User login
Study hints at a mechanism behind aggressive melanoma
that combined in vitro and animal model data.
The gene, ARID2, is a part of the switch/sucrose nonfermentable (SWI/SNF) complex, which maneuvers cellular structures called nucleosomes to make cellular DNA accessible. About 20% of human cancers have a mutation within the SWI/SNF complex.
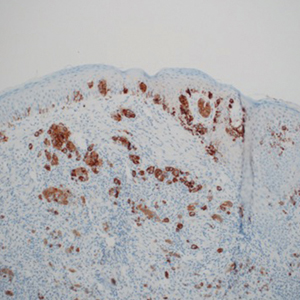
In the new study, published in Cell Reports, researchers reported that the ARID2 subunit was mutated in about 13% of melanoma patients identified through the Cancer Genome Atlas.
ARID2 mutations have been found in early melanoma lesions, which the authors suggested may play a role in early cancer cell dissemination. Other studies have shown SWI/SNF mutations, including ARID2 mutations, in melanoma metastases, especially the brain.
The researchers also found an up-regulation of synaptic pathways in melanoma cells as well as the Cancer Genome Atlas, which also suggests a potential role of ARID2 loss in metastasis or targeting the brain, since synaptic activation in cancer cells has been shown elsewhere to influence cell migration and survival in the brain.
“We look forward to future studies that investigate the role of the PBAF complex ... in order to better tailor treatments for melanoma patients,” wrote the study authors, who were led by Emily Bernstein, PhD, a professor in oncological sciences with the Icahn School of Medicine at Mount Sinai, New York.
The SWI/SNF complex includes a subcomplex that targets specific DNA sequences or chromatin reader domains. There are multiple versions of the targeting subcomplex, but two of the most frequently occurring are BAF and PBAF. The most commonly mutated subunit in melanoma is ARID2, which is part of PBAF, and contains an AT-rich region responsible for non–sequence-specific DNA interactions. There is evidence that it plays a role in tumor suppression. In mouse tumors, depletion of ARID2 is associated with increased sensitivity to immune checkpoint inhibition and destruction by T cells.
To better understand the role of ARID2 in tumor suppression, the researchers used CRISPR-Cas9 to create ARID2 deficiency in a known human metastatic melanoma cell line. They found there was reduced chromatin accessibility and accompanying gene expression among some PBAF and shared BAF-PBAF–occupied regions. There was also increased chromatin accessibility and gene expression in BAF-occupied regions, and these changes were associated with tumor aggression. In mice, they led to metastasis of distal organs.
This mechanism appears to be conserved between different melanoma cell lines, but deregulated transcriptional targets were different depending on the dominant transcription factors in the cell line. That suggests that the effect of ARID2 mutation or loss may be different depending on the stage of melanoma progression or level of invasiveness. “As melanoma comprises transcriptionally distinct, heterogeneous cell populations, we envision future studies utilizing single-cell methodologies to better understand the nuanced effects of ARID2 loss within subpopulations of cells in human melanoma tumors,” the authors wrote.
The study is limited by the fact that not all ARID2 mutations lead to complete loss of protein, and may lead instead to aberrant complexes.
The study was funded by the National Institutes of Health.
that combined in vitro and animal model data.
The gene, ARID2, is a part of the switch/sucrose nonfermentable (SWI/SNF) complex, which maneuvers cellular structures called nucleosomes to make cellular DNA accessible. About 20% of human cancers have a mutation within the SWI/SNF complex.
In the new study, published in Cell Reports, researchers reported that the ARID2 subunit was mutated in about 13% of melanoma patients identified through the Cancer Genome Atlas.
ARID2 mutations have been found in early melanoma lesions, which the authors suggested may play a role in early cancer cell dissemination. Other studies have shown SWI/SNF mutations, including ARID2 mutations, in melanoma metastases, especially the brain.
The researchers also found an up-regulation of synaptic pathways in melanoma cells as well as the Cancer Genome Atlas, which also suggests a potential role of ARID2 loss in metastasis or targeting the brain, since synaptic activation in cancer cells has been shown elsewhere to influence cell migration and survival in the brain.
“We look forward to future studies that investigate the role of the PBAF complex ... in order to better tailor treatments for melanoma patients,” wrote the study authors, who were led by Emily Bernstein, PhD, a professor in oncological sciences with the Icahn School of Medicine at Mount Sinai, New York.
The SWI/SNF complex includes a subcomplex that targets specific DNA sequences or chromatin reader domains. There are multiple versions of the targeting subcomplex, but two of the most frequently occurring are BAF and PBAF. The most commonly mutated subunit in melanoma is ARID2, which is part of PBAF, and contains an AT-rich region responsible for non–sequence-specific DNA interactions. There is evidence that it plays a role in tumor suppression. In mouse tumors, depletion of ARID2 is associated with increased sensitivity to immune checkpoint inhibition and destruction by T cells.
To better understand the role of ARID2 in tumor suppression, the researchers used CRISPR-Cas9 to create ARID2 deficiency in a known human metastatic melanoma cell line. They found there was reduced chromatin accessibility and accompanying gene expression among some PBAF and shared BAF-PBAF–occupied regions. There was also increased chromatin accessibility and gene expression in BAF-occupied regions, and these changes were associated with tumor aggression. In mice, they led to metastasis of distal organs.
This mechanism appears to be conserved between different melanoma cell lines, but deregulated transcriptional targets were different depending on the dominant transcription factors in the cell line. That suggests that the effect of ARID2 mutation or loss may be different depending on the stage of melanoma progression or level of invasiveness. “As melanoma comprises transcriptionally distinct, heterogeneous cell populations, we envision future studies utilizing single-cell methodologies to better understand the nuanced effects of ARID2 loss within subpopulations of cells in human melanoma tumors,” the authors wrote.
The study is limited by the fact that not all ARID2 mutations lead to complete loss of protein, and may lead instead to aberrant complexes.
The study was funded by the National Institutes of Health.
that combined in vitro and animal model data.
The gene, ARID2, is a part of the switch/sucrose nonfermentable (SWI/SNF) complex, which maneuvers cellular structures called nucleosomes to make cellular DNA accessible. About 20% of human cancers have a mutation within the SWI/SNF complex.
In the new study, published in Cell Reports, researchers reported that the ARID2 subunit was mutated in about 13% of melanoma patients identified through the Cancer Genome Atlas.
ARID2 mutations have been found in early melanoma lesions, which the authors suggested may play a role in early cancer cell dissemination. Other studies have shown SWI/SNF mutations, including ARID2 mutations, in melanoma metastases, especially the brain.
The researchers also found an up-regulation of synaptic pathways in melanoma cells as well as the Cancer Genome Atlas, which also suggests a potential role of ARID2 loss in metastasis or targeting the brain, since synaptic activation in cancer cells has been shown elsewhere to influence cell migration and survival in the brain.
“We look forward to future studies that investigate the role of the PBAF complex ... in order to better tailor treatments for melanoma patients,” wrote the study authors, who were led by Emily Bernstein, PhD, a professor in oncological sciences with the Icahn School of Medicine at Mount Sinai, New York.
The SWI/SNF complex includes a subcomplex that targets specific DNA sequences or chromatin reader domains. There are multiple versions of the targeting subcomplex, but two of the most frequently occurring are BAF and PBAF. The most commonly mutated subunit in melanoma is ARID2, which is part of PBAF, and contains an AT-rich region responsible for non–sequence-specific DNA interactions. There is evidence that it plays a role in tumor suppression. In mouse tumors, depletion of ARID2 is associated with increased sensitivity to immune checkpoint inhibition and destruction by T cells.
To better understand the role of ARID2 in tumor suppression, the researchers used CRISPR-Cas9 to create ARID2 deficiency in a known human metastatic melanoma cell line. They found there was reduced chromatin accessibility and accompanying gene expression among some PBAF and shared BAF-PBAF–occupied regions. There was also increased chromatin accessibility and gene expression in BAF-occupied regions, and these changes were associated with tumor aggression. In mice, they led to metastasis of distal organs.
This mechanism appears to be conserved between different melanoma cell lines, but deregulated transcriptional targets were different depending on the dominant transcription factors in the cell line. That suggests that the effect of ARID2 mutation or loss may be different depending on the stage of melanoma progression or level of invasiveness. “As melanoma comprises transcriptionally distinct, heterogeneous cell populations, we envision future studies utilizing single-cell methodologies to better understand the nuanced effects of ARID2 loss within subpopulations of cells in human melanoma tumors,” the authors wrote.
The study is limited by the fact that not all ARID2 mutations lead to complete loss of protein, and may lead instead to aberrant complexes.
The study was funded by the National Institutes of Health.
FROM CELL REPORTS
Field Cancerization in Dermatology: Updates on Treatment Considerations and Emerging Therapies
There has been increasing awareness of field cancerization in dermatology and how it relates to actinic damage, actinic keratoses (AKs), and the development of cutaneous squamous cell carcinomas (SCCs). The concept of field cancerization, which was first described in the context of oropharyngeal SCCs, attempted to explain the repeated observation of local recurrences that were instead multiple primary oropharyngeal SCCs occurring within a specific region of tissue. It was hypothesized that the tissue surrounding a malignancy also harbors irreversible oncogenic damage and therefore predisposes the surrounding tissue to developing further malignancy.1 The development of additional malignant lesions would be considered distinct from a true recurrence of the original malignancy.
Field cancerization may be partially explained by a genetic basis, as mutations in the tumor suppressor gene, TP53—the most frequently observed mutation in cutaneous SCCs—also is found in sun-exposed but clinically normal skin.2,3 The finding of oncogenic mutations in nonlesional skin supports the theory of field cancerization, in which a region contains multiple genetically altered populations, some of which may progress to cancer. Because there currently is no widely accepted clinical definition or validated clinical measurement of field cancerization in dermatology, it may be difficult for dermatologists to recognize which patients may be at risk for developing further malignancy in a potential area of field cancerization. Willenbrink et al4 updated the definition of field cancerization in dermatology as “multifocal clinical atypia characterized by AKs or SCCs in situ with or without invasive disease occurring in a field exposed to chronic UV radiation.” Managing patients with field cancerization can be challenging. Herein, we discuss updates to nonsurgical field-directed and lesion-directed therapies as well as other emerging therapies.
Field-Directed Therapies
Topical 5-fluorouracil (5-FU) and imiquimod cream 5% used as field-directed therapies help reduce the extent of AKs and actinic damage in areas of possible field cancerization.5 The addition of calcipotriol to topical 5-FU, which theoretically augments the skin’s T-cell antitumor response via the cytokine thymic stromal lymphopoietin, recently has been studied using short treatment courses resulting in an 87.8% reduction in AKs compared to a 26.3% reduction with topical 5-FU alone (when used twice daily for 4 days) and conferred a reduced risk of cutaneous SCCs 3 years after treatment (hazard ratio, 0.215 [95% CI, 0.048-0.972]; P=.032).6,7 Chemowraps using topical 5-FU may be considered in more difficult-to-treat areas of field cancerization with multiple AKs or keratinocyte carcinomas of the lower extremities.8 The routine use of chemowraps—weekly application of 5-FU covered with an occlusive dressing—may be limited by the inability to control the extent of epidermal damage and subsequent systemic absorption. Ingenol mebutate, which was approved for treatment of AKs in 2012, was removed from both the European and US markets in 2020 because the medication may paradoxically increase the long-term incidence of skin cancer.9
Meta-analysis has shown that photodynamic therapy (PDT) with aminolevulinic acid demonstrated complete AK clearance in 75.8% of patients (N=156)(95% CI, 55.4%-96.2%).10 A more recent method of PDT using natural sunlight as the activation source demonstrated AK clearance of 95.5%, and it appeared to be a less painful alternative to traditional PDT.11 Tacalcitol, another form of vitamin D, also has been shown to enhance the efficacy of PDT for AKs.12
Field-directed treatment with erbium:YAG and CO2 lasers, which physically remove the actinically damaged epidermis, have been shown to possibly be as efficacious as topical 5-FU and 30% trichloroacetic acid (TCA) but possibly inferior to PDT.13 There has been growing interest in laser-assisted therapy, in which an ablative fractional laser is used to generate microscopic channels to theoretically enhance the absorption of a topical medication. A meta-analysis of the use of laser-assisted therapy for photosensitizing agents in PDT demonstrated a 33% increased chance of AK clearance compared to PDT alone (P<.01).14
Lesion-Directed Therapies
Multiple KAs or cutaneous SCCs may develop in an area of field cancerization, and surgically treating these multiple lesions in a concentrated area may be challenging. Intralesional agents, including methotrexate, 5-FU, bleomycin, and interferon, are known treatments for KAs.15 Intralesional 5-FU (25 mg once weekly for 3–4 weeks) in particular produced complete resolution in 92% of cutaneous SCCs and may be optimal for multiple or rapidly growing lesions, especially on the extremities.16
Oral Therapies
Oral therapies are considered in high-risk patients with multiple or recurrent cutaneous SCCs or in those who are immunosuppressed. Two trials demonstrated that nicotinamide 500 mg twice daily for 4 and 12 months decreased AKs by 29% to 35% and 13% (average of 3–5 fewer AKs as compared to baseline), respectively.17,18 A meta-analysis found a reduction of cutaneous SCCs (rate ratio, 0.48 [95% CI, 0.26-0.88]; I2=67%; 552 patients, 5 trials), and given the favorable safety profile, nicotinamide can be considered for chemoprevention.19
Acitretin, shown to reduce AKs by 13.4% to 50%, is the primary oral chemoprevention recommended in transplant recipients.20 Interestingly, a recent meta-analysis failed to find significant differences between the efficacy of acitretin and nicotinamide.21 The tolerability of acitretin requires serious consideration, as 52.2% of patients withdrew due to adverse effects in one trial.22
Capecitabine (250–1150 mg twice daily), the oral form of 5-FU, decreased the incidence of AKs and cutaneous SCCs in 53% and 72% of transplant recipients, respectively.23 Although several reports observed paradoxical eruptions of AKs following capecitabine for other malignancies, this actually underscores the efficacy of capecitabine, as the newly emerged AKs resolved thereafter.24 Still, the evidence supporting capecitabine does not include any controlled studies.
Novel Therapies
In 2021, tirbanibulin ointment 1%, a Src tyrosine kinase inhibitor of tubulin polymerization that induces p53 expression and subsequent cell death, was approved by the US Food and Drug Administration for the treatment of AKs.25 Two trials reported AK clearance rates of 44% and 54% with application of tirbanibulin once daily for 5 days (vs 5% and 13%, respectively, with placebo, each with P<.001) at 2 months and a sustained clearance rate of 27% at 1 year. The predominant adverse effects were local skin reactions, including application-site pain, pruritus, mild erythema, or scaling. Unlike in other treatments such as 5-FU or cryotherapy, erosions, dyspigmentation, or scarring were not notably observed.
Intralesional talimogene laherparepvec (T-VEC), an oncolytic, genetically modified herpes simplex virus type 1 that incites antitumor immune responses, received US Food and Drug Administration approval in 2015 for the treatment of cutaneous and lymph node metastases of melanoma that are unable to be surgically resected. More recently, T-VEC has been investigated for oropharyngeal SCC. A phase 1 and phase 2 trial of 17 stage III/IV SCC patients receiving T-VEC and cisplatin demonstrated pathologic remission in 14 of 15 (93%) patients, with 82.4% survival at 29 months.26 A multicenter phase 1b trial of 36 patients with recurrent or metastatic head and neck SCCs treated with T-VEC and pembrolizumab exhibited a tolerable safety profile, and 5 cases had a partial response.27 However, phase 3 trials of T-VEC have yet to be pursued. Regarding its potential use for cutaneous SCCs, it has been reportedly used in a liver transplant recipient with metastatic cutaneous SCCs who received 2 doses of T-VEC (1 month apart) and attained remission of disease.28 There currently is a phase 2 trial examining the effectiveness of T-VEC in patients with cutaneous SCCs (ClinicalTrials.gov identifier NCT03714828).
Final Thoughts
It is important for dermatologists to bear in mind the possible role of field cancerization in their comprehensive care of patients at risk for multiple skin cancers. Management of areas of field cancerization can be challenging, particularly in patients who develop multiple KAs or cutaneous SCCs in a concentrated area and may need to involve different levels of treatment options, including field-directed therapies and lesion-directed therapies, as well as systemic chemoprevention.
- Braakhuis BJM, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Ashford BG, Clark J, Gupta R, et al. Reviewing the genetic alterations in high-risk cutaneous squamous cell carcinoma: a search for prognostic markers and therapeutic targets. Head Neck. 2017;39:1462-1469. doi:10.1002/hed.24765
- Albibas AA, Rose-Zerilli MJJ, Lai C, et al. Subclonal evolution of cancer-related gene mutations in p53 immunopositive patches in human skin. J Invest Dermatol. 2018;138:189-198. doi:10.1016/j.jid.2017.07.844
- Willenbrink TJ, Ruiz ES, Cornejo CM, et al. Field cancerization: definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol. 2020;83:709-717. doi:10.1016/j.jaad.2020.03.126
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi:10.1056/NEJMoa1811850
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116. doi:10.1172/JCI89820
- Rosenberg AR, Tabacchi M, Ngo KH, et al. Skin cancer precursor immunotherapy for squamous cell carcinoma prevention. JCI Insight. 2019;4:125476. doi:10.1172/jci.insight.125476
- Peuvrel L, Saint-Jean M, Quereux G, et al. 5-fluorouracil chemowraps for the treatment of multiple actinic keratoses. Eur J Dermatol. 2017;27:635-640. doi:10.1684/ejd.2017.3128
- Eisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:E209-E233. doi:10.1016/j.jaad.2021.02.082
- Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS One. 2014;9:E96829. doi:10.1371/journal.pone.0096829
- Zhu L, Wang P, Zhang G, et al. Conventional versus daylight photodynamic therapy for actinic keratosis: a randomized and prospective study in China. Photodiagnosis Photodyn Ther. 2018;24:366-371. doi:10.1016/j.pdpdt.2018.10.010
- Borgia F, Riso G, Catalano F, et al. Topical tacalcitol as neoadjuvant for photodynamic therapy of acral actinic keratoses: an intra-patient randomized study. Photodiagnosis Photodyn Ther. 2020;31:101803. doi:10.1016/j.pdpdt.2020.101803
- Tai F, Shah M, Pon K, et al. Laser resurfacing monotherapy for the treatment of actinic keratosis. J Cutan Med Surg. 2021;25:634-642. doi:10.1177/12034754211027515
- Steeb T, Schlager JG, Kohl C, et al. Laser-assisted photodynamic therapy for actinic keratosis: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:947-956. doi:10.1016/j.jaad.2018.09.021
- Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702. doi:10.1016/j.jaad.2009.09.048
- Maxfield L, Shah M, Schwartz C, et al. Intralesional 5-fluorouracil for the treatment of squamous cell carcinomas. J Am Acad Dermatol. 2021;84:1696-1697. doi:10.1016/j.jaad.2020.12.049
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi:10.1056/NEJMoa1506197
- Surjana D, Halliday GM, Martin AJ, et al. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J Invest Dermatol. 2012;132:1497-1500. doi:10.1038/jid.2011.459
- Mainville L, Smilga AS, Fortin PR. Effect of nicotinamide in skin cancer and actinic keratoses chemoprophylaxis, and adverse effects related to nicotinamide: a systematic review and meta-analysis [published online February 8, 2022]. J Cutan Med Surg. doi:10.1177/12034754221078201
- Massey PR, Schmults CD, Li SJ, et al. Consensus-based recommendations on the prevention of squamous cell carcinoma in solid organ transplant recipients: a Delphi Consensus Statement. JAMA Dermatol. 2021;157:1219-1226. doi:10.1001/jamadermatol.2021.3180
- Tee LY, Sultana R, Tam SYC, et al. Chemoprevention of keratinocyte carcinoma and actinic keratosis in solid-organ transplant recipients: systematic review and meta-analyses. J Am Acad Dermatol. 2021;84:528-530. doi:10.1016/j.jaad.2020.04.160
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273. doi:10.1046/j.1440-0960.2002.00613.x
- Schauder DM, Kim J, Nijhawan RI. Evaluation of the use of capecitabine for the treatment and prevention of actinic keratoses, squamous cell carcinoma, and basal cell carcinoma: a systematic review. JAMA Dermatol. 2020;156:1117-1124. doi:10.1001/jamadermatol.2020.2327
- Antoniolli LP, Escobar GF, Peruzzo J. Inflammatory actinic keratosis following capecitabine therapy. Dermatol Ther. 2020;33:E14082. doi:10.1111/dth.14082
- Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512-520. doi:10.1056/NEJMoa2024040
- Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16:4005-4015. doi:10.1158/1078-0432.CCR-10-0196
- Harrington KJ, Kong A, Mach N, et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): a multicenter, phase 1b study. Clin Cancer Res. 2020;26:5153-5161. doi:10.1158/1078-0432.CCR-20-1170
- Nguyen TA, Offner M, Hamid O, et al. Complete and sustained remission of metastatic cutaneous squamous cell carcinoma in a liver transplant patient treated with talimogene laherparepvec. Dermatol Surg. 2021;47:820-822. doi:10.1097/DSS.0000000000002739
There has been increasing awareness of field cancerization in dermatology and how it relates to actinic damage, actinic keratoses (AKs), and the development of cutaneous squamous cell carcinomas (SCCs). The concept of field cancerization, which was first described in the context of oropharyngeal SCCs, attempted to explain the repeated observation of local recurrences that were instead multiple primary oropharyngeal SCCs occurring within a specific region of tissue. It was hypothesized that the tissue surrounding a malignancy also harbors irreversible oncogenic damage and therefore predisposes the surrounding tissue to developing further malignancy.1 The development of additional malignant lesions would be considered distinct from a true recurrence of the original malignancy.
Field cancerization may be partially explained by a genetic basis, as mutations in the tumor suppressor gene, TP53—the most frequently observed mutation in cutaneous SCCs—also is found in sun-exposed but clinically normal skin.2,3 The finding of oncogenic mutations in nonlesional skin supports the theory of field cancerization, in which a region contains multiple genetically altered populations, some of which may progress to cancer. Because there currently is no widely accepted clinical definition or validated clinical measurement of field cancerization in dermatology, it may be difficult for dermatologists to recognize which patients may be at risk for developing further malignancy in a potential area of field cancerization. Willenbrink et al4 updated the definition of field cancerization in dermatology as “multifocal clinical atypia characterized by AKs or SCCs in situ with or without invasive disease occurring in a field exposed to chronic UV radiation.” Managing patients with field cancerization can be challenging. Herein, we discuss updates to nonsurgical field-directed and lesion-directed therapies as well as other emerging therapies.
Field-Directed Therapies
Topical 5-fluorouracil (5-FU) and imiquimod cream 5% used as field-directed therapies help reduce the extent of AKs and actinic damage in areas of possible field cancerization.5 The addition of calcipotriol to topical 5-FU, which theoretically augments the skin’s T-cell antitumor response via the cytokine thymic stromal lymphopoietin, recently has been studied using short treatment courses resulting in an 87.8% reduction in AKs compared to a 26.3% reduction with topical 5-FU alone (when used twice daily for 4 days) and conferred a reduced risk of cutaneous SCCs 3 years after treatment (hazard ratio, 0.215 [95% CI, 0.048-0.972]; P=.032).6,7 Chemowraps using topical 5-FU may be considered in more difficult-to-treat areas of field cancerization with multiple AKs or keratinocyte carcinomas of the lower extremities.8 The routine use of chemowraps—weekly application of 5-FU covered with an occlusive dressing—may be limited by the inability to control the extent of epidermal damage and subsequent systemic absorption. Ingenol mebutate, which was approved for treatment of AKs in 2012, was removed from both the European and US markets in 2020 because the medication may paradoxically increase the long-term incidence of skin cancer.9
Meta-analysis has shown that photodynamic therapy (PDT) with aminolevulinic acid demonstrated complete AK clearance in 75.8% of patients (N=156)(95% CI, 55.4%-96.2%).10 A more recent method of PDT using natural sunlight as the activation source demonstrated AK clearance of 95.5%, and it appeared to be a less painful alternative to traditional PDT.11 Tacalcitol, another form of vitamin D, also has been shown to enhance the efficacy of PDT for AKs.12
Field-directed treatment with erbium:YAG and CO2 lasers, which physically remove the actinically damaged epidermis, have been shown to possibly be as efficacious as topical 5-FU and 30% trichloroacetic acid (TCA) but possibly inferior to PDT.13 There has been growing interest in laser-assisted therapy, in which an ablative fractional laser is used to generate microscopic channels to theoretically enhance the absorption of a topical medication. A meta-analysis of the use of laser-assisted therapy for photosensitizing agents in PDT demonstrated a 33% increased chance of AK clearance compared to PDT alone (P<.01).14
Lesion-Directed Therapies
Multiple KAs or cutaneous SCCs may develop in an area of field cancerization, and surgically treating these multiple lesions in a concentrated area may be challenging. Intralesional agents, including methotrexate, 5-FU, bleomycin, and interferon, are known treatments for KAs.15 Intralesional 5-FU (25 mg once weekly for 3–4 weeks) in particular produced complete resolution in 92% of cutaneous SCCs and may be optimal for multiple or rapidly growing lesions, especially on the extremities.16
Oral Therapies
Oral therapies are considered in high-risk patients with multiple or recurrent cutaneous SCCs or in those who are immunosuppressed. Two trials demonstrated that nicotinamide 500 mg twice daily for 4 and 12 months decreased AKs by 29% to 35% and 13% (average of 3–5 fewer AKs as compared to baseline), respectively.17,18 A meta-analysis found a reduction of cutaneous SCCs (rate ratio, 0.48 [95% CI, 0.26-0.88]; I2=67%; 552 patients, 5 trials), and given the favorable safety profile, nicotinamide can be considered for chemoprevention.19
Acitretin, shown to reduce AKs by 13.4% to 50%, is the primary oral chemoprevention recommended in transplant recipients.20 Interestingly, a recent meta-analysis failed to find significant differences between the efficacy of acitretin and nicotinamide.21 The tolerability of acitretin requires serious consideration, as 52.2% of patients withdrew due to adverse effects in one trial.22
Capecitabine (250–1150 mg twice daily), the oral form of 5-FU, decreased the incidence of AKs and cutaneous SCCs in 53% and 72% of transplant recipients, respectively.23 Although several reports observed paradoxical eruptions of AKs following capecitabine for other malignancies, this actually underscores the efficacy of capecitabine, as the newly emerged AKs resolved thereafter.24 Still, the evidence supporting capecitabine does not include any controlled studies.
Novel Therapies
In 2021, tirbanibulin ointment 1%, a Src tyrosine kinase inhibitor of tubulin polymerization that induces p53 expression and subsequent cell death, was approved by the US Food and Drug Administration for the treatment of AKs.25 Two trials reported AK clearance rates of 44% and 54% with application of tirbanibulin once daily for 5 days (vs 5% and 13%, respectively, with placebo, each with P<.001) at 2 months and a sustained clearance rate of 27% at 1 year. The predominant adverse effects were local skin reactions, including application-site pain, pruritus, mild erythema, or scaling. Unlike in other treatments such as 5-FU or cryotherapy, erosions, dyspigmentation, or scarring were not notably observed.
Intralesional talimogene laherparepvec (T-VEC), an oncolytic, genetically modified herpes simplex virus type 1 that incites antitumor immune responses, received US Food and Drug Administration approval in 2015 for the treatment of cutaneous and lymph node metastases of melanoma that are unable to be surgically resected. More recently, T-VEC has been investigated for oropharyngeal SCC. A phase 1 and phase 2 trial of 17 stage III/IV SCC patients receiving T-VEC and cisplatin demonstrated pathologic remission in 14 of 15 (93%) patients, with 82.4% survival at 29 months.26 A multicenter phase 1b trial of 36 patients with recurrent or metastatic head and neck SCCs treated with T-VEC and pembrolizumab exhibited a tolerable safety profile, and 5 cases had a partial response.27 However, phase 3 trials of T-VEC have yet to be pursued. Regarding its potential use for cutaneous SCCs, it has been reportedly used in a liver transplant recipient with metastatic cutaneous SCCs who received 2 doses of T-VEC (1 month apart) and attained remission of disease.28 There currently is a phase 2 trial examining the effectiveness of T-VEC in patients with cutaneous SCCs (ClinicalTrials.gov identifier NCT03714828).
Final Thoughts
It is important for dermatologists to bear in mind the possible role of field cancerization in their comprehensive care of patients at risk for multiple skin cancers. Management of areas of field cancerization can be challenging, particularly in patients who develop multiple KAs or cutaneous SCCs in a concentrated area and may need to involve different levels of treatment options, including field-directed therapies and lesion-directed therapies, as well as systemic chemoprevention.
There has been increasing awareness of field cancerization in dermatology and how it relates to actinic damage, actinic keratoses (AKs), and the development of cutaneous squamous cell carcinomas (SCCs). The concept of field cancerization, which was first described in the context of oropharyngeal SCCs, attempted to explain the repeated observation of local recurrences that were instead multiple primary oropharyngeal SCCs occurring within a specific region of tissue. It was hypothesized that the tissue surrounding a malignancy also harbors irreversible oncogenic damage and therefore predisposes the surrounding tissue to developing further malignancy.1 The development of additional malignant lesions would be considered distinct from a true recurrence of the original malignancy.
Field cancerization may be partially explained by a genetic basis, as mutations in the tumor suppressor gene, TP53—the most frequently observed mutation in cutaneous SCCs—also is found in sun-exposed but clinically normal skin.2,3 The finding of oncogenic mutations in nonlesional skin supports the theory of field cancerization, in which a region contains multiple genetically altered populations, some of which may progress to cancer. Because there currently is no widely accepted clinical definition or validated clinical measurement of field cancerization in dermatology, it may be difficult for dermatologists to recognize which patients may be at risk for developing further malignancy in a potential area of field cancerization. Willenbrink et al4 updated the definition of field cancerization in dermatology as “multifocal clinical atypia characterized by AKs or SCCs in situ with or without invasive disease occurring in a field exposed to chronic UV radiation.” Managing patients with field cancerization can be challenging. Herein, we discuss updates to nonsurgical field-directed and lesion-directed therapies as well as other emerging therapies.
Field-Directed Therapies
Topical 5-fluorouracil (5-FU) and imiquimod cream 5% used as field-directed therapies help reduce the extent of AKs and actinic damage in areas of possible field cancerization.5 The addition of calcipotriol to topical 5-FU, which theoretically augments the skin’s T-cell antitumor response via the cytokine thymic stromal lymphopoietin, recently has been studied using short treatment courses resulting in an 87.8% reduction in AKs compared to a 26.3% reduction with topical 5-FU alone (when used twice daily for 4 days) and conferred a reduced risk of cutaneous SCCs 3 years after treatment (hazard ratio, 0.215 [95% CI, 0.048-0.972]; P=.032).6,7 Chemowraps using topical 5-FU may be considered in more difficult-to-treat areas of field cancerization with multiple AKs or keratinocyte carcinomas of the lower extremities.8 The routine use of chemowraps—weekly application of 5-FU covered with an occlusive dressing—may be limited by the inability to control the extent of epidermal damage and subsequent systemic absorption. Ingenol mebutate, which was approved for treatment of AKs in 2012, was removed from both the European and US markets in 2020 because the medication may paradoxically increase the long-term incidence of skin cancer.9
Meta-analysis has shown that photodynamic therapy (PDT) with aminolevulinic acid demonstrated complete AK clearance in 75.8% of patients (N=156)(95% CI, 55.4%-96.2%).10 A more recent method of PDT using natural sunlight as the activation source demonstrated AK clearance of 95.5%, and it appeared to be a less painful alternative to traditional PDT.11 Tacalcitol, another form of vitamin D, also has been shown to enhance the efficacy of PDT for AKs.12
Field-directed treatment with erbium:YAG and CO2 lasers, which physically remove the actinically damaged epidermis, have been shown to possibly be as efficacious as topical 5-FU and 30% trichloroacetic acid (TCA) but possibly inferior to PDT.13 There has been growing interest in laser-assisted therapy, in which an ablative fractional laser is used to generate microscopic channels to theoretically enhance the absorption of a topical medication. A meta-analysis of the use of laser-assisted therapy for photosensitizing agents in PDT demonstrated a 33% increased chance of AK clearance compared to PDT alone (P<.01).14
Lesion-Directed Therapies
Multiple KAs or cutaneous SCCs may develop in an area of field cancerization, and surgically treating these multiple lesions in a concentrated area may be challenging. Intralesional agents, including methotrexate, 5-FU, bleomycin, and interferon, are known treatments for KAs.15 Intralesional 5-FU (25 mg once weekly for 3–4 weeks) in particular produced complete resolution in 92% of cutaneous SCCs and may be optimal for multiple or rapidly growing lesions, especially on the extremities.16
Oral Therapies
Oral therapies are considered in high-risk patients with multiple or recurrent cutaneous SCCs or in those who are immunosuppressed. Two trials demonstrated that nicotinamide 500 mg twice daily for 4 and 12 months decreased AKs by 29% to 35% and 13% (average of 3–5 fewer AKs as compared to baseline), respectively.17,18 A meta-analysis found a reduction of cutaneous SCCs (rate ratio, 0.48 [95% CI, 0.26-0.88]; I2=67%; 552 patients, 5 trials), and given the favorable safety profile, nicotinamide can be considered for chemoprevention.19
Acitretin, shown to reduce AKs by 13.4% to 50%, is the primary oral chemoprevention recommended in transplant recipients.20 Interestingly, a recent meta-analysis failed to find significant differences between the efficacy of acitretin and nicotinamide.21 The tolerability of acitretin requires serious consideration, as 52.2% of patients withdrew due to adverse effects in one trial.22
Capecitabine (250–1150 mg twice daily), the oral form of 5-FU, decreased the incidence of AKs and cutaneous SCCs in 53% and 72% of transplant recipients, respectively.23 Although several reports observed paradoxical eruptions of AKs following capecitabine for other malignancies, this actually underscores the efficacy of capecitabine, as the newly emerged AKs resolved thereafter.24 Still, the evidence supporting capecitabine does not include any controlled studies.
Novel Therapies
In 2021, tirbanibulin ointment 1%, a Src tyrosine kinase inhibitor of tubulin polymerization that induces p53 expression and subsequent cell death, was approved by the US Food and Drug Administration for the treatment of AKs.25 Two trials reported AK clearance rates of 44% and 54% with application of tirbanibulin once daily for 5 days (vs 5% and 13%, respectively, with placebo, each with P<.001) at 2 months and a sustained clearance rate of 27% at 1 year. The predominant adverse effects were local skin reactions, including application-site pain, pruritus, mild erythema, or scaling. Unlike in other treatments such as 5-FU or cryotherapy, erosions, dyspigmentation, or scarring were not notably observed.
Intralesional talimogene laherparepvec (T-VEC), an oncolytic, genetically modified herpes simplex virus type 1 that incites antitumor immune responses, received US Food and Drug Administration approval in 2015 for the treatment of cutaneous and lymph node metastases of melanoma that are unable to be surgically resected. More recently, T-VEC has been investigated for oropharyngeal SCC. A phase 1 and phase 2 trial of 17 stage III/IV SCC patients receiving T-VEC and cisplatin demonstrated pathologic remission in 14 of 15 (93%) patients, with 82.4% survival at 29 months.26 A multicenter phase 1b trial of 36 patients with recurrent or metastatic head and neck SCCs treated with T-VEC and pembrolizumab exhibited a tolerable safety profile, and 5 cases had a partial response.27 However, phase 3 trials of T-VEC have yet to be pursued. Regarding its potential use for cutaneous SCCs, it has been reportedly used in a liver transplant recipient with metastatic cutaneous SCCs who received 2 doses of T-VEC (1 month apart) and attained remission of disease.28 There currently is a phase 2 trial examining the effectiveness of T-VEC in patients with cutaneous SCCs (ClinicalTrials.gov identifier NCT03714828).
Final Thoughts
It is important for dermatologists to bear in mind the possible role of field cancerization in their comprehensive care of patients at risk for multiple skin cancers. Management of areas of field cancerization can be challenging, particularly in patients who develop multiple KAs or cutaneous SCCs in a concentrated area and may need to involve different levels of treatment options, including field-directed therapies and lesion-directed therapies, as well as systemic chemoprevention.
- Braakhuis BJM, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Ashford BG, Clark J, Gupta R, et al. Reviewing the genetic alterations in high-risk cutaneous squamous cell carcinoma: a search for prognostic markers and therapeutic targets. Head Neck. 2017;39:1462-1469. doi:10.1002/hed.24765
- Albibas AA, Rose-Zerilli MJJ, Lai C, et al. Subclonal evolution of cancer-related gene mutations in p53 immunopositive patches in human skin. J Invest Dermatol. 2018;138:189-198. doi:10.1016/j.jid.2017.07.844
- Willenbrink TJ, Ruiz ES, Cornejo CM, et al. Field cancerization: definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol. 2020;83:709-717. doi:10.1016/j.jaad.2020.03.126
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi:10.1056/NEJMoa1811850
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116. doi:10.1172/JCI89820
- Rosenberg AR, Tabacchi M, Ngo KH, et al. Skin cancer precursor immunotherapy for squamous cell carcinoma prevention. JCI Insight. 2019;4:125476. doi:10.1172/jci.insight.125476
- Peuvrel L, Saint-Jean M, Quereux G, et al. 5-fluorouracil chemowraps for the treatment of multiple actinic keratoses. Eur J Dermatol. 2017;27:635-640. doi:10.1684/ejd.2017.3128
- Eisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:E209-E233. doi:10.1016/j.jaad.2021.02.082
- Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS One. 2014;9:E96829. doi:10.1371/journal.pone.0096829
- Zhu L, Wang P, Zhang G, et al. Conventional versus daylight photodynamic therapy for actinic keratosis: a randomized and prospective study in China. Photodiagnosis Photodyn Ther. 2018;24:366-371. doi:10.1016/j.pdpdt.2018.10.010
- Borgia F, Riso G, Catalano F, et al. Topical tacalcitol as neoadjuvant for photodynamic therapy of acral actinic keratoses: an intra-patient randomized study. Photodiagnosis Photodyn Ther. 2020;31:101803. doi:10.1016/j.pdpdt.2020.101803
- Tai F, Shah M, Pon K, et al. Laser resurfacing monotherapy for the treatment of actinic keratosis. J Cutan Med Surg. 2021;25:634-642. doi:10.1177/12034754211027515
- Steeb T, Schlager JG, Kohl C, et al. Laser-assisted photodynamic therapy for actinic keratosis: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:947-956. doi:10.1016/j.jaad.2018.09.021
- Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702. doi:10.1016/j.jaad.2009.09.048
- Maxfield L, Shah M, Schwartz C, et al. Intralesional 5-fluorouracil for the treatment of squamous cell carcinomas. J Am Acad Dermatol. 2021;84:1696-1697. doi:10.1016/j.jaad.2020.12.049
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi:10.1056/NEJMoa1506197
- Surjana D, Halliday GM, Martin AJ, et al. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J Invest Dermatol. 2012;132:1497-1500. doi:10.1038/jid.2011.459
- Mainville L, Smilga AS, Fortin PR. Effect of nicotinamide in skin cancer and actinic keratoses chemoprophylaxis, and adverse effects related to nicotinamide: a systematic review and meta-analysis [published online February 8, 2022]. J Cutan Med Surg. doi:10.1177/12034754221078201
- Massey PR, Schmults CD, Li SJ, et al. Consensus-based recommendations on the prevention of squamous cell carcinoma in solid organ transplant recipients: a Delphi Consensus Statement. JAMA Dermatol. 2021;157:1219-1226. doi:10.1001/jamadermatol.2021.3180
- Tee LY, Sultana R, Tam SYC, et al. Chemoprevention of keratinocyte carcinoma and actinic keratosis in solid-organ transplant recipients: systematic review and meta-analyses. J Am Acad Dermatol. 2021;84:528-530. doi:10.1016/j.jaad.2020.04.160
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273. doi:10.1046/j.1440-0960.2002.00613.x
- Schauder DM, Kim J, Nijhawan RI. Evaluation of the use of capecitabine for the treatment and prevention of actinic keratoses, squamous cell carcinoma, and basal cell carcinoma: a systematic review. JAMA Dermatol. 2020;156:1117-1124. doi:10.1001/jamadermatol.2020.2327
- Antoniolli LP, Escobar GF, Peruzzo J. Inflammatory actinic keratosis following capecitabine therapy. Dermatol Ther. 2020;33:E14082. doi:10.1111/dth.14082
- Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512-520. doi:10.1056/NEJMoa2024040
- Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16:4005-4015. doi:10.1158/1078-0432.CCR-10-0196
- Harrington KJ, Kong A, Mach N, et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): a multicenter, phase 1b study. Clin Cancer Res. 2020;26:5153-5161. doi:10.1158/1078-0432.CCR-20-1170
- Nguyen TA, Offner M, Hamid O, et al. Complete and sustained remission of metastatic cutaneous squamous cell carcinoma in a liver transplant patient treated with talimogene laherparepvec. Dermatol Surg. 2021;47:820-822. doi:10.1097/DSS.0000000000002739
- Braakhuis BJM, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Ashford BG, Clark J, Gupta R, et al. Reviewing the genetic alterations in high-risk cutaneous squamous cell carcinoma: a search for prognostic markers and therapeutic targets. Head Neck. 2017;39:1462-1469. doi:10.1002/hed.24765
- Albibas AA, Rose-Zerilli MJJ, Lai C, et al. Subclonal evolution of cancer-related gene mutations in p53 immunopositive patches in human skin. J Invest Dermatol. 2018;138:189-198. doi:10.1016/j.jid.2017.07.844
- Willenbrink TJ, Ruiz ES, Cornejo CM, et al. Field cancerization: definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol. 2020;83:709-717. doi:10.1016/j.jaad.2020.03.126
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi:10.1056/NEJMoa1811850
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116. doi:10.1172/JCI89820
- Rosenberg AR, Tabacchi M, Ngo KH, et al. Skin cancer precursor immunotherapy for squamous cell carcinoma prevention. JCI Insight. 2019;4:125476. doi:10.1172/jci.insight.125476
- Peuvrel L, Saint-Jean M, Quereux G, et al. 5-fluorouracil chemowraps for the treatment of multiple actinic keratoses. Eur J Dermatol. 2017;27:635-640. doi:10.1684/ejd.2017.3128
- Eisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:E209-E233. doi:10.1016/j.jaad.2021.02.082
- Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS One. 2014;9:E96829. doi:10.1371/journal.pone.0096829
- Zhu L, Wang P, Zhang G, et al. Conventional versus daylight photodynamic therapy for actinic keratosis: a randomized and prospective study in China. Photodiagnosis Photodyn Ther. 2018;24:366-371. doi:10.1016/j.pdpdt.2018.10.010
- Borgia F, Riso G, Catalano F, et al. Topical tacalcitol as neoadjuvant for photodynamic therapy of acral actinic keratoses: an intra-patient randomized study. Photodiagnosis Photodyn Ther. 2020;31:101803. doi:10.1016/j.pdpdt.2020.101803
- Tai F, Shah M, Pon K, et al. Laser resurfacing monotherapy for the treatment of actinic keratosis. J Cutan Med Surg. 2021;25:634-642. doi:10.1177/12034754211027515
- Steeb T, Schlager JG, Kohl C, et al. Laser-assisted photodynamic therapy for actinic keratosis: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:947-956. doi:10.1016/j.jaad.2018.09.021
- Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702. doi:10.1016/j.jaad.2009.09.048
- Maxfield L, Shah M, Schwartz C, et al. Intralesional 5-fluorouracil for the treatment of squamous cell carcinomas. J Am Acad Dermatol. 2021;84:1696-1697. doi:10.1016/j.jaad.2020.12.049
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi:10.1056/NEJMoa1506197
- Surjana D, Halliday GM, Martin AJ, et al. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J Invest Dermatol. 2012;132:1497-1500. doi:10.1038/jid.2011.459
- Mainville L, Smilga AS, Fortin PR. Effect of nicotinamide in skin cancer and actinic keratoses chemoprophylaxis, and adverse effects related to nicotinamide: a systematic review and meta-analysis [published online February 8, 2022]. J Cutan Med Surg. doi:10.1177/12034754221078201
- Massey PR, Schmults CD, Li SJ, et al. Consensus-based recommendations on the prevention of squamous cell carcinoma in solid organ transplant recipients: a Delphi Consensus Statement. JAMA Dermatol. 2021;157:1219-1226. doi:10.1001/jamadermatol.2021.3180
- Tee LY, Sultana R, Tam SYC, et al. Chemoprevention of keratinocyte carcinoma and actinic keratosis in solid-organ transplant recipients: systematic review and meta-analyses. J Am Acad Dermatol. 2021;84:528-530. doi:10.1016/j.jaad.2020.04.160
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273. doi:10.1046/j.1440-0960.2002.00613.x
- Schauder DM, Kim J, Nijhawan RI. Evaluation of the use of capecitabine for the treatment and prevention of actinic keratoses, squamous cell carcinoma, and basal cell carcinoma: a systematic review. JAMA Dermatol. 2020;156:1117-1124. doi:10.1001/jamadermatol.2020.2327
- Antoniolli LP, Escobar GF, Peruzzo J. Inflammatory actinic keratosis following capecitabine therapy. Dermatol Ther. 2020;33:E14082. doi:10.1111/dth.14082
- Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512-520. doi:10.1056/NEJMoa2024040
- Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16:4005-4015. doi:10.1158/1078-0432.CCR-10-0196
- Harrington KJ, Kong A, Mach N, et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): a multicenter, phase 1b study. Clin Cancer Res. 2020;26:5153-5161. doi:10.1158/1078-0432.CCR-20-1170
- Nguyen TA, Offner M, Hamid O, et al. Complete and sustained remission of metastatic cutaneous squamous cell carcinoma in a liver transplant patient treated with talimogene laherparepvec. Dermatol Surg. 2021;47:820-822. doi:10.1097/DSS.0000000000002739
Dodging potholes from cancer care to hospice transitions
I’m often in the position of caring for patients after they’ve stopped active cancer treatments, but before they’ve made the decision to enroll in hospice. They remain under my care until they feel emotionally ready, or until their care needs have escalated to the point in which hospice is unavoidable.
Jenny, a mom in her 50s with metastatic pancreatic cancer, stopped coming to the clinic. She lived about 40 minutes away from the clinic and was no longer receiving treatment. The car rides were painful and difficult for her. I held weekly video visits with her for 2 months before she eventually went to hospice and passed away. Before she died, she shared with me her sadness that her oncologist – who had taken care of her for 3 years – had “washed his hands of [me].” She rarely heard from him after their final conversation in the clinic when he informed her that she was no longer a candidate for further therapy. The sense of abandonment Jenny described was visceral and devastating. With her permission, I let her oncology team know how she felt and they reached out to her just 1 week before her death. After she died, her husband told me how meaningful it had been for the whole family to hear from Jenny’s oncologist who told them that she had done everything possible to fight her cancer and that “no stone was left unturned.” Her husband felt this final conversation provided Jenny with the closure she needed to pass away peacefully.
Transitioning from active therapy to symptom management
Switching gears from an all-out pursuit of active therapy to focusing on cancer symptoms is often a scary transition for patients and their families. The transition is often viewed as a movement away from hope and optimism to “giving up the fight.” Whether you agree with the warrior language or not, many patients still describe their journey in these terms and thus, experience enrollment in hospice as a sense of having failed.
The sense of failure can be compounded by feelings of abandonment by oncology providers when they are referred without much guidance or continuity through the hospice enrollment process. Unfortunately, the consequences of suboptimal hospice transitions can be damaging, especially for the mental health and well-being of the patient and their surviving loved ones.
When managed poorly, hospice transitions can easily lead to patient and family harm, which is a claim supported by research. A qualitative study published in 2019 included 92 caregivers of patients with terminal cancer. The authors found three common pathways for end-of-life transitions – a frictionless transition in which the patient and family are well prepared in advance by their oncologist; a more turbulent transition in which patient and family had direct conversations with their oncologist about the incurability of the disease and the lack of efficacy of further treatments, but were given no guidance on prognosis; and a third type of transition marked by abrupt shifts toward end-of-life care occurring in extremis and typically in the hospital.
In the latter two groups, caregivers felt their loved ones died very quickly after stopping treatment, taking them by surprise and leaving them rushing to put end-of-life care plans in place without much support from their oncologists. In the last group, caregivers shared they received their first prognostic information from the hospital or ICU doctor caring for their actively dying loved one, leaving them with a sense of anger and betrayal toward their oncologist for allowing them to be so ill-prepared.
A Japanese survey published in 2018 in The Oncologist of families of cancer patients who had passed away under hospice care over a 2-year period (2012-2014), found that about one-quarter felt abandoned by oncologists. Several factors that were associated with feeling either more or less abandonment. Spouses of patients, patients aged less than 60 years, and patients whose oncologists informed them that there was “nothing more to do” felt more abandoned by oncologists; whereas families for whom the oncologist provided reassurance about the trajectory of care, recommended hospice, and engaged with a palliative care team felt less abandoned by oncologists. Families who felt more abandoned had higher levels of depression and grief when measured with standardized instruments.
‘Don’t just put in the hospice order and walk away’
Fortunately, there are a few low-resource interventions that can improve the quality of care-to-hospice transitions and prevent the sense of abandonment felt by many patients and families.
First, don’t just put in the hospice order and walk away. Designate a staffer in your office to contact hospice directly, ensure all medical records are faxed and received, and update the patient and family on this progress throughout the transition. Taking care of details like these ensures the patient enrolls in hospice in a timely manner and reduces the chance the patient, who is likely to be quite sick at this point, will end up in the hospital despite your best efforts to get hospice involved.
Make sure the patient and family understand that you are still their oncologist and still available to them. If they want to continue care with you, have them name you as the “non–hospice-attending physician” so that you can continue to bill for telemedicine and office visits using the terminal diagnosis (with a billing modifier). This does not mean that you will be expected to manage the patient’s hospice problem list or respond to hospice nurse calls at 2 a.m. – the hospice doctor will still do this. It just ensures that patients do not receive a bill if you continue to see them.
If ongoing office or video visits are too much for the patient and family, consider assigning a member of your team to call the patient and family on a weekly basis to check in and offer support. A small 2018 pilot study aimed at improving communication found that when caregivers of advanced cancer patients transitioning to hospice received weekly supportive phone calls by a member of their oncology team (typically a nurse or nurse practitioner), they felt emotionally supported, had good continuity of care throughout the hospice enrollment, and appreciated the ability to have closure with their oncology team. In other words, a sense of abandonment was prevented and the patient-provider relationship was actually deepened through the transition.
These suggestions are not rocket science – they are simple, obvious ways to try to restore patient-centeredness to a transition that for providers can seem routine, but for patients and families is often the first time they have confronted the reality that death is approaching. That reality is terrifying and overwhelming. Patients and caregivers need our support more during hospice transitions than at any other point during their cancer journey – except perhaps at diagnosis.
As with Jenny, my patient who felt abandoned, all it took was a single call by her oncology team to restore the trust and heal the sense of feeling forsaken by the people who cared for her for years. Sometimes, even just one more phone call can feel like a lot to a chronically overburdened provider – but what a difference a simple call can make.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
I’m often in the position of caring for patients after they’ve stopped active cancer treatments, but before they’ve made the decision to enroll in hospice. They remain under my care until they feel emotionally ready, or until their care needs have escalated to the point in which hospice is unavoidable.
Jenny, a mom in her 50s with metastatic pancreatic cancer, stopped coming to the clinic. She lived about 40 minutes away from the clinic and was no longer receiving treatment. The car rides were painful and difficult for her. I held weekly video visits with her for 2 months before she eventually went to hospice and passed away. Before she died, she shared with me her sadness that her oncologist – who had taken care of her for 3 years – had “washed his hands of [me].” She rarely heard from him after their final conversation in the clinic when he informed her that she was no longer a candidate for further therapy. The sense of abandonment Jenny described was visceral and devastating. With her permission, I let her oncology team know how she felt and they reached out to her just 1 week before her death. After she died, her husband told me how meaningful it had been for the whole family to hear from Jenny’s oncologist who told them that she had done everything possible to fight her cancer and that “no stone was left unturned.” Her husband felt this final conversation provided Jenny with the closure she needed to pass away peacefully.
Transitioning from active therapy to symptom management
Switching gears from an all-out pursuit of active therapy to focusing on cancer symptoms is often a scary transition for patients and their families. The transition is often viewed as a movement away from hope and optimism to “giving up the fight.” Whether you agree with the warrior language or not, many patients still describe their journey in these terms and thus, experience enrollment in hospice as a sense of having failed.
The sense of failure can be compounded by feelings of abandonment by oncology providers when they are referred without much guidance or continuity through the hospice enrollment process. Unfortunately, the consequences of suboptimal hospice transitions can be damaging, especially for the mental health and well-being of the patient and their surviving loved ones.
When managed poorly, hospice transitions can easily lead to patient and family harm, which is a claim supported by research. A qualitative study published in 2019 included 92 caregivers of patients with terminal cancer. The authors found three common pathways for end-of-life transitions – a frictionless transition in which the patient and family are well prepared in advance by their oncologist; a more turbulent transition in which patient and family had direct conversations with their oncologist about the incurability of the disease and the lack of efficacy of further treatments, but were given no guidance on prognosis; and a third type of transition marked by abrupt shifts toward end-of-life care occurring in extremis and typically in the hospital.
In the latter two groups, caregivers felt their loved ones died very quickly after stopping treatment, taking them by surprise and leaving them rushing to put end-of-life care plans in place without much support from their oncologists. In the last group, caregivers shared they received their first prognostic information from the hospital or ICU doctor caring for their actively dying loved one, leaving them with a sense of anger and betrayal toward their oncologist for allowing them to be so ill-prepared.
A Japanese survey published in 2018 in The Oncologist of families of cancer patients who had passed away under hospice care over a 2-year period (2012-2014), found that about one-quarter felt abandoned by oncologists. Several factors that were associated with feeling either more or less abandonment. Spouses of patients, patients aged less than 60 years, and patients whose oncologists informed them that there was “nothing more to do” felt more abandoned by oncologists; whereas families for whom the oncologist provided reassurance about the trajectory of care, recommended hospice, and engaged with a palliative care team felt less abandoned by oncologists. Families who felt more abandoned had higher levels of depression and grief when measured with standardized instruments.
‘Don’t just put in the hospice order and walk away’
Fortunately, there are a few low-resource interventions that can improve the quality of care-to-hospice transitions and prevent the sense of abandonment felt by many patients and families.
First, don’t just put in the hospice order and walk away. Designate a staffer in your office to contact hospice directly, ensure all medical records are faxed and received, and update the patient and family on this progress throughout the transition. Taking care of details like these ensures the patient enrolls in hospice in a timely manner and reduces the chance the patient, who is likely to be quite sick at this point, will end up in the hospital despite your best efforts to get hospice involved.
Make sure the patient and family understand that you are still their oncologist and still available to them. If they want to continue care with you, have them name you as the “non–hospice-attending physician” so that you can continue to bill for telemedicine and office visits using the terminal diagnosis (with a billing modifier). This does not mean that you will be expected to manage the patient’s hospice problem list or respond to hospice nurse calls at 2 a.m. – the hospice doctor will still do this. It just ensures that patients do not receive a bill if you continue to see them.
If ongoing office or video visits are too much for the patient and family, consider assigning a member of your team to call the patient and family on a weekly basis to check in and offer support. A small 2018 pilot study aimed at improving communication found that when caregivers of advanced cancer patients transitioning to hospice received weekly supportive phone calls by a member of their oncology team (typically a nurse or nurse practitioner), they felt emotionally supported, had good continuity of care throughout the hospice enrollment, and appreciated the ability to have closure with their oncology team. In other words, a sense of abandonment was prevented and the patient-provider relationship was actually deepened through the transition.
These suggestions are not rocket science – they are simple, obvious ways to try to restore patient-centeredness to a transition that for providers can seem routine, but for patients and families is often the first time they have confronted the reality that death is approaching. That reality is terrifying and overwhelming. Patients and caregivers need our support more during hospice transitions than at any other point during their cancer journey – except perhaps at diagnosis.
As with Jenny, my patient who felt abandoned, all it took was a single call by her oncology team to restore the trust and heal the sense of feeling forsaken by the people who cared for her for years. Sometimes, even just one more phone call can feel like a lot to a chronically overburdened provider – but what a difference a simple call can make.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
I’m often in the position of caring for patients after they’ve stopped active cancer treatments, but before they’ve made the decision to enroll in hospice. They remain under my care until they feel emotionally ready, or until their care needs have escalated to the point in which hospice is unavoidable.
Jenny, a mom in her 50s with metastatic pancreatic cancer, stopped coming to the clinic. She lived about 40 minutes away from the clinic and was no longer receiving treatment. The car rides were painful and difficult for her. I held weekly video visits with her for 2 months before she eventually went to hospice and passed away. Before she died, she shared with me her sadness that her oncologist – who had taken care of her for 3 years – had “washed his hands of [me].” She rarely heard from him after their final conversation in the clinic when he informed her that she was no longer a candidate for further therapy. The sense of abandonment Jenny described was visceral and devastating. With her permission, I let her oncology team know how she felt and they reached out to her just 1 week before her death. After she died, her husband told me how meaningful it had been for the whole family to hear from Jenny’s oncologist who told them that she had done everything possible to fight her cancer and that “no stone was left unturned.” Her husband felt this final conversation provided Jenny with the closure she needed to pass away peacefully.
Transitioning from active therapy to symptom management
Switching gears from an all-out pursuit of active therapy to focusing on cancer symptoms is often a scary transition for patients and their families. The transition is often viewed as a movement away from hope and optimism to “giving up the fight.” Whether you agree with the warrior language or not, many patients still describe their journey in these terms and thus, experience enrollment in hospice as a sense of having failed.
The sense of failure can be compounded by feelings of abandonment by oncology providers when they are referred without much guidance or continuity through the hospice enrollment process. Unfortunately, the consequences of suboptimal hospice transitions can be damaging, especially for the mental health and well-being of the patient and their surviving loved ones.
When managed poorly, hospice transitions can easily lead to patient and family harm, which is a claim supported by research. A qualitative study published in 2019 included 92 caregivers of patients with terminal cancer. The authors found three common pathways for end-of-life transitions – a frictionless transition in which the patient and family are well prepared in advance by their oncologist; a more turbulent transition in which patient and family had direct conversations with their oncologist about the incurability of the disease and the lack of efficacy of further treatments, but were given no guidance on prognosis; and a third type of transition marked by abrupt shifts toward end-of-life care occurring in extremis and typically in the hospital.
In the latter two groups, caregivers felt their loved ones died very quickly after stopping treatment, taking them by surprise and leaving them rushing to put end-of-life care plans in place without much support from their oncologists. In the last group, caregivers shared they received their first prognostic information from the hospital or ICU doctor caring for their actively dying loved one, leaving them with a sense of anger and betrayal toward their oncologist for allowing them to be so ill-prepared.
A Japanese survey published in 2018 in The Oncologist of families of cancer patients who had passed away under hospice care over a 2-year period (2012-2014), found that about one-quarter felt abandoned by oncologists. Several factors that were associated with feeling either more or less abandonment. Spouses of patients, patients aged less than 60 years, and patients whose oncologists informed them that there was “nothing more to do” felt more abandoned by oncologists; whereas families for whom the oncologist provided reassurance about the trajectory of care, recommended hospice, and engaged with a palliative care team felt less abandoned by oncologists. Families who felt more abandoned had higher levels of depression and grief when measured with standardized instruments.
‘Don’t just put in the hospice order and walk away’
Fortunately, there are a few low-resource interventions that can improve the quality of care-to-hospice transitions and prevent the sense of abandonment felt by many patients and families.
First, don’t just put in the hospice order and walk away. Designate a staffer in your office to contact hospice directly, ensure all medical records are faxed and received, and update the patient and family on this progress throughout the transition. Taking care of details like these ensures the patient enrolls in hospice in a timely manner and reduces the chance the patient, who is likely to be quite sick at this point, will end up in the hospital despite your best efforts to get hospice involved.
Make sure the patient and family understand that you are still their oncologist and still available to them. If they want to continue care with you, have them name you as the “non–hospice-attending physician” so that you can continue to bill for telemedicine and office visits using the terminal diagnosis (with a billing modifier). This does not mean that you will be expected to manage the patient’s hospice problem list or respond to hospice nurse calls at 2 a.m. – the hospice doctor will still do this. It just ensures that patients do not receive a bill if you continue to see them.
If ongoing office or video visits are too much for the patient and family, consider assigning a member of your team to call the patient and family on a weekly basis to check in and offer support. A small 2018 pilot study aimed at improving communication found that when caregivers of advanced cancer patients transitioning to hospice received weekly supportive phone calls by a member of their oncology team (typically a nurse or nurse practitioner), they felt emotionally supported, had good continuity of care throughout the hospice enrollment, and appreciated the ability to have closure with their oncology team. In other words, a sense of abandonment was prevented and the patient-provider relationship was actually deepened through the transition.
These suggestions are not rocket science – they are simple, obvious ways to try to restore patient-centeredness to a transition that for providers can seem routine, but for patients and families is often the first time they have confronted the reality that death is approaching. That reality is terrifying and overwhelming. Patients and caregivers need our support more during hospice transitions than at any other point during their cancer journey – except perhaps at diagnosis.
As with Jenny, my patient who felt abandoned, all it took was a single call by her oncology team to restore the trust and heal the sense of feeling forsaken by the people who cared for her for years. Sometimes, even just one more phone call can feel like a lot to a chronically overburdened provider – but what a difference a simple call can make.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
Pick your sunscreen carefully: 75% don’t pass muster
Just in time for Memorial Day outings, a new report on sunscreens is out.
The news isn’t all sunny. , a nonprofit research and advocacy group that just issued its 16th annual Guide to Sunscreens.
In response, dermatologists, including the president of the American Academy of Dermatology, say that although some concerns have been raised about the safety of some sunscreen ingredients, sunscreens themselves remain an important tool in the fight against skin cancer. According to the Skin Cancer Foundation, 1 in 5 Americans will get skin cancer by age 70. Melanoma, the most deadly, has a 5-year survival rate of 99% if caught early.
2022 report
Overall, the Environmental Working Group found that about 1 in 4 sunscreens, or about 500 products, met their standards for providing adequate sun protection and avoiding ingredients linked to known health harms. Products meant for babies and children did slightly better, with about 1 in 3 meeting the standards. The group evaluated mineral sunscreens, also called physical sunscreens, and non-mineral sunscreens, also called chemical sunscreens. Mineral sunscreens contain zinc oxide or titanium dioxide and sit on the skin to deflect the sun’s rays. Chemical sunscreens, with ingredients such as oxybenzone or avobenzone, are partially absorbed into the skin.
Among the group’s concerns:
- The use of oxybenzone in the non-mineral sunscreens. About 30% of the non-mineral sunscreens have it, says Carla Burns, senior director for cosmetic science for the Environmental Working Group. Oxybenzone is a potential hormone disrupter and a skin sensitizer that may harm children and adults, she says. Some progress has been made, as the group found oxybenzone in 66% of the non-mineral sunscreens it reviewed in 2019. (The FDA is seeking more information on oxybenzone and many other sunscreen ingredients.)
- Contamination of sunscreens with benzene, which has been linked to leukemia and other blood disorders, according to the National Cancer Institute. But industry experts stress that that chemical is found in trace amounts in personal care products and does not pose a safety concern. “Benzene is a chemical that is ubiquitous in the environment and not an intentionally added ingredient in personal care products. People worldwide are exposed daily to benzene from indoor and outdoor sources, including air, drinking water, and food and beverages,” the Personal Care Products Council, an industry group, said in a statement.
- Protection from ultraviolet A (UVA) rays is often inadequate, according to research published last year by the Environmental Working Group.
Products on the ‘best’ list
The Environmental Working Group found that 282 recreational sunscreens met its criteria. Among them:
- Coral Safe Sunscreen Lotion, SPF 30
- Neutrogena Sheer Zinc Mineral Sunscreen Lotion, SPF 30
- Mad Hippie Facial Sunscreen Lotion, SPF 30+
The group chose 86 non-mineral sunscreens as better options, including:
- Alba Botanica Hawaiian Sunscreen Lotion, Aloe Vera, SPF 30
- Banana Boat Sport Ultra Sunscreen Stick, SPF 50+
- Black Girl Sunscreen Melanin Boosting Moisturizing Sunscreen Lotion, SPF 30
And 70 sunscreens made the kids’ best list, including:
- True Baby Everyday Play Sunscreen Lotion, SPF 30+
- Sun Biologic Kids’ Sunscreen Stick, SPF 30+
- Kiss My Face Organic Kids’ Defense Sunscreen Lotion, SPF 30
Industry response, FDA actions
In a statement, Alexandra Kowcz, chief scientist at the Personal Care Products Council, pointed out that “as part of a daily safe-sun regimen, sunscreen products help prevent sunburn and reduce skin cancer risk. It is unfortunate that as Americans spend more time outdoors, the Environmental Working Group’s (EWG) 2022 Guide to Sunscreens resorts to fear-mongering with misleading information that could keep consumers from using sunscreens altogether.”
The FDA has asked for more information about certain ingredients to further evaluate products, she says, and industry is working with the agency. The FDA says it is attempting to improve the quality, safety and effectiveness of over-the-counter sunscreen products. In September, 2021, the FDA issued a proposal for regulating OTC sunscreen products, as required under the CARES (Coronavirus Aid, Relief and Economic Security) Act. The effective date for the final order can’t be earlier than September 2022, the CARES Act says.
Dermatologists weigh in
“Every time something like this gets published, my patients come in hysterical,” says Michele Green, MD, a New York City dermatologist who reviewed the report for WebMD. She acknowledges that more research is needed on some sunscreen ingredients. “We really do not know the long-term consequence of oxybenzone,” she says.
Her advice: If her patients have melasma (a skin condition with brown patches on the face), she advises them to use both a chemical and a mineral sunscreen. “I don’t tell my patients in general not to use the chemical [sunscreens].”
For children, she says, the mineral sunscreens may be preferred. On her own children, who are teens, she uses the mineral sunscreens, due to possible concern about hormone disruption.
In a statement, Mark D. Kaufmann, MD, president of the American Academy of Dermatology, says that “sunscreen is an important part of a comprehensive sun protection strategy.”
Besides a broad-spectrum, water-resistant sunscreen with an SPF of 30 or higher for exposed skin, the academy recommends seeking shade and wearing sun-protective clothing to reduce skin cancer risk.
A version of this article first appeared on WebMD.com.
Just in time for Memorial Day outings, a new report on sunscreens is out.
The news isn’t all sunny. , a nonprofit research and advocacy group that just issued its 16th annual Guide to Sunscreens.
In response, dermatologists, including the president of the American Academy of Dermatology, say that although some concerns have been raised about the safety of some sunscreen ingredients, sunscreens themselves remain an important tool in the fight against skin cancer. According to the Skin Cancer Foundation, 1 in 5 Americans will get skin cancer by age 70. Melanoma, the most deadly, has a 5-year survival rate of 99% if caught early.
2022 report
Overall, the Environmental Working Group found that about 1 in 4 sunscreens, or about 500 products, met their standards for providing adequate sun protection and avoiding ingredients linked to known health harms. Products meant for babies and children did slightly better, with about 1 in 3 meeting the standards. The group evaluated mineral sunscreens, also called physical sunscreens, and non-mineral sunscreens, also called chemical sunscreens. Mineral sunscreens contain zinc oxide or titanium dioxide and sit on the skin to deflect the sun’s rays. Chemical sunscreens, with ingredients such as oxybenzone or avobenzone, are partially absorbed into the skin.
Among the group’s concerns:
- The use of oxybenzone in the non-mineral sunscreens. About 30% of the non-mineral sunscreens have it, says Carla Burns, senior director for cosmetic science for the Environmental Working Group. Oxybenzone is a potential hormone disrupter and a skin sensitizer that may harm children and adults, she says. Some progress has been made, as the group found oxybenzone in 66% of the non-mineral sunscreens it reviewed in 2019. (The FDA is seeking more information on oxybenzone and many other sunscreen ingredients.)
- Contamination of sunscreens with benzene, which has been linked to leukemia and other blood disorders, according to the National Cancer Institute. But industry experts stress that that chemical is found in trace amounts in personal care products and does not pose a safety concern. “Benzene is a chemical that is ubiquitous in the environment and not an intentionally added ingredient in personal care products. People worldwide are exposed daily to benzene from indoor and outdoor sources, including air, drinking water, and food and beverages,” the Personal Care Products Council, an industry group, said in a statement.
- Protection from ultraviolet A (UVA) rays is often inadequate, according to research published last year by the Environmental Working Group.
Products on the ‘best’ list
The Environmental Working Group found that 282 recreational sunscreens met its criteria. Among them:
- Coral Safe Sunscreen Lotion, SPF 30
- Neutrogena Sheer Zinc Mineral Sunscreen Lotion, SPF 30
- Mad Hippie Facial Sunscreen Lotion, SPF 30+
The group chose 86 non-mineral sunscreens as better options, including:
- Alba Botanica Hawaiian Sunscreen Lotion, Aloe Vera, SPF 30
- Banana Boat Sport Ultra Sunscreen Stick, SPF 50+
- Black Girl Sunscreen Melanin Boosting Moisturizing Sunscreen Lotion, SPF 30
And 70 sunscreens made the kids’ best list, including:
- True Baby Everyday Play Sunscreen Lotion, SPF 30+
- Sun Biologic Kids’ Sunscreen Stick, SPF 30+
- Kiss My Face Organic Kids’ Defense Sunscreen Lotion, SPF 30
Industry response, FDA actions
In a statement, Alexandra Kowcz, chief scientist at the Personal Care Products Council, pointed out that “as part of a daily safe-sun regimen, sunscreen products help prevent sunburn and reduce skin cancer risk. It is unfortunate that as Americans spend more time outdoors, the Environmental Working Group’s (EWG) 2022 Guide to Sunscreens resorts to fear-mongering with misleading information that could keep consumers from using sunscreens altogether.”
The FDA has asked for more information about certain ingredients to further evaluate products, she says, and industry is working with the agency. The FDA says it is attempting to improve the quality, safety and effectiveness of over-the-counter sunscreen products. In September, 2021, the FDA issued a proposal for regulating OTC sunscreen products, as required under the CARES (Coronavirus Aid, Relief and Economic Security) Act. The effective date for the final order can’t be earlier than September 2022, the CARES Act says.
Dermatologists weigh in
“Every time something like this gets published, my patients come in hysterical,” says Michele Green, MD, a New York City dermatologist who reviewed the report for WebMD. She acknowledges that more research is needed on some sunscreen ingredients. “We really do not know the long-term consequence of oxybenzone,” she says.
Her advice: If her patients have melasma (a skin condition with brown patches on the face), she advises them to use both a chemical and a mineral sunscreen. “I don’t tell my patients in general not to use the chemical [sunscreens].”
For children, she says, the mineral sunscreens may be preferred. On her own children, who are teens, she uses the mineral sunscreens, due to possible concern about hormone disruption.
In a statement, Mark D. Kaufmann, MD, president of the American Academy of Dermatology, says that “sunscreen is an important part of a comprehensive sun protection strategy.”
Besides a broad-spectrum, water-resistant sunscreen with an SPF of 30 or higher for exposed skin, the academy recommends seeking shade and wearing sun-protective clothing to reduce skin cancer risk.
A version of this article first appeared on WebMD.com.
Just in time for Memorial Day outings, a new report on sunscreens is out.
The news isn’t all sunny. , a nonprofit research and advocacy group that just issued its 16th annual Guide to Sunscreens.
In response, dermatologists, including the president of the American Academy of Dermatology, say that although some concerns have been raised about the safety of some sunscreen ingredients, sunscreens themselves remain an important tool in the fight against skin cancer. According to the Skin Cancer Foundation, 1 in 5 Americans will get skin cancer by age 70. Melanoma, the most deadly, has a 5-year survival rate of 99% if caught early.
2022 report
Overall, the Environmental Working Group found that about 1 in 4 sunscreens, or about 500 products, met their standards for providing adequate sun protection and avoiding ingredients linked to known health harms. Products meant for babies and children did slightly better, with about 1 in 3 meeting the standards. The group evaluated mineral sunscreens, also called physical sunscreens, and non-mineral sunscreens, also called chemical sunscreens. Mineral sunscreens contain zinc oxide or titanium dioxide and sit on the skin to deflect the sun’s rays. Chemical sunscreens, with ingredients such as oxybenzone or avobenzone, are partially absorbed into the skin.
Among the group’s concerns:
- The use of oxybenzone in the non-mineral sunscreens. About 30% of the non-mineral sunscreens have it, says Carla Burns, senior director for cosmetic science for the Environmental Working Group. Oxybenzone is a potential hormone disrupter and a skin sensitizer that may harm children and adults, she says. Some progress has been made, as the group found oxybenzone in 66% of the non-mineral sunscreens it reviewed in 2019. (The FDA is seeking more information on oxybenzone and many other sunscreen ingredients.)
- Contamination of sunscreens with benzene, which has been linked to leukemia and other blood disorders, according to the National Cancer Institute. But industry experts stress that that chemical is found in trace amounts in personal care products and does not pose a safety concern. “Benzene is a chemical that is ubiquitous in the environment and not an intentionally added ingredient in personal care products. People worldwide are exposed daily to benzene from indoor and outdoor sources, including air, drinking water, and food and beverages,” the Personal Care Products Council, an industry group, said in a statement.
- Protection from ultraviolet A (UVA) rays is often inadequate, according to research published last year by the Environmental Working Group.
Products on the ‘best’ list
The Environmental Working Group found that 282 recreational sunscreens met its criteria. Among them:
- Coral Safe Sunscreen Lotion, SPF 30
- Neutrogena Sheer Zinc Mineral Sunscreen Lotion, SPF 30
- Mad Hippie Facial Sunscreen Lotion, SPF 30+
The group chose 86 non-mineral sunscreens as better options, including:
- Alba Botanica Hawaiian Sunscreen Lotion, Aloe Vera, SPF 30
- Banana Boat Sport Ultra Sunscreen Stick, SPF 50+
- Black Girl Sunscreen Melanin Boosting Moisturizing Sunscreen Lotion, SPF 30
And 70 sunscreens made the kids’ best list, including:
- True Baby Everyday Play Sunscreen Lotion, SPF 30+
- Sun Biologic Kids’ Sunscreen Stick, SPF 30+
- Kiss My Face Organic Kids’ Defense Sunscreen Lotion, SPF 30
Industry response, FDA actions
In a statement, Alexandra Kowcz, chief scientist at the Personal Care Products Council, pointed out that “as part of a daily safe-sun regimen, sunscreen products help prevent sunburn and reduce skin cancer risk. It is unfortunate that as Americans spend more time outdoors, the Environmental Working Group’s (EWG) 2022 Guide to Sunscreens resorts to fear-mongering with misleading information that could keep consumers from using sunscreens altogether.”
The FDA has asked for more information about certain ingredients to further evaluate products, she says, and industry is working with the agency. The FDA says it is attempting to improve the quality, safety and effectiveness of over-the-counter sunscreen products. In September, 2021, the FDA issued a proposal for regulating OTC sunscreen products, as required under the CARES (Coronavirus Aid, Relief and Economic Security) Act. The effective date for the final order can’t be earlier than September 2022, the CARES Act says.
Dermatologists weigh in
“Every time something like this gets published, my patients come in hysterical,” says Michele Green, MD, a New York City dermatologist who reviewed the report for WebMD. She acknowledges that more research is needed on some sunscreen ingredients. “We really do not know the long-term consequence of oxybenzone,” she says.
Her advice: If her patients have melasma (a skin condition with brown patches on the face), she advises them to use both a chemical and a mineral sunscreen. “I don’t tell my patients in general not to use the chemical [sunscreens].”
For children, she says, the mineral sunscreens may be preferred. On her own children, who are teens, she uses the mineral sunscreens, due to possible concern about hormone disruption.
In a statement, Mark D. Kaufmann, MD, president of the American Academy of Dermatology, says that “sunscreen is an important part of a comprehensive sun protection strategy.”
Besides a broad-spectrum, water-resistant sunscreen with an SPF of 30 or higher for exposed skin, the academy recommends seeking shade and wearing sun-protective clothing to reduce skin cancer risk.
A version of this article first appeared on WebMD.com.
BRAF V600E Expression in Primary Melanoma and Its Association With Death: A Population-Based, Retrospective, Cross-Sectional Study
Approximately 50% of melanomas contain BRAF mutations, which occur in a greater proportion of melanomas found on sites of intermittent sun exposure.1BRAF-mutated melanomas have been associated with high levels of early-life ambient UV exposure, especially between ages 0 and 20 years.2 In addition, studies have shown that BRAF-mutated melanomas commonly are found on the trunk and extremities.1-3BRAF mutations also have been associated with younger age, superficial spreading subtype and low tumor thickness, absence of dermal melanocyte mitosis, low Ki-67 score, low phospho-histone H3 score, pigmented melanoma, advanced melanoma stage, and conjunctival melanoma.4-7BRAF mutations are found more frequently in metastatic melanoma lesions than primary melanomas, suggesting that BRAF mutations may be acquired during metastasis.8 Studies have shown different conclusions on the effect of BRAF mutation on melanoma-related death.5,9,10
The aim of this study was to identify trends in BRAF V600E–mutated melanoma according to age, sex, and melanoma-specific survival among Olmsted County, Minnesota, residents with a first diagnosis of melanoma at 18 to 60 years of age.
Methods
In total, 638 patients aged 18 to 60 years who resided in Olmsted County and had a first lifetime diagnosis of cutaneous melanoma between 1970 and 2009 were retrospectively identified as a part of the Rochester Epidemiology Project (REP). The REP is a health records linkage system that encompasses almost all sources of medical care available to the local population of Olmsted County.11 This study was approved by the Mayo Clinic Institutional Review Board (Rochester, Minnesota).
Of the 638 individuals identified in the REP, 536 had been seen at Mayo Clinic and thus potentially had tissue blocks available for the study of BRAF mutation expression. Of these 536 patients, 156 did not have sufficient residual tissue available. As a result, 380 (60%) of the original 638 patients had available blocks with sufficient tissue for immunohistochemical analysis of BRAF expression. Only primary cutaneous melanomas were included in the present study.
All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) for appropriateness of inclusion, which involved confirmation of the diagnosis of melanoma, histologic type of melanoma, and presence of sufficient residual tissue for immunohistochemical stains.
All specimens were originally diagnosed as malignant melanoma at the time of clinical care by at least 2 board-certified dermatopathologists. For the purposes of this study, all specimens were rereviewed for diagnostic accuracy. We required that specimens exhibit severe cytologic and architectural atypia as well as other features favoring melanoma, such as consumption of rete pegs, pagetosis, confluence of junctional melanocytes, evidence of regression, lack of maturation of melanocytes with descent into the dermis, or mitotic figures among the dermal melanocyte population.
The available tissue blocks were retrieved, sectioned, confirmed as melanoma, and stained with a mouse antihuman BRAF V600E monoclonal antibody (clone VE1; Spring Bioscience) to determine the presence of a BRAF V600E mutation. BRAF staining was evaluated in conjunction with a review of the associated slides stained with hematoxylin and eosin. Cytoplasmic staining of melanocytes for BRAF was graded as negative, focal or partial positive (<50% of tumor), or diffuse positive (>50% of tumor)(Figure 1). When a melanoma arose in association with a nevus, we considered only the melanoma component for BRAF staining. We categorized the histologic type as superficial spreading, nodular, or lentigo maligna, and the location as head and neck, trunk, or extremities.
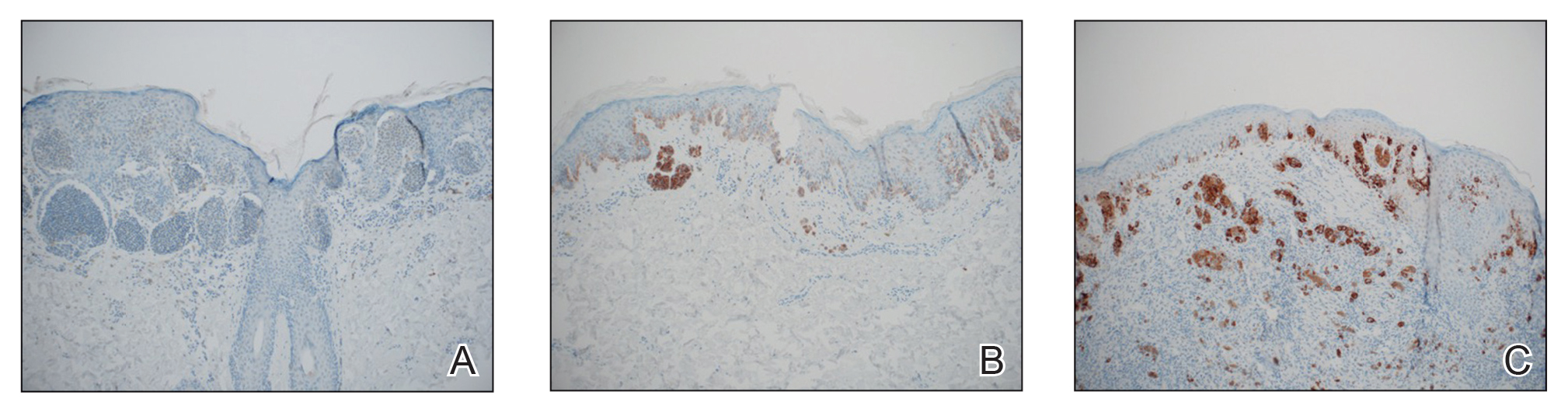
Patient characteristics and survival outcomes were gathered through the health record and included age, Breslow thickness, location, decade of diagnosis, histologic type, stage (ie, noninvasive, invasive, or advanced), and follow-up. Pathologic stage 0 was considered noninvasive; stages IA and IB, invasive; and stages IIA or higher, advanced.
Statistical Analysis—Comparisons between the group of patients in the study (n=380) and the group of patients excluded for the reasons stated above (n=258) as well as associations of mutant BRAF status (positive [partial positive and diffuse positive] vs negative) with patient age (young adults [age range, 18–39 years] and middle-aged adults [age range, 40–60 years]), sex, decade of diagnosis, location, histologic type, and stage were evaluated with Wilcoxon rank sum, χ2, Fisher exact, or Cochran-Armitage trend tests. Disease-specific survival and overall survival rates were estimated with the Kaplan-Meier method, and the duration of follow-up was calculated from the date of melanoma diagnosis to the date of death or the last follow-up. Associations of mutant BRAF expression status with death from melanoma and death from any cause were evaluated with Cox proportional hazard regression models and summarized with hazard ratio (HR) and 95% CI. Survival analyses were limited to patients with invasive or advanced disease. Statistical analyses were performed with SAS statistical software (SAS version 9.4). All tests were 2-sided, and P<.05 was considered statistically significant.
Results
Clinical and Tumor Characteristics—Of the 380 tissue specimens that underwent BRAF V600E analysis, 247 had negative staining; 106 had diffuse strong staining; and 27 had focal or partial staining. In total, 133 (35%) were positive, either partially or diffusely. The median age for patients who had negative staining was 45 years; for those with positive staining, it was 41 years (P=.07).
The patients who met inclusion criteria (n=380) were compared with those who were excluded (n=258)(eTable 1). The groups were similar on the basis of sex; age; and melanoma location, stage, and histologic subtype. However, some evidence showed that patients included in the study received the diagnosis of melanoma more recently (1970-1989, 13.2%; 1990-1999, 28.7%; 2000-2009, 58.2%) than those who were excluded (1970-1989, 24.7%; 1990-1999, 23.5%; 2000-2009, 51.8%)(P=.02).
BRAF V600E expression was more commonly found in superficial spreading (37.7%) and nodular melanomas (35.0%) than in situ melanomas (17.1%)(P=.01). Other characteristics of BRAF V600E expression are described in eTable 2. Overall, invasive and advanced melanomas were significantly more likely to harbor BRAF V600E expression than noninvasive melanomas (39.6% and 37.9%, respectively, vs 17.9%; P=.003). However, advanced melanomas more commonly expressed BRAF positivity among women, and invasive melanomas more commonly expressed BRAF positivity among men (eTable 2).
Survival—Survival analyses were limited to 297 patients with confirmed invasive or advanced disease. Of these, 180 (61%) had no BRAF V600E staining; 25 (8%) had partial staining; and 92 (31%) had diffuse positive staining. In total, 117 patients (39%) had a BRAF-mutated melanoma.
Among the patients still alive, the median (interquartile range [IQR]) duration of follow-up was 10.2 (7.0-16.8) years. Thirty-nine patients with invasive or advanced disease had died of any cause at a median (IQR) of 3.0 (1.3-10.2) years after diagnosis. In total, 26 patients died of melanoma at a median (IQR) follow-up of 2.5 (1.3-7.4) years after diagnosis. Eight women and 18 men died of malignant melanoma. Five deaths occurred because of malignant melanoma among patients aged 18 to 39 years, and 21 occurred among patients aged 40 to 60 years. In the 18- to 39-year-old group, all 5 deaths were among patients with a BRAF-positive melanoma. Estimated disease-specific survival rate (95% CI; number still at risk) at 5, 10, 15, and 20 years after diagnosis was 94% (91%-97%; 243), 91% (87%-95%; 142), 89% (85%-94%; 87), and 88% (83%-93%; 45), respectively.
In a univariable analysis, the HR for association of positive mutant BRAF expression with death of malignant melanoma was 1.84 (95% CI, 0.85-3.98; P=.12). No statistically significant interaction was observed between decade of diagnosis and BRAF expression (P=.60). However, the interaction between sex and BRAF expression was significant (P=.04), with increased risk of death from melanoma among women with BRAF-mutated melanoma (HR, 10.88; 95% CI, 1.34-88.41; P=.026) but not among men (HR 1.02; 95% CI, 0.40-2.64; P=.97)(Figures 2A and 2B). The HR for death from malignant melanoma among young adults aged 18 to 39 years with a BRAF-mutated melanoma was 16.4 (95% CI, 0.81-330.10; P=.068), whereas the HR among adults aged 40 to 60 years with a BRAF-mutated melanoma was 1.24 (95% CI, 0.52-2.98; P=.63)(Figures 2C and 2D).
BRAF V600E expression was not significantly associated with death from any cause (HR, 1.39; 95% CI, 0.74-2.61; P=.31) or with decade of diagnosis (P=.13). Similarly, BRAF expression was not associated with death from any cause according to sex (P=.31). However, a statistically significant interaction was seen between age at diagnosis and BRAF expression (P=.003). BRAF expression was significantly associated with death from any cause for adults aged 18 to 39 years (HR, 9.60; 95% CI, 1.15-80.00; P=.04). In comparison, no association of BRAF expression with death was observed for adults aged 40 to 60 years (HR, 0.99; 95% CI, 0.48-2.03; P=.98).
Comment
We found that melanomas with BRAF mutations were more likely in advanced and invasive melanoma. The frequency of BRAF mutations among melanomas that were considered advanced was higher in women than men. Although the number of deaths was limited, women with a melanoma with BRAF expression were more likely to die of melanoma, young adults with a BRAF-mutated melanoma had an almost 10-fold increased risk of dying from any cause, and middle-aged adults showed no increased risk of death. These findings suggest that young adults who are genetically prone to a BRAF-mutated melanoma could be at a disadvantage for all-cause mortality. Although this finding was significant, the 95% CI was large, and further studies would be warranted before sound conclusions could be made.
Melanoma has been increasing in incidence across all age groups in Olmsted County over the last 4 decades.12-14 However, our results show that the percentage of BRAF-mutated melanomas in this population has been stable over time, with no statistically significant difference by age or sex. Other confounding factors may have an influence, such as increased rates of early detection and diagnosis of melanoma in contemporary times. Our data suggest that patients included in the BRAF-mutation analysis study had received the diagnosis of melanoma more recently than those who were excluded from the study, which could be due to older melanomas being less likely to have adequate tissue specimens available for immunohistochemical staining/evaluation.
Prior research has shown that BRAF-mutated melanomas typically occur on the trunk and are more likely in individuals with more than 14 nevi on the back.2 In the present cohort, BRAF-positive melanomas had a predisposition toward the trunk but also were found on the head, neck, and extremities—areas that are more likely to have long-term sun damage. One suggestion is that 2 distinct pathways for melanoma development exist: one associated with a large number of melanocytic nevi (that is more prone to genetic mutations in melanocytes) and the other associated with long-term sun exposure.15,16 The combination of these hypotheses suggests that individuals who are prone to the development of large numbers of nevi may require sun exposure for the initial insult, but the development of melanoma may be carried out by other factors after this initial sun exposure insult, whereas individuals without large numbers of nevi who may have less genetic risk may require continued long-term sun exposure for melanoma to develop.17
Our study had limitations, including the small numbers of deaths overall and cause-specific deaths of metastatic melanoma, which limited our ability to conduct more extensive multivariable modeling. Also, the retrospective nature and time frame of looking back 4 decades did not allow us to have information sufficient to categorize some patients as having dysplastic nevus syndrome or not, which would be a potentially interesting variable to include in the analysis. Because the number of deaths in the 18- to 39-year-old cohort was only 5, further statistical comparison regarding tumor type and other variables pertaining to BRAF positivity were not possible. In addition, our data were collected from patients residing in a single geographic county (Olmsted County, Minnesota), which may limit generalizability. Lastly, BRAF V600E mutations were identified through immunostaining only, not molecular data, so it is possible some patients had false-negative immunohistochemistry findings and thus were not identified.
Conclusion
BRAF-mutated melanomas were found in 35% of our cohort, with no significant change in the percentage of melanomas with BRAF V600E mutations over the last 4 decades in this population. In addition, no differences or significant trends existed according to sex and BRAF-mutated melanoma development. Women with BRAF-mutated melanomas were more likely to die of metastatic melanoma than men, and young adults with BRAF-mutated melanomas had a higher all-cause mortality risk. Further research is needed to decipher what effect BRAF-mutated melanomas have on metastasis and cause-specific death in women as well as all-cause mortality in young adults.
Acknowledgment—The authors are indebted to Scientific Publications, Mayo Clinic (Rochester, Minnesota).
- Grimaldi AM, Cassidy PB, Leachmann S, et al. Novel approaches in melanoma prevention and therapy. Cancer Treat Res. 2014;159: 443-455.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Association between NRAS and BRAF mutational status and melanoma-specific survival among patients with higher-risk primary melanoma. JAMA Oncol. 2015;1:359-368.
- Liu W, Kelly JW, Trivett M, et al. Distinct clinical and pathological features are associated with the BRAF(T1799A(V600E)) mutation in primary melanoma. J Invest Dermatol. 2007;127:900-905.
- Kim SY, Kim SN, Hahn HJ, et al. Metaanalysis of BRAF mutations and clinicopathologic characteristics in primary melanoma. J Am Acad Dermatol. 2015;72:1036-1046.e2.
- Larsen AC, Dahl C, Dahmcke CM, et al. BRAF mutations in conjunctival melanoma: investigation of incidence, clinicopathological features, prognosis and paired premalignant lesions. Acta Ophthalmol. 2016;94:463-470.
- Shinozaki M, Fujimoto A, Morton DL, et al. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res. 2004;10:1753-1757.
- Heppt MV, Siepmann T, Engel J, et al. Prognostic significance of BRAF and NRAS mutations in melanoma: a German study from routine care. BMC Cancer. 2017;17:536.
- Mar VJ, Liu W, Devitt B, et al. The role of BRAF mutations in primary melanoma growth rate and survival. Br J Dermatol. 2015;173:76-82.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Whiteman DC, Parsons PG, Green AC. p53 expression and risk factors for cutaneous melanoma: a case-control study. Int J Cancer. 1998;77:843-848.
- Whiteman DC, Watt P, Purdie DM, et al. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95:806-812.
- Olsen CM, Zens MS, Green AC, et al. Biologic markers of sun exposure and melanoma risk in women: pooled case-control analysis. Int J Cancer. 2011;129:713-723.
Approximately 50% of melanomas contain BRAF mutations, which occur in a greater proportion of melanomas found on sites of intermittent sun exposure.1BRAF-mutated melanomas have been associated with high levels of early-life ambient UV exposure, especially between ages 0 and 20 years.2 In addition, studies have shown that BRAF-mutated melanomas commonly are found on the trunk and extremities.1-3BRAF mutations also have been associated with younger age, superficial spreading subtype and low tumor thickness, absence of dermal melanocyte mitosis, low Ki-67 score, low phospho-histone H3 score, pigmented melanoma, advanced melanoma stage, and conjunctival melanoma.4-7BRAF mutations are found more frequently in metastatic melanoma lesions than primary melanomas, suggesting that BRAF mutations may be acquired during metastasis.8 Studies have shown different conclusions on the effect of BRAF mutation on melanoma-related death.5,9,10
The aim of this study was to identify trends in BRAF V600E–mutated melanoma according to age, sex, and melanoma-specific survival among Olmsted County, Minnesota, residents with a first diagnosis of melanoma at 18 to 60 years of age.
Methods
In total, 638 patients aged 18 to 60 years who resided in Olmsted County and had a first lifetime diagnosis of cutaneous melanoma between 1970 and 2009 were retrospectively identified as a part of the Rochester Epidemiology Project (REP). The REP is a health records linkage system that encompasses almost all sources of medical care available to the local population of Olmsted County.11 This study was approved by the Mayo Clinic Institutional Review Board (Rochester, Minnesota).
Of the 638 individuals identified in the REP, 536 had been seen at Mayo Clinic and thus potentially had tissue blocks available for the study of BRAF mutation expression. Of these 536 patients, 156 did not have sufficient residual tissue available. As a result, 380 (60%) of the original 638 patients had available blocks with sufficient tissue for immunohistochemical analysis of BRAF expression. Only primary cutaneous melanomas were included in the present study.
All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) for appropriateness of inclusion, which involved confirmation of the diagnosis of melanoma, histologic type of melanoma, and presence of sufficient residual tissue for immunohistochemical stains.
All specimens were originally diagnosed as malignant melanoma at the time of clinical care by at least 2 board-certified dermatopathologists. For the purposes of this study, all specimens were rereviewed for diagnostic accuracy. We required that specimens exhibit severe cytologic and architectural atypia as well as other features favoring melanoma, such as consumption of rete pegs, pagetosis, confluence of junctional melanocytes, evidence of regression, lack of maturation of melanocytes with descent into the dermis, or mitotic figures among the dermal melanocyte population.
The available tissue blocks were retrieved, sectioned, confirmed as melanoma, and stained with a mouse antihuman BRAF V600E monoclonal antibody (clone VE1; Spring Bioscience) to determine the presence of a BRAF V600E mutation. BRAF staining was evaluated in conjunction with a review of the associated slides stained with hematoxylin and eosin. Cytoplasmic staining of melanocytes for BRAF was graded as negative, focal or partial positive (<50% of tumor), or diffuse positive (>50% of tumor)(Figure 1). When a melanoma arose in association with a nevus, we considered only the melanoma component for BRAF staining. We categorized the histologic type as superficial spreading, nodular, or lentigo maligna, and the location as head and neck, trunk, or extremities.

