User login
Treating frail patients requires ‘precision gerontology’
An estimated 15% of Americans over age 65 years who aren’t living in institutions are considered frail – a complex geriatric syndrome that raises the odds of disability, hospitalization, the need for nursing care, and death.
But while the word frailty may conjure images of wizened and weakened men and women, the clinical picture is far less clear.
“We’ve made a lot of progress in some ways, but still a lot of work to be done in others,” George A. Kuchel, MD, CM, the chair in geriatrics and gerontology and director of the UConn Center on Aging in Farmington, Conn., said at the annual meeting of the American College of Physicians.
“You have to be very careful about generalizations,” Dr. Kuchel said. “This is very important when you are thinking about managing it.”
One of the key take-home messages, Dr. Kuchel said, “and one of the first things I learned as a geriatrics fellow, is that when you have seen one older person, all you have seen is one older person.”
The second major take-home is that frailty is multifactorial – a critical consideration when it comes to managing elderly patients.
“Unlike other conditions, there is no single medication, there is no one single thing you can do – it is really multifactorial,” he said. “What it means is to match the components to target unique needs, and that is something that we are calling ‘precision gerontology,’ as opposed to precision medicine.”
The definitions of frailty vary but can involve increased vulnerability; enhanced risk of declining function, disability, and death; and a decline in functioning across multiple physiologic systems, accompanied by an increased vulnerability to stressors.
Key features that clinicians should emphasize include multifactorial etiology with each risk factor contributing only modestly:
- Multidimensional nature, with physical and psychosocial factors playing a part.
- Frailty represents an extreme consequence of the normal aging process.
- The process is dynamic, and individuals can fluctuate between frailty states.
Diagnosing frailty
Diagnosing frailty in the average clinical setting can be a challenge. Unlike other disorders, no single test or assessment tool exists for the condition. Most settings or patients, for example, do not even have the device to measure hand grip strength, Dr. Kuchel said. Other obstacles include a lack of time and reimbursement.
However, clinicians can quickly and easily assess patients for several warning signs, including the presence of multimorbidity (>5 diseases), slow walking speed (<1 m/sec), inability to climb a flight of stairs, and/or walk a block or rise from chair five times with arms folded.
“These are simple questions that can be asked by a medical assistant or even over the phone ahead of time,” he said.
Frailty and sarcopenia are closely linked but are not equivalent. As a result, dual-energy x-ray absorptiometry (DXA), which can measure both bone mineral density and muscle mass, is not a good assessment of frailty because muscle mass by itself is not necessarily tied to weakness. Instead, Dr. Kuchel said, measuring muscle function and quality is much more effective at identifying frail patients.
“Gait velocity is potentially the greatest single measure, and if there is one thing you should do with your patient, it is to check gait velocity,” Dr. Kuchel said. Researchers at his facility are working on radio technology identification-based device that allows for measuring gait when a patient walks down the hallway.
“Measuring gait should be the sixth vital sign, and you need to have that information in front of you when working with older patients,” he said. “We are working on integrating it into our system.”
Managing frailty
Although no single intervention for frailty exists, physical activity has been shown to delay its onset. Still, Dr. Kuchel said, clinicians can try a range of approaches, both biologic and social, to address the condition.
Assessing for and treating depression, for example, may help reduce frailty fatigue, as can stopping medications – including benzodiazepines, and corticosteroids – that might be worsening the condition. Another step is to check for low vitamin D levels and hypothyroidism, he said.
Some patients have unexplained anemia that could be corrected, as well as correcting basal and orthostatic hypotension, which can arise from overtreatment, Dr. Kuchel added.
People with HIV can experience accelerated aging, as can adults who were treated with chemotherapy and radiation as children. “We are also beginning to see some of this with long COVID, so there seems to be some overlap,” he said.
Finally, socioeconomic considerations include the possibility of elder neglect and/or abuse, and the effects of poverty on nutrition and the ability to pay for needed medications.
The bottom line, Dr. Kuchel said, is that managing frailty is possible, but doing so effectively may require stops and starts.
“Correct what is correctable, such as nutrition, vitamin D, depression, and stopping offending meds,” he said. “Match multicomponent interventions with deficits and interventions targeting health care systems will include better care coordination. A comprehensive geriatric assessment is important in the care of this geriatric syndrome.
Dr. Kuchel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An estimated 15% of Americans over age 65 years who aren’t living in institutions are considered frail – a complex geriatric syndrome that raises the odds of disability, hospitalization, the need for nursing care, and death.
But while the word frailty may conjure images of wizened and weakened men and women, the clinical picture is far less clear.
“We’ve made a lot of progress in some ways, but still a lot of work to be done in others,” George A. Kuchel, MD, CM, the chair in geriatrics and gerontology and director of the UConn Center on Aging in Farmington, Conn., said at the annual meeting of the American College of Physicians.
“You have to be very careful about generalizations,” Dr. Kuchel said. “This is very important when you are thinking about managing it.”
One of the key take-home messages, Dr. Kuchel said, “and one of the first things I learned as a geriatrics fellow, is that when you have seen one older person, all you have seen is one older person.”
The second major take-home is that frailty is multifactorial – a critical consideration when it comes to managing elderly patients.
“Unlike other conditions, there is no single medication, there is no one single thing you can do – it is really multifactorial,” he said. “What it means is to match the components to target unique needs, and that is something that we are calling ‘precision gerontology,’ as opposed to precision medicine.”
The definitions of frailty vary but can involve increased vulnerability; enhanced risk of declining function, disability, and death; and a decline in functioning across multiple physiologic systems, accompanied by an increased vulnerability to stressors.
Key features that clinicians should emphasize include multifactorial etiology with each risk factor contributing only modestly:
- Multidimensional nature, with physical and psychosocial factors playing a part.
- Frailty represents an extreme consequence of the normal aging process.
- The process is dynamic, and individuals can fluctuate between frailty states.
Diagnosing frailty
Diagnosing frailty in the average clinical setting can be a challenge. Unlike other disorders, no single test or assessment tool exists for the condition. Most settings or patients, for example, do not even have the device to measure hand grip strength, Dr. Kuchel said. Other obstacles include a lack of time and reimbursement.
However, clinicians can quickly and easily assess patients for several warning signs, including the presence of multimorbidity (>5 diseases), slow walking speed (<1 m/sec), inability to climb a flight of stairs, and/or walk a block or rise from chair five times with arms folded.
“These are simple questions that can be asked by a medical assistant or even over the phone ahead of time,” he said.
Frailty and sarcopenia are closely linked but are not equivalent. As a result, dual-energy x-ray absorptiometry (DXA), which can measure both bone mineral density and muscle mass, is not a good assessment of frailty because muscle mass by itself is not necessarily tied to weakness. Instead, Dr. Kuchel said, measuring muscle function and quality is much more effective at identifying frail patients.
“Gait velocity is potentially the greatest single measure, and if there is one thing you should do with your patient, it is to check gait velocity,” Dr. Kuchel said. Researchers at his facility are working on radio technology identification-based device that allows for measuring gait when a patient walks down the hallway.
“Measuring gait should be the sixth vital sign, and you need to have that information in front of you when working with older patients,” he said. “We are working on integrating it into our system.”
Managing frailty
Although no single intervention for frailty exists, physical activity has been shown to delay its onset. Still, Dr. Kuchel said, clinicians can try a range of approaches, both biologic and social, to address the condition.
Assessing for and treating depression, for example, may help reduce frailty fatigue, as can stopping medications – including benzodiazepines, and corticosteroids – that might be worsening the condition. Another step is to check for low vitamin D levels and hypothyroidism, he said.
Some patients have unexplained anemia that could be corrected, as well as correcting basal and orthostatic hypotension, which can arise from overtreatment, Dr. Kuchel added.
People with HIV can experience accelerated aging, as can adults who were treated with chemotherapy and radiation as children. “We are also beginning to see some of this with long COVID, so there seems to be some overlap,” he said.
Finally, socioeconomic considerations include the possibility of elder neglect and/or abuse, and the effects of poverty on nutrition and the ability to pay for needed medications.
The bottom line, Dr. Kuchel said, is that managing frailty is possible, but doing so effectively may require stops and starts.
“Correct what is correctable, such as nutrition, vitamin D, depression, and stopping offending meds,” he said. “Match multicomponent interventions with deficits and interventions targeting health care systems will include better care coordination. A comprehensive geriatric assessment is important in the care of this geriatric syndrome.
Dr. Kuchel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An estimated 15% of Americans over age 65 years who aren’t living in institutions are considered frail – a complex geriatric syndrome that raises the odds of disability, hospitalization, the need for nursing care, and death.
But while the word frailty may conjure images of wizened and weakened men and women, the clinical picture is far less clear.
“We’ve made a lot of progress in some ways, but still a lot of work to be done in others,” George A. Kuchel, MD, CM, the chair in geriatrics and gerontology and director of the UConn Center on Aging in Farmington, Conn., said at the annual meeting of the American College of Physicians.
“You have to be very careful about generalizations,” Dr. Kuchel said. “This is very important when you are thinking about managing it.”
One of the key take-home messages, Dr. Kuchel said, “and one of the first things I learned as a geriatrics fellow, is that when you have seen one older person, all you have seen is one older person.”
The second major take-home is that frailty is multifactorial – a critical consideration when it comes to managing elderly patients.
“Unlike other conditions, there is no single medication, there is no one single thing you can do – it is really multifactorial,” he said. “What it means is to match the components to target unique needs, and that is something that we are calling ‘precision gerontology,’ as opposed to precision medicine.”
The definitions of frailty vary but can involve increased vulnerability; enhanced risk of declining function, disability, and death; and a decline in functioning across multiple physiologic systems, accompanied by an increased vulnerability to stressors.
Key features that clinicians should emphasize include multifactorial etiology with each risk factor contributing only modestly:
- Multidimensional nature, with physical and psychosocial factors playing a part.
- Frailty represents an extreme consequence of the normal aging process.
- The process is dynamic, and individuals can fluctuate between frailty states.
Diagnosing frailty
Diagnosing frailty in the average clinical setting can be a challenge. Unlike other disorders, no single test or assessment tool exists for the condition. Most settings or patients, for example, do not even have the device to measure hand grip strength, Dr. Kuchel said. Other obstacles include a lack of time and reimbursement.
However, clinicians can quickly and easily assess patients for several warning signs, including the presence of multimorbidity (>5 diseases), slow walking speed (<1 m/sec), inability to climb a flight of stairs, and/or walk a block or rise from chair five times with arms folded.
“These are simple questions that can be asked by a medical assistant or even over the phone ahead of time,” he said.
Frailty and sarcopenia are closely linked but are not equivalent. As a result, dual-energy x-ray absorptiometry (DXA), which can measure both bone mineral density and muscle mass, is not a good assessment of frailty because muscle mass by itself is not necessarily tied to weakness. Instead, Dr. Kuchel said, measuring muscle function and quality is much more effective at identifying frail patients.
“Gait velocity is potentially the greatest single measure, and if there is one thing you should do with your patient, it is to check gait velocity,” Dr. Kuchel said. Researchers at his facility are working on radio technology identification-based device that allows for measuring gait when a patient walks down the hallway.
“Measuring gait should be the sixth vital sign, and you need to have that information in front of you when working with older patients,” he said. “We are working on integrating it into our system.”
Managing frailty
Although no single intervention for frailty exists, physical activity has been shown to delay its onset. Still, Dr. Kuchel said, clinicians can try a range of approaches, both biologic and social, to address the condition.
Assessing for and treating depression, for example, may help reduce frailty fatigue, as can stopping medications – including benzodiazepines, and corticosteroids – that might be worsening the condition. Another step is to check for low vitamin D levels and hypothyroidism, he said.
Some patients have unexplained anemia that could be corrected, as well as correcting basal and orthostatic hypotension, which can arise from overtreatment, Dr. Kuchel added.
People with HIV can experience accelerated aging, as can adults who were treated with chemotherapy and radiation as children. “We are also beginning to see some of this with long COVID, so there seems to be some overlap,” he said.
Finally, socioeconomic considerations include the possibility of elder neglect and/or abuse, and the effects of poverty on nutrition and the ability to pay for needed medications.
The bottom line, Dr. Kuchel said, is that managing frailty is possible, but doing so effectively may require stops and starts.
“Correct what is correctable, such as nutrition, vitamin D, depression, and stopping offending meds,” he said. “Match multicomponent interventions with deficits and interventions targeting health care systems will include better care coordination. A comprehensive geriatric assessment is important in the care of this geriatric syndrome.
Dr. Kuchel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM INTERNAL MEDICINE 2022
Long-COVID symptoms a serious challenge for older patients, physicians
Even mundane tasks such as making a meal can be exhausting for Louise Salant.
“I’m totally wiped out,” said the 71-year-old former private music instructor with asthma who lives in New York City and has been coping with debilitating symptoms of fatigue, shortness of breath, and gastrointestinal symptoms since recovering from a severe bout of COVID-19 2 years ago. “I just don’t have the energy.”
Ms. Salant is not alone. Many older people who contract COVID-19 experience prolonged symptoms of the disease. An analysis of Medicare Advantage claims data published in the BMJ found that about one-third of roughly 87,000 adults aged 65 in the database with a COVID-19 diagnosis sought care for persistent or new symptoms 21 or more days later.
That figure is about twice the rate of persistent COVID-19 related symptoms seen in a cohort of adults younger than age 65 with commercial insurance analyzed by the same group of researchers in a separate BMJ study. Compared with a 2020 comparator group of patients in this age cohort, these patients had a greater likelihood of respiratory failure, fatigue, hypertension, memory problems, kidney injury, mental health conditions, hypercoagulability, and cardiac rhythm disorders. When they compared post–COVID-19 symptoms to lasting symptoms of another serious viral disease – influenza – the researchers found that only respiratory failure, dementia, and post-viral fatigue were more common in the COVID-19 group.
“It became clear early in the pandemic that there is going to be a second pandemic related to all of the complications that we’ve seen related to COVID-19 infections,” said Ken Cohen, MD, executive director of translational research and national senior medical director for Optum Labs in Minnetonka, Minn., who coauthored the BMJ studies.
The results are among a growing body of evidence suggesting that older adults are at high risk of persistent post-COVID-19 symptoms.
Researchers in Rome, for example, found that 83% of 165 patients aged 65 or older who had been hospitalized for COVID-19 reported at least one lasting symptom – problems like fatigue, shortness of breath, joint pain, and coughing – in the months after hospitalization. One-third of those had two symptoms, and 46% had three or more.
A similar study in Norway found that two-thirds of patients aged 60 or older reported reduced health-related quality of life during follow-up visits 6 months after hospitalization for COVID-19. The most-reported impairments among those patients were the inability to perform the tasks of daily life, reduced mobility, and increased pain and discomfort.
Cognitive concerns
Mounting evidence indicates that COVID-19 may contribute to chronic cognitive impairment in older adults. A multisite U.S. study found that 28% of 817 adults presenting to emergency departments with COVID-19 had delirium and poorer outcomes. A Chinese case-control study that enrolled 1,438 individuals hospitalized in Wuhan for COVID-19, along with 438 of their uninfected spouses, found that 12% of COVID-19 survivors experienced cognitive impairment a year after discharge. Matteo Tosato, MD, PhD, head of the outpatient clinic for patients with long COVID symptoms at Gemelli Hospital in Rome, called those findings “very concerning.”
Jin Ho Han, MD, associate professor of emergency medicine at Vanderbilt University, Nashville, Tenn., said cognitive impairment is common after an acute illness, particularly in frail or vulnerable patients.
“Hospitalization and the acute illness itself accelerate cognitive decline,” said Dr. Han, and previous evidence links delirium with worsening cognition. He and his colleagues are studying the potential role of delirium in longer-term cognitive decline in older patients after COVID-19.
Dr. Han emphasized the importance of preventing COVID-19-related delirium through vaccines and other strategies to reduce exposure of older patients to the virus. “Once you have cognitive decline, there are no interventions to reverse it,” he said.
Alarm bells for long-term care
Experts expressed concern that the situation might be even worse for people living in long-term care facilities. Many already need assistance with tasks of daily living and could be particularly vulnerable to lasting effects of COVID-19, said Karl Steinberg, MD, president of the Society for Post-Acute and Long-Term Care Medicine. He estimated that roughly half of his patients who have had COVID-19, regardless of the severity of their symptoms, have endured some degree of functional decline.
“It’s common for long-term care facility residents to experience functional and cognitive decline, even after seemingly minor things, like a cold or a trip to the hospital,” Dr. Steinberg, who has been a medical director of long-term care facilities in San Diego County for more than 2 decades, told this news organization. “It makes it a little harder to determine whether the declines we’ve been seeing post COVID in these residents are attributable to post COVID versus just an accelerated step in their overall expected decline.”
The pandemic may have contributed to worse outcomes for people in long-term care facilities in several ways: the disease itself, its effects on health care delivery, and necessary preventive measures to protect long-term care residents from exposure to the virus.
“During the many months where family visits were prohibited, we saw people – whether they had COVID-19 or not – suffer major clinical, functional, cognitive declines or severe psychological symptoms,” Dr. Steinberg said.
He emphasized the importance of preventive measures such as vaccines and boosters in patients in long-term care facilities. He said the benefit of preventing lasting symptoms is often a strong motivator for family caregivers of people with dementia to get them vaccinated or boosted.
“It’s clear that vaccination and booster reduce the incidence of post-COVID symptoms,” he said. Almost all studies have been in younger cohorts, but he expects the benefits would also apply to older patients.
Easing symptoms and offering support
As with long COVID generally, many questions remain about the causes of lasting symptoms of COVID-19 in older patients, and how best to treat them. Dr. Tosato, who led the study of long-COVID patients in Rome, is focusing on inflammation as a critical factor in the condition. He and colleagues across Europe hope to answer some of them by launching a multicenter study of lasting COVID-19 symptoms.
In the meantime, Dr. Steinberg and Dr. Tosato said they are doing their best to evaluate and treat patients empirically.
“We pull from our armamentarium to treat system-specific symptoms,” Dr. Steinberg said. “We want to improve the quality of life and help each day be the best it can.”
Physicians in long-term care facilities might use medications such as antidepressants or nonpharmacologic approaches for patients experiencing depression symptoms. Families are also crucial in helping patients by bringing in home-cooked meals and encouraging loved ones who may be experiencing loss of taste or smell to eat, Dr. Steinberg said.
“We’ve seen with the return of families and loved ones visiting to some extent has alleviated some people’s symptoms, especially psychological ones,” he said.
Dr. Tosato said he and his colleagues start with an individualized, multidisciplinary assessment to determine what types of care may help. He noted that physicians might recommend medications or rehabilitative therapies depending on the patient’s needs.
“A personalized approach is key,” Dr. Tosato said. His study also found that the proportion of older patients experiencing symptoms declined over time – a glimmer of hope that many will recover.
Dr. Cohen emphasized the need for a multimodal rehabilitation, an evidence-based approach used to care for patients who survived hospitalization with severe COVID-19 – a group that has substantially higher rates of persistent symptoms. This approach includes cognitive rehabilitation, physical therapy, occupational therapy, and a graded exercise program.
Dr. Han and colleagues are studying potential therapies such as cognitive rehabilitation in adults who’ve experienced delirium. But until evidence-based treatments are available, they stress the role of support for patients with cognitive decline and their families.
“A lot of the work we do is teach patients and their families to compensate for newly acquired cognitive deficits from any illness, including COVID-19,” Dr. Han said.
Ms. Salant said she has experienced some improvement in her energy since her pulmonologist recommended a new inhaler based on her symptoms. Her sense of smell and taste, lost to the infection, returned after she received her first dose of a vaccine against COVID-19. She takes comfort in participating in Survivor Corps, a group of more than 170,000 COVID-19 survivors and their families who advocate for more scientific research on the disease.
She also expressed gratitude for the support she receives from her primary care physician, who she said has taken the time to learn more about the symptoms of long COVID, listens to her, and respects what she has to say.
“I have hope that I will keep getting better by baby steps,” Ms. Salant said.
Dr. Tosato, Dr. Steinberg, and Dr. Han have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Even mundane tasks such as making a meal can be exhausting for Louise Salant.
“I’m totally wiped out,” said the 71-year-old former private music instructor with asthma who lives in New York City and has been coping with debilitating symptoms of fatigue, shortness of breath, and gastrointestinal symptoms since recovering from a severe bout of COVID-19 2 years ago. “I just don’t have the energy.”
Ms. Salant is not alone. Many older people who contract COVID-19 experience prolonged symptoms of the disease. An analysis of Medicare Advantage claims data published in the BMJ found that about one-third of roughly 87,000 adults aged 65 in the database with a COVID-19 diagnosis sought care for persistent or new symptoms 21 or more days later.
That figure is about twice the rate of persistent COVID-19 related symptoms seen in a cohort of adults younger than age 65 with commercial insurance analyzed by the same group of researchers in a separate BMJ study. Compared with a 2020 comparator group of patients in this age cohort, these patients had a greater likelihood of respiratory failure, fatigue, hypertension, memory problems, kidney injury, mental health conditions, hypercoagulability, and cardiac rhythm disorders. When they compared post–COVID-19 symptoms to lasting symptoms of another serious viral disease – influenza – the researchers found that only respiratory failure, dementia, and post-viral fatigue were more common in the COVID-19 group.
“It became clear early in the pandemic that there is going to be a second pandemic related to all of the complications that we’ve seen related to COVID-19 infections,” said Ken Cohen, MD, executive director of translational research and national senior medical director for Optum Labs in Minnetonka, Minn., who coauthored the BMJ studies.
The results are among a growing body of evidence suggesting that older adults are at high risk of persistent post-COVID-19 symptoms.
Researchers in Rome, for example, found that 83% of 165 patients aged 65 or older who had been hospitalized for COVID-19 reported at least one lasting symptom – problems like fatigue, shortness of breath, joint pain, and coughing – in the months after hospitalization. One-third of those had two symptoms, and 46% had three or more.
A similar study in Norway found that two-thirds of patients aged 60 or older reported reduced health-related quality of life during follow-up visits 6 months after hospitalization for COVID-19. The most-reported impairments among those patients were the inability to perform the tasks of daily life, reduced mobility, and increased pain and discomfort.
Cognitive concerns
Mounting evidence indicates that COVID-19 may contribute to chronic cognitive impairment in older adults. A multisite U.S. study found that 28% of 817 adults presenting to emergency departments with COVID-19 had delirium and poorer outcomes. A Chinese case-control study that enrolled 1,438 individuals hospitalized in Wuhan for COVID-19, along with 438 of their uninfected spouses, found that 12% of COVID-19 survivors experienced cognitive impairment a year after discharge. Matteo Tosato, MD, PhD, head of the outpatient clinic for patients with long COVID symptoms at Gemelli Hospital in Rome, called those findings “very concerning.”
Jin Ho Han, MD, associate professor of emergency medicine at Vanderbilt University, Nashville, Tenn., said cognitive impairment is common after an acute illness, particularly in frail or vulnerable patients.
