User login
Age, smoking among leading cancer risk factors for SLE patients
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
FROM ARTHRITIS CARE & RESEARCH
COVID-19 impact: Less chemo, immune checkpoint inhibitors, and steroids
While neoadjuvant treatment recommendations were not strongly affected by the pandemic, about half of oncologists reported increased hesitancy over recommending frontline chemotherapy for metastatic disease, and a vast majority said they would recommend second- or third-line chemotherapy less often in the metastatic setting.
Most oncologists said they did not perform routine COVID-19 testing via reverse transcriptase–polymerase chain reaction (RT-PCR) before treating cancer patients. In fact, only 3% said they performed COVID-19 RT-PCR testing routinely.
Yüksel Ürün, MD, of Ankara (Turkey) University, and colleagues reported these findings in JCO Global Oncology.
The goal of the survey was to “understand readiness measures taken by oncologists to protect patients and health care workers from the novel coronavirus (COVID-19) and how their clinical decision-making was influenced by the pandemic,” the authors wrote.
The online survey was conducted among 343 oncologists from 28 countries. Responses were collected anonymously, a majority (71%) from university or academic centers, with 95% received between April 1 and April 29, 2020.
Use of telemedicine was common (80%) among respondents, as was use of surgical masks (90%) and personal protective equipment in general.
Only 33% of respondents described using N95 masks. However, the proportion of oncologists who had access to N95 masks while caring for patients known to have COVID-19, especially while doing invasive procedures such as intubation, bronchoscopy, and any airway-related manipulations, was not captured by the survey.
COVID testing and cancer treatment
Most respondents (58%) said they did not perform routine COVID-19 RT-PCR testing prior to administering systemic cancer treatment, with 39% stating they performed RT-PCR tests in selected patients, and 3% saying they performed such testing in all patients.
The survey indicated that hormonal treatments, tyrosine kinase inhibitors, and bone-modifying agents were considered relatively safe, but cytotoxic chemotherapy and immune therapies were not.
Nearly all oncologists said the pandemic would cause them to make no change to their recommendations regarding hormone therapy, and nearly 80% said they would make no changes regarding tyrosine kinase inhibitors or bone-modifying agents.
However, more than 90% of respondents said they would recommend cytotoxic chemotherapy less often, about 70% said they would recommend corticosteroids less often, and around 50% said they would recommend anti–programmed death-1/PD-ligand 1 or anti–cytotoxic T-lymphocyte–associated protein 4 antibodies less often.
The pandemic made most respondents more reluctant to recommend second- or third-line chemotherapy in the metastatic setting. About 80% and 70% of respondents, respectively, would recommend second- or third-line chemotherapy less often.
However, first-line chemotherapy for metastatic disease, as well as adjuvant and neoadjuvant therapy, were less affected. About 30% of respondents said they would recommend neoadjuvant therapy less often, and 50%-55% would recommend adjuvant therapy or frontline chemotherapy for metastatic disease less often.
Most respondents (78%) said they would use granulocyte colony–stimulating factor (G-CSF) more frequently during the pandemic.
The factors most likely to affect oncologists’ treatment decisions were patient age (81%) and concomitant disease (92%). Additionally, 80% of respondents’ treatment decisions were influenced by Eastern Cooperative Oncology Group performance status of 2 or higher, or the presence of chronic obstructive pulmonary disease.
Interpretation and implications
“These results highlight that, even in the early phases of COVID-19 – during which there was considerable uncertainty – basic core principles were guideposts for oncologists,” observed Aly-Khan Lalani, MD, of Juravinski Cancer Centre and McMaster University, Hamilton, Ont., who was not involved in this study.
“For example, [oncologists were] prioritizing strategies for treatments with the largest expected impact and carefully tailoring treatment according to patient comorbidities and performance status,” Dr. Lalani said.
Another oncologist who was not involved in the study expressed concern over reductions in adjuvant therapy supported by half of oncologists surveyed.
“Although benefits may be marginal in some cases, these are curative settings and especially warrant careful individual-level risk/benefit discussions,” said Kartik Sehgal, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston.
His concern extended as well to the small proportion (3%) of oncologists testing for COVID-19 in all patients. “Systematic testing is the need of the hour,” Dr. Sehgal said.
In their discussion of the findings, Dr. Ürün and colleagues noted a lack of consensus on monoclonal antibody and immunotherapy safety among surveyed oncologists. The steroids needed to manage severe immune-mediated toxicity with immune checkpoint inhibitors has led to some prescribing reluctance during the pandemic.
Immunosuppressive properties of immune checkpoint inhibitors also raise concern that they can increase COVID-19 severity. Studies are few, and findings to date are inconsistent with respect to the effect of immune checkpoint inhibitors on COVID-19 clinical course. However, a recently presented study suggested that immune checkpoint inhibitors do not increase the risk of death among cancer patients with COVID-19 (AACR: COVID-19 and Cancer, Abstract S02-01).
Dr. Ürün and colleagues noted that greater COVID-19 severity has been shown in patients with performance status greater than 1, hematologic malignancies, lung cancer, stage IV metastatic disease, chemotherapy within the prior 3 months, cancer treatment in the last 14 days, and the presence of chronic obstructive pulmonary disease. Nonmetastatic cancer has not been shown to affect COVID-19 severity, however.
Dr. Ürün and colleagues also underscored the need for research evidence to balance potential reductions in neutropenic complications with G-CSF (and therefore, reduced hospitalizations) with a theoretical risk of G-CSF–mediated pulmonary injury through its stimulation of an excessive immune response.
Finally, the authors urged oncologists to evaluate each proposed therapy’s risk/benefit ratio on an individual patient basis, and the team tasked the oncology community with gathering comprehensive, rigorous data.
There was no funding source declared for this study. Dr. Ürün and colleagues disclosed various relationships with many pharmaceutical companies, which included receiving research funding. Dr. Sehgal and Dr. Lalani reported no relevant conflicts.
SOURCE: Ürün Y et al. JCO Glob Oncol. 2020 Aug;6:1248-57.
While neoadjuvant treatment recommendations were not strongly affected by the pandemic, about half of oncologists reported increased hesitancy over recommending frontline chemotherapy for metastatic disease, and a vast majority said they would recommend second- or third-line chemotherapy less often in the metastatic setting.
Most oncologists said they did not perform routine COVID-19 testing via reverse transcriptase–polymerase chain reaction (RT-PCR) before treating cancer patients. In fact, only 3% said they performed COVID-19 RT-PCR testing routinely.
Yüksel Ürün, MD, of Ankara (Turkey) University, and colleagues reported these findings in JCO Global Oncology.
The goal of the survey was to “understand readiness measures taken by oncologists to protect patients and health care workers from the novel coronavirus (COVID-19) and how their clinical decision-making was influenced by the pandemic,” the authors wrote.
The online survey was conducted among 343 oncologists from 28 countries. Responses were collected anonymously, a majority (71%) from university or academic centers, with 95% received between April 1 and April 29, 2020.
Use of telemedicine was common (80%) among respondents, as was use of surgical masks (90%) and personal protective equipment in general.
Only 33% of respondents described using N95 masks. However, the proportion of oncologists who had access to N95 masks while caring for patients known to have COVID-19, especially while doing invasive procedures such as intubation, bronchoscopy, and any airway-related manipulations, was not captured by the survey.
COVID testing and cancer treatment
Most respondents (58%) said they did not perform routine COVID-19 RT-PCR testing prior to administering systemic cancer treatment, with 39% stating they performed RT-PCR tests in selected patients, and 3% saying they performed such testing in all patients.
The survey indicated that hormonal treatments, tyrosine kinase inhibitors, and bone-modifying agents were considered relatively safe, but cytotoxic chemotherapy and immune therapies were not.
Nearly all oncologists said the pandemic would cause them to make no change to their recommendations regarding hormone therapy, and nearly 80% said they would make no changes regarding tyrosine kinase inhibitors or bone-modifying agents.
However, more than 90% of respondents said they would recommend cytotoxic chemotherapy less often, about 70% said they would recommend corticosteroids less often, and around 50% said they would recommend anti–programmed death-1/PD-ligand 1 or anti–cytotoxic T-lymphocyte–associated protein 4 antibodies less often.
The pandemic made most respondents more reluctant to recommend second- or third-line chemotherapy in the metastatic setting. About 80% and 70% of respondents, respectively, would recommend second- or third-line chemotherapy less often.
However, first-line chemotherapy for metastatic disease, as well as adjuvant and neoadjuvant therapy, were less affected. About 30% of respondents said they would recommend neoadjuvant therapy less often, and 50%-55% would recommend adjuvant therapy or frontline chemotherapy for metastatic disease less often.
Most respondents (78%) said they would use granulocyte colony–stimulating factor (G-CSF) more frequently during the pandemic.
The factors most likely to affect oncologists’ treatment decisions were patient age (81%) and concomitant disease (92%). Additionally, 80% of respondents’ treatment decisions were influenced by Eastern Cooperative Oncology Group performance status of 2 or higher, or the presence of chronic obstructive pulmonary disease.
Interpretation and implications
“These results highlight that, even in the early phases of COVID-19 – during which there was considerable uncertainty – basic core principles were guideposts for oncologists,” observed Aly-Khan Lalani, MD, of Juravinski Cancer Centre and McMaster University, Hamilton, Ont., who was not involved in this study.
“For example, [oncologists were] prioritizing strategies for treatments with the largest expected impact and carefully tailoring treatment according to patient comorbidities and performance status,” Dr. Lalani said.
Another oncologist who was not involved in the study expressed concern over reductions in adjuvant therapy supported by half of oncologists surveyed.
“Although benefits may be marginal in some cases, these are curative settings and especially warrant careful individual-level risk/benefit discussions,” said Kartik Sehgal, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston.
His concern extended as well to the small proportion (3%) of oncologists testing for COVID-19 in all patients. “Systematic testing is the need of the hour,” Dr. Sehgal said.
In their discussion of the findings, Dr. Ürün and colleagues noted a lack of consensus on monoclonal antibody and immunotherapy safety among surveyed oncologists. The steroids needed to manage severe immune-mediated toxicity with immune checkpoint inhibitors has led to some prescribing reluctance during the pandemic.
Immunosuppressive properties of immune checkpoint inhibitors also raise concern that they can increase COVID-19 severity. Studies are few, and findings to date are inconsistent with respect to the effect of immune checkpoint inhibitors on COVID-19 clinical course. However, a recently presented study suggested that immune checkpoint inhibitors do not increase the risk of death among cancer patients with COVID-19 (AACR: COVID-19 and Cancer, Abstract S02-01).
Dr. Ürün and colleagues noted that greater COVID-19 severity has been shown in patients with performance status greater than 1, hematologic malignancies, lung cancer, stage IV metastatic disease, chemotherapy within the prior 3 months, cancer treatment in the last 14 days, and the presence of chronic obstructive pulmonary disease. Nonmetastatic cancer has not been shown to affect COVID-19 severity, however.
Dr. Ürün and colleagues also underscored the need for research evidence to balance potential reductions in neutropenic complications with G-CSF (and therefore, reduced hospitalizations) with a theoretical risk of G-CSF–mediated pulmonary injury through its stimulation of an excessive immune response.
Finally, the authors urged oncologists to evaluate each proposed therapy’s risk/benefit ratio on an individual patient basis, and the team tasked the oncology community with gathering comprehensive, rigorous data.
There was no funding source declared for this study. Dr. Ürün and colleagues disclosed various relationships with many pharmaceutical companies, which included receiving research funding. Dr. Sehgal and Dr. Lalani reported no relevant conflicts.
SOURCE: Ürün Y et al. JCO Glob Oncol. 2020 Aug;6:1248-57.
While neoadjuvant treatment recommendations were not strongly affected by the pandemic, about half of oncologists reported increased hesitancy over recommending frontline chemotherapy for metastatic disease, and a vast majority said they would recommend second- or third-line chemotherapy less often in the metastatic setting.
Most oncologists said they did not perform routine COVID-19 testing via reverse transcriptase–polymerase chain reaction (RT-PCR) before treating cancer patients. In fact, only 3% said they performed COVID-19 RT-PCR testing routinely.
Yüksel Ürün, MD, of Ankara (Turkey) University, and colleagues reported these findings in JCO Global Oncology.
The goal of the survey was to “understand readiness measures taken by oncologists to protect patients and health care workers from the novel coronavirus (COVID-19) and how their clinical decision-making was influenced by the pandemic,” the authors wrote.
The online survey was conducted among 343 oncologists from 28 countries. Responses were collected anonymously, a majority (71%) from university or academic centers, with 95% received between April 1 and April 29, 2020.
Use of telemedicine was common (80%) among respondents, as was use of surgical masks (90%) and personal protective equipment in general.
Only 33% of respondents described using N95 masks. However, the proportion of oncologists who had access to N95 masks while caring for patients known to have COVID-19, especially while doing invasive procedures such as intubation, bronchoscopy, and any airway-related manipulations, was not captured by the survey.
COVID testing and cancer treatment
Most respondents (58%) said they did not perform routine COVID-19 RT-PCR testing prior to administering systemic cancer treatment, with 39% stating they performed RT-PCR tests in selected patients, and 3% saying they performed such testing in all patients.
The survey indicated that hormonal treatments, tyrosine kinase inhibitors, and bone-modifying agents were considered relatively safe, but cytotoxic chemotherapy and immune therapies were not.
Nearly all oncologists said the pandemic would cause them to make no change to their recommendations regarding hormone therapy, and nearly 80% said they would make no changes regarding tyrosine kinase inhibitors or bone-modifying agents.
However, more than 90% of respondents said they would recommend cytotoxic chemotherapy less often, about 70% said they would recommend corticosteroids less often, and around 50% said they would recommend anti–programmed death-1/PD-ligand 1 or anti–cytotoxic T-lymphocyte–associated protein 4 antibodies less often.
The pandemic made most respondents more reluctant to recommend second- or third-line chemotherapy in the metastatic setting. About 80% and 70% of respondents, respectively, would recommend second- or third-line chemotherapy less often.
However, first-line chemotherapy for metastatic disease, as well as adjuvant and neoadjuvant therapy, were less affected. About 30% of respondents said they would recommend neoadjuvant therapy less often, and 50%-55% would recommend adjuvant therapy or frontline chemotherapy for metastatic disease less often.
Most respondents (78%) said they would use granulocyte colony–stimulating factor (G-CSF) more frequently during the pandemic.
The factors most likely to affect oncologists’ treatment decisions were patient age (81%) and concomitant disease (92%). Additionally, 80% of respondents’ treatment decisions were influenced by Eastern Cooperative Oncology Group performance status of 2 or higher, or the presence of chronic obstructive pulmonary disease.
Interpretation and implications
“These results highlight that, even in the early phases of COVID-19 – during which there was considerable uncertainty – basic core principles were guideposts for oncologists,” observed Aly-Khan Lalani, MD, of Juravinski Cancer Centre and McMaster University, Hamilton, Ont., who was not involved in this study.
“For example, [oncologists were] prioritizing strategies for treatments with the largest expected impact and carefully tailoring treatment according to patient comorbidities and performance status,” Dr. Lalani said.
Another oncologist who was not involved in the study expressed concern over reductions in adjuvant therapy supported by half of oncologists surveyed.
“Although benefits may be marginal in some cases, these are curative settings and especially warrant careful individual-level risk/benefit discussions,” said Kartik Sehgal, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston.
His concern extended as well to the small proportion (3%) of oncologists testing for COVID-19 in all patients. “Systematic testing is the need of the hour,” Dr. Sehgal said.
In their discussion of the findings, Dr. Ürün and colleagues noted a lack of consensus on monoclonal antibody and immunotherapy safety among surveyed oncologists. The steroids needed to manage severe immune-mediated toxicity with immune checkpoint inhibitors has led to some prescribing reluctance during the pandemic.
Immunosuppressive properties of immune checkpoint inhibitors also raise concern that they can increase COVID-19 severity. Studies are few, and findings to date are inconsistent with respect to the effect of immune checkpoint inhibitors on COVID-19 clinical course. However, a recently presented study suggested that immune checkpoint inhibitors do not increase the risk of death among cancer patients with COVID-19 (AACR: COVID-19 and Cancer, Abstract S02-01).
Dr. Ürün and colleagues noted that greater COVID-19 severity has been shown in patients with performance status greater than 1, hematologic malignancies, lung cancer, stage IV metastatic disease, chemotherapy within the prior 3 months, cancer treatment in the last 14 days, and the presence of chronic obstructive pulmonary disease. Nonmetastatic cancer has not been shown to affect COVID-19 severity, however.
Dr. Ürün and colleagues also underscored the need for research evidence to balance potential reductions in neutropenic complications with G-CSF (and therefore, reduced hospitalizations) with a theoretical risk of G-CSF–mediated pulmonary injury through its stimulation of an excessive immune response.
Finally, the authors urged oncologists to evaluate each proposed therapy’s risk/benefit ratio on an individual patient basis, and the team tasked the oncology community with gathering comprehensive, rigorous data.
There was no funding source declared for this study. Dr. Ürün and colleagues disclosed various relationships with many pharmaceutical companies, which included receiving research funding. Dr. Sehgal and Dr. Lalani reported no relevant conflicts.
SOURCE: Ürün Y et al. JCO Glob Oncol. 2020 Aug;6:1248-57.
FROM JCO GLOBAL ONCOLOGY
Scalp Wound Closures in Mohs Micrographic Surgery: A Survey of Staples vs Sutures
Limited data exist comparing staples and sutures for scalp closures during Mohs micrographic surgery (MMS). As a result, the closure method for these scalp wounds is based on surgeon preference without established consensus. The purpose of this study was to survey practicing Mohs surgeons on their scalp wound closure preferences as well as the clinical and economic variables that impact their decisions. Understanding practice habits can guide future trial design, with a goal of creating established criterion for MMS scalp wound closures.
Methods
An anonymous survey was distributed from April 2019 to June 2019 to fellowship-trained Mohs surgeons using an electronic mailing list from the American College of Mohs Surgery (ACMS). The 10-question survey was approved by the University of Kansas institutional review board and the executive committee of the ACMS. Surgeons were asked about their preferred method for scalp wound closure as well as clinical and economic variables that impacted those preferences. Respondents indicated their frequency of using deep sutures, epidermal sutures, and wound undermining on a sliding scale of 0% to 100%. Comparisons were made between practice habits, preferences, and surgeon demographics using t tests. Statistical significance was determined as P<.05.
Results
Sixty-eight ACMS fellowship-trained Mohs surgeons completed the survey. The average age of respondents was 45 years; 69.1% (n=47) of respondents were male, and 76.5% (n=52) practiced in a private setting (Table 1). Regardless of epidermal closure type, deep suture placement was used in an average (standard deviation [SD]) of 88.8% (19.5%) of cases overall, which did not statistically differ between years of Mohs experience or practice setting (Table 2). Wound undermining was performed in an average (SD) of 83.0% (24.3%) of cases overall and was more prevalent in private vs academic settings (87.6% [17.8%] vs 65.7% [35.0%]; P<.01). Epidermal sutures were used in an average (SD) of 27.1% (33.5%) of scalp wound cases overall. Surgeons with less experience (≤5 years) used them more frequently (average [SD], 42.7% [36.2%] of cases) than surgeons with more experience (≥16 years; average [SD], 18.8% [32.6%] of cases; P=.037). There was no significant difference between epidermal suture placement rates and practice setting (average [SD], 18.1% [28.1%] of cases for academic providers vs 30.0% [34.8%] of cases with private providers; P=.210).

