User login
Hair dye and cancer study ‘offers some reassurance’
Findings limited to White women in United States
The largest study of its kind has found no positive association between personal use of permanent hair dye and the risk for most cancers and cancer mortality.
The findings come from the Nurses’ Health Study, an ongoing prospective cohort study of more than 117,000 women who have been followed for 36 years and who did not have cancer at baseline.
The findings were published online on September 2 in the BMJ.
The results “offer some reassurance against concerns that personal use of permanent hair dyes might be associated with increased cancer risk or mortality,” write the investigators, with first author Yin Zhang, PhD, of Harvard Medical School, Boston.
The findings, which are limited to White women in the United States, indicate correlation, not causation, the authors emphasize.
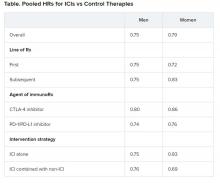
Nevertheless, the researchers found an increased risk for some cancers among hair dye users, especially with greater cumulative dose (200 or more uses during the study period). The risk was increased for basal cell carcinoma, breast cancer (specifically, estrogen receptor negative [ER–], progesterone receptor negative [PR–], and hormone receptor negative [ER–, PR–]), and ovarian cancer.
A British expert not involved in the study dismissed these findings. “The reported associations are very weak, and, given the number of associations reported in this manuscript, they are very likely to be chance findings,” commented Paul Pharoah, PhD, professor of cancer epidemiology at the University of Cambridge (England).
“For the cancers where an increase in risk is reported, the results are not compelling. Even if they were real findings, the associations may not be cause-and-effect, and, even if they were causal associations, the magnitude of the effects are so small that any risk would be trivial.
“In short, none of the findings reported in this manuscript suggest that women who use hair dye are putting themselves at increased risk of cancer,” he stated.
A U.S. researcher who has previously coauthored a study suggesting an association between hair dye and breast cancer agreed that the increases in risk reported in this current study are “small.” But they are “of interest,” especially for breast and ovarian cancer, said Alexandra White, PhD, of the National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, N.C.
Hair dyes include compounds that “are not just potential carcinogens but also act as endocrine disruptors,” she said in an interview.
“In both breast and ovarian cancer, we know that hormones play an important part in the etiology ... so it’s biologically plausible that you would see [these associations in the current study],” added Dr. White, who was approached for comment.
However, she added that, even with the “modest” 20%-28% increase in the relative risk for certain breast cancers linked to a heavy cumulative dose of dyes in the current study, “there doesn’t seem to be any strong association with any cancer type.”
But she also pointed out that the most outstanding risk association was among ER–/PR– breast cancers, which are the “most aggressive and difficult to treat,” and thus the new findings are “important.”
Dr. White is the lead author of a 2019 study that received a lot of media attention because it rang an alarm bell about hair dyes and breast cancer risk.
That study concluded that ever using permanent hair dye or hair straighteners was associated with a higher risk for breast cancer than never using them and that this higher risk was especially associated with Black women. However, the study participants were from the prospective Sister Study. The participants in that study had no history of breast cancer, but they each had at least one sister who did. This family history of breast cancer may represent selection bias.
With changes in the 1980s, even safer now?
The study of hair dyes and cancer has “major public health implications” because the use of hair dye is widespread, Dr. Zhang and colleagues write in their article. They estimate that 50% to 80% of women and 10% of men aged 40 years and older in the United States and Europe use hair dye.
Permanent hair dyes “pose the greatest potential concern,” they stated, adding that these account for approximately 80% of hair dyes used in the United States and Europe and an even higher percentage in Asia.
The International Agency for Research on Cancer classifies occupational exposure to hair dyes as probably carcinogenic, but the carcinogenicity resulting from personal use of hair dyes is not classifiable – thus, there is no warning about at-home usage.
Notably, there was “a huge and very important” change in hair dye ingredients in the 1980s after the Food and Drug Administration warned about some chemicals in permanent hair dyes and the cosmetic industry altered their formulas, lead author Dr. Zhang said.
However, the researchers could not analyze use before and after the changes because not enough women reported first use of permanent hair dye after 1980 (only 1890 of 117,200 participants).
“We could expect that the current ingredients should make it safer,” Dr. Zhang said.
Study details
The researchers report that ever-users of permanent hair dyes had no significant increases in risk for solid cancers (n = 20,805; hazard ratio, 0.98, 95% confidence interval, 0.96-1.01) or hematopoietic cancers overall (n = 1,807; HR, 1.00; 95% CI, 0.91-1.10) compared with nonusers.
Additionally, ever-users did not have an increased risk for most specific cancers or cancer-related death (n = 4,860; HR, 0.96; 95% CI, 0.91-1.02).
As noted above, there were some exceptions.
Basal cell carcinoma risk was slightly increased for ever-users (n = 22,560; HR, 1.05; 95% CI, 1.02-1.08). Cumulative dose (a calculation of duration and frequency) was positively associated with risk for ER– breast cancer, PR– breast cancer, ER–/PR– breast cancer, and ovarian cancer, with risk rising in accordance with the total amount of dye.
Notably, at a cumulative dose of ≥200 uses, there was a 20% increase in the relative risk for ER- breast cancer (n = 1521; HR, 1.20; 95% CI, 1.02-1.41; P value for trend, .03). At the same cumulative dose, there was a 28% increase in the relative risk for ER-/PR- breast cancer (n = 1287; HR, 1.28, 95% CI, 1.08-1.52; P value for trend, .006).
In addition, an increased risk for Hodgkin lymphoma was observed, but only for women with naturally dark hair (the calculation was based on 70 women, 24 of whom had dark hair).
In a press statement, senior author Eva Schernhammer, PhD, of Harvard and the Medical University of Vienna, said the results “justify further prospective validation.”
She also explained that there are many variables to consider in this research, including different populations and countries, different susceptibility genotypes, different exposure settings (personal use vs. occupational exposure), and different colors of the permanent hair dyes used (dark dyes vs. light dyes).
Geographic location is a particularly important variable, suggested the study authors.
They pointed out that Europe, but not the United States, banned some individual hair dye ingredients that were considered carcinogenic during both the 1980s and 2000s. One country has even tighter oversight: “The most restrictive regulation of hair dyes exists in Japan, where cosmetic products are considered equivalent to drugs.”
The study was funded by the Centers for Disease Control and Prevention and the National Institute for Occupational Safety and Health. The study authors and Dr. White have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Findings limited to White women in United States
Findings limited to White women in United States
The largest study of its kind has found no positive association between personal use of permanent hair dye and the risk for most cancers and cancer mortality.
The findings come from the Nurses’ Health Study, an ongoing prospective cohort study of more than 117,000 women who have been followed for 36 years and who did not have cancer at baseline.
The findings were published online on September 2 in the BMJ.
The results “offer some reassurance against concerns that personal use of permanent hair dyes might be associated with increased cancer risk or mortality,” write the investigators, with first author Yin Zhang, PhD, of Harvard Medical School, Boston.
The findings, which are limited to White women in the United States, indicate correlation, not causation, the authors emphasize.
Nevertheless, the researchers found an increased risk for some cancers among hair dye users, especially with greater cumulative dose (200 or more uses during the study period). The risk was increased for basal cell carcinoma, breast cancer (specifically, estrogen receptor negative [ER–], progesterone receptor negative [PR–], and hormone receptor negative [ER–, PR–]), and ovarian cancer.
A British expert not involved in the study dismissed these findings. “The reported associations are very weak, and, given the number of associations reported in this manuscript, they are very likely to be chance findings,” commented Paul Pharoah, PhD, professor of cancer epidemiology at the University of Cambridge (England).
“For the cancers where an increase in risk is reported, the results are not compelling. Even if they were real findings, the associations may not be cause-and-effect, and, even if they were causal associations, the magnitude of the effects are so small that any risk would be trivial.
“In short, none of the findings reported in this manuscript suggest that women who use hair dye are putting themselves at increased risk of cancer,” he stated.
A U.S. researcher who has previously coauthored a study suggesting an association between hair dye and breast cancer agreed that the increases in risk reported in this current study are “small.” But they are “of interest,” especially for breast and ovarian cancer, said Alexandra White, PhD, of the National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, N.C.
Hair dyes include compounds that “are not just potential carcinogens but also act as endocrine disruptors,” she said in an interview.
“In both breast and ovarian cancer, we know that hormones play an important part in the etiology ... so it’s biologically plausible that you would see [these associations in the current study],” added Dr. White, who was approached for comment.
However, she added that, even with the “modest” 20%-28% increase in the relative risk for certain breast cancers linked to a heavy cumulative dose of dyes in the current study, “there doesn’t seem to be any strong association with any cancer type.”
But she also pointed out that the most outstanding risk association was among ER–/PR– breast cancers, which are the “most aggressive and difficult to treat,” and thus the new findings are “important.”
Dr. White is the lead author of a 2019 study that received a lot of media attention because it rang an alarm bell about hair dyes and breast cancer risk.
That study concluded that ever using permanent hair dye or hair straighteners was associated with a higher risk for breast cancer than never using them and that this higher risk was especially associated with Black women. However, the study participants were from the prospective Sister Study. The participants in that study had no history of breast cancer, but they each had at least one sister who did. This family history of breast cancer may represent selection bias.
With changes in the 1980s, even safer now?
The study of hair dyes and cancer has “major public health implications” because the use of hair dye is widespread, Dr. Zhang and colleagues write in their article. They estimate that 50% to 80% of women and 10% of men aged 40 years and older in the United States and Europe use hair dye.
Permanent hair dyes “pose the greatest potential concern,” they stated, adding that these account for approximately 80% of hair dyes used in the United States and Europe and an even higher percentage in Asia.
The International Agency for Research on Cancer classifies occupational exposure to hair dyes as probably carcinogenic, but the carcinogenicity resulting from personal use of hair dyes is not classifiable – thus, there is no warning about at-home usage.
Notably, there was “a huge and very important” change in hair dye ingredients in the 1980s after the Food and Drug Administration warned about some chemicals in permanent hair dyes and the cosmetic industry altered their formulas, lead author Dr. Zhang said.
However, the researchers could not analyze use before and after the changes because not enough women reported first use of permanent hair dye after 1980 (only 1890 of 117,200 participants).
“We could expect that the current ingredients should make it safer,” Dr. Zhang said.
Study details
The researchers report that ever-users of permanent hair dyes had no significant increases in risk for solid cancers (n = 20,805; hazard ratio, 0.98, 95% confidence interval, 0.96-1.01) or hematopoietic cancers overall (n = 1,807; HR, 1.00; 95% CI, 0.91-1.10) compared with nonusers.
Additionally, ever-users did not have an increased risk for most specific cancers or cancer-related death (n = 4,860; HR, 0.96; 95% CI, 0.91-1.02).
As noted above, there were some exceptions.
Basal cell carcinoma risk was slightly increased for ever-users (n = 22,560; HR, 1.05; 95% CI, 1.02-1.08). Cumulative dose (a calculation of duration and frequency) was positively associated with risk for ER– breast cancer, PR– breast cancer, ER–/PR– breast cancer, and ovarian cancer, with risk rising in accordance with the total amount of dye.
Notably, at a cumulative dose of ≥200 uses, there was a 20% increase in the relative risk for ER- breast cancer (n = 1521; HR, 1.20; 95% CI, 1.02-1.41; P value for trend, .03). At the same cumulative dose, there was a 28% increase in the relative risk for ER-/PR- breast cancer (n = 1287; HR, 1.28, 95% CI, 1.08-1.52; P value for trend, .006).
In addition, an increased risk for Hodgkin lymphoma was observed, but only for women with naturally dark hair (the calculation was based on 70 women, 24 of whom had dark hair).
In a press statement, senior author Eva Schernhammer, PhD, of Harvard and the Medical University of Vienna, said the results “justify further prospective validation.”
She also explained that there are many variables to consider in this research, including different populations and countries, different susceptibility genotypes, different exposure settings (personal use vs. occupational exposure), and different colors of the permanent hair dyes used (dark dyes vs. light dyes).
Geographic location is a particularly important variable, suggested the study authors.
They pointed out that Europe, but not the United States, banned some individual hair dye ingredients that were considered carcinogenic during both the 1980s and 2000s. One country has even tighter oversight: “The most restrictive regulation of hair dyes exists in Japan, where cosmetic products are considered equivalent to drugs.”
The study was funded by the Centers for Disease Control and Prevention and the National Institute for Occupational Safety and Health. The study authors and Dr. White have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The largest study of its kind has found no positive association between personal use of permanent hair dye and the risk for most cancers and cancer mortality.
The findings come from the Nurses’ Health Study, an ongoing prospective cohort study of more than 117,000 women who have been followed for 36 years and who did not have cancer at baseline.
The findings were published online on September 2 in the BMJ.
The results “offer some reassurance against concerns that personal use of permanent hair dyes might be associated with increased cancer risk or mortality,” write the investigators, with first author Yin Zhang, PhD, of Harvard Medical School, Boston.
The findings, which are limited to White women in the United States, indicate correlation, not causation, the authors emphasize.
Nevertheless, the researchers found an increased risk for some cancers among hair dye users, especially with greater cumulative dose (200 or more uses during the study period). The risk was increased for basal cell carcinoma, breast cancer (specifically, estrogen receptor negative [ER–], progesterone receptor negative [PR–], and hormone receptor negative [ER–, PR–]), and ovarian cancer.
A British expert not involved in the study dismissed these findings. “The reported associations are very weak, and, given the number of associations reported in this manuscript, they are very likely to be chance findings,” commented Paul Pharoah, PhD, professor of cancer epidemiology at the University of Cambridge (England).
“For the cancers where an increase in risk is reported, the results are not compelling. Even if they were real findings, the associations may not be cause-and-effect, and, even if they were causal associations, the magnitude of the effects are so small that any risk would be trivial.
“In short, none of the findings reported in this manuscript suggest that women who use hair dye are putting themselves at increased risk of cancer,” he stated.
A U.S. researcher who has previously coauthored a study suggesting an association between hair dye and breast cancer agreed that the increases in risk reported in this current study are “small.” But they are “of interest,” especially for breast and ovarian cancer, said Alexandra White, PhD, of the National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, N.C.
Hair dyes include compounds that “are not just potential carcinogens but also act as endocrine disruptors,” she said in an interview.
“In both breast and ovarian cancer, we know that hormones play an important part in the etiology ... so it’s biologically plausible that you would see [these associations in the current study],” added Dr. White, who was approached for comment.
However, she added that, even with the “modest” 20%-28% increase in the relative risk for certain breast cancers linked to a heavy cumulative dose of dyes in the current study, “there doesn’t seem to be any strong association with any cancer type.”
But she also pointed out that the most outstanding risk association was among ER–/PR– breast cancers, which are the “most aggressive and difficult to treat,” and thus the new findings are “important.”
Dr. White is the lead author of a 2019 study that received a lot of media attention because it rang an alarm bell about hair dyes and breast cancer risk.
That study concluded that ever using permanent hair dye or hair straighteners was associated with a higher risk for breast cancer than never using them and that this higher risk was especially associated with Black women. However, the study participants were from the prospective Sister Study. The participants in that study had no history of breast cancer, but they each had at least one sister who did. This family history of breast cancer may represent selection bias.
