User login
Which solid organ transplant recipients face the highest risk of skin cancer?
BOSTON – .
White patients who meet these criteria should be screening within 2 years after transplant, while Black patients should be screened within 5 years after transplant, Ally-Khan Somani, MD, PhD, said at the annual meeting of the American Academy of Dermatology.
Dr. Somani, director of dermatologic surgery and the division of cutaneous oncology at Indiana University, Indianapolis, based his remarks on consensus screening guidelines assembled from three rounds of Delphi method surveys with 47 dermatologists and 37 transplant physicians, with the goal of establishing skin cancer screening recommendations for SOTRs. Among the dermatologists surveyed, 45% were Mohs surgeons and 55% were general dermatologists.
The panel recommended that the transplant team should perform risk assessment for SOTRs to risk stratify patients for skin cancer screening (high risk vs. low risk). They also proposed that dermatologists perform skin cancer screening by full-body skin examinations, and that SOTRs with a history of skin cancer should continue with routine skin cancer surveillance as recommended by their dermatologists.
Those at low risk for skin cancer include abdominal organ recipients, SOTR age of younger than 50 at time of transplant, and female gender. The guidelines recommend that White, Asian, and Hispanic patients who meet those criteria should be screened within 5 years after transplant, while no consensus was reached for Black patients who meet those criteria.
Based on posttransplant skin cancer incidence rates, risk is increased among males, Whites, thoracic organ recipients, and being age 50 or older, Dr. Somani said. “At our institution, we make sure there’s a good connection between our transplant teams and dermatologists. We recommend rapid referral for suspicious lesions and we educate patients and screen them within 1 year of transplant, or sooner for high-risk patients. Surveillance is increased to every 3 or 4 months for patients with a history of multiple or high-risk cancers or sooner, followed by routine surveillance as recommended by the patient’s dermatologist.”
To risk stratify patients on the development of their first skin cancer post transplantation, researchers developed the Skin and Ultraviolet Neoplasia Transplant Risk Assessment Calculator (SUNTRAC), a prediction tool with a freely available app. Data for the tool were drawn from the Transplant Skin Cancer Network study, a 5-year analysis of 6,340 adult recipients of a first solid organ transplant at 26 transplant centers in the United States. It generates a risk score for SOTRs (low, medium, high, or very high), which informs transplant care providers of a patient’s risk of skin cancer.
Dr. Somani disclosed that he has received grants and funding from Castle Biosciences. He is an adviser to Cook Biotech and a consultant to Sanara MedTech.
BOSTON – .
White patients who meet these criteria should be screening within 2 years after transplant, while Black patients should be screened within 5 years after transplant, Ally-Khan Somani, MD, PhD, said at the annual meeting of the American Academy of Dermatology.
Dr. Somani, director of dermatologic surgery and the division of cutaneous oncology at Indiana University, Indianapolis, based his remarks on consensus screening guidelines assembled from three rounds of Delphi method surveys with 47 dermatologists and 37 transplant physicians, with the goal of establishing skin cancer screening recommendations for SOTRs. Among the dermatologists surveyed, 45% were Mohs surgeons and 55% were general dermatologists.
The panel recommended that the transplant team should perform risk assessment for SOTRs to risk stratify patients for skin cancer screening (high risk vs. low risk). They also proposed that dermatologists perform skin cancer screening by full-body skin examinations, and that SOTRs with a history of skin cancer should continue with routine skin cancer surveillance as recommended by their dermatologists.
Those at low risk for skin cancer include abdominal organ recipients, SOTR age of younger than 50 at time of transplant, and female gender. The guidelines recommend that White, Asian, and Hispanic patients who meet those criteria should be screened within 5 years after transplant, while no consensus was reached for Black patients who meet those criteria.
Based on posttransplant skin cancer incidence rates, risk is increased among males, Whites, thoracic organ recipients, and being age 50 or older, Dr. Somani said. “At our institution, we make sure there’s a good connection between our transplant teams and dermatologists. We recommend rapid referral for suspicious lesions and we educate patients and screen them within 1 year of transplant, or sooner for high-risk patients. Surveillance is increased to every 3 or 4 months for patients with a history of multiple or high-risk cancers or sooner, followed by routine surveillance as recommended by the patient’s dermatologist.”
To risk stratify patients on the development of their first skin cancer post transplantation, researchers developed the Skin and Ultraviolet Neoplasia Transplant Risk Assessment Calculator (SUNTRAC), a prediction tool with a freely available app. Data for the tool were drawn from the Transplant Skin Cancer Network study, a 5-year analysis of 6,340 adult recipients of a first solid organ transplant at 26 transplant centers in the United States. It generates a risk score for SOTRs (low, medium, high, or very high), which informs transplant care providers of a patient’s risk of skin cancer.
Dr. Somani disclosed that he has received grants and funding from Castle Biosciences. He is an adviser to Cook Biotech and a consultant to Sanara MedTech.
BOSTON – .
White patients who meet these criteria should be screening within 2 years after transplant, while Black patients should be screened within 5 years after transplant, Ally-Khan Somani, MD, PhD, said at the annual meeting of the American Academy of Dermatology.
Dr. Somani, director of dermatologic surgery and the division of cutaneous oncology at Indiana University, Indianapolis, based his remarks on consensus screening guidelines assembled from three rounds of Delphi method surveys with 47 dermatologists and 37 transplant physicians, with the goal of establishing skin cancer screening recommendations for SOTRs. Among the dermatologists surveyed, 45% were Mohs surgeons and 55% were general dermatologists.
The panel recommended that the transplant team should perform risk assessment for SOTRs to risk stratify patients for skin cancer screening (high risk vs. low risk). They also proposed that dermatologists perform skin cancer screening by full-body skin examinations, and that SOTRs with a history of skin cancer should continue with routine skin cancer surveillance as recommended by their dermatologists.
Those at low risk for skin cancer include abdominal organ recipients, SOTR age of younger than 50 at time of transplant, and female gender. The guidelines recommend that White, Asian, and Hispanic patients who meet those criteria should be screened within 5 years after transplant, while no consensus was reached for Black patients who meet those criteria.
Based on posttransplant skin cancer incidence rates, risk is increased among males, Whites, thoracic organ recipients, and being age 50 or older, Dr. Somani said. “At our institution, we make sure there’s a good connection between our transplant teams and dermatologists. We recommend rapid referral for suspicious lesions and we educate patients and screen them within 1 year of transplant, or sooner for high-risk patients. Surveillance is increased to every 3 or 4 months for patients with a history of multiple or high-risk cancers or sooner, followed by routine surveillance as recommended by the patient’s dermatologist.”
To risk stratify patients on the development of their first skin cancer post transplantation, researchers developed the Skin and Ultraviolet Neoplasia Transplant Risk Assessment Calculator (SUNTRAC), a prediction tool with a freely available app. Data for the tool were drawn from the Transplant Skin Cancer Network study, a 5-year analysis of 6,340 adult recipients of a first solid organ transplant at 26 transplant centers in the United States. It generates a risk score for SOTRs (low, medium, high, or very high), which informs transplant care providers of a patient’s risk of skin cancer.
Dr. Somani disclosed that he has received grants and funding from Castle Biosciences. He is an adviser to Cook Biotech and a consultant to Sanara MedTech.
AT AAD 22
Age, skin cancer risks for ICI-induced bullous pemphigoid identified
that may result in treatment interruption or cessation.
Investigators in Boston report that among patients receiving ICIs, being aged 70 years or older and having skin cancer are both significant risk factors for bullous pemphigoid. On the plus side, ICI-induced bullous pemphigoid also appears to be a marker for improved tumor responses to therapy.
In a nested case-control study of 5,636 patients with cancer who received either a programmed death 1 inhibitor such as pembrolizumab (Keytruda) or nivolumab (Opdivo) or a cytotoxic T-lymphocyte–associated protein 4 inhibitor such as ipilimumab (Yervoy), 35 patients (0.6%) developed bullous pemphigoid. The study by Nicole R. LeBoeuf, MD, MPH, from Brigham and Women’s Hospital in Boston and colleagues was published online in JAMA Dermatology.
“What is interesting is that 0.6 is a small number, but we’re seeing bullous pemphigoid at considerably higher frequency than is expected in the general population,” Dr. LeBoeuf said in an interview.
And although bullous pemphigoid has the potential to disrupt ICI therapy, it also appears to be a marker for a favorable tumor response, the investigators found.
Their findings suggest that management of bullous pemphigoid for patients receiving ICIs should focus on early identification and management with therapies directed at the specific toxicity, Dr. LeBoeuf said.
“When you make a specific diagnosis like bullous pemphigoid, then you can treat that specific disease with very targeted therapies, such as omalizumab or dupilumab or rituximab – things that are not globally immune suppressing like steroid or other T-cell–depleting agents. Studies have shown that depleting B cells with anti-CD20 agents is not detrimental to immune checkpoint inhibitor therapy,” she said.
Dermatologic AEs common
About 40% of patients with cancer treated with ICIs experience immune-related dermatologic adverse events (AEs) that can range from mild rashes and hair and nail changes to uncommon but life-threatening complications, such as Stevens-Johnson syndrome, a form of toxic epidermal necrolysis, according to members of a European Academy of Dermatology and Venereology task force.
“The desirable, immune-mediated oncologic response is often achieved at the cost of immune-related adverse events (irAEs) that may potentially affect any organ system,” they wrote in a position statement on the management of ICI-derived dermatologic adverse events.
Dr. LeBoeuf and colleagues note that, while reported risk factors for idiopathic bullous pemphigoid include advanced age, type 2 diabetes, use of dipeptidyl peptidase-4 inhibitors, cerebrovascular disease, and neurocognitive disease, risk factors for bullous pemphigoid and other adverse dermatologic events associated with ICIs are less well known.
Study details
To identify risk factors for bullous pemphigoid in patients receiving ICI, the investigators performed a case-control study nested within a retrospective cohort study.
They evaluated records for all patients in the three Harvard-affiliated hospitals to identify patients with ICI-associated bullous pemphigoid from October 2014 through December 2020. Control persons were all patients in the Dana-Farber cancer registry who received ICIs during the study period.
The investigators chose age at ICI initiation (69 years and younger or 70 years and older), sex, ICI agents, and cancer type as potential risk factors.
They used propensity score matching based on age, cancer type, ICI agent, and number of ICI cycles to match two control persons with each case patient.
Of the 5,636 patients treated with ICIs during the study period, 35 (0.6%) developed bullous pemphigoid. The median age was 72.8 years, and 71.4% were men.
In a multivariate logistic regression model that included 2,955 patients with complete data in the cancer registry, factors significantly associated with developing bullous pemphigoid included age 70 years or older (odds ratio, 2.32; P = .01), having melanoma (OR, 3.21; P < .001), and having nonmelanoma skin cancer (OR, 8.32; P < .001).
In comparing the 35 case patients with their matched control patients, a complete or partial response at first restaging imaging was significantly associated with developing bullous pemphigoid (OR, 3.37; P = .01). In addition, there was a higher likelihood of tumor responses to ICIs among patients with bullous pemphigoid, compared with matched control patients (objective response rate, 82.9% vs. 61.4%; P = .03).
Prudent toxicity management
Ryan Sullivan, MD, who treats patients with skin cancer at Massachusetts General Hospital Cancer Center, Boston, but was not involved in the study, commented that the findings raise questions about the relationship between skin cancers and immune-related adverse events.
“It is compelling that bullous pemphigoid is a skin toxicity and is more common to happen in skin cancer patients,” he noted. “That’s a very interesting finding, and the reason that it’s interesting is that it’s harder to understand why a presumably antibody-mediated side effect would be more likely to have that cross-reactivity where the tumor started and where the toxicity happened,” he said in an interview.
He noted that the benefits of ICIs for patients with skin cancers far outweigh the risks of dermatologic adverse events such as bullous pemphigoid and that ICI-associated events require judicious management.
“This is true across the spectrum of toxicities: There are clear manifestations of toxicity that we should be more thoughtful about what’s driving them, more thoughtful about what it is, and more thoughtful about treating them, other than just pouring steroids into patients in industrial doses and hoping that everything’s going to be OK,” he said.
No funding source for the study was reported. Dr. LeBoeuf reported receiving grants from the National Institutes of Health National Cancer Institute during the conduct of the study and personal fees for serving as a consultant for several companies outside the study. Coauthor Arash Mostaghimi, MD, MPA, MPH, is associate editor of JAMA Dermatology but was not involved in study selection or evaluation for publication. Dr. Sullivan disclosed consulting for ICI makers Bristol-Myers Squibb and Merck.
A version of this article first appeared on Medscape.com.
that may result in treatment interruption or cessation.
Investigators in Boston report that among patients receiving ICIs, being aged 70 years or older and having skin cancer are both significant risk factors for bullous pemphigoid. On the plus side, ICI-induced bullous pemphigoid also appears to be a marker for improved tumor responses to therapy.
In a nested case-control study of 5,636 patients with cancer who received either a programmed death 1 inhibitor such as pembrolizumab (Keytruda) or nivolumab (Opdivo) or a cytotoxic T-lymphocyte–associated protein 4 inhibitor such as ipilimumab (Yervoy), 35 patients (0.6%) developed bullous pemphigoid. The study by Nicole R. LeBoeuf, MD, MPH, from Brigham and Women’s Hospital in Boston and colleagues was published online in JAMA Dermatology.
“What is interesting is that 0.6 is a small number, but we’re seeing bullous pemphigoid at considerably higher frequency than is expected in the general population,” Dr. LeBoeuf said in an interview.
And although bullous pemphigoid has the potential to disrupt ICI therapy, it also appears to be a marker for a favorable tumor response, the investigators found.
Their findings suggest that management of bullous pemphigoid for patients receiving ICIs should focus on early identification and management with therapies directed at the specific toxicity, Dr. LeBoeuf said.
“When you make a specific diagnosis like bullous pemphigoid, then you can treat that specific disease with very targeted therapies, such as omalizumab or dupilumab or rituximab – things that are not globally immune suppressing like steroid or other T-cell–depleting agents. Studies have shown that depleting B cells with anti-CD20 agents is not detrimental to immune checkpoint inhibitor therapy,” she said.
Dermatologic AEs common
About 40% of patients with cancer treated with ICIs experience immune-related dermatologic adverse events (AEs) that can range from mild rashes and hair and nail changes to uncommon but life-threatening complications, such as Stevens-Johnson syndrome, a form of toxic epidermal necrolysis, according to members of a European Academy of Dermatology and Venereology task force.
“The desirable, immune-mediated oncologic response is often achieved at the cost of immune-related adverse events (irAEs) that may potentially affect any organ system,” they wrote in a position statement on the management of ICI-derived dermatologic adverse events.
Dr. LeBoeuf and colleagues note that, while reported risk factors for idiopathic bullous pemphigoid include advanced age, type 2 diabetes, use of dipeptidyl peptidase-4 inhibitors, cerebrovascular disease, and neurocognitive disease, risk factors for bullous pemphigoid and other adverse dermatologic events associated with ICIs are less well known.
Study details
To identify risk factors for bullous pemphigoid in patients receiving ICI, the investigators performed a case-control study nested within a retrospective cohort study.
They evaluated records for all patients in the three Harvard-affiliated hospitals to identify patients with ICI-associated bullous pemphigoid from October 2014 through December 2020. Control persons were all patients in the Dana-Farber cancer registry who received ICIs during the study period.
The investigators chose age at ICI initiation (69 years and younger or 70 years and older), sex, ICI agents, and cancer type as potential risk factors.
They used propensity score matching based on age, cancer type, ICI agent, and number of ICI cycles to match two control persons with each case patient.
Of the 5,636 patients treated with ICIs during the study period, 35 (0.6%) developed bullous pemphigoid. The median age was 72.8 years, and 71.4% were men.
In a multivariate logistic regression model that included 2,955 patients with complete data in the cancer registry, factors significantly associated with developing bullous pemphigoid included age 70 years or older (odds ratio, 2.32; P = .01), having melanoma (OR, 3.21; P < .001), and having nonmelanoma skin cancer (OR, 8.32; P < .001).