Patient characteristics and survival outcomes were gathered through the health record and included age, Breslow thickness, location, decade of diagnosis, histologic type, stage (ie, noninvasive, invasive, or advanced), and follow-up. Pathologic stage 0 was considered noninvasive; stages IA and IB, invasive; and stages IIA or higher, advanced.
Statistical Analysis—Comparisons between the group of patients in the study (n=380) and the group of patients excluded for the reasons stated above (n=258) as well as associations of mutant BRAF status (positive [partial positive and diffuse positive] vs negative) with patient age (young adults [age range, 18–39 years] and middle-aged adults [age range, 40–60 years]), sex, decade of diagnosis, location, histologic type, and stage were evaluated with Wilcoxon rank sum, χ2, Fisher exact, or Cochran-Armitage trend tests. Disease-specific survival and overall survival rates were estimated with the Kaplan-Meier method, and the duration of follow-up was calculated from the date of melanoma diagnosis to the date of death or the last follow-up. Associations of mutant BRAF expression status with death from melanoma and death from any cause were evaluated with Cox proportional hazard regression models and summarized with hazard ratio (HR) and 95% CI. Survival analyses were limited to patients with invasive or advanced disease. Statistical analyses were performed with SAS statistical software (SAS version 9.4). All tests were 2-sided, and P<.05 was considered statistically significant.
Results
Clinical and Tumor Characteristics—Of the 380 tissue specimens that underwent BRAF V600E analysis, 247 had negative staining; 106 had diffuse strong staining; and 27 had focal or partial staining. In total, 133 (35%) were positive, either partially or diffusely. The median age for patients who had negative staining was 45 years; for those with positive staining, it was 41 years (P=.07).
The patients who met inclusion criteria (n=380) were compared with those who were excluded (n=258)(eTable 1). The groups were similar on the basis of sex; age; and melanoma location, stage, and histologic subtype. However, some evidence showed that patients included in the study received the diagnosis of melanoma more recently (1970-1989, 13.2%; 1990-1999, 28.7%; 2000-2009, 58.2%) than those who were excluded (1970-1989, 24.7%; 1990-1999, 23.5%; 2000-2009, 51.8%)(P=.02).
BRAF V600E expression was more commonly found in superficial spreading (37.7%) and nodular melanomas (35.0%) than in situ melanomas (17.1%)(P=.01). Other characteristics of BRAF V600E expression are described in eTable 2. Overall, invasive and advanced melanomas were significantly more likely to harbor BRAF V600E expression than noninvasive melanomas (39.6% and 37.9%, respectively, vs 17.9%; P=.003). However, advanced melanomas more commonly expressed BRAF positivity among women, and invasive melanomas more commonly expressed BRAF positivity among men (eTable 2).
Survival—Survival analyses were limited to 297 patients with confirmed invasive or advanced disease. Of these, 180 (61%) had no BRAF V600E staining; 25 (8%) had partial staining; and 92 (31%) had diffuse positive staining. In total, 117 patients (39%) had a BRAF-mutated melanoma.
Among the patients still alive, the median (interquartile range [IQR]) duration of follow-up was 10.2 (7.0-16.8) years. Thirty-nine patients with invasive or advanced disease had died of any cause at a median (IQR) of 3.0 (1.3-10.2) years after diagnosis. In total, 26 patients died of melanoma at a median (IQR) follow-up of 2.5 (1.3-7.4) years after diagnosis. Eight women and 18 men died of malignant melanoma. Five deaths occurred because of malignant melanoma among patients aged 18 to 39 years, and 21 occurred among patients aged 40 to 60 years. In the 18- to 39-year-old group, all 5 deaths were among patients with a BRAF-positive melanoma. Estimated disease-specific survival rate (95% CI; number still at risk) at 5, 10, 15, and 20 years after diagnosis was 94% (91%-97%; 243), 91% (87%-95%; 142), 89% (85%-94%; 87), and 88% (83%-93%; 45), respectively.
In a univariable analysis, the HR for association of positive mutant BRAF expression with death of malignant melanoma was 1.84 (95% CI, 0.85-3.98; P=.12). No statistically significant interaction was observed between decade of diagnosis and BRAF expression (P=.60). However, the interaction between sex and BRAF expression was significant (P=.04), with increased risk of death from melanoma among women with BRAF-mutated melanoma (HR, 10.88; 95% CI, 1.34-88.41; P=.026) but not among men (HR 1.02; 95% CI, 0.40-2.64; P=.97)(Figures 2A and 2B). The HR for death from malignant melanoma among young adults aged 18 to 39 years with a BRAF-mutated melanoma was 16.4 (95% CI, 0.81-330.10; P=.068), whereas the HR among adults aged 40 to 60 years with a BRAF-mutated melanoma was 1.24 (95% CI, 0.52-2.98; P=.63)(Figures 2C and 2D).

