User login
Adverse skin effects of cancer immunotherapy reviewed
Immune checkpoint inhibitors (ICIs) have unquestionably revolutionized the care of patients with malignant melanoma, non-small cell lung cancer, and other types of cancer.
, according to members of a European Academy of Dermatology and Venereology (EADV) task force.
“The desirable, immune-mediated oncologic response is often achieved at the cost of immune-related adverse events (irAEs) that may potentially affect any organ system,” they write in a position statement on the management of ICI-derived dermatologic adverse events.
Recommendations from the EADV “Dermatology for Cancer Patients” task force have been published in the Journal of the European Academy of Dermatology and Venereology.
Task force members developed the recommendations based on clinical experience from published data and came up with specific recommendations for treating cutaneous toxicities associated with dermatologic immune-related adverse events (dirAEs) that occur in patients receiving immunotherapy with an ICI.
ICIs include the cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) inhibitor ipilimumab (Yervoy, Bristol Myers Squibb), and inhibitors of programmed death protein 1 (PD-1) and its ligand (PD-L1), including nivolumab (Opdivo, Bristol Myers Squibb), pembrolizumab (Keytruda, Merck), and other agents.
“The basic principle of management is that the interventions should be tailored to serve the equilibrium between patients’ relief from the symptoms and signs of skin toxicity and the preservation of an unimpeded oncologic treatment,” they write.
The recommendations are in line with those included in a 2021 update of the American Society of Clinical Oncology (ASCO) guidelines on the management of irAEs in patients treated with ICIs across the whole range of organ systems, said Milan J. Anadkat, MD, professor of dermatology and director of dermatology clinical trials at Washington University School of Medicine, St. Louis. Dr. Anadkat was a coauthor of the ASCO guideline update.
Although the European recommendations focus only on dermatologic side effects of ICIs in patients with cancer, “that doesn’t diminish their importance. They do a good job of summarizing how to approach and how to manage it depending on the severity of the toxicities and the various types of toxicities,” he told this news organization.
Having a paper focused exclusively on the dermatologic side effects of ICIs allows the inclusion of photographs that can help clinicians identify specific conditions that may require referral to a dermatologist, he said.
Both Dr. Anadkat and the authors of the European recommendations noted that dermatologic irAEs are more common with CTLA-4 inhibition than with PD-1/PD-L1 inhibition.
“It has to do with where the target is,” Dr. Anadkat said. “CTLA-4 inhibition works on a central aspect of the immune system, so it’s a much less specific site, whereas PD-1 affects an interaction at the site of the tumor cell itself, so it’s a little more specific.”
Pruritus
ICI-induced pruritus can occur without apparent skin changes, they write, noting that in a recent study of patients with dirAEs, about one-third had isolated pruritus.
The task force members cite a meta-analysis indicating a pruritus incidence of 13.2% for patients treated with nivolumab and 20.2% for patients treated with pembrolizumab but respective grade 3 pruritus rates of only 0.5% and 2.3%. The reported incidence of pruritus with ipilimumab was 47% in a different study.
Recommended treatments include topical moisturizers with or without medium-to-high potency corticosteroids for grade 1 reactions, non-sedating histamines and/or GABA agonists such as pregabalin, or gabapentin for grade 2 pruritus, and suspension of ICIs until pruritus improves in patients with grade 3 pruritus.
Maculopapular rash
Maculopapular or eczema-like rashes may occur in up to 68% of patients who receive a CTLA-4 inhibitor and up to 20% of those who receive a PD1/PD-L1 inhibitor, the authors note. Rashes commonly appear within 3-6 weeks of initiating therapy.
“The clinical presentation is nonspecific and consists of a rapid onset of multiple minimally scaly, erythematous macules and papules, congregating into plaques. Lesions are mostly located on trunk and extensor surfaces of the extremities and the face is generally spared,” they write.
Maculopapular rashes are typically accompanied by itching but could be asymptomatic, they noted.
Mild (grade 1) rashes may respond to moisturizers and topical potent or super-potent corticosteroids. Patients with grade 2 rash should also receive oral antihistamines. Systemic corticosteroids may be considered for patients with grade 3 rashes but only after other dirAEs that may require specific management, such as psoriasis, are ruled out.
Psoriasis-like rash
The most common form of psoriasis seen in patients treated with ICIs is psoriasis vulgaris with plaques, but other clinical variants are also seen, the authors note.
“Topical agents (corticosteroids, Vitamin D analogues) are prescribed in Grades 1/2 and supplementary” to systemic treatment for patients with grade 3 or recalcitrant lesions, they write. “If skin-directed therapies fail to provide symptomatic control,” systemic treatment and narrow band UVB phototherapy “should be considered,” they add.
Evidence regarding the use of systemic therapies to treat psoriasis-like rash associated with ICIs is sparse. Acitretin can be safely used in patients with cancer. Low-dose methotrexate is also safe to use except in patients with non-melanoma skin cancers. Cyclosporine, however, should be avoided because of the potential for tumor-promoting effects, they emphasized.
The recommendations also cover treatment of lichen planus-like and vitiligo-like rashes, as well as hair and nail changes, autoimmune bullous disorders, and oral mucosal dirAEs.
In addition, the recommendations cover severe cutaneous adverse reactions as well as serious, potentially life-threatening dirAEs, including Stevens-Johnson syndrome/TEN, acute generalized exanthematous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome (DRESS/DIHS).
“The dose of corticosteroids may be adapted to the severity of DRESS. The therapeutic benefit of systemic corticosteroids in the management of SJS/TEN remains controversial, and some authors favor treatment with cyclosporine. However, the use of corticosteroids in this context of ICI treatment appears reasonable and should be proposed. Short courses of steroids seem also effective in AGEP,” the task force members write.
The recommendations did not have outside funding. Of the 19 authors, 6 disclosed relationships with various pharmaceutical companies, including AbbVie, Leo Pharma, Boehringer Ingelheim, Bristol Myers Squibb, and/or Janssen. Dr. Anadkat disclosed previous relationships with Merck, Bristol Myers Squibb, and current relationships with others.
A version of this article first appeared on Medscape.com.
Immune checkpoint inhibitors (ICIs) have unquestionably revolutionized the care of patients with malignant melanoma, non-small cell lung cancer, and other types of cancer.
, according to members of a European Academy of Dermatology and Venereology (EADV) task force.
“The desirable, immune-mediated oncologic response is often achieved at the cost of immune-related adverse events (irAEs) that may potentially affect any organ system,” they write in a position statement on the management of ICI-derived dermatologic adverse events.
Recommendations from the EADV “Dermatology for Cancer Patients” task force have been published in the Journal of the European Academy of Dermatology and Venereology.
Task force members developed the recommendations based on clinical experience from published data and came up with specific recommendations for treating cutaneous toxicities associated with dermatologic immune-related adverse events (dirAEs) that occur in patients receiving immunotherapy with an ICI.
ICIs include the cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) inhibitor ipilimumab (Yervoy, Bristol Myers Squibb), and inhibitors of programmed death protein 1 (PD-1) and its ligand (PD-L1), including nivolumab (Opdivo, Bristol Myers Squibb), pembrolizumab (Keytruda, Merck), and other agents.
“The basic principle of management is that the interventions should be tailored to serve the equilibrium between patients’ relief from the symptoms and signs of skin toxicity and the preservation of an unimpeded oncologic treatment,” they write.
The recommendations are in line with those included in a 2021 update of the American Society of Clinical Oncology (ASCO) guidelines on the management of irAEs in patients treated with ICIs across the whole range of organ systems, said Milan J. Anadkat, MD, professor of dermatology and director of dermatology clinical trials at Washington University School of Medicine, St. Louis. Dr. Anadkat was a coauthor of the ASCO guideline update.
Although the European recommendations focus only on dermatologic side effects of ICIs in patients with cancer, “that doesn’t diminish their importance. They do a good job of summarizing how to approach and how to manage it depending on the severity of the toxicities and the various types of toxicities,” he told this news organization.
Having a paper focused exclusively on the dermatologic side effects of ICIs allows the inclusion of photographs that can help clinicians identify specific conditions that may require referral to a dermatologist, he said.
Both Dr. Anadkat and the authors of the European recommendations noted that dermatologic irAEs are more common with CTLA-4 inhibition than with PD-1/PD-L1 inhibition.
“It has to do with where the target is,” Dr. Anadkat said. “CTLA-4 inhibition works on a central aspect of the immune system, so it’s a much less specific site, whereas PD-1 affects an interaction at the site of the tumor cell itself, so it’s a little more specific.”
Pruritus
ICI-induced pruritus can occur without apparent skin changes, they write, noting that in a recent study of patients with dirAEs, about one-third had isolated pruritus.
The task force members cite a meta-analysis indicating a pruritus incidence of 13.2% for patients treated with nivolumab and 20.2% for patients treated with pembrolizumab but respective grade 3 pruritus rates of only 0.5% and 2.3%. The reported incidence of pruritus with ipilimumab was 47% in a different study.
Recommended treatments include topical moisturizers with or without medium-to-high potency corticosteroids for grade 1 reactions, non-sedating histamines and/or GABA agonists such as pregabalin, or gabapentin for grade 2 pruritus, and suspension of ICIs until pruritus improves in patients with grade 3 pruritus.
Maculopapular rash
Maculopapular or eczema-like rashes may occur in up to 68% of patients who receive a CTLA-4 inhibitor and up to 20% of those who receive a PD1/PD-L1 inhibitor, the authors note. Rashes commonly appear within 3-6 weeks of initiating therapy.
“The clinical presentation is nonspecific and consists of a rapid onset of multiple minimally scaly, erythematous macules and papules, congregating into plaques. Lesions are mostly located on trunk and extensor surfaces of the extremities and the face is generally spared,” they write.
Maculopapular rashes are typically accompanied by itching but could be asymptomatic, they noted.
Mild (grade 1) rashes may respond to moisturizers and topical potent or super-potent corticosteroids. Patients with grade 2 rash should also receive oral antihistamines. Systemic corticosteroids may be considered for patients with grade 3 rashes but only after other dirAEs that may require specific management, such as psoriasis, are ruled out.
Psoriasis-like rash
The most common form of psoriasis seen in patients treated with ICIs is psoriasis vulgaris with plaques, but other clinical variants are also seen, the authors note.
“Topical agents (corticosteroids, Vitamin D analogues) are prescribed in Grades 1/2 and supplementary” to systemic treatment for patients with grade 3 or recalcitrant lesions, they write. “If skin-directed therapies fail to provide symptomatic control,” systemic treatment and narrow band UVB phototherapy “should be considered,” they add.
Evidence regarding the use of systemic therapies to treat psoriasis-like rash associated with ICIs is sparse. Acitretin can be safely used in patients with cancer. Low-dose methotrexate is also safe to use except in patients with non-melanoma skin cancers. Cyclosporine, however, should be avoided because of the potential for tumor-promoting effects, they emphasized.
The recommendations also cover treatment of lichen planus-like and vitiligo-like rashes, as well as hair and nail changes, autoimmune bullous disorders, and oral mucosal dirAEs.
In addition, the recommendations cover severe cutaneous adverse reactions as well as serious, potentially life-threatening dirAEs, including Stevens-Johnson syndrome/TEN, acute generalized exanthematous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome (DRESS/DIHS).
“The dose of corticosteroids may be adapted to the severity of DRESS. The therapeutic benefit of systemic corticosteroids in the management of SJS/TEN remains controversial, and some authors favor treatment with cyclosporine. However, the use of corticosteroids in this context of ICI treatment appears reasonable and should be proposed. Short courses of steroids seem also effective in AGEP,” the task force members write.
The recommendations did not have outside funding. Of the 19 authors, 6 disclosed relationships with various pharmaceutical companies, including AbbVie, Leo Pharma, Boehringer Ingelheim, Bristol Myers Squibb, and/or Janssen. Dr. Anadkat disclosed previous relationships with Merck, Bristol Myers Squibb, and current relationships with others.
A version of this article first appeared on Medscape.com.
Immune checkpoint inhibitors (ICIs) have unquestionably revolutionized the care of patients with malignant melanoma, non-small cell lung cancer, and other types of cancer.
, according to members of a European Academy of Dermatology and Venereology (EADV) task force.
“The desirable, immune-mediated oncologic response is often achieved at the cost of immune-related adverse events (irAEs) that may potentially affect any organ system,” they write in a position statement on the management of ICI-derived dermatologic adverse events.
Recommendations from the EADV “Dermatology for Cancer Patients” task force have been published in the Journal of the European Academy of Dermatology and Venereology.
Task force members developed the recommendations based on clinical experience from published data and came up with specific recommendations for treating cutaneous toxicities associated with dermatologic immune-related adverse events (dirAEs) that occur in patients receiving immunotherapy with an ICI.
ICIs include the cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) inhibitor ipilimumab (Yervoy, Bristol Myers Squibb), and inhibitors of programmed death protein 1 (PD-1) and its ligand (PD-L1), including nivolumab (Opdivo, Bristol Myers Squibb), pembrolizumab (Keytruda, Merck), and other agents.
“The basic principle of management is that the interventions should be tailored to serve the equilibrium between patients’ relief from the symptoms and signs of skin toxicity and the preservation of an unimpeded oncologic treatment,” they write.
The recommendations are in line with those included in a 2021 update of the American Society of Clinical Oncology (ASCO) guidelines on the management of irAEs in patients treated with ICIs across the whole range of organ systems, said Milan J. Anadkat, MD, professor of dermatology and director of dermatology clinical trials at Washington University School of Medicine, St. Louis. Dr. Anadkat was a coauthor of the ASCO guideline update.
Although the European recommendations focus only on dermatologic side effects of ICIs in patients with cancer, “that doesn’t diminish their importance. They do a good job of summarizing how to approach and how to manage it depending on the severity of the toxicities and the various types of toxicities,” he told this news organization.
Having a paper focused exclusively on the dermatologic side effects of ICIs allows the inclusion of photographs that can help clinicians identify specific conditions that may require referral to a dermatologist, he said.
Both Dr. Anadkat and the authors of the European recommendations noted that dermatologic irAEs are more common with CTLA-4 inhibition than with PD-1/PD-L1 inhibition.
“It has to do with where the target is,” Dr. Anadkat said. “CTLA-4 inhibition works on a central aspect of the immune system, so it’s a much less specific site, whereas PD-1 affects an interaction at the site of the tumor cell itself, so it’s a little more specific.”
Pruritus
ICI-induced pruritus can occur without apparent skin changes, they write, noting that in a recent study of patients with dirAEs, about one-third had isolated pruritus.
The task force members cite a meta-analysis indicating a pruritus incidence of 13.2% for patients treated with nivolumab and 20.2% for patients treated with pembrolizumab but respective grade 3 pruritus rates of only 0.5% and 2.3%. The reported incidence of pruritus with ipilimumab was 47% in a different study.
Recommended treatments include topical moisturizers with or without medium-to-high potency corticosteroids for grade 1 reactions, non-sedating histamines and/or GABA agonists such as pregabalin, or gabapentin for grade 2 pruritus, and suspension of ICIs until pruritus improves in patients with grade 3 pruritus.
Maculopapular rash
Maculopapular or eczema-like rashes may occur in up to 68% of patients who receive a CTLA-4 inhibitor and up to 20% of those who receive a PD1/PD-L1 inhibitor, the authors note. Rashes commonly appear within 3-6 weeks of initiating therapy.
“The clinical presentation is nonspecific and consists of a rapid onset of multiple minimally scaly, erythematous macules and papules, congregating into plaques. Lesions are mostly located on trunk and extensor surfaces of the extremities and the face is generally spared,” they write.
Maculopapular rashes are typically accompanied by itching but could be asymptomatic, they noted.
Mild (grade 1) rashes may respond to moisturizers and topical potent or super-potent corticosteroids. Patients with grade 2 rash should also receive oral antihistamines. Systemic corticosteroids may be considered for patients with grade 3 rashes but only after other dirAEs that may require specific management, such as psoriasis, are ruled out.
Psoriasis-like rash
The most common form of psoriasis seen in patients treated with ICIs is psoriasis vulgaris with plaques, but other clinical variants are also seen, the authors note.
“Topical agents (corticosteroids, Vitamin D analogues) are prescribed in Grades 1/2 and supplementary” to systemic treatment for patients with grade 3 or recalcitrant lesions, they write. “If skin-directed therapies fail to provide symptomatic control,” systemic treatment and narrow band UVB phototherapy “should be considered,” they add.
Evidence regarding the use of systemic therapies to treat psoriasis-like rash associated with ICIs is sparse. Acitretin can be safely used in patients with cancer. Low-dose methotrexate is also safe to use except in patients with non-melanoma skin cancers. Cyclosporine, however, should be avoided because of the potential for tumor-promoting effects, they emphasized.
The recommendations also cover treatment of lichen planus-like and vitiligo-like rashes, as well as hair and nail changes, autoimmune bullous disorders, and oral mucosal dirAEs.
In addition, the recommendations cover severe cutaneous adverse reactions as well as serious, potentially life-threatening dirAEs, including Stevens-Johnson syndrome/TEN, acute generalized exanthematous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome (DRESS/DIHS).
“The dose of corticosteroids may be adapted to the severity of DRESS. The therapeutic benefit of systemic corticosteroids in the management of SJS/TEN remains controversial, and some authors favor treatment with cyclosporine. However, the use of corticosteroids in this context of ICI treatment appears reasonable and should be proposed. Short courses of steroids seem also effective in AGEP,” the task force members write.
The recommendations did not have outside funding. Of the 19 authors, 6 disclosed relationships with various pharmaceutical companies, including AbbVie, Leo Pharma, Boehringer Ingelheim, Bristol Myers Squibb, and/or Janssen. Dr. Anadkat disclosed previous relationships with Merck, Bristol Myers Squibb, and current relationships with others.
A version of this article first appeared on Medscape.com.
Ways to lessen toxic effects of chemo in older adults
Age-related changes that potentiate adverse drug reactions include alterations in absorption, distribution, metabolism, and excretion. As such, older patients often require adjustments in medications to optimize safety and use. Medication adjustment is especially important for older patients on complex medication regimens for multiple conditions, such as those undergoing cancer treatment. Three recent high-quality randomized trials evaluated the use of geriatric assessment (GA) in older adults with cancer.1-3
Interdisciplinary GA can identify aging-related conditions associated with poor outcomes in older patients with cancer (e.g., toxic effects of chemotherapy) and provide recommendations aimed at improving health outcomes. The results of these trials suggest that interdisciplinary GA can improve care outcomes and oncologists’ communication for older adults with cancer, and should be considered an emerging standard of care.
Geriatric assessment and chemotherapy-related toxic effects
A cluster randomized trial1 at City of Hope National Medical Center conducted between August 2015 and February 2019 enrolled 613 participants and randomly assigned them to receive a GA-guided intervention or usual standard of care in a 2-to-1 ratio. Participants were eligible for the study if they were aged ≥65 years; had a diagnosis of solid malignant neoplasm of any stage; were starting a new chemotherapy regimen; and were fluent in English, Spanish, or Chinese.
