User login
Abaloparatide works in ‘ignored population’: Men with osteoporosis
San Diego – The anabolic osteoporosis treatment abaloparatide (Tymlos, Radius Health) works in men as well as women, new data indicate.
Findings from the Abaloparatide for the Treatment of Men With Osteoporosis (ATOM) randomized, double-blind, placebo-controlled, phase 3 study were presented last week at the American Association of Clinical Endocrinology (AACE) Annual Meeting 2022.
Abaloparatide, a subcutaneously administered parathyroid-hormone–related protein (PTHrP) analog, resulted in significant increases in bone mineral density by 12 months at the lumbar spine, total hip, and femoral neck, compared with placebo in men with osteoporosis, with no significant adverse effects.
“Osteoporosis is underdiagnosed in men. Abaloparatide is another option for an ignored population,” presenter Neil Binkley, MD, of the University of Wisconsin School of Medicine and Public Health Madison, said in an interview.
Abaloparatide was approved by the U.S. Food and Drug Administration in 2017 for the treatment of postmenopausal women at high risk for fracture due to a history of osteoporotic fracture or multiple fracture risk factors, or who haven’t responded to or are intolerant of other osteoporosis therapies.
While postmenopausal women have mainly been the focus in osteoporosis, men account for approximately 30% of the societal burden of osteoporosis and have greater fracture-related morbidity and mortality than women.
About one in four men over the age of 50 years will have a fragility fracture in their lifetime. Yet, they’re far less likely to be diagnosed or to be included in osteoporosis treatment trials, Dr. Binkley noted.
Asked to comment, session moderator Thanh D. Hoang, DO, told this news organization, “I think it’s a great option to treat osteoporosis, and now we have evidence for treating osteoporosis in men. Mostly the data have come from postmenopausal women.”
Screen men with hypogonadism or those taking steroids
“This new medication is an addition to the very limited number of treatments that we have when patients don’t respond to [initial] medications. To have another anabolic bone-forming medication is very, very good,” said Dr. Hoang, who is professor and program director of the Endocrinology Fellowship Program at Walter Reed National Military Medical Center, Bethesda, Maryland.
Radius Health filed a Supplemental New Drug Application with the FDA for abaloparatide (Tymlos) subcutaneous injection in men with osteoporosis at high risk for fracture in February. There is a 10-month review period.
Dr. Binkley advises bone screening for men who have conditions such as hypogonadism or who are taking glucocorticoids or chemotherapeutics.
But, he added, “I think that if we did nothing else good in the osteoporosis field, if we treated people after they fractured that would be a huge step forward. Even with a normal T score, when those people fracture, they [often] don’t have normal bone mineral density ... That’s a group of people we’re ignoring still. They’re not getting diagnosed, and they’re not getting treated.”
ATOM Study: Significant BMD increases at key sites
The approval of abaloparatide in women was based on the phase 3, 18-week ACTIVE trial of more than 2,000 high-risk women, in whom abaloparatide was associated with an 86% reduction in vertebral fracture incidence, compared with placebo, and also significantly greater reductions in nonvertebral fractures, compared with both placebo and teriparatide (Forteo, Eli Lilly).
The ATOM study involved a total of 228 men aged 40-85 years with primary or hypogonadism-associated osteoporosis randomized 2:1 to receive subcutaneous 80 μg abaloparatide or injected placebo daily for 12 months. All had T scores (based on male reference range) of ≤ −2.5 at the lumbar spine or hip, or ≤ −1.5 and with radiologic vertebral fracture or a history of low trauma nonvertebral fracture in the past 5 years, or T score ≤ −2.0 if older than 65 years.
Increases in bone mineral density from baseline were significantly greater with abaloparatide compared with placebo at the lumbar spine, total hip, and femoral neck at 3, 6, and 12 months. Mean percentage changes at 12 months were 8.5%, 2.1%, and 3.0%, for the three locations, respectively, compared with 1.2%, 0.01%, and 0.2% for placebo (all P ≤ .0001).
Three fractures occurred in those receiving placebo and one with abaloparatide.
For markers of bone turnover, median serum procollagen type I N-terminal propeptide (s-PINP) was 111.2 ng/mL after 1 month of abaloparatide treatment and 85.7 ng/mL at month 12. Median serum carboxy-terminal cross-linking telopeptide of type I collagen (s-CTX) was 0.48 ng/mL at month 6 and 0.45 ng/mL at month 12 in the abaloparatide group. Geometric mean relative to baseline s-PINP and s-CTX increased significantly at months 3, 6, and 12 (all P < .001 for relative treatment effect of abaloparatide vs. placebo).
The most commonly reported treatment-emergent adverse events were injection site erythema (12.8% vs. 5.1%), nasopharyngitis (8.7% vs. 7.6%), dizziness (8.7% vs. 1.3%), and arthralgia (6.7% vs. 1.3%), with abaloparatide versus placebo. Serious treatment-emergent adverse event rates were similar in both groups (5.4% vs. 5.1%). There was one death in the abaloparatide group, which was deemed unrelated to the drug.
Dr. Binkley has reported receiving consulting fees from Amgen and research support from Radius. Dr. Hoang has reported disclosures with Acella Pharmaceuticals and Horizon Therapeutics (no financial compensation).
A version of this article first appeared on Medscape.com.
San Diego – The anabolic osteoporosis treatment abaloparatide (Tymlos, Radius Health) works in men as well as women, new data indicate.
Findings from the Abaloparatide for the Treatment of Men With Osteoporosis (ATOM) randomized, double-blind, placebo-controlled, phase 3 study were presented last week at the American Association of Clinical Endocrinology (AACE) Annual Meeting 2022.
Abaloparatide, a subcutaneously administered parathyroid-hormone–related protein (PTHrP) analog, resulted in significant increases in bone mineral density by 12 months at the lumbar spine, total hip, and femoral neck, compared with placebo in men with osteoporosis, with no significant adverse effects.
“Osteoporosis is underdiagnosed in men. Abaloparatide is another option for an ignored population,” presenter Neil Binkley, MD, of the University of Wisconsin School of Medicine and Public Health Madison, said in an interview.
Abaloparatide was approved by the U.S. Food and Drug Administration in 2017 for the treatment of postmenopausal women at high risk for fracture due to a history of osteoporotic fracture or multiple fracture risk factors, or who haven’t responded to or are intolerant of other osteoporosis therapies.
While postmenopausal women have mainly been the focus in osteoporosis, men account for approximately 30% of the societal burden of osteoporosis and have greater fracture-related morbidity and mortality than women.
About one in four men over the age of 50 years will have a fragility fracture in their lifetime. Yet, they’re far less likely to be diagnosed or to be included in osteoporosis treatment trials, Dr. Binkley noted.
Asked to comment, session moderator Thanh D. Hoang, DO, told this news organization, “I think it’s a great option to treat osteoporosis, and now we have evidence for treating osteoporosis in men. Mostly the data have come from postmenopausal women.”
Screen men with hypogonadism or those taking steroids
“This new medication is an addition to the very limited number of treatments that we have when patients don’t respond to [initial] medications. To have another anabolic bone-forming medication is very, very good,” said Dr. Hoang, who is professor and program director of the Endocrinology Fellowship Program at Walter Reed National Military Medical Center, Bethesda, Maryland.
Radius Health filed a Supplemental New Drug Application with the FDA for abaloparatide (Tymlos) subcutaneous injection in men with osteoporosis at high risk for fracture in February. There is a 10-month review period.
Dr. Binkley advises bone screening for men who have conditions such as hypogonadism or who are taking glucocorticoids or chemotherapeutics.
But, he added, “I think that if we did nothing else good in the osteoporosis field, if we treated people after they fractured that would be a huge step forward. Even with a normal T score, when those people fracture, they [often] don’t have normal bone mineral density ... That’s a group of people we’re ignoring still. They’re not getting diagnosed, and they’re not getting treated.”
ATOM Study: Significant BMD increases at key sites
The approval of abaloparatide in women was based on the phase 3, 18-week ACTIVE trial of more than 2,000 high-risk women, in whom abaloparatide was associated with an 86% reduction in vertebral fracture incidence, compared with placebo, and also significantly greater reductions in nonvertebral fractures, compared with both placebo and teriparatide (Forteo, Eli Lilly).
The ATOM study involved a total of 228 men aged 40-85 years with primary or hypogonadism-associated osteoporosis randomized 2:1 to receive subcutaneous 80 μg abaloparatide or injected placebo daily for 12 months. All had T scores (based on male reference range) of ≤ −2.5 at the lumbar spine or hip, or ≤ −1.5 and with radiologic vertebral fracture or a history of low trauma nonvertebral fracture in the past 5 years, or T score ≤ −2.0 if older than 65 years.
Increases in bone mineral density from baseline were significantly greater with abaloparatide compared with placebo at the lumbar spine, total hip, and femoral neck at 3, 6, and 12 months. Mean percentage changes at 12 months were 8.5%, 2.1%, and 3.0%, for the three locations, respectively, compared with 1.2%, 0.01%, and 0.2% for placebo (all P ≤ .0001).
Three fractures occurred in those receiving placebo and one with abaloparatide.
For markers of bone turnover, median serum procollagen type I N-terminal propeptide (s-PINP) was 111.2 ng/mL after 1 month of abaloparatide treatment and 85.7 ng/mL at month 12. Median serum carboxy-terminal cross-linking telopeptide of type I collagen (s-CTX) was 0.48 ng/mL at month 6 and 0.45 ng/mL at month 12 in the abaloparatide group. Geometric mean relative to baseline s-PINP and s-CTX increased significantly at months 3, 6, and 12 (all P < .001 for relative treatment effect of abaloparatide vs. placebo).
The most commonly reported treatment-emergent adverse events were injection site erythema (12.8% vs. 5.1%), nasopharyngitis (8.7% vs. 7.6%), dizziness (8.7% vs. 1.3%), and arthralgia (6.7% vs. 1.3%), with abaloparatide versus placebo. Serious treatment-emergent adverse event rates were similar in both groups (5.4% vs. 5.1%). There was one death in the abaloparatide group, which was deemed unrelated to the drug.
Dr. Binkley has reported receiving consulting fees from Amgen and research support from Radius. Dr. Hoang has reported disclosures with Acella Pharmaceuticals and Horizon Therapeutics (no financial compensation).
A version of this article first appeared on Medscape.com.
San Diego – The anabolic osteoporosis treatment abaloparatide (Tymlos, Radius Health) works in men as well as women, new data indicate.
Findings from the Abaloparatide for the Treatment of Men With Osteoporosis (ATOM) randomized, double-blind, placebo-controlled, phase 3 study were presented last week at the American Association of Clinical Endocrinology (AACE) Annual Meeting 2022.
Abaloparatide, a subcutaneously administered parathyroid-hormone–related protein (PTHrP) analog, resulted in significant increases in bone mineral density by 12 months at the lumbar spine, total hip, and femoral neck, compared with placebo in men with osteoporosis, with no significant adverse effects.
“Osteoporosis is underdiagnosed in men. Abaloparatide is another option for an ignored population,” presenter Neil Binkley, MD, of the University of Wisconsin School of Medicine and Public Health Madison, said in an interview.
Abaloparatide was approved by the U.S. Food and Drug Administration in 2017 for the treatment of postmenopausal women at high risk for fracture due to a history of osteoporotic fracture or multiple fracture risk factors, or who haven’t responded to or are intolerant of other osteoporosis therapies.
While postmenopausal women have mainly been the focus in osteoporosis, men account for approximately 30% of the societal burden of osteoporosis and have greater fracture-related morbidity and mortality than women.
About one in four men over the age of 50 years will have a fragility fracture in their lifetime. Yet, they’re far less likely to be diagnosed or to be included in osteoporosis treatment trials, Dr. Binkley noted.
Asked to comment, session moderator Thanh D. Hoang, DO, told this news organization, “I think it’s a great option to treat osteoporosis, and now we have evidence for treating osteoporosis in men. Mostly the data have come from postmenopausal women.”
Screen men with hypogonadism or those taking steroids
“This new medication is an addition to the very limited number of treatments that we have when patients don’t respond to [initial] medications. To have another anabolic bone-forming medication is very, very good,” said Dr. Hoang, who is professor and program director of the Endocrinology Fellowship Program at Walter Reed National Military Medical Center, Bethesda, Maryland.
Radius Health filed a Supplemental New Drug Application with the FDA for abaloparatide (Tymlos) subcutaneous injection in men with osteoporosis at high risk for fracture in February. There is a 10-month review period.
Dr. Binkley advises bone screening for men who have conditions such as hypogonadism or who are taking glucocorticoids or chemotherapeutics.
But, he added, “I think that if we did nothing else good in the osteoporosis field, if we treated people after they fractured that would be a huge step forward. Even with a normal T score, when those people fracture, they [often] don’t have normal bone mineral density ... That’s a group of people we’re ignoring still. They’re not getting diagnosed, and they’re not getting treated.”
ATOM Study: Significant BMD increases at key sites
The approval of abaloparatide in women was based on the phase 3, 18-week ACTIVE trial of more than 2,000 high-risk women, in whom abaloparatide was associated with an 86% reduction in vertebral fracture incidence, compared with placebo, and also significantly greater reductions in nonvertebral fractures, compared with both placebo and teriparatide (Forteo, Eli Lilly).
The ATOM study involved a total of 228 men aged 40-85 years with primary or hypogonadism-associated osteoporosis randomized 2:1 to receive subcutaneous 80 μg abaloparatide or injected placebo daily for 12 months. All had T scores (based on male reference range) of ≤ −2.5 at the lumbar spine or hip, or ≤ −1.5 and with radiologic vertebral fracture or a history of low trauma nonvertebral fracture in the past 5 years, or T score ≤ −2.0 if older than 65 years.
Increases in bone mineral density from baseline were significantly greater with abaloparatide compared with placebo at the lumbar spine, total hip, and femoral neck at 3, 6, and 12 months. Mean percentage changes at 12 months were 8.5%, 2.1%, and 3.0%, for the three locations, respectively, compared with 1.2%, 0.01%, and 0.2% for placebo (all P ≤ .0001).
Three fractures occurred in those receiving placebo and one with abaloparatide.
For markers of bone turnover, median serum procollagen type I N-terminal propeptide (s-PINP) was 111.2 ng/mL after 1 month of abaloparatide treatment and 85.7 ng/mL at month 12. Median serum carboxy-terminal cross-linking telopeptide of type I collagen (s-CTX) was 0.48 ng/mL at month 6 and 0.45 ng/mL at month 12 in the abaloparatide group. Geometric mean relative to baseline s-PINP and s-CTX increased significantly at months 3, 6, and 12 (all P < .001 for relative treatment effect of abaloparatide vs. placebo).
The most commonly reported treatment-emergent adverse events were injection site erythema (12.8% vs. 5.1%), nasopharyngitis (8.7% vs. 7.6%), dizziness (8.7% vs. 1.3%), and arthralgia (6.7% vs. 1.3%), with abaloparatide versus placebo. Serious treatment-emergent adverse event rates were similar in both groups (5.4% vs. 5.1%). There was one death in the abaloparatide group, which was deemed unrelated to the drug.
Dr. Binkley has reported receiving consulting fees from Amgen and research support from Radius. Dr. Hoang has reported disclosures with Acella Pharmaceuticals and Horizon Therapeutics (no financial compensation).
A version of this article first appeared on Medscape.com.
AT AACE 2022
Study casts doubt on safety, efficacy of L-serine supplementation for AD
When given to patients with AD, L-serine supplements could be driving abnormally increased serine levels in the brain even higher, potentially accelerating neuronal death, according to study author Xu Chen, PhD, of the University of California, San Diego, and colleagues.
This conclusion conflicts with a 2020 study by Juliette Le Douce, PhD, and colleagues, who reported that oral L-serine supplementation may act as a “ready-to-use therapy” for AD, based on their findings that patients with AD had low levels of PHGDH, an enzyme necessary for synthesizing serine, and AD-like mice had low levels of serine.
Writing in Cell Metabolism, Dr. Chen and colleagues framed the present study, and their findings, in this context.
“In contrast to the work of Le Douce et al., here we report that PHGDH mRNA and protein levels are increased in the brains of two mouse models of AD and/or tauopathy, and are also progressively increased in human brains with no, early, and late AD pathology, as well as in people with no, asymptomatic, and symptomatic AD,” they wrote.
They suggested adjusting clinical recommendations for L-serine, the form of the amino acid commonly found in supplements. In the body, L-serine is converted to D-serine, which acts on the NMDA receptor (NMDAR).
‘Long-term use of D-serine contributes to neuronal death’ suggests research
“We feel oral L-serine as a ready-to-use therapy to AD warrants precaution,” Dr. Chen and colleagues wrote. “This is because despite being a cognitive enhancer, some [research] suggests that long-term use of D-serine contributes to neuronal death in AD through excitotoxicity. Furthermore, D-serine, as a co-agonist of NMDAR, would be expected to oppose NMDAR antagonists, which have proven clinical benefits in treating AD.”
According to principal author Sheng Zhong, PhD, of the University of California, San Diego, “Research is needed to test if targeting PHGDH can ameliorate cognitive decline in AD.”
Dr. Zhong also noted that the present findings support the “promise of using a specific RNA in blood as a biomarker for early detection of Alzheimer’s disease.” This approach is currently being validated at UCSD Shiley-Marcos Alzheimer’s Disease Research Center, he added.
Roles of PHGDH and serine in Alzheimer’s disease require further study
Commenting on both studies, Steve W. Barger, PhD, of the University of Arkansas for Medical Sciences, Little Rock, suggested that more work is needed to better understand the roles of PHGDH and serine in AD before clinical applications can be considered.
“In the end, these two studies fail to provide the clarity we need in designing evidence-based therapeutic hypotheses,” Dr. Barger said in an interview. “We still do not have a firm grasp on the role that D-serine plays in AD. Indeed, the evidence regarding even a single enzyme contributing to its levels is ambiguous.”
Dr. Barger, who has published extensively on the topic of neuronal death, with a particular focus on Alzheimer’s disease, noted that “determination of what happens to D-serine levels in AD has been of interest for decades,” but levels of the amino acid have been notoriously challenging to measure because “D-serine can disappear rapidly from the brain and its fluids after death.”
While Dr. Le Douce and colleagues did measure levels of serine in mice, Dr. Barger noted that the study by Dr. Chen and colleagues was conducted with more “quantitatively rigorous methods.” Even though Dr. Chen and colleagues “did not assay the levels of D-serine itself ... the implication of their findings is that PHGDH is poised to elevate this critical neurotransmitter,” leading to their conclusion that serine supplementation is “potentially dangerous.”
At this point, it may be too early to tell, according to Dr. Barger.
He suggested that conclusions drawn from PHGDH levels alone are “always limited,” and conclusions based on serine levels may be equally dubious, considering that the activities and effects of serine “are quite complex,” and may be influenced by other physiologic processes, including the effects of gut bacteria.
Instead, Dr. Barger suggested that changes in PHGDH and serine may be interpreted as signals coming from a more relevant process upstream: glucose metabolism.
“What we can say confidently is that the glucose metabolism that PHGDH connects to D-serine is most definitely a factor in AD,” he said. “Countless studies have documented what now appears to be a universal decline in glucose delivery to the cerebral cortex, even before frank dementia sets in.”
Dr. Barger noted that declining glucose delivery coincides with some of the earliest events in the development of AD, perhaps “linking accumulation of amyloid β-peptide to subsequent neurofibrillary tangles and tissue atrophy.”
Dr. Barger’s own work recently demonstrated that AD is associated with “an irregularity in the insertion of a specific glucose transporter (GLUT1) into the cell surface” of astrocytes.
“It could be more effective to direct therapeutic interventions at these events lying upstream of PHGDH or serine,” he concluded.
The study was partly supported by a Kreuger v. Wyeth research award. The investigators and Dr. Barger reported no conflicts of interest.
When given to patients with AD, L-serine supplements could be driving abnormally increased serine levels in the brain even higher, potentially accelerating neuronal death, according to study author Xu Chen, PhD, of the University of California, San Diego, and colleagues.
