94.9%, and 91.0% were still alive at the data cutoff. Overall survival rates were 98.7% at 12 months and 86.3% at 24 months.
Of 71 patients eligible for analysis, 23 experienced progression after completing pembrolizumab, at PFS rates at 12 and 24 months of 72.5% and 57.7%, respectively.
A total of 14 patients were given a second course of pembrolizumab, of whom six had a partial response and five had stable disease. At the data cutoff, five patients had completed 17 additional cycles and 11 were alive.
Pembro Approved at Fixed Dose
One notable aspect of the study is that patients in the pembrolizumab arm were given two different doses of the drug based on body weight, whereas the drug is approved in the United States at a fixed dose of 200 mg.
Herbst told Medscape Medical News he considers the 200-mg dose to be appropriate.
“I didn’t think that the 3-mg versus 10-mg dose per kg that we used in our study made much difference in an average-sized person,” he said, adding that the 200-mg dose “is something a little bit more than 3 mg/kg.”
“So I think that this is clearly the right dos, and I don’t think more would make any difference,” he said.
The study was funded by Merck, the manufacturer of pembrolizumab. Herbst has reported having a consulting or advisory role for many pharmaceutical companies. Other coauthors have also reported relationships with industry, and some of the authors are Merck employees. Peters has reported receiving education grants, providing consultation, attending advisory boards, and/or providing lectures for many pharmaceutical companies.
This article first appeared on Medscape.com.
More than a third (35%) of patients with relapsed non–small cell lung cancer (NSCLC) treated with pembrolizumab (Keytruda, Merck) were still alive at 3 years, according to long-term results from a pivotal clinical trial.
The results also showed that, among the 10% of patients who completed all 35 cycles of pembrolizumab, the 3-year overall survival was approximately 99%, with progression-free survival (PFS) at around 70%.
“It is too soon to say that pembrolizumab is a potential cure...and we know that it doesn’t work for all patients, but the agent remains very, very promising,” said lead investigator Roy Herbst, MD, PhD, Department of Medical Oncology, Yale Comprehensive Cancer Center, New Haven, Connecticut.
These new results come from the KEYNOTE-010 trial, conducted in more than 1000 patients with NSCLC who had progressed on chemotherapy, randomized to receive immunotherapy with pembrolizumab or chemotherapy with docetaxel.
The results were published online on February 20 in the Journal of Clinical Oncology and were previously presented at the 2018 annual meeting of the European Society of Medical Oncology.
Overall survival at 3 years was 35% in patients with PD-L1 expression ≥ 50% in the tumor, and 23% in those with PD-L1 ≥ 1%.
This compares with 3-year overall survival of 11-13% with docetaxel.
These results are “really extraordinary,” Herbst commented to Medscape Medical News.
The 3-year overall survival rate of 35% in patients with PD-L1 ≥ 50% “is huge,” he said. “It really shows the durability of the response.”
Herbst commented that the “almost 100%” survival at 3 years among patients who completed 35 cycles of pembrolizumab shows that this treatment period (of about 2 years) is “probably about the right time to treat.”
“Currently, the agent is being used in all potential settings, before any other treatment, after other treatment, and with other treatments,” he said.
“Our hope is to find the very best way to use pembrolizumab to treat individual lung cancer patients, assessing how much PD-L1 a tumor expresses, what stage the patient is in, as well as other variables and biomarkers we are working on. This is the story of tailored therapy,” Herbst said.
Approached for comment, Solange Peters, MD, PhD, Oncology Department, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, said that the results are “very good” and “confirm the paradigms we have been seeing in melanoma,” with good long-term control, which is “very reassuring.”
However, she told Medscape Medical News that the trial raises an important question: «How long do you need to expose your patient with lung cancer to immunotherapy in order to get this long-term control?»
She said the “good news” is that, for the 10% of patients who completed 2 years of treatment per protocol, almost all of them are still alive at 3 years, “which is not observed with chemotherapy.”
The question for Peters is “more about the definition of long-term control,” as it was seen that almost one in three patients nevertheless had some form of progression.
This suggests that you have a group of people “who are nicely controlled, you stop the drug, and 1 year later a third of them have progressed.”
Peters said: “So how long do you need to treat these patients? I would say I still don’t know.”
“If I were one of these patients probably I would still want to continue [on the drug]. Of course, some might have progressed even while remaining on the drug, but the proportion who would have progressed is probably smaller than this one.”
Responses on Re-introduction of Therapy
The study also allowed patients who had completed 35 cycles of pembrolizumab to be restarted on the drug if they experienced progression.
The team found that, among 14 patients, 43% had a partial response and 36% had stable disease.
Herbst highlighted this finding and told Medscape Medical News that this «could be very important to physicians because they might want to think about using the drug again» in patients who have progressed on it.
He believes that the progression was not because of any resistance per se but rather a slowing down of the adaptive immune response.
“It’s just that it needs a boost,” he said, while noting that tissue specimens will nevertheless be required to demonstrate the theory.
Peters agreed that these results are “very promising,” but questioned their overall significance, as it is “a very small number of patients” from a subset whose disease was controlled while on treatment and then progressed after stopping.
She also pointed out that, in another study in patients with lung cancer (CheckMate-153), some patients were rechallenged with immunotherapy after having stopped treatment at 1 year “with very poor results.”
Peters said studies in melanoma have shown “rechallenge can be useful in a significant proportion of patients, but still you have not demonstrated that stopping and rechallenging is the same as not stopping.”
