User login
Anemia of chronic kidney disease: When normalcy becomes undesirable
The last several years have seen increased debate over the appropriate hemoglobin target range when using erythropoiesis-stimulating agents (ESAs) to treat the anemia of chronic kidney disease and kidney failure. But several recent studies have raised alarms, and in November 2006 the US Food and Drug Administration (FDA) issued a new warning regarding the use of ESAs in renal disease.
For a perspective on the use of erythropoiesis-stimulating agents in cancer patients, see the related editorial.
This article will discuss the history of ESAs and the current guidelines for their use. ESAs are also indicated to treat anemia in patients undergoing cancer chemotherapy or surgery, but those uses will not be discussed in this article.
THE BENEFITS OF ESAs
The first ESA, Epogen, was approved by the FDA in 1989 to treat anemia associated with kidney disease.
Since then, ESAs have made a revolutionary change in the care of patients with kidney failure by allowing them to avoid blood transfusions, which were the norm, and by improving the quality of life, although the evidence for the latter is less compelling.1 The benefits of avoiding the use of blood products include a lower risk of reactions, lower cost, and avoiding sensitization of the human lymphocyte antigen (HLA) system in kidney transplant candidates.
To date, however, no randomized, placebo-controlled clinical trial with adequate power to detect a reduction in adverse clinical outcomes (hospitalizations, nonfatal cardiovascular events, or deaths) has assessed the effect of raising hemoglobin levels with ESAs in patients with chronic kidney disease or end-stage renal disease. Nevertheless, several small studies have shown ESAs to have favorable effects on surrogate end points, and an impressive amount of observational data have shown higher survival rates with higher hemoglobin levels.2–6
HOW HIGH SHOULD THE HEMOGLOBIN BE RAISED?
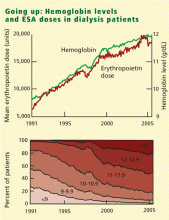
During ESA treatment, the FDA first approved a target hemoglobin range of 10 to 11 g/dL, and subsequently changed it to 10 to 12 g/dL in 1994. The National Kidney Foundation, in its 1997 practice guidelines, endorsed a target range of 11 to 12 g/dL.
The US Normal Hematocrit Study (1998) struck a sour note. In this study, 1,233 dialysis patients with cardiovascular disease were randomized to either a low hematocrit target (33%) or a normal hematocrit target (42%). The trial was stopped early when the investigators recognized that more patients in the normal-hematocrit group had died, that the difference was nearing statistical significance, and that continuing the study was unlikely to reveal a benefit in the normal-hematocrit group. Also of note, the incidence of vascular access thrombosis was higher in the normal-hematocrit group.8
In 2006 the National Kidney Foundation modified its 1997 guidelines, suggesting an upper hemoglobin boundary of 13 g/dL. But in early 2007 it retreated to a hemoglobin target range of 11–12 g/dL,9 after the simultaneous publication of two randomized controlled trials that found no improved outcomes with hemoglobin normalization, and some evidence of harm.10,11
The Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) trial randomized predialysis patients to a hemoglobin goal of either 11.3 g/dL or 13.5 g/dL. The trial was terminated early because the likelihood of benefit with the high hemoglobin goal was low. In fact, the higher-hemoglobin group had a higher incidence of the primary end point, ie, the composite of death, stroke, myocardial infarction, and hospitalization for congestive heart failure. Death and hospitalization for congestive heart failure were the main drivers of the difference in the composite end point between the groups. Quality of life was no better with the higher goal than with the lower goal.10
The Cardiovascular Risk Reduction by Early Anemia Treatment With Epoetin Beta (CREATE) trial11 found that the risk of cardiovascular events in predialysis patients was no lower when anemia was completely corrected (target hemoglobin range 13.0–15.0 g/dL) than with a goal of 10.5 to 11.5 g/dL. Moreover, renal function declined faster in the higher-goal group than in the lower-goal group. However, this study did show higher quality-of-life scores in the group with the higher hemoglobin goal.11
AN FDA ALERT
On November 16, 2006, the FDA issued an alert and required that ESA product labeling include a new boxed warning with the following information12:
- Use the lowest dose of an ESA (Procrit, Epogen, or Aranesp) that will gradually raise the hemoglobin concentration to the lowest level sufficient to avoid the need for blood transfusion.
- ESAs should not be given to treat symptoms of anemia or poor quality of life.
- Maintain the hemoglobin level in the target range of 10 to 12 g/dL.
- Decrease the dose if the hemoglobin level increases by more than 1 g/dL in any 2-week period.
ANOTHER LOOK AT THE DATA
In post hoc analyses, data from the US Normal Hematocrit and CHOIR studies were analyzed on an “as-treated” basis instead of on an intention-to-treat basis as originally reported.13,14 Although the original studies found no survival advantage (and perhaps harm) with higher hemoglobin targets (ie, by intention-to-treat analysis), when the investigators looked at the actual hemoglobin levels achieved, they found that event rates were higher with low hemoglobin levels.
Such discordant findings highlight the importance of randomized experimental designs to avoid bias due to confounding factors (measured and unmeasured) linked to both hemoglobin level and outcome. To reconcile the above findings, we offer the following observations:
- In each treatment group, event rates were higher among those who responded poorly to ESAs (hyporesponders). This finding undermines the intuitive assumption that higher achieved hemoglobin levels were causing volume-related events (congestive heart failure or pulmonary edema) and thrombotic events. Of note, rapid changes in hemoglobin levels in either direction further increased the frequency of events among hyporesponders (which might be associated with the more aggressive algorithm needed in the higher target group).
- Within each treatment group, the difference in event rates is unlikely to be explained by the variation in hemoglobin within its narrow range. Rather, it was mostly due to a higher burden of disease among the hyporesponders. This problem—called targeting bias—is peculiar to therapies that are adjusted according to a target level, eg, of serum hemoglobin.15 Therefore, any association of mortality with achieved hemoglobin within the individual target hemoglobin group is more likely due to other factors such as patient comorbidities.
- Patients assigned to the higher hemoglobin targets received more than just higher doses of ESAs: they also got more of other interventions such as intravenous iron supplementation. Therefore, the results of the trials reflect not only the target level achieved but also the independent effects of the study drug, the co-interventions, and the treatment algorithm.
TAKE-HOME POINTS
Partial correction of the anemia associated with kidney disease reduces transfusion requirements, but normalizing the hemoglobin level does not confer survival benefit and may be harmful. In accordance with the FDA recommendations and the available evidence, we agree that the goal for treating anemia associated with kidney disease should be partial correction: the upper boundary of hemoglobin should be 12 g/dL. However, transient trespasses beyond the upper boundary in day-to-day clinical practice should not trigger a panic response in the health care provider (as seen with hyperkalemia, for instance). Rather, they should result in appropriate and timely treatment adjustments.
Further efforts should explore the merits of treatment algorithms that minimize rapid changes in hemoglobin levels, as well as dose limitation of ESAs and co-interventions among hyporesponders.
- Eschbach JW, Abdulhadi MH, Browne JK, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med 1989; 111:992–1000.
- Ma JZ, Ebben J, Xia H, Collins AJ. Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol 1999; 10:610–619.
- Xue JL, St Peter WL, Ebben JP, Everson SE, Collins AJ. Anemia treatment in the pre-ESRD period and associated mortality in elderly patients. Am J Kidney Dis 2002; 40:1153–1161.
- Levin A, Thompson CR, Ethier J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis 1999; 34:125–134.
- Gouva C, Nikolopoulos P, Ioannidis JP, Siamopoulos KC. Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial. Kidney Int 2004; 66:753–760.
- Ritz E, Laville M, Bilous RW, et al. Target level for hemoglobin correction in patients with diabetes and CKD: primary results of the Anemia Correction in Diabetes (ACORD) Study. Am J Kidney Dis 2007; 49:194–207.
- KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis 2006; 47 suppl 3:S11–S145.
- Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339:584–590.
- KDOQI clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis 2007; 50:471–530.
- Singh AK, Szczech L, Tang KL, et al; CHOIR investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- US Food and Drug Administration. www.fda.gov/cder/drug/InfoSheets/HCP/RHE2007HCP.htm. Accessed 2/5/08.
- US Food and Drug Administration Advisory Committee briefing document. www.fda.gov/ohrms/dockets/AC/07/briefing/2007-4315b1-01-FDA.pdf. Accessed 2/5/08.
- Macdougall IC, Ritz E. The Normal Haematocrit Trial in patients with cardiac disease: are we any the less confused about target haemoglobin? Nephrol Dial Transplant 1998; 13:3030–3033.
- Greene T, Daugirdas J, Depner T, et al. Association of achieved dialysis dose with mortality in the hemodialysis study: an example of “dose-targeting bias.” J Am Soc Nephrol 2005; 16:3371–3380.
The last several years have seen increased debate over the appropriate hemoglobin target range when using erythropoiesis-stimulating agents (ESAs) to treat the anemia of chronic kidney disease and kidney failure. But several recent studies have raised alarms, and in November 2006 the US Food and Drug Administration (FDA) issued a new warning regarding the use of ESAs in renal disease.
For a perspective on the use of erythropoiesis-stimulating agents in cancer patients, see the related editorial.
This article will discuss the history of ESAs and the current guidelines for their use. ESAs are also indicated to treat anemia in patients undergoing cancer chemotherapy or surgery, but those uses will not be discussed in this article.
THE BENEFITS OF ESAs
The first ESA, Epogen, was approved by the FDA in 1989 to treat anemia associated with kidney disease.
Since then, ESAs have made a revolutionary change in the care of patients with kidney failure by allowing them to avoid blood transfusions, which were the norm, and by improving the quality of life, although the evidence for the latter is less compelling.1 The benefits of avoiding the use of blood products include a lower risk of reactions, lower cost, and avoiding sensitization of the human lymphocyte antigen (HLA) system in kidney transplant candidates.
To date, however, no randomized, placebo-controlled clinical trial with adequate power to detect a reduction in adverse clinical outcomes (hospitalizations, nonfatal cardiovascular events, or deaths) has assessed the effect of raising hemoglobin levels with ESAs in patients with chronic kidney disease or end-stage renal disease. Nevertheless, several small studies have shown ESAs to have favorable effects on surrogate end points, and an impressive amount of observational data have shown higher survival rates with higher hemoglobin levels.2–6
HOW HIGH SHOULD THE HEMOGLOBIN BE RAISED?
During ESA treatment, the FDA first approved a target hemoglobin range of 10 to 11 g/dL, and subsequently changed it to 10 to 12 g/dL in 1994. The National Kidney Foundation, in its 1997 practice guidelines, endorsed a target range of 11 to 12 g/dL.
The US Normal Hematocrit Study (1998) struck a sour note. In this study, 1,233 dialysis patients with cardiovascular disease were randomized to either a low hematocrit target (33%) or a normal hematocrit target (42%). The trial was stopped early when the investigators recognized that more patients in the normal-hematocrit group had died, that the difference was nearing statistical significance, and that continuing the study was unlikely to reveal a benefit in the normal-hematocrit group. Also of note, the incidence of vascular access thrombosis was higher in the normal-hematocrit group.8
In 2006 the National Kidney Foundation modified its 1997 guidelines, suggesting an upper hemoglobin boundary of 13 g/dL. But in early 2007 it retreated to a hemoglobin target range of 11–12 g/dL,9 after the simultaneous publication of two randomized controlled trials that found no improved outcomes with hemoglobin normalization, and some evidence of harm.10,11
The Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) trial randomized predialysis patients to a hemoglobin goal of either 11.3 g/dL or 13.5 g/dL. The trial was terminated early because the likelihood of benefit with the high hemoglobin goal was low. In fact, the higher-hemoglobin group had a higher incidence of the primary end point, ie, the composite of death, stroke, myocardial infarction, and hospitalization for congestive heart failure. Death and hospitalization for congestive heart failure were the main drivers of the difference in the composite end point between the groups. Quality of life was no better with the higher goal than with the lower goal.10
The Cardiovascular Risk Reduction by Early Anemia Treatment With Epoetin Beta (CREATE) trial11 found that the risk of cardiovascular events in predialysis patients was no lower when anemia was completely corrected (target hemoglobin range 13.0–15.0 g/dL) than with a goal of 10.5 to 11.5 g/dL. Moreover, renal function declined faster in the higher-goal group than in the lower-goal group. However, this study did show higher quality-of-life scores in the group with the higher hemoglobin goal.11
AN FDA ALERT
On November 16, 2006, the FDA issued an alert and required that ESA product labeling include a new boxed warning with the following information12:
- Use the lowest dose of an ESA (Procrit, Epogen, or Aranesp) that will gradually raise the hemoglobin concentration to the lowest level sufficient to avoid the need for blood transfusion.
- ESAs should not be given to treat symptoms of anemia or poor quality of life.
- Maintain the hemoglobin level in the target range of 10 to 12 g/dL.
- Decrease the dose if the hemoglobin level increases by more than 1 g/dL in any 2-week period.
ANOTHER LOOK AT THE DATA
In post hoc analyses, data from the US Normal Hematocrit and CHOIR studies were analyzed on an “as-treated” basis instead of on an intention-to-treat basis as originally reported.13,14 Although the original studies found no survival advantage (and perhaps harm) with higher hemoglobin targets (ie, by intention-to-treat analysis), when the investigators looked at the actual hemoglobin levels achieved, they found that event rates were higher with low hemoglobin levels.
Such discordant findings highlight the importance of randomized experimental designs to avoid bias due to confounding factors (measured and unmeasured) linked to both hemoglobin level and outcome. To reconcile the above findings, we offer the following observations:
- In each treatment group, event rates were higher among those who responded poorly to ESAs (hyporesponders). This finding undermines the intuitive assumption that higher achieved hemoglobin levels were causing volume-related events (congestive heart failure or pulmonary edema) and thrombotic events. Of note, rapid changes in hemoglobin levels in either direction further increased the frequency of events among hyporesponders (which might be associated with the more aggressive algorithm needed in the higher target group).
- Within each treatment group, the difference in event rates is unlikely to be explained by the variation in hemoglobin within its narrow range. Rather, it was mostly due to a higher burden of disease among the hyporesponders. This problem—called targeting bias—is peculiar to therapies that are adjusted according to a target level, eg, of serum hemoglobin.15 Therefore, any association of mortality with achieved hemoglobin within the individual target hemoglobin group is more likely due to other factors such as patient comorbidities.
- Patients assigned to the higher hemoglobin targets received more than just higher doses of ESAs: they also got more of other interventions such as intravenous iron supplementation. Therefore, the results of the trials reflect not only the target level achieved but also the independent effects of the study drug, the co-interventions, and the treatment algorithm.
TAKE-HOME POINTS
Partial correction of the anemia associated with kidney disease reduces transfusion requirements, but normalizing the hemoglobin level does not confer survival benefit and may be harmful. In accordance with the FDA recommendations and the available evidence, we agree that the goal for treating anemia associated with kidney disease should be partial correction: the upper boundary of hemoglobin should be 12 g/dL. However, transient trespasses beyond the upper boundary in day-to-day clinical practice should not trigger a panic response in the health care provider (as seen with hyperkalemia, for instance). Rather, they should result in appropriate and timely treatment adjustments.
Further efforts should explore the merits of treatment algorithms that minimize rapid changes in hemoglobin levels, as well as dose limitation of ESAs and co-interventions among hyporesponders.
The last several years have seen increased debate over the appropriate hemoglobin target range when using erythropoiesis-stimulating agents (ESAs) to treat the anemia of chronic kidney disease and kidney failure. But several recent studies have raised alarms, and in November 2006 the US Food and Drug Administration (FDA) issued a new warning regarding the use of ESAs in renal disease.
For a perspective on the use of erythropoiesis-stimulating agents in cancer patients, see the related editorial.
This article will discuss the history of ESAs and the current guidelines for their use. ESAs are also indicated to treat anemia in patients undergoing cancer chemotherapy or surgery, but those uses will not be discussed in this article.
THE BENEFITS OF ESAs
The first ESA, Epogen, was approved by the FDA in 1989 to treat anemia associated with kidney disease.
Since then, ESAs have made a revolutionary change in the care of patients with kidney failure by allowing them to avoid blood transfusions, which were the norm, and by improving the quality of life, although the evidence for the latter is less compelling.1 The benefits of avoiding the use of blood products include a lower risk of reactions, lower cost, and avoiding sensitization of the human lymphocyte antigen (HLA) system in kidney transplant candidates.
To date, however, no randomized, placebo-controlled clinical trial with adequate power to detect a reduction in adverse clinical outcomes (hospitalizations, nonfatal cardiovascular events, or deaths) has assessed the effect of raising hemoglobin levels with ESAs in patients with chronic kidney disease or end-stage renal disease. Nevertheless, several small studies have shown ESAs to have favorable effects on surrogate end points, and an impressive amount of observational data have shown higher survival rates with higher hemoglobin levels.2–6
HOW HIGH SHOULD THE HEMOGLOBIN BE RAISED?
During ESA treatment, the FDA first approved a target hemoglobin range of 10 to 11 g/dL, and subsequently changed it to 10 to 12 g/dL in 1994. The National Kidney Foundation, in its 1997 practice guidelines, endorsed a target range of 11 to 12 g/dL.
The US Normal Hematocrit Study (1998) struck a sour note. In this study, 1,233 dialysis patients with cardiovascular disease were randomized to either a low hematocrit target (33%) or a normal hematocrit target (42%). The trial was stopped early when the investigators recognized that more patients in the normal-hematocrit group had died, that the difference was nearing statistical significance, and that continuing the study was unlikely to reveal a benefit in the normal-hematocrit group. Also of note, the incidence of vascular access thrombosis was higher in the normal-hematocrit group.8
In 2006 the National Kidney Foundation modified its 1997 guidelines, suggesting an upper hemoglobin boundary of 13 g/dL. But in early 2007 it retreated to a hemoglobin target range of 11–12 g/dL,9 after the simultaneous publication of two randomized controlled trials that found no improved outcomes with hemoglobin normalization, and some evidence of harm.10,11
The Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) trial randomized predialysis patients to a hemoglobin goal of either 11.3 g/dL or 13.5 g/dL. The trial was terminated early because the likelihood of benefit with the high hemoglobin goal was low. In fact, the higher-hemoglobin group had a higher incidence of the primary end point, ie, the composite of death, stroke, myocardial infarction, and hospitalization for congestive heart failure. Death and hospitalization for congestive heart failure were the main drivers of the difference in the composite end point between the groups. Quality of life was no better with the higher goal than with the lower goal.10
The Cardiovascular Risk Reduction by Early Anemia Treatment With Epoetin Beta (CREATE) trial11 found that the risk of cardiovascular events in predialysis patients was no lower when anemia was completely corrected (target hemoglobin range 13.0–15.0 g/dL) than with a goal of 10.5 to 11.5 g/dL. Moreover, renal function declined faster in the higher-goal group than in the lower-goal group. However, this study did show higher quality-of-life scores in the group with the higher hemoglobin goal.11
AN FDA ALERT
On November 16, 2006, the FDA issued an alert and required that ESA product labeling include a new boxed warning with the following information12:
- Use the lowest dose of an ESA (Procrit, Epogen, or Aranesp) that will gradually raise the hemoglobin concentration to the lowest level sufficient to avoid the need for blood transfusion.
- ESAs should not be given to treat symptoms of anemia or poor quality of life.
- Maintain the hemoglobin level in the target range of 10 to 12 g/dL.
- Decrease the dose if the hemoglobin level increases by more than 1 g/dL in any 2-week period.
ANOTHER LOOK AT THE DATA
In post hoc analyses, data from the US Normal Hematocrit and CHOIR studies were analyzed on an “as-treated” basis instead of on an intention-to-treat basis as originally reported.13,14 Although the original studies found no survival advantage (and perhaps harm) with higher hemoglobin targets (ie, by intention-to-treat analysis), when the investigators looked at the actual hemoglobin levels achieved, they found that event rates were higher with low hemoglobin levels.
Such discordant findings highlight the importance of randomized experimental designs to avoid bias due to confounding factors (measured and unmeasured) linked to both hemoglobin level and outcome. To reconcile the above findings, we offer the following observations:
- In each treatment group, event rates were higher among those who responded poorly to ESAs (hyporesponders). This finding undermines the intuitive assumption that higher achieved hemoglobin levels were causing volume-related events (congestive heart failure or pulmonary edema) and thrombotic events. Of note, rapid changes in hemoglobin levels in either direction further increased the frequency of events among hyporesponders (which might be associated with the more aggressive algorithm needed in the higher target group).
- Within each treatment group, the difference in event rates is unlikely to be explained by the variation in hemoglobin within its narrow range. Rather, it was mostly due to a higher burden of disease among the hyporesponders. This problem—called targeting bias—is peculiar to therapies that are adjusted according to a target level, eg, of serum hemoglobin.15 Therefore, any association of mortality with achieved hemoglobin within the individual target hemoglobin group is more likely due to other factors such as patient comorbidities.
- Patients assigned to the higher hemoglobin targets received more than just higher doses of ESAs: they also got more of other interventions such as intravenous iron supplementation. Therefore, the results of the trials reflect not only the target level achieved but also the independent effects of the study drug, the co-interventions, and the treatment algorithm.
TAKE-HOME POINTS
Partial correction of the anemia associated with kidney disease reduces transfusion requirements, but normalizing the hemoglobin level does not confer survival benefit and may be harmful. In accordance with the FDA recommendations and the available evidence, we agree that the goal for treating anemia associated with kidney disease should be partial correction: the upper boundary of hemoglobin should be 12 g/dL. However, transient trespasses beyond the upper boundary in day-to-day clinical practice should not trigger a panic response in the health care provider (as seen with hyperkalemia, for instance). Rather, they should result in appropriate and timely treatment adjustments.
Further efforts should explore the merits of treatment algorithms that minimize rapid changes in hemoglobin levels, as well as dose limitation of ESAs and co-interventions among hyporesponders.
- Eschbach JW, Abdulhadi MH, Browne JK, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med 1989; 111:992–1000.
- Ma JZ, Ebben J, Xia H, Collins AJ. Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol 1999; 10:610–619.
- Xue JL, St Peter WL, Ebben JP, Everson SE, Collins AJ. Anemia treatment in the pre-ESRD period and associated mortality in elderly patients. Am J Kidney Dis 2002; 40:1153–1161.
- Levin A, Thompson CR, Ethier J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis 1999; 34:125–134.
- Gouva C, Nikolopoulos P, Ioannidis JP, Siamopoulos KC. Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial. Kidney Int 2004; 66:753–760.
- Ritz E, Laville M, Bilous RW, et al. Target level for hemoglobin correction in patients with diabetes and CKD: primary results of the Anemia Correction in Diabetes (ACORD) Study. Am J Kidney Dis 2007; 49:194–207.
- KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis 2006; 47 suppl 3:S11–S145.
- Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339:584–590.
- KDOQI clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis 2007; 50:471–530.
- Singh AK, Szczech L, Tang KL, et al; CHOIR investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- US Food and Drug Administration. www.fda.gov/cder/drug/InfoSheets/HCP/RHE2007HCP.htm. Accessed 2/5/08.
- US Food and Drug Administration Advisory Committee briefing document. www.fda.gov/ohrms/dockets/AC/07/briefing/2007-4315b1-01-FDA.pdf. Accessed 2/5/08.
- Macdougall IC, Ritz E. The Normal Haematocrit Trial in patients with cardiac disease: are we any the less confused about target haemoglobin? Nephrol Dial Transplant 1998; 13:3030–3033.
- Greene T, Daugirdas J, Depner T, et al. Association of achieved dialysis dose with mortality in the hemodialysis study: an example of “dose-targeting bias.” J Am Soc Nephrol 2005; 16:3371–3380.
- Eschbach JW, Abdulhadi MH, Browne JK, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med 1989; 111:992–1000.
- Ma JZ, Ebben J, Xia H, Collins AJ. Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol 1999; 10:610–619.
- Xue JL, St Peter WL, Ebben JP, Everson SE, Collins AJ. Anemia treatment in the pre-ESRD period and associated mortality in elderly patients. Am J Kidney Dis 2002; 40:1153–1161.
- Levin A, Thompson CR, Ethier J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis 1999; 34:125–134.
- Gouva C, Nikolopoulos P, Ioannidis JP, Siamopoulos KC. Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial. Kidney Int 2004; 66:753–760.
- Ritz E, Laville M, Bilous RW, et al. Target level for hemoglobin correction in patients with diabetes and CKD: primary results of the Anemia Correction in Diabetes (ACORD) Study. Am J Kidney Dis 2007; 49:194–207.
- KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis 2006; 47 suppl 3:S11–S145.
- Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339:584–590.
- KDOQI clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis 2007; 50:471–530.
- Singh AK, Szczech L, Tang KL, et al; CHOIR investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- US Food and Drug Administration. www.fda.gov/cder/drug/InfoSheets/HCP/RHE2007HCP.htm. Accessed 2/5/08.
- US Food and Drug Administration Advisory Committee briefing document. www.fda.gov/ohrms/dockets/AC/07/briefing/2007-4315b1-01-FDA.pdf. Accessed 2/5/08.
- Macdougall IC, Ritz E. The Normal Haematocrit Trial in patients with cardiac disease: are we any the less confused about target haemoglobin? Nephrol Dial Transplant 1998; 13:3030–3033.
- Greene T, Daugirdas J, Depner T, et al. Association of achieved dialysis dose with mortality in the hemodialysis study: an example of “dose-targeting bias.” J Am Soc Nephrol 2005; 16:3371–3380.
KEY POINTS
- ESAs reduce the need for blood transfusions and possibly improve quality of life.
- It is unclear if higher hemoglobin levels per se actually caused the adverse events in these trials. Event rates were highest in patients who responded poorly to ESAs.
- We concur with the FDA’s recommendation that the hemoglobin level be raised to no higher than 12 g/dL with ESAs in patients with chronic kidney disease or renal failure.
- Transient excursions of the hemoglobin level above 12 g/dL should not be a cause for panic. Rather, the next ESA dose should be reduced.
Statins and Kidney Disease
Chronic Kidney Disease Screening: Don't Miss This Vital Opportunity
Drugs help pass more ureteral stones
Prescribe tamsulosin (typically 0.4 mg daily) or nifedipine (typically 30 mg daily) for patients with lower ureteral calculi, to speed stone passage and to avoid surgical intervention
Strength of recommendation
A: Meta-analysis of randomized controlled trials
Singh A, Alter HJ, Littlepage A. A systematic review of medical therapy to facilitate passage of ureteral calculi. Ann Emerg Med. 2007; 50:552-563.1
ILLUSTRATIVE CASE
A 52-year-old man presents to your office for follow-up 2 days after he was seen in the ED and diagnosed with a distal ureteral calculus, his first. His pain is reasonably well controlled, but he has not yet passed the stone. Is there anything you can do to help him pass the stone?
Yes. Patients who are candidates for observation should be offered a trial of “medical expulsive therapy” using an α-antagonist or a calcium channel blocker. Until now, medical therapy for kidney stones consisted of pain relief only.
