User login
Noninvasive Ventilation Use Among Medicare Beneficiaries at the End of Life
Study Overview
Objective. To examine the trend of noninvasive and invasive mechanical ventilation at the end of life from 2000 to 2017.
Design. Observational population-based cohort study.
Setting and participants. The study was a population-based cohort study to examine the use of noninvasive and invasive mechanical ventilation among decedents. The study included a random 20% sample of Medicare beneficiaries older than 65 years who were hospitalized in the last 30 days of life and died between January 1, 2000, and December 31, 2017, except for the period October 1, 2015, to December 31, 2015, when the transition from International Classification of Diseases, Ninth Revision (ICD-9) to ICD-10 occurred. Beneficiaries with the primary admitting diagnosis of cardiac arrest or with preexisting tracheostomy were excluded because of expected requirements for ventilatory support. The sample included a total of 2,470,735 Medicare beneficiaries; mean age was 82.2 years, and 54.8% were female. Primary admitting diagnosis codes were used to identify 3 subcohorts: congestive heart failure, chronic obstructive pulmonary disease, and cancer; a fourth subcohort of dementia was identified using the primary admitting diagnosis code or the first 9 secondary diagnosis codes.
Main outcome measures. The study used procedure codes to identify the use of noninvasive ventilation, invasive mechanical ventilation, or none among decedents who were hospitalized in the last 30 days of life. Descriptive statistics to characterize variables by year of hospitalization and ventilatory support were calculated, and the rates of noninvasive and invasive mechanical ventilation use were tabulated. Other outcomes of interest include site of death (in-hospital death), hospice enrollment at death, and hospice enrollment in the last 3 days of life as measures of end-of- life care use. Multivariable logistic regressions were used to examine noninvasive and invasive mechanical ventilation use among decedents, and time trends were examined, with the pattern of use in year 2000 as reference. Subgroup analysis with the subcohort of patients with different diagnoses were conducted to examine trends.
Main results. From 2000 to 2017, 16.3% of decedents had invasive mechanical ventilation, 3.7% had noninvasive ventilation, and 1.0% had both noninvasive and invasive ventilation during their hospital stay. Compared to the reference year 2000, there was a 9-fold increase in noninvasive ventilation use, from 0.8% to 7.1% in 2017, and invasive mechanical ventilation use also increased slightly, from 15.0% to 18.5%. Compared to year 2000, decedents were 2.63 times and 1.04 times (adjusted odds ratio [OR]) more likely to receive noninvasive ventilation and invasive mechanical ventilation, respectively, in 2005, 7.87 times and 1.39 times more likely in 2011, and 11.84 times and 1.63 times more likely in 2017.
Subgroup analysis showed that for congestive heart failure and chronic obstructive pulmonary disease, the increase in noninvasive ventilation use mirrored the trend observed for the overall population, but the use of invasive mechanical ventilation did not increase from 2000 to 2017, with a rate of use of 11.1% versus 7.8% (adjusted OR, 1.07; 95% confidence interval [CI], 0.95-1.19) for congestive heart failure and 17.4% vs 13.2% (OR 1.03, 95% CI, 0.88-1.21) for chronic obstructive pulmonary disease. For the cancer and dementia subgroups, the increase in noninvasive ventilation use from 2000 to 2017 was accompanied by an increase in the use of invasive mechanical ventilation, with a rate of 6.2% versus 7.4% (OR, 1.40; 95% CI, 1.26-1.55) for decedents with cancer and a rate of 5.7% versus 6.2% (OR, 1.28; 95% CI, 1.17-1.41) for decedents with dementia. For other measures of end-of-life care, noninvasive ventilation use when compared to invasive mechanical ventilation use was associated with lower rates of in-hospital (acute care) deaths (50.3% vs 76.7%), hospice enrollment in the last 3 days of life (late hospice enrollment; 57.7% vs 63.0%), and higher rates of hospice enrollment at death (41.3% vs 20.0%).
Conclusion. There was an increase in the use of noninvasive ventilation from 2000 through 2017 among Medicare beneficiaries who died. The findings also suggest that the use of invasive mechanical ventilation did not increase among decedents with congestive heart failure and chronic obstructive pulmonary disease but increased among decedents with cancer and dementia.
Commentary
Noninvasive ventilation offers an alternative to invasive mechanical ventilation for providing ventilatory support for respiratory failure, and may offer benefits as it could avert adverse effects associated with invasive mechanical ventilation, particularly in the management of respiratory failure due to congestive heart failure and chronic obstructive pulmonary disease.1 There is evidence for potential benefits of use of noninvasive ventilation in other clinical scenarios, such as pneumonia in older adults with comorbidities, though its clinical utility is not as well established for other diseases.2
As noninvasive ventilation is introduced into clinical practice, it is not surprising that over the period of the study (2000 to 2017) that its use increased substantially. Advance directives that involve discussion of life-sustaining treatments, including in scenarios with respiratory failure, may also result in physician orders that specify whether an individual desires invasive mechanical ventilation versus other medical treatments, including noninvasive ventilation.3,4 By examining the temporal trends of use of noninvasive and invasive ventilation, this study reveals that invasive mechanical ventilation use among decedents with dementia and cancer has increased, despite increases in the use of noninvasive ventilation. It is important to understand further what would explain these temporal trends and whether the use of noninvasive and also invasive mechanical ventilation at the end of life represents appropriate care with clear goals or whether it may represent overuse. It is also less clear in the end-of-life care scenario what the goals of treatment with noninvasive ventilation would be, especially if it does not avert the use of invasive mechanical ventilation.
The study includes decedents only, thus limiting the ability to draw conclusions about clinically appropriate care.5 Further studies should examine a cohort of patients who have serious and life-threatening illness to examine the trends and potential effects of noninvasive ventilation on outcomes and utilization, as individuals who have improved and survived would not be included in this present decedent cohort.
Applications for Clinical Practice
This study highlights changes in the use of noninvasive and invasive ventilation over time and the different trends seen among subgroups with different diagnoses. For older adults with serious comorbid illness such as dementia, it is especially important to have discussions on advance directives so that care at the end of life is concordant with the patient’s wishes and that unnecessary, burdensome care can be averted. Further studies to understand and define the appropriate use of noninvasive and invasive mechanical ventilation for older adults with significant comorbidities who have serious, life-threatening illness are needed to ensure appropriate clinical treatment at the end of life.
–William W. Hung, MD, MPH
1. Lindenauer PK, Stefan MS, Shieh M et al. Outcomes associated with invasive and noninvasive ventilation a mong patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174:1982-993.
2. Johnson CS, Frei CR, Metersky ML, et al. Non-invasive mechanical ventilation and mortality in elderly immunocompromised patients hospitalized with pneumonia: a retrospective cohort study. BMC Pulm Med. 2014;14:7. Published 2014 Jan 27. doi:10.1186/1471-2466-14-7
3. Lee R, Brumbeck L, Sathitratanacheewin S, et al. Association of physician orders for life-sustaining treatment with icu admission among patients hospitalized near the end of life. JAMA. 2020;323:950-60.
4. Bomba P, Kemp M, Black J. POLST: An improvement over traditional advance directives. Cleveland Clinic J Med. 2012;79:457-464.
5. Duncan I, Ahmed T, Dove H, Maxwell TL. Medicare cost at end of life. Am J Hosp Palliat Care. 2019;36:705-710.
Study Overview
Objective. To examine the trend of noninvasive and invasive mechanical ventilation at the end of life from 2000 to 2017.
Design. Observational population-based cohort study.
Setting and participants. The study was a population-based cohort study to examine the use of noninvasive and invasive mechanical ventilation among decedents. The study included a random 20% sample of Medicare beneficiaries older than 65 years who were hospitalized in the last 30 days of life and died between January 1, 2000, and December 31, 2017, except for the period October 1, 2015, to December 31, 2015, when the transition from International Classification of Diseases, Ninth Revision (ICD-9) to ICD-10 occurred. Beneficiaries with the primary admitting diagnosis of cardiac arrest or with preexisting tracheostomy were excluded because of expected requirements for ventilatory support. The sample included a total of 2,470,735 Medicare beneficiaries; mean age was 82.2 years, and 54.8% were female. Primary admitting diagnosis codes were used to identify 3 subcohorts: congestive heart failure, chronic obstructive pulmonary disease, and cancer; a fourth subcohort of dementia was identified using the primary admitting diagnosis code or the first 9 secondary diagnosis codes.
Main outcome measures. The study used procedure codes to identify the use of noninvasive ventilation, invasive mechanical ventilation, or none among decedents who were hospitalized in the last 30 days of life. Descriptive statistics to characterize variables by year of hospitalization and ventilatory support were calculated, and the rates of noninvasive and invasive mechanical ventilation use were tabulated. Other outcomes of interest include site of death (in-hospital death), hospice enrollment at death, and hospice enrollment in the last 3 days of life as measures of end-of- life care use. Multivariable logistic regressions were used to examine noninvasive and invasive mechanical ventilation use among decedents, and time trends were examined, with the pattern of use in year 2000 as reference. Subgroup analysis with the subcohort of patients with different diagnoses were conducted to examine trends.
Main results. From 2000 to 2017, 16.3% of decedents had invasive mechanical ventilation, 3.7% had noninvasive ventilation, and 1.0% had both noninvasive and invasive ventilation during their hospital stay. Compared to the reference year 2000, there was a 9-fold increase in noninvasive ventilation use, from 0.8% to 7.1% in 2017, and invasive mechanical ventilation use also increased slightly, from 15.0% to 18.5%. Compared to year 2000, decedents were 2.63 times and 1.04 times (adjusted odds ratio [OR]) more likely to receive noninvasive ventilation and invasive mechanical ventilation, respectively, in 2005, 7.87 times and 1.39 times more likely in 2011, and 11.84 times and 1.63 times more likely in 2017.
Subgroup analysis showed that for congestive heart failure and chronic obstructive pulmonary disease, the increase in noninvasive ventilation use mirrored the trend observed for the overall population, but the use of invasive mechanical ventilation did not increase from 2000 to 2017, with a rate of use of 11.1% versus 7.8% (adjusted OR, 1.07; 95% confidence interval [CI], 0.95-1.19) for congestive heart failure and 17.4% vs 13.2% (OR 1.03, 95% CI, 0.88-1.21) for chronic obstructive pulmonary disease. For the cancer and dementia subgroups, the increase in noninvasive ventilation use from 2000 to 2017 was accompanied by an increase in the use of invasive mechanical ventilation, with a rate of 6.2% versus 7.4% (OR, 1.40; 95% CI, 1.26-1.55) for decedents with cancer and a rate of 5.7% versus 6.2% (OR, 1.28; 95% CI, 1.17-1.41) for decedents with dementia. For other measures of end-of-life care, noninvasive ventilation use when compared to invasive mechanical ventilation use was associated with lower rates of in-hospital (acute care) deaths (50.3% vs 76.7%), hospice enrollment in the last 3 days of life (late hospice enrollment; 57.7% vs 63.0%), and higher rates of hospice enrollment at death (41.3% vs 20.0%).
Conclusion. There was an increase in the use of noninvasive ventilation from 2000 through 2017 among Medicare beneficiaries who died. The findings also suggest that the use of invasive mechanical ventilation did not increase among decedents with congestive heart failure and chronic obstructive pulmonary disease but increased among decedents with cancer and dementia.
Commentary
Noninvasive ventilation offers an alternative to invasive mechanical ventilation for providing ventilatory support for respiratory failure, and may offer benefits as it could avert adverse effects associated with invasive mechanical ventilation, particularly in the management of respiratory failure due to congestive heart failure and chronic obstructive pulmonary disease.1 There is evidence for potential benefits of use of noninvasive ventilation in other clinical scenarios, such as pneumonia in older adults with comorbidities, though its clinical utility is not as well established for other diseases.2
As noninvasive ventilation is introduced into clinical practice, it is not surprising that over the period of the study (2000 to 2017) that its use increased substantially. Advance directives that involve discussion of life-sustaining treatments, including in scenarios with respiratory failure, may also result in physician orders that specify whether an individual desires invasive mechanical ventilation versus other medical treatments, including noninvasive ventilation.3,4 By examining the temporal trends of use of noninvasive and invasive ventilation, this study reveals that invasive mechanical ventilation use among decedents with dementia and cancer has increased, despite increases in the use of noninvasive ventilation. It is important to understand further what would explain these temporal trends and whether the use of noninvasive and also invasive mechanical ventilation at the end of life represents appropriate care with clear goals or whether it may represent overuse. It is also less clear in the end-of-life care scenario what the goals of treatment with noninvasive ventilation would be, especially if it does not avert the use of invasive mechanical ventilation.
The study includes decedents only, thus limiting the ability to draw conclusions about clinically appropriate care.5 Further studies should examine a cohort of patients who have serious and life-threatening illness to examine the trends and potential effects of noninvasive ventilation on outcomes and utilization, as individuals who have improved and survived would not be included in this present decedent cohort.
Applications for Clinical Practice
This study highlights changes in the use of noninvasive and invasive ventilation over time and the different trends seen among subgroups with different diagnoses. For older adults with serious comorbid illness such as dementia, it is especially important to have discussions on advance directives so that care at the end of life is concordant with the patient’s wishes and that unnecessary, burdensome care can be averted. Further studies to understand and define the appropriate use of noninvasive and invasive mechanical ventilation for older adults with significant comorbidities who have serious, life-threatening illness are needed to ensure appropriate clinical treatment at the end of life.
–William W. Hung, MD, MPH
Study Overview
Objective. To examine the trend of noninvasive and invasive mechanical ventilation at the end of life from 2000 to 2017.
Design. Observational population-based cohort study.
Setting and participants. The study was a population-based cohort study to examine the use of noninvasive and invasive mechanical ventilation among decedents. The study included a random 20% sample of Medicare beneficiaries older than 65 years who were hospitalized in the last 30 days of life and died between January 1, 2000, and December 31, 2017, except for the period October 1, 2015, to December 31, 2015, when the transition from International Classification of Diseases, Ninth Revision (ICD-9) to ICD-10 occurred. Beneficiaries with the primary admitting diagnosis of cardiac arrest or with preexisting tracheostomy were excluded because of expected requirements for ventilatory support. The sample included a total of 2,470,735 Medicare beneficiaries; mean age was 82.2 years, and 54.8% were female. Primary admitting diagnosis codes were used to identify 3 subcohorts: congestive heart failure, chronic obstructive pulmonary disease, and cancer; a fourth subcohort of dementia was identified using the primary admitting diagnosis code or the first 9 secondary diagnosis codes.
Main outcome measures. The study used procedure codes to identify the use of noninvasive ventilation, invasive mechanical ventilation, or none among decedents who were hospitalized in the last 30 days of life. Descriptive statistics to characterize variables by year of hospitalization and ventilatory support were calculated, and the rates of noninvasive and invasive mechanical ventilation use were tabulated. Other outcomes of interest include site of death (in-hospital death), hospice enrollment at death, and hospice enrollment in the last 3 days of life as measures of end-of- life care use. Multivariable logistic regressions were used to examine noninvasive and invasive mechanical ventilation use among decedents, and time trends were examined, with the pattern of use in year 2000 as reference. Subgroup analysis with the subcohort of patients with different diagnoses were conducted to examine trends.
Main results. From 2000 to 2017, 16.3% of decedents had invasive mechanical ventilation, 3.7% had noninvasive ventilation, and 1.0% had both noninvasive and invasive ventilation during their hospital stay. Compared to the reference year 2000, there was a 9-fold increase in noninvasive ventilation use, from 0.8% to 7.1% in 2017, and invasive mechanical ventilation use also increased slightly, from 15.0% to 18.5%. Compared to year 2000, decedents were 2.63 times and 1.04 times (adjusted odds ratio [OR]) more likely to receive noninvasive ventilation and invasive mechanical ventilation, respectively, in 2005, 7.87 times and 1.39 times more likely in 2011, and 11.84 times and 1.63 times more likely in 2017.
Subgroup analysis showed that for congestive heart failure and chronic obstructive pulmonary disease, the increase in noninvasive ventilation use mirrored the trend observed for the overall population, but the use of invasive mechanical ventilation did not increase from 2000 to 2017, with a rate of use of 11.1% versus 7.8% (adjusted OR, 1.07; 95% confidence interval [CI], 0.95-1.19) for congestive heart failure and 17.4% vs 13.2% (OR 1.03, 95% CI, 0.88-1.21) for chronic obstructive pulmonary disease. For the cancer and dementia subgroups, the increase in noninvasive ventilation use from 2000 to 2017 was accompanied by an increase in the use of invasive mechanical ventilation, with a rate of 6.2% versus 7.4% (OR, 1.40; 95% CI, 1.26-1.55) for decedents with cancer and a rate of 5.7% versus 6.2% (OR, 1.28; 95% CI, 1.17-1.41) for decedents with dementia. For other measures of end-of-life care, noninvasive ventilation use when compared to invasive mechanical ventilation use was associated with lower rates of in-hospital (acute care) deaths (50.3% vs 76.7%), hospice enrollment in the last 3 days of life (late hospice enrollment; 57.7% vs 63.0%), and higher rates of hospice enrollment at death (41.3% vs 20.0%).
Conclusion. There was an increase in the use of noninvasive ventilation from 2000 through 2017 among Medicare beneficiaries who died. The findings also suggest that the use of invasive mechanical ventilation did not increase among decedents with congestive heart failure and chronic obstructive pulmonary disease but increased among decedents with cancer and dementia.
Commentary
Noninvasive ventilation offers an alternative to invasive mechanical ventilation for providing ventilatory support for respiratory failure, and may offer benefits as it could avert adverse effects associated with invasive mechanical ventilation, particularly in the management of respiratory failure due to congestive heart failure and chronic obstructive pulmonary disease.1 There is evidence for potential benefits of use of noninvasive ventilation in other clinical scenarios, such as pneumonia in older adults with comorbidities, though its clinical utility is not as well established for other diseases.2
As noninvasive ventilation is introduced into clinical practice, it is not surprising that over the period of the study (2000 to 2017) that its use increased substantially. Advance directives that involve discussion of life-sustaining treatments, including in scenarios with respiratory failure, may also result in physician orders that specify whether an individual desires invasive mechanical ventilation versus other medical treatments, including noninvasive ventilation.3,4 By examining the temporal trends of use of noninvasive and invasive ventilation, this study reveals that invasive mechanical ventilation use among decedents with dementia and cancer has increased, despite increases in the use of noninvasive ventilation. It is important to understand further what would explain these temporal trends and whether the use of noninvasive and also invasive mechanical ventilation at the end of life represents appropriate care with clear goals or whether it may represent overuse. It is also less clear in the end-of-life care scenario what the goals of treatment with noninvasive ventilation would be, especially if it does not avert the use of invasive mechanical ventilation.
The study includes decedents only, thus limiting the ability to draw conclusions about clinically appropriate care.5 Further studies should examine a cohort of patients who have serious and life-threatening illness to examine the trends and potential effects of noninvasive ventilation on outcomes and utilization, as individuals who have improved and survived would not be included in this present decedent cohort.
Applications for Clinical Practice
This study highlights changes in the use of noninvasive and invasive ventilation over time and the different trends seen among subgroups with different diagnoses. For older adults with serious comorbid illness such as dementia, it is especially important to have discussions on advance directives so that care at the end of life is concordant with the patient’s wishes and that unnecessary, burdensome care can be averted. Further studies to understand and define the appropriate use of noninvasive and invasive mechanical ventilation for older adults with significant comorbidities who have serious, life-threatening illness are needed to ensure appropriate clinical treatment at the end of life.
–William W. Hung, MD, MPH
1. Lindenauer PK, Stefan MS, Shieh M et al. Outcomes associated with invasive and noninvasive ventilation a mong patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174:1982-993.
2. Johnson CS, Frei CR, Metersky ML, et al. Non-invasive mechanical ventilation and mortality in elderly immunocompromised patients hospitalized with pneumonia: a retrospective cohort study. BMC Pulm Med. 2014;14:7. Published 2014 Jan 27. doi:10.1186/1471-2466-14-7
3. Lee R, Brumbeck L, Sathitratanacheewin S, et al. Association of physician orders for life-sustaining treatment with icu admission among patients hospitalized near the end of life. JAMA. 2020;323:950-60.
4. Bomba P, Kemp M, Black J. POLST: An improvement over traditional advance directives. Cleveland Clinic J Med. 2012;79:457-464.
5. Duncan I, Ahmed T, Dove H, Maxwell TL. Medicare cost at end of life. Am J Hosp Palliat Care. 2019;36:705-710.
1. Lindenauer PK, Stefan MS, Shieh M et al. Outcomes associated with invasive and noninvasive ventilation a mong patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174:1982-993.
2. Johnson CS, Frei CR, Metersky ML, et al. Non-invasive mechanical ventilation and mortality in elderly immunocompromised patients hospitalized with pneumonia: a retrospective cohort study. BMC Pulm Med. 2014;14:7. Published 2014 Jan 27. doi:10.1186/1471-2466-14-7
3. Lee R, Brumbeck L, Sathitratanacheewin S, et al. Association of physician orders for life-sustaining treatment with icu admission among patients hospitalized near the end of life. JAMA. 2020;323:950-60.
4. Bomba P, Kemp M, Black J. POLST: An improvement over traditional advance directives. Cleveland Clinic J Med. 2012;79:457-464.
5. Duncan I, Ahmed T, Dove H, Maxwell TL. Medicare cost at end of life. Am J Hosp Palliat Care. 2019;36:705-710.
Patient-Centered Cancer Care: A Streamline Practice to MyHealtheVet Usage
BACKGROUND: Cancer care is often fragmented for the patient undergoing complex care. Cancer treatments such as chemotherapy and radiation require coordination with medical services and infusion centers. Past practices utilizing telephone communication limited care coordination. Before advances in technology, patients were restricted to obtain their health care information through conversation. Care coordination paired with patient-centered technology enables collaboration among the health care team and creates structure through visual displays of essential information.
OBJECTIVE: Our goals were to introduce technology and maximize enrollment for the patient by: (1) Establishing MyHealtheVet within the Hematology- Oncology department for increased patient support to memorialize communication. (2) Create access to care to better manage treatment of health-related issues. (3) Empower the patient to participate in managing their care.
METHODS: In October of 2017 the NF/SGVA began implementing MyHealtheVet . The Hematology-Oncology department had no formal process. By the Fall of 2019 the infusion room process was to enroll each patient or upgrade the patients’ account to premium status at the time of their first encounter with nursing during chemotherapy education.
RESULTS: MyHealtheVet usage and chemotherapy education appointments within the Hematology-Oncology department were surveyed. Over a two-year period, use in the Hematology-Oncology department at the NF/SGVA showed steady escalation of secure messaging as more oncology patients enrolled. When COVID19 arose in February 2020, the Hematology- Oncology department was already poised to handle care coordination for their patients during this crisis. With our established practices, coordination of care for patients in treatment well prepared to receive care and communication electronically through MyHealtheVet .
CONCLUSIONS: Personal health networks (PHN) have evolved and have become a novel solution to address challenges of the cancer care continuum. MyHealtheVet serves as a voice for the VA for navigating patient care coordination needs. My- HealtheVet is a clinical tool that functions locally using administrative features to involve, engage, and educate the patient with an array of resources. This enrollment process allowed us to remain patient- centered and well equipped to face COVID19 related access challenges as they quickly evolved in the spring of 2020. Pharmacy functions and Secure Messaging emerge as the strongest tools in our communication toolbox.
BACKGROUND: Cancer care is often fragmented for the patient undergoing complex care. Cancer treatments such as chemotherapy and radiation require coordination with medical services and infusion centers. Past practices utilizing telephone communication limited care coordination. Before advances in technology, patients were restricted to obtain their health care information through conversation. Care coordination paired with patient-centered technology enables collaboration among the health care team and creates structure through visual displays of essential information.
OBJECTIVE: Our goals were to introduce technology and maximize enrollment for the patient by: (1) Establishing MyHealtheVet within the Hematology- Oncology department for increased patient support to memorialize communication. (2) Create access to care to better manage treatment of health-related issues. (3) Empower the patient to participate in managing their care.
