User login
Lung disease raises mortality risk in older RA patients
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
FROM RHEUMATOLOGY
A Preoperative Transthoracic Echocardiography Protocol to Reduce Time to Hip Fracture Surgery
From Dignity Health Methodist Hospital of Sacramento Family Medicine Residency Program, Sacramento, CA (Dr. Oldach); Nationwide Children’s Hospital, Columbus, OH (Dr. Irwin); OhioHealth Research Institute, Columbus, OH (Dr. Pershing); Department of Clinical Transformation, OhioHealth, Columbus, OH (Dr. Zigmont and Dr. Gascon); and Department of Geriatrics, OhioHealth, Columbus, OH (Dr. Skully).
Abstract
Objective: An interdisciplinary committee was formed to identify factors contributing to surgical delays in urgent hip fracture repair at an urban, level 1 trauma center, with the goal of reducing preoperative time to less than 24 hours. Surgical optimization was identified as a primary, modifiable factor, as surgeons were reluctant to clear patients for surgery without cardiac consultation. Preoperative transthoracic echocardiogram (TTE) was recommended as a safe alternative to cardiac consultation in most patients.
Methods: A retrospective review was conducted for patients who underwent urgent hip fracture repair between January 2010 and April 2014 (n = 316). Time to medical optimization, time to surgery, hospital length of stay, and anesthesia induction were compared for 3 patient groups of interest: those who received (1) neither TTE nor cardiology consultation (ie, direct to surgery); (2) a preoperative TTE; or (3) preoperative cardiac consultation.
Results: There were significant between-group differences in medical optimization time (P = 0.001) and mean time to surgery (P < 0.001) when comparing the 3 groups of interest. Patients in the preoperative cardiac consult group had the longest times, followed by the TTE and direct-to-surgery groups. There were no differences in the type of induction agent used across treatment groups when stratifying by ejection fraction.
Conclusion: Preoperative TTE allows for decreased preoperative time compared to a cardiology consultation. It provides an easily implemented inter-departmental, intra-institutional intervention to decrease preoperative time in patients presenting with hip fractures.
Keywords: surgical delay; preoperative risk stratification; process improvement.
Hip fractures are common, expensive, and associated with poor outcomes.1,2 Ample literature suggests that morbidity, mortality, and cost of care may be reduced by minimizing surgical delays.3-5 While individual reports indicate mixed evidence, in a 2010 meta-analysis, surgery within 72 hours was associated with significant reductions in pneumonia and pressure sores, as well as a 19% reduction in all-cause mortality through 1 year.6 Additional reviews suggest evidence of improved patient outcomes (pain, length of stay, non-union, and/or mortality) when surgery occurs early, within 12 to 72 hours after injury.4,6,7 Regardless of the definition of “early surgery” used, surgical delay remains a challenge, often due to organizational factors, including admission day of the week and hospital staffing, and patient characteristics, such as comorbidities, echocardiographic findings, age, and insurance status.7-9
Among factors that contribute to surgical delays, the need for preoperative cardiovascular risk stratification is significantly modifiable.10 The American College of Cardiology (ACC)/American Heart Association (AHA) Task Force risk stratification framework for preoperative cardiac testing assists clinicians in determining surgical urgency, active cardiac conditions, cardiovascular risk factors, and functional capacity of each patient, and is well established for low- or intermediate-risk patients.11 Specifically, metabolic equivalents (METs) measurements are used to identify medically stable patients with good or excellent functional capacity versus poor or unknown functional status. Patients with ≥ 4 METs may proceed to surgery without further testing; patients with < 4 METs may either proceed with planned surgery or undergo additional testing. Patients with a perceived increased risk profile who require urgent or semi-urgent hip fracture repair may be confounded by disagreement about required preoperative cardiac testing.
At OhioHealth Grant Medical Center (GMC), an urban, level 1 trauma center, the consideration of further preoperative noninvasive testing frequently contributed to surgical delays. In 2009, hip fracture patients arriving to the emergency department (ED) waited an average of 51 hours before being transferred to the operating room (OR) for surgery. Presuming prompt surgery is both desirable and feasible, the Grant Hip Fracture Management Committee (GHFMC) was developed in order to expedite surgeries in hip fracture patients. The GHFMC recommended a preoperative hip fracture protocol, and the outcomes from protocol implementation are described in this article.
Methods
This study was approved by the OhioHealth Institutional Review Board, with a waiver of the informed consent requirement. Medical records from patients treated at GMC during the time period between January 2010 and April 2014 (ie, following implementation of GHFMC recommendations) were retrospectively reviewed to identify the extent to which the use of preoperative transthoracic echocardiography (TTE) reduced average time to surgery and total length of stay, compared to cardiac consultation. This chart review included 316 participants and was used to identify primary induction agent utilized, time to medical optimization, time to surgery, and total length of hospital stay.
Intervention
The GHFMC conducted a 9-month quality improvement project to decrease ED-to-OR time to less than 24 hours for hip fracture patients. The multidisciplinary committee consisted of physicians from orthopedic surgery, anesthesia, hospital medicine, and geriatrics, along with key administrators and nurse outcomes managers. While there is lack of complete clarity surrounding optimal surgical timing, the committee decided that surgery within 24 hours would be beneficial for the majority of patients and therefore was considered a prudent goal.
Based on identified barriers that contributed to surgical delays, several process improvement strategies were implemented, including admitting patients to the hospitalist service, engaging the orthopedic trauma team, and implementing pre- and postoperative protocols and order sets (eg, ED and pain management order sets). Specific emphasis was placed on establishing guidelines for determining medical optimization. In the absence of established guidelines, medical optimization was determined at the discretion of the attending physician. The necessity of preoperative cardiac assessment was based, in part, on physician concerns about determining safe anesthesia protocols and hemodynamically managing patients who may have occult heart disease, specifically those patients with low functional capacity (< 4 METs) and/or inability to accurately communicate their medical history.
Many hip fractures result from a fall, and it may be unclear whether the fall causing a fracture was purely mechanical or indicative of a distinct acute or chronic illness. As a result, many patients received cardiac consultations, with or without pharmacologic stress testing, adding another 24 to 36 hours to preoperative time. As invasive preoperative cardiac procedures generally result in surgical delays without improving outcomes,11 the committee recommended that clinicians reserve preoperative cardiac consultation for patients with active cardiac conditions.
In lieu of cardiac consultation, the committee suggested preoperative TTE. While use of TTE has not been shown to improve preoperative risk stratification in routine noncardiac surgeries, it has been shown to provide clinically useful information in patients at high risk for cardiac complications.11 There was consensus for incorporating preoperative TTE for several reasons: (1) the patients with hip fractures were not “routine,” and often did not have a reliable medical history; (2) a large percentage of patients had cardiac risk factors; (3) patients with undiagnosed aortic stenosis, severe left ventricular dysfunction, or severe pulmonary hypertension would likely have altered intraoperative fluid management; and (4) in supplanting cardiac consultations, TTE would likely expedite patients’ ED-to-OR times. Therefore, the GHFMC created a recommendation of ordering urgent TTE for patients who were unable to exercise at ≥ 4 METs but needed urgent hip fracture surgery.
In order to evaluate the success of the new protocol, the ED-to-OR times were calculated for a cohort of patients who underwent surgery for hip fracture following algorithm implementation.
Participants
A chart review was conducted for patients admitted to GMC between January 2010 and April 2014 for operative treatment of a hip fracture. Exclusion criteria included lack of radiologist-diagnosed hip fracture, periprosthetic hip fracture, or multiple traumas. Electronic patient charts were reviewed by investigators (KI and BO) using a standardized, electronic abstraction form for 3 groups of patients who (1) proceeded directly to planned surgery without TTE or cardiac consultation (direct-to-surgery group); (2) received preoperative TTE but not a cardiac consultation (TTE-only group); or (3) received preoperative cardiac consultation (cardiac consult group).
Measures
Demographics, comorbid conditions, MET score, anesthesia protocol, and in-hospital morbidity and mortality were extracted from medical charts. Medical optimization time was determined by the latest time stamp of 1 of the following: time that the final consulting specialist stated that the patient was stable for surgery; time that the hospitalist described the patient as being ready for surgery; time that the TTE report was certified by the reading cardiologist; or time that the hospitalist described the outcome of completed preoperative risk stratification. Time elapsed prior to medical optimization, surgery, and discharge were calculated using differences between the patient’s arrival date and time at the ED, first recorded time of medical optimization, surgical start time (from the surgical report), and discharge time, respectively.
To assess whether the TTE protocol may have affected anesthesia selection, the induction agent (etomidate or propofol) was abstracted from anesthesia reports and stratified by the ejection fraction of each patient: very low (≤ 35%), low (36%–50%), or normal (> 50%). Patients without an echocardiogram report were assumed to have a normal ejection fraction for this analysis.
Analysis
Descriptive statistics were produced using mean and standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. To determine whether statistically significant differences existed between the 3 groups, the Kruskal-Wallis test was used to compare skewed continuous variables, and Pearson’s chi-square test was used to compare categorical variables. Due to differences in baseline patient characteristics across the 3 treatment groups, inverse probability weights were used to adjust for group differences (using a multinomial logit treatment model) while comparing differences in outcome variables. This modeling strategy does not rely on any assumptions for the distribution of the outcome variable. Covariates were considered for inclusion in the treatment or outcome model if they were significantly associated (P < 0.05) with the group variable. Additionally, anesthetic agent (etomidate or propofol) was compared across the treatment groups after stratifying by ejection fraction to identify whether any differences existed in anesthesia regimen. Patients who were prescribed more than 1 anesthetic agent (n = 2) or an agent that was not of interest were removed from the analysis (n = 13). Stata (version 14) was used for analysis. All other missing data with respect to the tested variables were omitted in the analysis for that variable. Any disagreements about abstraction were resolved through consensus between the investigators.
Results
A total of 316 cases met inclusion criteria, including 108 direct-to-surgery patients, 143 preoperative TTE patients, and 65 cardiac consult patients. Patient demographics and preoperative characteristics are shown in Table 1. The average age for all patients was 76.5 years of age (SD, 12.89; IQR, 34-97); however, direct-to-surgery patients were significantly (P < 0.001) younger (71.2 years; SD, 14.2; interquartile range [IQR], 34-95 years) than TTE-only patients (79.0 years; SD, 11.5; IQR, 35-97 years) and cardiac consult patients (79.57 years; SD, 10.63; IQR, 49-97 years). The majority of patients were female (69.9%) and experienced a fall prior to admission (94%). Almost three-fourths of patients had 1 or more cardiac risk factors (73.7%), including history of congestive heart failure (CHF; 19%), coronary artery disease (CAD; 26.3%), chronic obstructive pulmonary disease (COPD; 19.3%), or aortic stenosis (AS; 3.5%). Due to between-group differences in these comorbid conditions, confounding factors were adjusted for in subsequent analyses.

As shown in Table 2, before adjustment for confounding factors, there were significant between-group differences in medical optimization time for patients in all 3 groups. After adjustment for treatment differences using age and number of comorbid diseases, and medical optimization time differences using age and COPD, fewer between-group differences were statistically significant. Patients who received a cardiac consult had an 18.44-hour longer medical optimization time compared to patients who went directly to surgery (29.136 vs 10.696 hours; P = 0.001). Optimization remained approximately 5 hours longer for the TTE-only group than for the direct-to-surgery group; however, this difference was not significant (P = 0.075).
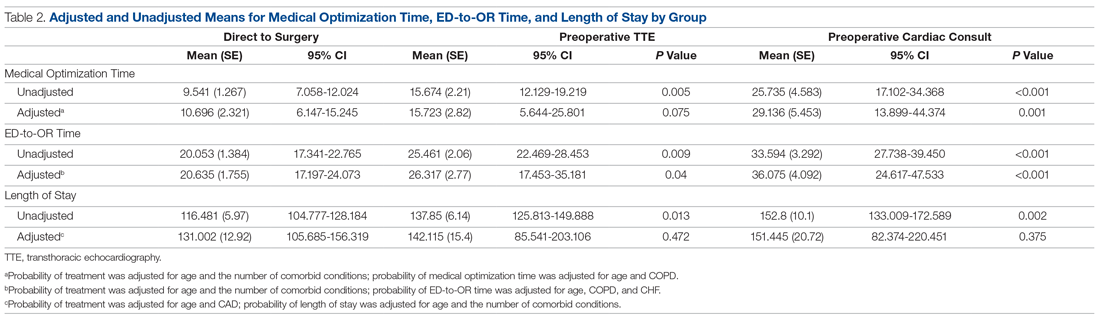
When comparing differences in ED-to-OR time for the 3 groups after adjusting the probability of treatment for age and the number of comorbid conditions, and adjusting the probability of ED-to-OR time for age, COPD, and CHF, significant differences remained in ED-to-OR times across all groups. Specifically, patients in the direct-to-surgery group experienced the shortest time (mean, 20.64 hours), compared to patients in the TTE-only group (mean, 26.32; P = 0.04) or patients in the cardiac consult group (mean, 36.08; P < 0.001). TTE-only patients had a longer time of 5.68 hours, compared to the direct-to-surgery group, and patients in the preoperative cardiac consult group were on average 15.44 hours longer than the direct-to-surgery group.
When comparing differences in the length of stay for the 3 groups before statistical adjustments, differences were observed; however, after removing the confounding factors related to treatment (age and CAD) and the outcome (age and the number of comorbid conditions), there were no statistically significant differences in the length of stay for the 3 groups. Average length of stay was 131 hours for direct-to-surgery patients, 142 hours for TTE-only patients, and 141 hours for cardiac consult patients.
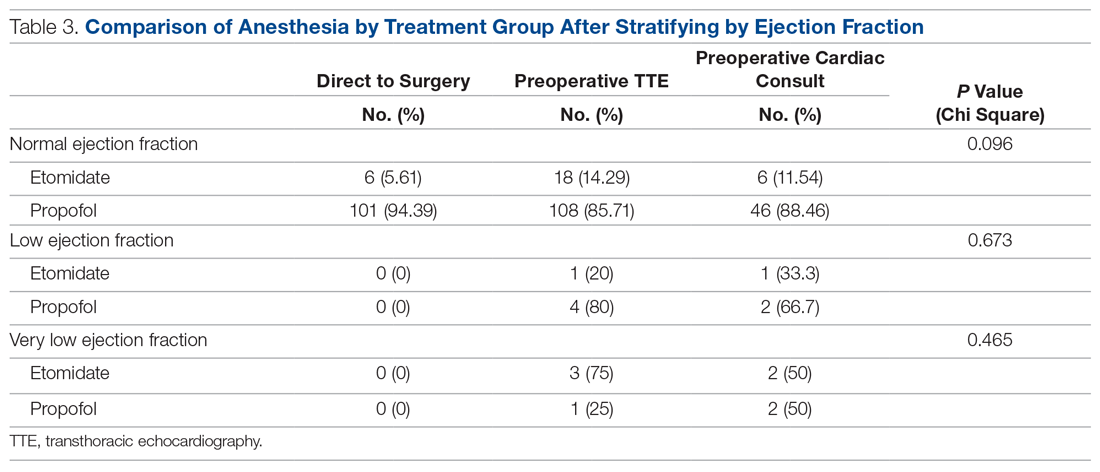
The use of different anesthetic agents was compared for patients in the 3 groups. The majority of patients in the study (87.7%) were given propofol, and there were no differences after stratifying by ejection fraction (Table 3).
Discussion
The GHFMC was created to reduce surgical delays for hip fracture. Medical optimization was considered a primary, modifiable factor given that surgeons were reluctant to proceed without a cardiac consult. To address this gap, the committee recommended a preoperative TTE for patients with low or unknown functional status. This threshold provides a quick and easy method for stratifying patients who previously required risk stratification by a cardiologist, which often resulted in surgery delays.
In their recommendations for implementation of hip fracture quality improvement projects, the Geriatric Fracture Center emphasizes the importance of multidisciplinary physician leadership along with standardization of approach across patients.12 This recommendation is supported by increasing evidence that orthogeriatric collaborations are associated with decreased mortality and length of stay.13 The GHFMC and subsequent interventions reflect this approach, allowing for collaboration to identify cross-disciplinary procedural barriers to care. In our institution, addressing identified procedural barriers to care was associated with a reduction in the average time to surgery from 51 hours to 25.3 hours.
Multiple approaches have been attempted to decrease presurgical time in hip fracture patients in various settings. Prehospital interventions, such as providing ambulances with checklists and ability to bypass the ED, have not been shown to decrease time to surgery for hip fracture patients, though similar strategies have been successful in other conditions, such as stroke.14,15 In-hospital procedures, such as implementation of a hip fracture protocol and reduction of preoperative interventions, have more consistently been found to decrease time to surgery and in-hospital mortality.16,17 However, reduced delays have not been found universally. Luttrell and Nana found that preoperative TTE resulted in approximately 30.8-hour delays from the ED to OR, compared to patients who did not receive a preoperative TTE.18 However, in that study hospitalists used TTE at their own discretion, and there may have been confounding factors contributing to delays. When used as part of a protocol targeting patients with poor or unknown functional capacity, we believe that preoperative TTE results in modest surgical delays yet provides clinically useful information about each patient.
ACC/AHA preoperative guidelines were updated after we implemented our intervention and now recommend that patients with poor or unknown functional capacity in whom stress testing will not influence care proceed to surgery “according to guideline-directed medical care.”11 While routine use of preoperative evaluation of left ventricular function is not recommended, assessing left ventricular function may be reasonable for patients with heart failure with a change in clinical status. Guidelines also recommend that patients with clinically suspected valvular stenosis undergo preoperative echocardiography.11
Limitations
This study has several limitations. First, due to resource limitations, a substantial period of time elapsed between implementation of the new protocol and the analysis of the data set. That is, the hip fracture protocol assessed in this paper occurred from January 2010 through April 2014, and final analysis of the data set occurred in April 2020. This limitation precludes our ability to formally assess any pre- or post-protocol changes in patient outcomes. Second, randomization was not used to create groups that were balanced in differing health characteristics (ie, patients with noncardiac-related surgeries, patients in different age groups); however, the use of inverse probability treatment regression analysis was a way to statistically address these between-group differences. Moreover, this study is limited by the factors that were measured; unmeasured factors cannot be accounted for. Third, health care providers working at the hospital during this time were aware of the goal to decrease presurgical time, possibly creating exaggerated effects compared to a blinded trial. Finally, although this intervention is likely translatable to other centers, these results represent the experiences of a single level 1 trauma center and may not be replicable elsewhere.
Conclusion
Preoperative TTE in lieu of cardiac consultation has several advantages. First, it requires interdepartmental collaboration for implementation, but can be implemented through a single hospital or hospital system. Unlike prehospital interventions, preoperative urgent TTE for patients with low functional capacity does not require the support of emergency medical technicians, ambulance services, or other hospitals in the region. Second, while costs are associated with TTE, they are offset by a reduction in expensive consultations with specialists, surgical delays, and longer lengths of stay. Third, despite likely increased ED-to-OR times compared to no intervention, urgent TTE decreases time to surgery compared with cardiology consultation. Prior to the GHFMC, the ED-to-OR time at our institution was 51 hours. In contrast, the mean time following the GHFMC-led protocol was less than half that, at 25.3 hours (SD, 19.1 hours). In fact, nearly two-thirds (65.2%) of the patients evaluated in this study underwent surgery within 24 hours of admission. This improvement in presurgical time was attributed, in part, to the implementation of preoperative TTE over cardiology consultations.
Acknowledgments: The authors thank Jenny Williams, RN, who was instrumental in obtaining the data set for analysis, and Shauna Ayres, MPH, from the OhioHealth Research Institute, who provided writing and technical assistance.
Corresponding author: Robert Skully, MD, OhioHealth Family Medicine Grant, 290 East Town St., Columbus, OH 43215; [email protected].
Funding: This work was supported by the OhioHealth Summer Research Externship Program.
Financial disclosures: None.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
2. Lewiecki EM, Wright NC, Curtis JR, et al. Hip fracture trends in the United States 2002 to 2015. Osteoporos Int. 2018;29:717-722.
3. Colais P, Di Martino M, Fusco D, et al. The effect of early surgery after hip fracture on 1-year mortality. BMC Geriatr. 2015;15:141.
4. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality after proximal femoral fracture: a retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg Am. 2015;97:1333-1339.
5. Judd KT, Christianson E. Expedited operative care of hip fractures results in significantly lower cost of treatment. Iowa Orthop J. 2015;35:62-64.
6. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182:1609-1616.
7. Ryan DJ, Yoshihara H, Yoneoka D, et al. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma. 2015;29:343-348.
8. Ricci WM, Brandt A, McAndrew C, Gardner MJ. Factors affecting delay to surgery and length of stay for patients with hip fracture. J Orthop Trauma. 2015;29:e109-e114.
9. Hagino T, Ochiai S, Senga S, et al. Efficacy of early surgery and causes of surgical delay in patients with hip fracture. J Orthop. 2015;12:142-146.
10. Rafiq A, Sklyar E, Bella JN. Cardiac evaluation and monitoring of patients undergoing noncardiac surgery. Health Serv Insights. 2017;9:1178632916686074.
11. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
12. Basu N, Natour M, Mounasamy V, Kates SL. Geriatric hip fracture management: keys to providing a successful program. Eur J Trauma Emerg Surg. 2016;42:565-569.
13. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28:e49-e55.
14. Tai YJ, Yan B. Minimising time to treatment: targeted strategies to minimise time to thrombolysis for acute ischaemic stroke. Intern Med J. 2013;43:1176-1182.
15. Larsson G, Stromberg RU, Rogmark C, Nilsdotter A. Prehospital fast track care for patients with hip fracture: Impact on time to surgery, hospital stay, post-operative complications and mortality a randomised, controlled trial. Injury. 2016;47:881-886.
16. Bohm E, Loucks L, Wittmeier K, et al. Reduced time to surgery improves mortality and length of stay following hip fracture: results from an intervention study in a Canadian health authority. Can J Surg. 2015;58:257-263.
17. Ventura C, Trombetti S, Pioli G, et al. Impact of multidisciplinary hip fracture program on timing of surgery in elderly patients. Osteoporos Int J. 2014;25:2591-2597.
18. Luttrell K, Nana A. Effect of preoperative transthoracic echocardiogram on mortality and surgical timing in elderly adults with hip fracture. J Am Geriatr Soc. 2015;63:2505-2509.
From Dignity Health Methodist Hospital of Sacramento Family Medicine Residency Program, Sacramento, CA (Dr. Oldach); Nationwide Children’s Hospital, Columbus, OH (Dr. Irwin); OhioHealth Research Institute, Columbus, OH (Dr. Pershing); Department of Clinical Transformation, OhioHealth, Columbus, OH (Dr. Zigmont and Dr. Gascon); and Department of Geriatrics, OhioHealth, Columbus, OH (Dr. Skully).
Abstract
Objective: An interdisciplinary committee was formed to identify factors contributing to surgical delays in urgent hip fracture repair at an urban, level 1 trauma center, with the goal of reducing preoperative time to less than 24 hours. Surgical optimization was identified as a primary, modifiable factor, as surgeons were reluctant to clear patients for surgery without cardiac consultation. Preoperative transthoracic echocardiogram (TTE) was recommended as a safe alternative to cardiac consultation in most patients.
