User login
Healthy Aging Project-Brain: A Psychoeducational and Motivational Group for Older Veterans
With a rapidly growing older adult population, increased attention has been given to cognitive changes that occur with age, with a focus on optimizing the cognitive health of aging individuals.1 Given the absence of pharmaceutical treatments to prevent cognitive decline, there is an increased need for health care systems to offer alternative or behavioral interventions that can mitigate the effects of cognitive decline in aging.
Notably, many individuals are able to maintain or even improve cognitive functioning throughout their lifespan, with some research implicating health behaviors as an important factor for promoting brain health with age. Specifically, sleep, exercise, eating habits, social engagement, and cognitive stimulation have been linked to improved cognitive functioning.2-8 In addition to the potential benefits for brain health, there is evidence that greater investment in attaining health goals is associated with subjective reports of higher well-being, fewer mental health symptoms, lower physical health stresses, decreased caregiver burden, and increased functional independence linked with longer independent living.9 The latter has a substantial financial impact, such that the positive consequence of increased independence is likely staving off the need for admission to assisted living and adult family homes, which can be costly.
Despite the role of health behaviors in brain aging and overall health and functioning, research indicates that only a small number of older adults (12.8%) follow recommended guidelines for healthy lifestyle factors.10 Education has been identified as one factor associated with the likelihood of engaging in positive health behaviors, prompting the delivery of health-education interventions. Most psychoeducational interventions have traditionally focused on one aspect of behavior change at a time (eg, sleep); however, Gross and colleaguesconducted a meta-analysis of cognitive interventions and in addition to the overall positive benefits (effect size 0.38), they also found suggestive evidence that interventions that combined multiple training strategies were associated with larger training gains (P = .04) after adjusting for multiple comparisons.11 For example, Miller and colleagues found a significant improvement on both subjective and objective measures of memory following a multicomponent approach that combined training in memory skills, stress reduction, nutrition, and physical activity.12
In addition to the potential positive impacts of health behaviors on brain health, findings suggest that targeted emphasis on health behavior change may have the potential to stave off mild cognitiveimpairment (MCI) or dementia even if for a short time. Given the increasing prevalence rates of MCI with age (6.7% in adults aged 60-64 years, reaching 25.2% in adults aged 80-84 years13) and dementia (prevalence of MCI converting to dementia is 18-40%14), as well as the corresponding emotional, financial, and family-oriented consequences (eg, impact on the well-being of family caregivers), the need for behavioral interventions that seek to optimize brain health is becoming increasingly apparent.
More than 9 million veterans are now aged ≥ 65 years.15 In addition to representing nearly half of all veterans and a sizable portion of aging adults in the US, older veterans are at increased risk of frailty, mortality, and high rates of chronic medical/mental health conditions that can lead to accelerated cognitive aging.6-17 Together, these conditions highlight the importance of developing comprehensive psychoeducational and behavioral interventions in this population. To address this need, we developed a novel psychoeducation and behavior change group called the Healthy Aging Project-Brain (HAP-B, pronounced “happy”). The HAP-B intervention was designed to promote healthy brain aging by using empirically supported health behavior change strategies, including education, personalized goal setting, and community support. The primary aim of this project was to develop and implement an intervention that was feasible and acceptable (eg, could be implemented in our setting, was appropriate for a veteran population) and to determine any positive outcomes/preliminary effects on overall health and well-being.
Methods
We recruited veterans aged ≥ 50 years through primary care clinics and self-referrals via flyers in the US Department of Veterans Affairs (VA) Puget Sound Health Care System (VAPSHCS), Seattle Division hospital. We targeted the “worried well” and welcomed veterans with MCI and mental health diagnoses. Notably, if there were significant mental health and/or substance use concerns, we encouraged veterans to seek focused care and stabilization prior to or concurrent with group participation. Exclusion criteria included presence of suicidality/homicidality, untreated or unstable substance use disorder, or a diagnosis of dementia. Exclusion criteria were assessed by the referring health care providers (HCPs), when appropriate, and through a health record review. Group facilitators used their clinical judgment to monitor participants if they began experiencing more severe cognitive impairment or acute mental health concerns. Although we did not encounter any of these instances, facilitators were prepared to discuss any concerns with the veteran and their referring HCP. Participants sampled were from 1 of 5 groups offered between January 2018 and March 2019. A waiver from the institutional review board was obtained after meeting criteria for quality improvement/quality assurance (QI/QA) for this study.
Procedures
At the initial stages of development, our team conducted a needs assessment to identify health-related areas where HCPs felt veterans would benefit from additional education and support. The needs assessment was conducted across primary care, geriatric extended care, and the Geriatric Research, Education, and Clinical Center (GRECC) at VAPSHCS. Combining the needs assessment results with the available research base, we identified sleep, physical activity, social engagement, and cognitive stimulation as areas for focus. Notably, although nutrition has been identified as an important factor in cognitive aging, a diet and nutrition class was already available to older veterans at the Seattle VA; hence, we chose to limit overlap by not covering this topic in our group.
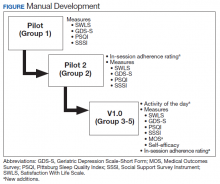
The group was offered on a quarterly basis as six 90-minute psychoeducational classes to allow time for didactics, discussion, and practice without overloading participants with information. Each group consisted of 4 to 9 veterans led by 2 cofacilitators. Group structure allowed for feedback and ideas from group members as well as accountability for engaging in behavior change. Cognitive functioning was not formally evaluated. Attendees were asked but not required to complete questionnaires before the classes began and again at completion. In addition at the completion of each group, feedback was collected from veterans and used to modify group content (Figure).
Two pilot groups were implemented in early and mid-2018 with iterative changes after each group. Then we revised the assessment battery and implemented the current version (v1.0), which was first offered in the fall of 2018 and was used with the final 3 groups. Noteworthy changes included weekly check-ins to assess use of health behavior logs and progress toward individual goals, additional pre-and postgroup measures, and in vivo skills practice relevant to the topic being discussed that day.
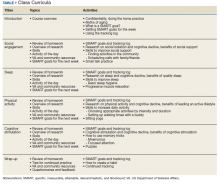
Each session began with a check-in, which included a review of daily logs and SMART (specific, measurable, attainable, relevant/realistic, and timebound) goals from the previous week.18 This allowed for praise/reinforcement of health behaviors as well as discussion of potential barriers. Second, an overview of research focusing on the relationship between aging, brain health, and the topic of the day was presented. As an example, in the discussion of social engagement, research was presented about the link between social isolation and cognitive decline; the indirect benefits of social support (eg, social support is linked to improved physical and mental health, which, in turn, is associated with less cognitive decline); and the direct benefits of social support (eg, high levels of emotional support are associated with better cognitive function) (Table 1).6
Next, facilitators reviewed skills and strategies to improve functioning in the topic of discussion. During the social engagement group, for example, facilitators discussed tips to improve social skills (eg, asking open-ended questions) and how to build social support into a daily routine (eg, scheduling weekly phone calls with family and friends). Following this discussion of skills, an activity was practiced, reinforcing learned material. During the social engagement group, veterans were invited to use small talk strategies with fellow group members. Finally, group sessions ended with each participant identifying a SMART goal for the coming week and troubleshooting potential barriers to success. SMART goals were kept broad, so veterans could choose a goal related to the topic discussed at the group that day (eg, scheduling a phone call with a friend twice in the coming week during the social engagement-focused group) or choose any other goal to focus on (eg, a sleep-related goal). Similarly, goals could change week to week, or could remain the same throughout the 6-week classes.
Measures
The questionnaires used for QI/QA analyses included the Satisfaction with Life Scale (SWLS); Geriatric Depression Scale-Short Form (GDS-S); Social Support Survey Instrument (SSSI); Pittsburg Sleep Quality Index (PSQI); Medical Outcomes Survey-Short Form (MOS-36 SF); and a self-efficacy scale (adapted from Huckans and colleagues for traumatic brain injury).19-24 Written feedback was collected at the end of the last group to assess perception of progress, self-perceived behavior change, what was helpful or unhelpful, and how likely the participants were to recommend the group to other veterans (0 to 3, very unlikely to very likely).
To promote consistency with other health and behavior change interventions at the VA, HAP-B used resources from the Whole Health model SMART goals. Research supports the use of self-monitoring techniques like SMART goals for behavior change.25
To facilitate skills practice and self-monitoring between classes, veterans were asked to complete 2 homework assignments. First, at the end of each group, each veteran identified a specific SMART goal to focus on and track in the coming week. Goals were unique to each veteran and allowed to change from week to week. Group discussion around SMART goals involved plans for how to address potential barriers; progress toward goals was discussed at the beginning of the following group. Second, veterans were asked to complete a worksheet used to track progress toward the weekly SMART goal and the specific health behaviors related to the 4 domains targeted by HAP-B. For example, when tracking sleep behaviors, veterans noted bedtime, waketime, number of times they woke up during the night, and length of daytime naps if applicable. Tracking logs were provided at the end of each class for personal purposes only. We asked veterans to rate themselves each week on whether they used the tracking sheet to monitor health behaviors; and how successful they were at accomplishing their previously identified SMART goal. We recorded responses on a 0 to 2 scale (0, not good; 1, fair; 2, good). This rating system was developed and implemented in later groups to promote self-monitoring, accountability, and discussion of potential barriers. However, due to the small sample that completed these ratings and the absence of objective corroborating data, these ratings were not included in the current analyses.
Every participant received a manual in binder format, which provided the didactic information for each group session, skills and strategies discussed in each session, and relevant resources in both the VA and community. For example, social engagement resources included information about volunteer opportunities, VA groups that focus on developing interpersonal skills, and recommendations from past group members on social events (eg, dance lessons at a senior center). We also developed a facilitator version of the manual in which we added comments and guidance on topics for discussion. Materials were developed with the goal of optimizing the ease of dissemination to other sites.
Results
Across the 5 groups, 31 veterans enrolled as participants and completed the initial intake measures, with an average of 6 participants per group (range 4-9). The majority (80%) attended at least 5 of the 6 classes. The mean
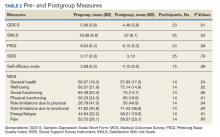
At the start of the class, the mean (SD) reports of participants were mild depressive symptoms 5.96 (3.8) on the GDS scale, moderate levels of self-efficacy 3.69 (0.5) on the self-efficacy scale, and moderate levels of satisfaction with life 18.08 (6.8) on the SWLS scale (Table 2). Data from 25 of 31 veterans who completed both pregroup and postgroup surveys were analyzed and paired samples t tests without corrections indicated a reduction in depressive symptoms (P = .01), improved self-efficacy (P = .08), and improved satisfaction with life (P = .03). There were no significant differences in self-reported sleep quality or perceived social support from pregroup to postgroup evaluations. Because the sample size was smaller for the MOS-36, which was not used until group 3, and the subscales are composed of few items each, we conducted exploratory analyses of the 8 MOS-36 subscales and found that well-being, physical functioning, role limitations due to physical and emotional functioning, and energy/fatigue significantly improved over time (Ps < .04).
Twenty-eight veterans provided written feedback following the final session. Qualitative feedback received at the completion of the group focused on participants’ desire for increased number of classes, longer sessions (eg, 2 participants recommended lengthening the group to 2 hours), and integrating mindfulness-based activities into each class. Participants rated themselves somewhat likely to very likely to recommend this group to other veterans (mean, 2.9 [SD, 0.4]).
Discussion
The ability and need to promote brain health with age is an emerging priority as our aging population grows. A growing body of evidence supports the role of health behaviors in healthy brain aging. Education and skills training in a group setting provides a supportive, cost-effective approach for increasing overall health in aging adults. Yet older adults are statistically less likely to engage in these behaviors on a regular basis. The current investigation provides preliminary support for a model of care that uses a comprehensive, experiential psychoeducational approach to facilitate behavior change in older adults. Our aim was to develop and implement an intervention that was feasible and acceptable to our older veterans and to determine any positive outcomes/preliminary effects on overall health and well-being.
Participants indicated that they enjoyed the group, learned new skills (per participant feedback and facilitator observation), and experienced improvements in mood, self-efficacy, and life satisfaction. Given the participants’ positive response to the group and its content, as well as continued referrals by HCPs to this group and low difficulty with ongoing recruitment, this program was deemed both feasible and acceptable in our veteran health care setting. Questions remain about the extent to which participants modified their health behaviors given that we did not collect objective measurements of behaviors (eg, time spent exercising), the duration of behavior change (ie, how long during and after the group were behaviors maintained), and the role of premorbid or concurrent characteristics that may moderate the effect of the intervention on health-related outcomes (eg, sleep quality, perceived social support, overall functioning, concurrent interventions, medications).
Strengths and Limitations
This study had a limited sample size and no control group. However, evidence of significant improvements in depressive symptoms, self-efficacy, and life satisfaction in the development groups without a control group is encouraging. This is particularly noteworthy given that older veterans as a group have higher rates of frailty and mortality than do other similarly aged counterparts.17An additional weakness is the absence of a brief cognitive assessment or other formal assessment as part of the inclusion/exclusion criteria. However, this program development project provides data from a realistic condition (recruited broadly and with few exclusions, offered in similar format as other VA classes), thus adding strength to the interpretation and possibly the generalizability of these findings.
Conclusions
Future directions include disseminating HAP-B materials and procedures across a variety of sites, both VA and non-VA. In line with this goal, we hope to increase sample size and sample diversity while optimizing protocol integrity during the exportation phase. With a greater sample size and power, we aim to examine the role of self-efficacy and other premorbid factors (eg, cognitive functioning at baseline) as mediators for observed changes in pre-/postmeasures and outcomes. We also hope to incorporate objective measures of behavior change, such as fitness trackers, heart rate/pulse monitors, and actigraphy for monitoring sleep. Finally, we are interested in conducting follow-up with past and future participants to detect changes that may occur with learning new skills following the completion of the group (eg, changes in sleep behavior that take time to take effect) and the extent to which participants continue to use the health behavior skills and strategies to maintain or enhance progress in behavioral goals. Finally, although this intervention was initially designed for use with older veterans receiving health care through the VA, we believe the concepts and work products described here can be used with older adults across a wide range of health care settings. Providers interested in trialing HAP-B at their local site are encouraged to contact the authors.
1. Jacobsen LA, Kent M, Lee M, Mather M. America’s aging population. Popul Bull. 2011;66(1):1-20.
2. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):85-592. doi:10.1093/sleep/33.5.585
3. Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;16:12-31. doi:10.1016/j.arr.2014.05.002
4. Middleton LE, Manini TM, Simonsick EM, et al. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med. 2011;171(14):1251-1257. doi:10.1001/archinternmed.2011.277
5. World Health Organization. Interventions on diet and physical activity: what works. https://www.who.int/dietphysicalactivity/whatworks/en/. Published 2009. Accessed June 19, 2020.
6. Seeman TE, Lusignolo TM, Albert M, Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychol. 2001;20(4):243-255. doi:10.1037//0278-6133.20.4.243
7. La Rue A. Healthy brain aging: role of cognitive reserve, cognitive stimulation and cognitive exercises. Clin Geriatr Med. 2010;26(1):99-111. doi:10.1016/j.cger.2009.11.003
8. Salthouse TA, Berish DE, Miles JD. The role of cognitive stimulation on the relations between age and cognitive functioning. Psychol Aging. 2002;17(4):548-557. doi:10.1037//0882-7974.17.4.548
9. Wrosch C, Schulz R, Heckhausen J. Health stresses and depressive symptomatology in the elderly: the importance of health engagement control strategies. Health Psychol. 2002;21(4):340-348. doi:10.1037//0278-6133.21.4.340
10. Pronk NP, Anderson LH, Crain AL, et al. Meeting recommendations for multiple healthy lifestyle factors: prevalence, clustering, and predictors among adolescent, adult, and senior health plan members. Am J Prev Med. 2004;27(suppl 2):25-33. doi:10.1016/j.amepre.2004.04.022
11. Gross AL, Parisi JM, Spira AP, et al. Memory training interventions for older adults: a meta-analysis. Aging Ment Health. 2012;16(6):722-734. doi:10.1080/13607863.2012.667783
12. Miller KJ, Siddarth P, Gaines JM, et al. The memory fitness program: cognitive effects of a healthy aging intervention. Am J Geriat Psychiatry. 2012;20(6):514-523. doi:10.1097/JGP.0b013e318227f821
13. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126-135. doi:10.1212/WNL.0000000000004826
14. Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367(9518):1262-1270. doi:10.1016/S0140-6736(06)68542-5
15. US Department of Veteran Affairs, National Center for Veteran Analysis and Statistics.Veteran population. 2020. https://www.va.gov/vetdata/Veteran_Population.asp. Updated May 21, 2020 . Accessed June 17, 2020.
16. Eibner C, Krull H, Brown K, et al. Current and projected characteristics and unique healthcare needs of the patient population served by the Department of Veterans Affairs. RAND Health Q. 2016;5(4):13.
17. Orkaby AR, Nussbaum L, Ho Y, et al. The burden of frailty among U.S. Veterans and its association with mortality, 2002-2012. J Gerontol A Biol Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
18. Doran GT. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manag Rev. 1981;70(11):35-36.
19. Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess. 1985;49(1):71-75. doi:10.1207/s15327752jpa4901-13
20. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5(1-2):165-173. doi:10.1300/J018v05n01_09
21. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705-714. doi:10.1016/0277-9536(91)90150-b
22. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. doi:10.1016/0165-1781(89)90047-4
23. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
24. Huckans M, Pavawalla S, Demadura T, et al. A pilot study examining effects of group-based cognitive strategy training treatment on self-reported cognitive problems, psychiatric symptoms, functioning, and compensatory strategy use in OIF/OEF combat veterans with persistent mild cognitive disorder and history of traumatic brain injury. J Rehabil Res Dev. 2010;47(1):43-60. doi:10.1682/jrrd.2009.02.0019
25. Pearson ES. Goal setting as a health behavior change strategy in overweight and obese adults: a systematic literature review examining intervention components. Patient Educ Couns. 2012;87(1):32-42. doi:10.1016/j.pec.2011.07.018
With a rapidly growing older adult population, increased attention has been given to cognitive changes that occur with age, with a focus on optimizing the cognitive health of aging individuals.1 Given the absence of pharmaceutical treatments to prevent cognitive decline, there is an increased need for health care systems to offer alternative or behavioral interventions that can mitigate the effects of cognitive decline in aging.
Notably, many individuals are able to maintain or even improve cognitive functioning throughout their lifespan, with some research implicating health behaviors as an important factor for promoting brain health with age. Specifically, sleep, exercise, eating habits, social engagement, and cognitive stimulation have been linked to improved cognitive functioning.2-8 In addition to the potential benefits for brain health, there is evidence that greater investment in attaining health goals is associated with subjective reports of higher well-being, fewer mental health symptoms, lower physical health stresses, decreased caregiver burden, and increased functional independence linked with longer independent living.9 The latter has a substantial financial impact, such that the positive consequence of increased independence is likely staving off the need for admission to assisted living and adult family homes, which can be costly.
Despite the role of health behaviors in brain aging and overall health and functioning, research indicates that only a small number of older adults (12.8%) follow recommended guidelines for healthy lifestyle factors.10 Education has been identified as one factor associated with the likelihood of engaging in positive health behaviors, prompting the delivery of health-education interventions. Most psychoeducational interventions have traditionally focused on one aspect of behavior change at a time (eg, sleep); however, Gross and colleaguesconducted a meta-analysis of cognitive interventions and in addition to the overall positive benefits (effect size 0.38), they also found suggestive evidence that interventions that combined multiple training strategies were associated with larger training gains (P = .04) after adjusting for multiple comparisons.11 For example, Miller and colleagues found a significant improvement on both subjective and objective measures of memory following a multicomponent approach that combined training in memory skills, stress reduction, nutrition, and physical activity.12
In addition to the potential positive impacts of health behaviors on brain health, findings suggest that targeted emphasis on health behavior change may have the potential to stave off mild cognitiveimpairment (MCI) or dementia even if for a short time. Given the increasing prevalence rates of MCI with age (6.7% in adults aged 60-64 years, reaching 25.2% in adults aged 80-84 years13) and dementia (prevalence of MCI converting to dementia is 18-40%14), as well as the corresponding emotional, financial, and family-oriented consequences (eg, impact on the well-being of family caregivers), the need for behavioral interventions that seek to optimize brain health is becoming increasingly apparent.
More than 9 million veterans are now aged ≥ 65 years.15 In addition to representing nearly half of all veterans and a sizable portion of aging adults in the US, older veterans are at increased risk of frailty, mortality, and high rates of chronic medical/mental health conditions that can lead to accelerated cognitive aging.6-17 Together, these conditions highlight the importance of developing comprehensive psychoeducational and behavioral interventions in this population. To address this need, we developed a novel psychoeducation and behavior change group called the Healthy Aging Project-Brain (HAP-B, pronounced “happy”). The HAP-B intervention was designed to promote healthy brain aging by using empirically supported health behavior change strategies, including education, personalized goal setting, and community support. The primary aim of this project was to develop and implement an intervention that was feasible and acceptable (eg, could be implemented in our setting, was appropriate for a veteran population) and to determine any positive outcomes/preliminary effects on overall health and well-being.
Methods
We recruited veterans aged ≥ 50 years through primary care clinics and self-referrals via flyers in the US Department of Veterans Affairs (VA) Puget Sound Health Care System (VAPSHCS), Seattle Division hospital. We targeted the “worried well” and welcomed veterans with MCI and mental health diagnoses. Notably, if there were significant mental health and/or substance use concerns, we encouraged veterans to seek focused care and stabilization prior to or concurrent with group participation. Exclusion criteria included presence of suicidality/homicidality, untreated or unstable substance use disorder, or a diagnosis of dementia. Exclusion criteria were assessed by the referring health care providers (HCPs), when appropriate, and through a health record review. Group facilitators used their clinical judgment to monitor participants if they began experiencing more severe cognitive impairment or acute mental health concerns. Although we did not encounter any of these instances, facilitators were prepared to discuss any concerns with the veteran and their referring HCP. Participants sampled were from 1 of 5 groups offered between January 2018 and March 2019. A waiver from the institutional review board was obtained after meeting criteria for quality improvement/quality assurance (QI/QA) for this study.
Procedures
At the initial stages of development, our team conducted a needs assessment to identify health-related areas where HCPs felt veterans would benefit from additional education and support. The needs assessment was conducted across primary care, geriatric extended care, and the Geriatric Research, Education, and Clinical Center (GRECC) at VAPSHCS. Combining the needs assessment results with the available research base, we identified sleep, physical activity, social engagement, and cognitive stimulation as areas for focus. Notably, although nutrition has been identified as an important factor in cognitive aging, a diet and nutrition class was already available to older veterans at the Seattle VA; hence, we chose to limit overlap by not covering this topic in our group.
The group was offered on a quarterly basis as six 90-minute psychoeducational classes to allow time for didactics, discussion, and practice without overloading participants with information. Each group consisted of 4 to 9 veterans led by 2 cofacilitators. Group structure allowed for feedback and ideas from group members as well as accountability for engaging in behavior change. Cognitive functioning was not formally evaluated. Attendees were asked but not required to complete questionnaires before the classes began and again at completion. In addition at the completion of each group, feedback was collected from veterans and used to modify group content (Figure).
Two pilot groups were implemented in early and mid-2018 with iterative changes after each group. Then we revised the assessment battery and implemented the current version (v1.0), which was first offered in the fall of 2018 and was used with the final 3 groups. Noteworthy changes included weekly check-ins to assess use of health behavior logs and progress toward individual goals, additional pre-and postgroup measures, and in vivo skills practice relevant to the topic being discussed that day.
Each session began with a check-in, which included a review of daily logs and SMART (specific, measurable, attainable, relevant/realistic, and timebound) goals from the previous week.18 This allowed for praise/reinforcement of health behaviors as well as discussion of potential barriers. Second, an overview of research focusing on the relationship between aging, brain health, and the topic of the day was presented. As an example, in the discussion of social engagement, research was presented about the link between social isolation and cognitive decline; the indirect benefits of social support (eg, social support is linked to improved physical and mental health, which, in turn, is associated with less cognitive decline); and the direct benefits of social support (eg, high levels of emotional support are associated with better cognitive function) (Table 1).6
Next, facilitators reviewed skills and strategies to improve functioning in the topic of discussion. During the social engagement group, for example, facilitators discussed tips to improve social skills (eg, asking open-ended questions) and how to build social support into a daily routine (eg, scheduling weekly phone calls with family and friends). Following this discussion of skills, an activity was practiced, reinforcing learned material. During the social engagement group, veterans were invited to use small talk strategies with fellow group members. Finally, group sessions ended with each participant identifying a SMART goal for the coming week and troubleshooting potential barriers to success. SMART goals were kept broad, so veterans could choose a goal related to the topic discussed at the group that day (eg, scheduling a phone call with a friend twice in the coming week during the social engagement-focused group) or choose any other goal to focus on (eg, a sleep-related goal). Similarly, goals could change week to week, or could remain the same throughout the 6-week classes.
