User login
Study identifies pandemic-related stressor in Parkinson’s disease
a team of researchers in the Netherlands reported, but they also identified meaningful targets for intervention.
Lisanne Dommershuijsen, MSc, a PhD candidate and researcher in epidemiology at the Erasmus University Medical Center in Rotterdam, the Netherlands, reported on a cross-sectional study of 833 participants with Parkinson’s disease in the PRIME-NL study at the International Congress of Parkinson’s Disease and Movement Disorders. The average age of participants was 70.2 and 38% were women.
“We studied targeted hypothetical interventions on COVID-19 stressors in people with Parkinson’s disease,” Ms. Dommershuijsen said. “This disruption in normal life caused considerable psychological stress in community-dwelling individuals. People with Parkinson’s disease might be especially vulnerable to this stress.
“For instance, because reduced levels of physical activity have worsened symptoms or because people with Parkinson’s often have difficulty with flexible [adaptations] to drastic and rapid changes in daily routines, such as those introduced by the COVID-19 pandemic, previous studies found that COVID-19 worsened depression and anxiety symptoms and reduced quality of life (QOL) in people with Parkinson’s disease,” Ms. Dommershuijsen said.
Hence, the goal of the study was to identify the most vulnerable subgroups in the Parkinson’s population and to suggest potential interventions to ameliorate these impacts, she said.
The study focused on eight different stressors that emerged in the pandemic: access to care, medicine and nursing services; loss of social contact; canceled social events; tension or conflict in the home; inability to perform physical activity or relax; and COVID-19 symptoms. The outcomes of interest were depression, as measured with the Beck Depression Inventory (BDI); anxiety, as measured with the Spielberger State-Trait Anxiety Inventory (STAI); and QOL, with the Parkinson’s Disease Quality of Life Questionnaire. The aggregate resulted in a scale of 0-40, with the mean stressor score in the study being 9.6, Ms. Dommershuijsen said.
The BDI and STAI scores for social stressors – loss of social contacts, social events canceled and tension or conflict at home – exceeded those for the so-called care stressors – problems accessing care, medication or nursing – she said, although all eight stressors yielded higher BDI and STAI scores across the board.
Vulnerable subgroups
“When we looked at vulnerable subgroups of people with Parkinson’s disease, we found more pronounced associations between the COVID-19 stress and mental health in women, in highly educated participants, and in participants with advanced Parkinson’s disease,” Ms. Dommershuijsen said. The impact on women and people with advanced disease is explainable, Ms. Dommershuijsen added in an interview; the former because depressive symptoms are more common in women, and the latter because loss of access to care impacts mental wellness.
“The finding that social stressors were more related to anxiety in highly educated people was surprising to us, given that depression in general is more common in people with a lower education,” she said in an interview. “One previous study of the general population suggested this might be related to expectations about available resources, but this findings and the possible explanation warrants further investigation.”
When the study stratified for coping strategies, the COVID-19 stressors had a smaller effect on depressive and anxiety symptoms in Parkinson’s disease patients prone to confrontive coping and planful problem solving, she said. “Whereas, we observed a larger effect of these stressors in people who are prone to using distancing or seeking social support as coping mechanisms,” Ms. Dommershuijsen said.
The researchers also created a model of a hypothetical 50% reduction in COVID-19 stressors among all study participants, but the effect wasn’t clinically relevant, Ms. Dommershuijsen said. However, in people with advanced Parkinson’s disease – that is, with an Movement Disorder Society–Unified Parkinson Disease Rating Scale score above median – the effect was clinically relevant in all outcomes.
The potential interventions the study identified were telemedicine via virtual consultations to alleviate care stressors, and virtual support groups and online classes to address social stressors. “However, a more personalized approach is needed to target tension or conflict at home, which was the most important social stressor influencing depression and anxiety symptoms in our study,” she said. “Social work can play an important role here.”
Asked to comment on the study, Roy Alcalay, MD, professor of neurology at Columbia University Irving Medical Center in New York, said in an interview that the findings align with his research on the impact of COVID-19 and related restrictions on people with Parkinson’s disease.
“The pandemic has affected people in different ways,” he said. “Initially very acutely, people just didn’t have access to doctors. There was also the acute question in movement disorders, but also in other diseases where the people with Parkinson’s disease are going to have the worse outcome when they have COVID-19.” Dr. Alcalay authored two recent papers on the impact of COVID-19 in people with Parkinson’s disease.
