User login
New cancer drugs may have saved more than 1.2 million Americans
Reductions in mortality were most notable for tumor types with relatively more approvals, including lung and breast cancer, melanoma, lymphoma, and leukemia.
A report from the American Cancer Society (ACS) estimated that, from 1991 to 2017, there were 2,902,200 total cancer deaths avoided from improvements in mortality from all potential sources.
The new findings, reported in the Journal of Medical Economics, suggest that drugs approved between 2000 and 2016 to treat the 15 most common cancer types helped to reduce mortality by 24% per 100,000 people.
“This study provides evidence that a significant share of that reduction from 2000 to 2016 was associated with the introduction of new therapies. The ACS report and other studies demonstrate that the improvements in lung cancer specifically are likely due to new treatments,” said lead study author Joanna P. MacEwan, MD, of PRECISIONheor in Los Angeles.
The findings contribute to a better understanding of whether increased spending on cancer drugs are worth the investment, according to the study authors.
“We provide evidence that the gains in survival measured in clinical trials are translating into health benefits for patients in the real world and confirm previous research that has also shown that new pharmaceutical treatments are associated with improved real-world survival outcomes for patients,” Dr. MacEwan said.
Full effect not yet observed
The researchers used a series of national data sets from sources including the Centers for Disease Control and Prevention; the U.S. Mortality Files by the National Center of Health Statistics; Survival, Epidemiology and End Results program; and United States Cancer Statistics.
The team calculated age-adjusted cancer mortality rates per year for the 15 most common tumor types and also looked at incident cases of cancer by tumor type, represented as per 100,000 people, for all ages, races, and genders.
The researchers then translated the change in cancer mortality in the U.S. from 2000 to 2016 associated with treatment stocks in each year into deaths averted per year.
Across the 16 years, mortality was down by 1,291,769 deaths. The following cancers had significant reductions in mortality: breast (n = 127,874), colorectal (n = 46,705), lung (n = 375,256), prostate (n = 476,210), gastric (n = 758), and renal (n = 739) cancers, as well as non-Hodgkin lymphoma (n = 48,836) and leukemia (n = 4,011).
Estimated mortality increased by 825 deaths in patients with thyroid cancer and 7,768 deaths for those with bladder cancer. These rises are likely due to the result of sparse drug approvals during this period – five for thyroid cancer and three for bladder cancer – Dr. MacEwan said. There were no approvals in liver or uterine cancer and few approvals in pancreatic and oral cancer.
The full effect of new drug introductions may not have been observed yet, Dr. MacEwan noted.
“There are fewer patients using the treatments for drugs approved in the later years of our study and less follow-up time to measure outcomes,” she said. “Over time, utilization of the newer therapies will likely increase and the full effect on mortality will be observed.”
Other factors at play
Multiple factors have led to the declines in mortality, said William G. Cance, MD, chief medical and scientific officer for the ACS, who was not involved in this study. “We are slowly sorting out the explanations in greater granularity.”
Dr. MacEwan said improved cancer screening may partially explain the decline in mortality in some tumor types.
“If screening in a particular tumor type improved during the study period and tumors were diagnosed earlier, then mortality for that tumor type may decline,” she said. “However, we did not find strong evidence to suggest that there were significant changes in screening during our study period. Breast cancer screening rates, for example, were stable over our study period.”
Cancer screening is not as strong an influence as it should be, Dr. Cance said.
“The lung cancer screening rate is low. In breast and colorectal cancers, we need to double down on earlier screening,” he said, noting that less than one-quarter of adults between ages 45 and 50 years are currently screened for colorectal cancer. The ACS recommends that people at average risk of colorectal cancer start regular screening at age 45.
More research is necessary to evaluate the relationship between drug approvals and cancer mortality, Dr. MacEwan said.
“Research directly linking utilization of new therapies to improved survival or reduced mortality in the real-world setting would more definitively demonstrate the impact of new treatments,” she said. “New therapies have improved outcomes for many patients and should continue to be considered as key elements of cancer treatment.”
“We need to continue to reduce tobacco smoking and improve on modifiable behaviors at the same time as we work on getting new drugs to cancer patients,” Dr. Cance said. “We are coming into an era of multiple new therapeutics, including targeted therapies, immunotherapies, and cellular therapies. Clinicians need to look closely at the trial data of new drugs and pay close attention to those that have the most mortality impact.”
“We also need equitable distribution of newer drugs,” Dr. Cance added. “They should be distributed to everybody who deserves them. Mortality is often impacted by social determinants of health.”
Funding for this research was provided by Pfizer. Study authors disclosed relationships, including employment, with Pfizer. Dr. Cance had no disclosures.
SOURCE: MacEwan JP et al. J Med Econ. 2020 Nov 9;1-12.
Reductions in mortality were most notable for tumor types with relatively more approvals, including lung and breast cancer, melanoma, lymphoma, and leukemia.
A report from the American Cancer Society (ACS) estimated that, from 1991 to 2017, there were 2,902,200 total cancer deaths avoided from improvements in mortality from all potential sources.
The new findings, reported in the Journal of Medical Economics, suggest that drugs approved between 2000 and 2016 to treat the 15 most common cancer types helped to reduce mortality by 24% per 100,000 people.
“This study provides evidence that a significant share of that reduction from 2000 to 2016 was associated with the introduction of new therapies. The ACS report and other studies demonstrate that the improvements in lung cancer specifically are likely due to new treatments,” said lead study author Joanna P. MacEwan, MD, of PRECISIONheor in Los Angeles.
The findings contribute to a better understanding of whether increased spending on cancer drugs are worth the investment, according to the study authors.
“We provide evidence that the gains in survival measured in clinical trials are translating into health benefits for patients in the real world and confirm previous research that has also shown that new pharmaceutical treatments are associated with improved real-world survival outcomes for patients,” Dr. MacEwan said.
Full effect not yet observed
The researchers used a series of national data sets from sources including the Centers for Disease Control and Prevention; the U.S. Mortality Files by the National Center of Health Statistics; Survival, Epidemiology and End Results program; and United States Cancer Statistics.
The team calculated age-adjusted cancer mortality rates per year for the 15 most common tumor types and also looked at incident cases of cancer by tumor type, represented as per 100,000 people, for all ages, races, and genders.
The researchers then translated the change in cancer mortality in the U.S. from 2000 to 2016 associated with treatment stocks in each year into deaths averted per year.
Across the 16 years, mortality was down by 1,291,769 deaths. The following cancers had significant reductions in mortality: breast (n = 127,874), colorectal (n = 46,705), lung (n = 375,256), prostate (n = 476,210), gastric (n = 758), and renal (n = 739) cancers, as well as non-Hodgkin lymphoma (n = 48,836) and leukemia (n = 4,011).
Estimated mortality increased by 825 deaths in patients with thyroid cancer and 7,768 deaths for those with bladder cancer. These rises are likely due to the result of sparse drug approvals during this period – five for thyroid cancer and three for bladder cancer – Dr. MacEwan said. There were no approvals in liver or uterine cancer and few approvals in pancreatic and oral cancer.
The full effect of new drug introductions may not have been observed yet, Dr. MacEwan noted.
“There are fewer patients using the treatments for drugs approved in the later years of our study and less follow-up time to measure outcomes,” she said. “Over time, utilization of the newer therapies will likely increase and the full effect on mortality will be observed.”
Other factors at play
Multiple factors have led to the declines in mortality, said William G. Cance, MD, chief medical and scientific officer for the ACS, who was not involved in this study. “We are slowly sorting out the explanations in greater granularity.”
Dr. MacEwan said improved cancer screening may partially explain the decline in mortality in some tumor types.
“If screening in a particular tumor type improved during the study period and tumors were diagnosed earlier, then mortality for that tumor type may decline,” she said. “However, we did not find strong evidence to suggest that there were significant changes in screening during our study period. Breast cancer screening rates, for example, were stable over our study period.”
Cancer screening is not as strong an influence as it should be, Dr. Cance said.
“The lung cancer screening rate is low. In breast and colorectal cancers, we need to double down on earlier screening,” he said, noting that less than one-quarter of adults between ages 45 and 50 years are currently screened for colorectal cancer. The ACS recommends that people at average risk of colorectal cancer start regular screening at age 45.
More research is necessary to evaluate the relationship between drug approvals and cancer mortality, Dr. MacEwan said.
“Research directly linking utilization of new therapies to improved survival or reduced mortality in the real-world setting would more definitively demonstrate the impact of new treatments,” she said. “New therapies have improved outcomes for many patients and should continue to be considered as key elements of cancer treatment.”
“We need to continue to reduce tobacco smoking and improve on modifiable behaviors at the same time as we work on getting new drugs to cancer patients,” Dr. Cance said. “We are coming into an era of multiple new therapeutics, including targeted therapies, immunotherapies, and cellular therapies. Clinicians need to look closely at the trial data of new drugs and pay close attention to those that have the most mortality impact.”
“We also need equitable distribution of newer drugs,” Dr. Cance added. “They should be distributed to everybody who deserves them. Mortality is often impacted by social determinants of health.”
Funding for this research was provided by Pfizer. Study authors disclosed relationships, including employment, with Pfizer. Dr. Cance had no disclosures.
SOURCE: MacEwan JP et al. J Med Econ. 2020 Nov 9;1-12.
Reductions in mortality were most notable for tumor types with relatively more approvals, including lung and breast cancer, melanoma, lymphoma, and leukemia.
A report from the American Cancer Society (ACS) estimated that, from 1991 to 2017, there were 2,902,200 total cancer deaths avoided from improvements in mortality from all potential sources.
The new findings, reported in the Journal of Medical Economics, suggest that drugs approved between 2000 and 2016 to treat the 15 most common cancer types helped to reduce mortality by 24% per 100,000 people.
“This study provides evidence that a significant share of that reduction from 2000 to 2016 was associated with the introduction of new therapies. The ACS report and other studies demonstrate that the improvements in lung cancer specifically are likely due to new treatments,” said lead study author Joanna P. MacEwan, MD, of PRECISIONheor in Los Angeles.
The findings contribute to a better understanding of whether increased spending on cancer drugs are worth the investment, according to the study authors.
“We provide evidence that the gains in survival measured in clinical trials are translating into health benefits for patients in the real world and confirm previous research that has also shown that new pharmaceutical treatments are associated with improved real-world survival outcomes for patients,” Dr. MacEwan said.
Full effect not yet observed
The researchers used a series of national data sets from sources including the Centers for Disease Control and Prevention; the U.S. Mortality Files by the National Center of Health Statistics; Survival, Epidemiology and End Results program; and United States Cancer Statistics.
The team calculated age-adjusted cancer mortality rates per year for the 15 most common tumor types and also looked at incident cases of cancer by tumor type, represented as per 100,000 people, for all ages, races, and genders.
The researchers then translated the change in cancer mortality in the U.S. from 2000 to 2016 associated with treatment stocks in each year into deaths averted per year.
Across the 16 years, mortality was down by 1,291,769 deaths. The following cancers had significant reductions in mortality: breast (n = 127,874), colorectal (n = 46,705), lung (n = 375,256), prostate (n = 476,210), gastric (n = 758), and renal (n = 739) cancers, as well as non-Hodgkin lymphoma (n = 48,836) and leukemia (n = 4,011).
Estimated mortality increased by 825 deaths in patients with thyroid cancer and 7,768 deaths for those with bladder cancer. These rises are likely due to the result of sparse drug approvals during this period – five for thyroid cancer and three for bladder cancer – Dr. MacEwan said. There were no approvals in liver or uterine cancer and few approvals in pancreatic and oral cancer.
The full effect of new drug introductions may not have been observed yet, Dr. MacEwan noted.
“There are fewer patients using the treatments for drugs approved in the later years of our study and less follow-up time to measure outcomes,” she said. “Over time, utilization of the newer therapies will likely increase and the full effect on mortality will be observed.”
Other factors at play
Multiple factors have led to the declines in mortality, said William G. Cance, MD, chief medical and scientific officer for the ACS, who was not involved in this study. “We are slowly sorting out the explanations in greater granularity.”
Dr. MacEwan said improved cancer screening may partially explain the decline in mortality in some tumor types.
“If screening in a particular tumor type improved during the study period and tumors were diagnosed earlier, then mortality for that tumor type may decline,” she said. “However, we did not find strong evidence to suggest that there were significant changes in screening during our study period. Breast cancer screening rates, for example, were stable over our study period.”
Cancer screening is not as strong an influence as it should be, Dr. Cance said.
“The lung cancer screening rate is low. In breast and colorectal cancers, we need to double down on earlier screening,” he said, noting that less than one-quarter of adults between ages 45 and 50 years are currently screened for colorectal cancer. The ACS recommends that people at average risk of colorectal cancer start regular screening at age 45.
More research is necessary to evaluate the relationship between drug approvals and cancer mortality, Dr. MacEwan said.
“Research directly linking utilization of new therapies to improved survival or reduced mortality in the real-world setting would more definitively demonstrate the impact of new treatments,” she said. “New therapies have improved outcomes for many patients and should continue to be considered as key elements of cancer treatment.”
“We need to continue to reduce tobacco smoking and improve on modifiable behaviors at the same time as we work on getting new drugs to cancer patients,” Dr. Cance said. “We are coming into an era of multiple new therapeutics, including targeted therapies, immunotherapies, and cellular therapies. Clinicians need to look closely at the trial data of new drugs and pay close attention to those that have the most mortality impact.”
“We also need equitable distribution of newer drugs,” Dr. Cance added. “They should be distributed to everybody who deserves them. Mortality is often impacted by social determinants of health.”
Funding for this research was provided by Pfizer. Study authors disclosed relationships, including employment, with Pfizer. Dr. Cance had no disclosures.
SOURCE: MacEwan JP et al. J Med Econ. 2020 Nov 9;1-12.
FROM JOURNAL OF MEDICAL ECONOMICS
What happened to melanoma care during COVID-19 sequestration
Initial evidence suggests that the , Rebecca I. Hartman, MD, MPH, said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medication Education.
This is not what National Comprehensive Cancer Network officials expected when they issued short-term recommendations on how to manage cutaneous melanoma during the first wave of the COVID-19 pandemic. Those recommendations for restriction of care, which Dr. Hartman characterized as “pretty significant changes from how we typically practice melanoma care in the U.S.,” came at a time when there was justifiable concern that the first COVID-19 surge would strain the U.S. health care system beyond the breaking point.
The rationale given for the NCCN recommendations was that most time-to-treat studies have shown no adverse patient outcomes for 90-day delays in treatment, even for thicker melanomas. But those studies, all retrospective, have been called into question. And the first real-world data on the impact of care restrictions during the lockdown, reported by Italian dermatologists, highlights adverse effects with potentially far-reaching consequences, noted Dr. Hartman, director of melanoma epidemiology at Brigham and Women’s Hospital and a dermatologist, Harvard University, Boston.
Analysis of the impact of lockdown-induced delays in melanoma care is not merely an academic exercise, she added. While everyone hopes that the spring 2020 COVID-19 shelter-in-place was a once-in-a-lifetime event, there’s no guarantee that will be the case. Moreover, the lockdown provides a natural experiment addressing the possible consequences of melanoma care delays on patient outcomes, a topic that for ethical reasons could never be addressed in a randomized trial.
The short-term NCCN recommendations included the use of excisional biopsies for melanoma diagnosis whenever possible; and delay of up to 3 months for wide local excision of in situ melanoma, any invasive melanoma with negative margins, and even T1 melanomas with positive margins provided the bulk of the lesion had been excised. The guidance also suggested delaying sentinel lymph node biopsy (SLNB), along with increased use of neoadjuvant therapy in patients with clinically palpable regional lymph nodes in order to delay surgery for up to 8 weeks. Single-agent systemic therapy at the least-frequent dosing was advised in order to minimize toxicity and reduce the need for additional health care resources: for example, nivolumab (Opdivo) at 480 mg every 4 weeks instead of every 2 weeks, and pembrolizumab (Keytruda) at 400 mg every 6 weeks, rather than every 3 weeks.
So, that’s what the NCCN recommended. Here’s what actually happened during shelter-in-place as captured in Dr. Hartman’s survey of 18 U.S. members of the Melanoma Prevention Working Group, all practicing dermatology in centers particularly hard-hit in the first wave of the pandemic: In-person new melanoma patient visits plunged from an average of 4.83 per week per provider to 0.83 per week. Telemedicine visits with new melanoma patients went from zero prepandemic to 0.67 visits per week per provider, which doesn’t come close to making up for the drop in in-person visits. Interestingly, two respondents reported turning to gene-expression profile testing for patient prognostication because of delays in SLNB.
Wide local excision was delayed by an average of 6 weeks in roughly one-third of melanoma patients with early tumor stage disease, regardless of margin status. For patients with stage T1b disease, wide local excision was typically performed on time during shelter-in-place; however, SLNB was delayed by an average of 5 weeks in 22% of patients with positive margins and 28% of those with negative margins. In contrast, 80% of patients with more advanced T2-T4 melanoma underwent on-schedule definitive management with wide local excision and SLNB, Dr. Hartman reported.
Critics have taken issue with the NCCN’s conclusion that most time-to-treatment studies show no harm arising from 90-day treatment delays. A review of the relevant published literature by Dr. Hartman’s Harvard colleagues, published in July, found that the evidence is mixed. “There is insufficient evidence to definitively conclude that delayed wide resection after gross removal of the primary melanoma is without harm,” they concluded in the review.
Spanish dermatologists performed a modeling study in order to estimate the potential impact of COVID-19 lockdowns on 5- and 10-year survival of melanoma patients. Using the growth rate of a random sample of 1,000 melanomas to model estimates of tumor thickness after various delays, coupled with American Joint Committee on Cancer survival data for different T stages, they estimated that 5-year survival would be reduced from 94.2% to 92.3% with a 90-day delay in diagnosis, and that 10-year survival would drop from 90.0% to 87.6%.
But that’s merely modeling. Francesco Ricci, MD, PhD, and colleagues from the melanoma unit at the Istituto Dermopatico dell’Immacolata, Rome, have provided a first look at the real-world impact of the lockdown. In the prelockdown period of January through March 9th, 2020, the referral center averaged 2.3 new melanoma diagnoses per day. During the Rome lockdown, from March 10th through May 3rd, this figure dropped to a mean of 0.6 melanoma diagnoses per day. Postlockdown, from May 4th to June 6th, the average climbed to 1.3 per day. The rate of newly diagnosed nodular melanoma was 5.5-fold greater postlockdown, compared with prelockdown; the rate of ulcerated melanoma was 4.9-fold greater.
“We can hypothesize that this may have been due to delays in diagnosis and care,” Dr. Hartman commented. “This is important because we know that nodular melanoma as well as ulceration tend to have a worse prognosis in terms of mortality.”
The mean Breslow thickness of newly diagnosed melanomas was 0.88 mm prelockdown, 0.66 mm during lockdown, and 1.96 mm postlockdown. The investigators speculated that the reduced Breslow thickness of melanomas diagnosed during lockdown might be explained by a greater willingness of more health-conscious people to defy the shelter-in-place instructions because of their concern about a suspicious skin lesion. “Though it is way too early to gauge the consequences of such diagnostic delay, should this issue be neglected, dermatologists and their patients may pay a higher price later with increased morbidity, mortality, and financial burden,” according to the investigators.
Dr. Hartman observed that it will be important to learn whether similar experiences occurred elsewhere during lockdown.
Another speaker, John M. Kirkwood, MD, said he has seen several melanoma patients referred from outside centers who had delays of up to 3 months in sentinel lymph node management of T2 and T3 tumors during lockdown who now have widespread metastatic disease.
“Now, is that anecdotal? I don’t know, it’s just worrisome to me,” commented Dr. Kirkwood, professor of medicine, dermatology, and translational science at the University of Pittsburgh.
Merrick Ross, MD, professor of surgical oncology at M.D. Anderson Cancer Center, Houston, recalled, “There was a period of time [during the lockdown] when we weren’t allowed to do certain elective procedures, if you want to call cancer surgery elective.”
“It’s too soon to talk about outcomes because a lot of patients are still in the process of being treated after what I would consider a significant delay in diagnosis,” the surgeon added.
An audience member asked if there will be an opportunity to see data on the damage done by delaying melanoma management as compared to lives saved through the lockdown for COVID-19. Dr. Ross replied that M.D. Anderson is in the midst of an institution-wide study analyzing the delay in diagnosis of a range of cancers.
“In our melanoma center it is absolutely clear, although we’re still collecting data, that the median tumor thickness is much higher since the lockdown,” Dr. Ross commented.
Dr. Hartman said she and her coinvestigators in the Melanoma Prevention Working Group are attempting to tally up the damage done via the lockdown by delaying melanoma diagnosis and treatment. But she agreed with the questioner that the most important thing is overall net lives saved through shelter-in-place.
“I’m sure that, separately, nondermatologists – perhaps infectious disease doctors and internists – are looking at how many lives were saved by the lockdown policy. So I do think all that data will come out,” Dr. Hartman predicted.
She reported having no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
SOURCE: Hartman, R. Cutaneous malignancies forum.
Initial evidence suggests that the , Rebecca I. Hartman, MD, MPH, said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medication Education.
This is not what National Comprehensive Cancer Network officials expected when they issued short-term recommendations on how to manage cutaneous melanoma during the first wave of the COVID-19 pandemic. Those recommendations for restriction of care, which Dr. Hartman characterized as “pretty significant changes from how we typically practice melanoma care in the U.S.,” came at a time when there was justifiable concern that the first COVID-19 surge would strain the U.S. health care system beyond the breaking point.
The rationale given for the NCCN recommendations was that most time-to-treat studies have shown no adverse patient outcomes for 90-day delays in treatment, even for thicker melanomas. But those studies, all retrospective, have been called into question. And the first real-world data on the impact of care restrictions during the lockdown, reported by Italian dermatologists, highlights adverse effects with potentially far-reaching consequences, noted Dr. Hartman, director of melanoma epidemiology at Brigham and Women’s Hospital and a dermatologist, Harvard University, Boston.
Analysis of the impact of lockdown-induced delays in melanoma care is not merely an academic exercise, she added. While everyone hopes that the spring 2020 COVID-19 shelter-in-place was a once-in-a-lifetime event, there’s no guarantee that will be the case. Moreover, the lockdown provides a natural experiment addressing the possible consequences of melanoma care delays on patient outcomes, a topic that for ethical reasons could never be addressed in a randomized trial.
The short-term NCCN recommendations included the use of excisional biopsies for melanoma diagnosis whenever possible; and delay of up to 3 months for wide local excision of in situ melanoma, any invasive melanoma with negative margins, and even T1 melanomas with positive margins provided the bulk of the lesion had been excised. The guidance also suggested delaying sentinel lymph node biopsy (SLNB), along with increased use of neoadjuvant therapy in patients with clinically palpable regional lymph nodes in order to delay surgery for up to 8 weeks. Single-agent systemic therapy at the least-frequent dosing was advised in order to minimize toxicity and reduce the need for additional health care resources: for example, nivolumab (Opdivo) at 480 mg every 4 weeks instead of every 2 weeks, and pembrolizumab (Keytruda) at 400 mg every 6 weeks, rather than every 3 weeks.