METHODS: In October of 2017 the NF/SGVA began implementing MyHealtheVet . The Hematology-Oncology department had no formal process. By the Fall of 2019 the infusion room process was to enroll each patient or upgrade the patients’ account to premium status at the time of their first encounter with nursing during chemotherapy education.
RESULTS: MyHealtheVet usage and chemotherapy education appointments within the Hematology-Oncology department were surveyed. Over a two-year period, use in the Hematology-Oncology department at the NF/SGVA showed steady escalation of secure messaging as more oncology patients enrolled. When COVID19 arose in February 2020, the Hematology- Oncology department was already poised to handle care coordination for their patients during this crisis. With our established practices, coordination of care for patients in treatment well prepared to receive care and communication electronically through MyHealtheVet .
CONCLUSIONS: Personal health networks (PHN) have evolved and have become a novel solution to address challenges of the cancer care continuum. MyHealtheVet serves as a voice for the VA for navigating patient care coordination needs. My- HealtheVet is a clinical tool that functions locally using administrative features to involve, engage, and educate the patient with an array of resources. This enrollment process allowed us to remain patient- centered and well equipped to face COVID19 related access challenges as they quickly evolved in the spring of 2020. Pharmacy functions and Secure Messaging emerge as the strongest tools in our communication toolbox.
BACKGROUND: Cancer care is often fragmented for the patient undergoing complex care. Cancer treatments such as chemotherapy and radiation require coordination with medical services and infusion centers. Past practices utilizing telephone communication limited care coordination. Before advances in technology, patients were restricted to obtain their health care information through conversation. Care coordination paired with patient-centered technology enables collaboration among the health care team and creates structure through visual displays of essential information.
OBJECTIVE: Our goals were to introduce technology and maximize enrollment for the patient by: (1) Establishing MyHealtheVet within the Hematology- Oncology department for increased patient support to memorialize communication. (2) Create access to care to better manage treatment of health-related issues. (3) Empower the patient to participate in managing their care.
METHODS: In October of 2017 the NF/SGVA began implementing MyHealtheVet . The Hematology-Oncology department had no formal process. By the Fall of 2019 the infusion room process was to enroll each patient or upgrade the patients’ account to premium status at the time of their first encounter with nursing during chemotherapy education.
RESULTS: MyHealtheVet usage and chemotherapy education appointments within the Hematology-Oncology department were surveyed. Over a two-year period, use in the Hematology-Oncology department at the NF/SGVA showed steady escalation of secure messaging as more oncology patients enrolled. When COVID19 arose in February 2020, the Hematology- Oncology department was already poised to handle care coordination for their patients during this crisis. With our established practices, coordination of care for patients in treatment well prepared to receive care and communication electronically through MyHealtheVet .
CONCLUSIONS: Personal health networks (PHN) have evolved and have become a novel solution to address challenges of the cancer care continuum. MyHealtheVet serves as a voice for the VA for navigating patient care coordination needs. My- HealtheVet is a clinical tool that functions locally using administrative features to involve, engage, and educate the patient with an array of resources. This enrollment process allowed us to remain patient- centered and well equipped to face COVID19 related access challenges as they quickly evolved in the spring of 2020. Pharmacy functions and Secure Messaging emerge as the strongest tools in our communication toolbox.
Oncology-Palliative Care Collaboration: Impact on Hospice Accession and End-of-Life Care
BACKGROUND: Timely hospice care in oncology improves end-of-life care, decreases hospitalizations, improves quality of life and satisfaction. However, collaborative practice of palliative care and oncology remains inconsistent, resulting in absent or delayed hospice services. Short hospice length of service is a marker of poor quality of care and end-user dissatisfaction. Most Americans desire end-of-life care in their homes; however, most of them receive their end-of-life care in hospitals.
HYPOTHESIS: A collaborative oncology-palliative care clinic model improves access to hospice care.
INTERVENTION: In January 2015, we implemented an integrated oncology-palliative care clinic model with the following elements:
(1) Pre-clinic “huddle” among palliative care and oncology staff to identify palliative care needs for patients;
(2) Shared palliative care and oncology clinic appointments;
(3) Introduction of palliative care for every new oncology clinic patient, for advance care planning;
(4) Concurrent oncology and palliative care follow-up for all high-risk patients (aggressive histology, progressing disease, etc.) for goals of care discussions and symptom management;
(5) Palliative care and oncology staff co-managing oncology patients enrolled in hospice care.
MEASUREMENTS: In December 2019, we conducted a retrospective review of all Veterans seen in oncologypalliative care clinic during FY2018-FY2019 with specific attention to community hospice referrals, hospice length of stay, and location of Veterans’ death.
RESULTS: Of a total of 189 Veterans seen in this clinic in FY18-FY19, at the time of review.
(1) 68 (36%) Veterans accessed hospice care.
(2) Of 71 deceased Veterans, 59 (83%) died on hospice (Medicare data: 50%).
(3) Average length of stay on hospice was 64 days (other studies: 48 days).
(4) Compared to other studies, our longer hospice stay was consistent across various cancers: lung (75 vs. 40 days), prostate (69 vs. 48 days), pancreas (40 vs. 32 days), colorectal (140 vs. 46 days).
(5) Of Veterans who died on hospice care, 30 (51%) died at home (other studies: 25%).
CONCLUSION: Our intervention improved access to hospice care in cancer care.
FUTURE IMPLICATIONS: (1) Impact of this intervention of cost of end-of-life care.
(2) Future innovative clinic models for delivery of collaborative comprehensive care for complex nee
BACKGROUND: Timely hospice care in oncology improves end-of-life care, decreases hospitalizations, improves quality of life and satisfaction. However, collaborative practice of palliative care and oncology remains inconsistent, resulting in absent or delayed hospice services. Short hospice length of service is a marker of poor quality of care and end-user dissatisfaction. Most Americans desire end-of-life care in their homes; however, most of them receive their end-of-life care in hospitals.
HYPOTHESIS: A collaborative oncology-palliative care clinic model improves access to hospice care.
INTERVENTION: In January 2015, we implemented an integrated oncology-palliative care clinic model with the following elements:
(1) Pre-clinic “huddle” among palliative care and oncology staff to identify palliative care needs for patients;
(2) Shared palliative care and oncology clinic appointments;
(3) Introduction of palliative care for every new oncology clinic patient, for advance care planning;
(4) Concurrent oncology and palliative care follow-up for all high-risk patients (aggressive histology, progressing disease, etc.) for goals of care discussions and symptom management;
(5) Palliative care and oncology staff co-managing oncology patients enrolled in hospice care.
MEASUREMENTS: In December 2019, we conducted a retrospective review of all Veterans seen in oncologypalliative care clinic during FY2018-FY2019 with specific attention to community hospice referrals, hospice length of stay, and location of Veterans’ death.
RESULTS: Of a total of 189 Veterans seen in this clinic in FY18-FY19, at the time of review.
(1) 68 (36%) Veterans accessed hospice care.
(2) Of 71 deceased Veterans, 59 (83%) died on hospice (Medicare data: 50%).
(3) Average length of stay on hospice was 64 days (other studies: 48 days).
(4) Compared to other studies, our longer hospice stay was consistent across various cancers: lung (75 vs. 40 days), prostate (69 vs. 48 days), pancreas (40 vs. 32 days), colorectal (140 vs. 46 days).
(5) Of Veterans who died on hospice care, 30 (51%) died at home (other studies: 25%).
CONCLUSION: Our intervention improved access to hospice care in cancer care.
FUTURE IMPLICATIONS: (1) Impact of this intervention of cost of end-of-life care.
(2) Future innovative clinic models for delivery of collaborative comprehensive care for complex nee
BACKGROUND: Timely hospice care in oncology improves end-of-life care, decreases hospitalizations, improves quality of life and satisfaction. However, collaborative practice of palliative care and oncology remains inconsistent, resulting in absent or delayed hospice services. Short hospice length of service is a marker of poor quality of care and end-user dissatisfaction. Most Americans desire end-of-life care in their homes; however, most of them receive their end-of-life care in hospitals.
HYPOTHESIS: A collaborative oncology-palliative care clinic model improves access to hospice care.
INTERVENTION: In January 2015, we implemented an integrated oncology-palliative care clinic model with the following elements:
(1) Pre-clinic “huddle” among palliative care and oncology staff to identify palliative care needs for patients;
(2) Shared palliative care and oncology clinic appointments;
(3) Introduction of palliative care for every new oncology clinic patient, for advance care planning;
(4) Concurrent oncology and palliative care follow-up for all high-risk patients (aggressive histology, progressing disease, etc.) for goals of care discussions and symptom management;
(5) Palliative care and oncology staff co-managing oncology patients enrolled in hospice care.
MEASUREMENTS: In December 2019, we conducted a retrospective review of all Veterans seen in oncologypalliative care clinic during FY2018-FY2019 with specific attention to community hospice referrals, hospice length of stay, and location of Veterans’ death.
RESULTS: Of a total of 189 Veterans seen in this clinic in FY18-FY19, at the time of review.
(1) 68 (36%) Veterans accessed hospice care.
(2) Of 71 deceased Veterans, 59 (83%) died on hospice (Medicare data: 50%).
(3) Average length of stay on hospice was 64 days (other studies: 48 days).
(4) Compared to other studies, our longer hospice stay was consistent across various cancers: lung (75 vs. 40 days), prostate (69 vs. 48 days), pancreas (40 vs. 32 days), colorectal (140 vs. 46 days).
(5) Of Veterans who died on hospice care, 30 (51%) died at home (other studies: 25%).
CONCLUSION: Our intervention improved access to hospice care in cancer care.
FUTURE IMPLICATIONS: (1) Impact of this intervention of cost of end-of-life care.
(2) Future innovative clinic models for delivery of collaborative comprehensive care for complex nee
Performance status, molecular testing key to metastatic cancer prognosis
according to Sam Brondfield, MD, MA, an inpatient medical oncologist at the University of California, San Francisco.
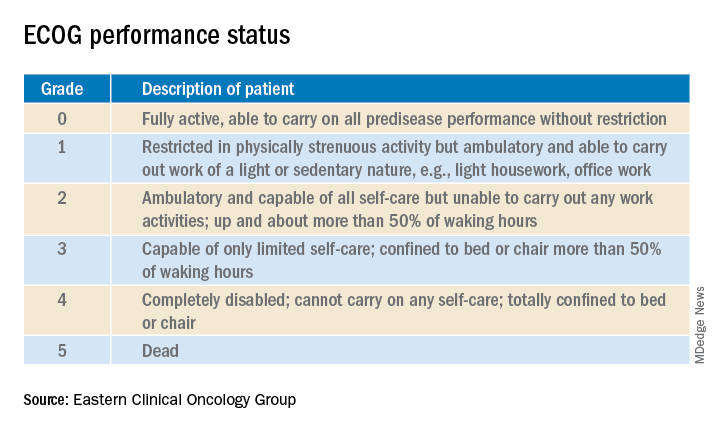
Oncologists have at their fingertips a voluminous and ever-growing body of clinical trials data to draw on for prognostication. Yet many hospitalists will be surprised to learn that this wealth of information is of little value in the inpatient settings where they work, he said at HM20 Virtual, hosted by the Society of Hospital Medicine.
“The applicability of clinical trials data to hospitalized patients is generally poor. That’s an important caveat to keep in mind,” Dr. Brondfield said.
Enrollment in clinical trials is usually restricted to patients with a score of 0 or 1 on the Eastern Clinical Oncology Group Performance Status, meaning their cancer is causing minimal or no disruption to their life (see graphic). Sometimes trials will include patients with a performance status of 2 on the ECOG scale, a tool developed nearly 40 years ago, but clinical trials virtually never enroll those with an ECOG status of 3 or 4. Yet most hospitalized patients with metastatic cancer have an ECOG performance status of 3 or worse. Thus, the clinical trials outcome data are of little relevance.
“In oncology the distinction between ECOG 2 and 3 is very important,” Dr. Brondfield emphasized.
When he talks about treatment options with hospitalized patients who have metastatic cancer and poor performance status – that is, ECOG 3 or 4 – he’ll often say: “Assuming you feel better and can go home, that’s when these clinical trial data may apply better to you.”
Dr. Brondfield cautioned against quoting the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) 5-year overall survival data when hospitalized patients with advanced cancer ask how long they have to live. For one thing, the national average 5-year overall survival figure is hardly an individualized assessment. Plus, oncology is a fast-moving field in which important treatment advances occur all the time, and the SEER data lag far behind. For example, when Dr. Brondfield recently looked up the current SEER 5-year survival for patients diagnosed with metastatic non–small cell lung cancer (NSCLC), the figure quoted was less than 6%, and it was drawn from data accrued in 2009-2015. That simply doesn’t reflect contemporary practice.
Indeed, it’s no longer true that the average survival of patients with metastatic NSCLC is less than a year. In the practice-changing KEYNOTE-189 randomized trial, which accrued participants in 2016-2017, the median overall survival of patients randomized to pembrolizumab (Keytruda) plus standard cytotoxic chemotherapy was 22 months, compared with 11 months with chemotherapy plus placebo (J Clin Oncol. 2020 May 10. doi: 10.1200/JCO.19.03136). As a result, immunotherapy with a programmed death–1 inhibitor such as pembrolizumab in combination with chemotherapy is now standard practice in patients with metastatic NSCLC without targetable mutations.
Performance status guides treatment decision-making
Hospitalists can help oncologists in decision-making regarding whether to offer palliative systemic therapy to patients with advanced metastatic cancer and poor performance status by determining whether that status is caused by the cancer itself or some other cause that’s not easily reversible, such as liver failure.
Take, for example, the inpatient with advanced SCLC. This is an aggressive and chemosensitive cancer. Dr. Brondfield said he is among many medical oncologists who are convinced that, if poor performance status in a patient with advanced SCLC is caused by the cancer itself, prompt initiation of inpatient chemotherapy should be recommended to elicit a response that improves quality of life and performance status in the short term. If, on the other hand, the poor performance status is caused by organ failure or some other issue that can’t easily be improved, hospice may be more appropriate.
“The contour of SCLC over time is that despite its treatment responsiveness it inevitably recurs. But with chemotherapy you can give people in this situation months of quality time, so we generally try to treat these sorts of patients,” Dr. Brondfield explained.
The National Comprehensive Cancer Network guidelines upon which oncologists rely leave lots of room for interpretation regarding the appropriateness of inpatient chemotherapy in patients with advanced cancer and poor patient performance status. Citing “knowledge that’s been passed down across oncology generations,” Dr. Brondfield said he and many of his colleagues believe early palliative supportive care rather than systemic cytotoxic cancer-directed therapy is appropriate for patients with poor performance status who have one of several specific relatively nonchemoresponsive types of metastatic cancer. These include esophageal, gastric, and head and neck cancers.
On the other hand, advanced SCLC isn’t the only type of metastatic cancer that’s so chemosensitive that he and many other oncologists believe aggressive chemotherapy should be offered even in the face of poor patient performance status attributable to the cancer itself.
Take, for example, colorectal cancer with no more than five metastases to the lung or liver, provided those metastases are treatable with resection or radiation. “Those patients are actually curable at a high rate. They have about a 30%-40% cure rate. So those patients, even if they have poor performance status, if we can get them up for surgery or radiation, we usually do try to treat them aggressively,” Dr. Brondfield said.
There are other often chemoresponsive metastatic cancers for which oncologists frequently recommend aggressive treatment to improve quality of life in patients with poor performance status. These cancers include aggressive lymphomas, which are actually often curable; multiple myeloma; testicular and germ cell cancers; NSCLC with a targetable mutation, which is often responsive to oral medications; and prostate and well-differentiated thyroid cancers, which can usually be treated with hormone- or iodine-based therapies rather than more toxic intravenous cytotoxic chemotherapy.
The impact of inpatient palliative chemotherapy in patients with poor performance status and advanced solid cancers not on the short list of highly chemosensitive cancers has not been well studied. A recent retrospective study of 228 such patients who received inpatient palliative chemotherapy at a large Brazilian academic medical center provided little reason for enthusiasm regarding the practice. Survival was short, with 30- and 60-day survival rates of 56% and 39%, respectively. Plus, 30% of patients were admitted to the ICU, where they received aggressive and costly end-of-life care. The investigators found these results suggestive of overprescribing of inpatient palliative chemotherapy (BMC Palliat Care. 2019 May 20;18[1]:42. doi: 10.1186/s12904-019-0427-4).
Of note, the investigators found in a multivariate analysis that an elevated bilirubin was associated with a 217% increased risk of 30-day mortality, and hypercalcemia was associated with a 119% increased risk.
“That’s something to take into account when these decisions are being made,” Dr. Brondfield advised.
In response to an audience comment that oncologists often seem overly optimistic about prognosis, Dr. Brondfield observed, “I think it’s very common for there to be a disagreement between the oncologist wanting to be aggressive for a sick inpatient and the hospitalist or generalist provider thinking: ‘This person looks way too sick for chemotherapy.’ ”
For this reason he is a firm believer in having multidisciplinary conversations regarding prognosis in challenging situations involving hospitalized patients with advanced cancer. An oncologist can bring to such discussions a detailed understanding of clinical trial and molecular data as well as information about the patient’s response to the first round of therapy. But lots of other factors are relevant to prognosis, including nutritional status, comorbidities, and the intuitive eyeball test of how a patient might do. The patient’s family, primary care provider, oncologist, the hospitalist, and the palliative care team will have perspectives of their own.
Molecular testing is now the norm in metastatic cancers
These days oncologists order molecular testing for most patients with metastatic carcinomas to determine eligibility for targeted therapy, suitability for participation in clinical trials, prognostication, and/or assistance in determining the site of origin if that’s unclear.
A single-pass fine needle aspiration biopsy doesn’t provide enough tissue for molecular testing. It’s therefore important to order initially a multipass fine needle aspiration to avoid the need for a repeat biopsy, which is uncomfortable for the patient and can delay diagnosis and treatment.
Dr. Brondfield advised waiting for molecular testing results to come in before trying to prognosticate in patients with a metastatic cancer for which targetable mutations might be present. Survival rates can vary substantially depending upon those test results. Take, for example, metastatic NSCLC: Just within the past year, clinical trials have been published reporting overall survival rates of 39 months in patients with treatable mutations in epidermal growth factor receptor, 42 months with anaplastic lymphoma kinase mutations, and 51 months in patients whose tumor signature features mutations in c-ros oncogene 1, as compared with 22 months with no targetable mutations in the KEYNOTE-189 trial.
“There’s a lot of heterogeneity around how metastatic tumors behave and respond to therapy. Not all metastatic cancers are the same,” the oncologist emphasized.
according to Sam Brondfield, MD, MA, an inpatient medical oncologist at the University of California, San Francisco.

Oncologists have at their fingertips a voluminous and ever-growing body of clinical trials data to draw on for prognostication. Yet many hospitalists will be surprised to learn that this wealth of information is of little value in the inpatient settings where they work, he said at HM20 Virtual, hosted by the Society of Hospital Medicine.
“The applicability of clinical trials data to hospitalized patients is generally poor. That’s an important caveat to keep in mind,” Dr. Brondfield said.
Enrollment in clinical trials is usually restricted to patients with a score of 0 or 1 on the Eastern Clinical Oncology Group Performance Status, meaning their cancer is causing minimal or no disruption to their life (see graphic). Sometimes trials will include patients with a performance status of 2 on the ECOG scale, a tool developed nearly 40 years ago, but clinical trials virtually never enroll those with an ECOG status of 3 or 4. Yet most hospitalized patients with metastatic cancer have an ECOG performance status of 3 or worse. Thus, the clinical trials outcome data are of little relevance.
“In oncology the distinction between ECOG 2 and 3 is very important,” Dr. Brondfield emphasized.
When he talks about treatment options with hospitalized patients who have metastatic cancer and poor performance status – that is, ECOG 3 or 4 – he’ll often say: “Assuming you feel better and can go home, that’s when these clinical trial data may apply better to you.”
Dr. Brondfield cautioned against quoting the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) 5-year overall survival data when hospitalized patients with advanced cancer ask how long they have to live. For one thing, the national average 5-year overall survival figure is hardly an individualized assessment. Plus, oncology is a fast-moving field in which important treatment advances occur all the time, and the SEER data lag far behind. For example, when Dr. Brondfield recently looked up the current SEER 5-year survival for patients diagnosed with metastatic non–small cell lung cancer (NSCLC), the figure quoted was less than 6%, and it was drawn from data accrued in 2009-2015. That simply doesn’t reflect contemporary practice.
Indeed, it’s no longer true that the average survival of patients with metastatic NSCLC is less than a year. In the practice-changing KEYNOTE-189 randomized trial, which accrued participants in 2016-2017, the median overall survival of patients randomized to pembrolizumab (Keytruda) plus standard cytotoxic chemotherapy was 22 months, compared with 11 months with chemotherapy plus placebo (J Clin Oncol. 2020 May 10. doi: 10.1200/JCO.19.03136). As a result, immunotherapy with a programmed death–1 inhibitor such as pembrolizumab in combination with chemotherapy is now standard practice in patients with metastatic NSCLC without targetable mutations.
Performance status guides treatment decision-making
Hospitalists can help oncologists in decision-making regarding whether to offer palliative systemic therapy to patients with advanced metastatic cancer and poor performance status by determining whether that status is caused by the cancer itself or some other cause that’s not easily reversible, such as liver failure.
Take, for example, the inpatient with advanced SCLC. This is an aggressive and chemosensitive cancer. Dr. Brondfield said he is among many medical oncologists who are convinced that, if poor performance status in a patient with advanced SCLC is caused by the cancer itself, prompt initiation of inpatient chemotherapy should be recommended to elicit a response that improves quality of life and performance status in the short term. If, on the other hand, the poor performance status is caused by organ failure or some other issue that can’t easily be improved, hospice may be more appropriate.
“The contour of SCLC over time is that despite its treatment responsiveness it inevitably recurs. But with chemotherapy you can give people in this situation months of quality time, so we generally try to treat these sorts of patients,” Dr. Brondfield explained.
The National Comprehensive Cancer Network guidelines upon which oncologists rely leave lots of room for interpretation regarding the appropriateness of inpatient chemotherapy in patients with advanced cancer and poor patient performance status. Citing “knowledge that’s been passed down across oncology generations,” Dr. Brondfield said he and many of his colleagues believe early palliative supportive care rather than systemic cytotoxic cancer-directed therapy is appropriate for patients with poor performance status who have one of several specific relatively nonchemoresponsive types of metastatic cancer. These include esophageal, gastric, and head and neck cancers.
On the other hand, advanced SCLC isn’t the only type of metastatic cancer that’s so chemosensitive that he and many other oncologists believe aggressive chemotherapy should be offered even in the face of poor patient performance status attributable to the cancer itself.
Take, for example, colorectal cancer with no more than five metastases to the lung or liver, provided those metastases are treatable with resection or radiation. “Those patients are actually curable at a high rate. They have about a 30%-40% cure rate. So those patients, even if they have poor performance status, if we can get them up for surgery or radiation, we usually do try to treat them aggressively,” Dr. Brondfield said.
There are other often chemoresponsive metastatic cancers for which oncologists frequently recommend aggressive treatment to improve quality of life in patients with poor performance status. These cancers include aggressive lymphomas, which are actually often curable; multiple myeloma; testicular and germ cell cancers; NSCLC with a targetable mutation, which is often responsive to oral medications; and prostate and well-differentiated thyroid cancers, which can usually be treated with hormone- or iodine-based therapies rather than more toxic intravenous cytotoxic chemotherapy.
The impact of inpatient palliative chemotherapy in patients with poor performance status and advanced solid cancers not on the short list of highly chemosensitive cancers has not been well studied. A recent retrospective study of 228 such patients who received inpatient palliative chemotherapy at a large Brazilian academic medical center provided little reason for enthusiasm regarding the practice. Survival was short, with 30- and 60-day survival rates of 56% and 39%, respectively. Plus, 30% of patients were admitted to the ICU, where they received aggressive and costly end-of-life care. The investigators found these results suggestive of overprescribing of inpatient palliative chemotherapy (BMC Palliat Care. 2019 May 20;18[1]:42. doi: 10.1186/s12904-019-0427-4).