Study Details
KEYNOTE-010 involved patients with NSCLC from 202 centers in 24 countries with stage IIIB/IV disease expressing PD-L1 who had experienced disease progression after at least two cycles of platinum-based chemotherapy.
They were randomized 1:1:1 to open-label pembrolizumab 2 mg/kg, pembrolizumab 10 mg/kg, or docetaxel 75 mg/m2 every 3 weeks.
Pembrolizumab was continued for 35 treatment cycles over 2 years and docetaxel was continued for the maximum duration allowed by local regulators.
Patients who stopped pembrolizumab after a complete response or completing all 35 cycles, and who subsequently experienced disease progression, could receive up to 17 additional cycles over 1 year if they had not received another anticancer therapy in the meantime.
Among the 1,034 patients originally recruited between August 2013 and February 2015, 691 were assigned to pembrolizumab at 3 mg/kg or 10 mg/kg and 343 to docetaxel.
For the intention-to-treat analysis in 1033 patients, the mean duration of follow-up was 42.6 months, with a median treatment duration of 3.5 months in the pembrolizumab group and 2.0 months in the docetaxel group.
Compared with docetaxel, pembrolizumab was associated with a significant reduction in the risk of death, at a hazard ratio of 0.53 in patients with PD-L1 ≥ 50% and 0.69 in those with PD-L1 ≥ 1% (both P < .0001).
In patients with PD-L1 ≥ 50%, median overall survival was 16.9 months in those given pembrolizumab and 8.2 months with docetaxel. Among those with PD-L1 ≥ 1%, median overall survival was 11.8 months with pembrolizumab versus 8.4 months with docetaxel.
Overall survival on Kaplan-Meier analysis was 34.5% with pembrolizumab and 12.7% with docetaxel in the PD-L1 ≥ 50% group, and 22.9% versus 11.0% in the PD-L1 ≥ 1% group.
PFS significantly improved with pembrolizumab versus docetaxel, at a hazard ratio of 0.57 (P < .00001) among patients with PD-L1 ≥ 50% and 0.83 (P < .005) in those with PD-L1 ≥ 1%.
In terms of safety, 17.7% of patients who completed 2 years of pembrolizumab had grade 3-5 treatment-related adverse events, compared with 16.6% among all pembrolizumab-treated patients and 36.6% of those given docetaxel.
The team reports that 79 patients completed 35 cycles of pembrolizumab, with a median follow-up of 43.4 months.
Compared with the overall patient group, these patients were less likely to be aged ≥ 65 years and to have received two or more prior treatment lines, although they were more likely to be current or former smokers and to have squamous tumor histology.
Patients who completed 35 cycles had an objective response rate of 94.9%, and 91.0% were still alive at the data cutoff. Overall survival rates were 98.7% at 12 months and 86.3% at 24 months.
Of 71 patients eligible for analysis, 23 experienced progression after completing pembrolizumab, at PFS rates at 12 and 24 months of 72.5% and 57.7%, respectively.
A total of 14 patients were given a second course of pembrolizumab, of whom six had a partial response and five had stable disease. At the data cutoff, five patients had completed 17 additional cycles and 11 were alive.
Pembro Approved at Fixed Dose
One notable aspect of the study is that patients in the pembrolizumab arm were given two different doses of the drug based on body weight, whereas the drug is approved in the United States at a fixed dose of 200 mg.
Herbst told Medscape Medical News he considers the 200-mg dose to be appropriate.
“I didn’t think that the 3-mg versus 10-mg dose per kg that we used in our study made much difference in an average-sized person,” he said, adding that the 200-mg dose “is something a little bit more than 3 mg/kg.”
“So I think that this is clearly the right dos, and I don’t think more would make any difference,” he said.
The study was funded by Merck, the manufacturer of pembrolizumab. Herbst has reported having a consulting or advisory role for many pharmaceutical companies. Other coauthors have also reported relationships with industry, and some of the authors are Merck employees. Peters has reported receiving education grants, providing consultation, attending advisory boards, and/or providing lectures for many pharmaceutical companies.
This article first appeared on Medscape.com.
FDA approves new drug for relapsed/refractory multiple myeloma
The U.S. Food and Drug Administration today approved isatuximab (Sarclisa, Sanofi) in combination with pomalidomide (Revlimid, Celgene) and dexamethasone for the treatment of adult patients with multiple myeloma who have received two or more prior therapies including lenalidomide and a proteasome inhibitor.
Isatuximab is an anti-CD38 monoclonal antibody administered by intravenous infusion that works by helping the immune system attack multiple myeloma cancer cells.
“While there is no cure for multiple myeloma, Sarclisa is now another CD38-directed treatment option added to the list of FDA-approved treatments of patients with multiple myeloma who have progressive disease after previous therapies,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
“In the clinical trial, there was a 40% reduction in the risk of disease progression or death with this therapy,” he added.
The new approval is based on results from ICARIA-MM, an open-label, randomized phase 3 clinical trial of isatuximab among 307 patients in this setting.
In the trial, at a median follow-up of 11.6 months, median progression-free survival was 11.5 months in the isatuximab-pomalidomide-dexamethasone group versus 6.5 months in the pomalidomide-dexamethasone group (hazard ratio, 0.60; P = .001), as reported last year. Overall response rates were 60.4% for the triplet-treated group versus 35.3% for the doublet-treated group.
The most common side effects for isatuximab included neutropenia, infusion-related reactions, pneumonia, upper respiratory tract infection, diarrhea, anemia, lymphopenia, and thrombocytopenia.
