User login
Dried blood spot tests show sensitivity as cCMV screen
Dried blood spot testing showed sensitivity comparable to saliva as a screening method for congenital cytomegalovirus infection in newborns, based on data from more than 12,000 newborns.
Congenital cytomegalovirus (cCMV) is a common congenital virus in the United States, but remains underrecognized, wrote Sheila C. Dollard, PhD, of the Centers for Disease Control and Prevention in Atlanta, and colleagues.
“Given the burden associated with cCMV and the proven benefits of treatment and early intervention for some affected infants, there has been growing interest in universal newborn screening,” but an ideal screening strategy has yet to be determined, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers screened 12,554 newborns in Minnesota, including 56 with confirmed CMV infection. The newborns were screened for cCMV via dried blood spots (DBS) and saliva collected 1-2 days after birth. The DBS were tested for CMV DNA via polymerase chain reaction (PCR) at the University of Minnesota (UMN) and the CDC.
The overall sensitivity rate was 85.7% for a combination of laboratory results from the UMN and the CDC, which had separate sensitivities of 73.2% and 76.8%, respectively.
The specificity of the combined results was 100.0% (100% from both UMN and CDC), the combined positive predictive value was 98.0% (100.0% from UMN, 97.7% from CDC), and the combined negative predictive value was 99.9% (99.9% from both UMN and CDC).
By comparison, saliva swab test results showed sensitivity of 92.9%, specificity of 99.9%, positive predictive value of 86.7%, and negative predictive value of 100.0%.
The study findings were limited by several factors including the false-positive and false-negative results from saliva screening. Overall, the false-positive rate was 0.06%, which is comparable to rates from other screening techniques, the researchers said. “The recent Food and Drug Administration approval of a point-of-care neonatal saliva CMV test (Meridian Bioscience), underscores the importance of further clarifying the role of false-positive saliva CMV test results and underscores the requirement for urine confirmation for diagnosis of cCMV,” they added.
However, the study findings support the acceptability and feasibility of cCMV screening, as parents reported generally positive attitudes about the process, the researchers said.
The study is ongoing, and designed to follow infants with confirmed cCMV for up to age 4 years to assess clinical outcomes, they added. “Diagnostic methods are always improving, and therefore, our results show the potential of DBS to provide low-cost CMV screening with smooth integration of sample collection, laboratory testing, and follow-up,” they concluded.
Findings lay foundation for widespread use
“By using enhanced PCR methods, Dollard et al. have rekindled the hope that NBDBS [newborn dried blood spots] testing may be a viable method for large-scale, universal newborn screening for congenital CMV,” Gail J. Demmler-Harrison, MD, of Texas Children’s Hospital, Houston, wrote in an accompanying editorial. Congenital CMV is a common infection, but accurate prevalence remains uncertain because not all newborns are tested, she noted. Detection of CMV currently may involve urine, saliva, and blood, but challenges to the use of these methods include “a variety of constantly evolving DNA detection methods,” she said.
Although urine and saliva samples have been proposed for universal screening, they would require the creation of new sample collection and testing programs. “The routine of collecting the NBDBS samples on all newborns and the logistics of routing them to central laboratories and then reporting results to caregivers is already in place and are strengths of NBDBS samples for universal newborn screening,” but had been limited by a less sensitive platform than urine or saliva, said Dr. Demmler-Harrison.
“The results in the study by Dollard et al. may be a total game changer for the NBDBS proponents,” she emphasized. “Furthermore, scientists who have adapted even more sensitive DNA detection assays, such as the loop-mediated isothermal assay for detection of DNA in clinical samples from newborns, may be able to adapt loop-mediated isothermal assay methodology to detect CMV DNA in NBDBS,” she added.
“By adapting the collection methods, by using optimal filter paper to enhance DNA adherence, by improving DNA elution procedures, and by developing novel amplification and detection methods, NBDBS may soon meet the challenge and reach the sensitivity and specificity necessary for universal screening for congenital CMV,” she concluded.
The study was supported by the CDC, the Minnesota Department of Health, the National Vaccine Program Office (U.S. federal government), and the University of South Carolina Disability Research and Dissemination Center.
Dr. Dollard and Dr. Demmler-Harrison had no financial conflicts to disclose.
Dried blood spot testing showed sensitivity comparable to saliva as a screening method for congenital cytomegalovirus infection in newborns, based on data from more than 12,000 newborns.
Congenital cytomegalovirus (cCMV) is a common congenital virus in the United States, but remains underrecognized, wrote Sheila C. Dollard, PhD, of the Centers for Disease Control and Prevention in Atlanta, and colleagues.
“Given the burden associated with cCMV and the proven benefits of treatment and early intervention for some affected infants, there has been growing interest in universal newborn screening,” but an ideal screening strategy has yet to be determined, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers screened 12,554 newborns in Minnesota, including 56 with confirmed CMV infection. The newborns were screened for cCMV via dried blood spots (DBS) and saliva collected 1-2 days after birth. The DBS were tested for CMV DNA via polymerase chain reaction (PCR) at the University of Minnesota (UMN) and the CDC.
The overall sensitivity rate was 85.7% for a combination of laboratory results from the UMN and the CDC, which had separate sensitivities of 73.2% and 76.8%, respectively.
The specificity of the combined results was 100.0% (100% from both UMN and CDC), the combined positive predictive value was 98.0% (100.0% from UMN, 97.7% from CDC), and the combined negative predictive value was 99.9% (99.9% from both UMN and CDC).
By comparison, saliva swab test results showed sensitivity of 92.9%, specificity of 99.9%, positive predictive value of 86.7%, and negative predictive value of 100.0%.
The study findings were limited by several factors including the false-positive and false-negative results from saliva screening. Overall, the false-positive rate was 0.06%, which is comparable to rates from other screening techniques, the researchers said. “The recent Food and Drug Administration approval of a point-of-care neonatal saliva CMV test (Meridian Bioscience), underscores the importance of further clarifying the role of false-positive saliva CMV test results and underscores the requirement for urine confirmation for diagnosis of cCMV,” they added.
However, the study findings support the acceptability and feasibility of cCMV screening, as parents reported generally positive attitudes about the process, the researchers said.
The study is ongoing, and designed to follow infants with confirmed cCMV for up to age 4 years to assess clinical outcomes, they added. “Diagnostic methods are always improving, and therefore, our results show the potential of DBS to provide low-cost CMV screening with smooth integration of sample collection, laboratory testing, and follow-up,” they concluded.
Findings lay foundation for widespread use
“By using enhanced PCR methods, Dollard et al. have rekindled the hope that NBDBS [newborn dried blood spots] testing may be a viable method for large-scale, universal newborn screening for congenital CMV,” Gail J. Demmler-Harrison, MD, of Texas Children’s Hospital, Houston, wrote in an accompanying editorial. Congenital CMV is a common infection, but accurate prevalence remains uncertain because not all newborns are tested, she noted. Detection of CMV currently may involve urine, saliva, and blood, but challenges to the use of these methods include “a variety of constantly evolving DNA detection methods,” she said.
Although urine and saliva samples have been proposed for universal screening, they would require the creation of new sample collection and testing programs. “The routine of collecting the NBDBS samples on all newborns and the logistics of routing them to central laboratories and then reporting results to caregivers is already in place and are strengths of NBDBS samples for universal newborn screening,” but had been limited by a less sensitive platform than urine or saliva, said Dr. Demmler-Harrison.
“The results in the study by Dollard et al. may be a total game changer for the NBDBS proponents,” she emphasized. “Furthermore, scientists who have adapted even more sensitive DNA detection assays, such as the loop-mediated isothermal assay for detection of DNA in clinical samples from newborns, may be able to adapt loop-mediated isothermal assay methodology to detect CMV DNA in NBDBS,” she added.
“By adapting the collection methods, by using optimal filter paper to enhance DNA adherence, by improving DNA elution procedures, and by developing novel amplification and detection methods, NBDBS may soon meet the challenge and reach the sensitivity and specificity necessary for universal screening for congenital CMV,” she concluded.
The study was supported by the CDC, the Minnesota Department of Health, the National Vaccine Program Office (U.S. federal government), and the University of South Carolina Disability Research and Dissemination Center.
Dr. Dollard and Dr. Demmler-Harrison had no financial conflicts to disclose.
Dried blood spot testing showed sensitivity comparable to saliva as a screening method for congenital cytomegalovirus infection in newborns, based on data from more than 12,000 newborns.
Congenital cytomegalovirus (cCMV) is a common congenital virus in the United States, but remains underrecognized, wrote Sheila C. Dollard, PhD, of the Centers for Disease Control and Prevention in Atlanta, and colleagues.
“Given the burden associated with cCMV and the proven benefits of treatment and early intervention for some affected infants, there has been growing interest in universal newborn screening,” but an ideal screening strategy has yet to be determined, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers screened 12,554 newborns in Minnesota, including 56 with confirmed CMV infection. The newborns were screened for cCMV via dried blood spots (DBS) and saliva collected 1-2 days after birth. The DBS were tested for CMV DNA via polymerase chain reaction (PCR) at the University of Minnesota (UMN) and the CDC.
The overall sensitivity rate was 85.7% for a combination of laboratory results from the UMN and the CDC, which had separate sensitivities of 73.2% and 76.8%, respectively.
The specificity of the combined results was 100.0% (100% from both UMN and CDC), the combined positive predictive value was 98.0% (100.0% from UMN, 97.7% from CDC), and the combined negative predictive value was 99.9% (99.9% from both UMN and CDC).
By comparison, saliva swab test results showed sensitivity of 92.9%, specificity of 99.9%, positive predictive value of 86.7%, and negative predictive value of 100.0%.
The study findings were limited by several factors including the false-positive and false-negative results from saliva screening. Overall, the false-positive rate was 0.06%, which is comparable to rates from other screening techniques, the researchers said. “The recent Food and Drug Administration approval of a point-of-care neonatal saliva CMV test (Meridian Bioscience), underscores the importance of further clarifying the role of false-positive saliva CMV test results and underscores the requirement for urine confirmation for diagnosis of cCMV,” they added.
However, the study findings support the acceptability and feasibility of cCMV screening, as parents reported generally positive attitudes about the process, the researchers said.
The study is ongoing, and designed to follow infants with confirmed cCMV for up to age 4 years to assess clinical outcomes, they added. “Diagnostic methods are always improving, and therefore, our results show the potential of DBS to provide low-cost CMV screening with smooth integration of sample collection, laboratory testing, and follow-up,” they concluded.
Findings lay foundation for widespread use
“By using enhanced PCR methods, Dollard et al. have rekindled the hope that NBDBS [newborn dried blood spots] testing may be a viable method for large-scale, universal newborn screening for congenital CMV,” Gail J. Demmler-Harrison, MD, of Texas Children’s Hospital, Houston, wrote in an accompanying editorial. Congenital CMV is a common infection, but accurate prevalence remains uncertain because not all newborns are tested, she noted. Detection of CMV currently may involve urine, saliva, and blood, but challenges to the use of these methods include “a variety of constantly evolving DNA detection methods,” she said.
Although urine and saliva samples have been proposed for universal screening, they would require the creation of new sample collection and testing programs. “The routine of collecting the NBDBS samples on all newborns and the logistics of routing them to central laboratories and then reporting results to caregivers is already in place and are strengths of NBDBS samples for universal newborn screening,” but had been limited by a less sensitive platform than urine or saliva, said Dr. Demmler-Harrison.
“The results in the study by Dollard et al. may be a total game changer for the NBDBS proponents,” she emphasized. “Furthermore, scientists who have adapted even more sensitive DNA detection assays, such as the loop-mediated isothermal assay for detection of DNA in clinical samples from newborns, may be able to adapt loop-mediated isothermal assay methodology to detect CMV DNA in NBDBS,” she added.
“By adapting the collection methods, by using optimal filter paper to enhance DNA adherence, by improving DNA elution procedures, and by developing novel amplification and detection methods, NBDBS may soon meet the challenge and reach the sensitivity and specificity necessary for universal screening for congenital CMV,” she concluded.
The study was supported by the CDC, the Minnesota Department of Health, the National Vaccine Program Office (U.S. federal government), and the University of South Carolina Disability Research and Dissemination Center.
Dr. Dollard and Dr. Demmler-Harrison had no financial conflicts to disclose.
FROM JAMA PEDIATRICS
New child COVID-19 cases decline as total passes 3 million
New COVID-19 cases in children continue to drop each week, but the total number of cases has now surpassed 3 million since the start of the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
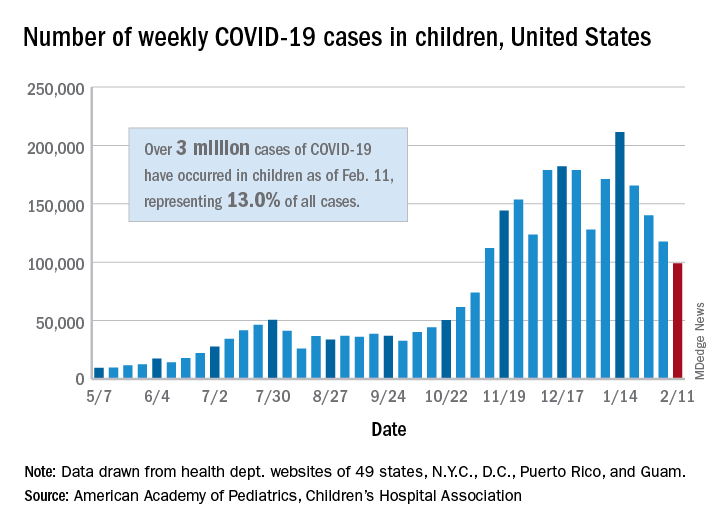
It was still enough, though, to bring the total to 3.03 million children infected with SARS-CoV-19 in the United States, the AAP and the CHA said in their weekly report.
The nation also hit a couple of other ignominious milestones. The cumulative rate of COVID-19 infection now stands at 4,030 per 100,000, so 4% of all children have been infected. Also, children represented 16.9% of all new cases for the week, which equals the highest proportion seen throughout the pandemic, based on data from health departments in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
There have been 241 COVID-19–related deaths in children so far, with 14 reported during the week of Feb. 5-11. Kansas just recorded its first pediatric death, which leaves 10 states that have had no fatalities. Texas, with 39 deaths, has had more than any other state, among the 43 that are reporting mortality by age, the AAP/CHA report showed.
New COVID-19 cases in children continue to drop each week, but the total number of cases has now surpassed 3 million since the start of the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

It was still enough, though, to bring the total to 3.03 million children infected with SARS-CoV-19 in the United States, the AAP and the CHA said in their weekly report.
The nation also hit a couple of other ignominious milestones. The cumulative rate of COVID-19 infection now stands at 4,030 per 100,000, so 4% of all children have been infected. Also, children represented 16.9% of all new cases for the week, which equals the highest proportion seen throughout the pandemic, based on data from health departments in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
There have been 241 COVID-19–related deaths in children so far, with 14 reported during the week of Feb. 5-11. Kansas just recorded its first pediatric death, which leaves 10 states that have had no fatalities. Texas, with 39 deaths, has had more than any other state, among the 43 that are reporting mortality by age, the AAP/CHA report showed.
New COVID-19 cases in children continue to drop each week, but the total number of cases has now surpassed 3 million since the start of the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

It was still enough, though, to bring the total to 3.03 million children infected with SARS-CoV-19 in the United States, the AAP and the CHA said in their weekly report.
The nation also hit a couple of other ignominious milestones. The cumulative rate of COVID-19 infection now stands at 4,030 per 100,000, so 4% of all children have been infected. Also, children represented 16.9% of all new cases for the week, which equals the highest proportion seen throughout the pandemic, based on data from health departments in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
There have been 241 COVID-19–related deaths in children so far, with 14 reported during the week of Feb. 5-11. Kansas just recorded its first pediatric death, which leaves 10 states that have had no fatalities. Texas, with 39 deaths, has had more than any other state, among the 43 that are reporting mortality by age, the AAP/CHA report showed.
One-third of health care workers leery of getting COVID-19 vaccine, survey shows
Moreover, 54% of direct care providers indicated that they would take the vaccine if offered, compared with 60% of noncare providers.
The findings come from what is believed to be the largest survey of health care provider attitudes toward COVID-19 vaccination, published online Jan. 25 in Clinical Infectious Diseases.
“We have shown that self-reported willingness to receive vaccination against COVID-19 differs by age, gender, race and hospital role, with physicians and research scientists showing the highest acceptance,” Jana Shaw, MD, MPH, State University of New York, Syracuse, N.Y, the study’s corresponding author, told this news organization. “Building trust in authorities and confidence in vaccines is a complex and time-consuming process that requires commitment and resources. We have to make those investments as hesitancy can severely undermine vaccination coverage. Because health care providers are members of our communities, it is possible that their views are shared by the public at large. Our findings can assist public health professionals as a starting point of discussion and engagement with communities to ensure that we vaccinate at least 80% of the public to end the pandemic.”
For the study, Dr. Shaw and her colleagues emailed an anonymous survey to 9,565 employees of State University of New York Upstate Medical University, Syracuse, an academic medical center that cares for an estimated 1.8 million people. The survey, which contained questions intended to evaluate attitudes, belief, and willingness to get vaccinated, took place between Nov. 23 and Dec. 5, about a week before the U.S. Food and Drug Administration granted the first emergency use authorization for the Pfizer-BioNTech BNT162b2 mRNA vaccine.
Survey recipients included physicians, nurse practitioners, physician assistants, nurses, pharmacists, medical and nursing students, allied health professionals, and nonclinical ancillary staff.
Of the 9,565 surveys sent, 5,287 responses were collected and used in the final analysis, for a response rate of 55%. The mean age of respondents was 43, 73% were female, 85% were White, 6% were Asian, 5% were Black/African American, and the rest were Native American, Native Hawaiian/Pacific Islander, or from other races. More than half of respondents (59%) reported that they provided direct patient care, and 32% said they provided care for patients with COVID-19.
Of all survey respondents, 58% expressed their intent to receive a COVID-19 vaccine, but this varied by their role in the health care system. For example, in response to the statement, “If a vaccine were offered free of charge, I would take it,” 80% of scientists and physicians agreed that they would, while colleagues in other roles were unsure whether they would take the vaccine, including 34% of registered nurses, 32% of allied health professionals, and 32% of master’s-level clinicians. These differences across roles were significant (P less than .001).
The researchers also found that direct patient care or care for COVID-19 patients was associated with lower vaccination intent. For example, 54% of direct care providers and 62% of non-care providers indicated they would take the vaccine if offered, compared with 52% of those who had provided care for COVID-19 patients vs. 61% of those who had not (P less than .001).
“This was a really surprising finding,” said Dr. Shaw, who is a pediatric infectious diseases physician at SUNY Upstate. “In general, one would expect that perceived severity of disease would lead to a greater desire to get vaccinated. Because our question did not address severity of disease, it is possible that we oversampled respondents who took care of patients with mild disease (i.e., in an outpatient setting). This could have led to an underestimation of disease severity and resulted in lower vaccination intent.”
A focus on rebuilding trust
Survey respondents who agreed or strongly agreed that they would accept a vaccine were older (a mean age of 44 years), compared with those who were not sure or who disagreed (a mean age of 42 vs. 38 years, respectively; P less than .001). In addition, fewer females agreed or strongly agreed that they would accept a vaccine (54% vs. 73% of males), whereas those who self-identified as Black/African American were least likely to want to get vaccinated, compared with those from other ethnic groups (31%, compared with 74% of Asians, 58% of Whites, and 39% of American Indians or Alaska Natives).
“We are deeply aware of the poor decisions scientists made in the past, which led to a prevailing skepticism and ‘feeling like guinea pigs’ among people of color, especially Black adults,” Dr. Shaw said. “Black adults are less likely, compared [with] White adults, to have confidence that scientists act in the public interest. Rebuilding trust will take time and has to start with addressing health care disparities. In addition, we need to acknowledge contributions of Black researchers to science. For example, until recently very few knew that the Moderna vaccine was developed [with the help of] Dr. Kizzmekia Corbett, who is Black.”
The top five main areas of unease that all respondents expressed about a COVID-19 vaccine were concern about adverse events/side effects (47%), efficacy (15%), rushed release (11%), safety (11%), and the research and authorization process (3%).
“I think it is important that fellow clinicians recognize that, in order to boost vaccine confidence we will need careful, individually tailored communication strategies,” Dr. Shaw said. “A consideration should be given to those [strategies] that utilize interpersonal channels that deliver leadership by example and leverage influencers in the institution to encourage wider adoption of vaccination.”
Aaron M. Milstone, MD, MHS, asked to comment on the research, recommended that health care workers advocate for the vaccine and encourage their patients, friends, and loved ones to get vaccinated. “Soon, COVID-19 will have taken more than half a million lives in the U.S.,” said Dr. Milstone, a pediatric epidemiologist at Johns Hopkins University, Baltimore. “Although vaccines can have side effects like fever and muscle aches, and very, very rare more serious side effects, the risks of dying from COVID are much greater than the risk of a serious vaccine reaction. The study’s authors shed light on the ongoing need for leaders of all communities to support the COVID vaccines, not just the scientific community, but religious leaders, political leaders, and community leaders.”
Addressing vaccine hesitancy
Informed by their own survey, Dr. Shaw and her colleagues have developed a plan to address vaccine hesitancy to ensure high vaccine uptake at SUNY Upstate. Those strategies include, but aren’t limited to, institution-wide forums for all employees on COVID-19 vaccine safety, risks, and benefits followed by Q&A sessions, grand rounds for providers summarizing clinical trial data on mRNA vaccines, development of an Ask COVID email line for staff to ask vaccine-related questions, and a detailed vaccine-specific FAQ document.