The ordeal of a first stone is all too common—the lifetime prevalence of kidney stones is 5.2%—and the probability of recurrence is about 50%.2,3
NHANES data show increasing prevalence between the periods 1976-1980 and 1988-1996.3 One fifth to one third of kidney stones require surgical intervention.4 In a cohort of 245 patients presenting to an ED in Canada, 50 (20%) required further procedures, including lithotripsy. Stones ≥ 6 mm in size were much less likely to pass (OR=10.7, 95% CI 4.6-24.8).5 The burden on the healthcare system is significant; there are approximately 2 million out-patient visits annually for this problem, and diagnosis and treatment costs about $2 billion annually.6
Watch and wait
The standard approach is a period of watchful waiting and pain control, with urgent urological referral for patients with evidence of upper urinary tract infection, high grade obstruction, inadequate pain or nausea control, or insufficient renal reserve.2,4 Most patients treated with watchful waiting pass their stone within 4 weeks. Any stones that don’t pass within 8 weeks are unlikely to pass spontaneously.2,7
Medical therapy has been proposed for decades
Medications that relax ureteral smooth muscle to help pass ureteral stones have been proposed for decades.8 Prior to 2000, however, only 1 randomized controlled trial (RCT) of medical therapy for ureteral stones had been published.9 A subsequent meta-analysis found 9 studies and showed that medical therapy did increase the chances that a stone would pass.10 The Singh meta-analysis found 13 subsequently published studies and nearly tripled the number of patients evaluated.
STUDY SUMMARY: A well-done meta-analysis
This meta-analysis is based on 16 studies of α-antagonists (most used tamsulosin) and 9 studies of nifedipine, a calcium channel blocker.1 The studies were identified by a comprehensive search strategy that included Medline, EMBASE, and the Cochrane Controlled Trials Register from January 1980 to January 2007. The authors included all randomized trials or controlled clinical trials of medical therapy for adults with acute ureteral colic.
The authors assessed the studies for quality using the Jadad scale, a validated scale of study quality. Higher scores represent better quality, including better documentation of randomization, blinding, and follow-up. The authors specified their planned sensitivity analyses, and used the random effects model to synthesize the results, which tends to provide a more conservative estimate of the effect.
In other words, this was a very well done meta-analysis.
Twenty-two studies met the inclusion criteria: 13 of α-antagonists, 6 of nifedipine, and 3 of both. In 13 of the 16 studies of α-antagonists, tamsulosin (Flomax) was the study drug. The results from the terazosin (Hytrin) and doxazosin (Cardura) studies were included with the tamsulosin studies. The Jadad quality scores of the 22 studies were fairly low, with a median of 2 (range of 0 to 3) on the 5-point scale. The most common deduction was because the study was not double-blinded.
Medical therapy makes sense
“Therapy using either α-antagonists or calcium channel blockers augments the stone expulsion rate compared to standard therapy for moderately sized distal ureteral stones.” 1 CT showing distal ureteral stone
α-Antagonist studies
These 16 studies enrolled 1235 patients with distal ureteral stones. Mean stone size ranged from 4.3 to 7.8 mm. α-Antagonists improved the stone expulsion rate (RR= 1.59, 95% CI 1.44-1.75; NNT=3.3).
The mean time to expulsion of the stone ranged from 2.7 to 14.2 days and duration of therapy ranged from 1 to 7 weeks. In the 9 trials that reported the time to stone expulsion, the stone came out between 2 and 6 days earlier than the control groups.
Adverse effects were reported in 4% of patients receiving the active medication; most were mild.
Nifedipine studies
There were 686 patients in the 9 trials of nifedipine. The mean stone size was 3.9 to 12.8 mm. Some studies included stones in the more proximal as well as the distal ureter.
Nifedipine treatment increased the rate of stone expulsion (RR=1.5, 95% CI 1.34-1.68; NNT=3.9). Time to stone expulsion was shorter in 7 of the 9 studies.
Adverse effects were reported in 15% of the patients. Most of these were mild— nausea, vomiting, asthenia, and dyspepsia.
WHAT’S NEW: Strong evidence for use of medical therapy
The new findings from the Singh meta-analysis reviewed in this PURL supports physicians who have already adopted this practice and should encourage usage by those who have not yet done so.
Inpatients in academic medical centers
There is a growing trend to use tamsulosin to facilitate passage of ureteral stones. The University Health System Consortium (www.uhc.org) has complete clinical data on inpatients with ureteral stones, from 64 academic medical centers and teaching hospitals, between 2003 and 2007. We used this database to analyze trends in the use of tamsulosin in 4300 inpatients with ureteral stones (ICD 9 code 5921).
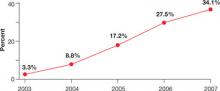
In 2003, only 3.3% of patients with a discharge diagnosis of ureteral stone received tamsulosin. In 2007, 34.1% of patients with ureteral stones discharged from these hospitals received tamsulosin, with similar rates of use when stratified by the specialty of the attending physician at discharge (family medicine, emergency medicine, internal medicine, urology) (FIGURE 1). We noted a wide range in the rate of adoption of this practice among academic medical centers: 48% in the centers with the highest rate of usage and 4.4% in the centers with the lowest rate.
FIGURE 1
% of inpatients in academic medical centers who received tamsulosin for ureteral stones, by year
Source: Unpublished data from the University Health System Consortium
Outpatients from a sample of US practices
The use of tamsulosin or nifedipine in outpatient practice was infrequent even 2 or 3 years ago. We used the National Ambulatory Medical Care Survey data (www.cdc.gov/nchs/about/major/ahcd/ahcd1.htm) from 2004 and 2005 (the most recent available), which provides a sample of all US outpatient practices. Only 7% of an estimated 1,345,000 patients diagnosed with ureteral stones were prescribed either tamsulosin or nifedipine, and urologists cared for most of those.
These unpublished data show that physicians in academic medical centers are increasingly adopting the practice of using tamsulosin or nifedipine for expulsion of ureteral stones, that urologists appear to be the first to begin using these medications in outpatients several years ago, and that this practice is being adopted actively in selected academic medical centers.
CAVEATS: Is either drug better? Too little data to tell
Our conclusion is that the strengths of this meta-analysis outweigh the weaknesses, the findings across studies are consistent, and the use of smooth-muscle relaxants for this indication makes sense from a mechanistic point of view.
The quality of a meta-analysis is only as good as the quality of the included studies, and, in this case, the overall quality of studies was not uniformly high. Median Jadad score, a summary measure of study quality, was 2, and the highest score was 3 (of a maximum of 5). The most common problem was lack of blinding, which can be critical in studies with subjective outcomes such as pain. We doubt that the lack of blinding led to any significant misclassification of outcome in this study, however.
Patients either passed the stone or they didn’t, or had a surgical intervention or not. It is reassuring that, when the best quality studies (Jadad score= 3) were analyzed separately, the results were equally good.
There have not been sufficient head-to-head trials to know if one is better than the other. We prefer α-antagonists because of the lower apparent side-effect profile. Our analysis of the UHC data shows that most of the physicians who are using medical therapy are using tamsulosin primarily for this diagnosis.
The majority of the patients in the studies included in the meta-analysis had been referred to a urologist. This raises the possibility that this treatment may not be as effective in patients with less severe symptoms for whom urological consultation is not necessary.
CHALLENGES TO IMPLEMENTATION: This change should be easy to put into practice
Tamsulosin is the best studied of the drugs, but also the most expensive. Based on the estimated number need to treat (NNT) of between 3 and 4 to prevent a surgical intervention and an estimated cost of around $90 for 1 month (www. drugstore.com, February 16, 2008), tamsulosin seems like a good investment to avoid surgical intervention.
The evidence for the other α-antagonists is consistent with that of tamsulosin, but there are fewer data, so it is not clear that the other agents will work as well.
Many people with renal colic are diagnosed and treated in the emergency department; they may not see their family physician until some time after the stone is diagnosed. It is unclear what effect this delay might have on medication effectiveness.
Neither tamsulosin nor nifedipine have an FDA indication for ureterolithiasis. However, they are prescribed commonly, and most physicians are familiar with their use and adverse-effect profiles.
Drugs used in the meta-analysis studies
α-Antagonists
Tamsulosin (Flomax)
Terazosin (Hytrin)
Doxazosin (Cardura)
Calcium channel blockers
Nifedipine (Adalat, Nifedical, Procardia)
Acknowledgement
We acknowledge Sofia Medvedev, PhD of the University HealthSystem Consortium (UHC) in Oak Brook, IL for analysis of the UHC Clinical Database and the National Ambulatory Medical Care Survey data.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Singh A, Alter HJ, Littlepage A. A systematic review of medical therapy to facilitate passage of ureteral calculi. Ann Emerg Med. 2007;50:552-563.
2. Teichman JM. Clinical practice. Acute renal colic from ureteral calculus. N Engl J Med. 2004;350:684-693.
3. Stamatelou KK, Francis ME, Jones CA, Nyberg LM. Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney International. 2003;63:1817-1823.
4. American Urological Association. Clinical Guidelines: Ureteral Calculi. Last updated 2007. Available at: http://www.auanet.org/guidelines/uretcal07.cfm. Accessed February 11, 2008.
5. Papa L, Stiell IG, Wells GA, Ball I, Battram E, Mahoney JE. Predicting intervention in renal colic patients after emergency department evaluation. Can J Emerg Med. 2005;7:78-86.
6. Pearle MS, Calhoun EA, Curhan GC. Urologic Diseases of America Project. Urologic diseases in America project: urolithiasis. J Urol. 2005;173:848-857.
7. Morse RM, Resnick MI. Ureteral calculi: natural history and treatment in an era of advanced technology. J Urol. 1991;145:263-265.
8. Peters HJ, Eckstein W. Possible pharmacological means of treating renal colic. Urol Res. 1975;3:55-59.
9. Borghi L, Meschi T, Amato F, Novarini A, Giannini A, Quarantelli C, et al. Nifedipine and methylpredniso-lone in facilitating ureteral stone passage: a randomized, double-blind, placebo-controlled study. J Urol. 1994;152:1095-1098.
10. Hollingsworth JM, Rogers MA, Kaufman SR, Bradford TJ, Saint S, Wei JT, et al. Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet. 2006;368:1171-1179.
Prescribe tamsulosin (typically 0.4 mg daily) or nifedipine (typically 30 mg daily) for patients with lower ureteral calculi, to speed stone passage and to avoid surgical intervention
Strength of recommendation
A: Meta-analysis of randomized controlled trials
Singh A, Alter HJ, Littlepage A. A systematic review of medical therapy to facilitate passage of ureteral calculi. Ann Emerg Med. 2007; 50:552-563.1
ILLUSTRATIVE CASE
A 52-year-old man presents to your office for follow-up 2 days after he was seen in the ED and diagnosed with a distal ureteral calculus, his first. His pain is reasonably well controlled, but he has not yet passed the stone. Is there anything you can do to help him pass the stone?
Yes. Patients who are candidates for observation should be offered a trial of “medical expulsive therapy” using an α-antagonist or a calcium channel blocker. Until now, medical therapy for kidney stones consisted of pain relief only.
The ordeal of a first stone is all too common—the lifetime prevalence of kidney stones is 5.2%—and the probability of recurrence is about 50%.2,3
NHANES data show increasing prevalence between the periods 1976-1980 and 1988-1996.3 One fifth to one third of kidney stones require surgical intervention.4 In a cohort of 245 patients presenting to an ED in Canada, 50 (20%) required further procedures, including lithotripsy. Stones ≥ 6 mm in size were much less likely to pass (OR=10.7, 95% CI 4.6-24.8).5 The burden on the healthcare system is significant; there are approximately 2 million out-patient visits annually for this problem, and diagnosis and treatment costs about $2 billion annually.6
Watch and wait
The standard approach is a period of watchful waiting and pain control, with urgent urological referral for patients with evidence of upper urinary tract infection, high grade obstruction, inadequate pain or nausea control, or insufficient renal reserve.2,4 Most patients treated with watchful waiting pass their stone within 4 weeks. Any stones that don’t pass within 8 weeks are unlikely to pass spontaneously.2,7
Medical therapy has been proposed for decades
Medications that relax ureteral smooth muscle to help pass ureteral stones have been proposed for decades.8 Prior to 2000, however, only 1 randomized controlled trial (RCT) of medical therapy for ureteral stones had been published.9 A subsequent meta-analysis found 9 studies and showed that medical therapy did increase the chances that a stone would pass.10 The Singh meta-analysis found 13 subsequently published studies and nearly tripled the number of patients evaluated.
STUDY SUMMARY: A well-done meta-analysis
This meta-analysis is based on 16 studies of α-antagonists (most used tamsulosin) and 9 studies of nifedipine, a calcium channel blocker.1 The studies were identified by a comprehensive search strategy that included Medline, EMBASE, and the Cochrane Controlled Trials Register from January 1980 to January 2007. The authors included all randomized trials or controlled clinical trials of medical therapy for adults with acute ureteral colic.
The authors assessed the studies for quality using the Jadad scale, a validated scale of study quality. Higher scores represent better quality, including better documentation of randomization, blinding, and follow-up. The authors specified their planned sensitivity analyses, and used the random effects model to synthesize the results, which tends to provide a more conservative estimate of the effect.
In other words, this was a very well done meta-analysis.
Twenty-two studies met the inclusion criteria: 13 of α-antagonists, 6 of nifedipine, and 3 of both. In 13 of the 16 studies of α-antagonists, tamsulosin (Flomax) was the study drug. The results from the terazosin (Hytrin) and doxazosin (Cardura) studies were included with the tamsulosin studies. The Jadad quality scores of the 22 studies were fairly low, with a median of 2 (range of 0 to 3) on the 5-point scale. The most common deduction was because the study was not double-blinded.
Medical therapy makes sense
“Therapy using either α-antagonists or calcium channel blockers augments the stone expulsion rate compared to standard therapy for moderately sized distal ureteral stones.” 1 CT showing distal ureteral stone
α-Antagonist studies
These 16 studies enrolled 1235 patients with distal ureteral stones. Mean stone size ranged from 4.3 to 7.8 mm. α-Antagonists improved the stone expulsion rate (RR= 1.59, 95% CI 1.44-1.75; NNT=3.3).
The mean time to expulsion of the stone ranged from 2.7 to 14.2 days and duration of therapy ranged from 1 to 7 weeks. In the 9 trials that reported the time to stone expulsion, the stone came out between 2 and 6 days earlier than the control groups.
Adverse effects were reported in 4% of patients receiving the active medication; most were mild.
Nifedipine studies
There were 686 patients in the 9 trials of nifedipine. The mean stone size was 3.9 to 12.8 mm. Some studies included stones in the more proximal as well as the distal ureter.
Nifedipine treatment increased the rate of stone expulsion (RR=1.5, 95% CI 1.34-1.68; NNT=3.9). Time to stone expulsion was shorter in 7 of the 9 studies.
Adverse effects were reported in 15% of the patients. Most of these were mild— nausea, vomiting, asthenia, and dyspepsia.
WHAT’S NEW: Strong evidence for use of medical therapy
The new findings from the Singh meta-analysis reviewed in this PURL supports physicians who have already adopted this practice and should encourage usage by those who have not yet done so.
Inpatients in academic medical centers
There is a growing trend to use tamsulosin to facilitate passage of ureteral stones. The University Health System Consortium (www.uhc.org) has complete clinical data on inpatients with ureteral stones, from 64 academic medical centers and teaching hospitals, between 2003 and 2007. We used this database to analyze trends in the use of tamsulosin in 4300 inpatients with ureteral stones (ICD 9 code 5921).
In 2003, only 3.3% of patients with a discharge diagnosis of ureteral stone received tamsulosin. In 2007, 34.1% of patients with ureteral stones discharged from these hospitals received tamsulosin, with similar rates of use when stratified by the specialty of the attending physician at discharge (family medicine, emergency medicine, internal medicine, urology) (FIGURE 1). We noted a wide range in the rate of adoption of this practice among academic medical centers: 48% in the centers with the highest rate of usage and 4.4% in the centers with the lowest rate.
FIGURE 1
% of inpatients in academic medical centers who received tamsulosin for ureteral stones, by year
Source: Unpublished data from the University Health System Consortium
Outpatients from a sample of US practices
The use of tamsulosin or nifedipine in outpatient practice was infrequent even 2 or 3 years ago. We used the National Ambulatory Medical Care Survey data (www.cdc.gov/nchs/about/major/ahcd/ahcd1.htm) from 2004 and 2005 (the most recent available), which provides a sample of all US outpatient practices. Only 7% of an estimated 1,345,000 patients diagnosed with ureteral stones were prescribed either tamsulosin or nifedipine, and urologists cared for most of those.
These unpublished data show that physicians in academic medical centers are increasingly adopting the practice of using tamsulosin or nifedipine for expulsion of ureteral stones, that urologists appear to be the first to begin using these medications in outpatients several years ago, and that this practice is being adopted actively in selected academic medical centers.
CAVEATS: Is either drug better? Too little data to tell
Our conclusion is that the strengths of this meta-analysis outweigh the weaknesses, the findings across studies are consistent, and the use of smooth-muscle relaxants for this indication makes sense from a mechanistic point of view.
The quality of a meta-analysis is only as good as the quality of the included studies, and, in this case, the overall quality of studies was not uniformly high. Median Jadad score, a summary measure of study quality, was 2, and the highest score was 3 (of a maximum of 5). The most common problem was lack of blinding, which can be critical in studies with subjective outcomes such as pain. We doubt that the lack of blinding led to any significant misclassification of outcome in this study, however.
Patients either passed the stone or they didn’t, or had a surgical intervention or not. It is reassuring that, when the best quality studies (Jadad score= 3) were analyzed separately, the results were equally good.
There have not been sufficient head-to-head trials to know if one is better than the other. We prefer α-antagonists because of the lower apparent side-effect profile. Our analysis of the UHC data shows that most of the physicians who are using medical therapy are using tamsulosin primarily for this diagnosis.
The majority of the patients in the studies included in the meta-analysis had been referred to a urologist. This raises the possibility that this treatment may not be as effective in patients with less severe symptoms for whom urological consultation is not necessary.
CHALLENGES TO IMPLEMENTATION: This change should be easy to put into practice
Tamsulosin is the best studied of the drugs, but also the most expensive. Based on the estimated number need to treat (NNT) of between 3 and 4 to prevent a surgical intervention and an estimated cost of around $90 for 1 month (www. drugstore.com, February 16, 2008), tamsulosin seems like a good investment to avoid surgical intervention.
The evidence for the other α-antagonists is consistent with that of tamsulosin, but there are fewer data, so it is not clear that the other agents will work as well.
Many people with renal colic are diagnosed and treated in the emergency department; they may not see their family physician until some time after the stone is diagnosed. It is unclear what effect this delay might have on medication effectiveness.
Neither tamsulosin nor nifedipine have an FDA indication for ureterolithiasis. However, they are prescribed commonly, and most physicians are familiar with their use and adverse-effect profiles.
Drugs used in the meta-analysis studies
α-Antagonists
Tamsulosin (Flomax)
Terazosin (Hytrin)
Doxazosin (Cardura)
Calcium channel blockers
Nifedipine (Adalat, Nifedical, Procardia)
Acknowledgement
We acknowledge Sofia Medvedev, PhD of the University HealthSystem Consortium (UHC) in Oak Brook, IL for analysis of the UHC Clinical Database and the National Ambulatory Medical Care Survey data.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
Prescribe tamsulosin (typically 0.4 mg daily) or nifedipine (typically 30 mg daily) for patients with lower ureteral calculi, to speed stone passage and to avoid surgical intervention
Strength of recommendation
A: Meta-analysis of randomized controlled trials
Singh A, Alter HJ, Littlepage A. A systematic review of medical therapy to facilitate passage of ureteral calculi. Ann Emerg Med. 2007; 50:552-563.1
ILLUSTRATIVE CASE
A 52-year-old man presents to your office for follow-up 2 days after he was seen in the ED and diagnosed with a distal ureteral calculus, his first. His pain is reasonably well controlled, but he has not yet passed the stone. Is there anything you can do to help him pass the stone?
Yes. Patients who are candidates for observation should be offered a trial of “medical expulsive therapy” using an α-antagonist or a calcium channel blocker. Until now, medical therapy for kidney stones consisted of pain relief only.
The ordeal of a first stone is all too common—the lifetime prevalence of kidney stones is 5.2%—and the probability of recurrence is about 50%.2,3
NHANES data show increasing prevalence between the periods 1976-1980 and 1988-1996.3 One fifth to one third of kidney stones require surgical intervention.4 In a cohort of 245 patients presenting to an ED in Canada, 50 (20%) required further procedures, including lithotripsy. Stones ≥ 6 mm in size were much less likely to pass (OR=10.7, 95% CI 4.6-24.8).5 The burden on the healthcare system is significant; there are approximately 2 million out-patient visits annually for this problem, and diagnosis and treatment costs about $2 billion annually.6
Watch and wait
The standard approach is a period of watchful waiting and pain control, with urgent urological referral for patients with evidence of upper urinary tract infection, high grade obstruction, inadequate pain or nausea control, or insufficient renal reserve.2,4 Most patients treated with watchful waiting pass their stone within 4 weeks. Any stones that don’t pass within 8 weeks are unlikely to pass spontaneously.2,7
Medical therapy has been proposed for decades
Medications that relax ureteral smooth muscle to help pass ureteral stones have been proposed for decades.8 Prior to 2000, however, only 1 randomized controlled trial (RCT) of medical therapy for ureteral stones had been published.9 A subsequent meta-analysis found 9 studies and showed that medical therapy did increase the chances that a stone would pass.10 The Singh meta-analysis found 13 subsequently published studies and nearly tripled the number of patients evaluated.
STUDY SUMMARY: A well-done meta-analysis
This meta-analysis is based on 16 studies of α-antagonists (most used tamsulosin) and 9 studies of nifedipine, a calcium channel blocker.1 The studies were identified by a comprehensive search strategy that included Medline, EMBASE, and the Cochrane Controlled Trials Register from January 1980 to January 2007. The authors included all randomized trials or controlled clinical trials of medical therapy for adults with acute ureteral colic.
The authors assessed the studies for quality using the Jadad scale, a validated scale of study quality. Higher scores represent better quality, including better documentation of randomization, blinding, and follow-up. The authors specified their planned sensitivity analyses, and used the random effects model to synthesize the results, which tends to provide a more conservative estimate of the effect.
In other words, this was a very well done meta-analysis.
Twenty-two studies met the inclusion criteria: 13 of α-antagonists, 6 of nifedipine, and 3 of both. In 13 of the 16 studies of α-antagonists, tamsulosin (Flomax) was the study drug. The results from the terazosin (Hytrin) and doxazosin (Cardura) studies were included with the tamsulosin studies. The Jadad quality scores of the 22 studies were fairly low, with a median of 2 (range of 0 to 3) on the 5-point scale. The most common deduction was because the study was not double-blinded.
Medical therapy makes sense
“Therapy using either α-antagonists or calcium channel blockers augments the stone expulsion rate compared to standard therapy for moderately sized distal ureteral stones.” 1 CT showing distal ureteral stone
α-Antagonist studies
These 16 studies enrolled 1235 patients with distal ureteral stones. Mean stone size ranged from 4.3 to 7.8 mm. α-Antagonists improved the stone expulsion rate (RR= 1.59, 95% CI 1.44-1.75; NNT=3.3).
The mean time to expulsion of the stone ranged from 2.7 to 14.2 days and duration of therapy ranged from 1 to 7 weeks. In the 9 trials that reported the time to stone expulsion, the stone came out between 2 and 6 days earlier than the control groups.
Adverse effects were reported in 4% of patients receiving the active medication; most were mild.
Nifedipine studies
There were 686 patients in the 9 trials of nifedipine. The mean stone size was 3.9 to 12.8 mm. Some studies included stones in the more proximal as well as the distal ureter.
Nifedipine treatment increased the rate of stone expulsion (RR=1.5, 95% CI 1.34-1.68; NNT=3.9). Time to stone expulsion was shorter in 7 of the 9 studies.
Adverse effects were reported in 15% of the patients. Most of these were mild— nausea, vomiting, asthenia, and dyspepsia.
WHAT’S NEW: Strong evidence for use of medical therapy
The new findings from the Singh meta-analysis reviewed in this PURL supports physicians who have already adopted this practice and should encourage usage by those who have not yet done so.
Inpatients in academic medical centers
There is a growing trend to use tamsulosin to facilitate passage of ureteral stones. The University Health System Consortium (www.uhc.org) has complete clinical data on inpatients with ureteral stones, from 64 academic medical centers and teaching hospitals, between 2003 and 2007. We used this database to analyze trends in the use of tamsulosin in 4300 inpatients with ureteral stones (ICD 9 code 5921).
In 2003, only 3.3% of patients with a discharge diagnosis of ureteral stone received tamsulosin. In 2007, 34.1% of patients with ureteral stones discharged from these hospitals received tamsulosin, with similar rates of use when stratified by the specialty of the attending physician at discharge (family medicine, emergency medicine, internal medicine, urology) (FIGURE 1). We noted a wide range in the rate of adoption of this practice among academic medical centers: 48% in the centers with the highest rate of usage and 4.4% in the centers with the lowest rate.
FIGURE 1
% of inpatients in academic medical centers who received tamsulosin for ureteral stones, by year
Source: Unpublished data from the University Health System Consortium
Outpatients from a sample of US practices
The use of tamsulosin or nifedipine in outpatient practice was infrequent even 2 or 3 years ago. We used the National Ambulatory Medical Care Survey data (www.cdc.gov/nchs/about/major/ahcd/ahcd1.htm) from 2004 and 2005 (the most recent available), which provides a sample of all US outpatient practices. Only 7% of an estimated 1,345,000 patients diagnosed with ureteral stones were prescribed either tamsulosin or nifedipine, and urologists cared for most of those.
These unpublished data show that physicians in academic medical centers are increasingly adopting the practice of using tamsulosin or nifedipine for expulsion of ureteral stones, that urologists appear to be the first to begin using these medications in outpatients several years ago, and that this practice is being adopted actively in selected academic medical centers.
CAVEATS: Is either drug better? Too little data to tell
Our conclusion is that the strengths of this meta-analysis outweigh the weaknesses, the findings across studies are consistent, and the use of smooth-muscle relaxants for this indication makes sense from a mechanistic point of view.
The quality of a meta-analysis is only as good as the quality of the included studies, and, in this case, the overall quality of studies was not uniformly high. Median Jadad score, a summary measure of study quality, was 2, and the highest score was 3 (of a maximum of 5). The most common problem was lack of blinding, which can be critical in studies with subjective outcomes such as pain. We doubt that the lack of blinding led to any significant misclassification of outcome in this study, however.
Patients either passed the stone or they didn’t, or had a surgical intervention or not. It is reassuring that, when the best quality studies (Jadad score= 3) were analyzed separately, the results were equally good.
There have not been sufficient head-to-head trials to know if one is better than the other. We prefer α-antagonists because of the lower apparent side-effect profile. Our analysis of the UHC data shows that most of the physicians who are using medical therapy are using tamsulosin primarily for this diagnosis.
The majority of the patients in the studies included in the meta-analysis had been referred to a urologist. This raises the possibility that this treatment may not be as effective in patients with less severe symptoms for whom urological consultation is not necessary.
CHALLENGES TO IMPLEMENTATION: This change should be easy to put into practice
Tamsulosin is the best studied of the drugs, but also the most expensive. Based on the estimated number need to treat (NNT) of between 3 and 4 to prevent a surgical intervention and an estimated cost of around $90 for 1 month (www. drugstore.com, February 16, 2008), tamsulosin seems like a good investment to avoid surgical intervention.
