User login
Is mild cognitive impairment reversible?
new research shows.
The investigators found individuals with these factors, which are all markers of cognitive reserve, had a significantly greater chance of reversion from MCI to normal cognition (NC) than progression from MCI to dementia.
In a cohort study of more than 600 women aged 75 years or older, about a third of those with MCI reverted to NC at some point during follow-up, which sends “an encouraging message,” study author Suzanne Tyas, PhD, associate professor, University of Waterloo (Ont.), said in an interview.
“That’s a positive thing for people to keep in mind when they’re thinking about prognosis. Some of these novel characteristics we’ve identified might be useful in thinking about how likely a particular patient might be to improve versus decline cognitively,” Dr. Tyas added.
The findings were published online Feb. 4, 2022, in the journal Neurology.
Highly educated cohort
As the population ages, the number of individuals experiencing age-related conditions, including dementia, increases. There is no cure for most dementia types so prevention is key – and preventing dementia requires understanding its risk factors, Dr. Tyas noted.
The analysis included participants from the Nun Study, a longitudinal study of aging and cognition among members of the School Sisters of Notre Dame in the United States. All were 75 and older at baseline, which was from 1991 to 1993; about 14.5% were older than 90 years.
Participants were generally highly educated, with 84.5% attaining an undergraduate or graduate degree. They also had a similar socioeconomic status, level of social supports, marital and reproductive history, and alcohol and tobacco use.
Researchers examined cognitive function at baseline and then about annually until death or end of the 12th round of assessments. They used five measures from the Consortium to Establish a Registry for Alzheimer’s Disease neuropsychological battery to categorize subjects into NC, MCI, or dementia: Delayed Word Recall, Verbal Fluency, Boston Naming, Constructional Praxis, and the Mini-Mental State Exam.
The current analysis focused on the 619 participants with data on apolipoprotein E (apo E) epsilon-4 genotyping and education. From convent archives, investigators also had access to the nuns’ early high school academic performance in English, Latin, algebra, and geometry.
“Typically we only have data for [overall] education. But I know from teaching that there’s a difference between people who just pass my courses and graduate with a university degree versus those who really excel,” Dr. Tyas said.
The researchers also assessed handwriting samples from before the participants entered the religious order. From these, they scored “idea density,” which is the number of ideas contained in the writing and “grammatical complexity,” which includes structure, use of clauses, subclauses, and so on.
Dementia not inevitable
Results showed 472 of the 619 participants had MCI during the study period. About 30.3% of these showed at least one reverse transition from MCI to NC during a mean follow-up of 8.6 years; 83.9% went on to develop dementia.
This shows converting from MCI to NC occurs relatively frequently, Dr. Tyas noted.
“This is encouraging because some people think that if they have a diagnosis of MCI they are inevitably going to decline to dementia,” she added.
The researchers also used complicated modeling of transition rates over time between NC, MCI, and dementia and adjusted for participants who died. They estimated relative risk of reversion versus progression for age, apo E, and potential cognitive reserve indicators.
Not surprisingly, younger age (90 years or less) and absence of apo E epsilon-4 allele contributed to a significantly higher rate for reversion from MCI to NC versus progression from MCI to dementia.
However, although age and apo E are known risk factors for dementia, these have not been examined in the context of whether individuals with MCI are more likely to improve or decline, said Dr. Tyas.
Higher educational attainment, the traditional indicator of cognitive reserve, was associated with a significantly higher relative risk for reversion from MCI to NC versus progression from MCI to dementia (RR, 2.6) for a bachelor’s degree versus less education.
There was a greater RR for even higher education after adjusting for age and apo E epsilon-4 status.
Language skills key
Interestingly, the investigators also found a significant association with good grades in high school English but not other subjects (RR for higher vs. lower English grades, 1.83; 95% confidence interval, 1.07-3.14).
In addition, they found both characteristics of written language skills (idea density and grammatical complexity) were significant predictors of conversion to NC.
“Those with high levels of idea density were four times more likely to improve to normal cognition than progress to dementia, and the effect was even stronger for grammatical structure. Those individuals with higher levels were almost six times more likely to improve than decline,” Dr. Tyas reported.
The RR for higher versus lower idea density was 3.93 (95% CI, 1.3-11.9) and the RR for higher versus lower grammatical complexity was 5.78 (95% CI, 1.56-21.42).
These new results could be useful when planning future clinical trials, Dr. Tyas noted. “MCI in some people is going to improve even without any treatment, and this should be taken into consideration when recruiting participants to a study and when interpreting the results.
“You don’t want something to look like it’s a benefit of the treatment when in fact these individuals would have just reverted on their own,” she added.
Research implications
Commenting on the findings, Claire Sexton, DPhil, director of scientific programs and outreach at the Alzheimer’s Association, noted that, in “this study of highly educated, older women,” transitions from MCI to NC “were about equally common” as transitions from MCI to dementia.
“As advances are made in early detection of dementia, and treatments are developed and marketed for people living with MCI, this article’s findings are important to inform discussions of prognosis with patients and [to the] design of clinical trials,” Dr. Sexton said.
The study was funded by the Canadian Institutes of Health Research and the Natural Sciences and Engineering Research Council of Canada. Funding for the Nun Study at the University of Kentucky was provided by the U.S. National Institute of Aging and the Kleberg Foundation. Dr. Tyas has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research shows.
The investigators found individuals with these factors, which are all markers of cognitive reserve, had a significantly greater chance of reversion from MCI to normal cognition (NC) than progression from MCI to dementia.
In a cohort study of more than 600 women aged 75 years or older, about a third of those with MCI reverted to NC at some point during follow-up, which sends “an encouraging message,” study author Suzanne Tyas, PhD, associate professor, University of Waterloo (Ont.), said in an interview.
“That’s a positive thing for people to keep in mind when they’re thinking about prognosis. Some of these novel characteristics we’ve identified might be useful in thinking about how likely a particular patient might be to improve versus decline cognitively,” Dr. Tyas added.
The findings were published online Feb. 4, 2022, in the journal Neurology.
Highly educated cohort
As the population ages, the number of individuals experiencing age-related conditions, including dementia, increases. There is no cure for most dementia types so prevention is key – and preventing dementia requires understanding its risk factors, Dr. Tyas noted.
The analysis included participants from the Nun Study, a longitudinal study of aging and cognition among members of the School Sisters of Notre Dame in the United States. All were 75 and older at baseline, which was from 1991 to 1993; about 14.5% were older than 90 years.
Participants were generally highly educated, with 84.5% attaining an undergraduate or graduate degree. They also had a similar socioeconomic status, level of social supports, marital and reproductive history, and alcohol and tobacco use.
Researchers examined cognitive function at baseline and then about annually until death or end of the 12th round of assessments. They used five measures from the Consortium to Establish a Registry for Alzheimer’s Disease neuropsychological battery to categorize subjects into NC, MCI, or dementia: Delayed Word Recall, Verbal Fluency, Boston Naming, Constructional Praxis, and the Mini-Mental State Exam.
The current analysis focused on the 619 participants with data on apolipoprotein E (apo E) epsilon-4 genotyping and education. From convent archives, investigators also had access to the nuns’ early high school academic performance in English, Latin, algebra, and geometry.
“Typically we only have data for [overall] education. But I know from teaching that there’s a difference between people who just pass my courses and graduate with a university degree versus those who really excel,” Dr. Tyas said.
The researchers also assessed handwriting samples from before the participants entered the religious order. From these, they scored “idea density,” which is the number of ideas contained in the writing and “grammatical complexity,” which includes structure, use of clauses, subclauses, and so on.
Dementia not inevitable
Results showed 472 of the 619 participants had MCI during the study period. About 30.3% of these showed at least one reverse transition from MCI to NC during a mean follow-up of 8.6 years; 83.9% went on to develop dementia.
This shows converting from MCI to NC occurs relatively frequently, Dr. Tyas noted.
“This is encouraging because some people think that if they have a diagnosis of MCI they are inevitably going to decline to dementia,” she added.
The researchers also used complicated modeling of transition rates over time between NC, MCI, and dementia and adjusted for participants who died. They estimated relative risk of reversion versus progression for age, apo E, and potential cognitive reserve indicators.
Not surprisingly, younger age (90 years or less) and absence of apo E epsilon-4 allele contributed to a significantly higher rate for reversion from MCI to NC versus progression from MCI to dementia.
However, although age and apo E are known risk factors for dementia, these have not been examined in the context of whether individuals with MCI are more likely to improve or decline, said Dr. Tyas.
Higher educational attainment, the traditional indicator of cognitive reserve, was associated with a significantly higher relative risk for reversion from MCI to NC versus progression from MCI to dementia (RR, 2.6) for a bachelor’s degree versus less education.
There was a greater RR for even higher education after adjusting for age and apo E epsilon-4 status.
Language skills key
Interestingly, the investigators also found a significant association with good grades in high school English but not other subjects (RR for higher vs. lower English grades, 1.83; 95% confidence interval, 1.07-3.14).
In addition, they found both characteristics of written language skills (idea density and grammatical complexity) were significant predictors of conversion to NC.
“Those with high levels of idea density were four times more likely to improve to normal cognition than progress to dementia, and the effect was even stronger for grammatical structure. Those individuals with higher levels were almost six times more likely to improve than decline,” Dr. Tyas reported.
The RR for higher versus lower idea density was 3.93 (95% CI, 1.3-11.9) and the RR for higher versus lower grammatical complexity was 5.78 (95% CI, 1.56-21.42).
These new results could be useful when planning future clinical trials, Dr. Tyas noted. “MCI in some people is going to improve even without any treatment, and this should be taken into consideration when recruiting participants to a study and when interpreting the results.
“You don’t want something to look like it’s a benefit of the treatment when in fact these individuals would have just reverted on their own,” she added.
Research implications
Commenting on the findings, Claire Sexton, DPhil, director of scientific programs and outreach at the Alzheimer’s Association, noted that, in “this study of highly educated, older women,” transitions from MCI to NC “were about equally common” as transitions from MCI to dementia.
“As advances are made in early detection of dementia, and treatments are developed and marketed for people living with MCI, this article’s findings are important to inform discussions of prognosis with patients and [to the] design of clinical trials,” Dr. Sexton said.
The study was funded by the Canadian Institutes of Health Research and the Natural Sciences and Engineering Research Council of Canada. Funding for the Nun Study at the University of Kentucky was provided by the U.S. National Institute of Aging and the Kleberg Foundation. Dr. Tyas has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research shows.
The investigators found individuals with these factors, which are all markers of cognitive reserve, had a significantly greater chance of reversion from MCI to normal cognition (NC) than progression from MCI to dementia.
In a cohort study of more than 600 women aged 75 years or older, about a third of those with MCI reverted to NC at some point during follow-up, which sends “an encouraging message,” study author Suzanne Tyas, PhD, associate professor, University of Waterloo (Ont.), said in an interview.
“That’s a positive thing for people to keep in mind when they’re thinking about prognosis. Some of these novel characteristics we’ve identified might be useful in thinking about how likely a particular patient might be to improve versus decline cognitively,” Dr. Tyas added.
The findings were published online Feb. 4, 2022, in the journal Neurology.
Highly educated cohort
As the population ages, the number of individuals experiencing age-related conditions, including dementia, increases. There is no cure for most dementia types so prevention is key – and preventing dementia requires understanding its risk factors, Dr. Tyas noted.
The analysis included participants from the Nun Study, a longitudinal study of aging and cognition among members of the School Sisters of Notre Dame in the United States. All were 75 and older at baseline, which was from 1991 to 1993; about 14.5% were older than 90 years.
Participants were generally highly educated, with 84.5% attaining an undergraduate or graduate degree. They also had a similar socioeconomic status, level of social supports, marital and reproductive history, and alcohol and tobacco use.
Researchers examined cognitive function at baseline and then about annually until death or end of the 12th round of assessments. They used five measures from the Consortium to Establish a Registry for Alzheimer’s Disease neuropsychological battery to categorize subjects into NC, MCI, or dementia: Delayed Word Recall, Verbal Fluency, Boston Naming, Constructional Praxis, and the Mini-Mental State Exam.
The current analysis focused on the 619 participants with data on apolipoprotein E (apo E) epsilon-4 genotyping and education. From convent archives, investigators also had access to the nuns’ early high school academic performance in English, Latin, algebra, and geometry.
“Typically we only have data for [overall] education. But I know from teaching that there’s a difference between people who just pass my courses and graduate with a university degree versus those who really excel,” Dr. Tyas said.
The researchers also assessed handwriting samples from before the participants entered the religious order. From these, they scored “idea density,” which is the number of ideas contained in the writing and “grammatical complexity,” which includes structure, use of clauses, subclauses, and so on.
Dementia not inevitable
Results showed 472 of the 619 participants had MCI during the study period. About 30.3% of these showed at least one reverse transition from MCI to NC during a mean follow-up of 8.6 years; 83.9% went on to develop dementia.
This shows converting from MCI to NC occurs relatively frequently, Dr. Tyas noted.
“This is encouraging because some people think that if they have a diagnosis of MCI they are inevitably going to decline to dementia,” she added.
The researchers also used complicated modeling of transition rates over time between NC, MCI, and dementia and adjusted for participants who died. They estimated relative risk of reversion versus progression for age, apo E, and potential cognitive reserve indicators.
Not surprisingly, younger age (90 years or less) and absence of apo E epsilon-4 allele contributed to a significantly higher rate for reversion from MCI to NC versus progression from MCI to dementia.
However, although age and apo E are known risk factors for dementia, these have not been examined in the context of whether individuals with MCI are more likely to improve or decline, said Dr. Tyas.
Higher educational attainment, the traditional indicator of cognitive reserve, was associated with a significantly higher relative risk for reversion from MCI to NC versus progression from MCI to dementia (RR, 2.6) for a bachelor’s degree versus less education.
There was a greater RR for even higher education after adjusting for age and apo E epsilon-4 status.
Language skills key
Interestingly, the investigators also found a significant association with good grades in high school English but not other subjects (RR for higher vs. lower English grades, 1.83; 95% confidence interval, 1.07-3.14).
In addition, they found both characteristics of written language skills (idea density and grammatical complexity) were significant predictors of conversion to NC.
“Those with high levels of idea density were four times more likely to improve to normal cognition than progress to dementia, and the effect was even stronger for grammatical structure. Those individuals with higher levels were almost six times more likely to improve than decline,” Dr. Tyas reported.
The RR for higher versus lower idea density was 3.93 (95% CI, 1.3-11.9) and the RR for higher versus lower grammatical complexity was 5.78 (95% CI, 1.56-21.42).
These new results could be useful when planning future clinical trials, Dr. Tyas noted. “MCI in some people is going to improve even without any treatment, and this should be taken into consideration when recruiting participants to a study and when interpreting the results.
“You don’t want something to look like it’s a benefit of the treatment when in fact these individuals would have just reverted on their own,” she added.
Research implications
Commenting on the findings, Claire Sexton, DPhil, director of scientific programs and outreach at the Alzheimer’s Association, noted that, in “this study of highly educated, older women,” transitions from MCI to NC “were about equally common” as transitions from MCI to dementia.
“As advances are made in early detection of dementia, and treatments are developed and marketed for people living with MCI, this article’s findings are important to inform discussions of prognosis with patients and [to the] design of clinical trials,” Dr. Sexton said.
The study was funded by the Canadian Institutes of Health Research and the Natural Sciences and Engineering Research Council of Canada. Funding for the Nun Study at the University of Kentucky was provided by the U.S. National Institute of Aging and the Kleberg Foundation. Dr. Tyas has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Seniors face higher risk of other medical conditions after COVID-19
The findings of the observational study, which were published in the BMJ, show the risk of a new condition being triggered by COVID is more than twice as high in seniors, compared with younger patients. Plus, the researchers observed an even higher risk among those who were hospitalized, with nearly half (46%) of patients having developed new conditions after the acute COVID-19 infection period.
Respiratory failure with shortness of breath was the most common postacute sequela, but a wide range of heart, kidney, lung, liver, cognitive, mental health, and other conditions were diagnosed at least 3 weeks after initial infection and persisted beyond 30 days.
This is one of the first studies to specifically describe the incidence and severity of new conditions triggered by COVID-19 infection in a general sample of older adults, said study author Ken Cohen MD, FACP, executive director of translational research at Optum Labs and national senior medical director at Optum Care.
“Much of what has been published on the postacute sequelae of COVID-19 has been predominantly from a younger population, and many of the patients had been hospitalized,” Dr. Cohen noted. “This was the first study to focus on a large population of seniors, most of whom did not require hospitalization.”
Dr. Cohen and colleagues reviewed the health insurance records of more than 133,000 Medicare beneficiaries aged 65 or older who were diagnosed with COVID-19 before April 2020. They also matched individuals by age, race, sex, hospitalization status, and other factors to comparison groups without COVID-19 (one from 2020 and one from 2019), and to a group diagnosed with other lower respiratory tract viral infections before the pandemic.
Risk of developing new conditions was higher in hospitalized
After acute COVID-19 infection, 32% of seniors sought medical care for at least one new medical condition in 2020, compared with 21% of uninfected people in the same year.
The most commonly observed conditions included:
- Respiratory failure (7.55% higher risk).
- Fatigue (5.66% higher risk).
- High blood pressure (4.43% higher risk).
- Memory problems (2.63% higher risk).
- Kidney injury (2.59% higher risk).
- Mental health diagnoses (2.5% higher risk).
- Blood-clotting disorders (1.47 % higher risk).
- Heart rhythm disorders (2.9% higher risk).
The risk of developing new conditions was even higher among those 23,486 who were hospitalized in 2020. Those individuals showed a 23.6% higher risk for developing at least one new condition, compared with uninfected seniors in the same year. Also, patients older than 75 had a higher risk for neurological disorders, including dementia, encephalopathy, and memory problems. The researchers also found that respiratory failure and kidney injury were significantly more likely to affect men and Black patients.
When those who had COVID were compared with the group with other lower respiratory viral infections before the pandemic, only the risks of respiratory failure (2.39% higher), dementia (0.71% higher), and fatigue (0.18% higher) were higher.
Primary care providers can learn from these data to better evaluate and manage their geriatric patients with COVID-19 infection, said Amit Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, in an interview.
“We must assess older patients who have had COVID-19 for more than just improvement from the respiratory symptoms of COVID-19 in post-COVID follow-up visits,” he said. “Older individuals with frailty have vulnerability to subsequent complications from severe illnesses and it is common to see post-illness diagnoses, such as new diagnosis of delirium; dementia; or renal, respiratory, or cardiac issues that is precipitated by the original illness. This study confirms that this is likely the case with COVID-19 as well.
“Primary care physicians should be vigilant for these complications, including attention to the rehabilitation needs of older patients with longer-term postviral fatigue from COVID-19,” Dr. Shah added.
Data predates ‘Omicron wave’
It remains uncertain whether sequelae will differ with the Omicron variant, but the findings remain applicable, Dr. Cohen said.
“We know that illness from the Omicron variant is on average less severe in those that have been vaccinated. However, throughout the Omicron wave, individuals who have not been vaccinated continue to have significant rates of serious illness and hospitalization,” he said.
“Our findings showed that serious illness with hospitalization was associated with a higher rate of sequelae. It can therefore be inferred that the rates of sequelae seen in our study would continue to occur in unvaccinated individuals who contract Omicron, but might occur less frequently in vaccinated individuals who contract Omicron and have less severe illness.”
Dr. Cohen serves as a consultant for Pfizer. Dr. Shah has disclosed no relevant financial relationships.
The findings of the observational study, which were published in the BMJ, show the risk of a new condition being triggered by COVID is more than twice as high in seniors, compared with younger patients. Plus, the researchers observed an even higher risk among those who were hospitalized, with nearly half (46%) of patients having developed new conditions after the acute COVID-19 infection period.
Respiratory failure with shortness of breath was the most common postacute sequela, but a wide range of heart, kidney, lung, liver, cognitive, mental health, and other conditions were diagnosed at least 3 weeks after initial infection and persisted beyond 30 days.
This is one of the first studies to specifically describe the incidence and severity of new conditions triggered by COVID-19 infection in a general sample of older adults, said study author Ken Cohen MD, FACP, executive director of translational research at Optum Labs and national senior medical director at Optum Care.
“Much of what has been published on the postacute sequelae of COVID-19 has been predominantly from a younger population, and many of the patients had been hospitalized,” Dr. Cohen noted. “This was the first study to focus on a large population of seniors, most of whom did not require hospitalization.”
Dr. Cohen and colleagues reviewed the health insurance records of more than 133,000 Medicare beneficiaries aged 65 or older who were diagnosed with COVID-19 before April 2020. They also matched individuals by age, race, sex, hospitalization status, and other factors to comparison groups without COVID-19 (one from 2020 and one from 2019), and to a group diagnosed with other lower respiratory tract viral infections before the pandemic.
Risk of developing new conditions was higher in hospitalized
After acute COVID-19 infection, 32% of seniors sought medical care for at least one new medical condition in 2020, compared with 21% of uninfected people in the same year.
The most commonly observed conditions included:
- Respiratory failure (7.55% higher risk).
- Fatigue (5.66% higher risk).
- High blood pressure (4.43% higher risk).
- Memory problems (2.63% higher risk).
- Kidney injury (2.59% higher risk).
- Mental health diagnoses (2.5% higher risk).
- Blood-clotting disorders (1.47 % higher risk).
- Heart rhythm disorders (2.9% higher risk).
The risk of developing new conditions was even higher among those 23,486 who were hospitalized in 2020. Those individuals showed a 23.6% higher risk for developing at least one new condition, compared with uninfected seniors in the same year. Also, patients older than 75 had a higher risk for neurological disorders, including dementia, encephalopathy, and memory problems. The researchers also found that respiratory failure and kidney injury were significantly more likely to affect men and Black patients.
When those who had COVID were compared with the group with other lower respiratory viral infections before the pandemic, only the risks of respiratory failure (2.39% higher), dementia (0.71% higher), and fatigue (0.18% higher) were higher.
Primary care providers can learn from these data to better evaluate and manage their geriatric patients with COVID-19 infection, said Amit Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, in an interview.
“We must assess older patients who have had COVID-19 for more than just improvement from the respiratory symptoms of COVID-19 in post-COVID follow-up visits,” he said. “Older individuals with frailty have vulnerability to subsequent complications from severe illnesses and it is common to see post-illness diagnoses, such as new diagnosis of delirium; dementia; or renal, respiratory, or cardiac issues that is precipitated by the original illness. This study confirms that this is likely the case with COVID-19 as well.
“Primary care physicians should be vigilant for these complications, including attention to the rehabilitation needs of older patients with longer-term postviral fatigue from COVID-19,” Dr. Shah added.
Data predates ‘Omicron wave’
It remains uncertain whether sequelae will differ with the Omicron variant, but the findings remain applicable, Dr. Cohen said.
“We know that illness from the Omicron variant is on average less severe in those that have been vaccinated. However, throughout the Omicron wave, individuals who have not been vaccinated continue to have significant rates of serious illness and hospitalization,” he said.
“Our findings showed that serious illness with hospitalization was associated with a higher rate of sequelae. It can therefore be inferred that the rates of sequelae seen in our study would continue to occur in unvaccinated individuals who contract Omicron, but might occur less frequently in vaccinated individuals who contract Omicron and have less severe illness.”
Dr. Cohen serves as a consultant for Pfizer. Dr. Shah has disclosed no relevant financial relationships.
The findings of the observational study, which were published in the BMJ, show the risk of a new condition being triggered by COVID is more than twice as high in seniors, compared with younger patients. Plus, the researchers observed an even higher risk among those who were hospitalized, with nearly half (46%) of patients having developed new conditions after the acute COVID-19 infection period.
Respiratory failure with shortness of breath was the most common postacute sequela, but a wide range of heart, kidney, lung, liver, cognitive, mental health, and other conditions were diagnosed at least 3 weeks after initial infection and persisted beyond 30 days.
This is one of the first studies to specifically describe the incidence and severity of new conditions triggered by COVID-19 infection in a general sample of older adults, said study author Ken Cohen MD, FACP, executive director of translational research at Optum Labs and national senior medical director at Optum Care.
“Much of what has been published on the postacute sequelae of COVID-19 has been predominantly from a younger population, and many of the patients had been hospitalized,” Dr. Cohen noted. “This was the first study to focus on a large population of seniors, most of whom did not require hospitalization.”
Dr. Cohen and colleagues reviewed the health insurance records of more than 133,000 Medicare beneficiaries aged 65 or older who were diagnosed with COVID-19 before April 2020. They also matched individuals by age, race, sex, hospitalization status, and other factors to comparison groups without COVID-19 (one from 2020 and one from 2019), and to a group diagnosed with other lower respiratory tract viral infections before the pandemic.
Risk of developing new conditions was higher in hospitalized
After acute COVID-19 infection, 32% of seniors sought medical care for at least one new medical condition in 2020, compared with 21% of uninfected people in the same year.
The most commonly observed conditions included:
- Respiratory failure (7.55% higher risk).
- Fatigue (5.66% higher risk).
- High blood pressure (4.43% higher risk).
- Memory problems (2.63% higher risk).
- Kidney injury (2.59% higher risk).
- Mental health diagnoses (2.5% higher risk).
- Blood-clotting disorders (1.47 % higher risk).
- Heart rhythm disorders (2.9% higher risk).
The risk of developing new conditions was even higher among those 23,486 who were hospitalized in 2020. Those individuals showed a 23.6% higher risk for developing at least one new condition, compared with uninfected seniors in the same year. Also, patients older than 75 had a higher risk for neurological disorders, including dementia, encephalopathy, and memory problems. The researchers also found that respiratory failure and kidney injury were significantly more likely to affect men and Black patients.
When those who had COVID were compared with the group with other lower respiratory viral infections before the pandemic, only the risks of respiratory failure (2.39% higher), dementia (0.71% higher), and fatigue (0.18% higher) were higher.
Primary care providers can learn from these data to better evaluate and manage their geriatric patients with COVID-19 infection, said Amit Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, in an interview.
“We must assess older patients who have had COVID-19 for more than just improvement from the respiratory symptoms of COVID-19 in post-COVID follow-up visits,” he said. “Older individuals with frailty have vulnerability to subsequent complications from severe illnesses and it is common to see post-illness diagnoses, such as new diagnosis of delirium; dementia; or renal, respiratory, or cardiac issues that is precipitated by the original illness. This study confirms that this is likely the case with COVID-19 as well.
“Primary care physicians should be vigilant for these complications, including attention to the rehabilitation needs of older patients with longer-term postviral fatigue from COVID-19,” Dr. Shah added.
Data predates ‘Omicron wave’
It remains uncertain whether sequelae will differ with the Omicron variant, but the findings remain applicable, Dr. Cohen said.
“We know that illness from the Omicron variant is on average less severe in those that have been vaccinated. However, throughout the Omicron wave, individuals who have not been vaccinated continue to have significant rates of serious illness and hospitalization,” he said.
“Our findings showed that serious illness with hospitalization was associated with a higher rate of sequelae. It can therefore be inferred that the rates of sequelae seen in our study would continue to occur in unvaccinated individuals who contract Omicron, but might occur less frequently in vaccinated individuals who contract Omicron and have less severe illness.”
Dr. Cohen serves as a consultant for Pfizer. Dr. Shah has disclosed no relevant financial relationships.
FROM BMJ
Structural Ableism: Defining Standards of Care Amid Crisis and Inequity
Equitable Standards for All Patients in a Crisis
Health care delivered during a pandemic instantiates medicine’s perspectives on the value of human life in clinical scenarios where resource allocation is limited. The COVID-19 pandemic has fostered dialogue and debate around the ethical principles that underly such resource allocation, which generally balance (1) utilitarian optimization of resources, (2) equality or equity in health access, (3) the instrumental value of individuals as agents in society, and (4) prioritizing the “worst off” in their natural history of disease.1,2 State legislatures and health systems have responded to the challeges posed by COVID-19 by considering both the scarcity of intensive care resources, such as mechanical ventilation and hemodialysis, and the clinical criteria to be used for determining which patients should receive said resources. These crisis guidelines have yielded several concerning themes vis-à-vis equitable distribution of health care resources, particularly when the disability status of patients is considered alongside life-expectancy or quality of life.3
Crisis standards of care (CSC) prioritize population-level health under a utilitarian paradigm, explicitly maximizing “life-years” within a population of patients rather than the life of any individual patient.4 Debated during initial COVID surges, these CSC guidelines have recently been enacted at the state level in several settings, including Alaska and Idaho.5 In a setting with scarce intensive care resources, balancing health equity in access to these resources against population-based survival metrics has been a challenge for commissions considering CSC.6,7 This need for balance has further promoted systemic views of “disability,” raising concern for structural “ableism” and highlighting the need for greater “ability awareness” in clinicians’ continued professional learning.
Structural Ableism: Defining Perspectives to Address Health Equity
Ableism has been defined as “a system that places value on people’s bodies and minds, based on societally constructed ideas of normalcy, intelligence, excellence, and productivity…[and] leads to people and society determining who is valuable and worthy based on their appearance and/or their ability to satisfactorily [re]produce, excel, and ‘behave.’”8 Regarding CSC, concerns about systemic bias in guideline design were raised early by disability advocacy groups during comment periods.9,10 More broadly, concerns about ableism sit alongside many deeply rooted societal perspectives of disabled individuals as pitiable or, conversely, heroic for having “overcome” their disability in some way. As a physician who sits in a manual wheelchair with paraplegia and mobility impairment, I have equally been subject to inappropriate bias and inappropriate praise for living in a wheelchair. I have also wondered, alongside my patients living with different levels of mobility or ability, why others often view us as “worse off.” Addressing directly whether disabled individuals are “worse off,” disability rights attorney and advocate Harriet McBryde Johnson has articulated a predominant sentiment among persons living with unique or different abilities:
Are we “worse off”? I don’t think so. Not in any meaningful way. There are too many variables. For those of us with congenital conditions, disability shapes all we are. Those disabled later in life adapt. We take constraints that no one would choose and build rich and satisfying lives within them. We enjoy pleasures other people enjoy and pleasures peculiarly our own. We have something the world needs.11
Many physician colleagues have common, invisible diseases such as diabetes and heart disease; fewer colleagues share conditions that are as visible as my spinal cord injury, as readily apparent to patients upon my entry to their hospital rooms. This simultaneous and inescapable identity as both patient and provider has afforded me wonderful doctor-patient interactions, particularly with those patients who appreciate how my patient experience impacts my ability to partially understand theirs. However, this simultaneous identity as doctor and patient also informed my personal and professional concerns regarding structural ableism as I considered scoring my own acutely ill hospital medicine patients with CSC triage scores in April 2020.
As a practicing hospital medicine physician, I have been emboldened by the efforts of my fellow clinicians amid COVID-19; their efforts have reaffirmed all the reasons I pursued a career in medicine. However, when I heard my clinical colleagues’ first explanation of the Massachusetts CSC guidelines in April 2020, I raised my hand to ask whether the “life-years” to which the guidelines referred were quality-adjusted. My concern regarding the implicit use of quality-adjusted life years (QALY) or disability-adjusted life years in clinical decision-making and implementation of these guidelines was validated when no clinical leaders could address this question directly. Sitting on the CSC committee for my hospital during this time was an honor. However, it was disconcerting to hear many clinicians’ unease when estimating mean survival for common chronic diseases, ranging from end-stage renal disease to advanced heart failure. If my expert colleagues, clinical specialists in kidney and heart disease, could not confidently apply mean survival estimates to multimorbid hospital patients, then idiosyncratic clinical judgment was sure to have a heavy hand in any calculation of “life-years.” Thus, my primary concern was that clinicians using triage heuristics would be subject to bias, regardless of their intention, and negatively adjust for the quality of a disabled life in their CSC triage scoring. My secondary concern was that the CSC guidelines themselves included systemic bias against disabled individuals.
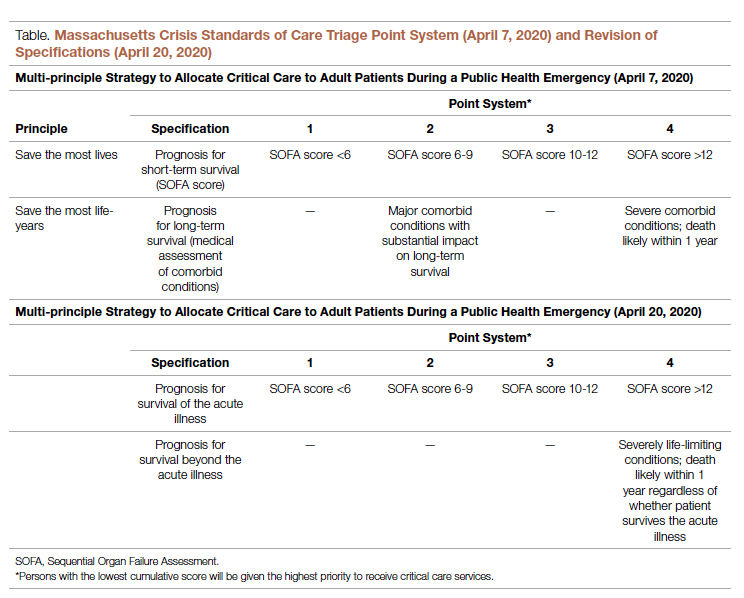
According to CSC schema, triage scores index heavily on Sequential Organ Failure Assessment (SOFA) scores to define short-term survival; SOFA scores are partially driven by the Glasgow Coma Scale (GCS). Following professional and public comment periods, CSC guidelines in Massachusetts were revised to, among other critical points of revision, change prognostic estimation via “life years” in favor of generic estimation of short-term survival (Table). I wondered, if I presented to an emergency department with severe COVID-19 and was scored with the GCS for the purpose of making a CSC ventilator triage decision, how would my complete paraplegia and lower-extremity motor impairment be accounted for by a clinician assessing “best motor response” in the GCS? The purpose of these scores is to act algorithmically, to guide clinicians whose cognitive load and time limitations may not allow for adjustment of these algorithms based on the individual patient in front of them. Individualization of clinical decisions is part of medicine’s art, but is difficult in the best of times and no easier during a crisis in care delivery. As CSC triage scores were amended and addended throughout 2020, I returned to the COVID wards, time and again wondering, “What have we learned about systemic bias and health inequity in the CSC process and the pandemic broadly, with specific regard to disability?”
Ability Awareness: Room for Our Improvement
Unfortunately, there is reason to believe that clinical judgment is impaired by structural ableism. In seminal work on this topic, Gerhart et al12 demonstrated that clinicians considered spinal cord injury (SCI) survivors to have low self-perceptions of worthiness, overall negative attitudes, and low self-esteem as compared to able-bodied individuals. However, surveyed SCI survivors generally had similar self-perceptions of worth and positivity as compared to ”able-bodied” clinicians.12 For providers who care for persons with disabilities, the majority (82.4%) have rated their disabled patients’ quality of life as worse.13 It is no wonder that patients with disabilities are more likely to feel that their doctor-patient relationship is impacted by lack of understanding, negative sentiment, or simple lack of listening.14 Generally, this poor doctor-patient relationship with disabled patients is exacerbated by poor exposure of medical trainees to disability education; only 34.2% of internal medicine residents recall any form of disability education in medical school, while only 52% of medical school deans report having disability educational content in their curricula.15,16 There is a similar lack of disability representation in the population of medical trainees themselves. While approximately 20% of the American population lives with a disability, less than 2% of American medical students have a disability.17-19
While representation of disabled populations in medical practice remains poor, disabled patients are generally less likely to receive age-appropriate prevention, appropriate access to care, and equal access to treatment.20-22 “Diagnostic overshadowing” refers to clinicians’ attribution of nonspecific signs or symptoms to a patient’s chronic disability as opposed to acute illness.23 This phenomenon has led to higher rates of preventable malignancy in disabled patients and misattribution of common somatic symptoms to intellectual disability.24,25 With this disparity in place as status quo for health care delivery to disabled populations, it is no surprise that certain portions of the disabled population have accounted for disproportionate mortality due to COVID-19.26,27Disability advocates have called for “nothing about us without us,” a phrase associated with the United Nations Convention on the Rights of Persons with Disabilities. Understanding the profound neurodiversity among several forms of sensory and cognitive disabilities, as well as the functional difference between cognitive disabilities, mobility impairment, and inability to meet one’s instrumental activities of daily living independently, others have proposed a unique approach to certain disabled populations in COVID care.28 My own perspective is that definite progress may require a more general understanding of the prevalence of disability by clinicians, both via medical training and by directly addressing health equity for disabled populations in such calculations as the CSC. Systemic ableism is apparent in our most common clinical scoring systems, ranging from the GCS and Functional Assessment Staging Table to the Eastern Cooperative Oncology Group and Karnofsky Performance Status scales. I have reexamined these scoring systems in my own understanding given their general equation of ambulation with ability or normalcy. As a doctor in a manual wheelchair who values greatly my personal quality of life and professional contribution to patient care, I worry that these scoring systems inherently discount my own equitable access to care. Individualization of patients’ particular abilities in the context of these scales must occur alongside evidence-based, guideline-directed management via these scoring systems.
Conclusion: Future Orientation
Updated CSC guidelines have accounted for the unique considerations of disabled patients by effectively caveating their scoring algorithms, directing clinicians via disclaimers to uniquely consider their disabled patients in clinical judgement. This is a first step, but it is also one that erodes the value of algorithms, which generally obviate more deliberative thinking and individualization. For our patients who lack certain abilities, as CSC continue to be activated in several states, we have an opportunity to pursue more inherently equitable solutions before further suffering accrues.29 By way of example, adaptations to scoring systems that leverage QALYs for value-based drug pricing indices have been proposed by organizations like the Institute for Clinical and Economic Review, which proposed the Equal-Value-of Life-Years-Gained framework to inform QALY-based arbitration of drug pricing.30 This is not a perfect rubric but instead represents an attempt to balance consideration of drugs, as has been done with ventilators during the pandemic, as a scare and expensive resource while addressing the just concerns of advocacy groups in structural ableism.
Resource stewardship during a crisis should not discount those states of human life that are perceived to be less desirable, particularly if they are not experienced as less desirable but are experienced uniquely. Instead, we should consider equitably measuring our intervention to match a patient’s needs, as we would dose-adjust a medication for renal function or consider minimally invasive procedures for multimorbid patients. COVID-19 has reflected our profession’s ethical adaptation during crisis as resources have become scarce; there is no better time to define solutions for health equity. We should now be concerned equally by the influence our personal biases have on our clinical practice and by the way in which these crisis standards will influence patients’ perception of and trust in their care providers during periods of perceived plentiful resources in the future. Health care resources are always limited, allocated according to societal values; if we value health equity for people of all abilities, then we will consider these abilities equitably as we pursue new standards for health care delivery.
Corresponding author: Gregory D. Snyder, MD, MBA, 2014 Washington Street, Newton, MA 02462; [email protected].
Disclosures: None.
1. Emanuel EJ, Persad G, Upshur R, et al. Fair Allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. doi:10.1056/NEJMsb2005114
2. Savulescu J, Persson I, Wilkinson D. Utilitarianism and the pandemic. Bioethics. 2020;34(6):620-632. doi:10.1111/bioe.12771
3. Mello MM, Persad G, White DB. Respecting disability rights - toward improved crisis standards of care. N Engl J Med. 2020;383(5):e26. doi: 10.1056/NEJMp2011997
4. The Commonwealth of Massachusetts Executive Office of Health and Human Services Department of Public Health. Crisis Standards of Care Planning Guidance for the COVID-19 Pandemic. April 7, 2020. https://d279m997dpfwgl.cloudfront.net/wp/2020/04/CSC_April-7_2020.pdf
5. Knowles H. Hospitals overwhelmed by covid are turning to ‘crisis standards of care.’ What does that mean? The Washington Post. September 21, 2021. Accessed January 24, 2022. https://www.washingtonpost.com/health/2021/09/22/crisis-standards-of-care/
6. Hick JL, Hanfling D, Wynia MK, Toner E. Crisis standards of care and COVID-19: What did we learn? How do we ensure equity? What should we do? NAM Perspect. 2021;2021:10.31478/202108e. doi:10.31478/202108e
7. Cleveland Manchanda EC, Sanky C, Appel JM. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID-19. J Racial Ethn Health Disparities. 2021;8(4):824-836. doi:10.1007/s40615-020-00840-5
8. Cleveland Manchanda EC, Sanky C, Appel JM. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID-19. J Racial Ethn Health Disparities. 2021;8(4):824-836. doi:10.1007/s40615-020-00840-5
9. Kukla E. My life is more ‘disposable’ during this pandemic. The New York Times. March 19, 2020. Accessed January 24, 2022. https://www.nytimes.com/2020/03/19/opinion/coronavirus-disabled-health-care.html
10. CPR and Coalition Partners Secure Important Changes in Massachusetts’ Crisis Standards of Care. Center for Public Representation. December 1, 2020. Accessed January 24, 2022. https://www.centerforpublicrep.org/news/cpr-and-coalition-partners-secure-important-changes-in-massachusetts-crisis-standards-of-care/
11. Johnson HM. Unspeakable conversations. The New York Times. February 16, 2003. Accessed January 24, 2022. https://www.nytimes.com/2003/02/16/magazine/unspeakable-conversations.html
12. Gerhart KA, Koziol-McLain J, Lowenstein SR, Whiteneck GG. Quality of life following spinal cord injury: knowledge and attitudes of emergency care providers. Ann Emerg Med. 1994;23(4):807-812. doi:10.1016/s0196-0644(94)70318-3
13. Iezzoni LI, Rao SR, Ressalam J, et al. Physicians’ perceptions of people with disability and their health care. Health Aff (Millwood). 2021;40(2):297-306. doi:10.1377/hlthaff.2020.01452
14. Smith DL. Disparities in patient-physician communication for persons with a disability from the 2006 Medical Expenditure Panel Survey (MEPS). Disabil Health J. 2009;2(4):206-215. doi:10.1016/j.dhjo.2009.06.002
15. Stillman MD, Ankam N, Mallow M, Capron M, Williams S. A survey of internal and family medicine residents: Assessment of disability-specific education and knowledge. Disabil Health J. 2021;14(2):101011. doi:10.1016/j.dhjo.2020.101011
16. Seidel E, Crowe S. The state of disability awareness in American medical schools. Am J Phys Med Rehabil. 2017;96(9):673-676. doi:10.1097/PHM.0000000000000719
17. Okoro CA, Hollis ND, Cyrus AC, Griffin-Blake S. Prevalence of disabilities and health care access by disability status and type among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887. doi:10.15585/mmwr.mm6732a3
18. Peacock G, Iezzoni LI, Harkin TR. Health care for Americans with disabilities--25 years after the ADA. N Engl J Med. 2015;373(10):892-893. doi:10.1056/NEJMp1508854
19. DeLisa JA, Thomas P. Physicians with disabilities and the physician workforce: a need to reassess our policies. Am J Phys Med Rehabil. 2005;84(1):5-11. doi:10.1097/01.phm.0000153323.28396.de
20. Disability and Health. Healthy People 2020. Accessed January 24, 2022. https://www.healthypeople.gov/2020/topics-objectives/topic/disability-and-health
21. Lagu T, Hannon NS, Rothberg MB, et al. Access to subspecialty care for patients with mobility impairment: a survey. Ann Intern Med. 2013;158(6):441-446. doi: 10.7326/0003-4819-158-6-201303190-00003
22. McCarthy EP, Ngo LH, Roetzheim RG, et al. Disparities in breast cancer treatment and survival for women with disabilities. Ann Intern Med. 2006;145(9):637-645. doi: 10.7326/0003-4819-145-9-200611070-00005
23. Javaid A, Nakata V, Michael D. Diagnostic overshadowing in learning disability: think beyond the disability. Prog Neurol Psychiatry. 2019;23:8-10.
24. Iezzoni LI, Rao SR, Agaronnik ND, El-Jawahri A. Cross-sectional analysis of the associations between four common cancers and disability. J Natl Compr Canc Netw. 2020;18(8):1031-1044. doi:10.6004/jnccn.2020.7551
25. Sanders JS, Keller S, Aravamuthan BR. Caring for individuals with intellectual and developmental disabilities in the COVID-19 crisis. Neurol Clin Pract. 2021;11(2):e174-e178. doi:10.1212/CPJ.0000000000000886
26. Landes SD, Turk MA, Formica MK, McDonald KE, Stevens JD. COVID-19 outcomes among people with intellectual and developmental disability living in residential group homes in New York State. Disabil Health J. 2020;13(4):100969. doi:10.1016/j.dhjo.2020.100969
27. Gleason J, Ross W, Fossi A, Blonksy H, Tobias J, Stephens M. The devastating impact of Covid-19 on individuals with intellectual disabilities in the United States. NEJM Catalyst. 2021.doi.org/10.1056/CAT.21.0051
28. Nankervis K, Chan J. Applying the CRPD to people with intellectual and developmental disability with behaviors of concern during COVID-19. J Policy Pract Intellect Disabil. 2021:10.1111/jppi.12374. doi:10.1111/jppi.12374
29. Alaska Department of Health and Social Services, Division of Public Health, Rural and Community Health Systems. Patient care strategies for scarce resource situations. Version 1. August 2021. Accessed November 11, 2021, https://dhss.alaska.gov/dph/Epi/id/SiteAssets/Pages/HumanCoV/SOA_DHSS_CrisisStandardsOfCare.pdf
30. Cost-effectiveness, the QALY, and the evlyg. ICER. May 21, 2021. Accessed January 24, 2022. https://icer.org/our-approach/methods-process/cost-effectiveness-the-qaly-and-the-evlyg/
Equitable Standards for All Patients in a Crisis
Health care delivered during a pandemic instantiates medicine’s perspectives on the value of human life in clinical scenarios where resource allocation is limited. The COVID-19 pandemic has fostered dialogue and debate around the ethical principles that underly such resource allocation, which generally balance (1) utilitarian optimization of resources, (2) equality or equity in health access, (3) the instrumental value of individuals as agents in society, and (4) prioritizing the “worst off” in their natural history of disease.1,2 State legislatures and health systems have responded to the challeges posed by COVID-19 by considering both the scarcity of intensive care resources, such as mechanical ventilation and hemodialysis, and the clinical criteria to be used for determining which patients should receive said resources. These crisis guidelines have yielded several concerning themes vis-à-vis equitable distribution of health care resources, particularly when the disability status of patients is considered alongside life-expectancy or quality of life.3
Crisis standards of care (CSC) prioritize population-level health under a utilitarian paradigm, explicitly maximizing “life-years” within a population of patients rather than the life of any individual patient.4 Debated during initial COVID surges, these CSC guidelines have recently been enacted at the state level in several settings, including Alaska and Idaho.5 In a setting with scarce intensive care resources, balancing health equity in access to these resources against population-based survival metrics has been a challenge for commissions considering CSC.6,7 This need for balance has further promoted systemic views of “disability,” raising concern for structural “ableism” and highlighting the need for greater “ability awareness” in clinicians’ continued professional learning.
Structural Ableism: Defining Perspectives to Address Health Equity
Ableism has been defined as “a system that places value on people’s bodies and minds, based on societally constructed ideas of normalcy, intelligence, excellence, and productivity…[and] leads to people and society determining who is valuable and worthy based on their appearance and/or their ability to satisfactorily [re]produce, excel, and ‘behave.’”8 Regarding CSC, concerns about systemic bias in guideline design were raised early by disability advocacy groups during comment periods.9,10 More broadly, concerns about ableism sit alongside many deeply rooted societal perspectives of disabled individuals as pitiable or, conversely, heroic for having “overcome” their disability in some way. As a physician who sits in a manual wheelchair with paraplegia and mobility impairment, I have equally been subject to inappropriate bias and inappropriate praise for living in a wheelchair. I have also wondered, alongside my patients living with different levels of mobility or ability, why others often view us as “worse off.” Addressing directly whether disabled individuals are “worse off,” disability rights attorney and advocate Harriet McBryde Johnson has articulated a predominant sentiment among persons living with unique or different abilities:
Are we “worse off”? I don’t think so. Not in any meaningful way. There are too many variables. For those of us with congenital conditions, disability shapes all we are. Those disabled later in life adapt. We take constraints that no one would choose and build rich and satisfying lives within them. We enjoy pleasures other people enjoy and pleasures peculiarly our own. We have something the world needs.11
Many physician colleagues have common, invisible diseases such as diabetes and heart disease; fewer colleagues share conditions that are as visible as my spinal cord injury, as readily apparent to patients upon my entry to their hospital rooms. This simultaneous and inescapable identity as both patient and provider has afforded me wonderful doctor-patient interactions, particularly with those patients who appreciate how my patient experience impacts my ability to partially understand theirs. However, this simultaneous identity as doctor and patient also informed my personal and professional concerns regarding structural ableism as I considered scoring my own acutely ill hospital medicine patients with CSC triage scores in April 2020.
As a practicing hospital medicine physician, I have been emboldened by the efforts of my fellow clinicians amid COVID-19; their efforts have reaffirmed all the reasons I pursued a career in medicine. However, when I heard my clinical colleagues’ first explanation of the Massachusetts CSC guidelines in April 2020, I raised my hand to ask whether the “life-years” to which the guidelines referred were quality-adjusted. My concern regarding the implicit use of quality-adjusted life years (QALY) or disability-adjusted life years in clinical decision-making and implementation of these guidelines was validated when no clinical leaders could address this question directly. Sitting on the CSC committee for my hospital during this time was an honor. However, it was disconcerting to hear many clinicians’ unease when estimating mean survival for common chronic diseases, ranging from end-stage renal disease to advanced heart failure. If my expert colleagues, clinical specialists in kidney and heart disease, could not confidently apply mean survival estimates to multimorbid hospital patients, then idiosyncratic clinical judgment was sure to have a heavy hand in any calculation of “life-years.” Thus, my primary concern was that clinicians using triage heuristics would be subject to bias, regardless of their intention, and negatively adjust for the quality of a disabled life in their CSC triage scoring. My secondary concern was that the CSC guidelines themselves included systemic bias against disabled individuals.
According to CSC schema, triage scores index heavily on Sequential Organ Failure Assessment (SOFA) scores to define short-term survival; SOFA scores are partially driven by the Glasgow Coma Scale (GCS). Following professional and public comment periods, CSC guidelines in Massachusetts were revised to, among other critical points of revision, change prognostic estimation via “life years” in favor of generic estimation of short-term survival (Table). I wondered, if I presented to an emergency department with severe COVID-19 and was scored with the GCS for the purpose of making a CSC ventilator triage decision, how would my complete paraplegia and lower-extremity motor impairment be accounted for by a clinician assessing “best motor response” in the GCS? The purpose of these scores is to act algorithmically, to guide clinicians whose cognitive load and time limitations may not allow for adjustment of these algorithms based on the individual patient in front of them. Individualization of clinical decisions is part of medicine’s art, but is difficult in the best of times and no easier during a crisis in care delivery. As CSC triage scores were amended and addended throughout 2020, I returned to the COVID wards, time and again wondering, “What have we learned about systemic bias and health inequity in the CSC process and the pandemic broadly, with specific regard to disability?”