“Hospitalization and the acute illness itself accelerate cognitive decline,” said Dr. Han, and previous evidence links delirium with worsening cognition. He and his colleagues are studying the potential role of delirium in longer-term cognitive decline in older patients after COVID-19.
Dr. Han emphasized the importance of preventing COVID-19-related delirium through vaccines and other strategies to reduce exposure of older patients to the virus. “Once you have cognitive decline, there are no interventions to reverse it,” he said.
Alarm bells for long-term care
Experts expressed concern that the situation might be even worse for people living in long-term care facilities. Many already need assistance with tasks of daily living and could be particularly vulnerable to lasting effects of COVID-19, said Karl Steinberg, MD, president of the Society for Post-Acute and Long-Term Care Medicine. He estimated that roughly half of his patients who have had COVID-19, regardless of the severity of their symptoms, have endured some degree of functional decline.
“It’s common for long-term care facility residents to experience functional and cognitive decline, even after seemingly minor things, like a cold or a trip to the hospital,” Dr. Steinberg, who has been a medical director of long-term care facilities in San Diego County for more than 2 decades, told this news organization. “It makes it a little harder to determine whether the declines we’ve been seeing post COVID in these residents are attributable to post COVID versus just an accelerated step in their overall expected decline.”
The pandemic may have contributed to worse outcomes for people in long-term care facilities in several ways: the disease itself, its effects on health care delivery, and necessary preventive measures to protect long-term care residents from exposure to the virus.
“During the many months where family visits were prohibited, we saw people – whether they had COVID-19 or not – suffer major clinical, functional, cognitive declines or severe psychological symptoms,” Dr. Steinberg said.
He emphasized the importance of preventive measures such as vaccines and boosters in patients in long-term care facilities. He said the benefit of preventing lasting symptoms is often a strong motivator for family caregivers of people with dementia to get them vaccinated or boosted.
“It’s clear that vaccination and booster reduce the incidence of post-COVID symptoms,” he said. Almost all studies have been in younger cohorts, but he expects the benefits would also apply to older patients.
Easing symptoms and offering support
As with long COVID generally, many questions remain about the causes of lasting symptoms of COVID-19 in older patients, and how best to treat them. Dr. Tosato, who led the study of long-COVID patients in Rome, is focusing on inflammation as a critical factor in the condition. He and colleagues across Europe hope to answer some of them by launching a multicenter study of lasting COVID-19 symptoms.
In the meantime, Dr. Steinberg and Dr. Tosato said they are doing their best to evaluate and treat patients empirically.
“We pull from our armamentarium to treat system-specific symptoms,” Dr. Steinberg said. “We want to improve the quality of life and help each day be the best it can.”
Physicians in long-term care facilities might use medications such as antidepressants or nonpharmacologic approaches for patients experiencing depression symptoms. Families are also crucial in helping patients by bringing in home-cooked meals and encouraging loved ones who may be experiencing loss of taste or smell to eat, Dr. Steinberg said.
“We’ve seen with the return of families and loved ones visiting to some extent has alleviated some people’s symptoms, especially psychological ones,” he said.
Dr. Tosato said he and his colleagues start with an individualized, multidisciplinary assessment to determine what types of care may help. He noted that physicians might recommend medications or rehabilitative therapies depending on the patient’s needs.
“A personalized approach is key,” Dr. Tosato said. His study also found that the proportion of older patients experiencing symptoms declined over time – a glimmer of hope that many will recover.
Dr. Cohen emphasized the need for a multimodal rehabilitation, an evidence-based approach used to care for patients who survived hospitalization with severe COVID-19 – a group that has substantially higher rates of persistent symptoms. This approach includes cognitive rehabilitation, physical therapy, occupational therapy, and a graded exercise program.
Dr. Han and colleagues are studying potential therapies such as cognitive rehabilitation in adults who’ve experienced delirium. But until evidence-based treatments are available, they stress the role of support for patients with cognitive decline and their families.
“A lot of the work we do is teach patients and their families to compensate for newly acquired cognitive deficits from any illness, including COVID-19,” Dr. Han said.
Ms. Salant said she has experienced some improvement in her energy since her pulmonologist recommended a new inhaler based on her symptoms. Her sense of smell and taste, lost to the infection, returned after she received her first dose of a vaccine against COVID-19. She takes comfort in participating in Survivor Corps, a group of more than 170,000 COVID-19 survivors and their families who advocate for more scientific research on the disease.
She also expressed gratitude for the support she receives from her primary care physician, who she said has taken the time to learn more about the symptoms of long COVID, listens to her, and respects what she has to say.
“I have hope that I will keep getting better by baby steps,” Ms. Salant said.
Dr. Tosato, Dr. Steinberg, and Dr. Han have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Even mundane tasks such as making a meal can be exhausting for Louise Salant.
“I’m totally wiped out,” said the 71-year-old former private music instructor with asthma who lives in New York City and has been coping with debilitating symptoms of fatigue, shortness of breath, and gastrointestinal symptoms since recovering from a severe bout of COVID-19 2 years ago. “I just don’t have the energy.”
Ms. Salant is not alone. Many older people who contract COVID-19 experience prolonged symptoms of the disease. An analysis of Medicare Advantage claims data published in the BMJ found that about one-third of roughly 87,000 adults aged 65 in the database with a COVID-19 diagnosis sought care for persistent or new symptoms 21 or more days later.
That figure is about twice the rate of persistent COVID-19 related symptoms seen in a cohort of adults younger than age 65 with commercial insurance analyzed by the same group of researchers in a separate BMJ study. Compared with a 2020 comparator group of patients in this age cohort, these patients had a greater likelihood of respiratory failure, fatigue, hypertension, memory problems, kidney injury, mental health conditions, hypercoagulability, and cardiac rhythm disorders. When they compared post–COVID-19 symptoms to lasting symptoms of another serious viral disease – influenza – the researchers found that only respiratory failure, dementia, and post-viral fatigue were more common in the COVID-19 group.
“It became clear early in the pandemic that there is going to be a second pandemic related to all of the complications that we’ve seen related to COVID-19 infections,” said Ken Cohen, MD, executive director of translational research and national senior medical director for Optum Labs in Minnetonka, Minn., who coauthored the BMJ studies.
The results are among a growing body of evidence suggesting that older adults are at high risk of persistent post-COVID-19 symptoms.
Researchers in Rome, for example, found that 83% of 165 patients aged 65 or older who had been hospitalized for COVID-19 reported at least one lasting symptom – problems like fatigue, shortness of breath, joint pain, and coughing – in the months after hospitalization. One-third of those had two symptoms, and 46% had three or more.
A similar study in Norway found that two-thirds of patients aged 60 or older reported reduced health-related quality of life during follow-up visits 6 months after hospitalization for COVID-19. The most-reported impairments among those patients were the inability to perform the tasks of daily life, reduced mobility, and increased pain and discomfort.
Cognitive concerns
Mounting evidence indicates that COVID-19 may contribute to chronic cognitive impairment in older adults. A multisite U.S. study found that 28% of 817 adults presenting to emergency departments with COVID-19 had delirium and poorer outcomes. A Chinese case-control study that enrolled 1,438 individuals hospitalized in Wuhan for COVID-19, along with 438 of their uninfected spouses, found that 12% of COVID-19 survivors experienced cognitive impairment a year after discharge. Matteo Tosato, MD, PhD, head of the outpatient clinic for patients with long COVID symptoms at Gemelli Hospital in Rome, called those findings “very concerning.”
Jin Ho Han, MD, associate professor of emergency medicine at Vanderbilt University, Nashville, Tenn., said cognitive impairment is common after an acute illness, particularly in frail or vulnerable patients.
“Hospitalization and the acute illness itself accelerate cognitive decline,” said Dr. Han, and previous evidence links delirium with worsening cognition. He and his colleagues are studying the potential role of delirium in longer-term cognitive decline in older patients after COVID-19.
Dr. Han emphasized the importance of preventing COVID-19-related delirium through vaccines and other strategies to reduce exposure of older patients to the virus. “Once you have cognitive decline, there are no interventions to reverse it,” he said.
Alarm bells for long-term care
Experts expressed concern that the situation might be even worse for people living in long-term care facilities. Many already need assistance with tasks of daily living and could be particularly vulnerable to lasting effects of COVID-19, said Karl Steinberg, MD, president of the Society for Post-Acute and Long-Term Care Medicine. He estimated that roughly half of his patients who have had COVID-19, regardless of the severity of their symptoms, have endured some degree of functional decline.
“It’s common for long-term care facility residents to experience functional and cognitive decline, even after seemingly minor things, like a cold or a trip to the hospital,” Dr. Steinberg, who has been a medical director of long-term care facilities in San Diego County for more than 2 decades, told this news organization. “It makes it a little harder to determine whether the declines we’ve been seeing post COVID in these residents are attributable to post COVID versus just an accelerated step in their overall expected decline.”
The pandemic may have contributed to worse outcomes for people in long-term care facilities in several ways: the disease itself, its effects on health care delivery, and necessary preventive measures to protect long-term care residents from exposure to the virus.
“During the many months where family visits were prohibited, we saw people – whether they had COVID-19 or not – suffer major clinical, functional, cognitive declines or severe psychological symptoms,” Dr. Steinberg said.
He emphasized the importance of preventive measures such as vaccines and boosters in patients in long-term care facilities. He said the benefit of preventing lasting symptoms is often a strong motivator for family caregivers of people with dementia to get them vaccinated or boosted.
“It’s clear that vaccination and booster reduce the incidence of post-COVID symptoms,” he said. Almost all studies have been in younger cohorts, but he expects the benefits would also apply to older patients.
Easing symptoms and offering support
As with long COVID generally, many questions remain about the causes of lasting symptoms of COVID-19 in older patients, and how best to treat them. Dr. Tosato, who led the study of long-COVID patients in Rome, is focusing on inflammation as a critical factor in the condition. He and colleagues across Europe hope to answer some of them by launching a multicenter study of lasting COVID-19 symptoms.
In the meantime, Dr. Steinberg and Dr. Tosato said they are doing their best to evaluate and treat patients empirically.
“We pull from our armamentarium to treat system-specific symptoms,” Dr. Steinberg said. “We want to improve the quality of life and help each day be the best it can.”
Physicians in long-term care facilities might use medications such as antidepressants or nonpharmacologic approaches for patients experiencing depression symptoms. Families are also crucial in helping patients by bringing in home-cooked meals and encouraging loved ones who may be experiencing loss of taste or smell to eat, Dr. Steinberg said.
“We’ve seen with the return of families and loved ones visiting to some extent has alleviated some people’s symptoms, especially psychological ones,” he said.
Dr. Tosato said he and his colleagues start with an individualized, multidisciplinary assessment to determine what types of care may help. He noted that physicians might recommend medications or rehabilitative therapies depending on the patient’s needs.
“A personalized approach is key,” Dr. Tosato said. His study also found that the proportion of older patients experiencing symptoms declined over time – a glimmer of hope that many will recover.
Dr. Cohen emphasized the need for a multimodal rehabilitation, an evidence-based approach used to care for patients who survived hospitalization with severe COVID-19 – a group that has substantially higher rates of persistent symptoms. This approach includes cognitive rehabilitation, physical therapy, occupational therapy, and a graded exercise program.
Dr. Han and colleagues are studying potential therapies such as cognitive rehabilitation in adults who’ve experienced delirium. But until evidence-based treatments are available, they stress the role of support for patients with cognitive decline and their families.
“A lot of the work we do is teach patients and their families to compensate for newly acquired cognitive deficits from any illness, including COVID-19,” Dr. Han said.
Ms. Salant said she has experienced some improvement in her energy since her pulmonologist recommended a new inhaler based on her symptoms. Her sense of smell and taste, lost to the infection, returned after she received her first dose of a vaccine against COVID-19. She takes comfort in participating in Survivor Corps, a group of more than 170,000 COVID-19 survivors and their families who advocate for more scientific research on the disease.
She also expressed gratitude for the support she receives from her primary care physician, who she said has taken the time to learn more about the symptoms of long COVID, listens to her, and respects what she has to say.
“I have hope that I will keep getting better by baby steps,” Ms. Salant said.
Dr. Tosato, Dr. Steinberg, and Dr. Han have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Virtual reality an ‘exciting opportunity’ for geriatric psychiatry
Researchers are increasingly turning their attention to virtual reality (VR) for the treatment of psychiatric disorders in older adults.
Recent studies have highlighted the usefulness of VR in treating depression and loneliness in older patients who may be socially isolated because of their age, comorbidities, or the COVID-19 pandemic.
“The unique capability of virtual reality to create an immersive and engaging setting is an exciting opportunity for geriatric psychiatry,” Harmehr Sekhon, PhD, postdoctoral research fellow, Lady Davis Institute/Jewish General Hospital, McGill University, Montreal, and McLean Hospital, Harvard Medical School, Boston, told this news organization.
, Dr. Sekhon said.
One novel approach involves using VR to administer a mindfulness intervention in older adults. Dr. Sekhon shared information on her own mindfulness study and on other developments in VR and telemedicine at the American Association for Geriatric Psychiatry annual meeting.
Potential bridging tool
As the population ages, the prevalence of mental health disorders increases. Telemedicine has proved to be a potential “bridge” to address the health care needs of older adults, Dr. Sekhon noted.
She cited her systematic review of telemedicine for older adults with dementia during COVID-19. Results showed that telemedicine was a “beneficial approach” to assisting these individuals and that it increased accessibility, said Dr. Sekhon.
In addition, a survey published last year showed that 87% of Americans in general want to continue using telehealth services after the pandemic. Most respondents agreed that telehealth had made it easier to get the care they needed. They also reported having received the same level of care via telehealth as with in-person care.
A growing body of research shows that VR has “positive influences on mood and well-being, cognition, pain management, [and] treatment of phobias in younger adults,” Dr. Sekhon said. She added that there is evidence that VR is feasible for older adults, with applications in cognitive disorders.
She cited a recent systematic review of 55 studies that assessed the impact of different types of VR on mental health in older adults. The results showed that VR could be helpful in screening for cognitive impairment – and it was comparable to some paper-based assessment. It was also useful as a training tool for those with cognitive impairment.
Examples of VR interventions that can be used to treat cognitive impairment include “virtual cities, kitchens, supermarkets,” Dr. Sekhon noted.
The technology is increasingly being used as a tool to deliver psychotherapy, in which patient engagement is “a key determinant” of outcomes, she added. “Virtual reality is a cutting-edge, engaging, and immersive technique to administer psychotherapy,” she said.
Such VR approaches are proving successful in older patients. Dr. Sekhon highlighted the case of an 85-year-old woman who engaged in ten sessions of psychodynamic psychotherapy that targeted persistent dysthymia and negativistic mood. The case was part of a proof-of-concept study published in the May issue of the American Journal of Geriatric Psychiatry.
Dr. Sekhon noted the intervention was well tolerated and was associated with minimal side effects.
VR-based meditation
Dr. Sekhon and her colleagues are now conducting a randomized controlled trial of VR meditation in older adults. VR-based meditation has been shown to increase relaxation and to decrease anxiety, sadness, and anger in younger adults. However, it has not been studied in the geriatric population.
The pilot study is assessing the feasibility and tolerability of VR meditation for older adults and its effects on stress, anxiety, depression, sleep, and quality of life. The study involves 30 adults aged 60 years and older.
Participants receive either 15-minute VR mindfulness meditation sessions twice a week for 4 weeks or are on a control wait list. The meditation sessions are user friendly and focus on breath meditation and body scans, Dr. Sekhon reported.
Because participants are older and balance is a concern, safety steps are incorporated into the sessions. “We ensure they’re doing this in a seated position, in a chair with arm rests, so that they’re very stable and there’s no risk of falls,” said Dr. Sekhon.
Another concern with VR is motion sickness, she noted. “It’s pretty minimal, but the best way we found so far is giving older adults time to adapt and feel comfortable with the VR,” she said. From the first session, participants learn how to put on the device and are checked to make sure they are comfortable with the process. To help them get used to everything, video and audio are not included during the first session.
Dr. Sekhon noted that results from the study are expected later this year.
In addition to mindfulness, researchers are using VR to deliver other established interventions, such as exposure therapy – and are implementing these approaches in varied environments, including long-term and palliative care settings.
VR-related technology is constantly improving and is becoming easier to use and more affordable, said Dr. Sekhon. She noted that the simplest devices that rely on smartphones cost as little as $15.
Although VR in older adults is promising, there are barriers to its adoption and use in research, she noted. For example, older adults may have cognitive, visual, or hearing impairments. They may have limited digital literacy, and/or they may not have access to the required technology.
These barriers can be overcome through workarounds, including providing instructional videos and digital literacy assistance via Zoom and working with community partners to facilitate study recruitment of older patients, Dr. Sekhon said.
Dr. Sekhon’s research is funded by the Canadian Institutes of Health Research and the Fonds de recherche du Quebec Sante.
A version of this article first appeared on Medscape.com.
Researchers are increasingly turning their attention to virtual reality (VR) for the treatment of psychiatric disorders in older adults.
Recent studies have highlighted the usefulness of VR in treating depression and loneliness in older patients who may be socially isolated because of their age, comorbidities, or the COVID-19 pandemic.
“The unique capability of virtual reality to create an immersive and engaging setting is an exciting opportunity for geriatric psychiatry,” Harmehr Sekhon, PhD, postdoctoral research fellow, Lady Davis Institute/Jewish General Hospital, McGill University, Montreal, and McLean Hospital, Harvard Medical School, Boston, told this news organization.
, Dr. Sekhon said.
One novel approach involves using VR to administer a mindfulness intervention in older adults. Dr. Sekhon shared information on her own mindfulness study and on other developments in VR and telemedicine at the American Association for Geriatric Psychiatry annual meeting.
Potential bridging tool
As the population ages, the prevalence of mental health disorders increases. Telemedicine has proved to be a potential “bridge” to address the health care needs of older adults, Dr. Sekhon noted.
She cited her systematic review of telemedicine for older adults with dementia during COVID-19. Results showed that telemedicine was a “beneficial approach” to assisting these individuals and that it increased accessibility, said Dr. Sekhon.
In addition, a survey published last year showed that 87% of Americans in general want to continue using telehealth services after the pandemic. Most respondents agreed that telehealth had made it easier to get the care they needed. They also reported having received the same level of care via telehealth as with in-person care.
A growing body of research shows that VR has “positive influences on mood and well-being, cognition, pain management, [and] treatment of phobias in younger adults,” Dr. Sekhon said. She added that there is evidence that VR is feasible for older adults, with applications in cognitive disorders.
She cited a recent systematic review of 55 studies that assessed the impact of different types of VR on mental health in older adults. The results showed that VR could be helpful in screening for cognitive impairment – and it was comparable to some paper-based assessment. It was also useful as a training tool for those with cognitive impairment.
Examples of VR interventions that can be used to treat cognitive impairment include “virtual cities, kitchens, supermarkets,” Dr. Sekhon noted.
The technology is increasingly being used as a tool to deliver psychotherapy, in which patient engagement is “a key determinant” of outcomes, she added. “Virtual reality is a cutting-edge, engaging, and immersive technique to administer psychotherapy,” she said.
Such VR approaches are proving successful in older patients. Dr. Sekhon highlighted the case of an 85-year-old woman who engaged in ten sessions of psychodynamic psychotherapy that targeted persistent dysthymia and negativistic mood. The case was part of a proof-of-concept study published in the May issue of the American Journal of Geriatric Psychiatry.
Dr. Sekhon noted the intervention was well tolerated and was associated with minimal side effects.
VR-based meditation
Dr. Sekhon and her colleagues are now conducting a randomized controlled trial of VR meditation in older adults. VR-based meditation has been shown to increase relaxation and to decrease anxiety, sadness, and anger in younger adults. However, it has not been studied in the geriatric population.
The pilot study is assessing the feasibility and tolerability of VR meditation for older adults and its effects on stress, anxiety, depression, sleep, and quality of life. The study involves 30 adults aged 60 years and older.
Participants receive either 15-minute VR mindfulness meditation sessions twice a week for 4 weeks or are on a control wait list. The meditation sessions are user friendly and focus on breath meditation and body scans, Dr. Sekhon reported.
Because participants are older and balance is a concern, safety steps are incorporated into the sessions. “We ensure they’re doing this in a seated position, in a chair with arm rests, so that they’re very stable and there’s no risk of falls,” said Dr. Sekhon.
Another concern with VR is motion sickness, she noted. “It’s pretty minimal, but the best way we found so far is giving older adults time to adapt and feel comfortable with the VR,” she said. From the first session, participants learn how to put on the device and are checked to make sure they are comfortable with the process. To help them get used to everything, video and audio are not included during the first session.
Dr. Sekhon noted that results from the study are expected later this year.
In addition to mindfulness, researchers are using VR to deliver other established interventions, such as exposure therapy – and are implementing these approaches in varied environments, including long-term and palliative care settings.
VR-related technology is constantly improving and is becoming easier to use and more affordable, said Dr. Sekhon. She noted that the simplest devices that rely on smartphones cost as little as $15.
Although VR in older adults is promising, there are barriers to its adoption and use in research, she noted. For example, older adults may have cognitive, visual, or hearing impairments. They may have limited digital literacy, and/or they may not have access to the required technology.
These barriers can be overcome through workarounds, including providing instructional videos and digital literacy assistance via Zoom and working with community partners to facilitate study recruitment of older patients, Dr. Sekhon said.
Dr. Sekhon’s research is funded by the Canadian Institutes of Health Research and the Fonds de recherche du Quebec Sante.
A version of this article first appeared on Medscape.com.
Researchers are increasingly turning their attention to virtual reality (VR) for the treatment of psychiatric disorders in older adults.
Recent studies have highlighted the usefulness of VR in treating depression and loneliness in older patients who may be socially isolated because of their age, comorbidities, or the COVID-19 pandemic.
“The unique capability of virtual reality to create an immersive and engaging setting is an exciting opportunity for geriatric psychiatry,” Harmehr Sekhon, PhD, postdoctoral research fellow, Lady Davis Institute/Jewish General Hospital, McGill University, Montreal, and McLean Hospital, Harvard Medical School, Boston, told this news organization.
, Dr. Sekhon said.
One novel approach involves using VR to administer a mindfulness intervention in older adults. Dr. Sekhon shared information on her own mindfulness study and on other developments in VR and telemedicine at the American Association for Geriatric Psychiatry annual meeting.
Potential bridging tool
As the population ages, the prevalence of mental health disorders increases. Telemedicine has proved to be a potential “bridge” to address the health care needs of older adults, Dr. Sekhon noted.
She cited her systematic review of telemedicine for older adults with dementia during COVID-19. Results showed that telemedicine was a “beneficial approach” to assisting these individuals and that it increased accessibility, said Dr. Sekhon.
