User login
A fish tale? More on that seafood, melanoma study
A recent study linking the consumption of fish to melanoma had sushi eaters looking up the number of the nearest dermatologist. But experts said the findings had several important limitations and that no one should change their diet based on the results.
“It wouldn’t impact my fish consumption at all,” said Sancy Leachman, MD, PhD, who directs the melanoma program at Oregon Health & Science University’s Knight Cancer Institute in Portland. “Fish are part of a healthy diet,” particularly if it replaces less healthy proteins such as beef.
Even the authors of the study advised caution when interpreting the findings.
“I wouldn’t encourage anyone to change their fish consumption habits just because of this paper,” said study leader Eunyoung Cho, ScD, an epidemiologist at Brown University, Providence, R.I. “Fish is cardioprotective and is related to reduced risk of developing certain cancers too.”
Solid findings or fishing expedition?
The study quickly generated headlines and was the most viewed article of the journal Cancer Causes & Control within a day of its publication.
Dr. Cho, who is on the editorial board of the journal, analyzed the results of a study funded by the National Institutes of Health and the Association for the Advancement of Retired Persons that began in the 1990s and explored possible links between fish consumption and developing different forms of cancer.
Previous research from this project had shown eating fish was associated with an increased risk of melanoma – but no other type of cancer.
Dr. Cho speculated that the risk is due to contaminants, such as mercury and arsenic, in fish. But she was curious to see if she could find a connection between the amount of fish consumed and the risk of developing skin cancer.
At the beginning of the study people reported how much fish they had consumed over the previous year, which varied widely by person. Then researchers tracked participants for 15 years, recording who developed cancer and who did not.
Dietary recall can be difficult; people often say they can’t remember what they ate yesterday, much less 1 year ago. Still, dietitian Clare Collins, PhD, of the University of Newcastle (Australia), noted that previous research showed that the dietary recall tool for this study is reliable.
NIH researchers never checked to see how their diet changed over time, as this was a study meant to observe changes in health status starting from a baseline point. The researchers assumed that the level of fish intake recorded at the beginning of the study remained steady.
Of 491,000 people tracked in the study, 5,000 developed malignant melanoma and another 3,300 developed melanoma cells on the skin surface. People who ate the most fish – an average of almost 43 g, or about 1.5 ounces, per day – were 23% more likely to develop melanoma than the lightest fish eaters, who averaged 3 g per day.
That risk is modest, Dr. Leachman said, especially for people with red hair who, she said, are 400% more likely to get melanoma than others. “A 23% increase, in the whole scheme of things, is not nearly as important as not getting sunburned if you’re a redhead,” Dr. Leachman said.
And what about the sun? Sun exposure is the principal risk factor for developing melanoma, and the researchers didn’t account for that factor. People who developed melanoma during the study may simply have spent more time in tanning beds or on the beach – or perhaps fishing and then eating their catch.
Dr. Cho and colleagues tried to consider the effects of solar radiation by looking at average sun exposure times in the places where the participants in the study were living when the research began. Using that framework, variation in sun exposure made no difference to melanoma risk, but Dr. Leachman said the technique isn’t foolproof.
“They assumed that they were going to get a certain amount of ultraviolet light just based on where they lived. We don’t know how long they lived there or if they really had ultraviolet exposure or not,” Dr. Leachman said. Someone in presumably less sunny Pennsylvania could get more sun than someone in sun-drenched Arizona depending on their lifestyle and habits.
The kind of study Dr. Cho published cannot account for individual behaviors related to sun exposure, a limitation her team readily acknowledges. Nor does it include information about moles or hair color – important considerations for skin cancers. It may be that redheads with moles who barely ate any fish developed melanoma at higher rates than anyone else, but the data don’t allow for that kind of analysis.
Dr. Cho’s group found that canned tuna and any type of fish that wasn’t fried were associated with a higher risk of developing melanoma, as people reported eating more of those products. However, people who said they ate greater quantities of fried fish had a lower risk of melanoma, a counterintuitive finding that she said warrants further research.
Given that the study showed only a modest chance of developing melanoma regardless of fish intake, and left so many questions unanswered, what was the point?
Other journals declined to publish this paper, Dr. Cho acknowledged, but she defended the article as a step toward better understanding the health impact of environmental contaminants in fish.
Dr. Leachman agreed. “These kinds of studies are very important to do. They have large data sets, where you can start to see trends that may be important,” she said. “They can help you identify things that might be related. These experiments are hypothesis generating.”
“I already published an article showing that total mercury level is related to skin cancer, and we know very well that in the U.S. fish consumption is the major source of mercury contamination,” Dr. Cho said. “So, I naturally thought that fish consumption may be associated with increased risk of skin cancer too.” Dr. Cho said she believed the findings confirm that hypothesis.
Dr. Cho said the next step would be to measure blood levels of different contaminants such as mercury and arsenic in people with melanoma, to determine which toxin is the biggest driver of melanoma. She said she plans to seek funding for that research.
Meanwhile, pass the salmon – but go light on the salt.
Dr. Cho and Dr. Leachman reported no relevant financial relationships. Dr. Cho is on the editorial board of Cancer Causes & Control.
A version of this article first appeared on Medscape.com.
A recent study linking the consumption of fish to melanoma had sushi eaters looking up the number of the nearest dermatologist. But experts said the findings had several important limitations and that no one should change their diet based on the results.
“It wouldn’t impact my fish consumption at all,” said Sancy Leachman, MD, PhD, who directs the melanoma program at Oregon Health & Science University’s Knight Cancer Institute in Portland. “Fish are part of a healthy diet,” particularly if it replaces less healthy proteins such as beef.
Even the authors of the study advised caution when interpreting the findings.
“I wouldn’t encourage anyone to change their fish consumption habits just because of this paper,” said study leader Eunyoung Cho, ScD, an epidemiologist at Brown University, Providence, R.I. “Fish is cardioprotective and is related to reduced risk of developing certain cancers too.”
Solid findings or fishing expedition?
The study quickly generated headlines and was the most viewed article of the journal Cancer Causes & Control within a day of its publication.
Dr. Cho, who is on the editorial board of the journal, analyzed the results of a study funded by the National Institutes of Health and the Association for the Advancement of Retired Persons that began in the 1990s and explored possible links between fish consumption and developing different forms of cancer.
Previous research from this project had shown eating fish was associated with an increased risk of melanoma – but no other type of cancer.
Dr. Cho speculated that the risk is due to contaminants, such as mercury and arsenic, in fish. But she was curious to see if she could find a connection between the amount of fish consumed and the risk of developing skin cancer.
At the beginning of the study people reported how much fish they had consumed over the previous year, which varied widely by person. Then researchers tracked participants for 15 years, recording who developed cancer and who did not.
Dietary recall can be difficult; people often say they can’t remember what they ate yesterday, much less 1 year ago. Still, dietitian Clare Collins, PhD, of the University of Newcastle (Australia), noted that previous research showed that the dietary recall tool for this study is reliable.
NIH researchers never checked to see how their diet changed over time, as this was a study meant to observe changes in health status starting from a baseline point. The researchers assumed that the level of fish intake recorded at the beginning of the study remained steady.
Of 491,000 people tracked in the study, 5,000 developed malignant melanoma and another 3,300 developed melanoma cells on the skin surface. People who ate the most fish – an average of almost 43 g, or about 1.5 ounces, per day – were 23% more likely to develop melanoma than the lightest fish eaters, who averaged 3 g per day.
That risk is modest, Dr. Leachman said, especially for people with red hair who, she said, are 400% more likely to get melanoma than others. “A 23% increase, in the whole scheme of things, is not nearly as important as not getting sunburned if you’re a redhead,” Dr. Leachman said.
And what about the sun? Sun exposure is the principal risk factor for developing melanoma, and the researchers didn’t account for that factor. People who developed melanoma during the study may simply have spent more time in tanning beds or on the beach – or perhaps fishing and then eating their catch.
Dr. Cho and colleagues tried to consider the effects of solar radiation by looking at average sun exposure times in the places where the participants in the study were living when the research began. Using that framework, variation in sun exposure made no difference to melanoma risk, but Dr. Leachman said the technique isn’t foolproof.
“They assumed that they were going to get a certain amount of ultraviolet light just based on where they lived. We don’t know how long they lived there or if they really had ultraviolet exposure or not,” Dr. Leachman said. Someone in presumably less sunny Pennsylvania could get more sun than someone in sun-drenched Arizona depending on their lifestyle and habits.
The kind of study Dr. Cho published cannot account for individual behaviors related to sun exposure, a limitation her team readily acknowledges. Nor does it include information about moles or hair color – important considerations for skin cancers. It may be that redheads with moles who barely ate any fish developed melanoma at higher rates than anyone else, but the data don’t allow for that kind of analysis.
Dr. Cho’s group found that canned tuna and any type of fish that wasn’t fried were associated with a higher risk of developing melanoma, as people reported eating more of those products. However, people who said they ate greater quantities of fried fish had a lower risk of melanoma, a counterintuitive finding that she said warrants further research.
Given that the study showed only a modest chance of developing melanoma regardless of fish intake, and left so many questions unanswered, what was the point?
Other journals declined to publish this paper, Dr. Cho acknowledged, but she defended the article as a step toward better understanding the health impact of environmental contaminants in fish.
Dr. Leachman agreed. “These kinds of studies are very important to do. They have large data sets, where you can start to see trends that may be important,” she said. “They can help you identify things that might be related. These experiments are hypothesis generating.”
“I already published an article showing that total mercury level is related to skin cancer, and we know very well that in the U.S. fish consumption is the major source of mercury contamination,” Dr. Cho said. “So, I naturally thought that fish consumption may be associated with increased risk of skin cancer too.” Dr. Cho said she believed the findings confirm that hypothesis.
Dr. Cho said the next step would be to measure blood levels of different contaminants such as mercury and arsenic in people with melanoma, to determine which toxin is the biggest driver of melanoma. She said she plans to seek funding for that research.
Meanwhile, pass the salmon – but go light on the salt.
Dr. Cho and Dr. Leachman reported no relevant financial relationships. Dr. Cho is on the editorial board of Cancer Causes & Control.
A version of this article first appeared on Medscape.com.
A recent study linking the consumption of fish to melanoma had sushi eaters looking up the number of the nearest dermatologist. But experts said the findings had several important limitations and that no one should change their diet based on the results.
“It wouldn’t impact my fish consumption at all,” said Sancy Leachman, MD, PhD, who directs the melanoma program at Oregon Health & Science University’s Knight Cancer Institute in Portland. “Fish are part of a healthy diet,” particularly if it replaces less healthy proteins such as beef.
Even the authors of the study advised caution when interpreting the findings.
“I wouldn’t encourage anyone to change their fish consumption habits just because of this paper,” said study leader Eunyoung Cho, ScD, an epidemiologist at Brown University, Providence, R.I. “Fish is cardioprotective and is related to reduced risk of developing certain cancers too.”
Solid findings or fishing expedition?
The study quickly generated headlines and was the most viewed article of the journal Cancer Causes & Control within a day of its publication.
Dr. Cho, who is on the editorial board of the journal, analyzed the results of a study funded by the National Institutes of Health and the Association for the Advancement of Retired Persons that began in the 1990s and explored possible links between fish consumption and developing different forms of cancer.
Previous research from this project had shown eating fish was associated with an increased risk of melanoma – but no other type of cancer.
Dr. Cho speculated that the risk is due to contaminants, such as mercury and arsenic, in fish. But she was curious to see if she could find a connection between the amount of fish consumed and the risk of developing skin cancer.
At the beginning of the study people reported how much fish they had consumed over the previous year, which varied widely by person. Then researchers tracked participants for 15 years, recording who developed cancer and who did not.
Dietary recall can be difficult; people often say they can’t remember what they ate yesterday, much less 1 year ago. Still, dietitian Clare Collins, PhD, of the University of Newcastle (Australia), noted that previous research showed that the dietary recall tool for this study is reliable.
NIH researchers never checked to see how their diet changed over time, as this was a study meant to observe changes in health status starting from a baseline point. The researchers assumed that the level of fish intake recorded at the beginning of the study remained steady.
Of 491,000 people tracked in the study, 5,000 developed malignant melanoma and another 3,300 developed melanoma cells on the skin surface. People who ate the most fish – an average of almost 43 g, or about 1.5 ounces, per day – were 23% more likely to develop melanoma than the lightest fish eaters, who averaged 3 g per day.
That risk is modest, Dr. Leachman said, especially for people with red hair who, she said, are 400% more likely to get melanoma than others. “A 23% increase, in the whole scheme of things, is not nearly as important as not getting sunburned if you’re a redhead,” Dr. Leachman said.
And what about the sun? Sun exposure is the principal risk factor for developing melanoma, and the researchers didn’t account for that factor. People who developed melanoma during the study may simply have spent more time in tanning beds or on the beach – or perhaps fishing and then eating their catch.
Dr. Cho and colleagues tried to consider the effects of solar radiation by looking at average sun exposure times in the places where the participants in the study were living when the research began. Using that framework, variation in sun exposure made no difference to melanoma risk, but Dr. Leachman said the technique isn’t foolproof.
“They assumed that they were going to get a certain amount of ultraviolet light just based on where they lived. We don’t know how long they lived there or if they really had ultraviolet exposure or not,” Dr. Leachman said. Someone in presumably less sunny Pennsylvania could get more sun than someone in sun-drenched Arizona depending on their lifestyle and habits.
The kind of study Dr. Cho published cannot account for individual behaviors related to sun exposure, a limitation her team readily acknowledges. Nor does it include information about moles or hair color – important considerations for skin cancers. It may be that redheads with moles who barely ate any fish developed melanoma at higher rates than anyone else, but the data don’t allow for that kind of analysis.
Dr. Cho’s group found that canned tuna and any type of fish that wasn’t fried were associated with a higher risk of developing melanoma, as people reported eating more of those products. However, people who said they ate greater quantities of fried fish had a lower risk of melanoma, a counterintuitive finding that she said warrants further research.
Given that the study showed only a modest chance of developing melanoma regardless of fish intake, and left so many questions unanswered, what was the point?
Other journals declined to publish this paper, Dr. Cho acknowledged, but she defended the article as a step toward better understanding the health impact of environmental contaminants in fish.
Dr. Leachman agreed. “These kinds of studies are very important to do. They have large data sets, where you can start to see trends that may be important,” she said. “They can help you identify things that might be related. These experiments are hypothesis generating.”
“I already published an article showing that total mercury level is related to skin cancer, and we know very well that in the U.S. fish consumption is the major source of mercury contamination,” Dr. Cho said. “So, I naturally thought that fish consumption may be associated with increased risk of skin cancer too.” Dr. Cho said she believed the findings confirm that hypothesis.
Dr. Cho said the next step would be to measure blood levels of different contaminants such as mercury and arsenic in people with melanoma, to determine which toxin is the biggest driver of melanoma. She said she plans to seek funding for that research.
Meanwhile, pass the salmon – but go light on the salt.
Dr. Cho and Dr. Leachman reported no relevant financial relationships. Dr. Cho is on the editorial board of Cancer Causes & Control.
A version of this article first appeared on Medscape.com.
Surgical site infections not increased in immunocompromised patients after Mohs surgery
, suggesting that antibiotic prophylaxis, which is often used for these patients, may not be necessary, according to new research.
The retrospective cohort study found that “immunosuppressed patients had similar infection rates as immunocompetent patients following Mohs micrographic surgery,” first author Tuyet A. Nguyen, MD, of the department of dermatology, Cedars-Sinai Medical Center, Los Angeles, told this news organization.
“Therefore, antibiotic prescribing patterns should not change simply due to immunosuppression. Furthermore, immunosuppressed patients appear to respond well to antibiotics and recover similarly to immunocompetent patients,” she said.
The study was presented at the annual meeting of the American College of Mohs Surgery.
Mohs surgery is increasingly being performed for patients who are immunosuppressed because of the higher incidence of skin cancer in this group of patients and their higher risk of more aggressive skin cancers.
Overall, the rate of surgical site infections following Mohs surgery generally ranges from 0.5% to 2.4%. However, research is lacking on the risk among patients who are immunosuppressed and on how effective the use of prophylactic antibiotics is for these patients.
For the retrospective study, Dr. Nguyen and her colleagues evaluated data on 5,886 patients who underwent Mohs surgery at Cedars-Sinai between October 2014 and August 2021. Among these patients, 741 (12.6%) were immunocompromised.
Causes of immunosuppression in the cohort included the following: immunosuppression after transplant surgery; having HIV, chronic myeloid leukemia, multiple myeloma, or other hematogenous forms of immunosuppression; or immunosuppression related to other conditions, such as chronic inflammatory diseases.
Overall, postprocedural infections occurred in 1.6% (95) of patients, a rate that mirrors that of the general population, Dr. Nguyen noted. No significant differences in surgical site infection rates were observed between immunocompromised patients (2.1%, n = 15) and those who were immunocompetent (1.6%, n = 80; P = .30).
Importantly, among those who were immunocompromised, the rates of infection were not significantly different between those who did receive antibiotics (3.0%, n = 8) and those who did not receive antibiotics (1.5%, n = 7; P = .19).
The lack of a difference in surgical site infection rates among those who did and those who did not receive antibiotics extended to the entire study population (2.0% vs. 1.4%; P = .12).
The study cohort mainly comprised immunosuppressed transplant patients, notably, heart, lung, and kidney transplant patients. However, “even in this population, we did not see a higher rate of infection,” senior author Nima M. Gharavi, MD, PhD, director of dermatologic surgery and Mohs micrographic surgery and associate professor of medicine and pathology and laboratory medicine at Cedars-Sinai Medical Center, said in an interview.
Yet the risk of infection among those patients has been shown to be high and of consequence. Data indicate that infections account for 13%-16% of deaths among kidney and heart transplant patients and up to 21% of deaths among lung transplant patients. The rate of mortality appears to parallel the level of immunosuppression, Dr. Nguyen explained.
Furthermore, up to 25% of patients who undergo heart and lung transplantation develop bacteremia.
In terms of why worse infections or bacteremia surgeries may not occur in association with Mohs, Dr. Nguyen speculated that, as opposed to other surgeries, those involving the skin may benefit from unique defense mechanisms.
“The skin is a complex system in its defense against foreign pathogens and infectious agents,” she explained during her presentation. “There is the physical barrier, the antimicrobial peptides, and an adaptive as well as innate immune response.”
“In immunosuppressed patients, with the decrease in adaptive immunity, it’s possible this loss is less important because the skin has such a robust immune system in general.”
In her presentation, Dr. Nguyen noted that “further studies are necessary to investigate why patients aren’t presenting with greater severity, and we plan to try to investigate whether the unique nature of skin-mediated immunity makes this organ less susceptible to severe or life-threatening infections in patients on immunosuppression.”
Of note, the rate of prophylactic antibiotic prescriptions was no higher for those who were and those who were not immunosuppressed (37.9% vs. 34.1%; P = .14), which Dr. Nguyen said is consistent with recommendations.
“Immunosuppression is not an indication for antibiotic use, and hence, we did not have a higher rate of antibiotics use in this population,” she told this news organization. However, a 2021 ACMS survey found that a high percentage of Mohs surgeons prescribe antibiotics for procedures in which antibiotics are not indicated so as to reduce the risk of infections and that immunosuppression is a common reason for doing so.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggesting that antibiotic prophylaxis, which is often used for these patients, may not be necessary, according to new research.
The retrospective cohort study found that “immunosuppressed patients had similar infection rates as immunocompetent patients following Mohs micrographic surgery,” first author Tuyet A. Nguyen, MD, of the department of dermatology, Cedars-Sinai Medical Center, Los Angeles, told this news organization.
“Therefore, antibiotic prescribing patterns should not change simply due to immunosuppression. Furthermore, immunosuppressed patients appear to respond well to antibiotics and recover similarly to immunocompetent patients,” she said.
The study was presented at the annual meeting of the American College of Mohs Surgery.
Mohs surgery is increasingly being performed for patients who are immunosuppressed because of the higher incidence of skin cancer in this group of patients and their higher risk of more aggressive skin cancers.
Overall, the rate of surgical site infections following Mohs surgery generally ranges from 0.5% to 2.4%. However, research is lacking on the risk among patients who are immunosuppressed and on how effective the use of prophylactic antibiotics is for these patients.
For the retrospective study, Dr. Nguyen and her colleagues evaluated data on 5,886 patients who underwent Mohs surgery at Cedars-Sinai between October 2014 and August 2021. Among these patients, 741 (12.6%) were immunocompromised.
Causes of immunosuppression in the cohort included the following: immunosuppression after transplant surgery; having HIV, chronic myeloid leukemia, multiple myeloma, or other hematogenous forms of immunosuppression; or immunosuppression related to other conditions, such as chronic inflammatory diseases.
Overall, postprocedural infections occurred in 1.6% (95) of patients, a rate that mirrors that of the general population, Dr. Nguyen noted. No significant differences in surgical site infection rates were observed between immunocompromised patients (2.1%, n = 15) and those who were immunocompetent (1.6%, n = 80; P = .30).
Importantly, among those who were immunocompromised, the rates of infection were not significantly different between those who did receive antibiotics (3.0%, n = 8) and those who did not receive antibiotics (1.5%, n = 7; P = .19).
The lack of a difference in surgical site infection rates among those who did and those who did not receive antibiotics extended to the entire study population (2.0% vs. 1.4%; P = .12).
The study cohort mainly comprised immunosuppressed transplant patients, notably, heart, lung, and kidney transplant patients. However, “even in this population, we did not see a higher rate of infection,” senior author Nima M. Gharavi, MD, PhD, director of dermatologic surgery and Mohs micrographic surgery and associate professor of medicine and pathology and laboratory medicine at Cedars-Sinai Medical Center, said in an interview.
Yet the risk of infection among those patients has been shown to be high and of consequence. Data indicate that infections account for 13%-16% of deaths among kidney and heart transplant patients and up to 21% of deaths among lung transplant patients. The rate of mortality appears to parallel the level of immunosuppression, Dr. Nguyen explained.
Furthermore, up to 25% of patients who undergo heart and lung transplantation develop bacteremia.
In terms of why worse infections or bacteremia surgeries may not occur in association with Mohs, Dr. Nguyen speculated that, as opposed to other surgeries, those involving the skin may benefit from unique defense mechanisms.
“The skin is a complex system in its defense against foreign pathogens and infectious agents,” she explained during her presentation. “There is the physical barrier, the antimicrobial peptides, and an adaptive as well as innate immune response.”
