User login
FDA: Gadolinium retention prompts new GBCA class warning, safety measures
Gadolinium-based contrast agents (GBCAs) used for MRI will now carry a warning regarding their potential retention in the bodies and brains of treated patients, according to the Food and Drug Administration.
The FDA is requiring the new class warning, along with other safety measures, based on evidence showing that trace amounts of gadolinium can be retained in the body for months to years after treatment.
Specifically, the agency will require that patients receiving GBCAs first receive a Medication Guide and that GBCA manufacturers conduct human and animal studies to further assess GBCA safety. At this time, the only known adverse health effect of gadolinium retention is nephrogenic systemic fibrosis, which affects a small subgroup of patients with pre-existing kidney failure. No causal association has been established between gadolinium retention and reported adverse events in those with normal kidney function.
The FDA recommended that health care professionals consider the retention characteristics of GBCAs for patients who may be at higher risk for retention, including those requiring multiple lifetime doses, pregnant women, children, and patients with inflammatory conditions, but stressed that, although repeated GBCA imaging studies should be minimized when possible, they should not be avoided or deferred when they are necessary. In the safety alert, the FDA noted that administration of the GBCAs Dotarem (gadoterate meglumine), Gadavist (gadobutrol), and ProHance (gadoteridol) produce the lowest gadolinium levels in the body, and the three agents leave similar gadolinium levels in the body.
The agency encourages reports of adverse events or side effects related to the use of GBCAs to its MedWatch Safety information and Adverse Event Reporting Program. Reports can be submitted online at www.fda.gov/MedWatch/report or by calling 1-800-332-1088 to request a preaddressed form that can be mailed or faxed to 1-800-FDA-0178.
Gadolinium-based contrast agents (GBCAs) used for MRI will now carry a warning regarding their potential retention in the bodies and brains of treated patients, according to the Food and Drug Administration.
The FDA is requiring the new class warning, along with other safety measures, based on evidence showing that trace amounts of gadolinium can be retained in the body for months to years after treatment.
Specifically, the agency will require that patients receiving GBCAs first receive a Medication Guide and that GBCA manufacturers conduct human and animal studies to further assess GBCA safety. At this time, the only known adverse health effect of gadolinium retention is nephrogenic systemic fibrosis, which affects a small subgroup of patients with pre-existing kidney failure. No causal association has been established between gadolinium retention and reported adverse events in those with normal kidney function.
The FDA recommended that health care professionals consider the retention characteristics of GBCAs for patients who may be at higher risk for retention, including those requiring multiple lifetime doses, pregnant women, children, and patients with inflammatory conditions, but stressed that, although repeated GBCA imaging studies should be minimized when possible, they should not be avoided or deferred when they are necessary. In the safety alert, the FDA noted that administration of the GBCAs Dotarem (gadoterate meglumine), Gadavist (gadobutrol), and ProHance (gadoteridol) produce the lowest gadolinium levels in the body, and the three agents leave similar gadolinium levels in the body.
The agency encourages reports of adverse events or side effects related to the use of GBCAs to its MedWatch Safety information and Adverse Event Reporting Program. Reports can be submitted online at www.fda.gov/MedWatch/report or by calling 1-800-332-1088 to request a preaddressed form that can be mailed or faxed to 1-800-FDA-0178.
Gadolinium-based contrast agents (GBCAs) used for MRI will now carry a warning regarding their potential retention in the bodies and brains of treated patients, according to the Food and Drug Administration.
The FDA is requiring the new class warning, along with other safety measures, based on evidence showing that trace amounts of gadolinium can be retained in the body for months to years after treatment.
Specifically, the agency will require that patients receiving GBCAs first receive a Medication Guide and that GBCA manufacturers conduct human and animal studies to further assess GBCA safety. At this time, the only known adverse health effect of gadolinium retention is nephrogenic systemic fibrosis, which affects a small subgroup of patients with pre-existing kidney failure. No causal association has been established between gadolinium retention and reported adverse events in those with normal kidney function.
The FDA recommended that health care professionals consider the retention characteristics of GBCAs for patients who may be at higher risk for retention, including those requiring multiple lifetime doses, pregnant women, children, and patients with inflammatory conditions, but stressed that, although repeated GBCA imaging studies should be minimized when possible, they should not be avoided or deferred when they are necessary. In the safety alert, the FDA noted that administration of the GBCAs Dotarem (gadoterate meglumine), Gadavist (gadobutrol), and ProHance (gadoteridol) produce the lowest gadolinium levels in the body, and the three agents leave similar gadolinium levels in the body.
The agency encourages reports of adverse events or side effects related to the use of GBCAs to its MedWatch Safety information and Adverse Event Reporting Program. Reports can be submitted online at www.fda.gov/MedWatch/report or by calling 1-800-332-1088 to request a preaddressed form that can be mailed or faxed to 1-800-FDA-0178.
What Are the Long-Term Neurologic Complications of Childhood Cancer?
KANSAS CITY, MO—As cancer treatment advances, the prevalence of childhood cancer survivors increases; as a result, physicians see more neurologic complications of surgery, chemotherapy, and radiation, according to an overview presented at the 46th Annual Meeting of the Child Neurology Society.
“There is an increasing impact of neurologic and neuropsychological toxicity that underscores the need for intervention and follow-up over the lifespan,” said Nicole Ullrich, MD, PhD, Director of Neurologic Neuro-Oncology at the Dana-Farber/Boston Children's Cancer and Blood Disorders Center.
Lifelong Effects
The prevalence of adult survivors of childhood cancer is one in 250 people, and there are more than 270,000 childhood cancer survivors in the United States. These cancer survivors “experience long-term toxicities affecting their respiratory system, cardiovascular system, cerebrovascular system, reproductive system, and a gamut of other late effects of treatment that we need to think about as they continue to advance into adulthood,” said Dr. Ullrich.
“Ninety-five percent of all cancer survivors will have a chronic health problem by the age of 45, and 80% will have a severe or life-threatening condition,” she said. The risk is greater for patients with CNS malignancies.
Childhood cancer survivors may develop physical, cognitive, and psychological issues later in life. These late effects have a significant impact on their quality and quantity of life. Neurologic issues can include headache, seizures, and stroke.
Headache and Seizures
Patients with elevated intracranial pressure or large tumors have an increased risk of headache. Nontumor causes of headache may include medications, such as antiemetic drugs and chemotherapy. Radiation therapy may cause acute radiation necrosis and long-term vascular issues. Supportive therapies, including steroids, antacids, and antinausea regimens, can cause headaches as well as sleep disruption, said Dr. Ullrich.
Approximately 15% to 25% of children with a brain tumor present with a seizure. Seizures are more often associated with low-grade tumors. Children with solid tumors or leukemia often have seizures without clinical or radiologic signs of a structural lesion. When a surgeon removes the tumor, there may be associated areas of dysplasia that surround the tumor, said Dr. Ullrich. Strategies such as electrocorticography or intraoperative monitoring can help identify epileptic zones for removal during surgery.
Potential causes of seizures include the tumor itself, surrounding edema, areas of cortical dysplasia, hyperexcitability related to neurotransmitters and glutamate levels, and scar formation that occurs after tumor resection. Individuals who have had cortically based or temporal lobe tumors or who have had incomplete resection or preexisting seizures before diagnosis have the highest risk of developing seizures, even years after completion of therapy.
EEG can help confirm seizures and distinguish between seizure types. It also can aid in the choice of an anticonvulsant. “We tend to lean more towards non-enzyme-inducing anticonvulsants in order to not interfere with concurrent chemotherapy,” said Dr. Ullrich. If seizures are acutely related to a drug or infection, Dr. Ullrich aims to withdraw anticonvulsants as soon as possible.
Surgery and Chemotherapy
Children who have had a gross total resection of the primary tumor may still be at risk for acute neurologic, neurosensory, and neuromotor issues, endocrine dysfunction, cerebellar mutism, and other neuropsychological deficits, said Dr. Ullrich.
Deficits and long-term effects of brain tumor treatment mainly depend on tumor location. Maximal tumor resection may cause hypopituitarism, vascular issues, and vision issues. Image-guided therapy can help surgeons remove most, if not all, of the tumor during the initial resection.
Chemotherapy-related neurologic effects are common, said Dr. Ullrich. One of the main side effects is chemotherapy-induced peripheral neuropathy (ie, any injury or inflammation to the peripheral nerve due to administration of a chemotherapeutic drug). Patients with peripheral neuropathy may exhibit changes in their gait, loss of reflexes, and sensory changes. Pediatric patients with Charcot-Marie-Tooth disease or other hereditary neuropathies have a greater risk of chemotherapy-induced peripheral neuropathy.
Radiation
Age at the time of radiation, radiation field, genetic predisposition, and total dose are all risk factors for radiation-induced cognitive injury. One study found that children younger than 7 had the most significant decline in overall IQ after radiation. This research led to a shift in the development of treatment protocols and inspired physicians to strive to decrease and eliminate the use of radiation in the youngest patients, said Dr. Ullrich.
Another consequence of radiation may be the development of secondary neoplasms. The mean interval between the time of radiation and the development of secondary tumors is around eight years. These secondary neoplasms resulting from radiation often have anaplastic features. Childhood cancer survivors should see a dermatologist to monitor radiated areas for skin cancer.
Stroke and SMART Syndrome
Stroke is increasingly recognized as a late consequence of cancer treatment, especially in patients who have been treated for leukemia and brain tumors. Studies have found that prior radiation is an independent predictor of stroke. Mueller et al found that pediatric cancer survivors with hypertension had a fourfold increased risk of stroke, compared with sibling controls.
“Screen for correctable risk factors such as hypertension, hypercholesterolemia, hyperlipidemia, obesity, and sedentary lifestyle,” said Dr. Ullrich.
SMART syndrome (stroke-like migraine attacks after radiation therapy) is another potential complication that can occur years after radiation therapy. The syndrome can present like a transient ischemic attack, and symptoms can last hours to days. This syndrome can be treated with aggressive preventive headache care.
Mitigation and Prevention
Neurologists can take steps to help prevent or ameliorate some of these late effects. The Children's Oncology Group has created Passport for Care, a tool that allows patients to share a summary of their cancer treatments and follow-up recommendations with their primary care providers. The Children's Oncology Group also has created long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers.
In addition, new and refined surgical techniques help detect and remove residual tumor after surgery. Proton beam radiation, intensity-modulated radiation therapy, and other approaches can help reduce doses of radiation, potentially reducing cognitive risks. “The goal is to shift the paradigm from just categorizing the late effects to mitigating them and actually preventing them in the first place,” said Dr. Ullrich.
—Erica Tricarico
Suggested Reading
Mueller S, Fullerton HJ, Stratton K, et al. Radiation, atherosclerotic risk factors and stroke risk in survivors of pediatric cancer: a report from the Childhood Cancer Survivor Study. Int J Radiat Oncol Biol Phys. 2013;86(4):649-655.
KANSAS CITY, MO—As cancer treatment advances, the prevalence of childhood cancer survivors increases; as a result, physicians see more neurologic complications of surgery, chemotherapy, and radiation, according to an overview presented at the 46th Annual Meeting of the Child Neurology Society.
“There is an increasing impact of neurologic and neuropsychological toxicity that underscores the need for intervention and follow-up over the lifespan,” said Nicole Ullrich, MD, PhD, Director of Neurologic Neuro-Oncology at the Dana-Farber/Boston Children's Cancer and Blood Disorders Center.
Lifelong Effects
The prevalence of adult survivors of childhood cancer is one in 250 people, and there are more than 270,000 childhood cancer survivors in the United States. These cancer survivors “experience long-term toxicities affecting their respiratory system, cardiovascular system, cerebrovascular system, reproductive system, and a gamut of other late effects of treatment that we need to think about as they continue to advance into adulthood,” said Dr. Ullrich.
“Ninety-five percent of all cancer survivors will have a chronic health problem by the age of 45, and 80% will have a severe or life-threatening condition,” she said. The risk is greater for patients with CNS malignancies.
Childhood cancer survivors may develop physical, cognitive, and psychological issues later in life. These late effects have a significant impact on their quality and quantity of life. Neurologic issues can include headache, seizures, and stroke.
Headache and Seizures
Patients with elevated intracranial pressure or large tumors have an increased risk of headache. Nontumor causes of headache may include medications, such as antiemetic drugs and chemotherapy. Radiation therapy may cause acute radiation necrosis and long-term vascular issues. Supportive therapies, including steroids, antacids, and antinausea regimens, can cause headaches as well as sleep disruption, said Dr. Ullrich.
Approximately 15% to 25% of children with a brain tumor present with a seizure. Seizures are more often associated with low-grade tumors. Children with solid tumors or leukemia often have seizures without clinical or radiologic signs of a structural lesion. When a surgeon removes the tumor, there may be associated areas of dysplasia that surround the tumor, said Dr. Ullrich. Strategies such as electrocorticography or intraoperative monitoring can help identify epileptic zones for removal during surgery.
Potential causes of seizures include the tumor itself, surrounding edema, areas of cortical dysplasia, hyperexcitability related to neurotransmitters and glutamate levels, and scar formation that occurs after tumor resection. Individuals who have had cortically based or temporal lobe tumors or who have had incomplete resection or preexisting seizures before diagnosis have the highest risk of developing seizures, even years after completion of therapy.
EEG can help confirm seizures and distinguish between seizure types. It also can aid in the choice of an anticonvulsant. “We tend to lean more towards non-enzyme-inducing anticonvulsants in order to not interfere with concurrent chemotherapy,” said Dr. Ullrich. If seizures are acutely related to a drug or infection, Dr. Ullrich aims to withdraw anticonvulsants as soon as possible.
Surgery and Chemotherapy
Children who have had a gross total resection of the primary tumor may still be at risk for acute neurologic, neurosensory, and neuromotor issues, endocrine dysfunction, cerebellar mutism, and other neuropsychological deficits, said Dr. Ullrich.
Deficits and long-term effects of brain tumor treatment mainly depend on tumor location. Maximal tumor resection may cause hypopituitarism, vascular issues, and vision issues. Image-guided therapy can help surgeons remove most, if not all, of the tumor during the initial resection.
Chemotherapy-related neurologic effects are common, said Dr. Ullrich. One of the main side effects is chemotherapy-induced peripheral neuropathy (ie, any injury or inflammation to the peripheral nerve due to administration of a chemotherapeutic drug). Patients with peripheral neuropathy may exhibit changes in their gait, loss of reflexes, and sensory changes. Pediatric patients with Charcot-Marie-Tooth disease or other hereditary neuropathies have a greater risk of chemotherapy-induced peripheral neuropathy.
