User login
Home visits: A practical approach
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
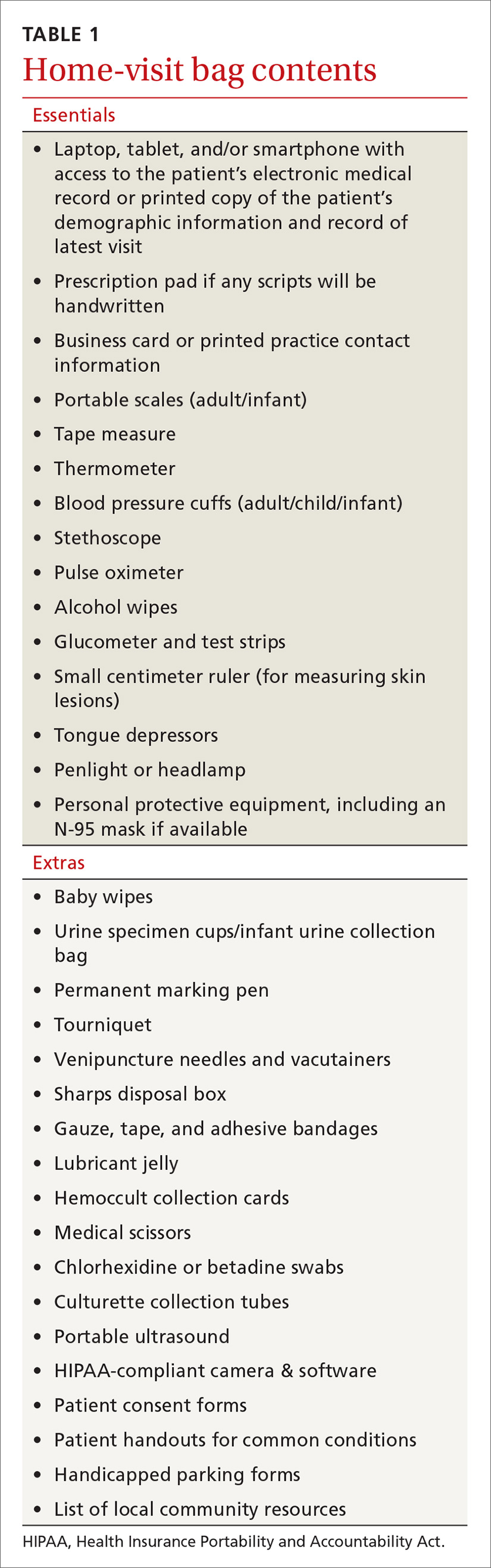
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.
Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
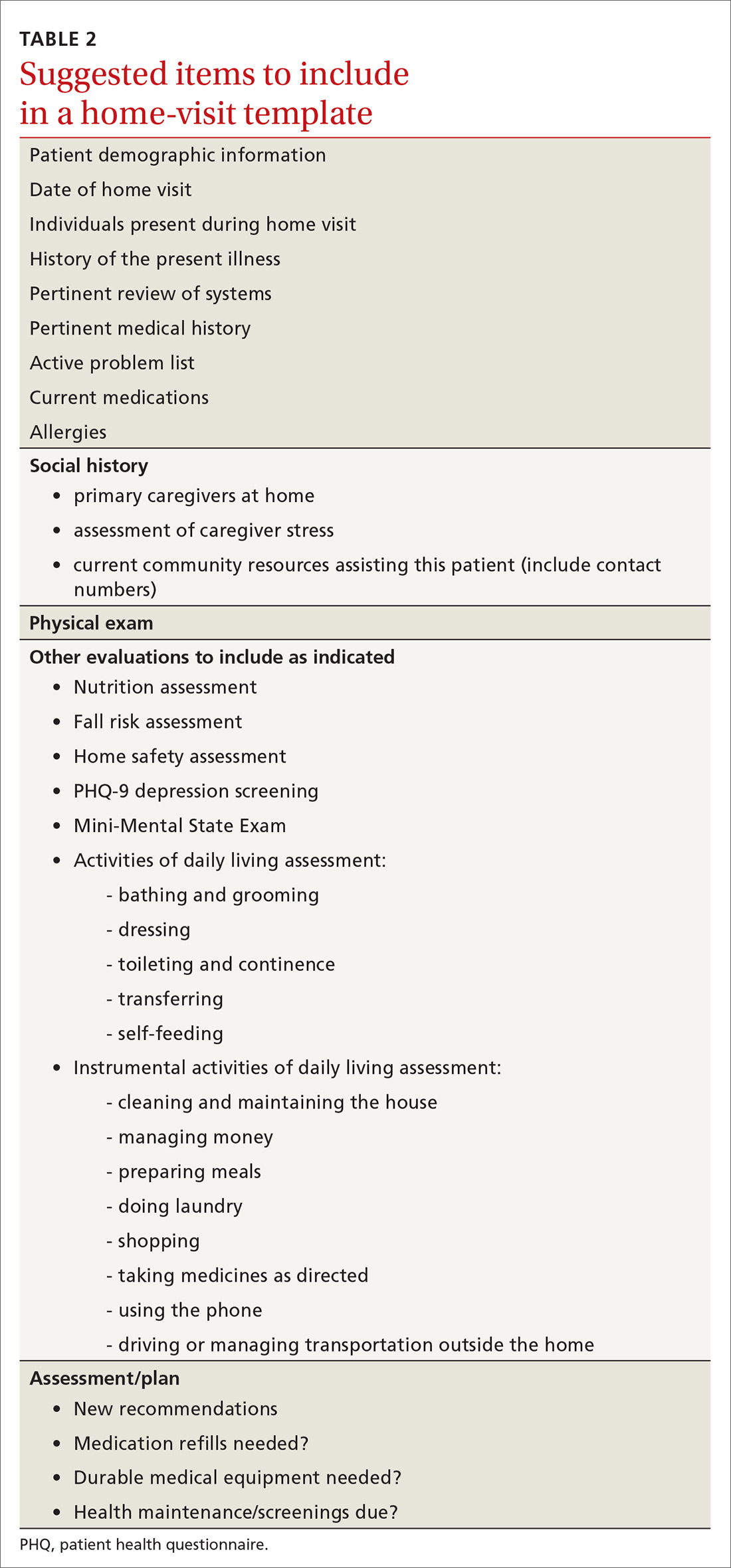
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.
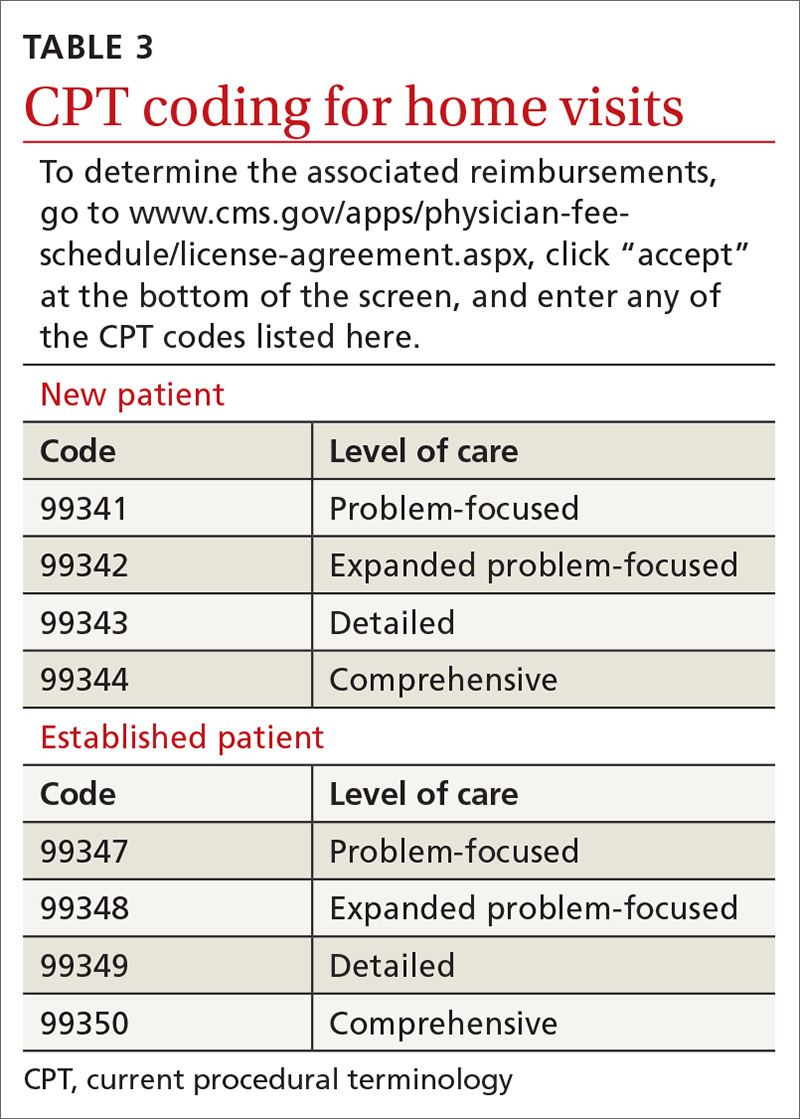
Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34
For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.

Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.

Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34

For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.

Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.

Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34

For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
PRACTICE RECOMMENDATIONS
❯ Consider incorporating home visits into the primary care of select vulnerable patients because doing so improves clinical outcomes, including mortality rates in neonates and elders. A
❯ Employ team-based home care and include community health workers, nurses, pharmacists, social workers, chaplains, and others. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Mortality higher in older adults hospitalized for IBD
Adults older than 65 years with inflammatory bowel diseases (IBD) had significantly higher rates of inpatient mortality, compared with those younger than 65 years, independent of factors including disease severity, based on data from more than 200,000 hospital admissions.
Older adults use a disproportionate share of health care resources, but data on outcomes among hospitalized older adults with gastrointestinal illness are limited, Jeffrey Schwartz, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, and colleagues wrote in the Journal of Clinical Gastroenterology.
“In particular, there remains a significant concern that elderly patients are more susceptible to the development of opportunistic infections and malignancy in the setting of biological therapy, which has evolved into the standard of care for IBD over the past 10 years,” they wrote.
In their study, the researchers identified 162,800 hospital admissions for Crohn’s disease and 96,450 admissions for ulcerative colitis. Of these, 20% and 30%, respectively, were older than 65 years, which the researchers designated as the geriatric group.
In a multivariate analysis, age older than 65 years was significantly associated with increased mortality in both Crohn’s disease (odds ratio, 3.47; 95% confidence interval, 2.72-4.44; P < .001) and ulcerative colitis (OR, 2.75; 95% CI, 2.16-3.49; P < .001). The association was independent of factors included comorbidities, admission type, hospital type, inpatient surgery, and IBD subtype.
The most frequent cause of death in both groups across all ages and disease subtypes was infections (approximately 80% for all groups). The total hospital length of stay was significantly longer for geriatric patients, compared with younger patients with Crohn’s disease, in multivariate analysis (average increase, 0.19 days; P = .009). The total charges also were significantly higher among geriatric Crohn’s disease patients, compared with younger patients (average increase, $2,467; P = .012). No significant differences in hospital stay or total charges appeared between geriatric and younger patients with ulcerative colitis.
The study findings were limited by several factors such as the inclusion of older patients with IBD who were hospitalized for other reasons and by the potential for increased mortality because of comorbidities among elderly patients, the researchers noted. However, the findings support the limited data from similar previous studies and showed greater inpatient mortality for older adults with IBD, compared with hospital inpatients overall.
“Given the high prevalence of IBD patients that require inpatient admission, as well as the rapidly aging nature of the U.S. population, further studies are needed targeting geriatric patients with UC [ulcerative colitis] and CD [Crohn’s disease] to improve their overall management and quality of care to determine if this mortality risk can be reduced,” they concluded.
Tune in to risks in older adults
The study is important because the percentage of the population older than 65 years has been increasing; “at the same time, we are seeing more elderly patients being newly diagnosed with Crohn’s disease and ulcerative colitis,” said Russell D. Cohen, MD, of the University of Chicago, in an interview. “These patients are more vulnerable to complications of the diseases, such as infections, as well as complications from the medications used to treat these diseases.” However, older adults are often excluded from clinical trials and even from many observational studies in IBD, he noted.
“We have known from past studies that infections such as sepsis are a leading cause of death in our IBD patients,” said Dr. Cohen. “It is also understandable that those patients who have had complicated courses and those with other comorbidities have a higher mortality rate. However, what was surprising in the current study is that, even when the authors controlled for these factors, the geriatric patients still had two and three-quarters to three and a half times the mortality than those who were younger.”
The take-home message for clinicians is that “the geriatric patient with IBD is at a much higher rate for inpatient mortality, most commonly from infectious complications, than younger patients,” Dr. Cohen emphasized. “Quicker attention to what may seem minor but could become a potentially life-threatening infection is imperative. Caution with the use of multiple immune suppressing medications in older patients is paramount, as is timely surgical intervention in IBD patients in whom medications simply are not working.”
Focus research on infection prevention, cost burden
“More research should be directed at finding out whether these deadly infections could be prevented, perhaps by preventative ‘prophylactic’ antibiotics in the elderly patients, especially those on multiple immunosuppressive agents,” said Dr. Cohen. “In addition, research into the undue cost burden that these patients place on our health care system and counter that with better access to the newer, safer biological therapies [most of which Medicare does not cover] rather than corticosteroids.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Cohen disclosed relationships with multiple companies including AbbVie, Bristol-Myers Squibb/Celgene, Eli Lilly, Gilead Sciences, Janssen, Pfizer, Takeda, and UCB Pharma.
SOURCE: Schwartz J et al. J Clin Gastroenterol. 2020 Nov 23. doi: 10.1097/MCG.0000000000001458.
Adults older than 65 years with inflammatory bowel diseases (IBD) had significantly higher rates of inpatient mortality, compared with those younger than 65 years, independent of factors including disease severity, based on data from more than 200,000 hospital admissions.
Older adults use a disproportionate share of health care resources, but data on outcomes among hospitalized older adults with gastrointestinal illness are limited, Jeffrey Schwartz, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, and colleagues wrote in the Journal of Clinical Gastroenterology.
“In particular, there remains a significant concern that elderly patients are more susceptible to the development of opportunistic infections and malignancy in the setting of biological therapy, which has evolved into the standard of care for IBD over the past 10 years,” they wrote.
In their study, the researchers identified 162,800 hospital admissions for Crohn’s disease and 96,450 admissions for ulcerative colitis. Of these, 20% and 30%, respectively, were older than 65 years, which the researchers designated as the geriatric group.
In a multivariate analysis, age older than 65 years was significantly associated with increased mortality in both Crohn’s disease (odds ratio, 3.47; 95% confidence interval, 2.72-4.44; P < .001) and ulcerative colitis (OR, 2.75; 95% CI, 2.16-3.49; P < .001). The association was independent of factors included comorbidities, admission type, hospital type, inpatient surgery, and IBD subtype.
The most frequent cause of death in both groups across all ages and disease subtypes was infections (approximately 80% for all groups). The total hospital length of stay was significantly longer for geriatric patients, compared with younger patients with Crohn’s disease, in multivariate analysis (average increase, 0.19 days; P = .009). The total charges also were significantly higher among geriatric Crohn’s disease patients, compared with younger patients (average increase, $2,467; P = .012). No significant differences in hospital stay or total charges appeared between geriatric and younger patients with ulcerative colitis.
The study findings were limited by several factors such as the inclusion of older patients with IBD who were hospitalized for other reasons and by the potential for increased mortality because of comorbidities among elderly patients, the researchers noted. However, the findings support the limited data from similar previous studies and showed greater inpatient mortality for older adults with IBD, compared with hospital inpatients overall.
“Given the high prevalence of IBD patients that require inpatient admission, as well as the rapidly aging nature of the U.S. population, further studies are needed targeting geriatric patients with UC [ulcerative colitis] and CD [Crohn’s disease] to improve their overall management and quality of care to determine if this mortality risk can be reduced,” they concluded.
Tune in to risks in older adults
The study is important because the percentage of the population older than 65 years has been increasing; “at the same time, we are seeing more elderly patients being newly diagnosed with Crohn’s disease and ulcerative colitis,” said Russell D. Cohen, MD, of the University of Chicago, in an interview. “These patients are more vulnerable to complications of the diseases, such as infections, as well as complications from the medications used to treat these diseases.” However, older adults are often excluded from clinical trials and even from many observational studies in IBD, he noted.
“We have known from past studies that infections such as sepsis are a leading cause of death in our IBD patients,” said Dr. Cohen. “It is also understandable that those patients who have had complicated courses and those with other comorbidities have a higher mortality rate. However, what was surprising in the current study is that, even when the authors controlled for these factors, the geriatric patients still had two and three-quarters to three and a half times the mortality than those who were younger.”
The take-home message for clinicians is that “the geriatric patient with IBD is at a much higher rate for inpatient mortality, most commonly from infectious complications, than younger patients,” Dr. Cohen emphasized. “Quicker attention to what may seem minor but could become a potentially life-threatening infection is imperative. Caution with the use of multiple immune suppressing medications in older patients is paramount, as is timely surgical intervention in IBD patients in whom medications simply are not working.”
Focus research on infection prevention, cost burden
“More research should be directed at finding out whether these deadly infections could be prevented, perhaps by preventative ‘prophylactic’ antibiotics in the elderly patients, especially those on multiple immunosuppressive agents,” said Dr. Cohen. “In addition, research into the undue cost burden that these patients place on our health care system and counter that with better access to the newer, safer biological therapies [most of which Medicare does not cover] rather than corticosteroids.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Cohen disclosed relationships with multiple companies including AbbVie, Bristol-Myers Squibb/Celgene, Eli Lilly, Gilead Sciences, Janssen, Pfizer, Takeda, and UCB Pharma.
SOURCE: Schwartz J et al. J Clin Gastroenterol. 2020 Nov 23. doi: 10.1097/MCG.0000000000001458.
Adults older than 65 years with inflammatory bowel diseases (IBD) had significantly higher rates of inpatient mortality, compared with those younger than 65 years, independent of factors including disease severity, based on data from more than 200,000 hospital admissions.
Older adults use a disproportionate share of health care resources, but data on outcomes among hospitalized older adults with gastrointestinal illness are limited, Jeffrey Schwartz, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, and colleagues wrote in the Journal of Clinical Gastroenterology.
“In particular, there remains a significant concern that elderly patients are more susceptible to the development of opportunistic infections and malignancy in the setting of biological therapy, which has evolved into the standard of care for IBD over the past 10 years,” they wrote.
In their study, the researchers identified 162,800 hospital admissions for Crohn’s disease and 96,450 admissions for ulcerative colitis. Of these, 20% and 30%, respectively, were older than 65 years, which the researchers designated as the geriatric group.
In a multivariate analysis, age older than 65 years was significantly associated with increased mortality in both Crohn’s disease (odds ratio, 3.47; 95% confidence interval, 2.72-4.44; P < .001) and ulcerative colitis (OR, 2.75; 95% CI, 2.16-3.49; P < .001). The association was independent of factors included comorbidities, admission type, hospital type, inpatient surgery, and IBD subtype.
The most frequent cause of death in both groups across all ages and disease subtypes was infections (approximately 80% for all groups). The total hospital length of stay was significantly longer for geriatric patients, compared with younger patients with Crohn’s disease, in multivariate analysis (average increase, 0.19 days; P = .009). The total charges also were significantly higher among geriatric Crohn’s disease patients, compared with younger patients (average increase, $2,467; P = .012). No significant differences in hospital stay or total charges appeared between geriatric and younger patients with ulcerative colitis.
The study findings were limited by several factors such as the inclusion of older patients with IBD who were hospitalized for other reasons and by the potential for increased mortality because of comorbidities among elderly patients, the researchers noted. However, the findings support the limited data from similar previous studies and showed greater inpatient mortality for older adults with IBD, compared with hospital inpatients overall.
“Given the high prevalence of IBD patients that require inpatient admission, as well as the rapidly aging nature of the U.S. population, further studies are needed targeting geriatric patients with UC [ulcerative colitis] and CD [Crohn’s disease] to improve their overall management and quality of care to determine if this mortality risk can be reduced,” they concluded.
Tune in to risks in older adults
The study is important because the percentage of the population older than 65 years has been increasing; “at the same time, we are seeing more elderly patients being newly diagnosed with Crohn’s disease and ulcerative colitis,” said Russell D. Cohen, MD, of the University of Chicago, in an interview. “These patients are more vulnerable to complications of the diseases, such as infections, as well as complications from the medications used to treat these diseases.” However, older adults are often excluded from clinical trials and even from many observational studies in IBD, he noted.
“We have known from past studies that infections such as sepsis are a leading cause of death in our IBD patients,” said Dr. Cohen. “It is also understandable that those patients who have had complicated courses and those with other comorbidities have a higher mortality rate. However, what was surprising in the current study is that, even when the authors controlled for these factors, the geriatric patients still had two and three-quarters to three and a half times the mortality than those who were younger.”
The take-home message for clinicians is that “the geriatric patient with IBD is at a much higher rate for inpatient mortality, most commonly from infectious complications, than younger patients,” Dr. Cohen emphasized. “Quicker attention to what may seem minor but could become a potentially life-threatening infection is imperative. Caution with the use of multiple immune suppressing medications in older patients is paramount, as is timely surgical intervention in IBD patients in whom medications simply are not working.”
Focus research on infection prevention, cost burden
“More research should be directed at finding out whether these deadly infections could be prevented, perhaps by preventative ‘prophylactic’ antibiotics in the elderly patients, especially those on multiple immunosuppressive agents,” said Dr. Cohen. “In addition, research into the undue cost burden that these patients place on our health care system and counter that with better access to the newer, safer biological therapies [most of which Medicare does not cover] rather than corticosteroids.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Cohen disclosed relationships with multiple companies including AbbVie, Bristol-Myers Squibb/Celgene, Eli Lilly, Gilead Sciences, Janssen, Pfizer, Takeda, and UCB Pharma.
SOURCE: Schwartz J et al. J Clin Gastroenterol. 2020 Nov 23. doi: 10.1097/MCG.0000000000001458.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
AGA publishes recommendations for managing IBD in elderly patients
The American Gastroenterological Association has published a Clinical Practice Update for management of inflammatory bowel disease (IBD) in elderly patients, including 15 best practice advice statements.
According to lead author Ashwin N. Ananthakrishnan, MD, MPH, of Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues, this topic is becoming increasingly relevant, as the population is aging, and prevalence of IBD among elderly is rising approximately 5% per year.
“Up to 15% of IBD in North America and Asia is diagnosed after the age of 60 years,” the investigators wrote in Gastroenterology.
Dr. Ananthakrishnan and colleagues noted that “care of elderly IBD patients poses unique challenges with respect to diagnosis and therapeutic decision-making.”
Challenges include greater frequency of comorbidities, increased risk of infection with anti–tumor necrosis factor therapy, increased risk of lymphoma with thiopurine therapy, greater likelihood of surgical complications, and, for Crohn’s disease, an elevated mortality rate, according to the update.
Another challenge is a lack of data.
“It should be noted that most clinical data to inform these practices are based on observational data or indirect evidence as elderly IBD patients comprise a very small proportion of subjects enrolled in IBD clinical trials or long-term pharmacovigilance initiatives,” the investigators wrote.
With this in mind, the update offers guidance for diagnosis, treatment, and ongoing health maintenance.
Diagnosis
Dr. Ananthakrishnan and colleagues first suggested that clinicians remain vigilant for IBD in elderly people, in consideration of the 15% prevalence rate in this subpopulation.
For elderly individuals with a low probability of IBD, the investigators recommended fecal calprotectin or lactoferrin to determine if endoscopy is needed. For elderly patients with chronic diarrhea or hematochezia, plus moderate to high suspicion of IBD, colorectal neoplasia, or microscopic colitis, they recommended colonoscopy.
Lastly, the expert panel suggested that elderly patients presenting with segmental left-sided colitis and diverticulosis may also have Crohn’s disease or IBD unclassified.
Treatment
The clinical practice update offers 10 best practice statements for treating elderly patients with IBD. There is a recurring emphasis on treatment personalization, which should be informed by patient goals and priorities, risk/presence of severe disease, chronological age, functional status, independence, comorbidities, frailty, and several other age-associated risk factors (e.g., venous thromboembolism).
Concerning specific therapies, the investigators cautioned against systemic corticosteroids for maintenance therapy; instead, nonsystemic corticosteroids (e.g., budesonide) are favored, or possibly early biological therapy if budesonide is not indicated. When selecting a biologic, Dr. Ananthakrishnan and colleagues recommended those associated with a lower risk of malignancy and infection (e.g., ustekinumab or vedolizumab).
The advantages of thiopurine monotherapy being oral, relatively inexpensive compared to biologicals and having a long track record of success in maintenance of remission must be balanced against the need for ongoing serological monitoring, and increased risk of some malignancies.
Finally, the expert panel recommended that all elderly patients receive multidisciplinary care, which may include primary care providers, mental health professionals, nutritionists, and other specialists. It may also be productive to consult with family and caregivers during treatment planning.
Health maintenance
The last two best practice advice statements concern health maintenance.
First, the investigators recommended that elderly patients with IBD adhere to vaccination schedules, including herpes zoster, pneumococcus, and influenza vaccines, ideally, before starting immunosuppression.
Second, Dr. Ananthakrishnan and colleagues advised that cessation of colorectal cancer surveillance may be considered in elderly patients with IBD; however, this decision should take into account a variety of factors, including comorbidities, age, life expectancy, likelihood of endoscopic resection, and surgical candidacy.
The review was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. The investigators disclosed relationships with Gilead, Sun Pharma, Kyn Therapeutics, and others.
SOURCE: Ananthakrishnan AN et al. Gastroenterology. 2020 Sep 30. doi: 10.1053/j.gastro.2020.08.060.
This story was updated on 12/4/2020.
The American Gastroenterological Association has published a Clinical Practice Update for management of inflammatory bowel disease (IBD) in elderly patients, including 15 best practice advice statements.
According to lead author Ashwin N. Ananthakrishnan, MD, MPH, of Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues, this topic is becoming increasingly relevant, as the population is aging, and prevalence of IBD among elderly is rising approximately 5% per year.
“Up to 15% of IBD in North America and Asia is diagnosed after the age of 60 years,” the investigators wrote in Gastroenterology.
Dr. Ananthakrishnan and colleagues noted that “care of elderly IBD patients poses unique challenges with respect to diagnosis and therapeutic decision-making.”
Challenges include greater frequency of comorbidities, increased risk of infection with anti–tumor necrosis factor therapy, increased risk of lymphoma with thiopurine therapy, greater likelihood of surgical complications, and, for Crohn’s disease, an elevated mortality rate, according to the update.
Another challenge is a lack of data.
“It should be noted that most clinical data to inform these practices are based on observational data or indirect evidence as elderly IBD patients comprise a very small proportion of subjects enrolled in IBD clinical trials or long-term pharmacovigilance initiatives,” the investigators wrote.
With this in mind, the update offers guidance for diagnosis, treatment, and ongoing health maintenance.
Diagnosis
Dr. Ananthakrishnan and colleagues first suggested that clinicians remain vigilant for IBD in elderly people, in consideration of the 15% prevalence rate in this subpopulation.
For elderly individuals with a low probability of IBD, the investigators recommended fecal calprotectin or lactoferrin to determine if endoscopy is needed. For elderly patients with chronic diarrhea or hematochezia, plus moderate to high suspicion of IBD, colorectal neoplasia, or microscopic colitis, they recommended colonoscopy.
Lastly, the expert panel suggested that elderly patients presenting with segmental left-sided colitis and diverticulosis may also have Crohn’s disease or IBD unclassified.