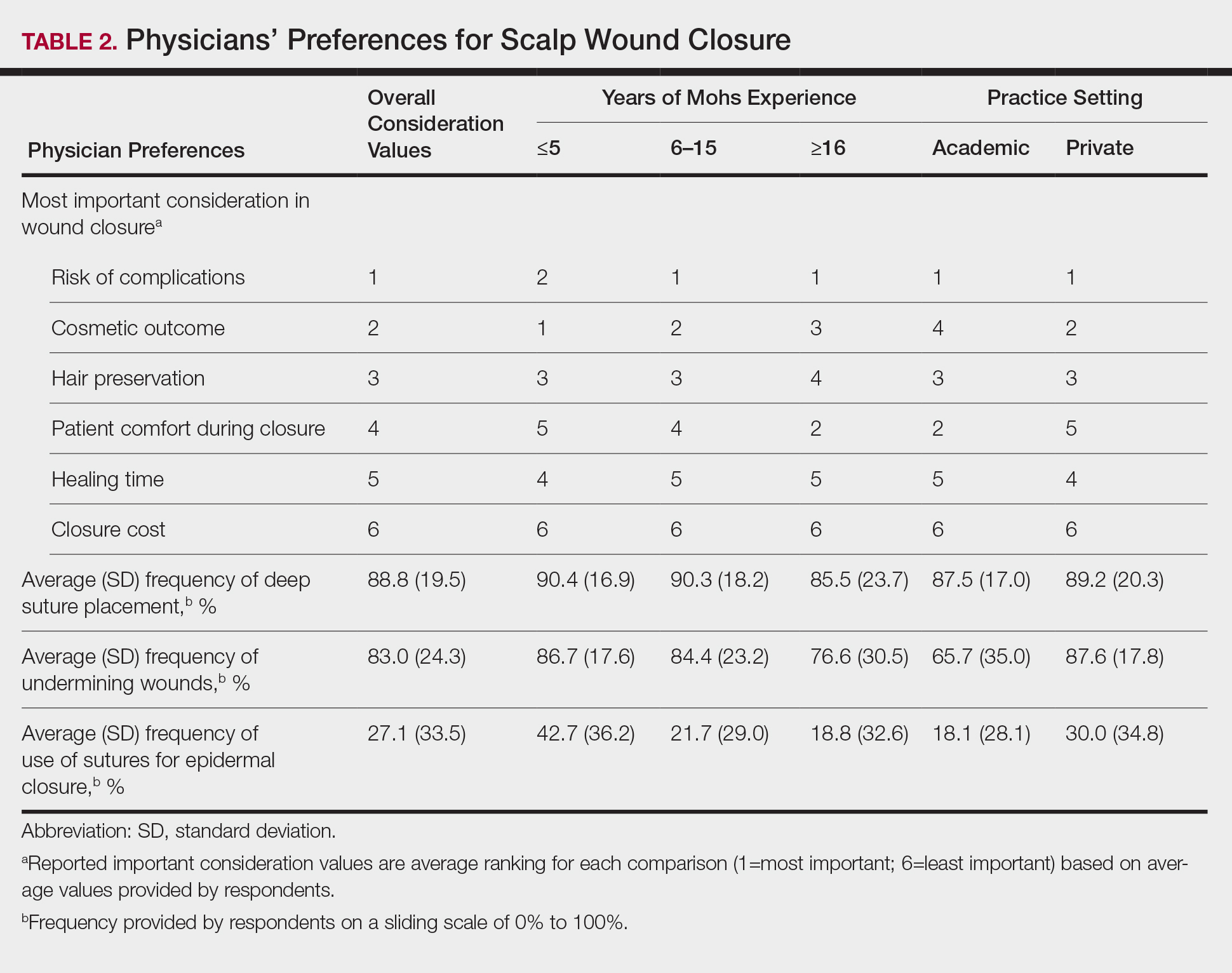
Clinical and economic factors that were most important during wound closure were ranked (beginning with most important) as the following: risk of complications, cosmetic outcome, hair preservation, patient comfort during closure, healing time, and closure cost. In all demographic cases, risk of complications was ranked 1 or 2 (1=most important; 6=least important) overall; cost was the least important factor overall (Table 2).
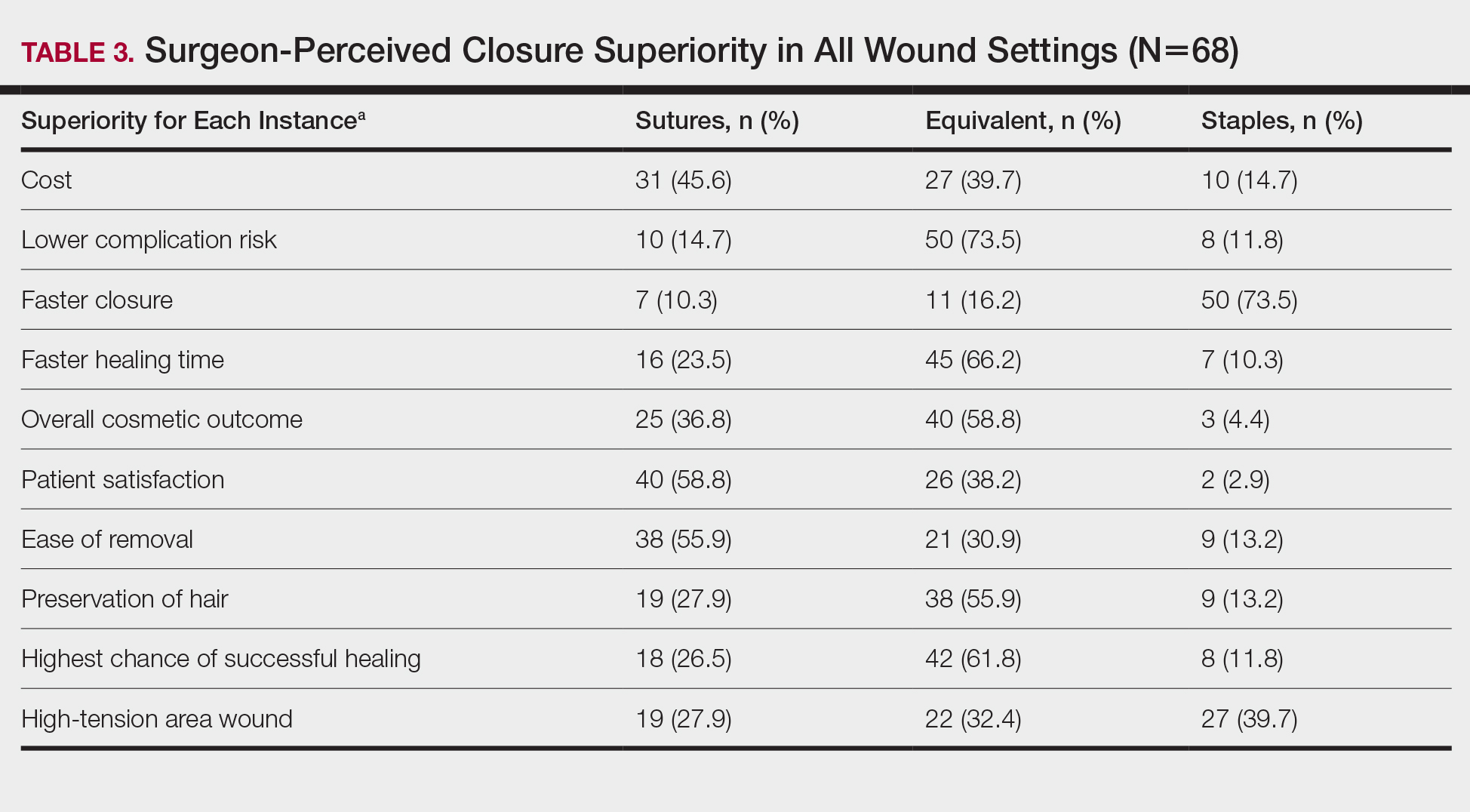
Surgeons perceived staples to be superior for speed of closure and for closing wounds in high-tension areas, whereas sutures were perceived as superior when considering cost of closure and ease of removal (Table 3). Successful healing rate, healing time, hair preservation, overall cosmetic outcome, and lower risk of complications were viewed as equivalent when comparing staples and sutures.
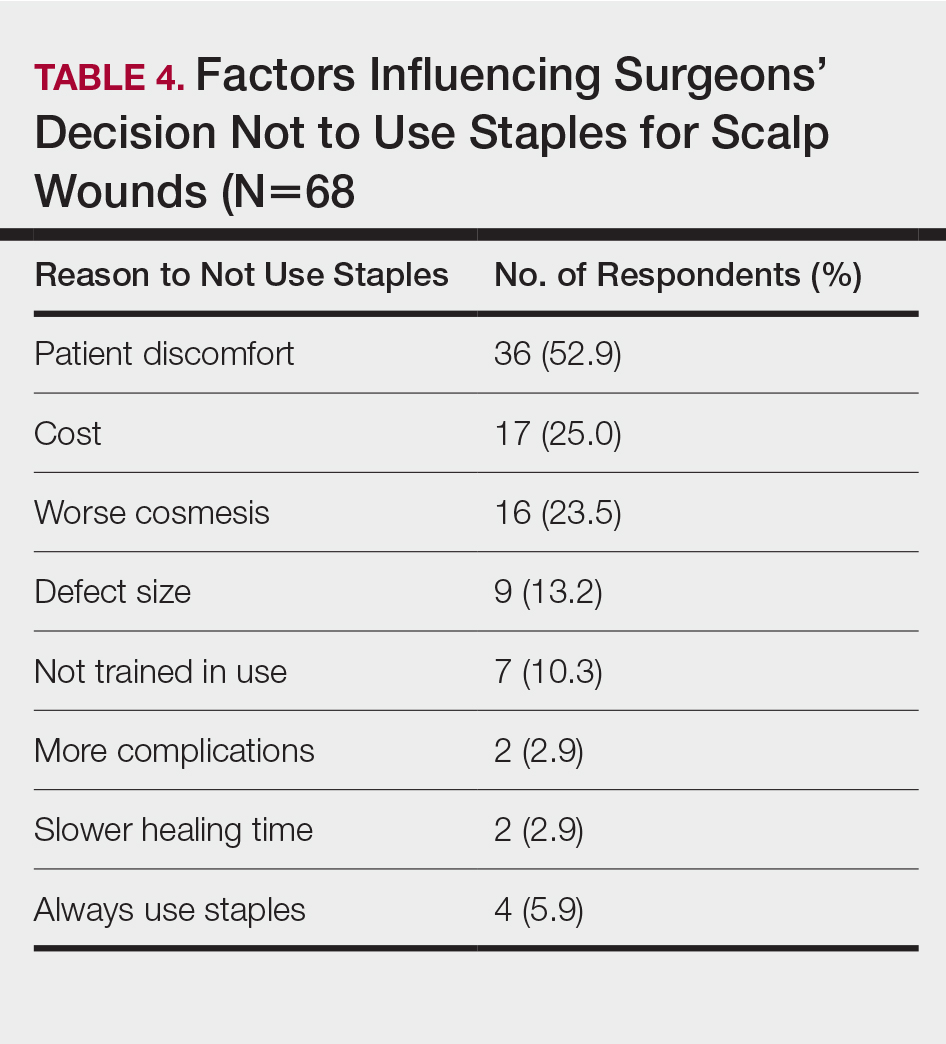
In cases in which surgeons did not use staples for closure, the most important factors for opting to not use them were patient discomfort (52.9% [n=36]), cost (25.0% [n=17]), and worse overall cosmetic outcome (23.5% [n=16])(Table 4). The most frequent locations outside of scalp wounds that physicians considered the use of staples for closure were the back (19.1% [n=13]), thigh (10.3% [n=7]), and shoulder (8.8% [n=6]).
Comment
Epidermal closure with sutures was reportedly used in an average of only 27.1% of scalp wound cases, with clinical factors such as cosmetic outcome, risk of complications, and closure time seen as either equivalent or inferior to staples. Our data suggest that surgeon closure perceptions generally are in agreement with established head and neck literature within different medical specialties that favor staple closures, particularly in high-tension areas.1 Interestingly, the most common reasons given for not using staples included patient discomfort, cost, and worse cosmetic outcomes, which are unsubstantiated with head and neck comparative studies.2-4
Although cost was the least important variable for determining closure type in our surveyed cohort, it is likely that the overall cost of closure is frequently underestimated. A higher material cost is noted with staples; however, the largest determinant of overall cost remains the surgeon’s time, which is reduced by factors of 10 or more when closing with staples.2,3 This difference—coupled with the unchanged cosmetic outcome and complication rates—makes staples more advantageous for high-tension scalp wounds.4 Moreover, the stapling technique is more reproducible than suturing, which requires more surgical skill and experience.
Limitations of this study include a lack of directly comparable data for staple and suture scalp wound closures. In addition, the small cohort of respondents in this preliminary study can serve to guide future studies.
Conclusion
Scalp wounds during MMS were most frequently closed using staples vs sutures, with the perception that these methods are equivalent in complication risk, cosmetic outcome, and overall patient satisfaction. These results agree with comparative literature for head and neck surgery and assist with establishing an epidemiologic baseline for future studies comparing their use during MMS.
- Ritchie AJ, Rocke LG. Staples versus sutures in the closure of scalp wounds: a prospective, double-blind, randomized trial. Injury. 1989;20:217-218.
- Batra J, Bekal RK, Byadgi S, et al. Comparison of skin staples and standard sutures for closing incisions after head and neck cancer surgery: a double-blind, randomized and prospective study. J Maxillofac Oral Surg. 2016;15:243-250.
- Kanegaye JT, Vance CW, Chan L, et al. Comparison of skin stapling devices and standard sutures for pediatric scalp lacerations: a randomized study of cost and time benefits. J Pediatr. 1997;130:808-813.
- Khan ANGA, Dayan PS, Miller S, et al. Cosmetic outcome of scalp wound closure with staples in the pediatric emergency department: a prospective, randomized trial. Pediatr Emerg Care. 2002;18:171-173.
Limited data exist comparing staples and sutures for scalp closures during Mohs micrographic surgery (MMS). As a result, the closure method for these scalp wounds is based on surgeon preference without established consensus. The purpose of this study was to survey practicing Mohs surgeons on their scalp wound closure preferences as well as the clinical and economic variables that impact their decisions. Understanding practice habits can guide future trial design, with a goal of creating established criterion for MMS scalp wound closures.
Methods
An anonymous survey was distributed from April 2019 to June 2019 to fellowship-trained Mohs surgeons using an electronic mailing list from the American College of Mohs Surgery (ACMS). The 10-question survey was approved by the University of Kansas institutional review board and the executive committee of the ACMS. Surgeons were asked about their preferred method for scalp wound closure as well as clinical and economic variables that impacted those preferences. Respondents indicated their frequency of using deep sutures, epidermal sutures, and wound undermining on a sliding scale of 0% to 100%. Comparisons were made between practice habits, preferences, and surgeon demographics using t tests. Statistical significance was determined as P<.05.
Results
Sixty-eight ACMS fellowship-trained Mohs surgeons completed the survey. The average age of respondents was 45 years; 69.1% (n=47) of respondents were male, and 76.5% (n=52) practiced in a private setting (Table 1). Regardless of epidermal closure type, deep suture placement was used in an average (standard deviation [SD]) of 88.8% (19.5%) of cases overall, which did not statistically differ between years of Mohs experience or practice setting (Table 2). Wound undermining was performed in an average (SD) of 83.0% (24.3%) of cases overall and was more prevalent in private vs academic settings (87.6% [17.8%] vs 65.7% [35.0%]; P<.01). Epidermal sutures were used in an average (SD) of 27.1% (33.5%) of scalp wound cases overall. Surgeons with less experience (≤5 years) used them more frequently (average [SD], 42.7% [36.2%] of cases) than surgeons with more experience (≥16 years; average [SD], 18.8% [32.6%] of cases; P=.037). There was no significant difference between epidermal suture placement rates and practice setting (average [SD], 18.1% [28.1%] of cases for academic providers vs 30.0% [34.8%] of cases with private providers; P=.210).


Clinical and economic factors that were most important during wound closure were ranked (beginning with most important) as the following: risk of complications, cosmetic outcome, hair preservation, patient comfort during closure, healing time, and closure cost. In all demographic cases, risk of complications was ranked 1 or 2 (1=most important; 6=least important) overall; cost was the least important factor overall (Table 2).
Surgeons perceived staples to be superior for speed of closure and for closing wounds in high-tension areas, whereas sutures were perceived as superior when considering cost of closure and ease of removal (Table 3). Successful healing rate, healing time, hair preservation, overall cosmetic outcome, and lower risk of complications were viewed as equivalent when comparing staples and sutures.

In cases in which surgeons did not use staples for closure, the most important factors for opting to not use them were patient discomfort (52.9% [n=36]), cost (25.0% [n=17]), and worse overall cosmetic outcome (23.5% [n=16])(Table 4). The most frequent locations outside of scalp wounds that physicians considered the use of staples for closure were the back (19.1% [n=13]), thigh (10.3% [n=7]), and shoulder (8.8% [n=6]).

Comment
Epidermal closure with sutures was reportedly used in an average of only 27.1% of scalp wound cases, with clinical factors such as cosmetic outcome, risk of complications, and closure time seen as either equivalent or inferior to staples. Our data suggest that surgeon closure perceptions generally are in agreement with established head and neck literature within different medical specialties that favor staple closures, particularly in high-tension areas.1 Interestingly, the most common reasons given for not using staples included patient discomfort, cost, and worse cosmetic outcomes, which are unsubstantiated with head and neck comparative studies.2-4
Although cost was the least important variable for determining closure type in our surveyed cohort, it is likely that the overall cost of closure is frequently underestimated. A higher material cost is noted with staples; however, the largest determinant of overall cost remains the surgeon’s time, which is reduced by factors of 10 or more when closing with staples.2,3 This difference—coupled with the unchanged cosmetic outcome and complication rates—makes staples more advantageous for high-tension scalp wounds.4 Moreover, the stapling technique is more reproducible than suturing, which requires more surgical skill and experience.
Limitations of this study include a lack of directly comparable data for staple and suture scalp wound closures. In addition, the small cohort of respondents in this preliminary study can serve to guide future studies.
Conclusion
Scalp wounds during MMS were most frequently closed using staples vs sutures, with the perception that these methods are equivalent in complication risk, cosmetic outcome, and overall patient satisfaction. These results agree with comparative literature for head and neck surgery and assist with establishing an epidemiologic baseline for future studies comparing their use during MMS.
Limited data exist comparing staples and sutures for scalp closures during Mohs micrographic surgery (MMS). As a result, the closure method for these scalp wounds is based on surgeon preference without established consensus. The purpose of this study was to survey practicing Mohs surgeons on their scalp wound closure preferences as well as the clinical and economic variables that impact their decisions. Understanding practice habits can guide future trial design, with a goal of creating established criterion for MMS scalp wound closures.
Methods
An anonymous survey was distributed from April 2019 to June 2019 to fellowship-trained Mohs surgeons using an electronic mailing list from the American College of Mohs Surgery (ACMS). The 10-question survey was approved by the University of Kansas institutional review board and the executive committee of the ACMS. Surgeons were asked about their preferred method for scalp wound closure as well as clinical and economic variables that impacted those preferences. Respondents indicated their frequency of using deep sutures, epidermal sutures, and wound undermining on a sliding scale of 0% to 100%. Comparisons were made between practice habits, preferences, and surgeon demographics using t tests. Statistical significance was determined as P<.05.