So, that’s what the NCCN recommended. Here’s what actually happened during shelter-in-place as captured in Dr. Hartman’s survey of 18 U.S. members of the Melanoma Prevention Working Group, all practicing dermatology in centers particularly hard-hit in the first wave of the pandemic: In-person new melanoma patient visits plunged from an average of 4.83 per week per provider to 0.83 per week. Telemedicine visits with new melanoma patients went from zero prepandemic to 0.67 visits per week per provider, which doesn’t come close to making up for the drop in in-person visits. Interestingly, two respondents reported turning to gene-expression profile testing for patient prognostication because of delays in SLNB.
Wide local excision was delayed by an average of 6 weeks in roughly one-third of melanoma patients with early tumor stage disease, regardless of margin status. For patients with stage T1b disease, wide local excision was typically performed on time during shelter-in-place; however, SLNB was delayed by an average of 5 weeks in 22% of patients with positive margins and 28% of those with negative margins. In contrast, 80% of patients with more advanced T2-T4 melanoma underwent on-schedule definitive management with wide local excision and SLNB, Dr. Hartman reported.
Critics have taken issue with the NCCN’s conclusion that most time-to-treatment studies show no harm arising from 90-day treatment delays. A review of the relevant published literature by Dr. Hartman’s Harvard colleagues, published in July, found that the evidence is mixed. “There is insufficient evidence to definitively conclude that delayed wide resection after gross removal of the primary melanoma is without harm,” they concluded in the review.
Spanish dermatologists performed a modeling study in order to estimate the potential impact of COVID-19 lockdowns on 5- and 10-year survival of melanoma patients. Using the growth rate of a random sample of 1,000 melanomas to model estimates of tumor thickness after various delays, coupled with American Joint Committee on Cancer survival data for different T stages, they estimated that 5-year survival would be reduced from 94.2% to 92.3% with a 90-day delay in diagnosis, and that 10-year survival would drop from 90.0% to 87.6%.
But that’s merely modeling. Francesco Ricci, MD, PhD, and colleagues from the melanoma unit at the Istituto Dermopatico dell’Immacolata, Rome, have provided a first look at the real-world impact of the lockdown. In the prelockdown period of January through March 9th, 2020, the referral center averaged 2.3 new melanoma diagnoses per day. During the Rome lockdown, from March 10th through May 3rd, this figure dropped to a mean of 0.6 melanoma diagnoses per day. Postlockdown, from May 4th to June 6th, the average climbed to 1.3 per day. The rate of newly diagnosed nodular melanoma was 5.5-fold greater postlockdown, compared with prelockdown; the rate of ulcerated melanoma was 4.9-fold greater.
“We can hypothesize that this may have been due to delays in diagnosis and care,” Dr. Hartman commented. “This is important because we know that nodular melanoma as well as ulceration tend to have a worse prognosis in terms of mortality.”
The mean Breslow thickness of newly diagnosed melanomas was 0.88 mm prelockdown, 0.66 mm during lockdown, and 1.96 mm postlockdown. The investigators speculated that the reduced Breslow thickness of melanomas diagnosed during lockdown might be explained by a greater willingness of more health-conscious people to defy the shelter-in-place instructions because of their concern about a suspicious skin lesion. “Though it is way too early to gauge the consequences of such diagnostic delay, should this issue be neglected, dermatologists and their patients may pay a higher price later with increased morbidity, mortality, and financial burden,” according to the investigators.
Dr. Hartman observed that it will be important to learn whether similar experiences occurred elsewhere during lockdown.
Another speaker, John M. Kirkwood, MD, said he has seen several melanoma patients referred from outside centers who had delays of up to 3 months in sentinel lymph node management of T2 and T3 tumors during lockdown who now have widespread metastatic disease.
“Now, is that anecdotal? I don’t know, it’s just worrisome to me,” commented Dr. Kirkwood, professor of medicine, dermatology, and translational science at the University of Pittsburgh.
Merrick Ross, MD, professor of surgical oncology at M.D. Anderson Cancer Center, Houston, recalled, “There was a period of time [during the lockdown] when we weren’t allowed to do certain elective procedures, if you want to call cancer surgery elective.”
“It’s too soon to talk about outcomes because a lot of patients are still in the process of being treated after what I would consider a significant delay in diagnosis,” the surgeon added.
An audience member asked if there will be an opportunity to see data on the damage done by delaying melanoma management as compared to lives saved through the lockdown for COVID-19. Dr. Ross replied that M.D. Anderson is in the midst of an institution-wide study analyzing the delay in diagnosis of a range of cancers.
“In our melanoma center it is absolutely clear, although we’re still collecting data, that the median tumor thickness is much higher since the lockdown,” Dr. Ross commented.
Dr. Hartman said she and her coinvestigators in the Melanoma Prevention Working Group are attempting to tally up the damage done via the lockdown by delaying melanoma diagnosis and treatment. But she agreed with the questioner that the most important thing is overall net lives saved through shelter-in-place.
“I’m sure that, separately, nondermatologists – perhaps infectious disease doctors and internists – are looking at how many lives were saved by the lockdown policy. So I do think all that data will come out,” Dr. Hartman predicted.
She reported having no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
SOURCE: Hartman, R. Cutaneous malignancies forum.
Initial evidence suggests that the , Rebecca I. Hartman, MD, MPH, said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medication Education.
This is not what National Comprehensive Cancer Network officials expected when they issued short-term recommendations on how to manage cutaneous melanoma during the first wave of the COVID-19 pandemic. Those recommendations for restriction of care, which Dr. Hartman characterized as “pretty significant changes from how we typically practice melanoma care in the U.S.,” came at a time when there was justifiable concern that the first COVID-19 surge would strain the U.S. health care system beyond the breaking point.
The rationale given for the NCCN recommendations was that most time-to-treat studies have shown no adverse patient outcomes for 90-day delays in treatment, even for thicker melanomas. But those studies, all retrospective, have been called into question. And the first real-world data on the impact of care restrictions during the lockdown, reported by Italian dermatologists, highlights adverse effects with potentially far-reaching consequences, noted Dr. Hartman, director of melanoma epidemiology at Brigham and Women’s Hospital and a dermatologist, Harvard University, Boston.
Analysis of the impact of lockdown-induced delays in melanoma care is not merely an academic exercise, she added. While everyone hopes that the spring 2020 COVID-19 shelter-in-place was a once-in-a-lifetime event, there’s no guarantee that will be the case. Moreover, the lockdown provides a natural experiment addressing the possible consequences of melanoma care delays on patient outcomes, a topic that for ethical reasons could never be addressed in a randomized trial.
The short-term NCCN recommendations included the use of excisional biopsies for melanoma diagnosis whenever possible; and delay of up to 3 months for wide local excision of in situ melanoma, any invasive melanoma with negative margins, and even T1 melanomas with positive margins provided the bulk of the lesion had been excised. The guidance also suggested delaying sentinel lymph node biopsy (SLNB), along with increased use of neoadjuvant therapy in patients with clinically palpable regional lymph nodes in order to delay surgery for up to 8 weeks. Single-agent systemic therapy at the least-frequent dosing was advised in order to minimize toxicity and reduce the need for additional health care resources: for example, nivolumab (Opdivo) at 480 mg every 4 weeks instead of every 2 weeks, and pembrolizumab (Keytruda) at 400 mg every 6 weeks, rather than every 3 weeks.
So, that’s what the NCCN recommended. Here’s what actually happened during shelter-in-place as captured in Dr. Hartman’s survey of 18 U.S. members of the Melanoma Prevention Working Group, all practicing dermatology in centers particularly hard-hit in the first wave of the pandemic: In-person new melanoma patient visits plunged from an average of 4.83 per week per provider to 0.83 per week. Telemedicine visits with new melanoma patients went from zero prepandemic to 0.67 visits per week per provider, which doesn’t come close to making up for the drop in in-person visits. Interestingly, two respondents reported turning to gene-expression profile testing for patient prognostication because of delays in SLNB.
Wide local excision was delayed by an average of 6 weeks in roughly one-third of melanoma patients with early tumor stage disease, regardless of margin status. For patients with stage T1b disease, wide local excision was typically performed on time during shelter-in-place; however, SLNB was delayed by an average of 5 weeks in 22% of patients with positive margins and 28% of those with negative margins. In contrast, 80% of patients with more advanced T2-T4 melanoma underwent on-schedule definitive management with wide local excision and SLNB, Dr. Hartman reported.
Critics have taken issue with the NCCN’s conclusion that most time-to-treatment studies show no harm arising from 90-day treatment delays. A review of the relevant published literature by Dr. Hartman’s Harvard colleagues, published in July, found that the evidence is mixed. “There is insufficient evidence to definitively conclude that delayed wide resection after gross removal of the primary melanoma is without harm,” they concluded in the review.
Spanish dermatologists performed a modeling study in order to estimate the potential impact of COVID-19 lockdowns on 5- and 10-year survival of melanoma patients. Using the growth rate of a random sample of 1,000 melanomas to model estimates of tumor thickness after various delays, coupled with American Joint Committee on Cancer survival data for different T stages, they estimated that 5-year survival would be reduced from 94.2% to 92.3% with a 90-day delay in diagnosis, and that 10-year survival would drop from 90.0% to 87.6%.
But that’s merely modeling. Francesco Ricci, MD, PhD, and colleagues from the melanoma unit at the Istituto Dermopatico dell’Immacolata, Rome, have provided a first look at the real-world impact of the lockdown. In the prelockdown period of January through March 9th, 2020, the referral center averaged 2.3 new melanoma diagnoses per day. During the Rome lockdown, from March 10th through May 3rd, this figure dropped to a mean of 0.6 melanoma diagnoses per day. Postlockdown, from May 4th to June 6th, the average climbed to 1.3 per day. The rate of newly diagnosed nodular melanoma was 5.5-fold greater postlockdown, compared with prelockdown; the rate of ulcerated melanoma was 4.9-fold greater.
“We can hypothesize that this may have been due to delays in diagnosis and care,” Dr. Hartman commented. “This is important because we know that nodular melanoma as well as ulceration tend to have a worse prognosis in terms of mortality.”
The mean Breslow thickness of newly diagnosed melanomas was 0.88 mm prelockdown, 0.66 mm during lockdown, and 1.96 mm postlockdown. The investigators speculated that the reduced Breslow thickness of melanomas diagnosed during lockdown might be explained by a greater willingness of more health-conscious people to defy the shelter-in-place instructions because of their concern about a suspicious skin lesion. “Though it is way too early to gauge the consequences of such diagnostic delay, should this issue be neglected, dermatologists and their patients may pay a higher price later with increased morbidity, mortality, and financial burden,” according to the investigators.
Dr. Hartman observed that it will be important to learn whether similar experiences occurred elsewhere during lockdown.
Another speaker, John M. Kirkwood, MD, said he has seen several melanoma patients referred from outside centers who had delays of up to 3 months in sentinel lymph node management of T2 and T3 tumors during lockdown who now have widespread metastatic disease.
“Now, is that anecdotal? I don’t know, it’s just worrisome to me,” commented Dr. Kirkwood, professor of medicine, dermatology, and translational science at the University of Pittsburgh.
Merrick Ross, MD, professor of surgical oncology at M.D. Anderson Cancer Center, Houston, recalled, “There was a period of time [during the lockdown] when we weren’t allowed to do certain elective procedures, if you want to call cancer surgery elective.”
“It’s too soon to talk about outcomes because a lot of patients are still in the process of being treated after what I would consider a significant delay in diagnosis,” the surgeon added.
An audience member asked if there will be an opportunity to see data on the damage done by delaying melanoma management as compared to lives saved through the lockdown for COVID-19. Dr. Ross replied that M.D. Anderson is in the midst of an institution-wide study analyzing the delay in diagnosis of a range of cancers.
“In our melanoma center it is absolutely clear, although we’re still collecting data, that the median tumor thickness is much higher since the lockdown,” Dr. Ross commented.
Dr. Hartman said she and her coinvestigators in the Melanoma Prevention Working Group are attempting to tally up the damage done via the lockdown by delaying melanoma diagnosis and treatment. But she agreed with the questioner that the most important thing is overall net lives saved through shelter-in-place.
“I’m sure that, separately, nondermatologists – perhaps infectious disease doctors and internists – are looking at how many lives were saved by the lockdown policy. So I do think all that data will come out,” Dr. Hartman predicted.
She reported having no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
SOURCE: Hartman, R. Cutaneous malignancies forum.
REPORTING FROM THE CUTANEOUS MALIGNANCIES FORUM
Intraoperative Tissue Expansion to Allow Primary Linear Closure of 2 Large Adjacent Surgical Defects
Practice Gap
Nonmelanoma skin cancers most commonly are found on the head and neck. In these locations, many of these malignancies will meet criteria to undergo treatment with Mohs micrographic surgery. It is becoming increasingly common for patients to have multiple lesions treated at the same time, and sometimes these lesions can be in close proximity to one another. The final size of the adjacent defects, along with the amount of normal tissue remaining between them, will determine how to best repair both defects.1 Many times, repair options are limited to the use of a larger and more extensive repair such as a flap or graft. We present a novel option to increase the options for surgical repair.
The Technique
We present a case of 2 large adjacent postsurgical defects where intraoperative tissue relaxation allowed for successful primary linear closure of both defects under notably decreased tension from baseline. A 70-year-old man presented for treatment of 2 adjacent invasive squamous cell carcinomas on the left temple and left frontal scalp. The initial lesion sizes were 2.0×1.0 and 2.0×2.0 cm, respectively. Mohs micrographic surgery was performed on both lesions, and the final defect sizes measured 2.0×1.4 and 3.0×1.6 cm, respectively. The island of normal tissue between the defects measured 2.3-cm wide. Different repair options were discussed with the patient, including allowing 1 or both lesions to heal via secondary intention, creating 1 large wound to repair with a full-thickness skin graft, using a large skin flap to cover both wounds, or utilizing a 2-to-Z flap.2 We also discussed using an intraoperative skin relaxation device to stretch the skin around 1 or both defects and close both defects in a linear fashion; the patient opted for the latter treatment option.
The left temple had adequate mobility to perform a primary closure oriented horizontally along the long axis of the defect. Although it would have been a simple repair for this lesion, the superior defect on the frontal scalp would have been subjected to increased downward tension. The scalp defect was already under considerable tension with limited tissue mobility, so closing the temple defect horizontally would have required repair of the scalp defect using a skin graft or leaving it open to heal on its own. Similarly, the force necessary to close the frontal scalp wound first would have prevented primary closure of the temple defect.
A SUTUREGARD ISR device (Sutureguard Medical Inc) was secured centrally over both defects at a 90° angle to one another to provide intraoperative tissue relaxation without undermining. The devices were held in place by a US Pharmacopeia 2-0 nylon suture and allowed to sit for 60 minutes (Figure 1).3
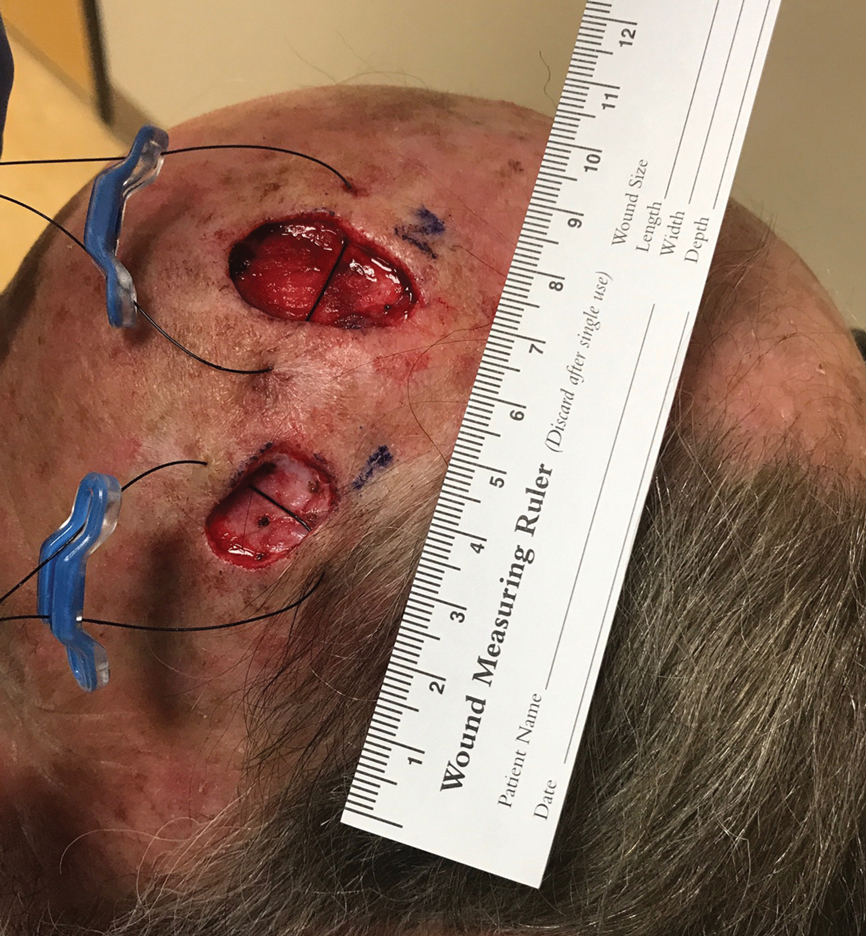
After 60 minutes, the temple defect had adequate relaxion to allow a standard layered intermediate closure in a vertical orientation along the hairline using 3-0 polyglactin 910 and 3-0 nylon. Although the scalp defect was not completely approximated, it was more than 60% smaller and able to be closed at both wound edges using the same layered approach. There was a central defect area approximately 4-mm wide that was left to heal by secondary intention (Figure 2). Undermining was not used to close either defect.

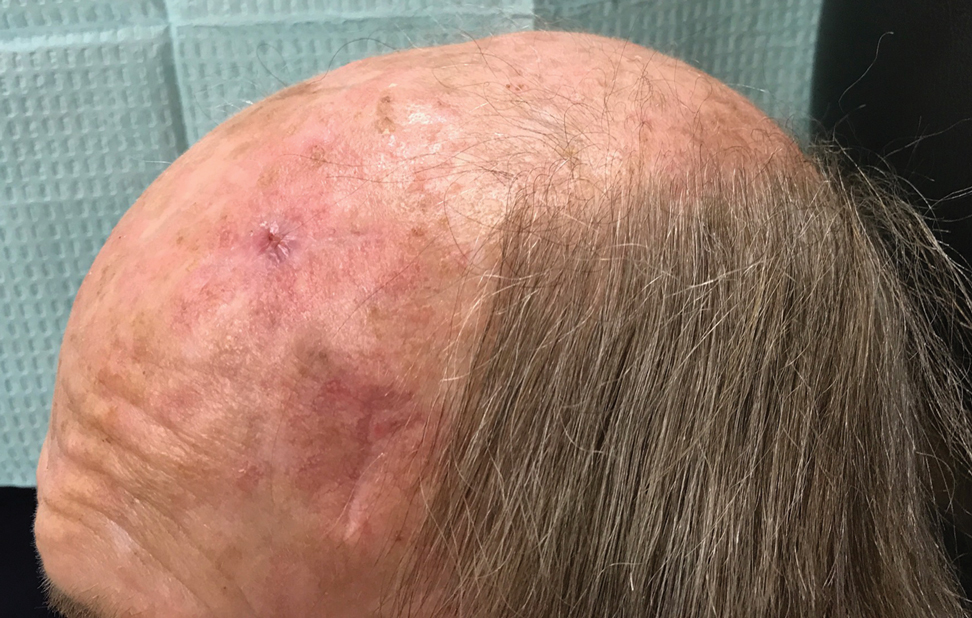
The patient tolerated the procedure well with minimal pain or discomfort. He followed standard postoperative care instructions and returned for suture removal after 14 days of healing. At the time of suture removal there were no complications. At 1-month follow-up the patient presented with excellent cosmetic results (Figure 3).
Practice Implications
The methods of repairing 2 adjacent postsurgical defects are numerous and vary depending on the size of the individual defects, the location of the defects, and the amount of normal skin remaining between them. Various methods of closure for the adjacent defects include healing by secondary intention, primary linear closure, skin grafts, skin flaps, creating 1 larger wound to be repaired, or a combination of these approaches.1,2,4,5
In our patient, closing the high-tension wound of the scalp would have prevented both wounds from being closed in a linear fashion without first stretching the tissue. Although Zitelli5 has cited that many wounds will heal well on their own despite a large size, many patients prefer the cosmetic appearance and shorter healing time of wounds that have been closed with sutures, particularly if those defects are greater than 8-mm wide. In contrast, patients preferred the cosmetic appearance of 4-mm wounds that healed via secondary intention.6 In our case, we closed the majority of the wound and left a small 4-mm-wide portion to heal on its own. The overall outcome was excellent and healed much quicker than leaving the entire scalp defect to heal by secondary intention.
The other methods of closure, such as a 2-to-Z flap, would have been difficult given the orientation of the lesions and the island between them.2 To create this flap, an extensive amount of undermining would have been necessary, leading to serious disruption of the blood and nerve supply and an increased risk for flap necrosis. Creating 1 large wound and repairing with a flap would have similar requirements and complications.
Intraoperative tissue relaxation can be used to allow primary closure of adjacent wounds without the need for undermining. Prior research has shown that 30 minutes of stress relaxation with 20 Newtons of applied tension yields a 65% reduction in wound-closure tension.7 Orienting the devices between 45° to 90° angles to one another creates opposing tension vectors so that the closure of one defect does not prevent the closure of the other defect. Even in cases in which the defects cannot be completely approximated, closing the wound edges to create a smaller central defect can decrease healing time and lead to an excellent cosmetic outcome without the need for a flap or graft.
The SUTUREGARD ISR suture retention bridge also is cost-effective for the surgeon and the patient. The device and suture-guide washer are included in a set that retails for $35 each or $300 for a box of 12.8 The suture most commonly used to secure the device in our practice is 2-0 nylon and retails for approximately $34 for a box of 12,9 which brings the total cost with the device to around $38 per use. The updated Current Procedural Terminology guidelines from the Centers for Medicare & Medicaid Services define that an intermediate repair requires a layered closure and may include, but does not require, limited undermining. A complex linear closure must meet criteria for an intermediate closure plus at least 1 additional criterion, such as exposure of cartilage, bone, or tendons within the defect; extensive undermining; wound-edge debridement; involvement of free margins; or use of a retention suture.10 Use of a suture retention bridge such as the SUTUREGARD ISR device and therefore a retention suture qualifies the repair as a complex linear closure. Overall, use of the device expands the surgeon’s choices for surgical closures and helps to limit the need for larger, more invasive repair procedures.
- McGinness JL, Parlette HL. A novel technique using a rotation flap for repairing adjacent surgical defects. Dermatol Surg. 2006;32:272-275.
- Blattner CM, Perry B, Young J, et al. 2-to-Z flap for reconstruction of adjacent skin defects. J Am Acad Dermatol. 2019;80:E77-E78.
- Blattner CM, Perry B, Young J, et al. The use of a suture retention device to enhance tissue expansion and healing in the repair of scalp and lower leg wounds. JAAD Case Rep. 2018;4:655-661.
- Zivony D, Siegle RJ. Burrow’s wedge advancement flaps for reconstruction of adjacent surgical defects. Dermatol Surg. 2002;28:1162-1164.
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106.
- Christenson LJ, Phillips PK, Weaver AL, et al. Primary closure vs second-intention treatment of skin punch biopsy sites: a randomized trial. Arch Dermatol. 2005;141:1093-1099.
- Lear W, Blattner CM, Mustoe TA, et al. In vivo stress relaxation of human scalp. J Mech Behav Biomed Mater. 2019;97:85-89.
