User login
Older IBD patients are most at risk of postdischarge VTE
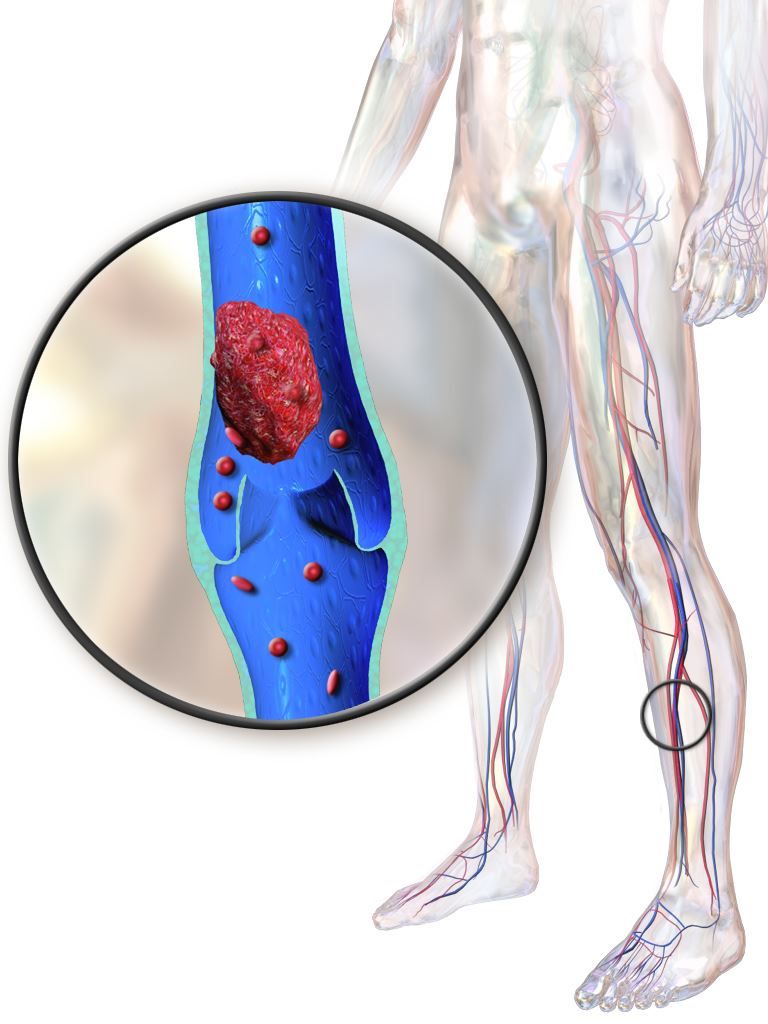
Hospitalized patients with inflammatory bowel diseases (IBD) are most likely to be readmitted for venous thromboembolism (VTE) within 60 days of discharge, according to a new study that analyzed 5 years of U.S. readmissions data.
“Given increased thrombotic risk postdischarge, as well as overall safety of VTE prophylaxis, extending prophylaxis for those at highest risk may have significant benefits,” wrote Adam S. Faye, MD, of Columbia University, and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine which IBD patients would be most in need of postdischarge VTE prophylaxis, as well as when to administer it, the researchers analyzed 2010-2014 data from the Nationwide Readmissions Database (NRD). They found a total of 872,122 index admissions for IBD patients; 4% of those patients had a prior VTE. Of the index admissions, 1,160 led to a VTE readmission within 90 days. Readmitted patients had a relatively equal proportion of ulcerative colitis (n = 522) and Crohn’s disease (n = 638).
More than 90% of VTE readmissions occurred within 60 days of discharge; the risk was highest over the first 10 days and then decreased in each ensuing 10-day period until a slight increase at the 81- to 90-day period. All patients over age 30 had higher rates of readmission than those of patients under age 18, with the highest risk in patients between the ages of 66 and 80 years (risk ratio 4.04; 95% confidence interval, 2.54-6.44, P less than .01). Women were at lower risk (RR 0.82; 95% CI, 0.73-0.92, P less than .01). Higher risks of readmission were also associated with being on Medicare (RR 1.39; 95% CI, 1.23-1.58, P less than .01) compared with being on private insurance and being cared for at a large hospital (RR 1.26; 95% CI, 1.04-1.52, P = .02) compared with a small hospital.
The highest risk of VTE readmission was associated with a prior history of VTE (RR 2.89; 95% CI, 2.40-3.48, P less than .01), having two or more comorbidities (RR 2.57; 95% CI, 2.11-3.12, P less than .01) and having a Clostridioides difficile infection as of index admission (RR 1.90; 95% CI, 1.51-2.38, P less than .01). In addition, increased risk was associated with being discharged to a nursing or care facility (RR 1.85; 95% CI, 1.56-2.20, P less than .01) or home with health services (RR 2.05; 95% CI, 1.78-2.38, P less than .01) compared with a routine discharge.
In their multivariable analysis, similar factors such as a history of VTE (adjusted RR 2.41; 95% CI, 1.99-2.90, P less than .01), two or more comorbidities (aRR 1.78; 95% CI, 1.44-2.20, P less than .01) and C. difficile infection (aRR 1.47; 95% CI, 1.17-1.85, P less than.01) continued to be associated with higher risk of VTE readmission.
Though they emphasized that the use of NRD data offered the impressive ability to “review over 15 million discharges across the U.S. annually,” Dr. Faye and coauthors acknowledged that their study did have limitations. These included the inability to verify via chart review the study’s outcomes and covariates. In addition, they were unable to assess potential contributing risk factors such as medication use, use of VTE prophylaxis during hospitalization, disease severity, and family history. Finally, though unlikely, they admitted the possibility that patients could be counted more than once if they were readmitted with a VTE each year of the study.
The authors reported being supported by grants from the National Institutes of Health and various pharmaceutical companies, as well as receiving honoraria and serving as consultants.
SOURCE: Faye AS et al. Clin Gastroenterol Hepatol. 2019 July 20. doi: 10.1016/j.cgh.2019.07.028.
Hospitalized patients with inflammatory bowel diseases (IBD) are most likely to be readmitted for venous thromboembolism (VTE) within 60 days of discharge, according to a new study that analyzed 5 years of U.S. readmissions data.
“Given increased thrombotic risk postdischarge, as well as overall safety of VTE prophylaxis, extending prophylaxis for those at highest risk may have significant benefits,” wrote Adam S. Faye, MD, of Columbia University, and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine which IBD patients would be most in need of postdischarge VTE prophylaxis, as well as when to administer it, the researchers analyzed 2010-2014 data from the Nationwide Readmissions Database (NRD). They found a total of 872,122 index admissions for IBD patients; 4% of those patients had a prior VTE. Of the index admissions, 1,160 led to a VTE readmission within 90 days. Readmitted patients had a relatively equal proportion of ulcerative colitis (n = 522) and Crohn’s disease (n = 638).
More than 90% of VTE readmissions occurred within 60 days of discharge; the risk was highest over the first 10 days and then decreased in each ensuing 10-day period until a slight increase at the 81- to 90-day period. All patients over age 30 had higher rates of readmission than those of patients under age 18, with the highest risk in patients between the ages of 66 and 80 years (risk ratio 4.04; 95% confidence interval, 2.54-6.44, P less than .01). Women were at lower risk (RR 0.82; 95% CI, 0.73-0.92, P less than .01). Higher risks of readmission were also associated with being on Medicare (RR 1.39; 95% CI, 1.23-1.58, P less than .01) compared with being on private insurance and being cared for at a large hospital (RR 1.26; 95% CI, 1.04-1.52, P = .02) compared with a small hospital.
The highest risk of VTE readmission was associated with a prior history of VTE (RR 2.89; 95% CI, 2.40-3.48, P less than .01), having two or more comorbidities (RR 2.57; 95% CI, 2.11-3.12, P less than .01) and having a Clostridioides difficile infection as of index admission (RR 1.90; 95% CI, 1.51-2.38, P less than .01). In addition, increased risk was associated with being discharged to a nursing or care facility (RR 1.85; 95% CI, 1.56-2.20, P less than .01) or home with health services (RR 2.05; 95% CI, 1.78-2.38, P less than .01) compared with a routine discharge.
In their multivariable analysis, similar factors such as a history of VTE (adjusted RR 2.41; 95% CI, 1.99-2.90, P less than .01), two or more comorbidities (aRR 1.78; 95% CI, 1.44-2.20, P less than .01) and C. difficile infection (aRR 1.47; 95% CI, 1.17-1.85, P less than.01) continued to be associated with higher risk of VTE readmission.
Though they emphasized that the use of NRD data offered the impressive ability to “review over 15 million discharges across the U.S. annually,” Dr. Faye and coauthors acknowledged that their study did have limitations. These included the inability to verify via chart review the study’s outcomes and covariates. In addition, they were unable to assess potential contributing risk factors such as medication use, use of VTE prophylaxis during hospitalization, disease severity, and family history. Finally, though unlikely, they admitted the possibility that patients could be counted more than once if they were readmitted with a VTE each year of the study.
The authors reported being supported by grants from the National Institutes of Health and various pharmaceutical companies, as well as receiving honoraria and serving as consultants.
SOURCE: Faye AS et al. Clin Gastroenterol Hepatol. 2019 July 20. doi: 10.1016/j.cgh.2019.07.028.
Hospitalized patients with inflammatory bowel diseases (IBD) are most likely to be readmitted for venous thromboembolism (VTE) within 60 days of discharge, according to a new study that analyzed 5 years of U.S. readmissions data.
“Given increased thrombotic risk postdischarge, as well as overall safety of VTE prophylaxis, extending prophylaxis for those at highest risk may have significant benefits,” wrote Adam S. Faye, MD, of Columbia University, and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine which IBD patients would be most in need of postdischarge VTE prophylaxis, as well as when to administer it, the researchers analyzed 2010-2014 data from the Nationwide Readmissions Database (NRD). They found a total of 872,122 index admissions for IBD patients; 4% of those patients had a prior VTE. Of the index admissions, 1,160 led to a VTE readmission within 90 days. Readmitted patients had a relatively equal proportion of ulcerative colitis (n = 522) and Crohn’s disease (n = 638).
More than 90% of VTE readmissions occurred within 60 days of discharge; the risk was highest over the first 10 days and then decreased in each ensuing 10-day period until a slight increase at the 81- to 90-day period. All patients over age 30 had higher rates of readmission than those of patients under age 18, with the highest risk in patients between the ages of 66 and 80 years (risk ratio 4.04; 95% confidence interval, 2.54-6.44, P less than .01). Women were at lower risk (RR 0.82; 95% CI, 0.73-0.92, P less than .01). Higher risks of readmission were also associated with being on Medicare (RR 1.39; 95% CI, 1.23-1.58, P less than .01) compared with being on private insurance and being cared for at a large hospital (RR 1.26; 95% CI, 1.04-1.52, P = .02) compared with a small hospital.
The highest risk of VTE readmission was associated with a prior history of VTE (RR 2.89; 95% CI, 2.40-3.48, P less than .01), having two or more comorbidities (RR 2.57; 95% CI, 2.11-3.12, P less than .01) and having a Clostridioides difficile infection as of index admission (RR 1.90; 95% CI, 1.51-2.38, P less than .01). In addition, increased risk was associated with being discharged to a nursing or care facility (RR 1.85; 95% CI, 1.56-2.20, P less than .01) or home with health services (RR 2.05; 95% CI, 1.78-2.38, P less than .01) compared with a routine discharge.
In their multivariable analysis, similar factors such as a history of VTE (adjusted RR 2.41; 95% CI, 1.99-2.90, P less than .01), two or more comorbidities (aRR 1.78; 95% CI, 1.44-2.20, P less than .01) and C. difficile infection (aRR 1.47; 95% CI, 1.17-1.85, P less than.01) continued to be associated with higher risk of VTE readmission.
Though they emphasized that the use of NRD data offered the impressive ability to “review over 15 million discharges across the U.S. annually,” Dr. Faye and coauthors acknowledged that their study did have limitations. These included the inability to verify via chart review the study’s outcomes and covariates. In addition, they were unable to assess potential contributing risk factors such as medication use, use of VTE prophylaxis during hospitalization, disease severity, and family history. Finally, though unlikely, they admitted the possibility that patients could be counted more than once if they were readmitted with a VTE each year of the study.
The authors reported being supported by grants from the National Institutes of Health and various pharmaceutical companies, as well as receiving honoraria and serving as consultants.
SOURCE: Faye AS et al. Clin Gastroenterol Hepatol. 2019 July 20. doi: 10.1016/j.cgh.2019.07.028.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Readmission for VTE in patients with inflammatory bowel diseases most often occurs within 60 days of discharge.
Major finding: The highest readmission risk was in patients between the ages of 66 and 80 (risk ratio 4.04; 95% confidence interval, 2.54-6.44, P less than .01).
Study details: A retrospective cohort study of 1,160 IBD patients who had VTE readmissions via 2010-2014 data from the Nationwide Readmissions Database.
Disclosures: The authors reported being supported by grants from the National Institutes of Health and various pharmaceutical companies, as well as receiving honoraria and serving as consultants.
Source: Faye AS et al. Clin Gastroenterol Hepatol. 2019 July 20. doi: 10.1016/j.cgh.2019.07.028.
Intranasal midazolam as first line for status epilepticus
BANGKOK – Lara Kay, MD, said at the International Epilepsy Congress.
Why? Because status epilepticus is a major medical emergency. It’s associated with substantial morbidity and mortality. And of the various factors that influence outcome in status epilepticus – including age, underlying etiology, and level of consciousness – only one is potentially within physician control: time to treatment, she noted at the congress sponsored by the International League Against Epilepsy.
“Time is brain,” observed Dr. Kay, a neurologist at the epilepsy center at University Hospital Frankfurt.
While intravenous benzodiazepines – for example, lorazepam at 2-4 mg – are widely accepted as the time-honored first-line treatment for status epilepticus, trying to place a line in a patient experiencing this emergency can be a tricky, time-consuming business. Multiple studies have demonstrated that various nonintravenous formulations of benzodiazepines, such as rectal diazepam or buccal or intramuscular midazolam, can be administered much faster and are as effective as intravenous benzodiazepines. But buccal midazolam is quite expensive in Germany, and the ready-to-use intramuscular midazolam applicator that’s available in the United States isn’t marketed in Germany. So several years ago Dr. Kay and her fellow neurologists started having their university hospital pharmacy manufacture intranasal midazolam.
Dr. Kay presented an observational study of 42 consecutive patients with status epilepticus who received intranasal midazolam as first-line treatment. The patients had a mean age of nearly 53 years and 23 were women. The starting dose was 2.5 mg per nostril, moving up to 5 mg per nostril after waiting 5 minutes in initial nonresponders.
Status epilepticus ceased both clinically and by EEG in 24 of the 42 patients, or 57%, in an average of 5 minutes after administration of the intranasal medication at a mean dose of 5.6 mg. Nonresponders received a mean dose of 7.5 mg. There were no significant differences between responders and nonresponders in terms of the proportion presenting with preexisting epilepsy or the epilepsy etiology. However, responders presented at a mean of 54 minutes in status epilepticus, while nonresponders had been in status for 17 minutes.
The 57% response rate with intranasal midazolam is comparable with other investigators’ reported success rates using other benzodiazepines and routes of administration, she noted.
Session cochair Gregory Krauss, MD, commented that he thought the Frankfurt neurologists may have been too cautious in their dosing of intranasal midazolam for status epilepticus.
“Often in the U.S. 5 mg is initially used in each nostril,” according to Dr. Krauss, professor of neurology at Johns Hopkins University, Baltimore.
Dr. Kay reported having no financial conflicts of interest regarding her study.
SOURCE: Kay L et al. IEC 2019, Abstract P029.
BANGKOK – Lara Kay, MD, said at the International Epilepsy Congress.
Why? Because status epilepticus is a major medical emergency. It’s associated with substantial morbidity and mortality. And of the various factors that influence outcome in status epilepticus – including age, underlying etiology, and level of consciousness – only one is potentially within physician control: time to treatment, she noted at the congress sponsored by the International League Against Epilepsy.
“Time is brain,” observed Dr. Kay, a neurologist at the epilepsy center at University Hospital Frankfurt.
While intravenous benzodiazepines – for example, lorazepam at 2-4 mg – are widely accepted as the time-honored first-line treatment for status epilepticus, trying to place a line in a patient experiencing this emergency can be a tricky, time-consuming business. Multiple studies have demonstrated that various nonintravenous formulations of benzodiazepines, such as rectal diazepam or buccal or intramuscular midazolam, can be administered much faster and are as effective as intravenous benzodiazepines. But buccal midazolam is quite expensive in Germany, and the ready-to-use intramuscular midazolam applicator that’s available in the United States isn’t marketed in Germany. So several years ago Dr. Kay and her fellow neurologists started having their university hospital pharmacy manufacture intranasal midazolam.
Dr. Kay presented an observational study of 42 consecutive patients with status epilepticus who received intranasal midazolam as first-line treatment. The patients had a mean age of nearly 53 years and 23 were women. The starting dose was 2.5 mg per nostril, moving up to 5 mg per nostril after waiting 5 minutes in initial nonresponders.
Status epilepticus ceased both clinically and by EEG in 24 of the 42 patients, or 57%, in an average of 5 minutes after administration of the intranasal medication at a mean dose of 5.6 mg. Nonresponders received a mean dose of 7.5 mg. There were no significant differences between responders and nonresponders in terms of the proportion presenting with preexisting epilepsy or the epilepsy etiology. However, responders presented at a mean of 54 minutes in status epilepticus, while nonresponders had been in status for 17 minutes.
The 57% response rate with intranasal midazolam is comparable with other investigators’ reported success rates using other benzodiazepines and routes of administration, she noted.
Session cochair Gregory Krauss, MD, commented that he thought the Frankfurt neurologists may have been too cautious in their dosing of intranasal midazolam for status epilepticus.
“Often in the U.S. 5 mg is initially used in each nostril,” according to Dr. Krauss, professor of neurology at Johns Hopkins University, Baltimore.
Dr. Kay reported having no financial conflicts of interest regarding her study.
SOURCE: Kay L et al. IEC 2019, Abstract P029.
BANGKOK – Lara Kay, MD, said at the International Epilepsy Congress.
Why? Because status epilepticus is a major medical emergency. It’s associated with substantial morbidity and mortality. And of the various factors that influence outcome in status epilepticus – including age, underlying etiology, and level of consciousness – only one is potentially within physician control: time to treatment, she noted at the congress sponsored by the International League Against Epilepsy.
“Time is brain,” observed Dr. Kay, a neurologist at the epilepsy center at University Hospital Frankfurt.
While intravenous benzodiazepines – for example, lorazepam at 2-4 mg – are widely accepted as the time-honored first-line treatment for status epilepticus, trying to place a line in a patient experiencing this emergency can be a tricky, time-consuming business. Multiple studies have demonstrated that various nonintravenous formulations of benzodiazepines, such as rectal diazepam or buccal or intramuscular midazolam, can be administered much faster and are as effective as intravenous benzodiazepines. But buccal midazolam is quite expensive in Germany, and the ready-to-use intramuscular midazolam applicator that’s available in the United States isn’t marketed in Germany. So several years ago Dr. Kay and her fellow neurologists started having their university hospital pharmacy manufacture intranasal midazolam.
Dr. Kay presented an observational study of 42 consecutive patients with status epilepticus who received intranasal midazolam as first-line treatment. The patients had a mean age of nearly 53 years and 23 were women. The starting dose was 2.5 mg per nostril, moving up to 5 mg per nostril after waiting 5 minutes in initial nonresponders.
Status epilepticus ceased both clinically and by EEG in 24 of the 42 patients, or 57%, in an average of 5 minutes after administration of the intranasal medication at a mean dose of 5.6 mg. Nonresponders received a mean dose of 7.5 mg. There were no significant differences between responders and nonresponders in terms of the proportion presenting with preexisting epilepsy or the epilepsy etiology. However, responders presented at a mean of 54 minutes in status epilepticus, while nonresponders had been in status for 17 minutes.
The 57% response rate with intranasal midazolam is comparable with other investigators’ reported success rates using other benzodiazepines and routes of administration, she noted.
Session cochair Gregory Krauss, MD, commented that he thought the Frankfurt neurologists may have been too cautious in their dosing of intranasal midazolam for status epilepticus.
“Often in the U.S. 5 mg is initially used in each nostril,” according to Dr. Krauss, professor of neurology at Johns Hopkins University, Baltimore.
Dr. Kay reported having no financial conflicts of interest regarding her study.
SOURCE: Kay L et al. IEC 2019, Abstract P029.
REPORTING FROM IEC 2019
Is Diagnosis Up in the Air?
ANSWER
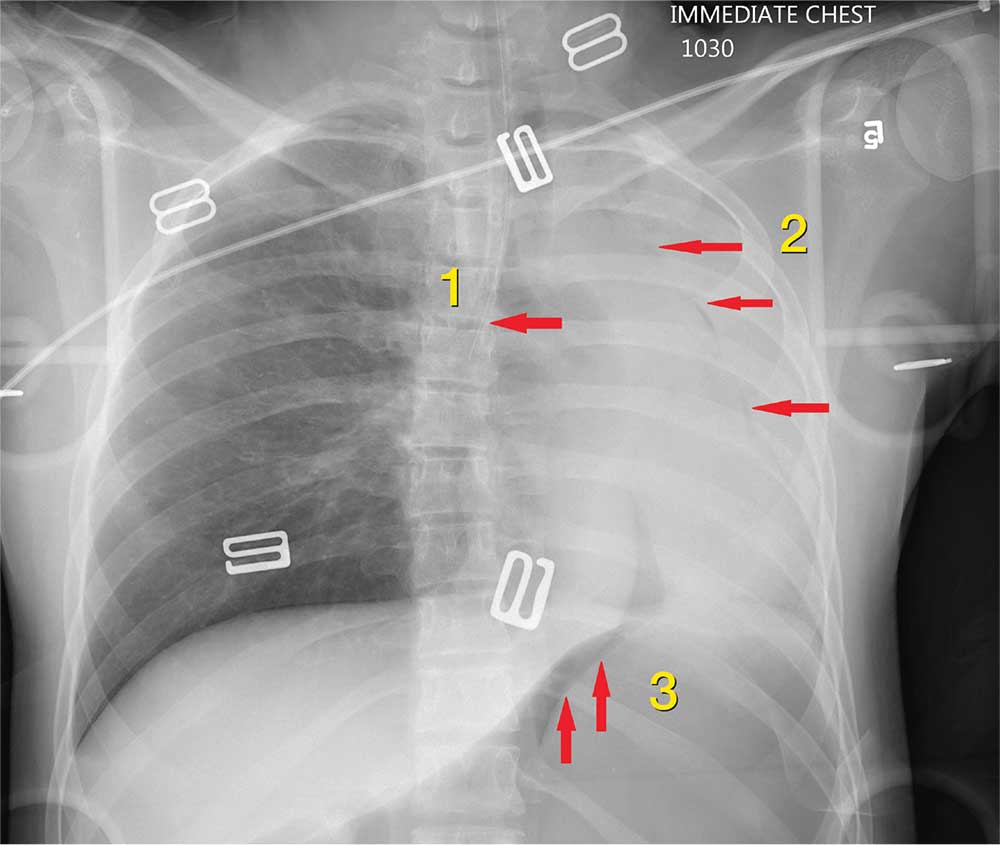
The radiograph shows 3 abnormalities:
(1) The endotracheal tube is in the right mainstem bronchus—a finding that in part leads to
(2) A whiteout of the left lung; the latter is due partly to collapse and atelectasis and partly to a possible pneumothorax.
(3) There is evidence of free air under the left hemidiaphragm, which is concerning for an intra-abdominal injury, such as a perforated viscus.
The patient’s endotracheal tube was partially withdrawn, and a left chest tube was placed. Subsequent CT of the abdomen confirmed the finding of free air. She was then taken to the operating room for an emergent laparotomy.

ANSWER
The radiograph shows 3 abnormalities:
(1) The endotracheal tube is in the right mainstem bronchus—a finding that in part leads to
(2) A whiteout of the left lung; the latter is due partly to collapse and atelectasis and partly to a possible pneumothorax.
(3) There is evidence of free air under the left hemidiaphragm, which is concerning for an intra-abdominal injury, such as a perforated viscus.
The patient’s endotracheal tube was partially withdrawn, and a left chest tube was placed. Subsequent CT of the abdomen confirmed the finding of free air. She was then taken to the operating room for an emergent laparotomy.