Radiation
Age at the time of radiation, radiation field, genetic predisposition, and total dose are all risk factors for radiation-induced cognitive injury. One study found that children younger than 7 had the most significant decline in overall IQ after radiation. This research led to a shift in the development of treatment protocols and inspired physicians to strive to decrease and eliminate the use of radiation in the youngest patients, said Dr. Ullrich.
Another consequence of radiation may be the development of secondary neoplasms. The mean interval between the time of radiation and the development of secondary tumors is around eight years. These secondary neoplasms resulting from radiation often have anaplastic features. Childhood cancer survivors should see a dermatologist to monitor radiated areas for skin cancer.
Stroke and SMART Syndrome
Stroke is increasingly recognized as a late consequence of cancer treatment, especially in patients who have been treated for leukemia and brain tumors. Studies have found that prior radiation is an independent predictor of stroke. Mueller et al found that pediatric cancer survivors with hypertension had a fourfold increased risk of stroke, compared with sibling controls.
“Screen for correctable risk factors such as hypertension, hypercholesterolemia, hyperlipidemia, obesity, and sedentary lifestyle,” said Dr. Ullrich.
SMART syndrome (stroke-like migraine attacks after radiation therapy) is another potential complication that can occur years after radiation therapy. The syndrome can present like a transient ischemic attack, and symptoms can last hours to days. This syndrome can be treated with aggressive preventive headache care.
Mitigation and Prevention
Neurologists can take steps to help prevent or ameliorate some of these late effects. The Children's Oncology Group has created Passport for Care, a tool that allows patients to share a summary of their cancer treatments and follow-up recommendations with their primary care providers. The Children's Oncology Group also has created long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers.
In addition, new and refined surgical techniques help detect and remove residual tumor after surgery. Proton beam radiation, intensity-modulated radiation therapy, and other approaches can help reduce doses of radiation, potentially reducing cognitive risks. “The goal is to shift the paradigm from just categorizing the late effects to mitigating them and actually preventing them in the first place,” said Dr. Ullrich.
—Erica Tricarico
Suggested Reading
Mueller S, Fullerton HJ, Stratton K, et al. Radiation, atherosclerotic risk factors and stroke risk in survivors of pediatric cancer: a report from the Childhood Cancer Survivor Study. Int J Radiat Oncol Biol Phys. 2013;86(4):649-655.
KANSAS CITY, MO—As cancer treatment advances, the prevalence of childhood cancer survivors increases; as a result, physicians see more neurologic complications of surgery, chemotherapy, and radiation, according to an overview presented at the 46th Annual Meeting of the Child Neurology Society.
“There is an increasing impact of neurologic and neuropsychological toxicity that underscores the need for intervention and follow-up over the lifespan,” said Nicole Ullrich, MD, PhD, Director of Neurologic Neuro-Oncology at the Dana-Farber/Boston Children's Cancer and Blood Disorders Center.
Lifelong Effects
The prevalence of adult survivors of childhood cancer is one in 250 people, and there are more than 270,000 childhood cancer survivors in the United States. These cancer survivors “experience long-term toxicities affecting their respiratory system, cardiovascular system, cerebrovascular system, reproductive system, and a gamut of other late effects of treatment that we need to think about as they continue to advance into adulthood,” said Dr. Ullrich.
“Ninety-five percent of all cancer survivors will have a chronic health problem by the age of 45, and 80% will have a severe or life-threatening condition,” she said. The risk is greater for patients with CNS malignancies.
Childhood cancer survivors may develop physical, cognitive, and psychological issues later in life. These late effects have a significant impact on their quality and quantity of life. Neurologic issues can include headache, seizures, and stroke.
Headache and Seizures
Patients with elevated intracranial pressure or large tumors have an increased risk of headache. Nontumor causes of headache may include medications, such as antiemetic drugs and chemotherapy. Radiation therapy may cause acute radiation necrosis and long-term vascular issues. Supportive therapies, including steroids, antacids, and antinausea regimens, can cause headaches as well as sleep disruption, said Dr. Ullrich.
Approximately 15% to 25% of children with a brain tumor present with a seizure. Seizures are more often associated with low-grade tumors. Children with solid tumors or leukemia often have seizures without clinical or radiologic signs of a structural lesion. When a surgeon removes the tumor, there may be associated areas of dysplasia that surround the tumor, said Dr. Ullrich. Strategies such as electrocorticography or intraoperative monitoring can help identify epileptic zones for removal during surgery.
Potential causes of seizures include the tumor itself, surrounding edema, areas of cortical dysplasia, hyperexcitability related to neurotransmitters and glutamate levels, and scar formation that occurs after tumor resection. Individuals who have had cortically based or temporal lobe tumors or who have had incomplete resection or preexisting seizures before diagnosis have the highest risk of developing seizures, even years after completion of therapy.
EEG can help confirm seizures and distinguish between seizure types. It also can aid in the choice of an anticonvulsant. “We tend to lean more towards non-enzyme-inducing anticonvulsants in order to not interfere with concurrent chemotherapy,” said Dr. Ullrich. If seizures are acutely related to a drug or infection, Dr. Ullrich aims to withdraw anticonvulsants as soon as possible.
Surgery and Chemotherapy
Children who have had a gross total resection of the primary tumor may still be at risk for acute neurologic, neurosensory, and neuromotor issues, endocrine dysfunction, cerebellar mutism, and other neuropsychological deficits, said Dr. Ullrich.
Deficits and long-term effects of brain tumor treatment mainly depend on tumor location. Maximal tumor resection may cause hypopituitarism, vascular issues, and vision issues. Image-guided therapy can help surgeons remove most, if not all, of the tumor during the initial resection.
Chemotherapy-related neurologic effects are common, said Dr. Ullrich. One of the main side effects is chemotherapy-induced peripheral neuropathy (ie, any injury or inflammation to the peripheral nerve due to administration of a chemotherapeutic drug). Patients with peripheral neuropathy may exhibit changes in their gait, loss of reflexes, and sensory changes. Pediatric patients with Charcot-Marie-Tooth disease or other hereditary neuropathies have a greater risk of chemotherapy-induced peripheral neuropathy.
Radiation
Age at the time of radiation, radiation field, genetic predisposition, and total dose are all risk factors for radiation-induced cognitive injury. One study found that children younger than 7 had the most significant decline in overall IQ after radiation. This research led to a shift in the development of treatment protocols and inspired physicians to strive to decrease and eliminate the use of radiation in the youngest patients, said Dr. Ullrich.
Another consequence of radiation may be the development of secondary neoplasms. The mean interval between the time of radiation and the development of secondary tumors is around eight years. These secondary neoplasms resulting from radiation often have anaplastic features. Childhood cancer survivors should see a dermatologist to monitor radiated areas for skin cancer.
Stroke and SMART Syndrome
Stroke is increasingly recognized as a late consequence of cancer treatment, especially in patients who have been treated for leukemia and brain tumors. Studies have found that prior radiation is an independent predictor of stroke. Mueller et al found that pediatric cancer survivors with hypertension had a fourfold increased risk of stroke, compared with sibling controls.
“Screen for correctable risk factors such as hypertension, hypercholesterolemia, hyperlipidemia, obesity, and sedentary lifestyle,” said Dr. Ullrich.
SMART syndrome (stroke-like migraine attacks after radiation therapy) is another potential complication that can occur years after radiation therapy. The syndrome can present like a transient ischemic attack, and symptoms can last hours to days. This syndrome can be treated with aggressive preventive headache care.
Mitigation and Prevention
Neurologists can take steps to help prevent or ameliorate some of these late effects. The Children's Oncology Group has created Passport for Care, a tool that allows patients to share a summary of their cancer treatments and follow-up recommendations with their primary care providers. The Children's Oncology Group also has created long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers.
In addition, new and refined surgical techniques help detect and remove residual tumor after surgery. Proton beam radiation, intensity-modulated radiation therapy, and other approaches can help reduce doses of radiation, potentially reducing cognitive risks. “The goal is to shift the paradigm from just categorizing the late effects to mitigating them and actually preventing them in the first place,” said Dr. Ullrich.
—Erica Tricarico
Suggested Reading
Mueller S, Fullerton HJ, Stratton K, et al. Radiation, atherosclerotic risk factors and stroke risk in survivors of pediatric cancer: a report from the Childhood Cancer Survivor Study. Int J Radiat Oncol Biol Phys. 2013;86(4):649-655.
Genomic Profiling May Improve Pediatric Brain Tumor Treatment
Genomic profiling of 282 pediatric gliomas detected genetic alterations in 96% of the cases, according to research published online ahead of print September 14 in the Oncologist. Information about genetic alterations may inform prognosis and help identify effective treatments, the researchers said.
Shakti Ramkissoon, MD, PhD, Associate Medical Director at Foundation Medicine in Morrisville, North Carolina, and colleagues studied 125 low-grade gliomas and 157 high-grade gliomas taken from children at medical centers in the US. Foundation Medicine, a genomic profiling company based in Cambridge, Massachusetts, supported the study, which is the largest to date of pediatric gliomas profiled by next-generation sequencing.
The investigators sequenced 315 cancer-related genes and 28 genes commonly rearranged in cancer. Patients’ median age was 11, and 50% were male.
The most frequently altered genes differed between low- and high-grade gliomas. In low-grade gliomas, BRAF was altered in 48% of cases. In addition, FGFR1 missense (17.6%), NF1 loss of function (8.8%), and TP53 (5.6%) mutations also were detected. Among high-grade gliomas, the genes most frequently mutated were TP53 (49%), H3F3A (37.6%), ATRX (24.2%), NF1 (22.2%), and PDGFRA (21.7%).
Studies indicate that low-grade gliomas with BRAF fusions, compared with BRAF mutations, have better outcomes, Dr. Ramkissoon said. “Therefore, determining the BRAF status for all pediatric low-grade gliomas is important for clinical management,” he said.
In addition, genetic mutations may highlight potential therapeutic targets. “Although surgical resection remains the most effective treatment option for pediatric low-grade gliomas, tumors located in eloquent areas not amenable to surgical resection (eg, motor cortex) require alternative therapeutic strategies,” the researchers said. “We report a multiply recurrent NF1-mutated pilocytic astrocytoma previously treated with surgery alone that now shows a remarkable response to dual inhibitor therapy (everolimus and trametinib) following three months of treatment.”
The study demonstrates that genomic profiling can be integrated into routine clinical practice, Dr. Ramkissoon said. “Comprehensive genomic profiling of pediatric gliomas provides objective data that promote diagnostic accuracy and enhance clinical decision-making,” the researchers concluded.
—Jake Remaly
Suggested Reading
Johnson A, Severson E, Gay L, et al. Comprehensive genomic profiling of 282 pediatric low- and high-grade gliomas reveals genomic drivers, tumor mutational burden, and hypermutation signatures. Oncologist. 2017 Sep 14 [Epub ahead of print].
Weller M, Weber RG, Willscher E, et al. Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol. 2015;129(5):679-693.
Genomic profiling of 282 pediatric gliomas detected genetic alterations in 96% of the cases, according to research published online ahead of print September 14 in the Oncologist. Information about genetic alterations may inform prognosis and help identify effective treatments, the researchers said.
Shakti Ramkissoon, MD, PhD, Associate Medical Director at Foundation Medicine in Morrisville, North Carolina, and colleagues studied 125 low-grade gliomas and 157 high-grade gliomas taken from children at medical centers in the US. Foundation Medicine, a genomic profiling company based in Cambridge, Massachusetts, supported the study, which is the largest to date of pediatric gliomas profiled by next-generation sequencing.
The investigators sequenced 315 cancer-related genes and 28 genes commonly rearranged in cancer. Patients’ median age was 11, and 50% were male.
The most frequently altered genes differed between low- and high-grade gliomas. In low-grade gliomas, BRAF was altered in 48% of cases. In addition, FGFR1 missense (17.6%), NF1 loss of function (8.8%), and TP53 (5.6%) mutations also were detected. Among high-grade gliomas, the genes most frequently mutated were TP53 (49%), H3F3A (37.6%), ATRX (24.2%), NF1 (22.2%), and PDGFRA (21.7%).
Studies indicate that low-grade gliomas with BRAF fusions, compared with BRAF mutations, have better outcomes, Dr. Ramkissoon said. “Therefore, determining the BRAF status for all pediatric low-grade gliomas is important for clinical management,” he said.
In addition, genetic mutations may highlight potential therapeutic targets. “Although surgical resection remains the most effective treatment option for pediatric low-grade gliomas, tumors located in eloquent areas not amenable to surgical resection (eg, motor cortex) require alternative therapeutic strategies,” the researchers said. “We report a multiply recurrent NF1-mutated pilocytic astrocytoma previously treated with surgery alone that now shows a remarkable response to dual inhibitor therapy (everolimus and trametinib) following three months of treatment.”
The study demonstrates that genomic profiling can be integrated into routine clinical practice, Dr. Ramkissoon said. “Comprehensive genomic profiling of pediatric gliomas provides objective data that promote diagnostic accuracy and enhance clinical decision-making,” the researchers concluded.
—Jake Remaly
Suggested Reading
Johnson A, Severson E, Gay L, et al. Comprehensive genomic profiling of 282 pediatric low- and high-grade gliomas reveals genomic drivers, tumor mutational burden, and hypermutation signatures. Oncologist. 2017 Sep 14 [Epub ahead of print].
Weller M, Weber RG, Willscher E, et al. Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol. 2015;129(5):679-693.
Genomic profiling of 282 pediatric gliomas detected genetic alterations in 96% of the cases, according to research published online ahead of print September 14 in the Oncologist. Information about genetic alterations may inform prognosis and help identify effective treatments, the researchers said.
Shakti Ramkissoon, MD, PhD, Associate Medical Director at Foundation Medicine in Morrisville, North Carolina, and colleagues studied 125 low-grade gliomas and 157 high-grade gliomas taken from children at medical centers in the US. Foundation Medicine, a genomic profiling company based in Cambridge, Massachusetts, supported the study, which is the largest to date of pediatric gliomas profiled by next-generation sequencing.
The investigators sequenced 315 cancer-related genes and 28 genes commonly rearranged in cancer. Patients’ median age was 11, and 50% were male.
