User login
Clinical Application and Limitations of the Fluorescence In Situ Hybridization (FISH) Assay in the Diagnosis and Management of Melanocytic Lesions: A Report of 3 Cases
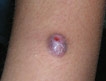
What Is Your Diagnosis? Spitzoid Melanoma
Kaposi's Sarcoma Returns: The Skinny Vodcast
Skin & Allergy News Managing Editor Amy Pfeiffer and Senior Editor Terry Rudd review hot news in dermatology with the experts in this month's newscast.
Highlights include an interview with Dr. Daniel M. Siegel about the AAD's political action committee, SkinPAC. And, Sherry Boschert talks to an HIV expert about the return of Kaposi's sarcoma.
Lastly, Dr. Lily Talakoub shares her best practices for treating adult acne.
Skin & Allergy News Managing Editor Amy Pfeiffer and Senior Editor Terry Rudd review hot news in dermatology with the experts in this month's newscast.
Highlights include an interview with Dr. Daniel M. Siegel about the AAD's political action committee, SkinPAC. And, Sherry Boschert talks to an HIV expert about the return of Kaposi's sarcoma.
Lastly, Dr. Lily Talakoub shares her best practices for treating adult acne.
Skin & Allergy News Managing Editor Amy Pfeiffer and Senior Editor Terry Rudd review hot news in dermatology with the experts in this month's newscast.
Highlights include an interview with Dr. Daniel M. Siegel about the AAD's political action committee, SkinPAC. And, Sherry Boschert talks to an HIV expert about the return of Kaposi's sarcoma.
Lastly, Dr. Lily Talakoub shares her best practices for treating adult acne.
SPECT/CT Before SLN Excision Improves Melanoma Survival
Single-photon emission computed tomography/computed tomography imaging before sentinel lymph node excision was associated with significantly higher disease-free survival rates in melanoma patients, according to the results of a new study published in JAMA Sept. 12.
In addition to less local relapse and a better 4-year progression-free survival, SPECT/CT was associated with the detection of more positive nodes, more sentinel lymph node-excision (SLNE) procedures performed in the head and neck area, and improved detection of positive nodes in obese patients than standard SLNE (JAMA 2012;308:1007-14).
SPECT/CT offers "the preoperative possibility of determining the exact location and visualization of the SLN, especially if the tracer signal is too weak for detection by the handheld gamma probe alone or the SLN is in the immediate vicinity of the remaining tracer depot," Dr. Ingo Stoffels and colleagues wrote. They noted that for 33 patients in the SPECT/CT cohort, the surgical approach was changed based on SPECT/CT findings.
Dr. Stoffels, of the University of Essen-Duisburg, Germany, and colleagues, analyzed a cohort of 403 patients with clinically negative lymph nodes. All patients underwent SLNE with (149) or without (254) preoperative SPECT/CT between 2003 and 2011 at a skin cancer treatment facility where, after 2008, preoperative SPECT/CT became the standard of care.
Dr. Stoffels and colleagues found that SPECT/CT allowed SLNEs in the head and neck more frequently (23.5% for SPECT/CT, compared with 2% for standard care). Also, more SLNs per patient were detected in the SPECT/CT cohort than in the SLNE alone cohort (2.40 vs. 1.87, respectively), and the number of positive SLNs per patient was also higher in the SPECT/CT cohort (0.34 vs. 0.21). The false-negative SLN rate was 6.8% in the SPECT/CT cohort and 23.8% in the SLNE alone cohort.
Importantly, the local relapse rate in the SPECT/CT cohort was lower than in the SLNE alone cohort (6.8% vs. 23.8%, respectively), and 4-year disease-free survival was higher in the SPECT/CT cohort than in the SLNE alone cohort (93.9% vs. 79.2%, respectively). However, overall survival did not differ between the cohorts.
Dr. Stoffels and colleagues also found that SPECT/CT improved detection of positive SLNs among patients with a body mass index of 30 or higher; 5 positive SLNs out of 20 were detected (25%) in 7 obese patients in the SPECT/CT cohort, compared with 4 positive SLNs out of 44 (9.1%) in 24 obese patients in the SLNE alone cohort.
"Our results demonstrate clear advantages of adding the described preoperative SLN imaging by SPECT/CT to the current practice of preoperative lymphoscintigraphy in patients with melanoma," the investigators wrote.
They acknowledged that the temporal separation of the two cohorts was a limitation of the study as it "could lead to a bias for the time-dependent end points."
The investigators received no outside funding for their research. A coauthor, Dr. Dirk Schadendorf, disclosed receiving consultancy fees, board membership, and lecture fees from Amgen, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, MSD, Novartis, and Roche.
The study by Dr. Stoffels and colleagues has several limitations worth mentioning, beginning with the inherent limitation in looking at groups undergoing procedures during two different time periods during which there may have been slight variations in surgical technique.
Second, the median follow-up was shorter in the SPECT/CT cohort (11 months), versus the standard lymphoscintigraphy cohort (35 months). Third, there were significant differences in patient characteristics between the two cohorts, such as fewer patients with head and neck primaries in the standard cohort than in the SPECT/CT cohort (6 vs. 32, respectively), and fewer obese patients in the SPECT/CT cohort. Finally, vital blue dye – routinely used by many centers internationally in conjunction with radioactive colloid dye for SLN localization – was not used by the investigators. Therefore, it is difficult to determine how the use of this second dye may have impacted the results.
These limitations aside, there is the strong suggestion from the data that the use of SPECT/CT –particularly in obese patients or in those with head and neck primaries – may help in the identification of sentinel nodes and, therefore, more accurately stage patients with melanoma.
Moreover, this technology may assist in surgical planning, allowing for more directed, and potentially smaller, surgical incisions. Further studies with larger patient numbers will be needed to further corroborate the findings.
Giorgos C. Karakousis, M.D., is an assistant professor of surgery at the Abramson Cancer Center, Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia. He had no relevant conflicts of interest to disclose.
The study by Dr. Stoffels and colleagues has several limitations worth mentioning, beginning with the inherent limitation in looking at groups undergoing procedures during two different time periods during which there may have been slight variations in surgical technique.
Second, the median follow-up was shorter in the SPECT/CT cohort (11 months), versus the standard lymphoscintigraphy cohort (35 months). Third, there were significant differences in patient characteristics between the two cohorts, such as fewer patients with head and neck primaries in the standard cohort than in the SPECT/CT cohort (6 vs. 32, respectively), and fewer obese patients in the SPECT/CT cohort. Finally, vital blue dye – routinely used by many centers internationally in conjunction with radioactive colloid dye for SLN localization – was not used by the investigators. Therefore, it is difficult to determine how the use of this second dye may have impacted the results.
These limitations aside, there is the strong suggestion from the data that the use of SPECT/CT –particularly in obese patients or in those with head and neck primaries – may help in the identification of sentinel nodes and, therefore, more accurately stage patients with melanoma.
Moreover, this technology may assist in surgical planning, allowing for more directed, and potentially smaller, surgical incisions. Further studies with larger patient numbers will be needed to further corroborate the findings.
Giorgos C. Karakousis, M.D., is an assistant professor of surgery at the Abramson Cancer Center, Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia. He had no relevant conflicts of interest to disclose.
The study by Dr. Stoffels and colleagues has several limitations worth mentioning, beginning with the inherent limitation in looking at groups undergoing procedures during two different time periods during which there may have been slight variations in surgical technique.