This conclusion conflicts with a 2020 study by Juliette Le Douce, PhD, and colleagues, who reported that oral L-serine supplementation may act as a “ready-to-use therapy” for AD, based on their findings that patients with AD had low levels of PHGDH, an enzyme necessary for synthesizing serine, and AD-like mice had low levels of serine.
Writing in Cell Metabolism, Dr. Chen and colleagues framed the present study, and their findings, in this context.
“In contrast to the work of Le Douce et al., here we report that PHGDH mRNA and protein levels are increased in the brains of two mouse models of AD and/or tauopathy, and are also progressively increased in human brains with no, early, and late AD pathology, as well as in people with no, asymptomatic, and symptomatic AD,” they wrote.
They suggested adjusting clinical recommendations for L-serine, the form of the amino acid commonly found in supplements. In the body, L-serine is converted to D-serine, which acts on the NMDA receptor (NMDAR).
‘Long-term use of D-serine contributes to neuronal death’ suggests research
“We feel oral L-serine as a ready-to-use therapy to AD warrants precaution,” Dr. Chen and colleagues wrote. “This is because despite being a cognitive enhancer, some [research] suggests that long-term use of D-serine contributes to neuronal death in AD through excitotoxicity. Furthermore, D-serine, as a co-agonist of NMDAR, would be expected to oppose NMDAR antagonists, which have proven clinical benefits in treating AD.”
According to principal author Sheng Zhong, PhD, of the University of California, San Diego, “Research is needed to test if targeting PHGDH can ameliorate cognitive decline in AD.”
Dr. Zhong also noted that the present findings support the “promise of using a specific RNA in blood as a biomarker for early detection of Alzheimer’s disease.” This approach is currently being validated at UCSD Shiley-Marcos Alzheimer’s Disease Research Center, he added.
Roles of PHGDH and serine in Alzheimer’s disease require further study
Commenting on both studies, Steve W. Barger, PhD, of the University of Arkansas for Medical Sciences, Little Rock, suggested that more work is needed to better understand the roles of PHGDH and serine in AD before clinical applications can be considered.
“In the end, these two studies fail to provide the clarity we need in designing evidence-based therapeutic hypotheses,” Dr. Barger said in an interview. “We still do not have a firm grasp on the role that D-serine plays in AD. Indeed, the evidence regarding even a single enzyme contributing to its levels is ambiguous.”
Dr. Barger, who has published extensively on the topic of neuronal death, with a particular focus on Alzheimer’s disease, noted that “determination of what happens to D-serine levels in AD has been of interest for decades,” but levels of the amino acid have been notoriously challenging to measure because “D-serine can disappear rapidly from the brain and its fluids after death.”
While Dr. Le Douce and colleagues did measure levels of serine in mice, Dr. Barger noted that the study by Dr. Chen and colleagues was conducted with more “quantitatively rigorous methods.” Even though Dr. Chen and colleagues “did not assay the levels of D-serine itself ... the implication of their findings is that PHGDH is poised to elevate this critical neurotransmitter,” leading to their conclusion that serine supplementation is “potentially dangerous.”
At this point, it may be too early to tell, according to Dr. Barger.
He suggested that conclusions drawn from PHGDH levels alone are “always limited,” and conclusions based on serine levels may be equally dubious, considering that the activities and effects of serine “are quite complex,” and may be influenced by other physiologic processes, including the effects of gut bacteria.
Instead, Dr. Barger suggested that changes in PHGDH and serine may be interpreted as signals coming from a more relevant process upstream: glucose metabolism.
“What we can say confidently is that the glucose metabolism that PHGDH connects to D-serine is most definitely a factor in AD,” he said. “Countless studies have documented what now appears to be a universal decline in glucose delivery to the cerebral cortex, even before frank dementia sets in.”
Dr. Barger noted that declining glucose delivery coincides with some of the earliest events in the development of AD, perhaps “linking accumulation of amyloid β-peptide to subsequent neurofibrillary tangles and tissue atrophy.”
Dr. Barger’s own work recently demonstrated that AD is associated with “an irregularity in the insertion of a specific glucose transporter (GLUT1) into the cell surface” of astrocytes.
“It could be more effective to direct therapeutic interventions at these events lying upstream of PHGDH or serine,” he concluded.
The study was partly supported by a Kreuger v. Wyeth research award. The investigators and Dr. Barger reported no conflicts of interest.
When given to patients with AD, L-serine supplements could be driving abnormally increased serine levels in the brain even higher, potentially accelerating neuronal death, according to study author Xu Chen, PhD, of the University of California, San Diego, and colleagues.
This conclusion conflicts with a 2020 study by Juliette Le Douce, PhD, and colleagues, who reported that oral L-serine supplementation may act as a “ready-to-use therapy” for AD, based on their findings that patients with AD had low levels of PHGDH, an enzyme necessary for synthesizing serine, and AD-like mice had low levels of serine.
Writing in Cell Metabolism, Dr. Chen and colleagues framed the present study, and their findings, in this context.
“In contrast to the work of Le Douce et al., here we report that PHGDH mRNA and protein levels are increased in the brains of two mouse models of AD and/or tauopathy, and are also progressively increased in human brains with no, early, and late AD pathology, as well as in people with no, asymptomatic, and symptomatic AD,” they wrote.
They suggested adjusting clinical recommendations for L-serine, the form of the amino acid commonly found in supplements. In the body, L-serine is converted to D-serine, which acts on the NMDA receptor (NMDAR).
‘Long-term use of D-serine contributes to neuronal death’ suggests research
“We feel oral L-serine as a ready-to-use therapy to AD warrants precaution,” Dr. Chen and colleagues wrote. “This is because despite being a cognitive enhancer, some [research] suggests that long-term use of D-serine contributes to neuronal death in AD through excitotoxicity. Furthermore, D-serine, as a co-agonist of NMDAR, would be expected to oppose NMDAR antagonists, which have proven clinical benefits in treating AD.”
According to principal author Sheng Zhong, PhD, of the University of California, San Diego, “Research is needed to test if targeting PHGDH can ameliorate cognitive decline in AD.”
Dr. Zhong also noted that the present findings support the “promise of using a specific RNA in blood as a biomarker for early detection of Alzheimer’s disease.” This approach is currently being validated at UCSD Shiley-Marcos Alzheimer’s Disease Research Center, he added.
Roles of PHGDH and serine in Alzheimer’s disease require further study
Commenting on both studies, Steve W. Barger, PhD, of the University of Arkansas for Medical Sciences, Little Rock, suggested that more work is needed to better understand the roles of PHGDH and serine in AD before clinical applications can be considered.
“In the end, these two studies fail to provide the clarity we need in designing evidence-based therapeutic hypotheses,” Dr. Barger said in an interview. “We still do not have a firm grasp on the role that D-serine plays in AD. Indeed, the evidence regarding even a single enzyme contributing to its levels is ambiguous.”
Dr. Barger, who has published extensively on the topic of neuronal death, with a particular focus on Alzheimer’s disease, noted that “determination of what happens to D-serine levels in AD has been of interest for decades,” but levels of the amino acid have been notoriously challenging to measure because “D-serine can disappear rapidly from the brain and its fluids after death.”
While Dr. Le Douce and colleagues did measure levels of serine in mice, Dr. Barger noted that the study by Dr. Chen and colleagues was conducted with more “quantitatively rigorous methods.” Even though Dr. Chen and colleagues “did not assay the levels of D-serine itself ... the implication of their findings is that PHGDH is poised to elevate this critical neurotransmitter,” leading to their conclusion that serine supplementation is “potentially dangerous.”
At this point, it may be too early to tell, according to Dr. Barger.
He suggested that conclusions drawn from PHGDH levels alone are “always limited,” and conclusions based on serine levels may be equally dubious, considering that the activities and effects of serine “are quite complex,” and may be influenced by other physiologic processes, including the effects of gut bacteria.
Instead, Dr. Barger suggested that changes in PHGDH and serine may be interpreted as signals coming from a more relevant process upstream: glucose metabolism.
“What we can say confidently is that the glucose metabolism that PHGDH connects to D-serine is most definitely a factor in AD,” he said. “Countless studies have documented what now appears to be a universal decline in glucose delivery to the cerebral cortex, even before frank dementia sets in.”
Dr. Barger noted that declining glucose delivery coincides with some of the earliest events in the development of AD, perhaps “linking accumulation of amyloid β-peptide to subsequent neurofibrillary tangles and tissue atrophy.”
Dr. Barger’s own work recently demonstrated that AD is associated with “an irregularity in the insertion of a specific glucose transporter (GLUT1) into the cell surface” of astrocytes.
“It could be more effective to direct therapeutic interventions at these events lying upstream of PHGDH or serine,” he concluded.
The study was partly supported by a Kreuger v. Wyeth research award. The investigators and Dr. Barger reported no conflicts of interest.
FROM CELL METABOLISM
Higher industriousness reduces risk of predementia syndrome in older adults
Higher industriousness was associated with a 25% reduced risk of concurrent motoric cognitive risk syndrome (MCR), based on data from approximately 6,000 individuals.
Previous research supports an association between conscientiousness and a lower risk of MCR, a form of predementia that involves slow gait speed and cognitive complaints, wrote Yannick Stephan, PhD, of the University of Montpellier (France), and colleagues. However, the specific facets of conscientiousness that impact MCR have not been examined.
In a study published in the Journal of Psychiatric Research, the authors reviewed data from 6,001 dementia-free adults aged 65-99 years who were enrolled in the Health and Retirement Study, a nationally representative longitudinal study of adults aged 50 years and older in the United States.
Baseline data were collected between 2008 and 2010, and participants were assessed for MCR at follow-up points during 2012-2014 and 2016-2018. Six facets of conscientiousness were assessed using a 24-item scale that has been used in previous studies. The six facets were industriousness, self-control, order, traditionalism, virtue, and responsibility. The researchers controlled for variables including demographic factors, cognition, physical activity, disease burden, depressive symptoms, and body mass index.
Overall, increased industriousness was significantly associated with a lower likelihood of concurrent MCR (odds ratio, 0.75) and a reduced risk of incident MCR (hazard ratio, 0.63,; P < .001 for both).
The conscientiousness facets of order, self-control, and responsibility also were associated with a lower likelihood of both concurrent and incident MCR, with ORs ranging from 0.82-0.88 for concurrent and HRs ranging from 0.72-0.82 for incident.
Traditionalism and virtue were significantly associated with a lower risk of incident MCR, but not concurrent MCR (HR, 0.84; P < .01 for both).
The mechanism of action for the association may be explained by several cognitive, health-related, behavioral, and psychological pathways, the researchers wrote. With regard to industriousness, the relationship could be partly explained by cognition, physical activity, disease burden, BMI, and depressive symptoms. However, industriousness also has been associated with a reduced risk of systemic inflammation, which may in turn reduce MCR risk. Also, data suggest that industriousness and MCR share a common genetic cause.
The study findings were limited by several factors including the observational design and the positive selection effect from patients with complete follow-up data, as these patients likely have higher levels of order, industriousness, and responsibility, the researchers noted. However, the results support those from previous studies and were strengthened by the large sample and examination of six facets of conscientiousness.
“This study thus provides a more detailed understanding of the specific components of conscientiousness that are associated with risk of MCR among older adults,” and the facets could be targeted in interventions to reduce both MCR and dementia, they concluded.
The Health and Retirement Study is supported by the National Institute on Aging and conducted by the University of Michigan. The current study was supported in part by the National Institutes of Health. The researchers had no financial conflicts to disclose.
Higher industriousness was associated with a 25% reduced risk of concurrent motoric cognitive risk syndrome (MCR), based on data from approximately 6,000 individuals.
Previous research supports an association between conscientiousness and a lower risk of MCR, a form of predementia that involves slow gait speed and cognitive complaints, wrote Yannick Stephan, PhD, of the University of Montpellier (France), and colleagues. However, the specific facets of conscientiousness that impact MCR have not been examined.
In a study published in the Journal of Psychiatric Research, the authors reviewed data from 6,001 dementia-free adults aged 65-99 years who were enrolled in the Health and Retirement Study, a nationally representative longitudinal study of adults aged 50 years and older in the United States.
Baseline data were collected between 2008 and 2010, and participants were assessed for MCR at follow-up points during 2012-2014 and 2016-2018. Six facets of conscientiousness were assessed using a 24-item scale that has been used in previous studies. The six facets were industriousness, self-control, order, traditionalism, virtue, and responsibility. The researchers controlled for variables including demographic factors, cognition, physical activity, disease burden, depressive symptoms, and body mass index.
Overall, increased industriousness was significantly associated with a lower likelihood of concurrent MCR (odds ratio, 0.75) and a reduced risk of incident MCR (hazard ratio, 0.63,; P < .001 for both).
The conscientiousness facets of order, self-control, and responsibility also were associated with a lower likelihood of both concurrent and incident MCR, with ORs ranging from 0.82-0.88 for concurrent and HRs ranging from 0.72-0.82 for incident.
Traditionalism and virtue were significantly associated with a lower risk of incident MCR, but not concurrent MCR (HR, 0.84; P < .01 for both).
The mechanism of action for the association may be explained by several cognitive, health-related, behavioral, and psychological pathways, the researchers wrote. With regard to industriousness, the relationship could be partly explained by cognition, physical activity, disease burden, BMI, and depressive symptoms. However, industriousness also has been associated with a reduced risk of systemic inflammation, which may in turn reduce MCR risk. Also, data suggest that industriousness and MCR share a common genetic cause.
The study findings were limited by several factors including the observational design and the positive selection effect from patients with complete follow-up data, as these patients likely have higher levels of order, industriousness, and responsibility, the researchers noted. However, the results support those from previous studies and were strengthened by the large sample and examination of six facets of conscientiousness.
“This study thus provides a more detailed understanding of the specific components of conscientiousness that are associated with risk of MCR among older adults,” and the facets could be targeted in interventions to reduce both MCR and dementia, they concluded.
The Health and Retirement Study is supported by the National Institute on Aging and conducted by the University of Michigan. The current study was supported in part by the National Institutes of Health. The researchers had no financial conflicts to disclose.
Higher industriousness was associated with a 25% reduced risk of concurrent motoric cognitive risk syndrome (MCR), based on data from approximately 6,000 individuals.
Previous research supports an association between conscientiousness and a lower risk of MCR, a form of predementia that involves slow gait speed and cognitive complaints, wrote Yannick Stephan, PhD, of the University of Montpellier (France), and colleagues. However, the specific facets of conscientiousness that impact MCR have not been examined.
In a study published in the Journal of Psychiatric Research, the authors reviewed data from 6,001 dementia-free adults aged 65-99 years who were enrolled in the Health and Retirement Study, a nationally representative longitudinal study of adults aged 50 years and older in the United States.
Baseline data were collected between 2008 and 2010, and participants were assessed for MCR at follow-up points during 2012-2014 and 2016-2018. Six facets of conscientiousness were assessed using a 24-item scale that has been used in previous studies. The six facets were industriousness, self-control, order, traditionalism, virtue, and responsibility. The researchers controlled for variables including demographic factors, cognition, physical activity, disease burden, depressive symptoms, and body mass index.
Overall, increased industriousness was significantly associated with a lower likelihood of concurrent MCR (odds ratio, 0.75) and a reduced risk of incident MCR (hazard ratio, 0.63,; P < .001 for both).
The conscientiousness facets of order, self-control, and responsibility also were associated with a lower likelihood of both concurrent and incident MCR, with ORs ranging from 0.82-0.88 for concurrent and HRs ranging from 0.72-0.82 for incident.
Traditionalism and virtue were significantly associated with a lower risk of incident MCR, but not concurrent MCR (HR, 0.84; P < .01 for both).
The mechanism of action for the association may be explained by several cognitive, health-related, behavioral, and psychological pathways, the researchers wrote. With regard to industriousness, the relationship could be partly explained by cognition, physical activity, disease burden, BMI, and depressive symptoms. However, industriousness also has been associated with a reduced risk of systemic inflammation, which may in turn reduce MCR risk. Also, data suggest that industriousness and MCR share a common genetic cause.
The study findings were limited by several factors including the observational design and the positive selection effect from patients with complete follow-up data, as these patients likely have higher levels of order, industriousness, and responsibility, the researchers noted. However, the results support those from previous studies and were strengthened by the large sample and examination of six facets of conscientiousness.
“This study thus provides a more detailed understanding of the specific components of conscientiousness that are associated with risk of MCR among older adults,” and the facets could be targeted in interventions to reduce both MCR and dementia, they concluded.
The Health and Retirement Study is supported by the National Institute on Aging and conducted by the University of Michigan. The current study was supported in part by the National Institutes of Health. The researchers had no financial conflicts to disclose.
FROM PSYCHIATRIC RESEARCH
Atypical knee pain
An 83-year-old woman, with an otherwise noncontributory past medical history, presented with chronic right knee pain. Over the prior 4 years, she had undergone evaluation by an outside physician and received several corticosteroid and hyaluronic acid intra-articular injections, without symptom resolution. She described the pain as a 4/10 at rest and as “severe” when climbing stairs and exercising. The pain was localized to her lower back and right groin and extended to her right knee. She also said that she found it difficult to put on her socks. An outside orthopedic surgeon recommended right total knee arthroplasty, prompting her to seek a second opinion.
Examination of her right knee was unrevealing. However, during the hip examination, there was a pronounced loss of range of motion and concordant pain reproduction with the FABER (combined flexion, abduction, external rotation) and FADIR (combined flexion, adduction, and internal rotation) maneuvers.