Treatment
The clinical practice update offers 10 best practice statements for treating elderly patients with IBD. There is a recurring emphasis on treatment personalization, which should be informed by patient goals and priorities, risk/presence of severe disease, chronological age, functional status, independence, comorbidities, frailty, and several other age-associated risk factors (e.g., venous thromboembolism).
Concerning specific therapies, the investigators cautioned against systemic corticosteroids for maintenance therapy; instead, nonsystemic corticosteroids (e.g., budesonide) are favored, or possibly early biological therapy if budesonide is not indicated. When selecting a biologic, Dr. Ananthakrishnan and colleagues recommended those associated with a lower risk of malignancy and infection (e.g., ustekinumab or vedolizumab).
The advantages of thiopurine monotherapy being oral, relatively inexpensive compared to biologicals and having a long track record of success in maintenance of remission must be balanced against the need for ongoing serological monitoring, and increased risk of some malignancies.
Finally, the expert panel recommended that all elderly patients receive multidisciplinary care, which may include primary care providers, mental health professionals, nutritionists, and other specialists. It may also be productive to consult with family and caregivers during treatment planning.
Health maintenance
The last two best practice advice statements concern health maintenance.
First, the investigators recommended that elderly patients with IBD adhere to vaccination schedules, including herpes zoster, pneumococcus, and influenza vaccines, ideally, before starting immunosuppression.
Second, Dr. Ananthakrishnan and colleagues advised that cessation of colorectal cancer surveillance may be considered in elderly patients with IBD; however, this decision should take into account a variety of factors, including comorbidities, age, life expectancy, likelihood of endoscopic resection, and surgical candidacy.
The review was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. The investigators disclosed relationships with Gilead, Sun Pharma, Kyn Therapeutics, and others.
SOURCE: Ananthakrishnan AN et al. Gastroenterology. 2020 Sep 30. doi: 10.1053/j.gastro.2020.08.060.
This story was updated on 12/4/2020.
The American Gastroenterological Association has published a Clinical Practice Update for management of inflammatory bowel disease (IBD) in elderly patients, including 15 best practice advice statements.
According to lead author Ashwin N. Ananthakrishnan, MD, MPH, of Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues, this topic is becoming increasingly relevant, as the population is aging, and prevalence of IBD among elderly is rising approximately 5% per year.
“Up to 15% of IBD in North America and Asia is diagnosed after the age of 60 years,” the investigators wrote in Gastroenterology.
Dr. Ananthakrishnan and colleagues noted that “care of elderly IBD patients poses unique challenges with respect to diagnosis and therapeutic decision-making.”
Challenges include greater frequency of comorbidities, increased risk of infection with anti–tumor necrosis factor therapy, increased risk of lymphoma with thiopurine therapy, greater likelihood of surgical complications, and, for Crohn’s disease, an elevated mortality rate, according to the update.
Another challenge is a lack of data.
“It should be noted that most clinical data to inform these practices are based on observational data or indirect evidence as elderly IBD patients comprise a very small proportion of subjects enrolled in IBD clinical trials or long-term pharmacovigilance initiatives,” the investigators wrote.
With this in mind, the update offers guidance for diagnosis, treatment, and ongoing health maintenance.
Diagnosis
Dr. Ananthakrishnan and colleagues first suggested that clinicians remain vigilant for IBD in elderly people, in consideration of the 15% prevalence rate in this subpopulation.
For elderly individuals with a low probability of IBD, the investigators recommended fecal calprotectin or lactoferrin to determine if endoscopy is needed. For elderly patients with chronic diarrhea or hematochezia, plus moderate to high suspicion of IBD, colorectal neoplasia, or microscopic colitis, they recommended colonoscopy.
Lastly, the expert panel suggested that elderly patients presenting with segmental left-sided colitis and diverticulosis may also have Crohn’s disease or IBD unclassified.
Treatment
The clinical practice update offers 10 best practice statements for treating elderly patients with IBD. There is a recurring emphasis on treatment personalization, which should be informed by patient goals and priorities, risk/presence of severe disease, chronological age, functional status, independence, comorbidities, frailty, and several other age-associated risk factors (e.g., venous thromboembolism).
Concerning specific therapies, the investigators cautioned against systemic corticosteroids for maintenance therapy; instead, nonsystemic corticosteroids (e.g., budesonide) are favored, or possibly early biological therapy if budesonide is not indicated. When selecting a biologic, Dr. Ananthakrishnan and colleagues recommended those associated with a lower risk of malignancy and infection (e.g., ustekinumab or vedolizumab).
The advantages of thiopurine monotherapy being oral, relatively inexpensive compared to biologicals and having a long track record of success in maintenance of remission must be balanced against the need for ongoing serological monitoring, and increased risk of some malignancies.
Finally, the expert panel recommended that all elderly patients receive multidisciplinary care, which may include primary care providers, mental health professionals, nutritionists, and other specialists. It may also be productive to consult with family and caregivers during treatment planning.
Health maintenance
The last two best practice advice statements concern health maintenance.
First, the investigators recommended that elderly patients with IBD adhere to vaccination schedules, including herpes zoster, pneumococcus, and influenza vaccines, ideally, before starting immunosuppression.
Second, Dr. Ananthakrishnan and colleagues advised that cessation of colorectal cancer surveillance may be considered in elderly patients with IBD; however, this decision should take into account a variety of factors, including comorbidities, age, life expectancy, likelihood of endoscopic resection, and surgical candidacy.
The review was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. The investigators disclosed relationships with Gilead, Sun Pharma, Kyn Therapeutics, and others.
SOURCE: Ananthakrishnan AN et al. Gastroenterology. 2020 Sep 30. doi: 10.1053/j.gastro.2020.08.060.
This story was updated on 12/4/2020.
FROM GASTROENTEROLOGY
Cognitive Behavioral Therapy Plus Placebo Is Inferior to NSAID Therapy for Arthritis Pain
Study Overview
Objective. To examine whether discontinuation of nonsteroidal anti-inflammatory drug (NSAID) therapy and initiation of telephone-based cognitive behavioral therapy (CBT) is not worse than continuation of NSAIDs in the management of arthritis pain.
Design. Randomized controlled trial with noninferiority design.
Setting and participants. This study was a multicenter trial conducted across 4 Veterans Affairs health care systems in Boston, Providence, Connecticut, and North Florida/South Georgia that started September 2013 and ended September 2018. Eligibility criteria included being age 20 years or older, radiographic evidence of knee osteoarthritis, and use of an NSAID for knee pain on most days of the month for at least the past 3 months. Exclusion criteria included significant hearing impairments that may impede the conduct of the trial, current opioid prescriptions excluding tramadol, contraindications to NSAID use, recent or scheduled intra-articular injections or surgery, comorbid conditions other than knee pain that limited walking, and bilateral knee replacements or pain only in the replaced knee. Concurrent use of tramadol and other non-NSAID analgesics was permitted.
A total of 490 participants took part in the 2-week run-in period where their NSAID regimen was discontinued and they were started on a standardized dose of the NSAID meloxicam 15 mg daily. During the run-in period, 126 participants were excluded for several reasons, including worsening pain and patient withdrawal, yielding 364 participants who were randomized to continue meloxicam treatment or placebo for 4 weeks with blinding.
Intervention. Subsequent to the 4-week phase 1 placebo controlled trial, participants in the placebo group were given CBT via telephone (unblinded) for 10 weeks, and the meloxicam group continued treatment with meloxicam for phase 2. The CBT group received 10 modules over 10 weeks in 30- to 45-minute telephone contacts with a psychologist using a treatment manual modified for knee osteoarthritis. These modules consisted of 1 introductory module, 8 pain coping skills modules (eg, deep breathing and visual imagery, progressive muscle relaxation, physical activity and bodily mechanics, identifying unhealthy thoughts, balancing unhealthy thoughts, managing stress, time-based pacing, and sleep hygiene), and a final module emphasizing skill consolidation and relapse prevention. Outcomes were assessed at the end of the phase 1 and phase 2 periods.
Main outcome measures. Main study outcome measures included pain as measured with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at 4 weeks. Secondary outcomes included the WOMAC pain score, disability score, and global impression of change after treatment at 14 weeks. The WOMAC pain scale ranges from 0 to 20, and consists of 5 questions regarding severity of pain during walking, stair use, lying in bed at night, sitting, and standing, with 0 indicating no pain; 1, mild pain; 2, moderate pain; 3, severe pain; and 4, very severe pain for each item. The WOMAC disability scale measures self-reported difficulty in performing tasks that reflect lower-extremity physical function, including climbing stairs, rising from a chair, walking, and other activities of daily living. The global impression of change after treatment was measured on a 5-point scale (where 1 indicates much better and 5 indicates much worse). The minimum clinically important difference of the WOMAC pain scale is 2, based on prior literature. With the noninferiority design, the margin was set as a score of 1.
Main results. The placebo group consisted of 180 participants, with an average age of 58.2 years (SD, 11.8 years); 89% of them were male. The meloxicam group consisted of 184 participants, with an average age of 58.6 years (SD, 10 years); 84% of them were male. The average body mass index was 33.9 and 33.4 in each group, respectively. For the primary outcome, the placebo group had a worse pain score than the meloxicam group at 4 weeks (difference of 1.4; 95% confidence interval, 0.8- 2.0). At 14 weeks, the placebo group (with CBT) had a worse pain score than the meloxicam group (difference of 0.8; 95% CI, 0.2-1.4). There was no statistically significant difference in the disability score or global impression of change after treatment score between the 2 groups. The observed difference in pain score did not, however, exceed the minimum clinically important difference.
Conclusion. Placebo treatment and CBT are inferior to NSAIDs in managing pain for patients with knee osteoarthritis. The difference in pain may not be clinically important, and there were no differences in function at 14 weeks.
Commentary
Osteoarthritis is a common chronic condition that causes pain and disability and is often treated with oral analgesics. NSAIDs, despite few high-quality trials demonstrating their efficacy, are among the most commonly used treatment for osteoarthritis pain.1 NSAID therapy, however, does have potential side effects, such as gastric reflux and renal dysfunction.2 This withdrawal trial with placebo control contributes further evidence of the effectiveness of NSAIDs on knee osteoarthritis, demonstrating that indeed NSAIDs improve pain scores to a greater degree than placebo treatment. Augmenting placebo treatment with nonpharmacologic CBT was inferior to NSAIDs in pain management. The authors pointed out that the difference in pain score may not be clinically important, and that lower-extremity function was not different between the groups, concluding that, despite the higher pain score, CBT could be a treatment option, particularly for those who may have difficulty tolerating NSAID treatment.
The study population had a number of chronic conditions in addition to having knee arthritis, and thus likely were taking multiple medications for chronic disease management. Use of multiple medications is associated with an increased risk of rug interactions and adverse effects of medications.3 Thus, this attempt to assess whether a nonpharmacologic alternative treatment is noninferior to a drug treatment is a step toward building the evidence base for deprescribing and enhancing medication safety.4 Previous studies have examined other nonpharmacologic treatments for knee arthritis, such as acupuncture,5 and it is worthwhile to consider combining nonpharmacological approaches as an alternative to oral analgesic medication use.
Applications for Clinical Practice
This study advances our understanding of the effect of NSAID use on knee osteoarthritis when compared to placebo with CBT. Although this is a negative study that failed to show that placebo combined with CBT is noninferior to NSAIDs, it did quantify the expected treatment effect of NSAIDs and showed that this effect likely is not clinically important and/or does not alter lower-extremity function. Further studies are needed to identify other nonpharmacologic approaches and test whether combinations of approaches are effective in the management of chronic pain from osteoarthritis.
–William W. Hung, MD, MPH
1. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150.
2. Pilotto A, Franceschi M, Leandro G, Di Mario F. NSAID and aspirin use by the elderly in general practice: effect on gastrointestinal symptoms and therapies. Drugs Aging. 2003;20:701-710.
3. Steinman MA. Polypharmacy-time to get beyond numbers. JAMA Intern Med. 2016;176:482-483.
4. Rashid R, Chang C, Niu F, et al. Evaluation of a pharmacist-managed nonsteroidal anti-inflammatory drugs deprescribing program in an integrated health care system. J Manag Care Spec Pharm. 2020;26:918-924.
5. Sun J, Zhao Y, Zhu R, et al. Acupotomy therapy for knee osteoarthritis pain: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:2168283.
Study Overview
Objective. To examine whether discontinuation of nonsteroidal anti-inflammatory drug (NSAID) therapy and initiation of telephone-based cognitive behavioral therapy (CBT) is not worse than continuation of NSAIDs in the management of arthritis pain.
Design. Randomized controlled trial with noninferiority design.
Setting and participants. This study was a multicenter trial conducted across 4 Veterans Affairs health care systems in Boston, Providence, Connecticut, and North Florida/South Georgia that started September 2013 and ended September 2018. Eligibility criteria included being age 20 years or older, radiographic evidence of knee osteoarthritis, and use of an NSAID for knee pain on most days of the month for at least the past 3 months. Exclusion criteria included significant hearing impairments that may impede the conduct of the trial, current opioid prescriptions excluding tramadol, contraindications to NSAID use, recent or scheduled intra-articular injections or surgery, comorbid conditions other than knee pain that limited walking, and bilateral knee replacements or pain only in the replaced knee. Concurrent use of tramadol and other non-NSAID analgesics was permitted.
A total of 490 participants took part in the 2-week run-in period where their NSAID regimen was discontinued and they were started on a standardized dose of the NSAID meloxicam 15 mg daily. During the run-in period, 126 participants were excluded for several reasons, including worsening pain and patient withdrawal, yielding 364 participants who were randomized to continue meloxicam treatment or placebo for 4 weeks with blinding.
Intervention. Subsequent to the 4-week phase 1 placebo controlled trial, participants in the placebo group were given CBT via telephone (unblinded) for 10 weeks, and the meloxicam group continued treatment with meloxicam for phase 2. The CBT group received 10 modules over 10 weeks in 30- to 45-minute telephone contacts with a psychologist using a treatment manual modified for knee osteoarthritis. These modules consisted of 1 introductory module, 8 pain coping skills modules (eg, deep breathing and visual imagery, progressive muscle relaxation, physical activity and bodily mechanics, identifying unhealthy thoughts, balancing unhealthy thoughts, managing stress, time-based pacing, and sleep hygiene), and a final module emphasizing skill consolidation and relapse prevention. Outcomes were assessed at the end of the phase 1 and phase 2 periods.
Main outcome measures. Main study outcome measures included pain as measured with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at 4 weeks. Secondary outcomes included the WOMAC pain score, disability score, and global impression of change after treatment at 14 weeks. The WOMAC pain scale ranges from 0 to 20, and consists of 5 questions regarding severity of pain during walking, stair use, lying in bed at night, sitting, and standing, with 0 indicating no pain; 1, mild pain; 2, moderate pain; 3, severe pain; and 4, very severe pain for each item. The WOMAC disability scale measures self-reported difficulty in performing tasks that reflect lower-extremity physical function, including climbing stairs, rising from a chair, walking, and other activities of daily living. The global impression of change after treatment was measured on a 5-point scale (where 1 indicates much better and 5 indicates much worse). The minimum clinically important difference of the WOMAC pain scale is 2, based on prior literature. With the noninferiority design, the margin was set as a score of 1.
Main results. The placebo group consisted of 180 participants, with an average age of 58.2 years (SD, 11.8 years); 89% of them were male. The meloxicam group consisted of 184 participants, with an average age of 58.6 years (SD, 10 years); 84% of them were male. The average body mass index was 33.9 and 33.4 in each group, respectively. For the primary outcome, the placebo group had a worse pain score than the meloxicam group at 4 weeks (difference of 1.4; 95% confidence interval, 0.8- 2.0). At 14 weeks, the placebo group (with CBT) had a worse pain score than the meloxicam group (difference of 0.8; 95% CI, 0.2-1.4). There was no statistically significant difference in the disability score or global impression of change after treatment score between the 2 groups. The observed difference in pain score did not, however, exceed the minimum clinically important difference.
Conclusion. Placebo treatment and CBT are inferior to NSAIDs in managing pain for patients with knee osteoarthritis. The difference in pain may not be clinically important, and there were no differences in function at 14 weeks.
Commentary
Osteoarthritis is a common chronic condition that causes pain and disability and is often treated with oral analgesics. NSAIDs, despite few high-quality trials demonstrating their efficacy, are among the most commonly used treatment for osteoarthritis pain.1 NSAID therapy, however, does have potential side effects, such as gastric reflux and renal dysfunction.2 This withdrawal trial with placebo control contributes further evidence of the effectiveness of NSAIDs on knee osteoarthritis, demonstrating that indeed NSAIDs improve pain scores to a greater degree than placebo treatment. Augmenting placebo treatment with nonpharmacologic CBT was inferior to NSAIDs in pain management. The authors pointed out that the difference in pain score may not be clinically important, and that lower-extremity function was not different between the groups, concluding that, despite the higher pain score, CBT could be a treatment option, particularly for those who may have difficulty tolerating NSAID treatment.
The study population had a number of chronic conditions in addition to having knee arthritis, and thus likely were taking multiple medications for chronic disease management. Use of multiple medications is associated with an increased risk of rug interactions and adverse effects of medications.3 Thus, this attempt to assess whether a nonpharmacologic alternative treatment is noninferior to a drug treatment is a step toward building the evidence base for deprescribing and enhancing medication safety.4 Previous studies have examined other nonpharmacologic treatments for knee arthritis, such as acupuncture,5 and it is worthwhile to consider combining nonpharmacological approaches as an alternative to oral analgesic medication use.
Applications for Clinical Practice
This study advances our understanding of the effect of NSAID use on knee osteoarthritis when compared to placebo with CBT. Although this is a negative study that failed to show that placebo combined with CBT is noninferior to NSAIDs, it did quantify the expected treatment effect of NSAIDs and showed that this effect likely is not clinically important and/or does not alter lower-extremity function. Further studies are needed to identify other nonpharmacologic approaches and test whether combinations of approaches are effective in the management of chronic pain from osteoarthritis.
–William W. Hung, MD, MPH
Study Overview
Objective. To examine whether discontinuation of nonsteroidal anti-inflammatory drug (NSAID) therapy and initiation of telephone-based cognitive behavioral therapy (CBT) is not worse than continuation of NSAIDs in the management of arthritis pain.
Design. Randomized controlled trial with noninferiority design.
Setting and participants. This study was a multicenter trial conducted across 4 Veterans Affairs health care systems in Boston, Providence, Connecticut, and North Florida/South Georgia that started September 2013 and ended September 2018. Eligibility criteria included being age 20 years or older, radiographic evidence of knee osteoarthritis, and use of an NSAID for knee pain on most days of the month for at least the past 3 months. Exclusion criteria included significant hearing impairments that may impede the conduct of the trial, current opioid prescriptions excluding tramadol, contraindications to NSAID use, recent or scheduled intra-articular injections or surgery, comorbid conditions other than knee pain that limited walking, and bilateral knee replacements or pain only in the replaced knee. Concurrent use of tramadol and other non-NSAID analgesics was permitted.
A total of 490 participants took part in the 2-week run-in period where their NSAID regimen was discontinued and they were started on a standardized dose of the NSAID meloxicam 15 mg daily. During the run-in period, 126 participants were excluded for several reasons, including worsening pain and patient withdrawal, yielding 364 participants who were randomized to continue meloxicam treatment or placebo for 4 weeks with blinding.
Intervention. Subsequent to the 4-week phase 1 placebo controlled trial, participants in the placebo group were given CBT via telephone (unblinded) for 10 weeks, and the meloxicam group continued treatment with meloxicam for phase 2. The CBT group received 10 modules over 10 weeks in 30- to 45-minute telephone contacts with a psychologist using a treatment manual modified for knee osteoarthritis. These modules consisted of 1 introductory module, 8 pain coping skills modules (eg, deep breathing and visual imagery, progressive muscle relaxation, physical activity and bodily mechanics, identifying unhealthy thoughts, balancing unhealthy thoughts, managing stress, time-based pacing, and sleep hygiene), and a final module emphasizing skill consolidation and relapse prevention. Outcomes were assessed at the end of the phase 1 and phase 2 periods.
Main outcome measures. Main study outcome measures included pain as measured with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at 4 weeks. Secondary outcomes included the WOMAC pain score, disability score, and global impression of change after treatment at 14 weeks. The WOMAC pain scale ranges from 0 to 20, and consists of 5 questions regarding severity of pain during walking, stair use, lying in bed at night, sitting, and standing, with 0 indicating no pain; 1, mild pain; 2, moderate pain; 3, severe pain; and 4, very severe pain for each item. The WOMAC disability scale measures self-reported difficulty in performing tasks that reflect lower-extremity physical function, including climbing stairs, rising from a chair, walking, and other activities of daily living. The global impression of change after treatment was measured on a 5-point scale (where 1 indicates much better and 5 indicates much worse). The minimum clinically important difference of the WOMAC pain scale is 2, based on prior literature. With the noninferiority design, the margin was set as a score of 1.
Main results. The placebo group consisted of 180 participants, with an average age of 58.2 years (SD, 11.8 years); 89% of them were male. The meloxicam group consisted of 184 participants, with an average age of 58.6 years (SD, 10 years); 84% of them were male. The average body mass index was 33.9 and 33.4 in each group, respectively. For the primary outcome, the placebo group had a worse pain score than the meloxicam group at 4 weeks (difference of 1.4; 95% confidence interval, 0.8- 2.0). At 14 weeks, the placebo group (with CBT) had a worse pain score than the meloxicam group (difference of 0.8; 95% CI, 0.2-1.4). There was no statistically significant difference in the disability score or global impression of change after treatment score between the 2 groups. The observed difference in pain score did not, however, exceed the minimum clinically important difference.
Conclusion. Placebo treatment and CBT are inferior to NSAIDs in managing pain for patients with knee osteoarthritis. The difference in pain may not be clinically important, and there were no differences in function at 14 weeks.
Commentary
Osteoarthritis is a common chronic condition that causes pain and disability and is often treated with oral analgesics. NSAIDs, despite few high-quality trials demonstrating their efficacy, are among the most commonly used treatment for osteoarthritis pain.1 NSAID therapy, however, does have potential side effects, such as gastric reflux and renal dysfunction.2 This withdrawal trial with placebo control contributes further evidence of the effectiveness of NSAIDs on knee osteoarthritis, demonstrating that indeed NSAIDs improve pain scores to a greater degree than placebo treatment. Augmenting placebo treatment with nonpharmacologic CBT was inferior to NSAIDs in pain management. The authors pointed out that the difference in pain score may not be clinically important, and that lower-extremity function was not different between the groups, concluding that, despite the higher pain score, CBT could be a treatment option, particularly for those who may have difficulty tolerating NSAID treatment.
The study population had a number of chronic conditions in addition to having knee arthritis, and thus likely were taking multiple medications for chronic disease management. Use of multiple medications is associated with an increased risk of rug interactions and adverse effects of medications.3 Thus, this attempt to assess whether a nonpharmacologic alternative treatment is noninferior to a drug treatment is a step toward building the evidence base for deprescribing and enhancing medication safety.4 Previous studies have examined other nonpharmacologic treatments for knee arthritis, such as acupuncture,5 and it is worthwhile to consider combining nonpharmacological approaches as an alternative to oral analgesic medication use.
Applications for Clinical Practice
This study advances our understanding of the effect of NSAID use on knee osteoarthritis when compared to placebo with CBT. Although this is a negative study that failed to show that placebo combined with CBT is noninferior to NSAIDs, it did quantify the expected treatment effect of NSAIDs and showed that this effect likely is not clinically important and/or does not alter lower-extremity function. Further studies are needed to identify other nonpharmacologic approaches and test whether combinations of approaches are effective in the management of chronic pain from osteoarthritis.
–William W. Hung, MD, MPH
1. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150.
2. Pilotto A, Franceschi M, Leandro G, Di Mario F. NSAID and aspirin use by the elderly in general practice: effect on gastrointestinal symptoms and therapies. Drugs Aging. 2003;20:701-710.
3. Steinman MA. Polypharmacy-time to get beyond numbers. JAMA Intern Med. 2016;176:482-483.
4. Rashid R, Chang C, Niu F, et al. Evaluation of a pharmacist-managed nonsteroidal anti-inflammatory drugs deprescribing program in an integrated health care system. J Manag Care Spec Pharm. 2020;26:918-924.
5. Sun J, Zhao Y, Zhu R, et al. Acupotomy therapy for knee osteoarthritis pain: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:2168283.
1. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150.
2. Pilotto A, Franceschi M, Leandro G, Di Mario F. NSAID and aspirin use by the elderly in general practice: effect on gastrointestinal symptoms and therapies. Drugs Aging. 2003;20:701-710.
3. Steinman MA. Polypharmacy-time to get beyond numbers. JAMA Intern Med. 2016;176:482-483.
4. Rashid R, Chang C, Niu F, et al. Evaluation of a pharmacist-managed nonsteroidal anti-inflammatory drugs deprescribing program in an integrated health care system. J Manag Care Spec Pharm. 2020;26:918-924.
5. Sun J, Zhao Y, Zhu R, et al. Acupotomy therapy for knee osteoarthritis pain: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:2168283.
Lung cancer CT scan is chance for ‘opportunistic’ osteoporosis check
Low-dose chest CT for lung cancer screening provides the opportunity to simultaneously screen patients for osteoporosis, detecting notably higher rates of osteoporosis in men than the traditional tool of DXA, research published in the Journal of Bone and Mineral Research shows.
“Our large-scale, multicenter study of bone density measured from routine low-dose CT scans demonstrated the great potential of using low-dose CT for the opportunistic screening of osteoporosis as an alternative to standard DXA scans,” said senior author Wei Tian, MD, of the Chinese Academy of Engineering and Peking University, in a press statement from the journal.
“Our study revealed the unexpectedly high prevalence of osteoporosis in men, which may impact on the management strategy of men in the future,” Dr. Tian added.
Josephine Therkildsen, MD, of Herning Hospital, Denmark, who has conducted similar research using cardiac CT scans, said the findings add important new insights into the issue of opportunistic screening.
“The results are highly interesting, as they show that low-dose CT-based opportunistic screening could identify a substantial number of patients with low lumbar bone mineral density (BMD) with the future potential to diagnose osteoporosis and initiate relevant treatment before a fracture occurs,” she told this news organization.
Perry J. Pickhardt, MD, chief of gastrointestinal imaging at the University of Wisconsin School of Medicine and Public Health in Madison, agrees. He said in an interview that CT scans of the chest and abdomen, commonly performed for a variety of clinical indications and widespread in most developed countries, can in fact be essential for the detection of a multitude of other concerns – yet are underused for those other purposes.
Use of CT in this way “would likely be very cost effective and clinically efficacious,” he said, adding: “We are seeing greatly increased interest in leveraging this extra information that is contained within every CT scan.” And, “Importantly, artificial intelligence advances now allow for automated approaches, which should allow for expanded use.”
Lung cancer CT scans shed light on osteoporosis prevalence
In the study, led by Xiaoguang Cheng, MD, PhD, of the department of radiology, Beijing Jishuitan Hospital, China, researchers examined lung cancer CT screening data from the prospective China Biobank Project to determine the prevalence of osteoporosis in China.
This included the thoracic low-dose CT scans of 69,095 adults, including 40,733 men and 28,362 women, taken between 2018 and 2019.
To screen for osteoporosis, they used quantitative CT software to evaluate lumbar spine (L1-L2) trabecular volume BMD (vBMD) and diagnostic criteria from the American College of Radiology. Using the vBMD measures from the CT imaging, they found the prevalence of osteoporosis among those over 50 years of age in the Chinese population to be 29% for women (49 million) and 13.5% for men (22.8 million).
