User login
Midlife cardiovascular conditions tied to greater cognitive decline in women
Even though men in midlife have more cardiovascular (CV) conditions and risk factors than women of the same age, women are more affected by these conditions in terms of cognitive decline, new research suggests.
Analyses of almost 1,400 participants in the population-based Mayo Clinic Study of Aging showed that diabetes, dyslipidemia, and coronary heart disease (CHD) all had stronger associations with global cognitive decline in women than in men.
“All men and women should be treated for cardiovascular risk factors and conditions, but this study really highlights the importance of very early and perhaps more aggressive treatment in women with these conditions,” co-investigator Michelle M. Mielke, PhD, professor of epidemiology and neurology, Mayo Clinic, Rochester, Minn., told this news organization.
The findings were published online Jan. 5 in Neurology.
Assessing sex differences
Most previous studies in this area have focused on CV risk factors in midlife in relation to late-life dementia (after age 75) or on late-life vascular risk factors and late-life dementia, Dr. Mielke noted.
However, a few recent studies have suggested vascular risk factors can affect cognition even in midlife. The current investigators sought to determine whether there are sex differences in these associations.
They assessed 1,857 nondemented participants aged 50 to 69 years from the Mayo Clinic Study on Aging. The mean education level was 14.9 years, and the mean body mass index (BMI) was 29.7.
Among the participants, 78.9% had at least one CV condition or risk factor, and the proportion was higher in men than women (83.4% vs. 74.5%; P < .0001).
Frequency of each individual CV condition or risk factor was also higher in men than women, and they had more years of education and higher BMI but took fewer medications.
Every 15 months, participants had an in-person interview and physical examination that included a neurologic assessment and short test of memory.
The neuropsychological battery included nine tests across four domains: memory, language, executive function, and visuospatial skills. Researchers calculated z-scores for these domains and for global cognition.
Multiple cognitive domains
Whereas this study evaluated multiple cognitive domains, most previous research has focused on global cognitive decline and/or decline in only one or two cognitive domains, the investigators note.
They collected information from medical records on CV conditions such as CHD, arrhythmias, congestive heart failure, peripheral vascular disease (PVD), and stroke; and CV risk factors such as hypertension, diabetes, dyslipidemia, smoking status, and BMI.
Because of the small number of patients with stroke and PVD, these were classified as “other cardiovascular conditions” in the statistical analysis.
Researchers adjusted for sex, age, years of education, depressive symptoms, comorbidities, medications, and apolipoprotein E (APOE) genotyping. The mean follow-up was 3 years and did not differ by sex.
As some participants didn’t have a follow-up visit, the current analysis included 1,394 individuals. Those without follow-up visits were younger, had less education and more comorbidities, and took more medications compared with those with a follow-up.
Results showed most CV conditions were more strongly associated with cognitive function among women than men. For example, CHD was associated with global decline only in women (P < .05).
CHD, diabetes, and dyslipidemia were associated with language decline in women only (all, P < .05), but congestive heart failure was significantly associated with language decline in men only.
Dr. Mielke cautioned about reading too much into the language results for women.
“It’s an intriguing finding and definitely we need to follow up on it,” she said. However, “more studies are needed to examine sex differences before we start saying it only has an effect on language.”
‘Treat aggressively and right away’
The researchers were somewhat surprised by the study findings. Because there is a higher prevalence of CV conditions and risk factors in men, they presumed men would be more affected by these conditions, said Dr. Mielke.
“But that’s not what we saw; we saw the reverse. It was actually the women who were affected more by these cardiovascular risk factors and conditions,” she said.
As midlife is when women enter menopause, fluctuating estrogen levels may help explain the differential impact on cognition among women. But Dr. Mielke said she wants to “move beyond” just looking at hormones.
She pointed out there are a variety of psychosocial factors that may also contribute to an imbalance in the cognitive impact of CV conditions on women.
“Midlife is when many women are still taking care of their children at home, are also taking care of their adult parents, and may be undergoing more stress while continuing to do a job,” Dr. Miekle said.
Structural brain development and genetics may also contribute to the greater effect on cognition in women, the investigators note.
Dr. Mielke stressed that the current study only identifies associations. “The next steps are to understand what some of the underlying mechanisms for this are,” she said.
In the meantime, these new results suggest middle-aged women with high blood pressure, cholesterol, or glucose measures “should be treated aggressively and right away” said Dr. Mielke.
“For example, for women who are just starting to become hypertensive, clinicians should treat them right away and not watch and wait.”
Study limitations cited include that its sample was limited to Olmsted County, Minnesota – so results may not be generalized to other populations. Also, as researchers combined PVD and stroke into one group, larger sample sizes are needed, especially for stroke. Another limitation was the study did not have information on duration of all CV conditions or risk factors.
Helpful for tailoring interventions?
Commenting on the study, Glen R. Finney, MD, director, Memory and Cognition Program, Geisinger Health Clinic, Wilkes-Barre, Pennsylvania, said the results are important.
“The more we understand about risk factors for the development of Alzheimer’s disease and related dementias, the better we understand how we can reduce the risks,” said Dr. Finney, who was not involved with the research.
Awareness that CV conditions are major risk factors in midlife has been “definitely rising,” said Dr. Finney. “Many studies originally were looking at late life and are now looking more at earlier in the disease process, and I think that’s important.”
Understanding how sex, ethnicity, and other demographic variables affect risks can help to “tailor interventions” for individual patients, he said.
The study was supported by the National Institutes of Health, the GHR Foundation, and the Rochester Epidemiology Project. Dr. Mielke is a consultant for Biogen and Brain Protection Company and is on the editorial boards of Neurology and Alzheimer’s and Dementia. Dr. Finney has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Even though men in midlife have more cardiovascular (CV) conditions and risk factors than women of the same age, women are more affected by these conditions in terms of cognitive decline, new research suggests.
Analyses of almost 1,400 participants in the population-based Mayo Clinic Study of Aging showed that diabetes, dyslipidemia, and coronary heart disease (CHD) all had stronger associations with global cognitive decline in women than in men.
“All men and women should be treated for cardiovascular risk factors and conditions, but this study really highlights the importance of very early and perhaps more aggressive treatment in women with these conditions,” co-investigator Michelle M. Mielke, PhD, professor of epidemiology and neurology, Mayo Clinic, Rochester, Minn., told this news organization.
The findings were published online Jan. 5 in Neurology.
Assessing sex differences
Most previous studies in this area have focused on CV risk factors in midlife in relation to late-life dementia (after age 75) or on late-life vascular risk factors and late-life dementia, Dr. Mielke noted.
However, a few recent studies have suggested vascular risk factors can affect cognition even in midlife. The current investigators sought to determine whether there are sex differences in these associations.
They assessed 1,857 nondemented participants aged 50 to 69 years from the Mayo Clinic Study on Aging. The mean education level was 14.9 years, and the mean body mass index (BMI) was 29.7.
Among the participants, 78.9% had at least one CV condition or risk factor, and the proportion was higher in men than women (83.4% vs. 74.5%; P < .0001).
Frequency of each individual CV condition or risk factor was also higher in men than women, and they had more years of education and higher BMI but took fewer medications.
Every 15 months, participants had an in-person interview and physical examination that included a neurologic assessment and short test of memory.
The neuropsychological battery included nine tests across four domains: memory, language, executive function, and visuospatial skills. Researchers calculated z-scores for these domains and for global cognition.
Multiple cognitive domains
Whereas this study evaluated multiple cognitive domains, most previous research has focused on global cognitive decline and/or decline in only one or two cognitive domains, the investigators note.
They collected information from medical records on CV conditions such as CHD, arrhythmias, congestive heart failure, peripheral vascular disease (PVD), and stroke; and CV risk factors such as hypertension, diabetes, dyslipidemia, smoking status, and BMI.
Because of the small number of patients with stroke and PVD, these were classified as “other cardiovascular conditions” in the statistical analysis.
Researchers adjusted for sex, age, years of education, depressive symptoms, comorbidities, medications, and apolipoprotein E (APOE) genotyping. The mean follow-up was 3 years and did not differ by sex.
As some participants didn’t have a follow-up visit, the current analysis included 1,394 individuals. Those without follow-up visits were younger, had less education and more comorbidities, and took more medications compared with those with a follow-up.
Results showed most CV conditions were more strongly associated with cognitive function among women than men. For example, CHD was associated with global decline only in women (P < .05).
CHD, diabetes, and dyslipidemia were associated with language decline in women only (all, P < .05), but congestive heart failure was significantly associated with language decline in men only.
Dr. Mielke cautioned about reading too much into the language results for women.
“It’s an intriguing finding and definitely we need to follow up on it,” she said. However, “more studies are needed to examine sex differences before we start saying it only has an effect on language.”
‘Treat aggressively and right away’
The researchers were somewhat surprised by the study findings. Because there is a higher prevalence of CV conditions and risk factors in men, they presumed men would be more affected by these conditions, said Dr. Mielke.
“But that’s not what we saw; we saw the reverse. It was actually the women who were affected more by these cardiovascular risk factors and conditions,” she said.
As midlife is when women enter menopause, fluctuating estrogen levels may help explain the differential impact on cognition among women. But Dr. Mielke said she wants to “move beyond” just looking at hormones.
She pointed out there are a variety of psychosocial factors that may also contribute to an imbalance in the cognitive impact of CV conditions on women.
“Midlife is when many women are still taking care of their children at home, are also taking care of their adult parents, and may be undergoing more stress while continuing to do a job,” Dr. Miekle said.
Structural brain development and genetics may also contribute to the greater effect on cognition in women, the investigators note.
Dr. Mielke stressed that the current study only identifies associations. “The next steps are to understand what some of the underlying mechanisms for this are,” she said.
In the meantime, these new results suggest middle-aged women with high blood pressure, cholesterol, or glucose measures “should be treated aggressively and right away” said Dr. Mielke.
“For example, for women who are just starting to become hypertensive, clinicians should treat them right away and not watch and wait.”
Study limitations cited include that its sample was limited to Olmsted County, Minnesota – so results may not be generalized to other populations. Also, as researchers combined PVD and stroke into one group, larger sample sizes are needed, especially for stroke. Another limitation was the study did not have information on duration of all CV conditions or risk factors.
Helpful for tailoring interventions?
Commenting on the study, Glen R. Finney, MD, director, Memory and Cognition Program, Geisinger Health Clinic, Wilkes-Barre, Pennsylvania, said the results are important.
“The more we understand about risk factors for the development of Alzheimer’s disease and related dementias, the better we understand how we can reduce the risks,” said Dr. Finney, who was not involved with the research.
Awareness that CV conditions are major risk factors in midlife has been “definitely rising,” said Dr. Finney. “Many studies originally were looking at late life and are now looking more at earlier in the disease process, and I think that’s important.”
Understanding how sex, ethnicity, and other demographic variables affect risks can help to “tailor interventions” for individual patients, he said.
The study was supported by the National Institutes of Health, the GHR Foundation, and the Rochester Epidemiology Project. Dr. Mielke is a consultant for Biogen and Brain Protection Company and is on the editorial boards of Neurology and Alzheimer’s and Dementia. Dr. Finney has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Even though men in midlife have more cardiovascular (CV) conditions and risk factors than women of the same age, women are more affected by these conditions in terms of cognitive decline, new research suggests.
Analyses of almost 1,400 participants in the population-based Mayo Clinic Study of Aging showed that diabetes, dyslipidemia, and coronary heart disease (CHD) all had stronger associations with global cognitive decline in women than in men.
“All men and women should be treated for cardiovascular risk factors and conditions, but this study really highlights the importance of very early and perhaps more aggressive treatment in women with these conditions,” co-investigator Michelle M. Mielke, PhD, professor of epidemiology and neurology, Mayo Clinic, Rochester, Minn., told this news organization.
The findings were published online Jan. 5 in Neurology.
Assessing sex differences
Most previous studies in this area have focused on CV risk factors in midlife in relation to late-life dementia (after age 75) or on late-life vascular risk factors and late-life dementia, Dr. Mielke noted.
However, a few recent studies have suggested vascular risk factors can affect cognition even in midlife. The current investigators sought to determine whether there are sex differences in these associations.
They assessed 1,857 nondemented participants aged 50 to 69 years from the Mayo Clinic Study on Aging. The mean education level was 14.9 years, and the mean body mass index (BMI) was 29.7.
Among the participants, 78.9% had at least one CV condition or risk factor, and the proportion was higher in men than women (83.4% vs. 74.5%; P < .0001).
Frequency of each individual CV condition or risk factor was also higher in men than women, and they had more years of education and higher BMI but took fewer medications.
Every 15 months, participants had an in-person interview and physical examination that included a neurologic assessment and short test of memory.
The neuropsychological battery included nine tests across four domains: memory, language, executive function, and visuospatial skills. Researchers calculated z-scores for these domains and for global cognition.
Multiple cognitive domains
Whereas this study evaluated multiple cognitive domains, most previous research has focused on global cognitive decline and/or decline in only one or two cognitive domains, the investigators note.
They collected information from medical records on CV conditions such as CHD, arrhythmias, congestive heart failure, peripheral vascular disease (PVD), and stroke; and CV risk factors such as hypertension, diabetes, dyslipidemia, smoking status, and BMI.
Because of the small number of patients with stroke and PVD, these were classified as “other cardiovascular conditions” in the statistical analysis.
Researchers adjusted for sex, age, years of education, depressive symptoms, comorbidities, medications, and apolipoprotein E (APOE) genotyping. The mean follow-up was 3 years and did not differ by sex.
As some participants didn’t have a follow-up visit, the current analysis included 1,394 individuals. Those without follow-up visits were younger, had less education and more comorbidities, and took more medications compared with those with a follow-up.
Results showed most CV conditions were more strongly associated with cognitive function among women than men. For example, CHD was associated with global decline only in women (P < .05).
CHD, diabetes, and dyslipidemia were associated with language decline in women only (all, P < .05), but congestive heart failure was significantly associated with language decline in men only.
Dr. Mielke cautioned about reading too much into the language results for women.
“It’s an intriguing finding and definitely we need to follow up on it,” she said. However, “more studies are needed to examine sex differences before we start saying it only has an effect on language.”
‘Treat aggressively and right away’
The researchers were somewhat surprised by the study findings. Because there is a higher prevalence of CV conditions and risk factors in men, they presumed men would be more affected by these conditions, said Dr. Mielke.
“But that’s not what we saw; we saw the reverse. It was actually the women who were affected more by these cardiovascular risk factors and conditions,” she said.
As midlife is when women enter menopause, fluctuating estrogen levels may help explain the differential impact on cognition among women. But Dr. Mielke said she wants to “move beyond” just looking at hormones.
She pointed out there are a variety of psychosocial factors that may also contribute to an imbalance in the cognitive impact of CV conditions on women.
“Midlife is when many women are still taking care of their children at home, are also taking care of their adult parents, and may be undergoing more stress while continuing to do a job,” Dr. Miekle said.
Structural brain development and genetics may also contribute to the greater effect on cognition in women, the investigators note.
Dr. Mielke stressed that the current study only identifies associations. “The next steps are to understand what some of the underlying mechanisms for this are,” she said.
In the meantime, these new results suggest middle-aged women with high blood pressure, cholesterol, or glucose measures “should be treated aggressively and right away” said Dr. Mielke.
“For example, for women who are just starting to become hypertensive, clinicians should treat them right away and not watch and wait.”
Study limitations cited include that its sample was limited to Olmsted County, Minnesota – so results may not be generalized to other populations. Also, as researchers combined PVD and stroke into one group, larger sample sizes are needed, especially for stroke. Another limitation was the study did not have information on duration of all CV conditions or risk factors.
Helpful for tailoring interventions?
Commenting on the study, Glen R. Finney, MD, director, Memory and Cognition Program, Geisinger Health Clinic, Wilkes-Barre, Pennsylvania, said the results are important.
“The more we understand about risk factors for the development of Alzheimer’s disease and related dementias, the better we understand how we can reduce the risks,” said Dr. Finney, who was not involved with the research.
Awareness that CV conditions are major risk factors in midlife has been “definitely rising,” said Dr. Finney. “Many studies originally were looking at late life and are now looking more at earlier in the disease process, and I think that’s important.”
Understanding how sex, ethnicity, and other demographic variables affect risks can help to “tailor interventions” for individual patients, he said.
The study was supported by the National Institutes of Health, the GHR Foundation, and the Rochester Epidemiology Project. Dr. Mielke is a consultant for Biogen and Brain Protection Company and is on the editorial boards of Neurology and Alzheimer’s and Dementia. Dr. Finney has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
At-home geriatric assessment offers cost-effective alternative to hospital
The comprehensive geriatric assessment (CGA) is an established strategy for guiding care of older adults in a hospital setting, but its use in other settings has not been well studied, Surya Singh, PhD, of the University of Oxford (England), and colleagues wrote in their paper published in Age and Ageing. Hospital at home is active treatment by health care professionals in the patient’s home for a condition that otherwise would require acute hospital inpatient care, for a limited time period.
Interest in providing health care in the home as an alternative to hospitalization is on the rise as a way to improve patient outcomes and reduce costs, but actual cost-effectiveness data on HAH interventions are limited, the authors said. “Wide scale implementation of such services has also been constrained by the practical difficulties of designing and delivering services that cut across primary and secondary care, might involve social care and require different workforce and funding arrangements.”
In this study, the researchers conducted a cost-effectiveness analysis alongside a randomized trial of an admission avoidance CGA hospital at home (CGAHAH) service as an alternative to hospital admission. They identified individuals aged 65 years and older who were living in the community but being considered for an unplanned hospital admission in the United Kingdom. A total of 700 individuals were randomized to CGAHAH and 355 to hospital care using a 2:1 ratio. Patients were assessed at baseline in the community or in an acute care setting before being transferred to CGAHAH service. These services included access to social workers, home care, district nursing, community rehabilitation, community mental health services and acute hospital services, such as diagnostic tests and transfer to hospital. The core workforce usually included consultant geriatricians, junior doctors, nurse practitioners, health care assistants or support workers, physiotherapists, occupational therapists, and community pharmacists. There were at least daily virtual ward rounds
Comparison between HAH and in-hospital groups
Patients in the CGAHAH group had a mean of 7.17 days of care, and those in hospital had a mean of 4.92 hospital days. At 6 months’ follow-up, the mean number of care days was 9.47 in the CGAHAH group and 10.58 in the hospital group, which was a nonsignificant difference.
“For complete cases, we found that allocation to CGAHAH resulted in 3 fewer days in hospital, a difference that was reduced to 1 day at 6 months follow-up,” the researchers wrote.
Overall, after adjusting for baseline variables, the health and social care costs after 6 months were less for CGAHAH than admission to hospital. The average cost differences between the two were approximately $3,000 or 2,265 pounds. The cost difference remained and increased to a mean difference of 2,840 pounds in favor of HAH after adding informal care/societal costs.
In addition, patients randomized to CGAHAH were less likely to have been admitted to long-term residential care at 6 months follow-up, compared with the hospital group; the mean days in residential care at 6 months were 3.43 and 6.14, respectively.
Both groups showed an approximate 15% decrease in measures of quality of life from baseline to 6 months, and no differences were noted in quality-adjusted survival between the groups.
Pandemic ‘has accelerated interest’ in HAH
“Health systems around the world are exploring alternatives to hospital admission, such as hospital at home, to act as a buffer to the increasing demand for hospital care,” corresponding author Sasha Shepperd, MSc, DPhil, said in an interview. “This is partly due to a growing older population with increased health needs, but also an emphasis on providing health care that limits a decline in capacity for the older population. Inevitably, the COVID-19 pandemic has accelerated interest in hospital at home to create additional acute health care capacity.”
The take home-message supports the home service option. “If you can access a hospital-at-home service, consider this as an option for older people who would otherwise be admitted to hospital and are eligible for hospital at home care. However, is important that the provision of hospital at home is adequately resourced, and that families and caregivers are supported,” she said.
“Barriers include delivering a different type of service that requires easy access to hospital services, including admission if required; a trained workforce to provide multidisciplinary care in a patient’s home; and ensuring a good fit with existing health and social care services,” Dr. Shepperd said.
Future research areas include the demands placed on caregivers from hospital-at-home services, and how the provision of hospital at home impacts hospital and community services, she added.
Findings support use of HAH
The data from the current study support the use of a hospital at home concept, especially in the geriatric age population, for acute health conditions that could be managed at home rather than acutely in a hospital-based environment,” Noel Deep, MD, emphasized in an interview.
Dr. Deep, who is a general internist in group practice in Antigo, Wisc., said he was not surprised by the study findings.
“I am a big proponent of the hospital at home approach to taking care of patients who can be safely and appropriately managed in the familiarity and comfort of their own home environment with help from physicians, nurses, and other home health care services,” he said. “It is a valuable option for appropriately screened and selected patients to be provided this approach to management of their acute health care situations.”
Primary care physicians should explore using HAH when faced with the decision of admitting an elderly individual to the hospital for management of an acute worsening of a chronic medical condition or a reversible acute illness, said Dr. Deep, who serves on the editorial advisory board of Internal Medicine News.
The current study reinforces previous studies and data showing the benefits of managing acute health problems of elderly individuals in their home environment. These benefits include “an opportunity to free up the emergency rooms and hospitals for providing care to those individuals who truly would be best served by being admitted to the hospital,” Dr. Deep explained. Home care for the elderly “would also lead to decreased utilization of the personal protective equipment and limit exposure of the vulnerable elderly individuals to the coronavirus. Primary care physicians should always explore this possibility of providing care to the patients in their homes if it is a viable option.
“While our practice environment [in the United States] is slightly different than that referenced in this article, many, if not almost all, of our primary care physicians provide care to the geriatric age population and provide assessment and management which would be comparable to this comprehensive geriatric assessment that is discussed in the article,” and many primary care physicians have seen similar results in outcomes that the study shows, said Dr. Deep. The available research and expert opinions are quite similar and agree upon the positive outcomes in terms of providing the CGAHAH approach.
Study is important but raises questions
The study is important because patient-centered, effective care should be the goal of any health system, William Golden, MD, of the University of Arkansas for Medical Sciences, Little Rock, said in an interview.
Dr. Golden also noted that the study raised a number of questions. How each patient entered the treatment protocol was not clear. “Similarly, it is not clear whether admission criteria and resource costs in England cross to the United States experience.”
“Having close follow up of patients at home as opposed to an ‘observation status’ could be a nice innovation, but more details are needed to consider implementation in a specific community setting,” he emphasized.
As for the clinical value of the study for primary care, “primary care professionals should welcome well-staffed alternatives to inpatient care for select patient presentations,” said Dr. Golden, who is also a member of the editorial advisory board of Internal Medicine News.
The current study does not identify the conditions that were treated at home and the logistics of delivering such services, which limits comparison with what experts have seen in practice in terms of outcomes using the CGAHAH, he said. “Interested practitioners would benefit from literature detailing the staffing and decision support tools that form the core framework of this innovation.”
Limitations and strengths of study, according to authors
The study findings were limited by several factors including the calculation of CGAHAH based on service budgets, rather than from collecting information on the actual resources used; potential errors in patients’ estimation of their informal care; and lack of data on a differential impact of CGAHAH for underserved communities, the researchers noted.
However, the results were strengthened by the large study population and randomized design, and support the value of CGAHAH, which addresses the need for management of multiple long-term conditions and the potential decline in functional and cognitive ability in older adults, they said. Providing CGAHAH as an alternative to admission to hospital for older people, with a focus on multidimensional assessment, is one option that might reduce reliance on hospitalization and residential care and at a lower cost.
The study was supported by the National Institute for Health Research, and several coauthors received individual grants from the NIHR, with no other financial conflicts to disclose. Dr. Golden and Dr. Deep had no financial conflicts to disclose.
The comprehensive geriatric assessment (CGA) is an established strategy for guiding care of older adults in a hospital setting, but its use in other settings has not been well studied, Surya Singh, PhD, of the University of Oxford (England), and colleagues wrote in their paper published in Age and Ageing. Hospital at home is active treatment by health care professionals in the patient’s home for a condition that otherwise would require acute hospital inpatient care, for a limited time period.
Interest in providing health care in the home as an alternative to hospitalization is on the rise as a way to improve patient outcomes and reduce costs, but actual cost-effectiveness data on HAH interventions are limited, the authors said. “Wide scale implementation of such services has also been constrained by the practical difficulties of designing and delivering services that cut across primary and secondary care, might involve social care and require different workforce and funding arrangements.”
In this study, the researchers conducted a cost-effectiveness analysis alongside a randomized trial of an admission avoidance CGA hospital at home (CGAHAH) service as an alternative to hospital admission. They identified individuals aged 65 years and older who were living in the community but being considered for an unplanned hospital admission in the United Kingdom. A total of 700 individuals were randomized to CGAHAH and 355 to hospital care using a 2:1 ratio. Patients were assessed at baseline in the community or in an acute care setting before being transferred to CGAHAH service. These services included access to social workers, home care, district nursing, community rehabilitation, community mental health services and acute hospital services, such as diagnostic tests and transfer to hospital. The core workforce usually included consultant geriatricians, junior doctors, nurse practitioners, health care assistants or support workers, physiotherapists, occupational therapists, and community pharmacists. There were at least daily virtual ward rounds
Comparison between HAH and in-hospital groups
Patients in the CGAHAH group had a mean of 7.17 days of care, and those in hospital had a mean of 4.92 hospital days. At 6 months’ follow-up, the mean number of care days was 9.47 in the CGAHAH group and 10.58 in the hospital group, which was a nonsignificant difference.
“For complete cases, we found that allocation to CGAHAH resulted in 3 fewer days in hospital, a difference that was reduced to 1 day at 6 months follow-up,” the researchers wrote.
Overall, after adjusting for baseline variables, the health and social care costs after 6 months were less for CGAHAH than admission to hospital. The average cost differences between the two were approximately $3,000 or 2,265 pounds. The cost difference remained and increased to a mean difference of 2,840 pounds in favor of HAH after adding informal care/societal costs.
In addition, patients randomized to CGAHAH were less likely to have been admitted to long-term residential care at 6 months follow-up, compared with the hospital group; the mean days in residential care at 6 months were 3.43 and 6.14, respectively.
Both groups showed an approximate 15% decrease in measures of quality of life from baseline to 6 months, and no differences were noted in quality-adjusted survival between the groups.
Pandemic ‘has accelerated interest’ in HAH
“Health systems around the world are exploring alternatives to hospital admission, such as hospital at home, to act as a buffer to the increasing demand for hospital care,” corresponding author Sasha Shepperd, MSc, DPhil, said in an interview. “This is partly due to a growing older population with increased health needs, but also an emphasis on providing health care that limits a decline in capacity for the older population. Inevitably, the COVID-19 pandemic has accelerated interest in hospital at home to create additional acute health care capacity.”
The take home-message supports the home service option. “If you can access a hospital-at-home service, consider this as an option for older people who would otherwise be admitted to hospital and are eligible for hospital at home care. However, is important that the provision of hospital at home is adequately resourced, and that families and caregivers are supported,” she said.
“Barriers include delivering a different type of service that requires easy access to hospital services, including admission if required; a trained workforce to provide multidisciplinary care in a patient’s home; and ensuring a good fit with existing health and social care services,” Dr. Shepperd said.
Future research areas include the demands placed on caregivers from hospital-at-home services, and how the provision of hospital at home impacts hospital and community services, she added.
Findings support use of HAH
The data from the current study support the use of a hospital at home concept, especially in the geriatric age population, for acute health conditions that could be managed at home rather than acutely in a hospital-based environment,” Noel Deep, MD, emphasized in an interview.
Dr. Deep, who is a general internist in group practice in Antigo, Wisc., said he was not surprised by the study findings.
“I am a big proponent of the hospital at home approach to taking care of patients who can be safely and appropriately managed in the familiarity and comfort of their own home environment with help from physicians, nurses, and other home health care services,” he said. “It is a valuable option for appropriately screened and selected patients to be provided this approach to management of their acute health care situations.”
Primary care physicians should explore using HAH when faced with the decision of admitting an elderly individual to the hospital for management of an acute worsening of a chronic medical condition or a reversible acute illness, said Dr. Deep, who serves on the editorial advisory board of Internal Medicine News.
The current study reinforces previous studies and data showing the benefits of managing acute health problems of elderly individuals in their home environment. These benefits include “an opportunity to free up the emergency rooms and hospitals for providing care to those individuals who truly would be best served by being admitted to the hospital,” Dr. Deep explained. Home care for the elderly “would also lead to decreased utilization of the personal protective equipment and limit exposure of the vulnerable elderly individuals to the coronavirus. Primary care physicians should always explore this possibility of providing care to the patients in their homes if it is a viable option.
“While our practice environment [in the United States] is slightly different than that referenced in this article, many, if not almost all, of our primary care physicians provide care to the geriatric age population and provide assessment and management which would be comparable to this comprehensive geriatric assessment that is discussed in the article,” and many primary care physicians have seen similar results in outcomes that the study shows, said Dr. Deep. The available research and expert opinions are quite similar and agree upon the positive outcomes in terms of providing the CGAHAH approach.
Study is important but raises questions
The study is important because patient-centered, effective care should be the goal of any health system, William Golden, MD, of the University of Arkansas for Medical Sciences, Little Rock, said in an interview.
Dr. Golden also noted that the study raised a number of questions. How each patient entered the treatment protocol was not clear. “Similarly, it is not clear whether admission criteria and resource costs in England cross to the United States experience.”
“Having close follow up of patients at home as opposed to an ‘observation status’ could be a nice innovation, but more details are needed to consider implementation in a specific community setting,” he emphasized.
As for the clinical value of the study for primary care, “primary care professionals should welcome well-staffed alternatives to inpatient care for select patient presentations,” said Dr. Golden, who is also a member of the editorial advisory board of Internal Medicine News.
The current study does not identify the conditions that were treated at home and the logistics of delivering such services, which limits comparison with what experts have seen in practice in terms of outcomes using the CGAHAH, he said. “Interested practitioners would benefit from literature detailing the staffing and decision support tools that form the core framework of this innovation.”
Limitations and strengths of study, according to authors
The study findings were limited by several factors including the calculation of CGAHAH based on service budgets, rather than from collecting information on the actual resources used; potential errors in patients’ estimation of their informal care; and lack of data on a differential impact of CGAHAH for underserved communities, the researchers noted.
However, the results were strengthened by the large study population and randomized design, and support the value of CGAHAH, which addresses the need for management of multiple long-term conditions and the potential decline in functional and cognitive ability in older adults, they said. Providing CGAHAH as an alternative to admission to hospital for older people, with a focus on multidimensional assessment, is one option that might reduce reliance on hospitalization and residential care and at a lower cost.
The study was supported by the National Institute for Health Research, and several coauthors received individual grants from the NIHR, with no other financial conflicts to disclose. Dr. Golden and Dr. Deep had no financial conflicts to disclose.
The comprehensive geriatric assessment (CGA) is an established strategy for guiding care of older adults in a hospital setting, but its use in other settings has not been well studied, Surya Singh, PhD, of the University of Oxford (England), and colleagues wrote in their paper published in Age and Ageing. Hospital at home is active treatment by health care professionals in the patient’s home for a condition that otherwise would require acute hospital inpatient care, for a limited time period.
Interest in providing health care in the home as an alternative to hospitalization is on the rise as a way to improve patient outcomes and reduce costs, but actual cost-effectiveness data on HAH interventions are limited, the authors said. “Wide scale implementation of such services has also been constrained by the practical difficulties of designing and delivering services that cut across primary and secondary care, might involve social care and require different workforce and funding arrangements.”
In this study, the researchers conducted a cost-effectiveness analysis alongside a randomized trial of an admission avoidance CGA hospital at home (CGAHAH) service as an alternative to hospital admission. They identified individuals aged 65 years and older who were living in the community but being considered for an unplanned hospital admission in the United Kingdom. A total of 700 individuals were randomized to CGAHAH and 355 to hospital care using a 2:1 ratio. Patients were assessed at baseline in the community or in an acute care setting before being transferred to CGAHAH service. These services included access to social workers, home care, district nursing, community rehabilitation, community mental health services and acute hospital services, such as diagnostic tests and transfer to hospital. The core workforce usually included consultant geriatricians, junior doctors, nurse practitioners, health care assistants or support workers, physiotherapists, occupational therapists, and community pharmacists. There were at least daily virtual ward rounds
Comparison between HAH and in-hospital groups
Patients in the CGAHAH group had a mean of 7.17 days of care, and those in hospital had a mean of 4.92 hospital days. At 6 months’ follow-up, the mean number of care days was 9.47 in the CGAHAH group and 10.58 in the hospital group, which was a nonsignificant difference.
“For complete cases, we found that allocation to CGAHAH resulted in 3 fewer days in hospital, a difference that was reduced to 1 day at 6 months follow-up,” the researchers wrote.
Overall, after adjusting for baseline variables, the health and social care costs after 6 months were less for CGAHAH than admission to hospital. The average cost differences between the two were approximately $3,000 or 2,265 pounds. The cost difference remained and increased to a mean difference of 2,840 pounds in favor of HAH after adding informal care/societal costs.
In addition, patients randomized to CGAHAH were less likely to have been admitted to long-term residential care at 6 months follow-up, compared with the hospital group; the mean days in residential care at 6 months were 3.43 and 6.14, respectively.
Both groups showed an approximate 15% decrease in measures of quality of life from baseline to 6 months, and no differences were noted in quality-adjusted survival between the groups.
Pandemic ‘has accelerated interest’ in HAH
“Health systems around the world are exploring alternatives to hospital admission, such as hospital at home, to act as a buffer to the increasing demand for hospital care,” corresponding author Sasha Shepperd, MSc, DPhil, said in an interview. “This is partly due to a growing older population with increased health needs, but also an emphasis on providing health care that limits a decline in capacity for the older population. Inevitably, the COVID-19 pandemic has accelerated interest in hospital at home to create additional acute health care capacity.”
The take home-message supports the home service option. “If you can access a hospital-at-home service, consider this as an option for older people who would otherwise be admitted to hospital and are eligible for hospital at home care. However, is important that the provision of hospital at home is adequately resourced, and that families and caregivers are supported,” she said.
“Barriers include delivering a different type of service that requires easy access to hospital services, including admission if required; a trained workforce to provide multidisciplinary care in a patient’s home; and ensuring a good fit with existing health and social care services,” Dr. Shepperd said.
Future research areas include the demands placed on caregivers from hospital-at-home services, and how the provision of hospital at home impacts hospital and community services, she added.
Findings support use of HAH
The data from the current study support the use of a hospital at home concept, especially in the geriatric age population, for acute health conditions that could be managed at home rather than acutely in a hospital-based environment,” Noel Deep, MD, emphasized in an interview.
Dr. Deep, who is a general internist in group practice in Antigo, Wisc., said he was not surprised by the study findings.
