User login
Mixed results on two treatments for erectile dysfunction
presented at the annual meeting of the American Urological Association.
In a single-blind prospective study that evaluated low-intensity shock-wave therapy, researchers randomly assigned 36 men with ED to receive mechanical therapy (n = 22) or sham treatment (n = 14) on their flaccid penises.
The patients in arm 1 of the study received three treatments of 5,000 shocks (4 Hz, 0.12 mJ/mm2) with the UroGold 1000 device (SoftWave) at weeks 0, 3, and 6. Those in arm 2 received a regimen of 5,000 shocks at week 0 and 3,000 at weeks 2 and 3, which was repeated 3 weeks later. Patients who completed sham treatment were unblinded and crossed over to the opposite arm for active treatment.
At weeks 20 and 32, the researchers assessed changes in gray-scale ultrasound erectile tissue homogeneity of the corpora cavernosa using visual grading scores as well as changes in color Duplex Doppler ultrasound assessments of artery blood flow parameters between baseline and follow-up.
Better blood flow – But is that enough?
After shock-wave therapy, more men experienced either improvements in or no worsening of blood flow parameters relative to baseline than after sham treatment. The decrease in end-diastolic volume was statistically significant for men in the active treatment arm 2 at week 32 (P = .003), according to the researchers.
The number of men whose visual grading scores for ultrasound gray-scale images improved in the proximal region was consistently higher with active treatment than with placebo (arm 1: 88.9% vs. 11.1%; arm 2: 40% vs. 20%), with statistical significance in arm 1 at weeks 20 (P = .005) and 32 (P = .001). Patients who received sham treatment and who subsequently received active shock-wave therapy also had improved scores on gray-scale ultrasound (arm 1: 33.3% vs. 11.1%; arm 2: 40% vs. 20%).
Scores on the International Index of Erectile Function (IIEF) were nominally higher for men in active treatment whose visual grading scores had improved compared with those who did not show improvement.
The most common adverse event was transient discomfort after the shock-wave treatment, according to the researchers.
The study provides “a glimpse into the concept” that the mechanotransduction from a shock wave results in biochemical changes, including “activation of stem cells within the corpus cavernosum,” said Irwin Goldstein, MD, director of San Diego Sexual Medicine and clinical professor of surgery at the University of California, San Diego, who led the trial. “If I can activate stem cells,” he added, “theoretically, I can improve the health of tissue.”
Dr. Goldstein noted that the study is the first to use before-and-after objective gray-scale ultrasound imaging along with color Doppler ultrasound. “We could see gray scale changes and peak systolic velocity changes even with a small group,” he said.
Dr. Goldstein added that the trial is the first in which zero energy was used in the sham phase instead of less energy than active treatment. With the sham treatment, there was no benefit on the gray scale, which he said is “very important.”
He said his team is in the process of submitting a proposal for a larger prospective trial to confirm the findings.
Although the results are promising, the study did not evaluate what matters most to men, said Louis Kuritzky, MD, a family medicine physician and assistant professor emeritus at HCA UCF Family Medicine Residency, in Gainesville, Fla.
“Men don’t care what the flow velocity is – they care [whether] they get an erection sufficient for penetration and completion of intercourse. That trial did not look at those endpoints. It looked at surrogates. Those are encouraging, but that’s not what I think a clinician would base their decision upon about whether or not a patient should possibly participate in shock therapy.”
Plasma injections a bust
The trial that assessed platelet-rich plasma was not encouraging. The results of the prospective, double-blind, randomized, placebo-controlled trial suggest that PRP is safe but not effective.
A proprietary version of the PRP injection is marketed as the “Priapus shot,” or the “P-shot,” despite a lack of solid evidence the therapy helps.
Brian Ledesma, MD, an andrology research fellow at the University of Miami, led the study, which received a “best abstract” award at the meeting. “We wanted to actually check and see – does this work or not?” Dr. Ledesma said of PRP injections generally.
Dr. Ledesma and his colleagues randomly assigned 61 men with mild to moderate ED to receive two intracavernosal injections of PRP 1 month apart (n = 28) or placebo treatment (n = 33). The primary outcome was change in IIEF score and the percentage of men meeting minimum clinically important difference (MCID) at 1 month. Complete data were available for 24 men who received PRP and for 28 who received placebo injections.
There was no significant difference in outcomes between the groups. IIEF scores changed from 17.4 (95% confidence interval [CI], –15.8 to 19.0) to 21 (95% CI, 17.9-24.0) for men who received PRP and from 18.6 (95% CI, 17.3-19.8) to 21.6 (95% CI, 19.1-24.1) for men in the placebo group (P = .756). Fourteen men (58.3%) in the PRP group, compared with 15 (53.6%) in the placebo group, met MCID. No differences were seen in mean penile Doppler parameters between PRP and placebo. The two adverse events reported in the trial were minor – a hematoma and “a new plaque that did not cause any curvature of the penis,” Dr. Ledesma said.
Platelet-rich plasma may be “really popular, but, objectively speaking, so far, we don’t have any evidence showing that it actually works.”
The study showed that “PRP was not more efficacious than placebo.” This treatment is “really popular, but, objectively speaking, so far, we don’t have any evidence showing that it actually works.”
Based on these findings, he said, “We would recommend sticking to the data primary care providers should tell their patients, ‘Don’t waste your money,’ because it’s pretty expensive.”
Dr. Kuritzky said more studies are needed for a definitive answer. “I think the results of PRP have been largely disappointing across most of the spheres of influence in which it’s been tried. So, it’s not so surprising to me that this trial would, again, not prove efficacious, but I’d have to hold judgment, dependent upon other trials,” he said.
Dr. Ledesma and his colleagues are conducting a prospective, randomized, double-blind trial “investigating whether PRP combined with shock-wave therapy could make a difference.” He said the trial, which is funded by the National Institutes of Health, is in the enrollment phase; results are expected in mid-2024.
Charles Runels, MD, who pioneered the P-Shot and other popular cosmetic procedures, defended the effectiveness of the injections.
“One of the legitimate criticisms of all of the review articles regarding PRP therapies in every field is that there is a significant variability in what people call PRP. The P-Shot represents a very specific protocol in the methods of preparing PRP, activating PRP, and injecting the PRP – all of which differ significantly from what was done in Ledesma’s study and which could account for their lack of results,” Dr. Runels said in an interview.
Dr. Runels added that “multiple studies” do show benefit of the injection of PRP for both erectile dysfunction and Peyronie’s disease and support the success of his protocol. “Also, all of our providers – there are over 3,000 people in our Cellular Medicine Association – offer money back to our patients if there are not satisfactory results,” he said.
Dr. Kuritzky said that when patients ask him about investigational treatments for ED, he tells them to stick to the more traditional approaches, such as phosphodiesterase type 5 inhibitors, intracorporeal injections, and vacuum devices.
But, he added, if other therapies are shown to be safe and effective “in a large population of men with diverse etiologies associated with their erectile dysfunction, including advanced age, diabetes, dyslipidemia, hypertension, cigarette smoking, then I think [they] could be recommended on a more consistent basis.”
Both studies were independently supported. Dr. Goldstein, Dr. Kuritzky, and Dr. Ledesma reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
presented at the annual meeting of the American Urological Association.
In a single-blind prospective study that evaluated low-intensity shock-wave therapy, researchers randomly assigned 36 men with ED to receive mechanical therapy (n = 22) or sham treatment (n = 14) on their flaccid penises.
The patients in arm 1 of the study received three treatments of 5,000 shocks (4 Hz, 0.12 mJ/mm2) with the UroGold 1000 device (SoftWave) at weeks 0, 3, and 6. Those in arm 2 received a regimen of 5,000 shocks at week 0 and 3,000 at weeks 2 and 3, which was repeated 3 weeks later. Patients who completed sham treatment were unblinded and crossed over to the opposite arm for active treatment.
At weeks 20 and 32, the researchers assessed changes in gray-scale ultrasound erectile tissue homogeneity of the corpora cavernosa using visual grading scores as well as changes in color Duplex Doppler ultrasound assessments of artery blood flow parameters between baseline and follow-up.
Better blood flow – But is that enough?
After shock-wave therapy, more men experienced either improvements in or no worsening of blood flow parameters relative to baseline than after sham treatment. The decrease in end-diastolic volume was statistically significant for men in the active treatment arm 2 at week 32 (P = .003), according to the researchers.
The number of men whose visual grading scores for ultrasound gray-scale images improved in the proximal region was consistently higher with active treatment than with placebo (arm 1: 88.9% vs. 11.1%; arm 2: 40% vs. 20%), with statistical significance in arm 1 at weeks 20 (P = .005) and 32 (P = .001). Patients who received sham treatment and who subsequently received active shock-wave therapy also had improved scores on gray-scale ultrasound (arm 1: 33.3% vs. 11.1%; arm 2: 40% vs. 20%).
Scores on the International Index of Erectile Function (IIEF) were nominally higher for men in active treatment whose visual grading scores had improved compared with those who did not show improvement.
The most common adverse event was transient discomfort after the shock-wave treatment, according to the researchers.
The study provides “a glimpse into the concept” that the mechanotransduction from a shock wave results in biochemical changes, including “activation of stem cells within the corpus cavernosum,” said Irwin Goldstein, MD, director of San Diego Sexual Medicine and clinical professor of surgery at the University of California, San Diego, who led the trial. “If I can activate stem cells,” he added, “theoretically, I can improve the health of tissue.”
Dr. Goldstein noted that the study is the first to use before-and-after objective gray-scale ultrasound imaging along with color Doppler ultrasound. “We could see gray scale changes and peak systolic velocity changes even with a small group,” he said.
Dr. Goldstein added that the trial is the first in which zero energy was used in the sham phase instead of less energy than active treatment. With the sham treatment, there was no benefit on the gray scale, which he said is “very important.”
He said his team is in the process of submitting a proposal for a larger prospective trial to confirm the findings.
Although the results are promising, the study did not evaluate what matters most to men, said Louis Kuritzky, MD, a family medicine physician and assistant professor emeritus at HCA UCF Family Medicine Residency, in Gainesville, Fla.
“Men don’t care what the flow velocity is – they care [whether] they get an erection sufficient for penetration and completion of intercourse. That trial did not look at those endpoints. It looked at surrogates. Those are encouraging, but that’s not what I think a clinician would base their decision upon about whether or not a patient should possibly participate in shock therapy.”
Plasma injections a bust
The trial that assessed platelet-rich plasma was not encouraging. The results of the prospective, double-blind, randomized, placebo-controlled trial suggest that PRP is safe but not effective.
A proprietary version of the PRP injection is marketed as the “Priapus shot,” or the “P-shot,” despite a lack of solid evidence the therapy helps.
Brian Ledesma, MD, an andrology research fellow at the University of Miami, led the study, which received a “best abstract” award at the meeting. “We wanted to actually check and see – does this work or not?” Dr. Ledesma said of PRP injections generally.
Dr. Ledesma and his colleagues randomly assigned 61 men with mild to moderate ED to receive two intracavernosal injections of PRP 1 month apart (n = 28) or placebo treatment (n = 33). The primary outcome was change in IIEF score and the percentage of men meeting minimum clinically important difference (MCID) at 1 month. Complete data were available for 24 men who received PRP and for 28 who received placebo injections.
There was no significant difference in outcomes between the groups. IIEF scores changed from 17.4 (95% confidence interval [CI], –15.8 to 19.0) to 21 (95% CI, 17.9-24.0) for men who received PRP and from 18.6 (95% CI, 17.3-19.8) to 21.6 (95% CI, 19.1-24.1) for men in the placebo group (P = .756). Fourteen men (58.3%) in the PRP group, compared with 15 (53.6%) in the placebo group, met MCID. No differences were seen in mean penile Doppler parameters between PRP and placebo. The two adverse events reported in the trial were minor – a hematoma and “a new plaque that did not cause any curvature of the penis,” Dr. Ledesma said.
Platelet-rich plasma may be “really popular, but, objectively speaking, so far, we don’t have any evidence showing that it actually works.”
The study showed that “PRP was not more efficacious than placebo.” This treatment is “really popular, but, objectively speaking, so far, we don’t have any evidence showing that it actually works.”
Based on these findings, he said, “We would recommend sticking to the data primary care providers should tell their patients, ‘Don’t waste your money,’ because it’s pretty expensive.”
Dr. Kuritzky said more studies are needed for a definitive answer. “I think the results of PRP have been largely disappointing across most of the spheres of influence in which it’s been tried. So, it’s not so surprising to me that this trial would, again, not prove efficacious, but I’d have to hold judgment, dependent upon other trials,” he said.
Dr. Ledesma and his colleagues are conducting a prospective, randomized, double-blind trial “investigating whether PRP combined with shock-wave therapy could make a difference.” He said the trial, which is funded by the National Institutes of Health, is in the enrollment phase; results are expected in mid-2024.
Charles Runels, MD, who pioneered the P-Shot and other popular cosmetic procedures, defended the effectiveness of the injections.
“One of the legitimate criticisms of all of the review articles regarding PRP therapies in every field is that there is a significant variability in what people call PRP. The P-Shot represents a very specific protocol in the methods of preparing PRP, activating PRP, and injecting the PRP – all of which differ significantly from what was done in Ledesma’s study and which could account for their lack of results,” Dr. Runels said in an interview.
Dr. Runels added that “multiple studies” do show benefit of the injection of PRP for both erectile dysfunction and Peyronie’s disease and support the success of his protocol. “Also, all of our providers – there are over 3,000 people in our Cellular Medicine Association – offer money back to our patients if there are not satisfactory results,” he said.
Dr. Kuritzky said that when patients ask him about investigational treatments for ED, he tells them to stick to the more traditional approaches, such as phosphodiesterase type 5 inhibitors, intracorporeal injections, and vacuum devices.
But, he added, if other therapies are shown to be safe and effective “in a large population of men with diverse etiologies associated with their erectile dysfunction, including advanced age, diabetes, dyslipidemia, hypertension, cigarette smoking, then I think [they] could be recommended on a more consistent basis.”
Both studies were independently supported. Dr. Goldstein, Dr. Kuritzky, and Dr. Ledesma reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
presented at the annual meeting of the American Urological Association.