With changes in the 1980s, even safer now?
The study of hair dyes and cancer has “major public health implications” because the use of hair dye is widespread, Dr. Zhang and colleagues write in their article. They estimate that 50% to 80% of women and 10% of men aged 40 years and older in the United States and Europe use hair dye.
Permanent hair dyes “pose the greatest potential concern,” they stated, adding that these account for approximately 80% of hair dyes used in the United States and Europe and an even higher percentage in Asia.
The International Agency for Research on Cancer classifies occupational exposure to hair dyes as probably carcinogenic, but the carcinogenicity resulting from personal use of hair dyes is not classifiable – thus, there is no warning about at-home usage.
Notably, there was “a huge and very important” change in hair dye ingredients in the 1980s after the Food and Drug Administration warned about some chemicals in permanent hair dyes and the cosmetic industry altered their formulas, lead author Dr. Zhang said.
However, the researchers could not analyze use before and after the changes because not enough women reported first use of permanent hair dye after 1980 (only 1890 of 117,200 participants).
“We could expect that the current ingredients should make it safer,” Dr. Zhang said.
Study details
The researchers report that ever-users of permanent hair dyes had no significant increases in risk for solid cancers (n = 20,805; hazard ratio, 0.98, 95% confidence interval, 0.96-1.01) or hematopoietic cancers overall (n = 1,807; HR, 1.00; 95% CI, 0.91-1.10) compared with nonusers.
Additionally, ever-users did not have an increased risk for most specific cancers or cancer-related death (n = 4,860; HR, 0.96; 95% CI, 0.91-1.02).
As noted above, there were some exceptions.
Basal cell carcinoma risk was slightly increased for ever-users (n = 22,560; HR, 1.05; 95% CI, 1.02-1.08). Cumulative dose (a calculation of duration and frequency) was positively associated with risk for ER– breast cancer, PR– breast cancer, ER–/PR– breast cancer, and ovarian cancer, with risk rising in accordance with the total amount of dye.
Notably, at a cumulative dose of ≥200 uses, there was a 20% increase in the relative risk for ER- breast cancer (n = 1521; HR, 1.20; 95% CI, 1.02-1.41; P value for trend, .03). At the same cumulative dose, there was a 28% increase in the relative risk for ER-/PR- breast cancer (n = 1287; HR, 1.28, 95% CI, 1.08-1.52; P value for trend, .006).
In addition, an increased risk for Hodgkin lymphoma was observed, but only for women with naturally dark hair (the calculation was based on 70 women, 24 of whom had dark hair).
In a press statement, senior author Eva Schernhammer, PhD, of Harvard and the Medical University of Vienna, said the results “justify further prospective validation.”
She also explained that there are many variables to consider in this research, including different populations and countries, different susceptibility genotypes, different exposure settings (personal use vs. occupational exposure), and different colors of the permanent hair dyes used (dark dyes vs. light dyes).
Geographic location is a particularly important variable, suggested the study authors.
They pointed out that Europe, but not the United States, banned some individual hair dye ingredients that were considered carcinogenic during both the 1980s and 2000s. One country has even tighter oversight: “The most restrictive regulation of hair dyes exists in Japan, where cosmetic products are considered equivalent to drugs.”
The study was funded by the Centers for Disease Control and Prevention and the National Institute for Occupational Safety and Health. The study authors and Dr. White have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Durable response 5 years after adjuvant combo in melanoma
Adjuvant therapy for patients with high-risk resected melanomas is now a standard of care, but the durability of the benefit gained from this treatment is still unclear.
New data show that the benefit is maintained over the longer term.
At 5 years, just over half of patients (52%) with advanced melanoma who had received a year of adjuvant therapy with two targeted agents were still alive and remained relapse free, compared with 36% of patients who received placebo.
The combination of the investigators concluded.
These data come from the COMBI-AD phase 3 trial and were published online in the New England Journal of Medicine.
“The treatment duration of this adjuvant therapy was 12 months; however, we do not know whether this is the optimal treatment duration,” said lead author Reinhard Dummer, MD, vice chairman, department of dermatology, University of Zürich Hospital. “Early biomarker results suggest that, in a subgroup, longer treatment durations might be necessary. In other patients, a shorter treatment could be sufficient.”
Richard Carvajal, MD, director of the Melanoma Service at New York–Presbyterian Hospital and Columbia University Medical Center, also in New York, said the new data “address prior concerns that any benefit achieved with targeted therapy in the adjuvant setting may be limited in duration.”
“Indeed, with active therapy, over 50% of patients are alive without relapse and 65% of patients are alive without the development of distant metastasis,” he said. “Although overall survival data remain immature, numerical improvement in survival is also reported.”
In an interview, Dr. Carvajal said that the plateaus observed with relapse and distant metastasis-free survival suggest that true disease cures are being achieved with treatment. “Based upon these results, the discussion of adjuvant therapeutic options should include a 12-month course of adjuvant dabrafenib and trametinib, as well as the option of adjuvant anti-PD-1 [programmed death–1] therapy.”
As for how the MEK-BRAF inhibitor combination compares with immunotherapy in this setting, he pointed out that, since there has been no head-to-head comparison of adjuvant targeted therapy and adjuvant nivolumab (Opdivo) or pembrolizumab (Keytruda), it is not possible to conclusively state that one regimen is more effective than another.
“For patients with resected BRAF-mutant melanoma at high risk of disease recurrence, we now have data demonstrating the clinical benefit for a course of adjuvant dabrafenib and trametinib, adjuvant nivolumab and adjuvant pembrolizumab,” said Dr. Carvajal.
“Although the efficacy of adjuvant ipilimumab [Yervoy] as well as adjuvant interferon have also been previously demonstrated, these agents are now appropriate for consideration in extremely rare clinical circumstances given the clinical efficacy and improved toxicity profile of single agent anti-PD-1 therapy.”
“The selection of the most appropriate adjuvant therapy should take into account the preferences of individual patients in terms of toxicity profile and drug administration considerations,” he added.
Study details
The COMBI-AD was a randomized, double-blind, placebo-controlled, phase 3 study conducted in 870 patients with high-risk, stage III, BRAF-V600E/K–mutant melanoma who were treatment naive. Participating patients had undergone surgical resection and had been disease free for ≤12 weeks.
Interim results from this study, reported in 2017, showed 1 year of oral adjuvant therapy with dabrafenib and trametinib provided a 53% lower risk for 3-year recurrence, compared with placebo.
Now, the investigators reported on the 5-year results for relapse-free survival and survival without distant metastasis. They noted that they were unable to analyze overall survival since the required number of events had not been reached.
Patients had been randomly assigned to receive 12 months of oral dabrafenib (at a dose of 150 mg twice daily) plus trametinib (2 mg once daily) or two matched placebos. Patients were followed for 60 months (5 years) for dabrafenib plus trametinib and 58 months for placebo.
At 5 years, the median relapse-free survival was not reached for patients who received the combination therapy group versus 16.6 months in the placebo group (hazard ratio for relapse or death, 0.51).
The percentage of patients who were alive without distant metastasis at 5 years was 65% in the dabrafenib plus trametinib group and 54% in the placebo arm (HR for distant metastasis or death, 0.55).
The hazard ratio for relapse-free survival favored dabrafenib plus trametinib across all patient subgroups that were evaluated in the study, and survival without distant metastasis showed a similar benefit for the combination regardless of disease stage.
Subsequent therapy was needed in 40% of patients who received dabrafenib plus trametinib and by 54% of those in the placebo group, with the most common treatments being immunotherapy in the combination-therapy group [26%] and small molecule–targeted therapy in the placebo group (35%).
A viable option
Dr. Dummer noted that, when this clinical trial was designed, all patients had to undergo aggressive surgery that involved lymph node dissection. “Nowadays, based on the lack of improvement on progression-free survival and overall survival, the surgical procedures are less aggressive and today we do not recommend aggressive lymph node dissection in patients that qualify for adjuvant therapy. In patients that do not have the BRAF mutation, there is the possibility of giving immunotherapy.”
He added that there is an urgent need for biomarkers that can identify early progression during adjuvant therapy. “Potentially, these patients would profit from immunotherapy alone or from combination using targeted therapy and immunotherapy,” Dr. Dummer said.
The study was funded by GlaxoSmithKline and Novartis. Dr. Dummer has declared multiple relationships with industry.
A version of this article originally appeared on Medscape.com.
Adjuvant therapy for patients with high-risk resected melanomas is now a standard of care, but the durability of the benefit gained from this treatment is still unclear.
New data show that the benefit is maintained over the longer term.
At 5 years, just over half of patients (52%) with advanced melanoma who had received a year of adjuvant therapy with two targeted agents were still alive and remained relapse free, compared with 36% of patients who received placebo.
The combination of the investigators concluded.
These data come from the COMBI-AD phase 3 trial and were published online in the New England Journal of Medicine.
“The treatment duration of this adjuvant therapy was 12 months; however, we do not know whether this is the optimal treatment duration,” said lead author Reinhard Dummer, MD, vice chairman, department of dermatology, University of Zürich Hospital. “Early biomarker results suggest that, in a subgroup, longer treatment durations might be necessary. In other patients, a shorter treatment could be sufficient.”
Richard Carvajal, MD, director of the Melanoma Service at New York–Presbyterian Hospital and Columbia University Medical Center, also in New York, said the new data “address prior concerns that any benefit achieved with targeted therapy in the adjuvant setting may be limited in duration.”
“Indeed, with active therapy, over 50% of patients are alive without relapse and 65% of patients are alive without the development of distant metastasis,” he said. “Although overall survival data remain immature, numerical improvement in survival is also reported.”
In an interview, Dr. Carvajal said that the plateaus observed with relapse and distant metastasis-free survival suggest that true disease cures are being achieved with treatment. “Based upon these results, the discussion of adjuvant therapeutic options should include a 12-month course of adjuvant dabrafenib and trametinib, as well as the option of adjuvant anti-PD-1 [programmed death–1] therapy.”
As for how the MEK-BRAF inhibitor combination compares with immunotherapy in this setting, he pointed out that, since there has been no head-to-head comparison of adjuvant targeted therapy and adjuvant nivolumab (Opdivo) or pembrolizumab (Keytruda), it is not possible to conclusively state that one regimen is more effective than another.
“For patients with resected BRAF-mutant melanoma at high risk of disease recurrence, we now have data demonstrating the clinical benefit for a course of adjuvant dabrafenib and trametinib, adjuvant nivolumab and adjuvant pembrolizumab,” said Dr. Carvajal.
“Although the efficacy of adjuvant ipilimumab [Yervoy] as well as adjuvant interferon have also been previously demonstrated, these agents are now appropriate for consideration in extremely rare clinical circumstances given the clinical efficacy and improved toxicity profile of single agent anti-PD-1 therapy.”
“The selection of the most appropriate adjuvant therapy should take into account the preferences of individual patients in terms of toxicity profile and drug administration considerations,” he added.
Study details
The COMBI-AD was a randomized, double-blind, placebo-controlled, phase 3 study conducted in 870 patients with high-risk, stage III, BRAF-V600E/K–mutant melanoma who were treatment naive. Participating patients had undergone surgical resection and had been disease free for ≤12 weeks.
Interim results from this study, reported in 2017, showed 1 year of oral adjuvant therapy with dabrafenib and trametinib provided a 53% lower risk for 3-year recurrence, compared with placebo.
Now, the investigators reported on the 5-year results for relapse-free survival and survival without distant metastasis. They noted that they were unable to analyze overall survival since the required number of events had not been reached.
Patients had been randomly assigned to receive 12 months of oral dabrafenib (at a dose of 150 mg twice daily) plus trametinib (2 mg once daily) or two matched placebos. Patients were followed for 60 months (5 years) for dabrafenib plus trametinib and 58 months for placebo.
At 5 years, the median relapse-free survival was not reached for patients who received the combination therapy group versus 16.6 months in the placebo group (hazard ratio for relapse or death, 0.51).
The percentage of patients who were alive without distant metastasis at 5 years was 65% in the dabrafenib plus trametinib group and 54% in the placebo arm (HR for distant metastasis or death, 0.55).
The hazard ratio for relapse-free survival favored dabrafenib plus trametinib across all patient subgroups that were evaluated in the study, and survival without distant metastasis showed a similar benefit for the combination regardless of disease stage.
Subsequent therapy was needed in 40% of patients who received dabrafenib plus trametinib and by 54% of those in the placebo group, with the most common treatments being immunotherapy in the combination-therapy group [26%] and small molecule–targeted therapy in the placebo group (35%).
A viable option
Dr. Dummer noted that, when this clinical trial was designed, all patients had to undergo aggressive surgery that involved lymph node dissection. “Nowadays, based on the lack of improvement on progression-free survival and overall survival, the surgical procedures are less aggressive and today we do not recommend aggressive lymph node dissection in patients that qualify for adjuvant therapy. In patients that do not have the BRAF mutation, there is the possibility of giving immunotherapy.”
He added that there is an urgent need for biomarkers that can identify early progression during adjuvant therapy. “Potentially, these patients would profit from immunotherapy alone or from combination using targeted therapy and immunotherapy,” Dr. Dummer said.
The study was funded by GlaxoSmithKline and Novartis. Dr. Dummer has declared multiple relationships with industry.
A version of this article originally appeared on Medscape.com.
Adjuvant therapy for patients with high-risk resected melanomas is now a standard of care, but the durability of the benefit gained from this treatment is still unclear.
New data show that the benefit is maintained over the longer term.
At 5 years, just over half of patients (52%) with advanced melanoma who had received a year of adjuvant therapy with two targeted agents were still alive and remained relapse free, compared with 36% of patients who received placebo.
The combination of the investigators concluded.
These data come from the COMBI-AD phase 3 trial and were published online in the New England Journal of Medicine.
“The treatment duration of this adjuvant therapy was 12 months; however, we do not know whether this is the optimal treatment duration,” said lead author Reinhard Dummer, MD, vice chairman, department of dermatology, University of Zürich Hospital. “Early biomarker results suggest that, in a subgroup, longer treatment durations might be necessary. In other patients, a shorter treatment could be sufficient.”
Richard Carvajal, MD, director of the Melanoma Service at New York–Presbyterian Hospital and Columbia University Medical Center, also in New York, said the new data “address prior concerns that any benefit achieved with targeted therapy in the adjuvant setting may be limited in duration.”
“Indeed, with active therapy, over 50% of patients are alive without relapse and 65% of patients are alive without the development of distant metastasis,” he said. “Although overall survival data remain immature, numerical improvement in survival is also reported.”
In an interview, Dr. Carvajal said that the plateaus observed with relapse and distant metastasis-free survival suggest that true disease cures are being achieved with treatment. “Based upon these results, the discussion of adjuvant therapeutic options should include a 12-month course of adjuvant dabrafenib and trametinib, as well as the option of adjuvant anti-PD-1 [programmed death–1] therapy.”
As for how the MEK-BRAF inhibitor combination compares with immunotherapy in this setting, he pointed out that, since there has been no head-to-head comparison of adjuvant targeted therapy and adjuvant nivolumab (Opdivo) or pembrolizumab (Keytruda), it is not possible to conclusively state that one regimen is more effective than another.