Ability Awareness: Room for Our Improvement
Unfortunately, there is reason to believe that clinical judgment is impaired by structural ableism. In seminal work on this topic, Gerhart et al12 demonstrated that clinicians considered spinal cord injury (SCI) survivors to have low self-perceptions of worthiness, overall negative attitudes, and low self-esteem as compared to able-bodied individuals. However, surveyed SCI survivors generally had similar self-perceptions of worth and positivity as compared to ”able-bodied” clinicians.12 For providers who care for persons with disabilities, the majority (82.4%) have rated their disabled patients’ quality of life as worse.13 It is no wonder that patients with disabilities are more likely to feel that their doctor-patient relationship is impacted by lack of understanding, negative sentiment, or simple lack of listening.14 Generally, this poor doctor-patient relationship with disabled patients is exacerbated by poor exposure of medical trainees to disability education; only 34.2% of internal medicine residents recall any form of disability education in medical school, while only 52% of medical school deans report having disability educational content in their curricula.15,16 There is a similar lack of disability representation in the population of medical trainees themselves. While approximately 20% of the American population lives with a disability, less than 2% of American medical students have a disability.17-19
While representation of disabled populations in medical practice remains poor, disabled patients are generally less likely to receive age-appropriate prevention, appropriate access to care, and equal access to treatment.20-22 “Diagnostic overshadowing” refers to clinicians’ attribution of nonspecific signs or symptoms to a patient’s chronic disability as opposed to acute illness.23 This phenomenon has led to higher rates of preventable malignancy in disabled patients and misattribution of common somatic symptoms to intellectual disability.24,25 With this disparity in place as status quo for health care delivery to disabled populations, it is no surprise that certain portions of the disabled population have accounted for disproportionate mortality due to COVID-19.26,27Disability advocates have called for “nothing about us without us,” a phrase associated with the United Nations Convention on the Rights of Persons with Disabilities. Understanding the profound neurodiversity among several forms of sensory and cognitive disabilities, as well as the functional difference between cognitive disabilities, mobility impairment, and inability to meet one’s instrumental activities of daily living independently, others have proposed a unique approach to certain disabled populations in COVID care.28 My own perspective is that definite progress may require a more general understanding of the prevalence of disability by clinicians, both via medical training and by directly addressing health equity for disabled populations in such calculations as the CSC. Systemic ableism is apparent in our most common clinical scoring systems, ranging from the GCS and Functional Assessment Staging Table to the Eastern Cooperative Oncology Group and Karnofsky Performance Status scales. I have reexamined these scoring systems in my own understanding given their general equation of ambulation with ability or normalcy. As a doctor in a manual wheelchair who values greatly my personal quality of life and professional contribution to patient care, I worry that these scoring systems inherently discount my own equitable access to care. Individualization of patients’ particular abilities in the context of these scales must occur alongside evidence-based, guideline-directed management via these scoring systems.
Conclusion: Future Orientation
Updated CSC guidelines have accounted for the unique considerations of disabled patients by effectively caveating their scoring algorithms, directing clinicians via disclaimers to uniquely consider their disabled patients in clinical judgement. This is a first step, but it is also one that erodes the value of algorithms, which generally obviate more deliberative thinking and individualization. For our patients who lack certain abilities, as CSC continue to be activated in several states, we have an opportunity to pursue more inherently equitable solutions before further suffering accrues.29 By way of example, adaptations to scoring systems that leverage QALYs for value-based drug pricing indices have been proposed by organizations like the Institute for Clinical and Economic Review, which proposed the Equal-Value-of Life-Years-Gained framework to inform QALY-based arbitration of drug pricing.30 This is not a perfect rubric but instead represents an attempt to balance consideration of drugs, as has been done with ventilators during the pandemic, as a scare and expensive resource while addressing the just concerns of advocacy groups in structural ableism.
Resource stewardship during a crisis should not discount those states of human life that are perceived to be less desirable, particularly if they are not experienced as less desirable but are experienced uniquely. Instead, we should consider equitably measuring our intervention to match a patient’s needs, as we would dose-adjust a medication for renal function or consider minimally invasive procedures for multimorbid patients. COVID-19 has reflected our profession’s ethical adaptation during crisis as resources have become scarce; there is no better time to define solutions for health equity. We should now be concerned equally by the influence our personal biases have on our clinical practice and by the way in which these crisis standards will influence patients’ perception of and trust in their care providers during periods of perceived plentiful resources in the future. Health care resources are always limited, allocated according to societal values; if we value health equity for people of all abilities, then we will consider these abilities equitably as we pursue new standards for health care delivery.
Corresponding author: Gregory D. Snyder, MD, MBA, 2014 Washington Street, Newton, MA 02462; [email protected].
Disclosures: None.
Equitable Standards for All Patients in a Crisis
Health care delivered during a pandemic instantiates medicine’s perspectives on the value of human life in clinical scenarios where resource allocation is limited. The COVID-19 pandemic has fostered dialogue and debate around the ethical principles that underly such resource allocation, which generally balance (1) utilitarian optimization of resources, (2) equality or equity in health access, (3) the instrumental value of individuals as agents in society, and (4) prioritizing the “worst off” in their natural history of disease.1,2 State legislatures and health systems have responded to the challeges posed by COVID-19 by considering both the scarcity of intensive care resources, such as mechanical ventilation and hemodialysis, and the clinical criteria to be used for determining which patients should receive said resources. These crisis guidelines have yielded several concerning themes vis-à-vis equitable distribution of health care resources, particularly when the disability status of patients is considered alongside life-expectancy or quality of life.3
Crisis standards of care (CSC) prioritize population-level health under a utilitarian paradigm, explicitly maximizing “life-years” within a population of patients rather than the life of any individual patient.4 Debated during initial COVID surges, these CSC guidelines have recently been enacted at the state level in several settings, including Alaska and Idaho.5 In a setting with scarce intensive care resources, balancing health equity in access to these resources against population-based survival metrics has been a challenge for commissions considering CSC.6,7 This need for balance has further promoted systemic views of “disability,” raising concern for structural “ableism” and highlighting the need for greater “ability awareness” in clinicians’ continued professional learning.
Structural Ableism: Defining Perspectives to Address Health Equity
Ableism has been defined as “a system that places value on people’s bodies and minds, based on societally constructed ideas of normalcy, intelligence, excellence, and productivity…[and] leads to people and society determining who is valuable and worthy based on their appearance and/or their ability to satisfactorily [re]produce, excel, and ‘behave.’”8 Regarding CSC, concerns about systemic bias in guideline design were raised early by disability advocacy groups during comment periods.9,10 More broadly, concerns about ableism sit alongside many deeply rooted societal perspectives of disabled individuals as pitiable or, conversely, heroic for having “overcome” their disability in some way. As a physician who sits in a manual wheelchair with paraplegia and mobility impairment, I have equally been subject to inappropriate bias and inappropriate praise for living in a wheelchair. I have also wondered, alongside my patients living with different levels of mobility or ability, why others often view us as “worse off.” Addressing directly whether disabled individuals are “worse off,” disability rights attorney and advocate Harriet McBryde Johnson has articulated a predominant sentiment among persons living with unique or different abilities:
Are we “worse off”? I don’t think so. Not in any meaningful way. There are too many variables. For those of us with congenital conditions, disability shapes all we are. Those disabled later in life adapt. We take constraints that no one would choose and build rich and satisfying lives within them. We enjoy pleasures other people enjoy and pleasures peculiarly our own. We have something the world needs.11
Many physician colleagues have common, invisible diseases such as diabetes and heart disease; fewer colleagues share conditions that are as visible as my spinal cord injury, as readily apparent to patients upon my entry to their hospital rooms. This simultaneous and inescapable identity as both patient and provider has afforded me wonderful doctor-patient interactions, particularly with those patients who appreciate how my patient experience impacts my ability to partially understand theirs. However, this simultaneous identity as doctor and patient also informed my personal and professional concerns regarding structural ableism as I considered scoring my own acutely ill hospital medicine patients with CSC triage scores in April 2020.
As a practicing hospital medicine physician, I have been emboldened by the efforts of my fellow clinicians amid COVID-19; their efforts have reaffirmed all the reasons I pursued a career in medicine. However, when I heard my clinical colleagues’ first explanation of the Massachusetts CSC guidelines in April 2020, I raised my hand to ask whether the “life-years” to which the guidelines referred were quality-adjusted. My concern regarding the implicit use of quality-adjusted life years (QALY) or disability-adjusted life years in clinical decision-making and implementation of these guidelines was validated when no clinical leaders could address this question directly. Sitting on the CSC committee for my hospital during this time was an honor. However, it was disconcerting to hear many clinicians’ unease when estimating mean survival for common chronic diseases, ranging from end-stage renal disease to advanced heart failure. If my expert colleagues, clinical specialists in kidney and heart disease, could not confidently apply mean survival estimates to multimorbid hospital patients, then idiosyncratic clinical judgment was sure to have a heavy hand in any calculation of “life-years.” Thus, my primary concern was that clinicians using triage heuristics would be subject to bias, regardless of their intention, and negatively adjust for the quality of a disabled life in their CSC triage scoring. My secondary concern was that the CSC guidelines themselves included systemic bias against disabled individuals.
According to CSC schema, triage scores index heavily on Sequential Organ Failure Assessment (SOFA) scores to define short-term survival; SOFA scores are partially driven by the Glasgow Coma Scale (GCS). Following professional and public comment periods, CSC guidelines in Massachusetts were revised to, among other critical points of revision, change prognostic estimation via “life years” in favor of generic estimation of short-term survival (Table). I wondered, if I presented to an emergency department with severe COVID-19 and was scored with the GCS for the purpose of making a CSC ventilator triage decision, how would my complete paraplegia and lower-extremity motor impairment be accounted for by a clinician assessing “best motor response” in the GCS? The purpose of these scores is to act algorithmically, to guide clinicians whose cognitive load and time limitations may not allow for adjustment of these algorithms based on the individual patient in front of them. Individualization of clinical decisions is part of medicine’s art, but is difficult in the best of times and no easier during a crisis in care delivery. As CSC triage scores were amended and addended throughout 2020, I returned to the COVID wards, time and again wondering, “What have we learned about systemic bias and health inequity in the CSC process and the pandemic broadly, with specific regard to disability?”