Methods: A retrospective review was conducted for patients who underwent urgent hip fracture repair between January 2010 and April 2014 (n = 316). Time to medical optimization, time to surgery, hospital length of stay, and anesthesia induction were compared for 3 patient groups of interest: those who received (1) neither TTE nor cardiology consultation (ie, direct to surgery); (2) a preoperative TTE; or (3) preoperative cardiac consultation.
Results: There were significant between-group differences in medical optimization time (P = 0.001) and mean time to surgery (P < 0.001) when comparing the 3 groups of interest. Patients in the preoperative cardiac consult group had the longest times, followed by the TTE and direct-to-surgery groups. There were no differences in the type of induction agent used across treatment groups when stratifying by ejection fraction.
Conclusion: Preoperative TTE allows for decreased preoperative time compared to a cardiology consultation. It provides an easily implemented inter-departmental, intra-institutional intervention to decrease preoperative time in patients presenting with hip fractures.
Keywords: surgical delay; preoperative risk stratification; process improvement.
Hip fractures are common, expensive, and associated with poor outcomes.1,2 Ample literature suggests that morbidity, mortality, and cost of care may be reduced by minimizing surgical delays.3-5 While individual reports indicate mixed evidence, in a 2010 meta-analysis, surgery within 72 hours was associated with significant reductions in pneumonia and pressure sores, as well as a 19% reduction in all-cause mortality through 1 year.6 Additional reviews suggest evidence of improved patient outcomes (pain, length of stay, non-union, and/or mortality) when surgery occurs early, within 12 to 72 hours after injury.4,6,7 Regardless of the definition of “early surgery” used, surgical delay remains a challenge, often due to organizational factors, including admission day of the week and hospital staffing, and patient characteristics, such as comorbidities, echocardiographic findings, age, and insurance status.7-9
Among factors that contribute to surgical delays, the need for preoperative cardiovascular risk stratification is significantly modifiable.10 The American College of Cardiology (ACC)/American Heart Association (AHA) Task Force risk stratification framework for preoperative cardiac testing assists clinicians in determining surgical urgency, active cardiac conditions, cardiovascular risk factors, and functional capacity of each patient, and is well established for low- or intermediate-risk patients.11 Specifically, metabolic equivalents (METs) measurements are used to identify medically stable patients with good or excellent functional capacity versus poor or unknown functional status. Patients with ≥ 4 METs may proceed to surgery without further testing; patients with < 4 METs may either proceed with planned surgery or undergo additional testing. Patients with a perceived increased risk profile who require urgent or semi-urgent hip fracture repair may be confounded by disagreement about required preoperative cardiac testing.
At OhioHealth Grant Medical Center (GMC), an urban, level 1 trauma center, the consideration of further preoperative noninvasive testing frequently contributed to surgical delays. In 2009, hip fracture patients arriving to the emergency department (ED) waited an average of 51 hours before being transferred to the operating room (OR) for surgery. Presuming prompt surgery is both desirable and feasible, the Grant Hip Fracture Management Committee (GHFMC) was developed in order to expedite surgeries in hip fracture patients. The GHFMC recommended a preoperative hip fracture protocol, and the outcomes from protocol implementation are described in this article.
Methods
This study was approved by the OhioHealth Institutional Review Board, with a waiver of the informed consent requirement. Medical records from patients treated at GMC during the time period between January 2010 and April 2014 (ie, following implementation of GHFMC recommendations) were retrospectively reviewed to identify the extent to which the use of preoperative transthoracic echocardiography (TTE) reduced average time to surgery and total length of stay, compared to cardiac consultation. This chart review included 316 participants and was used to identify primary induction agent utilized, time to medical optimization, time to surgery, and total length of hospital stay.
Intervention
The GHFMC conducted a 9-month quality improvement project to decrease ED-to-OR time to less than 24 hours for hip fracture patients. The multidisciplinary committee consisted of physicians from orthopedic surgery, anesthesia, hospital medicine, and geriatrics, along with key administrators and nurse outcomes managers. While there is lack of complete clarity surrounding optimal surgical timing, the committee decided that surgery within 24 hours would be beneficial for the majority of patients and therefore was considered a prudent goal.
Based on identified barriers that contributed to surgical delays, several process improvement strategies were implemented, including admitting patients to the hospitalist service, engaging the orthopedic trauma team, and implementing pre- and postoperative protocols and order sets (eg, ED and pain management order sets). Specific emphasis was placed on establishing guidelines for determining medical optimization. In the absence of established guidelines, medical optimization was determined at the discretion of the attending physician. The necessity of preoperative cardiac assessment was based, in part, on physician concerns about determining safe anesthesia protocols and hemodynamically managing patients who may have occult heart disease, specifically those patients with low functional capacity (< 4 METs) and/or inability to accurately communicate their medical history.
Many hip fractures result from a fall, and it may be unclear whether the fall causing a fracture was purely mechanical or indicative of a distinct acute or chronic illness. As a result, many patients received cardiac consultations, with or without pharmacologic stress testing, adding another 24 to 36 hours to preoperative time. As invasive preoperative cardiac procedures generally result in surgical delays without improving outcomes,11 the committee recommended that clinicians reserve preoperative cardiac consultation for patients with active cardiac conditions.
In lieu of cardiac consultation, the committee suggested preoperative TTE. While use of TTE has not been shown to improve preoperative risk stratification in routine noncardiac surgeries, it has been shown to provide clinically useful information in patients at high risk for cardiac complications.11 There was consensus for incorporating preoperative TTE for several reasons: (1) the patients with hip fractures were not “routine,” and often did not have a reliable medical history; (2) a large percentage of patients had cardiac risk factors; (3) patients with undiagnosed aortic stenosis, severe left ventricular dysfunction, or severe pulmonary hypertension would likely have altered intraoperative fluid management; and (4) in supplanting cardiac consultations, TTE would likely expedite patients’ ED-to-OR times. Therefore, the GHFMC created a recommendation of ordering urgent TTE for patients who were unable to exercise at ≥ 4 METs but needed urgent hip fracture surgery.
In order to evaluate the success of the new protocol, the ED-to-OR times were calculated for a cohort of patients who underwent surgery for hip fracture following algorithm implementation.
Participants
A chart review was conducted for patients admitted to GMC between January 2010 and April 2014 for operative treatment of a hip fracture. Exclusion criteria included lack of radiologist-diagnosed hip fracture, periprosthetic hip fracture, or multiple traumas. Electronic patient charts were reviewed by investigators (KI and BO) using a standardized, electronic abstraction form for 3 groups of patients who (1) proceeded directly to planned surgery without TTE or cardiac consultation (direct-to-surgery group); (2) received preoperative TTE but not a cardiac consultation (TTE-only group); or (3) received preoperative cardiac consultation (cardiac consult group).
Measures
Demographics, comorbid conditions, MET score, anesthesia protocol, and in-hospital morbidity and mortality were extracted from medical charts. Medical optimization time was determined by the latest time stamp of 1 of the following: time that the final consulting specialist stated that the patient was stable for surgery; time that the hospitalist described the patient as being ready for surgery; time that the TTE report was certified by the reading cardiologist; or time that the hospitalist described the outcome of completed preoperative risk stratification. Time elapsed prior to medical optimization, surgery, and discharge were calculated using differences between the patient’s arrival date and time at the ED, first recorded time of medical optimization, surgical start time (from the surgical report), and discharge time, respectively.
To assess whether the TTE protocol may have affected anesthesia selection, the induction agent (etomidate or propofol) was abstracted from anesthesia reports and stratified by the ejection fraction of each patient: very low (≤ 35%), low (36%–50%), or normal (> 50%). Patients without an echocardiogram report were assumed to have a normal ejection fraction for this analysis.
Analysis
Descriptive statistics were produced using mean and standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. To determine whether statistically significant differences existed between the 3 groups, the Kruskal-Wallis test was used to compare skewed continuous variables, and Pearson’s chi-square test was used to compare categorical variables. Due to differences in baseline patient characteristics across the 3 treatment groups, inverse probability weights were used to adjust for group differences (using a multinomial logit treatment model) while comparing differences in outcome variables. This modeling strategy does not rely on any assumptions for the distribution of the outcome variable. Covariates were considered for inclusion in the treatment or outcome model if they were significantly associated (P < 0.05) with the group variable. Additionally, anesthetic agent (etomidate or propofol) was compared across the treatment groups after stratifying by ejection fraction to identify whether any differences existed in anesthesia regimen. Patients who were prescribed more than 1 anesthetic agent (n = 2) or an agent that was not of interest were removed from the analysis (n = 13). Stata (version 14) was used for analysis. All other missing data with respect to the tested variables were omitted in the analysis for that variable. Any disagreements about abstraction were resolved through consensus between the investigators.
Results
A total of 316 cases met inclusion criteria, including 108 direct-to-surgery patients, 143 preoperative TTE patients, and 65 cardiac consult patients. Patient demographics and preoperative characteristics are shown in Table 1. The average age for all patients was 76.5 years of age (SD, 12.89; IQR, 34-97); however, direct-to-surgery patients were significantly (P < 0.001) younger (71.2 years; SD, 14.2; interquartile range [IQR], 34-95 years) than TTE-only patients (79.0 years; SD, 11.5; IQR, 35-97 years) and cardiac consult patients (79.57 years; SD, 10.63; IQR, 49-97 years). The majority of patients were female (69.9%) and experienced a fall prior to admission (94%). Almost three-fourths of patients had 1 or more cardiac risk factors (73.7%), including history of congestive heart failure (CHF; 19%), coronary artery disease (CAD; 26.3%), chronic obstructive pulmonary disease (COPD; 19.3%), or aortic stenosis (AS; 3.5%). Due to between-group differences in these comorbid conditions, confounding factors were adjusted for in subsequent analyses.

As shown in Table 2, before adjustment for confounding factors, there were significant between-group differences in medical optimization time for patients in all 3 groups. After adjustment for treatment differences using age and number of comorbid diseases, and medical optimization time differences using age and COPD, fewer between-group differences were statistically significant. Patients who received a cardiac consult had an 18.44-hour longer medical optimization time compared to patients who went directly to surgery (29.136 vs 10.696 hours; P = 0.001). Optimization remained approximately 5 hours longer for the TTE-only group than for the direct-to-surgery group; however, this difference was not significant (P = 0.075).

When comparing differences in ED-to-OR time for the 3 groups after adjusting the probability of treatment for age and the number of comorbid conditions, and adjusting the probability of ED-to-OR time for age, COPD, and CHF, significant differences remained in ED-to-OR times across all groups. Specifically, patients in the direct-to-surgery group experienced the shortest time (mean, 20.64 hours), compared to patients in the TTE-only group (mean, 26.32; P = 0.04) or patients in the cardiac consult group (mean, 36.08; P < 0.001). TTE-only patients had a longer time of 5.68 hours, compared to the direct-to-surgery group, and patients in the preoperative cardiac consult group were on average 15.44 hours longer than the direct-to-surgery group.
When comparing differences in the length of stay for the 3 groups before statistical adjustments, differences were observed; however, after removing the confounding factors related to treatment (age and CAD) and the outcome (age and the number of comorbid conditions), there were no statistically significant differences in the length of stay for the 3 groups. Average length of stay was 131 hours for direct-to-surgery patients, 142 hours for TTE-only patients, and 141 hours for cardiac consult patients.
The use of different anesthetic agents was compared for patients in the 3 groups. The majority of patients in the study (87.7%) were given propofol, and there were no differences after stratifying by ejection fraction (Table 3).

Discussion
The GHFMC was created to reduce surgical delays for hip fracture. Medical optimization was considered a primary, modifiable factor given that surgeons were reluctant to proceed without a cardiac consult. To address this gap, the committee recommended a preoperative TTE for patients with low or unknown functional status. This threshold provides a quick and easy method for stratifying patients who previously required risk stratification by a cardiologist, which often resulted in surgery delays.
In their recommendations for implementation of hip fracture quality improvement projects, the Geriatric Fracture Center emphasizes the importance of multidisciplinary physician leadership along with standardization of approach across patients.12 This recommendation is supported by increasing evidence that orthogeriatric collaborations are associated with decreased mortality and length of stay.13 The GHFMC and subsequent interventions reflect this approach, allowing for collaboration to identify cross-disciplinary procedural barriers to care. In our institution, addressing identified procedural barriers to care was associated with a reduction in the average time to surgery from 51 hours to 25.3 hours.
Multiple approaches have been attempted to decrease presurgical time in hip fracture patients in various settings. Prehospital interventions, such as providing ambulances with checklists and ability to bypass the ED, have not been shown to decrease time to surgery for hip fracture patients, though similar strategies have been successful in other conditions, such as stroke.14,15 In-hospital procedures, such as implementation of a hip fracture protocol and reduction of preoperative interventions, have more consistently been found to decrease time to surgery and in-hospital mortality.16,17 However, reduced delays have not been found universally. Luttrell and Nana found that preoperative TTE resulted in approximately 30.8-hour delays from the ED to OR, compared to patients who did not receive a preoperative TTE.18 However, in that study hospitalists used TTE at their own discretion, and there may have been confounding factors contributing to delays. When used as part of a protocol targeting patients with poor or unknown functional capacity, we believe that preoperative TTE results in modest surgical delays yet provides clinically useful information about each patient.
ACC/AHA preoperative guidelines were updated after we implemented our intervention and now recommend that patients with poor or unknown functional capacity in whom stress testing will not influence care proceed to surgery “according to guideline-directed medical care.”11 While routine use of preoperative evaluation of left ventricular function is not recommended, assessing left ventricular function may be reasonable for patients with heart failure with a change in clinical status. Guidelines also recommend that patients with clinically suspected valvular stenosis undergo preoperative echocardiography.11
Limitations
This study has several limitations. First, due to resource limitations, a substantial period of time elapsed between implementation of the new protocol and the analysis of the data set. That is, the hip fracture protocol assessed in this paper occurred from January 2010 through April 2014, and final analysis of the data set occurred in April 2020. This limitation precludes our ability to formally assess any pre- or post-protocol changes in patient outcomes. Second, randomization was not used to create groups that were balanced in differing health characteristics (ie, patients with noncardiac-related surgeries, patients in different age groups); however, the use of inverse probability treatment regression analysis was a way to statistically address these between-group differences. Moreover, this study is limited by the factors that were measured; unmeasured factors cannot be accounted for. Third, health care providers working at the hospital during this time were aware of the goal to decrease presurgical time, possibly creating exaggerated effects compared to a blinded trial. Finally, although this intervention is likely translatable to other centers, these results represent the experiences of a single level 1 trauma center and may not be replicable elsewhere.
Conclusion
Preoperative TTE in lieu of cardiac consultation has several advantages. First, it requires interdepartmental collaboration for implementation, but can be implemented through a single hospital or hospital system. Unlike prehospital interventions, preoperative urgent TTE for patients with low functional capacity does not require the support of emergency medical technicians, ambulance services, or other hospitals in the region. Second, while costs are associated with TTE, they are offset by a reduction in expensive consultations with specialists, surgical delays, and longer lengths of stay. Third, despite likely increased ED-to-OR times compared to no intervention, urgent TTE decreases time to surgery compared with cardiology consultation. Prior to the GHFMC, the ED-to-OR time at our institution was 51 hours. In contrast, the mean time following the GHFMC-led protocol was less than half that, at 25.3 hours (SD, 19.1 hours). In fact, nearly two-thirds (65.2%) of the patients evaluated in this study underwent surgery within 24 hours of admission. This improvement in presurgical time was attributed, in part, to the implementation of preoperative TTE over cardiology consultations.
Acknowledgments: The authors thank Jenny Williams, RN, who was instrumental in obtaining the data set for analysis, and Shauna Ayres, MPH, from the OhioHealth Research Institute, who provided writing and technical assistance.
Corresponding author: Robert Skully, MD, OhioHealth Family Medicine Grant, 290 East Town St., Columbus, OH 43215; [email protected].
Funding: This work was supported by the OhioHealth Summer Research Externship Program.
Financial disclosures: None.
From Dignity Health Methodist Hospital of Sacramento Family Medicine Residency Program, Sacramento, CA (Dr. Oldach); Nationwide Children’s Hospital, Columbus, OH (Dr. Irwin); OhioHealth Research Institute, Columbus, OH (Dr. Pershing); Department of Clinical Transformation, OhioHealth, Columbus, OH (Dr. Zigmont and Dr. Gascon); and Department of Geriatrics, OhioHealth, Columbus, OH (Dr. Skully).
Abstract
Objective: An interdisciplinary committee was formed to identify factors contributing to surgical delays in urgent hip fracture repair at an urban, level 1 trauma center, with the goal of reducing preoperative time to less than 24 hours. Surgical optimization was identified as a primary, modifiable factor, as surgeons were reluctant to clear patients for surgery without cardiac consultation. Preoperative transthoracic echocardiogram (TTE) was recommended as a safe alternative to cardiac consultation in most patients.
Methods: A retrospective review was conducted for patients who underwent urgent hip fracture repair between January 2010 and April 2014 (n = 316). Time to medical optimization, time to surgery, hospital length of stay, and anesthesia induction were compared for 3 patient groups of interest: those who received (1) neither TTE nor cardiology consultation (ie, direct to surgery); (2) a preoperative TTE; or (3) preoperative cardiac consultation.
Results: There were significant between-group differences in medical optimization time (P = 0.001) and mean time to surgery (P < 0.001) when comparing the 3 groups of interest. Patients in the preoperative cardiac consult group had the longest times, followed by the TTE and direct-to-surgery groups. There were no differences in the type of induction agent used across treatment groups when stratifying by ejection fraction.
Conclusion: Preoperative TTE allows for decreased preoperative time compared to a cardiology consultation. It provides an easily implemented inter-departmental, intra-institutional intervention to decrease preoperative time in patients presenting with hip fractures.
Keywords: surgical delay; preoperative risk stratification; process improvement.
Hip fractures are common, expensive, and associated with poor outcomes.1,2 Ample literature suggests that morbidity, mortality, and cost of care may be reduced by minimizing surgical delays.3-5 While individual reports indicate mixed evidence, in a 2010 meta-analysis, surgery within 72 hours was associated with significant reductions in pneumonia and pressure sores, as well as a 19% reduction in all-cause mortality through 1 year.6 Additional reviews suggest evidence of improved patient outcomes (pain, length of stay, non-union, and/or mortality) when surgery occurs early, within 12 to 72 hours after injury.4,6,7 Regardless of the definition of “early surgery” used, surgical delay remains a challenge, often due to organizational factors, including admission day of the week and hospital staffing, and patient characteristics, such as comorbidities, echocardiographic findings, age, and insurance status.7-9
Among factors that contribute to surgical delays, the need for preoperative cardiovascular risk stratification is significantly modifiable.10 The American College of Cardiology (ACC)/American Heart Association (AHA) Task Force risk stratification framework for preoperative cardiac testing assists clinicians in determining surgical urgency, active cardiac conditions, cardiovascular risk factors, and functional capacity of each patient, and is well established for low- or intermediate-risk patients.11 Specifically, metabolic equivalents (METs) measurements are used to identify medically stable patients with good or excellent functional capacity versus poor or unknown functional status. Patients with ≥ 4 METs may proceed to surgery without further testing; patients with < 4 METs may either proceed with planned surgery or undergo additional testing. Patients with a perceived increased risk profile who require urgent or semi-urgent hip fracture repair may be confounded by disagreement about required preoperative cardiac testing.
At OhioHealth Grant Medical Center (GMC), an urban, level 1 trauma center, the consideration of further preoperative noninvasive testing frequently contributed to surgical delays. In 2009, hip fracture patients arriving to the emergency department (ED) waited an average of 51 hours before being transferred to the operating room (OR) for surgery. Presuming prompt surgery is both desirable and feasible, the Grant Hip Fracture Management Committee (GHFMC) was developed in order to expedite surgeries in hip fracture patients. The GHFMC recommended a preoperative hip fracture protocol, and the outcomes from protocol implementation are described in this article.
Methods
This study was approved by the OhioHealth Institutional Review Board, with a waiver of the informed consent requirement. Medical records from patients treated at GMC during the time period between January 2010 and April 2014 (ie, following implementation of GHFMC recommendations) were retrospectively reviewed to identify the extent to which the use of preoperative transthoracic echocardiography (TTE) reduced average time to surgery and total length of stay, compared to cardiac consultation. This chart review included 316 participants and was used to identify primary induction agent utilized, time to medical optimization, time to surgery, and total length of hospital stay.
Intervention
The GHFMC conducted a 9-month quality improvement project to decrease ED-to-OR time to less than 24 hours for hip fracture patients. The multidisciplinary committee consisted of physicians from orthopedic surgery, anesthesia, hospital medicine, and geriatrics, along with key administrators and nurse outcomes managers. While there is lack of complete clarity surrounding optimal surgical timing, the committee decided that surgery within 24 hours would be beneficial for the majority of patients and therefore was considered a prudent goal.
Based on identified barriers that contributed to surgical delays, several process improvement strategies were implemented, including admitting patients to the hospitalist service, engaging the orthopedic trauma team, and implementing pre- and postoperative protocols and order sets (eg, ED and pain management order sets). Specific emphasis was placed on establishing guidelines for determining medical optimization. In the absence of established guidelines, medical optimization was determined at the discretion of the attending physician. The necessity of preoperative cardiac assessment was based, in part, on physician concerns about determining safe anesthesia protocols and hemodynamically managing patients who may have occult heart disease, specifically those patients with low functional capacity (< 4 METs) and/or inability to accurately communicate their medical history.
Many hip fractures result from a fall, and it may be unclear whether the fall causing a fracture was purely mechanical or indicative of a distinct acute or chronic illness. As a result, many patients received cardiac consultations, with or without pharmacologic stress testing, adding another 24 to 36 hours to preoperative time. As invasive preoperative cardiac procedures generally result in surgical delays without improving outcomes,11 the committee recommended that clinicians reserve preoperative cardiac consultation for patients with active cardiac conditions.
In lieu of cardiac consultation, the committee suggested preoperative TTE. While use of TTE has not been shown to improve preoperative risk stratification in routine noncardiac surgeries, it has been shown to provide clinically useful information in patients at high risk for cardiac complications.11 There was consensus for incorporating preoperative TTE for several reasons: (1) the patients with hip fractures were not “routine,” and often did not have a reliable medical history; (2) a large percentage of patients had cardiac risk factors; (3) patients with undiagnosed aortic stenosis, severe left ventricular dysfunction, or severe pulmonary hypertension would likely have altered intraoperative fluid management; and (4) in supplanting cardiac consultations, TTE would likely expedite patients’ ED-to-OR times. Therefore, the GHFMC created a recommendation of ordering urgent TTE for patients who were unable to exercise at ≥ 4 METs but needed urgent hip fracture surgery.
In order to evaluate the success of the new protocol, the ED-to-OR times were calculated for a cohort of patients who underwent surgery for hip fracture following algorithm implementation.
Participants
A chart review was conducted for patients admitted to GMC between January 2010 and April 2014 for operative treatment of a hip fracture. Exclusion criteria included lack of radiologist-diagnosed hip fracture, periprosthetic hip fracture, or multiple traumas. Electronic patient charts were reviewed by investigators (KI and BO) using a standardized, electronic abstraction form for 3 groups of patients who (1) proceeded directly to planned surgery without TTE or cardiac consultation (direct-to-surgery group); (2) received preoperative TTE but not a cardiac consultation (TTE-only group); or (3) received preoperative cardiac consultation (cardiac consult group).