BRAF V600E expression was not significantly associated with death from any cause (HR, 1.39; 95% CI, 0.74-2.61; P=.31) or with decade of diagnosis (P=.13). Similarly, BRAF expression was not associated with death from any cause according to sex (P=.31). However, a statistically significant interaction was seen between age at diagnosis and BRAF expression (P=.003). BRAF expression was significantly associated with death from any cause for adults aged 18 to 39 years (HR, 9.60; 95% CI, 1.15-80.00; P=.04). In comparison, no association of BRAF expression with death was observed for adults aged 40 to 60 years (HR, 0.99; 95% CI, 0.48-2.03; P=.98).
Comment
We found that melanomas with BRAF mutations were more likely in advanced and invasive melanoma. The frequency of BRAF mutations among melanomas that were considered advanced was higher in women than men. Although the number of deaths was limited, women with a melanoma with BRAF expression were more likely to die of melanoma, young adults with a BRAF-mutated melanoma had an almost 10-fold increased risk of dying from any cause, and middle-aged adults showed no increased risk of death. These findings suggest that young adults who are genetically prone to a BRAF-mutated melanoma could be at a disadvantage for all-cause mortality. Although this finding was significant, the 95% CI was large, and further studies would be warranted before sound conclusions could be made.
Melanoma has been increasing in incidence across all age groups in Olmsted County over the last 4 decades.12-14 However, our results show that the percentage of BRAF-mutated melanomas in this population has been stable over time, with no statistically significant difference by age or sex. Other confounding factors may have an influence, such as increased rates of early detection and diagnosis of melanoma in contemporary times. Our data suggest that patients included in the BRAF-mutation analysis study had received the diagnosis of melanoma more recently than those who were excluded from the study, which could be due to older melanomas being less likely to have adequate tissue specimens available for immunohistochemical staining/evaluation.
Prior research has shown that BRAF-mutated melanomas typically occur on the trunk and are more likely in individuals with more than 14 nevi on the back.2 In the present cohort, BRAF-positive melanomas had a predisposition toward the trunk but also were found on the head, neck, and extremities—areas that are more likely to have long-term sun damage. One suggestion is that 2 distinct pathways for melanoma development exist: one associated with a large number of melanocytic nevi (that is more prone to genetic mutations in melanocytes) and the other associated with long-term sun exposure.15,16 The combination of these hypotheses suggests that individuals who are prone to the development of large numbers of nevi may require sun exposure for the initial insult, but the development of melanoma may be carried out by other factors after this initial sun exposure insult, whereas individuals without large numbers of nevi who may have less genetic risk may require continued long-term sun exposure for melanoma to develop.17
Our study had limitations, including the small numbers of deaths overall and cause-specific deaths of metastatic melanoma, which limited our ability to conduct more extensive multivariable modeling. Also, the retrospective nature and time frame of looking back 4 decades did not allow us to have information sufficient to categorize some patients as having dysplastic nevus syndrome or not, which would be a potentially interesting variable to include in the analysis. Because the number of deaths in the 18- to 39-year-old cohort was only 5, further statistical comparison regarding tumor type and other variables pertaining to BRAF positivity were not possible. In addition, our data were collected from patients residing in a single geographic county (Olmsted County, Minnesota), which may limit generalizability. Lastly, BRAF V600E mutations were identified through immunostaining only, not molecular data, so it is possible some patients had false-negative immunohistochemistry findings and thus were not identified.
Conclusion
BRAF-mutated melanomas were found in 35% of our cohort, with no significant change in the percentage of melanomas with BRAF V600E mutations over the last 4 decades in this population. In addition, no differences or significant trends existed according to sex and BRAF-mutated melanoma development. Women with BRAF-mutated melanomas were more likely to die of metastatic melanoma than men, and young adults with BRAF-mutated melanomas had a higher all-cause mortality risk. Further research is needed to decipher what effect BRAF-mutated melanomas have on metastasis and cause-specific death in women as well as all-cause mortality in young adults.
Acknowledgment—The authors are indebted to Scientific Publications, Mayo Clinic (Rochester, Minnesota).
Approximately 50% of melanomas contain BRAF mutations, which occur in a greater proportion of melanomas found on sites of intermittent sun exposure.1BRAF-mutated melanomas have been associated with high levels of early-life ambient UV exposure, especially between ages 0 and 20 years.2 In addition, studies have shown that BRAF-mutated melanomas commonly are found on the trunk and extremities.1-3BRAF mutations also have been associated with younger age, superficial spreading subtype and low tumor thickness, absence of dermal melanocyte mitosis, low Ki-67 score, low phospho-histone H3 score, pigmented melanoma, advanced melanoma stage, and conjunctival melanoma.4-7BRAF mutations are found more frequently in metastatic melanoma lesions than primary melanomas, suggesting that BRAF mutations may be acquired during metastasis.8 Studies have shown different conclusions on the effect of BRAF mutation on melanoma-related death.5,9,10
The aim of this study was to identify trends in BRAF V600E–mutated melanoma according to age, sex, and melanoma-specific survival among Olmsted County, Minnesota, residents with a first diagnosis of melanoma at 18 to 60 years of age.
Methods
In total, 638 patients aged 18 to 60 years who resided in Olmsted County and had a first lifetime diagnosis of cutaneous melanoma between 1970 and 2009 were retrospectively identified as a part of the Rochester Epidemiology Project (REP). The REP is a health records linkage system that encompasses almost all sources of medical care available to the local population of Olmsted County.11 This study was approved by the Mayo Clinic Institutional Review Board (Rochester, Minnesota).
Of the 638 individuals identified in the REP, 536 had been seen at Mayo Clinic and thus potentially had tissue blocks available for the study of BRAF mutation expression. Of these 536 patients, 156 did not have sufficient residual tissue available. As a result, 380 (60%) of the original 638 patients had available blocks with sufficient tissue for immunohistochemical analysis of BRAF expression. Only primary cutaneous melanomas were included in the present study.
All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) for appropriateness of inclusion, which involved confirmation of the diagnosis of melanoma, histologic type of melanoma, and presence of sufficient residual tissue for immunohistochemical stains.
All specimens were originally diagnosed as malignant melanoma at the time of clinical care by at least 2 board-certified dermatopathologists. For the purposes of this study, all specimens were rereviewed for diagnostic accuracy. We required that specimens exhibit severe cytologic and architectural atypia as well as other features favoring melanoma, such as consumption of rete pegs, pagetosis, confluence of junctional melanocytes, evidence of regression, lack of maturation of melanocytes with descent into the dermis, or mitotic figures among the dermal melanocyte population.
The available tissue blocks were retrieved, sectioned, confirmed as melanoma, and stained with a mouse antihuman BRAF V600E monoclonal antibody (clone VE1; Spring Bioscience) to determine the presence of a BRAF V600E mutation. BRAF staining was evaluated in conjunction with a review of the associated slides stained with hematoxylin and eosin. Cytoplasmic staining of melanocytes for BRAF was graded as negative, focal or partial positive (<50% of tumor), or diffuse positive (>50% of tumor)(Figure 1). When a melanoma arose in association with a nevus, we considered only the melanoma component for BRAF staining. We categorized the histologic type as superficial spreading, nodular, or lentigo maligna, and the location as head and neck, trunk, or extremities.