In addition, a survey published last year showed that 87% of Americans in general want to continue using telehealth services after the pandemic. Most respondents agreed that telehealth had made it easier to get the care they needed. They also reported having received the same level of care via telehealth as with in-person care.
A growing body of research shows that VR has “positive influences on mood and well-being, cognition, pain management, [and] treatment of phobias in younger adults,” Dr. Sekhon said. She added that there is evidence that VR is feasible for older adults, with applications in cognitive disorders.
She cited a recent systematic review of 55 studies that assessed the impact of different types of VR on mental health in older adults. The results showed that VR could be helpful in screening for cognitive impairment – and it was comparable to some paper-based assessment. It was also useful as a training tool for those with cognitive impairment.
Examples of VR interventions that can be used to treat cognitive impairment include “virtual cities, kitchens, supermarkets,” Dr. Sekhon noted.
The technology is increasingly being used as a tool to deliver psychotherapy, in which patient engagement is “a key determinant” of outcomes, she added. “Virtual reality is a cutting-edge, engaging, and immersive technique to administer psychotherapy,” she said.
Such VR approaches are proving successful in older patients. Dr. Sekhon highlighted the case of an 85-year-old woman who engaged in ten sessions of psychodynamic psychotherapy that targeted persistent dysthymia and negativistic mood. The case was part of a proof-of-concept study published in the May issue of the American Journal of Geriatric Psychiatry.
Dr. Sekhon noted the intervention was well tolerated and was associated with minimal side effects.
VR-based meditation
Dr. Sekhon and her colleagues are now conducting a randomized controlled trial of VR meditation in older adults. VR-based meditation has been shown to increase relaxation and to decrease anxiety, sadness, and anger in younger adults. However, it has not been studied in the geriatric population.
The pilot study is assessing the feasibility and tolerability of VR meditation for older adults and its effects on stress, anxiety, depression, sleep, and quality of life. The study involves 30 adults aged 60 years and older.
Participants receive either 15-minute VR mindfulness meditation sessions twice a week for 4 weeks or are on a control wait list. The meditation sessions are user friendly and focus on breath meditation and body scans, Dr. Sekhon reported.
Because participants are older and balance is a concern, safety steps are incorporated into the sessions. “We ensure they’re doing this in a seated position, in a chair with arm rests, so that they’re very stable and there’s no risk of falls,” said Dr. Sekhon.
Another concern with VR is motion sickness, she noted. “It’s pretty minimal, but the best way we found so far is giving older adults time to adapt and feel comfortable with the VR,” she said. From the first session, participants learn how to put on the device and are checked to make sure they are comfortable with the process. To help them get used to everything, video and audio are not included during the first session.
Dr. Sekhon noted that results from the study are expected later this year.
In addition to mindfulness, researchers are using VR to deliver other established interventions, such as exposure therapy – and are implementing these approaches in varied environments, including long-term and palliative care settings.
VR-related technology is constantly improving and is becoming easier to use and more affordable, said Dr. Sekhon. She noted that the simplest devices that rely on smartphones cost as little as $15.
Although VR in older adults is promising, there are barriers to its adoption and use in research, she noted. For example, older adults may have cognitive, visual, or hearing impairments. They may have limited digital literacy, and/or they may not have access to the required technology.
These barriers can be overcome through workarounds, including providing instructional videos and digital literacy assistance via Zoom and working with community partners to facilitate study recruitment of older patients, Dr. Sekhon said.
Dr. Sekhon’s research is funded by the Canadian Institutes of Health Research and the Fonds de recherche du Quebec Sante.
A version of this article first appeared on Medscape.com.
FROM AAGP 2022
Atypical anxiety offers intervention target in Parkinson’s disease
Anxiety is common in Parkinson’s disease (PD) and has been shown to increase functional disability and decrease quality of life, but atypical presentations of anxiety are underrecognized and often undertreated in PD patients, wrote Nadeeka N. Dissanayaka, PhD, of the University of Queensland, Brisbane, Australia, and colleagues.
In a study published in the American Journal of Geriatric Psychiatry , the researchers conducted a systematic review of 60 studies to better characterize atypical PD-related anxiety. Fourteen studies involved Anxiety Not Otherwise Specified (NOS), 31 included fluctuating anxiety symptoms, and 22 included Fear of Falling (FOF).
Overall, the average prevalence rate for anxiety disorders in the PD population was 31%.
Anxiety NOS, fluctuating anxiety, and FOF accounted for a weighted mean prevalence of 14.9%, 34.19%, and 51.5%, respectively.
The symptomatology of anxiety NOS included psychological distress about the PD diagnosis, insecurity about the future, fear of losing control of motor and bodily functions, and social embarrassment. Clinically, anxiety NOS was associated with a range of factors including minor depression, on-off motor symptoms, muscle cramps, poor quality of life, and gait impairment.
The symptomatology of fluctuating anxiety was assessed in 9 studies of the “on” motor state and 16 studies of both “on” and “off.” Symptoms associated with the off state included panic attacks, feeling anxious or sad, and avoiding situations, as well as palpitations, dizziness, chills, and hot flashes.
Clinically, studies showed that anxiety was more severe in the off-medication state, and symptoms were reduced in the on state. Data from some studies showed that fluctuating anxiety was more common in PD patients who were female, and who had a younger age of PD onset and longer disease duration.
The symptomatology of FOF included associations between FOF and difficulty with walking and gait: Using a walker or other device, more frequent freezing in place, hesitation when turning, and slower speed while walking. Clinically, characteristics associated with FOF included older age, needing assistance for activities of daily living, a history of falls, and reduced quality of life.
The results of the review were limited by several factors including the varying assessment techniques, and the lack of data on treatment for atypical anxiety in PD, the researchers noted. “To our knowledge there are no treatment trials focused on Anxiety NOS,” and studies on the treatment of fluctuating anxiety and FOF are preliminary, they said.
However, the results support the need for early identification and classification of PD-related anxiety to improve treatment strategies and long-term outcomes, the researchers concluded. In the absence of evidence-based treatment strategies, “Given the heterogeneity of anxiety presentations in PD, the importance of tailoring interventions to meet the specific needs and unique symptom profiles of each individual cannot be overstated,” and routine screening of PD patients for anxiety every 6-12 months is recommended, they emphasized.
Dr. Dissanayaka disclosed support from the National Health and Medical Research Boosting Dementia Research Leadership Fellowship.
Anxiety is common in Parkinson’s disease (PD) and has been shown to increase functional disability and decrease quality of life, but atypical presentations of anxiety are underrecognized and often undertreated in PD patients, wrote Nadeeka N. Dissanayaka, PhD, of the University of Queensland, Brisbane, Australia, and colleagues.
In a study published in the American Journal of Geriatric Psychiatry , the researchers conducted a systematic review of 60 studies to better characterize atypical PD-related anxiety. Fourteen studies involved Anxiety Not Otherwise Specified (NOS), 31 included fluctuating anxiety symptoms, and 22 included Fear of Falling (FOF).
Overall, the average prevalence rate for anxiety disorders in the PD population was 31%.
Anxiety NOS, fluctuating anxiety, and FOF accounted for a weighted mean prevalence of 14.9%, 34.19%, and 51.5%, respectively.
The symptomatology of anxiety NOS included psychological distress about the PD diagnosis, insecurity about the future, fear of losing control of motor and bodily functions, and social embarrassment. Clinically, anxiety NOS was associated with a range of factors including minor depression, on-off motor symptoms, muscle cramps, poor quality of life, and gait impairment.
The symptomatology of fluctuating anxiety was assessed in 9 studies of the “on” motor state and 16 studies of both “on” and “off.” Symptoms associated with the off state included panic attacks, feeling anxious or sad, and avoiding situations, as well as palpitations, dizziness, chills, and hot flashes.
Clinically, studies showed that anxiety was more severe in the off-medication state, and symptoms were reduced in the on state. Data from some studies showed that fluctuating anxiety was more common in PD patients who were female, and who had a younger age of PD onset and longer disease duration.
The symptomatology of FOF included associations between FOF and difficulty with walking and gait: Using a walker or other device, more frequent freezing in place, hesitation when turning, and slower speed while walking. Clinically, characteristics associated with FOF included older age, needing assistance for activities of daily living, a history of falls, and reduced quality of life.
The results of the review were limited by several factors including the varying assessment techniques, and the lack of data on treatment for atypical anxiety in PD, the researchers noted. “To our knowledge there are no treatment trials focused on Anxiety NOS,” and studies on the treatment of fluctuating anxiety and FOF are preliminary, they said.
However, the results support the need for early identification and classification of PD-related anxiety to improve treatment strategies and long-term outcomes, the researchers concluded. In the absence of evidence-based treatment strategies, “Given the heterogeneity of anxiety presentations in PD, the importance of tailoring interventions to meet the specific needs and unique symptom profiles of each individual cannot be overstated,” and routine screening of PD patients for anxiety every 6-12 months is recommended, they emphasized.
Dr. Dissanayaka disclosed support from the National Health and Medical Research Boosting Dementia Research Leadership Fellowship.
Anxiety is common in Parkinson’s disease (PD) and has been shown to increase functional disability and decrease quality of life, but atypical presentations of anxiety are underrecognized and often undertreated in PD patients, wrote Nadeeka N. Dissanayaka, PhD, of the University of Queensland, Brisbane, Australia, and colleagues.
In a study published in the American Journal of Geriatric Psychiatry , the researchers conducted a systematic review of 60 studies to better characterize atypical PD-related anxiety. Fourteen studies involved Anxiety Not Otherwise Specified (NOS), 31 included fluctuating anxiety symptoms, and 22 included Fear of Falling (FOF).
Overall, the average prevalence rate for anxiety disorders in the PD population was 31%.
Anxiety NOS, fluctuating anxiety, and FOF accounted for a weighted mean prevalence of 14.9%, 34.19%, and 51.5%, respectively.
The symptomatology of anxiety NOS included psychological distress about the PD diagnosis, insecurity about the future, fear of losing control of motor and bodily functions, and social embarrassment. Clinically, anxiety NOS was associated with a range of factors including minor depression, on-off motor symptoms, muscle cramps, poor quality of life, and gait impairment.
The symptomatology of fluctuating anxiety was assessed in 9 studies of the “on” motor state and 16 studies of both “on” and “off.” Symptoms associated with the off state included panic attacks, feeling anxious or sad, and avoiding situations, as well as palpitations, dizziness, chills, and hot flashes.
Clinically, studies showed that anxiety was more severe in the off-medication state, and symptoms were reduced in the on state. Data from some studies showed that fluctuating anxiety was more common in PD patients who were female, and who had a younger age of PD onset and longer disease duration.
The symptomatology of FOF included associations between FOF and difficulty with walking and gait: Using a walker or other device, more frequent freezing in place, hesitation when turning, and slower speed while walking. Clinically, characteristics associated with FOF included older age, needing assistance for activities of daily living, a history of falls, and reduced quality of life.
The results of the review were limited by several factors including the varying assessment techniques, and the lack of data on treatment for atypical anxiety in PD, the researchers noted. “To our knowledge there are no treatment trials focused on Anxiety NOS,” and studies on the treatment of fluctuating anxiety and FOF are preliminary, they said.
However, the results support the need for early identification and classification of PD-related anxiety to improve treatment strategies and long-term outcomes, the researchers concluded. In the absence of evidence-based treatment strategies, “Given the heterogeneity of anxiety presentations in PD, the importance of tailoring interventions to meet the specific needs and unique symptom profiles of each individual cannot be overstated,” and routine screening of PD patients for anxiety every 6-12 months is recommended, they emphasized.
Dr. Dissanayaka disclosed support from the National Health and Medical Research Boosting Dementia Research Leadership Fellowship.
FROM THE AMERICAN JOURNAL OF GERIATRIC PSYCHIATRY
‘Alarming, unexpected’ rate of suicidal behavior in long-term care residents
In a meta-analysis that included 20 studies and more than 3 million total individuals living in long-term care (LTC), the prevalence rate for suicidal behavior was more than 6%. In addition, the most common of these behaviors was suicidal ideation.
The prevalence was much higher in women than in men.
These high rates underline the need for clinicians to exercise “extra caution” when assessing elderly people living in a long-term care facility, coinvestigator Syeda Beenish Bareeqa, MBBS, clinical researcher, Jinnah Medical and Dental College, Karachi, Pakistan, and research observer, University of Texas Southwestern Medical Center, Dallas, said in an interview.
“Missed diagnoses or undertreatment in this population can lead to deleterious health outcomes,” Dr. Bareeqa said.
The findings were presented at the annual meeting of the American Association for Geriatric Psychiatry.
Underdiagnosed, undertreated
In the United States, about 42% of adults 70 years and older will live in LTC, either in an assisted care facility or a nursing home, Dr. Bareeqa noted.
Although many LTC residents have a mood disorder, previous research shows that fewer than 25% of cases are diagnosed and treated, she said.
Dr. Bareeqa added that suicide – and its association with factors such as the COVID-19 pandemic, depression, and cyberbullying – is a topic of increasing interest to researchers. She and her colleagues wanted to investigate suicidal behaviors in the setting of LTC.
The researchers conducted a literature search for studies of suicidal behavior among LTC residents over aged 60 years. They examined general suicidal behavior and the most common subtypes: suicide ideation, suicide attempts, completed suicide, self-destructive behavior, and nonsuicidal self-injury.
The analysis included 20 studies and 3 million individuals living in LTC. The majority of the studies were conducted in the United States (n = 5) and Australia (n = 4).
Results showed an estimated suicidal behavior prevalence rate of 6.4% (.064; 95% confidence interval, .057 to .070), or 64 per 100,000 persons.
A rate this high is “alarming and unexpected,” said Dr. Bareeqa. She noted most of the studies included in the analysis were conducted in developed countries with advanced health care systems.
The World Health Organization reports the suicide rate per 100,000 older adults (aged 75 years and older) is 50 for men and 16 for women, but this is not stratified by living settings, Dr. Bareeqa noted.
Higher rates in women
In the current analysis, 5 of the 20 studies had low risk of bias, 14 had moderate risk, and 1 had high risk, Dr. Bareeqa reported.
In subgroup analyses, the researchers found much of the suicidal behavior was driven by studies out of Australia, where the prevalence of suicidal behaviors was 36.9% (95% CI, 9.2-64.7) vs. 1.4% in the U.S. (95% CI, 1.0-1.8).
Another surprising finding was the prevalence of suicidal behaviors among women (15.8%), which was much higher than among men (7.9%). “Male gender is a well-established risk factor for suicide in the medical literature but this is not the case in our study,” said Dr. Bareeqa.
In addition, the analysis showed suicidal ideation was the most common type of suicidal behavior. In a pooled population of around 2 million people in eight studies, the prevalence of suicidal ideation was 12%.
For psychiatric illnesses accompanying suicidal behavior, the prevalence of depression alone was 14.4%, which was much higher than the rate of 5.1% for multiple comorbidities – including depression, anxiety, obsessive-compulsive disorder, psychotic disorder, history of previous suicide attempt, delusion, delirium, and hallucination.
Although depression and other psychiatric conditions may help explain suicidal behavior in older adults, Dr. Bareeqa said physical illness also plays a major role.
“Illnesses like cancer or end-stage organ failure, which are quite common with advancing age, are debilitating and in some instances incurable. These medical problems create a breeding ground for mental health problems and can eventually lead to devastating outcomes such as suicide,” she said.
She noted the importance of a “multipronged approach” to prevent suicide among older people in LTC facilities.
In addition, her research team aims to assess the quality of care provided by LTC facilities. “Maybe we can get to the root of this problem and devise strategies to improve it,” she said.
‘Not uncommon’
In an interview with this news organization Rajesh R. Tampi, MBBS, professor and chairman, department of psychiatry, Creighton University and Catholic Health Initiatives Health Behavioral Health Services, Omaha, Neb., said the results suggest that, despite the risk for bias among the included studies, “suicidal behaviors are not uncommon among older adults in LTC.”
The analysis describes only associations “but does not indicate causality,” said Dr. Tampi, past president of the AAGP. He was not involved with the research.
Additional subgroup analyses should yield information on possible risk factors for suicidal behaviors in LTC, such as depression, anxiety, and chronic pain, he added.
A version of this article first appeared on Medscape.com.
In a meta-analysis that included 20 studies and more than 3 million total individuals living in long-term care (LTC), the prevalence rate for suicidal behavior was more than 6%. In addition, the most common of these behaviors was suicidal ideation.
The prevalence was much higher in women than in men.
These high rates underline the need for clinicians to exercise “extra caution” when assessing elderly people living in a long-term care facility, coinvestigator Syeda Beenish Bareeqa, MBBS, clinical researcher, Jinnah Medical and Dental College, Karachi, Pakistan, and research observer, University of Texas Southwestern Medical Center, Dallas, said in an interview.
“Missed diagnoses or undertreatment in this population can lead to deleterious health outcomes,” Dr. Bareeqa said.
The findings were presented at the annual meeting of the American Association for Geriatric Psychiatry.
Underdiagnosed, undertreated
In the United States, about 42% of adults 70 years and older will live in LTC, either in an assisted care facility or a nursing home, Dr. Bareeqa noted.
Although many LTC residents have a mood disorder, previous research shows that fewer than 25% of cases are diagnosed and treated, she said.
Dr. Bareeqa added that suicide – and its association with factors such as the COVID-19 pandemic, depression, and cyberbullying – is a topic of increasing interest to researchers. She and her colleagues wanted to investigate suicidal behaviors in the setting of LTC.
The researchers conducted a literature search for studies of suicidal behavior among LTC residents over aged 60 years. They examined general suicidal behavior and the most common subtypes: suicide ideation, suicide attempts, completed suicide, self-destructive behavior, and nonsuicidal self-injury.
The analysis included 20 studies and 3 million individuals living in LTC. The majority of the studies were conducted in the United States (n = 5) and Australia (n = 4).
Results showed an estimated suicidal behavior prevalence rate of 6.4% (.064; 95% confidence interval, .057 to .070), or 64 per 100,000 persons.
A rate this high is “alarming and unexpected,” said Dr. Bareeqa. She noted most of the studies included in the analysis were conducted in developed countries with advanced health care systems.
The World Health Organization reports the suicide rate per 100,000 older adults (aged 75 years and older) is 50 for men and 16 for women, but this is not stratified by living settings, Dr. Bareeqa noted.
Higher rates in women
In the current analysis, 5 of the 20 studies had low risk of bias, 14 had moderate risk, and 1 had high risk, Dr. Bareeqa reported.
In subgroup analyses, the researchers found much of the suicidal behavior was driven by studies out of Australia, where the prevalence of suicidal behaviors was 36.9% (95% CI, 9.2-64.7) vs. 1.4% in the U.S. (95% CI, 1.0-1.8).
Another surprising finding was the prevalence of suicidal behaviors among women (15.8%), which was much higher than among men (7.9%). “Male gender is a well-established risk factor for suicide in the medical literature but this is not the case in our study,” said Dr. Bareeqa.
In addition, the analysis showed suicidal ideation was the most common type of suicidal behavior. In a pooled population of around 2 million people in eight studies, the prevalence of suicidal ideation was 12%.
For psychiatric illnesses accompanying suicidal behavior, the prevalence of depression alone was 14.4%, which was much higher than the rate of 5.1% for multiple comorbidities – including depression, anxiety, obsessive-compulsive disorder, psychotic disorder, history of previous suicide attempt, delusion, delirium, and hallucination.
Although depression and other psychiatric conditions may help explain suicidal behavior in older adults, Dr. Bareeqa said physical illness also plays a major role.
“Illnesses like cancer or end-stage organ failure, which are quite common with advancing age, are debilitating and in some instances incurable. These medical problems create a breeding ground for mental health problems and can eventually lead to devastating outcomes such as suicide,” she said.
She noted the importance of a “multipronged approach” to prevent suicide among older people in LTC facilities.
In addition, her research team aims to assess the quality of care provided by LTC facilities. “Maybe we can get to the root of this problem and devise strategies to improve it,” she said.
‘Not uncommon’
In an interview with this news organization Rajesh R. Tampi, MBBS, professor and chairman, department of psychiatry, Creighton University and Catholic Health Initiatives Health Behavioral Health Services, Omaha, Neb., said the results suggest that, despite the risk for bias among the included studies, “suicidal behaviors are not uncommon among older adults in LTC.”
The analysis describes only associations “but does not indicate causality,” said Dr. Tampi, past president of the AAGP. He was not involved with the research.
Additional subgroup analyses should yield information on possible risk factors for suicidal behaviors in LTC, such as depression, anxiety, and chronic pain, he added.
A version of this article first appeared on Medscape.com.
In a meta-analysis that included 20 studies and more than 3 million total individuals living in long-term care (LTC), the prevalence rate for suicidal behavior was more than 6%. In addition, the most common of these behaviors was suicidal ideation.
The prevalence was much higher in women than in men.
These high rates underline the need for clinicians to exercise “extra caution” when assessing elderly people living in a long-term care facility, coinvestigator Syeda Beenish Bareeqa, MBBS, clinical researcher, Jinnah Medical and Dental College, Karachi, Pakistan, and research observer, University of Texas Southwestern Medical Center, Dallas, said in an interview.
“Missed diagnoses or undertreatment in this population can lead to deleterious health outcomes,” Dr. Bareeqa said.
The findings were presented at the annual meeting of the American Association for Geriatric Psychiatry.
Underdiagnosed, undertreated
In the United States, about 42% of adults 70 years and older will live in LTC, either in an assisted care facility or a nursing home, Dr. Bareeqa noted.
Although many LTC residents have a mood disorder, previous research shows that fewer than 25% of cases are diagnosed and treated, she said.
Dr. Bareeqa added that suicide – and its association with factors such as the COVID-19 pandemic, depression, and cyberbullying – is a topic of increasing interest to researchers. She and her colleagues wanted to investigate suicidal behaviors in the setting of LTC.
The researchers conducted a literature search for studies of suicidal behavior among LTC residents over aged 60 years. They examined general suicidal behavior and the most common subtypes: suicide ideation, suicide attempts, completed suicide, self-destructive behavior, and nonsuicidal self-injury.
The analysis included 20 studies and 3 million individuals living in LTC. The majority of the studies were conducted in the United States (n = 5) and Australia (n = 4).
Results showed an estimated suicidal behavior prevalence rate of 6.4% (.064; 95% confidence interval, .057 to .070), or 64 per 100,000 persons.
A rate this high is “alarming and unexpected,” said Dr. Bareeqa. She noted most of the studies included in the analysis were conducted in developed countries with advanced health care systems.
The World Health Organization reports the suicide rate per 100,000 older adults (aged 75 years and older) is 50 for men and 16 for women, but this is not stratified by living settings, Dr. Bareeqa noted.
Higher rates in women
In the current analysis, 5 of the 20 studies had low risk of bias, 14 had moderate risk, and 1 had high risk, Dr. Bareeqa reported.
