User login
COVID-19 and the psychological side effects of PPE
A few months ago, I published a short thought piece on the use of “sitters” with patients who were COVID-19 positive, or patients under investigation. In it, I recommended the use of telesitters for those who normally would warrant a human sitter, to decrease the discomfort of sitting in full personal protective equipment (PPE) (gown, mask, gloves, etc.) while monitoring a suicidal patient.
I received several queries, which I want to address here. In addition, I want to draw from my Army days in terms of the claustrophobia often experienced with PPE.
The first of the questions was about evidence-based practices. The second was about the discomfort of having sitters sit for many hours in the full gear.
I do not know of any evidence-based practices, but I hope we will develop them.
I agree that spending many hours in full PPE can be discomforting, which is why I wrote the essay.
As far as lessons learned from the Army time, I briefly learned how to wear a “gas mask” or Mission-Oriented Protective Posture (MOPP gear) while at Fort Bragg. We were run through the “gas chamber,” where sergeants released tear gas while we had the mask on. We were then asked to lift it up, and then tearing and sputtering, we could leave the small wooden building.
We wore the mask as part of our Army gear, usually on the right leg. After that, I mainly used the protective mask in its bag as a pillow when I was in the field.
Fast forward to August 1990. I arrived at Camp Casey, near the Korean demilitarized zone. Four days later, Saddam Hussein invaded Kuwait. The gas mask moved from a pillow to something we had to wear while doing 12-mile road marches in “full ruck.” In full ruck, you have your uniform on, with TA-50, knapsack, and weapon. No, I do not remember any more what TA-50 stands for, but essentially it is the webbing that holds your bullets and bandages.
Many could not tolerate it. They developed claustrophobia – sweating, air hunger, and panic. If stationed in the Gulf for Operation Desert Storm, they were evacuated home.
I wrote a couple of short articles on treatment of gas mask phobia.1,2 I basically advised desensitization. Start by watching TV in it for 5 minutes. Graduate to ironing your uniform in the mask. Go then to shorter runs. Work up to the 12-mile road march.
In my second tour in Korea, we had exercises where we simulated being hit by nerve agents and had to operate the hospital for days at a time in partial or full PPE. It was tough but we did it, and felt more confident about surviving attacks from North Korea.
So back to the pandemic present. I have gotten more used to my constant wearing of a surgical mask. I get anxious when I see others with masks below their noses.
The pandemic is not going away anytime soon, in my opinion. Furthermore, there are other viruses that are worse, such as Ebola. It is only a matter of time.
So, let us train with our PPE. If health care workers cannot tolerate them, use desensitization- and anxiety-reducing techniques to help them.
There are no easy answers here, in the time of the COVID pandemic. However, we owe it to ourselves, our patients, and society to do the best we can.
References
1. Ritchie EC. Milit Med. 1992 Feb;157(2):104-6.
2. Ritchie EC. Milit Med. 2001 Dec;166. Suppl. 2(1)83-4.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington. She has no disclosures and can be reached at [email protected].
A few months ago, I published a short thought piece on the use of “sitters” with patients who were COVID-19 positive, or patients under investigation. In it, I recommended the use of telesitters for those who normally would warrant a human sitter, to decrease the discomfort of sitting in full personal protective equipment (PPE) (gown, mask, gloves, etc.) while monitoring a suicidal patient.
I received several queries, which I want to address here. In addition, I want to draw from my Army days in terms of the claustrophobia often experienced with PPE.
The first of the questions was about evidence-based practices. The second was about the discomfort of having sitters sit for many hours in the full gear.
I do not know of any evidence-based practices, but I hope we will develop them.
I agree that spending many hours in full PPE can be discomforting, which is why I wrote the essay.
As far as lessons learned from the Army time, I briefly learned how to wear a “gas mask” or Mission-Oriented Protective Posture (MOPP gear) while at Fort Bragg. We were run through the “gas chamber,” where sergeants released tear gas while we had the mask on. We were then asked to lift it up, and then tearing and sputtering, we could leave the small wooden building.
We wore the mask as part of our Army gear, usually on the right leg. After that, I mainly used the protective mask in its bag as a pillow when I was in the field.
Fast forward to August 1990. I arrived at Camp Casey, near the Korean demilitarized zone. Four days later, Saddam Hussein invaded Kuwait. The gas mask moved from a pillow to something we had to wear while doing 12-mile road marches in “full ruck.” In full ruck, you have your uniform on, with TA-50, knapsack, and weapon. No, I do not remember any more what TA-50 stands for, but essentially it is the webbing that holds your bullets and bandages.
Many could not tolerate it. They developed claustrophobia – sweating, air hunger, and panic. If stationed in the Gulf for Operation Desert Storm, they were evacuated home.
I wrote a couple of short articles on treatment of gas mask phobia.1,2 I basically advised desensitization. Start by watching TV in it for 5 minutes. Graduate to ironing your uniform in the mask. Go then to shorter runs. Work up to the 12-mile road march.
In my second tour in Korea, we had exercises where we simulated being hit by nerve agents and had to operate the hospital for days at a time in partial or full PPE. It was tough but we did it, and felt more confident about surviving attacks from North Korea.
So back to the pandemic present. I have gotten more used to my constant wearing of a surgical mask. I get anxious when I see others with masks below their noses.
The pandemic is not going away anytime soon, in my opinion. Furthermore, there are other viruses that are worse, such as Ebola. It is only a matter of time.
So, let us train with our PPE. If health care workers cannot tolerate them, use desensitization- and anxiety-reducing techniques to help them.
There are no easy answers here, in the time of the COVID pandemic. However, we owe it to ourselves, our patients, and society to do the best we can.
References
1. Ritchie EC. Milit Med. 1992 Feb;157(2):104-6.
2. Ritchie EC. Milit Med. 2001 Dec;166. Suppl. 2(1)83-4.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington. She has no disclosures and can be reached at [email protected].
A few months ago, I published a short thought piece on the use of “sitters” with patients who were COVID-19 positive, or patients under investigation. In it, I recommended the use of telesitters for those who normally would warrant a human sitter, to decrease the discomfort of sitting in full personal protective equipment (PPE) (gown, mask, gloves, etc.) while monitoring a suicidal patient.
I received several queries, which I want to address here. In addition, I want to draw from my Army days in terms of the claustrophobia often experienced with PPE.
The first of the questions was about evidence-based practices. The second was about the discomfort of having sitters sit for many hours in the full gear.
I do not know of any evidence-based practices, but I hope we will develop them.
I agree that spending many hours in full PPE can be discomforting, which is why I wrote the essay.
As far as lessons learned from the Army time, I briefly learned how to wear a “gas mask” or Mission-Oriented Protective Posture (MOPP gear) while at Fort Bragg. We were run through the “gas chamber,” where sergeants released tear gas while we had the mask on. We were then asked to lift it up, and then tearing and sputtering, we could leave the small wooden building.
We wore the mask as part of our Army gear, usually on the right leg. After that, I mainly used the protective mask in its bag as a pillow when I was in the field.
Fast forward to August 1990. I arrived at Camp Casey, near the Korean demilitarized zone. Four days later, Saddam Hussein invaded Kuwait. The gas mask moved from a pillow to something we had to wear while doing 12-mile road marches in “full ruck.” In full ruck, you have your uniform on, with TA-50, knapsack, and weapon. No, I do not remember any more what TA-50 stands for, but essentially it is the webbing that holds your bullets and bandages.
Many could not tolerate it. They developed claustrophobia – sweating, air hunger, and panic. If stationed in the Gulf for Operation Desert Storm, they were evacuated home.
I wrote a couple of short articles on treatment of gas mask phobia.1,2 I basically advised desensitization. Start by watching TV in it for 5 minutes. Graduate to ironing your uniform in the mask. Go then to shorter runs. Work up to the 12-mile road march.
In my second tour in Korea, we had exercises where we simulated being hit by nerve agents and had to operate the hospital for days at a time in partial or full PPE. It was tough but we did it, and felt more confident about surviving attacks from North Korea.
So back to the pandemic present. I have gotten more used to my constant wearing of a surgical mask. I get anxious when I see others with masks below their noses.
The pandemic is not going away anytime soon, in my opinion. Furthermore, there are other viruses that are worse, such as Ebola. It is only a matter of time.
So, let us train with our PPE. If health care workers cannot tolerate them, use desensitization- and anxiety-reducing techniques to help them.
There are no easy answers here, in the time of the COVID pandemic. However, we owe it to ourselves, our patients, and society to do the best we can.
References
1. Ritchie EC. Milit Med. 1992 Feb;157(2):104-6.
2. Ritchie EC. Milit Med. 2001 Dec;166. Suppl. 2(1)83-4.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington. She has no disclosures and can be reached at [email protected].
Children and COVID-19: New cases may be leveling off
Growth in new pediatric COVID-19 cases has evened out in recent weeks, but children now represent 10% of all COVID-19 cases in the United States, and that measurement has been rising throughout the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
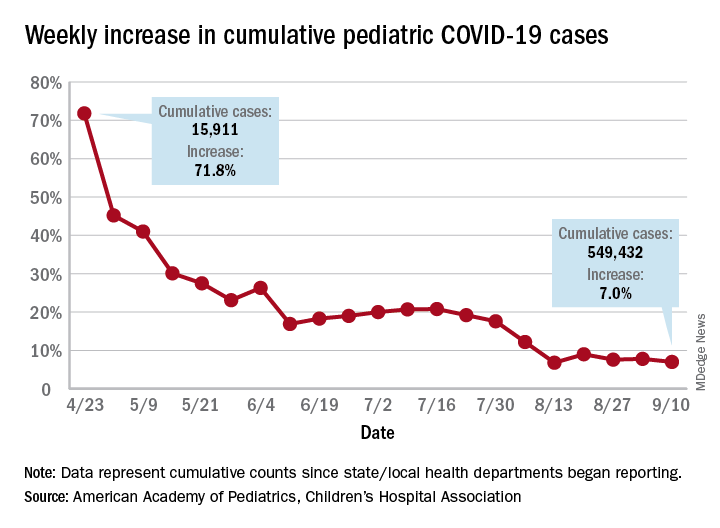
the AAP and the CHA said in the report, based on data from 49 states (New York City is included but not New York state), the District of Columbia, Puerto Rico, and Guam.
The weekly percentage of increase in the number of new cases has not reached double digits since early August and has been no higher than 7.8% over the last 3 weeks. The number of child COVID-19 cases, however, has finally reached 10% of the total for Americans of all ages, which stands at 5.49 million in the jurisdictions included in the report, the AHA and CHA reported.
Measures, however, continue to show low levels of severe illness in children, they noted, including the following:
- Child cases as a proportion of all COVID-19 hospitalizations: 1.7%.
- Hospitalization rate for children: 1.8%.
- Child deaths as a proportion of all deaths: 0.07%.
- Percent of child cases resulting in death: 0.01%.
The number of cumulative cases per 100,000 children is now up to 728.5 nationally, with a range by state that goes from 154.0 in Vermont to 1,670.3 in Tennessee, which is one of only two states reporting cases in those aged 0-20 years as children (the other is South Carolina). The age range for children is 0-17 or 0-19 for most other states, although Florida uses a range of 0-14, the report notes.
Other than Tennessee, there are 10 states with overall rates higher than 1,000 COVID-19 cases per 100,000 children, and there are nine states with cumulative totals over 15,000 cases (California is the highest with just over 75,000), according to the report.
Growth in new pediatric COVID-19 cases has evened out in recent weeks, but children now represent 10% of all COVID-19 cases in the United States, and that measurement has been rising throughout the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and the CHA said in the report, based on data from 49 states (New York City is included but not New York state), the District of Columbia, Puerto Rico, and Guam.
The weekly percentage of increase in the number of new cases has not reached double digits since early August and has been no higher than 7.8% over the last 3 weeks. The number of child COVID-19 cases, however, has finally reached 10% of the total for Americans of all ages, which stands at 5.49 million in the jurisdictions included in the report, the AHA and CHA reported.
Measures, however, continue to show low levels of severe illness in children, they noted, including the following:
- Child cases as a proportion of all COVID-19 hospitalizations: 1.7%.
- Hospitalization rate for children: 1.8%.
- Child deaths as a proportion of all deaths: 0.07%.
- Percent of child cases resulting in death: 0.01%.
The number of cumulative cases per 100,000 children is now up to 728.5 nationally, with a range by state that goes from 154.0 in Vermont to 1,670.3 in Tennessee, which is one of only two states reporting cases in those aged 0-20 years as children (the other is South Carolina). The age range for children is 0-17 or 0-19 for most other states, although Florida uses a range of 0-14, the report notes.
Other than Tennessee, there are 10 states with overall rates higher than 1,000 COVID-19 cases per 100,000 children, and there are nine states with cumulative totals over 15,000 cases (California is the highest with just over 75,000), according to the report.
Growth in new pediatric COVID-19 cases has evened out in recent weeks, but children now represent 10% of all COVID-19 cases in the United States, and that measurement has been rising throughout the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and the CHA said in the report, based on data from 49 states (New York City is included but not New York state), the District of Columbia, Puerto Rico, and Guam.
The weekly percentage of increase in the number of new cases has not reached double digits since early August and has been no higher than 7.8% over the last 3 weeks. The number of child COVID-19 cases, however, has finally reached 10% of the total for Americans of all ages, which stands at 5.49 million in the jurisdictions included in the report, the AHA and CHA reported.
Measures, however, continue to show low levels of severe illness in children, they noted, including the following:
- Child cases as a proportion of all COVID-19 hospitalizations: 1.7%.
- Hospitalization rate for children: 1.8%.
- Child deaths as a proportion of all deaths: 0.07%.
- Percent of child cases resulting in death: 0.01%.
The number of cumulative cases per 100,000 children is now up to 728.5 nationally, with a range by state that goes from 154.0 in Vermont to 1,670.3 in Tennessee, which is one of only two states reporting cases in those aged 0-20 years as children (the other is South Carolina). The age range for children is 0-17 or 0-19 for most other states, although Florida uses a range of 0-14, the report notes.
Other than Tennessee, there are 10 states with overall rates higher than 1,000 COVID-19 cases per 100,000 children, and there are nine states with cumulative totals over 15,000 cases (California is the highest with just over 75,000), according to the report.
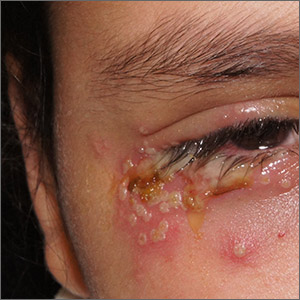
Painful periocular rash
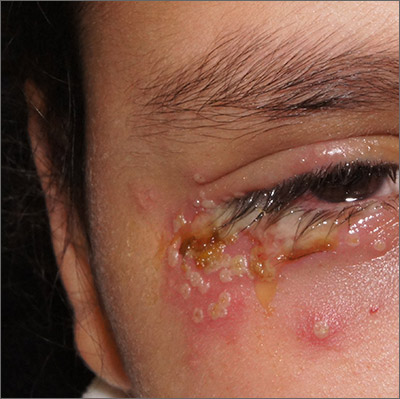
This patient was given a diagnosis of primary herpes simplex virus (HSV) based on the appearance of her eyelid. Swabs were performed for bacterial culture, and polymerase chain reaction (PCR) testing was done for HSV and varicella, but results were pending prior to her transfer to the Emergency Department (ED).
The patient was given a single dose of 800 mg oral acyclovir (200 mg/5mL) and 500 mg of oral cephalexin (250 mg/5mL) and referred to the ED for a more detailed eye exam and to exclude orbital erosions.
