User login
Momentous Melanoma Marker Modality?
Clarke et al published a study online on March 2 in the Journal of Cutaneous Pathology regarding a novel diagnostic test for melanoma. Using quantitative reverse transcriptase–polymerase chain reaction targeting 23 preselected genes, biopsy samples from a variety of melanocytic skin lesions—464 lesions in a training set and 437 lesions in a separate validation set—were analyzed. The test assigned a single numeric score favoring either benign or malignant with sensitivity and specificity of 89% and 93%, respectively (training set), and 90% and 91%, respectively (validation set), when compared to expert consensus dermatopathology review.
What’s the issue?
Any clinician who biopsies multiple melanocytic lesions per day daydreams about a modality that will consistently and accurately distinguish the neoplasms that haunt us the most: the ones with no clear diagnosis and the lesions where intra- and interdepartmental histopathology results vary across the board. In fact, a patient recently told me that I “missed” her “dangerous” melanoma when our dermatopathology and outside consultant opinions stated that the lesion was a dysplastic nevus. The patient personally took the slides to another institution where they were deemed an “evolving” melanoma in situ. Are they all correct? How do we know that something is evolving? Which tumors will eventually be the sinister ones? If we don’t know, then how can a patient understand his/her predicament? What’s a clinician to do?
Reassuringly, in perusing the exhibit hall at the 73rd Annual Meeting of the American Academy of Dermatology, the climate has shifted somewhat. A new zone of molecular and genetic technology has emerged between the rows of pharmaceutical innovation and office supply hardware. The reverse transcriptase–polymerase chain reaction melanoma diagnostic test distinguishes itself with its large study set and measurement parameters, as it quantifies gene expression. Other adjunctive diagnostic modalities have been proven useful in atypical melanocytic proliferations, such as fluorescence in situ hybridization, comparative genomic hybridization, and DNA microarray technology, with focus on physical chromosomal copy alterations; however, it seems as though this new test provides a functional measure and straightforward plus/minus result that may be more universally and objectively relevant and interpretable from a simple skin biopsy. Perhaps the diagnostic technology has now outpaced our limited and confusing vocabulary for melanocytic lesions. Nonetheless, further clinical follow-up, prospective prognostic data, and cost analysis will define its evolving role. How do you think this gene signature test will ultimately influence our interpretation of melanocytic biopsy results?
Clarke et al published a study online on March 2 in the Journal of Cutaneous Pathology regarding a novel diagnostic test for melanoma. Using quantitative reverse transcriptase–polymerase chain reaction targeting 23 preselected genes, biopsy samples from a variety of melanocytic skin lesions—464 lesions in a training set and 437 lesions in a separate validation set—were analyzed. The test assigned a single numeric score favoring either benign or malignant with sensitivity and specificity of 89% and 93%, respectively (training set), and 90% and 91%, respectively (validation set), when compared to expert consensus dermatopathology review.
What’s the issue?
Any clinician who biopsies multiple melanocytic lesions per day daydreams about a modality that will consistently and accurately distinguish the neoplasms that haunt us the most: the ones with no clear diagnosis and the lesions where intra- and interdepartmental histopathology results vary across the board. In fact, a patient recently told me that I “missed” her “dangerous” melanoma when our dermatopathology and outside consultant opinions stated that the lesion was a dysplastic nevus. The patient personally took the slides to another institution where they were deemed an “evolving” melanoma in situ. Are they all correct? How do we know that something is evolving? Which tumors will eventually be the sinister ones? If we don’t know, then how can a patient understand his/her predicament? What’s a clinician to do?
Reassuringly, in perusing the exhibit hall at the 73rd Annual Meeting of the American Academy of Dermatology, the climate has shifted somewhat. A new zone of molecular and genetic technology has emerged between the rows of pharmaceutical innovation and office supply hardware. The reverse transcriptase–polymerase chain reaction melanoma diagnostic test distinguishes itself with its large study set and measurement parameters, as it quantifies gene expression. Other adjunctive diagnostic modalities have been proven useful in atypical melanocytic proliferations, such as fluorescence in situ hybridization, comparative genomic hybridization, and DNA microarray technology, with focus on physical chromosomal copy alterations; however, it seems as though this new test provides a functional measure and straightforward plus/minus result that may be more universally and objectively relevant and interpretable from a simple skin biopsy. Perhaps the diagnostic technology has now outpaced our limited and confusing vocabulary for melanocytic lesions. Nonetheless, further clinical follow-up, prospective prognostic data, and cost analysis will define its evolving role. How do you think this gene signature test will ultimately influence our interpretation of melanocytic biopsy results?
Clarke et al published a study online on March 2 in the Journal of Cutaneous Pathology regarding a novel diagnostic test for melanoma. Using quantitative reverse transcriptase–polymerase chain reaction targeting 23 preselected genes, biopsy samples from a variety of melanocytic skin lesions—464 lesions in a training set and 437 lesions in a separate validation set—were analyzed. The test assigned a single numeric score favoring either benign or malignant with sensitivity and specificity of 89% and 93%, respectively (training set), and 90% and 91%, respectively (validation set), when compared to expert consensus dermatopathology review.
What’s the issue?
Any clinician who biopsies multiple melanocytic lesions per day daydreams about a modality that will consistently and accurately distinguish the neoplasms that haunt us the most: the ones with no clear diagnosis and the lesions where intra- and interdepartmental histopathology results vary across the board. In fact, a patient recently told me that I “missed” her “dangerous” melanoma when our dermatopathology and outside consultant opinions stated that the lesion was a dysplastic nevus. The patient personally took the slides to another institution where they were deemed an “evolving” melanoma in situ. Are they all correct? How do we know that something is evolving? Which tumors will eventually be the sinister ones? If we don’t know, then how can a patient understand his/her predicament? What’s a clinician to do?
Reassuringly, in perusing the exhibit hall at the 73rd Annual Meeting of the American Academy of Dermatology, the climate has shifted somewhat. A new zone of molecular and genetic technology has emerged between the rows of pharmaceutical innovation and office supply hardware. The reverse transcriptase–polymerase chain reaction melanoma diagnostic test distinguishes itself with its large study set and measurement parameters, as it quantifies gene expression. Other adjunctive diagnostic modalities have been proven useful in atypical melanocytic proliferations, such as fluorescence in situ hybridization, comparative genomic hybridization, and DNA microarray technology, with focus on physical chromosomal copy alterations; however, it seems as though this new test provides a functional measure and straightforward plus/minus result that may be more universally and objectively relevant and interpretable from a simple skin biopsy. Perhaps the diagnostic technology has now outpaced our limited and confusing vocabulary for melanocytic lesions. Nonetheless, further clinical follow-up, prospective prognostic data, and cost analysis will define its evolving role. How do you think this gene signature test will ultimately influence our interpretation of melanocytic biopsy results?
Intradermal ALA-PDT linked to long-term remission in BCC
KISSIMMEE, FLA. – Using a needle-free device to inject nodular basal cell carcinomas with intralesional 5-aminolevulinic acid before photodynamic therapy led to complete, years-long remissions and few side effects in a small case series.
“This approach represents an interesting alternative to Mohs, for sure,” Dr. Daniel Barolet said at the annual meeting of the American Society for Laser Medicine and Surgery. “The secret is in the injector nozzle, which lets you inject with multiple openings to get the best uniformity around the tumor.”
Mohs micrographic surgery remains the standard for basal cell carcinoma (BCC) in high-risk sites, and the number of Mohs surgeries has approximately doubled since 2001, said Dr. Barolet, adjunct professor of dermatology at McGill University in Montreal.
Mohs, however, can cause scarring, and BCCs recur in about 4% of patients. In contrast, photodynamic therapy (PDT) is associated with less scarring and pain, fewer complications, shorter recovery times, and lower costs, although the recurrence rate is about 14%, he noted.
Since PDT alone does not efficiently penetrate thick tumor volumes, it works best with pretreatment using agents such as aminolevulinic acid (ALA).
Using needles to inject the tumor, however, can cause pain, vascular damage, vasoconstriction, deep purpura, necrosis, and infection. “Because of this, no-needle injection is an interesting avenue for PDT,” he noted. Needle-free devices currently are used to inject insulin and to administer some vaccines. They are “virtually painless,” noninvasive, and tissue sparing, he said.