- SUTUREGARD purchasing facts. SUTUREGARD® Medical Inc website. https://suturegard.com/SUTUREGARD-Purchasing-Facts. Accessed October 15, 2020.
- Shop products: suture with needle McKesson nonabsorbable uncoated black suture monofilament nylon size 2-0 18 inch suture 1-needle 26 mm length 3/8 circle reverse cutting needle. McKesson website. https://mms.mckesson.com/catalog?query=1034509. Accessed October 15, 2020.
- Norris S. 2020 CPT updates to wound repair guidelines. Zotec Partners website. http://zotecpartners.com/resources/2020-cpt-updates-to-wound-repair-guidelines/. Published June 4, 2020. Accessed October 21, 2020.
Practice Gap
Nonmelanoma skin cancers most commonly are found on the head and neck. In these locations, many of these malignancies will meet criteria to undergo treatment with Mohs micrographic surgery. It is becoming increasingly common for patients to have multiple lesions treated at the same time, and sometimes these lesions can be in close proximity to one another. The final size of the adjacent defects, along with the amount of normal tissue remaining between them, will determine how to best repair both defects.1 Many times, repair options are limited to the use of a larger and more extensive repair such as a flap or graft. We present a novel option to increase the options for surgical repair.
The Technique
We present a case of 2 large adjacent postsurgical defects where intraoperative tissue relaxation allowed for successful primary linear closure of both defects under notably decreased tension from baseline. A 70-year-old man presented for treatment of 2 adjacent invasive squamous cell carcinomas on the left temple and left frontal scalp. The initial lesion sizes were 2.0×1.0 and 2.0×2.0 cm, respectively. Mohs micrographic surgery was performed on both lesions, and the final defect sizes measured 2.0×1.4 and 3.0×1.6 cm, respectively. The island of normal tissue between the defects measured 2.3-cm wide. Different repair options were discussed with the patient, including allowing 1 or both lesions to heal via secondary intention, creating 1 large wound to repair with a full-thickness skin graft, using a large skin flap to cover both wounds, or utilizing a 2-to-Z flap.2 We also discussed using an intraoperative skin relaxation device to stretch the skin around 1 or both defects and close both defects in a linear fashion; the patient opted for the latter treatment option.
The left temple had adequate mobility to perform a primary closure oriented horizontally along the long axis of the defect. Although it would have been a simple repair for this lesion, the superior defect on the frontal scalp would have been subjected to increased downward tension. The scalp defect was already under considerable tension with limited tissue mobility, so closing the temple defect horizontally would have required repair of the scalp defect using a skin graft or leaving it open to heal on its own. Similarly, the force necessary to close the frontal scalp wound first would have prevented primary closure of the temple defect.
A SUTUREGARD ISR device (Sutureguard Medical Inc) was secured centrally over both defects at a 90° angle to one another to provide intraoperative tissue relaxation without undermining. The devices were held in place by a US Pharmacopeia 2-0 nylon suture and allowed to sit for 60 minutes (Figure 1).3

After 60 minutes, the temple defect had adequate relaxion to allow a standard layered intermediate closure in a vertical orientation along the hairline using 3-0 polyglactin 910 and 3-0 nylon. Although the scalp defect was not completely approximated, it was more than 60% smaller and able to be closed at both wound edges using the same layered approach. There was a central defect area approximately 4-mm wide that was left to heal by secondary intention (Figure 2). Undermining was not used to close either defect.

The patient tolerated the procedure well with minimal pain or discomfort. He followed standard postoperative care instructions and returned for suture removal after 14 days of healing. At the time of suture removal there were no complications. At 1-month follow-up the patient presented with excellent cosmetic results (Figure 3).

Practice Implications
The methods of repairing 2 adjacent postsurgical defects are numerous and vary depending on the size of the individual defects, the location of the defects, and the amount of normal skin remaining between them. Various methods of closure for the adjacent defects include healing by secondary intention, primary linear closure, skin grafts, skin flaps, creating 1 larger wound to be repaired, or a combination of these approaches.1,2,4,5
In our patient, closing the high-tension wound of the scalp would have prevented both wounds from being closed in a linear fashion without first stretching the tissue. Although Zitelli5 has cited that many wounds will heal well on their own despite a large size, many patients prefer the cosmetic appearance and shorter healing time of wounds that have been closed with sutures, particularly if those defects are greater than 8-mm wide. In contrast, patients preferred the cosmetic appearance of 4-mm wounds that healed via secondary intention.6 In our case, we closed the majority of the wound and left a small 4-mm-wide portion to heal on its own. The overall outcome was excellent and healed much quicker than leaving the entire scalp defect to heal by secondary intention.
The other methods of closure, such as a 2-to-Z flap, would have been difficult given the orientation of the lesions and the island between them.2 To create this flap, an extensive amount of undermining would have been necessary, leading to serious disruption of the blood and nerve supply and an increased risk for flap necrosis. Creating 1 large wound and repairing with a flap would have similar requirements and complications.
Intraoperative tissue relaxation can be used to allow primary closure of adjacent wounds without the need for undermining. Prior research has shown that 30 minutes of stress relaxation with 20 Newtons of applied tension yields a 65% reduction in wound-closure tension.7 Orienting the devices between 45° to 90° angles to one another creates opposing tension vectors so that the closure of one defect does not prevent the closure of the other defect. Even in cases in which the defects cannot be completely approximated, closing the wound edges to create a smaller central defect can decrease healing time and lead to an excellent cosmetic outcome without the need for a flap or graft.
The SUTUREGARD ISR suture retention bridge also is cost-effective for the surgeon and the patient. The device and suture-guide washer are included in a set that retails for $35 each or $300 for a box of 12.8 The suture most commonly used to secure the device in our practice is 2-0 nylon and retails for approximately $34 for a box of 12,9 which brings the total cost with the device to around $38 per use. The updated Current Procedural Terminology guidelines from the Centers for Medicare & Medicaid Services define that an intermediate repair requires a layered closure and may include, but does not require, limited undermining. A complex linear closure must meet criteria for an intermediate closure plus at least 1 additional criterion, such as exposure of cartilage, bone, or tendons within the defect; extensive undermining; wound-edge debridement; involvement of free margins; or use of a retention suture.10 Use of a suture retention bridge such as the SUTUREGARD ISR device and therefore a retention suture qualifies the repair as a complex linear closure. Overall, use of the device expands the surgeon’s choices for surgical closures and helps to limit the need for larger, more invasive repair procedures.
Practice Gap
Nonmelanoma skin cancers most commonly are found on the head and neck. In these locations, many of these malignancies will meet criteria to undergo treatment with Mohs micrographic surgery. It is becoming increasingly common for patients to have multiple lesions treated at the same time, and sometimes these lesions can be in close proximity to one another. The final size of the adjacent defects, along with the amount of normal tissue remaining between them, will determine how to best repair both defects.1 Many times, repair options are limited to the use of a larger and more extensive repair such as a flap or graft. We present a novel option to increase the options for surgical repair.
The Technique
We present a case of 2 large adjacent postsurgical defects where intraoperative tissue relaxation allowed for successful primary linear closure of both defects under notably decreased tension from baseline. A 70-year-old man presented for treatment of 2 adjacent invasive squamous cell carcinomas on the left temple and left frontal scalp. The initial lesion sizes were 2.0×1.0 and 2.0×2.0 cm, respectively. Mohs micrographic surgery was performed on both lesions, and the final defect sizes measured 2.0×1.4 and 3.0×1.6 cm, respectively. The island of normal tissue between the defects measured 2.3-cm wide. Different repair options were discussed with the patient, including allowing 1 or both lesions to heal via secondary intention, creating 1 large wound to repair with a full-thickness skin graft, using a large skin flap to cover both wounds, or utilizing a 2-to-Z flap.2 We also discussed using an intraoperative skin relaxation device to stretch the skin around 1 or both defects and close both defects in a linear fashion; the patient opted for the latter treatment option.
The left temple had adequate mobility to perform a primary closure oriented horizontally along the long axis of the defect. Although it would have been a simple repair for this lesion, the superior defect on the frontal scalp would have been subjected to increased downward tension. The scalp defect was already under considerable tension with limited tissue mobility, so closing the temple defect horizontally would have required repair of the scalp defect using a skin graft or leaving it open to heal on its own. Similarly, the force necessary to close the frontal scalp wound first would have prevented primary closure of the temple defect.
A SUTUREGARD ISR device (Sutureguard Medical Inc) was secured centrally over both defects at a 90° angle to one another to provide intraoperative tissue relaxation without undermining. The devices were held in place by a US Pharmacopeia 2-0 nylon suture and allowed to sit for 60 minutes (Figure 1).3

After 60 minutes, the temple defect had adequate relaxion to allow a standard layered intermediate closure in a vertical orientation along the hairline using 3-0 polyglactin 910 and 3-0 nylon. Although the scalp defect was not completely approximated, it was more than 60% smaller and able to be closed at both wound edges using the same layered approach. There was a central defect area approximately 4-mm wide that was left to heal by secondary intention (Figure 2). Undermining was not used to close either defect.

The patient tolerated the procedure well with minimal pain or discomfort. He followed standard postoperative care instructions and returned for suture removal after 14 days of healing. At the time of suture removal there were no complications. At 1-month follow-up the patient presented with excellent cosmetic results (Figure 3).

Practice Implications
The methods of repairing 2 adjacent postsurgical defects are numerous and vary depending on the size of the individual defects, the location of the defects, and the amount of normal skin remaining between them. Various methods of closure for the adjacent defects include healing by secondary intention, primary linear closure, skin grafts, skin flaps, creating 1 larger wound to be repaired, or a combination of these approaches.1,2,4,5
In our patient, closing the high-tension wound of the scalp would have prevented both wounds from being closed in a linear fashion without first stretching the tissue. Although Zitelli5 has cited that many wounds will heal well on their own despite a large size, many patients prefer the cosmetic appearance and shorter healing time of wounds that have been closed with sutures, particularly if those defects are greater than 8-mm wide. In contrast, patients preferred the cosmetic appearance of 4-mm wounds that healed via secondary intention.6 In our case, we closed the majority of the wound and left a small 4-mm-wide portion to heal on its own. The overall outcome was excellent and healed much quicker than leaving the entire scalp defect to heal by secondary intention.
The other methods of closure, such as a 2-to-Z flap, would have been difficult given the orientation of the lesions and the island between them.2 To create this flap, an extensive amount of undermining would have been necessary, leading to serious disruption of the blood and nerve supply and an increased risk for flap necrosis. Creating 1 large wound and repairing with a flap would have similar requirements and complications.
Intraoperative tissue relaxation can be used to allow primary closure of adjacent wounds without the need for undermining. Prior research has shown that 30 minutes of stress relaxation with 20 Newtons of applied tension yields a 65% reduction in wound-closure tension.7 Orienting the devices between 45° to 90° angles to one another creates opposing tension vectors so that the closure of one defect does not prevent the closure of the other defect. Even in cases in which the defects cannot be completely approximated, closing the wound edges to create a smaller central defect can decrease healing time and lead to an excellent cosmetic outcome without the need for a flap or graft.
The SUTUREGARD ISR suture retention bridge also is cost-effective for the surgeon and the patient. The device and suture-guide washer are included in a set that retails for $35 each or $300 for a box of 12.8 The suture most commonly used to secure the device in our practice is 2-0 nylon and retails for approximately $34 for a box of 12,9 which brings the total cost with the device to around $38 per use. The updated Current Procedural Terminology guidelines from the Centers for Medicare & Medicaid Services define that an intermediate repair requires a layered closure and may include, but does not require, limited undermining. A complex linear closure must meet criteria for an intermediate closure plus at least 1 additional criterion, such as exposure of cartilage, bone, or tendons within the defect; extensive undermining; wound-edge debridement; involvement of free margins; or use of a retention suture.10 Use of a suture retention bridge such as the SUTUREGARD ISR device and therefore a retention suture qualifies the repair as a complex linear closure. Overall, use of the device expands the surgeon’s choices for surgical closures and helps to limit the need for larger, more invasive repair procedures.
- McGinness JL, Parlette HL. A novel technique using a rotation flap for repairing adjacent surgical defects. Dermatol Surg. 2006;32:272-275.
- Blattner CM, Perry B, Young J, et al. 2-to-Z flap for reconstruction of adjacent skin defects. J Am Acad Dermatol. 2019;80:E77-E78.
- Blattner CM, Perry B, Young J, et al. The use of a suture retention device to enhance tissue expansion and healing in the repair of scalp and lower leg wounds. JAAD Case Rep. 2018;4:655-661.
- Zivony D, Siegle RJ. Burrow’s wedge advancement flaps for reconstruction of adjacent surgical defects. Dermatol Surg. 2002;28:1162-1164.
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106.
- Christenson LJ, Phillips PK, Weaver AL, et al. Primary closure vs second-intention treatment of skin punch biopsy sites: a randomized trial. Arch Dermatol. 2005;141:1093-1099.
- Lear W, Blattner CM, Mustoe TA, et al. In vivo stress relaxation of human scalp. J Mech Behav Biomed Mater. 2019;97:85-89.
- SUTUREGARD purchasing facts. SUTUREGARD® Medical Inc website. https://suturegard.com/SUTUREGARD-Purchasing-Facts. Accessed October 15, 2020.
- Shop products: suture with needle McKesson nonabsorbable uncoated black suture monofilament nylon size 2-0 18 inch suture 1-needle 26 mm length 3/8 circle reverse cutting needle. McKesson website. https://mms.mckesson.com/catalog?query=1034509. Accessed October 15, 2020.
- Norris S. 2020 CPT updates to wound repair guidelines. Zotec Partners website. http://zotecpartners.com/resources/2020-cpt-updates-to-wound-repair-guidelines/. Published June 4, 2020. Accessed October 21, 2020.
- McGinness JL, Parlette HL. A novel technique using a rotation flap for repairing adjacent surgical defects. Dermatol Surg. 2006;32:272-275.
- Blattner CM, Perry B, Young J, et al. 2-to-Z flap for reconstruction of adjacent skin defects. J Am Acad Dermatol. 2019;80:E77-E78.
- Blattner CM, Perry B, Young J, et al. The use of a suture retention device to enhance tissue expansion and healing in the repair of scalp and lower leg wounds. JAAD Case Rep. 2018;4:655-661.
- Zivony D, Siegle RJ. Burrow’s wedge advancement flaps for reconstruction of adjacent surgical defects. Dermatol Surg. 2002;28:1162-1164.
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106.
- Christenson LJ, Phillips PK, Weaver AL, et al. Primary closure vs second-intention treatment of skin punch biopsy sites: a randomized trial. Arch Dermatol. 2005;141:1093-1099.
- Lear W, Blattner CM, Mustoe TA, et al. In vivo stress relaxation of human scalp. J Mech Behav Biomed Mater. 2019;97:85-89.
- SUTUREGARD purchasing facts. SUTUREGARD® Medical Inc website. https://suturegard.com/SUTUREGARD-Purchasing-Facts. Accessed October 15, 2020.
- Shop products: suture with needle McKesson nonabsorbable uncoated black suture monofilament nylon size 2-0 18 inch suture 1-needle 26 mm length 3/8 circle reverse cutting needle. McKesson website. https://mms.mckesson.com/catalog?query=1034509. Accessed October 15, 2020.
- Norris S. 2020 CPT updates to wound repair guidelines. Zotec Partners website. http://zotecpartners.com/resources/2020-cpt-updates-to-wound-repair-guidelines/. Published June 4, 2020. Accessed October 21, 2020.
Real-world results with checkpoint inhibitors found inferior to trial results
according to research published in JCO Clinical Cancer Informatics.
However, the research also suggests that real-world patients who receive ICIs achieve longer survival than patients on standard-of-care medications.
“Patients receiving ICIs in real-world practice may differ from those enrolled in trials in a variety of ways, including age, race, performance status, and comorbidity burden,” said study author Jerry S.H. Lee, PhD, of the University of Southern California, Los Angeles.
Dr. Lee noted that only 3%-4% of cancer patients participate in clinical trials. In fact, more than half of patients with melanoma and nearly three-quarters of those with non–small cell lung cancer (NSCLC) do not meet criteria for eligibility in clinical trials, he said.
To examine the discrepancies between real-world practice and clinical trials and to better understand which patients receive ICIs in clinical practice, Dr. Lee and colleagues conducted a retrospective analysis using electronic health record data from Veterans Administration (VA) facilities nationwide.
The researchers identified 11,888 cancer patients who were treated with ICIs. The cohort included patients who are underrepresented in pivotal clinical trials, including older, non-White, and/or higher disease-burdened patients.
The majority of patients were treated for NSCLC (51.1%), followed by melanoma (14.4%), renal cell carcinoma (RCC; 8.1%), squamous cell carcinoma of the head and neck (6.8%), urothelial cancer (6.4%), hepatocellular carcinoma (4.5%), and other less common cancer types (8.8%).
Overall survival by indication
In general, median overall survival (OS) in the VA cohort was inferior to median OS reported in clinical trials. However, patients treated with first-line nivolumab for melanoma and second-line pembrolizumab or nivolumab for NSCLC had similar OS in the real-world and trial data.
The researchers did not report exact OS numbers from clinical trials. However, they did report the exact numbers from the VA cohort and show OS differences between the VA cohort and clinical trials graphically.
Among patients in the VA cohort, the median OS was:
- 25.5 months in melanoma patients on first-line nivolumab
- 16.3 months in RCC patients receiving nivolumab in the second line or higher
- 14 months in RCC patients on first-line ipilimumab and nivolumab
- 10.6 months in NSCLC patients on first-line pembrolizumab
- 9.9 months in NSCLC patients receiving pembrolizumab or nivolumab in the second line or higher
- 9.1 months in NSCLC patients on first-line pembrolizumab and platinum-based chemotherapy
- 6.7 months in urothelial cancer patients receiving ICIs in the second line or higher.
A number of factors may have contributed to the shorter OS observed in the VA cohort, according to the researchers. The VA cohort is predominantly male, is older, and has a higher degree of comorbidity, compared with patients in clinical trials.
In addition, no data are available to determine the cause for discontinuation of therapy, and VA patients may have received ICIs after failing multiple lines of previous therapy, while clinical trials may limit patients to only one or two previous lines of therapy.
After stratifying VA patients by frailty status, the OS among non-frail patients was more similar to the OS reported in clinical trials.
“Real-world outcomes from the VA were more similar when adjusted for frailty, which shows the importance of patient diversity in clinical trials,” Dr. Lee said. He added that the definition of frailty among VA patients included potential injury during combat and therefore differs from a generic frailty definition.
ICIs vs. standard care
The researchers also found that VA patients treated with ICIs had longer OS, compared with a cohort of VA patients receiving standard-of-care therapies.
The median OS was as follows:
- In melanoma patients on first-line treatment – 39.29 months with nivolumab and 5.75 months with chemotherapy (P < .001).
- In RCC patients on first-line treatment – 14.01 months with ipilimumab plus nivolumab and 8.63 months with targeted therapy (P = .051).
- In RCC patients on second-line or greater treatment – 12.43 months with nivolumab and 8.09 months with everolimus (P < .001).
- In NSCLC patients on first-line therapy – 8.88 months with pembrolizumab and 6.38 months with a platinum doublet (P < .001).
- In NSCLC patients on first-line combination therapy – 10.59 months with pembrolizumab plus platinum chemotherapy and 6.38 months with a platinum doublet (P < .001).
- In NSCLC patients on second-line or greater therapy – 10.06 months with pembrolizumab or nivolumab and 6.41 months with docetaxel (P < .001).
- In urothelial cancer patients on second-line or greater therapy – 7.66 months with an ICI and 6.31 months with chemotherapy (P = .043).
Help for treatment decisions
“The real-world survival outcomes not only indicate the breadth of indications but also represent patients who tend not to be eligible for immunotherapy trials, based on their health status,” Dr. Lee said. “We hope this dataset of national-level experience provides practicing oncologists evidence to help patients and family members in the process of decision-making about therapy.”
Real-world data can also inform oncologists who face decisions on whether to prescribe or withhold ICIs and patients who face the financial burden of paying for ICIs, he said.
This dataset will be continually updated. The researchers have already added another 10,000 VA patients who have received immunotherapies in the year since the trial began.
“In a longitudinal way, we plan to examine what causes differences in outcomes and continue to find ways to extend care to veterans with a balance of high quality of life,” Dr. Lee said.
“Patients who participate in clinical trials are, on average, younger and healthier than the general population,” said Bora Youn, PhD, a senior biostatistician at Biogen in Cambridge, Mass., who was not involved in this study.
“In the case of immunotherapies, those with poor performance status and autoimmune conditions are often excluded from trials,” Dr. Youn added. “In the real world, these patients can also receive treatments, and clinicians often need to extrapolate the results from clinical trials. It is therefore important to collect real-world data to understand the effectiveness and safety of these therapies in patients with limited evidence.”
Dr. Youn led a real-world study, published in Cancer, of 1,256 Medicare recipients who were diagnosed with NSCLC and received ICI therapy.
“We found that factors associated with poor prognosis in general, such as squamous histology and failure of aggressive prior treatment, are also predictive of decreased survival among those who initiated immunotherapies. Yet, OS of older patients was relatively comparable to those observed in clinical trials,” Dr. Youn said.
“Understanding the real-world effectiveness of these treatments will help improve the evidence base, especially for those underrepresented in clinical trials. These studies can also help identify patients who are most likely to benefit from immunotherapies,” Dr. Youn added.
This study was supported by the VA Office of Research and Development Cooperative Studies Program. Dr. Lee and Dr. Youn disclosed no conflicts of interest.
SOURCE: Jennifer La et al. JCO Clinical Cancer Informatics. 2020:4:918-28.
according to research published in JCO Clinical Cancer Informatics.
However, the research also suggests that real-world patients who receive ICIs achieve longer survival than patients on standard-of-care medications.
“Patients receiving ICIs in real-world practice may differ from those enrolled in trials in a variety of ways, including age, race, performance status, and comorbidity burden,” said study author Jerry S.H. Lee, PhD, of the University of Southern California, Los Angeles.
Dr. Lee noted that only 3%-4% of cancer patients participate in clinical trials. In fact, more than half of patients with melanoma and nearly three-quarters of those with non–small cell lung cancer (NSCLC) do not meet criteria for eligibility in clinical trials, he said.
To examine the discrepancies between real-world practice and clinical trials and to better understand which patients receive ICIs in clinical practice, Dr. Lee and colleagues conducted a retrospective analysis using electronic health record data from Veterans Administration (VA) facilities nationwide.
The researchers identified 11,888 cancer patients who were treated with ICIs. The cohort included patients who are underrepresented in pivotal clinical trials, including older, non-White, and/or higher disease-burdened patients.
The majority of patients were treated for NSCLC (51.1%), followed by melanoma (14.4%), renal cell carcinoma (RCC; 8.1%), squamous cell carcinoma of the head and neck (6.8%), urothelial cancer (6.4%), hepatocellular carcinoma (4.5%), and other less common cancer types (8.8%).
Overall survival by indication
In general, median overall survival (OS) in the VA cohort was inferior to median OS reported in clinical trials. However, patients treated with first-line nivolumab for melanoma and second-line pembrolizumab or nivolumab for NSCLC had similar OS in the real-world and trial data.
The researchers did not report exact OS numbers from clinical trials. However, they did report the exact numbers from the VA cohort and show OS differences between the VA cohort and clinical trials graphically.