In a single-blind prospective study that evaluated low-intensity shock-wave therapy, researchers randomly assigned 36 men with ED to receive mechanical therapy (n = 22) or sham treatment (n = 14) on their flaccid penises.
The patients in arm 1 of the study received three treatments of 5,000 shocks (4 Hz, 0.12 mJ/mm2) with the UroGold 1000 device (SoftWave) at weeks 0, 3, and 6. Those in arm 2 received a regimen of 5,000 shocks at week 0 and 3,000 at weeks 2 and 3, which was repeated 3 weeks later. Patients who completed sham treatment were unblinded and crossed over to the opposite arm for active treatment.
At weeks 20 and 32, the researchers assessed changes in gray-scale ultrasound erectile tissue homogeneity of the corpora cavernosa using visual grading scores as well as changes in color Duplex Doppler ultrasound assessments of artery blood flow parameters between baseline and follow-up.
Better blood flow – But is that enough?
After shock-wave therapy, more men experienced either improvements in or no worsening of blood flow parameters relative to baseline than after sham treatment. The decrease in end-diastolic volume was statistically significant for men in the active treatment arm 2 at week 32 (P = .003), according to the researchers.
The number of men whose visual grading scores for ultrasound gray-scale images improved in the proximal region was consistently higher with active treatment than with placebo (arm 1: 88.9% vs. 11.1%; arm 2: 40% vs. 20%), with statistical significance in arm 1 at weeks 20 (P = .005) and 32 (P = .001). Patients who received sham treatment and who subsequently received active shock-wave therapy also had improved scores on gray-scale ultrasound (arm 1: 33.3% vs. 11.1%; arm 2: 40% vs. 20%).
Scores on the International Index of Erectile Function (IIEF) were nominally higher for men in active treatment whose visual grading scores had improved compared with those who did not show improvement.
The most common adverse event was transient discomfort after the shock-wave treatment, according to the researchers.
The study provides “a glimpse into the concept” that the mechanotransduction from a shock wave results in biochemical changes, including “activation of stem cells within the corpus cavernosum,” said Irwin Goldstein, MD, director of San Diego Sexual Medicine and clinical professor of surgery at the University of California, San Diego, who led the trial. “If I can activate stem cells,” he added, “theoretically, I can improve the health of tissue.”
Dr. Goldstein noted that the study is the first to use before-and-after objective gray-scale ultrasound imaging along with color Doppler ultrasound. “We could see gray scale changes and peak systolic velocity changes even with a small group,” he said.
Dr. Goldstein added that the trial is the first in which zero energy was used in the sham phase instead of less energy than active treatment. With the sham treatment, there was no benefit on the gray scale, which he said is “very important.”
He said his team is in the process of submitting a proposal for a larger prospective trial to confirm the findings.
Although the results are promising, the study did not evaluate what matters most to men, said Louis Kuritzky, MD, a family medicine physician and assistant professor emeritus at HCA UCF Family Medicine Residency, in Gainesville, Fla.
“Men don’t care what the flow velocity is – they care [whether] they get an erection sufficient for penetration and completion of intercourse. That trial did not look at those endpoints. It looked at surrogates. Those are encouraging, but that’s not what I think a clinician would base their decision upon about whether or not a patient should possibly participate in shock therapy.”
Plasma injections a bust
The trial that assessed platelet-rich plasma was not encouraging. The results of the prospective, double-blind, randomized, placebo-controlled trial suggest that PRP is safe but not effective.
A proprietary version of the PRP injection is marketed as the “Priapus shot,” or the “P-shot,” despite a lack of solid evidence the therapy helps.
Brian Ledesma, MD, an andrology research fellow at the University of Miami, led the study, which received a “best abstract” award at the meeting. “We wanted to actually check and see – does this work or not?” Dr. Ledesma said of PRP injections generally.
Dr. Ledesma and his colleagues randomly assigned 61 men with mild to moderate ED to receive two intracavernosal injections of PRP 1 month apart (n = 28) or placebo treatment (n = 33). The primary outcome was change in IIEF score and the percentage of men meeting minimum clinically important difference (MCID) at 1 month. Complete data were available for 24 men who received PRP and for 28 who received placebo injections.
There was no significant difference in outcomes between the groups. IIEF scores changed from 17.4 (95% confidence interval [CI], –15.8 to 19.0) to 21 (95% CI, 17.9-24.0) for men who received PRP and from 18.6 (95% CI, 17.3-19.8) to 21.6 (95% CI, 19.1-24.1) for men in the placebo group (P = .756). Fourteen men (58.3%) in the PRP group, compared with 15 (53.6%) in the placebo group, met MCID. No differences were seen in mean penile Doppler parameters between PRP and placebo. The two adverse events reported in the trial were minor – a hematoma and “a new plaque that did not cause any curvature of the penis,” Dr. Ledesma said.
Platelet-rich plasma may be “really popular, but, objectively speaking, so far, we don’t have any evidence showing that it actually works.”
The study showed that “PRP was not more efficacious than placebo.” This treatment is “really popular, but, objectively speaking, so far, we don’t have any evidence showing that it actually works.”
Based on these findings, he said, “We would recommend sticking to the data primary care providers should tell their patients, ‘Don’t waste your money,’ because it’s pretty expensive.”
Dr. Kuritzky said more studies are needed for a definitive answer. “I think the results of PRP have been largely disappointing across most of the spheres of influence in which it’s been tried. So, it’s not so surprising to me that this trial would, again, not prove efficacious, but I’d have to hold judgment, dependent upon other trials,” he said.
Dr. Ledesma and his colleagues are conducting a prospective, randomized, double-blind trial “investigating whether PRP combined with shock-wave therapy could make a difference.” He said the trial, which is funded by the National Institutes of Health, is in the enrollment phase; results are expected in mid-2024.
Charles Runels, MD, who pioneered the P-Shot and other popular cosmetic procedures, defended the effectiveness of the injections.
“One of the legitimate criticisms of all of the review articles regarding PRP therapies in every field is that there is a significant variability in what people call PRP. The P-Shot represents a very specific protocol in the methods of preparing PRP, activating PRP, and injecting the PRP – all of which differ significantly from what was done in Ledesma’s study and which could account for their lack of results,” Dr. Runels said in an interview.
Dr. Runels added that “multiple studies” do show benefit of the injection of PRP for both erectile dysfunction and Peyronie’s disease and support the success of his protocol. “Also, all of our providers – there are over 3,000 people in our Cellular Medicine Association – offer money back to our patients if there are not satisfactory results,” he said.
Dr. Kuritzky said that when patients ask him about investigational treatments for ED, he tells them to stick to the more traditional approaches, such as phosphodiesterase type 5 inhibitors, intracorporeal injections, and vacuum devices.
But, he added, if other therapies are shown to be safe and effective “in a large population of men with diverse etiologies associated with their erectile dysfunction, including advanced age, diabetes, dyslipidemia, hypertension, cigarette smoking, then I think [they] could be recommended on a more consistent basis.”
Both studies were independently supported. Dr. Goldstein, Dr. Kuritzky, and Dr. Ledesma reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AUA 2023
Watching feasible for asymptomatic kidney stones
Many patients with asymptomatic renal stones can qualify for an active surveillance program, Swiss researchers report at the American Urological Association 2023 Annual Meeting.
Kevin Stritt, MD, chief resident in the urology department at Lausanne University Hospital, said kidney stones often pass without symptoms. But until now, data on the frequency of asymptomatic, spontaneous passage of stones have been lacking.
The new data come from the NOSTONE trial, a prospective, multicenter, double-blind, placebo-controlled randomized trial to assess the efficacy of hydrochlorothiazide in the prevention of recurrence in patients with recurrent calcium-containing kidney stones.
Dr. Stritt and colleagues evaluated the natural history of asymptomatic renal stones during a median follow-up of 35 months. “We found for the first time that a relevant number of kidney stone passages [39%] were asymptomatic, spontaneous stone passages,” Dr. Stritt told this news organization.
All asymptomatic spontaneous stone passages were analyzed in a comparison of the total number of kidney stones on low-dose, nonintravenous contrast CT imaging at the beginning and end of the 3-year follow-up.
Of the 403 stones passed spontaneously, 61% (245) were symptomatic stone passages and 39% (158) were asymptomatic stone passages, Dr. Stritt told this news organization.
Asymptomatic stones were a median size of 2.4 mm, and symptomatic stones were 2.15 mm, which was not significantly different (P = .366), according to the researchers. Dr. Stritt said the spontaneous passage of asymptomatic stones was largely influenced by a higher number of stones on CT imaging at randomization (P = .001) and a lower total stone volume (P = .001).
Ephrem Olweny, MD, an assistant professor of urology and section chief of endourology at Rush University Medical Center in Chicago, said previous studies have found that the rate of spontaneous passage of kidney stones ranges from 3% to 29%.
“But this secondary analysis of data from a prior multicenter prospective randomized trial offers higher-quality data that will be of value in guiding patient counseling,” Dr. Olweny said.
“Observation should be initially offered to these patients. However, patients should be informed that 52% are likely to develop symptoms, and some may indeed opt for preemptive surgical removal,” he added.
David Schulsinger, MD, an associate professor in the department of urology at Stony Brook (N.Y.) University Hospital, said the incidence of kidney stones has been increasing worldwide, affecting approximately 12% of men and 6% of women. Dehydration and diets high in sodium and calcium are major factors, he said.
Patients with a history of stones have a 50% risk of recurrence in the next 5 years, and an 80% risk in their lifetime, he added.
Dr. Schulsinger said the message from the Swiss study is that urologists can be “comfortable” watching small stones, those averaging 2.4 mm or less in size. “But if a patient has a 7- or 8-mm stone, you might be more inclined to manage that patient a little bit more aggressively.”
Roughly half of patients with stones less than 2 mm will pass it in about 8 days, he said.
Dr. Olweny noted that the study was a secondary analysis of data from a randomized controlled trial that evaluated the efficacy of thiazides in preventing the recurrence of calcium stones. “The original study was not specifically designed to look at asymptomatic stone passage rates for small renal stones, and therefore, the observed rates may not reflect the most precise estimates,” he said.
Dr. Stritt said his group has not studied the size limit of stones that pass spontaneously without symptoms. “This study could serve to construct recurrence prediction models based on medical history and stone burden on CT imaging. More well-designed research on this topic is urgently needed,” he said. “These results should encourage urologists to counsel patients about the possibility of an active surveillance strategy when smaller kidney stones are present.”
The author and independent commentators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many patients with asymptomatic renal stones can qualify for an active surveillance program, Swiss researchers report at the American Urological Association 2023 Annual Meeting.
Kevin Stritt, MD, chief resident in the urology department at Lausanne University Hospital, said kidney stones often pass without symptoms. But until now, data on the frequency of asymptomatic, spontaneous passage of stones have been lacking.
The new data come from the NOSTONE trial, a prospective, multicenter, double-blind, placebo-controlled randomized trial to assess the efficacy of hydrochlorothiazide in the prevention of recurrence in patients with recurrent calcium-containing kidney stones.
Dr. Stritt and colleagues evaluated the natural history of asymptomatic renal stones during a median follow-up of 35 months. “We found for the first time that a relevant number of kidney stone passages [39%] were asymptomatic, spontaneous stone passages,” Dr. Stritt told this news organization.
All asymptomatic spontaneous stone passages were analyzed in a comparison of the total number of kidney stones on low-dose, nonintravenous contrast CT imaging at the beginning and end of the 3-year follow-up.
Of the 403 stones passed spontaneously, 61% (245) were symptomatic stone passages and 39% (158) were asymptomatic stone passages, Dr. Stritt told this news organization.
Asymptomatic stones were a median size of 2.4 mm, and symptomatic stones were 2.15 mm, which was not significantly different (P = .366), according to the researchers. Dr. Stritt said the spontaneous passage of asymptomatic stones was largely influenced by a higher number of stones on CT imaging at randomization (P = .001) and a lower total stone volume (P = .001).
Ephrem Olweny, MD, an assistant professor of urology and section chief of endourology at Rush University Medical Center in Chicago, said previous studies have found that the rate of spontaneous passage of kidney stones ranges from 3% to 29%.
“But this secondary analysis of data from a prior multicenter prospective randomized trial offers higher-quality data that will be of value in guiding patient counseling,” Dr. Olweny said.
“Observation should be initially offered to these patients. However, patients should be informed that 52% are likely to develop symptoms, and some may indeed opt for preemptive surgical removal,” he added.
David Schulsinger, MD, an associate professor in the department of urology at Stony Brook (N.Y.) University Hospital, said the incidence of kidney stones has been increasing worldwide, affecting approximately 12% of men and 6% of women. Dehydration and diets high in sodium and calcium are major factors, he said.
Patients with a history of stones have a 50% risk of recurrence in the next 5 years, and an 80% risk in their lifetime, he added.
Dr. Schulsinger said the message from the Swiss study is that urologists can be “comfortable” watching small stones, those averaging 2.4 mm or less in size. “But if a patient has a 7- or 8-mm stone, you might be more inclined to manage that patient a little bit more aggressively.”
Roughly half of patients with stones less than 2 mm will pass it in about 8 days, he said.
Dr. Olweny noted that the study was a secondary analysis of data from a randomized controlled trial that evaluated the efficacy of thiazides in preventing the recurrence of calcium stones. “The original study was not specifically designed to look at asymptomatic stone passage rates for small renal stones, and therefore, the observed rates may not reflect the most precise estimates,” he said.
Dr. Stritt said his group has not studied the size limit of stones that pass spontaneously without symptoms. “This study could serve to construct recurrence prediction models based on medical history and stone burden on CT imaging. More well-designed research on this topic is urgently needed,” he said. “These results should encourage urologists to counsel patients about the possibility of an active surveillance strategy when smaller kidney stones are present.”
The author and independent commentators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many patients with asymptomatic renal stones can qualify for an active surveillance program, Swiss researchers report at the American Urological Association 2023 Annual Meeting.
Kevin Stritt, MD, chief resident in the urology department at Lausanne University Hospital, said kidney stones often pass without symptoms. But until now, data on the frequency of asymptomatic, spontaneous passage of stones have been lacking.
The new data come from the NOSTONE trial, a prospective, multicenter, double-blind, placebo-controlled randomized trial to assess the efficacy of hydrochlorothiazide in the prevention of recurrence in patients with recurrent calcium-containing kidney stones.