Ability Awareness: Room for Our Improvement
Unfortunately, there is reason to believe that clinical judgment is impaired by structural ableism. In seminal work on this topic, Gerhart et al12 demonstrated that clinicians considered spinal cord injury (SCI) survivors to have low self-perceptions of worthiness, overall negative attitudes, and low self-esteem as compared to able-bodied individuals. However, surveyed SCI survivors generally had similar self-perceptions of worth and positivity as compared to ”able-bodied” clinicians.12 For providers who care for persons with disabilities, the majority (82.4%) have rated their disabled patients’ quality of life as worse.13 It is no wonder that patients with disabilities are more likely to feel that their doctor-patient relationship is impacted by lack of understanding, negative sentiment, or simple lack of listening.14 Generally, this poor doctor-patient relationship with disabled patients is exacerbated by poor exposure of medical trainees to disability education; only 34.2% of internal medicine residents recall any form of disability education in medical school, while only 52% of medical school deans report having disability educational content in their curricula.15,16 There is a similar lack of disability representation in the population of medical trainees themselves. While approximately 20% of the American population lives with a disability, less than 2% of American medical students have a disability.17-19
While representation of disabled populations in medical practice remains poor, disabled patients are generally less likely to receive age-appropriate prevention, appropriate access to care, and equal access to treatment.20-22 “Diagnostic overshadowing” refers to clinicians’ attribution of nonspecific signs or symptoms to a patient’s chronic disability as opposed to acute illness.23 This phenomenon has led to higher rates of preventable malignancy in disabled patients and misattribution of common somatic symptoms to intellectual disability.24,25 With this disparity in place as status quo for health care delivery to disabled populations, it is no surprise that certain portions of the disabled population have accounted for disproportionate mortality due to COVID-19.26,27Disability advocates have called for “nothing about us without us,” a phrase associated with the United Nations Convention on the Rights of Persons with Disabilities. Understanding the profound neurodiversity among several forms of sensory and cognitive disabilities, as well as the functional difference between cognitive disabilities, mobility impairment, and inability to meet one’s instrumental activities of daily living independently, others have proposed a unique approach to certain disabled populations in COVID care.28 My own perspective is that definite progress may require a more general understanding of the prevalence of disability by clinicians, both via medical training and by directly addressing health equity for disabled populations in such calculations as the CSC. Systemic ableism is apparent in our most common clinical scoring systems, ranging from the GCS and Functional Assessment Staging Table to the Eastern Cooperative Oncology Group and Karnofsky Performance Status scales. I have reexamined these scoring systems in my own understanding given their general equation of ambulation with ability or normalcy. As a doctor in a manual wheelchair who values greatly my personal quality of life and professional contribution to patient care, I worry that these scoring systems inherently discount my own equitable access to care. Individualization of patients’ particular abilities in the context of these scales must occur alongside evidence-based, guideline-directed management via these scoring systems.
Conclusion: Future Orientation
Updated CSC guidelines have accounted for the unique considerations of disabled patients by effectively caveating their scoring algorithms, directing clinicians via disclaimers to uniquely consider their disabled patients in clinical judgement. This is a first step, but it is also one that erodes the value of algorithms, which generally obviate more deliberative thinking and individualization. For our patients who lack certain abilities, as CSC continue to be activated in several states, we have an opportunity to pursue more inherently equitable solutions before further suffering accrues.29 By way of example, adaptations to scoring systems that leverage QALYs for value-based drug pricing indices have been proposed by organizations like the Institute for Clinical and Economic Review, which proposed the Equal-Value-of Life-Years-Gained framework to inform QALY-based arbitration of drug pricing.30 This is not a perfect rubric but instead represents an attempt to balance consideration of drugs, as has been done with ventilators during the pandemic, as a scare and expensive resource while addressing the just concerns of advocacy groups in structural ableism.
Resource stewardship during a crisis should not discount those states of human life that are perceived to be less desirable, particularly if they are not experienced as less desirable but are experienced uniquely. Instead, we should consider equitably measuring our intervention to match a patient’s needs, as we would dose-adjust a medication for renal function or consider minimally invasive procedures for multimorbid patients. COVID-19 has reflected our profession’s ethical adaptation during crisis as resources have become scarce; there is no better time to define solutions for health equity. We should now be concerned equally by the influence our personal biases have on our clinical practice and by the way in which these crisis standards will influence patients’ perception of and trust in their care providers during periods of perceived plentiful resources in the future. Health care resources are always limited, allocated according to societal values; if we value health equity for people of all abilities, then we will consider these abilities equitably as we pursue new standards for health care delivery.
Corresponding author: Gregory D. Snyder, MD, MBA, 2014 Washington Street, Newton, MA 02462; [email protected].
Disclosures: None.
1. Emanuel EJ, Persad G, Upshur R, et al. Fair Allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. doi:10.1056/NEJMsb2005114
2. Savulescu J, Persson I, Wilkinson D. Utilitarianism and the pandemic. Bioethics. 2020;34(6):620-632. doi:10.1111/bioe.12771
3. Mello MM, Persad G, White DB. Respecting disability rights - toward improved crisis standards of care. N Engl J Med. 2020;383(5):e26. doi: 10.1056/NEJMp2011997
4. The Commonwealth of Massachusetts Executive Office of Health and Human Services Department of Public Health. Crisis Standards of Care Planning Guidance for the COVID-19 Pandemic. April 7, 2020. https://d279m997dpfwgl.cloudfront.net/wp/2020/04/CSC_April-7_2020.pdf
5. Knowles H. Hospitals overwhelmed by covid are turning to ‘crisis standards of care.’ What does that mean? The Washington Post. September 21, 2021. Accessed January 24, 2022. https://www.washingtonpost.com/health/2021/09/22/crisis-standards-of-care/
6. Hick JL, Hanfling D, Wynia MK, Toner E. Crisis standards of care and COVID-19: What did we learn? How do we ensure equity? What should we do? NAM Perspect. 2021;2021:10.31478/202108e. doi:10.31478/202108e
7. Cleveland Manchanda EC, Sanky C, Appel JM. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID-19. J Racial Ethn Health Disparities. 2021;8(4):824-836. doi:10.1007/s40615-020-00840-5
8. Cleveland Manchanda EC, Sanky C, Appel JM. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID-19. J Racial Ethn Health Disparities. 2021;8(4):824-836. doi:10.1007/s40615-020-00840-5
9. Kukla E. My life is more ‘disposable’ during this pandemic. The New York Times. March 19, 2020. Accessed January 24, 2022. https://www.nytimes.com/2020/03/19/opinion/coronavirus-disabled-health-care.html
10. CPR and Coalition Partners Secure Important Changes in Massachusetts’ Crisis Standards of Care. Center for Public Representation. December 1, 2020. Accessed January 24, 2022. https://www.centerforpublicrep.org/news/cpr-and-coalition-partners-secure-important-changes-in-massachusetts-crisis-standards-of-care/
11. Johnson HM. Unspeakable conversations. The New York Times. February 16, 2003. Accessed January 24, 2022. https://www.nytimes.com/2003/02/16/magazine/unspeakable-conversations.html
12. Gerhart KA, Koziol-McLain J, Lowenstein SR, Whiteneck GG. Quality of life following spinal cord injury: knowledge and attitudes of emergency care providers. Ann Emerg Med. 1994;23(4):807-812. doi:10.1016/s0196-0644(94)70318-3
13. Iezzoni LI, Rao SR, Ressalam J, et al. Physicians’ perceptions of people with disability and their health care. Health Aff (Millwood). 2021;40(2):297-306. doi:10.1377/hlthaff.2020.01452
14. Smith DL. Disparities in patient-physician communication for persons with a disability from the 2006 Medical Expenditure Panel Survey (MEPS). Disabil Health J. 2009;2(4):206-215. doi:10.1016/j.dhjo.2009.06.002
15. Stillman MD, Ankam N, Mallow M, Capron M, Williams S. A survey of internal and family medicine residents: Assessment of disability-specific education and knowledge. Disabil Health J. 2021;14(2):101011. doi:10.1016/j.dhjo.2020.101011
16. Seidel E, Crowe S. The state of disability awareness in American medical schools. Am J Phys Med Rehabil. 2017;96(9):673-676. doi:10.1097/PHM.0000000000000719
17. Okoro CA, Hollis ND, Cyrus AC, Griffin-Blake S. Prevalence of disabilities and health care access by disability status and type among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887. doi:10.15585/mmwr.mm6732a3
18. Peacock G, Iezzoni LI, Harkin TR. Health care for Americans with disabilities--25 years after the ADA. N Engl J Med. 2015;373(10):892-893. doi:10.1056/NEJMp1508854
19. DeLisa JA, Thomas P. Physicians with disabilities and the physician workforce: a need to reassess our policies. Am J Phys Med Rehabil. 2005;84(1):5-11. doi:10.1097/01.phm.0000153323.28396.de
20. Disability and Health. Healthy People 2020. Accessed January 24, 2022. https://www.healthypeople.gov/2020/topics-objectives/topic/disability-and-health
21. Lagu T, Hannon NS, Rothberg MB, et al. Access to subspecialty care for patients with mobility impairment: a survey. Ann Intern Med. 2013;158(6):441-446. doi: 10.7326/0003-4819-158-6-201303190-00003
22. McCarthy EP, Ngo LH, Roetzheim RG, et al. Disparities in breast cancer treatment and survival for women with disabilities. Ann Intern Med. 2006;145(9):637-645. doi: 10.7326/0003-4819-145-9-200611070-00005
23. Javaid A, Nakata V, Michael D. Diagnostic overshadowing in learning disability: think beyond the disability. Prog Neurol Psychiatry. 2019;23:8-10.
24. Iezzoni LI, Rao SR, Agaronnik ND, El-Jawahri A. Cross-sectional analysis of the associations between four common cancers and disability. J Natl Compr Canc Netw. 2020;18(8):1031-1044. doi:10.6004/jnccn.2020.7551
25. Sanders JS, Keller S, Aravamuthan BR. Caring for individuals with intellectual and developmental disabilities in the COVID-19 crisis. Neurol Clin Pract. 2021;11(2):e174-e178. doi:10.1212/CPJ.0000000000000886
26. Landes SD, Turk MA, Formica MK, McDonald KE, Stevens JD. COVID-19 outcomes among people with intellectual and developmental disability living in residential group homes in New York State. Disabil Health J. 2020;13(4):100969. doi:10.1016/j.dhjo.2020.100969
27. Gleason J, Ross W, Fossi A, Blonksy H, Tobias J, Stephens M. The devastating impact of Covid-19 on individuals with intellectual disabilities in the United States. NEJM Catalyst. 2021.doi.org/10.1056/CAT.21.0051
28. Nankervis K, Chan J. Applying the CRPD to people with intellectual and developmental disability with behaviors of concern during COVID-19. J Policy Pract Intellect Disabil. 2021:10.1111/jppi.12374. doi:10.1111/jppi.12374
29. Alaska Department of Health and Social Services, Division of Public Health, Rural and Community Health Systems. Patient care strategies for scarce resource situations. Version 1. August 2021. Accessed November 11, 2021, https://dhss.alaska.gov/dph/Epi/id/SiteAssets/Pages/HumanCoV/SOA_DHSS_CrisisStandardsOfCare.pdf
30. Cost-effectiveness, the QALY, and the evlyg. ICER. May 21, 2021. Accessed January 24, 2022. https://icer.org/our-approach/methods-process/cost-effectiveness-the-qaly-and-the-evlyg/
1. Emanuel EJ, Persad G, Upshur R, et al. Fair Allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. doi:10.1056/NEJMsb2005114
2. Savulescu J, Persson I, Wilkinson D. Utilitarianism and the pandemic. Bioethics. 2020;34(6):620-632. doi:10.1111/bioe.12771
3. Mello MM, Persad G, White DB. Respecting disability rights - toward improved crisis standards of care. N Engl J Med. 2020;383(5):e26. doi: 10.1056/NEJMp2011997
4. The Commonwealth of Massachusetts Executive Office of Health and Human Services Department of Public Health. Crisis Standards of Care Planning Guidance for the COVID-19 Pandemic. April 7, 2020. https://d279m997dpfwgl.cloudfront.net/wp/2020/04/CSC_April-7_2020.pdf
5. Knowles H. Hospitals overwhelmed by covid are turning to ‘crisis standards of care.’ What does that mean? The Washington Post. September 21, 2021. Accessed January 24, 2022. https://www.washingtonpost.com/health/2021/09/22/crisis-standards-of-care/
6. Hick JL, Hanfling D, Wynia MK, Toner E. Crisis standards of care and COVID-19: What did we learn? How do we ensure equity? What should we do? NAM Perspect. 2021;2021:10.31478/202108e. doi:10.31478/202108e
7. Cleveland Manchanda EC, Sanky C, Appel JM. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID-19. J Racial Ethn Health Disparities. 2021;8(4):824-836. doi:10.1007/s40615-020-00840-5
8. Cleveland Manchanda EC, Sanky C, Appel JM. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID-19. J Racial Ethn Health Disparities. 2021;8(4):824-836. doi:10.1007/s40615-020-00840-5
9. Kukla E. My life is more ‘disposable’ during this pandemic. The New York Times. March 19, 2020. Accessed January 24, 2022. https://www.nytimes.com/2020/03/19/opinion/coronavirus-disabled-health-care.html
10. CPR and Coalition Partners Secure Important Changes in Massachusetts’ Crisis Standards of Care. Center for Public Representation. December 1, 2020. Accessed January 24, 2022. https://www.centerforpublicrep.org/news/cpr-and-coalition-partners-secure-important-changes-in-massachusetts-crisis-standards-of-care/
11. Johnson HM. Unspeakable conversations. The New York Times. February 16, 2003. Accessed January 24, 2022. https://www.nytimes.com/2003/02/16/magazine/unspeakable-conversations.html
12. Gerhart KA, Koziol-McLain J, Lowenstein SR, Whiteneck GG. Quality of life following spinal cord injury: knowledge and attitudes of emergency care providers. Ann Emerg Med. 1994;23(4):807-812. doi:10.1016/s0196-0644(94)70318-3
13. Iezzoni LI, Rao SR, Ressalam J, et al. Physicians’ perceptions of people with disability and their health care. Health Aff (Millwood). 2021;40(2):297-306. doi:10.1377/hlthaff.2020.01452
14. Smith DL. Disparities in patient-physician communication for persons with a disability from the 2006 Medical Expenditure Panel Survey (MEPS). Disabil Health J. 2009;2(4):206-215. doi:10.1016/j.dhjo.2009.06.002
15. Stillman MD, Ankam N, Mallow M, Capron M, Williams S. A survey of internal and family medicine residents: Assessment of disability-specific education and knowledge. Disabil Health J. 2021;14(2):101011. doi:10.1016/j.dhjo.2020.101011
16. Seidel E, Crowe S. The state of disability awareness in American medical schools. Am J Phys Med Rehabil. 2017;96(9):673-676. doi:10.1097/PHM.0000000000000719
17. Okoro CA, Hollis ND, Cyrus AC, Griffin-Blake S. Prevalence of disabilities and health care access by disability status and type among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887. doi:10.15585/mmwr.mm6732a3
18. Peacock G, Iezzoni LI, Harkin TR. Health care for Americans with disabilities--25 years after the ADA. N Engl J Med. 2015;373(10):892-893. doi:10.1056/NEJMp1508854
19. DeLisa JA, Thomas P. Physicians with disabilities and the physician workforce: a need to reassess our policies. Am J Phys Med Rehabil. 2005;84(1):5-11. doi:10.1097/01.phm.0000153323.28396.de
20. Disability and Health. Healthy People 2020. Accessed January 24, 2022. https://www.healthypeople.gov/2020/topics-objectives/topic/disability-and-health
21. Lagu T, Hannon NS, Rothberg MB, et al. Access to subspecialty care for patients with mobility impairment: a survey. Ann Intern Med. 2013;158(6):441-446. doi: 10.7326/0003-4819-158-6-201303190-00003
22. McCarthy EP, Ngo LH, Roetzheim RG, et al. Disparities in breast cancer treatment and survival for women with disabilities. Ann Intern Med. 2006;145(9):637-645. doi: 10.7326/0003-4819-145-9-200611070-00005
23. Javaid A, Nakata V, Michael D. Diagnostic overshadowing in learning disability: think beyond the disability. Prog Neurol Psychiatry. 2019;23:8-10.
24. Iezzoni LI, Rao SR, Agaronnik ND, El-Jawahri A. Cross-sectional analysis of the associations between four common cancers and disability. J Natl Compr Canc Netw. 2020;18(8):1031-1044. doi:10.6004/jnccn.2020.7551
25. Sanders JS, Keller S, Aravamuthan BR. Caring for individuals with intellectual and developmental disabilities in the COVID-19 crisis. Neurol Clin Pract. 2021;11(2):e174-e178. doi:10.1212/CPJ.0000000000000886
26. Landes SD, Turk MA, Formica MK, McDonald KE, Stevens JD. COVID-19 outcomes among people with intellectual and developmental disability living in residential group homes in New York State. Disabil Health J. 2020;13(4):100969. doi:10.1016/j.dhjo.2020.100969
27. Gleason J, Ross W, Fossi A, Blonksy H, Tobias J, Stephens M. The devastating impact of Covid-19 on individuals with intellectual disabilities in the United States. NEJM Catalyst. 2021.doi.org/10.1056/CAT.21.0051
28. Nankervis K, Chan J. Applying the CRPD to people with intellectual and developmental disability with behaviors of concern during COVID-19. J Policy Pract Intellect Disabil. 2021:10.1111/jppi.12374. doi:10.1111/jppi.12374
29. Alaska Department of Health and Social Services, Division of Public Health, Rural and Community Health Systems. Patient care strategies for scarce resource situations. Version 1. August 2021. Accessed November 11, 2021, https://dhss.alaska.gov/dph/Epi/id/SiteAssets/Pages/HumanCoV/SOA_DHSS_CrisisStandardsOfCare.pdf
30. Cost-effectiveness, the QALY, and the evlyg. ICER. May 21, 2021. Accessed January 24, 2022. https://icer.org/our-approach/methods-process/cost-effectiveness-the-qaly-and-the-evlyg/
Intervention in Acute Hospital Unit Reduces Delirium Incidence for Older Adults, Has No Effect on Length of Stay, Other Complications
Study Overview
Objective: To examine the effect of the intervention “Eat Walk Engage,” a program that is designed to more consistently deliver age-friendly principles of care to older individuals in acute medical and surgical wards.
Design: This cluster randomized trial to examine the effect of an intervention in acute medical and surgical wards on older adults was conducted in 8 acute medical and surgical wards in 4 public hospitals in Australia from 2016 to 2017. To be eligible to participate in this trial, wards had to have the following: a patient population with 50% of patients aged 65 years and older; perceived alignment with hospital priorities; and nurse manager agreement to participation. Randomization was stratified by hospital, resulting in 4 wards with the intervention (a general medicine ward, an orthopedic ward, a general surgery ward, and a respiratory medicine ward) and 4 control wards (2 general medicine wards, a respiratory medicine ward, and a general surgery ward). Participants were consecutive inpatients aged 65 years or older who were admitted to the ward for at least 3 consecutive days during the study time period. Exclusion criteria included terminal or critical illness, severe cognitive impairment without a surrogate decision-maker, non-English speaking, or previously enrolled in the trial. Of a total of 453 patients who were eligible from the intervention wards, 188 were excluded and 6 died, yielding 259 participants in the intervention group. There were 413 patients eligible from the control wards, with 139 excluded and 3 deaths, yielding 271 participants in the control group.
Intervention: The intervention, called “Eat Walk Engage,” was developed to target older adults at risk for hospital-associated complications of delirium, functional decline, pressure injuries, falls, and incontinence, and aimed to improve care practices, environment, and culture to support age-friendly principles. This ward-based program delivered a structured improvement intervention through a site facilitator who is a nurse or allied health professional. The site facilitator identified opportunities for improvement using structured assessments of context, patient-experience interviews, and audits of care processes, and engaged an interdisciplinary working group from the intervention wards to participate in an hour-per-month meeting to develop plans for iterative improvements. Each site developed their own intervention plan; examples of interventions include shifting priorities to enable staff to increase the proportion of patients sitting in a chair for meals; designating the patient lounge as a walking destination to increase the proportion of time patients spend mobile; and using orientation boards and small groups to engage older patients in meaningful activities.
Main outcome measures: Study outcome measures included hospital-associated complications for older people, which is a composite of hospital-associated delirium, hospital-associated disability, hospital-associated incontinence, and fall or pressure injury during hospitalization. Delirium was assessed using the 3-minute diagnostic interview for Confusion Assessment Method (3D-CAM); hospital-associated disability was defined as new disability at discharge compared to 2 weeks prior to hospitalization. The primary outcome was defined as incidence of any complications and hospital length of stay. Secondary outcomes included incidence of individual complications, hospital discharge to facility, mortality at 6 months, and readmission for any cause at 6 months.
Main results: Patient characteristics for the intervention and control groups, respectively, were: 47% women with a mean age of 75.9 years (SD, 7.3), and 53% women with a mean age of 78.0 years (SD, 8.2). For the primary outcome, 46.4% of participants in the intervention group experienced any hospital complications compared with 51.8% in the control group (odds ratio [OR], 1.07; 95% CI, 0.71-1.61). The incidence of delirium was lower in the intervention group as compared with the control group (15.9% vs 31.4%; OR, 0.53; 95% CI, 0.31-0.90), while there were no other differences in the incidence rates of other complications. There was also no difference in hospital length of stay; median length of stay in the intervention group was 6 days (interquartile range [IQR], 4-9 days) compared with 7 days in the control group (IQR, 5-10), with an estimated mean difference in length of stay of 0.16 days (95% CI, –0.43 to 0.78 days). There was also no significant difference in mortality or all-cause readmission at 6 months.
Conclusion: The intervention “Eat Walk Engage” did not reduce hospital-associated complications overall or hospital length of stay, but it did reduce the incidence of hospital-associated delirium.
Commentary
Older adults, often with reduced physiologic reserve, when admitted to the hospital with an acute illness may be vulnerable to potential hazards of hospitalization, such as complications from prolonged periods of immobility, pressure injury, and delirium.1 Models of care in the inpatient setting to reduce these hazards, including the Acute Care for the Elderly model and the Mobile Acute Care for the Elderly Team model, have been examined in clinical trials.2,3 Specifically, models of care to prevent and treat delirium have been developed and tested over the past decade.4 The effect of these models in improving function, reducing complications, and reducing delirium incidence has been well documented. The present study adds to the literature by testing a model that utilizes implementation science methods to take into account real-world settings. In contrast with prior models-of-care studies, the implementation of the intervention at each ward was not prescriptive, but rather was developed in each ward in an iterative manner with stakeholder input. The advantage of this approach is that engagement of stakeholders at each intervention ward obtains buy-in from staff, mobilizing staff in a way that a prescriptive model of care may not; this ultimately may lead to longer-lasting change. The iterative approach also allows for the intervention to be adapted to conditions and settings over time. Other studies have taken this approach of using implementation science to drive change.5 Although the intervention in the present study failed to improve the primary outcome, it did reduce the incidence of delirium, which is a significant outcome and one that may confer considerable benefits to older adults under the model’s care.
A limitation of the intervention’s nonprescriptive approach is that, because of the variation of the interventions across sites, it is difficult to discern what elements drove the clinical outcomes. In addition, it would be challenging to consider what aspects of the intervention did not work should refinement or changes be needed. How one may measure fidelity to the intervention or how well a site implements the intervention and its relationship with clinical outcomes will need to be examined further.
Application for Clinical Practice
Clinicians look to effective models of care to improve clinical outcomes for older adults in the hospital. The intervention described in this study offers a real-world approach that may need less upfront investment than other recently studied models, such as the Acute Care for the Elderly model, which requires structural and staffing enhancements. Clinicians and health system leaders may consider implementing this model to improve the care delivered to older adults in the hospital as it may help reduce the incidence of delirium among the older adults they serve.
–William W. Hung, MD, MPH
Disclosures: None.
1. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. doi:10.7326/0003-4819-118-3-199302010-00011
2. Fox MT, Persaud M, Maimets I, et al. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta-analysis. J Am Geriatr Soc. 2012;60(12):2237-2245. doi:10.1111/jgs.12028
3. Hung WW, Ross JS, Farber J, Siu AL. Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA Intern Med. 2013;173(11):990-996. doi:10.1001/jamainternmed.2013.478
4. Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital Elder Life Program: systematic review and meta-analysis of effectiveness. Am J Geriatr Psychiatry. 2018;26(10):1015-1033. doi:10.1016/j.jagp.2018.06.007
5. Naughton C, Cummins H, de Foubert M, et al. Implementation of the Frailty Care Bundle (FCB) to promote mobilisation, nutrition and cognitive engagement in older people in acute care settings: protocol for an implementation science study. [version 1; peer review: 1 approved]. HRB Open Res. 2022;5:3. doi:10.12688/hrbopenres.134731
Study Overview
Objective: To examine the effect of the intervention “Eat Walk Engage,” a program that is designed to more consistently deliver age-friendly principles of care to older individuals in acute medical and surgical wards.
Design: This cluster randomized trial to examine the effect of an intervention in acute medical and surgical wards on older adults was conducted in 8 acute medical and surgical wards in 4 public hospitals in Australia from 2016 to 2017. To be eligible to participate in this trial, wards had to have the following: a patient population with 50% of patients aged 65 years and older; perceived alignment with hospital priorities; and nurse manager agreement to participation. Randomization was stratified by hospital, resulting in 4 wards with the intervention (a general medicine ward, an orthopedic ward, a general surgery ward, and a respiratory medicine ward) and 4 control wards (2 general medicine wards, a respiratory medicine ward, and a general surgery ward). Participants were consecutive inpatients aged 65 years or older who were admitted to the ward for at least 3 consecutive days during the study time period. Exclusion criteria included terminal or critical illness, severe cognitive impairment without a surrogate decision-maker, non-English speaking, or previously enrolled in the trial. Of a total of 453 patients who were eligible from the intervention wards, 188 were excluded and 6 died, yielding 259 participants in the intervention group. There were 413 patients eligible from the control wards, with 139 excluded and 3 deaths, yielding 271 participants in the control group.
Intervention: The intervention, called “Eat Walk Engage,” was developed to target older adults at risk for hospital-associated complications of delirium, functional decline, pressure injuries, falls, and incontinence, and aimed to improve care practices, environment, and culture to support age-friendly principles. This ward-based program delivered a structured improvement intervention through a site facilitator who is a nurse or allied health professional. The site facilitator identified opportunities for improvement using structured assessments of context, patient-experience interviews, and audits of care processes, and engaged an interdisciplinary working group from the intervention wards to participate in an hour-per-month meeting to develop plans for iterative improvements. Each site developed their own intervention plan; examples of interventions include shifting priorities to enable staff to increase the proportion of patients sitting in a chair for meals; designating the patient lounge as a walking destination to increase the proportion of time patients spend mobile; and using orientation boards and small groups to engage older patients in meaningful activities.
Main outcome measures: Study outcome measures included hospital-associated complications for older people, which is a composite of hospital-associated delirium, hospital-associated disability, hospital-associated incontinence, and fall or pressure injury during hospitalization. Delirium was assessed using the 3-minute diagnostic interview for Confusion Assessment Method (3D-CAM); hospital-associated disability was defined as new disability at discharge compared to 2 weeks prior to hospitalization. The primary outcome was defined as incidence of any complications and hospital length of stay. Secondary outcomes included incidence of individual complications, hospital discharge to facility, mortality at 6 months, and readmission for any cause at 6 months.
Main results: Patient characteristics for the intervention and control groups, respectively, were: 47% women with a mean age of 75.9 years (SD, 7.3), and 53% women with a mean age of 78.0 years (SD, 8.2). For the primary outcome, 46.4% of participants in the intervention group experienced any hospital complications compared with 51.8% in the control group (odds ratio [OR], 1.07; 95% CI, 0.71-1.61). The incidence of delirium was lower in the intervention group as compared with the control group (15.9% vs 31.4%; OR, 0.53; 95% CI, 0.31-0.90), while there were no other differences in the incidence rates of other complications. There was also no difference in hospital length of stay; median length of stay in the intervention group was 6 days (interquartile range [IQR], 4-9 days) compared with 7 days in the control group (IQR, 5-10), with an estimated mean difference in length of stay of 0.16 days (95% CI, –0.43 to 0.78 days). There was also no significant difference in mortality or all-cause readmission at 6 months.
Conclusion: The intervention “Eat Walk Engage” did not reduce hospital-associated complications overall or hospital length of stay, but it did reduce the incidence of hospital-associated delirium.
Commentary
Older adults, often with reduced physiologic reserve, when admitted to the hospital with an acute illness may be vulnerable to potential hazards of hospitalization, such as complications from prolonged periods of immobility, pressure injury, and delirium.1 Models of care in the inpatient setting to reduce these hazards, including the Acute Care for the Elderly model and the Mobile Acute Care for the Elderly Team model, have been examined in clinical trials.2,3 Specifically, models of care to prevent and treat delirium have been developed and tested over the past decade.4 The effect of these models in improving function, reducing complications, and reducing delirium incidence has been well documented. The present study adds to the literature by testing a model that utilizes implementation science methods to take into account real-world settings. In contrast with prior models-of-care studies, the implementation of the intervention at each ward was not prescriptive, but rather was developed in each ward in an iterative manner with stakeholder input. The advantage of this approach is that engagement of stakeholders at each intervention ward obtains buy-in from staff, mobilizing staff in a way that a prescriptive model of care may not; this ultimately may lead to longer-lasting change. The iterative approach also allows for the intervention to be adapted to conditions and settings over time. Other studies have taken this approach of using implementation science to drive change.5 Although the intervention in the present study failed to improve the primary outcome, it did reduce the incidence of delirium, which is a significant outcome and one that may confer considerable benefits to older adults under the model’s care.
A limitation of the intervention’s nonprescriptive approach is that, because of the variation of the interventions across sites, it is difficult to discern what elements drove the clinical outcomes. In addition, it would be challenging to consider what aspects of the intervention did not work should refinement or changes be needed. How one may measure fidelity to the intervention or how well a site implements the intervention and its relationship with clinical outcomes will need to be examined further.
Application for Clinical Practice
Clinicians look to effective models of care to improve clinical outcomes for older adults in the hospital. The intervention described in this study offers a real-world approach that may need less upfront investment than other recently studied models, such as the Acute Care for the Elderly model, which requires structural and staffing enhancements. Clinicians and health system leaders may consider implementing this model to improve the care delivered to older adults in the hospital as it may help reduce the incidence of delirium among the older adults they serve.
–William W. Hung, MD, MPH
Disclosures: None.
Study Overview
Objective: To examine the effect of the intervention “Eat Walk Engage,” a program that is designed to more consistently deliver age-friendly principles of care to older individuals in acute medical and surgical wards.
Design: This cluster randomized trial to examine the effect of an intervention in acute medical and surgical wards on older adults was conducted in 8 acute medical and surgical wards in 4 public hospitals in Australia from 2016 to 2017. To be eligible to participate in this trial, wards had to have the following: a patient population with 50% of patients aged 65 years and older; perceived alignment with hospital priorities; and nurse manager agreement to participation. Randomization was stratified by hospital, resulting in 4 wards with the intervention (a general medicine ward, an orthopedic ward, a general surgery ward, and a respiratory medicine ward) and 4 control wards (2 general medicine wards, a respiratory medicine ward, and a general surgery ward). Participants were consecutive inpatients aged 65 years or older who were admitted to the ward for at least 3 consecutive days during the study time period. Exclusion criteria included terminal or critical illness, severe cognitive impairment without a surrogate decision-maker, non-English speaking, or previously enrolled in the trial. Of a total of 453 patients who were eligible from the intervention wards, 188 were excluded and 6 died, yielding 259 participants in the intervention group. There were 413 patients eligible from the control wards, with 139 excluded and 3 deaths, yielding 271 participants in the control group.
Intervention: The intervention, called “Eat Walk Engage,” was developed to target older adults at risk for hospital-associated complications of delirium, functional decline, pressure injuries, falls, and incontinence, and aimed to improve care practices, environment, and culture to support age-friendly principles. This ward-based program delivered a structured improvement intervention through a site facilitator who is a nurse or allied health professional. The site facilitator identified opportunities for improvement using structured assessments of context, patient-experience interviews, and audits of care processes, and engaged an interdisciplinary working group from the intervention wards to participate in an hour-per-month meeting to develop plans for iterative improvements. Each site developed their own intervention plan; examples of interventions include shifting priorities to enable staff to increase the proportion of patients sitting in a chair for meals; designating the patient lounge as a walking destination to increase the proportion of time patients spend mobile; and using orientation boards and small groups to engage older patients in meaningful activities.
Main outcome measures: Study outcome measures included hospital-associated complications for older people, which is a composite of hospital-associated delirium, hospital-associated disability, hospital-associated incontinence, and fall or pressure injury during hospitalization. Delirium was assessed using the 3-minute diagnostic interview for Confusion Assessment Method (3D-CAM); hospital-associated disability was defined as new disability at discharge compared to 2 weeks prior to hospitalization. The primary outcome was defined as incidence of any complications and hospital length of stay. Secondary outcomes included incidence of individual complications, hospital discharge to facility, mortality at 6 months, and readmission for any cause at 6 months.
Main results: Patient characteristics for the intervention and control groups, respectively, were: 47% women with a mean age of 75.9 years (SD, 7.3), and 53% women with a mean age of 78.0 years (SD, 8.2). For the primary outcome, 46.4% of participants in the intervention group experienced any hospital complications compared with 51.8% in the control group (odds ratio [OR], 1.07; 95% CI, 0.71-1.61). The incidence of delirium was lower in the intervention group as compared with the control group (15.9% vs 31.4%; OR, 0.53; 95% CI, 0.31-0.90), while there were no other differences in the incidence rates of other complications. There was also no difference in hospital length of stay; median length of stay in the intervention group was 6 days (interquartile range [IQR], 4-9 days) compared with 7 days in the control group (IQR, 5-10), with an estimated mean difference in length of stay of 0.16 days (95% CI, –0.43 to 0.78 days). There was also no significant difference in mortality or all-cause readmission at 6 months.
Conclusion: The intervention “Eat Walk Engage” did not reduce hospital-associated complications overall or hospital length of stay, but it did reduce the incidence of hospital-associated delirium.
Commentary
Older adults, often with reduced physiologic reserve, when admitted to the hospital with an acute illness may be vulnerable to potential hazards of hospitalization, such as complications from prolonged periods of immobility, pressure injury, and delirium.1 Models of care in the inpatient setting to reduce these hazards, including the Acute Care for the Elderly model and the Mobile Acute Care for the Elderly Team model, have been examined in clinical trials.2,3 Specifically, models of care to prevent and treat delirium have been developed and tested over the past decade.4 The effect of these models in improving function, reducing complications, and reducing delirium incidence has been well documented. The present study adds to the literature by testing a model that utilizes implementation science methods to take into account real-world settings. In contrast with prior models-of-care studies, the implementation of the intervention at each ward was not prescriptive, but rather was developed in each ward in an iterative manner with stakeholder input. The advantage of this approach is that engagement of stakeholders at each intervention ward obtains buy-in from staff, mobilizing staff in a way that a prescriptive model of care may not; this ultimately may lead to longer-lasting change. The iterative approach also allows for the intervention to be adapted to conditions and settings over time. Other studies have taken this approach of using implementation science to drive change.5 Although the intervention in the present study failed to improve the primary outcome, it did reduce the incidence of delirium, which is a significant outcome and one that may confer considerable benefits to older adults under the model’s care.
A limitation of the intervention’s nonprescriptive approach is that, because of the variation of the interventions across sites, it is difficult to discern what elements drove the clinical outcomes. In addition, it would be challenging to consider what aspects of the intervention did not work should refinement or changes be needed. How one may measure fidelity to the intervention or how well a site implements the intervention and its relationship with clinical outcomes will need to be examined further.
Application for Clinical Practice
Clinicians look to effective models of care to improve clinical outcomes for older adults in the hospital. The intervention described in this study offers a real-world approach that may need less upfront investment than other recently studied models, such as the Acute Care for the Elderly model, which requires structural and staffing enhancements. Clinicians and health system leaders may consider implementing this model to improve the care delivered to older adults in the hospital as it may help reduce the incidence of delirium among the older adults they serve.
–William W. Hung, MD, MPH
Disclosures: None.
1. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. doi:10.7326/0003-4819-118-3-199302010-00011
2. Fox MT, Persaud M, Maimets I, et al. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta-analysis. J Am Geriatr Soc. 2012;60(12):2237-2245. doi:10.1111/jgs.12028
3. Hung WW, Ross JS, Farber J, Siu AL. Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA Intern Med. 2013;173(11):990-996. doi:10.1001/jamainternmed.2013.478
4. Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital Elder Life Program: systematic review and meta-analysis of effectiveness. Am J Geriatr Psychiatry. 2018;26(10):1015-1033. doi:10.1016/j.jagp.2018.06.007
5. Naughton C, Cummins H, de Foubert M, et al. Implementation of the Frailty Care Bundle (FCB) to promote mobilisation, nutrition and cognitive engagement in older people in acute care settings: protocol for an implementation science study. [version 1; peer review: 1 approved]. HRB Open Res. 2022;5:3. doi:10.12688/hrbopenres.134731
1. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. doi:10.7326/0003-4819-118-3-199302010-00011
2. Fox MT, Persaud M, Maimets I, et al. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta-analysis. J Am Geriatr Soc. 2012;60(12):2237-2245. doi:10.1111/jgs.12028
3. Hung WW, Ross JS, Farber J, Siu AL. Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA Intern Med. 2013;173(11):990-996. doi:10.1001/jamainternmed.2013.478
4. Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital Elder Life Program: systematic review and meta-analysis of effectiveness. Am J Geriatr Psychiatry. 2018;26(10):1015-1033. doi:10.1016/j.jagp.2018.06.007
5. Naughton C, Cummins H, de Foubert M, et al. Implementation of the Frailty Care Bundle (FCB) to promote mobilisation, nutrition and cognitive engagement in older people in acute care settings: protocol for an implementation science study. [version 1; peer review: 1 approved]. HRB Open Res. 2022;5:3. doi:10.12688/hrbopenres.134731
Pruritus in elderly patients: Not a diagnosis
that has appeared seemingly out of the blue.
“They ask: ‘What happened? Why did I get this? Everything was going so well and all of a sudden, I get this itchy rash that keeps me up every night,’ ” Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, said during the Revolutionizing Atopic Dermatitis symposium. “Is this elderly atopic dermatitis? Is that a real thing?”
But such patients often lack flexural involvement, which is a telltale sign of atopic dermatitis, “so I really struggle with making the diagnosis of new onset AD in the elderly,” he said, adding that existing medical literature on the topic is variable, with the use of terms that include chronic eczematous eruption of the elderly, chronic “eczematiform” eruption in the elderly, chronic eczematous eruption of the aged, eczematous dermatitis not otherwise specified, dermal hypersensitivity reaction, urticarial dermatitis, and eczematous drug eruptions.
“Pruritus of the elderly is not a diagnosis,” Dr. Simpson said. “That’s just a symptom with a million etiologies. Never put that as your assessment. You could put pruritic eruption or pruritus, but try to look for the cause.”
More than 50% of older patients have xerosis, according to a 2013 clinical review on pruritus in the elderly, by Timothy G. Berger, MD, and colleagues at the University of California, San Francisco, which includes advice on the evaluation and management of pruritus in this group of patients based on whether they have a rash or not. For a patient with no rash, Dr. Simpson said, the workup “includes ruling out xerosis, scabies, and effects of medications that could cause rash such as narcotics and Adderall; as well as a generalized pruritus workup including renal and hepatic function, blood count, and thyroid levels.”
In a separate analysis of pruritic elderly patients by the same authors, five rash-related diagnoses accounted for 75% of cases: eczematous dermatitis, lichen simplex/prurigo nodularis, subacute prurigo, transient acantholytic dermatosis, and neuropathic disorder. “Morphology of pruritus with rash is also important,” Dr. Simpson added. “Is it eczematous? Papular? Prurigo nodularis? This helps lead you in the right direction.”
Some case-control studies have shown that calcium channel blockers could be related to eczema in older patients.
“But there aren’t a lot of studies out there that show that when you stop your calcium channel blocker, your eczema gets better,” Dr. Simpson said. “I’m reluctant to stop medications to try to help their eczema. I haven’t had many good results doing that.”
In an abstract presented during the 2021 annual meeting of the Society of Investigative Dermatology, he and his colleagues prospectively reviewed 89 patients over age 65 who had been referred with new-onset eczema. Of these, 34 underwent drug cessation trials for 1-3 months. “Not one patient improved when they stopped medications,” Dr. Simpson said, but “multiple patients were hospitalized for discontinuing their cardiac and antihypertensive medications.” While this was a biased sample of patients coming to him with chronic eczema, “in my experience, if you have chronic eczema in an older patient, stopping medications is likely not going to help.”
Other diagnostic tips he offered included asking patients what skin products they’re using, considering patch testing, and considering biopsy to rule out cutaneous T-cell lymphoma or bullous pemphigoid. “If you’re not sure there’s a rash, you might need to do a pruritus workup,” he said. If an eczematous rash is present and no other cause is found, try treating it like AD, he added.
Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
that has appeared seemingly out of the blue.
“They ask: ‘What happened? Why did I get this? Everything was going so well and all of a sudden, I get this itchy rash that keeps me up every night,’ ” Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, said during the Revolutionizing Atopic Dermatitis symposium. “Is this elderly atopic dermatitis? Is that a real thing?”
But such patients often lack flexural involvement, which is a telltale sign of atopic dermatitis, “so I really struggle with making the diagnosis of new onset AD in the elderly,” he said, adding that existing medical literature on the topic is variable, with the use of terms that include chronic eczematous eruption of the elderly, chronic “eczematiform” eruption in the elderly, chronic eczematous eruption of the aged, eczematous dermatitis not otherwise specified, dermal hypersensitivity reaction, urticarial dermatitis, and eczematous drug eruptions.
“Pruritus of the elderly is not a diagnosis,” Dr. Simpson said. “That’s just a symptom with a million etiologies. Never put that as your assessment. You could put pruritic eruption or pruritus, but try to look for the cause.”
More than 50% of older patients have xerosis, according to a 2013 clinical review on pruritus in the elderly, by Timothy G. Berger, MD, and colleagues at the University of California, San Francisco, which includes advice on the evaluation and management of pruritus in this group of patients based on whether they have a rash or not. For a patient with no rash, Dr. Simpson said, the workup “includes ruling out xerosis, scabies, and effects of medications that could cause rash such as narcotics and Adderall; as well as a generalized pruritus workup including renal and hepatic function, blood count, and thyroid levels.”
In a separate analysis of pruritic elderly patients by the same authors, five rash-related diagnoses accounted for 75% of cases: eczematous dermatitis, lichen simplex/prurigo nodularis, subacute prurigo, transient acantholytic dermatosis, and neuropathic disorder. “Morphology of pruritus with rash is also important,” Dr. Simpson added. “Is it eczematous? Papular? Prurigo nodularis? This helps lead you in the right direction.”
Some case-control studies have shown that calcium channel blockers could be related to eczema in older patients.
“But there aren’t a lot of studies out there that show that when you stop your calcium channel blocker, your eczema gets better,” Dr. Simpson said. “I’m reluctant to stop medications to try to help their eczema. I haven’t had many good results doing that.”
In an abstract presented during the 2021 annual meeting of the Society of Investigative Dermatology, he and his colleagues prospectively reviewed 89 patients over age 65 who had been referred with new-onset eczema. Of these, 34 underwent drug cessation trials for 1-3 months. “Not one patient improved when they stopped medications,” Dr. Simpson said, but “multiple patients were hospitalized for discontinuing their cardiac and antihypertensive medications.” While this was a biased sample of patients coming to him with chronic eczema, “in my experience, if you have chronic eczema in an older patient, stopping medications is likely not going to help.”
Other diagnostic tips he offered included asking patients what skin products they’re using, considering patch testing, and considering biopsy to rule out cutaneous T-cell lymphoma or bullous pemphigoid. “If you’re not sure there’s a rash, you might need to do a pruritus workup,” he said. If an eczematous rash is present and no other cause is found, try treating it like AD, he added.
Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
that has appeared seemingly out of the blue.
“They ask: ‘What happened? Why did I get this? Everything was going so well and all of a sudden, I get this itchy rash that keeps me up every night,’ ” Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, said during the Revolutionizing Atopic Dermatitis symposium. “Is this elderly atopic dermatitis? Is that a real thing?”
But such patients often lack flexural involvement, which is a telltale sign of atopic dermatitis, “so I really struggle with making the diagnosis of new onset AD in the elderly,” he said, adding that existing medical literature on the topic is variable, with the use of terms that include chronic eczematous eruption of the elderly, chronic “eczematiform” eruption in the elderly, chronic eczematous eruption of the aged, eczematous dermatitis not otherwise specified, dermal hypersensitivity reaction, urticarial dermatitis, and eczematous drug eruptions.
“Pruritus of the elderly is not a diagnosis,” Dr. Simpson said. “That’s just a symptom with a million etiologies. Never put that as your assessment. You could put pruritic eruption or pruritus, but try to look for the cause.”
More than 50% of older patients have xerosis, according to a 2013 clinical review on pruritus in the elderly, by Timothy G. Berger, MD, and colleagues at the University of California, San Francisco, which includes advice on the evaluation and management of pruritus in this group of patients based on whether they have a rash or not. For a patient with no rash, Dr. Simpson said, the workup “includes ruling out xerosis, scabies, and effects of medications that could cause rash such as narcotics and Adderall; as well as a generalized pruritus workup including renal and hepatic function, blood count, and thyroid levels.”
In a separate analysis of pruritic elderly patients by the same authors, five rash-related diagnoses accounted for 75% of cases: eczematous dermatitis, lichen simplex/prurigo nodularis, subacute prurigo, transient acantholytic dermatosis, and neuropathic disorder. “Morphology of pruritus with rash is also important,” Dr. Simpson added. “Is it eczematous? Papular? Prurigo nodularis? This helps lead you in the right direction.”
Some case-control studies have shown that calcium channel blockers could be related to eczema in older patients.
“But there aren’t a lot of studies out there that show that when you stop your calcium channel blocker, your eczema gets better,” Dr. Simpson said. “I’m reluctant to stop medications to try to help their eczema. I haven’t had many good results doing that.”
In an abstract presented during the 2021 annual meeting of the Society of Investigative Dermatology, he and his colleagues prospectively reviewed 89 patients over age 65 who had been referred with new-onset eczema. Of these, 34 underwent drug cessation trials for 1-3 months. “Not one patient improved when they stopped medications,” Dr. Simpson said, but “multiple patients were hospitalized for discontinuing their cardiac and antihypertensive medications.” While this was a biased sample of patients coming to him with chronic eczema, “in my experience, if you have chronic eczema in an older patient, stopping medications is likely not going to help.”
Other diagnostic tips he offered included asking patients what skin products they’re using, considering patch testing, and considering biopsy to rule out cutaneous T-cell lymphoma or bullous pemphigoid. “If you’re not sure there’s a rash, you might need to do a pruritus workup,” he said. If an eczematous rash is present and no other cause is found, try treating it like AD, he added.
Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
FROM REVOLUTIONIZING AD 2021
Moderate-vigorous stepping seen to lower diabetes risk in older women
More steps per day, particularly at a higher intensity, may reduce the risk of type 2 diabetes in older women, based on a prospective cohort study.
The link between daily stepping and diabetes was not significantly modified by body mass index (BMI) or other common diabetes risk factors, suggesting that the relationship is highly generalizable, lead author Alexis C. Garduno, MPH, a PhD student at the University of California, San Diego, and colleagues reported.
“Physical activity is a key modifiable behavior for diabetes prevention and management,” the investigators wrote in Diabetes Care. “Many prevention studies have demonstrated that regular physical activity, along with improved diet, reduces the risk of diabetes in adults. ... To the best of our knowledge, there are few studies examining the association between objectively measured steps per day and incident diabetes in a community-based setting.”
To this end, the investigators analyzed data from 4,838 older, community-living women in the Objective Physical Activity and Cardiovascular Health Study. Upon enrollment, women were without physician-diagnosed diabetes and had a mean age of 78.9 years. For 1 week, participants wore ActiGraph GT3X+ accelerometers to measure steps per day, as well as step intensity, graded as light or moderate to vigorous.
The relationship between daily activity and diabetes was analyzed using three multivariate models: The first included race/ethnicity and age; the second also included family history of diabetes, education, physical functioning, self-rated health, smoking status, and alcohol consumption; and the third added BMI, “a potential mediator in the causal pathway between steps per day and diabetes,” the investigators wrote.
Participants took an average of 3,729 steps per day, divided roughly evenly between light and moderate to vigorous intensity.
After a median follow-up of 5.7 years, 8.1% of women developed diabetes. The least-adjusted model showed a 14% reduction in diabetes risk per 2,000 steps (hazard ratio, 0.86; 95% confidence interval, 0.80-0.92; P = .007), whereas the second model, adjusting for more confounding variables, showed a 12% reduction in diabetes risk per 2,000 steps (HR, 0.88; 95% CI, 0.78-1.00; P = .045).
The final model, which added BMI, showed a 10% reduction in risk, although it didn’t reach statistical significance (HR, 0.90; 95% CI, 0.80-1.02; P = .11). Furthermore, accelerated failure time models suggested that BMI did not significantly impact the link between steps and diabetes (proportion mediated, 17.7%;95% CI, –55.0 to 142.0; P = .09). Further analyses also found no significant interactions between BMI or other possible confounders.
“The steps per day–diabetes association was not modified by age, race/ethnicity, BMI, physical functioning, or family history of diabetes, which supports the generalizability of these findings to community-living older women,” the investigators wrote.
Increased stepping intensity also appeared to lower risk of diabetes. After adjusting for confounding variables, light stepping was not linked to reduced risk (HR, 0.97; 95% CI, 0.73-1.29; P = .83), whereas moderate to vigorous stepping reduced risk by 14% per 2,000 steps (HR, 0.86; 95% CI, 0.74-1.00; P = .04).
“This study provides evidence supporting an association between steps per day and lower incident diabetes,” the investigators concluded. “While further work is needed to identify whether there is a minimum number of steps per day that results in a clinically significant reduction of diabetes and to evaluate the role that step intensity plays in diabetes etiology for older adults, findings from this study suggest that moderate-vigorous–intensity steps may be more important than lower-intensity steps with respect to incident diabetes. Steps per day–based interventions are needed to advance diabetes prevention science in older adults.”
The study was supported by the National Institute on Aging, the National Institute of Diabetes and Digestive and Kidney Diseases, the Tobacco-Related Disease Research Program, and others. The investigators had no potential conflicts of interest.
More steps per day, particularly at a higher intensity, may reduce the risk of type 2 diabetes in older women, based on a prospective cohort study.
The link between daily stepping and diabetes was not significantly modified by body mass index (BMI) or other common diabetes risk factors, suggesting that the relationship is highly generalizable, lead author Alexis C. Garduno, MPH, a PhD student at the University of California, San Diego, and colleagues reported.
“Physical activity is a key modifiable behavior for diabetes prevention and management,” the investigators wrote in Diabetes Care. “Many prevention studies have demonstrated that regular physical activity, along with improved diet, reduces the risk of diabetes in adults. ... To the best of our knowledge, there are few studies examining the association between objectively measured steps per day and incident diabetes in a community-based setting.”
To this end, the investigators analyzed data from 4,838 older, community-living women in the Objective Physical Activity and Cardiovascular Health Study. Upon enrollment, women were without physician-diagnosed diabetes and had a mean age of 78.9 years. For 1 week, participants wore ActiGraph GT3X+ accelerometers to measure steps per day, as well as step intensity, graded as light or moderate to vigorous.
The relationship between daily activity and diabetes was analyzed using three multivariate models: The first included race/ethnicity and age; the second also included family history of diabetes, education, physical functioning, self-rated health, smoking status, and alcohol consumption; and the third added BMI, “a potential mediator in the causal pathway between steps per day and diabetes,” the investigators wrote.
Participants took an average of 3,729 steps per day, divided roughly evenly between light and moderate to vigorous intensity.
After a median follow-up of 5.7 years, 8.1% of women developed diabetes. The least-adjusted model showed a 14% reduction in diabetes risk per 2,000 steps (hazard ratio, 0.86; 95% confidence interval, 0.80-0.92; P = .007), whereas the second model, adjusting for more confounding variables, showed a 12% reduction in diabetes risk per 2,000 steps (HR, 0.88; 95% CI, 0.78-1.00; P = .045).
The final model, which added BMI, showed a 10% reduction in risk, although it didn’t reach statistical significance (HR, 0.90; 95% CI, 0.80-1.02; P = .11). Furthermore, accelerated failure time models suggested that BMI did not significantly impact the link between steps and diabetes (proportion mediated, 17.7%;95% CI, –55.0 to 142.0; P = .09). Further analyses also found no significant interactions between BMI or other possible confounders.
“The steps per day–diabetes association was not modified by age, race/ethnicity, BMI, physical functioning, or family history of diabetes, which supports the generalizability of these findings to community-living older women,” the investigators wrote.
Increased stepping intensity also appeared to lower risk of diabetes. After adjusting for confounding variables, light stepping was not linked to reduced risk (HR, 0.97; 95% CI, 0.73-1.29; P = .83), whereas moderate to vigorous stepping reduced risk by 14% per 2,000 steps (HR, 0.86; 95% CI, 0.74-1.00; P = .04).
“This study provides evidence supporting an association between steps per day and lower incident diabetes,” the investigators concluded. “While further work is needed to identify whether there is a minimum number of steps per day that results in a clinically significant reduction of diabetes and to evaluate the role that step intensity plays in diabetes etiology for older adults, findings from this study suggest that moderate-vigorous–intensity steps may be more important than lower-intensity steps with respect to incident diabetes. Steps per day–based interventions are needed to advance diabetes prevention science in older adults.”
The study was supported by the National Institute on Aging, the National Institute of Diabetes and Digestive and Kidney Diseases, the Tobacco-Related Disease Research Program, and others. The investigators had no potential conflicts of interest.
More steps per day, particularly at a higher intensity, may reduce the risk of type 2 diabetes in older women, based on a prospective cohort study.
The link between daily stepping and diabetes was not significantly modified by body mass index (BMI) or other common diabetes risk factors, suggesting that the relationship is highly generalizable, lead author Alexis C. Garduno, MPH, a PhD student at the University of California, San Diego, and colleagues reported.
“Physical activity is a key modifiable behavior for diabetes prevention and management,” the investigators wrote in Diabetes Care. “Many prevention studies have demonstrated that regular physical activity, along with improved diet, reduces the risk of diabetes in adults. ... To the best of our knowledge, there are few studies examining the association between objectively measured steps per day and incident diabetes in a community-based setting.”
To this end, the investigators analyzed data from 4,838 older, community-living women in the Objective Physical Activity and Cardiovascular Health Study. Upon enrollment, women were without physician-diagnosed diabetes and had a mean age of 78.9 years. For 1 week, participants wore ActiGraph GT3X+ accelerometers to measure steps per day, as well as step intensity, graded as light or moderate to vigorous.
The relationship between daily activity and diabetes was analyzed using three multivariate models: The first included race/ethnicity and age; the second also included family history of diabetes, education, physical functioning, self-rated health, smoking status, and alcohol consumption; and the third added BMI, “a potential mediator in the causal pathway between steps per day and diabetes,” the investigators wrote.
Participants took an average of 3,729 steps per day, divided roughly evenly between light and moderate to vigorous intensity.
After a median follow-up of 5.7 years, 8.1% of women developed diabetes. The least-adjusted model showed a 14% reduction in diabetes risk per 2,000 steps (hazard ratio, 0.86; 95% confidence interval, 0.80-0.92; P = .007), whereas the second model, adjusting for more confounding variables, showed a 12% reduction in diabetes risk per 2,000 steps (HR, 0.88; 95% CI, 0.78-1.00; P = .045).
The final model, which added BMI, showed a 10% reduction in risk, although it didn’t reach statistical significance (HR, 0.90; 95% CI, 0.80-1.02; P = .11). Furthermore, accelerated failure time models suggested that BMI did not significantly impact the link between steps and diabetes (proportion mediated, 17.7%;95% CI, –55.0 to 142.0; P = .09). Further analyses also found no significant interactions between BMI or other possible confounders.
“The steps per day–diabetes association was not modified by age, race/ethnicity, BMI, physical functioning, or family history of diabetes, which supports the generalizability of these findings to community-living older women,” the investigators wrote.
Increased stepping intensity also appeared to lower risk of diabetes. After adjusting for confounding variables, light stepping was not linked to reduced risk (HR, 0.97; 95% CI, 0.73-1.29; P = .83), whereas moderate to vigorous stepping reduced risk by 14% per 2,000 steps (HR, 0.86; 95% CI, 0.74-1.00; P = .04).
“This study provides evidence supporting an association between steps per day and lower incident diabetes,” the investigators concluded. “While further work is needed to identify whether there is a minimum number of steps per day that results in a clinically significant reduction of diabetes and to evaluate the role that step intensity plays in diabetes etiology for older adults, findings from this study suggest that moderate-vigorous–intensity steps may be more important than lower-intensity steps with respect to incident diabetes. Steps per day–based interventions are needed to advance diabetes prevention science in older adults.”
The study was supported by the National Institute on Aging, the National Institute of Diabetes and Digestive and Kidney Diseases, the Tobacco-Related Disease Research Program, and others. The investigators had no potential conflicts of interest.
FROM DIABETES CARE
Easing dementia caregiver burden, addressing interpersonal violence
The number of people with dementia globally is expected to reach 74.7 million by 2030 and 131.5 million by 2050.1 Because dementia is progressive, many patients will exhibit severe symptoms termed behavioral crises. Deteriorating interpersonal conduct and escalating antisocial acts result in an acquired sociopathy.2 Increasing cognitive impairment causes these patients to misunderstand intimate care and perceive it as a threat, often resulting in outbursts of violence against their caregivers.3
Available studies (TABLE4-17) make evident the incidence of interpersonal violence experienced by caregivers secondary to aggressive acts by patients with dementia. This violence ranges from verbal abuse, including racial slurs, to physical abuse—sometimes resulting in significant physical injury. Aggressive behavior by patients with dementia, resulting in violence towards their caregivers or partners, stems from progressive cognitive decline, which can make optimal care difficult. Such episodes may also impair the psychological and physical well-being of caregivers, increasing their risk of depression, anxiety, and even post-traumatic stress disorder (PTSD).18 The extent of the impact is also determined by the interpretation of the abuse by the caregivers themselves. One study suggested that the perception of aggressive or violent behavior as “normal” by a caregiver reduced the overall negative effect of the interactions.7Our review emphasizes the unintended burden that can fall to caregivers of patients with dementia. We also address the role of primary care providers (PCPs) in identifying these instances of violence and intervening appropriately by providing safety strategies, education, resources, and support.
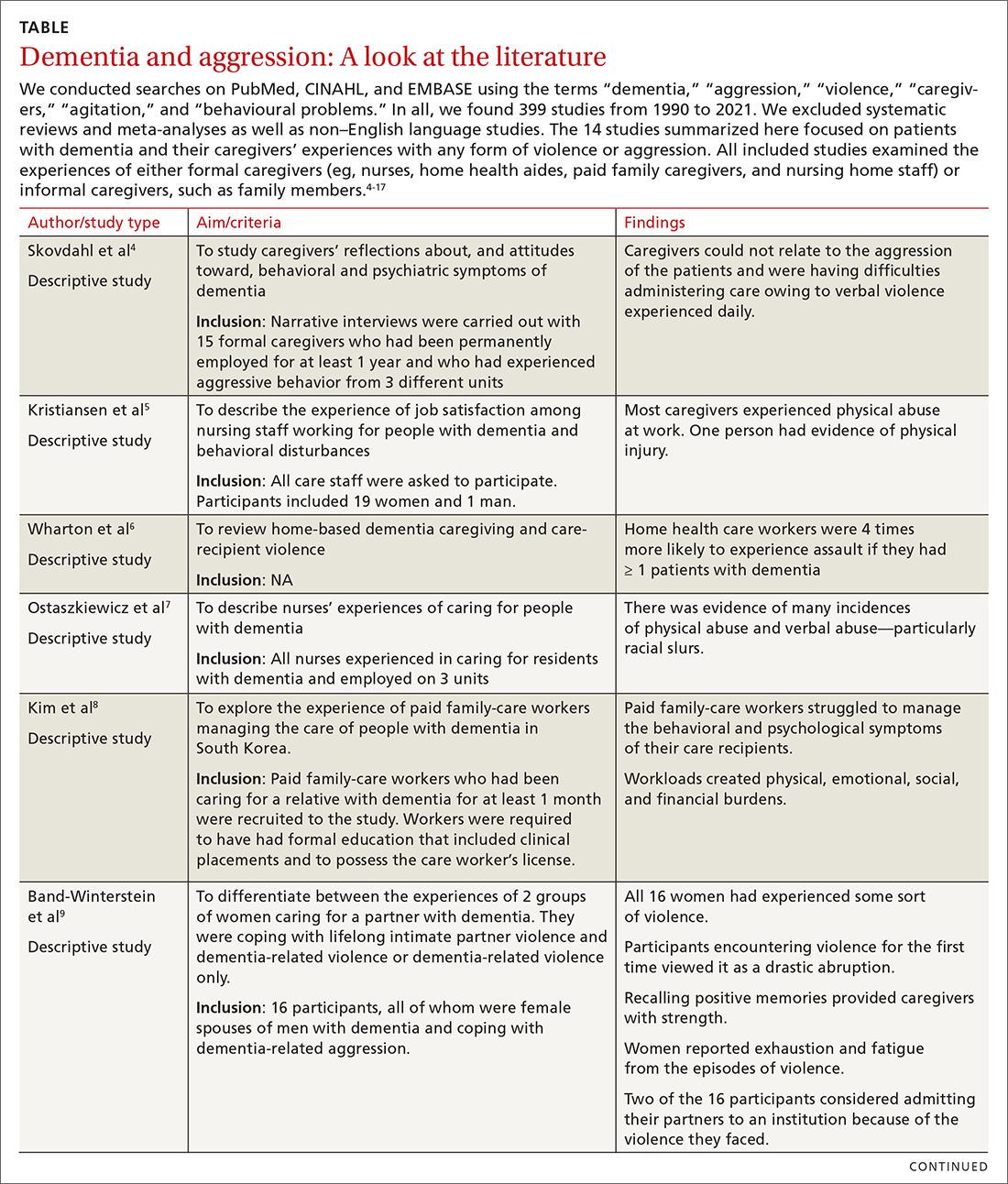
CASE
A 67-year-old man with a medical history of PTSD with depression, type 2 diabetes, alcohol use disorder/dependence, hypertension, and obstructive sleep apnea was brought to his PCP by his wife. She said he had recently been unable to keep appointment times, pay bills, or take his usual medications, venlafaxine and bupropion. She also said his PTSD symptoms had worsened. He was sleeping 12 to 14 hours per day and was increasingly irritable. The patient denied any concerns or changes in his behavior.
The PCP administered a Saint Louis University Mental Status (SLUMS) examination to screen for cognitive impairment.19 The patient scored 14/30 (less than 20 is indicative of dementia). He was unable to complete a simple math problem, recall any items from a list of 5, count in reverse, draw a clock correctly, or recall a full story. Throughout the exam, the patient demonstrated minimal effort and was often only able to complete a task after further prompting by the examiner.
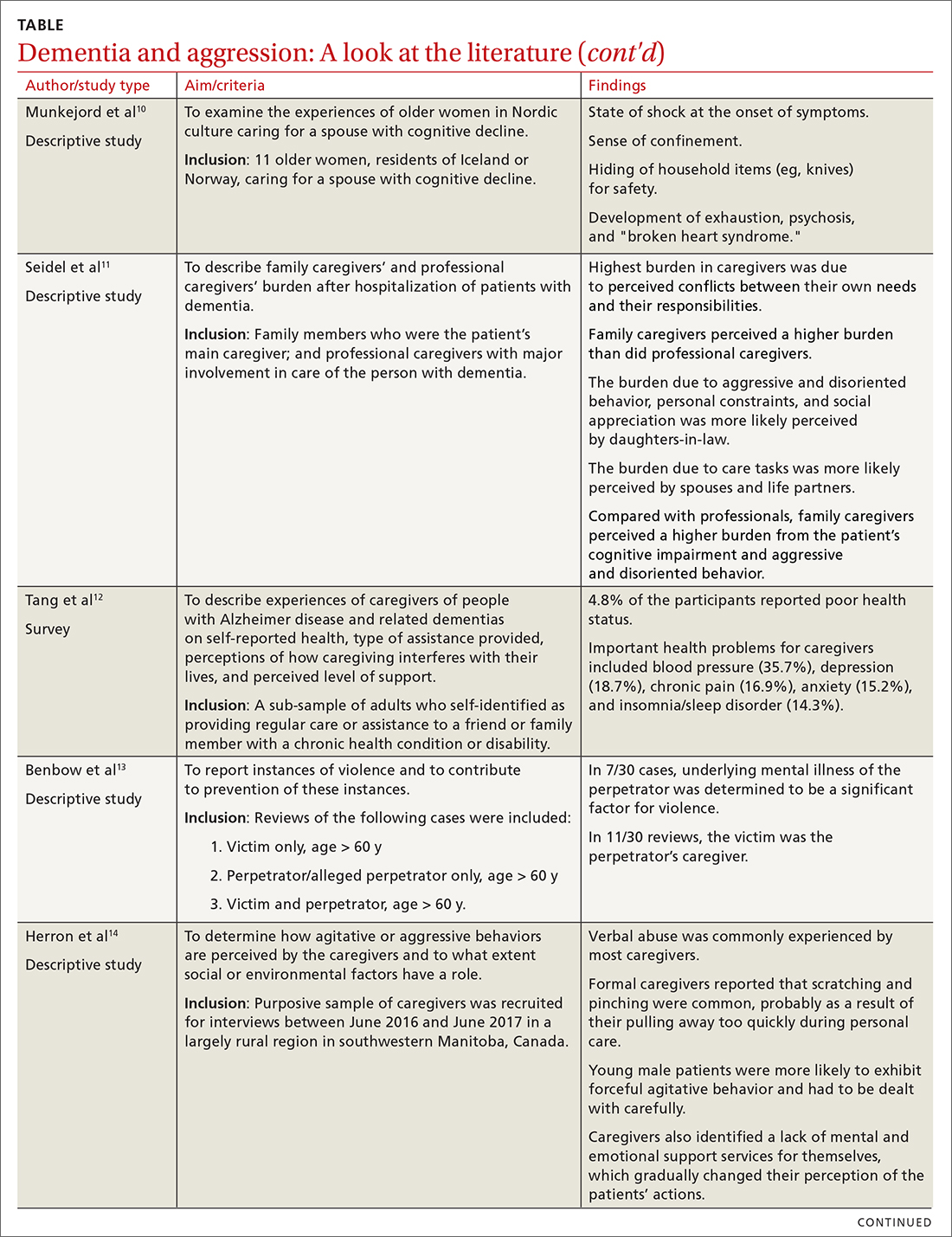
A computed tomography scan of the head revealed no signs of hemorrhage or damage. Thyroid-stimulating hormone levels and vitamin B12 levels were normal. A rapid plasma reagin test result was negative. The patient was given a diagnosis of Alzheimer disease. Donepezil was added to the patient’s medications, starting at 5 mg and then increased to 10 mg. His wife began to assist him with his tasks of daily living. His mood improved, and his wife noted he began to remember his appointments and take his medications with assistance.
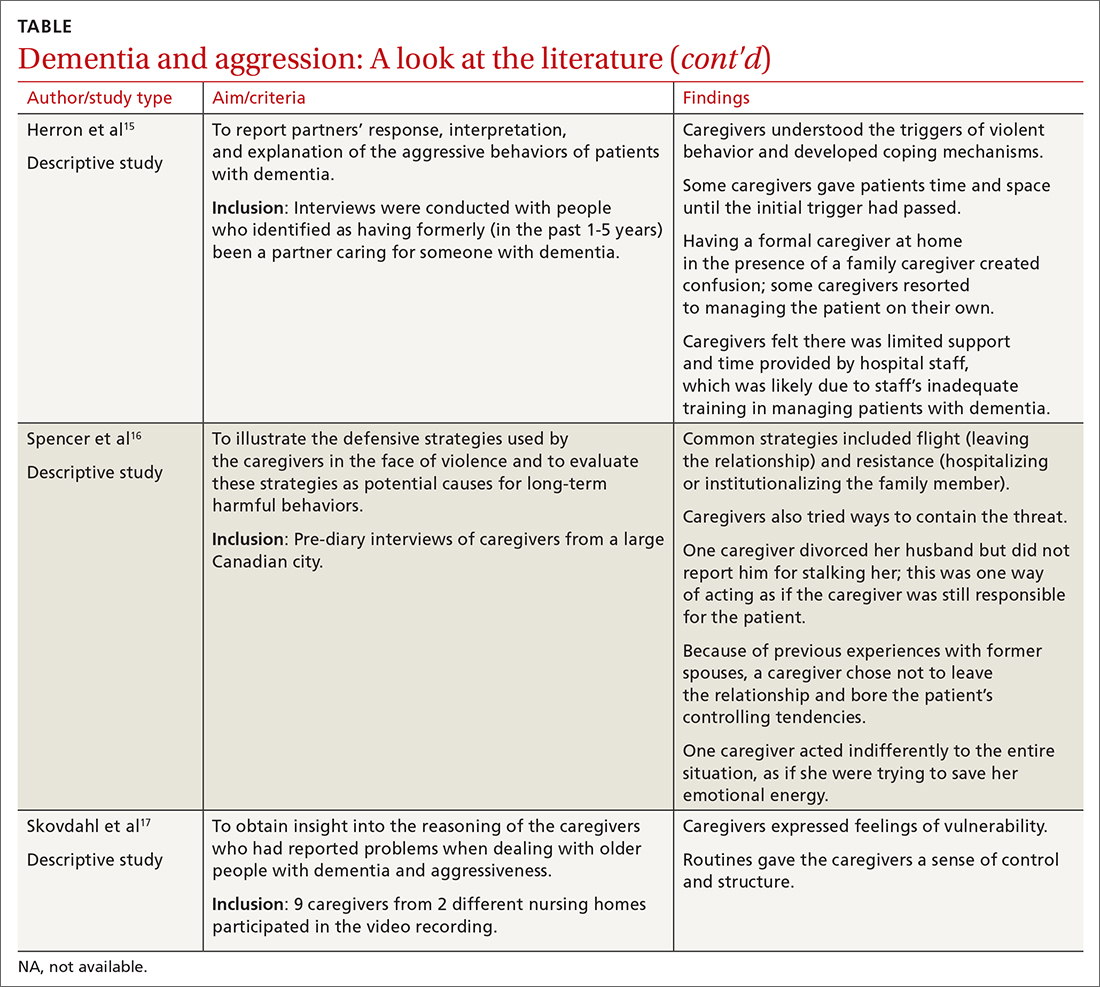
However, the patient’s irritability continued to escalate. He grew paranoid and accused his wife of mismanaging their money. This pattern steadily worsened over the course of 6 months. The situation escalated until one day the patient’s wife called a mental health hotline reporting that her husband was holding her hostage and threatening to kill her with a gun. He told her, “I can do something to you, and they won’t even find a fingernail. It doesn’t have to be with a gun either.” She was counseled to try to stay calm to avoid aggravating the situation and to go to a safe place and stay there until help arrived.
His memory had worsened to the point that he could not recall any events from the previous 2 years. He was paranoid about anyone entering his home and would not allow his deteriorating roof to be repaired or his yard to be maintained. He did not shower for weeks at a time. He slept holding a rifle and accused his wife of embezzlement.
Continue to: The patient was evaluated...
The patient was evaluated by another specialist, who assessed his SLUMS score to be 18/30. He increased the patient’s donepezil dose, initiated a bupropion taper, and added sertraline to the regimen. The PCP spoke to the patient’s wife regarding options for her safety including leaving the home, hiding firearms, and calling the police in cases of interpersonal violence. The wife said she did not want to pursue these options. She expressed worry that he might be harmed if he was uncooperative with the police and said there was no one except her to take care of him.
Caregivers struggle to care for their loved ones
Instances of personal violence lead to shock, astonishment, heartbreak, and fear. Anticipation of a recurrence of violence causes many partners and caregivers to feel exhausted, because there is minimal hope for any chance of improvement. There are a few exceptions, however, as our case will show. In addition to emotional exhaustion, there is also a never-ending sense of self-doubt, leading many caregivers to question their ability to handle their family member.20,21 Over time, this leads to caregiver burnout, leaving them unable to understand their family member’s aggression. The sudden loss of caregiver control in dealing with the patient may also result in the family member exhibiting behavioral changes reflecting emotional trauma. For caregivers who do not live with the patient, they may choose to make fewer or shorter visits—or not visit at all—because they fear being abused.7,22
Caregivers of patients with dementia often feel helpless and powerless once abrupt and drastic changes in personality lead to some form of interpersonal violence. Additionally, caregivers with a poor health status are more likely to have lower physical function and experience greater caregiving stress overall.23 Other factors increasing stress are longer years of caregiving and the severity of a patient’s dementia and functional impairment.23
Interventions to reduce caregiver burden
Many studies have assessed the role of different interventions to reduce caregiver burden, such as teaching them problem-solving skills, increasing their knowledge of dementia, recommending social resources, providing emotional support, changing caregiver perceptions of the care situation, introducing coping strategies, relying on strengths and experiences in caregiving, help-seeking, and engaging in activity programs.24-28 For Hispanic caregivers, a structured and self-paced online telenovela format has been effective in improving care and relieving caregiver stress.29 Online positive emotion regulators helped in significantly improving quality of life and physical health in the caregivers.30 In this last intervention, caregivers had 6 online sessions with a facilitator who taught them emotional regulation skills that included: noticing positive events, capitalizing on them, and feeling gratitude; practicing mindfulness; doing a positive reappraisal; acknowledging personal strengths and setting attainable goals; and performing acts of kindness. Empowerment programs have also shown significant improvement in the well-being of caregivers.31
Caregivers may reject support.
Continue to: These practical tips can help
These practical tips can help
Based on our review of the literature, we recommend offering the following supports to caregivers:
- Counsel caregivers early on in a patient’s dementia that behavior changes are likely and may be unpredictable. Explain that dementia can involve changes to personality and behavior as well as memory difficulties.33,34
- Describe resources for support, such as day programs for senior adults, insurance coverage for caregiver respite programs, and the Alzheimer’s Association (www.alz.org/). Encourage caregivers to seek general medical and mental health care for themselves. Caregivers should have opportunities and support to discuss their experiences and to be appropriately trained for the challenge of caring for a family member with dementia.35
- Encourage disclosure about abrupt changes in the patient’s behavior. This invites families to discuss issues with you and may make them more comfortable with such conversations.
- Involve ancillary services (eg, social worker) to plan for a higher level of care well in advance of it becoming necessary.
- Discuss safety strategies for the caregiver, including when it is appropriate to alter a patient’s set routines such as bedtimes and mealtimes.33,34
- Discuss when and how to involve law enforcement, if necessary.33,34 Emphasize the importance of removing firearms from the home as a safety measure. Although federal laws do not explicitly prohibit possession of arms by patients with neurologic damage, a few states mention “organic brain syndrome” or “dementia” as conditions prohibiting use or possession of firearms.36
- Suggest, as feasible, nonpharmacologic aids for the patient such as massage therapy, animal-assisted therapy, personalized interventions, music therapy, and light therapy.37 Prescribe medications to the patient to aid in behavior modification when appropriate.
- Screen caregivers and family members for signs of interpersonal violence. Take notice of changes in caregiver behavior or irregularity in attending follow-up appointments.
CASE
Over the next month, the patient’s symptoms further deteriorated. His PCP recommended hospitalization, but the patient and his wife declined. Magnetic resonance imaging of the patient’s brain revealed severe confluent and patchy regions of white matter and T2 signal hyperintensity, consistent with chronic microvascular ischemic disease. An old, small, left parietal lobe infarct was also noted.
One month later, the patient presented to the emergency department. His symptoms were largely unchanged, but his wife indicated that she could no longer live at home due to burnout. The patient’s medications were adjusted, but he was not admitted for inpatient care. His wife said they needed help at home, but the patient opposed the idea any time that it was mentioned.
A few weeks later, the patient presented for outpatient follow-up. He was delusional, believing that the government was compelling citizens to take sertraline in order to harm their mental health. He had also begun viewing online pornography in front of his wife and attempting to remove all of his money from the bank. He was prescribed aripiprazole 15 mg, and his symptoms began to improve. Soon after, however, he threatened to kill his grandson, then took all his Lasix pills (a 7-day supply) simultaneously. The patient denied that this was a suicide attempt.
Over the course of the next month, the patient began to report hearing voices. A neuropsychological evaluation confirmed a diagnosis of dementia with psychiatric symptoms due to neurologic injury. The patient was referred to a geriatric psychiatrist and continued to be managed medically. He was assigned a multidisciplinary team comprising palliative care, social work, and care management to assist in his care and provide support to the family. His behavior improved.
Continue to: At the time of this publication...
At the time of this publication, the patient’s irritability and paranoia had subsided and he had made no further threats to his family. He has allowed a home health aide into the house and has agreed to have his roof repaired. His wife still lives with him and assists him with activities of daily living.
Interprofessional teams are key
Caregiver burnout increases the risk of patient neglect or abuse, as individuals who have been the targets of aggressive behavior are more likely to leave demented patients unattended.8,16,23 Although tools are available to screen caregivers for depression and burnout, an important step forward would be to develop an interprofessional team to aid in identifying and closely following high-risk patient–caregiver groups. This continual and varied assessment of psychosocial stressors could help prevent the development of violent interactions. These teams would allow integration with the primary health care system by frequent and effective shared communication of knowledge, development of goals, and shared decision-making.38 Setting expectations, providing support, and discussing safety strategies can improve the health and welfare of caregivers and patients with dementia alike.
CORRESPONDENCE
Abu Baker Sheikh, MD, MSC 10-5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected].
1. Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol. 2017;13:327-339.
2. Cipriani G, Borin G, Vedovello M, et al. Sociopathic behavior and dementia. Acta Neurol Belg. 2013;113:111-115.
3. Cipriani G, Lucetti C, Danti S, et al. Violent and criminal manifestations in dementia patients. Geriatr Gerontol Int. 2016;16:541-549.
4. Skovdahl K, Kihlgren AL, Kihlgren M. Different attitudes when handling aggressive behaviour in dementia—narratives from two caregiver groups. Aging Ment Health. 2003;7:277-286.
5. Kristiansen L, Hellzén O, Asplund K. Swedish assistant nurses’ experiences of job satisfaction when caring for persons suffering from dementia and behavioural disturbances. An interview study. Int J Qualitat Stud Health Well-being. 2006;1:245-256.
6. Wharton TC, Ford BK. What is known about dementia care recipient violence and aggression against caregivers? J Gerontol Soc Work. 2014;57:460-477.
7. Ostaszkiewicz J, Lakhan P, O’Connell B, et al. Ongoing challenges responding to behavioural and psychological symptoms of dementia. Int Nurs Rev. 2015;62:506-516.
8. Kim J, De Bellis AM, Xiao LD. The experience of paid family-care workers of people with dementia in South Korea. Asian Nurs Res (Korean Soc Nurs Sci). 2018;12:34-41.
9. Band-Winterstein T, Avieli H. Women coping with a partner’s dementia-related violence: a qualitative study. J Nurs Scholarsh. 2019; 51:368-379.
10. Munkejord MC, Stefansdottir OA, Sveinbjarnardottir EK. Who cares for the carer? The suffering, struggles and unmet needs of older women caring for husbands living with cognitive decline. Int Pract Devel J. 2020;10:1-11.
11. Seidel D, Thyrian JR. Burden of caring for people with dementia - comparing family caregivers and professional caregivers. A descriptive study. J Multidiscip Healthc. 2019;12:655-663.
12. Tang W, Friedman DB, Kannaley K, et al. Experiences of caregivers by care recipient’s health condition: a study of caregivers for Alzheimer’s disease and related dementias versus other chronic conditions. Geriatr Nurs. 2019;40:181-184.
13. Benbow SM, Bhattacharyya S, Kingston P. Older adults and violence: an analysis of domestic homicide reviews in England involving adults over 60 years of age. Ageing Soc. 2018;39:1097-1121.
14. Herron RV, Wrathall MA. Putting responsive behaviours in place: examining how formal and informal carers understand the actions of people with dementia. Soc Sci Med. 2018;204:9-15.
15. Herron RV, Rosenberg MW. Responding to aggression and reactive behaviours in the home. Dementia (London). 2019;18:1328-1340.
16. Spencer D, Funk LM, Herron RV, et al. Fear, defensive strategies and caring for cognitively impaired family members. J Gerontol Soc Work. 2019;62:67-85.
17. Skovdahl K, Kihlgren AL, Kihlgren M. Dementia and aggressiveness: stimulated recall interviews with caregivers after video-recorded interactions. J Clin Nurs. 2004;13:515-525.
18. Needham I, Abderhalden C, Halfens RJ, et al. Non-somatic effects of patient aggression on nurses: a systematic review. J Adv Nurs. 2005;49:283-296.
19. Tariq SH, Tumosa N, Chibnall JT, et al. The Saint Louis University Mental Status (SLUMS) Examination for detecting mild cognitive impairment and dementia is more sensitive than the Mini-Mental Status Examination (MMSE) - a pilot study. Am J Geriatr Psych. 2006;14:900-910.
20. Janzen S, Zecevic AA, Kloseck M, et al. Managing agitation using nonpharmacological interventions for seniors with dementia. Am J Alzheimers Dis Other Demen. 2013;28:524-532.
21. Zeller A, Dassen T, Kok G, et al. Nursing home caregivers’ explanations for and coping strategies with residents’ aggression: a qualitative study. J Clin Nurs. 2011;20:2469-2478.
22. Alzheimer’s Society. Fix dementia care: homecare. Accessed December 28, 2021. https://www.alzheimers.org.uk/sites/default/files/migrate/downloads/fix_dementia_care_homecare_report.pdf
23. von Känel R, Mausbach BT, Dimsdale JE, et al. Refining caregiver vulnerability for clinical practice: determinants of self-rated health in spousal dementia caregivers. BMC Geriatr. 2019;19:18.
24. Chen HM, Huang MF, Yeh YC, et al. Effectiveness of coping strategies intervention on caregiver burden among caregivers of elderly patients with dementia. Psychogeriatrics. 2015; 15:20-25.
25. Wawrziczny E, Larochette C, Papo D, et al. A customized intervention for dementia caregivers: a quasi-experimental design. J Aging Health. 2019;31:1172-1195.
26. Gitlin LN, Piersol CV, Hodgson N, et al. Reducing neuropsychiatric symptoms in persons with dementia and associated burden in family caregivers using tailored activities: Design and methods of a randomized clinical trial. Contemp Clin Trials. 2016;49:92-102.
27. de Oliveira AM, Radanovic M, Homem de Mello PC, et al. An intervention to reduce neuropsychiatric symptoms and caregiver burden in dementia: preliminary results from a randomized trial of the tailored activity program-outpatient version. Int J Geriatr Psychiatry. 2019;34:1301-1307.
28. Livingston G, Barber J, Rapaport P, et al. Clinical effectiveness of a manual based coping strategy programme (START, STrAtegies for RelaTives) in promoting the mental health of carers of family members with dementia: pragmatic randomised controlled trial. BMJ. 2013;347:f6276.
29. Kajiyama B, Fernandez G, Carter EA, et al. Helping Hispanic dementia caregivers cope with stress using technology-based resources. Clin Gerontol. 2018;41:209-216.
30. Moskowitz JT, Cheung EO, Snowberg KE, et al. Randomized controlled trial of a facilitated online positive emotion regulation intervention for dementia caregivers. Health Psychol. 2019;38:391-402.
31. Yoon HK, Kim GS. An empowerment program for family caregivers of people with dementia. Public Health Nurs. 2020;37:222-233.
32. Zwingmann I, Dreier-Wolfgramm A, Esser A, et al. Why do family dementia caregivers reject caregiver support services? Analyzing types of rejection and associated health-impairments in a cluster-randomized controlled intervention trial. BMC Health Serv Res. 2020;20:121.
33. Nybakken S, Strandås M, Bondas T. Caregivers’ perceptions of aggressive behaviour in nursing home residents living with dementia: A meta-ethnography. J Adv Nurs. 2018;74:2713-2726.
34. Nakaishi L, Moss H, Weinstein M, et al. Exploring workplace violence among home care workers in a consumer-driven home health care program. Workplace Health Saf. 2013;61:441-450.
35. Medical Advisory Secretariat. Caregiver- and patient-directed interventions for dementia: an evidence-based analysis. Ont Health Technol Assess Ser. 2008;8:1-98.
36. Betz ME, McCourt AD, Vernick JS, et al. Firearms and dementia: clinical considerations. Ann Intern Med. 2018;169:47-49.
37. Leng M, Zhao Y, Wang Z. Comparative efficacy of non-pharmacological interventions on agitation in people with dementia: a systematic review and Bayesian network meta-analysis. Int J Nurs Stud. 2020;102:103489.
38. Morgan S, Pullon S, McKinlay E. Observation of interprofessional collaborative practice in primary care teams: an integrative literature review. Int J Nurs Stud. 2015;52:1217-1230.
The number of people with dementia globally is expected to reach 74.7 million by 2030 and 131.5 million by 2050.1 Because dementia is progressive, many patients will exhibit severe symptoms termed behavioral crises. Deteriorating interpersonal conduct and escalating antisocial acts result in an acquired sociopathy.2 Increasing cognitive impairment causes these patients to misunderstand intimate care and perceive it as a threat, often resulting in outbursts of violence against their caregivers.3
Available studies (TABLE4-17) make evident the incidence of interpersonal violence experienced by caregivers secondary to aggressive acts by patients with dementia. This violence ranges from verbal abuse, including racial slurs, to physical abuse—sometimes resulting in significant physical injury. Aggressive behavior by patients with dementia, resulting in violence towards their caregivers or partners, stems from progressive cognitive decline, which can make optimal care difficult. Such episodes may also impair the psychological and physical well-being of caregivers, increasing their risk of depression, anxiety, and even post-traumatic stress disorder (PTSD).18 The extent of the impact is also determined by the interpretation of the abuse by the caregivers themselves. One study suggested that the perception of aggressive or violent behavior as “normal” by a caregiver reduced the overall negative effect of the interactions.7Our review emphasizes the unintended burden that can fall to caregivers of patients with dementia. We also address the role of primary care providers (PCPs) in identifying these instances of violence and intervening appropriately by providing safety strategies, education, resources, and support.