Deaths because of treatment-related adverse events were reported for one patient (less than 1%) in the isatuximab-pomalidomide-dexamethasone group (sepsis) and two patients (1%) in the pomalidomide-dexamethasone group (pneumonia and urinary tract infection).
The drug can also cause serious side effects, including IV infusion-related reactions. In the case of a grade 3 or higher reaction, the drug should be permanently discontinued and health care professionals should institute appropriate medical management.
The FDA notes there have been higher incidences of second primary malignancies observed in a controlled clinical trial of patients with multiple myeloma receiving the drug.
The FDA also highlighted that laboratory test interference may be caused by isatuximab and that blood banks should be informed that patients are receiving the drug. Isatuximab may interfere with, for example, antibody screening for patients who need a blood transfusion. Isatuximab may also interfere with the assays used to monitor M-protein, which may impact the determination of complete response.
This article originally appeared on Medscape.com.
The U.S. Food and Drug Administration today approved isatuximab (Sarclisa, Sanofi) in combination with pomalidomide (Revlimid, Celgene) and dexamethasone for the treatment of adult patients with multiple myeloma who have received two or more prior therapies including lenalidomide and a proteasome inhibitor.
Isatuximab is an anti-CD38 monoclonal antibody administered by intravenous infusion that works by helping the immune system attack multiple myeloma cancer cells.
“While there is no cure for multiple myeloma, Sarclisa is now another CD38-directed treatment option added to the list of FDA-approved treatments of patients with multiple myeloma who have progressive disease after previous therapies,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
“In the clinical trial, there was a 40% reduction in the risk of disease progression or death with this therapy,” he added.
The new approval is based on results from ICARIA-MM, an open-label, randomized phase 3 clinical trial of isatuximab among 307 patients in this setting.
In the trial, at a median follow-up of 11.6 months, median progression-free survival was 11.5 months in the isatuximab-pomalidomide-dexamethasone group versus 6.5 months in the pomalidomide-dexamethasone group (hazard ratio, 0.60; P = .001), as reported last year. Overall response rates were 60.4% for the triplet-treated group versus 35.3% for the doublet-treated group.
The most common side effects for isatuximab included neutropenia, infusion-related reactions, pneumonia, upper respiratory tract infection, diarrhea, anemia, lymphopenia, and thrombocytopenia.
Deaths because of treatment-related adverse events were reported for one patient (less than 1%) in the isatuximab-pomalidomide-dexamethasone group (sepsis) and two patients (1%) in the pomalidomide-dexamethasone group (pneumonia and urinary tract infection).
The drug can also cause serious side effects, including IV infusion-related reactions. In the case of a grade 3 or higher reaction, the drug should be permanently discontinued and health care professionals should institute appropriate medical management.
The FDA notes there have been higher incidences of second primary malignancies observed in a controlled clinical trial of patients with multiple myeloma receiving the drug.
The FDA also highlighted that laboratory test interference may be caused by isatuximab and that blood banks should be informed that patients are receiving the drug. Isatuximab may interfere with, for example, antibody screening for patients who need a blood transfusion. Isatuximab may also interfere with the assays used to monitor M-protein, which may impact the determination of complete response.
This article originally appeared on Medscape.com.
The U.S. Food and Drug Administration today approved isatuximab (Sarclisa, Sanofi) in combination with pomalidomide (Revlimid, Celgene) and dexamethasone for the treatment of adult patients with multiple myeloma who have received two or more prior therapies including lenalidomide and a proteasome inhibitor.
Isatuximab is an anti-CD38 monoclonal antibody administered by intravenous infusion that works by helping the immune system attack multiple myeloma cancer cells.
“While there is no cure for multiple myeloma, Sarclisa is now another CD38-directed treatment option added to the list of FDA-approved treatments of patients with multiple myeloma who have progressive disease after previous therapies,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
“In the clinical trial, there was a 40% reduction in the risk of disease progression or death with this therapy,” he added.
The new approval is based on results from ICARIA-MM, an open-label, randomized phase 3 clinical trial of isatuximab among 307 patients in this setting.
In the trial, at a median follow-up of 11.6 months, median progression-free survival was 11.5 months in the isatuximab-pomalidomide-dexamethasone group versus 6.5 months in the pomalidomide-dexamethasone group (hazard ratio, 0.60; P = .001), as reported last year. Overall response rates were 60.4% for the triplet-treated group versus 35.3% for the doublet-treated group.
The most common side effects for isatuximab included neutropenia, infusion-related reactions, pneumonia, upper respiratory tract infection, diarrhea, anemia, lymphopenia, and thrombocytopenia.
Deaths because of treatment-related adverse events were reported for one patient (less than 1%) in the isatuximab-pomalidomide-dexamethasone group (sepsis) and two patients (1%) in the pomalidomide-dexamethasone group (pneumonia and urinary tract infection).
The drug can also cause serious side effects, including IV infusion-related reactions. In the case of a grade 3 or higher reaction, the drug should be permanently discontinued and health care professionals should institute appropriate medical management.
The FDA notes there have been higher incidences of second primary malignancies observed in a controlled clinical trial of patients with multiple myeloma receiving the drug.
The FDA also highlighted that laboratory test interference may be caused by isatuximab and that blood banks should be informed that patients are receiving the drug. Isatuximab may interfere with, for example, antibody screening for patients who need a blood transfusion. Isatuximab may also interfere with the assays used to monitor M-protein, which may impact the determination of complete response.
This article originally appeared on Medscape.com.