“In immunosuppressed patients, with the decrease in adaptive immunity, it’s possible this loss is less important because the skin has such a robust immune system in general.”
In her presentation, Dr. Nguyen noted that “further studies are necessary to investigate why patients aren’t presenting with greater severity, and we plan to try to investigate whether the unique nature of skin-mediated immunity makes this organ less susceptible to severe or life-threatening infections in patients on immunosuppression.”
Of note, the rate of prophylactic antibiotic prescriptions was no higher for those who were and those who were not immunosuppressed (37.9% vs. 34.1%; P = .14), which Dr. Nguyen said is consistent with recommendations.
“Immunosuppression is not an indication for antibiotic use, and hence, we did not have a higher rate of antibiotics use in this population,” she told this news organization. However, a 2021 ACMS survey found that a high percentage of Mohs surgeons prescribe antibiotics for procedures in which antibiotics are not indicated so as to reduce the risk of infections and that immunosuppression is a common reason for doing so.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggesting that antibiotic prophylaxis, which is often used for these patients, may not be necessary, according to new research.
The retrospective cohort study found that “immunosuppressed patients had similar infection rates as immunocompetent patients following Mohs micrographic surgery,” first author Tuyet A. Nguyen, MD, of the department of dermatology, Cedars-Sinai Medical Center, Los Angeles, told this news organization.
“Therefore, antibiotic prescribing patterns should not change simply due to immunosuppression. Furthermore, immunosuppressed patients appear to respond well to antibiotics and recover similarly to immunocompetent patients,” she said.
The study was presented at the annual meeting of the American College of Mohs Surgery.
Mohs surgery is increasingly being performed for patients who are immunosuppressed because of the higher incidence of skin cancer in this group of patients and their higher risk of more aggressive skin cancers.
Overall, the rate of surgical site infections following Mohs surgery generally ranges from 0.5% to 2.4%. However, research is lacking on the risk among patients who are immunosuppressed and on how effective the use of prophylactic antibiotics is for these patients.
For the retrospective study, Dr. Nguyen and her colleagues evaluated data on 5,886 patients who underwent Mohs surgery at Cedars-Sinai between October 2014 and August 2021. Among these patients, 741 (12.6%) were immunocompromised.
Causes of immunosuppression in the cohort included the following: immunosuppression after transplant surgery; having HIV, chronic myeloid leukemia, multiple myeloma, or other hematogenous forms of immunosuppression; or immunosuppression related to other conditions, such as chronic inflammatory diseases.
Overall, postprocedural infections occurred in 1.6% (95) of patients, a rate that mirrors that of the general population, Dr. Nguyen noted. No significant differences in surgical site infection rates were observed between immunocompromised patients (2.1%, n = 15) and those who were immunocompetent (1.6%, n = 80; P = .30).
Importantly, among those who were immunocompromised, the rates of infection were not significantly different between those who did receive antibiotics (3.0%, n = 8) and those who did not receive antibiotics (1.5%, n = 7; P = .19).
The lack of a difference in surgical site infection rates among those who did and those who did not receive antibiotics extended to the entire study population (2.0% vs. 1.4%; P = .12).
The study cohort mainly comprised immunosuppressed transplant patients, notably, heart, lung, and kidney transplant patients. However, “even in this population, we did not see a higher rate of infection,” senior author Nima M. Gharavi, MD, PhD, director of dermatologic surgery and Mohs micrographic surgery and associate professor of medicine and pathology and laboratory medicine at Cedars-Sinai Medical Center, said in an interview.
Yet the risk of infection among those patients has been shown to be high and of consequence. Data indicate that infections account for 13%-16% of deaths among kidney and heart transplant patients and up to 21% of deaths among lung transplant patients. The rate of mortality appears to parallel the level of immunosuppression, Dr. Nguyen explained.
Furthermore, up to 25% of patients who undergo heart and lung transplantation develop bacteremia.
In terms of why worse infections or bacteremia surgeries may not occur in association with Mohs, Dr. Nguyen speculated that, as opposed to other surgeries, those involving the skin may benefit from unique defense mechanisms.
“The skin is a complex system in its defense against foreign pathogens and infectious agents,” she explained during her presentation. “There is the physical barrier, the antimicrobial peptides, and an adaptive as well as innate immune response.”
“In immunosuppressed patients, with the decrease in adaptive immunity, it’s possible this loss is less important because the skin has such a robust immune system in general.”
In her presentation, Dr. Nguyen noted that “further studies are necessary to investigate why patients aren’t presenting with greater severity, and we plan to try to investigate whether the unique nature of skin-mediated immunity makes this organ less susceptible to severe or life-threatening infections in patients on immunosuppression.”
Of note, the rate of prophylactic antibiotic prescriptions was no higher for those who were and those who were not immunosuppressed (37.9% vs. 34.1%; P = .14), which Dr. Nguyen said is consistent with recommendations.
“Immunosuppression is not an indication for antibiotic use, and hence, we did not have a higher rate of antibiotics use in this population,” she told this news organization. However, a 2021 ACMS survey found that a high percentage of Mohs surgeons prescribe antibiotics for procedures in which antibiotics are not indicated so as to reduce the risk of infections and that immunosuppression is a common reason for doing so.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACMS ANNUAL MEETING
Mohs surgery in the elderly: The dilemma of when to treat
As increasing numbers of patients in their 80s, 90s, and even 100s present for possible Mohs micrographic surgery, surgeons are confronted with deciding when the risks of treatment may outweigh the benefits.
In one of two presentations at the annual meeting of the American College of Mohs Surgery that addressed this topic, Howard W. Rogers, MD, of Advanced Dermatology in Norwich, Conn., said that the crux of the issue is the concern not to undertreat. He noted that reduced access to dermatologic care during the pandemic has provided a stark lesson in the risks of delaying treatment in all age groups. “Mohs surgeons have all seen the consequences of delayed treatment due to the pandemic with enormous, destructive, and sometimes fatal cancers coming to the office in the last year,” he told this news organization.
“Pandemic-related treatment delay has caused increased suffering and morbidity for countless skin cancer patients across the U.S.,” he said. “In general, not treating skin cancer and hoping it’s not going to grow or having significant delays in treatment are a recipe for disastrous outcomes.”
That said, active monitoring may be appropriate “for select small cancers that tend to grow slowly in the very elderly,” added Dr. Rogers, the incoming ACMS president. Among the key situations where the benefits of active monitoring may outweigh the risks of surgery are small, slowly growing cancers, when frailty is an issue.
Frailty has been equated to compromised functionality, which can increase the risk of an array of complications, including prolonged wound healing and secondary complications stemming from immobility. The toll those issues can take on patients’ quality of life can be considerable, Dr. Rogers said.
When weighing treatment options with elderly patients, he emphasized that careful consideration should be given to whether the “time needed to benefit from a Mohs procedure is longer than the patient’s life expectancy.” Furthermore, a decision not to treat does not have to be the last word. “We need to have an honest dialogue on the consequences of nontreatment, but part of that should be that just because we don’t treat today, doesn’t mean we can’t treat it tomorrow, if necessary.”
Of note, he added, “more than 100,00 patients have surgery for basal cell carcinoma [BCC] in their last year of life.” And that figure will likely rise exponentially if population projections come to fruition, considering that the population of people over the age of 85 is predicted to increase to nearly 18 million in 2050, from 5.8 million in 2012, Dr. Rogers said.
Until more research emerges on how to best treat this age group, Dr. Rogers noted that experts recommend that for elderly patients, “treatment should be individualized with consideration of active monitoring of primary BCC that is not in the H-zone, asymptomatic, smaller than 1 cm, with treatment initiated if there is substantial growth or symptoms.” Ultimately, he urged surgeons to “be sensitive and treat our patients like ourselves or our family members.”
When appropriate – Mohs is safe in the very elderly
Taking on the issue in a separate presentation, Deborah MacFarlane, MD, professor of dermatology and head and neck surgery at MD Anderson Cancer Center, Houston, said that for skin cancer cases that warrant treatment, clinicians should not let age alone stand in the way of Mohs surgery.
The evidence of its safety in the elderly dates back to a paper published in 1997 that Dr. MacFarlane coauthored, describing Mohs surgery of BCCs, squamous cell cancers (SCCs), and melanomas among 115 patients aged 90 and older (average, 92.4 years) who had an average of 1.9 comorbid medical conditions, and were taking an average of 2.3 medications. “Overall, we had just one complication among the patients,” she said.
In a subsequent paper, Dr. MacFarlane and her colleagues found that age at the time of Mohs surgery, even in older patients, was unrelated to survival, stage of cancer, or the type of repair. “We have concluded that this rapidly growing segment of the population can undergo Mohs surgery and should not be relegated to less effective treatment out of fear of its affecting their survival,” Dr. MacFarlane said.
She agreed with the concern about frailty and hence functionality, which may need to be factored in when making a decision to perform Mohs surgery. “I think this is something we do intuitively anyway,” she added. “We’re going to offer Mohs to someone who we think will survive and who is in relatively good health,” Dr. MacFarlane noted.
The point is illustrated in a new multicenter study of 1,181 patients at 22 U.S. sites, aged 85 years and older with nonmelanoma skin cancer referred for Mohs surgery. In the study, published in JAMA Dermatology after the ACMS meeting, patients who had Mohs surgery were almost four times more likely to have high functional status (P < .001) and were more likely to have facial tumors (P < .001), compared with those who had an alternate surgery.
The main reasons provided by the surgeons for opting to treat with Mohs included a patient’s desire for treatment with a high cure rate (66%), good/excellent patient functional status for age (57%), and a high risk associated with the tumor based on histology (40%), noted Dr. MacFarlane, one of the authors.
She reiterated the point raised by Dr. Rogers that “this is something we’re going to increasingly face,” noting that people over 85 represent the fastest growing segment of the population. “I have more patients over the age of 100 than I’ve ever had before,” she said.
Nevertheless, her own experience with elderly patients speaks to the safety of Mohs surgery in this population: Dr. MacFarlane reported a review of her practice’s records of 171 patients aged 85 years and older between May 2016 and May 2022, who received 414 separate procedures, without a single complication.
Sharing many of Dr. Rogers’ concerns about using caution in at-risk patients, Dr. MacFarlane offered recommendations for the optimal treatment of elderly patients receiving Mohs, including handling tissue delicately, and “keep undermining to a minimum.” She noted that intermediate closures and full thickness skin grafts are ideal closures for the elderly, while flaps may be performed in selected robust skin. It is also important to involve caretakers from the onset, talk and listen to patients – and play their choice of music during treatment, she said.
Commenting on the debate, comoderator Nahid Y. Vidal, MD, of the department of dermatology, Mayo Clinic, Rochester, Minn., noted that the expanding older population is accompanied by increases in skin cancer, in addition to more immunosenescence that is related to development of infections, autoimmune disease, and malignant tumors.
“In our academic practice, as with both the reference speakers, we do frequently see elderly, and not uncommonly the super-elderly,” she told this news organization. “The take-home point for me is to treat your whole patient, not just the tumor,” considering social factors, frailty/spry factor, and preferences, “and to do the humanistic thing, while also remaining evidence based,” she said.
“Don’t assume that increased age translates to morbidity, worse outcomes, or futility of treatment,” she added. “Chances are, if [a patient] made it to 90 years old with only a few medications and few medical problems, they may make it to 100, so why put the patient at risk for metastasis and death from a treatable/curable skin cancer,” in the case of SCC, she said.
“By the same token, why not perform more conservative treatments such as ED&C [electrodesiccation and curettage] for very low-risk skin cancers in low-risk locations, such as a superficial basal cell carcinoma on the trunk?” Overall, instead of trying to determine how long a super-elderly individual will live, Dr. Vidal said that “it’s better to educate the patient, engage in a discussion about goals of care, and to make few assumptions.”
Dr. Rogers, Dr. MacFarlane, and Dr. Vidal report no disclosures.
A version of this article first appeared on Medscape.com.
As increasing numbers of patients in their 80s, 90s, and even 100s present for possible Mohs micrographic surgery, surgeons are confronted with deciding when the risks of treatment may outweigh the benefits.
In one of two presentations at the annual meeting of the American College of Mohs Surgery that addressed this topic, Howard W. Rogers, MD, of Advanced Dermatology in Norwich, Conn., said that the crux of the issue is the concern not to undertreat. He noted that reduced access to dermatologic care during the pandemic has provided a stark lesson in the risks of delaying treatment in all age groups. “Mohs surgeons have all seen the consequences of delayed treatment due to the pandemic with enormous, destructive, and sometimes fatal cancers coming to the office in the last year,” he told this news organization.
“Pandemic-related treatment delay has caused increased suffering and morbidity for countless skin cancer patients across the U.S.,” he said. “In general, not treating skin cancer and hoping it’s not going to grow or having significant delays in treatment are a recipe for disastrous outcomes.”
That said, active monitoring may be appropriate “for select small cancers that tend to grow slowly in the very elderly,” added Dr. Rogers, the incoming ACMS president. Among the key situations where the benefits of active monitoring may outweigh the risks of surgery are small, slowly growing cancers, when frailty is an issue.
Frailty has been equated to compromised functionality, which can increase the risk of an array of complications, including prolonged wound healing and secondary complications stemming from immobility. The toll those issues can take on patients’ quality of life can be considerable, Dr. Rogers said.
When weighing treatment options with elderly patients, he emphasized that careful consideration should be given to whether the “time needed to benefit from a Mohs procedure is longer than the patient’s life expectancy.” Furthermore, a decision not to treat does not have to be the last word. “We need to have an honest dialogue on the consequences of nontreatment, but part of that should be that just because we don’t treat today, doesn’t mean we can’t treat it tomorrow, if necessary.”
Of note, he added, “more than 100,00 patients have surgery for basal cell carcinoma [BCC] in their last year of life.” And that figure will likely rise exponentially if population projections come to fruition, considering that the population of people over the age of 85 is predicted to increase to nearly 18 million in 2050, from 5.8 million in 2012, Dr. Rogers said.
Until more research emerges on how to best treat this age group, Dr. Rogers noted that experts recommend that for elderly patients, “treatment should be individualized with consideration of active monitoring of primary BCC that is not in the H-zone, asymptomatic, smaller than 1 cm, with treatment initiated if there is substantial growth or symptoms.” Ultimately, he urged surgeons to “be sensitive and treat our patients like ourselves or our family members.”
When appropriate – Mohs is safe in the very elderly
Taking on the issue in a separate presentation, Deborah MacFarlane, MD, professor of dermatology and head and neck surgery at MD Anderson Cancer Center, Houston, said that for skin cancer cases that warrant treatment, clinicians should not let age alone stand in the way of Mohs surgery.
The evidence of its safety in the elderly dates back to a paper published in 1997 that Dr. MacFarlane coauthored, describing Mohs surgery of BCCs, squamous cell cancers (SCCs), and melanomas among 115 patients aged 90 and older (average, 92.4 years) who had an average of 1.9 comorbid medical conditions, and were taking an average of 2.3 medications. “Overall, we had just one complication among the patients,” she said.
In a subsequent paper, Dr. MacFarlane and her colleagues found that age at the time of Mohs surgery, even in older patients, was unrelated to survival, stage of cancer, or the type of repair. “We have concluded that this rapidly growing segment of the population can undergo Mohs surgery and should not be relegated to less effective treatment out of fear of its affecting their survival,” Dr. MacFarlane said.
She agreed with the concern about frailty and hence functionality, which may need to be factored in when making a decision to perform Mohs surgery. “I think this is something we do intuitively anyway,” she added. “We’re going to offer Mohs to someone who we think will survive and who is in relatively good health,” Dr. MacFarlane noted.
The point is illustrated in a new multicenter study of 1,181 patients at 22 U.S. sites, aged 85 years and older with nonmelanoma skin cancer referred for Mohs surgery. In the study, published in JAMA Dermatology after the ACMS meeting, patients who had Mohs surgery were almost four times more likely to have high functional status (P < .001) and were more likely to have facial tumors (P < .001), compared with those who had an alternate surgery.
The main reasons provided by the surgeons for opting to treat with Mohs included a patient’s desire for treatment with a high cure rate (66%), good/excellent patient functional status for age (57%), and a high risk associated with the tumor based on histology (40%), noted Dr. MacFarlane, one of the authors.
She reiterated the point raised by Dr. Rogers that “this is something we’re going to increasingly face,” noting that people over 85 represent the fastest growing segment of the population. “I have more patients over the age of 100 than I’ve ever had before,” she said.
Nevertheless, her own experience with elderly patients speaks to the safety of Mohs surgery in this population: Dr. MacFarlane reported a review of her practice’s records of 171 patients aged 85 years and older between May 2016 and May 2022, who received 414 separate procedures, without a single complication.
Sharing many of Dr. Rogers’ concerns about using caution in at-risk patients, Dr. MacFarlane offered recommendations for the optimal treatment of elderly patients receiving Mohs, including handling tissue delicately, and “keep undermining to a minimum.” She noted that intermediate closures and full thickness skin grafts are ideal closures for the elderly, while flaps may be performed in selected robust skin. It is also important to involve caretakers from the onset, talk and listen to patients – and play their choice of music during treatment, she said.
Commenting on the debate, comoderator Nahid Y. Vidal, MD, of the department of dermatology, Mayo Clinic, Rochester, Minn., noted that the expanding older population is accompanied by increases in skin cancer, in addition to more immunosenescence that is related to development of infections, autoimmune disease, and malignant tumors.
“In our academic practice, as with both the reference speakers, we do frequently see elderly, and not uncommonly the super-elderly,” she told this news organization. “The take-home point for me is to treat your whole patient, not just the tumor,” considering social factors, frailty/spry factor, and preferences, “and to do the humanistic thing, while also remaining evidence based,” she said.
“Don’t assume that increased age translates to morbidity, worse outcomes, or futility of treatment,” she added. “Chances are, if [a patient] made it to 90 years old with only a few medications and few medical problems, they may make it to 100, so why put the patient at risk for metastasis and death from a treatable/curable skin cancer,” in the case of SCC, she said.
“By the same token, why not perform more conservative treatments such as ED&C [electrodesiccation and curettage] for very low-risk skin cancers in low-risk locations, such as a superficial basal cell carcinoma on the trunk?” Overall, instead of trying to determine how long a super-elderly individual will live, Dr. Vidal said that “it’s better to educate the patient, engage in a discussion about goals of care, and to make few assumptions.”
Dr. Rogers, Dr. MacFarlane, and Dr. Vidal report no disclosures.
A version of this article first appeared on Medscape.com.
As increasing numbers of patients in their 80s, 90s, and even 100s present for possible Mohs micrographic surgery, surgeons are confronted with deciding when the risks of treatment may outweigh the benefits.
In one of two presentations at the annual meeting of the American College of Mohs Surgery that addressed this topic, Howard W. Rogers, MD, of Advanced Dermatology in Norwich, Conn., said that the crux of the issue is the concern not to undertreat. He noted that reduced access to dermatologic care during the pandemic has provided a stark lesson in the risks of delaying treatment in all age groups. “Mohs surgeons have all seen the consequences of delayed treatment due to the pandemic with enormous, destructive, and sometimes fatal cancers coming to the office in the last year,” he told this news organization.
“Pandemic-related treatment delay has caused increased suffering and morbidity for countless skin cancer patients across the U.S.,” he said. “In general, not treating skin cancer and hoping it’s not going to grow or having significant delays in treatment are a recipe for disastrous outcomes.”
That said, active monitoring may be appropriate “for select small cancers that tend to grow slowly in the very elderly,” added Dr. Rogers, the incoming ACMS president. Among the key situations where the benefits of active monitoring may outweigh the risks of surgery are small, slowly growing cancers, when frailty is an issue.
Frailty has been equated to compromised functionality, which can increase the risk of an array of complications, including prolonged wound healing and secondary complications stemming from immobility. The toll those issues can take on patients’ quality of life can be considerable, Dr. Rogers said.
When weighing treatment options with elderly patients, he emphasized that careful consideration should be given to whether the “time needed to benefit from a Mohs procedure is longer than the patient’s life expectancy.” Furthermore, a decision not to treat does not have to be the last word. “We need to have an honest dialogue on the consequences of nontreatment, but part of that should be that just because we don’t treat today, doesn’t mean we can’t treat it tomorrow, if necessary.”
Of note, he added, “more than 100,00 patients have surgery for basal cell carcinoma [BCC] in their last year of life.” And that figure will likely rise exponentially if population projections come to fruition, considering that the population of people over the age of 85 is predicted to increase to nearly 18 million in 2050, from 5.8 million in 2012, Dr. Rogers said.
Until more research emerges on how to best treat this age group, Dr. Rogers noted that experts recommend that for elderly patients, “treatment should be individualized with consideration of active monitoring of primary BCC that is not in the H-zone, asymptomatic, smaller than 1 cm, with treatment initiated if there is substantial growth or symptoms.” Ultimately, he urged surgeons to “be sensitive and treat our patients like ourselves or our family members.”
When appropriate – Mohs is safe in the very elderly
Taking on the issue in a separate presentation, Deborah MacFarlane, MD, professor of dermatology and head and neck surgery at MD Anderson Cancer Center, Houston, said that for skin cancer cases that warrant treatment, clinicians should not let age alone stand in the way of Mohs surgery.
The evidence of its safety in the elderly dates back to a paper published in 1997 that Dr. MacFarlane coauthored, describing Mohs surgery of BCCs, squamous cell cancers (SCCs), and melanomas among 115 patients aged 90 and older (average, 92.4 years) who had an average of 1.9 comorbid medical conditions, and were taking an average of 2.3 medications. “Overall, we had just one complication among the patients,” she said.
In a subsequent paper, Dr. MacFarlane and her colleagues found that age at the time of Mohs surgery, even in older patients, was unrelated to survival, stage of cancer, or the type of repair. “We have concluded that this rapidly growing segment of the population can undergo Mohs surgery and should not be relegated to less effective treatment out of fear of its affecting their survival,” Dr. MacFarlane said.
She agreed with the concern about frailty and hence functionality, which may need to be factored in when making a decision to perform Mohs surgery. “I think this is something we do intuitively anyway,” she added. “We’re going to offer Mohs to someone who we think will survive and who is in relatively good health,” Dr. MacFarlane noted.