The evidence for the other α-antagonists is consistent with that of tamsulosin, but there are fewer data, so it is not clear that the other agents will work as well.
Many people with renal colic are diagnosed and treated in the emergency department; they may not see their family physician until some time after the stone is diagnosed. It is unclear what effect this delay might have on medication effectiveness.
Neither tamsulosin nor nifedipine have an FDA indication for ureterolithiasis. However, they are prescribed commonly, and most physicians are familiar with their use and adverse-effect profiles.
Drugs used in the meta-analysis studies
α-Antagonists
Tamsulosin (Flomax)
Terazosin (Hytrin)
Doxazosin (Cardura)
Calcium channel blockers
Nifedipine (Adalat, Nifedical, Procardia)
Acknowledgement
We acknowledge Sofia Medvedev, PhD of the University HealthSystem Consortium (UHC) in Oak Brook, IL for analysis of the UHC Clinical Database and the National Ambulatory Medical Care Survey data.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Singh A, Alter HJ, Littlepage A. A systematic review of medical therapy to facilitate passage of ureteral calculi. Ann Emerg Med. 2007;50:552-563.
2. Teichman JM. Clinical practice. Acute renal colic from ureteral calculus. N Engl J Med. 2004;350:684-693.
3. Stamatelou KK, Francis ME, Jones CA, Nyberg LM. Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney International. 2003;63:1817-1823.
4. American Urological Association. Clinical Guidelines: Ureteral Calculi. Last updated 2007. Available at: http://www.auanet.org/guidelines/uretcal07.cfm. Accessed February 11, 2008.
5. Papa L, Stiell IG, Wells GA, Ball I, Battram E, Mahoney JE. Predicting intervention in renal colic patients after emergency department evaluation. Can J Emerg Med. 2005;7:78-86.
6. Pearle MS, Calhoun EA, Curhan GC. Urologic Diseases of America Project. Urologic diseases in America project: urolithiasis. J Urol. 2005;173:848-857.
7. Morse RM, Resnick MI. Ureteral calculi: natural history and treatment in an era of advanced technology. J Urol. 1991;145:263-265.
8. Peters HJ, Eckstein W. Possible pharmacological means of treating renal colic. Urol Res. 1975;3:55-59.
9. Borghi L, Meschi T, Amato F, Novarini A, Giannini A, Quarantelli C, et al. Nifedipine and methylpredniso-lone in facilitating ureteral stone passage: a randomized, double-blind, placebo-controlled study. J Urol. 1994;152:1095-1098.
10. Hollingsworth JM, Rogers MA, Kaufman SR, Bradford TJ, Saint S, Wei JT, et al. Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet. 2006;368:1171-1179.
1. Singh A, Alter HJ, Littlepage A. A systematic review of medical therapy to facilitate passage of ureteral calculi. Ann Emerg Med. 2007;50:552-563.
2. Teichman JM. Clinical practice. Acute renal colic from ureteral calculus. N Engl J Med. 2004;350:684-693.
3. Stamatelou KK, Francis ME, Jones CA, Nyberg LM. Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney International. 2003;63:1817-1823.
4. American Urological Association. Clinical Guidelines: Ureteral Calculi. Last updated 2007. Available at: http://www.auanet.org/guidelines/uretcal07.cfm. Accessed February 11, 2008.
5. Papa L, Stiell IG, Wells GA, Ball I, Battram E, Mahoney JE. Predicting intervention in renal colic patients after emergency department evaluation. Can J Emerg Med. 2005;7:78-86.
6. Pearle MS, Calhoun EA, Curhan GC. Urologic Diseases of America Project. Urologic diseases in America project: urolithiasis. J Urol. 2005;173:848-857.
7. Morse RM, Resnick MI. Ureteral calculi: natural history and treatment in an era of advanced technology. J Urol. 1991;145:263-265.
8. Peters HJ, Eckstein W. Possible pharmacological means of treating renal colic. Urol Res. 1975;3:55-59.
9. Borghi L, Meschi T, Amato F, Novarini A, Giannini A, Quarantelli C, et al. Nifedipine and methylpredniso-lone in facilitating ureteral stone passage: a randomized, double-blind, placebo-controlled study. J Urol. 1994;152:1095-1098.
10. Hollingsworth JM, Rogers MA, Kaufman SR, Bradford TJ, Saint S, Wei JT, et al. Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet. 2006;368:1171-1179.
Copyright © 2008 The Family Physicians Inquiries Network.
All rights reserved.
What is adequate hypertension control?
To the Editor: I read with interest the exchange of letters between Drs. Norenberg and Graves in the December 2007 issue,1,2 which followed Dr. Graves’ article in the October 2007 issue.3 Dr. Norenberg suggests that it is not always prudent to try to push systolic pressures below 140 mm Hg in the elderly, and Dr. Graves takes the position that physicians like Dr. Norenberg have been “too slow to adapt to evidence-based guidelines for quality of care.” I would like to focus on Dr. Graves’ reference to evidence-based guidelines for the treatment of systolic hypertension in the elderly.
Although there have been multiple published studies of the treatment of this disorder, none has achieved an average systolic blood pressure lower than 140. The Systolic Hypertension in the Elderly Program (SHEP)4 came closest with a final systolic blood pressure of 144. No study has ever documented the efficacy and safety of achieving systolic blood pressures less than 140 in a cohort of elderly patients, and there is substantial evidence that excessive lowering of diastolic blood pressure can be harmful.5,6
Many elderly patients can achieve the target referenced by Dr. Graves, and it is reasonable to expect physicians to continue to strive for that goal, but it would be unwise to push all seniors below 140 systolic. Consider the elderly patient with systolic hypertension who is on a robust three-drug regimen including a diuretic, with a blood pressure of 144/60 and with persistent but tolerable drug side effects. I am aware of no clinical trials that demonstrate that further lowering of this patient’s blood pressure would provide incremental benefit to outweigh the potential risks and costs of additional medications.
We need to be careful not to confuse evidence-based medicine with high-placed opinions, which can result in rigid approaches to treatment that are not in the best interest of our patients.
- Norenberg DD. What is adequate hypertension control? (Letter). Cleve Clin J Med 2007; 74:848.
- Graves JW. What is adequate hypertension control (In Reply). Cleve Clin J Med 2007; 74:848–849.
- Graves JW. What is adequate hypertension control? Having your dinner and dessert too. Cleve Clin J Med 2007; 74:748–754.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Somes GW, Pahor M, Shorr RI, Cushman WC, Applegate WB. The role of diastolic blood pressure when treating isolated systolic hypertension. Arch Intern Med 1991; 159:2004–2009.
- Fagard RH, Staessen JA, Thijs L, et al. On-treatment diastolic blood pressure and prognosis in systolic hypertension. Arch Intern Med 2007; 167:1884–1891.
To the Editor: I read with interest the exchange of letters between Drs. Norenberg and Graves in the December 2007 issue,1,2 which followed Dr. Graves’ article in the October 2007 issue.3 Dr. Norenberg suggests that it is not always prudent to try to push systolic pressures below 140 mm Hg in the elderly, and Dr. Graves takes the position that physicians like Dr. Norenberg have been “too slow to adapt to evidence-based guidelines for quality of care.” I would like to focus on Dr. Graves’ reference to evidence-based guidelines for the treatment of systolic hypertension in the elderly.
Although there have been multiple published studies of the treatment of this disorder, none has achieved an average systolic blood pressure lower than 140. The Systolic Hypertension in the Elderly Program (SHEP)4 came closest with a final systolic blood pressure of 144. No study has ever documented the efficacy and safety of achieving systolic blood pressures less than 140 in a cohort of elderly patients, and there is substantial evidence that excessive lowering of diastolic blood pressure can be harmful.5,6
Many elderly patients can achieve the target referenced by Dr. Graves, and it is reasonable to expect physicians to continue to strive for that goal, but it would be unwise to push all seniors below 140 systolic. Consider the elderly patient with systolic hypertension who is on a robust three-drug regimen including a diuretic, with a blood pressure of 144/60 and with persistent but tolerable drug side effects. I am aware of no clinical trials that demonstrate that further lowering of this patient’s blood pressure would provide incremental benefit to outweigh the potential risks and costs of additional medications.
We need to be careful not to confuse evidence-based medicine with high-placed opinions, which can result in rigid approaches to treatment that are not in the best interest of our patients.
To the Editor: I read with interest the exchange of letters between Drs. Norenberg and Graves in the December 2007 issue,1,2 which followed Dr. Graves’ article in the October 2007 issue.3 Dr. Norenberg suggests that it is not always prudent to try to push systolic pressures below 140 mm Hg in the elderly, and Dr. Graves takes the position that physicians like Dr. Norenberg have been “too slow to adapt to evidence-based guidelines for quality of care.” I would like to focus on Dr. Graves’ reference to evidence-based guidelines for the treatment of systolic hypertension in the elderly.
Although there have been multiple published studies of the treatment of this disorder, none has achieved an average systolic blood pressure lower than 140. The Systolic Hypertension in the Elderly Program (SHEP)4 came closest with a final systolic blood pressure of 144. No study has ever documented the efficacy and safety of achieving systolic blood pressures less than 140 in a cohort of elderly patients, and there is substantial evidence that excessive lowering of diastolic blood pressure can be harmful.5,6
Many elderly patients can achieve the target referenced by Dr. Graves, and it is reasonable to expect physicians to continue to strive for that goal, but it would be unwise to push all seniors below 140 systolic. Consider the elderly patient with systolic hypertension who is on a robust three-drug regimen including a diuretic, with a blood pressure of 144/60 and with persistent but tolerable drug side effects. I am aware of no clinical trials that demonstrate that further lowering of this patient’s blood pressure would provide incremental benefit to outweigh the potential risks and costs of additional medications.
We need to be careful not to confuse evidence-based medicine with high-placed opinions, which can result in rigid approaches to treatment that are not in the best interest of our patients.
- Norenberg DD. What is adequate hypertension control? (Letter). Cleve Clin J Med 2007; 74:848.
- Graves JW. What is adequate hypertension control (In Reply). Cleve Clin J Med 2007; 74:848–849.
- Graves JW. What is adequate hypertension control? Having your dinner and dessert too. Cleve Clin J Med 2007; 74:748–754.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Somes GW, Pahor M, Shorr RI, Cushman WC, Applegate WB. The role of diastolic blood pressure when treating isolated systolic hypertension. Arch Intern Med 1991; 159:2004–2009.
- Fagard RH, Staessen JA, Thijs L, et al. On-treatment diastolic blood pressure and prognosis in systolic hypertension. Arch Intern Med 2007; 167:1884–1891.
- Norenberg DD. What is adequate hypertension control? (Letter). Cleve Clin J Med 2007; 74:848.
- Graves JW. What is adequate hypertension control (In Reply). Cleve Clin J Med 2007; 74:848–849.
- Graves JW. What is adequate hypertension control? Having your dinner and dessert too. Cleve Clin J Med 2007; 74:748–754.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Somes GW, Pahor M, Shorr RI, Cushman WC, Applegate WB. The role of diastolic blood pressure when treating isolated systolic hypertension. Arch Intern Med 1991; 159:2004–2009.
- Fagard RH, Staessen JA, Thijs L, et al. On-treatment diastolic blood pressure and prognosis in systolic hypertension. Arch Intern Med 2007; 167:1884–1891.
In reply: What is adequate hypertension control?
In Reply: First, I am gratified by the tremendous interest in the care of the hypertensive patient that my article has generated. Dr. Norenberg and Dr. Kelleher are insightful clinicians, as evidenced by the issues that their letters raise. Secondly, as I am now 54 years old, SHEP’s definition of “elderly” as 60 years old and older appears less accurate to me! However, I think we might all agree that to date there has not been a trial with people 65 years old and younger that has not shown benefit to treatment of the blood pressure to less than 140/90 mm Hg.
I believe that Dr. Kelleher’s quest for more “evidence-based” data refers to treatment data in patients above that age. Hopefully, this quest will be answered by the results of the Hypertension in the Very Elderly Trial (HYVET).1 In this trial, 3,845 patients older than 80 years were treated to less than 140/90 mm Hg. On July 12, 2007, the trial was stopped by the data safety and monitoring board, with the expectation of published results at the European Society of Hypertension and International Society of Hypertension joint meeting in Berlin in 2008.
Third, I must remind the reader that in practicing evidence-based medicine, we clinicians always must interpret the results of double-blind placebo-controlled trials, which tell us the mean effect of a treatment, but apply this information to the individual patient seated in front of us. A recent study2 of individual blood pressure response to four forms of monotherapy showed that, in some patients, the blood pressure rose with hydrochlorothiazide instead of falling!
Fourth, Dr. Kelleher implies, correctly, that not all patients can reach the target of less than 140/90. In this regard I think the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)3 is very instructive. ALLHAT is the first trial ever to show improvement in the percent of people reaching goal blood pressure, rising from 52% to 63% during the 5-year study. ALLHAT shows us how good we can be and that we should not accept the failure to reach goal blood pressure in at least two-thirds of our patients.
The final and most important point is that the time for arguing the guideline recommendations4–6 based on our own opinion is past. Third-party payers and patients are demanding we meet those guidelines until new information suggests that they need to be altered. HYVET may force such an alteration, but until then Dr. Norenberg, Dr. Kelleher, and I must attempt to reach the target of less than 140/90 in the majority of our patients.
The final and most important point is that the time for arguing the guideline recommendations4–6 based on our own opinion is past. Third-party payers and patients are demanding we meet those guidelines until new information suggests that they need to be altered. HYVET may force such an alteration, but until then Dr. Norenberg, Dr. Kelleher, and I must attempt to reach the target of less than 140/90 in the majority of our patients.
- Imperial College London. Trial stops after stroke and mortality significantly reduced by blood pressure-lowering treatment for those aged 80 and over (Press Release). Accessed December 31, 2007. www.servier.com/pro/identification.asp.
- Hiltunen TP, Suonsyrja T, Hannila-Handelberg T, et al. Predictors of antihypertensive drug responses: initial data from a placebo-controlled, randomized, cross-over study with four antihypertensive drugs (The GENRES Study). Am J Hypertens 2007; 20:311–318.
- ALLHAT Collaborative Research Group. Major cardiovascular Events in hypertensive patients randomized to doxazosin vs chlorthalidone: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2000; 283:1967–1975. Correction in JAMA 2000; 288:2976.
- Khan NA, McAlister FA, Rabkin SW, et al Canadian Hypertension Education Program. The 2006 Canadian Hypertension Education Program recommendations for the management of hypertension: part II—therapy. Can J Cardiol 2006; 22:583–593.
- Chobanian AV, Bakris GL, Black HR, et al National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Mancia G, De Backer G, Dominiczak A, et al the Task Force For the Management of Arterial Hypertension of the European Society of Hypertension and the European Society of Cardiology. 2007 guidelines for the management of arterial hypertension. J Hypertens 2007; 25:1105–1187.
In Reply: First, I am gratified by the tremendous interest in the care of the hypertensive patient that my article has generated. Dr. Norenberg and Dr. Kelleher are insightful clinicians, as evidenced by the issues that their letters raise. Secondly, as I am now 54 years old, SHEP’s definition of “elderly” as 60 years old and older appears less accurate to me! However, I think we might all agree that to date there has not been a trial with people 65 years old and younger that has not shown benefit to treatment of the blood pressure to less than 140/90 mm Hg.
I believe that Dr. Kelleher’s quest for more “evidence-based” data refers to treatment data in patients above that age. Hopefully, this quest will be answered by the results of the Hypertension in the Very Elderly Trial (HYVET).1 In this trial, 3,845 patients older than 80 years were treated to less than 140/90 mm Hg. On July 12, 2007, the trial was stopped by the data safety and monitoring board, with the expectation of published results at the European Society of Hypertension and International Society of Hypertension joint meeting in Berlin in 2008.
Third, I must remind the reader that in practicing evidence-based medicine, we clinicians always must interpret the results of double-blind placebo-controlled trials, which tell us the mean effect of a treatment, but apply this information to the individual patient seated in front of us. A recent study2 of individual blood pressure response to four forms of monotherapy showed that, in some patients, the blood pressure rose with hydrochlorothiazide instead of falling!
Fourth, Dr. Kelleher implies, correctly, that not all patients can reach the target of less than 140/90. In this regard I think the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)3 is very instructive. ALLHAT is the first trial ever to show improvement in the percent of people reaching goal blood pressure, rising from 52% to 63% during the 5-year study. ALLHAT shows us how good we can be and that we should not accept the failure to reach goal blood pressure in at least two-thirds of our patients.
The final and most important point is that the time for arguing the guideline recommendations4–6 based on our own opinion is past. Third-party payers and patients are demanding we meet those guidelines until new information suggests that they need to be altered. HYVET may force such an alteration, but until then Dr. Norenberg, Dr. Kelleher, and I must attempt to reach the target of less than 140/90 in the majority of our patients.
The final and most important point is that the time for arguing the guideline recommendations4–6 based on our own opinion is past. Third-party payers and patients are demanding we meet those guidelines until new information suggests that they need to be altered. HYVET may force such an alteration, but until then Dr. Norenberg, Dr. Kelleher, and I must attempt to reach the target of less than 140/90 in the majority of our patients.
In Reply: First, I am gratified by the tremendous interest in the care of the hypertensive patient that my article has generated. Dr. Norenberg and Dr. Kelleher are insightful clinicians, as evidenced by the issues that their letters raise. Secondly, as I am now 54 years old, SHEP’s definition of “elderly” as 60 years old and older appears less accurate to me! However, I think we might all agree that to date there has not been a trial with people 65 years old and younger that has not shown benefit to treatment of the blood pressure to less than 140/90 mm Hg.
I believe that Dr. Kelleher’s quest for more “evidence-based” data refers to treatment data in patients above that age. Hopefully, this quest will be answered by the results of the Hypertension in the Very Elderly Trial (HYVET).1 In this trial, 3,845 patients older than 80 years were treated to less than 140/90 mm Hg. On July 12, 2007, the trial was stopped by the data safety and monitoring board, with the expectation of published results at the European Society of Hypertension and International Society of Hypertension joint meeting in Berlin in 2008.
Third, I must remind the reader that in practicing evidence-based medicine, we clinicians always must interpret the results of double-blind placebo-controlled trials, which tell us the mean effect of a treatment, but apply this information to the individual patient seated in front of us. A recent study2 of individual blood pressure response to four forms of monotherapy showed that, in some patients, the blood pressure rose with hydrochlorothiazide instead of falling!
Fourth, Dr. Kelleher implies, correctly, that not all patients can reach the target of less than 140/90. In this regard I think the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)3 is very instructive. ALLHAT is the first trial ever to show improvement in the percent of people reaching goal blood pressure, rising from 52% to 63% during the 5-year study. ALLHAT shows us how good we can be and that we should not accept the failure to reach goal blood pressure in at least two-thirds of our patients.
The final and most important point is that the time for arguing the guideline recommendations4–6 based on our own opinion is past. Third-party payers and patients are demanding we meet those guidelines until new information suggests that they need to be altered. HYVET may force such an alteration, but until then Dr. Norenberg, Dr. Kelleher, and I must attempt to reach the target of less than 140/90 in the majority of our patients.
The final and most important point is that the time for arguing the guideline recommendations4–6 based on our own opinion is past. Third-party payers and patients are demanding we meet those guidelines until new information suggests that they need to be altered. HYVET may force such an alteration, but until then Dr. Norenberg, Dr. Kelleher, and I must attempt to reach the target of less than 140/90 in the majority of our patients.
- Imperial College London. Trial stops after stroke and mortality significantly reduced by blood pressure-lowering treatment for those aged 80 and over (Press Release). Accessed December 31, 2007. www.servier.com/pro/identification.asp.
- Hiltunen TP, Suonsyrja T, Hannila-Handelberg T, et al. Predictors of antihypertensive drug responses: initial data from a placebo-controlled, randomized, cross-over study with four antihypertensive drugs (The GENRES Study). Am J Hypertens 2007; 20:311–318.
- ALLHAT Collaborative Research Group. Major cardiovascular Events in hypertensive patients randomized to doxazosin vs chlorthalidone: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2000; 283:1967–1975. Correction in JAMA 2000; 288:2976.
- Khan NA, McAlister FA, Rabkin SW, et al Canadian Hypertension Education Program. The 2006 Canadian Hypertension Education Program recommendations for the management of hypertension: part II—therapy. Can J Cardiol 2006; 22:583–593.
- Chobanian AV, Bakris GL, Black HR, et al National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Mancia G, De Backer G, Dominiczak A, et al the Task Force For the Management of Arterial Hypertension of the European Society of Hypertension and the European Society of Cardiology. 2007 guidelines for the management of arterial hypertension. J Hypertens 2007; 25:1105–1187.
- Imperial College London. Trial stops after stroke and mortality significantly reduced by blood pressure-lowering treatment for those aged 80 and over (Press Release). Accessed December 31, 2007. www.servier.com/pro/identification.asp.
- Hiltunen TP, Suonsyrja T, Hannila-Handelberg T, et al. Predictors of antihypertensive drug responses: initial data from a placebo-controlled, randomized, cross-over study with four antihypertensive drugs (The GENRES Study). Am J Hypertens 2007; 20:311–318.
- ALLHAT Collaborative Research Group. Major cardiovascular Events in hypertensive patients randomized to doxazosin vs chlorthalidone: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2000; 283:1967–1975. Correction in JAMA 2000; 288:2976.
- Khan NA, McAlister FA, Rabkin SW, et al Canadian Hypertension Education Program. The 2006 Canadian Hypertension Education Program recommendations for the management of hypertension: part II—therapy. Can J Cardiol 2006; 22:583–593.
- Chobanian AV, Bakris GL, Black HR, et al National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Mancia G, De Backer G, Dominiczak A, et al the Task Force For the Management of Arterial Hypertension of the European Society of Hypertension and the European Society of Cardiology. 2007 guidelines for the management of arterial hypertension. J Hypertens 2007; 25:1105–1187.
How to evaluate ‘dipstick hematuria’: What to do before you refer
Many people have some amount of blood in their urine, but relatively few have a serious problem.
In a population-based study in Rochester, Minnesota, red blood cells were found in the urine of 13% of symptom-free adults.1 In other studies, the figure ranged from 9% to 18%.2
Hematuria is sometimes detected during investigation of symptoms such as dysuria, urinary frequency, or flank pain. However, many referrals to urologists are made purely on the basis of the results of a dipstick urinalysis screening test in a patient without symptoms.
The United States Preventive Task Force3 discourages routine testing for hematuria to screen for bladder cancer in patients without symptoms, and the Canadian Task Force on the Periodic Health Examination4 and the American Urological Association (AUA)2,5 do not recommend it either. Nevertheless, approximately 40% of primary care physicians believe in it,6 although the number seems to be declining.7 A reason that dipstick testing is so popular is that it is an inexpensive and simple way to detect glucosuria and medical renal disease.8–11
The risk of significant disease in a patient with microhematuria but without symptoms is low, and the evaluation for hematuria can be costly and invasive. For an individual patient with a hemoglobin-positive dipstick test, the finding should not be ignored, but the patient does not necessarily need a complete evaluation. It is important to determine which patients require urologic studies and consultation, nephrologic evaluation, or no intervention at all.
This review addresses issues related to hematuria for the primary care physician, clarifies some of the important definitions, builds on these terms to delineate which patients should be referred to a urologist, and provides simple recommendations about ancillary studies and their potential role before urologic consultation. Understanding this information will ultimately assure appropriate management of patients without symptoms who have positive dipstick screening tests, leading to decreased use of costly and invasive tests and more appropriate long-term follow-up.
BASIC DEFINITIONS
Gross (macroscopic) hematuria is blood in the urine that is visible without microscopy. This condition almost always warrants urologic evaluation.2,5
Microscopic hematuria, or microhematuria, is the finding of red blood cells in the urine on microscopy. (In contrast, in dipstick hematuria—see below—blood cells may or may not be present in the urine.)
“Dipstick hematuria” and “dipstick microhematuria” are potential misnomers. The dip-stick test for hematuria is a nondiagnostic screening test. A positive result is simply a color change due to oxidation of a test-strip reagent; it does not confirm that blood cells are present. Factors that can cause a false-positive result on a dipstick test include hemoglobinuria, myoglobinuria, concentrated urine, menstrual blood in the urine sample, and rigorous exercise.12 Thus, the diagnosis of hematuria cannot be made by dipstick alone. Unless red blood cells are seen microscopically, the term microhematuria is inappropriate.
Of note, if the specific gravity of the urine is very low (< 1.007), microscopy can fail to detect urinary red blood cells, owing to cell lysis.2 This limitation can be overcome by restricting the patient’s fluid intake and then repeating the urinalysis.
Many patients with a positive dipstick oxidation reaction are labeled as having dip-stick hematuria although microscopic analysis would show that red blood cells are absent. Perhaps the term dipstick pseudohematuria would be more accurate. These patients will not benefit from a costly and invasive urologic workup, so it is crucial to distinguish them from patients with true microhematuria.
SIGNIFICANT HEMATURIA: ≥ 3 RBCs/HPF
Microhematuria is often intermittent, and many healthy patients occasionally have a few red blood cells in the urine.13 However, no cutoff point for the amount of hematuria can be used to rule out the possibility of cancer.14 To account for these complicating factors, the AUA Best Practice Policy Panel on Asymptomatic Hematuria considered the literature and expert opinions to define the amount of microhematuria warranting evaluation in patients with risk factors for significant urologic disease.2,5
The AUA panel defined microhematuria as an average of three or more red blood cells per high-power microscopic field (RBCs/HPF) in two out of three properly collected and prepared specimens. Urine should be collected midstream after wiping the urethral meatus with a disinfectant and voiding the initial portion of urine into the toilet. For proper preparation of the urine sample, 10 mL of urine should be centrifuged at 2,000 rpm for 5 minutes and the supernatant discarded. The sediment should then be resuspended in 0.5 to 1.0 mL of remaining urine, and a drop of this suspension should be examined microscopically. If contamination is suggested (ie, if squamous epithelial cells, bacteria, or both are present), one should consider collecting a specimen through catheterization.2,5
The AUA guidelines do not specify some of the details of management, such as the timing of subsequent microscopic urinalyses, but we recommend that all urinalyses to establish whether significant hematuria is present be done within 3 to 6 months of the screening dip-stick test. If a patient has no risk factors for cancer and has a negative result on the first microscopic urinalysis, the follow-up test can be performed in 1 year. Table 1 shows risk factors for significant urologic disease that warrant evaluation in patients with asymptomatic hematuria.5
Isolated urinary findings that might warrant evaluation by a nephrologist rather than a urologist include proteinuria, red cell casts, and dysmorphic red blood cells, especially if the serum creatinine level is elevated.2,5 Many medical renal conditions (eg, glomerulonephritis) and hemoglobinopathies (eg, sickle cell trait) can cause blood in the urine; when these conditions are accompanied by risk factors for urologic disease, urologic evaluation is indicated as well.
CLINICAL RELEVANCE OF HEMATURIA
Approximately 25% of cases of macroscopic hematuria are due to urologic cancers,14,15 and another 34% are due to other significant urologic diseases14—thus, the recommendation that patients with macroscopic hematuria be evaluated by a urologist. In contrast, in microhematuria, the rates of cancer are much lower, ranging between 1% and 10% in large studies.2,5
The urine dipstick test has been found to be 65% to 99% specific for the presence of blood cells, free hemoglobin, or myoglobin.2,5 If the true specificity is closer to the lower figure and all patients with a positive dipstick test were referred to a urologist, this would mean the urologic workup would be unnecessary in up to 35% of them, because the dipstick result would be falsely positive.