In addition, SUNY Upstate experts have engaged in numerous media interviews to provide education and updates on the benefits of vaccination to public and staff, stationary vaccine locations, and mobile COVID-19 vaccine carts. “To date, the COVID-19 vaccination process has been well received, and we anticipate strong vaccine uptake,” she said.
Dr. Shaw acknowledged certain limitations of the survey, including its cross-sectional design and the fact that it was conducted in a single health care system in the northeastern United States. “Thus, generalizability to other regions of the U.S. and other countries may be limited,” Dr. Shaw said. “The study was also conducted before EUA [emergency use authorization] was granted to either the Moderna or Pfizer-BioNTech vaccines. It is therefore likely that vaccine acceptance will change over time as more people get vaccinated.”
The authors have disclosed no relevant financial relationships. Dr. Milstone disclosed that he has received a research grant from Merck, but it is not related to vaccines.
A version of this article first appeared on Medscape.com.
Moreover, 54% of direct care providers indicated that they would take the vaccine if offered, compared with 60% of noncare providers.
The findings come from what is believed to be the largest survey of health care provider attitudes toward COVID-19 vaccination, published online Jan. 25 in Clinical Infectious Diseases.
“We have shown that self-reported willingness to receive vaccination against COVID-19 differs by age, gender, race and hospital role, with physicians and research scientists showing the highest acceptance,” Jana Shaw, MD, MPH, State University of New York, Syracuse, N.Y, the study’s corresponding author, told this news organization. “Building trust in authorities and confidence in vaccines is a complex and time-consuming process that requires commitment and resources. We have to make those investments as hesitancy can severely undermine vaccination coverage. Because health care providers are members of our communities, it is possible that their views are shared by the public at large. Our findings can assist public health professionals as a starting point of discussion and engagement with communities to ensure that we vaccinate at least 80% of the public to end the pandemic.”
For the study, Dr. Shaw and her colleagues emailed an anonymous survey to 9,565 employees of State University of New York Upstate Medical University, Syracuse, an academic medical center that cares for an estimated 1.8 million people. The survey, which contained questions intended to evaluate attitudes, belief, and willingness to get vaccinated, took place between Nov. 23 and Dec. 5, about a week before the U.S. Food and Drug Administration granted the first emergency use authorization for the Pfizer-BioNTech BNT162b2 mRNA vaccine.
Survey recipients included physicians, nurse practitioners, physician assistants, nurses, pharmacists, medical and nursing students, allied health professionals, and nonclinical ancillary staff.
Of the 9,565 surveys sent, 5,287 responses were collected and used in the final analysis, for a response rate of 55%. The mean age of respondents was 43, 73% were female, 85% were White, 6% were Asian, 5% were Black/African American, and the rest were Native American, Native Hawaiian/Pacific Islander, or from other races. More than half of respondents (59%) reported that they provided direct patient care, and 32% said they provided care for patients with COVID-19.
Of all survey respondents, 58% expressed their intent to receive a COVID-19 vaccine, but this varied by their role in the health care system. For example, in response to the statement, “If a vaccine were offered free of charge, I would take it,” 80% of scientists and physicians agreed that they would, while colleagues in other roles were unsure whether they would take the vaccine, including 34% of registered nurses, 32% of allied health professionals, and 32% of master’s-level clinicians. These differences across roles were significant (P less than .001).
The researchers also found that direct patient care or care for COVID-19 patients was associated with lower vaccination intent. For example, 54% of direct care providers and 62% of non-care providers indicated they would take the vaccine if offered, compared with 52% of those who had provided care for COVID-19 patients vs. 61% of those who had not (P less than .001).
“This was a really surprising finding,” said Dr. Shaw, who is a pediatric infectious diseases physician at SUNY Upstate. “In general, one would expect that perceived severity of disease would lead to a greater desire to get vaccinated. Because our question did not address severity of disease, it is possible that we oversampled respondents who took care of patients with mild disease (i.e., in an outpatient setting). This could have led to an underestimation of disease severity and resulted in lower vaccination intent.”
A focus on rebuilding trust
Survey respondents who agreed or strongly agreed that they would accept a vaccine were older (a mean age of 44 years), compared with those who were not sure or who disagreed (a mean age of 42 vs. 38 years, respectively; P less than .001). In addition, fewer females agreed or strongly agreed that they would accept a vaccine (54% vs. 73% of males), whereas those who self-identified as Black/African American were least likely to want to get vaccinated, compared with those from other ethnic groups (31%, compared with 74% of Asians, 58% of Whites, and 39% of American Indians or Alaska Natives).
“We are deeply aware of the poor decisions scientists made in the past, which led to a prevailing skepticism and ‘feeling like guinea pigs’ among people of color, especially Black adults,” Dr. Shaw said. “Black adults are less likely, compared [with] White adults, to have confidence that scientists act in the public interest. Rebuilding trust will take time and has to start with addressing health care disparities. In addition, we need to acknowledge contributions of Black researchers to science. For example, until recently very few knew that the Moderna vaccine was developed [with the help of] Dr. Kizzmekia Corbett, who is Black.”
The top five main areas of unease that all respondents expressed about a COVID-19 vaccine were concern about adverse events/side effects (47%), efficacy (15%), rushed release (11%), safety (11%), and the research and authorization process (3%).
“I think it is important that fellow clinicians recognize that, in order to boost vaccine confidence we will need careful, individually tailored communication strategies,” Dr. Shaw said. “A consideration should be given to those [strategies] that utilize interpersonal channels that deliver leadership by example and leverage influencers in the institution to encourage wider adoption of vaccination.”
Aaron M. Milstone, MD, MHS, asked to comment on the research, recommended that health care workers advocate for the vaccine and encourage their patients, friends, and loved ones to get vaccinated. “Soon, COVID-19 will have taken more than half a million lives in the U.S.,” said Dr. Milstone, a pediatric epidemiologist at Johns Hopkins University, Baltimore. “Although vaccines can have side effects like fever and muscle aches, and very, very rare more serious side effects, the risks of dying from COVID are much greater than the risk of a serious vaccine reaction. The study’s authors shed light on the ongoing need for leaders of all communities to support the COVID vaccines, not just the scientific community, but religious leaders, political leaders, and community leaders.”
Addressing vaccine hesitancy
Informed by their own survey, Dr. Shaw and her colleagues have developed a plan to address vaccine hesitancy to ensure high vaccine uptake at SUNY Upstate. Those strategies include, but aren’t limited to, institution-wide forums for all employees on COVID-19 vaccine safety, risks, and benefits followed by Q&A sessions, grand rounds for providers summarizing clinical trial data on mRNA vaccines, development of an Ask COVID email line for staff to ask vaccine-related questions, and a detailed vaccine-specific FAQ document.
In addition, SUNY Upstate experts have engaged in numerous media interviews to provide education and updates on the benefits of vaccination to public and staff, stationary vaccine locations, and mobile COVID-19 vaccine carts. “To date, the COVID-19 vaccination process has been well received, and we anticipate strong vaccine uptake,” she said.
Dr. Shaw acknowledged certain limitations of the survey, including its cross-sectional design and the fact that it was conducted in a single health care system in the northeastern United States. “Thus, generalizability to other regions of the U.S. and other countries may be limited,” Dr. Shaw said. “The study was also conducted before EUA [emergency use authorization] was granted to either the Moderna or Pfizer-BioNTech vaccines. It is therefore likely that vaccine acceptance will change over time as more people get vaccinated.”
The authors have disclosed no relevant financial relationships. Dr. Milstone disclosed that he has received a research grant from Merck, but it is not related to vaccines.
A version of this article first appeared on Medscape.com.
Moreover, 54% of direct care providers indicated that they would take the vaccine if offered, compared with 60% of noncare providers.
The findings come from what is believed to be the largest survey of health care provider attitudes toward COVID-19 vaccination, published online Jan. 25 in Clinical Infectious Diseases.
“We have shown that self-reported willingness to receive vaccination against COVID-19 differs by age, gender, race and hospital role, with physicians and research scientists showing the highest acceptance,” Jana Shaw, MD, MPH, State University of New York, Syracuse, N.Y, the study’s corresponding author, told this news organization. “Building trust in authorities and confidence in vaccines is a complex and time-consuming process that requires commitment and resources. We have to make those investments as hesitancy can severely undermine vaccination coverage. Because health care providers are members of our communities, it is possible that their views are shared by the public at large. Our findings can assist public health professionals as a starting point of discussion and engagement with communities to ensure that we vaccinate at least 80% of the public to end the pandemic.”
For the study, Dr. Shaw and her colleagues emailed an anonymous survey to 9,565 employees of State University of New York Upstate Medical University, Syracuse, an academic medical center that cares for an estimated 1.8 million people. The survey, which contained questions intended to evaluate attitudes, belief, and willingness to get vaccinated, took place between Nov. 23 and Dec. 5, about a week before the U.S. Food and Drug Administration granted the first emergency use authorization for the Pfizer-BioNTech BNT162b2 mRNA vaccine.
Survey recipients included physicians, nurse practitioners, physician assistants, nurses, pharmacists, medical and nursing students, allied health professionals, and nonclinical ancillary staff.
Of the 9,565 surveys sent, 5,287 responses were collected and used in the final analysis, for a response rate of 55%. The mean age of respondents was 43, 73% were female, 85% were White, 6% were Asian, 5% were Black/African American, and the rest were Native American, Native Hawaiian/Pacific Islander, or from other races. More than half of respondents (59%) reported that they provided direct patient care, and 32% said they provided care for patients with COVID-19.
Of all survey respondents, 58% expressed their intent to receive a COVID-19 vaccine, but this varied by their role in the health care system. For example, in response to the statement, “If a vaccine were offered free of charge, I would take it,” 80% of scientists and physicians agreed that they would, while colleagues in other roles were unsure whether they would take the vaccine, including 34% of registered nurses, 32% of allied health professionals, and 32% of master’s-level clinicians. These differences across roles were significant (P less than .001).
The researchers also found that direct patient care or care for COVID-19 patients was associated with lower vaccination intent. For example, 54% of direct care providers and 62% of non-care providers indicated they would take the vaccine if offered, compared with 52% of those who had provided care for COVID-19 patients vs. 61% of those who had not (P less than .001).
“This was a really surprising finding,” said Dr. Shaw, who is a pediatric infectious diseases physician at SUNY Upstate. “In general, one would expect that perceived severity of disease would lead to a greater desire to get vaccinated. Because our question did not address severity of disease, it is possible that we oversampled respondents who took care of patients with mild disease (i.e., in an outpatient setting). This could have led to an underestimation of disease severity and resulted in lower vaccination intent.”
A focus on rebuilding trust
Survey respondents who agreed or strongly agreed that they would accept a vaccine were older (a mean age of 44 years), compared with those who were not sure or who disagreed (a mean age of 42 vs. 38 years, respectively; P less than .001). In addition, fewer females agreed or strongly agreed that they would accept a vaccine (54% vs. 73% of males), whereas those who self-identified as Black/African American were least likely to want to get vaccinated, compared with those from other ethnic groups (31%, compared with 74% of Asians, 58% of Whites, and 39% of American Indians or Alaska Natives).
“We are deeply aware of the poor decisions scientists made in the past, which led to a prevailing skepticism and ‘feeling like guinea pigs’ among people of color, especially Black adults,” Dr. Shaw said. “Black adults are less likely, compared [with] White adults, to have confidence that scientists act in the public interest. Rebuilding trust will take time and has to start with addressing health care disparities. In addition, we need to acknowledge contributions of Black researchers to science. For example, until recently very few knew that the Moderna vaccine was developed [with the help of] Dr. Kizzmekia Corbett, who is Black.”
The top five main areas of unease that all respondents expressed about a COVID-19 vaccine were concern about adverse events/side effects (47%), efficacy (15%), rushed release (11%), safety (11%), and the research and authorization process (3%).
“I think it is important that fellow clinicians recognize that, in order to boost vaccine confidence we will need careful, individually tailored communication strategies,” Dr. Shaw said. “A consideration should be given to those [strategies] that utilize interpersonal channels that deliver leadership by example and leverage influencers in the institution to encourage wider adoption of vaccination.”
Aaron M. Milstone, MD, MHS, asked to comment on the research, recommended that health care workers advocate for the vaccine and encourage their patients, friends, and loved ones to get vaccinated. “Soon, COVID-19 will have taken more than half a million lives in the U.S.,” said Dr. Milstone, a pediatric epidemiologist at Johns Hopkins University, Baltimore. “Although vaccines can have side effects like fever and muscle aches, and very, very rare more serious side effects, the risks of dying from COVID are much greater than the risk of a serious vaccine reaction. The study’s authors shed light on the ongoing need for leaders of all communities to support the COVID vaccines, not just the scientific community, but religious leaders, political leaders, and community leaders.”
Addressing vaccine hesitancy
Informed by their own survey, Dr. Shaw and her colleagues have developed a plan to address vaccine hesitancy to ensure high vaccine uptake at SUNY Upstate. Those strategies include, but aren’t limited to, institution-wide forums for all employees on COVID-19 vaccine safety, risks, and benefits followed by Q&A sessions, grand rounds for providers summarizing clinical trial data on mRNA vaccines, development of an Ask COVID email line for staff to ask vaccine-related questions, and a detailed vaccine-specific FAQ document.
In addition, SUNY Upstate experts have engaged in numerous media interviews to provide education and updates on the benefits of vaccination to public and staff, stationary vaccine locations, and mobile COVID-19 vaccine carts. “To date, the COVID-19 vaccination process has been well received, and we anticipate strong vaccine uptake,” she said.
Dr. Shaw acknowledged certain limitations of the survey, including its cross-sectional design and the fact that it was conducted in a single health care system in the northeastern United States. “Thus, generalizability to other regions of the U.S. and other countries may be limited,” Dr. Shaw said. “The study was also conducted before EUA [emergency use authorization] was granted to either the Moderna or Pfizer-BioNTech vaccines. It is therefore likely that vaccine acceptance will change over time as more people get vaccinated.”
The authors have disclosed no relevant financial relationships. Dr. Milstone disclosed that he has received a research grant from Merck, but it is not related to vaccines.
A version of this article first appeared on Medscape.com.
Prospective data support delaying antibiotics for pediatric respiratory infections
For pediatric patients with respiratory tract infections (RTIs), immediately prescribing antibiotics may do more harm than good, based on prospective data from 436 children treated by primary care pediatricians in Spain.
In the largest trial of its kind to date, children who were immediately prescribed antibiotics showed no significant difference in symptom severity or duration from those who received a delayed prescription for antibiotics, or no prescription at all; yet those in the immediate-prescription group had a higher rate of gastrointestinal adverse events, reported lead author Gemma Mas-Dalmau, MD, of the Sant Pau Institute for Biomedical Research, Barcelona, and colleagues.
“Most RTIs are self-limiting, and antibiotics hardly alter the course of the condition, yet antibiotics are frequently prescribed for these conditions,” the investigators wrote in Pediatrics. “Antibiotic prescription for RTIs in children is especially considered to be inappropriately high.”
This clinical behavior is driven by several factors, according to Dr. Mas-Dalmau and colleagues, including limited diagnostics in primary care, pressure to meet parental expectations, and concern for possible complications if antibiotics are withheld or delayed.
In an accompanying editorial, Jeffrey S. Gerber, MD, PhD and Bonnie F. Offit, MD, of Children’s Hospital of Philadelphia, noted that “children in the United States receive more than one antibiotic prescription per year, driven largely by acute RTIs.”
Dr. Gerber and Dr. Offit noted that some RTIs are indeed caused by bacteria, and therefore benefit from antibiotics, but it’s “not always easy” to identify these cases.
“Primary care, urgent care, and emergency medicine clinicians have a hard job,” they wrote.
According to the Centers for Disease Control and Prevention, delayed prescription of antibiotics, in which a prescription is filled upon persistence or worsening of symptoms, can balance clinical caution and antibiotic stewardship.
“An example of this approach is acute otitis media, in which delayed prescribing has been shown to safely reduce antibiotic exposure,” wrote Dr. Gerber and Dr. Offit.
In a 2017 Cochrane systematic review of both adults and children with RTIs, antibiotic prescriptions, whether immediate, delayed, or not given at all, had no significant effect on most symptoms or complications. Although several randomized trials have evaluated delayed antibiotic prescriptions in children, Dr. Mas-Dalmau and colleagues described the current body of evidence as “scant.”
The present study built upon this knowledge base by prospectively following 436 children treated at 39 primary care centers in Spain from 2012 to 2016. Patients were between 2 and 14 years of age and presented for rhinosinusitis, pharyngitis, acute otitis media, or acute bronchitis. Inclusion in the study required the pediatrician to have “reasonable doubts about the need to prescribe an antibiotic.” Clinics with access to rapid streptococcal testing did not enroll patients with pharyngitis.
Patients were randomized in approximately equal groups to receive either immediate prescription of antibiotics, delayed prescription, or no prescription. In the delayed group, caregivers were advised to fill prescriptions if any of following three events occurred:
- No symptom improvement after a certain amount of days, depending on presenting complaint (acute otitis media, 4 days; pharyngitis, 7 days; acute rhinosinusitis, 15 days; acute bronchitis, 20 days).
- Temperature of at least 39° C after 24 hours, or at least 38° C but less than 39° C after 48 hours.
- Patient feeling “much worse.”
Primary outcomes were severity and duration of symptoms over 30 days, while secondary outcomes included antibiotic use over 30 days, additional unscheduled visits to primary care over 30 days, and parental satisfaction and beliefs regarding antibiotic efficacy.
In the final dataset, 148 patients received immediate antibiotic prescriptions, while 146 received delayed prescriptions, and 142 received no prescription. Rate of antibiotic use was highest in the immediate prescription group, at 96%, versus 25.3% in the delayed group and 12% among those who received no prescription upon first presentation (P < .001).
Although the mean duration of severe symptoms was longest in the delayed-prescription group, at 12.4 days, versus 10.9 days in the no-prescription group and 10.1 days in the immediate-prescription group, these differences were not statistically significant (P = .539). Median score for greatest severity of any symptom was also similar across groups. Secondary outcomes echoed this pattern, in which reconsultation rates and caregiver satisfaction were statistically similar regardless of treatment type.
In contrast, patients who received immediate antibiotic prescriptions had a significantly higher rate of gastrointestinal adverse events (8.8%) than those who received a delayed prescription (3.4%) or no prescription (2.8%; P = .037).
“Delayed antibiotic prescription is an efficacious and safe strategy for reducing inappropriate antibiotic treatment of uncomplicated RTIs in children when the doctor has reasonable doubts regarding the indication,” the investigators concluded. “[It] is therefore a useful tool for addressing the public health issue of bacterial resistance. However, no antibiotic prescription remains the recommended strategy when it is clear that antibiotics are not indicated, like in most cases of acute bronchitis.”
“These data are reassuring,” wrote Dr. Gerber and Dr. Offit; however, they went on to suggest that the data “might not substantially move the needle.”
“With rare exceptions, children with acute pharyngitis should first receive a group A streptococcal test,” they wrote. “If results are positive, all patients should get antibiotics; if results are negative, no one gets them. Acute bronchitis (whatever that is in children) is viral. Acute sinusitis with persistent symptoms (the most commonly diagnosed variety) already has a delayed option, and the current study ... was not powered for this outcome. We are left with acute otitis media, which dominated enrollment but already has an evidence-based guideline.”
Still, Dr. Gerber and Dr. Offit suggested that the findings should further encourage pediatricians to prescribe antibiotics judiciously, and when elected, to choose the shortest duration and narrowest spectrum possible.
In a joint comment, Rana El Feghaly, MD, MSCI, director of outpatient antibiotic stewardship at Children’s Mercy, Kansas City, and her colleague, Mary Anne Jackson, MD, noted that the findings are “in accordance” with the 2017 Cochrane review.
Dr. Feghaly and Dr. Jackson said that these new data provide greater support for conservative use of antibiotics, which is badly needed, considering approximately 50% of outpatient prescriptions are unnecessary or inappropriate .
Delayed antibiotic prescription is part of a multifaceted approach to the issue, they said, joining “communication skills training, antibiotic justification documentation, audit and feedback reporting with peer comparison, diagnostic stewardship, [and] the use of clinician education on practice-based guidelines.”
“Leveraging delayed antibiotic prescription may be an excellent way to combat antibiotic overuse in the outpatient setting, while avoiding provider and parental fear of the ‘no antibiotic’ approach,” Dr. Feghaly and Dr. Jackson said.
Karlyn Kinsella, MD, of Pediatric Associates of Cheshire, Conn., suggested that clinicians discuss these findings with parents who request antibiotics for “otitis, pharyngitis, bronchitis, or sinusitis.”
“We can cite this study that antibiotics have no effect on symptom duration or severity for these illnesses,” Dr. Kinsella said. “Of course, our clinical opinion in each case takes precedent.”
According to Dr. Kinsella, conversations with parents also need to cover reasonable expectations, as the study did, with clear time frames for each condition in which children should start to get better.
“I think this is really key in our anticipatory guidance so that patients know what to expect,” she said.
The study was funded by Instituto de Salud Carlos III, the European Union, and the Spanish Ministry of Health, Social Services, and Equality. The investigators and interviewees reported no conflicts of interest.
For pediatric patients with respiratory tract infections (RTIs), immediately prescribing antibiotics may do more harm than good, based on prospective data from 436 children treated by primary care pediatricians in Spain.