ANSWER
The radiograph shows 3 abnormalities:
(1) The endotracheal tube is in the right mainstem bronchus—a finding that in part leads to
(2) A whiteout of the left lung; the latter is due partly to collapse and atelectasis and partly to a possible pneumothorax.
(3) There is evidence of free air under the left hemidiaphragm, which is concerning for an intra-abdominal injury, such as a perforated viscus.
The patient’s endotracheal tube was partially withdrawn, and a left chest tube was placed. Subsequent CT of the abdomen confirmed the finding of free air. She was then taken to the operating room for an emergent laparotomy.
An air ambulance emergently transports a young woman from the scene of a motor vehicle collision to your facility. The details of the accident and age of the patient are unknown. The air ambulance crew had intubated her en route for a decreased level of responsiveness and for airway protection.
The patient is brought to your trauma bay, where you note an intubated female teenager who is unresponsive, with a Glasgow Coma Sc
Her pupils are equal and react bilaterally, albeit sluggishly. Her right leg was placed in an immobilizer by the air ambulance crew because they had noted a right thigh deformity.
Before completing your primary survey, you obtain a portable chest radiograph (shown). What is your impression?
Man, 46, With Wrist Laceration
A right hand–dominant 46-year-old man presents to the emergency department (ED) with a 1-cm laceration of his volar right wrist that occurred after he slipped on a wet floor while carrying a ceramic dish. The patient fell with his hand outstretched and landed on the dish as it broke against the floor. The patient has no pain but complains of tingling in his fingers. Past medical history is negative for diabetes, hypertension, or any neurologic disorders. Social history includes smoking one-half pack of cigarettes per day and drinking 6 to 10 12-oz beers each weekend. He works as a machinist.
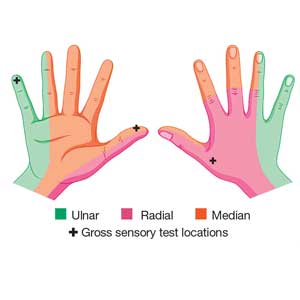
Physical examination shows no bony tenderness. There is a 1.0-cm transverse laceration at the base of the hand at the midline of the volar wrist crease. Flexion, extension, and strength of the fingers are intact, as are dull and sharp discrimination to the thumb and other fingers. A cotton-tip applicator is used for gross sensory testing. No other neuromuscular assessment of the hand is performed. An x-ray of the hand to rule out a fracture or ceramic foreign body is negative.
The wound is locally anesthetized with 1% xylocaine without epinephrine. The laceration is irrigated with normal saline solution and closed with 4-0 nylon sutures using conventional bedside-suturing technique. A sterile bandage is applied. After-care instructions include wound care and follow-up with the patient’s family physician in 1 week for suture removal.
The patient returns to the ED 4 days later, complaining of increased tingling and weakness of the thumb and index and middle fingers. Repeat neuromuscular examination shows decreased sensation and dull/sharp discrimination, and abnormal static 2-point discrimination of the thumb and index and middle fingers. Based on the location of the laceration, the follow-up provider suspects a median nerve injury. After a telephone consultation with a hand surgeon, the patient is told to come into the office in 2 days.
Subsequent follow-up by the hospital’s risk manager indicates that the hand surgeon found a transected median nerve, requiring surgery to repair it. The patient has resulting deficits in sensation and strength and requires extensive occupational therapy. The risk management team learns that the patient intends to file a malpractice suit.
DISCUSSION
Hand and finger injuries represent about 20% of ED visits and are among the most costly injuries for the employed population.1 Knife and glass lacerations of the fingers are most common.2 Failure to diagnose significant hand and finger injuries is also a major contributor to malpractice claims in the ED.3 It is imperative for the PA or NP working in a high-stress/high-volume environment to perform a thorough neuromuscular and vascular examination when encountering a traumatic hand injury or a laceration. This applies to all frontline practices, including urgent care, ED, and primary care and family practices.
Volar surface lacerations of the wrist and fingers are especially high risk.2 Small lacerations (< 2 cm for fingers and < 3 cm for wrist and forearm) may lead a provider to consider the injury minor; however, these have the greatest potential for missed significant deep injuries.2 Missed median nerve lacerations can result in major complications if not surgically repaired soon after the injury.4
Continue to: With our case patient...
With our case patient, a small glass cut at the volar wrist crease did not cause tendon lacerations or flexor deficits. The patient complained only of mild tingling to the fingers, and a detailed hand-and-finger examination was not performed to isolate further nerve injury.4
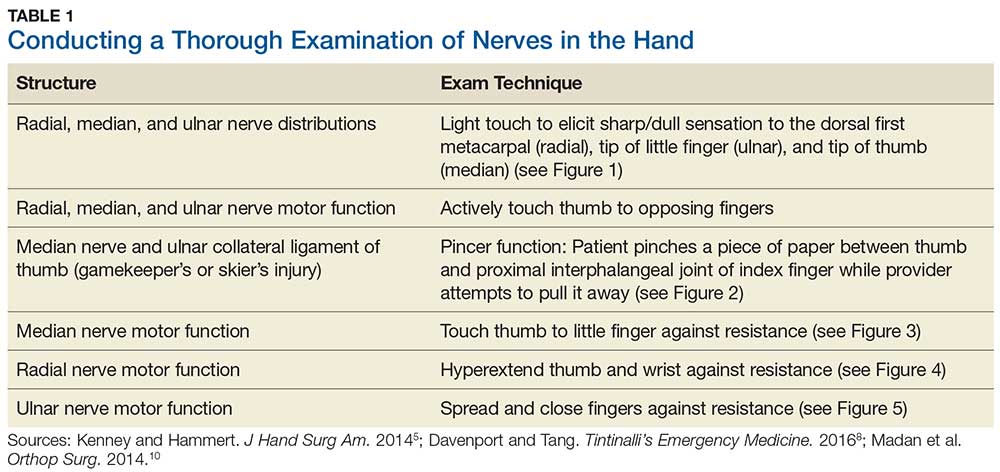
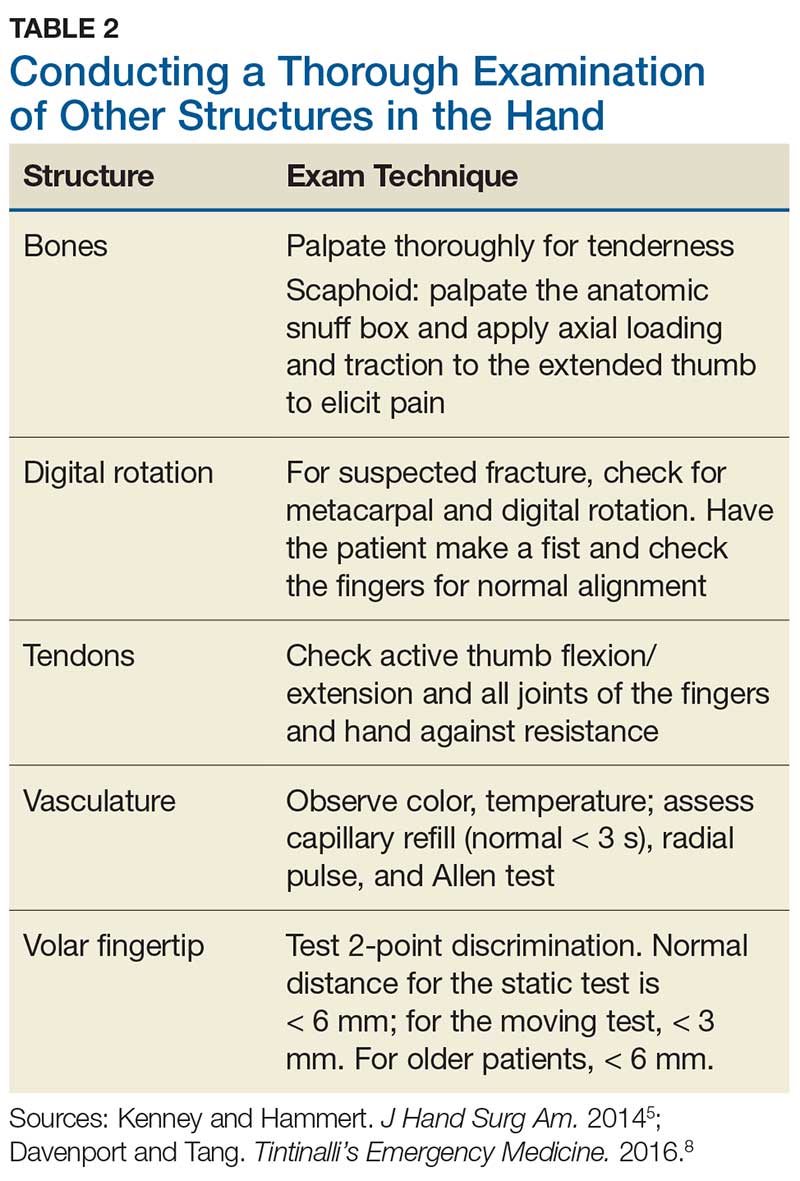
Although most nerve injuries result in a loss in sensory function, motor function must also be evaluated.5 With partial nerve lacerations, subtle loss of motor or sensory function can be missed by the examiner.4 It is imperative to conduct a thorough hand examination (outlined in Tables 1 and 2) to decrease the likelihood of missing a significant nerve or tendon injury.
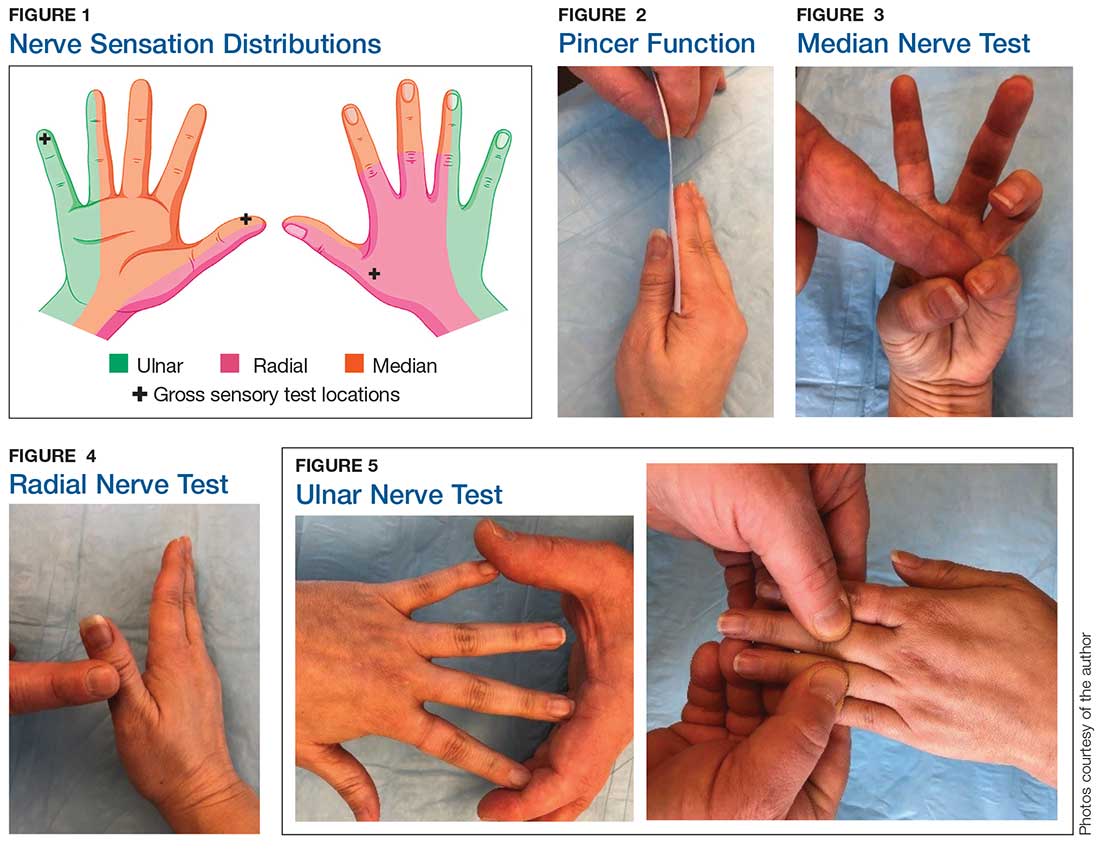
Sensory testing basics
Nerve laceration vs nerve compression disorder. It is important to distinguish sensory testing for a nerve injury or laceration from testing for a nerve compression disorder, such as carpal tunnel syndrome. When examining compression neuropathies, light touch, tuning fork vibration, and monofilament testing are used. When a nerve injury or laceration is suspected, light touch and 2-point discrimination are used.5 Static 2-point discrimination (also known as the Weber static test) will be immediately abnormal if a nerve is lacerated. In a nerve compression disorder, 2-point discrimination is decreased progressively.5
Sensory testing evidence
Comparing light touch, monofilament, and 2-point discrimination. As seen with our case patient, testing dull-sharp discrimination using the cotton-tip applicator for “dull” and the broken end of the wooden applicator stick for “sharp” may not be the most complete way to assess sensation in the hand and fingers. The physical examination should include light touch and 2-point discrimination.5
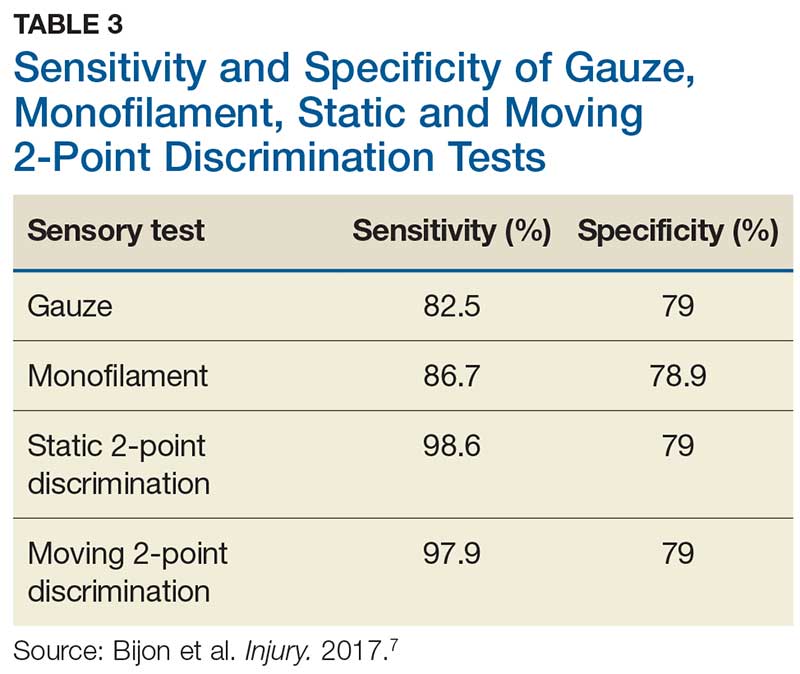
In one study, tests for sensation compared the gauze test (light touch), the static 2-point discrimination, the moving 2-point discrimination (m2PD; also known as the Weber dynamic test),6 and the monofilament test. The static and m2PD tests were statistically superior to the gauze and monofilament tests (see Table 3).7 Two-point discrimination abnormalities are detected immediately after a nerve is lacerated.5 This suggests performing 2-point discrimination, either moving or static, is superior to dull-sensation testing alone (gauze or cotton-tip applicator). This should be included in the motor and sensory examinations of the hand and fingers seen in Tables 1 and 2.
Continue to: Moving 2-point discrimination test
Moving 2-point discrimination test
The m2PD requires a 2-pointed instrument that can maintain a fixed 5 mm of width, such as a bent paperclip or EKG calipers. Commercially available devices specifically for 2-point discrimination can also be used.
When performing the m2PD test, the provider strokes 1 point in the proximal to distal direction in 5-mm increments on the finger and asks whether the patient feels “1 moving point.” The provider then holds 2 points and moves them in the proximal to distal direction in 5-mm increments and asks whether the patient feels “2 moving points.”
The m2PD test is then conducted comparing the ulnar and radial side of the injured finger with the ipsilateral noninjured finger. This should be done at least 4 times.8 The test is positive if there is a ≥ 2-mm difference between the affected and the unaffected side.7
Wound exploration
Data from a French insurance company indicate that 10% of ED malpractice claims in 2013 were related to inadequately examined hand lacerations. In an analysis of these claims, Mouton et al found that most injuries resulting in claims affected the thumb or the volar aspects of the fingers. Reasons for malpractice claims included residual stiffness, weakness, sensory deficit, retained foreign body, and wound infection. The researchers concluded that inadequate examination of hand wounds “carries a risk of lasting and sometimes severe residual impairment, and generates considerable societal costs.”3
In particular, small penetrating lacerations from broken glass or a knife should be considered high-risk injuries.2 In a study of small (< 2 cm) lacerations of the hand and fingers, 59% of the patients were found to have deep-structure injuries.2 Tuncali et al concluded that small lacerations increase the likelihood of missing deeper structural injuries because of failure to examine the wound.2 Furthermore, with glass lacerations, examiners tend to prioritize ruling out a foreign body and then fail to examine the wound. If a careful examination of the hand and fingers prompts suspicion of a tendon or nerve injury, referral to hand surgery for direct surgical exploration is indicated.
Continue to: CONCLUSION
CONCLUSION
Busy health care providers must be aware that approximately 10% to 15% of the negative outcomes in patient care result from diagnostic errors and are most common in the internal medicine, family medicine, and emergency medicine clinical environments.9 With hand and finger lacerations, small size can give a provider a false sense that the laceration is minor, resulting in a failure to diagnose a deeper injury (eg, tendon or nerve).1
When evaluating a traumatic injury or laceration to the hand or fingers, it is important to conduct a thorough sensory and motor examination. Experts recommend light touch and 2-point discrimination be included in the sensory exam to avoid missing nerve injuries. If a deeper structural injury is suspected, the patient should be referred to hand surgery and the wound surgically explored.2
1. Robinson LS, Sarkies M, Brown T, et al. Direct, indirect and intangible costs of acute hand and wrist injuries: a systematic review. Injury. 2016;47:2614-2626.
2. Tuncali D, Yavuz N, Terzioglu A, Aslan G. The rate of upper-extremity deep-structure injuries through small penetrating lacerations. Ann Plast Surg. 2005;55:146-148.
3. Mouton J, Houdre H, Beccari R, et al. Surgical exploration of hand wounds in the emergency room: preliminary study of 80 personal injury claims. Orthop Traumatol Surg Res. 2016;102:1009-1012.
4. Pederson WC. Median nerve injury and repair. J Hand Surg Am. 2014;39(6): 1216-1222.
5. Kenney RJ, Hammert WC. Physical examination of the hand. J Hand Surg Am. 2014;39(11):2324-2334.
6. Dellon AL. The moving two-point discrimination test: clinical evaluation of the quickly adapting fiber/receptor system. J Hand Surg. 1978;3(5):474-481.
7. Bijon C, Hidalgo-Diaz JJ, Chiara P, et al. Nerve injuries to the volar aspect of the hand: a comparison of the reliability of the Weber static test versus the gauze test. Injury. 2017;48:2582-2585.
8. Davenport M, Tang P. Injuries to the hand and digits. In: Tintinalli JE, Stapczynski J, Ma OJ, et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016:1667.
9. Croskerry P, Nimmo GR. Better clinical decision making and reducing diagnostic error. J R Coll Physicians Edinb. 2011;41:155-162.
10. Madan SS, Pai DR, Kaur A, Dixit R. Injury to the ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7.
A right hand–dominant 46-year-old man presents to the emergency department (ED) with a 1-cm laceration of his volar right wrist that occurred after he slipped on a wet floor while carrying a ceramic dish. The patient fell with his hand outstretched and landed on the dish as it broke against the floor. The patient has no pain but complains of tingling in his fingers. Past medical history is negative for diabetes, hypertension, or any neurologic disorders. Social history includes smoking one-half pack of cigarettes per day and drinking 6 to 10 12-oz beers each weekend. He works as a machinist.
Physical examination shows no bony tenderness. There is a 1.0-cm transverse laceration at the base of the hand at the midline of the volar wrist crease. Flexion, extension, and strength of the fingers are intact, as are dull and sharp discrimination to the thumb and other fingers. A cotton-tip applicator is used for gross sensory testing. No other neuromuscular assessment of the hand is performed. An x-ray of the hand to rule out a fracture or ceramic foreign body is negative.
The wound is locally anesthetized with 1% xylocaine without epinephrine. The laceration is irrigated with normal saline solution and closed with 4-0 nylon sutures using conventional bedside-suturing technique. A sterile bandage is applied. After-care instructions include wound care and follow-up with the patient’s family physician in 1 week for suture removal.
The patient returns to the ED 4 days later, complaining of increased tingling and weakness of the thumb and index and middle fingers. Repeat neuromuscular examination shows decreased sensation and dull/sharp discrimination, and abnormal static 2-point discrimination of the thumb and index and middle fingers. Based on the location of the laceration, the follow-up provider suspects a median nerve injury. After a telephone consultation with a hand surgeon, the patient is told to come into the office in 2 days.
Subsequent follow-up by the hospital’s risk manager indicates that the hand surgeon found a transected median nerve, requiring surgery to repair it. The patient has resulting deficits in sensation and strength and requires extensive occupational therapy. The risk management team learns that the patient intends to file a malpractice suit.
DISCUSSION
Hand and finger injuries represent about 20% of ED visits and are among the most costly injuries for the employed population.1 Knife and glass lacerations of the fingers are most common.2 Failure to diagnose significant hand and finger injuries is also a major contributor to malpractice claims in the ED.3 It is imperative for the PA or NP working in a high-stress/high-volume environment to perform a thorough neuromuscular and vascular examination when encountering a traumatic hand injury or a laceration. This applies to all frontline practices, including urgent care, ED, and primary care and family practices.
Volar surface lacerations of the wrist and fingers are especially high risk.2 Small lacerations (< 2 cm for fingers and < 3 cm for wrist and forearm) may lead a provider to consider the injury minor; however, these have the greatest potential for missed significant deep injuries.2 Missed median nerve lacerations can result in major complications if not surgically repaired soon after the injury.4
Continue to: With our case patient...
With our case patient, a small glass cut at the volar wrist crease did not cause tendon lacerations or flexor deficits. The patient complained only of mild tingling to the fingers, and a detailed hand-and-finger examination was not performed to isolate further nerve injury.4

Although most nerve injuries result in a loss in sensory function, motor function must also be evaluated.5 With partial nerve lacerations, subtle loss of motor or sensory function can be missed by the examiner.4 It is imperative to conduct a thorough hand examination (outlined in Tables 1 and 2) to decrease the likelihood of missing a significant nerve or tendon injury.

Sensory testing basics
Nerve laceration vs nerve compression disorder. It is important to distinguish sensory testing for a nerve injury or laceration from testing for a nerve compression disorder, such as carpal tunnel syndrome. When examining compression neuropathies, light touch, tuning fork vibration, and monofilament testing are used. When a nerve injury or laceration is suspected, light touch and 2-point discrimination are used.5 Static 2-point discrimination (also known as the Weber static test) will be immediately abnormal if a nerve is lacerated. In a nerve compression disorder, 2-point discrimination is decreased progressively.5

Sensory testing evidence
Comparing light touch, monofilament, and 2-point discrimination. As seen with our case patient, testing dull-sharp discrimination using the cotton-tip applicator for “dull” and the broken end of the wooden applicator stick for “sharp” may not be the most complete way to assess sensation in the hand and fingers. The physical examination should include light touch and 2-point discrimination.5
In one study, tests for sensation compared the gauze test (light touch), the static 2-point discrimination, the moving 2-point discrimination (m2PD; also known as the Weber dynamic test),6 and the monofilament test. The static and m2PD tests were statistically superior to the gauze and monofilament tests (see Table 3).7 Two-point discrimination abnormalities are detected immediately after a nerve is lacerated.5 This suggests performing 2-point discrimination, either moving or static, is superior to dull-sensation testing alone (gauze or cotton-tip applicator). This should be included in the motor and sensory examinations of the hand and fingers seen in Tables 1 and 2.