Second, the median follow-up was shorter in the SPECT/CT cohort (11 months), versus the standard lymphoscintigraphy cohort (35 months). Third, there were significant differences in patient characteristics between the two cohorts, such as fewer patients with head and neck primaries in the standard cohort than in the SPECT/CT cohort (6 vs. 32, respectively), and fewer obese patients in the SPECT/CT cohort. Finally, vital blue dye – routinely used by many centers internationally in conjunction with radioactive colloid dye for SLN localization – was not used by the investigators. Therefore, it is difficult to determine how the use of this second dye may have impacted the results.
These limitations aside, there is the strong suggestion from the data that the use of SPECT/CT –particularly in obese patients or in those with head and neck primaries – may help in the identification of sentinel nodes and, therefore, more accurately stage patients with melanoma.
Moreover, this technology may assist in surgical planning, allowing for more directed, and potentially smaller, surgical incisions. Further studies with larger patient numbers will be needed to further corroborate the findings.
Giorgos C. Karakousis, M.D., is an assistant professor of surgery at the Abramson Cancer Center, Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia. He had no relevant conflicts of interest to disclose.
Single-photon emission computed tomography/computed tomography imaging before sentinel lymph node excision was associated with significantly higher disease-free survival rates in melanoma patients, according to the results of a new study published in JAMA Sept. 12.
In addition to less local relapse and a better 4-year progression-free survival, SPECT/CT was associated with the detection of more positive nodes, more sentinel lymph node-excision (SLNE) procedures performed in the head and neck area, and improved detection of positive nodes in obese patients than standard SLNE (JAMA 2012;308:1007-14).
SPECT/CT offers "the preoperative possibility of determining the exact location and visualization of the SLN, especially if the tracer signal is too weak for detection by the handheld gamma probe alone or the SLN is in the immediate vicinity of the remaining tracer depot," Dr. Ingo Stoffels and colleagues wrote. They noted that for 33 patients in the SPECT/CT cohort, the surgical approach was changed based on SPECT/CT findings.
Dr. Stoffels, of the University of Essen-Duisburg, Germany, and colleagues, analyzed a cohort of 403 patients with clinically negative lymph nodes. All patients underwent SLNE with (149) or without (254) preoperative SPECT/CT between 2003 and 2011 at a skin cancer treatment facility where, after 2008, preoperative SPECT/CT became the standard of care.
Dr. Stoffels and colleagues found that SPECT/CT allowed SLNEs in the head and neck more frequently (23.5% for SPECT/CT, compared with 2% for standard care). Also, more SLNs per patient were detected in the SPECT/CT cohort than in the SLNE alone cohort (2.40 vs. 1.87, respectively), and the number of positive SLNs per patient was also higher in the SPECT/CT cohort (0.34 vs. 0.21). The false-negative SLN rate was 6.8% in the SPECT/CT cohort and 23.8% in the SLNE alone cohort.
Importantly, the local relapse rate in the SPECT/CT cohort was lower than in the SLNE alone cohort (6.8% vs. 23.8%, respectively), and 4-year disease-free survival was higher in the SPECT/CT cohort than in the SLNE alone cohort (93.9% vs. 79.2%, respectively). However, overall survival did not differ between the cohorts.
Dr. Stoffels and colleagues also found that SPECT/CT improved detection of positive SLNs among patients with a body mass index of 30 or higher; 5 positive SLNs out of 20 were detected (25%) in 7 obese patients in the SPECT/CT cohort, compared with 4 positive SLNs out of 44 (9.1%) in 24 obese patients in the SLNE alone cohort.
"Our results demonstrate clear advantages of adding the described preoperative SLN imaging by SPECT/CT to the current practice of preoperative lymphoscintigraphy in patients with melanoma," the investigators wrote.
They acknowledged that the temporal separation of the two cohorts was a limitation of the study as it "could lead to a bias for the time-dependent end points."
The investigators received no outside funding for their research. A coauthor, Dr. Dirk Schadendorf, disclosed receiving consultancy fees, board membership, and lecture fees from Amgen, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, MSD, Novartis, and Roche.
Single-photon emission computed tomography/computed tomography imaging before sentinel lymph node excision was associated with significantly higher disease-free survival rates in melanoma patients, according to the results of a new study published in JAMA Sept. 12.
In addition to less local relapse and a better 4-year progression-free survival, SPECT/CT was associated with the detection of more positive nodes, more sentinel lymph node-excision (SLNE) procedures performed in the head and neck area, and improved detection of positive nodes in obese patients than standard SLNE (JAMA 2012;308:1007-14).
SPECT/CT offers "the preoperative possibility of determining the exact location and visualization of the SLN, especially if the tracer signal is too weak for detection by the handheld gamma probe alone or the SLN is in the immediate vicinity of the remaining tracer depot," Dr. Ingo Stoffels and colleagues wrote. They noted that for 33 patients in the SPECT/CT cohort, the surgical approach was changed based on SPECT/CT findings.
Dr. Stoffels, of the University of Essen-Duisburg, Germany, and colleagues, analyzed a cohort of 403 patients with clinically negative lymph nodes. All patients underwent SLNE with (149) or without (254) preoperative SPECT/CT between 2003 and 2011 at a skin cancer treatment facility where, after 2008, preoperative SPECT/CT became the standard of care.
Dr. Stoffels and colleagues found that SPECT/CT allowed SLNEs in the head and neck more frequently (23.5% for SPECT/CT, compared with 2% for standard care). Also, more SLNs per patient were detected in the SPECT/CT cohort than in the SLNE alone cohort (2.40 vs. 1.87, respectively), and the number of positive SLNs per patient was also higher in the SPECT/CT cohort (0.34 vs. 0.21). The false-negative SLN rate was 6.8% in the SPECT/CT cohort and 23.8% in the SLNE alone cohort.
Importantly, the local relapse rate in the SPECT/CT cohort was lower than in the SLNE alone cohort (6.8% vs. 23.8%, respectively), and 4-year disease-free survival was higher in the SPECT/CT cohort than in the SLNE alone cohort (93.9% vs. 79.2%, respectively). However, overall survival did not differ between the cohorts.
Dr. Stoffels and colleagues also found that SPECT/CT improved detection of positive SLNs among patients with a body mass index of 30 or higher; 5 positive SLNs out of 20 were detected (25%) in 7 obese patients in the SPECT/CT cohort, compared with 4 positive SLNs out of 44 (9.1%) in 24 obese patients in the SLNE alone cohort.
"Our results demonstrate clear advantages of adding the described preoperative SLN imaging by SPECT/CT to the current practice of preoperative lymphoscintigraphy in patients with melanoma," the investigators wrote.
They acknowledged that the temporal separation of the two cohorts was a limitation of the study as it "could lead to a bias for the time-dependent end points."
The investigators received no outside funding for their research. A coauthor, Dr. Dirk Schadendorf, disclosed receiving consultancy fees, board membership, and lecture fees from Amgen, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, MSD, Novartis, and Roche.
FROM JAMA
Major Finding: The local relapse rate in the SPECT/CT cohort was lower than in the SLNE alone cohort (6.8% vs. 23.8%, respectively).
Data Source: A cohort of 403 patients with clinically negative lymph nodes who underwent SLNE with (149) or without (254) preoperative SPECT/CT between 2003 and 2011.
Disclosures: The investigators received no outside funding for their research. A coauthor, Dr. Dirk Schadendorf, disclosed receiving consultancy fees, board membership, and lecture fees from Amgen, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, MSD, Novartis, and Roche.
Medicare Okays Wound Plasma Gel for Clinical Trial Patients
The Centers for Medicare and Medicaid Services has announced coverage of platelet-rich plasma gel for chronic wounds, but only for patients who are enrolled in clinical trials that meet the agency’s criteria.