In subgroup analyses, the researchers found much of the suicidal behavior was driven by studies out of Australia, where the prevalence of suicidal behaviors was 36.9% (95% CI, 9.2-64.7) vs. 1.4% in the U.S. (95% CI, 1.0-1.8).
Another surprising finding was the prevalence of suicidal behaviors among women (15.8%), which was much higher than among men (7.9%). “Male gender is a well-established risk factor for suicide in the medical literature but this is not the case in our study,” said Dr. Bareeqa.
In addition, the analysis showed suicidal ideation was the most common type of suicidal behavior. In a pooled population of around 2 million people in eight studies, the prevalence of suicidal ideation was 12%.
For psychiatric illnesses accompanying suicidal behavior, the prevalence of depression alone was 14.4%, which was much higher than the rate of 5.1% for multiple comorbidities – including depression, anxiety, obsessive-compulsive disorder, psychotic disorder, history of previous suicide attempt, delusion, delirium, and hallucination.
Although depression and other psychiatric conditions may help explain suicidal behavior in older adults, Dr. Bareeqa said physical illness also plays a major role.
“Illnesses like cancer or end-stage organ failure, which are quite common with advancing age, are debilitating and in some instances incurable. These medical problems create a breeding ground for mental health problems and can eventually lead to devastating outcomes such as suicide,” she said.
She noted the importance of a “multipronged approach” to prevent suicide among older people in LTC facilities.
In addition, her research team aims to assess the quality of care provided by LTC facilities. “Maybe we can get to the root of this problem and devise strategies to improve it,” she said.
‘Not uncommon’
In an interview with this news organization Rajesh R. Tampi, MBBS, professor and chairman, department of psychiatry, Creighton University and Catholic Health Initiatives Health Behavioral Health Services, Omaha, Neb., said the results suggest that, despite the risk for bias among the included studies, “suicidal behaviors are not uncommon among older adults in LTC.”
The analysis describes only associations “but does not indicate causality,” said Dr. Tampi, past president of the AAGP. He was not involved with the research.
Additional subgroup analyses should yield information on possible risk factors for suicidal behaviors in LTC, such as depression, anxiety, and chronic pain, he added.
A version of this article first appeared on Medscape.com.
FROM AAGP 2022
Food for thought: Dangerous weight loss in an older adult
CASE Fixated on health and nutrition
At the insistence of her daughter, Ms. L, age 75, presents to the emergency department (ED) for self-neglect and severe weight loss, with a body mass index (BMI) of 13.5 kg/m2 (normal: 18.5 to 24.9 kg/m2). When asked why she is in the ED, Ms. L says she doesn’t know. She attributes her significant weight loss (approximately 20 pounds in the last few months) to gastroesophageal reflux disease (GERD). She constantly worries about her esophagus. She had been diagnosed with esophageal dysphagia 7 years ago after undergoing radiofrequency ablation for esophageal cancer. Ms. L fixates on the negative effects certain foods and ingredients might have on her stomach and esophagus.
Following transfer from the ED, Ms. L is involuntarily admitted to our inpatient unit. Although she acknowledges weight loss, she minimizes the severity of her illness and indicates she would like to gain weight, but only by eating healthy foods she is comfortable with, including kale, quinoa, and vegetables. Ms. L says that she has always been interested in “healthful foods” and that she “loves sugar,” but “it’s bad for you,” mentioning that “sugar fuels cancer.” She has daily thoughts about sugar causing cancer. Ms. L also mentions that she stopped eating flour, sugar, fried food, and oils because those foods affect her “stomach acid” and cause “pimples on my face and weight loss.” While in the inpatient unit, Ms. L requests a special diet and demands to know the origin and ingredients of the foods she is offered. She emphasizes that her esophageal cancer diagnosis and dysphagia exacerbate worries that certain foods cause cancer, and wants to continue her diet restrictions. Nonetheless, she says she wants to get healthy, and denies an intense fear of gaining weight or feeling fat.
HISTORY Multiple psychiatric diagnoses
Ms. L lives alone and enjoys spending time with her grandchildren, visiting museums, and listening to classical music. However, her family, social workers, and records from a previous psychiatric hospitalization reveal that Ms. L has a history of psychiatric illness and fears regarding certain types of foods for much of her adult life. Ms. L’s family also described a range of compulsive behaviors, including shoplifting, hoarding art, multiple plastic surgeries, and phases where Ms. L ate only frozen yogurt without sugar.
Ms. L’s daughter reported that Ms. L had seen a psychologist in the late 1990s for depression and had been diagnosed with obsessive-compulsive disorder (OCD) and attention deficit/hyperactivity disorder in the early 2000s. In 2006, during a depressive episode after her divorce, Ms. L had a suicide attempt with pills and alcohol, and was hospitalized. Records from that stay described a history of mood dysregulation with fears regarding food and nutrition. Ms. L was treated with aripiprazole 5 mg/d. A trial of trazodone 25 mg/d did not have any effect. When discharged, she was receiving lamotrigine 100 mg/d. However, her daughter believes she stopped taking all psychiatric medications shortly after discharge.
Her daughter says that in the past 2 years, Ms. L has seen multiple doctors for treatment of somatic gastrointestinal (GI) complaints. A 2018 note from a social worker indicated that Ms. L endorsed taking >80 supplements per day and constantly researched nutrition online. In the months leading to her current hospitalization, Ms. L suffered from severe self-neglect and fear regarding foods she felt were not healthy for her. She had stopped leaving her apartment.
Continue to: EVALUATION Poor insight, normal lab results...
EVALUATION Poor insight, normal lab results
During her evaluation, Ms. L appears cachectic and frail. She has a heavily constricted affect and is guarded, dismissive, and vague. Although her thought processes are linear and goal-directed, her insight into her condition is extremely poor and she appears surprised when clinicians inform her that her self-neglect would lead to death. Instead, Ms. L insists she is eating healthily and demonstrates severe anxiety in relation to her GI symptoms.
Ms. L is oriented to person, place, and time. She scores 27/30 on the Montreal Cognitive Assessment, indicating normal cognition. She denies any depressive symptoms or suicidal intent. She does not appear to be internally preoccupied and denies having auditory or visual hallucinations or manic symptoms.
A neurologic examination reveals that her cranial nerves are normal, and cerebellar function, strength, and sensory testing are intact. Her gait is steady and she walks without a walker. Despite her severely low BMI and recent history of self-neglect, Ms. L’s laboratory results are remarkably normal and show no liver, metabolic, or electrolyte abnormalities, no signs of infection, and normal vitamin B12 levels. She has slightly elevated creatinine and blood urea nitrogen levels, but a normal glomerular filtration rate.
Her medical history is significant for squamous cell esophageal cancer, treated with radiofrequency ablation. Although Ms. L is constantly worried about the recurrence of cancer, pathology reports demonstrate no esophageal dysplasia. However, she does show evidence of an approximately 1 cm × 1 cm mild, noncircumferential esophageal stenosis, likely resulting from radiofrequency ablation.
[polldaddy:11079394]
The authors’ observations
Several health- and physical symptom-related psychiatric disorders have overlapping features, which can complicate the differential diagnosis (Table 11). Ms. L presented to the ED with a severely low BMI of 13.5 kg/m2, obsessions regarding specific types of food, and preoccupations regarding her esophagus. Despite her extensive psychiatric history (including intense fears regarding food), we ruled out a primary psychotic disorder because she did not describe auditory or visual hallucinations and never appeared internally preoccupied. While her BMI and persistent minimization of the extent of her disease meet criteria for anorexia nervosa, she denied body dysmorphia and did not have any fear of gaining weight.
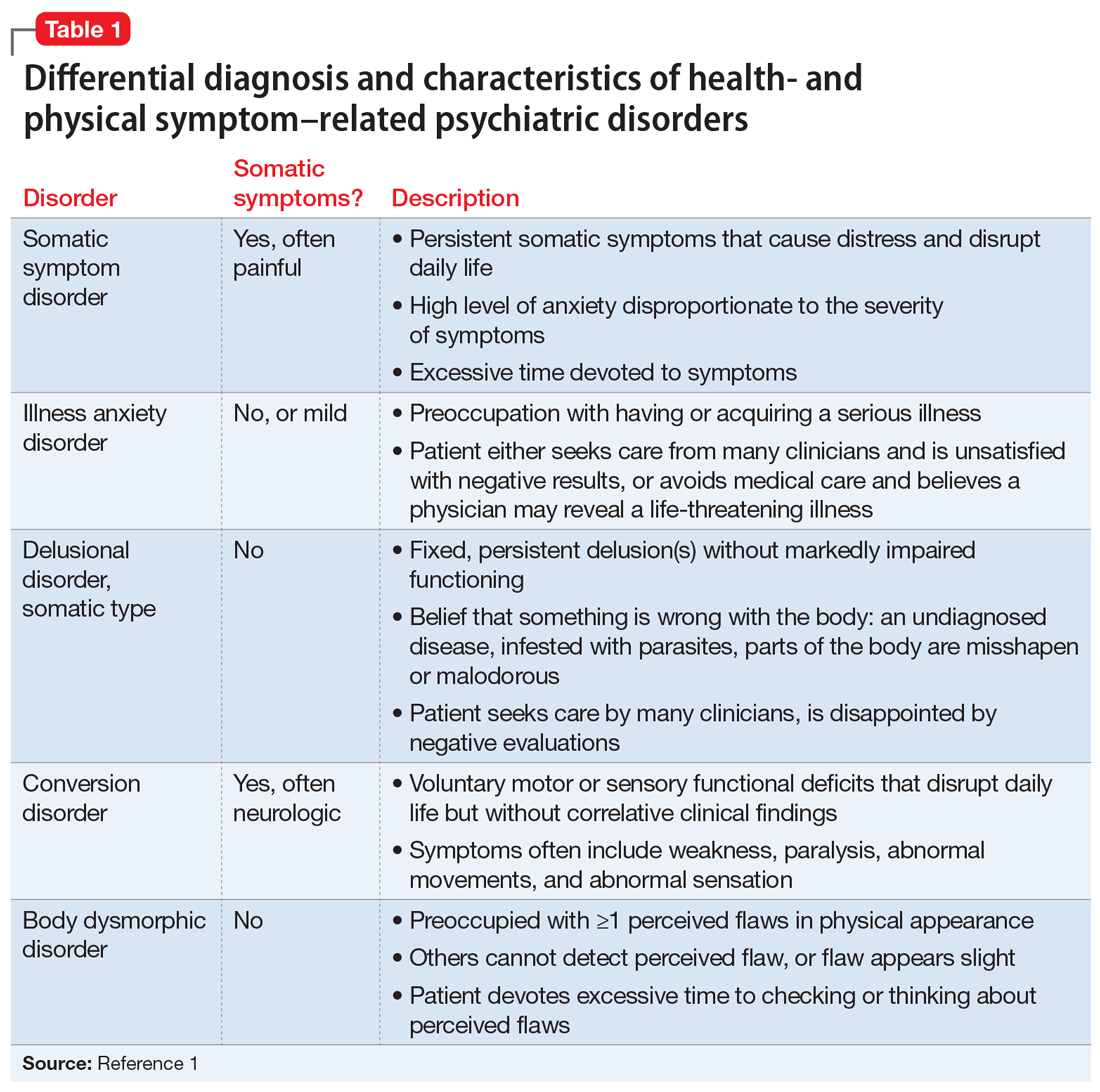
A central element of Ms. L’s presentation was her anxiety regarding how certain types of foods impact her health as well as her anxieties regarding her esophagus. While Ms. L was in remission from esophageal cancer and had a diagnosis of esophageal dysphagia, these preoccupations and obsessions regarding how certain types of foods affect her esophagus drove her to self-neglect and thus represent pathologic thought processes out of proportion to her symptoms. Illness anxiety disorder was considered because Ms. L met many of its criteria: preoccupation with having a serious illness, disproportionate preoccupation with somatic symptoms if they are present, extreme anxiety over health, and performance of health-related behaviors.1 However, illness anxiety disorder is a diagnosis of exclusion, and 1 criterion is that these symptoms cannot be explained by another mental disorder. We felt other diagnoses better fit Ms. L’s condition and ruled out illness anxiety disorder.
Ms. L’s long history of food and non-food–related obsessions and compulsions that interrupted her ability to perform daily activities were strongly suggestive for OCD. Additionally, her intense preoccupation, high level of anxiety, amount of time and energy spent seeking care for her esophagus and GERD symptoms, and the resulting significant disruption of daily life, met criteria for somatic symptom disorder (SSD). However, we did not believe that a diagnosis of OCD and SSD alone explained the entirety of Ms. L’s clinical picture. Despite ruling out anorexia nervosa, Ms. L nonetheless demonstrated disordered eating.
Avoidant/restrictive food intake disorder (ARFID) is an eating disorder in which patients restrict their diet and do not meet nutritional needs for any number of reasons, do not experience body dysmorphia, and do not fear weight gain.1 A common feature of ARFID is a fear of negative consequences from eating specific types of food.2 Table 21,2 summarizes additional clinical features of ARFID. Although ARFID is typically diagnosed in children and adolescents, particularly in individuals with autism with heightened sensory sensitivities, ARFID is also common among adult patients with GI disorders.3 In a retrospective chart review of 410 adults ages 18 to 90 (73% women) referred to a neurogastroenterology care center, 6.3% met the full criteria for ARFID and 17.3% had clinically significant avoidant or restrictive eating behaviors. Among patients with ARFID symptoms, 93% stated that a fear of GI symptoms was the driver of their avoidant or restrictive eating behaviors.2 Patients with GI diseases often develop dietary control and avoidance coping mechanisms to alleviate their symptoms.4 These strategies can exacerbate health anxieties and have a detrimental effect on mental health.5 Patients with GI disorders have a high degree of comorbidity with affective disorders, including anxiety disorders.6 These trends may arise from hypervigilance and the need to gain control over physical symptoms.7 Feeling a need for control, actions driven by anxiety and fear, and the need for compensatory behaviors are cardinal features of OCD and eating disorders.8 Multiple studies have demonstrated comorbidities between irritable bowel syndrome and eating disorders,9 SSD,10 and OCD.11 Taken together with observations that ARFID is also found in patients with GI disorders,2 these findings demonstrate that patients with a history of GI disease are at high risk of developing extreme health anxieties and behavioral coping strategies that can lead to disordered eating.
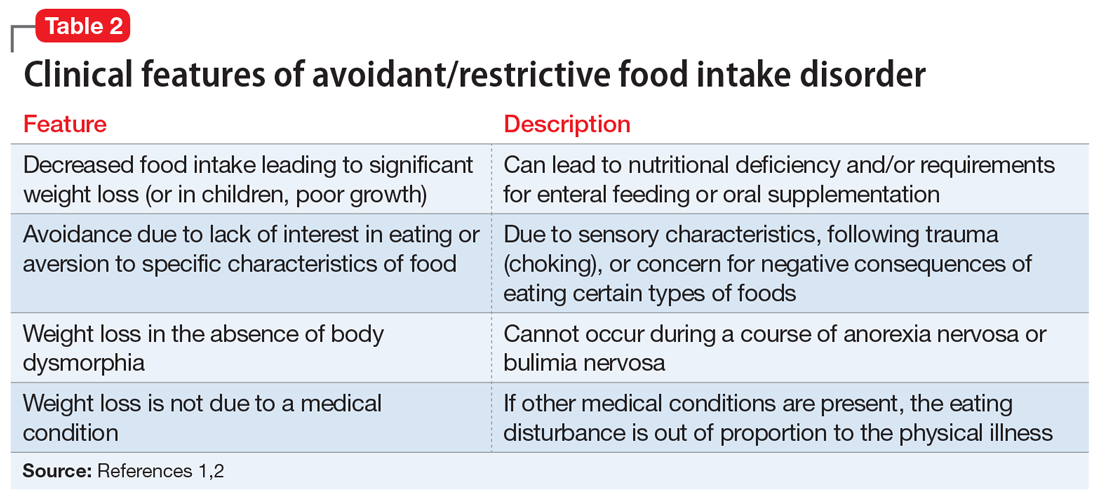
The rise in “healthy” eating materials online—particularly on social media—has created an atmosphere in which misinformation regarding diet and health is common and widespread. For patients with OCD and a predisposition to health anxiety, such as Ms. L, searching online for nutrition information and healthy living habits can exacerbate food-related anxieties and can lead to a pathological drive for purity and health.12Although not included in DSM-5, orthorexia nervosa was identified in 1997 as a proposed eating disorder best characterized as an obsession with healthy eating with associated restrictive behaviors.13 Patients with this disorder are rarely focused on losing weight, and orthorexic eating behaviors have been associated with both SSD and OCD.12,14 As in Ms. L’s case, patients with orthorexia nervosa demonstrate intrusive obsessions with nutrition, spend excessive amount of time researching nutrition, and fixate on food quality.12 Throughout Ms. L’s hospitalization, even as her food-related magical thinking symptoms improved, she constantly informed her care team that she had been “eating healthily” even though she was severely cachectic. Patients with SSD and OCD prone to health anxieties are at risk of developing pathologic food beliefs and dangerous eating behaviors. These patients may benefit from psychoeducation regarding nutrition and media literacy, which are components of effective eating disorder programs.15
[polldaddy:11079399]
Continue to: The authors' observations...
The authors’ observations
How do we approach the pharmacologic treatment of patients with co-occurring eating, somatic symptom, and anxiety disorders? Olanzapine facilitates recovery in children and adolescents with ARFID by promoting eating and weight gain, and decreasing symptoms of depression and anxiety.16 Patients with orthorexia nervosa also may benefit from treatment with olanzapine, which has decreased food-related fixations, magical thinking, and delusions regarding food.17 Further, orthorexic patients with ARFID have also been shown to respond to SSRIs due to those agents’ efficacy for treating intrusive thoughts, obsessions, and preoccupations from OCD and SSD.18,19 Thus, treating Ms. L’s symptoms with olanzapine and fluoxetine targeted the intersection of several diagnoses on our differential. Olanzapine’s propensity to cause weight gain is favorable in this population, particularly patients such as Ms. L, who do not exhibit body dysmorphia or fear of gaining weight.
OUTCOME Weight gain and fewer fears
Ms. L is prescribed olanzapine 5 mg/d and fluoxetine 20 mg/d. She gains 20.6 pounds in 4 weeks. Importantly, she endorses fewer fears related to foods and expands her palate to include foods she previously considered to be unhealthy, including white bread and farm-raised salmon. Further, she spends less time thinking about food and says she has less anxiety regarding the recurrence of GI symptoms.
1. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
2. Murray HB, Bailey AP, Keshishian AC. Prevalence and characteristics of avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Clin Gastroenterol Hepatol. 2020;18(9):1995-2002.e1.
3. Görmez A, Kılıç A, Kırpınar İ. Avoidant/restrictive food intake disorder: an adult case responding to cognitive behavioral therapy. Clinical Case Studies. 2018;17(6):443-452.
4. Reed-Knight B, Squires M, Chitkara DK, et al. Adolescents with irritable bowel syndrome report increased eating-associated symptoms, changes in dietary composition, and altered eating behaviors: a pilot comparison study to healthy adolescents. Neurogastroenterol Motil. 2016;28(12):1915-1920.
5. Melchior C, Desprez C, Riachi G, et al. Anxiety and depression profile is associated with eating disorders in patients with irritable bowel syndrome. Front Psychiatry. 2020;10:928.
6. Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. J Clin Psychiatry. 2001;62 Suppl 8:28-37.
7. Abraham S, Kellow J. Exploring eating disorder quality of life and functional gastrointestinal disorders among eating disorder patients. J Psychosom Res. 2011;70(4):372-377.
8. Swinbourne JM, Touyz SW. The co-morbidity of eating disorders and anxiety disorders: a review. Eur Eat Disord Rev. 2007;15(4):253-274.
9. Perkins SJ, Keville S, Schmidt U, et al. Eating disorders and irritable bowel syndrome: is there a link? J Psychosom Res. 2005;59(2):57-64.
10. Hausteiner-Wiehle C, Henningsen P. Irritable bowel syndrome: relations with functional, mental, and somatoform disorders. World J Gastroenterol. 2014;20(2):6024-6030.
11. Masand PS, Keuthen NJ, Gupta S, et al. Prevalence of irritable bowel syndrome in obsessive-compulsive disorder. CNS Spectr. 2006;11(1):21-25.
12. Koven NS, Abry AW. The clinical basis of orthorexia nervosa: emerging perspectives. Neuropsychiatr Dis Treat. 2015;11:385-394.
13. Bratman S. Health food junkie. Yoga Journal. 1997;136:42-50.
14. Barthels F, Müller R, Schüth T, et al. Orthorexic eating behavior in patients with somatoform disorders. Eat Weight Disord. 2021;26(1):135-143.
15. Ciao AC, Loth K, Neumark-Sztainer D. Preventing eating disorder pathology: common and unique features of successful eating disorders prevention programs. Curr Psychiatry Rep. 2014;16(7):453.
16. Brewerton TD, D’Agostino M. Adjunctive use of olanzapine in the treatment of avoidant restrictive food intake disorder in children and adolescents in an eating disorders program. J Child Adolesc Psychopharmacol. 2017;27(10):920-922.
17. Moroze RM, Dunn TM, Craig Holland J, et al. Microthinking about micronutrients: a case of transition from obsessions about healthy eating to near-fatal “orthorexia nervosa” and proposed diagnostic criteria. Psychosomatics. 2015;56(4):397-403.
18. Spettigue W, Norris ML, Santos A, et al. Treatment of children and adolescents with avoidant/restrictive food intake disorder: a case series examining the feasibility of family therapy and adjunctive treatments. J Eat Disord. 2018;6:20.
19. Niedzielski A, Kaźmierczak-Wojtaś N. Prevalence of Orthorexia Nervosa and Its Diagnostic Tools-A Literature Review. Int J Environ Res Public Health. 2021;18(10):5488. Published 2021 May 20. doi:10.3390/ijerph18105488 Prevalence of orthorexia nervosa and its diagnostic tools-a literature review. Int J Environ Res Public Health. 2021;18(10):5488.
CASE Fixated on health and nutrition
At the insistence of her daughter, Ms. L, age 75, presents to the emergency department (ED) for self-neglect and severe weight loss, with a body mass index (BMI) of 13.5 kg/m2 (normal: 18.5 to 24.9 kg/m2). When asked why she is in the ED, Ms. L says she doesn’t know. She attributes her significant weight loss (approximately 20 pounds in the last few months) to gastroesophageal reflux disease (GERD). She constantly worries about her esophagus. She had been diagnosed with esophageal dysphagia 7 years ago after undergoing radiofrequency ablation for esophageal cancer. Ms. L fixates on the negative effects certain foods and ingredients might have on her stomach and esophagus.