But that is not all. Most causes of hemoglobinuria or myoglobinuria are of limited clinical significance, except for rare conditions that are usually clinically obvious, such as severe burn injury. Further, remember that from 9% to 18% of patients without symptoms have red blood cells in the urine.2,5 In theory, if everyone in the United States had a dipstick test, this would be positive in patients with hematuria as well as in those with hemoglobinuria, myoglobinuria, and other false-positives; if everyone with a positive dipstick result were then referred to a urologist, a substantial portion of the population would receive an unnecessary urologic referral.
Urologic referral and evaluation in these patients not only wastes money: if they undergo imaging studies, they are exposed to radiation and contrast media, with their associated risks, and if they undergo cystoscopy, they face its attendant discomfort and risk of infection.
WHICH PATIENTS WITH HEMATURIA TO REFER TO A UROLOGIST
Gross hematuria
Red or tea-colored urine usually indicates gross hematuria. When there is any doubt—such as in the case of a color-blind patient—the presence of red blood cells can be confirmed or ruled out by a microscopic urinalysis.
Nearly all patients with an episode of gross hematuria should be referred to a urologist. The sole exception to this rule can be made when a woman younger than 40 years experiences gross hematuria in the classic setting of a culture-proven, symptomatic urinary tract infection (UTI) and her infection, symptoms, and hematuria all resolve completely with appropriate antibiotics.2,5 However, bleeding from cancer is classically intermittent. Therefore, one should not skip the urine culture and just prescribe antibiotics empirically: the patient might actually have cancer, but the supposed UTI may appear to resolve with antibiotic therapy. For the same reason, resolution of hematuria in any other setting does not obviate the need for referral.
Another scenario usually associated with a benign cause is bleeding after extreme physical activity—also known as “runner’s hematuria” or “march hematuria” (so named because it sometimes occurs in soldiers after a particularly grueling training march). Importantly, even in this situation, one should still be suspicious and probably refer the patient to a urologist: just because the patient has just run a marathon, it does not mean that he or she does not have cancer.
Depending on the character, timing, location, and many other characteristics of the patient’s bleeding, a variety of studies may or may not be necessary. For example, blood-spotting of the underpants might signify urethral bleeding, and imaging and cytologic studies might not be indicated. In view of the variability in presentation and workup, we recommend that the proper workup for these patients be determined by a urologist.
Symptomatic microhematuria
Patients with true microhematuria (three or more RBCs/HPF) accompanied by bothersome or worrisome symptoms should be referred to a urologist. In a study in Scotland, Sultana et al16 found that cancer was present in 6 (5%) of 126 patients with microhematuria without symptoms compared with 13 (10.5%) of 124 patients with microhematuria and irritative voiding symptoms; the difference, however, was not statistically significant.
Microscopic urinalysis should be part of the evaluation for flank pain or certain urinary symptoms such as frequency, urgency, retention, or dysuria; results of this test at the time of symptoms can later help the urologist distinguish the cause of the symptoms or hematuria. In addition, in combination with dipstick analysis, microscopic analysis can help distinguish patients with UTI or medical renal disease. If the evaluation suggests that the hematuria and symptoms are due to a UTI, then the findings on a repeat microscopic analysis, performed after the infection has cleared, should be normal. If hematuria, defined as three or more RBCs/HPF, persists in two of three subsequent urinalyses, then the guidelines mandate diagnostic evaluation even if the urinalysis subsequently becomes negative.
Asymptomatic hematuria
In symptom-free patients with dipstick hematuria found on a screening examination, it is crucial to confirm and document true microhematuria. Per the AUA guidelines, microhematuria worthy of urologic workup is the presence of three or more RBCs/HPF on at least two out of three microscopic urinalyses.2,5 Patients with dipstick pseudohematuria do not meet this criterion and will not benefit from a costly and invasive evaluation. Conversely, patients with higher levels of microhematuria, who have any risk factors for cancer, or who are anxious about the test results might benefit from urologic consultation before a second urinalysis to confirm the first, positive finding.
Many patients younger than age 40 with asymptomatic microhematuria but no other risk factors for urinary tract cancer can be followed conservatively. Khadra et al14 reported that only 1 of 143 patients younger than 40 years with microhematuria had cancer. Similarly, Jones et al17 found, in a prospective study, that no man younger than 40 years with microscopic hematuria had cancer.
Of note: gross or microscopic blood in the urine, even in the setting of anticoagulation, is a marker of urinary tract pathology such as cancer, stones, or infection. Just as patients on anticoagulation therapy who develop gastrointestinal bleeding need a gastrointestinal evaluation, those with hematuria require a urologic evaluation.2,5
STUDIES TO CONSIDER BEFORE CONSULTATION
In symptom-free patients, it is inappropriate to order laboratory or imaging tests on the basis of a dipstick test alone, without confirming that they actually have hematuria. When the blood is confirmed to be present by microscopic examination of centrifuged urine (as described above), benign causes such as UTI should be considered. If a patient does have a UTI with hematuria, urinalysis should be repeated once the infection has cleared up.
Imaging studies
For symptomatic microhematuria. Patients with acute symptoms of renal colic should undergo computed tomography (CT) in a “stone protocol” (without contrast, with 3- to 5-mm cuts of the abdomen and pelvis) to assess for urinary lithiasis. Pregnancy should always be ruled out before radiation exposure; renal ultrasonography is generally the first-choice imaging study for pregnant patients.
For asymptomatic microhematuria. Patients without the classic flank pain of urolithiasis should undergo more extensive studies. For patients at increased risk of cancer, such as heavy smokers, CT urography is the optimal imaging study and is the test least likely to necessitate other follow-up studies.18–20 Other imaging options, including ultrasonography and intravenous pyelography, incompletely assess the upper urinary tracts including both renal parenchyma and urothelial surfaces. CT urography has been shown to find more than 40% of hematuria-causing lesions missed by other studies.18 Because ordering alternative imaging first will often result in redundant studies, CT urography is the preferred initial imaging study in the evaluation of hematuria.
Before exposing a patient to contrast media, one should ascertain that he or she is not allergic to it. In addition, in patients at risk of contrast nephropathy (ie, those older than 60 years, with diabetes, or with preexisting medical renal disease), one should check the serum creatinine concentration. Magnetic resonance urography, a more expensive study, is as accurate as CT for diagnosing many urologic conditions, so it can be performed in lieu of CT urography in patients with renal insufficiency, iodine allergy, or any reason to avoid ionizing radiation. Some clinicians perform plain radiography of the kidneys, ureters, and bladder as well as ultrasonography in this setting, but determination of the appropriate alternative to CT urography, if required, is best left to the urologist.
Other tests
Cytologic testing of the urine can be valuable in patients with gross hematuria and in those with microhematuria who have risk factors for urinary tract cancer. Although its reported median sensitivity for malignancy is only 48%, a positive cytologic test is approximately 94% specific for malignancy.21 Other studies, such as the fluorescence in situ hybridization assay, and the nuclear matrix protein 22 test do not yet have a clear role in the diagnosis of urinary tract disease.22
However, in general, we caution non-urologists not to order special tumor marker or cytologic tests, or to do so only with careful forethought. Although these studies occasionally detect occult cancer in patients at high risk, an “atypical” finding on cytology or a positive tumor marker test can lead to inappropriate referral and unnecessary biopsy or other tests.
WHEN NOT TO REFER A PATIENT WITH HEMATURIA TO A UROLOGIST
Symptom-free patients with a positive dipstick hemoglobin test should not immediately be referred to a urologist: they should have a microscopic urinalysis first to determine whether they actually have microhematuria, unless microscopic laboratory services are unavailable. Only patients with documented true hematuria, as defined by the AUA guidelines, should be referred for urologic evaluation and diagnostic testing. Once a patient is referred for evaluation, the consultant is under clear pressure to perform a complete investigation to fulfill the expectations of the referring physician. Avoiding expensive unnecessary testing and referral in those without hematuria allows appropriate utilization of resources.
Patients with microhematuria associated with a UTI should have a repeat urinalysis after the UTI is successfully treated; if the hematuria clears with the infection, then the patient needs no further evaluation. Patients with dipstick pseudohematuria and significant proteinuria or a predominance of dysmorphic urinary blood cells might benefit from an evaluation by a nephrologist rather than a urologist.2 This is especially true if the patient has an elevated serum creatinine level.
ECONOMIC RELEVANCE
In our tertiary care urology clinic, approximately 75% of patients who are referred to us because of microhematuria have not had a microscopic urinalysis before coming here. On further evaluation, up to 75% of these patients are found to have dipstick pseudohematuria that did not actually require consultation or evaluation.23 It is possible that this occurs even more frequently in the general practice setting.
A Medicare level-4 urologic consultation for hematuria costs $170; the cumulative cost of unwarranted referrals is undoubtedly substantial. Even more money is wasted on CT urography, cytology, and other testing performed before urologic consultation in patients ultimately found not to have true hematuria. The economic and iatrogenic risks of evaluation cannot be justified in patients who do not exhibit findings that can be considered abnormal as defined in this article.
CONCLUSION
It is important to distinguish whether hematuria is microscopic or macroscopic, whether there are associated symptoms, and whether a patient has risk factors for significant urologic disease. While dipstick tests are sensitive, they do not reliably diagnose microhematuria, which is the microscopically proven presence of urinary red blood cells. Positive dipstick tests should always be followed by microscopic urinalysis; failure to do so can result in the unfortunate and unnecessary evaluation of dipstick pseudohematuria, a normal condition.
The AUA defines significant hematuria as three or more RBCs/HPF in two of three properly prepared specimens.2 This should determine whether a symptom-free patient needs urologic referral and evaluation for hematuria.
By following these principles, primary care physicians have a valuable opportunity to direct medical care, increase the efficiency of our health care system, and protect patients from the anxiety, costs, and risks of an unnecessary urologic workup.
- Mohr DN, Offord KP, Owen RA, Melton LJ. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986; 256:224–229.
- Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy—part I: definition, detection, prevalence, and etiology. Urology 2001; 57:599–603.
- US Preventive Services Task Force. Screening for Bladder Cancer, updated November 2004. Available at www.ahrq.gov/clinic/uspstf/uspsblad.htm.
- Logsetty S. Screening for bladder cancer. Canadian Task Force on the Periodic Health Examination. Canadian Guide to Clinical Preventive Health Care. 1994: Ottawa: Health Canada,826–836.
- Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: The American Urological Association Best Practice Policy—Part II: patient evaluation, cytology, voided markers, imaging cystoscopy, nephrology evaluation, and follow-up. Urology 2001; 57:604–610.
- Prochazka AV, Lundahl K, Pearson W, Oboler SK, Anderson RJ. Support of evidence-based guidelines for the annual physical examination: a survey of primary care providers. Arch Intern Med 2005; 165:1347–1352.
- Chacko KM, Feinberg LE. Laboratory screening at preventive health exams: trend of testing, 1978–2004. Am J Prev Med 2007; 32:59–62.
- Mariani AJ, Luangphinith S, Loo S, Scottolini A, Hodges CV. Dipstick chemical urinalysis: an accurate cost-effective screening test. J Urol 1984; 132:64–66.
- Murphy TE. The urinalysis—inexpensive and informative. J Insur Med 2004; 36:320–326.
- Woolhandler S, Pels RJ, Bor DH, Himmelstein DU, Lawrence RS. Dipstick urinalysis screening of asymptomatic adults for urinary tract disorders. I. Hematuria and proteinuria. JAMA 1989; 262:1214–1219.
- Pels RJ, Bor DH, Woolhandler S, Himmelstein DU, Lawrence RS. Dipstick urinalysis screening of asymptomatic adults for urinary tract disorders. II. Bacteriuria. JAMA 1989; 262:1221–1224.
- Gerber GS, Brendler CBWein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA. Evaluation of the urologic patient: history, physical examination, and urinalysis. Campbell-Walsh Urology 2007; 9th ed. Saunders Elsevier: Philadelphia:81–110.
- Cohen RA, Brown RS. Clinical practice. Microscopic hematuria. N Engl J Med 2003; 348:2330–2338.
- Khadra MH, Pickard RS, Charlton M, Powell PH, Neal DE. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol 2000; 163:524–527.
- Alishahi S, Byrne D, Goodman CM, Baxby K. Haematuria investigation based on a standard protocol: emphasis on the diagnosis of urological malignancy. J R Coll Surg Edinb 2002; 47:422–427.
- Sultana SR, Goodman CM, Byrne DJ, Baxby K. Microscopic haematuria: urological investigation using a standard protocol. Br J Urol 1996; 78:691–696.
- Jones DJ, Langstaff RJ, Holt SD, Morgans BT. The value of cystourethroscopy in the investigation of microscopic haematuria in adult males under 40 years. A prospective study of 100 patients. Br J Urol 1988; 62:541–545.
- Lang EK, Thomas R, Davis R, et al. Multiphasic helical computerized tomography for the assessment of microscopic hematuria: a prospective study. J Urol 2004; 171:237–243.
- Gray Sears CL, Ward JF, Sears ST, Puckett MF, Kane CJ, Amling CL. Prospective comparison of computerized tomography and excretory urography in the initial evaluation of asymptomatic microhematuria. J Urol 2002; 168:2457–2460.
- Liu W, Mortelé KJ, Silverman SG. Incidental extraurinary findings at MDCT urography in patients with hematuria: prevalence and impact on imaging costs. AJR Am J Roentgenol 2005; 185:1051–1056.
- van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol 2005; 47:736–748.
- Black PC, Brown GA, Dinney CP. Molecular markers of urothelial cancer and their use in the monitoring of superficial urothelial cancer. J Clin Oncol 2006; 24:5528–5535.
- Rao PR, Jones JS. Retrospective chart review of consultations for hematuria, 2004–2006. Unpublished data. Manuscript in progress.
Many people have some amount of blood in their urine, but relatively few have a serious problem.
In a population-based study in Rochester, Minnesota, red blood cells were found in the urine of 13% of symptom-free adults.1 In other studies, the figure ranged from 9% to 18%.2
Hematuria is sometimes detected during investigation of symptoms such as dysuria, urinary frequency, or flank pain. However, many referrals to urologists are made purely on the basis of the results of a dipstick urinalysis screening test in a patient without symptoms.
The United States Preventive Task Force3 discourages routine testing for hematuria to screen for bladder cancer in patients without symptoms, and the Canadian Task Force on the Periodic Health Examination4 and the American Urological Association (AUA)2,5 do not recommend it either. Nevertheless, approximately 40% of primary care physicians believe in it,6 although the number seems to be declining.7 A reason that dipstick testing is so popular is that it is an inexpensive and simple way to detect glucosuria and medical renal disease.8–11
The risk of significant disease in a patient with microhematuria but without symptoms is low, and the evaluation for hematuria can be costly and invasive. For an individual patient with a hemoglobin-positive dipstick test, the finding should not be ignored, but the patient does not necessarily need a complete evaluation. It is important to determine which patients require urologic studies and consultation, nephrologic evaluation, or no intervention at all.
This review addresses issues related to hematuria for the primary care physician, clarifies some of the important definitions, builds on these terms to delineate which patients should be referred to a urologist, and provides simple recommendations about ancillary studies and their potential role before urologic consultation. Understanding this information will ultimately assure appropriate management of patients without symptoms who have positive dipstick screening tests, leading to decreased use of costly and invasive tests and more appropriate long-term follow-up.
BASIC DEFINITIONS
Gross (macroscopic) hematuria is blood in the urine that is visible without microscopy. This condition almost always warrants urologic evaluation.2,5
Microscopic hematuria, or microhematuria, is the finding of red blood cells in the urine on microscopy. (In contrast, in dipstick hematuria—see below—blood cells may or may not be present in the urine.)
“Dipstick hematuria” and “dipstick microhematuria” are potential misnomers. The dip-stick test for hematuria is a nondiagnostic screening test. A positive result is simply a color change due to oxidation of a test-strip reagent; it does not confirm that blood cells are present. Factors that can cause a false-positive result on a dipstick test include hemoglobinuria, myoglobinuria, concentrated urine, menstrual blood in the urine sample, and rigorous exercise.12 Thus, the diagnosis of hematuria cannot be made by dipstick alone. Unless red blood cells are seen microscopically, the term microhematuria is inappropriate.
Of note, if the specific gravity of the urine is very low (< 1.007), microscopy can fail to detect urinary red blood cells, owing to cell lysis.2 This limitation can be overcome by restricting the patient’s fluid intake and then repeating the urinalysis.
Many patients with a positive dipstick oxidation reaction are labeled as having dip-stick hematuria although microscopic analysis would show that red blood cells are absent. Perhaps the term dipstick pseudohematuria would be more accurate. These patients will not benefit from a costly and invasive urologic workup, so it is crucial to distinguish them from patients with true microhematuria.
SIGNIFICANT HEMATURIA: ≥ 3 RBCs/HPF
Microhematuria is often intermittent, and many healthy patients occasionally have a few red blood cells in the urine.13 However, no cutoff point for the amount of hematuria can be used to rule out the possibility of cancer.14 To account for these complicating factors, the AUA Best Practice Policy Panel on Asymptomatic Hematuria considered the literature and expert opinions to define the amount of microhematuria warranting evaluation in patients with risk factors for significant urologic disease.2,5
The AUA panel defined microhematuria as an average of three or more red blood cells per high-power microscopic field (RBCs/HPF) in two out of three properly collected and prepared specimens. Urine should be collected midstream after wiping the urethral meatus with a disinfectant and voiding the initial portion of urine into the toilet. For proper preparation of the urine sample, 10 mL of urine should be centrifuged at 2,000 rpm for 5 minutes and the supernatant discarded. The sediment should then be resuspended in 0.5 to 1.0 mL of remaining urine, and a drop of this suspension should be examined microscopically. If contamination is suggested (ie, if squamous epithelial cells, bacteria, or both are present), one should consider collecting a specimen through catheterization.2,5
The AUA guidelines do not specify some of the details of management, such as the timing of subsequent microscopic urinalyses, but we recommend that all urinalyses to establish whether significant hematuria is present be done within 3 to 6 months of the screening dip-stick test. If a patient has no risk factors for cancer and has a negative result on the first microscopic urinalysis, the follow-up test can be performed in 1 year. Table 1 shows risk factors for significant urologic disease that warrant evaluation in patients with asymptomatic hematuria.5
Isolated urinary findings that might warrant evaluation by a nephrologist rather than a urologist include proteinuria, red cell casts, and dysmorphic red blood cells, especially if the serum creatinine level is elevated.2,5 Many medical renal conditions (eg, glomerulonephritis) and hemoglobinopathies (eg, sickle cell trait) can cause blood in the urine; when these conditions are accompanied by risk factors for urologic disease, urologic evaluation is indicated as well.
CLINICAL RELEVANCE OF HEMATURIA
Approximately 25% of cases of macroscopic hematuria are due to urologic cancers,14,15 and another 34% are due to other significant urologic diseases14—thus, the recommendation that patients with macroscopic hematuria be evaluated by a urologist. In contrast, in microhematuria, the rates of cancer are much lower, ranging between 1% and 10% in large studies.2,5
The urine dipstick test has been found to be 65% to 99% specific for the presence of blood cells, free hemoglobin, or myoglobin.2,5 If the true specificity is closer to the lower figure and all patients with a positive dipstick test were referred to a urologist, this would mean the urologic workup would be unnecessary in up to 35% of them, because the dipstick result would be falsely positive.
But that is not all. Most causes of hemoglobinuria or myoglobinuria are of limited clinical significance, except for rare conditions that are usually clinically obvious, such as severe burn injury. Further, remember that from 9% to 18% of patients without symptoms have red blood cells in the urine.2,5 In theory, if everyone in the United States had a dipstick test, this would be positive in patients with hematuria as well as in those with hemoglobinuria, myoglobinuria, and other false-positives; if everyone with a positive dipstick result were then referred to a urologist, a substantial portion of the population would receive an unnecessary urologic referral.
Urologic referral and evaluation in these patients not only wastes money: if they undergo imaging studies, they are exposed to radiation and contrast media, with their associated risks, and if they undergo cystoscopy, they face its attendant discomfort and risk of infection.
WHICH PATIENTS WITH HEMATURIA TO REFER TO A UROLOGIST
Gross hematuria
Red or tea-colored urine usually indicates gross hematuria. When there is any doubt—such as in the case of a color-blind patient—the presence of red blood cells can be confirmed or ruled out by a microscopic urinalysis.
Nearly all patients with an episode of gross hematuria should be referred to a urologist. The sole exception to this rule can be made when a woman younger than 40 years experiences gross hematuria in the classic setting of a culture-proven, symptomatic urinary tract infection (UTI) and her infection, symptoms, and hematuria all resolve completely with appropriate antibiotics.2,5 However, bleeding from cancer is classically intermittent. Therefore, one should not skip the urine culture and just prescribe antibiotics empirically: the patient might actually have cancer, but the supposed UTI may appear to resolve with antibiotic therapy. For the same reason, resolution of hematuria in any other setting does not obviate the need for referral.
Another scenario usually associated with a benign cause is bleeding after extreme physical activity—also known as “runner’s hematuria” or “march hematuria” (so named because it sometimes occurs in soldiers after a particularly grueling training march). Importantly, even in this situation, one should still be suspicious and probably refer the patient to a urologist: just because the patient has just run a marathon, it does not mean that he or she does not have cancer.
Depending on the character, timing, location, and many other characteristics of the patient’s bleeding, a variety of studies may or may not be necessary. For example, blood-spotting of the underpants might signify urethral bleeding, and imaging and cytologic studies might not be indicated. In view of the variability in presentation and workup, we recommend that the proper workup for these patients be determined by a urologist.
Symptomatic microhematuria
Patients with true microhematuria (three or more RBCs/HPF) accompanied by bothersome or worrisome symptoms should be referred to a urologist. In a study in Scotland, Sultana et al16 found that cancer was present in 6 (5%) of 126 patients with microhematuria without symptoms compared with 13 (10.5%) of 124 patients with microhematuria and irritative voiding symptoms; the difference, however, was not statistically significant.
Microscopic urinalysis should be part of the evaluation for flank pain or certain urinary symptoms such as frequency, urgency, retention, or dysuria; results of this test at the time of symptoms can later help the urologist distinguish the cause of the symptoms or hematuria. In addition, in combination with dipstick analysis, microscopic analysis can help distinguish patients with UTI or medical renal disease. If the evaluation suggests that the hematuria and symptoms are due to a UTI, then the findings on a repeat microscopic analysis, performed after the infection has cleared, should be normal. If hematuria, defined as three or more RBCs/HPF, persists in two of three subsequent urinalyses, then the guidelines mandate diagnostic evaluation even if the urinalysis subsequently becomes negative.
Asymptomatic hematuria
In symptom-free patients with dipstick hematuria found on a screening examination, it is crucial to confirm and document true microhematuria. Per the AUA guidelines, microhematuria worthy of urologic workup is the presence of three or more RBCs/HPF on at least two out of three microscopic urinalyses.2,5 Patients with dipstick pseudohematuria do not meet this criterion and will not benefit from a costly and invasive evaluation. Conversely, patients with higher levels of microhematuria, who have any risk factors for cancer, or who are anxious about the test results might benefit from urologic consultation before a second urinalysis to confirm the first, positive finding.
Many patients younger than age 40 with asymptomatic microhematuria but no other risk factors for urinary tract cancer can be followed conservatively. Khadra et al14 reported that only 1 of 143 patients younger than 40 years with microhematuria had cancer. Similarly, Jones et al17 found, in a prospective study, that no man younger than 40 years with microscopic hematuria had cancer.
Of note: gross or microscopic blood in the urine, even in the setting of anticoagulation, is a marker of urinary tract pathology such as cancer, stones, or infection. Just as patients on anticoagulation therapy who develop gastrointestinal bleeding need a gastrointestinal evaluation, those with hematuria require a urologic evaluation.2,5
STUDIES TO CONSIDER BEFORE CONSULTATION
In symptom-free patients, it is inappropriate to order laboratory or imaging tests on the basis of a dipstick test alone, without confirming that they actually have hematuria. When the blood is confirmed to be present by microscopic examination of centrifuged urine (as described above), benign causes such as UTI should be considered. If a patient does have a UTI with hematuria, urinalysis should be repeated once the infection has cleared up.
Imaging studies
For symptomatic microhematuria. Patients with acute symptoms of renal colic should undergo computed tomography (CT) in a “stone protocol” (without contrast, with 3- to 5-mm cuts of the abdomen and pelvis) to assess for urinary lithiasis. Pregnancy should always be ruled out before radiation exposure; renal ultrasonography is generally the first-choice imaging study for pregnant patients.
For asymptomatic microhematuria. Patients without the classic flank pain of urolithiasis should undergo more extensive studies. For patients at increased risk of cancer, such as heavy smokers, CT urography is the optimal imaging study and is the test least likely to necessitate other follow-up studies.18–20 Other imaging options, including ultrasonography and intravenous pyelography, incompletely assess the upper urinary tracts including both renal parenchyma and urothelial surfaces. CT urography has been shown to find more than 40% of hematuria-causing lesions missed by other studies.18 Because ordering alternative imaging first will often result in redundant studies, CT urography is the preferred initial imaging study in the evaluation of hematuria.
Before exposing a patient to contrast media, one should ascertain that he or she is not allergic to it. In addition, in patients at risk of contrast nephropathy (ie, those older than 60 years, with diabetes, or with preexisting medical renal disease), one should check the serum creatinine concentration. Magnetic resonance urography, a more expensive study, is as accurate as CT for diagnosing many urologic conditions, so it can be performed in lieu of CT urography in patients with renal insufficiency, iodine allergy, or any reason to avoid ionizing radiation. Some clinicians perform plain radiography of the kidneys, ureters, and bladder as well as ultrasonography in this setting, but determination of the appropriate alternative to CT urography, if required, is best left to the urologist.
Other tests
Cytologic testing of the urine can be valuable in patients with gross hematuria and in those with microhematuria who have risk factors for urinary tract cancer. Although its reported median sensitivity for malignancy is only 48%, a positive cytologic test is approximately 94% specific for malignancy.21 Other studies, such as the fluorescence in situ hybridization assay, and the nuclear matrix protein 22 test do not yet have a clear role in the diagnosis of urinary tract disease.22
However, in general, we caution non-urologists not to order special tumor marker or cytologic tests, or to do so only with careful forethought. Although these studies occasionally detect occult cancer in patients at high risk, an “atypical” finding on cytology or a positive tumor marker test can lead to inappropriate referral and unnecessary biopsy or other tests.
WHEN NOT TO REFER A PATIENT WITH HEMATURIA TO A UROLOGIST
Symptom-free patients with a positive dipstick hemoglobin test should not immediately be referred to a urologist: they should have a microscopic urinalysis first to determine whether they actually have microhematuria, unless microscopic laboratory services are unavailable. Only patients with documented true hematuria, as defined by the AUA guidelines, should be referred for urologic evaluation and diagnostic testing. Once a patient is referred for evaluation, the consultant is under clear pressure to perform a complete investigation to fulfill the expectations of the referring physician. Avoiding expensive unnecessary testing and referral in those without hematuria allows appropriate utilization of resources.