Measures
The questionnaires used for QI/QA analyses included the Satisfaction with Life Scale (SWLS); Geriatric Depression Scale-Short Form (GDS-S); Social Support Survey Instrument (SSSI); Pittsburg Sleep Quality Index (PSQI); Medical Outcomes Survey-Short Form (MOS-36 SF); and a self-efficacy scale (adapted from Huckans and colleagues for traumatic brain injury).19-24 Written feedback was collected at the end of the last group to assess perception of progress, self-perceived behavior change, what was helpful or unhelpful, and how likely the participants were to recommend the group to other veterans (0 to 3, very unlikely to very likely).
To promote consistency with other health and behavior change interventions at the VA, HAP-B used resources from the Whole Health model SMART goals. Research supports the use of self-monitoring techniques like SMART goals for behavior change.25
To facilitate skills practice and self-monitoring between classes, veterans were asked to complete 2 homework assignments. First, at the end of each group, each veteran identified a specific SMART goal to focus on and track in the coming week. Goals were unique to each veteran and allowed to change from week to week. Group discussion around SMART goals involved plans for how to address potential barriers; progress toward goals was discussed at the beginning of the following group. Second, veterans were asked to complete a worksheet used to track progress toward the weekly SMART goal and the specific health behaviors related to the 4 domains targeted by HAP-B. For example, when tracking sleep behaviors, veterans noted bedtime, waketime, number of times they woke up during the night, and length of daytime naps if applicable. Tracking logs were provided at the end of each class for personal purposes only. We asked veterans to rate themselves each week on whether they used the tracking sheet to monitor health behaviors; and how successful they were at accomplishing their previously identified SMART goal. We recorded responses on a 0 to 2 scale (0, not good; 1, fair; 2, good). This rating system was developed and implemented in later groups to promote self-monitoring, accountability, and discussion of potential barriers. However, due to the small sample that completed these ratings and the absence of objective corroborating data, these ratings were not included in the current analyses.
Every participant received a manual in binder format, which provided the didactic information for each group session, skills and strategies discussed in each session, and relevant resources in both the VA and community. For example, social engagement resources included information about volunteer opportunities, VA groups that focus on developing interpersonal skills, and recommendations from past group members on social events (eg, dance lessons at a senior center). We also developed a facilitator version of the manual in which we added comments and guidance on topics for discussion. Materials were developed with the goal of optimizing the ease of dissemination to other sites.
Results
Across the 5 groups, 31 veterans enrolled as participants and completed the initial intake measures, with an average of 6 participants per group (range 4-9). The majority (80%) attended at least 5 of the 6 classes. The mean
At the start of the class, the mean (SD) reports of participants were mild depressive symptoms 5.96 (3.8) on the GDS scale, moderate levels of self-efficacy 3.69 (0.5) on the self-efficacy scale, and moderate levels of satisfaction with life 18.08 (6.8) on the SWLS scale (Table 2). Data from 25 of 31 veterans who completed both pregroup and postgroup surveys were analyzed and paired samples t tests without corrections indicated a reduction in depressive symptoms (P = .01), improved self-efficacy (P = .08), and improved satisfaction with life (P = .03). There were no significant differences in self-reported sleep quality or perceived social support from pregroup to postgroup evaluations. Because the sample size was smaller for the MOS-36, which was not used until group 3, and the subscales are composed of few items each, we conducted exploratory analyses of the 8 MOS-36 subscales and found that well-being, physical functioning, role limitations due to physical and emotional functioning, and energy/fatigue significantly improved over time (Ps < .04).
Twenty-eight veterans provided written feedback following the final session. Qualitative feedback received at the completion of the group focused on participants’ desire for increased number of classes, longer sessions (eg, 2 participants recommended lengthening the group to 2 hours), and integrating mindfulness-based activities into each class. Participants rated themselves somewhat likely to very likely to recommend this group to other veterans (mean, 2.9 [SD, 0.4]).
Discussion
The ability and need to promote brain health with age is an emerging priority as our aging population grows. A growing body of evidence supports the role of health behaviors in healthy brain aging. Education and skills training in a group setting provides a supportive, cost-effective approach for increasing overall health in aging adults. Yet older adults are statistically less likely to engage in these behaviors on a regular basis. The current investigation provides preliminary support for a model of care that uses a comprehensive, experiential psychoeducational approach to facilitate behavior change in older adults. Our aim was to develop and implement an intervention that was feasible and acceptable to our older veterans and to determine any positive outcomes/preliminary effects on overall health and well-being.
Participants indicated that they enjoyed the group, learned new skills (per participant feedback and facilitator observation), and experienced improvements in mood, self-efficacy, and life satisfaction. Given the participants’ positive response to the group and its content, as well as continued referrals by HCPs to this group and low difficulty with ongoing recruitment, this program was deemed both feasible and acceptable in our veteran health care setting. Questions remain about the extent to which participants modified their health behaviors given that we did not collect objective measurements of behaviors (eg, time spent exercising), the duration of behavior change (ie, how long during and after the group were behaviors maintained), and the role of premorbid or concurrent characteristics that may moderate the effect of the intervention on health-related outcomes (eg, sleep quality, perceived social support, overall functioning, concurrent interventions, medications).
Strengths and Limitations
This study had a limited sample size and no control group. However, evidence of significant improvements in depressive symptoms, self-efficacy, and life satisfaction in the development groups without a control group is encouraging. This is particularly noteworthy given that older veterans as a group have higher rates of frailty and mortality than do other similarly aged counterparts.17An additional weakness is the absence of a brief cognitive assessment or other formal assessment as part of the inclusion/exclusion criteria. However, this program development project provides data from a realistic condition (recruited broadly and with few exclusions, offered in similar format as other VA classes), thus adding strength to the interpretation and possibly the generalizability of these findings.
Conclusions
Future directions include disseminating HAP-B materials and procedures across a variety of sites, both VA and non-VA. In line with this goal, we hope to increase sample size and sample diversity while optimizing protocol integrity during the exportation phase. With a greater sample size and power, we aim to examine the role of self-efficacy and other premorbid factors (eg, cognitive functioning at baseline) as mediators for observed changes in pre-/postmeasures and outcomes. We also hope to incorporate objective measures of behavior change, such as fitness trackers, heart rate/pulse monitors, and actigraphy for monitoring sleep. Finally, we are interested in conducting follow-up with past and future participants to detect changes that may occur with learning new skills following the completion of the group (eg, changes in sleep behavior that take time to take effect) and the extent to which participants continue to use the health behavior skills and strategies to maintain or enhance progress in behavioral goals. Finally, although this intervention was initially designed for use with older veterans receiving health care through the VA, we believe the concepts and work products described here can be used with older adults across a wide range of health care settings. Providers interested in trialing HAP-B at their local site are encouraged to contact the authors.
With a rapidly growing older adult population, increased attention has been given to cognitive changes that occur with age, with a focus on optimizing the cognitive health of aging individuals.1 Given the absence of pharmaceutical treatments to prevent cognitive decline, there is an increased need for health care systems to offer alternative or behavioral interventions that can mitigate the effects of cognitive decline in aging.
Notably, many individuals are able to maintain or even improve cognitive functioning throughout their lifespan, with some research implicating health behaviors as an important factor for promoting brain health with age. Specifically, sleep, exercise, eating habits, social engagement, and cognitive stimulation have been linked to improved cognitive functioning.2-8 In addition to the potential benefits for brain health, there is evidence that greater investment in attaining health goals is associated with subjective reports of higher well-being, fewer mental health symptoms, lower physical health stresses, decreased caregiver burden, and increased functional independence linked with longer independent living.9 The latter has a substantial financial impact, such that the positive consequence of increased independence is likely staving off the need for admission to assisted living and adult family homes, which can be costly.
Despite the role of health behaviors in brain aging and overall health and functioning, research indicates that only a small number of older adults (12.8%) follow recommended guidelines for healthy lifestyle factors.10 Education has been identified as one factor associated with the likelihood of engaging in positive health behaviors, prompting the delivery of health-education interventions. Most psychoeducational interventions have traditionally focused on one aspect of behavior change at a time (eg, sleep); however, Gross and colleaguesconducted a meta-analysis of cognitive interventions and in addition to the overall positive benefits (effect size 0.38), they also found suggestive evidence that interventions that combined multiple training strategies were associated with larger training gains (P = .04) after adjusting for multiple comparisons.11 For example, Miller and colleagues found a significant improvement on both subjective and objective measures of memory following a multicomponent approach that combined training in memory skills, stress reduction, nutrition, and physical activity.12
In addition to the potential positive impacts of health behaviors on brain health, findings suggest that targeted emphasis on health behavior change may have the potential to stave off mild cognitiveimpairment (MCI) or dementia even if for a short time. Given the increasing prevalence rates of MCI with age (6.7% in adults aged 60-64 years, reaching 25.2% in adults aged 80-84 years13) and dementia (prevalence of MCI converting to dementia is 18-40%14), as well as the corresponding emotional, financial, and family-oriented consequences (eg, impact on the well-being of family caregivers), the need for behavioral interventions that seek to optimize brain health is becoming increasingly apparent.
More than 9 million veterans are now aged ≥ 65 years.15 In addition to representing nearly half of all veterans and a sizable portion of aging adults in the US, older veterans are at increased risk of frailty, mortality, and high rates of chronic medical/mental health conditions that can lead to accelerated cognitive aging.6-17 Together, these conditions highlight the importance of developing comprehensive psychoeducational and behavioral interventions in this population. To address this need, we developed a novel psychoeducation and behavior change group called the Healthy Aging Project-Brain (HAP-B, pronounced “happy”). The HAP-B intervention was designed to promote healthy brain aging by using empirically supported health behavior change strategies, including education, personalized goal setting, and community support. The primary aim of this project was to develop and implement an intervention that was feasible and acceptable (eg, could be implemented in our setting, was appropriate for a veteran population) and to determine any positive outcomes/preliminary effects on overall health and well-being.
Methods
We recruited veterans aged ≥ 50 years through primary care clinics and self-referrals via flyers in the US Department of Veterans Affairs (VA) Puget Sound Health Care System (VAPSHCS), Seattle Division hospital. We targeted the “worried well” and welcomed veterans with MCI and mental health diagnoses. Notably, if there were significant mental health and/or substance use concerns, we encouraged veterans to seek focused care and stabilization prior to or concurrent with group participation. Exclusion criteria included presence of suicidality/homicidality, untreated or unstable substance use disorder, or a diagnosis of dementia. Exclusion criteria were assessed by the referring health care providers (HCPs), when appropriate, and through a health record review. Group facilitators used their clinical judgment to monitor participants if they began experiencing more severe cognitive impairment or acute mental health concerns. Although we did not encounter any of these instances, facilitators were prepared to discuss any concerns with the veteran and their referring HCP. Participants sampled were from 1 of 5 groups offered between January 2018 and March 2019. A waiver from the institutional review board was obtained after meeting criteria for quality improvement/quality assurance (QI/QA) for this study.
Procedures
At the initial stages of development, our team conducted a needs assessment to identify health-related areas where HCPs felt veterans would benefit from additional education and support. The needs assessment was conducted across primary care, geriatric extended care, and the Geriatric Research, Education, and Clinical Center (GRECC) at VAPSHCS. Combining the needs assessment results with the available research base, we identified sleep, physical activity, social engagement, and cognitive stimulation as areas for focus. Notably, although nutrition has been identified as an important factor in cognitive aging, a diet and nutrition class was already available to older veterans at the Seattle VA; hence, we chose to limit overlap by not covering this topic in our group.
The group was offered on a quarterly basis as six 90-minute psychoeducational classes to allow time for didactics, discussion, and practice without overloading participants with information. Each group consisted of 4 to 9 veterans led by 2 cofacilitators. Group structure allowed for feedback and ideas from group members as well as accountability for engaging in behavior change. Cognitive functioning was not formally evaluated. Attendees were asked but not required to complete questionnaires before the classes began and again at completion. In addition at the completion of each group, feedback was collected from veterans and used to modify group content (Figure).
Two pilot groups were implemented in early and mid-2018 with iterative changes after each group. Then we revised the assessment battery and implemented the current version (v1.0), which was first offered in the fall of 2018 and was used with the final 3 groups. Noteworthy changes included weekly check-ins to assess use of health behavior logs and progress toward individual goals, additional pre-and postgroup measures, and in vivo skills practice relevant to the topic being discussed that day.
Each session began with a check-in, which included a review of daily logs and SMART (specific, measurable, attainable, relevant/realistic, and timebound) goals from the previous week.18 This allowed for praise/reinforcement of health behaviors as well as discussion of potential barriers. Second, an overview of research focusing on the relationship between aging, brain health, and the topic of the day was presented. As an example, in the discussion of social engagement, research was presented about the link between social isolation and cognitive decline; the indirect benefits of social support (eg, social support is linked to improved physical and mental health, which, in turn, is associated with less cognitive decline); and the direct benefits of social support (eg, high levels of emotional support are associated with better cognitive function) (Table 1).6
Next, facilitators reviewed skills and strategies to improve functioning in the topic of discussion. During the social engagement group, for example, facilitators discussed tips to improve social skills (eg, asking open-ended questions) and how to build social support into a daily routine (eg, scheduling weekly phone calls with family and friends). Following this discussion of skills, an activity was practiced, reinforcing learned material. During the social engagement group, veterans were invited to use small talk strategies with fellow group members. Finally, group sessions ended with each participant identifying a SMART goal for the coming week and troubleshooting potential barriers to success. SMART goals were kept broad, so veterans could choose a goal related to the topic discussed at the group that day (eg, scheduling a phone call with a friend twice in the coming week during the social engagement-focused group) or choose any other goal to focus on (eg, a sleep-related goal). Similarly, goals could change week to week, or could remain the same throughout the 6-week classes.
Measures
The questionnaires used for QI/QA analyses included the Satisfaction with Life Scale (SWLS); Geriatric Depression Scale-Short Form (GDS-S); Social Support Survey Instrument (SSSI); Pittsburg Sleep Quality Index (PSQI); Medical Outcomes Survey-Short Form (MOS-36 SF); and a self-efficacy scale (adapted from Huckans and colleagues for traumatic brain injury).19-24 Written feedback was collected at the end of the last group to assess perception of progress, self-perceived behavior change, what was helpful or unhelpful, and how likely the participants were to recommend the group to other veterans (0 to 3, very unlikely to very likely).
To promote consistency with other health and behavior change interventions at the VA, HAP-B used resources from the Whole Health model SMART goals. Research supports the use of self-monitoring techniques like SMART goals for behavior change.25
To facilitate skills practice and self-monitoring between classes, veterans were asked to complete 2 homework assignments. First, at the end of each group, each veteran identified a specific SMART goal to focus on and track in the coming week. Goals were unique to each veteran and allowed to change from week to week. Group discussion around SMART goals involved plans for how to address potential barriers; progress toward goals was discussed at the beginning of the following group. Second, veterans were asked to complete a worksheet used to track progress toward the weekly SMART goal and the specific health behaviors related to the 4 domains targeted by HAP-B. For example, when tracking sleep behaviors, veterans noted bedtime, waketime, number of times they woke up during the night, and length of daytime naps if applicable. Tracking logs were provided at the end of each class for personal purposes only. We asked veterans to rate themselves each week on whether they used the tracking sheet to monitor health behaviors; and how successful they were at accomplishing their previously identified SMART goal. We recorded responses on a 0 to 2 scale (0, not good; 1, fair; 2, good). This rating system was developed and implemented in later groups to promote self-monitoring, accountability, and discussion of potential barriers. However, due to the small sample that completed these ratings and the absence of objective corroborating data, these ratings were not included in the current analyses.
Every participant received a manual in binder format, which provided the didactic information for each group session, skills and strategies discussed in each session, and relevant resources in both the VA and community. For example, social engagement resources included information about volunteer opportunities, VA groups that focus on developing interpersonal skills, and recommendations from past group members on social events (eg, dance lessons at a senior center). We also developed a facilitator version of the manual in which we added comments and guidance on topics for discussion. Materials were developed with the goal of optimizing the ease of dissemination to other sites.
Results
Across the 5 groups, 31 veterans enrolled as participants and completed the initial intake measures, with an average of 6 participants per group (range 4-9). The majority (80%) attended at least 5 of the 6 classes. The mean
At the start of the class, the mean (SD) reports of participants were mild depressive symptoms 5.96 (3.8) on the GDS scale, moderate levels of self-efficacy 3.69 (0.5) on the self-efficacy scale, and moderate levels of satisfaction with life 18.08 (6.8) on the SWLS scale (Table 2). Data from 25 of 31 veterans who completed both pregroup and postgroup surveys were analyzed and paired samples t tests without corrections indicated a reduction in depressive symptoms (P = .01), improved self-efficacy (P = .08), and improved satisfaction with life (P = .03). There were no significant differences in self-reported sleep quality or perceived social support from pregroup to postgroup evaluations. Because the sample size was smaller for the MOS-36, which was not used until group 3, and the subscales are composed of few items each, we conducted exploratory analyses of the 8 MOS-36 subscales and found that well-being, physical functioning, role limitations due to physical and emotional functioning, and energy/fatigue significantly improved over time (Ps < .04).
Twenty-eight veterans provided written feedback following the final session. Qualitative feedback received at the completion of the group focused on participants’ desire for increased number of classes, longer sessions (eg, 2 participants recommended lengthening the group to 2 hours), and integrating mindfulness-based activities into each class. Participants rated themselves somewhat likely to very likely to recommend this group to other veterans (mean, 2.9 [SD, 0.4]).
Discussion
The ability and need to promote brain health with age is an emerging priority as our aging population grows. A growing body of evidence supports the role of health behaviors in healthy brain aging. Education and skills training in a group setting provides a supportive, cost-effective approach for increasing overall health in aging adults. Yet older adults are statistically less likely to engage in these behaviors on a regular basis. The current investigation provides preliminary support for a model of care that uses a comprehensive, experiential psychoeducational approach to facilitate behavior change in older adults. Our aim was to develop and implement an intervention that was feasible and acceptable to our older veterans and to determine any positive outcomes/preliminary effects on overall health and well-being.
Participants indicated that they enjoyed the group, learned new skills (per participant feedback and facilitator observation), and experienced improvements in mood, self-efficacy, and life satisfaction. Given the participants’ positive response to the group and its content, as well as continued referrals by HCPs to this group and low difficulty with ongoing recruitment, this program was deemed both feasible and acceptable in our veteran health care setting. Questions remain about the extent to which participants modified their health behaviors given that we did not collect objective measurements of behaviors (eg, time spent exercising), the duration of behavior change (ie, how long during and after the group were behaviors maintained), and the role of premorbid or concurrent characteristics that may moderate the effect of the intervention on health-related outcomes (eg, sleep quality, perceived social support, overall functioning, concurrent interventions, medications).
Strengths and Limitations
This study had a limited sample size and no control group. However, evidence of significant improvements in depressive symptoms, self-efficacy, and life satisfaction in the development groups without a control group is encouraging. This is particularly noteworthy given that older veterans as a group have higher rates of frailty and mortality than do other similarly aged counterparts.17An additional weakness is the absence of a brief cognitive assessment or other formal assessment as part of the inclusion/exclusion criteria. However, this program development project provides data from a realistic condition (recruited broadly and with few exclusions, offered in similar format as other VA classes), thus adding strength to the interpretation and possibly the generalizability of these findings.
Conclusions
Future directions include disseminating HAP-B materials and procedures across a variety of sites, both VA and non-VA. In line with this goal, we hope to increase sample size and sample diversity while optimizing protocol integrity during the exportation phase. With a greater sample size and power, we aim to examine the role of self-efficacy and other premorbid factors (eg, cognitive functioning at baseline) as mediators for observed changes in pre-/postmeasures and outcomes. We also hope to incorporate objective measures of behavior change, such as fitness trackers, heart rate/pulse monitors, and actigraphy for monitoring sleep. Finally, we are interested in conducting follow-up with past and future participants to detect changes that may occur with learning new skills following the completion of the group (eg, changes in sleep behavior that take time to take effect) and the extent to which participants continue to use the health behavior skills and strategies to maintain or enhance progress in behavioral goals. Finally, although this intervention was initially designed for use with older veterans receiving health care through the VA, we believe the concepts and work products described here can be used with older adults across a wide range of health care settings. Providers interested in trialing HAP-B at their local site are encouraged to contact the authors.
1. Jacobsen LA, Kent M, Lee M, Mather M. America’s aging population. Popul Bull. 2011;66(1):1-20.
2. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):85-592. doi:10.1093/sleep/33.5.585
3. Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;16:12-31. doi:10.1016/j.arr.2014.05.002
4. Middleton LE, Manini TM, Simonsick EM, et al. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med. 2011;171(14):1251-1257. doi:10.1001/archinternmed.2011.277
5. World Health Organization. Interventions on diet and physical activity: what works. https://www.who.int/dietphysicalactivity/whatworks/en/. Published 2009. Accessed June 19, 2020.
6. Seeman TE, Lusignolo TM, Albert M, Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychol. 2001;20(4):243-255. doi:10.1037//0278-6133.20.4.243
7. La Rue A. Healthy brain aging: role of cognitive reserve, cognitive stimulation and cognitive exercises. Clin Geriatr Med. 2010;26(1):99-111. doi:10.1016/j.cger.2009.11.003
8. Salthouse TA, Berish DE, Miles JD. The role of cognitive stimulation on the relations between age and cognitive functioning. Psychol Aging. 2002;17(4):548-557. doi:10.1037//0882-7974.17.4.548
9. Wrosch C, Schulz R, Heckhausen J. Health stresses and depressive symptomatology in the elderly: the importance of health engagement control strategies. Health Psychol. 2002;21(4):340-348. doi:10.1037//0278-6133.21.4.340
10. Pronk NP, Anderson LH, Crain AL, et al. Meeting recommendations for multiple healthy lifestyle factors: prevalence, clustering, and predictors among adolescent, adult, and senior health plan members. Am J Prev Med. 2004;27(suppl 2):25-33. doi:10.1016/j.amepre.2004.04.022
11. Gross AL, Parisi JM, Spira AP, et al. Memory training interventions for older adults: a meta-analysis. Aging Ment Health. 2012;16(6):722-734. doi:10.1080/13607863.2012.667783
12. Miller KJ, Siddarth P, Gaines JM, et al. The memory fitness program: cognitive effects of a healthy aging intervention. Am J Geriat Psychiatry. 2012;20(6):514-523. doi:10.1097/JGP.0b013e318227f821
13. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126-135. doi:10.1212/WNL.0000000000004826
14. Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367(9518):1262-1270. doi:10.1016/S0140-6736(06)68542-5
15. US Department of Veteran Affairs, National Center for Veteran Analysis and Statistics.Veteran population. 2020. https://www.va.gov/vetdata/Veteran_Population.asp. Updated May 21, 2020 . Accessed June 17, 2020.
16. Eibner C, Krull H, Brown K, et al. Current and projected characteristics and unique healthcare needs of the patient population served by the Department of Veterans Affairs. RAND Health Q. 2016;5(4):13.
17. Orkaby AR, Nussbaum L, Ho Y, et al. The burden of frailty among U.S. Veterans and its association with mortality, 2002-2012. J Gerontol A Biol Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
18. Doran GT. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manag Rev. 1981;70(11):35-36.
19. Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess. 1985;49(1):71-75. doi:10.1207/s15327752jpa4901-13
20. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5(1-2):165-173. doi:10.1300/J018v05n01_09
21. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705-714. doi:10.1016/0277-9536(91)90150-b
22. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. doi:10.1016/0165-1781(89)90047-4
23. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
24. Huckans M, Pavawalla S, Demadura T, et al. A pilot study examining effects of group-based cognitive strategy training treatment on self-reported cognitive problems, psychiatric symptoms, functioning, and compensatory strategy use in OIF/OEF combat veterans with persistent mild cognitive disorder and history of traumatic brain injury. J Rehabil Res Dev. 2010;47(1):43-60. doi:10.1682/jrrd.2009.02.0019
25. Pearson ES. Goal setting as a health behavior change strategy in overweight and obese adults: a systematic literature review examining intervention components. Patient Educ Couns. 2012;87(1):32-42. doi:10.1016/j.pec.2011.07.018
1. Jacobsen LA, Kent M, Lee M, Mather M. America’s aging population. Popul Bull. 2011;66(1):1-20.
2. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):85-592. doi:10.1093/sleep/33.5.585
3. Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;16:12-31. doi:10.1016/j.arr.2014.05.002
4. Middleton LE, Manini TM, Simonsick EM, et al. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med. 2011;171(14):1251-1257. doi:10.1001/archinternmed.2011.277
5. World Health Organization. Interventions on diet and physical activity: what works. https://www.who.int/dietphysicalactivity/whatworks/en/. Published 2009. Accessed June 19, 2020.