To explore the potential role for needle-free injection in ALA-PDT, Dr. Barolot used a prototype high-speed jet to deliver intralesional 5-ALA in the nodular facial BCCs of four patients. He then performed photoactivation with a red light–emitting diode, with continuous wave at 630 nm, irradiance at 50 mW/cm2, and total fluence 50-100 J/cm2.
Patients had no evidence of clinical or histopathologic recurrence for up to 7 years after treatment, Dr. Barolet reported. They experienced mild crusting at treated sites for up to a week after treatment, but no other adverse effects. Two patients needed a second treatment 2 months after the initial treatment to achieve complete remission. “Excellent cosmesis was obtained,” he added, pointing to before and after photos that showed no evidence of lesions several months after treatment.
Multicenter clinical trials are needed to further evaluate the modality, but the preliminary data suggest that intralesional PDT is a reasonable alternative to Mohs for BCCs in high-risk body sites, as long as lesions are few in number and do not affect large areas of the body, Dr. Barolet said.
The modality is especially well suited to “tricky” areas of the body that are difficult to treat with Mohs, he said.
“Developing a user-friendly, disposable no-needle injector will make it much easier for users,” he added. For low-risk BCCs in low-risk sites, conventional treatments such as surgical excision remain the best option, he said.
Dr. Barolet reported no funding sources for the study and said he had no relevant financial disclosures.
KISSIMMEE, FLA. – Using a needle-free device to inject nodular basal cell carcinomas with intralesional 5-aminolevulinic acid before photodynamic therapy led to complete, years-long remissions and few side effects in a small case series.
“This approach represents an interesting alternative to Mohs, for sure,” Dr. Daniel Barolet said at the annual meeting of the American Society for Laser Medicine and Surgery. “The secret is in the injector nozzle, which lets you inject with multiple openings to get the best uniformity around the tumor.”
Mohs micrographic surgery remains the standard for basal cell carcinoma (BCC) in high-risk sites, and the number of Mohs surgeries has approximately doubled since 2001, said Dr. Barolet, adjunct professor of dermatology at McGill University in Montreal.
Mohs, however, can cause scarring, and BCCs recur in about 4% of patients. In contrast, photodynamic therapy (PDT) is associated with less scarring and pain, fewer complications, shorter recovery times, and lower costs, although the recurrence rate is about 14%, he noted.
Since PDT alone does not efficiently penetrate thick tumor volumes, it works best with pretreatment using agents such as aminolevulinic acid (ALA).
Using needles to inject the tumor, however, can cause pain, vascular damage, vasoconstriction, deep purpura, necrosis, and infection. “Because of this, no-needle injection is an interesting avenue for PDT,” he noted. Needle-free devices currently are used to inject insulin and to administer some vaccines. They are “virtually painless,” noninvasive, and tissue sparing, he said.
To explore the potential role for needle-free injection in ALA-PDT, Dr. Barolot used a prototype high-speed jet to deliver intralesional 5-ALA in the nodular facial BCCs of four patients. He then performed photoactivation with a red light–emitting diode, with continuous wave at 630 nm, irradiance at 50 mW/cm2, and total fluence 50-100 J/cm2.
Patients had no evidence of clinical or histopathologic recurrence for up to 7 years after treatment, Dr. Barolet reported. They experienced mild crusting at treated sites for up to a week after treatment, but no other adverse effects. Two patients needed a second treatment 2 months after the initial treatment to achieve complete remission. “Excellent cosmesis was obtained,” he added, pointing to before and after photos that showed no evidence of lesions several months after treatment.
Multicenter clinical trials are needed to further evaluate the modality, but the preliminary data suggest that intralesional PDT is a reasonable alternative to Mohs for BCCs in high-risk body sites, as long as lesions are few in number and do not affect large areas of the body, Dr. Barolet said.
The modality is especially well suited to “tricky” areas of the body that are difficult to treat with Mohs, he said.
“Developing a user-friendly, disposable no-needle injector will make it much easier for users,” he added. For low-risk BCCs in low-risk sites, conventional treatments such as surgical excision remain the best option, he said.
Dr. Barolet reported no funding sources for the study and said he had no relevant financial disclosures.
KISSIMMEE, FLA. – Using a needle-free device to inject nodular basal cell carcinomas with intralesional 5-aminolevulinic acid before photodynamic therapy led to complete, years-long remissions and few side effects in a small case series.
“This approach represents an interesting alternative to Mohs, for sure,” Dr. Daniel Barolet said at the annual meeting of the American Society for Laser Medicine and Surgery. “The secret is in the injector nozzle, which lets you inject with multiple openings to get the best uniformity around the tumor.”
Mohs micrographic surgery remains the standard for basal cell carcinoma (BCC) in high-risk sites, and the number of Mohs surgeries has approximately doubled since 2001, said Dr. Barolet, adjunct professor of dermatology at McGill University in Montreal.
Mohs, however, can cause scarring, and BCCs recur in about 4% of patients. In contrast, photodynamic therapy (PDT) is associated with less scarring and pain, fewer complications, shorter recovery times, and lower costs, although the recurrence rate is about 14%, he noted.
Since PDT alone does not efficiently penetrate thick tumor volumes, it works best with pretreatment using agents such as aminolevulinic acid (ALA).
Using needles to inject the tumor, however, can cause pain, vascular damage, vasoconstriction, deep purpura, necrosis, and infection. “Because of this, no-needle injection is an interesting avenue for PDT,” he noted. Needle-free devices currently are used to inject insulin and to administer some vaccines. They are “virtually painless,” noninvasive, and tissue sparing, he said.
To explore the potential role for needle-free injection in ALA-PDT, Dr. Barolot used a prototype high-speed jet to deliver intralesional 5-ALA in the nodular facial BCCs of four patients. He then performed photoactivation with a red light–emitting diode, with continuous wave at 630 nm, irradiance at 50 mW/cm2, and total fluence 50-100 J/cm2.
Patients had no evidence of clinical or histopathologic recurrence for up to 7 years after treatment, Dr. Barolet reported. They experienced mild crusting at treated sites for up to a week after treatment, but no other adverse effects. Two patients needed a second treatment 2 months after the initial treatment to achieve complete remission. “Excellent cosmesis was obtained,” he added, pointing to before and after photos that showed no evidence of lesions several months after treatment.
Multicenter clinical trials are needed to further evaluate the modality, but the preliminary data suggest that intralesional PDT is a reasonable alternative to Mohs for BCCs in high-risk body sites, as long as lesions are few in number and do not affect large areas of the body, Dr. Barolet said.
The modality is especially well suited to “tricky” areas of the body that are difficult to treat with Mohs, he said.
“Developing a user-friendly, disposable no-needle injector will make it much easier for users,” he added. For low-risk BCCs in low-risk sites, conventional treatments such as surgical excision remain the best option, he said.
Dr. Barolet reported no funding sources for the study and said he had no relevant financial disclosures.
AT LASER 2015
Key clinical point: Intralesional 5-ALA-PDT is a potential alternative to Mohs micrographic surgery for treating basal cell carcinomas in high-risk sites.
Major finding: Four treated patients experienced resolution of recurrent basal cell carcinomas for up to 7 years.
Data source: Series of four cases of recurrent nodular facial basal cell carcinomas.
Disclosures: Dr. Barolet reported no funding sources and declared no relevant financial disclosures.
Effectiveness and safety of ipilimumab therapy in advanced melanoma: evidence from clinical practice sites in the US
Class of 2015: New drugs projected to earn billions and billions
Of all drugs to be released in 2015, the melanoma drug Opdivo (nivolumab) is expected to have the brightest future, according to a report from Thomson Reuters.
With sales forecast to reach nearly $5.7 billion by 2019, Opdivo is at the head of a large 2015 “blockbuster” drug class. Opdivo is followed by a pair of drugs for the cardiovascular system: Praluent (alirocumab) for hypercholesterolemia with projected sales of $4.4 billion and LCZ-696 (sacubitril and valsartan) for chronic heart failure with projected 2019 sales of $3.7 billion, Thomson Reuters said.