Continue to: Moving 2-point discrimination test
Moving 2-point discrimination test
The m2PD requires a 2-pointed instrument that can maintain a fixed 5 mm of width, such as a bent paperclip or EKG calipers. Commercially available devices specifically for 2-point discrimination can also be used.
When performing the m2PD test, the provider strokes 1 point in the proximal to distal direction in 5-mm increments on the finger and asks whether the patient feels “1 moving point.” The provider then holds 2 points and moves them in the proximal to distal direction in 5-mm increments and asks whether the patient feels “2 moving points.”
The m2PD test is then conducted comparing the ulnar and radial side of the injured finger with the ipsilateral noninjured finger. This should be done at least 4 times.8 The test is positive if there is a ≥ 2-mm difference between the affected and the unaffected side.7
Wound exploration
Data from a French insurance company indicate that 10% of ED malpractice claims in 2013 were related to inadequately examined hand lacerations. In an analysis of these claims, Mouton et al found that most injuries resulting in claims affected the thumb or the volar aspects of the fingers. Reasons for malpractice claims included residual stiffness, weakness, sensory deficit, retained foreign body, and wound infection. The researchers concluded that inadequate examination of hand wounds “carries a risk of lasting and sometimes severe residual impairment, and generates considerable societal costs.”3
In particular, small penetrating lacerations from broken glass or a knife should be considered high-risk injuries.2 In a study of small (< 2 cm) lacerations of the hand and fingers, 59% of the patients were found to have deep-structure injuries.2 Tuncali et al concluded that small lacerations increase the likelihood of missing deeper structural injuries because of failure to examine the wound.2 Furthermore, with glass lacerations, examiners tend to prioritize ruling out a foreign body and then fail to examine the wound. If a careful examination of the hand and fingers prompts suspicion of a tendon or nerve injury, referral to hand surgery for direct surgical exploration is indicated.
Continue to: CONCLUSION
CONCLUSION
Busy health care providers must be aware that approximately 10% to 15% of the negative outcomes in patient care result from diagnostic errors and are most common in the internal medicine, family medicine, and emergency medicine clinical environments.9 With hand and finger lacerations, small size can give a provider a false sense that the laceration is minor, resulting in a failure to diagnose a deeper injury (eg, tendon or nerve).1
When evaluating a traumatic injury or laceration to the hand or fingers, it is important to conduct a thorough sensory and motor examination. Experts recommend light touch and 2-point discrimination be included in the sensory exam to avoid missing nerve injuries. If a deeper structural injury is suspected, the patient should be referred to hand surgery and the wound surgically explored.2
A right hand–dominant 46-year-old man presents to the emergency department (ED) with a 1-cm laceration of his volar right wrist that occurred after he slipped on a wet floor while carrying a ceramic dish. The patient fell with his hand outstretched and landed on the dish as it broke against the floor. The patient has no pain but complains of tingling in his fingers. Past medical history is negative for diabetes, hypertension, or any neurologic disorders. Social history includes smoking one-half pack of cigarettes per day and drinking 6 to 10 12-oz beers each weekend. He works as a machinist.
Physical examination shows no bony tenderness. There is a 1.0-cm transverse laceration at the base of the hand at the midline of the volar wrist crease. Flexion, extension, and strength of the fingers are intact, as are dull and sharp discrimination to the thumb and other fingers. A cotton-tip applicator is used for gross sensory testing. No other neuromuscular assessment of the hand is performed. An x-ray of the hand to rule out a fracture or ceramic foreign body is negative.
The wound is locally anesthetized with 1% xylocaine without epinephrine. The laceration is irrigated with normal saline solution and closed with 4-0 nylon sutures using conventional bedside-suturing technique. A sterile bandage is applied. After-care instructions include wound care and follow-up with the patient’s family physician in 1 week for suture removal.
The patient returns to the ED 4 days later, complaining of increased tingling and weakness of the thumb and index and middle fingers. Repeat neuromuscular examination shows decreased sensation and dull/sharp discrimination, and abnormal static 2-point discrimination of the thumb and index and middle fingers. Based on the location of the laceration, the follow-up provider suspects a median nerve injury. After a telephone consultation with a hand surgeon, the patient is told to come into the office in 2 days.
Subsequent follow-up by the hospital’s risk manager indicates that the hand surgeon found a transected median nerve, requiring surgery to repair it. The patient has resulting deficits in sensation and strength and requires extensive occupational therapy. The risk management team learns that the patient intends to file a malpractice suit.
DISCUSSION
Hand and finger injuries represent about 20% of ED visits and are among the most costly injuries for the employed population.1 Knife and glass lacerations of the fingers are most common.2 Failure to diagnose significant hand and finger injuries is also a major contributor to malpractice claims in the ED.3 It is imperative for the PA or NP working in a high-stress/high-volume environment to perform a thorough neuromuscular and vascular examination when encountering a traumatic hand injury or a laceration. This applies to all frontline practices, including urgent care, ED, and primary care and family practices.
Volar surface lacerations of the wrist and fingers are especially high risk.2 Small lacerations (< 2 cm for fingers and < 3 cm for wrist and forearm) may lead a provider to consider the injury minor; however, these have the greatest potential for missed significant deep injuries.2 Missed median nerve lacerations can result in major complications if not surgically repaired soon after the injury.4
Continue to: With our case patient...
With our case patient, a small glass cut at the volar wrist crease did not cause tendon lacerations or flexor deficits. The patient complained only of mild tingling to the fingers, and a detailed hand-and-finger examination was not performed to isolate further nerve injury.4

Although most nerve injuries result in a loss in sensory function, motor function must also be evaluated.5 With partial nerve lacerations, subtle loss of motor or sensory function can be missed by the examiner.4 It is imperative to conduct a thorough hand examination (outlined in Tables 1 and 2) to decrease the likelihood of missing a significant nerve or tendon injury.

Sensory testing basics
Nerve laceration vs nerve compression disorder. It is important to distinguish sensory testing for a nerve injury or laceration from testing for a nerve compression disorder, such as carpal tunnel syndrome. When examining compression neuropathies, light touch, tuning fork vibration, and monofilament testing are used. When a nerve injury or laceration is suspected, light touch and 2-point discrimination are used.5 Static 2-point discrimination (also known as the Weber static test) will be immediately abnormal if a nerve is lacerated. In a nerve compression disorder, 2-point discrimination is decreased progressively.5

Sensory testing evidence
Comparing light touch, monofilament, and 2-point discrimination. As seen with our case patient, testing dull-sharp discrimination using the cotton-tip applicator for “dull” and the broken end of the wooden applicator stick for “sharp” may not be the most complete way to assess sensation in the hand and fingers. The physical examination should include light touch and 2-point discrimination.5
In one study, tests for sensation compared the gauze test (light touch), the static 2-point discrimination, the moving 2-point discrimination (m2PD; also known as the Weber dynamic test),6 and the monofilament test. The static and m2PD tests were statistically superior to the gauze and monofilament tests (see Table 3).7 Two-point discrimination abnormalities are detected immediately after a nerve is lacerated.5 This suggests performing 2-point discrimination, either moving or static, is superior to dull-sensation testing alone (gauze or cotton-tip applicator). This should be included in the motor and sensory examinations of the hand and fingers seen in Tables 1 and 2.

Continue to: Moving 2-point discrimination test
Moving 2-point discrimination test
The m2PD requires a 2-pointed instrument that can maintain a fixed 5 mm of width, such as a bent paperclip or EKG calipers. Commercially available devices specifically for 2-point discrimination can also be used.
When performing the m2PD test, the provider strokes 1 point in the proximal to distal direction in 5-mm increments on the finger and asks whether the patient feels “1 moving point.” The provider then holds 2 points and moves them in the proximal to distal direction in 5-mm increments and asks whether the patient feels “2 moving points.”
The m2PD test is then conducted comparing the ulnar and radial side of the injured finger with the ipsilateral noninjured finger. This should be done at least 4 times.8 The test is positive if there is a ≥ 2-mm difference between the affected and the unaffected side.7
Wound exploration
Data from a French insurance company indicate that 10% of ED malpractice claims in 2013 were related to inadequately examined hand lacerations. In an analysis of these claims, Mouton et al found that most injuries resulting in claims affected the thumb or the volar aspects of the fingers. Reasons for malpractice claims included residual stiffness, weakness, sensory deficit, retained foreign body, and wound infection. The researchers concluded that inadequate examination of hand wounds “carries a risk of lasting and sometimes severe residual impairment, and generates considerable societal costs.”3
In particular, small penetrating lacerations from broken glass or a knife should be considered high-risk injuries.2 In a study of small (< 2 cm) lacerations of the hand and fingers, 59% of the patients were found to have deep-structure injuries.2 Tuncali et al concluded that small lacerations increase the likelihood of missing deeper structural injuries because of failure to examine the wound.2 Furthermore, with glass lacerations, examiners tend to prioritize ruling out a foreign body and then fail to examine the wound. If a careful examination of the hand and fingers prompts suspicion of a tendon or nerve injury, referral to hand surgery for direct surgical exploration is indicated.
Continue to: CONCLUSION
CONCLUSION
Busy health care providers must be aware that approximately 10% to 15% of the negative outcomes in patient care result from diagnostic errors and are most common in the internal medicine, family medicine, and emergency medicine clinical environments.9 With hand and finger lacerations, small size can give a provider a false sense that the laceration is minor, resulting in a failure to diagnose a deeper injury (eg, tendon or nerve).1
When evaluating a traumatic injury or laceration to the hand or fingers, it is important to conduct a thorough sensory and motor examination. Experts recommend light touch and 2-point discrimination be included in the sensory exam to avoid missing nerve injuries. If a deeper structural injury is suspected, the patient should be referred to hand surgery and the wound surgically explored.2
1. Robinson LS, Sarkies M, Brown T, et al. Direct, indirect and intangible costs of acute hand and wrist injuries: a systematic review. Injury. 2016;47:2614-2626.
2. Tuncali D, Yavuz N, Terzioglu A, Aslan G. The rate of upper-extremity deep-structure injuries through small penetrating lacerations. Ann Plast Surg. 2005;55:146-148.
3. Mouton J, Houdre H, Beccari R, et al. Surgical exploration of hand wounds in the emergency room: preliminary study of 80 personal injury claims. Orthop Traumatol Surg Res. 2016;102:1009-1012.
4. Pederson WC. Median nerve injury and repair. J Hand Surg Am. 2014;39(6): 1216-1222.
5. Kenney RJ, Hammert WC. Physical examination of the hand. J Hand Surg Am. 2014;39(11):2324-2334.
6. Dellon AL. The moving two-point discrimination test: clinical evaluation of the quickly adapting fiber/receptor system. J Hand Surg. 1978;3(5):474-481.
7. Bijon C, Hidalgo-Diaz JJ, Chiara P, et al. Nerve injuries to the volar aspect of the hand: a comparison of the reliability of the Weber static test versus the gauze test. Injury. 2017;48:2582-2585.
8. Davenport M, Tang P. Injuries to the hand and digits. In: Tintinalli JE, Stapczynski J, Ma OJ, et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016:1667.
9. Croskerry P, Nimmo GR. Better clinical decision making and reducing diagnostic error. J R Coll Physicians Edinb. 2011;41:155-162.
10. Madan SS, Pai DR, Kaur A, Dixit R. Injury to the ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7.
1. Robinson LS, Sarkies M, Brown T, et al. Direct, indirect and intangible costs of acute hand and wrist injuries: a systematic review. Injury. 2016;47:2614-2626.
2. Tuncali D, Yavuz N, Terzioglu A, Aslan G. The rate of upper-extremity deep-structure injuries through small penetrating lacerations. Ann Plast Surg. 2005;55:146-148.
3. Mouton J, Houdre H, Beccari R, et al. Surgical exploration of hand wounds in the emergency room: preliminary study of 80 personal injury claims. Orthop Traumatol Surg Res. 2016;102:1009-1012.
4. Pederson WC. Median nerve injury and repair. J Hand Surg Am. 2014;39(6): 1216-1222.
5. Kenney RJ, Hammert WC. Physical examination of the hand. J Hand Surg Am. 2014;39(11):2324-2334.
6. Dellon AL. The moving two-point discrimination test: clinical evaluation of the quickly adapting fiber/receptor system. J Hand Surg. 1978;3(5):474-481.
7. Bijon C, Hidalgo-Diaz JJ, Chiara P, et al. Nerve injuries to the volar aspect of the hand: a comparison of the reliability of the Weber static test versus the gauze test. Injury. 2017;48:2582-2585.
8. Davenport M, Tang P. Injuries to the hand and digits. In: Tintinalli JE, Stapczynski J, Ma OJ, et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016:1667.
9. Croskerry P, Nimmo GR. Better clinical decision making and reducing diagnostic error. J R Coll Physicians Edinb. 2011;41:155-162.
10. Madan SS, Pai DR, Kaur A, Dixit R. Injury to the ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7.
There’s Mischief Afoot

ANSWER
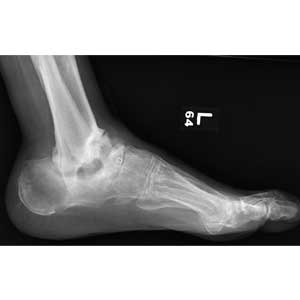
The radiograph demonstrates no evidence of an acute fracture or soft-tissue gas to suggest an abscess. Of note, though, within the tibiotalar joint, the patient has bony destruction and settling of the articular surfaces of both the distal tibia and fibula into the talus and calcaneus.
This finding is typically associated with neuropathic arthropathy (also known as a Charcot joint). This pathologic process is typically seen in a weight-bearing joint that develops progressive degeneration from chronic loss of sensation.

ANSWER
The radiograph demonstrates no evidence of an acute fracture or soft-tissue gas to suggest an abscess. Of note, though, within the tibiotalar joint, the patient has bony destruction and settling of the articular surfaces of both the distal tibia and fibula into the talus and calcaneus.
This finding is typically associated with neuropathic arthropathy (also known as a Charcot joint). This pathologic process is typically seen in a weight-bearing joint that develops progressive degeneration from chronic loss of sensation.

ANSWER
The radiograph demonstrates no evidence of an acute fracture or soft-tissue gas to suggest an abscess. Of note, though, within the tibiotalar joint, the patient has bony destruction and settling of the articular surfaces of both the distal tibia and fibula into the talus and calcaneus.
This finding is typically associated with neuropathic arthropathy (also known as a Charcot joint). This pathologic process is typically seen in a weight-bearing joint that develops progressive degeneration from chronic loss of sensation.