Of note, the investigators found in a multivariate analysis that an elevated bilirubin was associated with a 217% increased risk of 30-day mortality, and hypercalcemia was associated with a 119% increased risk.
“That’s something to take into account when these decisions are being made,” Dr. Brondfield advised.
In response to an audience comment that oncologists often seem overly optimistic about prognosis, Dr. Brondfield observed, “I think it’s very common for there to be a disagreement between the oncologist wanting to be aggressive for a sick inpatient and the hospitalist or generalist provider thinking: ‘This person looks way too sick for chemotherapy.’ ”
For this reason he is a firm believer in having multidisciplinary conversations regarding prognosis in challenging situations involving hospitalized patients with advanced cancer. An oncologist can bring to such discussions a detailed understanding of clinical trial and molecular data as well as information about the patient’s response to the first round of therapy. But lots of other factors are relevant to prognosis, including nutritional status, comorbidities, and the intuitive eyeball test of how a patient might do. The patient’s family, primary care provider, oncologist, the hospitalist, and the palliative care team will have perspectives of their own.
Molecular testing is now the norm in metastatic cancers
These days oncologists order molecular testing for most patients with metastatic carcinomas to determine eligibility for targeted therapy, suitability for participation in clinical trials, prognostication, and/or assistance in determining the site of origin if that’s unclear.
A single-pass fine needle aspiration biopsy doesn’t provide enough tissue for molecular testing. It’s therefore important to order initially a multipass fine needle aspiration to avoid the need for a repeat biopsy, which is uncomfortable for the patient and can delay diagnosis and treatment.
Dr. Brondfield advised waiting for molecular testing results to come in before trying to prognosticate in patients with a metastatic cancer for which targetable mutations might be present. Survival rates can vary substantially depending upon those test results. Take, for example, metastatic NSCLC: Just within the past year, clinical trials have been published reporting overall survival rates of 39 months in patients with treatable mutations in epidermal growth factor receptor, 42 months with anaplastic lymphoma kinase mutations, and 51 months in patients whose tumor signature features mutations in c-ros oncogene 1, as compared with 22 months with no targetable mutations in the KEYNOTE-189 trial.
“There’s a lot of heterogeneity around how metastatic tumors behave and respond to therapy. Not all metastatic cancers are the same,” the oncologist emphasized.
according to Sam Brondfield, MD, MA, an inpatient medical oncologist at the University of California, San Francisco.

Oncologists have at their fingertips a voluminous and ever-growing body of clinical trials data to draw on for prognostication. Yet many hospitalists will be surprised to learn that this wealth of information is of little value in the inpatient settings where they work, he said at HM20 Virtual, hosted by the Society of Hospital Medicine.
“The applicability of clinical trials data to hospitalized patients is generally poor. That’s an important caveat to keep in mind,” Dr. Brondfield said.
Enrollment in clinical trials is usually restricted to patients with a score of 0 or 1 on the Eastern Clinical Oncology Group Performance Status, meaning their cancer is causing minimal or no disruption to their life (see graphic). Sometimes trials will include patients with a performance status of 2 on the ECOG scale, a tool developed nearly 40 years ago, but clinical trials virtually never enroll those with an ECOG status of 3 or 4. Yet most hospitalized patients with metastatic cancer have an ECOG performance status of 3 or worse. Thus, the clinical trials outcome data are of little relevance.
“In oncology the distinction between ECOG 2 and 3 is very important,” Dr. Brondfield emphasized.
When he talks about treatment options with hospitalized patients who have metastatic cancer and poor performance status – that is, ECOG 3 or 4 – he’ll often say: “Assuming you feel better and can go home, that’s when these clinical trial data may apply better to you.”
Dr. Brondfield cautioned against quoting the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) 5-year overall survival data when hospitalized patients with advanced cancer ask how long they have to live. For one thing, the national average 5-year overall survival figure is hardly an individualized assessment. Plus, oncology is a fast-moving field in which important treatment advances occur all the time, and the SEER data lag far behind. For example, when Dr. Brondfield recently looked up the current SEER 5-year survival for patients diagnosed with metastatic non–small cell lung cancer (NSCLC), the figure quoted was less than 6%, and it was drawn from data accrued in 2009-2015. That simply doesn’t reflect contemporary practice.
Indeed, it’s no longer true that the average survival of patients with metastatic NSCLC is less than a year. In the practice-changing KEYNOTE-189 randomized trial, which accrued participants in 2016-2017, the median overall survival of patients randomized to pembrolizumab (Keytruda) plus standard cytotoxic chemotherapy was 22 months, compared with 11 months with chemotherapy plus placebo (J Clin Oncol. 2020 May 10. doi: 10.1200/JCO.19.03136). As a result, immunotherapy with a programmed death–1 inhibitor such as pembrolizumab in combination with chemotherapy is now standard practice in patients with metastatic NSCLC without targetable mutations.
Performance status guides treatment decision-making
Hospitalists can help oncologists in decision-making regarding whether to offer palliative systemic therapy to patients with advanced metastatic cancer and poor performance status by determining whether that status is caused by the cancer itself or some other cause that’s not easily reversible, such as liver failure.
Take, for example, the inpatient with advanced SCLC. This is an aggressive and chemosensitive cancer. Dr. Brondfield said he is among many medical oncologists who are convinced that, if poor performance status in a patient with advanced SCLC is caused by the cancer itself, prompt initiation of inpatient chemotherapy should be recommended to elicit a response that improves quality of life and performance status in the short term. If, on the other hand, the poor performance status is caused by organ failure or some other issue that can’t easily be improved, hospice may be more appropriate.
“The contour of SCLC over time is that despite its treatment responsiveness it inevitably recurs. But with chemotherapy you can give people in this situation months of quality time, so we generally try to treat these sorts of patients,” Dr. Brondfield explained.
The National Comprehensive Cancer Network guidelines upon which oncologists rely leave lots of room for interpretation regarding the appropriateness of inpatient chemotherapy in patients with advanced cancer and poor patient performance status. Citing “knowledge that’s been passed down across oncology generations,” Dr. Brondfield said he and many of his colleagues believe early palliative supportive care rather than systemic cytotoxic cancer-directed therapy is appropriate for patients with poor performance status who have one of several specific relatively nonchemoresponsive types of metastatic cancer. These include esophageal, gastric, and head and neck cancers.
On the other hand, advanced SCLC isn’t the only type of metastatic cancer that’s so chemosensitive that he and many other oncologists believe aggressive chemotherapy should be offered even in the face of poor patient performance status attributable to the cancer itself.
Take, for example, colorectal cancer with no more than five metastases to the lung or liver, provided those metastases are treatable with resection or radiation. “Those patients are actually curable at a high rate. They have about a 30%-40% cure rate. So those patients, even if they have poor performance status, if we can get them up for surgery or radiation, we usually do try to treat them aggressively,” Dr. Brondfield said.
There are other often chemoresponsive metastatic cancers for which oncologists frequently recommend aggressive treatment to improve quality of life in patients with poor performance status. These cancers include aggressive lymphomas, which are actually often curable; multiple myeloma; testicular and germ cell cancers; NSCLC with a targetable mutation, which is often responsive to oral medications; and prostate and well-differentiated thyroid cancers, which can usually be treated with hormone- or iodine-based therapies rather than more toxic intravenous cytotoxic chemotherapy.
The impact of inpatient palliative chemotherapy in patients with poor performance status and advanced solid cancers not on the short list of highly chemosensitive cancers has not been well studied. A recent retrospective study of 228 such patients who received inpatient palliative chemotherapy at a large Brazilian academic medical center provided little reason for enthusiasm regarding the practice. Survival was short, with 30- and 60-day survival rates of 56% and 39%, respectively. Plus, 30% of patients were admitted to the ICU, where they received aggressive and costly end-of-life care. The investigators found these results suggestive of overprescribing of inpatient palliative chemotherapy (BMC Palliat Care. 2019 May 20;18[1]:42. doi: 10.1186/s12904-019-0427-4).
Of note, the investigators found in a multivariate analysis that an elevated bilirubin was associated with a 217% increased risk of 30-day mortality, and hypercalcemia was associated with a 119% increased risk.
“That’s something to take into account when these decisions are being made,” Dr. Brondfield advised.
In response to an audience comment that oncologists often seem overly optimistic about prognosis, Dr. Brondfield observed, “I think it’s very common for there to be a disagreement between the oncologist wanting to be aggressive for a sick inpatient and the hospitalist or generalist provider thinking: ‘This person looks way too sick for chemotherapy.’ ”
For this reason he is a firm believer in having multidisciplinary conversations regarding prognosis in challenging situations involving hospitalized patients with advanced cancer. An oncologist can bring to such discussions a detailed understanding of clinical trial and molecular data as well as information about the patient’s response to the first round of therapy. But lots of other factors are relevant to prognosis, including nutritional status, comorbidities, and the intuitive eyeball test of how a patient might do. The patient’s family, primary care provider, oncologist, the hospitalist, and the palliative care team will have perspectives of their own.
Molecular testing is now the norm in metastatic cancers
These days oncologists order molecular testing for most patients with metastatic carcinomas to determine eligibility for targeted therapy, suitability for participation in clinical trials, prognostication, and/or assistance in determining the site of origin if that’s unclear.
A single-pass fine needle aspiration biopsy doesn’t provide enough tissue for molecular testing. It’s therefore important to order initially a multipass fine needle aspiration to avoid the need for a repeat biopsy, which is uncomfortable for the patient and can delay diagnosis and treatment.
Dr. Brondfield advised waiting for molecular testing results to come in before trying to prognosticate in patients with a metastatic cancer for which targetable mutations might be present. Survival rates can vary substantially depending upon those test results. Take, for example, metastatic NSCLC: Just within the past year, clinical trials have been published reporting overall survival rates of 39 months in patients with treatable mutations in epidermal growth factor receptor, 42 months with anaplastic lymphoma kinase mutations, and 51 months in patients whose tumor signature features mutations in c-ros oncogene 1, as compared with 22 months with no targetable mutations in the KEYNOTE-189 trial.
“There’s a lot of heterogeneity around how metastatic tumors behave and respond to therapy. Not all metastatic cancers are the same,” the oncologist emphasized.
FROM HM20 VIRTUAL
Consensus document reviews determination of brain death
The document, a result of the World Brain Death Project, surveys the clinical aspects of this determination, such as clinical testing, apnea testing, and the number of examinations required, as well as its social and legal aspects, including documentation, qualifications for making the determination, and religious attitudes toward BD/DNC.
The recommendations are the minimum criteria for BD/DNC, and countries and professional societies may choose to adopt stricter criteria, the authors noted. Seventeen supplements to the consensus statement contain detailed reports on topics the statement examines, including focuses on both adults and children.
“Perhaps the most important points of this project are, first, to show the worldwide acceptance of the concept of BD/DNC and what the minimum requirements are for BD/DNC,” said corresponding author Gene Sung, MD, MPH, director of the neurocritical care and stroke division at the University of Southern California, Los Angeles. Second, “this standard is centered around a clinical determination without the need for other testing.”
The consensus document and supplements were published online Aug. 3 in JAMA.
Comprehensive review
A lack of rigor has led to many differences in the determination of BD/DNC, said Dr. Sung. “Some of the variance that is common are the numbers of exams and examiners that are required and whether ancillary tests are required for determination of BD/DNC. In addition, a lot of guidelines and protocols that are in use are not thorough in detailing how to do the examinations and what to do in different circumstances.”
Professional societies such as the World Federation of Intensive and Critical Care recruited experts in BD/DNC to develop recommendations, which were based on relevant articles that they identified during a literature search. “We wanted to develop a fairly comprehensive document that, along with the 17 supplements, builds a foundation to show how to determine BD/DNC – what the minimum clinical criteria needed are and what to do in special circumstances,” Dr. Sung said.
Major sections of the statement include recommendations for the minimum clinical standards for the determination of BD/DNC in adults and children.
Determination must begin by establishing that the patient has sustained an irreversible brain injury that resulted in the loss of all brain function, according to the authors. Confounders such as pharmacologic paralysis and the effect of CNS depressant medications should be ruled out.
In addition, clinical evaluation must include an assessment for coma and an evaluation for brain stem areflexia. Among other criteria, the pupils should be fixed and nonresponsive to light, the face should not move in response to noxious cranial stimulation, and the gag and cough reflexes should be absent. Apnea testing is recommended to evaluate the responsiveness of respiratory centers in the medulla.
Although the definition of BD/DNC is the same in children as in adults, less evidence is available for the determination of BD/DNC in the very young. The authors thus advised a cautious approach to the evaluation of infants and younger children.
Recommendations vary by age and often require serial examinations, including apnea testing, they noted.
Ancillary testing
The consensus statement also reviews ancillary testing, which the authors recommend be required when the minimum clinical examination, including the apnea test, cannot be completed and when it is in the presence of confounding conditions that cannot be resolved.
The authors recommended digital subtraction angiography, radionuclide studies, and transcranial Doppler ultrasonography as ancillary tests based on blood flow in the brain. However, CT angiography and magnetic resonance angiography not be used.
A lack of guidance makes performing an apnea test in patients receiving extracorporeal membrane oxygenation (ECMO) challenging, according to the authors. Nevertheless, they recommended that the same principles of BD/DNC be applied to adults and children receiving ECMO.
They further recommended a period of preoxygenation before the apnea test, and the document describes in detail the method for administering this test to people receiving ECMO.
Another potentially challenging situation pointed out in the consensus document is the determination of BD/DNC in patients who have been treated with targeted temperature management. Therapeutic hypothermia, particularly if it is preceded or accompanied by sedation, can temporarily impair brain stem reflexes, thus mimicking BD/DNC.
The new document includes a flowchart and step-by-step recommendations as well as suggestions for determining BD/DNC under these circumstances.
Among document limitations acknowledged by the authors is the lack of high-quality data from randomized, controlled trials on which to base their recommendations.
In addition, economic, technological, or personnel limitations may reduce the available options for ancillary testing, they added. Also, the recommendations do not incorporate contributions from patients or social or religious groups, although the authors were mindful of their concerns.
To promote the national and international harmonization of BD/DNC criteria, “medical societies and countries can evaluate their own policies in relation to this document and fix any deficiencies,” Dr. Sung said.
“Many countries do not have any BD/DNC policies and can use the documents from this project to create their own. There may need to be discussions with legal, governmental, religious, and societal leaders to help understand and accept BD/DNC and to help enact policies in different communities,” he added.
Divergent definitions
The determination of death is not simply a scientific question, but also a philosophical, religious, and cultural question, wrote Robert D. Truog, MD, director of the Harvard Center for Bioethics, Boston, and colleagues in an accompanying editorial. Future research should consider cultural differences over these questions.
“Most important is that there be a clear and logical consistency between the definition of death and the tests that are used to diagnose it,” Dr. Truog said.
The concept of whole brain death was advanced as an equivalent to biological death, “such that, when the brain dies, the body literally disintegrates, just as it does after cardiac arrest,” but evidence indicates that this claim is untrue, Dr. Truog said. Current tests also do not diagnose the death of the whole brain.
Another hypothesis is that brain stem death represents the irreversible loss of consciousness and the capacity for spontaneous respiration.
“Instead of focusing on biology, [this definition] focuses on values and is based on the claim that when a person is in a state of irreversible apneic unconsciousness, we may consider them to be dead,” said Dr. Truog. He and his coeditorialists argued that the concept of whole brain death should be replaced with that of brain stem death.
“This report should be a call for our profession, as well as for federal and state lawmakers, to reform our laws so that they are consistent with our diagnostic criteria,” Dr. Truog said.
“The most straightforward way of doing this would be to change U.S. law and adopt the British standard of brain stem death, and then refine our testing to make the diagnosis of irreversible apneic unconsciousness as reliable and safe as possible,” he concluded.
The drafting of the consensus statement was not supported by outside funding. Dr. Sung reported no relevant financial relationships. Dr. Truog reported receiving compensation from Sanofi and Covance for participating in data and safety monitoring boards unrelated to the consensus document.
A version of this article originally appeared on Medscape.com.
The document, a result of the World Brain Death Project, surveys the clinical aspects of this determination, such as clinical testing, apnea testing, and the number of examinations required, as well as its social and legal aspects, including documentation, qualifications for making the determination, and religious attitudes toward BD/DNC.
The recommendations are the minimum criteria for BD/DNC, and countries and professional societies may choose to adopt stricter criteria, the authors noted. Seventeen supplements to the consensus statement contain detailed reports on topics the statement examines, including focuses on both adults and children.
“Perhaps the most important points of this project are, first, to show the worldwide acceptance of the concept of BD/DNC and what the minimum requirements are for BD/DNC,” said corresponding author Gene Sung, MD, MPH, director of the neurocritical care and stroke division at the University of Southern California, Los Angeles. Second, “this standard is centered around a clinical determination without the need for other testing.”
The consensus document and supplements were published online Aug. 3 in JAMA.
Comprehensive review
A lack of rigor has led to many differences in the determination of BD/DNC, said Dr. Sung. “Some of the variance that is common are the numbers of exams and examiners that are required and whether ancillary tests are required for determination of BD/DNC. In addition, a lot of guidelines and protocols that are in use are not thorough in detailing how to do the examinations and what to do in different circumstances.”
Professional societies such as the World Federation of Intensive and Critical Care recruited experts in BD/DNC to develop recommendations, which were based on relevant articles that they identified during a literature search. “We wanted to develop a fairly comprehensive document that, along with the 17 supplements, builds a foundation to show how to determine BD/DNC – what the minimum clinical criteria needed are and what to do in special circumstances,” Dr. Sung said.
Major sections of the statement include recommendations for the minimum clinical standards for the determination of BD/DNC in adults and children.
Determination must begin by establishing that the patient has sustained an irreversible brain injury that resulted in the loss of all brain function, according to the authors. Confounders such as pharmacologic paralysis and the effect of CNS depressant medications should be ruled out.
In addition, clinical evaluation must include an assessment for coma and an evaluation for brain stem areflexia. Among other criteria, the pupils should be fixed and nonresponsive to light, the face should not move in response to noxious cranial stimulation, and the gag and cough reflexes should be absent. Apnea testing is recommended to evaluate the responsiveness of respiratory centers in the medulla.
Although the definition of BD/DNC is the same in children as in adults, less evidence is available for the determination of BD/DNC in the very young. The authors thus advised a cautious approach to the evaluation of infants and younger children.
Recommendations vary by age and often require serial examinations, including apnea testing, they noted.
Ancillary testing
The consensus statement also reviews ancillary testing, which the authors recommend be required when the minimum clinical examination, including the apnea test, cannot be completed and when it is in the presence of confounding conditions that cannot be resolved.
The authors recommended digital subtraction angiography, radionuclide studies, and transcranial Doppler ultrasonography as ancillary tests based on blood flow in the brain. However, CT angiography and magnetic resonance angiography not be used.
A lack of guidance makes performing an apnea test in patients receiving extracorporeal membrane oxygenation (ECMO) challenging, according to the authors. Nevertheless, they recommended that the same principles of BD/DNC be applied to adults and children receiving ECMO.
They further recommended a period of preoxygenation before the apnea test, and the document describes in detail the method for administering this test to people receiving ECMO.
Another potentially challenging situation pointed out in the consensus document is the determination of BD/DNC in patients who have been treated with targeted temperature management. Therapeutic hypothermia, particularly if it is preceded or accompanied by sedation, can temporarily impair brain stem reflexes, thus mimicking BD/DNC.
The new document includes a flowchart and step-by-step recommendations as well as suggestions for determining BD/DNC under these circumstances.
Among document limitations acknowledged by the authors is the lack of high-quality data from randomized, controlled trials on which to base their recommendations.
In addition, economic, technological, or personnel limitations may reduce the available options for ancillary testing, they added. Also, the recommendations do not incorporate contributions from patients or social or religious groups, although the authors were mindful of their concerns.
To promote the national and international harmonization of BD/DNC criteria, “medical societies and countries can evaluate their own policies in relation to this document and fix any deficiencies,” Dr. Sung said.
“Many countries do not have any BD/DNC policies and can use the documents from this project to create their own. There may need to be discussions with legal, governmental, religious, and societal leaders to help understand and accept BD/DNC and to help enact policies in different communities,” he added.
Divergent definitions
The determination of death is not simply a scientific question, but also a philosophical, religious, and cultural question, wrote Robert D. Truog, MD, director of the Harvard Center for Bioethics, Boston, and colleagues in an accompanying editorial. Future research should consider cultural differences over these questions.
“Most important is that there be a clear and logical consistency between the definition of death and the tests that are used to diagnose it,” Dr. Truog said.
The concept of whole brain death was advanced as an equivalent to biological death, “such that, when the brain dies, the body literally disintegrates, just as it does after cardiac arrest,” but evidence indicates that this claim is untrue, Dr. Truog said. Current tests also do not diagnose the death of the whole brain.
Another hypothesis is that brain stem death represents the irreversible loss of consciousness and the capacity for spontaneous respiration.
“Instead of focusing on biology, [this definition] focuses on values and is based on the claim that when a person is in a state of irreversible apneic unconsciousness, we may consider them to be dead,” said Dr. Truog. He and his coeditorialists argued that the concept of whole brain death should be replaced with that of brain stem death.
“This report should be a call for our profession, as well as for federal and state lawmakers, to reform our laws so that they are consistent with our diagnostic criteria,” Dr. Truog said.
“The most straightforward way of doing this would be to change U.S. law and adopt the British standard of brain stem death, and then refine our testing to make the diagnosis of irreversible apneic unconsciousness as reliable and safe as possible,” he concluded.
The drafting of the consensus statement was not supported by outside funding. Dr. Sung reported no relevant financial relationships. Dr. Truog reported receiving compensation from Sanofi and Covance for participating in data and safety monitoring boards unrelated to the consensus document.
A version of this article originally appeared on Medscape.com.
The document, a result of the World Brain Death Project, surveys the clinical aspects of this determination, such as clinical testing, apnea testing, and the number of examinations required, as well as its social and legal aspects, including documentation, qualifications for making the determination, and religious attitudes toward BD/DNC.
The recommendations are the minimum criteria for BD/DNC, and countries and professional societies may choose to adopt stricter criteria, the authors noted. Seventeen supplements to the consensus statement contain detailed reports on topics the statement examines, including focuses on both adults and children.
“Perhaps the most important points of this project are, first, to show the worldwide acceptance of the concept of BD/DNC and what the minimum requirements are for BD/DNC,” said corresponding author Gene Sung, MD, MPH, director of the neurocritical care and stroke division at the University of Southern California, Los Angeles. Second, “this standard is centered around a clinical determination without the need for other testing.”
The consensus document and supplements were published online Aug. 3 in JAMA.
Comprehensive review
A lack of rigor has led to many differences in the determination of BD/DNC, said Dr. Sung. “Some of the variance that is common are the numbers of exams and examiners that are required and whether ancillary tests are required for determination of BD/DNC. In addition, a lot of guidelines and protocols that are in use are not thorough in detailing how to do the examinations and what to do in different circumstances.”
Professional societies such as the World Federation of Intensive and Critical Care recruited experts in BD/DNC to develop recommendations, which were based on relevant articles that they identified during a literature search. “We wanted to develop a fairly comprehensive document that, along with the 17 supplements, builds a foundation to show how to determine BD/DNC – what the minimum clinical criteria needed are and what to do in special circumstances,” Dr. Sung said.
Major sections of the statement include recommendations for the minimum clinical standards for the determination of BD/DNC in adults and children.
Determination must begin by establishing that the patient has sustained an irreversible brain injury that resulted in the loss of all brain function, according to the authors. Confounders such as pharmacologic paralysis and the effect of CNS depressant medications should be ruled out.
In addition, clinical evaluation must include an assessment for coma and an evaluation for brain stem areflexia. Among other criteria, the pupils should be fixed and nonresponsive to light, the face should not move in response to noxious cranial stimulation, and the gag and cough reflexes should be absent. Apnea testing is recommended to evaluate the responsiveness of respiratory centers in the medulla.