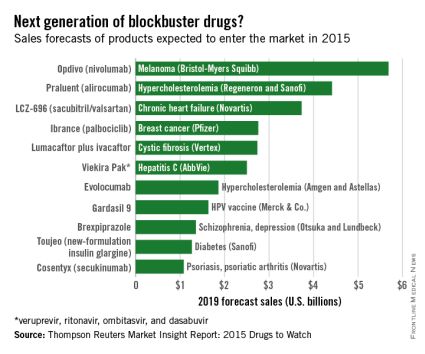
With estimated sales of $2.8 billion, the breast cancer drug Ibrance (palbociclib) is the second oncologic drug making the blockbuster list, with the first noncancer or non-CV drug – lumacaftor plus ivacaftor for cystic fibrosis – rounding out the Top 5 with projected sales of $2.7 billion by 2019.
Next comes Viekira Pak (ombitasvir, paritaprevir, and ritonavir tablets, copackaged with dasabuvir tablets), a hepatitis C virus drug with estimated 2019 sales of $2.5 billion, followed by the hypercholesterolemia/hyperlipidemia drug evolocumab, with projected sales of $1.9 billion. This $2.5 billion disparity between evolocumab and Praluent may be explained by Praluent’s arrival on the market a month sooner, and also because Praluent had a reduced rate of cardiac death, heart attack, and stroke in a phase III trial, a point likely to be relevant to most patients, according to the report.
Overall, 11 drugs are expected to reach $1 billion in sales by 2019, many more than the three blockbusters predicted from the 2014 stock of drugs. However, the two highest-selling new drugs from 2014, Sovaldi (sofosbuvir) and Harvoni (sofosbuvir plus ledipasvir) – both HCV drugs – are each predicted to reach sales of more than $10 billion by 2017, far exceeding anything from 2015, the report said.
The Thomson Reuters Market Insight Report used data collected from 2013 through early February 2015.
Of all drugs to be released in 2015, the melanoma drug Opdivo (nivolumab) is expected to have the brightest future, according to a report from Thomson Reuters.
With sales forecast to reach nearly $5.7 billion by 2019, Opdivo is at the head of a large 2015 “blockbuster” drug class. Opdivo is followed by a pair of drugs for the cardiovascular system: Praluent (alirocumab) for hypercholesterolemia with projected sales of $4.4 billion and LCZ-696 (sacubitril and valsartan) for chronic heart failure with projected 2019 sales of $3.7 billion, Thomson Reuters said.

With estimated sales of $2.8 billion, the breast cancer drug Ibrance (palbociclib) is the second oncologic drug making the blockbuster list, with the first noncancer or non-CV drug – lumacaftor plus ivacaftor for cystic fibrosis – rounding out the Top 5 with projected sales of $2.7 billion by 2019.
Next comes Viekira Pak (ombitasvir, paritaprevir, and ritonavir tablets, copackaged with dasabuvir tablets), a hepatitis C virus drug with estimated 2019 sales of $2.5 billion, followed by the hypercholesterolemia/hyperlipidemia drug evolocumab, with projected sales of $1.9 billion. This $2.5 billion disparity between evolocumab and Praluent may be explained by Praluent’s arrival on the market a month sooner, and also because Praluent had a reduced rate of cardiac death, heart attack, and stroke in a phase III trial, a point likely to be relevant to most patients, according to the report.
Overall, 11 drugs are expected to reach $1 billion in sales by 2019, many more than the three blockbusters predicted from the 2014 stock of drugs. However, the two highest-selling new drugs from 2014, Sovaldi (sofosbuvir) and Harvoni (sofosbuvir plus ledipasvir) – both HCV drugs – are each predicted to reach sales of more than $10 billion by 2017, far exceeding anything from 2015, the report said.
The Thomson Reuters Market Insight Report used data collected from 2013 through early February 2015.
Of all drugs to be released in 2015, the melanoma drug Opdivo (nivolumab) is expected to have the brightest future, according to a report from Thomson Reuters.
With sales forecast to reach nearly $5.7 billion by 2019, Opdivo is at the head of a large 2015 “blockbuster” drug class. Opdivo is followed by a pair of drugs for the cardiovascular system: Praluent (alirocumab) for hypercholesterolemia with projected sales of $4.4 billion and LCZ-696 (sacubitril and valsartan) for chronic heart failure with projected 2019 sales of $3.7 billion, Thomson Reuters said.

With estimated sales of $2.8 billion, the breast cancer drug Ibrance (palbociclib) is the second oncologic drug making the blockbuster list, with the first noncancer or non-CV drug – lumacaftor plus ivacaftor for cystic fibrosis – rounding out the Top 5 with projected sales of $2.7 billion by 2019.
Next comes Viekira Pak (ombitasvir, paritaprevir, and ritonavir tablets, copackaged with dasabuvir tablets), a hepatitis C virus drug with estimated 2019 sales of $2.5 billion, followed by the hypercholesterolemia/hyperlipidemia drug evolocumab, with projected sales of $1.9 billion. This $2.5 billion disparity between evolocumab and Praluent may be explained by Praluent’s arrival on the market a month sooner, and also because Praluent had a reduced rate of cardiac death, heart attack, and stroke in a phase III trial, a point likely to be relevant to most patients, according to the report.
Overall, 11 drugs are expected to reach $1 billion in sales by 2019, many more than the three blockbusters predicted from the 2014 stock of drugs. However, the two highest-selling new drugs from 2014, Sovaldi (sofosbuvir) and Harvoni (sofosbuvir plus ledipasvir) – both HCV drugs – are each predicted to reach sales of more than $10 billion by 2017, far exceeding anything from 2015, the report said.
The Thomson Reuters Market Insight Report used data collected from 2013 through early February 2015.
Pembrolizumab bests ipilimumab in advanced melanoma
Pembrolizumab was superior to ipilimumab, the standard of care, as first-line therapy for advanced melanoma in the phase III KEYNOTE-006 trial.
Pembrolizumab (Keytruda) hit all of its primary survival end points and nearly tripled response rates from 12% with ipilimumab (Yervoy) to 33% in the first frontline head-to-head comparison of the two immune checkpoint inhibitors.
Pembrolizumab reduced the risk of progression by 42% and the risk of death by 31% to 37%, compared with ipilimumab, study author Dr. Antoni Ribas reported at the annual meeting of the American Association for Cancer Research.
“We think that this data should change the paradigm of treatment for these patients, and the standard of care should quickly shift to giving PD-1 antibodies,” he said at a press briefing.
Pembrolizumab, a monoclonal antibody that inhibits programmed death receptor-1 (PD-1), is approved as second-line therapy for unresectable or metastatic melanoma after failing iplimumab or a BRAF inhibitor, if a BRAF V600 mutation is present.
Ipilimumab has been the gold standard against which everything else was measured, but “this is now expected to change the treatment landscape for melanoma. This is a very high impact trial,” Dr. Suzanne Topalian, director of the melanoma program at Johns Hopkins Sidney Kimmel Comprehensive Cancer Center in Baltimore, said during the briefing.
The 2011 approval of ipilimumab, a cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor, as first-line therapy for advanced melanoma was a landmark moment, she said, not only for melanoma because it was the first drug ever to show a survival advantage in a randomized trial, but also for immunotherapy because it was the first checkpoint blocker to show such a benefit.
Results of the KEYSTONE-006 trial, simultaneously published on line (N. Engl. J. Med. 2015. DOI: 10.1056/NEJMoa1503093), prompted the safety monitoring committee to recommend stopping the trial early and allowing ipilimumab patients to receive pembrolizumab.
Lead investigator Dr. Caroline Robert, head of dermatology at Institut Gustave-Roussy in Paris, said in a statement that she hoped the results would accelerate regulatory approval of pembrolizumab in Europe, where the drug is still not on the market.
KEYNOTE-006 included 834 patients with unresectable, ipilimumab-naive, stage III or IV melanoma treated with no more than one previous systemic therapy who were randomly assigned to 10 mg/kg pembrolizumab either every 2 weeks or every 3 weeks or four cycles of 3 mg/kg ipilimumab every 3 weeks, until disease progression or unacceptable toxicity. Treatment response was assessed 12 weeks after randomization and every 6 weeks thereafter per RECIST guideline v1.1 by central review and per immune-related response criteria by investigator review.
Two-thirds of patients were treatment naive, 79% had PD-ligand 1(PD-L1)-positive tumors, and 36% had BRAF V600-mutant tumors.