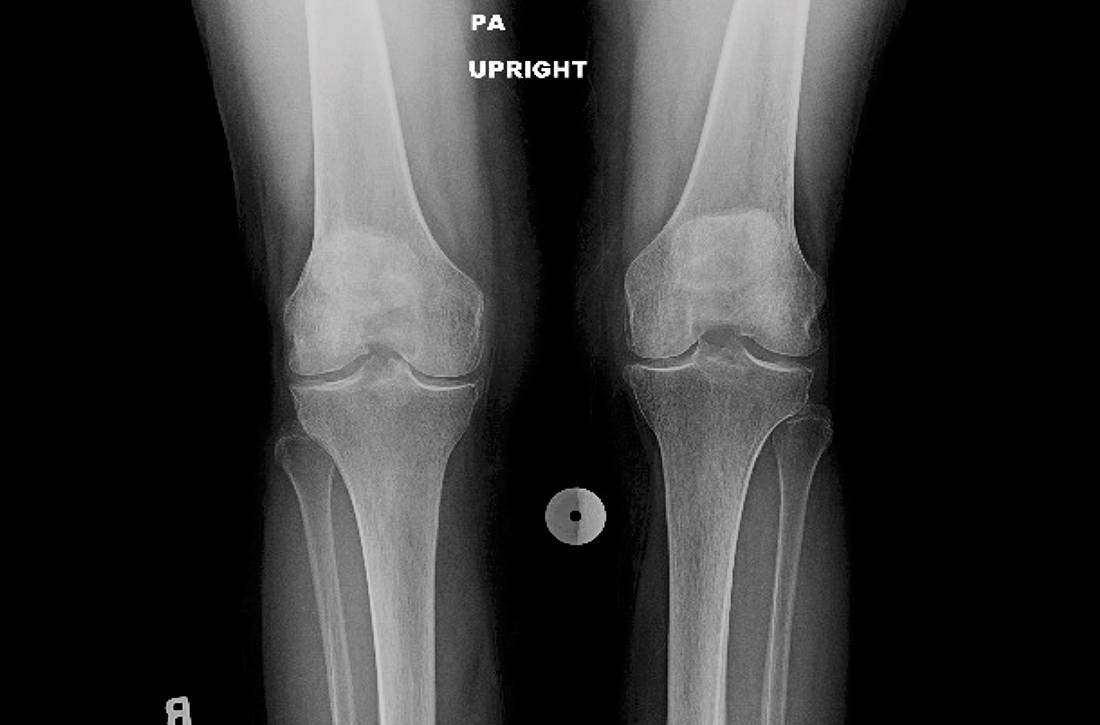
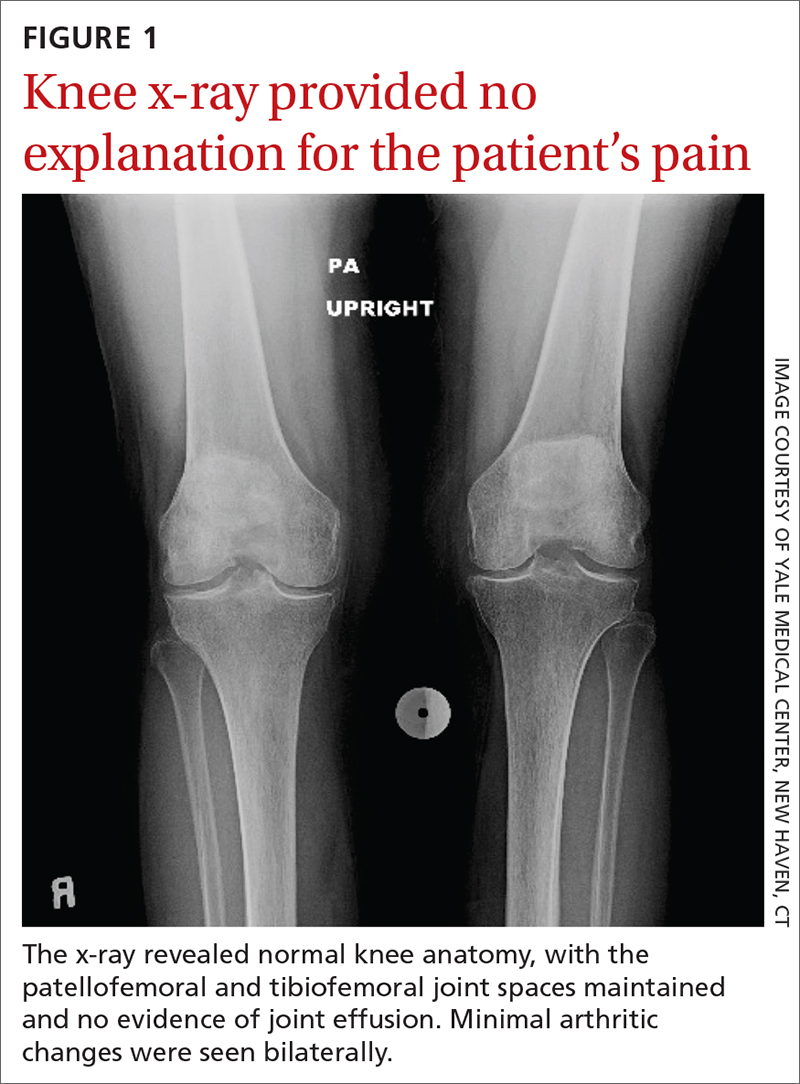
The patient’s extensive clinical and diagnostic history, combined with benign knee examination and imaging (FIGURE 1), ruled out isolated knee pathology.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Right hip OA with referred knee pain
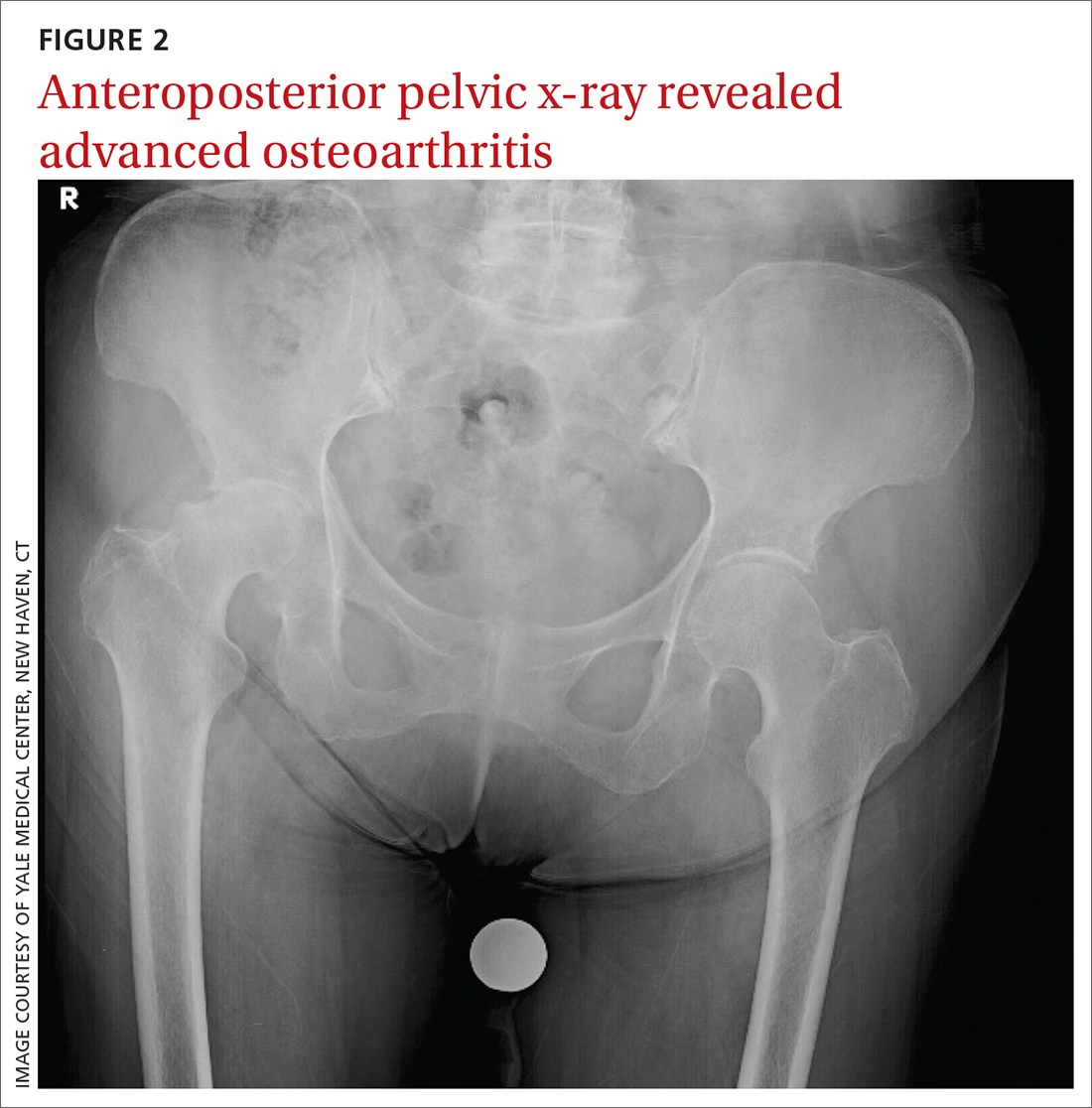
The patient’s history and physical exam prompted us to suspect right hip osteoarthritis (OA) with referred pain to the right knee. This suspicion was confirmed with hip radiographs (FIGURE 2), which revealed significant OA of the right hip, as evidenced by marked joint space narrowing, subchondral sclerosis, and osteophytes. There was also superior migration of the right femoral head relative to the acetabulum. Additionally, there was loss of sphericity of the right femoral head, suggesting avascular necrosis with collapse.
Hip and knee OA are among the most common causes of disability worldwide. Knee and hip pain are estimated to affect up to 27% and 15% of the general population, respectively.1,2 Referred knee pain secondary to hip pathology, also known as atypical knee pain, has been cited at highly variable rates, ranging from 2% to 27%.3
Eighty-six percent of patients with atypical knee pain experience a delay in diagnosis of more than 1 year.4 Half of these patients require the use of a wheelchair or walker for community navigation.4 These findings highlight the impact that a delay in diagnosis can have on the day-to-day quality of life for these patients. Also, delayed or missed diagnoses may have contributed to the doubling in the rate of knee replacement surgery from 2000 to 2010 and the reports that up to one-third of knee replacement surgeries did not meet appropriate criteria to be performed.5,6
Convergence confusion
Referred pain is likely explained by the convergence of nociceptive and non-nociceptive nerve fibers.7 Both of these fiber types conduct action potentials that terminate at second order neurons. Occasionally, nociceptive nerve fibers from different parts of the body (ie, knee and hip) terminate at the same second order fiber. At this point of convergence, higher brain centers lose their ability to discriminate the anatomic location of origin. This results in the perception of pain in a different location, where there is no intrinsic pathology.
Patients with hip OA report that the most common locations of pain are the groin, anterior thigh, buttock, anterior knee, and greater trochanter.3 One small study revealed that 85% of patients with referred pain who underwent total hip arthroplasty (THA) reported complete resolution of pain symptoms within 4 days of the procedure.3
Continue to: A comprehensive exam can reveal a different origin of pain
A comprehensive exam can reveal a different origin of pain
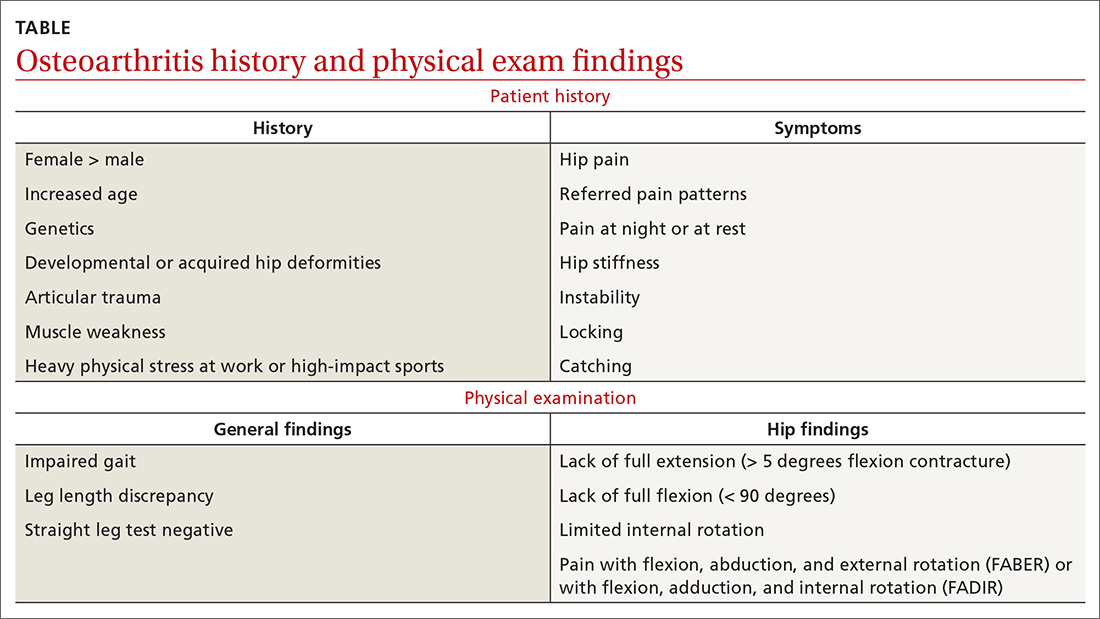
As with any musculoskeletal complaint, history and physical examination should include a focus on the joints proximal and distal to the purported joint of concern. When the hip is in consideration, historical inquiry should focus on degree and timeline of pain, stiffness, and traumatic history. Our patient reported difficulty donning socks, an excellent screening question to evaluate loss of range of motion in the hip. On physical examination, the FABER and FADIR maneuvers are quite specific to hip OA. A comprehensive list of history and physical examination findings can be found in the TABLE.
The differential includes a broad range of musculoskeletal diagnoses
The differential diagnosis for knee pain includes knee OA, spinopelvic pathology, infection, and rheumatologic disease.
Knee OA can be confirmed with knee radiographs, but one must also assess the joint above and below, as with all musculoskeletal complaints.
Spinopelvic pathology may be established with radiographs and a thorough nervous system exam.
Infection, such as septic arthritis or gout, can be diagnosed through radiographs, physical exam, and lab tests to evaluate white blood cell count, erythrocyte sedimentation rate, and C-reactive protein levels. High clinical suspicion may warrant a joint aspiration.
Continue to: Rheumatologic disease
Rheumatologic disease can be evaluated with a comprehensive physical exam, as well as lab work.
Management includes both surgical and nonsurgical options
Hip OA can be managed much like OA in other areas of the body. The Osteoarthritis Research Society International guidelines provide direction and insight concerning outpatient nonsurgical management.8 Weight loss and land-based, low-impact exercise programs are excellent first-line options. Second-line therapies include symptomatic management with systemic nonsteroidal anti-inflammatory drugs (NSAIDs) in patients without contraindications. (Topical NSAIDs, while useful in the treatment of knee OA, are not as effective for hip OA due to thickness of soft tissue in this area of the body.)
Patients who do not achieve symptomatic relief with these first- and second-line therapies may benefit from other nonoperative measures, such as intra-articular corticosteroid injections. If pain persists, patients may need a referral to an orthopedic surgeon to discuss surgical candidacy.
Following the x-ray, our patient received a fluoroscopic guided intra-articular hip joint anesthetic and corticosteroid injection. Her pain level went from a reported6/10 prior to the procedure to complete pain relief after it.
However, at her follow-up visit 4 weeks later, the patient reported return of functionally limiting pain. The orthopedic surgeon talked to the patient about the potential risks and benefits of THA. She elected to proceed with a right THA.
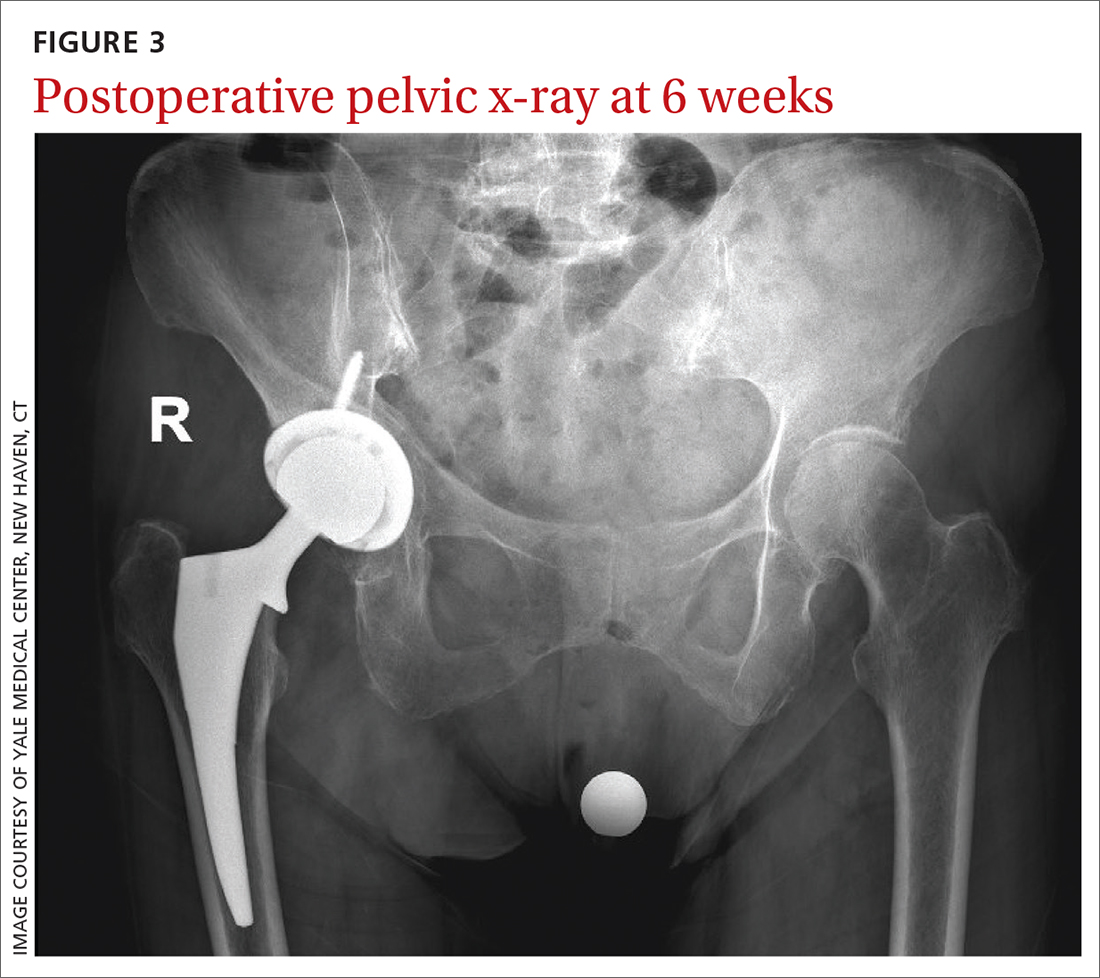
Six weeks after the surgery, the patient presented for follow-up with minimal hip pain and complete resolution of her knee pain (FIGURE 3). Functionally, she found it much easier to stand straight, and she was able to climb the stairs in her house independently.
1. Fernandes GS, Parekh SM, Moses J, et al. Prevalence of knee pain, radiographic osteoarthritis and arthroplasty in retired professional footballers compared with men in the general population: a cross-sectional study. Br J Sports Med. 2018;52:678-683. doi: 10.1136/bjsports-2017-097503
2. Christmas C, Crespo CJ, Franckowiak SC, et al. How common is hip pain among older adults? Results from the Third National Health and Nutrition Examination Survey. J Fam Pract. 2002;51:345-348.
3. Hsieh PH, Chang Y, Chen DW, et al. Pain distribution and response to total hip arthroplasty: a prospective observational study in 113 patients with end-stage hip disease. J Orthop Sci. 2012;17:213-218. doi: 10.1007/s00776-012-0204-1
4. Dibra FF, Prietao HA, Gray CF, et al. Don’t forget the hip! Hip arthritis masquerading as knee pain. Arthroplast Today. 2017;4:118-124. doi: 10.1016/j.artd.2017.06.008
5. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323-1330. doi: 10.1136/annrheumdis-2013-204763
6. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386-1397. doi: 10.2106/JBJS.N.01141
7. Sessle BJ. Central mechanisms of craniofacial musculoskeletal pain: a review. In: Graven-Nielsen T, Arendt-Nielsen L, Mense S, eds. Fundamentals of musculoskeletal pain. 1st ed. IASP Press; 2008:87-103.
8. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578-1589. doi: 10.1016/j.joca.2019.06.011
An 83-year-old woman, with an otherwise noncontributory past medical history, presented with chronic right knee pain. Over the prior 4 years, she had undergone evaluation by an outside physician and received several corticosteroid and hyaluronic acid intra-articular injections, without symptom resolution. She described the pain as a 4/10 at rest and as “severe” when climbing stairs and exercising. The pain was localized to her lower back and right groin and extended to her right knee. She also said that she found it difficult to put on her socks. An outside orthopedic surgeon recommended right total knee arthroplasty, prompting her to seek a second opinion.
Examination of her right knee was unrevealing. However, during the hip examination, there was a pronounced loss of range of motion and concordant pain reproduction with the FABER (combined flexion, abduction, external rotation) and FADIR (combined flexion, adduction, and internal rotation) maneuvers.
The patient’s extensive clinical and diagnostic history, combined with benign knee examination and imaging (FIGURE 1), ruled out isolated knee pathology.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Right hip OA with referred knee pain
The patient’s history and physical exam prompted us to suspect right hip osteoarthritis (OA) with referred pain to the right knee. This suspicion was confirmed with hip radiographs (FIGURE 2), which revealed significant OA of the right hip, as evidenced by marked joint space narrowing, subchondral sclerosis, and osteophytes. There was also superior migration of the right femoral head relative to the acetabulum. Additionally, there was loss of sphericity of the right femoral head, suggesting avascular necrosis with collapse.

Hip and knee OA are among the most common causes of disability worldwide. Knee and hip pain are estimated to affect up to 27% and 15% of the general population, respectively.1,2 Referred knee pain secondary to hip pathology, also known as atypical knee pain, has been cited at highly variable rates, ranging from 2% to 27%.3
Eighty-six percent of patients with atypical knee pain experience a delay in diagnosis of more than 1 year.4 Half of these patients require the use of a wheelchair or walker for community navigation.4 These findings highlight the impact that a delay in diagnosis can have on the day-to-day quality of life for these patients. Also, delayed or missed diagnoses may have contributed to the doubling in the rate of knee replacement surgery from 2000 to 2010 and the reports that up to one-third of knee replacement surgeries did not meet appropriate criteria to be performed.5,6
Convergence confusion
Referred pain is likely explained by the convergence of nociceptive and non-nociceptive nerve fibers.7 Both of these fiber types conduct action potentials that terminate at second order neurons. Occasionally, nociceptive nerve fibers from different parts of the body (ie, knee and hip) terminate at the same second order fiber. At this point of convergence, higher brain centers lose their ability to discriminate the anatomic location of origin. This results in the perception of pain in a different location, where there is no intrinsic pathology.