“For patients with resected BRAF-mutant melanoma at high risk of disease recurrence, we now have data demonstrating the clinical benefit for a course of adjuvant dabrafenib and trametinib, adjuvant nivolumab and adjuvant pembrolizumab,” said Dr. Carvajal.
“Although the efficacy of adjuvant ipilimumab [Yervoy] as well as adjuvant interferon have also been previously demonstrated, these agents are now appropriate for consideration in extremely rare clinical circumstances given the clinical efficacy and improved toxicity profile of single agent anti-PD-1 therapy.”
“The selection of the most appropriate adjuvant therapy should take into account the preferences of individual patients in terms of toxicity profile and drug administration considerations,” he added.
Study details
The COMBI-AD was a randomized, double-blind, placebo-controlled, phase 3 study conducted in 870 patients with high-risk, stage III, BRAF-V600E/K–mutant melanoma who were treatment naive. Participating patients had undergone surgical resection and had been disease free for ≤12 weeks.
Interim results from this study, reported in 2017, showed 1 year of oral adjuvant therapy with dabrafenib and trametinib provided a 53% lower risk for 3-year recurrence, compared with placebo.
Now, the investigators reported on the 5-year results for relapse-free survival and survival without distant metastasis. They noted that they were unable to analyze overall survival since the required number of events had not been reached.
Patients had been randomly assigned to receive 12 months of oral dabrafenib (at a dose of 150 mg twice daily) plus trametinib (2 mg once daily) or two matched placebos. Patients were followed for 60 months (5 years) for dabrafenib plus trametinib and 58 months for placebo.
At 5 years, the median relapse-free survival was not reached for patients who received the combination therapy group versus 16.6 months in the placebo group (hazard ratio for relapse or death, 0.51).
The percentage of patients who were alive without distant metastasis at 5 years was 65% in the dabrafenib plus trametinib group and 54% in the placebo arm (HR for distant metastasis or death, 0.55).
The hazard ratio for relapse-free survival favored dabrafenib plus trametinib across all patient subgroups that were evaluated in the study, and survival without distant metastasis showed a similar benefit for the combination regardless of disease stage.
Subsequent therapy was needed in 40% of patients who received dabrafenib plus trametinib and by 54% of those in the placebo group, with the most common treatments being immunotherapy in the combination-therapy group [26%] and small molecule–targeted therapy in the placebo group (35%).
A viable option
Dr. Dummer noted that, when this clinical trial was designed, all patients had to undergo aggressive surgery that involved lymph node dissection. “Nowadays, based on the lack of improvement on progression-free survival and overall survival, the surgical procedures are less aggressive and today we do not recommend aggressive lymph node dissection in patients that qualify for adjuvant therapy. In patients that do not have the BRAF mutation, there is the possibility of giving immunotherapy.”
He added that there is an urgent need for biomarkers that can identify early progression during adjuvant therapy. “Potentially, these patients would profit from immunotherapy alone or from combination using targeted therapy and immunotherapy,” Dr. Dummer said.
The study was funded by GlaxoSmithKline and Novartis. Dr. Dummer has declared multiple relationships with industry.
A version of this article originally appeared on Medscape.com.
Fatal pediatric melanomas diverse in presentation
results of a retrospective multicenter study showed.
“The most striking thing that we learned from this study is that pediatric melanoma can present in so many different ways, and it’s distinct from the adult population in that we see more presentations associated with congenital nevi, or spitz melanoma, which is a special class of pigmented lesions that looks a little different under the microscope,” Elena B. Hawryluk, MD, PhD, of the department of dermatology at Massachusetts General Hospital (MGH) and Harvard University, Boston, said in an interview. Dr. Hawryluk is lead author of the study, which was published online ahead of print in the Journal of the American Academy of Dermatology.
Dr. Hawryluk and colleagues at MGH and 11 other centers conducted a retrospective review of all cases of fatal pediatric melanoma among patients younger than 20 years diagnosed from late 1994 through early 2017.
They identified a total of 38 fatal cases over more than 2 decades. The cases were distinguished primarily by their heterogeneous clinical presentation and by the diversity of the patients, their precursor lesions, and the tumor histopathology, she said in an interview.
“We were surprised to find that patients with each of these presentations could end up with a fatal course, it wasn’t just all the adolescents, or all the patients with giant congenital nevi; it really presented quite diversely.”
Rare malignancy
Melanoma is far less common in the pediatric population than in adults, with an annual incidence of 18 per 1 million among adolescents aged 15-18 years, and 1 per 1 million in children under 10 years, the authors noted.
“Melanoma in children and adolescents often has distinct clinical presentations such as association with a congenital melanocytic nevus (CMN), spitzoid melanoma, or amelanotic melanoma, which are more rarely observed in adult melanoma patients. Unique pediatric-specific clinical detection criteria have been proposed to highlight these differences, such as a tendency to present amelanotically,” they wrote.
Factors associated with worse prognosis, such as higher Breslow thickness and mitotic index, are more frequently present at the time of diagnosis in children compared with adults, particularly those diagnosed before age 11 years.
“It is unclear if this difference is secondary to diagnostic delays due to low clinical suspicion, atypical clinical presentations, or more rapid tumor growth rate, as many childhood melanomas are of nodular or spitzoid subtypes,” Dr. Hawryluk and her coauthors wrote.
Study details
The investigators sought to characterize the clinical and histopathologic features of fatal pediatric melanomas.
They found that 21 of the 38 patients (57%) were of White heritage, 7 (19%) were of Hispanic or Latino background, 1 (3%) was of Asian lineage, and 1 each were of Black African American or Black Hispanic background. The remaining children were classified as “other” or did not have their ethnic backgrounds recorded.
The “striking prevalence” of Hispanic patients observed in the study is consistent with surveillance reports of an increasing incidence of melanoma among children of Hispanic background, they noted.
The mean age at diagnosis was 12.7 years, and the mean age at death was 15.6 years.
Of the 16 cases with known identifiable disease subtypes, 8 (50%) were nodular, 5 (31%) were superficial spreading, and 3 (19%) were spitzoid melanomas. Of the 38 fatal melanomas, 10 were thought to have originated from congenital melanocytic nevi.
Outlook improving
Recent therapeutic breakthroughs such as targeted agents and immunotherapy with checkpoint inhibitors augur well for children diagnosed with melanoma, Dr. Hawryluk said.
“Fortunately, it’s not superaggressive in children at high frequency, so we generally use adult algorithms to inform treatment decisions,” she said. “It’s just important to note that melanomas that arise in congenital nevi tend to have different driver mutations than those that arise in older patients who may have lots of sun exposure.”
“Nowadays, we’re lucky to have a lot of extra tests and workups so that, if a patient does have metastatic or advance disease, they can have a better genetic profile that would guide our choice of medications,” she added.
The study was supported by a Pediatric Dermatology Research Alliance Study Support grant and Society for Pediatric Dermatology, Pediatric Dermatology Research Alliance Pilot award. Dr. Hawryluk is supported by the Dermatology Foundation and the Harvard Medical School Eleanor and Miles Shore Fellowship award. The authors reported no conflicts of interest.
SOURCE: Hawryluk EB et al. J Am Acad Dermatol. 2020 Jul 1. doi: 10.1016/j.jaad.2020.06.1010.
results of a retrospective multicenter study showed.
“The most striking thing that we learned from this study is that pediatric melanoma can present in so many different ways, and it’s distinct from the adult population in that we see more presentations associated with congenital nevi, or spitz melanoma, which is a special class of pigmented lesions that looks a little different under the microscope,” Elena B. Hawryluk, MD, PhD, of the department of dermatology at Massachusetts General Hospital (MGH) and Harvard University, Boston, said in an interview. Dr. Hawryluk is lead author of the study, which was published online ahead of print in the Journal of the American Academy of Dermatology.
Dr. Hawryluk and colleagues at MGH and 11 other centers conducted a retrospective review of all cases of fatal pediatric melanoma among patients younger than 20 years diagnosed from late 1994 through early 2017.
They identified a total of 38 fatal cases over more than 2 decades. The cases were distinguished primarily by their heterogeneous clinical presentation and by the diversity of the patients, their precursor lesions, and the tumor histopathology, she said in an interview.
“We were surprised to find that patients with each of these presentations could end up with a fatal course, it wasn’t just all the adolescents, or all the patients with giant congenital nevi; it really presented quite diversely.”
Rare malignancy
Melanoma is far less common in the pediatric population than in adults, with an annual incidence of 18 per 1 million among adolescents aged 15-18 years, and 1 per 1 million in children under 10 years, the authors noted.
“Melanoma in children and adolescents often has distinct clinical presentations such as association with a congenital melanocytic nevus (CMN), spitzoid melanoma, or amelanotic melanoma, which are more rarely observed in adult melanoma patients. Unique pediatric-specific clinical detection criteria have been proposed to highlight these differences, such as a tendency to present amelanotically,” they wrote.
Factors associated with worse prognosis, such as higher Breslow thickness and mitotic index, are more frequently present at the time of diagnosis in children compared with adults, particularly those diagnosed before age 11 years.
“It is unclear if this difference is secondary to diagnostic delays due to low clinical suspicion, atypical clinical presentations, or more rapid tumor growth rate, as many childhood melanomas are of nodular or spitzoid subtypes,” Dr. Hawryluk and her coauthors wrote.
Study details
The investigators sought to characterize the clinical and histopathologic features of fatal pediatric melanomas.
They found that 21 of the 38 patients (57%) were of White heritage, 7 (19%) were of Hispanic or Latino background, 1 (3%) was of Asian lineage, and 1 each were of Black African American or Black Hispanic background. The remaining children were classified as “other” or did not have their ethnic backgrounds recorded.
The “striking prevalence” of Hispanic patients observed in the study is consistent with surveillance reports of an increasing incidence of melanoma among children of Hispanic background, they noted.
The mean age at diagnosis was 12.7 years, and the mean age at death was 15.6 years.
Of the 16 cases with known identifiable disease subtypes, 8 (50%) were nodular, 5 (31%) were superficial spreading, and 3 (19%) were spitzoid melanomas. Of the 38 fatal melanomas, 10 were thought to have originated from congenital melanocytic nevi.
Outlook improving
Recent therapeutic breakthroughs such as targeted agents and immunotherapy with checkpoint inhibitors augur well for children diagnosed with melanoma, Dr. Hawryluk said.
“Fortunately, it’s not superaggressive in children at high frequency, so we generally use adult algorithms to inform treatment decisions,” she said. “It’s just important to note that melanomas that arise in congenital nevi tend to have different driver mutations than those that arise in older patients who may have lots of sun exposure.”
“Nowadays, we’re lucky to have a lot of extra tests and workups so that, if a patient does have metastatic or advance disease, they can have a better genetic profile that would guide our choice of medications,” she added.
The study was supported by a Pediatric Dermatology Research Alliance Study Support grant and Society for Pediatric Dermatology, Pediatric Dermatology Research Alliance Pilot award. Dr. Hawryluk is supported by the Dermatology Foundation and the Harvard Medical School Eleanor and Miles Shore Fellowship award. The authors reported no conflicts of interest.
SOURCE: Hawryluk EB et al. J Am Acad Dermatol. 2020 Jul 1. doi: 10.1016/j.jaad.2020.06.1010.
results of a retrospective multicenter study showed.
“The most striking thing that we learned from this study is that pediatric melanoma can present in so many different ways, and it’s distinct from the adult population in that we see more presentations associated with congenital nevi, or spitz melanoma, which is a special class of pigmented lesions that looks a little different under the microscope,” Elena B. Hawryluk, MD, PhD, of the department of dermatology at Massachusetts General Hospital (MGH) and Harvard University, Boston, said in an interview. Dr. Hawryluk is lead author of the study, which was published online ahead of print in the Journal of the American Academy of Dermatology.
Dr. Hawryluk and colleagues at MGH and 11 other centers conducted a retrospective review of all cases of fatal pediatric melanoma among patients younger than 20 years diagnosed from late 1994 through early 2017.
They identified a total of 38 fatal cases over more than 2 decades. The cases were distinguished primarily by their heterogeneous clinical presentation and by the diversity of the patients, their precursor lesions, and the tumor histopathology, she said in an interview.
“We were surprised to find that patients with each of these presentations could end up with a fatal course, it wasn’t just all the adolescents, or all the patients with giant congenital nevi; it really presented quite diversely.”
Rare malignancy
Melanoma is far less common in the pediatric population than in adults, with an annual incidence of 18 per 1 million among adolescents aged 15-18 years, and 1 per 1 million in children under 10 years, the authors noted.
“Melanoma in children and adolescents often has distinct clinical presentations such as association with a congenital melanocytic nevus (CMN), spitzoid melanoma, or amelanotic melanoma, which are more rarely observed in adult melanoma patients. Unique pediatric-specific clinical detection criteria have been proposed to highlight these differences, such as a tendency to present amelanotically,” they wrote.
Factors associated with worse prognosis, such as higher Breslow thickness and mitotic index, are more frequently present at the time of diagnosis in children compared with adults, particularly those diagnosed before age 11 years.
“It is unclear if this difference is secondary to diagnostic delays due to low clinical suspicion, atypical clinical presentations, or more rapid tumor growth rate, as many childhood melanomas are of nodular or spitzoid subtypes,” Dr. Hawryluk and her coauthors wrote.
Study details
The investigators sought to characterize the clinical and histopathologic features of fatal pediatric melanomas.
They found that 21 of the 38 patients (57%) were of White heritage, 7 (19%) were of Hispanic or Latino background, 1 (3%) was of Asian lineage, and 1 each were of Black African American or Black Hispanic background. The remaining children were classified as “other” or did not have their ethnic backgrounds recorded.
The “striking prevalence” of Hispanic patients observed in the study is consistent with surveillance reports of an increasing incidence of melanoma among children of Hispanic background, they noted.
The mean age at diagnosis was 12.7 years, and the mean age at death was 15.6 years.
Of the 16 cases with known identifiable disease subtypes, 8 (50%) were nodular, 5 (31%) were superficial spreading, and 3 (19%) were spitzoid melanomas. Of the 38 fatal melanomas, 10 were thought to have originated from congenital melanocytic nevi.
Outlook improving
Recent therapeutic breakthroughs such as targeted agents and immunotherapy with checkpoint inhibitors augur well for children diagnosed with melanoma, Dr. Hawryluk said.
“Fortunately, it’s not superaggressive in children at high frequency, so we generally use adult algorithms to inform treatment decisions,” she said. “It’s just important to note that melanomas that arise in congenital nevi tend to have different driver mutations than those that arise in older patients who may have lots of sun exposure.”
“Nowadays, we’re lucky to have a lot of extra tests and workups so that, if a patient does have metastatic or advance disease, they can have a better genetic profile that would guide our choice of medications,” she added.
The study was supported by a Pediatric Dermatology Research Alliance Study Support grant and Society for Pediatric Dermatology, Pediatric Dermatology Research Alliance Pilot award. Dr. Hawryluk is supported by the Dermatology Foundation and the Harvard Medical School Eleanor and Miles Shore Fellowship award. The authors reported no conflicts of interest.