Patients with microhematuria associated with a UTI should have a repeat urinalysis after the UTI is successfully treated; if the hematuria clears with the infection, then the patient needs no further evaluation. Patients with dipstick pseudohematuria and significant proteinuria or a predominance of dysmorphic urinary blood cells might benefit from an evaluation by a nephrologist rather than a urologist.2 This is especially true if the patient has an elevated serum creatinine level.
ECONOMIC RELEVANCE
In our tertiary care urology clinic, approximately 75% of patients who are referred to us because of microhematuria have not had a microscopic urinalysis before coming here. On further evaluation, up to 75% of these patients are found to have dipstick pseudohematuria that did not actually require consultation or evaluation.23 It is possible that this occurs even more frequently in the general practice setting.
A Medicare level-4 urologic consultation for hematuria costs $170; the cumulative cost of unwarranted referrals is undoubtedly substantial. Even more money is wasted on CT urography, cytology, and other testing performed before urologic consultation in patients ultimately found not to have true hematuria. The economic and iatrogenic risks of evaluation cannot be justified in patients who do not exhibit findings that can be considered abnormal as defined in this article.
CONCLUSION
It is important to distinguish whether hematuria is microscopic or macroscopic, whether there are associated symptoms, and whether a patient has risk factors for significant urologic disease. While dipstick tests are sensitive, they do not reliably diagnose microhematuria, which is the microscopically proven presence of urinary red blood cells. Positive dipstick tests should always be followed by microscopic urinalysis; failure to do so can result in the unfortunate and unnecessary evaluation of dipstick pseudohematuria, a normal condition.
The AUA defines significant hematuria as three or more RBCs/HPF in two of three properly prepared specimens.2 This should determine whether a symptom-free patient needs urologic referral and evaluation for hematuria.
By following these principles, primary care physicians have a valuable opportunity to direct medical care, increase the efficiency of our health care system, and protect patients from the anxiety, costs, and risks of an unnecessary urologic workup.
Many people have some amount of blood in their urine, but relatively few have a serious problem.
In a population-based study in Rochester, Minnesota, red blood cells were found in the urine of 13% of symptom-free adults.1 In other studies, the figure ranged from 9% to 18%.2
Hematuria is sometimes detected during investigation of symptoms such as dysuria, urinary frequency, or flank pain. However, many referrals to urologists are made purely on the basis of the results of a dipstick urinalysis screening test in a patient without symptoms.
The United States Preventive Task Force3 discourages routine testing for hematuria to screen for bladder cancer in patients without symptoms, and the Canadian Task Force on the Periodic Health Examination4 and the American Urological Association (AUA)2,5 do not recommend it either. Nevertheless, approximately 40% of primary care physicians believe in it,6 although the number seems to be declining.7 A reason that dipstick testing is so popular is that it is an inexpensive and simple way to detect glucosuria and medical renal disease.8–11
The risk of significant disease in a patient with microhematuria but without symptoms is low, and the evaluation for hematuria can be costly and invasive. For an individual patient with a hemoglobin-positive dipstick test, the finding should not be ignored, but the patient does not necessarily need a complete evaluation. It is important to determine which patients require urologic studies and consultation, nephrologic evaluation, or no intervention at all.
This review addresses issues related to hematuria for the primary care physician, clarifies some of the important definitions, builds on these terms to delineate which patients should be referred to a urologist, and provides simple recommendations about ancillary studies and their potential role before urologic consultation. Understanding this information will ultimately assure appropriate management of patients without symptoms who have positive dipstick screening tests, leading to decreased use of costly and invasive tests and more appropriate long-term follow-up.
BASIC DEFINITIONS
Gross (macroscopic) hematuria is blood in the urine that is visible without microscopy. This condition almost always warrants urologic evaluation.2,5
Microscopic hematuria, or microhematuria, is the finding of red blood cells in the urine on microscopy. (In contrast, in dipstick hematuria—see below—blood cells may or may not be present in the urine.)
“Dipstick hematuria” and “dipstick microhematuria” are potential misnomers. The dip-stick test for hematuria is a nondiagnostic screening test. A positive result is simply a color change due to oxidation of a test-strip reagent; it does not confirm that blood cells are present. Factors that can cause a false-positive result on a dipstick test include hemoglobinuria, myoglobinuria, concentrated urine, menstrual blood in the urine sample, and rigorous exercise.12 Thus, the diagnosis of hematuria cannot be made by dipstick alone. Unless red blood cells are seen microscopically, the term microhematuria is inappropriate.
Of note, if the specific gravity of the urine is very low (< 1.007), microscopy can fail to detect urinary red blood cells, owing to cell lysis.2 This limitation can be overcome by restricting the patient’s fluid intake and then repeating the urinalysis.
Many patients with a positive dipstick oxidation reaction are labeled as having dip-stick hematuria although microscopic analysis would show that red blood cells are absent. Perhaps the term dipstick pseudohematuria would be more accurate. These patients will not benefit from a costly and invasive urologic workup, so it is crucial to distinguish them from patients with true microhematuria.
SIGNIFICANT HEMATURIA: ≥ 3 RBCs/HPF
Microhematuria is often intermittent, and many healthy patients occasionally have a few red blood cells in the urine.13 However, no cutoff point for the amount of hematuria can be used to rule out the possibility of cancer.14 To account for these complicating factors, the AUA Best Practice Policy Panel on Asymptomatic Hematuria considered the literature and expert opinions to define the amount of microhematuria warranting evaluation in patients with risk factors for significant urologic disease.2,5
The AUA panel defined microhematuria as an average of three or more red blood cells per high-power microscopic field (RBCs/HPF) in two out of three properly collected and prepared specimens. Urine should be collected midstream after wiping the urethral meatus with a disinfectant and voiding the initial portion of urine into the toilet. For proper preparation of the urine sample, 10 mL of urine should be centrifuged at 2,000 rpm for 5 minutes and the supernatant discarded. The sediment should then be resuspended in 0.5 to 1.0 mL of remaining urine, and a drop of this suspension should be examined microscopically. If contamination is suggested (ie, if squamous epithelial cells, bacteria, or both are present), one should consider collecting a specimen through catheterization.2,5
The AUA guidelines do not specify some of the details of management, such as the timing of subsequent microscopic urinalyses, but we recommend that all urinalyses to establish whether significant hematuria is present be done within 3 to 6 months of the screening dip-stick test. If a patient has no risk factors for cancer and has a negative result on the first microscopic urinalysis, the follow-up test can be performed in 1 year. Table 1 shows risk factors for significant urologic disease that warrant evaluation in patients with asymptomatic hematuria.5
Isolated urinary findings that might warrant evaluation by a nephrologist rather than a urologist include proteinuria, red cell casts, and dysmorphic red blood cells, especially if the serum creatinine level is elevated.2,5 Many medical renal conditions (eg, glomerulonephritis) and hemoglobinopathies (eg, sickle cell trait) can cause blood in the urine; when these conditions are accompanied by risk factors for urologic disease, urologic evaluation is indicated as well.
CLINICAL RELEVANCE OF HEMATURIA
Approximately 25% of cases of macroscopic hematuria are due to urologic cancers,14,15 and another 34% are due to other significant urologic diseases14—thus, the recommendation that patients with macroscopic hematuria be evaluated by a urologist. In contrast, in microhematuria, the rates of cancer are much lower, ranging between 1% and 10% in large studies.2,5
The urine dipstick test has been found to be 65% to 99% specific for the presence of blood cells, free hemoglobin, or myoglobin.2,5 If the true specificity is closer to the lower figure and all patients with a positive dipstick test were referred to a urologist, this would mean the urologic workup would be unnecessary in up to 35% of them, because the dipstick result would be falsely positive.
But that is not all. Most causes of hemoglobinuria or myoglobinuria are of limited clinical significance, except for rare conditions that are usually clinically obvious, such as severe burn injury. Further, remember that from 9% to 18% of patients without symptoms have red blood cells in the urine.2,5 In theory, if everyone in the United States had a dipstick test, this would be positive in patients with hematuria as well as in those with hemoglobinuria, myoglobinuria, and other false-positives; if everyone with a positive dipstick result were then referred to a urologist, a substantial portion of the population would receive an unnecessary urologic referral.
Urologic referral and evaluation in these patients not only wastes money: if they undergo imaging studies, they are exposed to radiation and contrast media, with their associated risks, and if they undergo cystoscopy, they face its attendant discomfort and risk of infection.
WHICH PATIENTS WITH HEMATURIA TO REFER TO A UROLOGIST
Gross hematuria
Red or tea-colored urine usually indicates gross hematuria. When there is any doubt—such as in the case of a color-blind patient—the presence of red blood cells can be confirmed or ruled out by a microscopic urinalysis.
Nearly all patients with an episode of gross hematuria should be referred to a urologist. The sole exception to this rule can be made when a woman younger than 40 years experiences gross hematuria in the classic setting of a culture-proven, symptomatic urinary tract infection (UTI) and her infection, symptoms, and hematuria all resolve completely with appropriate antibiotics.2,5 However, bleeding from cancer is classically intermittent. Therefore, one should not skip the urine culture and just prescribe antibiotics empirically: the patient might actually have cancer, but the supposed UTI may appear to resolve with antibiotic therapy. For the same reason, resolution of hematuria in any other setting does not obviate the need for referral.
Another scenario usually associated with a benign cause is bleeding after extreme physical activity—also known as “runner’s hematuria” or “march hematuria” (so named because it sometimes occurs in soldiers after a particularly grueling training march). Importantly, even in this situation, one should still be suspicious and probably refer the patient to a urologist: just because the patient has just run a marathon, it does not mean that he or she does not have cancer.
Depending on the character, timing, location, and many other characteristics of the patient’s bleeding, a variety of studies may or may not be necessary. For example, blood-spotting of the underpants might signify urethral bleeding, and imaging and cytologic studies might not be indicated. In view of the variability in presentation and workup, we recommend that the proper workup for these patients be determined by a urologist.
Symptomatic microhematuria
Patients with true microhematuria (three or more RBCs/HPF) accompanied by bothersome or worrisome symptoms should be referred to a urologist. In a study in Scotland, Sultana et al16 found that cancer was present in 6 (5%) of 126 patients with microhematuria without symptoms compared with 13 (10.5%) of 124 patients with microhematuria and irritative voiding symptoms; the difference, however, was not statistically significant.
Microscopic urinalysis should be part of the evaluation for flank pain or certain urinary symptoms such as frequency, urgency, retention, or dysuria; results of this test at the time of symptoms can later help the urologist distinguish the cause of the symptoms or hematuria. In addition, in combination with dipstick analysis, microscopic analysis can help distinguish patients with UTI or medical renal disease. If the evaluation suggests that the hematuria and symptoms are due to a UTI, then the findings on a repeat microscopic analysis, performed after the infection has cleared, should be normal. If hematuria, defined as three or more RBCs/HPF, persists in two of three subsequent urinalyses, then the guidelines mandate diagnostic evaluation even if the urinalysis subsequently becomes negative.
Asymptomatic hematuria
In symptom-free patients with dipstick hematuria found on a screening examination, it is crucial to confirm and document true microhematuria. Per the AUA guidelines, microhematuria worthy of urologic workup is the presence of three or more RBCs/HPF on at least two out of three microscopic urinalyses.2,5 Patients with dipstick pseudohematuria do not meet this criterion and will not benefit from a costly and invasive evaluation. Conversely, patients with higher levels of microhematuria, who have any risk factors for cancer, or who are anxious about the test results might benefit from urologic consultation before a second urinalysis to confirm the first, positive finding.
Many patients younger than age 40 with asymptomatic microhematuria but no other risk factors for urinary tract cancer can be followed conservatively. Khadra et al14 reported that only 1 of 143 patients younger than 40 years with microhematuria had cancer. Similarly, Jones et al17 found, in a prospective study, that no man younger than 40 years with microscopic hematuria had cancer.
Of note: gross or microscopic blood in the urine, even in the setting of anticoagulation, is a marker of urinary tract pathology such as cancer, stones, or infection. Just as patients on anticoagulation therapy who develop gastrointestinal bleeding need a gastrointestinal evaluation, those with hematuria require a urologic evaluation.2,5
STUDIES TO CONSIDER BEFORE CONSULTATION
In symptom-free patients, it is inappropriate to order laboratory or imaging tests on the basis of a dipstick test alone, without confirming that they actually have hematuria. When the blood is confirmed to be present by microscopic examination of centrifuged urine (as described above), benign causes such as UTI should be considered. If a patient does have a UTI with hematuria, urinalysis should be repeated once the infection has cleared up.
Imaging studies
For symptomatic microhematuria. Patients with acute symptoms of renal colic should undergo computed tomography (CT) in a “stone protocol” (without contrast, with 3- to 5-mm cuts of the abdomen and pelvis) to assess for urinary lithiasis. Pregnancy should always be ruled out before radiation exposure; renal ultrasonography is generally the first-choice imaging study for pregnant patients.
For asymptomatic microhematuria. Patients without the classic flank pain of urolithiasis should undergo more extensive studies. For patients at increased risk of cancer, such as heavy smokers, CT urography is the optimal imaging study and is the test least likely to necessitate other follow-up studies.18–20 Other imaging options, including ultrasonography and intravenous pyelography, incompletely assess the upper urinary tracts including both renal parenchyma and urothelial surfaces. CT urography has been shown to find more than 40% of hematuria-causing lesions missed by other studies.18 Because ordering alternative imaging first will often result in redundant studies, CT urography is the preferred initial imaging study in the evaluation of hematuria.
Before exposing a patient to contrast media, one should ascertain that he or she is not allergic to it. In addition, in patients at risk of contrast nephropathy (ie, those older than 60 years, with diabetes, or with preexisting medical renal disease), one should check the serum creatinine concentration. Magnetic resonance urography, a more expensive study, is as accurate as CT for diagnosing many urologic conditions, so it can be performed in lieu of CT urography in patients with renal insufficiency, iodine allergy, or any reason to avoid ionizing radiation. Some clinicians perform plain radiography of the kidneys, ureters, and bladder as well as ultrasonography in this setting, but determination of the appropriate alternative to CT urography, if required, is best left to the urologist.
Other tests
Cytologic testing of the urine can be valuable in patients with gross hematuria and in those with microhematuria who have risk factors for urinary tract cancer. Although its reported median sensitivity for malignancy is only 48%, a positive cytologic test is approximately 94% specific for malignancy.21 Other studies, such as the fluorescence in situ hybridization assay, and the nuclear matrix protein 22 test do not yet have a clear role in the diagnosis of urinary tract disease.22
However, in general, we caution non-urologists not to order special tumor marker or cytologic tests, or to do so only with careful forethought. Although these studies occasionally detect occult cancer in patients at high risk, an “atypical” finding on cytology or a positive tumor marker test can lead to inappropriate referral and unnecessary biopsy or other tests.
WHEN NOT TO REFER A PATIENT WITH HEMATURIA TO A UROLOGIST
Symptom-free patients with a positive dipstick hemoglobin test should not immediately be referred to a urologist: they should have a microscopic urinalysis first to determine whether they actually have microhematuria, unless microscopic laboratory services are unavailable. Only patients with documented true hematuria, as defined by the AUA guidelines, should be referred for urologic evaluation and diagnostic testing. Once a patient is referred for evaluation, the consultant is under clear pressure to perform a complete investigation to fulfill the expectations of the referring physician. Avoiding expensive unnecessary testing and referral in those without hematuria allows appropriate utilization of resources.
Patients with microhematuria associated with a UTI should have a repeat urinalysis after the UTI is successfully treated; if the hematuria clears with the infection, then the patient needs no further evaluation. Patients with dipstick pseudohematuria and significant proteinuria or a predominance of dysmorphic urinary blood cells might benefit from an evaluation by a nephrologist rather than a urologist.2 This is especially true if the patient has an elevated serum creatinine level.
ECONOMIC RELEVANCE
In our tertiary care urology clinic, approximately 75% of patients who are referred to us because of microhematuria have not had a microscopic urinalysis before coming here. On further evaluation, up to 75% of these patients are found to have dipstick pseudohematuria that did not actually require consultation or evaluation.23 It is possible that this occurs even more frequently in the general practice setting.
A Medicare level-4 urologic consultation for hematuria costs $170; the cumulative cost of unwarranted referrals is undoubtedly substantial. Even more money is wasted on CT urography, cytology, and other testing performed before urologic consultation in patients ultimately found not to have true hematuria. The economic and iatrogenic risks of evaluation cannot be justified in patients who do not exhibit findings that can be considered abnormal as defined in this article.
CONCLUSION
It is important to distinguish whether hematuria is microscopic or macroscopic, whether there are associated symptoms, and whether a patient has risk factors for significant urologic disease. While dipstick tests are sensitive, they do not reliably diagnose microhematuria, which is the microscopically proven presence of urinary red blood cells. Positive dipstick tests should always be followed by microscopic urinalysis; failure to do so can result in the unfortunate and unnecessary evaluation of dipstick pseudohematuria, a normal condition.
The AUA defines significant hematuria as three or more RBCs/HPF in two of three properly prepared specimens.2 This should determine whether a symptom-free patient needs urologic referral and evaluation for hematuria.
By following these principles, primary care physicians have a valuable opportunity to direct medical care, increase the efficiency of our health care system, and protect patients from the anxiety, costs, and risks of an unnecessary urologic workup.
- Mohr DN, Offord KP, Owen RA, Melton LJ. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986; 256:224–229.
- Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy—part I: definition, detection, prevalence, and etiology. Urology 2001; 57:599–603.
- US Preventive Services Task Force. Screening for Bladder Cancer, updated November 2004. Available at www.ahrq.gov/clinic/uspstf/uspsblad.htm.
- Logsetty S. Screening for bladder cancer. Canadian Task Force on the Periodic Health Examination. Canadian Guide to Clinical Preventive Health Care. 1994: Ottawa: Health Canada,826–836.
- Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: The American Urological Association Best Practice Policy—Part II: patient evaluation, cytology, voided markers, imaging cystoscopy, nephrology evaluation, and follow-up. Urology 2001; 57:604–610.
- Prochazka AV, Lundahl K, Pearson W, Oboler SK, Anderson RJ. Support of evidence-based guidelines for the annual physical examination: a survey of primary care providers. Arch Intern Med 2005; 165:1347–1352.
- Chacko KM, Feinberg LE. Laboratory screening at preventive health exams: trend of testing, 1978–2004. Am J Prev Med 2007; 32:59–62.
- Mariani AJ, Luangphinith S, Loo S, Scottolini A, Hodges CV. Dipstick chemical urinalysis: an accurate cost-effective screening test. J Urol 1984; 132:64–66.
- Murphy TE. The urinalysis—inexpensive and informative. J Insur Med 2004; 36:320–326.
- Woolhandler S, Pels RJ, Bor DH, Himmelstein DU, Lawrence RS. Dipstick urinalysis screening of asymptomatic adults for urinary tract disorders. I. Hematuria and proteinuria. JAMA 1989; 262:1214–1219.
- Pels RJ, Bor DH, Woolhandler S, Himmelstein DU, Lawrence RS. Dipstick urinalysis screening of asymptomatic adults for urinary tract disorders. II. Bacteriuria. JAMA 1989; 262:1221–1224.
- Gerber GS, Brendler CBWein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA. Evaluation of the urologic patient: history, physical examination, and urinalysis. Campbell-Walsh Urology 2007; 9th ed. Saunders Elsevier: Philadelphia:81–110.
- Cohen RA, Brown RS. Clinical practice. Microscopic hematuria. N Engl J Med 2003; 348:2330–2338.
- Khadra MH, Pickard RS, Charlton M, Powell PH, Neal DE. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol 2000; 163:524–527.
- Alishahi S, Byrne D, Goodman CM, Baxby K. Haematuria investigation based on a standard protocol: emphasis on the diagnosis of urological malignancy. J R Coll Surg Edinb 2002; 47:422–427.
- Sultana SR, Goodman CM, Byrne DJ, Baxby K. Microscopic haematuria: urological investigation using a standard protocol. Br J Urol 1996; 78:691–696.
- Jones DJ, Langstaff RJ, Holt SD, Morgans BT. The value of cystourethroscopy in the investigation of microscopic haematuria in adult males under 40 years. A prospective study of 100 patients. Br J Urol 1988; 62:541–545.
- Lang EK, Thomas R, Davis R, et al. Multiphasic helical computerized tomography for the assessment of microscopic hematuria: a prospective study. J Urol 2004; 171:237–243.
- Gray Sears CL, Ward JF, Sears ST, Puckett MF, Kane CJ, Amling CL. Prospective comparison of computerized tomography and excretory urography in the initial evaluation of asymptomatic microhematuria. J Urol 2002; 168:2457–2460.
- Liu W, Mortelé KJ, Silverman SG. Incidental extraurinary findings at MDCT urography in patients with hematuria: prevalence and impact on imaging costs. AJR Am J Roentgenol 2005; 185:1051–1056.
- van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol 2005; 47:736–748.
- Black PC, Brown GA, Dinney CP. Molecular markers of urothelial cancer and their use in the monitoring of superficial urothelial cancer. J Clin Oncol 2006; 24:5528–5535.
- Rao PR, Jones JS. Retrospective chart review of consultations for hematuria, 2004–2006. Unpublished data. Manuscript in progress.
- Mohr DN, Offord KP, Owen RA, Melton LJ. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986; 256:224–229.
- Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy—part I: definition, detection, prevalence, and etiology. Urology 2001; 57:599–603.
- US Preventive Services Task Force. Screening for Bladder Cancer, updated November 2004. Available at www.ahrq.gov/clinic/uspstf/uspsblad.htm.
- Logsetty S. Screening for bladder cancer. Canadian Task Force on the Periodic Health Examination. Canadian Guide to Clinical Preventive Health Care. 1994: Ottawa: Health Canada,826–836.
- Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: The American Urological Association Best Practice Policy—Part II: patient evaluation, cytology, voided markers, imaging cystoscopy, nephrology evaluation, and follow-up. Urology 2001; 57:604–610.
- Prochazka AV, Lundahl K, Pearson W, Oboler SK, Anderson RJ. Support of evidence-based guidelines for the annual physical examination: a survey of primary care providers. Arch Intern Med 2005; 165:1347–1352.
- Chacko KM, Feinberg LE. Laboratory screening at preventive health exams: trend of testing, 1978–2004. Am J Prev Med 2007; 32:59–62.
- Mariani AJ, Luangphinith S, Loo S, Scottolini A, Hodges CV. Dipstick chemical urinalysis: an accurate cost-effective screening test. J Urol 1984; 132:64–66.
- Murphy TE. The urinalysis—inexpensive and informative. J Insur Med 2004; 36:320–326.
- Woolhandler S, Pels RJ, Bor DH, Himmelstein DU, Lawrence RS. Dipstick urinalysis screening of asymptomatic adults for urinary tract disorders. I. Hematuria and proteinuria. JAMA 1989; 262:1214–1219.
- Pels RJ, Bor DH, Woolhandler S, Himmelstein DU, Lawrence RS. Dipstick urinalysis screening of asymptomatic adults for urinary tract disorders. II. Bacteriuria. JAMA 1989; 262:1221–1224.
- Gerber GS, Brendler CBWein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA. Evaluation of the urologic patient: history, physical examination, and urinalysis. Campbell-Walsh Urology 2007; 9th ed. Saunders Elsevier: Philadelphia:81–110.
- Cohen RA, Brown RS. Clinical practice. Microscopic hematuria. N Engl J Med 2003; 348:2330–2338.
- Khadra MH, Pickard RS, Charlton M, Powell PH, Neal DE. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol 2000; 163:524–527.
- Alishahi S, Byrne D, Goodman CM, Baxby K. Haematuria investigation based on a standard protocol: emphasis on the diagnosis of urological malignancy. J R Coll Surg Edinb 2002; 47:422–427.
- Sultana SR, Goodman CM, Byrne DJ, Baxby K. Microscopic haematuria: urological investigation using a standard protocol. Br J Urol 1996; 78:691–696.
- Jones DJ, Langstaff RJ, Holt SD, Morgans BT. The value of cystourethroscopy in the investigation of microscopic haematuria in adult males under 40 years. A prospective study of 100 patients. Br J Urol 1988; 62:541–545.
- Lang EK, Thomas R, Davis R, et al. Multiphasic helical computerized tomography for the assessment of microscopic hematuria: a prospective study. J Urol 2004; 171:237–243.
- Gray Sears CL, Ward JF, Sears ST, Puckett MF, Kane CJ, Amling CL. Prospective comparison of computerized tomography and excretory urography in the initial evaluation of asymptomatic microhematuria. J Urol 2002; 168:2457–2460.
- Liu W, Mortelé KJ, Silverman SG. Incidental extraurinary findings at MDCT urography in patients with hematuria: prevalence and impact on imaging costs. AJR Am J Roentgenol 2005; 185:1051–1056.
- van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol 2005; 47:736–748.
- Black PC, Brown GA, Dinney CP. Molecular markers of urothelial cancer and their use in the monitoring of superficial urothelial cancer. J Clin Oncol 2006; 24:5528–5535.
- Rao PR, Jones JS. Retrospective chart review of consultations for hematuria, 2004–2006. Unpublished data. Manuscript in progress.
KEY POINTS
- Dipstick tests by themselves do not confirm that hematuria is present; thus, “dipstick hematuria” is a potential misnomer. Patients without symptoms who have a positive dipstick test and negative microscopic urinalysis are better described as having dipstick pseudohematuria, a clinically insignificant finding.
- Significant hematuria is defined as three or more red blood cells per high-power field in a properly collected and centrifuged urine specimen; this is the definition that should dictate which patients require further urologic evaluation.
- Since the evaluation for hematuria usually includes cystoscopy and imaging studies, it is crucial to confirm that hematuria is truly present before initiating an invasive and costly evaluation.
Malpractice Chronicle
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Radiation Error After Surgery for Acinic Cancer
A woman in her 50s discovered a growth in her left parotid gland. Although the mass was not thought to be malignant, she elected to have it removed. Biopsy results led to a diagnosis of acinic cancer.
The patient underwent 13 radiation treatments with the defendant, a radiation oncologist, before it was discovered that the defendant had been radiating the right parotid gland instead of the surgical site at the left parotid gland.
The plaintiff claimed that radiation to the right parotid gland destroyed its ability to function, leaving her with a permanent dry mouth and diminished sense of taste. She also claimed that loss of the gland has caused oral and digestive health problems. The defendant admitted negligence in treating the wrong side of the patient’s face but disputed the damages in question.
According to a published report, a $250,000 verdict was returned.
Link Overlooked Between MSSA and Osteomyelitis
A teenage boy, born with sickle cell anemia, experienced vaso-occlusive crises once or twice each year. During these episodes, he would be treated with morphine, oxycodone with acetaminophen, and/or acetaminophen with codeine, along with IV hydration.
At age 16, the boy was admitted to the defendant hospital with a fever; he received a diagnosis of pneumonia, based on a chest x-ray. Of three blood cultures that were performed, one yielded positive results for methicillin-sensitive Staphylococcus aureus (MSSA). The patient underwent four days’ IV therapy with triple antibiotics, followed by a 10-day supply of oral antibiotics to be taken following discharge.
The next month, the patient was readmitted for three days at the defendant hospital with a three-week history of back pain. Six days after his second discharge, the boy returned to the defendant hospital, complaining once again of back pain. He was hospitalized for eight days. During both hospitalizations, the plaintiff was treated with pain medications and IV hydration.