In comparing the 35 case patients with their matched control patients, a complete or partial response at first restaging imaging was significantly associated with developing bullous pemphigoid (OR, 3.37; P = .01). In addition, there was a higher likelihood of tumor responses to ICIs among patients with bullous pemphigoid, compared with matched control patients (objective response rate, 82.9% vs. 61.4%; P = .03).
Prudent toxicity management
Ryan Sullivan, MD, who treats patients with skin cancer at Massachusetts General Hospital Cancer Center, Boston, but was not involved in the study, commented that the findings raise questions about the relationship between skin cancers and immune-related adverse events.
“It is compelling that bullous pemphigoid is a skin toxicity and is more common to happen in skin cancer patients,” he noted. “That’s a very interesting finding, and the reason that it’s interesting is that it’s harder to understand why a presumably antibody-mediated side effect would be more likely to have that cross-reactivity where the tumor started and where the toxicity happened,” he said in an interview.
He noted that the benefits of ICIs for patients with skin cancers far outweigh the risks of dermatologic adverse events such as bullous pemphigoid and that ICI-associated events require judicious management.
“This is true across the spectrum of toxicities: There are clear manifestations of toxicity that we should be more thoughtful about what’s driving them, more thoughtful about what it is, and more thoughtful about treating them, other than just pouring steroids into patients in industrial doses and hoping that everything’s going to be OK,” he said.
No funding source for the study was reported. Dr. LeBoeuf reported receiving grants from the National Institutes of Health National Cancer Institute during the conduct of the study and personal fees for serving as a consultant for several companies outside the study. Coauthor Arash Mostaghimi, MD, MPA, MPH, is associate editor of JAMA Dermatology but was not involved in study selection or evaluation for publication. Dr. Sullivan disclosed consulting for ICI makers Bristol-Myers Squibb and Merck.
A version of this article first appeared on Medscape.com.
that may result in treatment interruption or cessation.
Investigators in Boston report that among patients receiving ICIs, being aged 70 years or older and having skin cancer are both significant risk factors for bullous pemphigoid. On the plus side, ICI-induced bullous pemphigoid also appears to be a marker for improved tumor responses to therapy.
In a nested case-control study of 5,636 patients with cancer who received either a programmed death 1 inhibitor such as pembrolizumab (Keytruda) or nivolumab (Opdivo) or a cytotoxic T-lymphocyte–associated protein 4 inhibitor such as ipilimumab (Yervoy), 35 patients (0.6%) developed bullous pemphigoid. The study by Nicole R. LeBoeuf, MD, MPH, from Brigham and Women’s Hospital in Boston and colleagues was published online in JAMA Dermatology.
“What is interesting is that 0.6 is a small number, but we’re seeing bullous pemphigoid at considerably higher frequency than is expected in the general population,” Dr. LeBoeuf said in an interview.
And although bullous pemphigoid has the potential to disrupt ICI therapy, it also appears to be a marker for a favorable tumor response, the investigators found.
Their findings suggest that management of bullous pemphigoid for patients receiving ICIs should focus on early identification and management with therapies directed at the specific toxicity, Dr. LeBoeuf said.
“When you make a specific diagnosis like bullous pemphigoid, then you can treat that specific disease with very targeted therapies, such as omalizumab or dupilumab or rituximab – things that are not globally immune suppressing like steroid or other T-cell–depleting agents. Studies have shown that depleting B cells with anti-CD20 agents is not detrimental to immune checkpoint inhibitor therapy,” she said.
Dermatologic AEs common
About 40% of patients with cancer treated with ICIs experience immune-related dermatologic adverse events (AEs) that can range from mild rashes and hair and nail changes to uncommon but life-threatening complications, such as Stevens-Johnson syndrome, a form of toxic epidermal necrolysis, according to members of a European Academy of Dermatology and Venereology task force.
“The desirable, immune-mediated oncologic response is often achieved at the cost of immune-related adverse events (irAEs) that may potentially affect any organ system,” they wrote in a position statement on the management of ICI-derived dermatologic adverse events.
Dr. LeBoeuf and colleagues note that, while reported risk factors for idiopathic bullous pemphigoid include advanced age, type 2 diabetes, use of dipeptidyl peptidase-4 inhibitors, cerebrovascular disease, and neurocognitive disease, risk factors for bullous pemphigoid and other adverse dermatologic events associated with ICIs are less well known.
Study details
To identify risk factors for bullous pemphigoid in patients receiving ICI, the investigators performed a case-control study nested within a retrospective cohort study.
They evaluated records for all patients in the three Harvard-affiliated hospitals to identify patients with ICI-associated bullous pemphigoid from October 2014 through December 2020. Control persons were all patients in the Dana-Farber cancer registry who received ICIs during the study period.
The investigators chose age at ICI initiation (69 years and younger or 70 years and older), sex, ICI agents, and cancer type as potential risk factors.
They used propensity score matching based on age, cancer type, ICI agent, and number of ICI cycles to match two control persons with each case patient.
Of the 5,636 patients treated with ICIs during the study period, 35 (0.6%) developed bullous pemphigoid. The median age was 72.8 years, and 71.4% were men.
In a multivariate logistic regression model that included 2,955 patients with complete data in the cancer registry, factors significantly associated with developing bullous pemphigoid included age 70 years or older (odds ratio, 2.32; P = .01), having melanoma (OR, 3.21; P < .001), and having nonmelanoma skin cancer (OR, 8.32; P < .001).
In comparing the 35 case patients with their matched control patients, a complete or partial response at first restaging imaging was significantly associated with developing bullous pemphigoid (OR, 3.37; P = .01). In addition, there was a higher likelihood of tumor responses to ICIs among patients with bullous pemphigoid, compared with matched control patients (objective response rate, 82.9% vs. 61.4%; P = .03).
Prudent toxicity management
Ryan Sullivan, MD, who treats patients with skin cancer at Massachusetts General Hospital Cancer Center, Boston, but was not involved in the study, commented that the findings raise questions about the relationship between skin cancers and immune-related adverse events.
“It is compelling that bullous pemphigoid is a skin toxicity and is more common to happen in skin cancer patients,” he noted. “That’s a very interesting finding, and the reason that it’s interesting is that it’s harder to understand why a presumably antibody-mediated side effect would be more likely to have that cross-reactivity where the tumor started and where the toxicity happened,” he said in an interview.
He noted that the benefits of ICIs for patients with skin cancers far outweigh the risks of dermatologic adverse events such as bullous pemphigoid and that ICI-associated events require judicious management.
“This is true across the spectrum of toxicities: There are clear manifestations of toxicity that we should be more thoughtful about what’s driving them, more thoughtful about what it is, and more thoughtful about treating them, other than just pouring steroids into patients in industrial doses and hoping that everything’s going to be OK,” he said.
No funding source for the study was reported. Dr. LeBoeuf reported receiving grants from the National Institutes of Health National Cancer Institute during the conduct of the study and personal fees for serving as a consultant for several companies outside the study. Coauthor Arash Mostaghimi, MD, MPA, MPH, is associate editor of JAMA Dermatology but was not involved in study selection or evaluation for publication. Dr. Sullivan disclosed consulting for ICI makers Bristol-Myers Squibb and Merck.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Made-to-order TILs effective against metastatic melanoma
NEW ORLEANS – In just over one-third of patients with metastatic melanoma who had experienced disease progression while receiving multiple prior lines of therapy, including immunotherapy and targeted agents,
The product, called lifileucel, is custom made for each patient and utilizes tumor-infiltrating lymphocytes (TILs) extracted from tumor lesions. This approach differs from other cell-based therapies, such as chimeric antigen receptor (CAR) T cell therapy, which utilizes T cells collected from the patient’s blood.
The new results come from a phase 2 trial conducted in 66 patients with previously treated unresectable or metastatic melanoma who received a single dose of the product. The objective response rate was 36.4%.
“Lifileucel has demonstrated efficacy and durability of response for patients with metastatic melanoma and represents a viable therapeutic option warranting further investigation,” commented Jason Alan Chesney, MD, PhD, from the James Graham Brown Cancer Center, the University of Louisville (Ky.).
He presented the new data at the virtual American Association for Cancer Research (AACR) Annual Meeting 2021.
Customized cell therapy with TILs has been explored for the treatment of melanoma for more than a decade. Some researchers have reported durable response in 25% of patients.
However, “generalizing TIL therapy has been hampered by the complex and really not absolutely defined process for generating cells,” commented Philip Greenberg, MD, professor and head of the program in immunology in the Clinical Research Division of the Fred Hutchinson Cancer Center, Seattle, who was the invited discussant.
The current study demonstrates that cell generation can be performed at a centralized facility that has the required technical expertise. The patient-specific products are then disseminated to multiple centers, he said. The study also demonstrates that TILs can be successfully generated from tumor sites other than skin or lymph nodes.
“Toxicity was, however, significant, although it was generally manageable, and it did occur early, generally within the first 2 weeks,” he noted.
Patient-derived product
Lifileucel is a tailor-made immunotherapy product created from melanoma tumor tissues resected from lesions in skin, lymph nodes, liver, lung, peritoneum, musculoskeletal system, breast, or other visceral organs. The cells are shipped to a central manufacturing facility, whre the TILs are isolated, cultured, expanded, and reinvigorated. The cells are then harvested and cryopreserved. The process takes about 22 days. The cryopreserved product is then shipped back to the treating facility.
Prior to receiving the expanded and rejuvenated TILs, patients undergo myeloablative conditioning with cyclophosphamide followed by fludarabine. The TILs are then delivered in a single infusion, followed by administration of up to six doses of interleukin-2 (IL-2).
Details from clinical trial
At the meeting, Dr. Chesney reported details on the 66 patients in the trial. They had metastatic melanoma that was progressing on treatment. The mean number of prior lines of therapy was 3.3. All of the patients had received prior anti–programmed cell death protein–1 (PD-1) or programmed cell death–ligand-1 (PD-L1) agents; 53 had received a cytotoxic T lymphocyte protein 4 (CTLA-4) inhibitor; and 15 had received a BRAF/MEK inhibitor.
These patients had a mean of six baseline target and nontarget lesions, and 28 patients had liver and/or brain metastases.
Just over a third of patients (24 of 66, 36.4%) had an objective response; three patients had a complete response; and 21 had a partial response. In addition, 29 patients had stable disease, and nine experienced disease progression. Four patients had not undergone the first assessment at the time of data cutoff.
After a median follow-up of 28.1 months, the median duration of response was not reached. It ranged from 2.2 to > 35.2 months.
Since the data cutoff in April 2020, reduction of tumor burden has occurred in 50 of 62 evaluable patients. Reductions in the target lesion sum of diameters has occurred in 11 patients. In one patient, a partial response converted to a complete response 24 months after infusion, Dr. Chesney noted.
The mean number of TILs infused was 27.3 billion (27.3 x 109). Appropriate amounts of TILs were manufactured from tumor samples acquired across all sites, and reductions in target lesion sum of diameter were seen across the range of TIL total cell doses.
All patients experienced at least one adverse event of any grade; all but two experienced grade 3 or 4 adverse events. Two patients died, one as a result of intra-abdominal hemorrhage considered possibly related to TIL therapy, and one from acute respiratory failure deemed not related to TILs.
The most common grade 3 or 4 adverse events were thrombocytopenia, anemia, febrile neutropenia, neutropenia, hypophosphatemia, and lymphopenia.
“The adverse event profile was manageable and was consistent with the underlying and the known profiles of the nonmyeloblative depletion regimen and IL-2,” Dr. Chesney said.
The decreasing frequency of adverse events over time reflects the potential benefit of the one-time infusion, and no new safety risks have been identified during more than 2 years of follow-up, he added.
Remaining questions, next steps
Dr. Greenberg commented that the one of the limitations of the study is that the investigators did not characterize the TIL product.
“Studies have predicted that there’s a particular type of cell, a stemlike T cell, that’s responsible for mediating the efficacy,” he commented. He referred to research from Steven Rosenberg, MD, PhD, and colleagues at the National Cancer Institute, where TILs were first used in 2002.
Dr. Greenberg also raised the question of whether high-dose IL-2 was required post infusion, given that the patients were lymphodepleted before receiving lifileucel.
Future steps for TIL therapy, he said, should include identification of biomarkers for success or failure; strategies to enhance generation and expansion of tumor-reactive T cells; postinfusion strategies, such as using vaccines and/or checkpoint inhibitors to increase therapeutic activity; genetic modifications to enhance the function of TILs in the tumor microenvironment; and research into other tumor types that may be effectively treated with TILs.
The study was supported by Iovance Biotherapeutics. Dr. Chesney has received research funding from Iovance and other companies and has consulted for Amgen and Replimune. Dr. Greenberg has served on scientific advisory boards, has received grant/research support, and owns stock in several companies that do not include Iovance.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – In just over one-third of patients with metastatic melanoma who had experienced disease progression while receiving multiple prior lines of therapy, including immunotherapy and targeted agents,
The product, called lifileucel, is custom made for each patient and utilizes tumor-infiltrating lymphocytes (TILs) extracted from tumor lesions. This approach differs from other cell-based therapies, such as chimeric antigen receptor (CAR) T cell therapy, which utilizes T cells collected from the patient’s blood.
The new results come from a phase 2 trial conducted in 66 patients with previously treated unresectable or metastatic melanoma who received a single dose of the product. The objective response rate was 36.4%.
“Lifileucel has demonstrated efficacy and durability of response for patients with metastatic melanoma and represents a viable therapeutic option warranting further investigation,” commented Jason Alan Chesney, MD, PhD, from the James Graham Brown Cancer Center, the University of Louisville (Ky.).
He presented the new data at the virtual American Association for Cancer Research (AACR) Annual Meeting 2021.
Customized cell therapy with TILs has been explored for the treatment of melanoma for more than a decade. Some researchers have reported durable response in 25% of patients.
However, “generalizing TIL therapy has been hampered by the complex and really not absolutely defined process for generating cells,” commented Philip Greenberg, MD, professor and head of the program in immunology in the Clinical Research Division of the Fred Hutchinson Cancer Center, Seattle, who was the invited discussant.
The current study demonstrates that cell generation can be performed at a centralized facility that has the required technical expertise. The patient-specific products are then disseminated to multiple centers, he said. The study also demonstrates that TILs can be successfully generated from tumor sites other than skin or lymph nodes.
“Toxicity was, however, significant, although it was generally manageable, and it did occur early, generally within the first 2 weeks,” he noted.
Patient-derived product
Lifileucel is a tailor-made immunotherapy product created from melanoma tumor tissues resected from lesions in skin, lymph nodes, liver, lung, peritoneum, musculoskeletal system, breast, or other visceral organs. The cells are shipped to a central manufacturing facility, whre the TILs are isolated, cultured, expanded, and reinvigorated. The cells are then harvested and cryopreserved. The process takes about 22 days. The cryopreserved product is then shipped back to the treating facility.
Prior to receiving the expanded and rejuvenated TILs, patients undergo myeloablative conditioning with cyclophosphamide followed by fludarabine. The TILs are then delivered in a single infusion, followed by administration of up to six doses of interleukin-2 (IL-2).
Details from clinical trial
At the meeting, Dr. Chesney reported details on the 66 patients in the trial. They had metastatic melanoma that was progressing on treatment. The mean number of prior lines of therapy was 3.3. All of the patients had received prior anti–programmed cell death protein–1 (PD-1) or programmed cell death–ligand-1 (PD-L1) agents; 53 had received a cytotoxic T lymphocyte protein 4 (CTLA-4) inhibitor; and 15 had received a BRAF/MEK inhibitor.
These patients had a mean of six baseline target and nontarget lesions, and 28 patients had liver and/or brain metastases.
Just over a third of patients (24 of 66, 36.4%) had an objective response; three patients had a complete response; and 21 had a partial response. In addition, 29 patients had stable disease, and nine experienced disease progression. Four patients had not undergone the first assessment at the time of data cutoff.