SOURCE: Hawryluk EB et al. J Am Acad Dermatol. 2020 Jul 1. doi: 10.1016/j.jaad.2020.06.1010.
FROM JAAD
Melanoma experts say ‘no’ to routine gene profile testing
“The currently published evidence is insufficient to establish that routine use of GEP testing provides additional clinical value for melanoma staging and prognostication beyond available clinicopathologic variables,” they argued.
Patients must be protected “from potentially inaccurate testing that may provide a false sense of security or perceived increased risk” that could lead to the wrong decisions, they said in a consensus statement from the United States’ national Melanoma Prevention Working Group. The statement was published on July 29 in JAMA Dermatology.
The GEP test for melanoma that is available in the United States – DecisionDx-Melanoma from Castle Biosciences – checks the expression levels of 31 genes reported to be associated with melanoma metastasis and recurrence. It uses quantitative reverse transcriptase and polymerase chain reaction on RNA from formalin-fixed, paraffin-embedded biopsy specimens.
The test stratifies patients as being at low, intermediate, or high risk. It is marketed as a guide to whether to perform sentinel lymph node biopsies (SLNB) on patients age 55 years or older with tumors less than 2 mm deep and to decide what levels of follow-up, imaging, and adjuvant treatment are appropriate for tumors at least 0.3 mm deep.
Medicare reimburses at $7,193 per test for SLNB-eligible patients.
However, this test is not endorsed by the American Academy of Dermatology or National Comprehensive Cancer Network outside of studies because the evidence of benefit is not strong enough, the consensus authors noted.
Even so, use of the test is growing, with up to 10% of cutaneous melanomas now being tested in the United States.
Company welcomes “further discussions”
“To date, thousands of clinicians – over 4,200 US clinicians in the last 12 months – have utilized our GEP test for cutaneous melanoma in their patients after reviewing our clinical data and determining that our test provides clinically actionable information that complements current melanoma staging,” said Castle Biosciences Vice President of Research and Development Bob Cook, PhD, when asked for comment.
Citing company-funded studies, he said that “the strength of the existing evidence in support of these claims has undergone rigorous evaluation to obtain Medicare reimbursement.”
“We believe that the application of the test to help guide [the] decision to pursue SLNB has the potential to realize significant cost savings by reducing unnecessary SLNB procedures, particularly in the T1 population.”
Asked for a reaction to the consensus statement, Dr. Cook said in an interview: “We recently launched two prospective studies with multiple centers nationwide that will involve thousands of patients and provide additional data relating our tests to patient outcomes. ... We welcome further discussions to promote collaborative efforts with centers that are part of the [Melanoma Prevention Working Group] to improve patient outcomes.”
Cart before the horse
Medicare, although it reimburses the test, has its doubts. Due to the “low strength of evidence,” the Centers for Medicare & Medicaid Services said in their local coverage determination that continued reimbursement depends on demonstration of 95% or greater distant-metastasis–free survival and melanoma-specific survival at 3 years “in patients directed to no SLNB by the test compared to standard of care, and ... evidence of higher SLNB positivity in patients selected for this procedure by the test compared to standard of care.”
The statement hints at the Achilles’ heel of GEP in melanoma – that is, the lack of evidence that test results improve outcomes. This was the main concern of the consensus statement; the cart is before the horse.
One of the consensus authors, David Polsky, MD, PhD, professor of dermatologic oncology at New York University, New York City, said that “most of the data for this test come from retrospectively collected patient groups.” The prospective studies have been generally small, with no comparator group. “While they have shown some promise in intermediate thickness melanoma, they have not yet demonstrated utility for thin, stage I melanomas.”
First, do no harm
A new meta-analysis of over 800 patients with cutaneous melanoma tested by DecisionDx-Melanoma, published in JAMA Dermatology alongside the consensus statement, shows how the tests perform.
Among patients with a recurrence, DecisionDx-Melanoma correctly classified 82% with stage II disease but only 29% with stage I disease as high risk. Among those without recurrence, the test correctly classified 90% of stage I patients but only 44% with stage II disease as low risk.
Similar results were seen with the melanoma GEP test available in Europe, MelaGenix (NeraCare GmbH). This test was developed from a panel that was narrowed to seven protective genes and one high-risk gene using a training cohort of 125 cutaneous melanomas.
“The prognostic ability of GEP tests ... appeared to be poor at correctly identifying recurrence in patients with stage I disease, suggesting limited potential for clinical utility in these patients,” commented the meta-analysis authors, led by Michael Marchetti, MD, an assistant professor of dermatology at Weill Cornell Medical College in New York City.
“Unknown are the harms associated with a false-positive result, which were 10-fold more frequent than true-positive results in patients with stage I disease,” they pointed out.
“Further research is needed to define the incremental improvement in risk predictions provided by the test beyond ... all other known clinicopathologic factors,” which include patient sex, age, tumor location and thickness, ulceration, mitotic rate, lymphovascular invasion, microsatellites, and other factors proven to be linked to outcomes, they said.
Studies so far suggesting benefit have incorporated a few of those factors, but not all of them. For now, “it is not clear which patients should be tested or how to act on the results,” Dr. Marchetti and colleagues concluded.
Breast cancer standard of proof
Larger, prospective studies are needed to address whether GEP testing can replace SLNB to predict relapse “and [can identify] patients who could be spared surveillance imaging and/or benefit from adjuvant therapy,” wrote the consensus authors. Follow-up also needs to be long enough to detect delayed recurrence of thin melanomas, they added.
With more research, there is reason to hope that gene expression profiling will help in melanoma; it’s already standard of care in breast cancer, they pointed out.
On the hope front, one cohort study evaluated whether DecisionDx-Melanoma could identify patients at low risk for positive lymph nodes in T1/T2 disease who were eligible for biopsy. Only 1.6% of subjects who were aged 65 years or older and identified by the test as low risk had a positive node.
“This is a promising direction of investigation ... in a narrow, defined population,” noted authors led by Carrie Kovarik, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia, in an opinion piece last spring.
But still, until there’s “clear evidence that [DecisionDx-Melanoma] results affect patient outcomes, we should not use it to influence care decisions in patients with thin” melanomas. Dermatology “should expect the same standards” of proof as breast cancer, they wrote.
What to do right now?
Despite the marketing, “think twice before ordering GEP tests for” T1a melanomas is the message in an editorial that accompanies the consensus statement. The 5- and 10-year melanoma-specific survival rates are 99% and 98%, respectively. GEP tests are unlikely to change these estimates significantly. In fact, the new meta-analysis indicates “that there may be an approximately 12% misassignment rate in this population,” wrote editorialists Warren Chan, of Baylor College of Medicine, Houston and Hensin Tsao, MD, PhD, director of the melanoma genetics program at Massachusetts General Hospital, Boston.
“Even if you use GEP testing and discover a low-risk class assignment for a 2 mm thick melanoma, avoid the urge to bypass the sentinel lymph node discussion. ... Nodal sampling, for good reasons, remains part of all major guidelines and determines eligibility for adjuvant treatments. ... Many of us engaged in genomics research believe that accurate [melanoma] GEP will be developed in time, but better tools and greater tenacity are needed,” they wrote.
There was no industry funding for the consensus statement and meta-analysis. Authors on the consensus statement reported numerous ties to pharmaceutical and other companies, as listed in the paper.
A version of this article originally appeared on Medscape.com.
“The currently published evidence is insufficient to establish that routine use of GEP testing provides additional clinical value for melanoma staging and prognostication beyond available clinicopathologic variables,” they argued.
Patients must be protected “from potentially inaccurate testing that may provide a false sense of security or perceived increased risk” that could lead to the wrong decisions, they said in a consensus statement from the United States’ national Melanoma Prevention Working Group. The statement was published on July 29 in JAMA Dermatology.
The GEP test for melanoma that is available in the United States – DecisionDx-Melanoma from Castle Biosciences – checks the expression levels of 31 genes reported to be associated with melanoma metastasis and recurrence. It uses quantitative reverse transcriptase and polymerase chain reaction on RNA from formalin-fixed, paraffin-embedded biopsy specimens.
The test stratifies patients as being at low, intermediate, or high risk. It is marketed as a guide to whether to perform sentinel lymph node biopsies (SLNB) on patients age 55 years or older with tumors less than 2 mm deep and to decide what levels of follow-up, imaging, and adjuvant treatment are appropriate for tumors at least 0.3 mm deep.
Medicare reimburses at $7,193 per test for SLNB-eligible patients.
However, this test is not endorsed by the American Academy of Dermatology or National Comprehensive Cancer Network outside of studies because the evidence of benefit is not strong enough, the consensus authors noted.
Even so, use of the test is growing, with up to 10% of cutaneous melanomas now being tested in the United States.
Company welcomes “further discussions”
“To date, thousands of clinicians – over 4,200 US clinicians in the last 12 months – have utilized our GEP test for cutaneous melanoma in their patients after reviewing our clinical data and determining that our test provides clinically actionable information that complements current melanoma staging,” said Castle Biosciences Vice President of Research and Development Bob Cook, PhD, when asked for comment.
Citing company-funded studies, he said that “the strength of the existing evidence in support of these claims has undergone rigorous evaluation to obtain Medicare reimbursement.”
“We believe that the application of the test to help guide [the] decision to pursue SLNB has the potential to realize significant cost savings by reducing unnecessary SLNB procedures, particularly in the T1 population.”
Asked for a reaction to the consensus statement, Dr. Cook said in an interview: “We recently launched two prospective studies with multiple centers nationwide that will involve thousands of patients and provide additional data relating our tests to patient outcomes. ... We welcome further discussions to promote collaborative efforts with centers that are part of the [Melanoma Prevention Working Group] to improve patient outcomes.”
Cart before the horse
Medicare, although it reimburses the test, has its doubts. Due to the “low strength of evidence,” the Centers for Medicare & Medicaid Services said in their local coverage determination that continued reimbursement depends on demonstration of 95% or greater distant-metastasis–free survival and melanoma-specific survival at 3 years “in patients directed to no SLNB by the test compared to standard of care, and ... evidence of higher SLNB positivity in patients selected for this procedure by the test compared to standard of care.”
The statement hints at the Achilles’ heel of GEP in melanoma – that is, the lack of evidence that test results improve outcomes. This was the main concern of the consensus statement; the cart is before the horse.
One of the consensus authors, David Polsky, MD, PhD, professor of dermatologic oncology at New York University, New York City, said that “most of the data for this test come from retrospectively collected patient groups.” The prospective studies have been generally small, with no comparator group. “While they have shown some promise in intermediate thickness melanoma, they have not yet demonstrated utility for thin, stage I melanomas.”
First, do no harm
A new meta-analysis of over 800 patients with cutaneous melanoma tested by DecisionDx-Melanoma, published in JAMA Dermatology alongside the consensus statement, shows how the tests perform.
Among patients with a recurrence, DecisionDx-Melanoma correctly classified 82% with stage II disease but only 29% with stage I disease as high risk. Among those without recurrence, the test correctly classified 90% of stage I patients but only 44% with stage II disease as low risk.
Similar results were seen with the melanoma GEP test available in Europe, MelaGenix (NeraCare GmbH). This test was developed from a panel that was narrowed to seven protective genes and one high-risk gene using a training cohort of 125 cutaneous melanomas.
“The prognostic ability of GEP tests ... appeared to be poor at correctly identifying recurrence in patients with stage I disease, suggesting limited potential for clinical utility in these patients,” commented the meta-analysis authors, led by Michael Marchetti, MD, an assistant professor of dermatology at Weill Cornell Medical College in New York City.
“Unknown are the harms associated with a false-positive result, which were 10-fold more frequent than true-positive results in patients with stage I disease,” they pointed out.
“Further research is needed to define the incremental improvement in risk predictions provided by the test beyond ... all other known clinicopathologic factors,” which include patient sex, age, tumor location and thickness, ulceration, mitotic rate, lymphovascular invasion, microsatellites, and other factors proven to be linked to outcomes, they said.
Studies so far suggesting benefit have incorporated a few of those factors, but not all of them. For now, “it is not clear which patients should be tested or how to act on the results,” Dr. Marchetti and colleagues concluded.
Breast cancer standard of proof
Larger, prospective studies are needed to address whether GEP testing can replace SLNB to predict relapse “and [can identify] patients who could be spared surveillance imaging and/or benefit from adjuvant therapy,” wrote the consensus authors. Follow-up also needs to be long enough to detect delayed recurrence of thin melanomas, they added.
With more research, there is reason to hope that gene expression profiling will help in melanoma; it’s already standard of care in breast cancer, they pointed out.
On the hope front, one cohort study evaluated whether DecisionDx-Melanoma could identify patients at low risk for positive lymph nodes in T1/T2 disease who were eligible for biopsy. Only 1.6% of subjects who were aged 65 years or older and identified by the test as low risk had a positive node.
“This is a promising direction of investigation ... in a narrow, defined population,” noted authors led by Carrie Kovarik, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia, in an opinion piece last spring.
But still, until there’s “clear evidence that [DecisionDx-Melanoma] results affect patient outcomes, we should not use it to influence care decisions in patients with thin” melanomas. Dermatology “should expect the same standards” of proof as breast cancer, they wrote.
What to do right now?
Despite the marketing, “think twice before ordering GEP tests for” T1a melanomas is the message in an editorial that accompanies the consensus statement. The 5- and 10-year melanoma-specific survival rates are 99% and 98%, respectively. GEP tests are unlikely to change these estimates significantly. In fact, the new meta-analysis indicates “that there may be an approximately 12% misassignment rate in this population,” wrote editorialists Warren Chan, of Baylor College of Medicine, Houston and Hensin Tsao, MD, PhD, director of the melanoma genetics program at Massachusetts General Hospital, Boston.
“Even if you use GEP testing and discover a low-risk class assignment for a 2 mm thick melanoma, avoid the urge to bypass the sentinel lymph node discussion. ... Nodal sampling, for good reasons, remains part of all major guidelines and determines eligibility for adjuvant treatments. ... Many of us engaged in genomics research believe that accurate [melanoma] GEP will be developed in time, but better tools and greater tenacity are needed,” they wrote.
There was no industry funding for the consensus statement and meta-analysis. Authors on the consensus statement reported numerous ties to pharmaceutical and other companies, as listed in the paper.
A version of this article originally appeared on Medscape.com.
“The currently published evidence is insufficient to establish that routine use of GEP testing provides additional clinical value for melanoma staging and prognostication beyond available clinicopathologic variables,” they argued.
Patients must be protected “from potentially inaccurate testing that may provide a false sense of security or perceived increased risk” that could lead to the wrong decisions, they said in a consensus statement from the United States’ national Melanoma Prevention Working Group. The statement was published on July 29 in JAMA Dermatology.
The GEP test for melanoma that is available in the United States – DecisionDx-Melanoma from Castle Biosciences – checks the expression levels of 31 genes reported to be associated with melanoma metastasis and recurrence. It uses quantitative reverse transcriptase and polymerase chain reaction on RNA from formalin-fixed, paraffin-embedded biopsy specimens.