Although the definition of BD/DNC is the same in children as in adults, less evidence is available for the determination of BD/DNC in the very young. The authors thus advised a cautious approach to the evaluation of infants and younger children.
Recommendations vary by age and often require serial examinations, including apnea testing, they noted.
Ancillary testing
The consensus statement also reviews ancillary testing, which the authors recommend be required when the minimum clinical examination, including the apnea test, cannot be completed and when it is in the presence of confounding conditions that cannot be resolved.
The authors recommended digital subtraction angiography, radionuclide studies, and transcranial Doppler ultrasonography as ancillary tests based on blood flow in the brain. However, CT angiography and magnetic resonance angiography not be used.
A lack of guidance makes performing an apnea test in patients receiving extracorporeal membrane oxygenation (ECMO) challenging, according to the authors. Nevertheless, they recommended that the same principles of BD/DNC be applied to adults and children receiving ECMO.
They further recommended a period of preoxygenation before the apnea test, and the document describes in detail the method for administering this test to people receiving ECMO.
Another potentially challenging situation pointed out in the consensus document is the determination of BD/DNC in patients who have been treated with targeted temperature management. Therapeutic hypothermia, particularly if it is preceded or accompanied by sedation, can temporarily impair brain stem reflexes, thus mimicking BD/DNC.
The new document includes a flowchart and step-by-step recommendations as well as suggestions for determining BD/DNC under these circumstances.
Among document limitations acknowledged by the authors is the lack of high-quality data from randomized, controlled trials on which to base their recommendations.
In addition, economic, technological, or personnel limitations may reduce the available options for ancillary testing, they added. Also, the recommendations do not incorporate contributions from patients or social or religious groups, although the authors were mindful of their concerns.
To promote the national and international harmonization of BD/DNC criteria, “medical societies and countries can evaluate their own policies in relation to this document and fix any deficiencies,” Dr. Sung said.
“Many countries do not have any BD/DNC policies and can use the documents from this project to create their own. There may need to be discussions with legal, governmental, religious, and societal leaders to help understand and accept BD/DNC and to help enact policies in different communities,” he added.
Divergent definitions
The determination of death is not simply a scientific question, but also a philosophical, religious, and cultural question, wrote Robert D. Truog, MD, director of the Harvard Center for Bioethics, Boston, and colleagues in an accompanying editorial. Future research should consider cultural differences over these questions.
“Most important is that there be a clear and logical consistency between the definition of death and the tests that are used to diagnose it,” Dr. Truog said.
The concept of whole brain death was advanced as an equivalent to biological death, “such that, when the brain dies, the body literally disintegrates, just as it does after cardiac arrest,” but evidence indicates that this claim is untrue, Dr. Truog said. Current tests also do not diagnose the death of the whole brain.
Another hypothesis is that brain stem death represents the irreversible loss of consciousness and the capacity for spontaneous respiration.
“Instead of focusing on biology, [this definition] focuses on values and is based on the claim that when a person is in a state of irreversible apneic unconsciousness, we may consider them to be dead,” said Dr. Truog. He and his coeditorialists argued that the concept of whole brain death should be replaced with that of brain stem death.
“This report should be a call for our profession, as well as for federal and state lawmakers, to reform our laws so that they are consistent with our diagnostic criteria,” Dr. Truog said.
“The most straightforward way of doing this would be to change U.S. law and adopt the British standard of brain stem death, and then refine our testing to make the diagnosis of irreversible apneic unconsciousness as reliable and safe as possible,” he concluded.
The drafting of the consensus statement was not supported by outside funding. Dr. Sung reported no relevant financial relationships. Dr. Truog reported receiving compensation from Sanofi and Covance for participating in data and safety monitoring boards unrelated to the consensus document.
A version of this article originally appeared on Medscape.com.
Value of palliative care shines clearly in a crisis
Hospitalists have played a key role
For some palliative care professionals, the COVID-19 pandemic, particularly in viral hot spots like New York City, represents a “moment” that could lead to greater awareness of what this service offers to seriously ill patients in a crisis.
They say it has provided an opportunity to show what palliative care teams can contribute to the difficult circumstances of patients with severe symptoms, isolated and alone in quarantined hospitals, with poor survival rates, perhaps sedated for extended stays on scarce ventilators – and for their family members, who are able to visit them only virtually via telephone or tablet.
But it has also highlighted gaps – including insufficient staffing for some palliative care teams. Hospitalists and other clinicians in the hospital need to learn the basics of primary palliative care, such as how to communicate bad news, initiate goals of care conversations, and address common symptoms of serious illness, such as pain. That way, they could shoulder more of the demand for this kind of care when palliative care specialists are in short supply.
Hospitalists, some of whom also have pursued a specialization in palliative care, have played key roles in clarifying and redefining the new role for palliative care, whom it is meant for, and who should provide it. Central to this new role is the greater use of telemedicine – for talking to hospitalized patients without increasing viral exposure, for linking up with family members who can’t visit their loved ones in the hospital, and for helping frontline hospital staff who need a palliative care consultation – or just a chance to debrief on what they are seeing.
A pandemic wake-up call
Elizabeth Gundersen, MD, FHM, FAAHPM, director of the hospice and palliative medicine fellowship program at the Charles E. Schmidt College of Medicine at Florida Atlantic University (FAU) in Boca Raton, practiced hospital medicine for 10 years before pursuing a fellowship in hospice and palliative medicine and working as an academic palliative medicine physician. She calls the pandemic a wake-up call for gaps in care and all the things that weren’t working well in the health care system.
“Now we are seeing more clearly what’s lacking – or broken – and what we will carry forward from this experience into the post-COVID world,” she said. Some hospitalists do palliative care very well, and others don’t feel as comfortable in having these difficult conversations with patients. But in the uncertain course of the virus they get thrust into it.
Although FAU’s associated hospitals were not as inundated with COVID-19 patients in the early weeks of the pandemic as were other regions, the volume of other patients plummeted, Dr. Gundersen said, adding that “there’s still been incredible intensity and worry about the virus. For me, the basic role of palliative care hasn’t changed, and the phrase I have always used when introducing myself – ‘we’re an extra layer of support for the patient and family’ – still holds true,” she said.
“I try to make it clear to people that palliative care is not synonymous with end-of-life care. We don’t want people to think that a palliative care referral implies imminent death. The goal is not to get more people to have a do not attempt resuscitation (DNAR) order, but to determine the patient and family’s treatment goals and whether a DNAR order fits those goals.”
The tough conversations
Dr. Gundersen is cochair of SHM’s Palliative Care Special Interest Group, along with Rab Razzak, MD, clinical director of palliative medicine at University Hospitals Cleveland Medical Center, one of the hospitals affiliated with Case Western University in Cleveland. (Connect with them on Twitter: @Top_Gundersen and @rabrazzak.)
Dr. Razzak also transitioned from hospital medicine to palliative medicine 10 years ago. “As a hospitalist, I enjoyed the tough conversations and bringing the human element into my health care interactions,” he explained. “To me, palliative care is a philosophy of care that puts the person we call the patient at the center of the interaction, while we try to figure out how to best care for them as a person.”
When the pandemic hit, University Hospitals made 20 ICU beds available for COVID-19 patients, Dr. Razzak said. This unit has since been full but not overflowing, while overall hospital census went down. The palliative care team at the hospital includes four inpatient doctors, nurse practitioners, and a chaplain, as well as an outpatient team primarily focused on oncology.
“In some settings, palliative care has been at the forefront of difficult conversations, when things aren’t going well for the patient and there’s much uncertainty,” Dr. Razzak said. The interface between hospital medicine and palliative care can be complementary, he added. “We talk about primary palliative care, which we want every discipline to be able to do – lead meaningful conversations, help manage symptoms.”
The take-home message for hospitalists, he said, is to get training in how to have these discussions, using such resources as VitalTalk (https://www.vitaltalk.org/), a nonprofit organization that disseminates education in communication skills for difficult conversations, and the Center to Advance Palliative Care (www.capc.org) at Icahn School of Medicine at Mount Sinai in New York City. “Once you’ve mastered the conversation, it will get easier. But ask for help when you need it, and learn how to know when you need it.”
Dr. Gundersen added that hospital medicine groups and palliative care teams could reach out to each other and talk about what they did in the crisis and how they can work together in the future. She recommends frequent ongoing support and collaboration that could range from formal conferences or training sessions to informal team interactions, perhaps with sandwiches in the doctor’s lounge – provided that there’s room for social distancing. She has recently started giving talks in the community and grand rounds presentations in hospitals about palliative care.
Other approaches and applications
In New York City, the initial epicenter for the pandemic in the United States, the adult palliative care service of Columbia University Medical Center (CUMC) experienced a sevenfold increase in consultation requests at the apex of the crisis, said its director, Craig Blinderman, MD. That demand was impossible to meet with existing staff. So Dr. Blinderman and colleagues established a virtual consultation model, recruiting and deploying volunteer out-of-state palliative care specialists to staff it.
An eight-bed palliative care unit was opened at CUMC for COVID-19 patients whose surrogates had opted not to initiate or continue intubation or life-sustaining treatments. This helped to relieve some of the pressures on the ICUs while making it possible for in-person visits to the hospice unit by families – in full PPE. Palliative care staff were embedded in various units in the hospital.
A palliative care response team composed of a hospice and palliative medicine fellow and four psychiatry residents or fellows, based in the emergency department and with supervision from the palliative care team, provided time-critical goals of care conversations with families using telemedicine – and a forum for listening to their suffering. Dr. Blinderman and colleagues also have found time to write up their experience for medical journals.1,2
There’s no reason to think that hospitalists, with a little basic training, couldn’t be having these same goals of care conversations, Dr. Blinderman said. “But the fact that hospitalists, at the pandemic’s peak, along with ICU doctors, were seeing an unprecedented magnitude of dying on a daily basis generated a lot of moral distress for them.”
Palliative care professionals, because they engage with these issues in a different way, may be somewhat better equipped to deal with the sheer emotional demands when so many are dying, as at the peak of the surge in New York. “We don’t see dying as a failure on our part but an opportunity to relieve suffering,” Dr. Blinderman said. And the palliative care field also emphasizes the importance of self-care for its practitioners.
“How do we meet the incredible palliative care needs in the epicenter of a pandemic? That question also applies to other kinds of crises we could imagine, for example, climate-related disasters,” Dr. Blinderman said. “What lessons have we learned about the value of palliative care and how to start incorporating it more integrally into the delivery of hospital care? Here we showed that we could work collaboratively with our colleagues at other major medical centers, bringing together their expertise to help us when we didn’t have the bandwidth to meet the demand,” he said.
Scripts can help
“Also, it won’t make sense to just go back to normal (after the crisis fades),” Dr. Blinderman said. “We need to take a close look at how our society is functioning in the wake of the pandemic and the ways the health care system has failed us. We have learned that we’re all interconnected and we need to work together to serve our communities – locally and nationally – applying basic distributive justice.”
Could there be, for example, a national infrastructure for mobilizing and deploying palliative care resources to areas of greatest need, similar to what was done in New York?
At Northwestern Medicine in Chicago, a number of palliative care clinicians at the system’s hospitals worked together to develop scripts designed to help other clinicians start goals of care conversations with patients and families, for use in the hospital as well as in outpatient primary care and other settings, with results integrated into the system’s electronic health record.
Front-line clinicians may not have the time to ask for formal consults from palliative care because of high volume and rapidly changing patient status, explained Eytan Szmuilowicz, MD, director of the section of palliative medicine at Northwestern Memorial Hospital. Or they may not have access to specialty-level palliative care in their settings.
The scripts are aimed at primary care, emergency physicians, and hospitalists needing to consider critical care placement or attempted resuscitation and to ICU clinicians helping families make decisions about life-sustaining treatments. They also can help facilitate advance care planning discussions. An example is “CALMER,” a six-step mnemonic guide to promote goals of care discussions with hospitalized patients. For more information on these scripts, contact Dr. Szmuilowicz: [email protected].
Eerily quiet
The COVID-19 crisis has been quite a whirlwind for hospital medicine, said Jeanie Youngwerth, MD, a hospitalist and program director of the palliative care service at the University of Colorado in Denver, which was a significant viral hotspot early on.
“When it first started, things seemed to change almost overnight – starting on Friday, March 13. People had to take action right away to develop work flows and the technology to allow us to see as many patients as possible,” she said. By the time Monday came, it was a whole new ballgame.
Dr. Youngwerth and two colleagues worked quickly to develop inpatient telemedicine capacity where none existed. “We knew we would not be going into patients’ rooms, but most of our team showed up in the hospital to work with the primary care teams. Our job was to see what we could do that actually made a difference,” she said.
“The hospital became a very strange place. You’d walk down the hallway and it was eerily quiet. Everybody you came across was being so nice to each other.” Televisits became a powerful way to bring the human connection back to medical care.
“What we learned from families was that they were thirsting to have some kind of connection with their loved one, and to be able to talk about their loved one and who they were as a person,” she said. “We’d contact the family through video visits and then, when the family meeting ended, the nurse would bring an iPad into the patient’s room so the family could see their loved one on a ventilator. They would immediately start communicating with their loved one, praying aloud, singing, playing music. It would make a huge difference for the family – and for the staff.”
References
1. Nakagawa S et al. Pandemic palliative care consultations spanning state and institutional borders. J Am Geriatr Soc. 2020 May 22. doi: 10.1111/jgs.16643.
2. Lee J Abrukin L, Flores S. Early intervention of palliative care in the emergency department during the COVID-19 pandemic. JAMA Intern Med. 2020 Jun 5. doi: 10.1001/jamainternmed.2020.2713.
Hospitalists have played a key role
Hospitalists have played a key role
For some palliative care professionals, the COVID-19 pandemic, particularly in viral hot spots like New York City, represents a “moment” that could lead to greater awareness of what this service offers to seriously ill patients in a crisis.
They say it has provided an opportunity to show what palliative care teams can contribute to the difficult circumstances of patients with severe symptoms, isolated and alone in quarantined hospitals, with poor survival rates, perhaps sedated for extended stays on scarce ventilators – and for their family members, who are able to visit them only virtually via telephone or tablet.
But it has also highlighted gaps – including insufficient staffing for some palliative care teams. Hospitalists and other clinicians in the hospital need to learn the basics of primary palliative care, such as how to communicate bad news, initiate goals of care conversations, and address common symptoms of serious illness, such as pain. That way, they could shoulder more of the demand for this kind of care when palliative care specialists are in short supply.
Hospitalists, some of whom also have pursued a specialization in palliative care, have played key roles in clarifying and redefining the new role for palliative care, whom it is meant for, and who should provide it. Central to this new role is the greater use of telemedicine – for talking to hospitalized patients without increasing viral exposure, for linking up with family members who can’t visit their loved ones in the hospital, and for helping frontline hospital staff who need a palliative care consultation – or just a chance to debrief on what they are seeing.
A pandemic wake-up call
Elizabeth Gundersen, MD, FHM, FAAHPM, director of the hospice and palliative medicine fellowship program at the Charles E. Schmidt College of Medicine at Florida Atlantic University (FAU) in Boca Raton, practiced hospital medicine for 10 years before pursuing a fellowship in hospice and palliative medicine and working as an academic palliative medicine physician. She calls the pandemic a wake-up call for gaps in care and all the things that weren’t working well in the health care system.
“Now we are seeing more clearly what’s lacking – or broken – and what we will carry forward from this experience into the post-COVID world,” she said. Some hospitalists do palliative care very well, and others don’t feel as comfortable in having these difficult conversations with patients. But in the uncertain course of the virus they get thrust into it.
Although FAU’s associated hospitals were not as inundated with COVID-19 patients in the early weeks of the pandemic as were other regions, the volume of other patients plummeted, Dr. Gundersen said, adding that “there’s still been incredible intensity and worry about the virus. For me, the basic role of palliative care hasn’t changed, and the phrase I have always used when introducing myself – ‘we’re an extra layer of support for the patient and family’ – still holds true,” she said.
“I try to make it clear to people that palliative care is not synonymous with end-of-life care. We don’t want people to think that a palliative care referral implies imminent death. The goal is not to get more people to have a do not attempt resuscitation (DNAR) order, but to determine the patient and family’s treatment goals and whether a DNAR order fits those goals.”
The tough conversations
Dr. Gundersen is cochair of SHM’s Palliative Care Special Interest Group, along with Rab Razzak, MD, clinical director of palliative medicine at University Hospitals Cleveland Medical Center, one of the hospitals affiliated with Case Western University in Cleveland. (Connect with them on Twitter: @Top_Gundersen and @rabrazzak.)
Dr. Razzak also transitioned from hospital medicine to palliative medicine 10 years ago. “As a hospitalist, I enjoyed the tough conversations and bringing the human element into my health care interactions,” he explained. “To me, palliative care is a philosophy of care that puts the person we call the patient at the center of the interaction, while we try to figure out how to best care for them as a person.”
When the pandemic hit, University Hospitals made 20 ICU beds available for COVID-19 patients, Dr. Razzak said. This unit has since been full but not overflowing, while overall hospital census went down. The palliative care team at the hospital includes four inpatient doctors, nurse practitioners, and a chaplain, as well as an outpatient team primarily focused on oncology.
“In some settings, palliative care has been at the forefront of difficult conversations, when things aren’t going well for the patient and there’s much uncertainty,” Dr. Razzak said. The interface between hospital medicine and palliative care can be complementary, he added. “We talk about primary palliative care, which we want every discipline to be able to do – lead meaningful conversations, help manage symptoms.”
The take-home message for hospitalists, he said, is to get training in how to have these discussions, using such resources as VitalTalk (https://www.vitaltalk.org/), a nonprofit organization that disseminates education in communication skills for difficult conversations, and the Center to Advance Palliative Care (www.capc.org) at Icahn School of Medicine at Mount Sinai in New York City. “Once you’ve mastered the conversation, it will get easier. But ask for help when you need it, and learn how to know when you need it.”
Dr. Gundersen added that hospital medicine groups and palliative care teams could reach out to each other and talk about what they did in the crisis and how they can work together in the future. She recommends frequent ongoing support and collaboration that could range from formal conferences or training sessions to informal team interactions, perhaps with sandwiches in the doctor’s lounge – provided that there’s room for social distancing. She has recently started giving talks in the community and grand rounds presentations in hospitals about palliative care.
Other approaches and applications
In New York City, the initial epicenter for the pandemic in the United States, the adult palliative care service of Columbia University Medical Center (CUMC) experienced a sevenfold increase in consultation requests at the apex of the crisis, said its director, Craig Blinderman, MD. That demand was impossible to meet with existing staff. So Dr. Blinderman and colleagues established a virtual consultation model, recruiting and deploying volunteer out-of-state palliative care specialists to staff it.
An eight-bed palliative care unit was opened at CUMC for COVID-19 patients whose surrogates had opted not to initiate or continue intubation or life-sustaining treatments. This helped to relieve some of the pressures on the ICUs while making it possible for in-person visits to the hospice unit by families – in full PPE. Palliative care staff were embedded in various units in the hospital.
A palliative care response team composed of a hospice and palliative medicine fellow and four psychiatry residents or fellows, based in the emergency department and with supervision from the palliative care team, provided time-critical goals of care conversations with families using telemedicine – and a forum for listening to their suffering. Dr. Blinderman and colleagues also have found time to write up their experience for medical journals.1,2
There’s no reason to think that hospitalists, with a little basic training, couldn’t be having these same goals of care conversations, Dr. Blinderman said. “But the fact that hospitalists, at the pandemic’s peak, along with ICU doctors, were seeing an unprecedented magnitude of dying on a daily basis generated a lot of moral distress for them.”
Palliative care professionals, because they engage with these issues in a different way, may be somewhat better equipped to deal with the sheer emotional demands when so many are dying, as at the peak of the surge in New York. “We don’t see dying as a failure on our part but an opportunity to relieve suffering,” Dr. Blinderman said. And the palliative care field also emphasizes the importance of self-care for its practitioners.
“How do we meet the incredible palliative care needs in the epicenter of a pandemic? That question also applies to other kinds of crises we could imagine, for example, climate-related disasters,” Dr. Blinderman said. “What lessons have we learned about the value of palliative care and how to start incorporating it more integrally into the delivery of hospital care? Here we showed that we could work collaboratively with our colleagues at other major medical centers, bringing together their expertise to help us when we didn’t have the bandwidth to meet the demand,” he said.
Scripts can help
“Also, it won’t make sense to just go back to normal (after the crisis fades),” Dr. Blinderman said. “We need to take a close look at how our society is functioning in the wake of the pandemic and the ways the health care system has failed us. We have learned that we’re all interconnected and we need to work together to serve our communities – locally and nationally – applying basic distributive justice.”
Could there be, for example, a national infrastructure for mobilizing and deploying palliative care resources to areas of greatest need, similar to what was done in New York?
At Northwestern Medicine in Chicago, a number of palliative care clinicians at the system’s hospitals worked together to develop scripts designed to help other clinicians start goals of care conversations with patients and families, for use in the hospital as well as in outpatient primary care and other settings, with results integrated into the system’s electronic health record.
Front-line clinicians may not have the time to ask for formal consults from palliative care because of high volume and rapidly changing patient status, explained Eytan Szmuilowicz, MD, director of the section of palliative medicine at Northwestern Memorial Hospital. Or they may not have access to specialty-level palliative care in their settings.
The scripts are aimed at primary care, emergency physicians, and hospitalists needing to consider critical care placement or attempted resuscitation and to ICU clinicians helping families make decisions about life-sustaining treatments. They also can help facilitate advance care planning discussions. An example is “CALMER,” a six-step mnemonic guide to promote goals of care discussions with hospitalized patients. For more information on these scripts, contact Dr. Szmuilowicz: [email protected].
Eerily quiet
The COVID-19 crisis has been quite a whirlwind for hospital medicine, said Jeanie Youngwerth, MD, a hospitalist and program director of the palliative care service at the University of Colorado in Denver, which was a significant viral hotspot early on.
“When it first started, things seemed to change almost overnight – starting on Friday, March 13. People had to take action right away to develop work flows and the technology to allow us to see as many patients as possible,” she said. By the time Monday came, it was a whole new ballgame.
Dr. Youngwerth and two colleagues worked quickly to develop inpatient telemedicine capacity where none existed. “We knew we would not be going into patients’ rooms, but most of our team showed up in the hospital to work with the primary care teams. Our job was to see what we could do that actually made a difference,” she said.
“The hospital became a very strange place. You’d walk down the hallway and it was eerily quiet. Everybody you came across was being so nice to each other.” Televisits became a powerful way to bring the human connection back to medical care.
“What we learned from families was that they were thirsting to have some kind of connection with their loved one, and to be able to talk about their loved one and who they were as a person,” she said. “We’d contact the family through video visits and then, when the family meeting ended, the nurse would bring an iPad into the patient’s room so the family could see their loved one on a ventilator. They would immediately start communicating with their loved one, praying aloud, singing, playing music. It would make a huge difference for the family – and for the staff.”
References
1. Nakagawa S et al. Pandemic palliative care consultations spanning state and institutional borders. J Am Geriatr Soc. 2020 May 22. doi: 10.1111/jgs.16643.
2. Lee J Abrukin L, Flores S. Early intervention of palliative care in the emergency department during the COVID-19 pandemic. JAMA Intern Med. 2020 Jun 5. doi: 10.1001/jamainternmed.2020.2713.
For some palliative care professionals, the COVID-19 pandemic, particularly in viral hot spots like New York City, represents a “moment” that could lead to greater awareness of what this service offers to seriously ill patients in a crisis.
They say it has provided an opportunity to show what palliative care teams can contribute to the difficult circumstances of patients with severe symptoms, isolated and alone in quarantined hospitals, with poor survival rates, perhaps sedated for extended stays on scarce ventilators – and for their family members, who are able to visit them only virtually via telephone or tablet.
But it has also highlighted gaps – including insufficient staffing for some palliative care teams. Hospitalists and other clinicians in the hospital need to learn the basics of primary palliative care, such as how to communicate bad news, initiate goals of care conversations, and address common symptoms of serious illness, such as pain. That way, they could shoulder more of the demand for this kind of care when palliative care specialists are in short supply.
