User login
Modified Atkins diet beneficial in drug-resistant epilepsy
, new research shows.
In a randomized prospective study, the number of seizures per month dropped by more than half in one-quarter of patients following the high-fat, low-carb diet; and 5% of the group were free from all seizure activity after 6 months.
Both adults and adolescents reported benefits from the diet, which is a less strict version of a traditional ketogenic diet that many patients find difficult to follow. The modified Atkins diet includes foods such as leafy green vegetables and eggs, chicken, fish, bacon, and other animal proteins.
“The use of an exchange list and recipe booklet with local recipes and spices helped in the initiation of modified Atkins diet with the flexibility of meal choices and ease of administration,” said coinvestigator Manjari Tripathi, MD, DM, department of neurology, All India Institute of Medical Science, New Delhi.
“As items were everyday household ingredients in proportion to the requirement of the modified Atkins diet, this diet is possible in low-income countries also,” Dr. Tripathi added.
The findings were published online in the journal Neurology.
Low carbs, high benefit
The modified Atkins diet includes around 65% fat, 25% protein, and 10% carbohydrates. Unlike a traditional ketogenic diet, the modified Atkins diet includes no restrictions on protein, calories, or fluids.
Researchers have long known that ketogenic and Atkins diets are associated with reduced seizure activity in adolescents with epilepsy. But previous studies were small, and many were retrospective analyses.
The current investigators enrolled 160 patients (80 adults, 80 adolescents) aged 10-55 years whose epilepsy was not controlled despite using at least three antiseizure medications at maximum tolerated doses.
The intervention group received training in the modified Atkins diet and were given a food exchange list, sample menu, and recipe booklet. Carbohydrate intake was restricted to 20 grams per day.
Participants took supplemental multivitamins and minerals, kept a food diary, logged seizure activity, and measured urine ketone levels three times a day. They also received weekly check-up phone calls to ensure diet adherence.
The control group received a normal diet with no carbohydrate restrictions. All participants continued their prescribed antiseizure therapy throughout the trial.
Primary outcome met
The primary study outcome was a reduction in seizures of more than 50%. At 6 months, 26.2% of the intervention group had reached that goal, compared with just 2.5% of the control group (P < .001).
When the median number of seizures in the modified Atkins diet group was analyzed, the frequency dropped in the intervention group from 37.5 per month at baseline to 27.5 per month after 3 months of the modified Atkins diet and to 21.5 per month after 6 months.
Adding the modified Atkins diet had a larger effect on seizure activity in adults than in adolescents. At the end of 6 months, 36% of adolescents on the modified Atkins diet had more than a 50% reduction in seizures, while 57.1% of adults on the diet reached that level.
Quality-of-life scores were also higher in the intervention group.
By the end of the trial, 5% of patients on the modified Atkins diet had no seizure activity at all versus none of the control group. In fact, the median number of seizures increased in the control group during the study.
The mean morning and evening levels of urine ketosis in the intervention group were 58.3 ± 8.0 mg/dL and 62.2 ± 22.6 mg/dL, respectively, suggesting satisfactory diet adherence. There was no significant difference between groups in weight loss.
Dr. Tripathi noted that 33% of participants did not complete the study because of poor tolerance of the diet, lack of benefit, or the inability to follow up – in part due to COVID-19. However, she said tolerance of the modified Atkins diet was better than what has been reported with the ketogenic diet.
“Though the exact mechanism by which such a diet protects against seizures is unknown, there is evidence that it causes effects on intermediary metabolism that influences the dynamics of the major inhibitory and excitatory neurotransmitter systems in the brain,” Dr. Tripathi said.
Benefits outweigh cost
Commenting on the research findings, Mackenzie Cervenka, MD, professor of neurology and director of the Adult Epilepsy Diet Center at Johns Hopkins University, Baltimore, noted that the study is the first randomized controlled trial of this size to demonstrate a benefit from adding the modified Atkins diet to standard antiseizure therapy in treatment-resistant epilepsy.
“Importantly, the study also showed improvement in quality of life and behavior over standard-of-care therapies without significant adverse effects,” said Dr. Cervenka, who was not part of the research.
The investigators noted that the flexibility of the modified Atkins diet allows more variation in menu options and a greater intake of protein, making it easier to follow than a traditional ketogenic diet.
One area of debate, however, is whether these diets are manageable for individuals with low income. Poultry, meat, and fish, all of which are staples of a modified Atkins diet, can be more expensive than other high-carb options such as pasta and rice.
“While some of the foods such as protein sources that patients purchase when they are on a ketogenic diet therapy can be more expensive, if you take into account the cost of antiseizure medications and other antiseizure treatments, hospital visits, and missed work related to seizures, et cetera, the overall financial benefits of seizure reduction with incorporating a ketogenic diet therapy may outweigh these costs,” Dr. Cervenka said.
“There are also low-cost foods that can be used since there is a great deal of flexibility with a modified Atkins diet,” she added.
The study was funded by the Centre of Excellence for Epilepsy, which is funded by the Department of Biotechnology, Government of India. Dr. Tripathi and Dr. Cervenka report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
In a randomized prospective study, the number of seizures per month dropped by more than half in one-quarter of patients following the high-fat, low-carb diet; and 5% of the group were free from all seizure activity after 6 months.
Both adults and adolescents reported benefits from the diet, which is a less strict version of a traditional ketogenic diet that many patients find difficult to follow. The modified Atkins diet includes foods such as leafy green vegetables and eggs, chicken, fish, bacon, and other animal proteins.
“The use of an exchange list and recipe booklet with local recipes and spices helped in the initiation of modified Atkins diet with the flexibility of meal choices and ease of administration,” said coinvestigator Manjari Tripathi, MD, DM, department of neurology, All India Institute of Medical Science, New Delhi.
“As items were everyday household ingredients in proportion to the requirement of the modified Atkins diet, this diet is possible in low-income countries also,” Dr. Tripathi added.
The findings were published online in the journal Neurology.
Low carbs, high benefit
The modified Atkins diet includes around 65% fat, 25% protein, and 10% carbohydrates. Unlike a traditional ketogenic diet, the modified Atkins diet includes no restrictions on protein, calories, or fluids.
Researchers have long known that ketogenic and Atkins diets are associated with reduced seizure activity in adolescents with epilepsy. But previous studies were small, and many were retrospective analyses.
The current investigators enrolled 160 patients (80 adults, 80 adolescents) aged 10-55 years whose epilepsy was not controlled despite using at least three antiseizure medications at maximum tolerated doses.
The intervention group received training in the modified Atkins diet and were given a food exchange list, sample menu, and recipe booklet. Carbohydrate intake was restricted to 20 grams per day.
Participants took supplemental multivitamins and minerals, kept a food diary, logged seizure activity, and measured urine ketone levels three times a day. They also received weekly check-up phone calls to ensure diet adherence.
The control group received a normal diet with no carbohydrate restrictions. All participants continued their prescribed antiseizure therapy throughout the trial.
Primary outcome met
The primary study outcome was a reduction in seizures of more than 50%. At 6 months, 26.2% of the intervention group had reached that goal, compared with just 2.5% of the control group (P < .001).
When the median number of seizures in the modified Atkins diet group was analyzed, the frequency dropped in the intervention group from 37.5 per month at baseline to 27.5 per month after 3 months of the modified Atkins diet and to 21.5 per month after 6 months.
Adding the modified Atkins diet had a larger effect on seizure activity in adults than in adolescents. At the end of 6 months, 36% of adolescents on the modified Atkins diet had more than a 50% reduction in seizures, while 57.1% of adults on the diet reached that level.
Quality-of-life scores were also higher in the intervention group.
By the end of the trial, 5% of patients on the modified Atkins diet had no seizure activity at all versus none of the control group. In fact, the median number of seizures increased in the control group during the study.
The mean morning and evening levels of urine ketosis in the intervention group were 58.3 ± 8.0 mg/dL and 62.2 ± 22.6 mg/dL, respectively, suggesting satisfactory diet adherence. There was no significant difference between groups in weight loss.
Dr. Tripathi noted that 33% of participants did not complete the study because of poor tolerance of the diet, lack of benefit, or the inability to follow up – in part due to COVID-19. However, she said tolerance of the modified Atkins diet was better than what has been reported with the ketogenic diet.
“Though the exact mechanism by which such a diet protects against seizures is unknown, there is evidence that it causes effects on intermediary metabolism that influences the dynamics of the major inhibitory and excitatory neurotransmitter systems in the brain,” Dr. Tripathi said.
Benefits outweigh cost
Commenting on the research findings, Mackenzie Cervenka, MD, professor of neurology and director of the Adult Epilepsy Diet Center at Johns Hopkins University, Baltimore, noted that the study is the first randomized controlled trial of this size to demonstrate a benefit from adding the modified Atkins diet to standard antiseizure therapy in treatment-resistant epilepsy.
“Importantly, the study also showed improvement in quality of life and behavior over standard-of-care therapies without significant adverse effects,” said Dr. Cervenka, who was not part of the research.
The investigators noted that the flexibility of the modified Atkins diet allows more variation in menu options and a greater intake of protein, making it easier to follow than a traditional ketogenic diet.
One area of debate, however, is whether these diets are manageable for individuals with low income. Poultry, meat, and fish, all of which are staples of a modified Atkins diet, can be more expensive than other high-carb options such as pasta and rice.
“While some of the foods such as protein sources that patients purchase when they are on a ketogenic diet therapy can be more expensive, if you take into account the cost of antiseizure medications and other antiseizure treatments, hospital visits, and missed work related to seizures, et cetera, the overall financial benefits of seizure reduction with incorporating a ketogenic diet therapy may outweigh these costs,” Dr. Cervenka said.
“There are also low-cost foods that can be used since there is a great deal of flexibility with a modified Atkins diet,” she added.
The study was funded by the Centre of Excellence for Epilepsy, which is funded by the Department of Biotechnology, Government of India. Dr. Tripathi and Dr. Cervenka report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
In a randomized prospective study, the number of seizures per month dropped by more than half in one-quarter of patients following the high-fat, low-carb diet; and 5% of the group were free from all seizure activity after 6 months.
Both adults and adolescents reported benefits from the diet, which is a less strict version of a traditional ketogenic diet that many patients find difficult to follow. The modified Atkins diet includes foods such as leafy green vegetables and eggs, chicken, fish, bacon, and other animal proteins.
“The use of an exchange list and recipe booklet with local recipes and spices helped in the initiation of modified Atkins diet with the flexibility of meal choices and ease of administration,” said coinvestigator Manjari Tripathi, MD, DM, department of neurology, All India Institute of Medical Science, New Delhi.
“As items were everyday household ingredients in proportion to the requirement of the modified Atkins diet, this diet is possible in low-income countries also,” Dr. Tripathi added.
The findings were published online in the journal Neurology.
Low carbs, high benefit
The modified Atkins diet includes around 65% fat, 25% protein, and 10% carbohydrates. Unlike a traditional ketogenic diet, the modified Atkins diet includes no restrictions on protein, calories, or fluids.
Researchers have long known that ketogenic and Atkins diets are associated with reduced seizure activity in adolescents with epilepsy. But previous studies were small, and many were retrospective analyses.
The current investigators enrolled 160 patients (80 adults, 80 adolescents) aged 10-55 years whose epilepsy was not controlled despite using at least three antiseizure medications at maximum tolerated doses.
The intervention group received training in the modified Atkins diet and were given a food exchange list, sample menu, and recipe booklet. Carbohydrate intake was restricted to 20 grams per day.
Participants took supplemental multivitamins and minerals, kept a food diary, logged seizure activity, and measured urine ketone levels three times a day. They also received weekly check-up phone calls to ensure diet adherence.
The control group received a normal diet with no carbohydrate restrictions. All participants continued their prescribed antiseizure therapy throughout the trial.
Primary outcome met
The primary study outcome was a reduction in seizures of more than 50%. At 6 months, 26.2% of the intervention group had reached that goal, compared with just 2.5% of the control group (P < .001).
When the median number of seizures in the modified Atkins diet group was analyzed, the frequency dropped in the intervention group from 37.5 per month at baseline to 27.5 per month after 3 months of the modified Atkins diet and to 21.5 per month after 6 months.
Adding the modified Atkins diet had a larger effect on seizure activity in adults than in adolescents. At the end of 6 months, 36% of adolescents on the modified Atkins diet had more than a 50% reduction in seizures, while 57.1% of adults on the diet reached that level.
Quality-of-life scores were also higher in the intervention group.
By the end of the trial, 5% of patients on the modified Atkins diet had no seizure activity at all versus none of the control group. In fact, the median number of seizures increased in the control group during the study.
The mean morning and evening levels of urine ketosis in the intervention group were 58.3 ± 8.0 mg/dL and 62.2 ± 22.6 mg/dL, respectively, suggesting satisfactory diet adherence. There was no significant difference between groups in weight loss.
Dr. Tripathi noted that 33% of participants did not complete the study because of poor tolerance of the diet, lack of benefit, or the inability to follow up – in part due to COVID-19. However, she said tolerance of the modified Atkins diet was better than what has been reported with the ketogenic diet.
“Though the exact mechanism by which such a diet protects against seizures is unknown, there is evidence that it causes effects on intermediary metabolism that influences the dynamics of the major inhibitory and excitatory neurotransmitter systems in the brain,” Dr. Tripathi said.
Benefits outweigh cost
Commenting on the research findings, Mackenzie Cervenka, MD, professor of neurology and director of the Adult Epilepsy Diet Center at Johns Hopkins University, Baltimore, noted that the study is the first randomized controlled trial of this size to demonstrate a benefit from adding the modified Atkins diet to standard antiseizure therapy in treatment-resistant epilepsy.
“Importantly, the study also showed improvement in quality of life and behavior over standard-of-care therapies without significant adverse effects,” said Dr. Cervenka, who was not part of the research.
The investigators noted that the flexibility of the modified Atkins diet allows more variation in menu options and a greater intake of protein, making it easier to follow than a traditional ketogenic diet.
One area of debate, however, is whether these diets are manageable for individuals with low income. Poultry, meat, and fish, all of which are staples of a modified Atkins diet, can be more expensive than other high-carb options such as pasta and rice.
“While some of the foods such as protein sources that patients purchase when they are on a ketogenic diet therapy can be more expensive, if you take into account the cost of antiseizure medications and other antiseizure treatments, hospital visits, and missed work related to seizures, et cetera, the overall financial benefits of seizure reduction with incorporating a ketogenic diet therapy may outweigh these costs,” Dr. Cervenka said.
“There are also low-cost foods that can be used since there is a great deal of flexibility with a modified Atkins diet,” she added.
The study was funded by the Centre of Excellence for Epilepsy, which is funded by the Department of Biotechnology, Government of India. Dr. Tripathi and Dr. Cervenka report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Antiepileptic drugs tied to increased Parkinson’s disease risk
, new research suggests.
Drawing on data from the UK Biobank, investigators compared more than 1,400 individuals diagnosed with Parkinson’s disease with matched control persons and found a considerably higher risk of developing Parkinson’s disease among those who had taken AEDs in comparison with those who had not. There was a trend linking a greater number of AED prescriptions and multiple AEDs associated with a greater risk for Parkinson’s disease.
“We observed an association between the most commonly prescribed antiepileptic drugs in the U.K. and Parkinson’s disease using data from UK Biobank,” said senior author Alastair Noyce, PhD, professor of neurology and neuroepidemiology and honorary consultant neurologist, Queen Mary University of London.
“This is the first time that a comprehensive study of the link between AEDs and Parkinson’s disease has been undertaken,” said Dr. Noyce.
He added that the findings have no immediate clinical implications, “but further research is definitely needed, [as] this is an interesting observation made in a research setting.”
The study was published online in JAMA Neurology.
Plausible, but unclear link
Recent observational studies have found a “temporal association” between epilepsy and incident Parkinson’s disease, but the mechanism underlying this association is “unclear,” the authors wrote.
It is “plausible” that AEDs “may account for some or all of the apparent association between epilepsy and Parkinson’s disease” and that movement disorders are potential side effects of AEDs, but the association between AEDs and Parkinson’s disease has “not been well studied,” so it remains “unclear” whether AEDs play a role in the association.
“We have previously reported an association between epilepsy and Parkinson’s disease in several different datasets. Here, we wanted to see if it could be explained by an association with the drugs used to treat epilepsy rather than epilepsy per se,” Dr. Noyce explained.
Are AEDs the culprit?
The researchers used data from the UK Biobank, a longitudinal cohort study with more than 500,000 participants, as well as linked primary care medication data to conduct a nested case-control study to investigate this potential association. Participants ranged in age from 40 to 69 years and were recruited between 2006 and 2010.