The test stratifies patients as being at low, intermediate, or high risk. It is marketed as a guide to whether to perform sentinel lymph node biopsies (SLNB) on patients age 55 years or older with tumors less than 2 mm deep and to decide what levels of follow-up, imaging, and adjuvant treatment are appropriate for tumors at least 0.3 mm deep.
Medicare reimburses at $7,193 per test for SLNB-eligible patients.
However, this test is not endorsed by the American Academy of Dermatology or National Comprehensive Cancer Network outside of studies because the evidence of benefit is not strong enough, the consensus authors noted.
Even so, use of the test is growing, with up to 10% of cutaneous melanomas now being tested in the United States.
Company welcomes “further discussions”
“To date, thousands of clinicians – over 4,200 US clinicians in the last 12 months – have utilized our GEP test for cutaneous melanoma in their patients after reviewing our clinical data and determining that our test provides clinically actionable information that complements current melanoma staging,” said Castle Biosciences Vice President of Research and Development Bob Cook, PhD, when asked for comment.
Citing company-funded studies, he said that “the strength of the existing evidence in support of these claims has undergone rigorous evaluation to obtain Medicare reimbursement.”
“We believe that the application of the test to help guide [the] decision to pursue SLNB has the potential to realize significant cost savings by reducing unnecessary SLNB procedures, particularly in the T1 population.”
Asked for a reaction to the consensus statement, Dr. Cook said in an interview: “We recently launched two prospective studies with multiple centers nationwide that will involve thousands of patients and provide additional data relating our tests to patient outcomes. ... We welcome further discussions to promote collaborative efforts with centers that are part of the [Melanoma Prevention Working Group] to improve patient outcomes.”
Cart before the horse
Medicare, although it reimburses the test, has its doubts. Due to the “low strength of evidence,” the Centers for Medicare & Medicaid Services said in their local coverage determination that continued reimbursement depends on demonstration of 95% or greater distant-metastasis–free survival and melanoma-specific survival at 3 years “in patients directed to no SLNB by the test compared to standard of care, and ... evidence of higher SLNB positivity in patients selected for this procedure by the test compared to standard of care.”
The statement hints at the Achilles’ heel of GEP in melanoma – that is, the lack of evidence that test results improve outcomes. This was the main concern of the consensus statement; the cart is before the horse.
One of the consensus authors, David Polsky, MD, PhD, professor of dermatologic oncology at New York University, New York City, said that “most of the data for this test come from retrospectively collected patient groups.” The prospective studies have been generally small, with no comparator group. “While they have shown some promise in intermediate thickness melanoma, they have not yet demonstrated utility for thin, stage I melanomas.”
First, do no harm
A new meta-analysis of over 800 patients with cutaneous melanoma tested by DecisionDx-Melanoma, published in JAMA Dermatology alongside the consensus statement, shows how the tests perform.
Among patients with a recurrence, DecisionDx-Melanoma correctly classified 82% with stage II disease but only 29% with stage I disease as high risk. Among those without recurrence, the test correctly classified 90% of stage I patients but only 44% with stage II disease as low risk.
Similar results were seen with the melanoma GEP test available in Europe, MelaGenix (NeraCare GmbH). This test was developed from a panel that was narrowed to seven protective genes and one high-risk gene using a training cohort of 125 cutaneous melanomas.
“The prognostic ability of GEP tests ... appeared to be poor at correctly identifying recurrence in patients with stage I disease, suggesting limited potential for clinical utility in these patients,” commented the meta-analysis authors, led by Michael Marchetti, MD, an assistant professor of dermatology at Weill Cornell Medical College in New York City.
“Unknown are the harms associated with a false-positive result, which were 10-fold more frequent than true-positive results in patients with stage I disease,” they pointed out.
“Further research is needed to define the incremental improvement in risk predictions provided by the test beyond ... all other known clinicopathologic factors,” which include patient sex, age, tumor location and thickness, ulceration, mitotic rate, lymphovascular invasion, microsatellites, and other factors proven to be linked to outcomes, they said.
Studies so far suggesting benefit have incorporated a few of those factors, but not all of them. For now, “it is not clear which patients should be tested or how to act on the results,” Dr. Marchetti and colleagues concluded.
Breast cancer standard of proof
Larger, prospective studies are needed to address whether GEP testing can replace SLNB to predict relapse “and [can identify] patients who could be spared surveillance imaging and/or benefit from adjuvant therapy,” wrote the consensus authors. Follow-up also needs to be long enough to detect delayed recurrence of thin melanomas, they added.
With more research, there is reason to hope that gene expression profiling will help in melanoma; it’s already standard of care in breast cancer, they pointed out.
On the hope front, one cohort study evaluated whether DecisionDx-Melanoma could identify patients at low risk for positive lymph nodes in T1/T2 disease who were eligible for biopsy. Only 1.6% of subjects who were aged 65 years or older and identified by the test as low risk had a positive node.
“This is a promising direction of investigation ... in a narrow, defined population,” noted authors led by Carrie Kovarik, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia, in an opinion piece last spring.
But still, until there’s “clear evidence that [DecisionDx-Melanoma] results affect patient outcomes, we should not use it to influence care decisions in patients with thin” melanomas. Dermatology “should expect the same standards” of proof as breast cancer, they wrote.
What to do right now?
Despite the marketing, “think twice before ordering GEP tests for” T1a melanomas is the message in an editorial that accompanies the consensus statement. The 5- and 10-year melanoma-specific survival rates are 99% and 98%, respectively. GEP tests are unlikely to change these estimates significantly. In fact, the new meta-analysis indicates “that there may be an approximately 12% misassignment rate in this population,” wrote editorialists Warren Chan, of Baylor College of Medicine, Houston and Hensin Tsao, MD, PhD, director of the melanoma genetics program at Massachusetts General Hospital, Boston.
“Even if you use GEP testing and discover a low-risk class assignment for a 2 mm thick melanoma, avoid the urge to bypass the sentinel lymph node discussion. ... Nodal sampling, for good reasons, remains part of all major guidelines and determines eligibility for adjuvant treatments. ... Many of us engaged in genomics research believe that accurate [melanoma] GEP will be developed in time, but better tools and greater tenacity are needed,” they wrote.
There was no industry funding for the consensus statement and meta-analysis. Authors on the consensus statement reported numerous ties to pharmaceutical and other companies, as listed in the paper.
A version of this article originally appeared on Medscape.com.
VTE, sepsis risk increased among COVID-19 patients with cancer
, according to data from a registry study.
Researchers analyzed data on 5,556 patients with COVID-19 who had an inpatient or emergency encounter at Mount Sinai Health System (MSHS) in New York between March 1 and May 27, 2020. Patients were included in an anonymous MSHS COVID-19 registry.
There were 421 patients who had cancer: 96 with a hematologic malignancy and 325 with solid tumors.
After adjustment for age, gender, and number of comorbidities, the odds ratios for acute VTE and sepsis for patients with cancer (versus those without cancer) were 1.77 and 1.34, respectively. The adjusted odds ratio for mortality in cancer patients was 1.02.
The results remained “relatively consistent” after stratification by solid and nonsolid cancer types, with no significant difference in outcomes between those two groups, and results remained consistent in a propensity-matched model, according to Naomi Alpert, a biostatistician at Icahn School of Medicine at Mount Sinai, New York.
Ms. Alpert reported these findings at the AACR virtual meeting: COVID-19 and Cancer.
She noted that the cancer patients were older than the noncancer patients (mean age, 69.2 years vs. 63.8 years), and cancer patients were more likely to have two or more comorbid conditions (48.2% vs. 30.4%). Cancer patients also had significantly lower hemoglobin levels and red blood cell, platelet, and white blood cell counts (P < .01 for all).
“Low white blood cell count may be one of the reasons for higher risk of sepsis in cancer patients, as it may lead to a higher risk of infection,” Ms. Alpert said. “However, it’s not clear what role cancer therapies play in the risks of COVID-19 morbidity and mortality, so there is still quite a bit to learn.”
In fact, the findings are limited by a lack of information about cancer treatment, as the registry was not designed for that purpose, she noted.
Another study limitation is the short follow-up of a month or less in most patients, due, in part, to the novelty of COVID-19, but also to the lack of information on patients after they left the hospital.
“However, we had a very large sample size, with more than 400 cancer patients included, and, to our knowledge, this is the largest analysis of its kind to be done so far,” Ms. Alpert said. “In the future, it’s going to be very important to assess the effect of cancer therapies on COVID-19 complications and to see if prior therapies had any effect on outcomes.”
Longer follow-up would also be helpful for assessing the chronic effects of COVID-19 on cancer patients over time, she said. “It would be important to see whether some of these elevated risks of venous thromboembolism and sepsis are associated with longer-term mortality risks than what we were able to measure here,” she added.
Asked about the discrepancy between mortality in this study and those of larger registries, such as the COVID-19 and Cancer Consortium (CCC19) and TERAVOLT, Ms. Alpert noted that the current study included only patients who required hospitalization or emergency care.
“Our mortality rate was actually a bit higher than what was reported in some of the other studies,” she said. “We had about a 30% mortality rate in the cancer patients and about 25% for the noncancer patients, so ... we’re sort of looking at a subset of patients who we know are the sickest of the sick, which may explain some of the higher mortality that we’re seeing.”
Ms. Alpert reported having no disclosures.
SOURCE: Alpert N et al. AACR COVID-19 and Cancer, Abstract S12-02.
, according to data from a registry study.
Researchers analyzed data on 5,556 patients with COVID-19 who had an inpatient or emergency encounter at Mount Sinai Health System (MSHS) in New York between March 1 and May 27, 2020. Patients were included in an anonymous MSHS COVID-19 registry.
There were 421 patients who had cancer: 96 with a hematologic malignancy and 325 with solid tumors.
After adjustment for age, gender, and number of comorbidities, the odds ratios for acute VTE and sepsis for patients with cancer (versus those without cancer) were 1.77 and 1.34, respectively. The adjusted odds ratio for mortality in cancer patients was 1.02.
The results remained “relatively consistent” after stratification by solid and nonsolid cancer types, with no significant difference in outcomes between those two groups, and results remained consistent in a propensity-matched model, according to Naomi Alpert, a biostatistician at Icahn School of Medicine at Mount Sinai, New York.
Ms. Alpert reported these findings at the AACR virtual meeting: COVID-19 and Cancer.
She noted that the cancer patients were older than the noncancer patients (mean age, 69.2 years vs. 63.8 years), and cancer patients were more likely to have two or more comorbid conditions (48.2% vs. 30.4%). Cancer patients also had significantly lower hemoglobin levels and red blood cell, platelet, and white blood cell counts (P < .01 for all).
“Low white blood cell count may be one of the reasons for higher risk of sepsis in cancer patients, as it may lead to a higher risk of infection,” Ms. Alpert said. “However, it’s not clear what role cancer therapies play in the risks of COVID-19 morbidity and mortality, so there is still quite a bit to learn.”
In fact, the findings are limited by a lack of information about cancer treatment, as the registry was not designed for that purpose, she noted.
Another study limitation is the short follow-up of a month or less in most patients, due, in part, to the novelty of COVID-19, but also to the lack of information on patients after they left the hospital.
“However, we had a very large sample size, with more than 400 cancer patients included, and, to our knowledge, this is the largest analysis of its kind to be done so far,” Ms. Alpert said. “In the future, it’s going to be very important to assess the effect of cancer therapies on COVID-19 complications and to see if prior therapies had any effect on outcomes.”
Longer follow-up would also be helpful for assessing the chronic effects of COVID-19 on cancer patients over time, she said. “It would be important to see whether some of these elevated risks of venous thromboembolism and sepsis are associated with longer-term mortality risks than what we were able to measure here,” she added.
Asked about the discrepancy between mortality in this study and those of larger registries, such as the COVID-19 and Cancer Consortium (CCC19) and TERAVOLT, Ms. Alpert noted that the current study included only patients who required hospitalization or emergency care.
“Our mortality rate was actually a bit higher than what was reported in some of the other studies,” she said. “We had about a 30% mortality rate in the cancer patients and about 25% for the noncancer patients, so ... we’re sort of looking at a subset of patients who we know are the sickest of the sick, which may explain some of the higher mortality that we’re seeing.”
Ms. Alpert reported having no disclosures.
SOURCE: Alpert N et al. AACR COVID-19 and Cancer, Abstract S12-02.
, according to data from a registry study.
Researchers analyzed data on 5,556 patients with COVID-19 who had an inpatient or emergency encounter at Mount Sinai Health System (MSHS) in New York between March 1 and May 27, 2020. Patients were included in an anonymous MSHS COVID-19 registry.
There were 421 patients who had cancer: 96 with a hematologic malignancy and 325 with solid tumors.
After adjustment for age, gender, and number of comorbidities, the odds ratios for acute VTE and sepsis for patients with cancer (versus those without cancer) were 1.77 and 1.34, respectively. The adjusted odds ratio for mortality in cancer patients was 1.02.
The results remained “relatively consistent” after stratification by solid and nonsolid cancer types, with no significant difference in outcomes between those two groups, and results remained consistent in a propensity-matched model, according to Naomi Alpert, a biostatistician at Icahn School of Medicine at Mount Sinai, New York.
Ms. Alpert reported these findings at the AACR virtual meeting: COVID-19 and Cancer.
She noted that the cancer patients were older than the noncancer patients (mean age, 69.2 years vs. 63.8 years), and cancer patients were more likely to have two or more comorbid conditions (48.2% vs. 30.4%). Cancer patients also had significantly lower hemoglobin levels and red blood cell, platelet, and white blood cell counts (P < .01 for all).
“Low white blood cell count may be one of the reasons for higher risk of sepsis in cancer patients, as it may lead to a higher risk of infection,” Ms. Alpert said. “However, it’s not clear what role cancer therapies play in the risks of COVID-19 morbidity and mortality, so there is still quite a bit to learn.”
In fact, the findings are limited by a lack of information about cancer treatment, as the registry was not designed for that purpose, she noted.
Another study limitation is the short follow-up of a month or less in most patients, due, in part, to the novelty of COVID-19, but also to the lack of information on patients after they left the hospital.
“However, we had a very large sample size, with more than 400 cancer patients included, and, to our knowledge, this is the largest analysis of its kind to be done so far,” Ms. Alpert said. “In the future, it’s going to be very important to assess the effect of cancer therapies on COVID-19 complications and to see if prior therapies had any effect on outcomes.”
Longer follow-up would also be helpful for assessing the chronic effects of COVID-19 on cancer patients over time, she said. “It would be important to see whether some of these elevated risks of venous thromboembolism and sepsis are associated with longer-term mortality risks than what we were able to measure here,” she added.
Asked about the discrepancy between mortality in this study and those of larger registries, such as the COVID-19 and Cancer Consortium (CCC19) and TERAVOLT, Ms. Alpert noted that the current study included only patients who required hospitalization or emergency care.
“Our mortality rate was actually a bit higher than what was reported in some of the other studies,” she said. “We had about a 30% mortality rate in the cancer patients and about 25% for the noncancer patients, so ... we’re sort of looking at a subset of patients who we know are the sickest of the sick, which may explain some of the higher mortality that we’re seeing.”