The point is illustrated in a new multicenter study of 1,181 patients at 22 U.S. sites, aged 85 years and older with nonmelanoma skin cancer referred for Mohs surgery. In the study, published in JAMA Dermatology after the ACMS meeting, patients who had Mohs surgery were almost four times more likely to have high functional status (P < .001) and were more likely to have facial tumors (P < .001), compared with those who had an alternate surgery.
The main reasons provided by the surgeons for opting to treat with Mohs included a patient’s desire for treatment with a high cure rate (66%), good/excellent patient functional status for age (57%), and a high risk associated with the tumor based on histology (40%), noted Dr. MacFarlane, one of the authors.
She reiterated the point raised by Dr. Rogers that “this is something we’re going to increasingly face,” noting that people over 85 represent the fastest growing segment of the population. “I have more patients over the age of 100 than I’ve ever had before,” she said.
Nevertheless, her own experience with elderly patients speaks to the safety of Mohs surgery in this population: Dr. MacFarlane reported a review of her practice’s records of 171 patients aged 85 years and older between May 2016 and May 2022, who received 414 separate procedures, without a single complication.
Sharing many of Dr. Rogers’ concerns about using caution in at-risk patients, Dr. MacFarlane offered recommendations for the optimal treatment of elderly patients receiving Mohs, including handling tissue delicately, and “keep undermining to a minimum.” She noted that intermediate closures and full thickness skin grafts are ideal closures for the elderly, while flaps may be performed in selected robust skin. It is also important to involve caretakers from the onset, talk and listen to patients – and play their choice of music during treatment, she said.
Commenting on the debate, comoderator Nahid Y. Vidal, MD, of the department of dermatology, Mayo Clinic, Rochester, Minn., noted that the expanding older population is accompanied by increases in skin cancer, in addition to more immunosenescence that is related to development of infections, autoimmune disease, and malignant tumors.
“In our academic practice, as with both the reference speakers, we do frequently see elderly, and not uncommonly the super-elderly,” she told this news organization. “The take-home point for me is to treat your whole patient, not just the tumor,” considering social factors, frailty/spry factor, and preferences, “and to do the humanistic thing, while also remaining evidence based,” she said.
“Don’t assume that increased age translates to morbidity, worse outcomes, or futility of treatment,” she added. “Chances are, if [a patient] made it to 90 years old with only a few medications and few medical problems, they may make it to 100, so why put the patient at risk for metastasis and death from a treatable/curable skin cancer,” in the case of SCC, she said.
“By the same token, why not perform more conservative treatments such as ED&C [electrodesiccation and curettage] for very low-risk skin cancers in low-risk locations, such as a superficial basal cell carcinoma on the trunk?” Overall, instead of trying to determine how long a super-elderly individual will live, Dr. Vidal said that “it’s better to educate the patient, engage in a discussion about goals of care, and to make few assumptions.”
Dr. Rogers, Dr. MacFarlane, and Dr. Vidal report no disclosures.
A version of this article first appeared on Medscape.com.
FROM ACMS 2022
Adjunctive confocal microscopy found to reduce unnecessary skin excisions
results from a large randomized clinical trial showed.
“Skin cancer management exerts a sizable burden on health systems,” researchers led by Giovanni Pellacani, MD, wrote in an article published in JAMA Dermatology. “The systematic application of RCM in the triage of high-risk patients should improve diagnostic accuracy and reduce unnecessary excisions for histopathological diagnostic confirmation, thereby reducing costs, surgical waiting lists, and delayed diagnoses.”
However, they added, “the clinical application of RCM has mainly been limited to retrospective and prospective observational studies producing hypothetical estimates of clinical applicability without intention to affect clinical and therapeutic patient pathways.”
For the current study, Dr. Pellacani, who chairs the department of dermatology at Sapienza University, Rome, and colleagues hypothesized that RCM would reduce unnecessary excisions by more than 30% and would identify all melanoma lesions 0.5 mm or thinner at baseline. They enrolled 3,165 patients with suspect lesions from three dermatology referral centers between January 2017 and December 2019, with a mean follow-up of 9.6 months in the study. Participants were randomly assigned 1:1 to standard therapeutic care, which consisted of clinical and dermoscopy evaluation with or without adjunctive RCM, a novel noninvasive technology that provides in vivo imaging of the skin, with a high diagnostic accuracy.
Histopathologic examination of all excised lesions was performed at the pathology department of the referral center. Resulting information guided prospective clinical decision-making (excision or follow-up). The mean age of patients was 49 years, 49% were women, 21% had a personal history of melanoma, and 51% had Fitzpatrick phototype 2 skin.
When compared with standard therapeutic care only, adjunctive RCM was associated with a higher positive predictive value (18.9 vs. 33.3, respectively), lower benign to malignant ratio (3.7:1.0 vs. 1.8:1.0), and a reduction in the number needed to excise of 43.4% (5.3 vs. 3.0). In addition, all 15 lesions with delayed melanoma diagnoses were thinner than 0.5 mm. Of these, eight were diagnosed as melanoma in situ.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study, said that a strength of the analysis was its follow-up and histopathologic evaluation, “which are both essentially forms of feedback. Good, relevant feedback is necessary for all of us to improve.”
She pointed out that, while RCM does appear to reduce the number of benign lesions unnecessarily removed and increase the number of skin cancers appropriately excised, the authors acknowledged that they had at least 4 years of experience with RCM. “The study also does not address the time factor (the procedure takes about 7 minutes per lesion) and the financial cost of reflectance confocal microscopy, as compared to the cost of standard follow-up alone with an increased number of excisions.”
She added that the findings “are not yet applicable to general dermatology across the world, as the authors comment, given that reflectance confocal microscopy is not yet widely available.”
The Italian Ministry of Health supported the study. Neither the researchers nor Dr. Ko reported having relevant financial conflicts.
results from a large randomized clinical trial showed.
“Skin cancer management exerts a sizable burden on health systems,” researchers led by Giovanni Pellacani, MD, wrote in an article published in JAMA Dermatology. “The systematic application of RCM in the triage of high-risk patients should improve diagnostic accuracy and reduce unnecessary excisions for histopathological diagnostic confirmation, thereby reducing costs, surgical waiting lists, and delayed diagnoses.”
However, they added, “the clinical application of RCM has mainly been limited to retrospective and prospective observational studies producing hypothetical estimates of clinical applicability without intention to affect clinical and therapeutic patient pathways.”
For the current study, Dr. Pellacani, who chairs the department of dermatology at Sapienza University, Rome, and colleagues hypothesized that RCM would reduce unnecessary excisions by more than 30% and would identify all melanoma lesions 0.5 mm or thinner at baseline. They enrolled 3,165 patients with suspect lesions from three dermatology referral centers between January 2017 and December 2019, with a mean follow-up of 9.6 months in the study. Participants were randomly assigned 1:1 to standard therapeutic care, which consisted of clinical and dermoscopy evaluation with or without adjunctive RCM, a novel noninvasive technology that provides in vivo imaging of the skin, with a high diagnostic accuracy.
Histopathologic examination of all excised lesions was performed at the pathology department of the referral center. Resulting information guided prospective clinical decision-making (excision or follow-up). The mean age of patients was 49 years, 49% were women, 21% had a personal history of melanoma, and 51% had Fitzpatrick phototype 2 skin.
When compared with standard therapeutic care only, adjunctive RCM was associated with a higher positive predictive value (18.9 vs. 33.3, respectively), lower benign to malignant ratio (3.7:1.0 vs. 1.8:1.0), and a reduction in the number needed to excise of 43.4% (5.3 vs. 3.0). In addition, all 15 lesions with delayed melanoma diagnoses were thinner than 0.5 mm. Of these, eight were diagnosed as melanoma in situ.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study, said that a strength of the analysis was its follow-up and histopathologic evaluation, “which are both essentially forms of feedback. Good, relevant feedback is necessary for all of us to improve.”
She pointed out that, while RCM does appear to reduce the number of benign lesions unnecessarily removed and increase the number of skin cancers appropriately excised, the authors acknowledged that they had at least 4 years of experience with RCM. “The study also does not address the time factor (the procedure takes about 7 minutes per lesion) and the financial cost of reflectance confocal microscopy, as compared to the cost of standard follow-up alone with an increased number of excisions.”
She added that the findings “are not yet applicable to general dermatology across the world, as the authors comment, given that reflectance confocal microscopy is not yet widely available.”
The Italian Ministry of Health supported the study. Neither the researchers nor Dr. Ko reported having relevant financial conflicts.
results from a large randomized clinical trial showed.
“Skin cancer management exerts a sizable burden on health systems,” researchers led by Giovanni Pellacani, MD, wrote in an article published in JAMA Dermatology. “The systematic application of RCM in the triage of high-risk patients should improve diagnostic accuracy and reduce unnecessary excisions for histopathological diagnostic confirmation, thereby reducing costs, surgical waiting lists, and delayed diagnoses.”
However, they added, “the clinical application of RCM has mainly been limited to retrospective and prospective observational studies producing hypothetical estimates of clinical applicability without intention to affect clinical and therapeutic patient pathways.”
For the current study, Dr. Pellacani, who chairs the department of dermatology at Sapienza University, Rome, and colleagues hypothesized that RCM would reduce unnecessary excisions by more than 30% and would identify all melanoma lesions 0.5 mm or thinner at baseline. They enrolled 3,165 patients with suspect lesions from three dermatology referral centers between January 2017 and December 2019, with a mean follow-up of 9.6 months in the study. Participants were randomly assigned 1:1 to standard therapeutic care, which consisted of clinical and dermoscopy evaluation with or without adjunctive RCM, a novel noninvasive technology that provides in vivo imaging of the skin, with a high diagnostic accuracy.
Histopathologic examination of all excised lesions was performed at the pathology department of the referral center. Resulting information guided prospective clinical decision-making (excision or follow-up). The mean age of patients was 49 years, 49% were women, 21% had a personal history of melanoma, and 51% had Fitzpatrick phototype 2 skin.
When compared with standard therapeutic care only, adjunctive RCM was associated with a higher positive predictive value (18.9 vs. 33.3, respectively), lower benign to malignant ratio (3.7:1.0 vs. 1.8:1.0), and a reduction in the number needed to excise of 43.4% (5.3 vs. 3.0). In addition, all 15 lesions with delayed melanoma diagnoses were thinner than 0.5 mm. Of these, eight were diagnosed as melanoma in situ.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study, said that a strength of the analysis was its follow-up and histopathologic evaluation, “which are both essentially forms of feedback. Good, relevant feedback is necessary for all of us to improve.”
She pointed out that, while RCM does appear to reduce the number of benign lesions unnecessarily removed and increase the number of skin cancers appropriately excised, the authors acknowledged that they had at least 4 years of experience with RCM. “The study also does not address the time factor (the procedure takes about 7 minutes per lesion) and the financial cost of reflectance confocal microscopy, as compared to the cost of standard follow-up alone with an increased number of excisions.”
She added that the findings “are not yet applicable to general dermatology across the world, as the authors comment, given that reflectance confocal microscopy is not yet widely available.”
The Italian Ministry of Health supported the study. Neither the researchers nor Dr. Ko reported having relevant financial conflicts.
FROM JAMA DERMATOLOGY
Studies address ibrutinib bleeding risk in patients with CLL receiving Mohs surgery
Patients receiving , new research shows.
“Our cohort of CLL patients on ibrutinib had a two-times greater risk of bleeding complications relative to those on anticoagulants and a nearly 40-times greater risk of bleeding complications relative to those patients on no anticoagulants or CLL therapy,” Kelsey E. Hirotsu, MD, first author of one of two studies on the issue presented at the American College of Mohs Surgery annual meeting, told this news organization.
“It was definitely surprising to see this doubled risk with ibrutinib relative to anticoagulants, and certainly highlights the clinically relevant increased bleeding risk in patients on ibrutinib,” said Dr. Hirotsu, a Mohs micrographic surgery fellow in the department of dermatology, University of California, San Diego (UCSD).
With CLL associated with an increased risk for aggressive skin cancers, particularly squamous cell carcinoma, Mohs surgeons may commonly find themselves treating patients with these unique considerations. Surgical treatment of those cancers can be complicated not only because of potential underlying thrombocytopenia, which occurs in about 5% of untreated CLL patients, but also because of the increased risk for bleeding that is associated with the use of the Bruton tyrosine kinase inhibitor ibrutinib, commonly used for CLL.
While the nature of the increased bleeding-related complications among patients with CLL undergoing Mohs surgery has been documented in some case reports, evidence from larger studies has been lacking.
In one of the studies presented at the ACMS meeting, Dr. Hirotsu and her colleagues evaluated data on patients with CLL who underwent at least one Mohs surgery procedure at UCSD Dermatologic Surgery over 10 years. Of the 362 Mohs cases among 98 patients with CLL, 32 cases had at least one complication. Patients on anticoagulants, including antiplatelet agents, Coumadin, and direct oral anticoagulants (DOACs), not surprisingly, had higher rates of complications, particularly bleeding.
However, those treated with ibrutinib had the highest rates of complications among all of the patients (40.6%), with all of their complications involving bleeding-related events. In comparison, the complication rates, for instance, of patients treated with antiplatelets were 21.9%; Coumadin, 6.2%; and DOACs, 15.6%.
The incidence of bleeding-related complications among the cases in the ibrutinib-treated patients was 30.2% compared with 13.2% among those on blood thinners and no CLL therapy (relative risk [RR], 2.08; 95% confidence interval [CI], 0.85-5.11; P = .11). “Although not statistically significant, these results could trend toward significance with larger sample sizes,” Dr. Hirotsu said.
The risk for bleeding among patients on ibrutinib compared with patients on no medications, however, was significant, with a relative risk of 39.0 (95% CI, 2.35-646; P = .011).
Of note, among 12 patients on ibrutinib who experienced bleeding complications, 7 had previously undergone Mohs surgeries when they were not taking ibrutinib and no bleeding complications had occurred in those procedures. “This may further implicate ibrutinib as a cause of the bleeding-related complications,” Dr. Hirotsu said.
In investigating the role of thrombocytopenia at the time of Mohs surgery, the authors found that, among ibrutinib-treated patients who had no complications, 30% had thrombocytopenia, compared with 70% of those who did have bleeding while on ibrutinib at the time of surgery.
“It was interesting that thrombocytopenia is more common in ibrutinib patients with bleeding-related complications, but further research needs to be done to determine the clinical relevance and possible management implications,” Dr. Hirotsu said.
In a separate study presented at the meeting, 37 patients treated with ibrutinib for CLL while undergoing cutaneous surgery that included Mohs surgery and excisions had a significantly increased bleeding complication rate compared with a control group of 64 age- and sex-matched patients with CLL undergoing cutaneous surgery: 6 of 75 procedures (8%) versus 1 of 115 procedures (0.9%; P = .02).
Those with bleeding complications while on ibrutinib were all male, older (mean age, 82.7 vs. 73.0; P = .01), and had lower mean platelet counts (104 K/mcL vs. 150.5 K/mcL; P = .03).
There were no significant differences between the case and control groups in terms of anatomic site, type of procedure (Mohs versus excision), tumor diagnosis, lesion size, or type of reconstruction, while the control group was more likely to be on aspirin or other anticoagulants (P < .0001).
In an interview, senior author Nahid Y. Vidal, MD, a Mohs surgeon and dermatologic oncologist at the Mayo Clinic, Rochester, Minn., said that “the take-home message is that patients on ibrutinib should be considered higher risk for bleeding events, regardless of whether they are having a simpler surgery [excision] or more involved skin surgery procedure [Mohs with flap].”
Holding treatment
To offset the bleeding risk, Dr. Vidal notes that holding the treatment is considered safe and that the manufacturer recommends holding ibrutinib for at least 3-7 days pre- and post surgery, “depending on type of surgery and risk of bleeding.”
“In our institution, with the hematologist/oncologist’s input, we hold ibrutinib for 5 days preop and 3 days post op, and have not had bleed complications in these patients,” she said, noting that there were no bleeding events in the patients in the study when ibrutinib was held.
Likewise, Dr. Hirotsu noted that at her center at UCSD, patients on ibrutinib are asked during the preop call to hold treatment for 3 days before and after Mohs surgery – but are advised to discuss the decision with their hematologist/oncologist for approval.
The measure isn’t always successful in preventing bleeding, however, as seen in a case study describing two patients who experienced bleeding complications following Mohs surgery despite being taken off ibrutinib 3 days prior to the procedure.
The senior author of that study, Kira Minkis, MD, PhD, department of dermatology, Weill Cornell/New York Presbyterian, New York, told this news organization that her team concluded that in those cases ibrutinib perhaps should have been held longer than 3 days.
“In some cases, especially if the Mohs surgery is a large procedure with a more advanced reconstruction, such as a large flap, it might be more prudent to continue it longer than 3 days,” Dr. Minkis said. She noted that the high bleeding risk observed in the studies at ACMS was notable – but not unexpected.
“I’m not that surprised because if you look at the hematologic literature, the risk is indeed pretty significant, so it makes sense that it would also occur with Mohs surgeries,” she said.
She underscored that a 3-day hold of ibrutinib should be considered the minimum, “and in some cases, it should be held up to 7 days prior to surgery, depending on the specific surgery,” with the important caveat of consulting with the patient’s hematology team.
“Multidisciplinary decision-making is necessary for these cases, and the interruption of therapy should always be discussed with their hematology team,” she added. That said, Dr. Minkis noted that “I’ve never had a hematologist who had any concerns for withholding ibrutinib even for a week around the time of a surgery.”
Dr. Hirotsu, Dr. Vidal, and Dr. Minkis reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients receiving , new research shows.
“Our cohort of CLL patients on ibrutinib had a two-times greater risk of bleeding complications relative to those on anticoagulants and a nearly 40-times greater risk of bleeding complications relative to those patients on no anticoagulants or CLL therapy,” Kelsey E. Hirotsu, MD, first author of one of two studies on the issue presented at the American College of Mohs Surgery annual meeting, told this news organization.
“It was definitely surprising to see this doubled risk with ibrutinib relative to anticoagulants, and certainly highlights the clinically relevant increased bleeding risk in patients on ibrutinib,” said Dr. Hirotsu, a Mohs micrographic surgery fellow in the department of dermatology, University of California, San Diego (UCSD).
With CLL associated with an increased risk for aggressive skin cancers, particularly squamous cell carcinoma, Mohs surgeons may commonly find themselves treating patients with these unique considerations. Surgical treatment of those cancers can be complicated not only because of potential underlying thrombocytopenia, which occurs in about 5% of untreated CLL patients, but also because of the increased risk for bleeding that is associated with the use of the Bruton tyrosine kinase inhibitor ibrutinib, commonly used for CLL.
While the nature of the increased bleeding-related complications among patients with CLL undergoing Mohs surgery has been documented in some case reports, evidence from larger studies has been lacking.
In one of the studies presented at the ACMS meeting, Dr. Hirotsu and her colleagues evaluated data on patients with CLL who underwent at least one Mohs surgery procedure at UCSD Dermatologic Surgery over 10 years. Of the 362 Mohs cases among 98 patients with CLL, 32 cases had at least one complication. Patients on anticoagulants, including antiplatelet agents, Coumadin, and direct oral anticoagulants (DOACs), not surprisingly, had higher rates of complications, particularly bleeding.
However, those treated with ibrutinib had the highest rates of complications among all of the patients (40.6%), with all of their complications involving bleeding-related events. In comparison, the complication rates, for instance, of patients treated with antiplatelets were 21.9%; Coumadin, 6.2%; and DOACs, 15.6%.
The incidence of bleeding-related complications among the cases in the ibrutinib-treated patients was 30.2% compared with 13.2% among those on blood thinners and no CLL therapy (relative risk [RR], 2.08; 95% confidence interval [CI], 0.85-5.11; P = .11). “Although not statistically significant, these results could trend toward significance with larger sample sizes,” Dr. Hirotsu said.
The risk for bleeding among patients on ibrutinib compared with patients on no medications, however, was significant, with a relative risk of 39.0 (95% CI, 2.35-646; P = .011).
Of note, among 12 patients on ibrutinib who experienced bleeding complications, 7 had previously undergone Mohs surgeries when they were not taking ibrutinib and no bleeding complications had occurred in those procedures. “This may further implicate ibrutinib as a cause of the bleeding-related complications,” Dr. Hirotsu said.
In investigating the role of thrombocytopenia at the time of Mohs surgery, the authors found that, among ibrutinib-treated patients who had no complications, 30% had thrombocytopenia, compared with 70% of those who did have bleeding while on ibrutinib at the time of surgery.
“It was interesting that thrombocytopenia is more common in ibrutinib patients with bleeding-related complications, but further research needs to be done to determine the clinical relevance and possible management implications,” Dr. Hirotsu said.
In a separate study presented at the meeting, 37 patients treated with ibrutinib for CLL while undergoing cutaneous surgery that included Mohs surgery and excisions had a significantly increased bleeding complication rate compared with a control group of 64 age- and sex-matched patients with CLL undergoing cutaneous surgery: 6 of 75 procedures (8%) versus 1 of 115 procedures (0.9%; P = .02).
Those with bleeding complications while on ibrutinib were all male, older (mean age, 82.7 vs. 73.0; P = .01), and had lower mean platelet counts (104 K/mcL vs. 150.5 K/mcL; P = .03).
There were no significant differences between the case and control groups in terms of anatomic site, type of procedure (Mohs versus excision), tumor diagnosis, lesion size, or type of reconstruction, while the control group was more likely to be on aspirin or other anticoagulants (P < .0001).
In an interview, senior author Nahid Y. Vidal, MD, a Mohs surgeon and dermatologic oncologist at the Mayo Clinic, Rochester, Minn., said that “the take-home message is that patients on ibrutinib should be considered higher risk for bleeding events, regardless of whether they are having a simpler surgery [excision] or more involved skin surgery procedure [Mohs with flap].”
Holding treatment
To offset the bleeding risk, Dr. Vidal notes that holding the treatment is considered safe and that the manufacturer recommends holding ibrutinib for at least 3-7 days pre- and post surgery, “depending on type of surgery and risk of bleeding.”
“In our institution, with the hematologist/oncologist’s input, we hold ibrutinib for 5 days preop and 3 days post op, and have not had bleed complications in these patients,” she said, noting that there were no bleeding events in the patients in the study when ibrutinib was held.
Likewise, Dr. Hirotsu noted that at her center at UCSD, patients on ibrutinib are asked during the preop call to hold treatment for 3 days before and after Mohs surgery – but are advised to discuss the decision with their hematologist/oncologist for approval.