6. Seeman TE, Lusignolo TM, Albert M, Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychol. 2001;20(4):243-255. doi:10.1037//0278-6133.20.4.243
7. La Rue A. Healthy brain aging: role of cognitive reserve, cognitive stimulation and cognitive exercises. Clin Geriatr Med. 2010;26(1):99-111. doi:10.1016/j.cger.2009.11.003
8. Salthouse TA, Berish DE, Miles JD. The role of cognitive stimulation on the relations between age and cognitive functioning. Psychol Aging. 2002;17(4):548-557. doi:10.1037//0882-7974.17.4.548
9. Wrosch C, Schulz R, Heckhausen J. Health stresses and depressive symptomatology in the elderly: the importance of health engagement control strategies. Health Psychol. 2002;21(4):340-348. doi:10.1037//0278-6133.21.4.340
10. Pronk NP, Anderson LH, Crain AL, et al. Meeting recommendations for multiple healthy lifestyle factors: prevalence, clustering, and predictors among adolescent, adult, and senior health plan members. Am J Prev Med. 2004;27(suppl 2):25-33. doi:10.1016/j.amepre.2004.04.022
11. Gross AL, Parisi JM, Spira AP, et al. Memory training interventions for older adults: a meta-analysis. Aging Ment Health. 2012;16(6):722-734. doi:10.1080/13607863.2012.667783
12. Miller KJ, Siddarth P, Gaines JM, et al. The memory fitness program: cognitive effects of a healthy aging intervention. Am J Geriat Psychiatry. 2012;20(6):514-523. doi:10.1097/JGP.0b013e318227f821
13. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126-135. doi:10.1212/WNL.0000000000004826
14. Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367(9518):1262-1270. doi:10.1016/S0140-6736(06)68542-5
15. US Department of Veteran Affairs, National Center for Veteran Analysis and Statistics.Veteran population. 2020. https://www.va.gov/vetdata/Veteran_Population.asp. Updated May 21, 2020 . Accessed June 17, 2020.
16. Eibner C, Krull H, Brown K, et al. Current and projected characteristics and unique healthcare needs of the patient population served by the Department of Veterans Affairs. RAND Health Q. 2016;5(4):13.
17. Orkaby AR, Nussbaum L, Ho Y, et al. The burden of frailty among U.S. Veterans and its association with mortality, 2002-2012. J Gerontol A Biol Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
18. Doran GT. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manag Rev. 1981;70(11):35-36.
19. Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess. 1985;49(1):71-75. doi:10.1207/s15327752jpa4901-13
20. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5(1-2):165-173. doi:10.1300/J018v05n01_09
21. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705-714. doi:10.1016/0277-9536(91)90150-b
22. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. doi:10.1016/0165-1781(89)90047-4
23. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
24. Huckans M, Pavawalla S, Demadura T, et al. A pilot study examining effects of group-based cognitive strategy training treatment on self-reported cognitive problems, psychiatric symptoms, functioning, and compensatory strategy use in OIF/OEF combat veterans with persistent mild cognitive disorder and history of traumatic brain injury. J Rehabil Res Dev. 2010;47(1):43-60. doi:10.1682/jrrd.2009.02.0019
25. Pearson ES. Goal setting as a health behavior change strategy in overweight and obese adults: a systematic literature review examining intervention components. Patient Educ Couns. 2012;87(1):32-42. doi:10.1016/j.pec.2011.07.018
Does moderate drinking slow cognitive decline?
new research suggests. However, at least one expert urges caution in interpreting the findings.
Investigators found that consuming 10-14 alcoholic drinks per week had the strongest cognitive benefit. The findings “add more weight” to the growing body of research identifying beneficial cognitive effects of moderate alcohol consumption, said lead author, Ruiyuan Zhang, MD, of the department of epidemiology and biostatistics at the University of Georgia, Athens. However, Dr. Zhang emphasized that nondrinkers should not take up drinking to protect brain function, as alcohol can have negative effects.
The study was published online in JAMA Network Open.
Slower cognitive decline
The observational study was a secondary analysis of data from the Health and Retirement Study, a nationally representative U.S. survey of middle-aged and older adults. The survey, which began in 1992, is conducted every 2 years and collects health and economic data.
The current analysis used data from 1996 to 2008 and included information from individuals who participated in at least three surveys. The study included 19,887 participants, with a mean age 61.8 years. Most (60.1%) were women and white (85.2%). Mean follow-up was 9.1 years.
Researchers measured cognitive domains of mental status, word recall, and vocabulary. They also calculated a total cognition score, with higher scores indicating better cognitive abilities.
For each cognitive function measure, researchers categorized participants into a consistently low–trajectory group in which cognitive test scores from baseline through follow-up were consistently low or a consistently high–trajectory group, where cognitive test scores from baseline through follow-up were consistently high.
Based on self-reports, the investigators categorized participants as never drinkers (41.8%), former drinkers (39.5%), or current drinkers (18.7%). For current drinkers, researchers determined the number of drinking days per week and number of drinks per day. They further categorized these participants as low to moderate drinkers or heavy drinkers.
One drink was defined as a 12-ounce bottle of beer, a 5-ounce glass of wine, or a 1.5-ounce shot of spirits, said Dr. Zhang.
Women who consumed 8 or more drinks per week and men who drank 15 or more drinks per week were considered heavy drinkers. Other current drinkers were deemed low to moderate drinkers. Most current drinkers (85.2%) were low to moderate drinkers.
Other covariates included age, sex, race/ethnicity, years of education, marital status, tobacco smoking status, and body mass index.
Results showed moderate drinking was associated with relatively high cognitive test scores. After controlling for all covariates, compared with never drinkers, current low to moderate drinkers were significantly less likely to have consistently low trajectories for total cognitive score (odds ratio, 0.66; 95% confidence interval, 0.59-0.74), mental status (OR, 0.71; 95% CI, 0.63-0.81), word recall (OR, 0.74; 95% CI, 0.69-0.80), and vocabulary (OR, 0.64; 95% CI, 0.56-0.74) (all P < .001).
Former drinkers also had better cognitive outcomes for all cognitive domains. Heavy drinkers had lower odds of being in the consistently low trajectory group only for the vocabulary test.
Heavy drinking ‘risky’
Because few participants were deemed to be heavy drinkers, the power to identify an association between heavy drinking and cognitive function was limited. Dr. Zhang acknowledged, though he noted that heavy drinking is “risky.”
“We found that, after the drinking dosage passes the moderate level, the risk of low cognitive function increases very fast, which indicates that heavy drinking may harm cognitive function.” Limiting alcohol consumption “is still very important,” he said.
The associations of alcohol and cognitive functions differed by race/ethnicity. Low to moderate drinking was significantly associated with a lower odds of having a consistently low trajectory for all four cognitive function measures only among white participants.
A possible reason for this is that the study had so few African Americans (who made up only 14.8% of the sample), which limited the ability to identify relationships between alcohol intake and cognitive function, said Dr. Zhang. “Another reason is that the sensitivity to alcohol may be different between white and African American subjects.”
There was a significant U-shaped association between weekly amounts of alcohol and the odds of being in the consistently low–trajectory group for all cognitive functions. Depending on the function tested, the optimal number of weekly drinks ranged from 10-14.
Dr. Zhang noted that, when women were examined separately, alcohol consumption had a significant U-shaped relationship only with word recall, with the optimal dosage being around eight drinks.
U-shaped relationship an ‘important finding’
The U-shaped relationship is “an important finding,” said Dr. Zhang. “It shows that the human body may act differently to low and high doses of alcohol. Knowing why and how this happens is very important as it would help us understand how alcohol affects the function of the human body.”
Sensitivity analyses among participants with no chronic diseases showed the U-shaped association was still significant for scores of total word recall and vocabulary, but not for mental status or total cognition score.
The authors noted that 77.2% of participants had at least one chronic disease. They maintained that the association between alcohol consumption and cognitive function may be applicable both to healthy people and to those with a chronic disease.
The study also found that low to moderate drinkers had slower rates of cognitive decline over time for all cognition domains.
Although the mechanisms underlying the cognitive benefits of alcohol consumption are unclear, the authors believe it may be via cerebrovascular and cardiovascular pathways.
Alcohol may increase levels of brain-derived neurotrophic factor, a key regulator of neuronal plasticity and development in the dorsal striatum, they noted.
Balancing act
However, there’s also evidence that drinking, especially heavy drinking, increases the risk of hypertension, stroke, liver damage, and some cancers. “We think the role of alcohol drinking in cognitive function may be a balance of its beneficial and harmful effects on the cardiovascular system,” said Dr. Zhang.
“For the low to moderate drinker, the beneficial effects may outweigh the harmful effects on the small blood vessels in the brain. In this way, it could preserve cognition,” he added.
Dr. Zhang also noted that the study focused on middle-aged and older adults. “We can’t say whether or not moderate alcohol could benefit younger people” because they may have different characteristics, he said.
The findings of other studies examining the effects of alcohol on cognitive function are mixed. While studies have identified a beneficial effect, others have uncovered no, minimal, or adverse effects. This could be due to the use of different tests of cognitive function or different study populations, said Dr. Zhang.
A limitation of the current study was that assessment of alcohol consumption was based on self-report, which might have introduced recall bias. In addition, because individuals tend to underestimate their alcohol consumption, heavy drinkers could be misclassified as low to moderate drinkers, and low to moderate drinkers as former drinkers.
“This may make our study underestimate the association between low to moderate drinking and cognitive function,” said Dr. Zhang. In addition, alcohol consumption tended to change with time, and this change may be associated with other factors that led to changes in cognitive function, the authors noted.
Interpret with caution
Commenting on the study, Brent P. Forester, MD, chief of the Center of Excellence in Geriatric Psychiatry at McLean Hospital in Belmont, Mass., associate professor of psychiatry at Harvard Medical School, Boston, and a member of the American Psychiatric Association Council on Geriatric Psychiatry, said he views the study with some trepidation.
“As a clinician taking care of older adults, I would be very cautious about overinterpreting the beneficial effects of alcohol before we understand the mechanism better,” he said.
He noted that all of the risk factors associated with heart attack and stroke are also risk factors for Alzheimer’s disease and cognitive decline more broadly. “One of the issues here is how in the world does alcohol reduce cardiovascular and cerebrovascular risks, if you know it increases the risk of hypertension and stroke, regardless of dose.”
With regard to the possible impact of alcohol on brain-derived neurotrophic factor, Dr. Forester said, “it’s an interesting idea” but the actual mechanism is still unclear.
Even with dietary studies, such as those on the Mediterranean diet that include red wine, showing cognitive benefit, Dr. Forester said he’s still concerned about the adverse effects of alcohol on older people. These can include falls and sleep disturbances in addition to cognitive issues, and these effects can increase with age.
He was somewhat surprised at the level of alcohol that the study determined was beneficial. “Essentially, what they’re saying here is that, for men, it’s two drinks a day.” This could be “problematic” as two drinks per day can quickly escalate as individuals build tolerance.
He also pointed out that the study does not determine cause and effect, noting that it’s only an association.
Dr. Forester said the study raises a number of questions, including the type of alcohol study participants consumed and whether this has any impact on cognitive benefit. He also questioned whether the mediating effects of alcohol were associated with something that wasn’t measured, such as socioeconomic status.
Another question, he said, is what factors in individuals’ medical or psychiatric history determine whether they are more or less likely to benefit from low to moderate alcohol intake.
Perhaps alcohol should be recommended only for “select subpopulations” – for example, those who are healthy and have a family history of cognitive decline –but not for those with a history of substance abuse, including alcohol abuse, said Dr. Forester.
“For this population, the last thing you want to do is recommend alcohol to reduce risk of cognitive decline,” he cautioned.
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. The investigators and Dr. Forester have reported no relevant financial disclosures.
A version of this story originally appeared on Medscape.com.
new research suggests. However, at least one expert urges caution in interpreting the findings.
Investigators found that consuming 10-14 alcoholic drinks per week had the strongest cognitive benefit. The findings “add more weight” to the growing body of research identifying beneficial cognitive effects of moderate alcohol consumption, said lead author, Ruiyuan Zhang, MD, of the department of epidemiology and biostatistics at the University of Georgia, Athens. However, Dr. Zhang emphasized that nondrinkers should not take up drinking to protect brain function, as alcohol can have negative effects.
The study was published online in JAMA Network Open.
Slower cognitive decline
The observational study was a secondary analysis of data from the Health and Retirement Study, a nationally representative U.S. survey of middle-aged and older adults. The survey, which began in 1992, is conducted every 2 years and collects health and economic data.
The current analysis used data from 1996 to 2008 and included information from individuals who participated in at least three surveys. The study included 19,887 participants, with a mean age 61.8 years. Most (60.1%) were women and white (85.2%). Mean follow-up was 9.1 years.
Researchers measured cognitive domains of mental status, word recall, and vocabulary. They also calculated a total cognition score, with higher scores indicating better cognitive abilities.
For each cognitive function measure, researchers categorized participants into a consistently low–trajectory group in which cognitive test scores from baseline through follow-up were consistently low or a consistently high–trajectory group, where cognitive test scores from baseline through follow-up were consistently high.
Based on self-reports, the investigators categorized participants as never drinkers (41.8%), former drinkers (39.5%), or current drinkers (18.7%). For current drinkers, researchers determined the number of drinking days per week and number of drinks per day. They further categorized these participants as low to moderate drinkers or heavy drinkers.
One drink was defined as a 12-ounce bottle of beer, a 5-ounce glass of wine, or a 1.5-ounce shot of spirits, said Dr. Zhang.
Women who consumed 8 or more drinks per week and men who drank 15 or more drinks per week were considered heavy drinkers. Other current drinkers were deemed low to moderate drinkers. Most current drinkers (85.2%) were low to moderate drinkers.
Other covariates included age, sex, race/ethnicity, years of education, marital status, tobacco smoking status, and body mass index.
Results showed moderate drinking was associated with relatively high cognitive test scores. After controlling for all covariates, compared with never drinkers, current low to moderate drinkers were significantly less likely to have consistently low trajectories for total cognitive score (odds ratio, 0.66; 95% confidence interval, 0.59-0.74), mental status (OR, 0.71; 95% CI, 0.63-0.81), word recall (OR, 0.74; 95% CI, 0.69-0.80), and vocabulary (OR, 0.64; 95% CI, 0.56-0.74) (all P < .001).
Former drinkers also had better cognitive outcomes for all cognitive domains. Heavy drinkers had lower odds of being in the consistently low trajectory group only for the vocabulary test.
Heavy drinking ‘risky’
Because few participants were deemed to be heavy drinkers, the power to identify an association between heavy drinking and cognitive function was limited. Dr. Zhang acknowledged, though he noted that heavy drinking is “risky.”
“We found that, after the drinking dosage passes the moderate level, the risk of low cognitive function increases very fast, which indicates that heavy drinking may harm cognitive function.” Limiting alcohol consumption “is still very important,” he said.
The associations of alcohol and cognitive functions differed by race/ethnicity. Low to moderate drinking was significantly associated with a lower odds of having a consistently low trajectory for all four cognitive function measures only among white participants.
A possible reason for this is that the study had so few African Americans (who made up only 14.8% of the sample), which limited the ability to identify relationships between alcohol intake and cognitive function, said Dr. Zhang. “Another reason is that the sensitivity to alcohol may be different between white and African American subjects.”
There was a significant U-shaped association between weekly amounts of alcohol and the odds of being in the consistently low–trajectory group for all cognitive functions. Depending on the function tested, the optimal number of weekly drinks ranged from 10-14.
Dr. Zhang noted that, when women were examined separately, alcohol consumption had a significant U-shaped relationship only with word recall, with the optimal dosage being around eight drinks.
U-shaped relationship an ‘important finding’
The U-shaped relationship is “an important finding,” said Dr. Zhang. “It shows that the human body may act differently to low and high doses of alcohol. Knowing why and how this happens is very important as it would help us understand how alcohol affects the function of the human body.”
Sensitivity analyses among participants with no chronic diseases showed the U-shaped association was still significant for scores of total word recall and vocabulary, but not for mental status or total cognition score.
The authors noted that 77.2% of participants had at least one chronic disease. They maintained that the association between alcohol consumption and cognitive function may be applicable both to healthy people and to those with a chronic disease.
The study also found that low to moderate drinkers had slower rates of cognitive decline over time for all cognition domains.
Although the mechanisms underlying the cognitive benefits of alcohol consumption are unclear, the authors believe it may be via cerebrovascular and cardiovascular pathways.
Alcohol may increase levels of brain-derived neurotrophic factor, a key regulator of neuronal plasticity and development in the dorsal striatum, they noted.
Balancing act
However, there’s also evidence that drinking, especially heavy drinking, increases the risk of hypertension, stroke, liver damage, and some cancers. “We think the role of alcohol drinking in cognitive function may be a balance of its beneficial and harmful effects on the cardiovascular system,” said Dr. Zhang.
“For the low to moderate drinker, the beneficial effects may outweigh the harmful effects on the small blood vessels in the brain. In this way, it could preserve cognition,” he added.
Dr. Zhang also noted that the study focused on middle-aged and older adults. “We can’t say whether or not moderate alcohol could benefit younger people” because they may have different characteristics, he said.
The findings of other studies examining the effects of alcohol on cognitive function are mixed. While studies have identified a beneficial effect, others have uncovered no, minimal, or adverse effects. This could be due to the use of different tests of cognitive function or different study populations, said Dr. Zhang.
A limitation of the current study was that assessment of alcohol consumption was based on self-report, which might have introduced recall bias. In addition, because individuals tend to underestimate their alcohol consumption, heavy drinkers could be misclassified as low to moderate drinkers, and low to moderate drinkers as former drinkers.
“This may make our study underestimate the association between low to moderate drinking and cognitive function,” said Dr. Zhang. In addition, alcohol consumption tended to change with time, and this change may be associated with other factors that led to changes in cognitive function, the authors noted.
Interpret with caution
Commenting on the study, Brent P. Forester, MD, chief of the Center of Excellence in Geriatric Psychiatry at McLean Hospital in Belmont, Mass., associate professor of psychiatry at Harvard Medical School, Boston, and a member of the American Psychiatric Association Council on Geriatric Psychiatry, said he views the study with some trepidation.
“As a clinician taking care of older adults, I would be very cautious about overinterpreting the beneficial effects of alcohol before we understand the mechanism better,” he said.
He noted that all of the risk factors associated with heart attack and stroke are also risk factors for Alzheimer’s disease and cognitive decline more broadly. “One of the issues here is how in the world does alcohol reduce cardiovascular and cerebrovascular risks, if you know it increases the risk of hypertension and stroke, regardless of dose.”
With regard to the possible impact of alcohol on brain-derived neurotrophic factor, Dr. Forester said, “it’s an interesting idea” but the actual mechanism is still unclear.
Even with dietary studies, such as those on the Mediterranean diet that include red wine, showing cognitive benefit, Dr. Forester said he’s still concerned about the adverse effects of alcohol on older people. These can include falls and sleep disturbances in addition to cognitive issues, and these effects can increase with age.
He was somewhat surprised at the level of alcohol that the study determined was beneficial. “Essentially, what they’re saying here is that, for men, it’s two drinks a day.” This could be “problematic” as two drinks per day can quickly escalate as individuals build tolerance.
He also pointed out that the study does not determine cause and effect, noting that it’s only an association.
Dr. Forester said the study raises a number of questions, including the type of alcohol study participants consumed and whether this has any impact on cognitive benefit. He also questioned whether the mediating effects of alcohol were associated with something that wasn’t measured, such as socioeconomic status.
Another question, he said, is what factors in individuals’ medical or psychiatric history determine whether they are more or less likely to benefit from low to moderate alcohol intake.
Perhaps alcohol should be recommended only for “select subpopulations” – for example, those who are healthy and have a family history of cognitive decline –but not for those with a history of substance abuse, including alcohol abuse, said Dr. Forester.
“For this population, the last thing you want to do is recommend alcohol to reduce risk of cognitive decline,” he cautioned.
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. The investigators and Dr. Forester have reported no relevant financial disclosures.
A version of this story originally appeared on Medscape.com.
new research suggests. However, at least one expert urges caution in interpreting the findings.
Investigators found that consuming 10-14 alcoholic drinks per week had the strongest cognitive benefit. The findings “add more weight” to the growing body of research identifying beneficial cognitive effects of moderate alcohol consumption, said lead author, Ruiyuan Zhang, MD, of the department of epidemiology and biostatistics at the University of Georgia, Athens. However, Dr. Zhang emphasized that nondrinkers should not take up drinking to protect brain function, as alcohol can have negative effects.
The study was published online in JAMA Network Open.
Slower cognitive decline
The observational study was a secondary analysis of data from the Health and Retirement Study, a nationally representative U.S. survey of middle-aged and older adults. The survey, which began in 1992, is conducted every 2 years and collects health and economic data.
The current analysis used data from 1996 to 2008 and included information from individuals who participated in at least three surveys. The study included 19,887 participants, with a mean age 61.8 years. Most (60.1%) were women and white (85.2%). Mean follow-up was 9.1 years.
Researchers measured cognitive domains of mental status, word recall, and vocabulary. They also calculated a total cognition score, with higher scores indicating better cognitive abilities.
For each cognitive function measure, researchers categorized participants into a consistently low–trajectory group in which cognitive test scores from baseline through follow-up were consistently low or a consistently high–trajectory group, where cognitive test scores from baseline through follow-up were consistently high.
Based on self-reports, the investigators categorized participants as never drinkers (41.8%), former drinkers (39.5%), or current drinkers (18.7%). For current drinkers, researchers determined the number of drinking days per week and number of drinks per day. They further categorized these participants as low to moderate drinkers or heavy drinkers.
One drink was defined as a 12-ounce bottle of beer, a 5-ounce glass of wine, or a 1.5-ounce shot of spirits, said Dr. Zhang.
Women who consumed 8 or more drinks per week and men who drank 15 or more drinks per week were considered heavy drinkers. Other current drinkers were deemed low to moderate drinkers. Most current drinkers (85.2%) were low to moderate drinkers.
Other covariates included age, sex, race/ethnicity, years of education, marital status, tobacco smoking status, and body mass index.
Results showed moderate drinking was associated with relatively high cognitive test scores. After controlling for all covariates, compared with never drinkers, current low to moderate drinkers were significantly less likely to have consistently low trajectories for total cognitive score (odds ratio, 0.66; 95% confidence interval, 0.59-0.74), mental status (OR, 0.71; 95% CI, 0.63-0.81), word recall (OR, 0.74; 95% CI, 0.69-0.80), and vocabulary (OR, 0.64; 95% CI, 0.56-0.74) (all P < .001).
Former drinkers also had better cognitive outcomes for all cognitive domains. Heavy drinkers had lower odds of being in the consistently low trajectory group only for the vocabulary test.
Heavy drinking ‘risky’
Because few participants were deemed to be heavy drinkers, the power to identify an association between heavy drinking and cognitive function was limited. Dr. Zhang acknowledged, though he noted that heavy drinking is “risky.”
“We found that, after the drinking dosage passes the moderate level, the risk of low cognitive function increases very fast, which indicates that heavy drinking may harm cognitive function.” Limiting alcohol consumption “is still very important,” he said.
The associations of alcohol and cognitive functions differed by race/ethnicity. Low to moderate drinking was significantly associated with a lower odds of having a consistently low trajectory for all four cognitive function measures only among white participants.
A possible reason for this is that the study had so few African Americans (who made up only 14.8% of the sample), which limited the ability to identify relationships between alcohol intake and cognitive function, said Dr. Zhang. “Another reason is that the sensitivity to alcohol may be different between white and African American subjects.”
There was a significant U-shaped association between weekly amounts of alcohol and the odds of being in the consistently low–trajectory group for all cognitive functions. Depending on the function tested, the optimal number of weekly drinks ranged from 10-14.
Dr. Zhang noted that, when women were examined separately, alcohol consumption had a significant U-shaped relationship only with word recall, with the optimal dosage being around eight drinks.
U-shaped relationship an ‘important finding’
The U-shaped relationship is “an important finding,” said Dr. Zhang. “It shows that the human body may act differently to low and high doses of alcohol. Knowing why and how this happens is very important as it would help us understand how alcohol affects the function of the human body.”
Sensitivity analyses among participants with no chronic diseases showed the U-shaped association was still significant for scores of total word recall and vocabulary, but not for mental status or total cognition score.
The authors noted that 77.2% of participants had at least one chronic disease. They maintained that the association between alcohol consumption and cognitive function may be applicable both to healthy people and to those with a chronic disease.
The study also found that low to moderate drinkers had slower rates of cognitive decline over time for all cognition domains.
Although the mechanisms underlying the cognitive benefits of alcohol consumption are unclear, the authors believe it may be via cerebrovascular and cardiovascular pathways.
Alcohol may increase levels of brain-derived neurotrophic factor, a key regulator of neuronal plasticity and development in the dorsal striatum, they noted.
Balancing act
However, there’s also evidence that drinking, especially heavy drinking, increases the risk of hypertension, stroke, liver damage, and some cancers. “We think the role of alcohol drinking in cognitive function may be a balance of its beneficial and harmful effects on the cardiovascular system,” said Dr. Zhang.