The most frequently altered genes differed between low- and high-grade gliomas. In low-grade gliomas, BRAF was altered in 48% of cases. In addition, FGFR1 missense (17.6%), NF1 loss of function (8.8%), and TP53 (5.6%) mutations also were detected. Among high-grade gliomas, the genes most frequently mutated were TP53 (49%), H3F3A (37.6%), ATRX (24.2%), NF1 (22.2%), and PDGFRA (21.7%).
Studies indicate that low-grade gliomas with BRAF fusions, compared with BRAF mutations, have better outcomes, Dr. Ramkissoon said. “Therefore, determining the BRAF status for all pediatric low-grade gliomas is important for clinical management,” he said.
In addition, genetic mutations may highlight potential therapeutic targets. “Although surgical resection remains the most effective treatment option for pediatric low-grade gliomas, tumors located in eloquent areas not amenable to surgical resection (eg, motor cortex) require alternative therapeutic strategies,” the researchers said. “We report a multiply recurrent NF1-mutated pilocytic astrocytoma previously treated with surgery alone that now shows a remarkable response to dual inhibitor therapy (everolimus and trametinib) following three months of treatment.”
The study demonstrates that genomic profiling can be integrated into routine clinical practice, Dr. Ramkissoon said. “Comprehensive genomic profiling of pediatric gliomas provides objective data that promote diagnostic accuracy and enhance clinical decision-making,” the researchers concluded.
—Jake Remaly
Suggested Reading
Johnson A, Severson E, Gay L, et al. Comprehensive genomic profiling of 282 pediatric low- and high-grade gliomas reveals genomic drivers, tumor mutational burden, and hypermutation signatures. Oncologist. 2017 Sep 14 [Epub ahead of print].
Weller M, Weber RG, Willscher E, et al. Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol. 2015;129(5):679-693.
FDA approves biosimilar to bevacizumab
The Food and Drug Administration has approved a biosimilar to bevacizumab (Avastin) for the treatment of certain colorectal, lung, brain, kidney, and cervical cancers.
Bevacizumab-awwb is the first biosimilar approved in the United States for the treatment of cancer, the FDA said in a press release.
Approval is based on structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrate bevacizumab-awwb is biosimilar to bevacizumab, the FDA said.
• Metastatic colorectal cancer, in combination with intravenous 5-fluorouracil-based chemotherapy for first- or second-line treatment.
• Metastatic colorectal cancer, in combination with fluoropyrimidine-irinotecan–based or fluoropyrimidine-oxaliplatin–based chemotherapy for the second-line treatment of patients who have progressed on a first-line bevacizumab product–containing regimen.
• Non-squamous non–small cell lung cancer, in combination with carboplatin and paclitaxel for first line treatment of unresectable, locally advanced, recurrent, or metastatic disease.
• Glioblastoma with progressive disease following prior therapy, based on improvement in objective response rate.
• Metastatic renal cell carcinoma, in combination with interferon alfa.
• Cervical cancer that is persistent, recurrent, or metastatic, in combination with paclitaxel and cisplatin or paclitaxel and topotecan.
Common expected side effects of the biosimilar include epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Serious expected side effects include perforation or fistula, arterial and venous thromboembolic events, hypertension, posterior reversible encephalopathy syndrome, proteinuria, infusion-related reactions, and ovarian failure. Women who are pregnant should not take bevacizumab-awwb.
The biosimilar to bevacizumab carries a similar boxed warning regarding the increased risk of gastrointestinal perforations; surgery and wound healing complications; and severe or fatal pulmonary, gastrointestinal, central nervous system, and vaginal hemorrhage.
The biosimilar approval was granted to Amgen, which will market the drug under the trade name Mvasi.
The Food and Drug Administration has approved a biosimilar to bevacizumab (Avastin) for the treatment of certain colorectal, lung, brain, kidney, and cervical cancers.
Bevacizumab-awwb is the first biosimilar approved in the United States for the treatment of cancer, the FDA said in a press release.
Approval is based on structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrate bevacizumab-awwb is biosimilar to bevacizumab, the FDA said.
• Metastatic colorectal cancer, in combination with intravenous 5-fluorouracil-based chemotherapy for first- or second-line treatment.
• Metastatic colorectal cancer, in combination with fluoropyrimidine-irinotecan–based or fluoropyrimidine-oxaliplatin–based chemotherapy for the second-line treatment of patients who have progressed on a first-line bevacizumab product–containing regimen.
• Non-squamous non–small cell lung cancer, in combination with carboplatin and paclitaxel for first line treatment of unresectable, locally advanced, recurrent, or metastatic disease.
• Glioblastoma with progressive disease following prior therapy, based on improvement in objective response rate.
• Metastatic renal cell carcinoma, in combination with interferon alfa.
• Cervical cancer that is persistent, recurrent, or metastatic, in combination with paclitaxel and cisplatin or paclitaxel and topotecan.
Common expected side effects of the biosimilar include epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Serious expected side effects include perforation or fistula, arterial and venous thromboembolic events, hypertension, posterior reversible encephalopathy syndrome, proteinuria, infusion-related reactions, and ovarian failure. Women who are pregnant should not take bevacizumab-awwb.
The biosimilar to bevacizumab carries a similar boxed warning regarding the increased risk of gastrointestinal perforations; surgery and wound healing complications; and severe or fatal pulmonary, gastrointestinal, central nervous system, and vaginal hemorrhage.
The biosimilar approval was granted to Amgen, which will market the drug under the trade name Mvasi.
The Food and Drug Administration has approved a biosimilar to bevacizumab (Avastin) for the treatment of certain colorectal, lung, brain, kidney, and cervical cancers.
Bevacizumab-awwb is the first biosimilar approved in the United States for the treatment of cancer, the FDA said in a press release.
Approval is based on structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrate bevacizumab-awwb is biosimilar to bevacizumab, the FDA said.
• Metastatic colorectal cancer, in combination with intravenous 5-fluorouracil-based chemotherapy for first- or second-line treatment.
• Metastatic colorectal cancer, in combination with fluoropyrimidine-irinotecan–based or fluoropyrimidine-oxaliplatin–based chemotherapy for the second-line treatment of patients who have progressed on a first-line bevacizumab product–containing regimen.
• Non-squamous non–small cell lung cancer, in combination with carboplatin and paclitaxel for first line treatment of unresectable, locally advanced, recurrent, or metastatic disease.
• Glioblastoma with progressive disease following prior therapy, based on improvement in objective response rate.
• Metastatic renal cell carcinoma, in combination with interferon alfa.
• Cervical cancer that is persistent, recurrent, or metastatic, in combination with paclitaxel and cisplatin or paclitaxel and topotecan.
Common expected side effects of the biosimilar include epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Serious expected side effects include perforation or fistula, arterial and venous thromboembolic events, hypertension, posterior reversible encephalopathy syndrome, proteinuria, infusion-related reactions, and ovarian failure. Women who are pregnant should not take bevacizumab-awwb.
The biosimilar to bevacizumab carries a similar boxed warning regarding the increased risk of gastrointestinal perforations; surgery and wound healing complications; and severe or fatal pulmonary, gastrointestinal, central nervous system, and vaginal hemorrhage.
The biosimilar approval was granted to Amgen, which will market the drug under the trade name Mvasi.
Pembrolizumab and Nivolumab May Cause Neurologic Events
Approximately 3% of patients developed adverse neurologic events within 12 months of receiving nivolumab or pembrolizumab, according to the results of a single-center retrospective study published online ahead of print September 5 in JAMA Neurology.
These syndromes included myopathy, axonal thoracolumbar polyradiculopathy, severe demyelinating length-dependent peripheral neuropathy with axonal loss, asymmetric vasculitic neuropathy, cerebellar ataxia, autoimmune retinopathy, bilateral internuclear ophthalmoplegia, and headache, said Justin C. Kao, MBChB, a neurologist at Mayo Clinic in Rochester, Minnesota, and his coinvestigators.
Nivolumab and pembrolizumab are anti-programmed death–1 (PD-1) antibodies. In response to an increase in reports of neurologic events associated with anti–PD-1 therapy, the investigators searched the Mayo Cancer Pharmacy Database and identified 347 patients treated with pembrolizumab or nivolumab between 2014 and 2016. Ten patients (2.9%, two women) developed neurologic complications within 12 months of anti–PD-1 exposure. The median age was 71. None of their neurologic symptoms could be attributed directly to other treatments or to metastatic disease. Median modified Rankin Scale (mRS) score was 2.5. Symptom severity peaked at between one day and more than three months after starting anti–PD-1 treatment.
Stopping anti–PD-1 treatment and starting high-dose corticosteroids led to neurologic improvements (median mRS score, 2). A patient with necrotizing myopathy who had been receiving pembrolizumab for stage 4 melanoma developed extraocular, bulbar, and respiratory muscle weakness. These symptoms worsened over three weeks and did not respond to prednisone (80 mg/day) or to three sessions of plasmapheresis. The patient subsequently died.
If a patient on anti–PD-1 therapy develops neurologic symptoms, clinicians should promptly stop treatment and pursue a full workup, including electrodiagnostic studies and consideration of muscle or nerve biopsy to clarify the underlying pathophysiologic mechanisms, the researchers said. “If the clinical examination demonstrates severe clinical deficits at onset or worsens despite medication discontinuation, additional immune suppressant treatment should be considered,” they said.
—Amy Karon
Suggested Reading
Kao JC, Liao B, Markovic SN, et al. Neurological complications associated with anti-programmed death 1 (PD-1) antibodies. JAMA Neurol. 2017 Sep 5 [Epub ahead of print].
Approximately 3% of patients developed adverse neurologic events within 12 months of receiving nivolumab or pembrolizumab, according to the results of a single-center retrospective study published online ahead of print September 5 in JAMA Neurology.
These syndromes included myopathy, axonal thoracolumbar polyradiculopathy, severe demyelinating length-dependent peripheral neuropathy with axonal loss, asymmetric vasculitic neuropathy, cerebellar ataxia, autoimmune retinopathy, bilateral internuclear ophthalmoplegia, and headache, said Justin C. Kao, MBChB, a neurologist at Mayo Clinic in Rochester, Minnesota, and his coinvestigators.
Nivolumab and pembrolizumab are anti-programmed death–1 (PD-1) antibodies. In response to an increase in reports of neurologic events associated with anti–PD-1 therapy, the investigators searched the Mayo Cancer Pharmacy Database and identified 347 patients treated with pembrolizumab or nivolumab between 2014 and 2016. Ten patients (2.9%, two women) developed neurologic complications within 12 months of anti–PD-1 exposure. The median age was 71. None of their neurologic symptoms could be attributed directly to other treatments or to metastatic disease. Median modified Rankin Scale (mRS) score was 2.5. Symptom severity peaked at between one day and more than three months after starting anti–PD-1 treatment.
Stopping anti–PD-1 treatment and starting high-dose corticosteroids led to neurologic improvements (median mRS score, 2). A patient with necrotizing myopathy who had been receiving pembrolizumab for stage 4 melanoma developed extraocular, bulbar, and respiratory muscle weakness. These symptoms worsened over three weeks and did not respond to prednisone (80 mg/day) or to three sessions of plasmapheresis. The patient subsequently died.
If a patient on anti–PD-1 therapy develops neurologic symptoms, clinicians should promptly stop treatment and pursue a full workup, including electrodiagnostic studies and consideration of muscle or nerve biopsy to clarify the underlying pathophysiologic mechanisms, the researchers said. “If the clinical examination demonstrates severe clinical deficits at onset or worsens despite medication discontinuation, additional immune suppressant treatment should be considered,” they said.
—Amy Karon
Suggested Reading
Kao JC, Liao B, Markovic SN, et al. Neurological complications associated with anti-programmed death 1 (PD-1) antibodies. JAMA Neurol. 2017 Sep 5 [Epub ahead of print].
Approximately 3% of patients developed adverse neurologic events within 12 months of receiving nivolumab or pembrolizumab, according to the results of a single-center retrospective study published online ahead of print September 5 in JAMA Neurology.
These syndromes included myopathy, axonal thoracolumbar polyradiculopathy, severe demyelinating length-dependent peripheral neuropathy with axonal loss, asymmetric vasculitic neuropathy, cerebellar ataxia, autoimmune retinopathy, bilateral internuclear ophthalmoplegia, and headache, said Justin C. Kao, MBChB, a neurologist at Mayo Clinic in Rochester, Minnesota, and his coinvestigators.
Nivolumab and pembrolizumab are anti-programmed death–1 (PD-1) antibodies. In response to an increase in reports of neurologic events associated with anti–PD-1 therapy, the investigators searched the Mayo Cancer Pharmacy Database and identified 347 patients treated with pembrolizumab or nivolumab between 2014 and 2016. Ten patients (2.9%, two women) developed neurologic complications within 12 months of anti–PD-1 exposure. The median age was 71. None of their neurologic symptoms could be attributed directly to other treatments or to metastatic disease. Median modified Rankin Scale (mRS) score was 2.5. Symptom severity peaked at between one day and more than three months after starting anti–PD-1 treatment.
Stopping anti–PD-1 treatment and starting high-dose corticosteroids led to neurologic improvements (median mRS score, 2). A patient with necrotizing myopathy who had been receiving pembrolizumab for stage 4 melanoma developed extraocular, bulbar, and respiratory muscle weakness. These symptoms worsened over three weeks and did not respond to prednisone (80 mg/day) or to three sessions of plasmapheresis. The patient subsequently died.
If a patient on anti–PD-1 therapy develops neurologic symptoms, clinicians should promptly stop treatment and pursue a full workup, including electrodiagnostic studies and consideration of muscle or nerve biopsy to clarify the underlying pathophysiologic mechanisms, the researchers said. “If the clinical examination demonstrates severe clinical deficits at onset or worsens despite medication discontinuation, additional immune suppressant treatment should be considered,” they said.
—Amy Karon
Suggested Reading
Kao JC, Liao B, Markovic SN, et al. Neurological complications associated with anti-programmed death 1 (PD-1) antibodies. JAMA Neurol. 2017 Sep 5 [Epub ahead of print].
Pembrolizumab, nivolumab linked to 3% rate of neurologic events
Three percent of patients developed immune-related adverse neurologic events within 12 months of receiving nivolumab or pembrolizumab, according to the results of a single-center retrospective study.