Dr. Stritt and colleagues evaluated the natural history of asymptomatic renal stones during a median follow-up of 35 months. “We found for the first time that a relevant number of kidney stone passages [39%] were asymptomatic, spontaneous stone passages,” Dr. Stritt told this news organization.
All asymptomatic spontaneous stone passages were analyzed in a comparison of the total number of kidney stones on low-dose, nonintravenous contrast CT imaging at the beginning and end of the 3-year follow-up.
Of the 403 stones passed spontaneously, 61% (245) were symptomatic stone passages and 39% (158) were asymptomatic stone passages, Dr. Stritt told this news organization.
Asymptomatic stones were a median size of 2.4 mm, and symptomatic stones were 2.15 mm, which was not significantly different (P = .366), according to the researchers. Dr. Stritt said the spontaneous passage of asymptomatic stones was largely influenced by a higher number of stones on CT imaging at randomization (P = .001) and a lower total stone volume (P = .001).
Ephrem Olweny, MD, an assistant professor of urology and section chief of endourology at Rush University Medical Center in Chicago, said previous studies have found that the rate of spontaneous passage of kidney stones ranges from 3% to 29%.
“But this secondary analysis of data from a prior multicenter prospective randomized trial offers higher-quality data that will be of value in guiding patient counseling,” Dr. Olweny said.
“Observation should be initially offered to these patients. However, patients should be informed that 52% are likely to develop symptoms, and some may indeed opt for preemptive surgical removal,” he added.
David Schulsinger, MD, an associate professor in the department of urology at Stony Brook (N.Y.) University Hospital, said the incidence of kidney stones has been increasing worldwide, affecting approximately 12% of men and 6% of women. Dehydration and diets high in sodium and calcium are major factors, he said.
Patients with a history of stones have a 50% risk of recurrence in the next 5 years, and an 80% risk in their lifetime, he added.
Dr. Schulsinger said the message from the Swiss study is that urologists can be “comfortable” watching small stones, those averaging 2.4 mm or less in size. “But if a patient has a 7- or 8-mm stone, you might be more inclined to manage that patient a little bit more aggressively.”
Roughly half of patients with stones less than 2 mm will pass it in about 8 days, he said.
Dr. Olweny noted that the study was a secondary analysis of data from a randomized controlled trial that evaluated the efficacy of thiazides in preventing the recurrence of calcium stones. “The original study was not specifically designed to look at asymptomatic stone passage rates for small renal stones, and therefore, the observed rates may not reflect the most precise estimates,” he said.
Dr. Stritt said his group has not studied the size limit of stones that pass spontaneously without symptoms. “This study could serve to construct recurrence prediction models based on medical history and stone burden on CT imaging. More well-designed research on this topic is urgently needed,” he said. “These results should encourage urologists to counsel patients about the possibility of an active surveillance strategy when smaller kidney stones are present.”
The author and independent commentators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Young men at highest schizophrenia risk from cannabis abuse
A new study confirms the robust link between cannabis use and schizophrenia among men and women but suggests that young men may be especially susceptible to schizophrenia from cannabis abuse.
Of note,
“The entanglement of substance use disorders and mental illnesses is a major public health issue, requiring urgent action and support for people who need it,” study coauthor Nora Volkow, MD, director of the National Institute on Drug Abuse, said in a news release.
“As access to potent cannabis products continues to expand, it is crucial that we also expand prevention, screening, and treatment for people who may experience mental illnesses associated with cannabis use,” Dr. Volkow added.
The study was published online in Psychological Medicine.
A modifiable risk factor
The researchers analyzed Danish registry data spanning 5 decades and representing more than 6.9 million people in Denmark to estimate the population-level percentage of schizophrenia cases attributable to CUD.
A total of 60,563 participants were diagnosed with CUD. Three-quarters of cases were in men; there were 45,327 incident cases of schizophrenia during the study period.
The overall adjusted hazard ratio for CUD on schizophrenia was slightly higher among males than females (aHR, 2.42 vs. 2.02); however, among those aged 16 to 20 years, the adjusted incidence risk ratio for males was more than twice that for females (aIRR, 3.84 vs. 1.81).
The researchers estimate that, in 2021, about 15% of schizophrenia cases among males aged 16-49 could have been avoided by preventing CUD, compared with 4% among females in this age range.
For young men aged 21-30, the proportion of preventable schizophrenia cases related to CUD may be as high as 30%, the authors reported.
“Alongside the increasing evidence that CUD is a modifiable risk factor for schizophrenia, our findings underscore the importance of evidence-based strategies to regulate cannabis use and to effectively prevent, screen for, and treat CUD as well as schizophrenia,” the researchers wrote.
Legalization sends the wrong message
In a press statement, lead investigator Carsten Hjorthøj, PhD, with the University of Copenhagen, noted that “increases in the legalization of cannabis over the past few decades have made it one of the most frequently used psychoactive substances in the world, while also decreasing the public’s perception of its harm. This study adds to our growing understanding that cannabis use is not harmless, and that risks are not fixed at one point in time.”
In a prior study, Dr. Hjorthøj and colleagues found that the proportion of new schizophrenia cases attributable to CUD has consistently increased over the past 20 years.
“In my view, the association is most likely causative, at least to a large extent,” Dr. Hjorthøj said at the time this research was published.
“It is of course nearly impossible to use epidemiological studies to actually prove causation, but all the numbers behave exactly in the way that would be expected under the theory of causation,” Dr. Hjorthøj added.
The study received no specific funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study confirms the robust link between cannabis use and schizophrenia among men and women but suggests that young men may be especially susceptible to schizophrenia from cannabis abuse.
Of note,
“The entanglement of substance use disorders and mental illnesses is a major public health issue, requiring urgent action and support for people who need it,” study coauthor Nora Volkow, MD, director of the National Institute on Drug Abuse, said in a news release.
“As access to potent cannabis products continues to expand, it is crucial that we also expand prevention, screening, and treatment for people who may experience mental illnesses associated with cannabis use,” Dr. Volkow added.
The study was published online in Psychological Medicine.
A modifiable risk factor
The researchers analyzed Danish registry data spanning 5 decades and representing more than 6.9 million people in Denmark to estimate the population-level percentage of schizophrenia cases attributable to CUD.
A total of 60,563 participants were diagnosed with CUD. Three-quarters of cases were in men; there were 45,327 incident cases of schizophrenia during the study period.
The overall adjusted hazard ratio for CUD on schizophrenia was slightly higher among males than females (aHR, 2.42 vs. 2.02); however, among those aged 16 to 20 years, the adjusted incidence risk ratio for males was more than twice that for females (aIRR, 3.84 vs. 1.81).
The researchers estimate that, in 2021, about 15% of schizophrenia cases among males aged 16-49 could have been avoided by preventing CUD, compared with 4% among females in this age range.
For young men aged 21-30, the proportion of preventable schizophrenia cases related to CUD may be as high as 30%, the authors reported.
“Alongside the increasing evidence that CUD is a modifiable risk factor for schizophrenia, our findings underscore the importance of evidence-based strategies to regulate cannabis use and to effectively prevent, screen for, and treat CUD as well as schizophrenia,” the researchers wrote.
Legalization sends the wrong message
In a press statement, lead investigator Carsten Hjorthøj, PhD, with the University of Copenhagen, noted that “increases in the legalization of cannabis over the past few decades have made it one of the most frequently used psychoactive substances in the world, while also decreasing the public’s perception of its harm. This study adds to our growing understanding that cannabis use is not harmless, and that risks are not fixed at one point in time.”
In a prior study, Dr. Hjorthøj and colleagues found that the proportion of new schizophrenia cases attributable to CUD has consistently increased over the past 20 years.
“In my view, the association is most likely causative, at least to a large extent,” Dr. Hjorthøj said at the time this research was published.
“It is of course nearly impossible to use epidemiological studies to actually prove causation, but all the numbers behave exactly in the way that would be expected under the theory of causation,” Dr. Hjorthøj added.
The study received no specific funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study confirms the robust link between cannabis use and schizophrenia among men and women but suggests that young men may be especially susceptible to schizophrenia from cannabis abuse.
Of note,
“The entanglement of substance use disorders and mental illnesses is a major public health issue, requiring urgent action and support for people who need it,” study coauthor Nora Volkow, MD, director of the National Institute on Drug Abuse, said in a news release.
“As access to potent cannabis products continues to expand, it is crucial that we also expand prevention, screening, and treatment for people who may experience mental illnesses associated with cannabis use,” Dr. Volkow added.
The study was published online in Psychological Medicine.
A modifiable risk factor
The researchers analyzed Danish registry data spanning 5 decades and representing more than 6.9 million people in Denmark to estimate the population-level percentage of schizophrenia cases attributable to CUD.
A total of 60,563 participants were diagnosed with CUD. Three-quarters of cases were in men; there were 45,327 incident cases of schizophrenia during the study period.
The overall adjusted hazard ratio for CUD on schizophrenia was slightly higher among males than females (aHR, 2.42 vs. 2.02); however, among those aged 16 to 20 years, the adjusted incidence risk ratio for males was more than twice that for females (aIRR, 3.84 vs. 1.81).
The researchers estimate that, in 2021, about 15% of schizophrenia cases among males aged 16-49 could have been avoided by preventing CUD, compared with 4% among females in this age range.
For young men aged 21-30, the proportion of preventable schizophrenia cases related to CUD may be as high as 30%, the authors reported.
“Alongside the increasing evidence that CUD is a modifiable risk factor for schizophrenia, our findings underscore the importance of evidence-based strategies to regulate cannabis use and to effectively prevent, screen for, and treat CUD as well as schizophrenia,” the researchers wrote.
Legalization sends the wrong message
In a press statement, lead investigator Carsten Hjorthøj, PhD, with the University of Copenhagen, noted that “increases in the legalization of cannabis over the past few decades have made it one of the most frequently used psychoactive substances in the world, while also decreasing the public’s perception of its harm. This study adds to our growing understanding that cannabis use is not harmless, and that risks are not fixed at one point in time.”
In a prior study, Dr. Hjorthøj and colleagues found that the proportion of new schizophrenia cases attributable to CUD has consistently increased over the past 20 years.
“In my view, the association is most likely causative, at least to a large extent,” Dr. Hjorthøj said at the time this research was published.
“It is of course nearly impossible to use epidemiological studies to actually prove causation, but all the numbers behave exactly in the way that would be expected under the theory of causation,” Dr. Hjorthøj added.
The study received no specific funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PSYCHOLOGICAL MEDICINE
Prostate biopsies a laughing (gas) matter?
An old dog – nitrous oxide – can learn new tricks, managing pain in men undergoing transrectal biopsies, researchers reported at the annual meeting of the American Urological Association.
said Heidi Rayala, MD, PhD, assistant professor of surgery at Harvard Medical School, Boston, who helped conduct the study.
Nitrous oxide is best known as a pain medication and anesthetic during dental procedures and childbirth, after trauma, and in end-of-life care.
In the new study, Dr. Rayala and her colleagues at Harvard and Beth Israel-Deaconess Medical Center, Boston, randomly assigned 128 men to self-administered nitrous oxide (SANO) or oxygen as a placebo. Patients in the SANO group had a smaller change in post-biopsy pain score (Visual Analog Scale for pain, 0.43 vs. 1.03; P = .03) and lower odds of experiencing pain during the procedure (odds ratio, 0.45; confidence interval, 0.21-0.97; P = .04).
A comparison of anxiety scores in the two groups failed to find a statistically significant difference between SANO and placebo. However, more men who received nitrous oxide said they tolerated the procedure “better than expected” (61% vs. 41%; P = 0.02), according to the researchers.
Dr. Rayala said that the researchers used the Nitrouseal system (Sedation Systems), in which the patient holds a mask to their face and works with staff to adjust the gas levels to the desired amount. The system is governed to max out at 50% nitrous oxide, ensuring “minimal sedation concentrations, so anesthesia personnel are not required,” she said.
“At levels of less than 50%, patients respond normally to verbal commands and maintain normal airway reflexes,” Dr. Rayala added. “This provides an advantage in that patients do not require the presence of anesthesia personnel.” And because the body eliminates the gas within about 5 minutes, patients do not require an escort home, she said.
This system is also self-scavenging to protect the operating urologist and other personnel from environmental exposure to nitrous oxide.
Dr. Rayala said that three patients (2.3%) found the mask uncomfortable, but in follow-up studies the clinicians have done a better job of preparing patients for the feeling of the mask, making a marked difference. Headaches and nausea are the most commonly reported complaints at concentrations above 50%.
“We did not have patients report headaches or nausea in new study (by the BIDMC group),” she said. This study has been submitted for publication.
Clinicians outside the United States have been quicker to embrace nitrous oxide for prostate procedures.
In a randomized controlled trial, researchers in Australia found no significant improvement in pain scores at 15 minutes from the use of nitrous oxide during transrectal biopsies; however, improvements were seen in patient-reported discomfort, overall experience, and willingness to undergo repeat biopsies.
Stephen McCombie, MD, a consultant at Perth Urology Clinic, Australia, who has been adapting the nitrous oxide protocol for transrectal biopsies to transperineal procedures, said that the Beth Israel study “adds to the evidence to support adjunct use of mild inhalational anesthetics and analgesics during prostate biopsies to improve the patient experience of the procedure.”
He said that the role for these agents may grow with the global trend away from transrectal prostate biopsies and toward transperineal biopsies, largely driven by increasing rates of sepsis after transrectal biopsies.
“While transperineal biopsies can be more painful then transrectal biopsies when performed under local anesthesia, perhaps due to biopsies being taken through the highly sensate perineum as opposed to above the dentate line, optimization of the technique can significantly reduce the discomfort associated with the procedure, which may be further reduced with these agents,” Dr. McCombie said.
“Studies indicate that transperineal biopsies can be more painful than the traditional transrectal biopsies,” Dr. Rayala said. “We do offer transperineal biopsies at BIDMC, and we are gearing up to repeat the SANO study” for those patients.
Dr. Rayala and Dr. McCombie have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An old dog – nitrous oxide – can learn new tricks, managing pain in men undergoing transrectal biopsies, researchers reported at the annual meeting of the American Urological Association.
said Heidi Rayala, MD, PhD, assistant professor of surgery at Harvard Medical School, Boston, who helped conduct the study.