"The available evidence does not permit us to conclude that use of autologous PRP improves health outcomes in beneficiaries with chronic diabetic, pressure, and/or venous wounds," the agency wrote in its decision statement Therefore, trials are needed to prove effectiveness.
The decision comes after CMS rejection of coverage since 1992, according to Cytomedix of Gaithersburg, Md., which manufactures a device called AutoloGel that produces PRP from a patient’s own blood. "This determination reverses a nearly 20-year non-coverage determination for PRP and provides for an appropriate research study with practical study designs we are confident will demonstrate that patients treated with our autologous PRP product, the AutoloGel System, experience clinically significant health outcomes," Martin P. Rosendale, CEO of Cytomedix, said in a statement.
Cytomedix submitted another request in October 2011 for CMS review of its PRP therapy, which was approved under the coverage with evidence development (CED) program.
Under the program, services, equipment, and supplies are covered through Medicare and Medicaid while a product is further investigated. CMS noted in its decision that it "is evaluating PRP as a service, and not a specific system for administrating PRP."
In the PRP process, autologous blood is centrifuged to produce a concentrate of platelets and plasma proteins. Individual growth factors are not identified or separated, but additives are used to change the consistency.
Autologous PRP has had many uses over the years, including use as an adhesive in plastic surgery and as filler for acute wounds. It has also been used to treat amateur and professional athletes – such as golfer Tiger Woods, basketball player Kobe Bryant, and baseball star Alex Rodriguez – to help heal tendons and ligaments, and is growing in popularity in the sports world, according to recent consumer news reports.
According to the CMS, to continue being covered for wound healing PRP therapy must show complete wound healing in patients and a reduction of wound size or healing trajectory. It also must help a patient’s ability to return to previous function and normal activities. The studies have to be prospective and enroll Medicare beneficiaries with chronic nonhealing diabetic, pressure, and/or venous wounds who are receiving optimal care, in addition to the therapy.
The agency must receive all pertinent data by August 2014.
Cytomedix said that it has been in talks with CMS about trials. "We look forward to continuing to work with CMS in the coming weeks in further defining the specifics of the protocols for the research studies and clinical questions to be answered through the CED program," said Mr. Rosendale.
The Centers for Medicare and Medicaid Services has announced coverage of platelet-rich plasma gel for chronic wounds, but only for patients who are enrolled in clinical trials that meet the agency’s criteria.
"The available evidence does not permit us to conclude that use of autologous PRP improves health outcomes in beneficiaries with chronic diabetic, pressure, and/or venous wounds," the agency wrote in its decision statement Therefore, trials are needed to prove effectiveness.
The decision comes after CMS rejection of coverage since 1992, according to Cytomedix of Gaithersburg, Md., which manufactures a device called AutoloGel that produces PRP from a patient’s own blood. "This determination reverses a nearly 20-year non-coverage determination for PRP and provides for an appropriate research study with practical study designs we are confident will demonstrate that patients treated with our autologous PRP product, the AutoloGel System, experience clinically significant health outcomes," Martin P. Rosendale, CEO of Cytomedix, said in a statement.
Cytomedix submitted another request in October 2011 for CMS review of its PRP therapy, which was approved under the coverage with evidence development (CED) program.
Under the program, services, equipment, and supplies are covered through Medicare and Medicaid while a product is further investigated. CMS noted in its decision that it "is evaluating PRP as a service, and not a specific system for administrating PRP."
In the PRP process, autologous blood is centrifuged to produce a concentrate of platelets and plasma proteins. Individual growth factors are not identified or separated, but additives are used to change the consistency.
Autologous PRP has had many uses over the years, including use as an adhesive in plastic surgery and as filler for acute wounds. It has also been used to treat amateur and professional athletes – such as golfer Tiger Woods, basketball player Kobe Bryant, and baseball star Alex Rodriguez – to help heal tendons and ligaments, and is growing in popularity in the sports world, according to recent consumer news reports.
According to the CMS, to continue being covered for wound healing PRP therapy must show complete wound healing in patients and a reduction of wound size or healing trajectory. It also must help a patient’s ability to return to previous function and normal activities. The studies have to be prospective and enroll Medicare beneficiaries with chronic nonhealing diabetic, pressure, and/or venous wounds who are receiving optimal care, in addition to the therapy.
The agency must receive all pertinent data by August 2014.
Cytomedix said that it has been in talks with CMS about trials. "We look forward to continuing to work with CMS in the coming weeks in further defining the specifics of the protocols for the research studies and clinical questions to be answered through the CED program," said Mr. Rosendale.
The Centers for Medicare and Medicaid Services has announced coverage of platelet-rich plasma gel for chronic wounds, but only for patients who are enrolled in clinical trials that meet the agency’s criteria.
"The available evidence does not permit us to conclude that use of autologous PRP improves health outcomes in beneficiaries with chronic diabetic, pressure, and/or venous wounds," the agency wrote in its decision statement Therefore, trials are needed to prove effectiveness.
The decision comes after CMS rejection of coverage since 1992, according to Cytomedix of Gaithersburg, Md., which manufactures a device called AutoloGel that produces PRP from a patient’s own blood. "This determination reverses a nearly 20-year non-coverage determination for PRP and provides for an appropriate research study with practical study designs we are confident will demonstrate that patients treated with our autologous PRP product, the AutoloGel System, experience clinically significant health outcomes," Martin P. Rosendale, CEO of Cytomedix, said in a statement.
Cytomedix submitted another request in October 2011 for CMS review of its PRP therapy, which was approved under the coverage with evidence development (CED) program.
Under the program, services, equipment, and supplies are covered through Medicare and Medicaid while a product is further investigated. CMS noted in its decision that it "is evaluating PRP as a service, and not a specific system for administrating PRP."
In the PRP process, autologous blood is centrifuged to produce a concentrate of platelets and plasma proteins. Individual growth factors are not identified or separated, but additives are used to change the consistency.
Autologous PRP has had many uses over the years, including use as an adhesive in plastic surgery and as filler for acute wounds. It has also been used to treat amateur and professional athletes – such as golfer Tiger Woods, basketball player Kobe Bryant, and baseball star Alex Rodriguez – to help heal tendons and ligaments, and is growing in popularity in the sports world, according to recent consumer news reports.
According to the CMS, to continue being covered for wound healing PRP therapy must show complete wound healing in patients and a reduction of wound size or healing trajectory. It also must help a patient’s ability to return to previous function and normal activities. The studies have to be prospective and enroll Medicare beneficiaries with chronic nonhealing diabetic, pressure, and/or venous wounds who are receiving optimal care, in addition to the therapy.
The agency must receive all pertinent data by August 2014.
Cytomedix said that it has been in talks with CMS about trials. "We look forward to continuing to work with CMS in the coming weeks in further defining the specifics of the protocols for the research studies and clinical questions to be answered through the CED program," said Mr. Rosendale.
Vemurafenib After Ipilimumab Linked to Rash
CHICAGO – The sequencing and timing of two new targeted therapies for melanoma may have important implications for the development of serious skin toxicity, according to one center’s experience.
Investigators at Memorial Sloan-Kettering Cancer Center in New York retrospectively identified 16 patients treated there for BRAFV600E-mutant metastatic melanoma who received vemurafenib (Zelboraf) after ipilimumab (Yervoy).
Vemurafenib is an inhibitor of the BRAF kinase. Ipilimumab blocks cytotoxic T-lymphocyte–associated antigen 4 (CTLA 4), which normally acts as a key checkpoint or brake in the immune system.