After a median follow-up of 28.1 months, the median duration of response was not reached. It ranged from 2.2 to > 35.2 months.
Since the data cutoff in April 2020, reduction of tumor burden has occurred in 50 of 62 evaluable patients. Reductions in the target lesion sum of diameters has occurred in 11 patients. In one patient, a partial response converted to a complete response 24 months after infusion, Dr. Chesney noted.
The mean number of TILs infused was 27.3 billion (27.3 x 109). Appropriate amounts of TILs were manufactured from tumor samples acquired across all sites, and reductions in target lesion sum of diameter were seen across the range of TIL total cell doses.
All patients experienced at least one adverse event of any grade; all but two experienced grade 3 or 4 adverse events. Two patients died, one as a result of intra-abdominal hemorrhage considered possibly related to TIL therapy, and one from acute respiratory failure deemed not related to TILs.
The most common grade 3 or 4 adverse events were thrombocytopenia, anemia, febrile neutropenia, neutropenia, hypophosphatemia, and lymphopenia.
“The adverse event profile was manageable and was consistent with the underlying and the known profiles of the nonmyeloblative depletion regimen and IL-2,” Dr. Chesney said.
The decreasing frequency of adverse events over time reflects the potential benefit of the one-time infusion, and no new safety risks have been identified during more than 2 years of follow-up, he added.
Remaining questions, next steps
Dr. Greenberg commented that the one of the limitations of the study is that the investigators did not characterize the TIL product.
“Studies have predicted that there’s a particular type of cell, a stemlike T cell, that’s responsible for mediating the efficacy,” he commented. He referred to research from Steven Rosenberg, MD, PhD, and colleagues at the National Cancer Institute, where TILs were first used in 2002.
Dr. Greenberg also raised the question of whether high-dose IL-2 was required post infusion, given that the patients were lymphodepleted before receiving lifileucel.
Future steps for TIL therapy, he said, should include identification of biomarkers for success or failure; strategies to enhance generation and expansion of tumor-reactive T cells; postinfusion strategies, such as using vaccines and/or checkpoint inhibitors to increase therapeutic activity; genetic modifications to enhance the function of TILs in the tumor microenvironment; and research into other tumor types that may be effectively treated with TILs.
The study was supported by Iovance Biotherapeutics. Dr. Chesney has received research funding from Iovance and other companies and has consulted for Amgen and Replimune. Dr. Greenberg has served on scientific advisory boards, has received grant/research support, and owns stock in several companies that do not include Iovance.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – In just over one-third of patients with metastatic melanoma who had experienced disease progression while receiving multiple prior lines of therapy, including immunotherapy and targeted agents,
The product, called lifileucel, is custom made for each patient and utilizes tumor-infiltrating lymphocytes (TILs) extracted from tumor lesions. This approach differs from other cell-based therapies, such as chimeric antigen receptor (CAR) T cell therapy, which utilizes T cells collected from the patient’s blood.
The new results come from a phase 2 trial conducted in 66 patients with previously treated unresectable or metastatic melanoma who received a single dose of the product. The objective response rate was 36.4%.
“Lifileucel has demonstrated efficacy and durability of response for patients with metastatic melanoma and represents a viable therapeutic option warranting further investigation,” commented Jason Alan Chesney, MD, PhD, from the James Graham Brown Cancer Center, the University of Louisville (Ky.).
He presented the new data at the virtual American Association for Cancer Research (AACR) Annual Meeting 2021.
Customized cell therapy with TILs has been explored for the treatment of melanoma for more than a decade. Some researchers have reported durable response in 25% of patients.
However, “generalizing TIL therapy has been hampered by the complex and really not absolutely defined process for generating cells,” commented Philip Greenberg, MD, professor and head of the program in immunology in the Clinical Research Division of the Fred Hutchinson Cancer Center, Seattle, who was the invited discussant.
The current study demonstrates that cell generation can be performed at a centralized facility that has the required technical expertise. The patient-specific products are then disseminated to multiple centers, he said. The study also demonstrates that TILs can be successfully generated from tumor sites other than skin or lymph nodes.
“Toxicity was, however, significant, although it was generally manageable, and it did occur early, generally within the first 2 weeks,” he noted.
Patient-derived product
Lifileucel is a tailor-made immunotherapy product created from melanoma tumor tissues resected from lesions in skin, lymph nodes, liver, lung, peritoneum, musculoskeletal system, breast, or other visceral organs. The cells are shipped to a central manufacturing facility, whre the TILs are isolated, cultured, expanded, and reinvigorated. The cells are then harvested and cryopreserved. The process takes about 22 days. The cryopreserved product is then shipped back to the treating facility.
Prior to receiving the expanded and rejuvenated TILs, patients undergo myeloablative conditioning with cyclophosphamide followed by fludarabine. The TILs are then delivered in a single infusion, followed by administration of up to six doses of interleukin-2 (IL-2).
Details from clinical trial
At the meeting, Dr. Chesney reported details on the 66 patients in the trial. They had metastatic melanoma that was progressing on treatment. The mean number of prior lines of therapy was 3.3. All of the patients had received prior anti–programmed cell death protein–1 (PD-1) or programmed cell death–ligand-1 (PD-L1) agents; 53 had received a cytotoxic T lymphocyte protein 4 (CTLA-4) inhibitor; and 15 had received a BRAF/MEK inhibitor.
These patients had a mean of six baseline target and nontarget lesions, and 28 patients had liver and/or brain metastases.
Just over a third of patients (24 of 66, 36.4%) had an objective response; three patients had a complete response; and 21 had a partial response. In addition, 29 patients had stable disease, and nine experienced disease progression. Four patients had not undergone the first assessment at the time of data cutoff.
After a median follow-up of 28.1 months, the median duration of response was not reached. It ranged from 2.2 to > 35.2 months.
Since the data cutoff in April 2020, reduction of tumor burden has occurred in 50 of 62 evaluable patients. Reductions in the target lesion sum of diameters has occurred in 11 patients. In one patient, a partial response converted to a complete response 24 months after infusion, Dr. Chesney noted.
The mean number of TILs infused was 27.3 billion (27.3 x 109). Appropriate amounts of TILs were manufactured from tumor samples acquired across all sites, and reductions in target lesion sum of diameter were seen across the range of TIL total cell doses.
All patients experienced at least one adverse event of any grade; all but two experienced grade 3 or 4 adverse events. Two patients died, one as a result of intra-abdominal hemorrhage considered possibly related to TIL therapy, and one from acute respiratory failure deemed not related to TILs.
The most common grade 3 or 4 adverse events were thrombocytopenia, anemia, febrile neutropenia, neutropenia, hypophosphatemia, and lymphopenia.
“The adverse event profile was manageable and was consistent with the underlying and the known profiles of the nonmyeloblative depletion regimen and IL-2,” Dr. Chesney said.
The decreasing frequency of adverse events over time reflects the potential benefit of the one-time infusion, and no new safety risks have been identified during more than 2 years of follow-up, he added.
Remaining questions, next steps
Dr. Greenberg commented that the one of the limitations of the study is that the investigators did not characterize the TIL product.
“Studies have predicted that there’s a particular type of cell, a stemlike T cell, that’s responsible for mediating the efficacy,” he commented. He referred to research from Steven Rosenberg, MD, PhD, and colleagues at the National Cancer Institute, where TILs were first used in 2002.
Dr. Greenberg also raised the question of whether high-dose IL-2 was required post infusion, given that the patients were lymphodepleted before receiving lifileucel.
Future steps for TIL therapy, he said, should include identification of biomarkers for success or failure; strategies to enhance generation and expansion of tumor-reactive T cells; postinfusion strategies, such as using vaccines and/or checkpoint inhibitors to increase therapeutic activity; genetic modifications to enhance the function of TILs in the tumor microenvironment; and research into other tumor types that may be effectively treated with TILs.
The study was supported by Iovance Biotherapeutics. Dr. Chesney has received research funding from Iovance and other companies and has consulted for Amgen and Replimune. Dr. Greenberg has served on scientific advisory boards, has received grant/research support, and owns stock in several companies that do not include Iovance.
A version of this article first appeared on Medscape.com.
AT AACR 2022
Melanoma screening study stokes overdiagnosis debate
new research shows.
Without a corresponding decrease in melanoma mortality, an increase in the detection of those thin melanomas “raises the concern that early detection efforts, such as visual skin screening, may result in overdiagnosis,” the study authors wrote. “The value of a cancer screening program should most rigorously be measured not by the number of new, early cancers detected, but by its impact on the development of late-stage disease and its associated morbidity, cost, and mortality.”
The research, published in JAMA Dermatology, has reignited the controversy over the benefits and harms of primary care skin cancer screening, garnering two editorials that reflect different sides of the debate.
In one, Robert A. Swerlick, MD, pointed out that, “despite public messaging to the contrary, to my knowledge there is no evidence that routine skin examinations have any effect on melanoma mortality.
“The stage shift to smaller tumors should not be viewed as success and is very strong evidence of overdiagnosis,” wrote Dr. Swerlick, of the department of dermatology, Emory University, Atlanta.
The other editorial, however, argued that routine screening saves lives. “Most melanoma deaths are because of stage I disease, with an estimated 3%-15% of thin melanomas (≤ 1 mm) being lethal,” wrote a trio of editorialists from Oregon Health & Science University, Portland.
When considering the high mutation rate associated with melanoma and the current limits of treatment options, early diagnosis becomes “particularly important and counterbalances the risk of overdiagnosis,” the editorialists asserted.
Primary care screening study
The new findings come from an observational study of a quality improvement initiative conducted at the University of Pittsburgh Medical Center system between 2014 and 2018, in which primary care clinicians were offered training in melanoma identification through skin examination and were encouraged to offer annual skin cancer screening to patients aged 35 years and older.
Of 595,799 eligible patients, 144,851 (24.3%) were screened at least once during the study period. Those who received screening were more likely than unscreened patients to be older (median age, 59 vs. 55 years), women, and non-Hispanic White persons.
During a follow-up of 5 years, the researchers found that patients who received screening were significantly more likely than unscreened patients to be diagnosed with in situ melanoma (incidence, 30.4 vs. 14.4; hazard ratio, 2.6; P < .001) or thin invasive melanoma (incidence, 24.5 vs. 16.1; HR, 1.8; P < .001), after adjusting for factors that included age, sex, and race.
The screened patients were also more likely than unscreened patients to be diagnosed with in situ interval melanomas, defined as melanomas occurring at least 60 days after initial screening (incidence, 26.7 vs. 12.9; HR, 2.1; P < .001), as well as thin invasive interval melanomas (incidence, 18.5 vs. 14.4; HR, 1.3; P = .03).
The 60-day interval was included to account for the possible time to referral to a specialist for definitive diagnosis, the authors explained.
The incidence of the detection of melanomas thicker than 4 mm was lower in screened versus unscreened patients, but the difference was not statistically significant for all melanomas (2.7 vs. 3.3; HR, 0.8; P = .38) or interval melanomas (1.5 vs. 2.7; HR, 0.6; P = .15).
Experts weigh in
Although the follow-up period was of 5 years, not all patients were followed that long after undergoing screening. For instance, for some patients, follow-up occurred only 1 year after they had been screened.
The study’s senior author, Laura K. Ferris, MD, PhD, of the department of dermatology, University of Pittsburgh, noted that a longer follow-up could shift the results.
“When you look at the curves in our figures, you do start to see them separate more and more over time for the thicker melanomas,” Dr. Ferris said in an interview. “I do suspect that, if we followed patients longer, we might start to see a more significant difference.”
The findings nevertheless add to evidence that although routine screening substantially increases the detection of melanomas overall, these melanomas are often not the ones doctors are most worried about or that increase a person’s risk of mortality, Dr. Ferris noted.
When it comes to melanoma screening, balancing the risks and benefits is key. One major downside, Dr. Ferris said, is in regard to the burden such screening could place on the health care system, with potentially unproductive screenings causing delays in care for patients with more urgent needs.
“We are undersupplied in the dermatology workforce, and there is often a long wait to see dermatologists, so we really want to make sure, as trained professionals, that patients have access to us,” she said. “If we’re doing something that doesn’t have proven benefit and is increasing the wait time, that will come at the expense of other patients’ access.”
Costs involved in skin biopsies and excisions of borderline lesions as well as the potential to increase patients’ anxiety represent other important considerations, Dr. Ferris noted.
However, Sancy A. Leachman, MD, PhD, a coauthor of the editorial in favor of screening, said in an interview that “at the individual level, there are an almost infinite number of individual circumstances that could lead a person to decide that the potential benefits outweigh the harms.”
According to Dr. Leachman, who is chair of the department of dermatology, Oregon Health & Science University, these individual priorities may not align with those of the various decision-makers or with guidelines, such as those from the U.S. Preventive Services Task Force, which gives visual skin cancer screening of asymptomatic patients an “I” rating, indicating “insufficient evidence.”
“Many federal agencies and payer groups focus on minimizing costs and optimizing outcomes,” Dr. Leachman and coauthors wrote. As the only professional advocates for individual patients, physicians “have a responsibility to assure that the best interests of patients are served.”
The study was funded by the University of Pittsburgh Melanoma and Skin Cancer Program. Dr. Ferris and Dr. Swerlick disclosed no relevant financial relationships. Dr. Leachman is the principal investigator for War on Melanoma, an early-detection program in Oregon.
A version of this article first appeared on Medscape.com.
new research shows.
Without a corresponding decrease in melanoma mortality, an increase in the detection of those thin melanomas “raises the concern that early detection efforts, such as visual skin screening, may result in overdiagnosis,” the study authors wrote. “The value of a cancer screening program should most rigorously be measured not by the number of new, early cancers detected, but by its impact on the development of late-stage disease and its associated morbidity, cost, and mortality.”
The research, published in JAMA Dermatology, has reignited the controversy over the benefits and harms of primary care skin cancer screening, garnering two editorials that reflect different sides of the debate.
In one, Robert A. Swerlick, MD, pointed out that, “despite public messaging to the contrary, to my knowledge there is no evidence that routine skin examinations have any effect on melanoma mortality.
“The stage shift to smaller tumors should not be viewed as success and is very strong evidence of overdiagnosis,” wrote Dr. Swerlick, of the department of dermatology, Emory University, Atlanta.
The other editorial, however, argued that routine screening saves lives. “Most melanoma deaths are because of stage I disease, with an estimated 3%-15% of thin melanomas (≤ 1 mm) being lethal,” wrote a trio of editorialists from Oregon Health & Science University, Portland.
When considering the high mutation rate associated with melanoma and the current limits of treatment options, early diagnosis becomes “particularly important and counterbalances the risk of overdiagnosis,” the editorialists asserted.
Primary care screening study
The new findings come from an observational study of a quality improvement initiative conducted at the University of Pittsburgh Medical Center system between 2014 and 2018, in which primary care clinicians were offered training in melanoma identification through skin examination and were encouraged to offer annual skin cancer screening to patients aged 35 years and older.
Of 595,799 eligible patients, 144,851 (24.3%) were screened at least once during the study period. Those who received screening were more likely than unscreened patients to be older (median age, 59 vs. 55 years), women, and non-Hispanic White persons.
During a follow-up of 5 years, the researchers found that patients who received screening were significantly more likely than unscreened patients to be diagnosed with in situ melanoma (incidence, 30.4 vs. 14.4; hazard ratio, 2.6; P < .001) or thin invasive melanoma (incidence, 24.5 vs. 16.1; HR, 1.8; P < .001), after adjusting for factors that included age, sex, and race.
The screened patients were also more likely than unscreened patients to be diagnosed with in situ interval melanomas, defined as melanomas occurring at least 60 days after initial screening (incidence, 26.7 vs. 12.9; HR, 2.1; P < .001), as well as thin invasive interval melanomas (incidence, 18.5 vs. 14.4; HR, 1.3; P = .03).
The 60-day interval was included to account for the possible time to referral to a specialist for definitive diagnosis, the authors explained.