Among patients in the VA cohort, the median OS was:
- 25.5 months in melanoma patients on first-line nivolumab
- 16.3 months in RCC patients receiving nivolumab in the second line or higher
- 14 months in RCC patients on first-line ipilimumab and nivolumab
- 10.6 months in NSCLC patients on first-line pembrolizumab
- 9.9 months in NSCLC patients receiving pembrolizumab or nivolumab in the second line or higher
- 9.1 months in NSCLC patients on first-line pembrolizumab and platinum-based chemotherapy
- 6.7 months in urothelial cancer patients receiving ICIs in the second line or higher.
A number of factors may have contributed to the shorter OS observed in the VA cohort, according to the researchers. The VA cohort is predominantly male, is older, and has a higher degree of comorbidity, compared with patients in clinical trials.
In addition, no data are available to determine the cause for discontinuation of therapy, and VA patients may have received ICIs after failing multiple lines of previous therapy, while clinical trials may limit patients to only one or two previous lines of therapy.
After stratifying VA patients by frailty status, the OS among non-frail patients was more similar to the OS reported in clinical trials.
“Real-world outcomes from the VA were more similar when adjusted for frailty, which shows the importance of patient diversity in clinical trials,” Dr. Lee said. He added that the definition of frailty among VA patients included potential injury during combat and therefore differs from a generic frailty definition.
ICIs vs. standard care
The researchers also found that VA patients treated with ICIs had longer OS, compared with a cohort of VA patients receiving standard-of-care therapies.
The median OS was as follows:
- In melanoma patients on first-line treatment – 39.29 months with nivolumab and 5.75 months with chemotherapy (P < .001).
- In RCC patients on first-line treatment – 14.01 months with ipilimumab plus nivolumab and 8.63 months with targeted therapy (P = .051).
- In RCC patients on second-line or greater treatment – 12.43 months with nivolumab and 8.09 months with everolimus (P < .001).
- In NSCLC patients on first-line therapy – 8.88 months with pembrolizumab and 6.38 months with a platinum doublet (P < .001).
- In NSCLC patients on first-line combination therapy – 10.59 months with pembrolizumab plus platinum chemotherapy and 6.38 months with a platinum doublet (P < .001).
- In NSCLC patients on second-line or greater therapy – 10.06 months with pembrolizumab or nivolumab and 6.41 months with docetaxel (P < .001).
- In urothelial cancer patients on second-line or greater therapy – 7.66 months with an ICI and 6.31 months with chemotherapy (P = .043).
Help for treatment decisions
“The real-world survival outcomes not only indicate the breadth of indications but also represent patients who tend not to be eligible for immunotherapy trials, based on their health status,” Dr. Lee said. “We hope this dataset of national-level experience provides practicing oncologists evidence to help patients and family members in the process of decision-making about therapy.”
Real-world data can also inform oncologists who face decisions on whether to prescribe or withhold ICIs and patients who face the financial burden of paying for ICIs, he said.
This dataset will be continually updated. The researchers have already added another 10,000 VA patients who have received immunotherapies in the year since the trial began.
“In a longitudinal way, we plan to examine what causes differences in outcomes and continue to find ways to extend care to veterans with a balance of high quality of life,” Dr. Lee said.
“Patients who participate in clinical trials are, on average, younger and healthier than the general population,” said Bora Youn, PhD, a senior biostatistician at Biogen in Cambridge, Mass., who was not involved in this study.
“In the case of immunotherapies, those with poor performance status and autoimmune conditions are often excluded from trials,” Dr. Youn added. “In the real world, these patients can also receive treatments, and clinicians often need to extrapolate the results from clinical trials. It is therefore important to collect real-world data to understand the effectiveness and safety of these therapies in patients with limited evidence.”
Dr. Youn led a real-world study, published in Cancer, of 1,256 Medicare recipients who were diagnosed with NSCLC and received ICI therapy.
“We found that factors associated with poor prognosis in general, such as squamous histology and failure of aggressive prior treatment, are also predictive of decreased survival among those who initiated immunotherapies. Yet, OS of older patients was relatively comparable to those observed in clinical trials,” Dr. Youn said.
“Understanding the real-world effectiveness of these treatments will help improve the evidence base, especially for those underrepresented in clinical trials. These studies can also help identify patients who are most likely to benefit from immunotherapies,” Dr. Youn added.
This study was supported by the VA Office of Research and Development Cooperative Studies Program. Dr. Lee and Dr. Youn disclosed no conflicts of interest.
SOURCE: Jennifer La et al. JCO Clinical Cancer Informatics. 2020:4:918-28.
according to research published in JCO Clinical Cancer Informatics.
However, the research also suggests that real-world patients who receive ICIs achieve longer survival than patients on standard-of-care medications.
“Patients receiving ICIs in real-world practice may differ from those enrolled in trials in a variety of ways, including age, race, performance status, and comorbidity burden,” said study author Jerry S.H. Lee, PhD, of the University of Southern California, Los Angeles.
Dr. Lee noted that only 3%-4% of cancer patients participate in clinical trials. In fact, more than half of patients with melanoma and nearly three-quarters of those with non–small cell lung cancer (NSCLC) do not meet criteria for eligibility in clinical trials, he said.
To examine the discrepancies between real-world practice and clinical trials and to better understand which patients receive ICIs in clinical practice, Dr. Lee and colleagues conducted a retrospective analysis using electronic health record data from Veterans Administration (VA) facilities nationwide.
The researchers identified 11,888 cancer patients who were treated with ICIs. The cohort included patients who are underrepresented in pivotal clinical trials, including older, non-White, and/or higher disease-burdened patients.
The majority of patients were treated for NSCLC (51.1%), followed by melanoma (14.4%), renal cell carcinoma (RCC; 8.1%), squamous cell carcinoma of the head and neck (6.8%), urothelial cancer (6.4%), hepatocellular carcinoma (4.5%), and other less common cancer types (8.8%).
Overall survival by indication
In general, median overall survival (OS) in the VA cohort was inferior to median OS reported in clinical trials. However, patients treated with first-line nivolumab for melanoma and second-line pembrolizumab or nivolumab for NSCLC had similar OS in the real-world and trial data.
The researchers did not report exact OS numbers from clinical trials. However, they did report the exact numbers from the VA cohort and show OS differences between the VA cohort and clinical trials graphically.
Among patients in the VA cohort, the median OS was:
- 25.5 months in melanoma patients on first-line nivolumab
- 16.3 months in RCC patients receiving nivolumab in the second line or higher
- 14 months in RCC patients on first-line ipilimumab and nivolumab
- 10.6 months in NSCLC patients on first-line pembrolizumab
- 9.9 months in NSCLC patients receiving pembrolizumab or nivolumab in the second line or higher
- 9.1 months in NSCLC patients on first-line pembrolizumab and platinum-based chemotherapy
- 6.7 months in urothelial cancer patients receiving ICIs in the second line or higher.
A number of factors may have contributed to the shorter OS observed in the VA cohort, according to the researchers. The VA cohort is predominantly male, is older, and has a higher degree of comorbidity, compared with patients in clinical trials.
In addition, no data are available to determine the cause for discontinuation of therapy, and VA patients may have received ICIs after failing multiple lines of previous therapy, while clinical trials may limit patients to only one or two previous lines of therapy.
After stratifying VA patients by frailty status, the OS among non-frail patients was more similar to the OS reported in clinical trials.
“Real-world outcomes from the VA were more similar when adjusted for frailty, which shows the importance of patient diversity in clinical trials,” Dr. Lee said. He added that the definition of frailty among VA patients included potential injury during combat and therefore differs from a generic frailty definition.
ICIs vs. standard care
The researchers also found that VA patients treated with ICIs had longer OS, compared with a cohort of VA patients receiving standard-of-care therapies.
The median OS was as follows:
- In melanoma patients on first-line treatment – 39.29 months with nivolumab and 5.75 months with chemotherapy (P < .001).
- In RCC patients on first-line treatment – 14.01 months with ipilimumab plus nivolumab and 8.63 months with targeted therapy (P = .051).
- In RCC patients on second-line or greater treatment – 12.43 months with nivolumab and 8.09 months with everolimus (P < .001).
- In NSCLC patients on first-line therapy – 8.88 months with pembrolizumab and 6.38 months with a platinum doublet (P < .001).
- In NSCLC patients on first-line combination therapy – 10.59 months with pembrolizumab plus platinum chemotherapy and 6.38 months with a platinum doublet (P < .001).
- In NSCLC patients on second-line or greater therapy – 10.06 months with pembrolizumab or nivolumab and 6.41 months with docetaxel (P < .001).
- In urothelial cancer patients on second-line or greater therapy – 7.66 months with an ICI and 6.31 months with chemotherapy (P = .043).
Help for treatment decisions
“The real-world survival outcomes not only indicate the breadth of indications but also represent patients who tend not to be eligible for immunotherapy trials, based on their health status,” Dr. Lee said. “We hope this dataset of national-level experience provides practicing oncologists evidence to help patients and family members in the process of decision-making about therapy.”
Real-world data can also inform oncologists who face decisions on whether to prescribe or withhold ICIs and patients who face the financial burden of paying for ICIs, he said.
This dataset will be continually updated. The researchers have already added another 10,000 VA patients who have received immunotherapies in the year since the trial began.
“In a longitudinal way, we plan to examine what causes differences in outcomes and continue to find ways to extend care to veterans with a balance of high quality of life,” Dr. Lee said.
“Patients who participate in clinical trials are, on average, younger and healthier than the general population,” said Bora Youn, PhD, a senior biostatistician at Biogen in Cambridge, Mass., who was not involved in this study.
“In the case of immunotherapies, those with poor performance status and autoimmune conditions are often excluded from trials,” Dr. Youn added. “In the real world, these patients can also receive treatments, and clinicians often need to extrapolate the results from clinical trials. It is therefore important to collect real-world data to understand the effectiveness and safety of these therapies in patients with limited evidence.”
Dr. Youn led a real-world study, published in Cancer, of 1,256 Medicare recipients who were diagnosed with NSCLC and received ICI therapy.
“We found that factors associated with poor prognosis in general, such as squamous histology and failure of aggressive prior treatment, are also predictive of decreased survival among those who initiated immunotherapies. Yet, OS of older patients was relatively comparable to those observed in clinical trials,” Dr. Youn said.
“Understanding the real-world effectiveness of these treatments will help improve the evidence base, especially for those underrepresented in clinical trials. These studies can also help identify patients who are most likely to benefit from immunotherapies,” Dr. Youn added.
This study was supported by the VA Office of Research and Development Cooperative Studies Program. Dr. Lee and Dr. Youn disclosed no conflicts of interest.
SOURCE: Jennifer La et al. JCO Clinical Cancer Informatics. 2020:4:918-28.
FROM JCO CLINICAL CANCER INFORMATICS
Lower BP and better tumor control with drug combo?
It’s not ready for the clinic, but new research suggests that angiotensin receptor II blockers (ARBs) widely used to treat hypertension may improve responses to cancer immunotherapy agents targeted against the programmed death-1/ligand-1 (PD-1/PD-L1) pathway.
That conclusion comes from an observational study of 597 patients with more than 3 dozen different cancer types treated in clinical trials at the US National Institutes of Health. Investigators found that both objective response rates and 3-year overall survival (OS) rates were significantly higher for patients treated with a PD-1/PD-L1 inhibitor who were on ARBs, compared with patients who weren’t taking the antihypertensive agents.
An association was also seen between higher ORR and OS rates for patients taking ACE inhibitors, but it was not statistically significant, reported Julius Strauss, MD, from the Center for Cancer Research at the National Cancer Institute in Bethesda, Md.
All study patients received PD-1/PD-L1 inhibitors, and the ORR for patients treated with ARBs was 33.8%, compared with 19.5% for those treated with ACE inhibitors, and 17% for those who took neither drug. The respective complete response (CR) rates were 11.3%, 3.7%, and 3.1%.
Strauss discussed the data during an online briefing prior to his presentation of the findings during the 32nd EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics, which is taking place virtually.
Several early studies have suggested that angiotensin II, in addition to its effect on blood pressure, can also affect cancer growth by leading to downstream production of two proteins: vascular endothelial growth factor (VEGF) and transforming growth factor–beta (TGF-beta), he explained.
“Both of these [proteins] have been linked to cancer growth and cancer resistance to immune system attack,” Strauss observed.
He also discussed the mechanics of possible effects. Angiotensin II increases VEGF and TGF-beta through binding to the AT1 receptor, but has the opposite effect when it binds to the AT2 receptor, resulting in a decrease in both of the growth factors, he added.
ACE inhibitors prevent the conversion of angiotensin I to angiotensin II, with the result being that the drugs indirectly block both the AT1 and AT2 receptors.
In contrast, ARBs block only the AT1 receptor and leave the AT2 counter-regulatory receptor alone, said Strauss.
More data, including on overall survival
Strauss and colleagues examined whether ACE inhibitors and/or ARBs could have an effect on the response to PD-1/PD-L1 immune checkpoint inhibitors delivered with or without other immunotherapies, such as anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) checkpoint inhibitors, or targeted agents such as tyrosine kinase inhibitors (TKIs).
They pooled data on 597 patients receiving PD-1/PD-L1 inhibitors in clinical trials for various cancers, including 71 receiving concomitant ARBs, 82 receiving an ACE inhibitor, and 444 who were not receiving either class of antihypertensives.
The above-mentioned improvement in ORR with ARBs compared with patients not receiving the drug was statistically significant (P = .001), as was the improvement in CR rates (P = .002). In contrast, neither ORR nor CR were significantly better with patients on ACE inhibitors compared with patients not taking these drugs.
In multiple regression analysis controlling for age, gender, body mass index (BMI), tumor type, and additional therapies given, the superior ORR and CR rates with ARBs remained (P = .039 and .002, respectively), while there continued to be no significant additional benefit with ACE inhibitors.
The median overall survival was 35.2 months for patients on ARBs, 26.2 months for those on ACE inhibitors, and 18.8 months for patients on neither drug. The respective 3-year OS rates were 48.1%, 37.2%, and 31.5%, with the difference between the ARB and no-drug groups being significant (P = .0078).
In regression analysis controlling for the factors mentioned before, the OS advantage with ARBs but not ACE inhibitors remained significant (P = .006 for ARBs, and .078 for ACE inhibitors).
Strauss emphasized that further study is needed to determine if AT1 blockade can improve outcomes when combined anti-PD-1/PD-L1-based therapy.
It might be reasonable for patients who are taking ACE inhibitors to control blood pressure and are also receiving immunotherapy with a PD-1/PD-L1 inhibitor to be switched to an ARB if it is deemed safe and if further research bears it out, said Strauss in response to a question from Medscape Medical News.
Hypothesis-generating study
Meeting cochair Emiliano Calvo, MD, PhD, from Hospital de Madrid Norte Sanchinarro in Madrid, who attended the media briefing but was not involved in the study, commented that hypothesis-generating research using drugs already on the market for other indications adds important value to cancer therapy.
James Gulley, MD, PhD, from the Center for Cancer Research at the NCI, also a meeting cochair, agreed with Calvo.
“Thinking about utilizing the data that already exists to really get hypothesis-generating questions, it also opens up the possibility for real-world data, real-world evidence from these big datasets from [electronic medical records] that we could really interrogate and understand what we might see and get these hypothesis-generating findings that we could then prospectively evaluate,” Gulley said.
The research was funded by the National Cancer Institute. Strauss and Gulley are National Cancer Institute employees. Calvo disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
It’s not ready for the clinic, but new research suggests that angiotensin receptor II blockers (ARBs) widely used to treat hypertension may improve responses to cancer immunotherapy agents targeted against the programmed death-1/ligand-1 (PD-1/PD-L1) pathway.
That conclusion comes from an observational study of 597 patients with more than 3 dozen different cancer types treated in clinical trials at the US National Institutes of Health. Investigators found that both objective response rates and 3-year overall survival (OS) rates were significantly higher for patients treated with a PD-1/PD-L1 inhibitor who were on ARBs, compared with patients who weren’t taking the antihypertensive agents.
An association was also seen between higher ORR and OS rates for patients taking ACE inhibitors, but it was not statistically significant, reported Julius Strauss, MD, from the Center for Cancer Research at the National Cancer Institute in Bethesda, Md.
All study patients received PD-1/PD-L1 inhibitors, and the ORR for patients treated with ARBs was 33.8%, compared with 19.5% for those treated with ACE inhibitors, and 17% for those who took neither drug. The respective complete response (CR) rates were 11.3%, 3.7%, and 3.1%.
Strauss discussed the data during an online briefing prior to his presentation of the findings during the 32nd EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics, which is taking place virtually.
Several early studies have suggested that angiotensin II, in addition to its effect on blood pressure, can also affect cancer growth by leading to downstream production of two proteins: vascular endothelial growth factor (VEGF) and transforming growth factor–beta (TGF-beta), he explained.
“Both of these [proteins] have been linked to cancer growth and cancer resistance to immune system attack,” Strauss observed.
He also discussed the mechanics of possible effects. Angiotensin II increases VEGF and TGF-beta through binding to the AT1 receptor, but has the opposite effect when it binds to the AT2 receptor, resulting in a decrease in both of the growth factors, he added.
ACE inhibitors prevent the conversion of angiotensin I to angiotensin II, with the result being that the drugs indirectly block both the AT1 and AT2 receptors.
In contrast, ARBs block only the AT1 receptor and leave the AT2 counter-regulatory receptor alone, said Strauss.
More data, including on overall survival
Strauss and colleagues examined whether ACE inhibitors and/or ARBs could have an effect on the response to PD-1/PD-L1 immune checkpoint inhibitors delivered with or without other immunotherapies, such as anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) checkpoint inhibitors, or targeted agents such as tyrosine kinase inhibitors (TKIs).
They pooled data on 597 patients receiving PD-1/PD-L1 inhibitors in clinical trials for various cancers, including 71 receiving concomitant ARBs, 82 receiving an ACE inhibitor, and 444 who were not receiving either class of antihypertensives.
The above-mentioned improvement in ORR with ARBs compared with patients not receiving the drug was statistically significant (P = .001), as was the improvement in CR rates (P = .002). In contrast, neither ORR nor CR were significantly better with patients on ACE inhibitors compared with patients not taking these drugs.
In multiple regression analysis controlling for age, gender, body mass index (BMI), tumor type, and additional therapies given, the superior ORR and CR rates with ARBs remained (P = .039 and .002, respectively), while there continued to be no significant additional benefit with ACE inhibitors.
The median overall survival was 35.2 months for patients on ARBs, 26.2 months for those on ACE inhibitors, and 18.8 months for patients on neither drug. The respective 3-year OS rates were 48.1%, 37.2%, and 31.5%, with the difference between the ARB and no-drug groups being significant (P = .0078).
In regression analysis controlling for the factors mentioned before, the OS advantage with ARBs but not ACE inhibitors remained significant (P = .006 for ARBs, and .078 for ACE inhibitors).
Strauss emphasized that further study is needed to determine if AT1 blockade can improve outcomes when combined anti-PD-1/PD-L1-based therapy.
It might be reasonable for patients who are taking ACE inhibitors to control blood pressure and are also receiving immunotherapy with a PD-1/PD-L1 inhibitor to be switched to an ARB if it is deemed safe and if further research bears it out, said Strauss in response to a question from Medscape Medical News.
Hypothesis-generating study
Meeting cochair Emiliano Calvo, MD, PhD, from Hospital de Madrid Norte Sanchinarro in Madrid, who attended the media briefing but was not involved in the study, commented that hypothesis-generating research using drugs already on the market for other indications adds important value to cancer therapy.
James Gulley, MD, PhD, from the Center for Cancer Research at the NCI, also a meeting cochair, agreed with Calvo.
“Thinking about utilizing the data that already exists to really get hypothesis-generating questions, it also opens up the possibility for real-world data, real-world evidence from these big datasets from [electronic medical records] that we could really interrogate and understand what we might see and get these hypothesis-generating findings that we could then prospectively evaluate,” Gulley said.
The research was funded by the National Cancer Institute. Strauss and Gulley are National Cancer Institute employees. Calvo disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
It’s not ready for the clinic, but new research suggests that angiotensin receptor II blockers (ARBs) widely used to treat hypertension may improve responses to cancer immunotherapy agents targeted against the programmed death-1/ligand-1 (PD-1/PD-L1) pathway.
That conclusion comes from an observational study of 597 patients with more than 3 dozen different cancer types treated in clinical trials at the US National Institutes of Health. Investigators found that both objective response rates and 3-year overall survival (OS) rates were significantly higher for patients treated with a PD-1/PD-L1 inhibitor who were on ARBs, compared with patients who weren’t taking the antihypertensive agents.
An association was also seen between higher ORR and OS rates for patients taking ACE inhibitors, but it was not statistically significant, reported Julius Strauss, MD, from the Center for Cancer Research at the National Cancer Institute in Bethesda, Md.
All study patients received PD-1/PD-L1 inhibitors, and the ORR for patients treated with ARBs was 33.8%, compared with 19.5% for those treated with ACE inhibitors, and 17% for those who took neither drug. The respective complete response (CR) rates were 11.3%, 3.7%, and 3.1%.
Strauss discussed the data during an online briefing prior to his presentation of the findings during the 32nd EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics, which is taking place virtually.
Several early studies have suggested that angiotensin II, in addition to its effect on blood pressure, can also affect cancer growth by leading to downstream production of two proteins: vascular endothelial growth factor (VEGF) and transforming growth factor–beta (TGF-beta), he explained.
“Both of these [proteins] have been linked to cancer growth and cancer resistance to immune system attack,” Strauss observed.
He also discussed the mechanics of possible effects. Angiotensin II increases VEGF and TGF-beta through binding to the AT1 receptor, but has the opposite effect when it binds to the AT2 receptor, resulting in a decrease in both of the growth factors, he added.
ACE inhibitors prevent the conversion of angiotensin I to angiotensin II, with the result being that the drugs indirectly block both the AT1 and AT2 receptors.
In contrast, ARBs block only the AT1 receptor and leave the AT2 counter-regulatory receptor alone, said Strauss.
More data, including on overall survival
Strauss and colleagues examined whether ACE inhibitors and/or ARBs could have an effect on the response to PD-1/PD-L1 immune checkpoint inhibitors delivered with or without other immunotherapies, such as anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) checkpoint inhibitors, or targeted agents such as tyrosine kinase inhibitors (TKIs).
They pooled data on 597 patients receiving PD-1/PD-L1 inhibitors in clinical trials for various cancers, including 71 receiving concomitant ARBs, 82 receiving an ACE inhibitor, and 444 who were not receiving either class of antihypertensives.
The above-mentioned improvement in ORR with ARBs compared with patients not receiving the drug was statistically significant (P = .001), as was the improvement in CR rates (P = .002). In contrast, neither ORR nor CR were significantly better with patients on ACE inhibitors compared with patients not taking these drugs.
In multiple regression analysis controlling for age, gender, body mass index (BMI), tumor type, and additional therapies given, the superior ORR and CR rates with ARBs remained (P = .039 and .002, respectively), while there continued to be no significant additional benefit with ACE inhibitors.
The median overall survival was 35.2 months for patients on ARBs, 26.2 months for those on ACE inhibitors, and 18.8 months for patients on neither drug. The respective 3-year OS rates were 48.1%, 37.2%, and 31.5%, with the difference between the ARB and no-drug groups being significant (P = .0078).