Four of the patients (25%) developed a grade 3 maculopapular rash, according to data reported in a poster session at the annual meeting of the American Society of Clinical Oncology.
Biopsy findings suggested these were drug hypersensitivity reactions, and analyses showed that grade 3 rash was much more likely when vemurafenib was given within 1 month of stopping ipilimumab as compared with later (100% vs. 8%, P = .007).
"It’s interesting to speculate that loss of checkpoint inhibition by ipilimumab might predispose patients to drug reactions," lead investigator Dr. James J. Harding commented in an interview, while cautioning that the study was very small and retrospective.
"The take-home message is these agents, both of which improve overall survival, will be used in sequence. It’s not clear if there is a benefit of sequencing one before the other or combining them – that will be studied prospectively," he noted, as in the case of an ongoing phase I-II trial looking at the two drugs together (NCT01400451).
"Until more data are available, it’s possible that there may be a significant maculopapular rash if you give vemurafenib within a month of ipilimumab. In almost all cases, a dose interruption followed by dose reduction is acceptable," he added.
"One thing that people need to remember is that if you give vemurafenib after ipilimumab, you are giving a combination therapy because the ipilimumab half-life is 2 weeks," noted discussant Dr. Mario Sznol, vice-chief of medical oncology with the Yale Medical Group in New Haven, CT.
"I would have hoped that we would have seen really dramatic antitumor effects with this combination, especially in the patients who were treated soon after their last dose of ipilimumab. And in fact that’s not what we saw," he added. "I don’t think this curve [waterfall plot] looks much better than what we would have seen with vemurafenib alone in this population of patients," with no apparent difference for patients receiving vemurafenib within 45 days of ipilimumab and the rest.
"So it’s just a warning that there will be sequence issues and toxicity interactions, and we really need to know the biology when we combine these agents," Dr. Sznol concluded. "We may do better with this combination, but we may not. We may need to use this in combination with other agents."
Of the 16 patients studied, 13 (81%) developed any-grade skin rash on vemurafenib, making this by far the most common adverse event observed. (For comparison, the rate of skin rash with vemurafenib was 37% in the BRIM-3 trial and 52% in the BRIM-2 trial.)
The cases of grade 3 rash developed within 6-8 days of starting vemurafenib and began as a pruritic eruption on the neck or chest that rapidly expanded to involve the back, trunk, and extremities. The incidence seen was triple that in the BRIM-3 trial (25% vs. 8%, P = .02).
Biopsies, performed in two of the four patients, revealed spongiotic and perivascular dermatitis with eosinophils, consistent with drug hypersensitivity reaction.
Although the time elapsed since the prior ipilimumab influenced the development of grade 3 rash, the dose of prior ipilimumab, number of doses, and immune-related adverse events did not.
None of the rashes progressed to anaphylaxis or Stevens-Johnson syndrome. Steroids appeared to be largely ineffective, according to Dr. Harding; one patient developed the rash while already taking steroids, and another was given steroids with little to no improvement.
"We essentially stopped the vemurafenib and then redosed it 11 days later [after the rash resolved]. And, with the exception of one patient, all of the patients tolerated it well and were able to continue," he reported.
The objective overall response rate with vemurafenib was 50%, similar to what was seen in the prior phase II and III trials of the drug.
Dr. Harding disclosed no relevant conflicts of interest. Dr. Sznol disclosed that he is a consultant to Abbott Laboratories, Anaeropharma, BioVex, Bristol-Myers Squibb, Genesis Biopharma, Genzyme, and Prometheus; receives honoraria from Prometheus; and receives research funding from Bristol-Myers Squibb.
CHICAGO – The sequencing and timing of two new targeted therapies for melanoma may have important implications for the development of serious skin toxicity, according to one center’s experience.
Investigators at Memorial Sloan-Kettering Cancer Center in New York retrospectively identified 16 patients treated there for BRAFV600E-mutant metastatic melanoma who received vemurafenib (Zelboraf) after ipilimumab (Yervoy).
Vemurafenib is an inhibitor of the BRAF kinase. Ipilimumab blocks cytotoxic T-lymphocyte–associated antigen 4 (CTLA 4), which normally acts as a key checkpoint or brake in the immune system.
Four of the patients (25%) developed a grade 3 maculopapular rash, according to data reported in a poster session at the annual meeting of the American Society of Clinical Oncology.
Biopsy findings suggested these were drug hypersensitivity reactions, and analyses showed that grade 3 rash was much more likely when vemurafenib was given within 1 month of stopping ipilimumab as compared with later (100% vs. 8%, P = .007).
"It’s interesting to speculate that loss of checkpoint inhibition by ipilimumab might predispose patients to drug reactions," lead investigator Dr. James J. Harding commented in an interview, while cautioning that the study was very small and retrospective.
"The take-home message is these agents, both of which improve overall survival, will be used in sequence. It’s not clear if there is a benefit of sequencing one before the other or combining them – that will be studied prospectively," he noted, as in the case of an ongoing phase I-II trial looking at the two drugs together (NCT01400451).
"Until more data are available, it’s possible that there may be a significant maculopapular rash if you give vemurafenib within a month of ipilimumab. In almost all cases, a dose interruption followed by dose reduction is acceptable," he added.
"One thing that people need to remember is that if you give vemurafenib after ipilimumab, you are giving a combination therapy because the ipilimumab half-life is 2 weeks," noted discussant Dr. Mario Sznol, vice-chief of medical oncology with the Yale Medical Group in New Haven, CT.
"I would have hoped that we would have seen really dramatic antitumor effects with this combination, especially in the patients who were treated soon after their last dose of ipilimumab. And in fact that’s not what we saw," he added. "I don’t think this curve [waterfall plot] looks much better than what we would have seen with vemurafenib alone in this population of patients," with no apparent difference for patients receiving vemurafenib within 45 days of ipilimumab and the rest.
"So it’s just a warning that there will be sequence issues and toxicity interactions, and we really need to know the biology when we combine these agents," Dr. Sznol concluded. "We may do better with this combination, but we may not. We may need to use this in combination with other agents."
Of the 16 patients studied, 13 (81%) developed any-grade skin rash on vemurafenib, making this by far the most common adverse event observed. (For comparison, the rate of skin rash with vemurafenib was 37% in the BRIM-3 trial and 52% in the BRIM-2 trial.)
The cases of grade 3 rash developed within 6-8 days of starting vemurafenib and began as a pruritic eruption on the neck or chest that rapidly expanded to involve the back, trunk, and extremities. The incidence seen was triple that in the BRIM-3 trial (25% vs. 8%, P = .02).
Biopsies, performed in two of the four patients, revealed spongiotic and perivascular dermatitis with eosinophils, consistent with drug hypersensitivity reaction.
Although the time elapsed since the prior ipilimumab influenced the development of grade 3 rash, the dose of prior ipilimumab, number of doses, and immune-related adverse events did not.
None of the rashes progressed to anaphylaxis or Stevens-Johnson syndrome. Steroids appeared to be largely ineffective, according to Dr. Harding; one patient developed the rash while already taking steroids, and another was given steroids with little to no improvement.
"We essentially stopped the vemurafenib and then redosed it 11 days later [after the rash resolved]. And, with the exception of one patient, all of the patients tolerated it well and were able to continue," he reported.
The objective overall response rate with vemurafenib was 50%, similar to what was seen in the prior phase II and III trials of the drug.
Dr. Harding disclosed no relevant conflicts of interest. Dr. Sznol disclosed that he is a consultant to Abbott Laboratories, Anaeropharma, BioVex, Bristol-Myers Squibb, Genesis Biopharma, Genzyme, and Prometheus; receives honoraria from Prometheus; and receives research funding from Bristol-Myers Squibb.