The intervention included a GA at baseline followed by assessments focused on six common areas: sleep problems, problems with eating and feeding, incontinence, confusion, evidence of falls, and skin breakdown. An interdisciplinary team (oncologist, nurse practitioner, pharmacist, physical therapist, occupational therapist, social worker, and nutritionist) performed the assessment and developed a plan of care. Interventions were multifactorial and could include referral to specialists; recommendations for medication changes; symptom management; nutritional intervention with diet recommendations and supplementation; and interventions targeting social, spiritual, and functional well-being. Follow-up by a nurse practitioner continued until completion of chemotherapy or 6 months after starting chemotherapy, whichever was earlier.
The primary outcome was grade 3 or higher chemotherapy-related toxic effects using National Cancer Institute criteria, and secondary outcomes were advance directive completion, emergency room visits and unplanned hospitalizations, and survival up to 12 months. Results showed a 10% absolute reduction in the incidence of grade 3 or higher toxic effects (P = .02), with a number needed to treat of 10. Advance directive completion also increased by 15%, but no differences were observed for other outcomes. This study offers high-quality evidence that a GA-based intervention can reduce toxic effects of chemotherapy regimens for older adults with cancer.
Geriatric assessment in community oncology practices
A recent study by Supriya G. Mohile, MD, and colleagues2 is the first nationwide multicenter clinical trial to demonstrate the effects of GA and GA-guided management. This study was conducted in 40 oncology practices from the University of Rochester National Cancer Institute Community Oncology Research Program network. Centers were randomly assigned to intervention or usual care (362 patients treated by 68 oncologists in the intervention group and 371 patients treated by 91 oncologists in the usual-care group). Eligibility criteria were age ≥70 years; impairment in at least one GA domain other than polypharmacy; incurable advanced solid tumor or lymphoma with a plan to start new cancer treatment with a high risk for toxic effects within 4 weeks; and English language fluency. Both study groups underwent a baseline GA that assessed patients’ physical performance, functional status, comorbidity, cognition, nutrition, social support, polypharmacy, and psychological status. For the intervention group, a summary and management recommendations were provided to the treating oncologists.
The primary outcome was grade 3 or higher toxic effects within 3 months of starting a new regimen; secondary outcomes included treatment intensity and survival and GA outcomes within 3 months. A smaller proportion of patients in the intervention group experienced toxicity (51% vs. 71%), with an absolute risk reduction of 20%. Patients in the intervention group also had fewer falls and a greater reduction in medications used; there were no other differences in secondary outcomes. This study offers very strong and generalizable evidence that incorporating GA in the care of older adults with cancer at risk for toxicity can reduce toxicity as well as improve other outcomes, such as falls and polypharmacy.
Geriatric assessment and oncologist-patient communication
A secondary analysis3 of data from Dr. Mohile and colleagues2 evaluated the effect of GA-guided recommendations on oncologist-patient communication regarding comorbidities. Patients (n = 541) included in this analysis were 76.6 years of age on average and had 3.2 (standard deviation, 1.9) comorbid conditions. All patients underwent GA, but only oncologists in the intervention arm received GA-based recommendations. Clinical encounters between oncologist and patient immediately following the GA were audio recorded and analyzed to examine communication between oncologists and participants as it relates to chronic comorbid conditions.
In the intervention arm, more discussions regarding comorbidities took place, and more participants’ concerns about comorbidities were acknowledged. More importantly, participants in the intervention group were 2.4 times more likely to have their concerns about comorbidities addressed through referral or education, compared with the usual-care group (P = .004). Moreover, 41% of oncologists in the intervention arm modified dosage or cancer treatment schedule because of concern about tolerability or comorbidities. This study demonstrates beneficial effects of GA in increasing communication and perhaps consideration of comorbidities of older adults when planning cancer treatment.
Dr. Hung is professor of geriatrics and palliative care at Mount Sinai Hospital, New York. He disclosed no relevant conflicts of interest.
References
1. Li D et al. JAMA Oncol. 2021;7:e214158.
2. Mohile SG et al. Lancet. 2021;398:1894-1904.
3. Kleckner AS et al. JCO Oncol Pract. 2022;18:e9-19.
A version of this article first appeared on Medscape.com.
Age-related changes that potentiate adverse drug reactions include alterations in absorption, distribution, metabolism, and excretion. As such, older patients often require adjustments in medications to optimize safety and use. Medication adjustment is especially important for older patients on complex medication regimens for multiple conditions, such as those undergoing cancer treatment. Three recent high-quality randomized trials evaluated the use of geriatric assessment (GA) in older adults with cancer.1-3
Interdisciplinary GA can identify aging-related conditions associated with poor outcomes in older patients with cancer (e.g., toxic effects of chemotherapy) and provide recommendations aimed at improving health outcomes. The results of these trials suggest that interdisciplinary GA can improve care outcomes and oncologists’ communication for older adults with cancer, and should be considered an emerging standard of care.
Geriatric assessment and chemotherapy-related toxic effects
A cluster randomized trial1 at City of Hope National Medical Center conducted between August 2015 and February 2019 enrolled 613 participants and randomly assigned them to receive a GA-guided intervention or usual standard of care in a 2-to-1 ratio. Participants were eligible for the study if they were aged ≥65 years; had a diagnosis of solid malignant neoplasm of any stage; were starting a new chemotherapy regimen; and were fluent in English, Spanish, or Chinese.
The intervention included a GA at baseline followed by assessments focused on six common areas: sleep problems, problems with eating and feeding, incontinence, confusion, evidence of falls, and skin breakdown. An interdisciplinary team (oncologist, nurse practitioner, pharmacist, physical therapist, occupational therapist, social worker, and nutritionist) performed the assessment and developed a plan of care. Interventions were multifactorial and could include referral to specialists; recommendations for medication changes; symptom management; nutritional intervention with diet recommendations and supplementation; and interventions targeting social, spiritual, and functional well-being. Follow-up by a nurse practitioner continued until completion of chemotherapy or 6 months after starting chemotherapy, whichever was earlier.
The primary outcome was grade 3 or higher chemotherapy-related toxic effects using National Cancer Institute criteria, and secondary outcomes were advance directive completion, emergency room visits and unplanned hospitalizations, and survival up to 12 months. Results showed a 10% absolute reduction in the incidence of grade 3 or higher toxic effects (P = .02), with a number needed to treat of 10. Advance directive completion also increased by 15%, but no differences were observed for other outcomes. This study offers high-quality evidence that a GA-based intervention can reduce toxic effects of chemotherapy regimens for older adults with cancer.
Geriatric assessment in community oncology practices
A recent study by Supriya G. Mohile, MD, and colleagues2 is the first nationwide multicenter clinical trial to demonstrate the effects of GA and GA-guided management. This study was conducted in 40 oncology practices from the University of Rochester National Cancer Institute Community Oncology Research Program network. Centers were randomly assigned to intervention or usual care (362 patients treated by 68 oncologists in the intervention group and 371 patients treated by 91 oncologists in the usual-care group). Eligibility criteria were age ≥70 years; impairment in at least one GA domain other than polypharmacy; incurable advanced solid tumor or lymphoma with a plan to start new cancer treatment with a high risk for toxic effects within 4 weeks; and English language fluency. Both study groups underwent a baseline GA that assessed patients’ physical performance, functional status, comorbidity, cognition, nutrition, social support, polypharmacy, and psychological status. For the intervention group, a summary and management recommendations were provided to the treating oncologists.
The primary outcome was grade 3 or higher toxic effects within 3 months of starting a new regimen; secondary outcomes included treatment intensity and survival and GA outcomes within 3 months. A smaller proportion of patients in the intervention group experienced toxicity (51% vs. 71%), with an absolute risk reduction of 20%. Patients in the intervention group also had fewer falls and a greater reduction in medications used; there were no other differences in secondary outcomes. This study offers very strong and generalizable evidence that incorporating GA in the care of older adults with cancer at risk for toxicity can reduce toxicity as well as improve other outcomes, such as falls and polypharmacy.
Geriatric assessment and oncologist-patient communication
A secondary analysis3 of data from Dr. Mohile and colleagues2 evaluated the effect of GA-guided recommendations on oncologist-patient communication regarding comorbidities. Patients (n = 541) included in this analysis were 76.6 years of age on average and had 3.2 (standard deviation, 1.9) comorbid conditions. All patients underwent GA, but only oncologists in the intervention arm received GA-based recommendations. Clinical encounters between oncologist and patient immediately following the GA were audio recorded and analyzed to examine communication between oncologists and participants as it relates to chronic comorbid conditions.
In the intervention arm, more discussions regarding comorbidities took place, and more participants’ concerns about comorbidities were acknowledged. More importantly, participants in the intervention group were 2.4 times more likely to have their concerns about comorbidities addressed through referral or education, compared with the usual-care group (P = .004). Moreover, 41% of oncologists in the intervention arm modified dosage or cancer treatment schedule because of concern about tolerability or comorbidities. This study demonstrates beneficial effects of GA in increasing communication and perhaps consideration of comorbidities of older adults when planning cancer treatment.
Dr. Hung is professor of geriatrics and palliative care at Mount Sinai Hospital, New York. He disclosed no relevant conflicts of interest.
References
1. Li D et al. JAMA Oncol. 2021;7:e214158.
2. Mohile SG et al. Lancet. 2021;398:1894-1904.
3. Kleckner AS et al. JCO Oncol Pract. 2022;18:e9-19.
A version of this article first appeared on Medscape.com.
Age-related changes that potentiate adverse drug reactions include alterations in absorption, distribution, metabolism, and excretion. As such, older patients often require adjustments in medications to optimize safety and use. Medication adjustment is especially important for older patients on complex medication regimens for multiple conditions, such as those undergoing cancer treatment. Three recent high-quality randomized trials evaluated the use of geriatric assessment (GA) in older adults with cancer.1-3
Interdisciplinary GA can identify aging-related conditions associated with poor outcomes in older patients with cancer (e.g., toxic effects of chemotherapy) and provide recommendations aimed at improving health outcomes. The results of these trials suggest that interdisciplinary GA can improve care outcomes and oncologists’ communication for older adults with cancer, and should be considered an emerging standard of care.
Geriatric assessment and chemotherapy-related toxic effects
A cluster randomized trial1 at City of Hope National Medical Center conducted between August 2015 and February 2019 enrolled 613 participants and randomly assigned them to receive a GA-guided intervention or usual standard of care in a 2-to-1 ratio. Participants were eligible for the study if they were aged ≥65 years; had a diagnosis of solid malignant neoplasm of any stage; were starting a new chemotherapy regimen; and were fluent in English, Spanish, or Chinese.
The intervention included a GA at baseline followed by assessments focused on six common areas: sleep problems, problems with eating and feeding, incontinence, confusion, evidence of falls, and skin breakdown. An interdisciplinary team (oncologist, nurse practitioner, pharmacist, physical therapist, occupational therapist, social worker, and nutritionist) performed the assessment and developed a plan of care. Interventions were multifactorial and could include referral to specialists; recommendations for medication changes; symptom management; nutritional intervention with diet recommendations and supplementation; and interventions targeting social, spiritual, and functional well-being. Follow-up by a nurse practitioner continued until completion of chemotherapy or 6 months after starting chemotherapy, whichever was earlier.
The primary outcome was grade 3 or higher chemotherapy-related toxic effects using National Cancer Institute criteria, and secondary outcomes were advance directive completion, emergency room visits and unplanned hospitalizations, and survival up to 12 months. Results showed a 10% absolute reduction in the incidence of grade 3 or higher toxic effects (P = .02), with a number needed to treat of 10. Advance directive completion also increased by 15%, but no differences were observed for other outcomes. This study offers high-quality evidence that a GA-based intervention can reduce toxic effects of chemotherapy regimens for older adults with cancer.
Geriatric assessment in community oncology practices
A recent study by Supriya G. Mohile, MD, and colleagues2 is the first nationwide multicenter clinical trial to demonstrate the effects of GA and GA-guided management. This study was conducted in 40 oncology practices from the University of Rochester National Cancer Institute Community Oncology Research Program network. Centers were randomly assigned to intervention or usual care (362 patients treated by 68 oncologists in the intervention group and 371 patients treated by 91 oncologists in the usual-care group). Eligibility criteria were age ≥70 years; impairment in at least one GA domain other than polypharmacy; incurable advanced solid tumor or lymphoma with a plan to start new cancer treatment with a high risk for toxic effects within 4 weeks; and English language fluency. Both study groups underwent a baseline GA that assessed patients’ physical performance, functional status, comorbidity, cognition, nutrition, social support, polypharmacy, and psychological status. For the intervention group, a summary and management recommendations were provided to the treating oncologists.
The primary outcome was grade 3 or higher toxic effects within 3 months of starting a new regimen; secondary outcomes included treatment intensity and survival and GA outcomes within 3 months. A smaller proportion of patients in the intervention group experienced toxicity (51% vs. 71%), with an absolute risk reduction of 20%. Patients in the intervention group also had fewer falls and a greater reduction in medications used; there were no other differences in secondary outcomes. This study offers very strong and generalizable evidence that incorporating GA in the care of older adults with cancer at risk for toxicity can reduce toxicity as well as improve other outcomes, such as falls and polypharmacy.
Geriatric assessment and oncologist-patient communication
A secondary analysis3 of data from Dr. Mohile and colleagues2 evaluated the effect of GA-guided recommendations on oncologist-patient communication regarding comorbidities. Patients (n = 541) included in this analysis were 76.6 years of age on average and had 3.2 (standard deviation, 1.9) comorbid conditions. All patients underwent GA, but only oncologists in the intervention arm received GA-based recommendations. Clinical encounters between oncologist and patient immediately following the GA were audio recorded and analyzed to examine communication between oncologists and participants as it relates to chronic comorbid conditions.
In the intervention arm, more discussions regarding comorbidities took place, and more participants’ concerns about comorbidities were acknowledged. More importantly, participants in the intervention group were 2.4 times more likely to have their concerns about comorbidities addressed through referral or education, compared with the usual-care group (P = .004). Moreover, 41% of oncologists in the intervention arm modified dosage or cancer treatment schedule because of concern about tolerability or comorbidities. This study demonstrates beneficial effects of GA in increasing communication and perhaps consideration of comorbidities of older adults when planning cancer treatment.
Dr. Hung is professor of geriatrics and palliative care at Mount Sinai Hospital, New York. He disclosed no relevant conflicts of interest.
References
1. Li D et al. JAMA Oncol. 2021;7:e214158.
2. Mohile SG et al. Lancet. 2021;398:1894-1904.
3. Kleckner AS et al. JCO Oncol Pract. 2022;18:e9-19.
A version of this article first appeared on Medscape.com.
Complex link between gut microbiome and immunotherapy response in advanced melanoma
A large-scale than previously thought.
Overall, researchers identified a panel of species, including Roseburia spp. and Akkermansia muciniphila, associated with responses to ICI therapy. However, no single species was a “fully consistent biomarker” across the studies, the authors explain.
This “machine learning analysis confirmed the link between the microbiome and overall response rates (ORRs) and progression-free survival (PFS) with ICIs but also revealed limited reproducibility of microbiome-based signatures across cohorts,” Karla A. Lee, PhD, a clinical research fellow at King’s College London, and colleagues report. The results suggest that “the microbiome is predictive of response in some, but not all, cohorts.”
The findings were published online Feb. 28 in Nature Medicine.
Despite recent advances in targeted therapies for melanoma, less than half of the those who receive a single-agent ICI respond, and those who receive combination ICI therapy often suffer from severe drug toxicity problems. That is why finding patients more likely to respond to a single-agent ICI has become a priority.
Previous studies have identified the gut microbiome as “a potential biomarker of response, as well as a therapeutic target” in melanoma and other malignancies, but “little consensus exists on which microbiome characteristics are associated with treatment responses in the human setting,” the authors explain.
To further clarify the microbiome–immunotherapy relationship, the researchers performed metagenomic sequencing of stool samples collected from 165 ICI-naive patients with unresectable stage III or IV cutaneous melanoma from 5 observational cohorts in the Netherlands, United Kingdom, and Spain. These data were integrated with 147 samples from publicly available datasets.
First, the authors highlighted the variability in findings across these observational studies. For instance, they analyzed stool samples from one UK-based observational study of patients with melanoma (PRIMM-UK) and found a small but statistically significant difference in the microbiome composition of immunotherapy responders versus nonresponders (P = .05) but did not find such an association in a parallel study in the Netherlands (PRIMM-NL, P = .61).
The investigators also explored biomarkers of response across different cohorts and found several standouts. In trials using ORR as an endpoint, two uncultivated Roseburia species (CAG:182 and CAG:471) were associated with responses to ICIs. For patients with available PFS data, Phascolarctobacterium succinatutens and Lactobacillus vaginalis were “enriched in responders” across 7 datasets and significant in 3 of the 8 meta-analysis approaches. A muciniphila and Dorea formicigenerans were also associated with ORR and PFS at 12 months in several meta-analyses.
However, “no single bacterium was a fully consistent biomarker of response across all datasets,” the authors wrote.
Still, the findings could have important implications for the more than 50% of patients with advanced melanoma who don’t respond to single-agent ICI therapy.
“Our study shows that studying the microbiome is important to improve and personalize immunotherapy treatments for melanoma,” study coauthor Nicola Segata, PhD, principal investigator in the Laboratory of Computational Metagenomics, University of Trento, Italy, said in a press release. “However, it also suggests that because of the person-to-person variability of the gut microbiome, even larger studies must be carried out to understand the specific gut microbial features that are more likely to lead to a positive response to immunotherapy.”
Coauthor Tim Spector, PhD, head of the Department of Twin Research & Genetic Epidemiology at King’s College London, added that “the ultimate goal is to identify which specific features of the microbiome are directly influencing the clinical benefits of immunotherapy to exploit these features in new personalized approaches to support cancer immunotherapy.”
In the meantime, he said, “this study highlights the potential impact of good diet and gut health on chances of survival in patients undergoing immunotherapy.”
This study was coordinated by King’s College London, CIBIO Department of the University of Trento and European Institute of Oncology in Italy, and the University of Groningen in the Netherlands, and was funded by the Seerave Foundation. Dr. Lee, Dr. Segata, and Dr. Spector have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large-scale than previously thought.
Overall, researchers identified a panel of species, including Roseburia spp. and Akkermansia muciniphila, associated with responses to ICI therapy. However, no single species was a “fully consistent biomarker” across the studies, the authors explain.
This “machine learning analysis confirmed the link between the microbiome and overall response rates (ORRs) and progression-free survival (PFS) with ICIs but also revealed limited reproducibility of microbiome-based signatures across cohorts,” Karla A. Lee, PhD, a clinical research fellow at King’s College London, and colleagues report. The results suggest that “the microbiome is predictive of response in some, but not all, cohorts.”
The findings were published online Feb. 28 in Nature Medicine.