A 70-year-old man presents for evaluation of left foot pain, redness, and swelling. He reports injuring the foot a week ago; he went to the emergency department for evaluation of the cut he had sustained, which required stapling.
The patient has a chronic foot ulcer for which a home health aide provides wound care and dressing changes. His medical history is significant for hypertension, stroke with chronic left-sided weakness, congestive heart failure, and chronic renal insufficiency. He admits to daily tobacco use, and his medical record reflects a history of drug use.
On physical exam, you note an elderly, chronically ill male in no obvious distress. His vital signs are stable, and he is afebrile. Inspection of his left foot shows generalized swelling and redness. Good distal pulses are appreciated. On the dorsal aspect, there is a healing wound with a single staple present. On the heel is a 2-cm stage 2 ulcer with some scant purulent drainage.
Bloodwork and a radiograph of the left foot are ordered; lateral view is shown. What is your impression?
Giant cell arteritis: An updated review of an old disease
Giant cell arteritis (GCA) is a systemic vasculitis involving medium-sized and large arteries, most commonly the temporal, ophthalmic, occipital, vertebral, posterior ciliary, and proximal vertebral arteries. Moreover, involvement of the ophthalmic artery and its branches results in loss of vision. GCA can also involve the aorta and its proximal branches, especially in the upper extremities.
GCA is the most common systemic vasculitis in adults. It occurs almost exclusively in patients over age 50 and affects women more than men. It is most frequent in populations of northern European ancestry, especially Scandinavian. In a retrospective cohort study in Norway, the average annual cumulative incidence rate of GCA was 16.7 per 100,000 people over age 50.1 Risk factors include older age, history of smoking, current smoking, early menopause, and, possibly, stress-related disorders.2
PATHOGENESIS IS NOT COMPLETELY UNDERSTOOD
The pathogenesis of GCA is not completely understood, but there is evidence of immune activation in the arterial wall leading to activation of macrophages and formation of multinucleated giant cells (which may not always be present in biopsies).
The most relevant cytokines in the ongoing pathogenesis are still being defined, but the presence of interferon gamma and interleukin 6 (IL-6) seem to be critical for the expression of the disease. The primary immunogenic triggers for the elaboration of these cytokines and the arteritis remain elusive.
A SPECTRUM OF PRESENTATIONS
The initial symptoms of GCA may be vague, such as malaise, fever, and night sweats, and are likely due to systemic inflammation. Features of vascular involvement include headache, scalp tenderness, and jaw claudication (cramping pain in the jaw while chewing).
A less common but serious feature associated with GCA is partial or complete vision loss affecting 1 or both eyes.3 Some patients suddenly go completely blind without any visual prodrome.
Overlapping GCA phenotypes exist, with a spectrum of presentations that include classic cranial arteritis, extracranial GCA (also called large-vessel GCA), and polymyalgia rheumatica.2
Cranial GCA, the best-characterized clinical presentation, causes symptoms such as headache or signs such as tenderness of the temporal artery. On examination, the temporal arteries may be tender or nodular, and the pulses may be felt above the zygomatic arch, above and in front of the tragus of the ear. About two-thirds of patients with cranial GCA present with new-onset headache, most often in the temporal area, but possibly anywhere throughout the head.
Visual disturbance, jaw claudication, and tongue pain are less common but, if present, increase the likelihood of this diagnosis.2
Large-vessel involvement in GCA is common and refers to involvement of the aorta and its proximal branches. Imaging methods used in diagnosing large-vessel GCA include color Doppler ultrasonography, computed tomography with angiography, magnetic resonance imaging with angiography, and positron emission tomography. In some centers, such imaging is performed in all patients diagnosed with GCA to survey for large-vessel involvement.
Depending on the imaging study, large-vessel involvement has been found in 30% to 80% of cases of GCA.4,5 It is often associated with nonspecific symptoms such as fever, weight loss, chills, and malaise, but it can also cause more specific symptoms such as unilateral extremity claudication. In contrast to patients with cranial GCA, patients with large-vessel GCA were younger at onset, less likely to have headaches, and more likely to have arm claudication at presentation.6 Aortitis of the ascending aorta can occur with a histopathologic pattern of GCA but without the clinical stigmata of GCA.
The finding of aortitis should prompt the clinician to question the patient about other symptoms of GCA and to order imaging of the whole vascular tree. Ultrasonography and biopsy of the temporal arteries can be considered. Whether idiopathic aortitis is part of the GCA spectrum remains to be seen.
Laboratory tests often show anemia, leukocytosis, and thrombocytosis. Acute-phase reactants such as C-reactive protein and the erythrocyte sedimentation rate are often elevated. The sedimentation rate often exceeds 50 mm/hour and sometimes 100 mm/hour.
In 2 retrospective studies, the number of patients with GCA whose sedimentation rate was less than 50 mm/hour ranged between 5% and 11%.7,8 However, a small percentage of patients with GCA have normal inflammatory markers. Therefore, if the suspicion for GCA is high, treatment should be started and biopsy pursued.9 In patients with paraproteinemia or other causes of a spuriously elevated or low erythrocyte sedimentation rate, C-reactive protein is a more reliable test.
Polymyalgia rheumatica is another rheumatologic condition that can occur independently or in conjunction with GCA. It is characterized by stiffness and pain in the proximal joints such as the hips and shoulders, typically worse in the morning and better with activity. Although the patient may subjectively feel weak, a close neurologic examination will reveal normal muscle strength.
Polymyalgia rheumatica is observed in 40% to 60% of patients with GCA at the time of diagnosis; 16% to 21% of patients with polymyalgia rheumatica may develop GCA, especially if untreated.2,10
Differential diagnosis
Other vasculitides (eg, Takayasu arteritis) can also present with unexplained fever, anemia, and constitutional symptoms.
Infection should be considered if fever is present. An infectious disease accompanied by fever, headache, and elevated inflammatory markers can mimic GCA.
Nonarteritic anterior ischemic optic neuropathy can present with sudden vision loss, prompting concern for underlying GCA. Risk factors include hypertension and diabetes mellitus; other features of GCA, including elevated inflammatory markers, are generally absent.
TEMPORAL ARTERY BIOPSY: THE GOLD STANDARD FOR DIAGNOSIS
Temporal artery biopsy remains the standard to confirm the diagnosis. However, because inflammation in the temporal arteries can affect some segments but not others, biopsy results on conventional hematoxylin and eosin staining can be falsely negative in patients with GCA. In one study,11 the mean sensitivity of unilateral temporal artery biopsy was 86.9%.
Typical positive histologic findings are inflammation with panarteritis, CD4-positive lymphocytes, macrophages, giant cells, and fragmentation of the internal elastic lamina.12
When GCA is suspected, treatment with glucocorticoids should be started immediately and biopsy performed as soon as possible. Delaying biopsy for 14 days or more may not affect the accuracy of biopsy study.13 Treatment should never be withheld while awaiting the results of biopsy study.
Biopsy is usually performed unilaterally, on the same side as the symptoms or abnormal findings on examination. Bilateral temporal artery biopsy is also performed and compared with unilateral biopsy; this approach increases the diagnostic yield by about 5%.14
IMAGING
In patients with suspected GCA, imaging is recommended early to complement the clinical criteria for the diagnosis of GCA.15 Positron emission tomography, computed tomography angiography, magnetic resonance angiography, or Doppler ultrasonography can reveal inflammation of the arteries in the proximal upper or lower limbs or the aorta.2
In patients with suspected cranial GCA, ultrasonography of the temporal and axillary arteries is recommended first. If ultrasonography is not available or is inconclusive, high-resolution magnetic resonance imaging of the cranial arteries can be used as an alternative. Computed tomography and positron emission tomography of the cranial arteries are not recommended.
In patients with suspected large-vessel GCA, ultrasonography, positron emission tomography, computed tomography, and magnetic resonance imaging may be used to screen for vessel wall inflammation, edema, and luminal narrowing in extracranial arteries. Ultrasonography is of limited value in assessing aortitis.
Color duplex ultrasonography can be applied to assess for vascular inflammation of the temporal or large arteries. The typical finding of the “halo” sign, a hypoechoic ring around the arterial lumen, represents the inflammation-induced thickening of the arterial wall. The “compression sign,” the persistence of the “halo” during compression of the vessel lumen by the ultrasound probe, has high specificity for the diagnosis.16
Ultrasonography of suspected GCA has yielded sensitivities of 55% to 100% and specificities of 78% to 100%. However, its sensitivity depends on the user’s level of expertise, so it should be done only in medical centers with a high number of GCA cases and with highly experienced sonographers. High-resolution magnetic resonance imaging is an alternative to ultrasonography and has shown similar sensitivity and specificity.3
TREATMENT WITH GLUCOCORTICOIDS
Glucocorticoids remain the standard for treatment of GCA. The therapeutic effect of glucocorticoids in GCA has been established by years of clinical experience, but has never been proven in a placebo-controlled trial. When started appropriately and expeditiously, glucocorticoids produce exquisite resolution of signs and symptoms and prevent the serious complication of vision loss. Rapid resolution of symptoms is so typical of GCA that if the patient’s symptoms persist more than a few days after starting a glucocorticoid, the diagnosis of GCA should be reconsidered.
In a retrospective study of 245 patients with biopsy-proven GCA treated with glucocorticoids, 34 had permanent loss of sight.17 In 32 (94%) of the 34, the vision loss occurred before glucocorticoids were started. Of the remaining 2 patients, 1 lost vision 8 days into treatment, and the other lost vision 3 years after diagnosis and 1 year after discontinuation of glucocorticoids.
In a series of 144 patients with biopsy-proven GCA, 51 had no vision loss at presentation and no vision loss after starting glucocorticoids, and 93 had vision loss at presentation. In the latter group, symptoms worsened within 5 days of starting glucocorticoids in 9 patients.18 If vision was intact at the time of presentation, prompt initiation of glucocorticoids reduced the risk of vision loss to less than 1%.
High doses, slowly tapered
The European League Against Rheumatism recommends early initiation of high-dose glucocorticoids for patients with large-vessel vasculitis,19 and it also recommends glucocorticoids for patients with polymyalgia rheumatica.20 The optimal initial and tapering dosage has never been formally evaluated, but regimens have been devised on the basis of expert opinion.21
For patients with GCA who do not have vision loss at the time of diagnosis, the initial dose is prednisone 1 mg/kg or its equivalent daily for 2 to 4 weeks, after which it is tapered.21 If the initial dosage is prednisone 60 mg orally daily for 2 to 4 weeks, our practice is to taper it to 50 mg daily for 2 weeks, then 40 mg daily for 2 weeks. Then, it is decreased by 5 mg every 2 weeks until it is 20 mg daily, and then by 2.5 mg every 2 weeks until it is 10 mg orally daily. Thereafter, the dosage is decreased by 1 mg every 2 to 4 weeks.
For patients with GCA who experience transient vision loss or diplopia at the time of diagnosis, intravenous pulse glucocorticoid therapy should be initiated to reduce the risk of vision loss as rapidly as possible.22 A typical pulse regimen is methylprednisolone 1 g intravenously daily for 3 days. Though not rigorously validated in studies, such an approach is used to avoid vision impairment due to GCA, which is rarely reversible.
RELAPSE OF DISEASE
Suspect a relapse of GCA if the patient’s initial symptoms recur, if inflammatory markers become elevated, or if classic symptoms of GCA or polymyalgia rheumatica occur. Elevations in inflammatory markers do not definitely indicate a flare of GCA, but they should trigger close monitoring of the patient’s symptoms.
Relapse is treated by increasing the glucocorticoid dosage as appropriate to the nature of the relapse. If vision is affected or the patient has symptoms of GCA, then increments of 30 to 60 mg of prednisone are warranted, whereas if the patient has symptoms of polymyalgia rheumatica, then increments of 5 to 10 mg of prednisone are usually used.
The incidence of relapses of GCA in multiple tertiary care centers has been reported to vary between 34% and 75%.23,24 Most relapses occur at prednisone dosages of less than 20 mg orally daily and within the first year after diagnosis. The most common symptoms are limb ischemia, jaw claudication, constitutional symptoms, headaches, and polymyalgia rheumatica. In a review of 286 patients,25 213 (74%) had at least 1 relapse. The first relapse occurred in the first year in 50%, by 2 years in 68%, and by 5 years in 79%.
ADVERSE EFFECTS OF GLUCOCORTICOIDS
In high doses, glucocorticoids have well-known adverse effects. In a population-based study of 120 patients, each patient treated with glucocorticoids experienced at least 1 adverse effect (cataract, fracture, infection, osteonecrosis, diabetes, hypertension, weight gain, capillary fragility, or hair loss).26 The effects were related to aging and cumulative dosage of prednisone but not to the initial dosage.
Glucocorticoids can affect many organs and systems:
- Eyes (cataracts, increased intraocular pressure, exophthalmos)
- Heart (premature atherosclerotic disease, hypertension, fluid retention, hyperlipidemia, arrhythmias)
- Gastrointestinal system (ulcer, gastrointestinal bleeding, gastritis, visceral perforation, hepatic steatosis, acute pancreatitis)
- Bone and muscle (osteopenia, osteoporosis, osteonecrosis, myopathy)
- Brain (mood disorder, psychosis, memory impairment)
- Endocrine system (hyperglycemia, hypothalamic-pituitary-adrenal axis suppression)
- Immune system (immunosuppression, leading to infection and leukocytosis).
Patients receiving a glucocorticoid dose equivalent to 20 mg or more of prednisone daily for 1 month or more who also have another cause of immunocompromise need prophylaxis against Pneumocystis jirovecii pneumonia.27 They should also receive appropriate immunizations before starting glucocorticoids. Live-virus vaccines should not be given to these patients until they have been off glucocorticoids for 1 month.
Glucocorticoids and bone loss
Glucocorticoids are associated with bone loss and fracture, which can occur within the first few months of use and with dosages as low as 2.5 to 7.5 mg orally daily.28 Therefore, glucocorticoid-induced bone loss has to be treated aggressively, particularly in patients who are older and have a history of fragility fracture.
For patients with GCA who need glucocorticoids in doses greater than 5 mg orally daily for more than 3 months, the following measures are advised to decrease the risk of bone loss:
- Weight-bearing exercise
- Smoking cessation
- Moderation in alcohol intake
- Measures to prevent falls29
- Supplementation with 1,200 mg of calcium and 800 IU of vitamin D.30
Pharmacologic therapy should be initiated in men over age 50 who have established osteoporosis and in postmenopausal women with established osteoporosis or osteopenia. For men over age 50 with established osteopenia, risk assessment with the glucocorticoid-corrected FRAX score (www.sheffield.ac.uk/FRAX) should be performed to identify those at high risk in whom pharmacologic therapy is warranted.31
Bisphosphonates are the first-line therapy for glucocorticoid-induced osteoporosis.32
Teriparatide is the second-line therapy and is used in patients who cannot tolerate bisphosphonates or other osteoporosis therapies, and in those who have severe osteoporosis, with T scores of –3.5 and below if they have not had a fracture, and –2.5 and below if they have had a fragility fracture.33
Denosumab, a monoclonal antibody to an osteoclast differentiating factor, may be beneficial for some patients with glucocorticoid-induced osteoporosis.34
To assess the efficacy of therapy, measuring bone mineral density at baseline and at 1 year of therapy is recommended. If density is stable or improved, then repeating the measurement at 2- to 3-year intervals is suggested.
TOCILIZUMAB: A STEROID-SPARING MEDICATION
Due to the adverse effects of long-term use of glucocorticoids and high rates of relapse, there is a pressing need for medications that are more efficacious and less toxic to treat GCA.
The European League Against Rheumatism, in its 2009 management guidelines for large-vessel vasculitis, recommend using an adjunctive immunosuppressant agent.19 In the case of GCA, they recommend using methotrexate 10 to 15 mg/week, which has shown modest evidence of reducing the relapse rate and lowering the cumulative doses of glucocorticoids needed.35,36
Studies of tumor necrosis factor inhibitors and abatacept have not yielded significant reductions in the relapse rate or decreased cumulative doses of prednisone.37,38
Advances in treatment for GCA have stagnated, but recent trials39,40 have evaluated the IL-6 receptor alpha inhibitor tocilizumab, given the central role of IL-6 in the pathogenesis of GCA. Case reports have revealed rapid induction and maintenance of remission in GCA using tocilizumab.41,42
Villiger et al39 performed a randomized, placebo-controlled trial to study the efficacy and safety of tocilizumab in induction and maintenance of disease remission in 30 patients with newly diagnosed GCA. The primary outcome, complete remission at 12 weeks, was achieved in 85% of patients who received tocilizumab plus tapered prednisolone, compared with 40% of patients who received placebo plus tapering prednisolone. The tocilizumab group also had favorable results in secondary outcomes including relapse-free survival at 52 weeks, time to first relapse after induction of remission, and cumulative dose of prednisolone.
The GiACTA trial. Stone et al40 studied the effect of tocilizumab on rates of relapse during glucocorticoid tapering in 251 GCA patients over the course of 52 weeks. Patients were randomized in a 2:1:1:1 ratio to 4 treatment groups:
- Tocilizumab weekly plus prednisone, with prednisone tapered over 26 weeks
- Tocilizumab every other week plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 52 weeks.
The primary outcome was the rate of sustained glucocorticoid-free remission at 52 weeks. Secondary outcomes included the remission rate, the cumulative glucocorticoid dose, and safety measures. At 52 weeks, the rates of sustained remission were:
- 56% with tocilizumab weekly
- 53% with tocilizumab every other week
- 14% with placebo plus 26-week prednisone taper
- 18% with placebo plus 52-week taper.
Differences between the active treatment groups and the placebo groups were statistically significant (P < .001).
The cumulative dose of prednisone in tocilizumab recipients was significantly less than in placebo recipients. Rates of adverse events were similar. Ultimately, the study showed that tocilizumab, either weekly or every other week, was more effective than prednisone alone at sustaining glucocorticoid-free remission in patients with GCA.
However, the study also raised questions about tocilizumab’s toxic effect profile and its long-term efficacy, as well as who are the optimal candidates for this therapy. Data on long-term use of tocilizumab are primarily taken from its use in rheumatoid arthritis.43 As of this writing, Stone et al are conducting an open-label trial to help provide long-term safety and efficacy data in patients with GCA. In the meantime, we must extrapolate data from the long-term use of tocilizumab in rheumatoid arthritis.
Tocilizumab and lower gastrointestinal tract perforation
One of the major adverse effects of long-term use of tocilizumab is lower gastrointestinal tract perforation.
Xie et al,44 in 2016, reported that the risk of perforation in patients on tocilizumab for rheumatoid arthritis was more than 2 times higher than in patients taking a tumor necrosis factor inhibitor. However, the absolute rates of perforation were low overall, roughly 1 to 3 per 1,000 patient-years in the tocilizumab group. Risk factors for perforation included older age, history of diverticulitis or other gastrointestinal tract condition, and prednisone doses of 7.5 mg or more a day.
Does tocilizumab prevent blindness?
Another consideration is that tocilizumab may not prevent optic neuropathy. In the GiACTA trial, 1 patient in the group receiving tocilizumab every other week developed optic neuropathy.40 Prednisone had been completely tapered off at the time, and the condition resolved when glucocorticoids were restarted. Thus, it is unknown if tocilizumab would be effective on its own without concomitant use of glucocorticoids.
Vision loss is one of the most severe complications of GCA, and it is still unclear whether tocilizumab can prevent vision loss in GCA. Also, we still have no data on the effect of tocilizumab on histopathologic findings, and whether biopsy yield diminishes over time. We hope future studies will help guide us in this regard.
No guidelines on tocilizumab yet
Clinical guidelines on the appropriate use of tocilizumab in GCA are lacking. The American College of Rheumatology and the European League Against Rheumatism have yet to publish updated guidelines with comments on use of tocilizumab. Therefore, it is unclear if tocilizumab is a first-line treatment in GCA, as its efficacy alone without glucocorticoids and its long-term safety in GCA patients have not been studied.
Treatment with tocilizumab should be individualized; it should be considered in patients who have had adverse effects from glucocorticoids, and in patients who experience a flare or cannot have their glucocorticoid dose lowered to an appropriate range.
The optimal duration of tocilizumab therapy is also unknown. However, using the GiACTA study as a rough guide, we try to limit its use to 1 year until additional data are available.
Patients on IL-6 inhibition may have suppressed C-reactive protein regardless of disease activity.43 Therefore, this laboratory value may not be reliable in determining active disease in patients on tocilizumab.
The GiACTA trial has shown an impressive improvement in the relapse-free remission period in patients with GCA taking tocilizumab. However, much work needs to be done to define the safety of this medication and determine which patients should be started on it. In the meantime, we recommend starting high-dose glucocorticoid therapy as soon as the diagnosis of GCA is suspected. In patients who do not tolerate glucocorticoids or whose disease flares during glucocorticoid taper, we recommend starting treatment with tocilizumab either once a week or every other week for at least 1 year.
- Brekke LK, Diamantopoulos AP, Fevang BT, Aßmus J, Esperø E, Gjesdal CG. Incidence of giant cell arteritis in Western Norway 1972–2012: a retrospective cohort study. Arthritis Res Ther 2017; 19(1):278. doi:10.1186/s13075-017-1479-6
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford) 2017; 56(4):506–515. doi:10.1093/rheumatology/kew273
- Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med 2014; 371(17):1653. doi:10.1056/NEJMc1409206
- Ghinoi A, Pipitone N, Nicolini A, et al. Large-vessel involvement in recent-onset giant cell arteritis: a case-control colour-Doppler sonography study. Rheumatology (Oxford) 2012; 51(4):730–734. doi:10.1093/rheumatology/ker329
- Prieto-González S, Depetris M, García-Martínez A, et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case-control study. Ann Rheum Dis 2014; 73(7):1388–1392. doi:10.1136/annrheumdis-2013-204572
- Brack A, Martinez-Taboada V, Stanson A, Goronzy JJ, Weyand CM. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum 1999; 42(2):311–317. doi:10.1002/1529-0131(199902)42:2<311::AID-ANR14>3.0.CO;2-F
- Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurence in a population-based study. Arthritis Rheum 2001; 45(2):140–145. doi:10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
- Liozon E, Jauberteau-Marchan MO, Ly K, Loustaud-Ratti V, Soria P, Vidal E. Giant cell arteritis with a low erythrocyte sedimentation rate: comments on the article by Salvarani and Hunder. Arthritis Rheum 2002; 47(6):692–694. doi:10.1002/art.10809
- Yu-Wai-Man P, Dayan MR. Giant cell arteritis with normal inflammatory markers. Acta Ophthalmol Scand 2007; 85(4):460. doi:10.1111/j.1600-0420.2006.00864.x
- Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA 2016; 315(22):2442–2458. doi:10.1001/jama.2016.5444
- Niederkohr RD, Levin LA. Management of the patient with suspected temporal arteritis a decision-analytic approach. Ophthalmology 2005; 112(5):744–756. doi:10.1016/j.ophtha.2005.01.031
- Bowling K, Rait J, Atkinson J, Srinivas G. Temporal artery biopsy in the diagnosis of giant cell arteritis: does the end justify the means? Ann Med Surg (Lond) 2017; 20:1–5. doi:10.1016/j.amsu.2017.06.020
- Daily B, Dassow P, Haynes J, Nashelsky J. Giant cell arteritis: biopsy after corticosteroid initiation. Am Fam Physician 2017; 95(2):116–117. pmid:28084703
- Durling B, Toren A, Patel V, Gilberg S, Weis E, Jordan D. Incidence of discordant temporal artery biopsy in the diagnosis of giant cell arteritis. Can J Ophthalmol 2014; 49(2):157–161. doi:10.1016/j.jcjo.2013.12.008
- Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018; 77(5):636–643. doi:10.1136/annrheumdis-2017-212649
- Aschwanden M, Imfeld S, Staub D, et al. The ultrasound compression sign to diagnose temporal giant cell arteritis shows an excellent interobserver agreement. Clin Exp Rheumatol 2015; 33(2 suppl 89):S-113–S-115. pmid:26016760
- Aiello PD, Trautmann JC, McPhee TJ, Kunselman AR, Hunder GG. Visual prognosis in giant cell arteritis. Ophthalmology 1993; 100(4):550–555. pmid:8479714
- Hayreh SS, Zimmerman B. Visual deterioration in giant cell arteritis patients while on high doses of corticosteroid therapy. Ophthalmology 2003; 110(6):1204–1215. doi:10.1016/S0161-6420(03)00228-8
- Mukhtyar C, Guillevin L, Cid MC, et al; European Vasculitis Study Group. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2009; 68(3):318–323. doi:10.1136/ard.2008.088351
- Dejaco C, Singh YP, Perel P, et al; European League Against Rheumatism; American College of Rheumatology. 2015 recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis 2015; 74(10):1799–1807. doi:10.1136/annrheumdis-2015-207492
- Bienvenu B, Ly KH, Lambert M, et al; Groupe d’Étude Français des Artérites des gros Vaisseaux, under the Aegis of the Filière des Maladies Auto-Immunes et Auto-Inflammatoires Rares. Management of giant cell arteritis: recommendations of the French Study Group for Large Vessel Vasculitis (GEFA). Rev Med Interne 2016; 37(3):154–165. doi:10.1016/j.revmed.2015.12.015
- Hayreh SS, Biousse V. Treatment of acute visual loss in giant cell arteritis: should we prescribe high-dose intravenous steroids or just oral steroids? J Neuroophthalmol 2012; 32(3):278–287. doi:10.1097/WNO.0b013e3182688218
- Restuccia G, Boiardi L, Cavazza A, et al. Flares in biopsy-proven giant cell arteritis in Northern Italy: characteristics and predictors in a long-term follow-up study. Medicine (Baltimore) 2016; 95(19):e3524. doi:10.1097/MD.0000000000003524
- Kermani TA, Warrington KJ, Cuthbertson D, et al; Vasculitis Clinical Research Consortium. Disease relapses among patients with giant cell arteritis: a prospective, longitudinal cohort study. J Rheumatol 2015; 42(7):1213–1217. doi:10.3899/jrheum.141347
- Labarca C, Koster MJ, Crowson CS, et al. Predictors of relapse and treatment outcomes in biopsy-proven giant cell arteritis: a retrospective cohort study. Rheumatology (Oxford) 2016; 55(2):347–356. doi:10.1093/rheumatology/kev348
- Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum 2003; 49(5):703–708. doi:10.1002/art.11388
- Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis 2002; 34(8):1098–1107. doi:10.1086/339548
- van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 2002; 13(10):777–787. doi:10.1007/s001980200108
- Heffernan MP, Saag KG, Robinson JK, Callen JP. Prevention of osteoporosis associated with chronic glucocorticoid therapy. JAMA 2006; 295(11):1300–1303. pmid:16541489
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2017; 69(8):1095–1110. doi:10.1002/acr.23279
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res 201; 62(11):1515–1526. doi:10.1002/acr.20295
- Allen CS, Yeung JH, Vandermeer B, Homik J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev 2016; 10:CD001347. doi:10.1002/14651858.CD001347.pub2
- Carpinteri R, Porcelli T, Mejia C, et al. Glucocorticoid-induced osteoporosis and parathyroid hormone. J Endocrinol Invest 2010; 33(suppl 7):16–21. pmid:20938221
- Saag KG, Wagman RB, Geusens P, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol 2018; 6(6):445–454. doi:10.1016/S2213-8587(18)30075-5
- Hoffman GS, Cid MC, Hellmann DB, et al; International Network for the Study of Systemic Vasculitides. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002; 46(5):1309–1318. doi:10.1002/art.10262
- Spiera RF, Mitnick HJ, Kupersmith M, et al. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol 2001; 19(5):495–501. pmid:11579707
- Hoffman GS, Cid MC, Rendt-Zagar KE, et al; Infliximab-GCA Study Group. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007; 146(9):621–630. pmid:17470830
- Langford CA, Cuthbertson D, Ytterberg SR, et al; Vasculitis Clinical Research Consortium. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol 2017; 69(4):837–845. doi:10.1002/art.40044
- Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2016; 387(10031):1921–1927. doi:10.1016/S0140-6736(16)00560-2
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Oliveira F, Butendieck RR, Ginsburg WW, Parikh K, Abril A. Tocilizumab, an effective treatment for relapsing giant cell arteritis. Clin Exp Rheumatol 2014; 32(3 suppl 82):S76–S78. pmid:24854376
- Loricera J, Blanco R, Hernández JL, et al. Tocilizumab in giant cell arteritis: multicenter open-label study of 22 patients. Semin Arthritis Rheum 2015; 44(6):717–723. doi:10.1016/j.semarthrit.2014.12.005
- Tamaki H, Hajj-Ali RA. Tocilizumab for giant cell arteritis—a new giant step in an old disease. JAMA Neurol 2018; 75(2):145–146. doi:10.1001/jamaneurol.2017.3811
- Xie F, Yun H, Bernatsky S, Curtis JR. Risk for gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologics. Arthritis Rheumatol 2016; 68(11):2612–2617. doi:10.1002/art.39761
Giant cell arteritis (GCA) is a systemic vasculitis involving medium-sized and large arteries, most commonly the temporal, ophthalmic, occipital, vertebral, posterior ciliary, and proximal vertebral arteries. Moreover, involvement of the ophthalmic artery and its branches results in loss of vision. GCA can also involve the aorta and its proximal branches, especially in the upper extremities.
GCA is the most common systemic vasculitis in adults. It occurs almost exclusively in patients over age 50 and affects women more than men. It is most frequent in populations of northern European ancestry, especially Scandinavian. In a retrospective cohort study in Norway, the average annual cumulative incidence rate of GCA was 16.7 per 100,000 people over age 50.1 Risk factors include older age, history of smoking, current smoking, early menopause, and, possibly, stress-related disorders.2
PATHOGENESIS IS NOT COMPLETELY UNDERSTOOD
The pathogenesis of GCA is not completely understood, but there is evidence of immune activation in the arterial wall leading to activation of macrophages and formation of multinucleated giant cells (which may not always be present in biopsies).
The most relevant cytokines in the ongoing pathogenesis are still being defined, but the presence of interferon gamma and interleukin 6 (IL-6) seem to be critical for the expression of the disease. The primary immunogenic triggers for the elaboration of these cytokines and the arteritis remain elusive.
A SPECTRUM OF PRESENTATIONS
The initial symptoms of GCA may be vague, such as malaise, fever, and night sweats, and are likely due to systemic inflammation. Features of vascular involvement include headache, scalp tenderness, and jaw claudication (cramping pain in the jaw while chewing).
A less common but serious feature associated with GCA is partial or complete vision loss affecting 1 or both eyes.3 Some patients suddenly go completely blind without any visual prodrome.
Overlapping GCA phenotypes exist, with a spectrum of presentations that include classic cranial arteritis, extracranial GCA (also called large-vessel GCA), and polymyalgia rheumatica.2
Cranial GCA, the best-characterized clinical presentation, causes symptoms such as headache or signs such as tenderness of the temporal artery. On examination, the temporal arteries may be tender or nodular, and the pulses may be felt above the zygomatic arch, above and in front of the tragus of the ear. About two-thirds of patients with cranial GCA present with new-onset headache, most often in the temporal area, but possibly anywhere throughout the head.
Visual disturbance, jaw claudication, and tongue pain are less common but, if present, increase the likelihood of this diagnosis.2
Large-vessel involvement in GCA is common and refers to involvement of the aorta and its proximal branches. Imaging methods used in diagnosing large-vessel GCA include color Doppler ultrasonography, computed tomography with angiography, magnetic resonance imaging with angiography, and positron emission tomography. In some centers, such imaging is performed in all patients diagnosed with GCA to survey for large-vessel involvement.
Depending on the imaging study, large-vessel involvement has been found in 30% to 80% of cases of GCA.4,5 It is often associated with nonspecific symptoms such as fever, weight loss, chills, and malaise, but it can also cause more specific symptoms such as unilateral extremity claudication. In contrast to patients with cranial GCA, patients with large-vessel GCA were younger at onset, less likely to have headaches, and more likely to have arm claudication at presentation.6 Aortitis of the ascending aorta can occur with a histopathologic pattern of GCA but without the clinical stigmata of GCA.
The finding of aortitis should prompt the clinician to question the patient about other symptoms of GCA and to order imaging of the whole vascular tree. Ultrasonography and biopsy of the temporal arteries can be considered. Whether idiopathic aortitis is part of the GCA spectrum remains to be seen.
Laboratory tests often show anemia, leukocytosis, and thrombocytosis. Acute-phase reactants such as C-reactive protein and the erythrocyte sedimentation rate are often elevated. The sedimentation rate often exceeds 50 mm/hour and sometimes 100 mm/hour.
In 2 retrospective studies, the number of patients with GCA whose sedimentation rate was less than 50 mm/hour ranged between 5% and 11%.7,8 However, a small percentage of patients with GCA have normal inflammatory markers. Therefore, if the suspicion for GCA is high, treatment should be started and biopsy pursued.9 In patients with paraproteinemia or other causes of a spuriously elevated or low erythrocyte sedimentation rate, C-reactive protein is a more reliable test.
Polymyalgia rheumatica is another rheumatologic condition that can occur independently or in conjunction with GCA. It is characterized by stiffness and pain in the proximal joints such as the hips and shoulders, typically worse in the morning and better with activity. Although the patient may subjectively feel weak, a close neurologic examination will reveal normal muscle strength.
Polymyalgia rheumatica is observed in 40% to 60% of patients with GCA at the time of diagnosis; 16% to 21% of patients with polymyalgia rheumatica may develop GCA, especially if untreated.2,10
Differential diagnosis
Other vasculitides (eg, Takayasu arteritis) can also present with unexplained fever, anemia, and constitutional symptoms.
Infection should be considered if fever is present. An infectious disease accompanied by fever, headache, and elevated inflammatory markers can mimic GCA.
Nonarteritic anterior ischemic optic neuropathy can present with sudden vision loss, prompting concern for underlying GCA. Risk factors include hypertension and diabetes mellitus; other features of GCA, including elevated inflammatory markers, are generally absent.
TEMPORAL ARTERY BIOPSY: THE GOLD STANDARD FOR DIAGNOSIS
Temporal artery biopsy remains the standard to confirm the diagnosis. However, because inflammation in the temporal arteries can affect some segments but not others, biopsy results on conventional hematoxylin and eosin staining can be falsely negative in patients with GCA. In one study,11 the mean sensitivity of unilateral temporal artery biopsy was 86.9%.
Typical positive histologic findings are inflammation with panarteritis, CD4-positive lymphocytes, macrophages, giant cells, and fragmentation of the internal elastic lamina.12
When GCA is suspected, treatment with glucocorticoids should be started immediately and biopsy performed as soon as possible. Delaying biopsy for 14 days or more may not affect the accuracy of biopsy study.13 Treatment should never be withheld while awaiting the results of biopsy study.
Biopsy is usually performed unilaterally, on the same side as the symptoms or abnormal findings on examination. Bilateral temporal artery biopsy is also performed and compared with unilateral biopsy; this approach increases the diagnostic yield by about 5%.14
IMAGING
In patients with suspected GCA, imaging is recommended early to complement the clinical criteria for the diagnosis of GCA.15 Positron emission tomography, computed tomography angiography, magnetic resonance angiography, or Doppler ultrasonography can reveal inflammation of the arteries in the proximal upper or lower limbs or the aorta.2
In patients with suspected cranial GCA, ultrasonography of the temporal and axillary arteries is recommended first. If ultrasonography is not available or is inconclusive, high-resolution magnetic resonance imaging of the cranial arteries can be used as an alternative. Computed tomography and positron emission tomography of the cranial arteries are not recommended.
In patients with suspected large-vessel GCA, ultrasonography, positron emission tomography, computed tomography, and magnetic resonance imaging may be used to screen for vessel wall inflammation, edema, and luminal narrowing in extracranial arteries. Ultrasonography is of limited value in assessing aortitis.