CHICAGO – The sequencing and timing of two new targeted therapies for melanoma may have important implications for the development of serious skin toxicity, according to one center’s experience.
Investigators at Memorial Sloan-Kettering Cancer Center in New York retrospectively identified 16 patients treated there for BRAFV600E-mutant metastatic melanoma who received vemurafenib (Zelboraf) after ipilimumab (Yervoy).
Vemurafenib is an inhibitor of the BRAF kinase. Ipilimumab blocks cytotoxic T-lymphocyte–associated antigen 4 (CTLA 4), which normally acts as a key checkpoint or brake in the immune system.
Four of the patients (25%) developed a grade 3 maculopapular rash, according to data reported in a poster session at the annual meeting of the American Society of Clinical Oncology.
Biopsy findings suggested these were drug hypersensitivity reactions, and analyses showed that grade 3 rash was much more likely when vemurafenib was given within 1 month of stopping ipilimumab as compared with later (100% vs. 8%, P = .007).
"It’s interesting to speculate that loss of checkpoint inhibition by ipilimumab might predispose patients to drug reactions," lead investigator Dr. James J. Harding commented in an interview, while cautioning that the study was very small and retrospective.
"The take-home message is these agents, both of which improve overall survival, will be used in sequence. It’s not clear if there is a benefit of sequencing one before the other or combining them – that will be studied prospectively," he noted, as in the case of an ongoing phase I-II trial looking at the two drugs together (NCT01400451).
"Until more data are available, it’s possible that there may be a significant maculopapular rash if you give vemurafenib within a month of ipilimumab. In almost all cases, a dose interruption followed by dose reduction is acceptable," he added.
"One thing that people need to remember is that if you give vemurafenib after ipilimumab, you are giving a combination therapy because the ipilimumab half-life is 2 weeks," noted discussant Dr. Mario Sznol, vice-chief of medical oncology with the Yale Medical Group in New Haven, CT.
"I would have hoped that we would have seen really dramatic antitumor effects with this combination, especially in the patients who were treated soon after their last dose of ipilimumab. And in fact that’s not what we saw," he added. "I don’t think this curve [waterfall plot] looks much better than what we would have seen with vemurafenib alone in this population of patients," with no apparent difference for patients receiving vemurafenib within 45 days of ipilimumab and the rest.
"So it’s just a warning that there will be sequence issues and toxicity interactions, and we really need to know the biology when we combine these agents," Dr. Sznol concluded. "We may do better with this combination, but we may not. We may need to use this in combination with other agents."
Of the 16 patients studied, 13 (81%) developed any-grade skin rash on vemurafenib, making this by far the most common adverse event observed. (For comparison, the rate of skin rash with vemurafenib was 37% in the BRIM-3 trial and 52% in the BRIM-2 trial.)
The cases of grade 3 rash developed within 6-8 days of starting vemurafenib and began as a pruritic eruption on the neck or chest that rapidly expanded to involve the back, trunk, and extremities. The incidence seen was triple that in the BRIM-3 trial (25% vs. 8%, P = .02).
Biopsies, performed in two of the four patients, revealed spongiotic and perivascular dermatitis with eosinophils, consistent with drug hypersensitivity reaction.
Although the time elapsed since the prior ipilimumab influenced the development of grade 3 rash, the dose of prior ipilimumab, number of doses, and immune-related adverse events did not.
None of the rashes progressed to anaphylaxis or Stevens-Johnson syndrome. Steroids appeared to be largely ineffective, according to Dr. Harding; one patient developed the rash while already taking steroids, and another was given steroids with little to no improvement.
"We essentially stopped the vemurafenib and then redosed it 11 days later [after the rash resolved]. And, with the exception of one patient, all of the patients tolerated it well and were able to continue," he reported.
The objective overall response rate with vemurafenib was 50%, similar to what was seen in the prior phase II and III trials of the drug.
Dr. Harding disclosed no relevant conflicts of interest. Dr. Sznol disclosed that he is a consultant to Abbott Laboratories, Anaeropharma, BioVex, Bristol-Myers Squibb, Genesis Biopharma, Genzyme, and Prometheus; receives honoraria from Prometheus; and receives research funding from Bristol-Myers Squibb.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY
Major Finding: Four patients (25%) developed grade 3 maculopapular skin rash that histologically had features of a drug hypersensitivity rash.
Data Source: A single-center retrospective case series of 16 patients with BRAFV600E-mutant metastatic melanoma treated with vemurafenib after ipilimumab
Disclosures: Dr. Harding disclosed no relevant conflicts of interest. Dr. Sznol disclosed that he is a consultant to Abbott Laboratories, Anaeropharma Science, BioVex, Bristol-Myers Squibb, Genesis Biopharma, Genzyme, and Prometheus; receives honoraria from Prometheus; and receives research funding from Bristol-Myers Squibb.
Kaposi's Sarcoma Makes Unwelcome Return
Kaposi's sarcoma is making a comeback in aging HIV patients, even among those with well-controlled disease. Dr. Kieron S. Leslie, an associate clinical professor of dermatology at the University of California, San Francisco, discusses the cancer's return and its implications for patients with HIV.
Kaposi's sarcoma is making a comeback in aging HIV patients, even among those with well-controlled disease. Dr. Kieron S. Leslie, an associate clinical professor of dermatology at the University of California, San Francisco, discusses the cancer's return and its implications for patients with HIV.
Kaposi's sarcoma is making a comeback in aging HIV patients, even among those with well-controlled disease. Dr. Kieron S. Leslie, an associate clinical professor of dermatology at the University of California, San Francisco, discusses the cancer's return and its implications for patients with HIV.
Skin Cancer Drug Steps Into New Alzheimer's Study
The first human trial of bexarotene, the skin cancer drug shown to clear beta-amyloid brain plaques in mice with Alzheimer’s-like pathology, will commence sometime in September.
The phase Ib trial will randomize 12 healthy subjects to placebo or bexarotene (Targretin), Gary E. Landreth, Ph.D., said in an interview.
Researchers will obtain hourly samples of cerebrospinal fluid and blood for 36 hours after administration of the drug, looking for signs that bexarotene increases natural clearance of the beta-amyloid protein, said Dr. Landreth of Case Western Reserve University, Cleveland.
"If our hypothesis is correct, the drug should accelerate the synthesis and steady-state levels of apolipoprotein E," a protein that helps break down soluble beta-amyloid, he said. Hungry microglia are bexarotene’s second punch; in mice, the drug activated these cells to scavenge beta-amyloid plaques.
If these two events also occur in humans, Dr. Landreth said, "we will have a reason to go forward" into phase II trials.
Media stories about Dr. Landreth’s research sparked a firestorm of dialogue on Alzheimer’s Internet forums and message boards this spring. Families discussed traveling to Canada for bexarotene, and begging their family doctors for a prescription.
Two opinion pieces in the Aug. 9 issue of the New England Journal of Medicine tackled the thorny problem physicians may face when desperate families come looking for answers.
"Writing an off-label prescription is completely permissible and would fulfill family members’ desire to try anything to help their loved ones," wrote Dr. Steven D. Pearson of Massachusetts General Hospital’s Institute for Technology Assessment, Boston, and his colleagues. But in the case of bexarotene, they asked, "is it the right thing to do?"
In the second editorial, Frank M. LaFerla, Ph.D., of the University of California, Irvine, warned against overenthusiasm: "Only a well-designed and carefully executed clinical trial will reveal whether this class of drug lives up to its promise. Until such trials are performed, it would be a mistake to offer this treatment to Alzheimer’s patients."