Interestingly, the osteoporosis prevalence rate among women was comparable to estimates in the population derived from DXA (29.1%); however, the rate in men was twice that estimated from DXA scans (6.5%).
Decreases in trabecular vBMD with age were observed in both genders. However, declines were steeper among women, who had higher peak trabecular vBMD (185.4 mg/cm3), compared with men (176.6 mg/cm3) at age 30-34 years, but significantly lower measures (62.4 mg/cm3) than men (92.1 mg/cm3) at age 80 years.
The prevalence of osteoporosis in women increased from 2.8% at age 50-54 years to 79.8% at age 85 or older, while in men, the prevalence was 3.2% at age 50-54 years and 44.1% at age 85 or older.
“This is the first study to establish Chinese reference data for vBMD using opportunistic screening from low-dose chest CT in a large population cohort,” the authors write.
“The opportunistic screening of osteoporosis using low-dose CT is clinically feasible and requires no additional exposure to ionizing radiation.”
In addition, no additional equipment or patient time was required, suggesting that “this approach has potential for opportunistic screening for osteoporosis.”
They note, however, that further cohort studies are needed to assess clinical utility of this method.
CT ‘likely a more accurate measure’ of volumetric BMD
Dr. Pickhardt said the differences in osteoporosis prevalence observed between DXA and CT-derived measures in men likely reflect the greater accuracy of CT.
“DXA is a planar technique with a number of drawbacks,” he said in an interview. “CT provides a more direct volumetric measure and is likely a more accurate method for BMD assessment.”
He speculated that the greater differences between DXA versus CT seen in men than women “may relate to sex differences in cortical bone of vertebral bodies, which cannot be separated from the underlying trabecular bone with DXA (whereas CT directly measures the inner trabecular bone).”
The authors note that, although areal BMD (aBMD) derived from DXA is required for osteoporosis diagnosis according to World Health Organization criteria, “trabecular vBMD derived from CT can be also used for diagnosis based on thresholds published by the American College of Radiology of 120 mg/cm3 and 80 mg/cm3 to define osteopenia and osteoporosis, respectively, thresholds that were subsequently confirmed for the Chinese population.”
Furthermore, vBMD has been shown in some studies to be more strongly related to fracture risk, compared with DXA aBMD measures.
Importantly, in another recent study involving 9,223 adults, Dr. Pickhardt and colleagues reported that bone and muscle biomarkers derived from CT were comparable to the Fracture Risk Assessment Tool score for the presymptomatic prediction of future osteoporotic fractures.
Dr. Pickhardt is an advisor to Bracco Imaging and Zebra Medical Vision. Dr. Therkildsen has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Low-dose chest CT for lung cancer screening provides the opportunity to simultaneously screen patients for osteoporosis, detecting notably higher rates of osteoporosis in men than the traditional tool of DXA, research published in the Journal of Bone and Mineral Research shows.
“Our large-scale, multicenter study of bone density measured from routine low-dose CT scans demonstrated the great potential of using low-dose CT for the opportunistic screening of osteoporosis as an alternative to standard DXA scans,” said senior author Wei Tian, MD, of the Chinese Academy of Engineering and Peking University, in a press statement from the journal.
“Our study revealed the unexpectedly high prevalence of osteoporosis in men, which may impact on the management strategy of men in the future,” Dr. Tian added.
Josephine Therkildsen, MD, of Herning Hospital, Denmark, who has conducted similar research using cardiac CT scans, said the findings add important new insights into the issue of opportunistic screening.
“The results are highly interesting, as they show that low-dose CT-based opportunistic screening could identify a substantial number of patients with low lumbar bone mineral density (BMD) with the future potential to diagnose osteoporosis and initiate relevant treatment before a fracture occurs,” she told this news organization.
Perry J. Pickhardt, MD, chief of gastrointestinal imaging at the University of Wisconsin School of Medicine and Public Health in Madison, agrees. He said in an interview that CT scans of the chest and abdomen, commonly performed for a variety of clinical indications and widespread in most developed countries, can in fact be essential for the detection of a multitude of other concerns – yet are underused for those other purposes.
Use of CT in this way “would likely be very cost effective and clinically efficacious,” he said, adding: “We are seeing greatly increased interest in leveraging this extra information that is contained within every CT scan.” And, “Importantly, artificial intelligence advances now allow for automated approaches, which should allow for expanded use.”
Lung cancer CT scans shed light on osteoporosis prevalence
In the study, led by Xiaoguang Cheng, MD, PhD, of the department of radiology, Beijing Jishuitan Hospital, China, researchers examined lung cancer CT screening data from the prospective China Biobank Project to determine the prevalence of osteoporosis in China.
This included the thoracic low-dose CT scans of 69,095 adults, including 40,733 men and 28,362 women, taken between 2018 and 2019.
To screen for osteoporosis, they used quantitative CT software to evaluate lumbar spine (L1-L2) trabecular volume BMD (vBMD) and diagnostic criteria from the American College of Radiology. Using the vBMD measures from the CT imaging, they found the prevalence of osteoporosis among those over 50 years of age in the Chinese population to be 29% for women (49 million) and 13.5% for men (22.8 million).
Interestingly, the osteoporosis prevalence rate among women was comparable to estimates in the population derived from DXA (29.1%); however, the rate in men was twice that estimated from DXA scans (6.5%).
Decreases in trabecular vBMD with age were observed in both genders. However, declines were steeper among women, who had higher peak trabecular vBMD (185.4 mg/cm3), compared with men (176.6 mg/cm3) at age 30-34 years, but significantly lower measures (62.4 mg/cm3) than men (92.1 mg/cm3) at age 80 years.
The prevalence of osteoporosis in women increased from 2.8% at age 50-54 years to 79.8% at age 85 or older, while in men, the prevalence was 3.2% at age 50-54 years and 44.1% at age 85 or older.
“This is the first study to establish Chinese reference data for vBMD using opportunistic screening from low-dose chest CT in a large population cohort,” the authors write.
“The opportunistic screening of osteoporosis using low-dose CT is clinically feasible and requires no additional exposure to ionizing radiation.”
In addition, no additional equipment or patient time was required, suggesting that “this approach has potential for opportunistic screening for osteoporosis.”
They note, however, that further cohort studies are needed to assess clinical utility of this method.
CT ‘likely a more accurate measure’ of volumetric BMD
Dr. Pickhardt said the differences in osteoporosis prevalence observed between DXA and CT-derived measures in men likely reflect the greater accuracy of CT.
“DXA is a planar technique with a number of drawbacks,” he said in an interview. “CT provides a more direct volumetric measure and is likely a more accurate method for BMD assessment.”
He speculated that the greater differences between DXA versus CT seen in men than women “may relate to sex differences in cortical bone of vertebral bodies, which cannot be separated from the underlying trabecular bone with DXA (whereas CT directly measures the inner trabecular bone).”
The authors note that, although areal BMD (aBMD) derived from DXA is required for osteoporosis diagnosis according to World Health Organization criteria, “trabecular vBMD derived from CT can be also used for diagnosis based on thresholds published by the American College of Radiology of 120 mg/cm3 and 80 mg/cm3 to define osteopenia and osteoporosis, respectively, thresholds that were subsequently confirmed for the Chinese population.”
Furthermore, vBMD has been shown in some studies to be more strongly related to fracture risk, compared with DXA aBMD measures.
Importantly, in another recent study involving 9,223 adults, Dr. Pickhardt and colleagues reported that bone and muscle biomarkers derived from CT were comparable to the Fracture Risk Assessment Tool score for the presymptomatic prediction of future osteoporotic fractures.
Dr. Pickhardt is an advisor to Bracco Imaging and Zebra Medical Vision. Dr. Therkildsen has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Low-dose chest CT for lung cancer screening provides the opportunity to simultaneously screen patients for osteoporosis, detecting notably higher rates of osteoporosis in men than the traditional tool of DXA, research published in the Journal of Bone and Mineral Research shows.
“Our large-scale, multicenter study of bone density measured from routine low-dose CT scans demonstrated the great potential of using low-dose CT for the opportunistic screening of osteoporosis as an alternative to standard DXA scans,” said senior author Wei Tian, MD, of the Chinese Academy of Engineering and Peking University, in a press statement from the journal.
“Our study revealed the unexpectedly high prevalence of osteoporosis in men, which may impact on the management strategy of men in the future,” Dr. Tian added.
Josephine Therkildsen, MD, of Herning Hospital, Denmark, who has conducted similar research using cardiac CT scans, said the findings add important new insights into the issue of opportunistic screening.
“The results are highly interesting, as they show that low-dose CT-based opportunistic screening could identify a substantial number of patients with low lumbar bone mineral density (BMD) with the future potential to diagnose osteoporosis and initiate relevant treatment before a fracture occurs,” she told this news organization.
Perry J. Pickhardt, MD, chief of gastrointestinal imaging at the University of Wisconsin School of Medicine and Public Health in Madison, agrees. He said in an interview that CT scans of the chest and abdomen, commonly performed for a variety of clinical indications and widespread in most developed countries, can in fact be essential for the detection of a multitude of other concerns – yet are underused for those other purposes.
Use of CT in this way “would likely be very cost effective and clinically efficacious,” he said, adding: “We are seeing greatly increased interest in leveraging this extra information that is contained within every CT scan.” And, “Importantly, artificial intelligence advances now allow for automated approaches, which should allow for expanded use.”
Lung cancer CT scans shed light on osteoporosis prevalence
In the study, led by Xiaoguang Cheng, MD, PhD, of the department of radiology, Beijing Jishuitan Hospital, China, researchers examined lung cancer CT screening data from the prospective China Biobank Project to determine the prevalence of osteoporosis in China.
This included the thoracic low-dose CT scans of 69,095 adults, including 40,733 men and 28,362 women, taken between 2018 and 2019.
To screen for osteoporosis, they used quantitative CT software to evaluate lumbar spine (L1-L2) trabecular volume BMD (vBMD) and diagnostic criteria from the American College of Radiology. Using the vBMD measures from the CT imaging, they found the prevalence of osteoporosis among those over 50 years of age in the Chinese population to be 29% for women (49 million) and 13.5% for men (22.8 million).
Interestingly, the osteoporosis prevalence rate among women was comparable to estimates in the population derived from DXA (29.1%); however, the rate in men was twice that estimated from DXA scans (6.5%).
Decreases in trabecular vBMD with age were observed in both genders. However, declines were steeper among women, who had higher peak trabecular vBMD (185.4 mg/cm3), compared with men (176.6 mg/cm3) at age 30-34 years, but significantly lower measures (62.4 mg/cm3) than men (92.1 mg/cm3) at age 80 years.
The prevalence of osteoporosis in women increased from 2.8% at age 50-54 years to 79.8% at age 85 or older, while in men, the prevalence was 3.2% at age 50-54 years and 44.1% at age 85 or older.
“This is the first study to establish Chinese reference data for vBMD using opportunistic screening from low-dose chest CT in a large population cohort,” the authors write.
“The opportunistic screening of osteoporosis using low-dose CT is clinically feasible and requires no additional exposure to ionizing radiation.”
In addition, no additional equipment or patient time was required, suggesting that “this approach has potential for opportunistic screening for osteoporosis.”
They note, however, that further cohort studies are needed to assess clinical utility of this method.
CT ‘likely a more accurate measure’ of volumetric BMD
Dr. Pickhardt said the differences in osteoporosis prevalence observed between DXA and CT-derived measures in men likely reflect the greater accuracy of CT.
“DXA is a planar technique with a number of drawbacks,” he said in an interview. “CT provides a more direct volumetric measure and is likely a more accurate method for BMD assessment.”
He speculated that the greater differences between DXA versus CT seen in men than women “may relate to sex differences in cortical bone of vertebral bodies, which cannot be separated from the underlying trabecular bone with DXA (whereas CT directly measures the inner trabecular bone).”
The authors note that, although areal BMD (aBMD) derived from DXA is required for osteoporosis diagnosis according to World Health Organization criteria, “trabecular vBMD derived from CT can be also used for diagnosis based on thresholds published by the American College of Radiology of 120 mg/cm3 and 80 mg/cm3 to define osteopenia and osteoporosis, respectively, thresholds that were subsequently confirmed for the Chinese population.”
Furthermore, vBMD has been shown in some studies to be more strongly related to fracture risk, compared with DXA aBMD measures.
Importantly, in another recent study involving 9,223 adults, Dr. Pickhardt and colleagues reported that bone and muscle biomarkers derived from CT were comparable to the Fracture Risk Assessment Tool score for the presymptomatic prediction of future osteoporotic fractures.
Dr. Pickhardt is an advisor to Bracco Imaging and Zebra Medical Vision. Dr. Therkildsen has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Improving Primary Care Fall Risk Management: Adoption of Practice Changes After a Geriatric Mini-Fellowship
From the Senior Health Program, Providence Health & Services, Oregon, Portland, OR.
Abstract
Background: Approximately 51 million adults in the United States are 65 years of age or older, yet few geriatric-trained primary care providers (PCP) serve this population. The Age-Friendly Health System framework, consisting of evidence-based 4M care (Mobility, Medication, Mentation, and what Matters), encourages all PCPs to assess mobility in older adults.
Objective: To improve PCP knowledge, confidence, and clinical practice in assessing and managing fall risk.
Methods: A 1-week educational session focusing on mobility (part of a 4-week Geriatric Mini-Fellowship) for 6 selected PCPs from a large health care system was conducted to increase knowledge and ability to address fall risk in older adults. The week included learning and practicing a Fall Risk Management Plan (FRMP) algorithm, including planning for their own practice changes. Pre- and post-test surveys assessed changes in knowledge and confidence. Patient data were compared 12 months before and after training to evaluate PCP adoption of FRMP components.
Results: The training increased provider knowledge and confidence. The trained PCPs were 1.7 times more likely to screen for fall risk; 3.6 times more likely to discuss fall risk; and 5.8 times more likely to assess orthostatic blood pressure in their 65+ patients after the mini-fellowship. In high-risk patients, they were 4.1 times more likely to discuss fall risk and 6.3 times more likely to assess orthostatic blood pressure than their nontrained peers. Changes in physical therapy referral rates were not observed.
Conclusions: In-depth, skills-based geriatric educational sessions improved PCPs’ knowledge and confidence and also improved their fall risk management practices for their older patients.
Keywords: geriatrics; guidelines; Age-Friendly Health System; 4M; workforce training; practice change; fellowship.
The US population is aging rapidly. People aged 85 years and older are the largest-growing segment of the US population, and this segment is expected to increase by 123% by 2040.1 Caregiving needs increase with age as older adults develop more chronic conditions, such as hypertension, heart disease, arthritis, and dementia. However, even with increasing morbidity and dependence, a majority of older adults still live in the community rather than in institutional settings.2 These older adults seek medical care more frequently than younger people, with about 22% of patients 75 years and older having 10 or more health care visits in the previous 12 months. By 2040, nearly a quarter of the US population is expected to be 65 or older, with many of these older adults seeking regular primary care from providers who do not have formal training in the care of a population with multiple complex, chronic health conditions and increased caregiving needs.1
Despite this growing demand for health care professionals trained in the care of older adults, access to these types of clinicians is limited. In 2018, there were roughly 7000 certified geriatricians, with only 3600 of them practicing full-time.3,4 Similarly, of 290,000 certified nurse practitioners (NPs), about 9% of them have geriatric certification.5 Geriatricians, medical doctors trained in the care of older adults, and geriatric-trained NPs are part of a cadre of a geriatric-trained workforce that provides unique expertise in caring for older adults with chronic and advanced illness. They know how to manage multiple, complex geriatric syndromes like falls, dementia, and polypharmacy; understand and maximize team-based care; and focus on caring for an older person with a goal-centered versus a disease-centered approach.6
Broadly, geriatric care includes a spectrum of adults, from those who are aging healthfully to those who are the frailest. Research has suggested that approximately 30% of older adults need care by a geriatric-trained clinician, with the oldest and frailest patients needing more clinician time for assessment and treatment, care coordination, and coaching of caregivers.7 With this assumption in mind, it is projected that by 2025, there will be a national shortage of 26,980 geriatricians, with the western United States disproportionately affected by this shortage.4Rather than lamenting this shortage, Tinetti recommends a new path forward: “Our mission should not be to train enough geriatricians to provide direct care, but rather to ensure that every clinician caring for older adults is competent in geriatric principles and practices.”8 Sometimes called ”geriatricizing,” the idea is to use existing geriatric providers as a small elite training force to infuse geriatric principles and skills across their colleagues in primary care and other disciplines.8,9 Efforts of the American Geriatrics Society (AGS), with support from the John A. Hartford Foundation (JAHF), have been successful in developing geriatric training across multiple specialties, including surgery, orthopedics, and emergency medicine (www.americangeriatrics.org/programs/geriatrics-specialists-initiative).
The Age-Friendly Health System and 4M Model
To help augment this idea of equipping health care systems and their clinicians with more readily available geriatric knowledge, skills, and tools, the JAHF, along with the Institute for Healthcare Improvement (IHI), created the Age-Friendly Health System (AFHS) paradigm in 2015.10 Using the 4M model, the AFHS initiative established a set of evidence-based geriatric priorities and interventions meant to improve the care of older adults, reduce harm and duplication, and provide a framework for engaging leadership, clinical teams, and operational systems across inpatient and ambulatory settings.11 Mobility, including fall risk screening and intervention, is 1 of the 4M foundational elements of the Age-Friendly model. In addition to Mobility, the 4M model also includes 3 other key geriatric domains: Mentation (dementia, depression, and delirium), Medication (high-risk medications, polypharmacy, and deprescribing), and What Matters (goals of care conversations and understanding quality of life for older patients).11 The 4M initiative encourages adoption of a geriatric lens that looks across chronic conditions and accounts for the interplay among geriatric syndromes, such as falls, cognitive impairment, and frailty, in order to provide care better tailored to what the patient needs and desires.12 IHI and JAHF have targeted the adoption of the 4M model by 20% of US health care systems by 2020.11
Mini-Fellowship and Mobility Week
To bolster geriatric skills among community-based primary care providers (PCPs), we initiated a Geriatric Mini-Fellowship, a 4-week condensed curriculum taught over 6 months. Each week focuses on 1 of the age-friendly 4Ms, with the goal of increasing the knowledge, self-efficacy, skills, and competencies of the participating PCPs (called “fellow” hereafter) and at the same time, equipping each to become a champion of geriatric practice. This article focuses on the Mobility week, the second week of the mini-fellowship, and the effect of the week on the fellows’ practice changes.
To construct the Mobility week’s curriculum with a focus on the ambulatory setting, we relied upon national evidence-based work in fall risk management. The Centers for Disease Control and Prevention (CDC) has made fall risk screening and management in primary care a high priority. Using the clinical practice guidelines for managing fall risk developed by the American and British Geriatrics Societies (AGS/BGS), the CDC developed the Stopping Elderly Accidents, Deaths, and Injuries (STEADI) toolkit.13 Foundational to the toolkit is the validated 12-item Stay Independent falls screening questionnaire (STEADI questionnaire).14 Patients who score 4 or higher (out of a total score of 14) on the questionnaire are considered at increased risk of falling. The CDC has developed a clinical algorithm that guides clinical teams through screening and assessment to help identify appropriate interventions to target specific risk factors. Research has clearly established that a multifactorial approach to fall risk intervention can be successful in reducing fall risk by as much as 25%.15-17
The significant morbidity and mortality caused by falls make training nongeriatrician clinicians on how to better address fall risk imperative. More than 25% of older adults fall each year.18 These falls contribute to rising rates of fall-related deaths,19 emergency department (ED) visits,20 and hospital readmissions.21 Initiatives like the AFHS focus on mobility and the CDC’s development of supporting clinical materials22 aim to improve primary care adoption of fall risk screening and intervention practices.23,24 The epidemic of falls must compel all PCPs, not just those practicing geriatrics, to make discussing and addressing fall risk and falls a priority.
Methods
Setting
This project took place as part of a regional primary care effort in Oregon. Providence Health & Services-Oregon is part of a multi-state integrated health care system in the western United States whose PCPs serve more than 80,000 patients aged 65 years and older per year; these patients comprise 38% of the system’s office visits each year. Regionally, there are 47 family and internal medicine clinics employing roughly 290 providers (physicians, NPs, and physician assistants). The organization has only 4 PCPs trained in geriatrics and does not offer any geriatric clinical consultation services. Six PCPs from different clinics, representing both rural and urban settings, are chosen to participate in the geriatric mini-fellowship each year.
This project was conducted as a quality improvement initiative within the organization and did not constitute human subjects research. It was not conducted under the oversight of the Institutional Review Board.
Intervention
The mini-fellowship was taught in 4 1-week blocks between April and October 2018, with a curriculum designed to be interactive and practical. The faculty was intentionally interdisciplinary to teach and model team-based practice. Each week participants were excused from their clinical practice. Approximately 160 hours of continuing medical education credits were awarded for the full mini-fellowship. As part of each weekly session, a performance improvement project (PIP) focused on that week’s topic (1 of the 4Ms) was developed by the fellow and their team members to incorporate the mini-fellowship learnings into their clinic workflows. Fellows also had 2 hours per week of dedicated administration time for a year, outside the fellowship, to work on their PIP and 4M practice changes within their clinic.
Provider Education
The week for mobility training comprised 4 daylong sessions. The first 2 days were spent learning about the epidemiology of falls; risk factors for falling; how to conduct a thorough history and assessment of fall risk; and how to create a prioritized Fall Risk Management Plan (FRMP) to decrease a patient’s individual fall risk through tailored interventions. The FRMP was adapted from the CDC STEADI toolkit.13 Core faculty were 2 geriatric-trained providers (NP and physician) and a physical therapist (PT) specializing in fall prevention.
On the third day, fellows took part in a simulated fall risk clinic, in which older adults volunteered to be patient partners, providing an opportunity to apply learnings from days 1 and 2. The clinic included the fellow observing a PT complete a mobility assessment and a pharmacist conduct a high-risk medication review. The fellow synthesized the findings of the mobility assessment and medication review, as well as their own history and assessment, to create a summary of fall risk recommendations to discuss with their volunteer patient partner. The fellows were observed and evaluated in their skills by their patient partner, course faculty, and another fellow. The patient partners, and their assigned fellow, also participated in a 45-minute fall risk presentation, led by a nurse.
On the fourth day, the fellows were joined by select clinic partners, including nurses, pharmacists, and/or medical assistants. The session included discussions among each fellow’s clinical team regarding the current state of fall risk efforts at their clinic, an analysis of barriers, and identification of opportunities to improve workflows and screening rates. Each fellow took with them an action plan tailored to their clinic to improve fall risk management practices, starting with the fellow’s own practice.
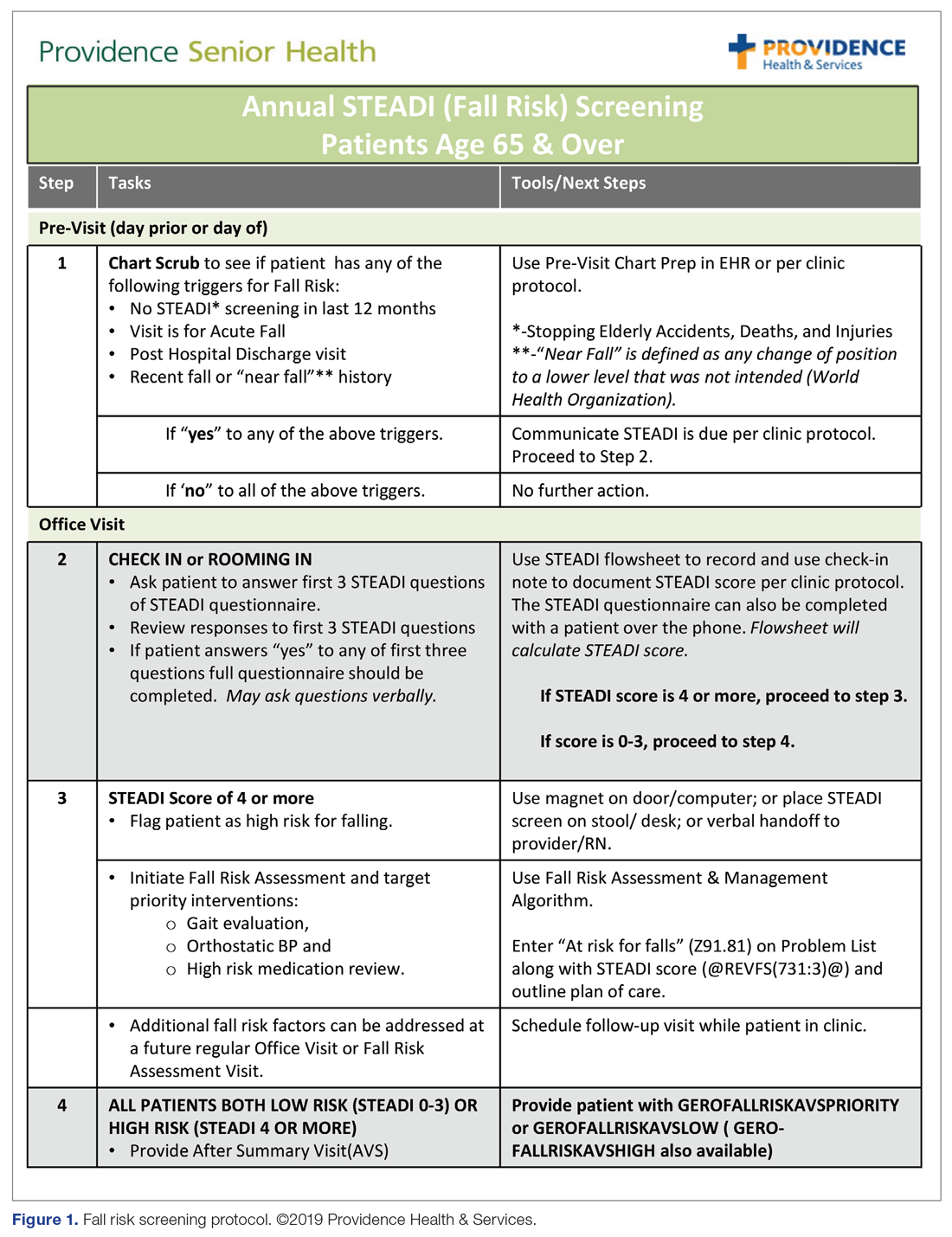
Fall Risk Management Plan
The educational sessions introduced the fellows to the FRMP. The FRMP, adapted from the STEADI toolkit, includes a process for fall risk screening (Figure 1) and stratifying a patient’s risk based on their STEADI score in order to promote 3 priority assessments (gait evaluation with PT referral if appropriate; orthostatic blood pressure; and high-risk medication review; Figure 2). Initial actions based on these priority assessments were followed over time, with additional fall risk interventions added as clinically indicated.25 The FRMP is intended to be used during routine office visits, Medicare annual wellness visits, or office visits focused on fall risk or related medical disorders (ie, fall risk visits.)