Patient characteristics and survival outcomes were gathered through the health record and included age, Breslow thickness, location, decade of diagnosis, histologic type, stage (ie, noninvasive, invasive, or advanced), and follow-up. Pathologic stage 0 was considered noninvasive; stages IA and IB, invasive; and stages IIA or higher, advanced.
Statistical Analysis—Comparisons between the group of patients in the study (n=380) and the group of patients excluded for the reasons stated above (n=258) as well as associations of mutant BRAF status (positive [partial positive and diffuse positive] vs negative) with patient age (young adults [age range, 18–39 years] and middle-aged adults [age range, 40–60 years]), sex, decade of diagnosis, location, histologic type, and stage were evaluated with Wilcoxon rank sum, χ2, Fisher exact, or Cochran-Armitage trend tests. Disease-specific survival and overall survival rates were estimated with the Kaplan-Meier method, and the duration of follow-up was calculated from the date of melanoma diagnosis to the date of death or the last follow-up. Associations of mutant BRAF expression status with death from melanoma and death from any cause were evaluated with Cox proportional hazard regression models and summarized with hazard ratio (HR) and 95% CI. Survival analyses were limited to patients with invasive or advanced disease. Statistical analyses were performed with SAS statistical software (SAS version 9.4). All tests were 2-sided, and P<.05 was considered statistically significant.
Results
Clinical and Tumor Characteristics—Of the 380 tissue specimens that underwent BRAF V600E analysis, 247 had negative staining; 106 had diffuse strong staining; and 27 had focal or partial staining. In total, 133 (35%) were positive, either partially or diffusely. The median age for patients who had negative staining was 45 years; for those with positive staining, it was 41 years (P=.07).
The patients who met inclusion criteria (n=380) were compared with those who were excluded (n=258)(eTable 1). The groups were similar on the basis of sex; age; and melanoma location, stage, and histologic subtype. However, some evidence showed that patients included in the study received the diagnosis of melanoma more recently (1970-1989, 13.2%; 1990-1999, 28.7%; 2000-2009, 58.2%) than those who were excluded (1970-1989, 24.7%; 1990-1999, 23.5%; 2000-2009, 51.8%)(P=.02).
BRAF V600E expression was more commonly found in superficial spreading (37.7%) and nodular melanomas (35.0%) than in situ melanomas (17.1%)(P=.01). Other characteristics of BRAF V600E expression are described in eTable 2. Overall, invasive and advanced melanomas were significantly more likely to harbor BRAF V600E expression than noninvasive melanomas (39.6% and 37.9%, respectively, vs 17.9%; P=.003). However, advanced melanomas more commonly expressed BRAF positivity among women, and invasive melanomas more commonly expressed BRAF positivity among men (eTable 2).
Survival—Survival analyses were limited to 297 patients with confirmed invasive or advanced disease. Of these, 180 (61%) had no BRAF V600E staining; 25 (8%) had partial staining; and 92 (31%) had diffuse positive staining. In total, 117 patients (39%) had a BRAF-mutated melanoma.
Among the patients still alive, the median (interquartile range [IQR]) duration of follow-up was 10.2 (7.0-16.8) years. Thirty-nine patients with invasive or advanced disease had died of any cause at a median (IQR) of 3.0 (1.3-10.2) years after diagnosis. In total, 26 patients died of melanoma at a median (IQR) follow-up of 2.5 (1.3-7.4) years after diagnosis. Eight women and 18 men died of malignant melanoma. Five deaths occurred because of malignant melanoma among patients aged 18 to 39 years, and 21 occurred among patients aged 40 to 60 years. In the 18- to 39-year-old group, all 5 deaths were among patients with a BRAF-positive melanoma. Estimated disease-specific survival rate (95% CI; number still at risk) at 5, 10, 15, and 20 years after diagnosis was 94% (91%-97%; 243), 91% (87%-95%; 142), 89% (85%-94%; 87), and 88% (83%-93%; 45), respectively.
In a univariable analysis, the HR for association of positive mutant BRAF expression with death of malignant melanoma was 1.84 (95% CI, 0.85-3.98; P=.12). No statistically significant interaction was observed between decade of diagnosis and BRAF expression (P=.60). However, the interaction between sex and BRAF expression was significant (P=.04), with increased risk of death from melanoma among women with BRAF-mutated melanoma (HR, 10.88; 95% CI, 1.34-88.41; P=.026) but not among men (HR 1.02; 95% CI, 0.40-2.64; P=.97)(Figures 2A and 2B). The HR for death from malignant melanoma among young adults aged 18 to 39 years with a BRAF-mutated melanoma was 16.4 (95% CI, 0.81-330.10; P=.068), whereas the HR among adults aged 40 to 60 years with a BRAF-mutated melanoma was 1.24 (95% CI, 0.52-2.98; P=.63)(Figures 2C and 2D).