Despite recent advances in targeted therapies for melanoma, less than half of the those who receive a single-agent ICI respond, and those who receive combination ICI therapy often suffer from severe drug toxicity problems. That is why finding patients more likely to respond to a single-agent ICI has become a priority.
Previous studies have identified the gut microbiome as “a potential biomarker of response, as well as a therapeutic target” in melanoma and other malignancies, but “little consensus exists on which microbiome characteristics are associated with treatment responses in the human setting,” the authors explain.
To further clarify the microbiome–immunotherapy relationship, the researchers performed metagenomic sequencing of stool samples collected from 165 ICI-naive patients with unresectable stage III or IV cutaneous melanoma from 5 observational cohorts in the Netherlands, United Kingdom, and Spain. These data were integrated with 147 samples from publicly available datasets.
First, the authors highlighted the variability in findings across these observational studies. For instance, they analyzed stool samples from one UK-based observational study of patients with melanoma (PRIMM-UK) and found a small but statistically significant difference in the microbiome composition of immunotherapy responders versus nonresponders (P = .05) but did not find such an association in a parallel study in the Netherlands (PRIMM-NL, P = .61).
The investigators also explored biomarkers of response across different cohorts and found several standouts. In trials using ORR as an endpoint, two uncultivated Roseburia species (CAG:182 and CAG:471) were associated with responses to ICIs. For patients with available PFS data, Phascolarctobacterium succinatutens and Lactobacillus vaginalis were “enriched in responders” across 7 datasets and significant in 3 of the 8 meta-analysis approaches. A muciniphila and Dorea formicigenerans were also associated with ORR and PFS at 12 months in several meta-analyses.
However, “no single bacterium was a fully consistent biomarker of response across all datasets,” the authors wrote.
Still, the findings could have important implications for the more than 50% of patients with advanced melanoma who don’t respond to single-agent ICI therapy.
“Our study shows that studying the microbiome is important to improve and personalize immunotherapy treatments for melanoma,” study coauthor Nicola Segata, PhD, principal investigator in the Laboratory of Computational Metagenomics, University of Trento, Italy, said in a press release. “However, it also suggests that because of the person-to-person variability of the gut microbiome, even larger studies must be carried out to understand the specific gut microbial features that are more likely to lead to a positive response to immunotherapy.”
Coauthor Tim Spector, PhD, head of the Department of Twin Research & Genetic Epidemiology at King’s College London, added that “the ultimate goal is to identify which specific features of the microbiome are directly influencing the clinical benefits of immunotherapy to exploit these features in new personalized approaches to support cancer immunotherapy.”
In the meantime, he said, “this study highlights the potential impact of good diet and gut health on chances of survival in patients undergoing immunotherapy.”
This study was coordinated by King’s College London, CIBIO Department of the University of Trento and European Institute of Oncology in Italy, and the University of Groningen in the Netherlands, and was funded by the Seerave Foundation. Dr. Lee, Dr. Segata, and Dr. Spector have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large-scale than previously thought.
Overall, researchers identified a panel of species, including Roseburia spp. and Akkermansia muciniphila, associated with responses to ICI therapy. However, no single species was a “fully consistent biomarker” across the studies, the authors explain.
This “machine learning analysis confirmed the link between the microbiome and overall response rates (ORRs) and progression-free survival (PFS) with ICIs but also revealed limited reproducibility of microbiome-based signatures across cohorts,” Karla A. Lee, PhD, a clinical research fellow at King’s College London, and colleagues report. The results suggest that “the microbiome is predictive of response in some, but not all, cohorts.”
The findings were published online Feb. 28 in Nature Medicine.
Despite recent advances in targeted therapies for melanoma, less than half of the those who receive a single-agent ICI respond, and those who receive combination ICI therapy often suffer from severe drug toxicity problems. That is why finding patients more likely to respond to a single-agent ICI has become a priority.
Previous studies have identified the gut microbiome as “a potential biomarker of response, as well as a therapeutic target” in melanoma and other malignancies, but “little consensus exists on which microbiome characteristics are associated with treatment responses in the human setting,” the authors explain.
To further clarify the microbiome–immunotherapy relationship, the researchers performed metagenomic sequencing of stool samples collected from 165 ICI-naive patients with unresectable stage III or IV cutaneous melanoma from 5 observational cohorts in the Netherlands, United Kingdom, and Spain. These data were integrated with 147 samples from publicly available datasets.
First, the authors highlighted the variability in findings across these observational studies. For instance, they analyzed stool samples from one UK-based observational study of patients with melanoma (PRIMM-UK) and found a small but statistically significant difference in the microbiome composition of immunotherapy responders versus nonresponders (P = .05) but did not find such an association in a parallel study in the Netherlands (PRIMM-NL, P = .61).
The investigators also explored biomarkers of response across different cohorts and found several standouts. In trials using ORR as an endpoint, two uncultivated Roseburia species (CAG:182 and CAG:471) were associated with responses to ICIs. For patients with available PFS data, Phascolarctobacterium succinatutens and Lactobacillus vaginalis were “enriched in responders” across 7 datasets and significant in 3 of the 8 meta-analysis approaches. A muciniphila and Dorea formicigenerans were also associated with ORR and PFS at 12 months in several meta-analyses.
However, “no single bacterium was a fully consistent biomarker of response across all datasets,” the authors wrote.
Still, the findings could have important implications for the more than 50% of patients with advanced melanoma who don’t respond to single-agent ICI therapy.
“Our study shows that studying the microbiome is important to improve and personalize immunotherapy treatments for melanoma,” study coauthor Nicola Segata, PhD, principal investigator in the Laboratory of Computational Metagenomics, University of Trento, Italy, said in a press release. “However, it also suggests that because of the person-to-person variability of the gut microbiome, even larger studies must be carried out to understand the specific gut microbial features that are more likely to lead to a positive response to immunotherapy.”
Coauthor Tim Spector, PhD, head of the Department of Twin Research & Genetic Epidemiology at King’s College London, added that “the ultimate goal is to identify which specific features of the microbiome are directly influencing the clinical benefits of immunotherapy to exploit these features in new personalized approaches to support cancer immunotherapy.”
In the meantime, he said, “this study highlights the potential impact of good diet and gut health on chances of survival in patients undergoing immunotherapy.”
This study was coordinated by King’s College London, CIBIO Department of the University of Trento and European Institute of Oncology in Italy, and the University of Groningen in the Netherlands, and was funded by the Seerave Foundation. Dr. Lee, Dr. Segata, and Dr. Spector have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
At-Home Treatment of Pigmented Lesions With a Zinc Chloride Preparation
To the Editor:
Zinc chloride originally was used by Dr. Frederic Mohs as an in vivo tissue fixative during the early phases of Mohs micrographic surgery.1 Although this technique has since been replaced with fresh frozen tissue fixation, zinc chloride still is found in topical preparations that are readily available to patients. Specifically, black salve describes variably composed topical preparations that share the common ingredients zinc chloride and Sanguinaria canadensis (bloodroot).2 Patients self-treat with these unregulated compounds, but the majority do not have their lesions evaluated by a clinician prior to use and are unaware of the potential risks.3-5 Products containing zinc chloride and S canadensis that are not marketed as black salve present a new problem for the dermatology community.
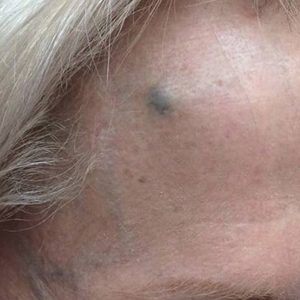
A 73-year-old man presented to our dermatology clinic for the focused evaluation of scaly lesions on the face and nose. At this visit, it was recommended he undergo a total-body skin examination for skin cancer screening given his age and substantial photodamage.
Physical examination revealed more than 20 superficial, 3- to 10-mm scars predominantly over the trunk. One scar over the left mid-back had a large, 1.2-cm peripheral rim of dark brown pigment that was clinically concerning for a melanocytic neoplasm. Shave removal of this lesion was performed. Histologic examination showed melanoma in situ with a central scar. The central scar spanned the depth of the dermis, and the melanocytic component was absent in this area, raising the question if prior biopsy or treatment had been performed on this lesion. During a discussion of the results with the patient, he was questioned about prior biopsy or treatment of this lesion. He reported prior use of a topical all-natural cream containing zinc chloride and S canadensis that he purchased online, which he had used to treat this lesion as well as numerous presumed moles.
The trend of at-home mole removal products containing the traditional ingredients in black salve seems to be one of rapidly shifting product availability as well as a departure from marketing items as black salve. Many prior black salve products are no longer available.4 The product that our patient used is a topical cream marketed as a treatment for moles and skin tags.6 Despite not being marketed as black salve, it does contain zinc chloride and S canadensis. The product’s website highlights these ingredients as being a safe and effective treatment for mole removal, with claims that the product will remove the mole or skin tag without irritating the surrounding skin and can be safely used anywhere on the body without scarring.6 A Google search at the time this article was written using the term skin tag remover revealed similar products marketed as all-natural “skin tag remover and mole corrector creams.” These similar products containing zinc chloride and S canadensis were available in the United States at the time of our initial research but have since been removed and only are available outside of the United States.7
Prior reports of melanoma masked by zinc chloride and S canadensis described the use of topical agents marketed as black salve. This new wave of products marketed as all-natural creams makes continued education on the available products and their associated risks necessary for clinicians. The lack of US Food and Drug Administration oversight for these products and their frequent introduction and discontinuation in the market makes keeping updated even more challenging. Because many patients self-treat without prior evaluation by a health care provider, treatment with these products can lead to a delay in diagnosis or inaccurate staging due to scars from the chemical destruction, both of which may have occurred in our patient.5 Until these products become regulated by the US Food and Drug Administration, it is imperative that clinicians continue to educate their patients on the lack of documented benefit and clear risks of their use as well as remain up-to-date on product trends.
- Cohen DK. Mohs micrographic surgery: past, present, and future. Dermatol Surg. 2019;45:329-339. doi:10.1097/DSS.0000000000001701
- Eastman KL. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289. doi:10.1089/acm.2012.0377
- Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80. doi:10.5826/dpc.0403a16
- McDaniel S. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
- Clark JJ. Community perceptions about the use of black salve. J Am Acad Dermatol. 2016;74:1021-1023. doi:10.1016/j.jaad.2015.10.016
- Skinprov Cream. Skinprov. Accessed February 22, 2022. https://skinprov.net
- HaloDerm. HaloDerm Inc. Accessed February 22, 2022. https://haloderm.com/
To the Editor:
Zinc chloride originally was used by Dr. Frederic Mohs as an in vivo tissue fixative during the early phases of Mohs micrographic surgery.1 Although this technique has since been replaced with fresh frozen tissue fixation, zinc chloride still is found in topical preparations that are readily available to patients. Specifically, black salve describes variably composed topical preparations that share the common ingredients zinc chloride and Sanguinaria canadensis (bloodroot).2 Patients self-treat with these unregulated compounds, but the majority do not have their lesions evaluated by a clinician prior to use and are unaware of the potential risks.3-5 Products containing zinc chloride and S canadensis that are not marketed as black salve present a new problem for the dermatology community.
A 73-year-old man presented to our dermatology clinic for the focused evaluation of scaly lesions on the face and nose. At this visit, it was recommended he undergo a total-body skin examination for skin cancer screening given his age and substantial photodamage.
Physical examination revealed more than 20 superficial, 3- to 10-mm scars predominantly over the trunk. One scar over the left mid-back had a large, 1.2-cm peripheral rim of dark brown pigment that was clinically concerning for a melanocytic neoplasm. Shave removal of this lesion was performed. Histologic examination showed melanoma in situ with a central scar. The central scar spanned the depth of the dermis, and the melanocytic component was absent in this area, raising the question if prior biopsy or treatment had been performed on this lesion. During a discussion of the results with the patient, he was questioned about prior biopsy or treatment of this lesion. He reported prior use of a topical all-natural cream containing zinc chloride and S canadensis that he purchased online, which he had used to treat this lesion as well as numerous presumed moles.
The trend of at-home mole removal products containing the traditional ingredients in black salve seems to be one of rapidly shifting product availability as well as a departure from marketing items as black salve. Many prior black salve products are no longer available.4 The product that our patient used is a topical cream marketed as a treatment for moles and skin tags.6 Despite not being marketed as black salve, it does contain zinc chloride and S canadensis. The product’s website highlights these ingredients as being a safe and effective treatment for mole removal, with claims that the product will remove the mole or skin tag without irritating the surrounding skin and can be safely used anywhere on the body without scarring.6 A Google search at the time this article was written using the term skin tag remover revealed similar products marketed as all-natural “skin tag remover and mole corrector creams.” These similar products containing zinc chloride and S canadensis were available in the United States at the time of our initial research but have since been removed and only are available outside of the United States.7
Prior reports of melanoma masked by zinc chloride and S canadensis described the use of topical agents marketed as black salve. This new wave of products marketed as all-natural creams makes continued education on the available products and their associated risks necessary for clinicians. The lack of US Food and Drug Administration oversight for these products and their frequent introduction and discontinuation in the market makes keeping updated even more challenging. Because many patients self-treat without prior evaluation by a health care provider, treatment with these products can lead to a delay in diagnosis or inaccurate staging due to scars from the chemical destruction, both of which may have occurred in our patient.5 Until these products become regulated by the US Food and Drug Administration, it is imperative that clinicians continue to educate their patients on the lack of documented benefit and clear risks of their use as well as remain up-to-date on product trends.
To the Editor:
Zinc chloride originally was used by Dr. Frederic Mohs as an in vivo tissue fixative during the early phases of Mohs micrographic surgery.1 Although this technique has since been replaced with fresh frozen tissue fixation, zinc chloride still is found in topical preparations that are readily available to patients. Specifically, black salve describes variably composed topical preparations that share the common ingredients zinc chloride and Sanguinaria canadensis (bloodroot).2 Patients self-treat with these unregulated compounds, but the majority do not have their lesions evaluated by a clinician prior to use and are unaware of the potential risks.3-5 Products containing zinc chloride and S canadensis that are not marketed as black salve present a new problem for the dermatology community.
A 73-year-old man presented to our dermatology clinic for the focused evaluation of scaly lesions on the face and nose. At this visit, it was recommended he undergo a total-body skin examination for skin cancer screening given his age and substantial photodamage.
Physical examination revealed more than 20 superficial, 3- to 10-mm scars predominantly over the trunk. One scar over the left mid-back had a large, 1.2-cm peripheral rim of dark brown pigment that was clinically concerning for a melanocytic neoplasm. Shave removal of this lesion was performed. Histologic examination showed melanoma in situ with a central scar. The central scar spanned the depth of the dermis, and the melanocytic component was absent in this area, raising the question if prior biopsy or treatment had been performed on this lesion. During a discussion of the results with the patient, he was questioned about prior biopsy or treatment of this lesion. He reported prior use of a topical all-natural cream containing zinc chloride and S canadensis that he purchased online, which he had used to treat this lesion as well as numerous presumed moles.
The trend of at-home mole removal products containing the traditional ingredients in black salve seems to be one of rapidly shifting product availability as well as a departure from marketing items as black salve. Many prior black salve products are no longer available.4 The product that our patient used is a topical cream marketed as a treatment for moles and skin tags.6 Despite not being marketed as black salve, it does contain zinc chloride and S canadensis. The product’s website highlights these ingredients as being a safe and effective treatment for mole removal, with claims that the product will remove the mole or skin tag without irritating the surrounding skin and can be safely used anywhere on the body without scarring.6 A Google search at the time this article was written using the term skin tag remover revealed similar products marketed as all-natural “skin tag remover and mole corrector creams.” These similar products containing zinc chloride and S canadensis were available in the United States at the time of our initial research but have since been removed and only are available outside of the United States.7
Prior reports of melanoma masked by zinc chloride and S canadensis described the use of topical agents marketed as black salve. This new wave of products marketed as all-natural creams makes continued education on the available products and their associated risks necessary for clinicians. The lack of US Food and Drug Administration oversight for these products and their frequent introduction and discontinuation in the market makes keeping updated even more challenging. Because many patients self-treat without prior evaluation by a health care provider, treatment with these products can lead to a delay in diagnosis or inaccurate staging due to scars from the chemical destruction, both of which may have occurred in our patient.5 Until these products become regulated by the US Food and Drug Administration, it is imperative that clinicians continue to educate their patients on the lack of documented benefit and clear risks of their use as well as remain up-to-date on product trends.
- Cohen DK. Mohs micrographic surgery: past, present, and future. Dermatol Surg. 2019;45:329-339. doi:10.1097/DSS.0000000000001701
- Eastman KL. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289. doi:10.1089/acm.2012.0377
- Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80. doi:10.5826/dpc.0403a16
- McDaniel S. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
- Clark JJ. Community perceptions about the use of black salve. J Am Acad Dermatol. 2016;74:1021-1023. doi:10.1016/j.jaad.2015.10.016
- Skinprov Cream. Skinprov. Accessed February 22, 2022. https://skinprov.net
- HaloDerm. HaloDerm Inc. Accessed February 22, 2022. https://haloderm.com/
- Cohen DK. Mohs micrographic surgery: past, present, and future. Dermatol Surg. 2019;45:329-339. doi:10.1097/DSS.0000000000001701
- Eastman KL. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289. doi:10.1089/acm.2012.0377
- Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80. doi:10.5826/dpc.0403a16
- McDaniel S. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
- Clark JJ. Community perceptions about the use of black salve. J Am Acad Dermatol. 2016;74:1021-1023. doi:10.1016/j.jaad.2015.10.016
- Skinprov Cream. Skinprov. Accessed February 22, 2022. https://skinprov.net
- HaloDerm. HaloDerm Inc. Accessed February 22, 2022. https://haloderm.com/
Practice Points
- Zinc chloride preparations are readily available over the counter and unregulated.
- Patients may attempt to self-treat pigmented lesions based on claims they see online.
- When asking patients about prior treatments, it may be prudent to specifically ask about over-the-counter products and their ingredients.
High early recurrence rates with Merkel cell carcinoma
, and more than half of all patients with stage IV disease will have a recurrence within 1 year of definitive therapy, results of a new study show.
A study of 618 patients with MCC who were enrolled in a Seattle-based data repository shows that among all patients the 5-year recurrence rate was 40%. The risk of recurrence within the first year was 11% for patients with pathologic stage I disease, 33% for those with stage IIA/IIB disease, 45% for those with stage IIIB disease, and 58% for patients with pathologic stage IV MCC.
Approximately 95% of all recurrences happened within 3 years of the initial diagnosis, report Aubriana McEvoy, MD, from the University of Washington in Seattle, and colleagues.
“This cohort study indicates that the highest yield (and likely most cost-effective) time period for detecting MCC recurrence is 1-3 years after diagnosis,” they write in a study published online in JAMA Dermatology.
The estimated annual incidence of MCC in the United States in 2018 was 2,000 according to the American Cancer Society. The annual incidence rate is rising rapidly, however, and is estimated to reach 3,284 by 2025, McEvoy and colleagues write.