“I am a big proponent of the hospital at home approach to taking care of patients who can be safely and appropriately managed in the familiarity and comfort of their own home environment with help from physicians, nurses, and other home health care services,” he said. “It is a valuable option for appropriately screened and selected patients to be provided this approach to management of their acute health care situations.”
Primary care physicians should explore using HAH when faced with the decision of admitting an elderly individual to the hospital for management of an acute worsening of a chronic medical condition or a reversible acute illness, said Dr. Deep, who serves on the editorial advisory board of Internal Medicine News.
The current study reinforces previous studies and data showing the benefits of managing acute health problems of elderly individuals in their home environment. These benefits include “an opportunity to free up the emergency rooms and hospitals for providing care to those individuals who truly would be best served by being admitted to the hospital,” Dr. Deep explained. Home care for the elderly “would also lead to decreased utilization of the personal protective equipment and limit exposure of the vulnerable elderly individuals to the coronavirus. Primary care physicians should always explore this possibility of providing care to the patients in their homes if it is a viable option.
“While our practice environment [in the United States] is slightly different than that referenced in this article, many, if not almost all, of our primary care physicians provide care to the geriatric age population and provide assessment and management which would be comparable to this comprehensive geriatric assessment that is discussed in the article,” and many primary care physicians have seen similar results in outcomes that the study shows, said Dr. Deep. The available research and expert opinions are quite similar and agree upon the positive outcomes in terms of providing the CGAHAH approach.
Study is important but raises questions
The study is important because patient-centered, effective care should be the goal of any health system, William Golden, MD, of the University of Arkansas for Medical Sciences, Little Rock, said in an interview.
Dr. Golden also noted that the study raised a number of questions. How each patient entered the treatment protocol was not clear. “Similarly, it is not clear whether admission criteria and resource costs in England cross to the United States experience.”
“Having close follow up of patients at home as opposed to an ‘observation status’ could be a nice innovation, but more details are needed to consider implementation in a specific community setting,” he emphasized.
As for the clinical value of the study for primary care, “primary care professionals should welcome well-staffed alternatives to inpatient care for select patient presentations,” said Dr. Golden, who is also a member of the editorial advisory board of Internal Medicine News.
The current study does not identify the conditions that were treated at home and the logistics of delivering such services, which limits comparison with what experts have seen in practice in terms of outcomes using the CGAHAH, he said. “Interested practitioners would benefit from literature detailing the staffing and decision support tools that form the core framework of this innovation.”
Limitations and strengths of study, according to authors
The study findings were limited by several factors including the calculation of CGAHAH based on service budgets, rather than from collecting information on the actual resources used; potential errors in patients’ estimation of their informal care; and lack of data on a differential impact of CGAHAH for underserved communities, the researchers noted.
However, the results were strengthened by the large study population and randomized design, and support the value of CGAHAH, which addresses the need for management of multiple long-term conditions and the potential decline in functional and cognitive ability in older adults, they said. Providing CGAHAH as an alternative to admission to hospital for older people, with a focus on multidimensional assessment, is one option that might reduce reliance on hospitalization and residential care and at a lower cost.
The study was supported by the National Institute for Health Research, and several coauthors received individual grants from the NIHR, with no other financial conflicts to disclose. Dr. Golden and Dr. Deep had no financial conflicts to disclose.
FROM AGE AND AGEING
Frail COPD patients at high risk of disability and death
, a prospective cohort study of community-dwelling adults has shown.
“Frailty, a widely recognized geriatric syndrome characterized by multidimensional functional decline in bio-psycho-social factors, is associated with functional disability and mortality,” senior author Tze Pin Ng, MD, National University of Singapore, and colleagues explain.“Our results ... suggest that beyond traditional prognostic markers such as FEV1% (forced expiratory volume in 1 second) and dyspnea, the physical frailty phenotype provides additional useful prognostic information on future risks of disability and mortality,” the authors suggest.
The study was published online Dec. 12 in the journal CHEST®.
SLAS-1 and SLAS-2
Data from the Singapore Longitudinal Ageing Study (SLAS-1) and SLAS-2 were collected and analyzed. SLAS-1 recruited 2,804 participants 55 years of age and older from Sept. 2003 through Dec. 2004, while SLAS-2 recruited 3,270 participants of the same age between March 2009 and June 2013. “Follow-up visits and assessments were conducted approximately 3-5 years apart,” the investigators noted.
Mortality was determined at a mean of 9.5 years of follow-up for SLAS-1 participants and a mean of 6.5 years’ follow-up for SLAS-2 participants. A total of 4,627 participants were eventually included in the analysis, of whom 1,162 patients had COPD and 3,465 patients did not. COPD was classified as mild if FEV1% was greater than or equal to 80%; moderate if FEV1% was greater than or equal to 50% to less than 80%, and severe if FEV1% was less than 50%.
Frailty in turn was based on five clinical criteria, including weakness, slowness, low physical activity, exhaustion, and shrinking. Participants were classified as frail if they met three or more of these criteria and prefrail if they met one or two criteria.
Adverse health outcomes were judged on the basis of instrumental or basic activities of daily living (IADL/ADL), while disability was judged by self-reported difficulties in or requiring assistance with at least one IADL or ADL.
Frail or prefrail
Almost half of the participants were frail or prefrail, as the authors reported, while 25% had COPD. Among the participants with COPD, 30% had moderate to severe COPD, 6.4% had dyspnea, and almost half had prefrailty, while approximately 7% were classified as frail.
This percentage was 86% higher than it was for participants without COPD, among whom just 3.2% were assessed as frail, at an odds ratio of 1.86 (95% CI, 1.35-2.56). Further adjustments for possible confounders reduced the gap between frail COPD and frail non-COPD participants, but frailty remained significantly associated with COPD, at an OR of 1.61 (95% CI, 1.15-2.26), the investigators note.
Furthermore, compared to those without COPD, a diagnosis of COPD without and with dyspnea was associated with a 1.5- and 4.2-fold increase in prevalent frailty (95% CI, 1.04-2.08; 1.84-9.19), respectively, although not with prefrailty. Again, adjusting for multiple confounders, FEV1%, dyspnea, and both prefrailty and frailty were associated with an approximately twofold higher prevalence of IADL/ADL disability, while the prevalence of IADL/ADL disability for participants with COPD was approximately fourfold higher in those with co-occurring FEV1% less than 80% with either prefrailty, frailty, or dyspnea.
Furthermore, the presence of prefrailty or frailty in combination with a lower FEV1% or dyspnea was associated with a 3.7- to 3.8-fold increased risk of having an IADL or ADL disability.
Frailty and mortality
Some 1,116 participants with COPD were followed for a mean of 2,981 days for mortality outcomes. Both FEV1% less than 50% and the presence of prefrailty and frailty almost doubled the risk of mortality, at an adjusted hazard ratio of 1.8 (95% CI, 1.24-2.68) compared to patients with an FEV1% greater than or equal to 80%. In combination with either FEV1% less than 80% or prefrailty/frailty, dyspnea almost more than doubled the risk of mortality, at an HR of 2.4 for both combinations.
“However, the mortality risk of participants with COPD was highest among those with FEV1% less than 80% and prefrailty/frailty,” the authors note, more than tripling mortality risk at an adjusted HR of 3.25 (95% CI, 1.97-5.36). Interestingly, FEV1 less than 80% and prefrailty/frailty – both alone and in combination – were also associated with a twofold to fourfold increased risk of IADL or ADL disability in participants without COPD but were less strongly associated with mortality.
Researchers then went on to create a summary risk score containing all relevant variables with values ranging from 0 to 5. The highest risk category of 3 to 5 was associated with a 7- to 8.5-fold increased risk for IADL and ADL disability and mortality among participants with COPD, and that risk remained high after adjusting for multiple confounders.
Interestingly, frailty did not significantly predict mortality in women, while dyspnea did not significantly predict mortality in men. “Recognition and assessment of physical frailty in addition to FEV1% and dyspnea would allow for more accurate identification and targeted treatment of COPD at risk of future adverse outcomes,” the authors suggest.
Frailty scoring system
Asked to comment on the study, Sachin Gupta, MD, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif., noted that the current study adds to the body of literature that outcomes in patients with COPD depend as much on objectively measured variables as on qualitative measures. “By applying a frailty scoring system, these researchers were able to categorize frailty and study its impact on patient characteristics and outcomes,” he told this news organization in an email.
The summary risk assessment tool developed and assessed is familiar: It carries parallels to the widely utilized BODE Index, replacing body mass index and 6-minute walk distance with the frailty scale, he added. “Findings from this study support the idea that what meets the eye in face-to-face visits – frailty – can be codified and be part of a tool that is predictive of outcomes,” Dr. Gupta underscored.
The authors had no conflicts of interest to declare. Dr. Gupta disclosed that he is also an employee and shareholder at Genentech.
A version of this article first appeared on Medscape.com.
, a prospective cohort study of community-dwelling adults has shown.
“Frailty, a widely recognized geriatric syndrome characterized by multidimensional functional decline in bio-psycho-social factors, is associated with functional disability and mortality,” senior author Tze Pin Ng, MD, National University of Singapore, and colleagues explain.“Our results ... suggest that beyond traditional prognostic markers such as FEV1% (forced expiratory volume in 1 second) and dyspnea, the physical frailty phenotype provides additional useful prognostic information on future risks of disability and mortality,” the authors suggest.
The study was published online Dec. 12 in the journal CHEST®.
SLAS-1 and SLAS-2
Data from the Singapore Longitudinal Ageing Study (SLAS-1) and SLAS-2 were collected and analyzed. SLAS-1 recruited 2,804 participants 55 years of age and older from Sept. 2003 through Dec. 2004, while SLAS-2 recruited 3,270 participants of the same age between March 2009 and June 2013. “Follow-up visits and assessments were conducted approximately 3-5 years apart,” the investigators noted.
Mortality was determined at a mean of 9.5 years of follow-up for SLAS-1 participants and a mean of 6.5 years’ follow-up for SLAS-2 participants. A total of 4,627 participants were eventually included in the analysis, of whom 1,162 patients had COPD and 3,465 patients did not. COPD was classified as mild if FEV1% was greater than or equal to 80%; moderate if FEV1% was greater than or equal to 50% to less than 80%, and severe if FEV1% was less than 50%.
Frailty in turn was based on five clinical criteria, including weakness, slowness, low physical activity, exhaustion, and shrinking. Participants were classified as frail if they met three or more of these criteria and prefrail if they met one or two criteria.
Adverse health outcomes were judged on the basis of instrumental or basic activities of daily living (IADL/ADL), while disability was judged by self-reported difficulties in or requiring assistance with at least one IADL or ADL.
Frail or prefrail
Almost half of the participants were frail or prefrail, as the authors reported, while 25% had COPD. Among the participants with COPD, 30% had moderate to severe COPD, 6.4% had dyspnea, and almost half had prefrailty, while approximately 7% were classified as frail.
This percentage was 86% higher than it was for participants without COPD, among whom just 3.2% were assessed as frail, at an odds ratio of 1.86 (95% CI, 1.35-2.56). Further adjustments for possible confounders reduced the gap between frail COPD and frail non-COPD participants, but frailty remained significantly associated with COPD, at an OR of 1.61 (95% CI, 1.15-2.26), the investigators note.
Furthermore, compared to those without COPD, a diagnosis of COPD without and with dyspnea was associated with a 1.5- and 4.2-fold increase in prevalent frailty (95% CI, 1.04-2.08; 1.84-9.19), respectively, although not with prefrailty. Again, adjusting for multiple confounders, FEV1%, dyspnea, and both prefrailty and frailty were associated with an approximately twofold higher prevalence of IADL/ADL disability, while the prevalence of IADL/ADL disability for participants with COPD was approximately fourfold higher in those with co-occurring FEV1% less than 80% with either prefrailty, frailty, or dyspnea.
Furthermore, the presence of prefrailty or frailty in combination with a lower FEV1% or dyspnea was associated with a 3.7- to 3.8-fold increased risk of having an IADL or ADL disability.
Frailty and mortality
Some 1,116 participants with COPD were followed for a mean of 2,981 days for mortality outcomes. Both FEV1% less than 50% and the presence of prefrailty and frailty almost doubled the risk of mortality, at an adjusted hazard ratio of 1.8 (95% CI, 1.24-2.68) compared to patients with an FEV1% greater than or equal to 80%. In combination with either FEV1% less than 80% or prefrailty/frailty, dyspnea almost more than doubled the risk of mortality, at an HR of 2.4 for both combinations.
“However, the mortality risk of participants with COPD was highest among those with FEV1% less than 80% and prefrailty/frailty,” the authors note, more than tripling mortality risk at an adjusted HR of 3.25 (95% CI, 1.97-5.36). Interestingly, FEV1 less than 80% and prefrailty/frailty – both alone and in combination – were also associated with a twofold to fourfold increased risk of IADL or ADL disability in participants without COPD but were less strongly associated with mortality.
Researchers then went on to create a summary risk score containing all relevant variables with values ranging from 0 to 5. The highest risk category of 3 to 5 was associated with a 7- to 8.5-fold increased risk for IADL and ADL disability and mortality among participants with COPD, and that risk remained high after adjusting for multiple confounders.
Interestingly, frailty did not significantly predict mortality in women, while dyspnea did not significantly predict mortality in men. “Recognition and assessment of physical frailty in addition to FEV1% and dyspnea would allow for more accurate identification and targeted treatment of COPD at risk of future adverse outcomes,” the authors suggest.
Frailty scoring system
Asked to comment on the study, Sachin Gupta, MD, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif., noted that the current study adds to the body of literature that outcomes in patients with COPD depend as much on objectively measured variables as on qualitative measures. “By applying a frailty scoring system, these researchers were able to categorize frailty and study its impact on patient characteristics and outcomes,” he told this news organization in an email.
The summary risk assessment tool developed and assessed is familiar: It carries parallels to the widely utilized BODE Index, replacing body mass index and 6-minute walk distance with the frailty scale, he added. “Findings from this study support the idea that what meets the eye in face-to-face visits – frailty – can be codified and be part of a tool that is predictive of outcomes,” Dr. Gupta underscored.
The authors had no conflicts of interest to declare. Dr. Gupta disclosed that he is also an employee and shareholder at Genentech.
A version of this article first appeared on Medscape.com.
, a prospective cohort study of community-dwelling adults has shown.
“Frailty, a widely recognized geriatric syndrome characterized by multidimensional functional decline in bio-psycho-social factors, is associated with functional disability and mortality,” senior author Tze Pin Ng, MD, National University of Singapore, and colleagues explain.“Our results ... suggest that beyond traditional prognostic markers such as FEV1% (forced expiratory volume in 1 second) and dyspnea, the physical frailty phenotype provides additional useful prognostic information on future risks of disability and mortality,” the authors suggest.
The study was published online Dec. 12 in the journal CHEST®.
SLAS-1 and SLAS-2
Data from the Singapore Longitudinal Ageing Study (SLAS-1) and SLAS-2 were collected and analyzed. SLAS-1 recruited 2,804 participants 55 years of age and older from Sept. 2003 through Dec. 2004, while SLAS-2 recruited 3,270 participants of the same age between March 2009 and June 2013. “Follow-up visits and assessments were conducted approximately 3-5 years apart,” the investigators noted.
Mortality was determined at a mean of 9.5 years of follow-up for SLAS-1 participants and a mean of 6.5 years’ follow-up for SLAS-2 participants. A total of 4,627 participants were eventually included in the analysis, of whom 1,162 patients had COPD and 3,465 patients did not. COPD was classified as mild if FEV1% was greater than or equal to 80%; moderate if FEV1% was greater than or equal to 50% to less than 80%, and severe if FEV1% was less than 50%.
Frailty in turn was based on five clinical criteria, including weakness, slowness, low physical activity, exhaustion, and shrinking. Participants were classified as frail if they met three or more of these criteria and prefrail if they met one or two criteria.
Adverse health outcomes were judged on the basis of instrumental or basic activities of daily living (IADL/ADL), while disability was judged by self-reported difficulties in or requiring assistance with at least one IADL or ADL.
Frail or prefrail
Almost half of the participants were frail or prefrail, as the authors reported, while 25% had COPD. Among the participants with COPD, 30% had moderate to severe COPD, 6.4% had dyspnea, and almost half had prefrailty, while approximately 7% were classified as frail.
This percentage was 86% higher than it was for participants without COPD, among whom just 3.2% were assessed as frail, at an odds ratio of 1.86 (95% CI, 1.35-2.56). Further adjustments for possible confounders reduced the gap between frail COPD and frail non-COPD participants, but frailty remained significantly associated with COPD, at an OR of 1.61 (95% CI, 1.15-2.26), the investigators note.
Furthermore, compared to those without COPD, a diagnosis of COPD without and with dyspnea was associated with a 1.5- and 4.2-fold increase in prevalent frailty (95% CI, 1.04-2.08; 1.84-9.19), respectively, although not with prefrailty. Again, adjusting for multiple confounders, FEV1%, dyspnea, and both prefrailty and frailty were associated with an approximately twofold higher prevalence of IADL/ADL disability, while the prevalence of IADL/ADL disability for participants with COPD was approximately fourfold higher in those with co-occurring FEV1% less than 80% with either prefrailty, frailty, or dyspnea.
Furthermore, the presence of prefrailty or frailty in combination with a lower FEV1% or dyspnea was associated with a 3.7- to 3.8-fold increased risk of having an IADL or ADL disability.
Frailty and mortality
Some 1,116 participants with COPD were followed for a mean of 2,981 days for mortality outcomes. Both FEV1% less than 50% and the presence of prefrailty and frailty almost doubled the risk of mortality, at an adjusted hazard ratio of 1.8 (95% CI, 1.24-2.68) compared to patients with an FEV1% greater than or equal to 80%. In combination with either FEV1% less than 80% or prefrailty/frailty, dyspnea almost more than doubled the risk of mortality, at an HR of 2.4 for both combinations.
“However, the mortality risk of participants with COPD was highest among those with FEV1% less than 80% and prefrailty/frailty,” the authors note, more than tripling mortality risk at an adjusted HR of 3.25 (95% CI, 1.97-5.36). Interestingly, FEV1 less than 80% and prefrailty/frailty – both alone and in combination – were also associated with a twofold to fourfold increased risk of IADL or ADL disability in participants without COPD but were less strongly associated with mortality.
Researchers then went on to create a summary risk score containing all relevant variables with values ranging from 0 to 5. The highest risk category of 3 to 5 was associated with a 7- to 8.5-fold increased risk for IADL and ADL disability and mortality among participants with COPD, and that risk remained high after adjusting for multiple confounders.
Interestingly, frailty did not significantly predict mortality in women, while dyspnea did not significantly predict mortality in men. “Recognition and assessment of physical frailty in addition to FEV1% and dyspnea would allow for more accurate identification and targeted treatment of COPD at risk of future adverse outcomes,” the authors suggest.
Frailty scoring system
Asked to comment on the study, Sachin Gupta, MD, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif., noted that the current study adds to the body of literature that outcomes in patients with COPD depend as much on objectively measured variables as on qualitative measures. “By applying a frailty scoring system, these researchers were able to categorize frailty and study its impact on patient characteristics and outcomes,” he told this news organization in an email.
The summary risk assessment tool developed and assessed is familiar: It carries parallels to the widely utilized BODE Index, replacing body mass index and 6-minute walk distance with the frailty scale, he added. “Findings from this study support the idea that what meets the eye in face-to-face visits – frailty – can be codified and be part of a tool that is predictive of outcomes,” Dr. Gupta underscored.
The authors had no conflicts of interest to declare. Dr. Gupta disclosed that he is also an employee and shareholder at Genentech.
A version of this article first appeared on Medscape.com.
FROM CHEST
New data support a causal role for depression in Alzheimer’s
Researchers have known for some time that depression is associated with Alzheimer’s disease (AD), but a causal link has been elusive. Now, using newly available data, they have uncovered genetic evidence of a causal role for depression in AD.
As depression typically affects those in early or midlife and dementia often occurs in later life, “it’s fascinating to see a connection between the two brain illnesses that manifest in different time windows,” coinvestigator Aliza P. Wingo, MD, associate professor of psychiatry and behavioral science, Emory University, Atlanta, said in an interview.
“If we can treat the depression early on, we may help reduce risk for dementia for our patients later in life,” Dr. Wingo said.
The findings were published online Dec. 16, 2021, in Biological Psychiatry.
Postmortem data
The investigators, who are all from the Emory University Center for Neurodegenerative Disease, wanted to clarify the genetic basis underlying the association between the established link between depression and dementia risk.
They used data from the largest and most recent genomewide association studies (GWAS). These included a 2019 analysis of depression among 807,553 individuals and a 2019 study of AD among 455,258 individuals, all of European ancestry. For sensitivity analyses, they used results from two additional AD GWAS.
The researchers also accessed postmortem brain samples from participants in the Religious Orders Study (ROS) and the Rush Memory and Aging Project (MAP). These participants were cognitively normal at enrollment, underwent annual clinical evaluations, and agreed to donate their brains.
They also assessed brain samples donated by participants in the Banner Sun Health Research Institute longitudinal study of healthy aging, Alzheimer’s, and Parkinson’s disease.
The brain samples allowed researchers to use deep brain proteomic data to help determine molecular links between depression and AD.
After quality control, the analysis included 8,356 proteins in 391 ROS/MAP participants and 7,854 proteins in 196 Banner participants.
suggesting the two conditions have a shared genetic basis.
The investigators also applied a framework called “Mendelian randomization” to determine causality between depression and AD.
After assessing the effect of 115 independent single-nucleotide polymorphisms (SNPs) from the GWAS of depression, they uncovered significant evidence “that the SNPs cause depression, which in turn cause AD,” said Dr. Wingo.
One-way relationship
The researchers conducted the same analysis on 61 significant SNPs from the GWAS of AD but did not find evidence to conclude AD causes depression.
“We found genetic evidence supporting a causal role of depression in AD but not vice versa,” Dr. Wingo said.
In addition, the investigators identified 75 brain transcripts (messenger RNA) and 28 brain proteins regulated by the depression-predisposing genetic variants. Of these, 46 brain transcripts and seven proteins were significantly associated with at least one AD feature – for example, beta-amyloid, tau tangles, and cognitive trajectory.
“These findings support the notion that the depression risk variants contribute to AD via regulating expression of their corresponding transcripts in the brain,” the investigators wrote.
It is only recently that large enough studies have allowed researchers sufficient power to reach these conclusions, coinvestigator Thomas Wingo, MD, said in an interview.
These additional “insights” into the relationship between depression and AD might “motivate” clinicians more to screen for and treat depressive symptoms, Dr. Aliza Wingo noted.
The new results also have implications for developing therapeutics to treat depression, she said. “If we target the genes, the brain proteins, that are shared risk between depression and AD, the medications that target that gene might mitigate risk for AD later on.”
However, the investigators advised caution. “A lot of this is still unknown,” said Dr. Thomas Wingo.
For example, it is not clear whether successfully treating depression mitigates the eventual risk of dementia, which is “a very important topic of inquiry and one we continue to work on,” he said, adding that a significant number of patients do not respond well to existing antidepressants such as SSRIs.
Need for further research
Commenting on the findings, Claire Sexton, DPhil, director of scientific programs and outreach, Alzheimer’s Association, said the study contributes to the debate about whether depression increases risk for AD, whether AD increases risk for depression, or both.
“These newly published findings strengthen our understanding of the role of depression as a risk factor for Alzheimer’s dementia,” said Dr. Sexton, who was not involved with the research.
While experts do not yet fully understand the impact of treating depression on dementia risk, “the findings emphasize the importance of assessing mental health status, particularly depression, and getting it properly diagnosed and treated in a timely manner,” she said.
However, she agreed more research in this area is needed. “Importantly, these findings need replication in broader, more diverse study populations,” Dr. Sexton said.
A study funded by the Alzheimer’s Association may provide more information on the link between depression and AD. It will investigate whether machine learning, an advanced computer science technique, can better predict cognitive decline, compared with traditional methods.
Over a period of 6 months, researchers will collect smartphone conversations from 225 older adults with dementia, mild cognitive impairment, or no cognitive impairment. They will also have data from cognitive tests, brain scans, and biomarkers such as cerebrospinal fluid samples to study brain changes associated with AD.
The novel method of analysis should be able to identify subtle differences in speech quality to indicate which depressive symptoms an individual might be experiencing.
“The study could help us further understand the potential impact of depression in the risk of developing dementia,” said Dr. Sexton.
Dr. Aliza Wingo and Dr. Thomas Wingo reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers have known for some time that depression is associated with Alzheimer’s disease (AD), but a causal link has been elusive. Now, using newly available data, they have uncovered genetic evidence of a causal role for depression in AD.
As depression typically affects those in early or midlife and dementia often occurs in later life, “it’s fascinating to see a connection between the two brain illnesses that manifest in different time windows,” coinvestigator Aliza P. Wingo, MD, associate professor of psychiatry and behavioral science, Emory University, Atlanta, said in an interview.
“If we can treat the depression early on, we may help reduce risk for dementia for our patients later in life,” Dr. Wingo said.
The findings were published online Dec. 16, 2021, in Biological Psychiatry.
Postmortem data
The investigators, who are all from the Emory University Center for Neurodegenerative Disease, wanted to clarify the genetic basis underlying the association between the established link between depression and dementia risk.
They used data from the largest and most recent genomewide association studies (GWAS). These included a 2019 analysis of depression among 807,553 individuals and a 2019 study of AD among 455,258 individuals, all of European ancestry. For sensitivity analyses, they used results from two additional AD GWAS.
The researchers also accessed postmortem brain samples from participants in the Religious Orders Study (ROS) and the Rush Memory and Aging Project (MAP). These participants were cognitively normal at enrollment, underwent annual clinical evaluations, and agreed to donate their brains.
They also assessed brain samples donated by participants in the Banner Sun Health Research Institute longitudinal study of healthy aging, Alzheimer’s, and Parkinson’s disease.
The brain samples allowed researchers to use deep brain proteomic data to help determine molecular links between depression and AD.
After quality control, the analysis included 8,356 proteins in 391 ROS/MAP participants and 7,854 proteins in 196 Banner participants.
suggesting the two conditions have a shared genetic basis.
The investigators also applied a framework called “Mendelian randomization” to determine causality between depression and AD.
After assessing the effect of 115 independent single-nucleotide polymorphisms (SNPs) from the GWAS of depression, they uncovered significant evidence “that the SNPs cause depression, which in turn cause AD,” said Dr. Wingo.
One-way relationship
The researchers conducted the same analysis on 61 significant SNPs from the GWAS of AD but did not find evidence to conclude AD causes depression.
“We found genetic evidence supporting a causal role of depression in AD but not vice versa,” Dr. Wingo said.
In addition, the investigators identified 75 brain transcripts (messenger RNA) and 28 brain proteins regulated by the depression-predisposing genetic variants. Of these, 46 brain transcripts and seven proteins were significantly associated with at least one AD feature – for example, beta-amyloid, tau tangles, and cognitive trajectory.
“These findings support the notion that the depression risk variants contribute to AD via regulating expression of their corresponding transcripts in the brain,” the investigators wrote.
It is only recently that large enough studies have allowed researchers sufficient power to reach these conclusions, coinvestigator Thomas Wingo, MD, said in an interview.
These additional “insights” into the relationship between depression and AD might “motivate” clinicians more to screen for and treat depressive symptoms, Dr. Aliza Wingo noted.
The new results also have implications for developing therapeutics to treat depression, she said. “If we target the genes, the brain proteins, that are shared risk between depression and AD, the medications that target that gene might mitigate risk for AD later on.”
However, the investigators advised caution. “A lot of this is still unknown,” said Dr. Thomas Wingo.
For example, it is not clear whether successfully treating depression mitigates the eventual risk of dementia, which is “a very important topic of inquiry and one we continue to work on,” he said, adding that a significant number of patients do not respond well to existing antidepressants such as SSRIs.
Need for further research
Commenting on the findings, Claire Sexton, DPhil, director of scientific programs and outreach, Alzheimer’s Association, said the study contributes to the debate about whether depression increases risk for AD, whether AD increases risk for depression, or both.
“These newly published findings strengthen our understanding of the role of depression as a risk factor for Alzheimer’s dementia,” said Dr. Sexton, who was not involved with the research.
While experts do not yet fully understand the impact of treating depression on dementia risk, “the findings emphasize the importance of assessing mental health status, particularly depression, and getting it properly diagnosed and treated in a timely manner,” she said.
However, she agreed more research in this area is needed. “Importantly, these findings need replication in broader, more diverse study populations,” Dr. Sexton said.
A study funded by the Alzheimer’s Association may provide more information on the link between depression and AD. It will investigate whether machine learning, an advanced computer science technique, can better predict cognitive decline, compared with traditional methods.
Over a period of 6 months, researchers will collect smartphone conversations from 225 older adults with dementia, mild cognitive impairment, or no cognitive impairment. They will also have data from cognitive tests, brain scans, and biomarkers such as cerebrospinal fluid samples to study brain changes associated with AD.
The novel method of analysis should be able to identify subtle differences in speech quality to indicate which depressive symptoms an individual might be experiencing.
“The study could help us further understand the potential impact of depression in the risk of developing dementia,” said Dr. Sexton.
Dr. Aliza Wingo and Dr. Thomas Wingo reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers have known for some time that depression is associated with Alzheimer’s disease (AD), but a causal link has been elusive. Now, using newly available data, they have uncovered genetic evidence of a causal role for depression in AD.
As depression typically affects those in early or midlife and dementia often occurs in later life, “it’s fascinating to see a connection between the two brain illnesses that manifest in different time windows,” coinvestigator Aliza P. Wingo, MD, associate professor of psychiatry and behavioral science, Emory University, Atlanta, said in an interview.
“If we can treat the depression early on, we may help reduce risk for dementia for our patients later in life,” Dr. Wingo said.
The findings were published online Dec. 16, 2021, in Biological Psychiatry.
Postmortem data
The investigators, who are all from the Emory University Center for Neurodegenerative Disease, wanted to clarify the genetic basis underlying the association between the established link between depression and dementia risk.
They used data from the largest and most recent genomewide association studies (GWAS). These included a 2019 analysis of depression among 807,553 individuals and a 2019 study of AD among 455,258 individuals, all of European ancestry. For sensitivity analyses, they used results from two additional AD GWAS.
The researchers also accessed postmortem brain samples from participants in the Religious Orders Study (ROS) and the Rush Memory and Aging Project (MAP). These participants were cognitively normal at enrollment, underwent annual clinical evaluations, and agreed to donate their brains.
They also assessed brain samples donated by participants in the Banner Sun Health Research Institute longitudinal study of healthy aging, Alzheimer’s, and Parkinson’s disease.
The brain samples allowed researchers to use deep brain proteomic data to help determine molecular links between depression and AD.
After quality control, the analysis included 8,356 proteins in 391 ROS/MAP participants and 7,854 proteins in 196 Banner participants.
suggesting the two conditions have a shared genetic basis.
The investigators also applied a framework called “Mendelian randomization” to determine causality between depression and AD.
After assessing the effect of 115 independent single-nucleotide polymorphisms (SNPs) from the GWAS of depression, they uncovered significant evidence “that the SNPs cause depression, which in turn cause AD,” said Dr. Wingo.
One-way relationship
The researchers conducted the same analysis on 61 significant SNPs from the GWAS of AD but did not find evidence to conclude AD causes depression.
“We found genetic evidence supporting a causal role of depression in AD but not vice versa,” Dr. Wingo said.
In addition, the investigators identified 75 brain transcripts (messenger RNA) and 28 brain proteins regulated by the depression-predisposing genetic variants. Of these, 46 brain transcripts and seven proteins were significantly associated with at least one AD feature – for example, beta-amyloid, tau tangles, and cognitive trajectory.
“These findings support the notion that the depression risk variants contribute to AD via regulating expression of their corresponding transcripts in the brain,” the investigators wrote.
It is only recently that large enough studies have allowed researchers sufficient power to reach these conclusions, coinvestigator Thomas Wingo, MD, said in an interview.
These additional “insights” into the relationship between depression and AD might “motivate” clinicians more to screen for and treat depressive symptoms, Dr. Aliza Wingo noted.
The new results also have implications for developing therapeutics to treat depression, she said. “If we target the genes, the brain proteins, that are shared risk between depression and AD, the medications that target that gene might mitigate risk for AD later on.”
However, the investigators advised caution. “A lot of this is still unknown,” said Dr. Thomas Wingo.
For example, it is not clear whether successfully treating depression mitigates the eventual risk of dementia, which is “a very important topic of inquiry and one we continue to work on,” he said, adding that a significant number of patients do not respond well to existing antidepressants such as SSRIs.
Need for further research
Commenting on the findings, Claire Sexton, DPhil, director of scientific programs and outreach, Alzheimer’s Association, said the study contributes to the debate about whether depression increases risk for AD, whether AD increases risk for depression, or both.
“These newly published findings strengthen our understanding of the role of depression as a risk factor for Alzheimer’s dementia,” said Dr. Sexton, who was not involved with the research.
While experts do not yet fully understand the impact of treating depression on dementia risk, “the findings emphasize the importance of assessing mental health status, particularly depression, and getting it properly diagnosed and treated in a timely manner,” she said.
However, she agreed more research in this area is needed. “Importantly, these findings need replication in broader, more diverse study populations,” Dr. Sexton said.
A study funded by the Alzheimer’s Association may provide more information on the link between depression and AD. It will investigate whether machine learning, an advanced computer science technique, can better predict cognitive decline, compared with traditional methods.
Over a period of 6 months, researchers will collect smartphone conversations from 225 older adults with dementia, mild cognitive impairment, or no cognitive impairment. They will also have data from cognitive tests, brain scans, and biomarkers such as cerebrospinal fluid samples to study brain changes associated with AD.
The novel method of analysis should be able to identify subtle differences in speech quality to indicate which depressive symptoms an individual might be experiencing.
“The study could help us further understand the potential impact of depression in the risk of developing dementia,” said Dr. Sexton.
Dr. Aliza Wingo and Dr. Thomas Wingo reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BIOLOGICAL PSYCHIATRY
Atopic dermatitis can be especially burdensome in the elderly
During the Revolutionizing Atopic Dermatitis virtual symposium, Katrina Abuabara, MD, highlighted the epidemiology and burden of AD among older adults. She began by noting that the disease peaks in infancy and older adulthood. In an analysis that she and her colleagues made of physician-diagnosed AD among more than 8.6 million patients in the United Kingdom between 1994 and 2013, the mean prevalence in a given year was 12.3% among those aged 0-17 years, 5.1% among those age 18-74 years, and 8.7% among those age 75 and older.