CASE
A 67-year-old man with a medical history of PTSD with depression, type 2 diabetes, alcohol use disorder/dependence, hypertension, and obstructive sleep apnea was brought to his PCP by his wife. She said he had recently been unable to keep appointment times, pay bills, or take his usual medications, venlafaxine and bupropion. She also said his PTSD symptoms had worsened. He was sleeping 12 to 14 hours per day and was increasingly irritable. The patient denied any concerns or changes in his behavior.
The PCP administered a Saint Louis University Mental Status (SLUMS) examination to screen for cognitive impairment.19 The patient scored 14/30 (less than 20 is indicative of dementia). He was unable to complete a simple math problem, recall any items from a list of 5, count in reverse, draw a clock correctly, or recall a full story. Throughout the exam, the patient demonstrated minimal effort and was often only able to complete a task after further prompting by the examiner.

A computed tomography scan of the head revealed no signs of hemorrhage or damage. Thyroid-stimulating hormone levels and vitamin B12 levels were normal. A rapid plasma reagin test result was negative. The patient was given a diagnosis of Alzheimer disease. Donepezil was added to the patient’s medications, starting at 5 mg and then increased to 10 mg. His wife began to assist him with his tasks of daily living. His mood improved, and his wife noted he began to remember his appointments and take his medications with assistance.

However, the patient’s irritability continued to escalate. He grew paranoid and accused his wife of mismanaging their money. This pattern steadily worsened over the course of 6 months. The situation escalated until one day the patient’s wife called a mental health hotline reporting that her husband was holding her hostage and threatening to kill her with a gun. He told her, “I can do something to you, and they won’t even find a fingernail. It doesn’t have to be with a gun either.” She was counseled to try to stay calm to avoid aggravating the situation and to go to a safe place and stay there until help arrived.
His memory had worsened to the point that he could not recall any events from the previous 2 years. He was paranoid about anyone entering his home and would not allow his deteriorating roof to be repaired or his yard to be maintained. He did not shower for weeks at a time. He slept holding a rifle and accused his wife of embezzlement.
Continue to: The patient was evaluated...
The patient was evaluated by another specialist, who assessed his SLUMS score to be 18/30. He increased the patient’s donepezil dose, initiated a bupropion taper, and added sertraline to the regimen. The PCP spoke to the patient’s wife regarding options for her safety including leaving the home, hiding firearms, and calling the police in cases of interpersonal violence. The wife said she did not want to pursue these options. She expressed worry that he might be harmed if he was uncooperative with the police and said there was no one except her to take care of him.
Caregivers struggle to care for their loved ones
Instances of personal violence lead to shock, astonishment, heartbreak, and fear. Anticipation of a recurrence of violence causes many partners and caregivers to feel exhausted, because there is minimal hope for any chance of improvement. There are a few exceptions, however, as our case will show. In addition to emotional exhaustion, there is also a never-ending sense of self-doubt, leading many caregivers to question their ability to handle their family member.20,21 Over time, this leads to caregiver burnout, leaving them unable to understand their family member’s aggression. The sudden loss of caregiver control in dealing with the patient may also result in the family member exhibiting behavioral changes reflecting emotional trauma. For caregivers who do not live with the patient, they may choose to make fewer or shorter visits—or not visit at all—because they fear being abused.7,22
Caregivers of patients with dementia often feel helpless and powerless once abrupt and drastic changes in personality lead to some form of interpersonal violence. Additionally, caregivers with a poor health status are more likely to have lower physical function and experience greater caregiving stress overall.23 Other factors increasing stress are longer years of caregiving and the severity of a patient’s dementia and functional impairment.23
Interventions to reduce caregiver burden
Many studies have assessed the role of different interventions to reduce caregiver burden, such as teaching them problem-solving skills, increasing their knowledge of dementia, recommending social resources, providing emotional support, changing caregiver perceptions of the care situation, introducing coping strategies, relying on strengths and experiences in caregiving, help-seeking, and engaging in activity programs.24-28 For Hispanic caregivers, a structured and self-paced online telenovela format has been effective in improving care and relieving caregiver stress.29 Online positive emotion regulators helped in significantly improving quality of life and physical health in the caregivers.30 In this last intervention, caregivers had 6 online sessions with a facilitator who taught them emotional regulation skills that included: noticing positive events, capitalizing on them, and feeling gratitude; practicing mindfulness; doing a positive reappraisal; acknowledging personal strengths and setting attainable goals; and performing acts of kindness. Empowerment programs have also shown significant improvement in the well-being of caregivers.31
Caregivers may reject support.
Continue to: These practical tips can help
These practical tips can help
Based on our review of the literature, we recommend offering the following supports to caregivers:
- Counsel caregivers early on in a patient’s dementia that behavior changes are likely and may be unpredictable. Explain that dementia can involve changes to personality and behavior as well as memory difficulties.33,34
- Describe resources for support, such as day programs for senior adults, insurance coverage for caregiver respite programs, and the Alzheimer’s Association (www.alz.org/). Encourage caregivers to seek general medical and mental health care for themselves. Caregivers should have opportunities and support to discuss their experiences and to be appropriately trained for the challenge of caring for a family member with dementia.35
- Encourage disclosure about abrupt changes in the patient’s behavior. This invites families to discuss issues with you and may make them more comfortable with such conversations.
- Involve ancillary services (eg, social worker) to plan for a higher level of care well in advance of it becoming necessary.
- Discuss safety strategies for the caregiver, including when it is appropriate to alter a patient’s set routines such as bedtimes and mealtimes.33,34
- Discuss when and how to involve law enforcement, if necessary.33,34 Emphasize the importance of removing firearms from the home as a safety measure. Although federal laws do not explicitly prohibit possession of arms by patients with neurologic damage, a few states mention “organic brain syndrome” or “dementia” as conditions prohibiting use or possession of firearms.36
- Suggest, as feasible, nonpharmacologic aids for the patient such as massage therapy, animal-assisted therapy, personalized interventions, music therapy, and light therapy.37 Prescribe medications to the patient to aid in behavior modification when appropriate.
- Screen caregivers and family members for signs of interpersonal violence. Take notice of changes in caregiver behavior or irregularity in attending follow-up appointments.
CASE
Over the next month, the patient’s symptoms further deteriorated. His PCP recommended hospitalization, but the patient and his wife declined. Magnetic resonance imaging of the patient’s brain revealed severe confluent and patchy regions of white matter and T2 signal hyperintensity, consistent with chronic microvascular ischemic disease. An old, small, left parietal lobe infarct was also noted.
One month later, the patient presented to the emergency department. His symptoms were largely unchanged, but his wife indicated that she could no longer live at home due to burnout. The patient’s medications were adjusted, but he was not admitted for inpatient care. His wife said they needed help at home, but the patient opposed the idea any time that it was mentioned.
A few weeks later, the patient presented for outpatient follow-up. He was delusional, believing that the government was compelling citizens to take sertraline in order to harm their mental health. He had also begun viewing online pornography in front of his wife and attempting to remove all of his money from the bank. He was prescribed aripiprazole 15 mg, and his symptoms began to improve. Soon after, however, he threatened to kill his grandson, then took all his Lasix pills (a 7-day supply) simultaneously. The patient denied that this was a suicide attempt.
Over the course of the next month, the patient began to report hearing voices. A neuropsychological evaluation confirmed a diagnosis of dementia with psychiatric symptoms due to neurologic injury. The patient was referred to a geriatric psychiatrist and continued to be managed medically. He was assigned a multidisciplinary team comprising palliative care, social work, and care management to assist in his care and provide support to the family. His behavior improved.
Continue to: At the time of this publication...
At the time of this publication, the patient’s irritability and paranoia had subsided and he had made no further threats to his family. He has allowed a home health aide into the house and has agreed to have his roof repaired. His wife still lives with him and assists him with activities of daily living.
Interprofessional teams are key
Caregiver burnout increases the risk of patient neglect or abuse, as individuals who have been the targets of aggressive behavior are more likely to leave demented patients unattended.8,16,23 Although tools are available to screen caregivers for depression and burnout, an important step forward would be to develop an interprofessional team to aid in identifying and closely following high-risk patient–caregiver groups. This continual and varied assessment of psychosocial stressors could help prevent the development of violent interactions. These teams would allow integration with the primary health care system by frequent and effective shared communication of knowledge, development of goals, and shared decision-making.38 Setting expectations, providing support, and discussing safety strategies can improve the health and welfare of caregivers and patients with dementia alike.
CORRESPONDENCE
Abu Baker Sheikh, MD, MSC 10-5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected].
The number of people with dementia globally is expected to reach 74.7 million by 2030 and 131.5 million by 2050.1 Because dementia is progressive, many patients will exhibit severe symptoms termed behavioral crises. Deteriorating interpersonal conduct and escalating antisocial acts result in an acquired sociopathy.2 Increasing cognitive impairment causes these patients to misunderstand intimate care and perceive it as a threat, often resulting in outbursts of violence against their caregivers.3
Available studies (TABLE4-17) make evident the incidence of interpersonal violence experienced by caregivers secondary to aggressive acts by patients with dementia. This violence ranges from verbal abuse, including racial slurs, to physical abuse—sometimes resulting in significant physical injury. Aggressive behavior by patients with dementia, resulting in violence towards their caregivers or partners, stems from progressive cognitive decline, which can make optimal care difficult. Such episodes may also impair the psychological and physical well-being of caregivers, increasing their risk of depression, anxiety, and even post-traumatic stress disorder (PTSD).18 The extent of the impact is also determined by the interpretation of the abuse by the caregivers themselves. One study suggested that the perception of aggressive or violent behavior as “normal” by a caregiver reduced the overall negative effect of the interactions.7Our review emphasizes the unintended burden that can fall to caregivers of patients with dementia. We also address the role of primary care providers (PCPs) in identifying these instances of violence and intervening appropriately by providing safety strategies, education, resources, and support.

CASE
A 67-year-old man with a medical history of PTSD with depression, type 2 diabetes, alcohol use disorder/dependence, hypertension, and obstructive sleep apnea was brought to his PCP by his wife. She said he had recently been unable to keep appointment times, pay bills, or take his usual medications, venlafaxine and bupropion. She also said his PTSD symptoms had worsened. He was sleeping 12 to 14 hours per day and was increasingly irritable. The patient denied any concerns or changes in his behavior.
The PCP administered a Saint Louis University Mental Status (SLUMS) examination to screen for cognitive impairment.19 The patient scored 14/30 (less than 20 is indicative of dementia). He was unable to complete a simple math problem, recall any items from a list of 5, count in reverse, draw a clock correctly, or recall a full story. Throughout the exam, the patient demonstrated minimal effort and was often only able to complete a task after further prompting by the examiner.

A computed tomography scan of the head revealed no signs of hemorrhage or damage. Thyroid-stimulating hormone levels and vitamin B12 levels were normal. A rapid plasma reagin test result was negative. The patient was given a diagnosis of Alzheimer disease. Donepezil was added to the patient’s medications, starting at 5 mg and then increased to 10 mg. His wife began to assist him with his tasks of daily living. His mood improved, and his wife noted he began to remember his appointments and take his medications with assistance.