HSV classically causes clustered vesicles on an erythematous base. Superinfection with skin flora can cause pustules instead of vesicles. Severe complications of HSV can include widespread skin involvement, eczema herpeticum, local destruction, central nervous system involvement, throat infections (affecting airway and oral intake), and dissemination in immunocompromised hosts. Ocular or periorbital infections increase the risk of keratitis, corneal ulcers, and loss of sight. Viral involvement of the cornea is best seen with fluorescein staining.
In cases like this one, PCR is the preferred method of testing over viral cultures or serology, given its speed, accuracy, and temporal relevance. Ophthalmology referral is warranted, although it should not delay treatment. Topical and oral antivirals are both effective when treating corneal disease; patient preference should be considered.
Most cases of HSV may resolve without treatment; however, treatment started while vesicles are present and within 72 hours of infection may shorten the time of viral replication and prevent progression to stromal involvement.
After a 12-hour wait in the ED, this patient was seen by an ophthalmology resident who did not observe orbital erosions but did note umbilication and misdiagnosed molluscum contagiosum. Umbilication is not pathognomonic for molluscum; few experienced in diagnosing molluscum contagiosum would make this error.
The patient was instructed to stop the acyclovir. Two days later when the PCR came back positive for HSV-1 and the bacterial culture confirmed growth of superimposed Staphylococcus aureus, the patient had been lost to follow-up. A better approach would have been for the ophthalmology resident to continue the acyclovir until PCR excluded herpetic disease.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Barker NH. Ocular herpes simplex. BMJ Clin Evid. 2008;2008:0707.

This patient was given a diagnosis of primary herpes simplex virus (HSV) based on the appearance of her eyelid. Swabs were performed for bacterial culture, and polymerase chain reaction (PCR) testing was done for HSV and varicella, but results were pending prior to her transfer to the Emergency Department (ED).
The patient was given a single dose of 800 mg oral acyclovir (200 mg/5mL) and 500 mg of oral cephalexin (250 mg/5mL) and referred to the ED for a more detailed eye exam and to exclude orbital erosions.
HSV classically causes clustered vesicles on an erythematous base. Superinfection with skin flora can cause pustules instead of vesicles. Severe complications of HSV can include widespread skin involvement, eczema herpeticum, local destruction, central nervous system involvement, throat infections (affecting airway and oral intake), and dissemination in immunocompromised hosts. Ocular or periorbital infections increase the risk of keratitis, corneal ulcers, and loss of sight. Viral involvement of the cornea is best seen with fluorescein staining.
In cases like this one, PCR is the preferred method of testing over viral cultures or serology, given its speed, accuracy, and temporal relevance. Ophthalmology referral is warranted, although it should not delay treatment. Topical and oral antivirals are both effective when treating corneal disease; patient preference should be considered.
Most cases of HSV may resolve without treatment; however, treatment started while vesicles are present and within 72 hours of infection may shorten the time of viral replication and prevent progression to stromal involvement.
After a 12-hour wait in the ED, this patient was seen by an ophthalmology resident who did not observe orbital erosions but did note umbilication and misdiagnosed molluscum contagiosum. Umbilication is not pathognomonic for molluscum; few experienced in diagnosing molluscum contagiosum would make this error.
The patient was instructed to stop the acyclovir. Two days later when the PCR came back positive for HSV-1 and the bacterial culture confirmed growth of superimposed Staphylococcus aureus, the patient had been lost to follow-up. A better approach would have been for the ophthalmology resident to continue the acyclovir until PCR excluded herpetic disease.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

This patient was given a diagnosis of primary herpes simplex virus (HSV) based on the appearance of her eyelid. Swabs were performed for bacterial culture, and polymerase chain reaction (PCR) testing was done for HSV and varicella, but results were pending prior to her transfer to the Emergency Department (ED).
The patient was given a single dose of 800 mg oral acyclovir (200 mg/5mL) and 500 mg of oral cephalexin (250 mg/5mL) and referred to the ED for a more detailed eye exam and to exclude orbital erosions.
HSV classically causes clustered vesicles on an erythematous base. Superinfection with skin flora can cause pustules instead of vesicles. Severe complications of HSV can include widespread skin involvement, eczema herpeticum, local destruction, central nervous system involvement, throat infections (affecting airway and oral intake), and dissemination in immunocompromised hosts. Ocular or periorbital infections increase the risk of keratitis, corneal ulcers, and loss of sight. Viral involvement of the cornea is best seen with fluorescein staining.
In cases like this one, PCR is the preferred method of testing over viral cultures or serology, given its speed, accuracy, and temporal relevance. Ophthalmology referral is warranted, although it should not delay treatment. Topical and oral antivirals are both effective when treating corneal disease; patient preference should be considered.
Most cases of HSV may resolve without treatment; however, treatment started while vesicles are present and within 72 hours of infection may shorten the time of viral replication and prevent progression to stromal involvement.
After a 12-hour wait in the ED, this patient was seen by an ophthalmology resident who did not observe orbital erosions but did note umbilication and misdiagnosed molluscum contagiosum. Umbilication is not pathognomonic for molluscum; few experienced in diagnosing molluscum contagiosum would make this error.
The patient was instructed to stop the acyclovir. Two days later when the PCR came back positive for HSV-1 and the bacterial culture confirmed growth of superimposed Staphylococcus aureus, the patient had been lost to follow-up. A better approach would have been for the ophthalmology resident to continue the acyclovir until PCR excluded herpetic disease.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Barker NH. Ocular herpes simplex. BMJ Clin Evid. 2008;2008:0707.
Barker NH. Ocular herpes simplex. BMJ Clin Evid. 2008;2008:0707.
Tough to tell COVID from smoke inhalation symptoms — And flu season’s coming
The patients walk into Dr. Melissa Marshall’s community clinics in Northern California with the telltale symptoms. They’re having trouble breathing. It may even hurt to inhale. They’ve got a cough, and the sore throat is definitely there.
A straight case of COVID-19? Not so fast. This is wildfire country.
Up and down the West Coast, hospitals and health facilities are reporting an influx of patients with problems most likely related to smoke inhalation. As fires rage largely uncontrolled amid dry heat and high winds, smoke and ash are billowing and settling on coastal areas like San Francisco and cities and towns hundreds of miles inland as well, turning the sky orange or gray and making even ordinary breathing difficult.
But that, Marshall said, is only part of the challenge.
“Obviously, there’s overlap in the symptoms,” said Marshall, the CEO of CommuniCare, a collection of six clinics in Yolo County, near Sacramento, that treats mostly underinsured and uninsured patients. “Any time someone comes in with even some of those symptoms, we ask ourselves, ‘Is it COVID?’ At the end of the day, clinically speaking, I still want to rule out the virus.”
The protocol is to treat the symptoms, whatever their cause, while recommending that the patient quarantine until test results for the virus come back, she said.
It is a scene playing out in numerous hospitals. Administrators and physicians, finely attuned to COVID-19’s ability to spread quickly and wreak havoc, simply won’t take a chance when they recognize symptoms that could emanate from the virus.
“We’ve seen an increase in patients presenting to the emergency department with respiratory distress,” said Dr. Nanette Mickiewicz, president and CEO of Dominican Hospital in Santa Cruz. “As this can also be a symptom of COVID-19, we’re treating these patients as we would any person under investigation for coronavirus until we can rule them out through our screening process.” During the workup, symptoms that are more specific to COVID-19, like fever, would become apparent.
For the workers at Dominican, the issue moved to the top of the list quickly. Santa Cruz and San Mateo counties have borne the brunt of the CZU Lightning Complex fires, which as of Sept. 10 had burned more than 86,000 acres, destroying 1,100 structures and threatening more than 7,600 others. Nearly a month after they began, the fires were approximately 84% contained, but thousands of people remained evacuated.
Dominican, a Dignity Health hospital, is “open, safe and providing care,” Mickiewicz said. Multiple tents erected outside the building serve as an extension of its ER waiting room. They also are used to perform what has come to be understood as an essential role: separating those with symptoms of COVID-19 from those without.
At the two Solano County hospitals operated by NorthBay Healthcare, the path of some of the wildfires prompted officials to review their evacuation procedures, said spokesperson Steve Huddleston. They ultimately avoided the need to evacuate patients, and new ones arrived with COVID-like symptoms that may actually have been from smoke inhalation.
Huddleston said NorthBay’s intake process “calls for anyone with COVID characteristics to be handled as [a] patient under investigation for COVID, which means they’re separated, screened and managed by staff in special PPE.” At the two hospitals, which have handled nearly 200 COVID cases so far, the protocol is well established.
Hospitals in California, though not under siege in most cases, are dealing with multiple issues they might typically face only sporadically. In Napa County, Adventist Health St. Helena Hospital evacuated 51 patients on a single August night as a fire approached, moving them to 10 other facilities according to their needs and bed space. After a 10-day closure, the hospital was allowed to reopen as evacuation orders were lifted, the fire having been contained some distance away.
The wildfires are also taking a personal toll on health care workers. CommuniCare’s Marshall lost her family’s home in rural Winters, along with 20 acres of olive trees and other plantings that surrounded it, in the Aug. 19 fires that swept through Solano County.
“They called it a ‘firenado,’ ” Marshall said. An apparent confluence of three fires raged out of control, demolishing thousands of acres. With her family safely accounted for and temporary housing arranged by a friend, she returned to work. “Our clinics interact with a very vulnerable population,” she said, “and this is a critical time for them.”
While she pondered how her family would rebuild, the CEO was faced with another immediate crisis: the clinic’s shortage of supplies. Last month, CommuniCare got down to 19 COVID test kits on hand, and ran so low on swabs “that we were literally turning to our veterinary friends for reinforcements,” the doctor said. The clinic’s COVID test results, meanwhile, were taking nearly two weeks to be returned from an overwhelmed outside lab, rendering contact tracing almost useless.
Those situations have been addressed, at least temporarily, Marshall said. But although the West Coast is in the most dangerous time of year for wildfires, generally September to December, another complication for health providers lies on the horizon: flu season.
The Southern Hemisphere, whose influenza trends during our summer months typically predict what’s to come for the U.S., has had very little of the disease this year, presumably because of restricted travel, social distancing and face masks. But it’s too early to be sure what the U.S. flu season will entail.
“You can start to see some cases of the flu in late October,” said Marshall, “and the reality is that it’s going to carry a number of characteristics that could also be symptomatic of COVID. And nothing changes: You have to rule it out, just to eliminate the risk.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This KHN story first published on California Healthline, a service of the California Health Care Foundation.
The patients walk into Dr. Melissa Marshall’s community clinics in Northern California with the telltale symptoms. They’re having trouble breathing. It may even hurt to inhale. They’ve got a cough, and the sore throat is definitely there.
A straight case of COVID-19? Not so fast. This is wildfire country.
Up and down the West Coast, hospitals and health facilities are reporting an influx of patients with problems most likely related to smoke inhalation. As fires rage largely uncontrolled amid dry heat and high winds, smoke and ash are billowing and settling on coastal areas like San Francisco and cities and towns hundreds of miles inland as well, turning the sky orange or gray and making even ordinary breathing difficult.
But that, Marshall said, is only part of the challenge.
“Obviously, there’s overlap in the symptoms,” said Marshall, the CEO of CommuniCare, a collection of six clinics in Yolo County, near Sacramento, that treats mostly underinsured and uninsured patients. “Any time someone comes in with even some of those symptoms, we ask ourselves, ‘Is it COVID?’ At the end of the day, clinically speaking, I still want to rule out the virus.”
The protocol is to treat the symptoms, whatever their cause, while recommending that the patient quarantine until test results for the virus come back, she said.
It is a scene playing out in numerous hospitals. Administrators and physicians, finely attuned to COVID-19’s ability to spread quickly and wreak havoc, simply won’t take a chance when they recognize symptoms that could emanate from the virus.
“We’ve seen an increase in patients presenting to the emergency department with respiratory distress,” said Dr. Nanette Mickiewicz, president and CEO of Dominican Hospital in Santa Cruz. “As this can also be a symptom of COVID-19, we’re treating these patients as we would any person under investigation for coronavirus until we can rule them out through our screening process.” During the workup, symptoms that are more specific to COVID-19, like fever, would become apparent.
For the workers at Dominican, the issue moved to the top of the list quickly. Santa Cruz and San Mateo counties have borne the brunt of the CZU Lightning Complex fires, which as of Sept. 10 had burned more than 86,000 acres, destroying 1,100 structures and threatening more than 7,600 others. Nearly a month after they began, the fires were approximately 84% contained, but thousands of people remained evacuated.
Dominican, a Dignity Health hospital, is “open, safe and providing care,” Mickiewicz said. Multiple tents erected outside the building serve as an extension of its ER waiting room. They also are used to perform what has come to be understood as an essential role: separating those with symptoms of COVID-19 from those without.
At the two Solano County hospitals operated by NorthBay Healthcare, the path of some of the wildfires prompted officials to review their evacuation procedures, said spokesperson Steve Huddleston. They ultimately avoided the need to evacuate patients, and new ones arrived with COVID-like symptoms that may actually have been from smoke inhalation.
Huddleston said NorthBay’s intake process “calls for anyone with COVID characteristics to be handled as [a] patient under investigation for COVID, which means they’re separated, screened and managed by staff in special PPE.” At the two hospitals, which have handled nearly 200 COVID cases so far, the protocol is well established.
Hospitals in California, though not under siege in most cases, are dealing with multiple issues they might typically face only sporadically. In Napa County, Adventist Health St. Helena Hospital evacuated 51 patients on a single August night as a fire approached, moving them to 10 other facilities according to their needs and bed space. After a 10-day closure, the hospital was allowed to reopen as evacuation orders were lifted, the fire having been contained some distance away.
The wildfires are also taking a personal toll on health care workers. CommuniCare’s Marshall lost her family’s home in rural Winters, along with 20 acres of olive trees and other plantings that surrounded it, in the Aug. 19 fires that swept through Solano County.
“They called it a ‘firenado,’ ” Marshall said. An apparent confluence of three fires raged out of control, demolishing thousands of acres. With her family safely accounted for and temporary housing arranged by a friend, she returned to work. “Our clinics interact with a very vulnerable population,” she said, “and this is a critical time for them.”
While she pondered how her family would rebuild, the CEO was faced with another immediate crisis: the clinic’s shortage of supplies. Last month, CommuniCare got down to 19 COVID test kits on hand, and ran so low on swabs “that we were literally turning to our veterinary friends for reinforcements,” the doctor said. The clinic’s COVID test results, meanwhile, were taking nearly two weeks to be returned from an overwhelmed outside lab, rendering contact tracing almost useless.
Those situations have been addressed, at least temporarily, Marshall said. But although the West Coast is in the most dangerous time of year for wildfires, generally September to December, another complication for health providers lies on the horizon: flu season.
The Southern Hemisphere, whose influenza trends during our summer months typically predict what’s to come for the U.S., has had very little of the disease this year, presumably because of restricted travel, social distancing and face masks. But it’s too early to be sure what the U.S. flu season will entail.
“You can start to see some cases of the flu in late October,” said Marshall, “and the reality is that it’s going to carry a number of characteristics that could also be symptomatic of COVID. And nothing changes: You have to rule it out, just to eliminate the risk.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This KHN story first published on California Healthline, a service of the California Health Care Foundation.