“We saw what we expected in early infancy with very high rates of active disease,” said Dr. Abuabara, associate professor of dermatology and epidemiology at the University of California, San Francisco. “We also saw a second peak in older adulthood. This was more surprising to us because the disease hadn’t been as well studied in this population.” Researchers who analyzed data from the Global Burden of Disease Study, which evaluates disease-related morbidity and mortality worldwide, found a somewhat attenuated peak but a similar trend around the world. Its authors ranked AD as 15th among all nonfatal diseases.
In a separate analysis, Dr. Abuabara and colleagues evaluated records of more than 9.1 million primary care patients in the United Kingdom between 1994 and 2013, and who were followed for an average of 6 years. They examined AD activity and found that, based on doctor visits and prescriptions, AD appeared to be active in 48% of those aged 0-17 years, compared with 42% of those aged 18-74 years, and 60% of those aged 75 years and older. “Also, when we looked at the distribution of active disease in older adults, we saw that those who were older had more severe disease,” she said. When they evaluated the prevalence of AD by sociodemographic factors, AD increased with age among older adults (adjusted odd ratio, 1.06), while it decreased by 14% annually among children. In addition, female older adults had about three-fourths the odds of prevalent disease as their male counterparts (aOR, 0.73).
“We also looked at rural and urban differences and found that across ages it was more common in urban as compared to rural populations,” she said. “As for socioeconomic status, it tends to be more common among those of higher socioeconomic status in children and in the older adult group.”
In a study that drew from medical records of 3.85 million primary care patients in the United Kingdom, AD was more common in Asian and Black ethnic groups than in people of White ethnicity. In addition, higher socioeconomic status was associated with a greater incidence of eczema in infants aged younger than 2 years, but the reverse was seen for all other age groups.
To identify subtypes of atopic eczema based on patterns of disease activity through mid-adulthood, Dr. Abuabara and colleagues evaluated members of two population-based birth cohorts: the 1958 National Childhood Development Study and the 1970 British Cohort Study. The patients were classified into one of four patters of disease activity followed to age 50: rare/none, increasing, decreasing, and high. “We found that there was the early-onset decreasing subgroup, which tend to have a lower probability of AD over time,” Dr. Abuabara said. “We also found that there was a small subgroup that had a constant high probability of AD over time. But we were surprised to find a subgroup with increasing probability over time. This was a fairly sizable subgroup.”
In an earlier study, she and her colleagues examined whether there were differences based on whether people had adult-onset or childhood-onset disease in the same two cohorts of U.K. patients. Those with childhood-onset disease had stronger associations with known genetic risk factors and they tended to be of higher socioeconomic status. “They also tended to have more asthma and other allergic comorbidities,” Dr. Abuabara said. “On the other hand, the adult-onset group [after age 23] were more likely to be female, more likely to be smokers, and tended to have lower childhood socioeconomic status.”
According to the best available evidence, she continued, there is good data on higher relative risk of osteoporosis/fractures and dementia specifically among older adults with AD, and good data on associations with cardiometabolic disease and atopic disease among adults overall, as well as data showing that AD does not seem to be associated with cancer overall. In a study conducted by Jonathan I. Silverberg, MD, PhD, MPH, and Mohammed S. Shaheen, JD, the researchers used physician-diagnosed AD to investigate the associations of osteopenia and osteoporosis in two large U.S. databases: the 2006-2012 Nationwide Emergency Department Sample (NEDS) database and 2002-2012 National Inpatient Sample (NIS). Among patients aged 50 years and older, AD was associated with a higher odds of osteoporosis in NEDS (aOR, 1.31) and NIS (aOR, 1.25) and osteopenia in NEDS (aOR, 1.86).
In a separate matched cohort study, Dr. Abuabara and colleagues used U.K. primary care patient data to evaluate the association between AD and fracture and whether fracture risk varies with AD severity. Overall, they observed a 10% increase in fracture risk among people with AD, compared with those without, especially those of the hip, spine, pelvis, and wrist. “We found that there was a dose-response effect,” she said. “Those with more severe eczema had a much higher risk of fractures. When we looked at different age groups, we found a similar increased risk in the oldest adults as in younger adults.”
In a longitudinal cohort study of primary care medical records from more than 1.1 million individuals in the United Kingdom, AD was associated with an increased risk of vascular dementia (hazard ratio, 1.88), Alzheimer’s disease (HR, 1.69, and other/unspecified dementia (HR, 1.48; .269). “We found a nice dose response, where people with more severe AD had higher rates of dementia,” Dr. Abuabara said. Results from a more recent, smaller study of patients in Taiwan also found an increased risk between AD and the risk of dementia, but not a dose-response effect, likely because of a much smaller sample size.
Mounting research suggests that the risk for cardiovascular disease is also elevated in patients with AD. “There is some variability in the literature, but I think it’s important that when we’re talking about atopic dermatitis to think about the heterogeneity of the disease,” Dr. Abuabara said. In a meta-analysis and systematic review of 19 studies on the topic, she and her colleagues found that AD was associated with an increased risk of myocardial infarction (relative risk, 1.12), stroke (RR, 1.10), ischemic stroke (RR, 1.17), angina (RR, 1.18), and heart failure (RR, 1.26). “For all the different [cardiovascular disease] outcomes there was increasing risk with increasing disease severity,” she said.
She reported that UCSF receives research funding from Pfizer and Cosmetique Active International. She also receives consulting fees from Target RWE.
During the Revolutionizing Atopic Dermatitis virtual symposium, Katrina Abuabara, MD, highlighted the epidemiology and burden of AD among older adults. She began by noting that the disease peaks in infancy and older adulthood. In an analysis that she and her colleagues made of physician-diagnosed AD among more than 8.6 million patients in the United Kingdom between 1994 and 2013, the mean prevalence in a given year was 12.3% among those aged 0-17 years, 5.1% among those age 18-74 years, and 8.7% among those age 75 and older.
“We saw what we expected in early infancy with very high rates of active disease,” said Dr. Abuabara, associate professor of dermatology and epidemiology at the University of California, San Francisco. “We also saw a second peak in older adulthood. This was more surprising to us because the disease hadn’t been as well studied in this population.” Researchers who analyzed data from the Global Burden of Disease Study, which evaluates disease-related morbidity and mortality worldwide, found a somewhat attenuated peak but a similar trend around the world. Its authors ranked AD as 15th among all nonfatal diseases.
In a separate analysis, Dr. Abuabara and colleagues evaluated records of more than 9.1 million primary care patients in the United Kingdom between 1994 and 2013, and who were followed for an average of 6 years. They examined AD activity and found that, based on doctor visits and prescriptions, AD appeared to be active in 48% of those aged 0-17 years, compared with 42% of those aged 18-74 years, and 60% of those aged 75 years and older. “Also, when we looked at the distribution of active disease in older adults, we saw that those who were older had more severe disease,” she said. When they evaluated the prevalence of AD by sociodemographic factors, AD increased with age among older adults (adjusted odd ratio, 1.06), while it decreased by 14% annually among children. In addition, female older adults had about three-fourths the odds of prevalent disease as their male counterparts (aOR, 0.73).
“We also looked at rural and urban differences and found that across ages it was more common in urban as compared to rural populations,” she said. “As for socioeconomic status, it tends to be more common among those of higher socioeconomic status in children and in the older adult group.”
In a study that drew from medical records of 3.85 million primary care patients in the United Kingdom, AD was more common in Asian and Black ethnic groups than in people of White ethnicity. In addition, higher socioeconomic status was associated with a greater incidence of eczema in infants aged younger than 2 years, but the reverse was seen for all other age groups.
To identify subtypes of atopic eczema based on patterns of disease activity through mid-adulthood, Dr. Abuabara and colleagues evaluated members of two population-based birth cohorts: the 1958 National Childhood Development Study and the 1970 British Cohort Study. The patients were classified into one of four patters of disease activity followed to age 50: rare/none, increasing, decreasing, and high. “We found that there was the early-onset decreasing subgroup, which tend to have a lower probability of AD over time,” Dr. Abuabara said. “We also found that there was a small subgroup that had a constant high probability of AD over time. But we were surprised to find a subgroup with increasing probability over time. This was a fairly sizable subgroup.”
In an earlier study, she and her colleagues examined whether there were differences based on whether people had adult-onset or childhood-onset disease in the same two cohorts of U.K. patients. Those with childhood-onset disease had stronger associations with known genetic risk factors and they tended to be of higher socioeconomic status. “They also tended to have more asthma and other allergic comorbidities,” Dr. Abuabara said. “On the other hand, the adult-onset group [after age 23] were more likely to be female, more likely to be smokers, and tended to have lower childhood socioeconomic status.”
According to the best available evidence, she continued, there is good data on higher relative risk of osteoporosis/fractures and dementia specifically among older adults with AD, and good data on associations with cardiometabolic disease and atopic disease among adults overall, as well as data showing that AD does not seem to be associated with cancer overall. In a study conducted by Jonathan I. Silverberg, MD, PhD, MPH, and Mohammed S. Shaheen, JD, the researchers used physician-diagnosed AD to investigate the associations of osteopenia and osteoporosis in two large U.S. databases: the 2006-2012 Nationwide Emergency Department Sample (NEDS) database and 2002-2012 National Inpatient Sample (NIS). Among patients aged 50 years and older, AD was associated with a higher odds of osteoporosis in NEDS (aOR, 1.31) and NIS (aOR, 1.25) and osteopenia in NEDS (aOR, 1.86).
In a separate matched cohort study, Dr. Abuabara and colleagues used U.K. primary care patient data to evaluate the association between AD and fracture and whether fracture risk varies with AD severity. Overall, they observed a 10% increase in fracture risk among people with AD, compared with those without, especially those of the hip, spine, pelvis, and wrist. “We found that there was a dose-response effect,” she said. “Those with more severe eczema had a much higher risk of fractures. When we looked at different age groups, we found a similar increased risk in the oldest adults as in younger adults.”
In a longitudinal cohort study of primary care medical records from more than 1.1 million individuals in the United Kingdom, AD was associated with an increased risk of vascular dementia (hazard ratio, 1.88), Alzheimer’s disease (HR, 1.69, and other/unspecified dementia (HR, 1.48; .269). “We found a nice dose response, where people with more severe AD had higher rates of dementia,” Dr. Abuabara said. Results from a more recent, smaller study of patients in Taiwan also found an increased risk between AD and the risk of dementia, but not a dose-response effect, likely because of a much smaller sample size.
Mounting research suggests that the risk for cardiovascular disease is also elevated in patients with AD. “There is some variability in the literature, but I think it’s important that when we’re talking about atopic dermatitis to think about the heterogeneity of the disease,” Dr. Abuabara said. In a meta-analysis and systematic review of 19 studies on the topic, she and her colleagues found that AD was associated with an increased risk of myocardial infarction (relative risk, 1.12), stroke (RR, 1.10), ischemic stroke (RR, 1.17), angina (RR, 1.18), and heart failure (RR, 1.26). “For all the different [cardiovascular disease] outcomes there was increasing risk with increasing disease severity,” she said.
She reported that UCSF receives research funding from Pfizer and Cosmetique Active International. She also receives consulting fees from Target RWE.
During the Revolutionizing Atopic Dermatitis virtual symposium, Katrina Abuabara, MD, highlighted the epidemiology and burden of AD among older adults. She began by noting that the disease peaks in infancy and older adulthood. In an analysis that she and her colleagues made of physician-diagnosed AD among more than 8.6 million patients in the United Kingdom between 1994 and 2013, the mean prevalence in a given year was 12.3% among those aged 0-17 years, 5.1% among those age 18-74 years, and 8.7% among those age 75 and older.
“We saw what we expected in early infancy with very high rates of active disease,” said Dr. Abuabara, associate professor of dermatology and epidemiology at the University of California, San Francisco. “We also saw a second peak in older adulthood. This was more surprising to us because the disease hadn’t been as well studied in this population.” Researchers who analyzed data from the Global Burden of Disease Study, which evaluates disease-related morbidity and mortality worldwide, found a somewhat attenuated peak but a similar trend around the world. Its authors ranked AD as 15th among all nonfatal diseases.
In a separate analysis, Dr. Abuabara and colleagues evaluated records of more than 9.1 million primary care patients in the United Kingdom between 1994 and 2013, and who were followed for an average of 6 years. They examined AD activity and found that, based on doctor visits and prescriptions, AD appeared to be active in 48% of those aged 0-17 years, compared with 42% of those aged 18-74 years, and 60% of those aged 75 years and older. “Also, when we looked at the distribution of active disease in older adults, we saw that those who were older had more severe disease,” she said. When they evaluated the prevalence of AD by sociodemographic factors, AD increased with age among older adults (adjusted odd ratio, 1.06), while it decreased by 14% annually among children. In addition, female older adults had about three-fourths the odds of prevalent disease as their male counterparts (aOR, 0.73).
“We also looked at rural and urban differences and found that across ages it was more common in urban as compared to rural populations,” she said. “As for socioeconomic status, it tends to be more common among those of higher socioeconomic status in children and in the older adult group.”
In a study that drew from medical records of 3.85 million primary care patients in the United Kingdom, AD was more common in Asian and Black ethnic groups than in people of White ethnicity. In addition, higher socioeconomic status was associated with a greater incidence of eczema in infants aged younger than 2 years, but the reverse was seen for all other age groups.
To identify subtypes of atopic eczema based on patterns of disease activity through mid-adulthood, Dr. Abuabara and colleagues evaluated members of two population-based birth cohorts: the 1958 National Childhood Development Study and the 1970 British Cohort Study. The patients were classified into one of four patters of disease activity followed to age 50: rare/none, increasing, decreasing, and high. “We found that there was the early-onset decreasing subgroup, which tend to have a lower probability of AD over time,” Dr. Abuabara said. “We also found that there was a small subgroup that had a constant high probability of AD over time. But we were surprised to find a subgroup with increasing probability over time. This was a fairly sizable subgroup.”
In an earlier study, she and her colleagues examined whether there were differences based on whether people had adult-onset or childhood-onset disease in the same two cohorts of U.K. patients. Those with childhood-onset disease had stronger associations with known genetic risk factors and they tended to be of higher socioeconomic status. “They also tended to have more asthma and other allergic comorbidities,” Dr. Abuabara said. “On the other hand, the adult-onset group [after age 23] were more likely to be female, more likely to be smokers, and tended to have lower childhood socioeconomic status.”
According to the best available evidence, she continued, there is good data on higher relative risk of osteoporosis/fractures and dementia specifically among older adults with AD, and good data on associations with cardiometabolic disease and atopic disease among adults overall, as well as data showing that AD does not seem to be associated with cancer overall. In a study conducted by Jonathan I. Silverberg, MD, PhD, MPH, and Mohammed S. Shaheen, JD, the researchers used physician-diagnosed AD to investigate the associations of osteopenia and osteoporosis in two large U.S. databases: the 2006-2012 Nationwide Emergency Department Sample (NEDS) database and 2002-2012 National Inpatient Sample (NIS). Among patients aged 50 years and older, AD was associated with a higher odds of osteoporosis in NEDS (aOR, 1.31) and NIS (aOR, 1.25) and osteopenia in NEDS (aOR, 1.86).
In a separate matched cohort study, Dr. Abuabara and colleagues used U.K. primary care patient data to evaluate the association between AD and fracture and whether fracture risk varies with AD severity. Overall, they observed a 10% increase in fracture risk among people with AD, compared with those without, especially those of the hip, spine, pelvis, and wrist. “We found that there was a dose-response effect,” she said. “Those with more severe eczema had a much higher risk of fractures. When we looked at different age groups, we found a similar increased risk in the oldest adults as in younger adults.”
In a longitudinal cohort study of primary care medical records from more than 1.1 million individuals in the United Kingdom, AD was associated with an increased risk of vascular dementia (hazard ratio, 1.88), Alzheimer’s disease (HR, 1.69, and other/unspecified dementia (HR, 1.48; .269). “We found a nice dose response, where people with more severe AD had higher rates of dementia,” Dr. Abuabara said. Results from a more recent, smaller study of patients in Taiwan also found an increased risk between AD and the risk of dementia, but not a dose-response effect, likely because of a much smaller sample size.
Mounting research suggests that the risk for cardiovascular disease is also elevated in patients with AD. “There is some variability in the literature, but I think it’s important that when we’re talking about atopic dermatitis to think about the heterogeneity of the disease,” Dr. Abuabara said. In a meta-analysis and systematic review of 19 studies on the topic, she and her colleagues found that AD was associated with an increased risk of myocardial infarction (relative risk, 1.12), stroke (RR, 1.10), ischemic stroke (RR, 1.17), angina (RR, 1.18), and heart failure (RR, 1.26). “For all the different [cardiovascular disease] outcomes there was increasing risk with increasing disease severity,” she said.
She reported that UCSF receives research funding from Pfizer and Cosmetique Active International. She also receives consulting fees from Target RWE.
FROM REVOLUTIONIZING AD 2021
Epilepsy in older adults: Misdiagnosis and case complexity are common
, a neurologist told an audience at the annual meeting of the American Epilepsy Society. She urged colleagues to focus on possible interactions with other neurological conditions, consider various complicating factors, and embrace a team strategy.
“There are lots of nuances,” said Rebecca O’Dwyer, MD, an adult epilepsy specialist with Rush Epilepsy Center in Chicago. “It takes a lot of time and requires a multidisciplinary approach. Taking care of older individuals with epilepsy truly is a team sport.”
According to a 2014 report highlighted by Dr. O’Dwyer, “nearly 25% of new-onset seizures occur after age 65. The incidence of epilepsy in this age group is almost twice the rate in children, and in people over age 80, it is triple the rate in children.”
Research suggests it can take up to 2 years to correctly diagnose epilepsy in older people, Dr. O’Dwyer said, and nearly two-thirds of cases may be misdiagnosed. “Some of it is just limited awareness. There’s this perception in the public that epilepsy is something that occurs in younger adults or young children, and that when you come to a certain age, you cannot have epilepsy. Also, there are differences in the clinical manifestations of their seizures, and many comorbid possibilities could also present in similar fashion to epilepsy. Some of our usual tools that we use to come to the diagnosis such as EEG are also known to be less sensitive in this age group.”
According to the 2014 report, research finds that the elderly are much more likely than young adults to have postictal sleepiness or unresponsiveness and seizures manifesting as brief moments of subtle confusion. They’re much less likely to have epileptic aura and generalized tonic seizures.
“An epileptic seizure in an older adult tends to be less dramatic with fewer motor manifestations, and they often tend to be monophasic. They may be so subtle that they’re missed by family members and other medical providers,” Dr. O’Dwyer said. “I had a patient whose seizure consisted of her tapping her left shoulder. She had been doing this for at least 6 months, and she came to my clinic after her daughter realized that she was a little confused afterward. She’d already seen a behavioral neurologist and been given the diagnosis of dementia. We were fortunate enough to catch one of these episodes while we were doing an EEG, and we diagnosed her with focal epilepsy. With one antiseizure medication, we stopped the seizures, and her memory came back.”
Make sure to take detailed histories and keep an eye out for descriptions of behaviors that are episodic but perhaps not typical of seizures, she said.
Epilepsy can be misdiagnosed as a variety of conditions, she said, such as syncope, Alzheimer’s disease, stroke, Parkinson’s disease, and atrial fibrillation. “When you do diagnose somebody older with new-onset epilepsy, you should work them up for a stroke. Because we know that within the first 4 weeks after their first seizure the likelihood that they could have a stroke is three times higher.”
It’s also possible that neurological conditions can be followed by new-onset epilepsy, she said, making dementia even worse. Low-dose antiepileptic drugs can be helpful in these patients.
But seniors are especially vulnerable to side effects of antiepileptic drugs such as sedation, dizziness, and cardiac-conduction abnormalities. “You must adhere to the mantra of going low and going slow because they are exquisitely susceptible,” Dr. O’Dwyer said.
She recommends lamotrigine, which is well tolerated with helpful mood-stabilizing effects, and levetiracetam, which attenuates cognitive decline in dementia but may cause side effects such as irritable mood. Zonisamide is showing promise in patients with parkinsonian syndromes, she said, and it may be helpful to maximize drugs that patients are already taking such as gabapentin or pregabalin.
Finally, Dr. O’Dwyer urged colleagues to work in teams that include caregivers, primary care doctors, social workers, and pharmacists. “Sometimes in all this,” she said, “my job is the easiest.”
Dr. O’Dwyer discloses research support from the Shapiro Foundation.
, a neurologist told an audience at the annual meeting of the American Epilepsy Society. She urged colleagues to focus on possible interactions with other neurological conditions, consider various complicating factors, and embrace a team strategy.
“There are lots of nuances,” said Rebecca O’Dwyer, MD, an adult epilepsy specialist with Rush Epilepsy Center in Chicago. “It takes a lot of time and requires a multidisciplinary approach. Taking care of older individuals with epilepsy truly is a team sport.”
According to a 2014 report highlighted by Dr. O’Dwyer, “nearly 25% of new-onset seizures occur after age 65. The incidence of epilepsy in this age group is almost twice the rate in children, and in people over age 80, it is triple the rate in children.”
Research suggests it can take up to 2 years to correctly diagnose epilepsy in older people, Dr. O’Dwyer said, and nearly two-thirds of cases may be misdiagnosed. “Some of it is just limited awareness. There’s this perception in the public that epilepsy is something that occurs in younger adults or young children, and that when you come to a certain age, you cannot have epilepsy. Also, there are differences in the clinical manifestations of their seizures, and many comorbid possibilities could also present in similar fashion to epilepsy. Some of our usual tools that we use to come to the diagnosis such as EEG are also known to be less sensitive in this age group.”
According to the 2014 report, research finds that the elderly are much more likely than young adults to have postictal sleepiness or unresponsiveness and seizures manifesting as brief moments of subtle confusion. They’re much less likely to have epileptic aura and generalized tonic seizures.
“An epileptic seizure in an older adult tends to be less dramatic with fewer motor manifestations, and they often tend to be monophasic. They may be so subtle that they’re missed by family members and other medical providers,” Dr. O’Dwyer said. “I had a patient whose seizure consisted of her tapping her left shoulder. She had been doing this for at least 6 months, and she came to my clinic after her daughter realized that she was a little confused afterward. She’d already seen a behavioral neurologist and been given the diagnosis of dementia. We were fortunate enough to catch one of these episodes while we were doing an EEG, and we diagnosed her with focal epilepsy. With one antiseizure medication, we stopped the seizures, and her memory came back.”
Make sure to take detailed histories and keep an eye out for descriptions of behaviors that are episodic but perhaps not typical of seizures, she said.
Epilepsy can be misdiagnosed as a variety of conditions, she said, such as syncope, Alzheimer’s disease, stroke, Parkinson’s disease, and atrial fibrillation. “When you do diagnose somebody older with new-onset epilepsy, you should work them up for a stroke. Because we know that within the first 4 weeks after their first seizure the likelihood that they could have a stroke is three times higher.”
It’s also possible that neurological conditions can be followed by new-onset epilepsy, she said, making dementia even worse. Low-dose antiepileptic drugs can be helpful in these patients.
But seniors are especially vulnerable to side effects of antiepileptic drugs such as sedation, dizziness, and cardiac-conduction abnormalities. “You must adhere to the mantra of going low and going slow because they are exquisitely susceptible,” Dr. O’Dwyer said.
She recommends lamotrigine, which is well tolerated with helpful mood-stabilizing effects, and levetiracetam, which attenuates cognitive decline in dementia but may cause side effects such as irritable mood. Zonisamide is showing promise in patients with parkinsonian syndromes, she said, and it may be helpful to maximize drugs that patients are already taking such as gabapentin or pregabalin.
Finally, Dr. O’Dwyer urged colleagues to work in teams that include caregivers, primary care doctors, social workers, and pharmacists. “Sometimes in all this,” she said, “my job is the easiest.”
Dr. O’Dwyer discloses research support from the Shapiro Foundation.
, a neurologist told an audience at the annual meeting of the American Epilepsy Society. She urged colleagues to focus on possible interactions with other neurological conditions, consider various complicating factors, and embrace a team strategy.
“There are lots of nuances,” said Rebecca O’Dwyer, MD, an adult epilepsy specialist with Rush Epilepsy Center in Chicago. “It takes a lot of time and requires a multidisciplinary approach. Taking care of older individuals with epilepsy truly is a team sport.”
According to a 2014 report highlighted by Dr. O’Dwyer, “nearly 25% of new-onset seizures occur after age 65. The incidence of epilepsy in this age group is almost twice the rate in children, and in people over age 80, it is triple the rate in children.”
Research suggests it can take up to 2 years to correctly diagnose epilepsy in older people, Dr. O’Dwyer said, and nearly two-thirds of cases may be misdiagnosed. “Some of it is just limited awareness. There’s this perception in the public that epilepsy is something that occurs in younger adults or young children, and that when you come to a certain age, you cannot have epilepsy. Also, there are differences in the clinical manifestations of their seizures, and many comorbid possibilities could also present in similar fashion to epilepsy. Some of our usual tools that we use to come to the diagnosis such as EEG are also known to be less sensitive in this age group.”
According to the 2014 report, research finds that the elderly are much more likely than young adults to have postictal sleepiness or unresponsiveness and seizures manifesting as brief moments of subtle confusion. They’re much less likely to have epileptic aura and generalized tonic seizures.
“An epileptic seizure in an older adult tends to be less dramatic with fewer motor manifestations, and they often tend to be monophasic. They may be so subtle that they’re missed by family members and other medical providers,” Dr. O’Dwyer said. “I had a patient whose seizure consisted of her tapping her left shoulder. She had been doing this for at least 6 months, and she came to my clinic after her daughter realized that she was a little confused afterward. She’d already seen a behavioral neurologist and been given the diagnosis of dementia. We were fortunate enough to catch one of these episodes while we were doing an EEG, and we diagnosed her with focal epilepsy. With one antiseizure medication, we stopped the seizures, and her memory came back.”
Make sure to take detailed histories and keep an eye out for descriptions of behaviors that are episodic but perhaps not typical of seizures, she said.
Epilepsy can be misdiagnosed as a variety of conditions, she said, such as syncope, Alzheimer’s disease, stroke, Parkinson’s disease, and atrial fibrillation. “When you do diagnose somebody older with new-onset epilepsy, you should work them up for a stroke. Because we know that within the first 4 weeks after their first seizure the likelihood that they could have a stroke is three times higher.”
It’s also possible that neurological conditions can be followed by new-onset epilepsy, she said, making dementia even worse. Low-dose antiepileptic drugs can be helpful in these patients.
But seniors are especially vulnerable to side effects of antiepileptic drugs such as sedation, dizziness, and cardiac-conduction abnormalities. “You must adhere to the mantra of going low and going slow because they are exquisitely susceptible,” Dr. O’Dwyer said.
She recommends lamotrigine, which is well tolerated with helpful mood-stabilizing effects, and levetiracetam, which attenuates cognitive decline in dementia but may cause side effects such as irritable mood. Zonisamide is showing promise in patients with parkinsonian syndromes, she said, and it may be helpful to maximize drugs that patients are already taking such as gabapentin or pregabalin.
Finally, Dr. O’Dwyer urged colleagues to work in teams that include caregivers, primary care doctors, social workers, and pharmacists. “Sometimes in all this,” she said, “my job is the easiest.”
Dr. O’Dwyer discloses research support from the Shapiro Foundation.
FROM AES 2021
Sleep disturbances more profound in older adults with atopic dermatitis
especially trouble staying asleep.
Those are key findings from a cross-sectional study that Jaya Manjunath, BS, and Jonathan I. Silverberg, MD, PhD, MPH, presented during a poster session at the Revolutionizing Atopic Dermatitis symposium.
“Atopic dermatitis is a chronic, pruritic skin disease associated with sleep disturbance and fatigue affecting adults of all ages,” they wrote. “When caring for geriatric patients, several factors such as sleep disturbance, polypharmacy, cognition, social support, and mobility should be considered. However, little is known about the characteristics of atopic dermatitis in the geriatric population.”
Ms. Manjunath, a student at George Washington University, Washington, and Dr. Silverberg, director of clinical research in the department of dermatology at GWU, recruited patients with AD aged 18 years and older diagnosed by Hanifin-Rajka criteria who were evaluated at an academic medical center between 2014 and 2019. They underwent full body skin exams and completed electronic questionnaires. AD severity was assessed with the Eczema Area and Severity Index (EASI), Scoring Atopic Dermatitis (SCORAD) total and itch subscores, Investigator’s Global Assessment (IGA), patient-reported Global Assessment of AD severity, and the Patient-Oriented Eczema Measure (POEM).
The researchers also assessed the frequency of sleep disturbances, including difficulty falling asleep and staying asleep, and used multivariable logistic regression models to evaluate associations of age (65 and older vs. 18-64 years) with AD severity, sleep disturbance or fatigue, controlling for total POEM score, sex, and race.
Using adjusted odds ratios, Ms. Manjunath and Dr. Silverberg found that being 65 or older was not associated with AD severity on the EASI (adjusted odds ratio, 1.47); total SCORAD (aOR, 1.10), and itch subscore (aOR, 1.00); IGA (aOR, 1.87); patient-reported Global Assessment of AD severity (aOR, 0.80), or the patient-oriented eczema measure (aOR, 0.55), associations that were not statistically significant.
However, the researchers found that older adult age was associated with an increased number of nights of sleep disturbance from AD in the past week (aOR, 2.14; P = .0142), as well as increased fatigue in the past 7 days (aOR, 1.81; P = .0313), trouble sleeping in the past 7 days (aOR, 1.98; P = .0118), and trouble staying asleep in the past 7 days (aOR, 2.26; P = .0030), but not with difficulty falling asleep in the last 7 days (aOR, 1.16; P = .5996).
“Future studies are needed to determine why geriatric AD patients have increased sleep disturbance and optimal interventions to address their sleep disturbance,” the researchers concluded.
The study was supported by the Agency for Healthcare Research and Quality, the Dermatology Foundation, and by an unrestricted grant from Galderma. Ms. Manjunath disclosed no relevant financial relationships. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
A version of this article first appeared on Medscape.com.
especially trouble staying asleep.
Those are key findings from a cross-sectional study that Jaya Manjunath, BS, and Jonathan I. Silverberg, MD, PhD, MPH, presented during a poster session at the Revolutionizing Atopic Dermatitis symposium.
“Atopic dermatitis is a chronic, pruritic skin disease associated with sleep disturbance and fatigue affecting adults of all ages,” they wrote. “When caring for geriatric patients, several factors such as sleep disturbance, polypharmacy, cognition, social support, and mobility should be considered. However, little is known about the characteristics of atopic dermatitis in the geriatric population.”
Ms. Manjunath, a student at George Washington University, Washington, and Dr. Silverberg, director of clinical research in the department of dermatology at GWU, recruited patients with AD aged 18 years and older diagnosed by Hanifin-Rajka criteria who were evaluated at an academic medical center between 2014 and 2019. They underwent full body skin exams and completed electronic questionnaires. AD severity was assessed with the Eczema Area and Severity Index (EASI), Scoring Atopic Dermatitis (SCORAD) total and itch subscores, Investigator’s Global Assessment (IGA), patient-reported Global Assessment of AD severity, and the Patient-Oriented Eczema Measure (POEM).
The researchers also assessed the frequency of sleep disturbances, including difficulty falling asleep and staying asleep, and used multivariable logistic regression models to evaluate associations of age (65 and older vs. 18-64 years) with AD severity, sleep disturbance or fatigue, controlling for total POEM score, sex, and race.
Using adjusted odds ratios, Ms. Manjunath and Dr. Silverberg found that being 65 or older was not associated with AD severity on the EASI (adjusted odds ratio, 1.47); total SCORAD (aOR, 1.10), and itch subscore (aOR, 1.00); IGA (aOR, 1.87); patient-reported Global Assessment of AD severity (aOR, 0.80), or the patient-oriented eczema measure (aOR, 0.55), associations that were not statistically significant.
However, the researchers found that older adult age was associated with an increased number of nights of sleep disturbance from AD in the past week (aOR, 2.14; P = .0142), as well as increased fatigue in the past 7 days (aOR, 1.81; P = .0313), trouble sleeping in the past 7 days (aOR, 1.98; P = .0118), and trouble staying asleep in the past 7 days (aOR, 2.26; P = .0030), but not with difficulty falling asleep in the last 7 days (aOR, 1.16; P = .5996).
“Future studies are needed to determine why geriatric AD patients have increased sleep disturbance and optimal interventions to address their sleep disturbance,” the researchers concluded.
The study was supported by the Agency for Healthcare Research and Quality, the Dermatology Foundation, and by an unrestricted grant from Galderma. Ms. Manjunath disclosed no relevant financial relationships. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
A version of this article first appeared on Medscape.com.
especially trouble staying asleep.
Those are key findings from a cross-sectional study that Jaya Manjunath, BS, and Jonathan I. Silverberg, MD, PhD, MPH, presented during a poster session at the Revolutionizing Atopic Dermatitis symposium.
“Atopic dermatitis is a chronic, pruritic skin disease associated with sleep disturbance and fatigue affecting adults of all ages,” they wrote. “When caring for geriatric patients, several factors such as sleep disturbance, polypharmacy, cognition, social support, and mobility should be considered. However, little is known about the characteristics of atopic dermatitis in the geriatric population.”
Ms. Manjunath, a student at George Washington University, Washington, and Dr. Silverberg, director of clinical research in the department of dermatology at GWU, recruited patients with AD aged 18 years and older diagnosed by Hanifin-Rajka criteria who were evaluated at an academic medical center between 2014 and 2019. They underwent full body skin exams and completed electronic questionnaires. AD severity was assessed with the Eczema Area and Severity Index (EASI), Scoring Atopic Dermatitis (SCORAD) total and itch subscores, Investigator’s Global Assessment (IGA), patient-reported Global Assessment of AD severity, and the Patient-Oriented Eczema Measure (POEM).
The researchers also assessed the frequency of sleep disturbances, including difficulty falling asleep and staying asleep, and used multivariable logistic regression models to evaluate associations of age (65 and older vs. 18-64 years) with AD severity, sleep disturbance or fatigue, controlling for total POEM score, sex, and race.
Using adjusted odds ratios, Ms. Manjunath and Dr. Silverberg found that being 65 or older was not associated with AD severity on the EASI (adjusted odds ratio, 1.47); total SCORAD (aOR, 1.10), and itch subscore (aOR, 1.00); IGA (aOR, 1.87); patient-reported Global Assessment of AD severity (aOR, 0.80), or the patient-oriented eczema measure (aOR, 0.55), associations that were not statistically significant.
However, the researchers found that older adult age was associated with an increased number of nights of sleep disturbance from AD in the past week (aOR, 2.14; P = .0142), as well as increased fatigue in the past 7 days (aOR, 1.81; P = .0313), trouble sleeping in the past 7 days (aOR, 1.98; P = .0118), and trouble staying asleep in the past 7 days (aOR, 2.26; P = .0030), but not with difficulty falling asleep in the last 7 days (aOR, 1.16; P = .5996).