The researchers compared 1,433 individuals diagnosed with Parkinson’s disease with 8,598 control persons who were matched in a 6:1 ratio for age, sex, race, ethnicity, and socioeconomic status (median [interquartile range] age, 71 [65-75] years; 60.9% men; 97.5% White).
Of those with Parkinson’s disease, 4.3% had been prescribed an AED prior to the date of their being diagnosed with Parkinson’s disease, compared with 2.5% in the control group; 4.4% had been diagnosed with epilepsy, compared with 1% of the control persons.
The strongest evidence was for the association between lamotrigine, levetiracetam, and sodium valproate and Parkinson’s disease. There was “weaker evidence” for carbamazepine, although all the AEDs were associated with a higher risk of Parkinson’s disease.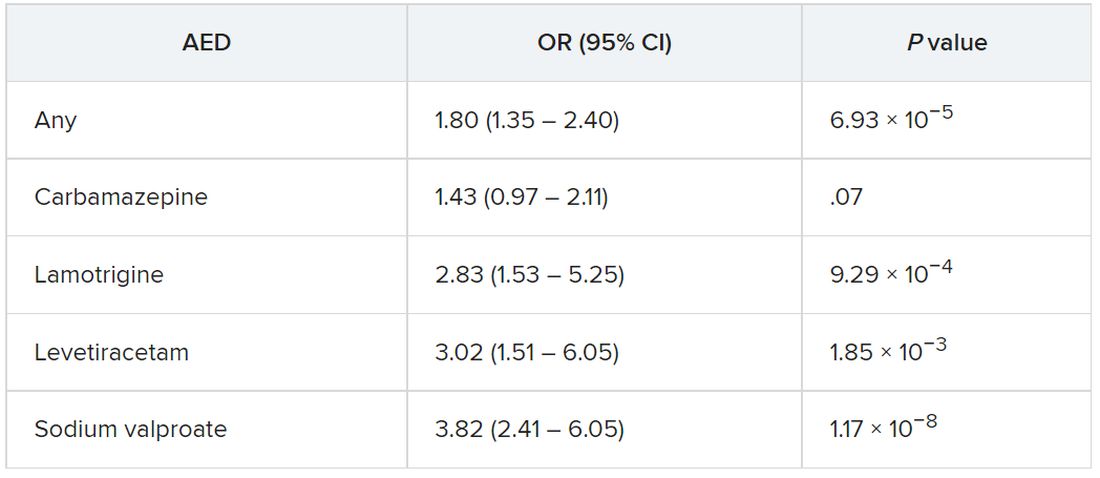
The odds of incident Parkinson’s disease were higher among those who were prescribed one or more AEDs and among individuals who were issued a higher number of prescriptions, the authors reported.
It is possible that it is the epilepsy itself that is associated with the risk of Parkinson’s disease, rather than the drugs, and that “likely explains part of the association we are seeing,” said Dr. Noyce.
“The bottom line is that more research into the links between epilepsy – and drugs used to treat epilepsy – and Parkinson’s disease is needed,” he said.
Moreover, “only with time will we work out whether the findings hold any real clinical relevance,” he added.
Alternative explanations
Commenting on the research, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, said, “It has been established in prior research that there is an association between epilepsy and Parkinson’s disease.” The current study “shows that having had a prescription written for one of four antiepileptic medications was associated with subsequently receiving a diagnosis of Parkinson’s disease.”
Although one possible conclusion is that the AEDs themselves increase the risk of developing Parkinson’s disease, “there seem to be other alternative explanations as to why a person who had been prescribed AEDs has an increased risk of receiving a diagnosis of Parkinson’s disease,” said Dr. Gilbert, an associate professor of neurology at Bellevue Hospital Center, New York, who was not involved with the current study.
For example, pre-motor changes in the brain of persons with Parkinson’s disease “may increase the risk of requiring an AED by potentially increasing the risk of having a seizure,” and “changes in the brain caused by the seizures for which AEDs are prescribed may increase the risk of Parkinson’s disease.”
Moreover, psychiatric changes related to Parkinson’s disease may have led to the prescription for AEDs, because at least two of the AEDs are also prescribed for mood stabilization, Dr. Gilbert suggested.
“An unanswered question that the paper acknowledges is, what about people who receive AEDs for reasons other than seizures? Do they also have an increased risk of Parkinson’s disease? This would be an interesting population to focus on because it would remove the link between AEDs and seizure and focus on the association between AEDs and Parkinson’s disease,” Dr. Gilbert said.
She emphasized that people who take AEDs for seizures “should not jump to the conclusion that they must come off these medications so as not to increase their risk of developing Parkinson’s disease.” She noted that having seizures “can be dangerous – injuries can occur during a seizure, and if a seizure can’t be stopped or a number occur in rapid succession, brain injury may result.”
For these reasons, people with “a tendency to have seizures need to protect themselves with AEDs” and “should certainly reach out to their neurologists with any questions,” Dr. Gilbert said.
The Preventive Neurology Unit is funded by Barts Charity. The Apocrita High Performance Cluster facility, supported by Queen Mary University London Research–IT Services, was used for this research. Dr. Noyce has received grants from Barts Charity, Parkinson’s UK, Cure Parkinson’s, the Michael J. Fox Foundation, Innovate UK, Solvemed, and Alchemab and personal fees from AstraZeneca, AbbVie, Zambon, BIAL, uMedeor, Alchemab, Britannia, and Charco Neurotech outside the submitted work. The other authors’ disclosures are listed on the original article. Dr. Gilbert reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Drawing on data from the UK Biobank, investigators compared more than 1,400 individuals diagnosed with Parkinson’s disease with matched control persons and found a considerably higher risk of developing Parkinson’s disease among those who had taken AEDs in comparison with those who had not. There was a trend linking a greater number of AED prescriptions and multiple AEDs associated with a greater risk for Parkinson’s disease.
“We observed an association between the most commonly prescribed antiepileptic drugs in the U.K. and Parkinson’s disease using data from UK Biobank,” said senior author Alastair Noyce, PhD, professor of neurology and neuroepidemiology and honorary consultant neurologist, Queen Mary University of London.
“This is the first time that a comprehensive study of the link between AEDs and Parkinson’s disease has been undertaken,” said Dr. Noyce.
He added that the findings have no immediate clinical implications, “but further research is definitely needed, [as] this is an interesting observation made in a research setting.”
The study was published online in JAMA Neurology.
Plausible, but unclear link
Recent observational studies have found a “temporal association” between epilepsy and incident Parkinson’s disease, but the mechanism underlying this association is “unclear,” the authors wrote.
It is “plausible” that AEDs “may account for some or all of the apparent association between epilepsy and Parkinson’s disease” and that movement disorders are potential side effects of AEDs, but the association between AEDs and Parkinson’s disease has “not been well studied,” so it remains “unclear” whether AEDs play a role in the association.
“We have previously reported an association between epilepsy and Parkinson’s disease in several different datasets. Here, we wanted to see if it could be explained by an association with the drugs used to treat epilepsy rather than epilepsy per se,” Dr. Noyce explained.
Are AEDs the culprit?
The researchers used data from the UK Biobank, a longitudinal cohort study with more than 500,000 participants, as well as linked primary care medication data to conduct a nested case-control study to investigate this potential association. Participants ranged in age from 40 to 69 years and were recruited between 2006 and 2010.
The researchers compared 1,433 individuals diagnosed with Parkinson’s disease with 8,598 control persons who were matched in a 6:1 ratio for age, sex, race, ethnicity, and socioeconomic status (median [interquartile range] age, 71 [65-75] years; 60.9% men; 97.5% White).
Of those with Parkinson’s disease, 4.3% had been prescribed an AED prior to the date of their being diagnosed with Parkinson’s disease, compared with 2.5% in the control group; 4.4% had been diagnosed with epilepsy, compared with 1% of the control persons.
The strongest evidence was for the association between lamotrigine, levetiracetam, and sodium valproate and Parkinson’s disease. There was “weaker evidence” for carbamazepine, although all the AEDs were associated with a higher risk of Parkinson’s disease.
The odds of incident Parkinson’s disease were higher among those who were prescribed one or more AEDs and among individuals who were issued a higher number of prescriptions, the authors reported.
It is possible that it is the epilepsy itself that is associated with the risk of Parkinson’s disease, rather than the drugs, and that “likely explains part of the association we are seeing,” said Dr. Noyce.
“The bottom line is that more research into the links between epilepsy – and drugs used to treat epilepsy – and Parkinson’s disease is needed,” he said.
Moreover, “only with time will we work out whether the findings hold any real clinical relevance,” he added.
Alternative explanations
Commenting on the research, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, said, “It has been established in prior research that there is an association between epilepsy and Parkinson’s disease.” The current study “shows that having had a prescription written for one of four antiepileptic medications was associated with subsequently receiving a diagnosis of Parkinson’s disease.”
Although one possible conclusion is that the AEDs themselves increase the risk of developing Parkinson’s disease, “there seem to be other alternative explanations as to why a person who had been prescribed AEDs has an increased risk of receiving a diagnosis of Parkinson’s disease,” said Dr. Gilbert, an associate professor of neurology at Bellevue Hospital Center, New York, who was not involved with the current study.
For example, pre-motor changes in the brain of persons with Parkinson’s disease “may increase the risk of requiring an AED by potentially increasing the risk of having a seizure,” and “changes in the brain caused by the seizures for which AEDs are prescribed may increase the risk of Parkinson’s disease.”
Moreover, psychiatric changes related to Parkinson’s disease may have led to the prescription for AEDs, because at least two of the AEDs are also prescribed for mood stabilization, Dr. Gilbert suggested.
“An unanswered question that the paper acknowledges is, what about people who receive AEDs for reasons other than seizures? Do they also have an increased risk of Parkinson’s disease? This would be an interesting population to focus on because it would remove the link between AEDs and seizure and focus on the association between AEDs and Parkinson’s disease,” Dr. Gilbert said.
She emphasized that people who take AEDs for seizures “should not jump to the conclusion that they must come off these medications so as not to increase their risk of developing Parkinson’s disease.” She noted that having seizures “can be dangerous – injuries can occur during a seizure, and if a seizure can’t be stopped or a number occur in rapid succession, brain injury may result.”
For these reasons, people with “a tendency to have seizures need to protect themselves with AEDs” and “should certainly reach out to their neurologists with any questions,” Dr. Gilbert said.
The Preventive Neurology Unit is funded by Barts Charity. The Apocrita High Performance Cluster facility, supported by Queen Mary University London Research–IT Services, was used for this research. Dr. Noyce has received grants from Barts Charity, Parkinson’s UK, Cure Parkinson’s, the Michael J. Fox Foundation, Innovate UK, Solvemed, and Alchemab and personal fees from AstraZeneca, AbbVie, Zambon, BIAL, uMedeor, Alchemab, Britannia, and Charco Neurotech outside the submitted work. The other authors’ disclosures are listed on the original article. Dr. Gilbert reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Drawing on data from the UK Biobank, investigators compared more than 1,400 individuals diagnosed with Parkinson’s disease with matched control persons and found a considerably higher risk of developing Parkinson’s disease among those who had taken AEDs in comparison with those who had not. There was a trend linking a greater number of AED prescriptions and multiple AEDs associated with a greater risk for Parkinson’s disease.
“We observed an association between the most commonly prescribed antiepileptic drugs in the U.K. and Parkinson’s disease using data from UK Biobank,” said senior author Alastair Noyce, PhD, professor of neurology and neuroepidemiology and honorary consultant neurologist, Queen Mary University of London.
“This is the first time that a comprehensive study of the link between AEDs and Parkinson’s disease has been undertaken,” said Dr. Noyce.
He added that the findings have no immediate clinical implications, “but further research is definitely needed, [as] this is an interesting observation made in a research setting.”
The study was published online in JAMA Neurology.
Plausible, but unclear link
Recent observational studies have found a “temporal association” between epilepsy and incident Parkinson’s disease, but the mechanism underlying this association is “unclear,” the authors wrote.
It is “plausible” that AEDs “may account for some or all of the apparent association between epilepsy and Parkinson’s disease” and that movement disorders are potential side effects of AEDs, but the association between AEDs and Parkinson’s disease has “not been well studied,” so it remains “unclear” whether AEDs play a role in the association.
“We have previously reported an association between epilepsy and Parkinson’s disease in several different datasets. Here, we wanted to see if it could be explained by an association with the drugs used to treat epilepsy rather than epilepsy per se,” Dr. Noyce explained.
Are AEDs the culprit?
The researchers used data from the UK Biobank, a longitudinal cohort study with more than 500,000 participants, as well as linked primary care medication data to conduct a nested case-control study to investigate this potential association. Participants ranged in age from 40 to 69 years and were recruited between 2006 and 2010.
The researchers compared 1,433 individuals diagnosed with Parkinson’s disease with 8,598 control persons who were matched in a 6:1 ratio for age, sex, race, ethnicity, and socioeconomic status (median [interquartile range] age, 71 [65-75] years; 60.9% men; 97.5% White).
Of those with Parkinson’s disease, 4.3% had been prescribed an AED prior to the date of their being diagnosed with Parkinson’s disease, compared with 2.5% in the control group; 4.4% had been diagnosed with epilepsy, compared with 1% of the control persons.
The strongest evidence was for the association between lamotrigine, levetiracetam, and sodium valproate and Parkinson’s disease. There was “weaker evidence” for carbamazepine, although all the AEDs were associated with a higher risk of Parkinson’s disease.
The odds of incident Parkinson’s disease were higher among those who were prescribed one or more AEDs and among individuals who were issued a higher number of prescriptions, the authors reported.
It is possible that it is the epilepsy itself that is associated with the risk of Parkinson’s disease, rather than the drugs, and that “likely explains part of the association we are seeing,” said Dr. Noyce.
“The bottom line is that more research into the links between epilepsy – and drugs used to treat epilepsy – and Parkinson’s disease is needed,” he said.
Moreover, “only with time will we work out whether the findings hold any real clinical relevance,” he added.
Alternative explanations
Commenting on the research, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, said, “It has been established in prior research that there is an association between epilepsy and Parkinson’s disease.” The current study “shows that having had a prescription written for one of four antiepileptic medications was associated with subsequently receiving a diagnosis of Parkinson’s disease.”
Although one possible conclusion is that the AEDs themselves increase the risk of developing Parkinson’s disease, “there seem to be other alternative explanations as to why a person who had been prescribed AEDs has an increased risk of receiving a diagnosis of Parkinson’s disease,” said Dr. Gilbert, an associate professor of neurology at Bellevue Hospital Center, New York, who was not involved with the current study.
For example, pre-motor changes in the brain of persons with Parkinson’s disease “may increase the risk of requiring an AED by potentially increasing the risk of having a seizure,” and “changes in the brain caused by the seizures for which AEDs are prescribed may increase the risk of Parkinson’s disease.”
Moreover, psychiatric changes related to Parkinson’s disease may have led to the prescription for AEDs, because at least two of the AEDs are also prescribed for mood stabilization, Dr. Gilbert suggested.
“An unanswered question that the paper acknowledges is, what about people who receive AEDs for reasons other than seizures? Do they also have an increased risk of Parkinson’s disease? This would be an interesting population to focus on because it would remove the link between AEDs and seizure and focus on the association between AEDs and Parkinson’s disease,” Dr. Gilbert said.
She emphasized that people who take AEDs for seizures “should not jump to the conclusion that they must come off these medications so as not to increase their risk of developing Parkinson’s disease.” She noted that having seizures “can be dangerous – injuries can occur during a seizure, and if a seizure can’t be stopped or a number occur in rapid succession, brain injury may result.”
For these reasons, people with “a tendency to have seizures need to protect themselves with AEDs” and “should certainly reach out to their neurologists with any questions,” Dr. Gilbert said.
The Preventive Neurology Unit is funded by Barts Charity. The Apocrita High Performance Cluster facility, supported by Queen Mary University London Research–IT Services, was used for this research. Dr. Noyce has received grants from Barts Charity, Parkinson’s UK, Cure Parkinson’s, the Michael J. Fox Foundation, Innovate UK, Solvemed, and Alchemab and personal fees from AstraZeneca, AbbVie, Zambon, BIAL, uMedeor, Alchemab, Britannia, and Charco Neurotech outside the submitted work. The other authors’ disclosures are listed on the original article. Dr. Gilbert reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Seizures in dementia hasten decline and death
NASHVILLE, TENN. – , according to a multicenter study presented at the 2022 annual meeting of the American Epilepsy Society.