The incidence of the detection of melanomas thicker than 4 mm was lower in screened versus unscreened patients, but the difference was not statistically significant for all melanomas (2.7 vs. 3.3; HR, 0.8; P = .38) or interval melanomas (1.5 vs. 2.7; HR, 0.6; P = .15).
Experts weigh in
Although the follow-up period was of 5 years, not all patients were followed that long after undergoing screening. For instance, for some patients, follow-up occurred only 1 year after they had been screened.
The study’s senior author, Laura K. Ferris, MD, PhD, of the department of dermatology, University of Pittsburgh, noted that a longer follow-up could shift the results.
“When you look at the curves in our figures, you do start to see them separate more and more over time for the thicker melanomas,” Dr. Ferris said in an interview. “I do suspect that, if we followed patients longer, we might start to see a more significant difference.”
The findings nevertheless add to evidence that although routine screening substantially increases the detection of melanomas overall, these melanomas are often not the ones doctors are most worried about or that increase a person’s risk of mortality, Dr. Ferris noted.
When it comes to melanoma screening, balancing the risks and benefits is key. One major downside, Dr. Ferris said, is in regard to the burden such screening could place on the health care system, with potentially unproductive screenings causing delays in care for patients with more urgent needs.
“We are undersupplied in the dermatology workforce, and there is often a long wait to see dermatologists, so we really want to make sure, as trained professionals, that patients have access to us,” she said. “If we’re doing something that doesn’t have proven benefit and is increasing the wait time, that will come at the expense of other patients’ access.”
Costs involved in skin biopsies and excisions of borderline lesions as well as the potential to increase patients’ anxiety represent other important considerations, Dr. Ferris noted.
However, Sancy A. Leachman, MD, PhD, a coauthor of the editorial in favor of screening, said in an interview that “at the individual level, there are an almost infinite number of individual circumstances that could lead a person to decide that the potential benefits outweigh the harms.”
According to Dr. Leachman, who is chair of the department of dermatology, Oregon Health & Science University, these individual priorities may not align with those of the various decision-makers or with guidelines, such as those from the U.S. Preventive Services Task Force, which gives visual skin cancer screening of asymptomatic patients an “I” rating, indicating “insufficient evidence.”
“Many federal agencies and payer groups focus on minimizing costs and optimizing outcomes,” Dr. Leachman and coauthors wrote. As the only professional advocates for individual patients, physicians “have a responsibility to assure that the best interests of patients are served.”
The study was funded by the University of Pittsburgh Melanoma and Skin Cancer Program. Dr. Ferris and Dr. Swerlick disclosed no relevant financial relationships. Dr. Leachman is the principal investigator for War on Melanoma, an early-detection program in Oregon.
A version of this article first appeared on Medscape.com.
new research shows.
Without a corresponding decrease in melanoma mortality, an increase in the detection of those thin melanomas “raises the concern that early detection efforts, such as visual skin screening, may result in overdiagnosis,” the study authors wrote. “The value of a cancer screening program should most rigorously be measured not by the number of new, early cancers detected, but by its impact on the development of late-stage disease and its associated morbidity, cost, and mortality.”
The research, published in JAMA Dermatology, has reignited the controversy over the benefits and harms of primary care skin cancer screening, garnering two editorials that reflect different sides of the debate.
In one, Robert A. Swerlick, MD, pointed out that, “despite public messaging to the contrary, to my knowledge there is no evidence that routine skin examinations have any effect on melanoma mortality.
“The stage shift to smaller tumors should not be viewed as success and is very strong evidence of overdiagnosis,” wrote Dr. Swerlick, of the department of dermatology, Emory University, Atlanta.
The other editorial, however, argued that routine screening saves lives. “Most melanoma deaths are because of stage I disease, with an estimated 3%-15% of thin melanomas (≤ 1 mm) being lethal,” wrote a trio of editorialists from Oregon Health & Science University, Portland.
When considering the high mutation rate associated with melanoma and the current limits of treatment options, early diagnosis becomes “particularly important and counterbalances the risk of overdiagnosis,” the editorialists asserted.
Primary care screening study
The new findings come from an observational study of a quality improvement initiative conducted at the University of Pittsburgh Medical Center system between 2014 and 2018, in which primary care clinicians were offered training in melanoma identification through skin examination and were encouraged to offer annual skin cancer screening to patients aged 35 years and older.
Of 595,799 eligible patients, 144,851 (24.3%) were screened at least once during the study period. Those who received screening were more likely than unscreened patients to be older (median age, 59 vs. 55 years), women, and non-Hispanic White persons.
During a follow-up of 5 years, the researchers found that patients who received screening were significantly more likely than unscreened patients to be diagnosed with in situ melanoma (incidence, 30.4 vs. 14.4; hazard ratio, 2.6; P < .001) or thin invasive melanoma (incidence, 24.5 vs. 16.1; HR, 1.8; P < .001), after adjusting for factors that included age, sex, and race.
The screened patients were also more likely than unscreened patients to be diagnosed with in situ interval melanomas, defined as melanomas occurring at least 60 days after initial screening (incidence, 26.7 vs. 12.9; HR, 2.1; P < .001), as well as thin invasive interval melanomas (incidence, 18.5 vs. 14.4; HR, 1.3; P = .03).
The 60-day interval was included to account for the possible time to referral to a specialist for definitive diagnosis, the authors explained.
The incidence of the detection of melanomas thicker than 4 mm was lower in screened versus unscreened patients, but the difference was not statistically significant for all melanomas (2.7 vs. 3.3; HR, 0.8; P = .38) or interval melanomas (1.5 vs. 2.7; HR, 0.6; P = .15).
Experts weigh in
Although the follow-up period was of 5 years, not all patients were followed that long after undergoing screening. For instance, for some patients, follow-up occurred only 1 year after they had been screened.
The study’s senior author, Laura K. Ferris, MD, PhD, of the department of dermatology, University of Pittsburgh, noted that a longer follow-up could shift the results.
“When you look at the curves in our figures, you do start to see them separate more and more over time for the thicker melanomas,” Dr. Ferris said in an interview. “I do suspect that, if we followed patients longer, we might start to see a more significant difference.”
The findings nevertheless add to evidence that although routine screening substantially increases the detection of melanomas overall, these melanomas are often not the ones doctors are most worried about or that increase a person’s risk of mortality, Dr. Ferris noted.
When it comes to melanoma screening, balancing the risks and benefits is key. One major downside, Dr. Ferris said, is in regard to the burden such screening could place on the health care system, with potentially unproductive screenings causing delays in care for patients with more urgent needs.
“We are undersupplied in the dermatology workforce, and there is often a long wait to see dermatologists, so we really want to make sure, as trained professionals, that patients have access to us,” she said. “If we’re doing something that doesn’t have proven benefit and is increasing the wait time, that will come at the expense of other patients’ access.”
Costs involved in skin biopsies and excisions of borderline lesions as well as the potential to increase patients’ anxiety represent other important considerations, Dr. Ferris noted.
However, Sancy A. Leachman, MD, PhD, a coauthor of the editorial in favor of screening, said in an interview that “at the individual level, there are an almost infinite number of individual circumstances that could lead a person to decide that the potential benefits outweigh the harms.”
According to Dr. Leachman, who is chair of the department of dermatology, Oregon Health & Science University, these individual priorities may not align with those of the various decision-makers or with guidelines, such as those from the U.S. Preventive Services Task Force, which gives visual skin cancer screening of asymptomatic patients an “I” rating, indicating “insufficient evidence.”
“Many federal agencies and payer groups focus on minimizing costs and optimizing outcomes,” Dr. Leachman and coauthors wrote. As the only professional advocates for individual patients, physicians “have a responsibility to assure that the best interests of patients are served.”
The study was funded by the University of Pittsburgh Melanoma and Skin Cancer Program. Dr. Ferris and Dr. Swerlick disclosed no relevant financial relationships. Dr. Leachman is the principal investigator for War on Melanoma, an early-detection program in Oregon.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
PLA testing brings nuance to the diagnosis of early-stage melanoma
BOSTON – Although
One such test, the Pigmented Lesional Assay (PLA) uses adhesive patches applied to lesions of concern at the bedside to extract RNA from the stratum corneum to help determine the risk for melanoma.
At the annual meeting of the American Academy of Dermatology, Caroline C. Kim, MD, director of melanoma and pigmented lesion clinics at Newton Wellesley Dermatology, Wellesley Hills, Mass., and Tufts Medical Center, Boston, spoke about the PLA, which uses genetic expression profiling to measure the expression level of specific genes that are associated with melanoma: PRAME (preferentially expressed antigen in melanoma) and LINC00518 (LINC). There are four possible results of the test: Aberrant expression of both LINC and PRAME (high risk); aberrant expression of a single gene (moderate risk); aberrant expression of neither gene (low risk); or inconclusive.
Validation data have shown a sensitivity of 91% and a specificity of 69% for the PLA, with a 99% negative predictive value; so a lesion that tested negative by PLA has a less than 1% chance of being melanoma. In addition, a study published in 2020 found that the addition of TERT (telomerase reverse transcriptase) mutation analyses increased the sensitivity of the PLA to 97%.
While the high negative predictive value is helpful to consider in clinical scenarios to rule-out melanoma for borderline lesions, one must consider the positive predictive value as well and how this may impact clinical care, Dr. Kim said. In a study examining outcomes of 381 lesions, 51 were PLA positive (single or double) and were biopsied, of which 19 (37%) revealed a melanoma diagnosis. In a large U.S. registry study of 3,418 lesions, 324 lesions that were PLA double positive were biopsied, with 18.7% revealing a melanoma diagnosis.
“No test is perfect, and this applies to PLA, even if you get a double-positive or double-negative test result,” Dr. Kim said. “You want to make sure that patients are aware of false positives and negatives. However, PLA could be an additional piece of data to inform your decision to proceed with biopsy on select borderline suspicious pigmented lesions. More studies are needed to better understand the approach to single- and double-positive PLA results.”
The PLA kit contains adhesive patches and supplies and a FedEx envelope for return to DermTech, the test’s manufacturer, for processing. The patches can be applied to lesions at least 4 mm in diameter; multiple kits are recommended for those greater than 16 mm in diameter. The test is not validated for lesions located on mucous membranes, palms, soles, nails, or on ulcerated or bleeding lesions, nor for those that have been previously biopsied. It is also not validated for use in pediatric patients or in those with skin types IV or higher. Results are returned in 2-3 days. If insurance does not cover the test, the cost to the patient is approximately $50 per lesion or a maximum of $150, according to Dr. Kim.
Use in clinical practice
In Dr. Kim’s clinical experience, the PLA can be considered for suspicious pigmented lesions on cosmetically sensitive areas and for suspicious lesions in areas difficult to biopsy or excise. For example, she discussed the case of a 72-year-old woman with a family history of melanoma, who presented to her clinic with a longstanding pigmented lesion on her right upper and lower eyelids that had previously been treated with laser. She had undergone multiple prior biopsies over 12 years, which caused mild to moderate atypical melanocytic proliferation. The PLA result was double negative for PRAME and LINC in her upper and lower eyelid, “which provided reassurance to the patient,” Dr. Kim said. The patient continues to be followed closely for any clinical changes.
Another patient, a 67-year-old woman, was referred to Dr. Kim from out of state for a teledermatology visit early in the COVID-19 pandemic. The patient had a lesion on her right calf that was hard, raised, and pink, did not resemble other lesions on her body, and had been present for a few weeks. “Her husband had recently passed away from brain cancer and she was very concerned about melanoma,” Dr. Kim recalled. “She lived alone, and the adult son was with her during the teledermatology call to assist. The patient asked about the PLA test, and given her difficulty going to a medical office at the time, we agreed to help her with this test.” The patient and her son arranged another teledermatology visit with Dr. Kim after receiving the kit in the mail from DermTech, and Dr. Kim coached them on how to properly administer the test. The results came back as PRAME negative and LINC positive. A biopsy with a local provider was recommended and the pathology results showed an inflamed seborrheic keratosis.
“This case exemplifies a false-positive result. We should be sure to make patients aware of this possibility,” Dr. Kim said.
Incorporating PLA into clinical practice requires certain workflow considerations, with paperwork to fill out in addition to performing the adhesive test, collection of insurance information, mailing the kit via FedEx, retrieving the results, and following up with the patient, said Dr. Kim. “For select borderline pigmented lesions, I discuss the rationale of the test with patients, the possibility of false-positive and false-negative results and the need to return for a biopsy when there is positive result. Clinical follow-up is recommended for negative results. There is also the possibility of charge to the patient if the test is not covered by their insurance.”
Skin biopsy still the gold standard
Despite the availability of the PLA as an assessment tool, Dr. Kim emphasized that skin biopsy remains the gold standard for diagnosing melanoma. “Future prospective randomized clinical trials are needed to examine the role of genetic expression profiling in staging and managing patients,” she said.
In 2019, she and her colleagues surveyed 42 pigmented lesion experts in the United States about why they ordered one of three molecular tests on the market or not and how results affected patient treatment. The proportion of clinicians who ordered the tests ranged from 21% to 29%. The top 2 reasons respondents chose for not ordering the PLA test specifically were: “Feel that further validation studies are necessary” (20%) and “do not feel it would be useful in my practice” (18%).
Results of a larger follow-up survey on usage patterns of PLA of both pigmented lesion experts and general clinicians on this topic are expected to be published shortly.
Dr. Kim reported having no disclosures related to her presentation.
BOSTON – Although
One such test, the Pigmented Lesional Assay (PLA) uses adhesive patches applied to lesions of concern at the bedside to extract RNA from the stratum corneum to help determine the risk for melanoma.
At the annual meeting of the American Academy of Dermatology, Caroline C. Kim, MD, director of melanoma and pigmented lesion clinics at Newton Wellesley Dermatology, Wellesley Hills, Mass., and Tufts Medical Center, Boston, spoke about the PLA, which uses genetic expression profiling to measure the expression level of specific genes that are associated with melanoma: PRAME (preferentially expressed antigen in melanoma) and LINC00518 (LINC). There are four possible results of the test: Aberrant expression of both LINC and PRAME (high risk); aberrant expression of a single gene (moderate risk); aberrant expression of neither gene (low risk); or inconclusive.
Validation data have shown a sensitivity of 91% and a specificity of 69% for the PLA, with a 99% negative predictive value; so a lesion that tested negative by PLA has a less than 1% chance of being melanoma. In addition, a study published in 2020 found that the addition of TERT (telomerase reverse transcriptase) mutation analyses increased the sensitivity of the PLA to 97%.
While the high negative predictive value is helpful to consider in clinical scenarios to rule-out melanoma for borderline lesions, one must consider the positive predictive value as well and how this may impact clinical care, Dr. Kim said. In a study examining outcomes of 381 lesions, 51 were PLA positive (single or double) and were biopsied, of which 19 (37%) revealed a melanoma diagnosis. In a large U.S. registry study of 3,418 lesions, 324 lesions that were PLA double positive were biopsied, with 18.7% revealing a melanoma diagnosis.
“No test is perfect, and this applies to PLA, even if you get a double-positive or double-negative test result,” Dr. Kim said. “You want to make sure that patients are aware of false positives and negatives. However, PLA could be an additional piece of data to inform your decision to proceed with biopsy on select borderline suspicious pigmented lesions. More studies are needed to better understand the approach to single- and double-positive PLA results.”
The PLA kit contains adhesive patches and supplies and a FedEx envelope for return to DermTech, the test’s manufacturer, for processing. The patches can be applied to lesions at least 4 mm in diameter; multiple kits are recommended for those greater than 16 mm in diameter. The test is not validated for lesions located on mucous membranes, palms, soles, nails, or on ulcerated or bleeding lesions, nor for those that have been previously biopsied. It is also not validated for use in pediatric patients or in those with skin types IV or higher. Results are returned in 2-3 days. If insurance does not cover the test, the cost to the patient is approximately $50 per lesion or a maximum of $150, according to Dr. Kim.