Although MCC is known to have high recurrence rates and is associated with a higher mortality rate than malignant melanoma, recurrence rate data are not captured by either the Surveillance, Epidemiology, and End Results (SEER) database or by the National Cancer Database. As a result, estimates of recurrence rates with MCC have been all over the map, ranging from 27% to 77%, depending on the population studied.
But as senior author Paul Nghiem, MD, PhD, professor and chair of dermatology at the University of Washington, Seattle, told this news organization, recurrence rates over time in their study were remarkably consistent.
“The biggest surprise to me was that, when we broke our nearly 20-year cohort into three 5- or 6-year chunks, every one of the groups had a 40% recurrence rate, within 1%. So we feel really confident that’s the right number,” he said.
Dr. Nghiem and colleagues report that, in contrast to patients with MCC, approximately 19% of patients with melanoma will have a recurrence, as will an estimated 5%-9% of patients with squamous cell carcinoma and 1%-10% of patients with basal cell carcinoma.
The fact that recurrence rates of MCC have remained stable over time despite presumed improvements in definitive therapy is disappointing, Dr. Nghiem acknowledged. He noted that it’s still unclear whether immunotherapy will have the same dramatic effect on survival rates for patients with MCC as it has for patients with malignant melanoma.
The high recurrence rates following definitive therapy for patients with early-stage disease was a novel finding, commented Shawn Demehri, MD, PhD, director of the high-risk skin cancer clinic at Massachusetts General Hospital in Boston.
“When you’re looking at patients with stage I or stage II, and they have definitive surgery but still have recurrences at a higher rate than melanoma, it brings home the point that these are among the most aggressive tumors of the skin,” he said in an interview.
The high recurrence rates seen with MCC are attributable to a variety of factors.
“This is a rare cancer of mostly older individuals with a lot of comorbidities, and also a cancer that, even though it is a primary cancer, might be detected a little later than even a melanoma primary tumor, just because of the nature of the neuroendocrine tumor cells,” he said.
Dr. Demehri was not involved in the study.
Prospective cohort
The study cohort consisted of 618 patients with MCC. The median age of the patients was 69, and 227 (37%) were women. The patients were enrolled within 6 months of their diagnosis in the prospective data repository from 2003 through 2019. Of this group, 223 had a recurrence of MCC.
As noted, there was a high risk of recurrence within 1 year, ranging from 11% for patients with pathologic stage I tumors to 58% for those with stage IV disease, and 95% of all recurrences occurred within 3 years of definitive therapy.
To get a better picture of the natural history of MCC recurrence, the investigators studied a cohort of patients with pathologically confirmed MCC who were prospectively enrolled from January 2003 through April 2019 in a data repository maintained at the University of Washington.
In addition to disease stage, factors associated with increased recurrence risk in univariable analyses include immunosuppression (hazard ratio, 2.4; P < .001), male sex (HR, 1.9; P < .001), known primary lesion among patients with clinically detectable nodal disease (HR, 2.3; P = .001), and older age (HR, 1.1, P = .06 for each 10-year increase).
Of the 187 patients in the cohort who died during the study, 121 died from MCC. At 4 years after diagnosis, MCC-specific survival rates were 95% for patients with pathologic stage I, 84% with stage IIA/IIB, 80% with stage IIIA, 58% with stage IIIB, and 41% with stage IV.
Evidence supports close monitoring within the first 3 years for patients with stage I-II MCC. Local recurrence within or adjacent to the primary tumor scar was associated with a 5-year MCC-specific survival rate of 85%, compared with 88% of patients with stage I or II disease who did not have recurrences.
“Because more than 90% of MCC recurrences arise within 3 years, it is appropriate to adjust surveillance intensity accordingly. Stage- and time-specific recurrence data can assist in appropriately focusing surveillance resources on patients and time intervals in which recurrence risk is highest,” the authors wrote.
“If you’re a patient who has not had your cancer come back for 3, 4, or 5 years, you can really cut down on the intensity of your follow-up and scans,” Dr. Nghiem said.
“We do now have two excellent blood tests that are working very well, and we have really good ways to detect the cancer coming back early, and that’s important, because we have potentially curative therapies that tend to work better if you catch the cancer early,” he said.
The study was supported by the National Institutes of Health. Dr. Nghiem reported personal fees and institutional support outside the study from several companies and patents for Merkel cell therapies with the University of Washington and University of Denmark. Dr. Demehri has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, and more than half of all patients with stage IV disease will have a recurrence within 1 year of definitive therapy, results of a new study show.
A study of 618 patients with MCC who were enrolled in a Seattle-based data repository shows that among all patients the 5-year recurrence rate was 40%. The risk of recurrence within the first year was 11% for patients with pathologic stage I disease, 33% for those with stage IIA/IIB disease, 45% for those with stage IIIB disease, and 58% for patients with pathologic stage IV MCC.
Approximately 95% of all recurrences happened within 3 years of the initial diagnosis, report Aubriana McEvoy, MD, from the University of Washington in Seattle, and colleagues.
“This cohort study indicates that the highest yield (and likely most cost-effective) time period for detecting MCC recurrence is 1-3 years after diagnosis,” they write in a study published online in JAMA Dermatology.
The estimated annual incidence of MCC in the United States in 2018 was 2,000 according to the American Cancer Society. The annual incidence rate is rising rapidly, however, and is estimated to reach 3,284 by 2025, McEvoy and colleagues write.
Although MCC is known to have high recurrence rates and is associated with a higher mortality rate than malignant melanoma, recurrence rate data are not captured by either the Surveillance, Epidemiology, and End Results (SEER) database or by the National Cancer Database. As a result, estimates of recurrence rates with MCC have been all over the map, ranging from 27% to 77%, depending on the population studied.
But as senior author Paul Nghiem, MD, PhD, professor and chair of dermatology at the University of Washington, Seattle, told this news organization, recurrence rates over time in their study were remarkably consistent.
“The biggest surprise to me was that, when we broke our nearly 20-year cohort into three 5- or 6-year chunks, every one of the groups had a 40% recurrence rate, within 1%. So we feel really confident that’s the right number,” he said.
Dr. Nghiem and colleagues report that, in contrast to patients with MCC, approximately 19% of patients with melanoma will have a recurrence, as will an estimated 5%-9% of patients with squamous cell carcinoma and 1%-10% of patients with basal cell carcinoma.
The fact that recurrence rates of MCC have remained stable over time despite presumed improvements in definitive therapy is disappointing, Dr. Nghiem acknowledged. He noted that it’s still unclear whether immunotherapy will have the same dramatic effect on survival rates for patients with MCC as it has for patients with malignant melanoma.
The high recurrence rates following definitive therapy for patients with early-stage disease was a novel finding, commented Shawn Demehri, MD, PhD, director of the high-risk skin cancer clinic at Massachusetts General Hospital in Boston.
“When you’re looking at patients with stage I or stage II, and they have definitive surgery but still have recurrences at a higher rate than melanoma, it brings home the point that these are among the most aggressive tumors of the skin,” he said in an interview.
The high recurrence rates seen with MCC are attributable to a variety of factors.
“This is a rare cancer of mostly older individuals with a lot of comorbidities, and also a cancer that, even though it is a primary cancer, might be detected a little later than even a melanoma primary tumor, just because of the nature of the neuroendocrine tumor cells,” he said.
Dr. Demehri was not involved in the study.
Prospective cohort
The study cohort consisted of 618 patients with MCC. The median age of the patients was 69, and 227 (37%) were women. The patients were enrolled within 6 months of their diagnosis in the prospective data repository from 2003 through 2019. Of this group, 223 had a recurrence of MCC.
As noted, there was a high risk of recurrence within 1 year, ranging from 11% for patients with pathologic stage I tumors to 58% for those with stage IV disease, and 95% of all recurrences occurred within 3 years of definitive therapy.
To get a better picture of the natural history of MCC recurrence, the investigators studied a cohort of patients with pathologically confirmed MCC who were prospectively enrolled from January 2003 through April 2019 in a data repository maintained at the University of Washington.
In addition to disease stage, factors associated with increased recurrence risk in univariable analyses include immunosuppression (hazard ratio, 2.4; P < .001), male sex (HR, 1.9; P < .001), known primary lesion among patients with clinically detectable nodal disease (HR, 2.3; P = .001), and older age (HR, 1.1, P = .06 for each 10-year increase).
Of the 187 patients in the cohort who died during the study, 121 died from MCC. At 4 years after diagnosis, MCC-specific survival rates were 95% for patients with pathologic stage I, 84% with stage IIA/IIB, 80% with stage IIIA, 58% with stage IIIB, and 41% with stage IV.
Evidence supports close monitoring within the first 3 years for patients with stage I-II MCC. Local recurrence within or adjacent to the primary tumor scar was associated with a 5-year MCC-specific survival rate of 85%, compared with 88% of patients with stage I or II disease who did not have recurrences.
“Because more than 90% of MCC recurrences arise within 3 years, it is appropriate to adjust surveillance intensity accordingly. Stage- and time-specific recurrence data can assist in appropriately focusing surveillance resources on patients and time intervals in which recurrence risk is highest,” the authors wrote.
“If you’re a patient who has not had your cancer come back for 3, 4, or 5 years, you can really cut down on the intensity of your follow-up and scans,” Dr. Nghiem said.
“We do now have two excellent blood tests that are working very well, and we have really good ways to detect the cancer coming back early, and that’s important, because we have potentially curative therapies that tend to work better if you catch the cancer early,” he said.
The study was supported by the National Institutes of Health. Dr. Nghiem reported personal fees and institutional support outside the study from several companies and patents for Merkel cell therapies with the University of Washington and University of Denmark. Dr. Demehri has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, and more than half of all patients with stage IV disease will have a recurrence within 1 year of definitive therapy, results of a new study show.
A study of 618 patients with MCC who were enrolled in a Seattle-based data repository shows that among all patients the 5-year recurrence rate was 40%. The risk of recurrence within the first year was 11% for patients with pathologic stage I disease, 33% for those with stage IIA/IIB disease, 45% for those with stage IIIB disease, and 58% for patients with pathologic stage IV MCC.
Approximately 95% of all recurrences happened within 3 years of the initial diagnosis, report Aubriana McEvoy, MD, from the University of Washington in Seattle, and colleagues.
“This cohort study indicates that the highest yield (and likely most cost-effective) time period for detecting MCC recurrence is 1-3 years after diagnosis,” they write in a study published online in JAMA Dermatology.
The estimated annual incidence of MCC in the United States in 2018 was 2,000 according to the American Cancer Society. The annual incidence rate is rising rapidly, however, and is estimated to reach 3,284 by 2025, McEvoy and colleagues write.
Although MCC is known to have high recurrence rates and is associated with a higher mortality rate than malignant melanoma, recurrence rate data are not captured by either the Surveillance, Epidemiology, and End Results (SEER) database or by the National Cancer Database. As a result, estimates of recurrence rates with MCC have been all over the map, ranging from 27% to 77%, depending on the population studied.
But as senior author Paul Nghiem, MD, PhD, professor and chair of dermatology at the University of Washington, Seattle, told this news organization, recurrence rates over time in their study were remarkably consistent.
“The biggest surprise to me was that, when we broke our nearly 20-year cohort into three 5- or 6-year chunks, every one of the groups had a 40% recurrence rate, within 1%. So we feel really confident that’s the right number,” he said.
Dr. Nghiem and colleagues report that, in contrast to patients with MCC, approximately 19% of patients with melanoma will have a recurrence, as will an estimated 5%-9% of patients with squamous cell carcinoma and 1%-10% of patients with basal cell carcinoma.
The fact that recurrence rates of MCC have remained stable over time despite presumed improvements in definitive therapy is disappointing, Dr. Nghiem acknowledged. He noted that it’s still unclear whether immunotherapy will have the same dramatic effect on survival rates for patients with MCC as it has for patients with malignant melanoma.
The high recurrence rates following definitive therapy for patients with early-stage disease was a novel finding, commented Shawn Demehri, MD, PhD, director of the high-risk skin cancer clinic at Massachusetts General Hospital in Boston.
“When you’re looking at patients with stage I or stage II, and they have definitive surgery but still have recurrences at a higher rate than melanoma, it brings home the point that these are among the most aggressive tumors of the skin,” he said in an interview.
The high recurrence rates seen with MCC are attributable to a variety of factors.
“This is a rare cancer of mostly older individuals with a lot of comorbidities, and also a cancer that, even though it is a primary cancer, might be detected a little later than even a melanoma primary tumor, just because of the nature of the neuroendocrine tumor cells,” he said.
Dr. Demehri was not involved in the study.
Prospective cohort
The study cohort consisted of 618 patients with MCC. The median age of the patients was 69, and 227 (37%) were women. The patients were enrolled within 6 months of their diagnosis in the prospective data repository from 2003 through 2019. Of this group, 223 had a recurrence of MCC.
As noted, there was a high risk of recurrence within 1 year, ranging from 11% for patients with pathologic stage I tumors to 58% for those with stage IV disease, and 95% of all recurrences occurred within 3 years of definitive therapy.
To get a better picture of the natural history of MCC recurrence, the investigators studied a cohort of patients with pathologically confirmed MCC who were prospectively enrolled from January 2003 through April 2019 in a data repository maintained at the University of Washington.
In addition to disease stage, factors associated with increased recurrence risk in univariable analyses include immunosuppression (hazard ratio, 2.4; P < .001), male sex (HR, 1.9; P < .001), known primary lesion among patients with clinically detectable nodal disease (HR, 2.3; P = .001), and older age (HR, 1.1, P = .06 for each 10-year increase).
Of the 187 patients in the cohort who died during the study, 121 died from MCC. At 4 years after diagnosis, MCC-specific survival rates were 95% for patients with pathologic stage I, 84% with stage IIA/IIB, 80% with stage IIIA, 58% with stage IIIB, and 41% with stage IV.
Evidence supports close monitoring within the first 3 years for patients with stage I-II MCC. Local recurrence within or adjacent to the primary tumor scar was associated with a 5-year MCC-specific survival rate of 85%, compared with 88% of patients with stage I or II disease who did not have recurrences.
“Because more than 90% of MCC recurrences arise within 3 years, it is appropriate to adjust surveillance intensity accordingly. Stage- and time-specific recurrence data can assist in appropriately focusing surveillance resources on patients and time intervals in which recurrence risk is highest,” the authors wrote.
“If you’re a patient who has not had your cancer come back for 3, 4, or 5 years, you can really cut down on the intensity of your follow-up and scans,” Dr. Nghiem said.
“We do now have two excellent blood tests that are working very well, and we have really good ways to detect the cancer coming back early, and that’s important, because we have potentially curative therapies that tend to work better if you catch the cancer early,” he said.
The study was supported by the National Institutes of Health. Dr. Nghiem reported personal fees and institutional support outside the study from several companies and patents for Merkel cell therapies with the University of Washington and University of Denmark. Dr. Demehri has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Survey: Artificial intelligence finds support among dermatologists
according to the results of a small survey.
Just 9% of the 90 respondents acknowledged that they have used AI in their practices, while 81% said they had not, and 10% weren’t sure or didn’t know. Despite that lack of familiarity, however, “many embrace the potential positive benefits, such as reducing misdiagnoses” and a majority (94.5%) “would use it at least in certain scenarios,” Vishal A. Patel, MD, and associates said in the Journal of Drugs in Dermatology.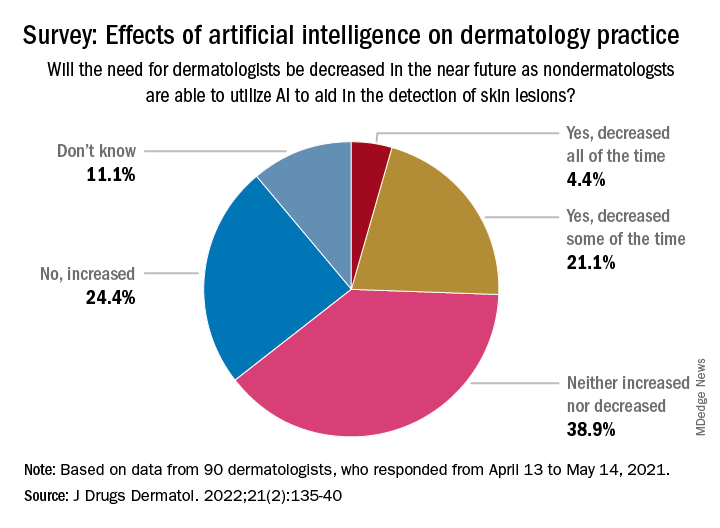
Dermatologists aged 40 years and under were more likely to have used AI previously: 15% reported previous experience, compared with 4% of those over age 40 – but the difference in “age did not have a significant effect on perception of AI,” the investigators noted, adding that most of the dermatologists over 40 believe “that AI would be most beneficial and used for detection of malignant skin lesions.”
The survey also asked about ways the respondents would use AI to help their patients. Almost two-thirds of respondents (66%) chose analysis and management of electronic health records “for research purposes to improve patient outcomes,” compared with 56% who chose identifying unknown/screening skin lesions “with a list of differential diagnoses,” 32% who chose telemedicine, and 26% who chose primary surveys of skin, said Dr. Patel, director of cutaneous oncology at the George Washington University Cancer Center in Washington, and coauthors.
The respondents were fairly evenly split when asked about the possible impact of nondermatologists using AI in the near future to detect skin lesions, such as melanomas, on the need for dermatologists. Just over a quarter said that the need for dermatologists will be decreased all (about 4.4%) or some (about 21.1%) of the time, and 24.4% said that the need will be increased, with the largest share (39.9%) of respondents choosing the middle ground: neither increased or decreased, the investigators reported.
The survey form was emailed to 850 members of the Orlando Dermatology, Aesthetic & Surgical Conference listserv, with responses accepted from April 13 to May 14, 2021. The investigators noted that the response rate was low enough to be a limiting factor, making selection bias “by those with a particular interest in the topic” a possibility.
No funding sources for the study were disclosed. Dr. Patel disclosed that he is chief medical officer for Lazarus AI, the other authors had no disclosures listed.
according to the results of a small survey.
Just 9% of the 90 respondents acknowledged that they have used AI in their practices, while 81% said they had not, and 10% weren’t sure or didn’t know. Despite that lack of familiarity, however, “many embrace the potential positive benefits, such as reducing misdiagnoses” and a majority (94.5%) “would use it at least in certain scenarios,” Vishal A. Patel, MD, and associates said in the Journal of Drugs in Dermatology.
Dermatologists aged 40 years and under were more likely to have used AI previously: 15% reported previous experience, compared with 4% of those over age 40 – but the difference in “age did not have a significant effect on perception of AI,” the investigators noted, adding that most of the dermatologists over 40 believe “that AI would be most beneficial and used for detection of malignant skin lesions.”
The survey also asked about ways the respondents would use AI to help their patients. Almost two-thirds of respondents (66%) chose analysis and management of electronic health records “for research purposes to improve patient outcomes,” compared with 56% who chose identifying unknown/screening skin lesions “with a list of differential diagnoses,” 32% who chose telemedicine, and 26% who chose primary surveys of skin, said Dr. Patel, director of cutaneous oncology at the George Washington University Cancer Center in Washington, and coauthors.