These syndromes included myopathy, axonal thoracolumbar polyradiculopathy, severe demyelinating length-dependent peripheral neuropathy with axonal loss, a facial diplegic variant of Guillain-Barré syndrome, asymmetric vasculitic neuropathy, cerebellar ataxia with dysarthria, autoimmune retinopathy, bilateral internuclear ophthalmoplegia, and headache, reported Justin C. Kao, MD, of Mayo Clinic, Rochester, Minn., and his coinvestigators. Most patients improved after stopping treatment and starting corticosteroids, but one patient developed necrotizing myopathy and died after withdrawal of ventilator support.
Nivolumab and pembrolizumab, which inhibit the programmed death–1 (PD-1) receptor, are approved for treating metastatic melanoma, non–small-cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, head and neck cancers, and urothelial carcinoma. In response to a surge in reports of neurologic events associated with anti–PD-1 therapy, the investigators searched the Mayo Clinic pharmacy database and identified 347 patients treated with pembrolizumab or nivolumab between 2014 and 2016. Ten patients (2.9%) developed neurologic complications within 12 months of anti–PD-1 exposure, including eight men and two women. The median age was 71 years. None of their neurologic symptoms could be directly attributed to other treatments or to metastatic disease. Most had mild to moderate disability, with modified Rankin Scale (mRS) scores of 2, and symptom severity peaked between 1 day and more than 3 months after starting anti–PD-1 treatment (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1912).
Stopping anti–PD-1 treatment and starting high-dose corticosteroids led to substantial neurologic improvements (mRS scores, 0-3), except in the case of fatal necrotizing myopathy, the researchers said. That patient, who was receiving pembrolizumab for stage 4 melanoma, developed extraocular, bulbar, and proximal limb girdle weakness that worsened over a period of 3 weeks and did not respond to prednisone (80 mg daily) or to three sessions of plasmapheresis.
If a patient on anti–PD-1 therapy develops neurologic symptoms, clinicians should promptly stop treatment and pursue a full work-up, including electrodiagnostic studies and consideration of muscle or nerve biopsy to clarify underlying pathophysiologic mechanisms, the researchers said. “If the clinical examination demonstrates severe clinical deficits at onset or worsens despite medication discontinuation, additional immune suppressant treatment should be considered,” they said. They recommended prednisone (1 mg/kg) with a taper over a 1-month period. Intravenous immunoglobulin therapy or plasma exchange may be warranted if patients continue to worsen, they said.
The investigators did not report external funding sources. Mr. Kao had no disclosures. Two coinvestigators disclosed ties to the American Association of Neuromuscular & Electrodiagnostic Medicine, the American Academy of Neurology, the Continuum: Lifelong Learning in Neurology, Ionis Pharmaceuticals, Alnylam, and Oxford University Press. The remaining coinvestigators reported having no conflicts of interest.
Neurologic symptoms have been and continue to be one of the most common reasons for admission to a cancer center. Neurotoxic chemotherapy, direct invasion of cancer, and other neurologic complications of treatment contribute to the substantial cross talk between oncologists and neurologists. Over the past 5 years, oncology has witnessed an explosion of new immunotherapeutics that are revolutionizing drug development and patient care in oncology today. In contrast to traditional chemotherapy, which targets rapidly dividing cancer cells and can lead to adverse effects in other organs with rapid cell turnover, immunotherapies target and activate the immune system, potentially leading to a wide range of inflammatory and immune-mediated adverse events, including those in the nervous system.
Only 5 of the 10 patients described by Kao et al. experienced nonneurologic immune-related adverse events, suggesting that neurologic complications may be the only defining symptom of an immune-related reaction. Consultation calls from the cancer center are all too familiar for neurologists, and this pattern appears likely to persist in the era of immunotherapy. The horizon of new checkpoint targets continues to expand, and combination therapies are beginning to emerge. Neurologists and oncologists need to be aware of the important checkpoints ahead in patient care.
Roy E. Strowd III, MD, is with the section on hematology and oncology, department of neurology and internal medicine, Wake Forest University, Winston-Salem, N.C. He reported having no conflicts of interest. These comments are excerpted from his editorial (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1916).
Neurologic symptoms have been and continue to be one of the most common reasons for admission to a cancer center. Neurotoxic chemotherapy, direct invasion of cancer, and other neurologic complications of treatment contribute to the substantial cross talk between oncologists and neurologists. Over the past 5 years, oncology has witnessed an explosion of new immunotherapeutics that are revolutionizing drug development and patient care in oncology today. In contrast to traditional chemotherapy, which targets rapidly dividing cancer cells and can lead to adverse effects in other organs with rapid cell turnover, immunotherapies target and activate the immune system, potentially leading to a wide range of inflammatory and immune-mediated adverse events, including those in the nervous system.
Only 5 of the 10 patients described by Kao et al. experienced nonneurologic immune-related adverse events, suggesting that neurologic complications may be the only defining symptom of an immune-related reaction. Consultation calls from the cancer center are all too familiar for neurologists, and this pattern appears likely to persist in the era of immunotherapy. The horizon of new checkpoint targets continues to expand, and combination therapies are beginning to emerge. Neurologists and oncologists need to be aware of the important checkpoints ahead in patient care.
Roy E. Strowd III, MD, is with the section on hematology and oncology, department of neurology and internal medicine, Wake Forest University, Winston-Salem, N.C. He reported having no conflicts of interest. These comments are excerpted from his editorial (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1916).
Neurologic symptoms have been and continue to be one of the most common reasons for admission to a cancer center. Neurotoxic chemotherapy, direct invasion of cancer, and other neurologic complications of treatment contribute to the substantial cross talk between oncologists and neurologists. Over the past 5 years, oncology has witnessed an explosion of new immunotherapeutics that are revolutionizing drug development and patient care in oncology today. In contrast to traditional chemotherapy, which targets rapidly dividing cancer cells and can lead to adverse effects in other organs with rapid cell turnover, immunotherapies target and activate the immune system, potentially leading to a wide range of inflammatory and immune-mediated adverse events, including those in the nervous system.
Only 5 of the 10 patients described by Kao et al. experienced nonneurologic immune-related adverse events, suggesting that neurologic complications may be the only defining symptom of an immune-related reaction. Consultation calls from the cancer center are all too familiar for neurologists, and this pattern appears likely to persist in the era of immunotherapy. The horizon of new checkpoint targets continues to expand, and combination therapies are beginning to emerge. Neurologists and oncologists need to be aware of the important checkpoints ahead in patient care.
Roy E. Strowd III, MD, is with the section on hematology and oncology, department of neurology and internal medicine, Wake Forest University, Winston-Salem, N.C. He reported having no conflicts of interest. These comments are excerpted from his editorial (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1916).
Three percent of patients developed immune-related adverse neurologic events within 12 months of receiving nivolumab or pembrolizumab, according to the results of a single-center retrospective study.
These syndromes included myopathy, axonal thoracolumbar polyradiculopathy, severe demyelinating length-dependent peripheral neuropathy with axonal loss, a facial diplegic variant of Guillain-Barré syndrome, asymmetric vasculitic neuropathy, cerebellar ataxia with dysarthria, autoimmune retinopathy, bilateral internuclear ophthalmoplegia, and headache, reported Justin C. Kao, MD, of Mayo Clinic, Rochester, Minn., and his coinvestigators. Most patients improved after stopping treatment and starting corticosteroids, but one patient developed necrotizing myopathy and died after withdrawal of ventilator support.
Nivolumab and pembrolizumab, which inhibit the programmed death–1 (PD-1) receptor, are approved for treating metastatic melanoma, non–small-cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, head and neck cancers, and urothelial carcinoma. In response to a surge in reports of neurologic events associated with anti–PD-1 therapy, the investigators searched the Mayo Clinic pharmacy database and identified 347 patients treated with pembrolizumab or nivolumab between 2014 and 2016. Ten patients (2.9%) developed neurologic complications within 12 months of anti–PD-1 exposure, including eight men and two women. The median age was 71 years. None of their neurologic symptoms could be directly attributed to other treatments or to metastatic disease. Most had mild to moderate disability, with modified Rankin Scale (mRS) scores of 2, and symptom severity peaked between 1 day and more than 3 months after starting anti–PD-1 treatment (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1912).
Stopping anti–PD-1 treatment and starting high-dose corticosteroids led to substantial neurologic improvements (mRS scores, 0-3), except in the case of fatal necrotizing myopathy, the researchers said. That patient, who was receiving pembrolizumab for stage 4 melanoma, developed extraocular, bulbar, and proximal limb girdle weakness that worsened over a period of 3 weeks and did not respond to prednisone (80 mg daily) or to three sessions of plasmapheresis.
If a patient on anti–PD-1 therapy develops neurologic symptoms, clinicians should promptly stop treatment and pursue a full work-up, including electrodiagnostic studies and consideration of muscle or nerve biopsy to clarify underlying pathophysiologic mechanisms, the researchers said. “If the clinical examination demonstrates severe clinical deficits at onset or worsens despite medication discontinuation, additional immune suppressant treatment should be considered,” they said. They recommended prednisone (1 mg/kg) with a taper over a 1-month period. Intravenous immunoglobulin therapy or plasma exchange may be warranted if patients continue to worsen, they said.
The investigators did not report external funding sources. Mr. Kao had no disclosures. Two coinvestigators disclosed ties to the American Association of Neuromuscular & Electrodiagnostic Medicine, the American Academy of Neurology, the Continuum: Lifelong Learning in Neurology, Ionis Pharmaceuticals, Alnylam, and Oxford University Press. The remaining coinvestigators reported having no conflicts of interest.
Three percent of patients developed immune-related adverse neurologic events within 12 months of receiving nivolumab or pembrolizumab, according to the results of a single-center retrospective study.
These syndromes included myopathy, axonal thoracolumbar polyradiculopathy, severe demyelinating length-dependent peripheral neuropathy with axonal loss, a facial diplegic variant of Guillain-Barré syndrome, asymmetric vasculitic neuropathy, cerebellar ataxia with dysarthria, autoimmune retinopathy, bilateral internuclear ophthalmoplegia, and headache, reported Justin C. Kao, MD, of Mayo Clinic, Rochester, Minn., and his coinvestigators. Most patients improved after stopping treatment and starting corticosteroids, but one patient developed necrotizing myopathy and died after withdrawal of ventilator support.
Nivolumab and pembrolizumab, which inhibit the programmed death–1 (PD-1) receptor, are approved for treating metastatic melanoma, non–small-cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, head and neck cancers, and urothelial carcinoma. In response to a surge in reports of neurologic events associated with anti–PD-1 therapy, the investigators searched the Mayo Clinic pharmacy database and identified 347 patients treated with pembrolizumab or nivolumab between 2014 and 2016. Ten patients (2.9%) developed neurologic complications within 12 months of anti–PD-1 exposure, including eight men and two women. The median age was 71 years. None of their neurologic symptoms could be directly attributed to other treatments or to metastatic disease. Most had mild to moderate disability, with modified Rankin Scale (mRS) scores of 2, and symptom severity peaked between 1 day and more than 3 months after starting anti–PD-1 treatment (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1912).
Stopping anti–PD-1 treatment and starting high-dose corticosteroids led to substantial neurologic improvements (mRS scores, 0-3), except in the case of fatal necrotizing myopathy, the researchers said. That patient, who was receiving pembrolizumab for stage 4 melanoma, developed extraocular, bulbar, and proximal limb girdle weakness that worsened over a period of 3 weeks and did not respond to prednisone (80 mg daily) or to three sessions of plasmapheresis.
If a patient on anti–PD-1 therapy develops neurologic symptoms, clinicians should promptly stop treatment and pursue a full work-up, including electrodiagnostic studies and consideration of muscle or nerve biopsy to clarify underlying pathophysiologic mechanisms, the researchers said. “If the clinical examination demonstrates severe clinical deficits at onset or worsens despite medication discontinuation, additional immune suppressant treatment should be considered,” they said. They recommended prednisone (1 mg/kg) with a taper over a 1-month period. Intravenous immunoglobulin therapy or plasma exchange may be warranted if patients continue to worsen, they said.
The investigators did not report external funding sources. Mr. Kao had no disclosures. Two coinvestigators disclosed ties to the American Association of Neuromuscular & Electrodiagnostic Medicine, the American Academy of Neurology, the Continuum: Lifelong Learning in Neurology, Ionis Pharmaceuticals, Alnylam, and Oxford University Press. The remaining coinvestigators reported having no conflicts of interest.
FROM JAMA NEUROLOGY
Key clinical point: Watch for immune-related adverse effects of nivolumab and pembrolizumab.
Major finding: Ten of 347 patients (2.9%) developed subacute neurologic immune-related adverse events, typically neuromuscular syndromes.
Data source: A single-center, retrospective cohort study of 347 patients who received pembrolizumab or nivolumab for metastatic melanoma or solid tumors.
Disclosures: The investigators did not report external funding sources. Mr. Kao had no disclosures. Two coinvestigators disclosed ties to the American Association of Neuromuscular & Electrodiagnostic Medicine, the American Academy of Neurology, the Continuum: Lifelong Learning in Neurology, Ionis Pharmaceuticals, Alnylam, and Oxford University Press. The remaining coinvestigators reported having no conflicts of interest.
Glioblastoma: Prognosis is poor, but new therapies are emerging
The questioning of former FBI director James B. Comey by Sen. John McCain (R-Ariz.) during a June 8 Senate Intelligence Committee hearing raised more than a few eyebrows; Sen. McCain seemed confused and disoriented, at one point referring to Mr. Comey as “President Comey,” but a possible medical explanation emerged soon after.
On July 14, Sen. McCain, 80, underwent surgery to remove a 5-cm blood clot that had been discovered above his left eye during a physical, and on July 19, the Mayo Clinic in Phoenix, where he had undergone the procedure, announced at his request that, “subsequent tissue pathology revealed that a primary brain tumor known as a glioblastoma was associated with the blood clot.”
Glioblastoma features
While Sen. McCain’s symptoms can’t necessarily be attributed to the glioblastoma, it is not unusual for glioblastoma patients to present with some sort of neurologic deficit, such as speech issues, unilateral weakness, or confusion, according to Eudocia Quant Lee, MD, a neuro-oncologist at Dana-Farber Cancer Institute, Boston.
Neuro-oncologist Manmeet Singh Ahluwalia, MD, of the Cleveland Clinic said seizures, persistent headaches, double or blurred vision, and changes in ability to think and learn can also be presenting symptoms.