“When we compared patients with seizures with those who did not have seizures, we found that patients with seizures were more likely to have more severe cognitive impairment; they were more likely to have physical dependence and so worse functional outcomes; and they also had higher mortality rates at a younger age,” lead study author Ifrah Zawar, MD, an assistant professor of neurology at the University of Virginia, Charlottesville, said in an interview.
“The average age of mortality for seizure patients was around 72 years and the average age of mortality for nonseizure patients was around 79 years, so there was a 7- to 8-year difference in mortality,” she said.
Seizures make matters worse
The study analyzed data on 26,425 patients with dementia, 374 (1.4%) of whom had seizures, collected from 2005 to 2021 at 39 Alzheimer’s disease centers in the United States. Patients who had seizures were significantly younger when cognitive decline began (ages 62.9 vs. 68.4 years, P < .001) and died younger (72.99 vs. 79.72 years, P < .001).
The study also found a number of factors associated with active seizures, including a history of dominant Alzheimer’s disease mutation (odds ratio, 5.55; P < .001), stroke (OR, 3.17; P < .001), transient ischemic attack (OR, 1.72; P = .003), traumatic brain injury (OR, 1.92; P < .001), Parkinson’s disease (OR, 1.79; P = .025), active depression (OR, 1.61; P < .001) and lower education (OR, 0.97; P =.043).
After the study made adjustments for sex and other associated factors, it found that patients with seizures were still at a 76% higher risk of dying younger (hazard ratio, 1.76; P < .001).
The study also determined that patients with seizures had worse functional assessment scores and were more likely to be physically dependent on others (OR, 2.52; P < .001). Seizure patients also performed worse on Mini-Mental Status Examination (18.50 vs. 22.88; P < .001) and Clinical Dementia Rating-Sum of boxes (7.95 vs. 4.28; P < .001) after adjusting for age and duration of cognitive decline.
A tip for caregivers
Dr. Zawar acknowledged that differentiating seizures from transient bouts of confusion in people with dementia can be difficult for family members and caregivers, but she offered advice to help them do so. “If they notice any unusual confusion or any altered mentation which is episodic in nature,” she said, “they should bring it to the neurologist’s attention as early as possible, because there are studies that have shown the diagnosis of seizures is delayed, and if they are treated in time they can be well-controlled.” Electroencephalography can also confirm the presence of seizures, she added.
Double whammy
One limitation of this study is the lack of details on the types of seizures the participants had along with the inconsistency of EEGs performed on the study population. “In future studies, I would like to have more EEG data on the types of seizures and the frequency of seizures to assess these factors further,” Dr. Zawar said.
Having more detailed information on the seizures would make the findings more valuable, Andrew J. Cole, MD, director of the epilepsy service at Massachusetts General Hospital in Boston said in an interview. “We know a lot about clinically apparent seizures, as witnessed by this paper, but we still don’t know a whole lot about clinically silent or cryptic or nighttime-only seizures that maybe no one would really recognize as such unless they were specifically looking for them, and this paper doesn’t address that issue,” he said.
While the finding that patients with other neurologic diseases have more seizures even if they also have Alzheimer’s disease isn’t “a huge surprise,” Dr. Cole added. “On the other hand, the paper is important because it shows us that in the course of having Alzheimer’s disease, having seizures also makes your outcome worse, the speed of progression faster, and it complicates the management and living with this disease, and they make that point quite clear.”
Dr. Zawar and Dr. Cole have no relevant disclosures.
NASHVILLE, TENN. – , according to a multicenter study presented at the 2022 annual meeting of the American Epilepsy Society.
“When we compared patients with seizures with those who did not have seizures, we found that patients with seizures were more likely to have more severe cognitive impairment; they were more likely to have physical dependence and so worse functional outcomes; and they also had higher mortality rates at a younger age,” lead study author Ifrah Zawar, MD, an assistant professor of neurology at the University of Virginia, Charlottesville, said in an interview.
“The average age of mortality for seizure patients was around 72 years and the average age of mortality for nonseizure patients was around 79 years, so there was a 7- to 8-year difference in mortality,” she said.
Seizures make matters worse
The study analyzed data on 26,425 patients with dementia, 374 (1.4%) of whom had seizures, collected from 2005 to 2021 at 39 Alzheimer’s disease centers in the United States. Patients who had seizures were significantly younger when cognitive decline began (ages 62.9 vs. 68.4 years, P < .001) and died younger (72.99 vs. 79.72 years, P < .001).
The study also found a number of factors associated with active seizures, including a history of dominant Alzheimer’s disease mutation (odds ratio, 5.55; P < .001), stroke (OR, 3.17; P < .001), transient ischemic attack (OR, 1.72; P = .003), traumatic brain injury (OR, 1.92; P < .001), Parkinson’s disease (OR, 1.79; P = .025), active depression (OR, 1.61; P < .001) and lower education (OR, 0.97; P =.043).
After the study made adjustments for sex and other associated factors, it found that patients with seizures were still at a 76% higher risk of dying younger (hazard ratio, 1.76; P < .001).
The study also determined that patients with seizures had worse functional assessment scores and were more likely to be physically dependent on others (OR, 2.52; P < .001). Seizure patients also performed worse on Mini-Mental Status Examination (18.50 vs. 22.88; P < .001) and Clinical Dementia Rating-Sum of boxes (7.95 vs. 4.28; P < .001) after adjusting for age and duration of cognitive decline.
A tip for caregivers
Dr. Zawar acknowledged that differentiating seizures from transient bouts of confusion in people with dementia can be difficult for family members and caregivers, but she offered advice to help them do so. “If they notice any unusual confusion or any altered mentation which is episodic in nature,” she said, “they should bring it to the neurologist’s attention as early as possible, because there are studies that have shown the diagnosis of seizures is delayed, and if they are treated in time they can be well-controlled.” Electroencephalography can also confirm the presence of seizures, she added.
Double whammy
One limitation of this study is the lack of details on the types of seizures the participants had along with the inconsistency of EEGs performed on the study population. “In future studies, I would like to have more EEG data on the types of seizures and the frequency of seizures to assess these factors further,” Dr. Zawar said.
Having more detailed information on the seizures would make the findings more valuable, Andrew J. Cole, MD, director of the epilepsy service at Massachusetts General Hospital in Boston said in an interview. “We know a lot about clinically apparent seizures, as witnessed by this paper, but we still don’t know a whole lot about clinically silent or cryptic or nighttime-only seizures that maybe no one would really recognize as such unless they were specifically looking for them, and this paper doesn’t address that issue,” he said.
While the finding that patients with other neurologic diseases have more seizures even if they also have Alzheimer’s disease isn’t “a huge surprise,” Dr. Cole added. “On the other hand, the paper is important because it shows us that in the course of having Alzheimer’s disease, having seizures also makes your outcome worse, the speed of progression faster, and it complicates the management and living with this disease, and they make that point quite clear.”
Dr. Zawar and Dr. Cole have no relevant disclosures.
NASHVILLE, TENN. – , according to a multicenter study presented at the 2022 annual meeting of the American Epilepsy Society.
“When we compared patients with seizures with those who did not have seizures, we found that patients with seizures were more likely to have more severe cognitive impairment; they were more likely to have physical dependence and so worse functional outcomes; and they also had higher mortality rates at a younger age,” lead study author Ifrah Zawar, MD, an assistant professor of neurology at the University of Virginia, Charlottesville, said in an interview.
“The average age of mortality for seizure patients was around 72 years and the average age of mortality for nonseizure patients was around 79 years, so there was a 7- to 8-year difference in mortality,” she said.
Seizures make matters worse
The study analyzed data on 26,425 patients with dementia, 374 (1.4%) of whom had seizures, collected from 2005 to 2021 at 39 Alzheimer’s disease centers in the United States. Patients who had seizures were significantly younger when cognitive decline began (ages 62.9 vs. 68.4 years, P < .001) and died younger (72.99 vs. 79.72 years, P < .001).
The study also found a number of factors associated with active seizures, including a history of dominant Alzheimer’s disease mutation (odds ratio, 5.55; P < .001), stroke (OR, 3.17; P < .001), transient ischemic attack (OR, 1.72; P = .003), traumatic brain injury (OR, 1.92; P < .001), Parkinson’s disease (OR, 1.79; P = .025), active depression (OR, 1.61; P < .001) and lower education (OR, 0.97; P =.043).
After the study made adjustments for sex and other associated factors, it found that patients with seizures were still at a 76% higher risk of dying younger (hazard ratio, 1.76; P < .001).
The study also determined that patients with seizures had worse functional assessment scores and were more likely to be physically dependent on others (OR, 2.52; P < .001). Seizure patients also performed worse on Mini-Mental Status Examination (18.50 vs. 22.88; P < .001) and Clinical Dementia Rating-Sum of boxes (7.95 vs. 4.28; P < .001) after adjusting for age and duration of cognitive decline.
A tip for caregivers
Dr. Zawar acknowledged that differentiating seizures from transient bouts of confusion in people with dementia can be difficult for family members and caregivers, but she offered advice to help them do so. “If they notice any unusual confusion or any altered mentation which is episodic in nature,” she said, “they should bring it to the neurologist’s attention as early as possible, because there are studies that have shown the diagnosis of seizures is delayed, and if they are treated in time they can be well-controlled.” Electroencephalography can also confirm the presence of seizures, she added.
Double whammy
One limitation of this study is the lack of details on the types of seizures the participants had along with the inconsistency of EEGs performed on the study population. “In future studies, I would like to have more EEG data on the types of seizures and the frequency of seizures to assess these factors further,” Dr. Zawar said.
Having more detailed information on the seizures would make the findings more valuable, Andrew J. Cole, MD, director of the epilepsy service at Massachusetts General Hospital in Boston said in an interview. “We know a lot about clinically apparent seizures, as witnessed by this paper, but we still don’t know a whole lot about clinically silent or cryptic or nighttime-only seizures that maybe no one would really recognize as such unless they were specifically looking for them, and this paper doesn’t address that issue,” he said.
While the finding that patients with other neurologic diseases have more seizures even if they also have Alzheimer’s disease isn’t “a huge surprise,” Dr. Cole added. “On the other hand, the paper is important because it shows us that in the course of having Alzheimer’s disease, having seizures also makes your outcome worse, the speed of progression faster, and it complicates the management and living with this disease, and they make that point quite clear.”
Dr. Zawar and Dr. Cole have no relevant disclosures.
AT AES 2022
Three antiseizure medications join list for newborn risks
NASHVILLE, TENN. – A study of more than 4 million births over 20 years in five Scandinavian countries has reported that three antiseizure medications should be used with caution in women of child-bearing age because they were associated with low birth weights.
In results presented at the annual meeting of the American Epilepsy Society, Jakob Christensen, MD, DSc, PhD, a professor at Aarhus University Hospital in Denmark, said that the study found that
“Because we have this large data set we were able to confirm the suspicion that’s been raised in the past that these drugs may be associated with low birth weight,” Dr. Christensen said in an interview.
The study analyzed records from population-based registers of 4.5 million births in Denmark, Finland, Iceland, Norway, and Sweden between 1996 and 2017, known as the SCAN-AED project. The researchers analyzed the association between prenatal use of antiseizure medications and birth weight, defining low birth weight as less than 5.5 pounds and small for gestational age as being in the lowest 10th percentile for sex, country, and gestational weight at birth.
The antiseizure medications and adjusted odds ratios for risk of low birth rate were:
- Carbamazepine, 1.44 (95% confidence interval [CI], 1.21-1.71).
- Oxcarbazepine, 1.32 (95% CI, 1.03-1.69).
- Topiramate, 1.60 (95% CI, 1.15-2.24).
- Pregabalin, 1.23 (95% CI, 1.02-1.48).
- Clobazam, 4.36 (95% CI, 1.66-11.45).
The odds ratios for being born small for gestational age were:
- Carbamazepine, 1.25 (95% CI, 1.11-1.41).
- Oxcarbazepine, 1.48 (95% CI, 1.27-1.73).
- Topiramate, 1.52 (95% CI, 1.20-1.91).
“Prenatal exposure to carbamazepine, oxcarbazepine, and topiramate were associated with all estimates of adverse birth weight outcomes, thus confirming results from preclinical studies in animals and previous smaller studies in humans,” Dr. Christensen said.
He noted a lack of evidence for newer medications because their use was relatively low over the 20 years of the study. “However, for drugs like lamotrigine where we have a high number of exposed children, the finding of no association with low birth weight is reassuring, indicating the drug is safe,” Dr. Christensen said.
Use with caution
This study adds supportive evidence for expanding the list of antiseizure medications associated with small for gestational age infants, Elizabeth Gerard, MD, director of the Women with Epilepsy Program and associate professor of neurology at Northwestern University in Chicago, said in an interview.
“Previous clinical trials demonstrated that topiramate and zonisamide as well as phenobarbital were associated with small for gestational age,” she said. “This study added to the list carbamazepine and oxcarbazepine. Previously it wasn’t clear from clinical data but there were some hints that carbamazepine and oxcarbazepine might be associated with small for gestational age, but this is the first study to present robust data that carbamazepine and oxcarbazepine are associated with small for gestational age infants as well.”
She noted that these drugs can be used cautiously in women of child-bearing age and pregnant women. “I think these lines of evidence suggest that women with epilepsy should be more carefully monitored, at least with these high-quality, standard-of-care drugs, for fetal growth monitoring and perhaps most of them, especially those on at-risk drugs, should have detailed growth gradings,” Dr. Gerard said. Pregnant women on these antiseizure medications should have ultrasound beginning at 24 weeks gestation to monitor fetal growth, she said.
The NordForsk Nordic Program and Health and Welfare and the Independent Research Fund Denmark provided funding for the study. Dr. Christensen disclosed financial relationships with Union Chimique Belge Nordic and Eisai. Dr. Gerard disclosed relationships with Xenon Pharmaceuticals and Eisai.
NASHVILLE, TENN. – A study of more than 4 million births over 20 years in five Scandinavian countries has reported that three antiseizure medications should be used with caution in women of child-bearing age because they were associated with low birth weights.
In results presented at the annual meeting of the American Epilepsy Society, Jakob Christensen, MD, DSc, PhD, a professor at Aarhus University Hospital in Denmark, said that the study found that
“Because we have this large data set we were able to confirm the suspicion that’s been raised in the past that these drugs may be associated with low birth weight,” Dr. Christensen said in an interview.
The study analyzed records from population-based registers of 4.5 million births in Denmark, Finland, Iceland, Norway, and Sweden between 1996 and 2017, known as the SCAN-AED project. The researchers analyzed the association between prenatal use of antiseizure medications and birth weight, defining low birth weight as less than 5.5 pounds and small for gestational age as being in the lowest 10th percentile for sex, country, and gestational weight at birth.
The antiseizure medications and adjusted odds ratios for risk of low birth rate were:
- Carbamazepine, 1.44 (95% confidence interval [CI], 1.21-1.71).
- Oxcarbazepine, 1.32 (95% CI, 1.03-1.69).
- Topiramate, 1.60 (95% CI, 1.15-2.24).
- Pregabalin, 1.23 (95% CI, 1.02-1.48).
- Clobazam, 4.36 (95% CI, 1.66-11.45).
The odds ratios for being born small for gestational age were:
- Carbamazepine, 1.25 (95% CI, 1.11-1.41).
- Oxcarbazepine, 1.48 (95% CI, 1.27-1.73).
- Topiramate, 1.52 (95% CI, 1.20-1.91).
“Prenatal exposure to carbamazepine, oxcarbazepine, and topiramate were associated with all estimates of adverse birth weight outcomes, thus confirming results from preclinical studies in animals and previous smaller studies in humans,” Dr. Christensen said.
He noted a lack of evidence for newer medications because their use was relatively low over the 20 years of the study. “However, for drugs like lamotrigine where we have a high number of exposed children, the finding of no association with low birth weight is reassuring, indicating the drug is safe,” Dr. Christensen said.
Use with caution
This study adds supportive evidence for expanding the list of antiseizure medications associated with small for gestational age infants, Elizabeth Gerard, MD, director of the Women with Epilepsy Program and associate professor of neurology at Northwestern University in Chicago, said in an interview.
“Previous clinical trials demonstrated that topiramate and zonisamide as well as phenobarbital were associated with small for gestational age,” she said. “This study added to the list carbamazepine and oxcarbazepine. Previously it wasn’t clear from clinical data but there were some hints that carbamazepine and oxcarbazepine might be associated with small for gestational age, but this is the first study to present robust data that carbamazepine and oxcarbazepine are associated with small for gestational age infants as well.”