BRAF V600E expression was not significantly associated with death from any cause (HR, 1.39; 95% CI, 0.74-2.61; P=.31) or with decade of diagnosis (P=.13). Similarly, BRAF expression was not associated with death from any cause according to sex (P=.31). However, a statistically significant interaction was seen between age at diagnosis and BRAF expression (P=.003). BRAF expression was significantly associated with death from any cause for adults aged 18 to 39 years (HR, 9.60; 95% CI, 1.15-80.00; P=.04). In comparison, no association of BRAF expression with death was observed for adults aged 40 to 60 years (HR, 0.99; 95% CI, 0.48-2.03; P=.98).
Comment
We found that melanomas with BRAF mutations were more likely in advanced and invasive melanoma. The frequency of BRAF mutations among melanomas that were considered advanced was higher in women than men. Although the number of deaths was limited, women with a melanoma with BRAF expression were more likely to die of melanoma, young adults with a BRAF-mutated melanoma had an almost 10-fold increased risk of dying from any cause, and middle-aged adults showed no increased risk of death. These findings suggest that young adults who are genetically prone to a BRAF-mutated melanoma could be at a disadvantage for all-cause mortality. Although this finding was significant, the 95% CI was large, and further studies would be warranted before sound conclusions could be made.
Melanoma has been increasing in incidence across all age groups in Olmsted County over the last 4 decades.12-14 However, our results show that the percentage of BRAF-mutated melanomas in this population has been stable over time, with no statistically significant difference by age or sex. Other confounding factors may have an influence, such as increased rates of early detection and diagnosis of melanoma in contemporary times. Our data suggest that patients included in the BRAF-mutation analysis study had received the diagnosis of melanoma more recently than those who were excluded from the study, which could be due to older melanomas being less likely to have adequate tissue specimens available for immunohistochemical staining/evaluation.
Prior research has shown that BRAF-mutated melanomas typically occur on the trunk and are more likely in individuals with more than 14 nevi on the back.2 In the present cohort, BRAF-positive melanomas had a predisposition toward the trunk but also were found on the head, neck, and extremities—areas that are more likely to have long-term sun damage. One suggestion is that 2 distinct pathways for melanoma development exist: one associated with a large number of melanocytic nevi (that is more prone to genetic mutations in melanocytes) and the other associated with long-term sun exposure.15,16 The combination of these hypotheses suggests that individuals who are prone to the development of large numbers of nevi may require sun exposure for the initial insult, but the development of melanoma may be carried out by other factors after this initial sun exposure insult, whereas individuals without large numbers of nevi who may have less genetic risk may require continued long-term sun exposure for melanoma to develop.17
Our study had limitations, including the small numbers of deaths overall and cause-specific deaths of metastatic melanoma, which limited our ability to conduct more extensive multivariable modeling. Also, the retrospective nature and time frame of looking back 4 decades did not allow us to have information sufficient to categorize some patients as having dysplastic nevus syndrome or not, which would be a potentially interesting variable to include in the analysis. Because the number of deaths in the 18- to 39-year-old cohort was only 5, further statistical comparison regarding tumor type and other variables pertaining to BRAF positivity were not possible. In addition, our data were collected from patients residing in a single geographic county (Olmsted County, Minnesota), which may limit generalizability. Lastly, BRAF V600E mutations were identified through immunostaining only, not molecular data, so it is possible some patients had false-negative immunohistochemistry findings and thus were not identified.
Conclusion
BRAF-mutated melanomas were found in 35% of our cohort, with no significant change in the percentage of melanomas with BRAF V600E mutations over the last 4 decades in this population. In addition, no differences or significant trends existed according to sex and BRAF-mutated melanoma development. Women with BRAF-mutated melanomas were more likely to die of metastatic melanoma than men, and young adults with BRAF-mutated melanomas had a higher all-cause mortality risk. Further research is needed to decipher what effect BRAF-mutated melanomas have on metastasis and cause-specific death in women as well as all-cause mortality in young adults.
Acknowledgment—The authors are indebted to Scientific Publications, Mayo Clinic (Rochester, Minnesota).
- Grimaldi AM, Cassidy PB, Leachmann S, et al. Novel approaches in melanoma prevention and therapy. Cancer Treat Res. 2014;159: 443-455.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Association between NRAS and BRAF mutational status and melanoma-specific survival among patients with higher-risk primary melanoma. JAMA Oncol. 2015;1:359-368.
- Liu W, Kelly JW, Trivett M, et al. Distinct clinical and pathological features are associated with the BRAF(T1799A(V600E)) mutation in primary melanoma. J Invest Dermatol. 2007;127:900-905.
- Kim SY, Kim SN, Hahn HJ, et al. Metaanalysis of BRAF mutations and clinicopathologic characteristics in primary melanoma. J Am Acad Dermatol. 2015;72:1036-1046.e2.
- Larsen AC, Dahl C, Dahmcke CM, et al. BRAF mutations in conjunctival melanoma: investigation of incidence, clinicopathological features, prognosis and paired premalignant lesions. Acta Ophthalmol. 2016;94:463-470.
- Shinozaki M, Fujimoto A, Morton DL, et al. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res. 2004;10:1753-1757.
- Heppt MV, Siepmann T, Engel J, et al. Prognostic significance of BRAF and NRAS mutations in melanoma: a German study from routine care. BMC Cancer. 2017;17:536.
- Mar VJ, Liu W, Devitt B, et al. The role of BRAF mutations in primary melanoma growth rate and survival. Br J Dermatol. 2015;173:76-82.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Whiteman DC, Parsons PG, Green AC. p53 expression and risk factors for cutaneous melanoma: a case-control study. Int J Cancer. 1998;77:843-848.
- Whiteman DC, Watt P, Purdie DM, et al. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95:806-812.
- Olsen CM, Zens MS, Green AC, et al. Biologic markers of sun exposure and melanoma risk in women: pooled case-control analysis. Int J Cancer. 2011;129:713-723.
- Grimaldi AM, Cassidy PB, Leachmann S, et al. Novel approaches in melanoma prevention and therapy. Cancer Treat Res. 2014;159: 443-455.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Association between NRAS and BRAF mutational status and melanoma-specific survival among patients with higher-risk primary melanoma. JAMA Oncol. 2015;1:359-368.
- Liu W, Kelly JW, Trivett M, et al. Distinct clinical and pathological features are associated with the BRAF(T1799A(V600E)) mutation in primary melanoma. J Invest Dermatol. 2007;127:900-905.
- Kim SY, Kim SN, Hahn HJ, et al. Metaanalysis of BRAF mutations and clinicopathologic characteristics in primary melanoma. J Am Acad Dermatol. 2015;72:1036-1046.e2.
- Larsen AC, Dahl C, Dahmcke CM, et al. BRAF mutations in conjunctival melanoma: investigation of incidence, clinicopathological features, prognosis and paired premalignant lesions. Acta Ophthalmol. 2016;94:463-470.
- Shinozaki M, Fujimoto A, Morton DL, et al. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res. 2004;10:1753-1757.
- Heppt MV, Siepmann T, Engel J, et al. Prognostic significance of BRAF and NRAS mutations in melanoma: a German study from routine care. BMC Cancer. 2017;17:536.
- Mar VJ, Liu W, Devitt B, et al. The role of BRAF mutations in primary melanoma growth rate and survival. Br J Dermatol. 2015;173:76-82.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Whiteman DC, Parsons PG, Green AC. p53 expression and risk factors for cutaneous melanoma: a case-control study. Int J Cancer. 1998;77:843-848.
- Whiteman DC, Watt P, Purdie DM, et al. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95:806-812.
- Olsen CM, Zens MS, Green AC, et al. Biologic markers of sun exposure and melanoma risk in women: pooled case-control analysis. Int J Cancer. 2011;129:713-723.
Practice Points
- Approximately 50% of melanomas contain BRAF mutations; the effects on survival are unclear.
- Women with BRAF-mutated melanoma are at increased risk for death from melanoma.
- BRAF expression is associated with death of any cause for adults aged 18 to 39 years.
Impact of the COVID-19 Pandemic on Characteristics of Cutaneous Tumors Treated by Mohs Micrographic Surgery
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.
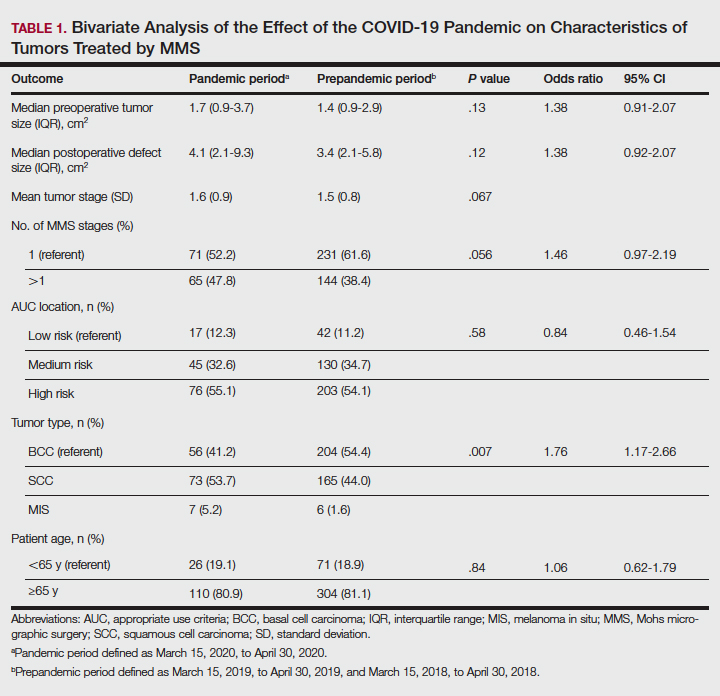
Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).
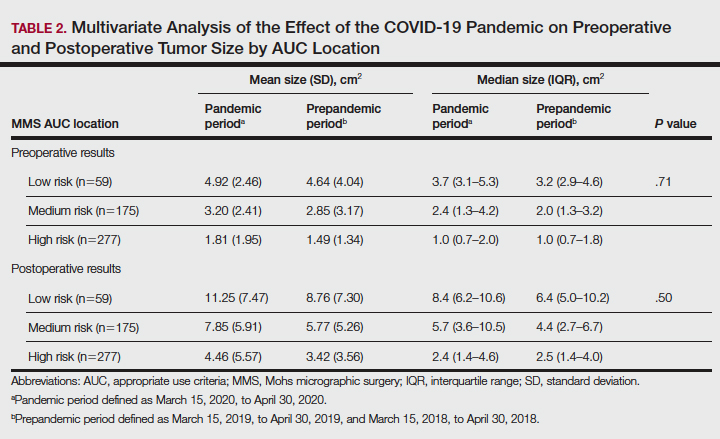
Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.

Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).

Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.

Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).

Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
Practice Points
- Mohs surgeons should follow best practice guidelines dictated by our governing professional societies in selecting skin cancers for treatment by Mohs micrographic surgery (MMS) during the COVID-19 pandemic and beyond.
- The COVID-19 pandemic has impacted the characteristics of skin cancers treated by MMS, largely driven by new guidelines.
- Changing characteristics of skin cancers treated by MMS are of clinical significance, potentially affecting the extent of reconstructive surgery, cost, operating time, and future tumor characteristics.
Reflectance Confocal Microscopy Findings in a Small-Diameter Invasive Melanoma
Melanomas have been designated as small melanomas or micromelanomas according to their long-axis diameter (<6 mm and ≤3 mm, respectively).1-3 Because small-diameter melanomas also have the potential to metastasize, particularly if nodular, early diagnosis can be highly rewarding. Deep melanomas with small diameters may have the same potential for metastasis as large-diameter melanomas. In this context, dermoscopy, digital dermoscopic monitoring, and total-body photography are useful in clinical practice. However, these techniques are of limited utility for small, dermoscopic feature–poor melanomas. Conversely, less than 10% of changing lesions, which are spotted via digital dermoscopic surveillance, turn out to be melanomas; therefore, simply removing all changing lesions may result in many unnecessary excisions of benign lesions.4
In vivo reflectance confocal microscopy (RCM) is an advanced technique that allows recognition of the architectural and cellular details of pigmented lesions. Reflectance confocal microscopy has the potential to reduce the rate of unnecessary excisions and to diminish the risk for missing a melanoma.5-7 In meta-analyses, RCM sensitivity was reported as 90% to 93% and specificity was reported as 78% to 82% in detecting melanoma.8,9
We describe a case that highlights the potential role of RCM in the diagnosis of small-diameter melanomas.
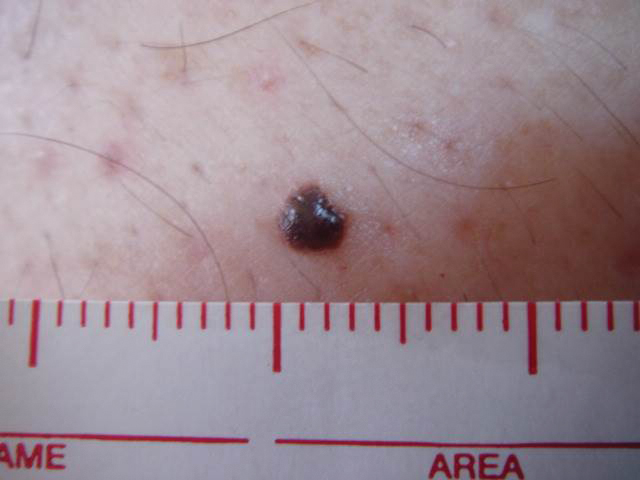
Case Report
A 57-year-old man with Fitzpatrick skin type III presented to the dermato-oncology unit for evaluation of multiple nevi. He was otherwise healthy and denied a history of skin cancer. Total-body skin examination with dermoscopy was performed, and several mildly atypical lesions were identified. We decided to perform digital dermoscopic monitoring. The patient’s 6-month monitoring appointment had been scheduled, but he did not arrive for the follow-up visit until 10 months after the initial examination. A lesion on the left arm, which initially was 1.5 mm in diameter, had enlarged. It was now a dark brown–gray papule with a 2.5-mm diameter (Figure 1). Dermoscopy revealed grayish globules/dots at the center of the lesion, reticular gray-blue areas, and few milialike cysts; at the periphery, a narrow rim of brownish delicate pigment network also was seen (Figure 2). The clinical and dermoscopic differential diagnosis was either an atypical nevus or an early melanoma. For a more precise diagnosis before excision, the lesion was evaluated with RCM, which takes 10 to 15 minutes to perform.
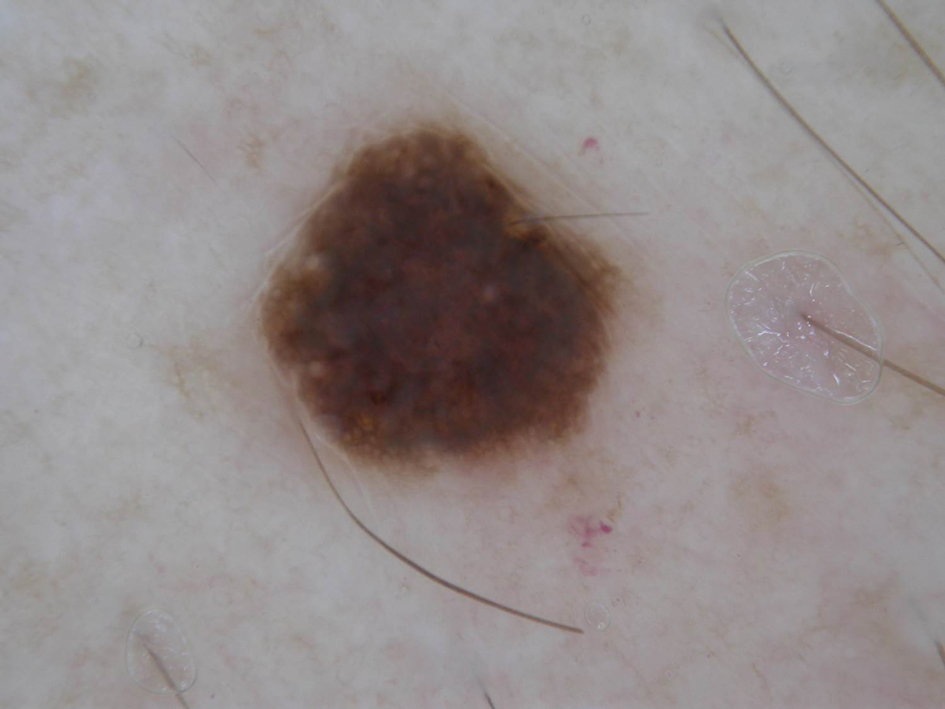
Under RCM at the epidermis level, there was a cobblestone pattern that showed a focus with mild disarrangement and few small, roundish, nucleated cells (Figure 3). A mosaic image, akin to low-magnification microscopy that enables overview of the entire lesion, at the level of the dermoepidermal junction (DEJ) showed an overall irregular meshwork pattern. Higher-magnification optical sections showed marked and diffuse (extending >10% of lesion area) architectural disorder with confluent junctional nests that were irregular to bizarre in shape and uneven in size and spacing as well as edged and nonedged papillae. At the superficial dermal level, atypical bright nucleated cells (>5 cells/mm2) were observed (Figure 4). Bright dots and/or plump bright cells within papillae also were observed. These RCM findings were highly suggestive for melanoma.
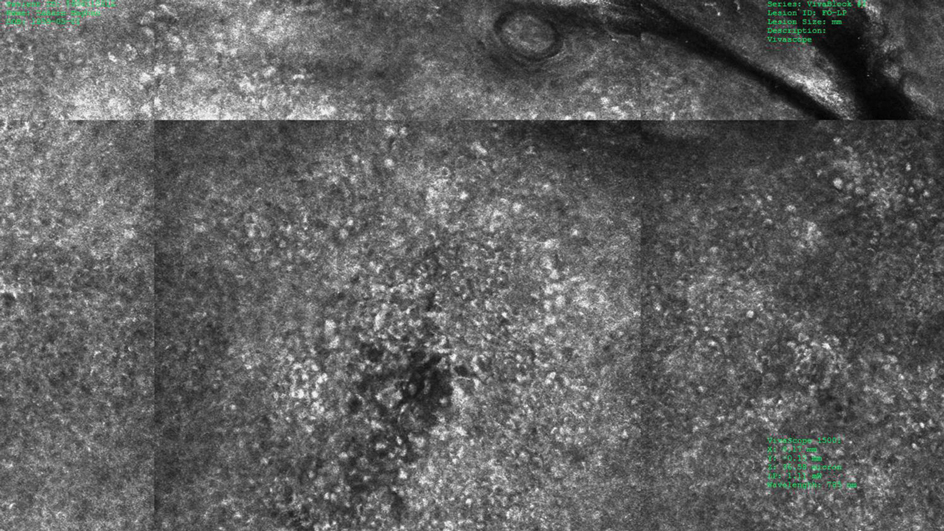
Histopathology showed an asymmetric, junctional, lentiginous, and nested proliferation of atypical epithelioid melanocytes, with few melanocytes in a pagetoid spread. There were small nests of atypical epithelioid melanocytes at the superficial dermis extending to a depth of 0.3 mm. The atypical epithelioid melanocytes displayed angulated hyperchromatic nuclei with conspicuous nucleoli and dusty brown cytoplasm. There was notable inflammation and pigment incontinence at the dermis. There was no evidence of ulceration or mitosis at the dermal component. The diagnosis of a pT1a malignant melanoma was reported (Figure 5).
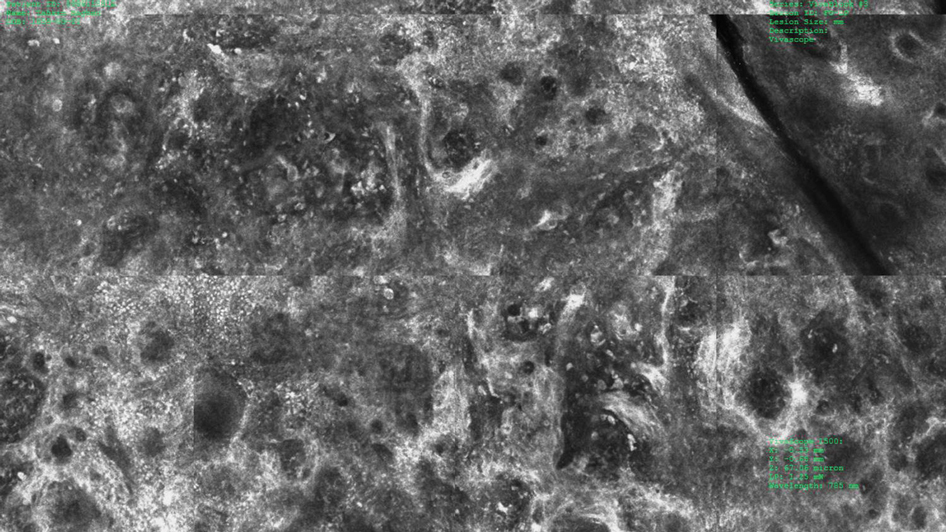
Comment
A small but enlarging dark gray papule with reticular gray-blue areas under dermoscopy in a 57-year-old man is obviously suspicious for melanoma. In daily practice, this type of small-diameter melanoma is difficult to diagnose with high confidence. We balance our aim to diagnose melanomas early with the need to reduce unnecessary excisions. Reflectance confocal microscopy may allow the clinician to arrive at the correct diagnosis and management decision with confidence before excision of the lesion.
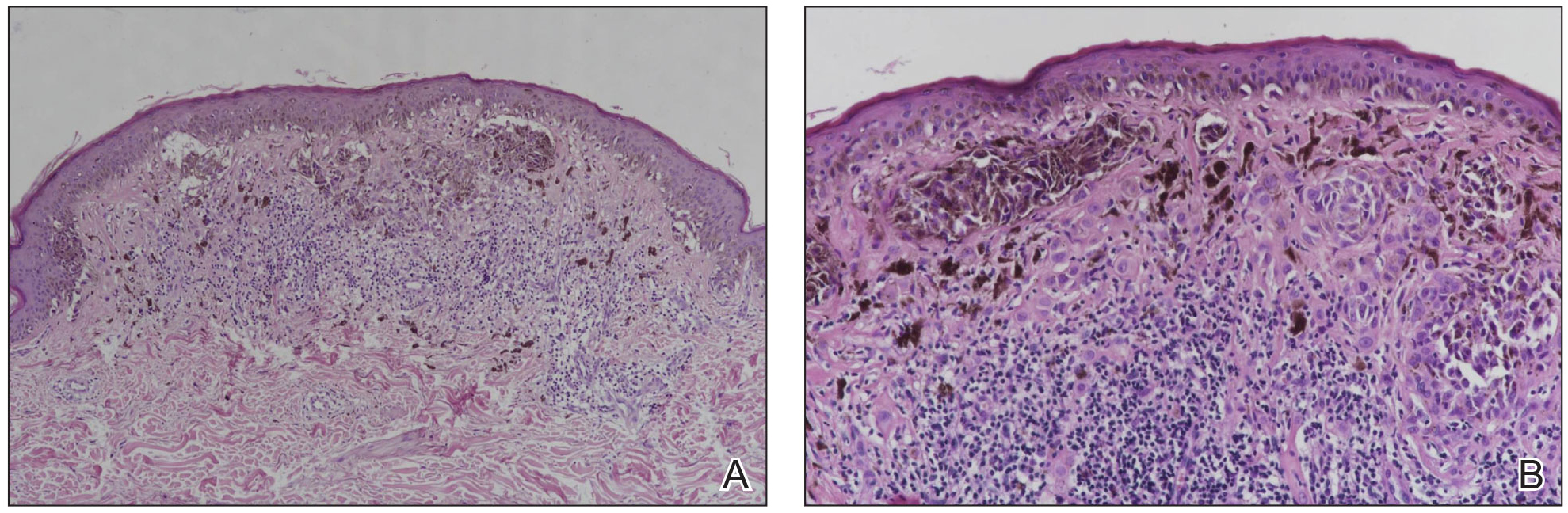
The distinction of a small-diameter melanoma from a nevus via RCM relies on evaluation of the architectural and cellular features. Findings on RCM in small-diameter melanomas have been scarcely reported in the literature; Pupelli et al10 evaluated small melanomas with a diameter of 2 to 5 mm. Among these small-diameter melanomas, the RCM features suggestive for melanomas were the presence of cytologic atypia with cellular pleomorphism, architectural disorder with irregular nests, at least 5 pagetoid cells/mm2, dendrites or tangled lines (ie, short fine lines with no visible nucleus interlacing with the adjacent keratinocytes) within the epidermis, and atypical roundish cells at the DEJ.10
The distinction between an atypical nevus and a small-diameter melanoma using RCM occasionally may be challenging.11 Pellacani et al12 reported an algorithm to distinguish melanoma from atypical nevi. According to this algorithm, when at least 1 of the architectural atypia features (irregular junctional nests, short interconnections between junctional nests, and nonhomogeneous cellularity within junctional nests) and at least 1 of the cytologic atypia features (round pagetoid cells or atypical cells at the DEJ) are observed simultaneously, the lesion is diagnosed as a dysplastic nevus or a melanoma in the first step. In the second step, the RCM diagnosis of melanoma requires at least 1 of 3 parameters: roundish pagetoid cells encompassing at least 50% of the lesional area at the spinous layer, atypical cells involving at least 50% of the lesional area at the DEJ level, and nonedged papillae involving at least 10% of the lesional area.12 Accordingly, our case corresponded with these RCM criteria for a melanoma, given that there were irregular junctional nests, atypical cells at the DEJ, and nonedged papillae involving at least 10% of the lesion.
The current limitations of RCM are the high cost of the device (approximately $58,125–$139,400 for different models), the amount of time needed to train staff in RCM units (seminars, congresses, and special courses organized by the International Confocal Working Group), and the amount of time needed for evaluation of individual lesions (15–20 minutes). However, RCM can be valuable in the clinical diagnosis of difficult lesions, as seen in our case.
Conclusion
Our case highlights the benefit of RCM in allowing the confident diagnosis and correct management of a small-diameter melanoma that turned out to be a melanoma with 0.3-mm Breslow thickness. Even so, histopathologic evaluation remains the gold standard for the diagnosis of melanoma.
- Bergman R, Katz I, Lichtig C, et al. Malignant melanomas with histologic diameters less than 6 mm. J Am Acad Dermatol. 1992;26:462-466.
- Bono A, Tolomio E, Trincone S, et al. Micro-melanoma detection: a clinical study on 206 consecutive cases of pigmented skin lesions with a diameter < or = 3 mm. Br J Dermatol. 2006;155:570-573.
- Bono A, Bartoli C, Baldi M, et al. Micro-melanoma detection. a clinical study on 22 cases of melanoma with a diameter equal to or less than 3 mm. Tumori. 2004;90:128-131.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Ferrari B, Pupelli G, Farnetani F, et al. Dermoscopic difficult lesions: an objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J Eur Acad Dermatol Venereol. 2015;29:1135-1140.
- Dinnes J, Deeks JJ, Saleh D, et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD013190.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Pupelli G, Longo C, Veneziano L, et al. Small-diameter melanocytic lesions: morphological analysis by means of in vivo confocal microscopy. Br J Dermatol. 2013;168:1027-1033.
- Carrera C, Marghoob AA. Discriminating nevi from melanomas: clues and pitfalls. Dermatol Clin. 2016;34:395-409.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
Melanomas have been designated as small melanomas or micromelanomas according to their long-axis diameter (<6 mm and ≤3 mm, respectively).1-3 Because small-diameter melanomas also have the potential to metastasize, particularly if nodular, early diagnosis can be highly rewarding. Deep melanomas with small diameters may have the same potential for metastasis as large-diameter melanomas. In this context, dermoscopy, digital dermoscopic monitoring, and total-body photography are useful in clinical practice. However, these techniques are of limited utility for small, dermoscopic feature–poor melanomas. Conversely, less than 10% of changing lesions, which are spotted via digital dermoscopic surveillance, turn out to be melanomas; therefore, simply removing all changing lesions may result in many unnecessary excisions of benign lesions.4
In vivo reflectance confocal microscopy (RCM) is an advanced technique that allows recognition of the architectural and cellular details of pigmented lesions. Reflectance confocal microscopy has the potential to reduce the rate of unnecessary excisions and to diminish the risk for missing a melanoma.5-7 In meta-analyses, RCM sensitivity was reported as 90% to 93% and specificity was reported as 78% to 82% in detecting melanoma.8,9
We describe a case that highlights the potential role of RCM in the diagnosis of small-diameter melanomas.

Case Report
A 57-year-old man with Fitzpatrick skin type III presented to the dermato-oncology unit for evaluation of multiple nevi. He was otherwise healthy and denied a history of skin cancer. Total-body skin examination with dermoscopy was performed, and several mildly atypical lesions were identified. We decided to perform digital dermoscopic monitoring. The patient’s 6-month monitoring appointment had been scheduled, but he did not arrive for the follow-up visit until 10 months after the initial examination. A lesion on the left arm, which initially was 1.5 mm in diameter, had enlarged. It was now a dark brown–gray papule with a 2.5-mm diameter (Figure 1). Dermoscopy revealed grayish globules/dots at the center of the lesion, reticular gray-blue areas, and few milialike cysts; at the periphery, a narrow rim of brownish delicate pigment network also was seen (Figure 2). The clinical and dermoscopic differential diagnosis was either an atypical nevus or an early melanoma. For a more precise diagnosis before excision, the lesion was evaluated with RCM, which takes 10 to 15 minutes to perform.

Under RCM at the epidermis level, there was a cobblestone pattern that showed a focus with mild disarrangement and few small, roundish, nucleated cells (Figure 3). A mosaic image, akin to low-magnification microscopy that enables overview of the entire lesion, at the level of the dermoepidermal junction (DEJ) showed an overall irregular meshwork pattern. Higher-magnification optical sections showed marked and diffuse (extending >10% of lesion area) architectural disorder with confluent junctional nests that were irregular to bizarre in shape and uneven in size and spacing as well as edged and nonedged papillae. At the superficial dermal level, atypical bright nucleated cells (>5 cells/mm2) were observed (Figure 4). Bright dots and/or plump bright cells within papillae also were observed. These RCM findings were highly suggestive for melanoma.

Histopathology showed an asymmetric, junctional, lentiginous, and nested proliferation of atypical epithelioid melanocytes, with few melanocytes in a pagetoid spread. There were small nests of atypical epithelioid melanocytes at the superficial dermis extending to a depth of 0.3 mm. The atypical epithelioid melanocytes displayed angulated hyperchromatic nuclei with conspicuous nucleoli and dusty brown cytoplasm. There was notable inflammation and pigment incontinence at the dermis. There was no evidence of ulceration or mitosis at the dermal component. The diagnosis of a pT1a malignant melanoma was reported (Figure 5).

Comment
A small but enlarging dark gray papule with reticular gray-blue areas under dermoscopy in a 57-year-old man is obviously suspicious for melanoma. In daily practice, this type of small-diameter melanoma is difficult to diagnose with high confidence. We balance our aim to diagnose melanomas early with the need to reduce unnecessary excisions. Reflectance confocal microscopy may allow the clinician to arrive at the correct diagnosis and management decision with confidence before excision of the lesion.