Ms. Alpert reported having no disclosures.
SOURCE: Alpert N et al. AACR COVID-19 and Cancer, Abstract S12-02.
FROM AACR: COVID-19 AND CANCER
Immunotherapy should not be withheld because of sex, age, or PS
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
Aspirin may accelerate cancer progression in older adults
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
FROM JOURNAL OF THE NATIONAL CANCER INSTITUTE
Age, smoking among leading cancer risk factors for SLE patients
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
FROM ARTHRITIS CARE & RESEARCH
COVID-19 impact: Less chemo, immune checkpoint inhibitors, and steroids
While neoadjuvant treatment recommendations were not strongly affected by the pandemic, about half of oncologists reported increased hesitancy over recommending frontline chemotherapy for metastatic disease, and a vast majority said they would recommend second- or third-line chemotherapy less often in the metastatic setting.
Most oncologists said they did not perform routine COVID-19 testing via reverse transcriptase–polymerase chain reaction (RT-PCR) before treating cancer patients. In fact, only 3% said they performed COVID-19 RT-PCR testing routinely.
Yüksel Ürün, MD, of Ankara (Turkey) University, and colleagues reported these findings in JCO Global Oncology.
The goal of the survey was to “understand readiness measures taken by oncologists to protect patients and health care workers from the novel coronavirus (COVID-19) and how their clinical decision-making was influenced by the pandemic,” the authors wrote.
The online survey was conducted among 343 oncologists from 28 countries. Responses were collected anonymously, a majority (71%) from university or academic centers, with 95% received between April 1 and April 29, 2020.
Use of telemedicine was common (80%) among respondents, as was use of surgical masks (90%) and personal protective equipment in general.
Only 33% of respondents described using N95 masks. However, the proportion of oncologists who had access to N95 masks while caring for patients known to have COVID-19, especially while doing invasive procedures such as intubation, bronchoscopy, and any airway-related manipulations, was not captured by the survey.
COVID testing and cancer treatment
Most respondents (58%) said they did not perform routine COVID-19 RT-PCR testing prior to administering systemic cancer treatment, with 39% stating they performed RT-PCR tests in selected patients, and 3% saying they performed such testing in all patients.
The survey indicated that hormonal treatments, tyrosine kinase inhibitors, and bone-modifying agents were considered relatively safe, but cytotoxic chemotherapy and immune therapies were not.
Nearly all oncologists said the pandemic would cause them to make no change to their recommendations regarding hormone therapy, and nearly 80% said they would make no changes regarding tyrosine kinase inhibitors or bone-modifying agents.
However, more than 90% of respondents said they would recommend cytotoxic chemotherapy less often, about 70% said they would recommend corticosteroids less often, and around 50% said they would recommend anti–programmed death-1/PD-ligand 1 or anti–cytotoxic T-lymphocyte–associated protein 4 antibodies less often.
The pandemic made most respondents more reluctant to recommend second- or third-line chemotherapy in the metastatic setting. About 80% and 70% of respondents, respectively, would recommend second- or third-line chemotherapy less often.
However, first-line chemotherapy for metastatic disease, as well as adjuvant and neoadjuvant therapy, were less affected. About 30% of respondents said they would recommend neoadjuvant therapy less often, and 50%-55% would recommend adjuvant therapy or frontline chemotherapy for metastatic disease less often.
Most respondents (78%) said they would use granulocyte colony–stimulating factor (G-CSF) more frequently during the pandemic.
The factors most likely to affect oncologists’ treatment decisions were patient age (81%) and concomitant disease (92%). Additionally, 80% of respondents’ treatment decisions were influenced by Eastern Cooperative Oncology Group performance status of 2 or higher, or the presence of chronic obstructive pulmonary disease.
Interpretation and implications
“These results highlight that, even in the early phases of COVID-19 – during which there was considerable uncertainty – basic core principles were guideposts for oncologists,” observed Aly-Khan Lalani, MD, of Juravinski Cancer Centre and McMaster University, Hamilton, Ont., who was not involved in this study.
“For example, [oncologists were] prioritizing strategies for treatments with the largest expected impact and carefully tailoring treatment according to patient comorbidities and performance status,” Dr. Lalani said.
Another oncologist who was not involved in the study expressed concern over reductions in adjuvant therapy supported by half of oncologists surveyed.
“Although benefits may be marginal in some cases, these are curative settings and especially warrant careful individual-level risk/benefit discussions,” said Kartik Sehgal, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston.
His concern extended as well to the small proportion (3%) of oncologists testing for COVID-19 in all patients. “Systematic testing is the need of the hour,” Dr. Sehgal said.
In their discussion of the findings, Dr. Ürün and colleagues noted a lack of consensus on monoclonal antibody and immunotherapy safety among surveyed oncologists. The steroids needed to manage severe immune-mediated toxicity with immune checkpoint inhibitors has led to some prescribing reluctance during the pandemic.
Immunosuppressive properties of immune checkpoint inhibitors also raise concern that they can increase COVID-19 severity. Studies are few, and findings to date are inconsistent with respect to the effect of immune checkpoint inhibitors on COVID-19 clinical course. However, a recently presented study suggested that immune checkpoint inhibitors do not increase the risk of death among cancer patients with COVID-19 (AACR: COVID-19 and Cancer, Abstract S02-01).
Dr. Ürün and colleagues noted that greater COVID-19 severity has been shown in patients with performance status greater than 1, hematologic malignancies, lung cancer, stage IV metastatic disease, chemotherapy within the prior 3 months, cancer treatment in the last 14 days, and the presence of chronic obstructive pulmonary disease. Nonmetastatic cancer has not been shown to affect COVID-19 severity, however.
Dr. Ürün and colleagues also underscored the need for research evidence to balance potential reductions in neutropenic complications with G-CSF (and therefore, reduced hospitalizations) with a theoretical risk of G-CSF–mediated pulmonary injury through its stimulation of an excessive immune response.
Finally, the authors urged oncologists to evaluate each proposed therapy’s risk/benefit ratio on an individual patient basis, and the team tasked the oncology community with gathering comprehensive, rigorous data.
There was no funding source declared for this study. Dr. Ürün and colleagues disclosed various relationships with many pharmaceutical companies, which included receiving research funding. Dr. Sehgal and Dr. Lalani reported no relevant conflicts.
SOURCE: Ürün Y et al. JCO Glob Oncol. 2020 Aug;6:1248-57.
While neoadjuvant treatment recommendations were not strongly affected by the pandemic, about half of oncologists reported increased hesitancy over recommending frontline chemotherapy for metastatic disease, and a vast majority said they would recommend second- or third-line chemotherapy less often in the metastatic setting.
Most oncologists said they did not perform routine COVID-19 testing via reverse transcriptase–polymerase chain reaction (RT-PCR) before treating cancer patients. In fact, only 3% said they performed COVID-19 RT-PCR testing routinely.
Yüksel Ürün, MD, of Ankara (Turkey) University, and colleagues reported these findings in JCO Global Oncology.
The goal of the survey was to “understand readiness measures taken by oncologists to protect patients and health care workers from the novel coronavirus (COVID-19) and how their clinical decision-making was influenced by the pandemic,” the authors wrote.
The online survey was conducted among 343 oncologists from 28 countries. Responses were collected anonymously, a majority (71%) from university or academic centers, with 95% received between April 1 and April 29, 2020.
Use of telemedicine was common (80%) among respondents, as was use of surgical masks (90%) and personal protective equipment in general.
Only 33% of respondents described using N95 masks. However, the proportion of oncologists who had access to N95 masks while caring for patients known to have COVID-19, especially while doing invasive procedures such as intubation, bronchoscopy, and any airway-related manipulations, was not captured by the survey.
COVID testing and cancer treatment
Most respondents (58%) said they did not perform routine COVID-19 RT-PCR testing prior to administering systemic cancer treatment, with 39% stating they performed RT-PCR tests in selected patients, and 3% saying they performed such testing in all patients.
The survey indicated that hormonal treatments, tyrosine kinase inhibitors, and bone-modifying agents were considered relatively safe, but cytotoxic chemotherapy and immune therapies were not.
Nearly all oncologists said the pandemic would cause them to make no change to their recommendations regarding hormone therapy, and nearly 80% said they would make no changes regarding tyrosine kinase inhibitors or bone-modifying agents.
However, more than 90% of respondents said they would recommend cytotoxic chemotherapy less often, about 70% said they would recommend corticosteroids less often, and around 50% said they would recommend anti–programmed death-1/PD-ligand 1 or anti–cytotoxic T-lymphocyte–associated protein 4 antibodies less often.
The pandemic made most respondents more reluctant to recommend second- or third-line chemotherapy in the metastatic setting. About 80% and 70% of respondents, respectively, would recommend second- or third-line chemotherapy less often.
However, first-line chemotherapy for metastatic disease, as well as adjuvant and neoadjuvant therapy, were less affected. About 30% of respondents said they would recommend neoadjuvant therapy less often, and 50%-55% would recommend adjuvant therapy or frontline chemotherapy for metastatic disease less often.
Most respondents (78%) said they would use granulocyte colony–stimulating factor (G-CSF) more frequently during the pandemic.
The factors most likely to affect oncologists’ treatment decisions were patient age (81%) and concomitant disease (92%). Additionally, 80% of respondents’ treatment decisions were influenced by Eastern Cooperative Oncology Group performance status of 2 or higher, or the presence of chronic obstructive pulmonary disease.
Interpretation and implications
“These results highlight that, even in the early phases of COVID-19 – during which there was considerable uncertainty – basic core principles were guideposts for oncologists,” observed Aly-Khan Lalani, MD, of Juravinski Cancer Centre and McMaster University, Hamilton, Ont., who was not involved in this study.
“For example, [oncologists were] prioritizing strategies for treatments with the largest expected impact and carefully tailoring treatment according to patient comorbidities and performance status,” Dr. Lalani said.
Another oncologist who was not involved in the study expressed concern over reductions in adjuvant therapy supported by half of oncologists surveyed.
“Although benefits may be marginal in some cases, these are curative settings and especially warrant careful individual-level risk/benefit discussions,” said Kartik Sehgal, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston.
His concern extended as well to the small proportion (3%) of oncologists testing for COVID-19 in all patients. “Systematic testing is the need of the hour,” Dr. Sehgal said.
In their discussion of the findings, Dr. Ürün and colleagues noted a lack of consensus on monoclonal antibody and immunotherapy safety among surveyed oncologists. The steroids needed to manage severe immune-mediated toxicity with immune checkpoint inhibitors has led to some prescribing reluctance during the pandemic.
Immunosuppressive properties of immune checkpoint inhibitors also raise concern that they can increase COVID-19 severity. Studies are few, and findings to date are inconsistent with respect to the effect of immune checkpoint inhibitors on COVID-19 clinical course. However, a recently presented study suggested that immune checkpoint inhibitors do not increase the risk of death among cancer patients with COVID-19 (AACR: COVID-19 and Cancer, Abstract S02-01).
Dr. Ürün and colleagues noted that greater COVID-19 severity has been shown in patients with performance status greater than 1, hematologic malignancies, lung cancer, stage IV metastatic disease, chemotherapy within the prior 3 months, cancer treatment in the last 14 days, and the presence of chronic obstructive pulmonary disease. Nonmetastatic cancer has not been shown to affect COVID-19 severity, however.
Dr. Ürün and colleagues also underscored the need for research evidence to balance potential reductions in neutropenic complications with G-CSF (and therefore, reduced hospitalizations) with a theoretical risk of G-CSF–mediated pulmonary injury through its stimulation of an excessive immune response.
Finally, the authors urged oncologists to evaluate each proposed therapy’s risk/benefit ratio on an individual patient basis, and the team tasked the oncology community with gathering comprehensive, rigorous data.
There was no funding source declared for this study. Dr. Ürün and colleagues disclosed various relationships with many pharmaceutical companies, which included receiving research funding. Dr. Sehgal and Dr. Lalani reported no relevant conflicts.
SOURCE: Ürün Y et al. JCO Glob Oncol. 2020 Aug;6:1248-57.
While neoadjuvant treatment recommendations were not strongly affected by the pandemic, about half of oncologists reported increased hesitancy over recommending frontline chemotherapy for metastatic disease, and a vast majority said they would recommend second- or third-line chemotherapy less often in the metastatic setting.
Most oncologists said they did not perform routine COVID-19 testing via reverse transcriptase–polymerase chain reaction (RT-PCR) before treating cancer patients. In fact, only 3% said they performed COVID-19 RT-PCR testing routinely.
Yüksel Ürün, MD, of Ankara (Turkey) University, and colleagues reported these findings in JCO Global Oncology.
The goal of the survey was to “understand readiness measures taken by oncologists to protect patients and health care workers from the novel coronavirus (COVID-19) and how their clinical decision-making was influenced by the pandemic,” the authors wrote.
The online survey was conducted among 343 oncologists from 28 countries. Responses were collected anonymously, a majority (71%) from university or academic centers, with 95% received between April 1 and April 29, 2020.
Use of telemedicine was common (80%) among respondents, as was use of surgical masks (90%) and personal protective equipment in general.
Only 33% of respondents described using N95 masks. However, the proportion of oncologists who had access to N95 masks while caring for patients known to have COVID-19, especially while doing invasive procedures such as intubation, bronchoscopy, and any airway-related manipulations, was not captured by the survey.
COVID testing and cancer treatment
Most respondents (58%) said they did not perform routine COVID-19 RT-PCR testing prior to administering systemic cancer treatment, with 39% stating they performed RT-PCR tests in selected patients, and 3% saying they performed such testing in all patients.
The survey indicated that hormonal treatments, tyrosine kinase inhibitors, and bone-modifying agents were considered relatively safe, but cytotoxic chemotherapy and immune therapies were not.
Nearly all oncologists said the pandemic would cause them to make no change to their recommendations regarding hormone therapy, and nearly 80% said they would make no changes regarding tyrosine kinase inhibitors or bone-modifying agents.
However, more than 90% of respondents said they would recommend cytotoxic chemotherapy less often, about 70% said they would recommend corticosteroids less often, and around 50% said they would recommend anti–programmed death-1/PD-ligand 1 or anti–cytotoxic T-lymphocyte–associated protein 4 antibodies less often.
The pandemic made most respondents more reluctant to recommend second- or third-line chemotherapy in the metastatic setting. About 80% and 70% of respondents, respectively, would recommend second- or third-line chemotherapy less often.
However, first-line chemotherapy for metastatic disease, as well as adjuvant and neoadjuvant therapy, were less affected. About 30% of respondents said they would recommend neoadjuvant therapy less often, and 50%-55% would recommend adjuvant therapy or frontline chemotherapy for metastatic disease less often.
Most respondents (78%) said they would use granulocyte colony–stimulating factor (G-CSF) more frequently during the pandemic.
The factors most likely to affect oncologists’ treatment decisions were patient age (81%) and concomitant disease (92%). Additionally, 80% of respondents’ treatment decisions were influenced by Eastern Cooperative Oncology Group performance status of 2 or higher, or the presence of chronic obstructive pulmonary disease.