She noted that these drugs can be used cautiously in women of child-bearing age and pregnant women. “I think these lines of evidence suggest that women with epilepsy should be more carefully monitored, at least with these high-quality, standard-of-care drugs, for fetal growth monitoring and perhaps most of them, especially those on at-risk drugs, should have detailed growth gradings,” Dr. Gerard said. Pregnant women on these antiseizure medications should have ultrasound beginning at 24 weeks gestation to monitor fetal growth, she said.
The NordForsk Nordic Program and Health and Welfare and the Independent Research Fund Denmark provided funding for the study. Dr. Christensen disclosed financial relationships with Union Chimique Belge Nordic and Eisai. Dr. Gerard disclosed relationships with Xenon Pharmaceuticals and Eisai.
NASHVILLE, TENN. – A study of more than 4 million births over 20 years in five Scandinavian countries has reported that three antiseizure medications should be used with caution in women of child-bearing age because they were associated with low birth weights.
In results presented at the annual meeting of the American Epilepsy Society, Jakob Christensen, MD, DSc, PhD, a professor at Aarhus University Hospital in Denmark, said that the study found that
“Because we have this large data set we were able to confirm the suspicion that’s been raised in the past that these drugs may be associated with low birth weight,” Dr. Christensen said in an interview.
The study analyzed records from population-based registers of 4.5 million births in Denmark, Finland, Iceland, Norway, and Sweden between 1996 and 2017, known as the SCAN-AED project. The researchers analyzed the association between prenatal use of antiseizure medications and birth weight, defining low birth weight as less than 5.5 pounds and small for gestational age as being in the lowest 10th percentile for sex, country, and gestational weight at birth.
The antiseizure medications and adjusted odds ratios for risk of low birth rate were:
- Carbamazepine, 1.44 (95% confidence interval [CI], 1.21-1.71).
- Oxcarbazepine, 1.32 (95% CI, 1.03-1.69).
- Topiramate, 1.60 (95% CI, 1.15-2.24).
- Pregabalin, 1.23 (95% CI, 1.02-1.48).
- Clobazam, 4.36 (95% CI, 1.66-11.45).
The odds ratios for being born small for gestational age were:
- Carbamazepine, 1.25 (95% CI, 1.11-1.41).
- Oxcarbazepine, 1.48 (95% CI, 1.27-1.73).
- Topiramate, 1.52 (95% CI, 1.20-1.91).
“Prenatal exposure to carbamazepine, oxcarbazepine, and topiramate were associated with all estimates of adverse birth weight outcomes, thus confirming results from preclinical studies in animals and previous smaller studies in humans,” Dr. Christensen said.
He noted a lack of evidence for newer medications because their use was relatively low over the 20 years of the study. “However, for drugs like lamotrigine where we have a high number of exposed children, the finding of no association with low birth weight is reassuring, indicating the drug is safe,” Dr. Christensen said.
Use with caution
This study adds supportive evidence for expanding the list of antiseizure medications associated with small for gestational age infants, Elizabeth Gerard, MD, director of the Women with Epilepsy Program and associate professor of neurology at Northwestern University in Chicago, said in an interview.
“Previous clinical trials demonstrated that topiramate and zonisamide as well as phenobarbital were associated with small for gestational age,” she said. “This study added to the list carbamazepine and oxcarbazepine. Previously it wasn’t clear from clinical data but there were some hints that carbamazepine and oxcarbazepine might be associated with small for gestational age, but this is the first study to present robust data that carbamazepine and oxcarbazepine are associated with small for gestational age infants as well.”
She noted that these drugs can be used cautiously in women of child-bearing age and pregnant women. “I think these lines of evidence suggest that women with epilepsy should be more carefully monitored, at least with these high-quality, standard-of-care drugs, for fetal growth monitoring and perhaps most of them, especially those on at-risk drugs, should have detailed growth gradings,” Dr. Gerard said. Pregnant women on these antiseizure medications should have ultrasound beginning at 24 weeks gestation to monitor fetal growth, she said.
The NordForsk Nordic Program and Health and Welfare and the Independent Research Fund Denmark provided funding for the study. Dr. Christensen disclosed financial relationships with Union Chimique Belge Nordic and Eisai. Dr. Gerard disclosed relationships with Xenon Pharmaceuticals and Eisai.
AT AES 2022
‘Striking’ rate of mental health comorbidities in epilepsy
NASHVILLE, TENN. – , new research reveals.
“We hope these results inspire epileptologists and neurologists to both recognize and screen for suicide ideation and behaviors in their adolescent patients,” said study investigator Hadley Greenwood, a third-year medical student at New York University.
The new data should also encourage providers “to become more comfortable” providing support to patients, “be that by increasing their familiarity with prescribing different antidepressants or by being well versed in how to connect patients to resources within their community,” said Mr. Greenwood.
The findings were presented here at the annual meeting of the American Epilepsy Society.
Little research
Previous studies have reported on the prevalence of suicidality as well as depression and anxiety among adults with epilepsy. “We wanted to look at adolescents because there’s much less in the literature out there about psychiatric comorbidity, and specifically suicidality, in this population,” said Mr. Greenwood.
Researchers used data from the Human Epilepsy Project, a study that collected data from 34 sites in the United States, Canada, Europe, and Australia from 2012 to 2017.
From a cohort of more than 400 participants, researchers identified 67 patients aged 11-17 years who were enrolled within 4 months of starting treatment for focal epilepsy.
Participants completed the Columbia–Suicide Severity Rating Scale (C-SSRS) at enrollment and at follow-ups over 36 months. The C-SSRS measures suicidal ideation and severity, said Mr. Greenwood.
“It’s scaled from passive suicide ideation, such as thoughts of ‘I wish I were dead’ without active intent, all the way up to active suicidal ideation with a plan and intent.”
Researchers were able to distinguish individuals with passive suicide ideation from those with more serious intentions, said Mr. Greenwood. They used medical records to evaluate the prevalence of suicidal ideation and behavior.
The investigators found that more than one in five (20.9%) teens endorsed any lifetime suicide ideation. This, said Mr. Greenwood, is “roughly equivalent” to the prevalence reported earlier in the adult cohort of the Human Epilepsy Project (21.6%).
‘Striking’ rate
The fact that one in five adolescents had any lifetime suicide ideation is “definitely a striking number,” said Mr. Greenwood.
Researchers found that 15% of patients experienced active suicide ideation, 7.5% exhibited preparatory or suicidal behaviors, and 3% had made a prior suicide attempt.
All of these percentages increased at 3 years: Thirty-one percent for suicide ideation; 25% for active suicide behavior, 15% for preparatory or suicide behaviors, and 5% for prior suicide attempt.
The fact that nearly one in three adolescents endorsed suicide ideation at 3 years is another “striking” finding, said Mr. Greenwood.
Of the 53 adolescents who had never had suicide ideation at the time of enrollment, 7 endorsed new-onset suicide ideation in the follow-up period. Five of 14 who had had suicide ideation at some point prior to enrollment continued to endorse it.
“The value of the study is identifying the prevalence and identifying the significant number of adolescents with epilepsy who are endorsing either suicide ideation or suicidal behaviors,” said Mr. Greenwood.
The researchers found that among younger teens (aged 11–14 years) rates of suicide ideation were higher than among their older counterparts (aged 15–17 years).
The study does not shed light on the biological connection between epilepsy and suicidality, but Mr. Greenwood noted that prior research has suggested a bidirectional relationship.
“Depression and other psychiatric comorbidities might exist prior to epileptic activity and actually predispose to epileptic activity.”
Mr. Greenwood noted that suicide ideation has “spiked” recently across the general population, and so it’s difficult to compare the prevalence in her study with “today’s prevalence.”
However, other research generally shows that the suicide ideation rate in the general adolescent population is much lower than in teens with epilepsy.
Unique aspects of the current study are that it reports suicide ideation and behaviors at around the time of an epilepsy diagnosis and documents how suicidality progresses or resolves over time, said Mr. Greenwood.
Underdiagnosed, undertreated
Commenting on the research, Elizabeth Donner, MD, director of the comprehensive epilepsy program, Hospital for Sick Children, and associate professor, department of pediatrics, University of Toronto, said a “key point” from the study is that the suicidality rate among teens with epilepsy exceeds that of children not living with epilepsy.
“We are significantly underdiagnosing and undertreating the mental health comorbidities in epilepsy,” said Dr. Donner. “Epilepsy is a brain disease and so are mental health disorders, so it shouldn’t come as any surprise that they coexist in individuals with epilepsy.”
The new results contribute to what is already known about the significant mortality rates among persons with epilepsy, said Dr. Donner. She referred to a 2018 study that showed that people with epilepsy were 3.5 times more likely to die by suicide.
Other research has shown that people with epilepsy are 10 times more likely to die by drowning, mostly in the bathtub, said Dr. Donner.
“You would think that we’re educating these people about risks related to their epilepsy, but either the messages don’t get through, or they don’t know how to keep themselves safe,” she said.
“This needs to be seen in a bigger picture, and the bigger picture is we need to recognize comorbid mental health issues; we need to address them once recognized; and then we need to counsel and support people to live safely with their epilepsy.
The study received funding from the Epilepsy Study Consortium, Finding a Cure for Epilepsy and Seizures (FACES) and other related foundations, UCB, Pfizer, Eisai, Lundbeck, and Sunovion. Mr. Greenwood and Dr. Donner report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – , new research reveals.
“We hope these results inspire epileptologists and neurologists to both recognize and screen for suicide ideation and behaviors in their adolescent patients,” said study investigator Hadley Greenwood, a third-year medical student at New York University.
The new data should also encourage providers “to become more comfortable” providing support to patients, “be that by increasing their familiarity with prescribing different antidepressants or by being well versed in how to connect patients to resources within their community,” said Mr. Greenwood.
The findings were presented here at the annual meeting of the American Epilepsy Society.
Little research
Previous studies have reported on the prevalence of suicidality as well as depression and anxiety among adults with epilepsy. “We wanted to look at adolescents because there’s much less in the literature out there about psychiatric comorbidity, and specifically suicidality, in this population,” said Mr. Greenwood.
Researchers used data from the Human Epilepsy Project, a study that collected data from 34 sites in the United States, Canada, Europe, and Australia from 2012 to 2017.
From a cohort of more than 400 participants, researchers identified 67 patients aged 11-17 years who were enrolled within 4 months of starting treatment for focal epilepsy.
Participants completed the Columbia–Suicide Severity Rating Scale (C-SSRS) at enrollment and at follow-ups over 36 months. The C-SSRS measures suicidal ideation and severity, said Mr. Greenwood.
“It’s scaled from passive suicide ideation, such as thoughts of ‘I wish I were dead’ without active intent, all the way up to active suicidal ideation with a plan and intent.”
Researchers were able to distinguish individuals with passive suicide ideation from those with more serious intentions, said Mr. Greenwood. They used medical records to evaluate the prevalence of suicidal ideation and behavior.
The investigators found that more than one in five (20.9%) teens endorsed any lifetime suicide ideation. This, said Mr. Greenwood, is “roughly equivalent” to the prevalence reported earlier in the adult cohort of the Human Epilepsy Project (21.6%).
‘Striking’ rate
The fact that one in five adolescents had any lifetime suicide ideation is “definitely a striking number,” said Mr. Greenwood.
Researchers found that 15% of patients experienced active suicide ideation, 7.5% exhibited preparatory or suicidal behaviors, and 3% had made a prior suicide attempt.
All of these percentages increased at 3 years: Thirty-one percent for suicide ideation; 25% for active suicide behavior, 15% for preparatory or suicide behaviors, and 5% for prior suicide attempt.
The fact that nearly one in three adolescents endorsed suicide ideation at 3 years is another “striking” finding, said Mr. Greenwood.
Of the 53 adolescents who had never had suicide ideation at the time of enrollment, 7 endorsed new-onset suicide ideation in the follow-up period. Five of 14 who had had suicide ideation at some point prior to enrollment continued to endorse it.
“The value of the study is identifying the prevalence and identifying the significant number of adolescents with epilepsy who are endorsing either suicide ideation or suicidal behaviors,” said Mr. Greenwood.
The researchers found that among younger teens (aged 11–14 years) rates of suicide ideation were higher than among their older counterparts (aged 15–17 years).
The study does not shed light on the biological connection between epilepsy and suicidality, but Mr. Greenwood noted that prior research has suggested a bidirectional relationship.
“Depression and other psychiatric comorbidities might exist prior to epileptic activity and actually predispose to epileptic activity.”
Mr. Greenwood noted that suicide ideation has “spiked” recently across the general population, and so it’s difficult to compare the prevalence in her study with “today’s prevalence.”
However, other research generally shows that the suicide ideation rate in the general adolescent population is much lower than in teens with epilepsy.
Unique aspects of the current study are that it reports suicide ideation and behaviors at around the time of an epilepsy diagnosis and documents how suicidality progresses or resolves over time, said Mr. Greenwood.
Underdiagnosed, undertreated
Commenting on the research, Elizabeth Donner, MD, director of the comprehensive epilepsy program, Hospital for Sick Children, and associate professor, department of pediatrics, University of Toronto, said a “key point” from the study is that the suicidality rate among teens with epilepsy exceeds that of children not living with epilepsy.
“We are significantly underdiagnosing and undertreating the mental health comorbidities in epilepsy,” said Dr. Donner. “Epilepsy is a brain disease and so are mental health disorders, so it shouldn’t come as any surprise that they coexist in individuals with epilepsy.”
The new results contribute to what is already known about the significant mortality rates among persons with epilepsy, said Dr. Donner. She referred to a 2018 study that showed that people with epilepsy were 3.5 times more likely to die by suicide.
Other research has shown that people with epilepsy are 10 times more likely to die by drowning, mostly in the bathtub, said Dr. Donner.
“You would think that we’re educating these people about risks related to their epilepsy, but either the messages don’t get through, or they don’t know how to keep themselves safe,” she said.
“This needs to be seen in a bigger picture, and the bigger picture is we need to recognize comorbid mental health issues; we need to address them once recognized; and then we need to counsel and support people to live safely with their epilepsy.
The study received funding from the Epilepsy Study Consortium, Finding a Cure for Epilepsy and Seizures (FACES) and other related foundations, UCB, Pfizer, Eisai, Lundbeck, and Sunovion. Mr. Greenwood and Dr. Donner report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – , new research reveals.
“We hope these results inspire epileptologists and neurologists to both recognize and screen for suicide ideation and behaviors in their adolescent patients,” said study investigator Hadley Greenwood, a third-year medical student at New York University.
The new data should also encourage providers “to become more comfortable” providing support to patients, “be that by increasing their familiarity with prescribing different antidepressants or by being well versed in how to connect patients to resources within their community,” said Mr. Greenwood.
The findings were presented here at the annual meeting of the American Epilepsy Society.
Little research
Previous studies have reported on the prevalence of suicidality as well as depression and anxiety among adults with epilepsy. “We wanted to look at adolescents because there’s much less in the literature out there about psychiatric comorbidity, and specifically suicidality, in this population,” said Mr. Greenwood.
Researchers used data from the Human Epilepsy Project, a study that collected data from 34 sites in the United States, Canada, Europe, and Australia from 2012 to 2017.
From a cohort of more than 400 participants, researchers identified 67 patients aged 11-17 years who were enrolled within 4 months of starting treatment for focal epilepsy.
Participants completed the Columbia–Suicide Severity Rating Scale (C-SSRS) at enrollment and at follow-ups over 36 months. The C-SSRS measures suicidal ideation and severity, said Mr. Greenwood.
“It’s scaled from passive suicide ideation, such as thoughts of ‘I wish I were dead’ without active intent, all the way up to active suicidal ideation with a plan and intent.”
Researchers were able to distinguish individuals with passive suicide ideation from those with more serious intentions, said Mr. Greenwood. They used medical records to evaluate the prevalence of suicidal ideation and behavior.
The investigators found that more than one in five (20.9%) teens endorsed any lifetime suicide ideation. This, said Mr. Greenwood, is “roughly equivalent” to the prevalence reported earlier in the adult cohort of the Human Epilepsy Project (21.6%).
‘Striking’ rate
The fact that one in five adolescents had any lifetime suicide ideation is “definitely a striking number,” said Mr. Greenwood.
Researchers found that 15% of patients experienced active suicide ideation, 7.5% exhibited preparatory or suicidal behaviors, and 3% had made a prior suicide attempt.
All of these percentages increased at 3 years: Thirty-one percent for suicide ideation; 25% for active suicide behavior, 15% for preparatory or suicide behaviors, and 5% for prior suicide attempt.
The fact that nearly one in three adolescents endorsed suicide ideation at 3 years is another “striking” finding, said Mr. Greenwood.