“Future studies are needed to determine why geriatric AD patients have increased sleep disturbance and optimal interventions to address their sleep disturbance,” the researchers concluded.
The study was supported by the Agency for Healthcare Research and Quality, the Dermatology Foundation, and by an unrestricted grant from Galderma. Ms. Manjunath disclosed no relevant financial relationships. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
A version of this article first appeared on Medscape.com.
FROM REVOLUTIONIZING AD 2021
A Practical Approach for Primary Care Practitioners to Evaluate and Manage Lower Urinary Tract Symptoms and Benign Prostatic Hyperplasia
Lower urinary tract symptoms (LUTS)are common and tend to increase in frequency with age. Managing LUTS can be complicated, requires an informed discussion between the primary care practitioner (PCP) and patient, and is best achieved by a thorough understanding of the many medical and surgical options available. Over the past 3 decades, medications have become the most common therapy; but recently, newer minimally invasive surgeries have challenged this paradigm. This article provides a comprehensive review for PCPs regarding the evaluation and management of LUTS in men and when to consider a urology referral.
Benign prostatic hyperplasia (BPH) and LUTS are common clinical encounters for most PCPs. About 50% of men will develop LUTS associated with BPH, and symptoms associated with these conditions increase as men age.1,2 Studies have estimated that 90% of men aged 45 to 80 years demonstrate some symptoms of LUTS.3 Strong genetic influence seems to suggest heritability, but BPH also occurs in sporadic forms and is heavily influenced by androgens.4
BPH is a histologic diagnosis, whereas LUTS consists of complex symptomatology related to both static or dynamic components.1 The enlarged prostate gland obstructs the urethra, simultaneously causing an increase in muscle tone and resistance at the bladder neck and prostatic urethra, leading to increased resistance to urine flow. As a result, there is a thickening of the detrusor muscles in the bladder wall and an overall decreased compliance. Urine becomes stored under increased pressure. These changes result in a weak or intermittent urine stream, incomplete emptying of the bladder, postvoid dribble, hesitancy, and irritative symptoms, such as urgency, frequency, and nocturia.
For many patients, BPH associated with LUTS is a quality of life (QOL) issue. The stigma associated with these symptoms often leads to delays in patients seeking care. Many patients do not seek treatment until symptoms have become so severe that changes in bladder health are often irreversible. Early intervention can dramatically improve a patient’s QOL. Also, early intervention has the potential to reduce overall health care expenditures. BPH-related spending exceeds $1 billion each year in the Medicare program alone.5
PCPs are in a unique position to help many patients who present with early-stage LUTS. Given the substantial impact this disease has on QOL, early recognition of symptoms and prompt treatment play a major role. Paramount to this effort is awareness and understanding of various treatments, their advantages, and adverse effects (AEs). This article highlights evidence-based evaluation and treatment of BPH/LUTS for PCPs who treat veterans and recommendations as to when to refer a patient to a urologist.
Evaluation of LUTS and BPH
Evaluation begins with a thorough medical history and physical examination. Particular attention should focus on ruling out other causes of LUTS, such as a urinary tract infection (UTI), acute prostatitis, malignancy, bladder dysfunction, neurogenic bladder, and other obstructive pathology, such as urethral stricture disease. The differential diagnosis of LUTS includes BPH, UTI, bladder neck obstruction, urethral stricture, bladder stones, polydipsia, overactive bladder (OAB), nocturnal polyuria, neurologic disease, genitourinary malignancy, renal failure, and acute/chronic urinary retention.6
Relevant medical history influencing urinary symptoms includes diabetes mellitus, underlying neurologic diseases, previous trauma, sexually transmitted infections, and certain medications. Symptom severity may be obtained using a validated questionnaire, such as the International Prostate Symptom Score (IPSS), which also aids clinicians in assessing the impact of LUTS on QOL. Additionally, urinary frequency or volume records (voiding diary) may help establish the severity of the patient’s symptoms and provide insight into other potential causes for LUTS. Patients with BPH often have concurrent erectile dysfunction (ED) or other sexual dysfunction symptoms. Patients should be evaluated for baseline sexual dysfunction before the initiation of treatment as many therapies worsen symptoms of ED or ejaculatory dysfunction.
A comprehensive physical examination with a focus on the genitourinary system should, at minimum, assess for abnormalities of the urethral meatus, prepuce, penis, groin nodes, and prior surgical scars. A digital rectal examination also should be performed. Although controversial, a digital rectal examination for prostate cancer screening may provide a rough estimate of prostate size, help rule out prostatitis, and detect incident prostate nodules. Prostate size does not necessarily correlate well with the degree of urinary obstruction or LUTS but is an important consideration when deciding among different therapies.1
Laboratory and Adjunctive Tests
A urinalysis with microscopy helps identify other potential causes for urinary symptoms, including infection, proteinuria, or glucosuria. In patients who present with gross or microscopic hematuria, additional consideration should be given to bladder calculi and genitourinary cancer.2 When a reversible source for the hematuria is not identified, these patients require referral to a urologist for a hematuria evaluation.
There is some controversy regarding prostate specific antigen (PSA) testing. Most professional organizations advocate for a shared decision-making approach before testing. The American Cancer Society recommends this informed discussion occur between the patient and the PCP for men aged > 50 years at average risk, men aged > 45 years at high risk of developing prostate cancer (African Americans or first-degree relative with early prostate cancer diagnosis), and aged 40 years for men with more than one first-degree relative with an early prostate cancer diagnosis.7
Adjunctive tests include postvoid residual (PVR), cystoscopy, uroflowmetry, urodynamics, and transrectal ultrasound. However, these are mostly performed by urologists. In some patients with bladder decompensation after prolonged partial bladder outlet obstruction, urodynamics may be used by urologists to determine whether a patient may benefit from an outlet obstruction procedure. Ordering additional imaging or serum studies for the assessment of LUTS is rarely helpful.
Treatment
Treatment includes management with or without lifestyle modification, medication administration, and surgical therapy. New to this paradigm are in-office minimally invasive surgical options. The goal of treatment is not only to reduce patient symptoms and improve QOL, but also to prevent the secondary sequala of urinary retention, bladder failure, and eventual renal impairment.7A basic understanding of these treatments can aid PCPs with appropriate patient counseling and urologic referral.8
Lifestyle and Behavior Modification
Behavior modification is the starting point for all patients with LUTS. Lifestyle modifications for LUTS include avoiding substances that exacerbate symptoms, such as α-agonists (decongestants), caffeine, alcohol, spicy/acidic foods, chocolate, and soda. These substances are known to be bladder irritants. Common medications contributing to LUTS include antidepressants, decongestants, antihistamines, bronchodilators, anticholinergics, and sympathomimetics. To decrease nocturia, behavioral modifications include limiting evening fluid intake, timed diuretic administration for patients already on a diuretic, and elevating legs 1 hour before bedtime. Counseling obese patients to lose weight and increasing physical activity have been linked to reduced LUTS.9 Other behavioral techniques include double voiding: a technique where patients void normally then change positions and return to void to empty the bladder. Another technique is timed voiding: Many patients have impaired sensation when the bladder is full. These patients are encouraged to void at regular intervals.
Complementary and Alternative Medicine
Multiple nutraceutical compounds claim improved urinary health and symptom reduction. These compounds are marketed to patients with little regulation and oversight since supplements are not regulated or held to the same standard as prescription medications. The most popular nutraceutical for prostate health and LUTS is saw palmetto. Despite its common usage for the treatment of LUTS, little data support saw palmetto health claims. In 2012, a systematic review of 32 randomized trials including 5666 patients compared saw palmetto with a placebo. The study found no difference in urinary symptom scores, urinary flow, or prostate size.10,11 Other phytotherapy compounds often considered for urinary symptoms include stinging nettle extract and β-sitosterol compounds. The mechanism of action of these agents is unknown and efficacy data are lacking.
Historically, acupuncture and pelvic floor physical therapy have been used successfully for OAB symptoms. A meta-analysis found positive beneficial effects of acupuncture compared with a sham control for short- and medium-term follow-up in both IPSS and urine flow rates in some studies; however, when combining the studies for more statistical power, the benefits were less clear.12 Physical therapists with specialized training and certification in pelvic health can incorporate certain bladder training techniques. These include voiding positional changes (double voiding and postvoid urethral milking) and timed voiding.13,14 These interventions often address etiologies of LUTS for which medical therapies are not effective as the sole treatment option.
Medication Management
Medical management includes α-blockers, 5-α-reductase inhibitors (5-α-RIs), antimuscarinic or anticholinergic medicines, β-3 agonists, and phosphodiesterase inhibitors (Table). These medications work independently as well as synergistically. The use of medications to improve symptoms must be balanced against potential AEs and the consequences of a lifetime of drug usage, which can be additive.15,16
First-line pharmacological therapy for BPH is α-blockers, which work by blocking α1A receptors in the prostate and bladder neck, leading to smooth muscle relaxation, increased diameter of the channel, and improved urinary flow. α-receptors in the bladder neck and prostate are expressed with increased frequency with age and are a potential cause for worsening symptoms as men age. Studies demonstrate that these medications reduce symptoms by 30 to 40% and increase flow rates by 16 to 25%.17 Commonly prescribed α-blockers include tamsulosin, alfuzosin, silodosin, doxazosin, and terazosin. Doxazosin and terazosin require dose titrations because they may cause significant hypotension. Orthostatic hypotension typically improves with time and is avoided if the patient takes the medication at bedtime. Both doxazosin and terazosin are on the American Geriatric Society’s Beers Criteria list and should be avoided in older patients.18 Tamsulosin, alfuzosin, and silodosin have a standardized dosing regimen and lower rates of hypotension. Significant AEs include ejaculation dysfunction, nasal congestion, and orthostatic hypotension. Duan and colleagues have linked tamsulosin with dementia. However, this association is not causal and further studies are necessary.19,20 Patients who have taken these agents also are at risk for intraoperative floppy iris syndrome (IFIS). Permanent visual problems can arise if the intraoperative management is not managed to account for IFIS. These medications have a rapid onset of action and work immediately. However, to reach maximum benefit, patients must take the medication for several weeks. Unfortunately, up to one-third of patients will have no improvement with α-blocker therapy, and many patients will discontinue these medications because of significant AEs.6,21
5-α-RIs (finasteride and dutasteride) inhibit the conversion of testosterone to more potent dihydrotestosterone. They effectively reduce prostate volume by 25 to 30%.22 The results occur slowly and can take 6 to 12 months to reach the desired outcome. These medications are effective in men with larger prostates and not as effective in men with smaller prostates.23 These medications can improve urinary flow rates by about 10%, reduce IPSS scores by 20 to 30%, reduce the risk of urinary retention by 50%, and reduce the progression of BPH to the point where surgery is required by 50%.24 Furthermore, 5-α-RIs lower PSA by > 50% after 12 months of treatment.25
A baseline PSA should be established before administration and after 6 months of treatment. Any increase in the PSA even if the level is within normal limits should be evaluated for prostate cancer. Sarkar and colleagues recently published a study evaluating prostate cancer diagnosis in patients treated with 5-α-RI and found there was a delay in diagnosing prostate cancer in this population. Controversy also exists as to the potential of these medications increasing the risk for high-grade prostate cancer, which has led to a US Food and Drug Administration (FDA) warning. AEs include decreased libido (1.5%), ejaculatory dysfunction (3.4%), gynecomastia (1.3%), and/or ED (1.6%).26-28 A recent study evaluating 5-α-RIs demonstrated about a 2-fold increased risk of depression.29
There are well-established studies that note increased effectiveness when using combined α-blocker therapy with 5-α-RI medications. The Medical Therapy of Prostate Symptoms (MTOPS) and Combination Avodart and Tamsulosin (CombAT) trials showed that the combination of both medications was more effective in improving voiding symptoms and flow rates than either agent alone.15,16 Combination therapy resulted in a 66% reduction in disease progression, 81% reduction in urinary retention, and a 67% reduction in the need for surgery compared with placebo.
Anticholinergic medication use in BPH with LUTS is well established, and their use is often combined with other therapies. Anticholinergics work by inhibiting muscarinic M3 receptors to reduce detrusor muscle contraction. This effectively decreases bladder contractions and delays the desire to void. Kaplan and colleagues showed that tolterodine significantly improved a patient’s QOL when added to α-blocker therapy.30 Patients reported a positive outcome at 12 weeks, which resulted in a reduction in urgency incontinence, urgency, nocturia, and the overall number of voiding episodes within 24 hours.
β-3 agonists are a class of medications for OAB; mirabegron and vibegron have proven effective in reducing similar symptoms. In phase 3 clinical trials, mirabegron improved urinary incontinence episodes by 50% and reduced the number of voids in 24 hours.31 Mirabegron is well tolerated and avoids many common anticholinergic effects.32 Vibegron is the newest medication in the class and could soon become the preferred agent given it does not have cytochrome P450 interactions and does not cause hypertension like mirabegron.33
Anticholinergics should be used with caution in patients with a history of urinary retention, elevated after-void residual, or other medications with known anticholinergic effects. AEs include sedation, confusion, dry mouth, constipation, and potential falls in older patients.18 Recent studies have noted an association with dementia in the prolonged use of these medications in older patients and should be used cautiously.20
Phosphodiesterase-5 enzyme inhibitors (PDE-5) are adjunctive medications shown to improve LUTS. This class of medication is prescribed mostly for ED. However, tadalafil 5 mg taken daily also is FDA approved for the treatment of LUTS secondary to BPH given its prolonged half-life. The exact mechanism for improved BPH symptoms is unknown. Possibly the effects are due to an increase mediated by PDE-5 in cyclic guanosine monophosphate (cGMP), which increases smooth muscle relaxation and tissue perfusion of the prostate and bladder.34 There have been limited studies on objective improvement in uroflowmetry parameters compared with other treatments. The daily dosing of tadalafil should not be prescribed in men with a creatinine clearance < 30 mL/min.29 Tadalafil is not considered a first-line agent and is usually reserved for patients who experience ED in addition to BPH. When initiating BPH pharmacologic therapy, the PCP should be aware of adherence and high discontinuation rates.35
Surgical Treatments
Surgical treatments are often delayed out of fear of potential AEs or considered a last resort when symptoms are too severe.36 Early intervention is required to prevent irreversible deleterious changes to detrusor muscle structure and function (Figure). Patients fear urinary incontinence, ED or ejaculatory dysfunction, and anesthesia complications associated with surgical interventions.6,37 Multiple studies show that patients fare better with early surgical intervention, experiencing improved IPSS scores, urinary flow, and QOL. The following is an overview of the most popular procedures.
Prostatic urethral lift (PUL) using the UroLift System is an FDA-approved, minimally-invasive treatment of LUTS secondary to BPH. This procedure treats prostates < 80 g with an absent median lobe.6,21,38 Permanent implants are placed per the prostatic urethra to displace obstructing prostate tissue laterally. This opens the urethra directly without cutting, heating, or removing any prostate tissue. This procedure is minimally invasive, often done in the office as an outpatient procedure, and offers better symptom relief than medication with a lower risk profile than transurethral resection of the prostate (TURP).39,40 The LIFT study was a multicenter, randomized, blinded trial; patients were randomized 2:1 to undergo UroLift or a sham operation. At 3 years, average improvements were statistically significant for total IPSS reduction (41%), QOL improvement (49%), and improved maximum flow rates by (51%).41 Risk for urinary incontinence is low, and the procedure has been shown to preserve erectile and ejaculatory function. Furthermore, patients report significant improvement in their QOL without the need for medications. Surgical retreatment rates at 5 years are 13.6%, with an additional 10.7% of subjects back on medication therapy with α-blockers or 5-α-RIs.42
Water vapor thermal therapy or Rez¯um uses steam as thermal energy to destroy obstructing prostate tissue and relieve the obstruction.43 The procedure differs from older conductive heat thermotherapies because the steam penetrates prostate zonal anatomy without affecting areas outside the targeted treatment zone. The procedure is done in the office with local anesthesia and provides long-lasting relief of LUTS with minimal risks. Following the procedure, patients require an indwelling urethral catheter for 3 to 7 days, and most patients begin to experience symptom improvement 2 to 4 weeks following the procedure.44 The procedure received FDA approval in 2015. Four-year data show significant improvement in maximal flow rate (50%), IPSS (47%), and QOL (43%).45 Surgical retreatment rates were 4.4%. Criticisms of this treatment include patient discomfort with the office procedure, the requirement for an indwelling catheter for a short period, and lack of long-term outcomes data. Guidelines support use in prostate volumes > 80 g with or without median lobe anatomy.
TURP is the gold standard to which other treatments are compared.46 The surgery is performed in the operating room where urologists use a rigid cystoscope and resection element to effectively carve out and cauterize obstructing prostate tissue. Patients typically recover for a short period with an indwelling urethral catheter that is often removed 12 to 24 hours after surgery. New research points out that despite increasing mean age (55% of patients are aged > 70 years with associated comorbidities), the morbidity of TURP was < 1% and mortality rate of 0 to 0.3%.47 Postoperative complications include bleeding that requires a transfusion (3%), retrograde ejaculation (65%), and rare urinary incontinence (2%).47 Surgical retreatment rates for patients following a TURP are approximately 13 to 15% at 8 years.34
Laser surgery for BPH includes multiple techniques: photovaporization of the prostate using a Greenlight XPS laser, holmium laser ablation, and holmium laser enucleation (HoLEP). Proponents of these treatments cite lower bleeding risks compared with TURP, but the operation is largely surgeon dependent on the technology chosen. Most studies comparing these technologies with TURP show similar outcomes of IPSS reports, quality of life improvements, and complications.
Patients with extremely large prostates, > 100 g or 4 times the normal size, pose a unique challenge to surgical treatment. Historically, patients were treated with an open simple prostatectomy operation or staged TURP procedures. Today, urologists use newer, safer ways to treat these patients. Both HoLEP and robot-assisted simple prostatectomy work well in relieving urinary symptoms with lower complications compared with older open surgery. Other minimally invasive procedures, such as prostatic artery embolism, have been described for the treatment of BPH specifically in men who may be unfit for surgery.48Future treatments are constantly evolving. Many unanswered questions remain about BPH and the role of inflammation, metabolic dysfunction, obesity, and other genetic factors driving BPH and prostate growth. Pharmaceutical opportunities exist in mechanisms aimed to reduce prostate growth, induce cellular apoptosis, as well as other drugs to reduce bladder symptoms. Newer, minimally invasive therapies also will become more readily available, such as Aquablation, which is the first FDA-granted surgical robot for the autonomous removal of prostatic tissue due to BPH.49 However, the goal of all future therapies should include the balance of alleviating disruptive symptoms while demonstrating a favorable risk profile. Many men discontinue taking medications, yet few present for surgery. Most concerning is the significant population of men who will develop irreversible bladder dysfunction while waiting for the perfect treatment. There are many opportunities for an effective treatment that is less invasive than surgery, provides durable relief, has minimal AEs, and is affordable.
Conclusions
There is no perfect treatment for patients with LUTS. All interventions have potential AEs and associated complications. Medications are often started as first-line therapy but are often discontinued at the onset of significant AEs. This process is often repeated. Many patients will try different medications without any significant improvement in their symptoms or short-term relief, which results in the gradual progression of the disease.
The PCP plays a significant role in the initial evaluation and management of BPH. These frontline clinicians can recognize patients who may already be experiencing sequela of prolonged bladder outlet obstruction and refer these men to urologists promptly. Counseling patients about their treatment options is an important duty for all PCPs.
A clear understanding of the available treatment options will help PCPs counsel patients appropriately about lifestyle modification, medications, and surgical treatment options for their symptoms. The treatment of this disorder is a rapidly evolving topic with the constant introduction of new technologies and medications, which are certain to continue to play an important role for PCPs and urologists.
1. Roehrborn CG. Benign prostatic hyperplasia: an overview. Rev Urol. 2005;7 Suppl 9(Suppl 9):S3-S14
2. McVary KT. Clinical manifestations and diagnostic evaluation of benign prostatic hyperplasia. UpToDate. Updated November 18, 2021. Accessed November 23, 2021. https:// www.uptodate.com/contents/clinical-manifestations-and -diagnostic-evaluation-of-benign-prostatic-hyperplasia
3. McVary KT. BPH: epidemiology and comorbidities. Am J Manag Care. 2006;12(5 Suppl):S122-S128.
4. Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol. 2011;8(1):29-41. doi:10.1038/nrurol.2010.207
5. Rensing AJ, Kuxhausen A, Vetter J, Strope SA. Differences in the treatment of benign prostatic hyperplasia: comparing the primary care physician and the urologist. Urol Pract. 2017;4(3):193-199. doi:10.1016/j.urpr.2016.07.002
6. Foster HE, Barry MJ, Dahm P, et al. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline. J Urol. 2018;200(3):612- 619. doi:10.1016/j.juro.2018.05.048
7. Landau A, Welliver C. Analyzing and characterizing why men seek care for lower urinary tract symptoms. Curr Urol Rep. 2020;21(12):58. Published 2020 Oct 30. doi:10.1007/s11934-020-01006-w
8. Das AK, Leong JY, Roehrborn CG. Office-based therapies for benign prostatic hyperplasia: a review and update. Can J Urol. 2019;26(4 Suppl 1):2-7.
9. Parsons JK, Sarma AV, McVary K, Wei JT. Obesity and benign prostatic hyperplasia: clinical connections, emerging etiological paradigms and future directions. J Urol. 2013;189(1 Suppl):S102-S106. doi:10.1016/j.juro.2012.11.029
10. Pattanaik S, Mavuduru RS, Panda A, et al. Phosphodiesterase inhibitors for lower urinary tract symptoms consistent with benign prostatic hyperplasia. Cochrane Database Syst Rev. 2018;11(11):CD010060. Published 2018 Nov 16. doi:10.1002/14651858.CD010060.pub2
11. McVary KT. Medical treatment of benign prostatic hyperplasia. UpToDate. Updated October 4, 2021. Accessed November 23, 2021. https://www.uptodate.com/contents /medical-treatment-of-benign-prostatic-hyperplasia
12. Zhang W, Ma L, Bauer BA, Liu Z, Lu Y. Acupuncture for benign prostatic hyperplasia: A systematic review and metaanalysis. PLoS One. 2017;12(4):e0174586. Published 2017 Apr 4. doi:10.1371/journal.pone.0174586
13. Newman DK, Guzzo T, Lee D, Jayadevappa R. An evidence- based strategy for the conservative management of the male patient with incontinence. Curr Opin Urol. 2014;24(6):553-559. doi:10.1097/MOU.0000000000000115
14. Newman DK, Wein AJ. Office-based behavioral therapy for management of incontinence and other pelvic disorders. Urol Clin North Am. 2013;40(4):613-635. doi:10.1016/j.ucl.2013.07.010
15. McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349(25):2387-2398. doi:10.1056/NEJMoa030656
16. Roehrborn CG, Barkin J, Siami P, et al. Clinical outcomes after combined therapy with dutasteride plus tamsulosin or either monotherapy in men with benign prostatic hyperplasia (BPH) by baseline characteristics: 4-year results from the randomized, double-blind Combination of Avodart and Tamsulosin (CombAT) trial. BJU Int. 2011;107(6):946-954. doi:10.1111/j.1464-410X.2011.10124.x
17. Djavan B, Marberger M. A meta-analysis on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol. 1999;36(1):1-13. doi:10.1159/000019919
18. By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. doi:10.1111/jgs.13702
19. Duan Y, Grady JJ, Albertsen PC, Helen Wu Z. Tamsulosin and the risk of dementia in older men with benign prostatic hyperplasia. Pharmacoepidemiol Drug Saf. 2018;27(3):340- 348. doi:10.1002/pds.4361
20. Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic drug exposure and the risk of dementia: a nested case-control study. JAMA Intern Med. 2019;179(8):1084-1093. doi:10.1001/jamainternmed.2019.0677
21. Parsons JK, Dahm P, Köhler TS, Lerner LB, Wilt TJ. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline amendment 2020. J Urol. 2020;204(4):799-804. doi:10.1097/JU.0000000000001298
22. Smith AB, Carson CC. Finasteride in the treatment of patients with benign prostatic hyperplasia: a review. Ther Clin Risk Manag. 2009;5(3):535-545. doi:10.2147/tcrm.s6195
23. Andriole GL, Guess HA, Epstein JI, et al. Treatment with finasteride preserves usefulness of prostate-specific antigen in the detection of prostate cancer: results of a randomized, double-blind, placebo-controlled clinical trial. PLESS Study Group. Proscar Long-term Efficacy and Safety Study. Urology. 1998;52(2):195-202. doi:10.1016/s0090-4295(98)00184-8
24. McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med. 1998;338(9):557-563. doi:10.1056/NEJM199802263380901
25. Rittmaster RS. 5alpha-reductase inhibitors in benign prostatic hyperplasia and prostate cancer risk reduction. Best Pract Res Clin Endocrinol Metab. 2008;22(2):389-402. doi:10.1016/j.beem.2008.01.016
26. La Torre A, Giupponi G, Duffy D, Conca A, Cai T, Scardigli A. Sexual dysfunction related to drugs: a critical review. Part V: α-blocker and 5-ARI drugs. Pharmacopsychiatry. 2016;49(1):3-13. doi:10.1055/s-0035-1565100
27. Corona G, Tirabassi G, Santi D, et al. Sexual dysfunction in subjects treated with inhibitors of 5α-reductase for benign prostatic hyperplasia: a comprehensive review and meta-analysis. Andrology. 2017;5(4):671-678. doi:10.1111/andr.12353
28. Trost L, Saitz TR, Hellstrom WJ. Side effects of 5-alpha reductase inhibitors: a comprehensive review. Sex Med Rev. 2013;1(1):24-41. doi:10.1002/smrj.3
29. Welk B, McArthur E, Ordon M, Anderson KK, Hayward J, Dixon S. Association of suicidality and depression with 5α-reductase inhibitors. JAMA Intern Med. 2017;177(5):683-691. doi:10.1001/jamainternmed.2017.0089
30. Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial [published correction appears in JAMA. 2007 Mar 21:297(11):1195] [published correction appears in JAMA. 2007 Oct 24;298(16):1864]. JAMA. 2006;296(19):2319-2328. doi:10.1001/jama.296.19.2319
31. Nitti VW, Auerbach S, Martin N, Calhoun A, Lee M, Herschorn S. Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol. 2013;189(4):1388-1395. doi:10.1016/j.juro.2012.10.017
32. Chapple CR, Cardozo L, Nitti VW, Siddiqui E, Michel MC. Mirabegron in overactive bladder: a review of efficacy, safety, and tolerability. Neurourol Urodyn. 2014;33(1):17-30. doi:10.1002/nau.22505
33. Rutman MP, King JR, Bennett N, Ankrom W, Mudd PN. PD14-01 once-daily vibegron, a novel oral β3 agonist does not inhibit CYP2D6, a common pathway for drug metabolism in patients on OAB medications. J Urol. 2019;201(Suppl 4):e231. doi:10.1097/01.JU.0000555478.73162.19
34. Bo K, Frawley HC, Haylen BT, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Neurourol Urodyn. 2017;36(2):221- 244. doi:10.1002/nau.23107
35. Cindolo L, Pirozzi L, Fanizza C, et al. Drug adherence and clinical outcomes for patients under pharmacological therapy for lower urinary tract symptoms related to benign prostatic hyperplasia: population-based cohort study. Eur Urol. 2015;68(3):418-425. doi:10.1016/j.eururo.2014.11.006
36. Ruhaiyem ME, Alshehri AA, Saade M, Shoabi TA, Zahoor H, Tawfeeq NA. Fear of going under general anesthesia: a cross-sectional study. Saudi J Anaesth. 2016;10(3):317- 321. doi:10.4103/1658-354X.179094
37. Hashim MJ. Patient-centered communication: basic skills. Am Fam Physician. 2017;95(1):29-34.
38. Roehrborn CG, Barkin J, Gange SN, et al. Five year results of the prospective randomized controlled prostatic urethral L.I.F.T. study. Can J Urol. 2017;24(3):8802-8813.
39. Gratzke C, Barber N, Speakman MJ, et al. Prostatic urethral lift vs transurethral resection of the prostate: 2-year results of the BPH6 prospective, multicentre, randomized study. BJU Int. 2017;119(5):767-775.doi:10.1111/bju.13714
40. Sønksen J, Barber NJ, Speakman MJ, et al. Prospective, randomized, multinational study of prostatic urethral lift versus transurethral resection of the prostate: 12-month results from the BPH6 study. Eur Urol. 2015;68(4):643-652. doi:10.1016/j.eururo.2015.04.024
41. Roehrborn CG, Gange SN, Shore ND, et al. The prostatic urethral lift for the treatment of lower urinary tract symptoms associated with prostate enlargement due to benign prostatic hyperplasia: the L.I.F.T. Study. J Urol. 2013;190(6):2161-2167. doi:10.1016/j.juro.2013.05.116
42. McNicholas TA. Benign prostatic hyperplasia and new treatment options - a critical appraisal of the UroLift system. Med Devices (Auckl). 2016;9:115-123. Published 2016 May 19. doi:10.2147/MDER.S60780
43. McVary KT, Rogers T, Roehrborn CG. Rezuˉm Water Vapor thermal therapy for lower urinary tract symptoms associated with benign prostatic hyperplasia: 4-year results from randomized controlled study. Urology. 2019;126:171-179. doi:10.1016/j.urology.2018.12.041
44. Bole R, Gopalakrishna A, Kuang R, et al. Comparative postoperative outcomes of Rezˉum prostate ablation in patients with large versus small glands. J Endourol. 2020;34(7):778-781. doi:10.1089/end.2020.0177
45. Darson MF, Alexander EE, Schiffman ZJ, et al. Procedural techniques and multicenter postmarket experience using minimally invasive convective radiofrequency thermal therapy with Rezˉum system for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Res Rep Urol. 2017;9:159-168. Published 2017 Aug 21. doi:10.2147/RRU.S143679
46. Baazeem A, Elhilali MM. Surgical management of benign prostatic hyperplasia: current evidence. Nat Clin Pract Urol. 2008;5(10):540-549. doi:10.1038/ncpuro1214
47. Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP)- -incidence, management, and prevention. Eur Urol. 2006;50(5):969-980. doi:10.1016/j.eururo.2005.12.042
48. Abt D, Schmid HP, Speakman MJ. Reasons to consider prostatic artery embolization. World J Urol. 2021;39(7):2301-2306. doi:10.1007/s00345-021-03601-z
49. Nguyen DD, Barber N, Bidair M, et al. Waterjet Ablation Therapy for Endoscopic Resection of prostate tissue trial (WATER) vs WATER II: comparing Aquablation therapy for benign prostatic hyperplasia in30-80and80-150mLprostates. BJUInt. 2020;125(1):112-122. doi:10.1111/bju.14917.
Lower urinary tract symptoms (LUTS)are common and tend to increase in frequency with age. Managing LUTS can be complicated, requires an informed discussion between the primary care practitioner (PCP) and patient, and is best achieved by a thorough understanding of the many medical and surgical options available. Over the past 3 decades, medications have become the most common therapy; but recently, newer minimally invasive surgeries have challenged this paradigm. This article provides a comprehensive review for PCPs regarding the evaluation and management of LUTS in men and when to consider a urology referral.
Benign prostatic hyperplasia (BPH) and LUTS are common clinical encounters for most PCPs. About 50% of men will develop LUTS associated with BPH, and symptoms associated with these conditions increase as men age.1,2 Studies have estimated that 90% of men aged 45 to 80 years demonstrate some symptoms of LUTS.3 Strong genetic influence seems to suggest heritability, but BPH also occurs in sporadic forms and is heavily influenced by androgens.4
BPH is a histologic diagnosis, whereas LUTS consists of complex symptomatology related to both static or dynamic components.1 The enlarged prostate gland obstructs the urethra, simultaneously causing an increase in muscle tone and resistance at the bladder neck and prostatic urethra, leading to increased resistance to urine flow. As a result, there is a thickening of the detrusor muscles in the bladder wall and an overall decreased compliance. Urine becomes stored under increased pressure. These changes result in a weak or intermittent urine stream, incomplete emptying of the bladder, postvoid dribble, hesitancy, and irritative symptoms, such as urgency, frequency, and nocturia.
For many patients, BPH associated with LUTS is a quality of life (QOL) issue. The stigma associated with these symptoms often leads to delays in patients seeking care. Many patients do not seek treatment until symptoms have become so severe that changes in bladder health are often irreversible. Early intervention can dramatically improve a patient’s QOL. Also, early intervention has the potential to reduce overall health care expenditures. BPH-related spending exceeds $1 billion each year in the Medicare program alone.5
PCPs are in a unique position to help many patients who present with early-stage LUTS. Given the substantial impact this disease has on QOL, early recognition of symptoms and prompt treatment play a major role. Paramount to this effort is awareness and understanding of various treatments, their advantages, and adverse effects (AEs). This article highlights evidence-based evaluation and treatment of BPH/LUTS for PCPs who treat veterans and recommendations as to when to refer a patient to a urologist.
Evaluation of LUTS and BPH
Evaluation begins with a thorough medical history and physical examination. Particular attention should focus on ruling out other causes of LUTS, such as a urinary tract infection (UTI), acute prostatitis, malignancy, bladder dysfunction, neurogenic bladder, and other obstructive pathology, such as urethral stricture disease. The differential diagnosis of LUTS includes BPH, UTI, bladder neck obstruction, urethral stricture, bladder stones, polydipsia, overactive bladder (OAB), nocturnal polyuria, neurologic disease, genitourinary malignancy, renal failure, and acute/chronic urinary retention.6
Relevant medical history influencing urinary symptoms includes diabetes mellitus, underlying neurologic diseases, previous trauma, sexually transmitted infections, and certain medications. Symptom severity may be obtained using a validated questionnaire, such as the International Prostate Symptom Score (IPSS), which also aids clinicians in assessing the impact of LUTS on QOL. Additionally, urinary frequency or volume records (voiding diary) may help establish the severity of the patient’s symptoms and provide insight into other potential causes for LUTS. Patients with BPH often have concurrent erectile dysfunction (ED) or other sexual dysfunction symptoms. Patients should be evaluated for baseline sexual dysfunction before the initiation of treatment as many therapies worsen symptoms of ED or ejaculatory dysfunction.