Measures
Demographics, comorbid conditions, MET score, anesthesia protocol, and in-hospital morbidity and mortality were extracted from medical charts. Medical optimization time was determined by the latest time stamp of 1 of the following: time that the final consulting specialist stated that the patient was stable for surgery; time that the hospitalist described the patient as being ready for surgery; time that the TTE report was certified by the reading cardiologist; or time that the hospitalist described the outcome of completed preoperative risk stratification. Time elapsed prior to medical optimization, surgery, and discharge were calculated using differences between the patient’s arrival date and time at the ED, first recorded time of medical optimization, surgical start time (from the surgical report), and discharge time, respectively.
To assess whether the TTE protocol may have affected anesthesia selection, the induction agent (etomidate or propofol) was abstracted from anesthesia reports and stratified by the ejection fraction of each patient: very low (≤ 35%), low (36%–50%), or normal (> 50%). Patients without an echocardiogram report were assumed to have a normal ejection fraction for this analysis.
Analysis
Descriptive statistics were produced using mean and standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. To determine whether statistically significant differences existed between the 3 groups, the Kruskal-Wallis test was used to compare skewed continuous variables, and Pearson’s chi-square test was used to compare categorical variables. Due to differences in baseline patient characteristics across the 3 treatment groups, inverse probability weights were used to adjust for group differences (using a multinomial logit treatment model) while comparing differences in outcome variables. This modeling strategy does not rely on any assumptions for the distribution of the outcome variable. Covariates were considered for inclusion in the treatment or outcome model if they were significantly associated (P < 0.05) with the group variable. Additionally, anesthetic agent (etomidate or propofol) was compared across the treatment groups after stratifying by ejection fraction to identify whether any differences existed in anesthesia regimen. Patients who were prescribed more than 1 anesthetic agent (n = 2) or an agent that was not of interest were removed from the analysis (n = 13). Stata (version 14) was used for analysis. All other missing data with respect to the tested variables were omitted in the analysis for that variable. Any disagreements about abstraction were resolved through consensus between the investigators.
Results
A total of 316 cases met inclusion criteria, including 108 direct-to-surgery patients, 143 preoperative TTE patients, and 65 cardiac consult patients. Patient demographics and preoperative characteristics are shown in Table 1. The average age for all patients was 76.5 years of age (SD, 12.89; IQR, 34-97); however, direct-to-surgery patients were significantly (P < 0.001) younger (71.2 years; SD, 14.2; interquartile range [IQR], 34-95 years) than TTE-only patients (79.0 years; SD, 11.5; IQR, 35-97 years) and cardiac consult patients (79.57 years; SD, 10.63; IQR, 49-97 years). The majority of patients were female (69.9%) and experienced a fall prior to admission (94%). Almost three-fourths of patients had 1 or more cardiac risk factors (73.7%), including history of congestive heart failure (CHF; 19%), coronary artery disease (CAD; 26.3%), chronic obstructive pulmonary disease (COPD; 19.3%), or aortic stenosis (AS; 3.5%). Due to between-group differences in these comorbid conditions, confounding factors were adjusted for in subsequent analyses.

As shown in Table 2, before adjustment for confounding factors, there were significant between-group differences in medical optimization time for patients in all 3 groups. After adjustment for treatment differences using age and number of comorbid diseases, and medical optimization time differences using age and COPD, fewer between-group differences were statistically significant. Patients who received a cardiac consult had an 18.44-hour longer medical optimization time compared to patients who went directly to surgery (29.136 vs 10.696 hours; P = 0.001). Optimization remained approximately 5 hours longer for the TTE-only group than for the direct-to-surgery group; however, this difference was not significant (P = 0.075).

When comparing differences in ED-to-OR time for the 3 groups after adjusting the probability of treatment for age and the number of comorbid conditions, and adjusting the probability of ED-to-OR time for age, COPD, and CHF, significant differences remained in ED-to-OR times across all groups. Specifically, patients in the direct-to-surgery group experienced the shortest time (mean, 20.64 hours), compared to patients in the TTE-only group (mean, 26.32; P = 0.04) or patients in the cardiac consult group (mean, 36.08; P < 0.001). TTE-only patients had a longer time of 5.68 hours, compared to the direct-to-surgery group, and patients in the preoperative cardiac consult group were on average 15.44 hours longer than the direct-to-surgery group.
When comparing differences in the length of stay for the 3 groups before statistical adjustments, differences were observed; however, after removing the confounding factors related to treatment (age and CAD) and the outcome (age and the number of comorbid conditions), there were no statistically significant differences in the length of stay for the 3 groups. Average length of stay was 131 hours for direct-to-surgery patients, 142 hours for TTE-only patients, and 141 hours for cardiac consult patients.
The use of different anesthetic agents was compared for patients in the 3 groups. The majority of patients in the study (87.7%) were given propofol, and there were no differences after stratifying by ejection fraction (Table 3).

Discussion
The GHFMC was created to reduce surgical delays for hip fracture. Medical optimization was considered a primary, modifiable factor given that surgeons were reluctant to proceed without a cardiac consult. To address this gap, the committee recommended a preoperative TTE for patients with low or unknown functional status. This threshold provides a quick and easy method for stratifying patients who previously required risk stratification by a cardiologist, which often resulted in surgery delays.
In their recommendations for implementation of hip fracture quality improvement projects, the Geriatric Fracture Center emphasizes the importance of multidisciplinary physician leadership along with standardization of approach across patients.12 This recommendation is supported by increasing evidence that orthogeriatric collaborations are associated with decreased mortality and length of stay.13 The GHFMC and subsequent interventions reflect this approach, allowing for collaboration to identify cross-disciplinary procedural barriers to care. In our institution, addressing identified procedural barriers to care was associated with a reduction in the average time to surgery from 51 hours to 25.3 hours.
Multiple approaches have been attempted to decrease presurgical time in hip fracture patients in various settings. Prehospital interventions, such as providing ambulances with checklists and ability to bypass the ED, have not been shown to decrease time to surgery for hip fracture patients, though similar strategies have been successful in other conditions, such as stroke.14,15 In-hospital procedures, such as implementation of a hip fracture protocol and reduction of preoperative interventions, have more consistently been found to decrease time to surgery and in-hospital mortality.16,17 However, reduced delays have not been found universally. Luttrell and Nana found that preoperative TTE resulted in approximately 30.8-hour delays from the ED to OR, compared to patients who did not receive a preoperative TTE.18 However, in that study hospitalists used TTE at their own discretion, and there may have been confounding factors contributing to delays. When used as part of a protocol targeting patients with poor or unknown functional capacity, we believe that preoperative TTE results in modest surgical delays yet provides clinically useful information about each patient.
ACC/AHA preoperative guidelines were updated after we implemented our intervention and now recommend that patients with poor or unknown functional capacity in whom stress testing will not influence care proceed to surgery “according to guideline-directed medical care.”11 While routine use of preoperative evaluation of left ventricular function is not recommended, assessing left ventricular function may be reasonable for patients with heart failure with a change in clinical status. Guidelines also recommend that patients with clinically suspected valvular stenosis undergo preoperative echocardiography.11
Limitations
This study has several limitations. First, due to resource limitations, a substantial period of time elapsed between implementation of the new protocol and the analysis of the data set. That is, the hip fracture protocol assessed in this paper occurred from January 2010 through April 2014, and final analysis of the data set occurred in April 2020. This limitation precludes our ability to formally assess any pre- or post-protocol changes in patient outcomes. Second, randomization was not used to create groups that were balanced in differing health characteristics (ie, patients with noncardiac-related surgeries, patients in different age groups); however, the use of inverse probability treatment regression analysis was a way to statistically address these between-group differences. Moreover, this study is limited by the factors that were measured; unmeasured factors cannot be accounted for. Third, health care providers working at the hospital during this time were aware of the goal to decrease presurgical time, possibly creating exaggerated effects compared to a blinded trial. Finally, although this intervention is likely translatable to other centers, these results represent the experiences of a single level 1 trauma center and may not be replicable elsewhere.
Conclusion
Preoperative TTE in lieu of cardiac consultation has several advantages. First, it requires interdepartmental collaboration for implementation, but can be implemented through a single hospital or hospital system. Unlike prehospital interventions, preoperative urgent TTE for patients with low functional capacity does not require the support of emergency medical technicians, ambulance services, or other hospitals in the region. Second, while costs are associated with TTE, they are offset by a reduction in expensive consultations with specialists, surgical delays, and longer lengths of stay. Third, despite likely increased ED-to-OR times compared to no intervention, urgent TTE decreases time to surgery compared with cardiology consultation. Prior to the GHFMC, the ED-to-OR time at our institution was 51 hours. In contrast, the mean time following the GHFMC-led protocol was less than half that, at 25.3 hours (SD, 19.1 hours). In fact, nearly two-thirds (65.2%) of the patients evaluated in this study underwent surgery within 24 hours of admission. This improvement in presurgical time was attributed, in part, to the implementation of preoperative TTE over cardiology consultations.
Acknowledgments: The authors thank Jenny Williams, RN, who was instrumental in obtaining the data set for analysis, and Shauna Ayres, MPH, from the OhioHealth Research Institute, who provided writing and technical assistance.
Corresponding author: Robert Skully, MD, OhioHealth Family Medicine Grant, 290 East Town St., Columbus, OH 43215; [email protected].
Funding: This work was supported by the OhioHealth Summer Research Externship Program.
Financial disclosures: None.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
2. Lewiecki EM, Wright NC, Curtis JR, et al. Hip fracture trends in the United States 2002 to 2015. Osteoporos Int. 2018;29:717-722.
3. Colais P, Di Martino M, Fusco D, et al. The effect of early surgery after hip fracture on 1-year mortality. BMC Geriatr. 2015;15:141.
4. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality after proximal femoral fracture: a retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg Am. 2015;97:1333-1339.
5. Judd KT, Christianson E. Expedited operative care of hip fractures results in significantly lower cost of treatment. Iowa Orthop J. 2015;35:62-64.
6. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182:1609-1616.
7. Ryan DJ, Yoshihara H, Yoneoka D, et al. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma. 2015;29:343-348.
8. Ricci WM, Brandt A, McAndrew C, Gardner MJ. Factors affecting delay to surgery and length of stay for patients with hip fracture. J Orthop Trauma. 2015;29:e109-e114.
9. Hagino T, Ochiai S, Senga S, et al. Efficacy of early surgery and causes of surgical delay in patients with hip fracture. J Orthop. 2015;12:142-146.
10. Rafiq A, Sklyar E, Bella JN. Cardiac evaluation and monitoring of patients undergoing noncardiac surgery. Health Serv Insights. 2017;9:1178632916686074.
11. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
12. Basu N, Natour M, Mounasamy V, Kates SL. Geriatric hip fracture management: keys to providing a successful program. Eur J Trauma Emerg Surg. 2016;42:565-569.
13. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28:e49-e55.
14. Tai YJ, Yan B. Minimising time to treatment: targeted strategies to minimise time to thrombolysis for acute ischaemic stroke. Intern Med J. 2013;43:1176-1182.
15. Larsson G, Stromberg RU, Rogmark C, Nilsdotter A. Prehospital fast track care for patients with hip fracture: Impact on time to surgery, hospital stay, post-operative complications and mortality a randomised, controlled trial. Injury. 2016;47:881-886.
16. Bohm E, Loucks L, Wittmeier K, et al. Reduced time to surgery improves mortality and length of stay following hip fracture: results from an intervention study in a Canadian health authority. Can J Surg. 2015;58:257-263.
17. Ventura C, Trombetti S, Pioli G, et al. Impact of multidisciplinary hip fracture program on timing of surgery in elderly patients. Osteoporos Int J. 2014;25:2591-2597.
18. Luttrell K, Nana A. Effect of preoperative transthoracic echocardiogram on mortality and surgical timing in elderly adults with hip fracture. J Am Geriatr Soc. 2015;63:2505-2509.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
2. Lewiecki EM, Wright NC, Curtis JR, et al. Hip fracture trends in the United States 2002 to 2015. Osteoporos Int. 2018;29:717-722.
3. Colais P, Di Martino M, Fusco D, et al. The effect of early surgery after hip fracture on 1-year mortality. BMC Geriatr. 2015;15:141.
4. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality after proximal femoral fracture: a retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg Am. 2015;97:1333-1339.
5. Judd KT, Christianson E. Expedited operative care of hip fractures results in significantly lower cost of treatment. Iowa Orthop J. 2015;35:62-64.
6. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182:1609-1616.
7. Ryan DJ, Yoshihara H, Yoneoka D, et al. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma. 2015;29:343-348.
8. Ricci WM, Brandt A, McAndrew C, Gardner MJ. Factors affecting delay to surgery and length of stay for patients with hip fracture. J Orthop Trauma. 2015;29:e109-e114.
9. Hagino T, Ochiai S, Senga S, et al. Efficacy of early surgery and causes of surgical delay in patients with hip fracture. J Orthop. 2015;12:142-146.
10. Rafiq A, Sklyar E, Bella JN. Cardiac evaluation and monitoring of patients undergoing noncardiac surgery. Health Serv Insights. 2017;9:1178632916686074.
11. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
12. Basu N, Natour M, Mounasamy V, Kates SL. Geriatric hip fracture management: keys to providing a successful program. Eur J Trauma Emerg Surg. 2016;42:565-569.
13. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28:e49-e55.
14. Tai YJ, Yan B. Minimising time to treatment: targeted strategies to minimise time to thrombolysis for acute ischaemic stroke. Intern Med J. 2013;43:1176-1182.
15. Larsson G, Stromberg RU, Rogmark C, Nilsdotter A. Prehospital fast track care for patients with hip fracture: Impact on time to surgery, hospital stay, post-operative complications and mortality a randomised, controlled trial. Injury. 2016;47:881-886.
16. Bohm E, Loucks L, Wittmeier K, et al. Reduced time to surgery improves mortality and length of stay following hip fracture: results from an intervention study in a Canadian health authority. Can J Surg. 2015;58:257-263.
17. Ventura C, Trombetti S, Pioli G, et al. Impact of multidisciplinary hip fracture program on timing of surgery in elderly patients. Osteoporos Int J. 2014;25:2591-2597.
18. Luttrell K, Nana A. Effect of preoperative transthoracic echocardiogram on mortality and surgical timing in elderly adults with hip fracture. J Am Geriatr Soc. 2015;63:2505-2509.
Noninvasive Ventilation Use Among Medicare Beneficiaries at the End of Life
Study Overview
Objective. To examine the trend of noninvasive and invasive mechanical ventilation at the end of life from 2000 to 2017.
Design. Observational population-based cohort study.
Setting and participants. The study was a population-based cohort study to examine the use of noninvasive and invasive mechanical ventilation among decedents. The study included a random 20% sample of Medicare beneficiaries older than 65 years who were hospitalized in the last 30 days of life and died between January 1, 2000, and December 31, 2017, except for the period October 1, 2015, to December 31, 2015, when the transition from International Classification of Diseases, Ninth Revision (ICD-9) to ICD-10 occurred. Beneficiaries with the primary admitting diagnosis of cardiac arrest or with preexisting tracheostomy were excluded because of expected requirements for ventilatory support. The sample included a total of 2,470,735 Medicare beneficiaries; mean age was 82.2 years, and 54.8% were female. Primary admitting diagnosis codes were used to identify 3 subcohorts: congestive heart failure, chronic obstructive pulmonary disease, and cancer; a fourth subcohort of dementia was identified using the primary admitting diagnosis code or the first 9 secondary diagnosis codes.
Main outcome measures. The study used procedure codes to identify the use of noninvasive ventilation, invasive mechanical ventilation, or none among decedents who were hospitalized in the last 30 days of life. Descriptive statistics to characterize variables by year of hospitalization and ventilatory support were calculated, and the rates of noninvasive and invasive mechanical ventilation use were tabulated. Other outcomes of interest include site of death (in-hospital death), hospice enrollment at death, and hospice enrollment in the last 3 days of life as measures of end-of- life care use. Multivariable logistic regressions were used to examine noninvasive and invasive mechanical ventilation use among decedents, and time trends were examined, with the pattern of use in year 2000 as reference. Subgroup analysis with the subcohort of patients with different diagnoses were conducted to examine trends.
Main results. From 2000 to 2017, 16.3% of decedents had invasive mechanical ventilation, 3.7% had noninvasive ventilation, and 1.0% had both noninvasive and invasive ventilation during their hospital stay. Compared to the reference year 2000, there was a 9-fold increase in noninvasive ventilation use, from 0.8% to 7.1% in 2017, and invasive mechanical ventilation use also increased slightly, from 15.0% to 18.5%. Compared to year 2000, decedents were 2.63 times and 1.04 times (adjusted odds ratio [OR]) more likely to receive noninvasive ventilation and invasive mechanical ventilation, respectively, in 2005, 7.87 times and 1.39 times more likely in 2011, and 11.84 times and 1.63 times more likely in 2017.
Subgroup analysis showed that for congestive heart failure and chronic obstructive pulmonary disease, the increase in noninvasive ventilation use mirrored the trend observed for the overall population, but the use of invasive mechanical ventilation did not increase from 2000 to 2017, with a rate of use of 11.1% versus 7.8% (adjusted OR, 1.07; 95% confidence interval [CI], 0.95-1.19) for congestive heart failure and 17.4% vs 13.2% (OR 1.03, 95% CI, 0.88-1.21) for chronic obstructive pulmonary disease. For the cancer and dementia subgroups, the increase in noninvasive ventilation use from 2000 to 2017 was accompanied by an increase in the use of invasive mechanical ventilation, with a rate of 6.2% versus 7.4% (OR, 1.40; 95% CI, 1.26-1.55) for decedents with cancer and a rate of 5.7% versus 6.2% (OR, 1.28; 95% CI, 1.17-1.41) for decedents with dementia. For other measures of end-of-life care, noninvasive ventilation use when compared to invasive mechanical ventilation use was associated with lower rates of in-hospital (acute care) deaths (50.3% vs 76.7%), hospice enrollment in the last 3 days of life (late hospice enrollment; 57.7% vs 63.0%), and higher rates of hospice enrollment at death (41.3% vs 20.0%).
Conclusion. There was an increase in the use of noninvasive ventilation from 2000 through 2017 among Medicare beneficiaries who died. The findings also suggest that the use of invasive mechanical ventilation did not increase among decedents with congestive heart failure and chronic obstructive pulmonary disease but increased among decedents with cancer and dementia.
Commentary
Noninvasive ventilation offers an alternative to invasive mechanical ventilation for providing ventilatory support for respiratory failure, and may offer benefits as it could avert adverse effects associated with invasive mechanical ventilation, particularly in the management of respiratory failure due to congestive heart failure and chronic obstructive pulmonary disease.1 There is evidence for potential benefits of use of noninvasive ventilation in other clinical scenarios, such as pneumonia in older adults with comorbidities, though its clinical utility is not as well established for other diseases.2
As noninvasive ventilation is introduced into clinical practice, it is not surprising that over the period of the study (2000 to 2017) that its use increased substantially. Advance directives that involve discussion of life-sustaining treatments, including in scenarios with respiratory failure, may also result in physician orders that specify whether an individual desires invasive mechanical ventilation versus other medical treatments, including noninvasive ventilation.3,4 By examining the temporal trends of use of noninvasive and invasive ventilation, this study reveals that invasive mechanical ventilation use among decedents with dementia and cancer has increased, despite increases in the use of noninvasive ventilation. It is important to understand further what would explain these temporal trends and whether the use of noninvasive and also invasive mechanical ventilation at the end of life represents appropriate care with clear goals or whether it may represent overuse. It is also less clear in the end-of-life care scenario what the goals of treatment with noninvasive ventilation would be, especially if it does not avert the use of invasive mechanical ventilation.
The study includes decedents only, thus limiting the ability to draw conclusions about clinically appropriate care.5 Further studies should examine a cohort of patients who have serious and life-threatening illness to examine the trends and potential effects of noninvasive ventilation on outcomes and utilization, as individuals who have improved and survived would not be included in this present decedent cohort.
Applications for Clinical Practice
This study highlights changes in the use of noninvasive and invasive ventilation over time and the different trends seen among subgroups with different diagnoses. For older adults with serious comorbid illness such as dementia, it is especially important to have discussions on advance directives so that care at the end of life is concordant with the patient’s wishes and that unnecessary, burdensome care can be averted. Further studies to understand and define the appropriate use of noninvasive and invasive mechanical ventilation for older adults with significant comorbidities who have serious, life-threatening illness are needed to ensure appropriate clinical treatment at the end of life.
–William W. Hung, MD, MPH
1. Lindenauer PK, Stefan MS, Shieh M et al. Outcomes associated with invasive and noninvasive ventilation a mong patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174:1982-993.
2. Johnson CS, Frei CR, Metersky ML, et al. Non-invasive mechanical ventilation and mortality in elderly immunocompromised patients hospitalized with pneumonia: a retrospective cohort study. BMC Pulm Med. 2014;14:7. Published 2014 Jan 27. doi:10.1186/1471-2466-14-7
3. Lee R, Brumbeck L, Sathitratanacheewin S, et al. Association of physician orders for life-sustaining treatment with icu admission among patients hospitalized near the end of life. JAMA. 2020;323:950-60.
4. Bomba P, Kemp M, Black J. POLST: An improvement over traditional advance directives. Cleveland Clinic J Med. 2012;79:457-464.
5. Duncan I, Ahmed T, Dove H, Maxwell TL. Medicare cost at end of life. Am J Hosp Palliat Care. 2019;36:705-710.
Study Overview
Objective. To examine the trend of noninvasive and invasive mechanical ventilation at the end of life from 2000 to 2017.
Design. Observational population-based cohort study.
Setting and participants. The study was a population-based cohort study to examine the use of noninvasive and invasive mechanical ventilation among decedents. The study included a random 20% sample of Medicare beneficiaries older than 65 years who were hospitalized in the last 30 days of life and died between January 1, 2000, and December 31, 2017, except for the period October 1, 2015, to December 31, 2015, when the transition from International Classification of Diseases, Ninth Revision (ICD-9) to ICD-10 occurred. Beneficiaries with the primary admitting diagnosis of cardiac arrest or with preexisting tracheostomy were excluded because of expected requirements for ventilatory support. The sample included a total of 2,470,735 Medicare beneficiaries; mean age was 82.2 years, and 54.8% were female. Primary admitting diagnosis codes were used to identify 3 subcohorts: congestive heart failure, chronic obstructive pulmonary disease, and cancer; a fourth subcohort of dementia was identified using the primary admitting diagnosis code or the first 9 secondary diagnosis codes.
Main outcome measures. The study used procedure codes to identify the use of noninvasive ventilation, invasive mechanical ventilation, or none among decedents who were hospitalized in the last 30 days of life. Descriptive statistics to characterize variables by year of hospitalization and ventilatory support were calculated, and the rates of noninvasive and invasive mechanical ventilation use were tabulated. Other outcomes of interest include site of death (in-hospital death), hospice enrollment at death, and hospice enrollment in the last 3 days of life as measures of end-of- life care use. Multivariable logistic regressions were used to examine noninvasive and invasive mechanical ventilation use among decedents, and time trends were examined, with the pattern of use in year 2000 as reference. Subgroup analysis with the subcohort of patients with different diagnoses were conducted to examine trends.