Of the 53 adolescents who had never had suicide ideation at the time of enrollment, 7 endorsed new-onset suicide ideation in the follow-up period. Five of 14 who had had suicide ideation at some point prior to enrollment continued to endorse it.
“The value of the study is identifying the prevalence and identifying the significant number of adolescents with epilepsy who are endorsing either suicide ideation or suicidal behaviors,” said Mr. Greenwood.
The researchers found that among younger teens (aged 11–14 years) rates of suicide ideation were higher than among their older counterparts (aged 15–17 years).
The study does not shed light on the biological connection between epilepsy and suicidality, but Mr. Greenwood noted that prior research has suggested a bidirectional relationship.
“Depression and other psychiatric comorbidities might exist prior to epileptic activity and actually predispose to epileptic activity.”
Mr. Greenwood noted that suicide ideation has “spiked” recently across the general population, and so it’s difficult to compare the prevalence in her study with “today’s prevalence.”
However, other research generally shows that the suicide ideation rate in the general adolescent population is much lower than in teens with epilepsy.
Unique aspects of the current study are that it reports suicide ideation and behaviors at around the time of an epilepsy diagnosis and documents how suicidality progresses or resolves over time, said Mr. Greenwood.
Underdiagnosed, undertreated
Commenting on the research, Elizabeth Donner, MD, director of the comprehensive epilepsy program, Hospital for Sick Children, and associate professor, department of pediatrics, University of Toronto, said a “key point” from the study is that the suicidality rate among teens with epilepsy exceeds that of children not living with epilepsy.
“We are significantly underdiagnosing and undertreating the mental health comorbidities in epilepsy,” said Dr. Donner. “Epilepsy is a brain disease and so are mental health disorders, so it shouldn’t come as any surprise that they coexist in individuals with epilepsy.”
The new results contribute to what is already known about the significant mortality rates among persons with epilepsy, said Dr. Donner. She referred to a 2018 study that showed that people with epilepsy were 3.5 times more likely to die by suicide.
Other research has shown that people with epilepsy are 10 times more likely to die by drowning, mostly in the bathtub, said Dr. Donner.
“You would think that we’re educating these people about risks related to their epilepsy, but either the messages don’t get through, or they don’t know how to keep themselves safe,” she said.
“This needs to be seen in a bigger picture, and the bigger picture is we need to recognize comorbid mental health issues; we need to address them once recognized; and then we need to counsel and support people to live safely with their epilepsy.
The study received funding from the Epilepsy Study Consortium, Finding a Cure for Epilepsy and Seizures (FACES) and other related foundations, UCB, Pfizer, Eisai, Lundbeck, and Sunovion. Mr. Greenwood and Dr. Donner report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AES 2022
Significant racial disparities persist in status epilepticus
NASHVILLE, Tenn. – Investigators found that among Black patients with status epilepticus, the hospitalization rate was twice that of their White counterparts. Other findings reveal age and income disparities.
“The results suggest that racial minorities, those with a lower income, and the elderly are an appropriate target to improve health outcomes and reduce health inequality,” said Gabriela Tantillo Sepúlveda, MD, assistant professor of neurology, Baylor College of Medicine, Houston.
The findings were presented at the annual meeting of the American Epilepsy Society.
An examination of outcomes
Status epilepticus is associated with high rates of morbidity and mortality. Disparities in epilepsy care have previously been described, but little attention has been paid to the contribution of disparities to status epilepticus care and associated outcomes.
Researchers used 2010-2019 data from the Nationwide Inpatient Sample, a database covering a cross-section of hospitalizations in 48 states and the District of Columbia. From relevant diagnostic codes, they calculated status epilepticus prevalence as the rate per 10,000 hospitalizations and stratified this by demographics.
Over the study period, investigators identified 486,861 status epilepticus hospitalizations, most (71.3%) at urban teaching hospitals.
Status epilepticus prevalence was highest for non-Hispanic Black patients, at 27.3, followed by non-Hispanic others, at 16.1, Hispanic patients, at 15.8, and non-Hispanic-White patients, at 13.7 (P < .01).
The finding that Black patients had double the rate as White patients was “definitely surprising,” said Dr. Tantillo Sepúlveda.
Research over the past 20 years revealed similar disparities related to status epilepticus, “so it’s upsetting that these disparities have persisted. Unfortunately, we still have a lot of work to do to reduce health inequalities,” she said.
The investigators found that the prevalence of status epilepticus was higher in the lowest-income quartile, compared with the highest (18.7 vs. 14; P < .01).
Need for physician advocacy
Unlike previous studies, this research assessed various interventions in different age groups and showed that the likelihood of intubation, tracheostomy, gastrostomy, and in-hospital mortality increased with age.
For example, compared with the reference group (patients aged 18-39 years), the odds of intubation were 1.22 (95% confidence interval, 1.16-1.27) for those aged 40-59 years and 1.48 (95% CI, 1.42-1.54) for those aged 60-79. Those aged 80 and older were most likely to be intubated, at an odds ratio of 1.5 (95% CI, 1.43-1.58).
Elderly patients were most likely to undergo tracheostomy (OR, 2.0; 95% CI, 1.75-2.27), gastrostomy (OR, 3.37; 95% CI, 2.97-3.83), and to experience in-hospital mortality (OR, 6.51; 95% CI, 5.95-7.13), compared with the youngest patients.
These intervention rates also varied by racial/ethnic groups. Minority populations, particularly Black people, had higher odds of tracheostomy and gastrostomy, compared with non-Hispanic White persons.
The odds of undergoing electroencephalography monitoring progressively rose as income level increased (OR, 1.47; 95% CI, 1.34-1.62) for the highest income quartile versus the lowest quartile. The odds of undergoing EEG monitoring were also higher at urban teaching hospitals than at rural hospitals.
Tackling these disparities in this patient population include increasing resources, personnel, and health education aimed at minorities, low-income patients, and the elderly, said Dr. Tantillo Sepúlveda. She added that more research is needed “to determine the most effective ways of accomplishing this goal.”
The medical community can help reduce disparities, said Dr. Tantillo Sepúlveda, by working to improve health literacy, to reduce stigma associated with seizures, and to increase awareness of seizure risk factors.
They can also work to expand access to outpatient neurology clinics, epilepsy monitoring units, and epilepsy surgery. “Ethnic and racial minorities are less likely to receive epilepsy surgery for temporal lobe epilepsy, which has been shown to improve quality of life and reduce seizure burden,” Dr. Tantillo Sepúlveda noted.
Across-the-board problem
Commenting on the research, Daniel Lowenstein, MD, professor of neurology, University of California, San Francisco, said the findings aren’t at all surprising. “It’s yet another piece of evidence on what has now become a rather voluminous literature that documents the very significant disparities that exist in our health care system,” said Dr. Lowenstein. “There’s just a huge literature on ‘name your disease and you’ll see the disparities.’ ”
Disparities exist, for example, in diagnosing breast cancer and prostate cancer, in the treatment of stroke and in related outcomes, and there is a well-documented “big disparity” in the approach to pain control among patients presenting at the emergency department, said Dr. Lowenstein.
However, he doesn’t know how disparities in epilepsy and specifically in status epilepticus, compared with disparities regarding other diseases and disorders. He noted that in the case of epilepsy, the situation is likely exacerbated by the stigma associated with that disease.
Dr. Lowenstein agreed that clinicians should play a role in reversing disparities. “We as physicians have a responsibility to be a voice for change in our health care system.”
The study was supported by the Center of Excellence for health equity, training, and research at the Baylor College of Medicine. Dr. Tantillo Sepúlveda and Dr. Lowenstein report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NASHVILLE, Tenn. – Investigators found that among Black patients with status epilepticus, the hospitalization rate was twice that of their White counterparts. Other findings reveal age and income disparities.
“The results suggest that racial minorities, those with a lower income, and the elderly are an appropriate target to improve health outcomes and reduce health inequality,” said Gabriela Tantillo Sepúlveda, MD, assistant professor of neurology, Baylor College of Medicine, Houston.
The findings were presented at the annual meeting of the American Epilepsy Society.
An examination of outcomes
Status epilepticus is associated with high rates of morbidity and mortality. Disparities in epilepsy care have previously been described, but little attention has been paid to the contribution of disparities to status epilepticus care and associated outcomes.
Researchers used 2010-2019 data from the Nationwide Inpatient Sample, a database covering a cross-section of hospitalizations in 48 states and the District of Columbia. From relevant diagnostic codes, they calculated status epilepticus prevalence as the rate per 10,000 hospitalizations and stratified this by demographics.
Over the study period, investigators identified 486,861 status epilepticus hospitalizations, most (71.3%) at urban teaching hospitals.
Status epilepticus prevalence was highest for non-Hispanic Black patients, at 27.3, followed by non-Hispanic others, at 16.1, Hispanic patients, at 15.8, and non-Hispanic-White patients, at 13.7 (P < .01).
The finding that Black patients had double the rate as White patients was “definitely surprising,” said Dr. Tantillo Sepúlveda.
Research over the past 20 years revealed similar disparities related to status epilepticus, “so it’s upsetting that these disparities have persisted. Unfortunately, we still have a lot of work to do to reduce health inequalities,” she said.
The investigators found that the prevalence of status epilepticus was higher in the lowest-income quartile, compared with the highest (18.7 vs. 14; P < .01).
Need for physician advocacy
Unlike previous studies, this research assessed various interventions in different age groups and showed that the likelihood of intubation, tracheostomy, gastrostomy, and in-hospital mortality increased with age.
For example, compared with the reference group (patients aged 18-39 years), the odds of intubation were 1.22 (95% confidence interval, 1.16-1.27) for those aged 40-59 years and 1.48 (95% CI, 1.42-1.54) for those aged 60-79. Those aged 80 and older were most likely to be intubated, at an odds ratio of 1.5 (95% CI, 1.43-1.58).
Elderly patients were most likely to undergo tracheostomy (OR, 2.0; 95% CI, 1.75-2.27), gastrostomy (OR, 3.37; 95% CI, 2.97-3.83), and to experience in-hospital mortality (OR, 6.51; 95% CI, 5.95-7.13), compared with the youngest patients.
These intervention rates also varied by racial/ethnic groups. Minority populations, particularly Black people, had higher odds of tracheostomy and gastrostomy, compared with non-Hispanic White persons.
The odds of undergoing electroencephalography monitoring progressively rose as income level increased (OR, 1.47; 95% CI, 1.34-1.62) for the highest income quartile versus the lowest quartile. The odds of undergoing EEG monitoring were also higher at urban teaching hospitals than at rural hospitals.
Tackling these disparities in this patient population include increasing resources, personnel, and health education aimed at minorities, low-income patients, and the elderly, said Dr. Tantillo Sepúlveda. She added that more research is needed “to determine the most effective ways of accomplishing this goal.”
The medical community can help reduce disparities, said Dr. Tantillo Sepúlveda, by working to improve health literacy, to reduce stigma associated with seizures, and to increase awareness of seizure risk factors.
They can also work to expand access to outpatient neurology clinics, epilepsy monitoring units, and epilepsy surgery. “Ethnic and racial minorities are less likely to receive epilepsy surgery for temporal lobe epilepsy, which has been shown to improve quality of life and reduce seizure burden,” Dr. Tantillo Sepúlveda noted.
Across-the-board problem
Commenting on the research, Daniel Lowenstein, MD, professor of neurology, University of California, San Francisco, said the findings aren’t at all surprising. “It’s yet another piece of evidence on what has now become a rather voluminous literature that documents the very significant disparities that exist in our health care system,” said Dr. Lowenstein. “There’s just a huge literature on ‘name your disease and you’ll see the disparities.’ ”
Disparities exist, for example, in diagnosing breast cancer and prostate cancer, in the treatment of stroke and in related outcomes, and there is a well-documented “big disparity” in the approach to pain control among patients presenting at the emergency department, said Dr. Lowenstein.
However, he doesn’t know how disparities in epilepsy and specifically in status epilepticus, compared with disparities regarding other diseases and disorders. He noted that in the case of epilepsy, the situation is likely exacerbated by the stigma associated with that disease.
Dr. Lowenstein agreed that clinicians should play a role in reversing disparities. “We as physicians have a responsibility to be a voice for change in our health care system.”
The study was supported by the Center of Excellence for health equity, training, and research at the Baylor College of Medicine. Dr. Tantillo Sepúlveda and Dr. Lowenstein report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NASHVILLE, Tenn. – Investigators found that among Black patients with status epilepticus, the hospitalization rate was twice that of their White counterparts. Other findings reveal age and income disparities.
“The results suggest that racial minorities, those with a lower income, and the elderly are an appropriate target to improve health outcomes and reduce health inequality,” said Gabriela Tantillo Sepúlveda, MD, assistant professor of neurology, Baylor College of Medicine, Houston.
The findings were presented at the annual meeting of the American Epilepsy Society.
An examination of outcomes
Status epilepticus is associated with high rates of morbidity and mortality. Disparities in epilepsy care have previously been described, but little attention has been paid to the contribution of disparities to status epilepticus care and associated outcomes.
Researchers used 2010-2019 data from the Nationwide Inpatient Sample, a database covering a cross-section of hospitalizations in 48 states and the District of Columbia. From relevant diagnostic codes, they calculated status epilepticus prevalence as the rate per 10,000 hospitalizations and stratified this by demographics.
Over the study period, investigators identified 486,861 status epilepticus hospitalizations, most (71.3%) at urban teaching hospitals.
Status epilepticus prevalence was highest for non-Hispanic Black patients, at 27.3, followed by non-Hispanic others, at 16.1, Hispanic patients, at 15.8, and non-Hispanic-White patients, at 13.7 (P < .01).
The finding that Black patients had double the rate as White patients was “definitely surprising,” said Dr. Tantillo Sepúlveda.
Research over the past 20 years revealed similar disparities related to status epilepticus, “so it’s upsetting that these disparities have persisted. Unfortunately, we still have a lot of work to do to reduce health inequalities,” she said.
The investigators found that the prevalence of status epilepticus was higher in the lowest-income quartile, compared with the highest (18.7 vs. 14; P < .01).
Need for physician advocacy
Unlike previous studies, this research assessed various interventions in different age groups and showed that the likelihood of intubation, tracheostomy, gastrostomy, and in-hospital mortality increased with age.
For example, compared with the reference group (patients aged 18-39 years), the odds of intubation were 1.22 (95% confidence interval, 1.16-1.27) for those aged 40-59 years and 1.48 (95% CI, 1.42-1.54) for those aged 60-79. Those aged 80 and older were most likely to be intubated, at an odds ratio of 1.5 (95% CI, 1.43-1.58).
Elderly patients were most likely to undergo tracheostomy (OR, 2.0; 95% CI, 1.75-2.27), gastrostomy (OR, 3.37; 95% CI, 2.97-3.83), and to experience in-hospital mortality (OR, 6.51; 95% CI, 5.95-7.13), compared with the youngest patients.
These intervention rates also varied by racial/ethnic groups. Minority populations, particularly Black people, had higher odds of tracheostomy and gastrostomy, compared with non-Hispanic White persons.
The odds of undergoing electroencephalography monitoring progressively rose as income level increased (OR, 1.47; 95% CI, 1.34-1.62) for the highest income quartile versus the lowest quartile. The odds of undergoing EEG monitoring were also higher at urban teaching hospitals than at rural hospitals.
Tackling these disparities in this patient population include increasing resources, personnel, and health education aimed at minorities, low-income patients, and the elderly, said Dr. Tantillo Sepúlveda. She added that more research is needed “to determine the most effective ways of accomplishing this goal.”
The medical community can help reduce disparities, said Dr. Tantillo Sepúlveda, by working to improve health literacy, to reduce stigma associated with seizures, and to increase awareness of seizure risk factors.
They can also work to expand access to outpatient neurology clinics, epilepsy monitoring units, and epilepsy surgery. “Ethnic and racial minorities are less likely to receive epilepsy surgery for temporal lobe epilepsy, which has been shown to improve quality of life and reduce seizure burden,” Dr. Tantillo Sepúlveda noted.
Across-the-board problem
Commenting on the research, Daniel Lowenstein, MD, professor of neurology, University of California, San Francisco, said the findings aren’t at all surprising. “It’s yet another piece of evidence on what has now become a rather voluminous literature that documents the very significant disparities that exist in our health care system,” said Dr. Lowenstein. “There’s just a huge literature on ‘name your disease and you’ll see the disparities.’ ”
Disparities exist, for example, in diagnosing breast cancer and prostate cancer, in the treatment of stroke and in related outcomes, and there is a well-documented “big disparity” in the approach to pain control among patients presenting at the emergency department, said Dr. Lowenstein.