Hospitalists, some of whom also have pursued a specialization in palliative care, have played key roles in clarifying and redefining the new role for palliative care, whom it is meant for, and who should provide it. Central to this new role is the greater use of telemedicine – for talking to hospitalized patients without increasing viral exposure, for linking up with family members who can’t visit their loved ones in the hospital, and for helping frontline hospital staff who need a palliative care consultation – or just a chance to debrief on what they are seeing.
A pandemic wake-up call
Elizabeth Gundersen, MD, FHM, FAAHPM, director of the hospice and palliative medicine fellowship program at the Charles E. Schmidt College of Medicine at Florida Atlantic University (FAU) in Boca Raton, practiced hospital medicine for 10 years before pursuing a fellowship in hospice and palliative medicine and working as an academic palliative medicine physician. She calls the pandemic a wake-up call for gaps in care and all the things that weren’t working well in the health care system.
“Now we are seeing more clearly what’s lacking – or broken – and what we will carry forward from this experience into the post-COVID world,” she said. Some hospitalists do palliative care very well, and others don’t feel as comfortable in having these difficult conversations with patients. But in the uncertain course of the virus they get thrust into it.
Although FAU’s associated hospitals were not as inundated with COVID-19 patients in the early weeks of the pandemic as were other regions, the volume of other patients plummeted, Dr. Gundersen said, adding that “there’s still been incredible intensity and worry about the virus. For me, the basic role of palliative care hasn’t changed, and the phrase I have always used when introducing myself – ‘we’re an extra layer of support for the patient and family’ – still holds true,” she said.
“I try to make it clear to people that palliative care is not synonymous with end-of-life care. We don’t want people to think that a palliative care referral implies imminent death. The goal is not to get more people to have a do not attempt resuscitation (DNAR) order, but to determine the patient and family’s treatment goals and whether a DNAR order fits those goals.”
The tough conversations
Dr. Gundersen is cochair of SHM’s Palliative Care Special Interest Group, along with Rab Razzak, MD, clinical director of palliative medicine at University Hospitals Cleveland Medical Center, one of the hospitals affiliated with Case Western University in Cleveland. (Connect with them on Twitter: @Top_Gundersen and @rabrazzak.)
Dr. Razzak also transitioned from hospital medicine to palliative medicine 10 years ago. “As a hospitalist, I enjoyed the tough conversations and bringing the human element into my health care interactions,” he explained. “To me, palliative care is a philosophy of care that puts the person we call the patient at the center of the interaction, while we try to figure out how to best care for them as a person.”
When the pandemic hit, University Hospitals made 20 ICU beds available for COVID-19 patients, Dr. Razzak said. This unit has since been full but not overflowing, while overall hospital census went down. The palliative care team at the hospital includes four inpatient doctors, nurse practitioners, and a chaplain, as well as an outpatient team primarily focused on oncology.
“In some settings, palliative care has been at the forefront of difficult conversations, when things aren’t going well for the patient and there’s much uncertainty,” Dr. Razzak said. The interface between hospital medicine and palliative care can be complementary, he added. “We talk about primary palliative care, which we want every discipline to be able to do – lead meaningful conversations, help manage symptoms.”
The take-home message for hospitalists, he said, is to get training in how to have these discussions, using such resources as VitalTalk (https://www.vitaltalk.org/), a nonprofit organization that disseminates education in communication skills for difficult conversations, and the Center to Advance Palliative Care (www.capc.org) at Icahn School of Medicine at Mount Sinai in New York City. “Once you’ve mastered the conversation, it will get easier. But ask for help when you need it, and learn how to know when you need it.”
Dr. Gundersen added that hospital medicine groups and palliative care teams could reach out to each other and talk about what they did in the crisis and how they can work together in the future. She recommends frequent ongoing support and collaboration that could range from formal conferences or training sessions to informal team interactions, perhaps with sandwiches in the doctor’s lounge – provided that there’s room for social distancing. She has recently started giving talks in the community and grand rounds presentations in hospitals about palliative care.
Other approaches and applications
In New York City, the initial epicenter for the pandemic in the United States, the adult palliative care service of Columbia University Medical Center (CUMC) experienced a sevenfold increase in consultation requests at the apex of the crisis, said its director, Craig Blinderman, MD. That demand was impossible to meet with existing staff. So Dr. Blinderman and colleagues established a virtual consultation model, recruiting and deploying volunteer out-of-state palliative care specialists to staff it.
An eight-bed palliative care unit was opened at CUMC for COVID-19 patients whose surrogates had opted not to initiate or continue intubation or life-sustaining treatments. This helped to relieve some of the pressures on the ICUs while making it possible for in-person visits to the hospice unit by families – in full PPE. Palliative care staff were embedded in various units in the hospital.
A palliative care response team composed of a hospice and palliative medicine fellow and four psychiatry residents or fellows, based in the emergency department and with supervision from the palliative care team, provided time-critical goals of care conversations with families using telemedicine – and a forum for listening to their suffering. Dr. Blinderman and colleagues also have found time to write up their experience for medical journals.1,2
There’s no reason to think that hospitalists, with a little basic training, couldn’t be having these same goals of care conversations, Dr. Blinderman said. “But the fact that hospitalists, at the pandemic’s peak, along with ICU doctors, were seeing an unprecedented magnitude of dying on a daily basis generated a lot of moral distress for them.”
Palliative care professionals, because they engage with these issues in a different way, may be somewhat better equipped to deal with the sheer emotional demands when so many are dying, as at the peak of the surge in New York. “We don’t see dying as a failure on our part but an opportunity to relieve suffering,” Dr. Blinderman said. And the palliative care field also emphasizes the importance of self-care for its practitioners.
“How do we meet the incredible palliative care needs in the epicenter of a pandemic? That question also applies to other kinds of crises we could imagine, for example, climate-related disasters,” Dr. Blinderman said. “What lessons have we learned about the value of palliative care and how to start incorporating it more integrally into the delivery of hospital care? Here we showed that we could work collaboratively with our colleagues at other major medical centers, bringing together their expertise to help us when we didn’t have the bandwidth to meet the demand,” he said.
Scripts can help
“Also, it won’t make sense to just go back to normal (after the crisis fades),” Dr. Blinderman said. “We need to take a close look at how our society is functioning in the wake of the pandemic and the ways the health care system has failed us. We have learned that we’re all interconnected and we need to work together to serve our communities – locally and nationally – applying basic distributive justice.”
Could there be, for example, a national infrastructure for mobilizing and deploying palliative care resources to areas of greatest need, similar to what was done in New York?
At Northwestern Medicine in Chicago, a number of palliative care clinicians at the system’s hospitals worked together to develop scripts designed to help other clinicians start goals of care conversations with patients and families, for use in the hospital as well as in outpatient primary care and other settings, with results integrated into the system’s electronic health record.
Front-line clinicians may not have the time to ask for formal consults from palliative care because of high volume and rapidly changing patient status, explained Eytan Szmuilowicz, MD, director of the section of palliative medicine at Northwestern Memorial Hospital. Or they may not have access to specialty-level palliative care in their settings.
The scripts are aimed at primary care, emergency physicians, and hospitalists needing to consider critical care placement or attempted resuscitation and to ICU clinicians helping families make decisions about life-sustaining treatments. They also can help facilitate advance care planning discussions. An example is “CALMER,” a six-step mnemonic guide to promote goals of care discussions with hospitalized patients. For more information on these scripts, contact Dr. Szmuilowicz: [email protected].
Eerily quiet
The COVID-19 crisis has been quite a whirlwind for hospital medicine, said Jeanie Youngwerth, MD, a hospitalist and program director of the palliative care service at the University of Colorado in Denver, which was a significant viral hotspot early on.
“When it first started, things seemed to change almost overnight – starting on Friday, March 13. People had to take action right away to develop work flows and the technology to allow us to see as many patients as possible,” she said. By the time Monday came, it was a whole new ballgame.
Dr. Youngwerth and two colleagues worked quickly to develop inpatient telemedicine capacity where none existed. “We knew we would not be going into patients’ rooms, but most of our team showed up in the hospital to work with the primary care teams. Our job was to see what we could do that actually made a difference,” she said.
“The hospital became a very strange place. You’d walk down the hallway and it was eerily quiet. Everybody you came across was being so nice to each other.” Televisits became a powerful way to bring the human connection back to medical care.
“What we learned from families was that they were thirsting to have some kind of connection with their loved one, and to be able to talk about their loved one and who they were as a person,” she said. “We’d contact the family through video visits and then, when the family meeting ended, the nurse would bring an iPad into the patient’s room so the family could see their loved one on a ventilator. They would immediately start communicating with their loved one, praying aloud, singing, playing music. It would make a huge difference for the family – and for the staff.”
References
1. Nakagawa S et al. Pandemic palliative care consultations spanning state and institutional borders. J Am Geriatr Soc. 2020 May 22. doi: 10.1111/jgs.16643.
2. Lee J Abrukin L, Flores S. Early intervention of palliative care in the emergency department during the COVID-19 pandemic. JAMA Intern Med. 2020 Jun 5. doi: 10.1001/jamainternmed.2020.2713.
Early palliative care fails to improve QOL in advanced heart failure
A new palliative care intervention for U.S. patients with advanced heart failure did not improve quality of life or mood after 16 weeks of participation in a randomized trial.
“Future analyses and studies will examine both the patient factors and intervention components to find the right palliative care dose, for the right patient, at the right time,” wrote Marie A. Bakitas, DNSc, of the University of Alabama at Birmingham, and coauthors. The study was published in JAMA Internal Medicine.
“My first reaction is disappointment,” Larry Allen, MD, of the University of Colorado in Denver, said in an interview. “We had hoped to see the ENABLE program, which had been successful in cancer, translate to the heart failure setting.”
Improvement of palliative care in heart failure patients might rest on who needs it most
“One thing to note,” Dr. Allen added in an interview, “is that, in this population of patients, some of the measures they were trying to improve were already relatively mild to start with. It may not be that the intervention didn’t help but that they picked a patient population that wasn’t particularly in need. If you treat someone who doesn’t have a problem, it’s hard to make them better.”
In a separate interview, Dr. Bakitas acknowledged a similar sentiment. “We were a little surprised until we looked at our sample,” she said. “We realized that we had recruited all these very high-functioning, good quality-of-life patients. What we then did was look at a subsample of patients who had low quality of life at baseline. Low and behold, the intervention had an effect. The patients who started with a poor quality of life had a statistically and clinically significant benefit. Their KCCQ score increased by over 5 points.”
As for next steps. Dr. Bakitas noted that they’re twofold: “One is refining the patient population who can benefit, and the second is working on the intervention and figuring out which pieces are the ones that provide the most benefit.
“Because of logistics and practical issues, not everyone in the study got all the intervention that they should have. Think of it like a drug trial; if someone misses a pill, they don’t get the full dose that we thought would work. We need to make sure our interventions have the right pieces in place. We don’t want to develop a great intervention that’s not practical for patients.”
Study design and outcomes
To determine the benefits of early palliative care for patients with heart failure, the researchers developed the ENABLE CHF-PC (Educate, Nurture, Advise, Before Life Ends Comprehensive Heartcare for Patients and Caregivers) intervention. This nurse-led program includes an in-person consultant followed by six telehealth nurse coaching sessions lasting 30-40 minutes and then monthly follow-up calls through either 48 weeks or the patient’s death.
To test the effectiveness of their intervention after 16 weeks, the researchers launched a two-site, single-blind randomized clinical trial made up of 415 patients who were 50 years or older with advanced heart failure. Among the patients, 53% were men and the mean age was 64 years; 55% were African American, 26% lived in a rural area, and 46% had a high school education or less. The average length of time since heart failure diagnosis was 5.1 years.
Patients were randomized evenly to receive either the ENABLE CHF-PC intervention (208) or usual care. The primary outcomes were quality of life (QOL), which was measured by the heart failure–specific 23-item Kansas City Cardiomyopathy Questionnaire (KCCQ) and the 14-item Functional Assessment of Chronic Illness Therapy–Palliative-14 (FACIT Pal-14), and mood, which was measured by the 14-item Hospital Anxiety and Depression Scale (HADS). Pain was measured via 3-item pain intensity and 2-item pain interference scales.
Effect size was measured as Cohen d or d-equivalent, where a small effect is 0.2, medium is 0.5, and large is about 0.854.
At baseline, the mean KCCQ score of 52.6 at baseline indicated a “fairly good” QOL across all patients. After 16 weeks, the mean KCCQ score improved 3.9 points in the intervention group, compared with 2.3 points in the usual care group (d = 0.07; [95% confidence interval, –0.09-0.24]). In addition, the mean FACIT-Pal-14 score improved 1.4 points in the intervention group compared to 0.2 points in the usual care group (d = 0.12 [95% CI, –0.03-0.28]). Only small differences were observed between groups regarding anxiety and depression, but pain intensity (difference, –2.8; SE, 0.9; d = –0.26 [95% CI, –0.43-0.09]) and pain interference (difference, –2.3; SE, 1; d = –0.21 [95% CI, –0.40 to –0.02]) demonstrated a statistically significant and clinically important decrease.
As heart failure care evolves, so must palliative care
Though the study and intervention developed by Dr. Bakitas and colleagues is commendable, it is only somewhat surprising that it did not drastically improve patients’ quality of life, Nathan E. Goldstein, MD, of the Icahn School of Medicine at Mount Sinai in New York, wrote in an accompanying editorial.
He noted several reasons for the lack of improvement, including a large proportion of patients still being in the early stages of the disease. Ultimately, however, he wonders if innovation in heart failure care ultimately impacted the study while it was occurring. Medications and technological advancements evolve rapidly in this field, he said, especially over the course of a 3-year study period.
To continue this work and produce real benefits in patients with advanced heart failure, Dr. Goldstein emphasized the need for “dynamic palliative care interventions that can adapt to the constantly changing landscape of the patient’s needs caused by the underlying nature of the disease, as well as the innovations in the field of cardiology.”
The authors acknowledged their study’s limitations, including data attrition at 16 weeks that was higher than expected – a turn of events they attributed to “unique socioeconomic factors … and lack of regular health care appointments” among some participants. In addition, a minority of patients were unable to stick to the study protocol, which has led the researchers to begin investigating video alternatives to in-person consultation.
The study was supported by the National Institutes of Health/National Institutes of Nursing Research. Four of the authors reported received grants from the National Institutes of Nursing Research outside the submitted work or during the study. Dr. Goldstein reported no conflicts of interest.
SOURCE: Bakitas MA et al. JAMA Intern Med. 2020 July 27. doi: 10.1001/jamainternmed.2020.2861.
A new palliative care intervention for U.S. patients with advanced heart failure did not improve quality of life or mood after 16 weeks of participation in a randomized trial.
“Future analyses and studies will examine both the patient factors and intervention components to find the right palliative care dose, for the right patient, at the right time,” wrote Marie A. Bakitas, DNSc, of the University of Alabama at Birmingham, and coauthors. The study was published in JAMA Internal Medicine.
“My first reaction is disappointment,” Larry Allen, MD, of the University of Colorado in Denver, said in an interview. “We had hoped to see the ENABLE program, which had been successful in cancer, translate to the heart failure setting.”
Improvement of palliative care in heart failure patients might rest on who needs it most
“One thing to note,” Dr. Allen added in an interview, “is that, in this population of patients, some of the measures they were trying to improve were already relatively mild to start with. It may not be that the intervention didn’t help but that they picked a patient population that wasn’t particularly in need. If you treat someone who doesn’t have a problem, it’s hard to make them better.”
In a separate interview, Dr. Bakitas acknowledged a similar sentiment. “We were a little surprised until we looked at our sample,” she said. “We realized that we had recruited all these very high-functioning, good quality-of-life patients. What we then did was look at a subsample of patients who had low quality of life at baseline. Low and behold, the intervention had an effect. The patients who started with a poor quality of life had a statistically and clinically significant benefit. Their KCCQ score increased by over 5 points.”
As for next steps. Dr. Bakitas noted that they’re twofold: “One is refining the patient population who can benefit, and the second is working on the intervention and figuring out which pieces are the ones that provide the most benefit.
“Because of logistics and practical issues, not everyone in the study got all the intervention that they should have. Think of it like a drug trial; if someone misses a pill, they don’t get the full dose that we thought would work. We need to make sure our interventions have the right pieces in place. We don’t want to develop a great intervention that’s not practical for patients.”
Study design and outcomes
To determine the benefits of early palliative care for patients with heart failure, the researchers developed the ENABLE CHF-PC (Educate, Nurture, Advise, Before Life Ends Comprehensive Heartcare for Patients and Caregivers) intervention. This nurse-led program includes an in-person consultant followed by six telehealth nurse coaching sessions lasting 30-40 minutes and then monthly follow-up calls through either 48 weeks or the patient’s death.
To test the effectiveness of their intervention after 16 weeks, the researchers launched a two-site, single-blind randomized clinical trial made up of 415 patients who were 50 years or older with advanced heart failure. Among the patients, 53% were men and the mean age was 64 years; 55% were African American, 26% lived in a rural area, and 46% had a high school education or less. The average length of time since heart failure diagnosis was 5.1 years.
Patients were randomized evenly to receive either the ENABLE CHF-PC intervention (208) or usual care. The primary outcomes were quality of life (QOL), which was measured by the heart failure–specific 23-item Kansas City Cardiomyopathy Questionnaire (KCCQ) and the 14-item Functional Assessment of Chronic Illness Therapy–Palliative-14 (FACIT Pal-14), and mood, which was measured by the 14-item Hospital Anxiety and Depression Scale (HADS). Pain was measured via 3-item pain intensity and 2-item pain interference scales.
Effect size was measured as Cohen d or d-equivalent, where a small effect is 0.2, medium is 0.5, and large is about 0.854.
At baseline, the mean KCCQ score of 52.6 at baseline indicated a “fairly good” QOL across all patients. After 16 weeks, the mean KCCQ score improved 3.9 points in the intervention group, compared with 2.3 points in the usual care group (d = 0.07; [95% confidence interval, –0.09-0.24]). In addition, the mean FACIT-Pal-14 score improved 1.4 points in the intervention group compared to 0.2 points in the usual care group (d = 0.12 [95% CI, –0.03-0.28]). Only small differences were observed between groups regarding anxiety and depression, but pain intensity (difference, –2.8; SE, 0.9; d = –0.26 [95% CI, –0.43-0.09]) and pain interference (difference, –2.3; SE, 1; d = –0.21 [95% CI, –0.40 to –0.02]) demonstrated a statistically significant and clinically important decrease.
As heart failure care evolves, so must palliative care
Though the study and intervention developed by Dr. Bakitas and colleagues is commendable, it is only somewhat surprising that it did not drastically improve patients’ quality of life, Nathan E. Goldstein, MD, of the Icahn School of Medicine at Mount Sinai in New York, wrote in an accompanying editorial.
He noted several reasons for the lack of improvement, including a large proportion of patients still being in the early stages of the disease. Ultimately, however, he wonders if innovation in heart failure care ultimately impacted the study while it was occurring. Medications and technological advancements evolve rapidly in this field, he said, especially over the course of a 3-year study period.
To continue this work and produce real benefits in patients with advanced heart failure, Dr. Goldstein emphasized the need for “dynamic palliative care interventions that can adapt to the constantly changing landscape of the patient’s needs caused by the underlying nature of the disease, as well as the innovations in the field of cardiology.”
The authors acknowledged their study’s limitations, including data attrition at 16 weeks that was higher than expected – a turn of events they attributed to “unique socioeconomic factors … and lack of regular health care appointments” among some participants. In addition, a minority of patients were unable to stick to the study protocol, which has led the researchers to begin investigating video alternatives to in-person consultation.
The study was supported by the National Institutes of Health/National Institutes of Nursing Research. Four of the authors reported received grants from the National Institutes of Nursing Research outside the submitted work or during the study. Dr. Goldstein reported no conflicts of interest.
SOURCE: Bakitas MA et al. JAMA Intern Med. 2020 July 27. doi: 10.1001/jamainternmed.2020.2861.
A new palliative care intervention for U.S. patients with advanced heart failure did not improve quality of life or mood after 16 weeks of participation in a randomized trial.
“Future analyses and studies will examine both the patient factors and intervention components to find the right palliative care dose, for the right patient, at the right time,” wrote Marie A. Bakitas, DNSc, of the University of Alabama at Birmingham, and coauthors. The study was published in JAMA Internal Medicine.
“My first reaction is disappointment,” Larry Allen, MD, of the University of Colorado in Denver, said in an interview. “We had hoped to see the ENABLE program, which had been successful in cancer, translate to the heart failure setting.”
Improvement of palliative care in heart failure patients might rest on who needs it most
“One thing to note,” Dr. Allen added in an interview, “is that, in this population of patients, some of the measures they were trying to improve were already relatively mild to start with. It may not be that the intervention didn’t help but that they picked a patient population that wasn’t particularly in need. If you treat someone who doesn’t have a problem, it’s hard to make them better.”
In a separate interview, Dr. Bakitas acknowledged a similar sentiment. “We were a little surprised until we looked at our sample,” she said. “We realized that we had recruited all these very high-functioning, good quality-of-life patients. What we then did was look at a subsample of patients who had low quality of life at baseline. Low and behold, the intervention had an effect. The patients who started with a poor quality of life had a statistically and clinically significant benefit. Their KCCQ score increased by over 5 points.”
As for next steps. Dr. Bakitas noted that they’re twofold: “One is refining the patient population who can benefit, and the second is working on the intervention and figuring out which pieces are the ones that provide the most benefit.
“Because of logistics and practical issues, not everyone in the study got all the intervention that they should have. Think of it like a drug trial; if someone misses a pill, they don’t get the full dose that we thought would work. We need to make sure our interventions have the right pieces in place. We don’t want to develop a great intervention that’s not practical for patients.”
Study design and outcomes
To determine the benefits of early palliative care for patients with heart failure, the researchers developed the ENABLE CHF-PC (Educate, Nurture, Advise, Before Life Ends Comprehensive Heartcare for Patients and Caregivers) intervention. This nurse-led program includes an in-person consultant followed by six telehealth nurse coaching sessions lasting 30-40 minutes and then monthly follow-up calls through either 48 weeks or the patient’s death.
To test the effectiveness of their intervention after 16 weeks, the researchers launched a two-site, single-blind randomized clinical trial made up of 415 patients who were 50 years or older with advanced heart failure. Among the patients, 53% were men and the mean age was 64 years; 55% were African American, 26% lived in a rural area, and 46% had a high school education or less. The average length of time since heart failure diagnosis was 5.1 years.
Patients were randomized evenly to receive either the ENABLE CHF-PC intervention (208) or usual care. The primary outcomes were quality of life (QOL), which was measured by the heart failure–specific 23-item Kansas City Cardiomyopathy Questionnaire (KCCQ) and the 14-item Functional Assessment of Chronic Illness Therapy–Palliative-14 (FACIT Pal-14), and mood, which was measured by the 14-item Hospital Anxiety and Depression Scale (HADS). Pain was measured via 3-item pain intensity and 2-item pain interference scales.
Effect size was measured as Cohen d or d-equivalent, where a small effect is 0.2, medium is 0.5, and large is about 0.854.
At baseline, the mean KCCQ score of 52.6 at baseline indicated a “fairly good” QOL across all patients. After 16 weeks, the mean KCCQ score improved 3.9 points in the intervention group, compared with 2.3 points in the usual care group (d = 0.07; [95% confidence interval, –0.09-0.24]). In addition, the mean FACIT-Pal-14 score improved 1.4 points in the intervention group compared to 0.2 points in the usual care group (d = 0.12 [95% CI, –0.03-0.28]). Only small differences were observed between groups regarding anxiety and depression, but pain intensity (difference, –2.8; SE, 0.9; d = –0.26 [95% CI, –0.43-0.09]) and pain interference (difference, –2.3; SE, 1; d = –0.21 [95% CI, –0.40 to –0.02]) demonstrated a statistically significant and clinically important decrease.