Use in clinical practice
In Dr. Kim’s clinical experience, the PLA can be considered for suspicious pigmented lesions on cosmetically sensitive areas and for suspicious lesions in areas difficult to biopsy or excise. For example, she discussed the case of a 72-year-old woman with a family history of melanoma, who presented to her clinic with a longstanding pigmented lesion on her right upper and lower eyelids that had previously been treated with laser. She had undergone multiple prior biopsies over 12 years, which caused mild to moderate atypical melanocytic proliferation. The PLA result was double negative for PRAME and LINC in her upper and lower eyelid, “which provided reassurance to the patient,” Dr. Kim said. The patient continues to be followed closely for any clinical changes.
Another patient, a 67-year-old woman, was referred to Dr. Kim from out of state for a teledermatology visit early in the COVID-19 pandemic. The patient had a lesion on her right calf that was hard, raised, and pink, did not resemble other lesions on her body, and had been present for a few weeks. “Her husband had recently passed away from brain cancer and she was very concerned about melanoma,” Dr. Kim recalled. “She lived alone, and the adult son was with her during the teledermatology call to assist. The patient asked about the PLA test, and given her difficulty going to a medical office at the time, we agreed to help her with this test.” The patient and her son arranged another teledermatology visit with Dr. Kim after receiving the kit in the mail from DermTech, and Dr. Kim coached them on how to properly administer the test. The results came back as PRAME negative and LINC positive. A biopsy with a local provider was recommended and the pathology results showed an inflamed seborrheic keratosis.
“This case exemplifies a false-positive result. We should be sure to make patients aware of this possibility,” Dr. Kim said.
Incorporating PLA into clinical practice requires certain workflow considerations, with paperwork to fill out in addition to performing the adhesive test, collection of insurance information, mailing the kit via FedEx, retrieving the results, and following up with the patient, said Dr. Kim. “For select borderline pigmented lesions, I discuss the rationale of the test with patients, the possibility of false-positive and false-negative results and the need to return for a biopsy when there is positive result. Clinical follow-up is recommended for negative results. There is also the possibility of charge to the patient if the test is not covered by their insurance.”
Skin biopsy still the gold standard
Despite the availability of the PLA as an assessment tool, Dr. Kim emphasized that skin biopsy remains the gold standard for diagnosing melanoma. “Future prospective randomized clinical trials are needed to examine the role of genetic expression profiling in staging and managing patients,” she said.
In 2019, she and her colleagues surveyed 42 pigmented lesion experts in the United States about why they ordered one of three molecular tests on the market or not and how results affected patient treatment. The proportion of clinicians who ordered the tests ranged from 21% to 29%. The top 2 reasons respondents chose for not ordering the PLA test specifically were: “Feel that further validation studies are necessary” (20%) and “do not feel it would be useful in my practice” (18%).
Results of a larger follow-up survey on usage patterns of PLA of both pigmented lesion experts and general clinicians on this topic are expected to be published shortly.
Dr. Kim reported having no disclosures related to her presentation.
BOSTON – Although
One such test, the Pigmented Lesional Assay (PLA) uses adhesive patches applied to lesions of concern at the bedside to extract RNA from the stratum corneum to help determine the risk for melanoma.
At the annual meeting of the American Academy of Dermatology, Caroline C. Kim, MD, director of melanoma and pigmented lesion clinics at Newton Wellesley Dermatology, Wellesley Hills, Mass., and Tufts Medical Center, Boston, spoke about the PLA, which uses genetic expression profiling to measure the expression level of specific genes that are associated with melanoma: PRAME (preferentially expressed antigen in melanoma) and LINC00518 (LINC). There are four possible results of the test: Aberrant expression of both LINC and PRAME (high risk); aberrant expression of a single gene (moderate risk); aberrant expression of neither gene (low risk); or inconclusive.
Validation data have shown a sensitivity of 91% and a specificity of 69% for the PLA, with a 99% negative predictive value; so a lesion that tested negative by PLA has a less than 1% chance of being melanoma. In addition, a study published in 2020 found that the addition of TERT (telomerase reverse transcriptase) mutation analyses increased the sensitivity of the PLA to 97%.
While the high negative predictive value is helpful to consider in clinical scenarios to rule-out melanoma for borderline lesions, one must consider the positive predictive value as well and how this may impact clinical care, Dr. Kim said. In a study examining outcomes of 381 lesions, 51 were PLA positive (single or double) and were biopsied, of which 19 (37%) revealed a melanoma diagnosis. In a large U.S. registry study of 3,418 lesions, 324 lesions that were PLA double positive were biopsied, with 18.7% revealing a melanoma diagnosis.
“No test is perfect, and this applies to PLA, even if you get a double-positive or double-negative test result,” Dr. Kim said. “You want to make sure that patients are aware of false positives and negatives. However, PLA could be an additional piece of data to inform your decision to proceed with biopsy on select borderline suspicious pigmented lesions. More studies are needed to better understand the approach to single- and double-positive PLA results.”
The PLA kit contains adhesive patches and supplies and a FedEx envelope for return to DermTech, the test’s manufacturer, for processing. The patches can be applied to lesions at least 4 mm in diameter; multiple kits are recommended for those greater than 16 mm in diameter. The test is not validated for lesions located on mucous membranes, palms, soles, nails, or on ulcerated or bleeding lesions, nor for those that have been previously biopsied. It is also not validated for use in pediatric patients or in those with skin types IV or higher. Results are returned in 2-3 days. If insurance does not cover the test, the cost to the patient is approximately $50 per lesion or a maximum of $150, according to Dr. Kim.
Use in clinical practice
In Dr. Kim’s clinical experience, the PLA can be considered for suspicious pigmented lesions on cosmetically sensitive areas and for suspicious lesions in areas difficult to biopsy or excise. For example, she discussed the case of a 72-year-old woman with a family history of melanoma, who presented to her clinic with a longstanding pigmented lesion on her right upper and lower eyelids that had previously been treated with laser. She had undergone multiple prior biopsies over 12 years, which caused mild to moderate atypical melanocytic proliferation. The PLA result was double negative for PRAME and LINC in her upper and lower eyelid, “which provided reassurance to the patient,” Dr. Kim said. The patient continues to be followed closely for any clinical changes.
Another patient, a 67-year-old woman, was referred to Dr. Kim from out of state for a teledermatology visit early in the COVID-19 pandemic. The patient had a lesion on her right calf that was hard, raised, and pink, did not resemble other lesions on her body, and had been present for a few weeks. “Her husband had recently passed away from brain cancer and she was very concerned about melanoma,” Dr. Kim recalled. “She lived alone, and the adult son was with her during the teledermatology call to assist. The patient asked about the PLA test, and given her difficulty going to a medical office at the time, we agreed to help her with this test.” The patient and her son arranged another teledermatology visit with Dr. Kim after receiving the kit in the mail from DermTech, and Dr. Kim coached them on how to properly administer the test. The results came back as PRAME negative and LINC positive. A biopsy with a local provider was recommended and the pathology results showed an inflamed seborrheic keratosis.
“This case exemplifies a false-positive result. We should be sure to make patients aware of this possibility,” Dr. Kim said.
Incorporating PLA into clinical practice requires certain workflow considerations, with paperwork to fill out in addition to performing the adhesive test, collection of insurance information, mailing the kit via FedEx, retrieving the results, and following up with the patient, said Dr. Kim. “For select borderline pigmented lesions, I discuss the rationale of the test with patients, the possibility of false-positive and false-negative results and the need to return for a biopsy when there is positive result. Clinical follow-up is recommended for negative results. There is also the possibility of charge to the patient if the test is not covered by their insurance.”
Skin biopsy still the gold standard
Despite the availability of the PLA as an assessment tool, Dr. Kim emphasized that skin biopsy remains the gold standard for diagnosing melanoma. “Future prospective randomized clinical trials are needed to examine the role of genetic expression profiling in staging and managing patients,” she said.
In 2019, she and her colleagues surveyed 42 pigmented lesion experts in the United States about why they ordered one of three molecular tests on the market or not and how results affected patient treatment. The proportion of clinicians who ordered the tests ranged from 21% to 29%. The top 2 reasons respondents chose for not ordering the PLA test specifically were: “Feel that further validation studies are necessary” (20%) and “do not feel it would be useful in my practice” (18%).
Results of a larger follow-up survey on usage patterns of PLA of both pigmented lesion experts and general clinicians on this topic are expected to be published shortly.
Dr. Kim reported having no disclosures related to her presentation.
AT AAD 22
Study finds discrepancies in biopsy decisions, diagnoses based on skin type
BOSTON – compared with White patients, new research shows.
“Our findings suggest diagnostic biases based on skin color exist in dermatology practice,” lead author Loren Krueger, MD, assistant professor in the department of dermatology, Emory University School of Medicine, Atlanta, said at the Annual Skin of Color Society Scientific Symposium. “A lower likelihood of biopsy of malignancy in darker skin types could contribute to disparities in cutaneous malignancies,” she added.
Disparities in dermatologic care among Black patients, compared with White patients, have been well documented. Recent evidence includes a 2020 study that showed significant shortcomings among medical students in correctly diagnosing squamous cell carcinoma, urticaria, and atopic dermatitis for patients with skin of color.
“It’s no secret that our images do not accurately or in the right quantity include skin of color,” Dr. Krueger said. “Yet few papers talk about how these biases actually impact our care. Importantly, this study demonstrates that diagnostic bias develops as early as the medical student level.”
To further investigate the role of skin color in the assessment of neoplastic and inflammatory skin conditions and decisions to perform biopsy, Dr. Krueger and her colleagues surveyed 144 dermatology residents and attending dermatologists to evaluate their clinical decisionmaking skills in assessing skin conditions for patients with lighter skin and those with darker skin. Almost 80% (113) provided complete responses and were included in the study.
For the survey, participants were shown photos of 10 neoplastic and 10 inflammatory skin conditions. Each image was matched in lighter (skin types I-II) and darker (skin types IV-VI) skinned patients in random order. Participants were asked to identify the suspected underlying etiology (neoplastic–benign, neoplastic–malignant, papulosquamous, lichenoid, infectious, bullous, or no suspected etiology) and whether they would choose to perform biopsy for the pictured condition.
Overall, their responses showed a slightly higher probability of recommending a biopsy for patients with skin types IV-V (odds ratio, 1.18; P = .054).
However, respondents were more than twice as likely to recommend a biopsy for benign neoplasms for patients with skin of color, compared with those with lighter skin types (OR, 2.57; P < .0001). They were significantly less likely to recommend a biopsy for a malignant neoplasm for patients with skin of color (OR, 0.42; P < .0001).
In addition, the correct etiology was much more commonly missed in diagnosing patients with skin of color, even after adjusting for years in dermatology practice (OR, 0.569; P < .0001).
Conversely, respondents were significantly less likely to recommend a biopsy for benign neoplasms and were more likely to recommend a biopsy for malignant neoplasms among White patients. Etiology was more commonly correct.
The findings underscore that “for skin of color patients, you’re more likely to have a benign neoplasm biopsied, you’re less likely to have a malignant neoplasm biopsied, and more often, your etiology may be missed,” Dr. Krueger said at the meeting.
Of note, while 45% of respondents were dermatology residents or fellows, 20.4% had 1-5 years of experience, and about 28% had 10 to more than 25 years of experience.
And while 75% of the dermatology residents, fellows, and attendings were White, there was no difference in the probability of correctly identifying the underlying etiology in dark or light skin types based on the provider’s self-identified race.
Importantly, the patterns in the study of diagnostic discrepancies are reflected in broader dermatologic outcomes. The 5-year melanoma survival rate is 74.1% among Black patients and 92.9% among White patients. Dr. Krueger referred to data showing that only 52.6% of Black patients have stage I melanoma at diagnosis, whereas among White patients, the rate is much higher, at 75.9%.
“We know skin malignancy can be more aggressive and late-stage in skin of color populations, leading to increased morbidity and later stage at initial diagnosis,” Dr. Krueger told this news organization. “We routinely attribute this to limited access to care and lack of awareness on skin malignancy. However, we have no evidence on how we, as dermatologists, may be playing a role.”
Furthermore, the decision to perform biopsy or not can affect the size and stage at diagnosis of a cutaneous malignancy, she noted.
Key changes needed to prevent the disparities – and their implications – should start at the training level, she emphasized. “I would love to see increased photo representation in training materials – this is a great place to start,” Dr. Krueger said.
In addition, “encouraging medical students, residents, and dermatologists to learn from skin of color experts is vital,” she said. “We should also provide hands-on experience and training with diverse patient populations.”
The first step to addressing biases “is to acknowledge they exist,” Dr. Krueger added. “I am hopeful this inspires others to continue to investigate these biases, as well as how we can eliminate them.”
The study was funded by the Rudin Resident Research Award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – compared with White patients, new research shows.
“Our findings suggest diagnostic biases based on skin color exist in dermatology practice,” lead author Loren Krueger, MD, assistant professor in the department of dermatology, Emory University School of Medicine, Atlanta, said at the Annual Skin of Color Society Scientific Symposium. “A lower likelihood of biopsy of malignancy in darker skin types could contribute to disparities in cutaneous malignancies,” she added.
Disparities in dermatologic care among Black patients, compared with White patients, have been well documented. Recent evidence includes a 2020 study that showed significant shortcomings among medical students in correctly diagnosing squamous cell carcinoma, urticaria, and atopic dermatitis for patients with skin of color.
“It’s no secret that our images do not accurately or in the right quantity include skin of color,” Dr. Krueger said. “Yet few papers talk about how these biases actually impact our care. Importantly, this study demonstrates that diagnostic bias develops as early as the medical student level.”
To further investigate the role of skin color in the assessment of neoplastic and inflammatory skin conditions and decisions to perform biopsy, Dr. Krueger and her colleagues surveyed 144 dermatology residents and attending dermatologists to evaluate their clinical decisionmaking skills in assessing skin conditions for patients with lighter skin and those with darker skin. Almost 80% (113) provided complete responses and were included in the study.
For the survey, participants were shown photos of 10 neoplastic and 10 inflammatory skin conditions. Each image was matched in lighter (skin types I-II) and darker (skin types IV-VI) skinned patients in random order. Participants were asked to identify the suspected underlying etiology (neoplastic–benign, neoplastic–malignant, papulosquamous, lichenoid, infectious, bullous, or no suspected etiology) and whether they would choose to perform biopsy for the pictured condition.
Overall, their responses showed a slightly higher probability of recommending a biopsy for patients with skin types IV-V (odds ratio, 1.18; P = .054).
However, respondents were more than twice as likely to recommend a biopsy for benign neoplasms for patients with skin of color, compared with those with lighter skin types (OR, 2.57; P < .0001). They were significantly less likely to recommend a biopsy for a malignant neoplasm for patients with skin of color (OR, 0.42; P < .0001).
In addition, the correct etiology was much more commonly missed in diagnosing patients with skin of color, even after adjusting for years in dermatology practice (OR, 0.569; P < .0001).
Conversely, respondents were significantly less likely to recommend a biopsy for benign neoplasms and were more likely to recommend a biopsy for malignant neoplasms among White patients. Etiology was more commonly correct.
The findings underscore that “for skin of color patients, you’re more likely to have a benign neoplasm biopsied, you’re less likely to have a malignant neoplasm biopsied, and more often, your etiology may be missed,” Dr. Krueger said at the meeting.
Of note, while 45% of respondents were dermatology residents or fellows, 20.4% had 1-5 years of experience, and about 28% had 10 to more than 25 years of experience.
And while 75% of the dermatology residents, fellows, and attendings were White, there was no difference in the probability of correctly identifying the underlying etiology in dark or light skin types based on the provider’s self-identified race.
Importantly, the patterns in the study of diagnostic discrepancies are reflected in broader dermatologic outcomes. The 5-year melanoma survival rate is 74.1% among Black patients and 92.9% among White patients. Dr. Krueger referred to data showing that only 52.6% of Black patients have stage I melanoma at diagnosis, whereas among White patients, the rate is much higher, at 75.9%.