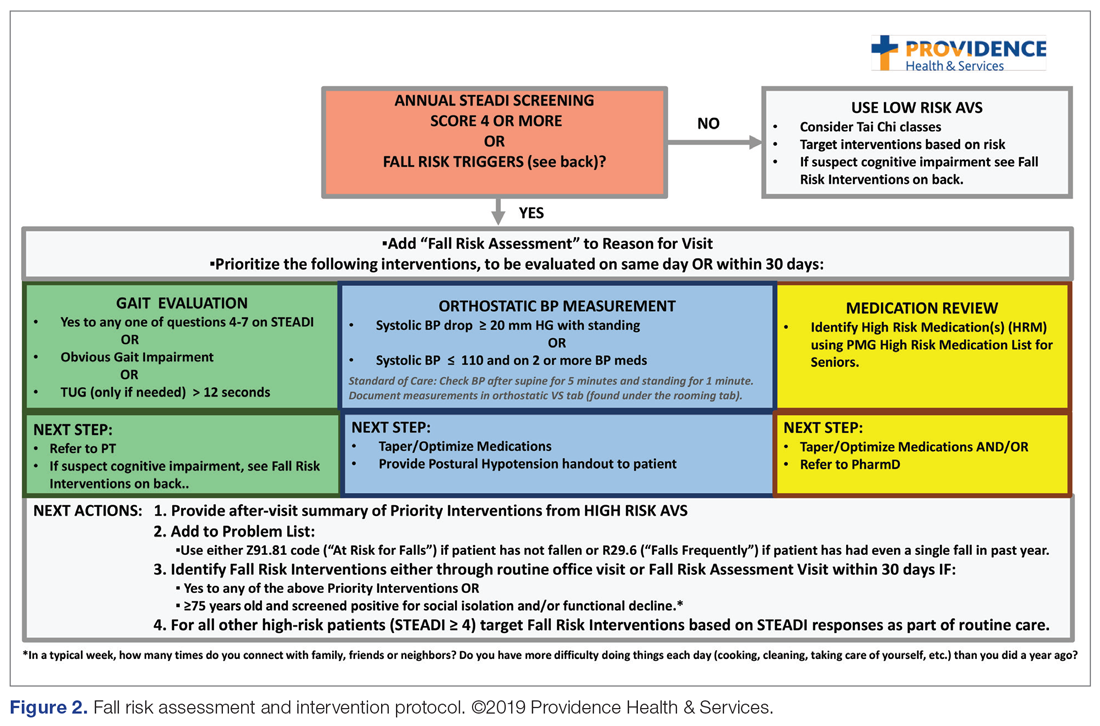
Providers and their teams were encouraged to spread out fall-related conversations with their patients over multiple visits, since many patients have multiple fall risk factors at play, in addition to other chronic medical issues, and since many interventions often require behavior changes on the part of the patient. Providers also had access to fall-related electronic health record (EHR) templates as well as a comprehensive, internal fall risk management website that included assessment tools, evidence-based resources, and patient handouts.
Assessment and Measurements
We assessed provider knowledge and comfort in their fall risk evaluation and management skills before and after the educational intervention using an 11-item multiple-choice questionnaire and a 4-item confidence questionnaire. The confidence questions used a 7-point Likert scale, with 0 indicating “no confidence” and 7 indicating ”lots of confidence.” The questions were administered via a paper survey. Qualitative comments were derived from evaluations completed at the end of the week.
The fellows’ practice of fall risk screening and management was studied from May 2018, at the completion of Mobility week, to May 2019 for the post-intervention period. A 1-year timeframe before May 2018 was used as the pre-intervention period. Eligible visit types, during which we assumed fall risk was discussed, were any office visits for patients 65+ completed by the patients’ PCPs that used fall risk as a reason for the visit or had a fall-related diagnosis code. Fall risk visits performed by other clinic providers were not counted.
Of those patients who had fall risk screenings completed and were determined to be high risk (STEADI score ≥ 4), data were analyzed to determine whether these patients had any fall-related follow-up visits to their PCP within 60 days of the STEADI screening. For these high-risk patients, data were studied to understand whether orthostatic blood pressure measurements were performed (as documented in a flowsheet) and whether a PT referral was placed. These data were compared with those from providers who practiced in clinics within the same system but who did not participate in the mini-fellowship. Data were obtained from the organization’s EHR. Additional data were measured to evaluate patterns of deprescribing of select high-risk medications, but these data are not included in this analysis.
Analysis
A paired-samples t test was used to measure changes in provider confidence levels. Data were aggregated across fellows, resulting in a mean. A chi-square test of independence was performed to examine the relationship between rates of FRMP adoption by select provider groups. Analysis included a pre- and post-intervention assessment of the fellows’ adoption of FRMP practices, as well as a comparison between the fellows’ practice patterns and those of a control group of PCPs in the organization’s other clinics who did not participate in the mini-fellowship (nontrained control group). Excluded from the control group were providers from the same clinic as the fellows; providers in clinics with a geriatric-trained provider on staff; and clinics outside of the Portland metro and Medford service areas. We used an alpha level of 0.05 for all statistical tests.
Data from 5 providers were included in the analysis of the FRMP adoption. The sixth provider changed practice settings from the clinic to the ED after completing the fellowship; her patient data were not included in the FRMP part of the analysis. EHR data included data on all visits of patients 65+, as well as data for just those 65+ patients who had been identified as being at high risk to fall based on a STEADI score of 4 or higher.
Results
Provider Questionnaire
All 6 providers responded to the pre-intervention and post-intervention tests. For the knowledge questions, fellows, as a composite, correctly answered 57% of the questions before the intervention and 79% after the intervention. Provider confidence level in delivering fall risk care was measured prior to the training (mean, 4.12 [SD, 0.62]) and at the end of the training (mean, 6.47 [SD, 0.45]), demonstrating a significant increase in confidence (t (5) = –10.46, P < 0.001).
Qualitative Comments
Providers also had the opportunity to provide comments on their experience during the Mobility week and at the end of 1 year. In general, the simulated interdisciplinary fall risk clinic was highly rated (“the highlight of the week”) as a practical strategy to embed learning principles. One fellow commented, “Putting the learning into practice helps solidify it in my brain.” Fellows also appreciated the opportunity to learn and meet with their clinic colleagues to begin work on a fall-risk focused PIP and to “have a framework for what to do for people who screen positive [for fall risk].”
FRMP Adoption
A comparison of the care the fellows provided to their patients 65+ in the 12 months pre- and post-training shows the fellows demonstrated significant changes in practice patterns. The fellows were 1.7 times more likely to screen for fall risk; 3.6 times more likely to discuss fall risk; and 5.8 times more likely to check orthostatic blood pressure than prior to the mini-fellowship (Table 1). 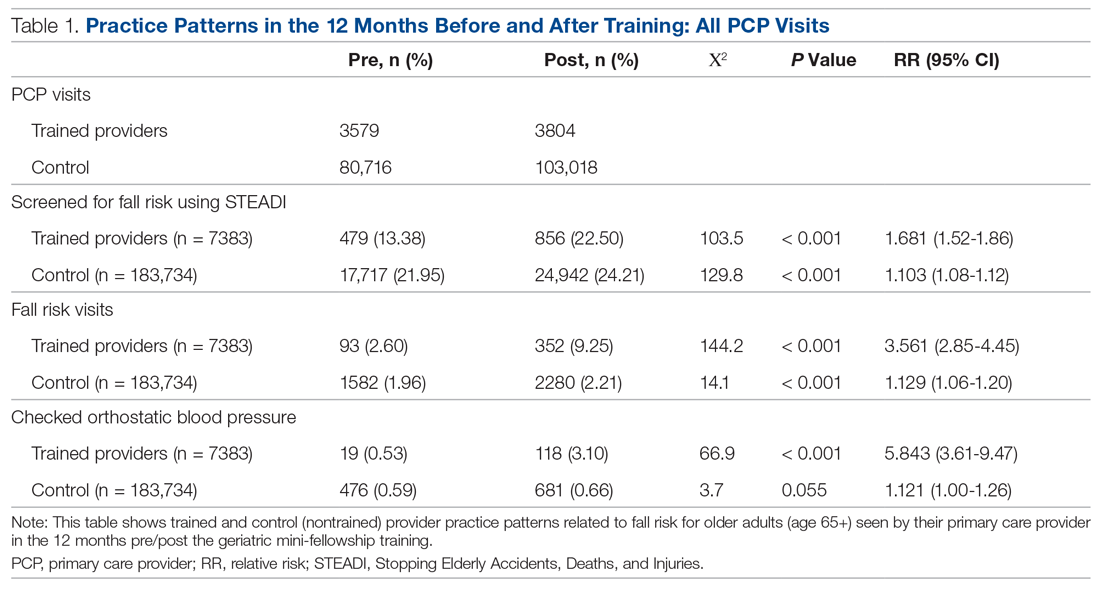
The control providers also demonstrated significant increases in fall risk screening and discussion of fall risk between the pre- and post-intervention periods; however, the relative risk (RR) was between 1.10 and 1.13 for this group. For the control group, checking orthostatic blood pressure did not significantly change. In the 12 months after training (Table 2), the fellows were 4.2 times more likely to discuss fall risk and almost 5 times more likely to check orthostatic blood pressure than their nontrained peers for all of their patients 65+, regardless of their risk to fall.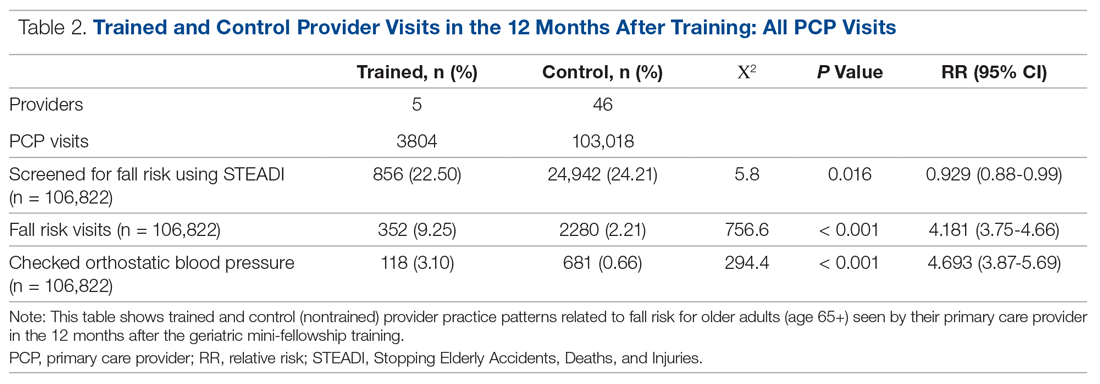
As shown in Table 3, for those patients determined to be at high risk of falling (STEADI score ≥ 4), fellows showed statistically significant increases in fall risk visits (RR, 3.02) and assessment of orthostatic blood pressure (RR, 10.68) before and after the mini-fellowship. The control providers did not show any changes in practice patterns between the pre- and post-period among patients at high risk to fall. 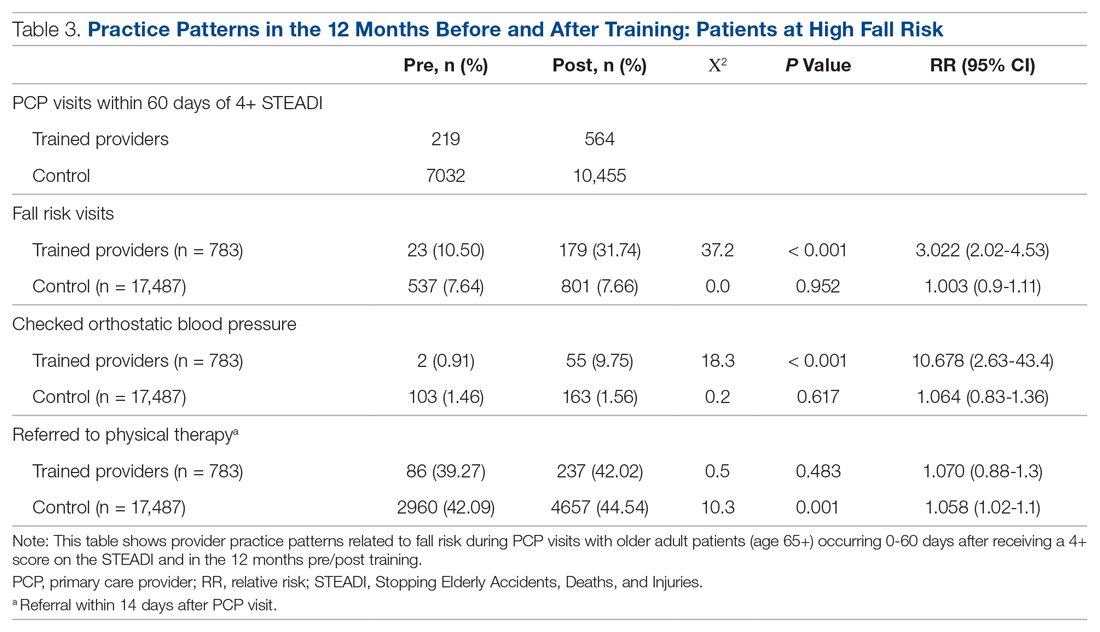
Neither the fellows nor the control group showed changes in patterns of referral to PT. In comparing the 2 groups in the 12 months after training (Table 4), for their patients at risk of falling, the fellows were 4 times more likely to complete fall risk visits and over 6 times more likely to assess orthostatic blood pressure than their nontrained peers. Subgroup analysis of the 75+ population revealed similar trends and significance, but these results are not included here.
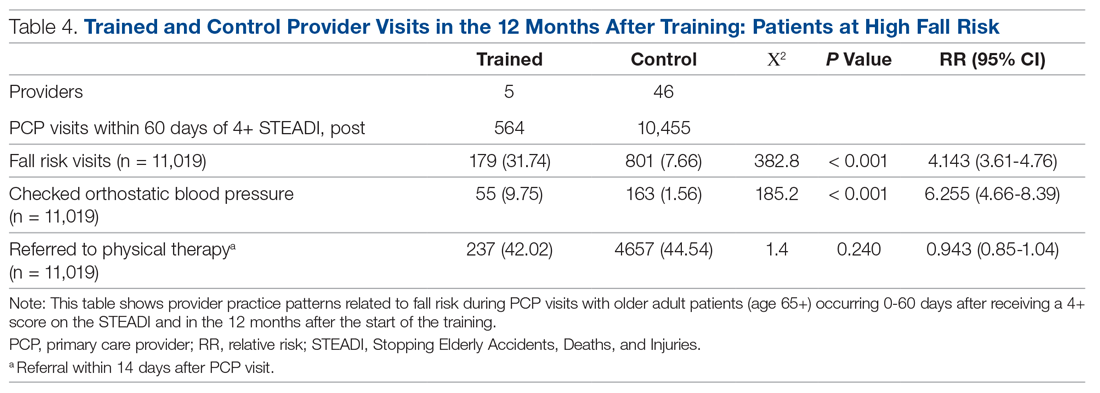
Discussion
This study aimed to improve not only providers’ knowledge and confidence in caring for older adults at increased risk to fall, but also their clinical practice in assessing and managing fall risk. In addition to improved knowledge and confidence, we found that the fellows increased their discussion of fall risk (through fall risk visits) and their assessment of orthostatic blood pressure for all of their patients, not just for those identified at increased risk to fall. This improvement held true for the fellows themselves before and after the intervention, but also as compared to their nontrained peers. These practice improvements for all of their 65+ patients, not just those identified as being at high risk to fall, are especially important, since studies indicate that early screening and intervention can help identify people at risk and prevent future falls.15
We were surprised that there were no significant differences in PT referrals made by the trained fellows, but this finding may have been confounded by the fact that the data included all PT referrals, regardless of diagnosis, not just those referrals that were fall-related. Furthermore, our baseline PT referral rates, at 39% for the intervention group and 42% for the control group, are higher than national data when looking at rehabilitation use by older adults.26
In comparison to a study evaluating the occurrence of fall risk–related clinical practice in primary care before any fall-related educational intervention, orthostatics were checked less frequently in our study (10% versus 30%) and there were fewer PT referrals (42%–44% versus 53%).27 However, the Phelan study took place in patients who had actually had a fall, rather than just having a higher risk for a fall, and was based on detailed chart review. Other studies23,24 found higher rates of fall risk interventions, but did not break out PT referrals specifically.
In terms of the educational intervention itself, most studies of geriatric education interventions have measured changes in knowledge, confidence, or self-efficacy as they relate to geriatric competence,28-30 and do not measure practice change as an outcome outside of intent to change or self-reported practice change.31,32 In general, practice change or longer-term health care–related outcomes have not been studied. Additionally, a range of dosages of educational interventions has been studied, from 1-hour lunchtime presentations23,32 to half-day29 or several half-day workshops,28 up to 160 hours over 10 months30 or 5 weekends over 6 months.31 The duration of our entire intervention at 160 hours over 6 months would be considered on the upper end of dosing relative to these studies, with our Mobility week intervention comprising 32 hours during 1 week. In the Warshaw study, despite 107 1-hour sessions being taught to over 60 physicians in 16 practices over 4 years, only 2 practices ultimately initiated any practice change projects.32 We believe that only curricula that embed practice change skills and opportunities, at a significant enough dose, can actually impact practice change in a sustainable manner.
Knowledge and skill acquisition among individual providers does not take place to a sufficient degree in the current health care arena, which is focused on productivity and short visit times. Consistent with other studies, we included interdisciplinary members of the primary care team for part of the mini-fellowship, although other studies used models that train across disciplines for the entirety of the learning experience.28-30,33 Our educational model was strengthened by including other professionals to provide some of the education and model the ideal geriatric team, including PT, occupational therapy, and pharmacy, for the week on mobility.
Most studies exploring interventions through geriatric educational initiatives are conducted within academic institutions, with a primary focus on physician faculty and, by extension, their teaching of residents and others.34,35 We believe our integrated model, which is steeped in community-based primary care practices like Lam’s,31 offers the greatest outreach to large community-based care systems and their patients. Training providers to work with their teams to change their own practices first gives skills and expertise that help further establish them as geriatric champions within their practices, laying the groundwork for more widespread practice change at their clinics.
Limitations
In addition to the limitations described above relating to the capture of PT referrals, other limitations included the relatively short time period for follow-up data as well as the small size of the intervention group. However, we found value in the instructional depth that the small group size allowed.
While the nontrained providers did show some improvement during the same period, we believe the relative risk was not clinically significant. We suspect that the larger health system efforts to standardize screening of patients 65+ across all clinics as a core quality metric confounded these results. The data analysis also included only fall-related patient visits that occurred with a provider who was that patient’s PCP, which could have missed visits done by other PCP colleagues, RNs, or pharmacists in the same clinic, thus undercounting the true number of fall-related visits. Furthermore, counting of fall-related interventions relied upon providers documenting consistently in the EHR, which could also lead to under-represention of fall risk clinical efforts.
The data presented, while encouraging, do not reflect clinic-wide practice change patterns and are considered only proximate outcomes rather than more long-term or cost-related outcomes, as would be captured by fall-related utilization measures like emergency room visits and hospitalizations. We expect to evaluate the broader impact and these value-based outcomes in the future. All providers and teams were from the same health care system, which may not allow our results to transfer to other organizations or regions of clinical practice.
Summary
This study demonstrates that an intensive mini-fellowship model of geriatrics training improved both knowledge and confidence in the realm of fall risk assessment and intervention among PCPs who had not been formally trained in geriatrics. More importantly, the training improved the fall-related care of their patients at increased risk to fall, but also of all of their older patients, with improvements in care measured up to a year after the mini-fellowship. Although this article only describes the work done as part of the Mobility aim of the 4M AFHS model, we believe the entire mini-fellowship curriculum offers the opportunity to “geriatricize” clinicians and their teams in learning geriatric principles and skills that they can translate into their practice in a sustainable way, as Tinetti encourages.8 Future study to evaluate other process outcomes more precisely, such as PT, as well as cost- and value-based outcomes, and the influence of trained providers on their clinic partners, will further establish the value proposition of targeted, disseminated, intensive geriatrics training of primary care clinicians as a strategy of age-friendly health systems as they work to improve the care of their older adults.
Acknowledgment: We are grateful for the dedication and hard work of the 2018 Geriatric Mini-Fellowship fellows at Providence Health & Services-Oregon who made this article possible. Thanks to Drs. Stephanie Cha, Emily Puukka-Clark, Laurie Dutkiewicz, Cara Ellis, Deb Frost, Jordan Roth, and Subhechchha Shah for promoting the AFHS work within their Providence Medical Group clinics and to PMG leadership and the fellows’ clinical teams for supporting the fellows, the AFHS work, and their older patients.
Corresponding author: Colleen M. Casey, PhD, ANP-BC, Providence Health & Services, Senior Health Program, 4400 NE Halsey, 5th Floor, Portland, OR 97213; [email protected].
Financial disclosures: None.
1. US Department of Health and Human Services. 2018 Profile of Older Americans. Administration on Aging. April 2018.
2. Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA. The population 65 years and older in the United States: 2016. Washington, DC: US Census Bureau; 2018.
3. American Board of Medicine Specialties. 2017-2018 ABMS Board Certification Report. https://www.abms.org/board-certification/abms-board-certification-report/. Accessed November 3, 2020.
4. US Department of Health and Human Services, Health Resources and Services Administration, National Center for Health Workforce Analysis. National and regional projections of supply and demand for geriatricians: 2013-2025. Rockville, MD: US Department of Health and Human Services; 2007.
5. American Association of Nurse Practitioners, NP Facts: The Voice of the Nurse Practitioner. 2020. https://storage.aanp.org/www/documents/NPFacts__080420.pdf.
6. Tinetti ME, Naik AD, Dodson JA, Moving from disease-centered to patient goals-directed care for patients with multiple chronic conditions: patient value-based care. JAMA Cardiol. 2016;1:9-10.
7. Fried LP, Hall WJ. Editorial: leading on behalf of an aging society. J Am Geriatr Soc. 2008;56:1791-1795.
8. Tinetti M. Mainstream or extinction: can defining who we are save geriatrics? J Am Geriatr Soc. 2016;64:1400-1404.
9. Jafari P, Kostas T, Levine S, et al. ECHO-Chicago Geriatrics: using telementoring to “geriatricize” the primary care workforce. Gerontol Geriatr Educ. 2020;41:333-341.
10. Fulmer T, Mate KS, Berman A. The Age-Friendly Health System imperative. J Am Geriatr Soc. 2018;66:22-24.
11. Mate KS, Berman A, Laderman M, et al. Creating Age-Friendly Health Systems - A vision for better care of older adults. Healthc (Amst). 2018;6:4-6.
12. Tinetti ME, et al. Patient priority-directed decision making and care for older adults with multiple chronic conditions. Clin Geriatr Med. 2016;32:261-275.
13. Stevens JA, Phelan EA. Development of STEADI: a fall prevention resource for health care providers. Health Promot Pract. 2013;14:706-714.
14. Rubenstein LZ, et al. Validating an evidence-based, self-rated fall risk questionnaire (FRQ) for older adults. J Safety Res. 2011;42:493-499.
15. Grossman DC, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319: 1696-1704.
16. Tricco AC, Thomas SM, Veroniki AA, et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta-analysis. JAMA. 2017;318:1687-1699.
17. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012(9):CD007146.
18. Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:993-998.
19. Burns E, Kakara R. Deaths from falls among persons aged >=65 Years - United States, 2007-2016. MMWR Morb Mortal Wkly Rep. 2018;67:509-514.
20. Shankar KN, Liu SW, Ganz DA. Trends and characteristics of emergency department visits for fall-related injuries in older adults, 2003-2010. West J Emerg Med. 2017;18:785-793.
21. Hoffman GJ, et al. Posthospital fall injuries and 30-day readmissions in adults 65 years and older. JAMA Netw Open. 2019;2:e194276.
22. Eckstrom E, Parker EM, Shakya I, Lee R. Coordinated care plan to prevent older adult falls. 2018. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2018.
23. Eckstrom E, Parker EM, Lambert GH, et al. Implementing STEADI in academic primary care to address older adult fall risk. Innov Aging. 2017;1:igx028.
24. Johnston YA, Bergen G, Bauer M, et al. Implementation of the stopping elderly accidents, deaths, and injuries initiative in primary care: an outcome evaluation. Gerontologist. 2019;59:1182-1191.
25. Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am. 2015;99:281-293.
26. Gell NM, Mroz TM, Patel KV. Rehabilitation services use and patient-reported outcomes among older adults in the United States. Arch Phys Med Rehabil. 2017;98:2221-2227.e3.
27. Phelan EA, Aerts S, Dowler D, et al. Adoption of evidence-based fall prevention practices in primary care for older adults with a history of falls. Front Public Health. 2016;4:190.
28. Solberg LB, Carter CS, Solberg LM. Geriatric care boot camp series: interprofessional education for a new training paradigm. Geriatr Nurs. 2019;40:579-583.
29. Solberg LB, Solberg LM, Carter CS. Geriatric care boot cAMP: an interprofessional education program for healthcare professionals. J Am Geriatr Soc. 2015;63:997-1001.
30. Coogle CL, Hackett L, Owens MG, et al. Perceived self-efficacy gains following an interprofessional faculty development programme in geriatrics education. J Interprof Care. 2016;30:483-492.
31. Lam R, Lee L, Tazkarji B, et al. Five-weekend care of the elderly certificate course: continuing professional development activity for family physicians. Can Fam Physician. 2015;61:e135-141.
32. Warshaw GA, Modawal A, Kues J, et al. Community physician education in geriatrics: applying the assessing care of vulnerable elders model with a multisite primary care group. J Am Geriatr Soc. 2010;58:1780-1785.
33. Solai LK, Kumar K, Mulvaney E, et al. Geriatric mental healthcare training: a mini-fellowship approach to interprofessional assessment and management of geriatric mental health issues. Am J Geriatr Psychiatry. 2019;27:706-711.
34. Christmas C, Park E, Schmaltz H, et al. A model intensive course in geriatric teaching for non-geriatrician educators. J Gen Intern Med. 2008;23:1048-1052.
35. Heflin MT, Bragg EJ, Fernandez H, et al. The Donald W. Reynolds Consortium for Faculty Development to Advance Geriatrics Education (FD~AGE): a model for dissemination of subspecialty educational expertise. Acad Med. 2012;87:618-626.
From the Senior Health Program, Providence Health & Services, Oregon, Portland, OR.
Abstract
Background: Approximately 51 million adults in the United States are 65 years of age or older, yet few geriatric-trained primary care providers (PCP) serve this population. The Age-Friendly Health System framework, consisting of evidence-based 4M care (Mobility, Medication, Mentation, and what Matters), encourages all PCPs to assess mobility in older adults.
Objective: To improve PCP knowledge, confidence, and clinical practice in assessing and managing fall risk.
Methods: A 1-week educational session focusing on mobility (part of a 4-week Geriatric Mini-Fellowship) for 6 selected PCPs from a large health care system was conducted to increase knowledge and ability to address fall risk in older adults. The week included learning and practicing a Fall Risk Management Plan (FRMP) algorithm, including planning for their own practice changes. Pre- and post-test surveys assessed changes in knowledge and confidence. Patient data were compared 12 months before and after training to evaluate PCP adoption of FRMP components.
Results: The training increased provider knowledge and confidence. The trained PCPs were 1.7 times more likely to screen for fall risk; 3.6 times more likely to discuss fall risk; and 5.8 times more likely to assess orthostatic blood pressure in their 65+ patients after the mini-fellowship. In high-risk patients, they were 4.1 times more likely to discuss fall risk and 6.3 times more likely to assess orthostatic blood pressure than their nontrained peers. Changes in physical therapy referral rates were not observed.
Conclusions: In-depth, skills-based geriatric educational sessions improved PCPs’ knowledge and confidence and also improved their fall risk management practices for their older patients.
Keywords: geriatrics; guidelines; Age-Friendly Health System; 4M; workforce training; practice change; fellowship.