A comprehensive physical examination with a focus on the genitourinary system should, at minimum, assess for abnormalities of the urethral meatus, prepuce, penis, groin nodes, and prior surgical scars. A digital rectal examination also should be performed. Although controversial, a digital rectal examination for prostate cancer screening may provide a rough estimate of prostate size, help rule out prostatitis, and detect incident prostate nodules. Prostate size does not necessarily correlate well with the degree of urinary obstruction or LUTS but is an important consideration when deciding among different therapies.1
Laboratory and Adjunctive Tests
A urinalysis with microscopy helps identify other potential causes for urinary symptoms, including infection, proteinuria, or glucosuria. In patients who present with gross or microscopic hematuria, additional consideration should be given to bladder calculi and genitourinary cancer.2 When a reversible source for the hematuria is not identified, these patients require referral to a urologist for a hematuria evaluation.
There is some controversy regarding prostate specific antigen (PSA) testing. Most professional organizations advocate for a shared decision-making approach before testing. The American Cancer Society recommends this informed discussion occur between the patient and the PCP for men aged > 50 years at average risk, men aged > 45 years at high risk of developing prostate cancer (African Americans or first-degree relative with early prostate cancer diagnosis), and aged 40 years for men with more than one first-degree relative with an early prostate cancer diagnosis.7
Adjunctive tests include postvoid residual (PVR), cystoscopy, uroflowmetry, urodynamics, and transrectal ultrasound. However, these are mostly performed by urologists. In some patients with bladder decompensation after prolonged partial bladder outlet obstruction, urodynamics may be used by urologists to determine whether a patient may benefit from an outlet obstruction procedure. Ordering additional imaging or serum studies for the assessment of LUTS is rarely helpful.
Treatment
Treatment includes management with or without lifestyle modification, medication administration, and surgical therapy. New to this paradigm are in-office minimally invasive surgical options. The goal of treatment is not only to reduce patient symptoms and improve QOL, but also to prevent the secondary sequala of urinary retention, bladder failure, and eventual renal impairment.7A basic understanding of these treatments can aid PCPs with appropriate patient counseling and urologic referral.8
Lifestyle and Behavior Modification
Behavior modification is the starting point for all patients with LUTS. Lifestyle modifications for LUTS include avoiding substances that exacerbate symptoms, such as α-agonists (decongestants), caffeine, alcohol, spicy/acidic foods, chocolate, and soda. These substances are known to be bladder irritants. Common medications contributing to LUTS include antidepressants, decongestants, antihistamines, bronchodilators, anticholinergics, and sympathomimetics. To decrease nocturia, behavioral modifications include limiting evening fluid intake, timed diuretic administration for patients already on a diuretic, and elevating legs 1 hour before bedtime. Counseling obese patients to lose weight and increasing physical activity have been linked to reduced LUTS.9 Other behavioral techniques include double voiding: a technique where patients void normally then change positions and return to void to empty the bladder. Another technique is timed voiding: Many patients have impaired sensation when the bladder is full. These patients are encouraged to void at regular intervals.
Complementary and Alternative Medicine
Multiple nutraceutical compounds claim improved urinary health and symptom reduction. These compounds are marketed to patients with little regulation and oversight since supplements are not regulated or held to the same standard as prescription medications. The most popular nutraceutical for prostate health and LUTS is saw palmetto. Despite its common usage for the treatment of LUTS, little data support saw palmetto health claims. In 2012, a systematic review of 32 randomized trials including 5666 patients compared saw palmetto with a placebo. The study found no difference in urinary symptom scores, urinary flow, or prostate size.10,11 Other phytotherapy compounds often considered for urinary symptoms include stinging nettle extract and β-sitosterol compounds. The mechanism of action of these agents is unknown and efficacy data are lacking.
Historically, acupuncture and pelvic floor physical therapy have been used successfully for OAB symptoms. A meta-analysis found positive beneficial effects of acupuncture compared with a sham control for short- and medium-term follow-up in both IPSS and urine flow rates in some studies; however, when combining the studies for more statistical power, the benefits were less clear.12 Physical therapists with specialized training and certification in pelvic health can incorporate certain bladder training techniques. These include voiding positional changes (double voiding and postvoid urethral milking) and timed voiding.13,14 These interventions often address etiologies of LUTS for which medical therapies are not effective as the sole treatment option.
Medication Management
Medical management includes α-blockers, 5-α-reductase inhibitors (5-α-RIs), antimuscarinic or anticholinergic medicines, β-3 agonists, and phosphodiesterase inhibitors (Table). These medications work independently as well as synergistically. The use of medications to improve symptoms must be balanced against potential AEs and the consequences of a lifetime of drug usage, which can be additive.15,16
First-line pharmacological therapy for BPH is α-blockers, which work by blocking α1A receptors in the prostate and bladder neck, leading to smooth muscle relaxation, increased diameter of the channel, and improved urinary flow. α-receptors in the bladder neck and prostate are expressed with increased frequency with age and are a potential cause for worsening symptoms as men age. Studies demonstrate that these medications reduce symptoms by 30 to 40% and increase flow rates by 16 to 25%.17 Commonly prescribed α-blockers include tamsulosin, alfuzosin, silodosin, doxazosin, and terazosin. Doxazosin and terazosin require dose titrations because they may cause significant hypotension. Orthostatic hypotension typically improves with time and is avoided if the patient takes the medication at bedtime. Both doxazosin and terazosin are on the American Geriatric Society’s Beers Criteria list and should be avoided in older patients.18 Tamsulosin, alfuzosin, and silodosin have a standardized dosing regimen and lower rates of hypotension. Significant AEs include ejaculation dysfunction, nasal congestion, and orthostatic hypotension. Duan and colleagues have linked tamsulosin with dementia. However, this association is not causal and further studies are necessary.19,20 Patients who have taken these agents also are at risk for intraoperative floppy iris syndrome (IFIS). Permanent visual problems can arise if the intraoperative management is not managed to account for IFIS. These medications have a rapid onset of action and work immediately. However, to reach maximum benefit, patients must take the medication for several weeks. Unfortunately, up to one-third of patients will have no improvement with α-blocker therapy, and many patients will discontinue these medications because of significant AEs.6,21
5-α-RIs (finasteride and dutasteride) inhibit the conversion of testosterone to more potent dihydrotestosterone. They effectively reduce prostate volume by 25 to 30%.22 The results occur slowly and can take 6 to 12 months to reach the desired outcome. These medications are effective in men with larger prostates and not as effective in men with smaller prostates.23 These medications can improve urinary flow rates by about 10%, reduce IPSS scores by 20 to 30%, reduce the risk of urinary retention by 50%, and reduce the progression of BPH to the point where surgery is required by 50%.24 Furthermore, 5-α-RIs lower PSA by > 50% after 12 months of treatment.25
A baseline PSA should be established before administration and after 6 months of treatment. Any increase in the PSA even if the level is within normal limits should be evaluated for prostate cancer. Sarkar and colleagues recently published a study evaluating prostate cancer diagnosis in patients treated with 5-α-RI and found there was a delay in diagnosing prostate cancer in this population. Controversy also exists as to the potential of these medications increasing the risk for high-grade prostate cancer, which has led to a US Food and Drug Administration (FDA) warning. AEs include decreased libido (1.5%), ejaculatory dysfunction (3.4%), gynecomastia (1.3%), and/or ED (1.6%).26-28 A recent study evaluating 5-α-RIs demonstrated about a 2-fold increased risk of depression.29
There are well-established studies that note increased effectiveness when using combined α-blocker therapy with 5-α-RI medications. The Medical Therapy of Prostate Symptoms (MTOPS) and Combination Avodart and Tamsulosin (CombAT) trials showed that the combination of both medications was more effective in improving voiding symptoms and flow rates than either agent alone.15,16 Combination therapy resulted in a 66% reduction in disease progression, 81% reduction in urinary retention, and a 67% reduction in the need for surgery compared with placebo.
Anticholinergic medication use in BPH with LUTS is well established, and their use is often combined with other therapies. Anticholinergics work by inhibiting muscarinic M3 receptors to reduce detrusor muscle contraction. This effectively decreases bladder contractions and delays the desire to void. Kaplan and colleagues showed that tolterodine significantly improved a patient’s QOL when added to α-blocker therapy.30 Patients reported a positive outcome at 12 weeks, which resulted in a reduction in urgency incontinence, urgency, nocturia, and the overall number of voiding episodes within 24 hours.
β-3 agonists are a class of medications for OAB; mirabegron and vibegron have proven effective in reducing similar symptoms. In phase 3 clinical trials, mirabegron improved urinary incontinence episodes by 50% and reduced the number of voids in 24 hours.31 Mirabegron is well tolerated and avoids many common anticholinergic effects.32 Vibegron is the newest medication in the class and could soon become the preferred agent given it does not have cytochrome P450 interactions and does not cause hypertension like mirabegron.33
Anticholinergics should be used with caution in patients with a history of urinary retention, elevated after-void residual, or other medications with known anticholinergic effects. AEs include sedation, confusion, dry mouth, constipation, and potential falls in older patients.18 Recent studies have noted an association with dementia in the prolonged use of these medications in older patients and should be used cautiously.20
Phosphodiesterase-5 enzyme inhibitors (PDE-5) are adjunctive medications shown to improve LUTS. This class of medication is prescribed mostly for ED. However, tadalafil 5 mg taken daily also is FDA approved for the treatment of LUTS secondary to BPH given its prolonged half-life. The exact mechanism for improved BPH symptoms is unknown. Possibly the effects are due to an increase mediated by PDE-5 in cyclic guanosine monophosphate (cGMP), which increases smooth muscle relaxation and tissue perfusion of the prostate and bladder.34 There have been limited studies on objective improvement in uroflowmetry parameters compared with other treatments. The daily dosing of tadalafil should not be prescribed in men with a creatinine clearance < 30 mL/min.29 Tadalafil is not considered a first-line agent and is usually reserved for patients who experience ED in addition to BPH. When initiating BPH pharmacologic therapy, the PCP should be aware of adherence and high discontinuation rates.35
Surgical Treatments
Surgical treatments are often delayed out of fear of potential AEs or considered a last resort when symptoms are too severe.36 Early intervention is required to prevent irreversible deleterious changes to detrusor muscle structure and function (Figure). Patients fear urinary incontinence, ED or ejaculatory dysfunction, and anesthesia complications associated with surgical interventions.6,37 Multiple studies show that patients fare better with early surgical intervention, experiencing improved IPSS scores, urinary flow, and QOL. The following is an overview of the most popular procedures.
Prostatic urethral lift (PUL) using the UroLift System is an FDA-approved, minimally-invasive treatment of LUTS secondary to BPH. This procedure treats prostates < 80 g with an absent median lobe.6,21,38 Permanent implants are placed per the prostatic urethra to displace obstructing prostate tissue laterally. This opens the urethra directly without cutting, heating, or removing any prostate tissue. This procedure is minimally invasive, often done in the office as an outpatient procedure, and offers better symptom relief than medication with a lower risk profile than transurethral resection of the prostate (TURP).39,40 The LIFT study was a multicenter, randomized, blinded trial; patients were randomized 2:1 to undergo UroLift or a sham operation. At 3 years, average improvements were statistically significant for total IPSS reduction (41%), QOL improvement (49%), and improved maximum flow rates by (51%).41 Risk for urinary incontinence is low, and the procedure has been shown to preserve erectile and ejaculatory function. Furthermore, patients report significant improvement in their QOL without the need for medications. Surgical retreatment rates at 5 years are 13.6%, with an additional 10.7% of subjects back on medication therapy with α-blockers or 5-α-RIs.42
Water vapor thermal therapy or Rez¯um uses steam as thermal energy to destroy obstructing prostate tissue and relieve the obstruction.43 The procedure differs from older conductive heat thermotherapies because the steam penetrates prostate zonal anatomy without affecting areas outside the targeted treatment zone. The procedure is done in the office with local anesthesia and provides long-lasting relief of LUTS with minimal risks. Following the procedure, patients require an indwelling urethral catheter for 3 to 7 days, and most patients begin to experience symptom improvement 2 to 4 weeks following the procedure.44 The procedure received FDA approval in 2015. Four-year data show significant improvement in maximal flow rate (50%), IPSS (47%), and QOL (43%).45 Surgical retreatment rates were 4.4%. Criticisms of this treatment include patient discomfort with the office procedure, the requirement for an indwelling catheter for a short period, and lack of long-term outcomes data. Guidelines support use in prostate volumes > 80 g with or without median lobe anatomy.
TURP is the gold standard to which other treatments are compared.46 The surgery is performed in the operating room where urologists use a rigid cystoscope and resection element to effectively carve out and cauterize obstructing prostate tissue. Patients typically recover for a short period with an indwelling urethral catheter that is often removed 12 to 24 hours after surgery. New research points out that despite increasing mean age (55% of patients are aged > 70 years with associated comorbidities), the morbidity of TURP was < 1% and mortality rate of 0 to 0.3%.47 Postoperative complications include bleeding that requires a transfusion (3%), retrograde ejaculation (65%), and rare urinary incontinence (2%).47 Surgical retreatment rates for patients following a TURP are approximately 13 to 15% at 8 years.34
Laser surgery for BPH includes multiple techniques: photovaporization of the prostate using a Greenlight XPS laser, holmium laser ablation, and holmium laser enucleation (HoLEP). Proponents of these treatments cite lower bleeding risks compared with TURP, but the operation is largely surgeon dependent on the technology chosen. Most studies comparing these technologies with TURP show similar outcomes of IPSS reports, quality of life improvements, and complications.
Patients with extremely large prostates, > 100 g or 4 times the normal size, pose a unique challenge to surgical treatment. Historically, patients were treated with an open simple prostatectomy operation or staged TURP procedures. Today, urologists use newer, safer ways to treat these patients. Both HoLEP and robot-assisted simple prostatectomy work well in relieving urinary symptoms with lower complications compared with older open surgery. Other minimally invasive procedures, such as prostatic artery embolism, have been described for the treatment of BPH specifically in men who may be unfit for surgery.48Future treatments are constantly evolving. Many unanswered questions remain about BPH and the role of inflammation, metabolic dysfunction, obesity, and other genetic factors driving BPH and prostate growth. Pharmaceutical opportunities exist in mechanisms aimed to reduce prostate growth, induce cellular apoptosis, as well as other drugs to reduce bladder symptoms. Newer, minimally invasive therapies also will become more readily available, such as Aquablation, which is the first FDA-granted surgical robot for the autonomous removal of prostatic tissue due to BPH.49 However, the goal of all future therapies should include the balance of alleviating disruptive symptoms while demonstrating a favorable risk profile. Many men discontinue taking medications, yet few present for surgery. Most concerning is the significant population of men who will develop irreversible bladder dysfunction while waiting for the perfect treatment. There are many opportunities for an effective treatment that is less invasive than surgery, provides durable relief, has minimal AEs, and is affordable.
Conclusions
There is no perfect treatment for patients with LUTS. All interventions have potential AEs and associated complications. Medications are often started as first-line therapy but are often discontinued at the onset of significant AEs. This process is often repeated. Many patients will try different medications without any significant improvement in their symptoms or short-term relief, which results in the gradual progression of the disease.
The PCP plays a significant role in the initial evaluation and management of BPH. These frontline clinicians can recognize patients who may already be experiencing sequela of prolonged bladder outlet obstruction and refer these men to urologists promptly. Counseling patients about their treatment options is an important duty for all PCPs.
A clear understanding of the available treatment options will help PCPs counsel patients appropriately about lifestyle modification, medications, and surgical treatment options for their symptoms. The treatment of this disorder is a rapidly evolving topic with the constant introduction of new technologies and medications, which are certain to continue to play an important role for PCPs and urologists.
Lower urinary tract symptoms (LUTS)are common and tend to increase in frequency with age. Managing LUTS can be complicated, requires an informed discussion between the primary care practitioner (PCP) and patient, and is best achieved by a thorough understanding of the many medical and surgical options available. Over the past 3 decades, medications have become the most common therapy; but recently, newer minimally invasive surgeries have challenged this paradigm. This article provides a comprehensive review for PCPs regarding the evaluation and management of LUTS in men and when to consider a urology referral.
Benign prostatic hyperplasia (BPH) and LUTS are common clinical encounters for most PCPs. About 50% of men will develop LUTS associated with BPH, and symptoms associated with these conditions increase as men age.1,2 Studies have estimated that 90% of men aged 45 to 80 years demonstrate some symptoms of LUTS.3 Strong genetic influence seems to suggest heritability, but BPH also occurs in sporadic forms and is heavily influenced by androgens.4
BPH is a histologic diagnosis, whereas LUTS consists of complex symptomatology related to both static or dynamic components.1 The enlarged prostate gland obstructs the urethra, simultaneously causing an increase in muscle tone and resistance at the bladder neck and prostatic urethra, leading to increased resistance to urine flow. As a result, there is a thickening of the detrusor muscles in the bladder wall and an overall decreased compliance. Urine becomes stored under increased pressure. These changes result in a weak or intermittent urine stream, incomplete emptying of the bladder, postvoid dribble, hesitancy, and irritative symptoms, such as urgency, frequency, and nocturia.
For many patients, BPH associated with LUTS is a quality of life (QOL) issue. The stigma associated with these symptoms often leads to delays in patients seeking care. Many patients do not seek treatment until symptoms have become so severe that changes in bladder health are often irreversible. Early intervention can dramatically improve a patient’s QOL. Also, early intervention has the potential to reduce overall health care expenditures. BPH-related spending exceeds $1 billion each year in the Medicare program alone.5
PCPs are in a unique position to help many patients who present with early-stage LUTS. Given the substantial impact this disease has on QOL, early recognition of symptoms and prompt treatment play a major role. Paramount to this effort is awareness and understanding of various treatments, their advantages, and adverse effects (AEs). This article highlights evidence-based evaluation and treatment of BPH/LUTS for PCPs who treat veterans and recommendations as to when to refer a patient to a urologist.
Evaluation of LUTS and BPH
Evaluation begins with a thorough medical history and physical examination. Particular attention should focus on ruling out other causes of LUTS, such as a urinary tract infection (UTI), acute prostatitis, malignancy, bladder dysfunction, neurogenic bladder, and other obstructive pathology, such as urethral stricture disease. The differential diagnosis of LUTS includes BPH, UTI, bladder neck obstruction, urethral stricture, bladder stones, polydipsia, overactive bladder (OAB), nocturnal polyuria, neurologic disease, genitourinary malignancy, renal failure, and acute/chronic urinary retention.6
Relevant medical history influencing urinary symptoms includes diabetes mellitus, underlying neurologic diseases, previous trauma, sexually transmitted infections, and certain medications. Symptom severity may be obtained using a validated questionnaire, such as the International Prostate Symptom Score (IPSS), which also aids clinicians in assessing the impact of LUTS on QOL. Additionally, urinary frequency or volume records (voiding diary) may help establish the severity of the patient’s symptoms and provide insight into other potential causes for LUTS. Patients with BPH often have concurrent erectile dysfunction (ED) or other sexual dysfunction symptoms. Patients should be evaluated for baseline sexual dysfunction before the initiation of treatment as many therapies worsen symptoms of ED or ejaculatory dysfunction.
A comprehensive physical examination with a focus on the genitourinary system should, at minimum, assess for abnormalities of the urethral meatus, prepuce, penis, groin nodes, and prior surgical scars. A digital rectal examination also should be performed. Although controversial, a digital rectal examination for prostate cancer screening may provide a rough estimate of prostate size, help rule out prostatitis, and detect incident prostate nodules. Prostate size does not necessarily correlate well with the degree of urinary obstruction or LUTS but is an important consideration when deciding among different therapies.1
Laboratory and Adjunctive Tests
A urinalysis with microscopy helps identify other potential causes for urinary symptoms, including infection, proteinuria, or glucosuria. In patients who present with gross or microscopic hematuria, additional consideration should be given to bladder calculi and genitourinary cancer.2 When a reversible source for the hematuria is not identified, these patients require referral to a urologist for a hematuria evaluation.
There is some controversy regarding prostate specific antigen (PSA) testing. Most professional organizations advocate for a shared decision-making approach before testing. The American Cancer Society recommends this informed discussion occur between the patient and the PCP for men aged > 50 years at average risk, men aged > 45 years at high risk of developing prostate cancer (African Americans or first-degree relative with early prostate cancer diagnosis), and aged 40 years for men with more than one first-degree relative with an early prostate cancer diagnosis.7
Adjunctive tests include postvoid residual (PVR), cystoscopy, uroflowmetry, urodynamics, and transrectal ultrasound. However, these are mostly performed by urologists. In some patients with bladder decompensation after prolonged partial bladder outlet obstruction, urodynamics may be used by urologists to determine whether a patient may benefit from an outlet obstruction procedure. Ordering additional imaging or serum studies for the assessment of LUTS is rarely helpful.
Treatment
Treatment includes management with or without lifestyle modification, medication administration, and surgical therapy. New to this paradigm are in-office minimally invasive surgical options. The goal of treatment is not only to reduce patient symptoms and improve QOL, but also to prevent the secondary sequala of urinary retention, bladder failure, and eventual renal impairment.7A basic understanding of these treatments can aid PCPs with appropriate patient counseling and urologic referral.8
Lifestyle and Behavior Modification
Behavior modification is the starting point for all patients with LUTS. Lifestyle modifications for LUTS include avoiding substances that exacerbate symptoms, such as α-agonists (decongestants), caffeine, alcohol, spicy/acidic foods, chocolate, and soda. These substances are known to be bladder irritants. Common medications contributing to LUTS include antidepressants, decongestants, antihistamines, bronchodilators, anticholinergics, and sympathomimetics. To decrease nocturia, behavioral modifications include limiting evening fluid intake, timed diuretic administration for patients already on a diuretic, and elevating legs 1 hour before bedtime. Counseling obese patients to lose weight and increasing physical activity have been linked to reduced LUTS.9 Other behavioral techniques include double voiding: a technique where patients void normally then change positions and return to void to empty the bladder. Another technique is timed voiding: Many patients have impaired sensation when the bladder is full. These patients are encouraged to void at regular intervals.
Complementary and Alternative Medicine
Multiple nutraceutical compounds claim improved urinary health and symptom reduction. These compounds are marketed to patients with little regulation and oversight since supplements are not regulated or held to the same standard as prescription medications. The most popular nutraceutical for prostate health and LUTS is saw palmetto. Despite its common usage for the treatment of LUTS, little data support saw palmetto health claims. In 2012, a systematic review of 32 randomized trials including 5666 patients compared saw palmetto with a placebo. The study found no difference in urinary symptom scores, urinary flow, or prostate size.10,11 Other phytotherapy compounds often considered for urinary symptoms include stinging nettle extract and β-sitosterol compounds. The mechanism of action of these agents is unknown and efficacy data are lacking.
Historically, acupuncture and pelvic floor physical therapy have been used successfully for OAB symptoms. A meta-analysis found positive beneficial effects of acupuncture compared with a sham control for short- and medium-term follow-up in both IPSS and urine flow rates in some studies; however, when combining the studies for more statistical power, the benefits were less clear.12 Physical therapists with specialized training and certification in pelvic health can incorporate certain bladder training techniques. These include voiding positional changes (double voiding and postvoid urethral milking) and timed voiding.13,14 These interventions often address etiologies of LUTS for which medical therapies are not effective as the sole treatment option.
Medication Management
Medical management includes α-blockers, 5-α-reductase inhibitors (5-α-RIs), antimuscarinic or anticholinergic medicines, β-3 agonists, and phosphodiesterase inhibitors (Table). These medications work independently as well as synergistically. The use of medications to improve symptoms must be balanced against potential AEs and the consequences of a lifetime of drug usage, which can be additive.15,16
First-line pharmacological therapy for BPH is α-blockers, which work by blocking α1A receptors in the prostate and bladder neck, leading to smooth muscle relaxation, increased diameter of the channel, and improved urinary flow. α-receptors in the bladder neck and prostate are expressed with increased frequency with age and are a potential cause for worsening symptoms as men age. Studies demonstrate that these medications reduce symptoms by 30 to 40% and increase flow rates by 16 to 25%.17 Commonly prescribed α-blockers include tamsulosin, alfuzosin, silodosin, doxazosin, and terazosin. Doxazosin and terazosin require dose titrations because they may cause significant hypotension. Orthostatic hypotension typically improves with time and is avoided if the patient takes the medication at bedtime. Both doxazosin and terazosin are on the American Geriatric Society’s Beers Criteria list and should be avoided in older patients.18 Tamsulosin, alfuzosin, and silodosin have a standardized dosing regimen and lower rates of hypotension. Significant AEs include ejaculation dysfunction, nasal congestion, and orthostatic hypotension. Duan and colleagues have linked tamsulosin with dementia. However, this association is not causal and further studies are necessary.19,20 Patients who have taken these agents also are at risk for intraoperative floppy iris syndrome (IFIS). Permanent visual problems can arise if the intraoperative management is not managed to account for IFIS. These medications have a rapid onset of action and work immediately. However, to reach maximum benefit, patients must take the medication for several weeks. Unfortunately, up to one-third of patients will have no improvement with α-blocker therapy, and many patients will discontinue these medications because of significant AEs.6,21
5-α-RIs (finasteride and dutasteride) inhibit the conversion of testosterone to more potent dihydrotestosterone. They effectively reduce prostate volume by 25 to 30%.22 The results occur slowly and can take 6 to 12 months to reach the desired outcome. These medications are effective in men with larger prostates and not as effective in men with smaller prostates.23 These medications can improve urinary flow rates by about 10%, reduce IPSS scores by 20 to 30%, reduce the risk of urinary retention by 50%, and reduce the progression of BPH to the point where surgery is required by 50%.24 Furthermore, 5-α-RIs lower PSA by > 50% after 12 months of treatment.25
A baseline PSA should be established before administration and after 6 months of treatment. Any increase in the PSA even if the level is within normal limits should be evaluated for prostate cancer. Sarkar and colleagues recently published a study evaluating prostate cancer diagnosis in patients treated with 5-α-RI and found there was a delay in diagnosing prostate cancer in this population. Controversy also exists as to the potential of these medications increasing the risk for high-grade prostate cancer, which has led to a US Food and Drug Administration (FDA) warning. AEs include decreased libido (1.5%), ejaculatory dysfunction (3.4%), gynecomastia (1.3%), and/or ED (1.6%).26-28 A recent study evaluating 5-α-RIs demonstrated about a 2-fold increased risk of depression.29
There are well-established studies that note increased effectiveness when using combined α-blocker therapy with 5-α-RI medications. The Medical Therapy of Prostate Symptoms (MTOPS) and Combination Avodart and Tamsulosin (CombAT) trials showed that the combination of both medications was more effective in improving voiding symptoms and flow rates than either agent alone.15,16 Combination therapy resulted in a 66% reduction in disease progression, 81% reduction in urinary retention, and a 67% reduction in the need for surgery compared with placebo.
Anticholinergic medication use in BPH with LUTS is well established, and their use is often combined with other therapies. Anticholinergics work by inhibiting muscarinic M3 receptors to reduce detrusor muscle contraction. This effectively decreases bladder contractions and delays the desire to void. Kaplan and colleagues showed that tolterodine significantly improved a patient’s QOL when added to α-blocker therapy.30 Patients reported a positive outcome at 12 weeks, which resulted in a reduction in urgency incontinence, urgency, nocturia, and the overall number of voiding episodes within 24 hours.
β-3 agonists are a class of medications for OAB; mirabegron and vibegron have proven effective in reducing similar symptoms. In phase 3 clinical trials, mirabegron improved urinary incontinence episodes by 50% and reduced the number of voids in 24 hours.31 Mirabegron is well tolerated and avoids many common anticholinergic effects.32 Vibegron is the newest medication in the class and could soon become the preferred agent given it does not have cytochrome P450 interactions and does not cause hypertension like mirabegron.33
Anticholinergics should be used with caution in patients with a history of urinary retention, elevated after-void residual, or other medications with known anticholinergic effects. AEs include sedation, confusion, dry mouth, constipation, and potential falls in older patients.18 Recent studies have noted an association with dementia in the prolonged use of these medications in older patients and should be used cautiously.20
Phosphodiesterase-5 enzyme inhibitors (PDE-5) are adjunctive medications shown to improve LUTS. This class of medication is prescribed mostly for ED. However, tadalafil 5 mg taken daily also is FDA approved for the treatment of LUTS secondary to BPH given its prolonged half-life. The exact mechanism for improved BPH symptoms is unknown. Possibly the effects are due to an increase mediated by PDE-5 in cyclic guanosine monophosphate (cGMP), which increases smooth muscle relaxation and tissue perfusion of the prostate and bladder.34 There have been limited studies on objective improvement in uroflowmetry parameters compared with other treatments. The daily dosing of tadalafil should not be prescribed in men with a creatinine clearance < 30 mL/min.29 Tadalafil is not considered a first-line agent and is usually reserved for patients who experience ED in addition to BPH. When initiating BPH pharmacologic therapy, the PCP should be aware of adherence and high discontinuation rates.35
Surgical Treatments
Surgical treatments are often delayed out of fear of potential AEs or considered a last resort when symptoms are too severe.36 Early intervention is required to prevent irreversible deleterious changes to detrusor muscle structure and function (Figure). Patients fear urinary incontinence, ED or ejaculatory dysfunction, and anesthesia complications associated with surgical interventions.6,37 Multiple studies show that patients fare better with early surgical intervention, experiencing improved IPSS scores, urinary flow, and QOL. The following is an overview of the most popular procedures.
Prostatic urethral lift (PUL) using the UroLift System is an FDA-approved, minimally-invasive treatment of LUTS secondary to BPH. This procedure treats prostates < 80 g with an absent median lobe.6,21,38 Permanent implants are placed per the prostatic urethra to displace obstructing prostate tissue laterally. This opens the urethra directly without cutting, heating, or removing any prostate tissue. This procedure is minimally invasive, often done in the office as an outpatient procedure, and offers better symptom relief than medication with a lower risk profile than transurethral resection of the prostate (TURP).39,40 The LIFT study was a multicenter, randomized, blinded trial; patients were randomized 2:1 to undergo UroLift or a sham operation. At 3 years, average improvements were statistically significant for total IPSS reduction (41%), QOL improvement (49%), and improved maximum flow rates by (51%).41 Risk for urinary incontinence is low, and the procedure has been shown to preserve erectile and ejaculatory function. Furthermore, patients report significant improvement in their QOL without the need for medications. Surgical retreatment rates at 5 years are 13.6%, with an additional 10.7% of subjects back on medication therapy with α-blockers or 5-α-RIs.42
Water vapor thermal therapy or Rez¯um uses steam as thermal energy to destroy obstructing prostate tissue and relieve the obstruction.43 The procedure differs from older conductive heat thermotherapies because the steam penetrates prostate zonal anatomy without affecting areas outside the targeted treatment zone. The procedure is done in the office with local anesthesia and provides long-lasting relief of LUTS with minimal risks. Following the procedure, patients require an indwelling urethral catheter for 3 to 7 days, and most patients begin to experience symptom improvement 2 to 4 weeks following the procedure.44 The procedure received FDA approval in 2015. Four-year data show significant improvement in maximal flow rate (50%), IPSS (47%), and QOL (43%).45 Surgical retreatment rates were 4.4%. Criticisms of this treatment include patient discomfort with the office procedure, the requirement for an indwelling catheter for a short period, and lack of long-term outcomes data. Guidelines support use in prostate volumes > 80 g with or without median lobe anatomy.
TURP is the gold standard to which other treatments are compared.46 The surgery is performed in the operating room where urologists use a rigid cystoscope and resection element to effectively carve out and cauterize obstructing prostate tissue. Patients typically recover for a short period with an indwelling urethral catheter that is often removed 12 to 24 hours after surgery. New research points out that despite increasing mean age (55% of patients are aged > 70 years with associated comorbidities), the morbidity of TURP was < 1% and mortality rate of 0 to 0.3%.47 Postoperative complications include bleeding that requires a transfusion (3%), retrograde ejaculation (65%), and rare urinary incontinence (2%).47 Surgical retreatment rates for patients following a TURP are approximately 13 to 15% at 8 years.34
Laser surgery for BPH includes multiple techniques: photovaporization of the prostate using a Greenlight XPS laser, holmium laser ablation, and holmium laser enucleation (HoLEP). Proponents of these treatments cite lower bleeding risks compared with TURP, but the operation is largely surgeon dependent on the technology chosen. Most studies comparing these technologies with TURP show similar outcomes of IPSS reports, quality of life improvements, and complications.
Patients with extremely large prostates, > 100 g or 4 times the normal size, pose a unique challenge to surgical treatment. Historically, patients were treated with an open simple prostatectomy operation or staged TURP procedures. Today, urologists use newer, safer ways to treat these patients. Both HoLEP and robot-assisted simple prostatectomy work well in relieving urinary symptoms with lower complications compared with older open surgery. Other minimally invasive procedures, such as prostatic artery embolism, have been described for the treatment of BPH specifically in men who may be unfit for surgery.48Future treatments are constantly evolving. Many unanswered questions remain about BPH and the role of inflammation, metabolic dysfunction, obesity, and other genetic factors driving BPH and prostate growth. Pharmaceutical opportunities exist in mechanisms aimed to reduce prostate growth, induce cellular apoptosis, as well as other drugs to reduce bladder symptoms. Newer, minimally invasive therapies also will become more readily available, such as Aquablation, which is the first FDA-granted surgical robot for the autonomous removal of prostatic tissue due to BPH.49 However, the goal of all future therapies should include the balance of alleviating disruptive symptoms while demonstrating a favorable risk profile. Many men discontinue taking medications, yet few present for surgery. Most concerning is the significant population of men who will develop irreversible bladder dysfunction while waiting for the perfect treatment. There are many opportunities for an effective treatment that is less invasive than surgery, provides durable relief, has minimal AEs, and is affordable.
Conclusions
There is no perfect treatment for patients with LUTS. All interventions have potential AEs and associated complications. Medications are often started as first-line therapy but are often discontinued at the onset of significant AEs. This process is often repeated. Many patients will try different medications without any significant improvement in their symptoms or short-term relief, which results in the gradual progression of the disease.
The PCP plays a significant role in the initial evaluation and management of BPH. These frontline clinicians can recognize patients who may already be experiencing sequela of prolonged bladder outlet obstruction and refer these men to urologists promptly. Counseling patients about their treatment options is an important duty for all PCPs.
A clear understanding of the available treatment options will help PCPs counsel patients appropriately about lifestyle modification, medications, and surgical treatment options for their symptoms. The treatment of this disorder is a rapidly evolving topic with the constant introduction of new technologies and medications, which are certain to continue to play an important role for PCPs and urologists.