The measure isn’t always successful in preventing bleeding, however, as seen in a case study describing two patients who experienced bleeding complications following Mohs surgery despite being taken off ibrutinib 3 days prior to the procedure.
The senior author of that study, Kira Minkis, MD, PhD, department of dermatology, Weill Cornell/New York Presbyterian, New York, told this news organization that her team concluded that in those cases ibrutinib perhaps should have been held longer than 3 days.
“In some cases, especially if the Mohs surgery is a large procedure with a more advanced reconstruction, such as a large flap, it might be more prudent to continue it longer than 3 days,” Dr. Minkis said. She noted that the high bleeding risk observed in the studies at ACMS was notable – but not unexpected.
“I’m not that surprised because if you look at the hematologic literature, the risk is indeed pretty significant, so it makes sense that it would also occur with Mohs surgeries,” she said.
She underscored that a 3-day hold of ibrutinib should be considered the minimum, “and in some cases, it should be held up to 7 days prior to surgery, depending on the specific surgery,” with the important caveat of consulting with the patient’s hematology team.
“Multidisciplinary decision-making is necessary for these cases, and the interruption of therapy should always be discussed with their hematology team,” she added. That said, Dr. Minkis noted that “I’ve never had a hematologist who had any concerns for withholding ibrutinib even for a week around the time of a surgery.”
Dr. Hirotsu, Dr. Vidal, and Dr. Minkis reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients receiving , new research shows.
“Our cohort of CLL patients on ibrutinib had a two-times greater risk of bleeding complications relative to those on anticoagulants and a nearly 40-times greater risk of bleeding complications relative to those patients on no anticoagulants or CLL therapy,” Kelsey E. Hirotsu, MD, first author of one of two studies on the issue presented at the American College of Mohs Surgery annual meeting, told this news organization.
“It was definitely surprising to see this doubled risk with ibrutinib relative to anticoagulants, and certainly highlights the clinically relevant increased bleeding risk in patients on ibrutinib,” said Dr. Hirotsu, a Mohs micrographic surgery fellow in the department of dermatology, University of California, San Diego (UCSD).
With CLL associated with an increased risk for aggressive skin cancers, particularly squamous cell carcinoma, Mohs surgeons may commonly find themselves treating patients with these unique considerations. Surgical treatment of those cancers can be complicated not only because of potential underlying thrombocytopenia, which occurs in about 5% of untreated CLL patients, but also because of the increased risk for bleeding that is associated with the use of the Bruton tyrosine kinase inhibitor ibrutinib, commonly used for CLL.
While the nature of the increased bleeding-related complications among patients with CLL undergoing Mohs surgery has been documented in some case reports, evidence from larger studies has been lacking.
In one of the studies presented at the ACMS meeting, Dr. Hirotsu and her colleagues evaluated data on patients with CLL who underwent at least one Mohs surgery procedure at UCSD Dermatologic Surgery over 10 years. Of the 362 Mohs cases among 98 patients with CLL, 32 cases had at least one complication. Patients on anticoagulants, including antiplatelet agents, Coumadin, and direct oral anticoagulants (DOACs), not surprisingly, had higher rates of complications, particularly bleeding.
However, those treated with ibrutinib had the highest rates of complications among all of the patients (40.6%), with all of their complications involving bleeding-related events. In comparison, the complication rates, for instance, of patients treated with antiplatelets were 21.9%; Coumadin, 6.2%; and DOACs, 15.6%.
The incidence of bleeding-related complications among the cases in the ibrutinib-treated patients was 30.2% compared with 13.2% among those on blood thinners and no CLL therapy (relative risk [RR], 2.08; 95% confidence interval [CI], 0.85-5.11; P = .11). “Although not statistically significant, these results could trend toward significance with larger sample sizes,” Dr. Hirotsu said.
The risk for bleeding among patients on ibrutinib compared with patients on no medications, however, was significant, with a relative risk of 39.0 (95% CI, 2.35-646; P = .011).
Of note, among 12 patients on ibrutinib who experienced bleeding complications, 7 had previously undergone Mohs surgeries when they were not taking ibrutinib and no bleeding complications had occurred in those procedures. “This may further implicate ibrutinib as a cause of the bleeding-related complications,” Dr. Hirotsu said.
In investigating the role of thrombocytopenia at the time of Mohs surgery, the authors found that, among ibrutinib-treated patients who had no complications, 30% had thrombocytopenia, compared with 70% of those who did have bleeding while on ibrutinib at the time of surgery.
“It was interesting that thrombocytopenia is more common in ibrutinib patients with bleeding-related complications, but further research needs to be done to determine the clinical relevance and possible management implications,” Dr. Hirotsu said.
In a separate study presented at the meeting, 37 patients treated with ibrutinib for CLL while undergoing cutaneous surgery that included Mohs surgery and excisions had a significantly increased bleeding complication rate compared with a control group of 64 age- and sex-matched patients with CLL undergoing cutaneous surgery: 6 of 75 procedures (8%) versus 1 of 115 procedures (0.9%; P = .02).
Those with bleeding complications while on ibrutinib were all male, older (mean age, 82.7 vs. 73.0; P = .01), and had lower mean platelet counts (104 K/mcL vs. 150.5 K/mcL; P = .03).
There were no significant differences between the case and control groups in terms of anatomic site, type of procedure (Mohs versus excision), tumor diagnosis, lesion size, or type of reconstruction, while the control group was more likely to be on aspirin or other anticoagulants (P < .0001).
In an interview, senior author Nahid Y. Vidal, MD, a Mohs surgeon and dermatologic oncologist at the Mayo Clinic, Rochester, Minn., said that “the take-home message is that patients on ibrutinib should be considered higher risk for bleeding events, regardless of whether they are having a simpler surgery [excision] or more involved skin surgery procedure [Mohs with flap].”
Holding treatment
To offset the bleeding risk, Dr. Vidal notes that holding the treatment is considered safe and that the manufacturer recommends holding ibrutinib for at least 3-7 days pre- and post surgery, “depending on type of surgery and risk of bleeding.”
“In our institution, with the hematologist/oncologist’s input, we hold ibrutinib for 5 days preop and 3 days post op, and have not had bleed complications in these patients,” she said, noting that there were no bleeding events in the patients in the study when ibrutinib was held.
Likewise, Dr. Hirotsu noted that at her center at UCSD, patients on ibrutinib are asked during the preop call to hold treatment for 3 days before and after Mohs surgery – but are advised to discuss the decision with their hematologist/oncologist for approval.
The measure isn’t always successful in preventing bleeding, however, as seen in a case study describing two patients who experienced bleeding complications following Mohs surgery despite being taken off ibrutinib 3 days prior to the procedure.
The senior author of that study, Kira Minkis, MD, PhD, department of dermatology, Weill Cornell/New York Presbyterian, New York, told this news organization that her team concluded that in those cases ibrutinib perhaps should have been held longer than 3 days.
“In some cases, especially if the Mohs surgery is a large procedure with a more advanced reconstruction, such as a large flap, it might be more prudent to continue it longer than 3 days,” Dr. Minkis said. She noted that the high bleeding risk observed in the studies at ACMS was notable – but not unexpected.
“I’m not that surprised because if you look at the hematologic literature, the risk is indeed pretty significant, so it makes sense that it would also occur with Mohs surgeries,” she said.
She underscored that a 3-day hold of ibrutinib should be considered the minimum, “and in some cases, it should be held up to 7 days prior to surgery, depending on the specific surgery,” with the important caveat of consulting with the patient’s hematology team.
“Multidisciplinary decision-making is necessary for these cases, and the interruption of therapy should always be discussed with their hematology team,” she added. That said, Dr. Minkis noted that “I’ve never had a hematologist who had any concerns for withholding ibrutinib even for a week around the time of a surgery.”
Dr. Hirotsu, Dr. Vidal, and Dr. Minkis reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE ACMS ANNUAL MEETING
Topical tranexamic acid reduces postop bleeding following Mohs surgery
The use of adjunctive , in a double-blind, randomized, controlled trial.
The findings suggest that “topical TXA application is an inexpensive and easy topical preventative measure to consider adding to the wound care of granulating defects in the setting of Mohs micrographic surgery,” first author Brianna Castillo, MD, chief dermatology resident at the University of Missouri, Columbia, told this news organization.
The study results were presented at the annual meeting of the American College of Mohs Surgery.
In wound healing by second intent after Mohs micrographic surgery, postoperative bleeding is common and can lead to patient distress, as well as return visits or emergency care, resulting in additional health care costs, Dr. Castillo said.
Topical TXA, an antifibrinolytic, synthetic lysine analogue that prevents blood clots from breaking down, is commonly used in surgical settings including cardiothoracic, orthopedic, gynecologic, oral, and trauma surgery, showing no increased risk of thrombotic events. However, its use is relatively new in dermatology.
TXA is approved by the Food and Drug Administration only as an oral formulation for menorrhagia in women and as a short-term preventative measure for hemophilia; however, other formulations are available for topical and subcutaneous uses, Dr. Castillo noted.
To evaluate the potential benefits of the treatment in postsurgical Mohs microsurgery bleeding, Dr. Castillo and colleagues enrolled 124 patients undergoing the surgery between October 2020 and December 2021 who had surgical defects deemed appropriate for second intention healing.
The patients were randomized to groups of 62 patients each to receive normal saline-soaked Telfa pads applied to the wound bed upon completion of surgery or TXA 25 mg/mL at a volume of 1 mL/cm2-soaked Telfa pads to the wound bed upon completion of the surgery.
In both groups, a standard pressure dressing was placed on top of the Telfa pads.
Most participants were men (90 vs. 34 patients), 45 were taking antiplatelet therapy, and 20 were taking anticoagulants, and in all cases, patients were similarly randomized in the two groups. Most of the surgical defects were on the head and neck or an extremity, and most (74) were under 2 cm.
All patients were provided with instructions to apply pressure to their wounds and to report bleeding complications. They were interviewed by phone 3 days following their surgeries regarding postoperative bleeding and any potential issues relating to the TXA treatment.
In follow-up interviews, six patients in the placebo group (9.7%) reported active bleeding from their wounds within 48 hours of surgery, with one patient requiring an intervention, while there were no reports of bleeding in the TXA group (P = .028). No side effects were reported in either group.
In the setting of Mohs micrographic surgery, subcutaneous TXA has previously been studied as an intraoperative hemostatic agent, with bleeding measured prior to the second layer or closure, Dr. Castillo explained. However, “no studies have evaluated topical TXA with the aim to reduce postoperative bleeding in the setting of Mohs micrographic surgery,” she said.
Dr. Castillo noted that topical TXA is relatively inexpensive and typically available in hospital pharmacies. “It’s only about $7 per vial of 10 ccs and we do dilute it,” she noted during the session. “It has a pretty good shelf-life and does not have to be refrigerated.”
“We have implemented this into our practice at the University of Missouri,” she added.
Commenting on the study, M. Laurin Council, MD, associate professor of dermatology in the division of dermatology, department of internal medicine, Washington University, St. Louis, noted that second intention healing is “an excellent option for certain patients after skin cancer removal.
“One problem with this method, however, is that postsurgical wounds may bleed in the hours after a procedure, [and] this can be incredibly distressing to patients and their families,” she told this news organization.
“The study presented here shows great promise for the drug TXA for preventing postsurgical bleeding in this subset of patients,” said Dr. Council, director of dermatologic surgery and director of micrographic surgery and the dermatologic oncology fellowship at Washington University.
Commenting that “the results are impressive,” she noted the study had some limitations. “This is a small pilot study, and we don’t know about confounding factors in each group, such as the proportion of patients who are on blood thinners or who have low platelets, and therefore trouble clotting, for example.”
The authors have reported no relevant financial relationships. Dr. Council has consulted for AbbVie, Castle Biosciences, and Sanofi-Genzyme/Regeneron; however, the consulting was not relevant to the current study.
A version of this article first appeared on Medscape.com.
The use of adjunctive , in a double-blind, randomized, controlled trial.
The findings suggest that “topical TXA application is an inexpensive and easy topical preventative measure to consider adding to the wound care of granulating defects in the setting of Mohs micrographic surgery,” first author Brianna Castillo, MD, chief dermatology resident at the University of Missouri, Columbia, told this news organization.
The study results were presented at the annual meeting of the American College of Mohs Surgery.
In wound healing by second intent after Mohs micrographic surgery, postoperative bleeding is common and can lead to patient distress, as well as return visits or emergency care, resulting in additional health care costs, Dr. Castillo said.
Topical TXA, an antifibrinolytic, synthetic lysine analogue that prevents blood clots from breaking down, is commonly used in surgical settings including cardiothoracic, orthopedic, gynecologic, oral, and trauma surgery, showing no increased risk of thrombotic events. However, its use is relatively new in dermatology.
TXA is approved by the Food and Drug Administration only as an oral formulation for menorrhagia in women and as a short-term preventative measure for hemophilia; however, other formulations are available for topical and subcutaneous uses, Dr. Castillo noted.
To evaluate the potential benefits of the treatment in postsurgical Mohs microsurgery bleeding, Dr. Castillo and colleagues enrolled 124 patients undergoing the surgery between October 2020 and December 2021 who had surgical defects deemed appropriate for second intention healing.
The patients were randomized to groups of 62 patients each to receive normal saline-soaked Telfa pads applied to the wound bed upon completion of surgery or TXA 25 mg/mL at a volume of 1 mL/cm2-soaked Telfa pads to the wound bed upon completion of the surgery.
In both groups, a standard pressure dressing was placed on top of the Telfa pads.
Most participants were men (90 vs. 34 patients), 45 were taking antiplatelet therapy, and 20 were taking anticoagulants, and in all cases, patients were similarly randomized in the two groups. Most of the surgical defects were on the head and neck or an extremity, and most (74) were under 2 cm.
All patients were provided with instructions to apply pressure to their wounds and to report bleeding complications. They were interviewed by phone 3 days following their surgeries regarding postoperative bleeding and any potential issues relating to the TXA treatment.
In follow-up interviews, six patients in the placebo group (9.7%) reported active bleeding from their wounds within 48 hours of surgery, with one patient requiring an intervention, while there were no reports of bleeding in the TXA group (P = .028). No side effects were reported in either group.
In the setting of Mohs micrographic surgery, subcutaneous TXA has previously been studied as an intraoperative hemostatic agent, with bleeding measured prior to the second layer or closure, Dr. Castillo explained. However, “no studies have evaluated topical TXA with the aim to reduce postoperative bleeding in the setting of Mohs micrographic surgery,” she said.
Dr. Castillo noted that topical TXA is relatively inexpensive and typically available in hospital pharmacies. “It’s only about $7 per vial of 10 ccs and we do dilute it,” she noted during the session. “It has a pretty good shelf-life and does not have to be refrigerated.”
“We have implemented this into our practice at the University of Missouri,” she added.
Commenting on the study, M. Laurin Council, MD, associate professor of dermatology in the division of dermatology, department of internal medicine, Washington University, St. Louis, noted that second intention healing is “an excellent option for certain patients after skin cancer removal.
“One problem with this method, however, is that postsurgical wounds may bleed in the hours after a procedure, [and] this can be incredibly distressing to patients and their families,” she told this news organization.
“The study presented here shows great promise for the drug TXA for preventing postsurgical bleeding in this subset of patients,” said Dr. Council, director of dermatologic surgery and director of micrographic surgery and the dermatologic oncology fellowship at Washington University.
Commenting that “the results are impressive,” she noted the study had some limitations. “This is a small pilot study, and we don’t know about confounding factors in each group, such as the proportion of patients who are on blood thinners or who have low platelets, and therefore trouble clotting, for example.”
The authors have reported no relevant financial relationships. Dr. Council has consulted for AbbVie, Castle Biosciences, and Sanofi-Genzyme/Regeneron; however, the consulting was not relevant to the current study.
A version of this article first appeared on Medscape.com.
The use of adjunctive , in a double-blind, randomized, controlled trial.
The findings suggest that “topical TXA application is an inexpensive and easy topical preventative measure to consider adding to the wound care of granulating defects in the setting of Mohs micrographic surgery,” first author Brianna Castillo, MD, chief dermatology resident at the University of Missouri, Columbia, told this news organization.
The study results were presented at the annual meeting of the American College of Mohs Surgery.
In wound healing by second intent after Mohs micrographic surgery, postoperative bleeding is common and can lead to patient distress, as well as return visits or emergency care, resulting in additional health care costs, Dr. Castillo said.
Topical TXA, an antifibrinolytic, synthetic lysine analogue that prevents blood clots from breaking down, is commonly used in surgical settings including cardiothoracic, orthopedic, gynecologic, oral, and trauma surgery, showing no increased risk of thrombotic events. However, its use is relatively new in dermatology.
TXA is approved by the Food and Drug Administration only as an oral formulation for menorrhagia in women and as a short-term preventative measure for hemophilia; however, other formulations are available for topical and subcutaneous uses, Dr. Castillo noted.
To evaluate the potential benefits of the treatment in postsurgical Mohs microsurgery bleeding, Dr. Castillo and colleagues enrolled 124 patients undergoing the surgery between October 2020 and December 2021 who had surgical defects deemed appropriate for second intention healing.
The patients were randomized to groups of 62 patients each to receive normal saline-soaked Telfa pads applied to the wound bed upon completion of surgery or TXA 25 mg/mL at a volume of 1 mL/cm2-soaked Telfa pads to the wound bed upon completion of the surgery.
In both groups, a standard pressure dressing was placed on top of the Telfa pads.
Most participants were men (90 vs. 34 patients), 45 were taking antiplatelet therapy, and 20 were taking anticoagulants, and in all cases, patients were similarly randomized in the two groups. Most of the surgical defects were on the head and neck or an extremity, and most (74) were under 2 cm.
All patients were provided with instructions to apply pressure to their wounds and to report bleeding complications. They were interviewed by phone 3 days following their surgeries regarding postoperative bleeding and any potential issues relating to the TXA treatment.
In follow-up interviews, six patients in the placebo group (9.7%) reported active bleeding from their wounds within 48 hours of surgery, with one patient requiring an intervention, while there were no reports of bleeding in the TXA group (P = .028). No side effects were reported in either group.
In the setting of Mohs micrographic surgery, subcutaneous TXA has previously been studied as an intraoperative hemostatic agent, with bleeding measured prior to the second layer or closure, Dr. Castillo explained. However, “no studies have evaluated topical TXA with the aim to reduce postoperative bleeding in the setting of Mohs micrographic surgery,” she said.
Dr. Castillo noted that topical TXA is relatively inexpensive and typically available in hospital pharmacies. “It’s only about $7 per vial of 10 ccs and we do dilute it,” she noted during the session. “It has a pretty good shelf-life and does not have to be refrigerated.”
“We have implemented this into our practice at the University of Missouri,” she added.
Commenting on the study, M. Laurin Council, MD, associate professor of dermatology in the division of dermatology, department of internal medicine, Washington University, St. Louis, noted that second intention healing is “an excellent option for certain patients after skin cancer removal.
“One problem with this method, however, is that postsurgical wounds may bleed in the hours after a procedure, [and] this can be incredibly distressing to patients and their families,” she told this news organization.
“The study presented here shows great promise for the drug TXA for preventing postsurgical bleeding in this subset of patients,” said Dr. Council, director of dermatologic surgery and director of micrographic surgery and the dermatologic oncology fellowship at Washington University.
Commenting that “the results are impressive,” she noted the study had some limitations. “This is a small pilot study, and we don’t know about confounding factors in each group, such as the proportion of patients who are on blood thinners or who have low platelets, and therefore trouble clotting, for example.”
The authors have reported no relevant financial relationships. Dr. Council has consulted for AbbVie, Castle Biosciences, and Sanofi-Genzyme/Regeneron; however, the consulting was not relevant to the current study.
A version of this article first appeared on Medscape.com.
FROM THE ACMS ANNUAL MEETING
Conjunctival Melanoma of the Left Lower Eyelid
To the Editor:
A 58-year-old man with a pigmented lesion on the left lower eyelid was referred to the oculoplastic clinic by an outside ophthalmologist. The patient had noticed the lesion growing over the course of the last 4 to 5 months. He reported scant amounts of blood and discharge coming from the nose and left eye the week prior, which persisted for 3 days. He had no associated pain or discomfort. A slit-lamp examination revealed a pigmented left lower eyelid lesion measuring 20×15 mm with telangiectasia and an eyelid margin abnormality with no palpable lymphadenopathy. The patient was diagnosed with clinical stage T3N0M0 malignant conjunctival melanoma of the left eyelid based on the American Joint Committee on Cancer classification. It is thought to have originated from conjunctival primary acquired melanosis (PAM). The T3 stage is defined as malignant melanoma with local invasion; the lesion involved the eyelid and puncta as well as canalicular portions of the lacrimal drainage system.1 The bloody discharge was attributed to the involvement of the canalicular system, which drains tears from the eye to the nose. Melanomas can bleed, so any bloody discharge from the eye also will come through the ipsilateral nasal passage. Oncology evaluated the lesion to help determine the stage, and they found no lymph node involvement or brain, neck, chest, abdominal, or pelvic metastasis by computed tomography and magnetic resonance imaging. Sentinel lymph node biopsy was discussed with head and neck oncology specialists and was ultimately not performed per the recommendation from the Head and Neck Oncology Board; it is not a common modality for managing conjunctival melanoma because it has not been shown to alter morbidity and mortality.
The entire eyelid from the medial canthus to the lateral canthus was removed without touching the pigmented mass to ensure a “no-touch” technique removal of the mass. The no-touch technique primarily is utilized to decrease the likelihood of instrumental seeding of healthy tissues or the vascular system.2 This technique focuses on preventing any direct manipulation of the tumor and avoiding an incisional biopsy as well as removal of the tumor en bloc. The margins were cutaneous—3 mm lateral to the lateral canthus, 5 mm below the lid margin, and 3 mm medial to the medial canthus—with dissection of the medial tissue from the orbital rim and lacrimal sac fossa. The lacrimal sac and lower canaliculus were then resected. The conjunctiva 5 mm inferior to the pigmented mass and the entire palpebral conjunctiva was resected to the inferior fornix across the entire palpebral conjunctiva of the lower eyelid (Figure). The eyelid and lacrimal portions were removed as a unit. Essentially, the entire lower eyelid (full thickness), including the lateral canthus, medial canthus, canaliculus, and lacrimal sac, were removed en bloc. The final tumor staging after tissue evaluation by pathology and systemic evaluation by oncology was pT3N0bM0.