Main results. From 2000 to 2017, 16.3% of decedents had invasive mechanical ventilation, 3.7% had noninvasive ventilation, and 1.0% had both noninvasive and invasive ventilation during their hospital stay. Compared to the reference year 2000, there was a 9-fold increase in noninvasive ventilation use, from 0.8% to 7.1% in 2017, and invasive mechanical ventilation use also increased slightly, from 15.0% to 18.5%. Compared to year 2000, decedents were 2.63 times and 1.04 times (adjusted odds ratio [OR]) more likely to receive noninvasive ventilation and invasive mechanical ventilation, respectively, in 2005, 7.87 times and 1.39 times more likely in 2011, and 11.84 times and 1.63 times more likely in 2017.
Subgroup analysis showed that for congestive heart failure and chronic obstructive pulmonary disease, the increase in noninvasive ventilation use mirrored the trend observed for the overall population, but the use of invasive mechanical ventilation did not increase from 2000 to 2017, with a rate of use of 11.1% versus 7.8% (adjusted OR, 1.07; 95% confidence interval [CI], 0.95-1.19) for congestive heart failure and 17.4% vs 13.2% (OR 1.03, 95% CI, 0.88-1.21) for chronic obstructive pulmonary disease. For the cancer and dementia subgroups, the increase in noninvasive ventilation use from 2000 to 2017 was accompanied by an increase in the use of invasive mechanical ventilation, with a rate of 6.2% versus 7.4% (OR, 1.40; 95% CI, 1.26-1.55) for decedents with cancer and a rate of 5.7% versus 6.2% (OR, 1.28; 95% CI, 1.17-1.41) for decedents with dementia. For other measures of end-of-life care, noninvasive ventilation use when compared to invasive mechanical ventilation use was associated with lower rates of in-hospital (acute care) deaths (50.3% vs 76.7%), hospice enrollment in the last 3 days of life (late hospice enrollment; 57.7% vs 63.0%), and higher rates of hospice enrollment at death (41.3% vs 20.0%).
Conclusion. There was an increase in the use of noninvasive ventilation from 2000 through 2017 among Medicare beneficiaries who died. The findings also suggest that the use of invasive mechanical ventilation did not increase among decedents with congestive heart failure and chronic obstructive pulmonary disease but increased among decedents with cancer and dementia.
Commentary
Noninvasive ventilation offers an alternative to invasive mechanical ventilation for providing ventilatory support for respiratory failure, and may offer benefits as it could avert adverse effects associated with invasive mechanical ventilation, particularly in the management of respiratory failure due to congestive heart failure and chronic obstructive pulmonary disease.1 There is evidence for potential benefits of use of noninvasive ventilation in other clinical scenarios, such as pneumonia in older adults with comorbidities, though its clinical utility is not as well established for other diseases.2
As noninvasive ventilation is introduced into clinical practice, it is not surprising that over the period of the study (2000 to 2017) that its use increased substantially. Advance directives that involve discussion of life-sustaining treatments, including in scenarios with respiratory failure, may also result in physician orders that specify whether an individual desires invasive mechanical ventilation versus other medical treatments, including noninvasive ventilation.3,4 By examining the temporal trends of use of noninvasive and invasive ventilation, this study reveals that invasive mechanical ventilation use among decedents with dementia and cancer has increased, despite increases in the use of noninvasive ventilation. It is important to understand further what would explain these temporal trends and whether the use of noninvasive and also invasive mechanical ventilation at the end of life represents appropriate care with clear goals or whether it may represent overuse. It is also less clear in the end-of-life care scenario what the goals of treatment with noninvasive ventilation would be, especially if it does not avert the use of invasive mechanical ventilation.
The study includes decedents only, thus limiting the ability to draw conclusions about clinically appropriate care.5 Further studies should examine a cohort of patients who have serious and life-threatening illness to examine the trends and potential effects of noninvasive ventilation on outcomes and utilization, as individuals who have improved and survived would not be included in this present decedent cohort.
Applications for Clinical Practice
This study highlights changes in the use of noninvasive and invasive ventilation over time and the different trends seen among subgroups with different diagnoses. For older adults with serious comorbid illness such as dementia, it is especially important to have discussions on advance directives so that care at the end of life is concordant with the patient’s wishes and that unnecessary, burdensome care can be averted. Further studies to understand and define the appropriate use of noninvasive and invasive mechanical ventilation for older adults with significant comorbidities who have serious, life-threatening illness are needed to ensure appropriate clinical treatment at the end of life.
–William W. Hung, MD, MPH
Study Overview
Objective. To examine the trend of noninvasive and invasive mechanical ventilation at the end of life from 2000 to 2017.
Design. Observational population-based cohort study.
Setting and participants. The study was a population-based cohort study to examine the use of noninvasive and invasive mechanical ventilation among decedents. The study included a random 20% sample of Medicare beneficiaries older than 65 years who were hospitalized in the last 30 days of life and died between January 1, 2000, and December 31, 2017, except for the period October 1, 2015, to December 31, 2015, when the transition from International Classification of Diseases, Ninth Revision (ICD-9) to ICD-10 occurred. Beneficiaries with the primary admitting diagnosis of cardiac arrest or with preexisting tracheostomy were excluded because of expected requirements for ventilatory support. The sample included a total of 2,470,735 Medicare beneficiaries; mean age was 82.2 years, and 54.8% were female. Primary admitting diagnosis codes were used to identify 3 subcohorts: congestive heart failure, chronic obstructive pulmonary disease, and cancer; a fourth subcohort of dementia was identified using the primary admitting diagnosis code or the first 9 secondary diagnosis codes.
Main outcome measures. The study used procedure codes to identify the use of noninvasive ventilation, invasive mechanical ventilation, or none among decedents who were hospitalized in the last 30 days of life. Descriptive statistics to characterize variables by year of hospitalization and ventilatory support were calculated, and the rates of noninvasive and invasive mechanical ventilation use were tabulated. Other outcomes of interest include site of death (in-hospital death), hospice enrollment at death, and hospice enrollment in the last 3 days of life as measures of end-of- life care use. Multivariable logistic regressions were used to examine noninvasive and invasive mechanical ventilation use among decedents, and time trends were examined, with the pattern of use in year 2000 as reference. Subgroup analysis with the subcohort of patients with different diagnoses were conducted to examine trends.
Main results. From 2000 to 2017, 16.3% of decedents had invasive mechanical ventilation, 3.7% had noninvasive ventilation, and 1.0% had both noninvasive and invasive ventilation during their hospital stay. Compared to the reference year 2000, there was a 9-fold increase in noninvasive ventilation use, from 0.8% to 7.1% in 2017, and invasive mechanical ventilation use also increased slightly, from 15.0% to 18.5%. Compared to year 2000, decedents were 2.63 times and 1.04 times (adjusted odds ratio [OR]) more likely to receive noninvasive ventilation and invasive mechanical ventilation, respectively, in 2005, 7.87 times and 1.39 times more likely in 2011, and 11.84 times and 1.63 times more likely in 2017.
Subgroup analysis showed that for congestive heart failure and chronic obstructive pulmonary disease, the increase in noninvasive ventilation use mirrored the trend observed for the overall population, but the use of invasive mechanical ventilation did not increase from 2000 to 2017, with a rate of use of 11.1% versus 7.8% (adjusted OR, 1.07; 95% confidence interval [CI], 0.95-1.19) for congestive heart failure and 17.4% vs 13.2% (OR 1.03, 95% CI, 0.88-1.21) for chronic obstructive pulmonary disease. For the cancer and dementia subgroups, the increase in noninvasive ventilation use from 2000 to 2017 was accompanied by an increase in the use of invasive mechanical ventilation, with a rate of 6.2% versus 7.4% (OR, 1.40; 95% CI, 1.26-1.55) for decedents with cancer and a rate of 5.7% versus 6.2% (OR, 1.28; 95% CI, 1.17-1.41) for decedents with dementia. For other measures of end-of-life care, noninvasive ventilation use when compared to invasive mechanical ventilation use was associated with lower rates of in-hospital (acute care) deaths (50.3% vs 76.7%), hospice enrollment in the last 3 days of life (late hospice enrollment; 57.7% vs 63.0%), and higher rates of hospice enrollment at death (41.3% vs 20.0%).
Conclusion. There was an increase in the use of noninvasive ventilation from 2000 through 2017 among Medicare beneficiaries who died. The findings also suggest that the use of invasive mechanical ventilation did not increase among decedents with congestive heart failure and chronic obstructive pulmonary disease but increased among decedents with cancer and dementia.
Commentary
Noninvasive ventilation offers an alternative to invasive mechanical ventilation for providing ventilatory support for respiratory failure, and may offer benefits as it could avert adverse effects associated with invasive mechanical ventilation, particularly in the management of respiratory failure due to congestive heart failure and chronic obstructive pulmonary disease.1 There is evidence for potential benefits of use of noninvasive ventilation in other clinical scenarios, such as pneumonia in older adults with comorbidities, though its clinical utility is not as well established for other diseases.2
As noninvasive ventilation is introduced into clinical practice, it is not surprising that over the period of the study (2000 to 2017) that its use increased substantially. Advance directives that involve discussion of life-sustaining treatments, including in scenarios with respiratory failure, may also result in physician orders that specify whether an individual desires invasive mechanical ventilation versus other medical treatments, including noninvasive ventilation.3,4 By examining the temporal trends of use of noninvasive and invasive ventilation, this study reveals that invasive mechanical ventilation use among decedents with dementia and cancer has increased, despite increases in the use of noninvasive ventilation. It is important to understand further what would explain these temporal trends and whether the use of noninvasive and also invasive mechanical ventilation at the end of life represents appropriate care with clear goals or whether it may represent overuse. It is also less clear in the end-of-life care scenario what the goals of treatment with noninvasive ventilation would be, especially if it does not avert the use of invasive mechanical ventilation.
The study includes decedents only, thus limiting the ability to draw conclusions about clinically appropriate care.5 Further studies should examine a cohort of patients who have serious and life-threatening illness to examine the trends and potential effects of noninvasive ventilation on outcomes and utilization, as individuals who have improved and survived would not be included in this present decedent cohort.
Applications for Clinical Practice
This study highlights changes in the use of noninvasive and invasive ventilation over time and the different trends seen among subgroups with different diagnoses. For older adults with serious comorbid illness such as dementia, it is especially important to have discussions on advance directives so that care at the end of life is concordant with the patient’s wishes and that unnecessary, burdensome care can be averted. Further studies to understand and define the appropriate use of noninvasive and invasive mechanical ventilation for older adults with significant comorbidities who have serious, life-threatening illness are needed to ensure appropriate clinical treatment at the end of life.
–William W. Hung, MD, MPH
1. Lindenauer PK, Stefan MS, Shieh M et al. Outcomes associated with invasive and noninvasive ventilation a mong patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174:1982-993.
2. Johnson CS, Frei CR, Metersky ML, et al. Non-invasive mechanical ventilation and mortality in elderly immunocompromised patients hospitalized with pneumonia: a retrospective cohort study. BMC Pulm Med. 2014;14:7. Published 2014 Jan 27. doi:10.1186/1471-2466-14-7
3. Lee R, Brumbeck L, Sathitratanacheewin S, et al. Association of physician orders for life-sustaining treatment with icu admission among patients hospitalized near the end of life. JAMA. 2020;323:950-60.
4. Bomba P, Kemp M, Black J. POLST: An improvement over traditional advance directives. Cleveland Clinic J Med. 2012;79:457-464.
5. Duncan I, Ahmed T, Dove H, Maxwell TL. Medicare cost at end of life. Am J Hosp Palliat Care. 2019;36:705-710.
1. Lindenauer PK, Stefan MS, Shieh M et al. Outcomes associated with invasive and noninvasive ventilation a mong patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174:1982-993.
2. Johnson CS, Frei CR, Metersky ML, et al. Non-invasive mechanical ventilation and mortality in elderly immunocompromised patients hospitalized with pneumonia: a retrospective cohort study. BMC Pulm Med. 2014;14:7. Published 2014 Jan 27. doi:10.1186/1471-2466-14-7
3. Lee R, Brumbeck L, Sathitratanacheewin S, et al. Association of physician orders for life-sustaining treatment with icu admission among patients hospitalized near the end of life. JAMA. 2020;323:950-60.
4. Bomba P, Kemp M, Black J. POLST: An improvement over traditional advance directives. Cleveland Clinic J Med. 2012;79:457-464.
5. Duncan I, Ahmed T, Dove H, Maxwell TL. Medicare cost at end of life. Am J Hosp Palliat Care. 2019;36:705-710.
PCPs play a small part in low-value care spending
according to a brief report published online Jan. 18 in Annals of Internal Medicine.
However, one expert said there are better ways to curb low-value care than focusing on which specialties are guilty of the practice.
Analyzing a 20% random sample of Medicare Part B claims, Aaron Baum, PhD, with the Icahn School of Medicine at Mount Sinai, New York, and colleagues found that the services primary care physicians performed or ordered made up on average 8.3% of the low-value care their patients received (interquartile range, 3.9%-15.1%; 95th percentile, 35.6%) and their referrals made up 15.4% (IQR, 6.3%-26.4%; 95th percentile, 44.6%).
By specialty, cardiology had the worst record with 27% of all spending on low-value services ($1.8 billion) attributed to that specialty. Yet, of the 25 highest-spending specialties in the report, 12 of them were associated with 1% or less than 1% each of all low-value spending, indicating the waste was widely distributed.
Dr. Baum said in an interview that though there are some PCPs guilty of high spending on low-value services, overall, most primary care physicians’ low-value services add up to only 0.3% of Part B spending. He noted that Part B spending is about one-third of all Medicare spending.
Primary care is often thought to be at the core of care management and spending and PCPs are often seen as the gatekeepers, but this analysis suggests that efforts to make big differences in curtailing low-value spending might be more effective elsewhere.
“There’s only so much spending you can reduce by changing primary care physicians’ services that they directly perform,” Dr. Baum said.
Low-value care is costly, can be harmful
Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, said in an interview that the report adds confirmation to previous research that has consistently shown low-value care is “extremely common, very costly, and provided by primary care providers and specialists alike.” He noted that it can also be harmful.
“The math is simple,” he said. “If we want to improve coverage and lower patient costs for essential services like visits, diagnostic tests, and drugs, we have to reduce spending on those services that do not make Americans any healthier.”
The study ranked 31 clinical services judged to be low value by physician societies, Medicare and clinical guidelines, and their use among beneficiaries enrolled between 2007 and 2014. Here’s how the top six low-value services compare.
Dr. Fendrick said a weakness of the paper is the years of the data (2007-2014). Some of the criteria around low-value care have changed since then. The age that a prostate-specific antigen test becomes low-value is now 70 years, for instance, instead of 75. He added that some of the figures attributed to non-PCP providers appear out of date.
Dr. Fendrick said, “I understand that there are Medicare patients who end up at a gastroenterologist or surgeon’s office to get colorectal cancer screening, but it would be very hard for me to believe that half of stress tests and over half of colon cancer screening over [age] 85 [years] and half of PSA for people over 75 did not have some type of referring clinicians involved. I certainly don’t think that would be the case in 2020-2021.”
Dr. Baum said those years were the latest years available for the data points needed for this analysis, but he and his colleagues were working to update the data for future publication.
Dr. Fendrick said not much has changed in recent years in terms of waste on low-value care, even with campaigns such as Choosing Wisely dedicated to identifying low-value services or procedures in each specialty.
“I believe there’s not a particular group of clinicians one way or the other who are actually doing any better now than they were 7 years ago,” he said. He would rather focus less on which specialties are associated with the most low-value care and more on the underlying policies that encourage low-value care.
“If you’re going to get paid for doing a stress test and get paid nothing or significantly less if you don’t, the incentives are in the wrong direction,” he said.
Dr. Fendrick said the pandemic era provides an opportunity to eliminate low-value care because use of those services has dropped drastically as resources have been diverted to COVID-19 patients and many services have been delayed or canceled.
He said he has been pushing an approach that providers should be paid more after the pandemic “to do the things we want them to do.”
As an example, he said, instead of paying $886 million on colonoscopies for people over the age of 85, “why don’t we put a policy in place that would make it better for patients by lowering cost sharing and better for providers by paying them more to do the service on the people who need it as opposed to the people who don’t?”
The research was funded by the American Board of Family Medicine Foundation. Dr. Baum and a coauthor reported receiving personal fees from American Board of Family Medicine Foundation during the conduct of the study. Another coauthor reported receiving personal fees from Collective Health, HealthRight 360, PLOS Medicine, and the New England Journal of Medicine, outside the submitted work. Dr. Fendrick disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a brief report published online Jan. 18 in Annals of Internal Medicine.
However, one expert said there are better ways to curb low-value care than focusing on which specialties are guilty of the practice.
Analyzing a 20% random sample of Medicare Part B claims, Aaron Baum, PhD, with the Icahn School of Medicine at Mount Sinai, New York, and colleagues found that the services primary care physicians performed or ordered made up on average 8.3% of the low-value care their patients received (interquartile range, 3.9%-15.1%; 95th percentile, 35.6%) and their referrals made up 15.4% (IQR, 6.3%-26.4%; 95th percentile, 44.6%).
By specialty, cardiology had the worst record with 27% of all spending on low-value services ($1.8 billion) attributed to that specialty. Yet, of the 25 highest-spending specialties in the report, 12 of them were associated with 1% or less than 1% each of all low-value spending, indicating the waste was widely distributed.
Dr. Baum said in an interview that though there are some PCPs guilty of high spending on low-value services, overall, most primary care physicians’ low-value services add up to only 0.3% of Part B spending. He noted that Part B spending is about one-third of all Medicare spending.
Primary care is often thought to be at the core of care management and spending and PCPs are often seen as the gatekeepers, but this analysis suggests that efforts to make big differences in curtailing low-value spending might be more effective elsewhere.
“There’s only so much spending you can reduce by changing primary care physicians’ services that they directly perform,” Dr. Baum said.
Low-value care is costly, can be harmful
Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, said in an interview that the report adds confirmation to previous research that has consistently shown low-value care is “extremely common, very costly, and provided by primary care providers and specialists alike.” He noted that it can also be harmful.
“The math is simple,” he said. “If we want to improve coverage and lower patient costs for essential services like visits, diagnostic tests, and drugs, we have to reduce spending on those services that do not make Americans any healthier.”
The study ranked 31 clinical services judged to be low value by physician societies, Medicare and clinical guidelines, and their use among beneficiaries enrolled between 2007 and 2014. Here’s how the top six low-value services compare.
Dr. Fendrick said a weakness of the paper is the years of the data (2007-2014). Some of the criteria around low-value care have changed since then. The age that a prostate-specific antigen test becomes low-value is now 70 years, for instance, instead of 75. He added that some of the figures attributed to non-PCP providers appear out of date.
Dr. Fendrick said, “I understand that there are Medicare patients who end up at a gastroenterologist or surgeon’s office to get colorectal cancer screening, but it would be very hard for me to believe that half of stress tests and over half of colon cancer screening over [age] 85 [years] and half of PSA for people over 75 did not have some type of referring clinicians involved. I certainly don’t think that would be the case in 2020-2021.”
Dr. Baum said those years were the latest years available for the data points needed for this analysis, but he and his colleagues were working to update the data for future publication.
Dr. Fendrick said not much has changed in recent years in terms of waste on low-value care, even with campaigns such as Choosing Wisely dedicated to identifying low-value services or procedures in each specialty.
“I believe there’s not a particular group of clinicians one way or the other who are actually doing any better now than they were 7 years ago,” he said. He would rather focus less on which specialties are associated with the most low-value care and more on the underlying policies that encourage low-value care.
“If you’re going to get paid for doing a stress test and get paid nothing or significantly less if you don’t, the incentives are in the wrong direction,” he said.
Dr. Fendrick said the pandemic era provides an opportunity to eliminate low-value care because use of those services has dropped drastically as resources have been diverted to COVID-19 patients and many services have been delayed or canceled.
He said he has been pushing an approach that providers should be paid more after the pandemic “to do the things we want them to do.”
As an example, he said, instead of paying $886 million on colonoscopies for people over the age of 85, “why don’t we put a policy in place that would make it better for patients by lowering cost sharing and better for providers by paying them more to do the service on the people who need it as opposed to the people who don’t?”
The research was funded by the American Board of Family Medicine Foundation. Dr. Baum and a coauthor reported receiving personal fees from American Board of Family Medicine Foundation during the conduct of the study. Another coauthor reported receiving personal fees from Collective Health, HealthRight 360, PLOS Medicine, and the New England Journal of Medicine, outside the submitted work. Dr. Fendrick disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a brief report published online Jan. 18 in Annals of Internal Medicine.
However, one expert said there are better ways to curb low-value care than focusing on which specialties are guilty of the practice.
Analyzing a 20% random sample of Medicare Part B claims, Aaron Baum, PhD, with the Icahn School of Medicine at Mount Sinai, New York, and colleagues found that the services primary care physicians performed or ordered made up on average 8.3% of the low-value care their patients received (interquartile range, 3.9%-15.1%; 95th percentile, 35.6%) and their referrals made up 15.4% (IQR, 6.3%-26.4%; 95th percentile, 44.6%).
By specialty, cardiology had the worst record with 27% of all spending on low-value services ($1.8 billion) attributed to that specialty. Yet, of the 25 highest-spending specialties in the report, 12 of them were associated with 1% or less than 1% each of all low-value spending, indicating the waste was widely distributed.
Dr. Baum said in an interview that though there are some PCPs guilty of high spending on low-value services, overall, most primary care physicians’ low-value services add up to only 0.3% of Part B spending. He noted that Part B spending is about one-third of all Medicare spending.
Primary care is often thought to be at the core of care management and spending and PCPs are often seen as the gatekeepers, but this analysis suggests that efforts to make big differences in curtailing low-value spending might be more effective elsewhere.
“There’s only so much spending you can reduce by changing primary care physicians’ services that they directly perform,” Dr. Baum said.
Low-value care is costly, can be harmful
Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, said in an interview that the report adds confirmation to previous research that has consistently shown low-value care is “extremely common, very costly, and provided by primary care providers and specialists alike.” He noted that it can also be harmful.
“The math is simple,” he said. “If we want to improve coverage and lower patient costs for essential services like visits, diagnostic tests, and drugs, we have to reduce spending on those services that do not make Americans any healthier.”
The study ranked 31 clinical services judged to be low value by physician societies, Medicare and clinical guidelines, and their use among beneficiaries enrolled between 2007 and 2014. Here’s how the top six low-value services compare.
Dr. Fendrick said a weakness of the paper is the years of the data (2007-2014). Some of the criteria around low-value care have changed since then. The age that a prostate-specific antigen test becomes low-value is now 70 years, for instance, instead of 75. He added that some of the figures attributed to non-PCP providers appear out of date.
Dr. Fendrick said, “I understand that there are Medicare patients who end up at a gastroenterologist or surgeon’s office to get colorectal cancer screening, but it would be very hard for me to believe that half of stress tests and over half of colon cancer screening over [age] 85 [years] and half of PSA for people over 75 did not have some type of referring clinicians involved. I certainly don’t think that would be the case in 2020-2021.”
Dr. Baum said those years were the latest years available for the data points needed for this analysis, but he and his colleagues were working to update the data for future publication.
Dr. Fendrick said not much has changed in recent years in terms of waste on low-value care, even with campaigns such as Choosing Wisely dedicated to identifying low-value services or procedures in each specialty.