1. Roehrborn CG. Benign prostatic hyperplasia: an overview. Rev Urol. 2005;7 Suppl 9(Suppl 9):S3-S14
2. McVary KT. Clinical manifestations and diagnostic evaluation of benign prostatic hyperplasia. UpToDate. Updated November 18, 2021. Accessed November 23, 2021. https:// www.uptodate.com/contents/clinical-manifestations-and -diagnostic-evaluation-of-benign-prostatic-hyperplasia
3. McVary KT. BPH: epidemiology and comorbidities. Am J Manag Care. 2006;12(5 Suppl):S122-S128.
4. Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol. 2011;8(1):29-41. doi:10.1038/nrurol.2010.207
5. Rensing AJ, Kuxhausen A, Vetter J, Strope SA. Differences in the treatment of benign prostatic hyperplasia: comparing the primary care physician and the urologist. Urol Pract. 2017;4(3):193-199. doi:10.1016/j.urpr.2016.07.002
6. Foster HE, Barry MJ, Dahm P, et al. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline. J Urol. 2018;200(3):612- 619. doi:10.1016/j.juro.2018.05.048
7. Landau A, Welliver C. Analyzing and characterizing why men seek care for lower urinary tract symptoms. Curr Urol Rep. 2020;21(12):58. Published 2020 Oct 30. doi:10.1007/s11934-020-01006-w
8. Das AK, Leong JY, Roehrborn CG. Office-based therapies for benign prostatic hyperplasia: a review and update. Can J Urol. 2019;26(4 Suppl 1):2-7.
9. Parsons JK, Sarma AV, McVary K, Wei JT. Obesity and benign prostatic hyperplasia: clinical connections, emerging etiological paradigms and future directions. J Urol. 2013;189(1 Suppl):S102-S106. doi:10.1016/j.juro.2012.11.029
10. Pattanaik S, Mavuduru RS, Panda A, et al. Phosphodiesterase inhibitors for lower urinary tract symptoms consistent with benign prostatic hyperplasia. Cochrane Database Syst Rev. 2018;11(11):CD010060. Published 2018 Nov 16. doi:10.1002/14651858.CD010060.pub2
11. McVary KT. Medical treatment of benign prostatic hyperplasia. UpToDate. Updated October 4, 2021. Accessed November 23, 2021. https://www.uptodate.com/contents /medical-treatment-of-benign-prostatic-hyperplasia
12. Zhang W, Ma L, Bauer BA, Liu Z, Lu Y. Acupuncture for benign prostatic hyperplasia: A systematic review and metaanalysis. PLoS One. 2017;12(4):e0174586. Published 2017 Apr 4. doi:10.1371/journal.pone.0174586
13. Newman DK, Guzzo T, Lee D, Jayadevappa R. An evidence- based strategy for the conservative management of the male patient with incontinence. Curr Opin Urol. 2014;24(6):553-559. doi:10.1097/MOU.0000000000000115
14. Newman DK, Wein AJ. Office-based behavioral therapy for management of incontinence and other pelvic disorders. Urol Clin North Am. 2013;40(4):613-635. doi:10.1016/j.ucl.2013.07.010
15. McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349(25):2387-2398. doi:10.1056/NEJMoa030656
16. Roehrborn CG, Barkin J, Siami P, et al. Clinical outcomes after combined therapy with dutasteride plus tamsulosin or either monotherapy in men with benign prostatic hyperplasia (BPH) by baseline characteristics: 4-year results from the randomized, double-blind Combination of Avodart and Tamsulosin (CombAT) trial. BJU Int. 2011;107(6):946-954. doi:10.1111/j.1464-410X.2011.10124.x
17. Djavan B, Marberger M. A meta-analysis on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol. 1999;36(1):1-13. doi:10.1159/000019919
18. By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. doi:10.1111/jgs.13702
19. Duan Y, Grady JJ, Albertsen PC, Helen Wu Z. Tamsulosin and the risk of dementia in older men with benign prostatic hyperplasia. Pharmacoepidemiol Drug Saf. 2018;27(3):340- 348. doi:10.1002/pds.4361
20. Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic drug exposure and the risk of dementia: a nested case-control study. JAMA Intern Med. 2019;179(8):1084-1093. doi:10.1001/jamainternmed.2019.0677
21. Parsons JK, Dahm P, Köhler TS, Lerner LB, Wilt TJ. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline amendment 2020. J Urol. 2020;204(4):799-804. doi:10.1097/JU.0000000000001298
22. Smith AB, Carson CC. Finasteride in the treatment of patients with benign prostatic hyperplasia: a review. Ther Clin Risk Manag. 2009;5(3):535-545. doi:10.2147/tcrm.s6195
23. Andriole GL, Guess HA, Epstein JI, et al. Treatment with finasteride preserves usefulness of prostate-specific antigen in the detection of prostate cancer: results of a randomized, double-blind, placebo-controlled clinical trial. PLESS Study Group. Proscar Long-term Efficacy and Safety Study. Urology. 1998;52(2):195-202. doi:10.1016/s0090-4295(98)00184-8
24. McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med. 1998;338(9):557-563. doi:10.1056/NEJM199802263380901
25. Rittmaster RS. 5alpha-reductase inhibitors in benign prostatic hyperplasia and prostate cancer risk reduction. Best Pract Res Clin Endocrinol Metab. 2008;22(2):389-402. doi:10.1016/j.beem.2008.01.016
26. La Torre A, Giupponi G, Duffy D, Conca A, Cai T, Scardigli A. Sexual dysfunction related to drugs: a critical review. Part V: α-blocker and 5-ARI drugs. Pharmacopsychiatry. 2016;49(1):3-13. doi:10.1055/s-0035-1565100
27. Corona G, Tirabassi G, Santi D, et al. Sexual dysfunction in subjects treated with inhibitors of 5α-reductase for benign prostatic hyperplasia: a comprehensive review and meta-analysis. Andrology. 2017;5(4):671-678. doi:10.1111/andr.12353
28. Trost L, Saitz TR, Hellstrom WJ. Side effects of 5-alpha reductase inhibitors: a comprehensive review. Sex Med Rev. 2013;1(1):24-41. doi:10.1002/smrj.3
29. Welk B, McArthur E, Ordon M, Anderson KK, Hayward J, Dixon S. Association of suicidality and depression with 5α-reductase inhibitors. JAMA Intern Med. 2017;177(5):683-691. doi:10.1001/jamainternmed.2017.0089
30. Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial [published correction appears in JAMA. 2007 Mar 21:297(11):1195] [published correction appears in JAMA. 2007 Oct 24;298(16):1864]. JAMA. 2006;296(19):2319-2328. doi:10.1001/jama.296.19.2319
31. Nitti VW, Auerbach S, Martin N, Calhoun A, Lee M, Herschorn S. Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol. 2013;189(4):1388-1395. doi:10.1016/j.juro.2012.10.017
32. Chapple CR, Cardozo L, Nitti VW, Siddiqui E, Michel MC. Mirabegron in overactive bladder: a review of efficacy, safety, and tolerability. Neurourol Urodyn. 2014;33(1):17-30. doi:10.1002/nau.22505
33. Rutman MP, King JR, Bennett N, Ankrom W, Mudd PN. PD14-01 once-daily vibegron, a novel oral β3 agonist does not inhibit CYP2D6, a common pathway for drug metabolism in patients on OAB medications. J Urol. 2019;201(Suppl 4):e231. doi:10.1097/01.JU.0000555478.73162.19
34. Bo K, Frawley HC, Haylen BT, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Neurourol Urodyn. 2017;36(2):221- 244. doi:10.1002/nau.23107
35. Cindolo L, Pirozzi L, Fanizza C, et al. Drug adherence and clinical outcomes for patients under pharmacological therapy for lower urinary tract symptoms related to benign prostatic hyperplasia: population-based cohort study. Eur Urol. 2015;68(3):418-425. doi:10.1016/j.eururo.2014.11.006
36. Ruhaiyem ME, Alshehri AA, Saade M, Shoabi TA, Zahoor H, Tawfeeq NA. Fear of going under general anesthesia: a cross-sectional study. Saudi J Anaesth. 2016;10(3):317- 321. doi:10.4103/1658-354X.179094
37. Hashim MJ. Patient-centered communication: basic skills. Am Fam Physician. 2017;95(1):29-34.
38. Roehrborn CG, Barkin J, Gange SN, et al. Five year results of the prospective randomized controlled prostatic urethral L.I.F.T. study. Can J Urol. 2017;24(3):8802-8813.
39. Gratzke C, Barber N, Speakman MJ, et al. Prostatic urethral lift vs transurethral resection of the prostate: 2-year results of the BPH6 prospective, multicentre, randomized study. BJU Int. 2017;119(5):767-775.doi:10.1111/bju.13714
40. Sønksen J, Barber NJ, Speakman MJ, et al. Prospective, randomized, multinational study of prostatic urethral lift versus transurethral resection of the prostate: 12-month results from the BPH6 study. Eur Urol. 2015;68(4):643-652. doi:10.1016/j.eururo.2015.04.024
41. Roehrborn CG, Gange SN, Shore ND, et al. The prostatic urethral lift for the treatment of lower urinary tract symptoms associated with prostate enlargement due to benign prostatic hyperplasia: the L.I.F.T. Study. J Urol. 2013;190(6):2161-2167. doi:10.1016/j.juro.2013.05.116
42. McNicholas TA. Benign prostatic hyperplasia and new treatment options - a critical appraisal of the UroLift system. Med Devices (Auckl). 2016;9:115-123. Published 2016 May 19. doi:10.2147/MDER.S60780
43. McVary KT, Rogers T, Roehrborn CG. Rezuˉm Water Vapor thermal therapy for lower urinary tract symptoms associated with benign prostatic hyperplasia: 4-year results from randomized controlled study. Urology. 2019;126:171-179. doi:10.1016/j.urology.2018.12.041
44. Bole R, Gopalakrishna A, Kuang R, et al. Comparative postoperative outcomes of Rezˉum prostate ablation in patients with large versus small glands. J Endourol. 2020;34(7):778-781. doi:10.1089/end.2020.0177
45. Darson MF, Alexander EE, Schiffman ZJ, et al. Procedural techniques and multicenter postmarket experience using minimally invasive convective radiofrequency thermal therapy with Rezˉum system for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Res Rep Urol. 2017;9:159-168. Published 2017 Aug 21. doi:10.2147/RRU.S143679
46. Baazeem A, Elhilali MM. Surgical management of benign prostatic hyperplasia: current evidence. Nat Clin Pract Urol. 2008;5(10):540-549. doi:10.1038/ncpuro1214
47. Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP)- -incidence, management, and prevention. Eur Urol. 2006;50(5):969-980. doi:10.1016/j.eururo.2005.12.042
48. Abt D, Schmid HP, Speakman MJ. Reasons to consider prostatic artery embolization. World J Urol. 2021;39(7):2301-2306. doi:10.1007/s00345-021-03601-z
49. Nguyen DD, Barber N, Bidair M, et al. Waterjet Ablation Therapy for Endoscopic Resection of prostate tissue trial (WATER) vs WATER II: comparing Aquablation therapy for benign prostatic hyperplasia in30-80and80-150mLprostates. BJUInt. 2020;125(1):112-122. doi:10.1111/bju.14917.
1. Roehrborn CG. Benign prostatic hyperplasia: an overview. Rev Urol. 2005;7 Suppl 9(Suppl 9):S3-S14
2. McVary KT. Clinical manifestations and diagnostic evaluation of benign prostatic hyperplasia. UpToDate. Updated November 18, 2021. Accessed November 23, 2021. https:// www.uptodate.com/contents/clinical-manifestations-and -diagnostic-evaluation-of-benign-prostatic-hyperplasia
3. McVary KT. BPH: epidemiology and comorbidities. Am J Manag Care. 2006;12(5 Suppl):S122-S128.
4. Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol. 2011;8(1):29-41. doi:10.1038/nrurol.2010.207
5. Rensing AJ, Kuxhausen A, Vetter J, Strope SA. Differences in the treatment of benign prostatic hyperplasia: comparing the primary care physician and the urologist. Urol Pract. 2017;4(3):193-199. doi:10.1016/j.urpr.2016.07.002
6. Foster HE, Barry MJ, Dahm P, et al. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline. J Urol. 2018;200(3):612- 619. doi:10.1016/j.juro.2018.05.048
7. Landau A, Welliver C. Analyzing and characterizing why men seek care for lower urinary tract symptoms. Curr Urol Rep. 2020;21(12):58. Published 2020 Oct 30. doi:10.1007/s11934-020-01006-w
8. Das AK, Leong JY, Roehrborn CG. Office-based therapies for benign prostatic hyperplasia: a review and update. Can J Urol. 2019;26(4 Suppl 1):2-7.
9. Parsons JK, Sarma AV, McVary K, Wei JT. Obesity and benign prostatic hyperplasia: clinical connections, emerging etiological paradigms and future directions. J Urol. 2013;189(1 Suppl):S102-S106. doi:10.1016/j.juro.2012.11.029
10. Pattanaik S, Mavuduru RS, Panda A, et al. Phosphodiesterase inhibitors for lower urinary tract symptoms consistent with benign prostatic hyperplasia. Cochrane Database Syst Rev. 2018;11(11):CD010060. Published 2018 Nov 16. doi:10.1002/14651858.CD010060.pub2
11. McVary KT. Medical treatment of benign prostatic hyperplasia. UpToDate. Updated October 4, 2021. Accessed November 23, 2021. https://www.uptodate.com/contents /medical-treatment-of-benign-prostatic-hyperplasia
12. Zhang W, Ma L, Bauer BA, Liu Z, Lu Y. Acupuncture for benign prostatic hyperplasia: A systematic review and metaanalysis. PLoS One. 2017;12(4):e0174586. Published 2017 Apr 4. doi:10.1371/journal.pone.0174586
13. Newman DK, Guzzo T, Lee D, Jayadevappa R. An evidence- based strategy for the conservative management of the male patient with incontinence. Curr Opin Urol. 2014;24(6):553-559. doi:10.1097/MOU.0000000000000115
14. Newman DK, Wein AJ. Office-based behavioral therapy for management of incontinence and other pelvic disorders. Urol Clin North Am. 2013;40(4):613-635. doi:10.1016/j.ucl.2013.07.010
15. McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349(25):2387-2398. doi:10.1056/NEJMoa030656
16. Roehrborn CG, Barkin J, Siami P, et al. Clinical outcomes after combined therapy with dutasteride plus tamsulosin or either monotherapy in men with benign prostatic hyperplasia (BPH) by baseline characteristics: 4-year results from the randomized, double-blind Combination of Avodart and Tamsulosin (CombAT) trial. BJU Int. 2011;107(6):946-954. doi:10.1111/j.1464-410X.2011.10124.x
17. Djavan B, Marberger M. A meta-analysis on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol. 1999;36(1):1-13. doi:10.1159/000019919
18. By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. doi:10.1111/jgs.13702
19. Duan Y, Grady JJ, Albertsen PC, Helen Wu Z. Tamsulosin and the risk of dementia in older men with benign prostatic hyperplasia. Pharmacoepidemiol Drug Saf. 2018;27(3):340- 348. doi:10.1002/pds.4361
20. Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic drug exposure and the risk of dementia: a nested case-control study. JAMA Intern Med. 2019;179(8):1084-1093. doi:10.1001/jamainternmed.2019.0677
21. Parsons JK, Dahm P, Köhler TS, Lerner LB, Wilt TJ. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline amendment 2020. J Urol. 2020;204(4):799-804. doi:10.1097/JU.0000000000001298
22. Smith AB, Carson CC. Finasteride in the treatment of patients with benign prostatic hyperplasia: a review. Ther Clin Risk Manag. 2009;5(3):535-545. doi:10.2147/tcrm.s6195
23. Andriole GL, Guess HA, Epstein JI, et al. Treatment with finasteride preserves usefulness of prostate-specific antigen in the detection of prostate cancer: results of a randomized, double-blind, placebo-controlled clinical trial. PLESS Study Group. Proscar Long-term Efficacy and Safety Study. Urology. 1998;52(2):195-202. doi:10.1016/s0090-4295(98)00184-8
24. McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med. 1998;338(9):557-563. doi:10.1056/NEJM199802263380901
25. Rittmaster RS. 5alpha-reductase inhibitors in benign prostatic hyperplasia and prostate cancer risk reduction. Best Pract Res Clin Endocrinol Metab. 2008;22(2):389-402. doi:10.1016/j.beem.2008.01.016
26. La Torre A, Giupponi G, Duffy D, Conca A, Cai T, Scardigli A. Sexual dysfunction related to drugs: a critical review. Part V: α-blocker and 5-ARI drugs. Pharmacopsychiatry. 2016;49(1):3-13. doi:10.1055/s-0035-1565100
27. Corona G, Tirabassi G, Santi D, et al. Sexual dysfunction in subjects treated with inhibitors of 5α-reductase for benign prostatic hyperplasia: a comprehensive review and meta-analysis. Andrology. 2017;5(4):671-678. doi:10.1111/andr.12353
28. Trost L, Saitz TR, Hellstrom WJ. Side effects of 5-alpha reductase inhibitors: a comprehensive review. Sex Med Rev. 2013;1(1):24-41. doi:10.1002/smrj.3
29. Welk B, McArthur E, Ordon M, Anderson KK, Hayward J, Dixon S. Association of suicidality and depression with 5α-reductase inhibitors. JAMA Intern Med. 2017;177(5):683-691. doi:10.1001/jamainternmed.2017.0089
30. Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial [published correction appears in JAMA. 2007 Mar 21:297(11):1195] [published correction appears in JAMA. 2007 Oct 24;298(16):1864]. JAMA. 2006;296(19):2319-2328. doi:10.1001/jama.296.19.2319
31. Nitti VW, Auerbach S, Martin N, Calhoun A, Lee M, Herschorn S. Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol. 2013;189(4):1388-1395. doi:10.1016/j.juro.2012.10.017
32. Chapple CR, Cardozo L, Nitti VW, Siddiqui E, Michel MC. Mirabegron in overactive bladder: a review of efficacy, safety, and tolerability. Neurourol Urodyn. 2014;33(1):17-30. doi:10.1002/nau.22505
33. Rutman MP, King JR, Bennett N, Ankrom W, Mudd PN. PD14-01 once-daily vibegron, a novel oral β3 agonist does not inhibit CYP2D6, a common pathway for drug metabolism in patients on OAB medications. J Urol. 2019;201(Suppl 4):e231. doi:10.1097/01.JU.0000555478.73162.19
34. Bo K, Frawley HC, Haylen BT, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Neurourol Urodyn. 2017;36(2):221- 244. doi:10.1002/nau.23107
35. Cindolo L, Pirozzi L, Fanizza C, et al. Drug adherence and clinical outcomes for patients under pharmacological therapy for lower urinary tract symptoms related to benign prostatic hyperplasia: population-based cohort study. Eur Urol. 2015;68(3):418-425. doi:10.1016/j.eururo.2014.11.006
36. Ruhaiyem ME, Alshehri AA, Saade M, Shoabi TA, Zahoor H, Tawfeeq NA. Fear of going under general anesthesia: a cross-sectional study. Saudi J Anaesth. 2016;10(3):317- 321. doi:10.4103/1658-354X.179094
37. Hashim MJ. Patient-centered communication: basic skills. Am Fam Physician. 2017;95(1):29-34.
38. Roehrborn CG, Barkin J, Gange SN, et al. Five year results of the prospective randomized controlled prostatic urethral L.I.F.T. study. Can J Urol. 2017;24(3):8802-8813.
39. Gratzke C, Barber N, Speakman MJ, et al. Prostatic urethral lift vs transurethral resection of the prostate: 2-year results of the BPH6 prospective, multicentre, randomized study. BJU Int. 2017;119(5):767-775.doi:10.1111/bju.13714
40. Sønksen J, Barber NJ, Speakman MJ, et al. Prospective, randomized, multinational study of prostatic urethral lift versus transurethral resection of the prostate: 12-month results from the BPH6 study. Eur Urol. 2015;68(4):643-652. doi:10.1016/j.eururo.2015.04.024
41. Roehrborn CG, Gange SN, Shore ND, et al. The prostatic urethral lift for the treatment of lower urinary tract symptoms associated with prostate enlargement due to benign prostatic hyperplasia: the L.I.F.T. Study. J Urol. 2013;190(6):2161-2167. doi:10.1016/j.juro.2013.05.116
42. McNicholas TA. Benign prostatic hyperplasia and new treatment options - a critical appraisal of the UroLift system. Med Devices (Auckl). 2016;9:115-123. Published 2016 May 19. doi:10.2147/MDER.S60780
43. McVary KT, Rogers T, Roehrborn CG. Rezuˉm Water Vapor thermal therapy for lower urinary tract symptoms associated with benign prostatic hyperplasia: 4-year results from randomized controlled study. Urology. 2019;126:171-179. doi:10.1016/j.urology.2018.12.041
44. Bole R, Gopalakrishna A, Kuang R, et al. Comparative postoperative outcomes of Rezˉum prostate ablation in patients with large versus small glands. J Endourol. 2020;34(7):778-781. doi:10.1089/end.2020.0177
45. Darson MF, Alexander EE, Schiffman ZJ, et al. Procedural techniques and multicenter postmarket experience using minimally invasive convective radiofrequency thermal therapy with Rezˉum system for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Res Rep Urol. 2017;9:159-168. Published 2017 Aug 21. doi:10.2147/RRU.S143679
46. Baazeem A, Elhilali MM. Surgical management of benign prostatic hyperplasia: current evidence. Nat Clin Pract Urol. 2008;5(10):540-549. doi:10.1038/ncpuro1214
47. Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP)- -incidence, management, and prevention. Eur Urol. 2006;50(5):969-980. doi:10.1016/j.eururo.2005.12.042
48. Abt D, Schmid HP, Speakman MJ. Reasons to consider prostatic artery embolization. World J Urol. 2021;39(7):2301-2306. doi:10.1007/s00345-021-03601-z
49. Nguyen DD, Barber N, Bidair M, et al. Waterjet Ablation Therapy for Endoscopic Resection of prostate tissue trial (WATER) vs WATER II: comparing Aquablation therapy for benign prostatic hyperplasia in30-80and80-150mLprostates. BJUInt. 2020;125(1):112-122. doi:10.1111/bju.14917.
Write an exercise Rx to improve patients' cardiorespiratory fitness
It is well-known that per capita health care spending in the United States is more than twice the average in other developed countries1; nevertheless, the overall health care ranking of the US is near the bottom compared to other countries in this group.2 Much of the reason for this poor relative showing lies in the fact that the US has employed a somewhat traditional fee-for-service health care model that does not incentivize efforts to promote health and wellness or prevent chronic disease. The paradigm of promoting physical activity for its disease-preventing and treatment benefits has not been well-integrated in the US health care system.
In this article, we endeavor to provide better understanding of the barriers that keep family physicians from routinely promoting physical activity in clinical practice; define tools and resources that can be used in the clinical setting to promote physical activity; and delineate areas for future work.
Glaring hole in US physical activity education
Many primary care physicians feel underprepared to prescribe or motivate patients to exercise. The reason for that lack of preparedness likely relates to a medical education system that does not spend time preparing physicians to perform this critical task. A study showed that, on average, medical schools require only 8 hours of physical activity education in their curriculum during the 4 years of schooling.3 Likewise, the average primary care residency program offers only 3 hours of didactic training on physical activity, nutrition, and obesity.4 The problem extends to sports medicine fellowship training, in which a 2019 survey showed that 63% of fellows were never taught how to write an exercise prescription in their training program.5
Without education on physical activity, medical students, residents, and fellows are woefully underprepared to realize the therapeutic value of physical activity in patient care, comprehend current physical activity guidelines, appropriately motivate patients to engage in exercise, and competently discuss exercise prescriptions in different disease states. Throughout their training, it is imperative for medical professionals to be educated on the social determinants of health, which include the conditions in which people live, work, and play. These environmental variables can contribute to health inequities that create additional barriers to improvement in physical fitness.6
National guidelines on physical activity
The 2018 National Physical Activity Guidelines detail recommendations for children, adolescents, adults, and special populations.7 The guidelines define physical activity as bodily movement produced by skeletal muscles that result in energy expenditure above resting baseline levels, and includes all types, intensities, and domains of activity. Exercise is a subset of physical activity characterized as planned, structured, repetitive, and designed to improve or maintain physical fitness, physical performance, or health.
Highlights from the 2018 guidelines include7:
- Preschool-aged children (3 to 5 years of age) should be physically active throughout the day, with as much as 3 hours per day of physical activity of all intensities—light, moderate, and vigorous.
- Older children and adolescents (6 to 17 years) should accumulate 60 minutes per day of moderate-to-vigorous physical activity, including aerobic, muscle-strengthening, and bone-strengthening activities.
- Adults of all ages should achieve approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week, along with at least 2 days per week of muscle-strengthening activities. Other types of physical activity include flexibility, balance, bone-strengthening, and mind–body exercises.
3-step framework for enhancing physical activity counseling
Merely knowing that physical activity is healthy is not enough, during a patient encounter, to increase the level of physical activity. Therefore, it is imperative to learn and adopt a framework that has proved to yield successful outcomes. The Screening, Brief Intervention, and Referral to Treatment (SBIRT) framework, which has predominantly been used to change patient behavior related to alcohol and substance use, is now being utilized by some providers to promote physical activity.8 We apply the SBIRT approach in this article, although research is lacking on its clinical utility and outcome measures.
Continue to: SBIRT
SBIRT: Screening
An office visit provides an opportunity to understand a patient’s level of physical activity. Often, understanding a patient’s baseline level of activity is only asked during a thorough social history, which might not be performed during patient encounters. As physical activity is the primary determinant of cardiorespiratory fitness (CRF), some health care systems have begun delineating physical activity levels as a vital sign to ensure that the assessment of physical activity is a standard part of every clinical encounter. At a minimum, this serves as a prompt and provides an opportunity to start a conversation around improving physical activity levels when guidelines are not being met.
The exercise vital sign. Assessment and documentation of physical activity in the electronic health record are not yet standardized; however, Kaiser Permanente health plans have implemented the exercise vital sign, or EVS, in its HealthConnect (Epic Systems) electronic health record. The EVS incorporates information about a patient’s:
- days per week of moderate-to-strenuous exercise (eg, a brisk walk)
- minutes per day, on average, of exercise at this level.
The physical activity vital sign. Intermountain Healthcare implemented the physical activity vital sign, or PAVS, in its iCentra (Cerner Corp.) electronic health record. The 3-question PAVS assessment asks:
- On average, how many days of the week do you perform physical activity or exercise?
- On average, how many total minutes of physical activity or exercise do you perform on those days?
- How would you describe the intensity of your physical activity or exercise: Light (ie, a casual walk)? Moderate (a brisk walk)? Or vigorous (jogging)?
PAVS includes a fourth data point: The physician–user documents whether the patient was counseled to start, increase, maintain, or modify physical activity or exercise.
EVS and the PAVS have demonstrated validity.9-11
Continue to: Cardiorespiratory fitness as a vital sign
Cardiorespiratory fitness as a vital sign. In 2016, the American Heart Association (AHA) asserted the importance of assessing CRF as a clinical vital sign.12 CRF is commonly expressed as maximal oxygen consumption (VO2max = O2 mL/kg/min) and measured through cardiopulmonary exercise testing (CPET), considered the gold standard by combining conventional graded exercise testing with ventilatory expired gas analysis. CPET is more objective and precise than equations estimating CRF that are derived from peak work rate. AHA recommended that efforts to improve CRF should become standard in clinical encounters, explaining that even a small increase in CRF (eg, 1 or 2 metabolic equivalentsa [METs]) is associated with a considerably (10% to 30%) lower rate of adverse cardiovascular events.12
De Souza de Silva and colleagues revealed an association between each 1-MET increase in CRF and per-person annual health care cost savings (adjusted for age and presence of cardiovascular disease) of $3272 (normal-weight patients), $4252 (overweight), and $6103 (obese).13 In its 2016 scientific statement on CRF as a vital sign, AHA listed several methods of estimating CRF and concluded that, although CPET involves a higher level of training, proficiency, equipment, and, therefore, cost, the independent and additive information obtained justifies its use in many patients.12
CASE
Mary Q, 68 years of age, presents for an annual well-woman examination. Body mass index is 32; resting heart rate (HR), 73 bpm; and blood pressure, 126/74 mm Hg. She reports being inactive, except for light walking every day with her dog around the neighborhood, which takes them approximately 15 minutes. She denies any history or signs and symptoms of cardiovascular, metabolic, or renal disease.
You consider 3 questions before taking next steps regarding increasing Ms. Q’s activity level:
- What is her PAVS?
- Does she need medical clearance before starting an exercise program?
- What would an evidence-based cardiovascular exercise prescription for Ms. Q look like?
SBIRT: Brief intervention
When a patient does not meet the recommended level of physical activity, you have an opportunity to deliver a brief intervention. To do this effectively, you must have adequate understanding of the patient’s receptivity for change. The transtheoretical, or Stages of Change, model proposes that a person typically goes through 5 stages of growth—pre-contemplation, contemplation, preparation, action, and maintenance—in the process of lifestyle modification. This model highlights the different approaches to exercise adoption and maintenance that need to be taken, based on a given patient’s stage at the moment.
Continue to: Using this framework...
Using this framework, you can help patients realize intrinsic motivation that can facilitate progression through each stage, utilizing techniques such as motivational interviewing—so-called change talk—to increase self-efficacy.14TABLE 115 provides examples of motivational interviewing techniques that can be used during a patient encounter to improve health behaviors, such as physical activity.
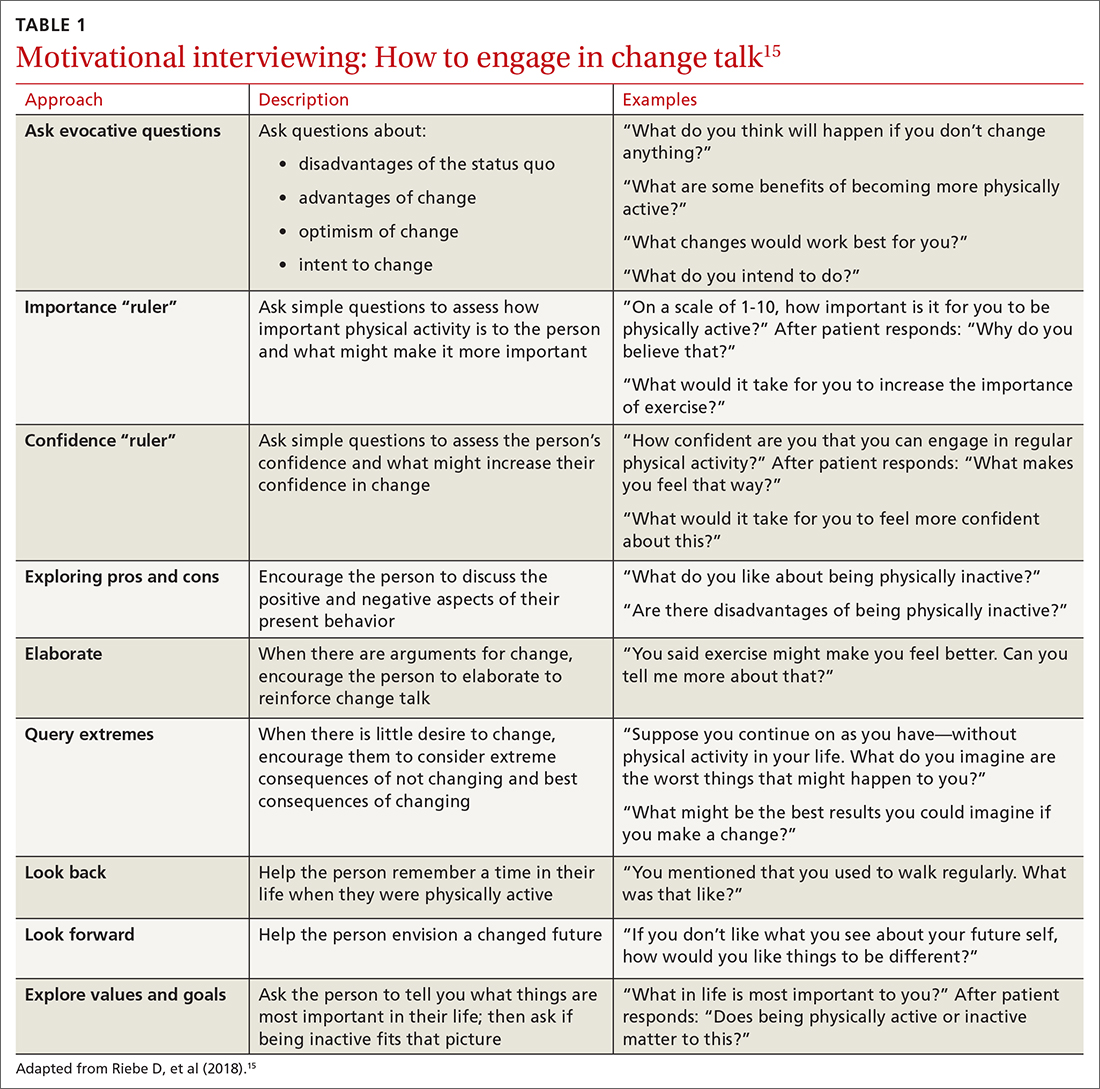
Writing the exercise prescription
A patient who wants to increase their level of physical activity should be offered a formal exercise prescription, which has been shown to increase the level of physical activity, particularly in older patients. In fact, a study conducted in Spain in the practices of family physicians found that older patients who received a physical activity prescription increased their activity by 131 minutes per week; and compared to control patients, they doubled the minutes per week devoted to moderate or vigorous physical activity.16
FITT-VP. The basics of a cardiovascular exercise prescription can be found in the FITT-VP (Frequency, Intensity, Time, Type, Volume, and [monitoring of] Progression) framework (TABLE 217-19). For most patients, this model includes 3 to 5 days per week of moderate-to-vigorous physical activity for 30 to 60 minutes per session. For patients with established chronic disease, physical activity provides health benefits but might require modification. Disease-specific patient handouts for exercise can be downloaded, at no cost, through the American College of Sports Medicine (ACSM) “Exercise Is Medicine” program, which can be found at: www.exerciseismedicine.org/support_page.php/rx-for-health-series.