Color duplex ultrasonography can be applied to assess for vascular inflammation of the temporal or large arteries. The typical finding of the “halo” sign, a hypoechoic ring around the arterial lumen, represents the inflammation-induced thickening of the arterial wall. The “compression sign,” the persistence of the “halo” during compression of the vessel lumen by the ultrasound probe, has high specificity for the diagnosis.16
Ultrasonography of suspected GCA has yielded sensitivities of 55% to 100% and specificities of 78% to 100%. However, its sensitivity depends on the user’s level of expertise, so it should be done only in medical centers with a high number of GCA cases and with highly experienced sonographers. High-resolution magnetic resonance imaging is an alternative to ultrasonography and has shown similar sensitivity and specificity.3
TREATMENT WITH GLUCOCORTICOIDS
Glucocorticoids remain the standard for treatment of GCA. The therapeutic effect of glucocorticoids in GCA has been established by years of clinical experience, but has never been proven in a placebo-controlled trial. When started appropriately and expeditiously, glucocorticoids produce exquisite resolution of signs and symptoms and prevent the serious complication of vision loss. Rapid resolution of symptoms is so typical of GCA that if the patient’s symptoms persist more than a few days after starting a glucocorticoid, the diagnosis of GCA should be reconsidered.
In a retrospective study of 245 patients with biopsy-proven GCA treated with glucocorticoids, 34 had permanent loss of sight.17 In 32 (94%) of the 34, the vision loss occurred before glucocorticoids were started. Of the remaining 2 patients, 1 lost vision 8 days into treatment, and the other lost vision 3 years after diagnosis and 1 year after discontinuation of glucocorticoids.
In a series of 144 patients with biopsy-proven GCA, 51 had no vision loss at presentation and no vision loss after starting glucocorticoids, and 93 had vision loss at presentation. In the latter group, symptoms worsened within 5 days of starting glucocorticoids in 9 patients.18 If vision was intact at the time of presentation, prompt initiation of glucocorticoids reduced the risk of vision loss to less than 1%.
High doses, slowly tapered
The European League Against Rheumatism recommends early initiation of high-dose glucocorticoids for patients with large-vessel vasculitis,19 and it also recommends glucocorticoids for patients with polymyalgia rheumatica.20 The optimal initial and tapering dosage has never been formally evaluated, but regimens have been devised on the basis of expert opinion.21
For patients with GCA who do not have vision loss at the time of diagnosis, the initial dose is prednisone 1 mg/kg or its equivalent daily for 2 to 4 weeks, after which it is tapered.21 If the initial dosage is prednisone 60 mg orally daily for 2 to 4 weeks, our practice is to taper it to 50 mg daily for 2 weeks, then 40 mg daily for 2 weeks. Then, it is decreased by 5 mg every 2 weeks until it is 20 mg daily, and then by 2.5 mg every 2 weeks until it is 10 mg orally daily. Thereafter, the dosage is decreased by 1 mg every 2 to 4 weeks.
For patients with GCA who experience transient vision loss or diplopia at the time of diagnosis, intravenous pulse glucocorticoid therapy should be initiated to reduce the risk of vision loss as rapidly as possible.22 A typical pulse regimen is methylprednisolone 1 g intravenously daily for 3 days. Though not rigorously validated in studies, such an approach is used to avoid vision impairment due to GCA, which is rarely reversible.
RELAPSE OF DISEASE
Suspect a relapse of GCA if the patient’s initial symptoms recur, if inflammatory markers become elevated, or if classic symptoms of GCA or polymyalgia rheumatica occur. Elevations in inflammatory markers do not definitely indicate a flare of GCA, but they should trigger close monitoring of the patient’s symptoms.
Relapse is treated by increasing the glucocorticoid dosage as appropriate to the nature of the relapse. If vision is affected or the patient has symptoms of GCA, then increments of 30 to 60 mg of prednisone are warranted, whereas if the patient has symptoms of polymyalgia rheumatica, then increments of 5 to 10 mg of prednisone are usually used.
The incidence of relapses of GCA in multiple tertiary care centers has been reported to vary between 34% and 75%.23,24 Most relapses occur at prednisone dosages of less than 20 mg orally daily and within the first year after diagnosis. The most common symptoms are limb ischemia, jaw claudication, constitutional symptoms, headaches, and polymyalgia rheumatica. In a review of 286 patients,25 213 (74%) had at least 1 relapse. The first relapse occurred in the first year in 50%, by 2 years in 68%, and by 5 years in 79%.
ADVERSE EFFECTS OF GLUCOCORTICOIDS
In high doses, glucocorticoids have well-known adverse effects. In a population-based study of 120 patients, each patient treated with glucocorticoids experienced at least 1 adverse effect (cataract, fracture, infection, osteonecrosis, diabetes, hypertension, weight gain, capillary fragility, or hair loss).26 The effects were related to aging and cumulative dosage of prednisone but not to the initial dosage.
Glucocorticoids can affect many organs and systems:
- Eyes (cataracts, increased intraocular pressure, exophthalmos)
- Heart (premature atherosclerotic disease, hypertension, fluid retention, hyperlipidemia, arrhythmias)
- Gastrointestinal system (ulcer, gastrointestinal bleeding, gastritis, visceral perforation, hepatic steatosis, acute pancreatitis)
- Bone and muscle (osteopenia, osteoporosis, osteonecrosis, myopathy)
- Brain (mood disorder, psychosis, memory impairment)
- Endocrine system (hyperglycemia, hypothalamic-pituitary-adrenal axis suppression)
- Immune system (immunosuppression, leading to infection and leukocytosis).
Patients receiving a glucocorticoid dose equivalent to 20 mg or more of prednisone daily for 1 month or more who also have another cause of immunocompromise need prophylaxis against Pneumocystis jirovecii pneumonia.27 They should also receive appropriate immunizations before starting glucocorticoids. Live-virus vaccines should not be given to these patients until they have been off glucocorticoids for 1 month.
Glucocorticoids and bone loss
Glucocorticoids are associated with bone loss and fracture, which can occur within the first few months of use and with dosages as low as 2.5 to 7.5 mg orally daily.28 Therefore, glucocorticoid-induced bone loss has to be treated aggressively, particularly in patients who are older and have a history of fragility fracture.
For patients with GCA who need glucocorticoids in doses greater than 5 mg orally daily for more than 3 months, the following measures are advised to decrease the risk of bone loss:
- Weight-bearing exercise
- Smoking cessation
- Moderation in alcohol intake
- Measures to prevent falls29
- Supplementation with 1,200 mg of calcium and 800 IU of vitamin D.30
Pharmacologic therapy should be initiated in men over age 50 who have established osteoporosis and in postmenopausal women with established osteoporosis or osteopenia. For men over age 50 with established osteopenia, risk assessment with the glucocorticoid-corrected FRAX score (www.sheffield.ac.uk/FRAX) should be performed to identify those at high risk in whom pharmacologic therapy is warranted.31
Bisphosphonates are the first-line therapy for glucocorticoid-induced osteoporosis.32
Teriparatide is the second-line therapy and is used in patients who cannot tolerate bisphosphonates or other osteoporosis therapies, and in those who have severe osteoporosis, with T scores of –3.5 and below if they have not had a fracture, and –2.5 and below if they have had a fragility fracture.33
Denosumab, a monoclonal antibody to an osteoclast differentiating factor, may be beneficial for some patients with glucocorticoid-induced osteoporosis.34
To assess the efficacy of therapy, measuring bone mineral density at baseline and at 1 year of therapy is recommended. If density is stable or improved, then repeating the measurement at 2- to 3-year intervals is suggested.
TOCILIZUMAB: A STEROID-SPARING MEDICATION
Due to the adverse effects of long-term use of glucocorticoids and high rates of relapse, there is a pressing need for medications that are more efficacious and less toxic to treat GCA.
The European League Against Rheumatism, in its 2009 management guidelines for large-vessel vasculitis, recommend using an adjunctive immunosuppressant agent.19 In the case of GCA, they recommend using methotrexate 10 to 15 mg/week, which has shown modest evidence of reducing the relapse rate and lowering the cumulative doses of glucocorticoids needed.35,36
Studies of tumor necrosis factor inhibitors and abatacept have not yielded significant reductions in the relapse rate or decreased cumulative doses of prednisone.37,38
Advances in treatment for GCA have stagnated, but recent trials39,40 have evaluated the IL-6 receptor alpha inhibitor tocilizumab, given the central role of IL-6 in the pathogenesis of GCA. Case reports have revealed rapid induction and maintenance of remission in GCA using tocilizumab.41,42
Villiger et al39 performed a randomized, placebo-controlled trial to study the efficacy and safety of tocilizumab in induction and maintenance of disease remission in 30 patients with newly diagnosed GCA. The primary outcome, complete remission at 12 weeks, was achieved in 85% of patients who received tocilizumab plus tapered prednisolone, compared with 40% of patients who received placebo plus tapering prednisolone. The tocilizumab group also had favorable results in secondary outcomes including relapse-free survival at 52 weeks, time to first relapse after induction of remission, and cumulative dose of prednisolone.
The GiACTA trial. Stone et al40 studied the effect of tocilizumab on rates of relapse during glucocorticoid tapering in 251 GCA patients over the course of 52 weeks. Patients were randomized in a 2:1:1:1 ratio to 4 treatment groups:
- Tocilizumab weekly plus prednisone, with prednisone tapered over 26 weeks
- Tocilizumab every other week plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 52 weeks.
The primary outcome was the rate of sustained glucocorticoid-free remission at 52 weeks. Secondary outcomes included the remission rate, the cumulative glucocorticoid dose, and safety measures. At 52 weeks, the rates of sustained remission were:
- 56% with tocilizumab weekly
- 53% with tocilizumab every other week
- 14% with placebo plus 26-week prednisone taper
- 18% with placebo plus 52-week taper.
Differences between the active treatment groups and the placebo groups were statistically significant (P < .001).
The cumulative dose of prednisone in tocilizumab recipients was significantly less than in placebo recipients. Rates of adverse events were similar. Ultimately, the study showed that tocilizumab, either weekly or every other week, was more effective than prednisone alone at sustaining glucocorticoid-free remission in patients with GCA.
However, the study also raised questions about tocilizumab’s toxic effect profile and its long-term efficacy, as well as who are the optimal candidates for this therapy. Data on long-term use of tocilizumab are primarily taken from its use in rheumatoid arthritis.43 As of this writing, Stone et al are conducting an open-label trial to help provide long-term safety and efficacy data in patients with GCA. In the meantime, we must extrapolate data from the long-term use of tocilizumab in rheumatoid arthritis.
Tocilizumab and lower gastrointestinal tract perforation
One of the major adverse effects of long-term use of tocilizumab is lower gastrointestinal tract perforation.
Xie et al,44 in 2016, reported that the risk of perforation in patients on tocilizumab for rheumatoid arthritis was more than 2 times higher than in patients taking a tumor necrosis factor inhibitor. However, the absolute rates of perforation were low overall, roughly 1 to 3 per 1,000 patient-years in the tocilizumab group. Risk factors for perforation included older age, history of diverticulitis or other gastrointestinal tract condition, and prednisone doses of 7.5 mg or more a day.
Does tocilizumab prevent blindness?
Another consideration is that tocilizumab may not prevent optic neuropathy. In the GiACTA trial, 1 patient in the group receiving tocilizumab every other week developed optic neuropathy.40 Prednisone had been completely tapered off at the time, and the condition resolved when glucocorticoids were restarted. Thus, it is unknown if tocilizumab would be effective on its own without concomitant use of glucocorticoids.
Vision loss is one of the most severe complications of GCA, and it is still unclear whether tocilizumab can prevent vision loss in GCA. Also, we still have no data on the effect of tocilizumab on histopathologic findings, and whether biopsy yield diminishes over time. We hope future studies will help guide us in this regard.
No guidelines on tocilizumab yet
Clinical guidelines on the appropriate use of tocilizumab in GCA are lacking. The American College of Rheumatology and the European League Against Rheumatism have yet to publish updated guidelines with comments on use of tocilizumab. Therefore, it is unclear if tocilizumab is a first-line treatment in GCA, as its efficacy alone without glucocorticoids and its long-term safety in GCA patients have not been studied.
Treatment with tocilizumab should be individualized; it should be considered in patients who have had adverse effects from glucocorticoids, and in patients who experience a flare or cannot have their glucocorticoid dose lowered to an appropriate range.
The optimal duration of tocilizumab therapy is also unknown. However, using the GiACTA study as a rough guide, we try to limit its use to 1 year until additional data are available.
Patients on IL-6 inhibition may have suppressed C-reactive protein regardless of disease activity.43 Therefore, this laboratory value may not be reliable in determining active disease in patients on tocilizumab.
The GiACTA trial has shown an impressive improvement in the relapse-free remission period in patients with GCA taking tocilizumab. However, much work needs to be done to define the safety of this medication and determine which patients should be started on it. In the meantime, we recommend starting high-dose glucocorticoid therapy as soon as the diagnosis of GCA is suspected. In patients who do not tolerate glucocorticoids or whose disease flares during glucocorticoid taper, we recommend starting treatment with tocilizumab either once a week or every other week for at least 1 year.
Giant cell arteritis (GCA) is a systemic vasculitis involving medium-sized and large arteries, most commonly the temporal, ophthalmic, occipital, vertebral, posterior ciliary, and proximal vertebral arteries. Moreover, involvement of the ophthalmic artery and its branches results in loss of vision. GCA can also involve the aorta and its proximal branches, especially in the upper extremities.
GCA is the most common systemic vasculitis in adults. It occurs almost exclusively in patients over age 50 and affects women more than men. It is most frequent in populations of northern European ancestry, especially Scandinavian. In a retrospective cohort study in Norway, the average annual cumulative incidence rate of GCA was 16.7 per 100,000 people over age 50.1 Risk factors include older age, history of smoking, current smoking, early menopause, and, possibly, stress-related disorders.2
PATHOGENESIS IS NOT COMPLETELY UNDERSTOOD
The pathogenesis of GCA is not completely understood, but there is evidence of immune activation in the arterial wall leading to activation of macrophages and formation of multinucleated giant cells (which may not always be present in biopsies).
The most relevant cytokines in the ongoing pathogenesis are still being defined, but the presence of interferon gamma and interleukin 6 (IL-6) seem to be critical for the expression of the disease. The primary immunogenic triggers for the elaboration of these cytokines and the arteritis remain elusive.
A SPECTRUM OF PRESENTATIONS
The initial symptoms of GCA may be vague, such as malaise, fever, and night sweats, and are likely due to systemic inflammation. Features of vascular involvement include headache, scalp tenderness, and jaw claudication (cramping pain in the jaw while chewing).
A less common but serious feature associated with GCA is partial or complete vision loss affecting 1 or both eyes.3 Some patients suddenly go completely blind without any visual prodrome.
Overlapping GCA phenotypes exist, with a spectrum of presentations that include classic cranial arteritis, extracranial GCA (also called large-vessel GCA), and polymyalgia rheumatica.2
Cranial GCA, the best-characterized clinical presentation, causes symptoms such as headache or signs such as tenderness of the temporal artery. On examination, the temporal arteries may be tender or nodular, and the pulses may be felt above the zygomatic arch, above and in front of the tragus of the ear. About two-thirds of patients with cranial GCA present with new-onset headache, most often in the temporal area, but possibly anywhere throughout the head.
Visual disturbance, jaw claudication, and tongue pain are less common but, if present, increase the likelihood of this diagnosis.2
Large-vessel involvement in GCA is common and refers to involvement of the aorta and its proximal branches. Imaging methods used in diagnosing large-vessel GCA include color Doppler ultrasonography, computed tomography with angiography, magnetic resonance imaging with angiography, and positron emission tomography. In some centers, such imaging is performed in all patients diagnosed with GCA to survey for large-vessel involvement.
Depending on the imaging study, large-vessel involvement has been found in 30% to 80% of cases of GCA.4,5 It is often associated with nonspecific symptoms such as fever, weight loss, chills, and malaise, but it can also cause more specific symptoms such as unilateral extremity claudication. In contrast to patients with cranial GCA, patients with large-vessel GCA were younger at onset, less likely to have headaches, and more likely to have arm claudication at presentation.6 Aortitis of the ascending aorta can occur with a histopathologic pattern of GCA but without the clinical stigmata of GCA.
The finding of aortitis should prompt the clinician to question the patient about other symptoms of GCA and to order imaging of the whole vascular tree. Ultrasonography and biopsy of the temporal arteries can be considered. Whether idiopathic aortitis is part of the GCA spectrum remains to be seen.
Laboratory tests often show anemia, leukocytosis, and thrombocytosis. Acute-phase reactants such as C-reactive protein and the erythrocyte sedimentation rate are often elevated. The sedimentation rate often exceeds 50 mm/hour and sometimes 100 mm/hour.
In 2 retrospective studies, the number of patients with GCA whose sedimentation rate was less than 50 mm/hour ranged between 5% and 11%.7,8 However, a small percentage of patients with GCA have normal inflammatory markers. Therefore, if the suspicion for GCA is high, treatment should be started and biopsy pursued.9 In patients with paraproteinemia or other causes of a spuriously elevated or low erythrocyte sedimentation rate, C-reactive protein is a more reliable test.
Polymyalgia rheumatica is another rheumatologic condition that can occur independently or in conjunction with GCA. It is characterized by stiffness and pain in the proximal joints such as the hips and shoulders, typically worse in the morning and better with activity. Although the patient may subjectively feel weak, a close neurologic examination will reveal normal muscle strength.
Polymyalgia rheumatica is observed in 40% to 60% of patients with GCA at the time of diagnosis; 16% to 21% of patients with polymyalgia rheumatica may develop GCA, especially if untreated.2,10
Differential diagnosis
Other vasculitides (eg, Takayasu arteritis) can also present with unexplained fever, anemia, and constitutional symptoms.
Infection should be considered if fever is present. An infectious disease accompanied by fever, headache, and elevated inflammatory markers can mimic GCA.
Nonarteritic anterior ischemic optic neuropathy can present with sudden vision loss, prompting concern for underlying GCA. Risk factors include hypertension and diabetes mellitus; other features of GCA, including elevated inflammatory markers, are generally absent.
TEMPORAL ARTERY BIOPSY: THE GOLD STANDARD FOR DIAGNOSIS
Temporal artery biopsy remains the standard to confirm the diagnosis. However, because inflammation in the temporal arteries can affect some segments but not others, biopsy results on conventional hematoxylin and eosin staining can be falsely negative in patients with GCA. In one study,11 the mean sensitivity of unilateral temporal artery biopsy was 86.9%.
Typical positive histologic findings are inflammation with panarteritis, CD4-positive lymphocytes, macrophages, giant cells, and fragmentation of the internal elastic lamina.12
When GCA is suspected, treatment with glucocorticoids should be started immediately and biopsy performed as soon as possible. Delaying biopsy for 14 days or more may not affect the accuracy of biopsy study.13 Treatment should never be withheld while awaiting the results of biopsy study.
Biopsy is usually performed unilaterally, on the same side as the symptoms or abnormal findings on examination. Bilateral temporal artery biopsy is also performed and compared with unilateral biopsy; this approach increases the diagnostic yield by about 5%.14
IMAGING
In patients with suspected GCA, imaging is recommended early to complement the clinical criteria for the diagnosis of GCA.15 Positron emission tomography, computed tomography angiography, magnetic resonance angiography, or Doppler ultrasonography can reveal inflammation of the arteries in the proximal upper or lower limbs or the aorta.2
In patients with suspected cranial GCA, ultrasonography of the temporal and axillary arteries is recommended first. If ultrasonography is not available or is inconclusive, high-resolution magnetic resonance imaging of the cranial arteries can be used as an alternative. Computed tomography and positron emission tomography of the cranial arteries are not recommended.
In patients with suspected large-vessel GCA, ultrasonography, positron emission tomography, computed tomography, and magnetic resonance imaging may be used to screen for vessel wall inflammation, edema, and luminal narrowing in extracranial arteries. Ultrasonography is of limited value in assessing aortitis.
Color duplex ultrasonography can be applied to assess for vascular inflammation of the temporal or large arteries. The typical finding of the “halo” sign, a hypoechoic ring around the arterial lumen, represents the inflammation-induced thickening of the arterial wall. The “compression sign,” the persistence of the “halo” during compression of the vessel lumen by the ultrasound probe, has high specificity for the diagnosis.16
Ultrasonography of suspected GCA has yielded sensitivities of 55% to 100% and specificities of 78% to 100%. However, its sensitivity depends on the user’s level of expertise, so it should be done only in medical centers with a high number of GCA cases and with highly experienced sonographers. High-resolution magnetic resonance imaging is an alternative to ultrasonography and has shown similar sensitivity and specificity.3
TREATMENT WITH GLUCOCORTICOIDS
Glucocorticoids remain the standard for treatment of GCA. The therapeutic effect of glucocorticoids in GCA has been established by years of clinical experience, but has never been proven in a placebo-controlled trial. When started appropriately and expeditiously, glucocorticoids produce exquisite resolution of signs and symptoms and prevent the serious complication of vision loss. Rapid resolution of symptoms is so typical of GCA that if the patient’s symptoms persist more than a few days after starting a glucocorticoid, the diagnosis of GCA should be reconsidered.
In a retrospective study of 245 patients with biopsy-proven GCA treated with glucocorticoids, 34 had permanent loss of sight.17 In 32 (94%) of the 34, the vision loss occurred before glucocorticoids were started. Of the remaining 2 patients, 1 lost vision 8 days into treatment, and the other lost vision 3 years after diagnosis and 1 year after discontinuation of glucocorticoids.
In a series of 144 patients with biopsy-proven GCA, 51 had no vision loss at presentation and no vision loss after starting glucocorticoids, and 93 had vision loss at presentation. In the latter group, symptoms worsened within 5 days of starting glucocorticoids in 9 patients.18 If vision was intact at the time of presentation, prompt initiation of glucocorticoids reduced the risk of vision loss to less than 1%.
High doses, slowly tapered
The European League Against Rheumatism recommends early initiation of high-dose glucocorticoids for patients with large-vessel vasculitis,19 and it also recommends glucocorticoids for patients with polymyalgia rheumatica.20 The optimal initial and tapering dosage has never been formally evaluated, but regimens have been devised on the basis of expert opinion.21
For patients with GCA who do not have vision loss at the time of diagnosis, the initial dose is prednisone 1 mg/kg or its equivalent daily for 2 to 4 weeks, after which it is tapered.21 If the initial dosage is prednisone 60 mg orally daily for 2 to 4 weeks, our practice is to taper it to 50 mg daily for 2 weeks, then 40 mg daily for 2 weeks. Then, it is decreased by 5 mg every 2 weeks until it is 20 mg daily, and then by 2.5 mg every 2 weeks until it is 10 mg orally daily. Thereafter, the dosage is decreased by 1 mg every 2 to 4 weeks.
For patients with GCA who experience transient vision loss or diplopia at the time of diagnosis, intravenous pulse glucocorticoid therapy should be initiated to reduce the risk of vision loss as rapidly as possible.22 A typical pulse regimen is methylprednisolone 1 g intravenously daily for 3 days. Though not rigorously validated in studies, such an approach is used to avoid vision impairment due to GCA, which is rarely reversible.
RELAPSE OF DISEASE
Suspect a relapse of GCA if the patient’s initial symptoms recur, if inflammatory markers become elevated, or if classic symptoms of GCA or polymyalgia rheumatica occur. Elevations in inflammatory markers do not definitely indicate a flare of GCA, but they should trigger close monitoring of the patient’s symptoms.
Relapse is treated by increasing the glucocorticoid dosage as appropriate to the nature of the relapse. If vision is affected or the patient has symptoms of GCA, then increments of 30 to 60 mg of prednisone are warranted, whereas if the patient has symptoms of polymyalgia rheumatica, then increments of 5 to 10 mg of prednisone are usually used.
The incidence of relapses of GCA in multiple tertiary care centers has been reported to vary between 34% and 75%.23,24 Most relapses occur at prednisone dosages of less than 20 mg orally daily and within the first year after diagnosis. The most common symptoms are limb ischemia, jaw claudication, constitutional symptoms, headaches, and polymyalgia rheumatica. In a review of 286 patients,25 213 (74%) had at least 1 relapse. The first relapse occurred in the first year in 50%, by 2 years in 68%, and by 5 years in 79%.
ADVERSE EFFECTS OF GLUCOCORTICOIDS
In high doses, glucocorticoids have well-known adverse effects. In a population-based study of 120 patients, each patient treated with glucocorticoids experienced at least 1 adverse effect (cataract, fracture, infection, osteonecrosis, diabetes, hypertension, weight gain, capillary fragility, or hair loss).26 The effects were related to aging and cumulative dosage of prednisone but not to the initial dosage.
Glucocorticoids can affect many organs and systems:
- Eyes (cataracts, increased intraocular pressure, exophthalmos)
- Heart (premature atherosclerotic disease, hypertension, fluid retention, hyperlipidemia, arrhythmias)
- Gastrointestinal system (ulcer, gastrointestinal bleeding, gastritis, visceral perforation, hepatic steatosis, acute pancreatitis)
- Bone and muscle (osteopenia, osteoporosis, osteonecrosis, myopathy)
- Brain (mood disorder, psychosis, memory impairment)
- Endocrine system (hyperglycemia, hypothalamic-pituitary-adrenal axis suppression)
- Immune system (immunosuppression, leading to infection and leukocytosis).
Patients receiving a glucocorticoid dose equivalent to 20 mg or more of prednisone daily for 1 month or more who also have another cause of immunocompromise need prophylaxis against Pneumocystis jirovecii pneumonia.27 They should also receive appropriate immunizations before starting glucocorticoids. Live-virus vaccines should not be given to these patients until they have been off glucocorticoids for 1 month.
Glucocorticoids and bone loss
Glucocorticoids are associated with bone loss and fracture, which can occur within the first few months of use and with dosages as low as 2.5 to 7.5 mg orally daily.28 Therefore, glucocorticoid-induced bone loss has to be treated aggressively, particularly in patients who are older and have a history of fragility fracture.
For patients with GCA who need glucocorticoids in doses greater than 5 mg orally daily for more than 3 months, the following measures are advised to decrease the risk of bone loss:
- Weight-bearing exercise
- Smoking cessation
- Moderation in alcohol intake
- Measures to prevent falls29
- Supplementation with 1,200 mg of calcium and 800 IU of vitamin D.30
Pharmacologic therapy should be initiated in men over age 50 who have established osteoporosis and in postmenopausal women with established osteoporosis or osteopenia. For men over age 50 with established osteopenia, risk assessment with the glucocorticoid-corrected FRAX score (www.sheffield.ac.uk/FRAX) should be performed to identify those at high risk in whom pharmacologic therapy is warranted.31
Bisphosphonates are the first-line therapy for glucocorticoid-induced osteoporosis.32
Teriparatide is the second-line therapy and is used in patients who cannot tolerate bisphosphonates or other osteoporosis therapies, and in those who have severe osteoporosis, with T scores of –3.5 and below if they have not had a fracture, and –2.5 and below if they have had a fragility fracture.33
Denosumab, a monoclonal antibody to an osteoclast differentiating factor, may be beneficial for some patients with glucocorticoid-induced osteoporosis.34
To assess the efficacy of therapy, measuring bone mineral density at baseline and at 1 year of therapy is recommended. If density is stable or improved, then repeating the measurement at 2- to 3-year intervals is suggested.
TOCILIZUMAB: A STEROID-SPARING MEDICATION
Due to the adverse effects of long-term use of glucocorticoids and high rates of relapse, there is a pressing need for medications that are more efficacious and less toxic to treat GCA.
The European League Against Rheumatism, in its 2009 management guidelines for large-vessel vasculitis, recommend using an adjunctive immunosuppressant agent.19 In the case of GCA, they recommend using methotrexate 10 to 15 mg/week, which has shown modest evidence of reducing the relapse rate and lowering the cumulative doses of glucocorticoids needed.35,36
Studies of tumor necrosis factor inhibitors and abatacept have not yielded significant reductions in the relapse rate or decreased cumulative doses of prednisone.37,38
Advances in treatment for GCA have stagnated, but recent trials39,40 have evaluated the IL-6 receptor alpha inhibitor tocilizumab, given the central role of IL-6 in the pathogenesis of GCA. Case reports have revealed rapid induction and maintenance of remission in GCA using tocilizumab.41,42
Villiger et al39 performed a randomized, placebo-controlled trial to study the efficacy and safety of tocilizumab in induction and maintenance of disease remission in 30 patients with newly diagnosed GCA. The primary outcome, complete remission at 12 weeks, was achieved in 85% of patients who received tocilizumab plus tapered prednisolone, compared with 40% of patients who received placebo plus tapering prednisolone. The tocilizumab group also had favorable results in secondary outcomes including relapse-free survival at 52 weeks, time to first relapse after induction of remission, and cumulative dose of prednisolone.
The GiACTA trial. Stone et al40 studied the effect of tocilizumab on rates of relapse during glucocorticoid tapering in 251 GCA patients over the course of 52 weeks. Patients were randomized in a 2:1:1:1 ratio to 4 treatment groups:
- Tocilizumab weekly plus prednisone, with prednisone tapered over 26 weeks
- Tocilizumab every other week plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 52 weeks.
The primary outcome was the rate of sustained glucocorticoid-free remission at 52 weeks. Secondary outcomes included the remission rate, the cumulative glucocorticoid dose, and safety measures. At 52 weeks, the rates of sustained remission were:
- 56% with tocilizumab weekly
- 53% with tocilizumab every other week
- 14% with placebo plus 26-week prednisone taper
- 18% with placebo plus 52-week taper.
Differences between the active treatment groups and the placebo groups were statistically significant (P < .001).
The cumulative dose of prednisone in tocilizumab recipients was significantly less than in placebo recipients. Rates of adverse events were similar. Ultimately, the study showed that tocilizumab, either weekly or every other week, was more effective than prednisone alone at sustaining glucocorticoid-free remission in patients with GCA.
However, the study also raised questions about tocilizumab’s toxic effect profile and its long-term efficacy, as well as who are the optimal candidates for this therapy. Data on long-term use of tocilizumab are primarily taken from its use in rheumatoid arthritis.43 As of this writing, Stone et al are conducting an open-label trial to help provide long-term safety and efficacy data in patients with GCA. In the meantime, we must extrapolate data from the long-term use of tocilizumab in rheumatoid arthritis.
Tocilizumab and lower gastrointestinal tract perforation
One of the major adverse effects of long-term use of tocilizumab is lower gastrointestinal tract perforation.
Xie et al,44 in 2016, reported that the risk of perforation in patients on tocilizumab for rheumatoid arthritis was more than 2 times higher than in patients taking a tumor necrosis factor inhibitor. However, the absolute rates of perforation were low overall, roughly 1 to 3 per 1,000 patient-years in the tocilizumab group. Risk factors for perforation included older age, history of diverticulitis or other gastrointestinal tract condition, and prednisone doses of 7.5 mg or more a day.
Does tocilizumab prevent blindness?
Another consideration is that tocilizumab may not prevent optic neuropathy. In the GiACTA trial, 1 patient in the group receiving tocilizumab every other week developed optic neuropathy.40 Prednisone had been completely tapered off at the time, and the condition resolved when glucocorticoids were restarted. Thus, it is unknown if tocilizumab would be effective on its own without concomitant use of glucocorticoids.
Vision loss is one of the most severe complications of GCA, and it is still unclear whether tocilizumab can prevent vision loss in GCA. Also, we still have no data on the effect of tocilizumab on histopathologic findings, and whether biopsy yield diminishes over time. We hope future studies will help guide us in this regard.
No guidelines on tocilizumab yet
Clinical guidelines on the appropriate use of tocilizumab in GCA are lacking. The American College of Rheumatology and the European League Against Rheumatism have yet to publish updated guidelines with comments on use of tocilizumab. Therefore, it is unclear if tocilizumab is a first-line treatment in GCA, as its efficacy alone without glucocorticoids and its long-term safety in GCA patients have not been studied.
Treatment with tocilizumab should be individualized; it should be considered in patients who have had adverse effects from glucocorticoids, and in patients who experience a flare or cannot have their glucocorticoid dose lowered to an appropriate range.
The optimal duration of tocilizumab therapy is also unknown. However, using the GiACTA study as a rough guide, we try to limit its use to 1 year until additional data are available.
Patients on IL-6 inhibition may have suppressed C-reactive protein regardless of disease activity.43 Therefore, this laboratory value may not be reliable in determining active disease in patients on tocilizumab.
The GiACTA trial has shown an impressive improvement in the relapse-free remission period in patients with GCA taking tocilizumab. However, much work needs to be done to define the safety of this medication and determine which patients should be started on it. In the meantime, we recommend starting high-dose glucocorticoid therapy as soon as the diagnosis of GCA is suspected. In patients who do not tolerate glucocorticoids or whose disease flares during glucocorticoid taper, we recommend starting treatment with tocilizumab either once a week or every other week for at least 1 year.
- Brekke LK, Diamantopoulos AP, Fevang BT, Aßmus J, Esperø E, Gjesdal CG. Incidence of giant cell arteritis in Western Norway 1972–2012: a retrospective cohort study. Arthritis Res Ther 2017; 19(1):278. doi:10.1186/s13075-017-1479-6
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford) 2017; 56(4):506–515. doi:10.1093/rheumatology/kew273
- Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med 2014; 371(17):1653. doi:10.1056/NEJMc1409206
- Ghinoi A, Pipitone N, Nicolini A, et al. Large-vessel involvement in recent-onset giant cell arteritis: a case-control colour-Doppler sonography study. Rheumatology (Oxford) 2012; 51(4):730–734. doi:10.1093/rheumatology/ker329
- Prieto-González S, Depetris M, García-Martínez A, et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case-control study. Ann Rheum Dis 2014; 73(7):1388–1392. doi:10.1136/annrheumdis-2013-204572
- Brack A, Martinez-Taboada V, Stanson A, Goronzy JJ, Weyand CM. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum 1999; 42(2):311–317. doi:10.1002/1529-0131(199902)42:2<311::AID-ANR14>3.0.CO;2-F
- Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurence in a population-based study. Arthritis Rheum 2001; 45(2):140–145. doi:10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
- Liozon E, Jauberteau-Marchan MO, Ly K, Loustaud-Ratti V, Soria P, Vidal E. Giant cell arteritis with a low erythrocyte sedimentation rate: comments on the article by Salvarani and Hunder. Arthritis Rheum 2002; 47(6):692–694. doi:10.1002/art.10809
- Yu-Wai-Man P, Dayan MR. Giant cell arteritis with normal inflammatory markers. Acta Ophthalmol Scand 2007; 85(4):460. doi:10.1111/j.1600-0420.2006.00864.x
- Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA 2016; 315(22):2442–2458. doi:10.1001/jama.2016.5444
- Niederkohr RD, Levin LA. Management of the patient with suspected temporal arteritis a decision-analytic approach. Ophthalmology 2005; 112(5):744–756. doi:10.1016/j.ophtha.2005.01.031
- Bowling K, Rait J, Atkinson J, Srinivas G. Temporal artery biopsy in the diagnosis of giant cell arteritis: does the end justify the means? Ann Med Surg (Lond) 2017; 20:1–5. doi:10.1016/j.amsu.2017.06.020
- Daily B, Dassow P, Haynes J, Nashelsky J. Giant cell arteritis: biopsy after corticosteroid initiation. Am Fam Physician 2017; 95(2):116–117. pmid:28084703