Dr. Landreth agrees. Although relatively benign, bexarotene can cause hypothyroidism and dyslipidemia in cancer patients. Although volunteers in the upcoming trial will almost certainly experience neither of these, "there is no guarantee at all that it would be safe in an Alzheimer’s population," he said. "I have absolutely no idea what could happen when this drug interacts with a diseased brain. If you remove the plaque, then what happens? Do you end up with holes in the brain? This is the question that keeps me up at night."
Dr. Landreth and his colleague, Paige Cramer, hold a U.S. provisional patent application for bexarotene as a potential therapy for Alzheimer’s disease and have founded ReXceptor Inc., which has licensing options from the university to use bexarotene to treat Alzheimer’s disease.
The first human trial of bexarotene, the skin cancer drug shown to clear beta-amyloid brain plaques in mice with Alzheimer’s-like pathology, will commence sometime in September.
The phase Ib trial will randomize 12 healthy subjects to placebo or bexarotene (Targretin), Gary E. Landreth, Ph.D., said in an interview.
Researchers will obtain hourly samples of cerebrospinal fluid and blood for 36 hours after administration of the drug, looking for signs that bexarotene increases natural clearance of the beta-amyloid protein, said Dr. Landreth of Case Western Reserve University, Cleveland.
"If our hypothesis is correct, the drug should accelerate the synthesis and steady-state levels of apolipoprotein E," a protein that helps break down soluble beta-amyloid, he said. Hungry microglia are bexarotene’s second punch; in mice, the drug activated these cells to scavenge beta-amyloid plaques.
If these two events also occur in humans, Dr. Landreth said, "we will have a reason to go forward" into phase II trials.
Media stories about Dr. Landreth’s research sparked a firestorm of dialogue on Alzheimer’s Internet forums and message boards this spring. Families discussed traveling to Canada for bexarotene, and begging their family doctors for a prescription.
Two opinion pieces in the Aug. 9 issue of the New England Journal of Medicine tackled the thorny problem physicians may face when desperate families come looking for answers.
"Writing an off-label prescription is completely permissible and would fulfill family members’ desire to try anything to help their loved ones," wrote Dr. Steven D. Pearson of Massachusetts General Hospital’s Institute for Technology Assessment, Boston, and his colleagues. But in the case of bexarotene, they asked, "is it the right thing to do?"
In the second editorial, Frank M. LaFerla, Ph.D., of the University of California, Irvine, warned against overenthusiasm: "Only a well-designed and carefully executed clinical trial will reveal whether this class of drug lives up to its promise. Until such trials are performed, it would be a mistake to offer this treatment to Alzheimer’s patients."
Dr. Landreth agrees. Although relatively benign, bexarotene can cause hypothyroidism and dyslipidemia in cancer patients. Although volunteers in the upcoming trial will almost certainly experience neither of these, "there is no guarantee at all that it would be safe in an Alzheimer’s population," he said. "I have absolutely no idea what could happen when this drug interacts with a diseased brain. If you remove the plaque, then what happens? Do you end up with holes in the brain? This is the question that keeps me up at night."
Dr. Landreth and his colleague, Paige Cramer, hold a U.S. provisional patent application for bexarotene as a potential therapy for Alzheimer’s disease and have founded ReXceptor Inc., which has licensing options from the university to use bexarotene to treat Alzheimer’s disease.
The first human trial of bexarotene, the skin cancer drug shown to clear beta-amyloid brain plaques in mice with Alzheimer’s-like pathology, will commence sometime in September.
The phase Ib trial will randomize 12 healthy subjects to placebo or bexarotene (Targretin), Gary E. Landreth, Ph.D., said in an interview.
Researchers will obtain hourly samples of cerebrospinal fluid and blood for 36 hours after administration of the drug, looking for signs that bexarotene increases natural clearance of the beta-amyloid protein, said Dr. Landreth of Case Western Reserve University, Cleveland.
"If our hypothesis is correct, the drug should accelerate the synthesis and steady-state levels of apolipoprotein E," a protein that helps break down soluble beta-amyloid, he said. Hungry microglia are bexarotene’s second punch; in mice, the drug activated these cells to scavenge beta-amyloid plaques.
If these two events also occur in humans, Dr. Landreth said, "we will have a reason to go forward" into phase II trials.
Media stories about Dr. Landreth’s research sparked a firestorm of dialogue on Alzheimer’s Internet forums and message boards this spring. Families discussed traveling to Canada for bexarotene, and begging their family doctors for a prescription.
Two opinion pieces in the Aug. 9 issue of the New England Journal of Medicine tackled the thorny problem physicians may face when desperate families come looking for answers.
"Writing an off-label prescription is completely permissible and would fulfill family members’ desire to try anything to help their loved ones," wrote Dr. Steven D. Pearson of Massachusetts General Hospital’s Institute for Technology Assessment, Boston, and his colleagues. But in the case of bexarotene, they asked, "is it the right thing to do?"
In the second editorial, Frank M. LaFerla, Ph.D., of the University of California, Irvine, warned against overenthusiasm: "Only a well-designed and carefully executed clinical trial will reveal whether this class of drug lives up to its promise. Until such trials are performed, it would be a mistake to offer this treatment to Alzheimer’s patients."
Dr. Landreth agrees. Although relatively benign, bexarotene can cause hypothyroidism and dyslipidemia in cancer patients. Although volunteers in the upcoming trial will almost certainly experience neither of these, "there is no guarantee at all that it would be safe in an Alzheimer’s population," he said. "I have absolutely no idea what could happen when this drug interacts with a diseased brain. If you remove the plaque, then what happens? Do you end up with holes in the brain? This is the question that keeps me up at night."
Dr. Landreth and his colleague, Paige Cramer, hold a U.S. provisional patent application for bexarotene as a potential therapy for Alzheimer’s disease and have founded ReXceptor Inc., which has licensing options from the university to use bexarotene to treat Alzheimer’s disease.
KIT Inhibition Promising for Select Few
ORLANDO – Patients who express a KIT genetic abnormality represent a small minority of those with melanoma, but there is a lot of interest in the development of specific inhibitors, said Dr. Richard D. Carvajal at the annual meeting of the Florida Society of Dermatology and Dermatologic Surgery.
Studies from Dr. Carvajal and other researchers indicate that inhibitors could significantly prolong median progression-free survival for patients who have amplification, overexpression, or a mutation of this genetic driver of melanoma.
In his phase II study of Novartis’ imatinib (Gleevec) for patients with melanoma and KIT alterations, 4 of 25 evaluable patients responded, for an overall durable response rate of 16%. Median progression-free survival was "pretty modest at 3 to 3.5 months," Dr. Carvajal said. Two patients had a complete response to therapy, and another two achieved a partial response (JAMA 2011;305:2327-34).
"So the question is: Can we better select patients likely to respond?" Dr. Carvajal asked at the meeting. "Certainly this is not vemurafenib [Zelboraf], where we get a response in 50% of patients. It could be due to variability in KIT mutations," said Dr. Carvajal, a medical oncologist specializing in melanoma and sarcomas at Memorial Sloan-Kettering Cancer Center in New York.
Only 3% of patients with melanoma harbor a KIT mutation. "You have to have a mutation in KIT; otherwise, the patient is not going to respond to imatinib," he said. The study was preceded by three negative clinical trials in unselected patients (before the mutation was identified).
In another phase II study enrolling only patients with KIT alterations, investigators reported that 10 of 43 patients responded to treatment with imatinib, for a 23% overall response rate (J. Clin. Oncol. 2011;29:2904-9).