However, the patient’s irritability continued to escalate. He grew paranoid and accused his wife of mismanaging their money. This pattern steadily worsened over the course of 6 months. The situation escalated until one day the patient’s wife called a mental health hotline reporting that her husband was holding her hostage and threatening to kill her with a gun. He told her, “I can do something to you, and they won’t even find a fingernail. It doesn’t have to be with a gun either.” She was counseled to try to stay calm to avoid aggravating the situation and to go to a safe place and stay there until help arrived.
His memory had worsened to the point that he could not recall any events from the previous 2 years. He was paranoid about anyone entering his home and would not allow his deteriorating roof to be repaired or his yard to be maintained. He did not shower for weeks at a time. He slept holding a rifle and accused his wife of embezzlement.
Continue to: The patient was evaluated...
The patient was evaluated by another specialist, who assessed his SLUMS score to be 18/30. He increased the patient’s donepezil dose, initiated a bupropion taper, and added sertraline to the regimen. The PCP spoke to the patient’s wife regarding options for her safety including leaving the home, hiding firearms, and calling the police in cases of interpersonal violence. The wife said she did not want to pursue these options. She expressed worry that he might be harmed if he was uncooperative with the police and said there was no one except her to take care of him.
Caregivers struggle to care for their loved ones
Instances of personal violence lead to shock, astonishment, heartbreak, and fear. Anticipation of a recurrence of violence causes many partners and caregivers to feel exhausted, because there is minimal hope for any chance of improvement. There are a few exceptions, however, as our case will show. In addition to emotional exhaustion, there is also a never-ending sense of self-doubt, leading many caregivers to question their ability to handle their family member.20,21 Over time, this leads to caregiver burnout, leaving them unable to understand their family member’s aggression. The sudden loss of caregiver control in dealing with the patient may also result in the family member exhibiting behavioral changes reflecting emotional trauma. For caregivers who do not live with the patient, they may choose to make fewer or shorter visits—or not visit at all—because they fear being abused.7,22
Caregivers of patients with dementia often feel helpless and powerless once abrupt and drastic changes in personality lead to some form of interpersonal violence. Additionally, caregivers with a poor health status are more likely to have lower physical function and experience greater caregiving stress overall.23 Other factors increasing stress are longer years of caregiving and the severity of a patient’s dementia and functional impairment.23
Interventions to reduce caregiver burden
Many studies have assessed the role of different interventions to reduce caregiver burden, such as teaching them problem-solving skills, increasing their knowledge of dementia, recommending social resources, providing emotional support, changing caregiver perceptions of the care situation, introducing coping strategies, relying on strengths and experiences in caregiving, help-seeking, and engaging in activity programs.24-28 For Hispanic caregivers, a structured and self-paced online telenovela format has been effective in improving care and relieving caregiver stress.29 Online positive emotion regulators helped in significantly improving quality of life and physical health in the caregivers.30 In this last intervention, caregivers had 6 online sessions with a facilitator who taught them emotional regulation skills that included: noticing positive events, capitalizing on them, and feeling gratitude; practicing mindfulness; doing a positive reappraisal; acknowledging personal strengths and setting attainable goals; and performing acts of kindness. Empowerment programs have also shown significant improvement in the well-being of caregivers.31
Caregivers may reject support.
Continue to: These practical tips can help
These practical tips can help
Based on our review of the literature, we recommend offering the following supports to caregivers:
- Counsel caregivers early on in a patient’s dementia that behavior changes are likely and may be unpredictable. Explain that dementia can involve changes to personality and behavior as well as memory difficulties.33,34
- Describe resources for support, such as day programs for senior adults, insurance coverage for caregiver respite programs, and the Alzheimer’s Association (www.alz.org/). Encourage caregivers to seek general medical and mental health care for themselves. Caregivers should have opportunities and support to discuss their experiences and to be appropriately trained for the challenge of caring for a family member with dementia.35
- Encourage disclosure about abrupt changes in the patient’s behavior. This invites families to discuss issues with you and may make them more comfortable with such conversations.
- Involve ancillary services (eg, social worker) to plan for a higher level of care well in advance of it becoming necessary.
- Discuss safety strategies for the caregiver, including when it is appropriate to alter a patient’s set routines such as bedtimes and mealtimes.33,34
- Discuss when and how to involve law enforcement, if necessary.33,34 Emphasize the importance of removing firearms from the home as a safety measure. Although federal laws do not explicitly prohibit possession of arms by patients with neurologic damage, a few states mention “organic brain syndrome” or “dementia” as conditions prohibiting use or possession of firearms.36
- Suggest, as feasible, nonpharmacologic aids for the patient such as massage therapy, animal-assisted therapy, personalized interventions, music therapy, and light therapy.37 Prescribe medications to the patient to aid in behavior modification when appropriate.
- Screen caregivers and family members for signs of interpersonal violence. Take notice of changes in caregiver behavior or irregularity in attending follow-up appointments.
CASE
Over the next month, the patient’s symptoms further deteriorated. His PCP recommended hospitalization, but the patient and his wife declined. Magnetic resonance imaging of the patient’s brain revealed severe confluent and patchy regions of white matter and T2 signal hyperintensity, consistent with chronic microvascular ischemic disease. An old, small, left parietal lobe infarct was also noted.
One month later, the patient presented to the emergency department. His symptoms were largely unchanged, but his wife indicated that she could no longer live at home due to burnout. The patient’s medications were adjusted, but he was not admitted for inpatient care. His wife said they needed help at home, but the patient opposed the idea any time that it was mentioned.
A few weeks later, the patient presented for outpatient follow-up. He was delusional, believing that the government was compelling citizens to take sertraline in order to harm their mental health. He had also begun viewing online pornography in front of his wife and attempting to remove all of his money from the bank. He was prescribed aripiprazole 15 mg, and his symptoms began to improve. Soon after, however, he threatened to kill his grandson, then took all his Lasix pills (a 7-day supply) simultaneously. The patient denied that this was a suicide attempt.
Over the course of the next month, the patient began to report hearing voices. A neuropsychological evaluation confirmed a diagnosis of dementia with psychiatric symptoms due to neurologic injury. The patient was referred to a geriatric psychiatrist and continued to be managed medically. He was assigned a multidisciplinary team comprising palliative care, social work, and care management to assist in his care and provide support to the family. His behavior improved.
Continue to: At the time of this publication...
At the time of this publication, the patient’s irritability and paranoia had subsided and he had made no further threats to his family. He has allowed a home health aide into the house and has agreed to have his roof repaired. His wife still lives with him and assists him with activities of daily living.
Interprofessional teams are key
Caregiver burnout increases the risk of patient neglect or abuse, as individuals who have been the targets of aggressive behavior are more likely to leave demented patients unattended.8,16,23 Although tools are available to screen caregivers for depression and burnout, an important step forward would be to develop an interprofessional team to aid in identifying and closely following high-risk patient–caregiver groups. This continual and varied assessment of psychosocial stressors could help prevent the development of violent interactions. These teams would allow integration with the primary health care system by frequent and effective shared communication of knowledge, development of goals, and shared decision-making.38 Setting expectations, providing support, and discussing safety strategies can improve the health and welfare of caregivers and patients with dementia alike.
CORRESPONDENCE
Abu Baker Sheikh, MD, MSC 10-5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected].
1. Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol. 2017;13:327-339.
2. Cipriani G, Borin G, Vedovello M, et al. Sociopathic behavior and dementia. Acta Neurol Belg. 2013;113:111-115.
3. Cipriani G, Lucetti C, Danti S, et al. Violent and criminal manifestations in dementia patients. Geriatr Gerontol Int. 2016;16:541-549.
4. Skovdahl K, Kihlgren AL, Kihlgren M. Different attitudes when handling aggressive behaviour in dementia—narratives from two caregiver groups. Aging Ment Health. 2003;7:277-286.
5. Kristiansen L, Hellzén O, Asplund K. Swedish assistant nurses’ experiences of job satisfaction when caring for persons suffering from dementia and behavioural disturbances. An interview study. Int J Qualitat Stud Health Well-being. 2006;1:245-256.
6. Wharton TC, Ford BK. What is known about dementia care recipient violence and aggression against caregivers? J Gerontol Soc Work. 2014;57:460-477.
7. Ostaszkiewicz J, Lakhan P, O’Connell B, et al. Ongoing challenges responding to behavioural and psychological symptoms of dementia. Int Nurs Rev. 2015;62:506-516.
8. Kim J, De Bellis AM, Xiao LD. The experience of paid family-care workers of people with dementia in South Korea. Asian Nurs Res (Korean Soc Nurs Sci). 2018;12:34-41.
9. Band-Winterstein T, Avieli H. Women coping with a partner’s dementia-related violence: a qualitative study. J Nurs Scholarsh. 2019; 51:368-379.
10. Munkejord MC, Stefansdottir OA, Sveinbjarnardottir EK. Who cares for the carer? The suffering, struggles and unmet needs of older women caring for husbands living with cognitive decline. Int Pract Devel J. 2020;10:1-11.
11. Seidel D, Thyrian JR. Burden of caring for people with dementia - comparing family caregivers and professional caregivers. A descriptive study. J Multidiscip Healthc. 2019;12:655-663.
12. Tang W, Friedman DB, Kannaley K, et al. Experiences of caregivers by care recipient’s health condition: a study of caregivers for Alzheimer’s disease and related dementias versus other chronic conditions. Geriatr Nurs. 2019;40:181-184.
13. Benbow SM, Bhattacharyya S, Kingston P. Older adults and violence: an analysis of domestic homicide reviews in England involving adults over 60 years of age. Ageing Soc. 2018;39:1097-1121.
14. Herron RV, Wrathall MA. Putting responsive behaviours in place: examining how formal and informal carers understand the actions of people with dementia. Soc Sci Med. 2018;204:9-15.
15. Herron RV, Rosenberg MW. Responding to aggression and reactive behaviours in the home. Dementia (London). 2019;18:1328-1340.
16. Spencer D, Funk LM, Herron RV, et al. Fear, defensive strategies and caring for cognitively impaired family members. J Gerontol Soc Work. 2019;62:67-85.
17. Skovdahl K, Kihlgren AL, Kihlgren M. Dementia and aggressiveness: stimulated recall interviews with caregivers after video-recorded interactions. J Clin Nurs. 2004;13:515-525.
18. Needham I, Abderhalden C, Halfens RJ, et al. Non-somatic effects of patient aggression on nurses: a systematic review. J Adv Nurs. 2005;49:283-296.
19. Tariq SH, Tumosa N, Chibnall JT, et al. The Saint Louis University Mental Status (SLUMS) Examination for detecting mild cognitive impairment and dementia is more sensitive than the Mini-Mental Status Examination (MMSE) - a pilot study. Am J Geriatr Psych. 2006;14:900-910.
20. Janzen S, Zecevic AA, Kloseck M, et al. Managing agitation using nonpharmacological interventions for seniors with dementia. Am J Alzheimers Dis Other Demen. 2013;28:524-532.
21. Zeller A, Dassen T, Kok G, et al. Nursing home caregivers’ explanations for and coping strategies with residents’ aggression: a qualitative study. J Clin Nurs. 2011;20:2469-2478.
22. Alzheimer’s Society. Fix dementia care: homecare. Accessed December 28, 2021. https://www.alzheimers.org.uk/sites/default/files/migrate/downloads/fix_dementia_care_homecare_report.pdf
23. von Känel R, Mausbach BT, Dimsdale JE, et al. Refining caregiver vulnerability for clinical practice: determinants of self-rated health in spousal dementia caregivers. BMC Geriatr. 2019;19:18.
24. Chen HM, Huang MF, Yeh YC, et al. Effectiveness of coping strategies intervention on caregiver burden among caregivers of elderly patients with dementia. Psychogeriatrics. 2015; 15:20-25.
25. Wawrziczny E, Larochette C, Papo D, et al. A customized intervention for dementia caregivers: a quasi-experimental design. J Aging Health. 2019;31:1172-1195.
26. Gitlin LN, Piersol CV, Hodgson N, et al. Reducing neuropsychiatric symptoms in persons with dementia and associated burden in family caregivers using tailored activities: Design and methods of a randomized clinical trial. Contemp Clin Trials. 2016;49:92-102.
27. de Oliveira AM, Radanovic M, Homem de Mello PC, et al. An intervention to reduce neuropsychiatric symptoms and caregiver burden in dementia: preliminary results from a randomized trial of the tailored activity program-outpatient version. Int J Geriatr Psychiatry. 2019;34:1301-1307.
28. Livingston G, Barber J, Rapaport P, et al. Clinical effectiveness of a manual based coping strategy programme (START, STrAtegies for RelaTives) in promoting the mental health of carers of family members with dementia: pragmatic randomised controlled trial. BMJ. 2013;347:f6276.
29. Kajiyama B, Fernandez G, Carter EA, et al. Helping Hispanic dementia caregivers cope with stress using technology-based resources. Clin Gerontol. 2018;41:209-216.
30. Moskowitz JT, Cheung EO, Snowberg KE, et al. Randomized controlled trial of a facilitated online positive emotion regulation intervention for dementia caregivers. Health Psychol. 2019;38:391-402.
31. Yoon HK, Kim GS. An empowerment program for family caregivers of people with dementia. Public Health Nurs. 2020;37:222-233.
32. Zwingmann I, Dreier-Wolfgramm A, Esser A, et al. Why do family dementia caregivers reject caregiver support services? Analyzing types of rejection and associated health-impairments in a cluster-randomized controlled intervention trial. BMC Health Serv Res. 2020;20:121.
33. Nybakken S, Strandås M, Bondas T. Caregivers’ perceptions of aggressive behaviour in nursing home residents living with dementia: A meta-ethnography. J Adv Nurs. 2018;74:2713-2726.
34. Nakaishi L, Moss H, Weinstein M, et al. Exploring workplace violence among home care workers in a consumer-driven home health care program. Workplace Health Saf. 2013;61:441-450.
35. Medical Advisory Secretariat. Caregiver- and patient-directed interventions for dementia: an evidence-based analysis. Ont Health Technol Assess Ser. 2008;8:1-98.
36. Betz ME, McCourt AD, Vernick JS, et al. Firearms and dementia: clinical considerations. Ann Intern Med. 2018;169:47-49.
37. Leng M, Zhao Y, Wang Z. Comparative efficacy of non-pharmacological interventions on agitation in people with dementia: a systematic review and Bayesian network meta-analysis. Int J Nurs Stud. 2020;102:103489.
38. Morgan S, Pullon S, McKinlay E. Observation of interprofessional collaborative practice in primary care teams: an integrative literature review. Int J Nurs Stud. 2015;52:1217-1230.
1. Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol. 2017;13:327-339.
2. Cipriani G, Borin G, Vedovello M, et al. Sociopathic behavior and dementia. Acta Neurol Belg. 2013;113:111-115.
3. Cipriani G, Lucetti C, Danti S, et al. Violent and criminal manifestations in dementia patients. Geriatr Gerontol Int. 2016;16:541-549.
4. Skovdahl K, Kihlgren AL, Kihlgren M. Different attitudes when handling aggressive behaviour in dementia—narratives from two caregiver groups. Aging Ment Health. 2003;7:277-286.
5. Kristiansen L, Hellzén O, Asplund K. Swedish assistant nurses’ experiences of job satisfaction when caring for persons suffering from dementia and behavioural disturbances. An interview study. Int J Qualitat Stud Health Well-being. 2006;1:245-256.
6. Wharton TC, Ford BK. What is known about dementia care recipient violence and aggression against caregivers? J Gerontol Soc Work. 2014;57:460-477.
7. Ostaszkiewicz J, Lakhan P, O’Connell B, et al. Ongoing challenges responding to behavioural and psychological symptoms of dementia. Int Nurs Rev. 2015;62:506-516.
8. Kim J, De Bellis AM, Xiao LD. The experience of paid family-care workers of people with dementia in South Korea. Asian Nurs Res (Korean Soc Nurs Sci). 2018;12:34-41.
9. Band-Winterstein T, Avieli H. Women coping with a partner’s dementia-related violence: a qualitative study. J Nurs Scholarsh. 2019; 51:368-379.
10. Munkejord MC, Stefansdottir OA, Sveinbjarnardottir EK. Who cares for the carer? The suffering, struggles and unmet needs of older women caring for husbands living with cognitive decline. Int Pract Devel J. 2020;10:1-11.
11. Seidel D, Thyrian JR. Burden of caring for people with dementia - comparing family caregivers and professional caregivers. A descriptive study. J Multidiscip Healthc. 2019;12:655-663.
12. Tang W, Friedman DB, Kannaley K, et al. Experiences of caregivers by care recipient’s health condition: a study of caregivers for Alzheimer’s disease and related dementias versus other chronic conditions. Geriatr Nurs. 2019;40:181-184.
13. Benbow SM, Bhattacharyya S, Kingston P. Older adults and violence: an analysis of domestic homicide reviews in England involving adults over 60 years of age. Ageing Soc. 2018;39:1097-1121.
14. Herron RV, Wrathall MA. Putting responsive behaviours in place: examining how formal and informal carers understand the actions of people with dementia. Soc Sci Med. 2018;204:9-15.
15. Herron RV, Rosenberg MW. Responding to aggression and reactive behaviours in the home. Dementia (London). 2019;18:1328-1340.
16. Spencer D, Funk LM, Herron RV, et al. Fear, defensive strategies and caring for cognitively impaired family members. J Gerontol Soc Work. 2019;62:67-85.
17. Skovdahl K, Kihlgren AL, Kihlgren M. Dementia and aggressiveness: stimulated recall interviews with caregivers after video-recorded interactions. J Clin Nurs. 2004;13:515-525.
18. Needham I, Abderhalden C, Halfens RJ, et al. Non-somatic effects of patient aggression on nurses: a systematic review. J Adv Nurs. 2005;49:283-296.
19. Tariq SH, Tumosa N, Chibnall JT, et al. The Saint Louis University Mental Status (SLUMS) Examination for detecting mild cognitive impairment and dementia is more sensitive than the Mini-Mental Status Examination (MMSE) - a pilot study. Am J Geriatr Psych. 2006;14:900-910.
20. Janzen S, Zecevic AA, Kloseck M, et al. Managing agitation using nonpharmacological interventions for seniors with dementia. Am J Alzheimers Dis Other Demen. 2013;28:524-532.
21. Zeller A, Dassen T, Kok G, et al. Nursing home caregivers’ explanations for and coping strategies with residents’ aggression: a qualitative study. J Clin Nurs. 2011;20:2469-2478.
22. Alzheimer’s Society. Fix dementia care: homecare. Accessed December 28, 2021. https://www.alzheimers.org.uk/sites/default/files/migrate/downloads/fix_dementia_care_homecare_report.pdf
23. von Känel R, Mausbach BT, Dimsdale JE, et al. Refining caregiver vulnerability for clinical practice: determinants of self-rated health in spousal dementia caregivers. BMC Geriatr. 2019;19:18.
24. Chen HM, Huang MF, Yeh YC, et al. Effectiveness of coping strategies intervention on caregiver burden among caregivers of elderly patients with dementia. Psychogeriatrics. 2015; 15:20-25.
25. Wawrziczny E, Larochette C, Papo D, et al. A customized intervention for dementia caregivers: a quasi-experimental design. J Aging Health. 2019;31:1172-1195.
26. Gitlin LN, Piersol CV, Hodgson N, et al. Reducing neuropsychiatric symptoms in persons with dementia and associated burden in family caregivers using tailored activities: Design and methods of a randomized clinical trial. Contemp Clin Trials. 2016;49:92-102.
27. de Oliveira AM, Radanovic M, Homem de Mello PC, et al. An intervention to reduce neuropsychiatric symptoms and caregiver burden in dementia: preliminary results from a randomized trial of the tailored activity program-outpatient version. Int J Geriatr Psychiatry. 2019;34:1301-1307.
28. Livingston G, Barber J, Rapaport P, et al. Clinical effectiveness of a manual based coping strategy programme (START, STrAtegies for RelaTives) in promoting the mental health of carers of family members with dementia: pragmatic randomised controlled trial. BMJ. 2013;347:f6276.
29. Kajiyama B, Fernandez G, Carter EA, et al. Helping Hispanic dementia caregivers cope with stress using technology-based resources. Clin Gerontol. 2018;41:209-216.
30. Moskowitz JT, Cheung EO, Snowberg KE, et al. Randomized controlled trial of a facilitated online positive emotion regulation intervention for dementia caregivers. Health Psychol. 2019;38:391-402.
31. Yoon HK, Kim GS. An empowerment program for family caregivers of people with dementia. Public Health Nurs. 2020;37:222-233.
32. Zwingmann I, Dreier-Wolfgramm A, Esser A, et al. Why do family dementia caregivers reject caregiver support services? Analyzing types of rejection and associated health-impairments in a cluster-randomized controlled intervention trial. BMC Health Serv Res. 2020;20:121.
33. Nybakken S, Strandås M, Bondas T. Caregivers’ perceptions of aggressive behaviour in nursing home residents living with dementia: A meta-ethnography. J Adv Nurs. 2018;74:2713-2726.
34. Nakaishi L, Moss H, Weinstein M, et al. Exploring workplace violence among home care workers in a consumer-driven home health care program. Workplace Health Saf. 2013;61:441-450.
35. Medical Advisory Secretariat. Caregiver- and patient-directed interventions for dementia: an evidence-based analysis. Ont Health Technol Assess Ser. 2008;8:1-98.
36. Betz ME, McCourt AD, Vernick JS, et al. Firearms and dementia: clinical considerations. Ann Intern Med. 2018;169:47-49.
37. Leng M, Zhao Y, Wang Z. Comparative efficacy of non-pharmacological interventions on agitation in people with dementia: a systematic review and Bayesian network meta-analysis. Int J Nurs Stud. 2020;102:103489.
38. Morgan S, Pullon S, McKinlay E. Observation of interprofessional collaborative practice in primary care teams: an integrative literature review. Int J Nurs Stud. 2015;52:1217-1230.
PRACTICE RECOMMENDATIONS
› Screen caregivers and family members of patients with dementia for signs of interpersonal violence. C
› Counsel caregivers early on that behavior changes in patients with dementia are likely and may be unpredictable. C
› Discuss safety strategies for the caregiver, including when it is appropriate to alter routines such as bedtimes and meals. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
How to identify balance disorders and reduce fall risk
CASE Mr. J, a 75-year-old man, presents to your family practice reporting that he feels increasingly unsteady and slow while walking. He fell twice last year, without resulting injury. He now worries about tripping while walking around the house and relies on his spouse to run errands.
Clearly, Mr. J is experiencing a problem with balance. What management approach should you undertake to prevent him from falling?