“We know skin malignancy can be more aggressive and late-stage in skin of color populations, leading to increased morbidity and later stage at initial diagnosis,” Dr. Krueger told this news organization. “We routinely attribute this to limited access to care and lack of awareness on skin malignancy. However, we have no evidence on how we, as dermatologists, may be playing a role.”
Furthermore, the decision to perform biopsy or not can affect the size and stage at diagnosis of a cutaneous malignancy, she noted.
Key changes needed to prevent the disparities – and their implications – should start at the training level, she emphasized. “I would love to see increased photo representation in training materials – this is a great place to start,” Dr. Krueger said.
In addition, “encouraging medical students, residents, and dermatologists to learn from skin of color experts is vital,” she said. “We should also provide hands-on experience and training with diverse patient populations.”
The first step to addressing biases “is to acknowledge they exist,” Dr. Krueger added. “I am hopeful this inspires others to continue to investigate these biases, as well as how we can eliminate them.”
The study was funded by the Rudin Resident Research Award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – compared with White patients, new research shows.
“Our findings suggest diagnostic biases based on skin color exist in dermatology practice,” lead author Loren Krueger, MD, assistant professor in the department of dermatology, Emory University School of Medicine, Atlanta, said at the Annual Skin of Color Society Scientific Symposium. “A lower likelihood of biopsy of malignancy in darker skin types could contribute to disparities in cutaneous malignancies,” she added.
Disparities in dermatologic care among Black patients, compared with White patients, have been well documented. Recent evidence includes a 2020 study that showed significant shortcomings among medical students in correctly diagnosing squamous cell carcinoma, urticaria, and atopic dermatitis for patients with skin of color.
“It’s no secret that our images do not accurately or in the right quantity include skin of color,” Dr. Krueger said. “Yet few papers talk about how these biases actually impact our care. Importantly, this study demonstrates that diagnostic bias develops as early as the medical student level.”
To further investigate the role of skin color in the assessment of neoplastic and inflammatory skin conditions and decisions to perform biopsy, Dr. Krueger and her colleagues surveyed 144 dermatology residents and attending dermatologists to evaluate their clinical decisionmaking skills in assessing skin conditions for patients with lighter skin and those with darker skin. Almost 80% (113) provided complete responses and were included in the study.
For the survey, participants were shown photos of 10 neoplastic and 10 inflammatory skin conditions. Each image was matched in lighter (skin types I-II) and darker (skin types IV-VI) skinned patients in random order. Participants were asked to identify the suspected underlying etiology (neoplastic–benign, neoplastic–malignant, papulosquamous, lichenoid, infectious, bullous, or no suspected etiology) and whether they would choose to perform biopsy for the pictured condition.
Overall, their responses showed a slightly higher probability of recommending a biopsy for patients with skin types IV-V (odds ratio, 1.18; P = .054).
However, respondents were more than twice as likely to recommend a biopsy for benign neoplasms for patients with skin of color, compared with those with lighter skin types (OR, 2.57; P < .0001). They were significantly less likely to recommend a biopsy for a malignant neoplasm for patients with skin of color (OR, 0.42; P < .0001).
In addition, the correct etiology was much more commonly missed in diagnosing patients with skin of color, even after adjusting for years in dermatology practice (OR, 0.569; P < .0001).
Conversely, respondents were significantly less likely to recommend a biopsy for benign neoplasms and were more likely to recommend a biopsy for malignant neoplasms among White patients. Etiology was more commonly correct.
The findings underscore that “for skin of color patients, you’re more likely to have a benign neoplasm biopsied, you’re less likely to have a malignant neoplasm biopsied, and more often, your etiology may be missed,” Dr. Krueger said at the meeting.
Of note, while 45% of respondents were dermatology residents or fellows, 20.4% had 1-5 years of experience, and about 28% had 10 to more than 25 years of experience.
And while 75% of the dermatology residents, fellows, and attendings were White, there was no difference in the probability of correctly identifying the underlying etiology in dark or light skin types based on the provider’s self-identified race.
Importantly, the patterns in the study of diagnostic discrepancies are reflected in broader dermatologic outcomes. The 5-year melanoma survival rate is 74.1% among Black patients and 92.9% among White patients. Dr. Krueger referred to data showing that only 52.6% of Black patients have stage I melanoma at diagnosis, whereas among White patients, the rate is much higher, at 75.9%.
“We know skin malignancy can be more aggressive and late-stage in skin of color populations, leading to increased morbidity and later stage at initial diagnosis,” Dr. Krueger told this news organization. “We routinely attribute this to limited access to care and lack of awareness on skin malignancy. However, we have no evidence on how we, as dermatologists, may be playing a role.”
Furthermore, the decision to perform biopsy or not can affect the size and stage at diagnosis of a cutaneous malignancy, she noted.
Key changes needed to prevent the disparities – and their implications – should start at the training level, she emphasized. “I would love to see increased photo representation in training materials – this is a great place to start,” Dr. Krueger said.
In addition, “encouraging medical students, residents, and dermatologists to learn from skin of color experts is vital,” she said. “We should also provide hands-on experience and training with diverse patient populations.”
The first step to addressing biases “is to acknowledge they exist,” Dr. Krueger added. “I am hopeful this inspires others to continue to investigate these biases, as well as how we can eliminate them.”
The study was funded by the Rudin Resident Research Award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Global melanoma incidence high and on the rise
Even by cautious calculations,
An estimated 325,000 people worldwide received a new diagnosis of cutaneous melanoma in 2020, and if present trends continue, the incidence of new cases is predicted to increase by about 50% in 2040, with melanoma deaths expected to rise by almost 70%, Melina Arnold, PhD, from the Cancer Surveillance Branch of the International Agency for Research on Cancer in Lyon, France, and colleagues reported.
“Melanoma is the most lethal form of skin cancer; this epidemiological assessment found a heavy public health and economic burden, and our projections suggest that it will remain so in the coming decades,” they wrote in a study published online in JAMA Dermatology.
In an accompanying editorial, Mavis Obeng-Kusi, MPharm and Ivo Abraham, PhD from the Center for Health Outcomes and PharmacoEconomic Research at the University of Arizona, Tucson, commented that the findings are “sobering,” but may substantially underestimate the gravity of the problem in low- and middle-income countries (LMIC).
“The study by Arnold et al. brings to the fore a public health concern that requires global attention and initiates conversations particularly related to LMIC settings, where the incidence and mortality of melanoma is thought to be minimal and for which preventive measures may be insufficient,” they wrote.
Down Under nations lead
Dr. Arnold and colleagues looked at data on age-standardized melanoma incidence and mortality rates per 100,000 person-years (PY) by country, each of 20 world regions as defined by the United Nations, and according to the UN’s four-tier Human Development Index, which stratifies countries into low-, medium-, high-, and very high–income categories.
As noted previously, the researchers estimated that there were 325,000 new melanoma cases worldwide in 2020 (174,000 cases in males and 151,000 in females). There were 57,000 estimated melanoma deaths the same year (32,000 in males and 25,000 in females.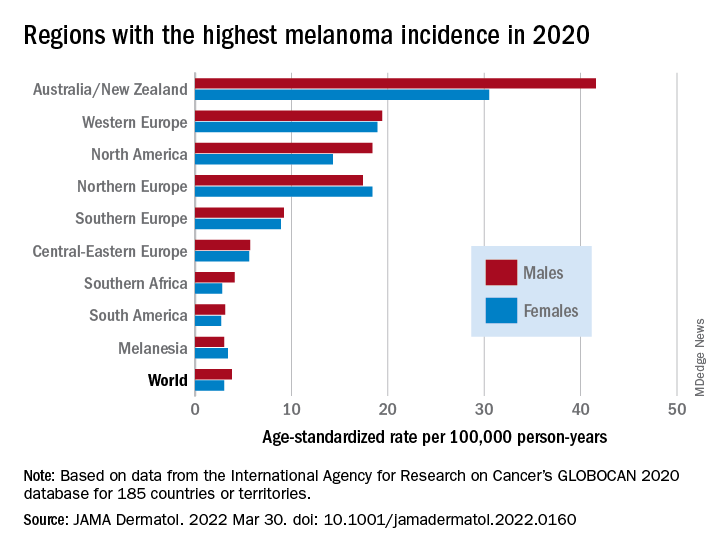
The highest incidence rates were seen in Australia and New Zealand, at 42 per 100,000 PY among males and 31 per 100,000 PY in females, followed by Western Europe with 19 per 100,000 PY in both males and females, North America with 18 and 14 cases per 100,000 PY in males and females respectively, and Northern Europe, with 17 per 100,000 PY in males, and 18 per 100,000 PY in females.
In contrast, in most African and Asian countries melanoma was rare, with rates commonly less than 1 per 100,000 PY, the investigators noted.
The melanoma mortality rate was highest in New Zealand, at 5 per 100,000 PY. Mortality rates worldwide varied less widely than incidence rates. In most other regions of the world, mortality rates were “much lower,” ranging between 0.2-1.0 per 100,000 PY, they wrote.
The authors estimated that, if 2020 rates remain stable, the global burden from melanoma in 2040 will increase to approximately 510,000 new cases and 96,000 deaths.
Public health efforts needed
In their editorial, Ms. Obeng-Kusi and Dr. Abraham pointed out that the study was hampered by the limited availability of cancer data from LMICs, leading the authors to estimate incidence and mortality rates based on proxy data, such as statistical modeling or averaged rates from neighboring countries.
They emphasized the need for going beyond the statistics: “Specific to cutaneous melanoma data, what is most important globally, knowing the exact numbers of cases and deaths or understanding the order of magnitude of the present and future epidemiology? No doubt the latter. Melanoma can be treated more easily if caught at earlier stages.”
Projections such as those provided by Dr. Arnold and colleagues could help to raise awareness of the importance of decreasing exposure to UV radiation, which accounts for three-fourths of all incident melanomas, the editorialists said.
The study was funded in part by a grant to coauthor Anna E. Cust, PhD, MPH. Dr. Cust reported receiving a fellowship from the Australian National Health and Medical Research Council outside the submitted work. Dr. Arnold had no conflicts of interested to disclose. Dr. Abraham reported financial relationships with various entities. Ms. Obeng-Kusi had no disclosures.
Even by cautious calculations,
An estimated 325,000 people worldwide received a new diagnosis of cutaneous melanoma in 2020, and if present trends continue, the incidence of new cases is predicted to increase by about 50% in 2040, with melanoma deaths expected to rise by almost 70%, Melina Arnold, PhD, from the Cancer Surveillance Branch of the International Agency for Research on Cancer in Lyon, France, and colleagues reported.
“Melanoma is the most lethal form of skin cancer; this epidemiological assessment found a heavy public health and economic burden, and our projections suggest that it will remain so in the coming decades,” they wrote in a study published online in JAMA Dermatology.
In an accompanying editorial, Mavis Obeng-Kusi, MPharm and Ivo Abraham, PhD from the Center for Health Outcomes and PharmacoEconomic Research at the University of Arizona, Tucson, commented that the findings are “sobering,” but may substantially underestimate the gravity of the problem in low- and middle-income countries (LMIC).
“The study by Arnold et al. brings to the fore a public health concern that requires global attention and initiates conversations particularly related to LMIC settings, where the incidence and mortality of melanoma is thought to be minimal and for which preventive measures may be insufficient,” they wrote.
Down Under nations lead
Dr. Arnold and colleagues looked at data on age-standardized melanoma incidence and mortality rates per 100,000 person-years (PY) by country, each of 20 world regions as defined by the United Nations, and according to the UN’s four-tier Human Development Index, which stratifies countries into low-, medium-, high-, and very high–income categories.
As noted previously, the researchers estimated that there were 325,000 new melanoma cases worldwide in 2020 (174,000 cases in males and 151,000 in females). There were 57,000 estimated melanoma deaths the same year (32,000 in males and 25,000 in females.
The highest incidence rates were seen in Australia and New Zealand, at 42 per 100,000 PY among males and 31 per 100,000 PY in females, followed by Western Europe with 19 per 100,000 PY in both males and females, North America with 18 and 14 cases per 100,000 PY in males and females respectively, and Northern Europe, with 17 per 100,000 PY in males, and 18 per 100,000 PY in females.
In contrast, in most African and Asian countries melanoma was rare, with rates commonly less than 1 per 100,000 PY, the investigators noted.
The melanoma mortality rate was highest in New Zealand, at 5 per 100,000 PY. Mortality rates worldwide varied less widely than incidence rates. In most other regions of the world, mortality rates were “much lower,” ranging between 0.2-1.0 per 100,000 PY, they wrote.
The authors estimated that, if 2020 rates remain stable, the global burden from melanoma in 2040 will increase to approximately 510,000 new cases and 96,000 deaths.
Public health efforts needed
In their editorial, Ms. Obeng-Kusi and Dr. Abraham pointed out that the study was hampered by the limited availability of cancer data from LMICs, leading the authors to estimate incidence and mortality rates based on proxy data, such as statistical modeling or averaged rates from neighboring countries.
They emphasized the need for going beyond the statistics: “Specific to cutaneous melanoma data, what is most important globally, knowing the exact numbers of cases and deaths or understanding the order of magnitude of the present and future epidemiology? No doubt the latter. Melanoma can be treated more easily if caught at earlier stages.”
Projections such as those provided by Dr. Arnold and colleagues could help to raise awareness of the importance of decreasing exposure to UV radiation, which accounts for three-fourths of all incident melanomas, the editorialists said.
The study was funded in part by a grant to coauthor Anna E. Cust, PhD, MPH. Dr. Cust reported receiving a fellowship from the Australian National Health and Medical Research Council outside the submitted work. Dr. Arnold had no conflicts of interested to disclose. Dr. Abraham reported financial relationships with various entities. Ms. Obeng-Kusi had no disclosures.
Even by cautious calculations,
An estimated 325,000 people worldwide received a new diagnosis of cutaneous melanoma in 2020, and if present trends continue, the incidence of new cases is predicted to increase by about 50% in 2040, with melanoma deaths expected to rise by almost 70%, Melina Arnold, PhD, from the Cancer Surveillance Branch of the International Agency for Research on Cancer in Lyon, France, and colleagues reported.
“Melanoma is the most lethal form of skin cancer; this epidemiological assessment found a heavy public health and economic burden, and our projections suggest that it will remain so in the coming decades,” they wrote in a study published online in JAMA Dermatology.
In an accompanying editorial, Mavis Obeng-Kusi, MPharm and Ivo Abraham, PhD from the Center for Health Outcomes and PharmacoEconomic Research at the University of Arizona, Tucson, commented that the findings are “sobering,” but may substantially underestimate the gravity of the problem in low- and middle-income countries (LMIC).
“The study by Arnold et al. brings to the fore a public health concern that requires global attention and initiates conversations particularly related to LMIC settings, where the incidence and mortality of melanoma is thought to be minimal and for which preventive measures may be insufficient,” they wrote.
Down Under nations lead
Dr. Arnold and colleagues looked at data on age-standardized melanoma incidence and mortality rates per 100,000 person-years (PY) by country, each of 20 world regions as defined by the United Nations, and according to the UN’s four-tier Human Development Index, which stratifies countries into low-, medium-, high-, and very high–income categories.
As noted previously, the researchers estimated that there were 325,000 new melanoma cases worldwide in 2020 (174,000 cases in males and 151,000 in females). There were 57,000 estimated melanoma deaths the same year (32,000 in males and 25,000 in females.
The highest incidence rates were seen in Australia and New Zealand, at 42 per 100,000 PY among males and 31 per 100,000 PY in females, followed by Western Europe with 19 per 100,000 PY in both males and females, North America with 18 and 14 cases per 100,000 PY in males and females respectively, and Northern Europe, with 17 per 100,000 PY in males, and 18 per 100,000 PY in females.
In contrast, in most African and Asian countries melanoma was rare, with rates commonly less than 1 per 100,000 PY, the investigators noted.