A week after his return home, the patient developed excruciating pain; he was unable to walk and became short of breath. He was taken to a different hospital, where x-rays and MRI revealed a fracture and collapse of the T7 vertebra. He underwent surgical repair with insertion of a plate and screws. Following a needle biopsy performed on day 8 of this hospitalization, a diagnosis was made of osteomyelitis of the spine. A biopsy revealed MSSA of the same type found in the boy’s blood culture during the initial admission. He was hospitalized for six weeks.
The plaintiff alleged negligence in the defendant’s failure to make a timely diagnosis of osteomyelitis. He claimed that osteomyelitis should have been considered when the blood culture was reported, and that his last discharge from the defendant hospital should not have occurred because he was unable to walk. The plaintiff claimed that he will develop arthritis as he grows older and will be at higher risk for lung and heart problems.
According to the defendant, the plaintiff reported that his pain had continued to decrease before his third discharge, that he was walking in the hallways of the defendant hospital before that discharge, and that he was able to walk into the nondefendant hospital. The defendant further claimed that the plaintiff’s discharge instructions after each hospitalization included follow-up at a hematology clinic, but he did not comply. The defendant also claimed that the plaintiff had severe osteoporosis secondary to sickle cell anemia, that his chronic illness was associated with a reduced life expectancy, and that any lung or heart problems that might develop would be the result of sickle cell anemia, not osteomyelitis.
A settlement of $925,000 was negotiated.
Long Wait for Diagnostic Testing
In May 2001, a 38-year-old Arizona man developed fever, chills, muscle aches, and cough. His family physician made a diagnosis of pneumonia. Late that summer, the patient developed headaches and a variety of other symptoms, including pain in the cervical spine, weight loss, and intermittent fevers. By October, the man was also complaining of fatigue, headaches, fever, and an unintended 20-lb weight loss. He was seen by a neurologist in November.
In early 2002, the patient was experiencing persistent headaches and vomiting and additional weight loss. One day in February, when the man was unable to find his way home, he went to a hospital. After being triaged, he was taken to his family physician by his wife. The plaintiff was seen by an internist two weeks later.
In March, the plaintiff went to a large, not-for-profit teaching hospital, where he was seen by a physician assistant. He was then seen by a rheumatologist in April.
In June 2002, the patient’s family physician ordered a spinal tap. A diagnosis of coccidioidomycosis (“valley fever meningitis”) was made. The man was treated and discharged from the hospital.
In July, he was readmitted, then transferred to another hospital, where he was treated until November. He then received home health care visits until June 2003, when a brain shunt was inserted to treat hydrocephalus. Later that year, the man had a recurrence of severe headaches. These were attributed to shunt malfunction, which necessitated insertion of a new shunt.
The plaintiff claimed that he had sustained extensive and debilitating neurologic injuries and brain damage. He has right-side facial paralysis, vision problems that require an eye patch, and vestibular dysfunction. He is unable to grip items and requires several prescription medications.
According to a published report, the plaintiff’s insurer, the family physician, and the hospitals involved settled prior to trial for confidential amounts. At trial, the plaintiff alleged negligence in his having been seen by a PA instead of a physician, in the PA’s failure to consult with his supervising physician, and in his failure to order proper testing or to obtain consultations.
The remaining defendants argued that the plaintiff was instructed to return for follow-up in order for a treatment plan to be formulated. The defendants also claimed that the plaintiff’s insurer denied authorization for testing and the plaintiff declined to self-pay. The defendants argued that if the plaintiff had followed up, abnormal findings on his subsequent examination would have prompted additional testing. The defendants also claimed that even if the patient’s condition had been diagnosed in March or April 2002, he still would have developed hydrocephalus and required a shunt.
A defense verdict was returned for the remaining defendants.
Renal Failure After Histoplasmosis Is Misdiagnosed
Eight years after undergoing transplantation with one of her father’s kidneys, a 29-year-old woman in relatively good health developed a mass in her right armpit. Her nephrologist (also her primary care provider) prescribed antibiotics in October 2001.
In November, when the lesion had not improved, the patient was referred to a surgeon, who removed the mass and sent it to the defendant pathologist for evaluation. The pathologist made a diagnosis of hidradenitis suppurative, an inflammation of the sweat glands.
The patient’s condition worsened. In January, she was admitted to the ICU, where she was diagnosed with histoplasmosis. Systemic complications, combined with the effects of the medications she needed, caused her kidney to fail. She recovered from histoplasmosis, but in April 2002, she needed to begin dialysis. By December, she was strong enough to undergo a second kidney transplant; this time, her mother was the donor. Complications arose, however, which resulted in the patient’s death in March 2003.
The plaintiff alleged negligence in the pathologist’s failure to diagnose histoplasmosis in a timely fashion. The defendant pathologist maintained that the tissue samples did not require him to conduct special stains to look for histoplasmosis. He also claimed that the decedent was already in renal failure and that the first transplanted kidney would have failed in any event.
According to a published report, a $5,924,141 verdict was returned, after which the parties involved reached a confidential settlement.
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Radiation Error After Surgery for Acinic Cancer
A woman in her 50s discovered a growth in her left parotid gland. Although the mass was not thought to be malignant, she elected to have it removed. Biopsy results led to a diagnosis of acinic cancer.
The patient underwent 13 radiation treatments with the defendant, a radiation oncologist, before it was discovered that the defendant had been radiating the right parotid gland instead of the surgical site at the left parotid gland.
The plaintiff claimed that radiation to the right parotid gland destroyed its ability to function, leaving her with a permanent dry mouth and diminished sense of taste. She also claimed that loss of the gland has caused oral and digestive health problems. The defendant admitted negligence in treating the wrong side of the patient’s face but disputed the damages in question.
According to a published report, a $250,000 verdict was returned.
Link Overlooked Between MSSA and Osteomyelitis
A teenage boy, born with sickle cell anemia, experienced vaso-occlusive crises once or twice each year. During these episodes, he would be treated with morphine, oxycodone with acetaminophen, and/or acetaminophen with codeine, along with IV hydration.
At age 16, the boy was admitted to the defendant hospital with a fever; he received a diagnosis of pneumonia, based on a chest x-ray. Of three blood cultures that were performed, one yielded positive results for methicillin-sensitive Staphylococcus aureus (MSSA). The patient underwent four days’ IV therapy with triple antibiotics, followed by a 10-day supply of oral antibiotics to be taken following discharge.
The next month, the patient was readmitted for three days at the defendant hospital with a three-week history of back pain. Six days after his second discharge, the boy returned to the defendant hospital, complaining once again of back pain. He was hospitalized for eight days. During both hospitalizations, the plaintiff was treated with pain medications and IV hydration.
A week after his return home, the patient developed excruciating pain; he was unable to walk and became short of breath. He was taken to a different hospital, where x-rays and MRI revealed a fracture and collapse of the T7 vertebra. He underwent surgical repair with insertion of a plate and screws. Following a needle biopsy performed on day 8 of this hospitalization, a diagnosis was made of osteomyelitis of the spine. A biopsy revealed MSSA of the same type found in the boy’s blood culture during the initial admission. He was hospitalized for six weeks.
The plaintiff alleged negligence in the defendant’s failure to make a timely diagnosis of osteomyelitis. He claimed that osteomyelitis should have been considered when the blood culture was reported, and that his last discharge from the defendant hospital should not have occurred because he was unable to walk. The plaintiff claimed that he will develop arthritis as he grows older and will be at higher risk for lung and heart problems.
According to the defendant, the plaintiff reported that his pain had continued to decrease before his third discharge, that he was walking in the hallways of the defendant hospital before that discharge, and that he was able to walk into the nondefendant hospital. The defendant further claimed that the plaintiff’s discharge instructions after each hospitalization included follow-up at a hematology clinic, but he did not comply. The defendant also claimed that the plaintiff had severe osteoporosis secondary to sickle cell anemia, that his chronic illness was associated with a reduced life expectancy, and that any lung or heart problems that might develop would be the result of sickle cell anemia, not osteomyelitis.
A settlement of $925,000 was negotiated.
Long Wait for Diagnostic Testing
In May 2001, a 38-year-old Arizona man developed fever, chills, muscle aches, and cough. His family physician made a diagnosis of pneumonia. Late that summer, the patient developed headaches and a variety of other symptoms, including pain in the cervical spine, weight loss, and intermittent fevers. By October, the man was also complaining of fatigue, headaches, fever, and an unintended 20-lb weight loss. He was seen by a neurologist in November.
In early 2002, the patient was experiencing persistent headaches and vomiting and additional weight loss. One day in February, when the man was unable to find his way home, he went to a hospital. After being triaged, he was taken to his family physician by his wife. The plaintiff was seen by an internist two weeks later.
In March, the plaintiff went to a large, not-for-profit teaching hospital, where he was seen by a physician assistant. He was then seen by a rheumatologist in April.
In June 2002, the patient’s family physician ordered a spinal tap. A diagnosis of coccidioidomycosis (“valley fever meningitis”) was made. The man was treated and discharged from the hospital.
In July, he was readmitted, then transferred to another hospital, where he was treated until November. He then received home health care visits until June 2003, when a brain shunt was inserted to treat hydrocephalus. Later that year, the man had a recurrence of severe headaches. These were attributed to shunt malfunction, which necessitated insertion of a new shunt.
The plaintiff claimed that he had sustained extensive and debilitating neurologic injuries and brain damage. He has right-side facial paralysis, vision problems that require an eye patch, and vestibular dysfunction. He is unable to grip items and requires several prescription medications.
According to a published report, the plaintiff’s insurer, the family physician, and the hospitals involved settled prior to trial for confidential amounts. At trial, the plaintiff alleged negligence in his having been seen by a PA instead of a physician, in the PA’s failure to consult with his supervising physician, and in his failure to order proper testing or to obtain consultations.
The remaining defendants argued that the plaintiff was instructed to return for follow-up in order for a treatment plan to be formulated. The defendants also claimed that the plaintiff’s insurer denied authorization for testing and the plaintiff declined to self-pay. The defendants argued that if the plaintiff had followed up, abnormal findings on his subsequent examination would have prompted additional testing. The defendants also claimed that even if the patient’s condition had been diagnosed in March or April 2002, he still would have developed hydrocephalus and required a shunt.
A defense verdict was returned for the remaining defendants.
Renal Failure After Histoplasmosis Is Misdiagnosed
Eight years after undergoing transplantation with one of her father’s kidneys, a 29-year-old woman in relatively good health developed a mass in her right armpit. Her nephrologist (also her primary care provider) prescribed antibiotics in October 2001.
In November, when the lesion had not improved, the patient was referred to a surgeon, who removed the mass and sent it to the defendant pathologist for evaluation. The pathologist made a diagnosis of hidradenitis suppurative, an inflammation of the sweat glands.
The patient’s condition worsened. In January, she was admitted to the ICU, where she was diagnosed with histoplasmosis. Systemic complications, combined with the effects of the medications she needed, caused her kidney to fail. She recovered from histoplasmosis, but in April 2002, she needed to begin dialysis. By December, she was strong enough to undergo a second kidney transplant; this time, her mother was the donor. Complications arose, however, which resulted in the patient’s death in March 2003.
The plaintiff alleged negligence in the pathologist’s failure to diagnose histoplasmosis in a timely fashion. The defendant pathologist maintained that the tissue samples did not require him to conduct special stains to look for histoplasmosis. He also claimed that the decedent was already in renal failure and that the first transplanted kidney would have failed in any event.
According to a published report, a $5,924,141 verdict was returned, after which the parties involved reached a confidential settlement.
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Radiation Error After Surgery for Acinic Cancer
A woman in her 50s discovered a growth in her left parotid gland. Although the mass was not thought to be malignant, she elected to have it removed. Biopsy results led to a diagnosis of acinic cancer.
The patient underwent 13 radiation treatments with the defendant, a radiation oncologist, before it was discovered that the defendant had been radiating the right parotid gland instead of the surgical site at the left parotid gland.
The plaintiff claimed that radiation to the right parotid gland destroyed its ability to function, leaving her with a permanent dry mouth and diminished sense of taste. She also claimed that loss of the gland has caused oral and digestive health problems. The defendant admitted negligence in treating the wrong side of the patient’s face but disputed the damages in question.
According to a published report, a $250,000 verdict was returned.
Link Overlooked Between MSSA and Osteomyelitis
A teenage boy, born with sickle cell anemia, experienced vaso-occlusive crises once or twice each year. During these episodes, he would be treated with morphine, oxycodone with acetaminophen, and/or acetaminophen with codeine, along with IV hydration.
At age 16, the boy was admitted to the defendant hospital with a fever; he received a diagnosis of pneumonia, based on a chest x-ray. Of three blood cultures that were performed, one yielded positive results for methicillin-sensitive Staphylococcus aureus (MSSA). The patient underwent four days’ IV therapy with triple antibiotics, followed by a 10-day supply of oral antibiotics to be taken following discharge.
The next month, the patient was readmitted for three days at the defendant hospital with a three-week history of back pain. Six days after his second discharge, the boy returned to the defendant hospital, complaining once again of back pain. He was hospitalized for eight days. During both hospitalizations, the plaintiff was treated with pain medications and IV hydration.
A week after his return home, the patient developed excruciating pain; he was unable to walk and became short of breath. He was taken to a different hospital, where x-rays and MRI revealed a fracture and collapse of the T7 vertebra. He underwent surgical repair with insertion of a plate and screws. Following a needle biopsy performed on day 8 of this hospitalization, a diagnosis was made of osteomyelitis of the spine. A biopsy revealed MSSA of the same type found in the boy’s blood culture during the initial admission. He was hospitalized for six weeks.
The plaintiff alleged negligence in the defendant’s failure to make a timely diagnosis of osteomyelitis. He claimed that osteomyelitis should have been considered when the blood culture was reported, and that his last discharge from the defendant hospital should not have occurred because he was unable to walk. The plaintiff claimed that he will develop arthritis as he grows older and will be at higher risk for lung and heart problems.
According to the defendant, the plaintiff reported that his pain had continued to decrease before his third discharge, that he was walking in the hallways of the defendant hospital before that discharge, and that he was able to walk into the nondefendant hospital. The defendant further claimed that the plaintiff’s discharge instructions after each hospitalization included follow-up at a hematology clinic, but he did not comply. The defendant also claimed that the plaintiff had severe osteoporosis secondary to sickle cell anemia, that his chronic illness was associated with a reduced life expectancy, and that any lung or heart problems that might develop would be the result of sickle cell anemia, not osteomyelitis.
A settlement of $925,000 was negotiated.
Long Wait for Diagnostic Testing
In May 2001, a 38-year-old Arizona man developed fever, chills, muscle aches, and cough. His family physician made a diagnosis of pneumonia. Late that summer, the patient developed headaches and a variety of other symptoms, including pain in the cervical spine, weight loss, and intermittent fevers. By October, the man was also complaining of fatigue, headaches, fever, and an unintended 20-lb weight loss. He was seen by a neurologist in November.
In early 2002, the patient was experiencing persistent headaches and vomiting and additional weight loss. One day in February, when the man was unable to find his way home, he went to a hospital. After being triaged, he was taken to his family physician by his wife. The plaintiff was seen by an internist two weeks later.
In March, the plaintiff went to a large, not-for-profit teaching hospital, where he was seen by a physician assistant. He was then seen by a rheumatologist in April.
In June 2002, the patient’s family physician ordered a spinal tap. A diagnosis of coccidioidomycosis (“valley fever meningitis”) was made. The man was treated and discharged from the hospital.
In July, he was readmitted, then transferred to another hospital, where he was treated until November. He then received home health care visits until June 2003, when a brain shunt was inserted to treat hydrocephalus. Later that year, the man had a recurrence of severe headaches. These were attributed to shunt malfunction, which necessitated insertion of a new shunt.
The plaintiff claimed that he had sustained extensive and debilitating neurologic injuries and brain damage. He has right-side facial paralysis, vision problems that require an eye patch, and vestibular dysfunction. He is unable to grip items and requires several prescription medications.
According to a published report, the plaintiff’s insurer, the family physician, and the hospitals involved settled prior to trial for confidential amounts. At trial, the plaintiff alleged negligence in his having been seen by a PA instead of a physician, in the PA’s failure to consult with his supervising physician, and in his failure to order proper testing or to obtain consultations.
The remaining defendants argued that the plaintiff was instructed to return for follow-up in order for a treatment plan to be formulated. The defendants also claimed that the plaintiff’s insurer denied authorization for testing and the plaintiff declined to self-pay. The defendants argued that if the plaintiff had followed up, abnormal findings on his subsequent examination would have prompted additional testing. The defendants also claimed that even if the patient’s condition had been diagnosed in March or April 2002, he still would have developed hydrocephalus and required a shunt.
A defense verdict was returned for the remaining defendants.
Renal Failure After Histoplasmosis Is Misdiagnosed
Eight years after undergoing transplantation with one of her father’s kidneys, a 29-year-old woman in relatively good health developed a mass in her right armpit. Her nephrologist (also her primary care provider) prescribed antibiotics in October 2001.
In November, when the lesion had not improved, the patient was referred to a surgeon, who removed the mass and sent it to the defendant pathologist for evaluation. The pathologist made a diagnosis of hidradenitis suppurative, an inflammation of the sweat glands.
The patient’s condition worsened. In January, she was admitted to the ICU, where she was diagnosed with histoplasmosis. Systemic complications, combined with the effects of the medications she needed, caused her kidney to fail. She recovered from histoplasmosis, but in April 2002, she needed to begin dialysis. By December, she was strong enough to undergo a second kidney transplant; this time, her mother was the donor. Complications arose, however, which resulted in the patient’s death in March 2003.
The plaintiff alleged negligence in the pathologist’s failure to diagnose histoplasmosis in a timely fashion. The defendant pathologist maintained that the tissue samples did not require him to conduct special stains to look for histoplasmosis. He also claimed that the decedent was already in renal failure and that the first transplanted kidney would have failed in any event.
According to a published report, a $5,924,141 verdict was returned, after which the parties involved reached a confidential settlement.
Preventing and managing diabetic complications in elderly patients
In elderly patients, as in all patients, diabetes is much more than the blood glucose level. However, in elderly patients the disease accelerates other common conditions of that population and markedly complicates their management.
Hypertension, coronary artery disease, and cerebrovascular attacks are more common in patients with diabetes.1 Longitudinal studies of elderly and middle-aged people with diabetes show increased rates of cognitive decline and dementia.2–4 Depression, urinary incontinence, and falls are also more common in elderly patients with diabetes. Physical disability is also increased: women with diabetes are half as likely to be able to manage ordinary physical tasks such as walking, climbing stairs, and doing housework as women without diabetes.5
In an earlier paper in this journal,6 we reviewed the management of diabetes per se in elderly patients. In the pages that follow, we review the management of its associated conditions.
HEART RISK TRUMPS BLOOD SUGAR
Coronary artery disease is by far the leading cause of death in elderly people with diabetes: 40% to 50% of patients with type 2 diabetes die of cardiac disease.7–9 The conventional risk factors—hypertension, hyperlipidemia, smoking, and diabetes—remain risk factors throughout old age. Risk reduction should focus on treating hypertension and dyslipidemia, smoking cessation, aspirin therapy, and exercise. While glycemic control reduces the risk of microvascular complications (eg, diabetic retinopathy and nephropathy) after about 8 years of treatment, benefits from control of elevated blood pressure and cholesterol occur after only 2 to 3 years.
Tight control of hypertension confers significant benefit
The United Kingdom Prospective Diabetes Study (UKPDS)10 found that patients who had tight control of blood pressure (mean treated blood pressure 144/82 mm Hg) had 24% fewer diabetes-related end points, 32% fewer diabetes-related deaths, 44% fewer strokes, a 34% reduced risk of deterioration of retinopathy, and a 47% reduced risk of visual deterioration than patients who had usual control (mean treated blood pressure 157/87 mm Hg). The benefit of treating hypertension outweighed the benefits of tight glycemic control.
A strong focus on blood pressure control should be a major focus of any treatment program. The American Geriatrics Society goal for blood pressure is less than 140/80 mm Hg if tolerated. Others have proposed more stringent targets.
Lipid control
Lipid control is integral to managing elderly patients with diabetes. In the Cholesterol and Recurrent Events trial11 and the Heart Protection Study,12 the cardiovascular benefits of reducing serum low-density lipoprotein cholesterol (LDL-C) levels were similar in elderly and younger patients with diabetes. In a meta-analysis of secondary prevention trials, absolute risk reduction was greatest in subjects older than 65 years with either diabetes or diastolic hypertension.
The American Diabetes Association,13 the American Geriatrics Society,14 and the Department of Veterans Affairs15,16 have all set a goal for serum LDL-C of less than 100 mg/dL. In addition, the American Diabetes Association has set goal levels for triglycerides (< 150 mg/dL) and high-density lipoprotein cholesterol (> 40 mg/dL).
Glycemic control
The importance of tight glycemic control in preventing coronary heart disease in the elderly is somewhat controversial. Treatment guidelines for elderly patients with diabetes are mainly extrapolated from the UKPDS, in which patients were a mean of 54 years old at the start of the study. After 10 years, the mean hemoglobin A1c levels were 7.9% in patients receiving conventional control and 7.0% in patients with intensive therapy. Every 1% reduction in hemoglobin A1c was associated with a 37% decline in microvascular complications of diabetes, a 14% decline in myocardial infarctions, and a 21% decline in any diabetes-related outcome.17
In the original trial,18 the rate of myocardial infarction was 17.4% in the conventional treatment group vs 14.7% in the intensive group (P = .052), and the risk of stroke did not differ. No thresholds for realizing benefits from reducing fasting glucose or hemoglobin A1c levels were detected.
A recent cohort study involving about 10,000 participants aged 45 to 79 years found that the risk of cardiovascular disease and death from any cause increased continuously with increasing hemoglobin A1c levels in people with or without diabetes.19 However, the impact of treatment remains to be clarified. The Action to Control Cardiovascular Risk in Diabetes trial will address this question (and others), but results will not be available for several years.
RETINOPATHY IS A MAJOR CAUSE OF BLINDNESS
Diabetic retinopathy, a leading cause of blindness in the United States, is perhaps the most threatening of the chronic microvascular complications of diabetes for elderly patients. The strongest predictor of retinopathy is the duration of diabetes.20–22 Retinopathy is classified as being nonproliferative, preproliferative, or proliferative.
Ischemia is believed to be the major cause of diabetic retinopathy, and glucose control has been shown to be of major benefit. A study of young adults with type 1 diabetes found that intensive therapy reduced the risk of developing retinopathy by 76% and slowed the progression of retinopathy by 54%. Comparable data for patients with type 2 diabetes are lacking.
Of some concern is a study in which retinopathy progressed more rapidly during the first year of aggressive insulin therapy in elderly patients with diabetes and baseline retinopathy.23 Further research is needed to identify which subgroups would benefit most from aggressive glycemic control.
In addition to specific ophthalmologic treatment, managing cardiovascular risk factors may reduce the progression of retinopathy: each cardiovascular risk factor has been found to also be a risk factor for retinopathy. Hypertension is an independent risk factor for any retinopathy, and its tight control reduces progression.20,24 Aspirin therapy has not been found to confer either risk or benefit.25,26
Although guidelines typically call for yearly ophthalmic examinations to screen for retinopathy, whether this is cost-effective has been questioned.27,28 But people older than 65 years with diabetes also have twice the risk of developing cataracts and three times the risk of developing glaucoma than those without diabetes. Considering the effects of visual loss on quality of life as well as the subsequent higher risk of accidents, eye examinations by an ophthalmologist at the time of diagnosis and annually thereafter are recommended. Tight glycemic and blood pressure control remains the cornerstone in the primary prevention of diabetic retinopathy. Panretinal and focal retinal laser photocoagulation reduces the risk of visual loss in patients with severe retinopathy and macular edema, respectively.29
NEUROPATHY PRESENTS IN MANY FORMS
Neuropathy is a particularly distressing complication and can lead to loss of sleep, limitation of activity, and depression.26,30,31 Diabetic neuropathies include focal neuropathies (entrapment syndromes and mono-neuropathies), polyneuropathy, and autonomic neuropathy.
Distal symmetric polyneuropathy (“glove and stocking” sensory symptoms) is the most common neuropathy of elderly people with diabetes. Pain, which can interrupt sleep and limit activity, can be treated with the anticonvulsants gabapentin (Gabarone, Neurontin), phenytoin (Dilantin, Phenytek) and carbamazepine (Carbatrol, Epitol, Equetro, Tegretol), and with tricyclic antidepressants. However, the anticholinergic effects of tricyclic antidepressants limit their use in older patients. Newer agents, such as duloxetine (Cymbalta) and pregabalin (Lyrica) show promise.30,31 Dysesthesia of a burning quality is sometimes treated with topical capsaicin or with oral mexiletine (Mexitil), although their role in treating older patients is not well established.
Patients with distal sensory polyneuropathy are predisposed to develop Charcot joints, which may mimic gout or degenerative joint disease. Plain radiography of the foot can help differentiate these diseases. Distal sensory polyneuropathy also predisposes patients to neuropathic foot ulcer, the leading cause of foot amputation in the United States.32
Feet should be inspected at each office visit. Testing sensation with a monofilament detects sensory neuropathy. Patients should be encouraged to examine their feet daily. Therapeutic shoes, prescribed by a podiatrist and individually designed to prevent blisters, calluses, and ulcers, are covered by Medicare for peripheral neuropathy if any of the following are also present: callus formation, poor circulation, foot deformity, or a history of foot callus, ulcer, or amputation (partial or complete). Medicare will pay for one pair of shoes plus three pairs of inserts per year.
Proximal motor neuropathy (diabetic amyotrophy) primarily affects elderly patients. It begins with unilateral thigh pain, which becomes bilateral and progresses to proximal muscle weakness and wasting. Distal symmetric polyneuropathy may also be present. Treatment includes glycemic control (usually with insulin) and physical therapy. Some forms of amyotrophy respond to immunotherapy.
Autonomic neuropathy, although not painful, can be the most life-threatening form of diabetic neuropathy.33 Tachycardia increases the risk of sudden death, while postural hypotension increases the risk of syncope, falling, and injury. Other forms of autonomic neuropathy include neurogenic bladder, sexual dysfunction, gastropathy (which is particularly sensitive to glycemic control), enteropathy, and gustatory sweating. Patients with autonomic neuropathy are more likely to have hypoglycemic unawareness.
NEPHROPATHY CAN PROGRESS RAPIDLY
Elderly patients with diabetes are especially at risk of developing nephropathy, which progresses from microalbuminuria to overt proteinuria to renal insufficiency and end-stage renal disease. Nephropathy may develop over a shorter time than the typical 10 to 20 years in younger patients. Independent risk factors for proteinuria and renal insufficiency include poor glycemic control over many years, hypertension, longer duration of diabetes, male sex, high serum total cholesterol levels, and smoking. Elderly patients are also at risk of renal insults such as receiving intravenous iodinated contrast agents in the course of radiologic procedures, nephrotoxic drugs, and comorbid illness such as congestive heart failure.