The patients walk into Dr. Melissa Marshall’s community clinics in Northern California with the telltale symptoms. They’re having trouble breathing. It may even hurt to inhale. They’ve got a cough, and the sore throat is definitely there.
A straight case of COVID-19? Not so fast. This is wildfire country.
Up and down the West Coast, hospitals and health facilities are reporting an influx of patients with problems most likely related to smoke inhalation. As fires rage largely uncontrolled amid dry heat and high winds, smoke and ash are billowing and settling on coastal areas like San Francisco and cities and towns hundreds of miles inland as well, turning the sky orange or gray and making even ordinary breathing difficult.
But that, Marshall said, is only part of the challenge.
“Obviously, there’s overlap in the symptoms,” said Marshall, the CEO of CommuniCare, a collection of six clinics in Yolo County, near Sacramento, that treats mostly underinsured and uninsured patients. “Any time someone comes in with even some of those symptoms, we ask ourselves, ‘Is it COVID?’ At the end of the day, clinically speaking, I still want to rule out the virus.”
The protocol is to treat the symptoms, whatever their cause, while recommending that the patient quarantine until test results for the virus come back, she said.
It is a scene playing out in numerous hospitals. Administrators and physicians, finely attuned to COVID-19’s ability to spread quickly and wreak havoc, simply won’t take a chance when they recognize symptoms that could emanate from the virus.
“We’ve seen an increase in patients presenting to the emergency department with respiratory distress,” said Dr. Nanette Mickiewicz, president and CEO of Dominican Hospital in Santa Cruz. “As this can also be a symptom of COVID-19, we’re treating these patients as we would any person under investigation for coronavirus until we can rule them out through our screening process.” During the workup, symptoms that are more specific to COVID-19, like fever, would become apparent.
For the workers at Dominican, the issue moved to the top of the list quickly. Santa Cruz and San Mateo counties have borne the brunt of the CZU Lightning Complex fires, which as of Sept. 10 had burned more than 86,000 acres, destroying 1,100 structures and threatening more than 7,600 others. Nearly a month after they began, the fires were approximately 84% contained, but thousands of people remained evacuated.
Dominican, a Dignity Health hospital, is “open, safe and providing care,” Mickiewicz said. Multiple tents erected outside the building serve as an extension of its ER waiting room. They also are used to perform what has come to be understood as an essential role: separating those with symptoms of COVID-19 from those without.
At the two Solano County hospitals operated by NorthBay Healthcare, the path of some of the wildfires prompted officials to review their evacuation procedures, said spokesperson Steve Huddleston. They ultimately avoided the need to evacuate patients, and new ones arrived with COVID-like symptoms that may actually have been from smoke inhalation.
Huddleston said NorthBay’s intake process “calls for anyone with COVID characteristics to be handled as [a] patient under investigation for COVID, which means they’re separated, screened and managed by staff in special PPE.” At the two hospitals, which have handled nearly 200 COVID cases so far, the protocol is well established.
Hospitals in California, though not under siege in most cases, are dealing with multiple issues they might typically face only sporadically. In Napa County, Adventist Health St. Helena Hospital evacuated 51 patients on a single August night as a fire approached, moving them to 10 other facilities according to their needs and bed space. After a 10-day closure, the hospital was allowed to reopen as evacuation orders were lifted, the fire having been contained some distance away.
The wildfires are also taking a personal toll on health care workers. CommuniCare’s Marshall lost her family’s home in rural Winters, along with 20 acres of olive trees and other plantings that surrounded it, in the Aug. 19 fires that swept through Solano County.
“They called it a ‘firenado,’ ” Marshall said. An apparent confluence of three fires raged out of control, demolishing thousands of acres. With her family safely accounted for and temporary housing arranged by a friend, she returned to work. “Our clinics interact with a very vulnerable population,” she said, “and this is a critical time for them.”
While she pondered how her family would rebuild, the CEO was faced with another immediate crisis: the clinic’s shortage of supplies. Last month, CommuniCare got down to 19 COVID test kits on hand, and ran so low on swabs “that we were literally turning to our veterinary friends for reinforcements,” the doctor said. The clinic’s COVID test results, meanwhile, were taking nearly two weeks to be returned from an overwhelmed outside lab, rendering contact tracing almost useless.
Those situations have been addressed, at least temporarily, Marshall said. But although the West Coast is in the most dangerous time of year for wildfires, generally September to December, another complication for health providers lies on the horizon: flu season.
The Southern Hemisphere, whose influenza trends during our summer months typically predict what’s to come for the U.S., has had very little of the disease this year, presumably because of restricted travel, social distancing and face masks. But it’s too early to be sure what the U.S. flu season will entail.
“You can start to see some cases of the flu in late October,” said Marshall, “and the reality is that it’s going to carry a number of characteristics that could also be symptomatic of COVID. And nothing changes: You have to rule it out, just to eliminate the risk.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This KHN story first published on California Healthline, a service of the California Health Care Foundation.
Worry over family, friends the main driver of COVID-19 stress
Individuals are more worried about family members becoming ill with COVID-19 or about unknowingly transmitting the disease to family members than they are about contracting it themselves, results of a new survey show.
Investigators surveyed over 3,000 adults, using an online questionnaire. Of the respondents, about 20% were health care workers, and most were living in locations with active stay-at-home orders at the time of the survey.
Close to half of participants were worried about family members contracting the virus, one third were worried about unknowingly infecting others, and 20% were worried about contracting the virus themselves.
“We were a little surprised to see that people were more concerned about others than about themselves, specifically worrying about whether a family member would contract COVID-19 and whether they might unintentionally infect others,” lead author Ran Barzilay, MD, PhD, child and adolescent psychiatrist at the Children’s Hospital of Philadelphia (CHOP), told Medscape Medical News.
The study was published online August 20 in Translational Psychiatry.
Interactive platform
“The pandemic has provided a unique opportunity to study resilience in healthcare professionals and others,” said Barzilay, assistant professor at the Lifespan Brain Institute, a collaboration between CHOP and the University of Pennsylvania, under the directorship of Raquel Gur, MD, PhD.
“After the pandemic broke out in March, we launched a website in early April where we surveyed people for levels of resilience, mental health, and well-being during the outbreak,” he added.
Survey participants then shared it with their contacts.
“To date, over 7000 people have completed it – mostly from the US but also from Israel,” Barzilay said.
The survey was anonymous, but participants could choose to have follow-up contact. The survey included an interactive 21-item resilience questionnaire and an assessment of COVID-19-related items related to worries concerning the following: contracting, dying from, or currently having the illness; having a family member contract the illness; unknowingly infecting others; and experiencing significant financial burden.
A total of 1350 participants took a second survey on anxiety and depression that utilized the Generalized Anxiety Disorder–7 and the Patient Health Questionnaire–2.
“What makes the survey unique is that it’s not just a means of collecting data but also an interactive platform that gives participants immediate personalized feedback, based on their responses to the resilience and well-being surveys, with practical tips and recommendations for stress management and ways of boosting resilience,” Barzilay said.
Tend and befriend
Ten days into the survey, data were available on 3,042 participants (64% women, 54% with advanced education, 20.5% health care providers), who ranged in age from 18 to 70 years (mean [SD], 38.9 [11.9] years).
After accounting for covariates, the researchers found that participants reported more distress about family members contracting COVID-19 and about unknowingly infecting others than about getting COVID-19 themselves (48.5% and 36% vs. 19.9%, respectively; P < .0005).
Increased COVID-19-related worries were associated with 22% higher anxiety and 16.1% higher depression scores; women had higher scores than men on both.
Each 1-SD increase in the composite score of COVID-19 worries was associated with over twice the increased probability of generalized anxiety and depression (odds ratio, 2.23; 95% confidence interval, 1.88-2.65; and OR, 1.67; 95% CI, 1.41-1.98, respectively; for both, P < .001).
On the other hand, for every 1-SD increase in the resilience score, there was a 64.9% decrease in the possibility of screening positive for generalized anxiety disorder and a 69.3% decrease in the possibility of screening positive for depression (for both, P < .0001).
Compared to participants from Israel, US participants were “more stressed” about contracting, dying from, and currently having COVID-19 themselves. Overall, Israeli participants scored higher than US participants on the resilience scale.
Rates of anxiety and depression did not differ significantly between healthcare providers and others. Health care providers worried more about contracting COVID-19 themselves and worried less about finances after COVID-19.
The authors propose that survey participants were more worried about others than about themselves because of “prosocial behavior under stress” and “tend-and-befriend,” whereby, “in response to threat, humans tend to protect their close ones (tending) and seek out their social group for mutual defense (befriending).”
This type of altruistic behavior has been “described in acute situations throughout history” and has been “linked to mechanisms of resilience for overcoming adversity,” the authors indicate.
Demographic biases
Commenting on the findings for Medscape Medical News, Golnaz Tabibnia, PhD, a neuroscientist at the University of California, Irvine, who was not involved in the research, suggested that although higher resilience scores were associated with lower COVID-related worries, it is possible, “as the authors suggest, that having more resilience resources makes you less worried, but the causality could go the other direction as well, and less worry/rumination may lead to more resilience.”
Also commenting on the study for Medscape Medical News, Christiaan Vinkers, MD, PhD, a psychiatrist at the Amsterdam University Medical Center, Amsterdam, the Netherlands, said it was noteworthy that healthcare providers reported similar levels of mood and anxiety symptoms, compared to others.
“This is encouraging, as it suggests adequate resilience levels in professionals who work in the front lines of the COVID-19 pandemic,” he said.
Resilience occurs not only at the individual level but also at the community level, which may help explain the striking differences in COVID-19-related worries and anxiety between participants from the United States and Israel, Vinkers added.
E. Alison Holman, PhD, professor, Sue and Bill Gross School of Nursing, University of California, Irvine, noted that respondents were predominantly white, female, and had relatively high incomes, “suggesting strong demographic biases in those who chose to participate.”
Holman, who was not involved with the study, told Medscape Medical News that the “findings do not address the real impact of COVID-19 on the hardest-hit communities in America – poor, Black, and Latinx communities, where a large proportion of essential workers live.”
Barzilay acknowledged that, “unfortunately, because of the way the study was circulated, it did not reach minorities, which is one of the things we want to improve.”
The study is ongoing and has been translated into Spanish, French, and Hebrew. The team plans to collect data on diverse populations.
The study was supported by grants from the National Institute of Mental Health, the Lifespan Brain Institute of Children’s Hospital of Philadelphia, Penn Medicine, the University of Pennsylvania, and in part by the Zuckerman STEM Leadership Program. Barzilay serves on the scientific board and reports stock ownership in Taliaz Health. The other authors, Golnaz, Vinkers, and Holman have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Individuals are more worried about family members becoming ill with COVID-19 or about unknowingly transmitting the disease to family members than they are about contracting it themselves, results of a new survey show.
Investigators surveyed over 3,000 adults, using an online questionnaire. Of the respondents, about 20% were health care workers, and most were living in locations with active stay-at-home orders at the time of the survey.
Close to half of participants were worried about family members contracting the virus, one third were worried about unknowingly infecting others, and 20% were worried about contracting the virus themselves.
“We were a little surprised to see that people were more concerned about others than about themselves, specifically worrying about whether a family member would contract COVID-19 and whether they might unintentionally infect others,” lead author Ran Barzilay, MD, PhD, child and adolescent psychiatrist at the Children’s Hospital of Philadelphia (CHOP), told Medscape Medical News.
The study was published online August 20 in Translational Psychiatry.
Interactive platform
“The pandemic has provided a unique opportunity to study resilience in healthcare professionals and others,” said Barzilay, assistant professor at the Lifespan Brain Institute, a collaboration between CHOP and the University of Pennsylvania, under the directorship of Raquel Gur, MD, PhD.
“After the pandemic broke out in March, we launched a website in early April where we surveyed people for levels of resilience, mental health, and well-being during the outbreak,” he added.
Survey participants then shared it with their contacts.
“To date, over 7000 people have completed it – mostly from the US but also from Israel,” Barzilay said.
The survey was anonymous, but participants could choose to have follow-up contact. The survey included an interactive 21-item resilience questionnaire and an assessment of COVID-19-related items related to worries concerning the following: contracting, dying from, or currently having the illness; having a family member contract the illness; unknowingly infecting others; and experiencing significant financial burden.
A total of 1350 participants took a second survey on anxiety and depression that utilized the Generalized Anxiety Disorder–7 and the Patient Health Questionnaire–2.
“What makes the survey unique is that it’s not just a means of collecting data but also an interactive platform that gives participants immediate personalized feedback, based on their responses to the resilience and well-being surveys, with practical tips and recommendations for stress management and ways of boosting resilience,” Barzilay said.
Tend and befriend
Ten days into the survey, data were available on 3,042 participants (64% women, 54% with advanced education, 20.5% health care providers), who ranged in age from 18 to 70 years (mean [SD], 38.9 [11.9] years).
After accounting for covariates, the researchers found that participants reported more distress about family members contracting COVID-19 and about unknowingly infecting others than about getting COVID-19 themselves (48.5% and 36% vs. 19.9%, respectively; P < .0005).
Increased COVID-19-related worries were associated with 22% higher anxiety and 16.1% higher depression scores; women had higher scores than men on both.
Each 1-SD increase in the composite score of COVID-19 worries was associated with over twice the increased probability of generalized anxiety and depression (odds ratio, 2.23; 95% confidence interval, 1.88-2.65; and OR, 1.67; 95% CI, 1.41-1.98, respectively; for both, P < .001).
On the other hand, for every 1-SD increase in the resilience score, there was a 64.9% decrease in the possibility of screening positive for generalized anxiety disorder and a 69.3% decrease in the possibility of screening positive for depression (for both, P < .0001).
Compared to participants from Israel, US participants were “more stressed” about contracting, dying from, and currently having COVID-19 themselves. Overall, Israeli participants scored higher than US participants on the resilience scale.
Rates of anxiety and depression did not differ significantly between healthcare providers and others. Health care providers worried more about contracting COVID-19 themselves and worried less about finances after COVID-19.
The authors propose that survey participants were more worried about others than about themselves because of “prosocial behavior under stress” and “tend-and-befriend,” whereby, “in response to threat, humans tend to protect their close ones (tending) and seek out their social group for mutual defense (befriending).”
This type of altruistic behavior has been “described in acute situations throughout history” and has been “linked to mechanisms of resilience for overcoming adversity,” the authors indicate.
Demographic biases
Commenting on the findings for Medscape Medical News, Golnaz Tabibnia, PhD, a neuroscientist at the University of California, Irvine, who was not involved in the research, suggested that although higher resilience scores were associated with lower COVID-related worries, it is possible, “as the authors suggest, that having more resilience resources makes you less worried, but the causality could go the other direction as well, and less worry/rumination may lead to more resilience.”
Also commenting on the study for Medscape Medical News, Christiaan Vinkers, MD, PhD, a psychiatrist at the Amsterdam University Medical Center, Amsterdam, the Netherlands, said it was noteworthy that healthcare providers reported similar levels of mood and anxiety symptoms, compared to others.
“This is encouraging, as it suggests adequate resilience levels in professionals who work in the front lines of the COVID-19 pandemic,” he said.
Resilience occurs not only at the individual level but also at the community level, which may help explain the striking differences in COVID-19-related worries and anxiety between participants from the United States and Israel, Vinkers added.
E. Alison Holman, PhD, professor, Sue and Bill Gross School of Nursing, University of California, Irvine, noted that respondents were predominantly white, female, and had relatively high incomes, “suggesting strong demographic biases in those who chose to participate.”