“For the low to moderate drinker, the beneficial effects may outweigh the harmful effects on the small blood vessels in the brain. In this way, it could preserve cognition,” he added.
Dr. Zhang also noted that the study focused on middle-aged and older adults. “We can’t say whether or not moderate alcohol could benefit younger people” because they may have different characteristics, he said.
The findings of other studies examining the effects of alcohol on cognitive function are mixed. While studies have identified a beneficial effect, others have uncovered no, minimal, or adverse effects. This could be due to the use of different tests of cognitive function or different study populations, said Dr. Zhang.
A limitation of the current study was that assessment of alcohol consumption was based on self-report, which might have introduced recall bias. In addition, because individuals tend to underestimate their alcohol consumption, heavy drinkers could be misclassified as low to moderate drinkers, and low to moderate drinkers as former drinkers.
“This may make our study underestimate the association between low to moderate drinking and cognitive function,” said Dr. Zhang. In addition, alcohol consumption tended to change with time, and this change may be associated with other factors that led to changes in cognitive function, the authors noted.
Interpret with caution
Commenting on the study, Brent P. Forester, MD, chief of the Center of Excellence in Geriatric Psychiatry at McLean Hospital in Belmont, Mass., associate professor of psychiatry at Harvard Medical School, Boston, and a member of the American Psychiatric Association Council on Geriatric Psychiatry, said he views the study with some trepidation.
“As a clinician taking care of older adults, I would be very cautious about overinterpreting the beneficial effects of alcohol before we understand the mechanism better,” he said.
He noted that all of the risk factors associated with heart attack and stroke are also risk factors for Alzheimer’s disease and cognitive decline more broadly. “One of the issues here is how in the world does alcohol reduce cardiovascular and cerebrovascular risks, if you know it increases the risk of hypertension and stroke, regardless of dose.”
With regard to the possible impact of alcohol on brain-derived neurotrophic factor, Dr. Forester said, “it’s an interesting idea” but the actual mechanism is still unclear.
Even with dietary studies, such as those on the Mediterranean diet that include red wine, showing cognitive benefit, Dr. Forester said he’s still concerned about the adverse effects of alcohol on older people. These can include falls and sleep disturbances in addition to cognitive issues, and these effects can increase with age.
He was somewhat surprised at the level of alcohol that the study determined was beneficial. “Essentially, what they’re saying here is that, for men, it’s two drinks a day.” This could be “problematic” as two drinks per day can quickly escalate as individuals build tolerance.
He also pointed out that the study does not determine cause and effect, noting that it’s only an association.
Dr. Forester said the study raises a number of questions, including the type of alcohol study participants consumed and whether this has any impact on cognitive benefit. He also questioned whether the mediating effects of alcohol were associated with something that wasn’t measured, such as socioeconomic status.
Another question, he said, is what factors in individuals’ medical or psychiatric history determine whether they are more or less likely to benefit from low to moderate alcohol intake.
Perhaps alcohol should be recommended only for “select subpopulations” – for example, those who are healthy and have a family history of cognitive decline –but not for those with a history of substance abuse, including alcohol abuse, said Dr. Forester.
“For this population, the last thing you want to do is recommend alcohol to reduce risk of cognitive decline,” he cautioned.
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. The investigators and Dr. Forester have reported no relevant financial disclosures.
A version of this story originally appeared on Medscape.com.
Colonoscopy over age 75 should be ‘carefully considered’
Older individuals had a higher risk of complications 30 days after undergoing a colonoscopy as an outpatient procedure compared with a younger group of colorectal cancer screening–eligible individuals, according to recent research published in JAMA Network Open.
Natalia Causada-Calo, MD, MSc, division of gastroenterology, at St. Michael’s Hospital, University of Toronto, and colleagues performed a retrospective cohort study of 38,069 patients in Ontario administrative databases who underwent colonoscopy between April 2008 and September 2017. The patients included were older than 50 years (mean age, 65.2 years) with a majority (73.1%) undergoing their first colonoscopy. Those with inflammatory bowel disease and hereditary colorectal cancer syndromes were excluded. Researchers divided patients into groups based on age, placing patients aged 50-74 years into a colorectal cancer–screening eligible group (30,443 patients), and individuals 75 years or older into an “older cohort” (7,627 patients). Dr. Causada-Calo and colleagues analyzed 30-day admission to hospital or emergency department, and also examined 30-day all-cause mortality and incidence of colorectal cancer.
Among individuals in the older cohort, 515 of 7,627 patients (6.8%) experienced complications after colonoscopy compared with 795 of 30,443 patients (2.6%) in the screening-eligible cohort (P less than .001). Older age was an independent risk factor for postcolonoscopy complications, with individuals older than 75 years having twofold greater odds of complications after colonoscopy (odds ratio, 2.3; 95% confidence interval, 2.0-2.6) compared with individuals aged 50-74 years.
Other independent risk factors for complications included liver disease (OR, 4.7; 95% CI, 3.5-6.5), heart failure (OR, 3.4; 95% CI, 2.5-4.6), smoking history (OR, 3.2; 95% CI, 2.4-4.3), obesity (OR, 2.3; 95% CI, 1.2-4.2), chronic kidney disease (OR, 1.8; 95% CI, 1.1-3.0), cardiac arrhythmia (OR, 1.7; 95% CI, 1.2-2.2), anemia (OR, 1.4; 95% CI, 1.2-1.7), and hypertension (OR, 1.2; 95% CI, 1.0-1.5). Individuals who had previously undergone colonoscopy had a lower risk of complications after the procedure (OR, 0.9; 95% CI, 0.7-1.0).
There was a significantly higher incidence of colorectal cancer treated with surgery in the older group (119 of 7,626; 1.6%) compared with the younger (144 of 30,443; 0.5%) group (P less than .001). Mortality from any cause was also significantly higher in the older group (20 patients; 0.2%) compared with the younger (39 patients; 0.1%) group (P less than .001).
“In accordance with our findings, the decision to perform colonoscopy should be considered carefully in older patients, particularly in the presence of comorbidities,” Dr. Causada-Calo and colleagues wrote.
Aasma Shaukat, MD, MPH, GI section chief at Minneapolis VA Health Care System and professor of medicine at University of Minnesota, said in an interview that screening colonoscopy in a population older than 75 years should be an individualized discussion with a patient who has minimal comorbidities, and the decision to move forward with a colonoscopy should be considered only if a patient’s life expectancy is at least 10 years.
“This study shows that diagnostic colonoscopy is associated with high risk of complications and quantifies the risk, to frame the discussion with the patient about going forward,” she said. “Colorectal cancers are slow growing. In individuals age 75 and older, competing health risks and risk of the colonoscopy often outweigh the small benefit they may derive. Older individuals should thus focus on other health priorities.”
Physicians should make their older patients aware that there is a risk for serious adverse events, including death, which increases after age 75. “[The] risk-benefit ratio for performing colonoscopy needs to carefully weighed,” Dr. Shaukat said. “[T]he patient should be presented with options, including the option of no screening.”
The American Cancer Society advocates “for individualized decision-making regarding screening for individuals after 75 but [does] not give any firm recommendations,” while the U.S. Preventive Services Task Force noted in its recommendations on colorectal cancer screening that the “harms are large and benefits are small” after 75 years of age, and choice to screen for colorectal cancer in that age group is an individual one, Dr. Shaukat said.
Robert A. Smith, PhD, senior vice president of cancer screening at the American Cancer Society, said in an interview that while colonoscopy is the dominant screening test for colorectal cancer, it is not known how often physicians and their older patients discuss noninvasive colorectal cancer screening methods. Noninvasive screening methods such as a high-sensitivity stool test should be a consideration even for older adults with mild chronic conditions, “especially if they have a history of screening with negative results,” he said. “[A] history of regular screening with normal test results should be a basis for considering cessation of screening after age 75, or at least transition to a test with lower risks of complications.”
Future research in this area could use a hybrid model of screening, such as using different tests among various age groups or risk groups, to see whether an invasive or noninvasive method would lower a complication rate, Dr. Smith said. “Further, we need to have a greater understanding of when individuals can confidently stop getting screened, based on their underlying risk and history of prior screening results,” he noted.
Dr. Shaukat said future studies should focus on randomized trials for individuals 75 years and older to assess the benefits and harms of screening, “[d]eveloping risk stratification tools that factor in an individual’s risk of colon cancer, their life expectancy, and guide individualized decision making to undergo screening.”
Dr. Armstrong is the chair of the National Colon Cancer Screening Network for the Canadian Partnership Against Cancer and the past president of the Canadian Association of Gastroenterology. Dr. Albashir has received honoraria and speaker fees from Janssen, and grants from AbbVie and ATGen. Dr. Shaukat and Dr. Smith report no relevant conflicts of interest.
SOURCE: Causada-Calo N et al. JAMA Netw Open. 2020;3(6):e208958.
Older individuals had a higher risk of complications 30 days after undergoing a colonoscopy as an outpatient procedure compared with a younger group of colorectal cancer screening–eligible individuals, according to recent research published in JAMA Network Open.
Natalia Causada-Calo, MD, MSc, division of gastroenterology, at St. Michael’s Hospital, University of Toronto, and colleagues performed a retrospective cohort study of 38,069 patients in Ontario administrative databases who underwent colonoscopy between April 2008 and September 2017. The patients included were older than 50 years (mean age, 65.2 years) with a majority (73.1%) undergoing their first colonoscopy. Those with inflammatory bowel disease and hereditary colorectal cancer syndromes were excluded. Researchers divided patients into groups based on age, placing patients aged 50-74 years into a colorectal cancer–screening eligible group (30,443 patients), and individuals 75 years or older into an “older cohort” (7,627 patients). Dr. Causada-Calo and colleagues analyzed 30-day admission to hospital or emergency department, and also examined 30-day all-cause mortality and incidence of colorectal cancer.
Among individuals in the older cohort, 515 of 7,627 patients (6.8%) experienced complications after colonoscopy compared with 795 of 30,443 patients (2.6%) in the screening-eligible cohort (P less than .001). Older age was an independent risk factor for postcolonoscopy complications, with individuals older than 75 years having twofold greater odds of complications after colonoscopy (odds ratio, 2.3; 95% confidence interval, 2.0-2.6) compared with individuals aged 50-74 years.
Other independent risk factors for complications included liver disease (OR, 4.7; 95% CI, 3.5-6.5), heart failure (OR, 3.4; 95% CI, 2.5-4.6), smoking history (OR, 3.2; 95% CI, 2.4-4.3), obesity (OR, 2.3; 95% CI, 1.2-4.2), chronic kidney disease (OR, 1.8; 95% CI, 1.1-3.0), cardiac arrhythmia (OR, 1.7; 95% CI, 1.2-2.2), anemia (OR, 1.4; 95% CI, 1.2-1.7), and hypertension (OR, 1.2; 95% CI, 1.0-1.5). Individuals who had previously undergone colonoscopy had a lower risk of complications after the procedure (OR, 0.9; 95% CI, 0.7-1.0).
There was a significantly higher incidence of colorectal cancer treated with surgery in the older group (119 of 7,626; 1.6%) compared with the younger (144 of 30,443; 0.5%) group (P less than .001). Mortality from any cause was also significantly higher in the older group (20 patients; 0.2%) compared with the younger (39 patients; 0.1%) group (P less than .001).
“In accordance with our findings, the decision to perform colonoscopy should be considered carefully in older patients, particularly in the presence of comorbidities,” Dr. Causada-Calo and colleagues wrote.
Aasma Shaukat, MD, MPH, GI section chief at Minneapolis VA Health Care System and professor of medicine at University of Minnesota, said in an interview that screening colonoscopy in a population older than 75 years should be an individualized discussion with a patient who has minimal comorbidities, and the decision to move forward with a colonoscopy should be considered only if a patient’s life expectancy is at least 10 years.
“This study shows that diagnostic colonoscopy is associated with high risk of complications and quantifies the risk, to frame the discussion with the patient about going forward,” she said. “Colorectal cancers are slow growing. In individuals age 75 and older, competing health risks and risk of the colonoscopy often outweigh the small benefit they may derive. Older individuals should thus focus on other health priorities.”
Physicians should make their older patients aware that there is a risk for serious adverse events, including death, which increases after age 75. “[The] risk-benefit ratio for performing colonoscopy needs to carefully weighed,” Dr. Shaukat said. “[T]he patient should be presented with options, including the option of no screening.”
The American Cancer Society advocates “for individualized decision-making regarding screening for individuals after 75 but [does] not give any firm recommendations,” while the U.S. Preventive Services Task Force noted in its recommendations on colorectal cancer screening that the “harms are large and benefits are small” after 75 years of age, and choice to screen for colorectal cancer in that age group is an individual one, Dr. Shaukat said.
Robert A. Smith, PhD, senior vice president of cancer screening at the American Cancer Society, said in an interview that while colonoscopy is the dominant screening test for colorectal cancer, it is not known how often physicians and their older patients discuss noninvasive colorectal cancer screening methods. Noninvasive screening methods such as a high-sensitivity stool test should be a consideration even for older adults with mild chronic conditions, “especially if they have a history of screening with negative results,” he said. “[A] history of regular screening with normal test results should be a basis for considering cessation of screening after age 75, or at least transition to a test with lower risks of complications.”
Future research in this area could use a hybrid model of screening, such as using different tests among various age groups or risk groups, to see whether an invasive or noninvasive method would lower a complication rate, Dr. Smith said. “Further, we need to have a greater understanding of when individuals can confidently stop getting screened, based on their underlying risk and history of prior screening results,” he noted.
Dr. Shaukat said future studies should focus on randomized trials for individuals 75 years and older to assess the benefits and harms of screening, “[d]eveloping risk stratification tools that factor in an individual’s risk of colon cancer, their life expectancy, and guide individualized decision making to undergo screening.”
Dr. Armstrong is the chair of the National Colon Cancer Screening Network for the Canadian Partnership Against Cancer and the past president of the Canadian Association of Gastroenterology. Dr. Albashir has received honoraria and speaker fees from Janssen, and grants from AbbVie and ATGen. Dr. Shaukat and Dr. Smith report no relevant conflicts of interest.
SOURCE: Causada-Calo N et al. JAMA Netw Open. 2020;3(6):e208958.
Older individuals had a higher risk of complications 30 days after undergoing a colonoscopy as an outpatient procedure compared with a younger group of colorectal cancer screening–eligible individuals, according to recent research published in JAMA Network Open.
Natalia Causada-Calo, MD, MSc, division of gastroenterology, at St. Michael’s Hospital, University of Toronto, and colleagues performed a retrospective cohort study of 38,069 patients in Ontario administrative databases who underwent colonoscopy between April 2008 and September 2017. The patients included were older than 50 years (mean age, 65.2 years) with a majority (73.1%) undergoing their first colonoscopy. Those with inflammatory bowel disease and hereditary colorectal cancer syndromes were excluded. Researchers divided patients into groups based on age, placing patients aged 50-74 years into a colorectal cancer–screening eligible group (30,443 patients), and individuals 75 years or older into an “older cohort” (7,627 patients). Dr. Causada-Calo and colleagues analyzed 30-day admission to hospital or emergency department, and also examined 30-day all-cause mortality and incidence of colorectal cancer.
Among individuals in the older cohort, 515 of 7,627 patients (6.8%) experienced complications after colonoscopy compared with 795 of 30,443 patients (2.6%) in the screening-eligible cohort (P less than .001). Older age was an independent risk factor for postcolonoscopy complications, with individuals older than 75 years having twofold greater odds of complications after colonoscopy (odds ratio, 2.3; 95% confidence interval, 2.0-2.6) compared with individuals aged 50-74 years.
Other independent risk factors for complications included liver disease (OR, 4.7; 95% CI, 3.5-6.5), heart failure (OR, 3.4; 95% CI, 2.5-4.6), smoking history (OR, 3.2; 95% CI, 2.4-4.3), obesity (OR, 2.3; 95% CI, 1.2-4.2), chronic kidney disease (OR, 1.8; 95% CI, 1.1-3.0), cardiac arrhythmia (OR, 1.7; 95% CI, 1.2-2.2), anemia (OR, 1.4; 95% CI, 1.2-1.7), and hypertension (OR, 1.2; 95% CI, 1.0-1.5). Individuals who had previously undergone colonoscopy had a lower risk of complications after the procedure (OR, 0.9; 95% CI, 0.7-1.0).
There was a significantly higher incidence of colorectal cancer treated with surgery in the older group (119 of 7,626; 1.6%) compared with the younger (144 of 30,443; 0.5%) group (P less than .001). Mortality from any cause was also significantly higher in the older group (20 patients; 0.2%) compared with the younger (39 patients; 0.1%) group (P less than .001).
“In accordance with our findings, the decision to perform colonoscopy should be considered carefully in older patients, particularly in the presence of comorbidities,” Dr. Causada-Calo and colleagues wrote.
Aasma Shaukat, MD, MPH, GI section chief at Minneapolis VA Health Care System and professor of medicine at University of Minnesota, said in an interview that screening colonoscopy in a population older than 75 years should be an individualized discussion with a patient who has minimal comorbidities, and the decision to move forward with a colonoscopy should be considered only if a patient’s life expectancy is at least 10 years.
“This study shows that diagnostic colonoscopy is associated with high risk of complications and quantifies the risk, to frame the discussion with the patient about going forward,” she said. “Colorectal cancers are slow growing. In individuals age 75 and older, competing health risks and risk of the colonoscopy often outweigh the small benefit they may derive. Older individuals should thus focus on other health priorities.”
Physicians should make their older patients aware that there is a risk for serious adverse events, including death, which increases after age 75. “[The] risk-benefit ratio for performing colonoscopy needs to carefully weighed,” Dr. Shaukat said. “[T]he patient should be presented with options, including the option of no screening.”
The American Cancer Society advocates “for individualized decision-making regarding screening for individuals after 75 but [does] not give any firm recommendations,” while the U.S. Preventive Services Task Force noted in its recommendations on colorectal cancer screening that the “harms are large and benefits are small” after 75 years of age, and choice to screen for colorectal cancer in that age group is an individual one, Dr. Shaukat said.
Robert A. Smith, PhD, senior vice president of cancer screening at the American Cancer Society, said in an interview that while colonoscopy is the dominant screening test for colorectal cancer, it is not known how often physicians and their older patients discuss noninvasive colorectal cancer screening methods. Noninvasive screening methods such as a high-sensitivity stool test should be a consideration even for older adults with mild chronic conditions, “especially if they have a history of screening with negative results,” he said. “[A] history of regular screening with normal test results should be a basis for considering cessation of screening after age 75, or at least transition to a test with lower risks of complications.”
Future research in this area could use a hybrid model of screening, such as using different tests among various age groups or risk groups, to see whether an invasive or noninvasive method would lower a complication rate, Dr. Smith said. “Further, we need to have a greater understanding of when individuals can confidently stop getting screened, based on their underlying risk and history of prior screening results,” he noted.
Dr. Shaukat said future studies should focus on randomized trials for individuals 75 years and older to assess the benefits and harms of screening, “[d]eveloping risk stratification tools that factor in an individual’s risk of colon cancer, their life expectancy, and guide individualized decision making to undergo screening.”
Dr. Armstrong is the chair of the National Colon Cancer Screening Network for the Canadian Partnership Against Cancer and the past president of the Canadian Association of Gastroenterology. Dr. Albashir has received honoraria and speaker fees from Janssen, and grants from AbbVie and ATGen. Dr. Shaukat and Dr. Smith report no relevant conflicts of interest.
SOURCE: Causada-Calo N et al. JAMA Netw Open. 2020;3(6):e208958.
FROM JAMA NETWORK OPEN
High-impact training can build bone in older women
Older adults, particularly postmenopausal women, are often advised to pursue low-impact, low-intensity exercise as a way to preserve joint health, but that approach might actually contribute to a decline in bone mineral density, researchers report.
Concerns about falls and fracture risk have led many clinicians to advise against higher-impact activities, like jumping, but that is exactly the type of activity that improves bone density and physical function, said Belinda Beck, PhD, professor at the Griffith University School of Allied Health Sciences in Southport, Australia.
“There has always been a quandary in terms of pursuing research on this,” she said in an interview. “We know from animal studies that bone only responds to high-intensity activity, but we worry about advising that for people with low bone mass, so instead we give them medications.”
“But not everyone likes to go on meds, they’re not 100% effective, and they’re not free of side effects,” said Beck, who is also the owner and director of The Bone Clinic in Brisbane, Australia.
In 2014, to assess whether high-intensity resistance and impact training (HiRIT) was a safe and effective way to improve bone mass, Beck and her colleagues conducted the LIFTMOR study of 101 postmenopausal women. The researchers showed that bone mineral density in the lumbar spine and femoral neck regions and functional performance measures were significantly better in the 49 participants randomized to HiRIT for 8 months than in the 52 randomized to low-intensity training.
Three years after the completion of LIFTMOR, the researchers looked at bone mineral density in 23 women from the HiRIT group in their retrospective observational study, the results of which were presented at the virtual American College of Sports Medicine 2020 Annual Meeting.
Ongoing gains were significantly better for the seven participants who continued with HiRIT (at least 25% compliance) than for the 16 who did not when looking at both bone mineral density of the lumbar spine (8.63% vs. 2.18%; P = .042) and femoral neck (3.67% vs. 2.85%; P = 0.14).
However, the women who discontinued HiRIT after 8 months maintained the gains in bone mineral density that they had achieved 3 years earlier.
Functional outcomes in the women who continued HiRIT were better than those in the women who did not, but the differences were not significant.
“The takeaway here is that this type of exercise appears to be a highly effective therapy to reduce risk of osteoporotic fracture, since it improves bone mass,” Beck said.
Jump more, lose less bone density
Given the widespread reluctance to suggest HiRIT-type activity to those with low bone mass, this research is significant, said Vanessa Yingling, PhD, from the Department of Kinesiology at California State University, East Bay.
“Once women hit 60, they’re somehow regarded as frail, but that becomes a self-fulfilling prophecy when we take this kinder, gentler approach to exercise,” Yingling said in an interview. “Building bone density in older adults is important, but maintaining current bone density is just as crucial. Without high-impact activity, we are likely to see decelerating density at a faster rate.”
The other key to the recent research is the functional testing, Yingling added. In addition to bone density measures, high-intensity activity can improve mobility and muscle strength, as the study noted.
This type of activity can be done in shorter bursts, making these workouts more efficient, she explained. For example, a Tabata high-intensity interval training session usually takes about 10 minutes, warm-up and cool-down included.
“A HiRIT workout even once or twice a week would likely improve function, strength, and bone density maintenance,” Beck said. “The result of that would be better fall prevention and potentially less medication usage for BMD issues.”
Both men and women can benefit from a HiRIT workout, Beck and Yingling said. Initially, supervision by a knowledgeable trainer or physical therapist is ideal, they added.
This article first appeared on Medscape.com.
Older adults, particularly postmenopausal women, are often advised to pursue low-impact, low-intensity exercise as a way to preserve joint health, but that approach might actually contribute to a decline in bone mineral density, researchers report.
Concerns about falls and fracture risk have led many clinicians to advise against higher-impact activities, like jumping, but that is exactly the type of activity that improves bone density and physical function, said Belinda Beck, PhD, professor at the Griffith University School of Allied Health Sciences in Southport, Australia.
“There has always been a quandary in terms of pursuing research on this,” she said in an interview. “We know from animal studies that bone only responds to high-intensity activity, but we worry about advising that for people with low bone mass, so instead we give them medications.”
“But not everyone likes to go on meds, they’re not 100% effective, and they’re not free of side effects,” said Beck, who is also the owner and director of The Bone Clinic in Brisbane, Australia.
In 2014, to assess whether high-intensity resistance and impact training (HiRIT) was a safe and effective way to improve bone mass, Beck and her colleagues conducted the LIFTMOR study of 101 postmenopausal women. The researchers showed that bone mineral density in the lumbar spine and femoral neck regions and functional performance measures were significantly better in the 49 participants randomized to HiRIT for 8 months than in the 52 randomized to low-intensity training.
Three years after the completion of LIFTMOR, the researchers looked at bone mineral density in 23 women from the HiRIT group in their retrospective observational study, the results of which were presented at the virtual American College of Sports Medicine 2020 Annual Meeting.
Ongoing gains were significantly better for the seven participants who continued with HiRIT (at least 25% compliance) than for the 16 who did not when looking at both bone mineral density of the lumbar spine (8.63% vs. 2.18%; P = .042) and femoral neck (3.67% vs. 2.85%; P = 0.14).
However, the women who discontinued HiRIT after 8 months maintained the gains in bone mineral density that they had achieved 3 years earlier.
Functional outcomes in the women who continued HiRIT were better than those in the women who did not, but the differences were not significant.
“The takeaway here is that this type of exercise appears to be a highly effective therapy to reduce risk of osteoporotic fracture, since it improves bone mass,” Beck said.
Jump more, lose less bone density
Given the widespread reluctance to suggest HiRIT-type activity to those with low bone mass, this research is significant, said Vanessa Yingling, PhD, from the Department of Kinesiology at California State University, East Bay.