Balance disorders are common in older people and drastically hinder quality of life.1-4 Patients often describe imbalance as vague symptoms: dizziness, unsteadiness, faintness, spinning sensations.5,6 Importantly, balance disorders disrupt normal gait and contribute to falls that are a major cause of disability and morbidity in older people. Almost 30% of people older than 65 years report 1 or more falls annually.7 Factors that increase the risk of falls include impaired mobility, previously reported falls, reduced psychological functioning, chronic medical conditions, and polypharmacy.7,8
The cause of any single case of imbalance is often multifactorial, resulting from dysfunction of multiple body systems (TABLE 17-56); in our clinical experience, most patients with imbalance and who are at risk of falls do not have a detectable deficit of the vestibular system. These alterations in function arise in 3 key systems—vision, proprioception, and vestibular function—which signal to, and are incorporated by, the cerebellum to mediate balance. Cognitive and neurologic decline are also factors in imbalance.
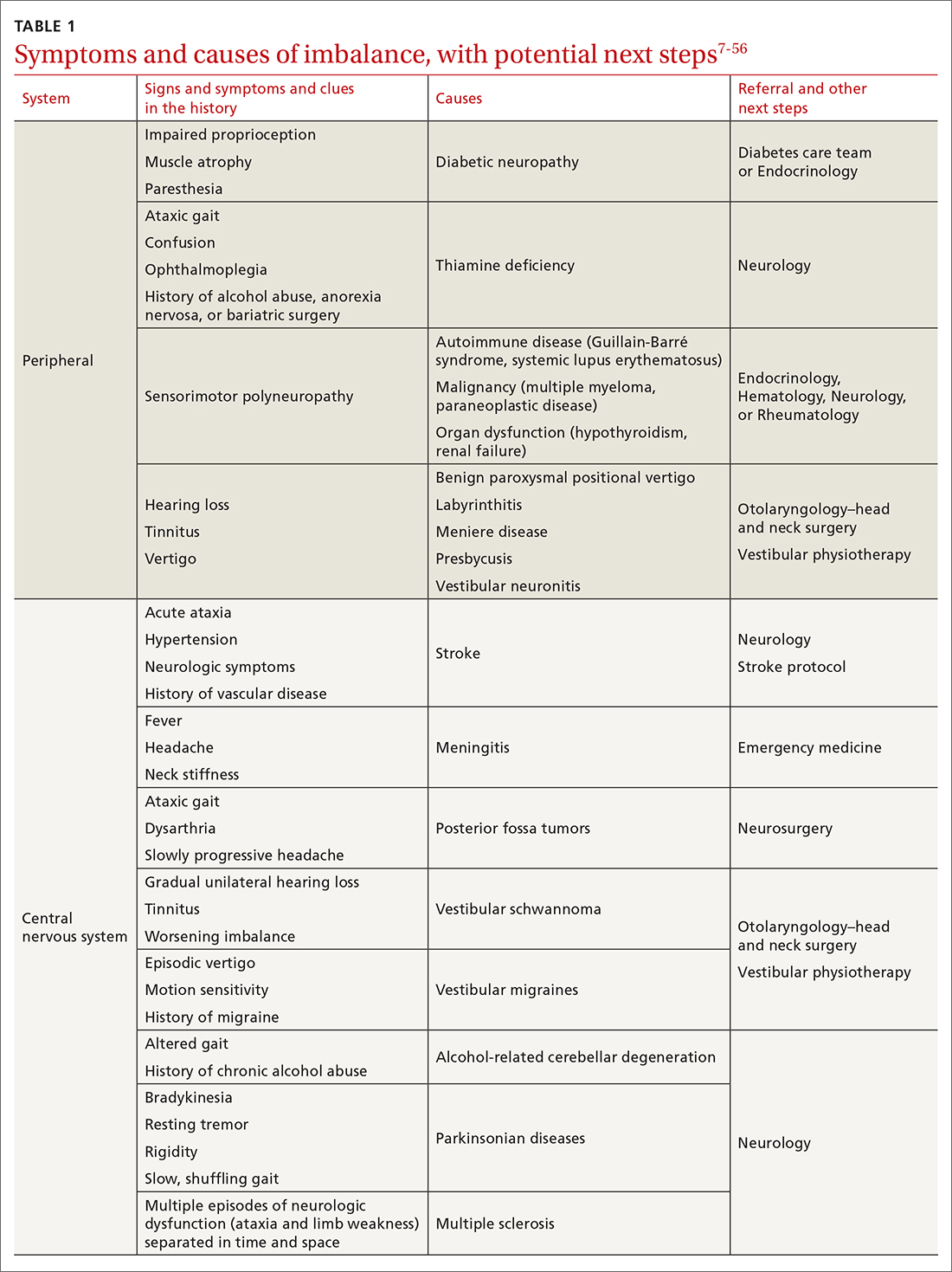
Considering that 20% of falls result in serious injury in older populations, it is important to identify balance disorders and implement preventive strategies to mitigate harmful consequences of falls on patients’ health and independence.7,57 In this article, we answer the question that the case presentation raises about the proper management approach to imbalance in family practice, including assessment of risk and rehabilitation strategies to reduce the risk of falls. Our insights and recommendations are based on our clinical experience and a review of the medical literature from the past 40 years.
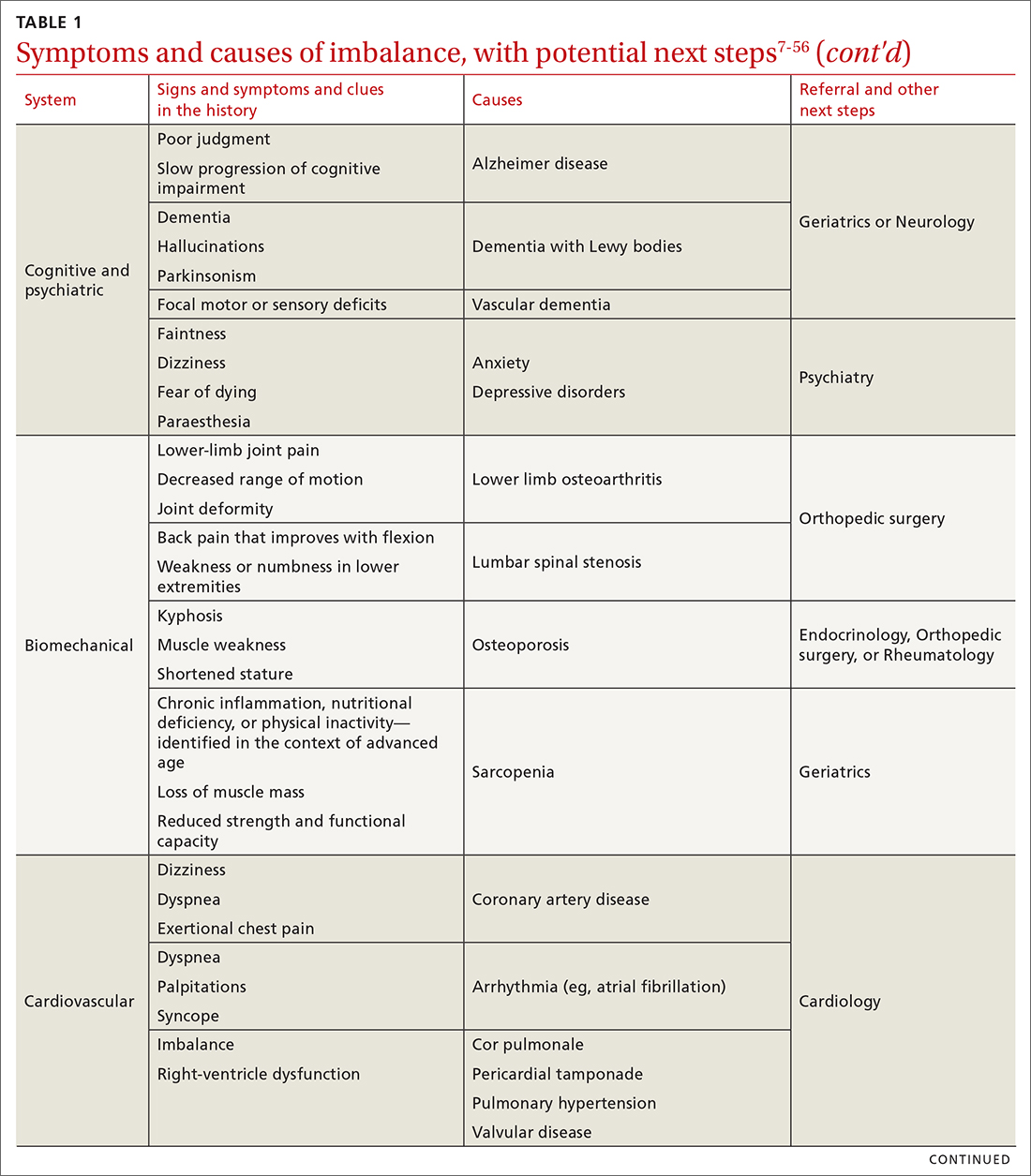
CASE Mr. J has a history of hypertension, age-related hearing loss, and osteoarthritis of the knees; he has not had surgery for the arthritis. His medications are antihypertensives and extra-strength acetaminophen for knee pain.
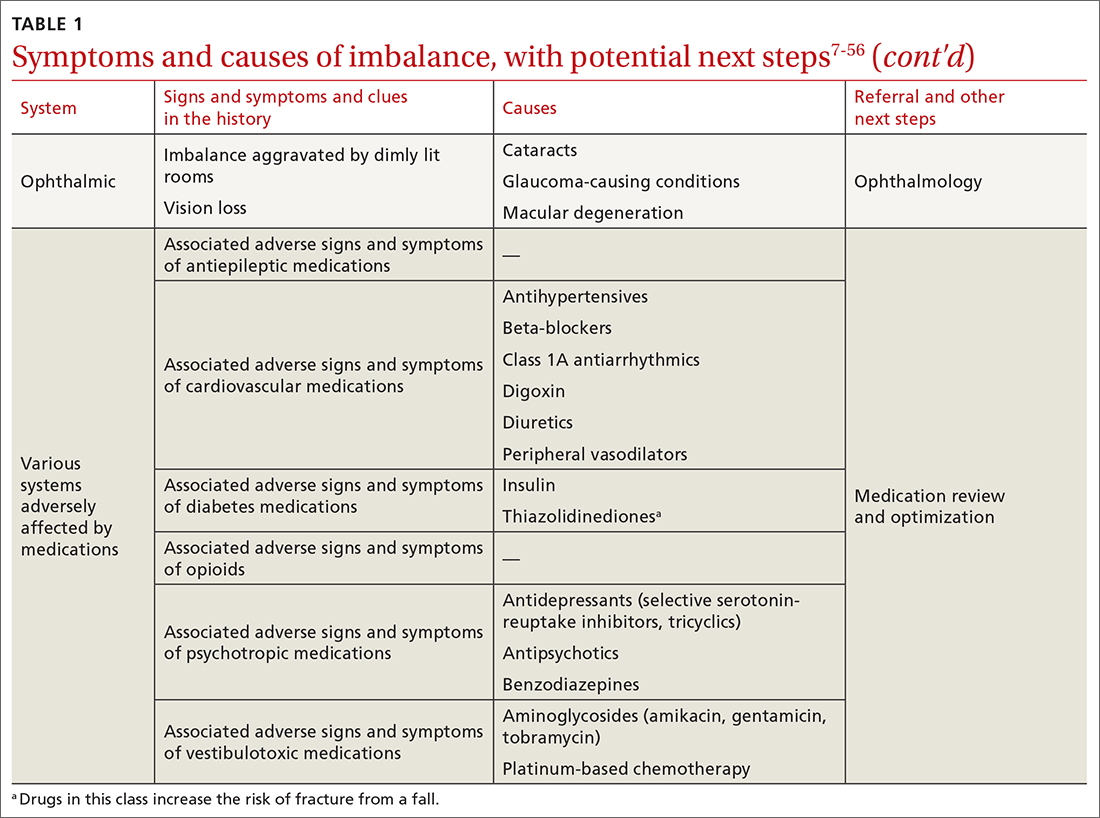
Making the diagnosis of a balance disorder
History
A thorough clinical history, often including a collateral history from caregivers, narrows the differential diagnosis. Information regarding onset, duration, timing, character, and previous episodes of imbalance is essential. Symptoms of imbalance are often challenging for the patient to describe: They might use terms such as vertigo or dizziness, when, in fact, on further questioning, they are describing balance difficulties. Inquiry into (1) their use of assistive walking devices and (2) development or exacerbation of neurologic, musculoskeletal, auditory, visual, and mood symptoms is necessary. Note the current level of their mobility, episodes of pain or fatigue, previous falls and associated injuries, fear of falling, balance confidence, and sensations that precede falls.58
Continue to: The medical and surgical histories
The medical and surgical histories are key pieces of information. The history of smoking, alcohol habits, and substance use is relevant.
A robust medication history is essential to evaluate a patient’s risk of falling. Polypharmacy—typically, defined as taking 4 or more medications—has been repeatedly associated with a heightened risk of falls.53,59-61 Moreover, a dose-dependent association between polypharmacy and hospitalization following falls has been identified, and demonstrates that taking 10 or more medications greatly increases the risk of hospitalization.59 Studies of polypharmacy cement the importance of inquiring about medication use when assessing imbalance, particularly in older patients.
Physical examination
A focused and detailed physical examination provides insight into systems that should be investigated:
- Obtain vital signs, including orthostatic vitals to test for orthostatic hypotension62; keep in mind that symptoms of orthostatic dizziness can occur without orthostatic hypotension.
- Examine gait, which can distinguish between causes of imbalance (TABLE 2).21,40,63-70
- Perform a cardiac examination.
- Assess visual acuity and visual fields; test for nystagmus and identify any optic-nerve and retinal abnormalities.
- Evaluate lower-limb sensation, proprioception, and motor function.
- Evaluate suspected vestibular dysfunction, including dysfunction with positional testing (the Dix-Hallpike maneuver71). The patient is taken from sitting to supine while the head is rotated 45° to the tested side by the examiner. As the patient moves into a supine position, the neck is extended 30° off the table and held for at least 30 seconds. The maneuver is positive if torsional nystagmus is noted while the head is held rotated during neck extension. The maneuver is negative if the patient reports dizziness, vertigo, unsteadiness, or “pressure in the head.” Torsional nystagmus must be present to confirm a diagnosis of benign paroxysmal positional vertigo.
- If you suspect a central nervous system cause of imbalance, assess the cranial nerves, coordination, strength, and, of course, balance.
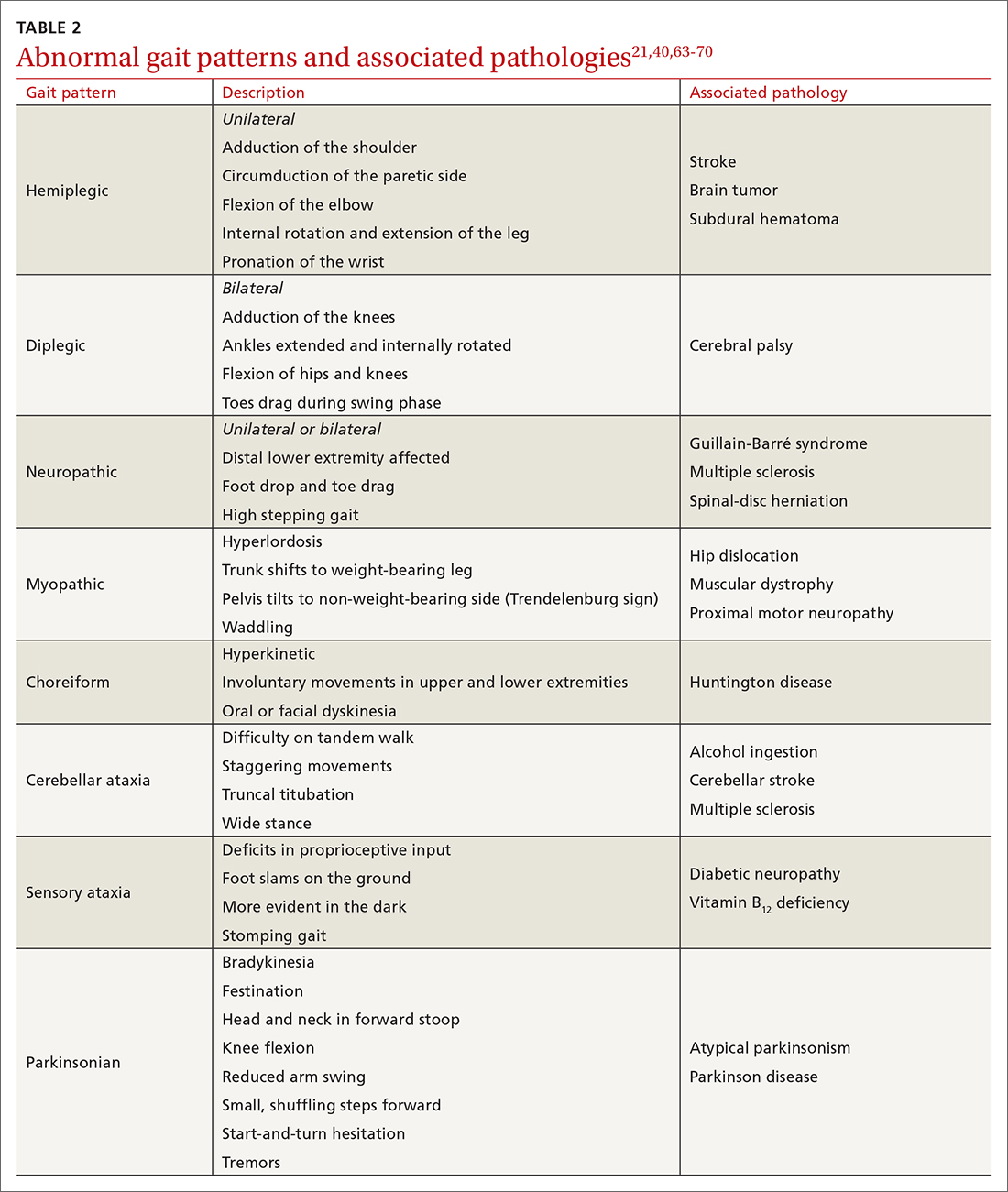
CASE
Mr. J’s physical examination showed normal vital signs without significant postural changes in blood pressure. Gait analysis revealed a slowed gait, with reduced range of motion in both knees over the entire gait cycle. Audiometry revealed symmetric moderate sensorineural hearing loss characteristic of presbycusis.
Diagnostic investigations
Consider focused investigations into imbalance based on the history and physical examination. We discourage overly broad testing and imaging; in primary care, cost and limited access to technology can bar robust investigations into causes of imbalance. However, identification of acute pathologies should prompt immediate referral to the emergency department. Furthermore, specific symptoms (TABLE 17-56) should prompt referral to specialists for assessment.
Continue to: In the emergency department...
In the emergency department and academic hospitals, key investigations can identify causes of imbalance:
- Electrocardiography and Holter monitoring test for cardiac arrhythmias.
- Echocardiography identifies structural abnormalities.
- Radiography and computed tomography are useful for detecting musculoskeletal abnormalities.
- Bone densitometry can identify osteoporosis.
- Head and spinal cord magnetic resonance imaging can be used to identify lesions of the central nervous system.
- Computed tomographic angiography of the head and neck is useful for identifying stroke, cerebral atrophy, and stenotic lesions of the carotid and vertebral arteries.
- Nerve conduction studies and levels of serum vitamin B12, hemoglobin A1C, thyroid-stimulating hormone, and random cortisol can uncover causes of peripheral neuropathy.
- Bedside cognitive screening tests can be used to measure cognitive decline.72
- Suspicion of vestibular disease requires audiometry and vestibular testing, including videonystagmography, head impulse testing, and vestibular evoked myogenic potentials.
In many cases of imbalance, no specific underlying correctable cause is discovered.
Management of imbalance
Pharmacotherapy
Targeted pharmacotherapy can be utilized in select clinical scenarios:
- Medical treatment of peripheral neuropathy should target the underlying condition.
- Cognitive behavioral therapy and antidepressants are useful for treating anxiety and depressive disorders.73
- Musculoskeletal pain can be managed with acetaminophen and topical nonsteroidal anti-inflammatory drugs (NSAIDs), using a short course of an oral NSAID when needed.74
- Cardiovascular disease management might include any of several classes of pharmacotherapy, including antiplatelet and lipid-lowering medications, antiarrhythmic drugs, and antihypertensive agents.
- Acute episodes of vertigo due to vestibular neuritis or labyrinthitis can be managed with an antiemetic.46
Surgical treatment
Surgery is infrequently considered for patients with imbalance. Examples of indications include microsurgical resection of vestibular schwannoma, resection of central nervous system tumors, lens replacement surgery for cataract, surgical management of severe spinal fracture, and hip or knee arthroplasty in select patients.
Tools for assessing the risk of falls
Scoring systems called falls risk assessment tools, or FRAT, have been developed to gauge a patient’s risk of falling. The various FRATs differ in specificity and sensitivity for predicting the risk of falls, and are typically designed for specific clinical environments, such as hospital inpatient care or long-term care facilities. Specifically, FRATs attempt to classify risk using sets of risk factors known to be associated with falls.
Continue to: Research abounds into...
Research abounds into the validity of commonly used FRATs across institutions, patient populations, and clinical environments:
The Johns Hopkins FRATa determines risk using metrics such as age, fall history, incontinence, cognition, mobility, and medications75; it is predominantly used for assessment in hospital inpatient units. This tool has been validated repeatedly.76,77
Peninsula Health FRATb stratifies patients in subacute and residential aged-care settings, based on risk factors that include recent falls, medications, psychological status, and cognition.78
FRAT-upc is a web-based tool that generates falls risk using risk factors that users input. This tool has been studied in the context of patients older than 65 years living in the community.79
Although FRATs are reasonably useful for predicting falls, their utility varies by patient population and clinical context. Moreover, it has been suggested that FRATs neglect environmental and personal factors when assessing risk by focusing primarily on bodily factors.80 Implementing a FRAT requires extensive consideration of the target population and should be accompanied by clinical judgment that is grounded in an individual patient’s circumstances.81
Continue to: Preventing falls in primary care
Preventing falls in primary care
An approach to preventing falls includes the development of individualized programs that account for frailty, a syndrome of physiologic decline associated with aging. Because frailty leads to diminished balance and mobility, a patient’s frailty index—determined using the 5 frailty phenotype criteria (exhaustion, weight loss, low physical activity, weakness, slowness)82 or the Canadian Study of Health and Aging Clinical Frailty Scale83—is a useful tool for predicting falls risk and readmission for falls following trauma-related injury. Prevention of falls in communities is critical for reducing mortality and allowing older people to maintain their independence and quality of life.
Exercise. In some areas, exercise and falls prevention programs are accessible to seniors.84 Community exercise programs that focus on balance retraining and muscle strengthening can reduce the risk of falls.73,85 The Choosing Wisely initiative of the ABIM [American Board of Internal Medicine] Foundation recommends that exercise programs be designed around an accurate functional baseline of the patient to avoid underdosed strength training.54
Multifactorial risk assessment in high-risk patients can reduce the rate of falls. Such an assessment includes examination of orthostatic blood pressure, vision and hearing, bone health, gait, activities of daily living, cognition, and environmental hazards, and enables provision of necessary interventions.73,86 Hearing amplification, specifically, correlates with enhanced postural control, slowed cognitive decline, and a reduced likelihood of falls.87-93 The mechanism behind improved balance performance might be reduced cognitive load through supporting a patient’s listening needs.88-90
Pharmacotherapy. Optimizing medications and performing a complete medication review before prescribing new medications is highly recommended to avoid unnecessary polypharmacy7,8,18,53-56 (TABLE 17-56).
Management of comorbidities associated with a higher risk of falls, including arthritis, cancer, stroke, diabetes, depression, kidney disease, chronic obstructive pulmonary disease, cognitive impairment, hypertension, and atrial fibrillation, is essential.94-96
Continue to: Home safety interventions
Home safety interventions, through occupational therapy, are important. These include removing unsafe mats and step-overs and installing nonslip strips on stairs, double-sided tape under mats, and handrails.73-97
Screening for risk of falls. The Centers for Disease Control and Prevention recommends that (1) all patients older than 65 years and (2) any patient presenting with an acute fall undergo screening for their risk of falls.98 When a patient is identified as at risk of falling, you can, when appropriate, assess modifiable risk factors and facilitate interventions.98 This strategy is supported by a 2018 statement from the US Preventive Services Task Force99 that recommends identifying high-risk patients who have:
- a history of falling
- a balance disturbance that causes a deficit of mobility or function
- poor performance on clinical tests, such as the 3-meter Timed Up and Go (TUG) assessment (www.cdc.gov/steadi/pdf/TUG_test-print.pdf).
An increased risk of falls should prompt you to refer the patient to community programs and physiotherapy in accordance with the individual’s personal goals99; a balance and vestibular physiotherapist is ideally positioned to accurately assess and manage patients at risk of falls. Specifically, the Task Force identified exercise programs and multifactorial interventions as being beneficial in preventing falls in high-risk older people.99
Balance assessment and rehabilitation in specialty centers
An individualized rehabilitation program aims to restore safe mobility by testing and addressing specific balance deficits, improving functional balance, and increasing balance confidence. Collaboration with colleagues from physiotherapy and occupational therapy aids in tailoring individualized programs.
Many tests are available to assess balance, determine the risk of falls, and guide rehabilitation:
- The timed 10-meter walk testd and the TUG test are simple assessments that measure functional mobility; both have normalized values for the risk of falls. A TUG time of ≥ 12 seconds suggests a high risk of falls.
- The 30-second chair stande evaluates functional lower-extremity strength in older patients. The test can indicate if lower-extremity strength is contributing to a patient’s imbalance.
- The modified clinical test of sensory interaction in balancef is a static balance test that measures the integrity of sensory inputs. The test can suggest if 1 or more sensory systems are compromised.
- The mini balance evaluation systems testg is similar: It can differentiate balance deficits by underlying system and allows individualization of a rehabilitation program.
- The functional gait assessmenth is a modification of the dynamic gait index that assesses postural stability during everyday dynamic activities, including tasks such as walking with head turns and pivots.
- The Berg Balance Scalei continues to be used extensively to assess balance.
Continue to: The mini balance evaluation systems test...
The mini balance evaluation systems test, functional gait index, and Berg Balance Scale all have normative age-graded values to predict fall risk.
CASE
Mr. J was referred for balance assessment and to a rehabilitation program. He underwent balance physiotherapy, including multifactorial balance assessment, joined a community exercise program, was fitted with hearing aids, and had his home environment optimized by an occupational therapist. (See examples of “home safety interventions” under “Preventing falls in primary care.”)
3 months later. Mr. J says he feels stronger on his feet. His knee pain has eased, and he is more confident walking around his home. He continues to engage in exercise programs and is comfortable running errands with his spouse.
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Division of OtolaryngologyHead and Neck Surgery, Queen’s University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected]
a www.hopkinsmedicine.org/institute_nursing/models_tools/jhfrat_acute%20care%20original_6_22_17.pdf
c www.ncbi.nlm.nih.gov/pmc/articles/PMC4376110/figure/figure14/?report=objectonly
e www.cdc.gov/steadi/pdf/STEADI-Assessment-30Sec-508.pdf
f www.mdapp.co/mctsib-modified-clinical-test-of-sensory-interaction-in-balance-calculator-404/
g www.sralab.org/sites/default/files/2017-07/MiniBEST_revised_final_3_8_13.pdf
1. Larocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient. 2011;4:189-201. doi: 10.2165/11591150-000000000-00000
2. TB, ZF, ES, et al. The relationship of balance disorders with falling, the effect of health problems, and social life on postural balance in the elderly living in a district in Turkey. Geriatrics (Basel). 2019;4:37. doi: 10.3390/geriatrics4020037
3. R, Sixt E, Landahl S, et al. Prevalence of dizziness and vertigo in an urban elderly population. J Vestib Res. 2004;14:47-52.
4. Sturnieks DL, St George R, Lord SR. Balance disorders in the elderly. Neurophysiol Clin. 2008;38:467-478. doi: 10.1016/j.neucli.2008.09.001
5. Boult C, Murphy J, Sloane P, et al. The relation of dizziness to functional decline. J Am Geriatr Soc. 1991;39:858-861. doi: 10.1111/j.1532-5415.1991.tb04451.x
6. Lin HW, Bhattacharyya N. Balance disorders in the elderly: epidemiology and functional impact. Laryngoscope. 2012;122:1858-1861. doi: 10.1002/lary.23376
7. Jia H, Lubetkin EI, DeMichele K, et al. Prevalence, risk factors, and burden of disease for falls and balance or walking problems among older adults in the U.S. Prev Med. 2019;126:105737. doi: 10.1016/j.ypmed.2019.05.025
8. Al-Momani M, Al-Momani F, Alghadir AH, et al. Factors related to gait and balance deficits in older adults. Clin Interv Aging. 2016;11:1043-1049. doi: 10.2147/CIA.S112282
9. Agrawal Y, Ward BK, Minor LB. Vestibular dysfunction: prevalence, impact and need for targeted treatment. J Vestib Res. 2013;23:113-117. doi: 10.3233/VES-130498
10. Altinsoy B, Erboy F, Tanriverdi H, et al. Syncope as a presentation of acute pulmonary embolism. Ther Clin Risk Manag. 2016;12:1023-1028. doi: 10.2147/TCRM.S105722
11. Belvederi Murri M, Triolo F, Coni A, et al. Instrumental assessment of balance and gait in depression: a systematic review. Psychiatry Res. 2020;284:112687. doi: 10.1016/j.psychres.2019.112687
12. Bhattacharyya N, Gubbels SP, Schwartz SR, et al. Clinical practice guideline: benign paroxysmal positional vertigo (update). Otolaryngol Head Neck Surg. 2017;156(suppl 3):S1-S47. doi: 10.1177/0194599816689667
13. S, Schwarm S, Grevenrath P, et al. Prevalence, aetiologies and prognosis of the symptom dizziness in primary care - a systematic review. BMC Fam Pract. 2018;19:33. doi: 10.1186/s12875-017-0695-0
14. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23:467-492. doi: 10.1128/CMR.00070-09
15. Chad DA. Lumbar spinal stenosis. Neurol Clin. 2007;25:407-418. doi: 10.1016/j.ncl.2007.01.003
16. Conrad BP, Shokat MS, Abbasi AZ, et al. Associations of self-report measures with gait, range of motion and proprioception in patients with lumbar spinal stenosis. Gait Posture. 2013;38:987-992. doi: 10.1016/j.gaitpost.2013.05.010
17. de Luna RA, Mihailovic A, Nguyen AM, et al. The association of glaucomatous visual field loss and balance. Transl Vis Sci Technol. 2017;6:8. doi: 10.1167/tvst.6.3.8
18. DiSogra RM. Common aminoglycosides and platinum-based ototoxic drugs: cochlear/vestibular side effects and incidence. Semin Hear. 2019;40:104-107. doi: 10.1055/s-0039-1684040
19. Ebersbach G, Moreau C, Gandor F, et al. Clinical syndromes: parkinsonian gait. Mov Disord. 2013;28:1552-1559. doi: 10.1002/mds.25675
20. Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S-1127S. doi: 10.3945/ajcn.2010.28608A
21. Filli L, Sutter T, Easthope CS, et al. Profiling walking dysfunction in multiple sclerosis: characterisation, classification and progression over time. Sci Rep. 2018;8:4984. doi: 10.1038/s41598-018-22676-0
22. Fritz NE, Kegelmeyer DA, Kloos AD, et al. Motor performance differentiates individuals with Lewy body dementia, Parkinson’s and Alzheimer’s disease. Gait Posture. 2016;50:1-7. doi: 10.1016/j.gaitpost.2016.08.009
23. Furman JM, Jacob RG. A clinical taxonomy of dizziness and anxiety in the otoneurological setting. J Anxiety Disord. 2001;15:9-26. doi: 10.1016/s0887-6185(00)00040-2
24. Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet Neurol. 2013;12:706-715. doi: 10.1016/S1474-4422(13)70107-8
25. Gerson LW, Jarjoura D, McCord G. Risk of imbalance in elderly people with impaired hearing or vision. Age Ageing. 1989;18:31-34. doi: 10.1093/ageing/18.1.31
26. Goudakos JK, Markou KD, Franco-Vidal V, et al. Corticosteroids in the treatment of vestibular neuritis: a systematic review and meta-analysis. Otol Neurotol. 2010;31:183-189. doi: 10.1097/MAO.0b013e3181ca843d
27. Green AD, CS, Bastian L, et al. Does this woman have osteoporosis? JAMA. 2004;292:2890-2900. doi: 10.1001/jama.292.23.2890
28. Hallemans A, Ortibus E, Meire F, et al. Low vision affects dynamic stability of gait. Gait Posture. 2010;32:547-551. doi: 10.1016/j.gaitpost.2010.07.018
29. Handelsman JA. Vestibulotoxicity: strategies for clinical diagnosis and rehabilitation. Int J Audiol. 2018;57(suppl 4):S99-S107. doi: 10.1080/14992027.2018.1468092
30. Head VA, Wakerley BR. Guillain-Barré syndrome in general practice: clinical features suggestive of early diagnosis. Br J Gen Pract. 2016;66:218-219. doi: 10.3399/bjgp16X684733
31. Helbostad JL, Vereijken B, Hesseberg K, et al. Altered vision destabilizes gait in older persons. Gait Posture. 2009;30:233-238. doi: 10.1016/j.gaitpost.2009.05.004
32. Hsu W-L, Chen C-Y, Tsauo J-Y, et al. Balance control in elderly people with osteoporosis. J Formos Med Assoc. 2014;113:334-339. doi: 10.1016/j.jfma.2014.02.006
33. Kim H-S, Yun DH, Yoo SD, et al. Balance control and knee osteoarthritis severity. Ann Rehabil Med. 2011;35:701-709. doi: 10.5535/arm.2011.35.5.701
34. Li L, Simonsick EM, Ferrucci L, et al. Hearing loss and gait speed among older adults in the United States. Gait Posture. 2013;38:25-29.
35. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88-100. doi: 10.1212/WNL.0000000000004058
36. Milner KA, Funk M, Richards S, et al. Gender differences in symptom presentation associated with coronary heart disease. Am J Cardiol. 1999;84:396-399. doi: 10.1016/s0002-9149(99)00322-7
37. Paillard T, F, Bru N, et al. The impact of time of day on the gait and balance control of Alzheimer’s patients. Chronobiol Int. 2016;33:161-168. doi: 10.3109/07420528.2015.1124885
38. Paldor I, Chen AS, Kaye AH. Growth rate of vestibular schwannoma. J Clin Neurosci. 2016;32:1-8. doi: 10.1016/j.jocn.2016.05.003
39. Picorelli AMA, Hatton AL, Gane EM, et al. Balance performance in older adults with hip osteoarthritis: a systematic review. Gait Posture. 2018;65:89-99. doi: 10.1016/j.gaitpost.2018.07.001
40. Raccagni C, Nonnekes J, Bloem BR, et al. Gait and postural disorders in parkinsonism: a clinical approach. J Neurol. 2020;267:3169-3176. doi: 10.1007/s00415-019-09382-1
41. Shanmugarajah PD, Hoggard N, Currie S, et al. Alcohol-related cerebellar degeneration: not all down to toxicity? Cerebellum Ataxias. 2016;3:17. doi: 10.1186/s40673-016-0055-1
42. Shih RY, Smirniotopoulos JG. Posterior fossa tumors in adult patients. Neuroimaging Clin N Am. 2016;26:493-510. doi: 10.1016/j.nic.2016.06.003
43. Smith EE. Clinical presentations and epidemiology of vascular dementia. Clin Sci (Lond). 2017;131:1059-1068. doi: 10.1042/CS20160607
44. Streur M, Ratcliffe SJ, Ball J, et al. Symptom clusters in adults with chronic atrial fibrillation. J Cardiovasc Nurs. 2017;32:296-303. doi: 10.1097/JCN.0000000000000344
45. Strupp M, M, JA. Peripheral vestibular disorders: an update. Curr Opin Neurol. 2019;32:165-173. doi: 10.1097/WCO.0000000000000649
46. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
47. Timar B, Timar R, L, et al. The impact of diabetic neuropathy on balance and on the risk of falls in patients with type 2 diabetes mellitus: a cross-sectional study. PLoS One. 2016;11:e0154654. doi: 10.1371/journal.pone.0154654
48. Walls R, Hockberger R, Gausche-Hill M. Peripheral nerve disorders. In: Rosen’s Emergency Medicine: Concepts and Clinical Practice. 9th ed. Elsevier, Inc; 2018:1307-1320.
49. Watson JC, Dyck PJB. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc. 2015;90:940-951. doi: 10.1016/j.mayocp.2015.05.004
50. Whitfield KC, Bourassa MW, Adamolekun B, et al. Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann N Y Acad Sci. 2018;1430:3-43. doi: 10.1111/nyas.13919
51. Wu V, Sykes EA, Beyea MM, et al. Approach to Meniere disease management. Can Fam Physician. 2019;65:463-467.
52. Yew KS, Cheng EM. Diagnosis of acute stroke. Am Fam Physician. 2015;91:528-536.
53. Seppala LJ, van de Glind EMM, Daams JG, et al; . Fall-risk-increasing drugs: a systematic review and meta-analysis: III. Others. J Am Med Dir Assoc. 2018;19:372.e1-372.e8. doi: 10.1016/j.jamda.2017.12.099
54. ABIM Foundation. Choosing wisely. Choosing Wisely website. 2021. Accessed November 11. 2021. www.choosingwisely.org/
55. Berlie HD, Garwood CL. Diabetes medications related to an increased risk of falls and fall-related morbidity in the elderly. Ann Pharmacother. 2010;44:712-717. doi: 10.1345/aph.1M551
56. Hartikainen S, E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci. 2007;62:1172-1181. doi: 10.1093/gerona/62.10.1172
57. Khanuja K, Joki J, Bachmann G, et al. Gait and balance in the aging population: Fall prevention using innovation and technology. Maturitas. 2018;110:51-56. doi: 10.1016/j.maturitas.2018.01.021
58. Salzman B. Gait and balance disorders in older adults. Am Fam Physician. 2010;82:61-68.
59. Zaninotto P, Huang YT, Di Gessa G, et al. Polypharmacy is a risk factor for hospital admission due to a fall: evidence from the English Longitudinal Study of Ageing. BMC Public Health. 2020;20:1804. doi: 10.1186/s12889-020-09920-x
60. Morin L, Calderon A, Welmer AK, et al. Polypharmacy and injurious falls in older adults: a nationwide nested case-control study. Clin Epidemiol. 2019;11:483-493. doi: 10.2147/CLEP.S201614
61. Dhalwani NN, Fahami R, Sathanapally H, et al. Association between polypharmacy and falls in older adults: a longitudinal study from England. BMJ Open. 2017;7:e016358. doi: 10.1136/bmjopen-2017-016358
62. Arnold AC, Raj SR. Orthostatic hypotension: a practical approach to investigation and management. Can J Cardiol. 2017;33:1725-1728. doi: 10.1016/j.cjca.2017.05.007
63. Alexander NB. Differential diagnosis of gait disorders in older adults. Clin Geriatr Med. 1996;12:689-703.
64. Baker JM. Gait disorders. Am J Med. 2018;131:602-607. doi: 10.1016/j.amjmed.2017.11.051
65. Cameron MH, Wagner JM. Gait abnormalities in multiple sclerosis: pathogenesis, evaluation, and advances in treatment. Curr Neurol Neurosci Rep. 2011;11:507-515. doi: 10.1007/s11910-011-0214-y
66. Chen C-L, Chen H-C, Tang SF-T, et al. Gait performance with compensatory adaptations in stroke patients with different degrees of motor recovery. Am J Phys Med Rehabil. 2003;82:925-935. doi: 10.1097/01.PHM.0000098040.13355.B5
67. Marsden J, Harris C. Cerebellar ataxia: pathophysiology and rehabilitation. Clin Rehabil. 2011;25:195-216. doi: 10.1177/0269215510382495
68. Mirek E, Filip M, W, et al. Three-dimensional trunk and lower limbs characteristics during gait in patients with Huntington’s disease. Front Neurosci. 2017;11:566. doi: 10.3389/fnins.2017.00566
69. Paramanandam V, Lizarraga KJ, Soh D, et al. Unusual gait disorders: a phenomenological approach and classification. Expert Rev Neurother. 2019;19:119-132. doi: 10.1080/14737175.2019.1562337
70. Sahyouni R, Goshtasbi K, Mahmoodi A, et al. Chronic subdural hematoma: a historical and clinical perspective. World Neurosurg. 2017;108:948-953. doi: 10.1016/j.wneu.2017.09.064
71. Talmud JD, Coffey R, Edemekong PF. Dix Hallpike maneuver. StatPearls [Internet]. StatPearls Publishing Updated September 5, 2021. Accessed December 6, 2021. www.ncbi.nlm.nih.gov/books/NBK459307/
72. Molnar FJ, Benjamin S, Hawkins SA, et al. One size does not fit all: choosing practical cognitive screening tools for your practice. J Am Geriatr Soc. 2020;68:2207-2213. doi: 10.1111/jgs.16713
73. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012:CD007146. doi: 10.1002/14651858.CD007146.pub3
74. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150. doi: 10.14336/AD.2017.0306
75. Poe SS, Cvach M, Dawson PB, Straus H, Hill EE. The Johns Hopkins Fall Risk Assessment Tool: postimplementation evaluation. J Nurs Care Qual. 2007;22:293-298. doi: 10.1097/01.NCQ.0000290408.74027.39
76. Poe SS, Dawson PB, Cvach M, et al. The Johns Hopkins Fall Risk Assessment Tool: a study of reliability and validity. J Nurs Care Qual. 2018;33:10-19. doi: 10.1097/NCQ.0000000000000301
77. Klinkenberg WD, Potter P. Validity of the Johns Hopkins Fall Risk Assessment Tool for predicting falls on inpatient medicine services. J Nurs Care Qual. 2017;32:108-113. doi: 10.1097/NCQ.0000000000000210
78. Stapleton C, Hough P, Oldmeadow L, et al. Four-item fall risk screening tool for subacute and residential aged care: the first step in fall prevention. Australas J Ageing. 2009;28:139-143. doi: 10.1111/j.1741-6612.2009.00375.x
79. Cattelani L, Palumbo P, Palmerini L, et al. FRAT-up, a Web-based fall-risk assessment tool for elderly people living in the community. J Med Internet Res. 2015;17:e41. doi: 10.2196/jmir.4064
80. De Clercq H, Naudé A, Bornman J. Factors included in adult fall risk assessment tools (FRATs): a systematic review. Ageing Soc. 2020;41:2558-2582. doi: 10.1017/S0144686X2000046X
81. Yap G, Melder A. Accuracy of validated falls risk assessment tools and clinical judgement. Centre for Clinical Effectiveness, Monash Innovation and Quality. Monash Health. February 5, 2020. Accessed November 11, 2021. https://monashhealth.org/wp-content/uploads/2019/01/Rapid-Review_Falls-risk-tools-FINAL.pdf
82. Chittrakul J, Siviroj P, Sungkarat S, et al. Physical frailty and fall risk in community-dwelling older adults: a cross-sectional study. J Aging Res. 2020;2020:3964973. doi: 10.1155/2020/3964973
83. Hatcher VH, Galet C, Lilienthal M, et al. Association of clinical frailty scores with hospital readmission for falls after index admission for trauma-related injury. JAMA Netw Open. 2019;2:e1912409. doi: 10.1001/jamanetworkopen.2019.12409
84. Exercise and fall prevention programs. Government of Ontario Ministry of Health. Updated April 9, 2019. Accessed November 11. 2021. www.ontario.ca/page/exercise-and-falls-prevention-programs
85. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1:CD012424. doi: 10.1002/14651858.CD012424.pub2
86. Hopewell S, Copsey B, Nicolson P, et al. Multifactorial interventions for preventing falls in older people living in the community: a systematic review and meta-analysis of 41 trials and almost 20 000 participants. Br J Sports Med. 2020;54:1340-1350. doi: 10.1136/bjsports-2019-100732
87. Jafari Z, Kolb BE, Mohajerani MH. Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev. 2019;56:100963. doi: 10.1016/j.arr.2019.100963
88. Griffiths TD, Lad M, Kumar S, et al. How can hearing loss cause dementia? Neuron. 2020;108:401-412. doi: 10.1016/j.neuron.2020.08.003
89. Martini A, Castiglione A, Bovo R, et al. Aging, cognitive load, dementia and hearing loss. Audiol Neurootol. 2014;19(suppl 1):2-5. doi: 10.1159/000371593
90. Vitkovic J, Le C, Lee S-L, et al. The contribution of hearing and hearing loss to balance control. Audiol Neurootol. 2016;21:195-202. doi: 10.1159/000445100
91. Maheu M, Behtani L, Nooristani M, et al. Vestibular function modulates the benefit of hearing aids in people with hearing loss during static postural control. Ear Hear. 2019;40:1418-1424. doi: 10.1097/AUD.0000000000000720
92. Negahban H, Bavarsad Cheshmeh Ali M, Nassadj G. Effect of hearing aids on static balance function in elderly with hearing loss. Gait Posture. 2017;58:126-129. doi: 10.1016/j.gaitpost.2017.07.112
93. Mahmoudi E, Basu T, Langa K, et al. Can hearing aids delay time to diagnosis of dementia, depression, or falls in older adults? J Am Geriatr Soc. 2019;67:2362-2369. doi: 10.1111/jgs.16109
94. Paliwal Y, Slattum PW, Ratliff SM. Chronic health conditions as a risk factor for falls among the community-dwelling US older adults: a zero-inflated regression modeling approach. Biomed Res Int. 2017;2017:5146378. doi: 10.1155/2017/5146378
95. Deandrea S, Lucenteforte E, Bravi F, et al. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010;21:658-668. doi: 10.1097/EDE.0b013e3181e89905
96. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51-61. doi: 10.1016/j.maturitas.2013.02.009
97. Stevens M, Holman CD, Bennett N. Preventing falls in older people: impact of an intervention to reduce environmental hazards in the home. J Am Geriatr Soc. 2001;49:1442-1447. doi: 10.1046/j.1532-5415.2001.4911235.x
98. Clinical resources. Centers for Disease Control and Prevention STEADI-Older Adult Fall Prevention website. 2020. Accessed November 12, 2021. www.cdc.gov/steadi/materials.html
99. ; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1696-1704. doi: 10.1001/jama.2018.3097
CASE Mr. J, a 75-year-old man, presents to your family practice reporting that he feels increasingly unsteady and slow while walking. He fell twice last year, without resulting injury. He now worries about tripping while walking around the house and relies on his spouse to run errands.
Clearly, Mr. J is experiencing a problem with balance. What management approach should you undertake to prevent him from falling?

Balance disorders are common in older people and drastically hinder quality of life.1-4 Patients often describe imbalance as vague symptoms: dizziness, unsteadiness, faintness, spinning sensations.5,6 Importantly, balance disorders disrupt normal gait and contribute to falls that are a major cause of disability and morbidity in older people. Almost 30% of people older than 65 years report 1 or more falls annually.7 Factors that increase the risk of falls include impaired mobility, previously reported falls, reduced psychological functioning, chronic medical conditions, and polypharmacy.7,8
The cause of any single case of imbalance is often multifactorial, resulting from dysfunction of multiple body systems (TABLE 17-56); in our clinical experience, most patients with imbalance and who are at risk of falls do not have a detectable deficit of the vestibular system. These alterations in function arise in 3 key systems—vision, proprioception, and vestibular function—which signal to, and are incorporated by, the cerebellum to mediate balance. Cognitive and neurologic decline are also factors in imbalance.

Considering that 20% of falls result in serious injury in older populations, it is important to identify balance disorders and implement preventive strategies to mitigate harmful consequences of falls on patients’ health and independence.7,57 In this article, we answer the question that the case presentation raises about the proper management approach to imbalance in family practice, including assessment of risk and rehabilitation strategies to reduce the risk of falls. Our insights and recommendations are based on our clinical experience and a review of the medical literature from the past 40 years.

CASE Mr. J has a history of hypertension, age-related hearing loss, and osteoarthritis of the knees; he has not had surgery for the arthritis. His medications are antihypertensives and extra-strength acetaminophen for knee pain.

Making the diagnosis of a balance disorder
History
A thorough clinical history, often including a collateral history from caregivers, narrows the differential diagnosis. Information regarding onset, duration, timing, character, and previous episodes of imbalance is essential. Symptoms of imbalance are often challenging for the patient to describe: They might use terms such as vertigo or dizziness, when, in fact, on further questioning, they are describing balance difficulties. Inquiry into (1) their use of assistive walking devices and (2) development or exacerbation of neurologic, musculoskeletal, auditory, visual, and mood symptoms is necessary. Note the current level of their mobility, episodes of pain or fatigue, previous falls and associated injuries, fear of falling, balance confidence, and sensations that precede falls.58
Continue to: The medical and surgical histories
The medical and surgical histories are key pieces of information. The history of smoking, alcohol habits, and substance use is relevant.
A robust medication history is essential to evaluate a patient’s risk of falling. Polypharmacy—typically, defined as taking 4 or more medications—has been repeatedly associated with a heightened risk of falls.53,59-61 Moreover, a dose-dependent association between polypharmacy and hospitalization following falls has been identified, and demonstrates that taking 10 or more medications greatly increases the risk of hospitalization.59 Studies of polypharmacy cement the importance of inquiring about medication use when assessing imbalance, particularly in older patients.
Physical examination
A focused and detailed physical examination provides insight into systems that should be investigated:
- Obtain vital signs, including orthostatic vitals to test for orthostatic hypotension62; keep in mind that symptoms of orthostatic dizziness can occur without orthostatic hypotension.
- Examine gait, which can distinguish between causes of imbalance (TABLE 2).21,40,63-70
- Perform a cardiac examination.
- Assess visual acuity and visual fields; test for nystagmus and identify any optic-nerve and retinal abnormalities.
- Evaluate lower-limb sensation, proprioception, and motor function.
- Evaluate suspected vestibular dysfunction, including dysfunction with positional testing (the Dix-Hallpike maneuver71). The patient is taken from sitting to supine while the head is rotated 45° to the tested side by the examiner. As the patient moves into a supine position, the neck is extended 30° off the table and held for at least 30 seconds. The maneuver is positive if torsional nystagmus is noted while the head is held rotated during neck extension. The maneuver is negative if the patient reports dizziness, vertigo, unsteadiness, or “pressure in the head.” Torsional nystagmus must be present to confirm a diagnosis of benign paroxysmal positional vertigo.
- If you suspect a central nervous system cause of imbalance, assess the cranial nerves, coordination, strength, and, of course, balance.

CASE
Mr. J’s physical examination showed normal vital signs without significant postural changes in blood pressure. Gait analysis revealed a slowed gait, with reduced range of motion in both knees over the entire gait cycle. Audiometry revealed symmetric moderate sensorineural hearing loss characteristic of presbycusis.
Diagnostic investigations
Consider focused investigations into imbalance based on the history and physical examination. We discourage overly broad testing and imaging; in primary care, cost and limited access to technology can bar robust investigations into causes of imbalance. However, identification of acute pathologies should prompt immediate referral to the emergency department. Furthermore, specific symptoms (TABLE 17-56) should prompt referral to specialists for assessment.
Continue to: In the emergency department...
In the emergency department and academic hospitals, key investigations can identify causes of imbalance:
- Electrocardiography and Holter monitoring test for cardiac arrhythmias.
- Echocardiography identifies structural abnormalities.
- Radiography and computed tomography are useful for detecting musculoskeletal abnormalities.
- Bone densitometry can identify osteoporosis.
- Head and spinal cord magnetic resonance imaging can be used to identify lesions of the central nervous system.
- Computed tomographic angiography of the head and neck is useful for identifying stroke, cerebral atrophy, and stenotic lesions of the carotid and vertebral arteries.
- Nerve conduction studies and levels of serum vitamin B12, hemoglobin A1C, thyroid-stimulating hormone, and random cortisol can uncover causes of peripheral neuropathy.
- Bedside cognitive screening tests can be used to measure cognitive decline.72
- Suspicion of vestibular disease requires audiometry and vestibular testing, including videonystagmography, head impulse testing, and vestibular evoked myogenic potentials.
In many cases of imbalance, no specific underlying correctable cause is discovered.
Management of imbalance
Pharmacotherapy
Targeted pharmacotherapy can be utilized in select clinical scenarios:
- Medical treatment of peripheral neuropathy should target the underlying condition.
- Cognitive behavioral therapy and antidepressants are useful for treating anxiety and depressive disorders.73
- Musculoskeletal pain can be managed with acetaminophen and topical nonsteroidal anti-inflammatory drugs (NSAIDs), using a short course of an oral NSAID when needed.74
- Cardiovascular disease management might include any of several classes of pharmacotherapy, including antiplatelet and lipid-lowering medications, antiarrhythmic drugs, and antihypertensive agents.
- Acute episodes of vertigo due to vestibular neuritis or labyrinthitis can be managed with an antiemetic.46
Surgical treatment
Surgery is infrequently considered for patients with imbalance. Examples of indications include microsurgical resection of vestibular schwannoma, resection of central nervous system tumors, lens replacement surgery for cataract, surgical management of severe spinal fracture, and hip or knee arthroplasty in select patients.
Tools for assessing the risk of falls
Scoring systems called falls risk assessment tools, or FRAT, have been developed to gauge a patient’s risk of falling. The various FRATs differ in specificity and sensitivity for predicting the risk of falls, and are typically designed for specific clinical environments, such as hospital inpatient care or long-term care facilities. Specifically, FRATs attempt to classify risk using sets of risk factors known to be associated with falls.
Continue to: Research abounds into...
Research abounds into the validity of commonly used FRATs across institutions, patient populations, and clinical environments:
The Johns Hopkins FRATa determines risk using metrics such as age, fall history, incontinence, cognition, mobility, and medications75; it is predominantly used for assessment in hospital inpatient units. This tool has been validated repeatedly.76,77
Peninsula Health FRATb stratifies patients in subacute and residential aged-care settings, based on risk factors that include recent falls, medications, psychological status, and cognition.78
FRAT-upc is a web-based tool that generates falls risk using risk factors that users input. This tool has been studied in the context of patients older than 65 years living in the community.79
Although FRATs are reasonably useful for predicting falls, their utility varies by patient population and clinical context. Moreover, it has been suggested that FRATs neglect environmental and personal factors when assessing risk by focusing primarily on bodily factors.80 Implementing a FRAT requires extensive consideration of the target population and should be accompanied by clinical judgment that is grounded in an individual patient’s circumstances.81
Continue to: Preventing falls in primary care
Preventing falls in primary care
An approach to preventing falls includes the development of individualized programs that account for frailty, a syndrome of physiologic decline associated with aging. Because frailty leads to diminished balance and mobility, a patient’s frailty index—determined using the 5 frailty phenotype criteria (exhaustion, weight loss, low physical activity, weakness, slowness)82 or the Canadian Study of Health and Aging Clinical Frailty Scale83—is a useful tool for predicting falls risk and readmission for falls following trauma-related injury. Prevention of falls in communities is critical for reducing mortality and allowing older people to maintain their independence and quality of life.
Exercise. In some areas, exercise and falls prevention programs are accessible to seniors.84 Community exercise programs that focus on balance retraining and muscle strengthening can reduce the risk of falls.73,85 The Choosing Wisely initiative of the ABIM [American Board of Internal Medicine] Foundation recommends that exercise programs be designed around an accurate functional baseline of the patient to avoid underdosed strength training.54
Multifactorial risk assessment in high-risk patients can reduce the rate of falls. Such an assessment includes examination of orthostatic blood pressure, vision and hearing, bone health, gait, activities of daily living, cognition, and environmental hazards, and enables provision of necessary interventions.73,86 Hearing amplification, specifically, correlates with enhanced postural control, slowed cognitive decline, and a reduced likelihood of falls.87-93 The mechanism behind improved balance performance might be reduced cognitive load through supporting a patient’s listening needs.88-90
Pharmacotherapy. Optimizing medications and performing a complete medication review before prescribing new medications is highly recommended to avoid unnecessary polypharmacy7,8,18,53-56 (TABLE 17-56).
Management of comorbidities associated with a higher risk of falls, including arthritis, cancer, stroke, diabetes, depression, kidney disease, chronic obstructive pulmonary disease, cognitive impairment, hypertension, and atrial fibrillation, is essential.94-96
Continue to: Home safety interventions
Home safety interventions, through occupational therapy, are important. These include removing unsafe mats and step-overs and installing nonslip strips on stairs, double-sided tape under mats, and handrails.73-97
Screening for risk of falls. The Centers for Disease Control and Prevention recommends that (1) all patients older than 65 years and (2) any patient presenting with an acute fall undergo screening for their risk of falls.98 When a patient is identified as at risk of falling, you can, when appropriate, assess modifiable risk factors and facilitate interventions.98 This strategy is supported by a 2018 statement from the US Preventive Services Task Force99 that recommends identifying high-risk patients who have:
- a history of falling
- a balance disturbance that causes a deficit of mobility or function
- poor performance on clinical tests, such as the 3-meter Timed Up and Go (TUG) assessment (www.cdc.gov/steadi/pdf/TUG_test-print.pdf).
An increased risk of falls should prompt you to refer the patient to community programs and physiotherapy in accordance with the individual’s personal goals99; a balance and vestibular physiotherapist is ideally positioned to accurately assess and manage patients at risk of falls. Specifically, the Task Force identified exercise programs and multifactorial interventions as being beneficial in preventing falls in high-risk older people.99
Balance assessment and rehabilitation in specialty centers
An individualized rehabilitation program aims to restore safe mobility by testing and addressing specific balance deficits, improving functional balance, and increasing balance confidence. Collaboration with colleagues from physiotherapy and occupational therapy aids in tailoring individualized programs.
Many tests are available to assess balance, determine the risk of falls, and guide rehabilitation:
- The timed 10-meter walk testd and the TUG test are simple assessments that measure functional mobility; both have normalized values for the risk of falls. A TUG time of ≥ 12 seconds suggests a high risk of falls.
- The 30-second chair stande evaluates functional lower-extremity strength in older patients. The test can indicate if lower-extremity strength is contributing to a patient’s imbalance.
- The modified clinical test of sensory interaction in balancef is a static balance test that measures the integrity of sensory inputs. The test can suggest if 1 or more sensory systems are compromised.
- The mini balance evaluation systems testg is similar: It can differentiate balance deficits by underlying system and allows individualization of a rehabilitation program.
- The functional gait assessmenth is a modification of the dynamic gait index that assesses postural stability during everyday dynamic activities, including tasks such as walking with head turns and pivots.
- The Berg Balance Scalei continues to be used extensively to assess balance.
Continue to: The mini balance evaluation systems test...
The mini balance evaluation systems test, functional gait index, and Berg Balance Scale all have normative age-graded values to predict fall risk.
CASE
Mr. J was referred for balance assessment and to a rehabilitation program. He underwent balance physiotherapy, including multifactorial balance assessment, joined a community exercise program, was fitted with hearing aids, and had his home environment optimized by an occupational therapist. (See examples of “home safety interventions” under “Preventing falls in primary care.”)
3 months later. Mr. J says he feels stronger on his feet. His knee pain has eased, and he is more confident walking around his home. He continues to engage in exercise programs and is comfortable running errands with his spouse.
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Division of OtolaryngologyHead and Neck Surgery, Queen’s University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected]
a www.hopkinsmedicine.org/institute_nursing/models_tools/jhfrat_acute%20care%20original_6_22_17.pdf
c www.ncbi.nlm.nih.gov/pmc/articles/PMC4376110/figure/figure14/?report=objectonly
e www.cdc.gov/steadi/pdf/STEADI-Assessment-30Sec-508.pdf
f www.mdapp.co/mctsib-modified-clinical-test-of-sensory-interaction-in-balance-calculator-404/
g www.sralab.org/sites/default/files/2017-07/MiniBEST_revised_final_3_8_13.pdf
CASE Mr. J, a 75-year-old man, presents to your family practice reporting that he feels increasingly unsteady and slow while walking. He fell twice last year, without resulting injury. He now worries about tripping while walking around the house and relies on his spouse to run errands.
Clearly, Mr. J is experiencing a problem with balance. What management approach should you undertake to prevent him from falling?