Results
Sixty-eight ACMS fellowship-trained Mohs surgeons completed the survey. The average age of respondents was 45 years; 69.1% (n=47) of respondents were male, and 76.5% (n=52) practiced in a private setting (Table 1). Regardless of epidermal closure type, deep suture placement was used in an average (standard deviation [SD]) of 88.8% (19.5%) of cases overall, which did not statistically differ between years of Mohs experience or practice setting (Table 2). Wound undermining was performed in an average (SD) of 83.0% (24.3%) of cases overall and was more prevalent in private vs academic settings (87.6% [17.8%] vs 65.7% [35.0%]; P<.01). Epidermal sutures were used in an average (SD) of 27.1% (33.5%) of scalp wound cases overall. Surgeons with less experience (≤5 years) used them more frequently (average [SD], 42.7% [36.2%] of cases) than surgeons with more experience (≥16 years; average [SD], 18.8% [32.6%] of cases; P=.037). There was no significant difference between epidermal suture placement rates and practice setting (average [SD], 18.1% [28.1%] of cases for academic providers vs 30.0% [34.8%] of cases with private providers; P=.210).


Clinical and economic factors that were most important during wound closure were ranked (beginning with most important) as the following: risk of complications, cosmetic outcome, hair preservation, patient comfort during closure, healing time, and closure cost. In all demographic cases, risk of complications was ranked 1 or 2 (1=most important; 6=least important) overall; cost was the least important factor overall (Table 2).
Surgeons perceived staples to be superior for speed of closure and for closing wounds in high-tension areas, whereas sutures were perceived as superior when considering cost of closure and ease of removal (Table 3). Successful healing rate, healing time, hair preservation, overall cosmetic outcome, and lower risk of complications were viewed as equivalent when comparing staples and sutures.

In cases in which surgeons did not use staples for closure, the most important factors for opting to not use them were patient discomfort (52.9% [n=36]), cost (25.0% [n=17]), and worse overall cosmetic outcome (23.5% [n=16])(Table 4). The most frequent locations outside of scalp wounds that physicians considered the use of staples for closure were the back (19.1% [n=13]), thigh (10.3% [n=7]), and shoulder (8.8% [n=6]).

Comment
Epidermal closure with sutures was reportedly used in an average of only 27.1% of scalp wound cases, with clinical factors such as cosmetic outcome, risk of complications, and closure time seen as either equivalent or inferior to staples. Our data suggest that surgeon closure perceptions generally are in agreement with established head and neck literature within different medical specialties that favor staple closures, particularly in high-tension areas.1 Interestingly, the most common reasons given for not using staples included patient discomfort, cost, and worse cosmetic outcomes, which are unsubstantiated with head and neck comparative studies.2-4
Although cost was the least important variable for determining closure type in our surveyed cohort, it is likely that the overall cost of closure is frequently underestimated. A higher material cost is noted with staples; however, the largest determinant of overall cost remains the surgeon’s time, which is reduced by factors of 10 or more when closing with staples.2,3 This difference—coupled with the unchanged cosmetic outcome and complication rates—makes staples more advantageous for high-tension scalp wounds.4 Moreover, the stapling technique is more reproducible than suturing, which requires more surgical skill and experience.
Limitations of this study include a lack of directly comparable data for staple and suture scalp wound closures. In addition, the small cohort of respondents in this preliminary study can serve to guide future studies.
Conclusion
Scalp wounds during MMS were most frequently closed using staples vs sutures, with the perception that these methods are equivalent in complication risk, cosmetic outcome, and overall patient satisfaction. These results agree with comparative literature for head and neck surgery and assist with establishing an epidemiologic baseline for future studies comparing their use during MMS.
- Ritchie AJ, Rocke LG. Staples versus sutures in the closure of scalp wounds: a prospective, double-blind, randomized trial. Injury. 1989;20:217-218.
- Batra J, Bekal RK, Byadgi S, et al. Comparison of skin staples and standard sutures for closing incisions after head and neck cancer surgery: a double-blind, randomized and prospective study. J Maxillofac Oral Surg. 2016;15:243-250.
- Kanegaye JT, Vance CW, Chan L, et al. Comparison of skin stapling devices and standard sutures for pediatric scalp lacerations: a randomized study of cost and time benefits. J Pediatr. 1997;130:808-813.
- Khan ANGA, Dayan PS, Miller S, et al. Cosmetic outcome of scalp wound closure with staples in the pediatric emergency department: a prospective, randomized trial. Pediatr Emerg Care. 2002;18:171-173.
- Ritchie AJ, Rocke LG. Staples versus sutures in the closure of scalp wounds: a prospective, double-blind, randomized trial. Injury. 1989;20:217-218.
- Batra J, Bekal RK, Byadgi S, et al. Comparison of skin staples and standard sutures for closing incisions after head and neck cancer surgery: a double-blind, randomized and prospective study. J Maxillofac Oral Surg. 2016;15:243-250.
- Kanegaye JT, Vance CW, Chan L, et al. Comparison of skin stapling devices and standard sutures for pediatric scalp lacerations: a randomized study of cost and time benefits. J Pediatr. 1997;130:808-813.
- Khan ANGA, Dayan PS, Miller S, et al. Cosmetic outcome of scalp wound closure with staples in the pediatric emergency department: a prospective, randomized trial. Pediatr Emerg Care. 2002;18:171-173.
Practice Points
- Scalp wounds present a unique challenge for closure during Mohs micrographic surgery due to the scalp's tendency to bleed, limited elasticity, and hair-bearing nature.
- Among fellowship-trained Mohs surgeons, scalp wounds were closed with staples more often than with epidermal sutures.
- Staples and sutures for scalp wounds were perceived to be equivalent in risk of complications, cosmetic outcome, and overall patient satisfaction.
- Compared to epidermal sutures, staples were perceived as advantageous in high-tension areas and for speed of closure.
Artificial intelligence matches cancer genotypes to patient phenotypes
Precision medicine is driven by technologies such as rapid genome sequencing and artificial intelligence (AI), according to a presentation at the AACR virtual meeting II.
AI can be applied to the sequencing information derived from advanced cancers to make highly personalized treatment recommendations for patients, said Olivier Elemento, PhD, of Weill Cornell Medicine, New York.
Dr. Elemento described such work during the opening plenary session of the meeting.
Dr. Elemento advocated for whole-genome sequencing (WGS) of metastatic sites, as it can reveal “branched evolution” as tumors progress from localized to metastatic (Nat Genet. 2016 Dec;48[12]:1490-9).
The metastases share common mutations with the primaries from which they arise but also develop their own mutational profiles, which facilitate site-of-origin-agnostic, predictive treatment choices.
As examples, Dr. Elemento mentioned HER2 amplification found in a patient with urothelial cancer (J Natl Compr Canc Netw. 2019 Mar 1;17[3]:194-200) and a patient with uterine serous carcinoma (Gynecol Oncol Rep. 2019 Feb 21;28:54-7), both of whom experienced long-lasting remissions to HER2-targeted therapy.
Dr. Elemento also noted that WGS can reveal complex structural variants in lung adenocarcinomas that lack alterations in the RTK/RAS/RAF pathway (unpublished data).
Application of machine learning
One study suggested that microRNA expression and machine learning can be used to identify malignant thyroid lesions (Clin Cancer Res. 2012 Apr 1;18[7]:2032-8). The approach diagnosed malignant lesions with 90% accuracy, 100% sensitivity, and 86% specificity.
Dr. Elemento and colleagues used a similar approach to predict response to immunotherapy in melanoma (unpublished data).
The idea was to mine the cancer genome and transcriptome, allowing for identification of signals from neoantigens, immune gene expression, immune cell composition, and T-cell receptor repertoires, Dr. Elemento said. Integrating these signals with clinical outcome data via machine learning technology enabled the researchers to predict immunotherapy response in malignant melanoma with nearly 90% accuracy.
AI and image analysis
Studies have indicated that AI can be applied to medical images to improve diagnosis and treatment. The approach has been shown to:
- Facilitate correct diagnoses of malignant skin lesions (Nature. 2017 Feb 2;542[7639]:115-8).
- Distinguish lung adenocarcinoma from squamous cell cancer with 100% accuracy (EBioMedicine. 2018 Jan;27:317-28).
- Recognize distinct breast cancer subtypes (ductal, lobular, mucinous, papillary) and biomarkers (bioRxiv 242818. doi: 10.1101/242818; EBioMedicine. 2018 Jan;27:317-28)
- Predict mesothelioma prognosis (Nat Med. 2019 Oct;25[10]:1519-25).
- Predict prostate biopsy results (unpublished data) and calculate Gleason scores that can predict survival in prostate cancer patients (AACR 2020, Abstract 867).
Drug development through applied AI
In another study, Dr. Elemento and colleagues used a Bayesian machine learning approach to predict targets of molecules without a known mechanism of action (Nat Commun. 2019 Nov 19;10[1]:5221).
The method involved using data on gene expression profiles, cell line viability, side effects in animals, and structures of the molecules. The researchers applied this method to a large library of orphan small molecules and found it could predict targets in about 40% of cases.
Of 24 AI-predicted microtubule-targeting molecules, 14 depolymerized microtubules in the lab. Five of these molecules were effective in cell lines that were resistant to other microtubule-targeted drugs.
Dr. Elemento went on to describe how Oncoceutics was developing an antineoplastic agent called ONC201, but the company lacked information about the agent’s target. Using AI, the target was identified as dopamine receptor 2 (DRD2; Clin Cancer Res. 2019 Apr 1;25[7]:2305-13).
With that information, Oncoceutics initiated trials of ONC201 in tumors expressing high levels of DRD2, including a highly resistant glioma (J Neurooncol. 2019 Oct;145[1]:97-105). Responses were seen, and ONC201 is now being tested against other DRD2-expressing cancers.
Challenges to acknowledge
Potential benefits of AI in the clinic are exciting, but there are many bench-to-bedside challenges.
A clinically obvious example of AI’s applications is radiographic image analysis. There is no biologic rationale for our RECIST “cut values” for partial response, minimal response, and stable disease.
If AI can measure subtle changes on imaging that correlate with tumor biology (i.e., radiomics), we stand a better chance of predicting treatment outcomes than we can with conventional measurements of shrinkage of arbitrarily selected “target lesions.”
A tremendous amount of work is needed to build the required large image banks. During that time, AI will only improve – and without the human risks of fatigue, inconsistency, or burnout.
Those human frailties notwithstanding, AI cannot substitute for the key discussions between patient and clinician regarding goals of care, trade-offs of risks and benefits, and shared decision-making regarding management options.
At least initially (but painfully), complex technologies like WGS and digital image analysis via AI may further disadvantage patients who are medically disadvantaged by geography or socioeconomic circumstances.
In the discussion period, AACR President Antoni Ribas, MD, of University of California, Los Angeles, asked whether AI can simulate crosstalk between gene pathways so that unique treatment combinations can be identified. Dr. Elemento said those simulations are the subject of ongoing investigation.
The theme of the opening plenary session at the AACR virtual meeting II was “Turning Science into Life-Saving Care.” Applications of AI to optimize personalized use of genomics, digital image analysis, and drug development show great promise for being among the technologies that can help to realize AACR’s thematic vision.
Dr. Elemento disclosed relationships with Volastra Therapeutics, OneThree Biotech, Owkin, Freenome, Genetic Intelligence, Acuamark Diagnostics, Eli Lilly, Janssen, and Sanofi.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Precision medicine is driven by technologies such as rapid genome sequencing and artificial intelligence (AI), according to a presentation at the AACR virtual meeting II.
AI can be applied to the sequencing information derived from advanced cancers to make highly personalized treatment recommendations for patients, said Olivier Elemento, PhD, of Weill Cornell Medicine, New York.
Dr. Elemento described such work during the opening plenary session of the meeting.
Dr. Elemento advocated for whole-genome sequencing (WGS) of metastatic sites, as it can reveal “branched evolution” as tumors progress from localized to metastatic (Nat Genet. 2016 Dec;48[12]:1490-9).
The metastases share common mutations with the primaries from which they arise but also develop their own mutational profiles, which facilitate site-of-origin-agnostic, predictive treatment choices.
As examples, Dr. Elemento mentioned HER2 amplification found in a patient with urothelial cancer (J Natl Compr Canc Netw. 2019 Mar 1;17[3]:194-200) and a patient with uterine serous carcinoma (Gynecol Oncol Rep. 2019 Feb 21;28:54-7), both of whom experienced long-lasting remissions to HER2-targeted therapy.
Dr. Elemento also noted that WGS can reveal complex structural variants in lung adenocarcinomas that lack alterations in the RTK/RAS/RAF pathway (unpublished data).
Application of machine learning
One study suggested that microRNA expression and machine learning can be used to identify malignant thyroid lesions (Clin Cancer Res. 2012 Apr 1;18[7]:2032-8). The approach diagnosed malignant lesions with 90% accuracy, 100% sensitivity, and 86% specificity.
Dr. Elemento and colleagues used a similar approach to predict response to immunotherapy in melanoma (unpublished data).
The idea was to mine the cancer genome and transcriptome, allowing for identification of signals from neoantigens, immune gene expression, immune cell composition, and T-cell receptor repertoires, Dr. Elemento said. Integrating these signals with clinical outcome data via machine learning technology enabled the researchers to predict immunotherapy response in malignant melanoma with nearly 90% accuracy.
AI and image analysis
Studies have indicated that AI can be applied to medical images to improve diagnosis and treatment. The approach has been shown to:
- Facilitate correct diagnoses of malignant skin lesions (Nature. 2017 Feb 2;542[7639]:115-8).
- Distinguish lung adenocarcinoma from squamous cell cancer with 100% accuracy (EBioMedicine. 2018 Jan;27:317-28).
- Recognize distinct breast cancer subtypes (ductal, lobular, mucinous, papillary) and biomarkers (bioRxiv 242818. doi: 10.1101/242818; EBioMedicine. 2018 Jan;27:317-28)
- Predict mesothelioma prognosis (Nat Med. 2019 Oct;25[10]:1519-25).
- Predict prostate biopsy results (unpublished data) and calculate Gleason scores that can predict survival in prostate cancer patients (AACR 2020, Abstract 867).
Drug development through applied AI
In another study, Dr. Elemento and colleagues used a Bayesian machine learning approach to predict targets of molecules without a known mechanism of action (Nat Commun. 2019 Nov 19;10[1]:5221).
The method involved using data on gene expression profiles, cell line viability, side effects in animals, and structures of the molecules. The researchers applied this method to a large library of orphan small molecules and found it could predict targets in about 40% of cases.
Of 24 AI-predicted microtubule-targeting molecules, 14 depolymerized microtubules in the lab. Five of these molecules were effective in cell lines that were resistant to other microtubule-targeted drugs.
Dr. Elemento went on to describe how Oncoceutics was developing an antineoplastic agent called ONC201, but the company lacked information about the agent’s target. Using AI, the target was identified as dopamine receptor 2 (DRD2; Clin Cancer Res. 2019 Apr 1;25[7]:2305-13).
With that information, Oncoceutics initiated trials of ONC201 in tumors expressing high levels of DRD2, including a highly resistant glioma (J Neurooncol. 2019 Oct;145[1]:97-105). Responses were seen, and ONC201 is now being tested against other DRD2-expressing cancers.
Challenges to acknowledge
Potential benefits of AI in the clinic are exciting, but there are many bench-to-bedside challenges.
A clinically obvious example of AI’s applications is radiographic image analysis. There is no biologic rationale for our RECIST “cut values” for partial response, minimal response, and stable disease.
If AI can measure subtle changes on imaging that correlate with tumor biology (i.e., radiomics), we stand a better chance of predicting treatment outcomes than we can with conventional measurements of shrinkage of arbitrarily selected “target lesions.”
A tremendous amount of work is needed to build the required large image banks. During that time, AI will only improve – and without the human risks of fatigue, inconsistency, or burnout.
Those human frailties notwithstanding, AI cannot substitute for the key discussions between patient and clinician regarding goals of care, trade-offs of risks and benefits, and shared decision-making regarding management options.
At least initially (but painfully), complex technologies like WGS and digital image analysis via AI may further disadvantage patients who are medically disadvantaged by geography or socioeconomic circumstances.
In the discussion period, AACR President Antoni Ribas, MD, of University of California, Los Angeles, asked whether AI can simulate crosstalk between gene pathways so that unique treatment combinations can be identified. Dr. Elemento said those simulations are the subject of ongoing investigation.
The theme of the opening plenary session at the AACR virtual meeting II was “Turning Science into Life-Saving Care.” Applications of AI to optimize personalized use of genomics, digital image analysis, and drug development show great promise for being among the technologies that can help to realize AACR’s thematic vision.
Dr. Elemento disclosed relationships with Volastra Therapeutics, OneThree Biotech, Owkin, Freenome, Genetic Intelligence, Acuamark Diagnostics, Eli Lilly, Janssen, and Sanofi.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Precision medicine is driven by technologies such as rapid genome sequencing and artificial intelligence (AI), according to a presentation at the AACR virtual meeting II.
AI can be applied to the sequencing information derived from advanced cancers to make highly personalized treatment recommendations for patients, said Olivier Elemento, PhD, of Weill Cornell Medicine, New York.
Dr. Elemento described such work during the opening plenary session of the meeting.
Dr. Elemento advocated for whole-genome sequencing (WGS) of metastatic sites, as it can reveal “branched evolution” as tumors progress from localized to metastatic (Nat Genet. 2016 Dec;48[12]:1490-9).
The metastases share common mutations with the primaries from which they arise but also develop their own mutational profiles, which facilitate site-of-origin-agnostic, predictive treatment choices.
As examples, Dr. Elemento mentioned HER2 amplification found in a patient with urothelial cancer (J Natl Compr Canc Netw. 2019 Mar 1;17[3]:194-200) and a patient with uterine serous carcinoma (Gynecol Oncol Rep. 2019 Feb 21;28:54-7), both of whom experienced long-lasting remissions to HER2-targeted therapy.
Dr. Elemento also noted that WGS can reveal complex structural variants in lung adenocarcinomas that lack alterations in the RTK/RAS/RAF pathway (unpublished data).
Application of machine learning
One study suggested that microRNA expression and machine learning can be used to identify malignant thyroid lesions (Clin Cancer Res. 2012 Apr 1;18[7]:2032-8). The approach diagnosed malignant lesions with 90% accuracy, 100% sensitivity, and 86% specificity.
Dr. Elemento and colleagues used a similar approach to predict response to immunotherapy in melanoma (unpublished data).
The idea was to mine the cancer genome and transcriptome, allowing for identification of signals from neoantigens, immune gene expression, immune cell composition, and T-cell receptor repertoires, Dr. Elemento said. Integrating these signals with clinical outcome data via machine learning technology enabled the researchers to predict immunotherapy response in malignant melanoma with nearly 90% accuracy.
AI and image analysis
Studies have indicated that AI can be applied to medical images to improve diagnosis and treatment. The approach has been shown to:
- Facilitate correct diagnoses of malignant skin lesions (Nature. 2017 Feb 2;542[7639]:115-8).
- Distinguish lung adenocarcinoma from squamous cell cancer with 100% accuracy (EBioMedicine. 2018 Jan;27:317-28).
- Recognize distinct breast cancer subtypes (ductal, lobular, mucinous, papillary) and biomarkers (bioRxiv 242818. doi: 10.1101/242818; EBioMedicine. 2018 Jan;27:317-28)
- Predict mesothelioma prognosis (Nat Med. 2019 Oct;25[10]:1519-25).