Nitrous oxide is best known as a pain medication and anesthetic during dental procedures and childbirth, after trauma, and in end-of-life care.
In the new study, Dr. Rayala and her colleagues at Harvard and Beth Israel-Deaconess Medical Center, Boston, randomly assigned 128 men to self-administered nitrous oxide (SANO) or oxygen as a placebo. Patients in the SANO group had a smaller change in post-biopsy pain score (Visual Analog Scale for pain, 0.43 vs. 1.03; P = .03) and lower odds of experiencing pain during the procedure (odds ratio, 0.45; confidence interval, 0.21-0.97; P = .04).
A comparison of anxiety scores in the two groups failed to find a statistically significant difference between SANO and placebo. However, more men who received nitrous oxide said they tolerated the procedure “better than expected” (61% vs. 41%; P = 0.02), according to the researchers.
Dr. Rayala said that the researchers used the Nitrouseal system (Sedation Systems), in which the patient holds a mask to their face and works with staff to adjust the gas levels to the desired amount. The system is governed to max out at 50% nitrous oxide, ensuring “minimal sedation concentrations, so anesthesia personnel are not required,” she said.
“At levels of less than 50%, patients respond normally to verbal commands and maintain normal airway reflexes,” Dr. Rayala added. “This provides an advantage in that patients do not require the presence of anesthesia personnel.” And because the body eliminates the gas within about 5 minutes, patients do not require an escort home, she said.
This system is also self-scavenging to protect the operating urologist and other personnel from environmental exposure to nitrous oxide.
Dr. Rayala said that three patients (2.3%) found the mask uncomfortable, but in follow-up studies the clinicians have done a better job of preparing patients for the feeling of the mask, making a marked difference. Headaches and nausea are the most commonly reported complaints at concentrations above 50%.
“We did not have patients report headaches or nausea in new study (by the BIDMC group),” she said. This study has been submitted for publication.
Clinicians outside the United States have been quicker to embrace nitrous oxide for prostate procedures.
In a randomized controlled trial, researchers in Australia found no significant improvement in pain scores at 15 minutes from the use of nitrous oxide during transrectal biopsies; however, improvements were seen in patient-reported discomfort, overall experience, and willingness to undergo repeat biopsies.
Stephen McCombie, MD, a consultant at Perth Urology Clinic, Australia, who has been adapting the nitrous oxide protocol for transrectal biopsies to transperineal procedures, said that the Beth Israel study “adds to the evidence to support adjunct use of mild inhalational anesthetics and analgesics during prostate biopsies to improve the patient experience of the procedure.”
He said that the role for these agents may grow with the global trend away from transrectal prostate biopsies and toward transperineal biopsies, largely driven by increasing rates of sepsis after transrectal biopsies.
“While transperineal biopsies can be more painful then transrectal biopsies when performed under local anesthesia, perhaps due to biopsies being taken through the highly sensate perineum as opposed to above the dentate line, optimization of the technique can significantly reduce the discomfort associated with the procedure, which may be further reduced with these agents,” Dr. McCombie said.
“Studies indicate that transperineal biopsies can be more painful than the traditional transrectal biopsies,” Dr. Rayala said. “We do offer transperineal biopsies at BIDMC, and we are gearing up to repeat the SANO study” for those patients.
Dr. Rayala and Dr. McCombie have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An old dog – nitrous oxide – can learn new tricks, managing pain in men undergoing transrectal biopsies, researchers reported at the annual meeting of the American Urological Association.
said Heidi Rayala, MD, PhD, assistant professor of surgery at Harvard Medical School, Boston, who helped conduct the study.
Nitrous oxide is best known as a pain medication and anesthetic during dental procedures and childbirth, after trauma, and in end-of-life care.
In the new study, Dr. Rayala and her colleagues at Harvard and Beth Israel-Deaconess Medical Center, Boston, randomly assigned 128 men to self-administered nitrous oxide (SANO) or oxygen as a placebo. Patients in the SANO group had a smaller change in post-biopsy pain score (Visual Analog Scale for pain, 0.43 vs. 1.03; P = .03) and lower odds of experiencing pain during the procedure (odds ratio, 0.45; confidence interval, 0.21-0.97; P = .04).
A comparison of anxiety scores in the two groups failed to find a statistically significant difference between SANO and placebo. However, more men who received nitrous oxide said they tolerated the procedure “better than expected” (61% vs. 41%; P = 0.02), according to the researchers.
Dr. Rayala said that the researchers used the Nitrouseal system (Sedation Systems), in which the patient holds a mask to their face and works with staff to adjust the gas levels to the desired amount. The system is governed to max out at 50% nitrous oxide, ensuring “minimal sedation concentrations, so anesthesia personnel are not required,” she said.
“At levels of less than 50%, patients respond normally to verbal commands and maintain normal airway reflexes,” Dr. Rayala added. “This provides an advantage in that patients do not require the presence of anesthesia personnel.” And because the body eliminates the gas within about 5 minutes, patients do not require an escort home, she said.
This system is also self-scavenging to protect the operating urologist and other personnel from environmental exposure to nitrous oxide.
Dr. Rayala said that three patients (2.3%) found the mask uncomfortable, but in follow-up studies the clinicians have done a better job of preparing patients for the feeling of the mask, making a marked difference. Headaches and nausea are the most commonly reported complaints at concentrations above 50%.
“We did not have patients report headaches or nausea in new study (by the BIDMC group),” she said. This study has been submitted for publication.
Clinicians outside the United States have been quicker to embrace nitrous oxide for prostate procedures.
In a randomized controlled trial, researchers in Australia found no significant improvement in pain scores at 15 minutes from the use of nitrous oxide during transrectal biopsies; however, improvements were seen in patient-reported discomfort, overall experience, and willingness to undergo repeat biopsies.
Stephen McCombie, MD, a consultant at Perth Urology Clinic, Australia, who has been adapting the nitrous oxide protocol for transrectal biopsies to transperineal procedures, said that the Beth Israel study “adds to the evidence to support adjunct use of mild inhalational anesthetics and analgesics during prostate biopsies to improve the patient experience of the procedure.”
He said that the role for these agents may grow with the global trend away from transrectal prostate biopsies and toward transperineal biopsies, largely driven by increasing rates of sepsis after transrectal biopsies.
“While transperineal biopsies can be more painful then transrectal biopsies when performed under local anesthesia, perhaps due to biopsies being taken through the highly sensate perineum as opposed to above the dentate line, optimization of the technique can significantly reduce the discomfort associated with the procedure, which may be further reduced with these agents,” Dr. McCombie said.
“Studies indicate that transperineal biopsies can be more painful than the traditional transrectal biopsies,” Dr. Rayala said. “We do offer transperineal biopsies at BIDMC, and we are gearing up to repeat the SANO study” for those patients.
Dr. Rayala and Dr. McCombie have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AUA 2023
Penile rash
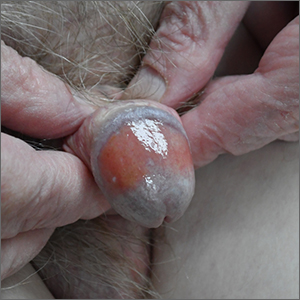
Biopsy revealed a lichenoid infiltrate in the upper dermis with thinning of the epidermis and a predominance of plasma cells. This finding, along with a red-orange, glossy lesion on the glans penis in an uncircumcised man is a classic presentation of Zoon balanitis.
Zoon balanitis is a chronic, idiopathic disorder that affects uncircumcised men who are middle-aged and older.1 Although the exact pathogenesis is unknown, it is hypothesized to be the result of chronic irritation due to poor hygiene/urine retention with prepuce dysfunction. The classic clinical presentation is an asymptomatic, well-defined, orange-to-red glazed patch with symmetric small red “cayenne pepper” spots contained within the glans penis and/or prepuce. Often there are symmetric “kissing lesions” where a second lesion of similar morphology is apparent on the prepuce where it meets the glans penis. While this disorder is typically asymptomatic, it can be associated with itching and/or burning.
There are several other inflammatory disorders in the differential for Zoon balanitis (erosive lichen planus, lichen sclerosus, psoriasis), but the most important consideration is penile intraepithelial carcinoma, specifically erythroplasia of Queyrat. Erythroplasia of Queyrat can be difficult to distinguish clinically and often requires a biopsy. Zoon balanitis will show a plasma cell predominant lichenoid infiltrate, whereas erythroplasia of Queyrat will show squamous cell carcinoma in situ.
It is important to note that Zoon balanitis is a clinicopathologic diagnosis and that zoonoid inflammation on biopsy is not pathognomonic, as this can also be seen in other inflammatory and neoplastic conditions. It is, therefore, advisable to follow up with patients with Zoon balanitis to ensure that the lesion is resolving and/or not getting worse.
Improved hygiene measures are the mainstay of treatment; circumcision is also effective.2 Both can help keep the glans clean and dry. In those with symptomatic disease, low-strength topical steroids including 1% hydrocortisone ointment or cream twice daily or topical calcineurin inhibitors (pimecrolimus 1% or tacrolimus 0.1%) twice daily can be used for symptom management.
Because this patient’s disease was asymptomatic, treatment was deferred. He was counseled to draw back the foreskin when urinating and to do the same while showering so that he could wash, then dry, the glans before returning the foreskin to its normal position. The patient is being monitored clinically.
Photo courtesy of Drew Mitchell, MD. Text courtesy of Drew Mitchell, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Mallon E, Hawkins D, Dinneen M, et al. Circumcision and genital dermatoses. Arch Dermatol. 2000;136:350-354. doi: 10.1001/archderm.136.3.350
2. Kumar B, Sharma R, Rajagopalan M, et al. Plasma cell balanitis: clinical and histopathological features—response to circumcision. Genitourin Med. 1995;71:32-34. doi: 10.1136/sti.71.1.32

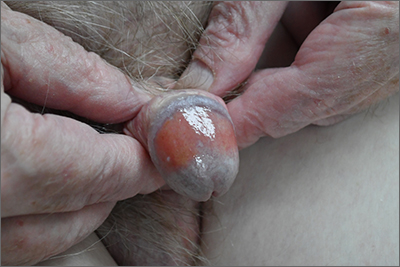
Biopsy revealed a lichenoid infiltrate in the upper dermis with thinning of the epidermis and a predominance of plasma cells. This finding, along with a red-orange, glossy lesion on the glans penis in an uncircumcised man is a classic presentation of Zoon balanitis.
Zoon balanitis is a chronic, idiopathic disorder that affects uncircumcised men who are middle-aged and older.1 Although the exact pathogenesis is unknown, it is hypothesized to be the result of chronic irritation due to poor hygiene/urine retention with prepuce dysfunction. The classic clinical presentation is an asymptomatic, well-defined, orange-to-red glazed patch with symmetric small red “cayenne pepper” spots contained within the glans penis and/or prepuce. Often there are symmetric “kissing lesions” where a second lesion of similar morphology is apparent on the prepuce where it meets the glans penis. While this disorder is typically asymptomatic, it can be associated with itching and/or burning.
There are several other inflammatory disorders in the differential for Zoon balanitis (erosive lichen planus, lichen sclerosus, psoriasis), but the most important consideration is penile intraepithelial carcinoma, specifically erythroplasia of Queyrat. Erythroplasia of Queyrat can be difficult to distinguish clinically and often requires a biopsy. Zoon balanitis will show a plasma cell predominant lichenoid infiltrate, whereas erythroplasia of Queyrat will show squamous cell carcinoma in situ.
It is important to note that Zoon balanitis is a clinicopathologic diagnosis and that zoonoid inflammation on biopsy is not pathognomonic, as this can also be seen in other inflammatory and neoplastic conditions. It is, therefore, advisable to follow up with patients with Zoon balanitis to ensure that the lesion is resolving and/or not getting worse.
Improved hygiene measures are the mainstay of treatment; circumcision is also effective.2 Both can help keep the glans clean and dry. In those with symptomatic disease, low-strength topical steroids including 1% hydrocortisone ointment or cream twice daily or topical calcineurin inhibitors (pimecrolimus 1% or tacrolimus 0.1%) twice daily can be used for symptom management.
Because this patient’s disease was asymptomatic, treatment was deferred. He was counseled to draw back the foreskin when urinating and to do the same while showering so that he could wash, then dry, the glans before returning the foreskin to its normal position. The patient is being monitored clinically.
Photo courtesy of Drew Mitchell, MD. Text courtesy of Drew Mitchell, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

Biopsy revealed a lichenoid infiltrate in the upper dermis with thinning of the epidermis and a predominance of plasma cells. This finding, along with a red-orange, glossy lesion on the glans penis in an uncircumcised man is a classic presentation of Zoon balanitis.
Zoon balanitis is a chronic, idiopathic disorder that affects uncircumcised men who are middle-aged and older.1 Although the exact pathogenesis is unknown, it is hypothesized to be the result of chronic irritation due to poor hygiene/urine retention with prepuce dysfunction. The classic clinical presentation is an asymptomatic, well-defined, orange-to-red glazed patch with symmetric small red “cayenne pepper” spots contained within the glans penis and/or prepuce. Often there are symmetric “kissing lesions” where a second lesion of similar morphology is apparent on the prepuce where it meets the glans penis. While this disorder is typically asymptomatic, it can be associated with itching and/or burning.
There are several other inflammatory disorders in the differential for Zoon balanitis (erosive lichen planus, lichen sclerosus, psoriasis), but the most important consideration is penile intraepithelial carcinoma, specifically erythroplasia of Queyrat. Erythroplasia of Queyrat can be difficult to distinguish clinically and often requires a biopsy. Zoon balanitis will show a plasma cell predominant lichenoid infiltrate, whereas erythroplasia of Queyrat will show squamous cell carcinoma in situ.
It is important to note that Zoon balanitis is a clinicopathologic diagnosis and that zoonoid inflammation on biopsy is not pathognomonic, as this can also be seen in other inflammatory and neoplastic conditions. It is, therefore, advisable to follow up with patients with Zoon balanitis to ensure that the lesion is resolving and/or not getting worse.
Improved hygiene measures are the mainstay of treatment; circumcision is also effective.2 Both can help keep the glans clean and dry. In those with symptomatic disease, low-strength topical steroids including 1% hydrocortisone ointment or cream twice daily or topical calcineurin inhibitors (pimecrolimus 1% or tacrolimus 0.1%) twice daily can be used for symptom management.