At the first interim analysis after a median follow-up of 8 months, 6-month progression-free survival rates were 47.3% for pembrolizumab every 2 weeks, 46.4% for pembrolizumab every 3 weeks, and 26.5% for ipilimumab (Hazard ratio, 0.58; P < .001), Dr. Antoni Ribas of the University of California Los Angeles Jonsson Comprehensive Cancer Center, reported.
The benefit was seen across all prespecified subgroups, including PD-L1-positive and PD-L1-negative tumors.
At the time of the analysis, responses by RECIST were ongoing in 89.4% of patients treated with pembrolizumab every 2 weeks, 96.7% on pembrolizumab every 3 weeks, and 88% given ipilumumab.
The median duration of response was 251 days in the pembrolizumab every 2 weeks-arm, but had not been reached in the other two arms.
There has been no evidence of resistance, and only a small minority, perhaps 5-10% of patients, have escape lesions or progress after response, he said.
At the second interim analysis after a median follow-up of 13.8 months, 1-year overall survival rates were 74% for pembrolizumab every 2 weeks (HR, 0.63; P = .0005), 68.4% for pembrolizumab every 3 weeks (HR, 0.69; P = .0036), and 58.2% for ipilimumab. The survival benefit extended to all subgroups, except the 18% of patients with PD-L1-negative tumors, although sample sizes were small and confidence intervals wide.
Efficacy and tolerability was similar for both pembrolizumab dosing schedules, Dr. Ribas said. Treatment-related grade 3-4 adverse events were lower in the pembrolizumab every 2 and 3 weeks arms than with ipilimumab (13.3% vs. 10.1% vs. 20%), despite exposure to pembrolizumab being nearly 3 times as long (164 days vs. 151.5 days vs. 50 days).
When asked how the findings would change his practice tomorrow, Dr. Ribas said pembrolizumab should be used first line but that he will continue to use ipilimumab, either alone or in combination with a PD-1 inhibitor, because it can give durable responses. The critical unanswered question of what the most effective sequence or combination of checkpoint inhibitors is will take years to answer.
“This is just the start,” he said. “This is amazing that single-agent checkpoint blockade gives these responses in melanoma and as you will see in lung cancer, but the reality is that there’s two-thirds of patients who do not respond and we have to do something about that.”
The study was funded by Merck Sharp & Dohme. Dr. Ribas is a consultant to Merck, with the honoraria paid to his institution. Dr. Robert is a consultant with honoraria for MSD, Bristol Myers Squibb, Roche, Novartis, GlaxoSmithKline, and Amgen.
Pembrolizumab was superior to ipilimumab, the standard of care, as first-line therapy for advanced melanoma in the phase III KEYNOTE-006 trial.
Pembrolizumab (Keytruda) hit all of its primary survival end points and nearly tripled response rates from 12% with ipilimumab (Yervoy) to 33% in the first frontline head-to-head comparison of the two immune checkpoint inhibitors.
Pembrolizumab reduced the risk of progression by 42% and the risk of death by 31% to 37%, compared with ipilimumab, study author Dr. Antoni Ribas reported at the annual meeting of the American Association for Cancer Research.
“We think that this data should change the paradigm of treatment for these patients, and the standard of care should quickly shift to giving PD-1 antibodies,” he said at a press briefing.
Pembrolizumab, a monoclonal antibody that inhibits programmed death receptor-1 (PD-1), is approved as second-line therapy for unresectable or metastatic melanoma after failing iplimumab or a BRAF inhibitor, if a BRAF V600 mutation is present.
Ipilimumab has been the gold standard against which everything else was measured, but “this is now expected to change the treatment landscape for melanoma. This is a very high impact trial,” Dr. Suzanne Topalian, director of the melanoma program at Johns Hopkins Sidney Kimmel Comprehensive Cancer Center in Baltimore, said during the briefing.
The 2011 approval of ipilimumab, a cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor, as first-line therapy for advanced melanoma was a landmark moment, she said, not only for melanoma because it was the first drug ever to show a survival advantage in a randomized trial, but also for immunotherapy because it was the first checkpoint blocker to show such a benefit.
Results of the KEYSTONE-006 trial, simultaneously published on line (N. Engl. J. Med. 2015. DOI: 10.1056/NEJMoa1503093), prompted the safety monitoring committee to recommend stopping the trial early and allowing ipilimumab patients to receive pembrolizumab.
Lead investigator Dr. Caroline Robert, head of dermatology at Institut Gustave-Roussy in Paris, said in a statement that she hoped the results would accelerate regulatory approval of pembrolizumab in Europe, where the drug is still not on the market.
KEYNOTE-006 included 834 patients with unresectable, ipilimumab-naive, stage III or IV melanoma treated with no more than one previous systemic therapy who were randomly assigned to 10 mg/kg pembrolizumab either every 2 weeks or every 3 weeks or four cycles of 3 mg/kg ipilimumab every 3 weeks, until disease progression or unacceptable toxicity. Treatment response was assessed 12 weeks after randomization and every 6 weeks thereafter per RECIST guideline v1.1 by central review and per immune-related response criteria by investigator review.
Two-thirds of patients were treatment naive, 79% had PD-ligand 1(PD-L1)-positive tumors, and 36% had BRAF V600-mutant tumors.
At the first interim analysis after a median follow-up of 8 months, 6-month progression-free survival rates were 47.3% for pembrolizumab every 2 weeks, 46.4% for pembrolizumab every 3 weeks, and 26.5% for ipilimumab (Hazard ratio, 0.58; P < .001), Dr. Antoni Ribas of the University of California Los Angeles Jonsson Comprehensive Cancer Center, reported.
The benefit was seen across all prespecified subgroups, including PD-L1-positive and PD-L1-negative tumors.
At the time of the analysis, responses by RECIST were ongoing in 89.4% of patients treated with pembrolizumab every 2 weeks, 96.7% on pembrolizumab every 3 weeks, and 88% given ipilumumab.
The median duration of response was 251 days in the pembrolizumab every 2 weeks-arm, but had not been reached in the other two arms.
There has been no evidence of resistance, and only a small minority, perhaps 5-10% of patients, have escape lesions or progress after response, he said.
At the second interim analysis after a median follow-up of 13.8 months, 1-year overall survival rates were 74% for pembrolizumab every 2 weeks (HR, 0.63; P = .0005), 68.4% for pembrolizumab every 3 weeks (HR, 0.69; P = .0036), and 58.2% for ipilimumab. The survival benefit extended to all subgroups, except the 18% of patients with PD-L1-negative tumors, although sample sizes were small and confidence intervals wide.
Efficacy and tolerability was similar for both pembrolizumab dosing schedules, Dr. Ribas said. Treatment-related grade 3-4 adverse events were lower in the pembrolizumab every 2 and 3 weeks arms than with ipilimumab (13.3% vs. 10.1% vs. 20%), despite exposure to pembrolizumab being nearly 3 times as long (164 days vs. 151.5 days vs. 50 days).
When asked how the findings would change his practice tomorrow, Dr. Ribas said pembrolizumab should be used first line but that he will continue to use ipilimumab, either alone or in combination with a PD-1 inhibitor, because it can give durable responses. The critical unanswered question of what the most effective sequence or combination of checkpoint inhibitors is will take years to answer.
“This is just the start,” he said. “This is amazing that single-agent checkpoint blockade gives these responses in melanoma and as you will see in lung cancer, but the reality is that there’s two-thirds of patients who do not respond and we have to do something about that.”
The study was funded by Merck Sharp & Dohme. Dr. Ribas is a consultant to Merck, with the honoraria paid to his institution. Dr. Robert is a consultant with honoraria for MSD, Bristol Myers Squibb, Roche, Novartis, GlaxoSmithKline, and Amgen.
Pembrolizumab was superior to ipilimumab, the standard of care, as first-line therapy for advanced melanoma in the phase III KEYNOTE-006 trial.
Pembrolizumab (Keytruda) hit all of its primary survival end points and nearly tripled response rates from 12% with ipilimumab (Yervoy) to 33% in the first frontline head-to-head comparison of the two immune checkpoint inhibitors.
Pembrolizumab reduced the risk of progression by 42% and the risk of death by 31% to 37%, compared with ipilimumab, study author Dr. Antoni Ribas reported at the annual meeting of the American Association for Cancer Research.
“We think that this data should change the paradigm of treatment for these patients, and the standard of care should quickly shift to giving PD-1 antibodies,” he said at a press briefing.