New guideline offers recommendations for reproductive health in patients with rheumatic diseases
A new guideline from the American College of Rheumatology offers the organization’s first clinical recommendations on how to manage reproductive health issues in patients with rheumatic and musculoskeletal diseases (RMDs).
“With the development of this guideline, the ACR recognizes the key role of clinical rheumatologists not only in managing disease activity but also in understanding the interactions of RMDs and their therapies in the context of reproductive health,” wrote Lisa R. Sammaritano, MD, of Weill Cornell Medicine and the Hospital for Special Surgery in New York, and coauthors. The guideline was published in Arthritis & Rheumatology.
To develop an evidence-based guideline on reproductive health in RMD patients, the researchers embarked on a systematic review of studies in areas like contraception, pregnancy and lactation, assisted reproductive technology (ART), fertility preservation, and hormone therapy. The guideline contains 12 ungraded good practice statements and 131 graded recommendations, all developed through the Grading of Recommendations Assessment, Development, and Evaluation methodology.
In counseling patients about these areas of care, the guideline says that rheumatologists and other clinicians “must collaborate with specialists in the fields of obstetrics-gynecology, maternal-fetal medicine, and reproductive endocrinology and infertility.”
“One thing this guideline does well is highlight the importance of involving maternal-fetal medicine colleagues,” Alison Cahill, MD, a professor in the department of women’s health at the University of Texas at Austin and a maternal-fetal medicine specialist within UT Health Austin’s Women’s Health Institute, said when asked for comment on the guideline. “We’re always very happy to see patients ahead of time who are planning pregnancy to be able to discuss what the care plan would look like. And specifically, to address medications, if required, for their rheumatologic care.
“As we learn more and more,” she added, “we’ve come to understand that most treatments and medications are actually safe or relatively safe to take in pregnancy. Certainly, the benefit of taking them outweighs any small or theoretic risks. On the flip side, the guideline does a nice job of highlighting the importance of good disease control, both at the time of conception and during pregnancy.”
Contraception
In regard to contraception, the guideline strongly recommends the use of effective contraceptives – with a conditional recommendation of IUDs or a subdermal progestin implant – in fertile women with a RMD who have neither systemic lupus erythematosus (SLE) nor positive antiphospholipid antibody (aPL). They also strongly recommend discussing the use of emergency contraception with all RMD patients.
For SLE patients, the guideline strongly recommends the use of effective contraceptives in those with stable or low disease activity who are not positive for aPL. They also strongly recommend progestin‐only or IUD contraceptives over combined estrogen‐progestin contraception. For aPL-positive patients, the guideline strongly recommends against combined estrogen‐progestin contraceptives and for levonorgestrel or copper IUDs or the progestin‐only pill.
Assisted reproductive technology
In regard to ART, the guideline strongly recommends proceeding as needed in aPL-negative women with uncomplicated, stable RMD who are on pregnancy‐compatible medications. They also strongly recommend deferring ART in any RMD patients with moderately or severely active disease.
For aPL-positive patients undergoing ART procedures, they strongly recommend prophylactic anticoagulation with heparin or low-molecular-weight heparin (LMWH) in women with obstetric antiphospholipid syndrome (APS) and therapeutic anticoagulation in women with thrombotic APS. In patients undergoing embryo and oocyte cryopreservation, they strongly recommend continuing immunosuppressive and biologic therapies – the exception being cyclophosphamide (CYC) – for anyone in stable condition.
Fertility preservation
In regard to fertility preservation in patients taking CYC, the guideline strongly suggests sperm cryopreservation as good practice prior to treatment. They also conditionally recommend monthly gonadotropin‐releasing hormone agonist cotherapy in premenopausal women with RMD.
Hormone therapy
In regard to menopause and hormone therapy, the guideline strongly suggests hormone therapy as good practice in postmenopausal women with RMD, without SLE or positive aPL, and who have severe vasomotor symptoms. Hormone therapy is conditionally recommended in patients with SLE, without positive aPL, and with no contraindications. For aPL-positive patients, they strongly recommend against hormone therapy in women with obstetric and/or thrombotic APS.
Pregnancy assessment and management
Among the many recommendations regarding pregnancy assessment and management, the guideline strongly suggests counseling women with RMD who are considering pregnancy to take into account the improved outcomes for pregnant women with low disease activity. They strongly recommend that women considering pregnancy should switch to pregnancy‐compatible medication and pause to assess its efficacy and tolerability before moving forward, along with strongly recommending that pregnant women with active disease initiate or continue a pregnancy‐compatible steroid‐sparing medication. They also recommend testing for anti‐Ro/SS-A and anti‐La/SS-B in women with SLE, Sjögren’s syndrome, systemic sclerosis, or rheumatoid arthritis, but only once and only before or early in the pregnancy.
For women with systemic sclerosis who develop scleroderma renal crisis during pregnancy, the authors strongly advise using ACE inhibitors or angiotensin receptor blockers “because the risk of maternal or fetal death with untreated disease is higher than the risk associated with use of these medications during pregnancy.”