“I believe there’s not a particular group of clinicians one way or the other who are actually doing any better now than they were 7 years ago,” he said. He would rather focus less on which specialties are associated with the most low-value care and more on the underlying policies that encourage low-value care.
“If you’re going to get paid for doing a stress test and get paid nothing or significantly less if you don’t, the incentives are in the wrong direction,” he said.
Dr. Fendrick said the pandemic era provides an opportunity to eliminate low-value care because use of those services has dropped drastically as resources have been diverted to COVID-19 patients and many services have been delayed or canceled.
He said he has been pushing an approach that providers should be paid more after the pandemic “to do the things we want them to do.”
As an example, he said, instead of paying $886 million on colonoscopies for people over the age of 85, “why don’t we put a policy in place that would make it better for patients by lowering cost sharing and better for providers by paying them more to do the service on the people who need it as opposed to the people who don’t?”
The research was funded by the American Board of Family Medicine Foundation. Dr. Baum and a coauthor reported receiving personal fees from American Board of Family Medicine Foundation during the conduct of the study. Another coauthor reported receiving personal fees from Collective Health, HealthRight 360, PLOS Medicine, and the New England Journal of Medicine, outside the submitted work. Dr. Fendrick disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Could an osteoporosis drug reduce need for hip revision surgery?
A single injection of denosumab (Prolia, Amgen), frequently used to treat osteoporosis, may reduce the need for revision surgery in patients with symptomatic osteolysis following total hip arthroplasty, a new proof-of-concept study suggests.
Aseptic loosening is the result of wear-induced osteolysis caused by the prosthetic hip and is a major contributor to the need for revision surgery in many parts of the world.
“The only established treatment for prosthesis-related osteolysis after joint replacement is revision surgery, which carries substantially greater morbidity and mortality than primary joint replacement,” Mohit M. Mahatma, MRes, of the University of Sheffield, England, and colleagues wrote in their article, published online Jan. 11 in The Lancet Rheumatology.
As well as an increased risk of infection and other complications, revision surgery is much more costly than a first-time operation, they added.
“The results of this proof-of-concept clinical trial indicate that denosumab is effective at reducing bone resorption activity within osteolytic lesion tissue and is well tolerated within the limitations of the single dose used here,” they concluded.
Commenting on the findings, Antonia Chen, MD, associate professor of orthopedic surgery, Harvard Medical School, Boston, emphasized that further studies are needed to assess the effectiveness of this strategy to reduce the need for hip revision surgery.
Nevertheless, “osteolysis is still unfortunately a problem we do have to deal with and we do not have any other way to prevent it,” she said in an interview. “So it’s a good start ... although further studies are definitely needed,” Dr. Chen added.
In an accompanying editorial, Hannu Aro, MD, Turku University Hospital in Finland, agreed: “Without a doubt, the trial is a breakthrough, but it represents only the first step in the development of pharmacological therapy aiming to slow, prevent, or even reverse the process of wear-induced periprosthetic osteolysis.”
Small single-center study
The phase 2, single-center, randomized, controlled trial involved 22 patients who had previously undergone hip replacement surgery at Sheffield Teaching Hospitals and were scheduled for revision surgery due to symptomatic osteolysis. They were randomized to a single subcutaneous injection of denosumab at a dose of 60 mg, or placebo, on their second hospital visit.
“The primary outcome was the between-group difference in the number of osteoclasts per mm of osteolytic membrane at the osteolytic membrane-bone interface at week 8,” the authors noted.
At this time point, there were 83% fewer osteoclasts at the interface in the denosumab group compared with placebo, at a median of 0.05 per mm in the treatment group compared with 0.30 per mm in the placebo group (P = .011).
Secondary histological outcomes were also significantly improved in favor of the denosumab group compared with placebo.
Potential to prevent half of all hip revision surgeries?
Patients who received denosumab also demonstrated an acute fall in serum and urinary markers of bone resorption following administration of the drug, reaching a nadir at week 4, which was maintained until revision surgery at week 8.
In contrast, “no change in these markers was observed in the placebo group [P < .0003 for all biomarkers],” the investigators noted. Rates of adverse events were comparable in both treatment groups.
As the authors explained, osteolysis occurs following joint replacement surgery when particles of plastic wear off from the prosthesis, triggering an immune reaction that attacks the bone around the implant, causing the joint to loosen.
“It is very clear from our bone biopsies and bone imaging that the [denosumab] injection stops the bone absorbing the microplastic particles from the replacement joint and therefore could prevent the bone from being eaten away and the need for revision surgery,” senior author Mark Wilkinson, MBChB, PhD, honorary consultant orthopedic surgeon, Sheffield Teaching Hospitals, said in a press release from his institution.
“This study is a significant breakthrough as we’ve demonstrated that there is a drug, already available and successful in the treatment of osteoporosis, that has the potential to prevent up to half of all revised replacement surgeries which are caused by osteolysis,” he added.
Dr. Wilkinson and coauthors said their results justify the need for future trials targeting earlier-stage disease to further test the use of denosumab to prevent or reduce the need for revision surgery.
In 2018, aseptic loosening accounted for over half of all revision procedures, as reported to the National Joint Registry in England and Wales.
Older polyethylene prostheses are the main culprit
Commenting further on the study, Dr. Chen noted that osteolysis still plagues orthopedic surgeons because the original polyethylene prostheses were not very good. A better prosthesis developed at Massachusetts General Hospital is made up of highly crossed-link polyethylene and still wears over time but to a much lesser extent than the older polyethylene prostheses.
Metal and ceramic prostheses also can induce osteolysis, but again to a much lesser extent than the older polyethylene implants.
“Any particle can technically cause osteolysis but plastic produces the most particles,” Dr. Chen explained. Although hip revision rates in the United States are low to begin with, aseptic loosening is still one of the main reasons that patients need to undergo revision surgery, she observed.
“A lot of patients are still living with the old plastic [implants] so there is still a need for something like this,” she stressed.
However, many questions about this potential new strategy remain to be answered, including when best to initiate treatment and how to manage patients at risk for osteolysis 20-30 years after they have received their original implant.
In his editorial, Dr. Aro said that serious adverse consequences often become evident 10-20 years after patients have undergone the original hip replacement procedures, when they are potentially less physically fit than they were at the time of the operation and thus less able to withstand the rigors of a difficult revision surgery.
“In this context, the concept of nonsurgical pharmacological treatment of periprosthetic osteolysis ... brings a new hope for the ever-increasing population of patients with total hip arthroplasty to avoid revision surgery,” Dr. Aro suggested.
However, Dr. Aro cautioned that reduction of bone turnover by antiresorptive agents such as denosumab has been associated with the development of atypical femoral fractures.
The study was funded by Amgen. Dr. Wilkinson has reported receiving a grant from Amgen. Dr. Chen has reported serving as a consultant for Striker and b-One Ortho. Dr. Aro has reported receiving a grant to his institution from Amgen Finland and the Academy of Finland. He has also served as a member of an advisory scientific board for Amgen Finland.
A version of this article first appeared on Medscape.com.
A single injection of denosumab (Prolia, Amgen), frequently used to treat osteoporosis, may reduce the need for revision surgery in patients with symptomatic osteolysis following total hip arthroplasty, a new proof-of-concept study suggests.
Aseptic loosening is the result of wear-induced osteolysis caused by the prosthetic hip and is a major contributor to the need for revision surgery in many parts of the world.
“The only established treatment for prosthesis-related osteolysis after joint replacement is revision surgery, which carries substantially greater morbidity and mortality than primary joint replacement,” Mohit M. Mahatma, MRes, of the University of Sheffield, England, and colleagues wrote in their article, published online Jan. 11 in The Lancet Rheumatology.
As well as an increased risk of infection and other complications, revision surgery is much more costly than a first-time operation, they added.
“The results of this proof-of-concept clinical trial indicate that denosumab is effective at reducing bone resorption activity within osteolytic lesion tissue and is well tolerated within the limitations of the single dose used here,” they concluded.
Commenting on the findings, Antonia Chen, MD, associate professor of orthopedic surgery, Harvard Medical School, Boston, emphasized that further studies are needed to assess the effectiveness of this strategy to reduce the need for hip revision surgery.
Nevertheless, “osteolysis is still unfortunately a problem we do have to deal with and we do not have any other way to prevent it,” she said in an interview. “So it’s a good start ... although further studies are definitely needed,” Dr. Chen added.
In an accompanying editorial, Hannu Aro, MD, Turku University Hospital in Finland, agreed: “Without a doubt, the trial is a breakthrough, but it represents only the first step in the development of pharmacological therapy aiming to slow, prevent, or even reverse the process of wear-induced periprosthetic osteolysis.”
Small single-center study
The phase 2, single-center, randomized, controlled trial involved 22 patients who had previously undergone hip replacement surgery at Sheffield Teaching Hospitals and were scheduled for revision surgery due to symptomatic osteolysis. They were randomized to a single subcutaneous injection of denosumab at a dose of 60 mg, or placebo, on their second hospital visit.
“The primary outcome was the between-group difference in the number of osteoclasts per mm of osteolytic membrane at the osteolytic membrane-bone interface at week 8,” the authors noted.
At this time point, there were 83% fewer osteoclasts at the interface in the denosumab group compared with placebo, at a median of 0.05 per mm in the treatment group compared with 0.30 per mm in the placebo group (P = .011).
Secondary histological outcomes were also significantly improved in favor of the denosumab group compared with placebo.
Potential to prevent half of all hip revision surgeries?
Patients who received denosumab also demonstrated an acute fall in serum and urinary markers of bone resorption following administration of the drug, reaching a nadir at week 4, which was maintained until revision surgery at week 8.
In contrast, “no change in these markers was observed in the placebo group [P < .0003 for all biomarkers],” the investigators noted. Rates of adverse events were comparable in both treatment groups.
As the authors explained, osteolysis occurs following joint replacement surgery when particles of plastic wear off from the prosthesis, triggering an immune reaction that attacks the bone around the implant, causing the joint to loosen.
“It is very clear from our bone biopsies and bone imaging that the [denosumab] injection stops the bone absorbing the microplastic particles from the replacement joint and therefore could prevent the bone from being eaten away and the need for revision surgery,” senior author Mark Wilkinson, MBChB, PhD, honorary consultant orthopedic surgeon, Sheffield Teaching Hospitals, said in a press release from his institution.
“This study is a significant breakthrough as we’ve demonstrated that there is a drug, already available and successful in the treatment of osteoporosis, that has the potential to prevent up to half of all revised replacement surgeries which are caused by osteolysis,” he added.
Dr. Wilkinson and coauthors said their results justify the need for future trials targeting earlier-stage disease to further test the use of denosumab to prevent or reduce the need for revision surgery.
In 2018, aseptic loosening accounted for over half of all revision procedures, as reported to the National Joint Registry in England and Wales.
Older polyethylene prostheses are the main culprit
Commenting further on the study, Dr. Chen noted that osteolysis still plagues orthopedic surgeons because the original polyethylene prostheses were not very good. A better prosthesis developed at Massachusetts General Hospital is made up of highly crossed-link polyethylene and still wears over time but to a much lesser extent than the older polyethylene prostheses.
Metal and ceramic prostheses also can induce osteolysis, but again to a much lesser extent than the older polyethylene implants.
“Any particle can technically cause osteolysis but plastic produces the most particles,” Dr. Chen explained. Although hip revision rates in the United States are low to begin with, aseptic loosening is still one of the main reasons that patients need to undergo revision surgery, she observed.
“A lot of patients are still living with the old plastic [implants] so there is still a need for something like this,” she stressed.
However, many questions about this potential new strategy remain to be answered, including when best to initiate treatment and how to manage patients at risk for osteolysis 20-30 years after they have received their original implant.
In his editorial, Dr. Aro said that serious adverse consequences often become evident 10-20 years after patients have undergone the original hip replacement procedures, when they are potentially less physically fit than they were at the time of the operation and thus less able to withstand the rigors of a difficult revision surgery.
“In this context, the concept of nonsurgical pharmacological treatment of periprosthetic osteolysis ... brings a new hope for the ever-increasing population of patients with total hip arthroplasty to avoid revision surgery,” Dr. Aro suggested.
However, Dr. Aro cautioned that reduction of bone turnover by antiresorptive agents such as denosumab has been associated with the development of atypical femoral fractures.
The study was funded by Amgen. Dr. Wilkinson has reported receiving a grant from Amgen. Dr. Chen has reported serving as a consultant for Striker and b-One Ortho. Dr. Aro has reported receiving a grant to his institution from Amgen Finland and the Academy of Finland. He has also served as a member of an advisory scientific board for Amgen Finland.
A version of this article first appeared on Medscape.com.
A single injection of denosumab (Prolia, Amgen), frequently used to treat osteoporosis, may reduce the need for revision surgery in patients with symptomatic osteolysis following total hip arthroplasty, a new proof-of-concept study suggests.
Aseptic loosening is the result of wear-induced osteolysis caused by the prosthetic hip and is a major contributor to the need for revision surgery in many parts of the world.
“The only established treatment for prosthesis-related osteolysis after joint replacement is revision surgery, which carries substantially greater morbidity and mortality than primary joint replacement,” Mohit M. Mahatma, MRes, of the University of Sheffield, England, and colleagues wrote in their article, published online Jan. 11 in The Lancet Rheumatology.
As well as an increased risk of infection and other complications, revision surgery is much more costly than a first-time operation, they added.
“The results of this proof-of-concept clinical trial indicate that denosumab is effective at reducing bone resorption activity within osteolytic lesion tissue and is well tolerated within the limitations of the single dose used here,” they concluded.
Commenting on the findings, Antonia Chen, MD, associate professor of orthopedic surgery, Harvard Medical School, Boston, emphasized that further studies are needed to assess the effectiveness of this strategy to reduce the need for hip revision surgery.
Nevertheless, “osteolysis is still unfortunately a problem we do have to deal with and we do not have any other way to prevent it,” she said in an interview. “So it’s a good start ... although further studies are definitely needed,” Dr. Chen added.
In an accompanying editorial, Hannu Aro, MD, Turku University Hospital in Finland, agreed: “Without a doubt, the trial is a breakthrough, but it represents only the first step in the development of pharmacological therapy aiming to slow, prevent, or even reverse the process of wear-induced periprosthetic osteolysis.”
Small single-center study
The phase 2, single-center, randomized, controlled trial involved 22 patients who had previously undergone hip replacement surgery at Sheffield Teaching Hospitals and were scheduled for revision surgery due to symptomatic osteolysis. They were randomized to a single subcutaneous injection of denosumab at a dose of 60 mg, or placebo, on their second hospital visit.
“The primary outcome was the between-group difference in the number of osteoclasts per mm of osteolytic membrane at the osteolytic membrane-bone interface at week 8,” the authors noted.
At this time point, there were 83% fewer osteoclasts at the interface in the denosumab group compared with placebo, at a median of 0.05 per mm in the treatment group compared with 0.30 per mm in the placebo group (P = .011).
Secondary histological outcomes were also significantly improved in favor of the denosumab group compared with placebo.
Potential to prevent half of all hip revision surgeries?
Patients who received denosumab also demonstrated an acute fall in serum and urinary markers of bone resorption following administration of the drug, reaching a nadir at week 4, which was maintained until revision surgery at week 8.
In contrast, “no change in these markers was observed in the placebo group [P < .0003 for all biomarkers],” the investigators noted. Rates of adverse events were comparable in both treatment groups.
As the authors explained, osteolysis occurs following joint replacement surgery when particles of plastic wear off from the prosthesis, triggering an immune reaction that attacks the bone around the implant, causing the joint to loosen.
“It is very clear from our bone biopsies and bone imaging that the [denosumab] injection stops the bone absorbing the microplastic particles from the replacement joint and therefore could prevent the bone from being eaten away and the need for revision surgery,” senior author Mark Wilkinson, MBChB, PhD, honorary consultant orthopedic surgeon, Sheffield Teaching Hospitals, said in a press release from his institution.
“This study is a significant breakthrough as we’ve demonstrated that there is a drug, already available and successful in the treatment of osteoporosis, that has the potential to prevent up to half of all revised replacement surgeries which are caused by osteolysis,” he added.
Dr. Wilkinson and coauthors said their results justify the need for future trials targeting earlier-stage disease to further test the use of denosumab to prevent or reduce the need for revision surgery.
In 2018, aseptic loosening accounted for over half of all revision procedures, as reported to the National Joint Registry in England and Wales.
Older polyethylene prostheses are the main culprit
Commenting further on the study, Dr. Chen noted that osteolysis still plagues orthopedic surgeons because the original polyethylene prostheses were not very good. A better prosthesis developed at Massachusetts General Hospital is made up of highly crossed-link polyethylene and still wears over time but to a much lesser extent than the older polyethylene prostheses.
Metal and ceramic prostheses also can induce osteolysis, but again to a much lesser extent than the older polyethylene implants.
“Any particle can technically cause osteolysis but plastic produces the most particles,” Dr. Chen explained. Although hip revision rates in the United States are low to begin with, aseptic loosening is still one of the main reasons that patients need to undergo revision surgery, she observed.
“A lot of patients are still living with the old plastic [implants] so there is still a need for something like this,” she stressed.
However, many questions about this potential new strategy remain to be answered, including when best to initiate treatment and how to manage patients at risk for osteolysis 20-30 years after they have received their original implant.
In his editorial, Dr. Aro said that serious adverse consequences often become evident 10-20 years after patients have undergone the original hip replacement procedures, when they are potentially less physically fit than they were at the time of the operation and thus less able to withstand the rigors of a difficult revision surgery.
“In this context, the concept of nonsurgical pharmacological treatment of periprosthetic osteolysis ... brings a new hope for the ever-increasing population of patients with total hip arthroplasty to avoid revision surgery,” Dr. Aro suggested.
However, Dr. Aro cautioned that reduction of bone turnover by antiresorptive agents such as denosumab has been associated with the development of atypical femoral fractures.
The study was funded by Amgen. Dr. Wilkinson has reported receiving a grant from Amgen. Dr. Chen has reported serving as a consultant for Striker and b-One Ortho. Dr. Aro has reported receiving a grant to his institution from Amgen Finland and the Academy of Finland. He has also served as a member of an advisory scientific board for Amgen Finland.
A version of this article first appeared on Medscape.com.
Early use of high-titer plasma may prevent severe COVID-19
Administering convalescent plasma that has high levels of antibodies against SARS-CoV-2 within the first 3 days of symptoms was associated with significantly lower chances of progression to severe COVID-19, new evidence demonstrates.
In a trial of 160 older adults with COVID-19, half of whom were randomly assigned to receive plasma and half to receive placebo infusion, treatment with high-titer plasma lowered the relative risk for severe disease by 48% in an intent-to-treat analysis.
“We now have evidence, in the context of a small but well-designed study, that convalescent plasma with high titers of antibody against SARS-CoV-2 administered in the first 3 days of mild symptoms to infected elderly reduces progression of illness and the rate of severe presentations,” senior author Fernando Polack, MD, said in an interview.
“Not any plasma, not any time,” added Dr. Polack, an infectious disease specialist and scientific director at Fundacion INFANT and professor of pediatrics at the University of Buenos Aires. The key, he said, is to select plasma in the upper 28th percentile of IgG antibody concentrations and to administer therapy prior to disease progression.
The study was published online Jan. 6 in The New England Journal of Medicine.
“It’s a very good study and approaches a different population from the PlasmAr study,” Ventura Simonovich, MD, chief of the clinical pharmacology section, Medical Clinic Service, Hospital Italiano de Buenos Aires, said in an interview. “This is the first published randomized controlled trial that shows real benefit in this [older adult] population, the most vulnerable in this disease,” he said.
Dr. Simonovich, who was not affiliated with the current study, was lead author of the PlasmAr trial, which was published in The New England Journal of Medicine Nov. 24, 2020. In that trial, the researchers evaluated adults aged 18 years and older and found no significant benefit with convalescent plasma treatment over placebo for patients with COVID-19 and severe pneumonia.
“We know antibodies work best when given early and in high dose. This is one of the rare reports that validates it in the outpatient setting,” David Sullivan, MD, professor of molecular biology and immunology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in an interview when asked to comment.
Dr. Sullivan pointed out that most previous studies on convalescent plasma focused on patients with COVID-19 who had severe cases late in the disease course.
Regarding the current study, he said, “The striking thing is treating people within 3 days of illness.”
A more cautious interpretation may be warranted, one expert said. “The study demonstrates the benefit of early intervention. There was a dose-dependent effect, with higher titers providing a greater benefit,” Manoj Menon, MD, MPH, a hematologist and oncologist at the University of Washington, Seattle, said in an interview.
“Taken together, the findings have biologic plausibility and produce more data on the role of convalescent plasma to a relevant age cohort,” he added.
However, Dr. Menon said: “Given the limited sample size, I do not think this study, although well conducted, definitively addresses the role of convalescent plasma for COVID-19. But it does merit additional study.”
A search for clear answers
Treatments that target the early stages of COVID-19 “remain elusive. Few strategies provide benefit, several have failed, and others are being evaluated,” the researchers noted. “In hospitalized patients with COVID-19, the infusion of convalescent plasma against SARS-CoV-2 late in the course of illness has not shown clear benefits and, consequently, the most appropriate antibody concentrations for effective treatment are unclear.”
To learn more, Dr. Polack and colleagues included patients with PCR-confirmed COVID-19 who were aged 75 years or older, regardless of comorbidities. They also included patients aged 65-74 years who had at least one underlying condition. Participants were enrolled at clinical sites or geriatric units in Argentina. The mean age was 77 years, and 62% were women.
In an intent-to-treat analysis, the primary outcome – severe respiratory disease – occurred in 16% of the plasma recipients, vs. 31% of the group that received placebo. The relative risk was 0.52 (95% confidence interval, 0.29-0.94; P = .03).
The number needed to treat to avoid a severe respiratory disease episode was 7 (95% CI, 4-50).
Life-threatening respiratory disease, a secondary outcome, occurred in four people in the plasma group, compared with 10 in the placebo group. Two patients in the treatment group and four patients in the placebo group died.
The researchers also ran a modified intent-to-treat analysis that excluded six participants who experienced severe respiratory disease prior to receiving plasma or placebo. In this analysis, efficacy of plasma therapy increased to 60%.
“Again, this finding suggests that early intervention is critical for efficacy,” the investigators noted.
The investigators, who are based in Argentina, defined their primary endpoint as a respiratory rate of 30 or more breaths per minute and/or an oxygen saturation of less than 93% while breathing ambient air.
Dr. Sullivan pointed out that this is equivalent to the threshold commonly used for hospitalizing people with COVID-19 in the United States. “So it’s equivalent to avoiding hospitalizations. The take-home is high-titer plasma prevents respiratory distress, which equals hospitalization for us.”
Dr. Sullivan is conducting similar research in the United States regarding the use of plasma for treatment or prevention. He and colleagues are evaluating adults aged 18-90 years, “not just the ones at highest risk for going to the hospital,” he said. Enrollment is ongoing.
An inexpensive therapy with global potential?
“Although our trial lacked the statistical power to discern long-term outcomes, the convalescent plasma group appeared to have better outcomes than the placebo group with respect to all secondary endpoints,” the researchers wrote. “Our findings underscore the need to return to the classic approach of treating acute viral infections early, and they define IgG targets that facilitate donor selection.”
Dr. Polack said, “This is an inexpensive solution to mitigate the burden of severe illness in the population most vulnerable to the virus: the elderly. And it has the attraction of being applicable not only in industrialized countries but in many areas of the developing world.”