“Then we see that, in addition to that question, there’s the question of even if they don’t have COVID-19, just the social distancing and the lack of access to health care, and specifically to physical and occupational therapy and other services, can be quite damaging,” he said.
What’s commendable about the study, he said, was that it just doesn’t highlight the problem. “They’re also highlighting potential solutions, that planful problem solving and coping strategies can be helpful to people.”
Neither Ms. Dommershuijsen nor Dr. Alcalay have any relevant relationships to disclose.
a team of researchers in the Netherlands reported, but they also identified meaningful targets for intervention.
Lisanne Dommershuijsen, MSc, a PhD candidate and researcher in epidemiology at the Erasmus University Medical Center in Rotterdam, the Netherlands, reported on a cross-sectional study of 833 participants with Parkinson’s disease in the PRIME-NL study at the International Congress of Parkinson’s Disease and Movement Disorders. The average age of participants was 70.2 and 38% were women.
“We studied targeted hypothetical interventions on COVID-19 stressors in people with Parkinson’s disease,” Ms. Dommershuijsen said. “This disruption in normal life caused considerable psychological stress in community-dwelling individuals. People with Parkinson’s disease might be especially vulnerable to this stress.
“For instance, because reduced levels of physical activity have worsened symptoms or because people with Parkinson’s often have difficulty with flexible [adaptations] to drastic and rapid changes in daily routines, such as those introduced by the COVID-19 pandemic, previous studies found that COVID-19 worsened depression and anxiety symptoms and reduced quality of life (QOL) in people with Parkinson’s disease,” Ms. Dommershuijsen said.
Hence, the goal of the study was to identify the most vulnerable subgroups in the Parkinson’s population and to suggest potential interventions to ameliorate these impacts, she said.
The study focused on eight different stressors that emerged in the pandemic: access to care, medicine and nursing services; loss of social contact; canceled social events; tension or conflict in the home; inability to perform physical activity or relax; and COVID-19 symptoms. The outcomes of interest were depression, as measured with the Beck Depression Inventory (BDI); anxiety, as measured with the Spielberger State-Trait Anxiety Inventory (STAI); and QOL, with the Parkinson’s Disease Quality of Life Questionnaire. The aggregate resulted in a scale of 0-40, with the mean stressor score in the study being 9.6, Ms. Dommershuijsen said.
The BDI and STAI scores for social stressors – loss of social contacts, social events canceled and tension or conflict at home – exceeded those for the so-called care stressors – problems accessing care, medication or nursing – she said, although all eight stressors yielded higher BDI and STAI scores across the board.
Vulnerable subgroups
“When we looked at vulnerable subgroups of people with Parkinson’s disease, we found more pronounced associations between the COVID-19 stress and mental health in women, in highly educated participants, and in participants with advanced Parkinson’s disease,” Ms. Dommershuijsen said. The impact on women and people with advanced disease is explainable, Ms. Dommershuijsen added in an interview; the former because depressive symptoms are more common in women, and the latter because loss of access to care impacts mental wellness.
“The finding that social stressors were more related to anxiety in highly educated people was surprising to us, given that depression in general is more common in people with a lower education,” she said in an interview. “One previous study of the general population suggested this might be related to expectations about available resources, but this findings and the possible explanation warrants further investigation.”
When the study stratified for coping strategies, the COVID-19 stressors had a smaller effect on depressive and anxiety symptoms in Parkinson’s disease patients prone to confrontive coping and planful problem solving, she said. “Whereas, we observed a larger effect of these stressors in people who are prone to using distancing or seeking social support as coping mechanisms,” Ms. Dommershuijsen said.
The researchers also created a model of a hypothetical 50% reduction in COVID-19 stressors among all study participants, but the effect wasn’t clinically relevant, Ms. Dommershuijsen said. However, in people with advanced Parkinson’s disease – that is, with an Movement Disorder Society–Unified Parkinson Disease Rating Scale score above median – the effect was clinically relevant in all outcomes.
The potential interventions the study identified were telemedicine via virtual consultations to alleviate care stressors, and virtual support groups and online classes to address social stressors. “However, a more personalized approach is needed to target tension or conflict at home, which was the most important social stressor influencing depression and anxiety symptoms in our study,” she said. “Social work can play an important role here.”
Asked to comment on the study, Roy Alcalay, MD, professor of neurology at Columbia University Irving Medical Center in New York, said in an interview that the findings align with his research on the impact of COVID-19 and related restrictions on people with Parkinson’s disease.