- Predict prostate biopsy results (unpublished data) and calculate Gleason scores that can predict survival in prostate cancer patients (AACR 2020, Abstract 867).
Drug development through applied AI
In another study, Dr. Elemento and colleagues used a Bayesian machine learning approach to predict targets of molecules without a known mechanism of action (Nat Commun. 2019 Nov 19;10[1]:5221).
The method involved using data on gene expression profiles, cell line viability, side effects in animals, and structures of the molecules. The researchers applied this method to a large library of orphan small molecules and found it could predict targets in about 40% of cases.
Of 24 AI-predicted microtubule-targeting molecules, 14 depolymerized microtubules in the lab. Five of these molecules were effective in cell lines that were resistant to other microtubule-targeted drugs.
Dr. Elemento went on to describe how Oncoceutics was developing an antineoplastic agent called ONC201, but the company lacked information about the agent’s target. Using AI, the target was identified as dopamine receptor 2 (DRD2; Clin Cancer Res. 2019 Apr 1;25[7]:2305-13).
With that information, Oncoceutics initiated trials of ONC201 in tumors expressing high levels of DRD2, including a highly resistant glioma (J Neurooncol. 2019 Oct;145[1]:97-105). Responses were seen, and ONC201 is now being tested against other DRD2-expressing cancers.
Challenges to acknowledge
Potential benefits of AI in the clinic are exciting, but there are many bench-to-bedside challenges.
A clinically obvious example of AI’s applications is radiographic image analysis. There is no biologic rationale for our RECIST “cut values” for partial response, minimal response, and stable disease.
If AI can measure subtle changes on imaging that correlate with tumor biology (i.e., radiomics), we stand a better chance of predicting treatment outcomes than we can with conventional measurements of shrinkage of arbitrarily selected “target lesions.”
A tremendous amount of work is needed to build the required large image banks. During that time, AI will only improve – and without the human risks of fatigue, inconsistency, or burnout.
Those human frailties notwithstanding, AI cannot substitute for the key discussions between patient and clinician regarding goals of care, trade-offs of risks and benefits, and shared decision-making regarding management options.
At least initially (but painfully), complex technologies like WGS and digital image analysis via AI may further disadvantage patients who are medically disadvantaged by geography or socioeconomic circumstances.
In the discussion period, AACR President Antoni Ribas, MD, of University of California, Los Angeles, asked whether AI can simulate crosstalk between gene pathways so that unique treatment combinations can be identified. Dr. Elemento said those simulations are the subject of ongoing investigation.
The theme of the opening plenary session at the AACR virtual meeting II was “Turning Science into Life-Saving Care.” Applications of AI to optimize personalized use of genomics, digital image analysis, and drug development show great promise for being among the technologies that can help to realize AACR’s thematic vision.
Dr. Elemento disclosed relationships with Volastra Therapeutics, OneThree Biotech, Owkin, Freenome, Genetic Intelligence, Acuamark Diagnostics, Eli Lilly, Janssen, and Sanofi.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM AACR 2020
Study: Immune checkpoint inhibitors don’t increase risk of death in cancer patients with COVID-19
The study included 113 cancer patients who had laboratory-confirmed COVID-19 within 12 months of receiving immune checkpoint inhibitor therapy. The patients did not receive chemotherapy within 3 months of testing positive for COVID-19.
In all, 33 patients were admitted to the hospital, including 6 who were admitted to the ICU, and 9 patients died.
“Nine out of 113 patients is a mortality rate of 8%, which is in the middle of the earlier reported rates for cancer patients in general [7.6%-12%],” said Aljosja Rogiers, MD, PhD, of the Melanoma Institute Australia in Sydney.
COVID-19 was the primary cause of death in seven of the patients, including three of those who were admitted to the ICU, Dr. Rogiers noted.
He reported these results during the AACR virtual meeting: COVID-19 and Cancer.
Study details
Patients in this study were treated at 19 hospitals in North America, Europe, and Australia, and the data cutoff was May 15, 2020. Most patients (64%) were treated in Europe, which was the epicenter for the COVID-19 pandemic at the time of data collection, Dr. Rogiers noted. A third of patients were in North America, and 3% were in Australia.
The patients’ median age was 63 years (range, 27-86 years). Most patients were men (65%), and most had Eastern Cooperative Oncology Group performance scores of 0-1 (90%).
The most common malignancies were melanoma (57%), non–small cell lung cancer (17%), and renal cell carcinoma (9%). Treatment was for early cancer in 26% of patients and for advanced cancer in 74%. Comorbidities included cardiovascular disease in 27% of patients, diabetes in 15%, pulmonary disease in 12%, and renal disease in 5%.
Immunosuppressive therapy equivalent to a prednisone dose of 10 mg or greater daily was given in 13% of patients, and other immunosuppressive therapies, such as infliximab, were given in 3%.
Among the 60% of patients with COVID-19 symptoms, 68% had fever, 59% had cough, 34% had dyspnea, and 15% had myalgia. Most of the 40% of asymptomatic patients were tested because they had COVID-19–positive contact, Dr. Rogiers noted.
Immune checkpoint inhibitor treatment included monotherapy with a programmed death–1/PD–ligand 1 inhibitor in 82% of patients, combination anti-PD-1 and anti-CTLA4 therapy in 13%, and other therapy – usually a checkpoint inhibitor combined with a different type of targeted agent – in 5%.
At the time of COVID-19 diagnosis, 30% of patients had achieved a partial response, complete response, or had no evidence of disease, 18% had stable disease, and 15% had progression. Response data were not available in 37% of cases, usually because treatment was only recently started prior to COVID-19 diagnosis, Dr. Rogiers said.
Treatments administered for COVID-19 included antibiotic therapy in 25% of patients, oxygen therapy in 20%, glucocorticoids in 10%, antiviral drugs in 6%, and intravenous immunoglobulin or anti–interleukin-6 in 2% each.
Among patients admitted to the ICU, 3% required mechanical ventilation, 2% had vasopressin, and 1% received renal replacement therapy.
At the data cutoff, 20 of 33 hospitalized patients (61%) had been discharged, and 4 (12%) were still in the hospital.
Mortality results
Nine patients died. The rate of death was 8% overall and 27% among hospitalized patients.
“The mortality rate of COVID-19 in the general population without comorbidities is about 1.4%,” Dr. Rogiers said. “For cancer patients, this is reported to be in the range of 7.6%-12%. To what extent patients on immune checkpoint inhibition are at a higher risk of mortality is currently unknown.”
Theoretically, immune checkpoint inhibition could either mitigate or exacerbate COVID-19 infection. It has been hypothesized that immune checkpoint inhibitors could increase the risk of severe acute lung injury or other complications of COVID-19, Dr. Rogiers said, explaining the rationale for the study.
The study shows that the patients who died had a median age of 72 years (range, 49-81 years), which is slightly higher than the median overall age of 63 years. Six patients were from North America, and three were from Italy.
“Two melanoma patients and two non–small cell lung cancer patients died,” Dr. Rogiers said. He noted that two other deaths were in patients with renal cell carcinoma, and three deaths were in other cancer types. All patients had advanced or metastatic disease.
Given that 57% of patients in the study had melanoma and 17% had NSCLC, this finding may indicate that COVID-19 has a slightly higher mortality rate in NSCLC patients than in melanoma patients, but the numbers are small, Dr. Rogiers said.
Notably, six of the patients who died were not admitted to the ICU. In four cases, this was because of underlying malignancy; in the other two cases, it was because of a constrained health care system, Dr. Rogiers said.
Overall, the findings show that the mortality rate of patients with COVID-19 and cancer treated with immune checkpoint inhibitors is similar to the mortality rate reported in the general cancer population, Dr. Rogiers said.
“Treatment with immune checkpoint inhibition does not seem to pose an additional mortality risk for cancer patients with COVID-19,” he concluded.
Dr. Rogiers reported having no conflicts of interest. There was no funding disclosed for the study.
SOURCE: Rogiers A et al. AACR: COVID-19 and Cancer, Abstract S02-01.
The study included 113 cancer patients who had laboratory-confirmed COVID-19 within 12 months of receiving immune checkpoint inhibitor therapy. The patients did not receive chemotherapy within 3 months of testing positive for COVID-19.
In all, 33 patients were admitted to the hospital, including 6 who were admitted to the ICU, and 9 patients died.
“Nine out of 113 patients is a mortality rate of 8%, which is in the middle of the earlier reported rates for cancer patients in general [7.6%-12%],” said Aljosja Rogiers, MD, PhD, of the Melanoma Institute Australia in Sydney.
COVID-19 was the primary cause of death in seven of the patients, including three of those who were admitted to the ICU, Dr. Rogiers noted.
He reported these results during the AACR virtual meeting: COVID-19 and Cancer.
Study details
Patients in this study were treated at 19 hospitals in North America, Europe, and Australia, and the data cutoff was May 15, 2020. Most patients (64%) were treated in Europe, which was the epicenter for the COVID-19 pandemic at the time of data collection, Dr. Rogiers noted. A third of patients were in North America, and 3% were in Australia.
The patients’ median age was 63 years (range, 27-86 years). Most patients were men (65%), and most had Eastern Cooperative Oncology Group performance scores of 0-1 (90%).
The most common malignancies were melanoma (57%), non–small cell lung cancer (17%), and renal cell carcinoma (9%). Treatment was for early cancer in 26% of patients and for advanced cancer in 74%. Comorbidities included cardiovascular disease in 27% of patients, diabetes in 15%, pulmonary disease in 12%, and renal disease in 5%.
Immunosuppressive therapy equivalent to a prednisone dose of 10 mg or greater daily was given in 13% of patients, and other immunosuppressive therapies, such as infliximab, were given in 3%.
Among the 60% of patients with COVID-19 symptoms, 68% had fever, 59% had cough, 34% had dyspnea, and 15% had myalgia. Most of the 40% of asymptomatic patients were tested because they had COVID-19–positive contact, Dr. Rogiers noted.
Immune checkpoint inhibitor treatment included monotherapy with a programmed death–1/PD–ligand 1 inhibitor in 82% of patients, combination anti-PD-1 and anti-CTLA4 therapy in 13%, and other therapy – usually a checkpoint inhibitor combined with a different type of targeted agent – in 5%.
At the time of COVID-19 diagnosis, 30% of patients had achieved a partial response, complete response, or had no evidence of disease, 18% had stable disease, and 15% had progression. Response data were not available in 37% of cases, usually because treatment was only recently started prior to COVID-19 diagnosis, Dr. Rogiers said.
Treatments administered for COVID-19 included antibiotic therapy in 25% of patients, oxygen therapy in 20%, glucocorticoids in 10%, antiviral drugs in 6%, and intravenous immunoglobulin or anti–interleukin-6 in 2% each.
Among patients admitted to the ICU, 3% required mechanical ventilation, 2% had vasopressin, and 1% received renal replacement therapy.
At the data cutoff, 20 of 33 hospitalized patients (61%) had been discharged, and 4 (12%) were still in the hospital.
Mortality results
Nine patients died. The rate of death was 8% overall and 27% among hospitalized patients.
“The mortality rate of COVID-19 in the general population without comorbidities is about 1.4%,” Dr. Rogiers said. “For cancer patients, this is reported to be in the range of 7.6%-12%. To what extent patients on immune checkpoint inhibition are at a higher risk of mortality is currently unknown.”
Theoretically, immune checkpoint inhibition could either mitigate or exacerbate COVID-19 infection. It has been hypothesized that immune checkpoint inhibitors could increase the risk of severe acute lung injury or other complications of COVID-19, Dr. Rogiers said, explaining the rationale for the study.
The study shows that the patients who died had a median age of 72 years (range, 49-81 years), which is slightly higher than the median overall age of 63 years. Six patients were from North America, and three were from Italy.
“Two melanoma patients and two non–small cell lung cancer patients died,” Dr. Rogiers said. He noted that two other deaths were in patients with renal cell carcinoma, and three deaths were in other cancer types. All patients had advanced or metastatic disease.
Given that 57% of patients in the study had melanoma and 17% had NSCLC, this finding may indicate that COVID-19 has a slightly higher mortality rate in NSCLC patients than in melanoma patients, but the numbers are small, Dr. Rogiers said.
Notably, six of the patients who died were not admitted to the ICU. In four cases, this was because of underlying malignancy; in the other two cases, it was because of a constrained health care system, Dr. Rogiers said.
Overall, the findings show that the mortality rate of patients with COVID-19 and cancer treated with immune checkpoint inhibitors is similar to the mortality rate reported in the general cancer population, Dr. Rogiers said.
“Treatment with immune checkpoint inhibition does not seem to pose an additional mortality risk for cancer patients with COVID-19,” he concluded.
Dr. Rogiers reported having no conflicts of interest. There was no funding disclosed for the study.
SOURCE: Rogiers A et al. AACR: COVID-19 and Cancer, Abstract S02-01.
The study included 113 cancer patients who had laboratory-confirmed COVID-19 within 12 months of receiving immune checkpoint inhibitor therapy. The patients did not receive chemotherapy within 3 months of testing positive for COVID-19.
In all, 33 patients were admitted to the hospital, including 6 who were admitted to the ICU, and 9 patients died.
“Nine out of 113 patients is a mortality rate of 8%, which is in the middle of the earlier reported rates for cancer patients in general [7.6%-12%],” said Aljosja Rogiers, MD, PhD, of the Melanoma Institute Australia in Sydney.
COVID-19 was the primary cause of death in seven of the patients, including three of those who were admitted to the ICU, Dr. Rogiers noted.
He reported these results during the AACR virtual meeting: COVID-19 and Cancer.
Study details
Patients in this study were treated at 19 hospitals in North America, Europe, and Australia, and the data cutoff was May 15, 2020. Most patients (64%) were treated in Europe, which was the epicenter for the COVID-19 pandemic at the time of data collection, Dr. Rogiers noted. A third of patients were in North America, and 3% were in Australia.
The patients’ median age was 63 years (range, 27-86 years). Most patients were men (65%), and most had Eastern Cooperative Oncology Group performance scores of 0-1 (90%).
The most common malignancies were melanoma (57%), non–small cell lung cancer (17%), and renal cell carcinoma (9%). Treatment was for early cancer in 26% of patients and for advanced cancer in 74%. Comorbidities included cardiovascular disease in 27% of patients, diabetes in 15%, pulmonary disease in 12%, and renal disease in 5%.
Immunosuppressive therapy equivalent to a prednisone dose of 10 mg or greater daily was given in 13% of patients, and other immunosuppressive therapies, such as infliximab, were given in 3%.
Among the 60% of patients with COVID-19 symptoms, 68% had fever, 59% had cough, 34% had dyspnea, and 15% had myalgia. Most of the 40% of asymptomatic patients were tested because they had COVID-19–positive contact, Dr. Rogiers noted.
Immune checkpoint inhibitor treatment included monotherapy with a programmed death–1/PD–ligand 1 inhibitor in 82% of patients, combination anti-PD-1 and anti-CTLA4 therapy in 13%, and other therapy – usually a checkpoint inhibitor combined with a different type of targeted agent – in 5%.
At the time of COVID-19 diagnosis, 30% of patients had achieved a partial response, complete response, or had no evidence of disease, 18% had stable disease, and 15% had progression. Response data were not available in 37% of cases, usually because treatment was only recently started prior to COVID-19 diagnosis, Dr. Rogiers said.
Treatments administered for COVID-19 included antibiotic therapy in 25% of patients, oxygen therapy in 20%, glucocorticoids in 10%, antiviral drugs in 6%, and intravenous immunoglobulin or anti–interleukin-6 in 2% each.
Among patients admitted to the ICU, 3% required mechanical ventilation, 2% had vasopressin, and 1% received renal replacement therapy.
At the data cutoff, 20 of 33 hospitalized patients (61%) had been discharged, and 4 (12%) were still in the hospital.
Mortality results
Nine patients died. The rate of death was 8% overall and 27% among hospitalized patients.
“The mortality rate of COVID-19 in the general population without comorbidities is about 1.4%,” Dr. Rogiers said. “For cancer patients, this is reported to be in the range of 7.6%-12%. To what extent patients on immune checkpoint inhibition are at a higher risk of mortality is currently unknown.”
Theoretically, immune checkpoint inhibition could either mitigate or exacerbate COVID-19 infection. It has been hypothesized that immune checkpoint inhibitors could increase the risk of severe acute lung injury or other complications of COVID-19, Dr. Rogiers said, explaining the rationale for the study.
The study shows that the patients who died had a median age of 72 years (range, 49-81 years), which is slightly higher than the median overall age of 63 years. Six patients were from North America, and three were from Italy.
“Two melanoma patients and two non–small cell lung cancer patients died,” Dr. Rogiers said. He noted that two other deaths were in patients with renal cell carcinoma, and three deaths were in other cancer types. All patients had advanced or metastatic disease.
Given that 57% of patients in the study had melanoma and 17% had NSCLC, this finding may indicate that COVID-19 has a slightly higher mortality rate in NSCLC patients than in melanoma patients, but the numbers are small, Dr. Rogiers said.
Notably, six of the patients who died were not admitted to the ICU. In four cases, this was because of underlying malignancy; in the other two cases, it was because of a constrained health care system, Dr. Rogiers said.
Overall, the findings show that the mortality rate of patients with COVID-19 and cancer treated with immune checkpoint inhibitors is similar to the mortality rate reported in the general cancer population, Dr. Rogiers said.
“Treatment with immune checkpoint inhibition does not seem to pose an additional mortality risk for cancer patients with COVID-19,” he concluded.
Dr. Rogiers reported having no conflicts of interest. There was no funding disclosed for the study.
SOURCE: Rogiers A et al. AACR: COVID-19 and Cancer, Abstract S02-01.
FROM AACR: COVID-19 AND CANCER
Risk Factors and Management of Skin Cancer Among Active-Duty Servicemembers and Veterans
Melanoma Risk for Servicemembers
Dr. Dunn: Active-duty jobs are quite diverse. We have had almost every civilian occupation category—everything from clerical to food service to outdoor construction workers. Federal service and active-duty military service could lead to assignments that involve high sunlight exposure and subsequently higher risk for melanoma and nonmelanoma skin cancer.
Dr. Miller: I found 2 articles on the topic. The first published in June 2018 reviewed melanoma and nonmelanoma skin cancers in the military.1 Riemenschneider and colleagues1 looked at 9 studies. Statistically, there was increased risk of melanoma associated with service and/or prisoner-of-war status. In World War II, they found tropical environments had the highest risk. And the highest rates were in the US Air Force.
The other article provided US Department of Defense data on skin cancer incidence rates, incidence rates of malignant melanoma in relation to years of military service overall, and the rates for differing military occupational groups.2 The researchers demonstrated that fixed-wing pilots and crew members had the highest rates of developing melanoma. The general trend was that the incidence rate was exponentially higher with more missions flown in relation to years of active service, which I thought was rather interesting.
For other occupational categories, the rate increase was not as great as those involved in aviation. Yes, it’s probably related to exposure. Flying at 40,000 feet on a transcontinental airplane trip is equivalent to the radiation dosage of a chest X-ray. Given all the training time and operational flying for the Air Force, it is anticipated that that mutagenic radiation would increase rates. An aircraft does not offer a lot of protection, especially in the cockpit.
We just had the anniversary of the Apollo 11 mission. Those astronauts received the equivalent of about 40 chest X-rays going to the moon and back. Exposure to UV and at higher altitudes cosmic radiation explains why we would see that more in Air Force personnel.