In the largest trial of its kind to date, children who were immediately prescribed antibiotics showed no significant difference in symptom severity or duration from those who received a delayed prescription for antibiotics, or no prescription at all; yet those in the immediate-prescription group had a higher rate of gastrointestinal adverse events, reported lead author Gemma Mas-Dalmau, MD, of the Sant Pau Institute for Biomedical Research, Barcelona, and colleagues.
“Most RTIs are self-limiting, and antibiotics hardly alter the course of the condition, yet antibiotics are frequently prescribed for these conditions,” the investigators wrote in Pediatrics. “Antibiotic prescription for RTIs in children is especially considered to be inappropriately high.”
This clinical behavior is driven by several factors, according to Dr. Mas-Dalmau and colleagues, including limited diagnostics in primary care, pressure to meet parental expectations, and concern for possible complications if antibiotics are withheld or delayed.
In an accompanying editorial, Jeffrey S. Gerber, MD, PhD and Bonnie F. Offit, MD, of Children’s Hospital of Philadelphia, noted that “children in the United States receive more than one antibiotic prescription per year, driven largely by acute RTIs.”
Dr. Gerber and Dr. Offit noted that some RTIs are indeed caused by bacteria, and therefore benefit from antibiotics, but it’s “not always easy” to identify these cases.
“Primary care, urgent care, and emergency medicine clinicians have a hard job,” they wrote.
According to the Centers for Disease Control and Prevention, delayed prescription of antibiotics, in which a prescription is filled upon persistence or worsening of symptoms, can balance clinical caution and antibiotic stewardship.
“An example of this approach is acute otitis media, in which delayed prescribing has been shown to safely reduce antibiotic exposure,” wrote Dr. Gerber and Dr. Offit.
In a 2017 Cochrane systematic review of both adults and children with RTIs, antibiotic prescriptions, whether immediate, delayed, or not given at all, had no significant effect on most symptoms or complications. Although several randomized trials have evaluated delayed antibiotic prescriptions in children, Dr. Mas-Dalmau and colleagues described the current body of evidence as “scant.”
The present study built upon this knowledge base by prospectively following 436 children treated at 39 primary care centers in Spain from 2012 to 2016. Patients were between 2 and 14 years of age and presented for rhinosinusitis, pharyngitis, acute otitis media, or acute bronchitis. Inclusion in the study required the pediatrician to have “reasonable doubts about the need to prescribe an antibiotic.” Clinics with access to rapid streptococcal testing did not enroll patients with pharyngitis.
Patients were randomized in approximately equal groups to receive either immediate prescription of antibiotics, delayed prescription, or no prescription. In the delayed group, caregivers were advised to fill prescriptions if any of following three events occurred:
- No symptom improvement after a certain amount of days, depending on presenting complaint (acute otitis media, 4 days; pharyngitis, 7 days; acute rhinosinusitis, 15 days; acute bronchitis, 20 days).
- Temperature of at least 39° C after 24 hours, or at least 38° C but less than 39° C after 48 hours.
- Patient feeling “much worse.”
Primary outcomes were severity and duration of symptoms over 30 days, while secondary outcomes included antibiotic use over 30 days, additional unscheduled visits to primary care over 30 days, and parental satisfaction and beliefs regarding antibiotic efficacy.
In the final dataset, 148 patients received immediate antibiotic prescriptions, while 146 received delayed prescriptions, and 142 received no prescription. Rate of antibiotic use was highest in the immediate prescription group, at 96%, versus 25.3% in the delayed group and 12% among those who received no prescription upon first presentation (P < .001).
Although the mean duration of severe symptoms was longest in the delayed-prescription group, at 12.4 days, versus 10.9 days in the no-prescription group and 10.1 days in the immediate-prescription group, these differences were not statistically significant (P = .539). Median score for greatest severity of any symptom was also similar across groups. Secondary outcomes echoed this pattern, in which reconsultation rates and caregiver satisfaction were statistically similar regardless of treatment type.
In contrast, patients who received immediate antibiotic prescriptions had a significantly higher rate of gastrointestinal adverse events (8.8%) than those who received a delayed prescription (3.4%) or no prescription (2.8%; P = .037).
“Delayed antibiotic prescription is an efficacious and safe strategy for reducing inappropriate antibiotic treatment of uncomplicated RTIs in children when the doctor has reasonable doubts regarding the indication,” the investigators concluded. “[It] is therefore a useful tool for addressing the public health issue of bacterial resistance. However, no antibiotic prescription remains the recommended strategy when it is clear that antibiotics are not indicated, like in most cases of acute bronchitis.”
“These data are reassuring,” wrote Dr. Gerber and Dr. Offit; however, they went on to suggest that the data “might not substantially move the needle.”
“With rare exceptions, children with acute pharyngitis should first receive a group A streptococcal test,” they wrote. “If results are positive, all patients should get antibiotics; if results are negative, no one gets them. Acute bronchitis (whatever that is in children) is viral. Acute sinusitis with persistent symptoms (the most commonly diagnosed variety) already has a delayed option, and the current study ... was not powered for this outcome. We are left with acute otitis media, which dominated enrollment but already has an evidence-based guideline.”
Still, Dr. Gerber and Dr. Offit suggested that the findings should further encourage pediatricians to prescribe antibiotics judiciously, and when elected, to choose the shortest duration and narrowest spectrum possible.
In a joint comment, Rana El Feghaly, MD, MSCI, director of outpatient antibiotic stewardship at Children’s Mercy, Kansas City, and her colleague, Mary Anne Jackson, MD, noted that the findings are “in accordance” with the 2017 Cochrane review.
Dr. Feghaly and Dr. Jackson said that these new data provide greater support for conservative use of antibiotics, which is badly needed, considering approximately 50% of outpatient prescriptions are unnecessary or inappropriate .
Delayed antibiotic prescription is part of a multifaceted approach to the issue, they said, joining “communication skills training, antibiotic justification documentation, audit and feedback reporting with peer comparison, diagnostic stewardship, [and] the use of clinician education on practice-based guidelines.”
“Leveraging delayed antibiotic prescription may be an excellent way to combat antibiotic overuse in the outpatient setting, while avoiding provider and parental fear of the ‘no antibiotic’ approach,” Dr. Feghaly and Dr. Jackson said.
Karlyn Kinsella, MD, of Pediatric Associates of Cheshire, Conn., suggested that clinicians discuss these findings with parents who request antibiotics for “otitis, pharyngitis, bronchitis, or sinusitis.”
“We can cite this study that antibiotics have no effect on symptom duration or severity for these illnesses,” Dr. Kinsella said. “Of course, our clinical opinion in each case takes precedent.”
According to Dr. Kinsella, conversations with parents also need to cover reasonable expectations, as the study did, with clear time frames for each condition in which children should start to get better.
“I think this is really key in our anticipatory guidance so that patients know what to expect,” she said.
The study was funded by Instituto de Salud Carlos III, the European Union, and the Spanish Ministry of Health, Social Services, and Equality. The investigators and interviewees reported no conflicts of interest.
For pediatric patients with respiratory tract infections (RTIs), immediately prescribing antibiotics may do more harm than good, based on prospective data from 436 children treated by primary care pediatricians in Spain.
In the largest trial of its kind to date, children who were immediately prescribed antibiotics showed no significant difference in symptom severity or duration from those who received a delayed prescription for antibiotics, or no prescription at all; yet those in the immediate-prescription group had a higher rate of gastrointestinal adverse events, reported lead author Gemma Mas-Dalmau, MD, of the Sant Pau Institute for Biomedical Research, Barcelona, and colleagues.
“Most RTIs are self-limiting, and antibiotics hardly alter the course of the condition, yet antibiotics are frequently prescribed for these conditions,” the investigators wrote in Pediatrics. “Antibiotic prescription for RTIs in children is especially considered to be inappropriately high.”
This clinical behavior is driven by several factors, according to Dr. Mas-Dalmau and colleagues, including limited diagnostics in primary care, pressure to meet parental expectations, and concern for possible complications if antibiotics are withheld or delayed.
In an accompanying editorial, Jeffrey S. Gerber, MD, PhD and Bonnie F. Offit, MD, of Children’s Hospital of Philadelphia, noted that “children in the United States receive more than one antibiotic prescription per year, driven largely by acute RTIs.”
Dr. Gerber and Dr. Offit noted that some RTIs are indeed caused by bacteria, and therefore benefit from antibiotics, but it’s “not always easy” to identify these cases.
“Primary care, urgent care, and emergency medicine clinicians have a hard job,” they wrote.
According to the Centers for Disease Control and Prevention, delayed prescription of antibiotics, in which a prescription is filled upon persistence or worsening of symptoms, can balance clinical caution and antibiotic stewardship.
“An example of this approach is acute otitis media, in which delayed prescribing has been shown to safely reduce antibiotic exposure,” wrote Dr. Gerber and Dr. Offit.
In a 2017 Cochrane systematic review of both adults and children with RTIs, antibiotic prescriptions, whether immediate, delayed, or not given at all, had no significant effect on most symptoms or complications. Although several randomized trials have evaluated delayed antibiotic prescriptions in children, Dr. Mas-Dalmau and colleagues described the current body of evidence as “scant.”
The present study built upon this knowledge base by prospectively following 436 children treated at 39 primary care centers in Spain from 2012 to 2016. Patients were between 2 and 14 years of age and presented for rhinosinusitis, pharyngitis, acute otitis media, or acute bronchitis. Inclusion in the study required the pediatrician to have “reasonable doubts about the need to prescribe an antibiotic.” Clinics with access to rapid streptococcal testing did not enroll patients with pharyngitis.
Patients were randomized in approximately equal groups to receive either immediate prescription of antibiotics, delayed prescription, or no prescription. In the delayed group, caregivers were advised to fill prescriptions if any of following three events occurred:
- No symptom improvement after a certain amount of days, depending on presenting complaint (acute otitis media, 4 days; pharyngitis, 7 days; acute rhinosinusitis, 15 days; acute bronchitis, 20 days).
- Temperature of at least 39° C after 24 hours, or at least 38° C but less than 39° C after 48 hours.
- Patient feeling “much worse.”
Primary outcomes were severity and duration of symptoms over 30 days, while secondary outcomes included antibiotic use over 30 days, additional unscheduled visits to primary care over 30 days, and parental satisfaction and beliefs regarding antibiotic efficacy.
In the final dataset, 148 patients received immediate antibiotic prescriptions, while 146 received delayed prescriptions, and 142 received no prescription. Rate of antibiotic use was highest in the immediate prescription group, at 96%, versus 25.3% in the delayed group and 12% among those who received no prescription upon first presentation (P < .001).
Although the mean duration of severe symptoms was longest in the delayed-prescription group, at 12.4 days, versus 10.9 days in the no-prescription group and 10.1 days in the immediate-prescription group, these differences were not statistically significant (P = .539). Median score for greatest severity of any symptom was also similar across groups. Secondary outcomes echoed this pattern, in which reconsultation rates and caregiver satisfaction were statistically similar regardless of treatment type.
In contrast, patients who received immediate antibiotic prescriptions had a significantly higher rate of gastrointestinal adverse events (8.8%) than those who received a delayed prescription (3.4%) or no prescription (2.8%; P = .037).
“Delayed antibiotic prescription is an efficacious and safe strategy for reducing inappropriate antibiotic treatment of uncomplicated RTIs in children when the doctor has reasonable doubts regarding the indication,” the investigators concluded. “[It] is therefore a useful tool for addressing the public health issue of bacterial resistance. However, no antibiotic prescription remains the recommended strategy when it is clear that antibiotics are not indicated, like in most cases of acute bronchitis.”
“These data are reassuring,” wrote Dr. Gerber and Dr. Offit; however, they went on to suggest that the data “might not substantially move the needle.”
“With rare exceptions, children with acute pharyngitis should first receive a group A streptococcal test,” they wrote. “If results are positive, all patients should get antibiotics; if results are negative, no one gets them. Acute bronchitis (whatever that is in children) is viral. Acute sinusitis with persistent symptoms (the most commonly diagnosed variety) already has a delayed option, and the current study ... was not powered for this outcome. We are left with acute otitis media, which dominated enrollment but already has an evidence-based guideline.”
Still, Dr. Gerber and Dr. Offit suggested that the findings should further encourage pediatricians to prescribe antibiotics judiciously, and when elected, to choose the shortest duration and narrowest spectrum possible.
In a joint comment, Rana El Feghaly, MD, MSCI, director of outpatient antibiotic stewardship at Children’s Mercy, Kansas City, and her colleague, Mary Anne Jackson, MD, noted that the findings are “in accordance” with the 2017 Cochrane review.
Dr. Feghaly and Dr. Jackson said that these new data provide greater support for conservative use of antibiotics, which is badly needed, considering approximately 50% of outpatient prescriptions are unnecessary or inappropriate .
Delayed antibiotic prescription is part of a multifaceted approach to the issue, they said, joining “communication skills training, antibiotic justification documentation, audit and feedback reporting with peer comparison, diagnostic stewardship, [and] the use of clinician education on practice-based guidelines.”
“Leveraging delayed antibiotic prescription may be an excellent way to combat antibiotic overuse in the outpatient setting, while avoiding provider and parental fear of the ‘no antibiotic’ approach,” Dr. Feghaly and Dr. Jackson said.
Karlyn Kinsella, MD, of Pediatric Associates of Cheshire, Conn., suggested that clinicians discuss these findings with parents who request antibiotics for “otitis, pharyngitis, bronchitis, or sinusitis.”
“We can cite this study that antibiotics have no effect on symptom duration or severity for these illnesses,” Dr. Kinsella said. “Of course, our clinical opinion in each case takes precedent.”
According to Dr. Kinsella, conversations with parents also need to cover reasonable expectations, as the study did, with clear time frames for each condition in which children should start to get better.
“I think this is really key in our anticipatory guidance so that patients know what to expect,” she said.
The study was funded by Instituto de Salud Carlos III, the European Union, and the Spanish Ministry of Health, Social Services, and Equality. The investigators and interviewees reported no conflicts of interest.
FROM PEDIATRICS
Zika vaccine candidate shows promise in phase 1 trial
in a phase 1 study.
Although Zika cases have declined in recent years, “geographic expansion of the Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” wrote Nadine C. Salisch, PhD, of Janssen Vaccines and Prevention, Leiden, the Netherlands, and colleagues in a paper published in Annals of Internal Medicine.
No vaccine against Zika is yet available, although more than 10 candidates have been studied in preclinical trials to date, they said.
The researchers randomized 100 healthy adult volunteers to an experimental Zika vaccine candidate known as Ad26.ZIKV.001 in either one-dose or two-dose regimens of 5x1010 viral particles (low dose) or 1x1011 viral particles (high dose) or placebo. Approximately half (55%) of the participants were women, and 72% were White.
Approximately 80% of patients in both two-dose groups showed antibody responses for a year after vaccination. Geometric mean titers (GMTs) reached peak of 823.4 in the low-dose/low-dose group and 961.5 in the high-dose/high-dose group. At day 365, the GMTs for these groups were 68.7 and 87.0, respectively.
A single high-dose vaccine achieved a similar level of neutralizing antibody titers, but lower peak neutralizing responses than the two-dose strategies, the researchers noted.
Most of the reported adverse events were mild to moderate, and short lived; the most common were injection site pain or tenderness, headache, and fatigue, the researchers said. After the first vaccination, 75% of participants in the low-dose groups, 88% of participants in high-dose groups, and 45% of participants receiving placebo reported local adverse events. In addition, 73%, 83%, and 40% of the participants in the low-dose, high-dose, and placebo groups, respectively, reported systemic adverse events. Reports were similar after the second vaccination. Two serious adverse events not related to vaccination were reported; one case of right lower lobe pneumonia and one case of incomplete spontaneous abortion.
The researchers also explored protective efficacy through a nonlethal mouse challenge model. “Transfer of 6 mg of IgG from Ad26.ZIKV.001 vaccines conferred complete protection from viremia in most recipient animals, with statistically significantly decreased breakthrough rates and cumulative viral loads per group compared with placebo,” they said.
The study findings were limited by the inability to assess safety and immunogenicity in an endemic area, the researchers noted. However, “Ad26.ZIKV.001 induces potent ZIKV-specific neutralizing responses with durability of at least 1 year, which supports further clinical development if an unmet medical need reemerges,” they said. “In addition, these data underscore the performance of the Ad26 vaccine platform, which Janssen is using for different infectious diseases, including COVID-19,” they noted.
Ad26 vector platform shows consistency
“Development of the investigational Janssen Zika vaccine candidate was initiated in 2015, and while the incidence of Zika virus has declined since the 2015-2016 outbreak, spread of the ‘carrier’ Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” lead author Dr. Salisch said in an interview. For this reason, researchers say the vaccine warrants further development should the need reemerge, she said.
“Our research has found that while a single higher-dose regimen had lower peak neutralizing responses than a two-dose regimen, it achieved a similar level of neutralizing antibody responses at 1 year, an encouraging finding that shows our vaccine may be a useful tool to curb Zika epidemics,” Dr. Salisch noted. “Previous experience with the Ad26 vector platform across our investigational vaccine programs have yielded similarly promising results, most recently with our investigational Janssen COVID-19 vaccine program, for which phase 3 data show a single-dose vaccine met all primary and key secondary endpoints,” she said.
“The biggest barrier [to further development of the candidate vaccine] is one that we actually consider ourselves fortunate to have: The very low incidence of reported Zika cases currently reported worldwide,” Dr. Salisch said. “However, the current Zika case rate can change at any time, and in the event the situation demands it, we are open to alternative regulatory pathways to help us glean the necessary insights on vaccine safety and efficacy to further advance the development of this candidate,” she emphasized.
As for additional research, “there are still questions surrounding Zika transmission and the pathomechanism of congenital Zika syndrome,” said Dr. Salisch. “Our hope is that a correlate of protection against Zika disease, and in particular against congenital Zika syndrome, can be identified,” she said.
Consider pregnant women in next phase of research
“A major hurdle in ZIKV vaccine development is the inability to conduct large efficacy studies in the absence of a current outbreak,” Ann Chahroudi, MD, of Emory University, Atlanta, and Sallie Permar, MD, of Weill Cornell Medicine, New York, wrote in an accompanying editorial.
The current study provided some efficacy data using a mouse model, but “these data are obviously not conclusive for human protection,” they said.
“A further challenge for ZIKV vaccine efficacy trials will be to demonstrate fetal protection from [congenital Zika syndrome] after adult immunization. There should be a clear plan to readily deploy phase 3 trials for the most promising vaccines to emerge from phase 1 and 2 in the event of an outbreak, as was implemented for Ebola, including infant follow-up,” they emphasized.
The editorialists noted that the study did not include pregnant women, who represent a major target for immunization, but they said that vaccination of pregnant women against other neonatal pathogens such as influenza and tetanus has been effective. “Candidate ZIKV vaccines proven safe in phase 1 trials should immediately be assessed for safety and efficacy in pregnant women,” they said. Although Zika infections are not at epidemic levels currently, resurgence remains a possibility and the coronavirus pandemic “has taught us that preparedness for emerging infections is crucial,” they said.
Zika vaccine research is a challenge worth pursuing
“It is important to continue Zika vaccine research because of the unpredictable nature of that infection,” Kevin Ault, MD, of the University of Kansas, Kansas City, said in an interview. “Several times Zika has gained a foothold in unexposed and vulnerable populations,” Dr. Ault said. “Additionally, there are some data about using this vector during pregnancy, and eventually this vaccine may prevent the birth defects associated with Zika infections during pregnancy, he noted.
Dr. Ault said he was not surprised by the study findings. “This is a promising early phase vaccine candidate, and this adenovirus vector has been used in other similar trials,” he said. Potential barriers to vaccine development include the challenge of conducting late phase clinical trials in pregnant women, he noted. “The relevant endpoint is going to be clinical disease, and one of the most critical populations is pregnant women,” he said. In addition, “later phase 3 trials would be conducted in a population where there is an ongoing Zika outbreak,” Dr. Ault emphasized.
The study was supported by Janssen Vaccines and Infectious Diseases.
Dr. Chahroudi had no financial conflicts to disclose. Dr. Permar disclosed grants from Merck and Moderna unrelated to the current study. Dr. Ault had no relevant financial conflicts to disclose; he has served as an adviser to the Centers for Disease Control and Prevention, the World Medical Association, the National Vaccine Program Office, and the National Institute for Allergy and Infectious Diseases. He is a fellow of the Infectious Disease Society of American and a fellow of ACOG.
in a phase 1 study.
Although Zika cases have declined in recent years, “geographic expansion of the Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” wrote Nadine C. Salisch, PhD, of Janssen Vaccines and Prevention, Leiden, the Netherlands, and colleagues in a paper published in Annals of Internal Medicine.
No vaccine against Zika is yet available, although more than 10 candidates have been studied in preclinical trials to date, they said.
The researchers randomized 100 healthy adult volunteers to an experimental Zika vaccine candidate known as Ad26.ZIKV.001 in either one-dose or two-dose regimens of 5x1010 viral particles (low dose) or 1x1011 viral particles (high dose) or placebo. Approximately half (55%) of the participants were women, and 72% were White.
Approximately 80% of patients in both two-dose groups showed antibody responses for a year after vaccination. Geometric mean titers (GMTs) reached peak of 823.4 in the low-dose/low-dose group and 961.5 in the high-dose/high-dose group. At day 365, the GMTs for these groups were 68.7 and 87.0, respectively.