“Once women hit 60, they’re somehow regarded as frail, but that becomes a self-fulfilling prophecy when we take this kinder, gentler approach to exercise,” Yingling said in an interview. “Building bone density in older adults is important, but maintaining current bone density is just as crucial. Without high-impact activity, we are likely to see decelerating density at a faster rate.”
The other key to the recent research is the functional testing, Yingling added. In addition to bone density measures, high-intensity activity can improve mobility and muscle strength, as the study noted.
This type of activity can be done in shorter bursts, making these workouts more efficient, she explained. For example, a Tabata high-intensity interval training session usually takes about 10 minutes, warm-up and cool-down included.
“A HiRIT workout even once or twice a week would likely improve function, strength, and bone density maintenance,” Beck said. “The result of that would be better fall prevention and potentially less medication usage for BMD issues.”
Both men and women can benefit from a HiRIT workout, Beck and Yingling said. Initially, supervision by a knowledgeable trainer or physical therapist is ideal, they added.
This article first appeared on Medscape.com.
Older adults, particularly postmenopausal women, are often advised to pursue low-impact, low-intensity exercise as a way to preserve joint health, but that approach might actually contribute to a decline in bone mineral density, researchers report.
Concerns about falls and fracture risk have led many clinicians to advise against higher-impact activities, like jumping, but that is exactly the type of activity that improves bone density and physical function, said Belinda Beck, PhD, professor at the Griffith University School of Allied Health Sciences in Southport, Australia.
“There has always been a quandary in terms of pursuing research on this,” she said in an interview. “We know from animal studies that bone only responds to high-intensity activity, but we worry about advising that for people with low bone mass, so instead we give them medications.”
“But not everyone likes to go on meds, they’re not 100% effective, and they’re not free of side effects,” said Beck, who is also the owner and director of The Bone Clinic in Brisbane, Australia.
In 2014, to assess whether high-intensity resistance and impact training (HiRIT) was a safe and effective way to improve bone mass, Beck and her colleagues conducted the LIFTMOR study of 101 postmenopausal women. The researchers showed that bone mineral density in the lumbar spine and femoral neck regions and functional performance measures were significantly better in the 49 participants randomized to HiRIT for 8 months than in the 52 randomized to low-intensity training.
Three years after the completion of LIFTMOR, the researchers looked at bone mineral density in 23 women from the HiRIT group in their retrospective observational study, the results of which were presented at the virtual American College of Sports Medicine 2020 Annual Meeting.
Ongoing gains were significantly better for the seven participants who continued with HiRIT (at least 25% compliance) than for the 16 who did not when looking at both bone mineral density of the lumbar spine (8.63% vs. 2.18%; P = .042) and femoral neck (3.67% vs. 2.85%; P = 0.14).
However, the women who discontinued HiRIT after 8 months maintained the gains in bone mineral density that they had achieved 3 years earlier.
Functional outcomes in the women who continued HiRIT were better than those in the women who did not, but the differences were not significant.
“The takeaway here is that this type of exercise appears to be a highly effective therapy to reduce risk of osteoporotic fracture, since it improves bone mass,” Beck said.
Jump more, lose less bone density
Given the widespread reluctance to suggest HiRIT-type activity to those with low bone mass, this research is significant, said Vanessa Yingling, PhD, from the Department of Kinesiology at California State University, East Bay.
“Once women hit 60, they’re somehow regarded as frail, but that becomes a self-fulfilling prophecy when we take this kinder, gentler approach to exercise,” Yingling said in an interview. “Building bone density in older adults is important, but maintaining current bone density is just as crucial. Without high-impact activity, we are likely to see decelerating density at a faster rate.”
The other key to the recent research is the functional testing, Yingling added. In addition to bone density measures, high-intensity activity can improve mobility and muscle strength, as the study noted.
This type of activity can be done in shorter bursts, making these workouts more efficient, she explained. For example, a Tabata high-intensity interval training session usually takes about 10 minutes, warm-up and cool-down included.
“A HiRIT workout even once or twice a week would likely improve function, strength, and bone density maintenance,” Beck said. “The result of that would be better fall prevention and potentially less medication usage for BMD issues.”
Both men and women can benefit from a HiRIT workout, Beck and Yingling said. Initially, supervision by a knowledgeable trainer or physical therapist is ideal, they added.
This article first appeared on Medscape.com.
Older adults boost muscle mass after bariatric surgery
Bariatric surgery may yield increases in muscle mass from baseline among older adults, findings from a small study suggest.
Although bariatric surgery can be used to treat obesity and related comorbidities in older adults, “here are concerns of excess loss of muscle mass after bariatric surgery, especially in elderly patients whose muscle tends to be less, compared to younger patients, at baseline,” wrote Moiz Dawood, MD, of Banner Gateway Medical Center, Gilbert, Ariz., and colleagues.
In a study presented in a poster at the virtual Annual Minimally Invasive Surgery Symposium sponsored by Global Academy for Medical Education, the researchers reviewed data from 89 adults older than 65 years (74% women) who underwent either laparoscopic sleeve gastrectomy (87 patients) or Roux-en-Y gastric bypass (2 patients) between May 2015 and March 2017.
At baseline, the average total body weight was 251 pounds and the average muscle mass percent was 50%. At 12 months after surgery, the average weight of the patients decreased to 197 pounds and the percentage of muscle mass increased to 55% (P < .001 for both).
The study findings were limited by the small sample size and retrospective design. However, the results support the benefits of bariatric surgery for older adults, not only with reductions in total body weight loss, but also increasing the total percentage of muscle mass, the researchers said.
The study is important in light of the ongoing discussion regarding the age limit for bariatric surgery, Dr. Dawood said in an interview. “Currently there is no upper age cutoff for patients who undergo bariatric surgery, and understanding the relationship between muscle mass and bariatric surgery would help in determining if there was a negative relationship,” he said.
“The results definitely point toward evidence that suggests that elderly patients do not lose muscle mass to a significant degree,” Dr. Dawood noted. “Muscle mass definitions and calculations also include variables such as weight and fat content. With the additional loss in weight after surgery, it was expected that the muscle mass composition would be affected,” he explained. “However, the results clearly show that even up to 1 year after surgery, older patients who lose weight do not lose significant weight from their muscle mass,” he noted.
The take-home message for clinicians, said Dr. Dawood, is “to understand that metabolic and bariatric surgery, when performed cohesively in a unified program that focuses on lifestyle and dietary changes, is the best way to achieve sustained weight loss.” He added, “this study indicates that physiologic changes that occur after weight loss surgery are not detrimental in the elderly population.”
Next steps for research include further studies in the elderly population to examine the physiologic changes that occur after weight loss surgery, said Dr. Dawood. “Being able to characterize the metabolic changes will help in answering the question of whether there is an upper age cut-off for patients undergoing bariatric surgery.”
Global Academy for Medical Education and this news organization are owned by the same parent company. The researchers had no relevant financial conflicts to disclose.
Bariatric surgery may yield increases in muscle mass from baseline among older adults, findings from a small study suggest.
Although bariatric surgery can be used to treat obesity and related comorbidities in older adults, “here are concerns of excess loss of muscle mass after bariatric surgery, especially in elderly patients whose muscle tends to be less, compared to younger patients, at baseline,” wrote Moiz Dawood, MD, of Banner Gateway Medical Center, Gilbert, Ariz., and colleagues.
In a study presented in a poster at the virtual Annual Minimally Invasive Surgery Symposium sponsored by Global Academy for Medical Education, the researchers reviewed data from 89 adults older than 65 years (74% women) who underwent either laparoscopic sleeve gastrectomy (87 patients) or Roux-en-Y gastric bypass (2 patients) between May 2015 and March 2017.
At baseline, the average total body weight was 251 pounds and the average muscle mass percent was 50%. At 12 months after surgery, the average weight of the patients decreased to 197 pounds and the percentage of muscle mass increased to 55% (P < .001 for both).
The study findings were limited by the small sample size and retrospective design. However, the results support the benefits of bariatric surgery for older adults, not only with reductions in total body weight loss, but also increasing the total percentage of muscle mass, the researchers said.
The study is important in light of the ongoing discussion regarding the age limit for bariatric surgery, Dr. Dawood said in an interview. “Currently there is no upper age cutoff for patients who undergo bariatric surgery, and understanding the relationship between muscle mass and bariatric surgery would help in determining if there was a negative relationship,” he said.
“The results definitely point toward evidence that suggests that elderly patients do not lose muscle mass to a significant degree,” Dr. Dawood noted. “Muscle mass definitions and calculations also include variables such as weight and fat content. With the additional loss in weight after surgery, it was expected that the muscle mass composition would be affected,” he explained. “However, the results clearly show that even up to 1 year after surgery, older patients who lose weight do not lose significant weight from their muscle mass,” he noted.
The take-home message for clinicians, said Dr. Dawood, is “to understand that metabolic and bariatric surgery, when performed cohesively in a unified program that focuses on lifestyle and dietary changes, is the best way to achieve sustained weight loss.” He added, “this study indicates that physiologic changes that occur after weight loss surgery are not detrimental in the elderly population.”
Next steps for research include further studies in the elderly population to examine the physiologic changes that occur after weight loss surgery, said Dr. Dawood. “Being able to characterize the metabolic changes will help in answering the question of whether there is an upper age cut-off for patients undergoing bariatric surgery.”
Global Academy for Medical Education and this news organization are owned by the same parent company. The researchers had no relevant financial conflicts to disclose.
Bariatric surgery may yield increases in muscle mass from baseline among older adults, findings from a small study suggest.
Although bariatric surgery can be used to treat obesity and related comorbidities in older adults, “here are concerns of excess loss of muscle mass after bariatric surgery, especially in elderly patients whose muscle tends to be less, compared to younger patients, at baseline,” wrote Moiz Dawood, MD, of Banner Gateway Medical Center, Gilbert, Ariz., and colleagues.
In a study presented in a poster at the virtual Annual Minimally Invasive Surgery Symposium sponsored by Global Academy for Medical Education, the researchers reviewed data from 89 adults older than 65 years (74% women) who underwent either laparoscopic sleeve gastrectomy (87 patients) or Roux-en-Y gastric bypass (2 patients) between May 2015 and March 2017.
At baseline, the average total body weight was 251 pounds and the average muscle mass percent was 50%. At 12 months after surgery, the average weight of the patients decreased to 197 pounds and the percentage of muscle mass increased to 55% (P < .001 for both).
The study findings were limited by the small sample size and retrospective design. However, the results support the benefits of bariatric surgery for older adults, not only with reductions in total body weight loss, but also increasing the total percentage of muscle mass, the researchers said.
The study is important in light of the ongoing discussion regarding the age limit for bariatric surgery, Dr. Dawood said in an interview. “Currently there is no upper age cutoff for patients who undergo bariatric surgery, and understanding the relationship between muscle mass and bariatric surgery would help in determining if there was a negative relationship,” he said.
“The results definitely point toward evidence that suggests that elderly patients do not lose muscle mass to a significant degree,” Dr. Dawood noted. “Muscle mass definitions and calculations also include variables such as weight and fat content. With the additional loss in weight after surgery, it was expected that the muscle mass composition would be affected,” he explained. “However, the results clearly show that even up to 1 year after surgery, older patients who lose weight do not lose significant weight from their muscle mass,” he noted.
The take-home message for clinicians, said Dr. Dawood, is “to understand that metabolic and bariatric surgery, when performed cohesively in a unified program that focuses on lifestyle and dietary changes, is the best way to achieve sustained weight loss.” He added, “this study indicates that physiologic changes that occur after weight loss surgery are not detrimental in the elderly population.”
Next steps for research include further studies in the elderly population to examine the physiologic changes that occur after weight loss surgery, said Dr. Dawood. “Being able to characterize the metabolic changes will help in answering the question of whether there is an upper age cut-off for patients undergoing bariatric surgery.”
Global Academy for Medical Education and this news organization are owned by the same parent company. The researchers had no relevant financial conflicts to disclose.
FROM MISS
What COVID-19 has taught us about senior care
Across the globe, there are marked differences in how countries responded to the COVID-19 outbreak, with varying degrees of success in limiting the spread of the virus. Some countries learned important lessons from previous outbreaks, including SARS and MERS, and put policies in place that contributed to lower infection and death rates from COVID-19 in these countries. Others struggled to respond appropriately to the outbreak.
The United States and most of the world was not affected significantly by SARS and MERS. Hence there is a need for different perspectives and observations on lessons that can be learned from this outbreak to help develop effective strategies and policies for the future. It also makes sense to focus intently on the demographic most affected by COVID-19 – the elderly.
Medical care, for the most part, is governed by protocols that clearly detail processes to be followed for the prevention and treatment of disease. Caring for older patients requires going above and beyond the protocols. That is one of the lessons learned from the COVID-19 pandemic – a wake-up call for a more proactive approach for at-risk patients, in this case everyone over the age of 60 years.
In this context, it is important for medical outreach to continue with the senior population long after the pandemic has run its course. Many seniors, particularly those susceptible to other illnesses or exhibiting ongoing issues, would benefit from a consistent and preplanned pattern of contacts by medical professionals and agencies that work with the aging population. These proactive follow-ups can facilitate prevention and treatment and, at the same time, reduce costs that would otherwise increase when health care is reactive.
Lessons in infectious disease containment
As COVID-19 spread globally, there were contrasting responses from individual countries in their efforts to contain the disease. Unfortunately, Italy suffered from its decision to lock down only specific regions of the country initially. The leadership in Italy may have ignored the advice of medical experts and been caught off guard by the intensity of the spread of COVID-19. In fact, they might not have taken strict actions right away because they did not want their responses to be viewed as an overreaction to the disease.
The government decided to shut down areas where the infection rates were high (“red zones”) rather than implement restrictions nationally. This may have inadvertently increased the spread as Italians vacated those “red zones” for other areas of the country not yet affected by COVID-19. Italy’s decentralized health care system also played a part in the effects of the disease, with some regions demonstrating more success in slowing the reach of the disease. According to an article in the Harvard Business Review, the neighboring regions of Lombardy and Veneto applied similar approaches to social distancing and retail closures. Veneto was more proactive, and its response to the outbreak was multipronged, including putting a “strong emphasis on home diagnosis and care” and “specific efforts to monitor and protect health care and other essential workers.” These measures most likely contributed to a slowdown of the spread of the disease in Veneto’s health care facilities, which lessened the load on medical providers.1
Conversely, Taiwan implemented proactive measures swiftly after learning about COVID-19. Taiwan was impacted adversely by the SARS outbreak in 2003 and, afterward, revised their medical policies and procedures to respond quickly to future infectious disease crises. In the beginning, little was known about COVID-19 or how it spread. However, Taiwan’s swift public health response to COVID-19 included early travel restrictions, patient screening, and quarantining of symptomatic patients. The government emphasized education and created real-time digital updates and alerts sent to their citizens, as well as partnering with media to broadcast crucial proactive health information and quickly disproving false information related to COVID-19. They coordinated with organizations throughout the country to increase supplies of personal protective equipment (PPE).2
Although countries and even cities within a country differ in terms of population demographics, health resources, government policies, and cultural practices, initial success stories have some similarities, including the following:
- Early travel restrictions from countries with positive cases, with some circumstances requiring compulsory quarantine periods and testing before entry.
- Extensive testing and proactive tracing of symptomatic cases early. Contacts of people testing positive were also tested, irrespective of being symptomatic or asymptomatic. If testing kits were unavailable, the contacts were self-quarantined.
- Emphasis on avoiding overburdening hospitals by having the public health infrastructure to divert people exhibiting symptoms, including using public health hotlines to send patients to dedicated testing sites and drive-through testing, rather than have patients presenting to emergency rooms and hospitals. This approach protected medical staff from exposure and allowed the focus to remain on treating severe symptomatic patients.
The vastly different response to the COVID-19 outbreak in these two countries illuminates the need for better preparation in the United States. At the onset of this outbreak, emergency room medical professionals, hospitalists, and outpatient primary care providers did not know how to screen for or treat this virus. Additionally, there was limited information on the most effective contact protocols for medical professionals, patients, and visitors. Finally, the lack of PPE and COVID-19 test kits hindered the U.S. response. Once the country is on the road to recovery from COVID-19, it is imperative to set the groundwork to prepare for future outbreaks and create mechanisms to quickly identify vulnerable populations when outbreaks occur.
Senior care in future infectious disease outbreaks
How can medical providers translate lessons learned from this outbreak into improving the quality of care for seniors? The National Institute on Aging (NIA) maintains a website with information about healthy aging. Seniors and their caregivers can use this website to learn more about chronic diseases, lifestyle modifications, disease prevention, and mental health.
In times of a pandemic, this website provides consistent and accurate information and education. One recommendation for reaching the elderly population during future outbreaks is for NIA to develop and implement strategies to increase the use of the website, including adding more audio and visual interfaces and developing a mobile app. Other recommendations for improving the quality of care for seniors include the following:
1. Identify which populations may be most affected when future outbreaks occur.
2. Consider nontraditional platforms, including social media, for communicating with the general population and for medical providers worldwide to learn from each other about new diseases, including the signs, symptoms, and treatment plans. Some medical professionals created specific WhatsApp groups to communicate, and the World Health Organization sent updated information about COVID-19 to anyone who texted them via WhatsApp.3
3. Create a checklist of signs and symptoms related to current infectious diseases and assess every vulnerable patient.
4. Share these guidelines with medical facilities that treat these populations, such as senior care, assisted living and rehabilitation facilities, hospitals, and outpatient treatment centers. Teach the staff at these medical facilities how to screen patients for signs and symptoms of the disease.
5. Implement social isolation strategies, travel and visitor restrictions, and testing and screening as soon as possible at these medical facilities.
6. Recognize that these strategies may affect the psychological and emotional well-being of seniors, increasing their risk for depression and anxiety and negatively affecting their immunity and mental health. Additionally, the use of PPE, either by the medical providers or the patient, may cause anxiety in seniors and those with mild cognitive impairment.
7. Encourage these medical facilities to improve coping strategies with older patients, such as incorporating communication technology that helps seniors stay connected with their families, and participating in physical and mental exercise, as well as religious activities.
8. Ask these medical facilities to create isolation or quarantine rooms for infected seniors.
9. Work with family members to proactively report to medical professionals any symptoms noticed in their senior relatives. Educate seniors to report symptoms earlier.
10. Offer incentives for medical professionals to conduct on-site testing in primary care offices or senior care facilities instead of sending patients to hospital emergency rooms for evaluation. This will only be effective if there are enough test kits available.
11. Urge insurance companies and Medicare to allow additional medical visits for screening vulnerable populations. Encourage the use of telemedicine in place of in-office visits (preferably billed at the same rate as an in-office visit) where appropriate, especially with nonambulatory patients or those with transportation issues. Many insurance companies, including Medicare, approved COVID-19–related coverage of telemedicine in place of office visits to limit the spread of the disease.
12. Provide community health care and integration and better coordination of local, state, and national health care.
13. Hold regular epidemic and pandemic preparedness exercises in every hospital, nursing home, and assisted living facility.
Proactive health care outreach
It is easier to identify the signs and symptoms of already identified infectious diseases as opposed to a novel one like COVID-19. The United States faced a steep learning curve with COVID-19. Hospitalists and other medical professionals were not able to learn about COVID-19 in a journal. At first, they did not know how to screen patients coming into the ER, how to protect staff, or what the treatment plan was for this new disease. As a result, the medical system experienced disorder and confusion. Investing in community health care and better coordination of local, state, and national health care resources is a priority.
The senior citizen population appears to be most vulnerable to this virus and may be just as vulnerable in future outbreaks. Yet the insights gained from this pandemic can lead to a more comprehensive outreach to senior patients and increased screenings for comorbidities and future contagious diseases. An emphasis on proactive health care and outreach for seniors, with a focus on identifying and treating comorbid conditions, improves the medical care system overall and may prevent or slow future community outbreaks.
Dr. Kasarla is a hospitalist with APOGEE Physicians at Wise Surgical at Parkway in Fort Worth, Tex. He did his internal medicine residency at Mercy Hospital & Medical Center, Chicago. Readers can contact him at [email protected]. Dr. Devireddy is a family physician at Positive Health Medical Center, Kingston, Jamaica. Contact him at [email protected].
References
1. Pisano GP et al. Lessons from Italy’s response to coronavirus. Harvard Business Review. 2020 Mar 27. https://hbr.org/2020/03/lessons-from-italys-response-to-coronavirus.
2. Tu C. Lessons from Taiwan’s experience with COVID-19. New Atlanticist. 2020 Apr 7. https://atlanticcouncil.org/blogs/new-atlanticist/lessons-from-taiwans-experience-with-covid-19/.
3. Newman LH. WhatsApp is at the center of coronavirus response. WIRED. 2020 Mar 20. https://www.wired.com/story/whatsapp-coronavirus-who-information-app/.
Across the globe, there are marked differences in how countries responded to the COVID-19 outbreak, with varying degrees of success in limiting the spread of the virus. Some countries learned important lessons from previous outbreaks, including SARS and MERS, and put policies in place that contributed to lower infection and death rates from COVID-19 in these countries. Others struggled to respond appropriately to the outbreak.
The United States and most of the world was not affected significantly by SARS and MERS. Hence there is a need for different perspectives and observations on lessons that can be learned from this outbreak to help develop effective strategies and policies for the future. It also makes sense to focus intently on the demographic most affected by COVID-19 – the elderly.
Medical care, for the most part, is governed by protocols that clearly detail processes to be followed for the prevention and treatment of disease. Caring for older patients requires going above and beyond the protocols. That is one of the lessons learned from the COVID-19 pandemic – a wake-up call for a more proactive approach for at-risk patients, in this case everyone over the age of 60 years.
In this context, it is important for medical outreach to continue with the senior population long after the pandemic has run its course. Many seniors, particularly those susceptible to other illnesses or exhibiting ongoing issues, would benefit from a consistent and preplanned pattern of contacts by medical professionals and agencies that work with the aging population. These proactive follow-ups can facilitate prevention and treatment and, at the same time, reduce costs that would otherwise increase when health care is reactive.
Lessons in infectious disease containment
As COVID-19 spread globally, there were contrasting responses from individual countries in their efforts to contain the disease. Unfortunately, Italy suffered from its decision to lock down only specific regions of the country initially. The leadership in Italy may have ignored the advice of medical experts and been caught off guard by the intensity of the spread of COVID-19. In fact, they might not have taken strict actions right away because they did not want their responses to be viewed as an overreaction to the disease.
The government decided to shut down areas where the infection rates were high (“red zones”) rather than implement restrictions nationally. This may have inadvertently increased the spread as Italians vacated those “red zones” for other areas of the country not yet affected by COVID-19. Italy’s decentralized health care system also played a part in the effects of the disease, with some regions demonstrating more success in slowing the reach of the disease. According to an article in the Harvard Business Review, the neighboring regions of Lombardy and Veneto applied similar approaches to social distancing and retail closures. Veneto was more proactive, and its response to the outbreak was multipronged, including putting a “strong emphasis on home diagnosis and care” and “specific efforts to monitor and protect health care and other essential workers.” These measures most likely contributed to a slowdown of the spread of the disease in Veneto’s health care facilities, which lessened the load on medical providers.1
Conversely, Taiwan implemented proactive measures swiftly after learning about COVID-19. Taiwan was impacted adversely by the SARS outbreak in 2003 and, afterward, revised their medical policies and procedures to respond quickly to future infectious disease crises. In the beginning, little was known about COVID-19 or how it spread. However, Taiwan’s swift public health response to COVID-19 included early travel restrictions, patient screening, and quarantining of symptomatic patients. The government emphasized education and created real-time digital updates and alerts sent to their citizens, as well as partnering with media to broadcast crucial proactive health information and quickly disproving false information related to COVID-19. They coordinated with organizations throughout the country to increase supplies of personal protective equipment (PPE).2
Although countries and even cities within a country differ in terms of population demographics, health resources, government policies, and cultural practices, initial success stories have some similarities, including the following:
- Early travel restrictions from countries with positive cases, with some circumstances requiring compulsory quarantine periods and testing before entry.
- Extensive testing and proactive tracing of symptomatic cases early. Contacts of people testing positive were also tested, irrespective of being symptomatic or asymptomatic. If testing kits were unavailable, the contacts were self-quarantined.
- Emphasis on avoiding overburdening hospitals by having the public health infrastructure to divert people exhibiting symptoms, including using public health hotlines to send patients to dedicated testing sites and drive-through testing, rather than have patients presenting to emergency rooms and hospitals. This approach protected medical staff from exposure and allowed the focus to remain on treating severe symptomatic patients.
The vastly different response to the COVID-19 outbreak in these two countries illuminates the need for better preparation in the United States. At the onset of this outbreak, emergency room medical professionals, hospitalists, and outpatient primary care providers did not know how to screen for or treat this virus. Additionally, there was limited information on the most effective contact protocols for medical professionals, patients, and visitors. Finally, the lack of PPE and COVID-19 test kits hindered the U.S. response. Once the country is on the road to recovery from COVID-19, it is imperative to set the groundwork to prepare for future outbreaks and create mechanisms to quickly identify vulnerable populations when outbreaks occur.