As heart failure care evolves, so must palliative care
Though the study and intervention developed by Dr. Bakitas and colleagues is commendable, it is only somewhat surprising that it did not drastically improve patients’ quality of life, Nathan E. Goldstein, MD, of the Icahn School of Medicine at Mount Sinai in New York, wrote in an accompanying editorial.
He noted several reasons for the lack of improvement, including a large proportion of patients still being in the early stages of the disease. Ultimately, however, he wonders if innovation in heart failure care ultimately impacted the study while it was occurring. Medications and technological advancements evolve rapidly in this field, he said, especially over the course of a 3-year study period.
To continue this work and produce real benefits in patients with advanced heart failure, Dr. Goldstein emphasized the need for “dynamic palliative care interventions that can adapt to the constantly changing landscape of the patient’s needs caused by the underlying nature of the disease, as well as the innovations in the field of cardiology.”
The authors acknowledged their study’s limitations, including data attrition at 16 weeks that was higher than expected – a turn of events they attributed to “unique socioeconomic factors … and lack of regular health care appointments” among some participants. In addition, a minority of patients were unable to stick to the study protocol, which has led the researchers to begin investigating video alternatives to in-person consultation.
The study was supported by the National Institutes of Health/National Institutes of Nursing Research. Four of the authors reported received grants from the National Institutes of Nursing Research outside the submitted work or during the study. Dr. Goldstein reported no conflicts of interest.
SOURCE: Bakitas MA et al. JAMA Intern Med. 2020 July 27. doi: 10.1001/jamainternmed.2020.2861.
FROM JAMA INTERNAL MEDICINE
An Advance Care Planning Video Program in Nursing Homes Did Not Reduce Hospital Transfer and Burdensome Treatment in Long-Stay Residents
Study Overview
Objective. To examine the effect of an advance care planning video intervention in nursing homes on resident outcomes of hospital transfer, burdensome treatment, and hospice enrollment.
Design. Pragmatic cluster randomized controlled trial.
Setting and participants. The study was conducted in 360 nursing homes located in 32 states across the United States. The facilities were owned by 2 for-profit nursing home chains; facilities with more than 50 beds were eligible to be included in the study. Facilities deemed by corporate leaders to have serious organizational problems or that lacked the ability to transfer electronic health records were excluded. The facilities, stratified by the primary outcome hospitalizations per 1000 person-days, were then randomized to intervention and control in a 1:2 ratio. Leaders from facilities in the intervention group received letters describing their selection to participate in the advance care planning video program, and all facilities invited agreed to participate. Participants (residents in nursing homes) were enrolled from February 1, 2016, to May 31, 2018. Each participant was followed for 12 months after enrollment. All residents living in intervention facilities were offered the opportunity to watch intervention videos. The target population of the study was residents with advanced illness, including advanced dementia or advanced cardiopulmonary disease, as defined by the Minimum Data Set (MDS) variables, who were aged 65 and older, were long-stay residents (100 days or more), and were enrolled as Medicare fee-for-service beneficiaries. Secondary analysis included residents without advanced illness meeting other criteria.
Intervention. The intervention consisted of a selection of 5 short videos (6 to 10 minutes each), which had been previously developed and tested in smaller randomized trials. These videos cover the topics of general goals of care, goals of care for advanced dementia, hospice, hospitalization, and advance care planning for healthy patients, and use narration and images of typical treatments representing intensive medical care, basic medical care, and comfort care. The video for goals of care for advanced dementia targeted proxies of residents rather than residents themselves.
The implementation strategy for the video program included using a program manager to oversee the organization of the program’s rollout (a manager for each for-profit nursing home chain) and 2 champions at each facility (typically social workers were tasked with showing videos to patients and families). Champions received training from the study investigators and the manager and were asked to choose and offer selected videos to residents or proxies within 7 days of admission or readmission, every 6 months during a resident’s stay, and when specific decisions occurred, such as transition to hospice care, and on special occasions, such as out-of-town family visits.
Video offering and use were captured through documentation by a facility champion using a report tool embedded in the facility’s electronic health record. Champions met with the facility’s program manager and study team to review reports of video use, identify residents who had not been shown a video, and problem-solve on how to reach these residents. Facilities in the control group used their usual procedures for advance care planning.
Main outcome measures. Study outcomes included hospitalization transfers per 1000 person-days alive among long-stay residents with advanced illness (primary outcome); proportion of residents with at least 1 hospital transfer; proportion of residents with at least 1 burdensome treatment; and hospice enrollment (secondary outcomes). Secondary outcomes also included hospitalization transfers for long-stay residents without advanced illness. Hospital transfers were identified using Medicare claims for admissions, emergency department visits, and observation stays. Burdensome treatments were identified from Medicare claims and MDS, including tube feeding, parenteral therapy, invasive mechanical intervention, and intensive care unit admission. Fidelity to video intervention was measured by the proportion of residents offered the videos and the proportion of residents shown the videos at least once during the study period.
Main results. A total of 360 facilities were included in the study, 119 intervention and 241 control facilities. For the primary outcome, 4171 residents with advanced illness were included in the intervention group and 8308 residents with advanced illness were included in the control group. The average age was 83.6 years in both groups. In the intervention and control groups, respectively, 71.2% and 70.5% were female, 78.4% and 81.5% were White, 68.6% and 70.1% had advanced dementia at baseline, and 35.4% and 33.4% had advanced congestive heart failure or chronic obstructive pulmonary disease at baseline. Approximately 34% of residents received hospice care at baseline. In the intervention and control groups, 43.9% and 45.3% of residents died during follow-up, and the average length of follow-up in each group was 253.1 days and 252.6 days, respectively.
For the primary outcome of hospital transfers per 1000 person-days alive, there were 3.7 episodes (standard error 0.2) in the intervention group and 3.9 episodes in the control group (standard error 0.3); the difference was not statistically significant. For residents without advanced illness, there also was no difference in the hospital transfer rate. For other secondary outcomes, the proportion of residents in the intervention and control groups with 1 or more hospital transfer was 40.9% and 41.6%, respectively; the proportion with 1 or more burdensome treatment was 9.6% and 10.7%; and hospice enrollment was 24.9% and 25.5%. None of these differences was statistically significant. In the intervention group, 55.6% of residents or proxies were offered the video intervention and 21.9% were shown the videos at least once. There was substantial variability in the proportion of residents in the intervention group who were shown videos.
Conclusion. The advance planning video program did not lead to a reduction in hospital transfer, burdensome treatment, or changes in hospice enrollment. Acceptance of the intervention by residents was variable, and this may have contributed to the null finding.
Commentary
Nursing home residents often have advanced illness and limited functional ability. Hospital transfers may be burdensome and of limited clinical benefit for these patients, particularly for those with advanced illness and limited life expectancy, and are associated with markers of poor quality of end-of-life care, such as increased rates of stage IV decubitus ulcer and feeding-tube use towards the end of life.1 Advance care planning is associated with less aggressive care towards the end of life for persons with advanced illness,2 which ultimately improves the quality of end-of-life care for these individuals. Prior interventions to improve advance care planning have had variable effects, while video-based interventions to improve advance care planning have shown promise.3
This pragmatic randomized trial assessed the effect of an advance care planning video program on important clinical outcomes for nursing home residents, particularly those with advanced illness. The results, however, are disappointing, as the video intervention failed to improve hospital transfer rate and burdensome treatment in this population. The negative results could be attributed to the limited adoption of the video intervention in the study, as only 21.9% of residents in the intervention group were actually exposed to the intervention. What is not reported, and is difficult to assess, is whether the video intervention led to advance care planning, as would be demonstrated by advance directive documentation and acceptance of goals of care of comfort. A per-protocol analysis may be considered to demonstrate if there is an effect on residents who were exposed to the intervention. Nonetheless, the low adoption rate of the intervention may prompt further investigation of factors limiting adoption and perhaps lead to a redesigned trial aimed at enhancing adoption, with consideration of use of implementation trial designs.
As pointed out by the study investigators, other changes to nursing home practices, specifically on hospital transfer, likely occurred during the study period. A number of national initiatives to reduce unnecessary hospital transfer from nursing homes have been introduced, and a reduction in hospital transfers occurred between 2011 and 20174; these initiatives could have impacted staff priorities and adoption of the study intervention relative to other co-occurring initiatives.
Applications for Clinical Practice
The authors of this study reported negative trial results, but their findings highlight important issues in conducting trials in the nursing home setting. Additional demonstration of actual effect on advance care planning discussions and documentation will further enhance our understanding of whether the intervention, as tested, yields changes in practice on advance care planning in nursing homes. The pragmatic clinical trial design used in this study accounts for real-world settings, but may have limited the study’s ability to account for and adjust for differences in staff, settings, and other conditions and factors that may impact adoption of and fidelity to the intervention. Quality improvement approaches, such as INTERACT, have targeted unnecessary hospital transfers and may yield positive results.5 Quality improvement approaches like INTERACT allow for a high degree of adaptation to local procedures and settings, which in clinical trials is difficult to do. However, in a real-world setting, such approaches may be necessary to improve care.
–William W. Hung, MD, MPH
1. Gozalo P, Teno JM, Mitchell SL, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365:1212-1221
2. Nichols LH, Bynum J, Iwashyna TJ, et al. Advance directives and nursing home stays associated with less aggressive end-of-life care for patients with severe dementia. Health Aff (Millwood). 2014;33:667-674.
3. Volandes AE, Paasche-Orlow MK, Barry MJ, et al. Video decision support tool for advance care planning in dementia: randomized controlled trial. BMJ. 2009;338:b2159.
4. McCarthy EP, Ogarek JA, Loomer L, et al. Hospital transfer rates among US nursing home residents with advanced illness before and after initiatives to reduce hospitalizations. JAMA Intern Med. 2020;180:385-394.
5. Rantz MJ, Popejoy L, Vogelsmeier, A et al. Successfully reducing hospitalizations of nursing home residents: results of the Missouri Quality Initiative. JAMA. 2017:18;960-966.
Study Overview
Objective. To examine the effect of an advance care planning video intervention in nursing homes on resident outcomes of hospital transfer, burdensome treatment, and hospice enrollment.
Design. Pragmatic cluster randomized controlled trial.
Setting and participants. The study was conducted in 360 nursing homes located in 32 states across the United States. The facilities were owned by 2 for-profit nursing home chains; facilities with more than 50 beds were eligible to be included in the study. Facilities deemed by corporate leaders to have serious organizational problems or that lacked the ability to transfer electronic health records were excluded. The facilities, stratified by the primary outcome hospitalizations per 1000 person-days, were then randomized to intervention and control in a 1:2 ratio. Leaders from facilities in the intervention group received letters describing their selection to participate in the advance care planning video program, and all facilities invited agreed to participate. Participants (residents in nursing homes) were enrolled from February 1, 2016, to May 31, 2018. Each participant was followed for 12 months after enrollment. All residents living in intervention facilities were offered the opportunity to watch intervention videos. The target population of the study was residents with advanced illness, including advanced dementia or advanced cardiopulmonary disease, as defined by the Minimum Data Set (MDS) variables, who were aged 65 and older, were long-stay residents (100 days or more), and were enrolled as Medicare fee-for-service beneficiaries. Secondary analysis included residents without advanced illness meeting other criteria.
Intervention. The intervention consisted of a selection of 5 short videos (6 to 10 minutes each), which had been previously developed and tested in smaller randomized trials. These videos cover the topics of general goals of care, goals of care for advanced dementia, hospice, hospitalization, and advance care planning for healthy patients, and use narration and images of typical treatments representing intensive medical care, basic medical care, and comfort care. The video for goals of care for advanced dementia targeted proxies of residents rather than residents themselves.
The implementation strategy for the video program included using a program manager to oversee the organization of the program’s rollout (a manager for each for-profit nursing home chain) and 2 champions at each facility (typically social workers were tasked with showing videos to patients and families). Champions received training from the study investigators and the manager and were asked to choose and offer selected videos to residents or proxies within 7 days of admission or readmission, every 6 months during a resident’s stay, and when specific decisions occurred, such as transition to hospice care, and on special occasions, such as out-of-town family visits.
Video offering and use were captured through documentation by a facility champion using a report tool embedded in the facility’s electronic health record. Champions met with the facility’s program manager and study team to review reports of video use, identify residents who had not been shown a video, and problem-solve on how to reach these residents. Facilities in the control group used their usual procedures for advance care planning.
Main outcome measures. Study outcomes included hospitalization transfers per 1000 person-days alive among long-stay residents with advanced illness (primary outcome); proportion of residents with at least 1 hospital transfer; proportion of residents with at least 1 burdensome treatment; and hospice enrollment (secondary outcomes). Secondary outcomes also included hospitalization transfers for long-stay residents without advanced illness. Hospital transfers were identified using Medicare claims for admissions, emergency department visits, and observation stays. Burdensome treatments were identified from Medicare claims and MDS, including tube feeding, parenteral therapy, invasive mechanical intervention, and intensive care unit admission. Fidelity to video intervention was measured by the proportion of residents offered the videos and the proportion of residents shown the videos at least once during the study period.
Main results. A total of 360 facilities were included in the study, 119 intervention and 241 control facilities. For the primary outcome, 4171 residents with advanced illness were included in the intervention group and 8308 residents with advanced illness were included in the control group. The average age was 83.6 years in both groups. In the intervention and control groups, respectively, 71.2% and 70.5% were female, 78.4% and 81.5% were White, 68.6% and 70.1% had advanced dementia at baseline, and 35.4% and 33.4% had advanced congestive heart failure or chronic obstructive pulmonary disease at baseline. Approximately 34% of residents received hospice care at baseline. In the intervention and control groups, 43.9% and 45.3% of residents died during follow-up, and the average length of follow-up in each group was 253.1 days and 252.6 days, respectively.
For the primary outcome of hospital transfers per 1000 person-days alive, there were 3.7 episodes (standard error 0.2) in the intervention group and 3.9 episodes in the control group (standard error 0.3); the difference was not statistically significant. For residents without advanced illness, there also was no difference in the hospital transfer rate. For other secondary outcomes, the proportion of residents in the intervention and control groups with 1 or more hospital transfer was 40.9% and 41.6%, respectively; the proportion with 1 or more burdensome treatment was 9.6% and 10.7%; and hospice enrollment was 24.9% and 25.5%. None of these differences was statistically significant. In the intervention group, 55.6% of residents or proxies were offered the video intervention and 21.9% were shown the videos at least once. There was substantial variability in the proportion of residents in the intervention group who were shown videos.
Conclusion. The advance planning video program did not lead to a reduction in hospital transfer, burdensome treatment, or changes in hospice enrollment. Acceptance of the intervention by residents was variable, and this may have contributed to the null finding.
Commentary
Nursing home residents often have advanced illness and limited functional ability. Hospital transfers may be burdensome and of limited clinical benefit for these patients, particularly for those with advanced illness and limited life expectancy, and are associated with markers of poor quality of end-of-life care, such as increased rates of stage IV decubitus ulcer and feeding-tube use towards the end of life.1 Advance care planning is associated with less aggressive care towards the end of life for persons with advanced illness,2 which ultimately improves the quality of end-of-life care for these individuals. Prior interventions to improve advance care planning have had variable effects, while video-based interventions to improve advance care planning have shown promise.3
This pragmatic randomized trial assessed the effect of an advance care planning video program on important clinical outcomes for nursing home residents, particularly those with advanced illness. The results, however, are disappointing, as the video intervention failed to improve hospital transfer rate and burdensome treatment in this population. The negative results could be attributed to the limited adoption of the video intervention in the study, as only 21.9% of residents in the intervention group were actually exposed to the intervention. What is not reported, and is difficult to assess, is whether the video intervention led to advance care planning, as would be demonstrated by advance directive documentation and acceptance of goals of care of comfort. A per-protocol analysis may be considered to demonstrate if there is an effect on residents who were exposed to the intervention. Nonetheless, the low adoption rate of the intervention may prompt further investigation of factors limiting adoption and perhaps lead to a redesigned trial aimed at enhancing adoption, with consideration of use of implementation trial designs.
As pointed out by the study investigators, other changes to nursing home practices, specifically on hospital transfer, likely occurred during the study period. A number of national initiatives to reduce unnecessary hospital transfer from nursing homes have been introduced, and a reduction in hospital transfers occurred between 2011 and 20174; these initiatives could have impacted staff priorities and adoption of the study intervention relative to other co-occurring initiatives.
Applications for Clinical Practice
The authors of this study reported negative trial results, but their findings highlight important issues in conducting trials in the nursing home setting. Additional demonstration of actual effect on advance care planning discussions and documentation will further enhance our understanding of whether the intervention, as tested, yields changes in practice on advance care planning in nursing homes. The pragmatic clinical trial design used in this study accounts for real-world settings, but may have limited the study’s ability to account for and adjust for differences in staff, settings, and other conditions and factors that may impact adoption of and fidelity to the intervention. Quality improvement approaches, such as INTERACT, have targeted unnecessary hospital transfers and may yield positive results.5 Quality improvement approaches like INTERACT allow for a high degree of adaptation to local procedures and settings, which in clinical trials is difficult to do. However, in a real-world setting, such approaches may be necessary to improve care.
–William W. Hung, MD, MPH
Study Overview
Objective. To examine the effect of an advance care planning video intervention in nursing homes on resident outcomes of hospital transfer, burdensome treatment, and hospice enrollment.
Design. Pragmatic cluster randomized controlled trial.
Setting and participants. The study was conducted in 360 nursing homes located in 32 states across the United States. The facilities were owned by 2 for-profit nursing home chains; facilities with more than 50 beds were eligible to be included in the study. Facilities deemed by corporate leaders to have serious organizational problems or that lacked the ability to transfer electronic health records were excluded. The facilities, stratified by the primary outcome hospitalizations per 1000 person-days, were then randomized to intervention and control in a 1:2 ratio. Leaders from facilities in the intervention group received letters describing their selection to participate in the advance care planning video program, and all facilities invited agreed to participate. Participants (residents in nursing homes) were enrolled from February 1, 2016, to May 31, 2018. Each participant was followed for 12 months after enrollment. All residents living in intervention facilities were offered the opportunity to watch intervention videos. The target population of the study was residents with advanced illness, including advanced dementia or advanced cardiopulmonary disease, as defined by the Minimum Data Set (MDS) variables, who were aged 65 and older, were long-stay residents (100 days or more), and were enrolled as Medicare fee-for-service beneficiaries. Secondary analysis included residents without advanced illness meeting other criteria.
Intervention. The intervention consisted of a selection of 5 short videos (6 to 10 minutes each), which had been previously developed and tested in smaller randomized trials. These videos cover the topics of general goals of care, goals of care for advanced dementia, hospice, hospitalization, and advance care planning for healthy patients, and use narration and images of typical treatments representing intensive medical care, basic medical care, and comfort care. The video for goals of care for advanced dementia targeted proxies of residents rather than residents themselves.
The implementation strategy for the video program included using a program manager to oversee the organization of the program’s rollout (a manager for each for-profit nursing home chain) and 2 champions at each facility (typically social workers were tasked with showing videos to patients and families). Champions received training from the study investigators and the manager and were asked to choose and offer selected videos to residents or proxies within 7 days of admission or readmission, every 6 months during a resident’s stay, and when specific decisions occurred, such as transition to hospice care, and on special occasions, such as out-of-town family visits.
Video offering and use were captured through documentation by a facility champion using a report tool embedded in the facility’s electronic health record. Champions met with the facility’s program manager and study team to review reports of video use, identify residents who had not been shown a video, and problem-solve on how to reach these residents. Facilities in the control group used their usual procedures for advance care planning.
Main outcome measures. Study outcomes included hospitalization transfers per 1000 person-days alive among long-stay residents with advanced illness (primary outcome); proportion of residents with at least 1 hospital transfer; proportion of residents with at least 1 burdensome treatment; and hospice enrollment (secondary outcomes). Secondary outcomes also included hospitalization transfers for long-stay residents without advanced illness. Hospital transfers were identified using Medicare claims for admissions, emergency department visits, and observation stays. Burdensome treatments were identified from Medicare claims and MDS, including tube feeding, parenteral therapy, invasive mechanical intervention, and intensive care unit admission. Fidelity to video intervention was measured by the proportion of residents offered the videos and the proportion of residents shown the videos at least once during the study period.
Main results. A total of 360 facilities were included in the study, 119 intervention and 241 control facilities. For the primary outcome, 4171 residents with advanced illness were included in the intervention group and 8308 residents with advanced illness were included in the control group. The average age was 83.6 years in both groups. In the intervention and control groups, respectively, 71.2% and 70.5% were female, 78.4% and 81.5% were White, 68.6% and 70.1% had advanced dementia at baseline, and 35.4% and 33.4% had advanced congestive heart failure or chronic obstructive pulmonary disease at baseline. Approximately 34% of residents received hospice care at baseline. In the intervention and control groups, 43.9% and 45.3% of residents died during follow-up, and the average length of follow-up in each group was 253.1 days and 252.6 days, respectively.
For the primary outcome of hospital transfers per 1000 person-days alive, there were 3.7 episodes (standard error 0.2) in the intervention group and 3.9 episodes in the control group (standard error 0.3); the difference was not statistically significant. For residents without advanced illness, there also was no difference in the hospital transfer rate. For other secondary outcomes, the proportion of residents in the intervention and control groups with 1 or more hospital transfer was 40.9% and 41.6%, respectively; the proportion with 1 or more burdensome treatment was 9.6% and 10.7%; and hospice enrollment was 24.9% and 25.5%. None of these differences was statistically significant. In the intervention group, 55.6% of residents or proxies were offered the video intervention and 21.9% were shown the videos at least once. There was substantial variability in the proportion of residents in the intervention group who were shown videos.
Conclusion. The advance planning video program did not lead to a reduction in hospital transfer, burdensome treatment, or changes in hospice enrollment. Acceptance of the intervention by residents was variable, and this may have contributed to the null finding.
Commentary
Nursing home residents often have advanced illness and limited functional ability. Hospital transfers may be burdensome and of limited clinical benefit for these patients, particularly for those with advanced illness and limited life expectancy, and are associated with markers of poor quality of end-of-life care, such as increased rates of stage IV decubitus ulcer and feeding-tube use towards the end of life.1 Advance care planning is associated with less aggressive care towards the end of life for persons with advanced illness,2 which ultimately improves the quality of end-of-life care for these individuals. Prior interventions to improve advance care planning have had variable effects, while video-based interventions to improve advance care planning have shown promise.3
This pragmatic randomized trial assessed the effect of an advance care planning video program on important clinical outcomes for nursing home residents, particularly those with advanced illness. The results, however, are disappointing, as the video intervention failed to improve hospital transfer rate and burdensome treatment in this population. The negative results could be attributed to the limited adoption of the video intervention in the study, as only 21.9% of residents in the intervention group were actually exposed to the intervention. What is not reported, and is difficult to assess, is whether the video intervention led to advance care planning, as would be demonstrated by advance directive documentation and acceptance of goals of care of comfort. A per-protocol analysis may be considered to demonstrate if there is an effect on residents who were exposed to the intervention. Nonetheless, the low adoption rate of the intervention may prompt further investigation of factors limiting adoption and perhaps lead to a redesigned trial aimed at enhancing adoption, with consideration of use of implementation trial designs.
As pointed out by the study investigators, other changes to nursing home practices, specifically on hospital transfer, likely occurred during the study period. A number of national initiatives to reduce unnecessary hospital transfer from nursing homes have been introduced, and a reduction in hospital transfers occurred between 2011 and 20174; these initiatives could have impacted staff priorities and adoption of the study intervention relative to other co-occurring initiatives.
Applications for Clinical Practice
The authors of this study reported negative trial results, but their findings highlight important issues in conducting trials in the nursing home setting. Additional demonstration of actual effect on advance care planning discussions and documentation will further enhance our understanding of whether the intervention, as tested, yields changes in practice on advance care planning in nursing homes. The pragmatic clinical trial design used in this study accounts for real-world settings, but may have limited the study’s ability to account for and adjust for differences in staff, settings, and other conditions and factors that may impact adoption of and fidelity to the intervention. Quality improvement approaches, such as INTERACT, have targeted unnecessary hospital transfers and may yield positive results.5 Quality improvement approaches like INTERACT allow for a high degree of adaptation to local procedures and settings, which in clinical trials is difficult to do. However, in a real-world setting, such approaches may be necessary to improve care.