The diagnosis of diabetic nephropathy is usually made clinically and not by renal biopsy. Diabetic nephropathy can be diagnosed with almost 100% specificity in type 1 diabetes and more than 85% specificity in type 2 diabetes by a urinary albumin excretion of more than 300 mg per day and an appropriate time course in the absence of other obvious causes of renal disease. The urinary albumin-to-creatinine ratio can be used to screen for microalbuminuria (the precursor of frank proteinuria and renal insufficiency). A value of more than 30 mg of albumin per gram of creatinine suggests that albumin excretion exceeds 30 mg and that microalbuminuria is present.
Prevention is a cornerstone of management. Good glycemic control reduces the risk of microalbuminuria, the progression of albuminuria, and the development of renal insufficiency. Lowering blood pressure reduces the decline in glomerular filtration rate and albuminuria. Angiotensin-converting enzyme (ACE) inhibitors reduce the rate of progression of proteinuria and reduce the rate of end-stage renal disease, although the data are stronger in patients with type 1 diabetes.34 When side effects such as cough limit the use of ACE inhibitors, angiotensin receptor blockers can be used as an alternative. Blood pressure should be controlled to reduce stroke and cardiovascular complications, regardless of whether microalbuminuria is present.35
End-stage renal disease in elderly patients with diabetes is becoming increasingly frequent. Nephropathy in older patients is different from that in younger patients. In elderly patients, the pathologic findings may suggest ischemia and hypertension, and the classic Kimmelstiel-Wilson lesions may be absent. Patients may present with end-stage renal disease following an episode of acute renal failure that does not resolve, which may occur after a radiologic procedure involving an iodinated contrast agent.
NONKETOTIC HYPEROSMOLAR COMA
Nonketotic hyperosmolar coma occurs predominantly in elderly patients with type 2 diabetes. Predisposing factors include dementia, infection, stroke, and myocardial infarction. Coma results from osmotic diuresis due to hyperglycemia and consequent dehydration. A drop in the glomerular filtration rate promotes further hyperglycemia and dehydration in a vicious circle. Glucose levels commonly reach 600 mg/dL or more, and serum osmolality often exceeds 320 mOsm/L. A fluid deficit of 5 to 10 L is typical.
Fluid replacement is the mainstay of treatment. Because free water is typically lost in an osmotic diuresis, 0.9% (normal) saline is usually given if hemodynamic instability is present or 0.45% (half-normal) saline otherwise. Insulin is also required, as is specific treatment of the precipitating cause, eg, infection. Ketoacidosis may also occur in the elderly.
Recovery from coma or improvement in mental status may lag behind correction of the serum osmolality and may take several days. Mortality rates can be high: severe hyperosmolarity, advanced age, and nursing home residence are the major risk factors for death.
INFECTIONS: SEVERE AND UNUSUAL
Elderly patients with diabetes are at increased risk of developing severe and unusual infections, particularly malignant external otitis. Necrotizing Pseudomonas aeruginosa infection initially involves the external ear canal and progresses to the mastoid air cells, the skull base, or temporal bone. The clinical presentation consists of fever, otalgia, otorrhea, and less commonly, cranial nerve palsy. Treatment involves surgical debridement and antibiotics.
Other infections associated with diabetes include rhinocerebral mucormycosis, necrotizing fasciitis, emphysematous cholecystitis, and emphysematous pyelonephritis. An elderly patient with diabetes is also at increased risk of renal papillary necrosis, which presents as insidious renal failure.
COGNITIVE IMPAIRMENT
Elderly people with diabetes are at increased risk of cognitive impairment, which poses a barrier to taking medications appropriately and performing other tasks of self-management.
Because dementia may go undetected, particularly in the early stages, cognitive function should be assessed in elderly patients when they fail to take therapy correctly or have frequent episodes of hypoglycemia, or if glycemic control deteriorates without an obvious explanation. Caregivers play a critical role in detecting and reporting early cognitive impairment.
DEPRESSION IS OFTEN UNDETECTED
Elderly patients with diabetes have a higher rate of depression than do age-matched controls, but it is commonly underdetected and undertreated.5,36 Depression has been associated with poor glycemic control, and treatment of depression is associated with improved control. Routine screening for depression should be performed; a variety of diagnostic instruments are available. Particular attention should be given to medications that are associated with depression.
POLYPHARMACY
Many elderly patients take multiple medications. Polypharmacy increases the risk of drug side effects, interactions, and nonadherence to taking medications.37–39 This problem is increased in diabetes, in which several medications are necessary to manage hyper-glycemia, hyperlipidemia, hypertension, and other associated conditions.
Patients should keep accurate medication lists, including over-the-counter medications, herbs, and nutritional supplements. Physicians should carefully review each medication to check if it is appropriate and used correctly.
FALLS
Elderly patients with diabetes mellitus are at increased risk of injurious falls, which are associated with high rates of complications, death, and functional decline.40,41 Risk factors include frailty and functional disability, visual impairment, peripheral or autonomic neuropathy, hypoglycemia, and polypharmacy.
Elderly patients should be screened for their risk of falls, and appropriate measures should be instituted. The American Geriatrics Society has guidelines for preventing falls in the elderly.41
URINARY INCONTINENCE
Elderly women with diabetes are at increased risk of developing urinary incontinence. Risk factors include autonomic neuropathy (causing either neurogenic bladder or fecal impaction), polyuria due to hyperglycemia, and urinary tract and vaginal infections. Although evidence is lacking that urinary incontinence affects glycemic control, assessing and treating the condition improves quality of life.
SUMMARY
Diabetes is a common problem in the elderly, accounting for considerable morbidity and mortality. In a large longitudinal analysis (> 50,000 patients), elderly persons newly diagnosed as having diabetes experienced high rates of complications during 10-year follow-up, far in excess of elderly persons without diabetes.42 Diabetes is underdiagnosed in the elderly and is frequently undertreated. Management of the elderly with diabetes presents unique challenges because of associated comorbidities, but with attention to detail and individualized approaches, quality and duration of life can be optimized. The greatest attention should be given to reduction of overall cardiovascular risk. Glycemic goals and the treatment regimens to achieve those goals should be individualized and chosen to control hyperglycemic symptoms and achieve the maximal glycemic control possible while minimizing the risk of hypoglycemia. Diabetes will continue to be a challenge to the patient, the physician, the care team, and the health care system.
- Gregg EW, Engelgau MM, Narayan V. Complications of diabetes in elderly people. BMJ 2002; 325:916–917.
- Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56:42–48.
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999; 53:1937–1942.
- Fontbonne A, Berr C, Ducimetiere P, Alperovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care 2001; 24:366–370.
- Gregg EW, Mangione CM, Cauley JA, et al. Diabetes and incidence of functional disability in older women. Diabetes Care 2002; 25:61–67.
- Hornick T, Aron DC. Managing diabetes in the elderly: go easy, individualize. Cleve Clin J Med 2008; 75:70–78.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bertoni AG, Krop JS, Anderson GF, Brancati FL. Diabetes-related morbidity and mortality in a national sample of U.S. elders. Diabetes Care 2002; 25:471–475.
- Bertoni AG, Kirk JK, Goff DC, Wagenknecht LE. Excess mortality related to diabetes mellitus in elderly Medicare beneficiaries. Ann Epidemiol 2004; 14:362–367.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the Cholesterol and Recurrent Events (CARE) trial. The CARE Investigators. Circulation 1998; 98:2513–2519.
- Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes. Lancet 2003; 361:2005–2016.
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2005; 28:S4–S36.
- Brown AF, Mangione CM, Saliba D, Sarkisian CA California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51:S265–S280.
- VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus in the Primary Care Setting 2003. Accessed January 4, 2008. www.oqp.med.va.gov/cpg/dm/DM3_cpg/content/introduction.htm.
- Pogach LM, Brietzke SA, Cowan CL, Conlin P, Walder DJ, Sawin CT VA/DoD Diabetes Guideline Development Group. Development of evidence-based clinical practice guidelines for diabetes: the Department of Veterans Affairs/Department of Defense guidelines initiative. Diabetes Care 2004; 27:B82–B89.
- Stratton IM, Asler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004; 141:413–420.
- Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM UK Prospective Diabetes Study Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol 2004; 122:1631–1640.
- Cahill M, Halley A, Codd M, et al. Prevalence of diabetic retinopathy in patients with diabetic mellitus diagnosed after the age of 70 years. Br J Opthalmol 1997; 81:218–222.
- Hirvela H, Laatikainen L. Diabetic retinopathy in people aged 70 years or older. The Oulu Eye Study. Br J Ophthalmol 1997; 81:214–217.
- Tovi J, Ingemansson SO, Engfeldt P. Insulin treatment of elderly type 2 diabetic patients: effects on retinopathy. Diabetes Metab 1998; 24:442–447.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002; 61:1086–1097.
- Kohner EM. Aspirin for diabetic retinopathy. BMJ 2003; 327:1060–1061.
- Greene DA, Stevens MJ, Feldman EL. Diabetic neuropathy: scope of the syndrome. Am J Med 1999; 107:2S–8S.
- Hutchinson A, McIntosh A, Peters J, et al. Effectiveness of screening and monitoring tests for diabetic retinopathy—a systematic review. Diabet Med 2000; 17:495–506.
- Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA 2000; 283:889–896.
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA 2007; 298:902–916.
- Argoff CE, Cole BE, Fishbain DA, Irving GA. Diabetic peripheral neuropathic pain: clinical and quality-of-life issues. Mayo Clin Proc 2006; 81:S3–S11.
- Wong MC, Chung JW, Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ 2007; 335:87: epubl June 11, 2007.
- Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower-extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care 1989; 12:24–31.
- Wheeler SG, Ahroni JH, Boyko EJ. Prospective study of autonomic neuropathy as a predictor of mortality in patients with diabetes. Diabetes Res Clin Pract 2002; 58:131–138.
- Brenner BM, Cooper ME, de Zeeuw D RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998; 317:713–720.
- Sinclair AJ, Girling AJ, Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. All Wales Research into Elderly (AWARE) Study. Diabetes Res Clin Pract 2000; 50:203–212.
- Moisan J, Gaudet M, Gregoire JP, Bouchard R. Non-compliance with drug treatment and reading difficulties with regard to prescription labelling among seniors. Gerontology 2002; 48:44–51.
- Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288:462–467.
- Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002; 25:1749–1754.
- American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. J Am Geriatr Soc 2001; 49:664–672.
- Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med 2007; 167:921–927.
In elderly patients, as in all patients, diabetes is much more than the blood glucose level. However, in elderly patients the disease accelerates other common conditions of that population and markedly complicates their management.
Hypertension, coronary artery disease, and cerebrovascular attacks are more common in patients with diabetes.1 Longitudinal studies of elderly and middle-aged people with diabetes show increased rates of cognitive decline and dementia.2–4 Depression, urinary incontinence, and falls are also more common in elderly patients with diabetes. Physical disability is also increased: women with diabetes are half as likely to be able to manage ordinary physical tasks such as walking, climbing stairs, and doing housework as women without diabetes.5
In an earlier paper in this journal,6 we reviewed the management of diabetes per se in elderly patients. In the pages that follow, we review the management of its associated conditions.
HEART RISK TRUMPS BLOOD SUGAR
Coronary artery disease is by far the leading cause of death in elderly people with diabetes: 40% to 50% of patients with type 2 diabetes die of cardiac disease.7–9 The conventional risk factors—hypertension, hyperlipidemia, smoking, and diabetes—remain risk factors throughout old age. Risk reduction should focus on treating hypertension and dyslipidemia, smoking cessation, aspirin therapy, and exercise. While glycemic control reduces the risk of microvascular complications (eg, diabetic retinopathy and nephropathy) after about 8 years of treatment, benefits from control of elevated blood pressure and cholesterol occur after only 2 to 3 years.
Tight control of hypertension confers significant benefit
The United Kingdom Prospective Diabetes Study (UKPDS)10 found that patients who had tight control of blood pressure (mean treated blood pressure 144/82 mm Hg) had 24% fewer diabetes-related end points, 32% fewer diabetes-related deaths, 44% fewer strokes, a 34% reduced risk of deterioration of retinopathy, and a 47% reduced risk of visual deterioration than patients who had usual control (mean treated blood pressure 157/87 mm Hg). The benefit of treating hypertension outweighed the benefits of tight glycemic control.
A strong focus on blood pressure control should be a major focus of any treatment program. The American Geriatrics Society goal for blood pressure is less than 140/80 mm Hg if tolerated. Others have proposed more stringent targets.
Lipid control
Lipid control is integral to managing elderly patients with diabetes. In the Cholesterol and Recurrent Events trial11 and the Heart Protection Study,12 the cardiovascular benefits of reducing serum low-density lipoprotein cholesterol (LDL-C) levels were similar in elderly and younger patients with diabetes. In a meta-analysis of secondary prevention trials, absolute risk reduction was greatest in subjects older than 65 years with either diabetes or diastolic hypertension.
The American Diabetes Association,13 the American Geriatrics Society,14 and the Department of Veterans Affairs15,16 have all set a goal for serum LDL-C of less than 100 mg/dL. In addition, the American Diabetes Association has set goal levels for triglycerides (< 150 mg/dL) and high-density lipoprotein cholesterol (> 40 mg/dL).
Glycemic control
The importance of tight glycemic control in preventing coronary heart disease in the elderly is somewhat controversial. Treatment guidelines for elderly patients with diabetes are mainly extrapolated from the UKPDS, in which patients were a mean of 54 years old at the start of the study. After 10 years, the mean hemoglobin A1c levels were 7.9% in patients receiving conventional control and 7.0% in patients with intensive therapy. Every 1% reduction in hemoglobin A1c was associated with a 37% decline in microvascular complications of diabetes, a 14% decline in myocardial infarctions, and a 21% decline in any diabetes-related outcome.17
In the original trial,18 the rate of myocardial infarction was 17.4% in the conventional treatment group vs 14.7% in the intensive group (P = .052), and the risk of stroke did not differ. No thresholds for realizing benefits from reducing fasting glucose or hemoglobin A1c levels were detected.
A recent cohort study involving about 10,000 participants aged 45 to 79 years found that the risk of cardiovascular disease and death from any cause increased continuously with increasing hemoglobin A1c levels in people with or without diabetes.19 However, the impact of treatment remains to be clarified. The Action to Control Cardiovascular Risk in Diabetes trial will address this question (and others), but results will not be available for several years.
RETINOPATHY IS A MAJOR CAUSE OF BLINDNESS
Diabetic retinopathy, a leading cause of blindness in the United States, is perhaps the most threatening of the chronic microvascular complications of diabetes for elderly patients. The strongest predictor of retinopathy is the duration of diabetes.20–22 Retinopathy is classified as being nonproliferative, preproliferative, or proliferative.
Ischemia is believed to be the major cause of diabetic retinopathy, and glucose control has been shown to be of major benefit. A study of young adults with type 1 diabetes found that intensive therapy reduced the risk of developing retinopathy by 76% and slowed the progression of retinopathy by 54%. Comparable data for patients with type 2 diabetes are lacking.
Of some concern is a study in which retinopathy progressed more rapidly during the first year of aggressive insulin therapy in elderly patients with diabetes and baseline retinopathy.23 Further research is needed to identify which subgroups would benefit most from aggressive glycemic control.
In addition to specific ophthalmologic treatment, managing cardiovascular risk factors may reduce the progression of retinopathy: each cardiovascular risk factor has been found to also be a risk factor for retinopathy. Hypertension is an independent risk factor for any retinopathy, and its tight control reduces progression.20,24 Aspirin therapy has not been found to confer either risk or benefit.25,26
Although guidelines typically call for yearly ophthalmic examinations to screen for retinopathy, whether this is cost-effective has been questioned.27,28 But people older than 65 years with diabetes also have twice the risk of developing cataracts and three times the risk of developing glaucoma than those without diabetes. Considering the effects of visual loss on quality of life as well as the subsequent higher risk of accidents, eye examinations by an ophthalmologist at the time of diagnosis and annually thereafter are recommended. Tight glycemic and blood pressure control remains the cornerstone in the primary prevention of diabetic retinopathy. Panretinal and focal retinal laser photocoagulation reduces the risk of visual loss in patients with severe retinopathy and macular edema, respectively.29
NEUROPATHY PRESENTS IN MANY FORMS
Neuropathy is a particularly distressing complication and can lead to loss of sleep, limitation of activity, and depression.26,30,31 Diabetic neuropathies include focal neuropathies (entrapment syndromes and mono-neuropathies), polyneuropathy, and autonomic neuropathy.
Distal symmetric polyneuropathy (“glove and stocking” sensory symptoms) is the most common neuropathy of elderly people with diabetes. Pain, which can interrupt sleep and limit activity, can be treated with the anticonvulsants gabapentin (Gabarone, Neurontin), phenytoin (Dilantin, Phenytek) and carbamazepine (Carbatrol, Epitol, Equetro, Tegretol), and with tricyclic antidepressants. However, the anticholinergic effects of tricyclic antidepressants limit their use in older patients. Newer agents, such as duloxetine (Cymbalta) and pregabalin (Lyrica) show promise.30,31 Dysesthesia of a burning quality is sometimes treated with topical capsaicin or with oral mexiletine (Mexitil), although their role in treating older patients is not well established.
Patients with distal sensory polyneuropathy are predisposed to develop Charcot joints, which may mimic gout or degenerative joint disease. Plain radiography of the foot can help differentiate these diseases. Distal sensory polyneuropathy also predisposes patients to neuropathic foot ulcer, the leading cause of foot amputation in the United States.32
Feet should be inspected at each office visit. Testing sensation with a monofilament detects sensory neuropathy. Patients should be encouraged to examine their feet daily. Therapeutic shoes, prescribed by a podiatrist and individually designed to prevent blisters, calluses, and ulcers, are covered by Medicare for peripheral neuropathy if any of the following are also present: callus formation, poor circulation, foot deformity, or a history of foot callus, ulcer, or amputation (partial or complete). Medicare will pay for one pair of shoes plus three pairs of inserts per year.
Proximal motor neuropathy (diabetic amyotrophy) primarily affects elderly patients. It begins with unilateral thigh pain, which becomes bilateral and progresses to proximal muscle weakness and wasting. Distal symmetric polyneuropathy may also be present. Treatment includes glycemic control (usually with insulin) and physical therapy. Some forms of amyotrophy respond to immunotherapy.
Autonomic neuropathy, although not painful, can be the most life-threatening form of diabetic neuropathy.33 Tachycardia increases the risk of sudden death, while postural hypotension increases the risk of syncope, falling, and injury. Other forms of autonomic neuropathy include neurogenic bladder, sexual dysfunction, gastropathy (which is particularly sensitive to glycemic control), enteropathy, and gustatory sweating. Patients with autonomic neuropathy are more likely to have hypoglycemic unawareness.
NEPHROPATHY CAN PROGRESS RAPIDLY
Elderly patients with diabetes are especially at risk of developing nephropathy, which progresses from microalbuminuria to overt proteinuria to renal insufficiency and end-stage renal disease. Nephropathy may develop over a shorter time than the typical 10 to 20 years in younger patients. Independent risk factors for proteinuria and renal insufficiency include poor glycemic control over many years, hypertension, longer duration of diabetes, male sex, high serum total cholesterol levels, and smoking. Elderly patients are also at risk of renal insults such as receiving intravenous iodinated contrast agents in the course of radiologic procedures, nephrotoxic drugs, and comorbid illness such as congestive heart failure.
The diagnosis of diabetic nephropathy is usually made clinically and not by renal biopsy. Diabetic nephropathy can be diagnosed with almost 100% specificity in type 1 diabetes and more than 85% specificity in type 2 diabetes by a urinary albumin excretion of more than 300 mg per day and an appropriate time course in the absence of other obvious causes of renal disease. The urinary albumin-to-creatinine ratio can be used to screen for microalbuminuria (the precursor of frank proteinuria and renal insufficiency). A value of more than 30 mg of albumin per gram of creatinine suggests that albumin excretion exceeds 30 mg and that microalbuminuria is present.
Prevention is a cornerstone of management. Good glycemic control reduces the risk of microalbuminuria, the progression of albuminuria, and the development of renal insufficiency. Lowering blood pressure reduces the decline in glomerular filtration rate and albuminuria. Angiotensin-converting enzyme (ACE) inhibitors reduce the rate of progression of proteinuria and reduce the rate of end-stage renal disease, although the data are stronger in patients with type 1 diabetes.34 When side effects such as cough limit the use of ACE inhibitors, angiotensin receptor blockers can be used as an alternative. Blood pressure should be controlled to reduce stroke and cardiovascular complications, regardless of whether microalbuminuria is present.35
End-stage renal disease in elderly patients with diabetes is becoming increasingly frequent. Nephropathy in older patients is different from that in younger patients. In elderly patients, the pathologic findings may suggest ischemia and hypertension, and the classic Kimmelstiel-Wilson lesions may be absent. Patients may present with end-stage renal disease following an episode of acute renal failure that does not resolve, which may occur after a radiologic procedure involving an iodinated contrast agent.
NONKETOTIC HYPEROSMOLAR COMA
Nonketotic hyperosmolar coma occurs predominantly in elderly patients with type 2 diabetes. Predisposing factors include dementia, infection, stroke, and myocardial infarction. Coma results from osmotic diuresis due to hyperglycemia and consequent dehydration. A drop in the glomerular filtration rate promotes further hyperglycemia and dehydration in a vicious circle. Glucose levels commonly reach 600 mg/dL or more, and serum osmolality often exceeds 320 mOsm/L. A fluid deficit of 5 to 10 L is typical.
Fluid replacement is the mainstay of treatment. Because free water is typically lost in an osmotic diuresis, 0.9% (normal) saline is usually given if hemodynamic instability is present or 0.45% (half-normal) saline otherwise. Insulin is also required, as is specific treatment of the precipitating cause, eg, infection. Ketoacidosis may also occur in the elderly.
Recovery from coma or improvement in mental status may lag behind correction of the serum osmolality and may take several days. Mortality rates can be high: severe hyperosmolarity, advanced age, and nursing home residence are the major risk factors for death.
INFECTIONS: SEVERE AND UNUSUAL
Elderly patients with diabetes are at increased risk of developing severe and unusual infections, particularly malignant external otitis. Necrotizing Pseudomonas aeruginosa infection initially involves the external ear canal and progresses to the mastoid air cells, the skull base, or temporal bone. The clinical presentation consists of fever, otalgia, otorrhea, and less commonly, cranial nerve palsy. Treatment involves surgical debridement and antibiotics.
Other infections associated with diabetes include rhinocerebral mucormycosis, necrotizing fasciitis, emphysematous cholecystitis, and emphysematous pyelonephritis. An elderly patient with diabetes is also at increased risk of renal papillary necrosis, which presents as insidious renal failure.
COGNITIVE IMPAIRMENT
Elderly people with diabetes are at increased risk of cognitive impairment, which poses a barrier to taking medications appropriately and performing other tasks of self-management.
Because dementia may go undetected, particularly in the early stages, cognitive function should be assessed in elderly patients when they fail to take therapy correctly or have frequent episodes of hypoglycemia, or if glycemic control deteriorates without an obvious explanation. Caregivers play a critical role in detecting and reporting early cognitive impairment.
DEPRESSION IS OFTEN UNDETECTED
Elderly patients with diabetes have a higher rate of depression than do age-matched controls, but it is commonly underdetected and undertreated.5,36 Depression has been associated with poor glycemic control, and treatment of depression is associated with improved control. Routine screening for depression should be performed; a variety of diagnostic instruments are available. Particular attention should be given to medications that are associated with depression.
POLYPHARMACY
Many elderly patients take multiple medications. Polypharmacy increases the risk of drug side effects, interactions, and nonadherence to taking medications.37–39 This problem is increased in diabetes, in which several medications are necessary to manage hyper-glycemia, hyperlipidemia, hypertension, and other associated conditions.
Patients should keep accurate medication lists, including over-the-counter medications, herbs, and nutritional supplements. Physicians should carefully review each medication to check if it is appropriate and used correctly.
FALLS
Elderly patients with diabetes mellitus are at increased risk of injurious falls, which are associated with high rates of complications, death, and functional decline.40,41 Risk factors include frailty and functional disability, visual impairment, peripheral or autonomic neuropathy, hypoglycemia, and polypharmacy.
Elderly patients should be screened for their risk of falls, and appropriate measures should be instituted. The American Geriatrics Society has guidelines for preventing falls in the elderly.41
URINARY INCONTINENCE
Elderly women with diabetes are at increased risk of developing urinary incontinence. Risk factors include autonomic neuropathy (causing either neurogenic bladder or fecal impaction), polyuria due to hyperglycemia, and urinary tract and vaginal infections. Although evidence is lacking that urinary incontinence affects glycemic control, assessing and treating the condition improves quality of life.
SUMMARY
Diabetes is a common problem in the elderly, accounting for considerable morbidity and mortality. In a large longitudinal analysis (> 50,000 patients), elderly persons newly diagnosed as having diabetes experienced high rates of complications during 10-year follow-up, far in excess of elderly persons without diabetes.42 Diabetes is underdiagnosed in the elderly and is frequently undertreated. Management of the elderly with diabetes presents unique challenges because of associated comorbidities, but with attention to detail and individualized approaches, quality and duration of life can be optimized. The greatest attention should be given to reduction of overall cardiovascular risk. Glycemic goals and the treatment regimens to achieve those goals should be individualized and chosen to control hyperglycemic symptoms and achieve the maximal glycemic control possible while minimizing the risk of hypoglycemia. Diabetes will continue to be a challenge to the patient, the physician, the care team, and the health care system.
In elderly patients, as in all patients, diabetes is much more than the blood glucose level. However, in elderly patients the disease accelerates other common conditions of that population and markedly complicates their management.
Hypertension, coronary artery disease, and cerebrovascular attacks are more common in patients with diabetes.1 Longitudinal studies of elderly and middle-aged people with diabetes show increased rates of cognitive decline and dementia.2–4 Depression, urinary incontinence, and falls are also more common in elderly patients with diabetes. Physical disability is also increased: women with diabetes are half as likely to be able to manage ordinary physical tasks such as walking, climbing stairs, and doing housework as women without diabetes.5
In an earlier paper in this journal,6 we reviewed the management of diabetes per se in elderly patients. In the pages that follow, we review the management of its associated conditions.
HEART RISK TRUMPS BLOOD SUGAR
Coronary artery disease is by far the leading cause of death in elderly people with diabetes: 40% to 50% of patients with type 2 diabetes die of cardiac disease.7–9 The conventional risk factors—hypertension, hyperlipidemia, smoking, and diabetes—remain risk factors throughout old age. Risk reduction should focus on treating hypertension and dyslipidemia, smoking cessation, aspirin therapy, and exercise. While glycemic control reduces the risk of microvascular complications (eg, diabetic retinopathy and nephropathy) after about 8 years of treatment, benefits from control of elevated blood pressure and cholesterol occur after only 2 to 3 years.
Tight control of hypertension confers significant benefit
The United Kingdom Prospective Diabetes Study (UKPDS)10 found that patients who had tight control of blood pressure (mean treated blood pressure 144/82 mm Hg) had 24% fewer diabetes-related end points, 32% fewer diabetes-related deaths, 44% fewer strokes, a 34% reduced risk of deterioration of retinopathy, and a 47% reduced risk of visual deterioration than patients who had usual control (mean treated blood pressure 157/87 mm Hg). The benefit of treating hypertension outweighed the benefits of tight glycemic control.
A strong focus on blood pressure control should be a major focus of any treatment program. The American Geriatrics Society goal for blood pressure is less than 140/80 mm Hg if tolerated. Others have proposed more stringent targets.