In regression analysis controlling for the factors mentioned before, the OS advantage with ARBs but not ACE inhibitors remained significant (P = .006 for ARBs, and .078 for ACE inhibitors).
Strauss emphasized that further study is needed to determine if AT1 blockade can improve outcomes when combined anti-PD-1/PD-L1-based therapy.
It might be reasonable for patients who are taking ACE inhibitors to control blood pressure and are also receiving immunotherapy with a PD-1/PD-L1 inhibitor to be switched to an ARB if it is deemed safe and if further research bears it out, said Strauss in response to a question from Medscape Medical News.
Hypothesis-generating study
Meeting cochair Emiliano Calvo, MD, PhD, from Hospital de Madrid Norte Sanchinarro in Madrid, who attended the media briefing but was not involved in the study, commented that hypothesis-generating research using drugs already on the market for other indications adds important value to cancer therapy.
James Gulley, MD, PhD, from the Center for Cancer Research at the NCI, also a meeting cochair, agreed with Calvo.
“Thinking about utilizing the data that already exists to really get hypothesis-generating questions, it also opens up the possibility for real-world data, real-world evidence from these big datasets from [electronic medical records] that we could really interrogate and understand what we might see and get these hypothesis-generating findings that we could then prospectively evaluate,” Gulley said.
The research was funded by the National Cancer Institute. Strauss and Gulley are National Cancer Institute employees. Calvo disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Are oncologists ready to confront a second wave of COVID-19?
Canceled appointments, postponed surgeries, and delayed cancer diagnoses – all are a recipe for exhaustion for oncologists around the world, struggling to reach and treat their patients during the pandemic. Physicians and their teams felt the pain as COVID-19 took its initial march around the globe.
“We saw the distress of people with cancer who could no longer get to anyone on the phone. Their medical visit was usually canceled. Their radiotherapy session was postponed or modified, and chemotherapy postponed,” says Axel Kahn, MD, chairman of the board of directors of La Ligue Nationale Contre le Cancer (National League Against Cancer). “In the vast majority of cases, cancer treatment can be postponed or readjusted, without affecting the patient’s chances of survival, but there has been a lot of anxiety because the patients do not know that.”
The stay-at-home factor was one that played out across many months during the first wave.
“I believe that the ‘stay-home’ message that we transmitted was rigorously followed by patients who should have come to the emergency room much earlier and who, therefore, were admitted with a much more deteriorated general condition than in non-COVID-19 times,” says Benjamín Domingo Arrué, MD, from the department of medical oncology at Hospital Universitari i Politècnic La Fe in Valencia, Spain.
And in Brazil, some of the impact from the initial hit of COVID-19 on oncology is only now being felt, according to Laura Testa, MD, head of breast medical oncology, Instituto do Câncer do Estado de São Paulo.
“We are starting to see a lot of cancer cases that didn’t show up at the beginning of the pandemic, but now they are arriving to us already in advanced stages,” she said. “These patients need hospital care. If the situation worsens and goes back to what we saw at the peak of the curve, I fear the public system won’t be able to treat properly the oncology patients that need hospital care and the patients with cancer who also have COVID-19.”
But even as health care worker fatigue and concerns linger, oncologists say that what they have learned in the last 6 months has helped them prepare as COVID-19 cases increase and a second global wave kicks up.
Lessons from the first wave
In the United States, COVID-19 hit different regions at different times and to different degrees. One of the areas hit first was Seattle.
“We jumped on top of this, we were evidence based, we put things in place very, very quickly,” said Julie Gralow, MD, professor at the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle.
“We did a really good job keeping COVID out of our cancer centers,” Dr. Gralow said. “We learned how to be super safe, and to keep symptomatic people out of the building, and to limit the extra people they could bring with them. It’s all about the number of contacts you have.”
The story was different, though, for oncologists in several other countries, and sometimes it varied immensely within each nation.
“We treated fewer patients with cancer during the first wave,” says Dirk Arnold, MD, medical director of the Asklepios Tumor Center Hamburg (Germany), in an interview. “In part, this was because staff were quarantined and because we had a completely different infrastructure in all of the hospitals. But also fewer patients with cancer came to the clinic at all. A lot of resources were directed toward COVID-19.”
In Spain, telemedicine helped keep up with visits, but other areas felt the effect of COVID-19 patient loads.
“At least in the oncology department of our center, we have practically maintained 100% of visits, mostly by telephone,” says Dr. Arrué, “but the reality is that our country has not yet been prepared for telemedicine.”
Laura Mezquita, MD, of the department of medical oncology at Hospital Clinic de Barcelona, describes a more dramatic situation: “We have seen how some of our patients, especially with metastatic disease, have been dismissed for intensive care and life-support treatments, as well as specific treatments against COVID-19 (tocilizumab, remdesivir, etc.) due to the general health collapse of the former wave,” she said. She adds that specific oncologic populations, such as those with thoracic tumors, have been more affected.
Distress among oncologists
Many oncologists are still feeling stressed and fatigued after the first wave, just as a second string of outbreaks is on its way.
A survey presented at last month’s ESMO 2020 Congress found that, in July-August, moral distress was reported by one-third of the oncologists who responded, and more than half reported a feeling of exhaustion.
“The tiredness and team exhaustion is noticeable,” said Dr. Arnold. “We recently had a task force discussion about what will happen when we have a second wave and how the department and our services will adapt. It was clear that those who were at the very front in the first wave had only a limited desire to do that again in the second wave.”
Another concern: COVID-19’s effect on staffing levels.
“We have a population of young caregivers who are affected by the COVID-19 disease with an absenteeism rate that is quite unprecedented,” said Sophie Beaupère, general delegate of Unicancer since January.
She said that, in general, the absenteeism rate in the cancer centers averages 5%-6%, depending on the year. But that rate is now skyrocketing.
Stop-start cycle for surgery
As caregivers quarantined around the world, more than 10% of patients with cancer had treatment canceled or delayed during the first wave of the pandemic, according to another survey from ESMO, involving 109 oncologists from 18 countries.
Difficulties were reported for surgeries by 34% of the centers, but also difficulties with delivering chemotherapy (22% of centers), radiotherapy (13.7%), and therapy with checkpoint inhibitors (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Stopping surgery is a real concern in France, noted Dr. Kahn, the National League Against Cancer chair. He says that in regions that were badly hit by COVID-19, “it was not possible to have access to the operating room for people who absolutely needed surgery; for example, patients with lung cancer that was still operable. Most of the recovery rooms were mobilized for resuscitation.”
There may be some solutions, suggested Thierry Breton, director general of the National Institute of Cancer in France. “We are getting prepared, with the health ministry, for a possible increase in hospital tension, which would lead to a situation where we would have to reschedule operations. Nationally, regionally, and locally, we are seeing how we can resume and prioritize surgeries that have not been done.”
Delays in cancer diagnosis
While COVID-19 affected treatment, many oncologists say the major impact of the first wave was a delay in diagnosing cancer. Some of this was a result of the suspension of cancer screening programs, but there was also fear among the general public about visiting clinics and hospitals during a pandemic.
“We didn’t do so well with cancer during the first wave here in the U.K.,” said Karol Sikora, PhD, MBBChir, professor of cancer medicine and founding dean at the University of Buckingham Medical School, London. “Cancer diagnostic pathways virtually stalled partly because patients didn’t seek help, but getting scans and biopsies was also very difficult. Even patients referred urgently under the ‘2-weeks-wait’ rule were turned down.”
In France, “the delay in diagnosis is indisputable,” said Dr. Kahn. “About 50% of the cancer diagnoses one would expect during this period were missed.”
“I am worried that there remains a major traffic jam that has not been caught up with, and, in the meantime, the health crisis is worsening,” he added.
In Seattle, Dr. Gralow said the first COVID-19 wave had little impact on treatment for breast cancer, but it was in screening for breast cancer “where things really got messed up.”
“Even though we’ve been fully ramped up again,” she said, concerns remain. To ensure that screening mammography is maintained, “we have spaced out the visits to keep our waiting rooms less populated, with a longer time between using the machine so we can clean it. To do this, we have extended operating hours and are now opening on Saturday.
“So we’re actually at 100% of our capacity, but I’m really nervous, though, that a lot of people put off their screening mammogram and aren’t going to come in and get it.
“Not only did people get the message to stay home and not do nonessential things, but I think a lot of people lost their health insurance when they lost their jobs,” she said, and without health insurance, they are not covered for cancer screening.
Looking ahead, with a plan
Many oncologists agree that access to care can and must be improved – and there were some positive moves.
“Some regimens changed during the first months of the pandemic, and I don’t see them going back to the way they were anytime soon,” said Dr. Testa. “The changes/adaptations that were made to minimize the chance of SARS-CoV-2 infection are still in place and will go on for a while. In this context, telemedicine helped a lot. The pandemic forced the stakeholders to step up and put it in place in March. And now it’s here to stay.”
The experience gained in the last several months has driven preparation for the next wave.
“We are not going to see the disorganization that we saw during the first wave,” said Florence Joly, MD, PhD, head of medical oncology at the Centre François Baclesse in Caen, France. “The difference between now and earlier this year is that COVID diagnostic tests are available. That was one of the problems in the first wave. We had no way to diagnose.”
On the East Coast of the United States, medical oncologist Charu Aggarwal, MD, MPH, is also optimistic: “I think we’re at a place where we can manage.”
“I believe if there was going to be a new wave of COVID-19 cases we would be: better psychologically prepared and better organized,” said Dr. Aggarwal, assistant professor of medicine in the hematology-oncology division at the University of Pennsylvania, Philadelphia. “We already have experience with all of the tools, we have telemedicine available, we have screening protocols available, we have testing, we are already universally masking, everyone’s hand-washing, so I do think that means we would be okay.”
Dr. Arnold agreed that “we are much better prepared than for the first wave, but … we have immense tasks in the area of patient management, the digitization of patient care, the clear allocation of resources when there is a second or third wave. In many areas of preparation, I believe, unfortunately, we are not as well positioned as we had actually hoped.”
The first wave of COVID hit cancer services in the United Kingdom particularly hard: One modeling study suggested that delays in cancer referrals will lead to thousands of additional deaths and tens of thousands of life-years lost.
“Cancer services are working at near normal levels now, but they are still fragile and could be severely compromised again if the NHS [National Health Service] gets flooded by COVID patients,” said Dr. Sikora.
The second wave may be different. “Although the number of infections has increased, the hospitalizations have only risen a little. Let’s see what happens,” he said in an interview. Since then, however, infections have continued to rise, and there has been an increase in hospitalizations. New social distancing measures in the United Kingdom were put into place on Oct. 12, with the aim of protecting the NHS from overload.
Dr. Arrué describes it this way: “The reality is that the ‘second wave’ has left behind the initial grief and shock that both patients and health professionals experienced when faced with something that, until now, we had only seen in the movies.” The second wave has led to new restrictions – including a partial lockdown since the beginning of October.
Dr. Aggarwal says her department recently had a conference with Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, about the impact of COVID-19 on oncology.
“I asked him what advice he’d give oncologists, and he said to go back to as much screening as you were doing previously as quickly as possible. That’s what must be relayed to our oncologists in the community – and also to primary care physicians – because they are often the ones who are ordering and championing the screening efforts.”
This article was originated by Aude Lecrubier, Medscape French edition, and developed by Zosia Chustecka, Medscape Oncology. With additional reporting by Kate Johnson, freelance medical journalist, Claudia Gottschling for Medscape Germany, Leoleli Schwartz for Medscape em português, Tim Locke for Medscape United Kingdom, and Carla Nieto Martínez, freelance medical journalist for Medscape Spanish edition.
This article first appeared on Medscape.com.
Canceled appointments, postponed surgeries, and delayed cancer diagnoses – all are a recipe for exhaustion for oncologists around the world, struggling to reach and treat their patients during the pandemic. Physicians and their teams felt the pain as COVID-19 took its initial march around the globe.
“We saw the distress of people with cancer who could no longer get to anyone on the phone. Their medical visit was usually canceled. Their radiotherapy session was postponed or modified, and chemotherapy postponed,” says Axel Kahn, MD, chairman of the board of directors of La Ligue Nationale Contre le Cancer (National League Against Cancer). “In the vast majority of cases, cancer treatment can be postponed or readjusted, without affecting the patient’s chances of survival, but there has been a lot of anxiety because the patients do not know that.”
The stay-at-home factor was one that played out across many months during the first wave.
“I believe that the ‘stay-home’ message that we transmitted was rigorously followed by patients who should have come to the emergency room much earlier and who, therefore, were admitted with a much more deteriorated general condition than in non-COVID-19 times,” says Benjamín Domingo Arrué, MD, from the department of medical oncology at Hospital Universitari i Politècnic La Fe in Valencia, Spain.
And in Brazil, some of the impact from the initial hit of COVID-19 on oncology is only now being felt, according to Laura Testa, MD, head of breast medical oncology, Instituto do Câncer do Estado de São Paulo.
“We are starting to see a lot of cancer cases that didn’t show up at the beginning of the pandemic, but now they are arriving to us already in advanced stages,” she said. “These patients need hospital care. If the situation worsens and goes back to what we saw at the peak of the curve, I fear the public system won’t be able to treat properly the oncology patients that need hospital care and the patients with cancer who also have COVID-19.”
But even as health care worker fatigue and concerns linger, oncologists say that what they have learned in the last 6 months has helped them prepare as COVID-19 cases increase and a second global wave kicks up.
Lessons from the first wave
In the United States, COVID-19 hit different regions at different times and to different degrees. One of the areas hit first was Seattle.
“We jumped on top of this, we were evidence based, we put things in place very, very quickly,” said Julie Gralow, MD, professor at the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle.
“We did a really good job keeping COVID out of our cancer centers,” Dr. Gralow said. “We learned how to be super safe, and to keep symptomatic people out of the building, and to limit the extra people they could bring with them. It’s all about the number of contacts you have.”
The story was different, though, for oncologists in several other countries, and sometimes it varied immensely within each nation.
“We treated fewer patients with cancer during the first wave,” says Dirk Arnold, MD, medical director of the Asklepios Tumor Center Hamburg (Germany), in an interview. “In part, this was because staff were quarantined and because we had a completely different infrastructure in all of the hospitals. But also fewer patients with cancer came to the clinic at all. A lot of resources were directed toward COVID-19.”
In Spain, telemedicine helped keep up with visits, but other areas felt the effect of COVID-19 patient loads.
“At least in the oncology department of our center, we have practically maintained 100% of visits, mostly by telephone,” says Dr. Arrué, “but the reality is that our country has not yet been prepared for telemedicine.”
Laura Mezquita, MD, of the department of medical oncology at Hospital Clinic de Barcelona, describes a more dramatic situation: “We have seen how some of our patients, especially with metastatic disease, have been dismissed for intensive care and life-support treatments, as well as specific treatments against COVID-19 (tocilizumab, remdesivir, etc.) due to the general health collapse of the former wave,” she said. She adds that specific oncologic populations, such as those with thoracic tumors, have been more affected.
Distress among oncologists
Many oncologists are still feeling stressed and fatigued after the first wave, just as a second string of outbreaks is on its way.
A survey presented at last month’s ESMO 2020 Congress found that, in July-August, moral distress was reported by one-third of the oncologists who responded, and more than half reported a feeling of exhaustion.
“The tiredness and team exhaustion is noticeable,” said Dr. Arnold. “We recently had a task force discussion about what will happen when we have a second wave and how the department and our services will adapt. It was clear that those who were at the very front in the first wave had only a limited desire to do that again in the second wave.”
Another concern: COVID-19’s effect on staffing levels.
“We have a population of young caregivers who are affected by the COVID-19 disease with an absenteeism rate that is quite unprecedented,” said Sophie Beaupère, general delegate of Unicancer since January.
She said that, in general, the absenteeism rate in the cancer centers averages 5%-6%, depending on the year. But that rate is now skyrocketing.
Stop-start cycle for surgery
As caregivers quarantined around the world, more than 10% of patients with cancer had treatment canceled or delayed during the first wave of the pandemic, according to another survey from ESMO, involving 109 oncologists from 18 countries.
Difficulties were reported for surgeries by 34% of the centers, but also difficulties with delivering chemotherapy (22% of centers), radiotherapy (13.7%), and therapy with checkpoint inhibitors (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Stopping surgery is a real concern in France, noted Dr. Kahn, the National League Against Cancer chair. He says that in regions that were badly hit by COVID-19, “it was not possible to have access to the operating room for people who absolutely needed surgery; for example, patients with lung cancer that was still operable. Most of the recovery rooms were mobilized for resuscitation.”
There may be some solutions, suggested Thierry Breton, director general of the National Institute of Cancer in France. “We are getting prepared, with the health ministry, for a possible increase in hospital tension, which would lead to a situation where we would have to reschedule operations. Nationally, regionally, and locally, we are seeing how we can resume and prioritize surgeries that have not been done.”
Delays in cancer diagnosis
While COVID-19 affected treatment, many oncologists say the major impact of the first wave was a delay in diagnosing cancer. Some of this was a result of the suspension of cancer screening programs, but there was also fear among the general public about visiting clinics and hospitals during a pandemic.
“We didn’t do so well with cancer during the first wave here in the U.K.,” said Karol Sikora, PhD, MBBChir, professor of cancer medicine and founding dean at the University of Buckingham Medical School, London. “Cancer diagnostic pathways virtually stalled partly because patients didn’t seek help, but getting scans and biopsies was also very difficult. Even patients referred urgently under the ‘2-weeks-wait’ rule were turned down.”
In France, “the delay in diagnosis is indisputable,” said Dr. Kahn. “About 50% of the cancer diagnoses one would expect during this period were missed.”
“I am worried that there remains a major traffic jam that has not been caught up with, and, in the meantime, the health crisis is worsening,” he added.
In Seattle, Dr. Gralow said the first COVID-19 wave had little impact on treatment for breast cancer, but it was in screening for breast cancer “where things really got messed up.”
“Even though we’ve been fully ramped up again,” she said, concerns remain. To ensure that screening mammography is maintained, “we have spaced out the visits to keep our waiting rooms less populated, with a longer time between using the machine so we can clean it. To do this, we have extended operating hours and are now opening on Saturday.
“So we’re actually at 100% of our capacity, but I’m really nervous, though, that a lot of people put off their screening mammogram and aren’t going to come in and get it.
“Not only did people get the message to stay home and not do nonessential things, but I think a lot of people lost their health insurance when they lost their jobs,” she said, and without health insurance, they are not covered for cancer screening.
Looking ahead, with a plan
Many oncologists agree that access to care can and must be improved – and there were some positive moves.
“Some regimens changed during the first months of the pandemic, and I don’t see them going back to the way they were anytime soon,” said Dr. Testa. “The changes/adaptations that were made to minimize the chance of SARS-CoV-2 infection are still in place and will go on for a while. In this context, telemedicine helped a lot. The pandemic forced the stakeholders to step up and put it in place in March. And now it’s here to stay.”
The experience gained in the last several months has driven preparation for the next wave.
“We are not going to see the disorganization that we saw during the first wave,” said Florence Joly, MD, PhD, head of medical oncology at the Centre François Baclesse in Caen, France. “The difference between now and earlier this year is that COVID diagnostic tests are available. That was one of the problems in the first wave. We had no way to diagnose.”
On the East Coast of the United States, medical oncologist Charu Aggarwal, MD, MPH, is also optimistic: “I think we’re at a place where we can manage.”
“I believe if there was going to be a new wave of COVID-19 cases we would be: better psychologically prepared and better organized,” said Dr. Aggarwal, assistant professor of medicine in the hematology-oncology division at the University of Pennsylvania, Philadelphia. “We already have experience with all of the tools, we have telemedicine available, we have screening protocols available, we have testing, we are already universally masking, everyone’s hand-washing, so I do think that means we would be okay.”
Dr. Arnold agreed that “we are much better prepared than for the first wave, but … we have immense tasks in the area of patient management, the digitization of patient care, the clear allocation of resources when there is a second or third wave. In many areas of preparation, I believe, unfortunately, we are not as well positioned as we had actually hoped.”
The first wave of COVID hit cancer services in the United Kingdom particularly hard: One modeling study suggested that delays in cancer referrals will lead to thousands of additional deaths and tens of thousands of life-years lost.
“Cancer services are working at near normal levels now, but they are still fragile and could be severely compromised again if the NHS [National Health Service] gets flooded by COVID patients,” said Dr. Sikora.
The second wave may be different. “Although the number of infections has increased, the hospitalizations have only risen a little. Let’s see what happens,” he said in an interview. Since then, however, infections have continued to rise, and there has been an increase in hospitalizations. New social distancing measures in the United Kingdom were put into place on Oct. 12, with the aim of protecting the NHS from overload.
Dr. Arrué describes it this way: “The reality is that the ‘second wave’ has left behind the initial grief and shock that both patients and health professionals experienced when faced with something that, until now, we had only seen in the movies.” The second wave has led to new restrictions – including a partial lockdown since the beginning of October.
Dr. Aggarwal says her department recently had a conference with Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, about the impact of COVID-19 on oncology.
“I asked him what advice he’d give oncologists, and he said to go back to as much screening as you were doing previously as quickly as possible. That’s what must be relayed to our oncologists in the community – and also to primary care physicians – because they are often the ones who are ordering and championing the screening efforts.”
This article was originated by Aude Lecrubier, Medscape French edition, and developed by Zosia Chustecka, Medscape Oncology. With additional reporting by Kate Johnson, freelance medical journalist, Claudia Gottschling for Medscape Germany, Leoleli Schwartz for Medscape em português, Tim Locke for Medscape United Kingdom, and Carla Nieto Martínez, freelance medical journalist for Medscape Spanish edition.
This article first appeared on Medscape.com.
Canceled appointments, postponed surgeries, and delayed cancer diagnoses – all are a recipe for exhaustion for oncologists around the world, struggling to reach and treat their patients during the pandemic. Physicians and their teams felt the pain as COVID-19 took its initial march around the globe.
“We saw the distress of people with cancer who could no longer get to anyone on the phone. Their medical visit was usually canceled. Their radiotherapy session was postponed or modified, and chemotherapy postponed,” says Axel Kahn, MD, chairman of the board of directors of La Ligue Nationale Contre le Cancer (National League Against Cancer). “In the vast majority of cases, cancer treatment can be postponed or readjusted, without affecting the patient’s chances of survival, but there has been a lot of anxiety because the patients do not know that.”
The stay-at-home factor was one that played out across many months during the first wave.
“I believe that the ‘stay-home’ message that we transmitted was rigorously followed by patients who should have come to the emergency room much earlier and who, therefore, were admitted with a much more deteriorated general condition than in non-COVID-19 times,” says Benjamín Domingo Arrué, MD, from the department of medical oncology at Hospital Universitari i Politècnic La Fe in Valencia, Spain.
And in Brazil, some of the impact from the initial hit of COVID-19 on oncology is only now being felt, according to Laura Testa, MD, head of breast medical oncology, Instituto do Câncer do Estado de São Paulo.
“We are starting to see a lot of cancer cases that didn’t show up at the beginning of the pandemic, but now they are arriving to us already in advanced stages,” she said. “These patients need hospital care. If the situation worsens and goes back to what we saw at the peak of the curve, I fear the public system won’t be able to treat properly the oncology patients that need hospital care and the patients with cancer who also have COVID-19.”
But even as health care worker fatigue and concerns linger, oncologists say that what they have learned in the last 6 months has helped them prepare as COVID-19 cases increase and a second global wave kicks up.