Dr. Bandino: At high altitude there is less ozone protecting you, although the shielding in a cockpit is better in modern aircraft. As an Air Force member, that was one of the first things I thought about was that an aviator has increased skin cancer risk. But it’s apt to think of military service in general as an occupational risk because there are so many contingency operations and deployments. Regarding sun exposure, sunscreen is provided nowadays and there is more sun awareness, but there is still a stigma and reluctance to apply the sunscreen. It leaves people’s skin feeling greasy, which is not ideal when one has to handle a firearm. It can also get in someone’s eyes and affect vision and performance during combat operations. In other words, there are many reasons that would reduce the desire to wear sunscreen and therefore increase exposure to the elements.
A great current example is coronavirus disease 2019 (COVID-19) operations. Although I’m a dermatologist and typically work inside, I’ve been tasked to run a COVID-19 screening tent in the middle of a field in San Antonio, and thus I’ve got to make sure I take my sunscreen out there every day. The general population may not have that variability in their work cycle and sudden change in occupational UV exposure.
Dr. Miller: I was deployed in a combat zone for operations Desert Shield and Desert Storm. I was with the 2nd Armored Division of the US Army deployed to the desert. There really wasn’t an emphasis on photoprotection. It’s just the logistics. The commanders have a lot more important things to think about, and that’s something, usually, that doesn’t get a high priority. The US military is deploying to more places near the equator, so from an operational sense, there’s probably something to brief the commanders about in terms of the long-term consequences of radiation exposure for military servicemembers.
Dr. Dunn: If you look at deployments over the past 2 decades, we have been putting tens of thousands of individuals in high UV exposure regions. Then you have to look at the long-term consequence of the increased incidence of skin cancer in those individuals. What is the cost of that when it comes to treatment of precancerous lesions and skin cancer throughout a life expectancy of 80-plus years?
Dr. Bandino: With most skin cancers there is such long lag time between exposures and development. I wish there were some better data and research out there that really showed whether military service truly is an independent risk factor or if it’s just specific occupation types within the military. I have family members who both work in contracting services and had served in the military. Would their skin cancer risk be the same as others who are doing similar jobs without the military service?
Dr. Dunn: I have had county employees present for skin cancer surgery and with them comes a form that relates to disability. For groundskeepers or police, we assumed that skin cancer is occupation related due to the patient’s increased sun exposure. Their cancers may be unrelated to their actual years of service, but it seems that many light-skinned individuals in the military are going to develop basal cell and squamous cell skin cancer in the coming decades, which likely is going to be attributed to their years of federal service, even though they may have had other significant recreational exposure outside of work. So, my gut feeling is that we are going to see skin cancer as a disability tied to federal service, which is going to cost us.
Dr. Logemann: Yes, I think there are always going to be confounders—what if the servicemembers used tanning beds, or they were avid surfers? It’s going to be difficult to always parse that out.
Dr. Miller: In talking about melanoma, you really have to parse out the subsets. Is it melanoma in situ, is it superficial, is it acral, is it nodular? They all have different initiation events.
Nodular melanomas probably don’t need UV light to initiate a tumor. Another risk factor is having more than 100 moles or many atypical moles, which puts that person in a higher risk category. Perhaps when soldiers, airmen, and navy personnel get inducted, they should be screened for their mole population because that is a risk factor for developing melanoma, and then we can intervene a little bit and have them watch their UV exposure.
Dr. Jarell: You can’t overstate the importance of how heterogeneous melanoma is as a disease. While there are clearly some types of melanoma that are caused by UV radiation, there are also many types that aren’t. We don’t understand why someone gets melanoma on the inner thigh, bottom of the foot, top of the sole, inside the mouth, or in the genital region—these aren’t places of high sun exposure.
Lentigo maligna, as an example, is clearly caused by UV radiation in most cases. But there are so many other different types of melanoma that you can’t just attribute to UV radiation, and so you get into this whole other discussion as to why people are getting melanoma—military or not.
Dr. Bandino: When volunteering for military service, there’s the DoDMERB (Department of Defense Medical Examination Review Board) system that screens individuals for medical issues incompatible with military service such as severe psoriasis or atopic dermatitis. But to my knowledge, the DoDMERB process focuses more on current or past issues and does little to investigate for future risk of disease. A cutaneous example would be assessing quantity of dysplastic nevi, Fitzpatrick scale 1 phenotype, and family history of melanoma to determine risk of developing melanoma in someone who may have more UV exposure during their military service than a civilian. This dermatological future risk assessment was certainly not something I was trained to do as a flight surgeon when performing basic trainee flight physicals prior to becoming a dermatologist.
Dr. Jarell: I am a little bit hard-pressed to generalize the military as high occupational risk for melanoma. There are clearly other professions—landscapers, fishermen—that are probably at much higher risk than, say, your general military all-comers. Us physicians in the military were probably not at increased risk compared to other physicians in the United States. We have to be careful not to go down a slippery slope and designate all MOSs (military occupational specialties) as at increased risk for skin cancer, in particular melanoma. Nonmelanoma skin cancer, such as basal cell and squamous cell carcinoma, is clearly related to the proportional amount of UV exposure. But melanoma is quite a diverse cancer that has many, many disparate etiologies.
Dr. Dunn: The entry physical into the military is an opportunity to make an impact on the number of nonmelanoma skin cancers that would arise in that population. There is an educational opportunity to tell inductees that nonmelanoma skin cancer is going to occur on convex surfaces of the sun-exposed skin—nose, ears, forehead, chin, tops of the shoulders. If offered sun protection for those areas and you stretch the potential impact of that information over tens of thousands of military members over decades, you might actually come up with a big number of people that not only decreases their morbidity but also dramatically decreased the cost to the system as a whole.
Dr. Jarell: You also have to factor in ethnicity and the role it plays in someone’s likelihood to get skin cancer—melanoma or nonmelanoma skin cancer. Darker-skinned people are at certainly decreased risk for different types of skin cancers.
Dr. Dunn: Yes, that would have to be part of the education and should be. If you have light skin and freckles, then you’re at much higher risk for nonmelanoma skin cancer and need to know the high-risk areas that can be protected by sunblock and clothing.
Dr. Logemann: One thing that might be a little bit unique in the military is that you’re living in San Antonio one minute, and then the next minute you’re over in Afghanistan with a different climate and different environment. When you’re deployed overseas, you might have a little bit less control over your situation; you might not have a lot of sunscreen in a field hospital in Afghanistan. Whereas if you were just living in San Antonio, you could go down to the store and buy it.
Dr. Miller: Is sunblock now encouraged or available to individuals in deployment situations or training situations where they’re going to have prolonged sun exposure every day? Is it part of the regimen, just like carrying extra water because of the risk for dehydration?
Dr. Logemann: To the best of my knowledge, it is not always included in your normal rations or uniform and it may be up to the servicemember to procure sunscreen.
Dr. Bandino: There have been improvements, and usually you at least have access to sunscreen. In many deployed locations, for example, you have the equivalent of a small PX (post exchange) or BX (base exchange), where they have a variety of products for sale from toothbrushes to flip-flops, and now also sunscreen. Of course, the type and quality of the sunscreen may not be that great. It’s likely going to be basic SPF (sun protection factor) 15 or 30 in small tubes. As a recent example, I participated in a humanitarian medical exercise in South America last summer and was actually issued sunscreen combined with DEET, which is great but it was only SPF 30. The combination product is a good idea for tropical locations, but in addition to people just not wanting to wear it, the DEET combination tends to burn and sting a little bit more; you can get a heat sensation from the DEET; and the DEET can damage plastic surfaces, which may not be ideal for deployed equipment.
The other problem is quantity. We all learned in residency the appropriate sunscreen quantity of at least 1 fl oz for the average adult body, and that’s what we counsel our patients on, but what they issued me was 1 small 2- to 3-fl oz tube. It fit in the palm of my hand, and that was my sunscreen for the trip.
So, I do think, even though there have been some improvements, much of sun protection will still fall on the individual servicemember. And, as mentioned, depending on your ethnicity, some people may need it more than others. But it is an area where there probably could be continued improvements.
Dr. Logemann: In addition to sunscreen, I think that maybe we should be taking into consideration some simple measures. For example, is it necessary for people to stand out in formation at 2
Dr. Dunn: I think we all kind of agree that the military service is diverse and that many of the subcategories of occupations within the military lead to increased sun exposure by mandate. We advise sun protection by physical barriers and sunblock.
Diagnosis of Skin Cancer Via Telemedicine
Dr. Dunn: I have friends who remain in the VA (US Department of Veterans Affairs) system, and they are involved with telemedicine in dermatology, which can reduce waiting time and increase the number of patients seen by the dermatologist. In-person and teledermatology visits now are available to servicemembers on active duty and retirees.
Dr. Bandino: At our residency program (San Antonio Uniformed Services Health Education Consortium), we’ve had asynchronous teledermatology for over a decade, even before I was a resident. We provide it primarily as a service for patients at small bases without access to dermatology. Some bases also use it as part of their prescreening process prior to authorizing an in-person dermatology consultation.
Certainly, with the coronavirus pandemic, civilian dermatology is seeing a boom in the teledermatology world that had been slowly increasing in popularity for the last few years. In our residency program, teledermatology has traditionally been just for active-duty servicemembers or their dependents, but now due to the coronavirus pandemic, our teledermatology services have significantly expanded to include adding synchronous capability. We have patients take pictures before their virtual appointment and/or FaceTime during the appointment. Even after the pandemic, there will likely be more integration of synchronous teledermatology going forward as we’re seeing some of the value. Of course, I’m sure we would all agree that accurate diagnosis of pigmented lesions can be very challenging with teledermatology, not to mention other diagnostic limitations. But I think there is still utility and it should only get better with time as technology improves. So, I’m hopeful that we can incorporate more of it in the military.
Dr. Logemann: I’m definitely aware that we have different telehealth opportunities available, even using some newer modalities that are command approved in recent weeks. My experience has been for more complicated dermatology, so people are in remote locations, and they’re being seen by a nondermatologist, and they have questions about how to approach management. But I’m not aware of telemedicine as a screening tool for skin cancer in the military or among my civilian colleagues. I would hope that it could be someday because we’re developing these total-body photography machines as well. It could be a way for a nondermatologist who identifies a lesion to have it triaged by a dermatologist. To say, “Oh yeah, that looks like a melanoma. They need to get in sooner vs later,” but not on a large-scale sort of screening modality.
Dr. Bandino: In my recent experience, it has definitely been a helpful triage tool. In the military, this form of triage can be particularly helpful if someone is overseas to determine whether he/she needs to evacuated and evaluated in-person right away.
Dr. Jarell: It’s been useful in looking at benign things. People have shown me in the past few weeks a lot of seborrheic keratoses and a lot of benign dermal nevus-type things, and I say, “Don’t worry about that.” And you can tell if the resolution is good enough. But a lot of people have shown me things in the past few weeks that have clearly been basal cell carcinoma, which we can probably let that ride out for a few more weeks, but I’m not sure if maybe somebody has an amelanotic melanoma. Maybe you need to come in and get that biopsied ASAP. Or something that looks like a melanoma. The patient should probably come in and get that biopsied.
Dr. Miller: I think we can rely on teledermatology. It’s all predicated on the resolution because we’re all trained in pattern recognition. I think it’s very useful to screen for things that look clinically benign. We have to understand that most dermatology is practiced by nondermatologists in the United States, and many studies show that their diagnostic accuracy is 20%, at best maybe 50%. So, they do need to reach out to a dermatologist and perhaps get some guidance on what to do. I think it could be a very useful tool if used appropriately.
Dr. Dunn: If used appropriately, teledermatology could function in a couple of ways. One, it could allow us to declare lesions to be wholly benign, and only should a lesion change would it need attention. The second is that it would allow us to accelerate the process of getting a patient to us—physically in front of us—for a biopsy if a suspicious lesion is seen. A by-product of that process would be that if patients who have wholly benign, nonworrisome lesions could be screened by telemedicine, then physical appointments where a patient is in front of the doctor would be more open. In other words, let’s say if 25% of all lesional visits could be declared benign via telemedicine that would allow dermatology to preserve its face-to-face appointments for patients who are more likely to have cancer and require procedures like skin biopsy.
Love it or hate it, I think we’re getting it no matter what now. Telemedicine creeped along forever and within 6 weeks it’s become ubiquitous. It’s phenomenal how fast we had to adapt to a system or perish in private practice. Sometimes these episodes that we go through have good consequences as well as bad consequences. Telemedicine probably has been needed for a long time and the insurers were not covering it very well, but suddenly a stay-at-home mandate has unveiled valuable technology—something that we probably should have been able to use more and be adequately reimbursed.
Surgical Treatment of Skin Cancer
Dr. Dunn: Treatment historically has been surgical for nonmelanoma and melanoma skin cancers. Some radiation devices have gained popularity again in the past decade or so, but excisional surgery remains the standard treatment for skin cancer. Nonmelanoma skin cancers almost all are probably treated surgically still, with a small percentage treated with superficial radiation.
Access to care is important to discuss. Are Mohs surgeons readily available, or are plastic surgeons, general surgeons, or vascular surgeons in the federal system contributing to the care of skin cancer? Are they doing excisional surgery after biopsies are done? Are they doing excisional biopsies with the intent of cure?
Dr. Logemann: For active duty, I don’t see any issues getting access to the medical center for Mohs micrographic surgery. Sometimes, if we have a lot of volume, some patients may get deferred to the network, but in my experience, it would not typically be an active-duty servicemember. An active-duty servicemember would get care rendered at one of the medical centers for Mohs surgery. Typically the active-duty–aged population isn’t getting much skin cancer. It certainly does happen, but most of the skin cancers frequently that are treated at medical centers are not infrequently retirees.
Dr. Bandino: Because of our residency program, we are required to have Mohs surgery capability to be ACGME (Accreditation Council for Graduate Medical Education) accredited. We typically have 3 Mohs surgeons, so we never have a problem with access.
In the military, I just refer cases to our Mohs surgeons and everything is taken care of in-house. In fact, this is an area where we may even have better access than the civilian world because there are no insurance hurdles or significant delay in care since our Mohs surgeons aren’t typically booked up for 3 to 4 months like many civilian Mohs surgeons. This is especially true for complex cases since we provide hospital-based care with all specialty services under the same umbrella. So, for example, if the Mohs surgeons have an extensive and complex case requiring multidisciplinary care such as ENT (ear, nose, and throat), facial plastics, or radiation-oncology, they’re all in-house with no insurance issues to navigate. This of course is not usual for most military bases and is only capable at bases attached to a large medical center. There are some similar scenarios in the civilian world with university medical centers and managed care organizations, but we may still have a slight advantage in accessibility and cost.
Dr. Dunn: There are guidelines from the National Comprehensive Cancer Network as to how to treat nonmelanoma and melanoma skin cancer. Almost all of them are surgical and almost all of them are safe, outpatient, local anesthetic procedures with a high cure rate. The vast majority of melanoma and nonmelanoma skin cancers can be handled safely and effectively with minimal morbidity and almost no known mortalities from the treatments themselves. Some of the cancers have been identified as high risk for metastasis and mortality, but they’re relatively uncommon still. The good news about skin cancer is that the risk of death remains very small.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel.J Am Acad Dermatol. 2018;78:1185-1192.
- Brundage JF, Williams VF, Stahlman S, et al. Incidence rates of malignant melanoma in relation to years of military service, overall and in selected military occupational groups, active component, U.S. Armed Forces, 2001-2015. MSMR. 2017;24:8-14.
Melanoma Risk for Servicemembers
Dr. Dunn: Active-duty jobs are quite diverse. We have had almost every civilian occupation category—everything from clerical to food service to outdoor construction workers. Federal service and active-duty military service could lead to assignments that involve high sunlight exposure and subsequently higher risk for melanoma and nonmelanoma skin cancer.
Dr. Miller: I found 2 articles on the topic. The first published in June 2018 reviewed melanoma and nonmelanoma skin cancers in the military.1 Riemenschneider and colleagues1 looked at 9 studies. Statistically, there was increased risk of melanoma associated with service and/or prisoner-of-war status. In World War II, they found tropical environments had the highest risk. And the highest rates were in the US Air Force.
The other article provided US Department of Defense data on skin cancer incidence rates, incidence rates of malignant melanoma in relation to years of military service overall, and the rates for differing military occupational groups.2 The researchers demonstrated that fixed-wing pilots and crew members had the highest rates of developing melanoma. The general trend was that the incidence rate was exponentially higher with more missions flown in relation to years of active service, which I thought was rather interesting.
For other occupational categories, the rate increase was not as great as those involved in aviation. Yes, it’s probably related to exposure. Flying at 40,000 feet on a transcontinental airplane trip is equivalent to the radiation dosage of a chest X-ray. Given all the training time and operational flying for the Air Force, it is anticipated that that mutagenic radiation would increase rates. An aircraft does not offer a lot of protection, especially in the cockpit.
We just had the anniversary of the Apollo 11 mission. Those astronauts received the equivalent of about 40 chest X-rays going to the moon and back. Exposure to UV and at higher altitudes cosmic radiation explains why we would see that more in Air Force personnel.
Dr. Bandino: At high altitude there is less ozone protecting you, although the shielding in a cockpit is better in modern aircraft. As an Air Force member, that was one of the first things I thought about was that an aviator has increased skin cancer risk. But it’s apt to think of military service in general as an occupational risk because there are so many contingency operations and deployments. Regarding sun exposure, sunscreen is provided nowadays and there is more sun awareness, but there is still a stigma and reluctance to apply the sunscreen. It leaves people’s skin feeling greasy, which is not ideal when one has to handle a firearm. It can also get in someone’s eyes and affect vision and performance during combat operations. In other words, there are many reasons that would reduce the desire to wear sunscreen and therefore increase exposure to the elements.
A great current example is coronavirus disease 2019 (COVID-19) operations. Although I’m a dermatologist and typically work inside, I’ve been tasked to run a COVID-19 screening tent in the middle of a field in San Antonio, and thus I’ve got to make sure I take my sunscreen out there every day. The general population may not have that variability in their work cycle and sudden change in occupational UV exposure.
Dr. Miller: I was deployed in a combat zone for operations Desert Shield and Desert Storm. I was with the 2nd Armored Division of the US Army deployed to the desert. There really wasn’t an emphasis on photoprotection. It’s just the logistics. The commanders have a lot more important things to think about, and that’s something, usually, that doesn’t get a high priority. The US military is deploying to more places near the equator, so from an operational sense, there’s probably something to brief the commanders about in terms of the long-term consequences of radiation exposure for military servicemembers.
Dr. Dunn: If you look at deployments over the past 2 decades, we have been putting tens of thousands of individuals in high UV exposure regions. Then you have to look at the long-term consequence of the increased incidence of skin cancer in those individuals. What is the cost of that when it comes to treatment of precancerous lesions and skin cancer throughout a life expectancy of 80-plus years?
Dr. Bandino: With most skin cancers there is such long lag time between exposures and development. I wish there were some better data and research out there that really showed whether military service truly is an independent risk factor or if it’s just specific occupation types within the military. I have family members who both work in contracting services and had served in the military. Would their skin cancer risk be the same as others who are doing similar jobs without the military service?
Dr. Dunn: I have had county employees present for skin cancer surgery and with them comes a form that relates to disability. For groundskeepers or police, we assumed that skin cancer is occupation related due to the patient’s increased sun exposure. Their cancers may be unrelated to their actual years of service, but it seems that many light-skinned individuals in the military are going to develop basal cell and squamous cell skin cancer in the coming decades, which likely is going to be attributed to their years of federal service, even though they may have had other significant recreational exposure outside of work. So, my gut feeling is that we are going to see skin cancer as a disability tied to federal service, which is going to cost us.