Balance disorders are common in older people and drastically hinder quality of life.1-4 Patients often describe imbalance as vague symptoms: dizziness, unsteadiness, faintness, spinning sensations.5,6 Importantly, balance disorders disrupt normal gait and contribute to falls that are a major cause of disability and morbidity in older people. Almost 30% of people older than 65 years report 1 or more falls annually.7 Factors that increase the risk of falls include impaired mobility, previously reported falls, reduced psychological functioning, chronic medical conditions, and polypharmacy.7,8
The cause of any single case of imbalance is often multifactorial, resulting from dysfunction of multiple body systems (TABLE 17-56); in our clinical experience, most patients with imbalance and who are at risk of falls do not have a detectable deficit of the vestibular system. These alterations in function arise in 3 key systems—vision, proprioception, and vestibular function—which signal to, and are incorporated by, the cerebellum to mediate balance. Cognitive and neurologic decline are also factors in imbalance.

Considering that 20% of falls result in serious injury in older populations, it is important to identify balance disorders and implement preventive strategies to mitigate harmful consequences of falls on patients’ health and independence.7,57 In this article, we answer the question that the case presentation raises about the proper management approach to imbalance in family practice, including assessment of risk and rehabilitation strategies to reduce the risk of falls. Our insights and recommendations are based on our clinical experience and a review of the medical literature from the past 40 years.

CASE Mr. J has a history of hypertension, age-related hearing loss, and osteoarthritis of the knees; he has not had surgery for the arthritis. His medications are antihypertensives and extra-strength acetaminophen for knee pain.

Making the diagnosis of a balance disorder
History
A thorough clinical history, often including a collateral history from caregivers, narrows the differential diagnosis. Information regarding onset, duration, timing, character, and previous episodes of imbalance is essential. Symptoms of imbalance are often challenging for the patient to describe: They might use terms such as vertigo or dizziness, when, in fact, on further questioning, they are describing balance difficulties. Inquiry into (1) their use of assistive walking devices and (2) development or exacerbation of neurologic, musculoskeletal, auditory, visual, and mood symptoms is necessary. Note the current level of their mobility, episodes of pain or fatigue, previous falls and associated injuries, fear of falling, balance confidence, and sensations that precede falls.58
Continue to: The medical and surgical histories
The medical and surgical histories are key pieces of information. The history of smoking, alcohol habits, and substance use is relevant.
A robust medication history is essential to evaluate a patient’s risk of falling. Polypharmacy—typically, defined as taking 4 or more medications—has been repeatedly associated with a heightened risk of falls.53,59-61 Moreover, a dose-dependent association between polypharmacy and hospitalization following falls has been identified, and demonstrates that taking 10 or more medications greatly increases the risk of hospitalization.59 Studies of polypharmacy cement the importance of inquiring about medication use when assessing imbalance, particularly in older patients.
Physical examination
A focused and detailed physical examination provides insight into systems that should be investigated:
- Obtain vital signs, including orthostatic vitals to test for orthostatic hypotension62; keep in mind that symptoms of orthostatic dizziness can occur without orthostatic hypotension.
- Examine gait, which can distinguish between causes of imbalance (TABLE 2).21,40,63-70
- Perform a cardiac examination.
- Assess visual acuity and visual fields; test for nystagmus and identify any optic-nerve and retinal abnormalities.
- Evaluate lower-limb sensation, proprioception, and motor function.
- Evaluate suspected vestibular dysfunction, including dysfunction with positional testing (the Dix-Hallpike maneuver71). The patient is taken from sitting to supine while the head is rotated 45° to the tested side by the examiner. As the patient moves into a supine position, the neck is extended 30° off the table and held for at least 30 seconds. The maneuver is positive if torsional nystagmus is noted while the head is held rotated during neck extension. The maneuver is negative if the patient reports dizziness, vertigo, unsteadiness, or “pressure in the head.” Torsional nystagmus must be present to confirm a diagnosis of benign paroxysmal positional vertigo.
- If you suspect a central nervous system cause of imbalance, assess the cranial nerves, coordination, strength, and, of course, balance.