–William W. Hung, MD, MPH
1. Gozalo P, Teno JM, Mitchell SL, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365:1212-1221
2. Nichols LH, Bynum J, Iwashyna TJ, et al. Advance directives and nursing home stays associated with less aggressive end-of-life care for patients with severe dementia. Health Aff (Millwood). 2014;33:667-674.
3. Volandes AE, Paasche-Orlow MK, Barry MJ, et al. Video decision support tool for advance care planning in dementia: randomized controlled trial. BMJ. 2009;338:b2159.
4. McCarthy EP, Ogarek JA, Loomer L, et al. Hospital transfer rates among US nursing home residents with advanced illness before and after initiatives to reduce hospitalizations. JAMA Intern Med. 2020;180:385-394.
5. Rantz MJ, Popejoy L, Vogelsmeier, A et al. Successfully reducing hospitalizations of nursing home residents: results of the Missouri Quality Initiative. JAMA. 2017:18;960-966.
1. Gozalo P, Teno JM, Mitchell SL, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365:1212-1221
2. Nichols LH, Bynum J, Iwashyna TJ, et al. Advance directives and nursing home stays associated with less aggressive end-of-life care for patients with severe dementia. Health Aff (Millwood). 2014;33:667-674.
3. Volandes AE, Paasche-Orlow MK, Barry MJ, et al. Video decision support tool for advance care planning in dementia: randomized controlled trial. BMJ. 2009;338:b2159.
4. McCarthy EP, Ogarek JA, Loomer L, et al. Hospital transfer rates among US nursing home residents with advanced illness before and after initiatives to reduce hospitalizations. JAMA Intern Med. 2020;180:385-394.
5. Rantz MJ, Popejoy L, Vogelsmeier, A et al. Successfully reducing hospitalizations of nursing home residents: results of the Missouri Quality Initiative. JAMA. 2017:18;960-966.
Psychiatry trainees drive COVID-19 palliative care in New York
As SARS-CoV-2 cases surged in New York this past spring, one hospital system met the growing demand for palliative care in COVID-19 patients in acute care and emergency settings by training and redeploying psychiatry trainees, producing 100 consultations during a crisis period. Developers of this program wrote about their experience in the Journal of Pain and Symptom Management.
Research shows that psychiatrists can play an important, complementary role in palliative care, but not many models have explored this in practice. Over a 45-day period in March and April, New York Presbyterian/Columbia University Irving Medical Center saw an influx of 7,600 COVID-19 patients. Many were critically ill, and palliative care needs skyrocketed. Initial efforts to install a palliative care team at the emergency department and a proactive consultation model in the step-down units failed to meet demand for consults.
COVID-19 patients present unique challenges. Their clinical trajectory is less clear than those with cancer or other illnesses, Daniel Shalev, MD, a fellow in hospice and palliative medicine at Columbia University/New York State Psychiatric Institute, New York, and the study’s first author, said in an interview. “Ethical and systems issues around distribution of scarce resources may inflect patients’ and physicians’ responses,” Dr. Shalev said. “And families may not be able to be at the bedside with patients.”
To rapidly expand the palliative care workforce and meet patient needs, Dr. Shalev and colleagues recruited 16 psychiatry trainees from NYP, Columbia University Irving Medical Center, and Weill Cornell Medicine to work at NYP/Columbia University Irving Medical Center’s section of adult palliative medicine. Senior general psychiatry residents, child and adolescent psychiatry fellows, addiction psychiatry fellows, and postresidency T32 research fellows became part of a psychiatry-palliative care liaison team, offering psychosocial support and care goal strategies to patients and families.
Already well-versed in serious illness communication and psychosocial aspects of medical illness, the residents and fellows received additional training and education about SARS-CoV-2 and goals-of-care conversations. Child and adolescent psychiatry fellows participated in a communication workshop about the virus at Weill Cornell Medicine.
Working closely with the medical center’s palliative care service, the liaison team did consults around the clock at the ED under the supervision of a consultation-liaison (C-L) psychiatrist specializing in primary palliative care skills. The team managed 16 cases a day during the peak of New York’s COVID-19 outbreak, operating on a rotating schedule of one to three shifts weekly. Some shifts took place remotely to reduce exposure to the virus.
“We were fortunate that New York Presbyterian was early and aggressive in ensuring all clinical staff had personal protective equipment” in the treatment of COVID-19 patients, Dr. Shalev said.
The C-L psychiatry coordinator served as a traffic controller of sorts, overseeing daily staffing changes, maintaining a psychiatry–palliative care liaison team–shared patient list, and ensuring follow-up and continuity on patient care. The rotating schedule freed up time for trainees to meet other research and outpatient obligations.
The liaison team held a meeting each morning and accompanied the adult palliative care service on its daily virtual rounds to help streamline case management and care coordination among the various palliative care channels. Modifications in personnel took place as cases started to recede. Overall, the team participated in 100 consultations.
The findings show that there is significant overlap in psychiatry and palliative care skill sets, Dr. Shalev said. “Furthermore, many patients benefiting from palliative care services have mental health needs. But there are gaps between psychiatry and palliative care, including a lack of collaboration and cross-training. Our model showed how easily our disciplines can work together to improve the care available to all patients,” he added.
Some things could have gone more smoothly. Working under the duress of a pandemic, project leaders didn’t have enough time to train and supervise the team about advanced symptom management. Psychiatry staff members also weren’t as comfortable with nonpsychiatric symptom management as serious illness communication and psychiatric symptom management. Dr. Shalev expects these growth areas to improve over time.
The model could easily translate to other facilities, he believes. As of this writing, the liaison team was transitioning to a longer-term assignment involving patients on mechanical ventilation and their families.
The program increased access to care during a time of limited resources,and successfully combined psychiatric and palliative services – two specialties that, at times, can have conflicting recommendations, noted Maria I. Lapid, MD, a professor of psychiatry at the Mayo Clinic in Rochester, Minn., and a faculty member of the Mayo Clinic Center for Palliative Medicine, who was not part of the study. As urgent training for psychiatric trainees proved useful in the current crisis, long-term psychiatric programs will need to explore and consider how to integrate palliative care training into the psychiatric curriculum.
“Not only is this relevant in the current pandemic, but this will continue to be relevant in the context of the rapidly aging population” in the United States, said Dr. Lapid.
Dr. Shalev and colleagues declared no conflicts of interest in their study. Their research received no funds or grants from public, commercial, or nonprofit agencies.
SOURCE: Shalev D et al. J Pain Symptom Manage. 2020 Jun 13. doi.org/10.1016/j.jpainsymman.2020.06.009.
As SARS-CoV-2 cases surged in New York this past spring, one hospital system met the growing demand for palliative care in COVID-19 patients in acute care and emergency settings by training and redeploying psychiatry trainees, producing 100 consultations during a crisis period. Developers of this program wrote about their experience in the Journal of Pain and Symptom Management.
Research shows that psychiatrists can play an important, complementary role in palliative care, but not many models have explored this in practice. Over a 45-day period in March and April, New York Presbyterian/Columbia University Irving Medical Center saw an influx of 7,600 COVID-19 patients. Many were critically ill, and palliative care needs skyrocketed. Initial efforts to install a palliative care team at the emergency department and a proactive consultation model in the step-down units failed to meet demand for consults.
COVID-19 patients present unique challenges. Their clinical trajectory is less clear than those with cancer or other illnesses, Daniel Shalev, MD, a fellow in hospice and palliative medicine at Columbia University/New York State Psychiatric Institute, New York, and the study’s first author, said in an interview. “Ethical and systems issues around distribution of scarce resources may inflect patients’ and physicians’ responses,” Dr. Shalev said. “And families may not be able to be at the bedside with patients.”
To rapidly expand the palliative care workforce and meet patient needs, Dr. Shalev and colleagues recruited 16 psychiatry trainees from NYP, Columbia University Irving Medical Center, and Weill Cornell Medicine to work at NYP/Columbia University Irving Medical Center’s section of adult palliative medicine. Senior general psychiatry residents, child and adolescent psychiatry fellows, addiction psychiatry fellows, and postresidency T32 research fellows became part of a psychiatry-palliative care liaison team, offering psychosocial support and care goal strategies to patients and families.
Already well-versed in serious illness communication and psychosocial aspects of medical illness, the residents and fellows received additional training and education about SARS-CoV-2 and goals-of-care conversations. Child and adolescent psychiatry fellows participated in a communication workshop about the virus at Weill Cornell Medicine.
Working closely with the medical center’s palliative care service, the liaison team did consults around the clock at the ED under the supervision of a consultation-liaison (C-L) psychiatrist specializing in primary palliative care skills. The team managed 16 cases a day during the peak of New York’s COVID-19 outbreak, operating on a rotating schedule of one to three shifts weekly. Some shifts took place remotely to reduce exposure to the virus.
“We were fortunate that New York Presbyterian was early and aggressive in ensuring all clinical staff had personal protective equipment” in the treatment of COVID-19 patients, Dr. Shalev said.
The C-L psychiatry coordinator served as a traffic controller of sorts, overseeing daily staffing changes, maintaining a psychiatry–palliative care liaison team–shared patient list, and ensuring follow-up and continuity on patient care. The rotating schedule freed up time for trainees to meet other research and outpatient obligations.
The liaison team held a meeting each morning and accompanied the adult palliative care service on its daily virtual rounds to help streamline case management and care coordination among the various palliative care channels. Modifications in personnel took place as cases started to recede. Overall, the team participated in 100 consultations.
The findings show that there is significant overlap in psychiatry and palliative care skill sets, Dr. Shalev said. “Furthermore, many patients benefiting from palliative care services have mental health needs. But there are gaps between psychiatry and palliative care, including a lack of collaboration and cross-training. Our model showed how easily our disciplines can work together to improve the care available to all patients,” he added.
Some things could have gone more smoothly. Working under the duress of a pandemic, project leaders didn’t have enough time to train and supervise the team about advanced symptom management. Psychiatry staff members also weren’t as comfortable with nonpsychiatric symptom management as serious illness communication and psychiatric symptom management. Dr. Shalev expects these growth areas to improve over time.
The model could easily translate to other facilities, he believes. As of this writing, the liaison team was transitioning to a longer-term assignment involving patients on mechanical ventilation and their families.
The program increased access to care during a time of limited resources,and successfully combined psychiatric and palliative services – two specialties that, at times, can have conflicting recommendations, noted Maria I. Lapid, MD, a professor of psychiatry at the Mayo Clinic in Rochester, Minn., and a faculty member of the Mayo Clinic Center for Palliative Medicine, who was not part of the study. As urgent training for psychiatric trainees proved useful in the current crisis, long-term psychiatric programs will need to explore and consider how to integrate palliative care training into the psychiatric curriculum.
“Not only is this relevant in the current pandemic, but this will continue to be relevant in the context of the rapidly aging population” in the United States, said Dr. Lapid.
Dr. Shalev and colleagues declared no conflicts of interest in their study. Their research received no funds or grants from public, commercial, or nonprofit agencies.
SOURCE: Shalev D et al. J Pain Symptom Manage. 2020 Jun 13. doi.org/10.1016/j.jpainsymman.2020.06.009.
As SARS-CoV-2 cases surged in New York this past spring, one hospital system met the growing demand for palliative care in COVID-19 patients in acute care and emergency settings by training and redeploying psychiatry trainees, producing 100 consultations during a crisis period. Developers of this program wrote about their experience in the Journal of Pain and Symptom Management.
Research shows that psychiatrists can play an important, complementary role in palliative care, but not many models have explored this in practice. Over a 45-day period in March and April, New York Presbyterian/Columbia University Irving Medical Center saw an influx of 7,600 COVID-19 patients. Many were critically ill, and palliative care needs skyrocketed. Initial efforts to install a palliative care team at the emergency department and a proactive consultation model in the step-down units failed to meet demand for consults.
COVID-19 patients present unique challenges. Their clinical trajectory is less clear than those with cancer or other illnesses, Daniel Shalev, MD, a fellow in hospice and palliative medicine at Columbia University/New York State Psychiatric Institute, New York, and the study’s first author, said in an interview. “Ethical and systems issues around distribution of scarce resources may inflect patients’ and physicians’ responses,” Dr. Shalev said. “And families may not be able to be at the bedside with patients.”
To rapidly expand the palliative care workforce and meet patient needs, Dr. Shalev and colleagues recruited 16 psychiatry trainees from NYP, Columbia University Irving Medical Center, and Weill Cornell Medicine to work at NYP/Columbia University Irving Medical Center’s section of adult palliative medicine. Senior general psychiatry residents, child and adolescent psychiatry fellows, addiction psychiatry fellows, and postresidency T32 research fellows became part of a psychiatry-palliative care liaison team, offering psychosocial support and care goal strategies to patients and families.
Already well-versed in serious illness communication and psychosocial aspects of medical illness, the residents and fellows received additional training and education about SARS-CoV-2 and goals-of-care conversations. Child and adolescent psychiatry fellows participated in a communication workshop about the virus at Weill Cornell Medicine.
Working closely with the medical center’s palliative care service, the liaison team did consults around the clock at the ED under the supervision of a consultation-liaison (C-L) psychiatrist specializing in primary palliative care skills. The team managed 16 cases a day during the peak of New York’s COVID-19 outbreak, operating on a rotating schedule of one to three shifts weekly. Some shifts took place remotely to reduce exposure to the virus.
“We were fortunate that New York Presbyterian was early and aggressive in ensuring all clinical staff had personal protective equipment” in the treatment of COVID-19 patients, Dr. Shalev said.
The C-L psychiatry coordinator served as a traffic controller of sorts, overseeing daily staffing changes, maintaining a psychiatry–palliative care liaison team–shared patient list, and ensuring follow-up and continuity on patient care. The rotating schedule freed up time for trainees to meet other research and outpatient obligations.
The liaison team held a meeting each morning and accompanied the adult palliative care service on its daily virtual rounds to help streamline case management and care coordination among the various palliative care channels. Modifications in personnel took place as cases started to recede. Overall, the team participated in 100 consultations.
The findings show that there is significant overlap in psychiatry and palliative care skill sets, Dr. Shalev said. “Furthermore, many patients benefiting from palliative care services have mental health needs. But there are gaps between psychiatry and palliative care, including a lack of collaboration and cross-training. Our model showed how easily our disciplines can work together to improve the care available to all patients,” he added.
Some things could have gone more smoothly. Working under the duress of a pandemic, project leaders didn’t have enough time to train and supervise the team about advanced symptom management. Psychiatry staff members also weren’t as comfortable with nonpsychiatric symptom management as serious illness communication and psychiatric symptom management. Dr. Shalev expects these growth areas to improve over time.
The model could easily translate to other facilities, he believes. As of this writing, the liaison team was transitioning to a longer-term assignment involving patients on mechanical ventilation and their families.
The program increased access to care during a time of limited resources,and successfully combined psychiatric and palliative services – two specialties that, at times, can have conflicting recommendations, noted Maria I. Lapid, MD, a professor of psychiatry at the Mayo Clinic in Rochester, Minn., and a faculty member of the Mayo Clinic Center for Palliative Medicine, who was not part of the study. As urgent training for psychiatric trainees proved useful in the current crisis, long-term psychiatric programs will need to explore and consider how to integrate palliative care training into the psychiatric curriculum.
“Not only is this relevant in the current pandemic, but this will continue to be relevant in the context of the rapidly aging population” in the United States, said Dr. Lapid.
Dr. Shalev and colleagues declared no conflicts of interest in their study. Their research received no funds or grants from public, commercial, or nonprofit agencies.
SOURCE: Shalev D et al. J Pain Symptom Manage. 2020 Jun 13. doi.org/10.1016/j.jpainsymman.2020.06.009.
With life in the balance, a pediatric palliative care program expands its work to adults
In late March of 2020, when it became clear that hospitals in the greater New York City area would face a capacity crisis in caring for seriously ill patients with COVID-19, members of the leadership team at the Children’s Hospital at Montefiore (CHAM) in the Bronx, N.Y., convened to draft a response plan.
The recommendations put into action that day included moving the hospital’s emergency department from the lower level to the fourth floor, increasing the age limit for patients seen in the ED from 21 years of age to 30 and freeing up an entire hospital floor and a half to accommodate the anticipated surge of patients with COVID-19 admitted to Montefiore’s interconnected adult hospital, according to Sarah E. Norris, MD.
“We made multiple moves all at once,” said Dr. Norris, director of pediatric palliative care at CHAM. “It struck everyone as logical that palliative care had to be expanded, because all of the news we had received as the surge came to New York from around the world was full of death and uncertainty, and would require thoughtful conversations about end-of-life wishes at critical times and how to really respect the person and understand their values.”
When Dr. Norris left the leadership team meeting, she returned to her office, put her face in her hands, and sobbed as she began to process the gravity of what was ahead. “I cried because I knew that so many families were going to suffer a heartbreak, no matter how much we could do,” she said.
Stitching the QUILT
Over the next few days, Dr. Norris began recruiting colleagues from the large Montefiore Health System – most of whom she did not know – who met criteria for work deployment to expand CHAM’s palliative care program of clinician to 27 clinicians consisting of pediatricians, nurse practitioners, and psychologists, to meet the projected needs of COVID-19 patients and their families.
Some candidates for the effort, known as the Quality in Life Team (QUILT), were 65 years of age or older, considered at high risk for developing COVID-19-related complications themselves. Others were immunocompromised or had medical conditions that would not allow them to have direct contact with COVID-19 patients. “There were also clinicians in other parts of our health system whose practice hours were going to be severely reduced,” said Dr. Norris, who is board-certified in general pediatrics and in hospice and palliative care medicine.
Once she assembled QUILT, members participated in a 1-day rapid training webinar covering the basics of palliative care and grief, and readied themselves for one of three roles: physicians to provide face-to-face palliative care in CHAM; supportive callers to provide support to patients with COVID-19 and their families between 12:00-8:00 p.m. each day; and bereavement callers to reach out to families who lost loved ones to COVID-19 and provide grief counseling for 3 weeks.
“This allows families to have at least two contacts a day from the hospital: one from the medical team that’s giving them technical, medical information, and another from members of the QUILT team,” Dr. Norris said. “We provide support for the worry, anxiety, and fear that we know creeps in when you’re separated from your family member, especially during a pandemic when you watch TV and there’s a death count rising.”
During her early meetings with QUILT members via Zoom or on the phone, Dr. Norris encouraged them to stretch their skill sets and mindsets as they shifted from caring for children and adolescents to mostly adults. “Pediatricians are all about family; that’s why we get into this,” she said. “We’re used to treating your kids, but then, suddenly, the parent becomes our patient, like in COVID-19, or the grandparent becomes our patient. We treat you all the same; you’re part of our family. There has been no adult who has died ‘within our house’ that has died alone. There has either been a staff member at their bedside, or when possible, a family member. We are witnessing life until the last breath here.”
‘They have no loved ones with them’
One day, members of CHAM’s medical team contacted Dr. Norris about a patient with COVID-19 who’d been cared for by Montefiore clinicians all of his young life. The boy’s mother, who did not speak English, was at his bedside in the ICU, and the clinicians asked Dr. Norris to speak with her by cell phone while they prepared him for intubation.
“We were looking at each other through a glass window wall in our ICU,” Dr. Norris recalled. “I talked to her the entire time the team worked to put him on the breathing machine, through an interpreter. I asked her to tell me about her son and about her family, and she did. We developed a warm relationship. After that, every day I would see her son through the glass window wall. Every couple of days, I would have the privilege of talking to his mother by phone. At one point, she asked me, ‘Dr. Norris, do you think his lungs will heal?’ I had to tell her no. Almost selfishly, I was relieved we were on the phone, because she cried, and so did I. When he died, she was able to be by his side.”
Frederick J. Kaskel, MD, PhD, joined QUILT as a supportive caller after being asked to go home during his on-call shift on St. Patrick’s Day at CHAM, where he serves as chief emeritus of nephrology. “I was told that I was deemed to be at high risk because of my age,” the 75-year-old said. “The next day, a junior person took over for me, and 2 days later she got sick with COVID-19. She’s fine but she was home for 3 weeks sick as a dog. It was scary.”
In his role as a supportive caller, Dr. Kaskel found himself engaged in his share of detective work, trying to find phone numbers of next of kin for patients hospitalized with COVID-19. “When they come into the ER, they may not have been with a loved one or a family member; they may have been brought in by an EMT,” he said. “Some of them speak little English and others have little documentation with them. It takes a lot of work to get phone numbers.”
Once Dr. Kaskel reaches a loved one by phone, he introduces himself as a member of the QUILT team. “I tell them I’m not calling to update the medical status but just to talk to them about their loved one,” he said. “Then I usually ask, ‘So, how are you doing with this? The stress is enormous, the uncertainties.’ Then they open up and express their fears. I’ve had a lot of people say, ‘we have no money, and I don’t know how we’re going to pay rent for the apartment. We have to line up for food.’ I also ask what they do to alleviate stress. One guy said, ‘I drink a lot, but I’m careful.’ ”
Dr. Kaskel, who is also a past president of the American Society of Pediatric Nephrology, applies that same personable approach in daily conversations with adult patients hospitalized at CHAM with COVID-19, the majority of whom are African Americans in their 30s, 40s, and 50s. “Invariably, they ask, ‘Has my loved one been updated as to my status?’ ” he said. “The second thing they often say is, ‘I’m worried about infecting other people, but I also worry if I’m going to get through this. I’m really afraid I’m going to die.’ I say, ‘You have a wonderful team keeping track of you. They’re seeing you all the time and making changes to your medicines.’ ”
When patients express their fear of dying from the virus, Dr. Kaskel asks them how they’re coping with that fear. Most tell him that they pray.
“If they don’t answer, I ask if they have any hobbies, like ‘Are you watching TV? Are you reading? Do you have your cell phone?’ ” he said. “Then they open up and say things like, ‘I’m listening to music on the cell phone,’ or ‘I’m FaceTiming with my loved ones.’ The use of FaceTime is crucial, because they are in a hospital, critically ill, potentially dying alone with strangers. This really hit me on the first day [of this work]. They have no loved ones with them. They have strangers: the CHAM nurses, the medical residents, the social workers, and the doctors.”
No hospital cheeseburgers
QUILT began its work on April 6, and at one time provided palliative care services for a peak of 92 mostly adult patients with COVID-19. The supportive callers made 249 individual connections with patients and family members by phone from April 6-13, 162 connections from April 13-19, and 130 connections from April 20-26, according to Dr. Norris. As of April 28, the CHAM inpatient census of patients aged 18 years and over with COVID-19 was 42, “and we’re making 130 connections by phone to patients and family members each day,” she said.
QUILT bereavement callers are following 30 families, providing 3 weeks of acute grief counseling from the date of death. “A sad truth is that, here in New York, our entire funeral, burial, cremation system is overwhelmed in volume,” Dr. Norris said. “Only half of the patients we’re following 3 weeks out have been able to have their family member buried or cremated; many are still waiting. What strikes me here is that pediatricians are often partners in care. With time, we’re partners in care in heartbreak, and in the occasional victory. We mourn patients who have died. We’ve had colleagues who died from COVID-19 right here at our hospital. But we stand together like a family.”
Dr. Norris recalled an older woman who came into CHAM’s ICU on a ventilator, critically ill from COVID-19. She called her husband at home every day with updates. “I got to know her husband, and I got to know her through him,” Dr. Norris said. “We talked every single day and she was able to graduate off of the breathing tube and out of the ICU, which was amazing.” The woman was moved to a floor in the adult hospital, but Dr. Norris continues to visit her and to provide her husband with updates, “because I’m devoted to them,” she said.