“The pandemic has affected people in different ways,” he said. “Initially very acutely, people just didn’t have access to doctors. There was also the acute question in movement disorders, but also in other diseases where the people with Parkinson’s disease are going to have the worse outcome when they have COVID-19.” Dr. Alcalay authored two recent papers on the impact of COVID-19 in people with Parkinson’s disease.
“Then we see that, in addition to that question, there’s the question of even if they don’t have COVID-19, just the social distancing and the lack of access to health care, and specifically to physical and occupational therapy and other services, can be quite damaging,” he said.
What’s commendable about the study, he said, was that it just doesn’t highlight the problem. “They’re also highlighting potential solutions, that planful problem solving and coping strategies can be helpful to people.”
Neither Ms. Dommershuijsen nor Dr. Alcalay have any relevant relationships to disclose.
a team of researchers in the Netherlands reported, but they also identified meaningful targets for intervention.
Lisanne Dommershuijsen, MSc, a PhD candidate and researcher in epidemiology at the Erasmus University Medical Center in Rotterdam, the Netherlands, reported on a cross-sectional study of 833 participants with Parkinson’s disease in the PRIME-NL study at the International Congress of Parkinson’s Disease and Movement Disorders. The average age of participants was 70.2 and 38% were women.
“We studied targeted hypothetical interventions on COVID-19 stressors in people with Parkinson’s disease,” Ms. Dommershuijsen said. “This disruption in normal life caused considerable psychological stress in community-dwelling individuals. People with Parkinson’s disease might be especially vulnerable to this stress.
“For instance, because reduced levels of physical activity have worsened symptoms or because people with Parkinson’s often have difficulty with flexible [adaptations] to drastic and rapid changes in daily routines, such as those introduced by the COVID-19 pandemic, previous studies found that COVID-19 worsened depression and anxiety symptoms and reduced quality of life (QOL) in people with Parkinson’s disease,” Ms. Dommershuijsen said.
Hence, the goal of the study was to identify the most vulnerable subgroups in the Parkinson’s population and to suggest potential interventions to ameliorate these impacts, she said.
The study focused on eight different stressors that emerged in the pandemic: access to care, medicine and nursing services; loss of social contact; canceled social events; tension or conflict in the home; inability to perform physical activity or relax; and COVID-19 symptoms. The outcomes of interest were depression, as measured with the Beck Depression Inventory (BDI); anxiety, as measured with the Spielberger State-Trait Anxiety Inventory (STAI); and QOL, with the Parkinson’s Disease Quality of Life Questionnaire. The aggregate resulted in a scale of 0-40, with the mean stressor score in the study being 9.6, Ms. Dommershuijsen said.
The BDI and STAI scores for social stressors – loss of social contacts, social events canceled and tension or conflict at home – exceeded those for the so-called care stressors – problems accessing care, medication or nursing – she said, although all eight stressors yielded higher BDI and STAI scores across the board.
Vulnerable subgroups
“When we looked at vulnerable subgroups of people with Parkinson’s disease, we found more pronounced associations between the COVID-19 stress and mental health in women, in highly educated participants, and in participants with advanced Parkinson’s disease,” Ms. Dommershuijsen said. The impact on women and people with advanced disease is explainable, Ms. Dommershuijsen added in an interview; the former because depressive symptoms are more common in women, and the latter because loss of access to care impacts mental wellness.
“The finding that social stressors were more related to anxiety in highly educated people was surprising to us, given that depression in general is more common in people with a lower education,” she said in an interview. “One previous study of the general population suggested this might be related to expectations about available resources, but this findings and the possible explanation warrants further investigation.”
When the study stratified for coping strategies, the COVID-19 stressors had a smaller effect on depressive and anxiety symptoms in Parkinson’s disease patients prone to confrontive coping and planful problem solving, she said. “Whereas, we observed a larger effect of these stressors in people who are prone to using distancing or seeking social support as coping mechanisms,” Ms. Dommershuijsen said.
The researchers also created a model of a hypothetical 50% reduction in COVID-19 stressors among all study participants, but the effect wasn’t clinically relevant, Ms. Dommershuijsen said. However, in people with advanced Parkinson’s disease – that is, with an Movement Disorder Society–Unified Parkinson Disease Rating Scale score above median – the effect was clinically relevant in all outcomes.
The potential interventions the study identified were telemedicine via virtual consultations to alleviate care stressors, and virtual support groups and online classes to address social stressors. “However, a more personalized approach is needed to target tension or conflict at home, which was the most important social stressor influencing depression and anxiety symptoms in our study,” she said. “Social work can play an important role here.”