Dr. Logemann: Yes, I think there are always going to be confounders—what if the servicemembers used tanning beds, or they were avid surfers? It’s going to be difficult to always parse that out.
Dr. Miller: In talking about melanoma, you really have to parse out the subsets. Is it melanoma in situ, is it superficial, is it acral, is it nodular? They all have different initiation events.
Nodular melanomas probably don’t need UV light to initiate a tumor. Another risk factor is having more than 100 moles or many atypical moles, which puts that person in a higher risk category. Perhaps when soldiers, airmen, and navy personnel get inducted, they should be screened for their mole population because that is a risk factor for developing melanoma, and then we can intervene a little bit and have them watch their UV exposure.
Dr. Jarell: You can’t overstate the importance of how heterogeneous melanoma is as a disease. While there are clearly some types of melanoma that are caused by UV radiation, there are also many types that aren’t. We don’t understand why someone gets melanoma on the inner thigh, bottom of the foot, top of the sole, inside the mouth, or in the genital region—these aren’t places of high sun exposure.
Lentigo maligna, as an example, is clearly caused by UV radiation in most cases. But there are so many other different types of melanoma that you can’t just attribute to UV radiation, and so you get into this whole other discussion as to why people are getting melanoma—military or not.
Dr. Bandino: When volunteering for military service, there’s the DoDMERB (Department of Defense Medical Examination Review Board) system that screens individuals for medical issues incompatible with military service such as severe psoriasis or atopic dermatitis. But to my knowledge, the DoDMERB process focuses more on current or past issues and does little to investigate for future risk of disease. A cutaneous example would be assessing quantity of dysplastic nevi, Fitzpatrick scale 1 phenotype, and family history of melanoma to determine risk of developing melanoma in someone who may have more UV exposure during their military service than a civilian. This dermatological future risk assessment was certainly not something I was trained to do as a flight surgeon when performing basic trainee flight physicals prior to becoming a dermatologist.
Dr. Jarell: I am a little bit hard-pressed to generalize the military as high occupational risk for melanoma. There are clearly other professions—landscapers, fishermen—that are probably at much higher risk than, say, your general military all-comers. Us physicians in the military were probably not at increased risk compared to other physicians in the United States. We have to be careful not to go down a slippery slope and designate all MOSs (military occupational specialties) as at increased risk for skin cancer, in particular melanoma. Nonmelanoma skin cancer, such as basal cell and squamous cell carcinoma, is clearly related to the proportional amount of UV exposure. But melanoma is quite a diverse cancer that has many, many disparate etiologies.
Dr. Dunn: The entry physical into the military is an opportunity to make an impact on the number of nonmelanoma skin cancers that would arise in that population. There is an educational opportunity to tell inductees that nonmelanoma skin cancer is going to occur on convex surfaces of the sun-exposed skin—nose, ears, forehead, chin, tops of the shoulders. If offered sun protection for those areas and you stretch the potential impact of that information over tens of thousands of military members over decades, you might actually come up with a big number of people that not only decreases their morbidity but also dramatically decreased the cost to the system as a whole.
Dr. Jarell: You also have to factor in ethnicity and the role it plays in someone’s likelihood to get skin cancer—melanoma or nonmelanoma skin cancer. Darker-skinned people are at certainly decreased risk for different types of skin cancers.
Dr. Dunn: Yes, that would have to be part of the education and should be. If you have light skin and freckles, then you’re at much higher risk for nonmelanoma skin cancer and need to know the high-risk areas that can be protected by sunblock and clothing.
Dr. Logemann: One thing that might be a little bit unique in the military is that you’re living in San Antonio one minute, and then the next minute you’re over in Afghanistan with a different climate and different environment. When you’re deployed overseas, you might have a little bit less control over your situation; you might not have a lot of sunscreen in a field hospital in Afghanistan. Whereas if you were just living in San Antonio, you could go down to the store and buy it.
Dr. Miller: Is sunblock now encouraged or available to individuals in deployment situations or training situations where they’re going to have prolonged sun exposure every day? Is it part of the regimen, just like carrying extra water because of the risk for dehydration?
Dr. Logemann: To the best of my knowledge, it is not always included in your normal rations or uniform and it may be up to the servicemember to procure sunscreen.
Dr. Bandino: There have been improvements, and usually you at least have access to sunscreen. In many deployed locations, for example, you have the equivalent of a small PX (post exchange) or BX (base exchange), where they have a variety of products for sale from toothbrushes to flip-flops, and now also sunscreen. Of course, the type and quality of the sunscreen may not be that great. It’s likely going to be basic SPF (sun protection factor) 15 or 30 in small tubes. As a recent example, I participated in a humanitarian medical exercise in South America last summer and was actually issued sunscreen combined with DEET, which is great but it was only SPF 30. The combination product is a good idea for tropical locations, but in addition to people just not wanting to wear it, the DEET combination tends to burn and sting a little bit more; you can get a heat sensation from the DEET; and the DEET can damage plastic surfaces, which may not be ideal for deployed equipment.
The other problem is quantity. We all learned in residency the appropriate sunscreen quantity of at least 1 fl oz for the average adult body, and that’s what we counsel our patients on, but what they issued me was 1 small 2- to 3-fl oz tube. It fit in the palm of my hand, and that was my sunscreen for the trip.
So, I do think, even though there have been some improvements, much of sun protection will still fall on the individual servicemember. And, as mentioned, depending on your ethnicity, some people may need it more than others. But it is an area where there probably could be continued improvements.
Dr. Logemann: In addition to sunscreen, I think that maybe we should be taking into consideration some simple measures. For example, is it necessary for people to stand out in formation at 2
Dr. Dunn: I think we all kind of agree that the military service is diverse and that many of the subcategories of occupations within the military lead to increased sun exposure by mandate. We advise sun protection by physical barriers and sunblock.
Diagnosis of Skin Cancer Via Telemedicine
Dr. Dunn: I have friends who remain in the VA (US Department of Veterans Affairs) system, and they are involved with telemedicine in dermatology, which can reduce waiting time and increase the number of patients seen by the dermatologist. In-person and teledermatology visits now are available to servicemembers on active duty and retirees.
Dr. Bandino: At our residency program (San Antonio Uniformed Services Health Education Consortium), we’ve had asynchronous teledermatology for over a decade, even before I was a resident. We provide it primarily as a service for patients at small bases without access to dermatology. Some bases also use it as part of their prescreening process prior to authorizing an in-person dermatology consultation.
Certainly, with the coronavirus pandemic, civilian dermatology is seeing a boom in the teledermatology world that had been slowly increasing in popularity for the last few years. In our residency program, teledermatology has traditionally been just for active-duty servicemembers or their dependents, but now due to the coronavirus pandemic, our teledermatology services have significantly expanded to include adding synchronous capability. We have patients take pictures before their virtual appointment and/or FaceTime during the appointment. Even after the pandemic, there will likely be more integration of synchronous teledermatology going forward as we’re seeing some of the value. Of course, I’m sure we would all agree that accurate diagnosis of pigmented lesions can be very challenging with teledermatology, not to mention other diagnostic limitations. But I think there is still utility and it should only get better with time as technology improves. So, I’m hopeful that we can incorporate more of it in the military.
Dr. Logemann: I’m definitely aware that we have different telehealth opportunities available, even using some newer modalities that are command approved in recent weeks. My experience has been for more complicated dermatology, so people are in remote locations, and they’re being seen by a nondermatologist, and they have questions about how to approach management. But I’m not aware of telemedicine as a screening tool for skin cancer in the military or among my civilian colleagues. I would hope that it could be someday because we’re developing these total-body photography machines as well. It could be a way for a nondermatologist who identifies a lesion to have it triaged by a dermatologist. To say, “Oh yeah, that looks like a melanoma. They need to get in sooner vs later,” but not on a large-scale sort of screening modality.
Dr. Bandino: In my recent experience, it has definitely been a helpful triage tool. In the military, this form of triage can be particularly helpful if someone is overseas to determine whether he/she needs to evacuated and evaluated in-person right away.
Dr. Jarell: It’s been useful in looking at benign things. People have shown me in the past few weeks a lot of seborrheic keratoses and a lot of benign dermal nevus-type things, and I say, “Don’t worry about that.” And you can tell if the resolution is good enough. But a lot of people have shown me things in the past few weeks that have clearly been basal cell carcinoma, which we can probably let that ride out for a few more weeks, but I’m not sure if maybe somebody has an amelanotic melanoma. Maybe you need to come in and get that biopsied ASAP. Or something that looks like a melanoma. The patient should probably come in and get that biopsied.
Dr. Miller: I think we can rely on teledermatology. It’s all predicated on the resolution because we’re all trained in pattern recognition. I think it’s very useful to screen for things that look clinically benign. We have to understand that most dermatology is practiced by nondermatologists in the United States, and many studies show that their diagnostic accuracy is 20%, at best maybe 50%. So, they do need to reach out to a dermatologist and perhaps get some guidance on what to do. I think it could be a very useful tool if used appropriately.
Dr. Dunn: If used appropriately, teledermatology could function in a couple of ways. One, it could allow us to declare lesions to be wholly benign, and only should a lesion change would it need attention. The second is that it would allow us to accelerate the process of getting a patient to us—physically in front of us—for a biopsy if a suspicious lesion is seen. A by-product of that process would be that if patients who have wholly benign, nonworrisome lesions could be screened by telemedicine, then physical appointments where a patient is in front of the doctor would be more open. In other words, let’s say if 25% of all lesional visits could be declared benign via telemedicine that would allow dermatology to preserve its face-to-face appointments for patients who are more likely to have cancer and require procedures like skin biopsy.
Love it or hate it, I think we’re getting it no matter what now. Telemedicine creeped along forever and within 6 weeks it’s become ubiquitous. It’s phenomenal how fast we had to adapt to a system or perish in private practice. Sometimes these episodes that we go through have good consequences as well as bad consequences. Telemedicine probably has been needed for a long time and the insurers were not covering it very well, but suddenly a stay-at-home mandate has unveiled valuable technology—something that we probably should have been able to use more and be adequately reimbursed.
Surgical Treatment of Skin Cancer
Dr. Dunn: Treatment historically has been surgical for nonmelanoma and melanoma skin cancers. Some radiation devices have gained popularity again in the past decade or so, but excisional surgery remains the standard treatment for skin cancer. Nonmelanoma skin cancers almost all are probably treated surgically still, with a small percentage treated with superficial radiation.
Access to care is important to discuss. Are Mohs surgeons readily available, or are plastic surgeons, general surgeons, or vascular surgeons in the federal system contributing to the care of skin cancer? Are they doing excisional surgery after biopsies are done? Are they doing excisional biopsies with the intent of cure?
Dr. Logemann: For active duty, I don’t see any issues getting access to the medical center for Mohs micrographic surgery. Sometimes, if we have a lot of volume, some patients may get deferred to the network, but in my experience, it would not typically be an active-duty servicemember. An active-duty servicemember would get care rendered at one of the medical centers for Mohs surgery. Typically the active-duty–aged population isn’t getting much skin cancer. It certainly does happen, but most of the skin cancers frequently that are treated at medical centers are not infrequently retirees.
Dr. Bandino: Because of our residency program, we are required to have Mohs surgery capability to be ACGME (Accreditation Council for Graduate Medical Education) accredited. We typically have 3 Mohs surgeons, so we never have a problem with access.
In the military, I just refer cases to our Mohs surgeons and everything is taken care of in-house. In fact, this is an area where we may even have better access than the civilian world because there are no insurance hurdles or significant delay in care since our Mohs surgeons aren’t typically booked up for 3 to 4 months like many civilian Mohs surgeons. This is especially true for complex cases since we provide hospital-based care with all specialty services under the same umbrella. So, for example, if the Mohs surgeons have an extensive and complex case requiring multidisciplinary care such as ENT (ear, nose, and throat), facial plastics, or radiation-oncology, they’re all in-house with no insurance issues to navigate. This of course is not usual for most military bases and is only capable at bases attached to a large medical center. There are some similar scenarios in the civilian world with university medical centers and managed care organizations, but we may still have a slight advantage in accessibility and cost.
Dr. Dunn: There are guidelines from the National Comprehensive Cancer Network as to how to treat nonmelanoma and melanoma skin cancer. Almost all of them are surgical and almost all of them are safe, outpatient, local anesthetic procedures with a high cure rate. The vast majority of melanoma and nonmelanoma skin cancers can be handled safely and effectively with minimal morbidity and almost no known mortalities from the treatments themselves. Some of the cancers have been identified as high risk for metastasis and mortality, but they’re relatively uncommon still. The good news about skin cancer is that the risk of death remains very small.
Melanoma Risk for Servicemembers
Dr. Dunn: Active-duty jobs are quite diverse. We have had almost every civilian occupation category—everything from clerical to food service to outdoor construction workers. Federal service and active-duty military service could lead to assignments that involve high sunlight exposure and subsequently higher risk for melanoma and nonmelanoma skin cancer.
Dr. Miller: I found 2 articles on the topic. The first published in June 2018 reviewed melanoma and nonmelanoma skin cancers in the military.1 Riemenschneider and colleagues1 looked at 9 studies. Statistically, there was increased risk of melanoma associated with service and/or prisoner-of-war status. In World War II, they found tropical environments had the highest risk. And the highest rates were in the US Air Force.
The other article provided US Department of Defense data on skin cancer incidence rates, incidence rates of malignant melanoma in relation to years of military service overall, and the rates for differing military occupational groups.2 The researchers demonstrated that fixed-wing pilots and crew members had the highest rates of developing melanoma. The general trend was that the incidence rate was exponentially higher with more missions flown in relation to years of active service, which I thought was rather interesting.
For other occupational categories, the rate increase was not as great as those involved in aviation. Yes, it’s probably related to exposure. Flying at 40,000 feet on a transcontinental airplane trip is equivalent to the radiation dosage of a chest X-ray. Given all the training time and operational flying for the Air Force, it is anticipated that that mutagenic radiation would increase rates. An aircraft does not offer a lot of protection, especially in the cockpit.
We just had the anniversary of the Apollo 11 mission. Those astronauts received the equivalent of about 40 chest X-rays going to the moon and back. Exposure to UV and at higher altitudes cosmic radiation explains why we would see that more in Air Force personnel.
Dr. Bandino: At high altitude there is less ozone protecting you, although the shielding in a cockpit is better in modern aircraft. As an Air Force member, that was one of the first things I thought about was that an aviator has increased skin cancer risk. But it’s apt to think of military service in general as an occupational risk because there are so many contingency operations and deployments. Regarding sun exposure, sunscreen is provided nowadays and there is more sun awareness, but there is still a stigma and reluctance to apply the sunscreen. It leaves people’s skin feeling greasy, which is not ideal when one has to handle a firearm. It can also get in someone’s eyes and affect vision and performance during combat operations. In other words, there are many reasons that would reduce the desire to wear sunscreen and therefore increase exposure to the elements.
A great current example is coronavirus disease 2019 (COVID-19) operations. Although I’m a dermatologist and typically work inside, I’ve been tasked to run a COVID-19 screening tent in the middle of a field in San Antonio, and thus I’ve got to make sure I take my sunscreen out there every day. The general population may not have that variability in their work cycle and sudden change in occupational UV exposure.
Dr. Miller: I was deployed in a combat zone for operations Desert Shield and Desert Storm. I was with the 2nd Armored Division of the US Army deployed to the desert. There really wasn’t an emphasis on photoprotection. It’s just the logistics. The commanders have a lot more important things to think about, and that’s something, usually, that doesn’t get a high priority. The US military is deploying to more places near the equator, so from an operational sense, there’s probably something to brief the commanders about in terms of the long-term consequences of radiation exposure for military servicemembers.
Dr. Dunn: If you look at deployments over the past 2 decades, we have been putting tens of thousands of individuals in high UV exposure regions. Then you have to look at the long-term consequence of the increased incidence of skin cancer in those individuals. What is the cost of that when it comes to treatment of precancerous lesions and skin cancer throughout a life expectancy of 80-plus years?
Dr. Bandino: With most skin cancers there is such long lag time between exposures and development. I wish there were some better data and research out there that really showed whether military service truly is an independent risk factor or if it’s just specific occupation types within the military. I have family members who both work in contracting services and had served in the military. Would their skin cancer risk be the same as others who are doing similar jobs without the military service?
Dr. Dunn: I have had county employees present for skin cancer surgery and with them comes a form that relates to disability. For groundskeepers or police, we assumed that skin cancer is occupation related due to the patient’s increased sun exposure. Their cancers may be unrelated to their actual years of service, but it seems that many light-skinned individuals in the military are going to develop basal cell and squamous cell skin cancer in the coming decades, which likely is going to be attributed to their years of federal service, even though they may have had other significant recreational exposure outside of work. So, my gut feeling is that we are going to see skin cancer as a disability tied to federal service, which is going to cost us.
Dr. Logemann: Yes, I think there are always going to be confounders—what if the servicemembers used tanning beds, or they were avid surfers? It’s going to be difficult to always parse that out.
Dr. Miller: In talking about melanoma, you really have to parse out the subsets. Is it melanoma in situ, is it superficial, is it acral, is it nodular? They all have different initiation events.
Nodular melanomas probably don’t need UV light to initiate a tumor. Another risk factor is having more than 100 moles or many atypical moles, which puts that person in a higher risk category. Perhaps when soldiers, airmen, and navy personnel get inducted, they should be screened for their mole population because that is a risk factor for developing melanoma, and then we can intervene a little bit and have them watch their UV exposure.
Dr. Jarell: You can’t overstate the importance of how heterogeneous melanoma is as a disease. While there are clearly some types of melanoma that are caused by UV radiation, there are also many types that aren’t. We don’t understand why someone gets melanoma on the inner thigh, bottom of the foot, top of the sole, inside the mouth, or in the genital region—these aren’t places of high sun exposure.
Lentigo maligna, as an example, is clearly caused by UV radiation in most cases. But there are so many other different types of melanoma that you can’t just attribute to UV radiation, and so you get into this whole other discussion as to why people are getting melanoma—military or not.
Dr. Bandino: When volunteering for military service, there’s the DoDMERB (Department of Defense Medical Examination Review Board) system that screens individuals for medical issues incompatible with military service such as severe psoriasis or atopic dermatitis. But to my knowledge, the DoDMERB process focuses more on current or past issues and does little to investigate for future risk of disease. A cutaneous example would be assessing quantity of dysplastic nevi, Fitzpatrick scale 1 phenotype, and family history of melanoma to determine risk of developing melanoma in someone who may have more UV exposure during their military service than a civilian. This dermatological future risk assessment was certainly not something I was trained to do as a flight surgeon when performing basic trainee flight physicals prior to becoming a dermatologist.
Dr. Jarell: I am a little bit hard-pressed to generalize the military as high occupational risk for melanoma. There are clearly other professions—landscapers, fishermen—that are probably at much higher risk than, say, your general military all-comers. Us physicians in the military were probably not at increased risk compared to other physicians in the United States. We have to be careful not to go down a slippery slope and designate all MOSs (military occupational specialties) as at increased risk for skin cancer, in particular melanoma. Nonmelanoma skin cancer, such as basal cell and squamous cell carcinoma, is clearly related to the proportional amount of UV exposure. But melanoma is quite a diverse cancer that has many, many disparate etiologies.