Convalescent plasma “is a potentially inexpensive alternative to monoclonal antibodies,” the researchers added. Furthermore, “early infusions of convalescent plasma can provide a bridge to recovery for at-risk patients until vaccines become widely available.”
Dr. Polack said the study findings did not surprise him. “We always thought that, as it has been the case in the past with many therapeutic strategies against respiratory and other viral infections, the earlier you treat, the better.
“We just hoped that within 72 hours of symptoms we would be treating early enough – remember that there is a 4- to 5-day incubation period that the virus leverages before the first symptom – and with enough antibody,” he added.
“We are glad it worked,” he said.
The study was supported by the Bill and Melinda Gates Foundation and by the Fundación INFANT Pandemic Fund. Dr. Polack, Dr. Simonovich, and Dr. Sullivan have disclosed various financial relationships industry.
A version of this article first appeared on Medscape.com.
Administering convalescent plasma that has high levels of antibodies against SARS-CoV-2 within the first 3 days of symptoms was associated with significantly lower chances of progression to severe COVID-19, new evidence demonstrates.
In a trial of 160 older adults with COVID-19, half of whom were randomly assigned to receive plasma and half to receive placebo infusion, treatment with high-titer plasma lowered the relative risk for severe disease by 48% in an intent-to-treat analysis.
“We now have evidence, in the context of a small but well-designed study, that convalescent plasma with high titers of antibody against SARS-CoV-2 administered in the first 3 days of mild symptoms to infected elderly reduces progression of illness and the rate of severe presentations,” senior author Fernando Polack, MD, said in an interview.
“Not any plasma, not any time,” added Dr. Polack, an infectious disease specialist and scientific director at Fundacion INFANT and professor of pediatrics at the University of Buenos Aires. The key, he said, is to select plasma in the upper 28th percentile of IgG antibody concentrations and to administer therapy prior to disease progression.
The study was published online Jan. 6 in The New England Journal of Medicine.
“It’s a very good study and approaches a different population from the PlasmAr study,” Ventura Simonovich, MD, chief of the clinical pharmacology section, Medical Clinic Service, Hospital Italiano de Buenos Aires, said in an interview. “This is the first published randomized controlled trial that shows real benefit in this [older adult] population, the most vulnerable in this disease,” he said.
Dr. Simonovich, who was not affiliated with the current study, was lead author of the PlasmAr trial, which was published in The New England Journal of Medicine Nov. 24, 2020. In that trial, the researchers evaluated adults aged 18 years and older and found no significant benefit with convalescent plasma treatment over placebo for patients with COVID-19 and severe pneumonia.
“We know antibodies work best when given early and in high dose. This is one of the rare reports that validates it in the outpatient setting,” David Sullivan, MD, professor of molecular biology and immunology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in an interview when asked to comment.
Dr. Sullivan pointed out that most previous studies on convalescent plasma focused on patients with COVID-19 who had severe cases late in the disease course.
Regarding the current study, he said, “The striking thing is treating people within 3 days of illness.”
A more cautious interpretation may be warranted, one expert said. “The study demonstrates the benefit of early intervention. There was a dose-dependent effect, with higher titers providing a greater benefit,” Manoj Menon, MD, MPH, a hematologist and oncologist at the University of Washington, Seattle, said in an interview.
“Taken together, the findings have biologic plausibility and produce more data on the role of convalescent plasma to a relevant age cohort,” he added.
However, Dr. Menon said: “Given the limited sample size, I do not think this study, although well conducted, definitively addresses the role of convalescent plasma for COVID-19. But it does merit additional study.”
A search for clear answers
Treatments that target the early stages of COVID-19 “remain elusive. Few strategies provide benefit, several have failed, and others are being evaluated,” the researchers noted. “In hospitalized patients with COVID-19, the infusion of convalescent plasma against SARS-CoV-2 late in the course of illness has not shown clear benefits and, consequently, the most appropriate antibody concentrations for effective treatment are unclear.”
To learn more, Dr. Polack and colleagues included patients with PCR-confirmed COVID-19 who were aged 75 years or older, regardless of comorbidities. They also included patients aged 65-74 years who had at least one underlying condition. Participants were enrolled at clinical sites or geriatric units in Argentina. The mean age was 77 years, and 62% were women.
In an intent-to-treat analysis, the primary outcome – severe respiratory disease – occurred in 16% of the plasma recipients, vs. 31% of the group that received placebo. The relative risk was 0.52 (95% confidence interval, 0.29-0.94; P = .03).
The number needed to treat to avoid a severe respiratory disease episode was 7 (95% CI, 4-50).
Life-threatening respiratory disease, a secondary outcome, occurred in four people in the plasma group, compared with 10 in the placebo group. Two patients in the treatment group and four patients in the placebo group died.
The researchers also ran a modified intent-to-treat analysis that excluded six participants who experienced severe respiratory disease prior to receiving plasma or placebo. In this analysis, efficacy of plasma therapy increased to 60%.
“Again, this finding suggests that early intervention is critical for efficacy,” the investigators noted.
The investigators, who are based in Argentina, defined their primary endpoint as a respiratory rate of 30 or more breaths per minute and/or an oxygen saturation of less than 93% while breathing ambient air.
Dr. Sullivan pointed out that this is equivalent to the threshold commonly used for hospitalizing people with COVID-19 in the United States. “So it’s equivalent to avoiding hospitalizations. The take-home is high-titer plasma prevents respiratory distress, which equals hospitalization for us.”
Dr. Sullivan is conducting similar research in the United States regarding the use of plasma for treatment or prevention. He and colleagues are evaluating adults aged 18-90 years, “not just the ones at highest risk for going to the hospital,” he said. Enrollment is ongoing.
An inexpensive therapy with global potential?
“Although our trial lacked the statistical power to discern long-term outcomes, the convalescent plasma group appeared to have better outcomes than the placebo group with respect to all secondary endpoints,” the researchers wrote. “Our findings underscore the need to return to the classic approach of treating acute viral infections early, and they define IgG targets that facilitate donor selection.”
Dr. Polack said, “This is an inexpensive solution to mitigate the burden of severe illness in the population most vulnerable to the virus: the elderly. And it has the attraction of being applicable not only in industrialized countries but in many areas of the developing world.”
Convalescent plasma “is a potentially inexpensive alternative to monoclonal antibodies,” the researchers added. Furthermore, “early infusions of convalescent plasma can provide a bridge to recovery for at-risk patients until vaccines become widely available.”
Dr. Polack said the study findings did not surprise him. “We always thought that, as it has been the case in the past with many therapeutic strategies against respiratory and other viral infections, the earlier you treat, the better.
“We just hoped that within 72 hours of symptoms we would be treating early enough – remember that there is a 4- to 5-day incubation period that the virus leverages before the first symptom – and with enough antibody,” he added.
“We are glad it worked,” he said.
The study was supported by the Bill and Melinda Gates Foundation and by the Fundación INFANT Pandemic Fund. Dr. Polack, Dr. Simonovich, and Dr. Sullivan have disclosed various financial relationships industry.
A version of this article first appeared on Medscape.com.
Administering convalescent plasma that has high levels of antibodies against SARS-CoV-2 within the first 3 days of symptoms was associated with significantly lower chances of progression to severe COVID-19, new evidence demonstrates.
In a trial of 160 older adults with COVID-19, half of whom were randomly assigned to receive plasma and half to receive placebo infusion, treatment with high-titer plasma lowered the relative risk for severe disease by 48% in an intent-to-treat analysis.
“We now have evidence, in the context of a small but well-designed study, that convalescent plasma with high titers of antibody against SARS-CoV-2 administered in the first 3 days of mild symptoms to infected elderly reduces progression of illness and the rate of severe presentations,” senior author Fernando Polack, MD, said in an interview.
“Not any plasma, not any time,” added Dr. Polack, an infectious disease specialist and scientific director at Fundacion INFANT and professor of pediatrics at the University of Buenos Aires. The key, he said, is to select plasma in the upper 28th percentile of IgG antibody concentrations and to administer therapy prior to disease progression.
The study was published online Jan. 6 in The New England Journal of Medicine.
“It’s a very good study and approaches a different population from the PlasmAr study,” Ventura Simonovich, MD, chief of the clinical pharmacology section, Medical Clinic Service, Hospital Italiano de Buenos Aires, said in an interview. “This is the first published randomized controlled trial that shows real benefit in this [older adult] population, the most vulnerable in this disease,” he said.
Dr. Simonovich, who was not affiliated with the current study, was lead author of the PlasmAr trial, which was published in The New England Journal of Medicine Nov. 24, 2020. In that trial, the researchers evaluated adults aged 18 years and older and found no significant benefit with convalescent plasma treatment over placebo for patients with COVID-19 and severe pneumonia.
“We know antibodies work best when given early and in high dose. This is one of the rare reports that validates it in the outpatient setting,” David Sullivan, MD, professor of molecular biology and immunology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in an interview when asked to comment.
Dr. Sullivan pointed out that most previous studies on convalescent plasma focused on patients with COVID-19 who had severe cases late in the disease course.
Regarding the current study, he said, “The striking thing is treating people within 3 days of illness.”
A more cautious interpretation may be warranted, one expert said. “The study demonstrates the benefit of early intervention. There was a dose-dependent effect, with higher titers providing a greater benefit,” Manoj Menon, MD, MPH, a hematologist and oncologist at the University of Washington, Seattle, said in an interview.
“Taken together, the findings have biologic plausibility and produce more data on the role of convalescent plasma to a relevant age cohort,” he added.
However, Dr. Menon said: “Given the limited sample size, I do not think this study, although well conducted, definitively addresses the role of convalescent plasma for COVID-19. But it does merit additional study.”
A search for clear answers
Treatments that target the early stages of COVID-19 “remain elusive. Few strategies provide benefit, several have failed, and others are being evaluated,” the researchers noted. “In hospitalized patients with COVID-19, the infusion of convalescent plasma against SARS-CoV-2 late in the course of illness has not shown clear benefits and, consequently, the most appropriate antibody concentrations for effective treatment are unclear.”
To learn more, Dr. Polack and colleagues included patients with PCR-confirmed COVID-19 who were aged 75 years or older, regardless of comorbidities. They also included patients aged 65-74 years who had at least one underlying condition. Participants were enrolled at clinical sites or geriatric units in Argentina. The mean age was 77 years, and 62% were women.
In an intent-to-treat analysis, the primary outcome – severe respiratory disease – occurred in 16% of the plasma recipients, vs. 31% of the group that received placebo. The relative risk was 0.52 (95% confidence interval, 0.29-0.94; P = .03).
The number needed to treat to avoid a severe respiratory disease episode was 7 (95% CI, 4-50).
Life-threatening respiratory disease, a secondary outcome, occurred in four people in the plasma group, compared with 10 in the placebo group. Two patients in the treatment group and four patients in the placebo group died.
The researchers also ran a modified intent-to-treat analysis that excluded six participants who experienced severe respiratory disease prior to receiving plasma or placebo. In this analysis, efficacy of plasma therapy increased to 60%.
“Again, this finding suggests that early intervention is critical for efficacy,” the investigators noted.
The investigators, who are based in Argentina, defined their primary endpoint as a respiratory rate of 30 or more breaths per minute and/or an oxygen saturation of less than 93% while breathing ambient air.
Dr. Sullivan pointed out that this is equivalent to the threshold commonly used for hospitalizing people with COVID-19 in the United States. “So it’s equivalent to avoiding hospitalizations. The take-home is high-titer plasma prevents respiratory distress, which equals hospitalization for us.”
Dr. Sullivan is conducting similar research in the United States regarding the use of plasma for treatment or prevention. He and colleagues are evaluating adults aged 18-90 years, “not just the ones at highest risk for going to the hospital,” he said. Enrollment is ongoing.
An inexpensive therapy with global potential?
“Although our trial lacked the statistical power to discern long-term outcomes, the convalescent plasma group appeared to have better outcomes than the placebo group with respect to all secondary endpoints,” the researchers wrote. “Our findings underscore the need to return to the classic approach of treating acute viral infections early, and they define IgG targets that facilitate donor selection.”
Dr. Polack said, “This is an inexpensive solution to mitigate the burden of severe illness in the population most vulnerable to the virus: the elderly. And it has the attraction of being applicable not only in industrialized countries but in many areas of the developing world.”
Convalescent plasma “is a potentially inexpensive alternative to monoclonal antibodies,” the researchers added. Furthermore, “early infusions of convalescent plasma can provide a bridge to recovery for at-risk patients until vaccines become widely available.”
Dr. Polack said the study findings did not surprise him. “We always thought that, as it has been the case in the past with many therapeutic strategies against respiratory and other viral infections, the earlier you treat, the better.
“We just hoped that within 72 hours of symptoms we would be treating early enough – remember that there is a 4- to 5-day incubation period that the virus leverages before the first symptom – and with enough antibody,” he added.
“We are glad it worked,” he said.
The study was supported by the Bill and Melinda Gates Foundation and by the Fundación INFANT Pandemic Fund. Dr. Polack, Dr. Simonovich, and Dr. Sullivan have disclosed various financial relationships industry.
A version of this article first appeared on Medscape.com.
Osteoporosis prevalence in PsA similar to general population
The rates of osteopenia and osteoporosis among individuals with psoriatic arthritis are comparable to those seen in the general population, research suggests.
The cohort study, published in Arthritis Care & Research, also found that clinicians are likely to refer patients for bone mineral density (BMD) testing based on osteoporosis risk factors or psoriatic arthritis disease severity markers.
Timothy S.H. Kwok, MD, of the University of Toronto, and coauthors wrote that previous research suggested a possible link between psoriatic arthritis and osteoporosis or osteopenia. However, no cohort studies appear to have examined this association.
The study involved 201 individuals with psoriatic arthritis attending a single specialist clinic, who were enrolled in a longitudinal study of psoriatic arthritis (PsA) and who were also referred for BMD testing with dual-energy x-ray absorptiometry.
Of these participants, 13% had a BMD in the osteoporotic range, 45% were in the osteopenic range, and 42% were in the normal range for BMD. The prevalence of osteoporosis observed in the general population aged 50 or above, observed in an earlier large prospective study, ranged from 7% to 16%, and osteopenia ranged from 27% to 46%.
“Our study suggests that patients with PsA have similar BMDs compared to the general population,” the authors wrote.
Researchers did note the suggestion that patients with polyarthritis had lower BMDs over time. Because of the small number of events, this did not achieve statistical significance, but “this relationship warrants further research, given that multiple cohort studies have independently demonstrated polyarticular onset of disease predicting clinical deformities and erosive disease in PsA,” they wrote.
They also saw that patients with increased body mass index had a significant 21% lower odds of having a BMD in the osteoporotic range, while those using biologics had a significant 83% lower odds.
Among participants with BMD scores in the osteopenic or osteoporotic range, these scores were seen in the lumbar spine in 63% of measurements, the femoral neck in 88%, and the total hip in 39%. Mean T-scores for the lumbar spine were –0.30±0.32, and for the femoral neck were –1.10±1.04 and the total hip, –0.45±0.42.
The study also examined what factors were associated with referral for BMD testing. They found that increasing age, menopause, elevated acute phase reactants, or use of biologics, methotrexate, and systemic glucocorticoids were associated with a higher likelihood of undergoing BMD testing.
Noting that the latest Canadian clinical practice guidelines on BMD testing advise that age, menopause, and use of systemic glucocorticoids use are risk factors that should prompt testing, the authors suggested clinicians were using a combination of traditional osteoporosis risk factors and markers of psoriatic disease severity to underpin their decision to refer.
However, they commented that none of the factors associated with a higher likelihood of having a BMD test were actually associated with lower BMD scores.
“This suggests that clinicians may be over-screening patients with PsA for osteopenia/osteoporosis, as they do not appear to be at baseline higher risk for lower BMD scores than the general population,” they wrote. “This is of importance, as there are currently no formal recommendations with regards to the optimal interval or time to commence BMD testing within the recent major PsA guidelines.”
The study was supported by a grant from the Krembil Foundation. No conflicts of interest were declared.
SOURCE: Kwok TSH et al. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24538.
The rates of osteopenia and osteoporosis among individuals with psoriatic arthritis are comparable to those seen in the general population, research suggests.
The cohort study, published in Arthritis Care & Research, also found that clinicians are likely to refer patients for bone mineral density (BMD) testing based on osteoporosis risk factors or psoriatic arthritis disease severity markers.
Timothy S.H. Kwok, MD, of the University of Toronto, and coauthors wrote that previous research suggested a possible link between psoriatic arthritis and osteoporosis or osteopenia. However, no cohort studies appear to have examined this association.
The study involved 201 individuals with psoriatic arthritis attending a single specialist clinic, who were enrolled in a longitudinal study of psoriatic arthritis (PsA) and who were also referred for BMD testing with dual-energy x-ray absorptiometry.
Of these participants, 13% had a BMD in the osteoporotic range, 45% were in the osteopenic range, and 42% were in the normal range for BMD. The prevalence of osteoporosis observed in the general population aged 50 or above, observed in an earlier large prospective study, ranged from 7% to 16%, and osteopenia ranged from 27% to 46%.
“Our study suggests that patients with PsA have similar BMDs compared to the general population,” the authors wrote.
Researchers did note the suggestion that patients with polyarthritis had lower BMDs over time. Because of the small number of events, this did not achieve statistical significance, but “this relationship warrants further research, given that multiple cohort studies have independently demonstrated polyarticular onset of disease predicting clinical deformities and erosive disease in PsA,” they wrote.
They also saw that patients with increased body mass index had a significant 21% lower odds of having a BMD in the osteoporotic range, while those using biologics had a significant 83% lower odds.
Among participants with BMD scores in the osteopenic or osteoporotic range, these scores were seen in the lumbar spine in 63% of measurements, the femoral neck in 88%, and the total hip in 39%. Mean T-scores for the lumbar spine were –0.30±0.32, and for the femoral neck were –1.10±1.04 and the total hip, –0.45±0.42.
The study also examined what factors were associated with referral for BMD testing. They found that increasing age, menopause, elevated acute phase reactants, or use of biologics, methotrexate, and systemic glucocorticoids were associated with a higher likelihood of undergoing BMD testing.
Noting that the latest Canadian clinical practice guidelines on BMD testing advise that age, menopause, and use of systemic glucocorticoids use are risk factors that should prompt testing, the authors suggested clinicians were using a combination of traditional osteoporosis risk factors and markers of psoriatic disease severity to underpin their decision to refer.
However, they commented that none of the factors associated with a higher likelihood of having a BMD test were actually associated with lower BMD scores.
“This suggests that clinicians may be over-screening patients with PsA for osteopenia/osteoporosis, as they do not appear to be at baseline higher risk for lower BMD scores than the general population,” they wrote. “This is of importance, as there are currently no formal recommendations with regards to the optimal interval or time to commence BMD testing within the recent major PsA guidelines.”
The study was supported by a grant from the Krembil Foundation. No conflicts of interest were declared.
SOURCE: Kwok TSH et al. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24538.
The rates of osteopenia and osteoporosis among individuals with psoriatic arthritis are comparable to those seen in the general population, research suggests.
The cohort study, published in Arthritis Care & Research, also found that clinicians are likely to refer patients for bone mineral density (BMD) testing based on osteoporosis risk factors or psoriatic arthritis disease severity markers.
Timothy S.H. Kwok, MD, of the University of Toronto, and coauthors wrote that previous research suggested a possible link between psoriatic arthritis and osteoporosis or osteopenia. However, no cohort studies appear to have examined this association.
The study involved 201 individuals with psoriatic arthritis attending a single specialist clinic, who were enrolled in a longitudinal study of psoriatic arthritis (PsA) and who were also referred for BMD testing with dual-energy x-ray absorptiometry.
Of these participants, 13% had a BMD in the osteoporotic range, 45% were in the osteopenic range, and 42% were in the normal range for BMD. The prevalence of osteoporosis observed in the general population aged 50 or above, observed in an earlier large prospective study, ranged from 7% to 16%, and osteopenia ranged from 27% to 46%.
“Our study suggests that patients with PsA have similar BMDs compared to the general population,” the authors wrote.
Researchers did note the suggestion that patients with polyarthritis had lower BMDs over time. Because of the small number of events, this did not achieve statistical significance, but “this relationship warrants further research, given that multiple cohort studies have independently demonstrated polyarticular onset of disease predicting clinical deformities and erosive disease in PsA,” they wrote.
They also saw that patients with increased body mass index had a significant 21% lower odds of having a BMD in the osteoporotic range, while those using biologics had a significant 83% lower odds.
Among participants with BMD scores in the osteopenic or osteoporotic range, these scores were seen in the lumbar spine in 63% of measurements, the femoral neck in 88%, and the total hip in 39%. Mean T-scores for the lumbar spine were –0.30±0.32, and for the femoral neck were –1.10±1.04 and the total hip, –0.45±0.42.
The study also examined what factors were associated with referral for BMD testing. They found that increasing age, menopause, elevated acute phase reactants, or use of biologics, methotrexate, and systemic glucocorticoids were associated with a higher likelihood of undergoing BMD testing.
Noting that the latest Canadian clinical practice guidelines on BMD testing advise that age, menopause, and use of systemic glucocorticoids use are risk factors that should prompt testing, the authors suggested clinicians were using a combination of traditional osteoporosis risk factors and markers of psoriatic disease severity to underpin their decision to refer.
However, they commented that none of the factors associated with a higher likelihood of having a BMD test were actually associated with lower BMD scores.
“This suggests that clinicians may be over-screening patients with PsA for osteopenia/osteoporosis, as they do not appear to be at baseline higher risk for lower BMD scores than the general population,” they wrote. “This is of importance, as there are currently no formal recommendations with regards to the optimal interval or time to commence BMD testing within the recent major PsA guidelines.”
The study was supported by a grant from the Krembil Foundation. No conflicts of interest were declared.
SOURCE: Kwok TSH et al. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24538.
FROM ARTHRITIS CARE & RESEARCH
Temper enthusiasm for long-term treatment with bisphosphonates?
Women treated with oral bisphosphonate drugs for osteoporosis for 5 years get no additional benefit – in terms of hip fracture risk – if the treatment is extended for another 5 years, new research shows.
“We found that hip fracture risk in women did not differ if women stopped bisphosphonate use after 5 years or stayed on the medication for 10 years,” coauthor Joan C. Lo, MD, Kaiser Permanente Northern California, Oakland, said in an interview.
The new study, published Dec. 7 in JAMA Network Open, did show a small benefit in continuing the treatment through 7 years vs. 5 years, but it wasn’t clear if this was significant.
“Whether there is a benefit to staying on the drug for 7 years needs to be further studied in randomized trials,” Dr. Lo stressed.
It is well established that oral bisphosphonates are effective in reducing the risk for fracture within the first 3-5 years of treatment; however, evidence on the effects of treatment beyond 5 years is lacking.
The most recent guidance from the American Society of Bone and Mineral Research (ASBMR) on the issue, which were released in 2015, recommends continuation of bisphosphonates beyond 5 years for high-risk patients, but it recommends a “drug holiday” for low-risk patients.
Study adds important new evidence
However, that guidance acknowledges that data are limited regarding long-term use. This large new study adds important new evidence to the discussion, Robert A. Adler, MD, who was a member of the ASBMR Task Force for the recent guidance, said in an interview.