The US population is aging rapidly. People aged 85 years and older are the largest-growing segment of the US population, and this segment is expected to increase by 123% by 2040.1 Caregiving needs increase with age as older adults develop more chronic conditions, such as hypertension, heart disease, arthritis, and dementia. However, even with increasing morbidity and dependence, a majority of older adults still live in the community rather than in institutional settings.2 These older adults seek medical care more frequently than younger people, with about 22% of patients 75 years and older having 10 or more health care visits in the previous 12 months. By 2040, nearly a quarter of the US population is expected to be 65 or older, with many of these older adults seeking regular primary care from providers who do not have formal training in the care of a population with multiple complex, chronic health conditions and increased caregiving needs.1
Despite this growing demand for health care professionals trained in the care of older adults, access to these types of clinicians is limited. In 2018, there were roughly 7000 certified geriatricians, with only 3600 of them practicing full-time.3,4 Similarly, of 290,000 certified nurse practitioners (NPs), about 9% of them have geriatric certification.5 Geriatricians, medical doctors trained in the care of older adults, and geriatric-trained NPs are part of a cadre of a geriatric-trained workforce that provides unique expertise in caring for older adults with chronic and advanced illness. They know how to manage multiple, complex geriatric syndromes like falls, dementia, and polypharmacy; understand and maximize team-based care; and focus on caring for an older person with a goal-centered versus a disease-centered approach.6
Broadly, geriatric care includes a spectrum of adults, from those who are aging healthfully to those who are the frailest. Research has suggested that approximately 30% of older adults need care by a geriatric-trained clinician, with the oldest and frailest patients needing more clinician time for assessment and treatment, care coordination, and coaching of caregivers.7 With this assumption in mind, it is projected that by 2025, there will be a national shortage of 26,980 geriatricians, with the western United States disproportionately affected by this shortage.4Rather than lamenting this shortage, Tinetti recommends a new path forward: “Our mission should not be to train enough geriatricians to provide direct care, but rather to ensure that every clinician caring for older adults is competent in geriatric principles and practices.”8 Sometimes called ”geriatricizing,” the idea is to use existing geriatric providers as a small elite training force to infuse geriatric principles and skills across their colleagues in primary care and other disciplines.8,9 Efforts of the American Geriatrics Society (AGS), with support from the John A. Hartford Foundation (JAHF), have been successful in developing geriatric training across multiple specialties, including surgery, orthopedics, and emergency medicine (www.americangeriatrics.org/programs/geriatrics-specialists-initiative).
The Age-Friendly Health System and 4M Model
To help augment this idea of equipping health care systems and their clinicians with more readily available geriatric knowledge, skills, and tools, the JAHF, along with the Institute for Healthcare Improvement (IHI), created the Age-Friendly Health System (AFHS) paradigm in 2015.10 Using the 4M model, the AFHS initiative established a set of evidence-based geriatric priorities and interventions meant to improve the care of older adults, reduce harm and duplication, and provide a framework for engaging leadership, clinical teams, and operational systems across inpatient and ambulatory settings.11 Mobility, including fall risk screening and intervention, is 1 of the 4M foundational elements of the Age-Friendly model. In addition to Mobility, the 4M model also includes 3 other key geriatric domains: Mentation (dementia, depression, and delirium), Medication (high-risk medications, polypharmacy, and deprescribing), and What Matters (goals of care conversations and understanding quality of life for older patients).11 The 4M initiative encourages adoption of a geriatric lens that looks across chronic conditions and accounts for the interplay among geriatric syndromes, such as falls, cognitive impairment, and frailty, in order to provide care better tailored to what the patient needs and desires.12 IHI and JAHF have targeted the adoption of the 4M model by 20% of US health care systems by 2020.11
Mini-Fellowship and Mobility Week
To bolster geriatric skills among community-based primary care providers (PCPs), we initiated a Geriatric Mini-Fellowship, a 4-week condensed curriculum taught over 6 months. Each week focuses on 1 of the age-friendly 4Ms, with the goal of increasing the knowledge, self-efficacy, skills, and competencies of the participating PCPs (called “fellow” hereafter) and at the same time, equipping each to become a champion of geriatric practice. This article focuses on the Mobility week, the second week of the mini-fellowship, and the effect of the week on the fellows’ practice changes.
To construct the Mobility week’s curriculum with a focus on the ambulatory setting, we relied upon national evidence-based work in fall risk management. The Centers for Disease Control and Prevention (CDC) has made fall risk screening and management in primary care a high priority. Using the clinical practice guidelines for managing fall risk developed by the American and British Geriatrics Societies (AGS/BGS), the CDC developed the Stopping Elderly Accidents, Deaths, and Injuries (STEADI) toolkit.13 Foundational to the toolkit is the validated 12-item Stay Independent falls screening questionnaire (STEADI questionnaire).14 Patients who score 4 or higher (out of a total score of 14) on the questionnaire are considered at increased risk of falling. The CDC has developed a clinical algorithm that guides clinical teams through screening and assessment to help identify appropriate interventions to target specific risk factors. Research has clearly established that a multifactorial approach to fall risk intervention can be successful in reducing fall risk by as much as 25%.15-17
The significant morbidity and mortality caused by falls make training nongeriatrician clinicians on how to better address fall risk imperative. More than 25% of older adults fall each year.18 These falls contribute to rising rates of fall-related deaths,19 emergency department (ED) visits,20 and hospital readmissions.21 Initiatives like the AFHS focus on mobility and the CDC’s development of supporting clinical materials22 aim to improve primary care adoption of fall risk screening and intervention practices.23,24 The epidemic of falls must compel all PCPs, not just those practicing geriatrics, to make discussing and addressing fall risk and falls a priority.
Methods
Setting
This project took place as part of a regional primary care effort in Oregon. Providence Health & Services-Oregon is part of a multi-state integrated health care system in the western United States whose PCPs serve more than 80,000 patients aged 65 years and older per year; these patients comprise 38% of the system’s office visits each year. Regionally, there are 47 family and internal medicine clinics employing roughly 290 providers (physicians, NPs, and physician assistants). The organization has only 4 PCPs trained in geriatrics and does not offer any geriatric clinical consultation services. Six PCPs from different clinics, representing both rural and urban settings, are chosen to participate in the geriatric mini-fellowship each year.
This project was conducted as a quality improvement initiative within the organization and did not constitute human subjects research. It was not conducted under the oversight of the Institutional Review Board.
Intervention
The mini-fellowship was taught in 4 1-week blocks between April and October 2018, with a curriculum designed to be interactive and practical. The faculty was intentionally interdisciplinary to teach and model team-based practice. Each week participants were excused from their clinical practice. Approximately 160 hours of continuing medical education credits were awarded for the full mini-fellowship. As part of each weekly session, a performance improvement project (PIP) focused on that week’s topic (1 of the 4Ms) was developed by the fellow and their team members to incorporate the mini-fellowship learnings into their clinic workflows. Fellows also had 2 hours per week of dedicated administration time for a year, outside the fellowship, to work on their PIP and 4M practice changes within their clinic.
Provider Education
The week for mobility training comprised 4 daylong sessions. The first 2 days were spent learning about the epidemiology of falls; risk factors for falling; how to conduct a thorough history and assessment of fall risk; and how to create a prioritized Fall Risk Management Plan (FRMP) to decrease a patient’s individual fall risk through tailored interventions. The FRMP was adapted from the CDC STEADI toolkit.13 Core faculty were 2 geriatric-trained providers (NP and physician) and a physical therapist (PT) specializing in fall prevention.
On the third day, fellows took part in a simulated fall risk clinic, in which older adults volunteered to be patient partners, providing an opportunity to apply learnings from days 1 and 2. The clinic included the fellow observing a PT complete a mobility assessment and a pharmacist conduct a high-risk medication review. The fellow synthesized the findings of the mobility assessment and medication review, as well as their own history and assessment, to create a summary of fall risk recommendations to discuss with their volunteer patient partner. The fellows were observed and evaluated in their skills by their patient partner, course faculty, and another fellow. The patient partners, and their assigned fellow, also participated in a 45-minute fall risk presentation, led by a nurse.
On the fourth day, the fellows were joined by select clinic partners, including nurses, pharmacists, and/or medical assistants. The session included discussions among each fellow’s clinical team regarding the current state of fall risk efforts at their clinic, an analysis of barriers, and identification of opportunities to improve workflows and screening rates. Each fellow took with them an action plan tailored to their clinic to improve fall risk management practices, starting with the fellow’s own practice.

Fall Risk Management Plan
The educational sessions introduced the fellows to the FRMP. The FRMP, adapted from the STEADI toolkit, includes a process for fall risk screening (Figure 1) and stratifying a patient’s risk based on their STEADI score in order to promote 3 priority assessments (gait evaluation with PT referral if appropriate; orthostatic blood pressure; and high-risk medication review; Figure 2). Initial actions based on these priority assessments were followed over time, with additional fall risk interventions added as clinically indicated.25 The FRMP is intended to be used during routine office visits, Medicare annual wellness visits, or office visits focused on fall risk or related medical disorders (ie, fall risk visits.)

Providers and their teams were encouraged to spread out fall-related conversations with their patients over multiple visits, since many patients have multiple fall risk factors at play, in addition to other chronic medical issues, and since many interventions often require behavior changes on the part of the patient. Providers also had access to fall-related electronic health record (EHR) templates as well as a comprehensive, internal fall risk management website that included assessment tools, evidence-based resources, and patient handouts.
Assessment and Measurements
We assessed provider knowledge and comfort in their fall risk evaluation and management skills before and after the educational intervention using an 11-item multiple-choice questionnaire and a 4-item confidence questionnaire. The confidence questions used a 7-point Likert scale, with 0 indicating “no confidence” and 7 indicating ”lots of confidence.” The questions were administered via a paper survey. Qualitative comments were derived from evaluations completed at the end of the week.
The fellows’ practice of fall risk screening and management was studied from May 2018, at the completion of Mobility week, to May 2019 for the post-intervention period. A 1-year timeframe before May 2018 was used as the pre-intervention period. Eligible visit types, during which we assumed fall risk was discussed, were any office visits for patients 65+ completed by the patients’ PCPs that used fall risk as a reason for the visit or had a fall-related diagnosis code. Fall risk visits performed by other clinic providers were not counted.
Of those patients who had fall risk screenings completed and were determined to be high risk (STEADI score ≥ 4), data were analyzed to determine whether these patients had any fall-related follow-up visits to their PCP within 60 days of the STEADI screening. For these high-risk patients, data were studied to understand whether orthostatic blood pressure measurements were performed (as documented in a flowsheet) and whether a PT referral was placed. These data were compared with those from providers who practiced in clinics within the same system but who did not participate in the mini-fellowship. Data were obtained from the organization’s EHR. Additional data were measured to evaluate patterns of deprescribing of select high-risk medications, but these data are not included in this analysis.
Analysis
A paired-samples t test was used to measure changes in provider confidence levels. Data were aggregated across fellows, resulting in a mean. A chi-square test of independence was performed to examine the relationship between rates of FRMP adoption by select provider groups. Analysis included a pre- and post-intervention assessment of the fellows’ adoption of FRMP practices, as well as a comparison between the fellows’ practice patterns and those of a control group of PCPs in the organization’s other clinics who did not participate in the mini-fellowship (nontrained control group). Excluded from the control group were providers from the same clinic as the fellows; providers in clinics with a geriatric-trained provider on staff; and clinics outside of the Portland metro and Medford service areas. We used an alpha level of 0.05 for all statistical tests.
Data from 5 providers were included in the analysis of the FRMP adoption. The sixth provider changed practice settings from the clinic to the ED after completing the fellowship; her patient data were not included in the FRMP part of the analysis. EHR data included data on all visits of patients 65+, as well as data for just those 65+ patients who had been identified as being at high risk to fall based on a STEADI score of 4 or higher.
Results
Provider Questionnaire
All 6 providers responded to the pre-intervention and post-intervention tests. For the knowledge questions, fellows, as a composite, correctly answered 57% of the questions before the intervention and 79% after the intervention. Provider confidence level in delivering fall risk care was measured prior to the training (mean, 4.12 [SD, 0.62]) and at the end of the training (mean, 6.47 [SD, 0.45]), demonstrating a significant increase in confidence (t (5) = –10.46, P < 0.001).
Qualitative Comments
Providers also had the opportunity to provide comments on their experience during the Mobility week and at the end of 1 year. In general, the simulated interdisciplinary fall risk clinic was highly rated (“the highlight of the week”) as a practical strategy to embed learning principles. One fellow commented, “Putting the learning into practice helps solidify it in my brain.” Fellows also appreciated the opportunity to learn and meet with their clinic colleagues to begin work on a fall-risk focused PIP and to “have a framework for what to do for people who screen positive [for fall risk].”
FRMP Adoption
A comparison of the care the fellows provided to their patients 65+ in the 12 months pre- and post-training shows the fellows demonstrated significant changes in practice patterns. The fellows were 1.7 times more likely to screen for fall risk; 3.6 times more likely to discuss fall risk; and 5.8 times more likely to check orthostatic blood pressure than prior to the mini-fellowship (Table 1). 
The control providers also demonstrated significant increases in fall risk screening and discussion of fall risk between the pre- and post-intervention periods; however, the relative risk (RR) was between 1.10 and 1.13 for this group. For the control group, checking orthostatic blood pressure did not significantly change. In the 12 months after training (Table 2), the fellows were 4.2 times more likely to discuss fall risk and almost 5 times more likely to check orthostatic blood pressure than their nontrained peers for all of their patients 65+, regardless of their risk to fall.
As shown in Table 3, for those patients determined to be at high risk of falling (STEADI score ≥ 4), fellows showed statistically significant increases in fall risk visits (RR, 3.02) and assessment of orthostatic blood pressure (RR, 10.68) before and after the mini-fellowship. The control providers did not show any changes in practice patterns between the pre- and post-period among patients at high risk to fall. 
Neither the fellows nor the control group showed changes in patterns of referral to PT. In comparing the 2 groups in the 12 months after training (Table 4), for their patients at risk of falling, the fellows were 4 times more likely to complete fall risk visits and over 6 times more likely to assess orthostatic blood pressure than their nontrained peers. Subgroup analysis of the 75+ population revealed similar trends and significance, but these results are not included here.

Discussion
This study aimed to improve not only providers’ knowledge and confidence in caring for older adults at increased risk to fall, but also their clinical practice in assessing and managing fall risk. In addition to improved knowledge and confidence, we found that the fellows increased their discussion of fall risk (through fall risk visits) and their assessment of orthostatic blood pressure for all of their patients, not just for those identified at increased risk to fall. This improvement held true for the fellows themselves before and after the intervention, but also as compared to their nontrained peers. These practice improvements for all of their 65+ patients, not just those identified as being at high risk to fall, are especially important, since studies indicate that early screening and intervention can help identify people at risk and prevent future falls.15
We were surprised that there were no significant differences in PT referrals made by the trained fellows, but this finding may have been confounded by the fact that the data included all PT referrals, regardless of diagnosis, not just those referrals that were fall-related. Furthermore, our baseline PT referral rates, at 39% for the intervention group and 42% for the control group, are higher than national data when looking at rehabilitation use by older adults.26
In comparison to a study evaluating the occurrence of fall risk–related clinical practice in primary care before any fall-related educational intervention, orthostatics were checked less frequently in our study (10% versus 30%) and there were fewer PT referrals (42%–44% versus 53%).27 However, the Phelan study took place in patients who had actually had a fall, rather than just having a higher risk for a fall, and was based on detailed chart review. Other studies23,24 found higher rates of fall risk interventions, but did not break out PT referrals specifically.
In terms of the educational intervention itself, most studies of geriatric education interventions have measured changes in knowledge, confidence, or self-efficacy as they relate to geriatric competence,28-30 and do not measure practice change as an outcome outside of intent to change or self-reported practice change.31,32 In general, practice change or longer-term health care–related outcomes have not been studied. Additionally, a range of dosages of educational interventions has been studied, from 1-hour lunchtime presentations23,32 to half-day29 or several half-day workshops,28 up to 160 hours over 10 months30 or 5 weekends over 6 months.31 The duration of our entire intervention at 160 hours over 6 months would be considered on the upper end of dosing relative to these studies, with our Mobility week intervention comprising 32 hours during 1 week. In the Warshaw study, despite 107 1-hour sessions being taught to over 60 physicians in 16 practices over 4 years, only 2 practices ultimately initiated any practice change projects.32 We believe that only curricula that embed practice change skills and opportunities, at a significant enough dose, can actually impact practice change in a sustainable manner.
Knowledge and skill acquisition among individual providers does not take place to a sufficient degree in the current health care arena, which is focused on productivity and short visit times. Consistent with other studies, we included interdisciplinary members of the primary care team for part of the mini-fellowship, although other studies used models that train across disciplines for the entirety of the learning experience.28-30,33 Our educational model was strengthened by including other professionals to provide some of the education and model the ideal geriatric team, including PT, occupational therapy, and pharmacy, for the week on mobility.
Most studies exploring interventions through geriatric educational initiatives are conducted within academic institutions, with a primary focus on physician faculty and, by extension, their teaching of residents and others.34,35 We believe our integrated model, which is steeped in community-based primary care practices like Lam’s,31 offers the greatest outreach to large community-based care systems and their patients. Training providers to work with their teams to change their own practices first gives skills and expertise that help further establish them as geriatric champions within their practices, laying the groundwork for more widespread practice change at their clinics.
Limitations
In addition to the limitations described above relating to the capture of PT referrals, other limitations included the relatively short time period for follow-up data as well as the small size of the intervention group. However, we found value in the instructional depth that the small group size allowed.
While the nontrained providers did show some improvement during the same period, we believe the relative risk was not clinically significant. We suspect that the larger health system efforts to standardize screening of patients 65+ across all clinics as a core quality metric confounded these results. The data analysis also included only fall-related patient visits that occurred with a provider who was that patient’s PCP, which could have missed visits done by other PCP colleagues, RNs, or pharmacists in the same clinic, thus undercounting the true number of fall-related visits. Furthermore, counting of fall-related interventions relied upon providers documenting consistently in the EHR, which could also lead to under-represention of fall risk clinical efforts.
The data presented, while encouraging, do not reflect clinic-wide practice change patterns and are considered only proximate outcomes rather than more long-term or cost-related outcomes, as would be captured by fall-related utilization measures like emergency room visits and hospitalizations. We expect to evaluate the broader impact and these value-based outcomes in the future. All providers and teams were from the same health care system, which may not allow our results to transfer to other organizations or regions of clinical practice.
Summary
This study demonstrates that an intensive mini-fellowship model of geriatrics training improved both knowledge and confidence in the realm of fall risk assessment and intervention among PCPs who had not been formally trained in geriatrics. More importantly, the training improved the fall-related care of their patients at increased risk to fall, but also of all of their older patients, with improvements in care measured up to a year after the mini-fellowship. Although this article only describes the work done as part of the Mobility aim of the 4M AFHS model, we believe the entire mini-fellowship curriculum offers the opportunity to “geriatricize” clinicians and their teams in learning geriatric principles and skills that they can translate into their practice in a sustainable way, as Tinetti encourages.8 Future study to evaluate other process outcomes more precisely, such as PT, as well as cost- and value-based outcomes, and the influence of trained providers on their clinic partners, will further establish the value proposition of targeted, disseminated, intensive geriatrics training of primary care clinicians as a strategy of age-friendly health systems as they work to improve the care of their older adults.
Acknowledgment: We are grateful for the dedication and hard work of the 2018 Geriatric Mini-Fellowship fellows at Providence Health & Services-Oregon who made this article possible. Thanks to Drs. Stephanie Cha, Emily Puukka-Clark, Laurie Dutkiewicz, Cara Ellis, Deb Frost, Jordan Roth, and Subhechchha Shah for promoting the AFHS work within their Providence Medical Group clinics and to PMG leadership and the fellows’ clinical teams for supporting the fellows, the AFHS work, and their older patients.
Corresponding author: Colleen M. Casey, PhD, ANP-BC, Providence Health & Services, Senior Health Program, 4400 NE Halsey, 5th Floor, Portland, OR 97213; [email protected].
Financial disclosures: None.
From the Senior Health Program, Providence Health & Services, Oregon, Portland, OR.
Abstract
Background: Approximately 51 million adults in the United States are 65 years of age or older, yet few geriatric-trained primary care providers (PCP) serve this population. The Age-Friendly Health System framework, consisting of evidence-based 4M care (Mobility, Medication, Mentation, and what Matters), encourages all PCPs to assess mobility in older adults.
Objective: To improve PCP knowledge, confidence, and clinical practice in assessing and managing fall risk.
Methods: A 1-week educational session focusing on mobility (part of a 4-week Geriatric Mini-Fellowship) for 6 selected PCPs from a large health care system was conducted to increase knowledge and ability to address fall risk in older adults. The week included learning and practicing a Fall Risk Management Plan (FRMP) algorithm, including planning for their own practice changes. Pre- and post-test surveys assessed changes in knowledge and confidence. Patient data were compared 12 months before and after training to evaluate PCP adoption of FRMP components.
Results: The training increased provider knowledge and confidence. The trained PCPs were 1.7 times more likely to screen for fall risk; 3.6 times more likely to discuss fall risk; and 5.8 times more likely to assess orthostatic blood pressure in their 65+ patients after the mini-fellowship. In high-risk patients, they were 4.1 times more likely to discuss fall risk and 6.3 times more likely to assess orthostatic blood pressure than their nontrained peers. Changes in physical therapy referral rates were not observed.
Conclusions: In-depth, skills-based geriatric educational sessions improved PCPs’ knowledge and confidence and also improved their fall risk management practices for their older patients.
Keywords: geriatrics; guidelines; Age-Friendly Health System; 4M; workforce training; practice change; fellowship.
The US population is aging rapidly. People aged 85 years and older are the largest-growing segment of the US population, and this segment is expected to increase by 123% by 2040.1 Caregiving needs increase with age as older adults develop more chronic conditions, such as hypertension, heart disease, arthritis, and dementia. However, even with increasing morbidity and dependence, a majority of older adults still live in the community rather than in institutional settings.2 These older adults seek medical care more frequently than younger people, with about 22% of patients 75 years and older having 10 or more health care visits in the previous 12 months. By 2040, nearly a quarter of the US population is expected to be 65 or older, with many of these older adults seeking regular primary care from providers who do not have formal training in the care of a population with multiple complex, chronic health conditions and increased caregiving needs.1
Despite this growing demand for health care professionals trained in the care of older adults, access to these types of clinicians is limited. In 2018, there were roughly 7000 certified geriatricians, with only 3600 of them practicing full-time.3,4 Similarly, of 290,000 certified nurse practitioners (NPs), about 9% of them have geriatric certification.5 Geriatricians, medical doctors trained in the care of older adults, and geriatric-trained NPs are part of a cadre of a geriatric-trained workforce that provides unique expertise in caring for older adults with chronic and advanced illness. They know how to manage multiple, complex geriatric syndromes like falls, dementia, and polypharmacy; understand and maximize team-based care; and focus on caring for an older person with a goal-centered versus a disease-centered approach.6
Broadly, geriatric care includes a spectrum of adults, from those who are aging healthfully to those who are the frailest. Research has suggested that approximately 30% of older adults need care by a geriatric-trained clinician, with the oldest and frailest patients needing more clinician time for assessment and treatment, care coordination, and coaching of caregivers.7 With this assumption in mind, it is projected that by 2025, there will be a national shortage of 26,980 geriatricians, with the western United States disproportionately affected by this shortage.4Rather than lamenting this shortage, Tinetti recommends a new path forward: “Our mission should not be to train enough geriatricians to provide direct care, but rather to ensure that every clinician caring for older adults is competent in geriatric principles and practices.”8 Sometimes called ”geriatricizing,” the idea is to use existing geriatric providers as a small elite training force to infuse geriatric principles and skills across their colleagues in primary care and other disciplines.8,9 Efforts of the American Geriatrics Society (AGS), with support from the John A. Hartford Foundation (JAHF), have been successful in developing geriatric training across multiple specialties, including surgery, orthopedics, and emergency medicine (www.americangeriatrics.org/programs/geriatrics-specialists-initiative).
The Age-Friendly Health System and 4M Model
To help augment this idea of equipping health care systems and their clinicians with more readily available geriatric knowledge, skills, and tools, the JAHF, along with the Institute for Healthcare Improvement (IHI), created the Age-Friendly Health System (AFHS) paradigm in 2015.10 Using the 4M model, the AFHS initiative established a set of evidence-based geriatric priorities and interventions meant to improve the care of older adults, reduce harm and duplication, and provide a framework for engaging leadership, clinical teams, and operational systems across inpatient and ambulatory settings.11 Mobility, including fall risk screening and intervention, is 1 of the 4M foundational elements of the Age-Friendly model. In addition to Mobility, the 4M model also includes 3 other key geriatric domains: Mentation (dementia, depression, and delirium), Medication (high-risk medications, polypharmacy, and deprescribing), and What Matters (goals of care conversations and understanding quality of life for older patients).11 The 4M initiative encourages adoption of a geriatric lens that looks across chronic conditions and accounts for the interplay among geriatric syndromes, such as falls, cognitive impairment, and frailty, in order to provide care better tailored to what the patient needs and desires.12 IHI and JAHF have targeted the adoption of the 4M model by 20% of US health care systems by 2020.11
Mini-Fellowship and Mobility Week
To bolster geriatric skills among community-based primary care providers (PCPs), we initiated a Geriatric Mini-Fellowship, a 4-week condensed curriculum taught over 6 months. Each week focuses on 1 of the age-friendly 4Ms, with the goal of increasing the knowledge, self-efficacy, skills, and competencies of the participating PCPs (called “fellow” hereafter) and at the same time, equipping each to become a champion of geriatric practice. This article focuses on the Mobility week, the second week of the mini-fellowship, and the effect of the week on the fellows’ practice changes.
To construct the Mobility week’s curriculum with a focus on the ambulatory setting, we relied upon national evidence-based work in fall risk management. The Centers for Disease Control and Prevention (CDC) has made fall risk screening and management in primary care a high priority. Using the clinical practice guidelines for managing fall risk developed by the American and British Geriatrics Societies (AGS/BGS), the CDC developed the Stopping Elderly Accidents, Deaths, and Injuries (STEADI) toolkit.13 Foundational to the toolkit is the validated 12-item Stay Independent falls screening questionnaire (STEADI questionnaire).14 Patients who score 4 or higher (out of a total score of 14) on the questionnaire are considered at increased risk of falling. The CDC has developed a clinical algorithm that guides clinical teams through screening and assessment to help identify appropriate interventions to target specific risk factors. Research has clearly established that a multifactorial approach to fall risk intervention can be successful in reducing fall risk by as much as 25%.15-17
The significant morbidity and mortality caused by falls make training nongeriatrician clinicians on how to better address fall risk imperative. More than 25% of older adults fall each year.18 These falls contribute to rising rates of fall-related deaths,19 emergency department (ED) visits,20 and hospital readmissions.21 Initiatives like the AFHS focus on mobility and the CDC’s development of supporting clinical materials22 aim to improve primary care adoption of fall risk screening and intervention practices.23,24 The epidemic of falls must compel all PCPs, not just those practicing geriatrics, to make discussing and addressing fall risk and falls a priority.
Methods
Setting
This project took place as part of a regional primary care effort in Oregon. Providence Health & Services-Oregon is part of a multi-state integrated health care system in the western United States whose PCPs serve more than 80,000 patients aged 65 years and older per year; these patients comprise 38% of the system’s office visits each year. Regionally, there are 47 family and internal medicine clinics employing roughly 290 providers (physicians, NPs, and physician assistants). The organization has only 4 PCPs trained in geriatrics and does not offer any geriatric clinical consultation services. Six PCPs from different clinics, representing both rural and urban settings, are chosen to participate in the geriatric mini-fellowship each year.