Holman, who was not involved with the study, told Medscape Medical News that the “findings do not address the real impact of COVID-19 on the hardest-hit communities in America – poor, Black, and Latinx communities, where a large proportion of essential workers live.”
Barzilay acknowledged that, “unfortunately, because of the way the study was circulated, it did not reach minorities, which is one of the things we want to improve.”
The study is ongoing and has been translated into Spanish, French, and Hebrew. The team plans to collect data on diverse populations.
The study was supported by grants from the National Institute of Mental Health, the Lifespan Brain Institute of Children’s Hospital of Philadelphia, Penn Medicine, the University of Pennsylvania, and in part by the Zuckerman STEM Leadership Program. Barzilay serves on the scientific board and reports stock ownership in Taliaz Health. The other authors, Golnaz, Vinkers, and Holman have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Individuals are more worried about family members becoming ill with COVID-19 or about unknowingly transmitting the disease to family members than they are about contracting it themselves, results of a new survey show.
Investigators surveyed over 3,000 adults, using an online questionnaire. Of the respondents, about 20% were health care workers, and most were living in locations with active stay-at-home orders at the time of the survey.
Close to half of participants were worried about family members contracting the virus, one third were worried about unknowingly infecting others, and 20% were worried about contracting the virus themselves.
“We were a little surprised to see that people were more concerned about others than about themselves, specifically worrying about whether a family member would contract COVID-19 and whether they might unintentionally infect others,” lead author Ran Barzilay, MD, PhD, child and adolescent psychiatrist at the Children’s Hospital of Philadelphia (CHOP), told Medscape Medical News.
The study was published online August 20 in Translational Psychiatry.
Interactive platform
“The pandemic has provided a unique opportunity to study resilience in healthcare professionals and others,” said Barzilay, assistant professor at the Lifespan Brain Institute, a collaboration between CHOP and the University of Pennsylvania, under the directorship of Raquel Gur, MD, PhD.
“After the pandemic broke out in March, we launched a website in early April where we surveyed people for levels of resilience, mental health, and well-being during the outbreak,” he added.
Survey participants then shared it with their contacts.
“To date, over 7000 people have completed it – mostly from the US but also from Israel,” Barzilay said.
The survey was anonymous, but participants could choose to have follow-up contact. The survey included an interactive 21-item resilience questionnaire and an assessment of COVID-19-related items related to worries concerning the following: contracting, dying from, or currently having the illness; having a family member contract the illness; unknowingly infecting others; and experiencing significant financial burden.
A total of 1350 participants took a second survey on anxiety and depression that utilized the Generalized Anxiety Disorder–7 and the Patient Health Questionnaire–2.
“What makes the survey unique is that it’s not just a means of collecting data but also an interactive platform that gives participants immediate personalized feedback, based on their responses to the resilience and well-being surveys, with practical tips and recommendations for stress management and ways of boosting resilience,” Barzilay said.
Tend and befriend
Ten days into the survey, data were available on 3,042 participants (64% women, 54% with advanced education, 20.5% health care providers), who ranged in age from 18 to 70 years (mean [SD], 38.9 [11.9] years).
After accounting for covariates, the researchers found that participants reported more distress about family members contracting COVID-19 and about unknowingly infecting others than about getting COVID-19 themselves (48.5% and 36% vs. 19.9%, respectively; P < .0005).
Increased COVID-19-related worries were associated with 22% higher anxiety and 16.1% higher depression scores; women had higher scores than men on both.
Each 1-SD increase in the composite score of COVID-19 worries was associated with over twice the increased probability of generalized anxiety and depression (odds ratio, 2.23; 95% confidence interval, 1.88-2.65; and OR, 1.67; 95% CI, 1.41-1.98, respectively; for both, P < .001).
On the other hand, for every 1-SD increase in the resilience score, there was a 64.9% decrease in the possibility of screening positive for generalized anxiety disorder and a 69.3% decrease in the possibility of screening positive for depression (for both, P < .0001).
Compared to participants from Israel, US participants were “more stressed” about contracting, dying from, and currently having COVID-19 themselves. Overall, Israeli participants scored higher than US participants on the resilience scale.
Rates of anxiety and depression did not differ significantly between healthcare providers and others. Health care providers worried more about contracting COVID-19 themselves and worried less about finances after COVID-19.
The authors propose that survey participants were more worried about others than about themselves because of “prosocial behavior under stress” and “tend-and-befriend,” whereby, “in response to threat, humans tend to protect their close ones (tending) and seek out their social group for mutual defense (befriending).”
This type of altruistic behavior has been “described in acute situations throughout history” and has been “linked to mechanisms of resilience for overcoming adversity,” the authors indicate.
Demographic biases
Commenting on the findings for Medscape Medical News, Golnaz Tabibnia, PhD, a neuroscientist at the University of California, Irvine, who was not involved in the research, suggested that although higher resilience scores were associated with lower COVID-related worries, it is possible, “as the authors suggest, that having more resilience resources makes you less worried, but the causality could go the other direction as well, and less worry/rumination may lead to more resilience.”
Also commenting on the study for Medscape Medical News, Christiaan Vinkers, MD, PhD, a psychiatrist at the Amsterdam University Medical Center, Amsterdam, the Netherlands, said it was noteworthy that healthcare providers reported similar levels of mood and anxiety symptoms, compared to others.
“This is encouraging, as it suggests adequate resilience levels in professionals who work in the front lines of the COVID-19 pandemic,” he said.
Resilience occurs not only at the individual level but also at the community level, which may help explain the striking differences in COVID-19-related worries and anxiety between participants from the United States and Israel, Vinkers added.
E. Alison Holman, PhD, professor, Sue and Bill Gross School of Nursing, University of California, Irvine, noted that respondents were predominantly white, female, and had relatively high incomes, “suggesting strong demographic biases in those who chose to participate.”
Holman, who was not involved with the study, told Medscape Medical News that the “findings do not address the real impact of COVID-19 on the hardest-hit communities in America – poor, Black, and Latinx communities, where a large proportion of essential workers live.”
Barzilay acknowledged that, “unfortunately, because of the way the study was circulated, it did not reach minorities, which is one of the things we want to improve.”
The study is ongoing and has been translated into Spanish, French, and Hebrew. The team plans to collect data on diverse populations.
The study was supported by grants from the National Institute of Mental Health, the Lifespan Brain Institute of Children’s Hospital of Philadelphia, Penn Medicine, the University of Pennsylvania, and in part by the Zuckerman STEM Leadership Program. Barzilay serves on the scientific board and reports stock ownership in Taliaz Health. The other authors, Golnaz, Vinkers, and Holman have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Disparities seen in COVID-19–related avoidance of care
In the early weeks and months of the COVID-19 pandemic, many people were trying to avoid the coronavirus by staying away from emergency rooms and medical offices. But how many people is “many”?
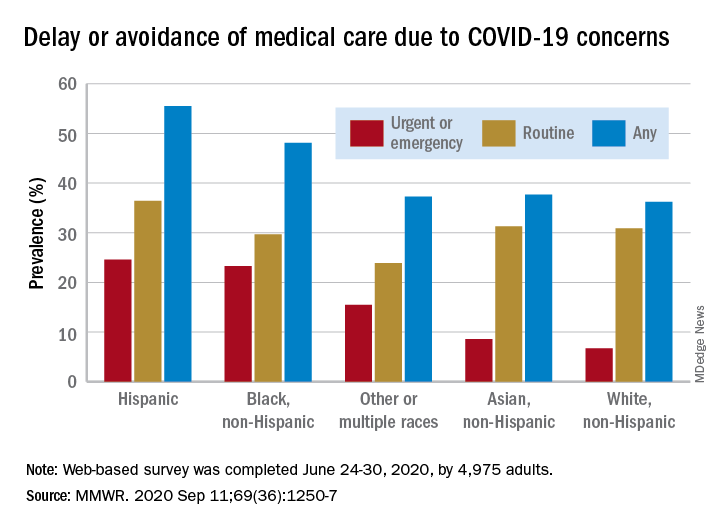
Turns out almost 41% of Americans delayed or avoided some form of medical care because of concerns about COVID-19, according to the results of a survey conducted June 24-30 by commercial survey company Qualtrics.
More specifically, the avoidance looks like this: 31.5% of the 4,975 adult respondents had avoided routine care and 12.0% had avoided urgent or emergency care, Mark E. Czeisler and associates said in the Morbidity and Mortality Weekly Report. The two categories were not mutually exclusive since respondents could select both routine care and urgent/emergency care.
There were, however, a number of significant disparities hidden among those numbers for the overall population. Blacks and Hispanics, with respective prevalences of 23.3% and 24.6%, were significantly more likely to delay or avoid urgent/emergency care than were Whites (6.7%), said Mr. Czeisler, a graduate student at Monash University, Melbourne, and associates.
Those differences “are especially concerning given increased COVID-19–associated mortality among Black adults and Hispanic adults,” they noted, adding that “age-adjusted COVID-19 hospitalization rates are approximately five times higher among Black persons and four times higher among Hispanic persons than” among Whites.
Other significant disparities in urgent/emergency care avoidance included the following:
- Unpaid caregivers for adults (29.8%) vs. noncaregivers (5.4%).
- Adults with two or more underlying conditions (22.7%) vs. those without such conditions (8.2%).
- Those with a disability (22.8%) vs. those without (8.9%).
- Those with health insurance (12.4%) vs. those without (7.8%).
The highest prevalence for all types of COVID-19–related delay and avoidance came from the adult caregivers (64.3%), followed by those with a disability (60.3%) and adults aged 18-24 years (57.2%). The lowest prevalence numbers were for adults with health insurance (24.8%) and those who were not caregivers for adults (32.2%), Mr. Czeisler and associates reported.
These reports of delayed and avoided care “might reflect adherence to community mitigation efforts such as stay-at-home orders, temporary closures of health facilities, or additional factors. However, if routine care avoidance were to be sustained, adults could miss opportunities for management of chronic conditions, receipt of routine vaccinations, or early detection of new conditions, which might worsen outcomes,” they wrote.
SOURCE: Czeisler ME et al. MMWR. 2020 Sep 11;69(36):1250-7.
In the early weeks and months of the COVID-19 pandemic, many people were trying to avoid the coronavirus by staying away from emergency rooms and medical offices. But how many people is “many”?

Turns out almost 41% of Americans delayed or avoided some form of medical care because of concerns about COVID-19, according to the results of a survey conducted June 24-30 by commercial survey company Qualtrics.
More specifically, the avoidance looks like this: 31.5% of the 4,975 adult respondents had avoided routine care and 12.0% had avoided urgent or emergency care, Mark E. Czeisler and associates said in the Morbidity and Mortality Weekly Report. The two categories were not mutually exclusive since respondents could select both routine care and urgent/emergency care.
There were, however, a number of significant disparities hidden among those numbers for the overall population. Blacks and Hispanics, with respective prevalences of 23.3% and 24.6%, were significantly more likely to delay or avoid urgent/emergency care than were Whites (6.7%), said Mr. Czeisler, a graduate student at Monash University, Melbourne, and associates.
Those differences “are especially concerning given increased COVID-19–associated mortality among Black adults and Hispanic adults,” they noted, adding that “age-adjusted COVID-19 hospitalization rates are approximately five times higher among Black persons and four times higher among Hispanic persons than” among Whites.
Other significant disparities in urgent/emergency care avoidance included the following:
- Unpaid caregivers for adults (29.8%) vs. noncaregivers (5.4%).
- Adults with two or more underlying conditions (22.7%) vs. those without such conditions (8.2%).
- Those with a disability (22.8%) vs. those without (8.9%).
- Those with health insurance (12.4%) vs. those without (7.8%).
The highest prevalence for all types of COVID-19–related delay and avoidance came from the adult caregivers (64.3%), followed by those with a disability (60.3%) and adults aged 18-24 years (57.2%). The lowest prevalence numbers were for adults with health insurance (24.8%) and those who were not caregivers for adults (32.2%), Mr. Czeisler and associates reported.
These reports of delayed and avoided care “might reflect adherence to community mitigation efforts such as stay-at-home orders, temporary closures of health facilities, or additional factors. However, if routine care avoidance were to be sustained, adults could miss opportunities for management of chronic conditions, receipt of routine vaccinations, or early detection of new conditions, which might worsen outcomes,” they wrote.
SOURCE: Czeisler ME et al. MMWR. 2020 Sep 11;69(36):1250-7.
In the early weeks and months of the COVID-19 pandemic, many people were trying to avoid the coronavirus by staying away from emergency rooms and medical offices. But how many people is “many”?

Turns out almost 41% of Americans delayed or avoided some form of medical care because of concerns about COVID-19, according to the results of a survey conducted June 24-30 by commercial survey company Qualtrics.
More specifically, the avoidance looks like this: 31.5% of the 4,975 adult respondents had avoided routine care and 12.0% had avoided urgent or emergency care, Mark E. Czeisler and associates said in the Morbidity and Mortality Weekly Report. The two categories were not mutually exclusive since respondents could select both routine care and urgent/emergency care.
There were, however, a number of significant disparities hidden among those numbers for the overall population. Blacks and Hispanics, with respective prevalences of 23.3% and 24.6%, were significantly more likely to delay or avoid urgent/emergency care than were Whites (6.7%), said Mr. Czeisler, a graduate student at Monash University, Melbourne, and associates.
Those differences “are especially concerning given increased COVID-19–associated mortality among Black adults and Hispanic adults,” they noted, adding that “age-adjusted COVID-19 hospitalization rates are approximately five times higher among Black persons and four times higher among Hispanic persons than” among Whites.
Other significant disparities in urgent/emergency care avoidance included the following:
- Unpaid caregivers for adults (29.8%) vs. noncaregivers (5.4%).
- Adults with two or more underlying conditions (22.7%) vs. those without such conditions (8.2%).
- Those with a disability (22.8%) vs. those without (8.9%).
- Those with health insurance (12.4%) vs. those without (7.8%).
The highest prevalence for all types of COVID-19–related delay and avoidance came from the adult caregivers (64.3%), followed by those with a disability (60.3%) and adults aged 18-24 years (57.2%). The lowest prevalence numbers were for adults with health insurance (24.8%) and those who were not caregivers for adults (32.2%), Mr. Czeisler and associates reported.
These reports of delayed and avoided care “might reflect adherence to community mitigation efforts such as stay-at-home orders, temporary closures of health facilities, or additional factors. However, if routine care avoidance were to be sustained, adults could miss opportunities for management of chronic conditions, receipt of routine vaccinations, or early detection of new conditions, which might worsen outcomes,” they wrote.
SOURCE: Czeisler ME et al. MMWR. 2020 Sep 11;69(36):1250-7.
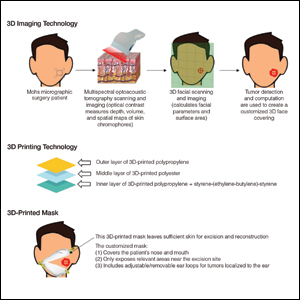
Use of 3D Technology to Support Dermatologists Returning to Practice Amid COVID-19
Coronavirus disease 2019 (COVID-19) has spread across all 7 continents, including 185 countries, and infected more than 21.9 million individuals worldwide as of August 18, 2020, according to the Johns Hopkins Coronavirus Resource Center. It has strained our health care system and affected all specialties, including dermatology. Dermatologists have taken important safety measures by canceling/deferring elective and nonemergency procedures and diagnosing/treating patients via telemedicine. Many residents and attending dermatologists have volunteered to care for COVID-19 inpatients and donated
N95 masks are necessary during the COVID-19 pandemic because they effectively filter at least 95% of 0.3-μm airborne particles and provide adequate face seals.1 3-Dimensional imaging integrated with 3D printers can be used to scan precise facial parameters (eg, jawline, nose) and account for facial hair density and length to produce comfortable tailored N95 masks and face seals.1,2 3-Dimensional printing utilizes robotics and
Face shields offer an additional layer of safety for the face and mucosae and also may provide longevity for N95 masks. Using synthetic polymers such as polycarbonate and polyethylene, 3D printers can be used to construct face shields via fused deposition modeling.1 These face shields may be worn over N95 masks and then can be sanitized and reused.