“[With the lack of recent research,] this new study from Kaiser Permanente is of great interest,” said Dr. Adler, chief of endocrinology and metabolism at Central Virginia Veterans Affairs Health Care System and professor of internal medicine and of epidemiology at Virginia Commonwealth University, Richmond.
“It is new data and suggests we might temper our enthusiasm for long-term treatment with bisphosphonates,” he said.
“Importantly, it is the first large observational trial and is closer to a real-world setting than a randomized controlled trial,” he said.
But, Dr. Adler emphasized: “The take-home message is that while this suggests that patients can probably be given a drug holiday for a couple of years ... they should be retested, and if they appear to be at an increased risk of fracture, they probably should restart again.
“Osteoporosis is a chronic disorder,” he emphasized. “It isn’t cured by any of our treatments, and as people get older, they are at a higher fracture risk.
“So we really need to follow our patients for a lifetime and reassess their fracture risk every couple of years – whether they are still on therapy or on a drug holiday.”
Possible that 7 years is better than 5 but remains to be proven
The new study involved data from Kaiser Permanente Northern and Southern California on 29,685 women who had completed 5 years of treatment with oral bisphosphonates, including alendronate, risedronate, or ibandronate, between 2002 and 2014.
Among the women, 11,105 (37%) continued taking the drugs beyond 5 years to 7 years, and 2,725 (9.2%) completed a total of 10 years of treatment.
Their median age was 71. Among those for whom bone mineral density data were available, 37% had osteoporosis after the first 5 years of treatment.
During these 5 years of treatment, 507 hip fractures occurred.
The cumulative incidence of hip fracture among for those who discontinued study therapy at entry, i.e., those who underwent treatment for 5 years, was 23.0 per 1,000 individuals.
After 7 years of treatment, the rate was 20.8 per 1000. For those who continued therapy for 10 years, the rate was 26.8 per 1000 individuals.
The rate in the 7-year treatment group was based on patients taking a 6-month drug holiday after the initial 5 years, but the results are hard to interpret, Dr. Lo said.
“It’s possible that 7 years is better than 5, but this is not a randomized trial, and some of the data analyses done in the study suggest more research should be done to look at a benefit after 7 years.
“At the end of the day, doctors and women need to decide at 5 years what an individual woman’s risk fracture risk is and determine if she should stay on the drug longer,” Dr. Lo emphasized.
Limitations: Subgroups not identified, adherence hard to assess
The uncertainty of any benefit of treatment with bisphosphonates beyond 5 years is further reflected in U.S. recommendations – the Food and Drug Administration has concluded on the basis of pooled data from the extension phase of major clinical trials that any advantages of treatment beyond 3-5 years are unclear.
Key limitations of the current study include the fact that the incidence of hip fracture was not evaluated in low-risk vs. high-risk subgroups; therefore, “these findings may not be applicable to older women at higher risk of osteoporotic fracture,” the authors wrote.
Furthermore, the study did not assess outcomes of fractures other than hip fractures, such as vertebral fractures, they noted.
Dr. Adler pointed out that another limitation is that adherence in the trial was defined as taking 60% of prescribed pills.
“I think this is the biggest weakness with the study,” he said. “Particularly with medications like oral bisphosphonates that don’t really make patients feel any different, it’s a real challenge to make sure patients continue to take these drugs properly.”
The findings should give some reassurance for patients who take a break from the drugs after 5 years. However, reassessment of their risk is critical, Dr. Adler reiterated.
The study was supported by a grant from the National Institute on Aging and the National Institute of Arthritis, Musculoskeletal, and Skin Diseases of the National Institutes of Health. The authors and Adler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women treated with oral bisphosphonate drugs for osteoporosis for 5 years get no additional benefit – in terms of hip fracture risk – if the treatment is extended for another 5 years, new research shows.
“We found that hip fracture risk in women did not differ if women stopped bisphosphonate use after 5 years or stayed on the medication for 10 years,” coauthor Joan C. Lo, MD, Kaiser Permanente Northern California, Oakland, said in an interview.
The new study, published Dec. 7 in JAMA Network Open, did show a small benefit in continuing the treatment through 7 years vs. 5 years, but it wasn’t clear if this was significant.
“Whether there is a benefit to staying on the drug for 7 years needs to be further studied in randomized trials,” Dr. Lo stressed.
It is well established that oral bisphosphonates are effective in reducing the risk for fracture within the first 3-5 years of treatment; however, evidence on the effects of treatment beyond 5 years is lacking.
The most recent guidance from the American Society of Bone and Mineral Research (ASBMR) on the issue, which were released in 2015, recommends continuation of bisphosphonates beyond 5 years for high-risk patients, but it recommends a “drug holiday” for low-risk patients.
Study adds important new evidence
However, that guidance acknowledges that data are limited regarding long-term use. This large new study adds important new evidence to the discussion, Robert A. Adler, MD, who was a member of the ASBMR Task Force for the recent guidance, said in an interview.
“[With the lack of recent research,] this new study from Kaiser Permanente is of great interest,” said Dr. Adler, chief of endocrinology and metabolism at Central Virginia Veterans Affairs Health Care System and professor of internal medicine and of epidemiology at Virginia Commonwealth University, Richmond.
“It is new data and suggests we might temper our enthusiasm for long-term treatment with bisphosphonates,” he said.
“Importantly, it is the first large observational trial and is closer to a real-world setting than a randomized controlled trial,” he said.
But, Dr. Adler emphasized: “The take-home message is that while this suggests that patients can probably be given a drug holiday for a couple of years ... they should be retested, and if they appear to be at an increased risk of fracture, they probably should restart again.
“Osteoporosis is a chronic disorder,” he emphasized. “It isn’t cured by any of our treatments, and as people get older, they are at a higher fracture risk.
“So we really need to follow our patients for a lifetime and reassess their fracture risk every couple of years – whether they are still on therapy or on a drug holiday.”
Possible that 7 years is better than 5 but remains to be proven
The new study involved data from Kaiser Permanente Northern and Southern California on 29,685 women who had completed 5 years of treatment with oral bisphosphonates, including alendronate, risedronate, or ibandronate, between 2002 and 2014.
Among the women, 11,105 (37%) continued taking the drugs beyond 5 years to 7 years, and 2,725 (9.2%) completed a total of 10 years of treatment.
Their median age was 71. Among those for whom bone mineral density data were available, 37% had osteoporosis after the first 5 years of treatment.
During these 5 years of treatment, 507 hip fractures occurred.
The cumulative incidence of hip fracture among for those who discontinued study therapy at entry, i.e., those who underwent treatment for 5 years, was 23.0 per 1,000 individuals.
After 7 years of treatment, the rate was 20.8 per 1000. For those who continued therapy for 10 years, the rate was 26.8 per 1000 individuals.
The rate in the 7-year treatment group was based on patients taking a 6-month drug holiday after the initial 5 years, but the results are hard to interpret, Dr. Lo said.
“It’s possible that 7 years is better than 5, but this is not a randomized trial, and some of the data analyses done in the study suggest more research should be done to look at a benefit after 7 years.
“At the end of the day, doctors and women need to decide at 5 years what an individual woman’s risk fracture risk is and determine if she should stay on the drug longer,” Dr. Lo emphasized.
Limitations: Subgroups not identified, adherence hard to assess
The uncertainty of any benefit of treatment with bisphosphonates beyond 5 years is further reflected in U.S. recommendations – the Food and Drug Administration has concluded on the basis of pooled data from the extension phase of major clinical trials that any advantages of treatment beyond 3-5 years are unclear.
Key limitations of the current study include the fact that the incidence of hip fracture was not evaluated in low-risk vs. high-risk subgroups; therefore, “these findings may not be applicable to older women at higher risk of osteoporotic fracture,” the authors wrote.
Furthermore, the study did not assess outcomes of fractures other than hip fractures, such as vertebral fractures, they noted.
Dr. Adler pointed out that another limitation is that adherence in the trial was defined as taking 60% of prescribed pills.
“I think this is the biggest weakness with the study,” he said. “Particularly with medications like oral bisphosphonates that don’t really make patients feel any different, it’s a real challenge to make sure patients continue to take these drugs properly.”
The findings should give some reassurance for patients who take a break from the drugs after 5 years. However, reassessment of their risk is critical, Dr. Adler reiterated.
The study was supported by a grant from the National Institute on Aging and the National Institute of Arthritis, Musculoskeletal, and Skin Diseases of the National Institutes of Health. The authors and Adler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women treated with oral bisphosphonate drugs for osteoporosis for 5 years get no additional benefit – in terms of hip fracture risk – if the treatment is extended for another 5 years, new research shows.
“We found that hip fracture risk in women did not differ if women stopped bisphosphonate use after 5 years or stayed on the medication for 10 years,” coauthor Joan C. Lo, MD, Kaiser Permanente Northern California, Oakland, said in an interview.
The new study, published Dec. 7 in JAMA Network Open, did show a small benefit in continuing the treatment through 7 years vs. 5 years, but it wasn’t clear if this was significant.
“Whether there is a benefit to staying on the drug for 7 years needs to be further studied in randomized trials,” Dr. Lo stressed.
It is well established that oral bisphosphonates are effective in reducing the risk for fracture within the first 3-5 years of treatment; however, evidence on the effects of treatment beyond 5 years is lacking.
The most recent guidance from the American Society of Bone and Mineral Research (ASBMR) on the issue, which were released in 2015, recommends continuation of bisphosphonates beyond 5 years for high-risk patients, but it recommends a “drug holiday” for low-risk patients.
Study adds important new evidence
However, that guidance acknowledges that data are limited regarding long-term use. This large new study adds important new evidence to the discussion, Robert A. Adler, MD, who was a member of the ASBMR Task Force for the recent guidance, said in an interview.
“[With the lack of recent research,] this new study from Kaiser Permanente is of great interest,” said Dr. Adler, chief of endocrinology and metabolism at Central Virginia Veterans Affairs Health Care System and professor of internal medicine and of epidemiology at Virginia Commonwealth University, Richmond.
“It is new data and suggests we might temper our enthusiasm for long-term treatment with bisphosphonates,” he said.
“Importantly, it is the first large observational trial and is closer to a real-world setting than a randomized controlled trial,” he said.
But, Dr. Adler emphasized: “The take-home message is that while this suggests that patients can probably be given a drug holiday for a couple of years ... they should be retested, and if they appear to be at an increased risk of fracture, they probably should restart again.
“Osteoporosis is a chronic disorder,” he emphasized. “It isn’t cured by any of our treatments, and as people get older, they are at a higher fracture risk.
“So we really need to follow our patients for a lifetime and reassess their fracture risk every couple of years – whether they are still on therapy or on a drug holiday.”
Possible that 7 years is better than 5 but remains to be proven
The new study involved data from Kaiser Permanente Northern and Southern California on 29,685 women who had completed 5 years of treatment with oral bisphosphonates, including alendronate, risedronate, or ibandronate, between 2002 and 2014.
Among the women, 11,105 (37%) continued taking the drugs beyond 5 years to 7 years, and 2,725 (9.2%) completed a total of 10 years of treatment.
Their median age was 71. Among those for whom bone mineral density data were available, 37% had osteoporosis after the first 5 years of treatment.
During these 5 years of treatment, 507 hip fractures occurred.
The cumulative incidence of hip fracture among for those who discontinued study therapy at entry, i.e., those who underwent treatment for 5 years, was 23.0 per 1,000 individuals.
After 7 years of treatment, the rate was 20.8 per 1000. For those who continued therapy for 10 years, the rate was 26.8 per 1000 individuals.
The rate in the 7-year treatment group was based on patients taking a 6-month drug holiday after the initial 5 years, but the results are hard to interpret, Dr. Lo said.
“It’s possible that 7 years is better than 5, but this is not a randomized trial, and some of the data analyses done in the study suggest more research should be done to look at a benefit after 7 years.
“At the end of the day, doctors and women need to decide at 5 years what an individual woman’s risk fracture risk is and determine if she should stay on the drug longer,” Dr. Lo emphasized.
Limitations: Subgroups not identified, adherence hard to assess
The uncertainty of any benefit of treatment with bisphosphonates beyond 5 years is further reflected in U.S. recommendations – the Food and Drug Administration has concluded on the basis of pooled data from the extension phase of major clinical trials that any advantages of treatment beyond 3-5 years are unclear.
Key limitations of the current study include the fact that the incidence of hip fracture was not evaluated in low-risk vs. high-risk subgroups; therefore, “these findings may not be applicable to older women at higher risk of osteoporotic fracture,” the authors wrote.
Furthermore, the study did not assess outcomes of fractures other than hip fractures, such as vertebral fractures, they noted.
Dr. Adler pointed out that another limitation is that adherence in the trial was defined as taking 60% of prescribed pills.
“I think this is the biggest weakness with the study,” he said. “Particularly with medications like oral bisphosphonates that don’t really make patients feel any different, it’s a real challenge to make sure patients continue to take these drugs properly.”
The findings should give some reassurance for patients who take a break from the drugs after 5 years. However, reassessment of their risk is critical, Dr. Adler reiterated.
The study was supported by a grant from the National Institute on Aging and the National Institute of Arthritis, Musculoskeletal, and Skin Diseases of the National Institutes of Health. The authors and Adler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CDC identifies next priority groups for COVID-19 vaccine
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention voted 13-1 for the recommendation. This builds on ACIP’s initial recommendation about which groups should be in the first wave of vaccinations, described as Phase 1a.
ACIP earlier recommended that Phase 1a include U.S. health care workers, a group of about 21 million people, and residents of long-term care facilities, a group of about 3 million.
On Dec. 20, ACIP said the next priority group, Phase 1b, should consist of what it called frontline essential workers, a group of about 30 million, and adults aged 75 years and older, a group of about 21 million. When overlap between the groups is taken into account, Phase 1b covers about 49 million people, according to the CDC.
Phase 1c then would include adults aged 65-74 years (a group of about 32 million), adults aged 16-64 years with high-risk medical conditions (a group of about 110 million), and essential workers who did not qualify for inclusion in Phase 1b (a group of about 57 million). With the overlap, Phase 1c would cover about 129 million.
The Food and Drug Administration recently granted emergency use authorizations for two COVID-19 vaccines, one developed by Pfizer-BioNTech and another from Moderna. Other companies, including Johnson & Johnson, have advanced their potential rival COVID-19 vaccines into late-stages of testing. To date, about 2.83 million doses of Pfizer’s COVID-19 vaccine have been distributed and 556,208 doses have been administered, according to the CDC.
But there will likely still be a period of months when competition for limited doses of COVID-19 vaccine will trigger difficult decisions. Current estimates indicate there will be enough supply to provide COVID-19 vaccines for 20 million people in December, 30 million people in January, and 50 million people in February, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases.
State governments and health systems will take ACIP’s recommendations into account as they roll out the initial supplies of COVID-19 vaccines.
There’s clearly wide latitude in these decisions. Recently, for example, many members of Congress tweeted photos of themselves getting COVID-19 vaccines, despite not falling into ACIP’s description of the Phase 1 group.
Difficult choices
All ACIP members described the Dec. 20 vote as a difficult decision. It forced them to choose among segments of the U.S. population that could benefit from early access to the limited supply of COVID-19 vaccines.
“For every group we add, it means we subtract a group. For every group we subtract, it means they don’t get the vaccine” for some months, said ACIP member Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn. “It’s incredibly humbling and heartbreaking.”
ACIP member Henry Bernstein, DO, who cast the lone dissenting vote, said he agreed with most of the panel’s recommendation. He said he fully supported the inclusion of adults aged 75 years and older and essential frontline workers in the second wave, Phase 1b. But he voted no because the data on COVID-19 morbidity and mortality for adults aged 65-74 years is similar enough to the older group to warrant their inclusion in the first wave.
“Therefore, inclusion of the 65- to 74-year-old group in Phase 1b made more sense to me,” said Dr. Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in New York.
As defined by the CDC, frontline essential workers included in phase 1b will be those commonly called “first responders,” such as firefighters and police officers. Also in this group are teachers, support staff, daycare providers, and those employed in grocery and agriculture industries. Others in this group would include U.S. Postal Service employees and transit workers.
ACIP panelists noted the difficulties that will emerge as government officials and leaders of health care organizations move to apply their guidance to real-world decisions about distributing a limited supply of COVID-19 vaccine. There’s a potential to worsen existing disparities in access to health care, as people with more income may find it easier to obtain proof that they qualify as having a high-risk condition, said José Romero, MD, the chair of ACIP.
Many people “don’t have access to medical care and can’t come up with a doctor’s note that says, ‘I have diabetes,’ ” he said.
ACIP panelists also noted in their deliberations that people may technically qualify for a priority group but have little risk, such as someone with a chronic medical condition who works from home.
And the risk for COVID-19 remains serious even for those who will ultimately fall into the phase 2 for vaccination. Young adults have suffered serious complications following COVID-19, such as stroke, that may alter their lives dramatically, ACIP member Dr. Talbot said, adding that she is reminded of this in her work.
“We need to be very cautious about saying, ‘Young adults will be fine,’ ” she said. “I spent the past week on back-up clinical call and have read these charts and have cried every day.”
The three ACIP members who had conflicts that prevented their voting were Robert L. Atmar, MD, who said he had participated in COVID-19 trials, including research on the Moderna vaccine; Sharon E. Frey, MD, who said that she had been involved with research on COVID-19 vaccines, including Moderna’s; and Paul Hunter, MD, who said he has received a grant from Pfizer for pneumococcal vaccines. The other panel members have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention voted 13-1 for the recommendation. This builds on ACIP’s initial recommendation about which groups should be in the first wave of vaccinations, described as Phase 1a.
ACIP earlier recommended that Phase 1a include U.S. health care workers, a group of about 21 million people, and residents of long-term care facilities, a group of about 3 million.
On Dec. 20, ACIP said the next priority group, Phase 1b, should consist of what it called frontline essential workers, a group of about 30 million, and adults aged 75 years and older, a group of about 21 million. When overlap between the groups is taken into account, Phase 1b covers about 49 million people, according to the CDC.
Phase 1c then would include adults aged 65-74 years (a group of about 32 million), adults aged 16-64 years with high-risk medical conditions (a group of about 110 million), and essential workers who did not qualify for inclusion in Phase 1b (a group of about 57 million). With the overlap, Phase 1c would cover about 129 million.
The Food and Drug Administration recently granted emergency use authorizations for two COVID-19 vaccines, one developed by Pfizer-BioNTech and another from Moderna. Other companies, including Johnson & Johnson, have advanced their potential rival COVID-19 vaccines into late-stages of testing. To date, about 2.83 million doses of Pfizer’s COVID-19 vaccine have been distributed and 556,208 doses have been administered, according to the CDC.
But there will likely still be a period of months when competition for limited doses of COVID-19 vaccine will trigger difficult decisions. Current estimates indicate there will be enough supply to provide COVID-19 vaccines for 20 million people in December, 30 million people in January, and 50 million people in February, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases.
State governments and health systems will take ACIP’s recommendations into account as they roll out the initial supplies of COVID-19 vaccines.
There’s clearly wide latitude in these decisions. Recently, for example, many members of Congress tweeted photos of themselves getting COVID-19 vaccines, despite not falling into ACIP’s description of the Phase 1 group.
Difficult choices
All ACIP members described the Dec. 20 vote as a difficult decision. It forced them to choose among segments of the U.S. population that could benefit from early access to the limited supply of COVID-19 vaccines.
“For every group we add, it means we subtract a group. For every group we subtract, it means they don’t get the vaccine” for some months, said ACIP member Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn. “It’s incredibly humbling and heartbreaking.”
ACIP member Henry Bernstein, DO, who cast the lone dissenting vote, said he agreed with most of the panel’s recommendation. He said he fully supported the inclusion of adults aged 75 years and older and essential frontline workers in the second wave, Phase 1b. But he voted no because the data on COVID-19 morbidity and mortality for adults aged 65-74 years is similar enough to the older group to warrant their inclusion in the first wave.
“Therefore, inclusion of the 65- to 74-year-old group in Phase 1b made more sense to me,” said Dr. Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in New York.
As defined by the CDC, frontline essential workers included in phase 1b will be those commonly called “first responders,” such as firefighters and police officers. Also in this group are teachers, support staff, daycare providers, and those employed in grocery and agriculture industries. Others in this group would include U.S. Postal Service employees and transit workers.
ACIP panelists noted the difficulties that will emerge as government officials and leaders of health care organizations move to apply their guidance to real-world decisions about distributing a limited supply of COVID-19 vaccine. There’s a potential to worsen existing disparities in access to health care, as people with more income may find it easier to obtain proof that they qualify as having a high-risk condition, said José Romero, MD, the chair of ACIP.
Many people “don’t have access to medical care and can’t come up with a doctor’s note that says, ‘I have diabetes,’ ” he said.
ACIP panelists also noted in their deliberations that people may technically qualify for a priority group but have little risk, such as someone with a chronic medical condition who works from home.
And the risk for COVID-19 remains serious even for those who will ultimately fall into the phase 2 for vaccination. Young adults have suffered serious complications following COVID-19, such as stroke, that may alter their lives dramatically, ACIP member Dr. Talbot said, adding that she is reminded of this in her work.
“We need to be very cautious about saying, ‘Young adults will be fine,’ ” she said. “I spent the past week on back-up clinical call and have read these charts and have cried every day.”
The three ACIP members who had conflicts that prevented their voting were Robert L. Atmar, MD, who said he had participated in COVID-19 trials, including research on the Moderna vaccine; Sharon E. Frey, MD, who said that she had been involved with research on COVID-19 vaccines, including Moderna’s; and Paul Hunter, MD, who said he has received a grant from Pfizer for pneumococcal vaccines. The other panel members have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention voted 13-1 for the recommendation. This builds on ACIP’s initial recommendation about which groups should be in the first wave of vaccinations, described as Phase 1a.
ACIP earlier recommended that Phase 1a include U.S. health care workers, a group of about 21 million people, and residents of long-term care facilities, a group of about 3 million.
On Dec. 20, ACIP said the next priority group, Phase 1b, should consist of what it called frontline essential workers, a group of about 30 million, and adults aged 75 years and older, a group of about 21 million. When overlap between the groups is taken into account, Phase 1b covers about 49 million people, according to the CDC.
Phase 1c then would include adults aged 65-74 years (a group of about 32 million), adults aged 16-64 years with high-risk medical conditions (a group of about 110 million), and essential workers who did not qualify for inclusion in Phase 1b (a group of about 57 million). With the overlap, Phase 1c would cover about 129 million.
The Food and Drug Administration recently granted emergency use authorizations for two COVID-19 vaccines, one developed by Pfizer-BioNTech and another from Moderna. Other companies, including Johnson & Johnson, have advanced their potential rival COVID-19 vaccines into late-stages of testing. To date, about 2.83 million doses of Pfizer’s COVID-19 vaccine have been distributed and 556,208 doses have been administered, according to the CDC.
But there will likely still be a period of months when competition for limited doses of COVID-19 vaccine will trigger difficult decisions. Current estimates indicate there will be enough supply to provide COVID-19 vaccines for 20 million people in December, 30 million people in January, and 50 million people in February, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases.
State governments and health systems will take ACIP’s recommendations into account as they roll out the initial supplies of COVID-19 vaccines.
There’s clearly wide latitude in these decisions. Recently, for example, many members of Congress tweeted photos of themselves getting COVID-19 vaccines, despite not falling into ACIP’s description of the Phase 1 group.