A single high-dose vaccine achieved a similar level of neutralizing antibody titers, but lower peak neutralizing responses than the two-dose strategies, the researchers noted.
Most of the reported adverse events were mild to moderate, and short lived; the most common were injection site pain or tenderness, headache, and fatigue, the researchers said. After the first vaccination, 75% of participants in the low-dose groups, 88% of participants in high-dose groups, and 45% of participants receiving placebo reported local adverse events. In addition, 73%, 83%, and 40% of the participants in the low-dose, high-dose, and placebo groups, respectively, reported systemic adverse events. Reports were similar after the second vaccination. Two serious adverse events not related to vaccination were reported; one case of right lower lobe pneumonia and one case of incomplete spontaneous abortion.
The researchers also explored protective efficacy through a nonlethal mouse challenge model. “Transfer of 6 mg of IgG from Ad26.ZIKV.001 vaccines conferred complete protection from viremia in most recipient animals, with statistically significantly decreased breakthrough rates and cumulative viral loads per group compared with placebo,” they said.
The study findings were limited by the inability to assess safety and immunogenicity in an endemic area, the researchers noted. However, “Ad26.ZIKV.001 induces potent ZIKV-specific neutralizing responses with durability of at least 1 year, which supports further clinical development if an unmet medical need reemerges,” they said. “In addition, these data underscore the performance of the Ad26 vaccine platform, which Janssen is using for different infectious diseases, including COVID-19,” they noted.
Ad26 vector platform shows consistency
“Development of the investigational Janssen Zika vaccine candidate was initiated in 2015, and while the incidence of Zika virus has declined since the 2015-2016 outbreak, spread of the ‘carrier’ Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” lead author Dr. Salisch said in an interview. For this reason, researchers say the vaccine warrants further development should the need reemerge, she said.
“Our research has found that while a single higher-dose regimen had lower peak neutralizing responses than a two-dose regimen, it achieved a similar level of neutralizing antibody responses at 1 year, an encouraging finding that shows our vaccine may be a useful tool to curb Zika epidemics,” Dr. Salisch noted. “Previous experience with the Ad26 vector platform across our investigational vaccine programs have yielded similarly promising results, most recently with our investigational Janssen COVID-19 vaccine program, for which phase 3 data show a single-dose vaccine met all primary and key secondary endpoints,” she said.
“The biggest barrier [to further development of the candidate vaccine] is one that we actually consider ourselves fortunate to have: The very low incidence of reported Zika cases currently reported worldwide,” Dr. Salisch said. “However, the current Zika case rate can change at any time, and in the event the situation demands it, we are open to alternative regulatory pathways to help us glean the necessary insights on vaccine safety and efficacy to further advance the development of this candidate,” she emphasized.
As for additional research, “there are still questions surrounding Zika transmission and the pathomechanism of congenital Zika syndrome,” said Dr. Salisch. “Our hope is that a correlate of protection against Zika disease, and in particular against congenital Zika syndrome, can be identified,” she said.
Consider pregnant women in next phase of research
“A major hurdle in ZIKV vaccine development is the inability to conduct large efficacy studies in the absence of a current outbreak,” Ann Chahroudi, MD, of Emory University, Atlanta, and Sallie Permar, MD, of Weill Cornell Medicine, New York, wrote in an accompanying editorial.
The current study provided some efficacy data using a mouse model, but “these data are obviously not conclusive for human protection,” they said.
“A further challenge for ZIKV vaccine efficacy trials will be to demonstrate fetal protection from [congenital Zika syndrome] after adult immunization. There should be a clear plan to readily deploy phase 3 trials for the most promising vaccines to emerge from phase 1 and 2 in the event of an outbreak, as was implemented for Ebola, including infant follow-up,” they emphasized.
The editorialists noted that the study did not include pregnant women, who represent a major target for immunization, but they said that vaccination of pregnant women against other neonatal pathogens such as influenza and tetanus has been effective. “Candidate ZIKV vaccines proven safe in phase 1 trials should immediately be assessed for safety and efficacy in pregnant women,” they said. Although Zika infections are not at epidemic levels currently, resurgence remains a possibility and the coronavirus pandemic “has taught us that preparedness for emerging infections is crucial,” they said.
Zika vaccine research is a challenge worth pursuing
“It is important to continue Zika vaccine research because of the unpredictable nature of that infection,” Kevin Ault, MD, of the University of Kansas, Kansas City, said in an interview. “Several times Zika has gained a foothold in unexposed and vulnerable populations,” Dr. Ault said. “Additionally, there are some data about using this vector during pregnancy, and eventually this vaccine may prevent the birth defects associated with Zika infections during pregnancy, he noted.
Dr. Ault said he was not surprised by the study findings. “This is a promising early phase vaccine candidate, and this adenovirus vector has been used in other similar trials,” he said. Potential barriers to vaccine development include the challenge of conducting late phase clinical trials in pregnant women, he noted. “The relevant endpoint is going to be clinical disease, and one of the most critical populations is pregnant women,” he said. In addition, “later phase 3 trials would be conducted in a population where there is an ongoing Zika outbreak,” Dr. Ault emphasized.
The study was supported by Janssen Vaccines and Infectious Diseases.
Dr. Chahroudi had no financial conflicts to disclose. Dr. Permar disclosed grants from Merck and Moderna unrelated to the current study. Dr. Ault had no relevant financial conflicts to disclose; he has served as an adviser to the Centers for Disease Control and Prevention, the World Medical Association, the National Vaccine Program Office, and the National Institute for Allergy and Infectious Diseases. He is a fellow of the Infectious Disease Society of American and a fellow of ACOG.
in a phase 1 study.
Although Zika cases have declined in recent years, “geographic expansion of the Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” wrote Nadine C. Salisch, PhD, of Janssen Vaccines and Prevention, Leiden, the Netherlands, and colleagues in a paper published in Annals of Internal Medicine.
No vaccine against Zika is yet available, although more than 10 candidates have been studied in preclinical trials to date, they said.
The researchers randomized 100 healthy adult volunteers to an experimental Zika vaccine candidate known as Ad26.ZIKV.001 in either one-dose or two-dose regimens of 5x1010 viral particles (low dose) or 1x1011 viral particles (high dose) or placebo. Approximately half (55%) of the participants were women, and 72% were White.
Approximately 80% of patients in both two-dose groups showed antibody responses for a year after vaccination. Geometric mean titers (GMTs) reached peak of 823.4 in the low-dose/low-dose group and 961.5 in the high-dose/high-dose group. At day 365, the GMTs for these groups were 68.7 and 87.0, respectively.
A single high-dose vaccine achieved a similar level of neutralizing antibody titers, but lower peak neutralizing responses than the two-dose strategies, the researchers noted.
Most of the reported adverse events were mild to moderate, and short lived; the most common were injection site pain or tenderness, headache, and fatigue, the researchers said. After the first vaccination, 75% of participants in the low-dose groups, 88% of participants in high-dose groups, and 45% of participants receiving placebo reported local adverse events. In addition, 73%, 83%, and 40% of the participants in the low-dose, high-dose, and placebo groups, respectively, reported systemic adverse events. Reports were similar after the second vaccination. Two serious adverse events not related to vaccination were reported; one case of right lower lobe pneumonia and one case of incomplete spontaneous abortion.
The researchers also explored protective efficacy through a nonlethal mouse challenge model. “Transfer of 6 mg of IgG from Ad26.ZIKV.001 vaccines conferred complete protection from viremia in most recipient animals, with statistically significantly decreased breakthrough rates and cumulative viral loads per group compared with placebo,” they said.
The study findings were limited by the inability to assess safety and immunogenicity in an endemic area, the researchers noted. However, “Ad26.ZIKV.001 induces potent ZIKV-specific neutralizing responses with durability of at least 1 year, which supports further clinical development if an unmet medical need reemerges,” they said. “In addition, these data underscore the performance of the Ad26 vaccine platform, which Janssen is using for different infectious diseases, including COVID-19,” they noted.
Ad26 vector platform shows consistency
“Development of the investigational Janssen Zika vaccine candidate was initiated in 2015, and while the incidence of Zika virus has declined since the 2015-2016 outbreak, spread of the ‘carrier’ Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” lead author Dr. Salisch said in an interview. For this reason, researchers say the vaccine warrants further development should the need reemerge, she said.
“Our research has found that while a single higher-dose regimen had lower peak neutralizing responses than a two-dose regimen, it achieved a similar level of neutralizing antibody responses at 1 year, an encouraging finding that shows our vaccine may be a useful tool to curb Zika epidemics,” Dr. Salisch noted. “Previous experience with the Ad26 vector platform across our investigational vaccine programs have yielded similarly promising results, most recently with our investigational Janssen COVID-19 vaccine program, for which phase 3 data show a single-dose vaccine met all primary and key secondary endpoints,” she said.
“The biggest barrier [to further development of the candidate vaccine] is one that we actually consider ourselves fortunate to have: The very low incidence of reported Zika cases currently reported worldwide,” Dr. Salisch said. “However, the current Zika case rate can change at any time, and in the event the situation demands it, we are open to alternative regulatory pathways to help us glean the necessary insights on vaccine safety and efficacy to further advance the development of this candidate,” she emphasized.
As for additional research, “there are still questions surrounding Zika transmission and the pathomechanism of congenital Zika syndrome,” said Dr. Salisch. “Our hope is that a correlate of protection against Zika disease, and in particular against congenital Zika syndrome, can be identified,” she said.
Consider pregnant women in next phase of research
“A major hurdle in ZIKV vaccine development is the inability to conduct large efficacy studies in the absence of a current outbreak,” Ann Chahroudi, MD, of Emory University, Atlanta, and Sallie Permar, MD, of Weill Cornell Medicine, New York, wrote in an accompanying editorial.
The current study provided some efficacy data using a mouse model, but “these data are obviously not conclusive for human protection,” they said.
“A further challenge for ZIKV vaccine efficacy trials will be to demonstrate fetal protection from [congenital Zika syndrome] after adult immunization. There should be a clear plan to readily deploy phase 3 trials for the most promising vaccines to emerge from phase 1 and 2 in the event of an outbreak, as was implemented for Ebola, including infant follow-up,” they emphasized.
The editorialists noted that the study did not include pregnant women, who represent a major target for immunization, but they said that vaccination of pregnant women against other neonatal pathogens such as influenza and tetanus has been effective. “Candidate ZIKV vaccines proven safe in phase 1 trials should immediately be assessed for safety and efficacy in pregnant women,” they said. Although Zika infections are not at epidemic levels currently, resurgence remains a possibility and the coronavirus pandemic “has taught us that preparedness for emerging infections is crucial,” they said.
Zika vaccine research is a challenge worth pursuing
“It is important to continue Zika vaccine research because of the unpredictable nature of that infection,” Kevin Ault, MD, of the University of Kansas, Kansas City, said in an interview. “Several times Zika has gained a foothold in unexposed and vulnerable populations,” Dr. Ault said. “Additionally, there are some data about using this vector during pregnancy, and eventually this vaccine may prevent the birth defects associated with Zika infections during pregnancy, he noted.
Dr. Ault said he was not surprised by the study findings. “This is a promising early phase vaccine candidate, and this adenovirus vector has been used in other similar trials,” he said. Potential barriers to vaccine development include the challenge of conducting late phase clinical trials in pregnant women, he noted. “The relevant endpoint is going to be clinical disease, and one of the most critical populations is pregnant women,” he said. In addition, “later phase 3 trials would be conducted in a population where there is an ongoing Zika outbreak,” Dr. Ault emphasized.
The study was supported by Janssen Vaccines and Infectious Diseases.
Dr. Chahroudi had no financial conflicts to disclose. Dr. Permar disclosed grants from Merck and Moderna unrelated to the current study. Dr. Ault had no relevant financial conflicts to disclose; he has served as an adviser to the Centers for Disease Control and Prevention, the World Medical Association, the National Vaccine Program Office, and the National Institute for Allergy and Infectious Diseases. He is a fellow of the Infectious Disease Society of American and a fellow of ACOG.
FROM ANNALS OF INTERNAL MEDICINE
Multifocal Annular Pink Plaques With a Central Violaceous Hue
The Diagnosis: Disseminated Erythema Chronicum Migrans
Empiric treatment with doxycycline 100 mg twice daily for 14 days was initiated for suspected early disseminated Lyme disease manifesting as disseminated multifocal erythema chronicum migrans (Figure). Lyme screening immunoassay and confirmatory IgM Western blot testing subsequently were found to be positive. The clinical history of recent travel to an endemic area and tick bite combined with the recent onset of multifocal erythema migrans lesions, systemic symptoms, elevated erythrocyte sedimentation rate, and positive Lyme serology supported the diagnosis of Lyme disease.
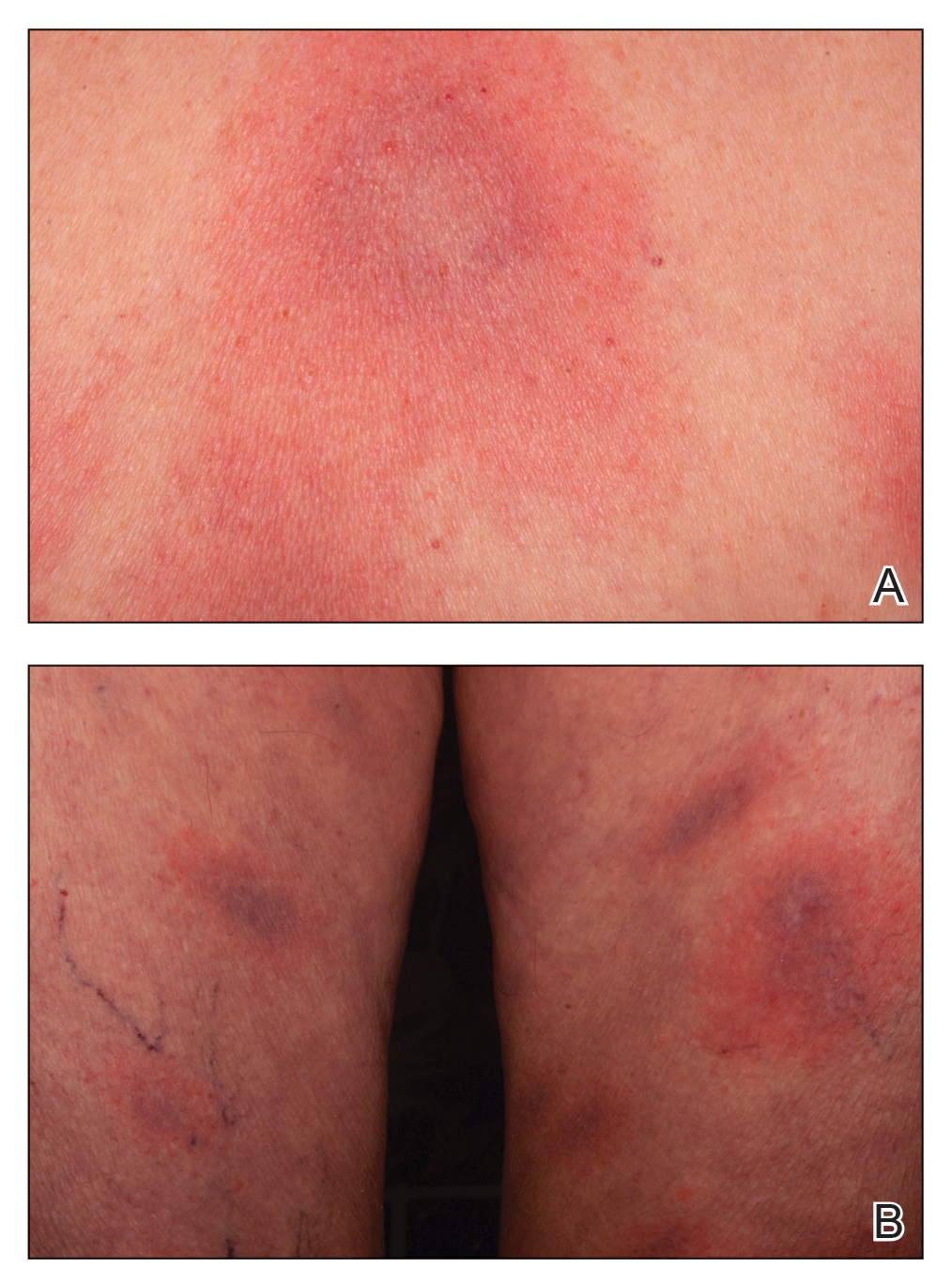
The appropriate clinical context and cutaneous morphology are key when considering the differential diagnosis for multifocal annular lesions. Several entities comprised the differential diagnosis considered in our patient. Sweet syndrome is a neutrophilic dermatosis that can present with fever and varying painful cutaneous lesions. It often is associated with certain medications, underlying illnesses, and infections.1 Our patient’s lesions were not painful, and she had no notable medical history, recent infections, or new medication use, making Sweet syndrome unlikely. A fixed drug eruption was low on the differential, as the patient denied starting any new medications within the 3 months prior to presentation. Erythema multiforme is an acute-onset immunemediated condition of the skin and mucous membranes that typically affects young adults and often is associated with infection (eg, herpes simplex virus, Mycoplasma pneumoniae) or medication use. Cutaneous lesions typically are self-limited, less than 3 cm targets with 3 concentric distinct color zones, often with central bullae or erosions. Although erythema multiforme was higher on the differential, it was less likely, as the patient lacked mucosal lesions and did not have symptoms of underlying herpetic or mycoplasma infection, and the clinical picture was more consistent with Lyme disease. Lastly, the failure for individual skin lesions to resolve within
24 hours excluded the diagnosis of urticaria.
Lyme disease is a tick-borne illness caused by 3 species of the Borrelia spirochete: Borrelia burgdorferi, Borrelia afzelii, and Borrelia garinii.2 In the United States, the disease predominantly is caused by B burgdorferi that is endemic in the upper Midwest and Northeast regions.3 There are 3 stages of Lyme disease: early localized, early disseminated, and late disseminated disease. Early localized disease typically presents with a characteristic single erythema migrans (EM) lesion 3 to 30 days following a bite by the tick Ixodes scapularis.2 The EM lesion gradually can enlarge over a period of several days, reaching up to 12 inches in diameter.2 Early disseminated disease can occur weeks to months following a tick bite and may present with systemic symptoms, multiple widespread
EM lesions, neurologic features such as meningitis or facial nerve palsy, and/or cardiac manifestations such as atrioventricular block or myocarditis. Late disseminated disease can present with chronic arthritis or encephalopathy after months to years if the disease is left untreated.4
Early localized Lyme disease can be diagnosed clinically if the characteristic EM lesion is present in a patient who visited an endemic area. Laboratory testing and Lyme serology are neither required nor recommended in these cases, as the lesion often appears before adequate time has lapsed to develop an adaptive immune response to the organism.5 In contrast, Lyme serology should be ordered in any patient who meets all of the following criteria: (1) patient lives in or has recently traveled to an area endemic for Lyme disease, (2) presence of a risk factor for tick exposure, and (3) symptoms consistent with early disseminated or late Lyme disease. Patients with signs of early or late disseminated disease typically are seropositive, as IgM antibodies can be detected within 2 weeks of onset of the EM lesion and IgG antibodies within 2 to 6 weeks.6 The Centers for Disease Control and Prevention recommends a 2-tiered approach when testing for Lyme disease.7 A screening test with high sensitivity such as an enzyme-linked immunosorbent assay or an immunofluorescence assay initially should be performed.7 If results of the screening test are equivocal or positive, secondary confirmatory testing should be performed via IgM, with or without IgG Western immunoblot assay.7 Biopsy with histologic evaluation can reveal nonspecific findings of vascular endothelial injury and increased mucin deposition. Patients with suspected Lyme disease should immediately be started on empiric treatment with doxycycline 100 mg twice daily for a minimum of 10 days (14–28 days if there is concern for dissemination) to prevent post-Lyme sequelae.5 Our patient’s cutaneous lesions responded to oral doxycycline.
- Sweet’s syndrome. Mayo Clinic. Accessed January 8, 2021. https://www.mayoclinic.org/diseases-conditions/sweets-syndrome /symptoms-causes/syc-20351117
- Steere AC. Lyme disease. N Engl J Med. 2001;345:115-125.
- Lyme disease maps: most recent year. Centers for Disease Control and Prevention. Updated November 22, 2019. Accessed January 8, 2021. https://www.cdc.gov/lyme/datasurveillance /maps-recent.html.
- Steere AC, Sikand VK. The present manifestations of Lyme disease and the outcomes of treatment. N Engl J Med. 2003;348:2472-2474.
- Sanchez E, Vannier E, Wormser GP, et al. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA. 2016;315:1767-1777.
- Shapiro ED. Borrelia burgdorferi (Lyme disease). Pediatr Rev. 2014; 35:500-509.
- Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 2019;68:703
The Diagnosis: Disseminated Erythema Chronicum Migrans
Empiric treatment with doxycycline 100 mg twice daily for 14 days was initiated for suspected early disseminated Lyme disease manifesting as disseminated multifocal erythema chronicum migrans (Figure). Lyme screening immunoassay and confirmatory IgM Western blot testing subsequently were found to be positive. The clinical history of recent travel to an endemic area and tick bite combined with the recent onset of multifocal erythema migrans lesions, systemic symptoms, elevated erythrocyte sedimentation rate, and positive Lyme serology supported the diagnosis of Lyme disease.