Dr. Dunn: The entry physical into the military is an opportunity to make an impact on the number of nonmelanoma skin cancers that would arise in that population. There is an educational opportunity to tell inductees that nonmelanoma skin cancer is going to occur on convex surfaces of the sun-exposed skin—nose, ears, forehead, chin, tops of the shoulders. If offered sun protection for those areas and you stretch the potential impact of that information over tens of thousands of military members over decades, you might actually come up with a big number of people that not only decreases their morbidity but also dramatically decreased the cost to the system as a whole.
Dr. Jarell: You also have to factor in ethnicity and the role it plays in someone’s likelihood to get skin cancer—melanoma or nonmelanoma skin cancer. Darker-skinned people are at certainly decreased risk for different types of skin cancers.
Dr. Dunn: Yes, that would have to be part of the education and should be. If you have light skin and freckles, then you’re at much higher risk for nonmelanoma skin cancer and need to know the high-risk areas that can be protected by sunblock and clothing.
Dr. Logemann: One thing that might be a little bit unique in the military is that you’re living in San Antonio one minute, and then the next minute you’re over in Afghanistan with a different climate and different environment. When you’re deployed overseas, you might have a little bit less control over your situation; you might not have a lot of sunscreen in a field hospital in Afghanistan. Whereas if you were just living in San Antonio, you could go down to the store and buy it.
Dr. Miller: Is sunblock now encouraged or available to individuals in deployment situations or training situations where they’re going to have prolonged sun exposure every day? Is it part of the regimen, just like carrying extra water because of the risk for dehydration?
Dr. Logemann: To the best of my knowledge, it is not always included in your normal rations or uniform and it may be up to the servicemember to procure sunscreen.
Dr. Bandino: There have been improvements, and usually you at least have access to sunscreen. In many deployed locations, for example, you have the equivalent of a small PX (post exchange) or BX (base exchange), where they have a variety of products for sale from toothbrushes to flip-flops, and now also sunscreen. Of course, the type and quality of the sunscreen may not be that great. It’s likely going to be basic SPF (sun protection factor) 15 or 30 in small tubes. As a recent example, I participated in a humanitarian medical exercise in South America last summer and was actually issued sunscreen combined with DEET, which is great but it was only SPF 30. The combination product is a good idea for tropical locations, but in addition to people just not wanting to wear it, the DEET combination tends to burn and sting a little bit more; you can get a heat sensation from the DEET; and the DEET can damage plastic surfaces, which may not be ideal for deployed equipment.
The other problem is quantity. We all learned in residency the appropriate sunscreen quantity of at least 1 fl oz for the average adult body, and that’s what we counsel our patients on, but what they issued me was 1 small 2- to 3-fl oz tube. It fit in the palm of my hand, and that was my sunscreen for the trip.
So, I do think, even though there have been some improvements, much of sun protection will still fall on the individual servicemember. And, as mentioned, depending on your ethnicity, some people may need it more than others. But it is an area where there probably could be continued improvements.
Dr. Logemann: In addition to sunscreen, I think that maybe we should be taking into consideration some simple measures. For example, is it necessary for people to stand out in formation at 2
Dr. Dunn: I think we all kind of agree that the military service is diverse and that many of the subcategories of occupations within the military lead to increased sun exposure by mandate. We advise sun protection by physical barriers and sunblock.
Diagnosis of Skin Cancer Via Telemedicine
Dr. Dunn: I have friends who remain in the VA (US Department of Veterans Affairs) system, and they are involved with telemedicine in dermatology, which can reduce waiting time and increase the number of patients seen by the dermatologist. In-person and teledermatology visits now are available to servicemembers on active duty and retirees.
Dr. Bandino: At our residency program (San Antonio Uniformed Services Health Education Consortium), we’ve had asynchronous teledermatology for over a decade, even before I was a resident. We provide it primarily as a service for patients at small bases without access to dermatology. Some bases also use it as part of their prescreening process prior to authorizing an in-person dermatology consultation.
Certainly, with the coronavirus pandemic, civilian dermatology is seeing a boom in the teledermatology world that had been slowly increasing in popularity for the last few years. In our residency program, teledermatology has traditionally been just for active-duty servicemembers or their dependents, but now due to the coronavirus pandemic, our teledermatology services have significantly expanded to include adding synchronous capability. We have patients take pictures before their virtual appointment and/or FaceTime during the appointment. Even after the pandemic, there will likely be more integration of synchronous teledermatology going forward as we’re seeing some of the value. Of course, I’m sure we would all agree that accurate diagnosis of pigmented lesions can be very challenging with teledermatology, not to mention other diagnostic limitations. But I think there is still utility and it should only get better with time as technology improves. So, I’m hopeful that we can incorporate more of it in the military.
Dr. Logemann: I’m definitely aware that we have different telehealth opportunities available, even using some newer modalities that are command approved in recent weeks. My experience has been for more complicated dermatology, so people are in remote locations, and they’re being seen by a nondermatologist, and they have questions about how to approach management. But I’m not aware of telemedicine as a screening tool for skin cancer in the military or among my civilian colleagues. I would hope that it could be someday because we’re developing these total-body photography machines as well. It could be a way for a nondermatologist who identifies a lesion to have it triaged by a dermatologist. To say, “Oh yeah, that looks like a melanoma. They need to get in sooner vs later,” but not on a large-scale sort of screening modality.
Dr. Bandino: In my recent experience, it has definitely been a helpful triage tool. In the military, this form of triage can be particularly helpful if someone is overseas to determine whether he/she needs to evacuated and evaluated in-person right away.
Dr. Jarell: It’s been useful in looking at benign things. People have shown me in the past few weeks a lot of seborrheic keratoses and a lot of benign dermal nevus-type things, and I say, “Don’t worry about that.” And you can tell if the resolution is good enough. But a lot of people have shown me things in the past few weeks that have clearly been basal cell carcinoma, which we can probably let that ride out for a few more weeks, but I’m not sure if maybe somebody has an amelanotic melanoma. Maybe you need to come in and get that biopsied ASAP. Or something that looks like a melanoma. The patient should probably come in and get that biopsied.
Dr. Miller: I think we can rely on teledermatology. It’s all predicated on the resolution because we’re all trained in pattern recognition. I think it’s very useful to screen for things that look clinically benign. We have to understand that most dermatology is practiced by nondermatologists in the United States, and many studies show that their diagnostic accuracy is 20%, at best maybe 50%. So, they do need to reach out to a dermatologist and perhaps get some guidance on what to do. I think it could be a very useful tool if used appropriately.
Dr. Dunn: If used appropriately, teledermatology could function in a couple of ways. One, it could allow us to declare lesions to be wholly benign, and only should a lesion change would it need attention. The second is that it would allow us to accelerate the process of getting a patient to us—physically in front of us—for a biopsy if a suspicious lesion is seen. A by-product of that process would be that if patients who have wholly benign, nonworrisome lesions could be screened by telemedicine, then physical appointments where a patient is in front of the doctor would be more open. In other words, let’s say if 25% of all lesional visits could be declared benign via telemedicine that would allow dermatology to preserve its face-to-face appointments for patients who are more likely to have cancer and require procedures like skin biopsy.
Love it or hate it, I think we’re getting it no matter what now. Telemedicine creeped along forever and within 6 weeks it’s become ubiquitous. It’s phenomenal how fast we had to adapt to a system or perish in private practice. Sometimes these episodes that we go through have good consequences as well as bad consequences. Telemedicine probably has been needed for a long time and the insurers were not covering it very well, but suddenly a stay-at-home mandate has unveiled valuable technology—something that we probably should have been able to use more and be adequately reimbursed.
Surgical Treatment of Skin Cancer
Dr. Dunn: Treatment historically has been surgical for nonmelanoma and melanoma skin cancers. Some radiation devices have gained popularity again in the past decade or so, but excisional surgery remains the standard treatment for skin cancer. Nonmelanoma skin cancers almost all are probably treated surgically still, with a small percentage treated with superficial radiation.
Access to care is important to discuss. Are Mohs surgeons readily available, or are plastic surgeons, general surgeons, or vascular surgeons in the federal system contributing to the care of skin cancer? Are they doing excisional surgery after biopsies are done? Are they doing excisional biopsies with the intent of cure?
Dr. Logemann: For active duty, I don’t see any issues getting access to the medical center for Mohs micrographic surgery. Sometimes, if we have a lot of volume, some patients may get deferred to the network, but in my experience, it would not typically be an active-duty servicemember. An active-duty servicemember would get care rendered at one of the medical centers for Mohs surgery. Typically the active-duty–aged population isn’t getting much skin cancer. It certainly does happen, but most of the skin cancers frequently that are treated at medical centers are not infrequently retirees.
Dr. Bandino: Because of our residency program, we are required to have Mohs surgery capability to be ACGME (Accreditation Council for Graduate Medical Education) accredited. We typically have 3 Mohs surgeons, so we never have a problem with access.
In the military, I just refer cases to our Mohs surgeons and everything is taken care of in-house. In fact, this is an area where we may even have better access than the civilian world because there are no insurance hurdles or significant delay in care since our Mohs surgeons aren’t typically booked up for 3 to 4 months like many civilian Mohs surgeons. This is especially true for complex cases since we provide hospital-based care with all specialty services under the same umbrella. So, for example, if the Mohs surgeons have an extensive and complex case requiring multidisciplinary care such as ENT (ear, nose, and throat), facial plastics, or radiation-oncology, they’re all in-house with no insurance issues to navigate. This of course is not usual for most military bases and is only capable at bases attached to a large medical center. There are some similar scenarios in the civilian world with university medical centers and managed care organizations, but we may still have a slight advantage in accessibility and cost.
Dr. Dunn: There are guidelines from the National Comprehensive Cancer Network as to how to treat nonmelanoma and melanoma skin cancer. Almost all of them are surgical and almost all of them are safe, outpatient, local anesthetic procedures with a high cure rate. The vast majority of melanoma and nonmelanoma skin cancers can be handled safely and effectively with minimal morbidity and almost no known mortalities from the treatments themselves. Some of the cancers have been identified as high risk for metastasis and mortality, but they’re relatively uncommon still. The good news about skin cancer is that the risk of death remains very small.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel.J Am Acad Dermatol. 2018;78:1185-1192.
- Brundage JF, Williams VF, Stahlman S, et al. Incidence rates of malignant melanoma in relation to years of military service, overall and in selected military occupational groups, active component, U.S. Armed Forces, 2001-2015. MSMR. 2017;24:8-14.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel.J Am Acad Dermatol. 2018;78:1185-1192.
- Brundage JF, Williams VF, Stahlman S, et al. Incidence rates of malignant melanoma in relation to years of military service, overall and in selected military occupational groups, active component, U.S. Armed Forces, 2001-2015. MSMR. 2017;24:8-14.
Hepatitis screening now for all patients with cancer on therapy
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
ASCO says ‘no’ to home infusions of cancer treatment, with exceptions
in a new policy statement issued July 31.
At the same time, it supports exceptions: namely, when individual physicians and patients, having jointly discussed risks and benefits, agree to have treatments administered in the home.
The new policy is limited to intravenous infusions of anticancer agents such as chemotherapy, monoclonal antibodies, and other drugs — administered by health care personnel. It does not refer to injections.
The policy was prompted by regulatory flexibilities from the Centers for Medicare & Medicaid Services made in response to the accelerating COVID-19 pandemic. “Among these flexibilities were new provisions that enabled providers to deliver care in a setting most appropriate – and safest – for individual patient circumstances,” which has “opened the path for potential increases in use of home infusion for anticancer therapy,” says ASCO.
“We’re not ready to endorse [chemo at home] as a general policy until we have evidence that it’s safe. At the same time, the policy gives physicians and patients autonomy to respond to whatever situation they find themselves in,” Stephen Grubbs, MD, ASCO’s senior director of clinical affairs, said in an interview.
“Antineoplastic drugs are effective at treating cancer but can be extremely toxic to normal human cells,” reads the statement, which was written by a group of about 25 professionals, including Grubbs and other ASCO staff as well as independent advisers.
“There is a paucity of evidence directly comparing the safety of chemotherapy infusions in the home and outpatient settings,” the ASCO policy explains.
ASCO’s policy acknowledges that there are data “from other countries demonstrating that ... home infusion can be safe, well-tolerated, and may be preferred by some patients.” But such data are limited and only apply “to certain circumstances and for specific agents,” it adds.
One US cancer center (in Philadelphia) already has an established chemo-at-home program and has seen an increase in its use during the pandemic, as reported by Medscape Medical News. Approached for comment, Justin Bekelman, MD, director of the Penn Center for Cancer Care Innovation in Philadelphia, interpreted the new ASCO policy in a positive light.
“Physicians at the Abramson Cancer Center of the University of Pennsylvania and ASCO agree – home-based cancer therapy with oncologist oversight and well-designed safety protocols can be a safe option for patients with cancer,” he said in a statement.
ASCO says its existing safety standards “may be difficult to satisfy in the home infusion context,” including for safely resolving life-threatening emergencies.
Grubbs said that in the worst-case scenario, such as anaphylaxis, “you can die from [it] if you don’t manage it quickly and properly.”
“When I was practicing, we always had a physician present right next to the infusion area because these are severe reactions that happen very quickly,” he said, adding that “several a year” occurred when he practiced full-time.
Also, chemotherapy spills are a “big deal” in the home, as clean-up may be complex and difficult, added Grubbs.
Data from ASCO’s PracticeNET program show that in the first months (March and April) of the COVID-19 pandemic, chemotherapy visits to infusion suites were not reduced in a dataset of 16 US practices, he noted. However, there are exceptions and variance based on location, Grubbs said, such as “hot spots” including New York City in April.
While the pandemic has no end in sight, ASCO issued a set of six recommendations for use of anticancer therapies infused in the home. First, they call for independent, publicly funded research to evaluate the safety and effectiveness of home infusion of anticancer therapy.
Next in importance, ASCO wants the current temporary regulation change from CMS due to the pandemic to end.
“CMS should not extend the temporary flexibility related to home infusion for Part B cancer drugs that was approved as part of their response to the public health emergency,” they state.
Even before the pandemic, changes were afoot. Under the 21st Century Cures Act, which was passed in 2019 and will be implemented in 2021, CMS instituted a permanent home infusion therapy services benefit, which includes anticancer therapies. It “remains to be seen what, if any, shift away from outpatient infusion facilities will occur,” observes ASCO in its policy statement.
This article first appeared on Medscape.com.
in a new policy statement issued July 31.
At the same time, it supports exceptions: namely, when individual physicians and patients, having jointly discussed risks and benefits, agree to have treatments administered in the home.
The new policy is limited to intravenous infusions of anticancer agents such as chemotherapy, monoclonal antibodies, and other drugs — administered by health care personnel. It does not refer to injections.
The policy was prompted by regulatory flexibilities from the Centers for Medicare & Medicaid Services made in response to the accelerating COVID-19 pandemic. “Among these flexibilities were new provisions that enabled providers to deliver care in a setting most appropriate – and safest – for individual patient circumstances,” which has “opened the path for potential increases in use of home infusion for anticancer therapy,” says ASCO.
“We’re not ready to endorse [chemo at home] as a general policy until we have evidence that it’s safe. At the same time, the policy gives physicians and patients autonomy to respond to whatever situation they find themselves in,” Stephen Grubbs, MD, ASCO’s senior director of clinical affairs, said in an interview.
“Antineoplastic drugs are effective at treating cancer but can be extremely toxic to normal human cells,” reads the statement, which was written by a group of about 25 professionals, including Grubbs and other ASCO staff as well as independent advisers.
“There is a paucity of evidence directly comparing the safety of chemotherapy infusions in the home and outpatient settings,” the ASCO policy explains.
ASCO’s policy acknowledges that there are data “from other countries demonstrating that ... home infusion can be safe, well-tolerated, and may be preferred by some patients.” But such data are limited and only apply “to certain circumstances and for specific agents,” it adds.
One US cancer center (in Philadelphia) already has an established chemo-at-home program and has seen an increase in its use during the pandemic, as reported by Medscape Medical News. Approached for comment, Justin Bekelman, MD, director of the Penn Center for Cancer Care Innovation in Philadelphia, interpreted the new ASCO policy in a positive light.
“Physicians at the Abramson Cancer Center of the University of Pennsylvania and ASCO agree – home-based cancer therapy with oncologist oversight and well-designed safety protocols can be a safe option for patients with cancer,” he said in a statement.
ASCO says its existing safety standards “may be difficult to satisfy in the home infusion context,” including for safely resolving life-threatening emergencies.
Grubbs said that in the worst-case scenario, such as anaphylaxis, “you can die from [it] if you don’t manage it quickly and properly.”
“When I was practicing, we always had a physician present right next to the infusion area because these are severe reactions that happen very quickly,” he said, adding that “several a year” occurred when he practiced full-time.
Also, chemotherapy spills are a “big deal” in the home, as clean-up may be complex and difficult, added Grubbs.
Data from ASCO’s PracticeNET program show that in the first months (March and April) of the COVID-19 pandemic, chemotherapy visits to infusion suites were not reduced in a dataset of 16 US practices, he noted. However, there are exceptions and variance based on location, Grubbs said, such as “hot spots” including New York City in April.
While the pandemic has no end in sight, ASCO issued a set of six recommendations for use of anticancer therapies infused in the home. First, they call for independent, publicly funded research to evaluate the safety and effectiveness of home infusion of anticancer therapy.
Next in importance, ASCO wants the current temporary regulation change from CMS due to the pandemic to end.
“CMS should not extend the temporary flexibility related to home infusion for Part B cancer drugs that was approved as part of their response to the public health emergency,” they state.
Even before the pandemic, changes were afoot. Under the 21st Century Cures Act, which was passed in 2019 and will be implemented in 2021, CMS instituted a permanent home infusion therapy services benefit, which includes anticancer therapies. It “remains to be seen what, if any, shift away from outpatient infusion facilities will occur,” observes ASCO in its policy statement.
This article first appeared on Medscape.com.
in a new policy statement issued July 31.
At the same time, it supports exceptions: namely, when individual physicians and patients, having jointly discussed risks and benefits, agree to have treatments administered in the home.
The new policy is limited to intravenous infusions of anticancer agents such as chemotherapy, monoclonal antibodies, and other drugs — administered by health care personnel. It does not refer to injections.
The policy was prompted by regulatory flexibilities from the Centers for Medicare & Medicaid Services made in response to the accelerating COVID-19 pandemic. “Among these flexibilities were new provisions that enabled providers to deliver care in a setting most appropriate – and safest – for individual patient circumstances,” which has “opened the path for potential increases in use of home infusion for anticancer therapy,” says ASCO.
“We’re not ready to endorse [chemo at home] as a general policy until we have evidence that it’s safe. At the same time, the policy gives physicians and patients autonomy to respond to whatever situation they find themselves in,” Stephen Grubbs, MD, ASCO’s senior director of clinical affairs, said in an interview.
“Antineoplastic drugs are effective at treating cancer but can be extremely toxic to normal human cells,” reads the statement, which was written by a group of about 25 professionals, including Grubbs and other ASCO staff as well as independent advisers.
“There is a paucity of evidence directly comparing the safety of chemotherapy infusions in the home and outpatient settings,” the ASCO policy explains.
ASCO’s policy acknowledges that there are data “from other countries demonstrating that ... home infusion can be safe, well-tolerated, and may be preferred by some patients.” But such data are limited and only apply “to certain circumstances and for specific agents,” it adds.