The respondents were fairly evenly split when asked about the possible impact of nondermatologists using AI in the near future to detect skin lesions, such as melanomas, on the need for dermatologists. Just over a quarter said that the need for dermatologists will be decreased all (about 4.4%) or some (about 21.1%) of the time, and 24.4% said that the need will be increased, with the largest share (39.9%) of respondents choosing the middle ground: neither increased or decreased, the investigators reported.
The survey form was emailed to 850 members of the Orlando Dermatology, Aesthetic & Surgical Conference listserv, with responses accepted from April 13 to May 14, 2021. The investigators noted that the response rate was low enough to be a limiting factor, making selection bias “by those with a particular interest in the topic” a possibility.
No funding sources for the study were disclosed. Dr. Patel disclosed that he is chief medical officer for Lazarus AI, the other authors had no disclosures listed.
according to the results of a small survey.
Just 9% of the 90 respondents acknowledged that they have used AI in their practices, while 81% said they had not, and 10% weren’t sure or didn’t know. Despite that lack of familiarity, however, “many embrace the potential positive benefits, such as reducing misdiagnoses” and a majority (94.5%) “would use it at least in certain scenarios,” Vishal A. Patel, MD, and associates said in the Journal of Drugs in Dermatology.
Dermatologists aged 40 years and under were more likely to have used AI previously: 15% reported previous experience, compared with 4% of those over age 40 – but the difference in “age did not have a significant effect on perception of AI,” the investigators noted, adding that most of the dermatologists over 40 believe “that AI would be most beneficial and used for detection of malignant skin lesions.”
The survey also asked about ways the respondents would use AI to help their patients. Almost two-thirds of respondents (66%) chose analysis and management of electronic health records “for research purposes to improve patient outcomes,” compared with 56% who chose identifying unknown/screening skin lesions “with a list of differential diagnoses,” 32% who chose telemedicine, and 26% who chose primary surveys of skin, said Dr. Patel, director of cutaneous oncology at the George Washington University Cancer Center in Washington, and coauthors.
The respondents were fairly evenly split when asked about the possible impact of nondermatologists using AI in the near future to detect skin lesions, such as melanomas, on the need for dermatologists. Just over a quarter said that the need for dermatologists will be decreased all (about 4.4%) or some (about 21.1%) of the time, and 24.4% said that the need will be increased, with the largest share (39.9%) of respondents choosing the middle ground: neither increased or decreased, the investigators reported.
The survey form was emailed to 850 members of the Orlando Dermatology, Aesthetic & Surgical Conference listserv, with responses accepted from April 13 to May 14, 2021. The investigators noted that the response rate was low enough to be a limiting factor, making selection bias “by those with a particular interest in the topic” a possibility.
No funding sources for the study were disclosed. Dr. Patel disclosed that he is chief medical officer for Lazarus AI, the other authors had no disclosures listed.
FROM JOURNAL OF DRUGS IN DERMATOLOGY
Why dermatologists should support artificial intelligence efforts
“AI is meant to be an enhancement strategy, a support tool to improve our diagnostic abilities,” Dr. Patel, a Mohs surgeon who is director of cutaneous oncology at the George Washington University Cancer Center, Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “Dermatologists should embrace AI and drive how it is utilized – be the captain of the plane (technology) and the passenger (patient). If we’re not in the forefront of the plane, we’re not to be able to dictate which way we are going with this.”
In 2019, a group of German researchers found that AI can improve accuracy and efficiency of specialists in classifying skin cancer based on dermoscopic images. “I really do believe this is going to be the future,” said Dr. Patel, who was not involved with the study. “Current research involves using supervised learning on known outcomes to determine inputs to predict them. In dermatology, think of identifying melanoma from clinical or dermoscopic images or predicting metastasis risk from digitized pathology slides.”
However, there are currently no universal guidelines on how large an AI dataset needs to be to yield accurate results. In the dermatology literature, most AI datasets range between 600 and 14,000 examples, Dr. Patel said, with a large study-specific variation in performance. “Misleading results can result from unanticipated training errors,” he said.
“The AI network may learn its intended task or an unrelated situational cue. For example, you can use great images to predict melanoma, but you may have an unintended poor outcome related to images that have, say, a ruler inside of them clustered within the melanoma diagnoses.” And unbeknown to the system’s developer, “the algorithm picks up that the ruler is predictive of an image being a melanoma and not the pigmented lesion itself.” In other words, the algorithm is only as good as the dataset being used, he said. “This is the key element, to ask what the dataset is that’s training the tool that you may one day use.”
Convolutional neural network
In 2017, a seminal study published in Nature showed that for classification of melanoma and epidermal lesions, a type of AI used in image processing known as a convolutional neural network (CNN) was on par with dermatologists and outperformed the average. For epidermal lesions, the network was one standard deviation higher above the average for dermatologists, while for melanocytic lesions, the network was just below one standard deviation above the average of the dermatologists. A CNN “clearly can perform well because it works on a different level than how our brains work,” Dr. Patel said.
In a separate study, a CNN trained to recognize melanoma in dermoscopic images was compared to 58 international dermatologists with varying levels of dermoscopy experience; 29% were “beginners,” with less than 2 years of experience; 19% were “skilled,” with 2-5 years of experience; and 52% were “experts,” with at least 5 years of experience. The analysis consisted of two experiments: In level I, dermatologists classified lesions based on dermoscopy only. In level II, dermatologists were provided dermoscopy, clinical images, and additional clinical information, while the CNN was trained on images only. The researchers found that most dermatologists were outperformed by the CNN. “Physicians of all different levels of training and experience may benefit from assistance by a CNN’s image classification,” they concluded.
Gene expression profiling
Another aspect of AI is gene expression profiling (GEP), which Dr. Patel defined as the evaluation of frequency and intensity of genetic activity at once to create a global picture of cellular function. “It’s AI that uses machine learning to evaluate genetic expression to assess lesion behavior,” he explained.
One GEP test on the market is the Pigmented Lesion Assay (PLA) from DermTech, a noninvasive test that looks at the expression of two genes to predict if a lesion is malignant or not. “Based on their validation set, they have shown some impressive numbers,” with sensitivities above 90%, and published registry data that have shown higher sensitivities “and even specificities above 90%,” he said.
“On the surface, it looks like this would be a useful test,” Dr. Patel said. A study published in 2021 looked at the evidence of applying real-world evidence with this test to see if results held up. Based on the authors’ analysis, he noted, “you would need a sensitivity and specificity of 95% to yield a positivity rate of 9.5% for the PLA test, which is what has been reported in real-world use. So, there’s a disconnect somewhere and we are not quite there yet.” That may be a result of the dataset itself not being as uniform between the validation and the training datasets, he continued. Also, the expression of certain genes is different “if you don’t have a clean input variable” of what the test is being used for, he added.
“If you’re not mirroring the dataset, you’re not going to get clean data,” he said. “So, if you’re using this on younger patients or for sun-damaged lesional skin or nonmelanocytic lesions around sun-damaged areas, there are variable expressions that may not be accurately captured by that algorithm. This might help explain the real-world variation that we’re seeing.”
Another GEP test in use is the 31-Gene Expression Profile Test for Melanoma, which evaluates gene expressions in melanoma tumors and what the behavior of that tumor may be. The test has been available for more than a decade “and there is a lot of speculation about its use,” Dr. Patel said. “A recent paper attempted to come up with an algorithm of how to use this, but there’s a lot of concern about the endpoints of what changes in management might result from this test. That is what we need to be thinking about. There’s a lot of back and forth about this.”
In 2020, authors of a consensus statement on prognostic GEP in cutaneous melanoma concluded that before GEP testing is routinely used, the clinical benefit in the management of patients with melanoma should be established through further clinical investigation. Dr. Patel recommended the accompanying editorial on GEP in melanoma, written by Hensin Tsao, MD, PhD, and Warren H. Chan, MS, in JAMA Dermatology.
In Dr. Patel’s opinion, T1a melanomas (0.8 mm, nonulcerated) do not need routine GEP, but the GEP test may be useful in cases that are in the “gray zone,” such as those with T1b or some borderline T2a melanomas (> 0.8 mm, < 1.2mm, nonulcerated, but with high mitosis, etc.); patients with unique coexisting conditions such as pregnancy, and patients who may not tolerate sentinel lymph node biopsy (SLNB) or adjuvant therapy.
Echoing sentiments expressed in the JAMA Dermatology editorial, he advised dermatologists to “remember your training and know the data. GEP predicting survival is not the same as SLNB positive rate. GEP should not replace standard guidelines in T2a and higher melanomas. Nodal sampling remains part of all major guidelines and determines adjuvant therapy.”
He cited the characterization of GEP in the editorial as “a powerful technology” that heralds the age of personalized medicine, but it is not ready for ubiquitous use. Prospective studies and time will lead to highly accurate tools.”
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
“AI is meant to be an enhancement strategy, a support tool to improve our diagnostic abilities,” Dr. Patel, a Mohs surgeon who is director of cutaneous oncology at the George Washington University Cancer Center, Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “Dermatologists should embrace AI and drive how it is utilized – be the captain of the plane (technology) and the passenger (patient). If we’re not in the forefront of the plane, we’re not to be able to dictate which way we are going with this.”
In 2019, a group of German researchers found that AI can improve accuracy and efficiency of specialists in classifying skin cancer based on dermoscopic images. “I really do believe this is going to be the future,” said Dr. Patel, who was not involved with the study. “Current research involves using supervised learning on known outcomes to determine inputs to predict them. In dermatology, think of identifying melanoma from clinical or dermoscopic images or predicting metastasis risk from digitized pathology slides.”
However, there are currently no universal guidelines on how large an AI dataset needs to be to yield accurate results. In the dermatology literature, most AI datasets range between 600 and 14,000 examples, Dr. Patel said, with a large study-specific variation in performance. “Misleading results can result from unanticipated training errors,” he said.
“The AI network may learn its intended task or an unrelated situational cue. For example, you can use great images to predict melanoma, but you may have an unintended poor outcome related to images that have, say, a ruler inside of them clustered within the melanoma diagnoses.” And unbeknown to the system’s developer, “the algorithm picks up that the ruler is predictive of an image being a melanoma and not the pigmented lesion itself.” In other words, the algorithm is only as good as the dataset being used, he said. “This is the key element, to ask what the dataset is that’s training the tool that you may one day use.”
Convolutional neural network
In 2017, a seminal study published in Nature showed that for classification of melanoma and epidermal lesions, a type of AI used in image processing known as a convolutional neural network (CNN) was on par with dermatologists and outperformed the average. For epidermal lesions, the network was one standard deviation higher above the average for dermatologists, while for melanocytic lesions, the network was just below one standard deviation above the average of the dermatologists. A CNN “clearly can perform well because it works on a different level than how our brains work,” Dr. Patel said.
In a separate study, a CNN trained to recognize melanoma in dermoscopic images was compared to 58 international dermatologists with varying levels of dermoscopy experience; 29% were “beginners,” with less than 2 years of experience; 19% were “skilled,” with 2-5 years of experience; and 52% were “experts,” with at least 5 years of experience. The analysis consisted of two experiments: In level I, dermatologists classified lesions based on dermoscopy only. In level II, dermatologists were provided dermoscopy, clinical images, and additional clinical information, while the CNN was trained on images only. The researchers found that most dermatologists were outperformed by the CNN. “Physicians of all different levels of training and experience may benefit from assistance by a CNN’s image classification,” they concluded.
Gene expression profiling
Another aspect of AI is gene expression profiling (GEP), which Dr. Patel defined as the evaluation of frequency and intensity of genetic activity at once to create a global picture of cellular function. “It’s AI that uses machine learning to evaluate genetic expression to assess lesion behavior,” he explained.
One GEP test on the market is the Pigmented Lesion Assay (PLA) from DermTech, a noninvasive test that looks at the expression of two genes to predict if a lesion is malignant or not. “Based on their validation set, they have shown some impressive numbers,” with sensitivities above 90%, and published registry data that have shown higher sensitivities “and even specificities above 90%,” he said.
“On the surface, it looks like this would be a useful test,” Dr. Patel said. A study published in 2021 looked at the evidence of applying real-world evidence with this test to see if results held up. Based on the authors’ analysis, he noted, “you would need a sensitivity and specificity of 95% to yield a positivity rate of 9.5% for the PLA test, which is what has been reported in real-world use. So, there’s a disconnect somewhere and we are not quite there yet.” That may be a result of the dataset itself not being as uniform between the validation and the training datasets, he continued. Also, the expression of certain genes is different “if you don’t have a clean input variable” of what the test is being used for, he added.
“If you’re not mirroring the dataset, you’re not going to get clean data,” he said. “So, if you’re using this on younger patients or for sun-damaged lesional skin or nonmelanocytic lesions around sun-damaged areas, there are variable expressions that may not be accurately captured by that algorithm. This might help explain the real-world variation that we’re seeing.”
Another GEP test in use is the 31-Gene Expression Profile Test for Melanoma, which evaluates gene expressions in melanoma tumors and what the behavior of that tumor may be. The test has been available for more than a decade “and there is a lot of speculation about its use,” Dr. Patel said. “A recent paper attempted to come up with an algorithm of how to use this, but there’s a lot of concern about the endpoints of what changes in management might result from this test. That is what we need to be thinking about. There’s a lot of back and forth about this.”
In 2020, authors of a consensus statement on prognostic GEP in cutaneous melanoma concluded that before GEP testing is routinely used, the clinical benefit in the management of patients with melanoma should be established through further clinical investigation. Dr. Patel recommended the accompanying editorial on GEP in melanoma, written by Hensin Tsao, MD, PhD, and Warren H. Chan, MS, in JAMA Dermatology.
In Dr. Patel’s opinion, T1a melanomas (0.8 mm, nonulcerated) do not need routine GEP, but the GEP test may be useful in cases that are in the “gray zone,” such as those with T1b or some borderline T2a melanomas (> 0.8 mm, < 1.2mm, nonulcerated, but with high mitosis, etc.); patients with unique coexisting conditions such as pregnancy, and patients who may not tolerate sentinel lymph node biopsy (SLNB) or adjuvant therapy.
Echoing sentiments expressed in the JAMA Dermatology editorial, he advised dermatologists to “remember your training and know the data. GEP predicting survival is not the same as SLNB positive rate. GEP should not replace standard guidelines in T2a and higher melanomas. Nodal sampling remains part of all major guidelines and determines adjuvant therapy.”
He cited the characterization of GEP in the editorial as “a powerful technology” that heralds the age of personalized medicine, but it is not ready for ubiquitous use. Prospective studies and time will lead to highly accurate tools.”
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
“AI is meant to be an enhancement strategy, a support tool to improve our diagnostic abilities,” Dr. Patel, a Mohs surgeon who is director of cutaneous oncology at the George Washington University Cancer Center, Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “Dermatologists should embrace AI and drive how it is utilized – be the captain of the plane (technology) and the passenger (patient). If we’re not in the forefront of the plane, we’re not to be able to dictate which way we are going with this.”
In 2019, a group of German researchers found that AI can improve accuracy and efficiency of specialists in classifying skin cancer based on dermoscopic images. “I really do believe this is going to be the future,” said Dr. Patel, who was not involved with the study. “Current research involves using supervised learning on known outcomes to determine inputs to predict them. In dermatology, think of identifying melanoma from clinical or dermoscopic images or predicting metastasis risk from digitized pathology slides.”
However, there are currently no universal guidelines on how large an AI dataset needs to be to yield accurate results. In the dermatology literature, most AI datasets range between 600 and 14,000 examples, Dr. Patel said, with a large study-specific variation in performance. “Misleading results can result from unanticipated training errors,” he said.
“The AI network may learn its intended task or an unrelated situational cue. For example, you can use great images to predict melanoma, but you may have an unintended poor outcome related to images that have, say, a ruler inside of them clustered within the melanoma diagnoses.” And unbeknown to the system’s developer, “the algorithm picks up that the ruler is predictive of an image being a melanoma and not the pigmented lesion itself.” In other words, the algorithm is only as good as the dataset being used, he said. “This is the key element, to ask what the dataset is that’s training the tool that you may one day use.”
Convolutional neural network
In 2017, a seminal study published in Nature showed that for classification of melanoma and epidermal lesions, a type of AI used in image processing known as a convolutional neural network (CNN) was on par with dermatologists and outperformed the average. For epidermal lesions, the network was one standard deviation higher above the average for dermatologists, while for melanocytic lesions, the network was just below one standard deviation above the average of the dermatologists. A CNN “clearly can perform well because it works on a different level than how our brains work,” Dr. Patel said.
In a separate study, a CNN trained to recognize melanoma in dermoscopic images was compared to 58 international dermatologists with varying levels of dermoscopy experience; 29% were “beginners,” with less than 2 years of experience; 19% were “skilled,” with 2-5 years of experience; and 52% were “experts,” with at least 5 years of experience. The analysis consisted of two experiments: In level I, dermatologists classified lesions based on dermoscopy only. In level II, dermatologists were provided dermoscopy, clinical images, and additional clinical information, while the CNN was trained on images only. The researchers found that most dermatologists were outperformed by the CNN. “Physicians of all different levels of training and experience may benefit from assistance by a CNN’s image classification,” they concluded.
Gene expression profiling
Another aspect of AI is gene expression profiling (GEP), which Dr. Patel defined as the evaluation of frequency and intensity of genetic activity at once to create a global picture of cellular function. “It’s AI that uses machine learning to evaluate genetic expression to assess lesion behavior,” he explained.
One GEP test on the market is the Pigmented Lesion Assay (PLA) from DermTech, a noninvasive test that looks at the expression of two genes to predict if a lesion is malignant or not. “Based on their validation set, they have shown some impressive numbers,” with sensitivities above 90%, and published registry data that have shown higher sensitivities “and even specificities above 90%,” he said.
“On the surface, it looks like this would be a useful test,” Dr. Patel said. A study published in 2021 looked at the evidence of applying real-world evidence with this test to see if results held up. Based on the authors’ analysis, he noted, “you would need a sensitivity and specificity of 95% to yield a positivity rate of 9.5% for the PLA test, which is what has been reported in real-world use. So, there’s a disconnect somewhere and we are not quite there yet.” That may be a result of the dataset itself not being as uniform between the validation and the training datasets, he continued. Also, the expression of certain genes is different “if you don’t have a clean input variable” of what the test is being used for, he added.
“If you’re not mirroring the dataset, you’re not going to get clean data,” he said. “So, if you’re using this on younger patients or for sun-damaged lesional skin or nonmelanocytic lesions around sun-damaged areas, there are variable expressions that may not be accurately captured by that algorithm. This might help explain the real-world variation that we’re seeing.”