However, he doesn’t know how disparities in epilepsy and specifically in status epilepticus, compared with disparities regarding other diseases and disorders. He noted that in the case of epilepsy, the situation is likely exacerbated by the stigma associated with that disease.
Dr. Lowenstein agreed that clinicians should play a role in reversing disparities. “We as physicians have a responsibility to be a voice for change in our health care system.”
The study was supported by the Center of Excellence for health equity, training, and research at the Baylor College of Medicine. Dr. Tantillo Sepúlveda and Dr. Lowenstein report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AES 2022
Study implicates myelin plasticity in absence seizures
NASHVILLE, TENN. – that seems to provoke dysregulation of the insulating layer surrounding nerve fibers, perpetuating a cycle of increasing nerve damage and more frequent seizures later on.
“This study was the first to demonstrate that, at least in some forms of epilepsy, myelin plasticity is part of the maladaptive plasticity response that underlines epilepsy progression,” Juliet Knowles, MD, PhD, assistant professor at Stanford (Calif.) University, said in an interview. She reported the findings at the 2022 annual meeting of the American Epilepsy Society.
Dr. Knowles and colleagues made their discovery using laboratory mice. They used an imaging technique known as qMTI – quantitative magnetization transfer in conjunction with diffusion MRI – to map changes in myelin sheath thickness, or myelin plasticity, in major white matter tracks of the brain.
“Over the last decade we’ve come to understand that myelin, which is the insulating substance that coats the projections of brain cells or neurons, is more dynamic than we used to think,” she said. “In fact, throughout life, myelin’s structure in some regions of the brain can be changed in response to neuro activity. It’s a newly appreciated form of brain plasticity.”
However, she said, myelin plasticity has mostly been studied in healthy brains; “We don’t know very much about what role myelin plasticity might play in disease states like epilepsy,” Dr. Knowles said. The study’s goal was to investigate myelin plasticity specifically in absence seizures.
“We hypothesized that maybe absence seizures prompt activity-dependent myelin plasticity, but that maybe seizure-induced myelin plasticity alters the way that brain networks act in a way that contributes to the disease process,” she said.
Maladaptive myelin plasticity
The researchers found that absence seizures were infrequent when they first started, but then they rapidly progressed. “Over a couple of weeks, they’ll go from having very few seizures to having many seizures per hour,” Dr. Knowles said.
Using qMTI, the researchers found increased myelin sheath thickness across the longitudinal extent of the anterior corpus callosum, but they found myelin sheath thickness unchanged in brain regions where absence seizures weren’t prominent.
They also found that genetically blocking activity-dependent myelination markedly decreased seizure progression and decreased ictal somatosensory electroencephalography (EEG) coherence. Conversely, blocking myelin plasticity had no effect on ictal EEG coherence between visual cortices connected by the posterior corpus callosum.
The next step for the researchers is to develop MRI methods to use in human studies, Dr. Knowles said.
“We are working on developing an imaging approach in these same animal models that we hope we can use also to study in a detailed way white matter plasticity in humans with epilepsy and we’re also continuing our studies in animal models to try to identify ways to target maladaptive myelin plasticity, which ultimately we hope will inform treatment of people with epilepsy,” Dr. Knowles said.
Of mice and men
Although this study used mice, Chris Dulla, PhD, associate professor and director of the neuroscience graduate program at Tufts University in Boston, said the finding is “probably pretty transferable” to humans.
“This is the first study that really showed it,” he said of the link between myelin changes and seizure frequency. “I think people have suspected it, but that’s why this is kind of a big deal because this is one of the first studies to show it conclusively.”
He offered suggestions for validating the findings in humans. “The first thing would be to do imaging studies in people where you can examine to see if those white matter tracks are altered in a similar way in people with epilepsy,” he said. “I think now this study gives us good reason to undertake the work that it would take to ask that question and answer it in the human brain.”
Dr. Knowles and Dr. Dulla have no relevant relationships to disclose.
NASHVILLE, TENN. – that seems to provoke dysregulation of the insulating layer surrounding nerve fibers, perpetuating a cycle of increasing nerve damage and more frequent seizures later on.
“This study was the first to demonstrate that, at least in some forms of epilepsy, myelin plasticity is part of the maladaptive plasticity response that underlines epilepsy progression,” Juliet Knowles, MD, PhD, assistant professor at Stanford (Calif.) University, said in an interview. She reported the findings at the 2022 annual meeting of the American Epilepsy Society.
Dr. Knowles and colleagues made their discovery using laboratory mice. They used an imaging technique known as qMTI – quantitative magnetization transfer in conjunction with diffusion MRI – to map changes in myelin sheath thickness, or myelin plasticity, in major white matter tracks of the brain.
“Over the last decade we’ve come to understand that myelin, which is the insulating substance that coats the projections of brain cells or neurons, is more dynamic than we used to think,” she said. “In fact, throughout life, myelin’s structure in some regions of the brain can be changed in response to neuro activity. It’s a newly appreciated form of brain plasticity.”
However, she said, myelin plasticity has mostly been studied in healthy brains; “We don’t know very much about what role myelin plasticity might play in disease states like epilepsy,” Dr. Knowles said. The study’s goal was to investigate myelin plasticity specifically in absence seizures.
“We hypothesized that maybe absence seizures prompt activity-dependent myelin plasticity, but that maybe seizure-induced myelin plasticity alters the way that brain networks act in a way that contributes to the disease process,” she said.
Maladaptive myelin plasticity
The researchers found that absence seizures were infrequent when they first started, but then they rapidly progressed. “Over a couple of weeks, they’ll go from having very few seizures to having many seizures per hour,” Dr. Knowles said.
Using qMTI, the researchers found increased myelin sheath thickness across the longitudinal extent of the anterior corpus callosum, but they found myelin sheath thickness unchanged in brain regions where absence seizures weren’t prominent.
They also found that genetically blocking activity-dependent myelination markedly decreased seizure progression and decreased ictal somatosensory electroencephalography (EEG) coherence. Conversely, blocking myelin plasticity had no effect on ictal EEG coherence between visual cortices connected by the posterior corpus callosum.
The next step for the researchers is to develop MRI methods to use in human studies, Dr. Knowles said.
“We are working on developing an imaging approach in these same animal models that we hope we can use also to study in a detailed way white matter plasticity in humans with epilepsy and we’re also continuing our studies in animal models to try to identify ways to target maladaptive myelin plasticity, which ultimately we hope will inform treatment of people with epilepsy,” Dr. Knowles said.
Of mice and men
Although this study used mice, Chris Dulla, PhD, associate professor and director of the neuroscience graduate program at Tufts University in Boston, said the finding is “probably pretty transferable” to humans.
“This is the first study that really showed it,” he said of the link between myelin changes and seizure frequency. “I think people have suspected it, but that’s why this is kind of a big deal because this is one of the first studies to show it conclusively.”
He offered suggestions for validating the findings in humans. “The first thing would be to do imaging studies in people where you can examine to see if those white matter tracks are altered in a similar way in people with epilepsy,” he said. “I think now this study gives us good reason to undertake the work that it would take to ask that question and answer it in the human brain.”
Dr. Knowles and Dr. Dulla have no relevant relationships to disclose.
NASHVILLE, TENN. – that seems to provoke dysregulation of the insulating layer surrounding nerve fibers, perpetuating a cycle of increasing nerve damage and more frequent seizures later on.
“This study was the first to demonstrate that, at least in some forms of epilepsy, myelin plasticity is part of the maladaptive plasticity response that underlines epilepsy progression,” Juliet Knowles, MD, PhD, assistant professor at Stanford (Calif.) University, said in an interview. She reported the findings at the 2022 annual meeting of the American Epilepsy Society.
Dr. Knowles and colleagues made their discovery using laboratory mice. They used an imaging technique known as qMTI – quantitative magnetization transfer in conjunction with diffusion MRI – to map changes in myelin sheath thickness, or myelin plasticity, in major white matter tracks of the brain.
“Over the last decade we’ve come to understand that myelin, which is the insulating substance that coats the projections of brain cells or neurons, is more dynamic than we used to think,” she said. “In fact, throughout life, myelin’s structure in some regions of the brain can be changed in response to neuro activity. It’s a newly appreciated form of brain plasticity.”
However, she said, myelin plasticity has mostly been studied in healthy brains; “We don’t know very much about what role myelin plasticity might play in disease states like epilepsy,” Dr. Knowles said. The study’s goal was to investigate myelin plasticity specifically in absence seizures.
“We hypothesized that maybe absence seizures prompt activity-dependent myelin plasticity, but that maybe seizure-induced myelin plasticity alters the way that brain networks act in a way that contributes to the disease process,” she said.
Maladaptive myelin plasticity
The researchers found that absence seizures were infrequent when they first started, but then they rapidly progressed. “Over a couple of weeks, they’ll go from having very few seizures to having many seizures per hour,” Dr. Knowles said.
Using qMTI, the researchers found increased myelin sheath thickness across the longitudinal extent of the anterior corpus callosum, but they found myelin sheath thickness unchanged in brain regions where absence seizures weren’t prominent.
They also found that genetically blocking activity-dependent myelination markedly decreased seizure progression and decreased ictal somatosensory electroencephalography (EEG) coherence. Conversely, blocking myelin plasticity had no effect on ictal EEG coherence between visual cortices connected by the posterior corpus callosum.
The next step for the researchers is to develop MRI methods to use in human studies, Dr. Knowles said.
“We are working on developing an imaging approach in these same animal models that we hope we can use also to study in a detailed way white matter plasticity in humans with epilepsy and we’re also continuing our studies in animal models to try to identify ways to target maladaptive myelin plasticity, which ultimately we hope will inform treatment of people with epilepsy,” Dr. Knowles said.
Of mice and men
Although this study used mice, Chris Dulla, PhD, associate professor and director of the neuroscience graduate program at Tufts University in Boston, said the finding is “probably pretty transferable” to humans.
“This is the first study that really showed it,” he said of the link between myelin changes and seizure frequency. “I think people have suspected it, but that’s why this is kind of a big deal because this is one of the first studies to show it conclusively.”
He offered suggestions for validating the findings in humans. “The first thing would be to do imaging studies in people where you can examine to see if those white matter tracks are altered in a similar way in people with epilepsy,” he said. “I think now this study gives us good reason to undertake the work that it would take to ask that question and answer it in the human brain.”
Dr. Knowles and Dr. Dulla have no relevant relationships to disclose.
AT AES 2022
Newer brand-name drugs fuel spending on antiseizure medications
NASHVILLE, TENN. – , pointing to a major shift to newer, costlier, brand-name drugs – a trend in spending that may not be sustainable, the lead author of a study of drug costs said.
The study, presented at the 2022 annual meeting of the American Epilepsy Society, evaluated claims data for prescriptions for common antiseizure medications in the Medicare Part D and Medicaid databases from 2012 to 2020. The study excluded gabapentin and pregabalin because they’re frequently prescribed for other indications in addition to epileptic seizures.
“We found that third-generation medications, even though they accounted for the smallest percentage of claims in 2020, took up the most astronomical portion of the money that was spent,” lead author Deepti Zutshi, MD, an associate professor of neurology at Wayne State University in Detroit, said in an interview.
The study found that Medicare Part D spending on antiseizure medications increased from $1.16 billion in 2012 to $2.68 billion in 2020. In Medicaid, spending followed a similar trend, increasing from $973 million in 2012 to $1.05 billion in 2020.
Analyzing Medicare/Medicaid claims data
The study categorized drugs two ways: by brand or generic; and by first, second, or third generation, Dr. Zutshi said. First-generation drugs include medications such as phenobarbital, phenytoin, valproate, and carbamazepine. Second-generation medications were released in the early 2000s and include medications such as lamotrigine and levetiracetam. Examples of third-generation drugs include lacosamide, vigabatrin, clobazam, and perampanel.
Prescribers shifted significantly to third-generation treatments, Dr. Zutshi said. In Medicare Part D, the total spent on third-generation antiseizure medications went from $124 million in 2012 to $1.08 billion in 2020, representing a quadrupling in percentage of costs, from 10.7% to 40.4%. The total number of claims for third-generation antiseizure medications was 240,000 in 2012 (1.3%) and 1.1 million in 2020 (4.4%).
When looking at brand versus generic, the total spent on brand-name antiseizure medications increased nearly threefold from $546 million in 2012 to $1.62 million in 2020, with the share of all funding spent on brand-name antiseizure medications jumping from 46.8% to 60.2%. However, the proportion of total claims for branded antiseizure medications actually dropped, from 9.24% in 2012 to 6.62% in 2020.
Medicaid trends followed a similar pattern. Third-generation antiseizure medications accounted for 1.7% of total claims in 2012 and 6% in 2020. Spending on third-generation antiseizure medications grew nearly eight times: from $147 million, or 15.1% of funding spent on antiseizure medications, in 2012 to $1.15 billion in 2020, a 56.1% share of costs. The total spend of branded antiseizure medications in Medicaid was $605 million in 2012 and $1.46 billion in 2020 – a jump in the share of total spending from 62.2% to 71.3%. As in Medicare Part D, the percentage of total claims for branded antiseizure medications in Medicaid also dropped from 2012 to 2020, from 12.1% to 6.8%.
Why the substantial increase in spending?
“The reason we are prescribing these more expensive medications may be that the third-generation medications have better side-effect profiles, improved safety and outcomes in pregnancy, or that they have less drug interactions with other medications,” Dr. Zutshi said.
That’s desirable for older patients on Medicare who are more likely to have comorbidities and be on other medications, or women of child-bearing age on Medicaid, Dr. Zutshi said. “But I don’t think people realize what the cost is to Medicare and Medicaid,” she said, “so this was a bit of a shocking finding in our paper when we looked at this. I wasn’t expecting to see the substantial increase of spending focusing on just a few medications.”
Neurologists and other providers have to be more aware of individual patients’ needs as well as cost when prescribing branded or third-generation antiseizure medications, Dr. Zutshi said. “We have to do what’s best for all of our patients, but it has to be sustainable. If not, we could start losing the ability to prescribe these medications in these vulnerable population groups, so we have to use them judiciously,” Dr. Zutshi said.
Controlling costs versus managing seizures
Timothy E. Welty, PharmD, a professor of pharmacy at Drake University in Des Moines, Iowa, noted some potential issues with the study’s methodology, namely that, while it excluded gabapentin and pregabalin, it did include other antiseizure medications that are used for other indications without accounting for them. Additionally, the pharmacy claims data the study used didn’t cross match with any diagnostic data.
Controlling drug costs is noteworthy, he said, but managing seizures is equally important. “You have to think not only in terms of preventing seizures and what impact that has on health care costs specifically, but what impact that has on overall costs to society,” Dr. Welty said. “Doing the best we can to get their seizures under control as quickly as possible has great benefits for the patient outside of health care costs.”
He added, “We just really need to educate pharmacists and decision makers within third-party payers, be it Medicare, Medicaid, private insurance, whatever, on the advances that are being made in the use of seizure medications to treat epilepsy and stop seizures, but it’s a far broader issue than just how many dollars are we spending on seizure medication.”
Dr. Zutshi and Dr. Welty have no relevant disclosures to report.
NASHVILLE, TENN. – , pointing to a major shift to newer, costlier, brand-name drugs – a trend in spending that may not be sustainable, the lead author of a study of drug costs said.
The study, presented at the 2022 annual meeting of the American Epilepsy Society, evaluated claims data for prescriptions for common antiseizure medications in the Medicare Part D and Medicaid databases from 2012 to 2020. The study excluded gabapentin and pregabalin because they’re frequently prescribed for other indications in addition to epileptic seizures.
“We found that third-generation medications, even though they accounted for the smallest percentage of claims in 2020, took up the most astronomical portion of the money that was spent,” lead author Deepti Zutshi, MD, an associate professor of neurology at Wayne State University in Detroit, said in an interview.
The study found that Medicare Part D spending on antiseizure medications increased from $1.16 billion in 2012 to $2.68 billion in 2020. In Medicaid, spending followed a similar trend, increasing from $973 million in 2012 to $1.05 billion in 2020.
Analyzing Medicare/Medicaid claims data
The study categorized drugs two ways: by brand or generic; and by first, second, or third generation, Dr. Zutshi said. First-generation drugs include medications such as phenobarbital, phenytoin, valproate, and carbamazepine. Second-generation medications were released in the early 2000s and include medications such as lamotrigine and levetiracetam. Examples of third-generation drugs include lacosamide, vigabatrin, clobazam, and perampanel.