The distinction of a small-diameter melanoma from a nevus via RCM relies on evaluation of the architectural and cellular features. Findings on RCM in small-diameter melanomas have been scarcely reported in the literature; Pupelli et al10 evaluated small melanomas with a diameter of 2 to 5 mm. Among these small-diameter melanomas, the RCM features suggestive for melanomas were the presence of cytologic atypia with cellular pleomorphism, architectural disorder with irregular nests, at least 5 pagetoid cells/mm2, dendrites or tangled lines (ie, short fine lines with no visible nucleus interlacing with the adjacent keratinocytes) within the epidermis, and atypical roundish cells at the DEJ.10
The distinction between an atypical nevus and a small-diameter melanoma using RCM occasionally may be challenging.11 Pellacani et al12 reported an algorithm to distinguish melanoma from atypical nevi. According to this algorithm, when at least 1 of the architectural atypia features (irregular junctional nests, short interconnections between junctional nests, and nonhomogeneous cellularity within junctional nests) and at least 1 of the cytologic atypia features (round pagetoid cells or atypical cells at the DEJ) are observed simultaneously, the lesion is diagnosed as a dysplastic nevus or a melanoma in the first step. In the second step, the RCM diagnosis of melanoma requires at least 1 of 3 parameters: roundish pagetoid cells encompassing at least 50% of the lesional area at the spinous layer, atypical cells involving at least 50% of the lesional area at the DEJ level, and nonedged papillae involving at least 10% of the lesional area.12 Accordingly, our case corresponded with these RCM criteria for a melanoma, given that there were irregular junctional nests, atypical cells at the DEJ, and nonedged papillae involving at least 10% of the lesion.
The current limitations of RCM are the high cost of the device (approximately $58,125–$139,400 for different models), the amount of time needed to train staff in RCM units (seminars, congresses, and special courses organized by the International Confocal Working Group), and the amount of time needed for evaluation of individual lesions (15–20 minutes). However, RCM can be valuable in the clinical diagnosis of difficult lesions, as seen in our case.
Conclusion
Our case highlights the benefit of RCM in allowing the confident diagnosis and correct management of a small-diameter melanoma that turned out to be a melanoma with 0.3-mm Breslow thickness. Even so, histopathologic evaluation remains the gold standard for the diagnosis of melanoma.
Melanomas have been designated as small melanomas or micromelanomas according to their long-axis diameter (<6 mm and ≤3 mm, respectively).1-3 Because small-diameter melanomas also have the potential to metastasize, particularly if nodular, early diagnosis can be highly rewarding. Deep melanomas with small diameters may have the same potential for metastasis as large-diameter melanomas. In this context, dermoscopy, digital dermoscopic monitoring, and total-body photography are useful in clinical practice. However, these techniques are of limited utility for small, dermoscopic feature–poor melanomas. Conversely, less than 10% of changing lesions, which are spotted via digital dermoscopic surveillance, turn out to be melanomas; therefore, simply removing all changing lesions may result in many unnecessary excisions of benign lesions.4
In vivo reflectance confocal microscopy (RCM) is an advanced technique that allows recognition of the architectural and cellular details of pigmented lesions. Reflectance confocal microscopy has the potential to reduce the rate of unnecessary excisions and to diminish the risk for missing a melanoma.5-7 In meta-analyses, RCM sensitivity was reported as 90% to 93% and specificity was reported as 78% to 82% in detecting melanoma.8,9
We describe a case that highlights the potential role of RCM in the diagnosis of small-diameter melanomas.

Case Report
A 57-year-old man with Fitzpatrick skin type III presented to the dermato-oncology unit for evaluation of multiple nevi. He was otherwise healthy and denied a history of skin cancer. Total-body skin examination with dermoscopy was performed, and several mildly atypical lesions were identified. We decided to perform digital dermoscopic monitoring. The patient’s 6-month monitoring appointment had been scheduled, but he did not arrive for the follow-up visit until 10 months after the initial examination. A lesion on the left arm, which initially was 1.5 mm in diameter, had enlarged. It was now a dark brown–gray papule with a 2.5-mm diameter (Figure 1). Dermoscopy revealed grayish globules/dots at the center of the lesion, reticular gray-blue areas, and few milialike cysts; at the periphery, a narrow rim of brownish delicate pigment network also was seen (Figure 2). The clinical and dermoscopic differential diagnosis was either an atypical nevus or an early melanoma. For a more precise diagnosis before excision, the lesion was evaluated with RCM, which takes 10 to 15 minutes to perform.

Under RCM at the epidermis level, there was a cobblestone pattern that showed a focus with mild disarrangement and few small, roundish, nucleated cells (Figure 3). A mosaic image, akin to low-magnification microscopy that enables overview of the entire lesion, at the level of the dermoepidermal junction (DEJ) showed an overall irregular meshwork pattern. Higher-magnification optical sections showed marked and diffuse (extending >10% of lesion area) architectural disorder with confluent junctional nests that were irregular to bizarre in shape and uneven in size and spacing as well as edged and nonedged papillae. At the superficial dermal level, atypical bright nucleated cells (>5 cells/mm2) were observed (Figure 4). Bright dots and/or plump bright cells within papillae also were observed. These RCM findings were highly suggestive for melanoma.

Histopathology showed an asymmetric, junctional, lentiginous, and nested proliferation of atypical epithelioid melanocytes, with few melanocytes in a pagetoid spread. There were small nests of atypical epithelioid melanocytes at the superficial dermis extending to a depth of 0.3 mm. The atypical epithelioid melanocytes displayed angulated hyperchromatic nuclei with conspicuous nucleoli and dusty brown cytoplasm. There was notable inflammation and pigment incontinence at the dermis. There was no evidence of ulceration or mitosis at the dermal component. The diagnosis of a pT1a malignant melanoma was reported (Figure 5).

Comment
A small but enlarging dark gray papule with reticular gray-blue areas under dermoscopy in a 57-year-old man is obviously suspicious for melanoma. In daily practice, this type of small-diameter melanoma is difficult to diagnose with high confidence. We balance our aim to diagnose melanomas early with the need to reduce unnecessary excisions. Reflectance confocal microscopy may allow the clinician to arrive at the correct diagnosis and management decision with confidence before excision of the lesion.

The distinction of a small-diameter melanoma from a nevus via RCM relies on evaluation of the architectural and cellular features. Findings on RCM in small-diameter melanomas have been scarcely reported in the literature; Pupelli et al10 evaluated small melanomas with a diameter of 2 to 5 mm. Among these small-diameter melanomas, the RCM features suggestive for melanomas were the presence of cytologic atypia with cellular pleomorphism, architectural disorder with irregular nests, at least 5 pagetoid cells/mm2, dendrites or tangled lines (ie, short fine lines with no visible nucleus interlacing with the adjacent keratinocytes) within the epidermis, and atypical roundish cells at the DEJ.10
The distinction between an atypical nevus and a small-diameter melanoma using RCM occasionally may be challenging.11 Pellacani et al12 reported an algorithm to distinguish melanoma from atypical nevi. According to this algorithm, when at least 1 of the architectural atypia features (irregular junctional nests, short interconnections between junctional nests, and nonhomogeneous cellularity within junctional nests) and at least 1 of the cytologic atypia features (round pagetoid cells or atypical cells at the DEJ) are observed simultaneously, the lesion is diagnosed as a dysplastic nevus or a melanoma in the first step. In the second step, the RCM diagnosis of melanoma requires at least 1 of 3 parameters: roundish pagetoid cells encompassing at least 50% of the lesional area at the spinous layer, atypical cells involving at least 50% of the lesional area at the DEJ level, and nonedged papillae involving at least 10% of the lesional area.12 Accordingly, our case corresponded with these RCM criteria for a melanoma, given that there were irregular junctional nests, atypical cells at the DEJ, and nonedged papillae involving at least 10% of the lesion.
The current limitations of RCM are the high cost of the device (approximately $58,125–$139,400 for different models), the amount of time needed to train staff in RCM units (seminars, congresses, and special courses organized by the International Confocal Working Group), and the amount of time needed for evaluation of individual lesions (15–20 minutes). However, RCM can be valuable in the clinical diagnosis of difficult lesions, as seen in our case.
Conclusion
Our case highlights the benefit of RCM in allowing the confident diagnosis and correct management of a small-diameter melanoma that turned out to be a melanoma with 0.3-mm Breslow thickness. Even so, histopathologic evaluation remains the gold standard for the diagnosis of melanoma.
- Bergman R, Katz I, Lichtig C, et al. Malignant melanomas with histologic diameters less than 6 mm. J Am Acad Dermatol. 1992;26:462-466.
- Bono A, Tolomio E, Trincone S, et al. Micro-melanoma detection: a clinical study on 206 consecutive cases of pigmented skin lesions with a diameter < or = 3 mm. Br J Dermatol. 2006;155:570-573.
- Bono A, Bartoli C, Baldi M, et al. Micro-melanoma detection. a clinical study on 22 cases of melanoma with a diameter equal to or less than 3 mm. Tumori. 2004;90:128-131.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Ferrari B, Pupelli G, Farnetani F, et al. Dermoscopic difficult lesions: an objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J Eur Acad Dermatol Venereol. 2015;29:1135-1140.
- Dinnes J, Deeks JJ, Saleh D, et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD013190.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Pupelli G, Longo C, Veneziano L, et al. Small-diameter melanocytic lesions: morphological analysis by means of in vivo confocal microscopy. Br J Dermatol. 2013;168:1027-1033.
- Carrera C, Marghoob AA. Discriminating nevi from melanomas: clues and pitfalls. Dermatol Clin. 2016;34:395-409.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
- Bergman R, Katz I, Lichtig C, et al. Malignant melanomas with histologic diameters less than 6 mm. J Am Acad Dermatol. 1992;26:462-466.
- Bono A, Tolomio E, Trincone S, et al. Micro-melanoma detection: a clinical study on 206 consecutive cases of pigmented skin lesions with a diameter < or = 3 mm. Br J Dermatol. 2006;155:570-573.
- Bono A, Bartoli C, Baldi M, et al. Micro-melanoma detection. a clinical study on 22 cases of melanoma with a diameter equal to or less than 3 mm. Tumori. 2004;90:128-131.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Ferrari B, Pupelli G, Farnetani F, et al. Dermoscopic difficult lesions: an objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J Eur Acad Dermatol Venereol. 2015;29:1135-1140.
- Dinnes J, Deeks JJ, Saleh D, et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD013190.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Pupelli G, Longo C, Veneziano L, et al. Small-diameter melanocytic lesions: morphological analysis by means of in vivo confocal microscopy. Br J Dermatol. 2013;168:1027-1033.
- Carrera C, Marghoob AA. Discriminating nevi from melanomas: clues and pitfalls. Dermatol Clin. 2016;34:395-409.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
Practice Points
- Melanomas with a long-axis diameter smaller than 6 mm are considered small melanomas, and those with diameters of 3 mm and smaller are considered micromelanomas; both are difficult to detect.
- Digital dermoscopic monitoring and reflectance confocal microscopy are important tools in detecting small melanomas.
Surgical Planning for Mohs Defect Reconstruction in the Digital Age
Practice Gap
An essential part of training for a micrographic surgery and dermatologic oncology fellowship and scope of practice involves planning and execution of reconstructive surgery for Mohs defects. Recently, a surgical pearl presented by Rickstrew and colleagues1 highlighted the use of different colored surgical marking pens and their benefit in a trainee-based environment.
Delineating multiple options for reconstruction with different colored markers on live patients allows fellows in-training to participate in surgical planning but introduces more markings or drawings that need to be wiped off during or after surgery, potentially prolonging operative time. Furthermore, the Rickstrew approach has the potential to (1) cause unnecessary emotional distress for the patient during surgical planning and (2) add to the cost of surgery with the purchase of various colors of surgical markers.
Technique
To improve patient experience and trainee education, we propose fine-tuning the colored marker approach by utilizing a digital drawing program for surgical planning prior to the procedure. We recommend Snip & Sketch—a free, readily accessible digital annotating application that runs on the Microsoft Windows 10 operating system (https://www.microsoft.com/en-us/p/snip-sketch/9mz95kl8mr0l#activetab=pivot:overviewtab)—to mark up screenshot photographs of postoperative Mohs defects from the electronic medical record.
Using Snip & Sketch, the fellow in-training can then use, for example, a green “digital pen” to draw on the captured image and plan their surgical repairs (Figure 1) without input from the attending physician. Different colored pens can be used to highlight nerves, vessels, relaxed skin tension lines, and tension vectors associated with flap movement.

Subsequently, the attending physician, using a different color digital pen—say, blue—can design alternative reconstructive options (Figure 1). Suture lines also can be drawn to outline the predicted appearance of surgical scars (Figure 2).

Then, the attending physician and fellow in-training brainstorm and discuss the advantages and disadvantages of each reconstructive option to determine the optimal approach to repairing the Mohs defect.
Advantages and Disadvantages
The main advantage of using a digital drawing program is that it is time-saving and cost-efficient. Digital planning also spares the patient undue anxiety from listening to the discussion on each repair option.
The primary downside of digital surgical planning is that it is 2-dimensional, thus providing an incomplete representation of a 3-dimensional cutaneous structure. In addition, skin laxity, flap mobility, and free-margin distortion cannot be fully appreciated on a 2-dimensional image.
Despite these drawbacks, digital surgical planning provides trainees with an active learning experience through a more collaborative and comprehensive discussion of reconstructive options.
Practice Implications
Active learning using an electronic device has been validated as a beneficial addition to Mohs micrographic surgery training.2 Utilizing a digitized annotating program for surgical planning increases the independence of trainees and allows immediate feedback from the attending physician. The synergy of digital technology and collaborative learning helps cultivate the next generation of confident and competent Mohs surgeons.
- Rickstrew J, Roberts E, Amarani A, et al. Different colored surgical marking pens for trainee education. J Am Acad Dermatol. 2021:S0190-9622(21)00226-7. doi:10.1016/j.jaad.2021.01.069
- Croley JA, Malone CH, Goodwin BP, et al. Mohs Surgical Reconstruction Educational Activity: a resident education tool. Adv Med Educ Pract. 2017;8:143-147. doi:10.2147/AMEP.S125454
Practice Gap
An essential part of training for a micrographic surgery and dermatologic oncology fellowship and scope of practice involves planning and execution of reconstructive surgery for Mohs defects. Recently, a surgical pearl presented by Rickstrew and colleagues1 highlighted the use of different colored surgical marking pens and their benefit in a trainee-based environment.
Delineating multiple options for reconstruction with different colored markers on live patients allows fellows in-training to participate in surgical planning but introduces more markings or drawings that need to be wiped off during or after surgery, potentially prolonging operative time. Furthermore, the Rickstrew approach has the potential to (1) cause unnecessary emotional distress for the patient during surgical planning and (2) add to the cost of surgery with the purchase of various colors of surgical markers.
Technique
To improve patient experience and trainee education, we propose fine-tuning the colored marker approach by utilizing a digital drawing program for surgical planning prior to the procedure. We recommend Snip & Sketch—a free, readily accessible digital annotating application that runs on the Microsoft Windows 10 operating system (https://www.microsoft.com/en-us/p/snip-sketch/9mz95kl8mr0l#activetab=pivot:overviewtab)—to mark up screenshot photographs of postoperative Mohs defects from the electronic medical record.
Using Snip & Sketch, the fellow in-training can then use, for example, a green “digital pen” to draw on the captured image and plan their surgical repairs (Figure 1) without input from the attending physician. Different colored pens can be used to highlight nerves, vessels, relaxed skin tension lines, and tension vectors associated with flap movement.

Subsequently, the attending physician, using a different color digital pen—say, blue—can design alternative reconstructive options (Figure 1). Suture lines also can be drawn to outline the predicted appearance of surgical scars (Figure 2).

Then, the attending physician and fellow in-training brainstorm and discuss the advantages and disadvantages of each reconstructive option to determine the optimal approach to repairing the Mohs defect.
Advantages and Disadvantages
The main advantage of using a digital drawing program is that it is time-saving and cost-efficient. Digital planning also spares the patient undue anxiety from listening to the discussion on each repair option.
The primary downside of digital surgical planning is that it is 2-dimensional, thus providing an incomplete representation of a 3-dimensional cutaneous structure. In addition, skin laxity, flap mobility, and free-margin distortion cannot be fully appreciated on a 2-dimensional image.
Despite these drawbacks, digital surgical planning provides trainees with an active learning experience through a more collaborative and comprehensive discussion of reconstructive options.
Practice Implications
Active learning using an electronic device has been validated as a beneficial addition to Mohs micrographic surgery training.2 Utilizing a digitized annotating program for surgical planning increases the independence of trainees and allows immediate feedback from the attending physician. The synergy of digital technology and collaborative learning helps cultivate the next generation of confident and competent Mohs surgeons.
Practice Gap
An essential part of training for a micrographic surgery and dermatologic oncology fellowship and scope of practice involves planning and execution of reconstructive surgery for Mohs defects. Recently, a surgical pearl presented by Rickstrew and colleagues1 highlighted the use of different colored surgical marking pens and their benefit in a trainee-based environment.
Delineating multiple options for reconstruction with different colored markers on live patients allows fellows in-training to participate in surgical planning but introduces more markings or drawings that need to be wiped off during or after surgery, potentially prolonging operative time. Furthermore, the Rickstrew approach has the potential to (1) cause unnecessary emotional distress for the patient during surgical planning and (2) add to the cost of surgery with the purchase of various colors of surgical markers.
Technique
To improve patient experience and trainee education, we propose fine-tuning the colored marker approach by utilizing a digital drawing program for surgical planning prior to the procedure. We recommend Snip & Sketch—a free, readily accessible digital annotating application that runs on the Microsoft Windows 10 operating system (https://www.microsoft.com/en-us/p/snip-sketch/9mz95kl8mr0l#activetab=pivot:overviewtab)—to mark up screenshot photographs of postoperative Mohs defects from the electronic medical record.
Using Snip & Sketch, the fellow in-training can then use, for example, a green “digital pen” to draw on the captured image and plan their surgical repairs (Figure 1) without input from the attending physician. Different colored pens can be used to highlight nerves, vessels, relaxed skin tension lines, and tension vectors associated with flap movement.

Subsequently, the attending physician, using a different color digital pen—say, blue—can design alternative reconstructive options (Figure 1). Suture lines also can be drawn to outline the predicted appearance of surgical scars (Figure 2).

Then, the attending physician and fellow in-training brainstorm and discuss the advantages and disadvantages of each reconstructive option to determine the optimal approach to repairing the Mohs defect.
Advantages and Disadvantages
The main advantage of using a digital drawing program is that it is time-saving and cost-efficient. Digital planning also spares the patient undue anxiety from listening to the discussion on each repair option.
The primary downside of digital surgical planning is that it is 2-dimensional, thus providing an incomplete representation of a 3-dimensional cutaneous structure. In addition, skin laxity, flap mobility, and free-margin distortion cannot be fully appreciated on a 2-dimensional image.
Despite these drawbacks, digital surgical planning provides trainees with an active learning experience through a more collaborative and comprehensive discussion of reconstructive options.
Practice Implications
Active learning using an electronic device has been validated as a beneficial addition to Mohs micrographic surgery training.2 Utilizing a digitized annotating program for surgical planning increases the independence of trainees and allows immediate feedback from the attending physician. The synergy of digital technology and collaborative learning helps cultivate the next generation of confident and competent Mohs surgeons.
- Rickstrew J, Roberts E, Amarani A, et al. Different colored surgical marking pens for trainee education. J Am Acad Dermatol. 2021:S0190-9622(21)00226-7. doi:10.1016/j.jaad.2021.01.069
- Croley JA, Malone CH, Goodwin BP, et al. Mohs Surgical Reconstruction Educational Activity: a resident education tool. Adv Med Educ Pract. 2017;8:143-147. doi:10.2147/AMEP.S125454
- Rickstrew J, Roberts E, Amarani A, et al. Different colored surgical marking pens for trainee education. J Am Acad Dermatol. 2021:S0190-9622(21)00226-7. doi:10.1016/j.jaad.2021.01.069
- Croley JA, Malone CH, Goodwin BP, et al. Mohs Surgical Reconstruction Educational Activity: a resident education tool. Adv Med Educ Pract. 2017;8:143-147. doi:10.2147/AMEP.S125454
Q&A with Hubert (Hugh) Greenway, MD
who was also recently selected as program director for cutaneous oncology at Scripps MD Anderson Cancer Center in San Diego. He is also a former president of the American College of Mohs Surgery.
After earning his medical degree from the Medical College of Georgia, Augusta, in 1974, Dr. Greenway was fellowship trained in Mohs skin cancer surgery by Frederic E. Mohs, MD, at the University of Wisconsin–Madison. He completed his dermatology residency at the Naval Medical Center San Diego and joined Scripps Clinic in 1983, where he launched the institution’s first Mohs surgery program, as well as a popular annual intensive course in superficial anatomy and cutaneous surgery that bears his name. He was also the first physician in the world to use interferon as a nonsurgical treatment of basal cell carcinoma.