The appropriate clinical context and cutaneous morphology are key when considering the differential diagnosis for multifocal annular lesions. Several entities comprised the differential diagnosis considered in our patient. Sweet syndrome is a neutrophilic dermatosis that can present with fever and varying painful cutaneous lesions. It often is associated with certain medications, underlying illnesses, and infections.1 Our patient’s lesions were not painful, and she had no notable medical history, recent infections, or new medication use, making Sweet syndrome unlikely. A fixed drug eruption was low on the differential, as the patient denied starting any new medications within the 3 months prior to presentation. Erythema multiforme is an acute-onset immunemediated condition of the skin and mucous membranes that typically affects young adults and often is associated with infection (eg, herpes simplex virus, Mycoplasma pneumoniae) or medication use. Cutaneous lesions typically are self-limited, less than 3 cm targets with 3 concentric distinct color zones, often with central bullae or erosions. Although erythema multiforme was higher on the differential, it was less likely, as the patient lacked mucosal lesions and did not have symptoms of underlying herpetic or mycoplasma infection, and the clinical picture was more consistent with Lyme disease. Lastly, the failure for individual skin lesions to resolve within
24 hours excluded the diagnosis of urticaria.
Lyme disease is a tick-borne illness caused by 3 species of the Borrelia spirochete: Borrelia burgdorferi, Borrelia afzelii, and Borrelia garinii.2 In the United States, the disease predominantly is caused by B burgdorferi that is endemic in the upper Midwest and Northeast regions.3 There are 3 stages of Lyme disease: early localized, early disseminated, and late disseminated disease. Early localized disease typically presents with a characteristic single erythema migrans (EM) lesion 3 to 30 days following a bite by the tick Ixodes scapularis.2 The EM lesion gradually can enlarge over a period of several days, reaching up to 12 inches in diameter.2 Early disseminated disease can occur weeks to months following a tick bite and may present with systemic symptoms, multiple widespread
EM lesions, neurologic features such as meningitis or facial nerve palsy, and/or cardiac manifestations such as atrioventricular block or myocarditis. Late disseminated disease can present with chronic arthritis or encephalopathy after months to years if the disease is left untreated.4
Early localized Lyme disease can be diagnosed clinically if the characteristic EM lesion is present in a patient who visited an endemic area. Laboratory testing and Lyme serology are neither required nor recommended in these cases, as the lesion often appears before adequate time has lapsed to develop an adaptive immune response to the organism.5 In contrast, Lyme serology should be ordered in any patient who meets all of the following criteria: (1) patient lives in or has recently traveled to an area endemic for Lyme disease, (2) presence of a risk factor for tick exposure, and (3) symptoms consistent with early disseminated or late Lyme disease. Patients with signs of early or late disseminated disease typically are seropositive, as IgM antibodies can be detected within 2 weeks of onset of the EM lesion and IgG antibodies within 2 to 6 weeks.6 The Centers for Disease Control and Prevention recommends a 2-tiered approach when testing for Lyme disease.7 A screening test with high sensitivity such as an enzyme-linked immunosorbent assay or an immunofluorescence assay initially should be performed.7 If results of the screening test are equivocal or positive, secondary confirmatory testing should be performed via IgM, with or without IgG Western immunoblot assay.7 Biopsy with histologic evaluation can reveal nonspecific findings of vascular endothelial injury and increased mucin deposition. Patients with suspected Lyme disease should immediately be started on empiric treatment with doxycycline 100 mg twice daily for a minimum of 10 days (14–28 days if there is concern for dissemination) to prevent post-Lyme sequelae.5 Our patient’s cutaneous lesions responded to oral doxycycline.
The Diagnosis: Disseminated Erythema Chronicum Migrans
Empiric treatment with doxycycline 100 mg twice daily for 14 days was initiated for suspected early disseminated Lyme disease manifesting as disseminated multifocal erythema chronicum migrans (Figure). Lyme screening immunoassay and confirmatory IgM Western blot testing subsequently were found to be positive. The clinical history of recent travel to an endemic area and tick bite combined with the recent onset of multifocal erythema migrans lesions, systemic symptoms, elevated erythrocyte sedimentation rate, and positive Lyme serology supported the diagnosis of Lyme disease.

The appropriate clinical context and cutaneous morphology are key when considering the differential diagnosis for multifocal annular lesions. Several entities comprised the differential diagnosis considered in our patient. Sweet syndrome is a neutrophilic dermatosis that can present with fever and varying painful cutaneous lesions. It often is associated with certain medications, underlying illnesses, and infections.1 Our patient’s lesions were not painful, and she had no notable medical history, recent infections, or new medication use, making Sweet syndrome unlikely. A fixed drug eruption was low on the differential, as the patient denied starting any new medications within the 3 months prior to presentation. Erythema multiforme is an acute-onset immunemediated condition of the skin and mucous membranes that typically affects young adults and often is associated with infection (eg, herpes simplex virus, Mycoplasma pneumoniae) or medication use. Cutaneous lesions typically are self-limited, less than 3 cm targets with 3 concentric distinct color zones, often with central bullae or erosions. Although erythema multiforme was higher on the differential, it was less likely, as the patient lacked mucosal lesions and did not have symptoms of underlying herpetic or mycoplasma infection, and the clinical picture was more consistent with Lyme disease. Lastly, the failure for individual skin lesions to resolve within
24 hours excluded the diagnosis of urticaria.
Lyme disease is a tick-borne illness caused by 3 species of the Borrelia spirochete: Borrelia burgdorferi, Borrelia afzelii, and Borrelia garinii.2 In the United States, the disease predominantly is caused by B burgdorferi that is endemic in the upper Midwest and Northeast regions.3 There are 3 stages of Lyme disease: early localized, early disseminated, and late disseminated disease. Early localized disease typically presents with a characteristic single erythema migrans (EM) lesion 3 to 30 days following a bite by the tick Ixodes scapularis.2 The EM lesion gradually can enlarge over a period of several days, reaching up to 12 inches in diameter.2 Early disseminated disease can occur weeks to months following a tick bite and may present with systemic symptoms, multiple widespread
EM lesions, neurologic features such as meningitis or facial nerve palsy, and/or cardiac manifestations such as atrioventricular block or myocarditis. Late disseminated disease can present with chronic arthritis or encephalopathy after months to years if the disease is left untreated.4
Early localized Lyme disease can be diagnosed clinically if the characteristic EM lesion is present in a patient who visited an endemic area. Laboratory testing and Lyme serology are neither required nor recommended in these cases, as the lesion often appears before adequate time has lapsed to develop an adaptive immune response to the organism.5 In contrast, Lyme serology should be ordered in any patient who meets all of the following criteria: (1) patient lives in or has recently traveled to an area endemic for Lyme disease, (2) presence of a risk factor for tick exposure, and (3) symptoms consistent with early disseminated or late Lyme disease. Patients with signs of early or late disseminated disease typically are seropositive, as IgM antibodies can be detected within 2 weeks of onset of the EM lesion and IgG antibodies within 2 to 6 weeks.6 The Centers for Disease Control and Prevention recommends a 2-tiered approach when testing for Lyme disease.7 A screening test with high sensitivity such as an enzyme-linked immunosorbent assay or an immunofluorescence assay initially should be performed.7 If results of the screening test are equivocal or positive, secondary confirmatory testing should be performed via IgM, with or without IgG Western immunoblot assay.7 Biopsy with histologic evaluation can reveal nonspecific findings of vascular endothelial injury and increased mucin deposition. Patients with suspected Lyme disease should immediately be started on empiric treatment with doxycycline 100 mg twice daily for a minimum of 10 days (14–28 days if there is concern for dissemination) to prevent post-Lyme sequelae.5 Our patient’s cutaneous lesions responded to oral doxycycline.
- Sweet’s syndrome. Mayo Clinic. Accessed January 8, 2021. https://www.mayoclinic.org/diseases-conditions/sweets-syndrome /symptoms-causes/syc-20351117
- Steere AC. Lyme disease. N Engl J Med. 2001;345:115-125.
- Lyme disease maps: most recent year. Centers for Disease Control and Prevention. Updated November 22, 2019. Accessed January 8, 2021. https://www.cdc.gov/lyme/datasurveillance /maps-recent.html.
- Steere AC, Sikand VK. The present manifestations of Lyme disease and the outcomes of treatment. N Engl J Med. 2003;348:2472-2474.
- Sanchez E, Vannier E, Wormser GP, et al. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA. 2016;315:1767-1777.
- Shapiro ED. Borrelia burgdorferi (Lyme disease). Pediatr Rev. 2014; 35:500-509.
- Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 2019;68:703
- Sweet’s syndrome. Mayo Clinic. Accessed January 8, 2021. https://www.mayoclinic.org/diseases-conditions/sweets-syndrome /symptoms-causes/syc-20351117
- Steere AC. Lyme disease. N Engl J Med. 2001;345:115-125.
- Lyme disease maps: most recent year. Centers for Disease Control and Prevention. Updated November 22, 2019. Accessed January 8, 2021. https://www.cdc.gov/lyme/datasurveillance /maps-recent.html.
- Steere AC, Sikand VK. The present manifestations of Lyme disease and the outcomes of treatment. N Engl J Med. 2003;348:2472-2474.
- Sanchez E, Vannier E, Wormser GP, et al. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA. 2016;315:1767-1777.
- Shapiro ED. Borrelia burgdorferi (Lyme disease). Pediatr Rev. 2014; 35:500-509.
- Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 2019;68:703
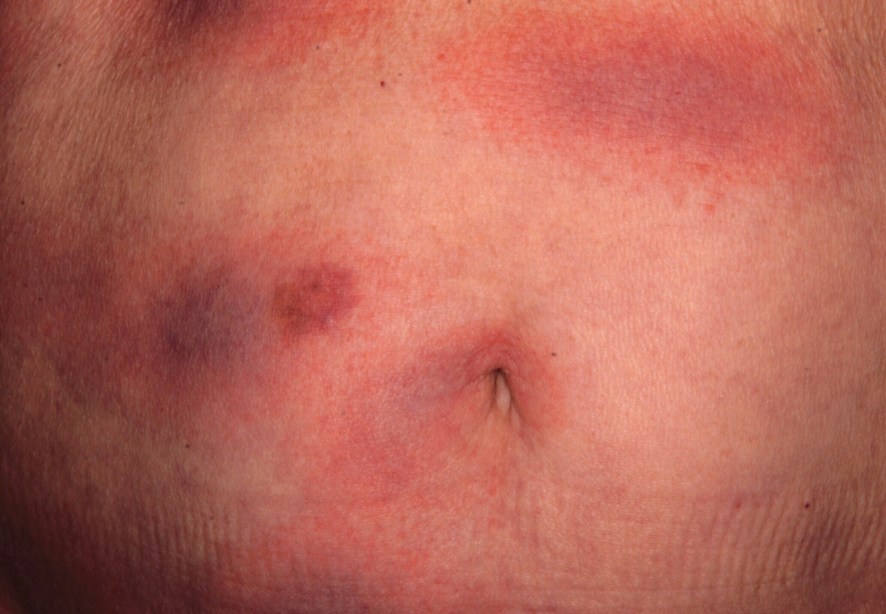
An otherwise healthy 78-year-old woman presented with a diffuse, mildly itchy rash of 5 days’ duration with associated fatigue, chills, decreased appetite, and nausea. She reported waking up with her arms “feeling like they weigh a ton.” She denied any pain, bleeding, or oozing and was unsure if new spots were continuing to develop. The patient reported having allergies to numerous medications but denied any new medications or recent illnesses. She had recently spent time on a farm in Minnesota, and upon further questioning she recalled a tick bite 2 months prior to presentation. She stated that she removed the nonengorged tick and that it could not have been attached for more than 24 hours. Her medical and family history were unremarkable. Physical examination showed multiple annular pink plaques with a central violaceous hue in a generalized distribution involving the face, trunk, arms, and legs with mild erythema of the palms. The plantar surfaces were clear, and there was no evidence of lymphadenopathy. The remainder of the physical examination and review of systems was negative. Laboratory screening was notable for an elevated erythrocyte sedimentation rate and C-reactive protein level with negative antinuclear antibodies.
The lost year – even for common respiratory viruses
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.
The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).
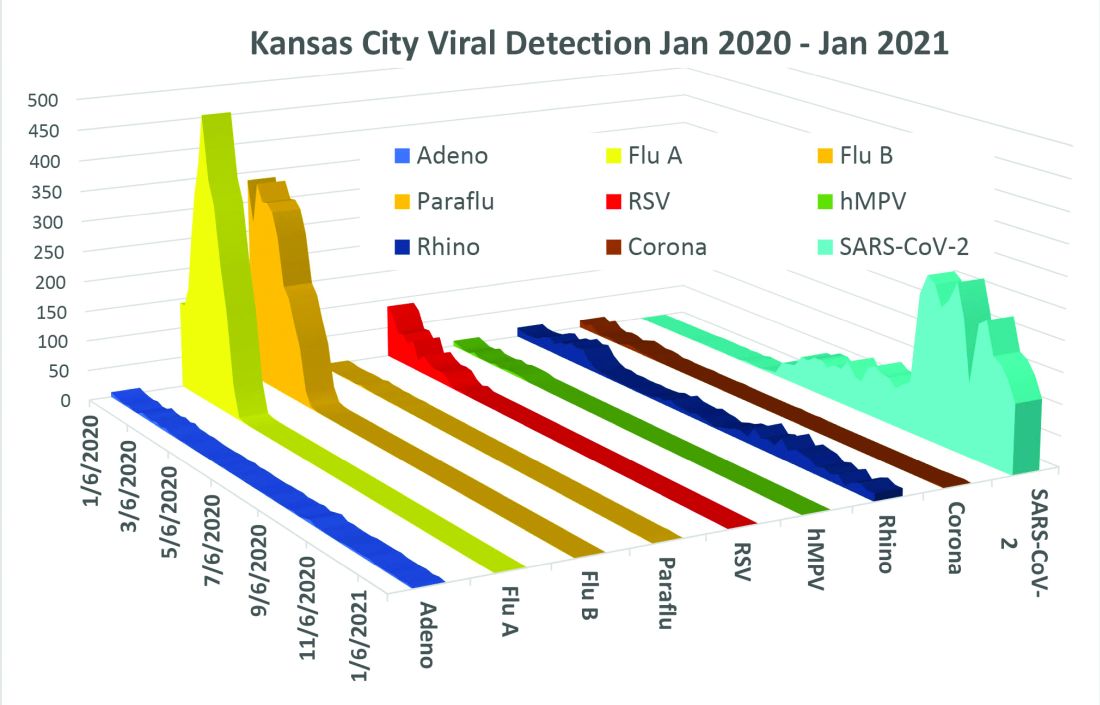
What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.

The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).

What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.

The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).

What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
Infectious diseases ‘giant’ John Bartlett: His ‘impact will endure’
The cause of death was not immediately disclosed.
Dr. Bartlett is remembered by colleagues for his wide range of infectious disease expertise, an ability to repeatedly predict emerging issues in the field, and for inspiring students and trainees to choose the same specialty.
“What I consistently found so extraordinary about John was his excitement for ID – the whole field. He had a wonderful sixth sense about what was going to be the next ‘big thing,’” Paul Edward Sax, MD, clinical director of the Infectious Disease Clinic at Brigham and Women’s Hospital in Boston, told this news organization.
“He thoroughly absorbed the emerging research on the topic and then provided the most wonderful clinical summaries,” Dr. Sax said. “His range of expert content areas was unbelievably broad.” Dr. Bartlett was “a true ID polymath.”
Dr. Bartlett was “a giant in the field of infectious diseases,” David Lee Thomas, MD, MPH, said in an interview. He agreed that Dr. Bartlett was a visionary who could anticipate the most exciting developments in the specialty.
Dr. Bartlett also “led the efforts to combat the foes, from HIV to antimicrobial resistance,” said Dr. Thomas, director of the division of infectious diseases and professor of medicine at Johns Hopkins University.
A pioneer in HIV research and care
Dr. Bartlett’s early research focused on anaerobic pulmonary and other infections, Bacteroides fragilis pathogenesis, and colitis caused by Clostridioides difficile.
Shortly after joining Johns Hopkins in 1980, he focused on HIV/AIDS research and caring for people with HIV. Dr. Bartlett led clinical trials of new treatments and developed years of HIV clinical treatment guidelines.
“Back when most hospitals, university medical centers, and ID divisions were running away from the AIDS epidemic, John took it on, both as a scientific priority and a moral imperative,” Dr. Sax writes in a blog post for NEJM Journal Watch. “With the help of Frank Polk and the Hopkins president, he established an outpatient AIDS clinic and an inpatient AIDS ward – both of which were way ahead of their time.”
In the same post, Dr. Sax points out that Dr. Bartlett was an expert in multiple areas – any one of which could be a sole career focus. “How many ID doctors are true experts in all of the following distinct topics? HIV, Clostridium difficile, respiratory tract infections, antimicrobial resistance, and anaerobic pulmonary infections.” Dr. Sax writes.
Expertise that defined an era
In a piece reviewing the long history of infectious disease medicine at Johns Hopkins published in Clinical Infectious Diseases in 2014, Paul Auwaerter, MD, and colleagues describe his tenure at the institution from 1980 to 2006 as “The Bartlett Era,” notable for the many advances he spearheaded.
“It is nearly impossible to find someone trained in infectious diseases in the past 30 years who has not been impacted by John Bartlett,” Dr. Auwaerter and colleagues note. “His tireless devotion to scholarship, teaching, and patient care remains an inspiration to his faculty members at Johns Hopkins, his colleagues, and coworkers around the world.”
Dr. Bartlett was not only a faculty member in the division of infectious diseases, he also helped establish it. When he joined Johns Hopkins, the infectious disease department featured just three faculty members with a research budget of less than $285,000. By the time he left 26 years later, the division had 44 faculty members on tenure track and a research budget exceeding $40 million.
Sharing memories via social media
Reactions to Dr. Bartlett’s passing on Twitter were swift.
“We have lost one of the greatest physicians I have ever met or had the privilege to learn from. Saddened to hear of Dr. John G. Bartlett’s passing. He inspired so many, including me, to choose the field of infectious diseases,” David Fisk, MD, infectious disease specialist in Santa Barbara, Calif., wrote on Twitter.
“John Bartlett just died – a true visionary and the classic ‘Renaissance’ person in clinical ID. Such a nice guy, too! His IDSA/IDWeek literature summaries (among other things) were amazing. We’ll miss him!” Dr. Sax tweeted on Jan. 19.
A colleague at Johns Hopkins, transplant infectious disease specialist Shmuel Shoham, MD, shared an anecdote about Dr. Bartlett on Twitter: “Year ago. My office is across from his. I ask him what he is doing. He tells me he is reviewing a file from the Vatican to adjudicate whether a miracle happened. True story.”
Infectious disease specialist Graeme Forrest, MBBS, also shared a story about Dr. Bartlett via Twitter. “He described to me in 2001 how the U.S. model of health care would not cope with a pandemic or serious bioterror attack as it’s not connected to disseminate information. How prescient from 20 years ago.”
Dr. Bartlett shared his expertise at many national and international infectious disease conferences over the years. He also authored 470 articles, 282 book chapters, and 61 editions of 14 books.
Dr. Bartlett was also a regular contributor to this news organization. For example, he shared his expertise in perspective pieces that addressed priorities in antibiotic stewardship, upcoming infectious disease predictions, and critical infectious disease topics in a three-part series.
Dr. Bartlett’s education includes a bachelor’s degree from Dartmouth College in Hanover, N.H., in 1959 and an MD from Upstate Medical Center in Syracuse, N.Y., in 1963. He did his first 2 years of residency at Brigham and Women’s Hospital.
He also served as an Army captain from 1965 to 1967, treating patients in fever wards in Vietnam. He then returned to the United States to finish his internal medicine training at the University of Alabama in 1968.
Dr. Bartlett completed his fellowship in infectious diseases at the University of California, Los Angeles. In 1975, he joined the faculty at Tufts University, Boston.
Leaving a legacy
Dr. Bartlett’s influence will likely live on in many ways at Johns Hopkins.
“John is a larger-than-life legend whose impact will endure and after whom we are so proud to have named our clinical service, The Bartlett Specialty Practice,” Dr. Thomas said.
The specialty practice clinic named for him has 23 exam rooms and features multidisciplinary care for people with HIV, hepatitis, bone infections, general infectious diseases, and more. Furthermore, friends, family, and colleagues joined forces to create the “Dr. John G. Bartlett HIV/AIDS Fund.”
They note that it is “only appropriate that we honor him by creating an endowment that will provide support for young trainees and junior faculty in the division, helping them transition to their independent careers.”
In addition to all his professional accomplishments, “He was also a genuinely nice person, approachable and humble,” Dr. Sax said. “We really lost a great one!”
A version of this article first appeared on Medscape.com.
The cause of death was not immediately disclosed.
Dr. Bartlett is remembered by colleagues for his wide range of infectious disease expertise, an ability to repeatedly predict emerging issues in the field, and for inspiring students and trainees to choose the same specialty.
“What I consistently found so extraordinary about John was his excitement for ID – the whole field. He had a wonderful sixth sense about what was going to be the next ‘big thing,’” Paul Edward Sax, MD, clinical director of the Infectious Disease Clinic at Brigham and Women’s Hospital in Boston, told this news organization.
“He thoroughly absorbed the emerging research on the topic and then provided the most wonderful clinical summaries,” Dr. Sax said. “His range of expert content areas was unbelievably broad.” Dr. Bartlett was “a true ID polymath.”
Dr. Bartlett was “a giant in the field of infectious diseases,” David Lee Thomas, MD, MPH, said in an interview. He agreed that Dr. Bartlett was a visionary who could anticipate the most exciting developments in the specialty.