Patients with hip OA report that the most common locations of pain are the groin, anterior thigh, buttock, anterior knee, and greater trochanter.3 One small study revealed that 85% of patients with referred pain who underwent total hip arthroplasty (THA) reported complete resolution of pain symptoms within 4 days of the procedure.3
Continue to: A comprehensive exam can reveal a different origin of pain
A comprehensive exam can reveal a different origin of pain
As with any musculoskeletal complaint, history and physical examination should include a focus on the joints proximal and distal to the purported joint of concern. When the hip is in consideration, historical inquiry should focus on degree and timeline of pain, stiffness, and traumatic history. Our patient reported difficulty donning socks, an excellent screening question to evaluate loss of range of motion in the hip. On physical examination, the FABER and FADIR maneuvers are quite specific to hip OA. A comprehensive list of history and physical examination findings can be found in the TABLE.

The differential includes a broad range of musculoskeletal diagnoses
The differential diagnosis for knee pain includes knee OA, spinopelvic pathology, infection, and rheumatologic disease.
Knee OA can be confirmed with knee radiographs, but one must also assess the joint above and below, as with all musculoskeletal complaints.
Spinopelvic pathology may be established with radiographs and a thorough nervous system exam.
Infection, such as septic arthritis or gout, can be diagnosed through radiographs, physical exam, and lab tests to evaluate white blood cell count, erythrocyte sedimentation rate, and C-reactive protein levels. High clinical suspicion may warrant a joint aspiration.
Continue to: Rheumatologic disease
Rheumatologic disease can be evaluated with a comprehensive physical exam, as well as lab work.
Management includes both surgical and nonsurgical options
Hip OA can be managed much like OA in other areas of the body. The Osteoarthritis Research Society International guidelines provide direction and insight concerning outpatient nonsurgical management.8 Weight loss and land-based, low-impact exercise programs are excellent first-line options. Second-line therapies include symptomatic management with systemic nonsteroidal anti-inflammatory drugs (NSAIDs) in patients without contraindications. (Topical NSAIDs, while useful in the treatment of knee OA, are not as effective for hip OA due to thickness of soft tissue in this area of the body.)
Patients who do not achieve symptomatic relief with these first- and second-line therapies may benefit from other nonoperative measures, such as intra-articular corticosteroid injections. If pain persists, patients may need a referral to an orthopedic surgeon to discuss surgical candidacy.
Following the x-ray, our patient received a fluoroscopic guided intra-articular hip joint anesthetic and corticosteroid injection. Her pain level went from a reported6/10 prior to the procedure to complete pain relief after it.
However, at her follow-up visit 4 weeks later, the patient reported return of functionally limiting pain. The orthopedic surgeon talked to the patient about the potential risks and benefits of THA. She elected to proceed with a right THA.
Six weeks after the surgery, the patient presented for follow-up with minimal hip pain and complete resolution of her knee pain (FIGURE 3). Functionally, she found it much easier to stand straight, and she was able to climb the stairs in her house independently.

An 83-year-old woman, with an otherwise noncontributory past medical history, presented with chronic right knee pain. Over the prior 4 years, she had undergone evaluation by an outside physician and received several corticosteroid and hyaluronic acid intra-articular injections, without symptom resolution. She described the pain as a 4/10 at rest and as “severe” when climbing stairs and exercising. The pain was localized to her lower back and right groin and extended to her right knee. She also said that she found it difficult to put on her socks. An outside orthopedic surgeon recommended right total knee arthroplasty, prompting her to seek a second opinion.
Examination of her right knee was unrevealing. However, during the hip examination, there was a pronounced loss of range of motion and concordant pain reproduction with the FABER (combined flexion, abduction, external rotation) and FADIR (combined flexion, adduction, and internal rotation) maneuvers.
The patient’s extensive clinical and diagnostic history, combined with benign knee examination and imaging (FIGURE 1), ruled out isolated knee pathology.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Right hip OA with referred knee pain
The patient’s history and physical exam prompted us to suspect right hip osteoarthritis (OA) with referred pain to the right knee. This suspicion was confirmed with hip radiographs (FIGURE 2), which revealed significant OA of the right hip, as evidenced by marked joint space narrowing, subchondral sclerosis, and osteophytes. There was also superior migration of the right femoral head relative to the acetabulum. Additionally, there was loss of sphericity of the right femoral head, suggesting avascular necrosis with collapse.

Hip and knee OA are among the most common causes of disability worldwide. Knee and hip pain are estimated to affect up to 27% and 15% of the general population, respectively.1,2 Referred knee pain secondary to hip pathology, also known as atypical knee pain, has been cited at highly variable rates, ranging from 2% to 27%.3
Eighty-six percent of patients with atypical knee pain experience a delay in diagnosis of more than 1 year.4 Half of these patients require the use of a wheelchair or walker for community navigation.4 These findings highlight the impact that a delay in diagnosis can have on the day-to-day quality of life for these patients. Also, delayed or missed diagnoses may have contributed to the doubling in the rate of knee replacement surgery from 2000 to 2010 and the reports that up to one-third of knee replacement surgeries did not meet appropriate criteria to be performed.5,6
Convergence confusion
Referred pain is likely explained by the convergence of nociceptive and non-nociceptive nerve fibers.7 Both of these fiber types conduct action potentials that terminate at second order neurons. Occasionally, nociceptive nerve fibers from different parts of the body (ie, knee and hip) terminate at the same second order fiber. At this point of convergence, higher brain centers lose their ability to discriminate the anatomic location of origin. This results in the perception of pain in a different location, where there is no intrinsic pathology.
Patients with hip OA report that the most common locations of pain are the groin, anterior thigh, buttock, anterior knee, and greater trochanter.3 One small study revealed that 85% of patients with referred pain who underwent total hip arthroplasty (THA) reported complete resolution of pain symptoms within 4 days of the procedure.3
Continue to: A comprehensive exam can reveal a different origin of pain
A comprehensive exam can reveal a different origin of pain
As with any musculoskeletal complaint, history and physical examination should include a focus on the joints proximal and distal to the purported joint of concern. When the hip is in consideration, historical inquiry should focus on degree and timeline of pain, stiffness, and traumatic history. Our patient reported difficulty donning socks, an excellent screening question to evaluate loss of range of motion in the hip. On physical examination, the FABER and FADIR maneuvers are quite specific to hip OA. A comprehensive list of history and physical examination findings can be found in the TABLE.

The differential includes a broad range of musculoskeletal diagnoses
The differential diagnosis for knee pain includes knee OA, spinopelvic pathology, infection, and rheumatologic disease.
Knee OA can be confirmed with knee radiographs, but one must also assess the joint above and below, as with all musculoskeletal complaints.
Spinopelvic pathology may be established with radiographs and a thorough nervous system exam.
Infection, such as septic arthritis or gout, can be diagnosed through radiographs, physical exam, and lab tests to evaluate white blood cell count, erythrocyte sedimentation rate, and C-reactive protein levels. High clinical suspicion may warrant a joint aspiration.
Continue to: Rheumatologic disease
Rheumatologic disease can be evaluated with a comprehensive physical exam, as well as lab work.
Management includes both surgical and nonsurgical options
Hip OA can be managed much like OA in other areas of the body. The Osteoarthritis Research Society International guidelines provide direction and insight concerning outpatient nonsurgical management.8 Weight loss and land-based, low-impact exercise programs are excellent first-line options. Second-line therapies include symptomatic management with systemic nonsteroidal anti-inflammatory drugs (NSAIDs) in patients without contraindications. (Topical NSAIDs, while useful in the treatment of knee OA, are not as effective for hip OA due to thickness of soft tissue in this area of the body.)
Patients who do not achieve symptomatic relief with these first- and second-line therapies may benefit from other nonoperative measures, such as intra-articular corticosteroid injections. If pain persists, patients may need a referral to an orthopedic surgeon to discuss surgical candidacy.
Following the x-ray, our patient received a fluoroscopic guided intra-articular hip joint anesthetic and corticosteroid injection. Her pain level went from a reported6/10 prior to the procedure to complete pain relief after it.
However, at her follow-up visit 4 weeks later, the patient reported return of functionally limiting pain. The orthopedic surgeon talked to the patient about the potential risks and benefits of THA. She elected to proceed with a right THA.
Six weeks after the surgery, the patient presented for follow-up with minimal hip pain and complete resolution of her knee pain (FIGURE 3). Functionally, she found it much easier to stand straight, and she was able to climb the stairs in her house independently.

1. Fernandes GS, Parekh SM, Moses J, et al. Prevalence of knee pain, radiographic osteoarthritis and arthroplasty in retired professional footballers compared with men in the general population: a cross-sectional study. Br J Sports Med. 2018;52:678-683. doi: 10.1136/bjsports-2017-097503
2. Christmas C, Crespo CJ, Franckowiak SC, et al. How common is hip pain among older adults? Results from the Third National Health and Nutrition Examination Survey. J Fam Pract. 2002;51:345-348.
3. Hsieh PH, Chang Y, Chen DW, et al. Pain distribution and response to total hip arthroplasty: a prospective observational study in 113 patients with end-stage hip disease. J Orthop Sci. 2012;17:213-218. doi: 10.1007/s00776-012-0204-1
4. Dibra FF, Prietao HA, Gray CF, et al. Don’t forget the hip! Hip arthritis masquerading as knee pain. Arthroplast Today. 2017;4:118-124. doi: 10.1016/j.artd.2017.06.008
5. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323-1330. doi: 10.1136/annrheumdis-2013-204763
6. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386-1397. doi: 10.2106/JBJS.N.01141
7. Sessle BJ. Central mechanisms of craniofacial musculoskeletal pain: a review. In: Graven-Nielsen T, Arendt-Nielsen L, Mense S, eds. Fundamentals of musculoskeletal pain. 1st ed. IASP Press; 2008:87-103.
8. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578-1589. doi: 10.1016/j.joca.2019.06.011
1. Fernandes GS, Parekh SM, Moses J, et al. Prevalence of knee pain, radiographic osteoarthritis and arthroplasty in retired professional footballers compared with men in the general population: a cross-sectional study. Br J Sports Med. 2018;52:678-683. doi: 10.1136/bjsports-2017-097503
2. Christmas C, Crespo CJ, Franckowiak SC, et al. How common is hip pain among older adults? Results from the Third National Health and Nutrition Examination Survey. J Fam Pract. 2002;51:345-348.
3. Hsieh PH, Chang Y, Chen DW, et al. Pain distribution and response to total hip arthroplasty: a prospective observational study in 113 patients with end-stage hip disease. J Orthop Sci. 2012;17:213-218. doi: 10.1007/s00776-012-0204-1
4. Dibra FF, Prietao HA, Gray CF, et al. Don’t forget the hip! Hip arthritis masquerading as knee pain. Arthroplast Today. 2017;4:118-124. doi: 10.1016/j.artd.2017.06.008
5. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323-1330. doi: 10.1136/annrheumdis-2013-204763
6. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386-1397. doi: 10.2106/JBJS.N.01141
7. Sessle BJ. Central mechanisms of craniofacial musculoskeletal pain: a review. In: Graven-Nielsen T, Arendt-Nielsen L, Mense S, eds. Fundamentals of musculoskeletal pain. 1st ed. IASP Press; 2008:87-103.
8. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578-1589. doi: 10.1016/j.joca.2019.06.011
Transvaginal mesh, native tissue repair have similar outcomes in 3-year trial
Transvaginal mesh was found to be safe and effective for patients with pelvic organ prolapse (POP) when compared with native tissue repair (NTR) in a 3-year trial.
Researchers, led by Bruce S. Kahn, MD, with the department of obstetrics & gynecology at Scripps Clinic in San Diego evaluated the two surgical treatment methods and published their findings in Obstetrics & Gynecology.
At completion of the 3-year follow-up in 2016, there were 401 participants in the transvaginal mesh group and 171 in the NTR group.
The prospective, nonrandomized, parallel-cohort, 27-site trial used a primary composite endpoint of anatomical success; subjective success (vaginal bulging); retreatment measures; and serious device-related or serious procedure-related adverse events.
The secondary endpoint was a composite outcome similar to the primary composite outcome but with anatomical success more stringently defined as POP quantification (POP-Q) point Ba < 0 and/or C < 0.
The secondary outcome was added to this trial because investigators had criticized the primary endpoint, set by the Food and Drug Administration, because it included anatomic outcome measures that were the same for inclusion criteria (POP-Q point Ba < 0 and/or C < 0.)
The secondary-outcome composite also included quality-of-life measures, mesh exposure, and mesh- and procedure-related complications.
Outcomes similar for both groups
The primary outcome demonstrated transvaginal mesh was not superior to native tissue repair (P =.056).
In the secondary outcome, superiority of transvaginal mesh over native tissue repair was shown (P =.009), with a propensity score–adjusted difference of 10.6% (90% confidence interval, 3.3%-17.9%) in favor of transvaginal mesh.
The authors noted that subjective success regarding vaginal bulging, which is important in patient satisfaction, was high and not statistically different between the two groups.
Additionally, transvaginal mesh repair was as safe as NTR regarding serious device-related and/or serious procedure-related side effects.
For the primary safety endpoint, 3.1% in the mesh group and 2.7% in the native tissue repair group experienced serious adverse events, demonstrating that mesh was noninferior to NTR.
Research results have been mixed
Unanswered questions surround surgical options for POP, which, the authors wrote, “affects 3%-6% of women based on symptoms and up to 50% of women based on vaginal examination.”
The FDA in 2011 issued 522 postmarket surveillance study orders for companies that market transvaginal mesh for POP.
Research results have varied and contentious debate has continued in the field. Some studies have shown that mesh has better subjective and objective outcomes than NTR in the anterior compartment. Others have found more complications with transvaginal mesh, such as mesh exposure and painful intercourse.
Complicating comparisons, early versions of the mesh used were larger and denser than today’s versions.
In this postmarket study, patients received either the Uphold LITE brand of transvaginal mesh or native tissue repair for surgical treatment of POP.
Expert: This study unlikely to change minds
In an accompanying editorial, John O.L. DeLancey, MD, professor of gynecology at the University of Michigan, Ann Arbor, pointed out that so far there’s been a lack of randomized trials that could answer whether mesh surgeries result in fewer symptoms or result in sufficient improvements in anatomy to justify their additional risk.
This study may not help with the decision. Dr. DeLancey wrote: “Will this study change the minds of either side of this debate? Probably not. The two sides are deeply entrenched in their positions.”
Two considerations are important in thinking about the issue, he said. Surgical outcomes for POP are “not as good as we would hope.” Also, many women have had serious complications with mesh operations.
He wrote: “Mesh litigation has resulted in more $8 billion in settlements, which is many times the $1 billion annual national cost of providing care for prolapse. Those of us who practice in referral centers have seen women with devastating problems, even though they probably represent a small fraction of cases.”
Dr. DeLancey highlighted some limitations of the study by Dr. Kahn and colleagues, especially regarding differences in the groups studied and the design of the study.
“For example,” he explained, “65% of individuals in the mesh-repair group had a prior hysterectomy as opposed to 30% in the native tissue repair group. In addition, some of the operations in the native tissue group are not typical choices; for example, hysteropexy was used for some patients and had a 47% failure rate.”
He said the all-or-nothing approach to surgical solutions may be clouding the debate – in other words mesh or no mesh for women as a group.
“Rather than asking whether mesh is better than no mesh, knowing which women (if any) stand to benefit from mesh is the critical question. We need to understand, for each woman, what structural failures exist so that we can target our interventions to correct them,” he wrote.
This study was sponsored by Boston Scientific. Dr. Kahn disclosed research support from Solaire, payments from AbbVie and Douchenay as a speaker, payments from Caldera and Cytuity (Boston Scientific) as a medical consultant, and payment from Johnson & Johnson as an expert witness. One coauthor disclosed that money was paid to her institution from Medtronic and Boston Scientific (both unrestricted educational grants for cadaveric lab). Another is chief medical officer at Axonics. One study coauthor receives research funding from Axonics and is a consultant for Group Dynamics, Medpace, and FirstThought. One coauthor received research support, is a consultant for Boston Scientific, and is an expert witness for Johnson & Johnson. Dr. DeLancey declared no relevant financial relationships.
Transvaginal mesh was found to be safe and effective for patients with pelvic organ prolapse (POP) when compared with native tissue repair (NTR) in a 3-year trial.
Researchers, led by Bruce S. Kahn, MD, with the department of obstetrics & gynecology at Scripps Clinic in San Diego evaluated the two surgical treatment methods and published their findings in Obstetrics & Gynecology.
At completion of the 3-year follow-up in 2016, there were 401 participants in the transvaginal mesh group and 171 in the NTR group.
The prospective, nonrandomized, parallel-cohort, 27-site trial used a primary composite endpoint of anatomical success; subjective success (vaginal bulging); retreatment measures; and serious device-related or serious procedure-related adverse events.
The secondary endpoint was a composite outcome similar to the primary composite outcome but with anatomical success more stringently defined as POP quantification (POP-Q) point Ba < 0 and/or C < 0.
The secondary outcome was added to this trial because investigators had criticized the primary endpoint, set by the Food and Drug Administration, because it included anatomic outcome measures that were the same for inclusion criteria (POP-Q point Ba < 0 and/or C < 0.)
The secondary-outcome composite also included quality-of-life measures, mesh exposure, and mesh- and procedure-related complications.
Outcomes similar for both groups
The primary outcome demonstrated transvaginal mesh was not superior to native tissue repair (P =.056).
In the secondary outcome, superiority of transvaginal mesh over native tissue repair was shown (P =.009), with a propensity score–adjusted difference of 10.6% (90% confidence interval, 3.3%-17.9%) in favor of transvaginal mesh.
The authors noted that subjective success regarding vaginal bulging, which is important in patient satisfaction, was high and not statistically different between the two groups.
Additionally, transvaginal mesh repair was as safe as NTR regarding serious device-related and/or serious procedure-related side effects.
For the primary safety endpoint, 3.1% in the mesh group and 2.7% in the native tissue repair group experienced serious adverse events, demonstrating that mesh was noninferior to NTR.
Research results have been mixed
Unanswered questions surround surgical options for POP, which, the authors wrote, “affects 3%-6% of women based on symptoms and up to 50% of women based on vaginal examination.”
The FDA in 2011 issued 522 postmarket surveillance study orders for companies that market transvaginal mesh for POP.
Research results have varied and contentious debate has continued in the field. Some studies have shown that mesh has better subjective and objective outcomes than NTR in the anterior compartment. Others have found more complications with transvaginal mesh, such as mesh exposure and painful intercourse.
Complicating comparisons, early versions of the mesh used were larger and denser than today’s versions.
In this postmarket study, patients received either the Uphold LITE brand of transvaginal mesh or native tissue repair for surgical treatment of POP.
Expert: This study unlikely to change minds
In an accompanying editorial, John O.L. DeLancey, MD, professor of gynecology at the University of Michigan, Ann Arbor, pointed out that so far there’s been a lack of randomized trials that could answer whether mesh surgeries result in fewer symptoms or result in sufficient improvements in anatomy to justify their additional risk.
This study may not help with the decision. Dr. DeLancey wrote: “Will this study change the minds of either side of this debate? Probably not. The two sides are deeply entrenched in their positions.”
Two considerations are important in thinking about the issue, he said. Surgical outcomes for POP are “not as good as we would hope.” Also, many women have had serious complications with mesh operations.
He wrote: “Mesh litigation has resulted in more $8 billion in settlements, which is many times the $1 billion annual national cost of providing care for prolapse. Those of us who practice in referral centers have seen women with devastating problems, even though they probably represent a small fraction of cases.”
Dr. DeLancey highlighted some limitations of the study by Dr. Kahn and colleagues, especially regarding differences in the groups studied and the design of the study.