A tarsoconjunctival (Hughes) flap from the upper eyelid was used to reconstruct the posterior lamella (tarsus/conjunctiva) of the lower eyelid, and a full-thickness skin graft harvested from the ipsilateral upper eyelid was used to reconstruct the anterior lamella (skin) of the lower eyelid. The reconstruction site was allowed to heal for 4 weeks before severing the tarsoconjunctival graft to allow the separation of the upper and lower eyelids. Adjunctive topical ophthalmic chemotherapy (mitomycin C 0.04%) was started 4 weeks after the last surgery. The medication was applied 4 times daily for 1 week and restarted after the conjunctival erythema and injection subsided, which was approximately 2.5 weeks, on average. The regimen of applying the medication (1 week on and 2.5 weeks off) was completed for 4 cycles. At 1 year follow-up after his diagnosis, the patient was without local recurrence or evidence of systemic metastasis. We plan to have him continue ophthalmic and oncologic evaluation every 3 to 4 months for the next 24 months, and then every 6 months for years 2 through 5.
Ocular melanoma can be further divided into uveal and conjunctival types, both arising from the respective tissue. Melanoma of the conjunctiva commonly arises from PAM with atypia, which is an acquired conjunctival pigmented lesion similar to a skin nevus that has the potential to become dysplastic. In a genetic analysis of 78 conjunctival melanomas, BRAF mutations were identified in 29% (23/78) of tumors, and NRAS mutations were detected in 18% (14/78) of tumors3; however, in our case, there were no BRAF or NRAS mutations detected. In a study of 84,836 cases that included a diagnosis of melanoma, ocular melanoma comprised 5.2% of melanomas, with cutaneous, mucosal, and unknown primary sites totaling the remaining percentage of melanomas. Of 4522 patients with ocular melanomas, 85% had uveal melanomas; 4.8% had conjunctival melanoma; and 10.2% were classified as other—comprised of cornea, not otherwise specified (NOS); retina; lacrimal gland; orbit, NOS; overlapping lesion of the eye; and eye, NOS.4 Melanomas of the uvea, including the ciliary body, choroid, and iris, result from a notably different pathogenesis than conjunctival melanoma, with the former being primarily associated with GNAQ and GNA mutations.3 Ciliary body and choroidal melanomas each have a different pathogenesis for occurrence, with choroidal melanoma being mostly from metastasis and ciliary body melanoma from mutations or metastasis.
Pigmented lesions on the conjunctiva or sclera arise from either melanocytes or nonmelanocytes and have a diverse differential diagnosis, including congenital melanosis, conjunctival nevi, PAM or secondary acquired melanosis, or conjunctival melanoma. The diagnosis of uveal melanoma should be based on fundoscopic examination by an experienced clinician. Uveal melanoma is unlike most other cancers in that diagnosis can be by clinical fundoscopic examination alone. Imaging studies such as ultrasound and fluorescein angiography can be performed for prognostication and characterization. Fine needle aspiration biopsy for molecular analysis is becoming more routine, but the results rarely affect the plan of care. Primary treatment of uveal melanoma should strive to preserve vision and prevent metastasis; however, a primary modality has yet to show notable results in decreasing distant disease spread or overall survival. Treatment of the primary tumor should involve consideration of tumor size, location, health of the patient, and patient preference.1,5
For patients with melanoma arising from the conjunctiva, initial management should focus on local disease control, including wide local excision to avoid seeding, supplemented with cryotherapy and alcohol epitheliectomy to the cornea to ensure local tumor extinction.2,6 Techniques including enucleation and orbital exenteration historically have been used for treatment of extensive disease, but this approach has not been associated with improvement in mortality and is a cause of notable morbidity.7,8 Sentinel lymph node biopsy has an established role in the management of cutaneous melanoma, but its use in the setting of conjunctival melanoma is controversial, with studies showing that up to 50% of patients with local recurrence can develop distant metastasis with no evidence of regional lymph node involvement.9,10 When the tumor is present at the surgical margins or in the case that lesions cannot be fully excised, adjuvant therapy may improve long-term control and prevent recurrence following surgical intervention. Mitomycin C 0.04% is the most commonly used topical chemotherapy agent because it has an established role in the treatment of PAM, but it remains adjuvant therapy for conjunctival melanoma due to the relatively poor outcomes when it is used for primary therapy.11
In one study, recurrence rates for conjunctival melanoma were 26%, 51%, and 65% at 5, 10, and 15 years, respectively.12 Risk factors for recurrence include increased tumor thickness, incomplete excision, positive margins, surgical excision without adjuvant therapy, and nonlimbal location.13 A multivariate analysis of 150 patients showed that the melanoma location not touching the limbus (P=.01) and pathologic evidence of tumor to the lateral margin (P=.02) were related to tumor recurrence, with relative risks (IQR) of 2.3 (1.2-4.6) and 2.9 (1.2-7.1), respectively. Careful surgical planning using wide microsurgical excisional biopsy emphasizing a no-touch technique as well as supplemental alcohol therapy for the cornea and conjunctiva is advised.12
- Aziz HA, Gastman BR, Singh AD. Management of conjunctival melanoma: critical assessment of sentinel lymph node biopsy. Ocul Concol Pathol. 2015;1:266-273. doi:10.1159/000381719
- Shields JA, Shields CL, De Potter P. Surgical management of circumscribed conjunctival melanomas. Ophthal Plast Reconstr Surg. 1998;14:208-215.
- Griewank KG, Westekemper H, Murali R, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19:3143-3152. doi:10.1158/1078-0432.CCR-13-0163
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer. 1998;83:1664-1678. doi:10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g
- Blum ES, Yang J, Komatsubara, KM, et al. Clinical management of uveal and conjunctival melanoma. Oncology (Williston Park). 2016;30:29-32, 34-43, 48.
- Kao A, Afshar A, Bloomer M, et al. Management of primary acquired melanosis, nevus, and conjunctival melanoma. Cancer Control. 2016;23:117-125.
- Paridaens AD, McCartney AC, Minassian DC, et al. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994;78:520-528.
- Norregaard JC, Gerner N, Jensen OA, et al. Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol. 1996;234:569-572.
- Esmaeli B, Wang X, Youssef A, et al. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology. 2001;108:2101-2105.
- Tuomaala S, Kivelä T. Metastatic pattern and survival in disseminated conjunctival melanoma: implications for sentinel lymph node biopsy. Ophthalmology. 2004;111:816-821.
- Demirci H, McCormick SA, Finger PT. Topical mitomycin chemotherapy for conjunctival malignant melanoma and primary acquired melanosis with atypia: clinical experience with histopathologic observations. Arch Ophthalmol. 2000;118:885-891.
- Shields CL. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Trans Am Ophthalmol Soc. 2000;98:471-492.
- Tuomaala S, Eskelin S, Tarkkanen A, et al. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. 2002;43:3399-3408.
To the Editor:
A 58-year-old man with a pigmented lesion on the left lower eyelid was referred to the oculoplastic clinic by an outside ophthalmologist. The patient had noticed the lesion growing over the course of the last 4 to 5 months. He reported scant amounts of blood and discharge coming from the nose and left eye the week prior, which persisted for 3 days. He had no associated pain or discomfort. A slit-lamp examination revealed a pigmented left lower eyelid lesion measuring 20×15 mm with telangiectasia and an eyelid margin abnormality with no palpable lymphadenopathy. The patient was diagnosed with clinical stage T3N0M0 malignant conjunctival melanoma of the left eyelid based on the American Joint Committee on Cancer classification. It is thought to have originated from conjunctival primary acquired melanosis (PAM). The T3 stage is defined as malignant melanoma with local invasion; the lesion involved the eyelid and puncta as well as canalicular portions of the lacrimal drainage system.1 The bloody discharge was attributed to the involvement of the canalicular system, which drains tears from the eye to the nose. Melanomas can bleed, so any bloody discharge from the eye also will come through the ipsilateral nasal passage. Oncology evaluated the lesion to help determine the stage, and they found no lymph node involvement or brain, neck, chest, abdominal, or pelvic metastasis by computed tomography and magnetic resonance imaging. Sentinel lymph node biopsy was discussed with head and neck oncology specialists and was ultimately not performed per the recommendation from the Head and Neck Oncology Board; it is not a common modality for managing conjunctival melanoma because it has not been shown to alter morbidity and mortality.
The entire eyelid from the medial canthus to the lateral canthus was removed without touching the pigmented mass to ensure a “no-touch” technique removal of the mass. The no-touch technique primarily is utilized to decrease the likelihood of instrumental seeding of healthy tissues or the vascular system.2 This technique focuses on preventing any direct manipulation of the tumor and avoiding an incisional biopsy as well as removal of the tumor en bloc. The margins were cutaneous—3 mm lateral to the lateral canthus, 5 mm below the lid margin, and 3 mm medial to the medial canthus—with dissection of the medial tissue from the orbital rim and lacrimal sac fossa. The lacrimal sac and lower canaliculus were then resected. The conjunctiva 5 mm inferior to the pigmented mass and the entire palpebral conjunctiva was resected to the inferior fornix across the entire palpebral conjunctiva of the lower eyelid (Figure). The eyelid and lacrimal portions were removed as a unit. Essentially, the entire lower eyelid (full thickness), including the lateral canthus, medial canthus, canaliculus, and lacrimal sac, were removed en bloc. The final tumor staging after tissue evaluation by pathology and systemic evaluation by oncology was pT3N0bM0.

A tarsoconjunctival (Hughes) flap from the upper eyelid was used to reconstruct the posterior lamella (tarsus/conjunctiva) of the lower eyelid, and a full-thickness skin graft harvested from the ipsilateral upper eyelid was used to reconstruct the anterior lamella (skin) of the lower eyelid. The reconstruction site was allowed to heal for 4 weeks before severing the tarsoconjunctival graft to allow the separation of the upper and lower eyelids. Adjunctive topical ophthalmic chemotherapy (mitomycin C 0.04%) was started 4 weeks after the last surgery. The medication was applied 4 times daily for 1 week and restarted after the conjunctival erythema and injection subsided, which was approximately 2.5 weeks, on average. The regimen of applying the medication (1 week on and 2.5 weeks off) was completed for 4 cycles. At 1 year follow-up after his diagnosis, the patient was without local recurrence or evidence of systemic metastasis. We plan to have him continue ophthalmic and oncologic evaluation every 3 to 4 months for the next 24 months, and then every 6 months for years 2 through 5.
Ocular melanoma can be further divided into uveal and conjunctival types, both arising from the respective tissue. Melanoma of the conjunctiva commonly arises from PAM with atypia, which is an acquired conjunctival pigmented lesion similar to a skin nevus that has the potential to become dysplastic. In a genetic analysis of 78 conjunctival melanomas, BRAF mutations were identified in 29% (23/78) of tumors, and NRAS mutations were detected in 18% (14/78) of tumors3; however, in our case, there were no BRAF or NRAS mutations detected. In a study of 84,836 cases that included a diagnosis of melanoma, ocular melanoma comprised 5.2% of melanomas, with cutaneous, mucosal, and unknown primary sites totaling the remaining percentage of melanomas. Of 4522 patients with ocular melanomas, 85% had uveal melanomas; 4.8% had conjunctival melanoma; and 10.2% were classified as other—comprised of cornea, not otherwise specified (NOS); retina; lacrimal gland; orbit, NOS; overlapping lesion of the eye; and eye, NOS.4 Melanomas of the uvea, including the ciliary body, choroid, and iris, result from a notably different pathogenesis than conjunctival melanoma, with the former being primarily associated with GNAQ and GNA mutations.3 Ciliary body and choroidal melanomas each have a different pathogenesis for occurrence, with choroidal melanoma being mostly from metastasis and ciliary body melanoma from mutations or metastasis.
Pigmented lesions on the conjunctiva or sclera arise from either melanocytes or nonmelanocytes and have a diverse differential diagnosis, including congenital melanosis, conjunctival nevi, PAM or secondary acquired melanosis, or conjunctival melanoma. The diagnosis of uveal melanoma should be based on fundoscopic examination by an experienced clinician. Uveal melanoma is unlike most other cancers in that diagnosis can be by clinical fundoscopic examination alone. Imaging studies such as ultrasound and fluorescein angiography can be performed for prognostication and characterization. Fine needle aspiration biopsy for molecular analysis is becoming more routine, but the results rarely affect the plan of care. Primary treatment of uveal melanoma should strive to preserve vision and prevent metastasis; however, a primary modality has yet to show notable results in decreasing distant disease spread or overall survival. Treatment of the primary tumor should involve consideration of tumor size, location, health of the patient, and patient preference.1,5
For patients with melanoma arising from the conjunctiva, initial management should focus on local disease control, including wide local excision to avoid seeding, supplemented with cryotherapy and alcohol epitheliectomy to the cornea to ensure local tumor extinction.2,6 Techniques including enucleation and orbital exenteration historically have been used for treatment of extensive disease, but this approach has not been associated with improvement in mortality and is a cause of notable morbidity.7,8 Sentinel lymph node biopsy has an established role in the management of cutaneous melanoma, but its use in the setting of conjunctival melanoma is controversial, with studies showing that up to 50% of patients with local recurrence can develop distant metastasis with no evidence of regional lymph node involvement.9,10 When the tumor is present at the surgical margins or in the case that lesions cannot be fully excised, adjuvant therapy may improve long-term control and prevent recurrence following surgical intervention. Mitomycin C 0.04% is the most commonly used topical chemotherapy agent because it has an established role in the treatment of PAM, but it remains adjuvant therapy for conjunctival melanoma due to the relatively poor outcomes when it is used for primary therapy.11
In one study, recurrence rates for conjunctival melanoma were 26%, 51%, and 65% at 5, 10, and 15 years, respectively.12 Risk factors for recurrence include increased tumor thickness, incomplete excision, positive margins, surgical excision without adjuvant therapy, and nonlimbal location.13 A multivariate analysis of 150 patients showed that the melanoma location not touching the limbus (P=.01) and pathologic evidence of tumor to the lateral margin (P=.02) were related to tumor recurrence, with relative risks (IQR) of 2.3 (1.2-4.6) and 2.9 (1.2-7.1), respectively. Careful surgical planning using wide microsurgical excisional biopsy emphasizing a no-touch technique as well as supplemental alcohol therapy for the cornea and conjunctiva is advised.12
To the Editor:
A 58-year-old man with a pigmented lesion on the left lower eyelid was referred to the oculoplastic clinic by an outside ophthalmologist. The patient had noticed the lesion growing over the course of the last 4 to 5 months. He reported scant amounts of blood and discharge coming from the nose and left eye the week prior, which persisted for 3 days. He had no associated pain or discomfort. A slit-lamp examination revealed a pigmented left lower eyelid lesion measuring 20×15 mm with telangiectasia and an eyelid margin abnormality with no palpable lymphadenopathy. The patient was diagnosed with clinical stage T3N0M0 malignant conjunctival melanoma of the left eyelid based on the American Joint Committee on Cancer classification. It is thought to have originated from conjunctival primary acquired melanosis (PAM). The T3 stage is defined as malignant melanoma with local invasion; the lesion involved the eyelid and puncta as well as canalicular portions of the lacrimal drainage system.1 The bloody discharge was attributed to the involvement of the canalicular system, which drains tears from the eye to the nose. Melanomas can bleed, so any bloody discharge from the eye also will come through the ipsilateral nasal passage. Oncology evaluated the lesion to help determine the stage, and they found no lymph node involvement or brain, neck, chest, abdominal, or pelvic metastasis by computed tomography and magnetic resonance imaging. Sentinel lymph node biopsy was discussed with head and neck oncology specialists and was ultimately not performed per the recommendation from the Head and Neck Oncology Board; it is not a common modality for managing conjunctival melanoma because it has not been shown to alter morbidity and mortality.
The entire eyelid from the medial canthus to the lateral canthus was removed without touching the pigmented mass to ensure a “no-touch” technique removal of the mass. The no-touch technique primarily is utilized to decrease the likelihood of instrumental seeding of healthy tissues or the vascular system.2 This technique focuses on preventing any direct manipulation of the tumor and avoiding an incisional biopsy as well as removal of the tumor en bloc. The margins were cutaneous—3 mm lateral to the lateral canthus, 5 mm below the lid margin, and 3 mm medial to the medial canthus—with dissection of the medial tissue from the orbital rim and lacrimal sac fossa. The lacrimal sac and lower canaliculus were then resected. The conjunctiva 5 mm inferior to the pigmented mass and the entire palpebral conjunctiva was resected to the inferior fornix across the entire palpebral conjunctiva of the lower eyelid (Figure). The eyelid and lacrimal portions were removed as a unit. Essentially, the entire lower eyelid (full thickness), including the lateral canthus, medial canthus, canaliculus, and lacrimal sac, were removed en bloc. The final tumor staging after tissue evaluation by pathology and systemic evaluation by oncology was pT3N0bM0.

A tarsoconjunctival (Hughes) flap from the upper eyelid was used to reconstruct the posterior lamella (tarsus/conjunctiva) of the lower eyelid, and a full-thickness skin graft harvested from the ipsilateral upper eyelid was used to reconstruct the anterior lamella (skin) of the lower eyelid. The reconstruction site was allowed to heal for 4 weeks before severing the tarsoconjunctival graft to allow the separation of the upper and lower eyelids. Adjunctive topical ophthalmic chemotherapy (mitomycin C 0.04%) was started 4 weeks after the last surgery. The medication was applied 4 times daily for 1 week and restarted after the conjunctival erythema and injection subsided, which was approximately 2.5 weeks, on average. The regimen of applying the medication (1 week on and 2.5 weeks off) was completed for 4 cycles. At 1 year follow-up after his diagnosis, the patient was without local recurrence or evidence of systemic metastasis. We plan to have him continue ophthalmic and oncologic evaluation every 3 to 4 months for the next 24 months, and then every 6 months for years 2 through 5.
Ocular melanoma can be further divided into uveal and conjunctival types, both arising from the respective tissue. Melanoma of the conjunctiva commonly arises from PAM with atypia, which is an acquired conjunctival pigmented lesion similar to a skin nevus that has the potential to become dysplastic. In a genetic analysis of 78 conjunctival melanomas, BRAF mutations were identified in 29% (23/78) of tumors, and NRAS mutations were detected in 18% (14/78) of tumors3; however, in our case, there were no BRAF or NRAS mutations detected. In a study of 84,836 cases that included a diagnosis of melanoma, ocular melanoma comprised 5.2% of melanomas, with cutaneous, mucosal, and unknown primary sites totaling the remaining percentage of melanomas. Of 4522 patients with ocular melanomas, 85% had uveal melanomas; 4.8% had conjunctival melanoma; and 10.2% were classified as other—comprised of cornea, not otherwise specified (NOS); retina; lacrimal gland; orbit, NOS; overlapping lesion of the eye; and eye, NOS.4 Melanomas of the uvea, including the ciliary body, choroid, and iris, result from a notably different pathogenesis than conjunctival melanoma, with the former being primarily associated with GNAQ and GNA mutations.3 Ciliary body and choroidal melanomas each have a different pathogenesis for occurrence, with choroidal melanoma being mostly from metastasis and ciliary body melanoma from mutations or metastasis.
Pigmented lesions on the conjunctiva or sclera arise from either melanocytes or nonmelanocytes and have a diverse differential diagnosis, including congenital melanosis, conjunctival nevi, PAM or secondary acquired melanosis, or conjunctival melanoma. The diagnosis of uveal melanoma should be based on fundoscopic examination by an experienced clinician. Uveal melanoma is unlike most other cancers in that diagnosis can be by clinical fundoscopic examination alone. Imaging studies such as ultrasound and fluorescein angiography can be performed for prognostication and characterization. Fine needle aspiration biopsy for molecular analysis is becoming more routine, but the results rarely affect the plan of care. Primary treatment of uveal melanoma should strive to preserve vision and prevent metastasis; however, a primary modality has yet to show notable results in decreasing distant disease spread or overall survival. Treatment of the primary tumor should involve consideration of tumor size, location, health of the patient, and patient preference.1,5
For patients with melanoma arising from the conjunctiva, initial management should focus on local disease control, including wide local excision to avoid seeding, supplemented with cryotherapy and alcohol epitheliectomy to the cornea to ensure local tumor extinction.2,6 Techniques including enucleation and orbital exenteration historically have been used for treatment of extensive disease, but this approach has not been associated with improvement in mortality and is a cause of notable morbidity.7,8 Sentinel lymph node biopsy has an established role in the management of cutaneous melanoma, but its use in the setting of conjunctival melanoma is controversial, with studies showing that up to 50% of patients with local recurrence can develop distant metastasis with no evidence of regional lymph node involvement.9,10 When the tumor is present at the surgical margins or in the case that lesions cannot be fully excised, adjuvant therapy may improve long-term control and prevent recurrence following surgical intervention. Mitomycin C 0.04% is the most commonly used topical chemotherapy agent because it has an established role in the treatment of PAM, but it remains adjuvant therapy for conjunctival melanoma due to the relatively poor outcomes when it is used for primary therapy.11
In one study, recurrence rates for conjunctival melanoma were 26%, 51%, and 65% at 5, 10, and 15 years, respectively.12 Risk factors for recurrence include increased tumor thickness, incomplete excision, positive margins, surgical excision without adjuvant therapy, and nonlimbal location.13 A multivariate analysis of 150 patients showed that the melanoma location not touching the limbus (P=.01) and pathologic evidence of tumor to the lateral margin (P=.02) were related to tumor recurrence, with relative risks (IQR) of 2.3 (1.2-4.6) and 2.9 (1.2-7.1), respectively. Careful surgical planning using wide microsurgical excisional biopsy emphasizing a no-touch technique as well as supplemental alcohol therapy for the cornea and conjunctiva is advised.12
- Aziz HA, Gastman BR, Singh AD. Management of conjunctival melanoma: critical assessment of sentinel lymph node biopsy. Ocul Concol Pathol. 2015;1:266-273. doi:10.1159/000381719
- Shields JA, Shields CL, De Potter P. Surgical management of circumscribed conjunctival melanomas. Ophthal Plast Reconstr Surg. 1998;14:208-215.