Dr. Bartlett also “led the efforts to combat the foes, from HIV to antimicrobial resistance,” said Dr. Thomas, director of the division of infectious diseases and professor of medicine at Johns Hopkins University.
A pioneer in HIV research and care
Dr. Bartlett’s early research focused on anaerobic pulmonary and other infections, Bacteroides fragilis pathogenesis, and colitis caused by Clostridioides difficile.
Shortly after joining Johns Hopkins in 1980, he focused on HIV/AIDS research and caring for people with HIV. Dr. Bartlett led clinical trials of new treatments and developed years of HIV clinical treatment guidelines.
“Back when most hospitals, university medical centers, and ID divisions were running away from the AIDS epidemic, John took it on, both as a scientific priority and a moral imperative,” Dr. Sax writes in a blog post for NEJM Journal Watch. “With the help of Frank Polk and the Hopkins president, he established an outpatient AIDS clinic and an inpatient AIDS ward – both of which were way ahead of their time.”
In the same post, Dr. Sax points out that Dr. Bartlett was an expert in multiple areas – any one of which could be a sole career focus. “How many ID doctors are true experts in all of the following distinct topics? HIV, Clostridium difficile, respiratory tract infections, antimicrobial resistance, and anaerobic pulmonary infections.” Dr. Sax writes.
Expertise that defined an era
In a piece reviewing the long history of infectious disease medicine at Johns Hopkins published in Clinical Infectious Diseases in 2014, Paul Auwaerter, MD, and colleagues describe his tenure at the institution from 1980 to 2006 as “The Bartlett Era,” notable for the many advances he spearheaded.
“It is nearly impossible to find someone trained in infectious diseases in the past 30 years who has not been impacted by John Bartlett,” Dr. Auwaerter and colleagues note. “His tireless devotion to scholarship, teaching, and patient care remains an inspiration to his faculty members at Johns Hopkins, his colleagues, and coworkers around the world.”
Dr. Bartlett was not only a faculty member in the division of infectious diseases, he also helped establish it. When he joined Johns Hopkins, the infectious disease department featured just three faculty members with a research budget of less than $285,000. By the time he left 26 years later, the division had 44 faculty members on tenure track and a research budget exceeding $40 million.
Sharing memories via social media
Reactions to Dr. Bartlett’s passing on Twitter were swift.
“We have lost one of the greatest physicians I have ever met or had the privilege to learn from. Saddened to hear of Dr. John G. Bartlett’s passing. He inspired so many, including me, to choose the field of infectious diseases,” David Fisk, MD, infectious disease specialist in Santa Barbara, Calif., wrote on Twitter.
“John Bartlett just died – a true visionary and the classic ‘Renaissance’ person in clinical ID. Such a nice guy, too! His IDSA/IDWeek literature summaries (among other things) were amazing. We’ll miss him!” Dr. Sax tweeted on Jan. 19.
A colleague at Johns Hopkins, transplant infectious disease specialist Shmuel Shoham, MD, shared an anecdote about Dr. Bartlett on Twitter: “Year ago. My office is across from his. I ask him what he is doing. He tells me he is reviewing a file from the Vatican to adjudicate whether a miracle happened. True story.”
Infectious disease specialist Graeme Forrest, MBBS, also shared a story about Dr. Bartlett via Twitter. “He described to me in 2001 how the U.S. model of health care would not cope with a pandemic or serious bioterror attack as it’s not connected to disseminate information. How prescient from 20 years ago.”
Dr. Bartlett shared his expertise at many national and international infectious disease conferences over the years. He also authored 470 articles, 282 book chapters, and 61 editions of 14 books.
Dr. Bartlett was also a regular contributor to this news organization. For example, he shared his expertise in perspective pieces that addressed priorities in antibiotic stewardship, upcoming infectious disease predictions, and critical infectious disease topics in a three-part series.
Dr. Bartlett’s education includes a bachelor’s degree from Dartmouth College in Hanover, N.H., in 1959 and an MD from Upstate Medical Center in Syracuse, N.Y., in 1963. He did his first 2 years of residency at Brigham and Women’s Hospital.
He also served as an Army captain from 1965 to 1967, treating patients in fever wards in Vietnam. He then returned to the United States to finish his internal medicine training at the University of Alabama in 1968.
Dr. Bartlett completed his fellowship in infectious diseases at the University of California, Los Angeles. In 1975, he joined the faculty at Tufts University, Boston.
Leaving a legacy
Dr. Bartlett’s influence will likely live on in many ways at Johns Hopkins.
“John is a larger-than-life legend whose impact will endure and after whom we are so proud to have named our clinical service, The Bartlett Specialty Practice,” Dr. Thomas said.
The specialty practice clinic named for him has 23 exam rooms and features multidisciplinary care for people with HIV, hepatitis, bone infections, general infectious diseases, and more. Furthermore, friends, family, and colleagues joined forces to create the “Dr. John G. Bartlett HIV/AIDS Fund.”
They note that it is “only appropriate that we honor him by creating an endowment that will provide support for young trainees and junior faculty in the division, helping them transition to their independent careers.”
In addition to all his professional accomplishments, “He was also a genuinely nice person, approachable and humble,” Dr. Sax said. “We really lost a great one!”
A version of this article first appeared on Medscape.com.
The cause of death was not immediately disclosed.
Dr. Bartlett is remembered by colleagues for his wide range of infectious disease expertise, an ability to repeatedly predict emerging issues in the field, and for inspiring students and trainees to choose the same specialty.
“What I consistently found so extraordinary about John was his excitement for ID – the whole field. He had a wonderful sixth sense about what was going to be the next ‘big thing,’” Paul Edward Sax, MD, clinical director of the Infectious Disease Clinic at Brigham and Women’s Hospital in Boston, told this news organization.
“He thoroughly absorbed the emerging research on the topic and then provided the most wonderful clinical summaries,” Dr. Sax said. “His range of expert content areas was unbelievably broad.” Dr. Bartlett was “a true ID polymath.”
Dr. Bartlett was “a giant in the field of infectious diseases,” David Lee Thomas, MD, MPH, said in an interview. He agreed that Dr. Bartlett was a visionary who could anticipate the most exciting developments in the specialty.
Dr. Bartlett also “led the efforts to combat the foes, from HIV to antimicrobial resistance,” said Dr. Thomas, director of the division of infectious diseases and professor of medicine at Johns Hopkins University.
A pioneer in HIV research and care
Dr. Bartlett’s early research focused on anaerobic pulmonary and other infections, Bacteroides fragilis pathogenesis, and colitis caused by Clostridioides difficile.
Shortly after joining Johns Hopkins in 1980, he focused on HIV/AIDS research and caring for people with HIV. Dr. Bartlett led clinical trials of new treatments and developed years of HIV clinical treatment guidelines.
“Back when most hospitals, university medical centers, and ID divisions were running away from the AIDS epidemic, John took it on, both as a scientific priority and a moral imperative,” Dr. Sax writes in a blog post for NEJM Journal Watch. “With the help of Frank Polk and the Hopkins president, he established an outpatient AIDS clinic and an inpatient AIDS ward – both of which were way ahead of their time.”
In the same post, Dr. Sax points out that Dr. Bartlett was an expert in multiple areas – any one of which could be a sole career focus. “How many ID doctors are true experts in all of the following distinct topics? HIV, Clostridium difficile, respiratory tract infections, antimicrobial resistance, and anaerobic pulmonary infections.” Dr. Sax writes.
Expertise that defined an era
In a piece reviewing the long history of infectious disease medicine at Johns Hopkins published in Clinical Infectious Diseases in 2014, Paul Auwaerter, MD, and colleagues describe his tenure at the institution from 1980 to 2006 as “The Bartlett Era,” notable for the many advances he spearheaded.
“It is nearly impossible to find someone trained in infectious diseases in the past 30 years who has not been impacted by John Bartlett,” Dr. Auwaerter and colleagues note. “His tireless devotion to scholarship, teaching, and patient care remains an inspiration to his faculty members at Johns Hopkins, his colleagues, and coworkers around the world.”
Dr. Bartlett was not only a faculty member in the division of infectious diseases, he also helped establish it. When he joined Johns Hopkins, the infectious disease department featured just three faculty members with a research budget of less than $285,000. By the time he left 26 years later, the division had 44 faculty members on tenure track and a research budget exceeding $40 million.
Sharing memories via social media
Reactions to Dr. Bartlett’s passing on Twitter were swift.
“We have lost one of the greatest physicians I have ever met or had the privilege to learn from. Saddened to hear of Dr. John G. Bartlett’s passing. He inspired so many, including me, to choose the field of infectious diseases,” David Fisk, MD, infectious disease specialist in Santa Barbara, Calif., wrote on Twitter.
“John Bartlett just died – a true visionary and the classic ‘Renaissance’ person in clinical ID. Such a nice guy, too! His IDSA/IDWeek literature summaries (among other things) were amazing. We’ll miss him!” Dr. Sax tweeted on Jan. 19.
A colleague at Johns Hopkins, transplant infectious disease specialist Shmuel Shoham, MD, shared an anecdote about Dr. Bartlett on Twitter: “Year ago. My office is across from his. I ask him what he is doing. He tells me he is reviewing a file from the Vatican to adjudicate whether a miracle happened. True story.”
Infectious disease specialist Graeme Forrest, MBBS, also shared a story about Dr. Bartlett via Twitter. “He described to me in 2001 how the U.S. model of health care would not cope with a pandemic or serious bioterror attack as it’s not connected to disseminate information. How prescient from 20 years ago.”
Dr. Bartlett shared his expertise at many national and international infectious disease conferences over the years. He also authored 470 articles, 282 book chapters, and 61 editions of 14 books.
Dr. Bartlett was also a regular contributor to this news organization. For example, he shared his expertise in perspective pieces that addressed priorities in antibiotic stewardship, upcoming infectious disease predictions, and critical infectious disease topics in a three-part series.
Dr. Bartlett’s education includes a bachelor’s degree from Dartmouth College in Hanover, N.H., in 1959 and an MD from Upstate Medical Center in Syracuse, N.Y., in 1963. He did his first 2 years of residency at Brigham and Women’s Hospital.
He also served as an Army captain from 1965 to 1967, treating patients in fever wards in Vietnam. He then returned to the United States to finish his internal medicine training at the University of Alabama in 1968.
Dr. Bartlett completed his fellowship in infectious diseases at the University of California, Los Angeles. In 1975, he joined the faculty at Tufts University, Boston.
Leaving a legacy
Dr. Bartlett’s influence will likely live on in many ways at Johns Hopkins.
“John is a larger-than-life legend whose impact will endure and after whom we are so proud to have named our clinical service, The Bartlett Specialty Practice,” Dr. Thomas said.
The specialty practice clinic named for him has 23 exam rooms and features multidisciplinary care for people with HIV, hepatitis, bone infections, general infectious diseases, and more. Furthermore, friends, family, and colleagues joined forces to create the “Dr. John G. Bartlett HIV/AIDS Fund.”
They note that it is “only appropriate that we honor him by creating an endowment that will provide support for young trainees and junior faculty in the division, helping them transition to their independent careers.”
In addition to all his professional accomplishments, “He was also a genuinely nice person, approachable and humble,” Dr. Sax said. “We really lost a great one!”
A version of this article first appeared on Medscape.com.
The changing brain signature of HIV
“This shift in subcortical signatures may be contributing to the increasing range of neuropsychiatric and cognitive outcomes,” write Neda Jahanshad, PhD, University of Southern California, Los Angeles, and colleagues.
The study was published online Jan. 15 in JAMA Network Open.
Brain signature of HIV
The researchers with the HIV Working Group within the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) consortium examined structural brain associations with CD4+ T cell counts and HIV viral load.
These clinical markers are the most consistently available in studies of HIV and generalize across demographically and clinically diverse HIV-infected individuals, they point out. However, the degree to which they capture central nervous system injury is not fully understood.
In this cross-sectional study of 1,203 HIV-infected adults from 13 HIV neuroimaging studies, a lower CD4+ T-cell count was associated with smaller hippocampal and thalamic volume independent of treatment status. However, in a subset of adults not on cART, a lower CD4+ T-cell count was associated with smaller putamen volume.
Across all participants, detectable viral load was associated with smaller hippocampal volume, but in the subset on cART, detectable viral load was also associated with smaller amygdala volume.
The findings indicate that plasma markers universally used to monitor immune function and response to treatment in patients with HIV infection are associated with subcortical brain volume.
“Our findings,” they add, “extend beyond the classically implicated regions of the basal ganglia and may represent a generalizable brain signature of HIV infection in the cART era.”
A limitation of the analysis is that most of the participants were men (n = 880, 73%). “A more extensive international effort assessing the neurologic effects of HIV infection in women is needed,” they conclude.
This analysis, they add, demonstrates the feasibility and utility of a global collaborative initiative to understand the neurologic signatures of HIV infection. They invite other HIV researchers to join the ENIGMA-HIV consortium.
“With a greater collaborative effort, we will be able to assess factors that may modulate neurologic outcomes, including cART treatment regimens, comorbidities, coinfections, substance use, socioeconomic factors, and demographic factors, as well as the functional implications of such structural brain differences, in well-powered analyses,” the researchers say.
“Understanding the neurobiological changes that may contribute to neuropsychiatric and cognitive outcomes in HIV-positive individuals is critical for identifying individuals at risk for neurologic symptoms, driving novel treatments that may protect the CNS, and monitoring treatment response,” they add.
Support for this research was provided by grants from the National Institutes of Health, the SA Medical Research Council, the National Health and Medical Research Council, and the European Research Council. Dr. Jahanshad received partial research support from Biogen for work unrelated to the topic of this article. A complete list of author disclosures is in the original article.
A version of this article first appeared on Medscape.com.
“This shift in subcortical signatures may be contributing to the increasing range of neuropsychiatric and cognitive outcomes,” write Neda Jahanshad, PhD, University of Southern California, Los Angeles, and colleagues.
The study was published online Jan. 15 in JAMA Network Open.
Brain signature of HIV
The researchers with the HIV Working Group within the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) consortium examined structural brain associations with CD4+ T cell counts and HIV viral load.
These clinical markers are the most consistently available in studies of HIV and generalize across demographically and clinically diverse HIV-infected individuals, they point out. However, the degree to which they capture central nervous system injury is not fully understood.
In this cross-sectional study of 1,203 HIV-infected adults from 13 HIV neuroimaging studies, a lower CD4+ T-cell count was associated with smaller hippocampal and thalamic volume independent of treatment status. However, in a subset of adults not on cART, a lower CD4+ T-cell count was associated with smaller putamen volume.
Across all participants, detectable viral load was associated with smaller hippocampal volume, but in the subset on cART, detectable viral load was also associated with smaller amygdala volume.
The findings indicate that plasma markers universally used to monitor immune function and response to treatment in patients with HIV infection are associated with subcortical brain volume.
“Our findings,” they add, “extend beyond the classically implicated regions of the basal ganglia and may represent a generalizable brain signature of HIV infection in the cART era.”
A limitation of the analysis is that most of the participants were men (n = 880, 73%). “A more extensive international effort assessing the neurologic effects of HIV infection in women is needed,” they conclude.
This analysis, they add, demonstrates the feasibility and utility of a global collaborative initiative to understand the neurologic signatures of HIV infection. They invite other HIV researchers to join the ENIGMA-HIV consortium.
“With a greater collaborative effort, we will be able to assess factors that may modulate neurologic outcomes, including cART treatment regimens, comorbidities, coinfections, substance use, socioeconomic factors, and demographic factors, as well as the functional implications of such structural brain differences, in well-powered analyses,” the researchers say.
“Understanding the neurobiological changes that may contribute to neuropsychiatric and cognitive outcomes in HIV-positive individuals is critical for identifying individuals at risk for neurologic symptoms, driving novel treatments that may protect the CNS, and monitoring treatment response,” they add.
Support for this research was provided by grants from the National Institutes of Health, the SA Medical Research Council, the National Health and Medical Research Council, and the European Research Council. Dr. Jahanshad received partial research support from Biogen for work unrelated to the topic of this article. A complete list of author disclosures is in the original article.
A version of this article first appeared on Medscape.com.
“This shift in subcortical signatures may be contributing to the increasing range of neuropsychiatric and cognitive outcomes,” write Neda Jahanshad, PhD, University of Southern California, Los Angeles, and colleagues.
The study was published online Jan. 15 in JAMA Network Open.
Brain signature of HIV
The researchers with the HIV Working Group within the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) consortium examined structural brain associations with CD4+ T cell counts and HIV viral load.
These clinical markers are the most consistently available in studies of HIV and generalize across demographically and clinically diverse HIV-infected individuals, they point out. However, the degree to which they capture central nervous system injury is not fully understood.
In this cross-sectional study of 1,203 HIV-infected adults from 13 HIV neuroimaging studies, a lower CD4+ T-cell count was associated with smaller hippocampal and thalamic volume independent of treatment status. However, in a subset of adults not on cART, a lower CD4+ T-cell count was associated with smaller putamen volume.
Across all participants, detectable viral load was associated with smaller hippocampal volume, but in the subset on cART, detectable viral load was also associated with smaller amygdala volume.
The findings indicate that plasma markers universally used to monitor immune function and response to treatment in patients with HIV infection are associated with subcortical brain volume.
“Our findings,” they add, “extend beyond the classically implicated regions of the basal ganglia and may represent a generalizable brain signature of HIV infection in the cART era.”
A limitation of the analysis is that most of the participants were men (n = 880, 73%). “A more extensive international effort assessing the neurologic effects of HIV infection in women is needed,” they conclude.
This analysis, they add, demonstrates the feasibility and utility of a global collaborative initiative to understand the neurologic signatures of HIV infection. They invite other HIV researchers to join the ENIGMA-HIV consortium.
“With a greater collaborative effort, we will be able to assess factors that may modulate neurologic outcomes, including cART treatment regimens, comorbidities, coinfections, substance use, socioeconomic factors, and demographic factors, as well as the functional implications of such structural brain differences, in well-powered analyses,” the researchers say.
“Understanding the neurobiological changes that may contribute to neuropsychiatric and cognitive outcomes in HIV-positive individuals is critical for identifying individuals at risk for neurologic symptoms, driving novel treatments that may protect the CNS, and monitoring treatment response,” they add.
Support for this research was provided by grants from the National Institutes of Health, the SA Medical Research Council, the National Health and Medical Research Council, and the European Research Council. Dr. Jahanshad received partial research support from Biogen for work unrelated to the topic of this article. A complete list of author disclosures is in the original article.
A version of this article first appeared on Medscape.com.
Scrub Typhus in Chile
To the Editor:
Scrub typhus (ST) is an infection caused by Orientia tsutsugamushi (genus Rickettsia), which is transmitted by the larvae of trombiculid mites, commonly called chiggers. The disease mainly has been described in Asia in an area known as the Tsutsugamushi Triangle, delineated by Pakistan, eastern Russia, and northern Australia. Although this classic distribution remains, recent reports have documented 1 case in the Arabian Peninsula1 and more than 16 cases in southern Chile.2-4 The first case in Chile was published in 2011 from Chiloé Island.2 To date, no other cases have been reported in the Americas.1-6
We describe a new case of ST from Chiloé Island and compare it to the first case reported in Chile in 2011.2 Both patients showed the typical clinical manifestation, but because ST has become an increasingly suspected disease in southern regions of Chile, new cases are now easily diagnosed. This infection is diagnosed mainly by skin lesions; therefore, dermatologists should be aware of this diagnosis when presented with a febrile rash.
A 67-year-old man from the city of Punta Arenas presented to the emergency department with a dark necrotic lesion on the right foot of 1 week’s duration. The patient later developed a generalized pruritic rash and fever. He also reported muscle pain, headache, cough, night sweats, and odynophagia. He reported recent travel to a rural area in the northern part of Chiloé Island, where he came into contact with firewood and participated in outdoor activities. He had no other relevant medical history.
Physical examination revealed a temperature of 38 °C and a macular rash, with some papules distributed mainly on the face, trunk, and proximal extremities (Figure 1). He had a necrotic eschar on the dorsum of the right foot, with an erythematous halo (tache noire)(Figure 2).