Following transfer from the ED, Ms. L is involuntarily admitted to our inpatient unit. Although she acknowledges weight loss, she minimizes the severity of her illness and indicates she would like to gain weight, but only by eating healthy foods she is comfortable with, including kale, quinoa, and vegetables. Ms. L says that she has always been interested in “healthful foods” and that she “loves sugar,” but “it’s bad for you,” mentioning that “sugar fuels cancer.” She has daily thoughts about sugar causing cancer. Ms. L also mentions that she stopped eating flour, sugar, fried food, and oils because those foods affect her “stomach acid” and cause “pimples on my face and weight loss.” While in the inpatient unit, Ms. L requests a special diet and demands to know the origin and ingredients of the foods she is offered. She emphasizes that her esophageal cancer diagnosis and dysphagia exacerbate worries that certain foods cause cancer, and wants to continue her diet restrictions. Nonetheless, she says she wants to get healthy, and denies an intense fear of gaining weight or feeling fat.
HISTORY Multiple psychiatric diagnoses
Ms. L lives alone and enjoys spending time with her grandchildren, visiting museums, and listening to classical music. However, her family, social workers, and records from a previous psychiatric hospitalization reveal that Ms. L has a history of psychiatric illness and fears regarding certain types of foods for much of her adult life. Ms. L’s family also described a range of compulsive behaviors, including shoplifting, hoarding art, multiple plastic surgeries, and phases where Ms. L ate only frozen yogurt without sugar.
Ms. L’s daughter reported that Ms. L had seen a psychologist in the late 1990s for depression and had been diagnosed with obsessive-compulsive disorder (OCD) and attention deficit/hyperactivity disorder in the early 2000s. In 2006, during a depressive episode after her divorce, Ms. L had a suicide attempt with pills and alcohol, and was hospitalized. Records from that stay described a history of mood dysregulation with fears regarding food and nutrition. Ms. L was treated with aripiprazole 5 mg/d. A trial of trazodone 25 mg/d did not have any effect. When discharged, she was receiving lamotrigine 100 mg/d. However, her daughter believes she stopped taking all psychiatric medications shortly after discharge.
Her daughter says that in the past 2 years, Ms. L has seen multiple doctors for treatment of somatic gastrointestinal (GI) complaints. A 2018 note from a social worker indicated that Ms. L endorsed taking >80 supplements per day and constantly researched nutrition online. In the months leading to her current hospitalization, Ms. L suffered from severe self-neglect and fear regarding foods she felt were not healthy for her. She had stopped leaving her apartment.
Continue to: EVALUATION Poor insight, normal lab results...
EVALUATION Poor insight, normal lab results
During her evaluation, Ms. L appears cachectic and frail. She has a heavily constricted affect and is guarded, dismissive, and vague. Although her thought processes are linear and goal-directed, her insight into her condition is extremely poor and she appears surprised when clinicians inform her that her self-neglect would lead to death. Instead, Ms. L insists she is eating healthily and demonstrates severe anxiety in relation to her GI symptoms.
Ms. L is oriented to person, place, and time. She scores 27/30 on the Montreal Cognitive Assessment, indicating normal cognition. She denies any depressive symptoms or suicidal intent. She does not appear to be internally preoccupied and denies having auditory or visual hallucinations or manic symptoms.
A neurologic examination reveals that her cranial nerves are normal, and cerebellar function, strength, and sensory testing are intact. Her gait is steady and she walks without a walker. Despite her severely low BMI and recent history of self-neglect, Ms. L’s laboratory results are remarkably normal and show no liver, metabolic, or electrolyte abnormalities, no signs of infection, and normal vitamin B12 levels. She has slightly elevated creatinine and blood urea nitrogen levels, but a normal glomerular filtration rate.
Her medical history is significant for squamous cell esophageal cancer, treated with radiofrequency ablation. Although Ms. L is constantly worried about the recurrence of cancer, pathology reports demonstrate no esophageal dysplasia. However, she does show evidence of an approximately 1 cm × 1 cm mild, noncircumferential esophageal stenosis, likely resulting from radiofrequency ablation.
[polldaddy:11079394]
The authors’ observations
Several health- and physical symptom-related psychiatric disorders have overlapping features, which can complicate the differential diagnosis (Table 11). Ms. L presented to the ED with a severely low BMI of 13.5 kg/m2, obsessions regarding specific types of food, and preoccupations regarding her esophagus. Despite her extensive psychiatric history (including intense fears regarding food), we ruled out a primary psychotic disorder because she did not describe auditory or visual hallucinations and never appeared internally preoccupied. While her BMI and persistent minimization of the extent of her disease meet criteria for anorexia nervosa, she denied body dysmorphia and did not have any fear of gaining weight.

A central element of Ms. L’s presentation was her anxiety regarding how certain types of foods impact her health as well as her anxieties regarding her esophagus. While Ms. L was in remission from esophageal cancer and had a diagnosis of esophageal dysphagia, these preoccupations and obsessions regarding how certain types of foods affect her esophagus drove her to self-neglect and thus represent pathologic thought processes out of proportion to her symptoms. Illness anxiety disorder was considered because Ms. L met many of its criteria: preoccupation with having a serious illness, disproportionate preoccupation with somatic symptoms if they are present, extreme anxiety over health, and performance of health-related behaviors.1 However, illness anxiety disorder is a diagnosis of exclusion, and 1 criterion is that these symptoms cannot be explained by another mental disorder. We felt other diagnoses better fit Ms. L’s condition and ruled out illness anxiety disorder.
Ms. L’s long history of food and non-food–related obsessions and compulsions that interrupted her ability to perform daily activities were strongly suggestive for OCD. Additionally, her intense preoccupation, high level of anxiety, amount of time and energy spent seeking care for her esophagus and GERD symptoms, and the resulting significant disruption of daily life, met criteria for somatic symptom disorder (SSD). However, we did not believe that a diagnosis of OCD and SSD alone explained the entirety of Ms. L’s clinical picture. Despite ruling out anorexia nervosa, Ms. L nonetheless demonstrated disordered eating.
Avoidant/restrictive food intake disorder (ARFID) is an eating disorder in which patients restrict their diet and do not meet nutritional needs for any number of reasons, do not experience body dysmorphia, and do not fear weight gain.1 A common feature of ARFID is a fear of negative consequences from eating specific types of food.2 Table 21,2 summarizes additional clinical features of ARFID. Although ARFID is typically diagnosed in children and adolescents, particularly in individuals with autism with heightened sensory sensitivities, ARFID is also common among adult patients with GI disorders.3 In a retrospective chart review of 410 adults ages 18 to 90 (73% women) referred to a neurogastroenterology care center, 6.3% met the full criteria for ARFID and 17.3% had clinically significant avoidant or restrictive eating behaviors. Among patients with ARFID symptoms, 93% stated that a fear of GI symptoms was the driver of their avoidant or restrictive eating behaviors.2 Patients with GI diseases often develop dietary control and avoidance coping mechanisms to alleviate their symptoms.4 These strategies can exacerbate health anxieties and have a detrimental effect on mental health.5 Patients with GI disorders have a high degree of comorbidity with affective disorders, including anxiety disorders.6 These trends may arise from hypervigilance and the need to gain control over physical symptoms.7 Feeling a need for control, actions driven by anxiety and fear, and the need for compensatory behaviors are cardinal features of OCD and eating disorders.8 Multiple studies have demonstrated comorbidities between irritable bowel syndrome and eating disorders,9 SSD,10 and OCD.11 Taken together with observations that ARFID is also found in patients with GI disorders,2 these findings demonstrate that patients with a history of GI disease are at high risk of developing extreme health anxieties and behavioral coping strategies that can lead to disordered eating.

The rise in “healthy” eating materials online—particularly on social media—has created an atmosphere in which misinformation regarding diet and health is common and widespread. For patients with OCD and a predisposition to health anxiety, such as Ms. L, searching online for nutrition information and healthy living habits can exacerbate food-related anxieties and can lead to a pathological drive for purity and health.12Although not included in DSM-5, orthorexia nervosa was identified in 1997 as a proposed eating disorder best characterized as an obsession with healthy eating with associated restrictive behaviors.13 Patients with this disorder are rarely focused on losing weight, and orthorexic eating behaviors have been associated with both SSD and OCD.12,14 As in Ms. L’s case, patients with orthorexia nervosa demonstrate intrusive obsessions with nutrition, spend excessive amount of time researching nutrition, and fixate on food quality.12 Throughout Ms. L’s hospitalization, even as her food-related magical thinking symptoms improved, she constantly informed her care team that she had been “eating healthily” even though she was severely cachectic. Patients with SSD and OCD prone to health anxieties are at risk of developing pathologic food beliefs and dangerous eating behaviors. These patients may benefit from psychoeducation regarding nutrition and media literacy, which are components of effective eating disorder programs.15
[polldaddy:11079399]
Continue to: The authors' observations...
The authors’ observations
How do we approach the pharmacologic treatment of patients with co-occurring eating, somatic symptom, and anxiety disorders? Olanzapine facilitates recovery in children and adolescents with ARFID by promoting eating and weight gain, and decreasing symptoms of depression and anxiety.16 Patients with orthorexia nervosa also may benefit from treatment with olanzapine, which has decreased food-related fixations, magical thinking, and delusions regarding food.17 Further, orthorexic patients with ARFID have also been shown to respond to SSRIs due to those agents’ efficacy for treating intrusive thoughts, obsessions, and preoccupations from OCD and SSD.18,19 Thus, treating Ms. L’s symptoms with olanzapine and fluoxetine targeted the intersection of several diagnoses on our differential. Olanzapine’s propensity to cause weight gain is favorable in this population, particularly patients such as Ms. L, who do not exhibit body dysmorphia or fear of gaining weight.
OUTCOME Weight gain and fewer fears
Ms. L is prescribed olanzapine 5 mg/d and fluoxetine 20 mg/d. She gains 20.6 pounds in 4 weeks. Importantly, she endorses fewer fears related to foods and expands her palate to include foods she previously considered to be unhealthy, including white bread and farm-raised salmon. Further, she spends less time thinking about food and says she has less anxiety regarding the recurrence of GI symptoms.
CASE Fixated on health and nutrition
At the insistence of her daughter, Ms. L, age 75, presents to the emergency department (ED) for self-neglect and severe weight loss, with a body mass index (BMI) of 13.5 kg/m2 (normal: 18.5 to 24.9 kg/m2). When asked why she is in the ED, Ms. L says she doesn’t know. She attributes her significant weight loss (approximately 20 pounds in the last few months) to gastroesophageal reflux disease (GERD). She constantly worries about her esophagus. She had been diagnosed with esophageal dysphagia 7 years ago after undergoing radiofrequency ablation for esophageal cancer. Ms. L fixates on the negative effects certain foods and ingredients might have on her stomach and esophagus.
Following transfer from the ED, Ms. L is involuntarily admitted to our inpatient unit. Although she acknowledges weight loss, she minimizes the severity of her illness and indicates she would like to gain weight, but only by eating healthy foods she is comfortable with, including kale, quinoa, and vegetables. Ms. L says that she has always been interested in “healthful foods” and that she “loves sugar,” but “it’s bad for you,” mentioning that “sugar fuels cancer.” She has daily thoughts about sugar causing cancer. Ms. L also mentions that she stopped eating flour, sugar, fried food, and oils because those foods affect her “stomach acid” and cause “pimples on my face and weight loss.” While in the inpatient unit, Ms. L requests a special diet and demands to know the origin and ingredients of the foods she is offered. She emphasizes that her esophageal cancer diagnosis and dysphagia exacerbate worries that certain foods cause cancer, and wants to continue her diet restrictions. Nonetheless, she says she wants to get healthy, and denies an intense fear of gaining weight or feeling fat.
HISTORY Multiple psychiatric diagnoses
Ms. L lives alone and enjoys spending time with her grandchildren, visiting museums, and listening to classical music. However, her family, social workers, and records from a previous psychiatric hospitalization reveal that Ms. L has a history of psychiatric illness and fears regarding certain types of foods for much of her adult life. Ms. L’s family also described a range of compulsive behaviors, including shoplifting, hoarding art, multiple plastic surgeries, and phases where Ms. L ate only frozen yogurt without sugar.
Ms. L’s daughter reported that Ms. L had seen a psychologist in the late 1990s for depression and had been diagnosed with obsessive-compulsive disorder (OCD) and attention deficit/hyperactivity disorder in the early 2000s. In 2006, during a depressive episode after her divorce, Ms. L had a suicide attempt with pills and alcohol, and was hospitalized. Records from that stay described a history of mood dysregulation with fears regarding food and nutrition. Ms. L was treated with aripiprazole 5 mg/d. A trial of trazodone 25 mg/d did not have any effect. When discharged, she was receiving lamotrigine 100 mg/d. However, her daughter believes she stopped taking all psychiatric medications shortly after discharge.
Her daughter says that in the past 2 years, Ms. L has seen multiple doctors for treatment of somatic gastrointestinal (GI) complaints. A 2018 note from a social worker indicated that Ms. L endorsed taking >80 supplements per day and constantly researched nutrition online. In the months leading to her current hospitalization, Ms. L suffered from severe self-neglect and fear regarding foods she felt were not healthy for her. She had stopped leaving her apartment.
Continue to: EVALUATION Poor insight, normal lab results...
EVALUATION Poor insight, normal lab results
During her evaluation, Ms. L appears cachectic and frail. She has a heavily constricted affect and is guarded, dismissive, and vague. Although her thought processes are linear and goal-directed, her insight into her condition is extremely poor and she appears surprised when clinicians inform her that her self-neglect would lead to death. Instead, Ms. L insists she is eating healthily and demonstrates severe anxiety in relation to her GI symptoms.
Ms. L is oriented to person, place, and time. She scores 27/30 on the Montreal Cognitive Assessment, indicating normal cognition. She denies any depressive symptoms or suicidal intent. She does not appear to be internally preoccupied and denies having auditory or visual hallucinations or manic symptoms.
A neurologic examination reveals that her cranial nerves are normal, and cerebellar function, strength, and sensory testing are intact. Her gait is steady and she walks without a walker. Despite her severely low BMI and recent history of self-neglect, Ms. L’s laboratory results are remarkably normal and show no liver, metabolic, or electrolyte abnormalities, no signs of infection, and normal vitamin B12 levels. She has slightly elevated creatinine and blood urea nitrogen levels, but a normal glomerular filtration rate.
Her medical history is significant for squamous cell esophageal cancer, treated with radiofrequency ablation. Although Ms. L is constantly worried about the recurrence of cancer, pathology reports demonstrate no esophageal dysplasia. However, she does show evidence of an approximately 1 cm × 1 cm mild, noncircumferential esophageal stenosis, likely resulting from radiofrequency ablation.
[polldaddy:11079394]
The authors’ observations
Several health- and physical symptom-related psychiatric disorders have overlapping features, which can complicate the differential diagnosis (Table 11). Ms. L presented to the ED with a severely low BMI of 13.5 kg/m2, obsessions regarding specific types of food, and preoccupations regarding her esophagus. Despite her extensive psychiatric history (including intense fears regarding food), we ruled out a primary psychotic disorder because she did not describe auditory or visual hallucinations and never appeared internally preoccupied. While her BMI and persistent minimization of the extent of her disease meet criteria for anorexia nervosa, she denied body dysmorphia and did not have any fear of gaining weight.

A central element of Ms. L’s presentation was her anxiety regarding how certain types of foods impact her health as well as her anxieties regarding her esophagus. While Ms. L was in remission from esophageal cancer and had a diagnosis of esophageal dysphagia, these preoccupations and obsessions regarding how certain types of foods affect her esophagus drove her to self-neglect and thus represent pathologic thought processes out of proportion to her symptoms. Illness anxiety disorder was considered because Ms. L met many of its criteria: preoccupation with having a serious illness, disproportionate preoccupation with somatic symptoms if they are present, extreme anxiety over health, and performance of health-related behaviors.1 However, illness anxiety disorder is a diagnosis of exclusion, and 1 criterion is that these symptoms cannot be explained by another mental disorder. We felt other diagnoses better fit Ms. L’s condition and ruled out illness anxiety disorder.
Ms. L’s long history of food and non-food–related obsessions and compulsions that interrupted her ability to perform daily activities were strongly suggestive for OCD. Additionally, her intense preoccupation, high level of anxiety, amount of time and energy spent seeking care for her esophagus and GERD symptoms, and the resulting significant disruption of daily life, met criteria for somatic symptom disorder (SSD). However, we did not believe that a diagnosis of OCD and SSD alone explained the entirety of Ms. L’s clinical picture. Despite ruling out anorexia nervosa, Ms. L nonetheless demonstrated disordered eating.
Avoidant/restrictive food intake disorder (ARFID) is an eating disorder in which patients restrict their diet and do not meet nutritional needs for any number of reasons, do not experience body dysmorphia, and do not fear weight gain.1 A common feature of ARFID is a fear of negative consequences from eating specific types of food.2 Table 21,2 summarizes additional clinical features of ARFID. Although ARFID is typically diagnosed in children and adolescents, particularly in individuals with autism with heightened sensory sensitivities, ARFID is also common among adult patients with GI disorders.3 In a retrospective chart review of 410 adults ages 18 to 90 (73% women) referred to a neurogastroenterology care center, 6.3% met the full criteria for ARFID and 17.3% had clinically significant avoidant or restrictive eating behaviors. Among patients with ARFID symptoms, 93% stated that a fear of GI symptoms was the driver of their avoidant or restrictive eating behaviors.2 Patients with GI diseases often develop dietary control and avoidance coping mechanisms to alleviate their symptoms.4 These strategies can exacerbate health anxieties and have a detrimental effect on mental health.5 Patients with GI disorders have a high degree of comorbidity with affective disorders, including anxiety disorders.6 These trends may arise from hypervigilance and the need to gain control over physical symptoms.7 Feeling a need for control, actions driven by anxiety and fear, and the need for compensatory behaviors are cardinal features of OCD and eating disorders.8 Multiple studies have demonstrated comorbidities between irritable bowel syndrome and eating disorders,9 SSD,10 and OCD.11 Taken together with observations that ARFID is also found in patients with GI disorders,2 these findings demonstrate that patients with a history of GI disease are at high risk of developing extreme health anxieties and behavioral coping strategies that can lead to disordered eating.

The rise in “healthy” eating materials online—particularly on social media—has created an atmosphere in which misinformation regarding diet and health is common and widespread. For patients with OCD and a predisposition to health anxiety, such as Ms. L, searching online for nutrition information and healthy living habits can exacerbate food-related anxieties and can lead to a pathological drive for purity and health.12Although not included in DSM-5, orthorexia nervosa was identified in 1997 as a proposed eating disorder best characterized as an obsession with healthy eating with associated restrictive behaviors.13 Patients with this disorder are rarely focused on losing weight, and orthorexic eating behaviors have been associated with both SSD and OCD.12,14 As in Ms. L’s case, patients with orthorexia nervosa demonstrate intrusive obsessions with nutrition, spend excessive amount of time researching nutrition, and fixate on food quality.12 Throughout Ms. L’s hospitalization, even as her food-related magical thinking symptoms improved, she constantly informed her care team that she had been “eating healthily” even though she was severely cachectic. Patients with SSD and OCD prone to health anxieties are at risk of developing pathologic food beliefs and dangerous eating behaviors. These patients may benefit from psychoeducation regarding nutrition and media literacy, which are components of effective eating disorder programs.15
[polldaddy:11079399]
Continue to: The authors' observations...
The authors’ observations
How do we approach the pharmacologic treatment of patients with co-occurring eating, somatic symptom, and anxiety disorders? Olanzapine facilitates recovery in children and adolescents with ARFID by promoting eating and weight gain, and decreasing symptoms of depression and anxiety.16 Patients with orthorexia nervosa also may benefit from treatment with olanzapine, which has decreased food-related fixations, magical thinking, and delusions regarding food.17 Further, orthorexic patients with ARFID have also been shown to respond to SSRIs due to those agents’ efficacy for treating intrusive thoughts, obsessions, and preoccupations from OCD and SSD.18,19 Thus, treating Ms. L’s symptoms with olanzapine and fluoxetine targeted the intersection of several diagnoses on our differential. Olanzapine’s propensity to cause weight gain is favorable in this population, particularly patients such as Ms. L, who do not exhibit body dysmorphia or fear of gaining weight.
OUTCOME Weight gain and fewer fears
Ms. L is prescribed olanzapine 5 mg/d and fluoxetine 20 mg/d. She gains 20.6 pounds in 4 weeks. Importantly, she endorses fewer fears related to foods and expands her palate to include foods she previously considered to be unhealthy, including white bread and farm-raised salmon. Further, she spends less time thinking about food and says she has less anxiety regarding the recurrence of GI symptoms.
1. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
2. Murray HB, Bailey AP, Keshishian AC. Prevalence and characteristics of avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Clin Gastroenterol Hepatol. 2020;18(9):1995-2002.e1.
3. Görmez A, Kılıç A, Kırpınar İ. Avoidant/restrictive food intake disorder: an adult case responding to cognitive behavioral therapy. Clinical Case Studies. 2018;17(6):443-452.
4. Reed-Knight B, Squires M, Chitkara DK, et al. Adolescents with irritable bowel syndrome report increased eating-associated symptoms, changes in dietary composition, and altered eating behaviors: a pilot comparison study to healthy adolescents. Neurogastroenterol Motil. 2016;28(12):1915-1920.
5. Melchior C, Desprez C, Riachi G, et al. Anxiety and depression profile is associated with eating disorders in patients with irritable bowel syndrome. Front Psychiatry. 2020;10:928.
6. Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. J Clin Psychiatry. 2001;62 Suppl 8:28-37.
7. Abraham S, Kellow J. Exploring eating disorder quality of life and functional gastrointestinal disorders among eating disorder patients. J Psychosom Res. 2011;70(4):372-377.
8. Swinbourne JM, Touyz SW. The co-morbidity of eating disorders and anxiety disorders: a review. Eur Eat Disord Rev. 2007;15(4):253-274.
9. Perkins SJ, Keville S, Schmidt U, et al. Eating disorders and irritable bowel syndrome: is there a link? J Psychosom Res. 2005;59(2):57-64.
10. Hausteiner-Wiehle C, Henningsen P. Irritable bowel syndrome: relations with functional, mental, and somatoform disorders. World J Gastroenterol. 2014;20(2):6024-6030.
11. Masand PS, Keuthen NJ, Gupta S, et al. Prevalence of irritable bowel syndrome in obsessive-compulsive disorder. CNS Spectr. 2006;11(1):21-25.
12. Koven NS, Abry AW. The clinical basis of orthorexia nervosa: emerging perspectives. Neuropsychiatr Dis Treat. 2015;11:385-394.
13. Bratman S. Health food junkie. Yoga Journal. 1997;136:42-50.
14. Barthels F, Müller R, Schüth T, et al. Orthorexic eating behavior in patients with somatoform disorders. Eat Weight Disord. 2021;26(1):135-143.
15. Ciao AC, Loth K, Neumark-Sztainer D. Preventing eating disorder pathology: common and unique features of successful eating disorders prevention programs. Curr Psychiatry Rep. 2014;16(7):453.