The melanoma mortality rate was highest in New Zealand, at 5 per 100,000 PY. Mortality rates worldwide varied less widely than incidence rates. In most other regions of the world, mortality rates were “much lower,” ranging between 0.2-1.0 per 100,000 PY, they wrote.
The authors estimated that, if 2020 rates remain stable, the global burden from melanoma in 2040 will increase to approximately 510,000 new cases and 96,000 deaths.
Public health efforts needed
In their editorial, Ms. Obeng-Kusi and Dr. Abraham pointed out that the study was hampered by the limited availability of cancer data from LMICs, leading the authors to estimate incidence and mortality rates based on proxy data, such as statistical modeling or averaged rates from neighboring countries.
They emphasized the need for going beyond the statistics: “Specific to cutaneous melanoma data, what is most important globally, knowing the exact numbers of cases and deaths or understanding the order of magnitude of the present and future epidemiology? No doubt the latter. Melanoma can be treated more easily if caught at earlier stages.”
Projections such as those provided by Dr. Arnold and colleagues could help to raise awareness of the importance of decreasing exposure to UV radiation, which accounts for three-fourths of all incident melanomas, the editorialists said.
The study was funded in part by a grant to coauthor Anna E. Cust, PhD, MPH. Dr. Cust reported receiving a fellowship from the Australian National Health and Medical Research Council outside the submitted work. Dr. Arnold had no conflicts of interested to disclose. Dr. Abraham reported financial relationships with various entities. Ms. Obeng-Kusi had no disclosures.
FROM JAMA DERMATOLOGY
Melanoma increasing, but is this overdiagnosis?
Melanoma has been increasing in incidence in the United States over the last few decades, but is this a true increase? Or is this a case of overdiagnosis, fueled by screening?
A new study argues the case for overdiagnosis.
commented lead author lead author Adewole Adamson, MD, an assistant professor of internal medicine, division of dermatology, at the University of Texas at Austin.
He posted this conclusion on Twitter after the study was published in JAMA Dermatology.
“The discrepancies in incidence and mortality trends found in this cohort study suggest considerable overdiagnosis of melanoma occurring among White patients in the U.S.,” the authors concluded.
They estimated that an estimated 59% of White women and 60% of White men with melanoma were overdiagnosed in 2014.
These results are similar to those from a recent study from Australia, which used a different method of assessing overdiagnosis. Those findings estimated that 54%-58% of melanoma cases represented overdiagnosis in Australia, Dr. Adamson noted.
“Our estimates shed light on the HUGE scope of this problem in the United States that we need to address,” Dr. Adamson commented on Twitter. “Calls for screening for melanoma in the general public will only push these numbers higher, and make patients out of healthy people.”
“Screening the general population for melanoma has never been shown to save lives and likely is responsible for the increase in melanoma overdiagnosis,” Dr. Adamson said in an interview. “Screening average- and/or low-risk patients is of low value and the harms may outweigh the theoretical benefits.”
Screening programs should be directed to those who may derive the most benefit. “Screening should be limited to high-risk patients such as older White men, patients with a lot of atypical nevi, heavy sun exposure, fair skin, and red hair,” he said. “Just like for other cancers, such as breast, prostate, and colorectal, there should be clear guidelines as to which populations to screen, as well as when to start and when to stop screening.”
Overdiagnosis is defined as the diagnosis of cancer that would never have caused any symptoms or problems in a patient’s lifetime. But therein lies the problem, explained Dr. Adamson. “Because we do not know which early, screen-detected skin cancers would be destined to progress, we are obligated to treat all of them.” There is evidence to suggest that melanoma in situ is not an obligate precursor lesion to invasive melanoma, similar to the situation in which not all ductal carcinoma in situ leads to invasive breast cancer. “It is possible that less aggressive management strategies could be the subject of future studies,” he said.
Patients out of healthy people
For their study, Dr. Adamson and colleagues compared rates of melanoma among White and Black patients. Melanoma is much less common among Black individuals, and they are also less likely to be screened. Additionally, screening rates among Black patients have remained more or less the same over the last decades, whereas screening has increased in White patients.
The team used trends in mortality as a result of melanoma in Black patients as a marker for improvements in medical care. From this, they estimated the expected mortality trends in White patients if medical care had not improved. This served as a marker for the change in true cancer occurrence. Overdiagnosis was calculated as the difference between observed incidence and estimated true cancer occurrence.
The incidence of melanoma rose dramatically among White patients from 1975 to 2014, increasing about fourfold in White women (incidence rate ratio, 4.01) and sixfold in White men (IRR, 5.97).
At the same time, there was much smaller increase (of less than 25%) in the incidence of melanoma in both Black women and Black men.
In that time period, melanoma-related mortality decreased approximately 25% in Black women and men; it remained stable in White women, but increased almost 50% in White men.
Had medical care not improved, estimated mortality would have increased 60% in White women and more than doubled in White men, the authors assert.
Guidelines needed
“Recognizing and addressing overdiagnosis is important,” said Anthony J. Olszanski, MD, RPh, associate professor, department of hematology/oncology at Fox Chase Cancer Center, Philadelphia, who was approached for comment on the paper.
That said, Dr. Olszanski noted that this particular study has important limitations. “It is, by nature, a retrospective study using data from the [Surveillance, Epidemiology, and End Results] database registry, limited to patients only in the U.S., and uses a control group of Black patients to estimate overdiagnosis in White patients. These important factors can certainly influence their findings. However, the paper also notes that White men have realized a true increase in diagnosis, backed by a notable increase in mortality.”
The findings should and do raise a number of provocative questions, Dr. Olszanski emphasized. “Should we curtail public screening? Should we mandate revised guidelines for biopsies or pathologic diagnosis?
“As a medical oncologist,” he continued, “I treat patients who clearly do not have benign disease and so it is easy for me to be biased toward aggressive screening. However, it is my opinion that we should develop guidelines aimed at lessening this apparent overdiagnosis.”
These guidelines should be based on prospective studies and would better define which lesions are most suspect and should be biopsied, which are rational for ongoing surveillance, and what pathologic features are most consistent with melanoma, he noted. “We also need to continue to educate the public, as all too often I see the patient who ignored a lesion that was changing over time. A changing lesion requires medical attention. Importantly, we likewise need to improve our commitment in educating the public about the risks of excessive ultraviolet radiation exposure and how to avoid it, as prevention continues to be a most prudent course.”
Screening catches disease early
Another expert approached for comment emphasized that identifying melanomas early on may prevent the need for aggressive therapy. “Many primary melanomas in the U.S. are diagnosed now at an early stage and are cured with surgery, and that hardly constitutes overdiagnosis,” said Jeffrey S. Weber, MD, PhD, deputy director of the Perlmutter Cancer Center and codirector of the melanoma research program, New York University Langone Health.
“In addition, the death rate from melanoma is likely decreased due to the advent of more effective therapies for metastatic disease, and the increasing use of adjuvant immune and targeted therapies that are highly effective at preventing relapse and undoubtedly at prolonging survival, but they have been approved only since 2017-2018,” he added.
This study was supported in part by the Robert Wood Johnson Foundation. Dr. Adamson and Dr. Olszanski disclosed no relevant financial relationships. Dr. Weber disclosed relationships with numerous pharmaceutical companies and holds equity in CytoMx, Biond, Neximmune, and Immunimax.
A version of this article first appeared on Medscape.com.
Melanoma has been increasing in incidence in the United States over the last few decades, but is this a true increase? Or is this a case of overdiagnosis, fueled by screening?
A new study argues the case for overdiagnosis.
commented lead author lead author Adewole Adamson, MD, an assistant professor of internal medicine, division of dermatology, at the University of Texas at Austin.
He posted this conclusion on Twitter after the study was published in JAMA Dermatology.
“The discrepancies in incidence and mortality trends found in this cohort study suggest considerable overdiagnosis of melanoma occurring among White patients in the U.S.,” the authors concluded.
They estimated that an estimated 59% of White women and 60% of White men with melanoma were overdiagnosed in 2014.
These results are similar to those from a recent study from Australia, which used a different method of assessing overdiagnosis. Those findings estimated that 54%-58% of melanoma cases represented overdiagnosis in Australia, Dr. Adamson noted.
“Our estimates shed light on the HUGE scope of this problem in the United States that we need to address,” Dr. Adamson commented on Twitter. “Calls for screening for melanoma in the general public will only push these numbers higher, and make patients out of healthy people.”
“Screening the general population for melanoma has never been shown to save lives and likely is responsible for the increase in melanoma overdiagnosis,” Dr. Adamson said in an interview. “Screening average- and/or low-risk patients is of low value and the harms may outweigh the theoretical benefits.”
Screening programs should be directed to those who may derive the most benefit. “Screening should be limited to high-risk patients such as older White men, patients with a lot of atypical nevi, heavy sun exposure, fair skin, and red hair,” he said. “Just like for other cancers, such as breast, prostate, and colorectal, there should be clear guidelines as to which populations to screen, as well as when to start and when to stop screening.”
Overdiagnosis is defined as the diagnosis of cancer that would never have caused any symptoms or problems in a patient’s lifetime. But therein lies the problem, explained Dr. Adamson. “Because we do not know which early, screen-detected skin cancers would be destined to progress, we are obligated to treat all of them.” There is evidence to suggest that melanoma in situ is not an obligate precursor lesion to invasive melanoma, similar to the situation in which not all ductal carcinoma in situ leads to invasive breast cancer. “It is possible that less aggressive management strategies could be the subject of future studies,” he said.
Patients out of healthy people
For their study, Dr. Adamson and colleagues compared rates of melanoma among White and Black patients. Melanoma is much less common among Black individuals, and they are also less likely to be screened. Additionally, screening rates among Black patients have remained more or less the same over the last decades, whereas screening has increased in White patients.
The team used trends in mortality as a result of melanoma in Black patients as a marker for improvements in medical care. From this, they estimated the expected mortality trends in White patients if medical care had not improved. This served as a marker for the change in true cancer occurrence. Overdiagnosis was calculated as the difference between observed incidence and estimated true cancer occurrence.
The incidence of melanoma rose dramatically among White patients from 1975 to 2014, increasing about fourfold in White women (incidence rate ratio, 4.01) and sixfold in White men (IRR, 5.97).
At the same time, there was much smaller increase (of less than 25%) in the incidence of melanoma in both Black women and Black men.
In that time period, melanoma-related mortality decreased approximately 25% in Black women and men; it remained stable in White women, but increased almost 50% in White men.
Had medical care not improved, estimated mortality would have increased 60% in White women and more than doubled in White men, the authors assert.
Guidelines needed
“Recognizing and addressing overdiagnosis is important,” said Anthony J. Olszanski, MD, RPh, associate professor, department of hematology/oncology at Fox Chase Cancer Center, Philadelphia, who was approached for comment on the paper.
That said, Dr. Olszanski noted that this particular study has important limitations. “It is, by nature, a retrospective study using data from the [Surveillance, Epidemiology, and End Results] database registry, limited to patients only in the U.S., and uses a control group of Black patients to estimate overdiagnosis in White patients. These important factors can certainly influence their findings. However, the paper also notes that White men have realized a true increase in diagnosis, backed by a notable increase in mortality.”
The findings should and do raise a number of provocative questions, Dr. Olszanski emphasized. “Should we curtail public screening? Should we mandate revised guidelines for biopsies or pathologic diagnosis?
“As a medical oncologist,” he continued, “I treat patients who clearly do not have benign disease and so it is easy for me to be biased toward aggressive screening. However, it is my opinion that we should develop guidelines aimed at lessening this apparent overdiagnosis.”
These guidelines should be based on prospective studies and would better define which lesions are most suspect and should be biopsied, which are rational for ongoing surveillance, and what pathologic features are most consistent with melanoma, he noted. “We also need to continue to educate the public, as all too often I see the patient who ignored a lesion that was changing over time. A changing lesion requires medical attention. Importantly, we likewise need to improve our commitment in educating the public about the risks of excessive ultraviolet radiation exposure and how to avoid it, as prevention continues to be a most prudent course.”
Screening catches disease early
Another expert approached for comment emphasized that identifying melanomas early on may prevent the need for aggressive therapy. “Many primary melanomas in the U.S. are diagnosed now at an early stage and are cured with surgery, and that hardly constitutes overdiagnosis,” said Jeffrey S. Weber, MD, PhD, deputy director of the Perlmutter Cancer Center and codirector of the melanoma research program, New York University Langone Health.
“In addition, the death rate from melanoma is likely decreased due to the advent of more effective therapies for metastatic disease, and the increasing use of adjuvant immune and targeted therapies that are highly effective at preventing relapse and undoubtedly at prolonging survival, but they have been approved only since 2017-2018,” he added.
This study was supported in part by the Robert Wood Johnson Foundation. Dr. Adamson and Dr. Olszanski disclosed no relevant financial relationships. Dr. Weber disclosed relationships with numerous pharmaceutical companies and holds equity in CytoMx, Biond, Neximmune, and Immunimax.
A version of this article first appeared on Medscape.com.
Melanoma has been increasing in incidence in the United States over the last few decades, but is this a true increase? Or is this a case of overdiagnosis, fueled by screening?
A new study argues the case for overdiagnosis.
commented lead author lead author Adewole Adamson, MD, an assistant professor of internal medicine, division of dermatology, at the University of Texas at Austin.
He posted this conclusion on Twitter after the study was published in JAMA Dermatology.
“The discrepancies in incidence and mortality trends found in this cohort study suggest considerable overdiagnosis of melanoma occurring among White patients in the U.S.,” the authors concluded.
They estimated that an estimated 59% of White women and 60% of White men with melanoma were overdiagnosed in 2014.
These results are similar to those from a recent study from Australia, which used a different method of assessing overdiagnosis. Those findings estimated that 54%-58% of melanoma cases represented overdiagnosis in Australia, Dr. Adamson noted.
“Our estimates shed light on the HUGE scope of this problem in the United States that we need to address,” Dr. Adamson commented on Twitter. “Calls for screening for melanoma in the general public will only push these numbers higher, and make patients out of healthy people.”
“Screening the general population for melanoma has never been shown to save lives and likely is responsible for the increase in melanoma overdiagnosis,” Dr. Adamson said in an interview. “Screening average- and/or low-risk patients is of low value and the harms may outweigh the theoretical benefits.”
Screening programs should be directed to those who may derive the most benefit. “Screening should be limited to high-risk patients such as older White men, patients with a lot of atypical nevi, heavy sun exposure, fair skin, and red hair,” he said. “Just like for other cancers, such as breast, prostate, and colorectal, there should be clear guidelines as to which populations to screen, as well as when to start and when to stop screening.”
Overdiagnosis is defined as the diagnosis of cancer that would never have caused any symptoms or problems in a patient’s lifetime. But therein lies the problem, explained Dr. Adamson. “Because we do not know which early, screen-detected skin cancers would be destined to progress, we are obligated to treat all of them.” There is evidence to suggest that melanoma in situ is not an obligate precursor lesion to invasive melanoma, similar to the situation in which not all ductal carcinoma in situ leads to invasive breast cancer. “It is possible that less aggressive management strategies could be the subject of future studies,” he said.
Patients out of healthy people
For their study, Dr. Adamson and colleagues compared rates of melanoma among White and Black patients. Melanoma is much less common among Black individuals, and they are also less likely to be screened. Additionally, screening rates among Black patients have remained more or less the same over the last decades, whereas screening has increased in White patients.
The team used trends in mortality as a result of melanoma in Black patients as a marker for improvements in medical care. From this, they estimated the expected mortality trends in White patients if medical care had not improved. This served as a marker for the change in true cancer occurrence. Overdiagnosis was calculated as the difference between observed incidence and estimated true cancer occurrence.
The incidence of melanoma rose dramatically among White patients from 1975 to 2014, increasing about fourfold in White women (incidence rate ratio, 4.01) and sixfold in White men (IRR, 5.97).
At the same time, there was much smaller increase (of less than 25%) in the incidence of melanoma in both Black women and Black men.