Lipid control
Lipid control is integral to managing elderly patients with diabetes. In the Cholesterol and Recurrent Events trial11 and the Heart Protection Study,12 the cardiovascular benefits of reducing serum low-density lipoprotein cholesterol (LDL-C) levels were similar in elderly and younger patients with diabetes. In a meta-analysis of secondary prevention trials, absolute risk reduction was greatest in subjects older than 65 years with either diabetes or diastolic hypertension.
The American Diabetes Association,13 the American Geriatrics Society,14 and the Department of Veterans Affairs15,16 have all set a goal for serum LDL-C of less than 100 mg/dL. In addition, the American Diabetes Association has set goal levels for triglycerides (< 150 mg/dL) and high-density lipoprotein cholesterol (> 40 mg/dL).
Glycemic control
The importance of tight glycemic control in preventing coronary heart disease in the elderly is somewhat controversial. Treatment guidelines for elderly patients with diabetes are mainly extrapolated from the UKPDS, in which patients were a mean of 54 years old at the start of the study. After 10 years, the mean hemoglobin A1c levels were 7.9% in patients receiving conventional control and 7.0% in patients with intensive therapy. Every 1% reduction in hemoglobin A1c was associated with a 37% decline in microvascular complications of diabetes, a 14% decline in myocardial infarctions, and a 21% decline in any diabetes-related outcome.17
In the original trial,18 the rate of myocardial infarction was 17.4% in the conventional treatment group vs 14.7% in the intensive group (P = .052), and the risk of stroke did not differ. No thresholds for realizing benefits from reducing fasting glucose or hemoglobin A1c levels were detected.
A recent cohort study involving about 10,000 participants aged 45 to 79 years found that the risk of cardiovascular disease and death from any cause increased continuously with increasing hemoglobin A1c levels in people with or without diabetes.19 However, the impact of treatment remains to be clarified. The Action to Control Cardiovascular Risk in Diabetes trial will address this question (and others), but results will not be available for several years.
RETINOPATHY IS A MAJOR CAUSE OF BLINDNESS
Diabetic retinopathy, a leading cause of blindness in the United States, is perhaps the most threatening of the chronic microvascular complications of diabetes for elderly patients. The strongest predictor of retinopathy is the duration of diabetes.20–22 Retinopathy is classified as being nonproliferative, preproliferative, or proliferative.
Ischemia is believed to be the major cause of diabetic retinopathy, and glucose control has been shown to be of major benefit. A study of young adults with type 1 diabetes found that intensive therapy reduced the risk of developing retinopathy by 76% and slowed the progression of retinopathy by 54%. Comparable data for patients with type 2 diabetes are lacking.
Of some concern is a study in which retinopathy progressed more rapidly during the first year of aggressive insulin therapy in elderly patients with diabetes and baseline retinopathy.23 Further research is needed to identify which subgroups would benefit most from aggressive glycemic control.
In addition to specific ophthalmologic treatment, managing cardiovascular risk factors may reduce the progression of retinopathy: each cardiovascular risk factor has been found to also be a risk factor for retinopathy. Hypertension is an independent risk factor for any retinopathy, and its tight control reduces progression.20,24 Aspirin therapy has not been found to confer either risk or benefit.25,26
Although guidelines typically call for yearly ophthalmic examinations to screen for retinopathy, whether this is cost-effective has been questioned.27,28 But people older than 65 years with diabetes also have twice the risk of developing cataracts and three times the risk of developing glaucoma than those without diabetes. Considering the effects of visual loss on quality of life as well as the subsequent higher risk of accidents, eye examinations by an ophthalmologist at the time of diagnosis and annually thereafter are recommended. Tight glycemic and blood pressure control remains the cornerstone in the primary prevention of diabetic retinopathy. Panretinal and focal retinal laser photocoagulation reduces the risk of visual loss in patients with severe retinopathy and macular edema, respectively.29
NEUROPATHY PRESENTS IN MANY FORMS
Neuropathy is a particularly distressing complication and can lead to loss of sleep, limitation of activity, and depression.26,30,31 Diabetic neuropathies include focal neuropathies (entrapment syndromes and mono-neuropathies), polyneuropathy, and autonomic neuropathy.
Distal symmetric polyneuropathy (“glove and stocking” sensory symptoms) is the most common neuropathy of elderly people with diabetes. Pain, which can interrupt sleep and limit activity, can be treated with the anticonvulsants gabapentin (Gabarone, Neurontin), phenytoin (Dilantin, Phenytek) and carbamazepine (Carbatrol, Epitol, Equetro, Tegretol), and with tricyclic antidepressants. However, the anticholinergic effects of tricyclic antidepressants limit their use in older patients. Newer agents, such as duloxetine (Cymbalta) and pregabalin (Lyrica) show promise.30,31 Dysesthesia of a burning quality is sometimes treated with topical capsaicin or with oral mexiletine (Mexitil), although their role in treating older patients is not well established.
Patients with distal sensory polyneuropathy are predisposed to develop Charcot joints, which may mimic gout or degenerative joint disease. Plain radiography of the foot can help differentiate these diseases. Distal sensory polyneuropathy also predisposes patients to neuropathic foot ulcer, the leading cause of foot amputation in the United States.32
Feet should be inspected at each office visit. Testing sensation with a monofilament detects sensory neuropathy. Patients should be encouraged to examine their feet daily. Therapeutic shoes, prescribed by a podiatrist and individually designed to prevent blisters, calluses, and ulcers, are covered by Medicare for peripheral neuropathy if any of the following are also present: callus formation, poor circulation, foot deformity, or a history of foot callus, ulcer, or amputation (partial or complete). Medicare will pay for one pair of shoes plus three pairs of inserts per year.
Proximal motor neuropathy (diabetic amyotrophy) primarily affects elderly patients. It begins with unilateral thigh pain, which becomes bilateral and progresses to proximal muscle weakness and wasting. Distal symmetric polyneuropathy may also be present. Treatment includes glycemic control (usually with insulin) and physical therapy. Some forms of amyotrophy respond to immunotherapy.
Autonomic neuropathy, although not painful, can be the most life-threatening form of diabetic neuropathy.33 Tachycardia increases the risk of sudden death, while postural hypotension increases the risk of syncope, falling, and injury. Other forms of autonomic neuropathy include neurogenic bladder, sexual dysfunction, gastropathy (which is particularly sensitive to glycemic control), enteropathy, and gustatory sweating. Patients with autonomic neuropathy are more likely to have hypoglycemic unawareness.
NEPHROPATHY CAN PROGRESS RAPIDLY
Elderly patients with diabetes are especially at risk of developing nephropathy, which progresses from microalbuminuria to overt proteinuria to renal insufficiency and end-stage renal disease. Nephropathy may develop over a shorter time than the typical 10 to 20 years in younger patients. Independent risk factors for proteinuria and renal insufficiency include poor glycemic control over many years, hypertension, longer duration of diabetes, male sex, high serum total cholesterol levels, and smoking. Elderly patients are also at risk of renal insults such as receiving intravenous iodinated contrast agents in the course of radiologic procedures, nephrotoxic drugs, and comorbid illness such as congestive heart failure.
The diagnosis of diabetic nephropathy is usually made clinically and not by renal biopsy. Diabetic nephropathy can be diagnosed with almost 100% specificity in type 1 diabetes and more than 85% specificity in type 2 diabetes by a urinary albumin excretion of more than 300 mg per day and an appropriate time course in the absence of other obvious causes of renal disease. The urinary albumin-to-creatinine ratio can be used to screen for microalbuminuria (the precursor of frank proteinuria and renal insufficiency). A value of more than 30 mg of albumin per gram of creatinine suggests that albumin excretion exceeds 30 mg and that microalbuminuria is present.
Prevention is a cornerstone of management. Good glycemic control reduces the risk of microalbuminuria, the progression of albuminuria, and the development of renal insufficiency. Lowering blood pressure reduces the decline in glomerular filtration rate and albuminuria. Angiotensin-converting enzyme (ACE) inhibitors reduce the rate of progression of proteinuria and reduce the rate of end-stage renal disease, although the data are stronger in patients with type 1 diabetes.34 When side effects such as cough limit the use of ACE inhibitors, angiotensin receptor blockers can be used as an alternative. Blood pressure should be controlled to reduce stroke and cardiovascular complications, regardless of whether microalbuminuria is present.35
End-stage renal disease in elderly patients with diabetes is becoming increasingly frequent. Nephropathy in older patients is different from that in younger patients. In elderly patients, the pathologic findings may suggest ischemia and hypertension, and the classic Kimmelstiel-Wilson lesions may be absent. Patients may present with end-stage renal disease following an episode of acute renal failure that does not resolve, which may occur after a radiologic procedure involving an iodinated contrast agent.
NONKETOTIC HYPEROSMOLAR COMA
Nonketotic hyperosmolar coma occurs predominantly in elderly patients with type 2 diabetes. Predisposing factors include dementia, infection, stroke, and myocardial infarction. Coma results from osmotic diuresis due to hyperglycemia and consequent dehydration. A drop in the glomerular filtration rate promotes further hyperglycemia and dehydration in a vicious circle. Glucose levels commonly reach 600 mg/dL or more, and serum osmolality often exceeds 320 mOsm/L. A fluid deficit of 5 to 10 L is typical.
Fluid replacement is the mainstay of treatment. Because free water is typically lost in an osmotic diuresis, 0.9% (normal) saline is usually given if hemodynamic instability is present or 0.45% (half-normal) saline otherwise. Insulin is also required, as is specific treatment of the precipitating cause, eg, infection. Ketoacidosis may also occur in the elderly.
Recovery from coma or improvement in mental status may lag behind correction of the serum osmolality and may take several days. Mortality rates can be high: severe hyperosmolarity, advanced age, and nursing home residence are the major risk factors for death.
INFECTIONS: SEVERE AND UNUSUAL
Elderly patients with diabetes are at increased risk of developing severe and unusual infections, particularly malignant external otitis. Necrotizing Pseudomonas aeruginosa infection initially involves the external ear canal and progresses to the mastoid air cells, the skull base, or temporal bone. The clinical presentation consists of fever, otalgia, otorrhea, and less commonly, cranial nerve palsy. Treatment involves surgical debridement and antibiotics.
Other infections associated with diabetes include rhinocerebral mucormycosis, necrotizing fasciitis, emphysematous cholecystitis, and emphysematous pyelonephritis. An elderly patient with diabetes is also at increased risk of renal papillary necrosis, which presents as insidious renal failure.
COGNITIVE IMPAIRMENT
Elderly people with diabetes are at increased risk of cognitive impairment, which poses a barrier to taking medications appropriately and performing other tasks of self-management.
Because dementia may go undetected, particularly in the early stages, cognitive function should be assessed in elderly patients when they fail to take therapy correctly or have frequent episodes of hypoglycemia, or if glycemic control deteriorates without an obvious explanation. Caregivers play a critical role in detecting and reporting early cognitive impairment.
DEPRESSION IS OFTEN UNDETECTED
Elderly patients with diabetes have a higher rate of depression than do age-matched controls, but it is commonly underdetected and undertreated.5,36 Depression has been associated with poor glycemic control, and treatment of depression is associated with improved control. Routine screening for depression should be performed; a variety of diagnostic instruments are available. Particular attention should be given to medications that are associated with depression.
POLYPHARMACY
Many elderly patients take multiple medications. Polypharmacy increases the risk of drug side effects, interactions, and nonadherence to taking medications.37–39 This problem is increased in diabetes, in which several medications are necessary to manage hyper-glycemia, hyperlipidemia, hypertension, and other associated conditions.
Patients should keep accurate medication lists, including over-the-counter medications, herbs, and nutritional supplements. Physicians should carefully review each medication to check if it is appropriate and used correctly.
FALLS
Elderly patients with diabetes mellitus are at increased risk of injurious falls, which are associated with high rates of complications, death, and functional decline.40,41 Risk factors include frailty and functional disability, visual impairment, peripheral or autonomic neuropathy, hypoglycemia, and polypharmacy.
Elderly patients should be screened for their risk of falls, and appropriate measures should be instituted. The American Geriatrics Society has guidelines for preventing falls in the elderly.41
URINARY INCONTINENCE
Elderly women with diabetes are at increased risk of developing urinary incontinence. Risk factors include autonomic neuropathy (causing either neurogenic bladder or fecal impaction), polyuria due to hyperglycemia, and urinary tract and vaginal infections. Although evidence is lacking that urinary incontinence affects glycemic control, assessing and treating the condition improves quality of life.
SUMMARY
Diabetes is a common problem in the elderly, accounting for considerable morbidity and mortality. In a large longitudinal analysis (> 50,000 patients), elderly persons newly diagnosed as having diabetes experienced high rates of complications during 10-year follow-up, far in excess of elderly persons without diabetes.42 Diabetes is underdiagnosed in the elderly and is frequently undertreated. Management of the elderly with diabetes presents unique challenges because of associated comorbidities, but with attention to detail and individualized approaches, quality and duration of life can be optimized. The greatest attention should be given to reduction of overall cardiovascular risk. Glycemic goals and the treatment regimens to achieve those goals should be individualized and chosen to control hyperglycemic symptoms and achieve the maximal glycemic control possible while minimizing the risk of hypoglycemia. Diabetes will continue to be a challenge to the patient, the physician, the care team, and the health care system.
- Gregg EW, Engelgau MM, Narayan V. Complications of diabetes in elderly people. BMJ 2002; 325:916–917.
- Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56:42–48.
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999; 53:1937–1942.
- Fontbonne A, Berr C, Ducimetiere P, Alperovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care 2001; 24:366–370.
- Gregg EW, Mangione CM, Cauley JA, et al. Diabetes and incidence of functional disability in older women. Diabetes Care 2002; 25:61–67.
- Hornick T, Aron DC. Managing diabetes in the elderly: go easy, individualize. Cleve Clin J Med 2008; 75:70–78.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bertoni AG, Krop JS, Anderson GF, Brancati FL. Diabetes-related morbidity and mortality in a national sample of U.S. elders. Diabetes Care 2002; 25:471–475.
- Bertoni AG, Kirk JK, Goff DC, Wagenknecht LE. Excess mortality related to diabetes mellitus in elderly Medicare beneficiaries. Ann Epidemiol 2004; 14:362–367.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the Cholesterol and Recurrent Events (CARE) trial. The CARE Investigators. Circulation 1998; 98:2513–2519.
- Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes. Lancet 2003; 361:2005–2016.
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2005; 28:S4–S36.
- Brown AF, Mangione CM, Saliba D, Sarkisian CA California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51:S265–S280.
- VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus in the Primary Care Setting 2003. Accessed January 4, 2008. www.oqp.med.va.gov/cpg/dm/DM3_cpg/content/introduction.htm.
- Pogach LM, Brietzke SA, Cowan CL, Conlin P, Walder DJ, Sawin CT VA/DoD Diabetes Guideline Development Group. Development of evidence-based clinical practice guidelines for diabetes: the Department of Veterans Affairs/Department of Defense guidelines initiative. Diabetes Care 2004; 27:B82–B89.
- Stratton IM, Asler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004; 141:413–420.
- Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM UK Prospective Diabetes Study Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol 2004; 122:1631–1640.
- Cahill M, Halley A, Codd M, et al. Prevalence of diabetic retinopathy in patients with diabetic mellitus diagnosed after the age of 70 years. Br J Opthalmol 1997; 81:218–222.
- Hirvela H, Laatikainen L. Diabetic retinopathy in people aged 70 years or older. The Oulu Eye Study. Br J Ophthalmol 1997; 81:214–217.
- Tovi J, Ingemansson SO, Engfeldt P. Insulin treatment of elderly type 2 diabetic patients: effects on retinopathy. Diabetes Metab 1998; 24:442–447.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002; 61:1086–1097.
- Kohner EM. Aspirin for diabetic retinopathy. BMJ 2003; 327:1060–1061.
- Greene DA, Stevens MJ, Feldman EL. Diabetic neuropathy: scope of the syndrome. Am J Med 1999; 107:2S–8S.
- Hutchinson A, McIntosh A, Peters J, et al. Effectiveness of screening and monitoring tests for diabetic retinopathy—a systematic review. Diabet Med 2000; 17:495–506.
- Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA 2000; 283:889–896.
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA 2007; 298:902–916.
- Argoff CE, Cole BE, Fishbain DA, Irving GA. Diabetic peripheral neuropathic pain: clinical and quality-of-life issues. Mayo Clin Proc 2006; 81:S3–S11.
- Wong MC, Chung JW, Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ 2007; 335:87: epubl June 11, 2007.
- Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower-extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care 1989; 12:24–31.
- Wheeler SG, Ahroni JH, Boyko EJ. Prospective study of autonomic neuropathy as a predictor of mortality in patients with diabetes. Diabetes Res Clin Pract 2002; 58:131–138.
- Brenner BM, Cooper ME, de Zeeuw D RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998; 317:713–720.
- Sinclair AJ, Girling AJ, Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. All Wales Research into Elderly (AWARE) Study. Diabetes Res Clin Pract 2000; 50:203–212.
- Moisan J, Gaudet M, Gregoire JP, Bouchard R. Non-compliance with drug treatment and reading difficulties with regard to prescription labelling among seniors. Gerontology 2002; 48:44–51.
- Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288:462–467.
- Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002; 25:1749–1754.
- American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. J Am Geriatr Soc 2001; 49:664–672.
- Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med 2007; 167:921–927.
- Gregg EW, Engelgau MM, Narayan V. Complications of diabetes in elderly people. BMJ 2002; 325:916–917.
- Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56:42–48.
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999; 53:1937–1942.
- Fontbonne A, Berr C, Ducimetiere P, Alperovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care 2001; 24:366–370.
- Gregg EW, Mangione CM, Cauley JA, et al. Diabetes and incidence of functional disability in older women. Diabetes Care 2002; 25:61–67.
- Hornick T, Aron DC. Managing diabetes in the elderly: go easy, individualize. Cleve Clin J Med 2008; 75:70–78.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bertoni AG, Krop JS, Anderson GF, Brancati FL. Diabetes-related morbidity and mortality in a national sample of U.S. elders. Diabetes Care 2002; 25:471–475.
- Bertoni AG, Kirk JK, Goff DC, Wagenknecht LE. Excess mortality related to diabetes mellitus in elderly Medicare beneficiaries. Ann Epidemiol 2004; 14:362–367.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the Cholesterol and Recurrent Events (CARE) trial. The CARE Investigators. Circulation 1998; 98:2513–2519.
- Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes. Lancet 2003; 361:2005–2016.
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2005; 28:S4–S36.
- Brown AF, Mangione CM, Saliba D, Sarkisian CA California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51:S265–S280.
- VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus in the Primary Care Setting 2003. Accessed January 4, 2008. www.oqp.med.va.gov/cpg/dm/DM3_cpg/content/introduction.htm.
- Pogach LM, Brietzke SA, Cowan CL, Conlin P, Walder DJ, Sawin CT VA/DoD Diabetes Guideline Development Group. Development of evidence-based clinical practice guidelines for diabetes: the Department of Veterans Affairs/Department of Defense guidelines initiative. Diabetes Care 2004; 27:B82–B89.
- Stratton IM, Asler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004; 141:413–420.
- Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM UK Prospective Diabetes Study Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol 2004; 122:1631–1640.
- Cahill M, Halley A, Codd M, et al. Prevalence of diabetic retinopathy in patients with diabetic mellitus diagnosed after the age of 70 years. Br J Opthalmol 1997; 81:218–222.
- Hirvela H, Laatikainen L. Diabetic retinopathy in people aged 70 years or older. The Oulu Eye Study. Br J Ophthalmol 1997; 81:214–217.
- Tovi J, Ingemansson SO, Engfeldt P. Insulin treatment of elderly type 2 diabetic patients: effects on retinopathy. Diabetes Metab 1998; 24:442–447.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002; 61:1086–1097.
- Kohner EM. Aspirin for diabetic retinopathy. BMJ 2003; 327:1060–1061.
- Greene DA, Stevens MJ, Feldman EL. Diabetic neuropathy: scope of the syndrome. Am J Med 1999; 107:2S–8S.
- Hutchinson A, McIntosh A, Peters J, et al. Effectiveness of screening and monitoring tests for diabetic retinopathy—a systematic review. Diabet Med 2000; 17:495–506.
- Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA 2000; 283:889–896.
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA 2007; 298:902–916.
- Argoff CE, Cole BE, Fishbain DA, Irving GA. Diabetic peripheral neuropathic pain: clinical and quality-of-life issues. Mayo Clin Proc 2006; 81:S3–S11.
- Wong MC, Chung JW, Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ 2007; 335:87: epubl June 11, 2007.
- Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower-extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care 1989; 12:24–31.
- Wheeler SG, Ahroni JH, Boyko EJ. Prospective study of autonomic neuropathy as a predictor of mortality in patients with diabetes. Diabetes Res Clin Pract 2002; 58:131–138.
- Brenner BM, Cooper ME, de Zeeuw D RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998; 317:713–720.
- Sinclair AJ, Girling AJ, Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. All Wales Research into Elderly (AWARE) Study. Diabetes Res Clin Pract 2000; 50:203–212.
- Moisan J, Gaudet M, Gregoire JP, Bouchard R. Non-compliance with drug treatment and reading difficulties with regard to prescription labelling among seniors. Gerontology 2002; 48:44–51.
- Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288:462–467.
- Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002; 25:1749–1754.
- American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. J Am Geriatr Soc 2001; 49:664–672.
- Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med 2007; 167:921–927.
KEY POINTS
- Compared with strict glycemic control, treating cardiovascular risk factors offers more benefit in a shorter time and should be a higher priority.
- Diabetic retinopathy is a leading cause of blindness. Yearly eye examinations are recommended.
- Elderly patients with diabetes are prone to rapidly progressive nephropathy, especially after receiving iodinated contrast agents. Good glycemic control and control of blood pressure, especially with angiotensin-converting enzyme inhibitors, reduce the risk and the rate of progression.
- Elderly patients with diabetes are at higher risk of cognitive decline, depression, and polypharmacy, all of which impede good diabetes management.
Surprisingly nonbenign imaging
As reviewed by Dr. Naim Issa and colleagues in this issue of the Journal, practices are changing with regard to choice of imaging techniques and contrast materials in patients with renal insufficiency. In 1 week, while on our inpatient rheumatology consultation service, I specifically commented in two patients’ charts that I would prefer to avoid the use of gadolinium because the patients had significant renal dysfunction. Since the patients did not yet need dialysis, standard contrast dye was also relatively contraindicated. It was a bit of a dilemma.
In an accompanying editorial, Dr. Jonathan Kay proposes that this pseudoscleroderma syndrome be called “gadolinium-associated systemic fibrosis (GASF).” Dr. Kay and colleagues have recently published an important study (Arthritis Rheum 2007; 56:3433–3441) in which they report that, with a focused physical examination, this often-unrecognized clinical syndrome can be diagnosed in 13% of patients undergoing chronic hemodialysis and that the diagnosis indicates a significant risk of death. They confirm the suggestion of earlier authors that exposure to gadolinium is a significant predisposing factor for the syndrome.
Gadolinium is not the first exogenous chemical trigger of a fibrosing syndrome to be identified: eg, bleomycin (Blenoxane) is a well-known trigger of pulmonary fibrosis. Pseudoscleroderma syndromes have been described after exposure to certain rapeseed oils and to impure preparations of tryptophan (the “eosinophilic-mayalgia syndrome”). The mechanisms of these reactions are not fully understood, and other than the accumulation of gadolinium in tissues in the setting of chronic renal disease, not much is known about the pathophysiology of GASF.
Guidelines will be proposed to try to limit the occurrence of this devastating syndrome. But we can only guess as to the glomerular filtration rate cutoff at which we should be most concerned, and we can only hope that acute dialysis after gadolinium exposure will be protective. In the meantime, we will need to revise our view that “MRI with contrast” is a benign test.
As reviewed by Dr. Naim Issa and colleagues in this issue of the Journal, practices are changing with regard to choice of imaging techniques and contrast materials in patients with renal insufficiency. In 1 week, while on our inpatient rheumatology consultation service, I specifically commented in two patients’ charts that I would prefer to avoid the use of gadolinium because the patients had significant renal dysfunction. Since the patients did not yet need dialysis, standard contrast dye was also relatively contraindicated. It was a bit of a dilemma.
In an accompanying editorial, Dr. Jonathan Kay proposes that this pseudoscleroderma syndrome be called “gadolinium-associated systemic fibrosis (GASF).” Dr. Kay and colleagues have recently published an important study (Arthritis Rheum 2007; 56:3433–3441) in which they report that, with a focused physical examination, this often-unrecognized clinical syndrome can be diagnosed in 13% of patients undergoing chronic hemodialysis and that the diagnosis indicates a significant risk of death. They confirm the suggestion of earlier authors that exposure to gadolinium is a significant predisposing factor for the syndrome.
Gadolinium is not the first exogenous chemical trigger of a fibrosing syndrome to be identified: eg, bleomycin (Blenoxane) is a well-known trigger of pulmonary fibrosis. Pseudoscleroderma syndromes have been described after exposure to certain rapeseed oils and to impure preparations of tryptophan (the “eosinophilic-mayalgia syndrome”). The mechanisms of these reactions are not fully understood, and other than the accumulation of gadolinium in tissues in the setting of chronic renal disease, not much is known about the pathophysiology of GASF.
Guidelines will be proposed to try to limit the occurrence of this devastating syndrome. But we can only guess as to the glomerular filtration rate cutoff at which we should be most concerned, and we can only hope that acute dialysis after gadolinium exposure will be protective. In the meantime, we will need to revise our view that “MRI with contrast” is a benign test.
As reviewed by Dr. Naim Issa and colleagues in this issue of the Journal, practices are changing with regard to choice of imaging techniques and contrast materials in patients with renal insufficiency. In 1 week, while on our inpatient rheumatology consultation service, I specifically commented in two patients’ charts that I would prefer to avoid the use of gadolinium because the patients had significant renal dysfunction. Since the patients did not yet need dialysis, standard contrast dye was also relatively contraindicated. It was a bit of a dilemma.
In an accompanying editorial, Dr. Jonathan Kay proposes that this pseudoscleroderma syndrome be called “gadolinium-associated systemic fibrosis (GASF).” Dr. Kay and colleagues have recently published an important study (Arthritis Rheum 2007; 56:3433–3441) in which they report that, with a focused physical examination, this often-unrecognized clinical syndrome can be diagnosed in 13% of patients undergoing chronic hemodialysis and that the diagnosis indicates a significant risk of death. They confirm the suggestion of earlier authors that exposure to gadolinium is a significant predisposing factor for the syndrome.
Gadolinium is not the first exogenous chemical trigger of a fibrosing syndrome to be identified: eg, bleomycin (Blenoxane) is a well-known trigger of pulmonary fibrosis. Pseudoscleroderma syndromes have been described after exposure to certain rapeseed oils and to impure preparations of tryptophan (the “eosinophilic-mayalgia syndrome”). The mechanisms of these reactions are not fully understood, and other than the accumulation of gadolinium in tissues in the setting of chronic renal disease, not much is known about the pathophysiology of GASF.
Guidelines will be proposed to try to limit the occurrence of this devastating syndrome. But we can only guess as to the glomerular filtration rate cutoff at which we should be most concerned, and we can only hope that acute dialysis after gadolinium exposure will be protective. In the meantime, we will need to revise our view that “MRI with contrast” is a benign test.