16. Brewerton TD, D’Agostino M. Adjunctive use of olanzapine in the treatment of avoidant restrictive food intake disorder in children and adolescents in an eating disorders program. J Child Adolesc Psychopharmacol. 2017;27(10):920-922.
17. Moroze RM, Dunn TM, Craig Holland J, et al. Microthinking about micronutrients: a case of transition from obsessions about healthy eating to near-fatal “orthorexia nervosa” and proposed diagnostic criteria. Psychosomatics. 2015;56(4):397-403.
18. Spettigue W, Norris ML, Santos A, et al. Treatment of children and adolescents with avoidant/restrictive food intake disorder: a case series examining the feasibility of family therapy and adjunctive treatments. J Eat Disord. 2018;6:20.
19. Niedzielski A, Kaźmierczak-Wojtaś N. Prevalence of Orthorexia Nervosa and Its Diagnostic Tools-A Literature Review. Int J Environ Res Public Health. 2021;18(10):5488. Published 2021 May 20. doi:10.3390/ijerph18105488 Prevalence of orthorexia nervosa and its diagnostic tools-a literature review. Int J Environ Res Public Health. 2021;18(10):5488.
1. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
2. Murray HB, Bailey AP, Keshishian AC. Prevalence and characteristics of avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Clin Gastroenterol Hepatol. 2020;18(9):1995-2002.e1.
3. Görmez A, Kılıç A, Kırpınar İ. Avoidant/restrictive food intake disorder: an adult case responding to cognitive behavioral therapy. Clinical Case Studies. 2018;17(6):443-452.
4. Reed-Knight B, Squires M, Chitkara DK, et al. Adolescents with irritable bowel syndrome report increased eating-associated symptoms, changes in dietary composition, and altered eating behaviors: a pilot comparison study to healthy adolescents. Neurogastroenterol Motil. 2016;28(12):1915-1920.
5. Melchior C, Desprez C, Riachi G, et al. Anxiety and depression profile is associated with eating disorders in patients with irritable bowel syndrome. Front Psychiatry. 2020;10:928.
6. Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. J Clin Psychiatry. 2001;62 Suppl 8:28-37.
7. Abraham S, Kellow J. Exploring eating disorder quality of life and functional gastrointestinal disorders among eating disorder patients. J Psychosom Res. 2011;70(4):372-377.
8. Swinbourne JM, Touyz SW. The co-morbidity of eating disorders and anxiety disorders: a review. Eur Eat Disord Rev. 2007;15(4):253-274.
9. Perkins SJ, Keville S, Schmidt U, et al. Eating disorders and irritable bowel syndrome: is there a link? J Psychosom Res. 2005;59(2):57-64.
10. Hausteiner-Wiehle C, Henningsen P. Irritable bowel syndrome: relations with functional, mental, and somatoform disorders. World J Gastroenterol. 2014;20(2):6024-6030.
11. Masand PS, Keuthen NJ, Gupta S, et al. Prevalence of irritable bowel syndrome in obsessive-compulsive disorder. CNS Spectr. 2006;11(1):21-25.
12. Koven NS, Abry AW. The clinical basis of orthorexia nervosa: emerging perspectives. Neuropsychiatr Dis Treat. 2015;11:385-394.
13. Bratman S. Health food junkie. Yoga Journal. 1997;136:42-50.
14. Barthels F, Müller R, Schüth T, et al. Orthorexic eating behavior in patients with somatoform disorders. Eat Weight Disord. 2021;26(1):135-143.
15. Ciao AC, Loth K, Neumark-Sztainer D. Preventing eating disorder pathology: common and unique features of successful eating disorders prevention programs. Curr Psychiatry Rep. 2014;16(7):453.
16. Brewerton TD, D’Agostino M. Adjunctive use of olanzapine in the treatment of avoidant restrictive food intake disorder in children and adolescents in an eating disorders program. J Child Adolesc Psychopharmacol. 2017;27(10):920-922.
17. Moroze RM, Dunn TM, Craig Holland J, et al. Microthinking about micronutrients: a case of transition from obsessions about healthy eating to near-fatal “orthorexia nervosa” and proposed diagnostic criteria. Psychosomatics. 2015;56(4):397-403.
18. Spettigue W, Norris ML, Santos A, et al. Treatment of children and adolescents with avoidant/restrictive food intake disorder: a case series examining the feasibility of family therapy and adjunctive treatments. J Eat Disord. 2018;6:20.
19. Niedzielski A, Kaźmierczak-Wojtaś N. Prevalence of Orthorexia Nervosa and Its Diagnostic Tools-A Literature Review. Int J Environ Res Public Health. 2021;18(10):5488. Published 2021 May 20. doi:10.3390/ijerph18105488 Prevalence of orthorexia nervosa and its diagnostic tools-a literature review. Int J Environ Res Public Health. 2021;18(10):5488.
Older adults with schizophrenia need ‘person-centered’ care
For example, individuals in a group characterized by substance use disorders (SUDs) had a depression prevalence of about 60% and relatively high death rates from unintentional injury and hepatitis.
“The health care needs of older adults with schizophrenia can vary widely, so aging persons with schizophrenia can’t be considered a uniform population,” study investigator Alison Hwong, MD, PhD, University of California, San Francisco, National Clinicians Scholars Program and San Francisco Veterans Affairs, told this news organization.
“For patients with multiple chronic conditions, we need to be proactive in coordinating specialty care. At the same time, we need novel models of person-centered care to help aging adults with schizophrenia live longer, healthier lives,” Dr. Hwong added.
The findings were presented as part of the American Association for Geriatric Psychiatry annual meeting.
Widening mortality gap
The life expectancy of patients with schizophrenia is lower by 8-15 years, compared with those without schizophrenia and this “mortality gap” has widened in recent years, Dr. Hwong noted. Those with schizophrenia also have high rates of health care utilization and high direct and indirect health care costs.
Most previous research looking at illness in schizophrenia focused on a single medical condition, “but by midlife, adults with schizophrenia may have multiple medical conditions,” said Dr. Hwong. “Little is known about multimorbidity in aging adults with schizophrenia and how that could be related to mortality outcomes.”
The study included 82,858 U.S. veterans aged 50 years and older who had at least one inpatient or two outpatient encounters associated with a diagnosis of schizophrenia in the previous 2 years. The study period ran from 2012 to 2018.
Using health care records and data linkages, researchers examined 20 common medical and psychiatric conditions other than schizophrenia that required medical attention. The investigators used the “latent class analysis” statistical model to assess differences across classes.
The study included three distinct patient classes: minimal morbidity (43% of the cohort), depression and medical comorbidity (34.2%), and SUDs and related conditions (22.8%).
The SUD group tended to be younger, with a mean age of 57.9 years versus 60.4 years for the minimal comorbidity group and 65.9 years for the depression group.
The SUD group was also less likely to be female (4.8% vs. 6.7% and 6%, respectively), less likely to be White, and more likely to be Black. This group was also less likely to be married and more likely to have a history of homelessness.
Disease prevalence rates
Results showed the minimal morbidity group had prevalence rates of less than 10% for all major conditions, except for tobacco dependence, which had a rate of 11.8%.
The depression and medical comorbidity group had very high prevalence rates (more than 20%) for heart attack, heart failure, stroke, cancer, dementia, arthritis, renal disease, sleep disorders, depression, and tobacco dependence. In addition, the rate was 60% for chronic obstructive pulmonary disease.
Participants in the SUD and related conditions group had rates of more than 70% for alcohol use disorder, other drug use disorders, and tobacco dependence. They also had high rates of COPD, hepatitis C, chronic pain, sleep disorders, depression, and PTSD.
On average, the SUD group was younger and may explain why they were less likely to have heart failure and renal disease, Dr. Hwong noted. These results may help inform treatment approaches, she added.
“For the group with largely substance use–related conditions, perhaps we can better address their needs with, for example, specific addiction and infectious disease services instead of a one-size-fits-all model,” said Dr. Hwong.
The investigators also examined mortality rates. Those in the depression and morbidity group had the highest rate of overall mortality; 47.5% of this class died during the observation period, compared with 27.2% of the SUD group.
More research is needed to understand why the mortality rate is so high in the depression and morbidity group, she said.
High rates of accidental death
The SUD group had the highest rates of death from accidents, possibly from overdoses, suicide, hepatitis C, and alcohol use–related deaths. “Their risks are very specific and appear largely related to substance use,” Dr. Hwong said.
The minimal comorbidity group showed the lowest rates of overall mortality rate (18%) and of cause-specific mortality for most of the included conditions.
Dr. Hwong noted she would like to study this class further. “I’m interested to know who are the people with schizophrenia who are thriving and are successfully aging – to learn what is going well for them.”
The researchers also plan to examine the subgroups in more detail to understand differences in treatments, health care utilization, and outcomes across groups. They are also interested in assessing other predictors of mortality outcomes in addition to multimorbidity.
One limitation of the study is that its cohort consisted of male veterans, so the findings may not be generalizable to other populations. In addition, these were observational data and so the results do not imply causality, Dr. Hwong said.
Dr. Hwong reported no relevant financial relationships, but she is supported by the VA and the UCSF National Clinician Scholars Program.
A version of this article first appeared on Medscape.com.
For example, individuals in a group characterized by substance use disorders (SUDs) had a depression prevalence of about 60% and relatively high death rates from unintentional injury and hepatitis.
“The health care needs of older adults with schizophrenia can vary widely, so aging persons with schizophrenia can’t be considered a uniform population,” study investigator Alison Hwong, MD, PhD, University of California, San Francisco, National Clinicians Scholars Program and San Francisco Veterans Affairs, told this news organization.
“For patients with multiple chronic conditions, we need to be proactive in coordinating specialty care. At the same time, we need novel models of person-centered care to help aging adults with schizophrenia live longer, healthier lives,” Dr. Hwong added.
The findings were presented as part of the American Association for Geriatric Psychiatry annual meeting.
Widening mortality gap
The life expectancy of patients with schizophrenia is lower by 8-15 years, compared with those without schizophrenia and this “mortality gap” has widened in recent years, Dr. Hwong noted. Those with schizophrenia also have high rates of health care utilization and high direct and indirect health care costs.
Most previous research looking at illness in schizophrenia focused on a single medical condition, “but by midlife, adults with schizophrenia may have multiple medical conditions,” said Dr. Hwong. “Little is known about multimorbidity in aging adults with schizophrenia and how that could be related to mortality outcomes.”
The study included 82,858 U.S. veterans aged 50 years and older who had at least one inpatient or two outpatient encounters associated with a diagnosis of schizophrenia in the previous 2 years. The study period ran from 2012 to 2018.
Using health care records and data linkages, researchers examined 20 common medical and psychiatric conditions other than schizophrenia that required medical attention. The investigators used the “latent class analysis” statistical model to assess differences across classes.
The study included three distinct patient classes: minimal morbidity (43% of the cohort), depression and medical comorbidity (34.2%), and SUDs and related conditions (22.8%).
The SUD group tended to be younger, with a mean age of 57.9 years versus 60.4 years for the minimal comorbidity group and 65.9 years for the depression group.
The SUD group was also less likely to be female (4.8% vs. 6.7% and 6%, respectively), less likely to be White, and more likely to be Black. This group was also less likely to be married and more likely to have a history of homelessness.
Disease prevalence rates
Results showed the minimal morbidity group had prevalence rates of less than 10% for all major conditions, except for tobacco dependence, which had a rate of 11.8%.
The depression and medical comorbidity group had very high prevalence rates (more than 20%) for heart attack, heart failure, stroke, cancer, dementia, arthritis, renal disease, sleep disorders, depression, and tobacco dependence. In addition, the rate was 60% for chronic obstructive pulmonary disease.
Participants in the SUD and related conditions group had rates of more than 70% for alcohol use disorder, other drug use disorders, and tobacco dependence. They also had high rates of COPD, hepatitis C, chronic pain, sleep disorders, depression, and PTSD.
On average, the SUD group was younger and may explain why they were less likely to have heart failure and renal disease, Dr. Hwong noted. These results may help inform treatment approaches, she added.
“For the group with largely substance use–related conditions, perhaps we can better address their needs with, for example, specific addiction and infectious disease services instead of a one-size-fits-all model,” said Dr. Hwong.
The investigators also examined mortality rates. Those in the depression and morbidity group had the highest rate of overall mortality; 47.5% of this class died during the observation period, compared with 27.2% of the SUD group.
More research is needed to understand why the mortality rate is so high in the depression and morbidity group, she said.
High rates of accidental death
The SUD group had the highest rates of death from accidents, possibly from overdoses, suicide, hepatitis C, and alcohol use–related deaths. “Their risks are very specific and appear largely related to substance use,” Dr. Hwong said.
The minimal comorbidity group showed the lowest rates of overall mortality rate (18%) and of cause-specific mortality for most of the included conditions.
Dr. Hwong noted she would like to study this class further. “I’m interested to know who are the people with schizophrenia who are thriving and are successfully aging – to learn what is going well for them.”
The researchers also plan to examine the subgroups in more detail to understand differences in treatments, health care utilization, and outcomes across groups. They are also interested in assessing other predictors of mortality outcomes in addition to multimorbidity.
One limitation of the study is that its cohort consisted of male veterans, so the findings may not be generalizable to other populations. In addition, these were observational data and so the results do not imply causality, Dr. Hwong said.
Dr. Hwong reported no relevant financial relationships, but she is supported by the VA and the UCSF National Clinician Scholars Program.
A version of this article first appeared on Medscape.com.
For example, individuals in a group characterized by substance use disorders (SUDs) had a depression prevalence of about 60% and relatively high death rates from unintentional injury and hepatitis.
“The health care needs of older adults with schizophrenia can vary widely, so aging persons with schizophrenia can’t be considered a uniform population,” study investigator Alison Hwong, MD, PhD, University of California, San Francisco, National Clinicians Scholars Program and San Francisco Veterans Affairs, told this news organization.
“For patients with multiple chronic conditions, we need to be proactive in coordinating specialty care. At the same time, we need novel models of person-centered care to help aging adults with schizophrenia live longer, healthier lives,” Dr. Hwong added.
The findings were presented as part of the American Association for Geriatric Psychiatry annual meeting.
Widening mortality gap
The life expectancy of patients with schizophrenia is lower by 8-15 years, compared with those without schizophrenia and this “mortality gap” has widened in recent years, Dr. Hwong noted. Those with schizophrenia also have high rates of health care utilization and high direct and indirect health care costs.
Most previous research looking at illness in schizophrenia focused on a single medical condition, “but by midlife, adults with schizophrenia may have multiple medical conditions,” said Dr. Hwong. “Little is known about multimorbidity in aging adults with schizophrenia and how that could be related to mortality outcomes.”
The study included 82,858 U.S. veterans aged 50 years and older who had at least one inpatient or two outpatient encounters associated with a diagnosis of schizophrenia in the previous 2 years. The study period ran from 2012 to 2018.
Using health care records and data linkages, researchers examined 20 common medical and psychiatric conditions other than schizophrenia that required medical attention. The investigators used the “latent class analysis” statistical model to assess differences across classes.
The study included three distinct patient classes: minimal morbidity (43% of the cohort), depression and medical comorbidity (34.2%), and SUDs and related conditions (22.8%).
The SUD group tended to be younger, with a mean age of 57.9 years versus 60.4 years for the minimal comorbidity group and 65.9 years for the depression group.
The SUD group was also less likely to be female (4.8% vs. 6.7% and 6%, respectively), less likely to be White, and more likely to be Black. This group was also less likely to be married and more likely to have a history of homelessness.
Disease prevalence rates
Results showed the minimal morbidity group had prevalence rates of less than 10% for all major conditions, except for tobacco dependence, which had a rate of 11.8%.
The depression and medical comorbidity group had very high prevalence rates (more than 20%) for heart attack, heart failure, stroke, cancer, dementia, arthritis, renal disease, sleep disorders, depression, and tobacco dependence. In addition, the rate was 60% for chronic obstructive pulmonary disease.
Participants in the SUD and related conditions group had rates of more than 70% for alcohol use disorder, other drug use disorders, and tobacco dependence. They also had high rates of COPD, hepatitis C, chronic pain, sleep disorders, depression, and PTSD.
On average, the SUD group was younger and may explain why they were less likely to have heart failure and renal disease, Dr. Hwong noted. These results may help inform treatment approaches, she added.
“For the group with largely substance use–related conditions, perhaps we can better address their needs with, for example, specific addiction and infectious disease services instead of a one-size-fits-all model,” said Dr. Hwong.
The investigators also examined mortality rates. Those in the depression and morbidity group had the highest rate of overall mortality; 47.5% of this class died during the observation period, compared with 27.2% of the SUD group.
More research is needed to understand why the mortality rate is so high in the depression and morbidity group, she said.
High rates of accidental death
The SUD group had the highest rates of death from accidents, possibly from overdoses, suicide, hepatitis C, and alcohol use–related deaths. “Their risks are very specific and appear largely related to substance use,” Dr. Hwong said.
The minimal comorbidity group showed the lowest rates of overall mortality rate (18%) and of cause-specific mortality for most of the included conditions.
Dr. Hwong noted she would like to study this class further. “I’m interested to know who are the people with schizophrenia who are thriving and are successfully aging – to learn what is going well for them.”
The researchers also plan to examine the subgroups in more detail to understand differences in treatments, health care utilization, and outcomes across groups. They are also interested in assessing other predictors of mortality outcomes in addition to multimorbidity.
One limitation of the study is that its cohort consisted of male veterans, so the findings may not be generalizable to other populations. In addition, these were observational data and so the results do not imply causality, Dr. Hwong said.
Dr. Hwong reported no relevant financial relationships, but she is supported by the VA and the UCSF National Clinician Scholars Program.
A version of this article first appeared on Medscape.com.
FROM AAGP 2022
TKA outcomes for age 80+ similar to younger patients
CHICAGO - Patients 80 years or older undergoing primary total knee arthroplasty (TKA) have similar odds of complications, compared with 65- to 79-year-old patients, an analysis of more than 1.7 million cases suggests.
Priscilla Varghese, MBA, MS, and an MD candidate at State University of New York, Brooklyn, led the research, presented at the American Academy of Orthopaedic Surgeons 2022 annual meeting.
Ms. Varghese’s team queried a Medicare claims database for the years 2005-2014 and analyzed information from 295,908 octogenarians and 1.4 million control patients aged 65-79 who received TKA.
Study group patients were randomly matched to controls in a 1:5 ratio according to gender and comorbidities, including chronic obstructive pulmonary disease, congestive heart failure, diabetes, peripheral vascular disease, and kidney failure.
Octogenarians were found to have higher incidence and odds of 90-day readmission rates (10.59% vs. 9.35%; odds ratio, 1.15; 95% confidence interval, 1.13-1.16; P < .0001).
Hospital stays were also longer (3.69 days ± 1.95 vs. 3.23 days ± 1.83; P < .0001), compared with controls.
Reassuring older patients
However, Ms. Varghese told this news organization she was surprised to find that the older group had equal incidence and odds of developing medical complications (1.26% vs. 1.26%; OR, 0.99; 95% CI, 0.96-1.03; P =.99).
“That’s a really important piece of information to have when we are advising 80-year-olds – to be able to say their risk of adverse outcomes is similar to someone who’s 10 years, 15 years younger,” she said. “It’s really reassuring.”
These results offer good news to older patients who might be hesitant to undergo the surgery, and good news in general as life expectancy increases and people stay active long into their later years, forecasting the need for more knee replacements.
The number of total knee replacements is expected to rise dramatically in the United States.
In a 2017 study published in Osteoarthritis Cartilage, the authors write, “the number of TKAs in the U.S., which already has the highest [incidence rate] of knee arthroplasty in the world, is expected to increase 143% by 2050.”
Thomas Fleeter, MD, an orthopedic surgeon practicing in Reston, Virginia, who was not involved in the study, told this news organization this study reinforces that “it’s OK to do knee replacements in elderly people; you just have to pick the right ones.”
He pointed out that the study also showed that the 80-and-older patients don’t have the added risk of loosening their mechanical components after the surgery, likely because they are less inclined than their younger counterparts to follow surgery with strenuous activities.
In a subanalysis, revision rates were also lower for the octogenarians (0.01% vs. 0.02% for controls).
Octogenarians who had TKA were found to have lower incidence and odds (1.6% vs. 1.93%; OR, 0.86; 95% CI, 0.83-0.88, P < .001) of implant-related complications, compared with the younger group.
The increased length of stay would be expected, Dr. Fleeter said, because those 80-plus may need a bit more help getting out of bed and may not have as much support at home.
A total knee replacement can have the substantial benefit of improving octogenarians’ ability to maintain their independence longer by facilitating driving or walking.
“It’s a small and manageable risk if you pick the right patients,” he said.
Demand for TKAs rises as population ages
As patients are living longer and wanting to maintain their mobility and as obesity rates are rising, more older patients will seek total knee replacements, especially since the payoff is high, Ms. Varghese noted.
“People who undergo this operation tend to show remarkable decreases in pain and increases in range of motion,” she said.
This study has the advantage of a more personalized look at risks of TKA because it stratifies age groups.
“The literature tends to look at the elderly population as one big cohort – 65 and older,” Ms. Varghese said. “We were able to provide patients more specific data.”
Ms. Varghese and Dr. Fleeter have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO - Patients 80 years or older undergoing primary total knee arthroplasty (TKA) have similar odds of complications, compared with 65- to 79-year-old patients, an analysis of more than 1.7 million cases suggests.
Priscilla Varghese, MBA, MS, and an MD candidate at State University of New York, Brooklyn, led the research, presented at the American Academy of Orthopaedic Surgeons 2022 annual meeting.
Ms. Varghese’s team queried a Medicare claims database for the years 2005-2014 and analyzed information from 295,908 octogenarians and 1.4 million control patients aged 65-79 who received TKA.
Study group patients were randomly matched to controls in a 1:5 ratio according to gender and comorbidities, including chronic obstructive pulmonary disease, congestive heart failure, diabetes, peripheral vascular disease, and kidney failure.
Octogenarians were found to have higher incidence and odds of 90-day readmission rates (10.59% vs. 9.35%; odds ratio, 1.15; 95% confidence interval, 1.13-1.16; P < .0001).
Hospital stays were also longer (3.69 days ± 1.95 vs. 3.23 days ± 1.83; P < .0001), compared with controls.
Reassuring older patients
However, Ms. Varghese told this news organization she was surprised to find that the older group had equal incidence and odds of developing medical complications (1.26% vs. 1.26%; OR, 0.99; 95% CI, 0.96-1.03; P =.99).
“That’s a really important piece of information to have when we are advising 80-year-olds – to be able to say their risk of adverse outcomes is similar to someone who’s 10 years, 15 years younger,” she said. “It’s really reassuring.”
These results offer good news to older patients who might be hesitant to undergo the surgery, and good news in general as life expectancy increases and people stay active long into their later years, forecasting the need for more knee replacements.