Asked to comment on the study, Roy Alcalay, MD, professor of neurology at Columbia University Irving Medical Center in New York, said in an interview that the findings align with his research on the impact of COVID-19 and related restrictions on people with Parkinson’s disease.
“The pandemic has affected people in different ways,” he said. “Initially very acutely, people just didn’t have access to doctors. There was also the acute question in movement disorders, but also in other diseases where the people with Parkinson’s disease are going to have the worse outcome when they have COVID-19.” Dr. Alcalay authored two recent papers on the impact of COVID-19 in people with Parkinson’s disease.
“Then we see that, in addition to that question, there’s the question of even if they don’t have COVID-19, just the social distancing and the lack of access to health care, and specifically to physical and occupational therapy and other services, can be quite damaging,” he said.
What’s commendable about the study, he said, was that it just doesn’t highlight the problem. “They’re also highlighting potential solutions, that planful problem solving and coping strategies can be helpful to people.”
Neither Ms. Dommershuijsen nor Dr. Alcalay have any relevant relationships to disclose.
FROM MDS VIRTUAL CONGRESS 2021
Sexual assault in women tied to increased stroke, dementia risk
Traumatic experiences, especially sexual assault, may put women at greater risk for poor brain health.
In the Ms Brain study, middle-aged women with trauma exposure had a greater volume of white matter hyperintensities (WMHs) than those without trauma. In addition, the differences persisted even after adjusting for depressive or post-traumatic stress symptoms.
WMHs are “an important indicator of small vessel disease in the brain and have been linked to future stroke risk, dementia risk, and mortality,” lead investigator Rebecca Thurston, PhD, from the University of Pittsburgh, told this news organization.
“What I take from this is, really, that sexual assault has implications for women’s health, far beyond exclusively mental health outcomes, but also for their cardiovascular health, as we have shown in other work and for their stroke and dementia risk as we are seeing in the present work,” Dr. Thurston added.
The study was presented at the North American Menopause Society (NAMS) Annual Meeting in Washington, D.C., and has been accepted for publication in the journal Brain Imaging and Behavior.
Beyond the usual suspects
As part of the study, 145 women (mean age, 59 years) free of clinical cardiovascular disease, stroke, or dementia provided their medical history, including history of traumatic experiences, depression, and post-traumatic stress disorder and underwent magnetic resonance brain imaging for WMHs.
More than two-thirds (68%) of the women reported at least one trauma, most commonly sexual assault (23%).
In multivariate analysis, women with trauma exposure had greater WMH volume than women without trauma (P = .01), with sexual assault most strongly associated with greater WMH volume (P = .02).
The associations persisted after adjusting for depressive or post-traumatic stress symptoms.
“A history of sexual assault was particularly related to white matter hyperintensities in the parietal lobe, and these kinds of white matter hyperintensities have been linked to Alzheimer’s disease in a fairly pronounced way,” Dr. Thurston said.
“When we think about risk factors for stroke, dementia, we need to think beyond exclusively our usual suspects and also think about women [who experienced] psychological trauma and experienced sexual assault in particular. So ask about it and consider it part of your screening regimen,” she added.
‘Burgeoning’ literature
Commenting on the findings, Charles Nemeroff, MD, PhD, professor and chair, department of psychiatry and behavioral sciences, Dell Medical School, University of Texas at Austin, and director of its Institute for Early Life Adversity Research, said the research adds to the “burgeoning literature on the long term neurobiological consequences of trauma and more specifically, sexual abuse, on brain imaging measures.”
“Our group and others reported several years ago that patients with mood disorders, more specifically bipolar disorder and major depression, had higher rates of WMH than matched controls. Those older studies did not control for a history of early life adversity such as childhood maltreatment,” Dr. Nemeroff said.
“In addition to this finding of increased WMH in subjects exposed to trauma is a very large literature documenting other central nervous system (CNS) changes in this population, including cortical thinning in certain brain areas and clearly an emerging finding that different forms of childhood maltreatment are associated with quite distinct structural brain alterations in adulthood,” he noted.
The study was supported by grants from the National Institutes of Health. Dr. Thurston and Dr. Nemeroff have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Traumatic experiences, especially sexual assault, may put women at greater risk for poor brain health.
In the Ms Brain study, middle-aged women with trauma exposure had a greater volume of white matter hyperintensities (WMHs) than those without trauma. In addition, the differences persisted even after adjusting for depressive or post-traumatic stress symptoms.