Because this patient’s disease was asymptomatic, treatment was deferred. He was counseled to draw back the foreskin when urinating and to do the same while showering so that he could wash, then dry, the glans before returning the foreskin to its normal position. The patient is being monitored clinically.
Photo courtesy of Drew Mitchell, MD. Text courtesy of Drew Mitchell, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Mallon E, Hawkins D, Dinneen M, et al. Circumcision and genital dermatoses. Arch Dermatol. 2000;136:350-354. doi: 10.1001/archderm.136.3.350
2. Kumar B, Sharma R, Rajagopalan M, et al. Plasma cell balanitis: clinical and histopathological features—response to circumcision. Genitourin Med. 1995;71:32-34. doi: 10.1136/sti.71.1.32
1. Mallon E, Hawkins D, Dinneen M, et al. Circumcision and genital dermatoses. Arch Dermatol. 2000;136:350-354. doi: 10.1001/archderm.136.3.350
2. Kumar B, Sharma R, Rajagopalan M, et al. Plasma cell balanitis: clinical and histopathological features—response to circumcision. Genitourin Med. 1995;71:32-34. doi: 10.1136/sti.71.1.32
Prostate cancer drug shortage leaves some with uncertainty
according to the Food and Drug Administration.
The therapy lutetium Lu 177 vipivotide tetraxetan (Pluvicto), approved in March 2022, will remain in limited supply until the drug’s manufacturer, Novartis, can ramp up production of the drug over the next 12 months.
In a letter in February, Novartis said it is giving priority to patients who have already started the regimen so they can “appropriately complete their course of therapy.” The manufacturer will not be taking any orders for new patients over the next 4-6 months, as they work to increase supply.
“We are operating our production site at full capacity to treat as many patients as possible, as quickly as possible,” Novartis said. “However, with a nuclear medicine like Pluvicto, there is no backup supply that we can draw from when we experience a delay.”
Pluvicto is currently made in small batches in the company’s manufacturing facility in Italy. The drug only has a 5-day window to reach its intended patient, after which time it cannot be used. Any disruption in the production or shipping process can create a delay.
Novartis said the facility in Italy is currently operating at full capacity and the company is “working to increase production capacity and supply” of the drug over the next 12 months at two new manufacturing sites in the United States.
The company also encountered supply problems with Pluvicto in 2022 after quality issues were discovered in the manufacturing process.
Currently, patients who are waiting for their first dose of Pluvicto will need to be rescheduled. The manufacturer will be reaching out to health care professionals with options for rescheduling.
Jonathan McConathy, MD, PhD, told The Wall Street Journal that “people will die from this shortage, for sure.”
Dr. McConathy, a radiologist at the University of Alabama at Birmingham who has consulted for Novartis, explained that some patients who would have benefited from the drug likely won’t receive it in time.
A version of this article first appeared on Medscape.com.
according to the Food and Drug Administration.
The therapy lutetium Lu 177 vipivotide tetraxetan (Pluvicto), approved in March 2022, will remain in limited supply until the drug’s manufacturer, Novartis, can ramp up production of the drug over the next 12 months.
In a letter in February, Novartis said it is giving priority to patients who have already started the regimen so they can “appropriately complete their course of therapy.” The manufacturer will not be taking any orders for new patients over the next 4-6 months, as they work to increase supply.
“We are operating our production site at full capacity to treat as many patients as possible, as quickly as possible,” Novartis said. “However, with a nuclear medicine like Pluvicto, there is no backup supply that we can draw from when we experience a delay.”
Pluvicto is currently made in small batches in the company’s manufacturing facility in Italy. The drug only has a 5-day window to reach its intended patient, after which time it cannot be used. Any disruption in the production or shipping process can create a delay.
Novartis said the facility in Italy is currently operating at full capacity and the company is “working to increase production capacity and supply” of the drug over the next 12 months at two new manufacturing sites in the United States.
The company also encountered supply problems with Pluvicto in 2022 after quality issues were discovered in the manufacturing process.
Currently, patients who are waiting for their first dose of Pluvicto will need to be rescheduled. The manufacturer will be reaching out to health care professionals with options for rescheduling.
Jonathan McConathy, MD, PhD, told The Wall Street Journal that “people will die from this shortage, for sure.”
Dr. McConathy, a radiologist at the University of Alabama at Birmingham who has consulted for Novartis, explained that some patients who would have benefited from the drug likely won’t receive it in time.
A version of this article first appeared on Medscape.com.
according to the Food and Drug Administration.
The therapy lutetium Lu 177 vipivotide tetraxetan (Pluvicto), approved in March 2022, will remain in limited supply until the drug’s manufacturer, Novartis, can ramp up production of the drug over the next 12 months.
In a letter in February, Novartis said it is giving priority to patients who have already started the regimen so they can “appropriately complete their course of therapy.” The manufacturer will not be taking any orders for new patients over the next 4-6 months, as they work to increase supply.
“We are operating our production site at full capacity to treat as many patients as possible, as quickly as possible,” Novartis said. “However, with a nuclear medicine like Pluvicto, there is no backup supply that we can draw from when we experience a delay.”
Pluvicto is currently made in small batches in the company’s manufacturing facility in Italy. The drug only has a 5-day window to reach its intended patient, after which time it cannot be used. Any disruption in the production or shipping process can create a delay.
Novartis said the facility in Italy is currently operating at full capacity and the company is “working to increase production capacity and supply” of the drug over the next 12 months at two new manufacturing sites in the United States.
The company also encountered supply problems with Pluvicto in 2022 after quality issues were discovered in the manufacturing process.
Currently, patients who are waiting for their first dose of Pluvicto will need to be rescheduled. The manufacturer will be reaching out to health care professionals with options for rescheduling.
Jonathan McConathy, MD, PhD, told The Wall Street Journal that “people will die from this shortage, for sure.”
Dr. McConathy, a radiologist at the University of Alabama at Birmingham who has consulted for Novartis, explained that some patients who would have benefited from the drug likely won’t receive it in time.
A version of this article first appeared on Medscape.com.
COVID can mimic prostate cancer symptoms
This patient has a strong likelihood of aggressive prostate cancer, right? If that same patient also presents with severe, burning bone pain with no precipitating trauma to the area and rest and over-the-counter painkillers are not helping, you’d think, “check for metastases,” right?
That patient was me in late January 2023.
As a research scientist member of the American Urological Association, I knew enough to know I had to consult my urologist ASAP.
With the above symptoms, I’ll admit I was scared. Fortunately, if that’s the right word, I was no stranger to a rapid, dramatic spike in PSA. In 2021 I was temporarily living in a new city, and I wanted to form a relationship with a good local urologist. The urologist that I was referred to gave me a thorough consultation, including a vigorous digital rectal exam (DRE) and sent me across the street for a blood draw.
To my shock, my PSA had spiked over 2 points, to 9.9 from 7.8 a few months earlier. I freaked. Had my 3-cm tumor burst out into an aggressive cancer? Research on PubMed provided an array of studies showing what could cause PSA to suddenly rise, including a DRE performed 72 hours before the blood draw.1 A week later, my PSA was back down to its normal 7.6.
But in January 2023, I had none of those previously reported experiences that could suddenly trigger a spike in PSA, like a DRE or riding on a thin bicycle seat for a few hours before the lab visit.
The COVID effect
I went back to PubMed and found a new circumstance that could cause a surge in PSA: COVID-19. A recent study2 of 91 men with benign prostatic hypertrophy by researchers in Turkey found that PSA spiked from 0 to 5 points during the COVID infection period and up to 2 points higher 3 months after the infection had cleared. I had tested positive for COVID-19 in mid-December 2022, 4 weeks before my 9.9 PSA reading.
Using Google translate, I communicated with the team in Turkey and found out that the PSA spike can last up to 6 months.
That study helps explain why my PSA dropped over 1.5 points to 8.5 just 2 weeks after the 9.9 reading, with the expectation that it would return to its previous normal of 7.8 within 6 months of infection with SARS-CoV-2. To be safe, my urologist scheduled another PSA test in May, along with an updated multiparametric MRI, which may be followed by an in-bore MRI-guided biopsy of the 3-cm tumor if the mass has enlarged.
COVID-19 pain
What about my burning bone pain in my upper right humerus and right rotator cuff that was not precipitated by trauma or strain? A radiograph found no evidence of metastasis, thank goodness. And my research showed that several studies3 have found that COVID-19 can cause burning musculoskeletal pain, including enthesopathy, which is what I had per the radiology report. So my PSA spike and searing pain were likely consequences of the infection.
To avoid the risk for a gross misdiagnosis after a radical spike in PSA, the informed urologist should ask the patient if he has had COVID-19 in the previous 6 months. Overlooking that question could lead to the wrong diagnostic decisions about a rapid jump in PSA or unexplained bone pain.
References
1. Bossens MM et al. Eur J Cancer. 1995;31A:682-5.
2. Cinislioglu AE et al. Urology. 2022;159:16-21.
3. Ciaffi J et al. Joint Bone Spine. 2021;88:105158.
Dr. Keller is founder of the Keller Research Institute, Jacksonville, Fla. He reported serving as a research scientist for the American Urological Association, serving on the advisory board of Active Surveillance Patient’s International, and serving on the boards of numerous nonprofit organizations.
A version of this article first appeared on Medscape.com.
This patient has a strong likelihood of aggressive prostate cancer, right? If that same patient also presents with severe, burning bone pain with no precipitating trauma to the area and rest and over-the-counter painkillers are not helping, you’d think, “check for metastases,” right?
That patient was me in late January 2023.
As a research scientist member of the American Urological Association, I knew enough to know I had to consult my urologist ASAP.
With the above symptoms, I’ll admit I was scared. Fortunately, if that’s the right word, I was no stranger to a rapid, dramatic spike in PSA. In 2021 I was temporarily living in a new city, and I wanted to form a relationship with a good local urologist. The urologist that I was referred to gave me a thorough consultation, including a vigorous digital rectal exam (DRE) and sent me across the street for a blood draw.
To my shock, my PSA had spiked over 2 points, to 9.9 from 7.8 a few months earlier. I freaked. Had my 3-cm tumor burst out into an aggressive cancer? Research on PubMed provided an array of studies showing what could cause PSA to suddenly rise, including a DRE performed 72 hours before the blood draw.1 A week later, my PSA was back down to its normal 7.6.
But in January 2023, I had none of those previously reported experiences that could suddenly trigger a spike in PSA, like a DRE or riding on a thin bicycle seat for a few hours before the lab visit.
The COVID effect
I went back to PubMed and found a new circumstance that could cause a surge in PSA: COVID-19. A recent study2 of 91 men with benign prostatic hypertrophy by researchers in Turkey found that PSA spiked from 0 to 5 points during the COVID infection period and up to 2 points higher 3 months after the infection had cleared. I had tested positive for COVID-19 in mid-December 2022, 4 weeks before my 9.9 PSA reading.
Using Google translate, I communicated with the team in Turkey and found out that the PSA spike can last up to 6 months.
That study helps explain why my PSA dropped over 1.5 points to 8.5 just 2 weeks after the 9.9 reading, with the expectation that it would return to its previous normal of 7.8 within 6 months of infection with SARS-CoV-2. To be safe, my urologist scheduled another PSA test in May, along with an updated multiparametric MRI, which may be followed by an in-bore MRI-guided biopsy of the 3-cm tumor if the mass has enlarged.
COVID-19 pain
What about my burning bone pain in my upper right humerus and right rotator cuff that was not precipitated by trauma or strain? A radiograph found no evidence of metastasis, thank goodness. And my research showed that several studies3 have found that COVID-19 can cause burning musculoskeletal pain, including enthesopathy, which is what I had per the radiology report. So my PSA spike and searing pain were likely consequences of the infection.
To avoid the risk for a gross misdiagnosis after a radical spike in PSA, the informed urologist should ask the patient if he has had COVID-19 in the previous 6 months. Overlooking that question could lead to the wrong diagnostic decisions about a rapid jump in PSA or unexplained bone pain.
References
1. Bossens MM et al. Eur J Cancer. 1995;31A:682-5.
2. Cinislioglu AE et al. Urology. 2022;159:16-21.
3. Ciaffi J et al. Joint Bone Spine. 2021;88:105158.
Dr. Keller is founder of the Keller Research Institute, Jacksonville, Fla. He reported serving as a research scientist for the American Urological Association, serving on the advisory board of Active Surveillance Patient’s International, and serving on the boards of numerous nonprofit organizations.
A version of this article first appeared on Medscape.com.
This patient has a strong likelihood of aggressive prostate cancer, right? If that same patient also presents with severe, burning bone pain with no precipitating trauma to the area and rest and over-the-counter painkillers are not helping, you’d think, “check for metastases,” right?
That patient was me in late January 2023.
As a research scientist member of the American Urological Association, I knew enough to know I had to consult my urologist ASAP.
With the above symptoms, I’ll admit I was scared. Fortunately, if that’s the right word, I was no stranger to a rapid, dramatic spike in PSA. In 2021 I was temporarily living in a new city, and I wanted to form a relationship with a good local urologist. The urologist that I was referred to gave me a thorough consultation, including a vigorous digital rectal exam (DRE) and sent me across the street for a blood draw.
To my shock, my PSA had spiked over 2 points, to 9.9 from 7.8 a few months earlier. I freaked. Had my 3-cm tumor burst out into an aggressive cancer? Research on PubMed provided an array of studies showing what could cause PSA to suddenly rise, including a DRE performed 72 hours before the blood draw.1 A week later, my PSA was back down to its normal 7.6.
But in January 2023, I had none of those previously reported experiences that could suddenly trigger a spike in PSA, like a DRE or riding on a thin bicycle seat for a few hours before the lab visit.
The COVID effect
I went back to PubMed and found a new circumstance that could cause a surge in PSA: COVID-19. A recent study2 of 91 men with benign prostatic hypertrophy by researchers in Turkey found that PSA spiked from 0 to 5 points during the COVID infection period and up to 2 points higher 3 months after the infection had cleared. I had tested positive for COVID-19 in mid-December 2022, 4 weeks before my 9.9 PSA reading.
Using Google translate, I communicated with the team in Turkey and found out that the PSA spike can last up to 6 months.
That study helps explain why my PSA dropped over 1.5 points to 8.5 just 2 weeks after the 9.9 reading, with the expectation that it would return to its previous normal of 7.8 within 6 months of infection with SARS-CoV-2. To be safe, my urologist scheduled another PSA test in May, along with an updated multiparametric MRI, which may be followed by an in-bore MRI-guided biopsy of the 3-cm tumor if the mass has enlarged.
COVID-19 pain
What about my burning bone pain in my upper right humerus and right rotator cuff that was not precipitated by trauma or strain? A radiograph found no evidence of metastasis, thank goodness. And my research showed that several studies3 have found that COVID-19 can cause burning musculoskeletal pain, including enthesopathy, which is what I had per the radiology report. So my PSA spike and searing pain were likely consequences of the infection.
To avoid the risk for a gross misdiagnosis after a radical spike in PSA, the informed urologist should ask the patient if he has had COVID-19 in the previous 6 months. Overlooking that question could lead to the wrong diagnostic decisions about a rapid jump in PSA or unexplained bone pain.
References
1. Bossens MM et al. Eur J Cancer. 1995;31A:682-5.
2. Cinislioglu AE et al. Urology. 2022;159:16-21.
3. Ciaffi J et al. Joint Bone Spine. 2021;88:105158.
Dr. Keller is founder of the Keller Research Institute, Jacksonville, Fla. He reported serving as a research scientist for the American Urological Association, serving on the advisory board of Active Surveillance Patient’s International, and serving on the boards of numerous nonprofit organizations.
A version of this article first appeared on Medscape.com.
Older men more at risk as dangerous falls rise for all seniors
When Senate Minority Leader Mitch McConnell (R-Ky.) fell recently at a dinner event in Washington, he unfortunately joined a large group of his senior citizen peers.
This wasn’t the first tumble the 81-year-old has taken. In 2019, he fell in his home, fracturing his shoulder. This time, he got a concussion and was recently released to an in-patient rehabilitation facility. While Sen. McConnell didn’t fracture his skull, in falling and hitting his head, he became part of an emerging statistic: One that reveals falls are more dangerous for senior men than senior women.
This new research, which appeared in the American Journal of Emergency Medicine, came as a surprise to lead researcher Scott Alter, MD, associate professor of emergency medicine at the Florida Atlantic University, Boca Raton.
“We always hear about lower bone density rates among females, so we didn’t expect to see males with more skull fractures,” he said.
Dr. Alter said that as a clinician in a southern Florida facility, his emergency department was the perfect study grounds to evaluate incoming geriatric patients due to falls. Older “patients are at higher risk of skull fractures and intercranial bleeding, and we wanted to look at any patient presenting with a head injury. Some 80% were fall related, however.”
The statistics bear out the fact that falls of all types are common among the elderly: Some 800,000 seniors wind up in the hospital each year because of falls.
The numbers show death rates from falls are on the rise in the senior citizen age group, too, up 30% from 2007 to 2016. Falls account for 70% of accidental deaths in people 75 and older. They are the leading cause of injury-related visits to emergency departments in the country, too.
Jennifer Stevens, MD, a gerontologist and executive director at Florida-based Abbey Delray South, is aware of the dire numbers and sees their consequences regularly. “The reasons seniors are at a high fall risk are many,” she said. “They include balance issues, declining strength, diseases like Parkinson’s and Alzheimer’s, side effects of their medications, and more.”
In addition, many seniors live in spaces that are not necessarily equipped for their limitations, and hazards exist all over their homes. Put together, and the risks for falls are everywhere. But there are steps seniors, their families, and even middle-aged people can take to mitigate and hopefully prevent dangerous falls.
Starting early
While in many cases the journey to lessen fall risks begins after a fall, the time to begin addressing the issue is long before you hit your senior years. Mary Therese Cole, a physical therapist and certified dementia practitioner at Manual Edge Physical Therapy in Colorado Springs, Colo., says that age 50 is a good time to start paying attention and addressing physical declines.
“This is an age where your vision might begin deteriorating,” she said. “It’s a big reason why elderly people trip and fall.”
As our brains begin to age in our middle years, the neural pathways from brain to extremities start to decline, too. The result is that many people stop picking up their feet as well as they used to do, making them more likely to trip.
“You’re not elderly yet, but you’re not a spring chicken, either,” Ms. Cole said. “Any issues you have now will only get worse if you’re not working on them.”
A good starting point in middle age, then, is to work on both strength training and balance exercises. A certified personal trainer or physical therapist can help get you on a program to ward off many of these declines.
If you’ve reached your later years, however, and are experiencing physical declines, it’s smart to check in with your primary care doctor for an assessment. “He or she can get your started on regular PT to evaluate any shortcomings and then address them,” Ms. Cole said.
She noted that when she’s working with senior patients, she’ll test their strength getting into and out of a chair, do a manual strength test to check on lower extremities, check their walking stride, and ask about conditions such as diabetes, former surgeries, and other conditions.
From there, Ms. Cole said she can write up a plan for the patient. Likewise, Dr. Stevens uses a program called Be Active that allows her to test seniors on a variety of measurements, including flexibility, balance, hand strength, and more.
“Then we match them with classes to address their shortcomings,” she said. “It’s critical that seniors have the ability to recover and not fall if they get knocked off balance.”
Beyond working on your physical limitations, taking a good look at your home is essential, too. “You can have an occupational therapist come to your home and do an evaluation,” Dr. Stevens said. “They can help you rearrange and reorganize for a safer environment.”
Big, common household fall hazards include throw rugs, lack of nightlights for middle-of-the-night visits to the bathroom, a lack of grab bars in the shower/bathtub, and furniture that blocks pathways.
For his part, Dr. Alter likes to point seniors and their doctors to the CDC’s STEADI program, which is aimed at stopping elderly accidents, deaths, and injuries.
“It includes screening for fall risk, assessing factors you can modify or improve, and more tools,” he said.
Dr. Alter also recommended seniors talk to their doctors about medications, particularly blood thinners.
“At a certain point, you need to weigh the benefits of disease prevention with the risk of injury if you fall,” he said. “The bleeding risk might be too high if the patient is at a high risk of falls.”
A version of this article originally appeared on WebMD.com.
When Senate Minority Leader Mitch McConnell (R-Ky.) fell recently at a dinner event in Washington, he unfortunately joined a large group of his senior citizen peers.
This wasn’t the first tumble the 81-year-old has taken. In 2019, he fell in his home, fracturing his shoulder. This time, he got a concussion and was recently released to an in-patient rehabilitation facility. While Sen. McConnell didn’t fracture his skull, in falling and hitting his head, he became part of an emerging statistic: One that reveals falls are more dangerous for senior men than senior women.
This new research, which appeared in the American Journal of Emergency Medicine, came as a surprise to lead researcher Scott Alter, MD, associate professor of emergency medicine at the Florida Atlantic University, Boca Raton.
“We always hear about lower bone density rates among females, so we didn’t expect to see males with more skull fractures,” he said.
Dr. Alter said that as a clinician in a southern Florida facility, his emergency department was the perfect study grounds to evaluate incoming geriatric patients due to falls. Older “patients are at higher risk of skull fractures and intercranial bleeding, and we wanted to look at any patient presenting with a head injury. Some 80% were fall related, however.”
The statistics bear out the fact that falls of all types are common among the elderly: Some 800,000 seniors wind up in the hospital each year because of falls.
The numbers show death rates from falls are on the rise in the senior citizen age group, too, up 30% from 2007 to 2016. Falls account for 70% of accidental deaths in people 75 and older. They are the leading cause of injury-related visits to emergency departments in the country, too.
Jennifer Stevens, MD, a gerontologist and executive director at Florida-based Abbey Delray South, is aware of the dire numbers and sees their consequences regularly. “The reasons seniors are at a high fall risk are many,” she said. “They include balance issues, declining strength, diseases like Parkinson’s and Alzheimer’s, side effects of their medications, and more.”
In addition, many seniors live in spaces that are not necessarily equipped for their limitations, and hazards exist all over their homes. Put together, and the risks for falls are everywhere. But there are steps seniors, their families, and even middle-aged people can take to mitigate and hopefully prevent dangerous falls.
Starting early
While in many cases the journey to lessen fall risks begins after a fall, the time to begin addressing the issue is long before you hit your senior years. Mary Therese Cole, a physical therapist and certified dementia practitioner at Manual Edge Physical Therapy in Colorado Springs, Colo., says that age 50 is a good time to start paying attention and addressing physical declines.
“This is an age where your vision might begin deteriorating,” she said. “It’s a big reason why elderly people trip and fall.”
As our brains begin to age in our middle years, the neural pathways from brain to extremities start to decline, too. The result is that many people stop picking up their feet as well as they used to do, making them more likely to trip.
“You’re not elderly yet, but you’re not a spring chicken, either,” Ms. Cole said. “Any issues you have now will only get worse if you’re not working on them.”
A good starting point in middle age, then, is to work on both strength training and balance exercises. A certified personal trainer or physical therapist can help get you on a program to ward off many of these declines.
If you’ve reached your later years, however, and are experiencing physical declines, it’s smart to check in with your primary care doctor for an assessment. “He or she can get your started on regular PT to evaluate any shortcomings and then address them,” Ms. Cole said.
She noted that when she’s working with senior patients, she’ll test their strength getting into and out of a chair, do a manual strength test to check on lower extremities, check their walking stride, and ask about conditions such as diabetes, former surgeries, and other conditions.
From there, Ms. Cole said she can write up a plan for the patient. Likewise, Dr. Stevens uses a program called Be Active that allows her to test seniors on a variety of measurements, including flexibility, balance, hand strength, and more.
“Then we match them with classes to address their shortcomings,” she said. “It’s critical that seniors have the ability to recover and not fall if they get knocked off balance.”
Beyond working on your physical limitations, taking a good look at your home is essential, too. “You can have an occupational therapist come to your home and do an evaluation,” Dr. Stevens said. “They can help you rearrange and reorganize for a safer environment.”
Big, common household fall hazards include throw rugs, lack of nightlights for middle-of-the-night visits to the bathroom, a lack of grab bars in the shower/bathtub, and furniture that blocks pathways.
For his part, Dr. Alter likes to point seniors and their doctors to the CDC’s STEADI program, which is aimed at stopping elderly accidents, deaths, and injuries.
“It includes screening for fall risk, assessing factors you can modify or improve, and more tools,” he said.
Dr. Alter also recommended seniors talk to their doctors about medications, particularly blood thinners.
“At a certain point, you need to weigh the benefits of disease prevention with the risk of injury if you fall,” he said. “The bleeding risk might be too high if the patient is at a high risk of falls.”
A version of this article originally appeared on WebMD.com.
When Senate Minority Leader Mitch McConnell (R-Ky.) fell recently at a dinner event in Washington, he unfortunately joined a large group of his senior citizen peers.
This wasn’t the first tumble the 81-year-old has taken. In 2019, he fell in his home, fracturing his shoulder. This time, he got a concussion and was recently released to an in-patient rehabilitation facility. While Sen. McConnell didn’t fracture his skull, in falling and hitting his head, he became part of an emerging statistic: One that reveals falls are more dangerous for senior men than senior women.
This new research, which appeared in the American Journal of Emergency Medicine, came as a surprise to lead researcher Scott Alter, MD, associate professor of emergency medicine at the Florida Atlantic University, Boca Raton.
“We always hear about lower bone density rates among females, so we didn’t expect to see males with more skull fractures,” he said.
Dr. Alter said that as a clinician in a southern Florida facility, his emergency department was the perfect study grounds to evaluate incoming geriatric patients due to falls. Older “patients are at higher risk of skull fractures and intercranial bleeding, and we wanted to look at any patient presenting with a head injury. Some 80% were fall related, however.”
The statistics bear out the fact that falls of all types are common among the elderly: Some 800,000 seniors wind up in the hospital each year because of falls.
The numbers show death rates from falls are on the rise in the senior citizen age group, too, up 30% from 2007 to 2016. Falls account for 70% of accidental deaths in people 75 and older. They are the leading cause of injury-related visits to emergency departments in the country, too.
Jennifer Stevens, MD, a gerontologist and executive director at Florida-based Abbey Delray South, is aware of the dire numbers and sees their consequences regularly. “The reasons seniors are at a high fall risk are many,” she said. “They include balance issues, declining strength, diseases like Parkinson’s and Alzheimer’s, side effects of their medications, and more.”
In addition, many seniors live in spaces that are not necessarily equipped for their limitations, and hazards exist all over their homes. Put together, and the risks for falls are everywhere. But there are steps seniors, their families, and even middle-aged people can take to mitigate and hopefully prevent dangerous falls.
Starting early
While in many cases the journey to lessen fall risks begins after a fall, the time to begin addressing the issue is long before you hit your senior years. Mary Therese Cole, a physical therapist and certified dementia practitioner at Manual Edge Physical Therapy in Colorado Springs, Colo., says that age 50 is a good time to start paying attention and addressing physical declines.
“This is an age where your vision might begin deteriorating,” she said. “It’s a big reason why elderly people trip and fall.”
As our brains begin to age in our middle years, the neural pathways from brain to extremities start to decline, too. The result is that many people stop picking up their feet as well as they used to do, making them more likely to trip.
“You’re not elderly yet, but you’re not a spring chicken, either,” Ms. Cole said. “Any issues you have now will only get worse if you’re not working on them.”
A good starting point in middle age, then, is to work on both strength training and balance exercises. A certified personal trainer or physical therapist can help get you on a program to ward off many of these declines.
If you’ve reached your later years, however, and are experiencing physical declines, it’s smart to check in with your primary care doctor for an assessment. “He or she can get your started on regular PT to evaluate any shortcomings and then address them,” Ms. Cole said.
She noted that when she’s working with senior patients, she’ll test their strength getting into and out of a chair, do a manual strength test to check on lower extremities, check their walking stride, and ask about conditions such as diabetes, former surgeries, and other conditions.
From there, Ms. Cole said she can write up a plan for the patient. Likewise, Dr. Stevens uses a program called Be Active that allows her to test seniors on a variety of measurements, including flexibility, balance, hand strength, and more.
“Then we match them with classes to address their shortcomings,” she said. “It’s critical that seniors have the ability to recover and not fall if they get knocked off balance.”
Beyond working on your physical limitations, taking a good look at your home is essential, too. “You can have an occupational therapist come to your home and do an evaluation,” Dr. Stevens said. “They can help you rearrange and reorganize for a safer environment.”
Big, common household fall hazards include throw rugs, lack of nightlights for middle-of-the-night visits to the bathroom, a lack of grab bars in the shower/bathtub, and furniture that blocks pathways.
For his part, Dr. Alter likes to point seniors and their doctors to the CDC’s STEADI program, which is aimed at stopping elderly accidents, deaths, and injuries.
“It includes screening for fall risk, assessing factors you can modify or improve, and more tools,” he said.
Dr. Alter also recommended seniors talk to their doctors about medications, particularly blood thinners.
“At a certain point, you need to weigh the benefits of disease prevention with the risk of injury if you fall,” he said. “The bleeding risk might be too high if the patient is at a high risk of falls.”
A version of this article originally appeared on WebMD.com.
Factors linked with increased VTE risk in COVID outpatients
Though VTE risk is well studied and significant in those hospitalized with COVID, little is known about the risk in the outpatient setting, said the authors of the new research published online in JAMA Network Open.
The study was conducted at two integrated health care delivery systems in northern and southern California. Data were gathered from the Kaiser Permanente Virtual Data Warehouse and electronic health records.
Nearly 400,000 patients studied
Researchers, led by Margaret Fang, MD, with the division of hospital medicine, University of California, San Francisco, identified 398,530 outpatients with COVID-19 from Jan. 1, 2020, through Jan. 31, 2021.
VTE risk was low overall for ambulatory COVID patients.
“It is a reassuring study,” Dr. Fang said in an interview.
The researchers found that the risk is highest in the first 30 days after COVID-19 diagnosis (unadjusted rate, 0.58; 95% confidence interval, 0.51-0.67 per 100 person-years vs. 0.09; 95% CI, 0.08-0.11 per 100 person-years after 30 days).
Factors linked with high VTE risk
They also found that several factors were linked with a higher risk of blood clots in the study population, including being at least 55 years old; being male; having a history of blood clots or thrombophilia; and a body mass index (BMI) of at least 30 kg/m2.
The authors write, “These findings may help identify subsets of patients with COVID-19 who could benefit from VTE preventive strategies and more intensive short-term surveillance.”
Are routine anticoagulants justified?
Previously, randomized clinical trials have found that hospitalized patients with moderate COVID-19 may benefit from therapeutically dosed heparin anticoagulants but that therapeutic anticoagulation had no net benefit – and perhaps could even harm – patients who were critically ill with COVID.
“[M]uch less is known about the optimal thromboprophylaxis strategy for people with milder presentations of COVID-19 who do not require hospitalization,” they write.
Mild COVID VTE risk similar to general population
The authors note that rates of blood clots linked with COVID-19 are not much higher than the average blood clot rate in the general population, which is about 0.1-0.2 per 100 person-years.
Therefore, the results don’t justify routine administration of anticoagulation given the costs, inconvenience, and bleeding risks, they acknowledge.
Dr. Fang told this publication that it’s hard to know what to tell patients, given the overall low VTE risk. She said their study wasn’t designed to advise when to give prophylaxis.
Physicians should inform patients of their higher risk
“We should tell our patients who fall into these risk categories that blood clot is a concern after the development of COVID, especially in those first 30 days. And some people might benefit from increased surveillance,” Dr. Fang said.
”I think this study would support ongoing studies that look at whether selected patients benefit from VTE prophylaxis, for example low-dose anticoagulants,” she said.
Dr. Fang said the subgroup factors they found increased risk of blood clots for all patients, not just COVID-19 patients. It’s not clear why factors such as being male may increase blood clot risk, though that is consistent with previous literature, but higher risk with higher BMI might be related to a combination of inflammation or decreased mobility, she said.
Unanswered questions
Robert H. Hopkins Jr., MD, says the study helps answer a couple of important questions – that the VTE risk in nonhospitalized COVID-19 patients is low and when and for which patients risk may be highest.
However, there are several unanswered questions that argue against routine initiation of anticoagulants, notes the professor of internal medicine and pediatrics chief, division of general internal medicine, at University of Arkansas for Medical Sciences, Little Rock.
One is the change in the COVID variant landscape.
“We do not know whether rates of VTE are same or lower or higher with current circulating variants,” Dr. Hopkins said.
The authors acknowledge this as a limitation. Study data predate Omicron and subvariants, which appear to lower clinical severity, so it’s unclear whether VTE risk is different in this Omicron era.
Dr. Hopkins added another unknown: “We do not know whether vaccination affects rates of VTE in ambulatory breakthrough infection.”
Dr. Hopkins and the authors also note the lack of a control group in the study, to better compare risk.
Coauthor Dr. Prasad reports consultant fees from EpiExcellence LLC outside the submitted work. Coauthor Dr. Go reports grants paid to the division of research, Kaiser Permanente Northern California, from CSL Behring, Novartis, Bristol Meyers Squibb/Pfizer Alliance, and Janssen outside the submitted work.
The research was funded through Patient-Centered Outcomes Research Institute.
Dr. Hopkins reports no relevant financial relationships.
Though VTE risk is well studied and significant in those hospitalized with COVID, little is known about the risk in the outpatient setting, said the authors of the new research published online in JAMA Network Open.
The study was conducted at two integrated health care delivery systems in northern and southern California. Data were gathered from the Kaiser Permanente Virtual Data Warehouse and electronic health records.
Nearly 400,000 patients studied
Researchers, led by Margaret Fang, MD, with the division of hospital medicine, University of California, San Francisco, identified 398,530 outpatients with COVID-19 from Jan. 1, 2020, through Jan. 31, 2021.
VTE risk was low overall for ambulatory COVID patients.
“It is a reassuring study,” Dr. Fang said in an interview.
The researchers found that the risk is highest in the first 30 days after COVID-19 diagnosis (unadjusted rate, 0.58; 95% confidence interval, 0.51-0.67 per 100 person-years vs. 0.09; 95% CI, 0.08-0.11 per 100 person-years after 30 days).
Factors linked with high VTE risk
They also found that several factors were linked with a higher risk of blood clots in the study population, including being at least 55 years old; being male; having a history of blood clots or thrombophilia; and a body mass index (BMI) of at least 30 kg/m2.
The authors write, “These findings may help identify subsets of patients with COVID-19 who could benefit from VTE preventive strategies and more intensive short-term surveillance.”
Are routine anticoagulants justified?
Previously, randomized clinical trials have found that hospitalized patients with moderate COVID-19 may benefit from therapeutically dosed heparin anticoagulants but that therapeutic anticoagulation had no net benefit – and perhaps could even harm – patients who were critically ill with COVID.
“[M]uch less is known about the optimal thromboprophylaxis strategy for people with milder presentations of COVID-19 who do not require hospitalization,” they write.
Mild COVID VTE risk similar to general population
The authors note that rates of blood clots linked with COVID-19 are not much higher than the average blood clot rate in the general population, which is about 0.1-0.2 per 100 person-years.
Therefore, the results don’t justify routine administration of anticoagulation given the costs, inconvenience, and bleeding risks, they acknowledge.
Dr. Fang told this publication that it’s hard to know what to tell patients, given the overall low VTE risk. She said their study wasn’t designed to advise when to give prophylaxis.
Physicians should inform patients of their higher risk
“We should tell our patients who fall into these risk categories that blood clot is a concern after the development of COVID, especially in those first 30 days. And some people might benefit from increased surveillance,” Dr. Fang said.
”I think this study would support ongoing studies that look at whether selected patients benefit from VTE prophylaxis, for example low-dose anticoagulants,” she said.
Dr. Fang said the subgroup factors they found increased risk of blood clots for all patients, not just COVID-19 patients. It’s not clear why factors such as being male may increase blood clot risk, though that is consistent with previous literature, but higher risk with higher BMI might be related to a combination of inflammation or decreased mobility, she said.
Unanswered questions
Robert H. Hopkins Jr., MD, says the study helps answer a couple of important questions – that the VTE risk in nonhospitalized COVID-19 patients is low and when and for which patients risk may be highest.
However, there are several unanswered questions that argue against routine initiation of anticoagulants, notes the professor of internal medicine and pediatrics chief, division of general internal medicine, at University of Arkansas for Medical Sciences, Little Rock.
One is the change in the COVID variant landscape.
“We do not know whether rates of VTE are same or lower or higher with current circulating variants,” Dr. Hopkins said.
The authors acknowledge this as a limitation. Study data predate Omicron and subvariants, which appear to lower clinical severity, so it’s unclear whether VTE risk is different in this Omicron era.
Dr. Hopkins added another unknown: “We do not know whether vaccination affects rates of VTE in ambulatory breakthrough infection.”
Dr. Hopkins and the authors also note the lack of a control group in the study, to better compare risk.
Coauthor Dr. Prasad reports consultant fees from EpiExcellence LLC outside the submitted work. Coauthor Dr. Go reports grants paid to the division of research, Kaiser Permanente Northern California, from CSL Behring, Novartis, Bristol Meyers Squibb/Pfizer Alliance, and Janssen outside the submitted work.
The research was funded through Patient-Centered Outcomes Research Institute.
Dr. Hopkins reports no relevant financial relationships.
Though VTE risk is well studied and significant in those hospitalized with COVID, little is known about the risk in the outpatient setting, said the authors of the new research published online in JAMA Network Open.
The study was conducted at two integrated health care delivery systems in northern and southern California. Data were gathered from the Kaiser Permanente Virtual Data Warehouse and electronic health records.
Nearly 400,000 patients studied
Researchers, led by Margaret Fang, MD, with the division of hospital medicine, University of California, San Francisco, identified 398,530 outpatients with COVID-19 from Jan. 1, 2020, through Jan. 31, 2021.
VTE risk was low overall for ambulatory COVID patients.
“It is a reassuring study,” Dr. Fang said in an interview.
The researchers found that the risk is highest in the first 30 days after COVID-19 diagnosis (unadjusted rate, 0.58; 95% confidence interval, 0.51-0.67 per 100 person-years vs. 0.09; 95% CI, 0.08-0.11 per 100 person-years after 30 days).
Factors linked with high VTE risk
They also found that several factors were linked with a higher risk of blood clots in the study population, including being at least 55 years old; being male; having a history of blood clots or thrombophilia; and a body mass index (BMI) of at least 30 kg/m2.
The authors write, “These findings may help identify subsets of patients with COVID-19 who could benefit from VTE preventive strategies and more intensive short-term surveillance.”
Are routine anticoagulants justified?
Previously, randomized clinical trials have found that hospitalized patients with moderate COVID-19 may benefit from therapeutically dosed heparin anticoagulants but that therapeutic anticoagulation had no net benefit – and perhaps could even harm – patients who were critically ill with COVID.
“[M]uch less is known about the optimal thromboprophylaxis strategy for people with milder presentations of COVID-19 who do not require hospitalization,” they write.
Mild COVID VTE risk similar to general population
The authors note that rates of blood clots linked with COVID-19 are not much higher than the average blood clot rate in the general population, which is about 0.1-0.2 per 100 person-years.
Therefore, the results don’t justify routine administration of anticoagulation given the costs, inconvenience, and bleeding risks, they acknowledge.
Dr. Fang told this publication that it’s hard to know what to tell patients, given the overall low VTE risk. She said their study wasn’t designed to advise when to give prophylaxis.
Physicians should inform patients of their higher risk
“We should tell our patients who fall into these risk categories that blood clot is a concern after the development of COVID, especially in those first 30 days. And some people might benefit from increased surveillance,” Dr. Fang said.
”I think this study would support ongoing studies that look at whether selected patients benefit from VTE prophylaxis, for example low-dose anticoagulants,” she said.
Dr. Fang said the subgroup factors they found increased risk of blood clots for all patients, not just COVID-19 patients. It’s not clear why factors such as being male may increase blood clot risk, though that is consistent with previous literature, but higher risk with higher BMI might be related to a combination of inflammation or decreased mobility, she said.
Unanswered questions
Robert H. Hopkins Jr., MD, says the study helps answer a couple of important questions – that the VTE risk in nonhospitalized COVID-19 patients is low and when and for which patients risk may be highest.
However, there are several unanswered questions that argue against routine initiation of anticoagulants, notes the professor of internal medicine and pediatrics chief, division of general internal medicine, at University of Arkansas for Medical Sciences, Little Rock.
One is the change in the COVID variant landscape.
“We do not know whether rates of VTE are same or lower or higher with current circulating variants,” Dr. Hopkins said.
The authors acknowledge this as a limitation. Study data predate Omicron and subvariants, which appear to lower clinical severity, so it’s unclear whether VTE risk is different in this Omicron era.
Dr. Hopkins added another unknown: “We do not know whether vaccination affects rates of VTE in ambulatory breakthrough infection.”
Dr. Hopkins and the authors also note the lack of a control group in the study, to better compare risk.
Coauthor Dr. Prasad reports consultant fees from EpiExcellence LLC outside the submitted work. Coauthor Dr. Go reports grants paid to the division of research, Kaiser Permanente Northern California, from CSL Behring, Novartis, Bristol Meyers Squibb/Pfizer Alliance, and Janssen outside the submitted work.
The research was funded through Patient-Centered Outcomes Research Institute.
Dr. Hopkins reports no relevant financial relationships.
FROM JAMA NETWORK OPEN
Digital rectal exam fails as screening tool for prostate cancer
, say investigators reporting the PROBASE study.
The study compared risk-adapted screening measures in men who had prostate-specific antigen (PSA) measured at age 45 with those who had PSA measurements plus DRE at age 50.
The results show that as a solitary screening tool, 99% of DREs did not raise suspicion for prostate cancer, and among the 57 cases where DRE did raise suspicion, only three men were found to have cancer, all of which were low-grade, reported Agne Krilaviciute, PhD, from the German Cancer Research Center in Heidelberg, and colleagues.
“We also see that the cancer detection rate by PSA is four times higher compared to the DRE detection. Around 18% of the tumors are located in the part of the prostate where DRE cannot detect them,” she said in an oral presentation at the European Association of Urology Congress.
The investigators found that the majority of prostate cancers that occurred in this relatively young population were International Society of Urological Pathology grade 1 (Gleason score 3 + 3 = 6) or grade 2 (Gleason 3 + 4 = 7). DRE yields positive results in only about 12% of cases of ISUP grade 1 or 2, they noted.
“We conclude that DRE as a solitary screening test does not lead to a significant PCa [prostate cancer] detection rate in young men,” Dr. Krilaviciute said.
Falling by the wayside
The study adds to the growing body of evidence that DRE may not be especially helpful as either a screening tool or when used in active surveillance of men with prostate cancer.
An international consensus panel found that DRE could be safely skipped for active surveillance when MRI and other more accurate and objective measures, such as biomarkers, are available.
A prostate cancer expert who was not involved in the PROBASE study told this news organization that when he was in medical school, it would have been considered a serious lapse of practice not to perform a DRE, but that things have changed considerably over the past several years.
“We have PSA now, we have technology with MRI, and the yield of digital rectal examination is very low,” commented Julio Pow-Sang, MD, chief of the genitourinary oncology program at Moffitt Cancer Center in Tampa, Fla.
“Empirically, it’s very rare to find positive cancer through rectal exam in this day and age of PSA,” he said, adding that the examination itself is highly subjective, with varying results depending on the skills of the particular examiner.
“I think that in time, with good studies like this, digital rectal exam specifically for prostate cancer is going to slowly fade away,” Dr. Pow-Sang said.
PROBASE results
PROBASE was a randomized screening study enrolling men at age 45 to test a risk-adapted screening strategy using a baseline PSA value with the additional offer of DRE in a large subcohort of participants.
The study was conducted in Germany, and the authors note that the “German statutory early detection program recommends DRE as a stand-alone screening test starting annually at age 45.”
The PROBASE investigators enrolled 46,495 men from February 2014 through December 2019.
Among the first 23,194 men enrolled, 6,537 underwent DRE at enrollment without a study PSA test.
In this group, 6,480 DREs (99%) were not suspicious for cancer, and 57 (1%) were. Of those with suspected prostate cancer, 37 underwent biopsy and 20 did not. Of those biopsied, only two were found to have prostate cancer. This translated into a cancer detection rate of 0.03% for DRE.
After a median of 6.6 years of follow-up, only one additional case of ISUP grade 2 prostate cancer was detected among the 6,357 men who had DREs at enrollment, translating into a prostate cancer detection rate of .05%.
The investigators also looked at men who suspicious DRE findings at baseline. They assumed that a DRE-detectable tumor at age 45 would still be manifest 5 years later and should be detectable with PSA at age 50. Of the 57 men with initially suspicious findings, 11 returned for PSA screening but refused biopsy, and of this group only one had an elevated PSA level. He then underwent biopsy, but the findings were negative.
Of those who underwent biopsy on the basis of DRE, 16 had prostatitis, 14 had benign prostatic hyperplasia, 1 had high-grade prostatic intraepithelial neoplasia, 1 had atypical small acinar proliferation, and 3 had equivocal findings.
In total, the investigators found 24 tumors among men screened with DRE. Of these, 3 occurred in men with results deemed suspicious and 21 were in men with unsuspicious digital exams. All of the tumors were ISUP grade 1, 2, or 3 tumors.
Among 245 men who had biopsies for a PSA level equal to or higher than 3 ng/mL, primarily Prostate Imaging Reporting and Data System (PI-RADS) 3-5 tumors, DRE findings at the time of biopsy were unsuspicious in about 82% of cases, Dr. Krilaviciute said.
“We also used MRI data to determine what proportion of tumors would be potentially detectable by DRE. We estimated that around 18% of tumors are located in the upper part of the prostate, which is not detectable by DRE,” she said. “Even excluding those tumors, still the DRE detection rate is low in palpable tumors.”
Although DRE performed better in higher-grade tumors, 80% of the tumors in the PROBASE participants were ISUP grade 1 or 2 and were likely to be undetected by DRE, she added.
“In Germany, the recommendations for the screening still include 45-year-olds to go with annual DRE. The PROBASE trial allowed us to evaluate for the first time what was the diagnostic performance for DRE at such a young age, and we see that 99% of men undergoing DRE have no suspicious findings, and among the 1% of suspicious findings having cancers extremely unlikely,” she said.
The study was supported by Deutsche Krebshilfe (German Cancer Aid). Dr. Krilaviciute and Dr. Pow-Sang reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
, say investigators reporting the PROBASE study.
The study compared risk-adapted screening measures in men who had prostate-specific antigen (PSA) measured at age 45 with those who had PSA measurements plus DRE at age 50.
The results show that as a solitary screening tool, 99% of DREs did not raise suspicion for prostate cancer, and among the 57 cases where DRE did raise suspicion, only three men were found to have cancer, all of which were low-grade, reported Agne Krilaviciute, PhD, from the German Cancer Research Center in Heidelberg, and colleagues.
“We also see that the cancer detection rate by PSA is four times higher compared to the DRE detection. Around 18% of the tumors are located in the part of the prostate where DRE cannot detect them,” she said in an oral presentation at the European Association of Urology Congress.
The investigators found that the majority of prostate cancers that occurred in this relatively young population were International Society of Urological Pathology grade 1 (Gleason score 3 + 3 = 6) or grade 2 (Gleason 3 + 4 = 7). DRE yields positive results in only about 12% of cases of ISUP grade 1 or 2, they noted.
“We conclude that DRE as a solitary screening test does not lead to a significant PCa [prostate cancer] detection rate in young men,” Dr. Krilaviciute said.
Falling by the wayside
The study adds to the growing body of evidence that DRE may not be especially helpful as either a screening tool or when used in active surveillance of men with prostate cancer.
An international consensus panel found that DRE could be safely skipped for active surveillance when MRI and other more accurate and objective measures, such as biomarkers, are available.
A prostate cancer expert who was not involved in the PROBASE study told this news organization that when he was in medical school, it would have been considered a serious lapse of practice not to perform a DRE, but that things have changed considerably over the past several years.
“We have PSA now, we have technology with MRI, and the yield of digital rectal examination is very low,” commented Julio Pow-Sang, MD, chief of the genitourinary oncology program at Moffitt Cancer Center in Tampa, Fla.
“Empirically, it’s very rare to find positive cancer through rectal exam in this day and age of PSA,” he said, adding that the examination itself is highly subjective, with varying results depending on the skills of the particular examiner.
“I think that in time, with good studies like this, digital rectal exam specifically for prostate cancer is going to slowly fade away,” Dr. Pow-Sang said.
PROBASE results
PROBASE was a randomized screening study enrolling men at age 45 to test a risk-adapted screening strategy using a baseline PSA value with the additional offer of DRE in a large subcohort of participants.
The study was conducted in Germany, and the authors note that the “German statutory early detection program recommends DRE as a stand-alone screening test starting annually at age 45.”
The PROBASE investigators enrolled 46,495 men from February 2014 through December 2019.
Among the first 23,194 men enrolled, 6,537 underwent DRE at enrollment without a study PSA test.
In this group, 6,480 DREs (99%) were not suspicious for cancer, and 57 (1%) were. Of those with suspected prostate cancer, 37 underwent biopsy and 20 did not. Of those biopsied, only two were found to have prostate cancer. This translated into a cancer detection rate of 0.03% for DRE.
After a median of 6.6 years of follow-up, only one additional case of ISUP grade 2 prostate cancer was detected among the 6,357 men who had DREs at enrollment, translating into a prostate cancer detection rate of .05%.
The investigators also looked at men who suspicious DRE findings at baseline. They assumed that a DRE-detectable tumor at age 45 would still be manifest 5 years later and should be detectable with PSA at age 50. Of the 57 men with initially suspicious findings, 11 returned for PSA screening but refused biopsy, and of this group only one had an elevated PSA level. He then underwent biopsy, but the findings were negative.
Of those who underwent biopsy on the basis of DRE, 16 had prostatitis, 14 had benign prostatic hyperplasia, 1 had high-grade prostatic intraepithelial neoplasia, 1 had atypical small acinar proliferation, and 3 had equivocal findings.
In total, the investigators found 24 tumors among men screened with DRE. Of these, 3 occurred in men with results deemed suspicious and 21 were in men with unsuspicious digital exams. All of the tumors were ISUP grade 1, 2, or 3 tumors.
Among 245 men who had biopsies for a PSA level equal to or higher than 3 ng/mL, primarily Prostate Imaging Reporting and Data System (PI-RADS) 3-5 tumors, DRE findings at the time of biopsy were unsuspicious in about 82% of cases, Dr. Krilaviciute said.
“We also used MRI data to determine what proportion of tumors would be potentially detectable by DRE. We estimated that around 18% of tumors are located in the upper part of the prostate, which is not detectable by DRE,” she said. “Even excluding those tumors, still the DRE detection rate is low in palpable tumors.”
Although DRE performed better in higher-grade tumors, 80% of the tumors in the PROBASE participants were ISUP grade 1 or 2 and were likely to be undetected by DRE, she added.
“In Germany, the recommendations for the screening still include 45-year-olds to go with annual DRE. The PROBASE trial allowed us to evaluate for the first time what was the diagnostic performance for DRE at such a young age, and we see that 99% of men undergoing DRE have no suspicious findings, and among the 1% of suspicious findings having cancers extremely unlikely,” she said.
The study was supported by Deutsche Krebshilfe (German Cancer Aid). Dr. Krilaviciute and Dr. Pow-Sang reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
, say investigators reporting the PROBASE study.
The study compared risk-adapted screening measures in men who had prostate-specific antigen (PSA) measured at age 45 with those who had PSA measurements plus DRE at age 50.
The results show that as a solitary screening tool, 99% of DREs did not raise suspicion for prostate cancer, and among the 57 cases where DRE did raise suspicion, only three men were found to have cancer, all of which were low-grade, reported Agne Krilaviciute, PhD, from the German Cancer Research Center in Heidelberg, and colleagues.
“We also see that the cancer detection rate by PSA is four times higher compared to the DRE detection. Around 18% of the tumors are located in the part of the prostate where DRE cannot detect them,” she said in an oral presentation at the European Association of Urology Congress.
The investigators found that the majority of prostate cancers that occurred in this relatively young population were International Society of Urological Pathology grade 1 (Gleason score 3 + 3 = 6) or grade 2 (Gleason 3 + 4 = 7). DRE yields positive results in only about 12% of cases of ISUP grade 1 or 2, they noted.
“We conclude that DRE as a solitary screening test does not lead to a significant PCa [prostate cancer] detection rate in young men,” Dr. Krilaviciute said.
Falling by the wayside
The study adds to the growing body of evidence that DRE may not be especially helpful as either a screening tool or when used in active surveillance of men with prostate cancer.
An international consensus panel found that DRE could be safely skipped for active surveillance when MRI and other more accurate and objective measures, such as biomarkers, are available.
A prostate cancer expert who was not involved in the PROBASE study told this news organization that when he was in medical school, it would have been considered a serious lapse of practice not to perform a DRE, but that things have changed considerably over the past several years.
“We have PSA now, we have technology with MRI, and the yield of digital rectal examination is very low,” commented Julio Pow-Sang, MD, chief of the genitourinary oncology program at Moffitt Cancer Center in Tampa, Fla.
“Empirically, it’s very rare to find positive cancer through rectal exam in this day and age of PSA,” he said, adding that the examination itself is highly subjective, with varying results depending on the skills of the particular examiner.
“I think that in time, with good studies like this, digital rectal exam specifically for prostate cancer is going to slowly fade away,” Dr. Pow-Sang said.
PROBASE results
PROBASE was a randomized screening study enrolling men at age 45 to test a risk-adapted screening strategy using a baseline PSA value with the additional offer of DRE in a large subcohort of participants.
The study was conducted in Germany, and the authors note that the “German statutory early detection program recommends DRE as a stand-alone screening test starting annually at age 45.”
The PROBASE investigators enrolled 46,495 men from February 2014 through December 2019.
Among the first 23,194 men enrolled, 6,537 underwent DRE at enrollment without a study PSA test.
In this group, 6,480 DREs (99%) were not suspicious for cancer, and 57 (1%) were. Of those with suspected prostate cancer, 37 underwent biopsy and 20 did not. Of those biopsied, only two were found to have prostate cancer. This translated into a cancer detection rate of 0.03% for DRE.
After a median of 6.6 years of follow-up, only one additional case of ISUP grade 2 prostate cancer was detected among the 6,357 men who had DREs at enrollment, translating into a prostate cancer detection rate of .05%.
The investigators also looked at men who suspicious DRE findings at baseline. They assumed that a DRE-detectable tumor at age 45 would still be manifest 5 years later and should be detectable with PSA at age 50. Of the 57 men with initially suspicious findings, 11 returned for PSA screening but refused biopsy, and of this group only one had an elevated PSA level. He then underwent biopsy, but the findings were negative.
Of those who underwent biopsy on the basis of DRE, 16 had prostatitis, 14 had benign prostatic hyperplasia, 1 had high-grade prostatic intraepithelial neoplasia, 1 had atypical small acinar proliferation, and 3 had equivocal findings.
In total, the investigators found 24 tumors among men screened with DRE. Of these, 3 occurred in men with results deemed suspicious and 21 were in men with unsuspicious digital exams. All of the tumors were ISUP grade 1, 2, or 3 tumors.
Among 245 men who had biopsies for a PSA level equal to or higher than 3 ng/mL, primarily Prostate Imaging Reporting and Data System (PI-RADS) 3-5 tumors, DRE findings at the time of biopsy were unsuspicious in about 82% of cases, Dr. Krilaviciute said.
“We also used MRI data to determine what proportion of tumors would be potentially detectable by DRE. We estimated that around 18% of tumors are located in the upper part of the prostate, which is not detectable by DRE,” she said. “Even excluding those tumors, still the DRE detection rate is low in palpable tumors.”
Although DRE performed better in higher-grade tumors, 80% of the tumors in the PROBASE participants were ISUP grade 1 or 2 and were likely to be undetected by DRE, she added.
“In Germany, the recommendations for the screening still include 45-year-olds to go with annual DRE. The PROBASE trial allowed us to evaluate for the first time what was the diagnostic performance for DRE at such a young age, and we see that 99% of men undergoing DRE have no suspicious findings, and among the 1% of suspicious findings having cancers extremely unlikely,” she said.
The study was supported by Deutsche Krebshilfe (German Cancer Aid). Dr. Krilaviciute and Dr. Pow-Sang reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM EAU 2023