The number of total knee replacements is expected to rise dramatically in the United States.
In a 2017 study published in Osteoarthritis Cartilage, the authors write, “the number of TKAs in the U.S., which already has the highest [incidence rate] of knee arthroplasty in the world, is expected to increase 143% by 2050.”
Thomas Fleeter, MD, an orthopedic surgeon practicing in Reston, Virginia, who was not involved in the study, told this news organization this study reinforces that “it’s OK to do knee replacements in elderly people; you just have to pick the right ones.”
He pointed out that the study also showed that the 80-and-older patients don’t have the added risk of loosening their mechanical components after the surgery, likely because they are less inclined than their younger counterparts to follow surgery with strenuous activities.
In a subanalysis, revision rates were also lower for the octogenarians (0.01% vs. 0.02% for controls).
Octogenarians who had TKA were found to have lower incidence and odds (1.6% vs. 1.93%; OR, 0.86; 95% CI, 0.83-0.88, P < .001) of implant-related complications, compared with the younger group.
The increased length of stay would be expected, Dr. Fleeter said, because those 80-plus may need a bit more help getting out of bed and may not have as much support at home.
A total knee replacement can have the substantial benefit of improving octogenarians’ ability to maintain their independence longer by facilitating driving or walking.
“It’s a small and manageable risk if you pick the right patients,” he said.
Demand for TKAs rises as population ages
As patients are living longer and wanting to maintain their mobility and as obesity rates are rising, more older patients will seek total knee replacements, especially since the payoff is high, Ms. Varghese noted.
“People who undergo this operation tend to show remarkable decreases in pain and increases in range of motion,” she said.
This study has the advantage of a more personalized look at risks of TKA because it stratifies age groups.
“The literature tends to look at the elderly population as one big cohort – 65 and older,” Ms. Varghese said. “We were able to provide patients more specific data.”
Ms. Varghese and Dr. Fleeter have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO - Patients 80 years or older undergoing primary total knee arthroplasty (TKA) have similar odds of complications, compared with 65- to 79-year-old patients, an analysis of more than 1.7 million cases suggests.
Priscilla Varghese, MBA, MS, and an MD candidate at State University of New York, Brooklyn, led the research, presented at the American Academy of Orthopaedic Surgeons 2022 annual meeting.
Ms. Varghese’s team queried a Medicare claims database for the years 2005-2014 and analyzed information from 295,908 octogenarians and 1.4 million control patients aged 65-79 who received TKA.
Study group patients were randomly matched to controls in a 1:5 ratio according to gender and comorbidities, including chronic obstructive pulmonary disease, congestive heart failure, diabetes, peripheral vascular disease, and kidney failure.
Octogenarians were found to have higher incidence and odds of 90-day readmission rates (10.59% vs. 9.35%; odds ratio, 1.15; 95% confidence interval, 1.13-1.16; P < .0001).
Hospital stays were also longer (3.69 days ± 1.95 vs. 3.23 days ± 1.83; P < .0001), compared with controls.
Reassuring older patients
However, Ms. Varghese told this news organization she was surprised to find that the older group had equal incidence and odds of developing medical complications (1.26% vs. 1.26%; OR, 0.99; 95% CI, 0.96-1.03; P =.99).
“That’s a really important piece of information to have when we are advising 80-year-olds – to be able to say their risk of adverse outcomes is similar to someone who’s 10 years, 15 years younger,” she said. “It’s really reassuring.”
These results offer good news to older patients who might be hesitant to undergo the surgery, and good news in general as life expectancy increases and people stay active long into their later years, forecasting the need for more knee replacements.
The number of total knee replacements is expected to rise dramatically in the United States.
In a 2017 study published in Osteoarthritis Cartilage, the authors write, “the number of TKAs in the U.S., which already has the highest [incidence rate] of knee arthroplasty in the world, is expected to increase 143% by 2050.”
Thomas Fleeter, MD, an orthopedic surgeon practicing in Reston, Virginia, who was not involved in the study, told this news organization this study reinforces that “it’s OK to do knee replacements in elderly people; you just have to pick the right ones.”
He pointed out that the study also showed that the 80-and-older patients don’t have the added risk of loosening their mechanical components after the surgery, likely because they are less inclined than their younger counterparts to follow surgery with strenuous activities.
In a subanalysis, revision rates were also lower for the octogenarians (0.01% vs. 0.02% for controls).
Octogenarians who had TKA were found to have lower incidence and odds (1.6% vs. 1.93%; OR, 0.86; 95% CI, 0.83-0.88, P < .001) of implant-related complications, compared with the younger group.
The increased length of stay would be expected, Dr. Fleeter said, because those 80-plus may need a bit more help getting out of bed and may not have as much support at home.
A total knee replacement can have the substantial benefit of improving octogenarians’ ability to maintain their independence longer by facilitating driving or walking.
“It’s a small and manageable risk if you pick the right patients,” he said.
Demand for TKAs rises as population ages
As patients are living longer and wanting to maintain their mobility and as obesity rates are rising, more older patients will seek total knee replacements, especially since the payoff is high, Ms. Varghese noted.
“People who undergo this operation tend to show remarkable decreases in pain and increases in range of motion,” she said.
This study has the advantage of a more personalized look at risks of TKA because it stratifies age groups.
“The literature tends to look at the elderly population as one big cohort – 65 and older,” Ms. Varghese said. “We were able to provide patients more specific data.”
Ms. Varghese and Dr. Fleeter have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Aducanumab and ARIA: Does the FDA’s prescribing label put patients at risk?
Specifically, the drug’s label calls for three MRI brain scans before, and during, the titration period. The problem is the trial data used for the drug’s approval by the U.S. Food and Drug Administration included five MRIs to screen for ARIA.
“We recommend proceeding as per the clinical trials,” said Meghan Riddle, MD, associate director, Memory and Aging program, Butler Hospital, and assistant professor of psychiatry and human behavior, Brown University, Providence, R.I.
Dr. Riddle shared her team’s clinical experience with aducanumab, as well as information on four ARIA cases from their clinic, during a presentation at the American Association for Geriatric Psychiatry (AAGP) 2022 Annual Meeting.
Significant safety risk?
As previously reported by this news organization, the FDA granted accelerated approval of aducanumab for AD last year.
ARIA is the most common risk associated with aducanumab and has two types: ARIA-E (with edema) and ARIA-H (with hemosiderin). These can co-occur, particularly in areas of high amyloid burden, Dr. Riddle noted during her presentation.
ARIA is often detected incidentally via MRI. Patients are usually asymptomatic, but when they do have symptoms, headache, dizziness, and vision changes are the most common complaints. However, these are generally mild, said Dr. Riddle.
Nevertheless, in some cases, there can be severe sequelae, including severe edema or bleeding and seizures, she added.
A major risk factor for ARIA is apolipoprotein 4 (APOE ε4) status. Carriers are twice as likely to develop ARIA as non-carriers.
“If you’re heterozygote for APOE ε4, you have about a 40% chance of developing ARIA, and if you’re homozygote, you have about a 66% chance of developing ARIA,” Dr. Riddle said.
Given the high rate of ARIA in APOE ε4 carriers, the team from Butler Hospital recommends APOE testing prior to treatment with aducanumab.
The risk for developing ARIA is highest within the year of dose titration, Dr. Riddle noted. The current FDA label recommends obtaining a recent brain MRI, within 1 year, and then scans before the 7th and 12th infusions. However, the protocol during the clinical trials of aducanumab included MRI at baseline and prior to the 5th, 7th, 9th, and 12th infusions.
Dr. Riddle’s group has opted to continue the research protocol with new patients. “There’s concern that the decreased MRI monitoring based on the current FDA label may pose a significant safety risk, particularly among those who we know are already at a higher risk of developing ARIA,” she said.
Dr. Riddle also shared how her team selects aducanumab candidates. They need to have mild cognitive impairment (MCI), a mini-mental state examination (MMSE) score of 24 to 30, and a recent MRI to review for eligibility and APOE testing.
The most common reason for treatment exclusion is advanced disease and comorbidity, such as stroke.
Once approved for treatment, patients receive monthly infusions titrated over 6 months – 1 mg/kg for 2 months, 3 mg/kg for 2 months, 6 mg/kg for 2 months, then 10 mg/kg.
Patients are monitored to ensure safety and tolerability and regular review of MRI findings. In addition, patients and their families receive ongoing education about the drug.
Dr. Riddle and her team permanently discontinue the aducanumab if patients develop microhemorrhage, more than one area of superficial siderosis, more than 10 microhemorrhages, more than two episodes of ARIA, or severe symptoms of ARIA.
Four cases
Of the 11 patients who were candidates for aducanumab treatment, four developed ARIA. All are APOE ε4 carriers, with two homozygotes and two heterozygotes. All had severe radiographic ARIA-E, with one developing ARIA-H.
“Importantly, they were all initially asymptomatic and the ARIA was just picked up on their regular surveillance MRI,” said Dr. Riddle. She added that the drug was discontinued in all four cases.
Three of the ARIA cases were detected prior to the 5th scan, which is “concerning,” said Dr. Riddle. “Based on the current FDA label of safety monitoring, they don’t recommend doing that MRI. So [clinicians] would have dosed through that ARIA, which could put someone at much greater risk of developing severe symptoms.”
In addition, 14 patients at the center are receiving treatment with aducanumab. However, at this point they have not yet received their first MRI screen.
Dr. Riddle noted that when patients are told they are not candidates for treatment, or when treatment is discontinued, they are upset. However, she added, there is also a substantial level of understanding.
“We have a very layered discussion that includes the simple fact that we still aren’t sure if this is going to provide any clinical benefit, that this decision [to approve the drug] was accelerated, and that data are still being gathered,” Dr. Riddle added.
Dr. Riddle noted that the risk of ARIA is highest during the dose titration period: “There’s a signal that once you get to the 10 mg/kg dose, that plateaus.”
None of the patients at her center have reached that 12-month treatment mark. “The current plan is to do the MRI at 12 months then to give serial MRIs but less frequently, and whether that’s at 6 months or annually is yet to be determined.”
“We’re kind of writing these protocols as information evolves,” Dr. Riddle said.
The Memory and Aging Program receives grants from NIH-ADNI, Alzheimer’s Association, Fain Family Foundation, Joukowsky Family Foundation, Winter Family, Rhode Island Foundation, Goodman Family Foundation, and Global Alzheimer Platform Foundation; and clinical trials include: Lilly, Biogen, Genentech, Avid, Roche, Eisai, and Novartis. Dr. Riddle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Specifically, the drug’s label calls for three MRI brain scans before, and during, the titration period. The problem is the trial data used for the drug’s approval by the U.S. Food and Drug Administration included five MRIs to screen for ARIA.
“We recommend proceeding as per the clinical trials,” said Meghan Riddle, MD, associate director, Memory and Aging program, Butler Hospital, and assistant professor of psychiatry and human behavior, Brown University, Providence, R.I.
Dr. Riddle shared her team’s clinical experience with aducanumab, as well as information on four ARIA cases from their clinic, during a presentation at the American Association for Geriatric Psychiatry (AAGP) 2022 Annual Meeting.
Significant safety risk?
As previously reported by this news organization, the FDA granted accelerated approval of aducanumab for AD last year.
ARIA is the most common risk associated with aducanumab and has two types: ARIA-E (with edema) and ARIA-H (with hemosiderin). These can co-occur, particularly in areas of high amyloid burden, Dr. Riddle noted during her presentation.
ARIA is often detected incidentally via MRI. Patients are usually asymptomatic, but when they do have symptoms, headache, dizziness, and vision changes are the most common complaints. However, these are generally mild, said Dr. Riddle.
Nevertheless, in some cases, there can be severe sequelae, including severe edema or bleeding and seizures, she added.
A major risk factor for ARIA is apolipoprotein 4 (APOE ε4) status. Carriers are twice as likely to develop ARIA as non-carriers.
“If you’re heterozygote for APOE ε4, you have about a 40% chance of developing ARIA, and if you’re homozygote, you have about a 66% chance of developing ARIA,” Dr. Riddle said.
Given the high rate of ARIA in APOE ε4 carriers, the team from Butler Hospital recommends APOE testing prior to treatment with aducanumab.
The risk for developing ARIA is highest within the year of dose titration, Dr. Riddle noted. The current FDA label recommends obtaining a recent brain MRI, within 1 year, and then scans before the 7th and 12th infusions. However, the protocol during the clinical trials of aducanumab included MRI at baseline and prior to the 5th, 7th, 9th, and 12th infusions.
Dr. Riddle’s group has opted to continue the research protocol with new patients. “There’s concern that the decreased MRI monitoring based on the current FDA label may pose a significant safety risk, particularly among those who we know are already at a higher risk of developing ARIA,” she said.
Dr. Riddle also shared how her team selects aducanumab candidates. They need to have mild cognitive impairment (MCI), a mini-mental state examination (MMSE) score of 24 to 30, and a recent MRI to review for eligibility and APOE testing.
The most common reason for treatment exclusion is advanced disease and comorbidity, such as stroke.
Once approved for treatment, patients receive monthly infusions titrated over 6 months – 1 mg/kg for 2 months, 3 mg/kg for 2 months, 6 mg/kg for 2 months, then 10 mg/kg.
Patients are monitored to ensure safety and tolerability and regular review of MRI findings. In addition, patients and their families receive ongoing education about the drug.
Dr. Riddle and her team permanently discontinue the aducanumab if patients develop microhemorrhage, more than one area of superficial siderosis, more than 10 microhemorrhages, more than two episodes of ARIA, or severe symptoms of ARIA.
Four cases
Of the 11 patients who were candidates for aducanumab treatment, four developed ARIA. All are APOE ε4 carriers, with two homozygotes and two heterozygotes. All had severe radiographic ARIA-E, with one developing ARIA-H.
“Importantly, they were all initially asymptomatic and the ARIA was just picked up on their regular surveillance MRI,” said Dr. Riddle. She added that the drug was discontinued in all four cases.
Three of the ARIA cases were detected prior to the 5th scan, which is “concerning,” said Dr. Riddle. “Based on the current FDA label of safety monitoring, they don’t recommend doing that MRI. So [clinicians] would have dosed through that ARIA, which could put someone at much greater risk of developing severe symptoms.”
In addition, 14 patients at the center are receiving treatment with aducanumab. However, at this point they have not yet received their first MRI screen.
Dr. Riddle noted that when patients are told they are not candidates for treatment, or when treatment is discontinued, they are upset. However, she added, there is also a substantial level of understanding.
“We have a very layered discussion that includes the simple fact that we still aren’t sure if this is going to provide any clinical benefit, that this decision [to approve the drug] was accelerated, and that data are still being gathered,” Dr. Riddle added.
Dr. Riddle noted that the risk of ARIA is highest during the dose titration period: “There’s a signal that once you get to the 10 mg/kg dose, that plateaus.”
None of the patients at her center have reached that 12-month treatment mark. “The current plan is to do the MRI at 12 months then to give serial MRIs but less frequently, and whether that’s at 6 months or annually is yet to be determined.”
“We’re kind of writing these protocols as information evolves,” Dr. Riddle said.
The Memory and Aging Program receives grants from NIH-ADNI, Alzheimer’s Association, Fain Family Foundation, Joukowsky Family Foundation, Winter Family, Rhode Island Foundation, Goodman Family Foundation, and Global Alzheimer Platform Foundation; and clinical trials include: Lilly, Biogen, Genentech, Avid, Roche, Eisai, and Novartis. Dr. Riddle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Specifically, the drug’s label calls for three MRI brain scans before, and during, the titration period. The problem is the trial data used for the drug’s approval by the U.S. Food and Drug Administration included five MRIs to screen for ARIA.
“We recommend proceeding as per the clinical trials,” said Meghan Riddle, MD, associate director, Memory and Aging program, Butler Hospital, and assistant professor of psychiatry and human behavior, Brown University, Providence, R.I.
Dr. Riddle shared her team’s clinical experience with aducanumab, as well as information on four ARIA cases from their clinic, during a presentation at the American Association for Geriatric Psychiatry (AAGP) 2022 Annual Meeting.
Significant safety risk?
As previously reported by this news organization, the FDA granted accelerated approval of aducanumab for AD last year.
ARIA is the most common risk associated with aducanumab and has two types: ARIA-E (with edema) and ARIA-H (with hemosiderin). These can co-occur, particularly in areas of high amyloid burden, Dr. Riddle noted during her presentation.
ARIA is often detected incidentally via MRI. Patients are usually asymptomatic, but when they do have symptoms, headache, dizziness, and vision changes are the most common complaints. However, these are generally mild, said Dr. Riddle.
Nevertheless, in some cases, there can be severe sequelae, including severe edema or bleeding and seizures, she added.
A major risk factor for ARIA is apolipoprotein 4 (APOE ε4) status. Carriers are twice as likely to develop ARIA as non-carriers.
“If you’re heterozygote for APOE ε4, you have about a 40% chance of developing ARIA, and if you’re homozygote, you have about a 66% chance of developing ARIA,” Dr. Riddle said.
Given the high rate of ARIA in APOE ε4 carriers, the team from Butler Hospital recommends APOE testing prior to treatment with aducanumab.
The risk for developing ARIA is highest within the year of dose titration, Dr. Riddle noted. The current FDA label recommends obtaining a recent brain MRI, within 1 year, and then scans before the 7th and 12th infusions. However, the protocol during the clinical trials of aducanumab included MRI at baseline and prior to the 5th, 7th, 9th, and 12th infusions.
Dr. Riddle’s group has opted to continue the research protocol with new patients. “There’s concern that the decreased MRI monitoring based on the current FDA label may pose a significant safety risk, particularly among those who we know are already at a higher risk of developing ARIA,” she said.
Dr. Riddle also shared how her team selects aducanumab candidates. They need to have mild cognitive impairment (MCI), a mini-mental state examination (MMSE) score of 24 to 30, and a recent MRI to review for eligibility and APOE testing.
The most common reason for treatment exclusion is advanced disease and comorbidity, such as stroke.
Once approved for treatment, patients receive monthly infusions titrated over 6 months – 1 mg/kg for 2 months, 3 mg/kg for 2 months, 6 mg/kg for 2 months, then 10 mg/kg.
Patients are monitored to ensure safety and tolerability and regular review of MRI findings. In addition, patients and their families receive ongoing education about the drug.
Dr. Riddle and her team permanently discontinue the aducanumab if patients develop microhemorrhage, more than one area of superficial siderosis, more than 10 microhemorrhages, more than two episodes of ARIA, or severe symptoms of ARIA.
Four cases
Of the 11 patients who were candidates for aducanumab treatment, four developed ARIA. All are APOE ε4 carriers, with two homozygotes and two heterozygotes. All had severe radiographic ARIA-E, with one developing ARIA-H.
“Importantly, they were all initially asymptomatic and the ARIA was just picked up on their regular surveillance MRI,” said Dr. Riddle. She added that the drug was discontinued in all four cases.
Three of the ARIA cases were detected prior to the 5th scan, which is “concerning,” said Dr. Riddle. “Based on the current FDA label of safety monitoring, they don’t recommend doing that MRI. So [clinicians] would have dosed through that ARIA, which could put someone at much greater risk of developing severe symptoms.”
In addition, 14 patients at the center are receiving treatment with aducanumab. However, at this point they have not yet received their first MRI screen.
Dr. Riddle noted that when patients are told they are not candidates for treatment, or when treatment is discontinued, they are upset. However, she added, there is also a substantial level of understanding.
“We have a very layered discussion that includes the simple fact that we still aren’t sure if this is going to provide any clinical benefit, that this decision [to approve the drug] was accelerated, and that data are still being gathered,” Dr. Riddle added.
Dr. Riddle noted that the risk of ARIA is highest during the dose titration period: “There’s a signal that once you get to the 10 mg/kg dose, that plateaus.”
None of the patients at her center have reached that 12-month treatment mark. “The current plan is to do the MRI at 12 months then to give serial MRIs but less frequently, and whether that’s at 6 months or annually is yet to be determined.”
“We’re kind of writing these protocols as information evolves,” Dr. Riddle said.
The Memory and Aging Program receives grants from NIH-ADNI, Alzheimer’s Association, Fain Family Foundation, Joukowsky Family Foundation, Winter Family, Rhode Island Foundation, Goodman Family Foundation, and Global Alzheimer Platform Foundation; and clinical trials include: Lilly, Biogen, Genentech, Avid, Roche, Eisai, and Novartis. Dr. Riddle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAGP 2022
Using a Real-Time Prediction Algorithm to Improve Sleep in the Hospital
Study Overview
Objective: This study evaluated whether a clinical-decision-support (CDS) tool that utilizes a real-time algorithm incorporating patient vital sign data can identify hospitalized patients who can forgo overnight vital sign checks and thus reduce delirium incidence.
Design: This was a parallel randomized clinical trial of adult inpatients admitted to the general medical service of a tertiary care academic medical center in the United States. The trial intervention consisted of a CDS notification in the electronic health record (EHR) that informed the physician if a patient had a high likelihood of nighttime vital signs within the reference ranges based on a logistic regression model of real-time patient data input. This notification provided the physician an opportunity to discontinue nighttime vital sign checks, dismiss the notification for 1 hour, or dismiss the notification until the next day.
Setting and participants: This clinical trial was conducted at the University of California, San Francisco Medical Center from March 11 to November 24, 2019. Participants included physicians who served on the primary team (eg, attending, resident) of 1699 patients on the general medical service who were outside of the intensive care unit (ICU). The hospital encounters were randomized (allocation ratio of 1:1) to sleep promotion vitals CDS (SPV CDS) intervention or usual care.
Main outcome and measures: The primary outcome was delirium as determined by bedside nurse assessment using the Nursing Delirium Screening Scale (Nu-DESC) recorded once per nursing shift. The Nu-DESC is a standardized delirium screening tool that defines delirium with a score ≥2. Secondary outcomes included sleep opportunity (ie, EHR-based sleep metrics that reflected the maximum time between iatrogenic interruptions, such as nighttime vital sign checks) and patient satisfaction (ie, patient satisfaction measured by standardized Hospital Consumer Assessment of Healthcare Providers and Systems [HCAHPS] survey). Potential balancing outcomes were assessed to ensure that reduced vital sign checks were not causing harms; these included ICU transfers, rapid response calls, and code blue alarms. All analyses were conducted on the basis of intention-to-treat.
Main results: A total of 3025 inpatient encounters were screened and 1930 encounters were randomized (966 SPV CDS intervention; 964 usual care). The randomized encounters consisted of 1699 patients; demographic factors between the 2 trial arms were similar. Specifically, the intervention arm included 566 men (59%) and mean (SD) age was 53 (15) years. The incidence of delirium was similar between the intervention and usual care arms: 108 (11%) vs 123 (13%) (P = .32). Compared to the usual care arm, the intervention arm had a higher mean (SD) number of sleep opportunity hours per night (4.95 [1.45] vs 4.57 [1.30], P < .001) and fewer nighttime vital sign checks (0.97 [0.95] vs 1.41 [0.86], P < .001). The post-discharge HCAHPS survey measuring patient satisfaction was completed by only 5% of patients (53 intervention, 49 usual care), and survey results were similar between the 2 arms (P = .86). In addition, safety outcomes including ICU transfers (49 [5%] vs 47 [5%], P = .92), rapid response calls (68 [7%] vs 55 [6%], P = .27), and code blue alarms (2 [0.2%] vs 9 [0.9%], P = .07) were similar between the study arms.