“For example,” he explained, “65% of individuals in the mesh-repair group had a prior hysterectomy as opposed to 30% in the native tissue repair group. In addition, some of the operations in the native tissue group are not typical choices; for example, hysteropexy was used for some patients and had a 47% failure rate.”
He said the all-or-nothing approach to surgical solutions may be clouding the debate – in other words mesh or no mesh for women as a group.
“Rather than asking whether mesh is better than no mesh, knowing which women (if any) stand to benefit from mesh is the critical question. We need to understand, for each woman, what structural failures exist so that we can target our interventions to correct them,” he wrote.
This study was sponsored by Boston Scientific. Dr. Kahn disclosed research support from Solaire, payments from AbbVie and Douchenay as a speaker, payments from Caldera and Cytuity (Boston Scientific) as a medical consultant, and payment from Johnson & Johnson as an expert witness. One coauthor disclosed that money was paid to her institution from Medtronic and Boston Scientific (both unrestricted educational grants for cadaveric lab). Another is chief medical officer at Axonics. One study coauthor receives research funding from Axonics and is a consultant for Group Dynamics, Medpace, and FirstThought. One coauthor received research support, is a consultant for Boston Scientific, and is an expert witness for Johnson & Johnson. Dr. DeLancey declared no relevant financial relationships.
Transvaginal mesh was found to be safe and effective for patients with pelvic organ prolapse (POP) when compared with native tissue repair (NTR) in a 3-year trial.
Researchers, led by Bruce S. Kahn, MD, with the department of obstetrics & gynecology at Scripps Clinic in San Diego evaluated the two surgical treatment methods and published their findings in Obstetrics & Gynecology.
At completion of the 3-year follow-up in 2016, there were 401 participants in the transvaginal mesh group and 171 in the NTR group.
The prospective, nonrandomized, parallel-cohort, 27-site trial used a primary composite endpoint of anatomical success; subjective success (vaginal bulging); retreatment measures; and serious device-related or serious procedure-related adverse events.
The secondary endpoint was a composite outcome similar to the primary composite outcome but with anatomical success more stringently defined as POP quantification (POP-Q) point Ba < 0 and/or C < 0.
The secondary outcome was added to this trial because investigators had criticized the primary endpoint, set by the Food and Drug Administration, because it included anatomic outcome measures that were the same for inclusion criteria (POP-Q point Ba < 0 and/or C < 0.)
The secondary-outcome composite also included quality-of-life measures, mesh exposure, and mesh- and procedure-related complications.
Outcomes similar for both groups
The primary outcome demonstrated transvaginal mesh was not superior to native tissue repair (P =.056).
In the secondary outcome, superiority of transvaginal mesh over native tissue repair was shown (P =.009), with a propensity score–adjusted difference of 10.6% (90% confidence interval, 3.3%-17.9%) in favor of transvaginal mesh.
The authors noted that subjective success regarding vaginal bulging, which is important in patient satisfaction, was high and not statistically different between the two groups.
Additionally, transvaginal mesh repair was as safe as NTR regarding serious device-related and/or serious procedure-related side effects.
For the primary safety endpoint, 3.1% in the mesh group and 2.7% in the native tissue repair group experienced serious adverse events, demonstrating that mesh was noninferior to NTR.
Research results have been mixed
Unanswered questions surround surgical options for POP, which, the authors wrote, “affects 3%-6% of women based on symptoms and up to 50% of women based on vaginal examination.”
The FDA in 2011 issued 522 postmarket surveillance study orders for companies that market transvaginal mesh for POP.
Research results have varied and contentious debate has continued in the field. Some studies have shown that mesh has better subjective and objective outcomes than NTR in the anterior compartment. Others have found more complications with transvaginal mesh, such as mesh exposure and painful intercourse.
Complicating comparisons, early versions of the mesh used were larger and denser than today’s versions.
In this postmarket study, patients received either the Uphold LITE brand of transvaginal mesh or native tissue repair for surgical treatment of POP.
Expert: This study unlikely to change minds
In an accompanying editorial, John O.L. DeLancey, MD, professor of gynecology at the University of Michigan, Ann Arbor, pointed out that so far there’s been a lack of randomized trials that could answer whether mesh surgeries result in fewer symptoms or result in sufficient improvements in anatomy to justify their additional risk.
This study may not help with the decision. Dr. DeLancey wrote: “Will this study change the minds of either side of this debate? Probably not. The two sides are deeply entrenched in their positions.”
Two considerations are important in thinking about the issue, he said. Surgical outcomes for POP are “not as good as we would hope.” Also, many women have had serious complications with mesh operations.
He wrote: “Mesh litigation has resulted in more $8 billion in settlements, which is many times the $1 billion annual national cost of providing care for prolapse. Those of us who practice in referral centers have seen women with devastating problems, even though they probably represent a small fraction of cases.”
Dr. DeLancey highlighted some limitations of the study by Dr. Kahn and colleagues, especially regarding differences in the groups studied and the design of the study.
“For example,” he explained, “65% of individuals in the mesh-repair group had a prior hysterectomy as opposed to 30% in the native tissue repair group. In addition, some of the operations in the native tissue group are not typical choices; for example, hysteropexy was used for some patients and had a 47% failure rate.”
He said the all-or-nothing approach to surgical solutions may be clouding the debate – in other words mesh or no mesh for women as a group.
“Rather than asking whether mesh is better than no mesh, knowing which women (if any) stand to benefit from mesh is the critical question. We need to understand, for each woman, what structural failures exist so that we can target our interventions to correct them,” he wrote.
This study was sponsored by Boston Scientific. Dr. Kahn disclosed research support from Solaire, payments from AbbVie and Douchenay as a speaker, payments from Caldera and Cytuity (Boston Scientific) as a medical consultant, and payment from Johnson & Johnson as an expert witness. One coauthor disclosed that money was paid to her institution from Medtronic and Boston Scientific (both unrestricted educational grants for cadaveric lab). Another is chief medical officer at Axonics. One study coauthor receives research funding from Axonics and is a consultant for Group Dynamics, Medpace, and FirstThought. One coauthor received research support, is a consultant for Boston Scientific, and is an expert witness for Johnson & Johnson. Dr. DeLancey declared no relevant financial relationships.
FROM OBSTETRICS & GYNECOLOGY
FDA clears diagnostic test for early Alzheimer’s
The Lumipulse G β-Amyloid Ratio 1-42/1-40 (Fujirebio Diagnostics) test detects amyloid plaques associated with AD in adults age 55 or older who are under investigation for AD and other causes of cognitive decline.
“The availability of an in vitro diagnostic test that can potentially eliminate the need for time-consuming and expensive [positron emission tomography (PET)] scans is great news for individuals and families concerned with the possibility of an Alzheimer’s disease diagnosis,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
“With the Lumipulse test, there is a new option that can typically be completed the same day and can give doctors the same information regarding brain amyloid status, without the radiation risk, to help determine if a patient’s cognitive impairment is due to Alzheimer’s disease,” he added.
In its statement, the FDA notes that there is an “unmet need for a reliable and safe test that can accurately identify patients with amyloid plaques consistent with Alzheimer’s disease.”
The agency goes on to state that this new test may eliminate the need to use PET brain scans, a “potentially costly and cumbersome option” to visualize amyloid plaques for the diagnosis of AD.
The Lumipulse test measures the ratio of β-amyloid 1-42 and β-amyloid 1-40 concentrations in human cerebral spinal fluid (CSF). A positive Lumipulse G β-amyloid Ratio (1-42/1-40) test result is consistent with the presence of amyloid plaques, similar to that revealed in a PET scan. A negative result is consistent with a negative amyloid PET scan result.
However, the FDA notes that the test is not a stand-alone assay and should be used in conjunction with other clinical evaluations and additional tests to determine treatment options.
The FDA reports that it evaluated the safety and efficacy of the test in a clinical study of 292 CSF samples from the Alzheimer’s Disease Neuroimaging Initiative sample bank.
The samples were tested by the Lumipulse G β-amyloid Ratio (1-42/1-40) and compared with amyloid PET scan results. In this clinical study, 97% of individuals with Lumipulse G β-amyloid Ratio (1-42/1-40) positive results had the presence of amyloid plaques by PET scan and 84% of individuals with negative results had a negative amyloid PET scan.
The risks associated with the Lumipulse G β-amyloid Ratio (1-42/1-40) test are mainly the possibility of false-positive and false-negative test results.
False-positive results, in conjunction with other clinical information, could lead to an inappropriate diagnosis of, and unnecessary treatment for AD.
False-negative test results could result in additional unnecessary diagnostic tests and potential delay in effective treatment for AD.
The FDA reviewed the device through the De Novo premarket review pathway, a regulatory pathway for low- to moderate-risk devices of a new type.
The agency says this action “creates a new regulatory classification, which means that subsequent devices of the same type with the same intended use may go through FDA’s 510(k) premarket process, whereby devices can obtain marketing authorization by demonstrating substantial equivalence to a predicate device.”
The Lumipulse G β-amyloid Ratio (1-42/1-40) was granted Breakthrough Device designation, a process designed to expedite the development and review of devices that may provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions.
A version of this article first appeared on Medscape.com.
The Lumipulse G β-Amyloid Ratio 1-42/1-40 (Fujirebio Diagnostics) test detects amyloid plaques associated with AD in adults age 55 or older who are under investigation for AD and other causes of cognitive decline.
“The availability of an in vitro diagnostic test that can potentially eliminate the need for time-consuming and expensive [positron emission tomography (PET)] scans is great news for individuals and families concerned with the possibility of an Alzheimer’s disease diagnosis,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
“With the Lumipulse test, there is a new option that can typically be completed the same day and can give doctors the same information regarding brain amyloid status, without the radiation risk, to help determine if a patient’s cognitive impairment is due to Alzheimer’s disease,” he added.
In its statement, the FDA notes that there is an “unmet need for a reliable and safe test that can accurately identify patients with amyloid plaques consistent with Alzheimer’s disease.”
The agency goes on to state that this new test may eliminate the need to use PET brain scans, a “potentially costly and cumbersome option” to visualize amyloid plaques for the diagnosis of AD.
The Lumipulse test measures the ratio of β-amyloid 1-42 and β-amyloid 1-40 concentrations in human cerebral spinal fluid (CSF). A positive Lumipulse G β-amyloid Ratio (1-42/1-40) test result is consistent with the presence of amyloid plaques, similar to that revealed in a PET scan. A negative result is consistent with a negative amyloid PET scan result.
However, the FDA notes that the test is not a stand-alone assay and should be used in conjunction with other clinical evaluations and additional tests to determine treatment options.
The FDA reports that it evaluated the safety and efficacy of the test in a clinical study of 292 CSF samples from the Alzheimer’s Disease Neuroimaging Initiative sample bank.
The samples were tested by the Lumipulse G β-amyloid Ratio (1-42/1-40) and compared with amyloid PET scan results. In this clinical study, 97% of individuals with Lumipulse G β-amyloid Ratio (1-42/1-40) positive results had the presence of amyloid plaques by PET scan and 84% of individuals with negative results had a negative amyloid PET scan.
The risks associated with the Lumipulse G β-amyloid Ratio (1-42/1-40) test are mainly the possibility of false-positive and false-negative test results.
False-positive results, in conjunction with other clinical information, could lead to an inappropriate diagnosis of, and unnecessary treatment for AD.
False-negative test results could result in additional unnecessary diagnostic tests and potential delay in effective treatment for AD.
The FDA reviewed the device through the De Novo premarket review pathway, a regulatory pathway for low- to moderate-risk devices of a new type.
The agency says this action “creates a new regulatory classification, which means that subsequent devices of the same type with the same intended use may go through FDA’s 510(k) premarket process, whereby devices can obtain marketing authorization by demonstrating substantial equivalence to a predicate device.”
The Lumipulse G β-amyloid Ratio (1-42/1-40) was granted Breakthrough Device designation, a process designed to expedite the development and review of devices that may provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions.
A version of this article first appeared on Medscape.com.
The Lumipulse G β-Amyloid Ratio 1-42/1-40 (Fujirebio Diagnostics) test detects amyloid plaques associated with AD in adults age 55 or older who are under investigation for AD and other causes of cognitive decline.
“The availability of an in vitro diagnostic test that can potentially eliminate the need for time-consuming and expensive [positron emission tomography (PET)] scans is great news for individuals and families concerned with the possibility of an Alzheimer’s disease diagnosis,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
“With the Lumipulse test, there is a new option that can typically be completed the same day and can give doctors the same information regarding brain amyloid status, without the radiation risk, to help determine if a patient’s cognitive impairment is due to Alzheimer’s disease,” he added.
In its statement, the FDA notes that there is an “unmet need for a reliable and safe test that can accurately identify patients with amyloid plaques consistent with Alzheimer’s disease.”
The agency goes on to state that this new test may eliminate the need to use PET brain scans, a “potentially costly and cumbersome option” to visualize amyloid plaques for the diagnosis of AD.
The Lumipulse test measures the ratio of β-amyloid 1-42 and β-amyloid 1-40 concentrations in human cerebral spinal fluid (CSF). A positive Lumipulse G β-amyloid Ratio (1-42/1-40) test result is consistent with the presence of amyloid plaques, similar to that revealed in a PET scan. A negative result is consistent with a negative amyloid PET scan result.
However, the FDA notes that the test is not a stand-alone assay and should be used in conjunction with other clinical evaluations and additional tests to determine treatment options.
The FDA reports that it evaluated the safety and efficacy of the test in a clinical study of 292 CSF samples from the Alzheimer’s Disease Neuroimaging Initiative sample bank.
The samples were tested by the Lumipulse G β-amyloid Ratio (1-42/1-40) and compared with amyloid PET scan results. In this clinical study, 97% of individuals with Lumipulse G β-amyloid Ratio (1-42/1-40) positive results had the presence of amyloid plaques by PET scan and 84% of individuals with negative results had a negative amyloid PET scan.
The risks associated with the Lumipulse G β-amyloid Ratio (1-42/1-40) test are mainly the possibility of false-positive and false-negative test results.
False-positive results, in conjunction with other clinical information, could lead to an inappropriate diagnosis of, and unnecessary treatment for AD.
False-negative test results could result in additional unnecessary diagnostic tests and potential delay in effective treatment for AD.
The FDA reviewed the device through the De Novo premarket review pathway, a regulatory pathway for low- to moderate-risk devices of a new type.
The agency says this action “creates a new regulatory classification, which means that subsequent devices of the same type with the same intended use may go through FDA’s 510(k) premarket process, whereby devices can obtain marketing authorization by demonstrating substantial equivalence to a predicate device.”
The Lumipulse G β-amyloid Ratio (1-42/1-40) was granted Breakthrough Device designation, a process designed to expedite the development and review of devices that may provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions.
A version of this article first appeared on Medscape.com.
Seven hours of sleep is ideal for middle aged and older
Sleep disturbances are common in older age, and previous studies have shown associations between too much or too little sleep and increased risk of cognitive decline, but the ideal amount of sleep for preserving mental health has not been well described, according to the authors of the new paper.
In the study published in Nature Aging, the team of researchers from China and the United Kingdom reviewed data from the UK Biobank, a national database of individuals in the United Kingdom that includes cognitive assessments, mental health questionnaires, and brain imaging data, as well as genetic information.
Sleep is important for physical and psychological health, and also serves a neuroprotective function by clearing waste products from the brain, lead author Yuzhu Li of Fudan University, Shanghai, China, and colleagues wrote.
The study population included 498,277 participants, aged 38-73 years, who completed touchscreen questionnaires about sleep duration between 2006 and 2010. The average age at baseline was 56.5 years, 54% were female, and the mean sleep duration was 7.15 hours.
The researchers also reviewed brain imaging data and genetic data from 39,692 participants in 2014 to examine the relationships between sleep duration and brain structure and between sleep duration and genetic risk. In addition, 156,884 participants completed an online follow-up mental health questionnaire in 2016-2017 to assess the longitudinal impact of sleep on mental health.
Both excessive and insufficient sleep was associated with impaired cognitive performance, evidenced by the U-shaped curve found by the researchers in their data analysis, which used quadratic associations.
Specific cognitive functions including pair matching, trail making, prospective memory, and reaction time were significantly impaired with too much or too little sleep, the researchers said. “This demonstrated the positive association of both insufficient and excessive sleep duration with inferior performance on cognitive tasks.”
When the researchers analyzed the association between sleep duration and mental health, sleep duration also showed a U-shaped association with symptoms of anxiety, depression, mental distress, mania, and self-harm, while well-being showed an inverted U-shape. All associations between sleep duration and mental health were statistically significant after controlling for confounding variables (P < .001).
On further analysis (using two-line tests), the researchers determined that consistent sleep duration of approximately 7 hours per night was optimal for cognitive performance and for good mental health.
The researchers also used neuroimaging data to examine the relationship between sleep duration and brain structure. Overall, greater changes were seen in the regions of the brain involved in cognitive processing and memory.
“The most significant cortical volumes nonlinearly associated with sleep duration included the precentral cortex, the superior frontal gyrus, the lateral orbitofrontal cortex, the pars orbitalis, the frontal pole, and the middle temporal cortex,” the researchers wrote (P < .05 for all).
The association between sleep duration and cognitive function diminished among individuals older than 65 years, compared with those aged approximately 40 years, which suggests that optimal sleep duration may be more beneficial in middle age, the researchers noted. However, no similar impact of age was seen for mental health. For brain structure, the nonlinear relationship between sleep duration and cortical volumes was greatest in those aged 44-59 years, and gradually flattened with older age.
Research supports sleep discussions with patients
“Primary care physicians can use this study in their discussions with middle-aged and older patients to recommend optimal sleep duration and measures to achieve this sleep target,” Noel Deep, MD, a general internist in group practice in Antigo, Wisc., who was not involved in the study, said in an interview.
“This study is important because it demonstrated that both inadequate and excessive sleep patterns were associated with cognitive and mental health changes,” said Dr. Deep. “It supported previous observations of cognitive decline and mental health disorders being linked to disturbed sleep. But this study was unique because it provides data supporting an optimal sleep duration of 7 hours and the ill effects of both insufficient and excessive sleep duration.
“The usual thought process has been to assume that older individuals may not require as much sleep as the younger individuals, but this study supports an optimal time duration of sleep of 7 hours that benefits the older individuals. It was also interesting to note the mental health effects caused by the inadequate and excessive sleep durations,” he added.
As for additional research, “I would like to look into the quality of the sleep, in addition to the duration of sleep,” said Dr. Deep. For example, whether the excessive sleep was caused by poor quality sleep or fragmented sleep leading to the structural and subsequent cognitive decline.
Study limitations
“The current study relied on self-reporting of the sleep duration and was not observed and recorded data,” Dr. Deep noted. “It would also be beneficial to not only rely on healthy volunteers reporting the sleep duration, but also obtain sleep data from individuals with known brain disorders.”
The study findings were limited by several other factors, including the use of total sleep duration only, without other measures of sleep hygiene, the researchers noted. More research is needed to investigate the mechanisms driving the association between too much and not enough sleep and poor mental health and cognitive function.
The study was supported by the National Key R&D Program of China, the Shanghai Municipal Science and Technology Major Project, the Shanghai Center for Brain Science and Brain-Inspired Technology, the 111 Project, the National Natural Sciences Foundation of China and the Shanghai Rising Star Program.
The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
Sleep disturbances are common in older age, and previous studies have shown associations between too much or too little sleep and increased risk of cognitive decline, but the ideal amount of sleep for preserving mental health has not been well described, according to the authors of the new paper.
In the study published in Nature Aging, the team of researchers from China and the United Kingdom reviewed data from the UK Biobank, a national database of individuals in the United Kingdom that includes cognitive assessments, mental health questionnaires, and brain imaging data, as well as genetic information.
Sleep is important for physical and psychological health, and also serves a neuroprotective function by clearing waste products from the brain, lead author Yuzhu Li of Fudan University, Shanghai, China, and colleagues wrote.
The study population included 498,277 participants, aged 38-73 years, who completed touchscreen questionnaires about sleep duration between 2006 and 2010. The average age at baseline was 56.5 years, 54% were female, and the mean sleep duration was 7.15 hours.