Pembrolizumab, a monoclonal antibody that inhibits programmed death receptor-1 (PD-1), is approved as second-line therapy for unresectable or metastatic melanoma after failing iplimumab or a BRAF inhibitor, if a BRAF V600 mutation is present.
Ipilimumab has been the gold standard against which everything else was measured, but “this is now expected to change the treatment landscape for melanoma. This is a very high impact trial,” Dr. Suzanne Topalian, director of the melanoma program at Johns Hopkins Sidney Kimmel Comprehensive Cancer Center in Baltimore, said during the briefing.
The 2011 approval of ipilimumab, a cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor, as first-line therapy for advanced melanoma was a landmark moment, she said, not only for melanoma because it was the first drug ever to show a survival advantage in a randomized trial, but also for immunotherapy because it was the first checkpoint blocker to show such a benefit.
Results of the KEYSTONE-006 trial, simultaneously published on line (N. Engl. J. Med. 2015. DOI: 10.1056/NEJMoa1503093), prompted the safety monitoring committee to recommend stopping the trial early and allowing ipilimumab patients to receive pembrolizumab.
Lead investigator Dr. Caroline Robert, head of dermatology at Institut Gustave-Roussy in Paris, said in a statement that she hoped the results would accelerate regulatory approval of pembrolizumab in Europe, where the drug is still not on the market.
KEYNOTE-006 included 834 patients with unresectable, ipilimumab-naive, stage III or IV melanoma treated with no more than one previous systemic therapy who were randomly assigned to 10 mg/kg pembrolizumab either every 2 weeks or every 3 weeks or four cycles of 3 mg/kg ipilimumab every 3 weeks, until disease progression or unacceptable toxicity. Treatment response was assessed 12 weeks after randomization and every 6 weeks thereafter per RECIST guideline v1.1 by central review and per immune-related response criteria by investigator review.
Two-thirds of patients were treatment naive, 79% had PD-ligand 1(PD-L1)-positive tumors, and 36% had BRAF V600-mutant tumors.
At the first interim analysis after a median follow-up of 8 months, 6-month progression-free survival rates were 47.3% for pembrolizumab every 2 weeks, 46.4% for pembrolizumab every 3 weeks, and 26.5% for ipilimumab (Hazard ratio, 0.58; P < .001), Dr. Antoni Ribas of the University of California Los Angeles Jonsson Comprehensive Cancer Center, reported.
The benefit was seen across all prespecified subgroups, including PD-L1-positive and PD-L1-negative tumors.
At the time of the analysis, responses by RECIST were ongoing in 89.4% of patients treated with pembrolizumab every 2 weeks, 96.7% on pembrolizumab every 3 weeks, and 88% given ipilumumab.
The median duration of response was 251 days in the pembrolizumab every 2 weeks-arm, but had not been reached in the other two arms.
There has been no evidence of resistance, and only a small minority, perhaps 5-10% of patients, have escape lesions or progress after response, he said.
At the second interim analysis after a median follow-up of 13.8 months, 1-year overall survival rates were 74% for pembrolizumab every 2 weeks (HR, 0.63; P = .0005), 68.4% for pembrolizumab every 3 weeks (HR, 0.69; P = .0036), and 58.2% for ipilimumab. The survival benefit extended to all subgroups, except the 18% of patients with PD-L1-negative tumors, although sample sizes were small and confidence intervals wide.
Efficacy and tolerability was similar for both pembrolizumab dosing schedules, Dr. Ribas said. Treatment-related grade 3-4 adverse events were lower in the pembrolizumab every 2 and 3 weeks arms than with ipilimumab (13.3% vs. 10.1% vs. 20%), despite exposure to pembrolizumab being nearly 3 times as long (164 days vs. 151.5 days vs. 50 days).
When asked how the findings would change his practice tomorrow, Dr. Ribas said pembrolizumab should be used first line but that he will continue to use ipilimumab, either alone or in combination with a PD-1 inhibitor, because it can give durable responses. The critical unanswered question of what the most effective sequence or combination of checkpoint inhibitors is will take years to answer.
“This is just the start,” he said. “This is amazing that single-agent checkpoint blockade gives these responses in melanoma and as you will see in lung cancer, but the reality is that there’s two-thirds of patients who do not respond and we have to do something about that.”
The study was funded by Merck Sharp & Dohme. Dr. Ribas is a consultant to Merck, with the honoraria paid to his institution. Dr. Robert is a consultant with honoraria for MSD, Bristol Myers Squibb, Roche, Novartis, GlaxoSmithKline, and Amgen.
FROM THE AACR ANNUAL MEETING
Key clinical point: Pembrolizumab was superior to ipilimumab, the standard of care, for first-line treatment of advanced melanoma.
Major finding: Pembrolizumab reduced the risk of progression by 42% and the risk of death by 31% to 37% compared with ipilimumab.
Data source: Phase III, randomized, open-label trial in 834 patients with advanced melanoma with no more than one prior systemic therapy.
Disclosures: The study was funded by Merck Sharp & Dohme. Dr. Ribas is a consultant to Merck, with the honoraria paid to his institution. Dr. Robert is a consultant with honoraria for MSD, Bristol-Myers Squibb, Roche, Novartis, GlaxoSmithKline, and Amgen.
Sleep disorders in patients with cancer
Sleep disturbances are common among patients with cancer for many reasons. Sleep problems can be present at any stage during treatment for cancer and in some patients, sleep disturbance may be the presenting symptoms that lead to the diagnosis of some types of cancer. Poor sleep impairs quality of life In people with cancer, but most do not specifically complain of sleep problems unless they are explicitly asked. Insomnia and fatigue are most common sleep disorders in this cohort, although primary sleep disorders, including obstructive sleep apnea and restless legs syndrome, which are common in the general population, have not been carefully studied in the oncology setting despite significant their impairment of quality of life.
Click on the PDF icon at the top of this introduction to read the full article.
disorder
Sleep disturbances are common among patients with cancer for many reasons. Sleep problems can be present at any stage during treatment for cancer and in some patients, sleep disturbance may be the presenting symptoms that lead to the diagnosis of some types of cancer. Poor sleep impairs quality of life In people with cancer, but most do not specifically complain of sleep problems unless they are explicitly asked. Insomnia and fatigue are most common sleep disorders in this cohort, although primary sleep disorders, including obstructive sleep apnea and restless legs syndrome, which are common in the general population, have not been carefully studied in the oncology setting despite significant their impairment of quality of life.
Click on the PDF icon at the top of this introduction to read the full article.
Sleep disturbances are common among patients with cancer for many reasons. Sleep problems can be present at any stage during treatment for cancer and in some patients, sleep disturbance may be the presenting symptoms that lead to the diagnosis of some types of cancer. Poor sleep impairs quality of life In people with cancer, but most do not specifically complain of sleep problems unless they are explicitly asked. Insomnia and fatigue are most common sleep disorders in this cohort, although primary sleep disorders, including obstructive sleep apnea and restless legs syndrome, which are common in the general population, have not been carefully studied in the oncology setting despite significant their impairment of quality of life.
Click on the PDF icon at the top of this introduction to read the full article.
disorder
disorder
Update on Melanoma Guidelines: Report From the AAD Meeting
Melanoma was an important topic at multiple sessions of the 73rd Annual Meeting of the American Academy of Dermatology (AAD) in San Francisco, California. Dr. Susan M. Swetter reviews the AAD and National Comprehensive Cancer Network recommendations for biopsy of pigmented suspicious lesions. She also discusses when sentinel lymph node biopsies are recommended and factors that would indicate a patient needs a sentinel lymph node biopsy. Dr. Swetter also outlines surgical margins for melanoma and emphasizes that these are clinical margins taken at the time of surgery, not histologic margins. She concludes with a review of the melanoma subtype lentigo maligna.
Melanoma was an important topic at multiple sessions of the 73rd Annual Meeting of the American Academy of Dermatology (AAD) in San Francisco, California. Dr. Susan M. Swetter reviews the AAD and National Comprehensive Cancer Network recommendations for biopsy of pigmented suspicious lesions. She also discusses when sentinel lymph node biopsies are recommended and factors that would indicate a patient needs a sentinel lymph node biopsy. Dr. Swetter also outlines surgical margins for melanoma and emphasizes that these are clinical margins taken at the time of surgery, not histologic margins. She concludes with a review of the melanoma subtype lentigo maligna.