- Durling B, Toren A, Patel V, Gilberg S, Weis E, Jordan D. Incidence of discordant temporal artery biopsy in the diagnosis of giant cell arteritis. Can J Ophthalmol 2014; 49(2):157–161. doi:10.1016/j.jcjo.2013.12.008
- Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018; 77(5):636–643. doi:10.1136/annrheumdis-2017-212649
- Aschwanden M, Imfeld S, Staub D, et al. The ultrasound compression sign to diagnose temporal giant cell arteritis shows an excellent interobserver agreement. Clin Exp Rheumatol 2015; 33(2 suppl 89):S-113–S-115. pmid:26016760
- Aiello PD, Trautmann JC, McPhee TJ, Kunselman AR, Hunder GG. Visual prognosis in giant cell arteritis. Ophthalmology 1993; 100(4):550–555. pmid:8479714
- Hayreh SS, Zimmerman B. Visual deterioration in giant cell arteritis patients while on high doses of corticosteroid therapy. Ophthalmology 2003; 110(6):1204–1215. doi:10.1016/S0161-6420(03)00228-8
- Mukhtyar C, Guillevin L, Cid MC, et al; European Vasculitis Study Group. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2009; 68(3):318–323. doi:10.1136/ard.2008.088351
- Dejaco C, Singh YP, Perel P, et al; European League Against Rheumatism; American College of Rheumatology. 2015 recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis 2015; 74(10):1799–1807. doi:10.1136/annrheumdis-2015-207492
- Bienvenu B, Ly KH, Lambert M, et al; Groupe d’Étude Français des Artérites des gros Vaisseaux, under the Aegis of the Filière des Maladies Auto-Immunes et Auto-Inflammatoires Rares. Management of giant cell arteritis: recommendations of the French Study Group for Large Vessel Vasculitis (GEFA). Rev Med Interne 2016; 37(3):154–165. doi:10.1016/j.revmed.2015.12.015
- Hayreh SS, Biousse V. Treatment of acute visual loss in giant cell arteritis: should we prescribe high-dose intravenous steroids or just oral steroids? J Neuroophthalmol 2012; 32(3):278–287. doi:10.1097/WNO.0b013e3182688218
- Restuccia G, Boiardi L, Cavazza A, et al. Flares in biopsy-proven giant cell arteritis in Northern Italy: characteristics and predictors in a long-term follow-up study. Medicine (Baltimore) 2016; 95(19):e3524. doi:10.1097/MD.0000000000003524
- Kermani TA, Warrington KJ, Cuthbertson D, et al; Vasculitis Clinical Research Consortium. Disease relapses among patients with giant cell arteritis: a prospective, longitudinal cohort study. J Rheumatol 2015; 42(7):1213–1217. doi:10.3899/jrheum.141347
- Labarca C, Koster MJ, Crowson CS, et al. Predictors of relapse and treatment outcomes in biopsy-proven giant cell arteritis: a retrospective cohort study. Rheumatology (Oxford) 2016; 55(2):347–356. doi:10.1093/rheumatology/kev348
- Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum 2003; 49(5):703–708. doi:10.1002/art.11388
- Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis 2002; 34(8):1098–1107. doi:10.1086/339548
- van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 2002; 13(10):777–787. doi:10.1007/s001980200108
- Heffernan MP, Saag KG, Robinson JK, Callen JP. Prevention of osteoporosis associated with chronic glucocorticoid therapy. JAMA 2006; 295(11):1300–1303. pmid:16541489
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2017; 69(8):1095–1110. doi:10.1002/acr.23279
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res 201; 62(11):1515–1526. doi:10.1002/acr.20295
- Allen CS, Yeung JH, Vandermeer B, Homik J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev 2016; 10:CD001347. doi:10.1002/14651858.CD001347.pub2
- Carpinteri R, Porcelli T, Mejia C, et al. Glucocorticoid-induced osteoporosis and parathyroid hormone. J Endocrinol Invest 2010; 33(suppl 7):16–21. pmid:20938221
- Saag KG, Wagman RB, Geusens P, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol 2018; 6(6):445–454. doi:10.1016/S2213-8587(18)30075-5
- Hoffman GS, Cid MC, Hellmann DB, et al; International Network for the Study of Systemic Vasculitides. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002; 46(5):1309–1318. doi:10.1002/art.10262
- Spiera RF, Mitnick HJ, Kupersmith M, et al. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol 2001; 19(5):495–501. pmid:11579707
- Hoffman GS, Cid MC, Rendt-Zagar KE, et al; Infliximab-GCA Study Group. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007; 146(9):621–630. pmid:17470830
- Langford CA, Cuthbertson D, Ytterberg SR, et al; Vasculitis Clinical Research Consortium. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol 2017; 69(4):837–845. doi:10.1002/art.40044
- Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2016; 387(10031):1921–1927. doi:10.1016/S0140-6736(16)00560-2
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Oliveira F, Butendieck RR, Ginsburg WW, Parikh K, Abril A. Tocilizumab, an effective treatment for relapsing giant cell arteritis. Clin Exp Rheumatol 2014; 32(3 suppl 82):S76–S78. pmid:24854376
- Loricera J, Blanco R, Hernández JL, et al. Tocilizumab in giant cell arteritis: multicenter open-label study of 22 patients. Semin Arthritis Rheum 2015; 44(6):717–723. doi:10.1016/j.semarthrit.2014.12.005
- Tamaki H, Hajj-Ali RA. Tocilizumab for giant cell arteritis—a new giant step in an old disease. JAMA Neurol 2018; 75(2):145–146. doi:10.1001/jamaneurol.2017.3811
- Xie F, Yun H, Bernatsky S, Curtis JR. Risk for gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologics. Arthritis Rheumatol 2016; 68(11):2612–2617. doi:10.1002/art.39761
- Brekke LK, Diamantopoulos AP, Fevang BT, Aßmus J, Esperø E, Gjesdal CG. Incidence of giant cell arteritis in Western Norway 1972–2012: a retrospective cohort study. Arthritis Res Ther 2017; 19(1):278. doi:10.1186/s13075-017-1479-6
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford) 2017; 56(4):506–515. doi:10.1093/rheumatology/kew273
- Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med 2014; 371(17):1653. doi:10.1056/NEJMc1409206
- Ghinoi A, Pipitone N, Nicolini A, et al. Large-vessel involvement in recent-onset giant cell arteritis: a case-control colour-Doppler sonography study. Rheumatology (Oxford) 2012; 51(4):730–734. doi:10.1093/rheumatology/ker329
- Prieto-González S, Depetris M, García-Martínez A, et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case-control study. Ann Rheum Dis 2014; 73(7):1388–1392. doi:10.1136/annrheumdis-2013-204572
- Brack A, Martinez-Taboada V, Stanson A, Goronzy JJ, Weyand CM. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum 1999; 42(2):311–317. doi:10.1002/1529-0131(199902)42:2<311::AID-ANR14>3.0.CO;2-F
- Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurence in a population-based study. Arthritis Rheum 2001; 45(2):140–145. doi:10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
- Liozon E, Jauberteau-Marchan MO, Ly K, Loustaud-Ratti V, Soria P, Vidal E. Giant cell arteritis with a low erythrocyte sedimentation rate: comments on the article by Salvarani and Hunder. Arthritis Rheum 2002; 47(6):692–694. doi:10.1002/art.10809
- Yu-Wai-Man P, Dayan MR. Giant cell arteritis with normal inflammatory markers. Acta Ophthalmol Scand 2007; 85(4):460. doi:10.1111/j.1600-0420.2006.00864.x
- Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA 2016; 315(22):2442–2458. doi:10.1001/jama.2016.5444
- Niederkohr RD, Levin LA. Management of the patient with suspected temporal arteritis a decision-analytic approach. Ophthalmology 2005; 112(5):744–756. doi:10.1016/j.ophtha.2005.01.031
- Bowling K, Rait J, Atkinson J, Srinivas G. Temporal artery biopsy in the diagnosis of giant cell arteritis: does the end justify the means? Ann Med Surg (Lond) 2017; 20:1–5. doi:10.1016/j.amsu.2017.06.020
- Daily B, Dassow P, Haynes J, Nashelsky J. Giant cell arteritis: biopsy after corticosteroid initiation. Am Fam Physician 2017; 95(2):116–117. pmid:28084703
- Durling B, Toren A, Patel V, Gilberg S, Weis E, Jordan D. Incidence of discordant temporal artery biopsy in the diagnosis of giant cell arteritis. Can J Ophthalmol 2014; 49(2):157–161. doi:10.1016/j.jcjo.2013.12.008
- Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018; 77(5):636–643. doi:10.1136/annrheumdis-2017-212649
- Aschwanden M, Imfeld S, Staub D, et al. The ultrasound compression sign to diagnose temporal giant cell arteritis shows an excellent interobserver agreement. Clin Exp Rheumatol 2015; 33(2 suppl 89):S-113–S-115. pmid:26016760
- Aiello PD, Trautmann JC, McPhee TJ, Kunselman AR, Hunder GG. Visual prognosis in giant cell arteritis. Ophthalmology 1993; 100(4):550–555. pmid:8479714
- Hayreh SS, Zimmerman B. Visual deterioration in giant cell arteritis patients while on high doses of corticosteroid therapy. Ophthalmology 2003; 110(6):1204–1215. doi:10.1016/S0161-6420(03)00228-8
- Mukhtyar C, Guillevin L, Cid MC, et al; European Vasculitis Study Group. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2009; 68(3):318–323. doi:10.1136/ard.2008.088351
- Dejaco C, Singh YP, Perel P, et al; European League Against Rheumatism; American College of Rheumatology. 2015 recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis 2015; 74(10):1799–1807. doi:10.1136/annrheumdis-2015-207492
- Bienvenu B, Ly KH, Lambert M, et al; Groupe d’Étude Français des Artérites des gros Vaisseaux, under the Aegis of the Filière des Maladies Auto-Immunes et Auto-Inflammatoires Rares. Management of giant cell arteritis: recommendations of the French Study Group for Large Vessel Vasculitis (GEFA). Rev Med Interne 2016; 37(3):154–165. doi:10.1016/j.revmed.2015.12.015
- Hayreh SS, Biousse V. Treatment of acute visual loss in giant cell arteritis: should we prescribe high-dose intravenous steroids or just oral steroids? J Neuroophthalmol 2012; 32(3):278–287. doi:10.1097/WNO.0b013e3182688218
- Restuccia G, Boiardi L, Cavazza A, et al. Flares in biopsy-proven giant cell arteritis in Northern Italy: characteristics and predictors in a long-term follow-up study. Medicine (Baltimore) 2016; 95(19):e3524. doi:10.1097/MD.0000000000003524
- Kermani TA, Warrington KJ, Cuthbertson D, et al; Vasculitis Clinical Research Consortium. Disease relapses among patients with giant cell arteritis: a prospective, longitudinal cohort study. J Rheumatol 2015; 42(7):1213–1217. doi:10.3899/jrheum.141347
- Labarca C, Koster MJ, Crowson CS, et al. Predictors of relapse and treatment outcomes in biopsy-proven giant cell arteritis: a retrospective cohort study. Rheumatology (Oxford) 2016; 55(2):347–356. doi:10.1093/rheumatology/kev348
- Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum 2003; 49(5):703–708. doi:10.1002/art.11388
- Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis 2002; 34(8):1098–1107. doi:10.1086/339548
- van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 2002; 13(10):777–787. doi:10.1007/s001980200108
- Heffernan MP, Saag KG, Robinson JK, Callen JP. Prevention of osteoporosis associated with chronic glucocorticoid therapy. JAMA 2006; 295(11):1300–1303. pmid:16541489
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2017; 69(8):1095–1110. doi:10.1002/acr.23279
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res 201; 62(11):1515–1526. doi:10.1002/acr.20295
- Allen CS, Yeung JH, Vandermeer B, Homik J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev 2016; 10:CD001347. doi:10.1002/14651858.CD001347.pub2
- Carpinteri R, Porcelli T, Mejia C, et al. Glucocorticoid-induced osteoporosis and parathyroid hormone. J Endocrinol Invest 2010; 33(suppl 7):16–21. pmid:20938221
- Saag KG, Wagman RB, Geusens P, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol 2018; 6(6):445–454. doi:10.1016/S2213-8587(18)30075-5
- Hoffman GS, Cid MC, Hellmann DB, et al; International Network for the Study of Systemic Vasculitides. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002; 46(5):1309–1318. doi:10.1002/art.10262
- Spiera RF, Mitnick HJ, Kupersmith M, et al. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol 2001; 19(5):495–501. pmid:11579707
- Hoffman GS, Cid MC, Rendt-Zagar KE, et al; Infliximab-GCA Study Group. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007; 146(9):621–630. pmid:17470830
- Langford CA, Cuthbertson D, Ytterberg SR, et al; Vasculitis Clinical Research Consortium. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol 2017; 69(4):837–845. doi:10.1002/art.40044
- Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2016; 387(10031):1921–1927. doi:10.1016/S0140-6736(16)00560-2
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Oliveira F, Butendieck RR, Ginsburg WW, Parikh K, Abril A. Tocilizumab, an effective treatment for relapsing giant cell arteritis. Clin Exp Rheumatol 2014; 32(3 suppl 82):S76–S78. pmid:24854376
- Loricera J, Blanco R, Hernández JL, et al. Tocilizumab in giant cell arteritis: multicenter open-label study of 22 patients. Semin Arthritis Rheum 2015; 44(6):717–723. doi:10.1016/j.semarthrit.2014.12.005
- Tamaki H, Hajj-Ali RA. Tocilizumab for giant cell arteritis—a new giant step in an old disease. JAMA Neurol 2018; 75(2):145–146. doi:10.1001/jamaneurol.2017.3811
- Xie F, Yun H, Bernatsky S, Curtis JR. Risk for gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologics. Arthritis Rheumatol 2016; 68(11):2612–2617. doi:10.1002/art.39761
KEY POINTS
- Giant cell arteritis can present with cranial symptoms, extracranial large-vessel involvement, or polymyalgia rheumatica.
- Temporal artery biopsy is the standard for diagnosis.
- Adverse effects of glucocorticoid treatment, particularly bone loss, need to be managed.
- In patients treated with glucocorticoids alone, the relapse rate is high when the drugs are tapered; thus, prolonged treatment is required.
Delayed Cardioversion Noninferior to Early Cardioversion in Recent-Onset Atrial Fibrillation
Study Overview
Objective. To assess whether immediate restoration of sinus rhythm is necessary in hemodynamically stable, recent onset (< 36 hr), symptomatic atrial fibrillation in the emergency department.
Design. Multicenter, randomized, open-label, noninferiority trial, RACE 7 ACWAS (Rate Control versus Electrical Cardioversion Trial 7--Acute Cardioversion versus Wait and See).
Setting and participants. 15 hospitals in the Netherlands, including 3 academic hospitals, 8 nonacademic teaching hospitals, and 4 nonteaching hospitals. Patients 18 years of age or older with recent-onset (< 36 hr), symptomatic atrial fibrillation without signs of myocardial ischemia or a history of persistent atrial fibrillation who presented to the emergency department were randomized in a 1:1 ratio to either a wait-and-see approach or early cardioversion. The wait-and-see approach consisted of the administration of rate-control medication, including intravenous or oral beta-adrenergic-receptor blocking agents, nondihydropyridine calcium-channel blockers, or digoxin to achieve a heart rate of 110 beats per minute or less and symptomatic relief. Patients were then discharged with an outpatient visit scheduled for the next day and a referral for cardioversion as close as possible to 48 hours after the onset of symptoms. The cardioconversion group received pharmacologic cardioversion with flecainide unless contraindicated, then electrical cardioversion was performed.
Main outcome measures. Primary outcome was the presence of sinus rhythm on electrocardiogram (ECG) recorded at the 4-week trial visit. Secondary endpoints included the duration of the index visit at the emergency department, emergency department visits related to atrial fibrillation, cardiovascular complications, and time until recurrence of atrial fibrillation.
Main results. From October 2014 through September 2018, 437 patients underwent randomization, with 218 patients assigned to the delayed cardioversion group and 219 to the early cardioversion group. Mean age was 65 years, and a majority of the patients (60%) were men (n = 261). The primary end point of the presence of sinus rhythm on the ECG recorded at the 4-week visit was present in 193 of 212 patients (91%) in the delayed cardioversion group and in 202 of 215 patients (94%) in the early cardioversion group. The –2.9 percentage points with confidence interval [CI] –8.2 to 2.2 (P = 0.005) met the criteria for the noninferiority of the wait-and-see approach.
For secondary outcomes, the median duration of the index visit was 120 minutes (range, 60 to 253) in the delayed cardioversion group and 158 minutes (range, 110 to 228) in the early cardioversion group. The median difference between the 2 groups was 30 minutes (95% CI, 6 to 51 minutes). There was no significant difference in cardiovascular complications between the 2 groups. Fourteen of 212 patients (7%) in the delayed cardioversion group and 14 of 215 patients (7%) in the early cardioversion group had subsequent visits to the emergency department because of a recurrence of atrial fibrillation. Telemetric ECG recordings were available for 335 of the 437 patients. Recurrence of atrial fibrillation occurred in 49 of the 164 (30%) patients in the delayed cardioversion group and 50 of the 171 (29%) patients in the early cardioversion group.
In terms of treatment, conversion to sinus rhythm within 48 hours occurred spontaneously in 150 of 218 patients (69%) in the delayed cardioversion group after receiving rate-control medications only. Of the 218 patients, 61 (28%) had delayed cardioversion (9 by pharmacologic and 52 by electrical cardioversion) as per protocol and achieved sinus rhythm within 48 hours. In the early cardioversion group, conversion to sinus rhythm occurred spontaneously in 36 of 219 patients (16%) before the initiation of the cardioversion and in 171 of 219 (78%) after cardioversion (83 by pharmacologic and 88 by electrical).
Conclusion. For patients with recent-onset, symptomatic atrial fibrillation, allowing a short time for spontaneous conversion to sinus rhythm is reasonable as demonstrated by this noninferiority study.
Commentary
Atrial fibrillation accounts for nearly 0.5% of all emergency department visits, and this number is increasing.1,2 Patients commonly undergo immediate restoration of sinus rhythm by means of pharmacologic or electrical cardioversion. However, it is questionable whether immediate restoration of sinus rhythm is necessary, as spontaneous conversion to sinus rhythm occurs frequently. In addition, the safety of cardioversion between 12 and 48 hours after the onset of atrial fibrillation is questionable.3,4
In this pragmatic trial, the findings suggest that rate-control therapy alone can achieve prompt symptom relief in almost all eligible patients, had a low risk of complications, and reduced the median length of stay in the emergency department to 2 hours. Independent of cardioversion strategy, the authors stressed the importance of management of stroke risk when patients present with atrial fibrillation to the emergency department. In this trial, 2 patients had cerebral embolism even though both were started on anticoagulation in the index visit. One patient from the delayed cardioversion group was on dabigatran after spontaneous conversion to sinus rhythm and had an event 5 days after the index visit. The other patient, from the early cardioversion group, was on rivaroxaban and had an event 10 days after electrical cardiology. In order for the results of this trial to be broadly applicable, exclusion of intraatrial thrombus on transesophageal echocardiography may be necessary when the onset of atrial fibrillation is not as clear.
There are several limitations of this study. First, this study included only 171 of the 3706 patients (4.6%) screened systematically at the 2 academic centers, but included 266 from 13 centers without systematic screening. The large amount of patients excluded from the controlled environment made the results less generalizable in the broader scope. Second, the reported incidence of recurrent atrial fibrillation within 4 weeks after randomization was an underestimation of the true recurrence rate since the trial used intermittent monitoring. Although the incidence of about 30% was similar between the 2 groups, the authors suggested that the probability of recurrence of atrial fibrillation was not affected by management approach during the acute event. Finally, for these results to be applicable in the general population, defined treatment algorithms and access to prompt follow-up are needed, and these may not be practical in other clinical settings.2,5
Applications for Clinical Practice
The current study demonstrated immediate cardioversion is not necessary for patients with recent-onset, symptomatic atrial fibrillation in the emergency department. Allowing a short time for spontaneous conversion to sinus rhythm is reasonable as long as the total time in atrial fibrillation is less than 48 hours. Special consideration for anticoagulation is critical because stroke has been associated with atrial fibrillation duration between 24 and 48 hours.
—Ka Ming Gordon Ngai, MD, MPH
1. Rozen G, Hosseini SM, Kaadan MI, et al. Emergency department visits for atrial fibrillation in the United States: trends in admission rates and economic burden from 2007 to 2014. J Am Heart Assoc. 2018;7(15):e009024.
2. Healey JS, McIntyre WF. The RACE to treat atrial fibrillation in the emergency department. N Engl J Med. 2019 Mar 18.
3. Andrade JM, Verma A, Mitchell LB, et al. 2018 Focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol. 2018;34:1371-1392.
4. Nuotio I, Hartikainen JE, Grönberg T, et al. Time to cardioversion for acute atrial fibrillation and thromboembolic complications. JAMA. 2014;312:647-649
5. Baugh CW, Clark CL, Wilson JW, et al. Creation and implementation of an outpatient pathway for atrial fibrillation in the emergency department setting: results of an expert panel. Acad Emerg Med. 2018;25:1065-1075.
Study Overview
Objective. To assess whether immediate restoration of sinus rhythm is necessary in hemodynamically stable, recent onset (< 36 hr), symptomatic atrial fibrillation in the emergency department.
Design. Multicenter, randomized, open-label, noninferiority trial, RACE 7 ACWAS (Rate Control versus Electrical Cardioversion Trial 7--Acute Cardioversion versus Wait and See).
Setting and participants. 15 hospitals in the Netherlands, including 3 academic hospitals, 8 nonacademic teaching hospitals, and 4 nonteaching hospitals. Patients 18 years of age or older with recent-onset (< 36 hr), symptomatic atrial fibrillation without signs of myocardial ischemia or a history of persistent atrial fibrillation who presented to the emergency department were randomized in a 1:1 ratio to either a wait-and-see approach or early cardioversion. The wait-and-see approach consisted of the administration of rate-control medication, including intravenous or oral beta-adrenergic-receptor blocking agents, nondihydropyridine calcium-channel blockers, or digoxin to achieve a heart rate of 110 beats per minute or less and symptomatic relief. Patients were then discharged with an outpatient visit scheduled for the next day and a referral for cardioversion as close as possible to 48 hours after the onset of symptoms. The cardioconversion group received pharmacologic cardioversion with flecainide unless contraindicated, then electrical cardioversion was performed.
Main outcome measures. Primary outcome was the presence of sinus rhythm on electrocardiogram (ECG) recorded at the 4-week trial visit. Secondary endpoints included the duration of the index visit at the emergency department, emergency department visits related to atrial fibrillation, cardiovascular complications, and time until recurrence of atrial fibrillation.
Main results. From October 2014 through September 2018, 437 patients underwent randomization, with 218 patients assigned to the delayed cardioversion group and 219 to the early cardioversion group. Mean age was 65 years, and a majority of the patients (60%) were men (n = 261). The primary end point of the presence of sinus rhythm on the ECG recorded at the 4-week visit was present in 193 of 212 patients (91%) in the delayed cardioversion group and in 202 of 215 patients (94%) in the early cardioversion group. The –2.9 percentage points with confidence interval [CI] –8.2 to 2.2 (P = 0.005) met the criteria for the noninferiority of the wait-and-see approach.
For secondary outcomes, the median duration of the index visit was 120 minutes (range, 60 to 253) in the delayed cardioversion group and 158 minutes (range, 110 to 228) in the early cardioversion group. The median difference between the 2 groups was 30 minutes (95% CI, 6 to 51 minutes). There was no significant difference in cardiovascular complications between the 2 groups. Fourteen of 212 patients (7%) in the delayed cardioversion group and 14 of 215 patients (7%) in the early cardioversion group had subsequent visits to the emergency department because of a recurrence of atrial fibrillation. Telemetric ECG recordings were available for 335 of the 437 patients. Recurrence of atrial fibrillation occurred in 49 of the 164 (30%) patients in the delayed cardioversion group and 50 of the 171 (29%) patients in the early cardioversion group.
In terms of treatment, conversion to sinus rhythm within 48 hours occurred spontaneously in 150 of 218 patients (69%) in the delayed cardioversion group after receiving rate-control medications only. Of the 218 patients, 61 (28%) had delayed cardioversion (9 by pharmacologic and 52 by electrical cardioversion) as per protocol and achieved sinus rhythm within 48 hours. In the early cardioversion group, conversion to sinus rhythm occurred spontaneously in 36 of 219 patients (16%) before the initiation of the cardioversion and in 171 of 219 (78%) after cardioversion (83 by pharmacologic and 88 by electrical).
Conclusion. For patients with recent-onset, symptomatic atrial fibrillation, allowing a short time for spontaneous conversion to sinus rhythm is reasonable as demonstrated by this noninferiority study.
Commentary
Atrial fibrillation accounts for nearly 0.5% of all emergency department visits, and this number is increasing.1,2 Patients commonly undergo immediate restoration of sinus rhythm by means of pharmacologic or electrical cardioversion. However, it is questionable whether immediate restoration of sinus rhythm is necessary, as spontaneous conversion to sinus rhythm occurs frequently. In addition, the safety of cardioversion between 12 and 48 hours after the onset of atrial fibrillation is questionable.3,4
In this pragmatic trial, the findings suggest that rate-control therapy alone can achieve prompt symptom relief in almost all eligible patients, had a low risk of complications, and reduced the median length of stay in the emergency department to 2 hours. Independent of cardioversion strategy, the authors stressed the importance of management of stroke risk when patients present with atrial fibrillation to the emergency department. In this trial, 2 patients had cerebral embolism even though both were started on anticoagulation in the index visit. One patient from the delayed cardioversion group was on dabigatran after spontaneous conversion to sinus rhythm and had an event 5 days after the index visit. The other patient, from the early cardioversion group, was on rivaroxaban and had an event 10 days after electrical cardiology. In order for the results of this trial to be broadly applicable, exclusion of intraatrial thrombus on transesophageal echocardiography may be necessary when the onset of atrial fibrillation is not as clear.
There are several limitations of this study. First, this study included only 171 of the 3706 patients (4.6%) screened systematically at the 2 academic centers, but included 266 from 13 centers without systematic screening. The large amount of patients excluded from the controlled environment made the results less generalizable in the broader scope. Second, the reported incidence of recurrent atrial fibrillation within 4 weeks after randomization was an underestimation of the true recurrence rate since the trial used intermittent monitoring. Although the incidence of about 30% was similar between the 2 groups, the authors suggested that the probability of recurrence of atrial fibrillation was not affected by management approach during the acute event. Finally, for these results to be applicable in the general population, defined treatment algorithms and access to prompt follow-up are needed, and these may not be practical in other clinical settings.2,5
Applications for Clinical Practice
The current study demonstrated immediate cardioversion is not necessary for patients with recent-onset, symptomatic atrial fibrillation in the emergency department. Allowing a short time for spontaneous conversion to sinus rhythm is reasonable as long as the total time in atrial fibrillation is less than 48 hours. Special consideration for anticoagulation is critical because stroke has been associated with atrial fibrillation duration between 24 and 48 hours.
—Ka Ming Gordon Ngai, MD, MPH
Study Overview
Objective. To assess whether immediate restoration of sinus rhythm is necessary in hemodynamically stable, recent onset (< 36 hr), symptomatic atrial fibrillation in the emergency department.
Design. Multicenter, randomized, open-label, noninferiority trial, RACE 7 ACWAS (Rate Control versus Electrical Cardioversion Trial 7--Acute Cardioversion versus Wait and See).
Setting and participants. 15 hospitals in the Netherlands, including 3 academic hospitals, 8 nonacademic teaching hospitals, and 4 nonteaching hospitals. Patients 18 years of age or older with recent-onset (< 36 hr), symptomatic atrial fibrillation without signs of myocardial ischemia or a history of persistent atrial fibrillation who presented to the emergency department were randomized in a 1:1 ratio to either a wait-and-see approach or early cardioversion. The wait-and-see approach consisted of the administration of rate-control medication, including intravenous or oral beta-adrenergic-receptor blocking agents, nondihydropyridine calcium-channel blockers, or digoxin to achieve a heart rate of 110 beats per minute or less and symptomatic relief. Patients were then discharged with an outpatient visit scheduled for the next day and a referral for cardioversion as close as possible to 48 hours after the onset of symptoms. The cardioconversion group received pharmacologic cardioversion with flecainide unless contraindicated, then electrical cardioversion was performed.
Main outcome measures. Primary outcome was the presence of sinus rhythm on electrocardiogram (ECG) recorded at the 4-week trial visit. Secondary endpoints included the duration of the index visit at the emergency department, emergency department visits related to atrial fibrillation, cardiovascular complications, and time until recurrence of atrial fibrillation.
Main results. From October 2014 through September 2018, 437 patients underwent randomization, with 218 patients assigned to the delayed cardioversion group and 219 to the early cardioversion group. Mean age was 65 years, and a majority of the patients (60%) were men (n = 261). The primary end point of the presence of sinus rhythm on the ECG recorded at the 4-week visit was present in 193 of 212 patients (91%) in the delayed cardioversion group and in 202 of 215 patients (94%) in the early cardioversion group. The –2.9 percentage points with confidence interval [CI] –8.2 to 2.2 (P = 0.005) met the criteria for the noninferiority of the wait-and-see approach.
For secondary outcomes, the median duration of the index visit was 120 minutes (range, 60 to 253) in the delayed cardioversion group and 158 minutes (range, 110 to 228) in the early cardioversion group. The median difference between the 2 groups was 30 minutes (95% CI, 6 to 51 minutes). There was no significant difference in cardiovascular complications between the 2 groups. Fourteen of 212 patients (7%) in the delayed cardioversion group and 14 of 215 patients (7%) in the early cardioversion group had subsequent visits to the emergency department because of a recurrence of atrial fibrillation. Telemetric ECG recordings were available for 335 of the 437 patients. Recurrence of atrial fibrillation occurred in 49 of the 164 (30%) patients in the delayed cardioversion group and 50 of the 171 (29%) patients in the early cardioversion group.
In terms of treatment, conversion to sinus rhythm within 48 hours occurred spontaneously in 150 of 218 patients (69%) in the delayed cardioversion group after receiving rate-control medications only. Of the 218 patients, 61 (28%) had delayed cardioversion (9 by pharmacologic and 52 by electrical cardioversion) as per protocol and achieved sinus rhythm within 48 hours. In the early cardioversion group, conversion to sinus rhythm occurred spontaneously in 36 of 219 patients (16%) before the initiation of the cardioversion and in 171 of 219 (78%) after cardioversion (83 by pharmacologic and 88 by electrical).
Conclusion. For patients with recent-onset, symptomatic atrial fibrillation, allowing a short time for spontaneous conversion to sinus rhythm is reasonable as demonstrated by this noninferiority study.
Commentary
Atrial fibrillation accounts for nearly 0.5% of all emergency department visits, and this number is increasing.1,2 Patients commonly undergo immediate restoration of sinus rhythm by means of pharmacologic or electrical cardioversion. However, it is questionable whether immediate restoration of sinus rhythm is necessary, as spontaneous conversion to sinus rhythm occurs frequently. In addition, the safety of cardioversion between 12 and 48 hours after the onset of atrial fibrillation is questionable.3,4
In this pragmatic trial, the findings suggest that rate-control therapy alone can achieve prompt symptom relief in almost all eligible patients, had a low risk of complications, and reduced the median length of stay in the emergency department to 2 hours. Independent of cardioversion strategy, the authors stressed the importance of management of stroke risk when patients present with atrial fibrillation to the emergency department. In this trial, 2 patients had cerebral embolism even though both were started on anticoagulation in the index visit. One patient from the delayed cardioversion group was on dabigatran after spontaneous conversion to sinus rhythm and had an event 5 days after the index visit. The other patient, from the early cardioversion group, was on rivaroxaban and had an event 10 days after electrical cardiology. In order for the results of this trial to be broadly applicable, exclusion of intraatrial thrombus on transesophageal echocardiography may be necessary when the onset of atrial fibrillation is not as clear.
There are several limitations of this study. First, this study included only 171 of the 3706 patients (4.6%) screened systematically at the 2 academic centers, but included 266 from 13 centers without systematic screening. The large amount of patients excluded from the controlled environment made the results less generalizable in the broader scope. Second, the reported incidence of recurrent atrial fibrillation within 4 weeks after randomization was an underestimation of the true recurrence rate since the trial used intermittent monitoring. Although the incidence of about 30% was similar between the 2 groups, the authors suggested that the probability of recurrence of atrial fibrillation was not affected by management approach during the acute event. Finally, for these results to be applicable in the general population, defined treatment algorithms and access to prompt follow-up are needed, and these may not be practical in other clinical settings.2,5
Applications for Clinical Practice
The current study demonstrated immediate cardioversion is not necessary for patients with recent-onset, symptomatic atrial fibrillation in the emergency department. Allowing a short time for spontaneous conversion to sinus rhythm is reasonable as long as the total time in atrial fibrillation is less than 48 hours. Special consideration for anticoagulation is critical because stroke has been associated with atrial fibrillation duration between 24 and 48 hours.
—Ka Ming Gordon Ngai, MD, MPH
1. Rozen G, Hosseini SM, Kaadan MI, et al. Emergency department visits for atrial fibrillation in the United States: trends in admission rates and economic burden from 2007 to 2014. J Am Heart Assoc. 2018;7(15):e009024.
2. Healey JS, McIntyre WF. The RACE to treat atrial fibrillation in the emergency department. N Engl J Med. 2019 Mar 18.
3. Andrade JM, Verma A, Mitchell LB, et al. 2018 Focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol. 2018;34:1371-1392.
4. Nuotio I, Hartikainen JE, Grönberg T, et al. Time to cardioversion for acute atrial fibrillation and thromboembolic complications. JAMA. 2014;312:647-649
5. Baugh CW, Clark CL, Wilson JW, et al. Creation and implementation of an outpatient pathway for atrial fibrillation in the emergency department setting: results of an expert panel. Acad Emerg Med. 2018;25:1065-1075.
1. Rozen G, Hosseini SM, Kaadan MI, et al. Emergency department visits for atrial fibrillation in the United States: trends in admission rates and economic burden from 2007 to 2014. J Am Heart Assoc. 2018;7(15):e009024.
2. Healey JS, McIntyre WF. The RACE to treat atrial fibrillation in the emergency department. N Engl J Med. 2019 Mar 18.
3. Andrade JM, Verma A, Mitchell LB, et al. 2018 Focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol. 2018;34:1371-1392.
4. Nuotio I, Hartikainen JE, Grönberg T, et al. Time to cardioversion for acute atrial fibrillation and thromboembolic complications. JAMA. 2014;312:647-649
5. Baugh CW, Clark CL, Wilson JW, et al. Creation and implementation of an outpatient pathway for atrial fibrillation in the emergency department setting: results of an expert panel. Acad Emerg Med. 2018;25:1065-1075.
When adolescents visit the ED, 10% leave with an opioid
although there was a small but significant decrease in prescriptions over that time, according to an analysis of two nationwide ambulatory care surveys.
For adolescents aged 13-17 years, 10.4% of ED visits were associated with a prescription for an opioid versus 1.6% among outpatient visits. There was a slight but significant decrease in the rate of opioid prescriptions in the ED setting over the study period, with an odds ratio of 0.95 (95% confidence interval, 0.92-0.97), but there was no significant change in the trend over time in the outpatient setting (OR, 1.02; 95% CI, 0.99-1.09), Joel D. Hudgins, MD, and associates reported in Pediatrics.
“Opioid prescribing in ambulatory care visits is particularly high in the ED setting and … certain diagnoses appear to be routinely treated with an opioid,” said Dr. Hudgins and associates from Boston Children’s Hospital.
The highest rates of opioid prescribing among adolescents visiting the ED involved dental disorders (60%) and acute injuries such as fractures of the clavicle (47%), ankle (38%), and metacarpals (36%). “However, when considering the total volume of opioid prescriptions dispensed [over 7.8 million during 2005-2015], certain common conditions, including abdominal pain, acute pharyngitis, urinary tract infection, and headache, contributed large numbers of prescriptions as well,” they added.
The study involved data from the National Hospital Ambulatory Medical Care Survey (hospital-based EDs) and the National Ambulatory Medical Care Survey (office-based practices), which both are conducted annually by the National Center for Health Statistics.
The senior investigator is supported by an award from the Burroughs Wellcome Fund by the Harvard-MIT Center for Regulatory Science. The authors said that they have no relevant financial relationships.
SOURCE: Hudgins JD et al. Pediatrics. 2019 June. doi: 10.1542/peds.2018-1578.
although there was a small but significant decrease in prescriptions over that time, according to an analysis of two nationwide ambulatory care surveys.