Among women with SLE, the recommendations strongly call for testing either before or early in pregnancy for anticardiolipin antibody, anti–beta2-glycoprotein I, or positive lupus anticoagulant, as well as initiating or continuing hydroxychloroquine (HCQ) if possible. Starting in the first trimester, the authors also conditionally recommend that SLE patients take low-dose aspirin daily
For pregnant women who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the guideline conditionally recommends prophylactic treatment with low-dose aspirin daily to protect against preeclampsia. When obstetric APS criteria are met, the guideline strongly advises combined treatment with daily low-dose aspirin and prophylactic-dose heparin (or LMWH), as well as prophylactic-dose anticoagulation for 6-12 weeks post partum. When patients have thrombotic APS, this combination treatment should contain heparin dose at a therapeutic level throughout pregnancy and postpartum. However, the authors conditionally recommend against giving low-dose aspirin plus prophylactic-dose heparin to women without obstetric APS. For refractory obstetric APS, the guideline also contains recommendations that are conditionally against treatment with intravenous immunoglobulin or an increased LMWH dose and strongly against adding prednisone to prophylactic-dose heparin or LMWH and low-dose aspirin. In pregnant patients with primary APS, the authors conditionally advise adding HCQ to prophylactic-dose heparin or LMWH and low-dose aspirin therapy. However, women with aPL who do not meet APS criteria or have another indication for HCQ are conditionally advised against prophylactic treatment with the antimalarial.
For women with Anti-Ro/SS-A and/or anti-La/SS-B antibodies in pregnancy, there is conditional advice to use HCQ. When there is no history of an infant with complete heart block or neonatal lupus erythematosus among women with these antibodies, the guideline conditionally advises serial fetal echocardiography (less often than weekly) starting between 16 and 18 weeks and continuing through 26 weeks, but this should be weekly when there is a prior history. Treatment with oral dexamethasone 4 mg daily is conditionally advised when there is echocardiographic evidence of fetal first- or second-degree heart block, but dexamethasone is not recommended when complete heart block is present.
Finally, in regard to medication use, the authors strongly recommend that men who are planning to be fathers continue on HCQ, azathioprine, 6‐mercaptopurine, colchicine, or tumor necrosis factor inhibitors. Conditional treatment recommendations for men planning for pregnancy include methotrexate, mycophenolate mofetil/mycophenolic acid (MMF), leflunomide, sulfasalazine, calcineurin inhibitors, and NSAIDs. They also strongly recommend that this group of men discontinue CYC and thalidomide.
Pregnant women are strongly recommended to discontinue methotrexate, leflunomide (with cholestyramine washout if there are detectable serum levels of its metabolite prior to pregnancy or as soon as it is confirmed), MMF, CYC, and thalidomide within 3 months prior to conception, and they strongly recommend HCQ (in women with SLE), azathioprine/6‐mercaptopurine, colchicine, or sulfasalazine for use throughout pregnancy. They strongly recommend a combination of low‐dose aspirin and prophylactic‐dose heparin for pregnant women with obstetric APS, along with low‐dose aspirin and therapeutic‐dose heparin for women with thrombotic APS throughout pregnancy and postpartum. However, for women with SLE and those who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the authors conditionally recommend low-dose aspirin starting in the first trimester.
The guideline suggests that women with RMD should be encouraged to breastfeed if they are willing and able; they also suggest that disease control be maintained through lactation‐compatible medications and that the risks and benefits be reviewed on a patient-by-patient basis. Treatment with HCQ, colchicine, sulfasalazine, rituximab, and all tumor necrosis factor inhibitors are strongly recommended as being compatible with breastfeeding, and they strongly recommend against using CYC, leflunomide, MMF, and thalidomide while breastfeeding.
The authors acknowledged the limitations of their guideline, including the literature review being conducted on studies involving adults and an “inability to include recommendations for uncommon but important clinical situations,” including those involving transgender patients and hormonal therapies.
The authors reported numerous potential conflicts of interest, including receiving research support, consulting fees, speaking fees, and honoraria from various pharmaceutical companies.
SOURCE: Sammaritano LR et al. Arthritis Rheumatol. 2020 Feb 23. doi: 10.1002/art.41191.
A new guideline from the American College of Rheumatology offers the organization’s first clinical recommendations on how to manage reproductive health issues in patients with rheumatic and musculoskeletal diseases (RMDs).
“With the development of this guideline, the ACR recognizes the key role of clinical rheumatologists not only in managing disease activity but also in understanding the interactions of RMDs and their therapies in the context of reproductive health,” wrote Lisa R. Sammaritano, MD, of Weill Cornell Medicine and the Hospital for Special Surgery in New York, and coauthors. The guideline was published in Arthritis & Rheumatology.
To develop an evidence-based guideline on reproductive health in RMD patients, the researchers embarked on a systematic review of studies in areas like contraception, pregnancy and lactation, assisted reproductive technology (ART), fertility preservation, and hormone therapy. The guideline contains 12 ungraded good practice statements and 131 graded recommendations, all developed through the Grading of Recommendations Assessment, Development, and Evaluation methodology.
In counseling patients about these areas of care, the guideline says that rheumatologists and other clinicians “must collaborate with specialists in the fields of obstetrics-gynecology, maternal-fetal medicine, and reproductive endocrinology and infertility.”
“One thing this guideline does well is highlight the importance of involving maternal-fetal medicine colleagues,” Alison Cahill, MD, a professor in the department of women’s health at the University of Texas at Austin and a maternal-fetal medicine specialist within UT Health Austin’s Women’s Health Institute, said when asked for comment on the guideline. “We’re always very happy to see patients ahead of time who are planning pregnancy to be able to discuss what the care plan would look like. And specifically, to address medications, if required, for their rheumatologic care.
“As we learn more and more,” she added, “we’ve come to understand that most treatments and medications are actually safe or relatively safe to take in pregnancy. Certainly, the benefit of taking them outweighs any small or theoretic risks. On the flip side, the guideline does a nice job of highlighting the importance of good disease control, both at the time of conception and during pregnancy.”