Mohs surgeons and staff may be at particularly high risk for COVID-19 infection due to their close proximity to the face during surgery, use of cautery, and prolonged time spent with patients while taking layers and suturing.

As dermatologists reopen and ramp up practice volume, there will be increased PPE requirements. Using 3D technology and imaging to produce N95 masks, face shields, and face coverings, we can offer effective diagnosis and treatment while optimizing safety for dermatologists, staff, and patients.
- Ishack S, Lipner SR. Applications of 3D printing technology to address COVID-19-related supply shortages [published online April 21, 2020]. Am J Med. 2020;133:771-773.
- Cai M, Li H, Shen S, et al. Customized design and 3D printing of face seal for an N95 filtering facepiece respirator. J Occup Environ Hyg. 2018;3:226-234.
- Ishack S, Lipner SR. A review of 3-dimensional skin bioprinting techniques: applications, approaches, and trends [published online March 17, 2020]. Dermatol Surg. doi:10.1097/DSS.0000000000002378.
- Banerjee SS, Burbine S, Shivaprakash NK, et al. 3D-printable PP/SEBS thermoplastic elastomeric blends: preparation and properties [published online February 17, 2019]. Polymers (Basel). doi:10.3390/polym11020347.
- Chuah SY, Attia ABE, Long V. Structural and functional 3D mapping of skin tumours with non-invasive multispectral optoacoustic tomography [published online November 2, 2016]. Skin Res Technol. 2017;23:221-226.
Coronavirus disease 2019 (COVID-19) has spread across all 7 continents, including 185 countries, and infected more than 21.9 million individuals worldwide as of August 18, 2020, according to the Johns Hopkins Coronavirus Resource Center. It has strained our health care system and affected all specialties, including dermatology. Dermatologists have taken important safety measures by canceling/deferring elective and nonemergency procedures and diagnosing/treating patients via telemedicine. Many residents and attending dermatologists have volunteered to care for COVID-19 inpatients and donated
N95 masks are necessary during the COVID-19 pandemic because they effectively filter at least 95% of 0.3-μm airborne particles and provide adequate face seals.1 3-Dimensional imaging integrated with 3D printers can be used to scan precise facial parameters (eg, jawline, nose) and account for facial hair density and length to produce comfortable tailored N95 masks and face seals.1,2 3-Dimensional printing utilizes robotics and
Face shields offer an additional layer of safety for the face and mucosae and also may provide longevity for N95 masks. Using synthetic polymers such as polycarbonate and polyethylene, 3D printers can be used to construct face shields via fused deposition modeling.1 These face shields may be worn over N95 masks and then can be sanitized and reused.
Mohs surgeons and staff may be at particularly high risk for COVID-19 infection due to their close proximity to the face during surgery, use of cautery, and prolonged time spent with patients while taking layers and suturing.

As dermatologists reopen and ramp up practice volume, there will be increased PPE requirements. Using 3D technology and imaging to produce N95 masks, face shields, and face coverings, we can offer effective diagnosis and treatment while optimizing safety for dermatologists, staff, and patients.
Coronavirus disease 2019 (COVID-19) has spread across all 7 continents, including 185 countries, and infected more than 21.9 million individuals worldwide as of August 18, 2020, according to the Johns Hopkins Coronavirus Resource Center. It has strained our health care system and affected all specialties, including dermatology. Dermatologists have taken important safety measures by canceling/deferring elective and nonemergency procedures and diagnosing/treating patients via telemedicine. Many residents and attending dermatologists have volunteered to care for COVID-19 inpatients and donated
N95 masks are necessary during the COVID-19 pandemic because they effectively filter at least 95% of 0.3-μm airborne particles and provide adequate face seals.1 3-Dimensional imaging integrated with 3D printers can be used to scan precise facial parameters (eg, jawline, nose) and account for facial hair density and length to produce comfortable tailored N95 masks and face seals.1,2 3-Dimensional printing utilizes robotics and
Face shields offer an additional layer of safety for the face and mucosae and also may provide longevity for N95 masks. Using synthetic polymers such as polycarbonate and polyethylene, 3D printers can be used to construct face shields via fused deposition modeling.1 These face shields may be worn over N95 masks and then can be sanitized and reused.
Mohs surgeons and staff may be at particularly high risk for COVID-19 infection due to their close proximity to the face during surgery, use of cautery, and prolonged time spent with patients while taking layers and suturing.

As dermatologists reopen and ramp up practice volume, there will be increased PPE requirements. Using 3D technology and imaging to produce N95 masks, face shields, and face coverings, we can offer effective diagnosis and treatment while optimizing safety for dermatologists, staff, and patients.
- Ishack S, Lipner SR. Applications of 3D printing technology to address COVID-19-related supply shortages [published online April 21, 2020]. Am J Med. 2020;133:771-773.
- Cai M, Li H, Shen S, et al. Customized design and 3D printing of face seal for an N95 filtering facepiece respirator. J Occup Environ Hyg. 2018;3:226-234.
- Ishack S, Lipner SR. A review of 3-dimensional skin bioprinting techniques: applications, approaches, and trends [published online March 17, 2020]. Dermatol Surg. doi:10.1097/DSS.0000000000002378.
- Banerjee SS, Burbine S, Shivaprakash NK, et al. 3D-printable PP/SEBS thermoplastic elastomeric blends: preparation and properties [published online February 17, 2019]. Polymers (Basel). doi:10.3390/polym11020347.
- Chuah SY, Attia ABE, Long V. Structural and functional 3D mapping of skin tumours with non-invasive multispectral optoacoustic tomography [published online November 2, 2016]. Skin Res Technol. 2017;23:221-226.
- Ishack S, Lipner SR. Applications of 3D printing technology to address COVID-19-related supply shortages [published online April 21, 2020]. Am J Med. 2020;133:771-773.
- Cai M, Li H, Shen S, et al. Customized design and 3D printing of face seal for an N95 filtering facepiece respirator. J Occup Environ Hyg. 2018;3:226-234.
- Ishack S, Lipner SR. A review of 3-dimensional skin bioprinting techniques: applications, approaches, and trends [published online March 17, 2020]. Dermatol Surg. doi:10.1097/DSS.0000000000002378.
- Banerjee SS, Burbine S, Shivaprakash NK, et al. 3D-printable PP/SEBS thermoplastic elastomeric blends: preparation and properties [published online February 17, 2019]. Polymers (Basel). doi:10.3390/polym11020347.
- Chuah SY, Attia ABE, Long V. Structural and functional 3D mapping of skin tumours with non-invasive multispectral optoacoustic tomography [published online November 2, 2016]. Skin Res Technol. 2017;23:221-226.
Practice Points
- Coronavirus disease 19 has overwhelmed our health care system and affected all specialties, including dermatology.
- There are concerns about shortages of personal protective equipment to safely care for patients.
- 3-Dimensional imaging and printing technologies can be harnessed to create face coverings and face shields for the dermatology outpatient setting.
What’s Eating You? Oriental Rat Flea (Xenopsylla cheopis)
A dult Siphonaptera (fleas) are highly adapted to life on the surface of their hosts. Their small 2- to 10-mm bodies are laterally flattened and wingless. They utilize particularly strong hind legs for jumping up to 150 times their body length and backward-directed spines on their legs and bodies for moving forward through fur, hair, and feathers. Xenopsylla cheopis , the oriental rat flea, lacks pronotal and genal combs and has a mesopleuron divided by internal scleritinization (Figure). These features differentiate the species from its close relatives, Ctenocephalides (cat and dog fleas), which have both sets of combs, as well as Pulex irritans (human flea), which do not have a divided mesopleuron. 1,2
Flea-borne infections are extremely important to public health and are present throughout the world. Further, humidity and warmth are essential for the life cycle of many species of fleas. Predicted global warming likely will increase their distribution, allowing the spread of diseases they carry into previously untouched areas.1 Therefore, it is important to continue to examine species that carry particularly dangerous pathogens, such as X cheopis.
Disease Vector
Xenopsylla cheopis primarily is known for being a vector in the transmission of Yersinia pestis, the etiologic agent of the plague. Plague occurs in 3 forms: bubonic, pneumonic, and septicemic. It has caused major epidemics throughout history, the most widely known being the Black Death, which lasted for 130 years, beginning in the 1330s in China and spreading into Europe where it wiped out one-third of the population. However, bubonic plague is thought to have caused documented outbreaks as early as 320
Between January 2010 and December 2015, 3248 cases of plague in humans were reported, resulting in 584 deaths worldwide.5 It is thought that the plague originated in Central Asia, and this area still is a focus of disease. However, the at-risk population is reduced to breeders and hunters of gerbils and marmots, the main reservoirs in the area. In Africa, 4 countries still regularly report cases, with Madagascar being the most severely affected country in the world.5 The Democratic Republic of the Congo, Uganda, and Tanzania also are affected. The Americas also experience the plague. There are sporadic cases of bubonic plague in the northwest corner of Peru, mostly in rural areas. In the western United States, plague circulates among wild rodents, resulting in several reported cases each year, with the most recent confirmed case noted in California in August 2020.5,6 Further adding to its relevance, Y pestis is one of several organisms most likely to be used as a biologic weapon.3,4
Due to the historical and continued significance of Y pestis, many studies have been performed over the decades regarding its association with X cheopis. It has been discovered that fleas transmit the bacteria to the host in 2 ways. The most well-defined form of transmission occurs after an incubation period of Y pestis in the flea for 7 to 31 days. During this time, the bacteria form a dense biofilm on a valve in the flea foregut—the proventriculus—interfering with its function, which allows regurgitation of the blood and the bacteria it contains into the bite site and consequently disease transmission. The proventriculus can become completely blocked in some fleas, preventing any blood from reaching the midgut and causing starvation. In these scenarios, the flea will make continuous attempts to feed, increasing transmission.7 The hemin storage gene, hms, encoding the second messenger molecule cyclic di-GMP plays a critical role in biofilm formation and proventricular blockage.8 The phosphoheptose isomerase gene, GmhA, also has been elucidated as crucial in late transmission due to its role in biofilm formation.9 Early-phase transmission, or biofilm-independent transmission, has been documented to occur as early as 3 hours after infection of the flea but can occur for up to 4 days.10 Historically, the importance of early-phase transmission has been overlooked. Research has shown that it likely is crucial to the epizootic transmission of the plague.10 As a result, the search has begun for genes that contribute to the maintenance of Y pestis in the flea vector during the first 4 days of colonization. It is thought that a key evolutionary development was the selective loss-of-function mutation in a gene essential for the activity of urease, an enzyme that causes acute oral toxicity and mortality in fleas.11,12 The Yersinia murine toxin gene, Ymt, also allows for early survival of Y pestis in the flea midgut by producing a phospholipase D that protects the bacteria from toxic by-products produced during digestion of blood.11,13 In addition, gene products that function in lipid A modification are crucial for the ability of Y pestis to resist the action of cationic antimicrobial peptides it produces, such as cecropin A and polymyxin B.13
Murine typhus, an acute febrile illness caused by Rickettsia typhi, is another disease that can be spread by oriental rat fleas. It has a worldwide distribution. In the United States, R typhi–induced rickettsia mainly is concentrated in suburban areas of Texas and California where it is thought to be mainly spread by Ctenocephalides, but it also is found in Hawaii where spread by X cheopis has been documented.14,15 The most common symptoms of rickettsia include fever, headache, arthralgia, and a characteristic rash that is pruritic and maculopapular, starting on the trunk and spreading peripherally but sparing the palms and soles. This rash occurs about a week after the onset of fever.14Rickettsia felis also has been isolated in the oriental rat flea. However, only a handful of cases of human disease caused by this bacterium have been reported throughout the world, with clinical similarity to murine typhus likely leading to underestimation of disease prevalence.15Bartonella and other species of bacteria also have been documented to be spread by X cheopis.16 Unfortunately, the interactions of X cheopis with these other bacteria are not as well studied as its relationship with Y pestis.
Adverse Reactions
A flea bite itself can cause discomfort. It begins as a punctate hemorrhagic area that develops a surrounding wheal within 30 minutes. Over the course of 1 to 2 days, a delayed reaction occurs and there is a transition to an extremely pruritic, papular lesion. Bites often occur in clusters and can persist for weeks.1
Prevention and Treatment
Control of host animals via extermination and proper sanitation can secondarily reduce the population of X cheopis. Direct pesticide control of the flea population also has been suggested to reduce flea-borne disease. However, insecticides cause a selective pressure on the flea population, leading to populations that are resistant to them. For example, the flea population in Madagascar developed resistance to DDT (dichlorodiphenyltrichloroethane), dieldrin, deltamethrin, and cyfluthrin after their widespread use.17 Further, a recent study revealed resistance of X cheopis populations to alphacypermethrin, lambda-cyhalothrin, and etofenprox, none of which were used in mass vector control, indicating that some cross-resistance mechanism between these and the extensively used insecticides may exist. With the development of widespread resistance to most pesticides, flea control in endemic areas is difficult. Insecticide targeting to fleas on rodents (eg, rodent bait containing insecticide) can allow for more targeted insecticide treatment, limiting the development of resistance.17 Recent development of a maceration protocol used to detect zoonotic pathogens in fleas in the field also will allow management with pesticides to be targeted geographically and temporally where infected vectors are located.18 Research of the interaction between vector, pathogen, and insect microbiome also should continue, as it may allow for development of biopesticides, limiting the use of chemical pesticides all together. The strategy is based on the introduction of microorganisms that can reduce vector lifespan or their ability to transmit pathogens.17
When flea-transmitted diseases do occur, treatment with antibiotics is advised. Early treatment of the plague with effective antibiotics such as streptomycin, gentamicin, tetracycline, or chloramphenicol for a minimum of 10 days is critical for survival. Additionally, patients with bubonic plague should be isolated for at least 2 days after administration of antibiotics, while patients with the pneumonic form should be isolated for 4 days into therapy to prevent the spread of disease. Prophylactic therapy for individuals who come into contact with infected individuals also is advised.4 Patients with murine typhus typically respond to doxycycline, tetracycline, or fluoroquinolones. The few cases of R felis–induced disease have responded to doxycycline. Of note, short courses of treatment of doxycycline are appropriate and safe in young children. The short (3–7 day) nature of the course limits the chances of teeth staining.14
- Bitam I, Dittmar K, Parola P, et al. Flea and flea-borne diseases. Int J Infect Dis. 2010;14:E667-E676.
- Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clin Microbiol Rev. 2014;27:48-67.
- Ligon BL. Plague: a review of its history and potential as a biological weapon. Semin Pediatr Infect Dis. 2006;17:161-170.
- Josko D. Yersinia pestis: still a plague in the 21st century. Clin Lab Sci. 2004;17:25-29.
- Plague around the world, 2010–2015. Wkly Epidemiol Rec. 2016;91:89-93.