Lessons from the first wave
In the United States, COVID-19 hit different regions at different times and to different degrees. One of the areas hit first was Seattle.
“We jumped on top of this, we were evidence based, we put things in place very, very quickly,” said Julie Gralow, MD, professor at the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle.
“We did a really good job keeping COVID out of our cancer centers,” Dr. Gralow said. “We learned how to be super safe, and to keep symptomatic people out of the building, and to limit the extra people they could bring with them. It’s all about the number of contacts you have.”
The story was different, though, for oncologists in several other countries, and sometimes it varied immensely within each nation.
“We treated fewer patients with cancer during the first wave,” says Dirk Arnold, MD, medical director of the Asklepios Tumor Center Hamburg (Germany), in an interview. “In part, this was because staff were quarantined and because we had a completely different infrastructure in all of the hospitals. But also fewer patients with cancer came to the clinic at all. A lot of resources were directed toward COVID-19.”
In Spain, telemedicine helped keep up with visits, but other areas felt the effect of COVID-19 patient loads.
“At least in the oncology department of our center, we have practically maintained 100% of visits, mostly by telephone,” says Dr. Arrué, “but the reality is that our country has not yet been prepared for telemedicine.”
Laura Mezquita, MD, of the department of medical oncology at Hospital Clinic de Barcelona, describes a more dramatic situation: “We have seen how some of our patients, especially with metastatic disease, have been dismissed for intensive care and life-support treatments, as well as specific treatments against COVID-19 (tocilizumab, remdesivir, etc.) due to the general health collapse of the former wave,” she said. She adds that specific oncologic populations, such as those with thoracic tumors, have been more affected.
Distress among oncologists
Many oncologists are still feeling stressed and fatigued after the first wave, just as a second string of outbreaks is on its way.
A survey presented at last month’s ESMO 2020 Congress found that, in July-August, moral distress was reported by one-third of the oncologists who responded, and more than half reported a feeling of exhaustion.
“The tiredness and team exhaustion is noticeable,” said Dr. Arnold. “We recently had a task force discussion about what will happen when we have a second wave and how the department and our services will adapt. It was clear that those who were at the very front in the first wave had only a limited desire to do that again in the second wave.”
Another concern: COVID-19’s effect on staffing levels.
“We have a population of young caregivers who are affected by the COVID-19 disease with an absenteeism rate that is quite unprecedented,” said Sophie Beaupère, general delegate of Unicancer since January.
She said that, in general, the absenteeism rate in the cancer centers averages 5%-6%, depending on the year. But that rate is now skyrocketing.
Stop-start cycle for surgery
As caregivers quarantined around the world, more than 10% of patients with cancer had treatment canceled or delayed during the first wave of the pandemic, according to another survey from ESMO, involving 109 oncologists from 18 countries.
Difficulties were reported for surgeries by 34% of the centers, but also difficulties with delivering chemotherapy (22% of centers), radiotherapy (13.7%), and therapy with checkpoint inhibitors (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Stopping surgery is a real concern in France, noted Dr. Kahn, the National League Against Cancer chair. He says that in regions that were badly hit by COVID-19, “it was not possible to have access to the operating room for people who absolutely needed surgery; for example, patients with lung cancer that was still operable. Most of the recovery rooms were mobilized for resuscitation.”
There may be some solutions, suggested Thierry Breton, director general of the National Institute of Cancer in France. “We are getting prepared, with the health ministry, for a possible increase in hospital tension, which would lead to a situation where we would have to reschedule operations. Nationally, regionally, and locally, we are seeing how we can resume and prioritize surgeries that have not been done.”
Delays in cancer diagnosis
While COVID-19 affected treatment, many oncologists say the major impact of the first wave was a delay in diagnosing cancer. Some of this was a result of the suspension of cancer screening programs, but there was also fear among the general public about visiting clinics and hospitals during a pandemic.
“We didn’t do so well with cancer during the first wave here in the U.K.,” said Karol Sikora, PhD, MBBChir, professor of cancer medicine and founding dean at the University of Buckingham Medical School, London. “Cancer diagnostic pathways virtually stalled partly because patients didn’t seek help, but getting scans and biopsies was also very difficult. Even patients referred urgently under the ‘2-weeks-wait’ rule were turned down.”
In France, “the delay in diagnosis is indisputable,” said Dr. Kahn. “About 50% of the cancer diagnoses one would expect during this period were missed.”
“I am worried that there remains a major traffic jam that has not been caught up with, and, in the meantime, the health crisis is worsening,” he added.
In Seattle, Dr. Gralow said the first COVID-19 wave had little impact on treatment for breast cancer, but it was in screening for breast cancer “where things really got messed up.”
“Even though we’ve been fully ramped up again,” she said, concerns remain. To ensure that screening mammography is maintained, “we have spaced out the visits to keep our waiting rooms less populated, with a longer time between using the machine so we can clean it. To do this, we have extended operating hours and are now opening on Saturday.
“So we’re actually at 100% of our capacity, but I’m really nervous, though, that a lot of people put off their screening mammogram and aren’t going to come in and get it.
“Not only did people get the message to stay home and not do nonessential things, but I think a lot of people lost their health insurance when they lost their jobs,” she said, and without health insurance, they are not covered for cancer screening.
Looking ahead, with a plan
Many oncologists agree that access to care can and must be improved – and there were some positive moves.
“Some regimens changed during the first months of the pandemic, and I don’t see them going back to the way they were anytime soon,” said Dr. Testa. “The changes/adaptations that were made to minimize the chance of SARS-CoV-2 infection are still in place and will go on for a while. In this context, telemedicine helped a lot. The pandemic forced the stakeholders to step up and put it in place in March. And now it’s here to stay.”
The experience gained in the last several months has driven preparation for the next wave.
“We are not going to see the disorganization that we saw during the first wave,” said Florence Joly, MD, PhD, head of medical oncology at the Centre François Baclesse in Caen, France. “The difference between now and earlier this year is that COVID diagnostic tests are available. That was one of the problems in the first wave. We had no way to diagnose.”
On the East Coast of the United States, medical oncologist Charu Aggarwal, MD, MPH, is also optimistic: “I think we’re at a place where we can manage.”
“I believe if there was going to be a new wave of COVID-19 cases we would be: better psychologically prepared and better organized,” said Dr. Aggarwal, assistant professor of medicine in the hematology-oncology division at the University of Pennsylvania, Philadelphia. “We already have experience with all of the tools, we have telemedicine available, we have screening protocols available, we have testing, we are already universally masking, everyone’s hand-washing, so I do think that means we would be okay.”
Dr. Arnold agreed that “we are much better prepared than for the first wave, but … we have immense tasks in the area of patient management, the digitization of patient care, the clear allocation of resources when there is a second or third wave. In many areas of preparation, I believe, unfortunately, we are not as well positioned as we had actually hoped.”
The first wave of COVID hit cancer services in the United Kingdom particularly hard: One modeling study suggested that delays in cancer referrals will lead to thousands of additional deaths and tens of thousands of life-years lost.
“Cancer services are working at near normal levels now, but they are still fragile and could be severely compromised again if the NHS [National Health Service] gets flooded by COVID patients,” said Dr. Sikora.
The second wave may be different. “Although the number of infections has increased, the hospitalizations have only risen a little. Let’s see what happens,” he said in an interview. Since then, however, infections have continued to rise, and there has been an increase in hospitalizations. New social distancing measures in the United Kingdom were put into place on Oct. 12, with the aim of protecting the NHS from overload.
Dr. Arrué describes it this way: “The reality is that the ‘second wave’ has left behind the initial grief and shock that both patients and health professionals experienced when faced with something that, until now, we had only seen in the movies.” The second wave has led to new restrictions – including a partial lockdown since the beginning of October.
Dr. Aggarwal says her department recently had a conference with Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, about the impact of COVID-19 on oncology.
“I asked him what advice he’d give oncologists, and he said to go back to as much screening as you were doing previously as quickly as possible. That’s what must be relayed to our oncologists in the community – and also to primary care physicians – because they are often the ones who are ordering and championing the screening efforts.”
This article was originated by Aude Lecrubier, Medscape French edition, and developed by Zosia Chustecka, Medscape Oncology. With additional reporting by Kate Johnson, freelance medical journalist, Claudia Gottschling for Medscape Germany, Leoleli Schwartz for Medscape em português, Tim Locke for Medscape United Kingdom, and Carla Nieto Martínez, freelance medical journalist for Medscape Spanish edition.
This article first appeared on Medscape.com.
Survey of Mohs surgeons highlights its use in invasive melanoma
of members of the American College of Mohs Surgery.
Of 513 survey participants, 40.9% reported using MMS to treat any subtype of melanoma. Most of these surgeons reported treating both lentigo maligna (97.5%) and other melanoma in situ (MIS) subtypes (91.4%). A slight majority – 58.6% – reported treating invasive T1 melanoma, and 20.5% reported treating invasive T2 and/or higher-stage melanoma with MMS.
The analysis, published in Dermatologic Surgery, was done by Spyros M. Siscos, MD, and a team of residents and faculty in the division of dermatology at the University of Kansas Medical Center, Kansas City.
It comes on the heels of an analysis of claims data for Mohs surgery, published last year in JAMA Dermatology, which showed a more than threefold increase in the use of Mohs surgery for melanoma from 2.6% of all surgical cases in 2001 to 7.9% in 2016.
With the increased use of MMS for treatment of melanoma, “Mohs surgeons who previously treated MIS with MMS may be increasingly doing so and/or expanding their scope of treatment to include invasive melanoma,” the University of Kansas investigators wrote.
That a slight majority now report treating invasive melanoma with MMS “may be due, in part, to upstaging during the MMS procedure and the increasing evidence demonstrating improved survival of early-invasive melanoma treated with MMS compared with [wide local excision],” as well as the advent of melanocytic immunohistochemical (IHC) stains, particularly melanoma antigen recognized by T-cells 1 (MART-1), they said. However, 29% of surveyed Mohs surgeons treating melanoma with MMS do not use IHC stains “despite growing evidence supporting” their use, the authors wrote.
The advent of IHC stains, particularly MART-1, has improved the accuracy of interpreting frozen sections of melanoma, they reported, noting that MMS without IHC has been associated with a recurrence rate as high as 33%. Of the 71% who reported using IHC stains, MART-1 was the primary IHC stain for virtually all of them (97.3%).
There was also variation in the number of surgeons who reported debulking MIS. Eighty-two percent take this approach, excising the clinically visible tumor before excising the initial Mohs stage – almost all with a scalpel. More than half of these surgeons – 58.5% – submit the entire debulked MIS specimen for permanent vertical sectioning (breadloafing) to evaluate for deeper tumor invasion.
The others reported submitting the entire debulked specimen for frozen vertical sectioning, or portions of the specimen for both permanent and frozen vertical sectioning. “It is unclear why a minority of surveyed Mohs surgeons reported not debulking MIS,” wrote Dr. Siscos and his colleagues.
The average margin size of the first Mohs stage for MIS was 4.96 ± 1.74 mm, which is at the lower end of the 0.5-1.0 cm range for wide local excision (WLE) recommended by the National Comprehensive Cancer Network (NCCN) and the American Academy of Dermatology (AAD), according to a clinical practice guideline. (The survey did not investigate initial margins for invasive melanoma treated with MMS.)
Jeremy R. Etzhorn, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and an author of a 2019 claims data analysis of excisional surgery practices for melanoma, said that the new survey findings – like the prior analysis – highlight the variability in approaches to using MMS for melanoma.
“Mohs for melanoma [seems] like a one-liner ... but really, there are [a lot] of different techniques that fall under that umbrella, if you parse out all the variations,” he said in an interview.
Per the 2016 claims analysis, he noted, IHC was used in less than 40% of Mohs surgery cases for melanoma, and there were wide geographic variations. “The biggest critique of Mohs surgery for melanoma over the last two decades has been that it’s hard to see the tumor,” he said. “But with the advent of IHC, that challenge was overcome.”
Surgical excision practices are evolving without the development of best practice guidelines, said Dr. Etzkorn, who is director of clinical research for the University of Pennsylvania dermatologic oncology center. Multisociety guidelines published in 2012 on appropriate use criteria for Mohs surgery do not offer specific recommendations on the use of MMS for invasive melanoma. Nor do guidelines from the AAD and the NCCN, he said.
“What this [new] study highlights and what’s being discussed amongst Moh’s surgeons” is that Mohs for melanoma “has be to be standardized” to some extent and then clinical trials conducted comparing Mohs to conventional excision. The studies that have been published in recent years comparing MMS with WLE for MIS and invasive melanoma are “not gold standard studies,” he said.
Practice guidelines then can be informed by high-quality evidence on its safety and efficacy, he said.
The 513 participants in the newly published survey represent a 31.5% response rate. Invasive T2 and/or higher stage melanoma was more likely to be treated with MMS in academic hospitals, compared with other practice settings (30.2% v. 18.1%), Dr. Siscos and his coauthors reported.
Participants who reported treating melanoma with MMS were more likely to report fellowship exposure and more likely to have received fellowship training on melanocytic IHC stains. The study “highlights the importance of fellowship exposure to MMS and IHC staining for melanoma,” the authors wrote, adding that postfellowship training opportunities in MMS and IHC staining for melanoma may help broaden its use among Mohs surgeons who received inadequate fellowship exposure.
Dr. Siscos and his colleagues reported no significant interest with commercial supporters. Dr. Etzkorn had no disclosures.
SOURCE: Siscos S et al. Dermatol Surg. 2020 Oct;46(10):1267-71.
of members of the American College of Mohs Surgery.
Of 513 survey participants, 40.9% reported using MMS to treat any subtype of melanoma. Most of these surgeons reported treating both lentigo maligna (97.5%) and other melanoma in situ (MIS) subtypes (91.4%). A slight majority – 58.6% – reported treating invasive T1 melanoma, and 20.5% reported treating invasive T2 and/or higher-stage melanoma with MMS.
The analysis, published in Dermatologic Surgery, was done by Spyros M. Siscos, MD, and a team of residents and faculty in the division of dermatology at the University of Kansas Medical Center, Kansas City.
It comes on the heels of an analysis of claims data for Mohs surgery, published last year in JAMA Dermatology, which showed a more than threefold increase in the use of Mohs surgery for melanoma from 2.6% of all surgical cases in 2001 to 7.9% in 2016.
With the increased use of MMS for treatment of melanoma, “Mohs surgeons who previously treated MIS with MMS may be increasingly doing so and/or expanding their scope of treatment to include invasive melanoma,” the University of Kansas investigators wrote.
That a slight majority now report treating invasive melanoma with MMS “may be due, in part, to upstaging during the MMS procedure and the increasing evidence demonstrating improved survival of early-invasive melanoma treated with MMS compared with [wide local excision],” as well as the advent of melanocytic immunohistochemical (IHC) stains, particularly melanoma antigen recognized by T-cells 1 (MART-1), they said. However, 29% of surveyed Mohs surgeons treating melanoma with MMS do not use IHC stains “despite growing evidence supporting” their use, the authors wrote.
The advent of IHC stains, particularly MART-1, has improved the accuracy of interpreting frozen sections of melanoma, they reported, noting that MMS without IHC has been associated with a recurrence rate as high as 33%. Of the 71% who reported using IHC stains, MART-1 was the primary IHC stain for virtually all of them (97.3%).
There was also variation in the number of surgeons who reported debulking MIS. Eighty-two percent take this approach, excising the clinically visible tumor before excising the initial Mohs stage – almost all with a scalpel. More than half of these surgeons – 58.5% – submit the entire debulked MIS specimen for permanent vertical sectioning (breadloafing) to evaluate for deeper tumor invasion.
The others reported submitting the entire debulked specimen for frozen vertical sectioning, or portions of the specimen for both permanent and frozen vertical sectioning. “It is unclear why a minority of surveyed Mohs surgeons reported not debulking MIS,” wrote Dr. Siscos and his colleagues.
The average margin size of the first Mohs stage for MIS was 4.96 ± 1.74 mm, which is at the lower end of the 0.5-1.0 cm range for wide local excision (WLE) recommended by the National Comprehensive Cancer Network (NCCN) and the American Academy of Dermatology (AAD), according to a clinical practice guideline. (The survey did not investigate initial margins for invasive melanoma treated with MMS.)
Jeremy R. Etzhorn, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and an author of a 2019 claims data analysis of excisional surgery practices for melanoma, said that the new survey findings – like the prior analysis – highlight the variability in approaches to using MMS for melanoma.
“Mohs for melanoma [seems] like a one-liner ... but really, there are [a lot] of different techniques that fall under that umbrella, if you parse out all the variations,” he said in an interview.
Per the 2016 claims analysis, he noted, IHC was used in less than 40% of Mohs surgery cases for melanoma, and there were wide geographic variations. “The biggest critique of Mohs surgery for melanoma over the last two decades has been that it’s hard to see the tumor,” he said. “But with the advent of IHC, that challenge was overcome.”
Surgical excision practices are evolving without the development of best practice guidelines, said Dr. Etzkorn, who is director of clinical research for the University of Pennsylvania dermatologic oncology center. Multisociety guidelines published in 2012 on appropriate use criteria for Mohs surgery do not offer specific recommendations on the use of MMS for invasive melanoma. Nor do guidelines from the AAD and the NCCN, he said.
“What this [new] study highlights and what’s being discussed amongst Moh’s surgeons” is that Mohs for melanoma “has be to be standardized” to some extent and then clinical trials conducted comparing Mohs to conventional excision. The studies that have been published in recent years comparing MMS with WLE for MIS and invasive melanoma are “not gold standard studies,” he said.
Practice guidelines then can be informed by high-quality evidence on its safety and efficacy, he said.
The 513 participants in the newly published survey represent a 31.5% response rate. Invasive T2 and/or higher stage melanoma was more likely to be treated with MMS in academic hospitals, compared with other practice settings (30.2% v. 18.1%), Dr. Siscos and his coauthors reported.
Participants who reported treating melanoma with MMS were more likely to report fellowship exposure and more likely to have received fellowship training on melanocytic IHC stains. The study “highlights the importance of fellowship exposure to MMS and IHC staining for melanoma,” the authors wrote, adding that postfellowship training opportunities in MMS and IHC staining for melanoma may help broaden its use among Mohs surgeons who received inadequate fellowship exposure.
Dr. Siscos and his colleagues reported no significant interest with commercial supporters. Dr. Etzkorn had no disclosures.
SOURCE: Siscos S et al. Dermatol Surg. 2020 Oct;46(10):1267-71.
of members of the American College of Mohs Surgery.
Of 513 survey participants, 40.9% reported using MMS to treat any subtype of melanoma. Most of these surgeons reported treating both lentigo maligna (97.5%) and other melanoma in situ (MIS) subtypes (91.4%). A slight majority – 58.6% – reported treating invasive T1 melanoma, and 20.5% reported treating invasive T2 and/or higher-stage melanoma with MMS.
The analysis, published in Dermatologic Surgery, was done by Spyros M. Siscos, MD, and a team of residents and faculty in the division of dermatology at the University of Kansas Medical Center, Kansas City.
It comes on the heels of an analysis of claims data for Mohs surgery, published last year in JAMA Dermatology, which showed a more than threefold increase in the use of Mohs surgery for melanoma from 2.6% of all surgical cases in 2001 to 7.9% in 2016.
With the increased use of MMS for treatment of melanoma, “Mohs surgeons who previously treated MIS with MMS may be increasingly doing so and/or expanding their scope of treatment to include invasive melanoma,” the University of Kansas investigators wrote.
That a slight majority now report treating invasive melanoma with MMS “may be due, in part, to upstaging during the MMS procedure and the increasing evidence demonstrating improved survival of early-invasive melanoma treated with MMS compared with [wide local excision],” as well as the advent of melanocytic immunohistochemical (IHC) stains, particularly melanoma antigen recognized by T-cells 1 (MART-1), they said. However, 29% of surveyed Mohs surgeons treating melanoma with MMS do not use IHC stains “despite growing evidence supporting” their use, the authors wrote.
The advent of IHC stains, particularly MART-1, has improved the accuracy of interpreting frozen sections of melanoma, they reported, noting that MMS without IHC has been associated with a recurrence rate as high as 33%. Of the 71% who reported using IHC stains, MART-1 was the primary IHC stain for virtually all of them (97.3%).
There was also variation in the number of surgeons who reported debulking MIS. Eighty-two percent take this approach, excising the clinically visible tumor before excising the initial Mohs stage – almost all with a scalpel. More than half of these surgeons – 58.5% – submit the entire debulked MIS specimen for permanent vertical sectioning (breadloafing) to evaluate for deeper tumor invasion.
The others reported submitting the entire debulked specimen for frozen vertical sectioning, or portions of the specimen for both permanent and frozen vertical sectioning. “It is unclear why a minority of surveyed Mohs surgeons reported not debulking MIS,” wrote Dr. Siscos and his colleagues.
The average margin size of the first Mohs stage for MIS was 4.96 ± 1.74 mm, which is at the lower end of the 0.5-1.0 cm range for wide local excision (WLE) recommended by the National Comprehensive Cancer Network (NCCN) and the American Academy of Dermatology (AAD), according to a clinical practice guideline. (The survey did not investigate initial margins for invasive melanoma treated with MMS.)
Jeremy R. Etzhorn, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and an author of a 2019 claims data analysis of excisional surgery practices for melanoma, said that the new survey findings – like the prior analysis – highlight the variability in approaches to using MMS for melanoma.
“Mohs for melanoma [seems] like a one-liner ... but really, there are [a lot] of different techniques that fall under that umbrella, if you parse out all the variations,” he said in an interview.
Per the 2016 claims analysis, he noted, IHC was used in less than 40% of Mohs surgery cases for melanoma, and there were wide geographic variations. “The biggest critique of Mohs surgery for melanoma over the last two decades has been that it’s hard to see the tumor,” he said. “But with the advent of IHC, that challenge was overcome.”
Surgical excision practices are evolving without the development of best practice guidelines, said Dr. Etzkorn, who is director of clinical research for the University of Pennsylvania dermatologic oncology center. Multisociety guidelines published in 2012 on appropriate use criteria for Mohs surgery do not offer specific recommendations on the use of MMS for invasive melanoma. Nor do guidelines from the AAD and the NCCN, he said.
“What this [new] study highlights and what’s being discussed amongst Moh’s surgeons” is that Mohs for melanoma “has be to be standardized” to some extent and then clinical trials conducted comparing Mohs to conventional excision. The studies that have been published in recent years comparing MMS with WLE for MIS and invasive melanoma are “not gold standard studies,” he said.
Practice guidelines then can be informed by high-quality evidence on its safety and efficacy, he said.
The 513 participants in the newly published survey represent a 31.5% response rate. Invasive T2 and/or higher stage melanoma was more likely to be treated with MMS in academic hospitals, compared with other practice settings (30.2% v. 18.1%), Dr. Siscos and his coauthors reported.
Participants who reported treating melanoma with MMS were more likely to report fellowship exposure and more likely to have received fellowship training on melanocytic IHC stains. The study “highlights the importance of fellowship exposure to MMS and IHC staining for melanoma,” the authors wrote, adding that postfellowship training opportunities in MMS and IHC staining for melanoma may help broaden its use among Mohs surgeons who received inadequate fellowship exposure.
Dr. Siscos and his colleagues reported no significant interest with commercial supporters. Dr. Etzkorn had no disclosures.