In that time period, melanoma-related mortality decreased approximately 25% in Black women and men; it remained stable in White women, but increased almost 50% in White men.
Had medical care not improved, estimated mortality would have increased 60% in White women and more than doubled in White men, the authors assert.
Guidelines needed
“Recognizing and addressing overdiagnosis is important,” said Anthony J. Olszanski, MD, RPh, associate professor, department of hematology/oncology at Fox Chase Cancer Center, Philadelphia, who was approached for comment on the paper.
That said, Dr. Olszanski noted that this particular study has important limitations. “It is, by nature, a retrospective study using data from the [Surveillance, Epidemiology, and End Results] database registry, limited to patients only in the U.S., and uses a control group of Black patients to estimate overdiagnosis in White patients. These important factors can certainly influence their findings. However, the paper also notes that White men have realized a true increase in diagnosis, backed by a notable increase in mortality.”
The findings should and do raise a number of provocative questions, Dr. Olszanski emphasized. “Should we curtail public screening? Should we mandate revised guidelines for biopsies or pathologic diagnosis?
“As a medical oncologist,” he continued, “I treat patients who clearly do not have benign disease and so it is easy for me to be biased toward aggressive screening. However, it is my opinion that we should develop guidelines aimed at lessening this apparent overdiagnosis.”
These guidelines should be based on prospective studies and would better define which lesions are most suspect and should be biopsied, which are rational for ongoing surveillance, and what pathologic features are most consistent with melanoma, he noted. “We also need to continue to educate the public, as all too often I see the patient who ignored a lesion that was changing over time. A changing lesion requires medical attention. Importantly, we likewise need to improve our commitment in educating the public about the risks of excessive ultraviolet radiation exposure and how to avoid it, as prevention continues to be a most prudent course.”
Screening catches disease early
Another expert approached for comment emphasized that identifying melanomas early on may prevent the need for aggressive therapy. “Many primary melanomas in the U.S. are diagnosed now at an early stage and are cured with surgery, and that hardly constitutes overdiagnosis,” said Jeffrey S. Weber, MD, PhD, deputy director of the Perlmutter Cancer Center and codirector of the melanoma research program, New York University Langone Health.
“In addition, the death rate from melanoma is likely decreased due to the advent of more effective therapies for metastatic disease, and the increasing use of adjuvant immune and targeted therapies that are highly effective at preventing relapse and undoubtedly at prolonging survival, but they have been approved only since 2017-2018,” he added.
This study was supported in part by the Robert Wood Johnson Foundation. Dr. Adamson and Dr. Olszanski disclosed no relevant financial relationships. Dr. Weber disclosed relationships with numerous pharmaceutical companies and holds equity in CytoMx, Biond, Neximmune, and Immunimax.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Few new cancer drugs replace current standards of care
, a new analysis shows.
Of more than 200 agents evaluated, most (42%) received approval as second-, third-, or later-line therapies.
“While there is justified enthusiasm for the high volume of new cancer drug approvals in oncology and malignant hematology, these approvals must be evaluated in the context of their use,” the authors note in a report published online March 15 in JAMA Network Open. Later-line drugs may, for instance, “benefit patients with few alternatives but also add to cost of care and further delay palliative and comfort services” compared to first-line therapies, which may alter “the treatment paradigm for a certain indication.”
The U.S. Food and Drug Administration approves several new cancer drugs each month, but it’s not clear how many transform the treatment landscape.
To investigate, David Benjamin, MD, with the Division of Hematology and Oncology, University of California, Irvine, and colleagues evaluated all 207 cancer drugs approved in the U.S. between May 1, 2016 and May 31, 2021.
The researchers found that only 28 drugs (14%) displaced the prior first-line standard of care for an indication.
Examples of these cancer drugs include alectinib for anaplastic lymphoma kinase rearrangement–positive metastatic non–small cell lung cancer (NSCLC), osimertinib for epidermal growth factor receptor exon 19 deletion or exon 21 L858R substitution NSCLC, atezolizumab plus bevacizumab for unresectable or metastatic hepatocellular carcinoma, and cabozantinib for advanced kidney cancer.
A total of 32 drugs (15%) were approved as first-line alternatives or new drugs. These drugs were approved for use in the first-line setting but did not necessarily replace the standard of care at the time of approval or were first-of-their-class therapies.
Examples of these drug approvals include apalutamide for nonmetastatic castrate-resistant prostate cancer, tepotinib for metastatic MET exon 14-skipping NSCLC, and avapritinib for unresectable or metastatic gastrointestinal stromal tumor with platelet-derived growth factor receptor alpha exon 18 variant, including D842V variant.
A total of 61 drugs (29%) were approved as add-on therapies for use in combination with a previously approved therapy or in the adjuvant or maintenance settings. These drugs “can only increase the cost of care,” the study team says.
Most new approvals (n = 86) were for use in second-, third- or later-line settings, often for patients for whom other treatment options had been exhausted.
The authors highlight disparities among approvals based on tumor type. Lung-related tumors received the most approvals (n = 37), followed by genitourinary tumors (n = 28), leukemia (n = 25), lymphoma (n = 22), breast cancer (n = 19), and gastrointestinal cancers (n = 14).
The authors note that cancer drugs considered new standards of care or approved as first-line setting alternatives could “provide market competition and work to lower cancer drug prices.”
The study was funded by a grant from Arnold Ventures.
A version of this article first appeared on Medscape.com.
, a new analysis shows.
Of more than 200 agents evaluated, most (42%) received approval as second-, third-, or later-line therapies.
“While there is justified enthusiasm for the high volume of new cancer drug approvals in oncology and malignant hematology, these approvals must be evaluated in the context of their use,” the authors note in a report published online March 15 in JAMA Network Open. Later-line drugs may, for instance, “benefit patients with few alternatives but also add to cost of care and further delay palliative and comfort services” compared to first-line therapies, which may alter “the treatment paradigm for a certain indication.”
The U.S. Food and Drug Administration approves several new cancer drugs each month, but it’s not clear how many transform the treatment landscape.
To investigate, David Benjamin, MD, with the Division of Hematology and Oncology, University of California, Irvine, and colleagues evaluated all 207 cancer drugs approved in the U.S. between May 1, 2016 and May 31, 2021.
The researchers found that only 28 drugs (14%) displaced the prior first-line standard of care for an indication.
Examples of these cancer drugs include alectinib for anaplastic lymphoma kinase rearrangement–positive metastatic non–small cell lung cancer (NSCLC), osimertinib for epidermal growth factor receptor exon 19 deletion or exon 21 L858R substitution NSCLC, atezolizumab plus bevacizumab for unresectable or metastatic hepatocellular carcinoma, and cabozantinib for advanced kidney cancer.
A total of 32 drugs (15%) were approved as first-line alternatives or new drugs. These drugs were approved for use in the first-line setting but did not necessarily replace the standard of care at the time of approval or were first-of-their-class therapies.
Examples of these drug approvals include apalutamide for nonmetastatic castrate-resistant prostate cancer, tepotinib for metastatic MET exon 14-skipping NSCLC, and avapritinib for unresectable or metastatic gastrointestinal stromal tumor with platelet-derived growth factor receptor alpha exon 18 variant, including D842V variant.
A total of 61 drugs (29%) were approved as add-on therapies for use in combination with a previously approved therapy or in the adjuvant or maintenance settings. These drugs “can only increase the cost of care,” the study team says.
Most new approvals (n = 86) were for use in second-, third- or later-line settings, often for patients for whom other treatment options had been exhausted.
The authors highlight disparities among approvals based on tumor type. Lung-related tumors received the most approvals (n = 37), followed by genitourinary tumors (n = 28), leukemia (n = 25), lymphoma (n = 22), breast cancer (n = 19), and gastrointestinal cancers (n = 14).
The authors note that cancer drugs considered new standards of care or approved as first-line setting alternatives could “provide market competition and work to lower cancer drug prices.”
The study was funded by a grant from Arnold Ventures.
A version of this article first appeared on Medscape.com.
, a new analysis shows.
Of more than 200 agents evaluated, most (42%) received approval as second-, third-, or later-line therapies.
“While there is justified enthusiasm for the high volume of new cancer drug approvals in oncology and malignant hematology, these approvals must be evaluated in the context of their use,” the authors note in a report published online March 15 in JAMA Network Open. Later-line drugs may, for instance, “benefit patients with few alternatives but also add to cost of care and further delay palliative and comfort services” compared to first-line therapies, which may alter “the treatment paradigm for a certain indication.”
The U.S. Food and Drug Administration approves several new cancer drugs each month, but it’s not clear how many transform the treatment landscape.
To investigate, David Benjamin, MD, with the Division of Hematology and Oncology, University of California, Irvine, and colleagues evaluated all 207 cancer drugs approved in the U.S. between May 1, 2016 and May 31, 2021.
The researchers found that only 28 drugs (14%) displaced the prior first-line standard of care for an indication.
Examples of these cancer drugs include alectinib for anaplastic lymphoma kinase rearrangement–positive metastatic non–small cell lung cancer (NSCLC), osimertinib for epidermal growth factor receptor exon 19 deletion or exon 21 L858R substitution NSCLC, atezolizumab plus bevacizumab for unresectable or metastatic hepatocellular carcinoma, and cabozantinib for advanced kidney cancer.
A total of 32 drugs (15%) were approved as first-line alternatives or new drugs. These drugs were approved for use in the first-line setting but did not necessarily replace the standard of care at the time of approval or were first-of-their-class therapies.
Examples of these drug approvals include apalutamide for nonmetastatic castrate-resistant prostate cancer, tepotinib for metastatic MET exon 14-skipping NSCLC, and avapritinib for unresectable or metastatic gastrointestinal stromal tumor with platelet-derived growth factor receptor alpha exon 18 variant, including D842V variant.
A total of 61 drugs (29%) were approved as add-on therapies for use in combination with a previously approved therapy or in the adjuvant or maintenance settings. These drugs “can only increase the cost of care,” the study team says.
Most new approvals (n = 86) were for use in second-, third- or later-line settings, often for patients for whom other treatment options had been exhausted.
The authors highlight disparities among approvals based on tumor type. Lung-related tumors received the most approvals (n = 37), followed by genitourinary tumors (n = 28), leukemia (n = 25), lymphoma (n = 22), breast cancer (n = 19), and gastrointestinal cancers (n = 14).
The authors note that cancer drugs considered new standards of care or approved as first-line setting alternatives could “provide market competition and work to lower cancer drug prices.”
The study was funded by a grant from Arnold Ventures.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
FDA approves new immunotherapy combo for metastatic melanoma
in adults and children 12 years or older, according to the drug’s manufacturer, Bristol-Myers Squibb.
Approval was based on the company’s RELATIVITY-047 trial, which found a median progression-free survival (PFS) of 10.1 months among 355 patients randomly assigned to the combination therapy compared with 4.6 months among 359 patients who received nivolumab alone (hazard ratio, 0.75; P = .0055).
In the combination therapy group, 18.9% of patients reported a grade 3/4 drug-related adverse event, compared with 9.7% in the nivolumab group; 14.6% of patients in the combination group had drug-related adverse events leading to discontinuation versus 6.7% of those receiving monotherapy, the company noted in a press release.
Relatlimab is the company’s third immune checkpoint inhibitor to reach the U.S. market, joining the PD-1 inhibitor nivolumab and the CTLA-4 blocker ipilimumab. Relatlimab targets LAG-3, a cell-surface receptor found on activated CD4+ T cells.
Nivolumab plus ipilimumab is currently the standard of care for previously untreated metastatic or inoperable melanoma. Both combinations produce similar PFS, but the incidence of grade 3/4 adverse events is higher with ipilimumab, according to a Jan. 6, 2022, editorial in the New England Journal of Medicine.
Musculoskeletal pain, fatigue, rash, pruritus, and diarrhea were the most common adverse reactions with combination nivolumab/relatlimab, occurring in 20% or more of RELATIVITY-047 trial participants.
Adrenal insufficiency, anemia, colitis, pneumonia, and myocardial infarction were the most frequent serious adverse reactions, but each occurred in less than 2% of patients. There were three fatal adverse events in the trial caused by hemophagocytic lymphohistiocytosis, acute lung edema, and pneumonitis.
The approved dosage is 480 mg nivolumab and 160 mg relatlimab administered intravenously every 4 weeks.
A version of this article first appeared on Medscape.com.
in adults and children 12 years or older, according to the drug’s manufacturer, Bristol-Myers Squibb.
Approval was based on the company’s RELATIVITY-047 trial, which found a median progression-free survival (PFS) of 10.1 months among 355 patients randomly assigned to the combination therapy compared with 4.6 months among 359 patients who received nivolumab alone (hazard ratio, 0.75; P = .0055).
In the combination therapy group, 18.9% of patients reported a grade 3/4 drug-related adverse event, compared with 9.7% in the nivolumab group; 14.6% of patients in the combination group had drug-related adverse events leading to discontinuation versus 6.7% of those receiving monotherapy, the company noted in a press release.
Relatlimab is the company’s third immune checkpoint inhibitor to reach the U.S. market, joining the PD-1 inhibitor nivolumab and the CTLA-4 blocker ipilimumab. Relatlimab targets LAG-3, a cell-surface receptor found on activated CD4+ T cells.
Nivolumab plus ipilimumab is currently the standard of care for previously untreated metastatic or inoperable melanoma. Both combinations produce similar PFS, but the incidence of grade 3/4 adverse events is higher with ipilimumab, according to a Jan. 6, 2022, editorial in the New England Journal of Medicine.
Musculoskeletal pain, fatigue, rash, pruritus, and diarrhea were the most common adverse reactions with combination nivolumab/relatlimab, occurring in 20% or more of RELATIVITY-047 trial participants.
Adrenal insufficiency, anemia, colitis, pneumonia, and myocardial infarction were the most frequent serious adverse reactions, but each occurred in less than 2% of patients. There were three fatal adverse events in the trial caused by hemophagocytic lymphohistiocytosis, acute lung edema, and pneumonitis.
The approved dosage is 480 mg nivolumab and 160 mg relatlimab administered intravenously every 4 weeks.
A version of this article first appeared on Medscape.com.
in adults and children 12 years or older, according to the drug’s manufacturer, Bristol-Myers Squibb.
Approval was based on the company’s RELATIVITY-047 trial, which found a median progression-free survival (PFS) of 10.1 months among 355 patients randomly assigned to the combination therapy compared with 4.6 months among 359 patients who received nivolumab alone (hazard ratio, 0.75; P = .0055).
In the combination therapy group, 18.9% of patients reported a grade 3/4 drug-related adverse event, compared with 9.7% in the nivolumab group; 14.6% of patients in the combination group had drug-related adverse events leading to discontinuation versus 6.7% of those receiving monotherapy, the company noted in a press release.
Relatlimab is the company’s third immune checkpoint inhibitor to reach the U.S. market, joining the PD-1 inhibitor nivolumab and the CTLA-4 blocker ipilimumab. Relatlimab targets LAG-3, a cell-surface receptor found on activated CD4+ T cells.
Nivolumab plus ipilimumab is currently the standard of care for previously untreated metastatic or inoperable melanoma. Both combinations produce similar PFS, but the incidence of grade 3/4 adverse events is higher with ipilimumab, according to a Jan. 6, 2022, editorial in the New England Journal of Medicine.
Musculoskeletal pain, fatigue, rash, pruritus, and diarrhea were the most common adverse reactions with combination nivolumab/relatlimab, occurring in 20% or more of RELATIVITY-047 trial participants.
Adrenal insufficiency, anemia, colitis, pneumonia, and myocardial infarction were the most frequent serious adverse reactions, but each occurred in less than 2% of patients. There were three fatal adverse events in the trial caused by hemophagocytic lymphohistiocytosis, acute lung edema, and pneumonitis.
The approved dosage is 480 mg nivolumab and 160 mg relatlimab administered intravenously every 4 weeks.
A version of this article first appeared on Medscape.com.