WMHs are “an important indicator of small vessel disease in the brain and have been linked to future stroke risk, dementia risk, and mortality,” lead investigator Rebecca Thurston, PhD, from the University of Pittsburgh, told this news organization.
“What I take from this is, really, that sexual assault has implications for women’s health, far beyond exclusively mental health outcomes, but also for their cardiovascular health, as we have shown in other work and for their stroke and dementia risk as we are seeing in the present work,” Dr. Thurston added.
The study was presented at the North American Menopause Society (NAMS) Annual Meeting in Washington, D.C., and has been accepted for publication in the journal Brain Imaging and Behavior.
Beyond the usual suspects
As part of the study, 145 women (mean age, 59 years) free of clinical cardiovascular disease, stroke, or dementia provided their medical history, including history of traumatic experiences, depression, and post-traumatic stress disorder and underwent magnetic resonance brain imaging for WMHs.
More than two-thirds (68%) of the women reported at least one trauma, most commonly sexual assault (23%).
In multivariate analysis, women with trauma exposure had greater WMH volume than women without trauma (P = .01), with sexual assault most strongly associated with greater WMH volume (P = .02).
The associations persisted after adjusting for depressive or post-traumatic stress symptoms.
“A history of sexual assault was particularly related to white matter hyperintensities in the parietal lobe, and these kinds of white matter hyperintensities have been linked to Alzheimer’s disease in a fairly pronounced way,” Dr. Thurston said.
“When we think about risk factors for stroke, dementia, we need to think beyond exclusively our usual suspects and also think about women [who experienced] psychological trauma and experienced sexual assault in particular. So ask about it and consider it part of your screening regimen,” she added.
‘Burgeoning’ literature
Commenting on the findings, Charles Nemeroff, MD, PhD, professor and chair, department of psychiatry and behavioral sciences, Dell Medical School, University of Texas at Austin, and director of its Institute for Early Life Adversity Research, said the research adds to the “burgeoning literature on the long term neurobiological consequences of trauma and more specifically, sexual abuse, on brain imaging measures.”
“Our group and others reported several years ago that patients with mood disorders, more specifically bipolar disorder and major depression, had higher rates of WMH than matched controls. Those older studies did not control for a history of early life adversity such as childhood maltreatment,” Dr. Nemeroff said.
“In addition to this finding of increased WMH in subjects exposed to trauma is a very large literature documenting other central nervous system (CNS) changes in this population, including cortical thinning in certain brain areas and clearly an emerging finding that different forms of childhood maltreatment are associated with quite distinct structural brain alterations in adulthood,” he noted.
The study was supported by grants from the National Institutes of Health. Dr. Thurston and Dr. Nemeroff have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Traumatic experiences, especially sexual assault, may put women at greater risk for poor brain health.
In the Ms Brain study, middle-aged women with trauma exposure had a greater volume of white matter hyperintensities (WMHs) than those without trauma. In addition, the differences persisted even after adjusting for depressive or post-traumatic stress symptoms.
WMHs are “an important indicator of small vessel disease in the brain and have been linked to future stroke risk, dementia risk, and mortality,” lead investigator Rebecca Thurston, PhD, from the University of Pittsburgh, told this news organization.
“What I take from this is, really, that sexual assault has implications for women’s health, far beyond exclusively mental health outcomes, but also for their cardiovascular health, as we have shown in other work and for their stroke and dementia risk as we are seeing in the present work,” Dr. Thurston added.
The study was presented at the North American Menopause Society (NAMS) Annual Meeting in Washington, D.C., and has been accepted for publication in the journal Brain Imaging and Behavior.
Beyond the usual suspects
As part of the study, 145 women (mean age, 59 years) free of clinical cardiovascular disease, stroke, or dementia provided their medical history, including history of traumatic experiences, depression, and post-traumatic stress disorder and underwent magnetic resonance brain imaging for WMHs.
More than two-thirds (68%) of the women reported at least one trauma, most commonly sexual assault (23%).
In multivariate analysis, women with trauma exposure had greater WMH volume than women without trauma (P = .01), with sexual assault most strongly associated with greater WMH volume (P = .02).
The associations persisted after adjusting for depressive or post-traumatic stress symptoms.
“A history of sexual assault was particularly related to white matter hyperintensities in the parietal lobe, and these kinds of white matter hyperintensities have been linked to Alzheimer’s disease in a fairly pronounced way,” Dr. Thurston said.