SOURCE: Siscos S et al. Dermatol Surg. 2020 Oct;46(10):1267-71.
FROM DERMATOLOGIC SURGERY
Clinical factors and treatment tied to COVID-19 mortality in cancer patients
according to two presentations at the European Society for Medical Oncology Virtual Congress 2020.
Two analyses of data from the COVID-19 and Cancer Consortium (CCC19) were presented at the meeting.
The data suggest that older age, male sex, more comorbidities, poor performance status, progressive cancer or multiple cancers, hematologic malignancy, and recent cancer therapy are all associated with higher mortality among patients with cancer and COVID-19. Anti-CD20 therapy is associated with an especially high mortality rate, according to an investigator.
Among hospitalized patients, increased absolute neutrophil count as well as abnormal D-dimer, high-sensitivity troponin, and C-reactive protein are associated with a higher risk of mortality.
Prior analyses of CCC19 data pointed to several factors associated with higher COVID-19 death rates, according to Petros Grivas, MD, PhD, of University of Washington, Seattle, who presented some CCC19 data at the meeting. However, the prior analyses were limited by weak statistical power and low event rates, Dr. Grivas said.
Clinical and laboratory factors: Abstract LBA72
The aim of Dr. Grivas’s analysis was to validate a priori identified demographic and clinicopathologic factors associated with 30-day all-cause mortality in patients with COVID-19 and cancer. Dr. Grivas and colleagues also explored the potential association between laboratory parameters and 30-day all-cause mortality.
The analysis included 3,899 patients with cancer and COVID-19 from 124 centers. Most centers are in the United States, but 4% are in Canada, and 2% are in Spain. About two-thirds of patients were 60 years of age or younger at baseline, half were men, 79% had solid tumors, and 21% had hematologic malignancies.
Cancer-specific factors associated with an increased risk of 30-day all-cause mortality were having progressive cancer (adjusted odds ratio, 2.9), receiving cancer therapy within 3 months (aOR, 1.2), having a hematologic versus solid tumor (aOR, 1.7), and having multiple malignancies (aOR, 1.5).
Clinical factors associated with an increased risk of 30-day all-cause mortality were Black versus White race (aOR, 1.5), older age (aOR, 1.7 per 10 years), three or more actively treated comorbidities (versus none; aOR, 2.1), and Eastern Cooperative Oncology Group performance status of 2 or more (versus 0; aOR, 4.6).
In hospitalized patients, several laboratory variables were associated with an increased risk of 30-day all-cause mortality. Having an absolute neutrophil count above the upper limit of normal doubled the risk (aOR, 2.0), while abnormal D-dimer, high-sensitivity troponin, and C-reactive protein all more than doubled the risk of mortality (aORs of 2.5, 2.5, and 2.4, respectively).
Further risk modeling with multivariable analysis will be performed after longer follow-up, Dr. Grivas noted.
Treatment-related outcomes: Abstract LBA71
An additional analysis of CCC19 data encompassed 3,654 patients. In this analysis, researchers investigated the correlation between timing of cancer treatment and COVID-19–related complications and 30-day mortality.
Mortality was highest among cancer patients treated 1-3 months prior to COVID-19 diagnosis, with all-cause mortality at 28%, said Trisha M. Wise-Draper, MD, PhD, of University of Cincinnati, when presenting the data at the meeting.
Rates for other complications (hospitalization, oxygen required, ICU admission, and mechanical ventilation) were similar regardless of treatment timing.
The unadjusted 30-day mortality rate was highest for patients treated most recently with chemoimmunotherapy (30%), followed by chemotherapy (18%), chemoradiotherapy (18%), and targeted therapy (17%).
The mortality rate was “particularly high,” at 50%, in patients receiving anti-CD20 therapy 1-3 months prior to COVID-19 diagnosis – the time period for which significant B-cell depletion develops, Dr. Wise-Draper observed.
An analysis of disease status among 1,449 patients treated within 3 months of COVID-19 diagnosis showed mortality risk increasing from 6% among patients in remission or with newly emergent disease, to 22% in patients with any active cancer, to 34% in those with progressing disease, Dr. Wise-Draper said.
Discussant Benjamin Solomon, MD, PhD, of Peter MacCallum Cancer Centre in Melbourne, made note of the high 30-day mortality rate seen in patients receiving anti-CD20 therapy as well as the elevated standardized mortality ratios with recent chemoimmunotherapy and targeted therapy.
“Although there are some limitations of this analysis, it provides the best data we have to date about the effects of treatment on early mortality in patients with COVID-19 and cancer. It points to a modest but heterogeneous effect of treatment on outcome, one which is likely to become clearer with larger cohorts and additional analysis,” Dr. Solomon said.
This research was funded by the American Cancer Society, Hope Foundation for Cancer Research, Jim and Carol O’Hare Fund, National Cancer Institute, National Human Genome Research Institute, Vanderbilt Institute for Clinical and Translational Research, and Fonds de Recherche du Quebec-Sante. Dr. Grivas disclosed relationships with many companies, but none are related to this work. Dr. Wise-Draper disclosed relationships with Merck, Bristol-Myers Squibb, Tesaro, GlaxoSmithKline, AstraZeneca, Shattuck Labs, and Rakuten. Dr. Solomon disclosed relationships with Amgen, AstraZeneca, Merck, Bristol-Myers Squibb, Novartis, Pfizer, and Roche-Genentech.
SOURCES: Grivas P et al. ESMO 2020, Abstract LBA72; Wise-Draper TM et al. ESMO 2020, Abstract LBA71.
according to two presentations at the European Society for Medical Oncology Virtual Congress 2020.
Two analyses of data from the COVID-19 and Cancer Consortium (CCC19) were presented at the meeting.
The data suggest that older age, male sex, more comorbidities, poor performance status, progressive cancer or multiple cancers, hematologic malignancy, and recent cancer therapy are all associated with higher mortality among patients with cancer and COVID-19. Anti-CD20 therapy is associated with an especially high mortality rate, according to an investigator.
Among hospitalized patients, increased absolute neutrophil count as well as abnormal D-dimer, high-sensitivity troponin, and C-reactive protein are associated with a higher risk of mortality.
Prior analyses of CCC19 data pointed to several factors associated with higher COVID-19 death rates, according to Petros Grivas, MD, PhD, of University of Washington, Seattle, who presented some CCC19 data at the meeting. However, the prior analyses were limited by weak statistical power and low event rates, Dr. Grivas said.
Clinical and laboratory factors: Abstract LBA72
The aim of Dr. Grivas’s analysis was to validate a priori identified demographic and clinicopathologic factors associated with 30-day all-cause mortality in patients with COVID-19 and cancer. Dr. Grivas and colleagues also explored the potential association between laboratory parameters and 30-day all-cause mortality.
The analysis included 3,899 patients with cancer and COVID-19 from 124 centers. Most centers are in the United States, but 4% are in Canada, and 2% are in Spain. About two-thirds of patients were 60 years of age or younger at baseline, half were men, 79% had solid tumors, and 21% had hematologic malignancies.
Cancer-specific factors associated with an increased risk of 30-day all-cause mortality were having progressive cancer (adjusted odds ratio, 2.9), receiving cancer therapy within 3 months (aOR, 1.2), having a hematologic versus solid tumor (aOR, 1.7), and having multiple malignancies (aOR, 1.5).
Clinical factors associated with an increased risk of 30-day all-cause mortality were Black versus White race (aOR, 1.5), older age (aOR, 1.7 per 10 years), three or more actively treated comorbidities (versus none; aOR, 2.1), and Eastern Cooperative Oncology Group performance status of 2 or more (versus 0; aOR, 4.6).
In hospitalized patients, several laboratory variables were associated with an increased risk of 30-day all-cause mortality. Having an absolute neutrophil count above the upper limit of normal doubled the risk (aOR, 2.0), while abnormal D-dimer, high-sensitivity troponin, and C-reactive protein all more than doubled the risk of mortality (aORs of 2.5, 2.5, and 2.4, respectively).
Further risk modeling with multivariable analysis will be performed after longer follow-up, Dr. Grivas noted.
Treatment-related outcomes: Abstract LBA71
An additional analysis of CCC19 data encompassed 3,654 patients. In this analysis, researchers investigated the correlation between timing of cancer treatment and COVID-19–related complications and 30-day mortality.
Mortality was highest among cancer patients treated 1-3 months prior to COVID-19 diagnosis, with all-cause mortality at 28%, said Trisha M. Wise-Draper, MD, PhD, of University of Cincinnati, when presenting the data at the meeting.
Rates for other complications (hospitalization, oxygen required, ICU admission, and mechanical ventilation) were similar regardless of treatment timing.
The unadjusted 30-day mortality rate was highest for patients treated most recently with chemoimmunotherapy (30%), followed by chemotherapy (18%), chemoradiotherapy (18%), and targeted therapy (17%).
The mortality rate was “particularly high,” at 50%, in patients receiving anti-CD20 therapy 1-3 months prior to COVID-19 diagnosis – the time period for which significant B-cell depletion develops, Dr. Wise-Draper observed.
An analysis of disease status among 1,449 patients treated within 3 months of COVID-19 diagnosis showed mortality risk increasing from 6% among patients in remission or with newly emergent disease, to 22% in patients with any active cancer, to 34% in those with progressing disease, Dr. Wise-Draper said.
Discussant Benjamin Solomon, MD, PhD, of Peter MacCallum Cancer Centre in Melbourne, made note of the high 30-day mortality rate seen in patients receiving anti-CD20 therapy as well as the elevated standardized mortality ratios with recent chemoimmunotherapy and targeted therapy.
“Although there are some limitations of this analysis, it provides the best data we have to date about the effects of treatment on early mortality in patients with COVID-19 and cancer. It points to a modest but heterogeneous effect of treatment on outcome, one which is likely to become clearer with larger cohorts and additional analysis,” Dr. Solomon said.
This research was funded by the American Cancer Society, Hope Foundation for Cancer Research, Jim and Carol O’Hare Fund, National Cancer Institute, National Human Genome Research Institute, Vanderbilt Institute for Clinical and Translational Research, and Fonds de Recherche du Quebec-Sante. Dr. Grivas disclosed relationships with many companies, but none are related to this work. Dr. Wise-Draper disclosed relationships with Merck, Bristol-Myers Squibb, Tesaro, GlaxoSmithKline, AstraZeneca, Shattuck Labs, and Rakuten. Dr. Solomon disclosed relationships with Amgen, AstraZeneca, Merck, Bristol-Myers Squibb, Novartis, Pfizer, and Roche-Genentech.
SOURCES: Grivas P et al. ESMO 2020, Abstract LBA72; Wise-Draper TM et al. ESMO 2020, Abstract LBA71.
according to two presentations at the European Society for Medical Oncology Virtual Congress 2020.
Two analyses of data from the COVID-19 and Cancer Consortium (CCC19) were presented at the meeting.
The data suggest that older age, male sex, more comorbidities, poor performance status, progressive cancer or multiple cancers, hematologic malignancy, and recent cancer therapy are all associated with higher mortality among patients with cancer and COVID-19. Anti-CD20 therapy is associated with an especially high mortality rate, according to an investigator.
Among hospitalized patients, increased absolute neutrophil count as well as abnormal D-dimer, high-sensitivity troponin, and C-reactive protein are associated with a higher risk of mortality.
Prior analyses of CCC19 data pointed to several factors associated with higher COVID-19 death rates, according to Petros Grivas, MD, PhD, of University of Washington, Seattle, who presented some CCC19 data at the meeting. However, the prior analyses were limited by weak statistical power and low event rates, Dr. Grivas said.
Clinical and laboratory factors: Abstract LBA72
The aim of Dr. Grivas’s analysis was to validate a priori identified demographic and clinicopathologic factors associated with 30-day all-cause mortality in patients with COVID-19 and cancer. Dr. Grivas and colleagues also explored the potential association between laboratory parameters and 30-day all-cause mortality.
The analysis included 3,899 patients with cancer and COVID-19 from 124 centers. Most centers are in the United States, but 4% are in Canada, and 2% are in Spain. About two-thirds of patients were 60 years of age or younger at baseline, half were men, 79% had solid tumors, and 21% had hematologic malignancies.
Cancer-specific factors associated with an increased risk of 30-day all-cause mortality were having progressive cancer (adjusted odds ratio, 2.9), receiving cancer therapy within 3 months (aOR, 1.2), having a hematologic versus solid tumor (aOR, 1.7), and having multiple malignancies (aOR, 1.5).
Clinical factors associated with an increased risk of 30-day all-cause mortality were Black versus White race (aOR, 1.5), older age (aOR, 1.7 per 10 years), three or more actively treated comorbidities (versus none; aOR, 2.1), and Eastern Cooperative Oncology Group performance status of 2 or more (versus 0; aOR, 4.6).
In hospitalized patients, several laboratory variables were associated with an increased risk of 30-day all-cause mortality. Having an absolute neutrophil count above the upper limit of normal doubled the risk (aOR, 2.0), while abnormal D-dimer, high-sensitivity troponin, and C-reactive protein all more than doubled the risk of mortality (aORs of 2.5, 2.5, and 2.4, respectively).
Further risk modeling with multivariable analysis will be performed after longer follow-up, Dr. Grivas noted.
Treatment-related outcomes: Abstract LBA71
An additional analysis of CCC19 data encompassed 3,654 patients. In this analysis, researchers investigated the correlation between timing of cancer treatment and COVID-19–related complications and 30-day mortality.
Mortality was highest among cancer patients treated 1-3 months prior to COVID-19 diagnosis, with all-cause mortality at 28%, said Trisha M. Wise-Draper, MD, PhD, of University of Cincinnati, when presenting the data at the meeting.
Rates for other complications (hospitalization, oxygen required, ICU admission, and mechanical ventilation) were similar regardless of treatment timing.
The unadjusted 30-day mortality rate was highest for patients treated most recently with chemoimmunotherapy (30%), followed by chemotherapy (18%), chemoradiotherapy (18%), and targeted therapy (17%).
The mortality rate was “particularly high,” at 50%, in patients receiving anti-CD20 therapy 1-3 months prior to COVID-19 diagnosis – the time period for which significant B-cell depletion develops, Dr. Wise-Draper observed.
An analysis of disease status among 1,449 patients treated within 3 months of COVID-19 diagnosis showed mortality risk increasing from 6% among patients in remission or with newly emergent disease, to 22% in patients with any active cancer, to 34% in those with progressing disease, Dr. Wise-Draper said.
Discussant Benjamin Solomon, MD, PhD, of Peter MacCallum Cancer Centre in Melbourne, made note of the high 30-day mortality rate seen in patients receiving anti-CD20 therapy as well as the elevated standardized mortality ratios with recent chemoimmunotherapy and targeted therapy.
“Although there are some limitations of this analysis, it provides the best data we have to date about the effects of treatment on early mortality in patients with COVID-19 and cancer. It points to a modest but heterogeneous effect of treatment on outcome, one which is likely to become clearer with larger cohorts and additional analysis,” Dr. Solomon said.
This research was funded by the American Cancer Society, Hope Foundation for Cancer Research, Jim and Carol O’Hare Fund, National Cancer Institute, National Human Genome Research Institute, Vanderbilt Institute for Clinical and Translational Research, and Fonds de Recherche du Quebec-Sante. Dr. Grivas disclosed relationships with many companies, but none are related to this work. Dr. Wise-Draper disclosed relationships with Merck, Bristol-Myers Squibb, Tesaro, GlaxoSmithKline, AstraZeneca, Shattuck Labs, and Rakuten. Dr. Solomon disclosed relationships with Amgen, AstraZeneca, Merck, Bristol-Myers Squibb, Novartis, Pfizer, and Roche-Genentech.
SOURCES: Grivas P et al. ESMO 2020, Abstract LBA72; Wise-Draper TM et al. ESMO 2020, Abstract LBA71.
FROM ESMO 2020
The scope of under- and overtreatment in older adults with cancer
Because of physiological changes with aging and differences in cancer biology, caring for older adults (OAs) with cancer requires careful assessment and planning.
Clark Dumontier, MD, of Brigham and Women’s Hospital in Boston, and colleagues sought to define the meaning of the terms “undertreatment” and “overtreatment” for OAs with cancer in a scoping literature review published in the Journal of Clinical Oncology.
Though OAs are typically defined as adults aged 65 years and older, in this review, the authors defined OAs as patients aged 60 years and older.
The authors theorized that a scoping review of papers about this patient population could provide clues about limitations in the oncology literature and guidance about patient management and future research. Despite comprising the majority of cancer patients, OAs are underrepresented in clinical trials.
About scoping reviews
Scoping reviews are used to identify existing evidence in a field, clarify concepts or definitions in the literature, survey how research on a topic is conducted, and identify knowledge gaps. In addition, scoping reviews summarize available evidence without answering a discrete research question.
Industry standards for scoping reviews have been established by the Johanna Briggs Institute and Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping reviews. According to these standards, scoping reviews should:
- Establish eligibility criteria with a rationale for each criterion clearly explained
- Search multiple databases in multiple languages
- Include “gray literature,” defined as studies that are unpublished or difficult to locate
- Have several independent reviewers screen titles and abstracts
- Ask multiple independent reviewers to review full text articles
- Present results with charts or diagrams that align with the review’s objective
- Graphically depict the decision process for including/excluding sources
- Identify implications for further research.
In their review, Dr. DuMontier and colleagues fulfilled many of the aforementioned criteria. The team searched three English-language databases for titles and abstracts that included the terms undertreatment and/or overtreatment, and were related to OAs with cancer, inclusive of all types of articles, cancer types, and treatments.
Definitions of undertreatment and overtreatment were extracted, and categories underlying these definitions were derived. Within a random subset of articles, two coauthors independently determined final categories of definitions and independently assigned those categories.
Findings and implications
To define OA, Dr. DuMontier and colleagues used a cutoff of 60 years or older. Articles mentioning undertreatment (n = 236), overtreatment (n = 71), or both (n = 51) met criteria for inclusion (n = 256), but only 14 articles (5.5%) explicitly provided formal definitions.
For most of the reviewed articles, the authors judged definitions from the surrounding context. In a random subset of 50 articles, there was a high level of agreement (87.1%; κ = 0.81) between two coauthors in independently assigning categories of definitions.
Undertreatment was applied to therapy that was less than recommended (148 articles; 62.7%) or less than recommended with worse outcomes (88 articles; 37.3%).
Overtreatment most commonly denoted intensive treatment of an OA in whom harms outweighed the benefits of treatment (38 articles; 53.5%) or intensive treatment of a cancer not expected to affect the OA during the patient’s remaining life (33 articles; 46.5%).
Overall, the authors found that undertreatment and overtreatment of OAs with cancer are imprecisely defined concepts. Formal geriatric assessment was recommended in just over half of articles, and only 26.2% recommended formal assessments of age-related vulnerabilities for management. The authors proposed definitions that accounted for both oncologic factors and geriatric domains.
Care of individual patients and clinical research
National Comprehensive Cancer Network (NCCN) guidelines for OAs with cancer recommend initial consideration of overall life expectancy. If a patient is a candidate for cancer treatment on that basis, the next recommended assessment is that of the patient’s capacity to understand the relevant information, appreciate the underlying values and overall medical situation, reason through decisions, and communicate a choice that is consistent with the patient’s articulated goals.
In the pretreatment evaluation of OAs in whom there are no concerns about tolerance to antineoplastic therapy, NCCN guidelines suggest geriatric screening with standardized tools and, if abnormal, comprehensive geriatric screening. The guidelines recommend considering alternative treatment options if nonmodifiable abnormalities are identified.
Referral to a geriatric clinical specialist, use of the Cancer and Aging Research Group’s Chemo Toxicity Calculator, and calculation of Chemotherapy Risk Assessment Scale for High-Age Patients score are specifically suggested if high-risk procedures (such as chemotherapy, radiation, or complex surgery, which most oncologists would consider to be “another day in the office”) are contemplated.
The American Society of Clinical Oncology (ASCO) guidelines for geriatric oncology are similarly detailed and endorse similar evaluations and management.
Employing disease-centric and geriatric domains
Dr. DuMontier and colleagues noted that, for OAs with comorbidity or psychosocial challenges, surrogate survival endpoints are unrelated to quality of life (QOL) outcomes. Nonetheless, QOL is valued by OAs at least as much as survival improvement.
Through no fault of their own, the authors’ conclusion that undertreatment and overtreatment are imperfectly defined concepts has a certain neutrality to it. However, the terms undertreatment and overtreatment are commonly used to signify that inappropriate treatment decisions were made. Therefore, the terms are inherently negative and pejorative.
As with most emotionally charged issues in oncology, it is ideal for professionals in our field to take charge when deficiencies exist. ASCO, NCCN, and the authors of this scoping review have provided a conceptual basis for doing so.
An integrated oncologist-geriatrician approach was shown to be effective in the randomized INTEGERATE trial, showing improved QOL, reduced hospital admissions, and reduced early treatment discontinuation from adverse events (ASCO 2020, Abstract 12011).
Therefore, those clinicians who have not formally, systematically, and routinely supplemented the traditional disease-centric endpoints with patient-centered criteria need to do so.
Similarly, a retrospective study published in JAMA Network Open demonstrated that geriatric and surgical comanagement of OAs with cancer was associated with significantly lower 90-day postoperative mortality and receipt of more supportive care services (physical therapy, occupational therapy, speech and swallow rehabilitation, and nutrition services), in comparison with management from the surgical service only.
These clinical and administrative changes will not only enhance patient management but also facilitate the clinical trials required to clarify optimal treatment intensity. As that occurs, we will be able to apply as much precision to the care of OAs with cancer as we do in other areas of cancer treatment.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Dumontier C et al. J Clin Oncol. 2020 Aug 1;38(22):2558-2569.
Because of physiological changes with aging and differences in cancer biology, caring for older adults (OAs) with cancer requires careful assessment and planning.
Clark Dumontier, MD, of Brigham and Women’s Hospital in Boston, and colleagues sought to define the meaning of the terms “undertreatment” and “overtreatment” for OAs with cancer in a scoping literature review published in the Journal of Clinical Oncology.
Though OAs are typically defined as adults aged 65 years and older, in this review, the authors defined OAs as patients aged 60 years and older.
The authors theorized that a scoping review of papers about this patient population could provide clues about limitations in the oncology literature and guidance about patient management and future research. Despite comprising the majority of cancer patients, OAs are underrepresented in clinical trials.
About scoping reviews
Scoping reviews are used to identify existing evidence in a field, clarify concepts or definitions in the literature, survey how research on a topic is conducted, and identify knowledge gaps. In addition, scoping reviews summarize available evidence without answering a discrete research question.
Industry standards for scoping reviews have been established by the Johanna Briggs Institute and Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping reviews. According to these standards, scoping reviews should:
- Establish eligibility criteria with a rationale for each criterion clearly explained
- Search multiple databases in multiple languages
- Include “gray literature,” defined as studies that are unpublished or difficult to locate
- Have several independent reviewers screen titles and abstracts
- Ask multiple independent reviewers to review full text articles
- Present results with charts or diagrams that align with the review’s objective
- Graphically depict the decision process for including/excluding sources
- Identify implications for further research.
In their review, Dr. DuMontier and colleagues fulfilled many of the aforementioned criteria. The team searched three English-language databases for titles and abstracts that included the terms undertreatment and/or overtreatment, and were related to OAs with cancer, inclusive of all types of articles, cancer types, and treatments.