Interpretation and implications
“These results highlight that, even in the early phases of COVID-19 – during which there was considerable uncertainty – basic core principles were guideposts for oncologists,” observed Aly-Khan Lalani, MD, of Juravinski Cancer Centre and McMaster University, Hamilton, Ont., who was not involved in this study.
“For example, [oncologists were] prioritizing strategies for treatments with the largest expected impact and carefully tailoring treatment according to patient comorbidities and performance status,” Dr. Lalani said.
Another oncologist who was not involved in the study expressed concern over reductions in adjuvant therapy supported by half of oncologists surveyed.
“Although benefits may be marginal in some cases, these are curative settings and especially warrant careful individual-level risk/benefit discussions,” said Kartik Sehgal, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston.
His concern extended as well to the small proportion (3%) of oncologists testing for COVID-19 in all patients. “Systematic testing is the need of the hour,” Dr. Sehgal said.
In their discussion of the findings, Dr. Ürün and colleagues noted a lack of consensus on monoclonal antibody and immunotherapy safety among surveyed oncologists. The steroids needed to manage severe immune-mediated toxicity with immune checkpoint inhibitors has led to some prescribing reluctance during the pandemic.
Immunosuppressive properties of immune checkpoint inhibitors also raise concern that they can increase COVID-19 severity. Studies are few, and findings to date are inconsistent with respect to the effect of immune checkpoint inhibitors on COVID-19 clinical course. However, a recently presented study suggested that immune checkpoint inhibitors do not increase the risk of death among cancer patients with COVID-19 (AACR: COVID-19 and Cancer, Abstract S02-01).
Dr. Ürün and colleagues noted that greater COVID-19 severity has been shown in patients with performance status greater than 1, hematologic malignancies, lung cancer, stage IV metastatic disease, chemotherapy within the prior 3 months, cancer treatment in the last 14 days, and the presence of chronic obstructive pulmonary disease. Nonmetastatic cancer has not been shown to affect COVID-19 severity, however.
Dr. Ürün and colleagues also underscored the need for research evidence to balance potential reductions in neutropenic complications with G-CSF (and therefore, reduced hospitalizations) with a theoretical risk of G-CSF–mediated pulmonary injury through its stimulation of an excessive immune response.
Finally, the authors urged oncologists to evaluate each proposed therapy’s risk/benefit ratio on an individual patient basis, and the team tasked the oncology community with gathering comprehensive, rigorous data.
There was no funding source declared for this study. Dr. Ürün and colleagues disclosed various relationships with many pharmaceutical companies, which included receiving research funding. Dr. Sehgal and Dr. Lalani reported no relevant conflicts.
SOURCE: Ürün Y et al. JCO Glob Oncol. 2020 Aug;6:1248-57.
FROM JCO GLOBAL ONCOLOGY
Scalp Wound Closures in Mohs Micrographic Surgery: A Survey of Staples vs Sutures
Limited data exist comparing staples and sutures for scalp closures during Mohs micrographic surgery (MMS). As a result, the closure method for these scalp wounds is based on surgeon preference without established consensus. The purpose of this study was to survey practicing Mohs surgeons on their scalp wound closure preferences as well as the clinical and economic variables that impact their decisions. Understanding practice habits can guide future trial design, with a goal of creating established criterion for MMS scalp wound closures.
Methods
An anonymous survey was distributed from April 2019 to June 2019 to fellowship-trained Mohs surgeons using an electronic mailing list from the American College of Mohs Surgery (ACMS). The 10-question survey was approved by the University of Kansas institutional review board and the executive committee of the ACMS. Surgeons were asked about their preferred method for scalp wound closure as well as clinical and economic variables that impacted those preferences. Respondents indicated their frequency of using deep sutures, epidermal sutures, and wound undermining on a sliding scale of 0% to 100%. Comparisons were made between practice habits, preferences, and surgeon demographics using t tests. Statistical significance was determined as P<.05.
Results
Sixty-eight ACMS fellowship-trained Mohs surgeons completed the survey. The average age of respondents was 45 years; 69.1% (n=47) of respondents were male, and 76.5% (n=52) practiced in a private setting (Table 1). Regardless of epidermal closure type, deep suture placement was used in an average (standard deviation [SD]) of 88.8% (19.5%) of cases overall, which did not statistically differ between years of Mohs experience or practice setting (Table 2). Wound undermining was performed in an average (SD) of 83.0% (24.3%) of cases overall and was more prevalent in private vs academic settings (87.6% [17.8%] vs 65.7% [35.0%]; P<.01). Epidermal sutures were used in an average (SD) of 27.1% (33.5%) of scalp wound cases overall. Surgeons with less experience (≤5 years) used them more frequently (average [SD], 42.7% [36.2%] of cases) than surgeons with more experience (≥16 years; average [SD], 18.8% [32.6%] of cases; P=.037). There was no significant difference between epidermal suture placement rates and practice setting (average [SD], 18.1% [28.1%] of cases for academic providers vs 30.0% [34.8%] of cases with private providers; P=.210).

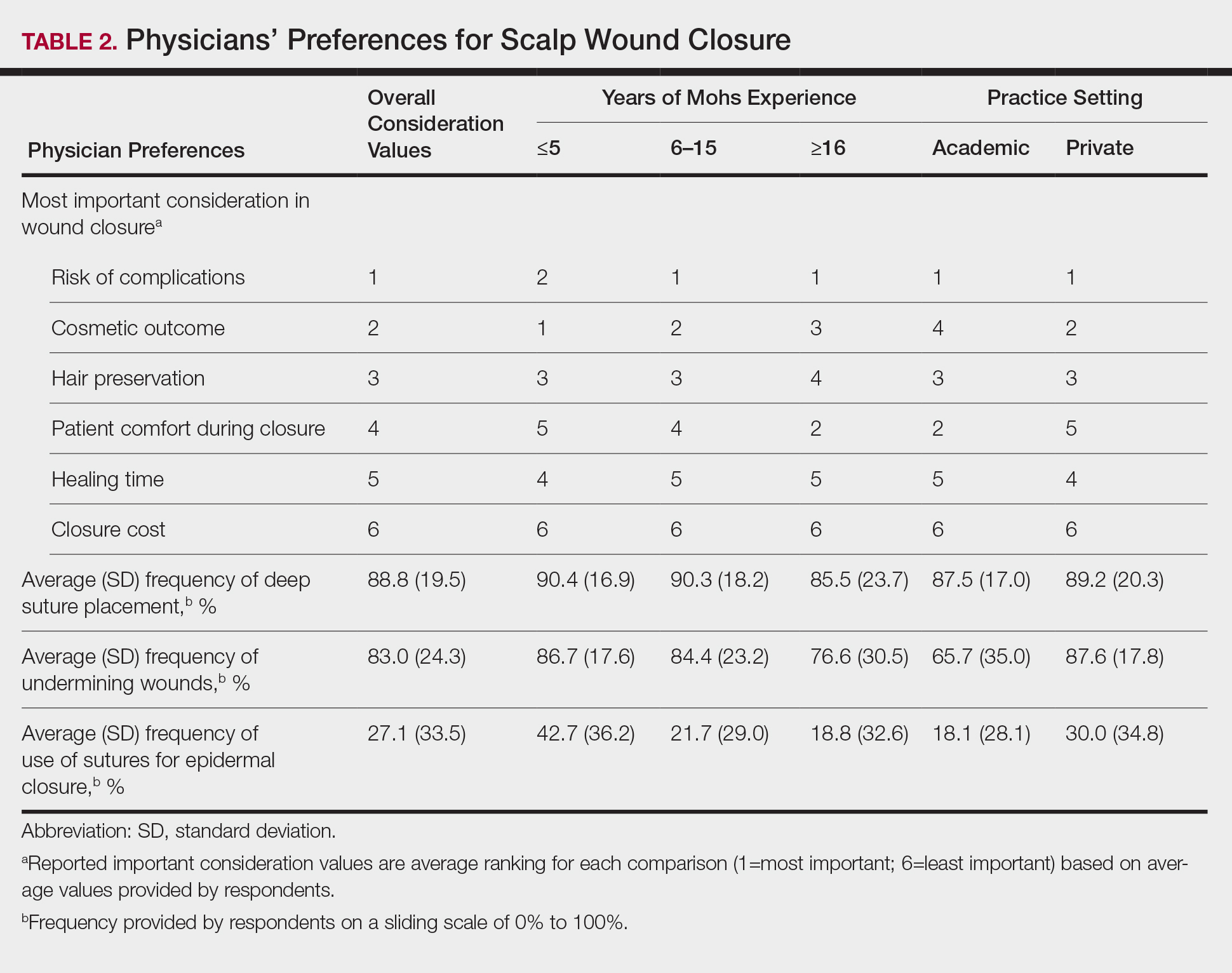
Clinical and economic factors that were most important during wound closure were ranked (beginning with most important) as the following: risk of complications, cosmetic outcome, hair preservation, patient comfort during closure, healing time, and closure cost. In all demographic cases, risk of complications was ranked 1 or 2 (1=most important; 6=least important) overall; cost was the least important factor overall (Table 2).
Surgeons perceived staples to be superior for speed of closure and for closing wounds in high-tension areas, whereas sutures were perceived as superior when considering cost of closure and ease of removal (Table 3). Successful healing rate, healing time, hair preservation, overall cosmetic outcome, and lower risk of complications were viewed as equivalent when comparing staples and sutures.
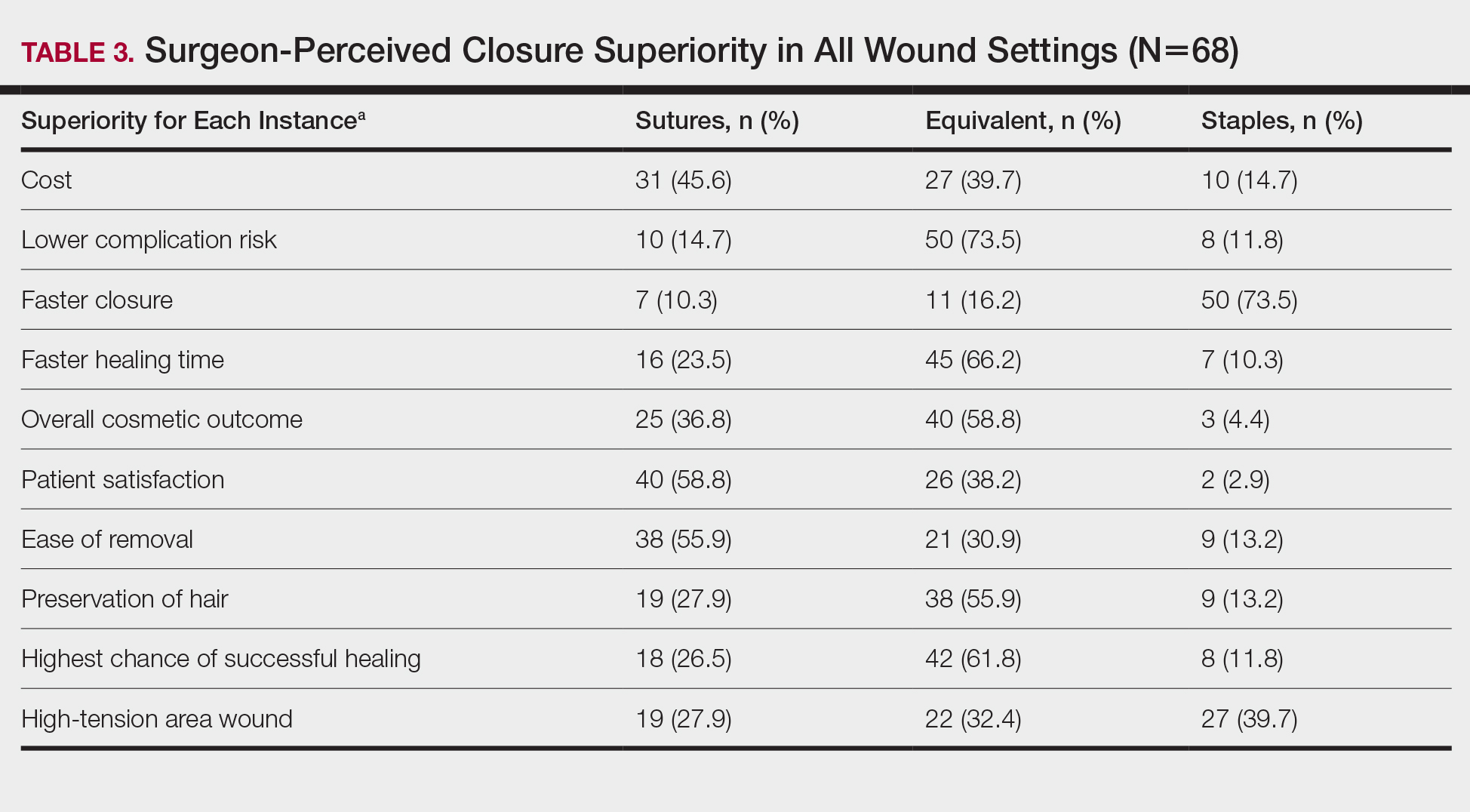
In cases in which surgeons did not use staples for closure, the most important factors for opting to not use them were patient discomfort (52.9% [n=36]), cost (25.0% [n=17]), and worse overall cosmetic outcome (23.5% [n=16])(Table 4). The most frequent locations outside of scalp wounds that physicians considered the use of staples for closure were the back (19.1% [n=13]), thigh (10.3% [n=7]), and shoulder (8.8% [n=6]).