Melanoma was an important topic at multiple sessions of the 73rd Annual Meeting of the American Academy of Dermatology (AAD) in San Francisco, California. Dr. Susan M. Swetter reviews the AAD and National Comprehensive Cancer Network recommendations for biopsy of pigmented suspicious lesions. She also discusses when sentinel lymph node biopsies are recommended and factors that would indicate a patient needs a sentinel lymph node biopsy. Dr. Swetter also outlines surgical margins for melanoma and emphasizes that these are clinical margins taken at the time of surgery, not histologic margins. She concludes with a review of the melanoma subtype lentigo maligna.
New melanoma therapies may break the bank
HOUSTON – Newer systemic therapies for metastatic malignant melanoma have resulted in significant gains in survival, but at a cost that may be unsustainable in the near future, according to Dr. Jeffrey E. Gershenwald.
Up to one-half of all expenses related to the treatment of malignant melanoma are accounted for by the care of patients with advanced disease, yet patients with distant metastases (stage IV disease) account for only about 2% of all patients, said Dr. Gershenwald, professor of surgical oncology at the University of Texas M.D. Anderson Cancer Center, Houston.
“How best can we achieve the right therapy for the right patient at the right time, and as we learn more and more about some of the therapies, particularly in melanoma, for the right length of time? We can’t really afford to give treatments in perpetuity, so we need to know how long they actually need to be delivered in order to have optimal value for the patient,” he said at the annual Society of Surgical Oncology Cancer Symposium.
Over the last 3 decades, and particularly over the last 5 years, there have been tremendous forward strides in therapy. In 1975, when dacarbazine became the standard of care for metastatic melanoma, it was associated with response rates of only about 6%-15%, durable responses in only 5%-15% of patients, and a median overall survival of about 6-9 months, Dr. Gershenwald reported.
Treatment toxicities, but not response rates, increased with the introduction of interleukin-2 in 1998, which for want of a better drug became the new preferred treatment.
But with the introduction of new systemic therapies, such as immune checkpoint inhibitors (ipilimumab [Yervoy], nivolumab [Opdivo], and pembrolizumab [Keytruda]) and targeted agents (vemurafenib [Zelboraf], dabrafenib [Tafinlar], and trametinib [Mekinist]), response rates have soared, resulting in an improvement in 1-year survival rates from about 30% to 35% in 1970 to as high as 80% in clinical trials in 2014.
Increased survival, higher costs
Dr. Gershenwald pointed to a recently published cost-effectiveness analysis of treatment strategies for BRAF-mutated metastatic melanoma. In it, the authors noted that vemurafenib costs $13,000 per month, translating into $207,000 for a patient with median survival. Patients for whom vemurafenib fails are often put on ipilimumab, at $150,000 per course.
The authors calculated that the incremental cost-effectiveness ratio (ICER) for vemurafenib compared with dacarbazine was nearly $354,993 per quality-adjusted life-year (QALY) gained, a figure that is more than threefold higher than widely accepted thresholds for cost-effective treatment ($50,000-$100,000 per QALY gained).
The ICER for firstline vemurafenib followed by ipilimumab was $158,139, still well above the accepted limits.
The authors of the cost analysis noted that the treatments could become cost effective if drug prices were to drop significantly, or if clinical trials could establish whether it was possible to achieve a durable response without continued therapy.
Going forward, clinicians will need to consider disease burden, including both the extent and growth rate of the disease, as well as the risk of recurrence, in deciding whether to use adjuvant therapies, Dr. Gershenwald said.
In addition, clinical choices will be based on disease biology, predictors of response (although few such predictors currently exist), the likelihood of resistance, and drug toxicities, quality of life, and ease of administration, he said.
Dr. Gershenwald disclosed serving on a Merck advisory board.
HOUSTON – Newer systemic therapies for metastatic malignant melanoma have resulted in significant gains in survival, but at a cost that may be unsustainable in the near future, according to Dr. Jeffrey E. Gershenwald.
Up to one-half of all expenses related to the treatment of malignant melanoma are accounted for by the care of patients with advanced disease, yet patients with distant metastases (stage IV disease) account for only about 2% of all patients, said Dr. Gershenwald, professor of surgical oncology at the University of Texas M.D. Anderson Cancer Center, Houston.
“How best can we achieve the right therapy for the right patient at the right time, and as we learn more and more about some of the therapies, particularly in melanoma, for the right length of time? We can’t really afford to give treatments in perpetuity, so we need to know how long they actually need to be delivered in order to have optimal value for the patient,” he said at the annual Society of Surgical Oncology Cancer Symposium.
Over the last 3 decades, and particularly over the last 5 years, there have been tremendous forward strides in therapy. In 1975, when dacarbazine became the standard of care for metastatic melanoma, it was associated with response rates of only about 6%-15%, durable responses in only 5%-15% of patients, and a median overall survival of about 6-9 months, Dr. Gershenwald reported.
Treatment toxicities, but not response rates, increased with the introduction of interleukin-2 in 1998, which for want of a better drug became the new preferred treatment.
But with the introduction of new systemic therapies, such as immune checkpoint inhibitors (ipilimumab [Yervoy], nivolumab [Opdivo], and pembrolizumab [Keytruda]) and targeted agents (vemurafenib [Zelboraf], dabrafenib [Tafinlar], and trametinib [Mekinist]), response rates have soared, resulting in an improvement in 1-year survival rates from about 30% to 35% in 1970 to as high as 80% in clinical trials in 2014.
Increased survival, higher costs
Dr. Gershenwald pointed to a recently published cost-effectiveness analysis of treatment strategies for BRAF-mutated metastatic melanoma. In it, the authors noted that vemurafenib costs $13,000 per month, translating into $207,000 for a patient with median survival. Patients for whom vemurafenib fails are often put on ipilimumab, at $150,000 per course.
The authors calculated that the incremental cost-effectiveness ratio (ICER) for vemurafenib compared with dacarbazine was nearly $354,993 per quality-adjusted life-year (QALY) gained, a figure that is more than threefold higher than widely accepted thresholds for cost-effective treatment ($50,000-$100,000 per QALY gained).
The ICER for firstline vemurafenib followed by ipilimumab was $158,139, still well above the accepted limits.
The authors of the cost analysis noted that the treatments could become cost effective if drug prices were to drop significantly, or if clinical trials could establish whether it was possible to achieve a durable response without continued therapy.
Going forward, clinicians will need to consider disease burden, including both the extent and growth rate of the disease, as well as the risk of recurrence, in deciding whether to use adjuvant therapies, Dr. Gershenwald said.
In addition, clinical choices will be based on disease biology, predictors of response (although few such predictors currently exist), the likelihood of resistance, and drug toxicities, quality of life, and ease of administration, he said.
Dr. Gershenwald disclosed serving on a Merck advisory board.
HOUSTON – Newer systemic therapies for metastatic malignant melanoma have resulted in significant gains in survival, but at a cost that may be unsustainable in the near future, according to Dr. Jeffrey E. Gershenwald.
Up to one-half of all expenses related to the treatment of malignant melanoma are accounted for by the care of patients with advanced disease, yet patients with distant metastases (stage IV disease) account for only about 2% of all patients, said Dr. Gershenwald, professor of surgical oncology at the University of Texas M.D. Anderson Cancer Center, Houston.
“How best can we achieve the right therapy for the right patient at the right time, and as we learn more and more about some of the therapies, particularly in melanoma, for the right length of time? We can’t really afford to give treatments in perpetuity, so we need to know how long they actually need to be delivered in order to have optimal value for the patient,” he said at the annual Society of Surgical Oncology Cancer Symposium.
Over the last 3 decades, and particularly over the last 5 years, there have been tremendous forward strides in therapy. In 1975, when dacarbazine became the standard of care for metastatic melanoma, it was associated with response rates of only about 6%-15%, durable responses in only 5%-15% of patients, and a median overall survival of about 6-9 months, Dr. Gershenwald reported.
Treatment toxicities, but not response rates, increased with the introduction of interleukin-2 in 1998, which for want of a better drug became the new preferred treatment.