Glioblastoma is the most common malignant primary brain tumor diagnosed in adults, with an estimated 12,000-13,000 new cases occurring each year in the United States. It is more common among older adults but can occur in younger patients. It arises in the brain and generally stays within the central nervous system, Dr. Lee explained, noting that it is much less common than lung cancer, breast cancer, and melanoma.
This is particularly true for older patients.
Prognosis and age
“We know, in general – as with most cancers – that the older you’re diagnosed with your cancer, the poorer your prognosis is,” she said, adding that other health issues and the ability to tolerate treatment can affect outcomes.
Outcomes also can be affected by type of surgery, functional status, extent of treatment, and molecular subtypes of the glioblastoma, Dr. Ahluwalia said.
Survival generally ranges about 14-18 months, although about 10% of patients live 5 years or longer.
The study, presented in a poster by Michael Weller, MD, of University Hospital and University of Zürich and his colleagues, also showed that, compared with the 398 older patients who survived less than 2 years (median, 6.2 months), those who survived longer had “more intensive up-front treatment and a trend toward higher initial Karnofsky performance scores as distinguishing clinical factors.”
In addition, molecular analyses showed more frequent O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation in those with longer survival, while isocitrate dehydrogenase (IDH) mutations were restricted to single patients.
“Collectively, our findings confirm that LTS is rare in elderly patients with glioblastoma and that clinical and tumor-associated molecular factors linked to LTS resemble those in standard-age patients, except for less common IDH mutation,” the investigators wrote.
Another abstract published online in conjunction with the ASCO annual meeting looked at outcomes, based on age and MGMT analysis, and similarly found that aggressive treatment with chemoradiation is associated with better outcomes in both younger and older patients.
In that study by Suryanarayan Mohapatra, MD, of Cleveland Clinic–Fairview Hospital, and his colleagues – including Dr. Ahluwalia – 567 of 1,165 patients were aged 65 years or older. The benefits of chemoradiation therapy, which was associated with a significantly lower risk of death vs. radiation therapy alone in the study, were more pronounced among the older patients (hazard ratio, 0.45 vs. 0.61 for those under age 65 years), but the difference did not reach statistical significance.
Dr. Mohapatra and his colleagues also showed that more aggressive therapy resulted in better overall and progression-free survival regardless of MGMT methylation status, but that there was no difference between the age groups on this measure. Overall and progression-free survival also were significantly better with gross-total resection and subtotal resection vs. biopsy only, and with diagnosis during 2009 and later vs. during 2007-2008. However, a difference between the two age groups was seen with respect to overall survival only among those diagnosed during 2009 or later, with a more prominent impact among the younger group, the investigators reported.
“Older individuals often get less aggressive treatment. However, based on the research, active and functional older patients should get aggressive treatment,” Dr. Ahluwalia said. “We advocate tailor-made treatment that takes into account patient condition, location of tumor, functional status, etc., in addition to patient age.”
Standard treatment approaches
The first step in the treatment of glioblastomas is maximal safe therapy, Dr. Lee said.
“You want to achieve as much of a resection as possible without leaving the patient with some sort of permanent neurologic deficit that could severely compromise the quality of their life,” she explained.
Sen. McCain’s tumor was “completely resected by imaging criteria,” according to the Mayo Clinic statement, which also noted that treatment options might include a combination of chemotherapy and radiation.
Indeed, the standard of care for glioblastomas after surgery is combined chemotherapy and radiation – typically given as approximately 6 weeks of radiation combined with oral temozolomide chemotherapy – followed by 6 monthly cycles of temozolomide, she explained.
Radiation is sometimes given for only 3 weeks, but this option is mainly reserved for elderly patients, she said, adding that trials in patients aged 65-70 years have shown that this shorter course of radiation can be equally effective but potentially less toxic.
Emerging treatment approaches
Another treatment that has shown promise involves the use of tumor-treating fields (TTFields) – a locoregionally delivered antimitotic treatment that disrupts cell division and organelle assembly.
A 2015 phase 3 trial showed that adding TTFields to maintenance temozolomide significantly prolonged progression-free and overall survival, Dr. Ahluwalia said.
Other studies, including two presented during poster sessions at the ASCO annual meeting, have shown a progression-free survival benefit with the addition of bevacizumab to the treatment regimen. One open-label phase 2 study showed that hypofractionated radiotherapy in combination with IV bevacizumab every 2 weeks vs. radiotherapy alone in newly diagnosed patients over age 65 years improved progression-free survival (median, 7.6 vs. 4.8 months), but not overall survival (median, 12.1 vs. 12.2 months).
Another phase 2 study presented by Phioanh (Leia) Nghiemphu, MD, of the University of California, Los Angeles, showed that in newly diagnosed patients aged 70 years and older, upfront treatment with bevacizumab and temozolomide was associated with promising survival benefits (overall survival, 12.3 months; progression-free survival, 5.1 months) and tolerable side effects. The best survival in multivariate analysis was in patients who received radiotherapy at progression; it was unclear whether the addition of bevacizumab led to a survival advantage, but it may have allowed delay of radiotherapy treatment, she noted.
“Although we have no cure for glioblastoma, treatments can control tumor growth for a period of time, and there are additional promising therapies emerging every day to treat this deadly cancer, she said in an interview. “There has been increasing interest in developing better therapies for the older patients with glioblastoma with less toxicity and still-robust survival, such as the addition of bevacizumab or a short course of radiotherapy with temozolomide chemotherapy.”
Dr. Ahluwalia encourages clinical trial participation for patients diagnosed with glioblastoma and noted that he is particularly excited about immunotherapy and targeted therapy trials.
Dr. Lee has served as consultant to Eli Lilly. Dr. Ahluwalia disclosed a financial relationship with multiple companies, including Novocure, which markets a TTFields device. Dr. Weller disclosed a financial relationships with multiple companies, including Novocure; Merck Sharp & Dohme, which markets temozolomide; and Roche, which markets bevacizumab. Dr. Nghiemphu has received research funding from Genentech/Roche and Novartis. Dr. Mohapatra reporting having no disclosures.
The questioning of former FBI director James B. Comey by Sen. John McCain (R-Ariz.) during a June 8 Senate Intelligence Committee hearing raised more than a few eyebrows; Sen. McCain seemed confused and disoriented, at one point referring to Mr. Comey as “President Comey,” but a possible medical explanation emerged soon after.
On July 14, Sen. McCain, 80, underwent surgery to remove a 5-cm blood clot that had been discovered above his left eye during a physical, and on July 19, the Mayo Clinic in Phoenix, where he had undergone the procedure, announced at his request that, “subsequent tissue pathology revealed that a primary brain tumor known as a glioblastoma was associated with the blood clot.”
Glioblastoma features
While Sen. McCain’s symptoms can’t necessarily be attributed to the glioblastoma, it is not unusual for glioblastoma patients to present with some sort of neurologic deficit, such as speech issues, unilateral weakness, or confusion, according to Eudocia Quant Lee, MD, a neuro-oncologist at Dana-Farber Cancer Institute, Boston.
Neuro-oncologist Manmeet Singh Ahluwalia, MD, of the Cleveland Clinic said seizures, persistent headaches, double or blurred vision, and changes in ability to think and learn can also be presenting symptoms.
Glioblastoma is the most common malignant primary brain tumor diagnosed in adults, with an estimated 12,000-13,000 new cases occurring each year in the United States. It is more common among older adults but can occur in younger patients. It arises in the brain and generally stays within the central nervous system, Dr. Lee explained, noting that it is much less common than lung cancer, breast cancer, and melanoma.
This is particularly true for older patients.
Prognosis and age
“We know, in general – as with most cancers – that the older you’re diagnosed with your cancer, the poorer your prognosis is,” she said, adding that other health issues and the ability to tolerate treatment can affect outcomes.
Outcomes also can be affected by type of surgery, functional status, extent of treatment, and molecular subtypes of the glioblastoma, Dr. Ahluwalia said.
Survival generally ranges about 14-18 months, although about 10% of patients live 5 years or longer.
The study, presented in a poster by Michael Weller, MD, of University Hospital and University of Zürich and his colleagues, also showed that, compared with the 398 older patients who survived less than 2 years (median, 6.2 months), those who survived longer had “more intensive up-front treatment and a trend toward higher initial Karnofsky performance scores as distinguishing clinical factors.”
In addition, molecular analyses showed more frequent O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation in those with longer survival, while isocitrate dehydrogenase (IDH) mutations were restricted to single patients.
“Collectively, our findings confirm that LTS is rare in elderly patients with glioblastoma and that clinical and tumor-associated molecular factors linked to LTS resemble those in standard-age patients, except for less common IDH mutation,” the investigators wrote.
Another abstract published online in conjunction with the ASCO annual meeting looked at outcomes, based on age and MGMT analysis, and similarly found that aggressive treatment with chemoradiation is associated with better outcomes in both younger and older patients.
In that study by Suryanarayan Mohapatra, MD, of Cleveland Clinic–Fairview Hospital, and his colleagues – including Dr. Ahluwalia – 567 of 1,165 patients were aged 65 years or older. The benefits of chemoradiation therapy, which was associated with a significantly lower risk of death vs. radiation therapy alone in the study, were more pronounced among the older patients (hazard ratio, 0.45 vs. 0.61 for those under age 65 years), but the difference did not reach statistical significance.
Dr. Mohapatra and his colleagues also showed that more aggressive therapy resulted in better overall and progression-free survival regardless of MGMT methylation status, but that there was no difference between the age groups on this measure. Overall and progression-free survival also were significantly better with gross-total resection and subtotal resection vs. biopsy only, and with diagnosis during 2009 and later vs. during 2007-2008. However, a difference between the two age groups was seen with respect to overall survival only among those diagnosed during 2009 or later, with a more prominent impact among the younger group, the investigators reported.
“Older individuals often get less aggressive treatment. However, based on the research, active and functional older patients should get aggressive treatment,” Dr. Ahluwalia said. “We advocate tailor-made treatment that takes into account patient condition, location of tumor, functional status, etc., in addition to patient age.”
Standard treatment approaches
The first step in the treatment of glioblastomas is maximal safe therapy, Dr. Lee said.
“You want to achieve as much of a resection as possible without leaving the patient with some sort of permanent neurologic deficit that could severely compromise the quality of their life,” she explained.
Sen. McCain’s tumor was “completely resected by imaging criteria,” according to the Mayo Clinic statement, which also noted that treatment options might include a combination of chemotherapy and radiation.
Indeed, the standard of care for glioblastomas after surgery is combined chemotherapy and radiation – typically given as approximately 6 weeks of radiation combined with oral temozolomide chemotherapy – followed by 6 monthly cycles of temozolomide, she explained.
Radiation is sometimes given for only 3 weeks, but this option is mainly reserved for elderly patients, she said, adding that trials in patients aged 65-70 years have shown that this shorter course of radiation can be equally effective but potentially less toxic.
Emerging treatment approaches
Another treatment that has shown promise involves the use of tumor-treating fields (TTFields) – a locoregionally delivered antimitotic treatment that disrupts cell division and organelle assembly.
A 2015 phase 3 trial showed that adding TTFields to maintenance temozolomide significantly prolonged progression-free and overall survival, Dr. Ahluwalia said.
Other studies, including two presented during poster sessions at the ASCO annual meeting, have shown a progression-free survival benefit with the addition of bevacizumab to the treatment regimen. One open-label phase 2 study showed that hypofractionated radiotherapy in combination with IV bevacizumab every 2 weeks vs. radiotherapy alone in newly diagnosed patients over age 65 years improved progression-free survival (median, 7.6 vs. 4.8 months), but not overall survival (median, 12.1 vs. 12.2 months).
Another phase 2 study presented by Phioanh (Leia) Nghiemphu, MD, of the University of California, Los Angeles, showed that in newly diagnosed patients aged 70 years and older, upfront treatment with bevacizumab and temozolomide was associated with promising survival benefits (overall survival, 12.3 months; progression-free survival, 5.1 months) and tolerable side effects. The best survival in multivariate analysis was in patients who received radiotherapy at progression; it was unclear whether the addition of bevacizumab led to a survival advantage, but it may have allowed delay of radiotherapy treatment, she noted.
“Although we have no cure for glioblastoma, treatments can control tumor growth for a period of time, and there are additional promising therapies emerging every day to treat this deadly cancer, she said in an interview. “There has been increasing interest in developing better therapies for the older patients with glioblastoma with less toxicity and still-robust survival, such as the addition of bevacizumab or a short course of radiotherapy with temozolomide chemotherapy.”
Dr. Ahluwalia encourages clinical trial participation for patients diagnosed with glioblastoma and noted that he is particularly excited about immunotherapy and targeted therapy trials.
Dr. Lee has served as consultant to Eli Lilly. Dr. Ahluwalia disclosed a financial relationship with multiple companies, including Novocure, which markets a TTFields device. Dr. Weller disclosed a financial relationships with multiple companies, including Novocure; Merck Sharp & Dohme, which markets temozolomide; and Roche, which markets bevacizumab. Dr. Nghiemphu has received research funding from Genentech/Roche and Novartis. Dr. Mohapatra reporting having no disclosures.
The questioning of former FBI director James B. Comey by Sen. John McCain (R-Ariz.) during a June 8 Senate Intelligence Committee hearing raised more than a few eyebrows; Sen. McCain seemed confused and disoriented, at one point referring to Mr. Comey as “President Comey,” but a possible medical explanation emerged soon after.
On July 14, Sen. McCain, 80, underwent surgery to remove a 5-cm blood clot that had been discovered above his left eye during a physical, and on July 19, the Mayo Clinic in Phoenix, where he had undergone the procedure, announced at his request that, “subsequent tissue pathology revealed that a primary brain tumor known as a glioblastoma was associated with the blood clot.”
Glioblastoma features
While Sen. McCain’s symptoms can’t necessarily be attributed to the glioblastoma, it is not unusual for glioblastoma patients to present with some sort of neurologic deficit, such as speech issues, unilateral weakness, or confusion, according to Eudocia Quant Lee, MD, a neuro-oncologist at Dana-Farber Cancer Institute, Boston.
Neuro-oncologist Manmeet Singh Ahluwalia, MD, of the Cleveland Clinic said seizures, persistent headaches, double or blurred vision, and changes in ability to think and learn can also be presenting symptoms.