“When we think about risk factors for stroke, dementia, we need to think beyond exclusively our usual suspects and also think about women [who experienced] psychological trauma and experienced sexual assault in particular. So ask about it and consider it part of your screening regimen,” she added.
‘Burgeoning’ literature
Commenting on the findings, Charles Nemeroff, MD, PhD, professor and chair, department of psychiatry and behavioral sciences, Dell Medical School, University of Texas at Austin, and director of its Institute for Early Life Adversity Research, said the research adds to the “burgeoning literature on the long term neurobiological consequences of trauma and more specifically, sexual abuse, on brain imaging measures.”
“Our group and others reported several years ago that patients with mood disorders, more specifically bipolar disorder and major depression, had higher rates of WMH than matched controls. Those older studies did not control for a history of early life adversity such as childhood maltreatment,” Dr. Nemeroff said.
“In addition to this finding of increased WMH in subjects exposed to trauma is a very large literature documenting other central nervous system (CNS) changes in this population, including cortical thinning in certain brain areas and clearly an emerging finding that different forms of childhood maltreatment are associated with quite distinct structural brain alterations in adulthood,” he noted.
The study was supported by grants from the National Institutes of Health. Dr. Thurston and Dr. Nemeroff have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Consensus statement warns against acetaminophen use during pregnancy
Pregnant women should use paracetamol/acetaminophen only with a medical indication and at the lowest effective dose for the shortest possible time, according to an international consensus statement published online Sept. 23 in Nature Reviews Endocrinology.
With global rates of use high and risks considered negligible, the expert panel of 13 U.S. and European authors call for focused research into how this analgesic and febrifuge may impair fetal development and lead to adverse outcomes in children. They outline several precautionary measures to be taken in the meantime.
According to first author and epidemiologist Ann Z. Bauer, ScD, a postdoctoral research fellow at the University of Massachusetts in Lowell, and colleagues, this drug is used by an estimated 65% of pregnant women in the United States, and more than 50% worldwide. It is currently the active ingredient in more than 600 prescription and nonprescription medications, including Tylenol, which historically has been deemed safe in all trimesters of pregnancy.
But a growing body of experimental and epidemiological evidence suggests prenatal exposure to paracetamol (N-acetyl-p-aminophenol, or APAP) might alter fetal development and elevate the risks of neurodevelopmental, reproductive and urogenital disorders in both sexes. Exposure in utero has been linked, for example, to potential behavioral problems in children.
The new recommendations are based on a review of experimental animal and cell-based research as well as human epidemiological data published from January 1995 to October 2020. The authors include clinicians, epidemiologists, and scientists specializing in toxicology, endocrinology, reproductive medicine and neurodevelopment.
Recommendations
Although the new guidance does not differ markedly from current advice, the authors believe stronger communication and greater awareness of risks are needed. In addition to restricting use of this medication to low doses for short periods when medically necessary, expectant mothers should receive counseling before conception or early in pregnancy. If uncertain about its use, they should consult their physicians or pharmacists.
In other recommendations, the panel said:
- The 2015 FDA Drug Safety Communication recommendations should be updated based on evaluation of all available scientific evidence.
- The European Medicines Agency Pharmacovigilance Risk Assessment Committee should review the most recent epidemiologic and experimental research and issue an updated Drug Safety Communication.
- Obstetric and gynecological associations should update their guidance after reviewing all available research.
- The Acetaminophen Awareness Coalition (“Know Your Dose” Campaign) should add standardized warnings and specifically advise pregnant women to forgo APAP unless it’s medically indicated.
- All sales of APAP-containing medications should be accompanied by recommendations specifically for use in pregnancy. This information should include warning labels on packaging, and if possible, APAP should be sold only in pharmacies (as in France).
Mechanism of action
APAP is an endocrine disruptor (Neuroscientist. 2020 Sep 11. doi: 10.1177/1073858420952046). “Chemicals that disrupt the endocrine system are concerning because they can interfere with the activity of endogenous hormones that are essential for healthy neurological, urogenital, and reproductive development,” researchers wrote.
“The precise mechanism is not clear but its toxicity is thought to be due mainly to hormone disruption,” Dr. Bauer said in an interview.
Moreover, APAP readily crosses the placenta and blood–brain barrier, and changes in APAP metabolism during pregnancy might make women and their fetuses more vulnerable to its toxic effects. For instance, the molar dose fraction of APAP converted to the oxidative metabolite N-acetyl-p-benzoquinone imine increases during pregnancy. In addition to its hepatotoxicity, this poisonous byproduct is thought to be a genotoxin that increases DNA cleavage by acting on the enzyme topoisomerase II.