Contraception
In regard to contraception, the guideline strongly recommends the use of effective contraceptives – with a conditional recommendation of IUDs or a subdermal progestin implant – in fertile women with a RMD who have neither systemic lupus erythematosus (SLE) nor positive antiphospholipid antibody (aPL). They also strongly recommend discussing the use of emergency contraception with all RMD patients.
For SLE patients, the guideline strongly recommends the use of effective contraceptives in those with stable or low disease activity who are not positive for aPL. They also strongly recommend progestin‐only or IUD contraceptives over combined estrogen‐progestin contraception. For aPL-positive patients, the guideline strongly recommends against combined estrogen‐progestin contraceptives and for levonorgestrel or copper IUDs or the progestin‐only pill.
Assisted reproductive technology
In regard to ART, the guideline strongly recommends proceeding as needed in aPL-negative women with uncomplicated, stable RMD who are on pregnancy‐compatible medications. They also strongly recommend deferring ART in any RMD patients with moderately or severely active disease.
For aPL-positive patients undergoing ART procedures, they strongly recommend prophylactic anticoagulation with heparin or low-molecular-weight heparin (LMWH) in women with obstetric antiphospholipid syndrome (APS) and therapeutic anticoagulation in women with thrombotic APS. In patients undergoing embryo and oocyte cryopreservation, they strongly recommend continuing immunosuppressive and biologic therapies – the exception being cyclophosphamide (CYC) – for anyone in stable condition.
Fertility preservation
In regard to fertility preservation in patients taking CYC, the guideline strongly suggests sperm cryopreservation as good practice prior to treatment. They also conditionally recommend monthly gonadotropin‐releasing hormone agonist cotherapy in premenopausal women with RMD.
Hormone therapy
In regard to menopause and hormone therapy, the guideline strongly suggests hormone therapy as good practice in postmenopausal women with RMD, without SLE or positive aPL, and who have severe vasomotor symptoms. Hormone therapy is conditionally recommended in patients with SLE, without positive aPL, and with no contraindications. For aPL-positive patients, they strongly recommend against hormone therapy in women with obstetric and/or thrombotic APS.
Pregnancy assessment and management
Among the many recommendations regarding pregnancy assessment and management, the guideline strongly suggests counseling women with RMD who are considering pregnancy to take into account the improved outcomes for pregnant women with low disease activity. They strongly recommend that women considering pregnancy should switch to pregnancy‐compatible medication and pause to assess its efficacy and tolerability before moving forward, along with strongly recommending that pregnant women with active disease initiate or continue a pregnancy‐compatible steroid‐sparing medication. They also recommend testing for anti‐Ro/SS-A and anti‐La/SS-B in women with SLE, Sjögren’s syndrome, systemic sclerosis, or rheumatoid arthritis, but only once and only before or early in the pregnancy.
For women with systemic sclerosis who develop scleroderma renal crisis during pregnancy, the authors strongly advise using ACE inhibitors or angiotensin receptor blockers “because the risk of maternal or fetal death with untreated disease is higher than the risk associated with use of these medications during pregnancy.”
Among women with SLE, the recommendations strongly call for testing either before or early in pregnancy for anticardiolipin antibody, anti–beta2-glycoprotein I, or positive lupus anticoagulant, as well as initiating or continuing hydroxychloroquine (HCQ) if possible. Starting in the first trimester, the authors also conditionally recommend that SLE patients take low-dose aspirin daily
For pregnant women who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the guideline conditionally recommends prophylactic treatment with low-dose aspirin daily to protect against preeclampsia. When obstetric APS criteria are met, the guideline strongly advises combined treatment with daily low-dose aspirin and prophylactic-dose heparin (or LMWH), as well as prophylactic-dose anticoagulation for 6-12 weeks post partum. When patients have thrombotic APS, this combination treatment should contain heparin dose at a therapeutic level throughout pregnancy and postpartum. However, the authors conditionally recommend against giving low-dose aspirin plus prophylactic-dose heparin to women without obstetric APS. For refractory obstetric APS, the guideline also contains recommendations that are conditionally against treatment with intravenous immunoglobulin or an increased LMWH dose and strongly against adding prednisone to prophylactic-dose heparin or LMWH and low-dose aspirin. In pregnant patients with primary APS, the authors conditionally advise adding HCQ to prophylactic-dose heparin or LMWH and low-dose aspirin therapy. However, women with aPL who do not meet APS criteria or have another indication for HCQ are conditionally advised against prophylactic treatment with the antimalarial.
For women with Anti-Ro/SS-A and/or anti-La/SS-B antibodies in pregnancy, there is conditional advice to use HCQ. When there is no history of an infant with complete heart block or neonatal lupus erythematosus among women with these antibodies, the guideline conditionally advises serial fetal echocardiography (less often than weekly) starting between 16 and 18 weeks and continuing through 26 weeks, but this should be weekly when there is a prior history. Treatment with oral dexamethasone 4 mg daily is conditionally advised when there is echocardiographic evidence of fetal first- or second-degree heart block, but dexamethasone is not recommended when complete heart block is present.
Finally, in regard to medication use, the authors strongly recommend that men who are planning to be fathers continue on HCQ, azathioprine, 6‐mercaptopurine, colchicine, or tumor necrosis factor inhibitors. Conditional treatment recommendations for men planning for pregnancy include methotrexate, mycophenolate mofetil/mycophenolic acid (MMF), leflunomide, sulfasalazine, calcineurin inhibitors, and NSAIDs. They also strongly recommend that this group of men discontinue CYC and thalidomide.