Glioblastoma is the most common malignant primary brain tumor diagnosed in adults, with an estimated 12,000-13,000 new cases occurring each year in the United States. It is more common among older adults but can occur in younger patients. It arises in the brain and generally stays within the central nervous system, Dr. Lee explained, noting that it is much less common than lung cancer, breast cancer, and melanoma.
This is particularly true for older patients.
Prognosis and age
“We know, in general – as with most cancers – that the older you’re diagnosed with your cancer, the poorer your prognosis is,” she said, adding that other health issues and the ability to tolerate treatment can affect outcomes.
Outcomes also can be affected by type of surgery, functional status, extent of treatment, and molecular subtypes of the glioblastoma, Dr. Ahluwalia said.
Survival generally ranges about 14-18 months, although about 10% of patients live 5 years or longer.
The study, presented in a poster by Michael Weller, MD, of University Hospital and University of Zürich and his colleagues, also showed that, compared with the 398 older patients who survived less than 2 years (median, 6.2 months), those who survived longer had “more intensive up-front treatment and a trend toward higher initial Karnofsky performance scores as distinguishing clinical factors.”
In addition, molecular analyses showed more frequent O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation in those with longer survival, while isocitrate dehydrogenase (IDH) mutations were restricted to single patients.
“Collectively, our findings confirm that LTS is rare in elderly patients with glioblastoma and that clinical and tumor-associated molecular factors linked to LTS resemble those in standard-age patients, except for less common IDH mutation,” the investigators wrote.
Another abstract published online in conjunction with the ASCO annual meeting looked at outcomes, based on age and MGMT analysis, and similarly found that aggressive treatment with chemoradiation is associated with better outcomes in both younger and older patients.
In that study by Suryanarayan Mohapatra, MD, of Cleveland Clinic–Fairview Hospital, and his colleagues – including Dr. Ahluwalia – 567 of 1,165 patients were aged 65 years or older. The benefits of chemoradiation therapy, which was associated with a significantly lower risk of death vs. radiation therapy alone in the study, were more pronounced among the older patients (hazard ratio, 0.45 vs. 0.61 for those under age 65 years), but the difference did not reach statistical significance.
Dr. Mohapatra and his colleagues also showed that more aggressive therapy resulted in better overall and progression-free survival regardless of MGMT methylation status, but that there was no difference between the age groups on this measure. Overall and progression-free survival also were significantly better with gross-total resection and subtotal resection vs. biopsy only, and with diagnosis during 2009 and later vs. during 2007-2008. However, a difference between the two age groups was seen with respect to overall survival only among those diagnosed during 2009 or later, with a more prominent impact among the younger group, the investigators reported.
“Older individuals often get less aggressive treatment. However, based on the research, active and functional older patients should get aggressive treatment,” Dr. Ahluwalia said. “We advocate tailor-made treatment that takes into account patient condition, location of tumor, functional status, etc., in addition to patient age.”
Standard treatment approaches
The first step in the treatment of glioblastomas is maximal safe therapy, Dr. Lee said.
“You want to achieve as much of a resection as possible without leaving the patient with some sort of permanent neurologic deficit that could severely compromise the quality of their life,” she explained.
Sen. McCain’s tumor was “completely resected by imaging criteria,” according to the Mayo Clinic statement, which also noted that treatment options might include a combination of chemotherapy and radiation.
Indeed, the standard of care for glioblastomas after surgery is combined chemotherapy and radiation – typically given as approximately 6 weeks of radiation combined with oral temozolomide chemotherapy – followed by 6 monthly cycles of temozolomide, she explained.
Radiation is sometimes given for only 3 weeks, but this option is mainly reserved for elderly patients, she said, adding that trials in patients aged 65-70 years have shown that this shorter course of radiation can be equally effective but potentially less toxic.
Emerging treatment approaches
Another treatment that has shown promise involves the use of tumor-treating fields (TTFields) – a locoregionally delivered antimitotic treatment that disrupts cell division and organelle assembly.
A 2015 phase 3 trial showed that adding TTFields to maintenance temozolomide significantly prolonged progression-free and overall survival, Dr. Ahluwalia said.
Other studies, including two presented during poster sessions at the ASCO annual meeting, have shown a progression-free survival benefit with the addition of bevacizumab to the treatment regimen. One open-label phase 2 study showed that hypofractionated radiotherapy in combination with IV bevacizumab every 2 weeks vs. radiotherapy alone in newly diagnosed patients over age 65 years improved progression-free survival (median, 7.6 vs. 4.8 months), but not overall survival (median, 12.1 vs. 12.2 months).
Another phase 2 study presented by Phioanh (Leia) Nghiemphu, MD, of the University of California, Los Angeles, showed that in newly diagnosed patients aged 70 years and older, upfront treatment with bevacizumab and temozolomide was associated with promising survival benefits (overall survival, 12.3 months; progression-free survival, 5.1 months) and tolerable side effects. The best survival in multivariate analysis was in patients who received radiotherapy at progression; it was unclear whether the addition of bevacizumab led to a survival advantage, but it may have allowed delay of radiotherapy treatment, she noted.
“Although we have no cure for glioblastoma, treatments can control tumor growth for a period of time, and there are additional promising therapies emerging every day to treat this deadly cancer, she said in an interview. “There has been increasing interest in developing better therapies for the older patients with glioblastoma with less toxicity and still-robust survival, such as the addition of bevacizumab or a short course of radiotherapy with temozolomide chemotherapy.”
Dr. Ahluwalia encourages clinical trial participation for patients diagnosed with glioblastoma and noted that he is particularly excited about immunotherapy and targeted therapy trials.
Dr. Lee has served as consultant to Eli Lilly. Dr. Ahluwalia disclosed a financial relationship with multiple companies, including Novocure, which markets a TTFields device. Dr. Weller disclosed a financial relationships with multiple companies, including Novocure; Merck Sharp & Dohme, which markets temozolomide; and Roche, which markets bevacizumab. Dr. Nghiemphu has received research funding from Genentech/Roche and Novartis. Dr. Mohapatra reporting having no disclosures.
Atezolizumab improves OS in NSCLC with brain metastases
GENEVA – Immune checkpoint inhibitors may improve survival in patients with non–small cell lung cancer (NSCLC) and brain metastases compared with chemotherapy, without adding unacceptable toxicities, pooled analyses of clinical trials suggest.
Among 1452 patients with NSCLC treated with the programmed death ligand-1 (PD-L1) inhibitor atezolizumab (Tecentriq), rates of treatment-related serious adverse events (SAEs) were similar between patients with brain metastases at baseline and those without brain metastases, reported Rimas V Lukas, MD, from the University of Chicago.
“Overall, I think that these results support the investigation of atezolizumab in non–small cell lung cancer patients with CNS metastases,” Dr. Lukas said at the European Lung Cancer Conference.
Lung cancer accounts for about 40%-50% of all cases of brain metastases, and approximately 20%-40% of patients with advanced NSCLC will develop metastases, which are associated with poor overall survival, according to Solange Peters, MD, from the Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland, the invited discussant.
To evaluate the safety and efficacy of the PD-L1 inhibitor in patients with brain metastases at baseline, Lukas et al. looked at pooled safety data on patients enrolled in one of five treatment studies with atezolizumab – PCD4989g; BIRCH, POPLAR, FIR, and OAK – and efficacy data from a subset of patients in OAK.
The pooled analysis included 1452 patients, 79 of whom (5%) had brain metastases at baseline. This analysis showed that, although there was a higher incidence of any neurological AE, treatment-related AE, or treatment–related neurologic AE among patients with brain metastases, treatment-related SAEs and treatment related neurological SAEs were similar between the two groups. The most common neurological AE was headache, reported by 8% of patients with metastases and 3% without.
The efficacy analysis, which included a cohort of the first 850 patients with or without brain metastases treated, showed a significant survival for atezolizumab in the OAK trial, with median overall survival of 20.1 months for patients with brain metastases assigned to the PD-L1 inhibitor, compared with 11.9 months for patients assigned to docetaxel. This translated into a hazard ratio for atezolizumab of 0.54 (P = .0279).
Among the 750 patients without baseline brain metastases in OAK, the respective OS rates were 13.0 months, vs. 9.4 months (HR, 0.75; P = .001).
Although it was not statistically significant, the risk for developing new central nervous system lesions also appeared to be lower with atezolizumab than with docetaxel, with a median time to new lesions not reached, vs. 9.5 months with docetaxel (HR, 0.42; 95% confidence interval, 0.15-1.18).
Among patients without baseline brain metastases, there was also a hint that atezolizumab could delay onset of CNS metastases, although the trend was not significant, the investigators found.
In her commentary on the study, Dr. Peters said that “checkpoint inhibitors demonstrate activity in the brain that remains to be quantified and prospectively compared to systemic activity in larger series, and these are very small series.”
“Limitations in duration and level of activity might exist, however, related to the blood brain barrier and the brain immune system characteristics,” she added.
The study was supported by F. Hoffmann-La Roche/Genentech, a member of the Roche Group. Dr. Lukas disclosed serving on advisory boards for AstraZeneca and Novocure and receiving honoraria from AbbVie. Two coauthors are employees of Genentech, and two are employed by Roche. The remaining author had no disclosures. Dr. Peters reported relationships with Bristol-Myers Squibb, F. Hoffmann-La Roche, Eli Lilly, AstraZeneca, Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Morphotek, Merrimack, Merck Sharp and Dohme, and Merck Serono.
GENEVA – Immune checkpoint inhibitors may improve survival in patients with non–small cell lung cancer (NSCLC) and brain metastases compared with chemotherapy, without adding unacceptable toxicities, pooled analyses of clinical trials suggest.
Among 1452 patients with NSCLC treated with the programmed death ligand-1 (PD-L1) inhibitor atezolizumab (Tecentriq), rates of treatment-related serious adverse events (SAEs) were similar between patients with brain metastases at baseline and those without brain metastases, reported Rimas V Lukas, MD, from the University of Chicago.
“Overall, I think that these results support the investigation of atezolizumab in non–small cell lung cancer patients with CNS metastases,” Dr. Lukas said at the European Lung Cancer Conference.
Lung cancer accounts for about 40%-50% of all cases of brain metastases, and approximately 20%-40% of patients with advanced NSCLC will develop metastases, which are associated with poor overall survival, according to Solange Peters, MD, from the Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland, the invited discussant.
To evaluate the safety and efficacy of the PD-L1 inhibitor in patients with brain metastases at baseline, Lukas et al. looked at pooled safety data on patients enrolled in one of five treatment studies with atezolizumab – PCD4989g; BIRCH, POPLAR, FIR, and OAK – and efficacy data from a subset of patients in OAK.
The pooled analysis included 1452 patients, 79 of whom (5%) had brain metastases at baseline. This analysis showed that, although there was a higher incidence of any neurological AE, treatment-related AE, or treatment–related neurologic AE among patients with brain metastases, treatment-related SAEs and treatment related neurological SAEs were similar between the two groups. The most common neurological AE was headache, reported by 8% of patients with metastases and 3% without.
The efficacy analysis, which included a cohort of the first 850 patients with or without brain metastases treated, showed a significant survival for atezolizumab in the OAK trial, with median overall survival of 20.1 months for patients with brain metastases assigned to the PD-L1 inhibitor, compared with 11.9 months for patients assigned to docetaxel. This translated into a hazard ratio for atezolizumab of 0.54 (P = .0279).
Among the 750 patients without baseline brain metastases in OAK, the respective OS rates were 13.0 months, vs. 9.4 months (HR, 0.75; P = .001).
Although it was not statistically significant, the risk for developing new central nervous system lesions also appeared to be lower with atezolizumab than with docetaxel, with a median time to new lesions not reached, vs. 9.5 months with docetaxel (HR, 0.42; 95% confidence interval, 0.15-1.18).
Among patients without baseline brain metastases, there was also a hint that atezolizumab could delay onset of CNS metastases, although the trend was not significant, the investigators found.
In her commentary on the study, Dr. Peters said that “checkpoint inhibitors demonstrate activity in the brain that remains to be quantified and prospectively compared to systemic activity in larger series, and these are very small series.”
“Limitations in duration and level of activity might exist, however, related to the blood brain barrier and the brain immune system characteristics,” she added.
The study was supported by F. Hoffmann-La Roche/Genentech, a member of the Roche Group. Dr. Lukas disclosed serving on advisory boards for AstraZeneca and Novocure and receiving honoraria from AbbVie. Two coauthors are employees of Genentech, and two are employed by Roche. The remaining author had no disclosures. Dr. Peters reported relationships with Bristol-Myers Squibb, F. Hoffmann-La Roche, Eli Lilly, AstraZeneca, Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Morphotek, Merrimack, Merck Sharp and Dohme, and Merck Serono.
GENEVA – Immune checkpoint inhibitors may improve survival in patients with non–small cell lung cancer (NSCLC) and brain metastases compared with chemotherapy, without adding unacceptable toxicities, pooled analyses of clinical trials suggest.
Among 1452 patients with NSCLC treated with the programmed death ligand-1 (PD-L1) inhibitor atezolizumab (Tecentriq), rates of treatment-related serious adverse events (SAEs) were similar between patients with brain metastases at baseline and those without brain metastases, reported Rimas V Lukas, MD, from the University of Chicago.
“Overall, I think that these results support the investigation of atezolizumab in non–small cell lung cancer patients with CNS metastases,” Dr. Lukas said at the European Lung Cancer Conference.
Lung cancer accounts for about 40%-50% of all cases of brain metastases, and approximately 20%-40% of patients with advanced NSCLC will develop metastases, which are associated with poor overall survival, according to Solange Peters, MD, from the Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland, the invited discussant.
To evaluate the safety and efficacy of the PD-L1 inhibitor in patients with brain metastases at baseline, Lukas et al. looked at pooled safety data on patients enrolled in one of five treatment studies with atezolizumab – PCD4989g; BIRCH, POPLAR, FIR, and OAK – and efficacy data from a subset of patients in OAK.
The pooled analysis included 1452 patients, 79 of whom (5%) had brain metastases at baseline. This analysis showed that, although there was a higher incidence of any neurological AE, treatment-related AE, or treatment–related neurologic AE among patients with brain metastases, treatment-related SAEs and treatment related neurological SAEs were similar between the two groups. The most common neurological AE was headache, reported by 8% of patients with metastases and 3% without.
The efficacy analysis, which included a cohort of the first 850 patients with or without brain metastases treated, showed a significant survival for atezolizumab in the OAK trial, with median overall survival of 20.1 months for patients with brain metastases assigned to the PD-L1 inhibitor, compared with 11.9 months for patients assigned to docetaxel. This translated into a hazard ratio for atezolizumab of 0.54 (P = .0279).