One US cancer center (in Philadelphia) already has an established chemo-at-home program and has seen an increase in its use during the pandemic, as reported by Medscape Medical News. Approached for comment, Justin Bekelman, MD, director of the Penn Center for Cancer Care Innovation in Philadelphia, interpreted the new ASCO policy in a positive light.
“Physicians at the Abramson Cancer Center of the University of Pennsylvania and ASCO agree – home-based cancer therapy with oncologist oversight and well-designed safety protocols can be a safe option for patients with cancer,” he said in a statement.
ASCO says its existing safety standards “may be difficult to satisfy in the home infusion context,” including for safely resolving life-threatening emergencies.
Grubbs said that in the worst-case scenario, such as anaphylaxis, “you can die from [it] if you don’t manage it quickly and properly.”
“When I was practicing, we always had a physician present right next to the infusion area because these are severe reactions that happen very quickly,” he said, adding that “several a year” occurred when he practiced full-time.
Also, chemotherapy spills are a “big deal” in the home, as clean-up may be complex and difficult, added Grubbs.
Data from ASCO’s PracticeNET program show that in the first months (March and April) of the COVID-19 pandemic, chemotherapy visits to infusion suites were not reduced in a dataset of 16 US practices, he noted. However, there are exceptions and variance based on location, Grubbs said, such as “hot spots” including New York City in April.
While the pandemic has no end in sight, ASCO issued a set of six recommendations for use of anticancer therapies infused in the home. First, they call for independent, publicly funded research to evaluate the safety and effectiveness of home infusion of anticancer therapy.
Next in importance, ASCO wants the current temporary regulation change from CMS due to the pandemic to end.
“CMS should not extend the temporary flexibility related to home infusion for Part B cancer drugs that was approved as part of their response to the public health emergency,” they state.
Even before the pandemic, changes were afoot. Under the 21st Century Cures Act, which was passed in 2019 and will be implemented in 2021, CMS instituted a permanent home infusion therapy services benefit, which includes anticancer therapies. It “remains to be seen what, if any, shift away from outpatient infusion facilities will occur,” observes ASCO in its policy statement.
This article first appeared on Medscape.com.
OK to treat many cancer patients despite pandemic, says ESMO
Not all are highly vulnerable to COVID-19
Another important recommendation is to stop labeling all patients with cancer as being vulnerable to infection with the virus as it can lead to inappropriate care with potential negative outcomes.
“Although it was reasonable to adopt over-protective measures for our patients at the outbreak of a novel infective disease which was not previously observed in humans, we now need to step away from the assumption that all cancer patients are vulnerable to COVID-19,” said first author of the consensus article Giuseppe Curigliano, MD, PhD, of the European Institute of Oncology, Milan, Italy, in a statement. “The implications have been important because for some patients treatment was delayed or interrupted over the last few months, and I believe that we will see the impact of this over-precautionary approach in the...future.”
The recommendations were issued by the European Society of Medical Oncology (ESMO) to help guide physicians in “optimizing the pathway to cancer care” as well as to improve outcomes during the pandemic. The recommendations were published online July 31 in Annals of Oncology.
Studies have found that patients with cancer face a higher risk of serious complications and death if they develop COVID-19. Data from the COVID-19 and Cancer Consortium registry, for example, showed that patients with progressing cancer and COVID-19 infection had a fivefold increase in the risk of 30-day mortality compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer.
But while this may be true for some patients, Curigliano and colleagues emphasize that individuals with cancer are not a heterogeneous group and that the term “cancer” itself represents myriad different diseases. The European experts note that current evidence suggests many patients with solid tumors are not more vulnerable to serious complications than the general population.
Thus, cancer prognoses vary considerably, and addressing all patients with cancer as being “COVID-19-vulnerable is probably neither reasonable nor informative,” say the authors.
Dramatic changes were initiated in cancer management for all cancer types, nevertheless, and although these changes seemed reasonable in an acute pandemic situation, note the authors, they were made in the absence of strong supportive evidence. Attempts to define the individualized risk for a given patient, taking into account their primary tumor subtype, stage, age, and gender, have been limited.
“Based on current evidence, only patients who are elderly, with multiple comorbidities, and receiving chemotherapy are vulnerable to the infection,” explained Curigliano.
However, on a positive note, a recently published prospective cohort study looked at approximately 800 patients with cancer – who had symptomatic COVID-19 – in the United Kingdom. The analysis showed no association at all between the risk for death and receiving chemotherapy or immunotherapy, points out Medscape commentator David Kerr, MD, of the University of Oxford, UK, in a recent commentary.
Key recommendations
An international consortium was established by ESMO, and the interdisciplinary expert panel consisted of 64 experts and one voting patient advocate. They agreed on 28 statements that can be used to help with many of the current clinical and technical areas of uncertainty that range from diagnosis to treatment decisions.
The following are several of the key recommendations:
- Patients with cancer who face the highest risk of severe COVID-19 are characterized by active and progressive cancer, advanced age, poor performance status, smoking status, comorbidities, and possibly type of cancer.
- Telehealth and digital health can be excellent tools for some types of care such as primary care triage and counseling, but meeting in person may be more effective for situations that include delivery of key cancer-related information and for patients with complex cancer needs.
- Prior to hospital admission, patients with cancer should be tested for COVID-19, if feasible, and if they are considered at high risk, regardless of symptoms or chest radiological findings.
- Patients with cancer and COVID-19 have a higher risk of thromboembolic events, and prophylaxis using low molecular weight or novel oral anticoagulants is recommended.
- Immune checkpoint inhibitors should not be withheld or delayed when there is a significant survival benefit, but use should be postponed in patients who test positive for COVID-19 until they recover.
- Use of high-dose steroids in patients with cancer infected with COVID-19 could potentially increase the risk of mortality, and a switch should be made to another immunosuppressant, if possible.
- The decision to use tyrosine kinase inhibitors (TKIs) of the PI3K/AKT/mTOR or RAS/RAF/MEK axis is complex, as they interfere with critical pathways involved in innate or adaptive immune responses. Stopping or withholding therapy depends on the risk-benefit balance, and the magnitude of benefit from the TKI needs to be considered.
The authors conclude that “ultimately, this set of statements will serve as a dynamic knowledge repository that will be better informed by accumulating data on SARS-CoV-2 biology, COVID-19 pandemic characteristics, on the risk of cancer patients for COVID-19 and its modulating factors, and finally, on optimal cancer care in the presence of the virus.”
No funding was reported for the current study. Several authors have disclosed relationships with industry, which are listed in the article.
This article first appeared on Medscape.com.
Not all are highly vulnerable to COVID-19
Not all are highly vulnerable to COVID-19
Another important recommendation is to stop labeling all patients with cancer as being vulnerable to infection with the virus as it can lead to inappropriate care with potential negative outcomes.
“Although it was reasonable to adopt over-protective measures for our patients at the outbreak of a novel infective disease which was not previously observed in humans, we now need to step away from the assumption that all cancer patients are vulnerable to COVID-19,” said first author of the consensus article Giuseppe Curigliano, MD, PhD, of the European Institute of Oncology, Milan, Italy, in a statement. “The implications have been important because for some patients treatment was delayed or interrupted over the last few months, and I believe that we will see the impact of this over-precautionary approach in the...future.”
The recommendations were issued by the European Society of Medical Oncology (ESMO) to help guide physicians in “optimizing the pathway to cancer care” as well as to improve outcomes during the pandemic. The recommendations were published online July 31 in Annals of Oncology.
Studies have found that patients with cancer face a higher risk of serious complications and death if they develop COVID-19. Data from the COVID-19 and Cancer Consortium registry, for example, showed that patients with progressing cancer and COVID-19 infection had a fivefold increase in the risk of 30-day mortality compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer.
But while this may be true for some patients, Curigliano and colleagues emphasize that individuals with cancer are not a heterogeneous group and that the term “cancer” itself represents myriad different diseases. The European experts note that current evidence suggests many patients with solid tumors are not more vulnerable to serious complications than the general population.
Thus, cancer prognoses vary considerably, and addressing all patients with cancer as being “COVID-19-vulnerable is probably neither reasonable nor informative,” say the authors.
Dramatic changes were initiated in cancer management for all cancer types, nevertheless, and although these changes seemed reasonable in an acute pandemic situation, note the authors, they were made in the absence of strong supportive evidence. Attempts to define the individualized risk for a given patient, taking into account their primary tumor subtype, stage, age, and gender, have been limited.
“Based on current evidence, only patients who are elderly, with multiple comorbidities, and receiving chemotherapy are vulnerable to the infection,” explained Curigliano.
However, on a positive note, a recently published prospective cohort study looked at approximately 800 patients with cancer – who had symptomatic COVID-19 – in the United Kingdom. The analysis showed no association at all between the risk for death and receiving chemotherapy or immunotherapy, points out Medscape commentator David Kerr, MD, of the University of Oxford, UK, in a recent commentary.
Key recommendations
An international consortium was established by ESMO, and the interdisciplinary expert panel consisted of 64 experts and one voting patient advocate. They agreed on 28 statements that can be used to help with many of the current clinical and technical areas of uncertainty that range from diagnosis to treatment decisions.
The following are several of the key recommendations:
- Patients with cancer who face the highest risk of severe COVID-19 are characterized by active and progressive cancer, advanced age, poor performance status, smoking status, comorbidities, and possibly type of cancer.
- Telehealth and digital health can be excellent tools for some types of care such as primary care triage and counseling, but meeting in person may be more effective for situations that include delivery of key cancer-related information and for patients with complex cancer needs.
- Prior to hospital admission, patients with cancer should be tested for COVID-19, if feasible, and if they are considered at high risk, regardless of symptoms or chest radiological findings.
- Patients with cancer and COVID-19 have a higher risk of thromboembolic events, and prophylaxis using low molecular weight or novel oral anticoagulants is recommended.
- Immune checkpoint inhibitors should not be withheld or delayed when there is a significant survival benefit, but use should be postponed in patients who test positive for COVID-19 until they recover.
- Use of high-dose steroids in patients with cancer infected with COVID-19 could potentially increase the risk of mortality, and a switch should be made to another immunosuppressant, if possible.
- The decision to use tyrosine kinase inhibitors (TKIs) of the PI3K/AKT/mTOR or RAS/RAF/MEK axis is complex, as they interfere with critical pathways involved in innate or adaptive immune responses. Stopping or withholding therapy depends on the risk-benefit balance, and the magnitude of benefit from the TKI needs to be considered.
The authors conclude that “ultimately, this set of statements will serve as a dynamic knowledge repository that will be better informed by accumulating data on SARS-CoV-2 biology, COVID-19 pandemic characteristics, on the risk of cancer patients for COVID-19 and its modulating factors, and finally, on optimal cancer care in the presence of the virus.”
No funding was reported for the current study. Several authors have disclosed relationships with industry, which are listed in the article.
This article first appeared on Medscape.com.
Another important recommendation is to stop labeling all patients with cancer as being vulnerable to infection with the virus as it can lead to inappropriate care with potential negative outcomes.
“Although it was reasonable to adopt over-protective measures for our patients at the outbreak of a novel infective disease which was not previously observed in humans, we now need to step away from the assumption that all cancer patients are vulnerable to COVID-19,” said first author of the consensus article Giuseppe Curigliano, MD, PhD, of the European Institute of Oncology, Milan, Italy, in a statement. “The implications have been important because for some patients treatment was delayed or interrupted over the last few months, and I believe that we will see the impact of this over-precautionary approach in the...future.”
The recommendations were issued by the European Society of Medical Oncology (ESMO) to help guide physicians in “optimizing the pathway to cancer care” as well as to improve outcomes during the pandemic. The recommendations were published online July 31 in Annals of Oncology.
Studies have found that patients with cancer face a higher risk of serious complications and death if they develop COVID-19. Data from the COVID-19 and Cancer Consortium registry, for example, showed that patients with progressing cancer and COVID-19 infection had a fivefold increase in the risk of 30-day mortality compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer.
But while this may be true for some patients, Curigliano and colleagues emphasize that individuals with cancer are not a heterogeneous group and that the term “cancer” itself represents myriad different diseases. The European experts note that current evidence suggests many patients with solid tumors are not more vulnerable to serious complications than the general population.
Thus, cancer prognoses vary considerably, and addressing all patients with cancer as being “COVID-19-vulnerable is probably neither reasonable nor informative,” say the authors.
Dramatic changes were initiated in cancer management for all cancer types, nevertheless, and although these changes seemed reasonable in an acute pandemic situation, note the authors, they were made in the absence of strong supportive evidence. Attempts to define the individualized risk for a given patient, taking into account their primary tumor subtype, stage, age, and gender, have been limited.
“Based on current evidence, only patients who are elderly, with multiple comorbidities, and receiving chemotherapy are vulnerable to the infection,” explained Curigliano.
However, on a positive note, a recently published prospective cohort study looked at approximately 800 patients with cancer – who had symptomatic COVID-19 – in the United Kingdom. The analysis showed no association at all between the risk for death and receiving chemotherapy or immunotherapy, points out Medscape commentator David Kerr, MD, of the University of Oxford, UK, in a recent commentary.
Key recommendations
An international consortium was established by ESMO, and the interdisciplinary expert panel consisted of 64 experts and one voting patient advocate. They agreed on 28 statements that can be used to help with many of the current clinical and technical areas of uncertainty that range from diagnosis to treatment decisions.
The following are several of the key recommendations:
- Patients with cancer who face the highest risk of severe COVID-19 are characterized by active and progressive cancer, advanced age, poor performance status, smoking status, comorbidities, and possibly type of cancer.
- Telehealth and digital health can be excellent tools for some types of care such as primary care triage and counseling, but meeting in person may be more effective for situations that include delivery of key cancer-related information and for patients with complex cancer needs.
- Prior to hospital admission, patients with cancer should be tested for COVID-19, if feasible, and if they are considered at high risk, regardless of symptoms or chest radiological findings.
- Patients with cancer and COVID-19 have a higher risk of thromboembolic events, and prophylaxis using low molecular weight or novel oral anticoagulants is recommended.
- Immune checkpoint inhibitors should not be withheld or delayed when there is a significant survival benefit, but use should be postponed in patients who test positive for COVID-19 until they recover.
- Use of high-dose steroids in patients with cancer infected with COVID-19 could potentially increase the risk of mortality, and a switch should be made to another immunosuppressant, if possible.
- The decision to use tyrosine kinase inhibitors (TKIs) of the PI3K/AKT/mTOR or RAS/RAF/MEK axis is complex, as they interfere with critical pathways involved in innate or adaptive immune responses. Stopping or withholding therapy depends on the risk-benefit balance, and the magnitude of benefit from the TKI needs to be considered.
The authors conclude that “ultimately, this set of statements will serve as a dynamic knowledge repository that will be better informed by accumulating data on SARS-CoV-2 biology, COVID-19 pandemic characteristics, on the risk of cancer patients for COVID-19 and its modulating factors, and finally, on optimal cancer care in the presence of the virus.”
No funding was reported for the current study. Several authors have disclosed relationships with industry, which are listed in the article.
This article first appeared on Medscape.com.
FDA approves triple drug combo for melanoma
The US Food and Drug Administration (FDA) has approved the triple-therapy combination of atezolizumab (Tecentriq) plus cobimetinib (Cotellic) and vemurafenib (Zelboraf) for the treatment of BRAF V600 mutation-positive advanced melanoma, according to a press statement from Genentech, which owns all three drugs.
This is the first melanoma indication for the PD-L1 inhibitor atezolizumab; the other two drugs, cobimetinib and vemurafenib, are a MEK- plus BRAF-inhibitor combination previously approved for BRAF-mutated melanoma.
The new approval is based on safety and efficacy results from the randomized, phase 3 IMspire150 study from patients with previously untreated BRAF V600 mutation-positive metastatic or unresectable locally advanced melanoma.
Progression-free survival (PFS), the primary endpoint, was improved by 4.5 months with the triple therapy compared to the doublet.
The addition of atezolizumab to cobimetinib and vemurafenib led to a longer median PFS of 15.1 months, compared to 10.6 months with placebo plus cobimetinib and vemurafenib (hazard ratio, 0.78; 95% CI, 0.63 – 0.97; P = .025).
The most common adverse reactions (rate ≥ 20%) in patients who received the triple combination were rash (75%), musculoskeletal pain (62%), fatigue (51%), hepatotoxicity (50%), pyrexia (49%), nausea (30%), pruritus (26%), edema (26%), stomatitis (23%), hypothyroidism (22%), and photosensitivity reaction (21%).
The review was conducted under Project Orbis, an initiative of the FDA Oncology Center of Excellence that facilitates concurrent submission and review of oncology products among international partners.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved the triple-therapy combination of atezolizumab (Tecentriq) plus cobimetinib (Cotellic) and vemurafenib (Zelboraf) for the treatment of BRAF V600 mutation-positive advanced melanoma, according to a press statement from Genentech, which owns all three drugs.
This is the first melanoma indication for the PD-L1 inhibitor atezolizumab; the other two drugs, cobimetinib and vemurafenib, are a MEK- plus BRAF-inhibitor combination previously approved for BRAF-mutated melanoma.
The new approval is based on safety and efficacy results from the randomized, phase 3 IMspire150 study from patients with previously untreated BRAF V600 mutation-positive metastatic or unresectable locally advanced melanoma.
Progression-free survival (PFS), the primary endpoint, was improved by 4.5 months with the triple therapy compared to the doublet.
The addition of atezolizumab to cobimetinib and vemurafenib led to a longer median PFS of 15.1 months, compared to 10.6 months with placebo plus cobimetinib and vemurafenib (hazard ratio, 0.78; 95% CI, 0.63 – 0.97; P = .025).
The most common adverse reactions (rate ≥ 20%) in patients who received the triple combination were rash (75%), musculoskeletal pain (62%), fatigue (51%), hepatotoxicity (50%), pyrexia (49%), nausea (30%), pruritus (26%), edema (26%), stomatitis (23%), hypothyroidism (22%), and photosensitivity reaction (21%).
The review was conducted under Project Orbis, an initiative of the FDA Oncology Center of Excellence that facilitates concurrent submission and review of oncology products among international partners.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved the triple-therapy combination of atezolizumab (Tecentriq) plus cobimetinib (Cotellic) and vemurafenib (Zelboraf) for the treatment of BRAF V600 mutation-positive advanced melanoma, according to a press statement from Genentech, which owns all three drugs.
This is the first melanoma indication for the PD-L1 inhibitor atezolizumab; the other two drugs, cobimetinib and vemurafenib, are a MEK- plus BRAF-inhibitor combination previously approved for BRAF-mutated melanoma.
The new approval is based on safety and efficacy results from the randomized, phase 3 IMspire150 study from patients with previously untreated BRAF V600 mutation-positive metastatic or unresectable locally advanced melanoma.
Progression-free survival (PFS), the primary endpoint, was improved by 4.5 months with the triple therapy compared to the doublet.
The addition of atezolizumab to cobimetinib and vemurafenib led to a longer median PFS of 15.1 months, compared to 10.6 months with placebo plus cobimetinib and vemurafenib (hazard ratio, 0.78; 95% CI, 0.63 – 0.97; P = .025).
The most common adverse reactions (rate ≥ 20%) in patients who received the triple combination were rash (75%), musculoskeletal pain (62%), fatigue (51%), hepatotoxicity (50%), pyrexia (49%), nausea (30%), pruritus (26%), edema (26%), stomatitis (23%), hypothyroidism (22%), and photosensitivity reaction (21%).
The review was conducted under Project Orbis, an initiative of the FDA Oncology Center of Excellence that facilitates concurrent submission and review of oncology products among international partners.
This article first appeared on Medscape.com.