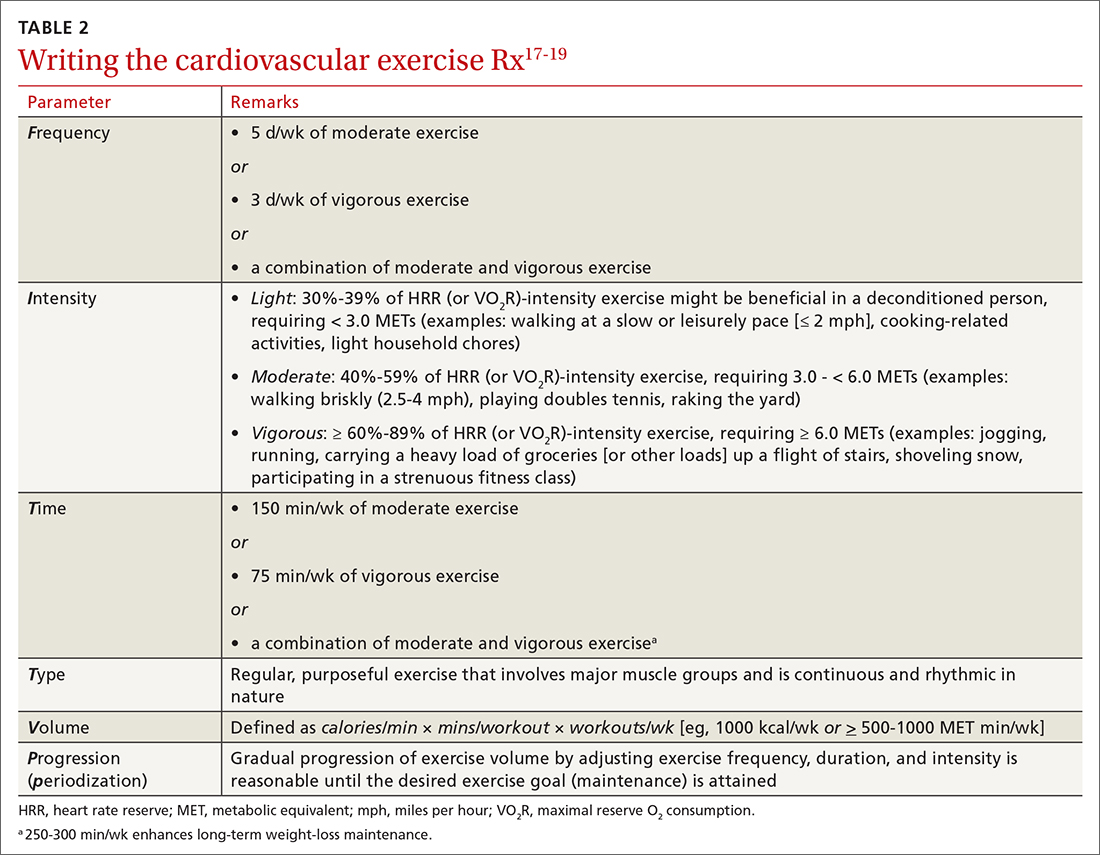
Determining intensity level. Although CPET is the gold standard for determining a patient’s target intensity level, such a test might be impracticable for a given patient. Surrogate markers of target intensity level can be obtained by measuring maximum HR (HRmax), using a well-known equation20:
HRmax = 220 – age
which is then multiplied by intensity range:
- light: 30%-39%
- moderate: 40%-59%
- vigorous: 60%-89%
or, more preferably, by calculating the HR training zone while accounting for HR at rest (HRrest). This is accomplished by calculating the HR reserve (HRR) (ie, HRR = HRmax – HRrest) and then calculating the target heart rate (THR)21:
THR = [HRR × %intensity] + HRrest
Continue to: The THR calculation...
The THR calculation is performed twice, once with a lower %intensity and again with a higher %intensity to develop a training zone based on HRR.
The HRR equation is more accurate than calculating HRmax from 220 – age, because HRR accounts for resting HR, which is often lower in people who are better conditioned.
Another method of calculating intensity for patients who are beginning a physical activity program is the rating of perceived exertion (RPE), which is graded on a scale of 6 to 20: Moderate exercise correlates with an RPE of 12 to 13 (“somewhat hard”); vigorous exercise correlates with an RPE of 14 to 16 (“hard”). By adding a zero to the rating on the RPE scale, the corresponding HR in a healthy adult can be estimated when they are performing an activity at that perceived intensity.22 Moderate exercise therefore correlates with a HR of 120 and 130 bpm.
The so-called talk test can also guide exercise intensity: Light-intensity activity correlates with an ability to sing; moderate-intensity physical activity likely allows the patient to still hold a conversation; and vigorous-intensity activity correlates with an inability to carry on a conversation while exercising.
An exercise prescription should be accompanied by a patient-derived goal, which can be reassessed during a follow-up visit. So-called SMART goals (Specific, Measurable, Achievable, Relevant, and Time-bound) are tools to help patients set personalized and realistic expectations for physical activity. Meeting the goal of approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week is ideal, but a patient needs to start where they are, at the moment, and gradually increase activity by setting what for them are realistic and sustainable goals.
Continue to: CASE
CASE
With a PAVS of 105 minutes (ie, 15 minutes per day × 7 days) of weekly light-to-moderate exercise walking her dog, Ms. Q does not satisfy current physical activity guidelines. She needs an exercise prescription to incorporate into her lifestyle (see “Cardiovascular exercise prescription,” at left).
First, based on ACSM pre-participation guidelines, Ms. Q does not need medical clearance before initiating light-to-moderate exercise and gradually progressing to vigorous-intensity exercise.
Second, in addition to walking the dog for 105 minutes a week, you:
- advise her to start walking for 10 minutes, 3 times per week, at a pace that keeps her HR at 97-104 bpm.
- encourage her to gradually increase the frequency or duration of her walks by no more than 10% per week.

SBIRT: Referral for treatment
When referring a patient to a fitness program or professional, it is essential to consider their preferences, resources, and environment.23 Community fitness partners are often an excellent referral option for a patient seeking guidance or structure for their exercise program. Using the ACSM ProFinder service, (www.acsm.org/get-stay-certified/find-a-pro) you can search for exercise professionals who have achieved the College’s Gold Standard credential.
Gym memberships or fitness programs might be part of the extra coverage offered by Medicare Advantage Plans, other Medicare health plans, or Medicare Supplement Insurance (Medigap) plans.24
Continue to: CASE
CASE
After providing Ms. Q with her exercise prescription, you refer her to a local gym that participates in the Silver Sneakers fitness and wellness program (for adults ≥ 65 years of age in eligible Medicare plans) to determine whether she qualifies to begin resistance and flexibility training, for which you will write a second exercise prescription (TABLE 317-19).
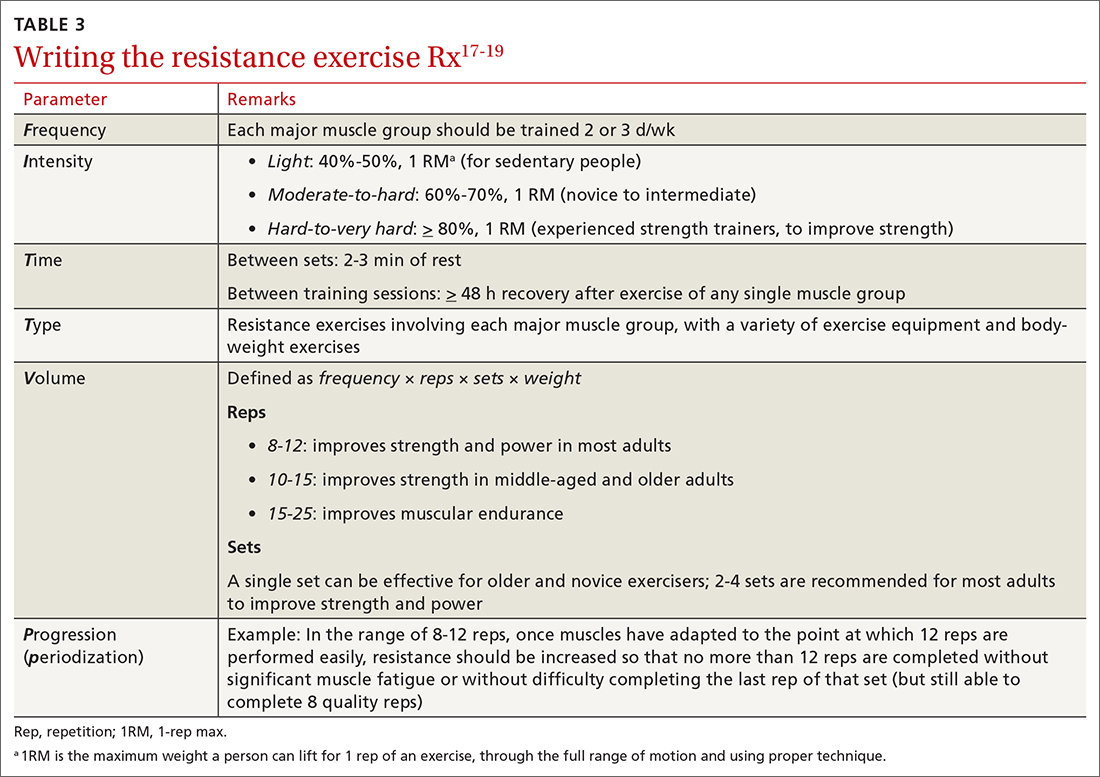
Pre-participation screening
Updated 2015 ACSM exercise pre-participation health screening recommendations attempt to decrease possible barriers to people who are becoming more physically active, by minimizing unnecessary referral to health care providers before they change their level of physical activity. ACSM recommendations on exercise clearance include this guidance25:
- For a patient who is asymptomatic and already physically active—regardless of whether they have known cardiovascular, metabolic, or renal disease—medical clearance is unnecessary for moderate-intensity exercise.
- Any patient who has been physically active and asymptomatic but who becomes symptomatic during exercise should immediately discontinue such activity and undergo medical evaluation.
- For a patient who is inactive, asymptomatic, and who does not have known cardiovascular, metabolic, or renal disease, medical clearance for light- or moderate-intensity exercise is unnecessary.
- For inactive, asymptomatic patients who have known cardiovascular, metabolic, or renal disease, medical clearance is recommended.
Digital health
Smartwatches and health apps (eg, CardioCoach, Fitbit, Garmin Connect, Nike Training Club, Strava, and Training Peaks) can provide workouts and offer patients the ability to collect information and even connect with other users through social media platforms. This information can be synced to Apple Health platforms for iPhones (www.apple.com/ios/health/) or through Google Fit (www.google.com/fit/) on Android devices. Primary care physicians who become familiar with health apps might find them useful for select patients who want to use technology to improve their physical activity level.
However, data on the value of using digital apps for increasing physical activity, in relation to their cost, are limited. Additional research is needed to assess their validity.
Billing and coding
For most patients, the physical activity assessment, prescription, and referral are performed in the context of treating another condition (eg, hypertension, type 2 diabetes, obesity, depression) or during a preventive health examination, and are typically covered without additional charge to the patient. An evaluation and management visit for an established patient could be used to bill if > 50% of the office visit was spent face-to-face with a physician, with patient counseling and coordination of care.
Continue to: Physicians and physical therapists...
Physicians and physical therapists can use the therapeutic exercise code (Current Procedural Terminology code 97110) when teaching patients exercises to develop muscle strength and endurance, joint range of motion, and flexibility26 (TABLE 426).
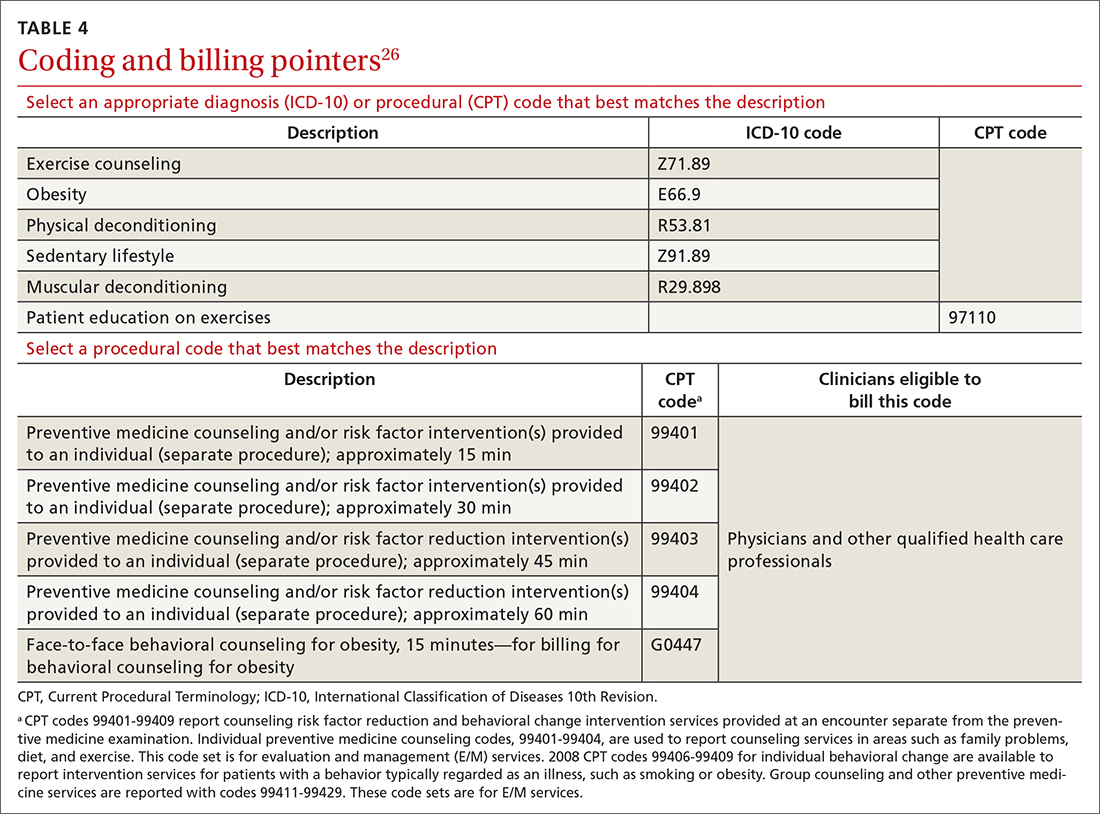
Conclusion
Physical activity and CRF are strong predictors of premature mortality, even compared to other risk factors, such as cigarette smoking, hypertension, hypercholesterolemia, and type 2 diabetes.27 Brief physical activity assessment and counseling is an efficient, effective, and cost-effective means to increase physical activity, and presents a unique opportunity for you to encourage lifestyle-based strategies for reducing cardiovascular risk.28
However, it is essential to meet patients where they are before trying to have them progress; it is therefore imperative to assess the individual patient’s level of activity using PAVS. With that information in hand, you can personalize physical activity advice; determine readiness for change and potential barriers for change; assist the patient in setting SMART goals; and arrange follow-up to assess adherence to the exercise prescription. Encourage the patient to call their health insurance plan to determine whether a gym membership or fitness program is covered.
Research is needed to evaluate the value of using digital apps, in light of their cost, to increase physical activity and improve CRF in a clinical setting. Prospective trials should be initiated to determine how routine implementation of CRF assessment in primary care alters the trajectory of clinical care. It is hoped that future research will answer the question: Would such an approach improve clinical outcomes and reduce health care expenditures?12
a Defined as O2 consumed while sitting at rest; equivalent to 3.5 mL of O2 × kg of body weight × min.
CORRESPONDENCE
Matthew Kampert, DO, MS, Sports Medicine, 5555 Transportation Boulevard, Cleveland, OH 44125; [email protected]
1. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024-1039. doi: 10.1001/jama.2018.1150
2. Tikkanen R, Abrams MK. U.S. health care from a global perspective, 2019: higher spending, worse outcomes? The Commonwealth Fund Website. January 30, 2020. Accessed November 16, 2021. www.commonwealthfund.org/publications/issue-briefs/2020/jan/us-health-care-global-perspective-2019
3. Stoutenberg M, Stasi S, Stamatakis E, et al. Physical activity training in US medical schools: preparing future physicians to engage in primary prevention. Phys Sportsmed. 2015;43:388-394. doi: 10.1080/00913847.2015.1084868
4. Antognoli EL, Seeholzer EL, Gullett H, et al. Primary care resident training for obesity, nutrition, and physical activity counseling: a mixed-methods study. Health Promot Pract. 2017;18:672-680. doi: 10.1177/1524839916658025
5. Asif IM, Drezner JA. Sports and exercise medicine education in the USA: call to action. Br J Sports Med. 2020;54:195-196. doi: 10.1136/bjsports-2019-101104
6. Douglas JA, Briones MD, Bauer EZ, et al. Social and environmental determinants of physical activity in urban parks: testing a neighborhood disorder model. Prev Med. 2018;109:119-124. doi: 10.1016/j.ypmed.2018.01.013
7. 2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: US Department of Health & Human Services; 2018. Accessed November 15, 2021. https://health.gov/sites/default/files/2019-09/PAG_Advisory_Committee_Report.pdf
8. Avis JL, Cave AL, Donaldson S, et al. Working with parents to prevent childhood obesity: protocol for a primary care-based ehealth study. JMIR Res Protoc. 2015;4:e35. doi:10.2196/resprot.4147
9. Ball TJ, Joy EA, Gren LH, et al. Concurrent validity of a self-reported physical activity ‘vital sign’ questionnaire with adult primary care patients. Prev Chronic Dis. 2016;13:e16. doi: 10.5888/pcd13.150228
10. Ball TJ, Joy EA, Gren LH, et al. Predictive validity of an adult physical activity “vital sign” recorded in electronic health records. J Phys Act Health. 2016;13:403-408. doi: 10.1123/jpah.2015-0210
11. Coleman KJ, Ngor E, Reynolds K, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc. 2012;44:2071-2076. doi: 10.1249/MSS.0b013e3182630ec1
12. Ross R, Blair SN, Arena R, et al; ; ; ; ; ; . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653-e699. doi: 10.1161/CIR.0000000000000461
13. de Souza de Silva CG, Kokkinos PP, Doom R, et al. Association between cardiorespiratory fitness, obesity, and health care costs: The Veterans Exercise Testing Study. Int J Obes (Lond). 2019;43:2225-2232. doi: 10.1038/s41366-018-0257-0
14. Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38-48. doi: 10.4278/0890-1171-12.1.38
15. Riebe D, Ehrman JK, Liguori G, et al. Methods for evoking change talk. In: ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Wolters Kluwer; 2018.
16. Grandes G, Sanchez A, Sanchez-Pinilla RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
17. McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: a review of concepts and evidence. Soc Sci Med. 2006;63:1011-1022. doi: 10.1016/j.socscimed.2006.03.012
18. Garber CE, Blissmer BE, Deschenes MR, et al; American College of Sports Medicine. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Position stand. Med Sci Sport Exerc. 2011;43:1334-1359. doi: 10.1249/MSS.0b013e318213fefb
19. Donnelly JE, Blair SN, Jakicic JM, et al; American College of Sports Medicine. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Position stand. Med Sci Sport Exerc. 2009;41:459-471. doi: 10.1249/MSS.0b013e3181949333
20. Fox SM 3rd, Naughton JP, Haskell WL. Physical activity and the prevention of coronary heart disease. Ann Clin Res. 1971;3:404-432.
21. Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307-315.
22. The Borg RPE scale. In: Borg G. Borg’s Perceived Exertion and Pain Scales. Human Kinetics; 1998:29-38.
23. Ratamess NA, Alvar BA, Evetoch TK, et al; American College of Sports Medicine. Progression models in resistance training for healthy adults. Position stand. Med Sci Sport Exerc. 2009;41:687-708. doi: 10.1249/MSS.0b013e3181915670
24. Gym memberships & fitness programs. Medicare.gov. Baltimore, MD: US Centers for Medicare and Medicaid Services. Accessed November 16, 2021. www.medicare.gov/coverage/gym-memberships-fitness-programs
25. Riebe D, Franklin BA, Thompson PD, et al. Updating ACSM’s recommendations for exercise preparticipation health screening. Med Sci Sports Exerc. 2015;47:2473-2479. doi: 10.1249/MSS.0000000000000664
26. Physical Activity Related Current Procedural Terminology (CPT®) Codes. Physical Activity Alliance website. Accessed November 16, 2021. https://paamovewithus.org/wp-content/uploads/2020/11/PAA-Physical-Activity-CPT-Codes-Nov-2020-AMA-Approved-Final-1.pdf
27. Blair SN. Physical inactivity: the biggest public health problem of the 21st century Br J Sports Med. 2009;43:1-2.
28. Vuori IM, Lavie CJ, Blair SN. Physical activity promotion in the health care system. Mayo Clin Proc. 2013;88:1446-1461. doi: 10.1016/j.mayocp.2013.08.020
It is well-known that per capita health care spending in the United States is more than twice the average in other developed countries1; nevertheless, the overall health care ranking of the US is near the bottom compared to other countries in this group.2 Much of the reason for this poor relative showing lies in the fact that the US has employed a somewhat traditional fee-for-service health care model that does not incentivize efforts to promote health and wellness or prevent chronic disease. The paradigm of promoting physical activity for its disease-preventing and treatment benefits has not been well-integrated in the US health care system.
In this article, we endeavor to provide better understanding of the barriers that keep family physicians from routinely promoting physical activity in clinical practice; define tools and resources that can be used in the clinical setting to promote physical activity; and delineate areas for future work.
Glaring hole in US physical activity education
Many primary care physicians feel underprepared to prescribe or motivate patients to exercise. The reason for that lack of preparedness likely relates to a medical education system that does not spend time preparing physicians to perform this critical task. A study showed that, on average, medical schools require only 8 hours of physical activity education in their curriculum during the 4 years of schooling.3 Likewise, the average primary care residency program offers only 3 hours of didactic training on physical activity, nutrition, and obesity.4 The problem extends to sports medicine fellowship training, in which a 2019 survey showed that 63% of fellows were never taught how to write an exercise prescription in their training program.5
Without education on physical activity, medical students, residents, and fellows are woefully underprepared to realize the therapeutic value of physical activity in patient care, comprehend current physical activity guidelines, appropriately motivate patients to engage in exercise, and competently discuss exercise prescriptions in different disease states. Throughout their training, it is imperative for medical professionals to be educated on the social determinants of health, which include the conditions in which people live, work, and play. These environmental variables can contribute to health inequities that create additional barriers to improvement in physical fitness.6
National guidelines on physical activity
The 2018 National Physical Activity Guidelines detail recommendations for children, adolescents, adults, and special populations.7 The guidelines define physical activity as bodily movement produced by skeletal muscles that result in energy expenditure above resting baseline levels, and includes all types, intensities, and domains of activity. Exercise is a subset of physical activity characterized as planned, structured, repetitive, and designed to improve or maintain physical fitness, physical performance, or health.
Highlights from the 2018 guidelines include7:
- Preschool-aged children (3 to 5 years of age) should be physically active throughout the day, with as much as 3 hours per day of physical activity of all intensities—light, moderate, and vigorous.
- Older children and adolescents (6 to 17 years) should accumulate 60 minutes per day of moderate-to-vigorous physical activity, including aerobic, muscle-strengthening, and bone-strengthening activities.
- Adults of all ages should achieve approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week, along with at least 2 days per week of muscle-strengthening activities. Other types of physical activity include flexibility, balance, bone-strengthening, and mind–body exercises.
3-step framework for enhancing physical activity counseling
Merely knowing that physical activity is healthy is not enough, during a patient encounter, to increase the level of physical activity. Therefore, it is imperative to learn and adopt a framework that has proved to yield successful outcomes. The Screening, Brief Intervention, and Referral to Treatment (SBIRT) framework, which has predominantly been used to change patient behavior related to alcohol and substance use, is now being utilized by some providers to promote physical activity.8 We apply the SBIRT approach in this article, although research is lacking on its clinical utility and outcome measures.
Continue to: SBIRT
SBIRT: Screening
An office visit provides an opportunity to understand a patient’s level of physical activity. Often, understanding a patient’s baseline level of activity is only asked during a thorough social history, which might not be performed during patient encounters. As physical activity is the primary determinant of cardiorespiratory fitness (CRF), some health care systems have begun delineating physical activity levels as a vital sign to ensure that the assessment of physical activity is a standard part of every clinical encounter. At a minimum, this serves as a prompt and provides an opportunity to start a conversation around improving physical activity levels when guidelines are not being met.
The exercise vital sign. Assessment and documentation of physical activity in the electronic health record are not yet standardized; however, Kaiser Permanente health plans have implemented the exercise vital sign, or EVS, in its HealthConnect (Epic Systems) electronic health record. The EVS incorporates information about a patient’s:
- days per week of moderate-to-strenuous exercise (eg, a brisk walk)
- minutes per day, on average, of exercise at this level.
The physical activity vital sign. Intermountain Healthcare implemented the physical activity vital sign, or PAVS, in its iCentra (Cerner Corp.) electronic health record. The 3-question PAVS assessment asks:
- On average, how many days of the week do you perform physical activity or exercise?
- On average, how many total minutes of physical activity or exercise do you perform on those days?
- How would you describe the intensity of your physical activity or exercise: Light (ie, a casual walk)? Moderate (a brisk walk)? Or vigorous (jogging)?
PAVS includes a fourth data point: The physician–user documents whether the patient was counseled to start, increase, maintain, or modify physical activity or exercise.
EVS and the PAVS have demonstrated validity.9-11
Continue to: Cardiorespiratory fitness as a vital sign
Cardiorespiratory fitness as a vital sign. In 2016, the American Heart Association (AHA) asserted the importance of assessing CRF as a clinical vital sign.12 CRF is commonly expressed as maximal oxygen consumption (VO2max = O2 mL/kg/min) and measured through cardiopulmonary exercise testing (CPET), considered the gold standard by combining conventional graded exercise testing with ventilatory expired gas analysis. CPET is more objective and precise than equations estimating CRF that are derived from peak work rate. AHA recommended that efforts to improve CRF should become standard in clinical encounters, explaining that even a small increase in CRF (eg, 1 or 2 metabolic equivalentsa [METs]) is associated with a considerably (10% to 30%) lower rate of adverse cardiovascular events.12
De Souza de Silva and colleagues revealed an association between each 1-MET increase in CRF and per-person annual health care cost savings (adjusted for age and presence of cardiovascular disease) of $3272 (normal-weight patients), $4252 (overweight), and $6103 (obese).13 In its 2016 scientific statement on CRF as a vital sign, AHA listed several methods of estimating CRF and concluded that, although CPET involves a higher level of training, proficiency, equipment, and, therefore, cost, the independent and additive information obtained justifies its use in many patients.12
CASE
Mary Q, 68 years of age, presents for an annual well-woman examination. Body mass index is 32; resting heart rate (HR), 73 bpm; and blood pressure, 126/74 mm Hg. She reports being inactive, except for light walking every day with her dog around the neighborhood, which takes them approximately 15 minutes. She denies any history or signs and symptoms of cardiovascular, metabolic, or renal disease.
You consider 3 questions before taking next steps regarding increasing Ms. Q’s activity level:
- What is her PAVS?
- Does she need medical clearance before starting an exercise program?
- What would an evidence-based cardiovascular exercise prescription for Ms. Q look like?
SBIRT: Brief intervention
When a patient does not meet the recommended level of physical activity, you have an opportunity to deliver a brief intervention. To do this effectively, you must have adequate understanding of the patient’s receptivity for change. The transtheoretical, or Stages of Change, model proposes that a person typically goes through 5 stages of growth—pre-contemplation, contemplation, preparation, action, and maintenance—in the process of lifestyle modification. This model highlights the different approaches to exercise adoption and maintenance that need to be taken, based on a given patient’s stage at the moment.
Continue to: Using this framework...
Using this framework, you can help patients realize intrinsic motivation that can facilitate progression through each stage, utilizing techniques such as motivational interviewing—so-called change talk—to increase self-efficacy.14TABLE 115 provides examples of motivational interviewing techniques that can be used during a patient encounter to improve health behaviors, such as physical activity.

Writing the exercise prescription
A patient who wants to increase their level of physical activity should be offered a formal exercise prescription, which has been shown to increase the level of physical activity, particularly in older patients. In fact, a study conducted in Spain in the practices of family physicians found that older patients who received a physical activity prescription increased their activity by 131 minutes per week; and compared to control patients, they doubled the minutes per week devoted to moderate or vigorous physical activity.16
FITT-VP. The basics of a cardiovascular exercise prescription can be found in the FITT-VP (Frequency, Intensity, Time, Type, Volume, and [monitoring of] Progression) framework (TABLE 217-19). For most patients, this model includes 3 to 5 days per week of moderate-to-vigorous physical activity for 30 to 60 minutes per session. For patients with established chronic disease, physical activity provides health benefits but might require modification. Disease-specific patient handouts for exercise can be downloaded, at no cost, through the American College of Sports Medicine (ACSM) “Exercise Is Medicine” program, which can be found at: www.exerciseismedicine.org/support_page.php/rx-for-health-series.

Determining intensity level. Although CPET is the gold standard for determining a patient’s target intensity level, such a test might be impracticable for a given patient. Surrogate markers of target intensity level can be obtained by measuring maximum HR (HRmax), using a well-known equation20:
HRmax = 220 – age
which is then multiplied by intensity range:
- light: 30%-39%
- moderate: 40%-59%
- vigorous: 60%-89%
or, more preferably, by calculating the HR training zone while accounting for HR at rest (HRrest). This is accomplished by calculating the HR reserve (HRR) (ie, HRR = HRmax – HRrest) and then calculating the target heart rate (THR)21:
THR = [HRR × %intensity] + HRrest
Continue to: The THR calculation...
The THR calculation is performed twice, once with a lower %intensity and again with a higher %intensity to develop a training zone based on HRR.
The HRR equation is more accurate than calculating HRmax from 220 – age, because HRR accounts for resting HR, which is often lower in people who are better conditioned.
Another method of calculating intensity for patients who are beginning a physical activity program is the rating of perceived exertion (RPE), which is graded on a scale of 6 to 20: Moderate exercise correlates with an RPE of 12 to 13 (“somewhat hard”); vigorous exercise correlates with an RPE of 14 to 16 (“hard”). By adding a zero to the rating on the RPE scale, the corresponding HR in a healthy adult can be estimated when they are performing an activity at that perceived intensity.22 Moderate exercise therefore correlates with a HR of 120 and 130 bpm.
The so-called talk test can also guide exercise intensity: Light-intensity activity correlates with an ability to sing; moderate-intensity physical activity likely allows the patient to still hold a conversation; and vigorous-intensity activity correlates with an inability to carry on a conversation while exercising.
An exercise prescription should be accompanied by a patient-derived goal, which can be reassessed during a follow-up visit. So-called SMART goals (Specific, Measurable, Achievable, Relevant, and Time-bound) are tools to help patients set personalized and realistic expectations for physical activity. Meeting the goal of approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week is ideal, but a patient needs to start where they are, at the moment, and gradually increase activity by setting what for them are realistic and sustainable goals.
Continue to: CASE
CASE
With a PAVS of 105 minutes (ie, 15 minutes per day × 7 days) of weekly light-to-moderate exercise walking her dog, Ms. Q does not satisfy current physical activity guidelines. She needs an exercise prescription to incorporate into her lifestyle (see “Cardiovascular exercise prescription,” at left).
First, based on ACSM pre-participation guidelines, Ms. Q does not need medical clearance before initiating light-to-moderate exercise and gradually progressing to vigorous-intensity exercise.
Second, in addition to walking the dog for 105 minutes a week, you:
- advise her to start walking for 10 minutes, 3 times per week, at a pace that keeps her HR at 97-104 bpm.
- encourage her to gradually increase the frequency or duration of her walks by no more than 10% per week.

SBIRT: Referral for treatment
When referring a patient to a fitness program or professional, it is essential to consider their preferences, resources, and environment.23 Community fitness partners are often an excellent referral option for a patient seeking guidance or structure for their exercise program. Using the ACSM ProFinder service, (www.acsm.org/get-stay-certified/find-a-pro) you can search for exercise professionals who have achieved the College’s Gold Standard credential.
Gym memberships or fitness programs might be part of the extra coverage offered by Medicare Advantage Plans, other Medicare health plans, or Medicare Supplement Insurance (Medigap) plans.24
Continue to: CASE
CASE
After providing Ms. Q with her exercise prescription, you refer her to a local gym that participates in the Silver Sneakers fitness and wellness program (for adults ≥ 65 years of age in eligible Medicare plans) to determine whether she qualifies to begin resistance and flexibility training, for which you will write a second exercise prescription (TABLE 317-19).

Pre-participation screening
Updated 2015 ACSM exercise pre-participation health screening recommendations attempt to decrease possible barriers to people who are becoming more physically active, by minimizing unnecessary referral to health care providers before they change their level of physical activity. ACSM recommendations on exercise clearance include this guidance25:
- For a patient who is asymptomatic and already physically active—regardless of whether they have known cardiovascular, metabolic, or renal disease—medical clearance is unnecessary for moderate-intensity exercise.
- Any patient who has been physically active and asymptomatic but who becomes symptomatic during exercise should immediately discontinue such activity and undergo medical evaluation.
- For a patient who is inactive, asymptomatic, and who does not have known cardiovascular, metabolic, or renal disease, medical clearance for light- or moderate-intensity exercise is unnecessary.
- For inactive, asymptomatic patients who have known cardiovascular, metabolic, or renal disease, medical clearance is recommended.
Digital health
Smartwatches and health apps (eg, CardioCoach, Fitbit, Garmin Connect, Nike Training Club, Strava, and Training Peaks) can provide workouts and offer patients the ability to collect information and even connect with other users through social media platforms. This information can be synced to Apple Health platforms for iPhones (www.apple.com/ios/health/) or through Google Fit (www.google.com/fit/) on Android devices. Primary care physicians who become familiar with health apps might find them useful for select patients who want to use technology to improve their physical activity level.
However, data on the value of using digital apps for increasing physical activity, in relation to their cost, are limited. Additional research is needed to assess their validity.
Billing and coding
For most patients, the physical activity assessment, prescription, and referral are performed in the context of treating another condition (eg, hypertension, type 2 diabetes, obesity, depression) or during a preventive health examination, and are typically covered without additional charge to the patient. An evaluation and management visit for an established patient could be used to bill if > 50% of the office visit was spent face-to-face with a physician, with patient counseling and coordination of care.
Continue to: Physicians and physical therapists...
Physicians and physical therapists can use the therapeutic exercise code (Current Procedural Terminology code 97110) when teaching patients exercises to develop muscle strength and endurance, joint range of motion, and flexibility26 (TABLE 426).

Conclusion
Physical activity and CRF are strong predictors of premature mortality, even compared to other risk factors, such as cigarette smoking, hypertension, hypercholesterolemia, and type 2 diabetes.27 Brief physical activity assessment and counseling is an efficient, effective, and cost-effective means to increase physical activity, and presents a unique opportunity for you to encourage lifestyle-based strategies for reducing cardiovascular risk.28
However, it is essential to meet patients where they are before trying to have them progress; it is therefore imperative to assess the individual patient’s level of activity using PAVS. With that information in hand, you can personalize physical activity advice; determine readiness for change and potential barriers for change; assist the patient in setting SMART goals; and arrange follow-up to assess adherence to the exercise prescription. Encourage the patient to call their health insurance plan to determine whether a gym membership or fitness program is covered.