For adolescents aged 13-17 years, 10.4% of ED visits were associated with a prescription for an opioid versus 1.6% among outpatient visits. There was a slight but significant decrease in the rate of opioid prescriptions in the ED setting over the study period, with an odds ratio of 0.95 (95% confidence interval, 0.92-0.97), but there was no significant change in the trend over time in the outpatient setting (OR, 1.02; 95% CI, 0.99-1.09), Joel D. Hudgins, MD, and associates reported in Pediatrics.
“Opioid prescribing in ambulatory care visits is particularly high in the ED setting and … certain diagnoses appear to be routinely treated with an opioid,” said Dr. Hudgins and associates from Boston Children’s Hospital.
The highest rates of opioid prescribing among adolescents visiting the ED involved dental disorders (60%) and acute injuries such as fractures of the clavicle (47%), ankle (38%), and metacarpals (36%). “However, when considering the total volume of opioid prescriptions dispensed [over 7.8 million during 2005-2015], certain common conditions, including abdominal pain, acute pharyngitis, urinary tract infection, and headache, contributed large numbers of prescriptions as well,” they added.
The study involved data from the National Hospital Ambulatory Medical Care Survey (hospital-based EDs) and the National Ambulatory Medical Care Survey (office-based practices), which both are conducted annually by the National Center for Health Statistics.
The senior investigator is supported by an award from the Burroughs Wellcome Fund by the Harvard-MIT Center for Regulatory Science. The authors said that they have no relevant financial relationships.
SOURCE: Hudgins JD et al. Pediatrics. 2019 June. doi: 10.1542/peds.2018-1578.
although there was a small but significant decrease in prescriptions over that time, according to an analysis of two nationwide ambulatory care surveys.