Definitions of undertreatment and overtreatment were extracted, and categories underlying these definitions were derived. Within a random subset of articles, two coauthors independently determined final categories of definitions and independently assigned those categories.
Findings and implications
To define OA, Dr. DuMontier and colleagues used a cutoff of 60 years or older. Articles mentioning undertreatment (n = 236), overtreatment (n = 71), or both (n = 51) met criteria for inclusion (n = 256), but only 14 articles (5.5%) explicitly provided formal definitions.
For most of the reviewed articles, the authors judged definitions from the surrounding context. In a random subset of 50 articles, there was a high level of agreement (87.1%; κ = 0.81) between two coauthors in independently assigning categories of definitions.
Undertreatment was applied to therapy that was less than recommended (148 articles; 62.7%) or less than recommended with worse outcomes (88 articles; 37.3%).
Overtreatment most commonly denoted intensive treatment of an OA in whom harms outweighed the benefits of treatment (38 articles; 53.5%) or intensive treatment of a cancer not expected to affect the OA during the patient’s remaining life (33 articles; 46.5%).
Overall, the authors found that undertreatment and overtreatment of OAs with cancer are imprecisely defined concepts. Formal geriatric assessment was recommended in just over half of articles, and only 26.2% recommended formal assessments of age-related vulnerabilities for management. The authors proposed definitions that accounted for both oncologic factors and geriatric domains.
Care of individual patients and clinical research
National Comprehensive Cancer Network (NCCN) guidelines for OAs with cancer recommend initial consideration of overall life expectancy. If a patient is a candidate for cancer treatment on that basis, the next recommended assessment is that of the patient’s capacity to understand the relevant information, appreciate the underlying values and overall medical situation, reason through decisions, and communicate a choice that is consistent with the patient’s articulated goals.
In the pretreatment evaluation of OAs in whom there are no concerns about tolerance to antineoplastic therapy, NCCN guidelines suggest geriatric screening with standardized tools and, if abnormal, comprehensive geriatric screening. The guidelines recommend considering alternative treatment options if nonmodifiable abnormalities are identified.
Referral to a geriatric clinical specialist, use of the Cancer and Aging Research Group’s Chemo Toxicity Calculator, and calculation of Chemotherapy Risk Assessment Scale for High-Age Patients score are specifically suggested if high-risk procedures (such as chemotherapy, radiation, or complex surgery, which most oncologists would consider to be “another day in the office”) are contemplated.
The American Society of Clinical Oncology (ASCO) guidelines for geriatric oncology are similarly detailed and endorse similar evaluations and management.
Employing disease-centric and geriatric domains
Dr. DuMontier and colleagues noted that, for OAs with comorbidity or psychosocial challenges, surrogate survival endpoints are unrelated to quality of life (QOL) outcomes. Nonetheless, QOL is valued by OAs at least as much as survival improvement.
Through no fault of their own, the authors’ conclusion that undertreatment and overtreatment are imperfectly defined concepts has a certain neutrality to it. However, the terms undertreatment and overtreatment are commonly used to signify that inappropriate treatment decisions were made. Therefore, the terms are inherently negative and pejorative.
As with most emotionally charged issues in oncology, it is ideal for professionals in our field to take charge when deficiencies exist. ASCO, NCCN, and the authors of this scoping review have provided a conceptual basis for doing so.
An integrated oncologist-geriatrician approach was shown to be effective in the randomized INTEGERATE trial, showing improved QOL, reduced hospital admissions, and reduced early treatment discontinuation from adverse events (ASCO 2020, Abstract 12011).
Therefore, those clinicians who have not formally, systematically, and routinely supplemented the traditional disease-centric endpoints with patient-centered criteria need to do so.
Similarly, a retrospective study published in JAMA Network Open demonstrated that geriatric and surgical comanagement of OAs with cancer was associated with significantly lower 90-day postoperative mortality and receipt of more supportive care services (physical therapy, occupational therapy, speech and swallow rehabilitation, and nutrition services), in comparison with management from the surgical service only.
These clinical and administrative changes will not only enhance patient management but also facilitate the clinical trials required to clarify optimal treatment intensity. As that occurs, we will be able to apply as much precision to the care of OAs with cancer as we do in other areas of cancer treatment.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Dumontier C et al. J Clin Oncol. 2020 Aug 1;38(22):2558-2569.
Because of physiological changes with aging and differences in cancer biology, caring for older adults (OAs) with cancer requires careful assessment and planning.
Clark Dumontier, MD, of Brigham and Women’s Hospital in Boston, and colleagues sought to define the meaning of the terms “undertreatment” and “overtreatment” for OAs with cancer in a scoping literature review published in the Journal of Clinical Oncology.
Though OAs are typically defined as adults aged 65 years and older, in this review, the authors defined OAs as patients aged 60 years and older.
The authors theorized that a scoping review of papers about this patient population could provide clues about limitations in the oncology literature and guidance about patient management and future research. Despite comprising the majority of cancer patients, OAs are underrepresented in clinical trials.
About scoping reviews
Scoping reviews are used to identify existing evidence in a field, clarify concepts or definitions in the literature, survey how research on a topic is conducted, and identify knowledge gaps. In addition, scoping reviews summarize available evidence without answering a discrete research question.
Industry standards for scoping reviews have been established by the Johanna Briggs Institute and Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping reviews. According to these standards, scoping reviews should:
- Establish eligibility criteria with a rationale for each criterion clearly explained
- Search multiple databases in multiple languages
- Include “gray literature,” defined as studies that are unpublished or difficult to locate
- Have several independent reviewers screen titles and abstracts
- Ask multiple independent reviewers to review full text articles
- Present results with charts or diagrams that align with the review’s objective
- Graphically depict the decision process for including/excluding sources
- Identify implications for further research.
In their review, Dr. DuMontier and colleagues fulfilled many of the aforementioned criteria. The team searched three English-language databases for titles and abstracts that included the terms undertreatment and/or overtreatment, and were related to OAs with cancer, inclusive of all types of articles, cancer types, and treatments.
Definitions of undertreatment and overtreatment were extracted, and categories underlying these definitions were derived. Within a random subset of articles, two coauthors independently determined final categories of definitions and independently assigned those categories.
Findings and implications
To define OA, Dr. DuMontier and colleagues used a cutoff of 60 years or older. Articles mentioning undertreatment (n = 236), overtreatment (n = 71), or both (n = 51) met criteria for inclusion (n = 256), but only 14 articles (5.5%) explicitly provided formal definitions.
For most of the reviewed articles, the authors judged definitions from the surrounding context. In a random subset of 50 articles, there was a high level of agreement (87.1%; κ = 0.81) between two coauthors in independently assigning categories of definitions.
Undertreatment was applied to therapy that was less than recommended (148 articles; 62.7%) or less than recommended with worse outcomes (88 articles; 37.3%).
Overtreatment most commonly denoted intensive treatment of an OA in whom harms outweighed the benefits of treatment (38 articles; 53.5%) or intensive treatment of a cancer not expected to affect the OA during the patient’s remaining life (33 articles; 46.5%).
Overall, the authors found that undertreatment and overtreatment of OAs with cancer are imprecisely defined concepts. Formal geriatric assessment was recommended in just over half of articles, and only 26.2% recommended formal assessments of age-related vulnerabilities for management. The authors proposed definitions that accounted for both oncologic factors and geriatric domains.
Care of individual patients and clinical research
National Comprehensive Cancer Network (NCCN) guidelines for OAs with cancer recommend initial consideration of overall life expectancy. If a patient is a candidate for cancer treatment on that basis, the next recommended assessment is that of the patient’s capacity to understand the relevant information, appreciate the underlying values and overall medical situation, reason through decisions, and communicate a choice that is consistent with the patient’s articulated goals.
In the pretreatment evaluation of OAs in whom there are no concerns about tolerance to antineoplastic therapy, NCCN guidelines suggest geriatric screening with standardized tools and, if abnormal, comprehensive geriatric screening. The guidelines recommend considering alternative treatment options if nonmodifiable abnormalities are identified.
Referral to a geriatric clinical specialist, use of the Cancer and Aging Research Group’s Chemo Toxicity Calculator, and calculation of Chemotherapy Risk Assessment Scale for High-Age Patients score are specifically suggested if high-risk procedures (such as chemotherapy, radiation, or complex surgery, which most oncologists would consider to be “another day in the office”) are contemplated.
The American Society of Clinical Oncology (ASCO) guidelines for geriatric oncology are similarly detailed and endorse similar evaluations and management.
Employing disease-centric and geriatric domains
Dr. DuMontier and colleagues noted that, for OAs with comorbidity or psychosocial challenges, surrogate survival endpoints are unrelated to quality of life (QOL) outcomes. Nonetheless, QOL is valued by OAs at least as much as survival improvement.
Through no fault of their own, the authors’ conclusion that undertreatment and overtreatment are imperfectly defined concepts has a certain neutrality to it. However, the terms undertreatment and overtreatment are commonly used to signify that inappropriate treatment decisions were made. Therefore, the terms are inherently negative and pejorative.
As with most emotionally charged issues in oncology, it is ideal for professionals in our field to take charge when deficiencies exist. ASCO, NCCN, and the authors of this scoping review have provided a conceptual basis for doing so.
An integrated oncologist-geriatrician approach was shown to be effective in the randomized INTEGERATE trial, showing improved QOL, reduced hospital admissions, and reduced early treatment discontinuation from adverse events (ASCO 2020, Abstract 12011).
Therefore, those clinicians who have not formally, systematically, and routinely supplemented the traditional disease-centric endpoints with patient-centered criteria need to do so.
Similarly, a retrospective study published in JAMA Network Open demonstrated that geriatric and surgical comanagement of OAs with cancer was associated with significantly lower 90-day postoperative mortality and receipt of more supportive care services (physical therapy, occupational therapy, speech and swallow rehabilitation, and nutrition services), in comparison with management from the surgical service only.
These clinical and administrative changes will not only enhance patient management but also facilitate the clinical trials required to clarify optimal treatment intensity. As that occurs, we will be able to apply as much precision to the care of OAs with cancer as we do in other areas of cancer treatment.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Dumontier C et al. J Clin Oncol. 2020 Aug 1;38(22):2558-2569.
Cancer disparities: One of the most pressing public health issues
“The burden of cancer is not shouldered equally by all segments of the U.S. population,” the AACR adds. “The adverse differences in cancer burden that exist among certain population groups are one of the most pressing public health challenges that we face in the United States.”
AACR president Antoni Ribas, MD, PhD, gave some examples of these disparities at a September 16 Congressional briefing that focused on the inaugural AACR Cancer Disparities Progress Report 2020.
He noted that:
- Black men have more than double the rate of death from prostate cancer compared with men of other racial and ethnic groups.
- Hispanic children are 24% more likely to develop leukemia than non-Hispanic children.
- Non-Hispanic Black children and adolescents with cancer are more than 50% more likely to die from the cancer than non-Hispanic white children and adolescents with cancer.
- Women of low socioeconomic status with early stage ovarian cancer are 50% less likely to receive recommended care than are women of high socioeconomic status.
- In addition to racial and ethnic minority groups, other populations that bear a disproportionate burden when it comes to cancer include individuals lacking adequate health insurance coverage, immigrants, those with disabilities, residents in rural areas, and members of the lesbian, gay, bisexual, and transgender communities.
“It is absolutely unacceptable that advances in cancer care and treatment are not benefiting everyone equally,” Ribas commented.
Making progress against cancer
Progress being made against cancer was highlighted in another publication, the annual AACR Cancer Progress Report 2020.
U.S. cancer deaths declined by 29% between 1991 and 2017, translating to nearly 3 million cancer deaths avoided, the report notes. In addition, 5-year survival rates for all cancers combined increased from 49% in the mid-1970s to 70% for patients diagnosed from 2010-2016.
Between August 2019 and July 31 of this year, the U.S. Food and Drug Administration approved 20 new anticancer drugs for various cancer types and 15 new indications for previously approved cancer drugs, marking the highest number of approvals in one 12-month period since AACR started producing these reports 10 years ago.
A continuing reduction in the cigarette smoking rate among US adults, which is now below 14%, is contributing greatly to declines in lung cancer rates, which have largely driven the improvements in cancer survival, the AACR noted.
This report also notes that progress has been made toward reducing cancer disparities. Overall disparities in cancer death rates among racial and ethnic groups are less pronounced now than they have been in the past two decades. For example, the overall cancer death rate for African American patients was 33% higher than for White patients in 1990 but just 14% higher in 2016.
However, both reports agree that more must be done to reduce cancer disparities even further.
They highlight initiatives that are underway, including:
- The draft guidance issued by the FDA to promote diversification of clinical trial populations.
- The National Institutes of Health’s (NIH’s) Continuing Umbrella of Research Experiences (CURE) program supporting underrepresented students and scientists along their academic and research career pathway.
- The Centers for Disease Control and Prevention’s Racial and Ethnic Approaches to Community Health (REACH) program, a grant-making program focused on encouraging preventive behaviors in underserved communities.
- The NIH’s All of Us program, which is gathering information from the genomes of 1 million healthy individuals with a focus on recruitment from historically underrepresented populations.
Ribas also announced that AACR has established a task force to focus on racial inequalities in cancer research.
Eliminating disparities would save money, argued John D. Carpten, PhD, from the University of Southern California, Los Angeles, who chaired the steering committee that developed the AACR Cancer Disparities Progress Report.
Carpten noted research showing that eliminating disparities for racial and ethnic minorities between 2003 and 2006 would have reduced health care costs by more than $1 trillion in the United States. This underscores the potentially far-reaching impact of efforts to eliminate disparities, he said.
“Without a doubt, socioeconomics and inequities in access to quality care represent major factors influencing cancer health disparities, and these disparities will persist until we address these issues” he said.
Both progress reports culminate in a call to action, largely focused on the need for “unwavering, bipartisan support from Congress, in the form of robust and sustained annual increases in funding for the NIH, NCI [National Cancer Institute], and FDA,” which is vital for accelerating the pace of progress.
The challenge is now compounded by the ongoing COVID-19 pandemic: Both progress reports note that racial and ethnic minorities, including African Americans, are not only affected disproportionately by cancer, but also by COVID-19, further highlighting the “stark inequities in health care.”
Ribas further called for action from national leadership and the scientific community.
“During this unprecedented time in our nation’s history, there is also a need for our nation’s leaders to take on a much bigger role in confronting and combating the structural and systemic racism that contributes to health disparities,” he said. The “pervasive racism and social injustices” that have contributed to disparities in both COVID-19 and cancer underscore the need for “the scientific community to step up and partner with Congress to assess and address this issue within the research community.”
This article first appeared on Medscape.com.
“The burden of cancer is not shouldered equally by all segments of the U.S. population,” the AACR adds. “The adverse differences in cancer burden that exist among certain population groups are one of the most pressing public health challenges that we face in the United States.”
AACR president Antoni Ribas, MD, PhD, gave some examples of these disparities at a September 16 Congressional briefing that focused on the inaugural AACR Cancer Disparities Progress Report 2020.
He noted that:
- Black men have more than double the rate of death from prostate cancer compared with men of other racial and ethnic groups.
- Hispanic children are 24% more likely to develop leukemia than non-Hispanic children.
- Non-Hispanic Black children and adolescents with cancer are more than 50% more likely to die from the cancer than non-Hispanic white children and adolescents with cancer.
- Women of low socioeconomic status with early stage ovarian cancer are 50% less likely to receive recommended care than are women of high socioeconomic status.
- In addition to racial and ethnic minority groups, other populations that bear a disproportionate burden when it comes to cancer include individuals lacking adequate health insurance coverage, immigrants, those with disabilities, residents in rural areas, and members of the lesbian, gay, bisexual, and transgender communities.
“It is absolutely unacceptable that advances in cancer care and treatment are not benefiting everyone equally,” Ribas commented.
Making progress against cancer
Progress being made against cancer was highlighted in another publication, the annual AACR Cancer Progress Report 2020.
U.S. cancer deaths declined by 29% between 1991 and 2017, translating to nearly 3 million cancer deaths avoided, the report notes. In addition, 5-year survival rates for all cancers combined increased from 49% in the mid-1970s to 70% for patients diagnosed from 2010-2016.
Between August 2019 and July 31 of this year, the U.S. Food and Drug Administration approved 20 new anticancer drugs for various cancer types and 15 new indications for previously approved cancer drugs, marking the highest number of approvals in one 12-month period since AACR started producing these reports 10 years ago.
A continuing reduction in the cigarette smoking rate among US adults, which is now below 14%, is contributing greatly to declines in lung cancer rates, which have largely driven the improvements in cancer survival, the AACR noted.
This report also notes that progress has been made toward reducing cancer disparities. Overall disparities in cancer death rates among racial and ethnic groups are less pronounced now than they have been in the past two decades. For example, the overall cancer death rate for African American patients was 33% higher than for White patients in 1990 but just 14% higher in 2016.
However, both reports agree that more must be done to reduce cancer disparities even further.
They highlight initiatives that are underway, including:
- The draft guidance issued by the FDA to promote diversification of clinical trial populations.
- The National Institutes of Health’s (NIH’s) Continuing Umbrella of Research Experiences (CURE) program supporting underrepresented students and scientists along their academic and research career pathway.
- The Centers for Disease Control and Prevention’s Racial and Ethnic Approaches to Community Health (REACH) program, a grant-making program focused on encouraging preventive behaviors in underserved communities.
- The NIH’s All of Us program, which is gathering information from the genomes of 1 million healthy individuals with a focus on recruitment from historically underrepresented populations.
Ribas also announced that AACR has established a task force to focus on racial inequalities in cancer research.
Eliminating disparities would save money, argued John D. Carpten, PhD, from the University of Southern California, Los Angeles, who chaired the steering committee that developed the AACR Cancer Disparities Progress Report.
Carpten noted research showing that eliminating disparities for racial and ethnic minorities between 2003 and 2006 would have reduced health care costs by more than $1 trillion in the United States. This underscores the potentially far-reaching impact of efforts to eliminate disparities, he said.
“Without a doubt, socioeconomics and inequities in access to quality care represent major factors influencing cancer health disparities, and these disparities will persist until we address these issues” he said.
Both progress reports culminate in a call to action, largely focused on the need for “unwavering, bipartisan support from Congress, in the form of robust and sustained annual increases in funding for the NIH, NCI [National Cancer Institute], and FDA,” which is vital for accelerating the pace of progress.
The challenge is now compounded by the ongoing COVID-19 pandemic: Both progress reports note that racial and ethnic minorities, including African Americans, are not only affected disproportionately by cancer, but also by COVID-19, further highlighting the “stark inequities in health care.”
Ribas further called for action from national leadership and the scientific community.
“During this unprecedented time in our nation’s history, there is also a need for our nation’s leaders to take on a much bigger role in confronting and combating the structural and systemic racism that contributes to health disparities,” he said. The “pervasive racism and social injustices” that have contributed to disparities in both COVID-19 and cancer underscore the need for “the scientific community to step up and partner with Congress to assess and address this issue within the research community.”
This article first appeared on Medscape.com.
“The burden of cancer is not shouldered equally by all segments of the U.S. population,” the AACR adds. “The adverse differences in cancer burden that exist among certain population groups are one of the most pressing public health challenges that we face in the United States.”
AACR president Antoni Ribas, MD, PhD, gave some examples of these disparities at a September 16 Congressional briefing that focused on the inaugural AACR Cancer Disparities Progress Report 2020.
He noted that:
- Black men have more than double the rate of death from prostate cancer compared with men of other racial and ethnic groups.
- Hispanic children are 24% more likely to develop leukemia than non-Hispanic children.
- Non-Hispanic Black children and adolescents with cancer are more than 50% more likely to die from the cancer than non-Hispanic white children and adolescents with cancer.
- Women of low socioeconomic status with early stage ovarian cancer are 50% less likely to receive recommended care than are women of high socioeconomic status.
- In addition to racial and ethnic minority groups, other populations that bear a disproportionate burden when it comes to cancer include individuals lacking adequate health insurance coverage, immigrants, those with disabilities, residents in rural areas, and members of the lesbian, gay, bisexual, and transgender communities.
“It is absolutely unacceptable that advances in cancer care and treatment are not benefiting everyone equally,” Ribas commented.
Making progress against cancer
Progress being made against cancer was highlighted in another publication, the annual AACR Cancer Progress Report 2020.
U.S. cancer deaths declined by 29% between 1991 and 2017, translating to nearly 3 million cancer deaths avoided, the report notes. In addition, 5-year survival rates for all cancers combined increased from 49% in the mid-1970s to 70% for patients diagnosed from 2010-2016.
Between August 2019 and July 31 of this year, the U.S. Food and Drug Administration approved 20 new anticancer drugs for various cancer types and 15 new indications for previously approved cancer drugs, marking the highest number of approvals in one 12-month period since AACR started producing these reports 10 years ago.
A continuing reduction in the cigarette smoking rate among US adults, which is now below 14%, is contributing greatly to declines in lung cancer rates, which have largely driven the improvements in cancer survival, the AACR noted.
This report also notes that progress has been made toward reducing cancer disparities. Overall disparities in cancer death rates among racial and ethnic groups are less pronounced now than they have been in the past two decades. For example, the overall cancer death rate for African American patients was 33% higher than for White patients in 1990 but just 14% higher in 2016.
However, both reports agree that more must be done to reduce cancer disparities even further.
They highlight initiatives that are underway, including:
- The draft guidance issued by the FDA to promote diversification of clinical trial populations.
- The National Institutes of Health’s (NIH’s) Continuing Umbrella of Research Experiences (CURE) program supporting underrepresented students and scientists along their academic and research career pathway.
- The Centers for Disease Control and Prevention’s Racial and Ethnic Approaches to Community Health (REACH) program, a grant-making program focused on encouraging preventive behaviors in underserved communities.
- The NIH’s All of Us program, which is gathering information from the genomes of 1 million healthy individuals with a focus on recruitment from historically underrepresented populations.
Ribas also announced that AACR has established a task force to focus on racial inequalities in cancer research.
Eliminating disparities would save money, argued John D. Carpten, PhD, from the University of Southern California, Los Angeles, who chaired the steering committee that developed the AACR Cancer Disparities Progress Report.
Carpten noted research showing that eliminating disparities for racial and ethnic minorities between 2003 and 2006 would have reduced health care costs by more than $1 trillion in the United States. This underscores the potentially far-reaching impact of efforts to eliminate disparities, he said.
“Without a doubt, socioeconomics and inequities in access to quality care represent major factors influencing cancer health disparities, and these disparities will persist until we address these issues” he said.
Both progress reports culminate in a call to action, largely focused on the need for “unwavering, bipartisan support from Congress, in the form of robust and sustained annual increases in funding for the NIH, NCI [National Cancer Institute], and FDA,” which is vital for accelerating the pace of progress.
The challenge is now compounded by the ongoing COVID-19 pandemic: Both progress reports note that racial and ethnic minorities, including African Americans, are not only affected disproportionately by cancer, but also by COVID-19, further highlighting the “stark inequities in health care.”
Ribas further called for action from national leadership and the scientific community.
“During this unprecedented time in our nation’s history, there is also a need for our nation’s leaders to take on a much bigger role in confronting and combating the structural and systemic racism that contributes to health disparities,” he said. The “pervasive racism and social injustices” that have contributed to disparities in both COVID-19 and cancer underscore the need for “the scientific community to step up and partner with Congress to assess and address this issue within the research community.”
This article first appeared on Medscape.com.