Another GEP test in use is the 31-Gene Expression Profile Test for Melanoma, which evaluates gene expressions in melanoma tumors and what the behavior of that tumor may be. The test has been available for more than a decade “and there is a lot of speculation about its use,” Dr. Patel said. “A recent paper attempted to come up with an algorithm of how to use this, but there’s a lot of concern about the endpoints of what changes in management might result from this test. That is what we need to be thinking about. There’s a lot of back and forth about this.”
In 2020, authors of a consensus statement on prognostic GEP in cutaneous melanoma concluded that before GEP testing is routinely used, the clinical benefit in the management of patients with melanoma should be established through further clinical investigation. Dr. Patel recommended the accompanying editorial on GEP in melanoma, written by Hensin Tsao, MD, PhD, and Warren H. Chan, MS, in JAMA Dermatology.
In Dr. Patel’s opinion, T1a melanomas (0.8 mm, nonulcerated) do not need routine GEP, but the GEP test may be useful in cases that are in the “gray zone,” such as those with T1b or some borderline T2a melanomas (> 0.8 mm, < 1.2mm, nonulcerated, but with high mitosis, etc.); patients with unique coexisting conditions such as pregnancy, and patients who may not tolerate sentinel lymph node biopsy (SLNB) or adjuvant therapy.
Echoing sentiments expressed in the JAMA Dermatology editorial, he advised dermatologists to “remember your training and know the data. GEP predicting survival is not the same as SLNB positive rate. GEP should not replace standard guidelines in T2a and higher melanomas. Nodal sampling remains part of all major guidelines and determines adjuvant therapy.”
He cited the characterization of GEP in the editorial as “a powerful technology” that heralds the age of personalized medicine, but it is not ready for ubiquitous use. Prospective studies and time will lead to highly accurate tools.”
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
FROM ODAC 2022
Pencil-core Granuloma Forming 62 Years After Initial Injury
To the Editor:
Trauma from a pencil tip can sometimes result in a fragment of lead being left embedded within the skin. Pencil lead is composed of 66% graphite carbon, 26% aluminum silicate, and 8% paraffin.1,2 While the toxicity of these individual elements is low, paraffin can cause nonallergic foreign-body reactions, aluminum silicate can induce epithelioid granulomatous reactions, and graphite has been reported to cause chronic granulomatous reactions in the lungs of those who work with graphite.2 Penetrating trauma with a pencil can result in the formation of a cutaneous granulomatous reaction that can sometimes occur years to decades after the initial injury.3,4 Several cases of pencil-core granulomas have been published, with lag times between the initial trauma and lesion growth as long as 58 years.1-10 The pencil-core granuloma may simulate malignant melanoma, as it presents clinically as a growing, darkly pigmented lesion, thus prompting biopsy. We present a case of a pencil-core granuloma that began to grow 62 years after the initial trauma.
A 72-year-old woman was referred to our clinic for evaluation of a dark nodule on the forehead. The lesion had been present since the age of 10 years, reportedly from an accidental stabbing with a pencil. The lesion had been flat, stable, and asymptomatic since the trauma occurred; however, the patient reported that approximately 9 months prior to presentation, it had started growing and became painful. Physical examination revealed a 1.0-cm, round, bluish-black nodule on the right superior forehead (Figure 1A). No satellite lesions or local lymphadenopathy were noted on general examination.
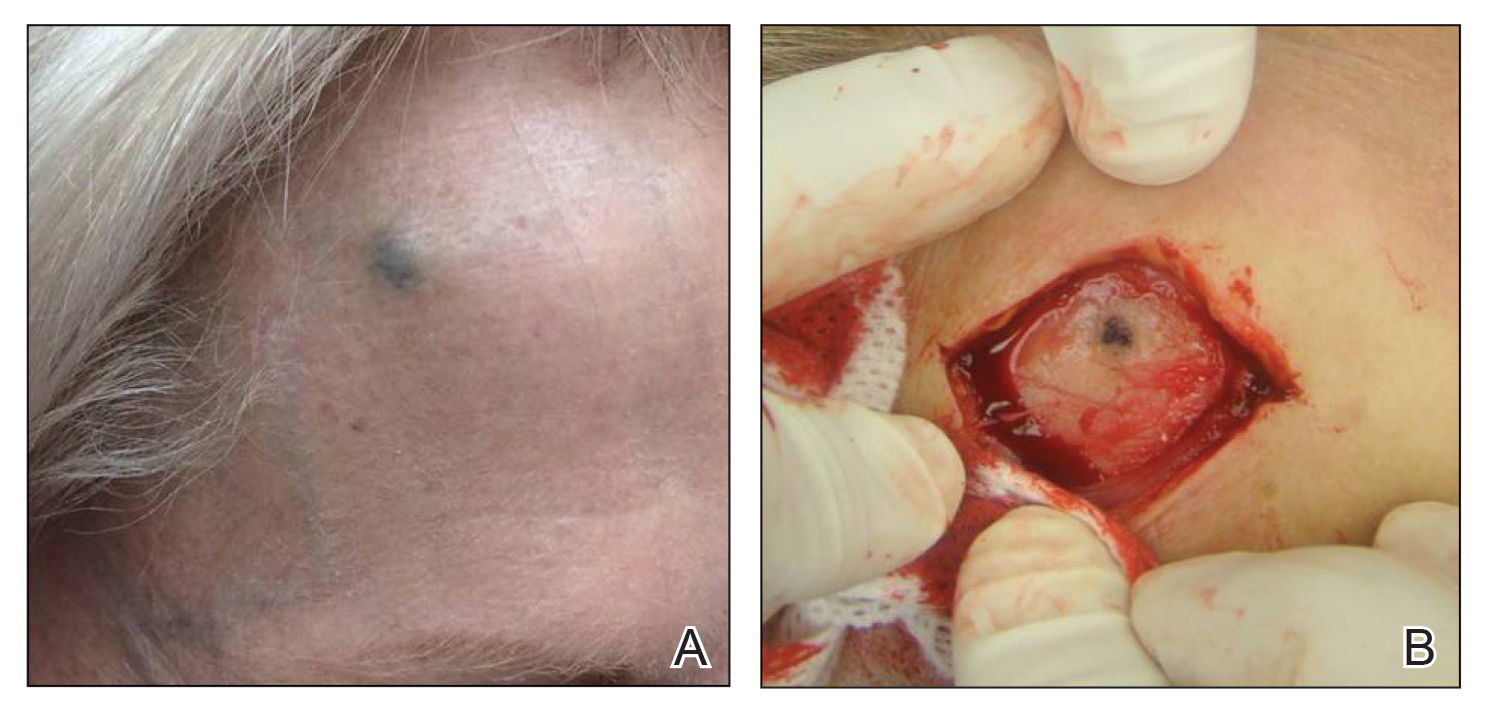
An elliptical excision of the lesion with 1-cm margins revealed a bluish-black mass extending through the dermis, through the frontalis muscle, and into the periosteum and frontal bone (Figure 1B). A No. 15 blade was then used to remove the remaining pigment from the outer table of the frontal bone. Histopathologic findings demonstrated a sarcoidal granulomatous dermatitis associated with abundant, nonpolarizable, black, granular pigment consistent with carbon tattoo. This foreign material was readily identifiable in large extracellular deposits and also within histiocytes, including numerous multinucleated giant cells (Figure 2). Immunostaining for MART-1 and SOX-10 antigens failed to demonstrate a melanocytic proliferation. These findings were consistent with a sarcoidal foreign-body granulomatous reaction to carbon tattoo following traumatic graphite implantation.
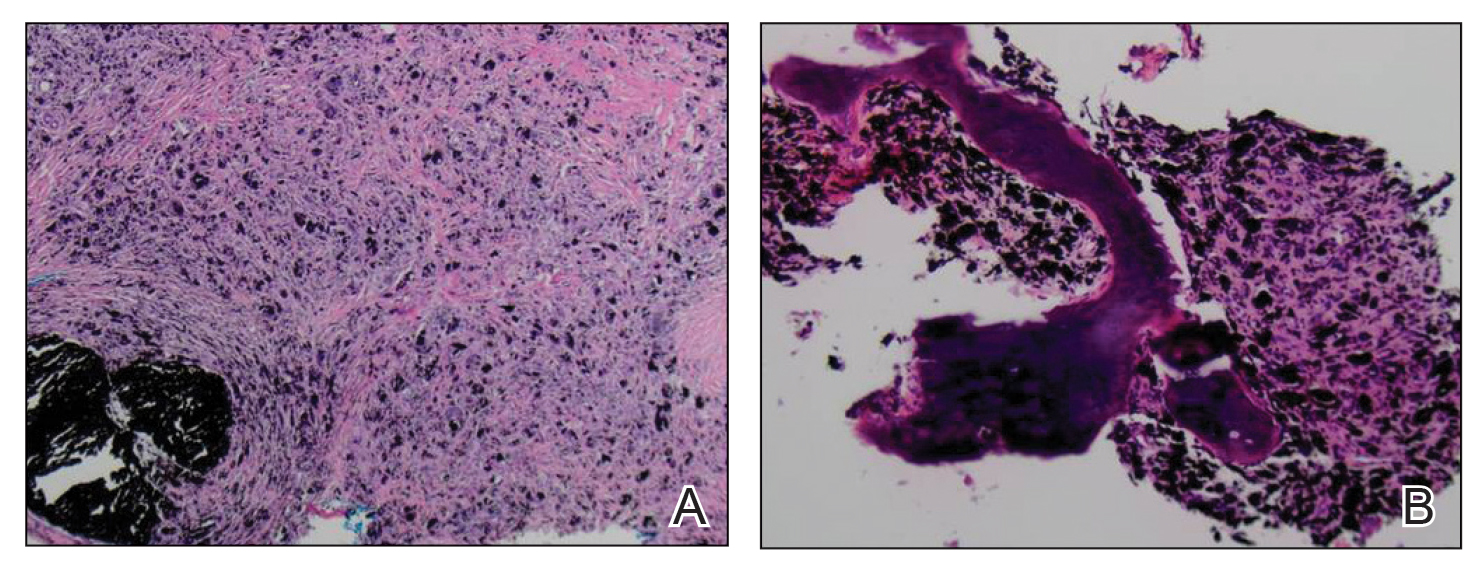
Granulomatous reactions to carbon tattoo may be sarcoidal (foreign-body granulomatous dermatitis), palisading, or rarely tuberculoid (caseating). Sarcoidal granulomatous tattoo reactions may occur in patients with sarcoidosis due to koebnerization, and histology alone is not discriminatory; however, in our patient, the absence of underlying sarcoidosis or clinical or histologic findings of sarcoidosis outside of the site of the pencil-core granuloma excluded that possibility.11 Pencil-core granulomas are characterized by a delayed foreign-body reaction to retained fragments of lead often years following a penetrating trauma with a pencil. Previous reports have described various lag times from injury to lesion growth of up to 58 years.1-10 Our patient claimed to have noticed the lesion growing and becoming painful only after a 62-year lag time following the initial trauma. To our knowledge, this is the longest lag time between the initial pencil injury and induction of the foreign-body reaction reported in the literature. Clinically, the lesion appeared and behaved very similar to a melanoma, prompting further treatment and evaluation.
It has been suggested that the lag period between the initial trauma and the rapid growth of the lesion may correspond to the amount of time required for the breakdown of the pencil lead to a critical size followed by the dispersal of those particles within the interstitium, where they can induce a granulomatous reaction.1,2,9 One case described a patient who reported that the growth and clinical change of the pencil-core granuloma only started when the patient accidentally hit the area where the trauma had occurred 31 years prior.1 This additional trauma may have caused further mechanical breakdown of the lead to set off the tissue reaction. In our case, the patient did not recall any additional trauma to the head prior to the onset of growth of the nodule on the forehead.
Our case indicates that carbon tattoo may be a possible sequela of a penetrating injury from a pencil with retained pencil lead fragments; however, many of these carbon tattoos may remain stable throughout the remainder of the patient’s life.
- Gormley RH, Kovach SJ III, Zhang PJ. Role for trauma in inducing pencil “lead” granuloma in the skin. J Am Acad Dermatol. 2010;62:1074-1075.
- Terasawa N, Kishimoto S, Kibe Y, et al. Graphite foreign body granuloma. Br J Dermatol. 1999;141:774-776.
- Fukunaga Y, Hashimoto I, Nakanishi H, et al. Pencil-core granuloma of the face: report of two rare cases. J Plast Reconstr Aesthet Surg. 2011;64:1235-1237.
- Aswani VH, Kim SL. Fifty-three years after a pencil puncture wound. Case Rep Dermatol. 2015;7:303-305.
- Taylor B, Frumkin A, Pitha JV. Delayed reaction to “lead” pencil simulating melanoma. Cutis. 1988;42:199-201.
- Granick MS, Erickson ER, Solomon MP. Pencil-core granuloma. Plast Reconstr Surg. 1992;89:136-138.
- Andreano J. Stump the experts. foreign body granuloma. J Dermatol Surg Oncol. 1992;18:277, 343.
- Yoshitatsu S, Takagi T. A case of giant pencil-core granuloma. J Dermatol. 2000;27:329-332.
- Hatano Y, Asada Y, Komada S, et al. A case of pencil core granuloma with an unusual temporal profile. Dermatology. 2000;201:151-153.
- Seitz IA, Silva BA, Schechter LS. Unusual sequela from a pencil stab wound reveals a retained graphite foreign body. Pediatr Emerg Care. 2014;30:568-570.
- Motaparthi K. Tattoo ink. In: Cockerell CJ, Hall BJ, eds. Nonneoplastic Dermatopathology. 2nd ed. Amirsys; 2016: 270.
To the Editor:
Trauma from a pencil tip can sometimes result in a fragment of lead being left embedded within the skin. Pencil lead is composed of 66% graphite carbon, 26% aluminum silicate, and 8% paraffin.1,2 While the toxicity of these individual elements is low, paraffin can cause nonallergic foreign-body reactions, aluminum silicate can induce epithelioid granulomatous reactions, and graphite has been reported to cause chronic granulomatous reactions in the lungs of those who work with graphite.2 Penetrating trauma with a pencil can result in the formation of a cutaneous granulomatous reaction that can sometimes occur years to decades after the initial injury.3,4 Several cases of pencil-core granulomas have been published, with lag times between the initial trauma and lesion growth as long as 58 years.1-10 The pencil-core granuloma may simulate malignant melanoma, as it presents clinically as a growing, darkly pigmented lesion, thus prompting biopsy. We present a case of a pencil-core granuloma that began to grow 62 years after the initial trauma.
A 72-year-old woman was referred to our clinic for evaluation of a dark nodule on the forehead. The lesion had been present since the age of 10 years, reportedly from an accidental stabbing with a pencil. The lesion had been flat, stable, and asymptomatic since the trauma occurred; however, the patient reported that approximately 9 months prior to presentation, it had started growing and became painful. Physical examination revealed a 1.0-cm, round, bluish-black nodule on the right superior forehead (Figure 1A). No satellite lesions or local lymphadenopathy were noted on general examination.

An elliptical excision of the lesion with 1-cm margins revealed a bluish-black mass extending through the dermis, through the frontalis muscle, and into the periosteum and frontal bone (Figure 1B). A No. 15 blade was then used to remove the remaining pigment from the outer table of the frontal bone. Histopathologic findings demonstrated a sarcoidal granulomatous dermatitis associated with abundant, nonpolarizable, black, granular pigment consistent with carbon tattoo. This foreign material was readily identifiable in large extracellular deposits and also within histiocytes, including numerous multinucleated giant cells (Figure 2). Immunostaining for MART-1 and SOX-10 antigens failed to demonstrate a melanocytic proliferation. These findings were consistent with a sarcoidal foreign-body granulomatous reaction to carbon tattoo following traumatic graphite implantation.

Granulomatous reactions to carbon tattoo may be sarcoidal (foreign-body granulomatous dermatitis), palisading, or rarely tuberculoid (caseating). Sarcoidal granulomatous tattoo reactions may occur in patients with sarcoidosis due to koebnerization, and histology alone is not discriminatory; however, in our patient, the absence of underlying sarcoidosis or clinical or histologic findings of sarcoidosis outside of the site of the pencil-core granuloma excluded that possibility.11 Pencil-core granulomas are characterized by a delayed foreign-body reaction to retained fragments of lead often years following a penetrating trauma with a pencil. Previous reports have described various lag times from injury to lesion growth of up to 58 years.1-10 Our patient claimed to have noticed the lesion growing and becoming painful only after a 62-year lag time following the initial trauma. To our knowledge, this is the longest lag time between the initial pencil injury and induction of the foreign-body reaction reported in the literature. Clinically, the lesion appeared and behaved very similar to a melanoma, prompting further treatment and evaluation.
It has been suggested that the lag period between the initial trauma and the rapid growth of the lesion may correspond to the amount of time required for the breakdown of the pencil lead to a critical size followed by the dispersal of those particles within the interstitium, where they can induce a granulomatous reaction.1,2,9 One case described a patient who reported that the growth and clinical change of the pencil-core granuloma only started when the patient accidentally hit the area where the trauma had occurred 31 years prior.1 This additional trauma may have caused further mechanical breakdown of the lead to set off the tissue reaction. In our case, the patient did not recall any additional trauma to the head prior to the onset of growth of the nodule on the forehead.
Our case indicates that carbon tattoo may be a possible sequela of a penetrating injury from a pencil with retained pencil lead fragments; however, many of these carbon tattoos may remain stable throughout the remainder of the patient’s life.
To the Editor:
Trauma from a pencil tip can sometimes result in a fragment of lead being left embedded within the skin. Pencil lead is composed of 66% graphite carbon, 26% aluminum silicate, and 8% paraffin.1,2 While the toxicity of these individual elements is low, paraffin can cause nonallergic foreign-body reactions, aluminum silicate can induce epithelioid granulomatous reactions, and graphite has been reported to cause chronic granulomatous reactions in the lungs of those who work with graphite.2 Penetrating trauma with a pencil can result in the formation of a cutaneous granulomatous reaction that can sometimes occur years to decades after the initial injury.3,4 Several cases of pencil-core granulomas have been published, with lag times between the initial trauma and lesion growth as long as 58 years.1-10 The pencil-core granuloma may simulate malignant melanoma, as it presents clinically as a growing, darkly pigmented lesion, thus prompting biopsy. We present a case of a pencil-core granuloma that began to grow 62 years after the initial trauma.
A 72-year-old woman was referred to our clinic for evaluation of a dark nodule on the forehead. The lesion had been present since the age of 10 years, reportedly from an accidental stabbing with a pencil. The lesion had been flat, stable, and asymptomatic since the trauma occurred; however, the patient reported that approximately 9 months prior to presentation, it had started growing and became painful. Physical examination revealed a 1.0-cm, round, bluish-black nodule on the right superior forehead (Figure 1A). No satellite lesions or local lymphadenopathy were noted on general examination.