Senior care in future infectious disease outbreaks
How can medical providers translate lessons learned from this outbreak into improving the quality of care for seniors? The National Institute on Aging (NIA) maintains a website with information about healthy aging. Seniors and their caregivers can use this website to learn more about chronic diseases, lifestyle modifications, disease prevention, and mental health.
In times of a pandemic, this website provides consistent and accurate information and education. One recommendation for reaching the elderly population during future outbreaks is for NIA to develop and implement strategies to increase the use of the website, including adding more audio and visual interfaces and developing a mobile app. Other recommendations for improving the quality of care for seniors include the following:
1. Identify which populations may be most affected when future outbreaks occur.
2. Consider nontraditional platforms, including social media, for communicating with the general population and for medical providers worldwide to learn from each other about new diseases, including the signs, symptoms, and treatment plans. Some medical professionals created specific WhatsApp groups to communicate, and the World Health Organization sent updated information about COVID-19 to anyone who texted them via WhatsApp.3
3. Create a checklist of signs and symptoms related to current infectious diseases and assess every vulnerable patient.
4. Share these guidelines with medical facilities that treat these populations, such as senior care, assisted living and rehabilitation facilities, hospitals, and outpatient treatment centers. Teach the staff at these medical facilities how to screen patients for signs and symptoms of the disease.
5. Implement social isolation strategies, travel and visitor restrictions, and testing and screening as soon as possible at these medical facilities.
6. Recognize that these strategies may affect the psychological and emotional well-being of seniors, increasing their risk for depression and anxiety and negatively affecting their immunity and mental health. Additionally, the use of PPE, either by the medical providers or the patient, may cause anxiety in seniors and those with mild cognitive impairment.
7. Encourage these medical facilities to improve coping strategies with older patients, such as incorporating communication technology that helps seniors stay connected with their families, and participating in physical and mental exercise, as well as religious activities.
8. Ask these medical facilities to create isolation or quarantine rooms for infected seniors.
9. Work with family members to proactively report to medical professionals any symptoms noticed in their senior relatives. Educate seniors to report symptoms earlier.
10. Offer incentives for medical professionals to conduct on-site testing in primary care offices or senior care facilities instead of sending patients to hospital emergency rooms for evaluation. This will only be effective if there are enough test kits available.
11. Urge insurance companies and Medicare to allow additional medical visits for screening vulnerable populations. Encourage the use of telemedicine in place of in-office visits (preferably billed at the same rate as an in-office visit) where appropriate, especially with nonambulatory patients or those with transportation issues. Many insurance companies, including Medicare, approved COVID-19–related coverage of telemedicine in place of office visits to limit the spread of the disease.
12. Provide community health care and integration and better coordination of local, state, and national health care.
13. Hold regular epidemic and pandemic preparedness exercises in every hospital, nursing home, and assisted living facility.
Proactive health care outreach
It is easier to identify the signs and symptoms of already identified infectious diseases as opposed to a novel one like COVID-19. The United States faced a steep learning curve with COVID-19. Hospitalists and other medical professionals were not able to learn about COVID-19 in a journal. At first, they did not know how to screen patients coming into the ER, how to protect staff, or what the treatment plan was for this new disease. As a result, the medical system experienced disorder and confusion. Investing in community health care and better coordination of local, state, and national health care resources is a priority.
The senior citizen population appears to be most vulnerable to this virus and may be just as vulnerable in future outbreaks. Yet the insights gained from this pandemic can lead to a more comprehensive outreach to senior patients and increased screenings for comorbidities and future contagious diseases. An emphasis on proactive health care and outreach for seniors, with a focus on identifying and treating comorbid conditions, improves the medical care system overall and may prevent or slow future community outbreaks.
Dr. Kasarla is a hospitalist with APOGEE Physicians at Wise Surgical at Parkway in Fort Worth, Tex. He did his internal medicine residency at Mercy Hospital & Medical Center, Chicago. Readers can contact him at [email protected]. Dr. Devireddy is a family physician at Positive Health Medical Center, Kingston, Jamaica. Contact him at [email protected].
References
1. Pisano GP et al. Lessons from Italy’s response to coronavirus. Harvard Business Review. 2020 Mar 27. https://hbr.org/2020/03/lessons-from-italys-response-to-coronavirus.
2. Tu C. Lessons from Taiwan’s experience with COVID-19. New Atlanticist. 2020 Apr 7. https://atlanticcouncil.org/blogs/new-atlanticist/lessons-from-taiwans-experience-with-covid-19/.
3. Newman LH. WhatsApp is at the center of coronavirus response. WIRED. 2020 Mar 20. https://www.wired.com/story/whatsapp-coronavirus-who-information-app/.
Across the globe, there are marked differences in how countries responded to the COVID-19 outbreak, with varying degrees of success in limiting the spread of the virus. Some countries learned important lessons from previous outbreaks, including SARS and MERS, and put policies in place that contributed to lower infection and death rates from COVID-19 in these countries. Others struggled to respond appropriately to the outbreak.
The United States and most of the world was not affected significantly by SARS and MERS. Hence there is a need for different perspectives and observations on lessons that can be learned from this outbreak to help develop effective strategies and policies for the future. It also makes sense to focus intently on the demographic most affected by COVID-19 – the elderly.
Medical care, for the most part, is governed by protocols that clearly detail processes to be followed for the prevention and treatment of disease. Caring for older patients requires going above and beyond the protocols. That is one of the lessons learned from the COVID-19 pandemic – a wake-up call for a more proactive approach for at-risk patients, in this case everyone over the age of 60 years.
In this context, it is important for medical outreach to continue with the senior population long after the pandemic has run its course. Many seniors, particularly those susceptible to other illnesses or exhibiting ongoing issues, would benefit from a consistent and preplanned pattern of contacts by medical professionals and agencies that work with the aging population. These proactive follow-ups can facilitate prevention and treatment and, at the same time, reduce costs that would otherwise increase when health care is reactive.
Lessons in infectious disease containment
As COVID-19 spread globally, there were contrasting responses from individual countries in their efforts to contain the disease. Unfortunately, Italy suffered from its decision to lock down only specific regions of the country initially. The leadership in Italy may have ignored the advice of medical experts and been caught off guard by the intensity of the spread of COVID-19. In fact, they might not have taken strict actions right away because they did not want their responses to be viewed as an overreaction to the disease.
The government decided to shut down areas where the infection rates were high (“red zones”) rather than implement restrictions nationally. This may have inadvertently increased the spread as Italians vacated those “red zones” for other areas of the country not yet affected by COVID-19. Italy’s decentralized health care system also played a part in the effects of the disease, with some regions demonstrating more success in slowing the reach of the disease. According to an article in the Harvard Business Review, the neighboring regions of Lombardy and Veneto applied similar approaches to social distancing and retail closures. Veneto was more proactive, and its response to the outbreak was multipronged, including putting a “strong emphasis on home diagnosis and care” and “specific efforts to monitor and protect health care and other essential workers.” These measures most likely contributed to a slowdown of the spread of the disease in Veneto’s health care facilities, which lessened the load on medical providers.1
Conversely, Taiwan implemented proactive measures swiftly after learning about COVID-19. Taiwan was impacted adversely by the SARS outbreak in 2003 and, afterward, revised their medical policies and procedures to respond quickly to future infectious disease crises. In the beginning, little was known about COVID-19 or how it spread. However, Taiwan’s swift public health response to COVID-19 included early travel restrictions, patient screening, and quarantining of symptomatic patients. The government emphasized education and created real-time digital updates and alerts sent to their citizens, as well as partnering with media to broadcast crucial proactive health information and quickly disproving false information related to COVID-19. They coordinated with organizations throughout the country to increase supplies of personal protective equipment (PPE).2
Although countries and even cities within a country differ in terms of population demographics, health resources, government policies, and cultural practices, initial success stories have some similarities, including the following:
- Early travel restrictions from countries with positive cases, with some circumstances requiring compulsory quarantine periods and testing before entry.
- Extensive testing and proactive tracing of symptomatic cases early. Contacts of people testing positive were also tested, irrespective of being symptomatic or asymptomatic. If testing kits were unavailable, the contacts were self-quarantined.
- Emphasis on avoiding overburdening hospitals by having the public health infrastructure to divert people exhibiting symptoms, including using public health hotlines to send patients to dedicated testing sites and drive-through testing, rather than have patients presenting to emergency rooms and hospitals. This approach protected medical staff from exposure and allowed the focus to remain on treating severe symptomatic patients.
The vastly different response to the COVID-19 outbreak in these two countries illuminates the need for better preparation in the United States. At the onset of this outbreak, emergency room medical professionals, hospitalists, and outpatient primary care providers did not know how to screen for or treat this virus. Additionally, there was limited information on the most effective contact protocols for medical professionals, patients, and visitors. Finally, the lack of PPE and COVID-19 test kits hindered the U.S. response. Once the country is on the road to recovery from COVID-19, it is imperative to set the groundwork to prepare for future outbreaks and create mechanisms to quickly identify vulnerable populations when outbreaks occur.
Senior care in future infectious disease outbreaks
How can medical providers translate lessons learned from this outbreak into improving the quality of care for seniors? The National Institute on Aging (NIA) maintains a website with information about healthy aging. Seniors and their caregivers can use this website to learn more about chronic diseases, lifestyle modifications, disease prevention, and mental health.
In times of a pandemic, this website provides consistent and accurate information and education. One recommendation for reaching the elderly population during future outbreaks is for NIA to develop and implement strategies to increase the use of the website, including adding more audio and visual interfaces and developing a mobile app. Other recommendations for improving the quality of care for seniors include the following:
1. Identify which populations may be most affected when future outbreaks occur.
2. Consider nontraditional platforms, including social media, for communicating with the general population and for medical providers worldwide to learn from each other about new diseases, including the signs, symptoms, and treatment plans. Some medical professionals created specific WhatsApp groups to communicate, and the World Health Organization sent updated information about COVID-19 to anyone who texted them via WhatsApp.3
3. Create a checklist of signs and symptoms related to current infectious diseases and assess every vulnerable patient.
4. Share these guidelines with medical facilities that treat these populations, such as senior care, assisted living and rehabilitation facilities, hospitals, and outpatient treatment centers. Teach the staff at these medical facilities how to screen patients for signs and symptoms of the disease.
5. Implement social isolation strategies, travel and visitor restrictions, and testing and screening as soon as possible at these medical facilities.
6. Recognize that these strategies may affect the psychological and emotional well-being of seniors, increasing their risk for depression and anxiety and negatively affecting their immunity and mental health. Additionally, the use of PPE, either by the medical providers or the patient, may cause anxiety in seniors and those with mild cognitive impairment.
7. Encourage these medical facilities to improve coping strategies with older patients, such as incorporating communication technology that helps seniors stay connected with their families, and participating in physical and mental exercise, as well as religious activities.
8. Ask these medical facilities to create isolation or quarantine rooms for infected seniors.
9. Work with family members to proactively report to medical professionals any symptoms noticed in their senior relatives. Educate seniors to report symptoms earlier.
10. Offer incentives for medical professionals to conduct on-site testing in primary care offices or senior care facilities instead of sending patients to hospital emergency rooms for evaluation. This will only be effective if there are enough test kits available.
11. Urge insurance companies and Medicare to allow additional medical visits for screening vulnerable populations. Encourage the use of telemedicine in place of in-office visits (preferably billed at the same rate as an in-office visit) where appropriate, especially with nonambulatory patients or those with transportation issues. Many insurance companies, including Medicare, approved COVID-19–related coverage of telemedicine in place of office visits to limit the spread of the disease.
12. Provide community health care and integration and better coordination of local, state, and national health care.
13. Hold regular epidemic and pandemic preparedness exercises in every hospital, nursing home, and assisted living facility.
Proactive health care outreach
It is easier to identify the signs and symptoms of already identified infectious diseases as opposed to a novel one like COVID-19. The United States faced a steep learning curve with COVID-19. Hospitalists and other medical professionals were not able to learn about COVID-19 in a journal. At first, they did not know how to screen patients coming into the ER, how to protect staff, or what the treatment plan was for this new disease. As a result, the medical system experienced disorder and confusion. Investing in community health care and better coordination of local, state, and national health care resources is a priority.
The senior citizen population appears to be most vulnerable to this virus and may be just as vulnerable in future outbreaks. Yet the insights gained from this pandemic can lead to a more comprehensive outreach to senior patients and increased screenings for comorbidities and future contagious diseases. An emphasis on proactive health care and outreach for seniors, with a focus on identifying and treating comorbid conditions, improves the medical care system overall and may prevent or slow future community outbreaks.
Dr. Kasarla is a hospitalist with APOGEE Physicians at Wise Surgical at Parkway in Fort Worth, Tex. He did his internal medicine residency at Mercy Hospital & Medical Center, Chicago. Readers can contact him at [email protected]. Dr. Devireddy is a family physician at Positive Health Medical Center, Kingston, Jamaica. Contact him at [email protected].
References
1. Pisano GP et al. Lessons from Italy’s response to coronavirus. Harvard Business Review. 2020 Mar 27. https://hbr.org/2020/03/lessons-from-italys-response-to-coronavirus.
2. Tu C. Lessons from Taiwan’s experience with COVID-19. New Atlanticist. 2020 Apr 7. https://atlanticcouncil.org/blogs/new-atlanticist/lessons-from-taiwans-experience-with-covid-19/.
3. Newman LH. WhatsApp is at the center of coronavirus response. WIRED. 2020 Mar 20. https://www.wired.com/story/whatsapp-coronavirus-who-information-app/.
Dairy doesn’t do a body good in midlife women
Dairy consumption does not improve bone mineral density (BMD) or reduce the risk of osteoporotic fracture in women starting menopause, a new analysis of the Study of Women’s Health Across the Nation (SWAN) indicates.
And this was regardless of baseline menopausal status, say Taylor Wallace, PhD, of George Mason University, Fairfax, Va., and colleagues in their article published online in Menopause.
“Our previous work indicated a potential premenopausal critical window in regard to the effectiveness of calcium supplements,” they noted.
Clifford Rosen, MD, professor of medicine, Tufts University, Boston, said in an interview that he believes the study reinforces earlier work that dairy intake in women aged 45-55 years does not affect the rate of bone loss or fractures.
“The SWAN study is longitudinal and with sufficient numbers to support their conclusion,” Dr. Rosen said.
SWAN study: White women consume the most dairy
As dairy is known to be one of the foremost sources of calcium, along with other bone beneficial nutrients, Dr. Wallace and colleagues decided to examine intake of this food type with long-term bone health using the SWAN data.
The SWAN bone substudy started in 1996 and involved 3,302 pre- or early perimenopausal women aged 42-53 years. The sample size for the annualized rate of BMD loss and fracture analysis involved 1955 women.
A modified food frequency questionnaire was used at baseline, at visit 5, and again at visit 9 to record daily dairy consumption, among many other food items.
“Women were classified into four dairy groups based on this cumulative average dairy intake,” Wallace and colleagues note. Intake was grouped into < 0.5 servings/day; 0.5 to < 1.5 servings/day; 1.5 to < 2.5 servings/day, and ≥ 2.5 servings/day.
“Non-Hispanic white individuals were more likely to consume higher amounts of dairy compared to African American, Chinese, and Japanese individuals,” the authors noted.
They found no significant differences for baseline age, body mass index, femoral neck and lumbar spine BMD, calcium supplement use, or fracture history by dairy intake group.
There were also no differences in the hazard ratios or relative risk of nontraumatic fractures by frequency of daily dairy intake.
Findings on dairy and bone are inconsistent
The authors caution that several factors should be taken into account when considering these new findings.
“First, dairy intake was low [overall] among SWAN participants, with 65% reporting consumption of < 1.5 servings/day,” they point out.
Dairy intake was also “particularly low” among racial groups other than whites, which may be due to higher rates of lactose intolerance among ethnic minorities, they speculate.
They previously reported that the use of calcium dietary supplements in SWAN was associated with a lower annualized rate of femoral neck BMD loss as well as BMD loss at the lumbar spine over 10 years of follow-up, mainly in women who were premenopausal at baseline.
But no associations were observed in the risk of bone fracture in any women in that analysis, regardless of menopausal status.
In this new analysis, there were no significant differences in calcium supplement use across the dairy intake groups.
Dr. Wallace and colleagues also noted that the relevance of dairy product intake for bone health has been in question as some observational studies have even “suggested consumption to be associated with an increased risk of fractures.”
The lead author of one of these studies, Karl Michaelsson, MD, PhD, of Uppsala (Sweden) University, said in an interview that his study had looked only at milk intake, and the lack of benefit on bone health from high milk consumption may not apply to all dairy products.
We “may need to look at different types of dairy products,” he said.
Summing up, Stephanie Faubion, MD, MBA, medical director of the North American Menopause Society, said the new SWAN findings do add to the evidence base, “albeit inconsistent ... suggesting a lack of benefit from dairy intake on BMD and fracture risk.”
Vitamin D data were not available; dairy may help in this respect
Dr. Rosen also noted that no information was available on vitamin D levels in patients involved in SWAN, which he believes is a limitation of the study.
Nevertheless, “it is important to recognize that elderly individuals who increase their dairy intake may have health benefits as recognized in the Nurses’ Health Study, possibly due to increased protein intake, higher vitamin D levels, or greater calcium intake,” he observed.
A randomized trial of enhanced dairy intake in long-term care residents is currently underway, which should provide answers for a much more vulnerable population than those in the SWAN cohort, Dr. Rosen concluded.
Dr. Wallace has reported serving on the scientific advisory board of the Vitamin Shoppe and has received research support from the National Dairy Council and scientific consulting fees from several food companies. Dr. Rosen has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Dairy consumption does not improve bone mineral density (BMD) or reduce the risk of osteoporotic fracture in women starting menopause, a new analysis of the Study of Women’s Health Across the Nation (SWAN) indicates.
And this was regardless of baseline menopausal status, say Taylor Wallace, PhD, of George Mason University, Fairfax, Va., and colleagues in their article published online in Menopause.
“Our previous work indicated a potential premenopausal critical window in regard to the effectiveness of calcium supplements,” they noted.
Clifford Rosen, MD, professor of medicine, Tufts University, Boston, said in an interview that he believes the study reinforces earlier work that dairy intake in women aged 45-55 years does not affect the rate of bone loss or fractures.
“The SWAN study is longitudinal and with sufficient numbers to support their conclusion,” Dr. Rosen said.
SWAN study: White women consume the most dairy
As dairy is known to be one of the foremost sources of calcium, along with other bone beneficial nutrients, Dr. Wallace and colleagues decided to examine intake of this food type with long-term bone health using the SWAN data.
The SWAN bone substudy started in 1996 and involved 3,302 pre- or early perimenopausal women aged 42-53 years. The sample size for the annualized rate of BMD loss and fracture analysis involved 1955 women.
A modified food frequency questionnaire was used at baseline, at visit 5, and again at visit 9 to record daily dairy consumption, among many other food items.
“Women were classified into four dairy groups based on this cumulative average dairy intake,” Wallace and colleagues note. Intake was grouped into < 0.5 servings/day; 0.5 to < 1.5 servings/day; 1.5 to < 2.5 servings/day, and ≥ 2.5 servings/day.
“Non-Hispanic white individuals were more likely to consume higher amounts of dairy compared to African American, Chinese, and Japanese individuals,” the authors noted.
They found no significant differences for baseline age, body mass index, femoral neck and lumbar spine BMD, calcium supplement use, or fracture history by dairy intake group.
There were also no differences in the hazard ratios or relative risk of nontraumatic fractures by frequency of daily dairy intake.
Findings on dairy and bone are inconsistent
The authors caution that several factors should be taken into account when considering these new findings.
“First, dairy intake was low [overall] among SWAN participants, with 65% reporting consumption of < 1.5 servings/day,” they point out.
Dairy intake was also “particularly low” among racial groups other than whites, which may be due to higher rates of lactose intolerance among ethnic minorities, they speculate.
They previously reported that the use of calcium dietary supplements in SWAN was associated with a lower annualized rate of femoral neck BMD loss as well as BMD loss at the lumbar spine over 10 years of follow-up, mainly in women who were premenopausal at baseline.
But no associations were observed in the risk of bone fracture in any women in that analysis, regardless of menopausal status.
In this new analysis, there were no significant differences in calcium supplement use across the dairy intake groups.
Dr. Wallace and colleagues also noted that the relevance of dairy product intake for bone health has been in question as some observational studies have even “suggested consumption to be associated with an increased risk of fractures.”
The lead author of one of these studies, Karl Michaelsson, MD, PhD, of Uppsala (Sweden) University, said in an interview that his study had looked only at milk intake, and the lack of benefit on bone health from high milk consumption may not apply to all dairy products.
We “may need to look at different types of dairy products,” he said.
Summing up, Stephanie Faubion, MD, MBA, medical director of the North American Menopause Society, said the new SWAN findings do add to the evidence base, “albeit inconsistent ... suggesting a lack of benefit from dairy intake on BMD and fracture risk.”
Vitamin D data were not available; dairy may help in this respect
Dr. Rosen also noted that no information was available on vitamin D levels in patients involved in SWAN, which he believes is a limitation of the study.
Nevertheless, “it is important to recognize that elderly individuals who increase their dairy intake may have health benefits as recognized in the Nurses’ Health Study, possibly due to increased protein intake, higher vitamin D levels, or greater calcium intake,” he observed.
A randomized trial of enhanced dairy intake in long-term care residents is currently underway, which should provide answers for a much more vulnerable population than those in the SWAN cohort, Dr. Rosen concluded.
Dr. Wallace has reported serving on the scientific advisory board of the Vitamin Shoppe and has received research support from the National Dairy Council and scientific consulting fees from several food companies. Dr. Rosen has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Dairy consumption does not improve bone mineral density (BMD) or reduce the risk of osteoporotic fracture in women starting menopause, a new analysis of the Study of Women’s Health Across the Nation (SWAN) indicates.
And this was regardless of baseline menopausal status, say Taylor Wallace, PhD, of George Mason University, Fairfax, Va., and colleagues in their article published online in Menopause.
“Our previous work indicated a potential premenopausal critical window in regard to the effectiveness of calcium supplements,” they noted.
Clifford Rosen, MD, professor of medicine, Tufts University, Boston, said in an interview that he believes the study reinforces earlier work that dairy intake in women aged 45-55 years does not affect the rate of bone loss or fractures.
“The SWAN study is longitudinal and with sufficient numbers to support their conclusion,” Dr. Rosen said.
SWAN study: White women consume the most dairy
As dairy is known to be one of the foremost sources of calcium, along with other bone beneficial nutrients, Dr. Wallace and colleagues decided to examine intake of this food type with long-term bone health using the SWAN data.
The SWAN bone substudy started in 1996 and involved 3,302 pre- or early perimenopausal women aged 42-53 years. The sample size for the annualized rate of BMD loss and fracture analysis involved 1955 women.
A modified food frequency questionnaire was used at baseline, at visit 5, and again at visit 9 to record daily dairy consumption, among many other food items.
“Women were classified into four dairy groups based on this cumulative average dairy intake,” Wallace and colleagues note. Intake was grouped into < 0.5 servings/day; 0.5 to < 1.5 servings/day; 1.5 to < 2.5 servings/day, and ≥ 2.5 servings/day.
“Non-Hispanic white individuals were more likely to consume higher amounts of dairy compared to African American, Chinese, and Japanese individuals,” the authors noted.
They found no significant differences for baseline age, body mass index, femoral neck and lumbar spine BMD, calcium supplement use, or fracture history by dairy intake group.
There were also no differences in the hazard ratios or relative risk of nontraumatic fractures by frequency of daily dairy intake.
Findings on dairy and bone are inconsistent
The authors caution that several factors should be taken into account when considering these new findings.
“First, dairy intake was low [overall] among SWAN participants, with 65% reporting consumption of < 1.5 servings/day,” they point out.
Dairy intake was also “particularly low” among racial groups other than whites, which may be due to higher rates of lactose intolerance among ethnic minorities, they speculate.
They previously reported that the use of calcium dietary supplements in SWAN was associated with a lower annualized rate of femoral neck BMD loss as well as BMD loss at the lumbar spine over 10 years of follow-up, mainly in women who were premenopausal at baseline.
But no associations were observed in the risk of bone fracture in any women in that analysis, regardless of menopausal status.
In this new analysis, there were no significant differences in calcium supplement use across the dairy intake groups.
Dr. Wallace and colleagues also noted that the relevance of dairy product intake for bone health has been in question as some observational studies have even “suggested consumption to be associated with an increased risk of fractures.”
The lead author of one of these studies, Karl Michaelsson, MD, PhD, of Uppsala (Sweden) University, said in an interview that his study had looked only at milk intake, and the lack of benefit on bone health from high milk consumption may not apply to all dairy products.