Prescribers shifted significantly to third-generation treatments, Dr. Zutshi said. In Medicare Part D, the total spent on third-generation antiseizure medications went from $124 million in 2012 to $1.08 billion in 2020, representing a quadrupling in percentage of costs, from 10.7% to 40.4%. The total number of claims for third-generation antiseizure medications was 240,000 in 2012 (1.3%) and 1.1 million in 2020 (4.4%).
When looking at brand versus generic, the total spent on brand-name antiseizure medications increased nearly threefold from $546 million in 2012 to $1.62 million in 2020, with the share of all funding spent on brand-name antiseizure medications jumping from 46.8% to 60.2%. However, the proportion of total claims for branded antiseizure medications actually dropped, from 9.24% in 2012 to 6.62% in 2020.
Medicaid trends followed a similar pattern. Third-generation antiseizure medications accounted for 1.7% of total claims in 2012 and 6% in 2020. Spending on third-generation antiseizure medications grew nearly eight times: from $147 million, or 15.1% of funding spent on antiseizure medications, in 2012 to $1.15 billion in 2020, a 56.1% share of costs. The total spend of branded antiseizure medications in Medicaid was $605 million in 2012 and $1.46 billion in 2020 – a jump in the share of total spending from 62.2% to 71.3%. As in Medicare Part D, the percentage of total claims for branded antiseizure medications in Medicaid also dropped from 2012 to 2020, from 12.1% to 6.8%.
Why the substantial increase in spending?
“The reason we are prescribing these more expensive medications may be that the third-generation medications have better side-effect profiles, improved safety and outcomes in pregnancy, or that they have less drug interactions with other medications,” Dr. Zutshi said.
That’s desirable for older patients on Medicare who are more likely to have comorbidities and be on other medications, or women of child-bearing age on Medicaid, Dr. Zutshi said. “But I don’t think people realize what the cost is to Medicare and Medicaid,” she said, “so this was a bit of a shocking finding in our paper when we looked at this. I wasn’t expecting to see the substantial increase of spending focusing on just a few medications.”
Neurologists and other providers have to be more aware of individual patients’ needs as well as cost when prescribing branded or third-generation antiseizure medications, Dr. Zutshi said. “We have to do what’s best for all of our patients, but it has to be sustainable. If not, we could start losing the ability to prescribe these medications in these vulnerable population groups, so we have to use them judiciously,” Dr. Zutshi said.
Controlling costs versus managing seizures
Timothy E. Welty, PharmD, a professor of pharmacy at Drake University in Des Moines, Iowa, noted some potential issues with the study’s methodology, namely that, while it excluded gabapentin and pregabalin, it did include other antiseizure medications that are used for other indications without accounting for them. Additionally, the pharmacy claims data the study used didn’t cross match with any diagnostic data.
Controlling drug costs is noteworthy, he said, but managing seizures is equally important. “You have to think not only in terms of preventing seizures and what impact that has on health care costs specifically, but what impact that has on overall costs to society,” Dr. Welty said. “Doing the best we can to get their seizures under control as quickly as possible has great benefits for the patient outside of health care costs.”
He added, “We just really need to educate pharmacists and decision makers within third-party payers, be it Medicare, Medicaid, private insurance, whatever, on the advances that are being made in the use of seizure medications to treat epilepsy and stop seizures, but it’s a far broader issue than just how many dollars are we spending on seizure medication.”
Dr. Zutshi and Dr. Welty have no relevant disclosures to report.
NASHVILLE, TENN. – , pointing to a major shift to newer, costlier, brand-name drugs – a trend in spending that may not be sustainable, the lead author of a study of drug costs said.
The study, presented at the 2022 annual meeting of the American Epilepsy Society, evaluated claims data for prescriptions for common antiseizure medications in the Medicare Part D and Medicaid databases from 2012 to 2020. The study excluded gabapentin and pregabalin because they’re frequently prescribed for other indications in addition to epileptic seizures.
“We found that third-generation medications, even though they accounted for the smallest percentage of claims in 2020, took up the most astronomical portion of the money that was spent,” lead author Deepti Zutshi, MD, an associate professor of neurology at Wayne State University in Detroit, said in an interview.
The study found that Medicare Part D spending on antiseizure medications increased from $1.16 billion in 2012 to $2.68 billion in 2020. In Medicaid, spending followed a similar trend, increasing from $973 million in 2012 to $1.05 billion in 2020.
Analyzing Medicare/Medicaid claims data
The study categorized drugs two ways: by brand or generic; and by first, second, or third generation, Dr. Zutshi said. First-generation drugs include medications such as phenobarbital, phenytoin, valproate, and carbamazepine. Second-generation medications were released in the early 2000s and include medications such as lamotrigine and levetiracetam. Examples of third-generation drugs include lacosamide, vigabatrin, clobazam, and perampanel.
Prescribers shifted significantly to third-generation treatments, Dr. Zutshi said. In Medicare Part D, the total spent on third-generation antiseizure medications went from $124 million in 2012 to $1.08 billion in 2020, representing a quadrupling in percentage of costs, from 10.7% to 40.4%. The total number of claims for third-generation antiseizure medications was 240,000 in 2012 (1.3%) and 1.1 million in 2020 (4.4%).
When looking at brand versus generic, the total spent on brand-name antiseizure medications increased nearly threefold from $546 million in 2012 to $1.62 million in 2020, with the share of all funding spent on brand-name antiseizure medications jumping from 46.8% to 60.2%. However, the proportion of total claims for branded antiseizure medications actually dropped, from 9.24% in 2012 to 6.62% in 2020.
Medicaid trends followed a similar pattern. Third-generation antiseizure medications accounted for 1.7% of total claims in 2012 and 6% in 2020. Spending on third-generation antiseizure medications grew nearly eight times: from $147 million, or 15.1% of funding spent on antiseizure medications, in 2012 to $1.15 billion in 2020, a 56.1% share of costs. The total spend of branded antiseizure medications in Medicaid was $605 million in 2012 and $1.46 billion in 2020 – a jump in the share of total spending from 62.2% to 71.3%. As in Medicare Part D, the percentage of total claims for branded antiseizure medications in Medicaid also dropped from 2012 to 2020, from 12.1% to 6.8%.
Why the substantial increase in spending?
“The reason we are prescribing these more expensive medications may be that the third-generation medications have better side-effect profiles, improved safety and outcomes in pregnancy, or that they have less drug interactions with other medications,” Dr. Zutshi said.
That’s desirable for older patients on Medicare who are more likely to have comorbidities and be on other medications, or women of child-bearing age on Medicaid, Dr. Zutshi said. “But I don’t think people realize what the cost is to Medicare and Medicaid,” she said, “so this was a bit of a shocking finding in our paper when we looked at this. I wasn’t expecting to see the substantial increase of spending focusing on just a few medications.”
Neurologists and other providers have to be more aware of individual patients’ needs as well as cost when prescribing branded or third-generation antiseizure medications, Dr. Zutshi said. “We have to do what’s best for all of our patients, but it has to be sustainable. If not, we could start losing the ability to prescribe these medications in these vulnerable population groups, so we have to use them judiciously,” Dr. Zutshi said.
Controlling costs versus managing seizures
Timothy E. Welty, PharmD, a professor of pharmacy at Drake University in Des Moines, Iowa, noted some potential issues with the study’s methodology, namely that, while it excluded gabapentin and pregabalin, it did include other antiseizure medications that are used for other indications without accounting for them. Additionally, the pharmacy claims data the study used didn’t cross match with any diagnostic data.
Controlling drug costs is noteworthy, he said, but managing seizures is equally important. “You have to think not only in terms of preventing seizures and what impact that has on health care costs specifically, but what impact that has on overall costs to society,” Dr. Welty said. “Doing the best we can to get their seizures under control as quickly as possible has great benefits for the patient outside of health care costs.”
He added, “We just really need to educate pharmacists and decision makers within third-party payers, be it Medicare, Medicaid, private insurance, whatever, on the advances that are being made in the use of seizure medications to treat epilepsy and stop seizures, but it’s a far broader issue than just how many dollars are we spending on seizure medication.”
Dr. Zutshi and Dr. Welty have no relevant disclosures to report.
AT AES 2022
More evidence in utero exposure to antiseizure meds safe for children’s cognition
NASHVILLE, TENN. – There is no negative impact of in utero exposure to antiseizure medications on children’s creativity, new research shows.
The results of this study, along with other research, suggest the risk for cognitive problems “is fairly low” overall for children of women with epilepsy taking lamotrigine or levetiracetam, study investigator, Kimford J. Meador, MD, professor, department of neurology & neurological sciences, Stanford (Calif.) University School of Medicine, told this news organization.
“This is another encouraging piece that’s showing these new drugs are safe with regard to cognition.”
The findings were presented at the annual meeting of the American Epilepsy Society.
Capturing creativity
Fetal exposure to antiseizure medications can produce adverse neurodevelopmental effects. These are typically assessed using measures such as general intelligence, verbal/nonverbal abilities, or additional educational needs.
However, these measures don’t capture creativity, which “is related to intelligence but not completely,” said Dr. Meador. “I have seen wonderful examples of creativity in people who have a lot of cognitive impairment.”
He referred to one of his patients with epilepsy who is “spectacularly good” at painting with watercolors, even though she has significant cognitive impairment.
The new analysis is part of the MONEAD study, a prospective, observational multicenter study examining pregnancy outcomes for both mother and child. It included pregnant women who were enrolled at under 20 weeks’ gestational age.
The women with epilepsy in the study were primarily on monotherapy (73%), and of these, 82% were on lamotrigine or levetiracetam. About 22% were on polytherapy, of which 42% were on dual therapy with lamotrigine and levetiracetam.
Fluency, originality
Researchers assessed the children of these women at age 4½ years using the Torrance Test of Creative Thinking-Figural (TTCT-F). This is a standardized assessment of creative thinking with index scores measuring such things as fluency, originality, abstractness, and elaboration.
Dr. Meador noted the research team used a shorter version of the test battery “so as to not wear out the families and kids.”
During the test, children were given lines of different shapes and asked to draw a picture using these lines. Dr. Meador pointed out the drawings ranged from quite basic to more intricate.
One child cleverly turned a few squiggly lines into a car. “I can look at this and say this kid’s going to do very well,” said Dr. Meador.
Investigators compared scores between 241 children of women with epilepsy (WWE) and 65 children of healthy women (HW). They adjusted for the mother’s IQ, education level, age at enrollment, gestation age at enrollment, post-birth average anxiety score, and the child’s ethnicity and sex.
Investigators found the mean TTCT-F scores did not differ significantly between the two groups: adjusted least squares mean of 89.5 (95% confidence interval, 86.7-92.3) for children of WWE, compared with adjusted least square mean of 92.0 (95% CI, 86.4-97.6) for children of HW.
Balancing act
The researchers haven’t looked at a dose effect in this current study, but Dr. Meador said it’s always “a balancing act” between giving enough of the drug to keep mothers from seizing, which affect both the mother and fetus, and giving as low a dose as possible to protect the fetus.
In addition, as medication levels change during pregnancy, he said he recommends that drug levels are monitored monthly so that medication can be adjusted as necessary.
Looking at what factors might predict creativity scores, researchers found children did less well creatively if their mother didn’t have a college degree (estimate –9.5; 95% CI, –17.9 to –1.2; P = .025).
“It looks like being in a home where the mother has had more education is going to have an impact on the kid’s thinking and creativity,” said Dr. Meador.
These new findings are consistent with a lack of differences in other cognitive abilities that Dr. Meador and his team found when the children were younger.
“At age 3, we did not find an overall difference in cognitive and verbal abilities and intelligence between the children of mothers with epilepsy and those of healthy women,” he said.
The researchers aim to assess cognitive and behavioral outcomes in these children when they are 6 years old.
Helpful information
Commenting on the findings, Stéphane Auvin, MD, PhD, chair of the department of pediatric neurology at the University of Paris, who co-moderated a platform session featuring the research, said the study “is an interesting measure of the impact of being exposed to antiseizure medications.”
Creativity is “complex,” he said. “It’s not only cognition; it could be things like behavior and impulsivity.”
The new information is “very helpful.” Focusing on something broader than just IQ “gives you a better picture of what’s going on.”
The study received funding from NIH, NINDS, and NICH. Dr. Meador has received grants from NIH/NINDS, NIH/NICHD, Veterans Administration, and Eisai. He has been a consultant for Epilepsy Consortium, Novartis, Supernus, Upsher Smith Labs, and UCB Pharma. Dr. Auvin reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – There is no negative impact of in utero exposure to antiseizure medications on children’s creativity, new research shows.
The results of this study, along with other research, suggest the risk for cognitive problems “is fairly low” overall for children of women with epilepsy taking lamotrigine or levetiracetam, study investigator, Kimford J. Meador, MD, professor, department of neurology & neurological sciences, Stanford (Calif.) University School of Medicine, told this news organization.
“This is another encouraging piece that’s showing these new drugs are safe with regard to cognition.”
The findings were presented at the annual meeting of the American Epilepsy Society.
Capturing creativity
Fetal exposure to antiseizure medications can produce adverse neurodevelopmental effects. These are typically assessed using measures such as general intelligence, verbal/nonverbal abilities, or additional educational needs.
However, these measures don’t capture creativity, which “is related to intelligence but not completely,” said Dr. Meador. “I have seen wonderful examples of creativity in people who have a lot of cognitive impairment.”
He referred to one of his patients with epilepsy who is “spectacularly good” at painting with watercolors, even though she has significant cognitive impairment.
The new analysis is part of the MONEAD study, a prospective, observational multicenter study examining pregnancy outcomes for both mother and child. It included pregnant women who were enrolled at under 20 weeks’ gestational age.
The women with epilepsy in the study were primarily on monotherapy (73%), and of these, 82% were on lamotrigine or levetiracetam. About 22% were on polytherapy, of which 42% were on dual therapy with lamotrigine and levetiracetam.
Fluency, originality
Researchers assessed the children of these women at age 4½ years using the Torrance Test of Creative Thinking-Figural (TTCT-F). This is a standardized assessment of creative thinking with index scores measuring such things as fluency, originality, abstractness, and elaboration.
Dr. Meador noted the research team used a shorter version of the test battery “so as to not wear out the families and kids.”
During the test, children were given lines of different shapes and asked to draw a picture using these lines. Dr. Meador pointed out the drawings ranged from quite basic to more intricate.
One child cleverly turned a few squiggly lines into a car. “I can look at this and say this kid’s going to do very well,” said Dr. Meador.
Investigators compared scores between 241 children of women with epilepsy (WWE) and 65 children of healthy women (HW). They adjusted for the mother’s IQ, education level, age at enrollment, gestation age at enrollment, post-birth average anxiety score, and the child’s ethnicity and sex.
Investigators found the mean TTCT-F scores did not differ significantly between the two groups: adjusted least squares mean of 89.5 (95% confidence interval, 86.7-92.3) for children of WWE, compared with adjusted least square mean of 92.0 (95% CI, 86.4-97.6) for children of HW.
Balancing act
The researchers haven’t looked at a dose effect in this current study, but Dr. Meador said it’s always “a balancing act” between giving enough of the drug to keep mothers from seizing, which affect both the mother and fetus, and giving as low a dose as possible to protect the fetus.
In addition, as medication levels change during pregnancy, he said he recommends that drug levels are monitored monthly so that medication can be adjusted as necessary.
Looking at what factors might predict creativity scores, researchers found children did less well creatively if their mother didn’t have a college degree (estimate –9.5; 95% CI, –17.9 to –1.2; P = .025).
“It looks like being in a home where the mother has had more education is going to have an impact on the kid’s thinking and creativity,” said Dr. Meador.
These new findings are consistent with a lack of differences in other cognitive abilities that Dr. Meador and his team found when the children were younger.
“At age 3, we did not find an overall difference in cognitive and verbal abilities and intelligence between the children of mothers with epilepsy and those of healthy women,” he said.
The researchers aim to assess cognitive and behavioral outcomes in these children when they are 6 years old.
Helpful information
Commenting on the findings, Stéphane Auvin, MD, PhD, chair of the department of pediatric neurology at the University of Paris, who co-moderated a platform session featuring the research, said the study “is an interesting measure of the impact of being exposed to antiseizure medications.”
Creativity is “complex,” he said. “It’s not only cognition; it could be things like behavior and impulsivity.”
The new information is “very helpful.” Focusing on something broader than just IQ “gives you a better picture of what’s going on.”