The researchers also reviewed brain imaging data and genetic data from 39,692 participants in 2014 to examine the relationships between sleep duration and brain structure and between sleep duration and genetic risk. In addition, 156,884 participants completed an online follow-up mental health questionnaire in 2016-2017 to assess the longitudinal impact of sleep on mental health.
Both excessive and insufficient sleep was associated with impaired cognitive performance, evidenced by the U-shaped curve found by the researchers in their data analysis, which used quadratic associations.
Specific cognitive functions including pair matching, trail making, prospective memory, and reaction time were significantly impaired with too much or too little sleep, the researchers said. “This demonstrated the positive association of both insufficient and excessive sleep duration with inferior performance on cognitive tasks.”
When the researchers analyzed the association between sleep duration and mental health, sleep duration also showed a U-shaped association with symptoms of anxiety, depression, mental distress, mania, and self-harm, while well-being showed an inverted U-shape. All associations between sleep duration and mental health were statistically significant after controlling for confounding variables (P < .001).
On further analysis (using two-line tests), the researchers determined that consistent sleep duration of approximately 7 hours per night was optimal for cognitive performance and for good mental health.
The researchers also used neuroimaging data to examine the relationship between sleep duration and brain structure. Overall, greater changes were seen in the regions of the brain involved in cognitive processing and memory.
“The most significant cortical volumes nonlinearly associated with sleep duration included the precentral cortex, the superior frontal gyrus, the lateral orbitofrontal cortex, the pars orbitalis, the frontal pole, and the middle temporal cortex,” the researchers wrote (P < .05 for all).
The association between sleep duration and cognitive function diminished among individuals older than 65 years, compared with those aged approximately 40 years, which suggests that optimal sleep duration may be more beneficial in middle age, the researchers noted. However, no similar impact of age was seen for mental health. For brain structure, the nonlinear relationship between sleep duration and cortical volumes was greatest in those aged 44-59 years, and gradually flattened with older age.
Research supports sleep discussions with patients
“Primary care physicians can use this study in their discussions with middle-aged and older patients to recommend optimal sleep duration and measures to achieve this sleep target,” Noel Deep, MD, a general internist in group practice in Antigo, Wisc., who was not involved in the study, said in an interview.
“This study is important because it demonstrated that both inadequate and excessive sleep patterns were associated with cognitive and mental health changes,” said Dr. Deep. “It supported previous observations of cognitive decline and mental health disorders being linked to disturbed sleep. But this study was unique because it provides data supporting an optimal sleep duration of 7 hours and the ill effects of both insufficient and excessive sleep duration.
“The usual thought process has been to assume that older individuals may not require as much sleep as the younger individuals, but this study supports an optimal time duration of sleep of 7 hours that benefits the older individuals. It was also interesting to note the mental health effects caused by the inadequate and excessive sleep durations,” he added.
As for additional research, “I would like to look into the quality of the sleep, in addition to the duration of sleep,” said Dr. Deep. For example, whether the excessive sleep was caused by poor quality sleep or fragmented sleep leading to the structural and subsequent cognitive decline.
Study limitations
“The current study relied on self-reporting of the sleep duration and was not observed and recorded data,” Dr. Deep noted. “It would also be beneficial to not only rely on healthy volunteers reporting the sleep duration, but also obtain sleep data from individuals with known brain disorders.”
The study findings were limited by several other factors, including the use of total sleep duration only, without other measures of sleep hygiene, the researchers noted. More research is needed to investigate the mechanisms driving the association between too much and not enough sleep and poor mental health and cognitive function.
The study was supported by the National Key R&D Program of China, the Shanghai Municipal Science and Technology Major Project, the Shanghai Center for Brain Science and Brain-Inspired Technology, the 111 Project, the National Natural Sciences Foundation of China and the Shanghai Rising Star Program.
The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
Sleep disturbances are common in older age, and previous studies have shown associations between too much or too little sleep and increased risk of cognitive decline, but the ideal amount of sleep for preserving mental health has not been well described, according to the authors of the new paper.
In the study published in Nature Aging, the team of researchers from China and the United Kingdom reviewed data from the UK Biobank, a national database of individuals in the United Kingdom that includes cognitive assessments, mental health questionnaires, and brain imaging data, as well as genetic information.
Sleep is important for physical and psychological health, and also serves a neuroprotective function by clearing waste products from the brain, lead author Yuzhu Li of Fudan University, Shanghai, China, and colleagues wrote.
The study population included 498,277 participants, aged 38-73 years, who completed touchscreen questionnaires about sleep duration between 2006 and 2010. The average age at baseline was 56.5 years, 54% were female, and the mean sleep duration was 7.15 hours.
The researchers also reviewed brain imaging data and genetic data from 39,692 participants in 2014 to examine the relationships between sleep duration and brain structure and between sleep duration and genetic risk. In addition, 156,884 participants completed an online follow-up mental health questionnaire in 2016-2017 to assess the longitudinal impact of sleep on mental health.
Both excessive and insufficient sleep was associated with impaired cognitive performance, evidenced by the U-shaped curve found by the researchers in their data analysis, which used quadratic associations.
Specific cognitive functions including pair matching, trail making, prospective memory, and reaction time were significantly impaired with too much or too little sleep, the researchers said. “This demonstrated the positive association of both insufficient and excessive sleep duration with inferior performance on cognitive tasks.”
When the researchers analyzed the association between sleep duration and mental health, sleep duration also showed a U-shaped association with symptoms of anxiety, depression, mental distress, mania, and self-harm, while well-being showed an inverted U-shape. All associations between sleep duration and mental health were statistically significant after controlling for confounding variables (P < .001).
On further analysis (using two-line tests), the researchers determined that consistent sleep duration of approximately 7 hours per night was optimal for cognitive performance and for good mental health.
The researchers also used neuroimaging data to examine the relationship between sleep duration and brain structure. Overall, greater changes were seen in the regions of the brain involved in cognitive processing and memory.
“The most significant cortical volumes nonlinearly associated with sleep duration included the precentral cortex, the superior frontal gyrus, the lateral orbitofrontal cortex, the pars orbitalis, the frontal pole, and the middle temporal cortex,” the researchers wrote (P < .05 for all).
The association between sleep duration and cognitive function diminished among individuals older than 65 years, compared with those aged approximately 40 years, which suggests that optimal sleep duration may be more beneficial in middle age, the researchers noted. However, no similar impact of age was seen for mental health. For brain structure, the nonlinear relationship between sleep duration and cortical volumes was greatest in those aged 44-59 years, and gradually flattened with older age.
Research supports sleep discussions with patients
“Primary care physicians can use this study in their discussions with middle-aged and older patients to recommend optimal sleep duration and measures to achieve this sleep target,” Noel Deep, MD, a general internist in group practice in Antigo, Wisc., who was not involved in the study, said in an interview.
“This study is important because it demonstrated that both inadequate and excessive sleep patterns were associated with cognitive and mental health changes,” said Dr. Deep. “It supported previous observations of cognitive decline and mental health disorders being linked to disturbed sleep. But this study was unique because it provides data supporting an optimal sleep duration of 7 hours and the ill effects of both insufficient and excessive sleep duration.
“The usual thought process has been to assume that older individuals may not require as much sleep as the younger individuals, but this study supports an optimal time duration of sleep of 7 hours that benefits the older individuals. It was also interesting to note the mental health effects caused by the inadequate and excessive sleep durations,” he added.
As for additional research, “I would like to look into the quality of the sleep, in addition to the duration of sleep,” said Dr. Deep. For example, whether the excessive sleep was caused by poor quality sleep or fragmented sleep leading to the structural and subsequent cognitive decline.
Study limitations
“The current study relied on self-reporting of the sleep duration and was not observed and recorded data,” Dr. Deep noted. “It would also be beneficial to not only rely on healthy volunteers reporting the sleep duration, but also obtain sleep data from individuals with known brain disorders.”
The study findings were limited by several other factors, including the use of total sleep duration only, without other measures of sleep hygiene, the researchers noted. More research is needed to investigate the mechanisms driving the association between too much and not enough sleep and poor mental health and cognitive function.
The study was supported by the National Key R&D Program of China, the Shanghai Municipal Science and Technology Major Project, the Shanghai Center for Brain Science and Brain-Inspired Technology, the 111 Project, the National Natural Sciences Foundation of China and the Shanghai Rising Star Program.
The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
FROM NATURE AGING
Deprescribing in older adults: An overview
Mr. J, age 73, has a 25-year history of generalized anxiety disorder and major depressive disorder. His medical history includes hypertension, hyperlipidemia, type 2 diabetes mellitus, hypothyroidism, osteoarthritis, insomnia, and allergic rhinitis. His last laboratory test results indicate his hemoglobin A1c, thyroid-stimulating hormone, low-density lipoprotein, and blood pressure measurements are at goal. He believes his conditions are well controlled but cites concerns about taking multiple medications each day and being able to afford his medications.
You review the list of Mr. J’s current prescription medications, which include alprazolam 0.5 mg/d, atorvastatin 40 mg/d, escitalopram 10 mg/d, levothyroxine 0.125 mg/d, lisinopril 20 mg/d, and metformin XR 1,000 mg/d. Mr. J reports taking over-the-counter (OTC) acetaminophen as needed for pain, diphenhydramine for insomnia, loratadine as needed for allergic rhinitis, and omeprazole for 2 years for indigestion. After further questioning, he also reports taking ginseng, milk thistle, a multivitamin, and, based on a friend’s recommendation, St John’s Wort (Table 1).
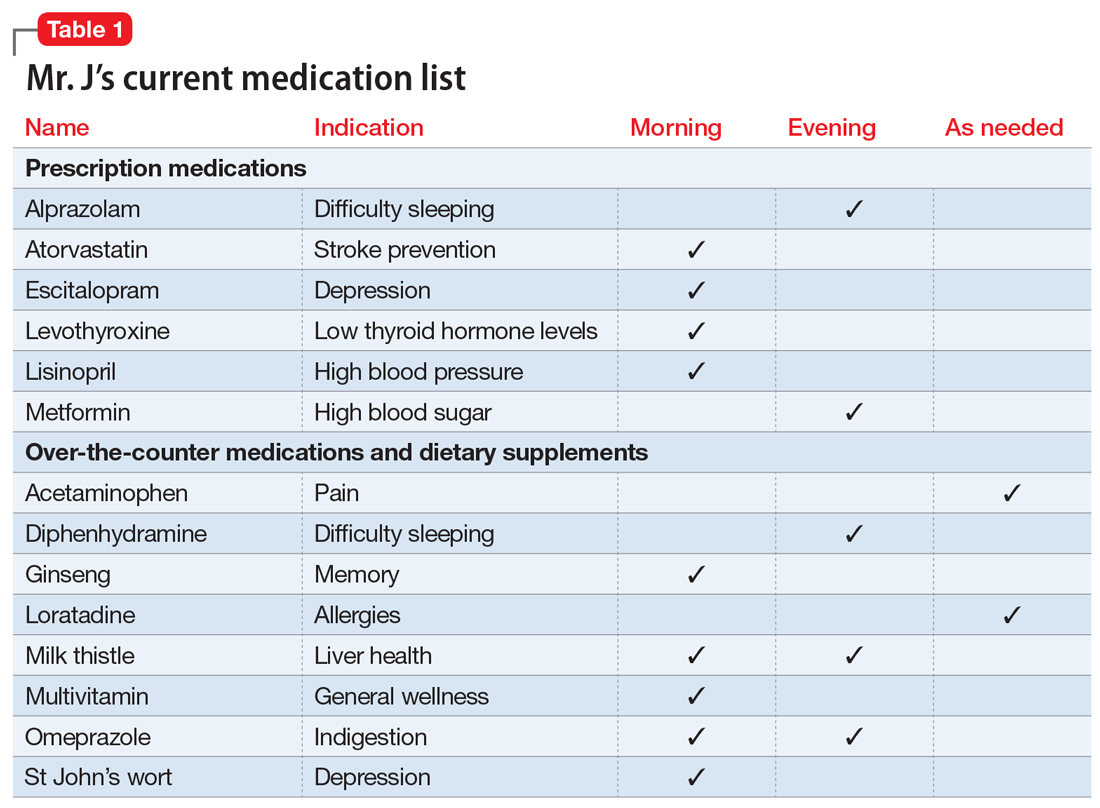
Similar to Mr. J, many older adults take multiple medications to manage chronic health conditions and promote their overall health. On average, 30% of older adults take ≥5 medications.1 Among commonly prescribed medications for these patients, an estimated 1 in 5 of may be inappropriate.1 Older adults have high rates of polypharmacy (often defined as taking ≥5 medications1), age-related physiological changes, increased number of comorbidities, and frailty, all of which can increase the risk of medication-related adverse events.2 As a result, older patients’ medications should be regularly evaluated to determine if each medication is appropriate to continue or should be tapered or stopped.
Deprescribing, in which medications are tapered or discontinued using a patient-centered approach, should be considered when a patient is no longer receiving benefit from a medication, or when the harm may exceed the benefit.1,3
Several researchers1,3 and organizations have published detailed descriptions of and guidelines for the process of deprescribing (see Related Resources). Here we provide a brief overview of this process (Figure1,3). The first step is to assemble a list of all prescription and OTC medications, herbal products, vitamins, or nutritional supplements the patient is taking. It is important to specifically ask patients about their use of nonprescription products, because these products are infrequently documented in medical records.
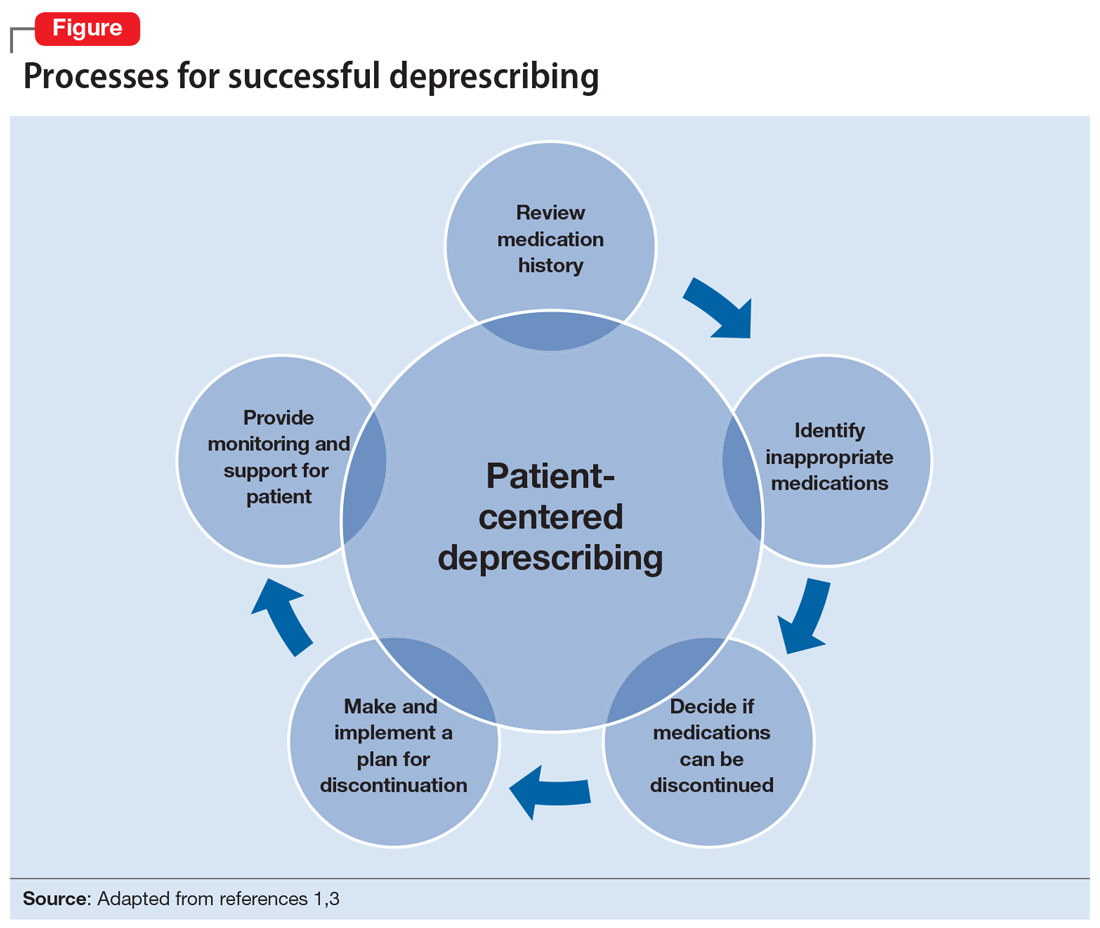
The second step is to evaluate the indication, effectiveness, safety, and patient’s adherence to each medication while beginning to consider opportunities to limit treatment burden and the risk of harm from medications. Ideally, this assessment should involve a patient-centered conversation that considers the patient’s goals, preferences, and treatment values. Many resources can be used to evaluate which medications might be inappropriate for an older adult. Two examples are the American Geriatrics Society Beers Criteria5 and STOPP/START criteria.6 By looking at these resources, you could identify that (for example) anticholinergic medications should be avoided in older patients due to an increased risk of adverse effects, change in cognitive status, and falls.5,6 These resources can aid in identifying, prioritizing, and deprescribing potentially harmful and/or inappropriate medications.
The next step is to decide whether any medications should be discontinued. Whenever possible, include the patient in this conversation, as they may have strong feelings about their current medication regimen. When there are multiple medications that can be discontinued, consider which medication to stop first based on potential harm, patient resistance, and other factors.
Continue to: Subsequently, work with...
Subsequently, work with the patient to create a plan for stopping or lowering the dose or frequency of the medication. These changes should be individualized based on the patient’s preferences as well as the properties of the medication. For example, some medications can be immediately discontinued, while others (eg, benzodiazepines) may need to be slowly tapered. It is important to consider if the patient will need to switch to a safer medication, change their behaviors (eg, lifestyle changes), or engage in alternative treatments (such as cognitive-behavioral therapy for insomnia) when they stop their current medication. Take an active role in monitoring your patient during this process, and encourage them to reach out to you or to their primary clinician if they have concerns.
CASE CONTINUED
Mr. J is a candidate for deprescribing because he has expressed concerns about his current regimen, and because he is taking potentially unsafe medications. The 2 medications he’s taking that may cause the most harm are diphenhydramine and alprazolam, due to the risk of cognitive impairment and falls. Through a patient-centered conversation, Mr. J says he is willing to stop diphenhydramine immediately and taper off the alprazolam over the next month, with the support of a tapering chart (Table 2). You explain to him that a long tapering of alprazolam may be necessary. He is willing to try good sleep hygiene practices and will put off starting trazodone as an alternative to diphenhydramine until he sees if it will be necessary. You make a note to follow up with him in 1 week to assess his insomnia and adherence to the new treatment plan. You also teach Mr. J that some of his supplements may interact with his prescription medications, such as St John’s Wort with escitalopram (ie, risk of serotonin syndrome) and ginseng with metformin (ie, risk for hypoglycemia). He says he doesn’t take ginseng, milk thistle, or St John’s Wort regularly, and because he feels they do not offer any benefit, he will stop taking them. He says that at his next visit with his primary care physician, he will bring up the idea of stopping omeprazole.