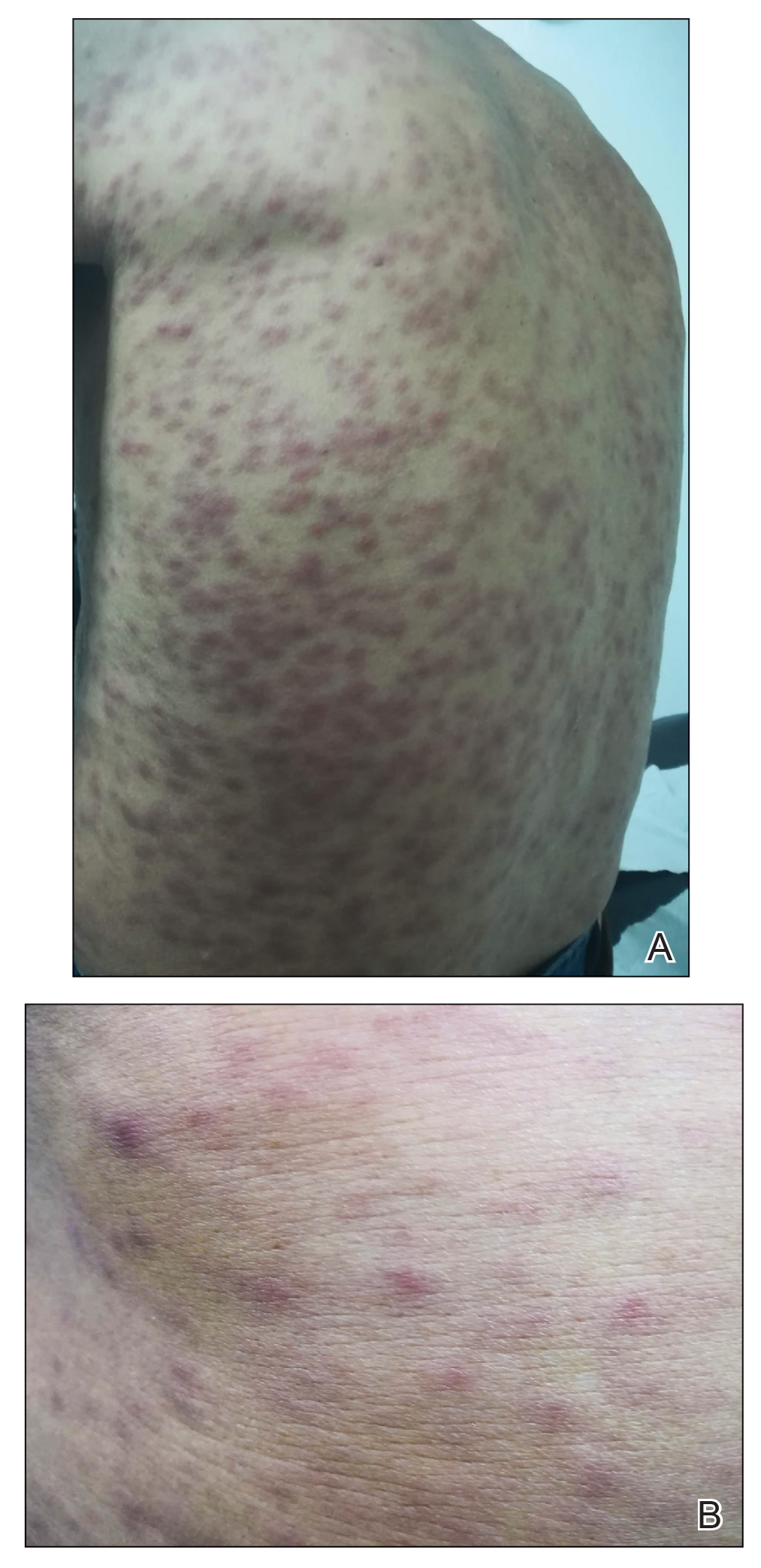
A complete blood cell count, urinalysis, and tests of hepatic and renal function were normal. C-reactive protein was elevated 18 times the normal value. Because of high awareness of ST in the region, eschar samples were taken and submitted for serologic testing and polymerase chain reaction (PCR) targeting the 16S rRNA Orientia gene. Empirical treatment with oral doxycycline 100 mg twice daily was started. Polymerase chain reaction analysis showed the presence of Orientia species, confirming the diagnosis of ST. The rash and eschar diminished considerably after 7 days of antibiotic treatment.
Scrub typhus is a high-impact disease in Asia, described mainly in an area known as the Tsutsugamushi Triangle. Recent reports show important epidemiologic changes in the distribution of the disease, with new published reports of cases outside this endemic area—1 in the Arabian peninsula1 and more than 16 in southern Chile.2-4
The disease begins with a painless, erythematous, and usually unnoticed papule at the site of the bite. After 48 to 72 hours, the papule changes to a necrotic form (tache noire), surrounded by a red halo that often is small, similar to a cigarette burn. This lesion is described in 20% to 90% of infected patients in different series.7 Two or 3 days later (1 to 3 weeks after exposure), high fever suddenly develops. Along with fever, a maculopapular rash distributed centrifugally develops, without compromise of the palms or soles. Patients frequently report headache and night sweating. Sometimes, ST is accompanied by muscle or joint pain, red eye, cough, and abdominal pain. Hearing loss and altered mental status less frequently have been reported.5,8
Common laboratory tests can be of use in diagnosis. An elevated C-reactive protein level and a slight to moderate increase in hepatic transaminases should be expected. Thrombocytopenia, leukopenia, and elevation of the lactate dehydrogenase level less frequently are present.5,9
Our case de1monstrated a typical presentation. The patient developed a febrile syndrome with a generalized rash and a tache noire–type eschar associated with muscle pain, headache, cough, night sweats, and odynophagia. Because of epidemiologic changes in the area, the familiar clinical findings, and laboratory confirmation, histologic studies were unnecessary. In cases in which the diagnosis is not evident, skin biopsy could be useful, as in the first case reported in Chile.2
In that first case, the patient initially was hospitalized because of a febrile syndrome; eventually, a necrotic eschar was noticed on his leg. He had been staying on Chiloé Island and reported being bitten by leeches on multiple occasions. Laboratory findings revealed only slightly raised levels of hepatic transaminases and alkaline phosphatase. After a more precise dermatologic evaluation, the eschar of a tache noire, combined with other clinical and laboratory findings, raised suspicion of ST. Because this entity had never been described in Chile, biopsy of the eschar was taken to consider other entities in the differential diagnosis. Biopsy showed necrotizing leukocytoclastic vasculitis in the dermis and subcutaneous tissue, perivascular inflammatory infiltrates comprising lymphocytes and macrophages, and rickettsial microorganisms inside endothelial cells under electron microscopic examination. The specimen was tested for the 16S ribosomal RNA Orientia gene; its presence confirmed the diagnosis.2
Classically, histology from the eschar shows signs of vasculitis and rickettsial microorganisms inside endothelial cells on electron microscopy.2,10 More recent publications describe important necrotic changes within keratinocytes as well as an inflammatory infiltrate comprising antigen-presenting cells, monocytes, macrophages, and dendritic cells. Using high-resolution thin sections with confocal laser scanning microscopy and staining of specific monoclonal antibodies against 56 kDa type-specific surface antigens, the bacteria were found inside antigen-presenting cells, many of them located perivascularly or passing through the endothelium.11
The causal agent in Asia is O tsutsugamushi, an obligate intracellular bacterium (genus Rickettsia). Orientia species are transmitted by larvae of trombiculid mites, commonly called chiggers. The reservoir is believed to be the same as with chiggers, in which some vertebrates become infected and trombiculid mites feed on them.12 Recent studies of Chilean cases have revealed the presence of a novel Orientia species, Candidatus Orientia chiloensis and its vector, trombiculid mites from the Herpetacarus species, Quadraseta species, and Paratrombicula species genera.13,14
A high seroprevalence of Orientia species in dogs was reported in the main cities of Chiloé Island. Rates were higher in rural settings and older dogs. Of 202 specimens, 21.3% were positive for IgG against Orientia species.15
In Chile, most cases of ST came from Chiloé Island; some reports of cases from continental Chilean regions have been published.6 Most cases have occurred in the context of activities that brought the patients in contact with plants and firewood in rural areas during the summer.3-6
The diagnosis of ST is eminently clinical, based on the triad of fever, macular or papular rash, and an inoculation necrotic eschar. The diagnosis is supported by epidemiologic facts and fast recovery after treatment is initiated.16 Although the diagnosis can be established based on a quick recovery in endemic countries, in areas such as Chile where incidence and distribution are not completely known, it is better to confirm the diagnosis with laboratory tests without delaying treatment. Several testing options exist, including serologic techniques (immunofluorescence or enzyme-linked immunosorbent assay), culture, and detection of the genetic material of Orientia species by PCR. Usually, IgM titers initially are negative, and IgG testing requires paired samples (acute and convalescent) to demonstrate seroconversion and therefore acute infection.17 Because culture requires a highly specialized laboratory, it is not frequently used. Polymerase chain reaction is recognized as the best confirmation method due to its high sensitivity and because it remains positive for a few days after treatment has been initiated. The specimen of choice is the eschar because of its high bacterial load. The base of the scar and the buffy coat are useful specimens when the eschar is unavailable.5,17-19
Due to potential complications of ST, empirical treatment with an antibiotic should be started based on clinical facts and never delayed because of diagnostic tests.18 Classically, ST is treated with a member of the tetracycline family, such as doxycycline, which provides a cure rate of 63% to 100% in ST.5
A 2017 systematic review of treatment options for this infection examined 11 studies from Southeast Asia, China, and South Korea (N=957).16 The review mainly compared doxycycline with azithromycin, chloramphenicol, and tetracycline. No significant difference in cure rate was noted in comparing doxycycline with any of the other 3 antibiotics; most of the studies examined were characterized by a moderate level of evidence. Regarding adverse effects, doxycycline showed a few more cases of gastrointestinal intolerance, and in 2 of 4 studies with chloramphenicol, patients presented with leukopenia.16 Several studies compared standard treatment (doxycycline) with rifampicin, telithromycin, erythromycin, and levofloxacin individually; similar cure rates were noted between doxycycline and each of those 4 agents.
Therapeutic failure in ST has been reported in several cases with the use of levofloxacin.20 Evidence for this novel antibiotic is still insufficient. Further studies are needed before rifampicin, telithromycin, erythromycin, or levofloxacin can be considered as options.Scrub typhus usually resolves within a few weeks. Left untreated, the disease can cause complications such as pneumonia, meningoencephalitis, renal failure, and even multiorgan failure and death. Without treatment, mortality is variable. A 2015 systematic review of mortality from untreated ST showed, on average, mortality of 6% (range, 0%–70%).21 When ST is treated, mortality falls to 0% to 30%.22 Cases reported in Chile have neither been lethal nor presented with severe complications.4,5
Scrub typhus is an infectious disease common in Asia, caused by O tsutsugamushi and transmitted by chiggers. It should be suspected when a febrile macular or papular rash and a tache noire appear. The diagnosis can be supported by laboratory findings, such as an elevated C-reactive protein level or a slight increase in the levels of hepatic transaminases, and response to treatment. The diagnosis is confirmed by serology or PCR of a specimen of the eschar. Empiric therapy with antibiotics is mandatory; doxycycline is the first option.
First described in Chile in 2011,2 ST was seen in a patient in whom disease was suspected because of clinical characteristics, laboratory and histologic findings, absence of prior reporting in South America, and confirmation with PCR targeting the 16S ribosomal RNA Orientia gene from specimens of the eschar. By 2020, 60 cases have been confirmed in Chile, not all of them published; there are no other reported cases in South America.
When comparing the first case in Chile2 with our case, we noted that both described classic clinical findings; however, the management approach and diagnostic challenges have evolved over time. Nowadays, ST is highly suspected, so it can be largely recognized and treated, which also provides better understanding of the nature of this disease in Chile. Because this infection is diagnosed mainly by characteristic cutaneous lesions, dermatologists should be aware of its epidemiology, clinical features, and transmission, and they should stay open to the possibility of this (until now) unusual diagnosis in South America.
Acknowledgments
The authors would like to thank the Chilean Rickettsia & Zoonosis Research Group (Thomas Weitzel, MD [Santiago, Chile]; Constanza Martínez-Valdebenito [Santiago, Chile]; and Gerardo Acosta-Jammet, DSc [Valdivia, Chile]), whose study in execution in the country allowed the detection of the case and confirmation by PCR. The authors also thank Juan Carlos Román, MD (Chiloé, Chile) who was part of the team that detected this case.
- Izzard L, Fuller A, Blacksell SD, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48:4404-4409.
- Balcells ME, Rabagliati R, García P, et al. Endemic scrub typhus-like illness, Chile. Emerg Infect Dis. 2011;17:1659-1663.
- Weitzel T, Dittrich S, López J, et al. Endemic scrub typhus in South America. N Engl J Med. 2016;375:954-961.
- Weitzel T, Acosta-Jamett G, Martínez-Valdebenito C, et al. Scrub typhus risk in travelers to southern Chile. Travel Med Infect Dis. 2019;29:78-79.
- Abarca K, Weitzel T, Martínez-Valdebenito C, et al. Scrub typhus, an emerging infectious disease in Chile. Rev Chilena Infectol. 2018;35:696-699.
- Weitzel T, Martínez-Valdebenito C, Acosta-Jamett G, et al. Scrub typhus in continental Chile, 2016-2018. Emerg Infect Dis. 2019;25:1214-1217.
- Guerrant RL, Walker DH, Weller PF, eds. Tropical Infectious Diseases: Principles, Pathogens and Practice. 3rd ed. Elsevier; 2011.
- Mahara F. Rickettsioses in Japan and the Far East. Ann N Y Acad Sci. 2006;1078:60-73.
- Salje J. Orientia tsutsugamushi: a neglected but fascinating obligate intracellular bacterial pathogen. PLoS Pathog. 2017;13:e1006657.
- Lee JS, Park MY, Kim YJ, et al. Histopathological features in both the eschar and erythematous lesions of tsutsugamushi disease: identification of CD30+ cell infiltration in tsutsugamushi disease. Am J Dermatopathol. 2009;31:551-556.
- Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, et al. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis. 2012;6:E1466.
- Walker DH. Scrub typhus—scientific neglect, ever-widening impact. N Engl J Med. 2016;375:913-915.
- Acosta-Jamett G, Martínez-Valdebenito C, Beltrami E, et al. Identification of trombiculid mites (Acari: Trombiculidae) on rodents from Chiloé Island and molecular evidence of infection with Orientia species [published online January 23, 2020]. PLoS Negl Trop Dis. doi:10.1371/journal.pntd.0007619
- Martínez-Valdebenito C, Angulo J, et al. Molecular description of a novel Orientia species causing scrub typhus in Chile. Emerg Infect Dis. 2020;26:2148-2156.
- Weitzel T, Jiang J, Acosta-Jamett G, et al. Canine seroprevalence to Orientia species in southern Chile: a cross-sectional survey on the Chiloé Island. PLoS One. 2018;13:e0200362.
- Wee I, Lo A, Rodrigo C. Drug treatment of scrub typhus: a systematic review and meta-analysis of controlled clinical trials. Trans R Soc Trop Med Hyg. 2017;111:336-344.
- Koh GCKW, Maude RJ, Paris DH, et al. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368-370.
- Weitzel T, Aylwin M, Martínez-Valdebenito C, et al. Imported scrub typhus: first case in South America and review of the literature. Trop Dis Travel Med Vaccines. 2018;4:10.
- Le Viet N, Laroche M, Thi Pham HL, et al. Use of eschar swabbing for the molecular diagnosis and genotyping of Orientia tsutsugamushi causing scrub typhus in Quang Nam province, Vietnam. 2017;11:e0005397.
- Jang HC, Choi SM, Jang MO, et al. Inappropriateness of quinolone in scrub typhus treatment due to gyrA mutation in Orientia tsutsugamushi Boryong strain. J Korean Med Sci. 2013;28:667-671.
- Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis. 2015;9:e0003971.
- Bonell A, Lubell Y, Newton PN, et al. Estimating the burden of scrub typhus: a systematic review. PLoS Negl Trop Dis. 2017;11:e0005838.
To the Editor:
Scrub typhus (ST) is an infection caused by Orientia tsutsugamushi (genus Rickettsia), which is transmitted by the larvae of trombiculid mites, commonly called chiggers. The disease mainly has been described in Asia in an area known as the Tsutsugamushi Triangle, delineated by Pakistan, eastern Russia, and northern Australia. Although this classic distribution remains, recent reports have documented 1 case in the Arabian Peninsula1 and more than 16 cases in southern Chile.2-4 The first case in Chile was published in 2011 from Chiloé Island.2 To date, no other cases have been reported in the Americas.1-6
We describe a new case of ST from Chiloé Island and compare it to the first case reported in Chile in 2011.2 Both patients showed the typical clinical manifestation, but because ST has become an increasingly suspected disease in southern regions of Chile, new cases are now easily diagnosed. This infection is diagnosed mainly by skin lesions; therefore, dermatologists should be aware of this diagnosis when presented with a febrile rash.
A 67-year-old man from the city of Punta Arenas presented to the emergency department with a dark necrotic lesion on the right foot of 1 week’s duration. The patient later developed a generalized pruritic rash and fever. He also reported muscle pain, headache, cough, night sweats, and odynophagia. He reported recent travel to a rural area in the northern part of Chiloé Island, where he came into contact with firewood and participated in outdoor activities. He had no other relevant medical history.
Physical examination revealed a temperature of 38 °C and a macular rash, with some papules distributed mainly on the face, trunk, and proximal extremities (Figure 1). He had a necrotic eschar on the dorsum of the right foot, with an erythematous halo (tache noire)(Figure 2).


A complete blood cell count, urinalysis, and tests of hepatic and renal function were normal. C-reactive protein was elevated 18 times the normal value. Because of high awareness of ST in the region, eschar samples were taken and submitted for serologic testing and polymerase chain reaction (PCR) targeting the 16S rRNA Orientia gene. Empirical treatment with oral doxycycline 100 mg twice daily was started. Polymerase chain reaction analysis showed the presence of Orientia species, confirming the diagnosis of ST. The rash and eschar diminished considerably after 7 days of antibiotic treatment.
Scrub typhus is a high-impact disease in Asia, described mainly in an area known as the Tsutsugamushi Triangle. Recent reports show important epidemiologic changes in the distribution of the disease, with new published reports of cases outside this endemic area—1 in the Arabian peninsula1 and more than 16 in southern Chile.2-4
The disease begins with a painless, erythematous, and usually unnoticed papule at the site of the bite. After 48 to 72 hours, the papule changes to a necrotic form (tache noire), surrounded by a red halo that often is small, similar to a cigarette burn. This lesion is described in 20% to 90% of infected patients in different series.7 Two or 3 days later (1 to 3 weeks after exposure), high fever suddenly develops. Along with fever, a maculopapular rash distributed centrifugally develops, without compromise of the palms or soles. Patients frequently report headache and night sweating. Sometimes, ST is accompanied by muscle or joint pain, red eye, cough, and abdominal pain. Hearing loss and altered mental status less frequently have been reported.5,8
Common laboratory tests can be of use in diagnosis. An elevated C-reactive protein level and a slight to moderate increase in hepatic transaminases should be expected. Thrombocytopenia, leukopenia, and elevation of the lactate dehydrogenase level less frequently are present.5,9
Our case de1monstrated a typical presentation. The patient developed a febrile syndrome with a generalized rash and a tache noire–type eschar associated with muscle pain, headache, cough, night sweats, and odynophagia. Because of epidemiologic changes in the area, the familiar clinical findings, and laboratory confirmation, histologic studies were unnecessary. In cases in which the diagnosis is not evident, skin biopsy could be useful, as in the first case reported in Chile.2
In that first case, the patient initially was hospitalized because of a febrile syndrome; eventually, a necrotic eschar was noticed on his leg. He had been staying on Chiloé Island and reported being bitten by leeches on multiple occasions. Laboratory findings revealed only slightly raised levels of hepatic transaminases and alkaline phosphatase. After a more precise dermatologic evaluation, the eschar of a tache noire, combined with other clinical and laboratory findings, raised suspicion of ST. Because this entity had never been described in Chile, biopsy of the eschar was taken to consider other entities in the differential diagnosis. Biopsy showed necrotizing leukocytoclastic vasculitis in the dermis and subcutaneous tissue, perivascular inflammatory infiltrates comprising lymphocytes and macrophages, and rickettsial microorganisms inside endothelial cells under electron microscopic examination. The specimen was tested for the 16S ribosomal RNA Orientia gene; its presence confirmed the diagnosis.2
Classically, histology from the eschar shows signs of vasculitis and rickettsial microorganisms inside endothelial cells on electron microscopy.2,10 More recent publications describe important necrotic changes within keratinocytes as well as an inflammatory infiltrate comprising antigen-presenting cells, monocytes, macrophages, and dendritic cells. Using high-resolution thin sections with confocal laser scanning microscopy and staining of specific monoclonal antibodies against 56 kDa type-specific surface antigens, the bacteria were found inside antigen-presenting cells, many of them located perivascularly or passing through the endothelium.11
The causal agent in Asia is O tsutsugamushi, an obligate intracellular bacterium (genus Rickettsia). Orientia species are transmitted by larvae of trombiculid mites, commonly called chiggers. The reservoir is believed to be the same as with chiggers, in which some vertebrates become infected and trombiculid mites feed on them.12 Recent studies of Chilean cases have revealed the presence of a novel Orientia species, Candidatus Orientia chiloensis and its vector, trombiculid mites from the Herpetacarus species, Quadraseta species, and Paratrombicula species genera.13,14
A high seroprevalence of Orientia species in dogs was reported in the main cities of Chiloé Island. Rates were higher in rural settings and older dogs. Of 202 specimens, 21.3% were positive for IgG against Orientia species.15
In Chile, most cases of ST came from Chiloé Island; some reports of cases from continental Chilean regions have been published.6 Most cases have occurred in the context of activities that brought the patients in contact with plants and firewood in rural areas during the summer.3-6
The diagnosis of ST is eminently clinical, based on the triad of fever, macular or papular rash, and an inoculation necrotic eschar. The diagnosis is supported by epidemiologic facts and fast recovery after treatment is initiated.16 Although the diagnosis can be established based on a quick recovery in endemic countries, in areas such as Chile where incidence and distribution are not completely known, it is better to confirm the diagnosis with laboratory tests without delaying treatment. Several testing options exist, including serologic techniques (immunofluorescence or enzyme-linked immunosorbent assay), culture, and detection of the genetic material of Orientia species by PCR. Usually, IgM titers initially are negative, and IgG testing requires paired samples (acute and convalescent) to demonstrate seroconversion and therefore acute infection.17 Because culture requires a highly specialized laboratory, it is not frequently used. Polymerase chain reaction is recognized as the best confirmation method due to its high sensitivity and because it remains positive for a few days after treatment has been initiated. The specimen of choice is the eschar because of its high bacterial load. The base of the scar and the buffy coat are useful specimens when the eschar is unavailable.5,17-19
Due to potential complications of ST, empirical treatment with an antibiotic should be started based on clinical facts and never delayed because of diagnostic tests.18 Classically, ST is treated with a member of the tetracycline family, such as doxycycline, which provides a cure rate of 63% to 100% in ST.5
A 2017 systematic review of treatment options for this infection examined 11 studies from Southeast Asia, China, and South Korea (N=957).16 The review mainly compared doxycycline with azithromycin, chloramphenicol, and tetracycline. No significant difference in cure rate was noted in comparing doxycycline with any of the other 3 antibiotics; most of the studies examined were characterized by a moderate level of evidence. Regarding adverse effects, doxycycline showed a few more cases of gastrointestinal intolerance, and in 2 of 4 studies with chloramphenicol, patients presented with leukopenia.16 Several studies compared standard treatment (doxycycline) with rifampicin, telithromycin, erythromycin, and levofloxacin individually; similar cure rates were noted between doxycycline and each of those 4 agents.