Conclusion: In this randomized clinical trial, a CDS tool utilizing a real-time prediction algorithm embedded in EHR did not reduce the incidence of delirium in hospitalized patients. However, this SPV CDS intervention helped physicians identify clinically stable patients who can forgo routine nighttime vital sign checks and facilitated greater opportunity for patients to sleep. These findings suggest that augmenting physician judgment using a real-time prediction algorithm can help to improve sleep opportunity without an accompanying increased risk of clinical decompensation during acute care.
Commentary
High-quality sleep is fundamental to health and well-being. Sleep deprivation and disorders are associated with many adverse health outcomes, including increased risks for obesity, diabetes, hypertension, myocardial infarction, and depression.1 In hospitalized patients who are acutely ill, restorative sleep is critical to facilitating recovery. However, poor sleep is exceedingly common in hospitalized patients and is associated with deleterious outcomes, such as high blood pressure, hyperglycemia, and delirium.2,3 Moreover, some of these adverse sleep-induced cardiometabolic outcomes, as well as sleep disruption itself, may persist after hospital discharge.4 Factors that precipitate interrupted sleep during hospitalization include iatrogenic causes such as frequent vital sign checks, nighttime procedures or early morning blood draws, and environmental factors such as loud ambient noise.3 Thus, a potential intervention to improve sleep quality in the hospital is to reduce nighttime interruptions such as frequent vital sign checks.
In the current study, Najafi and colleagues conducted a randomized trial to evaluate whether a CDS tool embedded in EHR, powered by a real-time prediction algorithm of patient data, can be utilized to identify patients in whom vital sign checks can be safely discontinued at nighttime. The authors found a modest but statistically significant reduction in the number of nighttime vital sign checks in patients who underwent the SPV CDS intervention, and a corresponding higher sleep opportunity per night in those who received the intervention. Importantly, this reduction in nighttime vital sign checks did not cause a higher risk of clinical decompensation as measured by ICU transfers, rapid response calls, or code blue alarms. Thus, the results demonstrated the feasibility of using a real-time, patient data-driven CDS tool to augment physician judgment in managing sleep disruption, an important hospital-associated stressor and a common hazard of hospitalization in older patients.
Delirium is a common clinical problem in hospitalized older patients that is associated with prolonged hospitalization, functional and cognitive decline, institutionalization, death, and increased health care costs.5 Despite a potential benefit of SPV CDS intervention in reducing vital sign checks and increasing sleep opportunity, this intervention did not reduce the incidence of delirium in hospitalized patients. This finding is not surprising given that delirium has a multifactorial etiology (eg, metabolic derangements, infections, medication side effects and drug toxicity, hospital environment). A small modification in nighttime vital sign checks and sleep opportunity may have limited impact on optimizing sleep quality and does not address other risk factors for delirium. As such, a multicomponent nonpharmacologic approach that includes sleep enhancement, early mobilization, feeding assistance, fluid repletion, infection prevention, and other interventions should guide delirium prevention in the hospital setting. The SPV CDS intervention may play a role in the delivery of a multifaceted, nonpharmacologic delirium prevention intervention in high-risk individuals.
Sleep disruption is one of the multiple hazards of hospitalization frequently experience by hospitalized older patients. Other hazards, or hospital-associated stressors, include mobility restriction (eg, physical restraints such as urinary catheters and intravenous lines, bed elevation and rails), malnourishment and dehydration (eg, frequent use of no-food-by-mouth order, lack of easy access to hydration), and pain (eg, poor pain control). Extended exposures to these stressors may lead to a maladaptive state called allostatic overload that transiently increases vulnerability to post-hospitalization adverse events, including emergency department use, hospital readmission, or death (ie, post-hospital syndrome).6 Thus, the optimization of sleep during hospitalization in vulnerable patients may have benefits that extend beyond delirium prevention. It is perceivable that a CDS tool embedded in EHR, powered by a real-time prediction algorithm of patient data, may be applied to reduce some of these hazards of hospitalization in addition to improving sleep opportunity.
Applications for Clinical Practice
Findings from the current study indicate that a CDS tool embedded in EHR that utilizes a real-time prediction algorithm of patient data may help to safely improve sleep opportunity in hospitalized patients. The participants in the current study were relatively young (53 [15] years). Given that age is a risk factor for delirium, the effects of this intervention on delirium prevention in the most susceptible population (ie, those over the age of 65) remain unknown and further investigation is warranted. Additional studies are needed to determine whether this approach yields similar results in geriatric patients and improves clinical outcomes.
—Fred Ko, MD
1. Institute of Medicine (US) Committee on Sleep Medicine and Research. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Colten HR, Altevogt BM, editors. National Academies Press (US); 2006.
2. Pilkington S. Causes and consequences of sleep deprivation in hospitalised patients. Nurs Stand. 2013;27(49):350-342. doi:10.7748/ns2013.08.27.49.35.e7649
3. Stewart NH, Arora VM. Sleep in hospitalized older adults. Sleep Med Clin. 2018;13(1):127-135. doi:10.1016/j.jsmc.2017.09.012
4. Altman MT, Knauert MP, Pisani MA. Sleep disturbance after hospitalization and critical illness: a systematic review. Ann Am Thorac Soc. 2017;14(9):1457-1468. doi:10.1513/AnnalsATS.201702-148SR
5. Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. JAMA. 2017;318(12):1161-1174. doi:10.1001/jama.2017.12067
6. Goldwater DS, Dharmarajan K, McEwan BS, Krumholz HM. Is posthospital syndrome a result of hospitalization-induced allostatic overload? J Hosp Med. 2018;13(5). doi:10.12788/jhm.2986
Study Overview
Objective: This study evaluated whether a clinical-decision-support (CDS) tool that utilizes a real-time algorithm incorporating patient vital sign data can identify hospitalized patients who can forgo overnight vital sign checks and thus reduce delirium incidence.
Design: This was a parallel randomized clinical trial of adult inpatients admitted to the general medical service of a tertiary care academic medical center in the United States. The trial intervention consisted of a CDS notification in the electronic health record (EHR) that informed the physician if a patient had a high likelihood of nighttime vital signs within the reference ranges based on a logistic regression model of real-time patient data input. This notification provided the physician an opportunity to discontinue nighttime vital sign checks, dismiss the notification for 1 hour, or dismiss the notification until the next day.
Setting and participants: This clinical trial was conducted at the University of California, San Francisco Medical Center from March 11 to November 24, 2019. Participants included physicians who served on the primary team (eg, attending, resident) of 1699 patients on the general medical service who were outside of the intensive care unit (ICU). The hospital encounters were randomized (allocation ratio of 1:1) to sleep promotion vitals CDS (SPV CDS) intervention or usual care.
Main outcome and measures: The primary outcome was delirium as determined by bedside nurse assessment using the Nursing Delirium Screening Scale (Nu-DESC) recorded once per nursing shift. The Nu-DESC is a standardized delirium screening tool that defines delirium with a score ≥2. Secondary outcomes included sleep opportunity (ie, EHR-based sleep metrics that reflected the maximum time between iatrogenic interruptions, such as nighttime vital sign checks) and patient satisfaction (ie, patient satisfaction measured by standardized Hospital Consumer Assessment of Healthcare Providers and Systems [HCAHPS] survey). Potential balancing outcomes were assessed to ensure that reduced vital sign checks were not causing harms; these included ICU transfers, rapid response calls, and code blue alarms. All analyses were conducted on the basis of intention-to-treat.
Main results: A total of 3025 inpatient encounters were screened and 1930 encounters were randomized (966 SPV CDS intervention; 964 usual care). The randomized encounters consisted of 1699 patients; demographic factors between the 2 trial arms were similar. Specifically, the intervention arm included 566 men (59%) and mean (SD) age was 53 (15) years. The incidence of delirium was similar between the intervention and usual care arms: 108 (11%) vs 123 (13%) (P = .32). Compared to the usual care arm, the intervention arm had a higher mean (SD) number of sleep opportunity hours per night (4.95 [1.45] vs 4.57 [1.30], P < .001) and fewer nighttime vital sign checks (0.97 [0.95] vs 1.41 [0.86], P < .001). The post-discharge HCAHPS survey measuring patient satisfaction was completed by only 5% of patients (53 intervention, 49 usual care), and survey results were similar between the 2 arms (P = .86). In addition, safety outcomes including ICU transfers (49 [5%] vs 47 [5%], P = .92), rapid response calls (68 [7%] vs 55 [6%], P = .27), and code blue alarms (2 [0.2%] vs 9 [0.9%], P = .07) were similar between the study arms.
Conclusion: In this randomized clinical trial, a CDS tool utilizing a real-time prediction algorithm embedded in EHR did not reduce the incidence of delirium in hospitalized patients. However, this SPV CDS intervention helped physicians identify clinically stable patients who can forgo routine nighttime vital sign checks and facilitated greater opportunity for patients to sleep. These findings suggest that augmenting physician judgment using a real-time prediction algorithm can help to improve sleep opportunity without an accompanying increased risk of clinical decompensation during acute care.
Commentary
High-quality sleep is fundamental to health and well-being. Sleep deprivation and disorders are associated with many adverse health outcomes, including increased risks for obesity, diabetes, hypertension, myocardial infarction, and depression.1 In hospitalized patients who are acutely ill, restorative sleep is critical to facilitating recovery. However, poor sleep is exceedingly common in hospitalized patients and is associated with deleterious outcomes, such as high blood pressure, hyperglycemia, and delirium.2,3 Moreover, some of these adverse sleep-induced cardiometabolic outcomes, as well as sleep disruption itself, may persist after hospital discharge.4 Factors that precipitate interrupted sleep during hospitalization include iatrogenic causes such as frequent vital sign checks, nighttime procedures or early morning blood draws, and environmental factors such as loud ambient noise.3 Thus, a potential intervention to improve sleep quality in the hospital is to reduce nighttime interruptions such as frequent vital sign checks.
In the current study, Najafi and colleagues conducted a randomized trial to evaluate whether a CDS tool embedded in EHR, powered by a real-time prediction algorithm of patient data, can be utilized to identify patients in whom vital sign checks can be safely discontinued at nighttime. The authors found a modest but statistically significant reduction in the number of nighttime vital sign checks in patients who underwent the SPV CDS intervention, and a corresponding higher sleep opportunity per night in those who received the intervention. Importantly, this reduction in nighttime vital sign checks did not cause a higher risk of clinical decompensation as measured by ICU transfers, rapid response calls, or code blue alarms. Thus, the results demonstrated the feasibility of using a real-time, patient data-driven CDS tool to augment physician judgment in managing sleep disruption, an important hospital-associated stressor and a common hazard of hospitalization in older patients.
Delirium is a common clinical problem in hospitalized older patients that is associated with prolonged hospitalization, functional and cognitive decline, institutionalization, death, and increased health care costs.5 Despite a potential benefit of SPV CDS intervention in reducing vital sign checks and increasing sleep opportunity, this intervention did not reduce the incidence of delirium in hospitalized patients. This finding is not surprising given that delirium has a multifactorial etiology (eg, metabolic derangements, infections, medication side effects and drug toxicity, hospital environment). A small modification in nighttime vital sign checks and sleep opportunity may have limited impact on optimizing sleep quality and does not address other risk factors for delirium. As such, a multicomponent nonpharmacologic approach that includes sleep enhancement, early mobilization, feeding assistance, fluid repletion, infection prevention, and other interventions should guide delirium prevention in the hospital setting. The SPV CDS intervention may play a role in the delivery of a multifaceted, nonpharmacologic delirium prevention intervention in high-risk individuals.
Sleep disruption is one of the multiple hazards of hospitalization frequently experience by hospitalized older patients. Other hazards, or hospital-associated stressors, include mobility restriction (eg, physical restraints such as urinary catheters and intravenous lines, bed elevation and rails), malnourishment and dehydration (eg, frequent use of no-food-by-mouth order, lack of easy access to hydration), and pain (eg, poor pain control). Extended exposures to these stressors may lead to a maladaptive state called allostatic overload that transiently increases vulnerability to post-hospitalization adverse events, including emergency department use, hospital readmission, or death (ie, post-hospital syndrome).6 Thus, the optimization of sleep during hospitalization in vulnerable patients may have benefits that extend beyond delirium prevention. It is perceivable that a CDS tool embedded in EHR, powered by a real-time prediction algorithm of patient data, may be applied to reduce some of these hazards of hospitalization in addition to improving sleep opportunity.
Applications for Clinical Practice
Findings from the current study indicate that a CDS tool embedded in EHR that utilizes a real-time prediction algorithm of patient data may help to safely improve sleep opportunity in hospitalized patients. The participants in the current study were relatively young (53 [15] years). Given that age is a risk factor for delirium, the effects of this intervention on delirium prevention in the most susceptible population (ie, those over the age of 65) remain unknown and further investigation is warranted. Additional studies are needed to determine whether this approach yields similar results in geriatric patients and improves clinical outcomes.
—Fred Ko, MD
Study Overview
Objective: This study evaluated whether a clinical-decision-support (CDS) tool that utilizes a real-time algorithm incorporating patient vital sign data can identify hospitalized patients who can forgo overnight vital sign checks and thus reduce delirium incidence.
Design: This was a parallel randomized clinical trial of adult inpatients admitted to the general medical service of a tertiary care academic medical center in the United States. The trial intervention consisted of a CDS notification in the electronic health record (EHR) that informed the physician if a patient had a high likelihood of nighttime vital signs within the reference ranges based on a logistic regression model of real-time patient data input. This notification provided the physician an opportunity to discontinue nighttime vital sign checks, dismiss the notification for 1 hour, or dismiss the notification until the next day.
Setting and participants: This clinical trial was conducted at the University of California, San Francisco Medical Center from March 11 to November 24, 2019. Participants included physicians who served on the primary team (eg, attending, resident) of 1699 patients on the general medical service who were outside of the intensive care unit (ICU). The hospital encounters were randomized (allocation ratio of 1:1) to sleep promotion vitals CDS (SPV CDS) intervention or usual care.
Main outcome and measures: The primary outcome was delirium as determined by bedside nurse assessment using the Nursing Delirium Screening Scale (Nu-DESC) recorded once per nursing shift. The Nu-DESC is a standardized delirium screening tool that defines delirium with a score ≥2. Secondary outcomes included sleep opportunity (ie, EHR-based sleep metrics that reflected the maximum time between iatrogenic interruptions, such as nighttime vital sign checks) and patient satisfaction (ie, patient satisfaction measured by standardized Hospital Consumer Assessment of Healthcare Providers and Systems [HCAHPS] survey). Potential balancing outcomes were assessed to ensure that reduced vital sign checks were not causing harms; these included ICU transfers, rapid response calls, and code blue alarms. All analyses were conducted on the basis of intention-to-treat.
Main results: A total of 3025 inpatient encounters were screened and 1930 encounters were randomized (966 SPV CDS intervention; 964 usual care). The randomized encounters consisted of 1699 patients; demographic factors between the 2 trial arms were similar. Specifically, the intervention arm included 566 men (59%) and mean (SD) age was 53 (15) years. The incidence of delirium was similar between the intervention and usual care arms: 108 (11%) vs 123 (13%) (P = .32). Compared to the usual care arm, the intervention arm had a higher mean (SD) number of sleep opportunity hours per night (4.95 [1.45] vs 4.57 [1.30], P < .001) and fewer nighttime vital sign checks (0.97 [0.95] vs 1.41 [0.86], P < .001). The post-discharge HCAHPS survey measuring patient satisfaction was completed by only 5% of patients (53 intervention, 49 usual care), and survey results were similar between the 2 arms (P = .86). In addition, safety outcomes including ICU transfers (49 [5%] vs 47 [5%], P = .92), rapid response calls (68 [7%] vs 55 [6%], P = .27), and code blue alarms (2 [0.2%] vs 9 [0.9%], P = .07) were similar between the study arms.
Conclusion: In this randomized clinical trial, a CDS tool utilizing a real-time prediction algorithm embedded in EHR did not reduce the incidence of delirium in hospitalized patients. However, this SPV CDS intervention helped physicians identify clinically stable patients who can forgo routine nighttime vital sign checks and facilitated greater opportunity for patients to sleep. These findings suggest that augmenting physician judgment using a real-time prediction algorithm can help to improve sleep opportunity without an accompanying increased risk of clinical decompensation during acute care.
Commentary
High-quality sleep is fundamental to health and well-being. Sleep deprivation and disorders are associated with many adverse health outcomes, including increased risks for obesity, diabetes, hypertension, myocardial infarction, and depression.1 In hospitalized patients who are acutely ill, restorative sleep is critical to facilitating recovery. However, poor sleep is exceedingly common in hospitalized patients and is associated with deleterious outcomes, such as high blood pressure, hyperglycemia, and delirium.2,3 Moreover, some of these adverse sleep-induced cardiometabolic outcomes, as well as sleep disruption itself, may persist after hospital discharge.4 Factors that precipitate interrupted sleep during hospitalization include iatrogenic causes such as frequent vital sign checks, nighttime procedures or early morning blood draws, and environmental factors such as loud ambient noise.3 Thus, a potential intervention to improve sleep quality in the hospital is to reduce nighttime interruptions such as frequent vital sign checks.
In the current study, Najafi and colleagues conducted a randomized trial to evaluate whether a CDS tool embedded in EHR, powered by a real-time prediction algorithm of patient data, can be utilized to identify patients in whom vital sign checks can be safely discontinued at nighttime. The authors found a modest but statistically significant reduction in the number of nighttime vital sign checks in patients who underwent the SPV CDS intervention, and a corresponding higher sleep opportunity per night in those who received the intervention. Importantly, this reduction in nighttime vital sign checks did not cause a higher risk of clinical decompensation as measured by ICU transfers, rapid response calls, or code blue alarms. Thus, the results demonstrated the feasibility of using a real-time, patient data-driven CDS tool to augment physician judgment in managing sleep disruption, an important hospital-associated stressor and a common hazard of hospitalization in older patients.
Delirium is a common clinical problem in hospitalized older patients that is associated with prolonged hospitalization, functional and cognitive decline, institutionalization, death, and increased health care costs.5 Despite a potential benefit of SPV CDS intervention in reducing vital sign checks and increasing sleep opportunity, this intervention did not reduce the incidence of delirium in hospitalized patients. This finding is not surprising given that delirium has a multifactorial etiology (eg, metabolic derangements, infections, medication side effects and drug toxicity, hospital environment). A small modification in nighttime vital sign checks and sleep opportunity may have limited impact on optimizing sleep quality and does not address other risk factors for delirium. As such, a multicomponent nonpharmacologic approach that includes sleep enhancement, early mobilization, feeding assistance, fluid repletion, infection prevention, and other interventions should guide delirium prevention in the hospital setting. The SPV CDS intervention may play a role in the delivery of a multifaceted, nonpharmacologic delirium prevention intervention in high-risk individuals.
Sleep disruption is one of the multiple hazards of hospitalization frequently experience by hospitalized older patients. Other hazards, or hospital-associated stressors, include mobility restriction (eg, physical restraints such as urinary catheters and intravenous lines, bed elevation and rails), malnourishment and dehydration (eg, frequent use of no-food-by-mouth order, lack of easy access to hydration), and pain (eg, poor pain control). Extended exposures to these stressors may lead to a maladaptive state called allostatic overload that transiently increases vulnerability to post-hospitalization adverse events, including emergency department use, hospital readmission, or death (ie, post-hospital syndrome).6 Thus, the optimization of sleep during hospitalization in vulnerable patients may have benefits that extend beyond delirium prevention. It is perceivable that a CDS tool embedded in EHR, powered by a real-time prediction algorithm of patient data, may be applied to reduce some of these hazards of hospitalization in addition to improving sleep opportunity.
Applications for Clinical Practice
Findings from the current study indicate that a CDS tool embedded in EHR that utilizes a real-time prediction algorithm of patient data may help to safely improve sleep opportunity in hospitalized patients. The participants in the current study were relatively young (53 [15] years). Given that age is a risk factor for delirium, the effects of this intervention on delirium prevention in the most susceptible population (ie, those over the age of 65) remain unknown and further investigation is warranted. Additional studies are needed to determine whether this approach yields similar results in geriatric patients and improves clinical outcomes.
—Fred Ko, MD
1. Institute of Medicine (US) Committee on Sleep Medicine and Research. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Colten HR, Altevogt BM, editors. National Academies Press (US); 2006.
2. Pilkington S. Causes and consequences of sleep deprivation in hospitalised patients. Nurs Stand. 2013;27(49):350-342. doi:10.7748/ns2013.08.27.49.35.e7649
3. Stewart NH, Arora VM. Sleep in hospitalized older adults. Sleep Med Clin. 2018;13(1):127-135. doi:10.1016/j.jsmc.2017.09.012
4. Altman MT, Knauert MP, Pisani MA. Sleep disturbance after hospitalization and critical illness: a systematic review. Ann Am Thorac Soc. 2017;14(9):1457-1468. doi:10.1513/AnnalsATS.201702-148SR
5. Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. JAMA. 2017;318(12):1161-1174. doi:10.1001/jama.2017.12067
6. Goldwater DS, Dharmarajan K, McEwan BS, Krumholz HM. Is posthospital syndrome a result of hospitalization-induced allostatic overload? J Hosp Med. 2018;13(5). doi:10.12788/jhm.2986
1. Institute of Medicine (US) Committee on Sleep Medicine and Research. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Colten HR, Altevogt BM, editors. National Academies Press (US); 2006.
2. Pilkington S. Causes and consequences of sleep deprivation in hospitalised patients. Nurs Stand. 2013;27(49):350-342. doi:10.7748/ns2013.08.27.49.35.e7649
3. Stewart NH, Arora VM. Sleep in hospitalized older adults. Sleep Med Clin. 2018;13(1):127-135. doi:10.1016/j.jsmc.2017.09.012
4. Altman MT, Knauert MP, Pisani MA. Sleep disturbance after hospitalization and critical illness: a systematic review. Ann Am Thorac Soc. 2017;14(9):1457-1468. doi:10.1513/AnnalsATS.201702-148SR
5. Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. JAMA. 2017;318(12):1161-1174. doi:10.1001/jama.2017.12067
6. Goldwater DS, Dharmarajan K, McEwan BS, Krumholz HM. Is posthospital syndrome a result of hospitalization-induced allostatic overload? J Hosp Med. 2018;13(5). doi:10.12788/jhm.2986