Pregnant women are strongly recommended to discontinue methotrexate, leflunomide (with cholestyramine washout if there are detectable serum levels of its metabolite prior to pregnancy or as soon as it is confirmed), MMF, CYC, and thalidomide within 3 months prior to conception, and they strongly recommend HCQ (in women with SLE), azathioprine/6‐mercaptopurine, colchicine, or sulfasalazine for use throughout pregnancy. They strongly recommend a combination of low‐dose aspirin and prophylactic‐dose heparin for pregnant women with obstetric APS, along with low‐dose aspirin and therapeutic‐dose heparin for women with thrombotic APS throughout pregnancy and postpartum. However, for women with SLE and those who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the authors conditionally recommend low-dose aspirin starting in the first trimester.
The guideline suggests that women with RMD should be encouraged to breastfeed if they are willing and able; they also suggest that disease control be maintained through lactation‐compatible medications and that the risks and benefits be reviewed on a patient-by-patient basis. Treatment with HCQ, colchicine, sulfasalazine, rituximab, and all tumor necrosis factor inhibitors are strongly recommended as being compatible with breastfeeding, and they strongly recommend against using CYC, leflunomide, MMF, and thalidomide while breastfeeding.
The authors acknowledged the limitations of their guideline, including the literature review being conducted on studies involving adults and an “inability to include recommendations for uncommon but important clinical situations,” including those involving transgender patients and hormonal therapies.
The authors reported numerous potential conflicts of interest, including receiving research support, consulting fees, speaking fees, and honoraria from various pharmaceutical companies.
SOURCE: Sammaritano LR et al. Arthritis Rheumatol. 2020 Feb 23. doi: 10.1002/art.41191.
A new guideline from the American College of Rheumatology offers the organization’s first clinical recommendations on how to manage reproductive health issues in patients with rheumatic and musculoskeletal diseases (RMDs).
“With the development of this guideline, the ACR recognizes the key role of clinical rheumatologists not only in managing disease activity but also in understanding the interactions of RMDs and their therapies in the context of reproductive health,” wrote Lisa R. Sammaritano, MD, of Weill Cornell Medicine and the Hospital for Special Surgery in New York, and coauthors. The guideline was published in Arthritis & Rheumatology.
To develop an evidence-based guideline on reproductive health in RMD patients, the researchers embarked on a systematic review of studies in areas like contraception, pregnancy and lactation, assisted reproductive technology (ART), fertility preservation, and hormone therapy. The guideline contains 12 ungraded good practice statements and 131 graded recommendations, all developed through the Grading of Recommendations Assessment, Development, and Evaluation methodology.
In counseling patients about these areas of care, the guideline says that rheumatologists and other clinicians “must collaborate with specialists in the fields of obstetrics-gynecology, maternal-fetal medicine, and reproductive endocrinology and infertility.”
“One thing this guideline does well is highlight the importance of involving maternal-fetal medicine colleagues,” Alison Cahill, MD, a professor in the department of women’s health at the University of Texas at Austin and a maternal-fetal medicine specialist within UT Health Austin’s Women’s Health Institute, said when asked for comment on the guideline. “We’re always very happy to see patients ahead of time who are planning pregnancy to be able to discuss what the care plan would look like. And specifically, to address medications, if required, for their rheumatologic care.
“As we learn more and more,” she added, “we’ve come to understand that most treatments and medications are actually safe or relatively safe to take in pregnancy. Certainly, the benefit of taking them outweighs any small or theoretic risks. On the flip side, the guideline does a nice job of highlighting the importance of good disease control, both at the time of conception and during pregnancy.”
Contraception
In regard to contraception, the guideline strongly recommends the use of effective contraceptives – with a conditional recommendation of IUDs or a subdermal progestin implant – in fertile women with a RMD who have neither systemic lupus erythematosus (SLE) nor positive antiphospholipid antibody (aPL). They also strongly recommend discussing the use of emergency contraception with all RMD patients.
For SLE patients, the guideline strongly recommends the use of effective contraceptives in those with stable or low disease activity who are not positive for aPL. They also strongly recommend progestin‐only or IUD contraceptives over combined estrogen‐progestin contraception. For aPL-positive patients, the guideline strongly recommends against combined estrogen‐progestin contraceptives and for levonorgestrel or copper IUDs or the progestin‐only pill.
Assisted reproductive technology
In regard to ART, the guideline strongly recommends proceeding as needed in aPL-negative women with uncomplicated, stable RMD who are on pregnancy‐compatible medications. They also strongly recommend deferring ART in any RMD patients with moderately or severely active disease.
For aPL-positive patients undergoing ART procedures, they strongly recommend prophylactic anticoagulation with heparin or low-molecular-weight heparin (LMWH) in women with obstetric antiphospholipid syndrome (APS) and therapeutic anticoagulation in women with thrombotic APS. In patients undergoing embryo and oocyte cryopreservation, they strongly recommend continuing immunosuppressive and biologic therapies – the exception being cyclophosphamide (CYC) – for anyone in stable condition.
Fertility preservation
In regard to fertility preservation in patients taking CYC, the guideline strongly suggests sperm cryopreservation as good practice prior to treatment. They also conditionally recommend monthly gonadotropin‐releasing hormone agonist cotherapy in premenopausal women with RMD.