Studies are underway with potential KIT inhibitors other than imatinib, including Pfizer’s sunitinib (Sutent), Novartis’ nilotinib (Tasigna), and Bristol-Myers Squibb’s dasatinib (Sprycel).
Sunitinib
Sunitinib is "reasonably promising" for melanoma patients with KIT mutations, said Dr. David Minor, of the California Pacific Center for Melanoma Research and Treatment at the University of California, San Francisco.
He and his colleagues conducted gene sequencing of tumors from 90 patients with advanced melanoma. The findings revealed that 11% featured a KIT mutation (Clin. Cancer Res. 2012:18:1457-63).
Ten of 12 patients treated with sunitinib were evaluable – 4 with KIT mutations and 6 with KIT amplification or overexpression. Although the mutation was a significant predictor of shortened survival time, treatment with sunitinib was associated with complete remission in 1 patient for 15 months and 2 partial responses for 1 and 7 months, he noted. A KIT mutation might be more clinically relevant because there were no complete responses and only one partial response in patients with KIT amplification or overexpression. Dr. Minor is director of inpatient oncology at the California Pacific Medical Center.
Nilotinib
Because only a small percentage of melanoma patients harbor a KIT mutation, conducting large efficacy studies remains a challenge, Dr. Carvajal said. "Indeed, a randomized, phase III trial of nilotinib versus DTIC [dacarbazine] in KIT-mutant melanoma was designed to be the definitive study demonstrating improved outcomes with nilotinib versus DTIC," he said. However, after patient accrual began for the TEAM (Tasigna Efficacy in Advanced Melanoma) trial in 2010, researchers reported difficulty enrolling a sufficient number of patients with metastatic or inoperable melanoma and a KIT mutation. Therefore, they redesigned the study into a single-arm phase II trial of nilotinib alone.
Researchers at the University of Pittsburgh also termed nilotinib a promising agent for melanoma patients with relevant KIT mutations in a review article (Expert Opin. Investig. Drugs 2012;21:861-9).
Dasatinib
Dr. Harriet Kluger and her colleagues at Yale University in New Haven, Conn., reported mixed efficacy for dasatinib in a phase II study (Cancer 2011;117:2202-8).
"At this point I would say that other than in the setting of patients with c-KIT mutations in their tumors, which is still being studied, dasatinib is not a promising agent for this disease," Dr. Kluger said in an interview. She is on the medical oncology faculty at Yale.
She and her colleagues studied 36 evaluable patients with stage 3 or 4 unresectable melanoma. Dasatinib was associated with two partial responses (24 weeks and 64 weeks) and three minor responses. Another patient who initially responded discontinued because of noncompliance. The median progression-free survival was 8 weeks, and the 6-month progression-free survival was 13%. Some activity of dasatinib was observed in a small subset of patients without KIT mutations, suggesting that more research is needed to identify predictive biomarkers for response to this inhibitor.
The KIT gene is located on chromosome 4 and codes for a family of proteins, the receptor tyrosine kinases. KIT function is essential for normal melanocyte genesis and migration. KIT protein signaling also is important for the development of reproductive germ cells, early hematopoietic stem cells, immune mast cells, and interstitial cells of Cajal located in the gastrointestinal tract.
Available data suggest that patients with mutations affecting exons 11 or 13 of KIT may be more likely to achieve clinical benefit with KIT inhibition, Dr. Carvajal said. He added that greater expression of the mutant KIT allele, compared with the wild type, may be a marker of tumors more "addicted" to KIT activation and that may be more susceptible to inhibition.
"This work is still fairly new," he said.
Dr. Carvajal is a consultant to Novartis. Dr. Minor and Dr. Kluger said that they had no relevant disclosures.
ORLANDO – Patients who express a KIT genetic abnormality represent a small minority of those with melanoma, but there is a lot of interest in the development of specific inhibitors, said Dr. Richard D. Carvajal at the annual meeting of the Florida Society of Dermatology and Dermatologic Surgery.
Studies from Dr. Carvajal and other researchers indicate that inhibitors could significantly prolong median progression-free survival for patients who have amplification, overexpression, or a mutation of this genetic driver of melanoma.
In his phase II study of Novartis’ imatinib (Gleevec) for patients with melanoma and KIT alterations, 4 of 25 evaluable patients responded, for an overall durable response rate of 16%. Median progression-free survival was "pretty modest at 3 to 3.5 months," Dr. Carvajal said. Two patients had a complete response to therapy, and another two achieved a partial response (JAMA 2011;305:2327-34).
"So the question is: Can we better select patients likely to respond?" Dr. Carvajal asked at the meeting. "Certainly this is not vemurafenib [Zelboraf], where we get a response in 50% of patients. It could be due to variability in KIT mutations," said Dr. Carvajal, a medical oncologist specializing in melanoma and sarcomas at Memorial Sloan-Kettering Cancer Center in New York.
Only 3% of patients with melanoma harbor a KIT mutation. "You have to have a mutation in KIT; otherwise, the patient is not going to respond to imatinib," he said. The study was preceded by three negative clinical trials in unselected patients (before the mutation was identified).
In another phase II study enrolling only patients with KIT alterations, investigators reported that 10 of 43 patients responded to treatment with imatinib, for a 23% overall response rate (J. Clin. Oncol. 2011;29:2904-9).
Studies are underway with potential KIT inhibitors other than imatinib, including Pfizer’s sunitinib (Sutent), Novartis’ nilotinib (Tasigna), and Bristol-Myers Squibb’s dasatinib (Sprycel).
Sunitinib
Sunitinib is "reasonably promising" for melanoma patients with KIT mutations, said Dr. David Minor, of the California Pacific Center for Melanoma Research and Treatment at the University of California, San Francisco.
He and his colleagues conducted gene sequencing of tumors from 90 patients with advanced melanoma. The findings revealed that 11% featured a KIT mutation (Clin. Cancer Res. 2012:18:1457-63).
Ten of 12 patients treated with sunitinib were evaluable – 4 with KIT mutations and 6 with KIT amplification or overexpression. Although the mutation was a significant predictor of shortened survival time, treatment with sunitinib was associated with complete remission in 1 patient for 15 months and 2 partial responses for 1 and 7 months, he noted. A KIT mutation might be more clinically relevant because there were no complete responses and only one partial response in patients with KIT amplification or overexpression. Dr. Minor is director of inpatient oncology at the California Pacific Medical Center.
Nilotinib
Because only a small percentage of melanoma patients harbor a KIT mutation, conducting large efficacy studies remains a challenge, Dr. Carvajal said. "Indeed, a randomized, phase III trial of nilotinib versus DTIC [dacarbazine] in KIT-mutant melanoma was designed to be the definitive study demonstrating improved outcomes with nilotinib versus DTIC," he said. However, after patient accrual began for the TEAM (Tasigna Efficacy in Advanced Melanoma) trial in 2010, researchers reported difficulty enrolling a sufficient number of patients with metastatic or inoperable melanoma and a KIT mutation. Therefore, they redesigned the study into a single-arm phase II trial of nilotinib alone.
Researchers at the University of Pittsburgh also termed nilotinib a promising agent for melanoma patients with relevant KIT mutations in a review article (Expert Opin. Investig. Drugs 2012;21:861-9).
Dasatinib
Dr. Harriet Kluger and her colleagues at Yale University in New Haven, Conn., reported mixed efficacy for dasatinib in a phase II study (Cancer 2011;117:2202-8).