This project was conducted as a quality improvement initiative within the organization and did not constitute human subjects research. It was not conducted under the oversight of the Institutional Review Board.
Intervention
The mini-fellowship was taught in 4 1-week blocks between April and October 2018, with a curriculum designed to be interactive and practical. The faculty was intentionally interdisciplinary to teach and model team-based practice. Each week participants were excused from their clinical practice. Approximately 160 hours of continuing medical education credits were awarded for the full mini-fellowship. As part of each weekly session, a performance improvement project (PIP) focused on that week’s topic (1 of the 4Ms) was developed by the fellow and their team members to incorporate the mini-fellowship learnings into their clinic workflows. Fellows also had 2 hours per week of dedicated administration time for a year, outside the fellowship, to work on their PIP and 4M practice changes within their clinic.
Provider Education
The week for mobility training comprised 4 daylong sessions. The first 2 days were spent learning about the epidemiology of falls; risk factors for falling; how to conduct a thorough history and assessment of fall risk; and how to create a prioritized Fall Risk Management Plan (FRMP) to decrease a patient’s individual fall risk through tailored interventions. The FRMP was adapted from the CDC STEADI toolkit.13 Core faculty were 2 geriatric-trained providers (NP and physician) and a physical therapist (PT) specializing in fall prevention.
On the third day, fellows took part in a simulated fall risk clinic, in which older adults volunteered to be patient partners, providing an opportunity to apply learnings from days 1 and 2. The clinic included the fellow observing a PT complete a mobility assessment and a pharmacist conduct a high-risk medication review. The fellow synthesized the findings of the mobility assessment and medication review, as well as their own history and assessment, to create a summary of fall risk recommendations to discuss with their volunteer patient partner. The fellows were observed and evaluated in their skills by their patient partner, course faculty, and another fellow. The patient partners, and their assigned fellow, also participated in a 45-minute fall risk presentation, led by a nurse.
On the fourth day, the fellows were joined by select clinic partners, including nurses, pharmacists, and/or medical assistants. The session included discussions among each fellow’s clinical team regarding the current state of fall risk efforts at their clinic, an analysis of barriers, and identification of opportunities to improve workflows and screening rates. Each fellow took with them an action plan tailored to their clinic to improve fall risk management practices, starting with the fellow’s own practice.

Fall Risk Management Plan
The educational sessions introduced the fellows to the FRMP. The FRMP, adapted from the STEADI toolkit, includes a process for fall risk screening (Figure 1) and stratifying a patient’s risk based on their STEADI score in order to promote 3 priority assessments (gait evaluation with PT referral if appropriate; orthostatic blood pressure; and high-risk medication review; Figure 2). Initial actions based on these priority assessments were followed over time, with additional fall risk interventions added as clinically indicated.25 The FRMP is intended to be used during routine office visits, Medicare annual wellness visits, or office visits focused on fall risk or related medical disorders (ie, fall risk visits.)

Providers and their teams were encouraged to spread out fall-related conversations with their patients over multiple visits, since many patients have multiple fall risk factors at play, in addition to other chronic medical issues, and since many interventions often require behavior changes on the part of the patient. Providers also had access to fall-related electronic health record (EHR) templates as well as a comprehensive, internal fall risk management website that included assessment tools, evidence-based resources, and patient handouts.
Assessment and Measurements
We assessed provider knowledge and comfort in their fall risk evaluation and management skills before and after the educational intervention using an 11-item multiple-choice questionnaire and a 4-item confidence questionnaire. The confidence questions used a 7-point Likert scale, with 0 indicating “no confidence” and 7 indicating ”lots of confidence.” The questions were administered via a paper survey. Qualitative comments were derived from evaluations completed at the end of the week.
The fellows’ practice of fall risk screening and management was studied from May 2018, at the completion of Mobility week, to May 2019 for the post-intervention period. A 1-year timeframe before May 2018 was used as the pre-intervention period. Eligible visit types, during which we assumed fall risk was discussed, were any office visits for patients 65+ completed by the patients’ PCPs that used fall risk as a reason for the visit or had a fall-related diagnosis code. Fall risk visits performed by other clinic providers were not counted.
Of those patients who had fall risk screenings completed and were determined to be high risk (STEADI score ≥ 4), data were analyzed to determine whether these patients had any fall-related follow-up visits to their PCP within 60 days of the STEADI screening. For these high-risk patients, data were studied to understand whether orthostatic blood pressure measurements were performed (as documented in a flowsheet) and whether a PT referral was placed. These data were compared with those from providers who practiced in clinics within the same system but who did not participate in the mini-fellowship. Data were obtained from the organization’s EHR. Additional data were measured to evaluate patterns of deprescribing of select high-risk medications, but these data are not included in this analysis.
Analysis
A paired-samples t test was used to measure changes in provider confidence levels. Data were aggregated across fellows, resulting in a mean. A chi-square test of independence was performed to examine the relationship between rates of FRMP adoption by select provider groups. Analysis included a pre- and post-intervention assessment of the fellows’ adoption of FRMP practices, as well as a comparison between the fellows’ practice patterns and those of a control group of PCPs in the organization’s other clinics who did not participate in the mini-fellowship (nontrained control group). Excluded from the control group were providers from the same clinic as the fellows; providers in clinics with a geriatric-trained provider on staff; and clinics outside of the Portland metro and Medford service areas. We used an alpha level of 0.05 for all statistical tests.
Data from 5 providers were included in the analysis of the FRMP adoption. The sixth provider changed practice settings from the clinic to the ED after completing the fellowship; her patient data were not included in the FRMP part of the analysis. EHR data included data on all visits of patients 65+, as well as data for just those 65+ patients who had been identified as being at high risk to fall based on a STEADI score of 4 or higher.
Results
Provider Questionnaire
All 6 providers responded to the pre-intervention and post-intervention tests. For the knowledge questions, fellows, as a composite, correctly answered 57% of the questions before the intervention and 79% after the intervention. Provider confidence level in delivering fall risk care was measured prior to the training (mean, 4.12 [SD, 0.62]) and at the end of the training (mean, 6.47 [SD, 0.45]), demonstrating a significant increase in confidence (t (5) = –10.46, P < 0.001).
Qualitative Comments
Providers also had the opportunity to provide comments on their experience during the Mobility week and at the end of 1 year. In general, the simulated interdisciplinary fall risk clinic was highly rated (“the highlight of the week”) as a practical strategy to embed learning principles. One fellow commented, “Putting the learning into practice helps solidify it in my brain.” Fellows also appreciated the opportunity to learn and meet with their clinic colleagues to begin work on a fall-risk focused PIP and to “have a framework for what to do for people who screen positive [for fall risk].”
FRMP Adoption
A comparison of the care the fellows provided to their patients 65+ in the 12 months pre- and post-training shows the fellows demonstrated significant changes in practice patterns. The fellows were 1.7 times more likely to screen for fall risk; 3.6 times more likely to discuss fall risk; and 5.8 times more likely to check orthostatic blood pressure than prior to the mini-fellowship (Table 1). 
The control providers also demonstrated significant increases in fall risk screening and discussion of fall risk between the pre- and post-intervention periods; however, the relative risk (RR) was between 1.10 and 1.13 for this group. For the control group, checking orthostatic blood pressure did not significantly change. In the 12 months after training (Table 2), the fellows were 4.2 times more likely to discuss fall risk and almost 5 times more likely to check orthostatic blood pressure than their nontrained peers for all of their patients 65+, regardless of their risk to fall.
As shown in Table 3, for those patients determined to be at high risk of falling (STEADI score ≥ 4), fellows showed statistically significant increases in fall risk visits (RR, 3.02) and assessment of orthostatic blood pressure (RR, 10.68) before and after the mini-fellowship. The control providers did not show any changes in practice patterns between the pre- and post-period among patients at high risk to fall. 
Neither the fellows nor the control group showed changes in patterns of referral to PT. In comparing the 2 groups in the 12 months after training (Table 4), for their patients at risk of falling, the fellows were 4 times more likely to complete fall risk visits and over 6 times more likely to assess orthostatic blood pressure than their nontrained peers. Subgroup analysis of the 75+ population revealed similar trends and significance, but these results are not included here.

Discussion
This study aimed to improve not only providers’ knowledge and confidence in caring for older adults at increased risk to fall, but also their clinical practice in assessing and managing fall risk. In addition to improved knowledge and confidence, we found that the fellows increased their discussion of fall risk (through fall risk visits) and their assessment of orthostatic blood pressure for all of their patients, not just for those identified at increased risk to fall. This improvement held true for the fellows themselves before and after the intervention, but also as compared to their nontrained peers. These practice improvements for all of their 65+ patients, not just those identified as being at high risk to fall, are especially important, since studies indicate that early screening and intervention can help identify people at risk and prevent future falls.15
We were surprised that there were no significant differences in PT referrals made by the trained fellows, but this finding may have been confounded by the fact that the data included all PT referrals, regardless of diagnosis, not just those referrals that were fall-related. Furthermore, our baseline PT referral rates, at 39% for the intervention group and 42% for the control group, are higher than national data when looking at rehabilitation use by older adults.26
In comparison to a study evaluating the occurrence of fall risk–related clinical practice in primary care before any fall-related educational intervention, orthostatics were checked less frequently in our study (10% versus 30%) and there were fewer PT referrals (42%–44% versus 53%).27 However, the Phelan study took place in patients who had actually had a fall, rather than just having a higher risk for a fall, and was based on detailed chart review. Other studies23,24 found higher rates of fall risk interventions, but did not break out PT referrals specifically.
In terms of the educational intervention itself, most studies of geriatric education interventions have measured changes in knowledge, confidence, or self-efficacy as they relate to geriatric competence,28-30 and do not measure practice change as an outcome outside of intent to change or self-reported practice change.31,32 In general, practice change or longer-term health care–related outcomes have not been studied. Additionally, a range of dosages of educational interventions has been studied, from 1-hour lunchtime presentations23,32 to half-day29 or several half-day workshops,28 up to 160 hours over 10 months30 or 5 weekends over 6 months.31 The duration of our entire intervention at 160 hours over 6 months would be considered on the upper end of dosing relative to these studies, with our Mobility week intervention comprising 32 hours during 1 week. In the Warshaw study, despite 107 1-hour sessions being taught to over 60 physicians in 16 practices over 4 years, only 2 practices ultimately initiated any practice change projects.32 We believe that only curricula that embed practice change skills and opportunities, at a significant enough dose, can actually impact practice change in a sustainable manner.
Knowledge and skill acquisition among individual providers does not take place to a sufficient degree in the current health care arena, which is focused on productivity and short visit times. Consistent with other studies, we included interdisciplinary members of the primary care team for part of the mini-fellowship, although other studies used models that train across disciplines for the entirety of the learning experience.28-30,33 Our educational model was strengthened by including other professionals to provide some of the education and model the ideal geriatric team, including PT, occupational therapy, and pharmacy, for the week on mobility.
Most studies exploring interventions through geriatric educational initiatives are conducted within academic institutions, with a primary focus on physician faculty and, by extension, their teaching of residents and others.34,35 We believe our integrated model, which is steeped in community-based primary care practices like Lam’s,31 offers the greatest outreach to large community-based care systems and their patients. Training providers to work with their teams to change their own practices first gives skills and expertise that help further establish them as geriatric champions within their practices, laying the groundwork for more widespread practice change at their clinics.
Limitations
In addition to the limitations described above relating to the capture of PT referrals, other limitations included the relatively short time period for follow-up data as well as the small size of the intervention group. However, we found value in the instructional depth that the small group size allowed.
While the nontrained providers did show some improvement during the same period, we believe the relative risk was not clinically significant. We suspect that the larger health system efforts to standardize screening of patients 65+ across all clinics as a core quality metric confounded these results. The data analysis also included only fall-related patient visits that occurred with a provider who was that patient’s PCP, which could have missed visits done by other PCP colleagues, RNs, or pharmacists in the same clinic, thus undercounting the true number of fall-related visits. Furthermore, counting of fall-related interventions relied upon providers documenting consistently in the EHR, which could also lead to under-represention of fall risk clinical efforts.
The data presented, while encouraging, do not reflect clinic-wide practice change patterns and are considered only proximate outcomes rather than more long-term or cost-related outcomes, as would be captured by fall-related utilization measures like emergency room visits and hospitalizations. We expect to evaluate the broader impact and these value-based outcomes in the future. All providers and teams were from the same health care system, which may not allow our results to transfer to other organizations or regions of clinical practice.
Summary
This study demonstrates that an intensive mini-fellowship model of geriatrics training improved both knowledge and confidence in the realm of fall risk assessment and intervention among PCPs who had not been formally trained in geriatrics. More importantly, the training improved the fall-related care of their patients at increased risk to fall, but also of all of their older patients, with improvements in care measured up to a year after the mini-fellowship. Although this article only describes the work done as part of the Mobility aim of the 4M AFHS model, we believe the entire mini-fellowship curriculum offers the opportunity to “geriatricize” clinicians and their teams in learning geriatric principles and skills that they can translate into their practice in a sustainable way, as Tinetti encourages.8 Future study to evaluate other process outcomes more precisely, such as PT, as well as cost- and value-based outcomes, and the influence of trained providers on their clinic partners, will further establish the value proposition of targeted, disseminated, intensive geriatrics training of primary care clinicians as a strategy of age-friendly health systems as they work to improve the care of their older adults.
Acknowledgment: We are grateful for the dedication and hard work of the 2018 Geriatric Mini-Fellowship fellows at Providence Health & Services-Oregon who made this article possible. Thanks to Drs. Stephanie Cha, Emily Puukka-Clark, Laurie Dutkiewicz, Cara Ellis, Deb Frost, Jordan Roth, and Subhechchha Shah for promoting the AFHS work within their Providence Medical Group clinics and to PMG leadership and the fellows’ clinical teams for supporting the fellows, the AFHS work, and their older patients.
Corresponding author: Colleen M. Casey, PhD, ANP-BC, Providence Health & Services, Senior Health Program, 4400 NE Halsey, 5th Floor, Portland, OR 97213; [email protected].
Financial disclosures: None.
1. US Department of Health and Human Services. 2018 Profile of Older Americans. Administration on Aging. April 2018.
2. Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA. The population 65 years and older in the United States: 2016. Washington, DC: US Census Bureau; 2018.
3. American Board of Medicine Specialties. 2017-2018 ABMS Board Certification Report. https://www.abms.org/board-certification/abms-board-certification-report/. Accessed November 3, 2020.
4. US Department of Health and Human Services, Health Resources and Services Administration, National Center for Health Workforce Analysis. National and regional projections of supply and demand for geriatricians: 2013-2025. Rockville, MD: US Department of Health and Human Services; 2007.
5. American Association of Nurse Practitioners, NP Facts: The Voice of the Nurse Practitioner. 2020. https://storage.aanp.org/www/documents/NPFacts__080420.pdf.
6. Tinetti ME, Naik AD, Dodson JA, Moving from disease-centered to patient goals-directed care for patients with multiple chronic conditions: patient value-based care. JAMA Cardiol. 2016;1:9-10.
7. Fried LP, Hall WJ. Editorial: leading on behalf of an aging society. J Am Geriatr Soc. 2008;56:1791-1795.
8. Tinetti M. Mainstream or extinction: can defining who we are save geriatrics? J Am Geriatr Soc. 2016;64:1400-1404.
9. Jafari P, Kostas T, Levine S, et al. ECHO-Chicago Geriatrics: using telementoring to “geriatricize” the primary care workforce. Gerontol Geriatr Educ. 2020;41:333-341.
10. Fulmer T, Mate KS, Berman A. The Age-Friendly Health System imperative. J Am Geriatr Soc. 2018;66:22-24.
11. Mate KS, Berman A, Laderman M, et al. Creating Age-Friendly Health Systems - A vision for better care of older adults. Healthc (Amst). 2018;6:4-6.
12. Tinetti ME, et al. Patient priority-directed decision making and care for older adults with multiple chronic conditions. Clin Geriatr Med. 2016;32:261-275.
13. Stevens JA, Phelan EA. Development of STEADI: a fall prevention resource for health care providers. Health Promot Pract. 2013;14:706-714.
14. Rubenstein LZ, et al. Validating an evidence-based, self-rated fall risk questionnaire (FRQ) for older adults. J Safety Res. 2011;42:493-499.
15. Grossman DC, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319: 1696-1704.
16. Tricco AC, Thomas SM, Veroniki AA, et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta-analysis. JAMA. 2017;318:1687-1699.
17. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012(9):CD007146.
18. Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:993-998.
19. Burns E, Kakara R. Deaths from falls among persons aged >=65 Years - United States, 2007-2016. MMWR Morb Mortal Wkly Rep. 2018;67:509-514.
20. Shankar KN, Liu SW, Ganz DA. Trends and characteristics of emergency department visits for fall-related injuries in older adults, 2003-2010. West J Emerg Med. 2017;18:785-793.
21. Hoffman GJ, et al. Posthospital fall injuries and 30-day readmissions in adults 65 years and older. JAMA Netw Open. 2019;2:e194276.
22. Eckstrom E, Parker EM, Shakya I, Lee R. Coordinated care plan to prevent older adult falls. 2018. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2018.
23. Eckstrom E, Parker EM, Lambert GH, et al. Implementing STEADI in academic primary care to address older adult fall risk. Innov Aging. 2017;1:igx028.
24. Johnston YA, Bergen G, Bauer M, et al. Implementation of the stopping elderly accidents, deaths, and injuries initiative in primary care: an outcome evaluation. Gerontologist. 2019;59:1182-1191.
25. Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am. 2015;99:281-293.
26. Gell NM, Mroz TM, Patel KV. Rehabilitation services use and patient-reported outcomes among older adults in the United States. Arch Phys Med Rehabil. 2017;98:2221-2227.e3.
27. Phelan EA, Aerts S, Dowler D, et al. Adoption of evidence-based fall prevention practices in primary care for older adults with a history of falls. Front Public Health. 2016;4:190.
28. Solberg LB, Carter CS, Solberg LM. Geriatric care boot camp series: interprofessional education for a new training paradigm. Geriatr Nurs. 2019;40:579-583.
29. Solberg LB, Solberg LM, Carter CS. Geriatric care boot cAMP: an interprofessional education program for healthcare professionals. J Am Geriatr Soc. 2015;63:997-1001.
30. Coogle CL, Hackett L, Owens MG, et al. Perceived self-efficacy gains following an interprofessional faculty development programme in geriatrics education. J Interprof Care. 2016;30:483-492.
31. Lam R, Lee L, Tazkarji B, et al. Five-weekend care of the elderly certificate course: continuing professional development activity for family physicians. Can Fam Physician. 2015;61:e135-141.
32. Warshaw GA, Modawal A, Kues J, et al. Community physician education in geriatrics: applying the assessing care of vulnerable elders model with a multisite primary care group. J Am Geriatr Soc. 2010;58:1780-1785.
33. Solai LK, Kumar K, Mulvaney E, et al. Geriatric mental healthcare training: a mini-fellowship approach to interprofessional assessment and management of geriatric mental health issues. Am J Geriatr Psychiatry. 2019;27:706-711.
34. Christmas C, Park E, Schmaltz H, et al. A model intensive course in geriatric teaching for non-geriatrician educators. J Gen Intern Med. 2008;23:1048-1052.
35. Heflin MT, Bragg EJ, Fernandez H, et al. The Donald W. Reynolds Consortium for Faculty Development to Advance Geriatrics Education (FD~AGE): a model for dissemination of subspecialty educational expertise. Acad Med. 2012;87:618-626.
1. US Department of Health and Human Services. 2018 Profile of Older Americans. Administration on Aging. April 2018.
2. Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA. The population 65 years and older in the United States: 2016. Washington, DC: US Census Bureau; 2018.
3. American Board of Medicine Specialties. 2017-2018 ABMS Board Certification Report. https://www.abms.org/board-certification/abms-board-certification-report/. Accessed November 3, 2020.
4. US Department of Health and Human Services, Health Resources and Services Administration, National Center for Health Workforce Analysis. National and regional projections of supply and demand for geriatricians: 2013-2025. Rockville, MD: US Department of Health and Human Services; 2007.
5. American Association of Nurse Practitioners, NP Facts: The Voice of the Nurse Practitioner. 2020. https://storage.aanp.org/www/documents/NPFacts__080420.pdf.
6. Tinetti ME, Naik AD, Dodson JA, Moving from disease-centered to patient goals-directed care for patients with multiple chronic conditions: patient value-based care. JAMA Cardiol. 2016;1:9-10.
7. Fried LP, Hall WJ. Editorial: leading on behalf of an aging society. J Am Geriatr Soc. 2008;56:1791-1795.
8. Tinetti M. Mainstream or extinction: can defining who we are save geriatrics? J Am Geriatr Soc. 2016;64:1400-1404.
9. Jafari P, Kostas T, Levine S, et al. ECHO-Chicago Geriatrics: using telementoring to “geriatricize” the primary care workforce. Gerontol Geriatr Educ. 2020;41:333-341.
10. Fulmer T, Mate KS, Berman A. The Age-Friendly Health System imperative. J Am Geriatr Soc. 2018;66:22-24.
11. Mate KS, Berman A, Laderman M, et al. Creating Age-Friendly Health Systems - A vision for better care of older adults. Healthc (Amst). 2018;6:4-6.
12. Tinetti ME, et al. Patient priority-directed decision making and care for older adults with multiple chronic conditions. Clin Geriatr Med. 2016;32:261-275.
13. Stevens JA, Phelan EA. Development of STEADI: a fall prevention resource for health care providers. Health Promot Pract. 2013;14:706-714.
14. Rubenstein LZ, et al. Validating an evidence-based, self-rated fall risk questionnaire (FRQ) for older adults. J Safety Res. 2011;42:493-499.
15. Grossman DC, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319: 1696-1704.
16. Tricco AC, Thomas SM, Veroniki AA, et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta-analysis. JAMA. 2017;318:1687-1699.
17. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012(9):CD007146.
18. Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:993-998.
19. Burns E, Kakara R. Deaths from falls among persons aged >=65 Years - United States, 2007-2016. MMWR Morb Mortal Wkly Rep. 2018;67:509-514.
20. Shankar KN, Liu SW, Ganz DA. Trends and characteristics of emergency department visits for fall-related injuries in older adults, 2003-2010. West J Emerg Med. 2017;18:785-793.
21. Hoffman GJ, et al. Posthospital fall injuries and 30-day readmissions in adults 65 years and older. JAMA Netw Open. 2019;2:e194276.
22. Eckstrom E, Parker EM, Shakya I, Lee R. Coordinated care plan to prevent older adult falls. 2018. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2018.
23. Eckstrom E, Parker EM, Lambert GH, et al. Implementing STEADI in academic primary care to address older adult fall risk. Innov Aging. 2017;1:igx028.
24. Johnston YA, Bergen G, Bauer M, et al. Implementation of the stopping elderly accidents, deaths, and injuries initiative in primary care: an outcome evaluation. Gerontologist. 2019;59:1182-1191.
25. Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am. 2015;99:281-293.
26. Gell NM, Mroz TM, Patel KV. Rehabilitation services use and patient-reported outcomes among older adults in the United States. Arch Phys Med Rehabil. 2017;98:2221-2227.e3.
27. Phelan EA, Aerts S, Dowler D, et al. Adoption of evidence-based fall prevention practices in primary care for older adults with a history of falls. Front Public Health. 2016;4:190.
28. Solberg LB, Carter CS, Solberg LM. Geriatric care boot camp series: interprofessional education for a new training paradigm. Geriatr Nurs. 2019;40:579-583.
29. Solberg LB, Solberg LM, Carter CS. Geriatric care boot cAMP: an interprofessional education program for healthcare professionals. J Am Geriatr Soc. 2015;63:997-1001.
30. Coogle CL, Hackett L, Owens MG, et al. Perceived self-efficacy gains following an interprofessional faculty development programme in geriatrics education. J Interprof Care. 2016;30:483-492.
31. Lam R, Lee L, Tazkarji B, et al. Five-weekend care of the elderly certificate course: continuing professional development activity for family physicians. Can Fam Physician. 2015;61:e135-141.
32. Warshaw GA, Modawal A, Kues J, et al. Community physician education in geriatrics: applying the assessing care of vulnerable elders model with a multisite primary care group. J Am Geriatr Soc. 2010;58:1780-1785.
33. Solai LK, Kumar K, Mulvaney E, et al. Geriatric mental healthcare training: a mini-fellowship approach to interprofessional assessment and management of geriatric mental health issues. Am J Geriatr Psychiatry. 2019;27:706-711.
34. Christmas C, Park E, Schmaltz H, et al. A model intensive course in geriatric teaching for non-geriatrician educators. J Gen Intern Med. 2008;23:1048-1052.
35. Heflin MT, Bragg EJ, Fernandez H, et al. The Donald W. Reynolds Consortium for Faculty Development to Advance Geriatrics Education (FD~AGE): a model for dissemination of subspecialty educational expertise. Acad Med. 2012;87:618-626.
Sedentary postmenopausal women have higher heart failure risk
The more time older women spent sitting or lying down, the more likely their risk of hospitalization for heart failure, based on data from more than 80,000 postmenopausal women.
The 2018 Physical Activity Guidelines show evidence of the impact of physical activity on reducing heart failure risk, but the association between activity, sedentary behavior (SB) and heart failure (HF) in older women in particular has not been well studied, wrote Michael J. LaMonte, PhD, MPH, of the State University of New York at Buffalo, and colleagues in a study published in Circulation: Heart Failure. “Given the high prevalence of prolonged sedentary time among U.S. adults aged 65 and older, among whom HF burden is substantial, understanding the role SB has in HF development is relevant to future HF prevention strategies,” the researchers wrote.
The researchers identified 80,982 women aged 50-79 years who were enrolled in the Women’s Health Initiative Observational Study, had no known HF, and could walk at least one block unassisted. The average follow-up period was 9 years, and a total of 1,402 women were hospitalized for heart failure during the period of time they were observed.
The time spent sedentary (combined sitting or lying down) was divided into tertiles of 6.5 hours or less, 6.6-9.5 hours, and more than 9.5 hours. Time spent sitting was divided into tertiles of 4.5 hours or less; 4.6-8.5 hours; and more than 8.5 hours.
Heart failure risk goes up with more down time
After controlling for multiple variables including age, race, education, income, smoking status alcohol use, menopausal hormone therapy, and hysterectomy status, the researchers found that patients in the second tertile for sedentary behavior had a significantly increased heart failure risk than patients in the first tertile for sedentary behavior. This risk was even greater for patients falling in the third tertile for sedentary behavior. Odds ratios were 1.00 (referent), 1.15, and 1.42 for the lowest to highest tertiles for total sedentary behavior, respectively, and 1.00 (referent), 1.14, and 1.54 for sitting (P < .001 for both total sedentary behavior and sitting only).
The trends remained significant after controlling for comorbidities including MI and coronary revascularization, and the associations were similar among categories of women with additional HF risk factors, including body mass index, diabetes, hypertension, and coronary heart disease.
Notably, the association between hours spent sitting or lying down and HF risk persisted even in women who met recommended activity levels, the researchers wrote.
The study findings were limited by the use of self-reports and by the inability to evaluate SB patterns or SB and HF subtypes, the researchers noted. However, the results were strengthened by the large sample size, use of time-varying SB exposure, and extensive controlling, and the data support the risk of increased SB on adverse cardiovascular outcomes.
“Results of this study underscore the need for effective strategies to reduce daily SB time, in addition to increasing recreational physical activity, as part of population efforts for HF prevention,” they concluded.
Clinicians know the value of a physically active lifestyle for heart health, said lead author Dr. LaMonte in a statement accompanying the study’s release. “However, our study clearly shows that we also need to increase efforts to reduce daily sedentary time and encourage adults to frequently interrupt their sedentary time. This does not necessarily require an extended bout of physical activity; it might simply be standing up for 5 minutes or standing and moving one’s feet in place.