But with the introduction of new systemic therapies, such as immune checkpoint inhibitors (ipilimumab [Yervoy], nivolumab [Opdivo], and pembrolizumab [Keytruda]) and targeted agents (vemurafenib [Zelboraf], dabrafenib [Tafinlar], and trametinib [Mekinist]), response rates have soared, resulting in an improvement in 1-year survival rates from about 30% to 35% in 1970 to as high as 80% in clinical trials in 2014.
Increased survival, higher costs
Dr. Gershenwald pointed to a recently published cost-effectiveness analysis of treatment strategies for BRAF-mutated metastatic melanoma. In it, the authors noted that vemurafenib costs $13,000 per month, translating into $207,000 for a patient with median survival. Patients for whom vemurafenib fails are often put on ipilimumab, at $150,000 per course.
The authors calculated that the incremental cost-effectiveness ratio (ICER) for vemurafenib compared with dacarbazine was nearly $354,993 per quality-adjusted life-year (QALY) gained, a figure that is more than threefold higher than widely accepted thresholds for cost-effective treatment ($50,000-$100,000 per QALY gained).
The ICER for firstline vemurafenib followed by ipilimumab was $158,139, still well above the accepted limits.
The authors of the cost analysis noted that the treatments could become cost effective if drug prices were to drop significantly, or if clinical trials could establish whether it was possible to achieve a durable response without continued therapy.
Going forward, clinicians will need to consider disease burden, including both the extent and growth rate of the disease, as well as the risk of recurrence, in deciding whether to use adjuvant therapies, Dr. Gershenwald said.
In addition, clinical choices will be based on disease biology, predictors of response (although few such predictors currently exist), the likelihood of resistance, and drug toxicities, quality of life, and ease of administration, he said.
Dr. Gershenwald disclosed serving on a Merck advisory board.
AT SSO 2015
Key clinical point: Immunotherapies and targeted agents for metastatic melanoma are effective but very costly.
Major finding: The incremental cost-effectiveness ratio for vemurafenib, compared with dacarbazine, was nearly $354,993 per quality-adjusted life-year gained.
Data source: A review of data on the efficacy and costs of therapy for metastatic malignant melanoma.
Disclosures: Dr. Gershenwald disclosed serving on a Merck advisory board.
Melanoma incidence drops for U.S. children and teens
The incidence of melanoma among American children and teens decreased by approximately 12% from 2004 to 2010, with the decline most notable for adolescents. The findings were published online in the Journal of Pediatrics.
In a review of data from the period of 2000-2010, Dr. Laura Campbell of Stanford (Calif.) University and her colleagues at Case Western Reserve University in Cleveland found an overall reduction in melanoma diagnoses of 11.58% per year for the period of 2004-2010 (J. Pediatr. 2015 [doi: 10.1016/j.jpeds. 2015.02.050]).
The study was conducted at Case Western Reserve University, and the researchers used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER-18) registry to examine trends in the incidence of pediatric melanoma.
Of note, the number of new melanoma cases decreased significantly (approximately 11%) among 15- to19-year-olds between 2003 and 2010. In addition, the overall incidence of melanoma decreased significantly (7%) among boys between 2000 and 2010.
The data revealed significant decreases for the number of new cases of melanoma on the trunk (15% per year from 2004 to 2010) and upper extremities (5% from 2000 to 2010).
Dr. Campbell and her colleagues determined that a melanoma diagnosis was equally likely for male and female patients, and was more common in older than in younger patients. White patients had by far the greatest incidence of melanoma, with 97% of the overall diagnoses; 90% of the cases were in non-Hispanic whites. Superficial spreading melanoma was the most common type of melanoma, at 31%, though nodular histology was seen almost as frequently in the 0- to 9-year-olds. This younger group was more likely to have thicker tumors, ulceration, lymph node involvement, and distant metastases.
Drawing on this large registry allowed researchers more confidence that they were identifying true trends in melanoma incidence, Dr. Campbell noted.
The reasons for this decrease, which stands in contrast to earlier data showing increased incidence rates of pediatric melanoma, were not examined in this study. However, Dr. Campbell drew on these earlier studies, as well as some international studies, to identify the potential contribution of public health campaigns advocating sun protection. These campaigns began in the 1990s in the United States, and would have benefited the 15- to 19-year olds in the SEER-18 data, in whom melanoma incidence decreased beginning in 2003. Some Swedish and Australian studies showing decreased melanoma cases were confounded by an immigration-driven decrease in the highest risk light-skinned population, noted Dr. Campbell; however, the quality of the SEER-18 data allowed researchers to account for this variable, she said.
Although the widespread adoption of sun-protective behaviors (wearing hats and protective clothing, using sunscreen appropriately, and avoiding midday sun exposure) may have accounted for some of the reduction in pediatric melanomas, other societal changes may have been at play.
“We hypothesize that there has been a shift in youth participating increasingly in indoor activities, such as television/electronic devices, which may be decreasing their UVR exposure,” Dr. Campbell said.
The incidence of melanoma among American children and teens decreased by approximately 12% from 2004 to 2010, with the decline most notable for adolescents. The findings were published online in the Journal of Pediatrics.
In a review of data from the period of 2000-2010, Dr. Laura Campbell of Stanford (Calif.) University and her colleagues at Case Western Reserve University in Cleveland found an overall reduction in melanoma diagnoses of 11.58% per year for the period of 2004-2010 (J. Pediatr. 2015 [doi: 10.1016/j.jpeds. 2015.02.050]).
The study was conducted at Case Western Reserve University, and the researchers used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER-18) registry to examine trends in the incidence of pediatric melanoma.
Of note, the number of new melanoma cases decreased significantly (approximately 11%) among 15- to19-year-olds between 2003 and 2010. In addition, the overall incidence of melanoma decreased significantly (7%) among boys between 2000 and 2010.
The data revealed significant decreases for the number of new cases of melanoma on the trunk (15% per year from 2004 to 2010) and upper extremities (5% from 2000 to 2010).
Dr. Campbell and her colleagues determined that a melanoma diagnosis was equally likely for male and female patients, and was more common in older than in younger patients. White patients had by far the greatest incidence of melanoma, with 97% of the overall diagnoses; 90% of the cases were in non-Hispanic whites. Superficial spreading melanoma was the most common type of melanoma, at 31%, though nodular histology was seen almost as frequently in the 0- to 9-year-olds. This younger group was more likely to have thicker tumors, ulceration, lymph node involvement, and distant metastases.
Drawing on this large registry allowed researchers more confidence that they were identifying true trends in melanoma incidence, Dr. Campbell noted.
The reasons for this decrease, which stands in contrast to earlier data showing increased incidence rates of pediatric melanoma, were not examined in this study. However, Dr. Campbell drew on these earlier studies, as well as some international studies, to identify the potential contribution of public health campaigns advocating sun protection. These campaigns began in the 1990s in the United States, and would have benefited the 15- to 19-year olds in the SEER-18 data, in whom melanoma incidence decreased beginning in 2003. Some Swedish and Australian studies showing decreased melanoma cases were confounded by an immigration-driven decrease in the highest risk light-skinned population, noted Dr. Campbell; however, the quality of the SEER-18 data allowed researchers to account for this variable, she said.
Although the widespread adoption of sun-protective behaviors (wearing hats and protective clothing, using sunscreen appropriately, and avoiding midday sun exposure) may have accounted for some of the reduction in pediatric melanomas, other societal changes may have been at play.
“We hypothesize that there has been a shift in youth participating increasingly in indoor activities, such as television/electronic devices, which may be decreasing their UVR exposure,” Dr. Campbell said.
The incidence of melanoma among American children and teens decreased by approximately 12% from 2004 to 2010, with the decline most notable for adolescents. The findings were published online in the Journal of Pediatrics.
In a review of data from the period of 2000-2010, Dr. Laura Campbell of Stanford (Calif.) University and her colleagues at Case Western Reserve University in Cleveland found an overall reduction in melanoma diagnoses of 11.58% per year for the period of 2004-2010 (J. Pediatr. 2015 [doi: 10.1016/j.jpeds. 2015.02.050]).
The study was conducted at Case Western Reserve University, and the researchers used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER-18) registry to examine trends in the incidence of pediatric melanoma.
Of note, the number of new melanoma cases decreased significantly (approximately 11%) among 15- to19-year-olds between 2003 and 2010. In addition, the overall incidence of melanoma decreased significantly (7%) among boys between 2000 and 2010.