Therapeutic failure in ST has been reported in several cases with the use of levofloxacin.20 Evidence for this novel antibiotic is still insufficient. Further studies are needed before rifampicin, telithromycin, erythromycin, or levofloxacin can be considered as options.Scrub typhus usually resolves within a few weeks. Left untreated, the disease can cause complications such as pneumonia, meningoencephalitis, renal failure, and even multiorgan failure and death. Without treatment, mortality is variable. A 2015 systematic review of mortality from untreated ST showed, on average, mortality of 6% (range, 0%–70%).21 When ST is treated, mortality falls to 0% to 30%.22 Cases reported in Chile have neither been lethal nor presented with severe complications.4,5
Scrub typhus is an infectious disease common in Asia, caused by O tsutsugamushi and transmitted by chiggers. It should be suspected when a febrile macular or papular rash and a tache noire appear. The diagnosis can be supported by laboratory findings, such as an elevated C-reactive protein level or a slight increase in the levels of hepatic transaminases, and response to treatment. The diagnosis is confirmed by serology or PCR of a specimen of the eschar. Empiric therapy with antibiotics is mandatory; doxycycline is the first option.
First described in Chile in 2011,2 ST was seen in a patient in whom disease was suspected because of clinical characteristics, laboratory and histologic findings, absence of prior reporting in South America, and confirmation with PCR targeting the 16S ribosomal RNA Orientia gene from specimens of the eschar. By 2020, 60 cases have been confirmed in Chile, not all of them published; there are no other reported cases in South America.
When comparing the first case in Chile2 with our case, we noted that both described classic clinical findings; however, the management approach and diagnostic challenges have evolved over time. Nowadays, ST is highly suspected, so it can be largely recognized and treated, which also provides better understanding of the nature of this disease in Chile. Because this infection is diagnosed mainly by characteristic cutaneous lesions, dermatologists should be aware of its epidemiology, clinical features, and transmission, and they should stay open to the possibility of this (until now) unusual diagnosis in South America.
Acknowledgments
The authors would like to thank the Chilean Rickettsia & Zoonosis Research Group (Thomas Weitzel, MD [Santiago, Chile]; Constanza Martínez-Valdebenito [Santiago, Chile]; and Gerardo Acosta-Jammet, DSc [Valdivia, Chile]), whose study in execution in the country allowed the detection of the case and confirmation by PCR. The authors also thank Juan Carlos Román, MD (Chiloé, Chile) who was part of the team that detected this case.
To the Editor:
Scrub typhus (ST) is an infection caused by Orientia tsutsugamushi (genus Rickettsia), which is transmitted by the larvae of trombiculid mites, commonly called chiggers. The disease mainly has been described in Asia in an area known as the Tsutsugamushi Triangle, delineated by Pakistan, eastern Russia, and northern Australia. Although this classic distribution remains, recent reports have documented 1 case in the Arabian Peninsula1 and more than 16 cases in southern Chile.2-4 The first case in Chile was published in 2011 from Chiloé Island.2 To date, no other cases have been reported in the Americas.1-6
We describe a new case of ST from Chiloé Island and compare it to the first case reported in Chile in 2011.2 Both patients showed the typical clinical manifestation, but because ST has become an increasingly suspected disease in southern regions of Chile, new cases are now easily diagnosed. This infection is diagnosed mainly by skin lesions; therefore, dermatologists should be aware of this diagnosis when presented with a febrile rash.
A 67-year-old man from the city of Punta Arenas presented to the emergency department with a dark necrotic lesion on the right foot of 1 week’s duration. The patient later developed a generalized pruritic rash and fever. He also reported muscle pain, headache, cough, night sweats, and odynophagia. He reported recent travel to a rural area in the northern part of Chiloé Island, where he came into contact with firewood and participated in outdoor activities. He had no other relevant medical history.
Physical examination revealed a temperature of 38 °C and a macular rash, with some papules distributed mainly on the face, trunk, and proximal extremities (Figure 1). He had a necrotic eschar on the dorsum of the right foot, with an erythematous halo (tache noire)(Figure 2).


A complete blood cell count, urinalysis, and tests of hepatic and renal function were normal. C-reactive protein was elevated 18 times the normal value. Because of high awareness of ST in the region, eschar samples were taken and submitted for serologic testing and polymerase chain reaction (PCR) targeting the 16S rRNA Orientia gene. Empirical treatment with oral doxycycline 100 mg twice daily was started. Polymerase chain reaction analysis showed the presence of Orientia species, confirming the diagnosis of ST. The rash and eschar diminished considerably after 7 days of antibiotic treatment.
Scrub typhus is a high-impact disease in Asia, described mainly in an area known as the Tsutsugamushi Triangle. Recent reports show important epidemiologic changes in the distribution of the disease, with new published reports of cases outside this endemic area—1 in the Arabian peninsula1 and more than 16 in southern Chile.2-4
The disease begins with a painless, erythematous, and usually unnoticed papule at the site of the bite. After 48 to 72 hours, the papule changes to a necrotic form (tache noire), surrounded by a red halo that often is small, similar to a cigarette burn. This lesion is described in 20% to 90% of infected patients in different series.7 Two or 3 days later (1 to 3 weeks after exposure), high fever suddenly develops. Along with fever, a maculopapular rash distributed centrifugally develops, without compromise of the palms or soles. Patients frequently report headache and night sweating. Sometimes, ST is accompanied by muscle or joint pain, red eye, cough, and abdominal pain. Hearing loss and altered mental status less frequently have been reported.5,8
Common laboratory tests can be of use in diagnosis. An elevated C-reactive protein level and a slight to moderate increase in hepatic transaminases should be expected. Thrombocytopenia, leukopenia, and elevation of the lactate dehydrogenase level less frequently are present.5,9
Our case de1monstrated a typical presentation. The patient developed a febrile syndrome with a generalized rash and a tache noire–type eschar associated with muscle pain, headache, cough, night sweats, and odynophagia. Because of epidemiologic changes in the area, the familiar clinical findings, and laboratory confirmation, histologic studies were unnecessary. In cases in which the diagnosis is not evident, skin biopsy could be useful, as in the first case reported in Chile.2
In that first case, the patient initially was hospitalized because of a febrile syndrome; eventually, a necrotic eschar was noticed on his leg. He had been staying on Chiloé Island and reported being bitten by leeches on multiple occasions. Laboratory findings revealed only slightly raised levels of hepatic transaminases and alkaline phosphatase. After a more precise dermatologic evaluation, the eschar of a tache noire, combined with other clinical and laboratory findings, raised suspicion of ST. Because this entity had never been described in Chile, biopsy of the eschar was taken to consider other entities in the differential diagnosis. Biopsy showed necrotizing leukocytoclastic vasculitis in the dermis and subcutaneous tissue, perivascular inflammatory infiltrates comprising lymphocytes and macrophages, and rickettsial microorganisms inside endothelial cells under electron microscopic examination. The specimen was tested for the 16S ribosomal RNA Orientia gene; its presence confirmed the diagnosis.2
Classically, histology from the eschar shows signs of vasculitis and rickettsial microorganisms inside endothelial cells on electron microscopy.2,10 More recent publications describe important necrotic changes within keratinocytes as well as an inflammatory infiltrate comprising antigen-presenting cells, monocytes, macrophages, and dendritic cells. Using high-resolution thin sections with confocal laser scanning microscopy and staining of specific monoclonal antibodies against 56 kDa type-specific surface antigens, the bacteria were found inside antigen-presenting cells, many of them located perivascularly or passing through the endothelium.11
The causal agent in Asia is O tsutsugamushi, an obligate intracellular bacterium (genus Rickettsia). Orientia species are transmitted by larvae of trombiculid mites, commonly called chiggers. The reservoir is believed to be the same as with chiggers, in which some vertebrates become infected and trombiculid mites feed on them.12 Recent studies of Chilean cases have revealed the presence of a novel Orientia species, Candidatus Orientia chiloensis and its vector, trombiculid mites from the Herpetacarus species, Quadraseta species, and Paratrombicula species genera.13,14
A high seroprevalence of Orientia species in dogs was reported in the main cities of Chiloé Island. Rates were higher in rural settings and older dogs. Of 202 specimens, 21.3% were positive for IgG against Orientia species.15
In Chile, most cases of ST came from Chiloé Island; some reports of cases from continental Chilean regions have been published.6 Most cases have occurred in the context of activities that brought the patients in contact with plants and firewood in rural areas during the summer.3-6
The diagnosis of ST is eminently clinical, based on the triad of fever, macular or papular rash, and an inoculation necrotic eschar. The diagnosis is supported by epidemiologic facts and fast recovery after treatment is initiated.16 Although the diagnosis can be established based on a quick recovery in endemic countries, in areas such as Chile where incidence and distribution are not completely known, it is better to confirm the diagnosis with laboratory tests without delaying treatment. Several testing options exist, including serologic techniques (immunofluorescence or enzyme-linked immunosorbent assay), culture, and detection of the genetic material of Orientia species by PCR. Usually, IgM titers initially are negative, and IgG testing requires paired samples (acute and convalescent) to demonstrate seroconversion and therefore acute infection.17 Because culture requires a highly specialized laboratory, it is not frequently used. Polymerase chain reaction is recognized as the best confirmation method due to its high sensitivity and because it remains positive for a few days after treatment has been initiated. The specimen of choice is the eschar because of its high bacterial load. The base of the scar and the buffy coat are useful specimens when the eschar is unavailable.5,17-19
Due to potential complications of ST, empirical treatment with an antibiotic should be started based on clinical facts and never delayed because of diagnostic tests.18 Classically, ST is treated with a member of the tetracycline family, such as doxycycline, which provides a cure rate of 63% to 100% in ST.5
A 2017 systematic review of treatment options for this infection examined 11 studies from Southeast Asia, China, and South Korea (N=957).16 The review mainly compared doxycycline with azithromycin, chloramphenicol, and tetracycline. No significant difference in cure rate was noted in comparing doxycycline with any of the other 3 antibiotics; most of the studies examined were characterized by a moderate level of evidence. Regarding adverse effects, doxycycline showed a few more cases of gastrointestinal intolerance, and in 2 of 4 studies with chloramphenicol, patients presented with leukopenia.16 Several studies compared standard treatment (doxycycline) with rifampicin, telithromycin, erythromycin, and levofloxacin individually; similar cure rates were noted between doxycycline and each of those 4 agents.
Therapeutic failure in ST has been reported in several cases with the use of levofloxacin.20 Evidence for this novel antibiotic is still insufficient. Further studies are needed before rifampicin, telithromycin, erythromycin, or levofloxacin can be considered as options.Scrub typhus usually resolves within a few weeks. Left untreated, the disease can cause complications such as pneumonia, meningoencephalitis, renal failure, and even multiorgan failure and death. Without treatment, mortality is variable. A 2015 systematic review of mortality from untreated ST showed, on average, mortality of 6% (range, 0%–70%).21 When ST is treated, mortality falls to 0% to 30%.22 Cases reported in Chile have neither been lethal nor presented with severe complications.4,5
Scrub typhus is an infectious disease common in Asia, caused by O tsutsugamushi and transmitted by chiggers. It should be suspected when a febrile macular or papular rash and a tache noire appear. The diagnosis can be supported by laboratory findings, such as an elevated C-reactive protein level or a slight increase in the levels of hepatic transaminases, and response to treatment. The diagnosis is confirmed by serology or PCR of a specimen of the eschar. Empiric therapy with antibiotics is mandatory; doxycycline is the first option.
First described in Chile in 2011,2 ST was seen in a patient in whom disease was suspected because of clinical characteristics, laboratory and histologic findings, absence of prior reporting in South America, and confirmation with PCR targeting the 16S ribosomal RNA Orientia gene from specimens of the eschar. By 2020, 60 cases have been confirmed in Chile, not all of them published; there are no other reported cases in South America.
When comparing the first case in Chile2 with our case, we noted that both described classic clinical findings; however, the management approach and diagnostic challenges have evolved over time. Nowadays, ST is highly suspected, so it can be largely recognized and treated, which also provides better understanding of the nature of this disease in Chile. Because this infection is diagnosed mainly by characteristic cutaneous lesions, dermatologists should be aware of its epidemiology, clinical features, and transmission, and they should stay open to the possibility of this (until now) unusual diagnosis in South America.
Acknowledgments
The authors would like to thank the Chilean Rickettsia & Zoonosis Research Group (Thomas Weitzel, MD [Santiago, Chile]; Constanza Martínez-Valdebenito [Santiago, Chile]; and Gerardo Acosta-Jammet, DSc [Valdivia, Chile]), whose study in execution in the country allowed the detection of the case and confirmation by PCR. The authors also thank Juan Carlos Román, MD (Chiloé, Chile) who was part of the team that detected this case.
- Izzard L, Fuller A, Blacksell SD, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48:4404-4409.
- Balcells ME, Rabagliati R, García P, et al. Endemic scrub typhus-like illness, Chile. Emerg Infect Dis. 2011;17:1659-1663.
- Weitzel T, Dittrich S, López J, et al. Endemic scrub typhus in South America. N Engl J Med. 2016;375:954-961.
- Weitzel T, Acosta-Jamett G, Martínez-Valdebenito C, et al. Scrub typhus risk in travelers to southern Chile. Travel Med Infect Dis. 2019;29:78-79.
- Abarca K, Weitzel T, Martínez-Valdebenito C, et al. Scrub typhus, an emerging infectious disease in Chile. Rev Chilena Infectol. 2018;35:696-699.
- Weitzel T, Martínez-Valdebenito C, Acosta-Jamett G, et al. Scrub typhus in continental Chile, 2016-2018. Emerg Infect Dis. 2019;25:1214-1217.
- Guerrant RL, Walker DH, Weller PF, eds. Tropical Infectious Diseases: Principles, Pathogens and Practice. 3rd ed. Elsevier; 2011.
- Mahara F. Rickettsioses in Japan and the Far East. Ann N Y Acad Sci. 2006;1078:60-73.
- Salje J. Orientia tsutsugamushi: a neglected but fascinating obligate intracellular bacterial pathogen. PLoS Pathog. 2017;13:e1006657.
- Lee JS, Park MY, Kim YJ, et al. Histopathological features in both the eschar and erythematous lesions of tsutsugamushi disease: identification of CD30+ cell infiltration in tsutsugamushi disease. Am J Dermatopathol. 2009;31:551-556.
- Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, et al. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis. 2012;6:E1466.
- Walker DH. Scrub typhus—scientific neglect, ever-widening impact. N Engl J Med. 2016;375:913-915.
- Acosta-Jamett G, Martínez-Valdebenito C, Beltrami E, et al. Identification of trombiculid mites (Acari: Trombiculidae) on rodents from Chiloé Island and molecular evidence of infection with Orientia species [published online January 23, 2020]. PLoS Negl Trop Dis. doi:10.1371/journal.pntd.0007619
- Martínez-Valdebenito C, Angulo J, et al. Molecular description of a novel Orientia species causing scrub typhus in Chile. Emerg Infect Dis. 2020;26:2148-2156.
- Weitzel T, Jiang J, Acosta-Jamett G, et al. Canine seroprevalence to Orientia species in southern Chile: a cross-sectional survey on the Chiloé Island. PLoS One. 2018;13:e0200362.
- Wee I, Lo A, Rodrigo C. Drug treatment of scrub typhus: a systematic review and meta-analysis of controlled clinical trials. Trans R Soc Trop Med Hyg. 2017;111:336-344.
- Koh GCKW, Maude RJ, Paris DH, et al. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368-370.
- Weitzel T, Aylwin M, Martínez-Valdebenito C, et al. Imported scrub typhus: first case in South America and review of the literature. Trop Dis Travel Med Vaccines. 2018;4:10.
- Le Viet N, Laroche M, Thi Pham HL, et al. Use of eschar swabbing for the molecular diagnosis and genotyping of Orientia tsutsugamushi causing scrub typhus in Quang Nam province, Vietnam. 2017;11:e0005397.
- Jang HC, Choi SM, Jang MO, et al. Inappropriateness of quinolone in scrub typhus treatment due to gyrA mutation in Orientia tsutsugamushi Boryong strain. J Korean Med Sci. 2013;28:667-671.
- Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis. 2015;9:e0003971.
- Bonell A, Lubell Y, Newton PN, et al. Estimating the burden of scrub typhus: a systematic review. PLoS Negl Trop Dis. 2017;11:e0005838.
- Izzard L, Fuller A, Blacksell SD, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48:4404-4409.
- Balcells ME, Rabagliati R, García P, et al. Endemic scrub typhus-like illness, Chile. Emerg Infect Dis. 2011;17:1659-1663.
- Weitzel T, Dittrich S, López J, et al. Endemic scrub typhus in South America. N Engl J Med. 2016;375:954-961.
- Weitzel T, Acosta-Jamett G, Martínez-Valdebenito C, et al. Scrub typhus risk in travelers to southern Chile. Travel Med Infect Dis. 2019;29:78-79.
- Abarca K, Weitzel T, Martínez-Valdebenito C, et al. Scrub typhus, an emerging infectious disease in Chile. Rev Chilena Infectol. 2018;35:696-699.
- Weitzel T, Martínez-Valdebenito C, Acosta-Jamett G, et al. Scrub typhus in continental Chile, 2016-2018. Emerg Infect Dis. 2019;25:1214-1217.
- Guerrant RL, Walker DH, Weller PF, eds. Tropical Infectious Diseases: Principles, Pathogens and Practice. 3rd ed. Elsevier; 2011.
- Mahara F. Rickettsioses in Japan and the Far East. Ann N Y Acad Sci. 2006;1078:60-73.
- Salje J. Orientia tsutsugamushi: a neglected but fascinating obligate intracellular bacterial pathogen. PLoS Pathog. 2017;13:e1006657.
- Lee JS, Park MY, Kim YJ, et al. Histopathological features in both the eschar and erythematous lesions of tsutsugamushi disease: identification of CD30+ cell infiltration in tsutsugamushi disease. Am J Dermatopathol. 2009;31:551-556.
- Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, et al. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis. 2012;6:E1466.
- Walker DH. Scrub typhus—scientific neglect, ever-widening impact. N Engl J Med. 2016;375:913-915.
- Acosta-Jamett G, Martínez-Valdebenito C, Beltrami E, et al. Identification of trombiculid mites (Acari: Trombiculidae) on rodents from Chiloé Island and molecular evidence of infection with Orientia species [published online January 23, 2020]. PLoS Negl Trop Dis. doi:10.1371/journal.pntd.0007619
- Martínez-Valdebenito C, Angulo J, et al. Molecular description of a novel Orientia species causing scrub typhus in Chile. Emerg Infect Dis. 2020;26:2148-2156.
- Weitzel T, Jiang J, Acosta-Jamett G, et al. Canine seroprevalence to Orientia species in southern Chile: a cross-sectional survey on the Chiloé Island. PLoS One. 2018;13:e0200362.
- Wee I, Lo A, Rodrigo C. Drug treatment of scrub typhus: a systematic review and meta-analysis of controlled clinical trials. Trans R Soc Trop Med Hyg. 2017;111:336-344.
- Koh GCKW, Maude RJ, Paris DH, et al. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368-370.
- Weitzel T, Aylwin M, Martínez-Valdebenito C, et al. Imported scrub typhus: first case in South America and review of the literature. Trop Dis Travel Med Vaccines. 2018;4:10.
- Le Viet N, Laroche M, Thi Pham HL, et al. Use of eschar swabbing for the molecular diagnosis and genotyping of Orientia tsutsugamushi causing scrub typhus in Quang Nam province, Vietnam. 2017;11:e0005397.
- Jang HC, Choi SM, Jang MO, et al. Inappropriateness of quinolone in scrub typhus treatment due to gyrA mutation in Orientia tsutsugamushi Boryong strain. J Korean Med Sci. 2013;28:667-671.
- Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis. 2015;9:e0003971.
- Bonell A, Lubell Y, Newton PN, et al. Estimating the burden of scrub typhus: a systematic review. PLoS Negl Trop Dis. 2017;11:e0005838.
Practice Points
- Scrub typhus is clinically suspected in patients who present with a febrile macular or papular rash and a characteristic necrotic eschar known as tache noire while residing in or traveling to rural areas.
- Scrub typhus can lead to serious complications. Due to its changing epidemiology, dermatologists outside the usual area of distribution should be aware in the event that new cases emerge.