- Griewank KG, Westekemper H, Murali R, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19:3143-3152. doi:10.1158/1078-0432.CCR-13-0163
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer. 1998;83:1664-1678. doi:10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g
- Blum ES, Yang J, Komatsubara, KM, et al. Clinical management of uveal and conjunctival melanoma. Oncology (Williston Park). 2016;30:29-32, 34-43, 48.
- Kao A, Afshar A, Bloomer M, et al. Management of primary acquired melanosis, nevus, and conjunctival melanoma. Cancer Control. 2016;23:117-125.
- Paridaens AD, McCartney AC, Minassian DC, et al. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994;78:520-528.
- Norregaard JC, Gerner N, Jensen OA, et al. Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol. 1996;234:569-572.
- Esmaeli B, Wang X, Youssef A, et al. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology. 2001;108:2101-2105.
- Tuomaala S, Kivelä T. Metastatic pattern and survival in disseminated conjunctival melanoma: implications for sentinel lymph node biopsy. Ophthalmology. 2004;111:816-821.
- Demirci H, McCormick SA, Finger PT. Topical mitomycin chemotherapy for conjunctival malignant melanoma and primary acquired melanosis with atypia: clinical experience with histopathologic observations. Arch Ophthalmol. 2000;118:885-891.
- Shields CL. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Trans Am Ophthalmol Soc. 2000;98:471-492.
- Tuomaala S, Eskelin S, Tarkkanen A, et al. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. 2002;43:3399-3408.
- Aziz HA, Gastman BR, Singh AD. Management of conjunctival melanoma: critical assessment of sentinel lymph node biopsy. Ocul Concol Pathol. 2015;1:266-273. doi:10.1159/000381719
- Shields JA, Shields CL, De Potter P. Surgical management of circumscribed conjunctival melanomas. Ophthal Plast Reconstr Surg. 1998;14:208-215.
- Griewank KG, Westekemper H, Murali R, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19:3143-3152. doi:10.1158/1078-0432.CCR-13-0163
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer. 1998;83:1664-1678. doi:10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g
- Blum ES, Yang J, Komatsubara, KM, et al. Clinical management of uveal and conjunctival melanoma. Oncology (Williston Park). 2016;30:29-32, 34-43, 48.
- Kao A, Afshar A, Bloomer M, et al. Management of primary acquired melanosis, nevus, and conjunctival melanoma. Cancer Control. 2016;23:117-125.
- Paridaens AD, McCartney AC, Minassian DC, et al. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994;78:520-528.
- Norregaard JC, Gerner N, Jensen OA, et al. Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol. 1996;234:569-572.
- Esmaeli B, Wang X, Youssef A, et al. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology. 2001;108:2101-2105.
- Tuomaala S, Kivelä T. Metastatic pattern and survival in disseminated conjunctival melanoma: implications for sentinel lymph node biopsy. Ophthalmology. 2004;111:816-821.
- Demirci H, McCormick SA, Finger PT. Topical mitomycin chemotherapy for conjunctival malignant melanoma and primary acquired melanosis with atypia: clinical experience with histopathologic observations. Arch Ophthalmol. 2000;118:885-891.
- Shields CL. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Trans Am Ophthalmol Soc. 2000;98:471-492.
- Tuomaala S, Eskelin S, Tarkkanen A, et al. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. 2002;43:3399-3408.
Practice Points
- Ophthalmologists should carefully examine palpebral and bulbar conjunctiva at each annual visit paying careful attention to pigmented nevi.
- Conjunctival abnormalities should be thoroughly documented via color photography to accurately follow for suspicious change.
Bupivacaine following Mohs surgery reduces opioid use, study finds
An injection of a randomized trial shows.
“Single-dose, in-office bupivacaine administration immediately following reconstructions known to be high risk for pain reduces postoperative narcotic use and acute pain during the time period when our patients have the highest levels of pain,” said first author Vanessa B. Voss, MD, of the University of Missouri–Columbia, who presented the findings at the annual meeting of the American College of Mohs Surgery.
“It was well tolerated, there were no adverse effects, and we recommend the consideration of using this in Mohs micrographic surgery reconstructions that are at the highest risk for pain,” she said.
Recent research has shown that Mohs micrographic surgeons have the highest rates of opioid prescribing of all dermatologists, with about 11% of patients undergoing a Mohs procedure prescribed the drugs for postoperative use, Dr. Voss explained.
Yet, with the ongoing opioid epidemic and even short courses of postoperative opioids placing patients at risk for addiction, the pressure is on to find alternative, nonaddictive strategies for the treatment of acute postoperative pain.
Bupivacaine is commonly used intraoperatively with other types of surgeries to reduce postoperative pain, with a favorable duration of action lasting up to 7 hours, compared with just 2-3 hours with lidocaine. And while its use in Mohs surgery is typically also intraoperative, along with lidocaine, the unique postoperative treatment approach in Mohs surgery has not been well studied, Dr. Voss noted.
To investigate, Dr. Voss and colleagues conducted the prospective, multicenter randomized trial, enrolling 174 patients undergoing Mohs micrographic surgery for skin cancer.
Patients were receiving complex flap reconstructions that have been specifically designated in an American Academy of Dermatology position statement to be high risk for pain following Mohs surgeries, and hence, more likely to involve prescriptions for opioids. These include reconstruction flaps of the scalp, ear, nose or lip, a wedge repair of the ear or lip, or a Mustarde cheek rotation flap.
The mean age of the patients was about 69 years, and about 65% were male. The two groups had no significant differences in demographics, tumor types, or repairs. They were randomized to receive either local injections of bupivacaine 0.5% (with no epinephrine) or placebo with sterile saline injection immediately following the procedure, with the total amount of injection standardized and dependent upon the flap surface area, ranging from 2.5 to 5 cm3.
For postoperative pain, all patients were prescribed acetaminophen 1,000 mg alternating with ibuprofen 400 mg, and tramadol, with instructions to only use tramadol as needed for breakthrough pain.
The reported use of narcotic analgesics by participants was significantly higher among those receiving placebo versus bupivacaine in the first 24 hours following surgery (odds ratio, 2.18; P = .03), as well as in the second 24 hours (OR, 2.18; P = .08) and at 48 hours combined (OR, 2.58; P < .01).
Those in the bupivacaine group also reported lower average pain scores, on a scale of 0-10, during the first 8-hour interval (mean difference, 1.6; P < .001). Importantly, overall, reports of pain medication use and the percentage of patients reporting pain under control were similar between groups, despite lower opioid use in the bupivacaine group.
“The percentage of patients reporting their pain to be under control was similar at all time intervals in both groups, so this means the bupivacaine group had their pain well-controlled despite fewer narcotics, with significant reductions in opioid use,” Dr. Voss noted.
Bupivacaine, though generally regarded as safe, has a reputation for being the most cardiotoxic of the local anesthetic agents; however, there were no such side effects reported in the study. Dr. Voss said the likely explanation is the use of low doses.
“In our study, we had no cardiotoxic effects when using up to 5 cc of 0.5%, which equates to 25 mg per patient,” she explained. This is considered a “very low dose,” since the maximum in the Food and Drug Administration pamphlet for local infiltration is 175 mg per patient every 3 hours, “yet is sufficient for reducing pain/narcotic use.”
She added that “surgeons must be careful to avoid accidental intravascular injection, which could increase risks of systemic toxicity, but this is very rare in the reconstruction settings described.”
Overall, the study suggests a potentially beneficial and unique nonopioid approach that is currently lacking for Mohs procedures associated with a high level of pain. “These findings offer a very effective intervention to reduce postoperative opioid use in this subset of patients,” Dr. Voss told this news organization. “There is not any other intervention that I am aware of to address this, although further study into other long-acting anesthetics may demonstrate similar effects.”
Commenting on the study, Justin J. Leitenberger, MD, session moderator, said that these “data could be impactful for reducing pain as well as the need for opioid medication after dermatologic surgery, both of which would be significant for our patients and public health outcomes.”
Among the challenges in treating pain following Mohs surgeries is that “every patient has a different pain threshold and expectation after surgery,” said Dr. Leitenberger, assistant professor of medicine and dermatology and codirector of dermatologic surgery, Mohs micrographic surgery, and laser and cosmetic surgery at Oregon Health & Science University, Portland.
“Patients undergoing larger repairs in tense areas of skin can experience increased pain and require prescription pain medication,” he said. “Bupivacaine, in this study, shows promise to provide longer lasting pain control from the surgical appointment and easier bridging to nonopioid pain control.”
Regarding the potential cardiotoxicities associated with the drug, Dr. Leitenberger agreed that the risks are low, and added that many surgeons have, in fact, switched to full use of bupivacaine, as opposed to combination with lidocaine, apparently without problems. “This is a small dose locally to the area after a procedure and I agree that the risks are minuscule,” he said.
“Of note, during national lidocaine shortages over the past few years, many practices transitioned to exclusive use of bupivacaine for the entire Mohs procedure, and [anecdotally], this transition did not result in toxicities that were reported,” Dr. Leitenberger said.
Commenting further, Vishal Patel, MD, assistant professor of dermatology and hematology/oncology at George Washington University and director of cutaneous oncology at the GW Cancer Center, both in Washington, also agreed that the benefits appear important. “The benefit from using bupivacaine is encouraging on multiple levels,” he said in an interview.
“Given all that we know about opioids and their negative side effect profile as well as their limited help in cutaneous surgery pain control, the use of long-acting anesthetics is an innovative and reasonable approach to provide pain control in the immediate postoperative window when patients tend to have the most pain,” said Dr. Patel, who is also director of dermatologic surgery at George Washington University.
“After this window, acetaminophen and ibuprofen, which have been shown when used in tandem in an alternating schedule to be superior to opioids, provides an effective pain regimen,” he said. “For larger and more pain-sensitive patients, this appears to be a promising combination.”
Dr. Voss, Dr. Leitenberger, and Dr. Patel have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An injection of a randomized trial shows.
“Single-dose, in-office bupivacaine administration immediately following reconstructions known to be high risk for pain reduces postoperative narcotic use and acute pain during the time period when our patients have the highest levels of pain,” said first author Vanessa B. Voss, MD, of the University of Missouri–Columbia, who presented the findings at the annual meeting of the American College of Mohs Surgery.
“It was well tolerated, there were no adverse effects, and we recommend the consideration of using this in Mohs micrographic surgery reconstructions that are at the highest risk for pain,” she said.
Recent research has shown that Mohs micrographic surgeons have the highest rates of opioid prescribing of all dermatologists, with about 11% of patients undergoing a Mohs procedure prescribed the drugs for postoperative use, Dr. Voss explained.
Yet, with the ongoing opioid epidemic and even short courses of postoperative opioids placing patients at risk for addiction, the pressure is on to find alternative, nonaddictive strategies for the treatment of acute postoperative pain.
Bupivacaine is commonly used intraoperatively with other types of surgeries to reduce postoperative pain, with a favorable duration of action lasting up to 7 hours, compared with just 2-3 hours with lidocaine. And while its use in Mohs surgery is typically also intraoperative, along with lidocaine, the unique postoperative treatment approach in Mohs surgery has not been well studied, Dr. Voss noted.
To investigate, Dr. Voss and colleagues conducted the prospective, multicenter randomized trial, enrolling 174 patients undergoing Mohs micrographic surgery for skin cancer.
Patients were receiving complex flap reconstructions that have been specifically designated in an American Academy of Dermatology position statement to be high risk for pain following Mohs surgeries, and hence, more likely to involve prescriptions for opioids. These include reconstruction flaps of the scalp, ear, nose or lip, a wedge repair of the ear or lip, or a Mustarde cheek rotation flap.
The mean age of the patients was about 69 years, and about 65% were male. The two groups had no significant differences in demographics, tumor types, or repairs. They were randomized to receive either local injections of bupivacaine 0.5% (with no epinephrine) or placebo with sterile saline injection immediately following the procedure, with the total amount of injection standardized and dependent upon the flap surface area, ranging from 2.5 to 5 cm3.
For postoperative pain, all patients were prescribed acetaminophen 1,000 mg alternating with ibuprofen 400 mg, and tramadol, with instructions to only use tramadol as needed for breakthrough pain.
The reported use of narcotic analgesics by participants was significantly higher among those receiving placebo versus bupivacaine in the first 24 hours following surgery (odds ratio, 2.18; P = .03), as well as in the second 24 hours (OR, 2.18; P = .08) and at 48 hours combined (OR, 2.58; P < .01).
Those in the bupivacaine group also reported lower average pain scores, on a scale of 0-10, during the first 8-hour interval (mean difference, 1.6; P < .001). Importantly, overall, reports of pain medication use and the percentage of patients reporting pain under control were similar between groups, despite lower opioid use in the bupivacaine group.
“The percentage of patients reporting their pain to be under control was similar at all time intervals in both groups, so this means the bupivacaine group had their pain well-controlled despite fewer narcotics, with significant reductions in opioid use,” Dr. Voss noted.
Bupivacaine, though generally regarded as safe, has a reputation for being the most cardiotoxic of the local anesthetic agents; however, there were no such side effects reported in the study. Dr. Voss said the likely explanation is the use of low doses.
“In our study, we had no cardiotoxic effects when using up to 5 cc of 0.5%, which equates to 25 mg per patient,” she explained. This is considered a “very low dose,” since the maximum in the Food and Drug Administration pamphlet for local infiltration is 175 mg per patient every 3 hours, “yet is sufficient for reducing pain/narcotic use.”
She added that “surgeons must be careful to avoid accidental intravascular injection, which could increase risks of systemic toxicity, but this is very rare in the reconstruction settings described.”
Overall, the study suggests a potentially beneficial and unique nonopioid approach that is currently lacking for Mohs procedures associated with a high level of pain. “These findings offer a very effective intervention to reduce postoperative opioid use in this subset of patients,” Dr. Voss told this news organization. “There is not any other intervention that I am aware of to address this, although further study into other long-acting anesthetics may demonstrate similar effects.”
Commenting on the study, Justin J. Leitenberger, MD, session moderator, said that these “data could be impactful for reducing pain as well as the need for opioid medication after dermatologic surgery, both of which would be significant for our patients and public health outcomes.”
Among the challenges in treating pain following Mohs surgeries is that “every patient has a different pain threshold and expectation after surgery,” said Dr. Leitenberger, assistant professor of medicine and dermatology and codirector of dermatologic surgery, Mohs micrographic surgery, and laser and cosmetic surgery at Oregon Health & Science University, Portland.
“Patients undergoing larger repairs in tense areas of skin can experience increased pain and require prescription pain medication,” he said. “Bupivacaine, in this study, shows promise to provide longer lasting pain control from the surgical appointment and easier bridging to nonopioid pain control.”
Regarding the potential cardiotoxicities associated with the drug, Dr. Leitenberger agreed that the risks are low, and added that many surgeons have, in fact, switched to full use of bupivacaine, as opposed to combination with lidocaine, apparently without problems. “This is a small dose locally to the area after a procedure and I agree that the risks are minuscule,” he said.
“Of note, during national lidocaine shortages over the past few years, many practices transitioned to exclusive use of bupivacaine for the entire Mohs procedure, and [anecdotally], this transition did not result in toxicities that were reported,” Dr. Leitenberger said.
Commenting further, Vishal Patel, MD, assistant professor of dermatology and hematology/oncology at George Washington University and director of cutaneous oncology at the GW Cancer Center, both in Washington, also agreed that the benefits appear important. “The benefit from using bupivacaine is encouraging on multiple levels,” he said in an interview.
“Given all that we know about opioids and their negative side effect profile as well as their limited help in cutaneous surgery pain control, the use of long-acting anesthetics is an innovative and reasonable approach to provide pain control in the immediate postoperative window when patients tend to have the most pain,” said Dr. Patel, who is also director of dermatologic surgery at George Washington University.
“After this window, acetaminophen and ibuprofen, which have been shown when used in tandem in an alternating schedule to be superior to opioids, provides an effective pain regimen,” he said. “For larger and more pain-sensitive patients, this appears to be a promising combination.”
Dr. Voss, Dr. Leitenberger, and Dr. Patel have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An injection of a randomized trial shows.
“Single-dose, in-office bupivacaine administration immediately following reconstructions known to be high risk for pain reduces postoperative narcotic use and acute pain during the time period when our patients have the highest levels of pain,” said first author Vanessa B. Voss, MD, of the University of Missouri–Columbia, who presented the findings at the annual meeting of the American College of Mohs Surgery.
“It was well tolerated, there were no adverse effects, and we recommend the consideration of using this in Mohs micrographic surgery reconstructions that are at the highest risk for pain,” she said.
Recent research has shown that Mohs micrographic surgeons have the highest rates of opioid prescribing of all dermatologists, with about 11% of patients undergoing a Mohs procedure prescribed the drugs for postoperative use, Dr. Voss explained.
Yet, with the ongoing opioid epidemic and even short courses of postoperative opioids placing patients at risk for addiction, the pressure is on to find alternative, nonaddictive strategies for the treatment of acute postoperative pain.
Bupivacaine is commonly used intraoperatively with other types of surgeries to reduce postoperative pain, with a favorable duration of action lasting up to 7 hours, compared with just 2-3 hours with lidocaine. And while its use in Mohs surgery is typically also intraoperative, along with lidocaine, the unique postoperative treatment approach in Mohs surgery has not been well studied, Dr. Voss noted.
To investigate, Dr. Voss and colleagues conducted the prospective, multicenter randomized trial, enrolling 174 patients undergoing Mohs micrographic surgery for skin cancer.
Patients were receiving complex flap reconstructions that have been specifically designated in an American Academy of Dermatology position statement to be high risk for pain following Mohs surgeries, and hence, more likely to involve prescriptions for opioids. These include reconstruction flaps of the scalp, ear, nose or lip, a wedge repair of the ear or lip, or a Mustarde cheek rotation flap.
The mean age of the patients was about 69 years, and about 65% were male. The two groups had no significant differences in demographics, tumor types, or repairs. They were randomized to receive either local injections of bupivacaine 0.5% (with no epinephrine) or placebo with sterile saline injection immediately following the procedure, with the total amount of injection standardized and dependent upon the flap surface area, ranging from 2.5 to 5 cm3.
For postoperative pain, all patients were prescribed acetaminophen 1,000 mg alternating with ibuprofen 400 mg, and tramadol, with instructions to only use tramadol as needed for breakthrough pain.
The reported use of narcotic analgesics by participants was significantly higher among those receiving placebo versus bupivacaine in the first 24 hours following surgery (odds ratio, 2.18; P = .03), as well as in the second 24 hours (OR, 2.18; P = .08) and at 48 hours combined (OR, 2.58; P < .01).
Those in the bupivacaine group also reported lower average pain scores, on a scale of 0-10, during the first 8-hour interval (mean difference, 1.6; P < .001). Importantly, overall, reports of pain medication use and the percentage of patients reporting pain under control were similar between groups, despite lower opioid use in the bupivacaine group.
“The percentage of patients reporting their pain to be under control was similar at all time intervals in both groups, so this means the bupivacaine group had their pain well-controlled despite fewer narcotics, with significant reductions in opioid use,” Dr. Voss noted.
Bupivacaine, though generally regarded as safe, has a reputation for being the most cardiotoxic of the local anesthetic agents; however, there were no such side effects reported in the study. Dr. Voss said the likely explanation is the use of low doses.
“In our study, we had no cardiotoxic effects when using up to 5 cc of 0.5%, which equates to 25 mg per patient,” she explained. This is considered a “very low dose,” since the maximum in the Food and Drug Administration pamphlet for local infiltration is 175 mg per patient every 3 hours, “yet is sufficient for reducing pain/narcotic use.”
She added that “surgeons must be careful to avoid accidental intravascular injection, which could increase risks of systemic toxicity, but this is very rare in the reconstruction settings described.”
Overall, the study suggests a potentially beneficial and unique nonopioid approach that is currently lacking for Mohs procedures associated with a high level of pain. “These findings offer a very effective intervention to reduce postoperative opioid use in this subset of patients,” Dr. Voss told this news organization. “There is not any other intervention that I am aware of to address this, although further study into other long-acting anesthetics may demonstrate similar effects.”
Commenting on the study, Justin J. Leitenberger, MD, session moderator, said that these “data could be impactful for reducing pain as well as the need for opioid medication after dermatologic surgery, both of which would be significant for our patients and public health outcomes.”
Among the challenges in treating pain following Mohs surgeries is that “every patient has a different pain threshold and expectation after surgery,” said Dr. Leitenberger, assistant professor of medicine and dermatology and codirector of dermatologic surgery, Mohs micrographic surgery, and laser and cosmetic surgery at Oregon Health & Science University, Portland.
“Patients undergoing larger repairs in tense areas of skin can experience increased pain and require prescription pain medication,” he said. “Bupivacaine, in this study, shows promise to provide longer lasting pain control from the surgical appointment and easier bridging to nonopioid pain control.”
Regarding the potential cardiotoxicities associated with the drug, Dr. Leitenberger agreed that the risks are low, and added that many surgeons have, in fact, switched to full use of bupivacaine, as opposed to combination with lidocaine, apparently without problems. “This is a small dose locally to the area after a procedure and I agree that the risks are minuscule,” he said.
“Of note, during national lidocaine shortages over the past few years, many practices transitioned to exclusive use of bupivacaine for the entire Mohs procedure, and [anecdotally], this transition did not result in toxicities that were reported,” Dr. Leitenberger said.
Commenting further, Vishal Patel, MD, assistant professor of dermatology and hematology/oncology at George Washington University and director of cutaneous oncology at the GW Cancer Center, both in Washington, also agreed that the benefits appear important. “The benefit from using bupivacaine is encouraging on multiple levels,” he said in an interview.
“Given all that we know about opioids and their negative side effect profile as well as their limited help in cutaneous surgery pain control, the use of long-acting anesthetics is an innovative and reasonable approach to provide pain control in the immediate postoperative window when patients tend to have the most pain,” said Dr. Patel, who is also director of dermatologic surgery at George Washington University.
“After this window, acetaminophen and ibuprofen, which have been shown when used in tandem in an alternating schedule to be superior to opioids, provides an effective pain regimen,” he said. “For larger and more pain-sensitive patients, this appears to be a promising combination.”
Dr. Voss, Dr. Leitenberger, and Dr. Patel have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACMS 2022
Skin Cancer Education in the Medical School Curriculum
To the Editor:
Skin cancer represents a notable health care burden of rising incidence.1-3 Nondermatologist health care providers play a key role in skin cancer screening through the use of skin cancer examination (SCE)1,4; however, several factors including poor diagnostic accuracy, low confidence, and lack of training have contributed to limited use of the SCE by these providers.4,5 Therefore, it is important to identify and implement changes in the medical school curriculum that can facilitate improved use of SCE in clinical practice. We sought to examine factors in the medical school curriculum that influence skin cancer education.