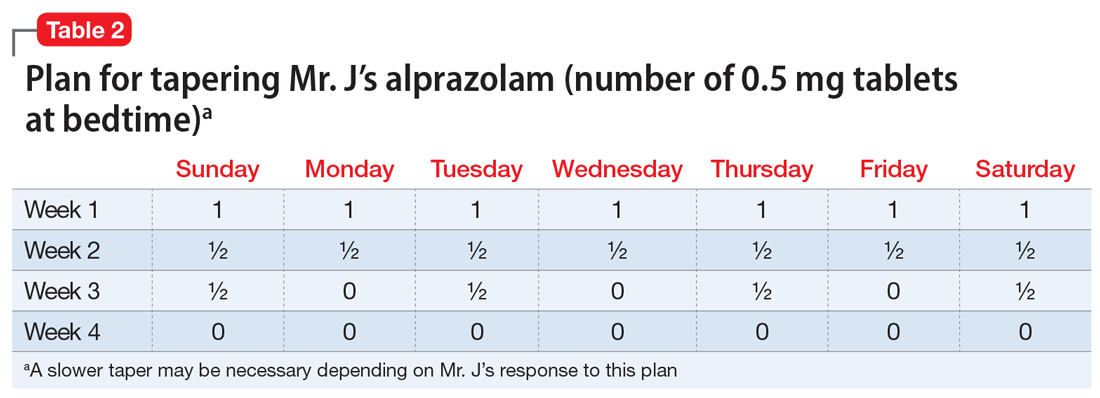
Related Resources
- Deprescribing.org. Deprescribing guidelines and algorithms. https://deprescribing.org/resources/deprescribing-guidelines-algorithms/
- US Deprescribing Research Network. Resources for Clinicians. https://deprescribingresearch.org/resources-2/resources-for-clinicians/
Drug Brand Names
Alprazolam • Xanax
Atorvastatin • Lipitor
Escitalopram • Lexapro
Levothyroxine • Synthroid
Lisinopril • Zestril
Metformin XR • Glucophage XR
Trazodone • Desyrel
1. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
2. Gibson G, Kennedy LH, Barlow G. Polypharmacy in older adults. Current Psychiatry. 2020;19(4):40-46.
3. Reeve E, Shakib S, Hendrix I, et al. Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process. Br J Clin Pharmcol. 2014;78(4):738-747.
4. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older: a systematic review. Drugs Aging. 2008;25(12):1021-1031.
5. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218.

Mr. J, age 73, has a 25-year history of generalized anxiety disorder and major depressive disorder. His medical history includes hypertension, hyperlipidemia, type 2 diabetes mellitus, hypothyroidism, osteoarthritis, insomnia, and allergic rhinitis. His last laboratory test results indicate his hemoglobin A1c, thyroid-stimulating hormone, low-density lipoprotein, and blood pressure measurements are at goal. He believes his conditions are well controlled but cites concerns about taking multiple medications each day and being able to afford his medications.
You review the list of Mr. J’s current prescription medications, which include alprazolam 0.5 mg/d, atorvastatin 40 mg/d, escitalopram 10 mg/d, levothyroxine 0.125 mg/d, lisinopril 20 mg/d, and metformin XR 1,000 mg/d. Mr. J reports taking over-the-counter (OTC) acetaminophen as needed for pain, diphenhydramine for insomnia, loratadine as needed for allergic rhinitis, and omeprazole for 2 years for indigestion. After further questioning, he also reports taking ginseng, milk thistle, a multivitamin, and, based on a friend’s recommendation, St John’s Wort (Table 1).

Similar to Mr. J, many older adults take multiple medications to manage chronic health conditions and promote their overall health. On average, 30% of older adults take ≥5 medications.1 Among commonly prescribed medications for these patients, an estimated 1 in 5 of may be inappropriate.1 Older adults have high rates of polypharmacy (often defined as taking ≥5 medications1), age-related physiological changes, increased number of comorbidities, and frailty, all of which can increase the risk of medication-related adverse events.2 As a result, older patients’ medications should be regularly evaluated to determine if each medication is appropriate to continue or should be tapered or stopped.
Deprescribing, in which medications are tapered or discontinued using a patient-centered approach, should be considered when a patient is no longer receiving benefit from a medication, or when the harm may exceed the benefit.1,3
Several researchers1,3 and organizations have published detailed descriptions of and guidelines for the process of deprescribing (see Related Resources). Here we provide a brief overview of this process (Figure1,3). The first step is to assemble a list of all prescription and OTC medications, herbal products, vitamins, or nutritional supplements the patient is taking. It is important to specifically ask patients about their use of nonprescription products, because these products are infrequently documented in medical records.

The second step is to evaluate the indication, effectiveness, safety, and patient’s adherence to each medication while beginning to consider opportunities to limit treatment burden and the risk of harm from medications. Ideally, this assessment should involve a patient-centered conversation that considers the patient’s goals, preferences, and treatment values. Many resources can be used to evaluate which medications might be inappropriate for an older adult. Two examples are the American Geriatrics Society Beers Criteria5 and STOPP/START criteria.6 By looking at these resources, you could identify that (for example) anticholinergic medications should be avoided in older patients due to an increased risk of adverse effects, change in cognitive status, and falls.5,6 These resources can aid in identifying, prioritizing, and deprescribing potentially harmful and/or inappropriate medications.
The next step is to decide whether any medications should be discontinued. Whenever possible, include the patient in this conversation, as they may have strong feelings about their current medication regimen. When there are multiple medications that can be discontinued, consider which medication to stop first based on potential harm, patient resistance, and other factors.
Continue to: Subsequently, work with...
Subsequently, work with the patient to create a plan for stopping or lowering the dose or frequency of the medication. These changes should be individualized based on the patient’s preferences as well as the properties of the medication. For example, some medications can be immediately discontinued, while others (eg, benzodiazepines) may need to be slowly tapered. It is important to consider if the patient will need to switch to a safer medication, change their behaviors (eg, lifestyle changes), or engage in alternative treatments (such as cognitive-behavioral therapy for insomnia) when they stop their current medication. Take an active role in monitoring your patient during this process, and encourage them to reach out to you or to their primary clinician if they have concerns.
CASE CONTINUED
Mr. J is a candidate for deprescribing because he has expressed concerns about his current regimen, and because he is taking potentially unsafe medications. The 2 medications he’s taking that may cause the most harm are diphenhydramine and alprazolam, due to the risk of cognitive impairment and falls. Through a patient-centered conversation, Mr. J says he is willing to stop diphenhydramine immediately and taper off the alprazolam over the next month, with the support of a tapering chart (Table 2). You explain to him that a long tapering of alprazolam may be necessary. He is willing to try good sleep hygiene practices and will put off starting trazodone as an alternative to diphenhydramine until he sees if it will be necessary. You make a note to follow up with him in 1 week to assess his insomnia and adherence to the new treatment plan. You also teach Mr. J that some of his supplements may interact with his prescription medications, such as St John’s Wort with escitalopram (ie, risk of serotonin syndrome) and ginseng with metformin (ie, risk for hypoglycemia). He says he doesn’t take ginseng, milk thistle, or St John’s Wort regularly, and because he feels they do not offer any benefit, he will stop taking them. He says that at his next visit with his primary care physician, he will bring up the idea of stopping omeprazole.

Related Resources
- Deprescribing.org. Deprescribing guidelines and algorithms. https://deprescribing.org/resources/deprescribing-guidelines-algorithms/
- US Deprescribing Research Network. Resources for Clinicians. https://deprescribingresearch.org/resources-2/resources-for-clinicians/
Drug Brand Names
Alprazolam • Xanax
Atorvastatin • Lipitor
Escitalopram • Lexapro
Levothyroxine • Synthroid
Lisinopril • Zestril
Metformin XR • Glucophage XR
Trazodone • Desyrel

Mr. J, age 73, has a 25-year history of generalized anxiety disorder and major depressive disorder. His medical history includes hypertension, hyperlipidemia, type 2 diabetes mellitus, hypothyroidism, osteoarthritis, insomnia, and allergic rhinitis. His last laboratory test results indicate his hemoglobin A1c, thyroid-stimulating hormone, low-density lipoprotein, and blood pressure measurements are at goal. He believes his conditions are well controlled but cites concerns about taking multiple medications each day and being able to afford his medications.
You review the list of Mr. J’s current prescription medications, which include alprazolam 0.5 mg/d, atorvastatin 40 mg/d, escitalopram 10 mg/d, levothyroxine 0.125 mg/d, lisinopril 20 mg/d, and metformin XR 1,000 mg/d. Mr. J reports taking over-the-counter (OTC) acetaminophen as needed for pain, diphenhydramine for insomnia, loratadine as needed for allergic rhinitis, and omeprazole for 2 years for indigestion. After further questioning, he also reports taking ginseng, milk thistle, a multivitamin, and, based on a friend’s recommendation, St John’s Wort (Table 1).

Similar to Mr. J, many older adults take multiple medications to manage chronic health conditions and promote their overall health. On average, 30% of older adults take ≥5 medications.1 Among commonly prescribed medications for these patients, an estimated 1 in 5 of may be inappropriate.1 Older adults have high rates of polypharmacy (often defined as taking ≥5 medications1), age-related physiological changes, increased number of comorbidities, and frailty, all of which can increase the risk of medication-related adverse events.2 As a result, older patients’ medications should be regularly evaluated to determine if each medication is appropriate to continue or should be tapered or stopped.
Deprescribing, in which medications are tapered or discontinued using a patient-centered approach, should be considered when a patient is no longer receiving benefit from a medication, or when the harm may exceed the benefit.1,3
Several researchers1,3 and organizations have published detailed descriptions of and guidelines for the process of deprescribing (see Related Resources). Here we provide a brief overview of this process (Figure1,3). The first step is to assemble a list of all prescription and OTC medications, herbal products, vitamins, or nutritional supplements the patient is taking. It is important to specifically ask patients about their use of nonprescription products, because these products are infrequently documented in medical records.

The second step is to evaluate the indication, effectiveness, safety, and patient’s adherence to each medication while beginning to consider opportunities to limit treatment burden and the risk of harm from medications. Ideally, this assessment should involve a patient-centered conversation that considers the patient’s goals, preferences, and treatment values. Many resources can be used to evaluate which medications might be inappropriate for an older adult. Two examples are the American Geriatrics Society Beers Criteria5 and STOPP/START criteria.6 By looking at these resources, you could identify that (for example) anticholinergic medications should be avoided in older patients due to an increased risk of adverse effects, change in cognitive status, and falls.5,6 These resources can aid in identifying, prioritizing, and deprescribing potentially harmful and/or inappropriate medications.
The next step is to decide whether any medications should be discontinued. Whenever possible, include the patient in this conversation, as they may have strong feelings about their current medication regimen. When there are multiple medications that can be discontinued, consider which medication to stop first based on potential harm, patient resistance, and other factors.
Continue to: Subsequently, work with...
Subsequently, work with the patient to create a plan for stopping or lowering the dose or frequency of the medication. These changes should be individualized based on the patient’s preferences as well as the properties of the medication. For example, some medications can be immediately discontinued, while others (eg, benzodiazepines) may need to be slowly tapered. It is important to consider if the patient will need to switch to a safer medication, change their behaviors (eg, lifestyle changes), or engage in alternative treatments (such as cognitive-behavioral therapy for insomnia) when they stop their current medication. Take an active role in monitoring your patient during this process, and encourage them to reach out to you or to their primary clinician if they have concerns.
CASE CONTINUED
Mr. J is a candidate for deprescribing because he has expressed concerns about his current regimen, and because he is taking potentially unsafe medications. The 2 medications he’s taking that may cause the most harm are diphenhydramine and alprazolam, due to the risk of cognitive impairment and falls. Through a patient-centered conversation, Mr. J says he is willing to stop diphenhydramine immediately and taper off the alprazolam over the next month, with the support of a tapering chart (Table 2). You explain to him that a long tapering of alprazolam may be necessary. He is willing to try good sleep hygiene practices and will put off starting trazodone as an alternative to diphenhydramine until he sees if it will be necessary. You make a note to follow up with him in 1 week to assess his insomnia and adherence to the new treatment plan. You also teach Mr. J that some of his supplements may interact with his prescription medications, such as St John’s Wort with escitalopram (ie, risk of serotonin syndrome) and ginseng with metformin (ie, risk for hypoglycemia). He says he doesn’t take ginseng, milk thistle, or St John’s Wort regularly, and because he feels they do not offer any benefit, he will stop taking them. He says that at his next visit with his primary care physician, he will bring up the idea of stopping omeprazole.

Related Resources
- Deprescribing.org. Deprescribing guidelines and algorithms. https://deprescribing.org/resources/deprescribing-guidelines-algorithms/
- US Deprescribing Research Network. Resources for Clinicians. https://deprescribingresearch.org/resources-2/resources-for-clinicians/
Drug Brand Names
Alprazolam • Xanax
Atorvastatin • Lipitor
Escitalopram • Lexapro
Levothyroxine • Synthroid
Lisinopril • Zestril
Metformin XR • Glucophage XR
Trazodone • Desyrel
1. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
2. Gibson G, Kennedy LH, Barlow G. Polypharmacy in older adults. Current Psychiatry. 2020;19(4):40-46.
3. Reeve E, Shakib S, Hendrix I, et al. Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process. Br J Clin Pharmcol. 2014;78(4):738-747.
4. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older: a systematic review. Drugs Aging. 2008;25(12):1021-1031.
5. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218.
1. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
2. Gibson G, Kennedy LH, Barlow G. Polypharmacy in older adults. Current Psychiatry. 2020;19(4):40-46.
3. Reeve E, Shakib S, Hendrix I, et al. Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process. Br J Clin Pharmcol. 2014;78(4):738-747.
4. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older: a systematic review. Drugs Aging. 2008;25(12):1021-1031.
5. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218.
‘Where does it hurt?’: Primary care tips for common ortho problems
Knee and shoulder pain are common complaints for patients in the primary care office.
But identifying the source of the pain can be complicated,
and an accurate diagnosis of the underlying cause of discomfort is key to appropriate management – whether that involves simple home care options of ice and rest or a recommendation for a follow-up with a specialist.
Speaking at the annual meeting of the American College of Physicians, Greg Nakamoto, MD, department of orthopedics, Virginia Mason Medical Center, Seattle, discussed common knee and shoulder problems that patients often present with in the primary care setting, and offered tips on diagnosis and appropriate management.
The most common conditions causing knee pain are osteoarthritis and meniscal tears. “The differential for knee pain is broad,” Dr. Nakamoto said. “You have to have a way to divide it down, such as if it’s acute or chronic.”
The initial workup has several key components. The first steps: Determine the location of the pain – anterior, medial, lateral, posterior – and then whether it stems from an injury or is atraumatic.
“If you have to ask one question – ask where it hurts,” he said. “And is it from an injury or just wear and tear? That helps me when deciding if surgery is needed.”
Pain in the knee generally localizes well to the site of pathology, and knee pain of acute traumatic onset requires more scrutiny for problems best treated with early surgery. “This also helps establish whether radiographic findings are due to injury or degeneration,” Dr. Nakamoto said. “The presence of swelling guides the need for anti-inflammatories or cortisone.”
Palpating for tenderness along the joint line is important, as is palpating above and below the joint line, Dr. Nakamoto said.
“Tenderness limited to the joint line, combined with a meniscal exam maneuver that reproduces joint-line pain, is suggestive of pain from meniscal pathology,” he said.
Imaging is an important component of evaluating knee symptoms, and the question often arises as to when to order an MRI.
Dr. Nakamoto offered the following scenario: If significant osteoarthritis is evident on weight-bearing x-ray, treat the patient for the condition. However, if little or no osteoarthritis appears on x-ray, and if the onset of symptoms was traumatic and both patient history and physical examination suggest a meniscal tear, order an MRI.
An early MRI also is needed if the patient has had either atraumatic or traumatic onset of symptoms and their history and physical exams are suspicious for a mechanically locked or locking meniscus. For suspicion of a ruptured quadriceps or patellar tendon or a stress fracture, an MRI is needed urgently.
An MRI would be ordered later if the patient’s symptoms have not improved significantly after 3 months of conservative management.
Dr. Nakamoto stressed how common undiagnosed meniscus tears are in the general population. A third of men aged 50-59 years and nearly 20% of women in that age group have a tear, he said. “That number goes up to 56% and 51% in men and women aged 70-90 years, and 61% of these tears were in patients who were asymptomatic in the last month.”
In the setting of osteoarthritis, 76% of asymptomatic patients had a meniscus tear, and 91% of patients with symptomatic osteoarthritis had a meniscus tear, he added.
Treating knee pain
Treatment will vary depending on the underlying etiology of pain. For a possible meniscus tear, the recommendation is for a conservative intervention with ice, ibuprofen, knee immobilizer, and crutches, with a follow-up appointment in a week.
Three types of injections also can help:
- Cortisone for osteoarthritis or meniscus tears, swelling, and inflammation, and prophylaxis against inflammation.
- Viscosupplementation (intra‐articular hyaluronic acid) for chronic, baseline osteoarthritis symptoms.
- Regenerative therapies (platelet-rich plasma, stem cells, etc.) are used primarily for osteoarthritis (these do not regrow cartilage, but some patients report decreased pain).
The data on injections are mixed, Dr. Nakamoto said. For example, the results of a 2015 Cochrane review on cortisone injections for osteoarthritis reported that the benefits were small to moderate at 4‐6 weeks, and small to none at 13 weeks.
“There is a lot of controversy for viscosupplementation despite all of the data on it,” he said. “But the recommendations from professional organizations are mixed.”
He noted that he has been using viscosupplementation since the 1990s, and some patients do benefit from it.
Shoulder pain
The most common causes of shoulder pain are adhesive capsulitis, rotator cuff tears and tendinopathy, and impingement.
As with knee pain, the same assessment routine largely applies.
First, pinpoint the location: Is the trouble spot the lateral shoulder and upper arm, the trapezial ridge, or the shoulder blade?
Next, assess pain on movement: Does the patient experience discomfort reaching overhead or behind the back, or moving at the glenohumeral joint/capsule and engaging the rotator cuff? Check for stiffness, weakness, and decreased range of motion in the rotator cuff.
Determine if the cause of the pain is traumatic or atraumatic and stems from an acute injury versus degeneration or overuse.
As with the knee, imaging is a major component of the assessment and typically involves the use of x-ray. An MRI may be required for evaluating full- and partial-thickness tears and when contemplating surgery.
MRI also is necessary for evaluating cases of acute, traumatic shoulder injury, and patients exhibiting disability suggestive of a rotator cuff tear in an otherwise healthy tendon.
Some pain can be treated with cortisone injections or regenerative therapies, which generally are given at the acromioclavicular or glenohumeral joints or in the subacromial space. A 2005 meta-analysis found that subacromial injections of corticosteroids are effective for improvement for rotator cuff tendinitis up to a 9‐month period.
Surgery may be warranted in some cases, Dr. Nakamoto said. These include adhesive capsulitis, rotator cuff tear, acute traumatic injury in an otherwise healthy tendon, and chronic (or acute-on-chronic) tears in a degenerative tendon following a trial of conservative therapy.
A version of this article first appeared on Medscape.com.
Knee and shoulder pain are common complaints for patients in the primary care office.
But identifying the source of the pain can be complicated,
and an accurate diagnosis of the underlying cause of discomfort is key to appropriate management – whether that involves simple home care options of ice and rest or a recommendation for a follow-up with a specialist.
Speaking at the annual meeting of the American College of Physicians, Greg Nakamoto, MD, department of orthopedics, Virginia Mason Medical Center, Seattle, discussed common knee and shoulder problems that patients often present with in the primary care setting, and offered tips on diagnosis and appropriate management.
The most common conditions causing knee pain are osteoarthritis and meniscal tears. “The differential for knee pain is broad,” Dr. Nakamoto said. “You have to have a way to divide it down, such as if it’s acute or chronic.”
The initial workup has several key components. The first steps: Determine the location of the pain – anterior, medial, lateral, posterior – and then whether it stems from an injury or is atraumatic.
“If you have to ask one question – ask where it hurts,” he said. “And is it from an injury or just wear and tear? That helps me when deciding if surgery is needed.”
Pain in the knee generally localizes well to the site of pathology, and knee pain of acute traumatic onset requires more scrutiny for problems best treated with early surgery. “This also helps establish whether radiographic findings are due to injury or degeneration,” Dr. Nakamoto said. “The presence of swelling guides the need for anti-inflammatories or cortisone.”
Palpating for tenderness along the joint line is important, as is palpating above and below the joint line, Dr. Nakamoto said.
“Tenderness limited to the joint line, combined with a meniscal exam maneuver that reproduces joint-line pain, is suggestive of pain from meniscal pathology,” he said.
Imaging is an important component of evaluating knee symptoms, and the question often arises as to when to order an MRI.
Dr. Nakamoto offered the following scenario: If significant osteoarthritis is evident on weight-bearing x-ray, treat the patient for the condition. However, if little or no osteoarthritis appears on x-ray, and if the onset of symptoms was traumatic and both patient history and physical examination suggest a meniscal tear, order an MRI.