“We do not have sufficient evidence on the best approach to recommend for interrupting sedentary time. However, accumulating data suggest that habitual activities such as steps taken during household and other activities of daily living are an important aspect of cardiovascular disease prevention and healthy aging,” Dr. LaMonte added.
Promote more movement and less sitting
“This is the first study to assess sedentary time and the risk for incident heart failure hospitalization in postmenopausal women,” said Robert H. Hopkins Jr., MD, of the University of Arkansas for Medical Sciences, Little Rock, in an interview.
“Heart failure is the cause of approximately 35% of cardiovascular mortalities in women, and sedentary behaviors are common in older adults,” he noted.
Kashif J. Piracha, MD, of Houston Methodist Willowbrook Hospital, agreed that there is a lack of existing data looking at the relationship between sedentary behavior and the risk of the development of heart failure in postmenopausal women. In an interview, he cited this as a reason “it was important to conduct this study.”
Dr. Hopkins added that he was not surprised by the study results “There are a number of studies which have demonstrated reduction in risk for heart failure in men and in combined populations of men and women with increased physical activity.” There are fewer data (but similar outcomes) in studies of men with increased levels of sedentary behaviors, he said.
“This study adds one more reason that other clinicians in primary care and me need to encourage our older patients to get up and move,” said Dr. Hopkins, who also serves on the editorial advisory board of Internal Medicine News. “Many of us have focused our efforts in the past on achieving exercise goals and this study provides a foundation for a recommendation that ‘it is not just about exercise;’ we need to also encourage our patients to minimize their time in sedentary pursuits in addition to exercise if we are to optimize their health into older age.”
Dr. Hopkins noted that the large size of the study was a strength, but the observational design and use of patient surveys were limitations.
“We need further studies to better tease out whether there are risk differences in different sedentary behavior patterns, whether this applies across heart failure with reduced ejection fraction versus heart failure with preserved ejection fraction, and whether there are additional ways we can mitigate these risks as our society ages,” he said.
Findings differ from California Men’s Health Study’s
“The results corroborate the fact that there is less risk of heart failure in physically active patients,” Dr. Piracha noted.
The message for clinicians is to encourage postmenopausal female patients to engage in physical activity as much as possible, said Dr. Piracha. “Also, it appears that in this population, even with good physical activity, prolonged sedentary behavior of more than 8.5 hours a day was still associated with a higher risk of incident HF hospitalization. Therefore, a case can be made to focus on carrying out physical activity with an intensity that can be sustained for longer, rather than shorter periods of time.”
Notably, the finding of increased HF hospitalization in women who reported high amounts of physical activity but were still sedentary for more than 8.5 hours a day “is contrary to what was seen in the California Men’s Health Study.” In that study, “men with high physical activity levels who also had prolonged sitting time did not have increased risk of HF hospitalization,” Dr. Piracha noted. “Further research is needed to elucidate what hormonal or other factors contribute to this difference.”
The new study was supported by the National Heart, Lung, and Blood Institute. The researchers had no financial conflicts to disclose. Dr. Hopkins and Dr. Piracha had no financial conflicts to disclose.
SOURCE: LaMonte MJ et al. Circ Heart Fail. 2020 Nov 24. doi: 10.1161/CIRCHEARTFAILURE.120.007508.
The more time older women spent sitting or lying down, the more likely their risk of hospitalization for heart failure, based on data from more than 80,000 postmenopausal women.
The 2018 Physical Activity Guidelines show evidence of the impact of physical activity on reducing heart failure risk, but the association between activity, sedentary behavior (SB) and heart failure (HF) in older women in particular has not been well studied, wrote Michael J. LaMonte, PhD, MPH, of the State University of New York at Buffalo, and colleagues in a study published in Circulation: Heart Failure. “Given the high prevalence of prolonged sedentary time among U.S. adults aged 65 and older, among whom HF burden is substantial, understanding the role SB has in HF development is relevant to future HF prevention strategies,” the researchers wrote.
The researchers identified 80,982 women aged 50-79 years who were enrolled in the Women’s Health Initiative Observational Study, had no known HF, and could walk at least one block unassisted. The average follow-up period was 9 years, and a total of 1,402 women were hospitalized for heart failure during the period of time they were observed.
The time spent sedentary (combined sitting or lying down) was divided into tertiles of 6.5 hours or less, 6.6-9.5 hours, and more than 9.5 hours. Time spent sitting was divided into tertiles of 4.5 hours or less; 4.6-8.5 hours; and more than 8.5 hours.
Heart failure risk goes up with more down time
After controlling for multiple variables including age, race, education, income, smoking status alcohol use, menopausal hormone therapy, and hysterectomy status, the researchers found that patients in the second tertile for sedentary behavior had a significantly increased heart failure risk than patients in the first tertile for sedentary behavior. This risk was even greater for patients falling in the third tertile for sedentary behavior. Odds ratios were 1.00 (referent), 1.15, and 1.42 for the lowest to highest tertiles for total sedentary behavior, respectively, and 1.00 (referent), 1.14, and 1.54 for sitting (P < .001 for both total sedentary behavior and sitting only).
The trends remained significant after controlling for comorbidities including MI and coronary revascularization, and the associations were similar among categories of women with additional HF risk factors, including body mass index, diabetes, hypertension, and coronary heart disease.
Notably, the association between hours spent sitting or lying down and HF risk persisted even in women who met recommended activity levels, the researchers wrote.
The study findings were limited by the use of self-reports and by the inability to evaluate SB patterns or SB and HF subtypes, the researchers noted. However, the results were strengthened by the large sample size, use of time-varying SB exposure, and extensive controlling, and the data support the risk of increased SB on adverse cardiovascular outcomes.
“Results of this study underscore the need for effective strategies to reduce daily SB time, in addition to increasing recreational physical activity, as part of population efforts for HF prevention,” they concluded.
Clinicians know the value of a physically active lifestyle for heart health, said lead author Dr. LaMonte in a statement accompanying the study’s release. “However, our study clearly shows that we also need to increase efforts to reduce daily sedentary time and encourage adults to frequently interrupt their sedentary time. This does not necessarily require an extended bout of physical activity; it might simply be standing up for 5 minutes or standing and moving one’s feet in place.
“We do not have sufficient evidence on the best approach to recommend for interrupting sedentary time. However, accumulating data suggest that habitual activities such as steps taken during household and other activities of daily living are an important aspect of cardiovascular disease prevention and healthy aging,” Dr. LaMonte added.
Promote more movement and less sitting
“This is the first study to assess sedentary time and the risk for incident heart failure hospitalization in postmenopausal women,” said Robert H. Hopkins Jr., MD, of the University of Arkansas for Medical Sciences, Little Rock, in an interview.
“Heart failure is the cause of approximately 35% of cardiovascular mortalities in women, and sedentary behaviors are common in older adults,” he noted.
Kashif J. Piracha, MD, of Houston Methodist Willowbrook Hospital, agreed that there is a lack of existing data looking at the relationship between sedentary behavior and the risk of the development of heart failure in postmenopausal women. In an interview, he cited this as a reason “it was important to conduct this study.”
Dr. Hopkins added that he was not surprised by the study results “There are a number of studies which have demonstrated reduction in risk for heart failure in men and in combined populations of men and women with increased physical activity.” There are fewer data (but similar outcomes) in studies of men with increased levels of sedentary behaviors, he said.
“This study adds one more reason that other clinicians in primary care and me need to encourage our older patients to get up and move,” said Dr. Hopkins, who also serves on the editorial advisory board of Internal Medicine News. “Many of us have focused our efforts in the past on achieving exercise goals and this study provides a foundation for a recommendation that ‘it is not just about exercise;’ we need to also encourage our patients to minimize their time in sedentary pursuits in addition to exercise if we are to optimize their health into older age.”
Dr. Hopkins noted that the large size of the study was a strength, but the observational design and use of patient surveys were limitations.
“We need further studies to better tease out whether there are risk differences in different sedentary behavior patterns, whether this applies across heart failure with reduced ejection fraction versus heart failure with preserved ejection fraction, and whether there are additional ways we can mitigate these risks as our society ages,” he said.
Findings differ from California Men’s Health Study’s
“The results corroborate the fact that there is less risk of heart failure in physically active patients,” Dr. Piracha noted.
The message for clinicians is to encourage postmenopausal female patients to engage in physical activity as much as possible, said Dr. Piracha. “Also, it appears that in this population, even with good physical activity, prolonged sedentary behavior of more than 8.5 hours a day was still associated with a higher risk of incident HF hospitalization. Therefore, a case can be made to focus on carrying out physical activity with an intensity that can be sustained for longer, rather than shorter periods of time.”
Notably, the finding of increased HF hospitalization in women who reported high amounts of physical activity but were still sedentary for more than 8.5 hours a day “is contrary to what was seen in the California Men’s Health Study.” In that study, “men with high physical activity levels who also had prolonged sitting time did not have increased risk of HF hospitalization,” Dr. Piracha noted. “Further research is needed to elucidate what hormonal or other factors contribute to this difference.”
The new study was supported by the National Heart, Lung, and Blood Institute. The researchers had no financial conflicts to disclose. Dr. Hopkins and Dr. Piracha had no financial conflicts to disclose.
SOURCE: LaMonte MJ et al. Circ Heart Fail. 2020 Nov 24. doi: 10.1161/CIRCHEARTFAILURE.120.007508.
The more time older women spent sitting or lying down, the more likely their risk of hospitalization for heart failure, based on data from more than 80,000 postmenopausal women.
The 2018 Physical Activity Guidelines show evidence of the impact of physical activity on reducing heart failure risk, but the association between activity, sedentary behavior (SB) and heart failure (HF) in older women in particular has not been well studied, wrote Michael J. LaMonte, PhD, MPH, of the State University of New York at Buffalo, and colleagues in a study published in Circulation: Heart Failure. “Given the high prevalence of prolonged sedentary time among U.S. adults aged 65 and older, among whom HF burden is substantial, understanding the role SB has in HF development is relevant to future HF prevention strategies,” the researchers wrote.
The researchers identified 80,982 women aged 50-79 years who were enrolled in the Women’s Health Initiative Observational Study, had no known HF, and could walk at least one block unassisted. The average follow-up period was 9 years, and a total of 1,402 women were hospitalized for heart failure during the period of time they were observed.
The time spent sedentary (combined sitting or lying down) was divided into tertiles of 6.5 hours or less, 6.6-9.5 hours, and more than 9.5 hours. Time spent sitting was divided into tertiles of 4.5 hours or less; 4.6-8.5 hours; and more than 8.5 hours.
Heart failure risk goes up with more down time
After controlling for multiple variables including age, race, education, income, smoking status alcohol use, menopausal hormone therapy, and hysterectomy status, the researchers found that patients in the second tertile for sedentary behavior had a significantly increased heart failure risk than patients in the first tertile for sedentary behavior. This risk was even greater for patients falling in the third tertile for sedentary behavior. Odds ratios were 1.00 (referent), 1.15, and 1.42 for the lowest to highest tertiles for total sedentary behavior, respectively, and 1.00 (referent), 1.14, and 1.54 for sitting (P < .001 for both total sedentary behavior and sitting only).
The trends remained significant after controlling for comorbidities including MI and coronary revascularization, and the associations were similar among categories of women with additional HF risk factors, including body mass index, diabetes, hypertension, and coronary heart disease.
Notably, the association between hours spent sitting or lying down and HF risk persisted even in women who met recommended activity levels, the researchers wrote.
The study findings were limited by the use of self-reports and by the inability to evaluate SB patterns or SB and HF subtypes, the researchers noted. However, the results were strengthened by the large sample size, use of time-varying SB exposure, and extensive controlling, and the data support the risk of increased SB on adverse cardiovascular outcomes.
“Results of this study underscore the need for effective strategies to reduce daily SB time, in addition to increasing recreational physical activity, as part of population efforts for HF prevention,” they concluded.
Clinicians know the value of a physically active lifestyle for heart health, said lead author Dr. LaMonte in a statement accompanying the study’s release. “However, our study clearly shows that we also need to increase efforts to reduce daily sedentary time and encourage adults to frequently interrupt their sedentary time. This does not necessarily require an extended bout of physical activity; it might simply be standing up for 5 minutes or standing and moving one’s feet in place.
“We do not have sufficient evidence on the best approach to recommend for interrupting sedentary time. However, accumulating data suggest that habitual activities such as steps taken during household and other activities of daily living are an important aspect of cardiovascular disease prevention and healthy aging,” Dr. LaMonte added.
Promote more movement and less sitting
“This is the first study to assess sedentary time and the risk for incident heart failure hospitalization in postmenopausal women,” said Robert H. Hopkins Jr., MD, of the University of Arkansas for Medical Sciences, Little Rock, in an interview.
“Heart failure is the cause of approximately 35% of cardiovascular mortalities in women, and sedentary behaviors are common in older adults,” he noted.
Kashif J. Piracha, MD, of Houston Methodist Willowbrook Hospital, agreed that there is a lack of existing data looking at the relationship between sedentary behavior and the risk of the development of heart failure in postmenopausal women. In an interview, he cited this as a reason “it was important to conduct this study.”
Dr. Hopkins added that he was not surprised by the study results “There are a number of studies which have demonstrated reduction in risk for heart failure in men and in combined populations of men and women with increased physical activity.” There are fewer data (but similar outcomes) in studies of men with increased levels of sedentary behaviors, he said.
“This study adds one more reason that other clinicians in primary care and me need to encourage our older patients to get up and move,” said Dr. Hopkins, who also serves on the editorial advisory board of Internal Medicine News. “Many of us have focused our efforts in the past on achieving exercise goals and this study provides a foundation for a recommendation that ‘it is not just about exercise;’ we need to also encourage our patients to minimize their time in sedentary pursuits in addition to exercise if we are to optimize their health into older age.”
Dr. Hopkins noted that the large size of the study was a strength, but the observational design and use of patient surveys were limitations.
“We need further studies to better tease out whether there are risk differences in different sedentary behavior patterns, whether this applies across heart failure with reduced ejection fraction versus heart failure with preserved ejection fraction, and whether there are additional ways we can mitigate these risks as our society ages,” he said.
Findings differ from California Men’s Health Study’s
“The results corroborate the fact that there is less risk of heart failure in physically active patients,” Dr. Piracha noted.
The message for clinicians is to encourage postmenopausal female patients to engage in physical activity as much as possible, said Dr. Piracha. “Also, it appears that in this population, even with good physical activity, prolonged sedentary behavior of more than 8.5 hours a day was still associated with a higher risk of incident HF hospitalization. Therefore, a case can be made to focus on carrying out physical activity with an intensity that can be sustained for longer, rather than shorter periods of time.”
Notably, the finding of increased HF hospitalization in women who reported high amounts of physical activity but were still sedentary for more than 8.5 hours a day “is contrary to what was seen in the California Men’s Health Study.” In that study, “men with high physical activity levels who also had prolonged sitting time did not have increased risk of HF hospitalization,” Dr. Piracha noted. “Further research is needed to elucidate what hormonal or other factors contribute to this difference.”
The new study was supported by the National Heart, Lung, and Blood Institute. The researchers had no financial conflicts to disclose. Dr. Hopkins and Dr. Piracha had no financial conflicts to disclose.
SOURCE: LaMonte MJ et al. Circ Heart Fail. 2020 Nov 24. doi: 10.1161/CIRCHEARTFAILURE.120.007508.
FROM CIRCULATION: HEART FAILURE
One step may improve auditory screening among older adults
according to a study published online Nov. 9 in the Annals of Family Medicine.
“Our findings demonstrate that using an electronic alert to prompt primary care clinicians to ask the single question, ‘Do you have difficulty with your hearing?’ to identify and refer appropriate at-risk patients for hearing testing is feasible and improves outcomes,” wrote Philip Zazove, MD, professor and chair, department of family medicine, University of Michigan Medical School, Ann Arbor, and colleagues.
Although hearing loss is known to be associated with an increased risk for a variety of health conditions, including hypertension, diabetes, dementia, and depression, the U.S. Preventive Services Task Force has concluded that there are insufficient data to evaluate the value of widespread screening.
To address that gap, Dr. Zazove and colleagues designed the Early Auditory Referral–Primary Care study. As part of the study, researchers added a hearing loss alert to the EMR systems of 10 family medicine clinics within two large health care systems, the University of Michigan (UM) and Beaumont Health (BH). Clinicians were educated on how to perform hearing loss screenings and the alerts were triggered to appear when clinicians evaluated patients 55 years or older who were being seen for non–hearing-related issues.
Between July 2016 and February 2019, 14,877 patients were enrolled in the study resulting in 36,701 encounters.
The researchers found that clinicians addressed the alert for 10,567 patients, resulting in an increase in referral rates from 3.2% at baseline to 14.4% in the UM system and from 0.7% to 4.7% in the BH system. For 26.2% of patients, the alert was not addressed at any encounter with the family clinician.
At the time of enrollment, patients were asked to complete a Hearing Handicap Index for the Elderly (HHI) questionnaire that was used to identify patients at risk for hearing loss. These results were blinded to clinicians. From the HHI data, available from 5,893 patients, the researchers found that 25.2% of patients had scores suggestive of hearing loss and that these patients had greater overall referral rates during the study period, compared with patients with lower scores (28% vs. 9.2%, respectively; P < .001).
Addressing hearing loss/communication challenges can improve health care utilization and improve quality of life for older patients, noted coauthor Michael McKee, MD, MPH, in an interview.
“This includes their relationships with significant others, better adherence to treatment plans, and possibly reducing their risk for cognitive decline,” Dr. McKee said.
While acknowledging that this type of alert should be relatively easy to implement in most EMR systems, “the issue of electronic medical record alert fatigue must be considered,” said Angela Shoup, PhD, FAAA, FNAP, president of the American Academy of Audiology and executive director of the University of Texas Callier Center for Communication Disorders in Dallas.
“Health care providers and information technology advisers are increasingly sensitive to the need to carefully curate alerts to ensure providers do not become so inundated that they miss important clinical decision support tools,” Dr. Shoup said in an interview.
“Tailoring the alert to specifically trigger only for the specified population, as noted in this article, is one technique recommended to help reduce EMR alert fatigue,” she noted.
The addition of this prompt for family clinicians “should increase the chances that hearing loss patients, who suffer substantial morbidity when untreated, will get better and earlier hearing healthcare with potentially fewer hospitalizations and improved quality of life,” Dr. Zazove and colleagues conclude.
Funding for this study was provided through a grant from the National Institute on Deafness and Other Communication Disorders (NIDCD). The authors and Dr. Shoup have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
according to a study published online Nov. 9 in the Annals of Family Medicine.
“Our findings demonstrate that using an electronic alert to prompt primary care clinicians to ask the single question, ‘Do you have difficulty with your hearing?’ to identify and refer appropriate at-risk patients for hearing testing is feasible and improves outcomes,” wrote Philip Zazove, MD, professor and chair, department of family medicine, University of Michigan Medical School, Ann Arbor, and colleagues.
Although hearing loss is known to be associated with an increased risk for a variety of health conditions, including hypertension, diabetes, dementia, and depression, the U.S. Preventive Services Task Force has concluded that there are insufficient data to evaluate the value of widespread screening.
To address that gap, Dr. Zazove and colleagues designed the Early Auditory Referral–Primary Care study. As part of the study, researchers added a hearing loss alert to the EMR systems of 10 family medicine clinics within two large health care systems, the University of Michigan (UM) and Beaumont Health (BH). Clinicians were educated on how to perform hearing loss screenings and the alerts were triggered to appear when clinicians evaluated patients 55 years or older who were being seen for non–hearing-related issues.
Between July 2016 and February 2019, 14,877 patients were enrolled in the study resulting in 36,701 encounters.
The researchers found that clinicians addressed the alert for 10,567 patients, resulting in an increase in referral rates from 3.2% at baseline to 14.4% in the UM system and from 0.7% to 4.7% in the BH system. For 26.2% of patients, the alert was not addressed at any encounter with the family clinician.
At the time of enrollment, patients were asked to complete a Hearing Handicap Index for the Elderly (HHI) questionnaire that was used to identify patients at risk for hearing loss. These results were blinded to clinicians. From the HHI data, available from 5,893 patients, the researchers found that 25.2% of patients had scores suggestive of hearing loss and that these patients had greater overall referral rates during the study period, compared with patients with lower scores (28% vs. 9.2%, respectively; P < .001).
Addressing hearing loss/communication challenges can improve health care utilization and improve quality of life for older patients, noted coauthor Michael McKee, MD, MPH, in an interview.
“This includes their relationships with significant others, better adherence to treatment plans, and possibly reducing their risk for cognitive decline,” Dr. McKee said.
While acknowledging that this type of alert should be relatively easy to implement in most EMR systems, “the issue of electronic medical record alert fatigue must be considered,” said Angela Shoup, PhD, FAAA, FNAP, president of the American Academy of Audiology and executive director of the University of Texas Callier Center for Communication Disorders in Dallas.
“Health care providers and information technology advisers are increasingly sensitive to the need to carefully curate alerts to ensure providers do not become so inundated that they miss important clinical decision support tools,” Dr. Shoup said in an interview.
“Tailoring the alert to specifically trigger only for the specified population, as noted in this article, is one technique recommended to help reduce EMR alert fatigue,” she noted.
The addition of this prompt for family clinicians “should increase the chances that hearing loss patients, who suffer substantial morbidity when untreated, will get better and earlier hearing healthcare with potentially fewer hospitalizations and improved quality of life,” Dr. Zazove and colleagues conclude.
Funding for this study was provided through a grant from the National Institute on Deafness and Other Communication Disorders (NIDCD). The authors and Dr. Shoup have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
according to a study published online Nov. 9 in the Annals of Family Medicine.
“Our findings demonstrate that using an electronic alert to prompt primary care clinicians to ask the single question, ‘Do you have difficulty with your hearing?’ to identify and refer appropriate at-risk patients for hearing testing is feasible and improves outcomes,” wrote Philip Zazove, MD, professor and chair, department of family medicine, University of Michigan Medical School, Ann Arbor, and colleagues.
Although hearing loss is known to be associated with an increased risk for a variety of health conditions, including hypertension, diabetes, dementia, and depression, the U.S. Preventive Services Task Force has concluded that there are insufficient data to evaluate the value of widespread screening.
To address that gap, Dr. Zazove and colleagues designed the Early Auditory Referral–Primary Care study. As part of the study, researchers added a hearing loss alert to the EMR systems of 10 family medicine clinics within two large health care systems, the University of Michigan (UM) and Beaumont Health (BH). Clinicians were educated on how to perform hearing loss screenings and the alerts were triggered to appear when clinicians evaluated patients 55 years or older who were being seen for non–hearing-related issues.
Between July 2016 and February 2019, 14,877 patients were enrolled in the study resulting in 36,701 encounters.
The researchers found that clinicians addressed the alert for 10,567 patients, resulting in an increase in referral rates from 3.2% at baseline to 14.4% in the UM system and from 0.7% to 4.7% in the BH system. For 26.2% of patients, the alert was not addressed at any encounter with the family clinician.
At the time of enrollment, patients were asked to complete a Hearing Handicap Index for the Elderly (HHI) questionnaire that was used to identify patients at risk for hearing loss. These results were blinded to clinicians. From the HHI data, available from 5,893 patients, the researchers found that 25.2% of patients had scores suggestive of hearing loss and that these patients had greater overall referral rates during the study period, compared with patients with lower scores (28% vs. 9.2%, respectively; P < .001).
Addressing hearing loss/communication challenges can improve health care utilization and improve quality of life for older patients, noted coauthor Michael McKee, MD, MPH, in an interview.
“This includes their relationships with significant others, better adherence to treatment plans, and possibly reducing their risk for cognitive decline,” Dr. McKee said.
While acknowledging that this type of alert should be relatively easy to implement in most EMR systems, “the issue of electronic medical record alert fatigue must be considered,” said Angela Shoup, PhD, FAAA, FNAP, president of the American Academy of Audiology and executive director of the University of Texas Callier Center for Communication Disorders in Dallas.
“Health care providers and information technology advisers are increasingly sensitive to the need to carefully curate alerts to ensure providers do not become so inundated that they miss important clinical decision support tools,” Dr. Shoup said in an interview.
“Tailoring the alert to specifically trigger only for the specified population, as noted in this article, is one technique recommended to help reduce EMR alert fatigue,” she noted.
The addition of this prompt for family clinicians “should increase the chances that hearing loss patients, who suffer substantial morbidity when untreated, will get better and earlier hearing healthcare with potentially fewer hospitalizations and improved quality of life,” Dr. Zazove and colleagues conclude.
Funding for this study was provided through a grant from the National Institute on Deafness and Other Communication Disorders (NIDCD). The authors and Dr. Shoup have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Osteoporosis underdiagnosed in older men with fracture
Osteoporosis is frequently underdiagnosed and undertreated in men before and even after they have experienced a fracture, according to research presented at the virtual annual meeting of the American College of Rheumatology.
“This is an important public health concern,” as fractures contribute significantly to morbidity and mortality, said Jeffrey Curtis, MD, MS, MPH, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham.
Men are often overlooked, he said, “because it’s misconstrued as a disease that mainly, if not only, affects Caucasian women,” despite the fact that 20%-25% of fractures occur in men.
Emerging evidence suggests that men who have bone fractures have worse outcomes than women, Dr. Curtis said.
Guidelines lacking
Consistent guidelines for osteoporosis screening among men are also lacking, leading to ambiguity and increased disease burden.
Researchers studied records for a 5% random sample of male Medicare fee-for-service beneficiaries (n = 9,876) aged at least 65 years with a closed fragility fracture between January 2010 and September 2014. Average age for the men with fractures was 77.9 years, and the most common sites of the fracture were the spine, hip, and ankle.
They looked back to see whether these men had been effectively screened and treated.
Very few had.
“We found that 92.8% of them did not have any diagnosis or treatment of osteoporosis at baseline,” Curtis said. On top of that, less than 6% of men had undergone any dual-energy x-ray absorptiometry (DEXA) or bone mineral testing in the 2 years prior to their fracture.
Even men who had high-risk factors for falls, such as those using beta-blockers, mobility impairment, or a history of opioid use, were unlikely to be screened, he said.
Dr. Curtis’s data show there was actually a decline in DEXA scans from 2012 to 2014, and that decline was particularly high in men aged 75 years and older who are more likely to be at risk for fracture.
In addition to underscreening and undertreating before the fracture, Dr. Curtis said, “The treatment patterns after the fracture were not much better.” In the year after the fracture, “only about 10% of these men had BMD [bone mineral density] testing. Only 9% were treated with an osteoporosis medication.”
“Importantly, about 7% of the men in this large cohort went on to have one or more fractures in the next year,” he added.
Reasons for undertreatment
Reasons for the poor rates of diagnosis and treatment may begin with patients not having symptoms. Therefore, they aren’t coming into doctors’ offices asking to be screened. “Even if they break bones, they may not know enough to ask how to prevent the next fracture,” Dr. Curtis said.
There’s a financial obstacle as well, Dr. Curtis explained. “U.S. legislation that provides population screening for Medicare patients really, for men, is quite dissimilar to the near-universal coverage for women. So many clinicians worry they won’t get reimbursed if they order DEXA in men for screening.”
Additionally, postfracture quality-of-care guidelines that are reimbursed as part of the Medicare Access and CHIP Reauthorization Act of 2015 and the Merit-based Incentive Payment System program specifically exclude men, he noted.
Better management of male osteoporosis, including early identification of at-risk individuals is clearly warranted, he said, so they can be screened and put on effective therapy.