Recently, physicians in the adult hospital consulted with Dr. Norris about the woman. “They were trying to figure out what to do with her next,” she said. “Could she go home, or did she need rehab? They said, ‘We called you, Dr. Norris, because her husband thinks he can take her home.’ We know that COVID-19 really weakens people, so I went over to see her myself. I thought, ‘No single person could take care of an adult so weak at home.’ So, I called her husband and said, ‘I’m here with your wife, and I have to tell you; if she were my mother, I couldn’t take her home today. I need you to trust me.’ He said, ‘OK. We trust you and know that you have her best interest at heart.’ ”
Dr. Kaskel relayed the story of an older patient who was slowly recovering from COVID-19. During a phone call, he asked the man if there was anything he wanted at that moment.
“He said, ‘I’d love to see my wife and my children and my grandkids. I know I’m going to see them again, but right now, doc, if you could get me a cheeseburger with lettuce and tomato and ketchup and French fries from outside of the hospital, I’d be the happiest man in the world.’
I said, ‘What’s the matter with the cheeseburger made at the hospital?’
He said, ‘No! They can’t make the cheeseburger I want.’
I promised him I’d relay that message to the social worker responsible for the patient. I told her please, if you buy this for him, I’ll pay you back.”
Self-care and the next chapter
Twice each week, QUILT members gather in front of their computer monitors for mandatory Zoom meetings facilitated by two psychologists to share challenges, best practices, and to discuss the difficult work they’re doing. “We meet, because you cannot help someone if you cannot help yourself,” Dr. Norris said. “We have been encouraged each and every meeting to practice self-compassion, and to recognize that things happen during a pandemic – some will be the best you can do.”
She described organizing and serving on QUILT as a grounding experience with important lessons for the delivery of health care after the pandemic subsides and the team members return to their respective practices. “I think we’ve all gained a greater sense of humility, and we understand that the badge I wear every day does not protect me from becoming a patient, or from having my own family fall ill,” she said. “Here, we think about it very simply: ‘I’m going to treat you like you’re part of my own family.’ ”
Dr. Kaskel said that serving on QUILT as a supportive caller is an experience he won’t soon forget.
“The human bond is so accessible if you accept it,” he said. “If someone is an introvert that might not be able to draw out a stranger on the phone, then [he or she] shouldn’t do this [work]. But the fact that you can make a bond with someone that you’re not even seeing in person and know that both sides of this phone call are getting good vibes, that’s a remarkable feeling that I never really knew before, because I’ve never really had to do that before. It brings up feelings like I had after 9/11 – a unified approach to surviving this as people, as a community, the idea that ‘we will get through this,’ even though it’s totally different than anything before. The idea that there’s still hope. Those are things you can’t put a price on.”
An article about how CHAM transformed to provide care to adult COVID-19 patients was published online May 4, 2020, in the Journal of Pediatrics: doi: 10.1016/j.jpeds.2020.04.060.
In late March of 2020, when it became clear that hospitals in the greater New York City area would face a capacity crisis in caring for seriously ill patients with COVID-19, members of the leadership team at the Children’s Hospital at Montefiore (CHAM) in the Bronx, N.Y., convened to draft a response plan.
The recommendations put into action that day included moving the hospital’s emergency department from the lower level to the fourth floor, increasing the age limit for patients seen in the ED from 21 years of age to 30 and freeing up an entire hospital floor and a half to accommodate the anticipated surge of patients with COVID-19 admitted to Montefiore’s interconnected adult hospital, according to Sarah E. Norris, MD.
“We made multiple moves all at once,” said Dr. Norris, director of pediatric palliative care at CHAM. “It struck everyone as logical that palliative care had to be expanded, because all of the news we had received as the surge came to New York from around the world was full of death and uncertainty, and would require thoughtful conversations about end-of-life wishes at critical times and how to really respect the person and understand their values.”
When Dr. Norris left the leadership team meeting, she returned to her office, put her face in her hands, and sobbed as she began to process the gravity of what was ahead. “I cried because I knew that so many families were going to suffer a heartbreak, no matter how much we could do,” she said.
Stitching the QUILT
Over the next few days, Dr. Norris began recruiting colleagues from the large Montefiore Health System – most of whom she did not know – who met criteria for work deployment to expand CHAM’s palliative care program of clinician to 27 clinicians consisting of pediatricians, nurse practitioners, and psychologists, to meet the projected needs of COVID-19 patients and their families.
Some candidates for the effort, known as the Quality in Life Team (QUILT), were 65 years of age or older, considered at high risk for developing COVID-19-related complications themselves. Others were immunocompromised or had medical conditions that would not allow them to have direct contact with COVID-19 patients. “There were also clinicians in other parts of our health system whose practice hours were going to be severely reduced,” said Dr. Norris, who is board-certified in general pediatrics and in hospice and palliative care medicine.
Once she assembled QUILT, members participated in a 1-day rapid training webinar covering the basics of palliative care and grief, and readied themselves for one of three roles: physicians to provide face-to-face palliative care in CHAM; supportive callers to provide support to patients with COVID-19 and their families between 12:00-8:00 p.m. each day; and bereavement callers to reach out to families who lost loved ones to COVID-19 and provide grief counseling for 3 weeks.
“This allows families to have at least two contacts a day from the hospital: one from the medical team that’s giving them technical, medical information, and another from members of the QUILT team,” Dr. Norris said. “We provide support for the worry, anxiety, and fear that we know creeps in when you’re separated from your family member, especially during a pandemic when you watch TV and there’s a death count rising.”
During her early meetings with QUILT members via Zoom or on the phone, Dr. Norris encouraged them to stretch their skill sets and mindsets as they shifted from caring for children and adolescents to mostly adults. “Pediatricians are all about family; that’s why we get into this,” she said. “We’re used to treating your kids, but then, suddenly, the parent becomes our patient, like in COVID-19, or the grandparent becomes our patient. We treat you all the same; you’re part of our family. There has been no adult who has died ‘within our house’ that has died alone. There has either been a staff member at their bedside, or when possible, a family member. We are witnessing life until the last breath here.”
‘They have no loved ones with them’
One day, members of CHAM’s medical team contacted Dr. Norris about a patient with COVID-19 who’d been cared for by Montefiore clinicians all of his young life. The boy’s mother, who did not speak English, was at his bedside in the ICU, and the clinicians asked Dr. Norris to speak with her by cell phone while they prepared him for intubation.
“We were looking at each other through a glass window wall in our ICU,” Dr. Norris recalled. “I talked to her the entire time the team worked to put him on the breathing machine, through an interpreter. I asked her to tell me about her son and about her family, and she did. We developed a warm relationship. After that, every day I would see her son through the glass window wall. Every couple of days, I would have the privilege of talking to his mother by phone. At one point, she asked me, ‘Dr. Norris, do you think his lungs will heal?’ I had to tell her no. Almost selfishly, I was relieved we were on the phone, because she cried, and so did I. When he died, she was able to be by his side.”
Frederick J. Kaskel, MD, PhD, joined QUILT as a supportive caller after being asked to go home during his on-call shift on St. Patrick’s Day at CHAM, where he serves as chief emeritus of nephrology. “I was told that I was deemed to be at high risk because of my age,” the 75-year-old said. “The next day, a junior person took over for me, and 2 days later she got sick with COVID-19. She’s fine but she was home for 3 weeks sick as a dog. It was scary.”
In his role as a supportive caller, Dr. Kaskel found himself engaged in his share of detective work, trying to find phone numbers of next of kin for patients hospitalized with COVID-19. “When they come into the ER, they may not have been with a loved one or a family member; they may have been brought in by an EMT,” he said. “Some of them speak little English and others have little documentation with them. It takes a lot of work to get phone numbers.”
Once Dr. Kaskel reaches a loved one by phone, he introduces himself as a member of the QUILT team. “I tell them I’m not calling to update the medical status but just to talk to them about their loved one,” he said. “Then I usually ask, ‘So, how are you doing with this? The stress is enormous, the uncertainties.’ Then they open up and express their fears. I’ve had a lot of people say, ‘we have no money, and I don’t know how we’re going to pay rent for the apartment. We have to line up for food.’ I also ask what they do to alleviate stress. One guy said, ‘I drink a lot, but I’m careful.’ ”
Dr. Kaskel, who is also a past president of the American Society of Pediatric Nephrology, applies that same personable approach in daily conversations with adult patients hospitalized at CHAM with COVID-19, the majority of whom are African Americans in their 30s, 40s, and 50s. “Invariably, they ask, ‘Has my loved one been updated as to my status?’ ” he said. “The second thing they often say is, ‘I’m worried about infecting other people, but I also worry if I’m going to get through this. I’m really afraid I’m going to die.’ I say, ‘You have a wonderful team keeping track of you. They’re seeing you all the time and making changes to your medicines.’ ”
When patients express their fear of dying from the virus, Dr. Kaskel asks them how they’re coping with that fear. Most tell him that they pray.
“If they don’t answer, I ask if they have any hobbies, like ‘Are you watching TV? Are you reading? Do you have your cell phone?’ ” he said. “Then they open up and say things like, ‘I’m listening to music on the cell phone,’ or ‘I’m FaceTiming with my loved ones.’ The use of FaceTime is crucial, because they are in a hospital, critically ill, potentially dying alone with strangers. This really hit me on the first day [of this work]. They have no loved ones with them. They have strangers: the CHAM nurses, the medical residents, the social workers, and the doctors.”
No hospital cheeseburgers
QUILT began its work on April 6, and at one time provided palliative care services for a peak of 92 mostly adult patients with COVID-19. The supportive callers made 249 individual connections with patients and family members by phone from April 6-13, 162 connections from April 13-19, and 130 connections from April 20-26, according to Dr. Norris. As of April 28, the CHAM inpatient census of patients aged 18 years and over with COVID-19 was 42, “and we’re making 130 connections by phone to patients and family members each day,” she said.
QUILT bereavement callers are following 30 families, providing 3 weeks of acute grief counseling from the date of death. “A sad truth is that, here in New York, our entire funeral, burial, cremation system is overwhelmed in volume,” Dr. Norris said. “Only half of the patients we’re following 3 weeks out have been able to have their family member buried or cremated; many are still waiting. What strikes me here is that pediatricians are often partners in care. With time, we’re partners in care in heartbreak, and in the occasional victory. We mourn patients who have died. We’ve had colleagues who died from COVID-19 right here at our hospital. But we stand together like a family.”
Dr. Norris recalled an older woman who came into CHAM’s ICU on a ventilator, critically ill from COVID-19. She called her husband at home every day with updates. “I got to know her husband, and I got to know her through him,” Dr. Norris said. “We talked every single day and she was able to graduate off of the breathing tube and out of the ICU, which was amazing.” The woman was moved to a floor in the adult hospital, but Dr. Norris continues to visit her and to provide her husband with updates, “because I’m devoted to them,” she said.
Recently, physicians in the adult hospital consulted with Dr. Norris about the woman. “They were trying to figure out what to do with her next,” she said. “Could she go home, or did she need rehab? They said, ‘We called you, Dr. Norris, because her husband thinks he can take her home.’ We know that COVID-19 really weakens people, so I went over to see her myself. I thought, ‘No single person could take care of an adult so weak at home.’ So, I called her husband and said, ‘I’m here with your wife, and I have to tell you; if she were my mother, I couldn’t take her home today. I need you to trust me.’ He said, ‘OK. We trust you and know that you have her best interest at heart.’ ”
Dr. Kaskel relayed the story of an older patient who was slowly recovering from COVID-19. During a phone call, he asked the man if there was anything he wanted at that moment.
“He said, ‘I’d love to see my wife and my children and my grandkids. I know I’m going to see them again, but right now, doc, if you could get me a cheeseburger with lettuce and tomato and ketchup and French fries from outside of the hospital, I’d be the happiest man in the world.’
I said, ‘What’s the matter with the cheeseburger made at the hospital?’
He said, ‘No! They can’t make the cheeseburger I want.’
I promised him I’d relay that message to the social worker responsible for the patient. I told her please, if you buy this for him, I’ll pay you back.”
Self-care and the next chapter
Twice each week, QUILT members gather in front of their computer monitors for mandatory Zoom meetings facilitated by two psychologists to share challenges, best practices, and to discuss the difficult work they’re doing. “We meet, because you cannot help someone if you cannot help yourself,” Dr. Norris said. “We have been encouraged each and every meeting to practice self-compassion, and to recognize that things happen during a pandemic – some will be the best you can do.”
She described organizing and serving on QUILT as a grounding experience with important lessons for the delivery of health care after the pandemic subsides and the team members return to their respective practices. “I think we’ve all gained a greater sense of humility, and we understand that the badge I wear every day does not protect me from becoming a patient, or from having my own family fall ill,” she said. “Here, we think about it very simply: ‘I’m going to treat you like you’re part of my own family.’ ”
Dr. Kaskel said that serving on QUILT as a supportive caller is an experience he won’t soon forget.
“The human bond is so accessible if you accept it,” he said. “If someone is an introvert that might not be able to draw out a stranger on the phone, then [he or she] shouldn’t do this [work]. But the fact that you can make a bond with someone that you’re not even seeing in person and know that both sides of this phone call are getting good vibes, that’s a remarkable feeling that I never really knew before, because I’ve never really had to do that before. It brings up feelings like I had after 9/11 – a unified approach to surviving this as people, as a community, the idea that ‘we will get through this,’ even though it’s totally different than anything before. The idea that there’s still hope. Those are things you can’t put a price on.”
An article about how CHAM transformed to provide care to adult COVID-19 patients was published online May 4, 2020, in the Journal of Pediatrics: doi: 10.1016/j.jpeds.2020.04.060.
In late March of 2020, when it became clear that hospitals in the greater New York City area would face a capacity crisis in caring for seriously ill patients with COVID-19, members of the leadership team at the Children’s Hospital at Montefiore (CHAM) in the Bronx, N.Y., convened to draft a response plan.
The recommendations put into action that day included moving the hospital’s emergency department from the lower level to the fourth floor, increasing the age limit for patients seen in the ED from 21 years of age to 30 and freeing up an entire hospital floor and a half to accommodate the anticipated surge of patients with COVID-19 admitted to Montefiore’s interconnected adult hospital, according to Sarah E. Norris, MD.
“We made multiple moves all at once,” said Dr. Norris, director of pediatric palliative care at CHAM. “It struck everyone as logical that palliative care had to be expanded, because all of the news we had received as the surge came to New York from around the world was full of death and uncertainty, and would require thoughtful conversations about end-of-life wishes at critical times and how to really respect the person and understand their values.”
When Dr. Norris left the leadership team meeting, she returned to her office, put her face in her hands, and sobbed as she began to process the gravity of what was ahead. “I cried because I knew that so many families were going to suffer a heartbreak, no matter how much we could do,” she said.
Stitching the QUILT
Over the next few days, Dr. Norris began recruiting colleagues from the large Montefiore Health System – most of whom she did not know – who met criteria for work deployment to expand CHAM’s palliative care program of clinician to 27 clinicians consisting of pediatricians, nurse practitioners, and psychologists, to meet the projected needs of COVID-19 patients and their families.
Some candidates for the effort, known as the Quality in Life Team (QUILT), were 65 years of age or older, considered at high risk for developing COVID-19-related complications themselves. Others were immunocompromised or had medical conditions that would not allow them to have direct contact with COVID-19 patients. “There were also clinicians in other parts of our health system whose practice hours were going to be severely reduced,” said Dr. Norris, who is board-certified in general pediatrics and in hospice and palliative care medicine.
Once she assembled QUILT, members participated in a 1-day rapid training webinar covering the basics of palliative care and grief, and readied themselves for one of three roles: physicians to provide face-to-face palliative care in CHAM; supportive callers to provide support to patients with COVID-19 and their families between 12:00-8:00 p.m. each day; and bereavement callers to reach out to families who lost loved ones to COVID-19 and provide grief counseling for 3 weeks.
“This allows families to have at least two contacts a day from the hospital: one from the medical team that’s giving them technical, medical information, and another from members of the QUILT team,” Dr. Norris said. “We provide support for the worry, anxiety, and fear that we know creeps in when you’re separated from your family member, especially during a pandemic when you watch TV and there’s a death count rising.”
During her early meetings with QUILT members via Zoom or on the phone, Dr. Norris encouraged them to stretch their skill sets and mindsets as they shifted from caring for children and adolescents to mostly adults. “Pediatricians are all about family; that’s why we get into this,” she said. “We’re used to treating your kids, but then, suddenly, the parent becomes our patient, like in COVID-19, or the grandparent becomes our patient. We treat you all the same; you’re part of our family. There has been no adult who has died ‘within our house’ that has died alone. There has either been a staff member at their bedside, or when possible, a family member. We are witnessing life until the last breath here.”
‘They have no loved ones with them’
One day, members of CHAM’s medical team contacted Dr. Norris about a patient with COVID-19 who’d been cared for by Montefiore clinicians all of his young life. The boy’s mother, who did not speak English, was at his bedside in the ICU, and the clinicians asked Dr. Norris to speak with her by cell phone while they prepared him for intubation.
“We were looking at each other through a glass window wall in our ICU,” Dr. Norris recalled. “I talked to her the entire time the team worked to put him on the breathing machine, through an interpreter. I asked her to tell me about her son and about her family, and she did. We developed a warm relationship. After that, every day I would see her son through the glass window wall. Every couple of days, I would have the privilege of talking to his mother by phone. At one point, she asked me, ‘Dr. Norris, do you think his lungs will heal?’ I had to tell her no. Almost selfishly, I was relieved we were on the phone, because she cried, and so did I. When he died, she was able to be by his side.”
Frederick J. Kaskel, MD, PhD, joined QUILT as a supportive caller after being asked to go home during his on-call shift on St. Patrick’s Day at CHAM, where he serves as chief emeritus of nephrology. “I was told that I was deemed to be at high risk because of my age,” the 75-year-old said. “The next day, a junior person took over for me, and 2 days later she got sick with COVID-19. She’s fine but she was home for 3 weeks sick as a dog. It was scary.”
In his role as a supportive caller, Dr. Kaskel found himself engaged in his share of detective work, trying to find phone numbers of next of kin for patients hospitalized with COVID-19. “When they come into the ER, they may not have been with a loved one or a family member; they may have been brought in by an EMT,” he said. “Some of them speak little English and others have little documentation with them. It takes a lot of work to get phone numbers.”
Once Dr. Kaskel reaches a loved one by phone, he introduces himself as a member of the QUILT team. “I tell them I’m not calling to update the medical status but just to talk to them about their loved one,” he said. “Then I usually ask, ‘So, how are you doing with this? The stress is enormous, the uncertainties.’ Then they open up and express their fears. I’ve had a lot of people say, ‘we have no money, and I don’t know how we’re going to pay rent for the apartment. We have to line up for food.’ I also ask what they do to alleviate stress. One guy said, ‘I drink a lot, but I’m careful.’ ”
Dr. Kaskel, who is also a past president of the American Society of Pediatric Nephrology, applies that same personable approach in daily conversations with adult patients hospitalized at CHAM with COVID-19, the majority of whom are African Americans in their 30s, 40s, and 50s. “Invariably, they ask, ‘Has my loved one been updated as to my status?’ ” he said. “The second thing they often say is, ‘I’m worried about infecting other people, but I also worry if I’m going to get through this. I’m really afraid I’m going to die.’ I say, ‘You have a wonderful team keeping track of you. They’re seeing you all the time and making changes to your medicines.’ ”
When patients express their fear of dying from the virus, Dr. Kaskel asks them how they’re coping with that fear. Most tell him that they pray.
“If they don’t answer, I ask if they have any hobbies, like ‘Are you watching TV? Are you reading? Do you have your cell phone?’ ” he said. “Then they open up and say things like, ‘I’m listening to music on the cell phone,’ or ‘I’m FaceTiming with my loved ones.’ The use of FaceTime is crucial, because they are in a hospital, critically ill, potentially dying alone with strangers. This really hit me on the first day [of this work]. They have no loved ones with them. They have strangers: the CHAM nurses, the medical residents, the social workers, and the doctors.”
No hospital cheeseburgers
QUILT began its work on April 6, and at one time provided palliative care services for a peak of 92 mostly adult patients with COVID-19. The supportive callers made 249 individual connections with patients and family members by phone from April 6-13, 162 connections from April 13-19, and 130 connections from April 20-26, according to Dr. Norris. As of April 28, the CHAM inpatient census of patients aged 18 years and over with COVID-19 was 42, “and we’re making 130 connections by phone to patients and family members each day,” she said.
QUILT bereavement callers are following 30 families, providing 3 weeks of acute grief counseling from the date of death. “A sad truth is that, here in New York, our entire funeral, burial, cremation system is overwhelmed in volume,” Dr. Norris said. “Only half of the patients we’re following 3 weeks out have been able to have their family member buried or cremated; many are still waiting. What strikes me here is that pediatricians are often partners in care. With time, we’re partners in care in heartbreak, and in the occasional victory. We mourn patients who have died. We’ve had colleagues who died from COVID-19 right here at our hospital. But we stand together like a family.”
Dr. Norris recalled an older woman who came into CHAM’s ICU on a ventilator, critically ill from COVID-19. She called her husband at home every day with updates. “I got to know her husband, and I got to know her through him,” Dr. Norris said. “We talked every single day and she was able to graduate off of the breathing tube and out of the ICU, which was amazing.” The woman was moved to a floor in the adult hospital, but Dr. Norris continues to visit her and to provide her husband with updates, “because I’m devoted to them,” she said.
Recently, physicians in the adult hospital consulted with Dr. Norris about the woman. “They were trying to figure out what to do with her next,” she said. “Could she go home, or did she need rehab? They said, ‘We called you, Dr. Norris, because her husband thinks he can take her home.’ We know that COVID-19 really weakens people, so I went over to see her myself. I thought, ‘No single person could take care of an adult so weak at home.’ So, I called her husband and said, ‘I’m here with your wife, and I have to tell you; if she were my mother, I couldn’t take her home today. I need you to trust me.’ He said, ‘OK. We trust you and know that you have her best interest at heart.’ ”
Dr. Kaskel relayed the story of an older patient who was slowly recovering from COVID-19. During a phone call, he asked the man if there was anything he wanted at that moment.
“He said, ‘I’d love to see my wife and my children and my grandkids. I know I’m going to see them again, but right now, doc, if you could get me a cheeseburger with lettuce and tomato and ketchup and French fries from outside of the hospital, I’d be the happiest man in the world.’
I said, ‘What’s the matter with the cheeseburger made at the hospital?’
He said, ‘No! They can’t make the cheeseburger I want.’
I promised him I’d relay that message to the social worker responsible for the patient. I told her please, if you buy this for him, I’ll pay you back.”
Self-care and the next chapter
Twice each week, QUILT members gather in front of their computer monitors for mandatory Zoom meetings facilitated by two psychologists to share challenges, best practices, and to discuss the difficult work they’re doing. “We meet, because you cannot help someone if you cannot help yourself,” Dr. Norris said. “We have been encouraged each and every meeting to practice self-compassion, and to recognize that things happen during a pandemic – some will be the best you can do.”
She described organizing and serving on QUILT as a grounding experience with important lessons for the delivery of health care after the pandemic subsides and the team members return to their respective practices. “I think we’ve all gained a greater sense of humility, and we understand that the badge I wear every day does not protect me from becoming a patient, or from having my own family fall ill,” she said. “Here, we think about it very simply: ‘I’m going to treat you like you’re part of my own family.’ ”
Dr. Kaskel said that serving on QUILT as a supportive caller is an experience he won’t soon forget.
“The human bond is so accessible if you accept it,” he said. “If someone is an introvert that might not be able to draw out a stranger on the phone, then [he or she] shouldn’t do this [work]. But the fact that you can make a bond with someone that you’re not even seeing in person and know that both sides of this phone call are getting good vibes, that’s a remarkable feeling that I never really knew before, because I’ve never really had to do that before. It brings up feelings like I had after 9/11 – a unified approach to surviving this as people, as a community, the idea that ‘we will get through this,’ even though it’s totally different than anything before. The idea that there’s still hope. Those are things you can’t put a price on.”
An article about how CHAM transformed to provide care to adult COVID-19 patients was published online May 4, 2020, in the Journal of Pediatrics: doi: 10.1016/j.jpeds.2020.04.060.