An early MRI also is needed if the patient has had either atraumatic or traumatic onset of symptoms and their history and physical exams are suspicious for a mechanically locked or locking meniscus. For suspicion of a ruptured quadriceps or patellar tendon or a stress fracture, an MRI is needed urgently.
An MRI would be ordered later if the patient’s symptoms have not improved significantly after 3 months of conservative management.
Dr. Nakamoto stressed how common undiagnosed meniscus tears are in the general population. A third of men aged 50-59 years and nearly 20% of women in that age group have a tear, he said. “That number goes up to 56% and 51% in men and women aged 70-90 years, and 61% of these tears were in patients who were asymptomatic in the last month.”
In the setting of osteoarthritis, 76% of asymptomatic patients had a meniscus tear, and 91% of patients with symptomatic osteoarthritis had a meniscus tear, he added.
Treating knee pain
Treatment will vary depending on the underlying etiology of pain. For a possible meniscus tear, the recommendation is for a conservative intervention with ice, ibuprofen, knee immobilizer, and crutches, with a follow-up appointment in a week.
Three types of injections also can help:
- Cortisone for osteoarthritis or meniscus tears, swelling, and inflammation, and prophylaxis against inflammation.
- Viscosupplementation (intra‐articular hyaluronic acid) for chronic, baseline osteoarthritis symptoms.
- Regenerative therapies (platelet-rich plasma, stem cells, etc.) are used primarily for osteoarthritis (these do not regrow cartilage, but some patients report decreased pain).
The data on injections are mixed, Dr. Nakamoto said. For example, the results of a 2015 Cochrane review on cortisone injections for osteoarthritis reported that the benefits were small to moderate at 4‐6 weeks, and small to none at 13 weeks.
“There is a lot of controversy for viscosupplementation despite all of the data on it,” he said. “But the recommendations from professional organizations are mixed.”
He noted that he has been using viscosupplementation since the 1990s, and some patients do benefit from it.
Shoulder pain
The most common causes of shoulder pain are adhesive capsulitis, rotator cuff tears and tendinopathy, and impingement.
As with knee pain, the same assessment routine largely applies.
First, pinpoint the location: Is the trouble spot the lateral shoulder and upper arm, the trapezial ridge, or the shoulder blade?
Next, assess pain on movement: Does the patient experience discomfort reaching overhead or behind the back, or moving at the glenohumeral joint/capsule and engaging the rotator cuff? Check for stiffness, weakness, and decreased range of motion in the rotator cuff.
Determine if the cause of the pain is traumatic or atraumatic and stems from an acute injury versus degeneration or overuse.
As with the knee, imaging is a major component of the assessment and typically involves the use of x-ray. An MRI may be required for evaluating full- and partial-thickness tears and when contemplating surgery.
MRI also is necessary for evaluating cases of acute, traumatic shoulder injury, and patients exhibiting disability suggestive of a rotator cuff tear in an otherwise healthy tendon.
Some pain can be treated with cortisone injections or regenerative therapies, which generally are given at the acromioclavicular or glenohumeral joints or in the subacromial space. A 2005 meta-analysis found that subacromial injections of corticosteroids are effective for improvement for rotator cuff tendinitis up to a 9‐month period.
Surgery may be warranted in some cases, Dr. Nakamoto said. These include adhesive capsulitis, rotator cuff tear, acute traumatic injury in an otherwise healthy tendon, and chronic (or acute-on-chronic) tears in a degenerative tendon following a trial of conservative therapy.
A version of this article first appeared on Medscape.com.
Knee and shoulder pain are common complaints for patients in the primary care office.
But identifying the source of the pain can be complicated,
and an accurate diagnosis of the underlying cause of discomfort is key to appropriate management – whether that involves simple home care options of ice and rest or a recommendation for a follow-up with a specialist.
Speaking at the annual meeting of the American College of Physicians, Greg Nakamoto, MD, department of orthopedics, Virginia Mason Medical Center, Seattle, discussed common knee and shoulder problems that patients often present with in the primary care setting, and offered tips on diagnosis and appropriate management.
The most common conditions causing knee pain are osteoarthritis and meniscal tears. “The differential for knee pain is broad,” Dr. Nakamoto said. “You have to have a way to divide it down, such as if it’s acute or chronic.”
The initial workup has several key components. The first steps: Determine the location of the pain – anterior, medial, lateral, posterior – and then whether it stems from an injury or is atraumatic.
“If you have to ask one question – ask where it hurts,” he said. “And is it from an injury or just wear and tear? That helps me when deciding if surgery is needed.”
Pain in the knee generally localizes well to the site of pathology, and knee pain of acute traumatic onset requires more scrutiny for problems best treated with early surgery. “This also helps establish whether radiographic findings are due to injury or degeneration,” Dr. Nakamoto said. “The presence of swelling guides the need for anti-inflammatories or cortisone.”
Palpating for tenderness along the joint line is important, as is palpating above and below the joint line, Dr. Nakamoto said.
“Tenderness limited to the joint line, combined with a meniscal exam maneuver that reproduces joint-line pain, is suggestive of pain from meniscal pathology,” he said.
Imaging is an important component of evaluating knee symptoms, and the question often arises as to when to order an MRI.
Dr. Nakamoto offered the following scenario: If significant osteoarthritis is evident on weight-bearing x-ray, treat the patient for the condition. However, if little or no osteoarthritis appears on x-ray, and if the onset of symptoms was traumatic and both patient history and physical examination suggest a meniscal tear, order an MRI.
An early MRI also is needed if the patient has had either atraumatic or traumatic onset of symptoms and their history and physical exams are suspicious for a mechanically locked or locking meniscus. For suspicion of a ruptured quadriceps or patellar tendon or a stress fracture, an MRI is needed urgently.
An MRI would be ordered later if the patient’s symptoms have not improved significantly after 3 months of conservative management.
Dr. Nakamoto stressed how common undiagnosed meniscus tears are in the general population. A third of men aged 50-59 years and nearly 20% of women in that age group have a tear, he said. “That number goes up to 56% and 51% in men and women aged 70-90 years, and 61% of these tears were in patients who were asymptomatic in the last month.”
In the setting of osteoarthritis, 76% of asymptomatic patients had a meniscus tear, and 91% of patients with symptomatic osteoarthritis had a meniscus tear, he added.
Treating knee pain
Treatment will vary depending on the underlying etiology of pain. For a possible meniscus tear, the recommendation is for a conservative intervention with ice, ibuprofen, knee immobilizer, and crutches, with a follow-up appointment in a week.
Three types of injections also can help:
- Cortisone for osteoarthritis or meniscus tears, swelling, and inflammation, and prophylaxis against inflammation.
- Viscosupplementation (intra‐articular hyaluronic acid) for chronic, baseline osteoarthritis symptoms.
- Regenerative therapies (platelet-rich plasma, stem cells, etc.) are used primarily for osteoarthritis (these do not regrow cartilage, but some patients report decreased pain).
The data on injections are mixed, Dr. Nakamoto said. For example, the results of a 2015 Cochrane review on cortisone injections for osteoarthritis reported that the benefits were small to moderate at 4‐6 weeks, and small to none at 13 weeks.
“There is a lot of controversy for viscosupplementation despite all of the data on it,” he said. “But the recommendations from professional organizations are mixed.”
He noted that he has been using viscosupplementation since the 1990s, and some patients do benefit from it.
Shoulder pain
The most common causes of shoulder pain are adhesive capsulitis, rotator cuff tears and tendinopathy, and impingement.
As with knee pain, the same assessment routine largely applies.
First, pinpoint the location: Is the trouble spot the lateral shoulder and upper arm, the trapezial ridge, or the shoulder blade?
Next, assess pain on movement: Does the patient experience discomfort reaching overhead or behind the back, or moving at the glenohumeral joint/capsule and engaging the rotator cuff? Check for stiffness, weakness, and decreased range of motion in the rotator cuff.
Determine if the cause of the pain is traumatic or atraumatic and stems from an acute injury versus degeneration or overuse.
As with the knee, imaging is a major component of the assessment and typically involves the use of x-ray. An MRI may be required for evaluating full- and partial-thickness tears and when contemplating surgery.
MRI also is necessary for evaluating cases of acute, traumatic shoulder injury, and patients exhibiting disability suggestive of a rotator cuff tear in an otherwise healthy tendon.
Some pain can be treated with cortisone injections or regenerative therapies, which generally are given at the acromioclavicular or glenohumeral joints or in the subacromial space. A 2005 meta-analysis found that subacromial injections of corticosteroids are effective for improvement for rotator cuff tendinitis up to a 9‐month period.
Surgery may be warranted in some cases, Dr. Nakamoto said. These include adhesive capsulitis, rotator cuff tear, acute traumatic injury in an otherwise healthy tendon, and chronic (or acute-on-chronic) tears in a degenerative tendon following a trial of conservative therapy.
A version of this article first appeared on Medscape.com.
FROM INTERNAL MEDICINE 2022
New blood biomarker to detect early dementia?
Investigators found that plasma concentrations of 2-aminoethyl dihydrogen phosphate and taurine could distinguish adults with early-stage Alzheimer’s disease from cognitively normal adults.
“Our biomarker for early-stage Alzheimer’s disease represents new thinking and is unique from the amyloid-beta and p-tau molecules that are currently being investigated to diagnose AD,” Sandra Banack, PhD, senior scientist, Brain Chemistry Labs, Jackson, Wyoming, told this news organization.
If further studies pan out, Dr. Banack said this biomarker could “easily be transformed into a test to aid clinical evaluations for Alzheimer’s disease.”
The study was published online in PLOS ONE.
New drug target?
The researchers measured concentrations of 2-aminoethyl dihydrogen phosphate and taurine in blood plasma samples in 25 patients (21 men; mean age, 71) with a clinical diagnosis of early-stage Alzheimer’s based on a Clinical Dementia Rating (CDR) score of 0.5, suggesting very mild cognitive impairment, and 25 healthy controls (20 men; mean age, 39).
The concentration of 2-aminoethyl dihydrogen phosphate, normalized by the concentration of taurine, reliably distinguished blood samples of early-stage Alzheimer’s patients from controls in a blinded analysis.
This biomarker “could lead to new understanding of [AD] and lead to new drug candidates,” Dr. Banack told this news organization.
The researchers note that 2-aminoethyl dihydrogen phosphate plays an important role in the structure and function of cellular membranes.
Physiologic effects of increased 2-aminoethyl dihydrogen phosphate concentrations in the blood are not known. However, in one study, concentrations of this molecule were found to be significantly lower in the temporal cortex, frontal cortex, and hippocampus (40%) in patients with Alzheimer’s disease, compared with controls.
“New biomarkers take time before they can be implemented in the clinic. The next step will be to repeat the experiments using a large sample size of AD patient blood samples,” Dr. Banack told this news organization.
The study team is looking to source a larger sample size of AD blood samples to replicate these findings. They are also examining this biomarker relative to other neurodegenerative diseases.
“If verified with larger sample sizes, the quantification of 2-aminoethyl dihydrogen phosphate could potentially assist in the diagnosis of early-stage Alzheimer’s disease when used in conjunction with the patient’s CDR score and other potential AD biomarkers,” Dr. Banack and colleagues say.
Caveats, cautionary notes
Commenting on the findings, Rebecca M. Edelmayer, PhD, Alzheimer’s Association senior director of scientific engagement, said the study is “interesting, though very small-scale and very preliminary.”
Dr. Edelmayer said one “major limitation” is that participants did not have their Alzheimer’s diagnosis confirmed with “gold standard biomarkers. They have been diagnosed based only on their cognitive and behavioral symptoms.”
She also cautioned that the study population is not representative – either of the general public or people living with Alzheimer’s disease.
For example, 41 out of all 50 samples are from men, “though we know women are disproportionately impacted by Alzheimer’s.”
“There is a mismatch in the age of the study groups,” Dr. Edelmayer noted. The mean age of controls in the study was 39 and the mean age of people with dementia was 71. Race or ethnicity and other demographic information is also unclear from the article.
“There is an urgent need for simple, inexpensive, noninvasive and easily available diagnostic tools for Alzheimer’s, such as a blood test. A simple blood test for Alzheimer’s would be a great advance for individuals with – and at risk for – the disease, families, doctors, and researchers,” Dr. Edelmayer said.
“Bottom line,” Dr. Edelmayer continued, “these results need to be further tested and verified in long-term, large-scale studies with diverse populations that are representative of those living with Alzheimer’s disease.”
This research was supported by the William Stamps Farish Fund and the Josephine P. & John J. Louis Foundation. Brain Chemistry Labs has applied for a patent related to this research. Dr. Edelmayer has no relevant disclosures.
A version of this article first appeared on Medscape.com.
Investigators found that plasma concentrations of 2-aminoethyl dihydrogen phosphate and taurine could distinguish adults with early-stage Alzheimer’s disease from cognitively normal adults.
“Our biomarker for early-stage Alzheimer’s disease represents new thinking and is unique from the amyloid-beta and p-tau molecules that are currently being investigated to diagnose AD,” Sandra Banack, PhD, senior scientist, Brain Chemistry Labs, Jackson, Wyoming, told this news organization.
If further studies pan out, Dr. Banack said this biomarker could “easily be transformed into a test to aid clinical evaluations for Alzheimer’s disease.”
The study was published online in PLOS ONE.
New drug target?
The researchers measured concentrations of 2-aminoethyl dihydrogen phosphate and taurine in blood plasma samples in 25 patients (21 men; mean age, 71) with a clinical diagnosis of early-stage Alzheimer’s based on a Clinical Dementia Rating (CDR) score of 0.5, suggesting very mild cognitive impairment, and 25 healthy controls (20 men; mean age, 39).
The concentration of 2-aminoethyl dihydrogen phosphate, normalized by the concentration of taurine, reliably distinguished blood samples of early-stage Alzheimer’s patients from controls in a blinded analysis.
This biomarker “could lead to new understanding of [AD] and lead to new drug candidates,” Dr. Banack told this news organization.
The researchers note that 2-aminoethyl dihydrogen phosphate plays an important role in the structure and function of cellular membranes.
Physiologic effects of increased 2-aminoethyl dihydrogen phosphate concentrations in the blood are not known. However, in one study, concentrations of this molecule were found to be significantly lower in the temporal cortex, frontal cortex, and hippocampus (40%) in patients with Alzheimer’s disease, compared with controls.
“New biomarkers take time before they can be implemented in the clinic. The next step will be to repeat the experiments using a large sample size of AD patient blood samples,” Dr. Banack told this news organization.
The study team is looking to source a larger sample size of AD blood samples to replicate these findings. They are also examining this biomarker relative to other neurodegenerative diseases.
“If verified with larger sample sizes, the quantification of 2-aminoethyl dihydrogen phosphate could potentially assist in the diagnosis of early-stage Alzheimer’s disease when used in conjunction with the patient’s CDR score and other potential AD biomarkers,” Dr. Banack and colleagues say.
Caveats, cautionary notes
Commenting on the findings, Rebecca M. Edelmayer, PhD, Alzheimer’s Association senior director of scientific engagement, said the study is “interesting, though very small-scale and very preliminary.”
Dr. Edelmayer said one “major limitation” is that participants did not have their Alzheimer’s diagnosis confirmed with “gold standard biomarkers. They have been diagnosed based only on their cognitive and behavioral symptoms.”
She also cautioned that the study population is not representative – either of the general public or people living with Alzheimer’s disease.
For example, 41 out of all 50 samples are from men, “though we know women are disproportionately impacted by Alzheimer’s.”
“There is a mismatch in the age of the study groups,” Dr. Edelmayer noted. The mean age of controls in the study was 39 and the mean age of people with dementia was 71. Race or ethnicity and other demographic information is also unclear from the article.
“There is an urgent need for simple, inexpensive, noninvasive and easily available diagnostic tools for Alzheimer’s, such as a blood test. A simple blood test for Alzheimer’s would be a great advance for individuals with – and at risk for – the disease, families, doctors, and researchers,” Dr. Edelmayer said.
“Bottom line,” Dr. Edelmayer continued, “these results need to be further tested and verified in long-term, large-scale studies with diverse populations that are representative of those living with Alzheimer’s disease.”
This research was supported by the William Stamps Farish Fund and the Josephine P. & John J. Louis Foundation. Brain Chemistry Labs has applied for a patent related to this research. Dr. Edelmayer has no relevant disclosures.
A version of this article first appeared on Medscape.com.
Investigators found that plasma concentrations of 2-aminoethyl dihydrogen phosphate and taurine could distinguish adults with early-stage Alzheimer’s disease from cognitively normal adults.
“Our biomarker for early-stage Alzheimer’s disease represents new thinking and is unique from the amyloid-beta and p-tau molecules that are currently being investigated to diagnose AD,” Sandra Banack, PhD, senior scientist, Brain Chemistry Labs, Jackson, Wyoming, told this news organization.
If further studies pan out, Dr. Banack said this biomarker could “easily be transformed into a test to aid clinical evaluations for Alzheimer’s disease.”
The study was published online in PLOS ONE.
New drug target?
The researchers measured concentrations of 2-aminoethyl dihydrogen phosphate and taurine in blood plasma samples in 25 patients (21 men; mean age, 71) with a clinical diagnosis of early-stage Alzheimer’s based on a Clinical Dementia Rating (CDR) score of 0.5, suggesting very mild cognitive impairment, and 25 healthy controls (20 men; mean age, 39).
The concentration of 2-aminoethyl dihydrogen phosphate, normalized by the concentration of taurine, reliably distinguished blood samples of early-stage Alzheimer’s patients from controls in a blinded analysis.
This biomarker “could lead to new understanding of [AD] and lead to new drug candidates,” Dr. Banack told this news organization.
The researchers note that 2-aminoethyl dihydrogen phosphate plays an important role in the structure and function of cellular membranes.
Physiologic effects of increased 2-aminoethyl dihydrogen phosphate concentrations in the blood are not known. However, in one study, concentrations of this molecule were found to be significantly lower in the temporal cortex, frontal cortex, and hippocampus (40%) in patients with Alzheimer’s disease, compared with controls.
“New biomarkers take time before they can be implemented in the clinic. The next step will be to repeat the experiments using a large sample size of AD patient blood samples,” Dr. Banack told this news organization.
The study team is looking to source a larger sample size of AD blood samples to replicate these findings. They are also examining this biomarker relative to other neurodegenerative diseases.
“If verified with larger sample sizes, the quantification of 2-aminoethyl dihydrogen phosphate could potentially assist in the diagnosis of early-stage Alzheimer’s disease when used in conjunction with the patient’s CDR score and other potential AD biomarkers,” Dr. Banack and colleagues say.
Caveats, cautionary notes
Commenting on the findings, Rebecca M. Edelmayer, PhD, Alzheimer’s Association senior director of scientific engagement, said the study is “interesting, though very small-scale and very preliminary.”
Dr. Edelmayer said one “major limitation” is that participants did not have their Alzheimer’s diagnosis confirmed with “gold standard biomarkers. They have been diagnosed based only on their cognitive and behavioral symptoms.”
She also cautioned that the study population is not representative – either of the general public or people living with Alzheimer’s disease.
For example, 41 out of all 50 samples are from men, “though we know women are disproportionately impacted by Alzheimer’s.”
“There is a mismatch in the age of the study groups,” Dr. Edelmayer noted. The mean age of controls in the study was 39 and the mean age of people with dementia was 71. Race or ethnicity and other demographic information is also unclear from the article.
“There is an urgent need for simple, inexpensive, noninvasive and easily available diagnostic tools for Alzheimer’s, such as a blood test. A simple blood test for Alzheimer’s would be a great advance for individuals with – and at risk for – the disease, families, doctors, and researchers,” Dr. Edelmayer said.
“Bottom line,” Dr. Edelmayer continued, “these results need to be further tested and verified in long-term, large-scale studies with diverse populations that are representative of those living with Alzheimer’s disease.”
This research was supported by the William Stamps Farish Fund and the Josephine P. & John J. Louis Foundation. Brain Chemistry Labs has applied for a patent related to this research. Dr. Edelmayer has no relevant disclosures.
A version of this article first appeared on Medscape.com.