The data revealed significant decreases for the number of new cases of melanoma on the trunk (15% per year from 2004 to 2010) and upper extremities (5% from 2000 to 2010).
Dr. Campbell and her colleagues determined that a melanoma diagnosis was equally likely for male and female patients, and was more common in older than in younger patients. White patients had by far the greatest incidence of melanoma, with 97% of the overall diagnoses; 90% of the cases were in non-Hispanic whites. Superficial spreading melanoma was the most common type of melanoma, at 31%, though nodular histology was seen almost as frequently in the 0- to 9-year-olds. This younger group was more likely to have thicker tumors, ulceration, lymph node involvement, and distant metastases.
Drawing on this large registry allowed researchers more confidence that they were identifying true trends in melanoma incidence, Dr. Campbell noted.
The reasons for this decrease, which stands in contrast to earlier data showing increased incidence rates of pediatric melanoma, were not examined in this study. However, Dr. Campbell drew on these earlier studies, as well as some international studies, to identify the potential contribution of public health campaigns advocating sun protection. These campaigns began in the 1990s in the United States, and would have benefited the 15- to 19-year olds in the SEER-18 data, in whom melanoma incidence decreased beginning in 2003. Some Swedish and Australian studies showing decreased melanoma cases were confounded by an immigration-driven decrease in the highest risk light-skinned population, noted Dr. Campbell; however, the quality of the SEER-18 data allowed researchers to account for this variable, she said.
Although the widespread adoption of sun-protective behaviors (wearing hats and protective clothing, using sunscreen appropriately, and avoiding midday sun exposure) may have accounted for some of the reduction in pediatric melanomas, other societal changes may have been at play.
“We hypothesize that there has been a shift in youth participating increasingly in indoor activities, such as television/electronic devices, which may be decreasing their UVR exposure,” Dr. Campbell said.
FROM THE JOURNAL OF PEDIATRICS
Key clinical point: The overall incidence of melanoma in American children and teens decreased from 2004 to 2010.
Major finding: Researchers identified 1,185 patients younger than 20 years of age with melanoma diagnoses during the period of 2000-2010, and noted a significant decrease of 11.58% per year in melanoma diagnoses from 2004 to 2010.
Data source: The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER-18) registry for 2000-2010.
Disclosures: The authors reported no conflicts of interest.
Medicare beneficiaries often experience delays before melanoma surgery
About 22% of Medicare beneficiaries experience a delay of longer than 1.5 months between melanoma diagnosis and surgery, according to findings from a retrospective cohort study.
Of 32,501 melanoma cases from the Surveillance, Epidemiology, and End Results (SEER)–Medicare database, about 77.7% underwent excision within 1.5 months of biopsy, and of those who underwent excision after 1.5 months, 8.1% experienced a delay of more than 3 months. The risk-adjusted incidence of surgical delay longer than 1.5 months was significantly increased for those aged 85 years and older, compared with those aged 65 years and younger (odds ratio, 1.28), for those with a prior melanoma (OR, 1.20), and for those with three or more Elixhauser comorbidities (OR, 1.18), Dr. Jason P. Lott of Yale University, New Haven, Conn. and his colleagues reported online April 8 in JAMA Dermatology.
The lowest risk of delay was among those who underwent biopsy and excision by dermatologists (probability, 16.5%), and the highest was among those with a biopsy performed by a nondermatologist and excised by a primary care physician (probability, 30.7%), the investigators said (JAMA Dermatol. 2015 April 8 [doi:10.1001/jamadermatol.2015.119]).
The study, which provides the first population-based estimates of melanoma surgery delay among Medicare beneficiaries, and which shows that delays are relatively common, highlights “opportunities for quality improvement in dermatologic care and suggests that efforts to minimize the delay of surgery for melanoma might focus on increased access to dermatologic expertise and enhanced coordination of care among different specialists,” the investigators concluded.
This study was supported by the Robert Wood Johnson Foundation and the P30 Cancer Center Support Grant at the Yale Comprehensive Cancer Center.
Dr. Lott reported having no disclosures. Coauthor Dr. Cary P. Gross received research grant support from Johnson & Johnson, Merck, and 21st Century Oncology.
About 22% of Medicare beneficiaries experience a delay of longer than 1.5 months between melanoma diagnosis and surgery, according to findings from a retrospective cohort study.
Of 32,501 melanoma cases from the Surveillance, Epidemiology, and End Results (SEER)–Medicare database, about 77.7% underwent excision within 1.5 months of biopsy, and of those who underwent excision after 1.5 months, 8.1% experienced a delay of more than 3 months. The risk-adjusted incidence of surgical delay longer than 1.5 months was significantly increased for those aged 85 years and older, compared with those aged 65 years and younger (odds ratio, 1.28), for those with a prior melanoma (OR, 1.20), and for those with three or more Elixhauser comorbidities (OR, 1.18), Dr. Jason P. Lott of Yale University, New Haven, Conn. and his colleagues reported online April 8 in JAMA Dermatology.
The lowest risk of delay was among those who underwent biopsy and excision by dermatologists (probability, 16.5%), and the highest was among those with a biopsy performed by a nondermatologist and excised by a primary care physician (probability, 30.7%), the investigators said (JAMA Dermatol. 2015 April 8 [doi:10.1001/jamadermatol.2015.119]).
The study, which provides the first population-based estimates of melanoma surgery delay among Medicare beneficiaries, and which shows that delays are relatively common, highlights “opportunities for quality improvement in dermatologic care and suggests that efforts to minimize the delay of surgery for melanoma might focus on increased access to dermatologic expertise and enhanced coordination of care among different specialists,” the investigators concluded.
This study was supported by the Robert Wood Johnson Foundation and the P30 Cancer Center Support Grant at the Yale Comprehensive Cancer Center.
Dr. Lott reported having no disclosures. Coauthor Dr. Cary P. Gross received research grant support from Johnson & Johnson, Merck, and 21st Century Oncology.
About 22% of Medicare beneficiaries experience a delay of longer than 1.5 months between melanoma diagnosis and surgery, according to findings from a retrospective cohort study.
Of 32,501 melanoma cases from the Surveillance, Epidemiology, and End Results (SEER)–Medicare database, about 77.7% underwent excision within 1.5 months of biopsy, and of those who underwent excision after 1.5 months, 8.1% experienced a delay of more than 3 months. The risk-adjusted incidence of surgical delay longer than 1.5 months was significantly increased for those aged 85 years and older, compared with those aged 65 years and younger (odds ratio, 1.28), for those with a prior melanoma (OR, 1.20), and for those with three or more Elixhauser comorbidities (OR, 1.18), Dr. Jason P. Lott of Yale University, New Haven, Conn. and his colleagues reported online April 8 in JAMA Dermatology.
The lowest risk of delay was among those who underwent biopsy and excision by dermatologists (probability, 16.5%), and the highest was among those with a biopsy performed by a nondermatologist and excised by a primary care physician (probability, 30.7%), the investigators said (JAMA Dermatol. 2015 April 8 [doi:10.1001/jamadermatol.2015.119]).
The study, which provides the first population-based estimates of melanoma surgery delay among Medicare beneficiaries, and which shows that delays are relatively common, highlights “opportunities for quality improvement in dermatologic care and suggests that efforts to minimize the delay of surgery for melanoma might focus on increased access to dermatologic expertise and enhanced coordination of care among different specialists,” the investigators concluded.
This study was supported by the Robert Wood Johnson Foundation and the P30 Cancer Center Support Grant at the Yale Comprehensive Cancer Center.
Dr. Lott reported having no disclosures. Coauthor Dr. Cary P. Gross received research grant support from Johnson & Johnson, Merck, and 21st Century Oncology.
FROM JAMA DERMATOLOGY
Key clinical point: Opportunities exist for improving the timely delivery of melanoma surgery for Medicare beneficiaries.
Major finding: A total of 22% of Medicare beneficiaries had a delay of more than 1.5 months between melanoma diagnosis and surgery.
Data source: A retrospective cohort study involving 32,501 melanoma cases.
Disclosures:This study was supported by the Robert Wood Johnson Foundation and the P30 Cancer Center Support Grant at the Yale Comprehensive Cancer Center. Dr. Lott reported having no disclosures. Coauthor Dr. Cary P. Gross received research grant support from Johnson & Johnson, Merck, and 21st Century Oncology.