The study received funding from NIH, NINDS, and NICH. Dr. Meador has received grants from NIH/NINDS, NIH/NICHD, Veterans Administration, and Eisai. He has been a consultant for Epilepsy Consortium, Novartis, Supernus, Upsher Smith Labs, and UCB Pharma. Dr. Auvin reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – There is no negative impact of in utero exposure to antiseizure medications on children’s creativity, new research shows.
The results of this study, along with other research, suggest the risk for cognitive problems “is fairly low” overall for children of women with epilepsy taking lamotrigine or levetiracetam, study investigator, Kimford J. Meador, MD, professor, department of neurology & neurological sciences, Stanford (Calif.) University School of Medicine, told this news organization.
“This is another encouraging piece that’s showing these new drugs are safe with regard to cognition.”
The findings were presented at the annual meeting of the American Epilepsy Society.
Capturing creativity
Fetal exposure to antiseizure medications can produce adverse neurodevelopmental effects. These are typically assessed using measures such as general intelligence, verbal/nonverbal abilities, or additional educational needs.
However, these measures don’t capture creativity, which “is related to intelligence but not completely,” said Dr. Meador. “I have seen wonderful examples of creativity in people who have a lot of cognitive impairment.”
He referred to one of his patients with epilepsy who is “spectacularly good” at painting with watercolors, even though she has significant cognitive impairment.
The new analysis is part of the MONEAD study, a prospective, observational multicenter study examining pregnancy outcomes for both mother and child. It included pregnant women who were enrolled at under 20 weeks’ gestational age.
The women with epilepsy in the study were primarily on monotherapy (73%), and of these, 82% were on lamotrigine or levetiracetam. About 22% were on polytherapy, of which 42% were on dual therapy with lamotrigine and levetiracetam.
Fluency, originality
Researchers assessed the children of these women at age 4½ years using the Torrance Test of Creative Thinking-Figural (TTCT-F). This is a standardized assessment of creative thinking with index scores measuring such things as fluency, originality, abstractness, and elaboration.
Dr. Meador noted the research team used a shorter version of the test battery “so as to not wear out the families and kids.”
During the test, children were given lines of different shapes and asked to draw a picture using these lines. Dr. Meador pointed out the drawings ranged from quite basic to more intricate.
One child cleverly turned a few squiggly lines into a car. “I can look at this and say this kid’s going to do very well,” said Dr. Meador.
Investigators compared scores between 241 children of women with epilepsy (WWE) and 65 children of healthy women (HW). They adjusted for the mother’s IQ, education level, age at enrollment, gestation age at enrollment, post-birth average anxiety score, and the child’s ethnicity and sex.
Investigators found the mean TTCT-F scores did not differ significantly between the two groups: adjusted least squares mean of 89.5 (95% confidence interval, 86.7-92.3) for children of WWE, compared with adjusted least square mean of 92.0 (95% CI, 86.4-97.6) for children of HW.
Balancing act
The researchers haven’t looked at a dose effect in this current study, but Dr. Meador said it’s always “a balancing act” between giving enough of the drug to keep mothers from seizing, which affect both the mother and fetus, and giving as low a dose as possible to protect the fetus.
In addition, as medication levels change during pregnancy, he said he recommends that drug levels are monitored monthly so that medication can be adjusted as necessary.
Looking at what factors might predict creativity scores, researchers found children did less well creatively if their mother didn’t have a college degree (estimate –9.5; 95% CI, –17.9 to –1.2; P = .025).
“It looks like being in a home where the mother has had more education is going to have an impact on the kid’s thinking and creativity,” said Dr. Meador.
These new findings are consistent with a lack of differences in other cognitive abilities that Dr. Meador and his team found when the children were younger.
“At age 3, we did not find an overall difference in cognitive and verbal abilities and intelligence between the children of mothers with epilepsy and those of healthy women,” he said.
The researchers aim to assess cognitive and behavioral outcomes in these children when they are 6 years old.
Helpful information
Commenting on the findings, Stéphane Auvin, MD, PhD, chair of the department of pediatric neurology at the University of Paris, who co-moderated a platform session featuring the research, said the study “is an interesting measure of the impact of being exposed to antiseizure medications.”
Creativity is “complex,” he said. “It’s not only cognition; it could be things like behavior and impulsivity.”
The new information is “very helpful.” Focusing on something broader than just IQ “gives you a better picture of what’s going on.”
The study received funding from NIH, NINDS, and NICH. Dr. Meador has received grants from NIH/NINDS, NIH/NICHD, Veterans Administration, and Eisai. He has been a consultant for Epilepsy Consortium, Novartis, Supernus, Upsher Smith Labs, and UCB Pharma. Dr. Auvin reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AES 2022
Strong two-way link between epilepsy and depression
, with implications for diagnosis and patient care. The findings “strongly support previous observations of a bidirectional association between these two brain disorders,” said Eva Bølling-Ladegaard, MD, a PhD student, department of clinical medicine (Neurology), Aarhus (Denmark) University.
“We add to the existing evidence in temporal range, showing that the increased risks of depression following epilepsy, and vice versa, are sustained over a much more extended time period than previously shown; that is, 20 years on both sides of receiving a diagnosis of the index disorder,” Ms. Bølling-Ladegaard said.
The study was published online in Neurology.
Epilepsy then depression
The researchers examined the magnitude and long-term temporal association between epilepsy and depression. They compared the risk of the two brain disorders following another chronic disorder (asthma) in a nationwide, register-based, matched cohort study.
In a population of more than 8.7 million people, they identified 139,014 persons with epilepsy (54% males; median age at diagnosis, 43 years), 219,990 with depression (37% males; median age at diagnosis, 43 years), and 358,821 with asthma (49% males; median age at diagnosis, 29 years).
The rate of developing depression was increased nearly twofold among people with epilepsy compared with the matched population who did not have epilepsy (adjusted hazard ratio, 1.88; 95% confidence interval, 1.82-1.95).
The rate of depression was highest during the first months and years after epilepsy diagnosis. It declined over time, yet remained significantly elevated throughout the 20+ years of observation.
The cumulative incidence of depression at 5 and 35 years’ follow-up in the epilepsy cohort was 1.37% and 6.05%, respectively, compared with 0.59% and 3.92% in the reference population.
The highest rate of depression after epilepsy was among individuals aged 40-59 years, and the lowest was among those aged 0-19 years at first epilepsy diagnosis.
Depression then epilepsy
The rate of developing epilepsy was increased more than twofold among patients with incident depression compared with the matched population who were without depression (aHR, 2.35; 95% CI, 2.25-2.44).
As in the opposite analysis, the rate of epilepsy was highest during the first months and years after depression diagnosis and declined over time.
The cumulative incidence of epilepsy at 5 and 35 years after depression diagnosis was 1.10% and 4.19%, respectively, compared with 0.32% and 2.06% in the reference population.
The rate of epilepsy was highest among those aged 0-19 years at time of first depression diagnosis and was lowest among those aged 80+ at first depression diagnosis.
For comparison, after asthma diagnosis, rates of depression and epilepsy were increased 1.63-fold (95% CI, 1.59-1.67) and 1.48-fold (95% CI, 1.44-1.53), respectively, compared with matched individuals without asthma.
Using admission with seizures as a proxy for treatment failure, the researchers observed an increased risk of treatment failure among people with epilepsy who were diagnosed with depression.
“Our results support previous findings indicating worse seizure outcomes in people with epilepsy and coexisting depression,” said Ms. Bølling-Ladegaard.
“Increased clinical awareness of the association between epilepsy and depression is therefore needed in order to increase the proportion of patients that receive appropriate treatment and improve outcomes for these patient groups,” she said.
Clinical implications
Reached for comment, Zulfi Haneef, MBBS, MD, associate professor of neurology, Baylor College of Medicine, Houston, noted that the link between epilepsy and depression is “well-known.”
“However, typically one thinks of epilepsy as leading to depression, not vice versa. Here they show the risk of epilepsy following depression to be high (highest of the risks given), which is thought provoking. However, association does not imply causation,” Dr. Haneef said.
“Prima facie, there is no biological rationale for depression to lead to epilepsy,” he said. He noted that some antidepressants can reduce the seizure threshold.
The findings do have implications for care, he said.
“For neurologists, this is another study that exhorts them to screen for depression and treat adequately in all patients with epilepsy,” Dr. Haneef said.
“For psychiatrists, this study may give guidance to watch more carefully for seizures in patients with depression, especially when using antidepressant medications that induce seizures.
“For the general public with either epilepsy or depression, it would help them be aware about this bidirectional association,” Dr. Haneef said.
The study was funded by the Lundbeck Foundation, the Danish Epilepsy Association, and the Novo Nordisk Foundation. Ms. Bølling-Ladegaard and Dr. Haneef have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, with implications for diagnosis and patient care. The findings “strongly support previous observations of a bidirectional association between these two brain disorders,” said Eva Bølling-Ladegaard, MD, a PhD student, department of clinical medicine (Neurology), Aarhus (Denmark) University.
“We add to the existing evidence in temporal range, showing that the increased risks of depression following epilepsy, and vice versa, are sustained over a much more extended time period than previously shown; that is, 20 years on both sides of receiving a diagnosis of the index disorder,” Ms. Bølling-Ladegaard said.
The study was published online in Neurology.
Epilepsy then depression
The researchers examined the magnitude and long-term temporal association between epilepsy and depression. They compared the risk of the two brain disorders following another chronic disorder (asthma) in a nationwide, register-based, matched cohort study.
In a population of more than 8.7 million people, they identified 139,014 persons with epilepsy (54% males; median age at diagnosis, 43 years), 219,990 with depression (37% males; median age at diagnosis, 43 years), and 358,821 with asthma (49% males; median age at diagnosis, 29 years).
The rate of developing depression was increased nearly twofold among people with epilepsy compared with the matched population who did not have epilepsy (adjusted hazard ratio, 1.88; 95% confidence interval, 1.82-1.95).
The rate of depression was highest during the first months and years after epilepsy diagnosis. It declined over time, yet remained significantly elevated throughout the 20+ years of observation.
The cumulative incidence of depression at 5 and 35 years’ follow-up in the epilepsy cohort was 1.37% and 6.05%, respectively, compared with 0.59% and 3.92% in the reference population.
The highest rate of depression after epilepsy was among individuals aged 40-59 years, and the lowest was among those aged 0-19 years at first epilepsy diagnosis.
Depression then epilepsy
The rate of developing epilepsy was increased more than twofold among patients with incident depression compared with the matched population who were without depression (aHR, 2.35; 95% CI, 2.25-2.44).
As in the opposite analysis, the rate of epilepsy was highest during the first months and years after depression diagnosis and declined over time.
The cumulative incidence of epilepsy at 5 and 35 years after depression diagnosis was 1.10% and 4.19%, respectively, compared with 0.32% and 2.06% in the reference population.
The rate of epilepsy was highest among those aged 0-19 years at time of first depression diagnosis and was lowest among those aged 80+ at first depression diagnosis.
For comparison, after asthma diagnosis, rates of depression and epilepsy were increased 1.63-fold (95% CI, 1.59-1.67) and 1.48-fold (95% CI, 1.44-1.53), respectively, compared with matched individuals without asthma.
Using admission with seizures as a proxy for treatment failure, the researchers observed an increased risk of treatment failure among people with epilepsy who were diagnosed with depression.
“Our results support previous findings indicating worse seizure outcomes in people with epilepsy and coexisting depression,” said Ms. Bølling-Ladegaard.
“Increased clinical awareness of the association between epilepsy and depression is therefore needed in order to increase the proportion of patients that receive appropriate treatment and improve outcomes for these patient groups,” she said.
Clinical implications
Reached for comment, Zulfi Haneef, MBBS, MD, associate professor of neurology, Baylor College of Medicine, Houston, noted that the link between epilepsy and depression is “well-known.”
“However, typically one thinks of epilepsy as leading to depression, not vice versa. Here they show the risk of epilepsy following depression to be high (highest of the risks given), which is thought provoking. However, association does not imply causation,” Dr. Haneef said.
“Prima facie, there is no biological rationale for depression to lead to epilepsy,” he said. He noted that some antidepressants can reduce the seizure threshold.
The findings do have implications for care, he said.
“For neurologists, this is another study that exhorts them to screen for depression and treat adequately in all patients with epilepsy,” Dr. Haneef said.
“For psychiatrists, this study may give guidance to watch more carefully for seizures in patients with depression, especially when using antidepressant medications that induce seizures.
“For the general public with either epilepsy or depression, it would help them be aware about this bidirectional association,” Dr. Haneef said.
The study was funded by the Lundbeck Foundation, the Danish Epilepsy Association, and the Novo Nordisk Foundation. Ms. Bølling-Ladegaard and Dr. Haneef have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, with implications for diagnosis and patient care. The findings “strongly support previous observations of a bidirectional association between these two brain disorders,” said Eva Bølling-Ladegaard, MD, a PhD student, department of clinical medicine (Neurology), Aarhus (Denmark) University.
“We add to the existing evidence in temporal range, showing that the increased risks of depression following epilepsy, and vice versa, are sustained over a much more extended time period than previously shown; that is, 20 years on both sides of receiving a diagnosis of the index disorder,” Ms. Bølling-Ladegaard said.
The study was published online in Neurology.
Epilepsy then depression
The researchers examined the magnitude and long-term temporal association between epilepsy and depression. They compared the risk of the two brain disorders following another chronic disorder (asthma) in a nationwide, register-based, matched cohort study.
In a population of more than 8.7 million people, they identified 139,014 persons with epilepsy (54% males; median age at diagnosis, 43 years), 219,990 with depression (37% males; median age at diagnosis, 43 years), and 358,821 with asthma (49% males; median age at diagnosis, 29 years).
The rate of developing depression was increased nearly twofold among people with epilepsy compared with the matched population who did not have epilepsy (adjusted hazard ratio, 1.88; 95% confidence interval, 1.82-1.95).
The rate of depression was highest during the first months and years after epilepsy diagnosis. It declined over time, yet remained significantly elevated throughout the 20+ years of observation.
The cumulative incidence of depression at 5 and 35 years’ follow-up in the epilepsy cohort was 1.37% and 6.05%, respectively, compared with 0.59% and 3.92% in the reference population.
The highest rate of depression after epilepsy was among individuals aged 40-59 years, and the lowest was among those aged 0-19 years at first epilepsy diagnosis.
Depression then epilepsy
The rate of developing epilepsy was increased more than twofold among patients with incident depression compared with the matched population who were without depression (aHR, 2.35; 95% CI, 2.25-2.44).
As in the opposite analysis, the rate of epilepsy was highest during the first months and years after depression diagnosis and declined over time.
The cumulative incidence of epilepsy at 5 and 35 years after depression diagnosis was 1.10% and 4.19%, respectively, compared with 0.32% and 2.06% in the reference population.
The rate of epilepsy was highest among those aged 0-19 years at time of first depression diagnosis and was lowest among those aged 80+ at first depression diagnosis.
For comparison, after asthma diagnosis, rates of depression and epilepsy were increased 1.63-fold (95% CI, 1.59-1.67) and 1.48-fold (95% CI, 1.44-1.53), respectively, compared with matched individuals without asthma.
Using admission with seizures as a proxy for treatment failure, the researchers observed an increased risk of treatment failure among people with epilepsy who were diagnosed with depression.
“Our results support previous findings indicating worse seizure outcomes in people with epilepsy and coexisting depression,” said Ms. Bølling-Ladegaard.
“Increased clinical awareness of the association between epilepsy and depression is therefore needed in order to increase the proportion of patients that receive appropriate treatment and improve outcomes for these patient groups,” she said.
Clinical implications
Reached for comment, Zulfi Haneef, MBBS, MD, associate professor of neurology, Baylor College of Medicine, Houston, noted that the link between epilepsy and depression is “well-known.”
“However, typically one thinks of epilepsy as leading to depression, not vice versa. Here they show the risk of epilepsy following depression to be high (highest of the risks given), which is thought provoking. However, association does not imply causation,” Dr. Haneef said.
“Prima facie, there is no biological rationale for depression to lead to epilepsy,” he said. He noted that some antidepressants can reduce the seizure threshold.
The findings do have implications for care, he said.
“For neurologists, this is another study that exhorts them to screen for depression and treat adequately in all patients with epilepsy,” Dr. Haneef said.
“For psychiatrists, this study may give guidance to watch more carefully for seizures in patients with depression, especially when using antidepressant medications that induce seizures.
“For the general public with either epilepsy or depression, it would help them be aware about this bidirectional association,” Dr. Haneef said.
The study was funded by the Lundbeck Foundation, the Danish Epilepsy Association, and the Novo Nordisk Foundation. Ms. Bølling-Ladegaard and Dr. Haneef have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY







