User login
Premenstrual Disorders and Perinatal Depression: A Two-Way Street
Premenstrual disorders (PMDs) and perinatal depression (PND) appear to have a bidirectional association, a Swedish national registry-based analysis found.
In women with PND, 2.9% had PMDs before pregnancy vs 0.6% in a matched cohort of unaffected women, according to an international team led by Quian Yang, MD, PhD, of the Institute of Environmental Medicine at the Karolinska Institutet in Stockholm, Sweden. Their study appears in PLoS Medicine.
“Preconception and maternity care providers should be aware of the risk of developing perinatal depression among women with a history of PMDs,” Dr. Yang said in an interview. “Healthcare providers may inform women with perinatal depression about the potential risk of PMDs when menstruation returns after childbirth.” She recommended screening as part of routine perinatal care to identify and treat the condition at an early stage. Counseling and medication may help prevent adverse consequences.
In other findings, the correlation with PMDs held for both prenatal and postnatal depression, regardless of any history of psychiatric disorders and also in full-sister comparisons, the authors noted, with a stronger correlation in the absence of psychiatric disorders (P for interaction <.001).
“Interestingly, we noted a stronger association between PMDs and subsequent PND than the association in the other direction, Dr. Yang said. And although many experience PMD symptom onset in adolescence, symptom worsening has been reported with increasing age and parity. “It is possible that women with milder premenstrual symptoms experienced worse symptoms after pregnancy and are therefore first diagnosed with PMD after pregnancy,” the authors hypothesized.
Both PMDs and PND share depressive symptomatology and onset coinciding with hormonal fluctuations, particularly estrogen and progesterone, suggesting a shared etiology, Dr. Yang explained. “It’s plausible that an abnormal response to natural hormone fluctuations predisposes women to both PMDs and PND. However, the underlying mechanism is complex, and future research is needed to reveal the underlying etiology.”
Affecting a majority of women of reproductive age to some degree, PMDs in certain women can cause significant functional impairment and, when severe, have been linked to increased risks of accidents and suicidal behavior. The psychological symptoms of the more serious form, premenstrual dysphoric disorder, for example, are associated with a 50%-78% lifetime risk for psychiatric disorders, including major depressive, dysthymic, seasonal affective, and generalized anxiety disorders, as well as suicidality.
Mood disorders are common in pregnancy and the postpartum period.
The Swedish Study
In 1.8 million singleton pregnancies in Sweden during 2001-2018, the investigators identified 84,949 women with PND and 849,482 unaffected women and individually matched them 10:1 by age and calendar year. Incident PND and PMDs were identified through clinical diagnoses or prescribed medications, and adjustment was made for such demographics as country of birth, educational level, region of residency, and cohabitation status.
In an initial matched-cohort case-control study with a mean follow-up of 6.9 years, PMDs were associated with a nearly five times higher risk of subsequent PND (odds ratio, 4.76; 95% CI, 4.52-5.01; P <.001).
In another matched cohort with a mean follow-up of 7.0 years, there were 4227 newly diagnosed PMDs in women with PND (incidence rate [IR], 7.6/1000 person-years) and 21,326 among controls (IR, 3.8/1000). Compared with matched controls, women with PND were at almost twice the risk of subsequent PMDs (hazard ratio, 1.81; 95% CI, 1.74-1.88; P <.001).
Commenting on the study but not involved in it, Bernard L. Harlow, PhD, a professor of epidemiology at Boston University School of Public Health in Massachusetts who specializes in epidemiologic studies of female reproductive disorders, said he was not surprised at these findings, which clearly support the need for PMD screening in mothers-to-be. “Anything that is easy to measure and noninvasive that will minimize the risk of postpartum depression should be part of the standard of care during the prenatal period.” As to safety: If treatment is indicated, he added, “studies have shown that the risk to the mother and child is much greater if the mother’s mood disorder is not controlled than any risk to the baby due to depression treatment.” But though PMDs may be predictive of PND, there are still barriers to actual PND care. A 2023 analysis reported that 65% of mothers-to-be who screened positive for metal health comorbidities were not referred for treatment.
Dr. Yang and colleagues acknowledged that their findings may not be generalizable to mild forms of these disorders since the data were based on clinical diagnoses and prescriptions.
The study was supported by the Chinese Scholarship Council, the Swedish Research Council for Health, Working Life and Welfare, the Karolinska Institutet, and the Icelandic Research Fund. The authors and Dr. Harlow had no relevant competing interests to disclose.
Premenstrual disorders (PMDs) and perinatal depression (PND) appear to have a bidirectional association, a Swedish national registry-based analysis found.
In women with PND, 2.9% had PMDs before pregnancy vs 0.6% in a matched cohort of unaffected women, according to an international team led by Quian Yang, MD, PhD, of the Institute of Environmental Medicine at the Karolinska Institutet in Stockholm, Sweden. Their study appears in PLoS Medicine.
“Preconception and maternity care providers should be aware of the risk of developing perinatal depression among women with a history of PMDs,” Dr. Yang said in an interview. “Healthcare providers may inform women with perinatal depression about the potential risk of PMDs when menstruation returns after childbirth.” She recommended screening as part of routine perinatal care to identify and treat the condition at an early stage. Counseling and medication may help prevent adverse consequences.
In other findings, the correlation with PMDs held for both prenatal and postnatal depression, regardless of any history of psychiatric disorders and also in full-sister comparisons, the authors noted, with a stronger correlation in the absence of psychiatric disorders (P for interaction <.001).
“Interestingly, we noted a stronger association between PMDs and subsequent PND than the association in the other direction, Dr. Yang said. And although many experience PMD symptom onset in adolescence, symptom worsening has been reported with increasing age and parity. “It is possible that women with milder premenstrual symptoms experienced worse symptoms after pregnancy and are therefore first diagnosed with PMD after pregnancy,” the authors hypothesized.
Both PMDs and PND share depressive symptomatology and onset coinciding with hormonal fluctuations, particularly estrogen and progesterone, suggesting a shared etiology, Dr. Yang explained. “It’s plausible that an abnormal response to natural hormone fluctuations predisposes women to both PMDs and PND. However, the underlying mechanism is complex, and future research is needed to reveal the underlying etiology.”
Affecting a majority of women of reproductive age to some degree, PMDs in certain women can cause significant functional impairment and, when severe, have been linked to increased risks of accidents and suicidal behavior. The psychological symptoms of the more serious form, premenstrual dysphoric disorder, for example, are associated with a 50%-78% lifetime risk for psychiatric disorders, including major depressive, dysthymic, seasonal affective, and generalized anxiety disorders, as well as suicidality.
Mood disorders are common in pregnancy and the postpartum period.
The Swedish Study
In 1.8 million singleton pregnancies in Sweden during 2001-2018, the investigators identified 84,949 women with PND and 849,482 unaffected women and individually matched them 10:1 by age and calendar year. Incident PND and PMDs were identified through clinical diagnoses or prescribed medications, and adjustment was made for such demographics as country of birth, educational level, region of residency, and cohabitation status.
In an initial matched-cohort case-control study with a mean follow-up of 6.9 years, PMDs were associated with a nearly five times higher risk of subsequent PND (odds ratio, 4.76; 95% CI, 4.52-5.01; P <.001).
In another matched cohort with a mean follow-up of 7.0 years, there were 4227 newly diagnosed PMDs in women with PND (incidence rate [IR], 7.6/1000 person-years) and 21,326 among controls (IR, 3.8/1000). Compared with matched controls, women with PND were at almost twice the risk of subsequent PMDs (hazard ratio, 1.81; 95% CI, 1.74-1.88; P <.001).
Commenting on the study but not involved in it, Bernard L. Harlow, PhD, a professor of epidemiology at Boston University School of Public Health in Massachusetts who specializes in epidemiologic studies of female reproductive disorders, said he was not surprised at these findings, which clearly support the need for PMD screening in mothers-to-be. “Anything that is easy to measure and noninvasive that will minimize the risk of postpartum depression should be part of the standard of care during the prenatal period.” As to safety: If treatment is indicated, he added, “studies have shown that the risk to the mother and child is much greater if the mother’s mood disorder is not controlled than any risk to the baby due to depression treatment.” But though PMDs may be predictive of PND, there are still barriers to actual PND care. A 2023 analysis reported that 65% of mothers-to-be who screened positive for metal health comorbidities were not referred for treatment.
Dr. Yang and colleagues acknowledged that their findings may not be generalizable to mild forms of these disorders since the data were based on clinical diagnoses and prescriptions.
The study was supported by the Chinese Scholarship Council, the Swedish Research Council for Health, Working Life and Welfare, the Karolinska Institutet, and the Icelandic Research Fund. The authors and Dr. Harlow had no relevant competing interests to disclose.
Premenstrual disorders (PMDs) and perinatal depression (PND) appear to have a bidirectional association, a Swedish national registry-based analysis found.
In women with PND, 2.9% had PMDs before pregnancy vs 0.6% in a matched cohort of unaffected women, according to an international team led by Quian Yang, MD, PhD, of the Institute of Environmental Medicine at the Karolinska Institutet in Stockholm, Sweden. Their study appears in PLoS Medicine.
“Preconception and maternity care providers should be aware of the risk of developing perinatal depression among women with a history of PMDs,” Dr. Yang said in an interview. “Healthcare providers may inform women with perinatal depression about the potential risk of PMDs when menstruation returns after childbirth.” She recommended screening as part of routine perinatal care to identify and treat the condition at an early stage. Counseling and medication may help prevent adverse consequences.
In other findings, the correlation with PMDs held for both prenatal and postnatal depression, regardless of any history of psychiatric disorders and also in full-sister comparisons, the authors noted, with a stronger correlation in the absence of psychiatric disorders (P for interaction <.001).
“Interestingly, we noted a stronger association between PMDs and subsequent PND than the association in the other direction, Dr. Yang said. And although many experience PMD symptom onset in adolescence, symptom worsening has been reported with increasing age and parity. “It is possible that women with milder premenstrual symptoms experienced worse symptoms after pregnancy and are therefore first diagnosed with PMD after pregnancy,” the authors hypothesized.
Both PMDs and PND share depressive symptomatology and onset coinciding with hormonal fluctuations, particularly estrogen and progesterone, suggesting a shared etiology, Dr. Yang explained. “It’s plausible that an abnormal response to natural hormone fluctuations predisposes women to both PMDs and PND. However, the underlying mechanism is complex, and future research is needed to reveal the underlying etiology.”
Affecting a majority of women of reproductive age to some degree, PMDs in certain women can cause significant functional impairment and, when severe, have been linked to increased risks of accidents and suicidal behavior. The psychological symptoms of the more serious form, premenstrual dysphoric disorder, for example, are associated with a 50%-78% lifetime risk for psychiatric disorders, including major depressive, dysthymic, seasonal affective, and generalized anxiety disorders, as well as suicidality.
Mood disorders are common in pregnancy and the postpartum period.
The Swedish Study
In 1.8 million singleton pregnancies in Sweden during 2001-2018, the investigators identified 84,949 women with PND and 849,482 unaffected women and individually matched them 10:1 by age and calendar year. Incident PND and PMDs were identified through clinical diagnoses or prescribed medications, and adjustment was made for such demographics as country of birth, educational level, region of residency, and cohabitation status.
In an initial matched-cohort case-control study with a mean follow-up of 6.9 years, PMDs were associated with a nearly five times higher risk of subsequent PND (odds ratio, 4.76; 95% CI, 4.52-5.01; P <.001).
In another matched cohort with a mean follow-up of 7.0 years, there were 4227 newly diagnosed PMDs in women with PND (incidence rate [IR], 7.6/1000 person-years) and 21,326 among controls (IR, 3.8/1000). Compared with matched controls, women with PND were at almost twice the risk of subsequent PMDs (hazard ratio, 1.81; 95% CI, 1.74-1.88; P <.001).
Commenting on the study but not involved in it, Bernard L. Harlow, PhD, a professor of epidemiology at Boston University School of Public Health in Massachusetts who specializes in epidemiologic studies of female reproductive disorders, said he was not surprised at these findings, which clearly support the need for PMD screening in mothers-to-be. “Anything that is easy to measure and noninvasive that will minimize the risk of postpartum depression should be part of the standard of care during the prenatal period.” As to safety: If treatment is indicated, he added, “studies have shown that the risk to the mother and child is much greater if the mother’s mood disorder is not controlled than any risk to the baby due to depression treatment.” But though PMDs may be predictive of PND, there are still barriers to actual PND care. A 2023 analysis reported that 65% of mothers-to-be who screened positive for metal health comorbidities were not referred for treatment.
Dr. Yang and colleagues acknowledged that their findings may not be generalizable to mild forms of these disorders since the data were based on clinical diagnoses and prescriptions.
The study was supported by the Chinese Scholarship Council, the Swedish Research Council for Health, Working Life and Welfare, the Karolinska Institutet, and the Icelandic Research Fund. The authors and Dr. Harlow had no relevant competing interests to disclose.
FROM PLOS MEDICINE
Moral Injury in Health Care: A Unified Definition and its Relationship to Burnout
Moral injury was identified by health care professionals (HCPs) as a driver of occupational distress prior to the COVID-19 pandemic, but the crisis expanded the appeal and investigation of the term.1 HCPs now consider moral injury an essential component of the framework to describe their distress, because using the term burnout alone fails to capture their full experience and has proven resistant to interventions.2 Moral injury goes beyond the transdiagnostic symptoms of exhaustion and cynicism and beyond operational, demand-resource mismatches that characterize burnout. It describes the frustration, anger, and helplessness associated with relational ruptures and the existential threats to a clinician’s professional identity as business interests erode their ability to put their patients’ needs ahead of corporate and health care system obligations.3
Proper characterization of moral injury in health care—separate from the military environments where it originated—is stymied by an ill-defined relationship between 2 definitions of the term and by an unclear relationship between moral injury and the long-standing body of scholarship in burnout. To clarify the concept, inform research agendas, and open avenues for more effective solutions to the crisis of HCP distress, we propose a unified conceptualization of moral injury and its association with burnout in health care.
CONTEXTUAL DISTINCTIONS
It is important to properly distinguish between the original use of moral injury in the military and its expanded use in civilian circumstances. Health care and the military are both professions whereupon donning the “uniform” of a physician—or soldier, sailor, airman, or marine—members must comport with strict expectations of behavior, including the refusal to engage in illegal actions or those contrary to professional ethics. Individuals in both professions acquire a highly specialized body of knowledge and enter an implied contract to provide critical services to society, specifically healing and protection, respectively. Members of both professions are trained to make complex judgments with integrity under conditions of technical and ethical uncertainty, upon which they take highly skilled action. Medical and military professionals must be free to act on their ethical principles, without confounding demands.4 However, the context of each profession’s commitment to society carries different moral implications.
The risk of moral injury is inherent in military service. The military promises protection with an implicit acknowledgment of the need to use lethal force to uphold the agreement. In contrast, HCPs promise healing and care. The military promises to protect our society, with an implicit acknowledgment of the need to use lethal force to uphold the agreement. Some military actions may inflict harm without the hope of benefitting an individual, and are therefore potentially morally injurious. The health care contract with society, promising healing and care, is devoid of inherent moral injury due to harm without potential individual benefit. Therefore, the presence of moral injury in health care settings are warning signs of a dysfunctional environment.
One complex example of the dysfunctional environments is illustrative. The military and health care are among the few industries where supply creates demand. For example, the more bad state actors there are, the more demand for the military. As we have seen since the 1950s, the more technology and therapeutics we create in health care, coupled with a larger share paid for by third parties, the greater the demand for and use of them.5 In a fee for service environment, corporate greed feeds on this reality. In most other environments, more technological and therapeutic options inevitably pit clinicians against multiple other factions: payers, who do not want to underwrite them; patients, who sometimes demand them without justification or later rail against spiraling health care costs; and administrators, especially in capitated systems, who watch their bottom lines erode. The moral injury risk in this instance demands a collective conversation among stakeholders regarding the structural determinants of health—how we choose to distribute limited resources. The intermediary of moral injury is a useful measure of the harm that results from ignoring or avoiding such challenges.
HARMONIZING DEFINITIONS
Moral injury is inherently nuanced. The 2 dominant definitions arise from work with combat veterans and create additional and perhaps unnecessary complexity. Unifying these 2 definitions eliminates inadvertent confusion, preventing the risk of unbridled interdisciplinary investigation which leads to a lack of precision in the meaning of moral injury and other related concepts, such as burnout.6
The first definition was developed by Jonathan Shay in 1994 and outlines 3 necessarycomponents, viewing the violator as a powerholder: (1) betrayal of what is right, (2) by someone who holds legitimate authority, (3) in a high stakes situation.7 Litz and colleagues describe moral injury another way: “Perpetrating, failing to prevent, bearing witness to, or learning about acts that transgress deeply held moral beliefs and expectations.”8 The violator is posited to be either the self or others.
Rather than representing “self” or “other” imposed moral injury, we propose the 2 definitions are related as exposure (ie, the perceived betrayal) and response (ie, the resulting transgression). An individual who experiences a betrayal by a legitimate authority has an opportunity to choose their response. They may acquiesce and transgress their moral beliefs (eg, their oath to provide ethical health care), or they could refuse, by speaking out, or in some way resisting the authority’s betrayal. The case of Ray Brovont is a useful illustration of reconciling the definitions (Box).9
Myriad factors—known as potentially morally injurious events—drive moral injury, such as resource-constrained decision making, witnessing the behaviors of colleagues that violate deeply held moral beliefs, questionable billing practices, and more. Each begins with a betrayal. Spotlighting the betrayal, refusing to perpetuate it, or taking actions toward change, may reduce the risk of experiencing moral injury.9 Conversely, acquiescing and transgressing one’s oath, the profession’s covenant with society, increases the risk of experiencing moral injury.8
Many HCPs believe they are not always free to resist betrayal, fearing retaliation, job loss, blacklisting, or worse. They feel constrained by debt accrued while receiving their education, being their household’s primary earner, community ties, practicing a niche specialty that requires working for a tertiary referral center, or perhaps believing the situation will be the same elsewhere. To not stand up or speak out is to choose complicity with corporate greed that uses HCPs to undermine their professional duties, which significantly increases the risk of experiencing moral injury.
MORAL INJURY AND BURNOUT
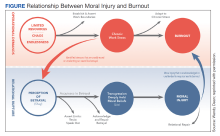
In addition to reconciling the definitions of moral injury, the relationship between moral injury and burnout are still being elucidated. We suggest that moral injury and burnout represent independent and potentially interrelated pathways to distress (Figure). Exposure to chronic, inconsonant, and transactional demands, which things like shorter work hours, better self-care, or improved health system operations might mitigate, manifests as burnout. In contrast, moral injury arises when a superior’s actions or a system’s policies and practices—such as justifiable but unnecessary testing, or referral restrictions to prevent revenue leakage—undermine one’s professional obligations to prioritize the patient’s best interest.
If concerns from HCPs about transactional demands are persistently dismissed, such inaction may be perceived as a betrayal, raising the risk of moral injury. Additionally, the resignation or helplessness of moral injury perceived as inescapable may present with emotional exhaustion, ineffectiveness, and depersonalization, all hallmarks of burnout. Both conditions can mediate and moderate the relationship between triggers for workplace distress and resulting psychological, physical, and existential harm.
CONCLUSIONS
Moral injury is increasingly recognized as a source of distress among HCPs, resulting from structural constraints on their ability to deliver optimal care and their own unwillingness to stand up for their patients, their oaths, and their professions.1 Unlike the military, where moral injury is inherent in the contract with society, moral injury in health care (and the relational rupture it connotes) is a signal of systemic dysfunction, fractured trust, and the need for relational repair.
Health care is at a crossroads, experiencing a workforce retention crisis while simultaneously predicting a significant increase in care needs by Baby Boomers over the next 3 decades.
Health care does not have the luxury of experimenting another 30 years with interventions that have limited impact. We must design a new generation of approaches, shaped by lessons learned from the pandemic while acknowledging that prepandemic standards were already failing the workforce. A unified definition of moral injury must be integrated to frame clinician distress alongside burnout, recentering ethical decision making, rather than profit, at the heart of health care. Harmonizing the definitions of moral injury and clarifying the relationship of moral injury with burnout reduces the need for further reinterpretations, allowing for more robust, easily comparable studies focused on identifying risk factors, as well as rapidly implementing effective mitigation strategies.
1. Griffin BJ, Weber MC, Hinkson KD, et al. Toward a dimensional contextual model of moral injury: a scoping review on healthcare workers. Curr Treat Options Psych. 2023;10:199-216. doi:10.1007/s40501-023-00296-4
2. National Academies of Sciences, Engineering, and Medicine; National Academy of Medicine; Committee on Systems Approaches to Improve Patient Care by Supporting Clinician Well-Being. Taking Action Against Clinician Burnout: A Systems Approach to Professional Well-Being. The National Academies Press; 2019. doi:10.17226/25521
3. Dean W, Talbot S, Dean A. Reframing clinician distress: moral injury not burnout. Fed Pract. 2019;36(9):400-402.
4. Gardner HE, Schulman LS. The professions in America today: crucial but fragile. Daedalus. 2005;134(3):13-18. doi:10.1162/0011526054622132
5. Fuchs VR. Major trends in the U.S. health economy since 1950. N Engl J Med. 2012;366(11):973-977. doi:10.1056/NEJMp1200478
6. Molendijk T. Warnings against romanticising moral injury. Br J Psychiatry. 2022;220(1):1-3. doi:10.1192/bjp.2021.114
7. Shay J. Moral injury. Psychoanalytic Psychol. 2014;31(2):182-191. doi:10.1037/a0036090
8. Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003
9. Brovont v KS-I Med. Servs., P.A., 622 SW3d 671 (Mo Ct App 2020).
Moral injury was identified by health care professionals (HCPs) as a driver of occupational distress prior to the COVID-19 pandemic, but the crisis expanded the appeal and investigation of the term.1 HCPs now consider moral injury an essential component of the framework to describe their distress, because using the term burnout alone fails to capture their full experience and has proven resistant to interventions.2 Moral injury goes beyond the transdiagnostic symptoms of exhaustion and cynicism and beyond operational, demand-resource mismatches that characterize burnout. It describes the frustration, anger, and helplessness associated with relational ruptures and the existential threats to a clinician’s professional identity as business interests erode their ability to put their patients’ needs ahead of corporate and health care system obligations.3
Proper characterization of moral injury in health care—separate from the military environments where it originated—is stymied by an ill-defined relationship between 2 definitions of the term and by an unclear relationship between moral injury and the long-standing body of scholarship in burnout. To clarify the concept, inform research agendas, and open avenues for more effective solutions to the crisis of HCP distress, we propose a unified conceptualization of moral injury and its association with burnout in health care.
CONTEXTUAL DISTINCTIONS
It is important to properly distinguish between the original use of moral injury in the military and its expanded use in civilian circumstances. Health care and the military are both professions whereupon donning the “uniform” of a physician—or soldier, sailor, airman, or marine—members must comport with strict expectations of behavior, including the refusal to engage in illegal actions or those contrary to professional ethics. Individuals in both professions acquire a highly specialized body of knowledge and enter an implied contract to provide critical services to society, specifically healing and protection, respectively. Members of both professions are trained to make complex judgments with integrity under conditions of technical and ethical uncertainty, upon which they take highly skilled action. Medical and military professionals must be free to act on their ethical principles, without confounding demands.4 However, the context of each profession’s commitment to society carries different moral implications.
The risk of moral injury is inherent in military service. The military promises protection with an implicit acknowledgment of the need to use lethal force to uphold the agreement. In contrast, HCPs promise healing and care. The military promises to protect our society, with an implicit acknowledgment of the need to use lethal force to uphold the agreement. Some military actions may inflict harm without the hope of benefitting an individual, and are therefore potentially morally injurious. The health care contract with society, promising healing and care, is devoid of inherent moral injury due to harm without potential individual benefit. Therefore, the presence of moral injury in health care settings are warning signs of a dysfunctional environment.
One complex example of the dysfunctional environments is illustrative. The military and health care are among the few industries where supply creates demand. For example, the more bad state actors there are, the more demand for the military. As we have seen since the 1950s, the more technology and therapeutics we create in health care, coupled with a larger share paid for by third parties, the greater the demand for and use of them.5 In a fee for service environment, corporate greed feeds on this reality. In most other environments, more technological and therapeutic options inevitably pit clinicians against multiple other factions: payers, who do not want to underwrite them; patients, who sometimes demand them without justification or later rail against spiraling health care costs; and administrators, especially in capitated systems, who watch their bottom lines erode. The moral injury risk in this instance demands a collective conversation among stakeholders regarding the structural determinants of health—how we choose to distribute limited resources. The intermediary of moral injury is a useful measure of the harm that results from ignoring or avoiding such challenges.
HARMONIZING DEFINITIONS
Moral injury is inherently nuanced. The 2 dominant definitions arise from work with combat veterans and create additional and perhaps unnecessary complexity. Unifying these 2 definitions eliminates inadvertent confusion, preventing the risk of unbridled interdisciplinary investigation which leads to a lack of precision in the meaning of moral injury and other related concepts, such as burnout.6
The first definition was developed by Jonathan Shay in 1994 and outlines 3 necessarycomponents, viewing the violator as a powerholder: (1) betrayal of what is right, (2) by someone who holds legitimate authority, (3) in a high stakes situation.7 Litz and colleagues describe moral injury another way: “Perpetrating, failing to prevent, bearing witness to, or learning about acts that transgress deeply held moral beliefs and expectations.”8 The violator is posited to be either the self or others.
Rather than representing “self” or “other” imposed moral injury, we propose the 2 definitions are related as exposure (ie, the perceived betrayal) and response (ie, the resulting transgression). An individual who experiences a betrayal by a legitimate authority has an opportunity to choose their response. They may acquiesce and transgress their moral beliefs (eg, their oath to provide ethical health care), or they could refuse, by speaking out, or in some way resisting the authority’s betrayal. The case of Ray Brovont is a useful illustration of reconciling the definitions (Box).9
Myriad factors—known as potentially morally injurious events—drive moral injury, such as resource-constrained decision making, witnessing the behaviors of colleagues that violate deeply held moral beliefs, questionable billing practices, and more. Each begins with a betrayal. Spotlighting the betrayal, refusing to perpetuate it, or taking actions toward change, may reduce the risk of experiencing moral injury.9 Conversely, acquiescing and transgressing one’s oath, the profession’s covenant with society, increases the risk of experiencing moral injury.8
Many HCPs believe they are not always free to resist betrayal, fearing retaliation, job loss, blacklisting, or worse. They feel constrained by debt accrued while receiving their education, being their household’s primary earner, community ties, practicing a niche specialty that requires working for a tertiary referral center, or perhaps believing the situation will be the same elsewhere. To not stand up or speak out is to choose complicity with corporate greed that uses HCPs to undermine their professional duties, which significantly increases the risk of experiencing moral injury.
MORAL INJURY AND BURNOUT
In addition to reconciling the definitions of moral injury, the relationship between moral injury and burnout are still being elucidated. We suggest that moral injury and burnout represent independent and potentially interrelated pathways to distress (Figure). Exposure to chronic, inconsonant, and transactional demands, which things like shorter work hours, better self-care, or improved health system operations might mitigate, manifests as burnout. In contrast, moral injury arises when a superior’s actions or a system’s policies and practices—such as justifiable but unnecessary testing, or referral restrictions to prevent revenue leakage—undermine one’s professional obligations to prioritize the patient’s best interest.
If concerns from HCPs about transactional demands are persistently dismissed, such inaction may be perceived as a betrayal, raising the risk of moral injury. Additionally, the resignation or helplessness of moral injury perceived as inescapable may present with emotional exhaustion, ineffectiveness, and depersonalization, all hallmarks of burnout. Both conditions can mediate and moderate the relationship between triggers for workplace distress and resulting psychological, physical, and existential harm.
CONCLUSIONS
Moral injury is increasingly recognized as a source of distress among HCPs, resulting from structural constraints on their ability to deliver optimal care and their own unwillingness to stand up for their patients, their oaths, and their professions.1 Unlike the military, where moral injury is inherent in the contract with society, moral injury in health care (and the relational rupture it connotes) is a signal of systemic dysfunction, fractured trust, and the need for relational repair.
Health care is at a crossroads, experiencing a workforce retention crisis while simultaneously predicting a significant increase in care needs by Baby Boomers over the next 3 decades.
Health care does not have the luxury of experimenting another 30 years with interventions that have limited impact. We must design a new generation of approaches, shaped by lessons learned from the pandemic while acknowledging that prepandemic standards were already failing the workforce. A unified definition of moral injury must be integrated to frame clinician distress alongside burnout, recentering ethical decision making, rather than profit, at the heart of health care. Harmonizing the definitions of moral injury and clarifying the relationship of moral injury with burnout reduces the need for further reinterpretations, allowing for more robust, easily comparable studies focused on identifying risk factors, as well as rapidly implementing effective mitigation strategies.
Moral injury was identified by health care professionals (HCPs) as a driver of occupational distress prior to the COVID-19 pandemic, but the crisis expanded the appeal and investigation of the term.1 HCPs now consider moral injury an essential component of the framework to describe their distress, because using the term burnout alone fails to capture their full experience and has proven resistant to interventions.2 Moral injury goes beyond the transdiagnostic symptoms of exhaustion and cynicism and beyond operational, demand-resource mismatches that characterize burnout. It describes the frustration, anger, and helplessness associated with relational ruptures and the existential threats to a clinician’s professional identity as business interests erode their ability to put their patients’ needs ahead of corporate and health care system obligations.3
Proper characterization of moral injury in health care—separate from the military environments where it originated—is stymied by an ill-defined relationship between 2 definitions of the term and by an unclear relationship between moral injury and the long-standing body of scholarship in burnout. To clarify the concept, inform research agendas, and open avenues for more effective solutions to the crisis of HCP distress, we propose a unified conceptualization of moral injury and its association with burnout in health care.
CONTEXTUAL DISTINCTIONS
It is important to properly distinguish between the original use of moral injury in the military and its expanded use in civilian circumstances. Health care and the military are both professions whereupon donning the “uniform” of a physician—or soldier, sailor, airman, or marine—members must comport with strict expectations of behavior, including the refusal to engage in illegal actions or those contrary to professional ethics. Individuals in both professions acquire a highly specialized body of knowledge and enter an implied contract to provide critical services to society, specifically healing and protection, respectively. Members of both professions are trained to make complex judgments with integrity under conditions of technical and ethical uncertainty, upon which they take highly skilled action. Medical and military professionals must be free to act on their ethical principles, without confounding demands.4 However, the context of each profession’s commitment to society carries different moral implications.
The risk of moral injury is inherent in military service. The military promises protection with an implicit acknowledgment of the need to use lethal force to uphold the agreement. In contrast, HCPs promise healing and care. The military promises to protect our society, with an implicit acknowledgment of the need to use lethal force to uphold the agreement. Some military actions may inflict harm without the hope of benefitting an individual, and are therefore potentially morally injurious. The health care contract with society, promising healing and care, is devoid of inherent moral injury due to harm without potential individual benefit. Therefore, the presence of moral injury in health care settings are warning signs of a dysfunctional environment.
One complex example of the dysfunctional environments is illustrative. The military and health care are among the few industries where supply creates demand. For example, the more bad state actors there are, the more demand for the military. As we have seen since the 1950s, the more technology and therapeutics we create in health care, coupled with a larger share paid for by third parties, the greater the demand for and use of them.5 In a fee for service environment, corporate greed feeds on this reality. In most other environments, more technological and therapeutic options inevitably pit clinicians against multiple other factions: payers, who do not want to underwrite them; patients, who sometimes demand them without justification or later rail against spiraling health care costs; and administrators, especially in capitated systems, who watch their bottom lines erode. The moral injury risk in this instance demands a collective conversation among stakeholders regarding the structural determinants of health—how we choose to distribute limited resources. The intermediary of moral injury is a useful measure of the harm that results from ignoring or avoiding such challenges.
HARMONIZING DEFINITIONS
Moral injury is inherently nuanced. The 2 dominant definitions arise from work with combat veterans and create additional and perhaps unnecessary complexity. Unifying these 2 definitions eliminates inadvertent confusion, preventing the risk of unbridled interdisciplinary investigation which leads to a lack of precision in the meaning of moral injury and other related concepts, such as burnout.6
The first definition was developed by Jonathan Shay in 1994 and outlines 3 necessarycomponents, viewing the violator as a powerholder: (1) betrayal of what is right, (2) by someone who holds legitimate authority, (3) in a high stakes situation.7 Litz and colleagues describe moral injury another way: “Perpetrating, failing to prevent, bearing witness to, or learning about acts that transgress deeply held moral beliefs and expectations.”8 The violator is posited to be either the self or others.
Rather than representing “self” or “other” imposed moral injury, we propose the 2 definitions are related as exposure (ie, the perceived betrayal) and response (ie, the resulting transgression). An individual who experiences a betrayal by a legitimate authority has an opportunity to choose their response. They may acquiesce and transgress their moral beliefs (eg, their oath to provide ethical health care), or they could refuse, by speaking out, or in some way resisting the authority’s betrayal. The case of Ray Brovont is a useful illustration of reconciling the definitions (Box).9
Myriad factors—known as potentially morally injurious events—drive moral injury, such as resource-constrained decision making, witnessing the behaviors of colleagues that violate deeply held moral beliefs, questionable billing practices, and more. Each begins with a betrayal. Spotlighting the betrayal, refusing to perpetuate it, or taking actions toward change, may reduce the risk of experiencing moral injury.9 Conversely, acquiescing and transgressing one’s oath, the profession’s covenant with society, increases the risk of experiencing moral injury.8
Many HCPs believe they are not always free to resist betrayal, fearing retaliation, job loss, blacklisting, or worse. They feel constrained by debt accrued while receiving their education, being their household’s primary earner, community ties, practicing a niche specialty that requires working for a tertiary referral center, or perhaps believing the situation will be the same elsewhere. To not stand up or speak out is to choose complicity with corporate greed that uses HCPs to undermine their professional duties, which significantly increases the risk of experiencing moral injury.
MORAL INJURY AND BURNOUT
In addition to reconciling the definitions of moral injury, the relationship between moral injury and burnout are still being elucidated. We suggest that moral injury and burnout represent independent and potentially interrelated pathways to distress (Figure). Exposure to chronic, inconsonant, and transactional demands, which things like shorter work hours, better self-care, or improved health system operations might mitigate, manifests as burnout. In contrast, moral injury arises when a superior’s actions or a system’s policies and practices—such as justifiable but unnecessary testing, or referral restrictions to prevent revenue leakage—undermine one’s professional obligations to prioritize the patient’s best interest.
If concerns from HCPs about transactional demands are persistently dismissed, such inaction may be perceived as a betrayal, raising the risk of moral injury. Additionally, the resignation or helplessness of moral injury perceived as inescapable may present with emotional exhaustion, ineffectiveness, and depersonalization, all hallmarks of burnout. Both conditions can mediate and moderate the relationship between triggers for workplace distress and resulting psychological, physical, and existential harm.
CONCLUSIONS
Moral injury is increasingly recognized as a source of distress among HCPs, resulting from structural constraints on their ability to deliver optimal care and their own unwillingness to stand up for their patients, their oaths, and their professions.1 Unlike the military, where moral injury is inherent in the contract with society, moral injury in health care (and the relational rupture it connotes) is a signal of systemic dysfunction, fractured trust, and the need for relational repair.
Health care is at a crossroads, experiencing a workforce retention crisis while simultaneously predicting a significant increase in care needs by Baby Boomers over the next 3 decades.
Health care does not have the luxury of experimenting another 30 years with interventions that have limited impact. We must design a new generation of approaches, shaped by lessons learned from the pandemic while acknowledging that prepandemic standards were already failing the workforce. A unified definition of moral injury must be integrated to frame clinician distress alongside burnout, recentering ethical decision making, rather than profit, at the heart of health care. Harmonizing the definitions of moral injury and clarifying the relationship of moral injury with burnout reduces the need for further reinterpretations, allowing for more robust, easily comparable studies focused on identifying risk factors, as well as rapidly implementing effective mitigation strategies.
1. Griffin BJ, Weber MC, Hinkson KD, et al. Toward a dimensional contextual model of moral injury: a scoping review on healthcare workers. Curr Treat Options Psych. 2023;10:199-216. doi:10.1007/s40501-023-00296-4
2. National Academies of Sciences, Engineering, and Medicine; National Academy of Medicine; Committee on Systems Approaches to Improve Patient Care by Supporting Clinician Well-Being. Taking Action Against Clinician Burnout: A Systems Approach to Professional Well-Being. The National Academies Press; 2019. doi:10.17226/25521
3. Dean W, Talbot S, Dean A. Reframing clinician distress: moral injury not burnout. Fed Pract. 2019;36(9):400-402.
4. Gardner HE, Schulman LS. The professions in America today: crucial but fragile. Daedalus. 2005;134(3):13-18. doi:10.1162/0011526054622132
5. Fuchs VR. Major trends in the U.S. health economy since 1950. N Engl J Med. 2012;366(11):973-977. doi:10.1056/NEJMp1200478
6. Molendijk T. Warnings against romanticising moral injury. Br J Psychiatry. 2022;220(1):1-3. doi:10.1192/bjp.2021.114
7. Shay J. Moral injury. Psychoanalytic Psychol. 2014;31(2):182-191. doi:10.1037/a0036090
8. Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003
9. Brovont v KS-I Med. Servs., P.A., 622 SW3d 671 (Mo Ct App 2020).
1. Griffin BJ, Weber MC, Hinkson KD, et al. Toward a dimensional contextual model of moral injury: a scoping review on healthcare workers. Curr Treat Options Psych. 2023;10:199-216. doi:10.1007/s40501-023-00296-4
2. National Academies of Sciences, Engineering, and Medicine; National Academy of Medicine; Committee on Systems Approaches to Improve Patient Care by Supporting Clinician Well-Being. Taking Action Against Clinician Burnout: A Systems Approach to Professional Well-Being. The National Academies Press; 2019. doi:10.17226/25521
3. Dean W, Talbot S, Dean A. Reframing clinician distress: moral injury not burnout. Fed Pract. 2019;36(9):400-402.
4. Gardner HE, Schulman LS. The professions in America today: crucial but fragile. Daedalus. 2005;134(3):13-18. doi:10.1162/0011526054622132
5. Fuchs VR. Major trends in the U.S. health economy since 1950. N Engl J Med. 2012;366(11):973-977. doi:10.1056/NEJMp1200478
6. Molendijk T. Warnings against romanticising moral injury. Br J Psychiatry. 2022;220(1):1-3. doi:10.1192/bjp.2021.114
7. Shay J. Moral injury. Psychoanalytic Psychol. 2014;31(2):182-191. doi:10.1037/a0036090
8. Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003
9. Brovont v KS-I Med. Servs., P.A., 622 SW3d 671 (Mo Ct App 2020).
Trauma, Racism Linked to Increased Suicide Risk in Black Men
One in three Black men in rural America experienced suicidal or death ideation (SDI) in the past week, new research showed.
A developmental model used in the study showed a direct association between experiences pertaining to threat, deprivation, and racial discrimination during childhood and suicide risk in adulthood, suggesting that a broad range of adverse experiences in early life may affect SDI risk among Black men.
“During the past 20-30 years, young Black men have evinced increasing levels of suicidal behavior and related cognitions,” lead author Steven Kogan, PhD, professor of family and consumer sciences at the University of Georgia, Athens, Georgia, and colleagues wrote.
“By controlling for depressive symptoms in assessing increases in SDI over time, our study’s design directly informed the extent to which social adversities affect SDI independent of other depressive problems,” they added.
The findings were published online in Cultural Diversity and Ethnic Minority Psychology.
Second Leading Cause of Death
Suicide is the second leading cause of death for Black Americans ages 15-24, according to the Centers for Disease Control and Prevention. The outlook is worse for Black men, whose death rate from suicide is about four times greater than for Black women.
Previous research suggests Black men are disproportionately exposed to social adversity, including poverty and discrimination, which may increase the risk for SDI. In addition, racial discrimination has been shown to increase the risks for depression, anxiety, and psychological distress among Black youth and adults.
But little research exists to better understand how these negative experiences affect vulnerability to SDI. The new study tested a model linking adversity during childhood and emerging exposure to racial discrimination to increases in suicidal thoughts.
Researchers analyzed data from 504 participants in the African American Men’s Project, which included a series of surveys completed by young men in rural Georgia at three different time points over a period of about 3 years.
Composite scores for childhood threat and deprivation were developed using the Adverse Childhood Experiences Scale and Childhood Trauma Questionnaire. Everyday discrimination was measured on the Schedule of Racist Events response scale.
To assess their experience with childhood threats, the men in the study, who were about 21 years old on average when they enrolled, were asked if they experienced a series of adverse childhood experiences and deprivation through age 16. Questions explored issues such as directly experiencing physical violence or witnessing abuse in the home and whether the men felt loved and “important or special” as children.
The investigators also asked the men about their experiences of racial discrimination, the quality of their relationships, their belief that aggression is a means of gaining respect, and their cynicism regarding romantic relationships.
Targeted Prevention
Overall, 33.6% of participants reported SDI in the previous week. A history of childhood threats and deprivation was associated with an increased likelihood of SDI (P < .001).
Researchers also found that a history of racial discrimination was significantly associated with the development of negative relational schemas, which are characterized by beliefs that other people are untrustworthy, uncaring, and/or hostile. Negative schemas were in turn associated with an increased risk for suicidal thoughts (P = .03).
“Clinical and preventive interventions for suicidality should target the influence of racism and adverse experiences and the negative relational schemas they induce,” the investigators noted.
“Policy efforts designed to dismantle systemic racism are critically needed. Interventions that address SDI, including programming designed to support Black men through their experiences with racial discrimination and processing of childhood experiences of adversity, may help young Black men resist the psychological impacts of racism, expand their positive support networks, and decrease their risk of SDI,” they added.
The study authors reported no funding sources or relevant financial relationships.
A version of this article appeared on Medscape.com.
One in three Black men in rural America experienced suicidal or death ideation (SDI) in the past week, new research showed.
A developmental model used in the study showed a direct association between experiences pertaining to threat, deprivation, and racial discrimination during childhood and suicide risk in adulthood, suggesting that a broad range of adverse experiences in early life may affect SDI risk among Black men.
“During the past 20-30 years, young Black men have evinced increasing levels of suicidal behavior and related cognitions,” lead author Steven Kogan, PhD, professor of family and consumer sciences at the University of Georgia, Athens, Georgia, and colleagues wrote.
“By controlling for depressive symptoms in assessing increases in SDI over time, our study’s design directly informed the extent to which social adversities affect SDI independent of other depressive problems,” they added.
The findings were published online in Cultural Diversity and Ethnic Minority Psychology.
Second Leading Cause of Death
Suicide is the second leading cause of death for Black Americans ages 15-24, according to the Centers for Disease Control and Prevention. The outlook is worse for Black men, whose death rate from suicide is about four times greater than for Black women.
Previous research suggests Black men are disproportionately exposed to social adversity, including poverty and discrimination, which may increase the risk for SDI. In addition, racial discrimination has been shown to increase the risks for depression, anxiety, and psychological distress among Black youth and adults.
But little research exists to better understand how these negative experiences affect vulnerability to SDI. The new study tested a model linking adversity during childhood and emerging exposure to racial discrimination to increases in suicidal thoughts.
Researchers analyzed data from 504 participants in the African American Men’s Project, which included a series of surveys completed by young men in rural Georgia at three different time points over a period of about 3 years.
Composite scores for childhood threat and deprivation were developed using the Adverse Childhood Experiences Scale and Childhood Trauma Questionnaire. Everyday discrimination was measured on the Schedule of Racist Events response scale.
To assess their experience with childhood threats, the men in the study, who were about 21 years old on average when they enrolled, were asked if they experienced a series of adverse childhood experiences and deprivation through age 16. Questions explored issues such as directly experiencing physical violence or witnessing abuse in the home and whether the men felt loved and “important or special” as children.
The investigators also asked the men about their experiences of racial discrimination, the quality of their relationships, their belief that aggression is a means of gaining respect, and their cynicism regarding romantic relationships.
Targeted Prevention
Overall, 33.6% of participants reported SDI in the previous week. A history of childhood threats and deprivation was associated with an increased likelihood of SDI (P < .001).
Researchers also found that a history of racial discrimination was significantly associated with the development of negative relational schemas, which are characterized by beliefs that other people are untrustworthy, uncaring, and/or hostile. Negative schemas were in turn associated with an increased risk for suicidal thoughts (P = .03).
“Clinical and preventive interventions for suicidality should target the influence of racism and adverse experiences and the negative relational schemas they induce,” the investigators noted.
“Policy efforts designed to dismantle systemic racism are critically needed. Interventions that address SDI, including programming designed to support Black men through their experiences with racial discrimination and processing of childhood experiences of adversity, may help young Black men resist the psychological impacts of racism, expand their positive support networks, and decrease their risk of SDI,” they added.
The study authors reported no funding sources or relevant financial relationships.
A version of this article appeared on Medscape.com.
One in three Black men in rural America experienced suicidal or death ideation (SDI) in the past week, new research showed.
A developmental model used in the study showed a direct association between experiences pertaining to threat, deprivation, and racial discrimination during childhood and suicide risk in adulthood, suggesting that a broad range of adverse experiences in early life may affect SDI risk among Black men.
“During the past 20-30 years, young Black men have evinced increasing levels of suicidal behavior and related cognitions,” lead author Steven Kogan, PhD, professor of family and consumer sciences at the University of Georgia, Athens, Georgia, and colleagues wrote.
“By controlling for depressive symptoms in assessing increases in SDI over time, our study’s design directly informed the extent to which social adversities affect SDI independent of other depressive problems,” they added.
The findings were published online in Cultural Diversity and Ethnic Minority Psychology.
Second Leading Cause of Death
Suicide is the second leading cause of death for Black Americans ages 15-24, according to the Centers for Disease Control and Prevention. The outlook is worse for Black men, whose death rate from suicide is about four times greater than for Black women.
Previous research suggests Black men are disproportionately exposed to social adversity, including poverty and discrimination, which may increase the risk for SDI. In addition, racial discrimination has been shown to increase the risks for depression, anxiety, and psychological distress among Black youth and adults.
But little research exists to better understand how these negative experiences affect vulnerability to SDI. The new study tested a model linking adversity during childhood and emerging exposure to racial discrimination to increases in suicidal thoughts.
Researchers analyzed data from 504 participants in the African American Men’s Project, which included a series of surveys completed by young men in rural Georgia at three different time points over a period of about 3 years.
Composite scores for childhood threat and deprivation were developed using the Adverse Childhood Experiences Scale and Childhood Trauma Questionnaire. Everyday discrimination was measured on the Schedule of Racist Events response scale.
To assess their experience with childhood threats, the men in the study, who were about 21 years old on average when they enrolled, were asked if they experienced a series of adverse childhood experiences and deprivation through age 16. Questions explored issues such as directly experiencing physical violence or witnessing abuse in the home and whether the men felt loved and “important or special” as children.
The investigators also asked the men about their experiences of racial discrimination, the quality of their relationships, their belief that aggression is a means of gaining respect, and their cynicism regarding romantic relationships.
Targeted Prevention
Overall, 33.6% of participants reported SDI in the previous week. A history of childhood threats and deprivation was associated with an increased likelihood of SDI (P < .001).
Researchers also found that a history of racial discrimination was significantly associated with the development of negative relational schemas, which are characterized by beliefs that other people are untrustworthy, uncaring, and/or hostile. Negative schemas were in turn associated with an increased risk for suicidal thoughts (P = .03).
“Clinical and preventive interventions for suicidality should target the influence of racism and adverse experiences and the negative relational schemas they induce,” the investigators noted.
“Policy efforts designed to dismantle systemic racism are critically needed. Interventions that address SDI, including programming designed to support Black men through their experiences with racial discrimination and processing of childhood experiences of adversity, may help young Black men resist the psychological impacts of racism, expand their positive support networks, and decrease their risk of SDI,” they added.
The study authors reported no funding sources or relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM CULTURAL DIVERSITY AND ETHNIC MINORITY PSYCHOLOGY
Will Your Next Prescription Be 20 Minutes of Nature a Day?
What if a walk in a green environment could reshape brains, recalibrate sense of time, and stave off mental health conditions? If the research trends are true, you might soon find yourself writing prescriptions of 20 minutes of nature per day.
In the wake of the pandemic, the British government allocated more than £5 million to pandemic recovery efforts that specifically involved green spaces. Since then, it has committed even more funding toward an expansive social prescribing program that connects patients to “link workers” who determine personal care needs and facilitate community and volunteer-based interventions. These can include group walking and volunteering to help out in community gardens or conservation efforts. Similar green programs can be found in Japan, where shinrin-yoku (forest bathing) was recently adopted as a national health strategy, and in the United States and Canada.
“Disconnection from nature is a major part of the health problems that we have on this planet,” said William Bird, a UK-based general practitioner, green prescriber, and CEO of Intelligent Health, which is geared toward building healthy, active, and connected communities. Dr. Bird received the prestigious Member of the Order of the British Empire (MBE) in 2010 for services related to physical activity and health.
“Our brains are designed to connect to nature ... and we haven’t lost that instinct,” he explained. “Once we are with birdsong and water flowing and greenery, cortisol levels drop, our central vagus nerve improves, our fight and flight [response] disappears, and we start to be more receptive to other people.”
Shifting Time Perception and Health
Ricardo A. Correia, PhD, a biologist and researcher at the University of Helsinki in Finland, said he believed that the mechanism for at least some of these outcomes might be differences in how time is perceived. In a perspective that appeared in March in People and Nature, Dr. Correia explored how the “services” that nature provides shift time perceptions and, in turn, regulate overall well-being.
“I reached the realization that there was some evidence for the shift in some of the dimensions that we use to make sense of time in urban vs natural environments,” he told this news organization.
Dr. Correia explained that human time perception facilitates understanding cause and effect, so we can act in a way that allows us to survive.
“Time perception in humans is really complex and multifaceted,” he said. “The way that we make sense of time is not directly attached to any sensory organ, but rather goes through a range of cognitive, emotional, and bodily processes, all of which vary from person to person.”
Dr. Correia pointed to evidence showing that time perception is shorter in urban environments and longer in natural ones. This, in turn, influences attention and attention restoration. “When we live in cities, we are exposed to similar sorts of demanding environments, increased time pressures, less time for oneself and for recreational purposes,” he said. “Ever-mounting pressure on daily demands plus processes we use to make sense of time, especially attention, means that we pay a cognitive toll.”
Dr. Correia posits that it might be possible to recalibrate time perception, but only by breaking the cycle of exposure.
“If we are always exposed to fast-paced lifestyles, we become attuned to them and get caught up in an endless loop.” This cycle can be broken, Dr. Correia explained, by increasing exposure to natural environments. This leads to positive emotions, a sense of being in the present, and a heightened sense of mindfulness, all of which help mitigate the physical and mental health outcomes commonly associated with time scarcity.
Brain-Mental Health Benefits
To date, there is quite a bit of research exploring the impacts of exposure to nature on the brain. For example, data have shown that adolescents raised exclusively in rural environments have a larger hippocampus and better spatial processing than children exclusively raised in cities. Other research demonstrated that spending just an hour in the forest led to a decline in amygdala activity in adults, whereas it remained stable after walking in an urban setting, underscoring the salutogenic effects on brain regions related to stress. There is also evidence from a 10-year longitudinal study of more than 2 million Welsh adults that highlights the value of proximity to green or blue (eg, lakes and rivers) spaces and common mental health conditions, with every additional 360 meters to the nearest green or blue space associated with 10% greater odds of anxiety and depression.
Dr. Bird said there has been a massive sea change in attitudes among general practitioners, who have come around to embracing the concept of nature as medicine. This shift among peers, who teased him in the 1990s about his green walking and conservation prescriptions, portends a bandwagon of epic proportions that could benefit patients. He said that he was especially hopeful that green prescriptions will become mainstream in certain conditions, especially those like depression and anxiety that are resistant to medication.
But Dr. Bird cautions that primary care professionals need to be mindful. “Patients need to know that it’s real science, otherwise they’ll think that they’re being pawned off or dismissed,” he said. “I try to put real evidence behind it and explain that there’s no contraindication. The main thing is to start where patients are, what they’re feeling, and what they need. Some people just don’t like nature,” he said.
A version of this article appeared on Medscape.com.
What if a walk in a green environment could reshape brains, recalibrate sense of time, and stave off mental health conditions? If the research trends are true, you might soon find yourself writing prescriptions of 20 minutes of nature per day.
In the wake of the pandemic, the British government allocated more than £5 million to pandemic recovery efforts that specifically involved green spaces. Since then, it has committed even more funding toward an expansive social prescribing program that connects patients to “link workers” who determine personal care needs and facilitate community and volunteer-based interventions. These can include group walking and volunteering to help out in community gardens or conservation efforts. Similar green programs can be found in Japan, where shinrin-yoku (forest bathing) was recently adopted as a national health strategy, and in the United States and Canada.
“Disconnection from nature is a major part of the health problems that we have on this planet,” said William Bird, a UK-based general practitioner, green prescriber, and CEO of Intelligent Health, which is geared toward building healthy, active, and connected communities. Dr. Bird received the prestigious Member of the Order of the British Empire (MBE) in 2010 for services related to physical activity and health.
“Our brains are designed to connect to nature ... and we haven’t lost that instinct,” he explained. “Once we are with birdsong and water flowing and greenery, cortisol levels drop, our central vagus nerve improves, our fight and flight [response] disappears, and we start to be more receptive to other people.”
Shifting Time Perception and Health
Ricardo A. Correia, PhD, a biologist and researcher at the University of Helsinki in Finland, said he believed that the mechanism for at least some of these outcomes might be differences in how time is perceived. In a perspective that appeared in March in People and Nature, Dr. Correia explored how the “services” that nature provides shift time perceptions and, in turn, regulate overall well-being.
“I reached the realization that there was some evidence for the shift in some of the dimensions that we use to make sense of time in urban vs natural environments,” he told this news organization.
Dr. Correia explained that human time perception facilitates understanding cause and effect, so we can act in a way that allows us to survive.
“Time perception in humans is really complex and multifaceted,” he said. “The way that we make sense of time is not directly attached to any sensory organ, but rather goes through a range of cognitive, emotional, and bodily processes, all of which vary from person to person.”
Dr. Correia pointed to evidence showing that time perception is shorter in urban environments and longer in natural ones. This, in turn, influences attention and attention restoration. “When we live in cities, we are exposed to similar sorts of demanding environments, increased time pressures, less time for oneself and for recreational purposes,” he said. “Ever-mounting pressure on daily demands plus processes we use to make sense of time, especially attention, means that we pay a cognitive toll.”
Dr. Correia posits that it might be possible to recalibrate time perception, but only by breaking the cycle of exposure.
“If we are always exposed to fast-paced lifestyles, we become attuned to them and get caught up in an endless loop.” This cycle can be broken, Dr. Correia explained, by increasing exposure to natural environments. This leads to positive emotions, a sense of being in the present, and a heightened sense of mindfulness, all of which help mitigate the physical and mental health outcomes commonly associated with time scarcity.
Brain-Mental Health Benefits
To date, there is quite a bit of research exploring the impacts of exposure to nature on the brain. For example, data have shown that adolescents raised exclusively in rural environments have a larger hippocampus and better spatial processing than children exclusively raised in cities. Other research demonstrated that spending just an hour in the forest led to a decline in amygdala activity in adults, whereas it remained stable after walking in an urban setting, underscoring the salutogenic effects on brain regions related to stress. There is also evidence from a 10-year longitudinal study of more than 2 million Welsh adults that highlights the value of proximity to green or blue (eg, lakes and rivers) spaces and common mental health conditions, with every additional 360 meters to the nearest green or blue space associated with 10% greater odds of anxiety and depression.
Dr. Bird said there has been a massive sea change in attitudes among general practitioners, who have come around to embracing the concept of nature as medicine. This shift among peers, who teased him in the 1990s about his green walking and conservation prescriptions, portends a bandwagon of epic proportions that could benefit patients. He said that he was especially hopeful that green prescriptions will become mainstream in certain conditions, especially those like depression and anxiety that are resistant to medication.
But Dr. Bird cautions that primary care professionals need to be mindful. “Patients need to know that it’s real science, otherwise they’ll think that they’re being pawned off or dismissed,” he said. “I try to put real evidence behind it and explain that there’s no contraindication. The main thing is to start where patients are, what they’re feeling, and what they need. Some people just don’t like nature,” he said.
A version of this article appeared on Medscape.com.
What if a walk in a green environment could reshape brains, recalibrate sense of time, and stave off mental health conditions? If the research trends are true, you might soon find yourself writing prescriptions of 20 minutes of nature per day.
In the wake of the pandemic, the British government allocated more than £5 million to pandemic recovery efforts that specifically involved green spaces. Since then, it has committed even more funding toward an expansive social prescribing program that connects patients to “link workers” who determine personal care needs and facilitate community and volunteer-based interventions. These can include group walking and volunteering to help out in community gardens or conservation efforts. Similar green programs can be found in Japan, where shinrin-yoku (forest bathing) was recently adopted as a national health strategy, and in the United States and Canada.
“Disconnection from nature is a major part of the health problems that we have on this planet,” said William Bird, a UK-based general practitioner, green prescriber, and CEO of Intelligent Health, which is geared toward building healthy, active, and connected communities. Dr. Bird received the prestigious Member of the Order of the British Empire (MBE) in 2010 for services related to physical activity and health.
“Our brains are designed to connect to nature ... and we haven’t lost that instinct,” he explained. “Once we are with birdsong and water flowing and greenery, cortisol levels drop, our central vagus nerve improves, our fight and flight [response] disappears, and we start to be more receptive to other people.”
Shifting Time Perception and Health
Ricardo A. Correia, PhD, a biologist and researcher at the University of Helsinki in Finland, said he believed that the mechanism for at least some of these outcomes might be differences in how time is perceived. In a perspective that appeared in March in People and Nature, Dr. Correia explored how the “services” that nature provides shift time perceptions and, in turn, regulate overall well-being.
“I reached the realization that there was some evidence for the shift in some of the dimensions that we use to make sense of time in urban vs natural environments,” he told this news organization.
Dr. Correia explained that human time perception facilitates understanding cause and effect, so we can act in a way that allows us to survive.
“Time perception in humans is really complex and multifaceted,” he said. “The way that we make sense of time is not directly attached to any sensory organ, but rather goes through a range of cognitive, emotional, and bodily processes, all of which vary from person to person.”
Dr. Correia pointed to evidence showing that time perception is shorter in urban environments and longer in natural ones. This, in turn, influences attention and attention restoration. “When we live in cities, we are exposed to similar sorts of demanding environments, increased time pressures, less time for oneself and for recreational purposes,” he said. “Ever-mounting pressure on daily demands plus processes we use to make sense of time, especially attention, means that we pay a cognitive toll.”
Dr. Correia posits that it might be possible to recalibrate time perception, but only by breaking the cycle of exposure.
“If we are always exposed to fast-paced lifestyles, we become attuned to them and get caught up in an endless loop.” This cycle can be broken, Dr. Correia explained, by increasing exposure to natural environments. This leads to positive emotions, a sense of being in the present, and a heightened sense of mindfulness, all of which help mitigate the physical and mental health outcomes commonly associated with time scarcity.
Brain-Mental Health Benefits
To date, there is quite a bit of research exploring the impacts of exposure to nature on the brain. For example, data have shown that adolescents raised exclusively in rural environments have a larger hippocampus and better spatial processing than children exclusively raised in cities. Other research demonstrated that spending just an hour in the forest led to a decline in amygdala activity in adults, whereas it remained stable after walking in an urban setting, underscoring the salutogenic effects on brain regions related to stress. There is also evidence from a 10-year longitudinal study of more than 2 million Welsh adults that highlights the value of proximity to green or blue (eg, lakes and rivers) spaces and common mental health conditions, with every additional 360 meters to the nearest green or blue space associated with 10% greater odds of anxiety and depression.
Dr. Bird said there has been a massive sea change in attitudes among general practitioners, who have come around to embracing the concept of nature as medicine. This shift among peers, who teased him in the 1990s about his green walking and conservation prescriptions, portends a bandwagon of epic proportions that could benefit patients. He said that he was especially hopeful that green prescriptions will become mainstream in certain conditions, especially those like depression and anxiety that are resistant to medication.
But Dr. Bird cautions that primary care professionals need to be mindful. “Patients need to know that it’s real science, otherwise they’ll think that they’re being pawned off or dismissed,” he said. “I try to put real evidence behind it and explain that there’s no contraindication. The main thing is to start where patients are, what they’re feeling, and what they need. Some people just don’t like nature,” he said.
A version of this article appeared on Medscape.com.
Is Measuring How Many Times Patients Get Screened for Depression Really a Reflection of Good Clinical Care?
Every time a patient visits Jason Connelly, MD, they must fill out a depression screening, thanks to a 2017 rule which mandates such assessments.
Providing a screening and, if needed, a follow-up plan means a patient may gain access to medication or cognitive-behavioral therapy that will improve their lives. But Dr. Connelly, a family medicine physician at Novant Health West Rowan Family Medicine in Cleveland, North Carolina, said the screening measure — and others like it that insurers and quality groups use to assess clinician performance — does not allow for enough flexibility.
For instance, he must follow-up with patients every 4 months, regardless of the severity of their depression.
“A lot of times when these are written for the purpose of measures, they don’t take into consideration the reality of clinical medicine,” Dr. Connelly, who is also a clinical physician executive with Novant, said. “There certainly needs to be room for the ability to specify the level of depression such that if it is mild, well, maybe that follow-up is at 6 months or 12 months or at patient discretion.”
A recent report from the American College of Physicians (ACP) supported Dr. Connelly’s view. The body looked at eight quality measures in primary care for patients with major depressive disorder (MDD) and found only one — a risk assessment for suicide — to be clinically meaningful and based on evidence.
The ACP panel said nearly all of the performance measures “lacked current clinical evidence, did not consider patient preferences, were not tested appropriately, or were outside a physician’s control.”
The group called for improvements in such assessments “to accurately assess the quality of clinical care” for patients with major depression.
Necessary Evil or Burdensome Time Suck?
The Centers for Medicare & Medicaid Services scores clinicians and health systems on the percentage of their patients who receive a screening during a visit; if the screening is positive, clinicians must document a follow-up plan using special manual entry codes.
Physicians say the process of meeting government standards for invalid measures can create unnecessary visits and physician paperwork, shrink monetary bonuses, and may not portray an accurate portrait of what best practice looks like in primary care for mental health. But many also said the program overall brings value to patients and provides a picture of how well they are practicing but only when measures are clinically relevant.
Standards ACP Used for Validating Depression Measurement
A committee with ACP used a modified appropriateness method from RAND and UCLA.
They weighed if a metric was evidence-based, methodologically sound, and clinically meaningful.
They rated each measure using a 9-point scale, including appropriate care, feasibility or applicability, and measure specifications.
A total of 11 committee members voted anonymously if each metric was a valid way of measuring individual clinicians, at the practice/system level, and health plan.
“There’s been such a flood of performance measurements that we can get sidetracked, diverted, and spend resources and effort on measurements that don’t improve care,” said Nick Fitterman, MD, chair of the ACP’s Performance Measurement Committee.
Primary care clinicians can choose from more than 60 metrics for 2024. Many involve caring for patients with mental illness or screening for those who could be underdiagnosed. Programs that certify health systems as providing quality care use the measures, in addition to the Merit-Based Incentive Payment System. Health systems choose six measures of quality to tie to their reimbursement — along with assessments of costs and use of technology.
In turn, Medicare adjusts its reimbursement based on how well a clinician’s numbers turn out and if they improved over time.
“You don’t get the benefit of the upside if you don’t meet the measure, so your payment is neutral and that can be significant from a broader system lens,” Dr. Connelly said. “Then you start to have to make decisions on what services do we then have to limit because we no longer have the financial capability.”
The implications for health systems and patient care are the reason ACP and clinicians are calling for some measures to be amended. Dr. Fitterman said his organization plans to work with CMS.
Implementing Measurement
At Bassett Health in New York, the health system uses the depression and follow-up plan measure to qualify for certification from the Health Resources and Services Administration as a patient-centered medical home, which the company uses in part to market itself to patients.
Amy Grace, MD, an attending physician in internal and family medicine at Bassett Health in Little Falls, New York, said if a patient refuses to take a depression screening, she will not meet the measure for that visit. But providing a screening is not always clinically appropriate, and some patients do not need a follow-up plan.
“If someone has just had a death in the family, they might answer the questions in a way that would be consistent with depression, but they’re experiencing grief as opposed to clinical depression,” Dr. Grace said.
Suggestions From ACP for Improvement in MDD Metrics
- Create and implement criteria for patients who do not need a follow-up plan based on clinician judgment.
- Add methods for clinicians to measure and indicate severity of MDD.
- Enable use of a wider array of evidence-based tools and screenings to screen for MDD.
- Allow clinicians to document changes in treatment plan.
Bassett is building into the electronic health record a button that documents the screening was not conducted and that it was not appropriate to administer that day. Of course, building these in-house options entails utilizing resources that smaller systems or independent groups of clinicians may lack.
Eric Wei, MD, senior vice president and chief quality officer at NYC Health + Hospitals in New York City, said the ACP report underscores that many measures, even beyond depression, must be improved.
“With burnout and cognitive overload of our providers, on top of the medicine and just trying to come to the right diagnosis and providing the right treatment and the best care experience, you have to remember all these quality metrics and make sure you put all these things in certain places in the electronic health record,” Dr. Wei said.
Still, Dr. Wei said that the annual rate of depression screening across 400,000 patients in his system is 91%. He and his team spent 6 years working to improve uptake among clinicians, and now, they have moved on to increasing rates of administration of the suicide assessment.
Each clinician uses a dashboard to track their individual metric performance, according to Ted Long, MD, senior vice president for ambulatory care and population health at NYC Health + Hospitals. Dr. Long said he is proud of the improvements he and his colleagues have made in catching undiagnosed depression and in other disease states.
At his primary care practice in the Bronx, nearly 9 out of 10 patients with hypertension have their condition under control, he said. How does he know? Measurement tracking.
“Knowing that when a new patient is in front of me with high blood pressure, that there’s a 9 out of 10 chance that after seeing me because of my clinic, not just because of me, I’m going to be able to keep them healthy by controlling their blood pressure, that’s very meaningful to me,” Dr. Long said. “I think that’s the other side: It enables me as a doctor to know that I’m delivering the highest quality of care to my patients.”
Takeaways for Depression Screening and Follow-Up in Clinical Settings
Just because a patient scores positive for the depression screener, a clinician should dig deeper before making a diagnosis.
Patients have the right to refuse a screener and their wishes should be respected.
Providing a screener may not be appropriate at every visit, such as for a patient with a sprained ankle or a potential respiratory infection where time is limited.
Clinicians can clarify within the measure that the patient did not have mental capacity on that visit to fill out the screener.
A version of this article appeared on Medscape.com.
Every time a patient visits Jason Connelly, MD, they must fill out a depression screening, thanks to a 2017 rule which mandates such assessments.
Providing a screening and, if needed, a follow-up plan means a patient may gain access to medication or cognitive-behavioral therapy that will improve their lives. But Dr. Connelly, a family medicine physician at Novant Health West Rowan Family Medicine in Cleveland, North Carolina, said the screening measure — and others like it that insurers and quality groups use to assess clinician performance — does not allow for enough flexibility.
For instance, he must follow-up with patients every 4 months, regardless of the severity of their depression.
“A lot of times when these are written for the purpose of measures, they don’t take into consideration the reality of clinical medicine,” Dr. Connelly, who is also a clinical physician executive with Novant, said. “There certainly needs to be room for the ability to specify the level of depression such that if it is mild, well, maybe that follow-up is at 6 months or 12 months or at patient discretion.”
A recent report from the American College of Physicians (ACP) supported Dr. Connelly’s view. The body looked at eight quality measures in primary care for patients with major depressive disorder (MDD) and found only one — a risk assessment for suicide — to be clinically meaningful and based on evidence.
The ACP panel said nearly all of the performance measures “lacked current clinical evidence, did not consider patient preferences, were not tested appropriately, or were outside a physician’s control.”
The group called for improvements in such assessments “to accurately assess the quality of clinical care” for patients with major depression.
Necessary Evil or Burdensome Time Suck?
The Centers for Medicare & Medicaid Services scores clinicians and health systems on the percentage of their patients who receive a screening during a visit; if the screening is positive, clinicians must document a follow-up plan using special manual entry codes.
Physicians say the process of meeting government standards for invalid measures can create unnecessary visits and physician paperwork, shrink monetary bonuses, and may not portray an accurate portrait of what best practice looks like in primary care for mental health. But many also said the program overall brings value to patients and provides a picture of how well they are practicing but only when measures are clinically relevant.
Standards ACP Used for Validating Depression Measurement
A committee with ACP used a modified appropriateness method from RAND and UCLA.
They weighed if a metric was evidence-based, methodologically sound, and clinically meaningful.
They rated each measure using a 9-point scale, including appropriate care, feasibility or applicability, and measure specifications.
A total of 11 committee members voted anonymously if each metric was a valid way of measuring individual clinicians, at the practice/system level, and health plan.
“There’s been such a flood of performance measurements that we can get sidetracked, diverted, and spend resources and effort on measurements that don’t improve care,” said Nick Fitterman, MD, chair of the ACP’s Performance Measurement Committee.
Primary care clinicians can choose from more than 60 metrics for 2024. Many involve caring for patients with mental illness or screening for those who could be underdiagnosed. Programs that certify health systems as providing quality care use the measures, in addition to the Merit-Based Incentive Payment System. Health systems choose six measures of quality to tie to their reimbursement — along with assessments of costs and use of technology.
In turn, Medicare adjusts its reimbursement based on how well a clinician’s numbers turn out and if they improved over time.
“You don’t get the benefit of the upside if you don’t meet the measure, so your payment is neutral and that can be significant from a broader system lens,” Dr. Connelly said. “Then you start to have to make decisions on what services do we then have to limit because we no longer have the financial capability.”
The implications for health systems and patient care are the reason ACP and clinicians are calling for some measures to be amended. Dr. Fitterman said his organization plans to work with CMS.
Implementing Measurement
At Bassett Health in New York, the health system uses the depression and follow-up plan measure to qualify for certification from the Health Resources and Services Administration as a patient-centered medical home, which the company uses in part to market itself to patients.
Amy Grace, MD, an attending physician in internal and family medicine at Bassett Health in Little Falls, New York, said if a patient refuses to take a depression screening, she will not meet the measure for that visit. But providing a screening is not always clinically appropriate, and some patients do not need a follow-up plan.
“If someone has just had a death in the family, they might answer the questions in a way that would be consistent with depression, but they’re experiencing grief as opposed to clinical depression,” Dr. Grace said.
Suggestions From ACP for Improvement in MDD Metrics
- Create and implement criteria for patients who do not need a follow-up plan based on clinician judgment.
- Add methods for clinicians to measure and indicate severity of MDD.
- Enable use of a wider array of evidence-based tools and screenings to screen for MDD.
- Allow clinicians to document changes in treatment plan.
Bassett is building into the electronic health record a button that documents the screening was not conducted and that it was not appropriate to administer that day. Of course, building these in-house options entails utilizing resources that smaller systems or independent groups of clinicians may lack.
Eric Wei, MD, senior vice president and chief quality officer at NYC Health + Hospitals in New York City, said the ACP report underscores that many measures, even beyond depression, must be improved.
“With burnout and cognitive overload of our providers, on top of the medicine and just trying to come to the right diagnosis and providing the right treatment and the best care experience, you have to remember all these quality metrics and make sure you put all these things in certain places in the electronic health record,” Dr. Wei said.
Still, Dr. Wei said that the annual rate of depression screening across 400,000 patients in his system is 91%. He and his team spent 6 years working to improve uptake among clinicians, and now, they have moved on to increasing rates of administration of the suicide assessment.
Each clinician uses a dashboard to track their individual metric performance, according to Ted Long, MD, senior vice president for ambulatory care and population health at NYC Health + Hospitals. Dr. Long said he is proud of the improvements he and his colleagues have made in catching undiagnosed depression and in other disease states.
At his primary care practice in the Bronx, nearly 9 out of 10 patients with hypertension have their condition under control, he said. How does he know? Measurement tracking.
“Knowing that when a new patient is in front of me with high blood pressure, that there’s a 9 out of 10 chance that after seeing me because of my clinic, not just because of me, I’m going to be able to keep them healthy by controlling their blood pressure, that’s very meaningful to me,” Dr. Long said. “I think that’s the other side: It enables me as a doctor to know that I’m delivering the highest quality of care to my patients.”
Takeaways for Depression Screening and Follow-Up in Clinical Settings
Just because a patient scores positive for the depression screener, a clinician should dig deeper before making a diagnosis.
Patients have the right to refuse a screener and their wishes should be respected.
Providing a screener may not be appropriate at every visit, such as for a patient with a sprained ankle or a potential respiratory infection where time is limited.
Clinicians can clarify within the measure that the patient did not have mental capacity on that visit to fill out the screener.
A version of this article appeared on Medscape.com.
Every time a patient visits Jason Connelly, MD, they must fill out a depression screening, thanks to a 2017 rule which mandates such assessments.
Providing a screening and, if needed, a follow-up plan means a patient may gain access to medication or cognitive-behavioral therapy that will improve their lives. But Dr. Connelly, a family medicine physician at Novant Health West Rowan Family Medicine in Cleveland, North Carolina, said the screening measure — and others like it that insurers and quality groups use to assess clinician performance — does not allow for enough flexibility.
For instance, he must follow-up with patients every 4 months, regardless of the severity of their depression.
“A lot of times when these are written for the purpose of measures, they don’t take into consideration the reality of clinical medicine,” Dr. Connelly, who is also a clinical physician executive with Novant, said. “There certainly needs to be room for the ability to specify the level of depression such that if it is mild, well, maybe that follow-up is at 6 months or 12 months or at patient discretion.”
A recent report from the American College of Physicians (ACP) supported Dr. Connelly’s view. The body looked at eight quality measures in primary care for patients with major depressive disorder (MDD) and found only one — a risk assessment for suicide — to be clinically meaningful and based on evidence.
The ACP panel said nearly all of the performance measures “lacked current clinical evidence, did not consider patient preferences, were not tested appropriately, or were outside a physician’s control.”
The group called for improvements in such assessments “to accurately assess the quality of clinical care” for patients with major depression.
Necessary Evil or Burdensome Time Suck?
The Centers for Medicare & Medicaid Services scores clinicians and health systems on the percentage of their patients who receive a screening during a visit; if the screening is positive, clinicians must document a follow-up plan using special manual entry codes.
Physicians say the process of meeting government standards for invalid measures can create unnecessary visits and physician paperwork, shrink monetary bonuses, and may not portray an accurate portrait of what best practice looks like in primary care for mental health. But many also said the program overall brings value to patients and provides a picture of how well they are practicing but only when measures are clinically relevant.
Standards ACP Used for Validating Depression Measurement
A committee with ACP used a modified appropriateness method from RAND and UCLA.
They weighed if a metric was evidence-based, methodologically sound, and clinically meaningful.
They rated each measure using a 9-point scale, including appropriate care, feasibility or applicability, and measure specifications.
A total of 11 committee members voted anonymously if each metric was a valid way of measuring individual clinicians, at the practice/system level, and health plan.
“There’s been such a flood of performance measurements that we can get sidetracked, diverted, and spend resources and effort on measurements that don’t improve care,” said Nick Fitterman, MD, chair of the ACP’s Performance Measurement Committee.
Primary care clinicians can choose from more than 60 metrics for 2024. Many involve caring for patients with mental illness or screening for those who could be underdiagnosed. Programs that certify health systems as providing quality care use the measures, in addition to the Merit-Based Incentive Payment System. Health systems choose six measures of quality to tie to their reimbursement — along with assessments of costs and use of technology.
In turn, Medicare adjusts its reimbursement based on how well a clinician’s numbers turn out and if they improved over time.
“You don’t get the benefit of the upside if you don’t meet the measure, so your payment is neutral and that can be significant from a broader system lens,” Dr. Connelly said. “Then you start to have to make decisions on what services do we then have to limit because we no longer have the financial capability.”
The implications for health systems and patient care are the reason ACP and clinicians are calling for some measures to be amended. Dr. Fitterman said his organization plans to work with CMS.
Implementing Measurement
At Bassett Health in New York, the health system uses the depression and follow-up plan measure to qualify for certification from the Health Resources and Services Administration as a patient-centered medical home, which the company uses in part to market itself to patients.
Amy Grace, MD, an attending physician in internal and family medicine at Bassett Health in Little Falls, New York, said if a patient refuses to take a depression screening, she will not meet the measure for that visit. But providing a screening is not always clinically appropriate, and some patients do not need a follow-up plan.
“If someone has just had a death in the family, they might answer the questions in a way that would be consistent with depression, but they’re experiencing grief as opposed to clinical depression,” Dr. Grace said.
Suggestions From ACP for Improvement in MDD Metrics
- Create and implement criteria for patients who do not need a follow-up plan based on clinician judgment.
- Add methods for clinicians to measure and indicate severity of MDD.
- Enable use of a wider array of evidence-based tools and screenings to screen for MDD.
- Allow clinicians to document changes in treatment plan.
Bassett is building into the electronic health record a button that documents the screening was not conducted and that it was not appropriate to administer that day. Of course, building these in-house options entails utilizing resources that smaller systems or independent groups of clinicians may lack.
Eric Wei, MD, senior vice president and chief quality officer at NYC Health + Hospitals in New York City, said the ACP report underscores that many measures, even beyond depression, must be improved.
“With burnout and cognitive overload of our providers, on top of the medicine and just trying to come to the right diagnosis and providing the right treatment and the best care experience, you have to remember all these quality metrics and make sure you put all these things in certain places in the electronic health record,” Dr. Wei said.
Still, Dr. Wei said that the annual rate of depression screening across 400,000 patients in his system is 91%. He and his team spent 6 years working to improve uptake among clinicians, and now, they have moved on to increasing rates of administration of the suicide assessment.
Each clinician uses a dashboard to track their individual metric performance, according to Ted Long, MD, senior vice president for ambulatory care and population health at NYC Health + Hospitals. Dr. Long said he is proud of the improvements he and his colleagues have made in catching undiagnosed depression and in other disease states.
At his primary care practice in the Bronx, nearly 9 out of 10 patients with hypertension have their condition under control, he said. How does he know? Measurement tracking.
“Knowing that when a new patient is in front of me with high blood pressure, that there’s a 9 out of 10 chance that after seeing me because of my clinic, not just because of me, I’m going to be able to keep them healthy by controlling their blood pressure, that’s very meaningful to me,” Dr. Long said. “I think that’s the other side: It enables me as a doctor to know that I’m delivering the highest quality of care to my patients.”
Takeaways for Depression Screening and Follow-Up in Clinical Settings
Just because a patient scores positive for the depression screener, a clinician should dig deeper before making a diagnosis.
Patients have the right to refuse a screener and their wishes should be respected.
Providing a screener may not be appropriate at every visit, such as for a patient with a sprained ankle or a potential respiratory infection where time is limited.
Clinicians can clarify within the measure that the patient did not have mental capacity on that visit to fill out the screener.
A version of this article appeared on Medscape.com.
For Some MDs, Long COVID Burnout Is a New Reality
Dhaval Desai, MD, was teaching his 4-year-old to ride a bike after another exhausting shift at the hospital during the summer after the first COVID-19 surge. He was putting on a happy face and forcing out a “Yay!” he did not feel. The pandemic had taken its toll, and he just wanted to lie down and be alone. Realizing that he was “scraping to find joy” was when he knew something was wrong.
“I was giving, giving, giving at work a lot, and I had little left to give at home,” said Dr. Desai, director of hospital medicine at Emory Saint Joseph’s Hospital and an assistant professor of medicine at Emory University in Atlanta, Georgia.
At work, he worried about his wife managing two kids — including a newborn — during the pandemic. At home, he stressed about work and the crush of patients with COVID the hospital was grappling to handle. He was exhausted, resentful, and angry, and it was jeopardizing what mattered most to him: His home life.
“It was all colliding…I realized, OK, I’m struggling,” he said.
Dr. Desai is one of thousands of physicians across the United States who have experienced burnout and depression, exacerbated by the pandemic. After 4 years, the impact is still being felt. Medscape’s 2024 annual report on this issue found that burnout and depression among doctors — while encouragingly better than the prior year — remain higher than before COVID. For doctors caring for patients with long COVID, those suffering from the debilitating aftereffects of an infection, the sense of helplessness when recovery is elusive can also weigh heavily.
Overall, more female physicians reported feeling burned out and depressed. Experts attributed this gap to issues including fewer women in supportive leadership and mentoring roles, compensation disparities, fewer career advancement opportunities, and more responsibilities caring for children and elders.
Multiple international studies and reports have highlighted the surge in burnout experienced by physicians and healthcare workers globally during the pandemic. Even before COVID, studies found the suicide rate among male and female US physicians was higher than the general population and higher than any other profession, including the military. The risk among female physicians, in particular, was 250%-400% higher.
“That’s really, on average, one a day, and that’s really unacceptable. No one should die by suicide, but a physician who knows the risks and knows that, should never do that,” said Dr. Desai about suicides overall among doctors.
The story of Lorna Breen had rattled Dr. Desai. Dr. Breen was a Manhattan physician who died by suicide in April 2020 after grappling with the city’s devastating first wave and then contracting COVID-19 herself. While Dr. Desai did not have thoughts of suicide, he was facing his own battles. Those experiences and the stigma around mental health prompted him to write his book, Burning Out on the Covid Front Lines: A Doctor’s Memoir of Fatherhood, Race and Perseverance in the Pandemic, with the hope that it can help others like him.
Mental Health Stigma
But despite the body of research and growing awareness toward addressing mental health among physicians, almost four in 10 doctors are wary of revealing their personal struggles.
More than half of those surveyed in the Medscape Medical News report said they had not consulted a mental health professional before and would not do so going forward either. The fear of tarnishing their reputation or even losing their license keeps doctors silent. Advocates and groups like the Dr. Lorna Breen Heroes’ Foundation are pushing for hospitals and healthcare systems to remove and rephrase invasive and stigmatizing language around mental health in licensure, credentialing, or insurance applications.
Burnout Triggers: Systemic Problems, Social Tensions
Burnout can make a person feel “depleted and used up” and is characterized by extreme tiredness, low energy, frustration about work, emotional distance or numbness, and difficulty with concentration, responsibilities, or creativity. It can make an individual feel helpless, alone, defeated, cynical, and without purpose and can also cause physical symptoms such as headaches, loss of appetite, insomnia, and body aches. Unaddressed, it can lead to depression, anxiety, and a variety of physical health issues.
“We can still be highly functional and not okay,” said Dr. Desai.
For doctors, burnout often builds over time from large and small systemic problems and inefficiencies, multiplied by a dozen or more patients each day: Not enough time for documentation, complicated paperwork, navigating byzantine health and insurance systems, and hitting roadblocks. The administrative work, combined with an enormous patient load, and staffing and resource shortages create barriers to care and cuts into the amount of time they can spend providing actual care.
These existing problems worsened as patients with COVID overwhelmed hospitals and clinics. At the same time, healthcare workers worried about caring for the sick, getting infected themselves, or having multiple staff falling ill at once. As each surge came and went, backlash, hostility, abuse, and even violence toward healthcare workers also increased. The discrimination some medical staff were subjected to compounded the burnout.
“When we’re not getting the support we need as physicians and healthcare workers, that adds to burnout, and I saw that in my colleagues,” said Dr. Desai.
Impact of Burnout
At the Mount Sinai Center for Post-COVID Care in New York City, doctors grapple with feelings of helplessness in caring for patients with long COVID who show little sign of recovery. That emotional toll can also be difficult, said director Zijian Chen, MD, who helped launch the clinic in May 2020.
“Sometimes you’re faced with patients who you’re trying to do everything for, but they’re not just not getting better,” said Dr. Chen. “It’s really frustrating because we want everybody to get better. So, there’s that lack of fulfillment there that can cause a sense of burnout.”
While the worst outcomes and death rates initially brought on by acute infections have lessened, long COVID clinics exemplify some of the ongoing challenges within healthcare. Many operate with insufficient financial and staffing resources despite wait-lists and a steady flow of new and returning patients. Even with the demand, a number of these clinics have shuttered, leaving patients without access to much-needed medical help.
“There are clinicians who are burning out. That is definitely something that I’ve seen,” said Monica Verduzco-Gutierrez, MD, a professor and chair of the Department of Rehabilitation Medicine at the University of Texas Health Science Center in San Antonio, Texas.
“[It] takes a lot of resources for a successful long COVID clinic. A lot of special funding may be drying up and couple that with clinicians burning out, then they’re going to shut their doors.”
And it’s not just long COVID clinics. Data have shown an overall exodus in healthcare, especially during the pandemic. One study found burnout was one of the “most impactful” predictors of a physician’s intention to leave the profession during the pandemic. The loss of talent and skills during a major health crisis can put the entire system under stress, with patients ultimately suffering from poorer care.
“Healthcare system fragility and the chaos is far worse than it was before. We are continuing to be forced to do more with less,” said Dr. Desai.
Alleviating Burnout
While it is difficult to assess whether burnout from the pandemic is transient, experts say this is an opportunity for health institutions to learn from these experiences and implement policies and actions that can help reduce the mental health strain on staff. One study found that changes made by organizations had a bigger positive impact on reducing burnout than individual changes.
Advocates say more support staff, more work flexibility, and higher compensation would significantly ease the burden that drives burnout and depression.
In addition, half the physicians surveyed in the Medscape Medical News report felt their employers were not acknowledging how pervasive burnout is at their workplace. Having a trusted peer or leader set an example by sharing his or her own challenging experiences and saying it›s time to address these struggles can be an enormously validating step forward, said Dr. Desai. Acknowledging his own difficulties was not only a huge weight off his shoulders but also helped surpris colleagues who sought him out for counsel.
“I’m not suggesting everybody get on medication,” he said. “But talking to a therapist, acknowledging there’s issues, restructuring your life to realize something’s off, and just knowing that you’re not alone? That’s huge.”
Dr. Desai said he still faces personal challenges but is in a much better place, doing well at work and at home. He talks to a therapist, is taking medication, and has developed better coping mechanisms. He is spending more time with his family, detaching for a few hours from work-related emails, learning to draw boundaries and say no, and trying to be more present and “intentional” in connecting with colleagues and patients.
“It’s okay to not be okay,” said Dr. Desai. “It’s okay to be vulnerable and acknowledge when we can’t do more.”
Are you in a crisis? Call or text 988 or text TALK to 741741. For immediate support for healthcare professionals, as well as resources for institutions and organizations, visit: afsp.org/suicide-prevention-for-healthcare-professionals/#facts-about-mental-health-and-suicide.
A version of this article appeared on Medscape.com.
Dhaval Desai, MD, was teaching his 4-year-old to ride a bike after another exhausting shift at the hospital during the summer after the first COVID-19 surge. He was putting on a happy face and forcing out a “Yay!” he did not feel. The pandemic had taken its toll, and he just wanted to lie down and be alone. Realizing that he was “scraping to find joy” was when he knew something was wrong.
“I was giving, giving, giving at work a lot, and I had little left to give at home,” said Dr. Desai, director of hospital medicine at Emory Saint Joseph’s Hospital and an assistant professor of medicine at Emory University in Atlanta, Georgia.
At work, he worried about his wife managing two kids — including a newborn — during the pandemic. At home, he stressed about work and the crush of patients with COVID the hospital was grappling to handle. He was exhausted, resentful, and angry, and it was jeopardizing what mattered most to him: His home life.
“It was all colliding…I realized, OK, I’m struggling,” he said.
Dr. Desai is one of thousands of physicians across the United States who have experienced burnout and depression, exacerbated by the pandemic. After 4 years, the impact is still being felt. Medscape’s 2024 annual report on this issue found that burnout and depression among doctors — while encouragingly better than the prior year — remain higher than before COVID. For doctors caring for patients with long COVID, those suffering from the debilitating aftereffects of an infection, the sense of helplessness when recovery is elusive can also weigh heavily.
Overall, more female physicians reported feeling burned out and depressed. Experts attributed this gap to issues including fewer women in supportive leadership and mentoring roles, compensation disparities, fewer career advancement opportunities, and more responsibilities caring for children and elders.
Multiple international studies and reports have highlighted the surge in burnout experienced by physicians and healthcare workers globally during the pandemic. Even before COVID, studies found the suicide rate among male and female US physicians was higher than the general population and higher than any other profession, including the military. The risk among female physicians, in particular, was 250%-400% higher.
“That’s really, on average, one a day, and that’s really unacceptable. No one should die by suicide, but a physician who knows the risks and knows that, should never do that,” said Dr. Desai about suicides overall among doctors.
The story of Lorna Breen had rattled Dr. Desai. Dr. Breen was a Manhattan physician who died by suicide in April 2020 after grappling with the city’s devastating first wave and then contracting COVID-19 herself. While Dr. Desai did not have thoughts of suicide, he was facing his own battles. Those experiences and the stigma around mental health prompted him to write his book, Burning Out on the Covid Front Lines: A Doctor’s Memoir of Fatherhood, Race and Perseverance in the Pandemic, with the hope that it can help others like him.
Mental Health Stigma
But despite the body of research and growing awareness toward addressing mental health among physicians, almost four in 10 doctors are wary of revealing their personal struggles.
More than half of those surveyed in the Medscape Medical News report said they had not consulted a mental health professional before and would not do so going forward either. The fear of tarnishing their reputation or even losing their license keeps doctors silent. Advocates and groups like the Dr. Lorna Breen Heroes’ Foundation are pushing for hospitals and healthcare systems to remove and rephrase invasive and stigmatizing language around mental health in licensure, credentialing, or insurance applications.
Burnout Triggers: Systemic Problems, Social Tensions
Burnout can make a person feel “depleted and used up” and is characterized by extreme tiredness, low energy, frustration about work, emotional distance or numbness, and difficulty with concentration, responsibilities, or creativity. It can make an individual feel helpless, alone, defeated, cynical, and without purpose and can also cause physical symptoms such as headaches, loss of appetite, insomnia, and body aches. Unaddressed, it can lead to depression, anxiety, and a variety of physical health issues.
“We can still be highly functional and not okay,” said Dr. Desai.
For doctors, burnout often builds over time from large and small systemic problems and inefficiencies, multiplied by a dozen or more patients each day: Not enough time for documentation, complicated paperwork, navigating byzantine health and insurance systems, and hitting roadblocks. The administrative work, combined with an enormous patient load, and staffing and resource shortages create barriers to care and cuts into the amount of time they can spend providing actual care.
These existing problems worsened as patients with COVID overwhelmed hospitals and clinics. At the same time, healthcare workers worried about caring for the sick, getting infected themselves, or having multiple staff falling ill at once. As each surge came and went, backlash, hostility, abuse, and even violence toward healthcare workers also increased. The discrimination some medical staff were subjected to compounded the burnout.
“When we’re not getting the support we need as physicians and healthcare workers, that adds to burnout, and I saw that in my colleagues,” said Dr. Desai.
Impact of Burnout
At the Mount Sinai Center for Post-COVID Care in New York City, doctors grapple with feelings of helplessness in caring for patients with long COVID who show little sign of recovery. That emotional toll can also be difficult, said director Zijian Chen, MD, who helped launch the clinic in May 2020.
“Sometimes you’re faced with patients who you’re trying to do everything for, but they’re not just not getting better,” said Dr. Chen. “It’s really frustrating because we want everybody to get better. So, there’s that lack of fulfillment there that can cause a sense of burnout.”
While the worst outcomes and death rates initially brought on by acute infections have lessened, long COVID clinics exemplify some of the ongoing challenges within healthcare. Many operate with insufficient financial and staffing resources despite wait-lists and a steady flow of new and returning patients. Even with the demand, a number of these clinics have shuttered, leaving patients without access to much-needed medical help.
“There are clinicians who are burning out. That is definitely something that I’ve seen,” said Monica Verduzco-Gutierrez, MD, a professor and chair of the Department of Rehabilitation Medicine at the University of Texas Health Science Center in San Antonio, Texas.
“[It] takes a lot of resources for a successful long COVID clinic. A lot of special funding may be drying up and couple that with clinicians burning out, then they’re going to shut their doors.”
And it’s not just long COVID clinics. Data have shown an overall exodus in healthcare, especially during the pandemic. One study found burnout was one of the “most impactful” predictors of a physician’s intention to leave the profession during the pandemic. The loss of talent and skills during a major health crisis can put the entire system under stress, with patients ultimately suffering from poorer care.
“Healthcare system fragility and the chaos is far worse than it was before. We are continuing to be forced to do more with less,” said Dr. Desai.
Alleviating Burnout
While it is difficult to assess whether burnout from the pandemic is transient, experts say this is an opportunity for health institutions to learn from these experiences and implement policies and actions that can help reduce the mental health strain on staff. One study found that changes made by organizations had a bigger positive impact on reducing burnout than individual changes.
Advocates say more support staff, more work flexibility, and higher compensation would significantly ease the burden that drives burnout and depression.
In addition, half the physicians surveyed in the Medscape Medical News report felt their employers were not acknowledging how pervasive burnout is at their workplace. Having a trusted peer or leader set an example by sharing his or her own challenging experiences and saying it›s time to address these struggles can be an enormously validating step forward, said Dr. Desai. Acknowledging his own difficulties was not only a huge weight off his shoulders but also helped surpris colleagues who sought him out for counsel.
“I’m not suggesting everybody get on medication,” he said. “But talking to a therapist, acknowledging there’s issues, restructuring your life to realize something’s off, and just knowing that you’re not alone? That’s huge.”
Dr. Desai said he still faces personal challenges but is in a much better place, doing well at work and at home. He talks to a therapist, is taking medication, and has developed better coping mechanisms. He is spending more time with his family, detaching for a few hours from work-related emails, learning to draw boundaries and say no, and trying to be more present and “intentional” in connecting with colleagues and patients.
“It’s okay to not be okay,” said Dr. Desai. “It’s okay to be vulnerable and acknowledge when we can’t do more.”
Are you in a crisis? Call or text 988 or text TALK to 741741. For immediate support for healthcare professionals, as well as resources for institutions and organizations, visit: afsp.org/suicide-prevention-for-healthcare-professionals/#facts-about-mental-health-and-suicide.
A version of this article appeared on Medscape.com.
Dhaval Desai, MD, was teaching his 4-year-old to ride a bike after another exhausting shift at the hospital during the summer after the first COVID-19 surge. He was putting on a happy face and forcing out a “Yay!” he did not feel. The pandemic had taken its toll, and he just wanted to lie down and be alone. Realizing that he was “scraping to find joy” was when he knew something was wrong.
“I was giving, giving, giving at work a lot, and I had little left to give at home,” said Dr. Desai, director of hospital medicine at Emory Saint Joseph’s Hospital and an assistant professor of medicine at Emory University in Atlanta, Georgia.
At work, he worried about his wife managing two kids — including a newborn — during the pandemic. At home, he stressed about work and the crush of patients with COVID the hospital was grappling to handle. He was exhausted, resentful, and angry, and it was jeopardizing what mattered most to him: His home life.
“It was all colliding…I realized, OK, I’m struggling,” he said.
Dr. Desai is one of thousands of physicians across the United States who have experienced burnout and depression, exacerbated by the pandemic. After 4 years, the impact is still being felt. Medscape’s 2024 annual report on this issue found that burnout and depression among doctors — while encouragingly better than the prior year — remain higher than before COVID. For doctors caring for patients with long COVID, those suffering from the debilitating aftereffects of an infection, the sense of helplessness when recovery is elusive can also weigh heavily.
Overall, more female physicians reported feeling burned out and depressed. Experts attributed this gap to issues including fewer women in supportive leadership and mentoring roles, compensation disparities, fewer career advancement opportunities, and more responsibilities caring for children and elders.
Multiple international studies and reports have highlighted the surge in burnout experienced by physicians and healthcare workers globally during the pandemic. Even before COVID, studies found the suicide rate among male and female US physicians was higher than the general population and higher than any other profession, including the military. The risk among female physicians, in particular, was 250%-400% higher.
“That’s really, on average, one a day, and that’s really unacceptable. No one should die by suicide, but a physician who knows the risks and knows that, should never do that,” said Dr. Desai about suicides overall among doctors.
The story of Lorna Breen had rattled Dr. Desai. Dr. Breen was a Manhattan physician who died by suicide in April 2020 after grappling with the city’s devastating first wave and then contracting COVID-19 herself. While Dr. Desai did not have thoughts of suicide, he was facing his own battles. Those experiences and the stigma around mental health prompted him to write his book, Burning Out on the Covid Front Lines: A Doctor’s Memoir of Fatherhood, Race and Perseverance in the Pandemic, with the hope that it can help others like him.
Mental Health Stigma
But despite the body of research and growing awareness toward addressing mental health among physicians, almost four in 10 doctors are wary of revealing their personal struggles.
More than half of those surveyed in the Medscape Medical News report said they had not consulted a mental health professional before and would not do so going forward either. The fear of tarnishing their reputation or even losing their license keeps doctors silent. Advocates and groups like the Dr. Lorna Breen Heroes’ Foundation are pushing for hospitals and healthcare systems to remove and rephrase invasive and stigmatizing language around mental health in licensure, credentialing, or insurance applications.
Burnout Triggers: Systemic Problems, Social Tensions
Burnout can make a person feel “depleted and used up” and is characterized by extreme tiredness, low energy, frustration about work, emotional distance or numbness, and difficulty with concentration, responsibilities, or creativity. It can make an individual feel helpless, alone, defeated, cynical, and without purpose and can also cause physical symptoms such as headaches, loss of appetite, insomnia, and body aches. Unaddressed, it can lead to depression, anxiety, and a variety of physical health issues.
“We can still be highly functional and not okay,” said Dr. Desai.
For doctors, burnout often builds over time from large and small systemic problems and inefficiencies, multiplied by a dozen or more patients each day: Not enough time for documentation, complicated paperwork, navigating byzantine health and insurance systems, and hitting roadblocks. The administrative work, combined with an enormous patient load, and staffing and resource shortages create barriers to care and cuts into the amount of time they can spend providing actual care.
These existing problems worsened as patients with COVID overwhelmed hospitals and clinics. At the same time, healthcare workers worried about caring for the sick, getting infected themselves, or having multiple staff falling ill at once. As each surge came and went, backlash, hostility, abuse, and even violence toward healthcare workers also increased. The discrimination some medical staff were subjected to compounded the burnout.
“When we’re not getting the support we need as physicians and healthcare workers, that adds to burnout, and I saw that in my colleagues,” said Dr. Desai.
Impact of Burnout
At the Mount Sinai Center for Post-COVID Care in New York City, doctors grapple with feelings of helplessness in caring for patients with long COVID who show little sign of recovery. That emotional toll can also be difficult, said director Zijian Chen, MD, who helped launch the clinic in May 2020.
“Sometimes you’re faced with patients who you’re trying to do everything for, but they’re not just not getting better,” said Dr. Chen. “It’s really frustrating because we want everybody to get better. So, there’s that lack of fulfillment there that can cause a sense of burnout.”
While the worst outcomes and death rates initially brought on by acute infections have lessened, long COVID clinics exemplify some of the ongoing challenges within healthcare. Many operate with insufficient financial and staffing resources despite wait-lists and a steady flow of new and returning patients. Even with the demand, a number of these clinics have shuttered, leaving patients without access to much-needed medical help.
“There are clinicians who are burning out. That is definitely something that I’ve seen,” said Monica Verduzco-Gutierrez, MD, a professor and chair of the Department of Rehabilitation Medicine at the University of Texas Health Science Center in San Antonio, Texas.
“[It] takes a lot of resources for a successful long COVID clinic. A lot of special funding may be drying up and couple that with clinicians burning out, then they’re going to shut their doors.”
And it’s not just long COVID clinics. Data have shown an overall exodus in healthcare, especially during the pandemic. One study found burnout was one of the “most impactful” predictors of a physician’s intention to leave the profession during the pandemic. The loss of talent and skills during a major health crisis can put the entire system under stress, with patients ultimately suffering from poorer care.
“Healthcare system fragility and the chaos is far worse than it was before. We are continuing to be forced to do more with less,” said Dr. Desai.
Alleviating Burnout
While it is difficult to assess whether burnout from the pandemic is transient, experts say this is an opportunity for health institutions to learn from these experiences and implement policies and actions that can help reduce the mental health strain on staff. One study found that changes made by organizations had a bigger positive impact on reducing burnout than individual changes.
Advocates say more support staff, more work flexibility, and higher compensation would significantly ease the burden that drives burnout and depression.
In addition, half the physicians surveyed in the Medscape Medical News report felt their employers were not acknowledging how pervasive burnout is at their workplace. Having a trusted peer or leader set an example by sharing his or her own challenging experiences and saying it›s time to address these struggles can be an enormously validating step forward, said Dr. Desai. Acknowledging his own difficulties was not only a huge weight off his shoulders but also helped surpris colleagues who sought him out for counsel.
“I’m not suggesting everybody get on medication,” he said. “But talking to a therapist, acknowledging there’s issues, restructuring your life to realize something’s off, and just knowing that you’re not alone? That’s huge.”
Dr. Desai said he still faces personal challenges but is in a much better place, doing well at work and at home. He talks to a therapist, is taking medication, and has developed better coping mechanisms. He is spending more time with his family, detaching for a few hours from work-related emails, learning to draw boundaries and say no, and trying to be more present and “intentional” in connecting with colleagues and patients.
“It’s okay to not be okay,” said Dr. Desai. “It’s okay to be vulnerable and acknowledge when we can’t do more.”
Are you in a crisis? Call or text 988 or text TALK to 741741. For immediate support for healthcare professionals, as well as resources for institutions and organizations, visit: afsp.org/suicide-prevention-for-healthcare-professionals/#facts-about-mental-health-and-suicide.
A version of this article appeared on Medscape.com.
Dogs Able to Sniff Out PTSD, Other Trauma, in Human Breath
Dogs can detect stress-related compounds in the breath of people experiencing early signs of trauma, including those with posttraumatic stress disorder (PTSD), a new proof-of-concept study suggested.
The research provides evidence that some service dogs with PTSD can be trained to detect episodes of pending distress through a person’s breath and perhaps prompt the individual to use coping skills to manage the episode.
“Ours is the first study to demonstrate that at least some dogs can detect putative stress-related volatile organic compounds in human breath that are associated with PTSD symptoms,” study author Laura Kiiroja, PhD candidate, department of psychology and neuroscience, faculty of science, Dalhousie University, Halifax, Nova Scotia, Canada, told this news organization.
The study was published online on March 28, 2024, in Frontiers of Allergy.
Heightened Sense of Smell
The lifetime prevalence of PTSD is about 8% in the general population, but data show it can reach 23% in veterans. In addition, many more trauma-exposed individuals experience subthreshold symptoms.
Research is investigating the application of dogs’ sense of smell — which is up to 100,000 times more sensitive than humans’ — to detect cancers, viruses, parasites, hypoglycemia, and seizures in humans.
The new study included 26 mostly civilian “donors” (mean age, 31 years; 18 females) who had experienced various types of trauma but had no severe mental illness. More than 50% met the criteria for PTSD.
Participants were recruited from a study examining neurocognitive mechanisms underlying the potential links between trauma and cannabis use. However, participants in the dog study abstained from using cannabis for at least 12 hours prior to the study experiments.
Breath Donors
Breath samples were collected via disposable medical-grade face masks at baseline and during ensuing experiments. In total, 40 breath sample sets were collected.
Two female companion dogs — Ivy, a red golden retriever, and Callie, a German shepherd/Belgian Malinois mix — were trained to identify target odors from the samples.
The animals were tested to determine whether they were able to discriminate between breath samples collected from these same “breath donors” during a relatively relaxed state and during induced stress testing which is known as the alternative forced choice discrimination test.
The dogs’ ability to discern trauma cues from breath samples of various individuals was tested by presenting one sample (baseline or trauma cue) at a time. The researchers used signal detection theory to evaluate the sensitivity and specificity of dogs in detecting human stress VOCs.
Investigators found the dogs had about a 90% accuracy rate across all sample sets in the discrimination experiment and 74% and 81% accuracy for Ivy and Callie, respectively, in the detection experiment.
“Our study contributed to the evidence showing that not only are dogs able to detect some physical health conditions in humans but also that some mental health conditions alter the released VOCs in a way that is detectable by dogs,” Ms. Kiiroja said.
Emotion Detectors
At baseline and during each cue exposure, donors reported their affect using the Positive and Negative Affect Schedule. Ivy’s performance correlated with the donors’ self-reported anxiety, and Callie’s performance correlated with the donors’ self-reported shame.
Based on these correlations, the researchers speculate Ivy detected VOCs that likely originated from the sympathetic-adrenomedullary axis, which involves adrenaline and noradrenaline.
VOCs detected by Callie likely originated in the hypothalamus-pituitary-adrenal axis, which involves cortisol and corticosterone. These two endocrine subsystems play a major role in reestablishing homeostasis in response to a stressor.
The results suggest some service dogs could alert to upcoming intrusion and hyperarousal symptoms even before physical signs manifest and before the person is even aware of the situation, said Ms. Kiiroja.
“This would enable earlier distraction and reminders to use skills learned in psychotherapy; this would have a better likelihood of increasing the efficacy of these skills and preventing further escalation of the arousal,” she said.
Most breath samples likely included both early and late stress VOCs, as the breath donors wore the trauma mask for a relatively long time, the authors noted. Future studies should test dogs’ olfactory acuity on samples collected minutes after the trauma cue, they added.
Another limitation is that all donors were regular cannabis users, so the results may not generalize to others. However, the fact the dogs demonstrated their detection ability even with cannabis users makes the proof-of-concept “more stringent,” Ms. Kiiroja said.
The goal of the study was to see if some dogs are capable of detecting stress VOCs from people with trauma histories in response to trauma cues, so the small number of dogs in the study isn’t a limitation, the authors noted.
‘Wonderful Work’
Commenting on the findings, Elspeth Ritchie, MD, chair of psychiatry, MedStar Washington Hospital Center, Washington, described the research as “wonderful work.” Dr. Ritchie, who was not a part of this study, has also studied PTSD supports dogs.
The study is yet another illustration of the “amazing things dogs can do ... not just for veterans but for people with mental illness.” They can be a source of comfort and help people manage their anxiety.
Training PTSD service dogs can be expensive, with some well-accredited organizations charging about $50,000 for an animal, Dr. Ritchie said. Training a dog to detect VOCs could also be costly, she added.
Although such research has increased in recent years, it’s unclear how it would be applied in practice. Identifying funding for this sort of study and designing trials would also be challenging, Dr. Ritchie added.
“The idea is good, but when you try to operationalize it, it gets tricky,” she said.
The fact that all donors in the study used cannabis is a confounding factor and raises the question of what else might confound the results, Dr. Ritchie added.
Dr. Ritchie emphasized that although ideally veterans would learn to recognize the onset of stress symptoms themselves, a dog could serve as a valuable companion in this process. “That’s precisely why this research should progress,” she said.
The authors and Dr. Ritchie reported no relevant disclosures.
A version of this article appeared on Medscape.com.
Dogs can detect stress-related compounds in the breath of people experiencing early signs of trauma, including those with posttraumatic stress disorder (PTSD), a new proof-of-concept study suggested.
The research provides evidence that some service dogs with PTSD can be trained to detect episodes of pending distress through a person’s breath and perhaps prompt the individual to use coping skills to manage the episode.
“Ours is the first study to demonstrate that at least some dogs can detect putative stress-related volatile organic compounds in human breath that are associated with PTSD symptoms,” study author Laura Kiiroja, PhD candidate, department of psychology and neuroscience, faculty of science, Dalhousie University, Halifax, Nova Scotia, Canada, told this news organization.
The study was published online on March 28, 2024, in Frontiers of Allergy.
Heightened Sense of Smell
The lifetime prevalence of PTSD is about 8% in the general population, but data show it can reach 23% in veterans. In addition, many more trauma-exposed individuals experience subthreshold symptoms.
Research is investigating the application of dogs’ sense of smell — which is up to 100,000 times more sensitive than humans’ — to detect cancers, viruses, parasites, hypoglycemia, and seizures in humans.
The new study included 26 mostly civilian “donors” (mean age, 31 years; 18 females) who had experienced various types of trauma but had no severe mental illness. More than 50% met the criteria for PTSD.
Participants were recruited from a study examining neurocognitive mechanisms underlying the potential links between trauma and cannabis use. However, participants in the dog study abstained from using cannabis for at least 12 hours prior to the study experiments.
Breath Donors
Breath samples were collected via disposable medical-grade face masks at baseline and during ensuing experiments. In total, 40 breath sample sets were collected.
Two female companion dogs — Ivy, a red golden retriever, and Callie, a German shepherd/Belgian Malinois mix — were trained to identify target odors from the samples.
The animals were tested to determine whether they were able to discriminate between breath samples collected from these same “breath donors” during a relatively relaxed state and during induced stress testing which is known as the alternative forced choice discrimination test.
The dogs’ ability to discern trauma cues from breath samples of various individuals was tested by presenting one sample (baseline or trauma cue) at a time. The researchers used signal detection theory to evaluate the sensitivity and specificity of dogs in detecting human stress VOCs.
Investigators found the dogs had about a 90% accuracy rate across all sample sets in the discrimination experiment and 74% and 81% accuracy for Ivy and Callie, respectively, in the detection experiment.
“Our study contributed to the evidence showing that not only are dogs able to detect some physical health conditions in humans but also that some mental health conditions alter the released VOCs in a way that is detectable by dogs,” Ms. Kiiroja said.
Emotion Detectors
At baseline and during each cue exposure, donors reported their affect using the Positive and Negative Affect Schedule. Ivy’s performance correlated with the donors’ self-reported anxiety, and Callie’s performance correlated with the donors’ self-reported shame.
Based on these correlations, the researchers speculate Ivy detected VOCs that likely originated from the sympathetic-adrenomedullary axis, which involves adrenaline and noradrenaline.
VOCs detected by Callie likely originated in the hypothalamus-pituitary-adrenal axis, which involves cortisol and corticosterone. These two endocrine subsystems play a major role in reestablishing homeostasis in response to a stressor.
The results suggest some service dogs could alert to upcoming intrusion and hyperarousal symptoms even before physical signs manifest and before the person is even aware of the situation, said Ms. Kiiroja.
“This would enable earlier distraction and reminders to use skills learned in psychotherapy; this would have a better likelihood of increasing the efficacy of these skills and preventing further escalation of the arousal,” she said.
Most breath samples likely included both early and late stress VOCs, as the breath donors wore the trauma mask for a relatively long time, the authors noted. Future studies should test dogs’ olfactory acuity on samples collected minutes after the trauma cue, they added.
Another limitation is that all donors were regular cannabis users, so the results may not generalize to others. However, the fact the dogs demonstrated their detection ability even with cannabis users makes the proof-of-concept “more stringent,” Ms. Kiiroja said.
The goal of the study was to see if some dogs are capable of detecting stress VOCs from people with trauma histories in response to trauma cues, so the small number of dogs in the study isn’t a limitation, the authors noted.
‘Wonderful Work’
Commenting on the findings, Elspeth Ritchie, MD, chair of psychiatry, MedStar Washington Hospital Center, Washington, described the research as “wonderful work.” Dr. Ritchie, who was not a part of this study, has also studied PTSD supports dogs.
The study is yet another illustration of the “amazing things dogs can do ... not just for veterans but for people with mental illness.” They can be a source of comfort and help people manage their anxiety.
Training PTSD service dogs can be expensive, with some well-accredited organizations charging about $50,000 for an animal, Dr. Ritchie said. Training a dog to detect VOCs could also be costly, she added.
Although such research has increased in recent years, it’s unclear how it would be applied in practice. Identifying funding for this sort of study and designing trials would also be challenging, Dr. Ritchie added.
“The idea is good, but when you try to operationalize it, it gets tricky,” she said.
The fact that all donors in the study used cannabis is a confounding factor and raises the question of what else might confound the results, Dr. Ritchie added.
Dr. Ritchie emphasized that although ideally veterans would learn to recognize the onset of stress symptoms themselves, a dog could serve as a valuable companion in this process. “That’s precisely why this research should progress,” she said.
The authors and Dr. Ritchie reported no relevant disclosures.
A version of this article appeared on Medscape.com.
Dogs can detect stress-related compounds in the breath of people experiencing early signs of trauma, including those with posttraumatic stress disorder (PTSD), a new proof-of-concept study suggested.
The research provides evidence that some service dogs with PTSD can be trained to detect episodes of pending distress through a person’s breath and perhaps prompt the individual to use coping skills to manage the episode.
“Ours is the first study to demonstrate that at least some dogs can detect putative stress-related volatile organic compounds in human breath that are associated with PTSD symptoms,” study author Laura Kiiroja, PhD candidate, department of psychology and neuroscience, faculty of science, Dalhousie University, Halifax, Nova Scotia, Canada, told this news organization.
The study was published online on March 28, 2024, in Frontiers of Allergy.
Heightened Sense of Smell
The lifetime prevalence of PTSD is about 8% in the general population, but data show it can reach 23% in veterans. In addition, many more trauma-exposed individuals experience subthreshold symptoms.
Research is investigating the application of dogs’ sense of smell — which is up to 100,000 times more sensitive than humans’ — to detect cancers, viruses, parasites, hypoglycemia, and seizures in humans.
The new study included 26 mostly civilian “donors” (mean age, 31 years; 18 females) who had experienced various types of trauma but had no severe mental illness. More than 50% met the criteria for PTSD.
Participants were recruited from a study examining neurocognitive mechanisms underlying the potential links between trauma and cannabis use. However, participants in the dog study abstained from using cannabis for at least 12 hours prior to the study experiments.
Breath Donors
Breath samples were collected via disposable medical-grade face masks at baseline and during ensuing experiments. In total, 40 breath sample sets were collected.
Two female companion dogs — Ivy, a red golden retriever, and Callie, a German shepherd/Belgian Malinois mix — were trained to identify target odors from the samples.
The animals were tested to determine whether they were able to discriminate between breath samples collected from these same “breath donors” during a relatively relaxed state and during induced stress testing which is known as the alternative forced choice discrimination test.
The dogs’ ability to discern trauma cues from breath samples of various individuals was tested by presenting one sample (baseline or trauma cue) at a time. The researchers used signal detection theory to evaluate the sensitivity and specificity of dogs in detecting human stress VOCs.
Investigators found the dogs had about a 90% accuracy rate across all sample sets in the discrimination experiment and 74% and 81% accuracy for Ivy and Callie, respectively, in the detection experiment.
“Our study contributed to the evidence showing that not only are dogs able to detect some physical health conditions in humans but also that some mental health conditions alter the released VOCs in a way that is detectable by dogs,” Ms. Kiiroja said.
Emotion Detectors
At baseline and during each cue exposure, donors reported their affect using the Positive and Negative Affect Schedule. Ivy’s performance correlated with the donors’ self-reported anxiety, and Callie’s performance correlated with the donors’ self-reported shame.
Based on these correlations, the researchers speculate Ivy detected VOCs that likely originated from the sympathetic-adrenomedullary axis, which involves adrenaline and noradrenaline.
VOCs detected by Callie likely originated in the hypothalamus-pituitary-adrenal axis, which involves cortisol and corticosterone. These two endocrine subsystems play a major role in reestablishing homeostasis in response to a stressor.
The results suggest some service dogs could alert to upcoming intrusion and hyperarousal symptoms even before physical signs manifest and before the person is even aware of the situation, said Ms. Kiiroja.
“This would enable earlier distraction and reminders to use skills learned in psychotherapy; this would have a better likelihood of increasing the efficacy of these skills and preventing further escalation of the arousal,” she said.
Most breath samples likely included both early and late stress VOCs, as the breath donors wore the trauma mask for a relatively long time, the authors noted. Future studies should test dogs’ olfactory acuity on samples collected minutes after the trauma cue, they added.
Another limitation is that all donors were regular cannabis users, so the results may not generalize to others. However, the fact the dogs demonstrated their detection ability even with cannabis users makes the proof-of-concept “more stringent,” Ms. Kiiroja said.
The goal of the study was to see if some dogs are capable of detecting stress VOCs from people with trauma histories in response to trauma cues, so the small number of dogs in the study isn’t a limitation, the authors noted.
‘Wonderful Work’
Commenting on the findings, Elspeth Ritchie, MD, chair of psychiatry, MedStar Washington Hospital Center, Washington, described the research as “wonderful work.” Dr. Ritchie, who was not a part of this study, has also studied PTSD supports dogs.
The study is yet another illustration of the “amazing things dogs can do ... not just for veterans but for people with mental illness.” They can be a source of comfort and help people manage their anxiety.
Training PTSD service dogs can be expensive, with some well-accredited organizations charging about $50,000 for an animal, Dr. Ritchie said. Training a dog to detect VOCs could also be costly, she added.
Although such research has increased in recent years, it’s unclear how it would be applied in practice. Identifying funding for this sort of study and designing trials would also be challenging, Dr. Ritchie added.
“The idea is good, but when you try to operationalize it, it gets tricky,” she said.
The fact that all donors in the study used cannabis is a confounding factor and raises the question of what else might confound the results, Dr. Ritchie added.
Dr. Ritchie emphasized that although ideally veterans would learn to recognize the onset of stress symptoms themselves, a dog could serve as a valuable companion in this process. “That’s precisely why this research should progress,” she said.
The authors and Dr. Ritchie reported no relevant disclosures.
A version of this article appeared on Medscape.com.
Why We Need to Know About Our Patients’ History of Trauma
This case is a little out of the ordinary, but we would love to find out how readers would handle it.
Diana is a 51-year-old woman with a history of depression, obesity, hypertension, type 2 diabetes, and coronary artery disease. She has come in for a routine visit for her chronic illnesses. She seems very distant and has a flat affect during the initial interview. When you ask about any recent stressful events, she begins crying and explains that her daughter was just deported, leaving behind a child and boyfriend.
Their country of origin suffers from chronic instability and violence. Diana’s father was murdered there, and Diana was the victim of sexual assault. “I escaped when I was 18, and I tried to never look back. Until now.” Diana is very worried about her daughter’s return to that country. “I don’t want her to have to endure what I have endured.”
You spend some time discussing the patient’s mental health burden and identify a counselor and online resources that might help. You wonder if Diana’s adverse childhood experiences (ACEs) might have contributed to some of her physical illnesses.
ACEs and Adult Health
One of the most pronounced and straightforward links is that between ACEs and depression. In the Southern Community Cohort Study of more than 38,200 US adults, the highest odds ratio between ACEs and chronic disease was for depression. Persons who reported more than three ACEs had about a twofold increase in the risk for depression compared with persons without ACEs. There was a monotonic increase in the risk for depression and other chronic illnesses as the burden of ACEs increased.
In another study from the United Kingdom, each additional ACE was associated with a significant 11% increase in the risk for incident diabetes during adulthood. Researchers found that both depression symptoms and cardiometabolic dysfunction mediated the effects of ACEs in promoting higher rates of diabetes.
Depression and diabetes are significant risk factors for coronary artery disease, so it is not surprising that ACEs are also associated with a higher risk for coronary events. A review by Godoy and colleagues described how ACEs promote neuroendocrine, autonomic, and inflammatory dysfunction, which in turn leads to higher rates of traditional cardiovascular risk factors such as diabetes and obesity. Ultimately, the presence of four or more ACEs is associated with more than a twofold higher risk for cardiovascular disease compared with no ACEs.
Many of the pathologic processes that promote cardiovascular disease also increase the risk for dementia. Could the reach of ACEs span decades to promote a higher risk for dementia among older adults? A study by Yuan and colleagues of 7222 Chinese adults suggests that the answer is yes. This study divided the cohort into persons with a history of no ACEs, household dysfunction during childhood, or mistreatment during childhood. Child mistreatment was associated with higher rates of diabetes, depression, and cardiovascular disease, as well as an odds ratio of 1.37 (95% CI, 1.12 to 1.68) for cognitive impairment.
The magnitude of the effects ACEs can have on well-being is reinforced by epidemiologic data surrounding ACEs. According to the US Centers for Disease Control and Prevention (CDC), 64% of US adults report at least one ACE and 17% experienced at least four ACEs. Risk factors for ACEs include being female, American Indian or Alaska Native, or unemployed.
How do we reduce the impact of ACEs? Prevention is key. The CDC estimates that nearly 2 million cases of adult heart disease and more than 20 million cases of adult depression could be avoided if ACEs were eliminated.
But what is the best means to pragmatically reduce ACEs in our current practice models? How do we discover a history of ACEs in patients, and what are the best practices in managing persons with a positive history? We will cover these critical subjects in a future article, but for now, please provide your own comments and pearls regarding the prevention and management of ACEs.
Dr. Vega, health sciences clinical professor, family medicine, University of California, Irvine, disclosed ties with GlaxoSmithKline and Johnson and Johnson. Ms. Hurtado, MD candidate, University of California, Irvine School of Medicine, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This case is a little out of the ordinary, but we would love to find out how readers would handle it.
Diana is a 51-year-old woman with a history of depression, obesity, hypertension, type 2 diabetes, and coronary artery disease. She has come in for a routine visit for her chronic illnesses. She seems very distant and has a flat affect during the initial interview. When you ask about any recent stressful events, she begins crying and explains that her daughter was just deported, leaving behind a child and boyfriend.
Their country of origin suffers from chronic instability and violence. Diana’s father was murdered there, and Diana was the victim of sexual assault. “I escaped when I was 18, and I tried to never look back. Until now.” Diana is very worried about her daughter’s return to that country. “I don’t want her to have to endure what I have endured.”
You spend some time discussing the patient’s mental health burden and identify a counselor and online resources that might help. You wonder if Diana’s adverse childhood experiences (ACEs) might have contributed to some of her physical illnesses.
ACEs and Adult Health
One of the most pronounced and straightforward links is that between ACEs and depression. In the Southern Community Cohort Study of more than 38,200 US adults, the highest odds ratio between ACEs and chronic disease was for depression. Persons who reported more than three ACEs had about a twofold increase in the risk for depression compared with persons without ACEs. There was a monotonic increase in the risk for depression and other chronic illnesses as the burden of ACEs increased.
In another study from the United Kingdom, each additional ACE was associated with a significant 11% increase in the risk for incident diabetes during adulthood. Researchers found that both depression symptoms and cardiometabolic dysfunction mediated the effects of ACEs in promoting higher rates of diabetes.
Depression and diabetes are significant risk factors for coronary artery disease, so it is not surprising that ACEs are also associated with a higher risk for coronary events. A review by Godoy and colleagues described how ACEs promote neuroendocrine, autonomic, and inflammatory dysfunction, which in turn leads to higher rates of traditional cardiovascular risk factors such as diabetes and obesity. Ultimately, the presence of four or more ACEs is associated with more than a twofold higher risk for cardiovascular disease compared with no ACEs.
Many of the pathologic processes that promote cardiovascular disease also increase the risk for dementia. Could the reach of ACEs span decades to promote a higher risk for dementia among older adults? A study by Yuan and colleagues of 7222 Chinese adults suggests that the answer is yes. This study divided the cohort into persons with a history of no ACEs, household dysfunction during childhood, or mistreatment during childhood. Child mistreatment was associated with higher rates of diabetes, depression, and cardiovascular disease, as well as an odds ratio of 1.37 (95% CI, 1.12 to 1.68) for cognitive impairment.
The magnitude of the effects ACEs can have on well-being is reinforced by epidemiologic data surrounding ACEs. According to the US Centers for Disease Control and Prevention (CDC), 64% of US adults report at least one ACE and 17% experienced at least four ACEs. Risk factors for ACEs include being female, American Indian or Alaska Native, or unemployed.
How do we reduce the impact of ACEs? Prevention is key. The CDC estimates that nearly 2 million cases of adult heart disease and more than 20 million cases of adult depression could be avoided if ACEs were eliminated.
But what is the best means to pragmatically reduce ACEs in our current practice models? How do we discover a history of ACEs in patients, and what are the best practices in managing persons with a positive history? We will cover these critical subjects in a future article, but for now, please provide your own comments and pearls regarding the prevention and management of ACEs.
Dr. Vega, health sciences clinical professor, family medicine, University of California, Irvine, disclosed ties with GlaxoSmithKline and Johnson and Johnson. Ms. Hurtado, MD candidate, University of California, Irvine School of Medicine, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This case is a little out of the ordinary, but we would love to find out how readers would handle it.
Diana is a 51-year-old woman with a history of depression, obesity, hypertension, type 2 diabetes, and coronary artery disease. She has come in for a routine visit for her chronic illnesses. She seems very distant and has a flat affect during the initial interview. When you ask about any recent stressful events, she begins crying and explains that her daughter was just deported, leaving behind a child and boyfriend.
Their country of origin suffers from chronic instability and violence. Diana’s father was murdered there, and Diana was the victim of sexual assault. “I escaped when I was 18, and I tried to never look back. Until now.” Diana is very worried about her daughter’s return to that country. “I don’t want her to have to endure what I have endured.”
You spend some time discussing the patient’s mental health burden and identify a counselor and online resources that might help. You wonder if Diana’s adverse childhood experiences (ACEs) might have contributed to some of her physical illnesses.
ACEs and Adult Health
One of the most pronounced and straightforward links is that between ACEs and depression. In the Southern Community Cohort Study of more than 38,200 US adults, the highest odds ratio between ACEs and chronic disease was for depression. Persons who reported more than three ACEs had about a twofold increase in the risk for depression compared with persons without ACEs. There was a monotonic increase in the risk for depression and other chronic illnesses as the burden of ACEs increased.
In another study from the United Kingdom, each additional ACE was associated with a significant 11% increase in the risk for incident diabetes during adulthood. Researchers found that both depression symptoms and cardiometabolic dysfunction mediated the effects of ACEs in promoting higher rates of diabetes.
Depression and diabetes are significant risk factors for coronary artery disease, so it is not surprising that ACEs are also associated with a higher risk for coronary events. A review by Godoy and colleagues described how ACEs promote neuroendocrine, autonomic, and inflammatory dysfunction, which in turn leads to higher rates of traditional cardiovascular risk factors such as diabetes and obesity. Ultimately, the presence of four or more ACEs is associated with more than a twofold higher risk for cardiovascular disease compared with no ACEs.
Many of the pathologic processes that promote cardiovascular disease also increase the risk for dementia. Could the reach of ACEs span decades to promote a higher risk for dementia among older adults? A study by Yuan and colleagues of 7222 Chinese adults suggests that the answer is yes. This study divided the cohort into persons with a history of no ACEs, household dysfunction during childhood, or mistreatment during childhood. Child mistreatment was associated with higher rates of diabetes, depression, and cardiovascular disease, as well as an odds ratio of 1.37 (95% CI, 1.12 to 1.68) for cognitive impairment.
The magnitude of the effects ACEs can have on well-being is reinforced by epidemiologic data surrounding ACEs. According to the US Centers for Disease Control and Prevention (CDC), 64% of US adults report at least one ACE and 17% experienced at least four ACEs. Risk factors for ACEs include being female, American Indian or Alaska Native, or unemployed.
How do we reduce the impact of ACEs? Prevention is key. The CDC estimates that nearly 2 million cases of adult heart disease and more than 20 million cases of adult depression could be avoided if ACEs were eliminated.
But what is the best means to pragmatically reduce ACEs in our current practice models? How do we discover a history of ACEs in patients, and what are the best practices in managing persons with a positive history? We will cover these critical subjects in a future article, but for now, please provide your own comments and pearls regarding the prevention and management of ACEs.
Dr. Vega, health sciences clinical professor, family medicine, University of California, Irvine, disclosed ties with GlaxoSmithKline and Johnson and Johnson. Ms. Hurtado, MD candidate, University of California, Irvine School of Medicine, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Expert Shares Her Phased Approach to Caring for Patients with Delusional Infestation
SAN DIEGO — In the clinical opinion of Jenny E. Murase, MD, .
“The fact that delusional infestation is a fixed, false belief [means] we will never agree with patients on the etiology by definition,” Dr. Murase, a dermatologist with the Palo Alto Foundation Medical Group, Mountain View, California, said at the annual meeting of the American Academy of Dermatology. “But somehow, we must come to some kind of an agreement on how to approach this therapeutically.”
Patients with delusional infestation (DI) often describe a cutaneous sensation of itching or crawling, biting, stinging — a pins and needles sensation. “Formication is when there’s a crawling sensation on the surface of the skin,” she said. “That’s something we can agree on — the fact that there is a shared understanding that they’re experiencing some kind of sensation in their skin.”
First described in 1894, several different terms have been used to describe DI in the past, including acarophobia, delusions of parasitosis, Ekbom syndrome, and Morgellons disease. The current term used for DI includes other animate or inanimate pathogens besides parasites.
The average dermatologist manages two to three patients with DI every 5 years, “so it’s not uncommon,” said Dr. Murase, who also holds a faculty position in the department of dermatology at the University of California, San Francisco. Females are about 2.5 times more likely to be affected compared with males, she said, and 8%-12% of patients with DI have a friend or relative who shares the symptom, and they often accompany them to the office visit. “Initially, you’re trying to determine if this a primary condition where it’s only the cutaneous condition the patient is experiencing, or if there is a secondary condition like an underlying psychiatric disorder or medical condition or drug use that contributes to the sensation,” she said.
According to a descriptive study of 115 patients with DI, 50% had at least one drug detected in hair samples, and nearly 60% had evidence of some cognitive impairment that could not be explained by deficits in IQ. Another study of 147 patients with DI seen at the Mayo Clinic between 2001 and 2007 found that 81% had a prior psychiatric condition and 26% had a shared psychotic disorder.
Phased Approach to Treatment
Dr. Murase discussed her phased approach to caring for patients with DI, based on a review article that she and colleagues published in the International Journal of Dermatology. Phase 1 involves preparing for the visit by asking staff to refer to patients with DI as VIPs and allowing them to talk freely about the sensation they’re experiencing. “The goal is to improve the patient’s condition, not to convince the patient that he or she is delusional,” Dr. Murase explained. “Many patients can’t distinguish between when they’re talking to the doctor and when they’re talking to a nurse or a nurse practitioner; they like to feel that they’re being heard and listened to.”
She also recommends scheduling patients with DI for the end of the day and arranging frequent follow-up visits. “Making them feel valued is the bottom line,” she emphasized. “Remember: They’re less likely to respect socially defined boundaries like time constraints, so you do have to set boundaries, and don’t take what they may say to you personally. You’re not going to be able to care for that individual unless you do that. They may appear defiant, frustrated, and angry, but the fact that they showed up in your office means that you can help that person.”
Phase 2 of care for these patients consists of building a therapeutic rapport by greeting them with a smile and positive attitude and using welcoming body language such as sitting side-by-side during the office visit as opposed to face-to-face, “so it’s a less aggressive approach,” she said. Next, ask about their goal with a question such as, “Is it more important for you to find the bug/virus or to improve your condition?”
During the visit, “you’re continually shifting from etiology — which they are desperate to understand — to a shared desire for treatment,” Dr. Murase said. “No one knows what causes DI and remember, in medicine we treat patients when the exact etiology is unknown. So, we’re not doing anything that differently. Focus on the effect that the symptoms are having on their life. Say something like, ‘it must be so miserable to be living this way. I really want to help you.’ ”
Phase 3 of care for patients with DI involves performing a thorough history and physical exam. The initial office visit should include a full body exam to rule out any underlying dermatologic condition that may be causing the sensation they’re complaining about. She cited a retrospective study of 108 patients who presented to the Mayo Clinic with DI as the main reason for their office visit. Of the 80 patients who had a biopsy, 61% had chronic dermatitis; 48% had excoriation, ulceration, or erosion; and 31% had nonspecific dermal inflammation.
Whether to perform a biopsy or not is controversial, Dr. Murase added, because it’s probably not going to change the clinical impression or diagnosis. “If you agree to do the biopsy, get a verbal contract with the patient,” she advised. “You might say, ‘We’re going to do this. You’re going to choose the site, we’re going to do a biopsy, but we are going to be in agreement here that, if we can’t find the etiology, that you will still be open to going on therapy.’ This is important because it establishes a therapeutic alliance.”
Since patients with DI often bring in their own specimens, she also recommends providing them with microscope glass slides without cover slips and asking them to use clear tape, not tape that is opaque or matted, to cover the specimen.
To rule out other illnesses and conditions that could be triggering the perceived DI, she said lab tests to consider include a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, calcium, hemoglobin A1c, vitamin B12, urinalysis, toxicology screen, HIV/hepatitis C, and rapid plasma reagin.
Starting Treatment
Phase 4 of care for patients with DI involves initiating therapy, which includes demonstrating empathy by reflecting on the detrimental effects of the patient’s reported sensations on their quality of life. “Emphasize that you are not questioning their experience, and that you don’t doubt that they feel things on their skin,” Dr. Murase said. “Recommend medications on an empirical or ‘trial and error’ pragmatic basis. I often tell patients, ‘I will never give up on you if you will never give up on me.’”
For treating patients with DI, her first-generation antipsychotic of choice is pimozide. She starts at a dose of 0.5 mg, building up to 2-3 mg once a day. Haloperidol is another option: 0.5 mg to start, building up to 1-5 mg every night at bedtime. “This requires monitoring for bone suppression via CBC and hypermetabolic complications via fasting lipids and HbA1c,” she said. “There is also an increased risk of prolonged QT with pimozide and risk of extrapyramidal symptoms and tardive dyskinesia.”
Second-generation antipsychotics to consider include risperidone (0.5 mg to start, building up to 102 mg at bedtime); olanzapine (2.5 mg to start, building up to 5-10 mg at bedtime); aripiprazole (2-5 mg to start, building up to 10-15 mg a day), and quetiapine (12.5 mg to start, building up to 200 mg at bedtime).
For all medical therapy she recommends starting patients with a low dose, increasing by 0.5 mg every 2-3 weeks, and let them be “stable and comfortable” for 3-4 months, and then taper down the dose by 0.5 mg every 2-4 weeks or more slowly. In the medical chart, Dr. Murase recommends avoiding use of the terms “psychosis” and “delusions.” Instead, “formication” (tactile hallucination of insects crawling on or within the skin) or “cutaneous dysesthesia” are better terms if patients access their records, she said.
Dr. Murase reported having no relevant disclosures.
SAN DIEGO — In the clinical opinion of Jenny E. Murase, MD, .
“The fact that delusional infestation is a fixed, false belief [means] we will never agree with patients on the etiology by definition,” Dr. Murase, a dermatologist with the Palo Alto Foundation Medical Group, Mountain View, California, said at the annual meeting of the American Academy of Dermatology. “But somehow, we must come to some kind of an agreement on how to approach this therapeutically.”
Patients with delusional infestation (DI) often describe a cutaneous sensation of itching or crawling, biting, stinging — a pins and needles sensation. “Formication is when there’s a crawling sensation on the surface of the skin,” she said. “That’s something we can agree on — the fact that there is a shared understanding that they’re experiencing some kind of sensation in their skin.”
First described in 1894, several different terms have been used to describe DI in the past, including acarophobia, delusions of parasitosis, Ekbom syndrome, and Morgellons disease. The current term used for DI includes other animate or inanimate pathogens besides parasites.
The average dermatologist manages two to three patients with DI every 5 years, “so it’s not uncommon,” said Dr. Murase, who also holds a faculty position in the department of dermatology at the University of California, San Francisco. Females are about 2.5 times more likely to be affected compared with males, she said, and 8%-12% of patients with DI have a friend or relative who shares the symptom, and they often accompany them to the office visit. “Initially, you’re trying to determine if this a primary condition where it’s only the cutaneous condition the patient is experiencing, or if there is a secondary condition like an underlying psychiatric disorder or medical condition or drug use that contributes to the sensation,” she said.
According to a descriptive study of 115 patients with DI, 50% had at least one drug detected in hair samples, and nearly 60% had evidence of some cognitive impairment that could not be explained by deficits in IQ. Another study of 147 patients with DI seen at the Mayo Clinic between 2001 and 2007 found that 81% had a prior psychiatric condition and 26% had a shared psychotic disorder.
Phased Approach to Treatment
Dr. Murase discussed her phased approach to caring for patients with DI, based on a review article that she and colleagues published in the International Journal of Dermatology. Phase 1 involves preparing for the visit by asking staff to refer to patients with DI as VIPs and allowing them to talk freely about the sensation they’re experiencing. “The goal is to improve the patient’s condition, not to convince the patient that he or she is delusional,” Dr. Murase explained. “Many patients can’t distinguish between when they’re talking to the doctor and when they’re talking to a nurse or a nurse practitioner; they like to feel that they’re being heard and listened to.”
She also recommends scheduling patients with DI for the end of the day and arranging frequent follow-up visits. “Making them feel valued is the bottom line,” she emphasized. “Remember: They’re less likely to respect socially defined boundaries like time constraints, so you do have to set boundaries, and don’t take what they may say to you personally. You’re not going to be able to care for that individual unless you do that. They may appear defiant, frustrated, and angry, but the fact that they showed up in your office means that you can help that person.”
Phase 2 of care for these patients consists of building a therapeutic rapport by greeting them with a smile and positive attitude and using welcoming body language such as sitting side-by-side during the office visit as opposed to face-to-face, “so it’s a less aggressive approach,” she said. Next, ask about their goal with a question such as, “Is it more important for you to find the bug/virus or to improve your condition?”
During the visit, “you’re continually shifting from etiology — which they are desperate to understand — to a shared desire for treatment,” Dr. Murase said. “No one knows what causes DI and remember, in medicine we treat patients when the exact etiology is unknown. So, we’re not doing anything that differently. Focus on the effect that the symptoms are having on their life. Say something like, ‘it must be so miserable to be living this way. I really want to help you.’ ”
Phase 3 of care for patients with DI involves performing a thorough history and physical exam. The initial office visit should include a full body exam to rule out any underlying dermatologic condition that may be causing the sensation they’re complaining about. She cited a retrospective study of 108 patients who presented to the Mayo Clinic with DI as the main reason for their office visit. Of the 80 patients who had a biopsy, 61% had chronic dermatitis; 48% had excoriation, ulceration, or erosion; and 31% had nonspecific dermal inflammation.
Whether to perform a biopsy or not is controversial, Dr. Murase added, because it’s probably not going to change the clinical impression or diagnosis. “If you agree to do the biopsy, get a verbal contract with the patient,” she advised. “You might say, ‘We’re going to do this. You’re going to choose the site, we’re going to do a biopsy, but we are going to be in agreement here that, if we can’t find the etiology, that you will still be open to going on therapy.’ This is important because it establishes a therapeutic alliance.”
Since patients with DI often bring in their own specimens, she also recommends providing them with microscope glass slides without cover slips and asking them to use clear tape, not tape that is opaque or matted, to cover the specimen.
To rule out other illnesses and conditions that could be triggering the perceived DI, she said lab tests to consider include a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, calcium, hemoglobin A1c, vitamin B12, urinalysis, toxicology screen, HIV/hepatitis C, and rapid plasma reagin.
Starting Treatment
Phase 4 of care for patients with DI involves initiating therapy, which includes demonstrating empathy by reflecting on the detrimental effects of the patient’s reported sensations on their quality of life. “Emphasize that you are not questioning their experience, and that you don’t doubt that they feel things on their skin,” Dr. Murase said. “Recommend medications on an empirical or ‘trial and error’ pragmatic basis. I often tell patients, ‘I will never give up on you if you will never give up on me.’”
For treating patients with DI, her first-generation antipsychotic of choice is pimozide. She starts at a dose of 0.5 mg, building up to 2-3 mg once a day. Haloperidol is another option: 0.5 mg to start, building up to 1-5 mg every night at bedtime. “This requires monitoring for bone suppression via CBC and hypermetabolic complications via fasting lipids and HbA1c,” she said. “There is also an increased risk of prolonged QT with pimozide and risk of extrapyramidal symptoms and tardive dyskinesia.”
Second-generation antipsychotics to consider include risperidone (0.5 mg to start, building up to 102 mg at bedtime); olanzapine (2.5 mg to start, building up to 5-10 mg at bedtime); aripiprazole (2-5 mg to start, building up to 10-15 mg a day), and quetiapine (12.5 mg to start, building up to 200 mg at bedtime).
For all medical therapy she recommends starting patients with a low dose, increasing by 0.5 mg every 2-3 weeks, and let them be “stable and comfortable” for 3-4 months, and then taper down the dose by 0.5 mg every 2-4 weeks or more slowly. In the medical chart, Dr. Murase recommends avoiding use of the terms “psychosis” and “delusions.” Instead, “formication” (tactile hallucination of insects crawling on or within the skin) or “cutaneous dysesthesia” are better terms if patients access their records, she said.
Dr. Murase reported having no relevant disclosures.
SAN DIEGO — In the clinical opinion of Jenny E. Murase, MD, .
“The fact that delusional infestation is a fixed, false belief [means] we will never agree with patients on the etiology by definition,” Dr. Murase, a dermatologist with the Palo Alto Foundation Medical Group, Mountain View, California, said at the annual meeting of the American Academy of Dermatology. “But somehow, we must come to some kind of an agreement on how to approach this therapeutically.”
Patients with delusional infestation (DI) often describe a cutaneous sensation of itching or crawling, biting, stinging — a pins and needles sensation. “Formication is when there’s a crawling sensation on the surface of the skin,” she said. “That’s something we can agree on — the fact that there is a shared understanding that they’re experiencing some kind of sensation in their skin.”
First described in 1894, several different terms have been used to describe DI in the past, including acarophobia, delusions of parasitosis, Ekbom syndrome, and Morgellons disease. The current term used for DI includes other animate or inanimate pathogens besides parasites.
The average dermatologist manages two to three patients with DI every 5 years, “so it’s not uncommon,” said Dr. Murase, who also holds a faculty position in the department of dermatology at the University of California, San Francisco. Females are about 2.5 times more likely to be affected compared with males, she said, and 8%-12% of patients with DI have a friend or relative who shares the symptom, and they often accompany them to the office visit. “Initially, you’re trying to determine if this a primary condition where it’s only the cutaneous condition the patient is experiencing, or if there is a secondary condition like an underlying psychiatric disorder or medical condition or drug use that contributes to the sensation,” she said.
According to a descriptive study of 115 patients with DI, 50% had at least one drug detected in hair samples, and nearly 60% had evidence of some cognitive impairment that could not be explained by deficits in IQ. Another study of 147 patients with DI seen at the Mayo Clinic between 2001 and 2007 found that 81% had a prior psychiatric condition and 26% had a shared psychotic disorder.
Phased Approach to Treatment
Dr. Murase discussed her phased approach to caring for patients with DI, based on a review article that she and colleagues published in the International Journal of Dermatology. Phase 1 involves preparing for the visit by asking staff to refer to patients with DI as VIPs and allowing them to talk freely about the sensation they’re experiencing. “The goal is to improve the patient’s condition, not to convince the patient that he or she is delusional,” Dr. Murase explained. “Many patients can’t distinguish between when they’re talking to the doctor and when they’re talking to a nurse or a nurse practitioner; they like to feel that they’re being heard and listened to.”
She also recommends scheduling patients with DI for the end of the day and arranging frequent follow-up visits. “Making them feel valued is the bottom line,” she emphasized. “Remember: They’re less likely to respect socially defined boundaries like time constraints, so you do have to set boundaries, and don’t take what they may say to you personally. You’re not going to be able to care for that individual unless you do that. They may appear defiant, frustrated, and angry, but the fact that they showed up in your office means that you can help that person.”
Phase 2 of care for these patients consists of building a therapeutic rapport by greeting them with a smile and positive attitude and using welcoming body language such as sitting side-by-side during the office visit as opposed to face-to-face, “so it’s a less aggressive approach,” she said. Next, ask about their goal with a question such as, “Is it more important for you to find the bug/virus or to improve your condition?”
During the visit, “you’re continually shifting from etiology — which they are desperate to understand — to a shared desire for treatment,” Dr. Murase said. “No one knows what causes DI and remember, in medicine we treat patients when the exact etiology is unknown. So, we’re not doing anything that differently. Focus on the effect that the symptoms are having on their life. Say something like, ‘it must be so miserable to be living this way. I really want to help you.’ ”
Phase 3 of care for patients with DI involves performing a thorough history and physical exam. The initial office visit should include a full body exam to rule out any underlying dermatologic condition that may be causing the sensation they’re complaining about. She cited a retrospective study of 108 patients who presented to the Mayo Clinic with DI as the main reason for their office visit. Of the 80 patients who had a biopsy, 61% had chronic dermatitis; 48% had excoriation, ulceration, or erosion; and 31% had nonspecific dermal inflammation.
Whether to perform a biopsy or not is controversial, Dr. Murase added, because it’s probably not going to change the clinical impression or diagnosis. “If you agree to do the biopsy, get a verbal contract with the patient,” she advised. “You might say, ‘We’re going to do this. You’re going to choose the site, we’re going to do a biopsy, but we are going to be in agreement here that, if we can’t find the etiology, that you will still be open to going on therapy.’ This is important because it establishes a therapeutic alliance.”
Since patients with DI often bring in their own specimens, she also recommends providing them with microscope glass slides without cover slips and asking them to use clear tape, not tape that is opaque or matted, to cover the specimen.
To rule out other illnesses and conditions that could be triggering the perceived DI, she said lab tests to consider include a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, calcium, hemoglobin A1c, vitamin B12, urinalysis, toxicology screen, HIV/hepatitis C, and rapid plasma reagin.
Starting Treatment
Phase 4 of care for patients with DI involves initiating therapy, which includes demonstrating empathy by reflecting on the detrimental effects of the patient’s reported sensations on their quality of life. “Emphasize that you are not questioning their experience, and that you don’t doubt that they feel things on their skin,” Dr. Murase said. “Recommend medications on an empirical or ‘trial and error’ pragmatic basis. I often tell patients, ‘I will never give up on you if you will never give up on me.’”
For treating patients with DI, her first-generation antipsychotic of choice is pimozide. She starts at a dose of 0.5 mg, building up to 2-3 mg once a day. Haloperidol is another option: 0.5 mg to start, building up to 1-5 mg every night at bedtime. “This requires monitoring for bone suppression via CBC and hypermetabolic complications via fasting lipids and HbA1c,” she said. “There is also an increased risk of prolonged QT with pimozide and risk of extrapyramidal symptoms and tardive dyskinesia.”
Second-generation antipsychotics to consider include risperidone (0.5 mg to start, building up to 102 mg at bedtime); olanzapine (2.5 mg to start, building up to 5-10 mg at bedtime); aripiprazole (2-5 mg to start, building up to 10-15 mg a day), and quetiapine (12.5 mg to start, building up to 200 mg at bedtime).
For all medical therapy she recommends starting patients with a low dose, increasing by 0.5 mg every 2-3 weeks, and let them be “stable and comfortable” for 3-4 months, and then taper down the dose by 0.5 mg every 2-4 weeks or more slowly. In the medical chart, Dr. Murase recommends avoiding use of the terms “psychosis” and “delusions.” Instead, “formication” (tactile hallucination of insects crawling on or within the skin) or “cutaneous dysesthesia” are better terms if patients access their records, she said.
Dr. Murase reported having no relevant disclosures.
FROM AAD 2024
What We’ve Learned About Remote Learning
I would have preferred to start this Letter reporting to you that the pandemic is fading out of sight in our rear view mirror. However, I think it is more accurate to say the pandemic is sitting in that blind spot off our passenger side rear fender. Unless you’re like one of those cars with “blind spot detection” blinking a warning, you probably aren’t giving the pandemic much thought. However, three journalists at The New York Times have taken this lull in the pandemic’s newsworthiness to consider the consequences of school closure and remote learning.
From what you may have read and heard, and possibly experienced firsthand, you have a sense that keeping children out of school has been awash in negatives. These journalists looked at all the data they could find and their article is replete with graphs and references. I will just summarize some of what they discovered.
“While poverty and other factors played a role, remote learning was a key driver in academic declines ...” They found there was a direct relationship between the length of school closure and the severity of academic skill loss. The journalists noted that “some time in school was better than no time.” And sadly, “most students have not caught up.”
Poverty played a significant role, with students in economically challenged communities experiencing steeper losses in academics. The reporters quoted Stanford Professor Sean F. Reardon, EdD, who has said “A community’s poverty rate and length of school closures had a ‘roughly equal’ effect.” Poorer school districts tended to continue remote learning longer than those in more well off communities.
At the very beginning of the pandemic, when we were floating in a sea of unknowns, the decision to close schools and take advantage of the new technology that made remote learning possible sounded like the best and maybe only option. However, looking back, Dr. Sean O’Leary, who helped craft AAP guidelines, admits “we probably kept schools closed longer than we should have.”
Early signs that children were not as likely as adults to get sick, and that students posed little threat to others in the school environment, were not taken seriously enough. Too much time and energy was wasted in deep cleaning even after it was clear the virus was airborne. Opening windows that had been painted shut would have been a much better investment.
As it became more apparent that school closures were not having the deterrent effect we had hoped for, there were still communities that resisted. The Times’ reporters noted that teachers’ unions and Democratic cities tended to be more cautious about reopening. And clearly there was political flavor to how communities responded. Masking is probably one of the best examples where emotions and politics colored our responses.
Are there things we could have done differently? One can certainly understand why teachers might have been cautious about returning to in-school learning. With more than a quarter of teachers in this country being older than 50 (16% over 55) and nearly 80% of elementary and middle school teachers self-reporting that they are obese or overweight, educators represent a group that we know now is more vulnerable to complications from COVID. In retrospect, had we understood more about the virus and the downsides of remote learning, the government could have offered paid leave to teachers who felt vulnerable. Then, by expediting the transition of the younger, less vulnerable college students in their final years of training into the workforce earlier could have kept schools open until we were up to speed with vaccines and treatment. But the water has spilled over the dam. We can hope that we as a nation have learned that making frequent evaluations of our strategies and being flexible enough to make changes will help in future pandemics. Unfortunately, those RNA viruses are fast mutators and clever adapters. Strategies we thought were working the first time may not succeed with new variants.
We have now learned that, in general, remote learning was a bust. My grandkids knew it at the time. It’s not just the learning piece. It’s about the social contact with peers that can provide comfort and support when the adults around at home may be anxious and depressed. School is a place you can be physically active away from 24/7 television at home. Adapting to going to school can be difficult for some young children in the beginning because of separation anxiety, but for the vast majority of children doing the school thing is a habit that is quickly rewarded and reinforced daily.
Children learn in school because they are rubbing elbows with other kids who are learning. While some peers may be distracting, the data suggest the distractions of home are far more of a problem. Most children I know were eager to get back in school because that’s where their friends were. But, getting back in the habit of going to school can be difficult for some, especially those who have been less successful in the past. Not surprisingly, the longer the hiatus the more difficult the reentry becomes.
The big lesson we mustn’t forget is that being in school is far more valuable than we ever imagined. And, when we are considering our options in future pandemics and natural disasters, we should be giving much more weight to in-school learning than we have in the past.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I would have preferred to start this Letter reporting to you that the pandemic is fading out of sight in our rear view mirror. However, I think it is more accurate to say the pandemic is sitting in that blind spot off our passenger side rear fender. Unless you’re like one of those cars with “blind spot detection” blinking a warning, you probably aren’t giving the pandemic much thought. However, three journalists at The New York Times have taken this lull in the pandemic’s newsworthiness to consider the consequences of school closure and remote learning.
From what you may have read and heard, and possibly experienced firsthand, you have a sense that keeping children out of school has been awash in negatives. These journalists looked at all the data they could find and their article is replete with graphs and references. I will just summarize some of what they discovered.
“While poverty and other factors played a role, remote learning was a key driver in academic declines ...” They found there was a direct relationship between the length of school closure and the severity of academic skill loss. The journalists noted that “some time in school was better than no time.” And sadly, “most students have not caught up.”
Poverty played a significant role, with students in economically challenged communities experiencing steeper losses in academics. The reporters quoted Stanford Professor Sean F. Reardon, EdD, who has said “A community’s poverty rate and length of school closures had a ‘roughly equal’ effect.” Poorer school districts tended to continue remote learning longer than those in more well off communities.
At the very beginning of the pandemic, when we were floating in a sea of unknowns, the decision to close schools and take advantage of the new technology that made remote learning possible sounded like the best and maybe only option. However, looking back, Dr. Sean O’Leary, who helped craft AAP guidelines, admits “we probably kept schools closed longer than we should have.”
Early signs that children were not as likely as adults to get sick, and that students posed little threat to others in the school environment, were not taken seriously enough. Too much time and energy was wasted in deep cleaning even after it was clear the virus was airborne. Opening windows that had been painted shut would have been a much better investment.
As it became more apparent that school closures were not having the deterrent effect we had hoped for, there were still communities that resisted. The Times’ reporters noted that teachers’ unions and Democratic cities tended to be more cautious about reopening. And clearly there was political flavor to how communities responded. Masking is probably one of the best examples where emotions and politics colored our responses.
Are there things we could have done differently? One can certainly understand why teachers might have been cautious about returning to in-school learning. With more than a quarter of teachers in this country being older than 50 (16% over 55) and nearly 80% of elementary and middle school teachers self-reporting that they are obese or overweight, educators represent a group that we know now is more vulnerable to complications from COVID. In retrospect, had we understood more about the virus and the downsides of remote learning, the government could have offered paid leave to teachers who felt vulnerable. Then, by expediting the transition of the younger, less vulnerable college students in their final years of training into the workforce earlier could have kept schools open until we were up to speed with vaccines and treatment. But the water has spilled over the dam. We can hope that we as a nation have learned that making frequent evaluations of our strategies and being flexible enough to make changes will help in future pandemics. Unfortunately, those RNA viruses are fast mutators and clever adapters. Strategies we thought were working the first time may not succeed with new variants.
We have now learned that, in general, remote learning was a bust. My grandkids knew it at the time. It’s not just the learning piece. It’s about the social contact with peers that can provide comfort and support when the adults around at home may be anxious and depressed. School is a place you can be physically active away from 24/7 television at home. Adapting to going to school can be difficult for some young children in the beginning because of separation anxiety, but for the vast majority of children doing the school thing is a habit that is quickly rewarded and reinforced daily.
Children learn in school because they are rubbing elbows with other kids who are learning. While some peers may be distracting, the data suggest the distractions of home are far more of a problem. Most children I know were eager to get back in school because that’s where their friends were. But, getting back in the habit of going to school can be difficult for some, especially those who have been less successful in the past. Not surprisingly, the longer the hiatus the more difficult the reentry becomes.
The big lesson we mustn’t forget is that being in school is far more valuable than we ever imagined. And, when we are considering our options in future pandemics and natural disasters, we should be giving much more weight to in-school learning than we have in the past.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I would have preferred to start this Letter reporting to you that the pandemic is fading out of sight in our rear view mirror. However, I think it is more accurate to say the pandemic is sitting in that blind spot off our passenger side rear fender. Unless you’re like one of those cars with “blind spot detection” blinking a warning, you probably aren’t giving the pandemic much thought. However, three journalists at The New York Times have taken this lull in the pandemic’s newsworthiness to consider the consequences of school closure and remote learning.
From what you may have read and heard, and possibly experienced firsthand, you have a sense that keeping children out of school has been awash in negatives. These journalists looked at all the data they could find and their article is replete with graphs and references. I will just summarize some of what they discovered.
“While poverty and other factors played a role, remote learning was a key driver in academic declines ...” They found there was a direct relationship between the length of school closure and the severity of academic skill loss. The journalists noted that “some time in school was better than no time.” And sadly, “most students have not caught up.”
Poverty played a significant role, with students in economically challenged communities experiencing steeper losses in academics. The reporters quoted Stanford Professor Sean F. Reardon, EdD, who has said “A community’s poverty rate and length of school closures had a ‘roughly equal’ effect.” Poorer school districts tended to continue remote learning longer than those in more well off communities.
At the very beginning of the pandemic, when we were floating in a sea of unknowns, the decision to close schools and take advantage of the new technology that made remote learning possible sounded like the best and maybe only option. However, looking back, Dr. Sean O’Leary, who helped craft AAP guidelines, admits “we probably kept schools closed longer than we should have.”
Early signs that children were not as likely as adults to get sick, and that students posed little threat to others in the school environment, were not taken seriously enough. Too much time and energy was wasted in deep cleaning even after it was clear the virus was airborne. Opening windows that had been painted shut would have been a much better investment.
As it became more apparent that school closures were not having the deterrent effect we had hoped for, there were still communities that resisted. The Times’ reporters noted that teachers’ unions and Democratic cities tended to be more cautious about reopening. And clearly there was political flavor to how communities responded. Masking is probably one of the best examples where emotions and politics colored our responses.
Are there things we could have done differently? One can certainly understand why teachers might have been cautious about returning to in-school learning. With more than a quarter of teachers in this country being older than 50 (16% over 55) and nearly 80% of elementary and middle school teachers self-reporting that they are obese or overweight, educators represent a group that we know now is more vulnerable to complications from COVID. In retrospect, had we understood more about the virus and the downsides of remote learning, the government could have offered paid leave to teachers who felt vulnerable. Then, by expediting the transition of the younger, less vulnerable college students in their final years of training into the workforce earlier could have kept schools open until we were up to speed with vaccines and treatment. But the water has spilled over the dam. We can hope that we as a nation have learned that making frequent evaluations of our strategies and being flexible enough to make changes will help in future pandemics. Unfortunately, those RNA viruses are fast mutators and clever adapters. Strategies we thought were working the first time may not succeed with new variants.
We have now learned that, in general, remote learning was a bust. My grandkids knew it at the time. It’s not just the learning piece. It’s about the social contact with peers that can provide comfort and support when the adults around at home may be anxious and depressed. School is a place you can be physically active away from 24/7 television at home. Adapting to going to school can be difficult for some young children in the beginning because of separation anxiety, but for the vast majority of children doing the school thing is a habit that is quickly rewarded and reinforced daily.
Children learn in school because they are rubbing elbows with other kids who are learning. While some peers may be distracting, the data suggest the distractions of home are far more of a problem. Most children I know were eager to get back in school because that’s where their friends were. But, getting back in the habit of going to school can be difficult for some, especially those who have been less successful in the past. Not surprisingly, the longer the hiatus the more difficult the reentry becomes.
The big lesson we mustn’t forget is that being in school is far more valuable than we ever imagined. And, when we are considering our options in future pandemics and natural disasters, we should be giving much more weight to in-school learning than we have in the past.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].