An elliptical excision of the lesion with 1-cm margins revealed a bluish-black mass extending through the dermis, through the frontalis muscle, and into the periosteum and frontal bone (Figure 1B). A No. 15 blade was then used to remove the remaining pigment from the outer table of the frontal bone. Histopathologic findings demonstrated a sarcoidal granulomatous dermatitis associated with abundant, nonpolarizable, black, granular pigment consistent with carbon tattoo. This foreign material was readily identifiable in large extracellular deposits and also within histiocytes, including numerous multinucleated giant cells (Figure 2). Immunostaining for MART-1 and SOX-10 antigens failed to demonstrate a melanocytic proliferation. These findings were consistent with a sarcoidal foreign-body granulomatous reaction to carbon tattoo following traumatic graphite implantation.

Granulomatous reactions to carbon tattoo may be sarcoidal (foreign-body granulomatous dermatitis), palisading, or rarely tuberculoid (caseating). Sarcoidal granulomatous tattoo reactions may occur in patients with sarcoidosis due to koebnerization, and histology alone is not discriminatory; however, in our patient, the absence of underlying sarcoidosis or clinical or histologic findings of sarcoidosis outside of the site of the pencil-core granuloma excluded that possibility.11 Pencil-core granulomas are characterized by a delayed foreign-body reaction to retained fragments of lead often years following a penetrating trauma with a pencil. Previous reports have described various lag times from injury to lesion growth of up to 58 years.1-10 Our patient claimed to have noticed the lesion growing and becoming painful only after a 62-year lag time following the initial trauma. To our knowledge, this is the longest lag time between the initial pencil injury and induction of the foreign-body reaction reported in the literature. Clinically, the lesion appeared and behaved very similar to a melanoma, prompting further treatment and evaluation.
It has been suggested that the lag period between the initial trauma and the rapid growth of the lesion may correspond to the amount of time required for the breakdown of the pencil lead to a critical size followed by the dispersal of those particles within the interstitium, where they can induce a granulomatous reaction.1,2,9 One case described a patient who reported that the growth and clinical change of the pencil-core granuloma only started when the patient accidentally hit the area where the trauma had occurred 31 years prior.1 This additional trauma may have caused further mechanical breakdown of the lead to set off the tissue reaction. In our case, the patient did not recall any additional trauma to the head prior to the onset of growth of the nodule on the forehead.
Our case indicates that carbon tattoo may be a possible sequela of a penetrating injury from a pencil with retained pencil lead fragments; however, many of these carbon tattoos may remain stable throughout the remainder of the patient’s life.
- Gormley RH, Kovach SJ III, Zhang PJ. Role for trauma in inducing pencil “lead” granuloma in the skin. J Am Acad Dermatol. 2010;62:1074-1075.
- Terasawa N, Kishimoto S, Kibe Y, et al. Graphite foreign body granuloma. Br J Dermatol. 1999;141:774-776.
- Fukunaga Y, Hashimoto I, Nakanishi H, et al. Pencil-core granuloma of the face: report of two rare cases. J Plast Reconstr Aesthet Surg. 2011;64:1235-1237.
- Aswani VH, Kim SL. Fifty-three years after a pencil puncture wound. Case Rep Dermatol. 2015;7:303-305.
- Taylor B, Frumkin A, Pitha JV. Delayed reaction to “lead” pencil simulating melanoma. Cutis. 1988;42:199-201.
- Granick MS, Erickson ER, Solomon MP. Pencil-core granuloma. Plast Reconstr Surg. 1992;89:136-138.
- Andreano J. Stump the experts. foreign body granuloma. J Dermatol Surg Oncol. 1992;18:277, 343.
- Yoshitatsu S, Takagi T. A case of giant pencil-core granuloma. J Dermatol. 2000;27:329-332.
- Hatano Y, Asada Y, Komada S, et al. A case of pencil core granuloma with an unusual temporal profile. Dermatology. 2000;201:151-153.
- Seitz IA, Silva BA, Schechter LS. Unusual sequela from a pencil stab wound reveals a retained graphite foreign body. Pediatr Emerg Care. 2014;30:568-570.
- Motaparthi K. Tattoo ink. In: Cockerell CJ, Hall BJ, eds. Nonneoplastic Dermatopathology. 2nd ed. Amirsys; 2016: 270.
- Gormley RH, Kovach SJ III, Zhang PJ. Role for trauma in inducing pencil “lead” granuloma in the skin. J Am Acad Dermatol. 2010;62:1074-1075.
- Terasawa N, Kishimoto S, Kibe Y, et al. Graphite foreign body granuloma. Br J Dermatol. 1999;141:774-776.
- Fukunaga Y, Hashimoto I, Nakanishi H, et al. Pencil-core granuloma of the face: report of two rare cases. J Plast Reconstr Aesthet Surg. 2011;64:1235-1237.
- Aswani VH, Kim SL. Fifty-three years after a pencil puncture wound. Case Rep Dermatol. 2015;7:303-305.
- Taylor B, Frumkin A, Pitha JV. Delayed reaction to “lead” pencil simulating melanoma. Cutis. 1988;42:199-201.
- Granick MS, Erickson ER, Solomon MP. Pencil-core granuloma. Plast Reconstr Surg. 1992;89:136-138.
- Andreano J. Stump the experts. foreign body granuloma. J Dermatol Surg Oncol. 1992;18:277, 343.
- Yoshitatsu S, Takagi T. A case of giant pencil-core granuloma. J Dermatol. 2000;27:329-332.
- Hatano Y, Asada Y, Komada S, et al. A case of pencil core granuloma with an unusual temporal profile. Dermatology. 2000;201:151-153.
- Seitz IA, Silva BA, Schechter LS. Unusual sequela from a pencil stab wound reveals a retained graphite foreign body. Pediatr Emerg Care. 2014;30:568-570.
- Motaparthi K. Tattoo ink. In: Cockerell CJ, Hall BJ, eds. Nonneoplastic Dermatopathology. 2nd ed. Amirsys; 2016: 270.
Practice Points
- Pencil-core granulomas can arise even decades after the lead is embedded in the skin.
- It is important to biopsy to confirm the diagnosis, as pencil-core granulomas can very closely mimic melanomas.
“I didn’t want to meet you.” Dispelling myths about palliative care
The names of health care professionals and patients cited within the dialogue text have been changed to protect their privacy.
but over the years I have come to realize that she was right – most people, including many within health care, don’t have a good appreciation of what palliative care is or how it can help patients and health care teams.
A recent national survey about cancer-related health information found that of more than 1,000 surveyed Americans, less than 30% professed any knowledge of palliative care. Of those who had some knowledge of palliative care, around 30% believed palliative care was synonymous with hospice.1 Another 15% believed that a patient would have to give up cancer-directed treatments to receive palliative care.1
It’s not giving up
This persistent belief that palliative care is equivalent to hospice, or is tantamount to “giving up,” is one of the most commonly held myths I encounter in everyday practice.
I knock on the exam door and walk in.
A small, trim woman in her late 50s is sitting in a chair, arms folded across her chest, face drawn in.
“Hi,” I start. “I’m Sarah, the palliative care nurse practitioner who works in this clinic. I work closely with Dr. Smith.”
Dr. Smith is the patient’s oncologist.
“I really didn’t want to meet you,” she says in a quiet voice, her eyes large with concern.
I don’t take it personally. Few patients really want to be in the position of needing to meet the palliative care team.
“I looked up palliative care on Google and saw the word hospice.”
“Yeah,” I say. “I hear that a lot. Well, I can reassure you that this isn’t hospice.
In this clinic, our focus is on your cancer symptoms, your treatment side effects, and your quality of life.”
She looks visibly relieved. “Quality of life,” she echoes. “I need more of that.”
“OK,” I say. “So, tell me what you’re struggling with the most right now.”
That’s how many palliative care visits start. I actually prefer if patients haven’t heard of palliative care because it allows me to frame it for them, rather than having to start by addressing a myth or a prior negative experience. Even when patients haven’t had a negative experience with palliative care per se, typically, if they’ve interacted with palliative care in the past, it’s usually because someone they loved died in a hospital setting and it is the memory of that terrible loss that becomes synonymous with their recollection of palliative care.
Many patients I meet have never seen another outpatient palliative care practitioner – and this makes sense – we are still too few and far between. Most established palliative care teams are hospital based and many patients seen in the community do not have easy access to palliative care teams where they receive oncologic care.2 As an embedded practitioner, I see patients in the same exam rooms and infusion centers where they receive their cancer therapies, so I’m effectively woven into the fabric of their oncology experience. Just being there in the cancer center allows me to be in the right place at the right time for the right patients and their care teams.
More than pain management
Another myth I tend to dispel a lot is that palliative care is just a euphemism for “pain management.” I have seen this less lately, but still occasionally in the chart I’ll see documented in a note, “patient is seeing palliative/pain management,” when a patient is seeing me or one of my colleagues. Unfortunately, when providers have limited or outdated views of what palliative care is or the value it brings to patient-centered cancer care, referrals to palliative care tend to be delayed.3
“I really think Ms. Lopez could benefit from seeing palliative care,” an oncology nurse practitioner says to an oncologist.
I’m standing nearby, about to see another patient in one of the exam rooms in our clinic.
“But I don’t think she’s ready. And besides, she doesn’t have any pain,” he says.
He turns to me quizzically. “What do you think?”
“Tell me about the patient,” I ask, taking a few steps in their direction.
“Well, she’s a 64-year-old woman with metastatic cancer.
She has a really poor appetite and is losing some weight.
Seems a bit down, kind of pessimistic about things.
Her scan showed some new growth, so guess I’m not surprised by that.”
“I might be able to help her with the appetite and the mood changes.
I can at least talk with her and see where she’s at,” I offer.
“Alright,” he says. “We’ll put the palliative referral in.”
He hesitates. “But are you sure you want to see her?
She doesn’t have any pain.” He sounds skeptical.
“Yeah, I mean, it sounds like she has symptoms that are bothering her, so I’d be happy to see her. She sounds completely appropriate for palliative care.”
I hear this assumption a lot – that palliative care is somehow equivalent to pain management and that unless a patient’s pain is severe, it’s not worth referring the patient to palliative care. Don’t get me wrong – we do a lot of pain management, but at its heart, palliative care is an interdisciplinary specialty focused on improving or maintaining quality of life for people with serious illness. Because the goal is so broad, care can take many shapes.4
In addition to pain, palliative care clinicians commonly treat nausea, shortness of breath, constipation or diarrhea, poor appetite, fatigue, anxiety, depression, and insomnia.
Palliative care is more than medical or nursing care
A related misconception about palliative care held by many lay people and health care workers alike is that palliative care is primarily medical or nursing care focused mostly on alleviating physical symptoms such as pain or nausea. This couldn’t be further from the truth.
We’ve been talking for a while.
Ms. Lopez tells me about her struggles to maintain her weight while undergoing chemotherapy. She has low-grade nausea that is impacting her ability and desire to eat more and didn’t think that her weight loss was severe enough to warrant taking medication.
We talk about how she may be able to use antinausea medication sparingly to alleviate nausea while also limiting side effects from the medications—which was a big concern for her.
I ask her what else is bothering her.
She tells me that she has always been a strong Catholic and even when life has gotten tough, her faith was never shaken – until now.
She is struggling to understand why she ended up with metastatic cancer at such a relatively young age—why would God do this to her?
She had plans for retirement that have since evaporated in the face of a foreshortened life.
Why did this happen to her of all people? She was completely healthy until her diagnosis.
Her face is wet with tears.
We talk a little about how a diagnosis like this can change so much of a person’s life and identity. I try to validate her experience. She’s clearly suffering from a sense that her life is not what she expected, and she is struggling to integrate how her future looks at this point.
I ask her what conversations with her priest have been like.
At this point you may be wondering where this conversation is going. Why are we talking about Ms. Lopez’s religion? Palliative care is best delivered through high functioning interdisciplinary teams that can include other supportive people in a patient’s life. We work in concert to try to bring comfort to a patient and their family.4 That support network can include nurses, physicians, social workers, and chaplains. In this case, Ms. Lopez had not yet reached out to her priest. She hasn’t had the time or energy to contact her priest given her symptoms.
“Can I contact your priest for you?
Maybe he can visit or call and chat with you?”
She nods and wipes tears away.
“That would be really nice,” she says. “I’d love it if he could pray with me.”
A few hours after the visit, I call Ms. Lopez’s priest.
I ask him to reach out to her and about her request for prayer.
He says he’s been thinking about her and that her presence has been missed at weekly Mass. He thanks me for the call and says he’ll call her tomorrow.
I say my own small prayer for Ms. Lopez and head home, the day’s work completed.
Sarah D'Ambruoso was born and raised in Maine. She completed her undergraduate and graduate nursing education at New York University and UCLA, respectively, and currently works as a palliative care nurse practitioner in an oncology clinic in Los Angeles.
References
1. Cheng BT et al. Patterns of palliative care beliefs among adults in the U.S.: Analysis of a National Cancer Database. J Pain Symptom Manage. 2019 Aug 10. doi: 10.1016/j.jpainsymman.2019.07.030.
2. Finlay E et al. Filling the gap: Creating an outpatient palliative care program in your institution. Am Soc Clin Oncol Educ Book. 2018 May 23. doi: 10.1200/EDBK_200775.
3. Von Roenn JH et al. Barriers and approaches to the successful integration of palliative care and oncology practice. J Natl Compr Canc Netw. 2013 Mar. doi: 10.6004/jnccn.2013.0209.
4. Ferrell BR et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2016 Oct 31. doi: 10.1200/JCO.2016.70.1474.
The names of health care professionals and patients cited within the dialogue text have been changed to protect their privacy.
but over the years I have come to realize that she was right – most people, including many within health care, don’t have a good appreciation of what palliative care is or how it can help patients and health care teams.
A recent national survey about cancer-related health information found that of more than 1,000 surveyed Americans, less than 30% professed any knowledge of palliative care. Of those who had some knowledge of palliative care, around 30% believed palliative care was synonymous with hospice.1 Another 15% believed that a patient would have to give up cancer-directed treatments to receive palliative care.1
It’s not giving up
This persistent belief that palliative care is equivalent to hospice, or is tantamount to “giving up,” is one of the most commonly held myths I encounter in everyday practice.
I knock on the exam door and walk in.
A small, trim woman in her late 50s is sitting in a chair, arms folded across her chest, face drawn in.
“Hi,” I start. “I’m Sarah, the palliative care nurse practitioner who works in this clinic. I work closely with Dr. Smith.”
Dr. Smith is the patient’s oncologist.
“I really didn’t want to meet you,” she says in a quiet voice, her eyes large with concern.
I don’t take it personally. Few patients really want to be in the position of needing to meet the palliative care team.
“I looked up palliative care on Google and saw the word hospice.”
“Yeah,” I say. “I hear that a lot. Well, I can reassure you that this isn’t hospice.
In this clinic, our focus is on your cancer symptoms, your treatment side effects, and your quality of life.”
She looks visibly relieved. “Quality of life,” she echoes. “I need more of that.”
“OK,” I say. “So, tell me what you’re struggling with the most right now.”
That’s how many palliative care visits start. I actually prefer if patients haven’t heard of palliative care because it allows me to frame it for them, rather than having to start by addressing a myth or a prior negative experience. Even when patients haven’t had a negative experience with palliative care per se, typically, if they’ve interacted with palliative care in the past, it’s usually because someone they loved died in a hospital setting and it is the memory of that terrible loss that becomes synonymous with their recollection of palliative care.
Many patients I meet have never seen another outpatient palliative care practitioner – and this makes sense – we are still too few and far between. Most established palliative care teams are hospital based and many patients seen in the community do not have easy access to palliative care teams where they receive oncologic care.2 As an embedded practitioner, I see patients in the same exam rooms and infusion centers where they receive their cancer therapies, so I’m effectively woven into the fabric of their oncology experience. Just being there in the cancer center allows me to be in the right place at the right time for the right patients and their care teams.
More than pain management
Another myth I tend to dispel a lot is that palliative care is just a euphemism for “pain management.” I have seen this less lately, but still occasionally in the chart I’ll see documented in a note, “patient is seeing palliative/pain management,” when a patient is seeing me or one of my colleagues. Unfortunately, when providers have limited or outdated views of what palliative care is or the value it brings to patient-centered cancer care, referrals to palliative care tend to be delayed.3
“I really think Ms. Lopez could benefit from seeing palliative care,” an oncology nurse practitioner says to an oncologist.
I’m standing nearby, about to see another patient in one of the exam rooms in our clinic.
“But I don’t think she’s ready. And besides, she doesn’t have any pain,” he says.
He turns to me quizzically. “What do you think?”
“Tell me about the patient,” I ask, taking a few steps in their direction.
“Well, she’s a 64-year-old woman with metastatic cancer.
She has a really poor appetite and is losing some weight.
Seems a bit down, kind of pessimistic about things.
Her scan showed some new growth, so guess I’m not surprised by that.”
“I might be able to help her with the appetite and the mood changes.
I can at least talk with her and see where she’s at,” I offer.
“Alright,” he says. “We’ll put the palliative referral in.”
He hesitates. “But are you sure you want to see her?
She doesn’t have any pain.” He sounds skeptical.
“Yeah, I mean, it sounds like she has symptoms that are bothering her, so I’d be happy to see her. She sounds completely appropriate for palliative care.”
I hear this assumption a lot – that palliative care is somehow equivalent to pain management and that unless a patient’s pain is severe, it’s not worth referring the patient to palliative care. Don’t get me wrong – we do a lot of pain management, but at its heart, palliative care is an interdisciplinary specialty focused on improving or maintaining quality of life for people with serious illness. Because the goal is so broad, care can take many shapes.4
In addition to pain, palliative care clinicians commonly treat nausea, shortness of breath, constipation or diarrhea, poor appetite, fatigue, anxiety, depression, and insomnia.
Palliative care is more than medical or nursing care
A related misconception about palliative care held by many lay people and health care workers alike is that palliative care is primarily medical or nursing care focused mostly on alleviating physical symptoms such as pain or nausea. This couldn’t be further from the truth.
We’ve been talking for a while.
Ms. Lopez tells me about her struggles to maintain her weight while undergoing chemotherapy. She has low-grade nausea that is impacting her ability and desire to eat more and didn’t think that her weight loss was severe enough to warrant taking medication.
We talk about how she may be able to use antinausea medication sparingly to alleviate nausea while also limiting side effects from the medications—which was a big concern for her.
I ask her what else is bothering her.
She tells me that she has always been a strong Catholic and even when life has gotten tough, her faith was never shaken – until now.
She is struggling to understand why she ended up with metastatic cancer at such a relatively young age—why would God do this to her?
She had plans for retirement that have since evaporated in the face of a foreshortened life.
Why did this happen to her of all people? She was completely healthy until her diagnosis.
Her face is wet with tears.
We talk a little about how a diagnosis like this can change so much of a person’s life and identity. I try to validate her experience. She’s clearly suffering from a sense that her life is not what she expected, and she is struggling to integrate how her future looks at this point.
I ask her what conversations with her priest have been like.