To date, Dr. Greenway has performed more than 41,000 Mohs surgery cases and has trained 61 fellows who practice in academic and clinical settings. In 2017, he received the Frederic E. Mohs Award from the ACMS at the college’s annual meeting. He is also a past CEO of Scripps Clinic. In this Q&A, Dr. Greenway opens up about what it was like to train with Dr. Mohs, what makes a good Mohs surgeon, and why he’s excited about the future of dermatology.
I understand that you first became interested in a medical career after meeting Dr. Carl Jones, a friend of your father who was your Scoutmaster in the Boy Scouts in Georgia. What about Dr. Jones inspired you to pursue a career in medicine?
Dr. Jones was an internist/allergist in Atlanta, where I grew up. His three sons and I were friends. My dad had dealt with several medical problems being injured in World War II and subsequently undergoing a couple of kidney transplantations, so I developed an interest in medicine personally. Even though Dr. Jones was a specialist, he started out as a family doctor like I did, so he was interested in the whole person and all of his or her medical problems as opposed to those related to his specialty only. I traveled with the Boy Scouts to camp at places like Valley Forge in Pennsylvania, and Dr. Jones was involved with the medical set-ups of those large events. That also contributed to my interest in medicine.
As part of your 9-year service in the U.S. Navy, you spent 2 years as the flight surgeon at NAS Atlanta/Dobbins Air Force Base. What was your most memorable experience from that assignment?
Dobbins is a large facility with two Lockheed plants, and the Air Force had built the medical clinic, which was staffed by the Navy. Getting to know some of the active-duty members of the Air Force, the Navy, and the National Guard, and their commitment to our country, was memorable. Jimmy Carter was the president in those days. When he would fly in Dobbins, one of my jobs as the flight surgeon was to be on base when Air Force One landed or departed. One night, we had a DC-9 commercial aircraft coming from Huntsville, Ala., to Atlanta that got caught in a thunderstorm a little above 30,000 feet. Both engines went out and the aircraft essentially became a glider. The pilots tried to land on our runway but unfortunately, they ended up 4 miles short. We were heavily involved in responding to the crash, which was a tragic event. I also learned to fly (second seat) different types of aircraft during my assignment at NAS Atlanta/Dobbins Air Force Base, everything from the large C-5s to Navy fighter jets and helicopters. Coincidentally, Dr. Jones was involved with a couple of free health clinics in Atlanta when I was stationed there. Every Tuesday night, my wife (who is a nurse) and I would volunteer at a clinic in Cabbagetown, which was one of the poorer areas of Atlanta. It was a chance to give back to a group of people who didn’t have a whole lot.
In the middle your dermatology residency at Naval Medical Center San Diego, you were selected by Dr. Mohs for fellowship training in Mohs skin cancer surgery at the University of Wisconsin–Madison. What do you remember most about your training with Dr. Mohs?
Dr. Mohs was a kind, humble man who had this great idea about skin cancer. He was not a dermatologist; he was a general surgeon. The technique he developed was originally called chemosurgery because he put a chemical onto the skin. This was known as the fixed-tissue technique. Then we had a fresh-tissue technique, where we did not use the chemical, but we were able to use local anesthesia right away. That developed into the Mohs surgery we know today. Dr. Mohs did not name it that; he was very humble, but he was very proud of his technique. He was also a very hard worker. On the first day of my fellowship, I started at 7 in the morning and ended at 7 at night. It was the same for the last day of my fellowship. He also had an excellent office staff, many of whom had worked with him for many years. Patients with difficult skin cancers traveled to Madison from all over the world because there weren’t that many Mohs surgery clinics in those days. During the latter part of my fellowship, Michael McCall, MD, and I had the opportunity to remove a skin cancer from the nose of Dr. Mohs. We presented the case at a national conference, and I titled the talk “Mohs Surgery for Mohs’ Nose.”
Early in your career Dr. Mohs asked you to take over his practice, but you accepted an offer to establish the first Mohs surgery office at Scripps in San Diego instead. What convinced you to head West?
After my fellowship, I returned to San Diego to complete my residency with the Navy, where we opened a Mohs surgery clinic. Dr. Mohs came out for the ribbon cutting. During that time, I was taking care of several patients that he had treated in Wisconsin. Through that my wife and I ended up going to dinner with Cecil and Ida Green, philanthropists who made several financial gifts to Scripps Clinic – and for whom Scripps Green Hospital is named. Cecil cofounded Texas Instruments and was knighted by Queen Elizabeth. During dinner, he suggested that I stay in San Diego for a year and work at Scripps after my residency assignment with the Navy. I agreed and have been here ever since.
What do you find most interesting about Mohs surgery?
In Mohs surgery, you’re able to provide not only surgical care to eliminate the tumor, but also the pathology and the reconstruction. That was interesting to me. Dr. Mohs was not that interested in reconstruction. He was more focused on the tumor, in part because with the original fixed-tissue technique you could not do the reconstruction. You had to wait for an extra layer of tissue to separate. But with the fresh-tissue technique, you were able to provide the reconstruction that day. Mohs surgery deals with a subset of tumors that are challenging to treat. That also spiked my academic and clinical interest.
In your opinion, what’s been the most important advance in Mohs surgery to date?
In recent years, immunology has come into play, so now we have teams of clinicians in dermatology, medical oncology, surgery, and other subspecialties providing patients the best of care. In the arena of Mohs surgery itself, in the 1980s, the American College of Mohs Surgery developed a 1-year fellowship program, which enabled us to train many men and women to practice Mohs surgery. Most of them are dermatologists.
Please complete the sentence: “You can tell a good Mohs surgeon by the way he/she ...”
Treats patients, is willing to spend time with them, and shows an interest in them. One of the things we should strive for is to let patients know that they as a person are important; it’s not just the melanoma on their nose. We’re not only dealing with a skin cancer; we’re dealing with a patient who has skin cancer.
For the past 39 years, you have led Hugh Greenway’s Superficial Anatomy and Cutaneous Surgery course, which takes place every January in San Diego. What’s been key to sustaining this training course for nearly 4 decades?
There have been many people involved in its success, so it’s not just me. When I first started my practice, there really was not a focus on anatomy in the general dermatologic community. Dermatologic surgery textbooks contained very little content on surgical anatomy so I developed an interest a putting together a course that would cover some of this material. I met with Terence Davidson, MD, an otolaryngologist who was dean of continuing medical education at the University of California, San Diego. The course includes lectures from experts in many subspecialties and hands-on laboratories using cadavers to work on anatomy and surgical techniques. After about 16 years of doing the course Dr. Davidson told me: “When we started this course, as a group, the head and neck surgeons were the best to do the reconstructions on the face with skin flaps and grafts and layered closures. But now, as a group, the dermatologists are best at doing that.” That’s what we want to hear in medical education.
During the peak of the COVID-19 pandemic, what were your most significant challenges from both a clinical and a personal standpoint?
I’m fortunate to practice at a place like Scripps, where there are many resources to look at what was happening with COVID-19. Clinically, we had to put a lot of things on hold, but we tried our best to keep our cancer patients in particular in the forefront of care. It has been a challenge, but fortunately we have been able to take care of patients after a brief timeout. Many of us remember the polio vaccine back in the 1950s. Having worked overseas and at missionary hospital where we had children die of measles because they were not vaccinated gave me a larger appreciation for the importance of vaccines. I recommend all young physicians who work with me to read, “The Great Influenza: The Story of the Deadliest Pandemic in History,” by John M. Barry, which recounts the 1918 flu epidemic.
Who inspires you most in your work today?
I don’t view what I do as work. Dr. Jones and Dr. Mohs continue to inspire me with what they accomplished during their careers. You have to love people and love patients. Every patient who comes to see me has a story, so I try to understand their story. One of the things I really enjoy is training the young fellows. We train three Mohs fellows per year at Scripps, and it’s a great challenge every day.
What development in dermatology are you most excited about in the next 5 years?
Dermatology will continue to evolve just like all other medical specialties. We’re going to see a large growth in telemedicine, and immunotherapy is playing a key role in dermatologic oncology. What excites me the most in medicine is the young people who enter the field willing to contribute their lives to helping others.
who was also recently selected as program director for cutaneous oncology at Scripps MD Anderson Cancer Center in San Diego. He is also a former president of the American College of Mohs Surgery.
After earning his medical degree from the Medical College of Georgia, Augusta, in 1974, Dr. Greenway was fellowship trained in Mohs skin cancer surgery by Frederic E. Mohs, MD, at the University of Wisconsin–Madison. He completed his dermatology residency at the Naval Medical Center San Diego and joined Scripps Clinic in 1983, where he launched the institution’s first Mohs surgery program, as well as a popular annual intensive course in superficial anatomy and cutaneous surgery that bears his name. He was also the first physician in the world to use interferon as a nonsurgical treatment of basal cell carcinoma.

To date, Dr. Greenway has performed more than 41,000 Mohs surgery cases and has trained 61 fellows who practice in academic and clinical settings. In 2017, he received the Frederic E. Mohs Award from the ACMS at the college’s annual meeting. He is also a past CEO of Scripps Clinic. In this Q&A, Dr. Greenway opens up about what it was like to train with Dr. Mohs, what makes a good Mohs surgeon, and why he’s excited about the future of dermatology.
I understand that you first became interested in a medical career after meeting Dr. Carl Jones, a friend of your father who was your Scoutmaster in the Boy Scouts in Georgia. What about Dr. Jones inspired you to pursue a career in medicine?
Dr. Jones was an internist/allergist in Atlanta, where I grew up. His three sons and I were friends. My dad had dealt with several medical problems being injured in World War II and subsequently undergoing a couple of kidney transplantations, so I developed an interest in medicine personally. Even though Dr. Jones was a specialist, he started out as a family doctor like I did, so he was interested in the whole person and all of his or her medical problems as opposed to those related to his specialty only. I traveled with the Boy Scouts to camp at places like Valley Forge in Pennsylvania, and Dr. Jones was involved with the medical set-ups of those large events. That also contributed to my interest in medicine.
As part of your 9-year service in the U.S. Navy, you spent 2 years as the flight surgeon at NAS Atlanta/Dobbins Air Force Base. What was your most memorable experience from that assignment?
Dobbins is a large facility with two Lockheed plants, and the Air Force had built the medical clinic, which was staffed by the Navy. Getting to know some of the active-duty members of the Air Force, the Navy, and the National Guard, and their commitment to our country, was memorable. Jimmy Carter was the president in those days. When he would fly in Dobbins, one of my jobs as the flight surgeon was to be on base when Air Force One landed or departed. One night, we had a DC-9 commercial aircraft coming from Huntsville, Ala., to Atlanta that got caught in a thunderstorm a little above 30,000 feet. Both engines went out and the aircraft essentially became a glider. The pilots tried to land on our runway but unfortunately, they ended up 4 miles short. We were heavily involved in responding to the crash, which was a tragic event. I also learned to fly (second seat) different types of aircraft during my assignment at NAS Atlanta/Dobbins Air Force Base, everything from the large C-5s to Navy fighter jets and helicopters. Coincidentally, Dr. Jones was involved with a couple of free health clinics in Atlanta when I was stationed there. Every Tuesday night, my wife (who is a nurse) and I would volunteer at a clinic in Cabbagetown, which was one of the poorer areas of Atlanta. It was a chance to give back to a group of people who didn’t have a whole lot.
In the middle your dermatology residency at Naval Medical Center San Diego, you were selected by Dr. Mohs for fellowship training in Mohs skin cancer surgery at the University of Wisconsin–Madison. What do you remember most about your training with Dr. Mohs?
Dr. Mohs was a kind, humble man who had this great idea about skin cancer. He was not a dermatologist; he was a general surgeon. The technique he developed was originally called chemosurgery because he put a chemical onto the skin. This was known as the fixed-tissue technique. Then we had a fresh-tissue technique, where we did not use the chemical, but we were able to use local anesthesia right away. That developed into the Mohs surgery we know today. Dr. Mohs did not name it that; he was very humble, but he was very proud of his technique. He was also a very hard worker. On the first day of my fellowship, I started at 7 in the morning and ended at 7 at night. It was the same for the last day of my fellowship. He also had an excellent office staff, many of whom had worked with him for many years. Patients with difficult skin cancers traveled to Madison from all over the world because there weren’t that many Mohs surgery clinics in those days. During the latter part of my fellowship, Michael McCall, MD, and I had the opportunity to remove a skin cancer from the nose of Dr. Mohs. We presented the case at a national conference, and I titled the talk “Mohs Surgery for Mohs’ Nose.”
Early in your career Dr. Mohs asked you to take over his practice, but you accepted an offer to establish the first Mohs surgery office at Scripps in San Diego instead. What convinced you to head West?
After my fellowship, I returned to San Diego to complete my residency with the Navy, where we opened a Mohs surgery clinic. Dr. Mohs came out for the ribbon cutting. During that time, I was taking care of several patients that he had treated in Wisconsin. Through that my wife and I ended up going to dinner with Cecil and Ida Green, philanthropists who made several financial gifts to Scripps Clinic – and for whom Scripps Green Hospital is named. Cecil cofounded Texas Instruments and was knighted by Queen Elizabeth. During dinner, he suggested that I stay in San Diego for a year and work at Scripps after my residency assignment with the Navy. I agreed and have been here ever since.
What do you find most interesting about Mohs surgery?
In Mohs surgery, you’re able to provide not only surgical care to eliminate the tumor, but also the pathology and the reconstruction. That was interesting to me. Dr. Mohs was not that interested in reconstruction. He was more focused on the tumor, in part because with the original fixed-tissue technique you could not do the reconstruction. You had to wait for an extra layer of tissue to separate. But with the fresh-tissue technique, you were able to provide the reconstruction that day. Mohs surgery deals with a subset of tumors that are challenging to treat. That also spiked my academic and clinical interest.
In your opinion, what’s been the most important advance in Mohs surgery to date?
In recent years, immunology has come into play, so now we have teams of clinicians in dermatology, medical oncology, surgery, and other subspecialties providing patients the best of care. In the arena of Mohs surgery itself, in the 1980s, the American College of Mohs Surgery developed a 1-year fellowship program, which enabled us to train many men and women to practice Mohs surgery. Most of them are dermatologists.
Please complete the sentence: “You can tell a good Mohs surgeon by the way he/she ...”
Treats patients, is willing to spend time with them, and shows an interest in them. One of the things we should strive for is to let patients know that they as a person are important; it’s not just the melanoma on their nose. We’re not only dealing with a skin cancer; we’re dealing with a patient who has skin cancer.
For the past 39 years, you have led Hugh Greenway’s Superficial Anatomy and Cutaneous Surgery course, which takes place every January in San Diego. What’s been key to sustaining this training course for nearly 4 decades?
There have been many people involved in its success, so it’s not just me. When I first started my practice, there really was not a focus on anatomy in the general dermatologic community. Dermatologic surgery textbooks contained very little content on surgical anatomy so I developed an interest a putting together a course that would cover some of this material. I met with Terence Davidson, MD, an otolaryngologist who was dean of continuing medical education at the University of California, San Diego. The course includes lectures from experts in many subspecialties and hands-on laboratories using cadavers to work on anatomy and surgical techniques. After about 16 years of doing the course Dr. Davidson told me: “When we started this course, as a group, the head and neck surgeons were the best to do the reconstructions on the face with skin flaps and grafts and layered closures. But now, as a group, the dermatologists are best at doing that.” That’s what we want to hear in medical education.
During the peak of the COVID-19 pandemic, what were your most significant challenges from both a clinical and a personal standpoint?
I’m fortunate to practice at a place like Scripps, where there are many resources to look at what was happening with COVID-19. Clinically, we had to put a lot of things on hold, but we tried our best to keep our cancer patients in particular in the forefront of care. It has been a challenge, but fortunately we have been able to take care of patients after a brief timeout. Many of us remember the polio vaccine back in the 1950s. Having worked overseas and at missionary hospital where we had children die of measles because they were not vaccinated gave me a larger appreciation for the importance of vaccines. I recommend all young physicians who work with me to read, “The Great Influenza: The Story of the Deadliest Pandemic in History,” by John M. Barry, which recounts the 1918 flu epidemic.
Who inspires you most in your work today?
I don’t view what I do as work. Dr. Jones and Dr. Mohs continue to inspire me with what they accomplished during their careers. You have to love people and love patients. Every patient who comes to see me has a story, so I try to understand their story. One of the things I really enjoy is training the young fellows. We train three Mohs fellows per year at Scripps, and it’s a great challenge every day.
What development in dermatology are you most excited about in the next 5 years?
Dermatology will continue to evolve just like all other medical specialties. We’re going to see a large growth in telemedicine, and immunotherapy is playing a key role in dermatologic oncology. What excites me the most in medicine is the young people who enter the field willing to contribute their lives to helping others.
who was also recently selected as program director for cutaneous oncology at Scripps MD Anderson Cancer Center in San Diego. He is also a former president of the American College of Mohs Surgery.
After earning his medical degree from the Medical College of Georgia, Augusta, in 1974, Dr. Greenway was fellowship trained in Mohs skin cancer surgery by Frederic E. Mohs, MD, at the University of Wisconsin–Madison. He completed his dermatology residency at the Naval Medical Center San Diego and joined Scripps Clinic in 1983, where he launched the institution’s first Mohs surgery program, as well as a popular annual intensive course in superficial anatomy and cutaneous surgery that bears his name. He was also the first physician in the world to use interferon as a nonsurgical treatment of basal cell carcinoma.

To date, Dr. Greenway has performed more than 41,000 Mohs surgery cases and has trained 61 fellows who practice in academic and clinical settings. In 2017, he received the Frederic E. Mohs Award from the ACMS at the college’s annual meeting. He is also a past CEO of Scripps Clinic. In this Q&A, Dr. Greenway opens up about what it was like to train with Dr. Mohs, what makes a good Mohs surgeon, and why he’s excited about the future of dermatology.
I understand that you first became interested in a medical career after meeting Dr. Carl Jones, a friend of your father who was your Scoutmaster in the Boy Scouts in Georgia. What about Dr. Jones inspired you to pursue a career in medicine?
Dr. Jones was an internist/allergist in Atlanta, where I grew up. His three sons and I were friends. My dad had dealt with several medical problems being injured in World War II and subsequently undergoing a couple of kidney transplantations, so I developed an interest in medicine personally. Even though Dr. Jones was a specialist, he started out as a family doctor like I did, so he was interested in the whole person and all of his or her medical problems as opposed to those related to his specialty only. I traveled with the Boy Scouts to camp at places like Valley Forge in Pennsylvania, and Dr. Jones was involved with the medical set-ups of those large events. That also contributed to my interest in medicine.
As part of your 9-year service in the U.S. Navy, you spent 2 years as the flight surgeon at NAS Atlanta/Dobbins Air Force Base. What was your most memorable experience from that assignment?
Dobbins is a large facility with two Lockheed plants, and the Air Force had built the medical clinic, which was staffed by the Navy. Getting to know some of the active-duty members of the Air Force, the Navy, and the National Guard, and their commitment to our country, was memorable. Jimmy Carter was the president in those days. When he would fly in Dobbins, one of my jobs as the flight surgeon was to be on base when Air Force One landed or departed. One night, we had a DC-9 commercial aircraft coming from Huntsville, Ala., to Atlanta that got caught in a thunderstorm a little above 30,000 feet. Both engines went out and the aircraft essentially became a glider. The pilots tried to land on our runway but unfortunately, they ended up 4 miles short. We were heavily involved in responding to the crash, which was a tragic event. I also learned to fly (second seat) different types of aircraft during my assignment at NAS Atlanta/Dobbins Air Force Base, everything from the large C-5s to Navy fighter jets and helicopters. Coincidentally, Dr. Jones was involved with a couple of free health clinics in Atlanta when I was stationed there. Every Tuesday night, my wife (who is a nurse) and I would volunteer at a clinic in Cabbagetown, which was one of the poorer areas of Atlanta. It was a chance to give back to a group of people who didn’t have a whole lot.
In the middle your dermatology residency at Naval Medical Center San Diego, you were selected by Dr. Mohs for fellowship training in Mohs skin cancer surgery at the University of Wisconsin–Madison. What do you remember most about your training with Dr. Mohs?
Dr. Mohs was a kind, humble man who had this great idea about skin cancer. He was not a dermatologist; he was a general surgeon. The technique he developed was originally called chemosurgery because he put a chemical onto the skin. This was known as the fixed-tissue technique. Then we had a fresh-tissue technique, where we did not use the chemical, but we were able to use local anesthesia right away. That developed into the Mohs surgery we know today. Dr. Mohs did not name it that; he was very humble, but he was very proud of his technique. He was also a very hard worker. On the first day of my fellowship, I started at 7 in the morning and ended at 7 at night. It was the same for the last day of my fellowship. He also had an excellent office staff, many of whom had worked with him for many years. Patients with difficult skin cancers traveled to Madison from all over the world because there weren’t that many Mohs surgery clinics in those days. During the latter part of my fellowship, Michael McCall, MD, and I had the opportunity to remove a skin cancer from the nose of Dr. Mohs. We presented the case at a national conference, and I titled the talk “Mohs Surgery for Mohs’ Nose.”
Early in your career Dr. Mohs asked you to take over his practice, but you accepted an offer to establish the first Mohs surgery office at Scripps in San Diego instead. What convinced you to head West?
After my fellowship, I returned to San Diego to complete my residency with the Navy, where we opened a Mohs surgery clinic. Dr. Mohs came out for the ribbon cutting. During that time, I was taking care of several patients that he had treated in Wisconsin. Through that my wife and I ended up going to dinner with Cecil and Ida Green, philanthropists who made several financial gifts to Scripps Clinic – and for whom Scripps Green Hospital is named. Cecil cofounded Texas Instruments and was knighted by Queen Elizabeth. During dinner, he suggested that I stay in San Diego for a year and work at Scripps after my residency assignment with the Navy. I agreed and have been here ever since.
What do you find most interesting about Mohs surgery?
In Mohs surgery, you’re able to provide not only surgical care to eliminate the tumor, but also the pathology and the reconstruction. That was interesting to me. Dr. Mohs was not that interested in reconstruction. He was more focused on the tumor, in part because with the original fixed-tissue technique you could not do the reconstruction. You had to wait for an extra layer of tissue to separate. But with the fresh-tissue technique, you were able to provide the reconstruction that day. Mohs surgery deals with a subset of tumors that are challenging to treat. That also spiked my academic and clinical interest.
In your opinion, what’s been the most important advance in Mohs surgery to date?
In recent years, immunology has come into play, so now we have teams of clinicians in dermatology, medical oncology, surgery, and other subspecialties providing patients the best of care. In the arena of Mohs surgery itself, in the 1980s, the American College of Mohs Surgery developed a 1-year fellowship program, which enabled us to train many men and women to practice Mohs surgery. Most of them are dermatologists.
Please complete the sentence: “You can tell a good Mohs surgeon by the way he/she ...”
Treats patients, is willing to spend time with them, and shows an interest in them. One of the things we should strive for is to let patients know that they as a person are important; it’s not just the melanoma on their nose. We’re not only dealing with a skin cancer; we’re dealing with a patient who has skin cancer.
For the past 39 years, you have led Hugh Greenway’s Superficial Anatomy and Cutaneous Surgery course, which takes place every January in San Diego. What’s been key to sustaining this training course for nearly 4 decades?
There have been many people involved in its success, so it’s not just me. When I first started my practice, there really was not a focus on anatomy in the general dermatologic community. Dermatologic surgery textbooks contained very little content on surgical anatomy so I developed an interest a putting together a course that would cover some of this material. I met with Terence Davidson, MD, an otolaryngologist who was dean of continuing medical education at the University of California, San Diego. The course includes lectures from experts in many subspecialties and hands-on laboratories using cadavers to work on anatomy and surgical techniques. After about 16 years of doing the course Dr. Davidson told me: “When we started this course, as a group, the head and neck surgeons were the best to do the reconstructions on the face with skin flaps and grafts and layered closures. But now, as a group, the dermatologists are best at doing that.” That’s what we want to hear in medical education.
During the peak of the COVID-19 pandemic, what were your most significant challenges from both a clinical and a personal standpoint?
I’m fortunate to practice at a place like Scripps, where there are many resources to look at what was happening with COVID-19. Clinically, we had to put a lot of things on hold, but we tried our best to keep our cancer patients in particular in the forefront of care. It has been a challenge, but fortunately we have been able to take care of patients after a brief timeout. Many of us remember the polio vaccine back in the 1950s. Having worked overseas and at missionary hospital where we had children die of measles because they were not vaccinated gave me a larger appreciation for the importance of vaccines. I recommend all young physicians who work with me to read, “The Great Influenza: The Story of the Deadliest Pandemic in History,” by John M. Barry, which recounts the 1918 flu epidemic.
Who inspires you most in your work today?
I don’t view what I do as work. Dr. Jones and Dr. Mohs continue to inspire me with what they accomplished during their careers. You have to love people and love patients. Every patient who comes to see me has a story, so I try to understand their story. One of the things I really enjoy is training the young fellows. We train three Mohs fellows per year at Scripps, and it’s a great challenge every day.
What development in dermatology are you most excited about in the next 5 years?
Dermatology will continue to evolve just like all other medical specialties. We’re going to see a large growth in telemedicine, and immunotherapy is playing a key role in dermatologic oncology. What excites me the most in medicine is the young people who enter the field willing to contribute their lives to helping others.
Melanoma
THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot in a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P<.05), followed by Hispanic (P<.05), Asian American/Native American/Pacific Islander (P<.05), and Black (P<.05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P=.015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P<.001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P=.07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi:10.1016/j.jaad.2001.05.034
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi:10.1001/archinte.166.17.1907
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi:10.1023/a:1018432632528
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi:10.1016/j. jaad.2004.05.005
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708. doi:10.1001/archderm.142.6.704
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi:10.1097/DSS.0000000000001759
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016)[published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi:10.1016/j. jaad.2020.08.097

THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot in a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P<.05), followed by Hispanic (P<.05), Asian American/Native American/Pacific Islander (P<.05), and Black (P<.05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P=.015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P<.001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P=.07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.

THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot in a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P<.05), followed by Hispanic (P<.05), Asian American/Native American/Pacific Islander (P<.05), and Black (P<.05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P=.015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P<.001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P=.07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi:10.1016/j.jaad.2001.05.034
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi:10.1001/archinte.166.17.1907
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi:10.1023/a:1018432632528
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi:10.1016/j. jaad.2004.05.005
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708. doi:10.1001/archderm.142.6.704
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi:10.1097/DSS.0000000000001759
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016)[published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi:10.1016/j. jaad.2020.08.097
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi:10.1016/j.jaad.2001.05.034
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi:10.1001/archinte.166.17.1907
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi:10.1023/a:1018432632528
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi:10.1016/j. jaad.2004.05.005
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708. doi:10.1001/archderm.142.6.704
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi:10.1097/DSS.0000000000001759
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016)[published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi:10.1016/j. jaad.2020.08.097