- Sullivan K. California confirms first human case of the plague in 5 years: what to know. NBC News website. https://www.nbcnews.com/health/health-news/california-confirms-first-human-case-bubonic-plague-5-years-what-n1237306. Published August 19, 2020. Accessed August 24, 2020.
- Hinnebusch BJ, Bland DM, Bosio CF, et al. Comparative ability of Oropsylla and Xenopsylla cheopis fleas to transmit Yersinia pestis by two different mechanisms. PLOS Negl Trop Dis. 2017;11:e0005276.
- Bobrov AG, Kirillina O, Vadyvaloo V, et al. The Yersinia pestis HmsCDE regulatory system is essential for blockage of the oriental rat flea (Xenopsylla cheopis), a classic plague vector. Environ Microbiol. 2015;17:947-959.
- Darby C, Ananth SL, Tan L, et al. Identification of gmhA, a Yersina pestis gene required for flea blockage, by using a Caenorhabditis elegans biofilm system. Infect Immun. 2005;73:7236-7242.
- Eisen RJ, Dennis DT, Gage KL. The role of early-phase transmission in the spread of Yersinia pestis. J Med Entomol. 2015;52:1183-1192.
- Carniel E. Subtle genetic modifications transformed an enteropathogen into a flea-borne pathogen. Proc Natl Acad Sci U S A. 2014;111:18409-18410.
- Chouikha I, Hinnebusch BJ. Silencing urease: a key evolutionary step that facilitated the adaptation of Yersinia pestis to the flea-borne transmission route. Proc Natl Acad Sci U S A. 2014;111:18709-19714.
- Aoyagi KL, Brooks BD, Bearden SW, et al. LPS modification promotes maintenance of Yersinia pestis in fleas. Microbiology. 2015;161:628-638.
- Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913-918.
- Eremeeva ME, Warashina WR, Sturgeon MM, et al. Rickettsia typhi and R. felis in rat fleas (Xenopsylla cheopis), Oahu, Hawaii. Emerg Infect Dis. 2018;14:1613-1615.
- Billeter SA, Gundi VAKB, Rood MP, et al. Molecular detection and identification of Bartonella species in Xenopsylla cheopis fleas (Siphonaptera: Pulicidae) collected from Rattus norvecus rats in Los Angeles, California. Appl Environ Microbiol. 2011;77:7850-7852.
- Miarinjara A, Boyer S. Current perspectives on plague vector control in Madagascar: susceptibility status of Xenopsylla cheopis to 12 insecticides. PLOS Negl Trop Dis. 2016;10:e0004414.
- Harrison GF, Scheirer JL, Melanson VR. Development and validation of an arthropod maceration protocol for zoonotic pathogen detection in mosquitoes and fleas. J Vector Ecol. 2014;40:83-89.
A dult Siphonaptera (fleas) are highly adapted to life on the surface of their hosts. Their small 2- to 10-mm bodies are laterally flattened and wingless. They utilize particularly strong hind legs for jumping up to 150 times their body length and backward-directed spines on their legs and bodies for moving forward through fur, hair, and feathers. Xenopsylla cheopis , the oriental rat flea, lacks pronotal and genal combs and has a mesopleuron divided by internal scleritinization (Figure). These features differentiate the species from its close relatives, Ctenocephalides (cat and dog fleas), which have both sets of combs, as well as Pulex irritans (human flea), which do not have a divided mesopleuron. 1,2

Flea-borne infections are extremely important to public health and are present throughout the world. Further, humidity and warmth are essential for the life cycle of many species of fleas. Predicted global warming likely will increase their distribution, allowing the spread of diseases they carry into previously untouched areas.1 Therefore, it is important to continue to examine species that carry particularly dangerous pathogens, such as X cheopis.
Disease Vector
Xenopsylla cheopis primarily is known for being a vector in the transmission of Yersinia pestis, the etiologic agent of the plague. Plague occurs in 3 forms: bubonic, pneumonic, and septicemic. It has caused major epidemics throughout history, the most widely known being the Black Death, which lasted for 130 years, beginning in the 1330s in China and spreading into Europe where it wiped out one-third of the population. However, bubonic plague is thought to have caused documented outbreaks as early as 320
Between January 2010 and December 2015, 3248 cases of plague in humans were reported, resulting in 584 deaths worldwide.5 It is thought that the plague originated in Central Asia, and this area still is a focus of disease. However, the at-risk population is reduced to breeders and hunters of gerbils and marmots, the main reservoirs in the area. In Africa, 4 countries still regularly report cases, with Madagascar being the most severely affected country in the world.5 The Democratic Republic of the Congo, Uganda, and Tanzania also are affected. The Americas also experience the plague. There are sporadic cases of bubonic plague in the northwest corner of Peru, mostly in rural areas. In the western United States, plague circulates among wild rodents, resulting in several reported cases each year, with the most recent confirmed case noted in California in August 2020.5,6 Further adding to its relevance, Y pestis is one of several organisms most likely to be used as a biologic weapon.3,4
Due to the historical and continued significance of Y pestis, many studies have been performed over the decades regarding its association with X cheopis. It has been discovered that fleas transmit the bacteria to the host in 2 ways. The most well-defined form of transmission occurs after an incubation period of Y pestis in the flea for 7 to 31 days. During this time, the bacteria form a dense biofilm on a valve in the flea foregut—the proventriculus—interfering with its function, which allows regurgitation of the blood and the bacteria it contains into the bite site and consequently disease transmission. The proventriculus can become completely blocked in some fleas, preventing any blood from reaching the midgut and causing starvation. In these scenarios, the flea will make continuous attempts to feed, increasing transmission.7 The hemin storage gene, hms, encoding the second messenger molecule cyclic di-GMP plays a critical role in biofilm formation and proventricular blockage.8 The phosphoheptose isomerase gene, GmhA, also has been elucidated as crucial in late transmission due to its role in biofilm formation.9 Early-phase transmission, or biofilm-independent transmission, has been documented to occur as early as 3 hours after infection of the flea but can occur for up to 4 days.10 Historically, the importance of early-phase transmission has been overlooked. Research has shown that it likely is crucial to the epizootic transmission of the plague.10 As a result, the search has begun for genes that contribute to the maintenance of Y pestis in the flea vector during the first 4 days of colonization. It is thought that a key evolutionary development was the selective loss-of-function mutation in a gene essential for the activity of urease, an enzyme that causes acute oral toxicity and mortality in fleas.11,12 The Yersinia murine toxin gene, Ymt, also allows for early survival of Y pestis in the flea midgut by producing a phospholipase D that protects the bacteria from toxic by-products produced during digestion of blood.11,13 In addition, gene products that function in lipid A modification are crucial for the ability of Y pestis to resist the action of cationic antimicrobial peptides it produces, such as cecropin A and polymyxin B.13
Murine typhus, an acute febrile illness caused by Rickettsia typhi, is another disease that can be spread by oriental rat fleas. It has a worldwide distribution. In the United States, R typhi–induced rickettsia mainly is concentrated in suburban areas of Texas and California where it is thought to be mainly spread by Ctenocephalides, but it also is found in Hawaii where spread by X cheopis has been documented.14,15 The most common symptoms of rickettsia include fever, headache, arthralgia, and a characteristic rash that is pruritic and maculopapular, starting on the trunk and spreading peripherally but sparing the palms and soles. This rash occurs about a week after the onset of fever.14Rickettsia felis also has been isolated in the oriental rat flea. However, only a handful of cases of human disease caused by this bacterium have been reported throughout the world, with clinical similarity to murine typhus likely leading to underestimation of disease prevalence.15Bartonella and other species of bacteria also have been documented to be spread by X cheopis.16 Unfortunately, the interactions of X cheopis with these other bacteria are not as well studied as its relationship with Y pestis.
Adverse Reactions
A flea bite itself can cause discomfort. It begins as a punctate hemorrhagic area that develops a surrounding wheal within 30 minutes. Over the course of 1 to 2 days, a delayed reaction occurs and there is a transition to an extremely pruritic, papular lesion. Bites often occur in clusters and can persist for weeks.1
Prevention and Treatment
Control of host animals via extermination and proper sanitation can secondarily reduce the population of X cheopis. Direct pesticide control of the flea population also has been suggested to reduce flea-borne disease. However, insecticides cause a selective pressure on the flea population, leading to populations that are resistant to them. For example, the flea population in Madagascar developed resistance to DDT (dichlorodiphenyltrichloroethane), dieldrin, deltamethrin, and cyfluthrin after their widespread use.17 Further, a recent study revealed resistance of X cheopis populations to alphacypermethrin, lambda-cyhalothrin, and etofenprox, none of which were used in mass vector control, indicating that some cross-resistance mechanism between these and the extensively used insecticides may exist. With the development of widespread resistance to most pesticides, flea control in endemic areas is difficult. Insecticide targeting to fleas on rodents (eg, rodent bait containing insecticide) can allow for more targeted insecticide treatment, limiting the development of resistance.17 Recent development of a maceration protocol used to detect zoonotic pathogens in fleas in the field also will allow management with pesticides to be targeted geographically and temporally where infected vectors are located.18 Research of the interaction between vector, pathogen, and insect microbiome also should continue, as it may allow for development of biopesticides, limiting the use of chemical pesticides all together. The strategy is based on the introduction of microorganisms that can reduce vector lifespan or their ability to transmit pathogens.17
When flea-transmitted diseases do occur, treatment with antibiotics is advised. Early treatment of the plague with effective antibiotics such as streptomycin, gentamicin, tetracycline, or chloramphenicol for a minimum of 10 days is critical for survival. Additionally, patients with bubonic plague should be isolated for at least 2 days after administration of antibiotics, while patients with the pneumonic form should be isolated for 4 days into therapy to prevent the spread of disease. Prophylactic therapy for individuals who come into contact with infected individuals also is advised.4 Patients with murine typhus typically respond to doxycycline, tetracycline, or fluoroquinolones. The few cases of R felis–induced disease have responded to doxycycline. Of note, short courses of treatment of doxycycline are appropriate and safe in young children. The short (3–7 day) nature of the course limits the chances of teeth staining.14
A dult Siphonaptera (fleas) are highly adapted to life on the surface of their hosts. Their small 2- to 10-mm bodies are laterally flattened and wingless. They utilize particularly strong hind legs for jumping up to 150 times their body length and backward-directed spines on their legs and bodies for moving forward through fur, hair, and feathers. Xenopsylla cheopis , the oriental rat flea, lacks pronotal and genal combs and has a mesopleuron divided by internal scleritinization (Figure). These features differentiate the species from its close relatives, Ctenocephalides (cat and dog fleas), which have both sets of combs, as well as Pulex irritans (human flea), which do not have a divided mesopleuron. 1,2

Flea-borne infections are extremely important to public health and are present throughout the world. Further, humidity and warmth are essential for the life cycle of many species of fleas. Predicted global warming likely will increase their distribution, allowing the spread of diseases they carry into previously untouched areas.1 Therefore, it is important to continue to examine species that carry particularly dangerous pathogens, such as X cheopis.
Disease Vector
Xenopsylla cheopis primarily is known for being a vector in the transmission of Yersinia pestis, the etiologic agent of the plague. Plague occurs in 3 forms: bubonic, pneumonic, and septicemic. It has caused major epidemics throughout history, the most widely known being the Black Death, which lasted for 130 years, beginning in the 1330s in China and spreading into Europe where it wiped out one-third of the population. However, bubonic plague is thought to have caused documented outbreaks as early as 320
Between January 2010 and December 2015, 3248 cases of plague in humans were reported, resulting in 584 deaths worldwide.5 It is thought that the plague originated in Central Asia, and this area still is a focus of disease. However, the at-risk population is reduced to breeders and hunters of gerbils and marmots, the main reservoirs in the area. In Africa, 4 countries still regularly report cases, with Madagascar being the most severely affected country in the world.5 The Democratic Republic of the Congo, Uganda, and Tanzania also are affected. The Americas also experience the plague. There are sporadic cases of bubonic plague in the northwest corner of Peru, mostly in rural areas. In the western United States, plague circulates among wild rodents, resulting in several reported cases each year, with the most recent confirmed case noted in California in August 2020.5,6 Further adding to its relevance, Y pestis is one of several organisms most likely to be used as a biologic weapon.3,4
Due to the historical and continued significance of Y pestis, many studies have been performed over the decades regarding its association with X cheopis. It has been discovered that fleas transmit the bacteria to the host in 2 ways. The most well-defined form of transmission occurs after an incubation period of Y pestis in the flea for 7 to 31 days. During this time, the bacteria form a dense biofilm on a valve in the flea foregut—the proventriculus—interfering with its function, which allows regurgitation of the blood and the bacteria it contains into the bite site and consequently disease transmission. The proventriculus can become completely blocked in some fleas, preventing any blood from reaching the midgut and causing starvation. In these scenarios, the flea will make continuous attempts to feed, increasing transmission.7 The hemin storage gene, hms, encoding the second messenger molecule cyclic di-GMP plays a critical role in biofilm formation and proventricular blockage.8 The phosphoheptose isomerase gene, GmhA, also has been elucidated as crucial in late transmission due to its role in biofilm formation.9 Early-phase transmission, or biofilm-independent transmission, has been documented to occur as early as 3 hours after infection of the flea but can occur for up to 4 days.10 Historically, the importance of early-phase transmission has been overlooked. Research has shown that it likely is crucial to the epizootic transmission of the plague.10 As a result, the search has begun for genes that contribute to the maintenance of Y pestis in the flea vector during the first 4 days of colonization. It is thought that a key evolutionary development was the selective loss-of-function mutation in a gene essential for the activity of urease, an enzyme that causes acute oral toxicity and mortality in fleas.11,12 The Yersinia murine toxin gene, Ymt, also allows for early survival of Y pestis in the flea midgut by producing a phospholipase D that protects the bacteria from toxic by-products produced during digestion of blood.11,13 In addition, gene products that function in lipid A modification are crucial for the ability of Y pestis to resist the action of cationic antimicrobial peptides it produces, such as cecropin A and polymyxin B.13
Murine typhus, an acute febrile illness caused by Rickettsia typhi, is another disease that can be spread by oriental rat fleas. It has a worldwide distribution. In the United States, R typhi–induced rickettsia mainly is concentrated in suburban areas of Texas and California where it is thought to be mainly spread by Ctenocephalides, but it also is found in Hawaii where spread by X cheopis has been documented.14,15 The most common symptoms of rickettsia include fever, headache, arthralgia, and a characteristic rash that is pruritic and maculopapular, starting on the trunk and spreading peripherally but sparing the palms and soles. This rash occurs about a week after the onset of fever.14Rickettsia felis also has been isolated in the oriental rat flea. However, only a handful of cases of human disease caused by this bacterium have been reported throughout the world, with clinical similarity to murine typhus likely leading to underestimation of disease prevalence.15Bartonella and other species of bacteria also have been documented to be spread by X cheopis.16 Unfortunately, the interactions of X cheopis with these other bacteria are not as well studied as its relationship with Y pestis.