At this point you may be wondering where this conversation is going. Why are we talking about Ms. Lopez’s religion? Palliative care is best delivered through high functioning interdisciplinary teams that can include other supportive people in a patient’s life. We work in concert to try to bring comfort to a patient and their family.4 That support network can include nurses, physicians, social workers, and chaplains. In this case, Ms. Lopez had not yet reached out to her priest. She hasn’t had the time or energy to contact her priest given her symptoms.
“Can I contact your priest for you?
Maybe he can visit or call and chat with you?”
She nods and wipes tears away.
“That would be really nice,” she says. “I’d love it if he could pray with me.”
A few hours after the visit, I call Ms. Lopez’s priest.
I ask him to reach out to her and about her request for prayer.
He says he’s been thinking about her and that her presence has been missed at weekly Mass. He thanks me for the call and says he’ll call her tomorrow.
I say my own small prayer for Ms. Lopez and head home, the day’s work completed.
Sarah D'Ambruoso was born and raised in Maine. She completed her undergraduate and graduate nursing education at New York University and UCLA, respectively, and currently works as a palliative care nurse practitioner in an oncology clinic in Los Angeles.
References
1. Cheng BT et al. Patterns of palliative care beliefs among adults in the U.S.: Analysis of a National Cancer Database. J Pain Symptom Manage. 2019 Aug 10. doi: 10.1016/j.jpainsymman.2019.07.030.
2. Finlay E et al. Filling the gap: Creating an outpatient palliative care program in your institution. Am Soc Clin Oncol Educ Book. 2018 May 23. doi: 10.1200/EDBK_200775.
3. Von Roenn JH et al. Barriers and approaches to the successful integration of palliative care and oncology practice. J Natl Compr Canc Netw. 2013 Mar. doi: 10.6004/jnccn.2013.0209.
4. Ferrell BR et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2016 Oct 31. doi: 10.1200/JCO.2016.70.1474.
The names of health care professionals and patients cited within the dialogue text have been changed to protect their privacy.
but over the years I have come to realize that she was right – most people, including many within health care, don’t have a good appreciation of what palliative care is or how it can help patients and health care teams.
A recent national survey about cancer-related health information found that of more than 1,000 surveyed Americans, less than 30% professed any knowledge of palliative care. Of those who had some knowledge of palliative care, around 30% believed palliative care was synonymous with hospice.1 Another 15% believed that a patient would have to give up cancer-directed treatments to receive palliative care.1
It’s not giving up
This persistent belief that palliative care is equivalent to hospice, or is tantamount to “giving up,” is one of the most commonly held myths I encounter in everyday practice.
I knock on the exam door and walk in.
A small, trim woman in her late 50s is sitting in a chair, arms folded across her chest, face drawn in.
“Hi,” I start. “I’m Sarah, the palliative care nurse practitioner who works in this clinic. I work closely with Dr. Smith.”
Dr. Smith is the patient’s oncologist.
“I really didn’t want to meet you,” she says in a quiet voice, her eyes large with concern.
I don’t take it personally. Few patients really want to be in the position of needing to meet the palliative care team.
“I looked up palliative care on Google and saw the word hospice.”
“Yeah,” I say. “I hear that a lot. Well, I can reassure you that this isn’t hospice.
In this clinic, our focus is on your cancer symptoms, your treatment side effects, and your quality of life.”
She looks visibly relieved. “Quality of life,” she echoes. “I need more of that.”
“OK,” I say. “So, tell me what you’re struggling with the most right now.”
That’s how many palliative care visits start. I actually prefer if patients haven’t heard of palliative care because it allows me to frame it for them, rather than having to start by addressing a myth or a prior negative experience. Even when patients haven’t had a negative experience with palliative care per se, typically, if they’ve interacted with palliative care in the past, it’s usually because someone they loved died in a hospital setting and it is the memory of that terrible loss that becomes synonymous with their recollection of palliative care.
Many patients I meet have never seen another outpatient palliative care practitioner – and this makes sense – we are still too few and far between. Most established palliative care teams are hospital based and many patients seen in the community do not have easy access to palliative care teams where they receive oncologic care.2 As an embedded practitioner, I see patients in the same exam rooms and infusion centers where they receive their cancer therapies, so I’m effectively woven into the fabric of their oncology experience. Just being there in the cancer center allows me to be in the right place at the right time for the right patients and their care teams.
More than pain management
Another myth I tend to dispel a lot is that palliative care is just a euphemism for “pain management.” I have seen this less lately, but still occasionally in the chart I’ll see documented in a note, “patient is seeing palliative/pain management,” when a patient is seeing me or one of my colleagues. Unfortunately, when providers have limited or outdated views of what palliative care is or the value it brings to patient-centered cancer care, referrals to palliative care tend to be delayed.3
“I really think Ms. Lopez could benefit from seeing palliative care,” an oncology nurse practitioner says to an oncologist.
I’m standing nearby, about to see another patient in one of the exam rooms in our clinic.
“But I don’t think she’s ready. And besides, she doesn’t have any pain,” he says.
He turns to me quizzically. “What do you think?”
“Tell me about the patient,” I ask, taking a few steps in their direction.
“Well, she’s a 64-year-old woman with metastatic cancer.
She has a really poor appetite and is losing some weight.
Seems a bit down, kind of pessimistic about things.
Her scan showed some new growth, so guess I’m not surprised by that.”
“I might be able to help her with the appetite and the mood changes.
I can at least talk with her and see where she’s at,” I offer.
“Alright,” he says. “We’ll put the palliative referral in.”
He hesitates. “But are you sure you want to see her?
She doesn’t have any pain.” He sounds skeptical.
“Yeah, I mean, it sounds like she has symptoms that are bothering her, so I’d be happy to see her. She sounds completely appropriate for palliative care.”
I hear this assumption a lot – that palliative care is somehow equivalent to pain management and that unless a patient’s pain is severe, it’s not worth referring the patient to palliative care. Don’t get me wrong – we do a lot of pain management, but at its heart, palliative care is an interdisciplinary specialty focused on improving or maintaining quality of life for people with serious illness. Because the goal is so broad, care can take many shapes.4
In addition to pain, palliative care clinicians commonly treat nausea, shortness of breath, constipation or diarrhea, poor appetite, fatigue, anxiety, depression, and insomnia.
Palliative care is more than medical or nursing care
A related misconception about palliative care held by many lay people and health care workers alike is that palliative care is primarily medical or nursing care focused mostly on alleviating physical symptoms such as pain or nausea. This couldn’t be further from the truth.
We’ve been talking for a while.
Ms. Lopez tells me about her struggles to maintain her weight while undergoing chemotherapy. She has low-grade nausea that is impacting her ability and desire to eat more and didn’t think that her weight loss was severe enough to warrant taking medication.
We talk about how she may be able to use antinausea medication sparingly to alleviate nausea while also limiting side effects from the medications—which was a big concern for her.
I ask her what else is bothering her.
She tells me that she has always been a strong Catholic and even when life has gotten tough, her faith was never shaken – until now.
She is struggling to understand why she ended up with metastatic cancer at such a relatively young age—why would God do this to her?
She had plans for retirement that have since evaporated in the face of a foreshortened life.
Why did this happen to her of all people? She was completely healthy until her diagnosis.
Her face is wet with tears.
We talk a little about how a diagnosis like this can change so much of a person’s life and identity. I try to validate her experience. She’s clearly suffering from a sense that her life is not what she expected, and she is struggling to integrate how her future looks at this point.
I ask her what conversations with her priest have been like.
At this point you may be wondering where this conversation is going. Why are we talking about Ms. Lopez’s religion? Palliative care is best delivered through high functioning interdisciplinary teams that can include other supportive people in a patient’s life. We work in concert to try to bring comfort to a patient and their family.4 That support network can include nurses, physicians, social workers, and chaplains. In this case, Ms. Lopez had not yet reached out to her priest. She hasn’t had the time or energy to contact her priest given her symptoms.
“Can I contact your priest for you?
Maybe he can visit or call and chat with you?”
She nods and wipes tears away.
“That would be really nice,” she says. “I’d love it if he could pray with me.”
A few hours after the visit, I call Ms. Lopez’s priest.
I ask him to reach out to her and about her request for prayer.
He says he’s been thinking about her and that her presence has been missed at weekly Mass. He thanks me for the call and says he’ll call her tomorrow.
I say my own small prayer for Ms. Lopez and head home, the day’s work completed.
Sarah D'Ambruoso was born and raised in Maine. She completed her undergraduate and graduate nursing education at New York University and UCLA, respectively, and currently works as a palliative care nurse practitioner in an oncology clinic in Los Angeles.
References
1. Cheng BT et al. Patterns of palliative care beliefs among adults in the U.S.: Analysis of a National Cancer Database. J Pain Symptom Manage. 2019 Aug 10. doi: 10.1016/j.jpainsymman.2019.07.030.
2. Finlay E et al. Filling the gap: Creating an outpatient palliative care program in your institution. Am Soc Clin Oncol Educ Book. 2018 May 23. doi: 10.1200/EDBK_200775.
3. Von Roenn JH et al. Barriers and approaches to the successful integration of palliative care and oncology practice. J Natl Compr Canc Netw. 2013 Mar. doi: 10.6004/jnccn.2013.0209.
4. Ferrell BR et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2016 Oct 31. doi: 10.1200/JCO.2016.70.1474.
Medicare NCDs hinder access to cancer biomarker testing for minorities
of data from patients with advanced non–small cell lung cancer (aNSCLC), metastatic colorectal cancer, metastatic breast cancer, or advanced melanoma. The finding was reported in JAMA Network Open.
Biomarker testing has become an essential tool in cancer care over the last decade. In 2011, for example, less than 1% of patients with aNSCLC, metastatic colorectal cancer, metastatic breast cancer, and advanced melanoma underwent NGS testing, but by 2019, 40% of patients with these cancers received the testing.
“Next-generation sequencing testing has become increasingly important because it enables identification of multiple biomarkers simultaneously and efficiently while minimizing the number of biopsies required,” wrote the authors, led by William B. Wong, PharmD, of Genentech.
It has been unknown whether for Medicare beneficiaries and the overall population, if the NCD affected health equity issues, the authors wrote. While increased use of appropriate targeted therapies facilitated by NGS testing is associated with improved survival rates in patients with advanced or metastatic cancer, variability in health care coverage policies has posed a significant barrier to obtaining NGS testing for cancer patients, specifically through policy coverage limitations. It has remained unclear if the NCD has influenced NGS testing coverage in insurance types (for example, Medicaid) encompassing a larger population of minority racial and ethnic groups often experiencing poorer care and outcomes.
The retrospective cohort analysis compared EHR data from 280 U.S. cancer clinics in the (800 sites of care) pre- versus post-NCD period for patients with aNSCLC, metastatic colorectal cancer, metastatic breast cancer, or advanced melanoma (January 2011–March 2020). Nearly 70% of all patients in the study were Medicare recipients who needed NCD approval to cover the cost of testing.
Among 92,687 patients (mean age, 66.6 years; 55.7% women), compared with Medicare beneficiaries, changes in pre- to post-NCD NGS testing trends were similar in commercially insured patients (odds ratio, 1.03; 95% CI, 0.98-1.08; P = .25). Pre- to post-NCD NGS testing trends increased at a slower rate among patients in assistance programs (OR, 0.93; 95% CI, 0.87-0.99; P = .03), compared with Medicare beneficiaries. The rate of increase for patients receiving Medicaid was not significantly different statistically compared with those receiving Medicare (OR, 0.92; 95% CI, 0.84-1.01; P = .07). Also, the NCD was not associated with racial and ethnic groups within Medicare beneficiaries alone or across all insurance types.
Compared with non-Hispanic White individuals, increases in average NGS use from the pre-NCD to post-NCD period were 14% lower (OR, 0.86; 95% CI, 0.74-0.99; P = .04) among African American and 23% lower (OR, 0.77; 95% CI, 0.62-0.96; P = .02) among Hispanic/Latino individuals; increases were similar, however, among Asian individuals and other races and ethnicities.
The authors observed that the post-NCD trend of increasing NGS testing seen in Medicare beneficiaries was similarly observed in those with commercial insurance. Testing rate differences, however, widened or were maintained after versus before the NCD in PAP (personal assistance program) and Medicaid beneficiaries relative to Medicare beneficiaries, suggesting that access to NGS testing did not improve equally across insurance types. Since Medicare coverage is determined at the state level, the authors urged research examining individual state coverage policies to further elucidate factors slowing uptake among Medicaid beneficiaries. “Additional efforts beyond coverage policies,” the authors concluded, “are needed to ensure equitable access to the benefits of precision medicine.”
The study was supported by Genentech.
of data from patients with advanced non–small cell lung cancer (aNSCLC), metastatic colorectal cancer, metastatic breast cancer, or advanced melanoma. The finding was reported in JAMA Network Open.
Biomarker testing has become an essential tool in cancer care over the last decade. In 2011, for example, less than 1% of patients with aNSCLC, metastatic colorectal cancer, metastatic breast cancer, and advanced melanoma underwent NGS testing, but by 2019, 40% of patients with these cancers received the testing.
“Next-generation sequencing testing has become increasingly important because it enables identification of multiple biomarkers simultaneously and efficiently while minimizing the number of biopsies required,” wrote the authors, led by William B. Wong, PharmD, of Genentech.
It has been unknown whether for Medicare beneficiaries and the overall population, if the NCD affected health equity issues, the authors wrote. While increased use of appropriate targeted therapies facilitated by NGS testing is associated with improved survival rates in patients with advanced or metastatic cancer, variability in health care coverage policies has posed a significant barrier to obtaining NGS testing for cancer patients, specifically through policy coverage limitations. It has remained unclear if the NCD has influenced NGS testing coverage in insurance types (for example, Medicaid) encompassing a larger population of minority racial and ethnic groups often experiencing poorer care and outcomes.
The retrospective cohort analysis compared EHR data from 280 U.S. cancer clinics in the (800 sites of care) pre- versus post-NCD period for patients with aNSCLC, metastatic colorectal cancer, metastatic breast cancer, or advanced melanoma (January 2011–March 2020). Nearly 70% of all patients in the study were Medicare recipients who needed NCD approval to cover the cost of testing.
Among 92,687 patients (mean age, 66.6 years; 55.7% women), compared with Medicare beneficiaries, changes in pre- to post-NCD NGS testing trends were similar in commercially insured patients (odds ratio, 1.03; 95% CI, 0.98-1.08; P = .25). Pre- to post-NCD NGS testing trends increased at a slower rate among patients in assistance programs (OR, 0.93; 95% CI, 0.87-0.99; P = .03), compared with Medicare beneficiaries. The rate of increase for patients receiving Medicaid was not significantly different statistically compared with those receiving Medicare (OR, 0.92; 95% CI, 0.84-1.01; P = .07). Also, the NCD was not associated with racial and ethnic groups within Medicare beneficiaries alone or across all insurance types.
Compared with non-Hispanic White individuals, increases in average NGS use from the pre-NCD to post-NCD period were 14% lower (OR, 0.86; 95% CI, 0.74-0.99; P = .04) among African American and 23% lower (OR, 0.77; 95% CI, 0.62-0.96; P = .02) among Hispanic/Latino individuals; increases were similar, however, among Asian individuals and other races and ethnicities.
The authors observed that the post-NCD trend of increasing NGS testing seen in Medicare beneficiaries was similarly observed in those with commercial insurance. Testing rate differences, however, widened or were maintained after versus before the NCD in PAP (personal assistance program) and Medicaid beneficiaries relative to Medicare beneficiaries, suggesting that access to NGS testing did not improve equally across insurance types. Since Medicare coverage is determined at the state level, the authors urged research examining individual state coverage policies to further elucidate factors slowing uptake among Medicaid beneficiaries. “Additional efforts beyond coverage policies,” the authors concluded, “are needed to ensure equitable access to the benefits of precision medicine.”
The study was supported by Genentech.
of data from patients with advanced non–small cell lung cancer (aNSCLC), metastatic colorectal cancer, metastatic breast cancer, or advanced melanoma. The finding was reported in JAMA Network Open.
Biomarker testing has become an essential tool in cancer care over the last decade. In 2011, for example, less than 1% of patients with aNSCLC, metastatic colorectal cancer, metastatic breast cancer, and advanced melanoma underwent NGS testing, but by 2019, 40% of patients with these cancers received the testing.
“Next-generation sequencing testing has become increasingly important because it enables identification of multiple biomarkers simultaneously and efficiently while minimizing the number of biopsies required,” wrote the authors, led by William B. Wong, PharmD, of Genentech.
It has been unknown whether for Medicare beneficiaries and the overall population, if the NCD affected health equity issues, the authors wrote. While increased use of appropriate targeted therapies facilitated by NGS testing is associated with improved survival rates in patients with advanced or metastatic cancer, variability in health care coverage policies has posed a significant barrier to obtaining NGS testing for cancer patients, specifically through policy coverage limitations. It has remained unclear if the NCD has influenced NGS testing coverage in insurance types (for example, Medicaid) encompassing a larger population of minority racial and ethnic groups often experiencing poorer care and outcomes.
The retrospective cohort analysis compared EHR data from 280 U.S. cancer clinics in the (800 sites of care) pre- versus post-NCD period for patients with aNSCLC, metastatic colorectal cancer, metastatic breast cancer, or advanced melanoma (January 2011–March 2020). Nearly 70% of all patients in the study were Medicare recipients who needed NCD approval to cover the cost of testing.
Among 92,687 patients (mean age, 66.6 years; 55.7% women), compared with Medicare beneficiaries, changes in pre- to post-NCD NGS testing trends were similar in commercially insured patients (odds ratio, 1.03; 95% CI, 0.98-1.08; P = .25). Pre- to post-NCD NGS testing trends increased at a slower rate among patients in assistance programs (OR, 0.93; 95% CI, 0.87-0.99; P = .03), compared with Medicare beneficiaries. The rate of increase for patients receiving Medicaid was not significantly different statistically compared with those receiving Medicare (OR, 0.92; 95% CI, 0.84-1.01; P = .07). Also, the NCD was not associated with racial and ethnic groups within Medicare beneficiaries alone or across all insurance types.
Compared with non-Hispanic White individuals, increases in average NGS use from the pre-NCD to post-NCD period were 14% lower (OR, 0.86; 95% CI, 0.74-0.99; P = .04) among African American and 23% lower (OR, 0.77; 95% CI, 0.62-0.96; P = .02) among Hispanic/Latino individuals; increases were similar, however, among Asian individuals and other races and ethnicities.
The authors observed that the post-NCD trend of increasing NGS testing seen in Medicare beneficiaries was similarly observed in those with commercial insurance. Testing rate differences, however, widened or were maintained after versus before the NCD in PAP (personal assistance program) and Medicaid beneficiaries relative to Medicare beneficiaries, suggesting that access to NGS testing did not improve equally across insurance types. Since Medicare coverage is determined at the state level, the authors urged research examining individual state coverage policies to further elucidate factors slowing uptake among Medicaid beneficiaries. “Additional efforts beyond coverage policies,” the authors concluded, “are needed to ensure equitable access to the benefits of precision medicine.”
The study was supported by Genentech.
FROM JAMA NETWORK OPEN