A voluntary electronic survey was distributed through class email and social media to all medical student classes at 4 medical schools (Figure). Responses were collected between March 2 and April 20, 2020. Survey items assessed demographics and curricular factors that influence skin cancer education.
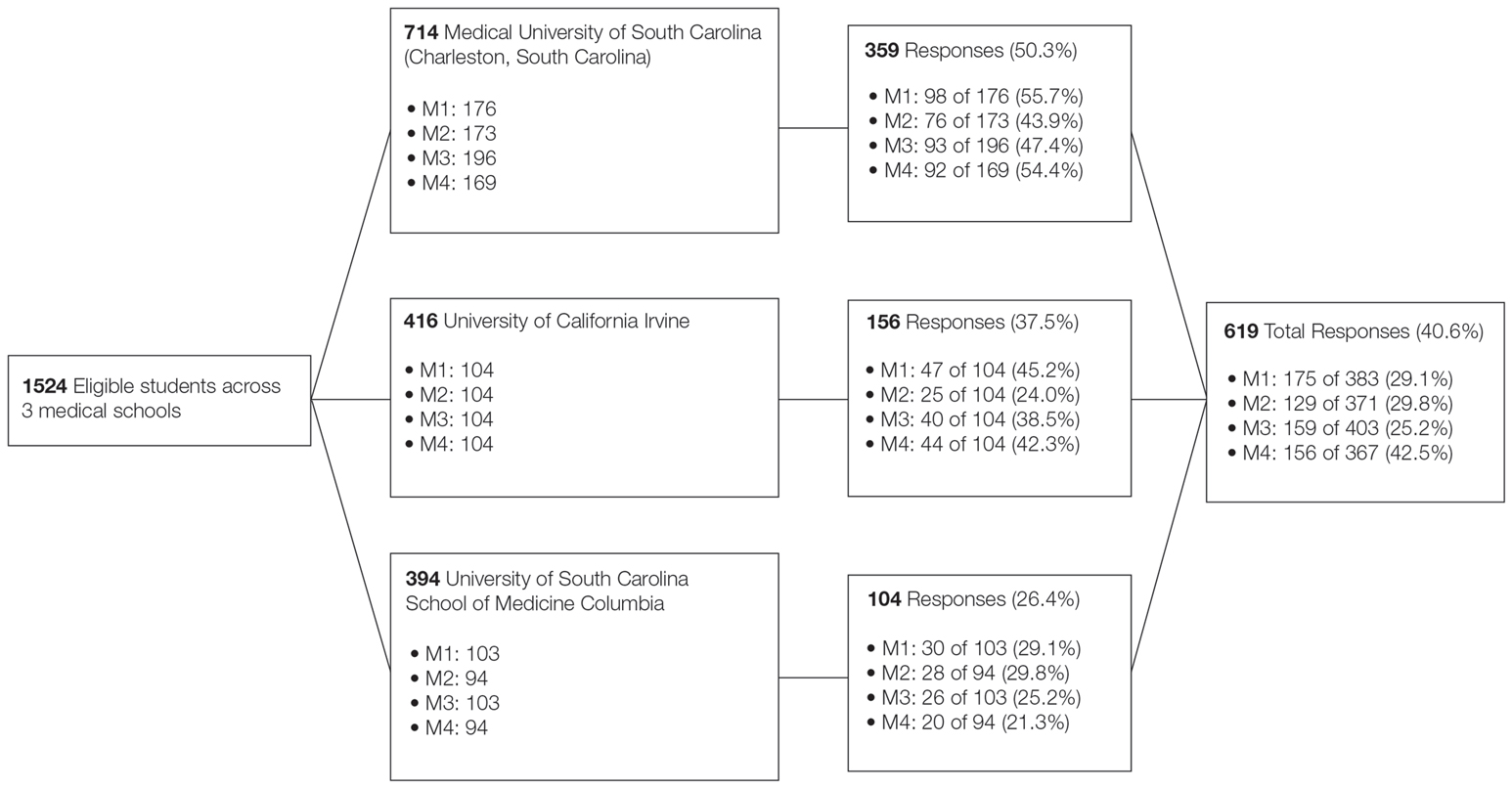
Knowledge of the clinical features of melanoma was assessed by asking participants to correctly identify at least 5 of 6 pigmented lesions as concerning or not concerning for melanoma. Confidence in performing the SCE—the primary outcome—was measured by dichotomizing a 4-point Likert-type scale (“very confident” and “moderately confident” against “slightly confident” and “not at all confident”).
Logistic regression was used to examine curricular factors associated with confidence; descriptive statistics were used for remaining analyses. Analyses were performed using SAS 9.4 statistical software. Prior to analysis, responses from the University of South Carolina School of Medicine Greenville were excluded because the response rate was less than 20%.
The survey was distributed to 1524 students; 619 (40.6%) answered at least 1 question, with a variable response rate to each item (eTable 1). Most respondents were female (351 [56.7%]); 438 (70.8%) were White.
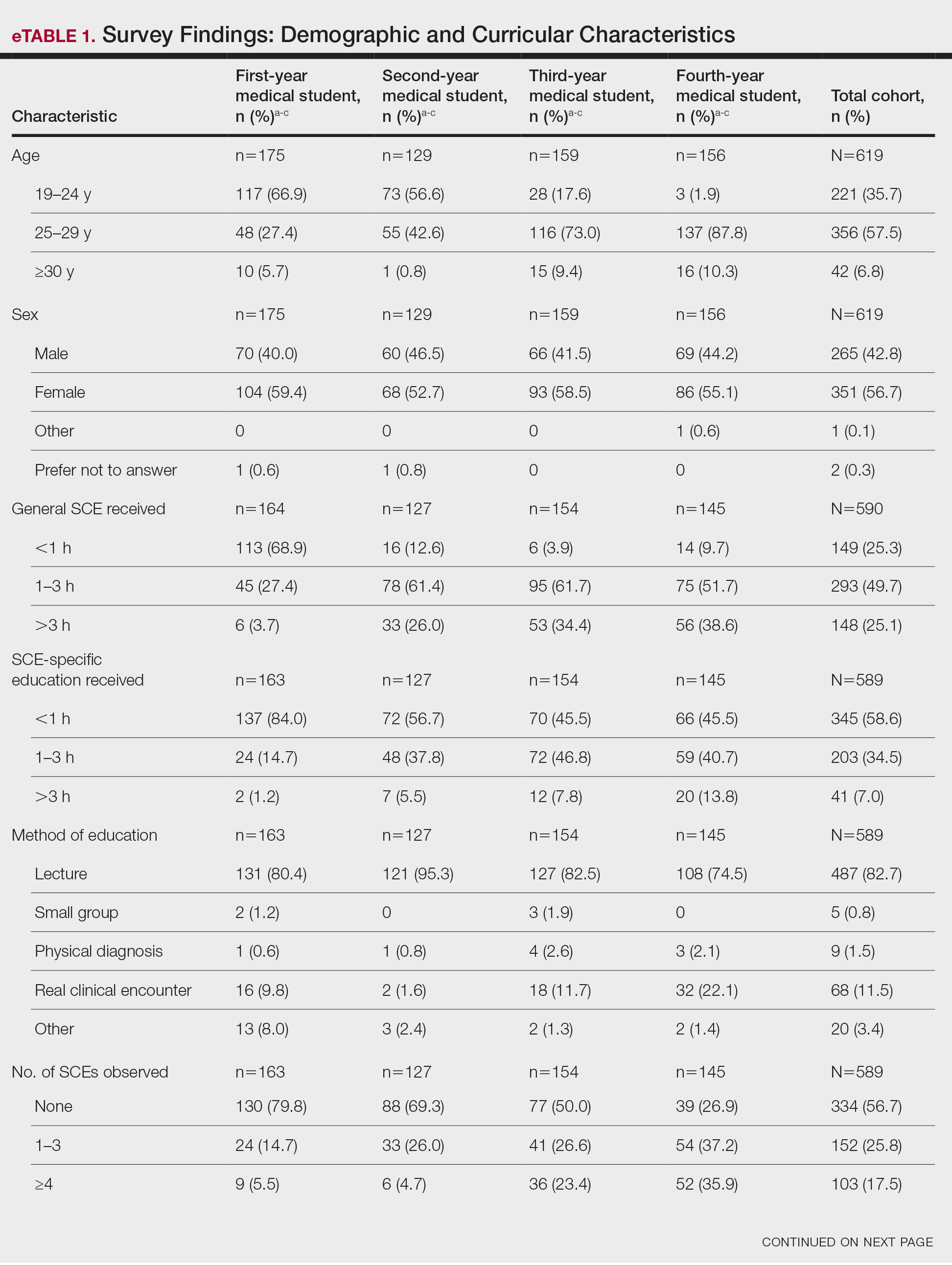
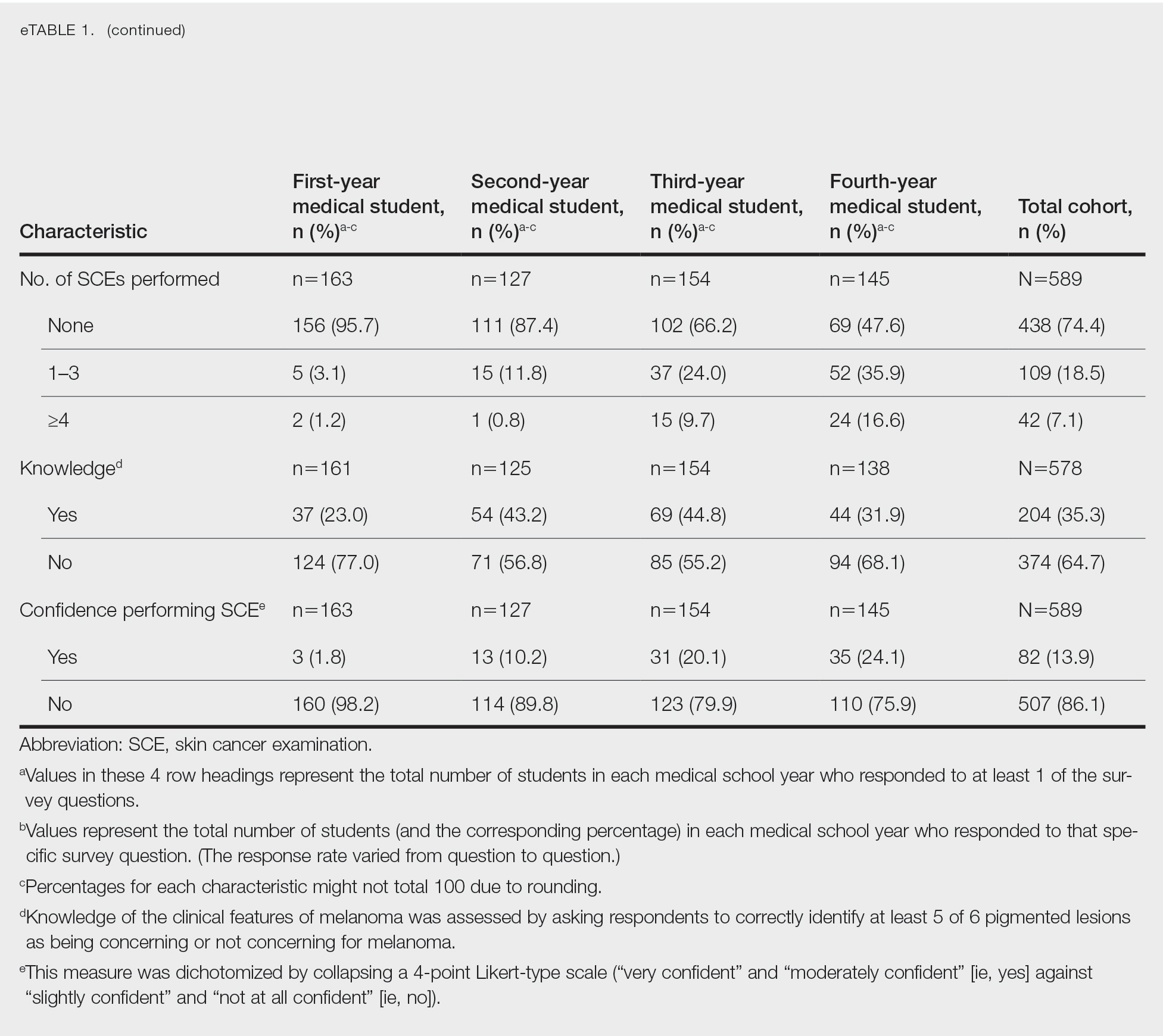
Most respondents said that they received 3 hours or less of general skin cancer (74.9%) or SCE-specific (93.0%) education by the end of their fourth year of medical training. Lecture was the most common method of instruction. Education was provided most often by dermatologists (48.6%), followed by general practice physicians (21.2%). Numerous (26.9%) fourth-year respondents reported that they had never observed SCE; even more (47.6%) had never performed SCE. Almost half of second- and third-year students (43.2% and 44.8%, respectively) considered themselves knowledgeable about the clinical features of melanoma, but only 31.9% of fourth-year students considered themselves knowledgeable.
Only 24.1% of fourth-year students reported confidence performing SCE (eTable 1). Students who received most of their instruction through real clinical encounters were 4.14 times more likely to be confident performing SCE than students who had been given lecture-based learning. Students who performed 1 to 3 SCE or 4 or more SCE were 3.02 and 32.25 times, respectively, more likely to be confident than students who had never performed SCE (eTable 2).
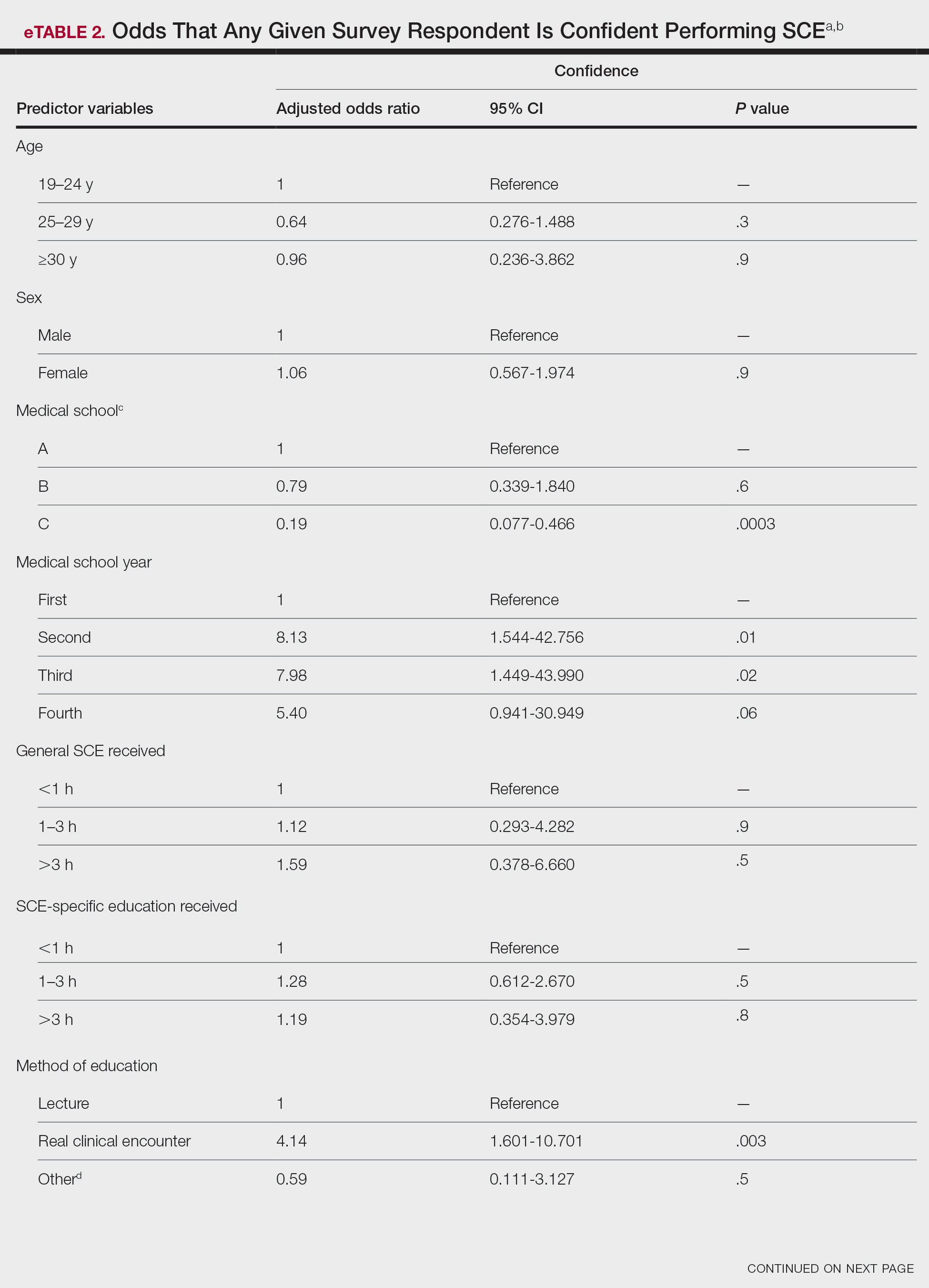
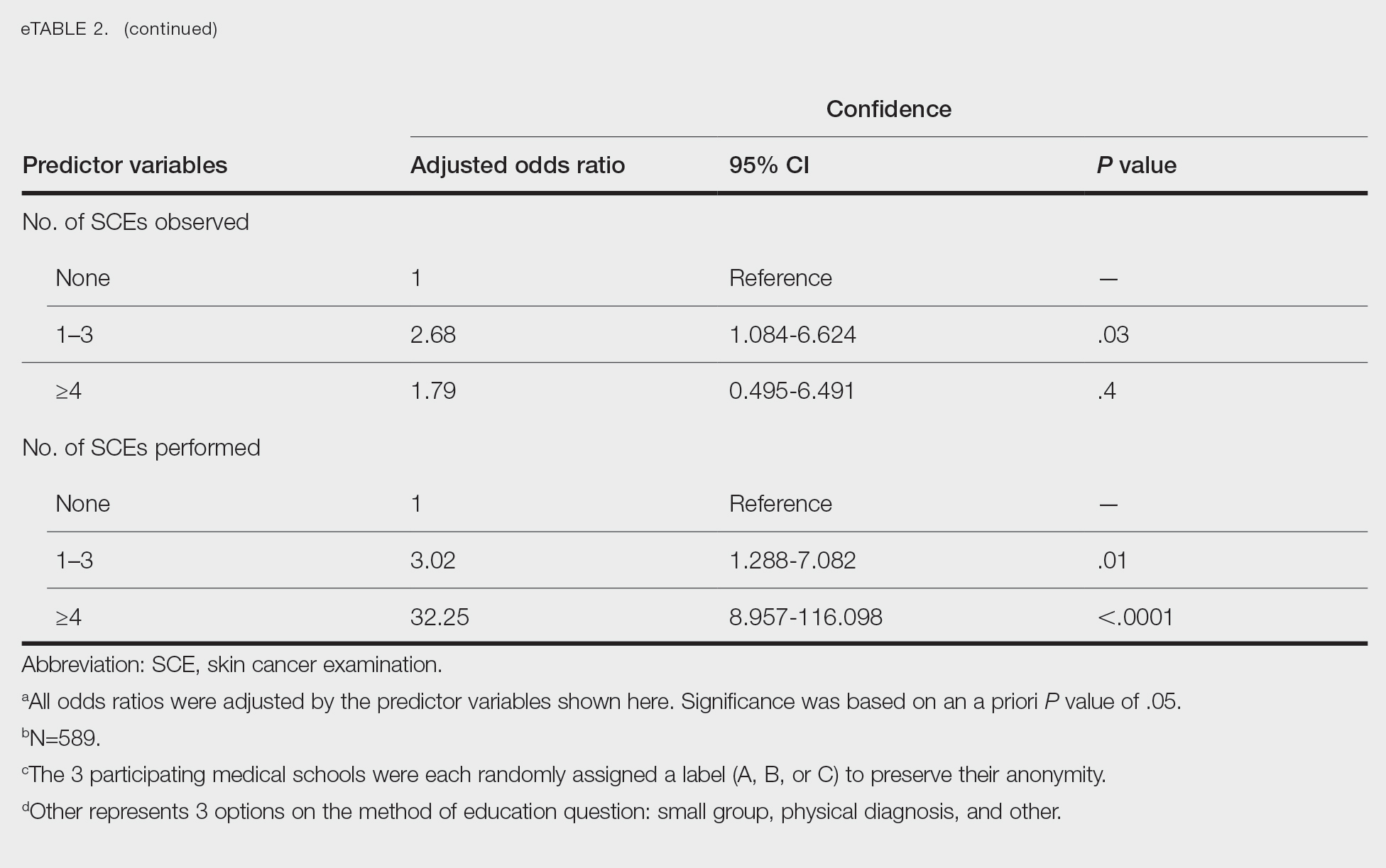
Consistent with a recent study,6 our results reflect the discrepancy between the burden and education of skin cancer. This is especially demonstrated by our cohort’s low confidence in performing SCE, a metric associated with both intention to perform and actual performance of SCE in practice.4,5 We also observed a downward trend in knowledge among students who were about to enter residency, potentially indicating the need for longitudinal training.
Given curricular time constraints, it is essential that medical schools implement changes in learning that will have the greatest impact. Although our results strongly support the efficacy of hands-on clinical training, exposure to dermatology in the second half of medical school training is limited nationwide.6 Concentrated efforts to increase clinical exposure might help prepare future physicians in all specialties to combat the burden of this disease.
Limitations of our study include the potential for selection and recall biases. Although our survey spanned multiple institutions in different regions of the United States, results might not be universally representative.
Acknowledgments—We thank Dirk Elston, MD, and Amy Wahlquist, MS (both from Charleston, South Carolina), who helped facilitate the survey on which our research is based. We also acknowledge the assistance of Philip Carmon, MD (Columbia, South Carolina); Julie Flugel (Columbia, South Carolina); Algimantas Simpson, MD (Columbia, South Carolina); Nathan Jasperse, MD (Irvine, California); Jeremy Teruel, MD (Charleston, South Carolina); Alan Snyder, MD, MSCR (Charleston, South Carolina); John Bosland (Charleston, South Carolina); and Daniel Spangler (Greenville, South Carolina).