Adverse Reactions
A flea bite itself can cause discomfort. It begins as a punctate hemorrhagic area that develops a surrounding wheal within 30 minutes. Over the course of 1 to 2 days, a delayed reaction occurs and there is a transition to an extremely pruritic, papular lesion. Bites often occur in clusters and can persist for weeks.1
Prevention and Treatment
Control of host animals via extermination and proper sanitation can secondarily reduce the population of X cheopis. Direct pesticide control of the flea population also has been suggested to reduce flea-borne disease. However, insecticides cause a selective pressure on the flea population, leading to populations that are resistant to them. For example, the flea population in Madagascar developed resistance to DDT (dichlorodiphenyltrichloroethane), dieldrin, deltamethrin, and cyfluthrin after their widespread use.17 Further, a recent study revealed resistance of X cheopis populations to alphacypermethrin, lambda-cyhalothrin, and etofenprox, none of which were used in mass vector control, indicating that some cross-resistance mechanism between these and the extensively used insecticides may exist. With the development of widespread resistance to most pesticides, flea control in endemic areas is difficult. Insecticide targeting to fleas on rodents (eg, rodent bait containing insecticide) can allow for more targeted insecticide treatment, limiting the development of resistance.17 Recent development of a maceration protocol used to detect zoonotic pathogens in fleas in the field also will allow management with pesticides to be targeted geographically and temporally where infected vectors are located.18 Research of the interaction between vector, pathogen, and insect microbiome also should continue, as it may allow for development of biopesticides, limiting the use of chemical pesticides all together. The strategy is based on the introduction of microorganisms that can reduce vector lifespan or their ability to transmit pathogens.17
When flea-transmitted diseases do occur, treatment with antibiotics is advised. Early treatment of the plague with effective antibiotics such as streptomycin, gentamicin, tetracycline, or chloramphenicol for a minimum of 10 days is critical for survival. Additionally, patients with bubonic plague should be isolated for at least 2 days after administration of antibiotics, while patients with the pneumonic form should be isolated for 4 days into therapy to prevent the spread of disease. Prophylactic therapy for individuals who come into contact with infected individuals also is advised.4 Patients with murine typhus typically respond to doxycycline, tetracycline, or fluoroquinolones. The few cases of R felis–induced disease have responded to doxycycline. Of note, short courses of treatment of doxycycline are appropriate and safe in young children. The short (3–7 day) nature of the course limits the chances of teeth staining.14
- Bitam I, Dittmar K, Parola P, et al. Flea and flea-borne diseases. Int J Infect Dis. 2010;14:E667-E676.
- Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clin Microbiol Rev. 2014;27:48-67.
- Ligon BL. Plague: a review of its history and potential as a biological weapon. Semin Pediatr Infect Dis. 2006;17:161-170.
- Josko D. Yersinia pestis: still a plague in the 21st century. Clin Lab Sci. 2004;17:25-29.
- Plague around the world, 2010–2015. Wkly Epidemiol Rec. 2016;91:89-93.
- Sullivan K. California confirms first human case of the plague in 5 years: what to know. NBC News website. https://www.nbcnews.com/health/health-news/california-confirms-first-human-case-bubonic-plague-5-years-what-n1237306. Published August 19, 2020. Accessed August 24, 2020.
- Hinnebusch BJ, Bland DM, Bosio CF, et al. Comparative ability of Oropsylla and Xenopsylla cheopis fleas to transmit Yersinia pestis by two different mechanisms. PLOS Negl Trop Dis. 2017;11:e0005276.
- Bobrov AG, Kirillina O, Vadyvaloo V, et al. The Yersinia pestis HmsCDE regulatory system is essential for blockage of the oriental rat flea (Xenopsylla cheopis), a classic plague vector. Environ Microbiol. 2015;17:947-959.
- Darby C, Ananth SL, Tan L, et al. Identification of gmhA, a Yersina pestis gene required for flea blockage, by using a Caenorhabditis elegans biofilm system. Infect Immun. 2005;73:7236-7242.
- Eisen RJ, Dennis DT, Gage KL. The role of early-phase transmission in the spread of Yersinia pestis. J Med Entomol. 2015;52:1183-1192.
- Carniel E. Subtle genetic modifications transformed an enteropathogen into a flea-borne pathogen. Proc Natl Acad Sci U S A. 2014;111:18409-18410.
- Chouikha I, Hinnebusch BJ. Silencing urease: a key evolutionary step that facilitated the adaptation of Yersinia pestis to the flea-borne transmission route. Proc Natl Acad Sci U S A. 2014;111:18709-19714.
- Aoyagi KL, Brooks BD, Bearden SW, et al. LPS modification promotes maintenance of Yersinia pestis in fleas. Microbiology. 2015;161:628-638.
- Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913-918.
- Eremeeva ME, Warashina WR, Sturgeon MM, et al. Rickettsia typhi and R. felis in rat fleas (Xenopsylla cheopis), Oahu, Hawaii. Emerg Infect Dis. 2018;14:1613-1615.
- Billeter SA, Gundi VAKB, Rood MP, et al. Molecular detection and identification of Bartonella species in Xenopsylla cheopis fleas (Siphonaptera: Pulicidae) collected from Rattus norvecus rats in Los Angeles, California. Appl Environ Microbiol. 2011;77:7850-7852.
- Miarinjara A, Boyer S. Current perspectives on plague vector control in Madagascar: susceptibility status of Xenopsylla cheopis to 12 insecticides. PLOS Negl Trop Dis. 2016;10:e0004414.
- Harrison GF, Scheirer JL, Melanson VR. Development and validation of an arthropod maceration protocol for zoonotic pathogen detection in mosquitoes and fleas. J Vector Ecol. 2014;40:83-89.
- Bitam I, Dittmar K, Parola P, et al. Flea and flea-borne diseases. Int J Infect Dis. 2010;14:E667-E676.
- Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clin Microbiol Rev. 2014;27:48-67.
- Ligon BL. Plague: a review of its history and potential as a biological weapon. Semin Pediatr Infect Dis. 2006;17:161-170.
- Josko D. Yersinia pestis: still a plague in the 21st century. Clin Lab Sci. 2004;17:25-29.
- Plague around the world, 2010–2015. Wkly Epidemiol Rec. 2016;91:89-93.
- Sullivan K. California confirms first human case of the plague in 5 years: what to know. NBC News website. https://www.nbcnews.com/health/health-news/california-confirms-first-human-case-bubonic-plague-5-years-what-n1237306. Published August 19, 2020. Accessed August 24, 2020.
- Hinnebusch BJ, Bland DM, Bosio CF, et al. Comparative ability of Oropsylla and Xenopsylla cheopis fleas to transmit Yersinia pestis by two different mechanisms. PLOS Negl Trop Dis. 2017;11:e0005276.
- Bobrov AG, Kirillina O, Vadyvaloo V, et al. The Yersinia pestis HmsCDE regulatory system is essential for blockage of the oriental rat flea (Xenopsylla cheopis), a classic plague vector. Environ Microbiol. 2015;17:947-959.
- Darby C, Ananth SL, Tan L, et al. Identification of gmhA, a Yersina pestis gene required for flea blockage, by using a Caenorhabditis elegans biofilm system. Infect Immun. 2005;73:7236-7242.
- Eisen RJ, Dennis DT, Gage KL. The role of early-phase transmission in the spread of Yersinia pestis. J Med Entomol. 2015;52:1183-1192.
- Carniel E. Subtle genetic modifications transformed an enteropathogen into a flea-borne pathogen. Proc Natl Acad Sci U S A. 2014;111:18409-18410.
- Chouikha I, Hinnebusch BJ. Silencing urease: a key evolutionary step that facilitated the adaptation of Yersinia pestis to the flea-borne transmission route. Proc Natl Acad Sci U S A. 2014;111:18709-19714.
- Aoyagi KL, Brooks BD, Bearden SW, et al. LPS modification promotes maintenance of Yersinia pestis in fleas. Microbiology. 2015;161:628-638.
- Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913-918.
- Eremeeva ME, Warashina WR, Sturgeon MM, et al. Rickettsia typhi and R. felis in rat fleas (Xenopsylla cheopis), Oahu, Hawaii. Emerg Infect Dis. 2018;14:1613-1615.
- Billeter SA, Gundi VAKB, Rood MP, et al. Molecular detection and identification of Bartonella species in Xenopsylla cheopis fleas (Siphonaptera: Pulicidae) collected from Rattus norvecus rats in Los Angeles, California. Appl Environ Microbiol. 2011;77:7850-7852.
- Miarinjara A, Boyer S. Current perspectives on plague vector control in Madagascar: susceptibility status of Xenopsylla cheopis to 12 insecticides. PLOS Negl Trop Dis. 2016;10:e0004414.
- Harrison GF, Scheirer JL, Melanson VR. Development and validation of an arthropod maceration protocol for zoonotic pathogen detection in mosquitoes and fleas. J Vector Ecol. 2014;40:83-89.
Practice Points
- Xenopsylla cheopis, the oriental rat flea, is most known for carrying Yersinia pestis, the causative agent of the plague; however, it also is a vector for other bacteria, such as Rickettsia typhi, the species responsible for most cases of murine typhus.
- Despite the perception that it largely is a historical illness, modern outbreaks of plague occur in many parts of the world each year. Because fleas thrive in warm humid weather, global warming threatens the spread of the oriental rat flea and its diseases into previously unaffected parts of the world.
- There has been an effort to control oriental rat flea populations, which unfortunately has been complicated by pesticide resistance in many flea populations. It is important to continue to research the oriental rat flea and the bacterial species it carries in the hopes of finding better methods of controlling the pests and therefore decreasing illness in humans.
- Health care providers should be vigilant in identifying symptoms of flea-borne illnesses. If a patient is displaying symptoms, prompt recognition and antibiotic therapy is critical, particularly for the plague.
Approximation of Alcohol-Based Hand Sanitizer Volume Using a Toothpaste Cap
Practice Gap
The Centers for Disease Control and Prevention recommends handwashing with soap and water or using alcohol-based hand sanitizers to prevent transmission of coronavirus disease 2019. Five steps are delineated for effective handwashing: wetting, lathering, scrubbing, rinsing, and drying. Although alcohol-based sanitizers may be perceived as more damaging to the skin, they are less likely to cause dermatitis than handwashing with soap and water.1 Instructions are precise for handwashing, while there are no recommendations for effective use of alcohol-based hand sanitizers. A common inquiry regarding alcohol-based hand sanitizers is the volume needed for efficacy without causing skin irritation.
The Technique
Approximately 1 mL of alcohol-based hand sanitizer is recommended by some manufacturers. However, abundant evidence refutes this recommendation, including a study that tested the microbial efficacy of alcohol-based sanitizers by volume. A volume of 2 mL was necessary to achieve the 2.0 log reduction of contaminants as required by the US Food and Drug Administration for antimicrobial efficacy.2 The precise measurement of hand sanitizer using a calibrated syringe before each use is impractical. Thus, we recommend using a screw-top toothpaste cap to assist in approximating the necessary volume (Figure). The cap holds approximately 1 mL of liquid as measured using a syringe; therefore, 2 caps filled with sanitizer should be used.

Practice Implications
The general public may be underutilizing hand sanitizer due to fear of excessive skin irritation or supply shortages, which will reduce efficacy. Patients and physicians can use this simple visual approximation to ensure adequate use of hand sanitizer volume.
- Stutz N, Becker D, Jappe U, et al. Nurses’ perceptions of the benefits and adverse effects of hand disinfection: alcohol-based hand rubs vs. hygienic handwashing: a multicentre questionnaire study with additional patch testing by the German Contact Dermatitis Research Group. Br J Dermatol. 2009;160:565-572.
- Kampf G, Ruselack S, Eggerstedt S, et al. Less and less-influence of volume on hand coverage and bactericidal efficacy in hand disinfection. BMC Infect Dis. 2013;13:472.
Practice Gap
The Centers for Disease Control and Prevention recommends handwashing with soap and water or using alcohol-based hand sanitizers to prevent transmission of coronavirus disease 2019. Five steps are delineated for effective handwashing: wetting, lathering, scrubbing, rinsing, and drying. Although alcohol-based sanitizers may be perceived as more damaging to the skin, they are less likely to cause dermatitis than handwashing with soap and water.1 Instructions are precise for handwashing, while there are no recommendations for effective use of alcohol-based hand sanitizers. A common inquiry regarding alcohol-based hand sanitizers is the volume needed for efficacy without causing skin irritation.
The Technique
Approximately 1 mL of alcohol-based hand sanitizer is recommended by some manufacturers. However, abundant evidence refutes this recommendation, including a study that tested the microbial efficacy of alcohol-based sanitizers by volume. A volume of 2 mL was necessary to achieve the 2.0 log reduction of contaminants as required by the US Food and Drug Administration for antimicrobial efficacy.2 The precise measurement of hand sanitizer using a calibrated syringe before each use is impractical. Thus, we recommend using a screw-top toothpaste cap to assist in approximating the necessary volume (Figure). The cap holds approximately 1 mL of liquid as measured using a syringe; therefore, 2 caps filled with sanitizer should be used.

Practice Implications
The general public may be underutilizing hand sanitizer due to fear of excessive skin irritation or supply shortages, which will reduce efficacy. Patients and physicians can use this simple visual approximation to ensure adequate use of hand sanitizer volume.
Practice Gap
The Centers for Disease Control and Prevention recommends handwashing with soap and water or using alcohol-based hand sanitizers to prevent transmission of coronavirus disease 2019. Five steps are delineated for effective handwashing: wetting, lathering, scrubbing, rinsing, and drying. Although alcohol-based sanitizers may be perceived as more damaging to the skin, they are less likely to cause dermatitis than handwashing with soap and water.1 Instructions are precise for handwashing, while there are no recommendations for effective use of alcohol-based hand sanitizers. A common inquiry regarding alcohol-based hand sanitizers is the volume needed for efficacy without causing skin irritation.
The Technique
Approximately 1 mL of alcohol-based hand sanitizer is recommended by some manufacturers. However, abundant evidence refutes this recommendation, including a study that tested the microbial efficacy of alcohol-based sanitizers by volume. A volume of 2 mL was necessary to achieve the 2.0 log reduction of contaminants as required by the US Food and Drug Administration for antimicrobial efficacy.2 The precise measurement of hand sanitizer using a calibrated syringe before each use is impractical. Thus, we recommend using a screw-top toothpaste cap to assist in approximating the necessary volume (Figure). The cap holds approximately 1 mL of liquid as measured using a syringe; therefore, 2 caps filled with sanitizer should be used.

Practice Implications
The general public may be underutilizing hand sanitizer due to fear of excessive skin irritation or supply shortages, which will reduce efficacy. Patients and physicians can use this simple visual approximation to ensure adequate use of hand sanitizer volume.
- Stutz N, Becker D, Jappe U, et al. Nurses’ perceptions of the benefits and adverse effects of hand disinfection: alcohol-based hand rubs vs. hygienic handwashing: a multicentre questionnaire study with additional patch testing by the German Contact Dermatitis Research Group. Br J Dermatol. 2009;160:565-572.
- Kampf G, Ruselack S, Eggerstedt S, et al. Less and less-influence of volume on hand coverage and bactericidal efficacy in hand disinfection. BMC Infect Dis. 2013;13:472.
- Stutz N, Becker D, Jappe U, et al. Nurses’ perceptions of the benefits and adverse effects of hand disinfection: alcohol-based hand rubs vs. hygienic handwashing: a multicentre questionnaire study with additional patch testing by the German Contact Dermatitis Research Group. Br J Dermatol. 2009;160:565-572.
- Kampf G, Ruselack S, Eggerstedt S, et al. Less and less-influence of volume on hand coverage and bactericidal efficacy in hand disinfection. BMC Infect Dis. 2013;13:472.
Social distancing impacts other infectious diseases
Diagnoses of 12 common pediatric infectious diseases in a large pediatric primary care network declined significantly in the weeks after COVID-19 social distancing (SD) was enacted in Massachusetts, compared with the same time period in 2019, an analysis of EHR data has shown.