Asked for her perspective on the statement, Kjersti Aagaard, MD, PhD, a professor of obstetrics and gynecology at Baylor College of Medicine and Texas Children’s Hospital in Houston, called the expert panel’s statement thoughtful and comprehensive, but she urged caution in interpreting the role of acetaminophen.
The challenge in linking any commonly used medication to adverse effects and congenital defects, she said, is “teasing out an association from causation. Given the commonality of the use of acetaminophen with the relative rarity of the outcomes, it is clear that not all cases of exposure result in adverse outcomes.”
As for judicious use, she said, one would be to reduce a high fever, which can cause miscarriage, neural tube defects, and potential heart disease in adulthood. Acetaminophen is the drug of choice in this case since nonsteroidal anti-inflammatory drugs such as ibuprofen are not recommended owing to their known risks to the fetal heart.
Dr. Aagaard emphasized that while acetaminophen use is temporally associated with learning and behavioral problems, and urogenital disorders at birth in male infants such as like hypospadias, so is exposure to multiple environmental chemicals and pollutants, as well as climate change. “It would be a real mistake with real life implications if we associated any congenital disease or disorder with a commonly used medication with known benefits if the true causal link lies elsewhere.”
She said the precautionary statements fall into the time-honored therapeutic principle of first do no harm. “However, the call for research action must be undertaken earnestly and sincerely.”
According to Dr. Bauer, the statement’s essential take-home message is that “physicians should educate themselves and educate women about what we’re learning about the risks of acetaminophen in pregnancy.” Risk can be minimized by using the lowest effective dose for the shortest time and only when medically indicated. “Pregnant women should speak to their physicians about acetaminophen. It’s about empowerment and making smart decisions,” she said.
This study received no specific funding. Coauthor Dr. R.T. Mitchell is supported by a UK Research Institute fellowship.
Pregnant women should use paracetamol/acetaminophen only with a medical indication and at the lowest effective dose for the shortest possible time, according to an international consensus statement published online Sept. 23 in Nature Reviews Endocrinology.
With global rates of use high and risks considered negligible, the expert panel of 13 U.S. and European authors call for focused research into how this analgesic and febrifuge may impair fetal development and lead to adverse outcomes in children. They outline several precautionary measures to be taken in the meantime.
According to first author and epidemiologist Ann Z. Bauer, ScD, a postdoctoral research fellow at the University of Massachusetts in Lowell, and colleagues, this drug is used by an estimated 65% of pregnant women in the United States, and more than 50% worldwide. It is currently the active ingredient in more than 600 prescription and nonprescription medications, including Tylenol, which historically has been deemed safe in all trimesters of pregnancy.
But a growing body of experimental and epidemiological evidence suggests prenatal exposure to paracetamol (N-acetyl-p-aminophenol, or APAP) might alter fetal development and elevate the risks of neurodevelopmental, reproductive and urogenital disorders in both sexes. Exposure in utero has been linked, for example, to potential behavioral problems in children.
The new recommendations are based on a review of experimental animal and cell-based research as well as human epidemiological data published from January 1995 to October 2020. The authors include clinicians, epidemiologists, and scientists specializing in toxicology, endocrinology, reproductive medicine and neurodevelopment.
Recommendations
Although the new guidance does not differ markedly from current advice, the authors believe stronger communication and greater awareness of risks are needed. In addition to restricting use of this medication to low doses for short periods when medically necessary, expectant mothers should receive counseling before conception or early in pregnancy. If uncertain about its use, they should consult their physicians or pharmacists.
In other recommendations, the panel said:
- The 2015 FDA Drug Safety Communication recommendations should be updated based on evaluation of all available scientific evidence.
- The European Medicines Agency Pharmacovigilance Risk Assessment Committee should review the most recent epidemiologic and experimental research and issue an updated Drug Safety Communication.
- Obstetric and gynecological associations should update their guidance after reviewing all available research.
- The Acetaminophen Awareness Coalition (“Know Your Dose” Campaign) should add standardized warnings and specifically advise pregnant women to forgo APAP unless it’s medically indicated.
- All sales of APAP-containing medications should be accompanied by recommendations specifically for use in pregnancy. This information should include warning labels on packaging, and if possible, APAP should be sold only in pharmacies (as in France).
Mechanism of action
APAP is an endocrine disruptor (Neuroscientist. 2020 Sep 11. doi: 10.1177/1073858420952046). “Chemicals that disrupt the endocrine system are concerning because they can interfere with the activity of endogenous hormones that are essential for healthy neurological, urogenital, and reproductive development,” researchers wrote.