Sonali Khandelwal, MD, a rheumatologist with Rush University Medical Center, Chicago, who was not part of the research, agreed.
She said in an interview that part of the problem is that diagnosis and treatment could come from a variety of specialists – endocrinologists, rheumatologists, orthopedists, and primary care physicians – and each may think it falls in another’s realm.
At Rush and some other sites nationally, she said, an alert is registered in electronic medical records flagging any patient who may need bone density screening based on age, medications, or history.
Rush University also has a fracture liaison service under which everyone hospitalized there who may have had a history of a fracture or is admitted with a fracture gets followed up with screening and treatment, “to capture those patients who may not have come through the system otherwise.”
She said guidelines have called for DEXA screening for men at age 70, but she said clinical screening should start younger – as young as 50 – for patients with conditions such as lupus, rheumatoid arthritis, hypogonadism, or those on chronic steroids.
Dr. Khandelwal said that, even when an insurance company doesn›t typically cover bone density screening for men, physicians can often make a case for reimbursement if the patient has a history of falls or fractures.
“In the long run, preventing a fracture is saving so much more money than when you get a fracture and end up in a hospital and have to go to a nursing home,” she said.
Dr. Curtis reported relationships with AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Janssen, Lilly, Myriad, Pfizer, Regeneron, Roche, UCB, Gilead Sciences, and Sanofi. Dr. Khandelwal reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Osteoporosis is frequently underdiagnosed and undertreated in men before and even after they have experienced a fracture, according to research presented at the virtual annual meeting of the American College of Rheumatology.
“This is an important public health concern,” as fractures contribute significantly to morbidity and mortality, said Jeffrey Curtis, MD, MS, MPH, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham.
Men are often overlooked, he said, “because it’s misconstrued as a disease that mainly, if not only, affects Caucasian women,” despite the fact that 20%-25% of fractures occur in men.
Emerging evidence suggests that men who have bone fractures have worse outcomes than women, Dr. Curtis said.
Guidelines lacking
Consistent guidelines for osteoporosis screening among men are also lacking, leading to ambiguity and increased disease burden.
Researchers studied records for a 5% random sample of male Medicare fee-for-service beneficiaries (n = 9,876) aged at least 65 years with a closed fragility fracture between January 2010 and September 2014. Average age for the men with fractures was 77.9 years, and the most common sites of the fracture were the spine, hip, and ankle.
They looked back to see whether these men had been effectively screened and treated.
Very few had.
“We found that 92.8% of them did not have any diagnosis or treatment of osteoporosis at baseline,” Curtis said. On top of that, less than 6% of men had undergone any dual-energy x-ray absorptiometry (DEXA) or bone mineral testing in the 2 years prior to their fracture.
Even men who had high-risk factors for falls, such as those using beta-blockers, mobility impairment, or a history of opioid use, were unlikely to be screened, he said.
Dr. Curtis’s data show there was actually a decline in DEXA scans from 2012 to 2014, and that decline was particularly high in men aged 75 years and older who are more likely to be at risk for fracture.
In addition to underscreening and undertreating before the fracture, Dr. Curtis said, “The treatment patterns after the fracture were not much better.” In the year after the fracture, “only about 10% of these men had BMD [bone mineral density] testing. Only 9% were treated with an osteoporosis medication.”
“Importantly, about 7% of the men in this large cohort went on to have one or more fractures in the next year,” he added.
Reasons for undertreatment
Reasons for the poor rates of diagnosis and treatment may begin with patients not having symptoms. Therefore, they aren’t coming into doctors’ offices asking to be screened. “Even if they break bones, they may not know enough to ask how to prevent the next fracture,” Dr. Curtis said.
There’s a financial obstacle as well, Dr. Curtis explained. “U.S. legislation that provides population screening for Medicare patients really, for men, is quite dissimilar to the near-universal coverage for women. So many clinicians worry they won’t get reimbursed if they order DEXA in men for screening.”
Additionally, postfracture quality-of-care guidelines that are reimbursed as part of the Medicare Access and CHIP Reauthorization Act of 2015 and the Merit-based Incentive Payment System program specifically exclude men, he noted.
Better management of male osteoporosis, including early identification of at-risk individuals is clearly warranted, he said, so they can be screened and put on effective therapy.
Sonali Khandelwal, MD, a rheumatologist with Rush University Medical Center, Chicago, who was not part of the research, agreed.
She said in an interview that part of the problem is that diagnosis and treatment could come from a variety of specialists – endocrinologists, rheumatologists, orthopedists, and primary care physicians – and each may think it falls in another’s realm.
At Rush and some other sites nationally, she said, an alert is registered in electronic medical records flagging any patient who may need bone density screening based on age, medications, or history.
Rush University also has a fracture liaison service under which everyone hospitalized there who may have had a history of a fracture or is admitted with a fracture gets followed up with screening and treatment, “to capture those patients who may not have come through the system otherwise.”
She said guidelines have called for DEXA screening for men at age 70, but she said clinical screening should start younger – as young as 50 – for patients with conditions such as lupus, rheumatoid arthritis, hypogonadism, or those on chronic steroids.
Dr. Khandelwal said that, even when an insurance company doesn›t typically cover bone density screening for men, physicians can often make a case for reimbursement if the patient has a history of falls or fractures.
“In the long run, preventing a fracture is saving so much more money than when you get a fracture and end up in a hospital and have to go to a nursing home,” she said.
Dr. Curtis reported relationships with AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Janssen, Lilly, Myriad, Pfizer, Regeneron, Roche, UCB, Gilead Sciences, and Sanofi. Dr. Khandelwal reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Osteoporosis is frequently underdiagnosed and undertreated in men before and even after they have experienced a fracture, according to research presented at the virtual annual meeting of the American College of Rheumatology.
“This is an important public health concern,” as fractures contribute significantly to morbidity and mortality, said Jeffrey Curtis, MD, MS, MPH, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham.
Men are often overlooked, he said, “because it’s misconstrued as a disease that mainly, if not only, affects Caucasian women,” despite the fact that 20%-25% of fractures occur in men.
Emerging evidence suggests that men who have bone fractures have worse outcomes than women, Dr. Curtis said.
Guidelines lacking
Consistent guidelines for osteoporosis screening among men are also lacking, leading to ambiguity and increased disease burden.
Researchers studied records for a 5% random sample of male Medicare fee-for-service beneficiaries (n = 9,876) aged at least 65 years with a closed fragility fracture between January 2010 and September 2014. Average age for the men with fractures was 77.9 years, and the most common sites of the fracture were the spine, hip, and ankle.
They looked back to see whether these men had been effectively screened and treated.
Very few had.
“We found that 92.8% of them did not have any diagnosis or treatment of osteoporosis at baseline,” Curtis said. On top of that, less than 6% of men had undergone any dual-energy x-ray absorptiometry (DEXA) or bone mineral testing in the 2 years prior to their fracture.
Even men who had high-risk factors for falls, such as those using beta-blockers, mobility impairment, or a history of opioid use, were unlikely to be screened, he said.
Dr. Curtis’s data show there was actually a decline in DEXA scans from 2012 to 2014, and that decline was particularly high in men aged 75 years and older who are more likely to be at risk for fracture.
In addition to underscreening and undertreating before the fracture, Dr. Curtis said, “The treatment patterns after the fracture were not much better.” In the year after the fracture, “only about 10% of these men had BMD [bone mineral density] testing. Only 9% were treated with an osteoporosis medication.”
“Importantly, about 7% of the men in this large cohort went on to have one or more fractures in the next year,” he added.
Reasons for undertreatment
Reasons for the poor rates of diagnosis and treatment may begin with patients not having symptoms. Therefore, they aren’t coming into doctors’ offices asking to be screened. “Even if they break bones, they may not know enough to ask how to prevent the next fracture,” Dr. Curtis said.
There’s a financial obstacle as well, Dr. Curtis explained. “U.S. legislation that provides population screening for Medicare patients really, for men, is quite dissimilar to the near-universal coverage for women. So many clinicians worry they won’t get reimbursed if they order DEXA in men for screening.”
Additionally, postfracture quality-of-care guidelines that are reimbursed as part of the Medicare Access and CHIP Reauthorization Act of 2015 and the Merit-based Incentive Payment System program specifically exclude men, he noted.
Better management of male osteoporosis, including early identification of at-risk individuals is clearly warranted, he said, so they can be screened and put on effective therapy.
Sonali Khandelwal, MD, a rheumatologist with Rush University Medical Center, Chicago, who was not part of the research, agreed.
She said in an interview that part of the problem is that diagnosis and treatment could come from a variety of specialists – endocrinologists, rheumatologists, orthopedists, and primary care physicians – and each may think it falls in another’s realm.
At Rush and some other sites nationally, she said, an alert is registered in electronic medical records flagging any patient who may need bone density screening based on age, medications, or history.
Rush University also has a fracture liaison service under which everyone hospitalized there who may have had a history of a fracture or is admitted with a fracture gets followed up with screening and treatment, “to capture those patients who may not have come through the system otherwise.”
She said guidelines have called for DEXA screening for men at age 70, but she said clinical screening should start younger – as young as 50 – for patients with conditions such as lupus, rheumatoid arthritis, hypogonadism, or those on chronic steroids.
Dr. Khandelwal said that, even when an insurance company doesn›t typically cover bone density screening for men, physicians can often make a case for reimbursement if the patient has a history of falls or fractures.
“In the long run, preventing a fracture is saving so much more money than when you get a fracture and end up in a hospital and have to go to a nursing home,” she said.
Dr. Curtis reported relationships with AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Janssen, Lilly, Myriad, Pfizer, Regeneron, Roche, UCB, Gilead Sciences, and Sanofi. Dr. Khandelwal reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Study advances personalized treatment for older breast cancer patients
Findings from the study were reported at the 12th European Breast Cancer Conference.
“Primary endocrine therapy is usually reserved for older, less fit, and frail women. Rates of use vary widely,” noted investigator Lynda Wyld, MBChB, PhD, of the University of Sheffield (England).
“Although there is no set threshold for who is suitable, some women are undoubtedly over- and undertreated for their breast cancer,” she added.
Dr. Wyld and colleagues undertook the Age Gap study among women older than 70 years with breast cancer recruited from 56 U.K. breast units during 2013-2018.
The main goals were to determine which women can be safely offered primary endocrine therapy as nonstandard care and to develop and test a tool to help women in this age group make treatment decisions.
The first component of the study was a multicenter, prospective cohort study of women with ER+ disease who were eligible for surgery. Results showed that breast cancer–specific mortality was greater with primary endocrine therapy than with surgery in the entire cohort. However, breast cancer–specific mortality was lower with primary endocrine therapy than with surgery in a cohort matched with propensity scores to achieve similar age, fitness, and frailty.
The second component of the study was a cluster-randomized controlled trial of women with operable breast cancer, most of whom had ER+ disease. Results showed that a decision support tool increased awareness of treatment options and readiness to decide. The tool also altered treatment choices, prompting a larger share of patients with ER+ disease to choose primary endocrine therapy.
Prospective cohort study
The prospective observational study was conducted in 2,854 women with ER+ disease who were eligible for surgery and treated in usual practice. Most women (n = 2,354) were treated with surgery (followed by antiestrogen therapy), while the rest received primary endocrine therapy (n = 500).
In the entire cohort, patients undergoing surgery were younger, had a lower level of comorbidity, and were less often frail. But these characteristics were generally similar in a propensity-matched cohort of 672 patients.
At a median follow-up of 52 months, overall and breast cancer–specific survival were significantly poorer with primary endocrine therapy versus surgery in the entire cohort but not in the propensity-matched cohort.
In the entire cohort, the breast cancer–specific mortality was 9.5% with primary endocrine therapy and 4.9% with surgery. In the propensity-matched cohort, breast cancer–specific mortality was 3.1% and 6.6%, respectively.
The overall mortality was 41.8% with primary endocrine therapy and 14.6% with surgery in the entire cohort, but the gap narrowed to 34.5% and 25.6%, respectively, in the propensity-matched cohort.
In the latter, “although there is a slight divergence in overall survival and it’s likely that with longer-term follow-up this will become significant, at the moment, it isn’t,” Dr. Wyld commented.
Curves for breast cancer–specific survival basically overlapped until 5 years, when surgery started to show an advantage. The rate of locoregional recurrence or progression was low and not significantly different by treatment.
None of the women in the entire cohort died from surgery. “But it’s worth bearing in mind that these were all women selected for surgery, who were thought to be fit for it by their surgeons. The least fit women in this cohort will have obviously been offered primary endocrine therapy,” Dr. Wyld cautioned.
Although 19% of patients had a surgical complication, only 2.1% had a systemic surgical complication.
Cluster-randomized controlled trial
In the cluster-randomized controlled trial, researchers compared a decision support tool to usual care. The tool was developed using U.K. registry data from almost 30,000 older women and input from women in this age group on their preferred format and method of presentation, according to Dr. Wyld.
The tool consists of an algorithm available to clinicians online (for input of tumor stage and biology, comorbidities, and functional status) plus a booklet and outcome sheets for patients to take home after discussions that can be personalized to their particulars.
Intention-to-treat analyses were based on 1,339 patients with operable breast cancer, 1,161 of whom had ER+ disease. Per-protocol analyses were based on the subset of 449 patients who were offered a choice between surgery and primary endocrine therapy, presumably because they were less fit and frailer.
Results showed that, at 6 months, mean scores for global quality of life on the EORTC questionnaire did not differ between decision support and usual care in the intention-to-treat population (69.0 vs. 68.9; P = .900), but scores were more favorable with decision support in the per-protocol population (70.7 vs. 66.8; P = .044).
The tool also altered treatment choices, with a larger share of ER+ patients choosing primary endocrine therapy (21.0% vs. 15.4%; P = .029) but still having similar disease outcomes.
Although ER+ patients in the decision support group more often selected primary endocrine therapy, at a median follow-up of 36 months, the groups did not differ significantly on overall survival, cause-specific survival, or time to recurrence in either intention-to-treat or per-protocol analyses.
Larger shares of women in the decision support group reported that they had adequate knowledge about the treatment options available to them (94% vs. 74%), were aware of the advantages and disadvantages of each option (91% vs. 76%), knew which option they preferred (96% vs. 91%), and were ready to make a decision (99% vs. 90%).
Applying results to practice
“Most women over the age of 70 are relatively fit, and the aim should be to treat them with surgery,” Dr. Wyld said. “For the less fit, a point is reached where the oncology benefits of surgery disappear and surgery may just cause harm. This threshold appears to be for women in their mid-80s with moderate to poor health.”
“Use of the Age Gap online tool may enhance shared decision-making for these women while increasing knowledge. And whilst it does seem to increase the use of primary endocrine therapy, this does not seem to have an adverse impact on survival at 36 months of follow-up,” she added.
“The study by Dr. Wyld and colleagues adds to the available literature regarding the scenarios in which some treatments may be omitted without impacting overall survival in older women with breast cancer,” Lesly A. Dossett, MD, of Michigan Medicine in Ann Arbor, commented in an interview.
In her own practice, Dr. Dossett emphasizes the generally favorable prognosis for older women with hormone receptor–positive breast cancer, she said. However, tools that help communicate risk and clarify the value of various therapies are welcome.
“The decision support tool appears to be a promising tool in helping to avoid treatments that are unlikely to benefit older women with breast cancer,” Dr. Dossett said. “The results will be widely applicable, as there is growing recognition that this patient population is at risk for overtreatment.”
The study was funded by the U.K. National Institute for Health Research programme grant for applied research. Dr. Wyld and Dr. Dossett said they had no relevant conflicts of interest.
SOURCES: Wyld L et al. EBCC-12 Virtual Congress. Abstract 8A and Abstract 8B.
Findings from the study were reported at the 12th European Breast Cancer Conference.
“Primary endocrine therapy is usually reserved for older, less fit, and frail women. Rates of use vary widely,” noted investigator Lynda Wyld, MBChB, PhD, of the University of Sheffield (England).
“Although there is no set threshold for who is suitable, some women are undoubtedly over- and undertreated for their breast cancer,” she added.
Dr. Wyld and colleagues undertook the Age Gap study among women older than 70 years with breast cancer recruited from 56 U.K. breast units during 2013-2018.
The main goals were to determine which women can be safely offered primary endocrine therapy as nonstandard care and to develop and test a tool to help women in this age group make treatment decisions.
The first component of the study was a multicenter, prospective cohort study of women with ER+ disease who were eligible for surgery. Results showed that breast cancer–specific mortality was greater with primary endocrine therapy than with surgery in the entire cohort. However, breast cancer–specific mortality was lower with primary endocrine therapy than with surgery in a cohort matched with propensity scores to achieve similar age, fitness, and frailty.
The second component of the study was a cluster-randomized controlled trial of women with operable breast cancer, most of whom had ER+ disease. Results showed that a decision support tool increased awareness of treatment options and readiness to decide. The tool also altered treatment choices, prompting a larger share of patients with ER+ disease to choose primary endocrine therapy.
Prospective cohort study
The prospective observational study was conducted in 2,854 women with ER+ disease who were eligible for surgery and treated in usual practice. Most women (n = 2,354) were treated with surgery (followed by antiestrogen therapy), while the rest received primary endocrine therapy (n = 500).
In the entire cohort, patients undergoing surgery were younger, had a lower level of comorbidity, and were less often frail. But these characteristics were generally similar in a propensity-matched cohort of 672 patients.
At a median follow-up of 52 months, overall and breast cancer–specific survival were significantly poorer with primary endocrine therapy versus surgery in the entire cohort but not in the propensity-matched cohort.
In the entire cohort, the breast cancer–specific mortality was 9.5% with primary endocrine therapy and 4.9% with surgery. In the propensity-matched cohort, breast cancer–specific mortality was 3.1% and 6.6%, respectively.
The overall mortality was 41.8% with primary endocrine therapy and 14.6% with surgery in the entire cohort, but the gap narrowed to 34.5% and 25.6%, respectively, in the propensity-matched cohort.
In the latter, “although there is a slight divergence in overall survival and it’s likely that with longer-term follow-up this will become significant, at the moment, it isn’t,” Dr. Wyld commented.
Curves for breast cancer–specific survival basically overlapped until 5 years, when surgery started to show an advantage. The rate of locoregional recurrence or progression was low and not significantly different by treatment.
None of the women in the entire cohort died from surgery. “But it’s worth bearing in mind that these were all women selected for surgery, who were thought to be fit for it by their surgeons. The least fit women in this cohort will have obviously been offered primary endocrine therapy,” Dr. Wyld cautioned.
Although 19% of patients had a surgical complication, only 2.1% had a systemic surgical complication.
Cluster-randomized controlled trial
In the cluster-randomized controlled trial, researchers compared a decision support tool to usual care. The tool was developed using U.K. registry data from almost 30,000 older women and input from women in this age group on their preferred format and method of presentation, according to Dr. Wyld.
The tool consists of an algorithm available to clinicians online (for input of tumor stage and biology, comorbidities, and functional status) plus a booklet and outcome sheets for patients to take home after discussions that can be personalized to their particulars.
Intention-to-treat analyses were based on 1,339 patients with operable breast cancer, 1,161 of whom had ER+ disease. Per-protocol analyses were based on the subset of 449 patients who were offered a choice between surgery and primary endocrine therapy, presumably because they were less fit and frailer.
Results showed that, at 6 months, mean scores for global quality of life on the EORTC questionnaire did not differ between decision support and usual care in the intention-to-treat population (69.0 vs. 68.9; P = .900), but scores were more favorable with decision support in the per-protocol population (70.7 vs. 66.8; P = .044).
The tool also altered treatment choices, with a larger share of ER+ patients choosing primary endocrine therapy (21.0% vs. 15.4%; P = .029) but still having similar disease outcomes.
Although ER+ patients in the decision support group more often selected primary endocrine therapy, at a median follow-up of 36 months, the groups did not differ significantly on overall survival, cause-specific survival, or time to recurrence in either intention-to-treat or per-protocol analyses.
Larger shares of women in the decision support group reported that they had adequate knowledge about the treatment options available to them (94% vs. 74%), were aware of the advantages and disadvantages of each option (91% vs. 76%), knew which option they preferred (96% vs. 91%), and were ready to make a decision (99% vs. 90%).
Applying results to practice
“Most women over the age of 70 are relatively fit, and the aim should be to treat them with surgery,” Dr. Wyld said. “For the less fit, a point is reached where the oncology benefits of surgery disappear and surgery may just cause harm. This threshold appears to be for women in their mid-80s with moderate to poor health.”
“Use of the Age Gap online tool may enhance shared decision-making for these women while increasing knowledge. And whilst it does seem to increase the use of primary endocrine therapy, this does not seem to have an adverse impact on survival at 36 months of follow-up,” she added.
“The study by Dr. Wyld and colleagues adds to the available literature regarding the scenarios in which some treatments may be omitted without impacting overall survival in older women with breast cancer,” Lesly A. Dossett, MD, of Michigan Medicine in Ann Arbor, commented in an interview.
In her own practice, Dr. Dossett emphasizes the generally favorable prognosis for older women with hormone receptor–positive breast cancer, she said. However, tools that help communicate risk and clarify the value of various therapies are welcome.
“The decision support tool appears to be a promising tool in helping to avoid treatments that are unlikely to benefit older women with breast cancer,” Dr. Dossett said. “The results will be widely applicable, as there is growing recognition that this patient population is at risk for overtreatment.”
The study was funded by the U.K. National Institute for Health Research programme grant for applied research. Dr. Wyld and Dr. Dossett said they had no relevant conflicts of interest.
SOURCES: Wyld L et al. EBCC-12 Virtual Congress. Abstract 8A and Abstract 8B.
Findings from the study were reported at the 12th European Breast Cancer Conference.
“Primary endocrine therapy is usually reserved for older, less fit, and frail women. Rates of use vary widely,” noted investigator Lynda Wyld, MBChB, PhD, of the University of Sheffield (England).
“Although there is no set threshold for who is suitable, some women are undoubtedly over- and undertreated for their breast cancer,” she added.
Dr. Wyld and colleagues undertook the Age Gap study among women older than 70 years with breast cancer recruited from 56 U.K. breast units during 2013-2018.
The main goals were to determine which women can be safely offered primary endocrine therapy as nonstandard care and to develop and test a tool to help women in this age group make treatment decisions.
The first component of the study was a multicenter, prospective cohort study of women with ER+ disease who were eligible for surgery. Results showed that breast cancer–specific mortality was greater with primary endocrine therapy than with surgery in the entire cohort. However, breast cancer–specific mortality was lower with primary endocrine therapy than with surgery in a cohort matched with propensity scores to achieve similar age, fitness, and frailty.
The second component of the study was a cluster-randomized controlled trial of women with operable breast cancer, most of whom had ER+ disease. Results showed that a decision support tool increased awareness of treatment options and readiness to decide. The tool also altered treatment choices, prompting a larger share of patients with ER+ disease to choose primary endocrine therapy.
Prospective cohort study
The prospective observational study was conducted in 2,854 women with ER+ disease who were eligible for surgery and treated in usual practice. Most women (n = 2,354) were treated with surgery (followed by antiestrogen therapy), while the rest received primary endocrine therapy (n = 500).
In the entire cohort, patients undergoing surgery were younger, had a lower level of comorbidity, and were less often frail. But these characteristics were generally similar in a propensity-matched cohort of 672 patients.
At a median follow-up of 52 months, overall and breast cancer–specific survival were significantly poorer with primary endocrine therapy versus surgery in the entire cohort but not in the propensity-matched cohort.
In the entire cohort, the breast cancer–specific mortality was 9.5% with primary endocrine therapy and 4.9% with surgery. In the propensity-matched cohort, breast cancer–specific mortality was 3.1% and 6.6%, respectively.
The overall mortality was 41.8% with primary endocrine therapy and 14.6% with surgery in the entire cohort, but the gap narrowed to 34.5% and 25.6%, respectively, in the propensity-matched cohort.
In the latter, “although there is a slight divergence in overall survival and it’s likely that with longer-term follow-up this will become significant, at the moment, it isn’t,” Dr. Wyld commented.
Curves for breast cancer–specific survival basically overlapped until 5 years, when surgery started to show an advantage. The rate of locoregional recurrence or progression was low and not significantly different by treatment.
None of the women in the entire cohort died from surgery. “But it’s worth bearing in mind that these were all women selected for surgery, who were thought to be fit for it by their surgeons. The least fit women in this cohort will have obviously been offered primary endocrine therapy,” Dr. Wyld cautioned.
Although 19% of patients had a surgical complication, only 2.1% had a systemic surgical complication.
Cluster-randomized controlled trial
In the cluster-randomized controlled trial, researchers compared a decision support tool to usual care. The tool was developed using U.K. registry data from almost 30,000 older women and input from women in this age group on their preferred format and method of presentation, according to Dr. Wyld.
The tool consists of an algorithm available to clinicians online (for input of tumor stage and biology, comorbidities, and functional status) plus a booklet and outcome sheets for patients to take home after discussions that can be personalized to their particulars.
Intention-to-treat analyses were based on 1,339 patients with operable breast cancer, 1,161 of whom had ER+ disease. Per-protocol analyses were based on the subset of 449 patients who were offered a choice between surgery and primary endocrine therapy, presumably because they were less fit and frailer.
Results showed that, at 6 months, mean scores for global quality of life on the EORTC questionnaire did not differ between decision support and usual care in the intention-to-treat population (69.0 vs. 68.9; P = .900), but scores were more favorable with decision support in the per-protocol population (70.7 vs. 66.8; P = .044).
The tool also altered treatment choices, with a larger share of ER+ patients choosing primary endocrine therapy (21.0% vs. 15.4%; P = .029) but still having similar disease outcomes.
Although ER+ patients in the decision support group more often selected primary endocrine therapy, at a median follow-up of 36 months, the groups did not differ significantly on overall survival, cause-specific survival, or time to recurrence in either intention-to-treat or per-protocol analyses.
Larger shares of women in the decision support group reported that they had adequate knowledge about the treatment options available to them (94% vs. 74%), were aware of the advantages and disadvantages of each option (91% vs. 76%), knew which option they preferred (96% vs. 91%), and were ready to make a decision (99% vs. 90%).
Applying results to practice
“Most women over the age of 70 are relatively fit, and the aim should be to treat them with surgery,” Dr. Wyld said. “For the less fit, a point is reached where the oncology benefits of surgery disappear and surgery may just cause harm. This threshold appears to be for women in their mid-80s with moderate to poor health.”
“Use of the Age Gap online tool may enhance shared decision-making for these women while increasing knowledge. And whilst it does seem to increase the use of primary endocrine therapy, this does not seem to have an adverse impact on survival at 36 months of follow-up,” she added.
“The study by Dr. Wyld and colleagues adds to the available literature regarding the scenarios in which some treatments may be omitted without impacting overall survival in older women with breast cancer,” Lesly A. Dossett, MD, of Michigan Medicine in Ann Arbor, commented in an interview.
In her own practice, Dr. Dossett emphasizes the generally favorable prognosis for older women with hormone receptor–positive breast cancer, she said. However, tools that help communicate risk and clarify the value of various therapies are welcome.
“The decision support tool appears to be a promising tool in helping to avoid treatments that are unlikely to benefit older women with breast cancer,” Dr. Dossett said. “The results will be widely applicable, as there is growing recognition that this patient population is at risk for overtreatment.”
The study was funded by the U.K. National Institute for Health Research programme grant for applied research. Dr. Wyld and Dr. Dossett said they had no relevant conflicts of interest.
SOURCES: Wyld L et al. EBCC-12 Virtual Congress. Abstract 8A and Abstract 8B.
FROM EBCC-12 VIRTUAL CONFERENCE