- Guy GP Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007-2011. Am J Prev Med. 2015;48:183-187. doi:10.1016/j.amepre.2014.08.036
- Paulson KG, Gupta D, Kim TS, et al. Age-specific incidence of melanoma in the United States. JAMA Dermatol. 2020;156:57-64. doi:10.1001/jamadermatol.2019.3353
- Lim HW, Collins SAB, Resneck JS Jr, et al. Contribution of health care factors to the burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:1151-1160.e21. doi:10.1016/j.jaad.2017.03.006
- Garg A, Wang J, Reddy SB, et al; Integrated Skin Exam Consortium. Curricular factors associated with medical students’ practice of the skin cancer examination: an educational enhancement initiative by the Integrated Skin Exam Consortium. JAMA Dermatol. 2014;150:850-855. doi:10.1001/jamadermatol.2013.8723
- Oliveria SA, Heneghan MK, Cushman LF, et al. Skin cancer screening by dermatologists, family practitioners, and internists: barriers and facilitating factors. Arch Dermatol. 2011;147:39-44. doi:10.1001/archdermatol.2010.414
- Cahn BA, Harper HE, Halverstam CP, et al. Current status of dermatologic education in US medical schools. JAMA Dermatol. 2020;156:468-470. doi:10.1001/jamadermatol.2020.0006
To the Editor:
Skin cancer represents a notable health care burden of rising incidence.1-3 Nondermatologist health care providers play a key role in skin cancer screening through the use of skin cancer examination (SCE)1,4; however, several factors including poor diagnostic accuracy, low confidence, and lack of training have contributed to limited use of the SCE by these providers.4,5 Therefore, it is important to identify and implement changes in the medical school curriculum that can facilitate improved use of SCE in clinical practice. We sought to examine factors in the medical school curriculum that influence skin cancer education.
A voluntary electronic survey was distributed through class email and social media to all medical student classes at 4 medical schools (Figure). Responses were collected between March 2 and April 20, 2020. Survey items assessed demographics and curricular factors that influence skin cancer education.

Knowledge of the clinical features of melanoma was assessed by asking participants to correctly identify at least 5 of 6 pigmented lesions as concerning or not concerning for melanoma. Confidence in performing the SCE—the primary outcome—was measured by dichotomizing a 4-point Likert-type scale (“very confident” and “moderately confident” against “slightly confident” and “not at all confident”).
Logistic regression was used to examine curricular factors associated with confidence; descriptive statistics were used for remaining analyses. Analyses were performed using SAS 9.4 statistical software. Prior to analysis, responses from the University of South Carolina School of Medicine Greenville were excluded because the response rate was less than 20%.
The survey was distributed to 1524 students; 619 (40.6%) answered at least 1 question, with a variable response rate to each item (eTable 1). Most respondents were female (351 [56.7%]); 438 (70.8%) were White.


Most respondents said that they received 3 hours or less of general skin cancer (74.9%) or SCE-specific (93.0%) education by the end of their fourth year of medical training. Lecture was the most common method of instruction. Education was provided most often by dermatologists (48.6%), followed by general practice physicians (21.2%). Numerous (26.9%) fourth-year respondents reported that they had never observed SCE; even more (47.6%) had never performed SCE. Almost half of second- and third-year students (43.2% and 44.8%, respectively) considered themselves knowledgeable about the clinical features of melanoma, but only 31.9% of fourth-year students considered themselves knowledgeable.
Only 24.1% of fourth-year students reported confidence performing SCE (eTable 1). Students who received most of their instruction through real clinical encounters were 4.14 times more likely to be confident performing SCE than students who had been given lecture-based learning. Students who performed 1 to 3 SCE or 4 or more SCE were 3.02 and 32.25 times, respectively, more likely to be confident than students who had never performed SCE (eTable 2).


Consistent with a recent study,6 our results reflect the discrepancy between the burden and education of skin cancer. This is especially demonstrated by our cohort’s low confidence in performing SCE, a metric associated with both intention to perform and actual performance of SCE in practice.4,5 We also observed a downward trend in knowledge among students who were about to enter residency, potentially indicating the need for longitudinal training.
Given curricular time constraints, it is essential that medical schools implement changes in learning that will have the greatest impact. Although our results strongly support the efficacy of hands-on clinical training, exposure to dermatology in the second half of medical school training is limited nationwide.6 Concentrated efforts to increase clinical exposure might help prepare future physicians in all specialties to combat the burden of this disease.
Limitations of our study include the potential for selection and recall biases. Although our survey spanned multiple institutions in different regions of the United States, results might not be universally representative.
Acknowledgments—We thank Dirk Elston, MD, and Amy Wahlquist, MS (both from Charleston, South Carolina), who helped facilitate the survey on which our research is based. We also acknowledge the assistance of Philip Carmon, MD (Columbia, South Carolina); Julie Flugel (Columbia, South Carolina); Algimantas Simpson, MD (Columbia, South Carolina); Nathan Jasperse, MD (Irvine, California); Jeremy Teruel, MD (Charleston, South Carolina); Alan Snyder, MD, MSCR (Charleston, South Carolina); John Bosland (Charleston, South Carolina); and Daniel Spangler (Greenville, South Carolina).
To the Editor:
Skin cancer represents a notable health care burden of rising incidence.1-3 Nondermatologist health care providers play a key role in skin cancer screening through the use of skin cancer examination (SCE)1,4; however, several factors including poor diagnostic accuracy, low confidence, and lack of training have contributed to limited use of the SCE by these providers.4,5 Therefore, it is important to identify and implement changes in the medical school curriculum that can facilitate improved use of SCE in clinical practice. We sought to examine factors in the medical school curriculum that influence skin cancer education.
A voluntary electronic survey was distributed through class email and social media to all medical student classes at 4 medical schools (Figure). Responses were collected between March 2 and April 20, 2020. Survey items assessed demographics and curricular factors that influence skin cancer education.

Knowledge of the clinical features of melanoma was assessed by asking participants to correctly identify at least 5 of 6 pigmented lesions as concerning or not concerning for melanoma. Confidence in performing the SCE—the primary outcome—was measured by dichotomizing a 4-point Likert-type scale (“very confident” and “moderately confident” against “slightly confident” and “not at all confident”).
Logistic regression was used to examine curricular factors associated with confidence; descriptive statistics were used for remaining analyses. Analyses were performed using SAS 9.4 statistical software. Prior to analysis, responses from the University of South Carolina School of Medicine Greenville were excluded because the response rate was less than 20%.
The survey was distributed to 1524 students; 619 (40.6%) answered at least 1 question, with a variable response rate to each item (eTable 1). Most respondents were female (351 [56.7%]); 438 (70.8%) were White.


Most respondents said that they received 3 hours or less of general skin cancer (74.9%) or SCE-specific (93.0%) education by the end of their fourth year of medical training. Lecture was the most common method of instruction. Education was provided most often by dermatologists (48.6%), followed by general practice physicians (21.2%). Numerous (26.9%) fourth-year respondents reported that they had never observed SCE; even more (47.6%) had never performed SCE. Almost half of second- and third-year students (43.2% and 44.8%, respectively) considered themselves knowledgeable about the clinical features of melanoma, but only 31.9% of fourth-year students considered themselves knowledgeable.
Only 24.1% of fourth-year students reported confidence performing SCE (eTable 1). Students who received most of their instruction through real clinical encounters were 4.14 times more likely to be confident performing SCE than students who had been given lecture-based learning. Students who performed 1 to 3 SCE or 4 or more SCE were 3.02 and 32.25 times, respectively, more likely to be confident than students who had never performed SCE (eTable 2).


Consistent with a recent study,6 our results reflect the discrepancy between the burden and education of skin cancer. This is especially demonstrated by our cohort’s low confidence in performing SCE, a metric associated with both intention to perform and actual performance of SCE in practice.4,5 We also observed a downward trend in knowledge among students who were about to enter residency, potentially indicating the need for longitudinal training.
Given curricular time constraints, it is essential that medical schools implement changes in learning that will have the greatest impact. Although our results strongly support the efficacy of hands-on clinical training, exposure to dermatology in the second half of medical school training is limited nationwide.6 Concentrated efforts to increase clinical exposure might help prepare future physicians in all specialties to combat the burden of this disease.
Limitations of our study include the potential for selection and recall biases. Although our survey spanned multiple institutions in different regions of the United States, results might not be universally representative.
Acknowledgments—We thank Dirk Elston, MD, and Amy Wahlquist, MS (both from Charleston, South Carolina), who helped facilitate the survey on which our research is based. We also acknowledge the assistance of Philip Carmon, MD (Columbia, South Carolina); Julie Flugel (Columbia, South Carolina); Algimantas Simpson, MD (Columbia, South Carolina); Nathan Jasperse, MD (Irvine, California); Jeremy Teruel, MD (Charleston, South Carolina); Alan Snyder, MD, MSCR (Charleston, South Carolina); John Bosland (Charleston, South Carolina); and Daniel Spangler (Greenville, South Carolina).
- Guy GP Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007-2011. Am J Prev Med. 2015;48:183-187. doi:10.1016/j.amepre.2014.08.036
- Paulson KG, Gupta D, Kim TS, et al. Age-specific incidence of melanoma in the United States. JAMA Dermatol. 2020;156:57-64. doi:10.1001/jamadermatol.2019.3353
- Lim HW, Collins SAB, Resneck JS Jr, et al. Contribution of health care factors to the burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:1151-1160.e21. doi:10.1016/j.jaad.2017.03.006
- Garg A, Wang J, Reddy SB, et al; Integrated Skin Exam Consortium. Curricular factors associated with medical students’ practice of the skin cancer examination: an educational enhancement initiative by the Integrated Skin Exam Consortium. JAMA Dermatol. 2014;150:850-855. doi:10.1001/jamadermatol.2013.8723
- Oliveria SA, Heneghan MK, Cushman LF, et al. Skin cancer screening by dermatologists, family practitioners, and internists: barriers and facilitating factors. Arch Dermatol. 2011;147:39-44. doi:10.1001/archdermatol.2010.414
- Cahn BA, Harper HE, Halverstam CP, et al. Current status of dermatologic education in US medical schools. JAMA Dermatol. 2020;156:468-470. doi:10.1001/jamadermatol.2020.0006
- Guy GP Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007-2011. Am J Prev Med. 2015;48:183-187. doi:10.1016/j.amepre.2014.08.036
- Paulson KG, Gupta D, Kim TS, et al. Age-specific incidence of melanoma in the United States. JAMA Dermatol. 2020;156:57-64. doi:10.1001/jamadermatol.2019.3353
- Lim HW, Collins SAB, Resneck JS Jr, et al. Contribution of health care factors to the burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:1151-1160.e21. doi:10.1016/j.jaad.2017.03.006
- Garg A, Wang J, Reddy SB, et al; Integrated Skin Exam Consortium. Curricular factors associated with medical students’ practice of the skin cancer examination: an educational enhancement initiative by the Integrated Skin Exam Consortium. JAMA Dermatol. 2014;150:850-855. doi:10.1001/jamadermatol.2013.8723
- Oliveria SA, Heneghan MK, Cushman LF, et al. Skin cancer screening by dermatologists, family practitioners, and internists: barriers and facilitating factors. Arch Dermatol. 2011;147:39-44. doi:10.1001/archdermatol.2010.414
- Cahn BA, Harper HE, Halverstam CP, et al. Current status of dermatologic education in US medical schools. JAMA Dermatol. 2020;156:468-470. doi:10.1001/jamadermatol.2020.0006
Practice Points
- Nondermatologist practitioners play a notable role in mitigating the health care burden of skin cancer by screening with the skin cancer examination.
- Exposure to the skin cancer examination should occur during medical school prior to graduates’ entering diverse specialties.
- Most medical students received relatively few hours of skin cancer education, and many never performed or even observed a skin cancer examination prior to graduating medical school.
- Increasing hands-on training and clinical exposure during medical school is imperative to adequately prepare future physicians.
Experts urge stopping melanoma trial because of failure and harm
New
The approach seemed promising, given the efficacy of PD-1 and PD-L1 inhibitors in metastatic melanoma, and the relatively short response times to BRAF and MEK inhibitors could potentially be supplemented by longer response times associated with PD-1 and PD-L1 inhibitors. The two categories also have different mechanisms of action and nonoverlapping toxicities, which led to an expectation that the combination would be well tolerated.
But the new study joins two previous randomized, controlled trials that also failed to show much clinical benefit. IMspire150 assigned BRAF V600–mutated melanoma patients to vemurafenib and cobimetinib plus the anti–PD-L1 antibody atezolizumab or placebo. The treatment arm had a small benefit in progression-free survival (hazard ratio, 0.78), which led to Food and Drug Administration approval of the combination, though there was no significant difference when the two cohorts were assessed by an independent review committee. The KEYNOTE-022 trial examined dabrafenib plus trametinib with or without the anti–PD-1 antibody pembrolizumab, and found no difference in investigator-assessed progression free survival.
The new study was published in the Journal of Clinical Oncology. In an accompanying editorial, Margaret K. Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center, and Paul B. Chapman, MD, of Weill Cornell Medicine, both in New York, speculated that the toxicity of the triplet combination might explain the latest failure, since patients in the triplet arm had more treatment interruptions and dose reductions than the doublet arm (32% received full-dose dabrafenib vs. 54% in the doublet arm), which may have undermined efficacy.
Citing the fact that there are now three randomized, controlled trials with discouraging results, “we believe that there are sufficient data now to be confident that the addition of anti–PD-1 or anti–PD-L1 antibodies to combination RAFi [RAF inhibitors] plus MEKi [MEK inhibitors] is not associated with a significant clinical benefit and should not be studied further in melanoma.
Moreover, “there is some evidence of harm,” the editorial authors wrote. “As the additional toxicity of triplet combination limited the delivery of combination RAFi plus MEKi therapy in COMBI-I. Focus should turn instead to optimizing doses and schedules of combination RAFi plus MEKi and checkpoint inhibitors, developing treatment strategies to overcome resistance to these therapies, and determining how best to sequence combination RAFi plus MEKi therapy and checkpoint inhibitors. Regarding the latter point, there are several sequential therapy trials currently underway in previously untreated patients with BRAF V600–mutated melanoma.”
In the study, patients were randomized to receive dabrafenib and trametinib plus the anti–PD receptor–1 antibody spartalizumab or placebo. After a median follow-up of 27.2 months, mean progression-free survival was 16.2 months in the spartalizumab arm and 12.0 months in the placebo arm (HR, 0.82; P = .042). The spartalizumab group had a 69% objective response rate versus 64% in the placebo group. 55% of the spartalizumab group experienced grade 3 or higher treatment-related adverse events, compared with 33% in the placebo group.
“These results do not support broad use of first-line immunotherapy plus targeted therapy combination, but they provide additional data toward understanding the optimal application of these therapeutic classes in patients with BRAF V600–mutant metastatic melanoma,” the authors of the study wrote.
The study was funded by F Hoffmann–La Roche and Genentech. Dr. Callahan has been employed at Bristol-Myers Squibb, Celgene, and Kleo Pharmaceuticals. Dr. Callahan has consulted for or advised AstraZeneca, Moderna Therapeutics, Merck, and Immunocore. Dr. Chapman has stock or ownership interest in Rgenix; has consulted for or advised Merck, Pfizer, and Black Diamond Therapeutics; and has received research funding from Genentech.
New
The approach seemed promising, given the efficacy of PD-1 and PD-L1 inhibitors in metastatic melanoma, and the relatively short response times to BRAF and MEK inhibitors could potentially be supplemented by longer response times associated with PD-1 and PD-L1 inhibitors. The two categories also have different mechanisms of action and nonoverlapping toxicities, which led to an expectation that the combination would be well tolerated.
But the new study joins two previous randomized, controlled trials that also failed to show much clinical benefit. IMspire150 assigned BRAF V600–mutated melanoma patients to vemurafenib and cobimetinib plus the anti–PD-L1 antibody atezolizumab or placebo. The treatment arm had a small benefit in progression-free survival (hazard ratio, 0.78), which led to Food and Drug Administration approval of the combination, though there was no significant difference when the two cohorts were assessed by an independent review committee. The KEYNOTE-022 trial examined dabrafenib plus trametinib with or without the anti–PD-1 antibody pembrolizumab, and found no difference in investigator-assessed progression free survival.
The new study was published in the Journal of Clinical Oncology. In an accompanying editorial, Margaret K. Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center, and Paul B. Chapman, MD, of Weill Cornell Medicine, both in New York, speculated that the toxicity of the triplet combination might explain the latest failure, since patients in the triplet arm had more treatment interruptions and dose reductions than the doublet arm (32% received full-dose dabrafenib vs. 54% in the doublet arm), which may have undermined efficacy.
Citing the fact that there are now three randomized, controlled trials with discouraging results, “we believe that there are sufficient data now to be confident that the addition of anti–PD-1 or anti–PD-L1 antibodies to combination RAFi [RAF inhibitors] plus MEKi [MEK inhibitors] is not associated with a significant clinical benefit and should not be studied further in melanoma.
Moreover, “there is some evidence of harm,” the editorial authors wrote. “As the additional toxicity of triplet combination limited the delivery of combination RAFi plus MEKi therapy in COMBI-I. Focus should turn instead to optimizing doses and schedules of combination RAFi plus MEKi and checkpoint inhibitors, developing treatment strategies to overcome resistance to these therapies, and determining how best to sequence combination RAFi plus MEKi therapy and checkpoint inhibitors. Regarding the latter point, there are several sequential therapy trials currently underway in previously untreated patients with BRAF V600–mutated melanoma.”
In the study, patients were randomized to receive dabrafenib and trametinib plus the anti–PD receptor–1 antibody spartalizumab or placebo. After a median follow-up of 27.2 months, mean progression-free survival was 16.2 months in the spartalizumab arm and 12.0 months in the placebo arm (HR, 0.82; P = .042). The spartalizumab group had a 69% objective response rate versus 64% in the placebo group. 55% of the spartalizumab group experienced grade 3 or higher treatment-related adverse events, compared with 33% in the placebo group.
“These results do not support broad use of first-line immunotherapy plus targeted therapy combination, but they provide additional data toward understanding the optimal application of these therapeutic classes in patients with BRAF V600–mutant metastatic melanoma,” the authors of the study wrote.
The study was funded by F Hoffmann–La Roche and Genentech. Dr. Callahan has been employed at Bristol-Myers Squibb, Celgene, and Kleo Pharmaceuticals. Dr. Callahan has consulted for or advised AstraZeneca, Moderna Therapeutics, Merck, and Immunocore. Dr. Chapman has stock or ownership interest in Rgenix; has consulted for or advised Merck, Pfizer, and Black Diamond Therapeutics; and has received research funding from Genentech.
New
The approach seemed promising, given the efficacy of PD-1 and PD-L1 inhibitors in metastatic melanoma, and the relatively short response times to BRAF and MEK inhibitors could potentially be supplemented by longer response times associated with PD-1 and PD-L1 inhibitors. The two categories also have different mechanisms of action and nonoverlapping toxicities, which led to an expectation that the combination would be well tolerated.
But the new study joins two previous randomized, controlled trials that also failed to show much clinical benefit. IMspire150 assigned BRAF V600–mutated melanoma patients to vemurafenib and cobimetinib plus the anti–PD-L1 antibody atezolizumab or placebo. The treatment arm had a small benefit in progression-free survival (hazard ratio, 0.78), which led to Food and Drug Administration approval of the combination, though there was no significant difference when the two cohorts were assessed by an independent review committee. The KEYNOTE-022 trial examined dabrafenib plus trametinib with or without the anti–PD-1 antibody pembrolizumab, and found no difference in investigator-assessed progression free survival.
The new study was published in the Journal of Clinical Oncology. In an accompanying editorial, Margaret K. Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center, and Paul B. Chapman, MD, of Weill Cornell Medicine, both in New York, speculated that the toxicity of the triplet combination might explain the latest failure, since patients in the triplet arm had more treatment interruptions and dose reductions than the doublet arm (32% received full-dose dabrafenib vs. 54% in the doublet arm), which may have undermined efficacy.
Citing the fact that there are now three randomized, controlled trials with discouraging results, “we believe that there are sufficient data now to be confident that the addition of anti–PD-1 or anti–PD-L1 antibodies to combination RAFi [RAF inhibitors] plus MEKi [MEK inhibitors] is not associated with a significant clinical benefit and should not be studied further in melanoma.
Moreover, “there is some evidence of harm,” the editorial authors wrote. “As the additional toxicity of triplet combination limited the delivery of combination RAFi plus MEKi therapy in COMBI-I. Focus should turn instead to optimizing doses and schedules of combination RAFi plus MEKi and checkpoint inhibitors, developing treatment strategies to overcome resistance to these therapies, and determining how best to sequence combination RAFi plus MEKi therapy and checkpoint inhibitors. Regarding the latter point, there are several sequential therapy trials currently underway in previously untreated patients with BRAF V600–mutated melanoma.”
In the study, patients were randomized to receive dabrafenib and trametinib plus the anti–PD receptor–1 antibody spartalizumab or placebo. After a median follow-up of 27.2 months, mean progression-free survival was 16.2 months in the spartalizumab arm and 12.0 months in the placebo arm (HR, 0.82; P = .042). The spartalizumab group had a 69% objective response rate versus 64% in the placebo group. 55% of the spartalizumab group experienced grade 3 or higher treatment-related adverse events, compared with 33% in the placebo group.
“These results do not support broad use of first-line immunotherapy plus targeted therapy combination, but they provide additional data toward understanding the optimal application of these therapeutic classes in patients with BRAF V600–mutant metastatic melanoma,” the authors of the study wrote.
The study was funded by F Hoffmann–La Roche and Genentech. Dr. Callahan has been employed at Bristol-Myers Squibb, Celgene, and Kleo Pharmaceuticals. Dr. Callahan has consulted for or advised AstraZeneca, Moderna Therapeutics, Merck, and Immunocore. Dr. Chapman has stock or ownership interest in Rgenix; has consulted for or advised Merck, Pfizer, and Black Diamond Therapeutics; and has received research funding from Genentech.
FROM THE JOURNAL OF CLINICAL ONCOLOGY