While declines in infectious disease transmission with SD are not surprising, “these data demonstrate the extent to which transmission of common pediatric infections can be altered when close contact with other children is eliminated,” Jonathan Hatoun, MD, MPH of the Pediatric Physicians’ Organization at Children’s in Brookline, Mass., and coauthors wrote in Pediatrics . “Notably, three of the studied diseases, namely, influenza, croup, and bronchiolitis, essentially disappeared with [social distancing].”
The researchers analyzed the weekly incidence of each diagnosis for similar calendar periods in 2019 and 2020. A pre-SD period was defined as week 1-9, starting on Jan. 1, and a post-SD period was defined as week 13-18. (The several-week gap represented an implementation period as social distancing was enacted in the state earlier in 2020, from a declared statewide state of emergency through school closures and stay-at-home advisories.)
To isolate the effect of widespread SD, they performed a “difference-in-differences regression analysis, with diagnosis count as a function of calendar year, time period (pre-SD versus post-SD) and the interaction between the two.” The Massachusetts pediatric network provides care for approximately 375,000 children in 100 locations around the state.
In their research brief, Dr. Hatoun and coauthors presented weekly rates expressed as diagnoses per 100,000 patients per day. The rate of bronchiolitis, for instance, was 18 and 8 in the pre- and post-SD–equivalent weeks of 2019, respectively, and 20 and 0.6 in the pre- and post-SD weeks of 2020. Their analysis showed the rate in the 2020 post-SD period to be 10 diagnoses per 100,000 patients per day lower than they would have expected based on the 2019 trend.
Rates of pneumonia, acute otitis media, and streptococcal pharyngitis were similarly 14, 85, and 31 diagnoses per 100,000 patients per day lower, respectively. The prevalence of each of the other conditions analyzed – the common cold, croup, gastroenteritis, nonstreptococcal pharyngitis, sinusitis, skin and soft tissue infections, and urinary tract infection (UTI) – also was significantly lower in the 2020 post-SD period than would be expected based on 2019 data (P < .001 for all diagnoses).
Putting things in perspective
“This study puts numbers to the sense that we have all had in pediatrics – that social distancing appears to have had a dramatic impact on the transmission of common childhood infectious diseases, especially other respiratory viral pathogens,” Audrey R. John, MD, PhD, chief of the division of pediatric infectious disease at Children’s Hospital of Philadelphia, said in an interview.
The authors acknowledged the possible role of families not seeking care, but said that a smaller decrease in diagnoses of UTI – generally not a contagious disease – “suggests that changes in care-seeking behavior had a relatively modest effect on the other observed declines.” (The rate of UTI for the pre- and post-SD periods was 3.3 and 3.7 per 100,000 patients per day in 2019, and 3.4 and 2.4 in 2020, for a difference in differences of –1.5).
In an accompanying editorial, David W. Kimberlin, MD and Erica C. Bjornstad, MD, PhD, MPH, of the University of Alabama at Birmingham, called the report “provocative” and wrote that similar observations of infections dropping during periods of isolation – namely, dramatic declines in influenza and other respiratory viruses in Seattle after a record snowstorm in 2019 – combined with findings from other modeling studies “suggest that the decline [reported in Boston] is indeed real” (Pediatrics 2020. doi: 10.1542/peds.2020-019232).
However, “we also now know that immunization rates for American children have plummeted since the onset of the SARS-CoV-2 pandemic [because of a] ... dramatic decrease in the use of health care during the first months of the pandemic,” they wrote. “Viewed through this lens,” the declines reported in Boston may reflect inflections going “undiagnosed and untreated.”
Ultimately, Dr. Kimberlin and Dr. Bjornstad said, “the verdict remains out.”
Dr. John said that she and others are “concerned about children not seeking care in a timely manner, and [concerned] that reductions in reported infections might be due to a lack of recognition rather than a lack of transmission.”
In Philadelphia, however, declines in admissions for asthma exacerbations, “which are often caused by respiratory viral infections, suggests that this may not be the case,” said Dr. John, who was asked to comment on the study.
In addition, she said, the Massachusetts data showing that UTI diagnoses “are nearly as common this year as in 2019” are “reassuring.”
Are there lessons for the future?
Coauthor Louis Vernacchio, MD, MSc, chief medical officer of the Pediatric Physicians’ Organization at Children’s network, said in an interview that beyond the pandemic, it’s likely that “more careful attention to proven infection control practices in daycares and schools could reduce the burden of common infectious diseases in children.”
Dr. John similarly sees a long-term value of quantifying the impact of social distancing. “We’ve always known [for instance] that bronchiolitis is the result of viral infection.” Findings like the Massachusetts data “will help us advise families who might be trying to protect their premature infants (at risk for severe bronchiolitis) through social distancing.”
The analysis covered both in-person and telemedicine encounters occurring on weekdays.
The authors of the research brief indicated they have no relevant financial disclosures and there was no external funding. The authors of the commentary also reported they have no relevant financial disclosures, and Dr. John said she had no relevant financial disclosures.
SOURCE: Hatoun J et al. Pediatrics. 2020. doi: 10.1542/peds.2020-006460.
Diagnoses of 12 common pediatric infectious diseases in a large pediatric primary care network declined significantly in the weeks after COVID-19 social distancing (SD) was enacted in Massachusetts, compared with the same time period in 2019, an analysis of EHR data has shown.
While declines in infectious disease transmission with SD are not surprising, “these data demonstrate the extent to which transmission of common pediatric infections can be altered when close contact with other children is eliminated,” Jonathan Hatoun, MD, MPH of the Pediatric Physicians’ Organization at Children’s in Brookline, Mass., and coauthors wrote in Pediatrics . “Notably, three of the studied diseases, namely, influenza, croup, and bronchiolitis, essentially disappeared with [social distancing].”
The researchers analyzed the weekly incidence of each diagnosis for similar calendar periods in 2019 and 2020. A pre-SD period was defined as week 1-9, starting on Jan. 1, and a post-SD period was defined as week 13-18. (The several-week gap represented an implementation period as social distancing was enacted in the state earlier in 2020, from a declared statewide state of emergency through school closures and stay-at-home advisories.)
To isolate the effect of widespread SD, they performed a “difference-in-differences regression analysis, with diagnosis count as a function of calendar year, time period (pre-SD versus post-SD) and the interaction between the two.” The Massachusetts pediatric network provides care for approximately 375,000 children in 100 locations around the state.
In their research brief, Dr. Hatoun and coauthors presented weekly rates expressed as diagnoses per 100,000 patients per day. The rate of bronchiolitis, for instance, was 18 and 8 in the pre- and post-SD–equivalent weeks of 2019, respectively, and 20 and 0.6 in the pre- and post-SD weeks of 2020. Their analysis showed the rate in the 2020 post-SD period to be 10 diagnoses per 100,000 patients per day lower than they would have expected based on the 2019 trend.
Rates of pneumonia, acute otitis media, and streptococcal pharyngitis were similarly 14, 85, and 31 diagnoses per 100,000 patients per day lower, respectively. The prevalence of each of the other conditions analyzed – the common cold, croup, gastroenteritis, nonstreptococcal pharyngitis, sinusitis, skin and soft tissue infections, and urinary tract infection (UTI) – also was significantly lower in the 2020 post-SD period than would be expected based on 2019 data (P < .001 for all diagnoses).
Putting things in perspective
“This study puts numbers to the sense that we have all had in pediatrics – that social distancing appears to have had a dramatic impact on the transmission of common childhood infectious diseases, especially other respiratory viral pathogens,” Audrey R. John, MD, PhD, chief of the division of pediatric infectious disease at Children’s Hospital of Philadelphia, said in an interview.
The authors acknowledged the possible role of families not seeking care, but said that a smaller decrease in diagnoses of UTI – generally not a contagious disease – “suggests that changes in care-seeking behavior had a relatively modest effect on the other observed declines.” (The rate of UTI for the pre- and post-SD periods was 3.3 and 3.7 per 100,000 patients per day in 2019, and 3.4 and 2.4 in 2020, for a difference in differences of –1.5).
In an accompanying editorial, David W. Kimberlin, MD and Erica C. Bjornstad, MD, PhD, MPH, of the University of Alabama at Birmingham, called the report “provocative” and wrote that similar observations of infections dropping during periods of isolation – namely, dramatic declines in influenza and other respiratory viruses in Seattle after a record snowstorm in 2019 – combined with findings from other modeling studies “suggest that the decline [reported in Boston] is indeed real” (Pediatrics 2020. doi: 10.1542/peds.2020-019232).
However, “we also now know that immunization rates for American children have plummeted since the onset of the SARS-CoV-2 pandemic [because of a] ... dramatic decrease in the use of health care during the first months of the pandemic,” they wrote. “Viewed through this lens,” the declines reported in Boston may reflect inflections going “undiagnosed and untreated.”
Ultimately, Dr. Kimberlin and Dr. Bjornstad said, “the verdict remains out.”
Dr. John said that she and others are “concerned about children not seeking care in a timely manner, and [concerned] that reductions in reported infections might be due to a lack of recognition rather than a lack of transmission.”
In Philadelphia, however, declines in admissions for asthma exacerbations, “which are often caused by respiratory viral infections, suggests that this may not be the case,” said Dr. John, who was asked to comment on the study.
In addition, she said, the Massachusetts data showing that UTI diagnoses “are nearly as common this year as in 2019” are “reassuring.”
Are there lessons for the future?
Coauthor Louis Vernacchio, MD, MSc, chief medical officer of the Pediatric Physicians’ Organization at Children’s network, said in an interview that beyond the pandemic, it’s likely that “more careful attention to proven infection control practices in daycares and schools could reduce the burden of common infectious diseases in children.”
Dr. John similarly sees a long-term value of quantifying the impact of social distancing. “We’ve always known [for instance] that bronchiolitis is the result of viral infection.” Findings like the Massachusetts data “will help us advise families who might be trying to protect their premature infants (at risk for severe bronchiolitis) through social distancing.”
The analysis covered both in-person and telemedicine encounters occurring on weekdays.
The authors of the research brief indicated they have no relevant financial disclosures and there was no external funding. The authors of the commentary also reported they have no relevant financial disclosures, and Dr. John said she had no relevant financial disclosures.
SOURCE: Hatoun J et al. Pediatrics. 2020. doi: 10.1542/peds.2020-006460.
Diagnoses of 12 common pediatric infectious diseases in a large pediatric primary care network declined significantly in the weeks after COVID-19 social distancing (SD) was enacted in Massachusetts, compared with the same time period in 2019, an analysis of EHR data has shown.
While declines in infectious disease transmission with SD are not surprising, “these data demonstrate the extent to which transmission of common pediatric infections can be altered when close contact with other children is eliminated,” Jonathan Hatoun, MD, MPH of the Pediatric Physicians’ Organization at Children’s in Brookline, Mass., and coauthors wrote in Pediatrics . “Notably, three of the studied diseases, namely, influenza, croup, and bronchiolitis, essentially disappeared with [social distancing].”
The researchers analyzed the weekly incidence of each diagnosis for similar calendar periods in 2019 and 2020. A pre-SD period was defined as week 1-9, starting on Jan. 1, and a post-SD period was defined as week 13-18. (The several-week gap represented an implementation period as social distancing was enacted in the state earlier in 2020, from a declared statewide state of emergency through school closures and stay-at-home advisories.)
To isolate the effect of widespread SD, they performed a “difference-in-differences regression analysis, with diagnosis count as a function of calendar year, time period (pre-SD versus post-SD) and the interaction between the two.” The Massachusetts pediatric network provides care for approximately 375,000 children in 100 locations around the state.
In their research brief, Dr. Hatoun and coauthors presented weekly rates expressed as diagnoses per 100,000 patients per day. The rate of bronchiolitis, for instance, was 18 and 8 in the pre- and post-SD–equivalent weeks of 2019, respectively, and 20 and 0.6 in the pre- and post-SD weeks of 2020. Their analysis showed the rate in the 2020 post-SD period to be 10 diagnoses per 100,000 patients per day lower than they would have expected based on the 2019 trend.
Rates of pneumonia, acute otitis media, and streptococcal pharyngitis were similarly 14, 85, and 31 diagnoses per 100,000 patients per day lower, respectively. The prevalence of each of the other conditions analyzed – the common cold, croup, gastroenteritis, nonstreptococcal pharyngitis, sinusitis, skin and soft tissue infections, and urinary tract infection (UTI) – also was significantly lower in the 2020 post-SD period than would be expected based on 2019 data (P < .001 for all diagnoses).
Putting things in perspective
“This study puts numbers to the sense that we have all had in pediatrics – that social distancing appears to have had a dramatic impact on the transmission of common childhood infectious diseases, especially other respiratory viral pathogens,” Audrey R. John, MD, PhD, chief of the division of pediatric infectious disease at Children’s Hospital of Philadelphia, said in an interview.
The authors acknowledged the possible role of families not seeking care, but said that a smaller decrease in diagnoses of UTI – generally not a contagious disease – “suggests that changes in care-seeking behavior had a relatively modest effect on the other observed declines.” (The rate of UTI for the pre- and post-SD periods was 3.3 and 3.7 per 100,000 patients per day in 2019, and 3.4 and 2.4 in 2020, for a difference in differences of –1.5).
In an accompanying editorial, David W. Kimberlin, MD and Erica C. Bjornstad, MD, PhD, MPH, of the University of Alabama at Birmingham, called the report “provocative” and wrote that similar observations of infections dropping during periods of isolation – namely, dramatic declines in influenza and other respiratory viruses in Seattle after a record snowstorm in 2019 – combined with findings from other modeling studies “suggest that the decline [reported in Boston] is indeed real” (Pediatrics 2020. doi: 10.1542/peds.2020-019232).
However, “we also now know that immunization rates for American children have plummeted since the onset of the SARS-CoV-2 pandemic [because of a] ... dramatic decrease in the use of health care during the first months of the pandemic,” they wrote. “Viewed through this lens,” the declines reported in Boston may reflect inflections going “undiagnosed and untreated.”
Ultimately, Dr. Kimberlin and Dr. Bjornstad said, “the verdict remains out.”
Dr. John said that she and others are “concerned about children not seeking care in a timely manner, and [concerned] that reductions in reported infections might be due to a lack of recognition rather than a lack of transmission.”
In Philadelphia, however, declines in admissions for asthma exacerbations, “which are often caused by respiratory viral infections, suggests that this may not be the case,” said Dr. John, who was asked to comment on the study.
In addition, she said, the Massachusetts data showing that UTI diagnoses “are nearly as common this year as in 2019” are “reassuring.”
Are there lessons for the future?
Coauthor Louis Vernacchio, MD, MSc, chief medical officer of the Pediatric Physicians’ Organization at Children’s network, said in an interview that beyond the pandemic, it’s likely that “more careful attention to proven infection control practices in daycares and schools could reduce the burden of common infectious diseases in children.”
Dr. John similarly sees a long-term value of quantifying the impact of social distancing. “We’ve always known [for instance] that bronchiolitis is the result of viral infection.” Findings like the Massachusetts data “will help us advise families who might be trying to protect their premature infants (at risk for severe bronchiolitis) through social distancing.”
The analysis covered both in-person and telemedicine encounters occurring on weekdays.
The authors of the research brief indicated they have no relevant financial disclosures and there was no external funding. The authors of the commentary also reported they have no relevant financial disclosures, and Dr. John said she had no relevant financial disclosures.
SOURCE: Hatoun J et al. Pediatrics. 2020. doi: 10.1542/peds.2020-006460.
FROM PEDIATRICS