Difficult choices
All ACIP members described the Dec. 20 vote as a difficult decision. It forced them to choose among segments of the U.S. population that could benefit from early access to the limited supply of COVID-19 vaccines.
“For every group we add, it means we subtract a group. For every group we subtract, it means they don’t get the vaccine” for some months, said ACIP member Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn. “It’s incredibly humbling and heartbreaking.”
ACIP member Henry Bernstein, DO, who cast the lone dissenting vote, said he agreed with most of the panel’s recommendation. He said he fully supported the inclusion of adults aged 75 years and older and essential frontline workers in the second wave, Phase 1b. But he voted no because the data on COVID-19 morbidity and mortality for adults aged 65-74 years is similar enough to the older group to warrant their inclusion in the first wave.
“Therefore, inclusion of the 65- to 74-year-old group in Phase 1b made more sense to me,” said Dr. Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in New York.
As defined by the CDC, frontline essential workers included in phase 1b will be those commonly called “first responders,” such as firefighters and police officers. Also in this group are teachers, support staff, daycare providers, and those employed in grocery and agriculture industries. Others in this group would include U.S. Postal Service employees and transit workers.
ACIP panelists noted the difficulties that will emerge as government officials and leaders of health care organizations move to apply their guidance to real-world decisions about distributing a limited supply of COVID-19 vaccine. There’s a potential to worsen existing disparities in access to health care, as people with more income may find it easier to obtain proof that they qualify as having a high-risk condition, said José Romero, MD, the chair of ACIP.
Many people “don’t have access to medical care and can’t come up with a doctor’s note that says, ‘I have diabetes,’ ” he said.
ACIP panelists also noted in their deliberations that people may technically qualify for a priority group but have little risk, such as someone with a chronic medical condition who works from home.
And the risk for COVID-19 remains serious even for those who will ultimately fall into the phase 2 for vaccination. Young adults have suffered serious complications following COVID-19, such as stroke, that may alter their lives dramatically, ACIP member Dr. Talbot said, adding that she is reminded of this in her work.
“We need to be very cautious about saying, ‘Young adults will be fine,’ ” she said. “I spent the past week on back-up clinical call and have read these charts and have cried every day.”
The three ACIP members who had conflicts that prevented their voting were Robert L. Atmar, MD, who said he had participated in COVID-19 trials, including research on the Moderna vaccine; Sharon E. Frey, MD, who said that she had been involved with research on COVID-19 vaccines, including Moderna’s; and Paul Hunter, MD, who said he has received a grant from Pfizer for pneumococcal vaccines. The other panel members have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Study links sleep meds and dementia risk in older adults
Sleep medications for older patients who report sleep problems may not be the best treatment given growing evidence of the link between these medications and the risk of incident dementia.
Adults aged 65 years and older who used sleep medications 5-7 days a week demonstrated a 30% increased risk of dementia, compared with those who did not use sleep medications, findings from a prospective study of 6,373 individuals show.
Adults aged 65 and older report a higher burden of sleep problems than other age groups, but major medical associations discourage the use of sleep medications by older adults because of growing evidence of a link between sleep medication use and cognitive decline, wrote Rebecca Robbins, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on this association among adults in the United States are limited, they said.
In a study published in Sleep Medicine, the researchers surveyed 6,373 adults aged 65 years and older who were enrolled in the nationally representative National Health and Aging Trends Study (NHATS). The majority of the participants were non-Hispanic White (71%), 59% were women, and 21% ranged in age from 70 to 74 years.
Participants responded to questions about routine sleep medication use. Routine was defined as “most nights” or “every night.” The data were collected for an 8-year period from 2011 to 2018. The study began in 2011, with a core interview administered annually.
Approximately 15% of the study population reported routine use of sleep medications. Overall, routine use of sleep medication was significantly associated with risk of incident dementia (hazard ratio, 1.30; P < .01) after controlling for multiple variables including age, sex, education level, and chronic conditions.
Dementia screening was conducted by participants rating their memory and then performing a memory-related activity (immediate and delayed 10-word recall) and other exercises to assess executive function and orientation. A separate eight-item informant screener was performed for patient proxies. The researcher noted, “Sensitivity of the NHATS probable dementia screening measure has been determined in previous research to be 66%, and specificity is 87%, with respect to a clinical dementia diagnosis.”
The study findings were limited by several factors including the use of self-reports, the lack of data on type or dose of sleep medication, and lack of data on the indication for the prescription, the researchers noted.
“Also, sleep medication use leads to worse performance on cognitive testing, such as the questionnaires used to screen for dementia in this study, and therefore could have resulted in a false diagnosis of dementia,” they added.
However, the results were strengthened by the large, nationally representative study population and support the need for quality geriatric care, the researchers said.
“Our findings provide further support and evidence that sleep medications are all too commonly administered, yet associated with greater risk for incident dementia, and that the U.S. health care system is in need of creative solutions for addressing poor sleep among older individuals,” they concluded.
Implications and alternatives
The study is important as the number of aging Americans increases, said Carolyn M. D’Ambrosio, MD, FCCP, of Brigham and Women’s Hospital and Harvard Medical School, Boston, in an interview. “In the elderly, inability to fall asleep or stay asleep are common issues that are brought to a health care provider,” she said. Dr. D’Ambrosio said she was not surprised by the study findings “as elderly patients often have sleep issues and sometimes a well-meaning health care provider gives them sleep medication to help. We have known that some of these sleep medications such as benzodiazepines affect cognitive performance,” she said.
Dr. D’Ambrosio said she avoids prescribing sleep medications for older adults if possible. “A deep dive into sleep habits, environment, and other things that disrupt sleep often gets to the problem rather than just masking it with a sleep medication,” she noted. Alternatives to improve sleep in older adults include exercise, exposure to bright light during the day, and good healthy sleep habits, all of which contribute to improved sleep in the elderly, said Dr. D’Ambrosio. She also recommends screening older adults for other issues that affect sleep, such as chronic pain.
The current study highlighted the association between sleep medication use and dementia, but it does not show causation, Dr. D’Ambrosio said. “So much more needs to be done to determine whether the sleep medications are causing worsening cognitive function long term, or if the dementia is starting but not yet diagnosed and the sleep medication is given but not the cause of the dementia, she noted.
Research gaps and treatment strategies
Older adults experiencing sleep difficulties may try various medications including pharmacologics (e.g., benzodiazepines), over-the-counter agents, such as diphenhydramine or doxylamine preparations, and/or herbal and nutritional supplements such as valerian or melatonin, said Mary Jo S. Farmer, MD, FCCP, of the University of Massachusetts Medical School–Baystate, Springfield, in an interview. “However, sleep medications, particularly benzodiazepines, are strongly discouraged by major medical associations including the American Geriatrics Society in part because of the growing evidence that use of sleep medications is associated with cognitive impairment and decline,” she said.
The current study results contribute to previous work demonstrating that both pharmacologic and nonpharmacologic sleep medication, although commonly administered, is associated with subsequent adverse outcomes in older adults, Dr. Farmer said. This association sets the stage for creative and different solutions for addressing poor sleep among older adults, such as behavioral treatments including cognitive-behavioral therapy, she noted.
Dr. Farmer said, “Areas for future research include exploring the causal link between prescription and/or over-the-counter sleep medication use and incident dementia in a randomized controlled trial,” she added.
“Another interesting opportunity for future research is to explore the indications for sleep medications among older adults since it has been shown in the general population that sleep difficulties represent only 12% of the indication for sleep medication prescriptions,” Dr. Farmer noted. “Future research could examine the strength of the underlying motivation to use sleep medication even in light of suggested long-term effects, and the effectiveness of other measures to avoid or minimize sleep difficulties,” she said.
“My experience is that the majority of ambulatory patients recently seen in sleep clinic want to avoid long-term use of sleep medications and will ask what other measures can be tried to consistently achieve a good night’s sleep without medication use,” Dr. Farmer said. “If medications are used, patients would rather try melatonin than a benzodiazepine. Many patients who come to sleep clinic with sleep medications already prescribed and are subsequently found to have sleep apnea and/or restless legs find that they no longer need sleep medication when these other medical conditions are appropriately diagnosed and managed,” she explained. “Finally, many patients tell me they feel less energetic upon awakening, almost feel hung over, and express being less sharp cognitively when taking pharmacologic sleep medication, whether for short or long periods of time, and therefore they want to avoid continuing with sleep medication use,” she said.
Dr. Farmer’s strategy for developing alternatives to sleep medications in older adults includes taking a careful history, including a complete list of medical problems, review of medications, and a thorough sleep history including usual time of sleep onset, awake time, and the frequency of daytime naps. “Tips for improving the quality of nighttime sleep may include adequately treating pain and other medical conditions such as heartburn, sleep apnea, and restless legs, creating a soothing environment to promote sleep by eliminating noise and bright lights, avoiding stimulant medications and substances such as caffeine and nicotine before bedtime, avoiding excessive amounts of alcohol, avoiding diuretics before bedtime, encouraging physical activity during the day, spending time in the sunlight as much as possible to help regulate the sleep cycle, limiting daytime naps, and establishing a regular sleep schedule,” she said.
The study was supported by National Institutes of Health awards K01HL150339, U54MD000538, K07AG052685, R01AG056531, R01AG056031. Lead author Dr. Robbins had no financial conflicts to disclose. Dr. D’Ambrosio disclosed serving as a section editor for sleep medicine for Dynamed and owning a patent on a circadian programming device. Dr. Farmer had no disclosures.
SOURCE: Robbins R et al. Sleep Med. 2020 Nov 11. doi: 10.1016/j.sleep.2020.11.004.
Sleep medications for older patients who report sleep problems may not be the best treatment given growing evidence of the link between these medications and the risk of incident dementia.
Adults aged 65 years and older who used sleep medications 5-7 days a week demonstrated a 30% increased risk of dementia, compared with those who did not use sleep medications, findings from a prospective study of 6,373 individuals show.
Adults aged 65 and older report a higher burden of sleep problems than other age groups, but major medical associations discourage the use of sleep medications by older adults because of growing evidence of a link between sleep medication use and cognitive decline, wrote Rebecca Robbins, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on this association among adults in the United States are limited, they said.
In a study published in Sleep Medicine, the researchers surveyed 6,373 adults aged 65 years and older who were enrolled in the nationally representative National Health and Aging Trends Study (NHATS). The majority of the participants were non-Hispanic White (71%), 59% were women, and 21% ranged in age from 70 to 74 years.
Participants responded to questions about routine sleep medication use. Routine was defined as “most nights” or “every night.” The data were collected for an 8-year period from 2011 to 2018. The study began in 2011, with a core interview administered annually.
Approximately 15% of the study population reported routine use of sleep medications. Overall, routine use of sleep medication was significantly associated with risk of incident dementia (hazard ratio, 1.30; P < .01) after controlling for multiple variables including age, sex, education level, and chronic conditions.
Dementia screening was conducted by participants rating their memory and then performing a memory-related activity (immediate and delayed 10-word recall) and other exercises to assess executive function and orientation. A separate eight-item informant screener was performed for patient proxies. The researcher noted, “Sensitivity of the NHATS probable dementia screening measure has been determined in previous research to be 66%, and specificity is 87%, with respect to a clinical dementia diagnosis.”
The study findings were limited by several factors including the use of self-reports, the lack of data on type or dose of sleep medication, and lack of data on the indication for the prescription, the researchers noted.
“Also, sleep medication use leads to worse performance on cognitive testing, such as the questionnaires used to screen for dementia in this study, and therefore could have resulted in a false diagnosis of dementia,” they added.
However, the results were strengthened by the large, nationally representative study population and support the need for quality geriatric care, the researchers said.
“Our findings provide further support and evidence that sleep medications are all too commonly administered, yet associated with greater risk for incident dementia, and that the U.S. health care system is in need of creative solutions for addressing poor sleep among older individuals,” they concluded.
Implications and alternatives
The study is important as the number of aging Americans increases, said Carolyn M. D’Ambrosio, MD, FCCP, of Brigham and Women’s Hospital and Harvard Medical School, Boston, in an interview. “In the elderly, inability to fall asleep or stay asleep are common issues that are brought to a health care provider,” she said. Dr. D’Ambrosio said she was not surprised by the study findings “as elderly patients often have sleep issues and sometimes a well-meaning health care provider gives them sleep medication to help. We have known that some of these sleep medications such as benzodiazepines affect cognitive performance,” she said.
Dr. D’Ambrosio said she avoids prescribing sleep medications for older adults if possible. “A deep dive into sleep habits, environment, and other things that disrupt sleep often gets to the problem rather than just masking it with a sleep medication,” she noted. Alternatives to improve sleep in older adults include exercise, exposure to bright light during the day, and good healthy sleep habits, all of which contribute to improved sleep in the elderly, said Dr. D’Ambrosio. She also recommends screening older adults for other issues that affect sleep, such as chronic pain.
The current study highlighted the association between sleep medication use and dementia, but it does not show causation, Dr. D’Ambrosio said. “So much more needs to be done to determine whether the sleep medications are causing worsening cognitive function long term, or if the dementia is starting but not yet diagnosed and the sleep medication is given but not the cause of the dementia, she noted.
Research gaps and treatment strategies
Older adults experiencing sleep difficulties may try various medications including pharmacologics (e.g., benzodiazepines), over-the-counter agents, such as diphenhydramine or doxylamine preparations, and/or herbal and nutritional supplements such as valerian or melatonin, said Mary Jo S. Farmer, MD, FCCP, of the University of Massachusetts Medical School–Baystate, Springfield, in an interview. “However, sleep medications, particularly benzodiazepines, are strongly discouraged by major medical associations including the American Geriatrics Society in part because of the growing evidence that use of sleep medications is associated with cognitive impairment and decline,” she said.
The current study results contribute to previous work demonstrating that both pharmacologic and nonpharmacologic sleep medication, although commonly administered, is associated with subsequent adverse outcomes in older adults, Dr. Farmer said. This association sets the stage for creative and different solutions for addressing poor sleep among older adults, such as behavioral treatments including cognitive-behavioral therapy, she noted.
Dr. Farmer said, “Areas for future research include exploring the causal link between prescription and/or over-the-counter sleep medication use and incident dementia in a randomized controlled trial,” she added.
“Another interesting opportunity for future research is to explore the indications for sleep medications among older adults since it has been shown in the general population that sleep difficulties represent only 12% of the indication for sleep medication prescriptions,” Dr. Farmer noted. “Future research could examine the strength of the underlying motivation to use sleep medication even in light of suggested long-term effects, and the effectiveness of other measures to avoid or minimize sleep difficulties,” she said.
“My experience is that the majority of ambulatory patients recently seen in sleep clinic want to avoid long-term use of sleep medications and will ask what other measures can be tried to consistently achieve a good night’s sleep without medication use,” Dr. Farmer said. “If medications are used, patients would rather try melatonin than a benzodiazepine. Many patients who come to sleep clinic with sleep medications already prescribed and are subsequently found to have sleep apnea and/or restless legs find that they no longer need sleep medication when these other medical conditions are appropriately diagnosed and managed,” she explained. “Finally, many patients tell me they feel less energetic upon awakening, almost feel hung over, and express being less sharp cognitively when taking pharmacologic sleep medication, whether for short or long periods of time, and therefore they want to avoid continuing with sleep medication use,” she said.
Dr. Farmer’s strategy for developing alternatives to sleep medications in older adults includes taking a careful history, including a complete list of medical problems, review of medications, and a thorough sleep history including usual time of sleep onset, awake time, and the frequency of daytime naps. “Tips for improving the quality of nighttime sleep may include adequately treating pain and other medical conditions such as heartburn, sleep apnea, and restless legs, creating a soothing environment to promote sleep by eliminating noise and bright lights, avoiding stimulant medications and substances such as caffeine and nicotine before bedtime, avoiding excessive amounts of alcohol, avoiding diuretics before bedtime, encouraging physical activity during the day, spending time in the sunlight as much as possible to help regulate the sleep cycle, limiting daytime naps, and establishing a regular sleep schedule,” she said.
The study was supported by National Institutes of Health awards K01HL150339, U54MD000538, K07AG052685, R01AG056531, R01AG056031. Lead author Dr. Robbins had no financial conflicts to disclose. Dr. D’Ambrosio disclosed serving as a section editor for sleep medicine for Dynamed and owning a patent on a circadian programming device. Dr. Farmer had no disclosures.
SOURCE: Robbins R et al. Sleep Med. 2020 Nov 11. doi: 10.1016/j.sleep.2020.11.004.
Sleep medications for older patients who report sleep problems may not be the best treatment given growing evidence of the link between these medications and the risk of incident dementia.
Adults aged 65 years and older who used sleep medications 5-7 days a week demonstrated a 30% increased risk of dementia, compared with those who did not use sleep medications, findings from a prospective study of 6,373 individuals show.
Adults aged 65 and older report a higher burden of sleep problems than other age groups, but major medical associations discourage the use of sleep medications by older adults because of growing evidence of a link between sleep medication use and cognitive decline, wrote Rebecca Robbins, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on this association among adults in the United States are limited, they said.
In a study published in Sleep Medicine, the researchers surveyed 6,373 adults aged 65 years and older who were enrolled in the nationally representative National Health and Aging Trends Study (NHATS). The majority of the participants were non-Hispanic White (71%), 59% were women, and 21% ranged in age from 70 to 74 years.
Participants responded to questions about routine sleep medication use. Routine was defined as “most nights” or “every night.” The data were collected for an 8-year period from 2011 to 2018. The study began in 2011, with a core interview administered annually.
Approximately 15% of the study population reported routine use of sleep medications. Overall, routine use of sleep medication was significantly associated with risk of incident dementia (hazard ratio, 1.30; P < .01) after controlling for multiple variables including age, sex, education level, and chronic conditions.
Dementia screening was conducted by participants rating their memory and then performing a memory-related activity (immediate and delayed 10-word recall) and other exercises to assess executive function and orientation. A separate eight-item informant screener was performed for patient proxies. The researcher noted, “Sensitivity of the NHATS probable dementia screening measure has been determined in previous research to be 66%, and specificity is 87%, with respect to a clinical dementia diagnosis.”
The study findings were limited by several factors including the use of self-reports, the lack of data on type or dose of sleep medication, and lack of data on the indication for the prescription, the researchers noted.
“Also, sleep medication use leads to worse performance on cognitive testing, such as the questionnaires used to screen for dementia in this study, and therefore could have resulted in a false diagnosis of dementia,” they added.
However, the results were strengthened by the large, nationally representative study population and support the need for quality geriatric care, the researchers said.
“Our findings provide further support and evidence that sleep medications are all too commonly administered, yet associated with greater risk for incident dementia, and that the U.S. health care system is in need of creative solutions for addressing poor sleep among older individuals,” they concluded.
Implications and alternatives
The study is important as the number of aging Americans increases, said Carolyn M. D’Ambrosio, MD, FCCP, of Brigham and Women’s Hospital and Harvard Medical School, Boston, in an interview. “In the elderly, inability to fall asleep or stay asleep are common issues that are brought to a health care provider,” she said. Dr. D’Ambrosio said she was not surprised by the study findings “as elderly patients often have sleep issues and sometimes a well-meaning health care provider gives them sleep medication to help. We have known that some of these sleep medications such as benzodiazepines affect cognitive performance,” she said.
Dr. D’Ambrosio said she avoids prescribing sleep medications for older adults if possible. “A deep dive into sleep habits, environment, and other things that disrupt sleep often gets to the problem rather than just masking it with a sleep medication,” she noted. Alternatives to improve sleep in older adults include exercise, exposure to bright light during the day, and good healthy sleep habits, all of which contribute to improved sleep in the elderly, said Dr. D’Ambrosio. She also recommends screening older adults for other issues that affect sleep, such as chronic pain.
The current study highlighted the association between sleep medication use and dementia, but it does not show causation, Dr. D’Ambrosio said. “So much more needs to be done to determine whether the sleep medications are causing worsening cognitive function long term, or if the dementia is starting but not yet diagnosed and the sleep medication is given but not the cause of the dementia, she noted.
Research gaps and treatment strategies
Older adults experiencing sleep difficulties may try various medications including pharmacologics (e.g., benzodiazepines), over-the-counter agents, such as diphenhydramine or doxylamine preparations, and/or herbal and nutritional supplements such as valerian or melatonin, said Mary Jo S. Farmer, MD, FCCP, of the University of Massachusetts Medical School–Baystate, Springfield, in an interview. “However, sleep medications, particularly benzodiazepines, are strongly discouraged by major medical associations including the American Geriatrics Society in part because of the growing evidence that use of sleep medications is associated with cognitive impairment and decline,” she said.
The current study results contribute to previous work demonstrating that both pharmacologic and nonpharmacologic sleep medication, although commonly administered, is associated with subsequent adverse outcomes in older adults, Dr. Farmer said. This association sets the stage for creative and different solutions for addressing poor sleep among older adults, such as behavioral treatments including cognitive-behavioral therapy, she noted.
Dr. Farmer said, “Areas for future research include exploring the causal link between prescription and/or over-the-counter sleep medication use and incident dementia in a randomized controlled trial,” she added.
“Another interesting opportunity for future research is to explore the indications for sleep medications among older adults since it has been shown in the general population that sleep difficulties represent only 12% of the indication for sleep medication prescriptions,” Dr. Farmer noted. “Future research could examine the strength of the underlying motivation to use sleep medication even in light of suggested long-term effects, and the effectiveness of other measures to avoid or minimize sleep difficulties,” she said.
“My experience is that the majority of ambulatory patients recently seen in sleep clinic want to avoid long-term use of sleep medications and will ask what other measures can be tried to consistently achieve a good night’s sleep without medication use,” Dr. Farmer said. “If medications are used, patients would rather try melatonin than a benzodiazepine. Many patients who come to sleep clinic with sleep medications already prescribed and are subsequently found to have sleep apnea and/or restless legs find that they no longer need sleep medication when these other medical conditions are appropriately diagnosed and managed,” she explained. “Finally, many patients tell me they feel less energetic upon awakening, almost feel hung over, and express being less sharp cognitively when taking pharmacologic sleep medication, whether for short or long periods of time, and therefore they want to avoid continuing with sleep medication use,” she said.
Dr. Farmer’s strategy for developing alternatives to sleep medications in older adults includes taking a careful history, including a complete list of medical problems, review of medications, and a thorough sleep history including usual time of sleep onset, awake time, and the frequency of daytime naps. “Tips for improving the quality of nighttime sleep may include adequately treating pain and other medical conditions such as heartburn, sleep apnea, and restless legs, creating a soothing environment to promote sleep by eliminating noise and bright lights, avoiding stimulant medications and substances such as caffeine and nicotine before bedtime, avoiding excessive amounts of alcohol, avoiding diuretics before bedtime, encouraging physical activity during the day, spending time in the sunlight as much as possible to help regulate the sleep cycle, limiting daytime naps, and establishing a regular sleep schedule,” she said.
The study was supported by National Institutes of Health awards K01HL150339, U54MD000538, K07AG052685, R01AG056531, R01AG056031. Lead author Dr. Robbins had no financial conflicts to disclose. Dr. D’Ambrosio disclosed serving as a section editor for sleep medicine for Dynamed and owning a patent on a circadian programming device. Dr. Farmer had no disclosures.
SOURCE: Robbins R et al. Sleep Med. 2020 Nov 11. doi: 10.1016/j.sleep.2020.11.004.
FROM SLEEP MEDICINE