Research is needed to evaluate the value of using digital apps, in light of their cost, to increase physical activity and improve CRF in a clinical setting. Prospective trials should be initiated to determine how routine implementation of CRF assessment in primary care alters the trajectory of clinical care. It is hoped that future research will answer the question: Would such an approach improve clinical outcomes and reduce health care expenditures?12
a Defined as O2 consumed while sitting at rest; equivalent to 3.5 mL of O2 × kg of body weight × min.
CORRESPONDENCE
Matthew Kampert, DO, MS, Sports Medicine, 5555 Transportation Boulevard, Cleveland, OH 44125; [email protected]
It is well-known that per capita health care spending in the United States is more than twice the average in other developed countries1; nevertheless, the overall health care ranking of the US is near the bottom compared to other countries in this group.2 Much of the reason for this poor relative showing lies in the fact that the US has employed a somewhat traditional fee-for-service health care model that does not incentivize efforts to promote health and wellness or prevent chronic disease. The paradigm of promoting physical activity for its disease-preventing and treatment benefits has not been well-integrated in the US health care system.
In this article, we endeavor to provide better understanding of the barriers that keep family physicians from routinely promoting physical activity in clinical practice; define tools and resources that can be used in the clinical setting to promote physical activity; and delineate areas for future work.
Glaring hole in US physical activity education
Many primary care physicians feel underprepared to prescribe or motivate patients to exercise. The reason for that lack of preparedness likely relates to a medical education system that does not spend time preparing physicians to perform this critical task. A study showed that, on average, medical schools require only 8 hours of physical activity education in their curriculum during the 4 years of schooling.3 Likewise, the average primary care residency program offers only 3 hours of didactic training on physical activity, nutrition, and obesity.4 The problem extends to sports medicine fellowship training, in which a 2019 survey showed that 63% of fellows were never taught how to write an exercise prescription in their training program.5
Without education on physical activity, medical students, residents, and fellows are woefully underprepared to realize the therapeutic value of physical activity in patient care, comprehend current physical activity guidelines, appropriately motivate patients to engage in exercise, and competently discuss exercise prescriptions in different disease states. Throughout their training, it is imperative for medical professionals to be educated on the social determinants of health, which include the conditions in which people live, work, and play. These environmental variables can contribute to health inequities that create additional barriers to improvement in physical fitness.6
National guidelines on physical activity
The 2018 National Physical Activity Guidelines detail recommendations for children, adolescents, adults, and special populations.7 The guidelines define physical activity as bodily movement produced by skeletal muscles that result in energy expenditure above resting baseline levels, and includes all types, intensities, and domains of activity. Exercise is a subset of physical activity characterized as planned, structured, repetitive, and designed to improve or maintain physical fitness, physical performance, or health.
Highlights from the 2018 guidelines include7:
- Preschool-aged children (3 to 5 years of age) should be physically active throughout the day, with as much as 3 hours per day of physical activity of all intensities—light, moderate, and vigorous.
- Older children and adolescents (6 to 17 years) should accumulate 60 minutes per day of moderate-to-vigorous physical activity, including aerobic, muscle-strengthening, and bone-strengthening activities.
- Adults of all ages should achieve approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week, along with at least 2 days per week of muscle-strengthening activities. Other types of physical activity include flexibility, balance, bone-strengthening, and mind–body exercises.
3-step framework for enhancing physical activity counseling
Merely knowing that physical activity is healthy is not enough, during a patient encounter, to increase the level of physical activity. Therefore, it is imperative to learn and adopt a framework that has proved to yield successful outcomes. The Screening, Brief Intervention, and Referral to Treatment (SBIRT) framework, which has predominantly been used to change patient behavior related to alcohol and substance use, is now being utilized by some providers to promote physical activity.8 We apply the SBIRT approach in this article, although research is lacking on its clinical utility and outcome measures.
Continue to: SBIRT
SBIRT: Screening
An office visit provides an opportunity to understand a patient’s level of physical activity. Often, understanding a patient’s baseline level of activity is only asked during a thorough social history, which might not be performed during patient encounters. As physical activity is the primary determinant of cardiorespiratory fitness (CRF), some health care systems have begun delineating physical activity levels as a vital sign to ensure that the assessment of physical activity is a standard part of every clinical encounter. At a minimum, this serves as a prompt and provides an opportunity to start a conversation around improving physical activity levels when guidelines are not being met.
The exercise vital sign. Assessment and documentation of physical activity in the electronic health record are not yet standardized; however, Kaiser Permanente health plans have implemented the exercise vital sign, or EVS, in its HealthConnect (Epic Systems) electronic health record. The EVS incorporates information about a patient’s:
- days per week of moderate-to-strenuous exercise (eg, a brisk walk)
- minutes per day, on average, of exercise at this level.
The physical activity vital sign. Intermountain Healthcare implemented the physical activity vital sign, or PAVS, in its iCentra (Cerner Corp.) electronic health record. The 3-question PAVS assessment asks:
- On average, how many days of the week do you perform physical activity or exercise?
- On average, how many total minutes of physical activity or exercise do you perform on those days?
- How would you describe the intensity of your physical activity or exercise: Light (ie, a casual walk)? Moderate (a brisk walk)? Or vigorous (jogging)?
PAVS includes a fourth data point: The physician–user documents whether the patient was counseled to start, increase, maintain, or modify physical activity or exercise.
EVS and the PAVS have demonstrated validity.9-11
Continue to: Cardiorespiratory fitness as a vital sign
Cardiorespiratory fitness as a vital sign. In 2016, the American Heart Association (AHA) asserted the importance of assessing CRF as a clinical vital sign.12 CRF is commonly expressed as maximal oxygen consumption (VO2max = O2 mL/kg/min) and measured through cardiopulmonary exercise testing (CPET), considered the gold standard by combining conventional graded exercise testing with ventilatory expired gas analysis. CPET is more objective and precise than equations estimating CRF that are derived from peak work rate. AHA recommended that efforts to improve CRF should become standard in clinical encounters, explaining that even a small increase in CRF (eg, 1 or 2 metabolic equivalentsa [METs]) is associated with a considerably (10% to 30%) lower rate of adverse cardiovascular events.12
De Souza de Silva and colleagues revealed an association between each 1-MET increase in CRF and per-person annual health care cost savings (adjusted for age and presence of cardiovascular disease) of $3272 (normal-weight patients), $4252 (overweight), and $6103 (obese).13 In its 2016 scientific statement on CRF as a vital sign, AHA listed several methods of estimating CRF and concluded that, although CPET involves a higher level of training, proficiency, equipment, and, therefore, cost, the independent and additive information obtained justifies its use in many patients.12
CASE
Mary Q, 68 years of age, presents for an annual well-woman examination. Body mass index is 32; resting heart rate (HR), 73 bpm; and blood pressure, 126/74 mm Hg. She reports being inactive, except for light walking every day with her dog around the neighborhood, which takes them approximately 15 minutes. She denies any history or signs and symptoms of cardiovascular, metabolic, or renal disease.
You consider 3 questions before taking next steps regarding increasing Ms. Q’s activity level:
- What is her PAVS?
- Does she need medical clearance before starting an exercise program?
- What would an evidence-based cardiovascular exercise prescription for Ms. Q look like?
SBIRT: Brief intervention
When a patient does not meet the recommended level of physical activity, you have an opportunity to deliver a brief intervention. To do this effectively, you must have adequate understanding of the patient’s receptivity for change. The transtheoretical, or Stages of Change, model proposes that a person typically goes through 5 stages of growth—pre-contemplation, contemplation, preparation, action, and maintenance—in the process of lifestyle modification. This model highlights the different approaches to exercise adoption and maintenance that need to be taken, based on a given patient’s stage at the moment.
Continue to: Using this framework...
Using this framework, you can help patients realize intrinsic motivation that can facilitate progression through each stage, utilizing techniques such as motivational interviewing—so-called change talk—to increase self-efficacy.14TABLE 115 provides examples of motivational interviewing techniques that can be used during a patient encounter to improve health behaviors, such as physical activity.

Writing the exercise prescription
A patient who wants to increase their level of physical activity should be offered a formal exercise prescription, which has been shown to increase the level of physical activity, particularly in older patients. In fact, a study conducted in Spain in the practices of family physicians found that older patients who received a physical activity prescription increased their activity by 131 minutes per week; and compared to control patients, they doubled the minutes per week devoted to moderate or vigorous physical activity.16
FITT-VP. The basics of a cardiovascular exercise prescription can be found in the FITT-VP (Frequency, Intensity, Time, Type, Volume, and [monitoring of] Progression) framework (TABLE 217-19). For most patients, this model includes 3 to 5 days per week of moderate-to-vigorous physical activity for 30 to 60 minutes per session. For patients with established chronic disease, physical activity provides health benefits but might require modification. Disease-specific patient handouts for exercise can be downloaded, at no cost, through the American College of Sports Medicine (ACSM) “Exercise Is Medicine” program, which can be found at: www.exerciseismedicine.org/support_page.php/rx-for-health-series.

Determining intensity level. Although CPET is the gold standard for determining a patient’s target intensity level, such a test might be impracticable for a given patient. Surrogate markers of target intensity level can be obtained by measuring maximum HR (HRmax), using a well-known equation20:
HRmax = 220 – age
which is then multiplied by intensity range:
- light: 30%-39%
- moderate: 40%-59%
- vigorous: 60%-89%
or, more preferably, by calculating the HR training zone while accounting for HR at rest (HRrest). This is accomplished by calculating the HR reserve (HRR) (ie, HRR = HRmax – HRrest) and then calculating the target heart rate (THR)21:
THR = [HRR × %intensity] + HRrest
Continue to: The THR calculation...
The THR calculation is performed twice, once with a lower %intensity and again with a higher %intensity to develop a training zone based on HRR.
The HRR equation is more accurate than calculating HRmax from 220 – age, because HRR accounts for resting HR, which is often lower in people who are better conditioned.
Another method of calculating intensity for patients who are beginning a physical activity program is the rating of perceived exertion (RPE), which is graded on a scale of 6 to 20: Moderate exercise correlates with an RPE of 12 to 13 (“somewhat hard”); vigorous exercise correlates with an RPE of 14 to 16 (“hard”). By adding a zero to the rating on the RPE scale, the corresponding HR in a healthy adult can be estimated when they are performing an activity at that perceived intensity.22 Moderate exercise therefore correlates with a HR of 120 and 130 bpm.
The so-called talk test can also guide exercise intensity: Light-intensity activity correlates with an ability to sing; moderate-intensity physical activity likely allows the patient to still hold a conversation; and vigorous-intensity activity correlates with an inability to carry on a conversation while exercising.
An exercise prescription should be accompanied by a patient-derived goal, which can be reassessed during a follow-up visit. So-called SMART goals (Specific, Measurable, Achievable, Relevant, and Time-bound) are tools to help patients set personalized and realistic expectations for physical activity. Meeting the goal of approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week is ideal, but a patient needs to start where they are, at the moment, and gradually increase activity by setting what for them are realistic and sustainable goals.
Continue to: CASE
CASE
With a PAVS of 105 minutes (ie, 15 minutes per day × 7 days) of weekly light-to-moderate exercise walking her dog, Ms. Q does not satisfy current physical activity guidelines. She needs an exercise prescription to incorporate into her lifestyle (see “Cardiovascular exercise prescription,” at left).
First, based on ACSM pre-participation guidelines, Ms. Q does not need medical clearance before initiating light-to-moderate exercise and gradually progressing to vigorous-intensity exercise.
Second, in addition to walking the dog for 105 minutes a week, you:
- advise her to start walking for 10 minutes, 3 times per week, at a pace that keeps her HR at 97-104 bpm.
- encourage her to gradually increase the frequency or duration of her walks by no more than 10% per week.

SBIRT: Referral for treatment
When referring a patient to a fitness program or professional, it is essential to consider their preferences, resources, and environment.23 Community fitness partners are often an excellent referral option for a patient seeking guidance or structure for their exercise program. Using the ACSM ProFinder service, (www.acsm.org/get-stay-certified/find-a-pro) you can search for exercise professionals who have achieved the College’s Gold Standard credential.
Gym memberships or fitness programs might be part of the extra coverage offered by Medicare Advantage Plans, other Medicare health plans, or Medicare Supplement Insurance (Medigap) plans.24
Continue to: CASE
CASE
After providing Ms. Q with her exercise prescription, you refer her to a local gym that participates in the Silver Sneakers fitness and wellness program (for adults ≥ 65 years of age in eligible Medicare plans) to determine whether she qualifies to begin resistance and flexibility training, for which you will write a second exercise prescription (TABLE 317-19).

Pre-participation screening
Updated 2015 ACSM exercise pre-participation health screening recommendations attempt to decrease possible barriers to people who are becoming more physically active, by minimizing unnecessary referral to health care providers before they change their level of physical activity. ACSM recommendations on exercise clearance include this guidance25:
- For a patient who is asymptomatic and already physically active—regardless of whether they have known cardiovascular, metabolic, or renal disease—medical clearance is unnecessary for moderate-intensity exercise.
- Any patient who has been physically active and asymptomatic but who becomes symptomatic during exercise should immediately discontinue such activity and undergo medical evaluation.
- For a patient who is inactive, asymptomatic, and who does not have known cardiovascular, metabolic, or renal disease, medical clearance for light- or moderate-intensity exercise is unnecessary.
- For inactive, asymptomatic patients who have known cardiovascular, metabolic, or renal disease, medical clearance is recommended.
Digital health
Smartwatches and health apps (eg, CardioCoach, Fitbit, Garmin Connect, Nike Training Club, Strava, and Training Peaks) can provide workouts and offer patients the ability to collect information and even connect with other users through social media platforms. This information can be synced to Apple Health platforms for iPhones (www.apple.com/ios/health/) or through Google Fit (www.google.com/fit/) on Android devices. Primary care physicians who become familiar with health apps might find them useful for select patients who want to use technology to improve their physical activity level.
However, data on the value of using digital apps for increasing physical activity, in relation to their cost, are limited. Additional research is needed to assess their validity.
Billing and coding
For most patients, the physical activity assessment, prescription, and referral are performed in the context of treating another condition (eg, hypertension, type 2 diabetes, obesity, depression) or during a preventive health examination, and are typically covered without additional charge to the patient. An evaluation and management visit for an established patient could be used to bill if > 50% of the office visit was spent face-to-face with a physician, with patient counseling and coordination of care.
Continue to: Physicians and physical therapists...
Physicians and physical therapists can use the therapeutic exercise code (Current Procedural Terminology code 97110) when teaching patients exercises to develop muscle strength and endurance, joint range of motion, and flexibility26 (TABLE 426).

Conclusion
Physical activity and CRF are strong predictors of premature mortality, even compared to other risk factors, such as cigarette smoking, hypertension, hypercholesterolemia, and type 2 diabetes.27 Brief physical activity assessment and counseling is an efficient, effective, and cost-effective means to increase physical activity, and presents a unique opportunity for you to encourage lifestyle-based strategies for reducing cardiovascular risk.28
However, it is essential to meet patients where they are before trying to have them progress; it is therefore imperative to assess the individual patient’s level of activity using PAVS. With that information in hand, you can personalize physical activity advice; determine readiness for change and potential barriers for change; assist the patient in setting SMART goals; and arrange follow-up to assess adherence to the exercise prescription. Encourage the patient to call their health insurance plan to determine whether a gym membership or fitness program is covered.
Research is needed to evaluate the value of using digital apps, in light of their cost, to increase physical activity and improve CRF in a clinical setting. Prospective trials should be initiated to determine how routine implementation of CRF assessment in primary care alters the trajectory of clinical care. It is hoped that future research will answer the question: Would such an approach improve clinical outcomes and reduce health care expenditures?12
a Defined as O2 consumed while sitting at rest; equivalent to 3.5 mL of O2 × kg of body weight × min.
CORRESPONDENCE
Matthew Kampert, DO, MS, Sports Medicine, 5555 Transportation Boulevard, Cleveland, OH 44125; [email protected]
1. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024-1039. doi: 10.1001/jama.2018.1150
2. Tikkanen R, Abrams MK. U.S. health care from a global perspective, 2019: higher spending, worse outcomes? The Commonwealth Fund Website. January 30, 2020. Accessed November 16, 2021. www.commonwealthfund.org/publications/issue-briefs/2020/jan/us-health-care-global-perspective-2019
3. Stoutenberg M, Stasi S, Stamatakis E, et al. Physical activity training in US medical schools: preparing future physicians to engage in primary prevention. Phys Sportsmed. 2015;43:388-394. doi: 10.1080/00913847.2015.1084868
4. Antognoli EL, Seeholzer EL, Gullett H, et al. Primary care resident training for obesity, nutrition, and physical activity counseling: a mixed-methods study. Health Promot Pract. 2017;18:672-680. doi: 10.1177/1524839916658025
5. Asif IM, Drezner JA. Sports and exercise medicine education in the USA: call to action. Br J Sports Med. 2020;54:195-196. doi: 10.1136/bjsports-2019-101104
6. Douglas JA, Briones MD, Bauer EZ, et al. Social and environmental determinants of physical activity in urban parks: testing a neighborhood disorder model. Prev Med. 2018;109:119-124. doi: 10.1016/j.ypmed.2018.01.013
7. 2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: US Department of Health & Human Services; 2018. Accessed November 15, 2021. https://health.gov/sites/default/files/2019-09/PAG_Advisory_Committee_Report.pdf
8. Avis JL, Cave AL, Donaldson S, et al. Working with parents to prevent childhood obesity: protocol for a primary care-based ehealth study. JMIR Res Protoc. 2015;4:e35. doi:10.2196/resprot.4147
9. Ball TJ, Joy EA, Gren LH, et al. Concurrent validity of a self-reported physical activity ‘vital sign’ questionnaire with adult primary care patients. Prev Chronic Dis. 2016;13:e16. doi: 10.5888/pcd13.150228
10. Ball TJ, Joy EA, Gren LH, et al. Predictive validity of an adult physical activity “vital sign” recorded in electronic health records. J Phys Act Health. 2016;13:403-408. doi: 10.1123/jpah.2015-0210
11. Coleman KJ, Ngor E, Reynolds K, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc. 2012;44:2071-2076. doi: 10.1249/MSS.0b013e3182630ec1
12. Ross R, Blair SN, Arena R, et al; ; ; ; ; ; . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653-e699. doi: 10.1161/CIR.0000000000000461
13. de Souza de Silva CG, Kokkinos PP, Doom R, et al. Association between cardiorespiratory fitness, obesity, and health care costs: The Veterans Exercise Testing Study. Int J Obes (Lond). 2019;43:2225-2232. doi: 10.1038/s41366-018-0257-0
14. Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38-48. doi: 10.4278/0890-1171-12.1.38
15. Riebe D, Ehrman JK, Liguori G, et al. Methods for evoking change talk. In: ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Wolters Kluwer; 2018.
16. Grandes G, Sanchez A, Sanchez-Pinilla RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
17. McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: a review of concepts and evidence. Soc Sci Med. 2006;63:1011-1022. doi: 10.1016/j.socscimed.2006.03.012
18. Garber CE, Blissmer BE, Deschenes MR, et al; American College of Sports Medicine. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Position stand. Med Sci Sport Exerc. 2011;43:1334-1359. doi: 10.1249/MSS.0b013e318213fefb
19. Donnelly JE, Blair SN, Jakicic JM, et al; American College of Sports Medicine. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Position stand. Med Sci Sport Exerc. 2009;41:459-471. doi: 10.1249/MSS.0b013e3181949333
20. Fox SM 3rd, Naughton JP, Haskell WL. Physical activity and the prevention of coronary heart disease. Ann Clin Res. 1971;3:404-432.
21. Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307-315.
22. The Borg RPE scale. In: Borg G. Borg’s Perceived Exertion and Pain Scales. Human Kinetics; 1998:29-38.
23. Ratamess NA, Alvar BA, Evetoch TK, et al; American College of Sports Medicine. Progression models in resistance training for healthy adults. Position stand. Med Sci Sport Exerc. 2009;41:687-708. doi: 10.1249/MSS.0b013e3181915670
24. Gym memberships & fitness programs. Medicare.gov. Baltimore, MD: US Centers for Medicare and Medicaid Services. Accessed November 16, 2021. www.medicare.gov/coverage/gym-memberships-fitness-programs
25. Riebe D, Franklin BA, Thompson PD, et al. Updating ACSM’s recommendations for exercise preparticipation health screening. Med Sci Sports Exerc. 2015;47:2473-2479. doi: 10.1249/MSS.0000000000000664
26. Physical Activity Related Current Procedural Terminology (CPT®) Codes. Physical Activity Alliance website. Accessed November 16, 2021. https://paamovewithus.org/wp-content/uploads/2020/11/PAA-Physical-Activity-CPT-Codes-Nov-2020-AMA-Approved-Final-1.pdf
27. Blair SN. Physical inactivity: the biggest public health problem of the 21st century Br J Sports Med. 2009;43:1-2.
28. Vuori IM, Lavie CJ, Blair SN. Physical activity promotion in the health care system. Mayo Clin Proc. 2013;88:1446-1461. doi: 10.1016/j.mayocp.2013.08.020
1. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024-1039. doi: 10.1001/jama.2018.1150
2. Tikkanen R, Abrams MK. U.S. health care from a global perspective, 2019: higher spending, worse outcomes? The Commonwealth Fund Website. January 30, 2020. Accessed November 16, 2021. www.commonwealthfund.org/publications/issue-briefs/2020/jan/us-health-care-global-perspective-2019
3. Stoutenberg M, Stasi S, Stamatakis E, et al. Physical activity training in US medical schools: preparing future physicians to engage in primary prevention. Phys Sportsmed. 2015;43:388-394. doi: 10.1080/00913847.2015.1084868
4. Antognoli EL, Seeholzer EL, Gullett H, et al. Primary care resident training for obesity, nutrition, and physical activity counseling: a mixed-methods study. Health Promot Pract. 2017;18:672-680. doi: 10.1177/1524839916658025
5. Asif IM, Drezner JA. Sports and exercise medicine education in the USA: call to action. Br J Sports Med. 2020;54:195-196. doi: 10.1136/bjsports-2019-101104
6. Douglas JA, Briones MD, Bauer EZ, et al. Social and environmental determinants of physical activity in urban parks: testing a neighborhood disorder model. Prev Med. 2018;109:119-124. doi: 10.1016/j.ypmed.2018.01.013
7. 2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: US Department of Health & Human Services; 2018. Accessed November 15, 2021. https://health.gov/sites/default/files/2019-09/PAG_Advisory_Committee_Report.pdf
8. Avis JL, Cave AL, Donaldson S, et al. Working with parents to prevent childhood obesity: protocol for a primary care-based ehealth study. JMIR Res Protoc. 2015;4:e35. doi:10.2196/resprot.4147
9. Ball TJ, Joy EA, Gren LH, et al. Concurrent validity of a self-reported physical activity ‘vital sign’ questionnaire with adult primary care patients. Prev Chronic Dis. 2016;13:e16. doi: 10.5888/pcd13.150228
10. Ball TJ, Joy EA, Gren LH, et al. Predictive validity of an adult physical activity “vital sign” recorded in electronic health records. J Phys Act Health. 2016;13:403-408. doi: 10.1123/jpah.2015-0210
11. Coleman KJ, Ngor E, Reynolds K, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc. 2012;44:2071-2076. doi: 10.1249/MSS.0b013e3182630ec1
12. Ross R, Blair SN, Arena R, et al; ; ; ; ; ; . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653-e699. doi: 10.1161/CIR.0000000000000461
13. de Souza de Silva CG, Kokkinos PP, Doom R, et al. Association between cardiorespiratory fitness, obesity, and health care costs: The Veterans Exercise Testing Study. Int J Obes (Lond). 2019;43:2225-2232. doi: 10.1038/s41366-018-0257-0
14. Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38-48. doi: 10.4278/0890-1171-12.1.38
15. Riebe D, Ehrman JK, Liguori G, et al. Methods for evoking change talk. In: ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Wolters Kluwer; 2018.
16. Grandes G, Sanchez A, Sanchez-Pinilla RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
17. McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: a review of concepts and evidence. Soc Sci Med. 2006;63:1011-1022. doi: 10.1016/j.socscimed.2006.03.012
18. Garber CE, Blissmer BE, Deschenes MR, et al; American College of Sports Medicine. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Position stand. Med Sci Sport Exerc. 2011;43:1334-1359. doi: 10.1249/MSS.0b013e318213fefb
19. Donnelly JE, Blair SN, Jakicic JM, et al; American College of Sports Medicine. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Position stand. Med Sci Sport Exerc. 2009;41:459-471. doi: 10.1249/MSS.0b013e3181949333
20. Fox SM 3rd, Naughton JP, Haskell WL. Physical activity and the prevention of coronary heart disease. Ann Clin Res. 1971;3:404-432.
21. Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307-315.
22. The Borg RPE scale. In: Borg G. Borg’s Perceived Exertion and Pain Scales. Human Kinetics; 1998:29-38.
23. Ratamess NA, Alvar BA, Evetoch TK, et al; American College of Sports Medicine. Progression models in resistance training for healthy adults. Position stand. Med Sci Sport Exerc. 2009;41:687-708. doi: 10.1249/MSS.0b013e3181915670
24. Gym memberships & fitness programs. Medicare.gov. Baltimore, MD: US Centers for Medicare and Medicaid Services. Accessed November 16, 2021. www.medicare.gov/coverage/gym-memberships-fitness-programs
25. Riebe D, Franklin BA, Thompson PD, et al. Updating ACSM’s recommendations for exercise preparticipation health screening. Med Sci Sports Exerc. 2015;47:2473-2479. doi: 10.1249/MSS.0000000000000664
26. Physical Activity Related Current Procedural Terminology (CPT®) Codes. Physical Activity Alliance website. Accessed November 16, 2021. https://paamovewithus.org/wp-content/uploads/2020/11/PAA-Physical-Activity-CPT-Codes-Nov-2020-AMA-Approved-Final-1.pdf
27. Blair SN. Physical inactivity: the biggest public health problem of the 21st century Br J Sports Med. 2009;43:1-2.
28. Vuori IM, Lavie CJ, Blair SN. Physical activity promotion in the health care system. Mayo Clin Proc. 2013;88:1446-1461. doi: 10.1016/j.mayocp.2013.08.020
PRACTICE RECOMMENDATIONS
› Encourage children and adolescents (6 to 17 years of age) to engage in 60 min of moderate-to-vigorous physical activity, including aerobic, muscle-strengthening, and bone-strengthening endeavors on most, if not all, days of the week. A
› Encourage adults to perform approximately 150 to 300 min of moderate or 75 to 150 min of vigorous physical activity (or an equivalent combination) per week, along with moderate-intensity muscle-strengthening activities on ≥ 2 days per week. A
› Counsel patients that even a small (eg, 1-2 metabolic equivalents) increase in cardiorespiratory fitness is associated with a 10% to 30% lower rate of adverse events. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Could Viagra help prevent Alzheimer’s?
published in the journal Nature Aging.
Patients who used the drug sildenafil, the generic name for Viagra, were 69% less likely to develop the disease than were nonusers.
“Sildenafil, which has been shown to significantly improve cognition and memory in preclinical models, presented as the best drug candidate,” Feixiong Cheng, PhD, the lead study author in the Cleveland Clinic’s Genomic Medicine Institute, said in a statement.
“Notably, we found that sildenafil use reduced the likelihood of Alzheimer’s in individuals with coronary artery disease, hypertension, and type 2 diabetes, all of which are comorbidities significantly associated with risk of the disease, as well as in those without,” he said.
Alzheimer’s, which is the most common form of age-related dementia, affects hundreds of millions of people worldwide. The disease is expected to affect nearly 14 million Americans by 2050. There is no approved treatment for it.
Dr. Cheng and colleagues at the Cleveland Clinic used a large gene-mapping network to analyze whether more than 1,600 Food and Drug Administration–approved drugs could work against Alzheimer’s. They gave higher scores to drugs that target both amyloid and tau proteins in the brain, which are two hallmarks of the disease. Sildenafil appeared at the top of the list.
Then the researchers used a database of health insurance claims for more than 7 million people in the U.S. to understand the relationship between sildenafil and Alzheimer’s disease outcomes. They compared sildenafil users to nonusers and found that those who used the drug were 69% less likely to have the neurodegenerative disease, even after 6 years of follow-up.
After that, the research team came up with a lab model that showed the sildenafil increased brain cell growth and targeted tau proteins. The lab model could indicate how the drug influences disease-related brain changes.
But Dr. Cheng cautioned against drawing strong conclusions. The study doesn’t demonstrate a causal relationship between sildenafil and Alzheimer’s disease. Researchers will need to conduct clinical trials with a placebo control to see how well the drug works.
Other researchers said the findings offer a new avenue for research but don’t yet provide solid answers.
“Being able to repurpose a drug already licensed for health conditions could help speed up the drug discovery process and bring about life-changing dementia treatments sooner,” Susan Kohlhaas, PhD, director of research at Alzheimer’s Research UK, told the Science Media Centre.
“Importantly, this research doesn’t prove that sildenafil is responsible for reducing dementia risk, or that it slows or stops the disease,” she continued. “If you want to discuss any treatments you are receiving, the first port of call is to speak to your doctor.”
And doctors won’t likely recommend it as a treatment just yet either.
“While these data are interesting scientifically, based on this study, I would not rush out to start taking sildenafil as a prevention for Alzheimer’s disease,” Tara Spires-Jones, PhD, deputy director of the Centre for Discovery Brain Sciences at the University of Edinburgh, told the Science Media Centre.
A version of this article first appeared on WebMD.com.
published in the journal Nature Aging.
Patients who used the drug sildenafil, the generic name for Viagra, were 69% less likely to develop the disease than were nonusers.
“Sildenafil, which has been shown to significantly improve cognition and memory in preclinical models, presented as the best drug candidate,” Feixiong Cheng, PhD, the lead study author in the Cleveland Clinic’s Genomic Medicine Institute, said in a statement.
“Notably, we found that sildenafil use reduced the likelihood of Alzheimer’s in individuals with coronary artery disease, hypertension, and type 2 diabetes, all of which are comorbidities significantly associated with risk of the disease, as well as in those without,” he said.
Alzheimer’s, which is the most common form of age-related dementia, affects hundreds of millions of people worldwide. The disease is expected to affect nearly 14 million Americans by 2050. There is no approved treatment for it.
Dr. Cheng and colleagues at the Cleveland Clinic used a large gene-mapping network to analyze whether more than 1,600 Food and Drug Administration–approved drugs could work against Alzheimer’s. They gave higher scores to drugs that target both amyloid and tau proteins in the brain, which are two hallmarks of the disease. Sildenafil appeared at the top of the list.
Then the researchers used a database of health insurance claims for more than 7 million people in the U.S. to understand the relationship between sildenafil and Alzheimer’s disease outcomes. They compared sildenafil users to nonusers and found that those who used the drug were 69% less likely to have the neurodegenerative disease, even after 6 years of follow-up.
After that, the research team came up with a lab model that showed the sildenafil increased brain cell growth and targeted tau proteins. The lab model could indicate how the drug influences disease-related brain changes.
But Dr. Cheng cautioned against drawing strong conclusions. The study doesn’t demonstrate a causal relationship between sildenafil and Alzheimer’s disease. Researchers will need to conduct clinical trials with a placebo control to see how well the drug works.
Other researchers said the findings offer a new avenue for research but don’t yet provide solid answers.
“Being able to repurpose a drug already licensed for health conditions could help speed up the drug discovery process and bring about life-changing dementia treatments sooner,” Susan Kohlhaas, PhD, director of research at Alzheimer’s Research UK, told the Science Media Centre.
“Importantly, this research doesn’t prove that sildenafil is responsible for reducing dementia risk, or that it slows or stops the disease,” she continued. “If you want to discuss any treatments you are receiving, the first port of call is to speak to your doctor.”
And doctors won’t likely recommend it as a treatment just yet either.
“While these data are interesting scientifically, based on this study, I would not rush out to start taking sildenafil as a prevention for Alzheimer’s disease,” Tara Spires-Jones, PhD, deputy director of the Centre for Discovery Brain Sciences at the University of Edinburgh, told the Science Media Centre.
A version of this article first appeared on WebMD.com.
published in the journal Nature Aging.
Patients who used the drug sildenafil, the generic name for Viagra, were 69% less likely to develop the disease than were nonusers.
“Sildenafil, which has been shown to significantly improve cognition and memory in preclinical models, presented as the best drug candidate,” Feixiong Cheng, PhD, the lead study author in the Cleveland Clinic’s Genomic Medicine Institute, said in a statement.
“Notably, we found that sildenafil use reduced the likelihood of Alzheimer’s in individuals with coronary artery disease, hypertension, and type 2 diabetes, all of which are comorbidities significantly associated with risk of the disease, as well as in those without,” he said.
Alzheimer’s, which is the most common form of age-related dementia, affects hundreds of millions of people worldwide. The disease is expected to affect nearly 14 million Americans by 2050. There is no approved treatment for it.
Dr. Cheng and colleagues at the Cleveland Clinic used a large gene-mapping network to analyze whether more than 1,600 Food and Drug Administration–approved drugs could work against Alzheimer’s. They gave higher scores to drugs that target both amyloid and tau proteins in the brain, which are two hallmarks of the disease. Sildenafil appeared at the top of the list.
Then the researchers used a database of health insurance claims for more than 7 million people in the U.S. to understand the relationship between sildenafil and Alzheimer’s disease outcomes. They compared sildenafil users to nonusers and found that those who used the drug were 69% less likely to have the neurodegenerative disease, even after 6 years of follow-up.
After that, the research team came up with a lab model that showed the sildenafil increased brain cell growth and targeted tau proteins. The lab model could indicate how the drug influences disease-related brain changes.
But Dr. Cheng cautioned against drawing strong conclusions. The study doesn’t demonstrate a causal relationship between sildenafil and Alzheimer’s disease. Researchers will need to conduct clinical trials with a placebo control to see how well the drug works.
Other researchers said the findings offer a new avenue for research but don’t yet provide solid answers.
“Being able to repurpose a drug already licensed for health conditions could help speed up the drug discovery process and bring about life-changing dementia treatments sooner,” Susan Kohlhaas, PhD, director of research at Alzheimer’s Research UK, told the Science Media Centre.
“Importantly, this research doesn’t prove that sildenafil is responsible for reducing dementia risk, or that it slows or stops the disease,” she continued. “If you want to discuss any treatments you are receiving, the first port of call is to speak to your doctor.”
And doctors won’t likely recommend it as a treatment just yet either.
“While these data are interesting scientifically, based on this study, I would not rush out to start taking sildenafil as a prevention for Alzheimer’s disease,” Tara Spires-Jones, PhD, deputy director of the Centre for Discovery Brain Sciences at the University of Edinburgh, told the Science Media Centre.
A version of this article first appeared on WebMD.com.
FROM NATURE AGING