We “may need to look at different types of dairy products,” he said.
Summing up, Stephanie Faubion, MD, MBA, medical director of the North American Menopause Society, said the new SWAN findings do add to the evidence base, “albeit inconsistent ... suggesting a lack of benefit from dairy intake on BMD and fracture risk.”
Vitamin D data were not available; dairy may help in this respect
Dr. Rosen also noted that no information was available on vitamin D levels in patients involved in SWAN, which he believes is a limitation of the study.
Nevertheless, “it is important to recognize that elderly individuals who increase their dairy intake may have health benefits as recognized in the Nurses’ Health Study, possibly due to increased protein intake, higher vitamin D levels, or greater calcium intake,” he observed.
A randomized trial of enhanced dairy intake in long-term care residents is currently underway, which should provide answers for a much more vulnerable population than those in the SWAN cohort, Dr. Rosen concluded.
Dr. Wallace has reported serving on the scientific advisory board of the Vitamin Shoppe and has received research support from the National Dairy Council and scientific consulting fees from several food companies. Dr. Rosen has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
How old is too old for statins?
ILLUSTRATIVE CASE
Ms. M is a 76-year-old woman with well-controlled type 2 diabetes mellitus for 10 years and well-controlled mild hypertension. She is otherwise healthy, and her mother lived to age 95. Ms. M has never smoked, has no previous history of vascular/cardiovascular disease, and drinks 1 glass of wine 2 to 3 times per week. Based on the American College of Cardiology (ACC) calculator, she was started on atorvastatin years ago. Is continued use of the medication of any benefit at her current age?
The 2018 American Heart Association (AHA)/ACC/Multi-Society cholesterol guidelines do not provide primary prevention recommendations for those older than age 75 years.3 Up to age 75, the guidelines recommend that patients with type 2 diabetes and a low-density lipoprotein cholesterol (LDL-C) level ≥ 70 mg/dL, as well as those without diabetes but with an LDL-C ≥ 70 mg/dL and a 10-year atherosclerotic cardiovascular disease (ASCVD) risk ≥ 10%, be started on medium-intensity statin therapy.
A 2018 consensus panel review of the current literature, sponsored by the National Institute on Aging and the National Heart, Lung, and Blood Institute, concluded that there was insufficient evidence regarding the benefits and harms of statins in older adults, especially those with comorbidities, and that there was a paucity of evidence about statin therapy outcomes (both adverse and beneficial) relevant to older adults.4
A review of all guidelines published since 2013 revealed that only the United Kingdom’s 2014 National Institute for Health and Care Excellence (NICE) guideline provides a strong, risk-based recommendation for initiating primary prevention with statins in patients > 75 years old.5 These recommendations are based on the QRISK2 calculator (which has since been updated to the QRISK3), which assigns everyone ages > 75 years a > 10% 10-year risk score. This provides a universal statin indication for anyone in the 76-to-84 age range.6
Both the ACC/AHA and US Preventive Services Task Force guidelines clearly state that there are too few data and inadequate evidence in people older than 75 for a strong, risk-based statin recommendation.5 The Canadian Cardiovascular Society guideline takes a similar stance, emphasizing that the recommended Framingham risk model is not well validated in people > 75 years.5
STUDY SUMMARIES
Two different looks at statin use in the elderly
A retrospective cohort study (N = 46,864; median follow-up, 5.6 years) examined whether statin treatment is associated with a reduction in atherosclerotic disease and mortality in old and very old adults with and without type 2 diabetes.1 Patients were enrolled from a large, anonymized national database in Spain. The researchers looked only at first-time users of statins and those without a statin prescription within the past 18 months.
Patients with previous ASCVD, type 1 diabetes, previous lipid-lowering treatment, dementia, cancer, or paralysis were excluded, as were those who were in residential care, were on dialysis, or had received an organ transplant. Patients were stratified by age (75-84 years and ≥ 85 years), diabetes status (with or without type 2 diabetes), and statin use (nonuser or new user).
Continue to: Results
Results. For patients with type 2 diabetes, the risk of ASCVD (a composite of coronary heart disease and stroke) was lower among those who took statins than among those who did not in the 75-to-84 group (hazard ratio [HR] = 0.76; 95% confidence interval [CI], 0.65-0.89; 1-year number needed to treat [NNT] = 164). Among those who took statins, there was also lower all-cause mortality (HR = 0.84; 95% CI, 0.75-0.94; 1-year NNT = 306). In those ages ≥ 85 years with diabetes, the statin group did not have a lower risk of ASCVD (HR = 0.82; 95% CI, 0.53-1.26) or all-cause mortality (HR = 1.05; 95% CI, 0.86-1.28).
For patients ages 75 to 84 years without diabetes, there was no difference in risk between groups for ASCVD (HR = 0.94; 95% CI, 0.86–1.04) or all-cause mortality (HR = 0.98; 95% CI, 0.91-1.05). In those ages ≥ 85 years without diabetes, there was also no difference between groups for ASCVD (HR = 1; 95% CI, 0.80-1.24) or for all-cause mortality (HR = 1; 95% CI, 0.90-1.11).
A 2019 meta-analysis of randomized controlled trials (RCTs) (n = 134,537) and RCT summary data (n = 12,705) evaluated the safety and efficacy of statin therapy in patients ages ≥ 55 years.2 In the group of patients ages > 75 years (n = 14,483; median follow-up, 4.9 years), each 1 mmol/L reduction in LDL-C was associated with significant decreased risk for major vascular events (risk ratio [RR] = 0.82; 95% CI, 0.70-0.95) and for major coronary events (RR = 0.82; 95% CI, 0.70-0.96).
In subgroup analysis by the presence or absence of previous vascular disease, there was a decreased risk per 1 mmol/L LDL-C reduction of major vascular events in patients with previous vascular disease (RR = 0.85; 95% CI, 0.73-0.98); however, there was not a significant effect in patients without previous vascular disease (RR = 0.92; 95% CI, 0.73-1.16).
WHAT’S NEW
Statins may be unnecessary in older adults without ASCVD or T2DM
Statin therapy reduces the risk of ASCVD and mortality in patients ages 75 to 84 with type 2 diabetes and in patients > 75 years with known vascular disease. However, statin therapy seems to provide no benefit in patients ages > 75 years without ASCVD or in patients ages ≥ 85 years without ASCVD, regardless of type 2 diabetes status.
Continue to: CAVEATS
CAVEATS
Retrospective cohort design leaves cause and effect equivocal
Even though the first study was large (with more than 46,000 patients) and the median follow-up was 5.6 years, it was a retrospective cohort study. While there is clearly an association between statin therapy and reduced ASCVD and all-cause mortality in patients with diabetes ages 75 to 84 years, cause and effect cannot be unequivocally stated. However, the meta-analysis, which included RCTs, confirms the benefit of statins in secondary prevention for older patients.
The cohort study did not look at adverse effects from statin therapy in this age group, but the data from the 2019 meta-analysis did not reveal any significant risk of myopathy.
CHALLENGES TO IMPLEMENTATION
Guidelines are lacking and discontinuing meds requires discussion
The lack of supporting guidelines to treat this age group with statins remains the largest barrier to implementation. Many patients may already be taking a statin, so a discussion about discontinuing medication will need to be initiated.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ramos R, Comas-Cufi M, Marti-Lluch R, et al. Statins for primary prevention of cardiovascular events and mortality in old and very old adults with and without type 2 diabetes: retrospective cohort study. BMJ. 2018;362:k3359.
2. Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomized controlled trials. Lancet. 2019;393:407-415.
3. Stone NJ, Grundy SM. The 2018 AHA/ACC/Multi-Society cholesterol guidelines: looking at past, present and future. Prog Cardiovasc Dis. 2019;62:375-383.
4. Singh S, Zieman S, Go AS, et al. Statins for primary prevention in older adults—moving towards evidence-based decision-making. J Am Geriatr Soc. 2018;66:2188-2196.
5. Mortensen MB, Falk E. Primary prevention with statins in the elderly. J Am Coll Cardiol. 2018;71:85-94.
6. ClinRisk. Welcome to the QRISK®3-2018 risk calculator. www.qrisk.org/three/. Accessed May 27, 2020.
ILLUSTRATIVE CASE
Ms. M is a 76-year-old woman with well-controlled type 2 diabetes mellitus for 10 years and well-controlled mild hypertension. She is otherwise healthy, and her mother lived to age 95. Ms. M has never smoked, has no previous history of vascular/cardiovascular disease, and drinks 1 glass of wine 2 to 3 times per week. Based on the American College of Cardiology (ACC) calculator, she was started on atorvastatin years ago. Is continued use of the medication of any benefit at her current age?
The 2018 American Heart Association (AHA)/ACC/Multi-Society cholesterol guidelines do not provide primary prevention recommendations for those older than age 75 years.3 Up to age 75, the guidelines recommend that patients with type 2 diabetes and a low-density lipoprotein cholesterol (LDL-C) level ≥ 70 mg/dL, as well as those without diabetes but with an LDL-C ≥ 70 mg/dL and a 10-year atherosclerotic cardiovascular disease (ASCVD) risk ≥ 10%, be started on medium-intensity statin therapy.
A 2018 consensus panel review of the current literature, sponsored by the National Institute on Aging and the National Heart, Lung, and Blood Institute, concluded that there was insufficient evidence regarding the benefits and harms of statins in older adults, especially those with comorbidities, and that there was a paucity of evidence about statin therapy outcomes (both adverse and beneficial) relevant to older adults.4
A review of all guidelines published since 2013 revealed that only the United Kingdom’s 2014 National Institute for Health and Care Excellence (NICE) guideline provides a strong, risk-based recommendation for initiating primary prevention with statins in patients > 75 years old.5 These recommendations are based on the QRISK2 calculator (which has since been updated to the QRISK3), which assigns everyone ages > 75 years a > 10% 10-year risk score. This provides a universal statin indication for anyone in the 76-to-84 age range.6
Both the ACC/AHA and US Preventive Services Task Force guidelines clearly state that there are too few data and inadequate evidence in people older than 75 for a strong, risk-based statin recommendation.5 The Canadian Cardiovascular Society guideline takes a similar stance, emphasizing that the recommended Framingham risk model is not well validated in people > 75 years.5
STUDY SUMMARIES
Two different looks at statin use in the elderly
A retrospective cohort study (N = 46,864; median follow-up, 5.6 years) examined whether statin treatment is associated with a reduction in atherosclerotic disease and mortality in old and very old adults with and without type 2 diabetes.1 Patients were enrolled from a large, anonymized national database in Spain. The researchers looked only at first-time users of statins and those without a statin prescription within the past 18 months.
Patients with previous ASCVD, type 1 diabetes, previous lipid-lowering treatment, dementia, cancer, or paralysis were excluded, as were those who were in residential care, were on dialysis, or had received an organ transplant. Patients were stratified by age (75-84 years and ≥ 85 years), diabetes status (with or without type 2 diabetes), and statin use (nonuser or new user).
Continue to: Results
Results. For patients with type 2 diabetes, the risk of ASCVD (a composite of coronary heart disease and stroke) was lower among those who took statins than among those who did not in the 75-to-84 group (hazard ratio [HR] = 0.76; 95% confidence interval [CI], 0.65-0.89; 1-year number needed to treat [NNT] = 164). Among those who took statins, there was also lower all-cause mortality (HR = 0.84; 95% CI, 0.75-0.94; 1-year NNT = 306). In those ages ≥ 85 years with diabetes, the statin group did not have a lower risk of ASCVD (HR = 0.82; 95% CI, 0.53-1.26) or all-cause mortality (HR = 1.05; 95% CI, 0.86-1.28).
For patients ages 75 to 84 years without diabetes, there was no difference in risk between groups for ASCVD (HR = 0.94; 95% CI, 0.86–1.04) or all-cause mortality (HR = 0.98; 95% CI, 0.91-1.05). In those ages ≥ 85 years without diabetes, there was also no difference between groups for ASCVD (HR = 1; 95% CI, 0.80-1.24) or for all-cause mortality (HR = 1; 95% CI, 0.90-1.11).
A 2019 meta-analysis of randomized controlled trials (RCTs) (n = 134,537) and RCT summary data (n = 12,705) evaluated the safety and efficacy of statin therapy in patients ages ≥ 55 years.2 In the group of patients ages > 75 years (n = 14,483; median follow-up, 4.9 years), each 1 mmol/L reduction in LDL-C was associated with significant decreased risk for major vascular events (risk ratio [RR] = 0.82; 95% CI, 0.70-0.95) and for major coronary events (RR = 0.82; 95% CI, 0.70-0.96).
In subgroup analysis by the presence or absence of previous vascular disease, there was a decreased risk per 1 mmol/L LDL-C reduction of major vascular events in patients with previous vascular disease (RR = 0.85; 95% CI, 0.73-0.98); however, there was not a significant effect in patients without previous vascular disease (RR = 0.92; 95% CI, 0.73-1.16).
WHAT’S NEW
Statins may be unnecessary in older adults without ASCVD or T2DM
Statin therapy reduces the risk of ASCVD and mortality in patients ages 75 to 84 with type 2 diabetes and in patients > 75 years with known vascular disease. However, statin therapy seems to provide no benefit in patients ages > 75 years without ASCVD or in patients ages ≥ 85 years without ASCVD, regardless of type 2 diabetes status.
Continue to: CAVEATS
CAVEATS
Retrospective cohort design leaves cause and effect equivocal
Even though the first study was large (with more than 46,000 patients) and the median follow-up was 5.6 years, it was a retrospective cohort study. While there is clearly an association between statin therapy and reduced ASCVD and all-cause mortality in patients with diabetes ages 75 to 84 years, cause and effect cannot be unequivocally stated. However, the meta-analysis, which included RCTs, confirms the benefit of statins in secondary prevention for older patients.
The cohort study did not look at adverse effects from statin therapy in this age group, but the data from the 2019 meta-analysis did not reveal any significant risk of myopathy.
CHALLENGES TO IMPLEMENTATION
Guidelines are lacking and discontinuing meds requires discussion
The lack of supporting guidelines to treat this age group with statins remains the largest barrier to implementation. Many patients may already be taking a statin, so a discussion about discontinuing medication will need to be initiated.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
Ms. M is a 76-year-old woman with well-controlled type 2 diabetes mellitus for 10 years and well-controlled mild hypertension. She is otherwise healthy, and her mother lived to age 95. Ms. M has never smoked, has no previous history of vascular/cardiovascular disease, and drinks 1 glass of wine 2 to 3 times per week. Based on the American College of Cardiology (ACC) calculator, she was started on atorvastatin years ago. Is continued use of the medication of any benefit at her current age?
The 2018 American Heart Association (AHA)/ACC/Multi-Society cholesterol guidelines do not provide primary prevention recommendations for those older than age 75 years.3 Up to age 75, the guidelines recommend that patients with type 2 diabetes and a low-density lipoprotein cholesterol (LDL-C) level ≥ 70 mg/dL, as well as those without diabetes but with an LDL-C ≥ 70 mg/dL and a 10-year atherosclerotic cardiovascular disease (ASCVD) risk ≥ 10%, be started on medium-intensity statin therapy.
A 2018 consensus panel review of the current literature, sponsored by the National Institute on Aging and the National Heart, Lung, and Blood Institute, concluded that there was insufficient evidence regarding the benefits and harms of statins in older adults, especially those with comorbidities, and that there was a paucity of evidence about statin therapy outcomes (both adverse and beneficial) relevant to older adults.4
A review of all guidelines published since 2013 revealed that only the United Kingdom’s 2014 National Institute for Health and Care Excellence (NICE) guideline provides a strong, risk-based recommendation for initiating primary prevention with statins in patients > 75 years old.5 These recommendations are based on the QRISK2 calculator (which has since been updated to the QRISK3), which assigns everyone ages > 75 years a > 10% 10-year risk score. This provides a universal statin indication for anyone in the 76-to-84 age range.6
Both the ACC/AHA and US Preventive Services Task Force guidelines clearly state that there are too few data and inadequate evidence in people older than 75 for a strong, risk-based statin recommendation.5 The Canadian Cardiovascular Society guideline takes a similar stance, emphasizing that the recommended Framingham risk model is not well validated in people > 75 years.5
STUDY SUMMARIES
Two different looks at statin use in the elderly
A retrospective cohort study (N = 46,864; median follow-up, 5.6 years) examined whether statin treatment is associated with a reduction in atherosclerotic disease and mortality in old and very old adults with and without type 2 diabetes.1 Patients were enrolled from a large, anonymized national database in Spain. The researchers looked only at first-time users of statins and those without a statin prescription within the past 18 months.
Patients with previous ASCVD, type 1 diabetes, previous lipid-lowering treatment, dementia, cancer, or paralysis were excluded, as were those who were in residential care, were on dialysis, or had received an organ transplant. Patients were stratified by age (75-84 years and ≥ 85 years), diabetes status (with or without type 2 diabetes), and statin use (nonuser or new user).
Continue to: Results
Results. For patients with type 2 diabetes, the risk of ASCVD (a composite of coronary heart disease and stroke) was lower among those who took statins than among those who did not in the 75-to-84 group (hazard ratio [HR] = 0.76; 95% confidence interval [CI], 0.65-0.89; 1-year number needed to treat [NNT] = 164). Among those who took statins, there was also lower all-cause mortality (HR = 0.84; 95% CI, 0.75-0.94; 1-year NNT = 306). In those ages ≥ 85 years with diabetes, the statin group did not have a lower risk of ASCVD (HR = 0.82; 95% CI, 0.53-1.26) or all-cause mortality (HR = 1.05; 95% CI, 0.86-1.28).
For patients ages 75 to 84 years without diabetes, there was no difference in risk between groups for ASCVD (HR = 0.94; 95% CI, 0.86–1.04) or all-cause mortality (HR = 0.98; 95% CI, 0.91-1.05). In those ages ≥ 85 years without diabetes, there was also no difference between groups for ASCVD (HR = 1; 95% CI, 0.80-1.24) or for all-cause mortality (HR = 1; 95% CI, 0.90-1.11).
A 2019 meta-analysis of randomized controlled trials (RCTs) (n = 134,537) and RCT summary data (n = 12,705) evaluated the safety and efficacy of statin therapy in patients ages ≥ 55 years.2 In the group of patients ages > 75 years (n = 14,483; median follow-up, 4.9 years), each 1 mmol/L reduction in LDL-C was associated with significant decreased risk for major vascular events (risk ratio [RR] = 0.82; 95% CI, 0.70-0.95) and for major coronary events (RR = 0.82; 95% CI, 0.70-0.96).
In subgroup analysis by the presence or absence of previous vascular disease, there was a decreased risk per 1 mmol/L LDL-C reduction of major vascular events in patients with previous vascular disease (RR = 0.85; 95% CI, 0.73-0.98); however, there was not a significant effect in patients without previous vascular disease (RR = 0.92; 95% CI, 0.73-1.16).
WHAT’S NEW
Statins may be unnecessary in older adults without ASCVD or T2DM
Statin therapy reduces the risk of ASCVD and mortality in patients ages 75 to 84 with type 2 diabetes and in patients > 75 years with known vascular disease. However, statin therapy seems to provide no benefit in patients ages > 75 years without ASCVD or in patients ages ≥ 85 years without ASCVD, regardless of type 2 diabetes status.
Continue to: CAVEATS
CAVEATS
Retrospective cohort design leaves cause and effect equivocal
Even though the first study was large (with more than 46,000 patients) and the median follow-up was 5.6 years, it was a retrospective cohort study. While there is clearly an association between statin therapy and reduced ASCVD and all-cause mortality in patients with diabetes ages 75 to 84 years, cause and effect cannot be unequivocally stated. However, the meta-analysis, which included RCTs, confirms the benefit of statins in secondary prevention for older patients.
The cohort study did not look at adverse effects from statin therapy in this age group, but the data from the 2019 meta-analysis did not reveal any significant risk of myopathy.
CHALLENGES TO IMPLEMENTATION
Guidelines are lacking and discontinuing meds requires discussion
The lack of supporting guidelines to treat this age group with statins remains the largest barrier to implementation. Many patients may already be taking a statin, so a discussion about discontinuing medication will need to be initiated.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ramos R, Comas-Cufi M, Marti-Lluch R, et al. Statins for primary prevention of cardiovascular events and mortality in old and very old adults with and without type 2 diabetes: retrospective cohort study. BMJ. 2018;362:k3359.
2. Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomized controlled trials. Lancet. 2019;393:407-415.
3. Stone NJ, Grundy SM. The 2018 AHA/ACC/Multi-Society cholesterol guidelines: looking at past, present and future. Prog Cardiovasc Dis. 2019;62:375-383.
4. Singh S, Zieman S, Go AS, et al. Statins for primary prevention in older adults—moving towards evidence-based decision-making. J Am Geriatr Soc. 2018;66:2188-2196.
5. Mortensen MB, Falk E. Primary prevention with statins in the elderly. J Am Coll Cardiol. 2018;71:85-94.
6. ClinRisk. Welcome to the QRISK®3-2018 risk calculator. www.qrisk.org/three/. Accessed May 27, 2020.
1. Ramos R, Comas-Cufi M, Marti-Lluch R, et al. Statins for primary prevention of cardiovascular events and mortality in old and very old adults with and without type 2 diabetes: retrospective cohort study. BMJ. 2018;362:k3359.
2. Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomized controlled trials. Lancet. 2019;393:407-415.
3. Stone NJ, Grundy SM. The 2018 AHA/ACC/Multi-Society cholesterol guidelines: looking at past, present and future. Prog Cardiovasc Dis. 2019;62:375-383.
4. Singh S, Zieman S, Go AS, et al. Statins for primary prevention in older adults—moving towards evidence-based decision-making. J Am Geriatr Soc. 2018;66:2188-2196.
5. Mortensen MB, Falk E. Primary prevention with statins in the elderly. J Am Coll Cardiol. 2018;71:85-94.
6. ClinRisk. Welcome to the QRISK®3-2018 risk calculator. www.qrisk.org/three/. Accessed May 27, 2020.
PRACTICE CHANGER
Do not start a statin in patients ages ≥ 75 years who do not have known vascular disease or type 2 diabetes; start or continue a statin in all patients ages 75 to 84 with type 2 diabetes to prevent cardiovascular events and mortality; and start or continue a statin in patients ages > 75 years who have known vascular occlusive disease.
STRENGTH OF RECOMMENDATION
B: Based on a meta-analysis of randomized controlled trials and a retrospective cohort study.
Ramos R, Comas-Cufi M, Marti-Lluch R, et al. Statins for primary prevention of cardiovascular events and mortality in old and very old adults with and without type 2 diabetes: retrospective cohort study. BMJ. 2018;362:k3359.1
Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomized controlled trials. Lancet. 2019;393:407-415.2
Acute lymphoblastic leukemia can be successfully treated in the frail elderly
A treatment schedule of very attenuated chemotherapy using standard drugs is feasible and effective in frail and elderly patients with acute lymphoblastic leukemia (ALL), according to a prospective study published in Clinical Lymphoma, Myeloma & Leukemia.
The study comprised 67 previously untreated patients with B- or T-lineage Philadelphia chromosome–negative ALL from 30 Spanish hospitals who were enrolled in the prospective, multicenter ALL-07FRAIL trial (NCT01358201) from the Spanish PETHEMA (Programa Español de Tratamientos en Hematologia) group from January 2008 to October 2019.
The median patient age in this analysis was 67 years and 51 patients (76%) were older than 70 years. The median Charlson Comorbidity Index was 5, with the main comorbidities being cardiovascular (47 patients), other neoplasia (24), diabetes (17), and very advanced age (>80 years; 12).
The attenuated treatment regimen consisted of a prephase with dexamethasone and intrathecal therapy with methotrexate was given for a maximum of 1 week. Then weekly induction therapy consisted of weekly vincristine (capped at 1 mg/week) and daily dexamethasone with a progressively decreasing dose along 4 weeks, as well as two additional doses of intrathecal methotrexate.
Those patients who achieved complete remission received maintenance therapy with mercaptopurine and methotrexate to complete 2 years of treatment. In addition, reinduction pulses with vincristine and dexamethasone were given every 3 months during the first year, according to Josep-Maria Ribera, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain and colleagues on behalf of the PETHEMA group of the Spanish Society of Hematology.
The complete remission rate was 54% (36/67 patients). The median disease-free survival and overall survival were 6.9 months and 7.6 months, respectively.
Of the 32 patients who initiated maintenance therapy, 5 patients died of infection (2), hemorrhage (2), and acute cognitive impairment (1), and 23 relapsed, with a cumulative incidence of relapse of 74% and a median time to relapse of 12.3 months.
The most frequent toxic events reported were hematologic (neutropenia 77% and thrombocytopenia 54%, of grade III-IV in all cases) followed by infections, metabolic (mainly hyperglycemia), and neurologic, according to the researchers.
“The lack of similar trials specifically directed to this frail population is one of the major strengths of this study, and we consider that this minimal chemotherapy approach could be used as a backbone for addition of immuno/targeted therapy in this subset of infirm patients,” the researchers concluded.