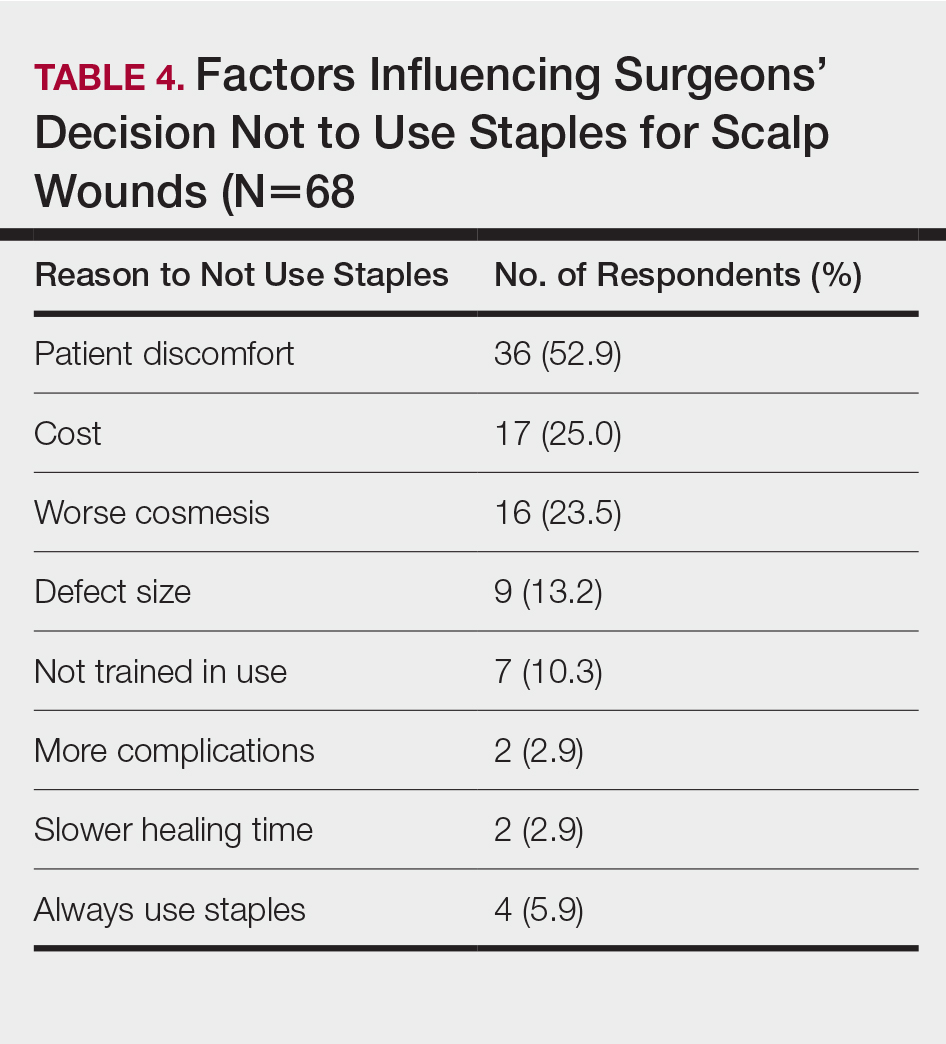
Comment
Epidermal closure with sutures was reportedly used in an average of only 27.1% of scalp wound cases, with clinical factors such as cosmetic outcome, risk of complications, and closure time seen as either equivalent or inferior to staples. Our data suggest that surgeon closure perceptions generally are in agreement with established head and neck literature within different medical specialties that favor staple closures, particularly in high-tension areas.1 Interestingly, the most common reasons given for not using staples included patient discomfort, cost, and worse cosmetic outcomes, which are unsubstantiated with head and neck comparative studies.2-4
Although cost was the least important variable for determining closure type in our surveyed cohort, it is likely that the overall cost of closure is frequently underestimated. A higher material cost is noted with staples; however, the largest determinant of overall cost remains the surgeon’s time, which is reduced by factors of 10 or more when closing with staples.2,3 This difference—coupled with the unchanged cosmetic outcome and complication rates—makes staples more advantageous for high-tension scalp wounds.4 Moreover, the stapling technique is more reproducible than suturing, which requires more surgical skill and experience.
Limitations of this study include a lack of directly comparable data for staple and suture scalp wound closures. In addition, the small cohort of respondents in this preliminary study can serve to guide future studies.
Conclusion
Scalp wounds during MMS were most frequently closed using staples vs sutures, with the perception that these methods are equivalent in complication risk, cosmetic outcome, and overall patient satisfaction. These results agree with comparative literature for head and neck surgery and assist with establishing an epidemiologic baseline for future studies comparing their use during MMS.
- Ritchie AJ, Rocke LG. Staples versus sutures in the closure of scalp wounds: a prospective, double-blind, randomized trial. Injury. 1989;20:217-218.
- Batra J, Bekal RK, Byadgi S, et al. Comparison of skin staples and standard sutures for closing incisions after head and neck cancer surgery: a double-blind, randomized and prospective study. J Maxillofac Oral Surg. 2016;15:243-250.
- Kanegaye JT, Vance CW, Chan L, et al. Comparison of skin stapling devices and standard sutures for pediatric scalp lacerations: a randomized study of cost and time benefits. J Pediatr. 1997;130:808-813.
- Khan ANGA, Dayan PS, Miller S, et al. Cosmetic outcome of scalp wound closure with staples in the pediatric emergency department: a prospective, randomized trial. Pediatr Emerg Care. 2002;18:171-173.
Limited data exist comparing staples and sutures for scalp closures during Mohs micrographic surgery (MMS). As a result, the closure method for these scalp wounds is based on surgeon preference without established consensus. The purpose of this study was to survey practicing Mohs surgeons on their scalp wound closure preferences as well as the clinical and economic variables that impact their decisions. Understanding practice habits can guide future trial design, with a goal of creating established criterion for MMS scalp wound closures.
Methods
An anonymous survey was distributed from April 2019 to June 2019 to fellowship-trained Mohs surgeons using an electronic mailing list from the American College of Mohs Surgery (ACMS). The 10-question survey was approved by the University of Kansas institutional review board and the executive committee of the ACMS. Surgeons were asked about their preferred method for scalp wound closure as well as clinical and economic variables that impacted those preferences. Respondents indicated their frequency of using deep sutures, epidermal sutures, and wound undermining on a sliding scale of 0% to 100%. Comparisons were made between practice habits, preferences, and surgeon demographics using t tests. Statistical significance was determined as P<.05.
Results
Sixty-eight ACMS fellowship-trained Mohs surgeons completed the survey. The average age of respondents was 45 years; 69.1% (n=47) of respondents were male, and 76.5% (n=52) practiced in a private setting (Table 1). Regardless of epidermal closure type, deep suture placement was used in an average (standard deviation [SD]) of 88.8% (19.5%) of cases overall, which did not statistically differ between years of Mohs experience or practice setting (Table 2). Wound undermining was performed in an average (SD) of 83.0% (24.3%) of cases overall and was more prevalent in private vs academic settings (87.6% [17.8%] vs 65.7% [35.0%]; P<.01). Epidermal sutures were used in an average (SD) of 27.1% (33.5%) of scalp wound cases overall. Surgeons with less experience (≤5 years) used them more frequently (average [SD], 42.7% [36.2%] of cases) than surgeons with more experience (≥16 years; average [SD], 18.8% [32.6%] of cases; P=.037). There was no significant difference between epidermal suture placement rates and practice setting (average [SD], 18.1% [28.1%] of cases for academic providers vs 30.0% [34.8%] of cases with private providers; P=.210).


Clinical and economic factors that were most important during wound closure were ranked (beginning with most important) as the following: risk of complications, cosmetic outcome, hair preservation, patient comfort during closure, healing time, and closure cost. In all demographic cases, risk of complications was ranked 1 or 2 (1=most important; 6=least important) overall; cost was the least important factor overall (Table 2).
Surgeons perceived staples to be superior for speed of closure and for closing wounds in high-tension areas, whereas sutures were perceived as superior when considering cost of closure and ease of removal (Table 3). Successful healing rate, healing time, hair preservation, overall cosmetic outcome, and lower risk of complications were viewed as equivalent when comparing staples and sutures.

In cases in which surgeons did not use staples for closure, the most important factors for opting to not use them were patient discomfort (52.9% [n=36]), cost (25.0% [n=17]), and worse overall cosmetic outcome (23.5% [n=16])(Table 4). The most frequent locations outside of scalp wounds that physicians considered the use of staples for closure were the back (19.1% [n=13]), thigh (10.3% [n=7]), and shoulder (8.8% [n=6]).

Comment
Epidermal closure with sutures was reportedly used in an average of only 27.1% of scalp wound cases, with clinical factors such as cosmetic outcome, risk of complications, and closure time seen as either equivalent or inferior to staples. Our data suggest that surgeon closure perceptions generally are in agreement with established head and neck literature within different medical specialties that favor staple closures, particularly in high-tension areas.1 Interestingly, the most common reasons given for not using staples included patient discomfort, cost, and worse cosmetic outcomes, which are unsubstantiated with head and neck comparative studies.2-4
Although cost was the least important variable for determining closure type in our surveyed cohort, it is likely that the overall cost of closure is frequently underestimated. A higher material cost is noted with staples; however, the largest determinant of overall cost remains the surgeon’s time, which is reduced by factors of 10 or more when closing with staples.2,3 This difference—coupled with the unchanged cosmetic outcome and complication rates—makes staples more advantageous for high-tension scalp wounds.4 Moreover, the stapling technique is more reproducible than suturing, which requires more surgical skill and experience.
Limitations of this study include a lack of directly comparable data for staple and suture scalp wound closures. In addition, the small cohort of respondents in this preliminary study can serve to guide future studies.
Conclusion
Scalp wounds during MMS were most frequently closed using staples vs sutures, with the perception that these methods are equivalent in complication risk, cosmetic outcome, and overall patient satisfaction. These results agree with comparative literature for head and neck surgery and assist with establishing an epidemiologic baseline for future studies comparing their use during MMS.
Limited data exist comparing staples and sutures for scalp closures during Mohs micrographic surgery (MMS). As a result, the closure method for these scalp wounds is based on surgeon preference without established consensus. The purpose of this study was to survey practicing Mohs surgeons on their scalp wound closure preferences as well as the clinical and economic variables that impact their decisions. Understanding practice habits can guide future trial design, with a goal of creating established criterion for MMS scalp wound closures.
Methods
An anonymous survey was distributed from April 2019 to June 2019 to fellowship-trained Mohs surgeons using an electronic mailing list from the American College of Mohs Surgery (ACMS). The 10-question survey was approved by the University of Kansas institutional review board and the executive committee of the ACMS. Surgeons were asked about their preferred method for scalp wound closure as well as clinical and economic variables that impacted those preferences. Respondents indicated their frequency of using deep sutures, epidermal sutures, and wound undermining on a sliding scale of 0% to 100%. Comparisons were made between practice habits, preferences, and surgeon demographics using t tests. Statistical significance was determined as P<.05.
Results
Sixty-eight ACMS fellowship-trained Mohs surgeons completed the survey. The average age of respondents was 45 years; 69.1% (n=47) of respondents were male, and 76.5% (n=52) practiced in a private setting (Table 1). Regardless of epidermal closure type, deep suture placement was used in an average (standard deviation [SD]) of 88.8% (19.5%) of cases overall, which did not statistically differ between years of Mohs experience or practice setting (Table 2). Wound undermining was performed in an average (SD) of 83.0% (24.3%) of cases overall and was more prevalent in private vs academic settings (87.6% [17.8%] vs 65.7% [35.0%]; P<.01). Epidermal sutures were used in an average (SD) of 27.1% (33.5%) of scalp wound cases overall. Surgeons with less experience (≤5 years) used them more frequently (average [SD], 42.7% [36.2%] of cases) than surgeons with more experience (≥16 years; average [SD], 18.8% [32.6%] of cases; P=.037). There was no significant difference between epidermal suture placement rates and practice setting (average [SD], 18.1% [28.1%] of cases for academic providers vs 30.0% [34.8%] of cases with private providers; P=.210).


Clinical and economic factors that were most important during wound closure were ranked (beginning with most important) as the following: risk of complications, cosmetic outcome, hair preservation, patient comfort during closure, healing time, and closure cost. In all demographic cases, risk of complications was ranked 1 or 2 (1=most important; 6=least important) overall; cost was the least important factor overall (Table 2).
Surgeons perceived staples to be superior for speed of closure and for closing wounds in high-tension areas, whereas sutures were perceived as superior when considering cost of closure and ease of removal (Table 3). Successful healing rate, healing time, hair preservation, overall cosmetic outcome, and lower risk of complications were viewed as equivalent when comparing staples and sutures.

In cases in which surgeons did not use staples for closure, the most important factors for opting to not use them were patient discomfort (52.9% [n=36]), cost (25.0% [n=17]), and worse overall cosmetic outcome (23.5% [n=16])(Table 4). The most frequent locations outside of scalp wounds that physicians considered the use of staples for closure were the back (19.1% [n=13]), thigh (10.3% [n=7]), and shoulder (8.8% [n=6]).

Comment
Epidermal closure with sutures was reportedly used in an average of only 27.1% of scalp wound cases, with clinical factors such as cosmetic outcome, risk of complications, and closure time seen as either equivalent or inferior to staples. Our data suggest that surgeon closure perceptions generally are in agreement with established head and neck literature within different medical specialties that favor staple closures, particularly in high-tension areas.1 Interestingly, the most common reasons given for not using staples included patient discomfort, cost, and worse cosmetic outcomes, which are unsubstantiated with head and neck comparative studies.2-4
Although cost was the least important variable for determining closure type in our surveyed cohort, it is likely that the overall cost of closure is frequently underestimated. A higher material cost is noted with staples; however, the largest determinant of overall cost remains the surgeon’s time, which is reduced by factors of 10 or more when closing with staples.2,3 This difference—coupled with the unchanged cosmetic outcome and complication rates—makes staples more advantageous for high-tension scalp wounds.4 Moreover, the stapling technique is more reproducible than suturing, which requires more surgical skill and experience.
Limitations of this study include a lack of directly comparable data for staple and suture scalp wound closures. In addition, the small cohort of respondents in this preliminary study can serve to guide future studies.
Conclusion
Scalp wounds during MMS were most frequently closed using staples vs sutures, with the perception that these methods are equivalent in complication risk, cosmetic outcome, and overall patient satisfaction. These results agree with comparative literature for head and neck surgery and assist with establishing an epidemiologic baseline for future studies comparing their use during MMS.
- Ritchie AJ, Rocke LG. Staples versus sutures in the closure of scalp wounds: a prospective, double-blind, randomized trial. Injury. 1989;20:217-218.
- Batra J, Bekal RK, Byadgi S, et al. Comparison of skin staples and standard sutures for closing incisions after head and neck cancer surgery: a double-blind, randomized and prospective study. J Maxillofac Oral Surg. 2016;15:243-250.
- Kanegaye JT, Vance CW, Chan L, et al. Comparison of skin stapling devices and standard sutures for pediatric scalp lacerations: a randomized study of cost and time benefits. J Pediatr. 1997;130:808-813.
- Khan ANGA, Dayan PS, Miller S, et al. Cosmetic outcome of scalp wound closure with staples in the pediatric emergency department: a prospective, randomized trial. Pediatr Emerg Care. 2002;18:171-173.
- Ritchie AJ, Rocke LG. Staples versus sutures in the closure of scalp wounds: a prospective, double-blind, randomized trial. Injury. 1989;20:217-218.
- Batra J, Bekal RK, Byadgi S, et al. Comparison of skin staples and standard sutures for closing incisions after head and neck cancer surgery: a double-blind, randomized and prospective study. J Maxillofac Oral Surg. 2016;15:243-250.
- Kanegaye JT, Vance CW, Chan L, et al. Comparison of skin stapling devices and standard sutures for pediatric scalp lacerations: a randomized study of cost and time benefits. J Pediatr. 1997;130:808-813.
- Khan ANGA, Dayan PS, Miller S, et al. Cosmetic outcome of scalp wound closure with staples in the pediatric emergency department: a prospective, randomized trial. Pediatr Emerg Care. 2002;18:171-173.
Practice Points
- Scalp wounds present a unique challenge for closure during Mohs micrographic surgery due to the scalp's tendency to bleed, limited elasticity, and hair-bearing nature.
- Among fellowship-trained Mohs surgeons, scalp wounds were closed with staples more often than with epidermal sutures.
- Staples and sutures for scalp wounds were perceived to be equivalent in risk of complications, cosmetic outcome, and overall patient satisfaction.
- Compared to epidermal sutures, staples were perceived as advantageous in high-tension areas and for speed of closure.