"At this point I would say that other than in the setting of patients with c-KIT mutations in their tumors, which is still being studied, dasatinib is not a promising agent for this disease," Dr. Kluger said in an interview. She is on the medical oncology faculty at Yale.
She and her colleagues studied 36 evaluable patients with stage 3 or 4 unresectable melanoma. Dasatinib was associated with two partial responses (24 weeks and 64 weeks) and three minor responses. Another patient who initially responded discontinued because of noncompliance. The median progression-free survival was 8 weeks, and the 6-month progression-free survival was 13%. Some activity of dasatinib was observed in a small subset of patients without KIT mutations, suggesting that more research is needed to identify predictive biomarkers for response to this inhibitor.
The KIT gene is located on chromosome 4 and codes for a family of proteins, the receptor tyrosine kinases. KIT function is essential for normal melanocyte genesis and migration. KIT protein signaling also is important for the development of reproductive germ cells, early hematopoietic stem cells, immune mast cells, and interstitial cells of Cajal located in the gastrointestinal tract.
Available data suggest that patients with mutations affecting exons 11 or 13 of KIT may be more likely to achieve clinical benefit with KIT inhibition, Dr. Carvajal said. He added that greater expression of the mutant KIT allele, compared with the wild type, may be a marker of tumors more "addicted" to KIT activation and that may be more susceptible to inhibition.
"This work is still fairly new," he said.
Dr. Carvajal is a consultant to Novartis. Dr. Minor and Dr. Kluger said that they had no relevant disclosures.
ORLANDO – Patients who express a KIT genetic abnormality represent a small minority of those with melanoma, but there is a lot of interest in the development of specific inhibitors, said Dr. Richard D. Carvajal at the annual meeting of the Florida Society of Dermatology and Dermatologic Surgery.
Studies from Dr. Carvajal and other researchers indicate that inhibitors could significantly prolong median progression-free survival for patients who have amplification, overexpression, or a mutation of this genetic driver of melanoma.
In his phase II study of Novartis’ imatinib (Gleevec) for patients with melanoma and KIT alterations, 4 of 25 evaluable patients responded, for an overall durable response rate of 16%. Median progression-free survival was "pretty modest at 3 to 3.5 months," Dr. Carvajal said. Two patients had a complete response to therapy, and another two achieved a partial response (JAMA 2011;305:2327-34).
"So the question is: Can we better select patients likely to respond?" Dr. Carvajal asked at the meeting. "Certainly this is not vemurafenib [Zelboraf], where we get a response in 50% of patients. It could be due to variability in KIT mutations," said Dr. Carvajal, a medical oncologist specializing in melanoma and sarcomas at Memorial Sloan-Kettering Cancer Center in New York.
Only 3% of patients with melanoma harbor a KIT mutation. "You have to have a mutation in KIT; otherwise, the patient is not going to respond to imatinib," he said. The study was preceded by three negative clinical trials in unselected patients (before the mutation was identified).
In another phase II study enrolling only patients with KIT alterations, investigators reported that 10 of 43 patients responded to treatment with imatinib, for a 23% overall response rate (J. Clin. Oncol. 2011;29:2904-9).
Studies are underway with potential KIT inhibitors other than imatinib, including Pfizer’s sunitinib (Sutent), Novartis’ nilotinib (Tasigna), and Bristol-Myers Squibb’s dasatinib (Sprycel).
Sunitinib
Sunitinib is "reasonably promising" for melanoma patients with KIT mutations, said Dr. David Minor, of the California Pacific Center for Melanoma Research and Treatment at the University of California, San Francisco.
He and his colleagues conducted gene sequencing of tumors from 90 patients with advanced melanoma. The findings revealed that 11% featured a KIT mutation (Clin. Cancer Res. 2012:18:1457-63).
Ten of 12 patients treated with sunitinib were evaluable – 4 with KIT mutations and 6 with KIT amplification or overexpression. Although the mutation was a significant predictor of shortened survival time, treatment with sunitinib was associated with complete remission in 1 patient for 15 months and 2 partial responses for 1 and 7 months, he noted. A KIT mutation might be more clinically relevant because there were no complete responses and only one partial response in patients with KIT amplification or overexpression. Dr. Minor is director of inpatient oncology at the California Pacific Medical Center.
Nilotinib
Because only a small percentage of melanoma patients harbor a KIT mutation, conducting large efficacy studies remains a challenge, Dr. Carvajal said. "Indeed, a randomized, phase III trial of nilotinib versus DTIC [dacarbazine] in KIT-mutant melanoma was designed to be the definitive study demonstrating improved outcomes with nilotinib versus DTIC," he said. However, after patient accrual began for the TEAM (Tasigna Efficacy in Advanced Melanoma) trial in 2010, researchers reported difficulty enrolling a sufficient number of patients with metastatic or inoperable melanoma and a KIT mutation. Therefore, they redesigned the study into a single-arm phase II trial of nilotinib alone.
Researchers at the University of Pittsburgh also termed nilotinib a promising agent for melanoma patients with relevant KIT mutations in a review article (Expert Opin. Investig. Drugs 2012;21:861-9).
Dasatinib
Dr. Harriet Kluger and her colleagues at Yale University in New Haven, Conn., reported mixed efficacy for dasatinib in a phase II study (Cancer 2011;117:2202-8).
"At this point I would say that other than in the setting of patients with c-KIT mutations in their tumors, which is still being studied, dasatinib is not a promising agent for this disease," Dr. Kluger said in an interview. She is on the medical oncology faculty at Yale.
She and her colleagues studied 36 evaluable patients with stage 3 or 4 unresectable melanoma. Dasatinib was associated with two partial responses (24 weeks and 64 weeks) and three minor responses. Another patient who initially responded discontinued because of noncompliance. The median progression-free survival was 8 weeks, and the 6-month progression-free survival was 13%. Some activity of dasatinib was observed in a small subset of patients without KIT mutations, suggesting that more research is needed to identify predictive biomarkers for response to this inhibitor.
The KIT gene is located on chromosome 4 and codes for a family of proteins, the receptor tyrosine kinases. KIT function is essential for normal melanocyte genesis and migration. KIT protein signaling also is important for the development of reproductive germ cells, early hematopoietic stem cells, immune mast cells, and interstitial cells of Cajal located in the gastrointestinal tract.
Available data suggest that patients with mutations affecting exons 11 or 13 of KIT may be more likely to achieve clinical benefit with KIT inhibition, Dr. Carvajal said. He added that greater expression of the mutant KIT allele, compared with the wild type, may be a marker of tumors more "addicted" to KIT activation and that may be more susceptible to inhibition.
"This work is still fairly new," he said.
Dr. Carvajal is a consultant to Novartis. Dr. Minor and Dr. Kluger said that they had no relevant disclosures.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE FLORIDA SOCIETY OF DERMATOLOGY AND DERMATOLOGIC SURGERY
Photoprotection for Preventing Skin Cancer and Premature Skin Aging
Dr. Hale discusses how regular sunscreen use can diminish the chances of developing future skin cancers and can slow the process of premature skin aging. For more information, read Dr. Hale's article in the May 2012 issue, "Sunscreens and Photoaging: An Update."
Dr. Hale discusses how regular sunscreen use can diminish the chances of developing future skin cancers and can slow the process of premature skin aging. For more information, read Dr. Hale's article in the May 2012 issue, "Sunscreens and Photoaging: An Update."
Dr. Hale discusses how regular sunscreen use can diminish the chances of developing future skin cancers and can slow the process of premature skin aging. For more information, read Dr. Hale's article in the May 2012 issue, "Sunscreens and Photoaging: An Update."