The study was supported by the CERCA Program/Generalitat de Catalunya and the Josep Carreras Leukemia Research Institute. The authors reported having no disclosures.
SOURCE: Ribera J-M et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 5. doi: 10.1016/j.clml.2020.03.011.
A treatment schedule of very attenuated chemotherapy using standard drugs is feasible and effective in frail and elderly patients with acute lymphoblastic leukemia (ALL), according to a prospective study published in Clinical Lymphoma, Myeloma & Leukemia.
The study comprised 67 previously untreated patients with B- or T-lineage Philadelphia chromosome–negative ALL from 30 Spanish hospitals who were enrolled in the prospective, multicenter ALL-07FRAIL trial (NCT01358201) from the Spanish PETHEMA (Programa Español de Tratamientos en Hematologia) group from January 2008 to October 2019.
The median patient age in this analysis was 67 years and 51 patients (76%) were older than 70 years. The median Charlson Comorbidity Index was 5, with the main comorbidities being cardiovascular (47 patients), other neoplasia (24), diabetes (17), and very advanced age (>80 years; 12).
The attenuated treatment regimen consisted of a prephase with dexamethasone and intrathecal therapy with methotrexate was given for a maximum of 1 week. Then weekly induction therapy consisted of weekly vincristine (capped at 1 mg/week) and daily dexamethasone with a progressively decreasing dose along 4 weeks, as well as two additional doses of intrathecal methotrexate.
Those patients who achieved complete remission received maintenance therapy with mercaptopurine and methotrexate to complete 2 years of treatment. In addition, reinduction pulses with vincristine and dexamethasone were given every 3 months during the first year, according to Josep-Maria Ribera, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain and colleagues on behalf of the PETHEMA group of the Spanish Society of Hematology.
The complete remission rate was 54% (36/67 patients). The median disease-free survival and overall survival were 6.9 months and 7.6 months, respectively.
Of the 32 patients who initiated maintenance therapy, 5 patients died of infection (2), hemorrhage (2), and acute cognitive impairment (1), and 23 relapsed, with a cumulative incidence of relapse of 74% and a median time to relapse of 12.3 months.
The most frequent toxic events reported were hematologic (neutropenia 77% and thrombocytopenia 54%, of grade III-IV in all cases) followed by infections, metabolic (mainly hyperglycemia), and neurologic, according to the researchers.
“The lack of similar trials specifically directed to this frail population is one of the major strengths of this study, and we consider that this minimal chemotherapy approach could be used as a backbone for addition of immuno/targeted therapy in this subset of infirm patients,” the researchers concluded.
The study was supported by the CERCA Program/Generalitat de Catalunya and the Josep Carreras Leukemia Research Institute. The authors reported having no disclosures.
SOURCE: Ribera J-M et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 5. doi: 10.1016/j.clml.2020.03.011.
A treatment schedule of very attenuated chemotherapy using standard drugs is feasible and effective in frail and elderly patients with acute lymphoblastic leukemia (ALL), according to a prospective study published in Clinical Lymphoma, Myeloma & Leukemia.
The study comprised 67 previously untreated patients with B- or T-lineage Philadelphia chromosome–negative ALL from 30 Spanish hospitals who were enrolled in the prospective, multicenter ALL-07FRAIL trial (NCT01358201) from the Spanish PETHEMA (Programa Español de Tratamientos en Hematologia) group from January 2008 to October 2019.
The median patient age in this analysis was 67 years and 51 patients (76%) were older than 70 years. The median Charlson Comorbidity Index was 5, with the main comorbidities being cardiovascular (47 patients), other neoplasia (24), diabetes (17), and very advanced age (>80 years; 12).
The attenuated treatment regimen consisted of a prephase with dexamethasone and intrathecal therapy with methotrexate was given for a maximum of 1 week. Then weekly induction therapy consisted of weekly vincristine (capped at 1 mg/week) and daily dexamethasone with a progressively decreasing dose along 4 weeks, as well as two additional doses of intrathecal methotrexate.
Those patients who achieved complete remission received maintenance therapy with mercaptopurine and methotrexate to complete 2 years of treatment. In addition, reinduction pulses with vincristine and dexamethasone were given every 3 months during the first year, according to Josep-Maria Ribera, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain and colleagues on behalf of the PETHEMA group of the Spanish Society of Hematology.
The complete remission rate was 54% (36/67 patients). The median disease-free survival and overall survival were 6.9 months and 7.6 months, respectively.
Of the 32 patients who initiated maintenance therapy, 5 patients died of infection (2), hemorrhage (2), and acute cognitive impairment (1), and 23 relapsed, with a cumulative incidence of relapse of 74% and a median time to relapse of 12.3 months.
The most frequent toxic events reported were hematologic (neutropenia 77% and thrombocytopenia 54%, of grade III-IV in all cases) followed by infections, metabolic (mainly hyperglycemia), and neurologic, according to the researchers.
“The lack of similar trials specifically directed to this frail population is one of the major strengths of this study, and we consider that this minimal chemotherapy approach could be used as a backbone for addition of immuno/targeted therapy in this subset of infirm patients,” the researchers concluded.
The study was supported by the CERCA Program/Generalitat de Catalunya and the Josep Carreras Leukemia Research Institute. The authors reported having no disclosures.
SOURCE: Ribera J-M et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 5. doi: 10.1016/j.clml.2020.03.011.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
COVID-19: Psychiatrists assess geriatric harm from social distancing
One of the greatest tragedies of the first wave of the COVID-19 pandemic has been the failure of health policy makers to anticipate and mitigate the enormous havoc the policy of social distancing would wreak on mental health and cognitive function in older persons, speakers agreed at a webinar on COVID-19, social distancing, and its impact on social and mental health in the elderly hosted by the International Psychogeriatric Association in collaboration with INTERDEM.
“Social distancing” is a two-edged sword: It is for now and the foreseeable future the only available effective strategy for protecting against infection in the older population most vulnerable to severe forms of COVID-19. Yet social distancing also has caused many elderly – particularly those in nursing homes and other long-term care facilities – to plunge into a profound experience of loneliness, isolation, distress, feelings of abandonment, anxiety, depression, and accelerated cognitive deterioration. And this needn’t have happened, the mental health professionals asserted.
“When are we going to get rid of the term ‘social distancing?’ ” asked IPA President William E. Reichman, MD. “Many have appreciated – including the World Health Organization – that the real issue is physical distancing to prevent contagion. And physical distancing doesn’t have to mean social distancing.”
Social connectedness between elderly persons and their peers and family members can be maintained and should be emphatically encouraged during the physical distancing required by the pandemic, said Myrra Vernooij-Dassen, PhD, of Radboud University in Nigmegen, the Netherlands, and chair of INTERDEM, a pan-European network of dementia researchers.
This can be achieved using readily available technologies, including the telephone and videoconferencing, as well as by creating opportunities for supervised masked visits between a family member and an elderly loved one in outdoor courtyards or gardens within long-term care facilities. And yet, as the pandemic seized hold in many parts of the world, family members were blocked from entry to these facilities, she observed.
Impact on mental health, cognition
Dr. Vernooij-Dassen noted that studies of previous quarantine periods as well as preliminary findings during the COVID-19 pandemic demonstrate an inverse relationship between social isolation measures and cognitive functioning in the elderly.
“ Conversely, epidemiologic data indicate that a socially integrated lifestyle had a favorable influence on cognitive functioning and could even delay onset of dementia,” she said.
INTERDEM is backing two ongoing studies evaluating the hypothesis that interventions fostering increased social interaction among elderly individuals can delay onset of dementia or favorably affect its course. The proposed mechanism of benefit is stimulation of brain plasticity to enhance cognitive reserve.
“This is a hypothesis of hope. We know that social interaction for humans is like water to plants – we really, really need it,” she explained.
Diego de Leo, MD, PhD, emeritus professor of psychiatry and former director of the Australian Institute for Suicide Research and Prevention at Griffith University in Brisbane, was living in hard-hit Padua, Italy, during the first surge of COVID-19. He described his anecdotal experience.
“What I hear from many Italian colleagues and friends and directors of mental health services is that emergency admissions related to mental disorders declined during the first wave of the COVID pandemic. For example, not many people attended emergency departments due to suicide attempts; there was a very marked decrease in the number of suicide attempts during the worst days of the pandemic,” he said.
People with psychiatric conditions were afraid to go to the hospital because they thought they would contract the infection and die there. That’s changing now, however.
“Now there is an increased number of admissions to mental health units. A new wave. It has been a U-shaped curve. And we’re now witnessing an increasing number of fatal suicides due to persistent fears, due to people imagining that there is no more room for them, and no more future for them from a financial point of view – which is the major negative outcome of this crisis. It will be a disaster for many families,” the psychiatrist continued.
A noteworthy phenomenon in northern Italy was that, when tablets were made available to nursing home residents in an effort to enhance their connectedness to the outside world, those with dementia often became so frustrated and confused by their difficulty in using the devices that they developed a hypokinetic delirium marked by refusal to eat or leave their bed, he reported.
It’s far too early to have reliable data on suicide trends in response to the pandemic, according to Dr. de Leo. But one thing is for sure: The strategy of social distancing employed to curb COVID-19 has increased the prevalence of known risk factors for suicide in older individuals, including loneliness, anxiety, and depression; increased alcohol use; and a perception of being a burden on society. Dr. de Leo directs a foundation dedicated to helping people experiencing traumatic bereavement, and in one recent week, the foundation was contacted by eight families in the province of Padua with a recent death by suicide apparently related to fallout from the COVID-19 pandemic. That’s an unusually high spike in suicide in a province with a population of 1 million.
“People probably preferred to end the agitation, the fear, the extreme anxiety about their destiny by deciding to prematurely truncate their life. That has been reported by nursing staff,” he said.
The Italian government has determined that, to date, 36% of all COVID-related deaths have occurred in people aged 85 years or older, and 84% of deaths were in individuals aged at least 70 years. And in Milan and the surrounding province of Lombardy, it’s estimated that COVID-19 has taken the lives of 25% of all nursing home residents. The North American experience has been uncomfortably similar.
“Almost 80% of COVID deaths in Canada have occurred in congregate settings,” observed Dr. Reichman, professor of psychiatry at the University of Toronto, and president and CEO of Baycrest Health Sciences, a geriatric research center.
“Certainly, the appalling number of deaths in nursing homes is the No. 1 horror of the pandemic,” declared Carmelle Peisah, MBBS, MD, a psychiatrist at the University of New South Wales in Kensington, Australia.
The fire next time
The conventional wisdom holds that COVID-19 has caused all sorts of mayhem in the delivery of elder care. Not so, in Dr. Reichman’s view.
“I would suggest that the pandemic has not caused many of the problems we talk about, it’s actually revealed problems that have always been there under the surface. For example, many older people, even before COVID-19, were socially isolated, socially distant. They had difficulty connecting with their relatives, difficulty accessing transportation to get to the store to buy food and see their doctors, and to interact with other older people,” the psychiatrist said.
“I would say as well that the pandemic didn’t cause the problems we’ve seen in long-term congregate senior care. The pandemic revealed them. We’ve had facilities where older people were severely crowded together, which compromises their quality of life, even when there’s not a pandemic. We’ve had difficulty staffing these kinds of environments with people that are paid an honest wage for the very hard work that they do. In many of these settings they’re inadequately trained, not only in infection prevention and control but in all other aspects of care. And the pandemic has revealed that many of these organizations are not properly funded. The government doesn’t support them well enough across jurisdictions, and they can’t raise enough philanthropic funds to provide the kind of quality of life that residents demand,” Dr. Reichman continued.
Could the pandemic spur improved elder care? His hope is that health care professionals, politicians, and society at large will learn from the devastation left by the first surge of the pandemic and will lobby for the resources necessary for much-needed improvements in geriatric care.
“We need to be better prepared should there be not only a second wave of this pandemic, but for other pandemics to come,” Dr. Reichman concluded.
The speakers indicated they had no financial conflicts regarding their presentations.
One of the greatest tragedies of the first wave of the COVID-19 pandemic has been the failure of health policy makers to anticipate and mitigate the enormous havoc the policy of social distancing would wreak on mental health and cognitive function in older persons, speakers agreed at a webinar on COVID-19, social distancing, and its impact on social and mental health in the elderly hosted by the International Psychogeriatric Association in collaboration with INTERDEM.
“Social distancing” is a two-edged sword: It is for now and the foreseeable future the only available effective strategy for protecting against infection in the older population most vulnerable to severe forms of COVID-19. Yet social distancing also has caused many elderly – particularly those in nursing homes and other long-term care facilities – to plunge into a profound experience of loneliness, isolation, distress, feelings of abandonment, anxiety, depression, and accelerated cognitive deterioration. And this needn’t have happened, the mental health professionals asserted.
“When are we going to get rid of the term ‘social distancing?’ ” asked IPA President William E. Reichman, MD. “Many have appreciated – including the World Health Organization – that the real issue is physical distancing to prevent contagion. And physical distancing doesn’t have to mean social distancing.”
Social connectedness between elderly persons and their peers and family members can be maintained and should be emphatically encouraged during the physical distancing required by the pandemic, said Myrra Vernooij-Dassen, PhD, of Radboud University in Nigmegen, the Netherlands, and chair of INTERDEM, a pan-European network of dementia researchers.
This can be achieved using readily available technologies, including the telephone and videoconferencing, as well as by creating opportunities for supervised masked visits between a family member and an elderly loved one in outdoor courtyards or gardens within long-term care facilities. And yet, as the pandemic seized hold in many parts of the world, family members were blocked from entry to these facilities, she observed.
Impact on mental health, cognition
Dr. Vernooij-Dassen noted that studies of previous quarantine periods as well as preliminary findings during the COVID-19 pandemic demonstrate an inverse relationship between social isolation measures and cognitive functioning in the elderly.
“ Conversely, epidemiologic data indicate that a socially integrated lifestyle had a favorable influence on cognitive functioning and could even delay onset of dementia,” she said.
INTERDEM is backing two ongoing studies evaluating the hypothesis that interventions fostering increased social interaction among elderly individuals can delay onset of dementia or favorably affect its course. The proposed mechanism of benefit is stimulation of brain plasticity to enhance cognitive reserve.
“This is a hypothesis of hope. We know that social interaction for humans is like water to plants – we really, really need it,” she explained.
Diego de Leo, MD, PhD, emeritus professor of psychiatry and former director of the Australian Institute for Suicide Research and Prevention at Griffith University in Brisbane, was living in hard-hit Padua, Italy, during the first surge of COVID-19. He described his anecdotal experience.
“What I hear from many Italian colleagues and friends and directors of mental health services is that emergency admissions related to mental disorders declined during the first wave of the COVID pandemic. For example, not many people attended emergency departments due to suicide attempts; there was a very marked decrease in the number of suicide attempts during the worst days of the pandemic,” he said.
People with psychiatric conditions were afraid to go to the hospital because they thought they would contract the infection and die there. That’s changing now, however.
“Now there is an increased number of admissions to mental health units. A new wave. It has been a U-shaped curve. And we’re now witnessing an increasing number of fatal suicides due to persistent fears, due to people imagining that there is no more room for them, and no more future for them from a financial point of view – which is the major negative outcome of this crisis. It will be a disaster for many families,” the psychiatrist continued.
A noteworthy phenomenon in northern Italy was that, when tablets were made available to nursing home residents in an effort to enhance their connectedness to the outside world, those with dementia often became so frustrated and confused by their difficulty in using the devices that they developed a hypokinetic delirium marked by refusal to eat or leave their bed, he reported.
It’s far too early to have reliable data on suicide trends in response to the pandemic, according to Dr. de Leo. But one thing is for sure: The strategy of social distancing employed to curb COVID-19 has increased the prevalence of known risk factors for suicide in older individuals, including loneliness, anxiety, and depression; increased alcohol use; and a perception of being a burden on society. Dr. de Leo directs a foundation dedicated to helping people experiencing traumatic bereavement, and in one recent week, the foundation was contacted by eight families in the province of Padua with a recent death by suicide apparently related to fallout from the COVID-19 pandemic. That’s an unusually high spike in suicide in a province with a population of 1 million.
“People probably preferred to end the agitation, the fear, the extreme anxiety about their destiny by deciding to prematurely truncate their life. That has been reported by nursing staff,” he said.
The Italian government has determined that, to date, 36% of all COVID-related deaths have occurred in people aged 85 years or older, and 84% of deaths were in individuals aged at least 70 years. And in Milan and the surrounding province of Lombardy, it’s estimated that COVID-19 has taken the lives of 25% of all nursing home residents. The North American experience has been uncomfortably similar.
“Almost 80% of COVID deaths in Canada have occurred in congregate settings,” observed Dr. Reichman, professor of psychiatry at the University of Toronto, and president and CEO of Baycrest Health Sciences, a geriatric research center.
“Certainly, the appalling number of deaths in nursing homes is the No. 1 horror of the pandemic,” declared Carmelle Peisah, MBBS, MD, a psychiatrist at the University of New South Wales in Kensington, Australia.
The fire next time
The conventional wisdom holds that COVID-19 has caused all sorts of mayhem in the delivery of elder care. Not so, in Dr. Reichman’s view.
“I would suggest that the pandemic has not caused many of the problems we talk about, it’s actually revealed problems that have always been there under the surface. For example, many older people, even before COVID-19, were socially isolated, socially distant. They had difficulty connecting with their relatives, difficulty accessing transportation to get to the store to buy food and see their doctors, and to interact with other older people,” the psychiatrist said.
“I would say as well that the pandemic didn’t cause the problems we’ve seen in long-term congregate senior care. The pandemic revealed them. We’ve had facilities where older people were severely crowded together, which compromises their quality of life, even when there’s not a pandemic. We’ve had difficulty staffing these kinds of environments with people that are paid an honest wage for the very hard work that they do. In many of these settings they’re inadequately trained, not only in infection prevention and control but in all other aspects of care. And the pandemic has revealed that many of these organizations are not properly funded. The government doesn’t support them well enough across jurisdictions, and they can’t raise enough philanthropic funds to provide the kind of quality of life that residents demand,” Dr. Reichman continued.
Could the pandemic spur improved elder care? His hope is that health care professionals, politicians, and society at large will learn from the devastation left by the first surge of the pandemic and will lobby for the resources necessary for much-needed improvements in geriatric care.
“We need to be better prepared should there be not only a second wave of this pandemic, but for other pandemics to come,” Dr. Reichman concluded.
The speakers indicated they had no financial conflicts regarding their presentations.
One of the greatest tragedies of the first wave of the COVID-19 pandemic has been the failure of health policy makers to anticipate and mitigate the enormous havoc the policy of social distancing would wreak on mental health and cognitive function in older persons, speakers agreed at a webinar on COVID-19, social distancing, and its impact on social and mental health in the elderly hosted by the International Psychogeriatric Association in collaboration with INTERDEM.
“Social distancing” is a two-edged sword: It is for now and the foreseeable future the only available effective strategy for protecting against infection in the older population most vulnerable to severe forms of COVID-19. Yet social distancing also has caused many elderly – particularly those in nursing homes and other long-term care facilities – to plunge into a profound experience of loneliness, isolation, distress, feelings of abandonment, anxiety, depression, and accelerated cognitive deterioration. And this needn’t have happened, the mental health professionals asserted.
“When are we going to get rid of the term ‘social distancing?’ ” asked IPA President William E. Reichman, MD. “Many have appreciated – including the World Health Organization – that the real issue is physical distancing to prevent contagion. And physical distancing doesn’t have to mean social distancing.”
Social connectedness between elderly persons and their peers and family members can be maintained and should be emphatically encouraged during the physical distancing required by the pandemic, said Myrra Vernooij-Dassen, PhD, of Radboud University in Nigmegen, the Netherlands, and chair of INTERDEM, a pan-European network of dementia researchers.
This can be achieved using readily available technologies, including the telephone and videoconferencing, as well as by creating opportunities for supervised masked visits between a family member and an elderly loved one in outdoor courtyards or gardens within long-term care facilities. And yet, as the pandemic seized hold in many parts of the world, family members were blocked from entry to these facilities, she observed.
Impact on mental health, cognition
Dr. Vernooij-Dassen noted that studies of previous quarantine periods as well as preliminary findings during the COVID-19 pandemic demonstrate an inverse relationship between social isolation measures and cognitive functioning in the elderly.
“ Conversely, epidemiologic data indicate that a socially integrated lifestyle had a favorable influence on cognitive functioning and could even delay onset of dementia,” she said.
INTERDEM is backing two ongoing studies evaluating the hypothesis that interventions fostering increased social interaction among elderly individuals can delay onset of dementia or favorably affect its course. The proposed mechanism of benefit is stimulation of brain plasticity to enhance cognitive reserve.
“This is a hypothesis of hope. We know that social interaction for humans is like water to plants – we really, really need it,” she explained.
Diego de Leo, MD, PhD, emeritus professor of psychiatry and former director of the Australian Institute for Suicide Research and Prevention at Griffith University in Brisbane, was living in hard-hit Padua, Italy, during the first surge of COVID-19. He described his anecdotal experience.
“What I hear from many Italian colleagues and friends and directors of mental health services is that emergency admissions related to mental disorders declined during the first wave of the COVID pandemic. For example, not many people attended emergency departments due to suicide attempts; there was a very marked decrease in the number of suicide attempts during the worst days of the pandemic,” he said.
People with psychiatric conditions were afraid to go to the hospital because they thought they would contract the infection and die there. That’s changing now, however.
“Now there is an increased number of admissions to mental health units. A new wave. It has been a U-shaped curve. And we’re now witnessing an increasing number of fatal suicides due to persistent fears, due to people imagining that there is no more room for them, and no more future for them from a financial point of view – which is the major negative outcome of this crisis. It will be a disaster for many families,” the psychiatrist continued.
A noteworthy phenomenon in northern Italy was that, when tablets were made available to nursing home residents in an effort to enhance their connectedness to the outside world, those with dementia often became so frustrated and confused by their difficulty in using the devices that they developed a hypokinetic delirium marked by refusal to eat or leave their bed, he reported.
It’s far too early to have reliable data on suicide trends in response to the pandemic, according to Dr. de Leo. But one thing is for sure: The strategy of social distancing employed to curb COVID-19 has increased the prevalence of known risk factors for suicide in older individuals, including loneliness, anxiety, and depression; increased alcohol use; and a perception of being a burden on society. Dr. de Leo directs a foundation dedicated to helping people experiencing traumatic bereavement, and in one recent week, the foundation was contacted by eight families in the province of Padua with a recent death by suicide apparently related to fallout from the COVID-19 pandemic. That’s an unusually high spike in suicide in a province with a population of 1 million.
“People probably preferred to end the agitation, the fear, the extreme anxiety about their destiny by deciding to prematurely truncate their life. That has been reported by nursing staff,” he said.
The Italian government has determined that, to date, 36% of all COVID-related deaths have occurred in people aged 85 years or older, and 84% of deaths were in individuals aged at least 70 years. And in Milan and the surrounding province of Lombardy, it’s estimated that COVID-19 has taken the lives of 25% of all nursing home residents. The North American experience has been uncomfortably similar.
“Almost 80% of COVID deaths in Canada have occurred in congregate settings,” observed Dr. Reichman, professor of psychiatry at the University of Toronto, and president and CEO of Baycrest Health Sciences, a geriatric research center.
“Certainly, the appalling number of deaths in nursing homes is the No. 1 horror of the pandemic,” declared Carmelle Peisah, MBBS, MD, a psychiatrist at the University of New South Wales in Kensington, Australia.
The fire next time
The conventional wisdom holds that COVID-19 has caused all sorts of mayhem in the delivery of elder care. Not so, in Dr. Reichman’s view.
“I would suggest that the pandemic has not caused many of the problems we talk about, it’s actually revealed problems that have always been there under the surface. For example, many older people, even before COVID-19, were socially isolated, socially distant. They had difficulty connecting with their relatives, difficulty accessing transportation to get to the store to buy food and see their doctors, and to interact with other older people,” the psychiatrist said.
“I would say as well that the pandemic didn’t cause the problems we’ve seen in long-term congregate senior care. The pandemic revealed them. We’ve had facilities where older people were severely crowded together, which compromises their quality of life, even when there’s not a pandemic. We’ve had difficulty staffing these kinds of environments with people that are paid an honest wage for the very hard work that they do. In many of these settings they’re inadequately trained, not only in infection prevention and control but in all other aspects of care. And the pandemic has revealed that many of these organizations are not properly funded. The government doesn’t support them well enough across jurisdictions, and they can’t raise enough philanthropic funds to provide the kind of quality of life that residents demand,” Dr. Reichman continued.
Could the pandemic spur improved elder care? His hope is that health care professionals, politicians, and society at large will learn from the devastation left by the first surge of the pandemic and will lobby for the resources necessary for much-needed improvements in geriatric care.
“We need to be better prepared should there be not only a second wave of this pandemic, but for other pandemics to come,” Dr. Reichman concluded.
The speakers indicated they had no financial conflicts regarding their presentations.