Among the 750 patients without baseline brain metastases in OAK, the respective OS rates were 13.0 months, vs. 9.4 months (HR, 0.75; P = .001).
Although it was not statistically significant, the risk for developing new central nervous system lesions also appeared to be lower with atezolizumab than with docetaxel, with a median time to new lesions not reached, vs. 9.5 months with docetaxel (HR, 0.42; 95% confidence interval, 0.15-1.18).
Among patients without baseline brain metastases, there was also a hint that atezolizumab could delay onset of CNS metastases, although the trend was not significant, the investigators found.
In her commentary on the study, Dr. Peters said that “checkpoint inhibitors demonstrate activity in the brain that remains to be quantified and prospectively compared to systemic activity in larger series, and these are very small series.”
“Limitations in duration and level of activity might exist, however, related to the blood brain barrier and the brain immune system characteristics,” she added.
The study was supported by F. Hoffmann-La Roche/Genentech, a member of the Roche Group. Dr. Lukas disclosed serving on advisory boards for AstraZeneca and Novocure and receiving honoraria from AbbVie. Two coauthors are employees of Genentech, and two are employed by Roche. The remaining author had no disclosures. Dr. Peters reported relationships with Bristol-Myers Squibb, F. Hoffmann-La Roche, Eli Lilly, AstraZeneca, Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Morphotek, Merrimack, Merck Sharp and Dohme, and Merck Serono.
Key clinical point: The PD-L1 inhibitor atezolizumab improved overall survival, vs. docetaxel, in patients with non–small cell lung cancer and brain metastases.
Major finding: The hazard ratio for overall survival was 0.54 for patients assigned to atezolizumab, vs. docetaxel, in the OAK trial.
Data source: Pooled safety analysis of 1452 patients and efficacy analysis of 850 patients with NSCLC with or without brain metastases.
Disclosures: The study was supported by F. Hoffmann-La Roche/Genentech, a member of the Roche Group. Dr. Lukas disclosed serving on advisory boards for AstraZeneca and Novocure and receiving honoraria from AbbVie. Two coauthors are employees of Genentech, and two are employed by Roche. The remaining author had no disclosures. Dr. Peters reported relationships with Bristol-Myers Squibb, F. Hoffmann-La Roche, Eli Lilly, AstraZeneca, Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Morphotek, Merrimack, Merck Sharp and Dohme, and Merck Serono.
Neuropathic pain puts cancer survivors out of work
AMSTERDAM – Five years after a cancer diagnosis, patients who report having chronic neuropathic pain are twice as likely to be out of work as patients who report having no neuropathic pain, authors of a large longitudinal study said.
“For middle-term cancer survivors, suffering from chronic neuropathic pain unfortunately predicts labor-market exit,” said Marc-Karim Bendiane, from Aix-Marseille University in Marseille, France.
Pain is still frequently underdiagnosed, poorly managed, and undertreated among cancer survivors, and there is a need for alternatives to analgesics for control of chronic neuropathic pain (CNP), Mr. Bendiane said at an annual congress sponsored by the European Cancer Organisation.
Mr. Bendiane and colleagues used data from VICAN, a longitudinal survey of issues of concern to cancer survivors 2 years and 5 years after a diagnosis. The cohort consists of patients diagnosed with cancers who comprise 88% of all cancer diagnoses in France, including cancers of the breast; colon and rectum; lip, oral cavity, and pharynx; kidney; cervix; endometrium; non-Hodgkin lymphoma; melanoma; thyroid; bladder; and prostate.
To assess CNP, the researchers used data from a seven-item questionnaire designed to identify neuropathic characteristics of pain experienced by patients in the 2 weeks prior to a comprehensive patient interview.
Of the 982 patients who were working at the time of diagnosis, 36% reported pain within the previous 2 weeks, and of this group, 79% had chronic pain of neuropathic origin. CNP was more common in women than in men (P less than .01); in college-educated people, compared with less-educated people (P less than .001); those who had undergone chemotherapy, compared with no chemotherapy (P less than .001); and those who had radiotherapy vs. no radiotherapy (P less than .001).
For each cancer site, the prevalence of CNP among 5-year cancer survivors was substantially higher than the overall prevalence in France of 7%. For example, 34% of patients with cancers of the cervix and endometrium reported CNP, as did 29.9% of patients who survived cancers of the lip, oral cavity, and pharynx, 32.1% of lung cancer survivors, and 32.7% of breast cancer survivors.
Five years after diagnosis, 22.6% of patients who had been employed in 2010 were out of work in 2015.
The presence of CNP was associated with a nearly twofold greater risk of unemployment (adjusted odds ratio, 1.96; P less than .001) in a multivariate logistic regression analysis comparing employed and unemployed patients and controlling for social and demographic characteristics, job characteristics at diagnosis, and medical factors such as tumor site, prognosis, and treatment type.
The French National Cancer Institute and INSERM, the National Institute for Research in Health and Medicine, supported the study. The investigators reported no conflicts of interest.
AMSTERDAM – Five years after a cancer diagnosis, patients who report having chronic neuropathic pain are twice as likely to be out of work as patients who report having no neuropathic pain, authors of a large longitudinal study said.
“For middle-term cancer survivors, suffering from chronic neuropathic pain unfortunately predicts labor-market exit,” said Marc-Karim Bendiane, from Aix-Marseille University in Marseille, France.
Pain is still frequently underdiagnosed, poorly managed, and undertreated among cancer survivors, and there is a need for alternatives to analgesics for control of chronic neuropathic pain (CNP), Mr. Bendiane said at an annual congress sponsored by the European Cancer Organisation.
Mr. Bendiane and colleagues used data from VICAN, a longitudinal survey of issues of concern to cancer survivors 2 years and 5 years after a diagnosis. The cohort consists of patients diagnosed with cancers who comprise 88% of all cancer diagnoses in France, including cancers of the breast; colon and rectum; lip, oral cavity, and pharynx; kidney; cervix; endometrium; non-Hodgkin lymphoma; melanoma; thyroid; bladder; and prostate.
To assess CNP, the researchers used data from a seven-item questionnaire designed to identify neuropathic characteristics of pain experienced by patients in the 2 weeks prior to a comprehensive patient interview.
Of the 982 patients who were working at the time of diagnosis, 36% reported pain within the previous 2 weeks, and of this group, 79% had chronic pain of neuropathic origin. CNP was more common in women than in men (P less than .01); in college-educated people, compared with less-educated people (P less than .001); those who had undergone chemotherapy, compared with no chemotherapy (P less than .001); and those who had radiotherapy vs. no radiotherapy (P less than .001).
For each cancer site, the prevalence of CNP among 5-year cancer survivors was substantially higher than the overall prevalence in France of 7%. For example, 34% of patients with cancers of the cervix and endometrium reported CNP, as did 29.9% of patients who survived cancers of the lip, oral cavity, and pharynx, 32.1% of lung cancer survivors, and 32.7% of breast cancer survivors.
Five years after diagnosis, 22.6% of patients who had been employed in 2010 were out of work in 2015.
The presence of CNP was associated with a nearly twofold greater risk of unemployment (adjusted odds ratio, 1.96; P less than .001) in a multivariate logistic regression analysis comparing employed and unemployed patients and controlling for social and demographic characteristics, job characteristics at diagnosis, and medical factors such as tumor site, prognosis, and treatment type.
The French National Cancer Institute and INSERM, the National Institute for Research in Health and Medicine, supported the study. The investigators reported no conflicts of interest.
AMSTERDAM – Five years after a cancer diagnosis, patients who report having chronic neuropathic pain are twice as likely to be out of work as patients who report having no neuropathic pain, authors of a large longitudinal study said.
“For middle-term cancer survivors, suffering from chronic neuropathic pain unfortunately predicts labor-market exit,” said Marc-Karim Bendiane, from Aix-Marseille University in Marseille, France.
Pain is still frequently underdiagnosed, poorly managed, and undertreated among cancer survivors, and there is a need for alternatives to analgesics for control of chronic neuropathic pain (CNP), Mr. Bendiane said at an annual congress sponsored by the European Cancer Organisation.
Mr. Bendiane and colleagues used data from VICAN, a longitudinal survey of issues of concern to cancer survivors 2 years and 5 years after a diagnosis. The cohort consists of patients diagnosed with cancers who comprise 88% of all cancer diagnoses in France, including cancers of the breast; colon and rectum; lip, oral cavity, and pharynx; kidney; cervix; endometrium; non-Hodgkin lymphoma; melanoma; thyroid; bladder; and prostate.
To assess CNP, the researchers used data from a seven-item questionnaire designed to identify neuropathic characteristics of pain experienced by patients in the 2 weeks prior to a comprehensive patient interview.
Of the 982 patients who were working at the time of diagnosis, 36% reported pain within the previous 2 weeks, and of this group, 79% had chronic pain of neuropathic origin. CNP was more common in women than in men (P less than .01); in college-educated people, compared with less-educated people (P less than .001); those who had undergone chemotherapy, compared with no chemotherapy (P less than .001); and those who had radiotherapy vs. no radiotherapy (P less than .001).
For each cancer site, the prevalence of CNP among 5-year cancer survivors was substantially higher than the overall prevalence in France of 7%. For example, 34% of patients with cancers of the cervix and endometrium reported CNP, as did 29.9% of patients who survived cancers of the lip, oral cavity, and pharynx, 32.1% of lung cancer survivors, and 32.7% of breast cancer survivors.
Five years after diagnosis, 22.6% of patients who had been employed in 2010 were out of work in 2015.
The presence of CNP was associated with a nearly twofold greater risk of unemployment (adjusted odds ratio, 1.96; P less than .001) in a multivariate logistic regression analysis comparing employed and unemployed patients and controlling for social and demographic characteristics, job characteristics at diagnosis, and medical factors such as tumor site, prognosis, and treatment type.
The French National Cancer Institute and INSERM, the National Institute for Research in Health and Medicine, supported the study. The investigators reported no conflicts of interest.
Key clinical point: Chronic neuropathic pain is a barrier to employment for many cancer survivors.
Major finding: Cancer survivors with chronic neuropathic pain were twice as likely to be unemployed 5 years after diagnosis as patients with no pain.
Data source: Longitudinal study of French cancer survivors.
Disclosures: The French National Cancer Institute and INSERM, the National Institute for Research in Health and Medicine, supported the study. The investigators reported no conflicts of interest.
Detecting Autoimmune Neural Antibodies Is Key to Preventing Misdiagnosis
LAS VEGAS—Advances in autoimmune neurology, a rapidly evolving field, have the potential to prevent misdiagnosis of many disorders, from multiple sclerosis to neurodegenerative diseases. The key: increased detection of autoimmune and paraneoplastic neural antibodies, said Sean J. Pittock, MD, at the American Academy of Neurology’s Fall 2016 Conference. The importance of autoimmune neurology “cannot be overstated because these are reversible, treatable conditions,” Dr. Pittock said. “If you miss them, you have done your patients a disservice.”
Dr. Pittock, Professor of Neurology, Director of the Neuroimmunology Laboratory, and Director of the Center for MS and Autoimmune Neurology at the Mayo Clinic in Rochester, Minnesota, said he works primarily “in the field of discovering novel antibodies that make sense to the clinician.” He outlined these antibodies by brain site (cortex, cerebellum, brainstem, hypothalamus, basal ganglia, and spinal cord), as well as associated disorders and type of antibodies (see table).
“Different antibodies tell you what cancers you should be looking for,” he said. The classic paraneoplastic antibodies have oncological associations with small cell and aerodigestive carcinomas, breast and gynecologic adenocarcinomas, Hodgkin lymphoma, and thymoma. The synaptic antibodies are associated with prostate, lung, thymic, and endometrial carcinomas, as well as with teratoma.
In his laboratory, Dr. Pittock uses a complex algorithm to test for 20 types of neural antibodies using diverse methodologies, from indirect immunofluorescence to cell-based and immunoprecipitation assays. Results are usually available within six to eight days. “We are moving towards a movement disorder evaluation, a CNS hyperexcitability evaluation, and a demyelinating disease evaluation,” he said.
“In paraneoplastic disorders, the tumors are often difficult to find,” Dr. Pittock said. “The immune system is having a dramatic impact on the tumor, and so these tumors are very small, so you may miss them.”
Dr. Pittock provided an overview of how clinicians might approach diagnosing autoimmune disorders. “For children, obviously, anaplastic diseases are rare, but if you have a patient with opsoclonus myoclonus, 50% of those children will have a cancer. For adolescents and young females, you always think about teratoma. If it is a young man, you really need to think about testicular tumors.”
The type of cancer defines what type of investigation is appropriate, he said. For example, due to low resolution, PET scans are not as useful for thymoma, teratoma, or testicular or gastrointestinal tumors. On the other hand, PET scans can help evaluate equivocal findings, such as pulmonary nodules or lymph nodes. With the exception of Medicare, however, PET scans are not generally covered by insurance. Medicare covers PET scanning under ICD-9 code V71.1, “observation for suspected malignant neoplasm,” and ICD-10, Z12, “encounter for screening for malignant neoplasm”; Z12.9, “site unspecified.”
At the Mayo Clinic, Dr. Pittock said they see five patients per week with NMDA-receptor encephalitis. To diagnose a possible autoimmune neurologic disorder, he asked that clinicians “please consider using the concept of immunotherapy as kind of a diagnostic test.” To do so, the patient should be treated acutely with IV methylprednisolone, IVIg, or plasma exchanges. If the patient improves, continue acute IV therapy and taper oral prednisone; other options include oral azathioprine or mycophenolate mofetil. If the patient does not improve, consider alternative acute therapy or no further therapy.
In one study that he and his colleagues conducted, 81% of patients who had failed antiepileptic drugs (AED) and were having daily seizures were treated with such a diagnostic test. Of these, 62% responded and 34% of patients became seizure-free. In contrast, Dr. Pittock said, patients treated with a third AED in a trial rarely experience meaningful improvement.
An autoimmune condition may also have a neuropsychiatric presentation. Dr. Pittock urged attendees to watch a recent film based on the 2013 book, “Brain on Fire: My Month of Madness,” by Susannah Cahalan, who went from psychosis to catatonia before a physician determined she had brain inflammation due to an autoimmune reaction.