For adolescents aged 13-17 years, 10.4% of ED visits were associated with a prescription for an opioid versus 1.6% among outpatient visits. There was a slight but significant decrease in the rate of opioid prescriptions in the ED setting over the study period, with an odds ratio of 0.95 (95% confidence interval, 0.92-0.97), but there was no significant change in the trend over time in the outpatient setting (OR, 1.02; 95% CI, 0.99-1.09), Joel D. Hudgins, MD, and associates reported in Pediatrics.
“Opioid prescribing in ambulatory care visits is particularly high in the ED setting and … certain diagnoses appear to be routinely treated with an opioid,” said Dr. Hudgins and associates from Boston Children’s Hospital.
The highest rates of opioid prescribing among adolescents visiting the ED involved dental disorders (60%) and acute injuries such as fractures of the clavicle (47%), ankle (38%), and metacarpals (36%). “However, when considering the total volume of opioid prescriptions dispensed [over 7.8 million during 2005-2015], certain common conditions, including abdominal pain, acute pharyngitis, urinary tract infection, and headache, contributed large numbers of prescriptions as well,” they added.
The study involved data from the National Hospital Ambulatory Medical Care Survey (hospital-based EDs) and the National Ambulatory Medical Care Survey (office-based practices), which both are conducted annually by the National Center for Health Statistics.
The senior investigator is supported by an award from the Burroughs Wellcome Fund by the Harvard-MIT Center for Regulatory Science. The authors said that they have no relevant financial relationships.
SOURCE: Hudgins JD et al. Pediatrics. 2019 June. doi: 10.1542/peds.2018-1578.
FROM PEDIATRICS
CABANA: Heart failure patients got biggest bang from AFib ablation
SAN FRANCISCO – Catheter ablation of atrial fibrillation (AFib) in the roughly one-third of patients with heart failure enrolled in the CABANA multicenter, randomized trial produced striking, statistically significant improvements both in the study’s primary, combined endpoint and in all-cause mortality in intention-to-treat analyses.
These findings, from prespecified secondary analyses, contrasted with the study’s overall result, which showed no benefit in the primary endpoint analysis in the total study population of 2,204 patients with AFib (JAMA. 2019 Apr 2;321[13]:1261-74). They are also at odds with the primary endpoint result in the two-thirds of enrolled patients without heart failure, which showed no significant between-group differences in these two outcome measures among the patients assigned to the catheter ablation arm and the study’s control, which was medical management arm.
Among the 778 AFib patients enrolled in CABANA with any form of heart failure (35% of the total study enrollment), the incidence of the study’s primary endpoint – the combined rate of death, disabling stroke, serious bleeding, or cardiac arrest during a median follow-up of slightly more than 4 years – was 36% lower among the catheter-ablated heart failure patients than in the heart failure patients assigned to medical treatment, according to an intention-to-treat analysis, which was a statistically significant difference. The incidence of all-cause mortality during follow-up was 43% lower in the ablated heart failure patients, compared with the controls, Douglas L. Packer, MD, said at the annual scientific sessions of the Heart Rhythm Society.
In contrast, among enrolled patients without heart failure, the intention-to-treat primary endpoint was 6% higher in the ablated patients, and all-cause mortality was a relative 27% higher, although neither difference was statistically significant.
It’s a “little surprising” that the results showed this much benefit in the patients with heart failure, said Dr. Packer, professor of medicine at the Mayo Clinic in Rochester, Minn., and lead investigator of the CABANA (Catheter Ablation vs Anti-Arrhythmic Drug Therapy for Atrial Fibrillation) trial. “I think these data confirm the results of the CASTLE-AF trial, but without some of the glitches some people have cited” about that study, such as concerns about a high level of patient selection in CASTLE-AF and its relatively modest number of enrolled patients, he said in an interview.
The CASTLE-AF (Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF) study, run entirely in patients with heart failure with reduced ejection fraction and AFib, showed a statistically significant improvement in patient survival and heart failure hospitalization after catheter ablation compared with medical management (N Engl J Med. 2018 Feb 1;378[5]:417-27). Prior to the CASTLE-AF report, results from several other small studies (J Interv Card Electrophysiol. 2018 Oct;53[1]:19-29), as well as those from the AATAC trial (Circulation. 2016 Apr 26;133[17]:1637-44), also showed consistent evidence for benefit from catheter ablation in patients with heart failure and AFib, noted CABANA coinvestigator Jonathan P. Piccini, MD, during a separate talk at the meeting.
“The improvement of cardiovascular outcomes with ablation in patients with heart failure and AFib is consistent across multiple trials, at least with respect to heart failure with reduced ejection fraction” concluded Dr. Piccini, a cardiac electrophysiologist at Duke University in Durham, N.C.
As a result of the new heart failure analysis, “I think the guidelines will change,” predicted Dr. Packer, with catheter ablation receiving a firmer endorsement for patients with heart failure the next time U.S. guidelines for heart failure and AFib management are updated. The findings say “there is substantial benefit of catheter ablation in heart failure patients, but I don’t think our findings lessen the utility of ablation in patients without heart failure,” he stressed. Even patients without heart failure showed reduction in AFib burden and improvement in quality of life that were similar to what was seen in the heart failure patients.
The new report from CABANA of benefit from AFib catheter ablation in patients with heart failure “absolutely advances the evidence,” commented Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern University in Chicago. “A number of us were quite circumspect about this based on the CASTLE-AF data, but the new CABANA analyses have addressed our anxiety that the CASTLE-AF results were just by chance.” The new CABANA analyses “may not confirm CASTLE-AF, but it enriches the conversation and makes it possible that we are seeing benefit in some patients with heart failure who get ablated.”
Dr. Yancy, who chaired the most recent update to the U.S. heart failure management guideline (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803) stopped short of saying that the cumulative evidence now supports a guideline change, but he acknowledged in an interview that the evidence could legitimately influence practice. Catheter ablation should now be “strongly considered” in patients with heart failure and AFib, he said, although he also had three qualifications for opting for this approach: Patients must already be on guideline-directed medical therapy for their heart failure, the catheter ablation needs to be performed by an experienced and skilled operator, and follow-up surveillance must focus on both the patient’s AFib and heart failure. “It’s absolutely appropriate to consider catheter ablation” for heart failure patients, but the evidence is not yet there for guideline change, Dr. Yancy concluded.
It remains uncertain why catheter ablation of AFib should be more effective in patients with heart failure than in those without. Dr. Packer speculated that one reason may be the heart rate reduction that AFib ablation produces may especially benefit heart failure patients. An additional helpful effect of ablation in heart failure patients may be reducing heart rate variability. Another notable finding of the new analysis was that 79% of patients with heart failure in CABANA had heart failure with preserved ejection fraction, with a left ventricular ejection fraction of at least 50%. “Getting rid of AFib in patients with heart failure with preserved ejection fraction will be more important than we have thought,” Dr. Packer said.
Other new CABANA analyses presented for the first time in separate talks at the meeting also showed that, while catheter ablation had no meaningful difference in effect on outcomes based on the sex of patients, both age and minority ethnic and racial status appeared to make a substantial difference. For CABANA’s primary endpoint, catheter ablation was especially effective for improving outcomes in patients 64 years old or younger, and the analysis showed a signal of possibly worse outcomes in patients who were at least 75 years old. The “substantially” better outcomes in minority-group patients represented the largest between-group difference among subgroups seen in CABANA and is a “big deal,” said Dr. Packer, who predicted that future catheter ablation use will likely rise in patients with heart failure, in younger patients, and in minority patients.
Dr. Piccini noted that, “it’s possible that CABANA identified some patient subgroups that do really well after ablation, but the problem is that, in the United States, we now often don’t treat” minority patients or those with reduced left ventricular ejection fractions with ablation, according to recent registry findings.
CABANA received partial funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. Dr. Packer has been a consultant to and/or received research funding from these four companies, as well as numerous drug and device companies, and has a financial interest in a licensed AFib mapping technology. Dr. Piccini has ties Boston Scientific, Medtronic, and numerous other drug and device companies, and disclosed an unspecified relationship with GlaxoSmithKline. Dr. Yancy disclosed an unspecified relationship with Abbott Laboratories.
SOURCE: Packer DL. Heart Rhythm 2019, Abstract S-AB14-06.
SAN FRANCISCO – Catheter ablation of atrial fibrillation (AFib) in the roughly one-third of patients with heart failure enrolled in the CABANA multicenter, randomized trial produced striking, statistically significant improvements both in the study’s primary, combined endpoint and in all-cause mortality in intention-to-treat analyses.
These findings, from prespecified secondary analyses, contrasted with the study’s overall result, which showed no benefit in the primary endpoint analysis in the total study population of 2,204 patients with AFib (JAMA. 2019 Apr 2;321[13]:1261-74). They are also at odds with the primary endpoint result in the two-thirds of enrolled patients without heart failure, which showed no significant between-group differences in these two outcome measures among the patients assigned to the catheter ablation arm and the study’s control, which was medical management arm.
Among the 778 AFib patients enrolled in CABANA with any form of heart failure (35% of the total study enrollment), the incidence of the study’s primary endpoint – the combined rate of death, disabling stroke, serious bleeding, or cardiac arrest during a median follow-up of slightly more than 4 years – was 36% lower among the catheter-ablated heart failure patients than in the heart failure patients assigned to medical treatment, according to an intention-to-treat analysis, which was a statistically significant difference. The incidence of all-cause mortality during follow-up was 43% lower in the ablated heart failure patients, compared with the controls, Douglas L. Packer, MD, said at the annual scientific sessions of the Heart Rhythm Society.
In contrast, among enrolled patients without heart failure, the intention-to-treat primary endpoint was 6% higher in the ablated patients, and all-cause mortality was a relative 27% higher, although neither difference was statistically significant.
It’s a “little surprising” that the results showed this much benefit in the patients with heart failure, said Dr. Packer, professor of medicine at the Mayo Clinic in Rochester, Minn., and lead investigator of the CABANA (Catheter Ablation vs Anti-Arrhythmic Drug Therapy for Atrial Fibrillation) trial. “I think these data confirm the results of the CASTLE-AF trial, but without some of the glitches some people have cited” about that study, such as concerns about a high level of patient selection in CASTLE-AF and its relatively modest number of enrolled patients, he said in an interview.
The CASTLE-AF (Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF) study, run entirely in patients with heart failure with reduced ejection fraction and AFib, showed a statistically significant improvement in patient survival and heart failure hospitalization after catheter ablation compared with medical management (N Engl J Med. 2018 Feb 1;378[5]:417-27). Prior to the CASTLE-AF report, results from several other small studies (J Interv Card Electrophysiol. 2018 Oct;53[1]:19-29), as well as those from the AATAC trial (Circulation. 2016 Apr 26;133[17]:1637-44), also showed consistent evidence for benefit from catheter ablation in patients with heart failure and AFib, noted CABANA coinvestigator Jonathan P. Piccini, MD, during a separate talk at the meeting.
“The improvement of cardiovascular outcomes with ablation in patients with heart failure and AFib is consistent across multiple trials, at least with respect to heart failure with reduced ejection fraction” concluded Dr. Piccini, a cardiac electrophysiologist at Duke University in Durham, N.C.
As a result of the new heart failure analysis, “I think the guidelines will change,” predicted Dr. Packer, with catheter ablation receiving a firmer endorsement for patients with heart failure the next time U.S. guidelines for heart failure and AFib management are updated. The findings say “there is substantial benefit of catheter ablation in heart failure patients, but I don’t think our findings lessen the utility of ablation in patients without heart failure,” he stressed. Even patients without heart failure showed reduction in AFib burden and improvement in quality of life that were similar to what was seen in the heart failure patients.
The new report from CABANA of benefit from AFib catheter ablation in patients with heart failure “absolutely advances the evidence,” commented Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern University in Chicago. “A number of us were quite circumspect about this based on the CASTLE-AF data, but the new CABANA analyses have addressed our anxiety that the CASTLE-AF results were just by chance.” The new CABANA analyses “may not confirm CASTLE-AF, but it enriches the conversation and makes it possible that we are seeing benefit in some patients with heart failure who get ablated.”
Dr. Yancy, who chaired the most recent update to the U.S. heart failure management guideline (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803) stopped short of saying that the cumulative evidence now supports a guideline change, but he acknowledged in an interview that the evidence could legitimately influence practice. Catheter ablation should now be “strongly considered” in patients with heart failure and AFib, he said, although he also had three qualifications for opting for this approach: Patients must already be on guideline-directed medical therapy for their heart failure, the catheter ablation needs to be performed by an experienced and skilled operator, and follow-up surveillance must focus on both the patient’s AFib and heart failure. “It’s absolutely appropriate to consider catheter ablation” for heart failure patients, but the evidence is not yet there for guideline change, Dr. Yancy concluded.
It remains uncertain why catheter ablation of AFib should be more effective in patients with heart failure than in those without. Dr. Packer speculated that one reason may be the heart rate reduction that AFib ablation produces may especially benefit heart failure patients. An additional helpful effect of ablation in heart failure patients may be reducing heart rate variability. Another notable finding of the new analysis was that 79% of patients with heart failure in CABANA had heart failure with preserved ejection fraction, with a left ventricular ejection fraction of at least 50%. “Getting rid of AFib in patients with heart failure with preserved ejection fraction will be more important than we have thought,” Dr. Packer said.
Other new CABANA analyses presented for the first time in separate talks at the meeting also showed that, while catheter ablation had no meaningful difference in effect on outcomes based on the sex of patients, both age and minority ethnic and racial status appeared to make a substantial difference. For CABANA’s primary endpoint, catheter ablation was especially effective for improving outcomes in patients 64 years old or younger, and the analysis showed a signal of possibly worse outcomes in patients who were at least 75 years old. The “substantially” better outcomes in minority-group patients represented the largest between-group difference among subgroups seen in CABANA and is a “big deal,” said Dr. Packer, who predicted that future catheter ablation use will likely rise in patients with heart failure, in younger patients, and in minority patients.
Dr. Piccini noted that, “it’s possible that CABANA identified some patient subgroups that do really well after ablation, but the problem is that, in the United States, we now often don’t treat” minority patients or those with reduced left ventricular ejection fractions with ablation, according to recent registry findings.
CABANA received partial funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. Dr. Packer has been a consultant to and/or received research funding from these four companies, as well as numerous drug and device companies, and has a financial interest in a licensed AFib mapping technology. Dr. Piccini has ties Boston Scientific, Medtronic, and numerous other drug and device companies, and disclosed an unspecified relationship with GlaxoSmithKline. Dr. Yancy disclosed an unspecified relationship with Abbott Laboratories.
SOURCE: Packer DL. Heart Rhythm 2019, Abstract S-AB14-06.
SAN FRANCISCO – Catheter ablation of atrial fibrillation (AFib) in the roughly one-third of patients with heart failure enrolled in the CABANA multicenter, randomized trial produced striking, statistically significant improvements both in the study’s primary, combined endpoint and in all-cause mortality in intention-to-treat analyses.
These findings, from prespecified secondary analyses, contrasted with the study’s overall result, which showed no benefit in the primary endpoint analysis in the total study population of 2,204 patients with AFib (JAMA. 2019 Apr 2;321[13]:1261-74). They are also at odds with the primary endpoint result in the two-thirds of enrolled patients without heart failure, which showed no significant between-group differences in these two outcome measures among the patients assigned to the catheter ablation arm and the study’s control, which was medical management arm.
Among the 778 AFib patients enrolled in CABANA with any form of heart failure (35% of the total study enrollment), the incidence of the study’s primary endpoint – the combined rate of death, disabling stroke, serious bleeding, or cardiac arrest during a median follow-up of slightly more than 4 years – was 36% lower among the catheter-ablated heart failure patients than in the heart failure patients assigned to medical treatment, according to an intention-to-treat analysis, which was a statistically significant difference. The incidence of all-cause mortality during follow-up was 43% lower in the ablated heart failure patients, compared with the controls, Douglas L. Packer, MD, said at the annual scientific sessions of the Heart Rhythm Society.
In contrast, among enrolled patients without heart failure, the intention-to-treat primary endpoint was 6% higher in the ablated patients, and all-cause mortality was a relative 27% higher, although neither difference was statistically significant.
It’s a “little surprising” that the results showed this much benefit in the patients with heart failure, said Dr. Packer, professor of medicine at the Mayo Clinic in Rochester, Minn., and lead investigator of the CABANA (Catheter Ablation vs Anti-Arrhythmic Drug Therapy for Atrial Fibrillation) trial. “I think these data confirm the results of the CASTLE-AF trial, but without some of the glitches some people have cited” about that study, such as concerns about a high level of patient selection in CASTLE-AF and its relatively modest number of enrolled patients, he said in an interview.
The CASTLE-AF (Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF) study, run entirely in patients with heart failure with reduced ejection fraction and AFib, showed a statistically significant improvement in patient survival and heart failure hospitalization after catheter ablation compared with medical management (N Engl J Med. 2018 Feb 1;378[5]:417-27). Prior to the CASTLE-AF report, results from several other small studies (J Interv Card Electrophysiol. 2018 Oct;53[1]:19-29), as well as those from the AATAC trial (Circulation. 2016 Apr 26;133[17]:1637-44), also showed consistent evidence for benefit from catheter ablation in patients with heart failure and AFib, noted CABANA coinvestigator Jonathan P. Piccini, MD, during a separate talk at the meeting.
“The improvement of cardiovascular outcomes with ablation in patients with heart failure and AFib is consistent across multiple trials, at least with respect to heart failure with reduced ejection fraction” concluded Dr. Piccini, a cardiac electrophysiologist at Duke University in Durham, N.C.
As a result of the new heart failure analysis, “I think the guidelines will change,” predicted Dr. Packer, with catheter ablation receiving a firmer endorsement for patients with heart failure the next time U.S. guidelines for heart failure and AFib management are updated. The findings say “there is substantial benefit of catheter ablation in heart failure patients, but I don’t think our findings lessen the utility of ablation in patients without heart failure,” he stressed. Even patients without heart failure showed reduction in AFib burden and improvement in quality of life that were similar to what was seen in the heart failure patients.
The new report from CABANA of benefit from AFib catheter ablation in patients with heart failure “absolutely advances the evidence,” commented Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern University in Chicago. “A number of us were quite circumspect about this based on the CASTLE-AF data, but the new CABANA analyses have addressed our anxiety that the CASTLE-AF results were just by chance.” The new CABANA analyses “may not confirm CASTLE-AF, but it enriches the conversation and makes it possible that we are seeing benefit in some patients with heart failure who get ablated.”
Dr. Yancy, who chaired the most recent update to the U.S. heart failure management guideline (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803) stopped short of saying that the cumulative evidence now supports a guideline change, but he acknowledged in an interview that the evidence could legitimately influence practice. Catheter ablation should now be “strongly considered” in patients with heart failure and AFib, he said, although he also had three qualifications for opting for this approach: Patients must already be on guideline-directed medical therapy for their heart failure, the catheter ablation needs to be performed by an experienced and skilled operator, and follow-up surveillance must focus on both the patient’s AFib and heart failure. “It’s absolutely appropriate to consider catheter ablation” for heart failure patients, but the evidence is not yet there for guideline change, Dr. Yancy concluded.
It remains uncertain why catheter ablation of AFib should be more effective in patients with heart failure than in those without. Dr. Packer speculated that one reason may be the heart rate reduction that AFib ablation produces may especially benefit heart failure patients. An additional helpful effect of ablation in heart failure patients may be reducing heart rate variability. Another notable finding of the new analysis was that 79% of patients with heart failure in CABANA had heart failure with preserved ejection fraction, with a left ventricular ejection fraction of at least 50%. “Getting rid of AFib in patients with heart failure with preserved ejection fraction will be more important than we have thought,” Dr. Packer said.
Other new CABANA analyses presented for the first time in separate talks at the meeting also showed that, while catheter ablation had no meaningful difference in effect on outcomes based on the sex of patients, both age and minority ethnic and racial status appeared to make a substantial difference. For CABANA’s primary endpoint, catheter ablation was especially effective for improving outcomes in patients 64 years old or younger, and the analysis showed a signal of possibly worse outcomes in patients who were at least 75 years old. The “substantially” better outcomes in minority-group patients represented the largest between-group difference among subgroups seen in CABANA and is a “big deal,” said Dr. Packer, who predicted that future catheter ablation use will likely rise in patients with heart failure, in younger patients, and in minority patients.
Dr. Piccini noted that, “it’s possible that CABANA identified some patient subgroups that do really well after ablation, but the problem is that, in the United States, we now often don’t treat” minority patients or those with reduced left ventricular ejection fractions with ablation, according to recent registry findings.
CABANA received partial funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. Dr. Packer has been a consultant to and/or received research funding from these four companies, as well as numerous drug and device companies, and has a financial interest in a licensed AFib mapping technology. Dr. Piccini has ties Boston Scientific, Medtronic, and numerous other drug and device companies, and disclosed an unspecified relationship with GlaxoSmithKline. Dr. Yancy disclosed an unspecified relationship with Abbott Laboratories.
SOURCE: Packer DL. Heart Rhythm 2019, Abstract S-AB14-06.
REPORTING FROM HEART RHYTHM 2019
Key clinical point: Catheter ablation for atrial fibrillation is especially effective in patients with heart failure.
Major finding: Heart failure patients treated with catheter ablation had a 36% relative cut in the primary endpoint, compared with control patients.
Study details: CABANA, a multicenter, randomized trial with 2,204 patients, including 778 patients with heart failure.
Disclosures: CABANA received partial funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. Dr. Packer has been a consultant to and/or received research funding from these four companies, as well as numerous drug and device companies, and has a financial interest in a licensed AFib mapping technology. Source: Packer DL. Heart Rhythm 2019, Abstract S-AB14-06.
Bundled payment for OA surgery linked to more emergency department visits
And therein lies a key lesson for health policy makers who have embraced bundled payments to reduce rising health care costs, Mayilee Canizares, PhD, observed at the OARSI 2019 World Congress.
In Ontario, with patients discharged sooner and directly to home, there was the negative impact of increased emergency department visits after surgery, Dr. Canizares, of the University Health Network in Toronto, said at OARSI 2019 World Congress, sponsored by the Osteoarthritis Research Society International. “Our findings highlight the importance of coordinating the appropriate support services as well as the need to continue assessing the optimal discharge care plan for osteoarthritis patients undergoing surgery.”
Dr. Canizares’ study of the Ontario-wide experience with orthopedic surgery for osteoarthritis during 2004-2016 received the OARSI 2019 award for the meeting’s top-rated study in clinical epidemiology/health services research.
Using administrative data from Canada’s national health care system, Dr. Canizares and her coinvestigators found that the number of individuals undergoing elective orthopedic surgery for osteoarthritis ballooned from 22,700 in 2004 to 41,900 in 2016, representing an increase from 246 to 381 procedures per 100,000 people. During this time, the mean length of stay declined from about 5 days to just under 3 days, the 30-day readmission rate dropped from 4.2% to 3.4%, and the rate of emergency department visits within 30 days post discharge rose steadily from 8.7% in 2004 to 14.1% in 2016.
Roughly half of the operations were total knee replacements and one-third were hip replacements. The profile of patients undergoing surgery changed little over the course of the 12-year study with the exception that in more recent years patients presented with more comorbidities: Indeed, three or more comorbid conditions were present in 2.9% of the surgical patients in 2004 compared to 4.2% in 2016.
In multivariate logistic regression analyses, patient characteristics didn’t explain the change over time in early readmission or unplanned emergency department visit rates. However, discharge disposition did: By 2014, more patients were being discharged home, and in nearly half of cases that was being done without support.
Dr. Canizares reported having no financial conflicts regarding her study, funded by the Toronto General and Western Hospital Foundation.
SOURCE: Canizares M. OARSI, Abstract 16.
And therein lies a key lesson for health policy makers who have embraced bundled payments to reduce rising health care costs, Mayilee Canizares, PhD, observed at the OARSI 2019 World Congress.
In Ontario, with patients discharged sooner and directly to home, there was the negative impact of increased emergency department visits after surgery, Dr. Canizares, of the University Health Network in Toronto, said at OARSI 2019 World Congress, sponsored by the Osteoarthritis Research Society International. “Our findings highlight the importance of coordinating the appropriate support services as well as the need to continue assessing the optimal discharge care plan for osteoarthritis patients undergoing surgery.”
Dr. Canizares’ study of the Ontario-wide experience with orthopedic surgery for osteoarthritis during 2004-2016 received the OARSI 2019 award for the meeting’s top-rated study in clinical epidemiology/health services research.
Using administrative data from Canada’s national health care system, Dr. Canizares and her coinvestigators found that the number of individuals undergoing elective orthopedic surgery for osteoarthritis ballooned from 22,700 in 2004 to 41,900 in 2016, representing an increase from 246 to 381 procedures per 100,000 people. During this time, the mean length of stay declined from about 5 days to just under 3 days, the 30-day readmission rate dropped from 4.2% to 3.4%, and the rate of emergency department visits within 30 days post discharge rose steadily from 8.7% in 2004 to 14.1% in 2016.
Roughly half of the operations were total knee replacements and one-third were hip replacements. The profile of patients undergoing surgery changed little over the course of the 12-year study with the exception that in more recent years patients presented with more comorbidities: Indeed, three or more comorbid conditions were present in 2.9% of the surgical patients in 2004 compared to 4.2% in 2016.
In multivariate logistic regression analyses, patient characteristics didn’t explain the change over time in early readmission or unplanned emergency department visit rates. However, discharge disposition did: By 2014, more patients were being discharged home, and in nearly half of cases that was being done without support.
Dr. Canizares reported having no financial conflicts regarding her study, funded by the Toronto General and Western Hospital Foundation.
SOURCE: Canizares M. OARSI, Abstract 16.
And therein lies a key lesson for health policy makers who have embraced bundled payments to reduce rising health care costs, Mayilee Canizares, PhD, observed at the OARSI 2019 World Congress.
In Ontario, with patients discharged sooner and directly to home, there was the negative impact of increased emergency department visits after surgery, Dr. Canizares, of the University Health Network in Toronto, said at OARSI 2019 World Congress, sponsored by the Osteoarthritis Research Society International. “Our findings highlight the importance of coordinating the appropriate support services as well as the need to continue assessing the optimal discharge care plan for osteoarthritis patients undergoing surgery.”
Dr. Canizares’ study of the Ontario-wide experience with orthopedic surgery for osteoarthritis during 2004-2016 received the OARSI 2019 award for the meeting’s top-rated study in clinical epidemiology/health services research.
Using administrative data from Canada’s national health care system, Dr. Canizares and her coinvestigators found that the number of individuals undergoing elective orthopedic surgery for osteoarthritis ballooned from 22,700 in 2004 to 41,900 in 2016, representing an increase from 246 to 381 procedures per 100,000 people. During this time, the mean length of stay declined from about 5 days to just under 3 days, the 30-day readmission rate dropped from 4.2% to 3.4%, and the rate of emergency department visits within 30 days post discharge rose steadily from 8.7% in 2004 to 14.1% in 2016.
Roughly half of the operations were total knee replacements and one-third were hip replacements. The profile of patients undergoing surgery changed little over the course of the 12-year study with the exception that in more recent years patients presented with more comorbidities: Indeed, three or more comorbid conditions were present in 2.9% of the surgical patients in 2004 compared to 4.2% in 2016.
In multivariate logistic regression analyses, patient characteristics didn’t explain the change over time in early readmission or unplanned emergency department visit rates. However, discharge disposition did: By 2014, more patients were being discharged home, and in nearly half of cases that was being done without support.
Dr. Canizares reported having no financial conflicts regarding her study, funded by the Toronto General and Western Hospital Foundation.
SOURCE: Canizares M. OARSI, Abstract 16.
REPORTING FROM OARSI 2019