Hormone therapy
In regard to menopause and hormone therapy, the guideline strongly suggests hormone therapy as good practice in postmenopausal women with RMD, without SLE or positive aPL, and who have severe vasomotor symptoms. Hormone therapy is conditionally recommended in patients with SLE, without positive aPL, and with no contraindications. For aPL-positive patients, they strongly recommend against hormone therapy in women with obstetric and/or thrombotic APS.
Pregnancy assessment and management
Among the many recommendations regarding pregnancy assessment and management, the guideline strongly suggests counseling women with RMD who are considering pregnancy to take into account the improved outcomes for pregnant women with low disease activity. They strongly recommend that women considering pregnancy should switch to pregnancy‐compatible medication and pause to assess its efficacy and tolerability before moving forward, along with strongly recommending that pregnant women with active disease initiate or continue a pregnancy‐compatible steroid‐sparing medication. They also recommend testing for anti‐Ro/SS-A and anti‐La/SS-B in women with SLE, Sjögren’s syndrome, systemic sclerosis, or rheumatoid arthritis, but only once and only before or early in the pregnancy.
For women with systemic sclerosis who develop scleroderma renal crisis during pregnancy, the authors strongly advise using ACE inhibitors or angiotensin receptor blockers “because the risk of maternal or fetal death with untreated disease is higher than the risk associated with use of these medications during pregnancy.”
Among women with SLE, the recommendations strongly call for testing either before or early in pregnancy for anticardiolipin antibody, anti–beta2-glycoprotein I, or positive lupus anticoagulant, as well as initiating or continuing hydroxychloroquine (HCQ) if possible. Starting in the first trimester, the authors also conditionally recommend that SLE patients take low-dose aspirin daily
For pregnant women who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the guideline conditionally recommends prophylactic treatment with low-dose aspirin daily to protect against preeclampsia. When obstetric APS criteria are met, the guideline strongly advises combined treatment with daily low-dose aspirin and prophylactic-dose heparin (or LMWH), as well as prophylactic-dose anticoagulation for 6-12 weeks post partum. When patients have thrombotic APS, this combination treatment should contain heparin dose at a therapeutic level throughout pregnancy and postpartum. However, the authors conditionally recommend against giving low-dose aspirin plus prophylactic-dose heparin to women without obstetric APS. For refractory obstetric APS, the guideline also contains recommendations that are conditionally against treatment with intravenous immunoglobulin or an increased LMWH dose and strongly against adding prednisone to prophylactic-dose heparin or LMWH and low-dose aspirin. In pregnant patients with primary APS, the authors conditionally advise adding HCQ to prophylactic-dose heparin or LMWH and low-dose aspirin therapy. However, women with aPL who do not meet APS criteria or have another indication for HCQ are conditionally advised against prophylactic treatment with the antimalarial.
For women with Anti-Ro/SS-A and/or anti-La/SS-B antibodies in pregnancy, there is conditional advice to use HCQ. When there is no history of an infant with complete heart block or neonatal lupus erythematosus among women with these antibodies, the guideline conditionally advises serial fetal echocardiography (less often than weekly) starting between 16 and 18 weeks and continuing through 26 weeks, but this should be weekly when there is a prior history. Treatment with oral dexamethasone 4 mg daily is conditionally advised when there is echocardiographic evidence of fetal first- or second-degree heart block, but dexamethasone is not recommended when complete heart block is present.
Finally, in regard to medication use, the authors strongly recommend that men who are planning to be fathers continue on HCQ, azathioprine, 6‐mercaptopurine, colchicine, or tumor necrosis factor inhibitors. Conditional treatment recommendations for men planning for pregnancy include methotrexate, mycophenolate mofetil/mycophenolic acid (MMF), leflunomide, sulfasalazine, calcineurin inhibitors, and NSAIDs. They also strongly recommend that this group of men discontinue CYC and thalidomide.
Pregnant women are strongly recommended to discontinue methotrexate, leflunomide (with cholestyramine washout if there are detectable serum levels of its metabolite prior to pregnancy or as soon as it is confirmed), MMF, CYC, and thalidomide within 3 months prior to conception, and they strongly recommend HCQ (in women with SLE), azathioprine/6‐mercaptopurine, colchicine, or sulfasalazine for use throughout pregnancy. They strongly recommend a combination of low‐dose aspirin and prophylactic‐dose heparin for pregnant women with obstetric APS, along with low‐dose aspirin and therapeutic‐dose heparin for women with thrombotic APS throughout pregnancy and postpartum. However, for women with SLE and those who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the authors conditionally recommend low-dose aspirin starting in the first trimester.
The guideline suggests that women with RMD should be encouraged to breastfeed if they are willing and able; they also suggest that disease control be maintained through lactation‐compatible medications and that the risks and benefits be reviewed on a patient-by-patient basis. Treatment with HCQ, colchicine, sulfasalazine, rituximab, and all tumor necrosis factor inhibitors are strongly recommended as being compatible with breastfeeding, and they strongly recommend against using CYC, leflunomide, MMF, and thalidomide while breastfeeding.
The authors acknowledged the limitations of their guideline, including the literature review being conducted on studies involving adults and an “inability to include recommendations for uncommon but important clinical situations,” including those involving transgender patients and hormonal therapies.
The authors reported numerous potential conflicts of interest, including receiving research support, consulting fees, speaking fees, and honoraria from various pharmaceutical companies.
SOURCE: Sammaritano LR et al. Arthritis Rheumatol. 2020 Feb 23. doi: 10.1002/art.41191.
FROM ARTHRITIS & RHEUMATOLOGY