“Many of these patients previously would have ended up in a psychiatric institution or in an ICU setting where, potentially, in the setting of intractable disease, they may have had their machine turned off, they may have died, when they have the potential to make a full recovery and, sometimes, spontaneously improve. The take-home message for this one is, if you want to investigate a patient with this, test spinal fluid.
“Autoimmune neurology is here to stay. It is a new field; it is very exciting; it has just been recognized by the American Academy of Neurology as having its own section,” Dr. Pittock said, and invited those interested to join.
Dr. Pittock has received research support from Alexion Pharmaceuticals, the Guthy-Jackson Charitable Foundation, and the National Institutes of Health.
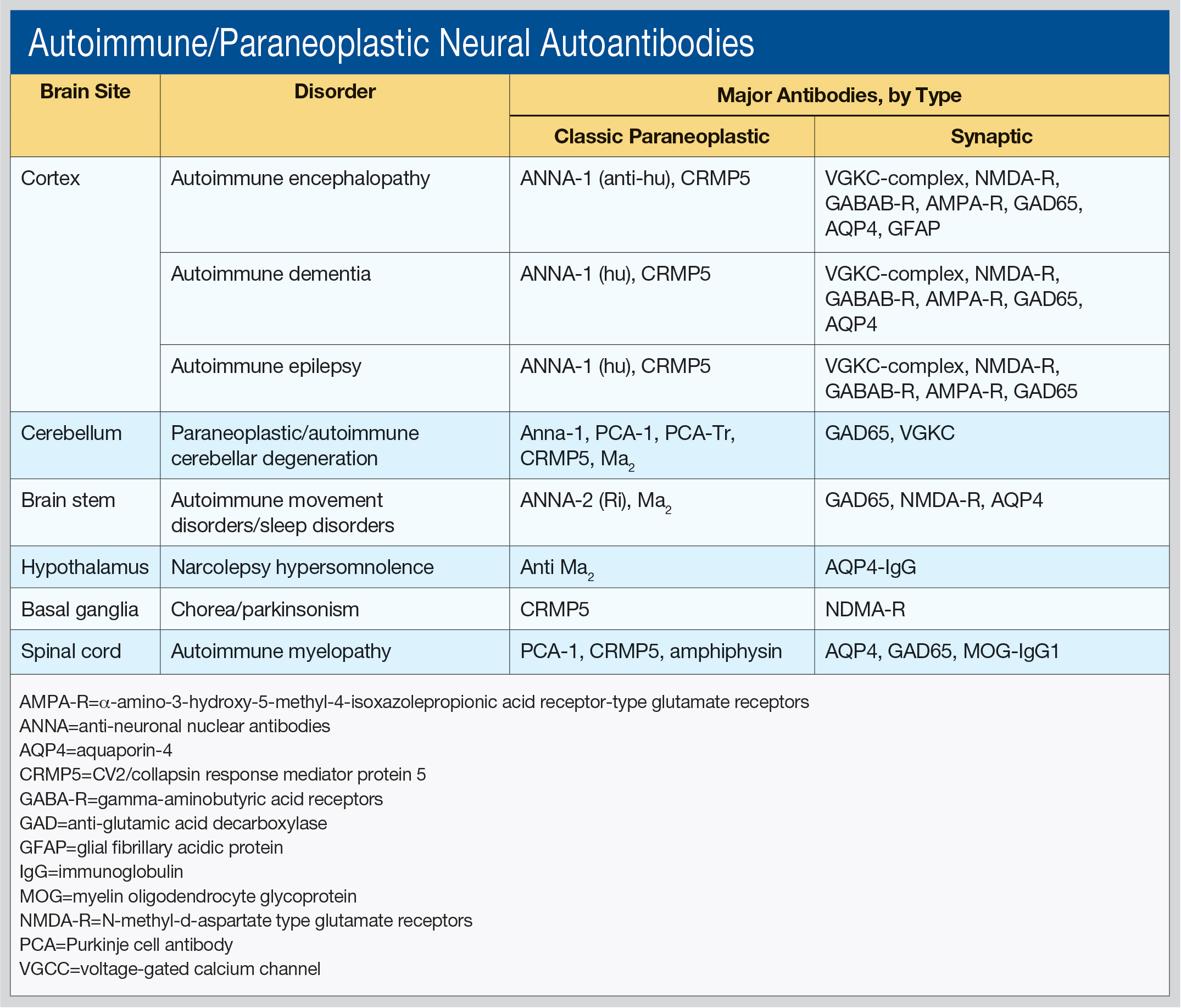
—Debra Hughes
Suggested Reading
Linnoila J, Pittock SJ. Autoantibody-associated central nervous system neurologic disorders. Semin Neurol. 2016;36(4):382-396.
Toledano M, Britton JW, McKeon A, et al. Utility of an immunotherapy trial in evaluating patients with presumed autoimmune epilepsy. Neurology. 2014;82(18):1578-1586.
LAS VEGAS—Advances in autoimmune neurology, a rapidly evolving field, have the potential to prevent misdiagnosis of many disorders, from multiple sclerosis to neurodegenerative diseases. The key: increased detection of autoimmune and paraneoplastic neural antibodies, said Sean J. Pittock, MD, at the American Academy of Neurology’s Fall 2016 Conference. The importance of autoimmune neurology “cannot be overstated because these are reversible, treatable conditions,” Dr. Pittock said. “If you miss them, you have done your patients a disservice.”
Dr. Pittock, Professor of Neurology, Director of the Neuroimmunology Laboratory, and Director of the Center for MS and Autoimmune Neurology at the Mayo Clinic in Rochester, Minnesota, said he works primarily “in the field of discovering novel antibodies that make sense to the clinician.” He outlined these antibodies by brain site (cortex, cerebellum, brainstem, hypothalamus, basal ganglia, and spinal cord), as well as associated disorders and type of antibodies (see table).
“Different antibodies tell you what cancers you should be looking for,” he said. The classic paraneoplastic antibodies have oncological associations with small cell and aerodigestive carcinomas, breast and gynecologic adenocarcinomas, Hodgkin lymphoma, and thymoma. The synaptic antibodies are associated with prostate, lung, thymic, and endometrial carcinomas, as well as with teratoma.
In his laboratory, Dr. Pittock uses a complex algorithm to test for 20 types of neural antibodies using diverse methodologies, from indirect immunofluorescence to cell-based and immunoprecipitation assays. Results are usually available within six to eight days. “We are moving towards a movement disorder evaluation, a CNS hyperexcitability evaluation, and a demyelinating disease evaluation,” he said.
“In paraneoplastic disorders, the tumors are often difficult to find,” Dr. Pittock said. “The immune system is having a dramatic impact on the tumor, and so these tumors are very small, so you may miss them.”
Dr. Pittock provided an overview of how clinicians might approach diagnosing autoimmune disorders. “For children, obviously, anaplastic diseases are rare, but if you have a patient with opsoclonus myoclonus, 50% of those children will have a cancer. For adolescents and young females, you always think about teratoma. If it is a young man, you really need to think about testicular tumors.”
The type of cancer defines what type of investigation is appropriate, he said. For example, due to low resolution, PET scans are not as useful for thymoma, teratoma, or testicular or gastrointestinal tumors. On the other hand, PET scans can help evaluate equivocal findings, such as pulmonary nodules or lymph nodes. With the exception of Medicare, however, PET scans are not generally covered by insurance. Medicare covers PET scanning under ICD-9 code V71.1, “observation for suspected malignant neoplasm,” and ICD-10, Z12, “encounter for screening for malignant neoplasm”; Z12.9, “site unspecified.”
At the Mayo Clinic, Dr. Pittock said they see five patients per week with NMDA-receptor encephalitis. To diagnose a possible autoimmune neurologic disorder, he asked that clinicians “please consider using the concept of immunotherapy as kind of a diagnostic test.” To do so, the patient should be treated acutely with IV methylprednisolone, IVIg, or plasma exchanges. If the patient improves, continue acute IV therapy and taper oral prednisone; other options include oral azathioprine or mycophenolate mofetil. If the patient does not improve, consider alternative acute therapy or no further therapy.
In one study that he and his colleagues conducted, 81% of patients who had failed antiepileptic drugs (AED) and were having daily seizures were treated with such a diagnostic test. Of these, 62% responded and 34% of patients became seizure-free. In contrast, Dr. Pittock said, patients treated with a third AED in a trial rarely experience meaningful improvement.
An autoimmune condition may also have a neuropsychiatric presentation. Dr. Pittock urged attendees to watch a recent film based on the 2013 book, “Brain on Fire: My Month of Madness,” by Susannah Cahalan, who went from psychosis to catatonia before a physician determined she had brain inflammation due to an autoimmune reaction.
“Many of these patients previously would have ended up in a psychiatric institution or in an ICU setting where, potentially, in the setting of intractable disease, they may have had their machine turned off, they may have died, when they have the potential to make a full recovery and, sometimes, spontaneously improve. The take-home message for this one is, if you want to investigate a patient with this, test spinal fluid.
“Autoimmune neurology is here to stay. It is a new field; it is very exciting; it has just been recognized by the American Academy of Neurology as having its own section,” Dr. Pittock said, and invited those interested to join.
Dr. Pittock has received research support from Alexion Pharmaceuticals, the Guthy-Jackson Charitable Foundation, and the National Institutes of Health.

—Debra Hughes
Suggested Reading
Linnoila J, Pittock SJ. Autoantibody-associated central nervous system neurologic disorders. Semin Neurol. 2016;36(4):382-396.
Toledano M, Britton JW, McKeon A, et al. Utility of an immunotherapy trial in evaluating patients with presumed autoimmune epilepsy. Neurology. 2014;82(18):1578-1586.
LAS VEGAS—Advances in autoimmune neurology, a rapidly evolving field, have the potential to prevent misdiagnosis of many disorders, from multiple sclerosis to neurodegenerative diseases. The key: increased detection of autoimmune and paraneoplastic neural antibodies, said Sean J. Pittock, MD, at the American Academy of Neurology’s Fall 2016 Conference. The importance of autoimmune neurology “cannot be overstated because these are reversible, treatable conditions,” Dr. Pittock said. “If you miss them, you have done your patients a disservice.”
Dr. Pittock, Professor of Neurology, Director of the Neuroimmunology Laboratory, and Director of the Center for MS and Autoimmune Neurology at the Mayo Clinic in Rochester, Minnesota, said he works primarily “in the field of discovering novel antibodies that make sense to the clinician.” He outlined these antibodies by brain site (cortex, cerebellum, brainstem, hypothalamus, basal ganglia, and spinal cord), as well as associated disorders and type of antibodies (see table).
“Different antibodies tell you what cancers you should be looking for,” he said. The classic paraneoplastic antibodies have oncological associations with small cell and aerodigestive carcinomas, breast and gynecologic adenocarcinomas, Hodgkin lymphoma, and thymoma. The synaptic antibodies are associated with prostate, lung, thymic, and endometrial carcinomas, as well as with teratoma.
In his laboratory, Dr. Pittock uses a complex algorithm to test for 20 types of neural antibodies using diverse methodologies, from indirect immunofluorescence to cell-based and immunoprecipitation assays. Results are usually available within six to eight days. “We are moving towards a movement disorder evaluation, a CNS hyperexcitability evaluation, and a demyelinating disease evaluation,” he said.
“In paraneoplastic disorders, the tumors are often difficult to find,” Dr. Pittock said. “The immune system is having a dramatic impact on the tumor, and so these tumors are very small, so you may miss them.”
Dr. Pittock provided an overview of how clinicians might approach diagnosing autoimmune disorders. “For children, obviously, anaplastic diseases are rare, but if you have a patient with opsoclonus myoclonus, 50% of those children will have a cancer. For adolescents and young females, you always think about teratoma. If it is a young man, you really need to think about testicular tumors.”
The type of cancer defines what type of investigation is appropriate, he said. For example, due to low resolution, PET scans are not as useful for thymoma, teratoma, or testicular or gastrointestinal tumors. On the other hand, PET scans can help evaluate equivocal findings, such as pulmonary nodules or lymph nodes. With the exception of Medicare, however, PET scans are not generally covered by insurance. Medicare covers PET scanning under ICD-9 code V71.1, “observation for suspected malignant neoplasm,” and ICD-10, Z12, “encounter for screening for malignant neoplasm”; Z12.9, “site unspecified.”
At the Mayo Clinic, Dr. Pittock said they see five patients per week with NMDA-receptor encephalitis. To diagnose a possible autoimmune neurologic disorder, he asked that clinicians “please consider using the concept of immunotherapy as kind of a diagnostic test.” To do so, the patient should be treated acutely with IV methylprednisolone, IVIg, or plasma exchanges. If the patient improves, continue acute IV therapy and taper oral prednisone; other options include oral azathioprine or mycophenolate mofetil. If the patient does not improve, consider alternative acute therapy or no further therapy.
In one study that he and his colleagues conducted, 81% of patients who had failed antiepileptic drugs (AED) and were having daily seizures were treated with such a diagnostic test. Of these, 62% responded and 34% of patients became seizure-free. In contrast, Dr. Pittock said, patients treated with a third AED in a trial rarely experience meaningful improvement.
An autoimmune condition may also have a neuropsychiatric presentation. Dr. Pittock urged attendees to watch a recent film based on the 2013 book, “Brain on Fire: My Month of Madness,” by Susannah Cahalan, who went from psychosis to catatonia before a physician determined she had brain inflammation due to an autoimmune reaction.
“Many of these patients previously would have ended up in a psychiatric institution or in an ICU setting where, potentially, in the setting of intractable disease, they may have had their machine turned off, they may have died, when they have the potential to make a full recovery and, sometimes, spontaneously improve. The take-home message for this one is, if you want to investigate a patient with this, test spinal fluid.
“Autoimmune neurology is here to stay. It is a new field; it is very exciting; it has just been recognized by the American Academy of Neurology as having its own section,” Dr. Pittock said, and invited those interested to join.
Dr. Pittock has received research support from Alexion Pharmaceuticals, the Guthy-Jackson Charitable Foundation, and the National Institutes of Health.

—Debra Hughes
Suggested Reading
Linnoila J, Pittock SJ. Autoantibody-associated central nervous system neurologic disorders. Semin Neurol. 2016;36(4):382-396.
Toledano M, Britton JW, McKeon A, et al. Utility of an immunotherapy trial in evaluating patients with presumed autoimmune epilepsy. Neurology. 2014;82(18):1578-1586.