CASE
Mr. J’s physical examination showed normal vital signs without significant postural changes in blood pressure. Gait analysis revealed a slowed gait, with reduced range of motion in both knees over the entire gait cycle. Audiometry revealed symmetric moderate sensorineural hearing loss characteristic of presbycusis.
Diagnostic investigations
Consider focused investigations into imbalance based on the history and physical examination. We discourage overly broad testing and imaging; in primary care, cost and limited access to technology can bar robust investigations into causes of imbalance. However, identification of acute pathologies should prompt immediate referral to the emergency department. Furthermore, specific symptoms (TABLE 17-56) should prompt referral to specialists for assessment.
Continue to: In the emergency department...
In the emergency department and academic hospitals, key investigations can identify causes of imbalance:
- Electrocardiography and Holter monitoring test for cardiac arrhythmias.
- Echocardiography identifies structural abnormalities.
- Radiography and computed tomography are useful for detecting musculoskeletal abnormalities.
- Bone densitometry can identify osteoporosis.
- Head and spinal cord magnetic resonance imaging can be used to identify lesions of the central nervous system.
- Computed tomographic angiography of the head and neck is useful for identifying stroke, cerebral atrophy, and stenotic lesions of the carotid and vertebral arteries.
- Nerve conduction studies and levels of serum vitamin B12, hemoglobin A1C, thyroid-stimulating hormone, and random cortisol can uncover causes of peripheral neuropathy.
- Bedside cognitive screening tests can be used to measure cognitive decline.72
- Suspicion of vestibular disease requires audiometry and vestibular testing, including videonystagmography, head impulse testing, and vestibular evoked myogenic potentials.
In many cases of imbalance, no specific underlying correctable cause is discovered.
Management of imbalance
Pharmacotherapy
Targeted pharmacotherapy can be utilized in select clinical scenarios:
- Medical treatment of peripheral neuropathy should target the underlying condition.
- Cognitive behavioral therapy and antidepressants are useful for treating anxiety and depressive disorders.73
- Musculoskeletal pain can be managed with acetaminophen and topical nonsteroidal anti-inflammatory drugs (NSAIDs), using a short course of an oral NSAID when needed.74
- Cardiovascular disease management might include any of several classes of pharmacotherapy, including antiplatelet and lipid-lowering medications, antiarrhythmic drugs, and antihypertensive agents.
- Acute episodes of vertigo due to vestibular neuritis or labyrinthitis can be managed with an antiemetic.46
Surgical treatment
Surgery is infrequently considered for patients with imbalance. Examples of indications include microsurgical resection of vestibular schwannoma, resection of central nervous system tumors, lens replacement surgery for cataract, surgical management of severe spinal fracture, and hip or knee arthroplasty in select patients.
Tools for assessing the risk of falls
Scoring systems called falls risk assessment tools, or FRAT, have been developed to gauge a patient’s risk of falling. The various FRATs differ in specificity and sensitivity for predicting the risk of falls, and are typically designed for specific clinical environments, such as hospital inpatient care or long-term care facilities. Specifically, FRATs attempt to classify risk using sets of risk factors known to be associated with falls.
Continue to: Research abounds into...
Research abounds into the validity of commonly used FRATs across institutions, patient populations, and clinical environments:
The Johns Hopkins FRATa determines risk using metrics such as age, fall history, incontinence, cognition, mobility, and medications75; it is predominantly used for assessment in hospital inpatient units. This tool has been validated repeatedly.76,77
Peninsula Health FRATb stratifies patients in subacute and residential aged-care settings, based on risk factors that include recent falls, medications, psychological status, and cognition.78
FRAT-upc is a web-based tool that generates falls risk using risk factors that users input. This tool has been studied in the context of patients older than 65 years living in the community.79
Although FRATs are reasonably useful for predicting falls, their utility varies by patient population and clinical context. Moreover, it has been suggested that FRATs neglect environmental and personal factors when assessing risk by focusing primarily on bodily factors.80 Implementing a FRAT requires extensive consideration of the target population and should be accompanied by clinical judgment that is grounded in an individual patient’s circumstances.81
Continue to: Preventing falls in primary care
Preventing falls in primary care
An approach to preventing falls includes the development of individualized programs that account for frailty, a syndrome of physiologic decline associated with aging. Because frailty leads to diminished balance and mobility, a patient’s frailty index—determined using the 5 frailty phenotype criteria (exhaustion, weight loss, low physical activity, weakness, slowness)82 or the Canadian Study of Health and Aging Clinical Frailty Scale83—is a useful tool for predicting falls risk and readmission for falls following trauma-related injury. Prevention of falls in communities is critical for reducing mortality and allowing older people to maintain their independence and quality of life.
Exercise. In some areas, exercise and falls prevention programs are accessible to seniors.84 Community exercise programs that focus on balance retraining and muscle strengthening can reduce the risk of falls.73,85 The Choosing Wisely initiative of the ABIM [American Board of Internal Medicine] Foundation recommends that exercise programs be designed around an accurate functional baseline of the patient to avoid underdosed strength training.54
Multifactorial risk assessment in high-risk patients can reduce the rate of falls. Such an assessment includes examination of orthostatic blood pressure, vision and hearing, bone health, gait, activities of daily living, cognition, and environmental hazards, and enables provision of necessary interventions.73,86 Hearing amplification, specifically, correlates with enhanced postural control, slowed cognitive decline, and a reduced likelihood of falls.87-93 The mechanism behind improved balance performance might be reduced cognitive load through supporting a patient’s listening needs.88-90
Pharmacotherapy. Optimizing medications and performing a complete medication review before prescribing new medications is highly recommended to avoid unnecessary polypharmacy7,8,18,53-56 (TABLE 17-56).
Management of comorbidities associated with a higher risk of falls, including arthritis, cancer, stroke, diabetes, depression, kidney disease, chronic obstructive pulmonary disease, cognitive impairment, hypertension, and atrial fibrillation, is essential.94-96
Continue to: Home safety interventions
Home safety interventions, through occupational therapy, are important. These include removing unsafe mats and step-overs and installing nonslip strips on stairs, double-sided tape under mats, and handrails.73-97
Screening for risk of falls. The Centers for Disease Control and Prevention recommends that (1) all patients older than 65 years and (2) any patient presenting with an acute fall undergo screening for their risk of falls.98 When a patient is identified as at risk of falling, you can, when appropriate, assess modifiable risk factors and facilitate interventions.98 This strategy is supported by a 2018 statement from the US Preventive Services Task Force99 that recommends identifying high-risk patients who have:
- a history of falling
- a balance disturbance that causes a deficit of mobility or function
- poor performance on clinical tests, such as the 3-meter Timed Up and Go (TUG) assessment (www.cdc.gov/steadi/pdf/TUG_test-print.pdf).
An increased risk of falls should prompt you to refer the patient to community programs and physiotherapy in accordance with the individual’s personal goals99; a balance and vestibular physiotherapist is ideally positioned to accurately assess and manage patients at risk of falls. Specifically, the Task Force identified exercise programs and multifactorial interventions as being beneficial in preventing falls in high-risk older people.99
Balance assessment and rehabilitation in specialty centers
An individualized rehabilitation program aims to restore safe mobility by testing and addressing specific balance deficits, improving functional balance, and increasing balance confidence. Collaboration with colleagues from physiotherapy and occupational therapy aids in tailoring individualized programs.
Many tests are available to assess balance, determine the risk of falls, and guide rehabilitation:
- The timed 10-meter walk testd and the TUG test are simple assessments that measure functional mobility; both have normalized values for the risk of falls. A TUG time of ≥ 12 seconds suggests a high risk of falls.
- The 30-second chair stande evaluates functional lower-extremity strength in older patients. The test can indicate if lower-extremity strength is contributing to a patient’s imbalance.
- The modified clinical test of sensory interaction in balancef is a static balance test that measures the integrity of sensory inputs. The test can suggest if 1 or more sensory systems are compromised.
- The mini balance evaluation systems testg is similar: It can differentiate balance deficits by underlying system and allows individualization of a rehabilitation program.
- The functional gait assessmenth is a modification of the dynamic gait index that assesses postural stability during everyday dynamic activities, including tasks such as walking with head turns and pivots.
- The Berg Balance Scalei continues to be used extensively to assess balance.
Continue to: The mini balance evaluation systems test...
The mini balance evaluation systems test, functional gait index, and Berg Balance Scale all have normative age-graded values to predict fall risk.
CASE
Mr. J was referred for balance assessment and to a rehabilitation program. He underwent balance physiotherapy, including multifactorial balance assessment, joined a community exercise program, was fitted with hearing aids, and had his home environment optimized by an occupational therapist. (See examples of “home safety interventions” under “Preventing falls in primary care.”)
3 months later. Mr. J says he feels stronger on his feet. His knee pain has eased, and he is more confident walking around his home. He continues to engage in exercise programs and is comfortable running errands with his spouse.
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Division of OtolaryngologyHead and Neck Surgery, Queen’s University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected]
a www.hopkinsmedicine.org/institute_nursing/models_tools/jhfrat_acute%20care%20original_6_22_17.pdf
c www.ncbi.nlm.nih.gov/pmc/articles/PMC4376110/figure/figure14/?report=objectonly
e www.cdc.gov/steadi/pdf/STEADI-Assessment-30Sec-508.pdf
f www.mdapp.co/mctsib-modified-clinical-test-of-sensory-interaction-in-balance-calculator-404/
g www.sralab.org/sites/default/files/2017-07/MiniBEST_revised_final_3_8_13.pdf
1. Larocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient. 2011;4:189-201. doi: 10.2165/11591150-000000000-00000
2. TB, ZF, ES, et al. The relationship of balance disorders with falling, the effect of health problems, and social life on postural balance in the elderly living in a district in Turkey. Geriatrics (Basel). 2019;4:37. doi: 10.3390/geriatrics4020037
3. R, Sixt E, Landahl S, et al. Prevalence of dizziness and vertigo in an urban elderly population. J Vestib Res. 2004;14:47-52.
4. Sturnieks DL, St George R, Lord SR. Balance disorders in the elderly. Neurophysiol Clin. 2008;38:467-478. doi: 10.1016/j.neucli.2008.09.001
5. Boult C, Murphy J, Sloane P, et al. The relation of dizziness to functional decline. J Am Geriatr Soc. 1991;39:858-861. doi: 10.1111/j.1532-5415.1991.tb04451.x
6. Lin HW, Bhattacharyya N. Balance disorders in the elderly: epidemiology and functional impact. Laryngoscope. 2012;122:1858-1861. doi: 10.1002/lary.23376
7. Jia H, Lubetkin EI, DeMichele K, et al. Prevalence, risk factors, and burden of disease for falls and balance or walking problems among older adults in the U.S. Prev Med. 2019;126:105737. doi: 10.1016/j.ypmed.2019.05.025
8. Al-Momani M, Al-Momani F, Alghadir AH, et al. Factors related to gait and balance deficits in older adults. Clin Interv Aging. 2016;11:1043-1049. doi: 10.2147/CIA.S112282
9. Agrawal Y, Ward BK, Minor LB. Vestibular dysfunction: prevalence, impact and need for targeted treatment. J Vestib Res. 2013;23:113-117. doi: 10.3233/VES-130498
10. Altinsoy B, Erboy F, Tanriverdi H, et al. Syncope as a presentation of acute pulmonary embolism. Ther Clin Risk Manag. 2016;12:1023-1028. doi: 10.2147/TCRM.S105722
11. Belvederi Murri M, Triolo F, Coni A, et al. Instrumental assessment of balance and gait in depression: a systematic review. Psychiatry Res. 2020;284:112687. doi: 10.1016/j.psychres.2019.112687
12. Bhattacharyya N, Gubbels SP, Schwartz SR, et al. Clinical practice guideline: benign paroxysmal positional vertigo (update). Otolaryngol Head Neck Surg. 2017;156(suppl 3):S1-S47. doi: 10.1177/0194599816689667
13. S, Schwarm S, Grevenrath P, et al. Prevalence, aetiologies and prognosis of the symptom dizziness in primary care - a systematic review. BMC Fam Pract. 2018;19:33. doi: 10.1186/s12875-017-0695-0
14. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23:467-492. doi: 10.1128/CMR.00070-09
15. Chad DA. Lumbar spinal stenosis. Neurol Clin. 2007;25:407-418. doi: 10.1016/j.ncl.2007.01.003
16. Conrad BP, Shokat MS, Abbasi AZ, et al. Associations of self-report measures with gait, range of motion and proprioception in patients with lumbar spinal stenosis. Gait Posture. 2013;38:987-992. doi: 10.1016/j.gaitpost.2013.05.010
17. de Luna RA, Mihailovic A, Nguyen AM, et al. The association of glaucomatous visual field loss and balance. Transl Vis Sci Technol. 2017;6:8. doi: 10.1167/tvst.6.3.8
18. DiSogra RM. Common aminoglycosides and platinum-based ototoxic drugs: cochlear/vestibular side effects and incidence. Semin Hear. 2019;40:104-107. doi: 10.1055/s-0039-1684040
19. Ebersbach G, Moreau C, Gandor F, et al. Clinical syndromes: parkinsonian gait. Mov Disord. 2013;28:1552-1559. doi: 10.1002/mds.25675
20. Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S-1127S. doi: 10.3945/ajcn.2010.28608A
21. Filli L, Sutter T, Easthope CS, et al. Profiling walking dysfunction in multiple sclerosis: characterisation, classification and progression over time. Sci Rep. 2018;8:4984. doi: 10.1038/s41598-018-22676-0
22. Fritz NE, Kegelmeyer DA, Kloos AD, et al. Motor performance differentiates individuals with Lewy body dementia, Parkinson’s and Alzheimer’s disease. Gait Posture. 2016;50:1-7. doi: 10.1016/j.gaitpost.2016.08.009
23. Furman JM, Jacob RG. A clinical taxonomy of dizziness and anxiety in the otoneurological setting. J Anxiety Disord. 2001;15:9-26. doi: 10.1016/s0887-6185(00)00040-2
24. Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet Neurol. 2013;12:706-715. doi: 10.1016/S1474-4422(13)70107-8
25. Gerson LW, Jarjoura D, McCord G. Risk of imbalance in elderly people with impaired hearing or vision. Age Ageing. 1989;18:31-34. doi: 10.1093/ageing/18.1.31
26. Goudakos JK, Markou KD, Franco-Vidal V, et al. Corticosteroids in the treatment of vestibular neuritis: a systematic review and meta-analysis. Otol Neurotol. 2010;31:183-189. doi: 10.1097/MAO.0b013e3181ca843d
27. Green AD, CS, Bastian L, et al. Does this woman have osteoporosis? JAMA. 2004;292:2890-2900. doi: 10.1001/jama.292.23.2890
28. Hallemans A, Ortibus E, Meire F, et al. Low vision affects dynamic stability of gait. Gait Posture. 2010;32:547-551. doi: 10.1016/j.gaitpost.2010.07.018
29. Handelsman JA. Vestibulotoxicity: strategies for clinical diagnosis and rehabilitation. Int J Audiol. 2018;57(suppl 4):S99-S107. doi: 10.1080/14992027.2018.1468092
30. Head VA, Wakerley BR. Guillain-Barré syndrome in general practice: clinical features suggestive of early diagnosis. Br J Gen Pract. 2016;66:218-219. doi: 10.3399/bjgp16X684733
31. Helbostad JL, Vereijken B, Hesseberg K, et al. Altered vision destabilizes gait in older persons. Gait Posture. 2009;30:233-238. doi: 10.1016/j.gaitpost.2009.05.004
32. Hsu W-L, Chen C-Y, Tsauo J-Y, et al. Balance control in elderly people with osteoporosis. J Formos Med Assoc. 2014;113:334-339. doi: 10.1016/j.jfma.2014.02.006
33. Kim H-S, Yun DH, Yoo SD, et al. Balance control and knee osteoarthritis severity. Ann Rehabil Med. 2011;35:701-709. doi: 10.5535/arm.2011.35.5.701
34. Li L, Simonsick EM, Ferrucci L, et al. Hearing loss and gait speed among older adults in the United States. Gait Posture. 2013;38:25-29.
35. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88-100. doi: 10.1212/WNL.0000000000004058
36. Milner KA, Funk M, Richards S, et al. Gender differences in symptom presentation associated with coronary heart disease. Am J Cardiol. 1999;84:396-399. doi: 10.1016/s0002-9149(99)00322-7
37. Paillard T, F, Bru N, et al. The impact of time of day on the gait and balance control of Alzheimer’s patients. Chronobiol Int. 2016;33:161-168. doi: 10.3109/07420528.2015.1124885
38. Paldor I, Chen AS, Kaye AH. Growth rate of vestibular schwannoma. J Clin Neurosci. 2016;32:1-8. doi: 10.1016/j.jocn.2016.05.003
39. Picorelli AMA, Hatton AL, Gane EM, et al. Balance performance in older adults with hip osteoarthritis: a systematic review. Gait Posture. 2018;65:89-99. doi: 10.1016/j.gaitpost.2018.07.001
40. Raccagni C, Nonnekes J, Bloem BR, et al. Gait and postural disorders in parkinsonism: a clinical approach. J Neurol. 2020;267:3169-3176. doi: 10.1007/s00415-019-09382-1
41. Shanmugarajah PD, Hoggard N, Currie S, et al. Alcohol-related cerebellar degeneration: not all down to toxicity? Cerebellum Ataxias. 2016;3:17. doi: 10.1186/s40673-016-0055-1
42. Shih RY, Smirniotopoulos JG. Posterior fossa tumors in adult patients. Neuroimaging Clin N Am. 2016;26:493-510. doi: 10.1016/j.nic.2016.06.003
43. Smith EE. Clinical presentations and epidemiology of vascular dementia. Clin Sci (Lond). 2017;131:1059-1068. doi: 10.1042/CS20160607
44. Streur M, Ratcliffe SJ, Ball J, et al. Symptom clusters in adults with chronic atrial fibrillation. J Cardiovasc Nurs. 2017;32:296-303. doi: 10.1097/JCN.0000000000000344
45. Strupp M, M, JA. Peripheral vestibular disorders: an update. Curr Opin Neurol. 2019;32:165-173. doi: 10.1097/WCO.0000000000000649
46. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
47. Timar B, Timar R, L, et al. The impact of diabetic neuropathy on balance and on the risk of falls in patients with type 2 diabetes mellitus: a cross-sectional study. PLoS One. 2016;11:e0154654. doi: 10.1371/journal.pone.0154654
48. Walls R, Hockberger R, Gausche-Hill M. Peripheral nerve disorders. In: Rosen’s Emergency Medicine: Concepts and Clinical Practice. 9th ed. Elsevier, Inc; 2018:1307-1320.
49. Watson JC, Dyck PJB. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc. 2015;90:940-951. doi: 10.1016/j.mayocp.2015.05.004
50. Whitfield KC, Bourassa MW, Adamolekun B, et al. Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann N Y Acad Sci. 2018;1430:3-43. doi: 10.1111/nyas.13919
51. Wu V, Sykes EA, Beyea MM, et al. Approach to Meniere disease management. Can Fam Physician. 2019;65:463-467.
52. Yew KS, Cheng EM. Diagnosis of acute stroke. Am Fam Physician. 2015;91:528-536.
53. Seppala LJ, van de Glind EMM, Daams JG, et al; . Fall-risk-increasing drugs: a systematic review and meta-analysis: III. Others. J Am Med Dir Assoc. 2018;19:372.e1-372.e8. doi: 10.1016/j.jamda.2017.12.099
54. ABIM Foundation. Choosing wisely. Choosing Wisely website. 2021. Accessed November 11. 2021. www.choosingwisely.org/
55. Berlie HD, Garwood CL. Diabetes medications related to an increased risk of falls and fall-related morbidity in the elderly. Ann Pharmacother. 2010;44:712-717. doi: 10.1345/aph.1M551
56. Hartikainen S, E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci. 2007;62:1172-1181. doi: 10.1093/gerona/62.10.1172
57. Khanuja K, Joki J, Bachmann G, et al. Gait and balance in the aging population: Fall prevention using innovation and technology. Maturitas. 2018;110:51-56. doi: 10.1016/j.maturitas.2018.01.021
58. Salzman B. Gait and balance disorders in older adults. Am Fam Physician. 2010;82:61-68.
59. Zaninotto P, Huang YT, Di Gessa G, et al. Polypharmacy is a risk factor for hospital admission due to a fall: evidence from the English Longitudinal Study of Ageing. BMC Public Health. 2020;20:1804. doi: 10.1186/s12889-020-09920-x
60. Morin L, Calderon A, Welmer AK, et al. Polypharmacy and injurious falls in older adults: a nationwide nested case-control study. Clin Epidemiol. 2019;11:483-493. doi: 10.2147/CLEP.S201614
61. Dhalwani NN, Fahami R, Sathanapally H, et al. Association between polypharmacy and falls in older adults: a longitudinal study from England. BMJ Open. 2017;7:e016358. doi: 10.1136/bmjopen-2017-016358
62. Arnold AC, Raj SR. Orthostatic hypotension: a practical approach to investigation and management. Can J Cardiol. 2017;33:1725-1728. doi: 10.1016/j.cjca.2017.05.007
63. Alexander NB. Differential diagnosis of gait disorders in older adults. Clin Geriatr Med. 1996;12:689-703.
64. Baker JM. Gait disorders. Am J Med. 2018;131:602-607. doi: 10.1016/j.amjmed.2017.11.051
65. Cameron MH, Wagner JM. Gait abnormalities in multiple sclerosis: pathogenesis, evaluation, and advances in treatment. Curr Neurol Neurosci Rep. 2011;11:507-515. doi: 10.1007/s11910-011-0214-y
66. Chen C-L, Chen H-C, Tang SF-T, et al. Gait performance with compensatory adaptations in stroke patients with different degrees of motor recovery. Am J Phys Med Rehabil. 2003;82:925-935. doi: 10.1097/01.PHM.0000098040.13355.B5
67. Marsden J, Harris C. Cerebellar ataxia: pathophysiology and rehabilitation. Clin Rehabil. 2011;25:195-216. doi: 10.1177/0269215510382495
68. Mirek E, Filip M, W, et al. Three-dimensional trunk and lower limbs characteristics during gait in patients with Huntington’s disease. Front Neurosci. 2017;11:566. doi: 10.3389/fnins.2017.00566
69. Paramanandam V, Lizarraga KJ, Soh D, et al. Unusual gait disorders: a phenomenological approach and classification. Expert Rev Neurother. 2019;19:119-132. doi: 10.1080/14737175.2019.1562337
70. Sahyouni R, Goshtasbi K, Mahmoodi A, et al. Chronic subdural hematoma: a historical and clinical perspective. World Neurosurg. 2017;108:948-953. doi: 10.1016/j.wneu.2017.09.064
71. Talmud JD, Coffey R, Edemekong PF. Dix Hallpike maneuver. StatPearls [Internet]. StatPearls Publishing Updated September 5, 2021. Accessed December 6, 2021. www.ncbi.nlm.nih.gov/books/NBK459307/
72. Molnar FJ, Benjamin S, Hawkins SA, et al. One size does not fit all: choosing practical cognitive screening tools for your practice. J Am Geriatr Soc. 2020;68:2207-2213. doi: 10.1111/jgs.16713
73. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012:CD007146. doi: 10.1002/14651858.CD007146.pub3
74. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150. doi: 10.14336/AD.2017.0306
75. Poe SS, Cvach M, Dawson PB, Straus H, Hill EE. The Johns Hopkins Fall Risk Assessment Tool: postimplementation evaluation. J Nurs Care Qual. 2007;22:293-298. doi: 10.1097/01.NCQ.0000290408.74027.39
76. Poe SS, Dawson PB, Cvach M, et al. The Johns Hopkins Fall Risk Assessment Tool: a study of reliability and validity. J Nurs Care Qual. 2018;33:10-19. doi: 10.1097/NCQ.0000000000000301
77. Klinkenberg WD, Potter P. Validity of the Johns Hopkins Fall Risk Assessment Tool for predicting falls on inpatient medicine services. J Nurs Care Qual. 2017;32:108-113. doi: 10.1097/NCQ.0000000000000210
78. Stapleton C, Hough P, Oldmeadow L, et al. Four-item fall risk screening tool for subacute and residential aged care: the first step in fall prevention. Australas J Ageing. 2009;28:139-143. doi: 10.1111/j.1741-6612.2009.00375.x
79. Cattelani L, Palumbo P, Palmerini L, et al. FRAT-up, a Web-based fall-risk assessment tool for elderly people living in the community. J Med Internet Res. 2015;17:e41. doi: 10.2196/jmir.4064
80. De Clercq H, Naudé A, Bornman J. Factors included in adult fall risk assessment tools (FRATs): a systematic review. Ageing Soc. 2020;41:2558-2582. doi: 10.1017/S0144686X2000046X
81. Yap G, Melder A. Accuracy of validated falls risk assessment tools and clinical judgement. Centre for Clinical Effectiveness, Monash Innovation and Quality. Monash Health. February 5, 2020. Accessed November 11, 2021. https://monashhealth.org/wp-content/uploads/2019/01/Rapid-Review_Falls-risk-tools-FINAL.pdf
82. Chittrakul J, Siviroj P, Sungkarat S, et al. Physical frailty and fall risk in community-dwelling older adults: a cross-sectional study. J Aging Res. 2020;2020:3964973. doi: 10.1155/2020/3964973
83. Hatcher VH, Galet C, Lilienthal M, et al. Association of clinical frailty scores with hospital readmission for falls after index admission for trauma-related injury. JAMA Netw Open. 2019;2:e1912409. doi: 10.1001/jamanetworkopen.2019.12409
84. Exercise and fall prevention programs. Government of Ontario Ministry of Health. Updated April 9, 2019. Accessed November 11. 2021. www.ontario.ca/page/exercise-and-falls-prevention-programs
85. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1:CD012424. doi: 10.1002/14651858.CD012424.pub2
86. Hopewell S, Copsey B, Nicolson P, et al. Multifactorial interventions for preventing falls in older people living in the community: a systematic review and meta-analysis of 41 trials and almost 20 000 participants. Br J Sports Med. 2020;54:1340-1350. doi: 10.1136/bjsports-2019-100732
87. Jafari Z, Kolb BE, Mohajerani MH. Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev. 2019;56:100963. doi: 10.1016/j.arr.2019.100963
88. Griffiths TD, Lad M, Kumar S, et al. How can hearing loss cause dementia? Neuron. 2020;108:401-412. doi: 10.1016/j.neuron.2020.08.003
89. Martini A, Castiglione A, Bovo R, et al. Aging, cognitive load, dementia and hearing loss. Audiol Neurootol. 2014;19(suppl 1):2-5. doi: 10.1159/000371593
90. Vitkovic J, Le C, Lee S-L, et al. The contribution of hearing and hearing loss to balance control. Audiol Neurootol. 2016;21:195-202. doi: 10.1159/000445100
91. Maheu M, Behtani L, Nooristani M, et al. Vestibular function modulates the benefit of hearing aids in people with hearing loss during static postural control. Ear Hear. 2019;40:1418-1424. doi: 10.1097/AUD.0000000000000720
92. Negahban H, Bavarsad Cheshmeh Ali M, Nassadj G. Effect of hearing aids on static balance function in elderly with hearing loss. Gait Posture. 2017;58:126-129. doi: 10.1016/j.gaitpost.2017.07.112
93. Mahmoudi E, Basu T, Langa K, et al. Can hearing aids delay time to diagnosis of dementia, depression, or falls in older adults? J Am Geriatr Soc. 2019;67:2362-2369. doi: 10.1111/jgs.16109
94. Paliwal Y, Slattum PW, Ratliff SM. Chronic health conditions as a risk factor for falls among the community-dwelling US older adults: a zero-inflated regression modeling approach. Biomed Res Int. 2017;2017:5146378. doi: 10.1155/2017/5146378
95. Deandrea S, Lucenteforte E, Bravi F, et al. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010;21:658-668. doi: 10.1097/EDE.0b013e3181e89905
96. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51-61. doi: 10.1016/j.maturitas.2013.02.009
97. Stevens M, Holman CD, Bennett N. Preventing falls in older people: impact of an intervention to reduce environmental hazards in the home. J Am Geriatr Soc. 2001;49:1442-1447. doi: 10.1046/j.1532-5415.2001.4911235.x
98. Clinical resources. Centers for Disease Control and Prevention STEADI-Older Adult Fall Prevention website. 2020. Accessed November 12, 2021. www.cdc.gov/steadi/materials.html
99. ; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1696-1704. doi: 10.1001/jama.2018.3097
1. Larocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient. 2011;4:189-201. doi: 10.2165/11591150-000000000-00000
2. TB, ZF, ES, et al. The relationship of balance disorders with falling, the effect of health problems, and social life on postural balance in the elderly living in a district in Turkey. Geriatrics (Basel). 2019;4:37. doi: 10.3390/geriatrics4020037
3. R, Sixt E, Landahl S, et al. Prevalence of dizziness and vertigo in an urban elderly population. J Vestib Res. 2004;14:47-52.
4. Sturnieks DL, St George R, Lord SR. Balance disorders in the elderly. Neurophysiol Clin. 2008;38:467-478. doi: 10.1016/j.neucli.2008.09.001
5. Boult C, Murphy J, Sloane P, et al. The relation of dizziness to functional decline. J Am Geriatr Soc. 1991;39:858-861. doi: 10.1111/j.1532-5415.1991.tb04451.x
6. Lin HW, Bhattacharyya N. Balance disorders in the elderly: epidemiology and functional impact. Laryngoscope. 2012;122:1858-1861. doi: 10.1002/lary.23376
7. Jia H, Lubetkin EI, DeMichele K, et al. Prevalence, risk factors, and burden of disease for falls and balance or walking problems among older adults in the U.S. Prev Med. 2019;126:105737. doi: 10.1016/j.ypmed.2019.05.025
8. Al-Momani M, Al-Momani F, Alghadir AH, et al. Factors related to gait and balance deficits in older adults. Clin Interv Aging. 2016;11:1043-1049. doi: 10.2147/CIA.S112282
9. Agrawal Y, Ward BK, Minor LB. Vestibular dysfunction: prevalence, impact and need for targeted treatment. J Vestib Res. 2013;23:113-117. doi: 10.3233/VES-130498
10. Altinsoy B, Erboy F, Tanriverdi H, et al. Syncope as a presentation of acute pulmonary embolism. Ther Clin Risk Manag. 2016;12:1023-1028. doi: 10.2147/TCRM.S105722
11. Belvederi Murri M, Triolo F, Coni A, et al. Instrumental assessment of balance and gait in depression: a systematic review. Psychiatry Res. 2020;284:112687. doi: 10.1016/j.psychres.2019.112687
12. Bhattacharyya N, Gubbels SP, Schwartz SR, et al. Clinical practice guideline: benign paroxysmal positional vertigo (update). Otolaryngol Head Neck Surg. 2017;156(suppl 3):S1-S47. doi: 10.1177/0194599816689667
13. S, Schwarm S, Grevenrath P, et al. Prevalence, aetiologies and prognosis of the symptom dizziness in primary care - a systematic review. BMC Fam Pract. 2018;19:33. doi: 10.1186/s12875-017-0695-0
14. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23:467-492. doi: 10.1128/CMR.00070-09
15. Chad DA. Lumbar spinal stenosis. Neurol Clin. 2007;25:407-418. doi: 10.1016/j.ncl.2007.01.003
16. Conrad BP, Shokat MS, Abbasi AZ, et al. Associations of self-report measures with gait, range of motion and proprioception in patients with lumbar spinal stenosis. Gait Posture. 2013;38:987-992. doi: 10.1016/j.gaitpost.2013.05.010
17. de Luna RA, Mihailovic A, Nguyen AM, et al. The association of glaucomatous visual field loss and balance. Transl Vis Sci Technol. 2017;6:8. doi: 10.1167/tvst.6.3.8
18. DiSogra RM. Common aminoglycosides and platinum-based ototoxic drugs: cochlear/vestibular side effects and incidence. Semin Hear. 2019;40:104-107. doi: 10.1055/s-0039-1684040
19. Ebersbach G, Moreau C, Gandor F, et al. Clinical syndromes: parkinsonian gait. Mov Disord. 2013;28:1552-1559. doi: 10.1002/mds.25675
20. Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S-1127S. doi: 10.3945/ajcn.2010.28608A
21. Filli L, Sutter T, Easthope CS, et al. Profiling walking dysfunction in multiple sclerosis: characterisation, classification and progression over time. Sci Rep. 2018;8:4984. doi: 10.1038/s41598-018-22676-0
22. Fritz NE, Kegelmeyer DA, Kloos AD, et al. Motor performance differentiates individuals with Lewy body dementia, Parkinson’s and Alzheimer’s disease. Gait Posture. 2016;50:1-7. doi: 10.1016/j.gaitpost.2016.08.009
23. Furman JM, Jacob RG. A clinical taxonomy of dizziness and anxiety in the otoneurological setting. J Anxiety Disord. 2001;15:9-26. doi: 10.1016/s0887-6185(00)00040-2
24. Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet Neurol. 2013;12:706-715. doi: 10.1016/S1474-4422(13)70107-8
25. Gerson LW, Jarjoura D, McCord G. Risk of imbalance in elderly people with impaired hearing or vision. Age Ageing. 1989;18:31-34. doi: 10.1093/ageing/18.1.31
26. Goudakos JK, Markou KD, Franco-Vidal V, et al. Corticosteroids in the treatment of vestibular neuritis: a systematic review and meta-analysis. Otol Neurotol. 2010;31:183-189. doi: 10.1097/MAO.0b013e3181ca843d
27. Green AD, CS, Bastian L, et al. Does this woman have osteoporosis? JAMA. 2004;292:2890-2900. doi: 10.1001/jama.292.23.2890
28. Hallemans A, Ortibus E, Meire F, et al. Low vision affects dynamic stability of gait. Gait Posture. 2010;32:547-551. doi: 10.1016/j.gaitpost.2010.07.018
29. Handelsman JA. Vestibulotoxicity: strategies for clinical diagnosis and rehabilitation. Int J Audiol. 2018;57(suppl 4):S99-S107. doi: 10.1080/14992027.2018.1468092
30. Head VA, Wakerley BR. Guillain-Barré syndrome in general practice: clinical features suggestive of early diagnosis. Br J Gen Pract. 2016;66:218-219. doi: 10.3399/bjgp16X684733
31. Helbostad JL, Vereijken B, Hesseberg K, et al. Altered vision destabilizes gait in older persons. Gait Posture. 2009;30:233-238. doi: 10.1016/j.gaitpost.2009.05.004
32. Hsu W-L, Chen C-Y, Tsauo J-Y, et al. Balance control in elderly people with osteoporosis. J Formos Med Assoc. 2014;113:334-339. doi: 10.1016/j.jfma.2014.02.006
33. Kim H-S, Yun DH, Yoo SD, et al. Balance control and knee osteoarthritis severity. Ann Rehabil Med. 2011;35:701-709. doi: 10.5535/arm.2011.35.5.701
34. Li L, Simonsick EM, Ferrucci L, et al. Hearing loss and gait speed among older adults in the United States. Gait Posture. 2013;38:25-29.
35. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88-100. doi: 10.1212/WNL.0000000000004058
36. Milner KA, Funk M, Richards S, et al. Gender differences in symptom presentation associated with coronary heart disease. Am J Cardiol. 1999;84:396-399. doi: 10.1016/s0002-9149(99)00322-7
37. Paillard T, F, Bru N, et al. The impact of time of day on the gait and balance control of Alzheimer’s patients. Chronobiol Int. 2016;33:161-168. doi: 10.3109/07420528.2015.1124885
38. Paldor I, Chen AS, Kaye AH. Growth rate of vestibular schwannoma. J Clin Neurosci. 2016;32:1-8. doi: 10.1016/j.jocn.2016.05.003
39. Picorelli AMA, Hatton AL, Gane EM, et al. Balance performance in older adults with hip osteoarthritis: a systematic review. Gait Posture. 2018;65:89-99. doi: 10.1016/j.gaitpost.2018.07.001
40. Raccagni C, Nonnekes J, Bloem BR, et al. Gait and postural disorders in parkinsonism: a clinical approach. J Neurol. 2020;267:3169-3176. doi: 10.1007/s00415-019-09382-1
41. Shanmugarajah PD, Hoggard N, Currie S, et al. Alcohol-related cerebellar degeneration: not all down to toxicity? Cerebellum Ataxias. 2016;3:17. doi: 10.1186/s40673-016-0055-1
42. Shih RY, Smirniotopoulos JG. Posterior fossa tumors in adult patients. Neuroimaging Clin N Am. 2016;26:493-510. doi: 10.1016/j.nic.2016.06.003
43. Smith EE. Clinical presentations and epidemiology of vascular dementia. Clin Sci (Lond). 2017;131:1059-1068. doi: 10.1042/CS20160607
44. Streur M, Ratcliffe SJ, Ball J, et al. Symptom clusters in adults with chronic atrial fibrillation. J Cardiovasc Nurs. 2017;32:296-303. doi: 10.1097/JCN.0000000000000344
45. Strupp M, M, JA. Peripheral vestibular disorders: an update. Curr Opin Neurol. 2019;32:165-173. doi: 10.1097/WCO.0000000000000649
46. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
47. Timar B, Timar R, L, et al. The impact of diabetic neuropathy on balance and on the risk of falls in patients with type 2 diabetes mellitus: a cross-sectional study. PLoS One. 2016;11:e0154654. doi: 10.1371/journal.pone.0154654
48. Walls R, Hockberger R, Gausche-Hill M. Peripheral nerve disorders. In: Rosen’s Emergency Medicine: Concepts and Clinical Practice. 9th ed. Elsevier, Inc; 2018:1307-1320.
49. Watson JC, Dyck PJB. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc. 2015;90:940-951. doi: 10.1016/j.mayocp.2015.05.004
50. Whitfield KC, Bourassa MW, Adamolekun B, et al. Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann N Y Acad Sci. 2018;1430:3-43. doi: 10.1111/nyas.13919
51. Wu V, Sykes EA, Beyea MM, et al. Approach to Meniere disease management. Can Fam Physician. 2019;65:463-467.
52. Yew KS, Cheng EM. Diagnosis of acute stroke. Am Fam Physician. 2015;91:528-536.
53. Seppala LJ, van de Glind EMM, Daams JG, et al; . Fall-risk-increasing drugs: a systematic review and meta-analysis: III. Others. J Am Med Dir Assoc. 2018;19:372.e1-372.e8. doi: 10.1016/j.jamda.2017.12.099
54. ABIM Foundation. Choosing wisely. Choosing Wisely website. 2021. Accessed November 11. 2021. www.choosingwisely.org/
55. Berlie HD, Garwood CL. Diabetes medications related to an increased risk of falls and fall-related morbidity in the elderly. Ann Pharmacother. 2010;44:712-717. doi: 10.1345/aph.1M551
56. Hartikainen S, E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci. 2007;62:1172-1181. doi: 10.1093/gerona/62.10.1172
57. Khanuja K, Joki J, Bachmann G, et al. Gait and balance in the aging population: Fall prevention using innovation and technology. Maturitas. 2018;110:51-56. doi: 10.1016/j.maturitas.2018.01.021
58. Salzman B. Gait and balance disorders in older adults. Am Fam Physician. 2010;82:61-68.
59. Zaninotto P, Huang YT, Di Gessa G, et al. Polypharmacy is a risk factor for hospital admission due to a fall: evidence from the English Longitudinal Study of Ageing. BMC Public Health. 2020;20:1804. doi: 10.1186/s12889-020-09920-x
60. Morin L, Calderon A, Welmer AK, et al. Polypharmacy and injurious falls in older adults: a nationwide nested case-control study. Clin Epidemiol. 2019;11:483-493. doi: 10.2147/CLEP.S201614
61. Dhalwani NN, Fahami R, Sathanapally H, et al. Association between polypharmacy and falls in older adults: a longitudinal study from England. BMJ Open. 2017;7:e016358. doi: 10.1136/bmjopen-2017-016358
62. Arnold AC, Raj SR. Orthostatic hypotension: a practical approach to investigation and management. Can J Cardiol. 2017;33:1725-1728. doi: 10.1016/j.cjca.2017.05.007
63. Alexander NB. Differential diagnosis of gait disorders in older adults. Clin Geriatr Med. 1996;12:689-703.
64. Baker JM. Gait disorders. Am J Med. 2018;131:602-607. doi: 10.1016/j.amjmed.2017.11.051
65. Cameron MH, Wagner JM. Gait abnormalities in multiple sclerosis: pathogenesis, evaluation, and advances in treatment. Curr Neurol Neurosci Rep. 2011;11:507-515. doi: 10.1007/s11910-011-0214-y
66. Chen C-L, Chen H-C, Tang SF-T, et al. Gait performance with compensatory adaptations in stroke patients with different degrees of motor recovery. Am J Phys Med Rehabil. 2003;82:925-935. doi: 10.1097/01.PHM.0000098040.13355.B5
67. Marsden J, Harris C. Cerebellar ataxia: pathophysiology and rehabilitation. Clin Rehabil. 2011;25:195-216. doi: 10.1177/0269215510382495
68. Mirek E, Filip M, W, et al. Three-dimensional trunk and lower limbs characteristics during gait in patients with Huntington’s disease. Front Neurosci. 2017;11:566. doi: 10.3389/fnins.2017.00566
69. Paramanandam V, Lizarraga KJ, Soh D, et al. Unusual gait disorders: a phenomenological approach and classification. Expert Rev Neurother. 2019;19:119-132. doi: 10.1080/14737175.2019.1562337
70. Sahyouni R, Goshtasbi K, Mahmoodi A, et al. Chronic subdural hematoma: a historical and clinical perspective. World Neurosurg. 2017;108:948-953. doi: 10.1016/j.wneu.2017.09.064
71. Talmud JD, Coffey R, Edemekong PF. Dix Hallpike maneuver. StatPearls [Internet]. StatPearls Publishing Updated September 5, 2021. Accessed December 6, 2021. www.ncbi.nlm.nih.gov/books/NBK459307/
72. Molnar FJ, Benjamin S, Hawkins SA, et al. One size does not fit all: choosing practical cognitive screening tools for your practice. J Am Geriatr Soc. 2020;68:2207-2213. doi: 10.1111/jgs.16713
73. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012:CD007146. doi: 10.1002/14651858.CD007146.pub3
74. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150. doi: 10.14336/AD.2017.0306
75. Poe SS, Cvach M, Dawson PB, Straus H, Hill EE. The Johns Hopkins Fall Risk Assessment Tool: postimplementation evaluation. J Nurs Care Qual. 2007;22:293-298. doi: 10.1097/01.NCQ.0000290408.74027.39
76. Poe SS, Dawson PB, Cvach M, et al. The Johns Hopkins Fall Risk Assessment Tool: a study of reliability and validity. J Nurs Care Qual. 2018;33:10-19. doi: 10.1097/NCQ.0000000000000301
77. Klinkenberg WD, Potter P. Validity of the Johns Hopkins Fall Risk Assessment Tool for predicting falls on inpatient medicine services. J Nurs Care Qual. 2017;32:108-113. doi: 10.1097/NCQ.0000000000000210
78. Stapleton C, Hough P, Oldmeadow L, et al. Four-item fall risk screening tool for subacute and residential aged care: the first step in fall prevention. Australas J Ageing. 2009;28:139-143. doi: 10.1111/j.1741-6612.2009.00375.x
79. Cattelani L, Palumbo P, Palmerini L, et al. FRAT-up, a Web-based fall-risk assessment tool for elderly people living in the community. J Med Internet Res. 2015;17:e41. doi: 10.2196/jmir.4064
80. De Clercq H, Naudé A, Bornman J. Factors included in adult fall risk assessment tools (FRATs): a systematic review. Ageing Soc. 2020;41:2558-2582. doi: 10.1017/S0144686X2000046X
81. Yap G, Melder A. Accuracy of validated falls risk assessment tools and clinical judgement. Centre for Clinical Effectiveness, Monash Innovation and Quality. Monash Health. February 5, 2020. Accessed November 11, 2021. https://monashhealth.org/wp-content/uploads/2019/01/Rapid-Review_Falls-risk-tools-FINAL.pdf
82. Chittrakul J, Siviroj P, Sungkarat S, et al. Physical frailty and fall risk in community-dwelling older adults: a cross-sectional study. J Aging Res. 2020;2020:3964973. doi: 10.1155/2020/3964973
83. Hatcher VH, Galet C, Lilienthal M, et al. Association of clinical frailty scores with hospital readmission for falls after index admission for trauma-related injury. JAMA Netw Open. 2019;2:e1912409. doi: 10.1001/jamanetworkopen.2019.12409
84. Exercise and fall prevention programs. Government of Ontario Ministry of Health. Updated April 9, 2019. Accessed November 11. 2021. www.ontario.ca/page/exercise-and-falls-prevention-programs
85. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1:CD012424. doi: 10.1002/14651858.CD012424.pub2
86. Hopewell S, Copsey B, Nicolson P, et al. Multifactorial interventions for preventing falls in older people living in the community: a systematic review and meta-analysis of 41 trials and almost 20 000 participants. Br J Sports Med. 2020;54:1340-1350. doi: 10.1136/bjsports-2019-100732
87. Jafari Z, Kolb BE, Mohajerani MH. Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev. 2019;56:100963. doi: 10.1016/j.arr.2019.100963
88. Griffiths TD, Lad M, Kumar S, et al. How can hearing loss cause dementia? Neuron. 2020;108:401-412. doi: 10.1016/j.neuron.2020.08.003
89. Martini A, Castiglione A, Bovo R, et al. Aging, cognitive load, dementia and hearing loss. Audiol Neurootol. 2014;19(suppl 1):2-5. doi: 10.1159/000371593
90. Vitkovic J, Le C, Lee S-L, et al. The contribution of hearing and hearing loss to balance control. Audiol Neurootol. 2016;21:195-202. doi: 10.1159/000445100
91. Maheu M, Behtani L, Nooristani M, et al. Vestibular function modulates the benefit of hearing aids in people with hearing loss during static postural control. Ear Hear. 2019;40:1418-1424. doi: 10.1097/AUD.0000000000000720
92. Negahban H, Bavarsad Cheshmeh Ali M, Nassadj G. Effect of hearing aids on static balance function in elderly with hearing loss. Gait Posture. 2017;58:126-129. doi: 10.1016/j.gaitpost.2017.07.112
93. Mahmoudi E, Basu T, Langa K, et al. Can hearing aids delay time to diagnosis of dementia, depression, or falls in older adults? J Am Geriatr Soc. 2019;67:2362-2369. doi: 10.1111/jgs.16109
94. Paliwal Y, Slattum PW, Ratliff SM. Chronic health conditions as a risk factor for falls among the community-dwelling US older adults: a zero-inflated regression modeling approach. Biomed Res Int. 2017;2017:5146378. doi: 10.1155/2017/5146378
95. Deandrea S, Lucenteforte E, Bravi F, et al. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010;21:658-668. doi: 10.1097/EDE.0b013e3181e89905
96. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51-61. doi: 10.1016/j.maturitas.2013.02.009
97. Stevens M, Holman CD, Bennett N. Preventing falls in older people: impact of an intervention to reduce environmental hazards in the home. J Am Geriatr Soc. 2001;49:1442-1447. doi: 10.1046/j.1532-5415.2001.4911235.x
98. Clinical resources. Centers for Disease Control and Prevention STEADI-Older Adult Fall Prevention website. 2020. Accessed November 12, 2021. www.cdc.gov/steadi/materials.html
99. ; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1696-1704. doi: 10.1001/jama.2018.3097
PRACTICE RECOMMENDATIONS
› Utilize a falls-prevention program for older patients that focuses on balance and functional exercises. A
› Perform a multifactorial assessment of the risk of falls in older patients that includes optimizing medications, managing comorbidities, and addressing environmental hazards. B
› Use a systems-based approach to presentations of imbalance to direct your clinical judgment and highlight the need for referral to specialists for management and rehabilitation. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
How safe is a drug holiday from bisphosphonates for osteoporosis?
Researchers found a small but greater risk of a hip fracture after 2 years of taking a “drug holiday” – stopping therapy – after long-term (≥3-year) use of one bisphosphonate, risedronate, versus another, alendronate.
The risk of a hip fracture after stopping either of these oral bisphosphonate osteoporosis drugs was similar until 2 years, suggesting that patients who take a drug holiday from risedronate should be revaluated before 2 years.
These top-line findings from a propensity-matched cohort study of older patients in Ontario, Canada, were reported at the annual American Society of Bone and Mineral Research (ASBMR) last fall.
The full study, led by Kaleen N. Hayes, PharmD, PhD, Brown University School of Public Health, Providence, R.I., was published online in the Annals of Internal Medicine.
“We emphasize that our results do not indicate that alendronate therapy should be preferred over risedronate therapy,” the researchers stress, as several real-world studies found a similar risk of fractures while patients were receiving either drug.
“The decision to initiate alendronate or risedronate therapy [the two most commonly prescribed bisphosphonates] is driven by the prescriber,” they note, adding that some patients may prefer risedronate because it is available as a monthly dose or a weekly delayed-release formula that does not require fasting.
“We found little difference in the association between risedronate versus alendronate drug holidays and hip fractures until approximately 2 years of not receiving therapy,” Dr. Hayes and colleagues summarize.
Over 3 years, risedronate drug holidays were associated with an 18% relative and 0.6% absolute increased risk for hip fracture compared with alendronate drug holidays.
“To further inform clinical decision-making on drug holidays,” they conclude, “future research should examine when to start and restart osteoporosis therapy on the basis of initial length and type of treatment, patient characteristics, and relative risk for hip fractures versus [atypical femoral fracture].”
Hip fracture risk with risedronate vs. alendronate drug holiday
Long-term bisphosphonate use is associated with a rare risk of osteonecrosis of the jaw or atypical femoral fractures. At the same time, bisphosphonates continue to have a therapeutic effect after therapy is discontinued.
Guidelines recommend that patients at low risk of fracture should therefore have a “drug holiday” after 3 to 5 years of bisphosphonate use and be reassessed 2 to 3 years later, largely based on the Fracture Intervention Trial Long-Term Extension (FLEX) study of alendronate. But risedronate has a shorter half-life, so it may provide shorter residual fracture protection.
Using Ontario administrative data, Dr. Hayes and associates identified more than 60,000 patients who were over aged 65, had received at least 3 years of continuous alendronate or risedronate, and had a subsequent 3-year drug holiday between 2000 and 2020.
They excluded patients who had a fracture or entered a nursing home within 120 days of starting a drug holiday who may have stopped the bisphosphonate due to declining health rather than a drug holiday.
Roughly half (55%) had been taking risedronate and 45% had been taking alendronate.
Using propensity scores, the researchers matched 25,077 patients who had been taking risedronate with an equal number who had been taking alendronate.
Most of the patients were women (82%) and were White.
They started the drug holiday when they were on average 81 years old, after taking the bisphosphonate for 5.9 years on average.
During the 3-year drug holiday, 915 of the 50,154 patients had hip fractures.
This was equivalent to 12.4 hip fractures per 1,000 patients per year during a risedronate holiday and 10.6 hip fractures per 1,000 patients per year during an alendronate holiday (hazard ratio, 1.18).
The risk of hip fracture was not significantly higher at 1 year (HR, 1.03) or at 2 years of a risedronate holiday versus an alendronate holiday (HR, 1.14).
However, the risk of a hip fracture was significantly higher at 2 to 3 years of a risedronate holiday than after an alendronate holiday (HR, 1.34).
There was no significant difference in the risk of any osteoporotic fracture overall (including hip, vertebrae, pelvis, ribs, forearm), however, during a 3-year risedronate versus alendronate drug holiday (HR, 1.07).
The research was supported by the Canadian Institutes of Health Research and Institute for Clinical Evaluative Sciences. Dr. Hayes was supported by a CIHR doctoral research award. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found a small but greater risk of a hip fracture after 2 years of taking a “drug holiday” – stopping therapy – after long-term (≥3-year) use of one bisphosphonate, risedronate, versus another, alendronate.
The risk of a hip fracture after stopping either of these oral bisphosphonate osteoporosis drugs was similar until 2 years, suggesting that patients who take a drug holiday from risedronate should be revaluated before 2 years.
These top-line findings from a propensity-matched cohort study of older patients in Ontario, Canada, were reported at the annual American Society of Bone and Mineral Research (ASBMR) last fall.
The full study, led by Kaleen N. Hayes, PharmD, PhD, Brown University School of Public Health, Providence, R.I., was published online in the Annals of Internal Medicine.
“We emphasize that our results do not indicate that alendronate therapy should be preferred over risedronate therapy,” the researchers stress, as several real-world studies found a similar risk of fractures while patients were receiving either drug.
“The decision to initiate alendronate or risedronate therapy [the two most commonly prescribed bisphosphonates] is driven by the prescriber,” they note, adding that some patients may prefer risedronate because it is available as a monthly dose or a weekly delayed-release formula that does not require fasting.
“We found little difference in the association between risedronate versus alendronate drug holidays and hip fractures until approximately 2 years of not receiving therapy,” Dr. Hayes and colleagues summarize.
Over 3 years, risedronate drug holidays were associated with an 18% relative and 0.6% absolute increased risk for hip fracture compared with alendronate drug holidays.
“To further inform clinical decision-making on drug holidays,” they conclude, “future research should examine when to start and restart osteoporosis therapy on the basis of initial length and type of treatment, patient characteristics, and relative risk for hip fractures versus [atypical femoral fracture].”
Hip fracture risk with risedronate vs. alendronate drug holiday
Long-term bisphosphonate use is associated with a rare risk of osteonecrosis of the jaw or atypical femoral fractures. At the same time, bisphosphonates continue to have a therapeutic effect after therapy is discontinued.
Guidelines recommend that patients at low risk of fracture should therefore have a “drug holiday” after 3 to 5 years of bisphosphonate use and be reassessed 2 to 3 years later, largely based on the Fracture Intervention Trial Long-Term Extension (FLEX) study of alendronate. But risedronate has a shorter half-life, so it may provide shorter residual fracture protection.
Using Ontario administrative data, Dr. Hayes and associates identified more than 60,000 patients who were over aged 65, had received at least 3 years of continuous alendronate or risedronate, and had a subsequent 3-year drug holiday between 2000 and 2020.
They excluded patients who had a fracture or entered a nursing home within 120 days of starting a drug holiday who may have stopped the bisphosphonate due to declining health rather than a drug holiday.
Roughly half (55%) had been taking risedronate and 45% had been taking alendronate.
Using propensity scores, the researchers matched 25,077 patients who had been taking risedronate with an equal number who had been taking alendronate.
Most of the patients were women (82%) and were White.
They started the drug holiday when they were on average 81 years old, after taking the bisphosphonate for 5.9 years on average.
During the 3-year drug holiday, 915 of the 50,154 patients had hip fractures.
This was equivalent to 12.4 hip fractures per 1,000 patients per year during a risedronate holiday and 10.6 hip fractures per 1,000 patients per year during an alendronate holiday (hazard ratio, 1.18).
The risk of hip fracture was not significantly higher at 1 year (HR, 1.03) or at 2 years of a risedronate holiday versus an alendronate holiday (HR, 1.14).
However, the risk of a hip fracture was significantly higher at 2 to 3 years of a risedronate holiday than after an alendronate holiday (HR, 1.34).
There was no significant difference in the risk of any osteoporotic fracture overall (including hip, vertebrae, pelvis, ribs, forearm), however, during a 3-year risedronate versus alendronate drug holiday (HR, 1.07).
The research was supported by the Canadian Institutes of Health Research and Institute for Clinical Evaluative Sciences. Dr. Hayes was supported by a CIHR doctoral research award. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found a small but greater risk of a hip fracture after 2 years of taking a “drug holiday” – stopping therapy – after long-term (≥3-year) use of one bisphosphonate, risedronate, versus another, alendronate.
The risk of a hip fracture after stopping either of these oral bisphosphonate osteoporosis drugs was similar until 2 years, suggesting that patients who take a drug holiday from risedronate should be revaluated before 2 years.
These top-line findings from a propensity-matched cohort study of older patients in Ontario, Canada, were reported at the annual American Society of Bone and Mineral Research (ASBMR) last fall.
The full study, led by Kaleen N. Hayes, PharmD, PhD, Brown University School of Public Health, Providence, R.I., was published online in the Annals of Internal Medicine.
“We emphasize that our results do not indicate that alendronate therapy should be preferred over risedronate therapy,” the researchers stress, as several real-world studies found a similar risk of fractures while patients were receiving either drug.
“The decision to initiate alendronate or risedronate therapy [the two most commonly prescribed bisphosphonates] is driven by the prescriber,” they note, adding that some patients may prefer risedronate because it is available as a monthly dose or a weekly delayed-release formula that does not require fasting.
“We found little difference in the association between risedronate versus alendronate drug holidays and hip fractures until approximately 2 years of not receiving therapy,” Dr. Hayes and colleagues summarize.
Over 3 years, risedronate drug holidays were associated with an 18% relative and 0.6% absolute increased risk for hip fracture compared with alendronate drug holidays.
“To further inform clinical decision-making on drug holidays,” they conclude, “future research should examine when to start and restart osteoporosis therapy on the basis of initial length and type of treatment, patient characteristics, and relative risk for hip fractures versus [atypical femoral fracture].”
Hip fracture risk with risedronate vs. alendronate drug holiday
Long-term bisphosphonate use is associated with a rare risk of osteonecrosis of the jaw or atypical femoral fractures. At the same time, bisphosphonates continue to have a therapeutic effect after therapy is discontinued.
Guidelines recommend that patients at low risk of fracture should therefore have a “drug holiday” after 3 to 5 years of bisphosphonate use and be reassessed 2 to 3 years later, largely based on the Fracture Intervention Trial Long-Term Extension (FLEX) study of alendronate. But risedronate has a shorter half-life, so it may provide shorter residual fracture protection.
Using Ontario administrative data, Dr. Hayes and associates identified more than 60,000 patients who were over aged 65, had received at least 3 years of continuous alendronate or risedronate, and had a subsequent 3-year drug holiday between 2000 and 2020.
They excluded patients who had a fracture or entered a nursing home within 120 days of starting a drug holiday who may have stopped the bisphosphonate due to declining health rather than a drug holiday.
Roughly half (55%) had been taking risedronate and 45% had been taking alendronate.
Using propensity scores, the researchers matched 25,077 patients who had been taking risedronate with an equal number who had been taking alendronate.
Most of the patients were women (82%) and were White.
They started the drug holiday when they were on average 81 years old, after taking the bisphosphonate for 5.9 years on average.
During the 3-year drug holiday, 915 of the 50,154 patients had hip fractures.
This was equivalent to 12.4 hip fractures per 1,000 patients per year during a risedronate holiday and 10.6 hip fractures per 1,000 patients per year during an alendronate holiday (hazard ratio, 1.18).
The risk of hip fracture was not significantly higher at 1 year (HR, 1.03) or at 2 years of a risedronate holiday versus an alendronate holiday (HR, 1.14).
However, the risk of a hip fracture was significantly higher at 2 to 3 years of a risedronate holiday than after an alendronate holiday (HR, 1.34).
There was no significant difference in the risk of any osteoporotic fracture overall (including hip, vertebrae, pelvis, ribs, forearm), however, during a 3-year risedronate versus alendronate drug holiday (HR, 1.07).
The research was supported by the Canadian Institutes of Health Research and Institute for Clinical Evaluative Sciences. Dr. Hayes was supported by a CIHR doctoral research award. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Health issues in women midlife linked with health decline at 65
Having specific health issues, including depressive symptoms and cardiovascular disease, as a middle-aged woman was associated with experiencing clinically important declines in health later in life, a new study finds.
The most predictive parameters of poorer health at age 65 were cardiovascular disease, clinically significant depressive symptoms, and current smoking. Osteoarthritis, lower education level, and higher body mass index (BMI) also were associated with poorer health status 10 years on, Daniel H. Solomon, MD, MPH and colleagues wrote in their observational study, which was published in JAMA Network Open.
Determining a patient’s score on a health-related quality of life measure based on these variables might be useful in clinical practice to recognize midlife patients at increased risk for later health deterioration, Dr. Solomon, of the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital, Boston, said in a statement. This measure is called the Short Form 36 (SF-36), and the researchers specifically focused on the physical component summary score (PCS) of this measure. The SF-36 is similar to the Framingham 10-year coronary heart disease risk prediction score, according to Dr. Solomon, who is a professor of medicine at Harvard Medical School, also in Boston.
Based on their risk scores, women could preemptively target modifiable risk factors before they enter old age, the investigators wrote.
“Age 55-65 may be a critical decade. A person’s health and factors during this period may set them on a path for their later adult years,” Dr. Solomon said in a statement. “The good news is that a large proportion of women at midlife are very stable and will not go on to experience declines. But being able to identify women at higher risk could help lead to interventions targeted to them.”
Study details
The study included a cohort of 1,091 women drawn from the 3,302-participant Study of Women’s Health Across the Nation (SWAN), a racially and ethnically diverse group enrolled from six U.S. sites at or immediately before transition to menopause and followed for 10 years from age 55 to 65. The study sample, consisting of 24.6% Black, 24% Japanese or Chinese, and 51.9% White, had a median baseline age of 54.8 years and median BMI of 27 kg/m2 at entry. The median baseline PCS score was 53.1 (interquartile range, 46.8-56.7).
Over 10 years, 206 (18.9%) of the women in the study experienced clinically important declines of at least 8 points in baseline characteristics at around age 55. The following were significantly associated with these declines:
- Having a higher BMI.
- Having osteoarthritis.
- Having a lower educational level.
- Being a current smoker.
- Having clinically significant depressive symptoms.
- Having cardiovascular disease.
- Having better (or higher) physical health and function score on the PCS.
The association between a higher PCS score and a greater decline might seem like an anomaly, Dr. Solomon said in an interview, but one interpretation of this finding is that women with higher or better scores at baseline have further to fall once other risk factors take effect.
With data analyzed from October 2020 to March 2021, the median 10-year change in PCS was –1.02 points, but 206 women experienced declines of 8 points or more.
Those with health declines were more likely to be Black and less likely to be Japanese. They were also more likely to have other comorbidities such as diabetes, hypertension, and osteoporosis, and to report less physical activity.
Scoring system should not replace individualized evaluation, outside expert said
Commenting on the findings, Margaret J. Nachtigall, MD, a clinical associate professor in the department of obstetrics and gynecology at New York University Langone Health, cautioned that a generalized scoring system should not replace individualized evaluation of women at midlife.
“I assess women around age 55 on a daily basis for health risk factors going forward. And while a number such as BMI can be helpful, I worry that reliance on a score could miss treating the individual,” Dr. Nachtigall said an interview. For instance, one woman might have a high BMI owing to greater muscle mass, which is heavy, while another may have a lower BMI but more fat-related weight, as well as exacerbating conditions such as hypertension that would elevate her risk. “You have to make the calculation for each person.”
Dr. Nachtigall, who was not involved in the SWAN analysis, noted, however, that a big-data scoring system might be a useful adjunct to individual patient evaluation in that “it would make physicians look at all these many risk factors to identify those prone to decline.”
Study includes racially diverse population
According to the authors, while other studies have identified similar and other risk factors such as poor sleep, most have not included such a racially diverse population and have focused on women already in their senior years when the window of opportunity may already have closed.
“As a clinician and epidemiologist, I often think about the window of opportunity at midlife, when people are vital, engaged, and resilient,” said Dr. Solomon in the statement. “If we can identify risk factors and determine who is at risk, we may be able to find interventions that can stave off health declines and help put people on a better health trajectory.”
Eric M. Ascher, DO, who practices family medicine at Lenox Hill Hospital in New York and was not involved in the SWAN research, agreed with Dr. Solomon.
“Doctors who treat chronic conditions often meet patients when they are already suffering from a medical problem,” he said in an interview. “It is key to decrease your risk factors before it is too late.”
Dr. Ascher added that many primary care providers already rely heavily on scoring systems when determining level of risk and type of intervention. “Any additional risk factor-scoring systems that are easy to implement and will prevent chronic diseases would be something providers would want to use with their patients.”
Detailed analyses of larger at-risk populations are needed to validate these risk factors and identify others, the authors said.
SWAN is supported by the National Institute on Aging, the National Institute of Nursing Research, and the National Institutes of Heath’s Office of Research on Women’s Health. Dr. Solomon reported financial ties to Amgen, AbbVie and Moderna, UpToDate, and Arthritis & Rheumatology; as well as serving on the board of directors for the Childhood Arthritis and Rheumatology Research Alliance and an advisory committee for the Food and Drug Administration outside of this work. Dr. Nachtigall and Dr. Ascher disclosed no conflicts of interest with regard to their comments.
Having specific health issues, including depressive symptoms and cardiovascular disease, as a middle-aged woman was associated with experiencing clinically important declines in health later in life, a new study finds.
The most predictive parameters of poorer health at age 65 were cardiovascular disease, clinically significant depressive symptoms, and current smoking. Osteoarthritis, lower education level, and higher body mass index (BMI) also were associated with poorer health status 10 years on, Daniel H. Solomon, MD, MPH and colleagues wrote in their observational study, which was published in JAMA Network Open.
Determining a patient’s score on a health-related quality of life measure based on these variables might be useful in clinical practice to recognize midlife patients at increased risk for later health deterioration, Dr. Solomon, of the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital, Boston, said in a statement. This measure is called the Short Form 36 (SF-36), and the researchers specifically focused on the physical component summary score (PCS) of this measure. The SF-36 is similar to the Framingham 10-year coronary heart disease risk prediction score, according to Dr. Solomon, who is a professor of medicine at Harvard Medical School, also in Boston.
Based on their risk scores, women could preemptively target modifiable risk factors before they enter old age, the investigators wrote.
“Age 55-65 may be a critical decade. A person’s health and factors during this period may set them on a path for their later adult years,” Dr. Solomon said in a statement. “The good news is that a large proportion of women at midlife are very stable and will not go on to experience declines. But being able to identify women at higher risk could help lead to interventions targeted to them.”
Study details
The study included a cohort of 1,091 women drawn from the 3,302-participant Study of Women’s Health Across the Nation (SWAN), a racially and ethnically diverse group enrolled from six U.S. sites at or immediately before transition to menopause and followed for 10 years from age 55 to 65. The study sample, consisting of 24.6% Black, 24% Japanese or Chinese, and 51.9% White, had a median baseline age of 54.8 years and median BMI of 27 kg/m2 at entry. The median baseline PCS score was 53.1 (interquartile range, 46.8-56.7).
Over 10 years, 206 (18.9%) of the women in the study experienced clinically important declines of at least 8 points in baseline characteristics at around age 55. The following were significantly associated with these declines:
- Having a higher BMI.
- Having osteoarthritis.
- Having a lower educational level.
- Being a current smoker.
- Having clinically significant depressive symptoms.
- Having cardiovascular disease.
- Having better (or higher) physical health and function score on the PCS.
The association between a higher PCS score and a greater decline might seem like an anomaly, Dr. Solomon said in an interview, but one interpretation of this finding is that women with higher or better scores at baseline have further to fall once other risk factors take effect.
With data analyzed from October 2020 to March 2021, the median 10-year change in PCS was –1.02 points, but 206 women experienced declines of 8 points or more.
Those with health declines were more likely to be Black and less likely to be Japanese. They were also more likely to have other comorbidities such as diabetes, hypertension, and osteoporosis, and to report less physical activity.
Scoring system should not replace individualized evaluation, outside expert said
Commenting on the findings, Margaret J. Nachtigall, MD, a clinical associate professor in the department of obstetrics and gynecology at New York University Langone Health, cautioned that a generalized scoring system should not replace individualized evaluation of women at midlife.
“I assess women around age 55 on a daily basis for health risk factors going forward. And while a number such as BMI can be helpful, I worry that reliance on a score could miss treating the individual,” Dr. Nachtigall said an interview. For instance, one woman might have a high BMI owing to greater muscle mass, which is heavy, while another may have a lower BMI but more fat-related weight, as well as exacerbating conditions such as hypertension that would elevate her risk. “You have to make the calculation for each person.”
Dr. Nachtigall, who was not involved in the SWAN analysis, noted, however, that a big-data scoring system might be a useful adjunct to individual patient evaluation in that “it would make physicians look at all these many risk factors to identify those prone to decline.”
Study includes racially diverse population
According to the authors, while other studies have identified similar and other risk factors such as poor sleep, most have not included such a racially diverse population and have focused on women already in their senior years when the window of opportunity may already have closed.
“As a clinician and epidemiologist, I often think about the window of opportunity at midlife, when people are vital, engaged, and resilient,” said Dr. Solomon in the statement. “If we can identify risk factors and determine who is at risk, we may be able to find interventions that can stave off health declines and help put people on a better health trajectory.”
Eric M. Ascher, DO, who practices family medicine at Lenox Hill Hospital in New York and was not involved in the SWAN research, agreed with Dr. Solomon.
“Doctors who treat chronic conditions often meet patients when they are already suffering from a medical problem,” he said in an interview. “It is key to decrease your risk factors before it is too late.”
Dr. Ascher added that many primary care providers already rely heavily on scoring systems when determining level of risk and type of intervention. “Any additional risk factor-scoring systems that are easy to implement and will prevent chronic diseases would be something providers would want to use with their patients.”
Detailed analyses of larger at-risk populations are needed to validate these risk factors and identify others, the authors said.
SWAN is supported by the National Institute on Aging, the National Institute of Nursing Research, and the National Institutes of Heath’s Office of Research on Women’s Health. Dr. Solomon reported financial ties to Amgen, AbbVie and Moderna, UpToDate, and Arthritis & Rheumatology; as well as serving on the board of directors for the Childhood Arthritis and Rheumatology Research Alliance and an advisory committee for the Food and Drug Administration outside of this work. Dr. Nachtigall and Dr. Ascher disclosed no conflicts of interest with regard to their comments.
Having specific health issues, including depressive symptoms and cardiovascular disease, as a middle-aged woman was associated with experiencing clinically important declines in health later in life, a new study finds.
The most predictive parameters of poorer health at age 65 were cardiovascular disease, clinically significant depressive symptoms, and current smoking. Osteoarthritis, lower education level, and higher body mass index (BMI) also were associated with poorer health status 10 years on, Daniel H. Solomon, MD, MPH and colleagues wrote in their observational study, which was published in JAMA Network Open.
Determining a patient’s score on a health-related quality of life measure based on these variables might be useful in clinical practice to recognize midlife patients at increased risk for later health deterioration, Dr. Solomon, of the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital, Boston, said in a statement. This measure is called the Short Form 36 (SF-36), and the researchers specifically focused on the physical component summary score (PCS) of this measure. The SF-36 is similar to the Framingham 10-year coronary heart disease risk prediction score, according to Dr. Solomon, who is a professor of medicine at Harvard Medical School, also in Boston.
Based on their risk scores, women could preemptively target modifiable risk factors before they enter old age, the investigators wrote.
“Age 55-65 may be a critical decade. A person’s health and factors during this period may set them on a path for their later adult years,” Dr. Solomon said in a statement. “The good news is that a large proportion of women at midlife are very stable and will not go on to experience declines. But being able to identify women at higher risk could help lead to interventions targeted to them.”
Study details
The study included a cohort of 1,091 women drawn from the 3,302-participant Study of Women’s Health Across the Nation (SWAN), a racially and ethnically diverse group enrolled from six U.S. sites at or immediately before transition to menopause and followed for 10 years from age 55 to 65. The study sample, consisting of 24.6% Black, 24% Japanese or Chinese, and 51.9% White, had a median baseline age of 54.8 years and median BMI of 27 kg/m2 at entry. The median baseline PCS score was 53.1 (interquartile range, 46.8-56.7).
Over 10 years, 206 (18.9%) of the women in the study experienced clinically important declines of at least 8 points in baseline characteristics at around age 55. The following were significantly associated with these declines:
- Having a higher BMI.
- Having osteoarthritis.
- Having a lower educational level.
- Being a current smoker.
- Having clinically significant depressive symptoms.
- Having cardiovascular disease.
- Having better (or higher) physical health and function score on the PCS.
The association between a higher PCS score and a greater decline might seem like an anomaly, Dr. Solomon said in an interview, but one interpretation of this finding is that women with higher or better scores at baseline have further to fall once other risk factors take effect.
With data analyzed from October 2020 to March 2021, the median 10-year change in PCS was –1.02 points, but 206 women experienced declines of 8 points or more.
Those with health declines were more likely to be Black and less likely to be Japanese. They were also more likely to have other comorbidities such as diabetes, hypertension, and osteoporosis, and to report less physical activity.
Scoring system should not replace individualized evaluation, outside expert said
Commenting on the findings, Margaret J. Nachtigall, MD, a clinical associate professor in the department of obstetrics and gynecology at New York University Langone Health, cautioned that a generalized scoring system should not replace individualized evaluation of women at midlife.
“I assess women around age 55 on a daily basis for health risk factors going forward. And while a number such as BMI can be helpful, I worry that reliance on a score could miss treating the individual,” Dr. Nachtigall said an interview. For instance, one woman might have a high BMI owing to greater muscle mass, which is heavy, while another may have a lower BMI but more fat-related weight, as well as exacerbating conditions such as hypertension that would elevate her risk. “You have to make the calculation for each person.”
Dr. Nachtigall, who was not involved in the SWAN analysis, noted, however, that a big-data scoring system might be a useful adjunct to individual patient evaluation in that “it would make physicians look at all these many risk factors to identify those prone to decline.”
Study includes racially diverse population
According to the authors, while other studies have identified similar and other risk factors such as poor sleep, most have not included such a racially diverse population and have focused on women already in their senior years when the window of opportunity may already have closed.
“As a clinician and epidemiologist, I often think about the window of opportunity at midlife, when people are vital, engaged, and resilient,” said Dr. Solomon in the statement. “If we can identify risk factors and determine who is at risk, we may be able to find interventions that can stave off health declines and help put people on a better health trajectory.”
Eric M. Ascher, DO, who practices family medicine at Lenox Hill Hospital in New York and was not involved in the SWAN research, agreed with Dr. Solomon.
“Doctors who treat chronic conditions often meet patients when they are already suffering from a medical problem,” he said in an interview. “It is key to decrease your risk factors before it is too late.”
Dr. Ascher added that many primary care providers already rely heavily on scoring systems when determining level of risk and type of intervention. “Any additional risk factor-scoring systems that are easy to implement and will prevent chronic diseases would be something providers would want to use with their patients.”
Detailed analyses of larger at-risk populations are needed to validate these risk factors and identify others, the authors said.
SWAN is supported by the National Institute on Aging, the National Institute of Nursing Research, and the National Institutes of Heath’s Office of Research on Women’s Health. Dr. Solomon reported financial ties to Amgen, AbbVie and Moderna, UpToDate, and Arthritis & Rheumatology; as well as serving on the board of directors for the Childhood Arthritis and Rheumatology Research Alliance and an advisory committee for the Food and Drug Administration outside of this work. Dr. Nachtigall and Dr. Ascher disclosed no conflicts of interest with regard to their comments.
FROM JAMA NETWORK OPEN