“The precise mechanism is not clear but its toxicity is thought to be due mainly to hormone disruption,” Dr. Bauer said in an interview.
Moreover, APAP readily crosses the placenta and blood–brain barrier, and changes in APAP metabolism during pregnancy might make women and their fetuses more vulnerable to its toxic effects. For instance, the molar dose fraction of APAP converted to the oxidative metabolite N-acetyl-p-benzoquinone imine increases during pregnancy. In addition to its hepatotoxicity, this poisonous byproduct is thought to be a genotoxin that increases DNA cleavage by acting on the enzyme topoisomerase II.
Asked for her perspective on the statement, Kjersti Aagaard, MD, PhD, a professor of obstetrics and gynecology at Baylor College of Medicine and Texas Children’s Hospital in Houston, called the expert panel’s statement thoughtful and comprehensive, but she urged caution in interpreting the role of acetaminophen.
The challenge in linking any commonly used medication to adverse effects and congenital defects, she said, is “teasing out an association from causation. Given the commonality of the use of acetaminophen with the relative rarity of the outcomes, it is clear that not all cases of exposure result in adverse outcomes.”
As for judicious use, she said, one would be to reduce a high fever, which can cause miscarriage, neural tube defects, and potential heart disease in adulthood. Acetaminophen is the drug of choice in this case since nonsteroidal anti-inflammatory drugs such as ibuprofen are not recommended owing to their known risks to the fetal heart.
Dr. Aagaard emphasized that while acetaminophen use is temporally associated with learning and behavioral problems, and urogenital disorders at birth in male infants such as like hypospadias, so is exposure to multiple environmental chemicals and pollutants, as well as climate change. “It would be a real mistake with real life implications if we associated any congenital disease or disorder with a commonly used medication with known benefits if the true causal link lies elsewhere.”
She said the precautionary statements fall into the time-honored therapeutic principle of first do no harm. “However, the call for research action must be undertaken earnestly and sincerely.”
According to Dr. Bauer, the statement’s essential take-home message is that “physicians should educate themselves and educate women about what we’re learning about the risks of acetaminophen in pregnancy.” Risk can be minimized by using the lowest effective dose for the shortest time and only when medically indicated. “Pregnant women should speak to their physicians about acetaminophen. It’s about empowerment and making smart decisions,” she said.
This study received no specific funding. Coauthor Dr. R.T. Mitchell is supported by a UK Research Institute fellowship.
Pregnant women should use paracetamol/acetaminophen only with a medical indication and at the lowest effective dose for the shortest possible time, according to an international consensus statement published online Sept. 23 in Nature Reviews Endocrinology.
With global rates of use high and risks considered negligible, the expert panel of 13 U.S. and European authors call for focused research into how this analgesic and febrifuge may impair fetal development and lead to adverse outcomes in children. They outline several precautionary measures to be taken in the meantime.
According to first author and epidemiologist Ann Z. Bauer, ScD, a postdoctoral research fellow at the University of Massachusetts in Lowell, and colleagues, this drug is used by an estimated 65% of pregnant women in the United States, and more than 50% worldwide. It is currently the active ingredient in more than 600 prescription and nonprescription medications, including Tylenol, which historically has been deemed safe in all trimesters of pregnancy.
But a growing body of experimental and epidemiological evidence suggests prenatal exposure to paracetamol (N-acetyl-p-aminophenol, or APAP) might alter fetal development and elevate the risks of neurodevelopmental, reproductive and urogenital disorders in both sexes. Exposure in utero has been linked, for example, to potential behavioral problems in children.
The new recommendations are based on a review of experimental animal and cell-based research as well as human epidemiological data published from January 1995 to October 2020. The authors include clinicians, epidemiologists, and scientists specializing in toxicology, endocrinology, reproductive medicine and neurodevelopment.
Recommendations
Although the new guidance does not differ markedly from current advice, the authors believe stronger communication and greater awareness of risks are needed. In addition to restricting use of this medication to low doses for short periods when medically necessary, expectant mothers should receive counseling before conception or early in pregnancy. If uncertain about its use, they should consult their physicians or pharmacists.
In other recommendations, the panel said:
- The 2015 FDA Drug Safety Communication recommendations should be updated based on evaluation of all available scientific evidence.
- The European Medicines Agency Pharmacovigilance Risk Assessment Committee should review the most recent epidemiologic and experimental research and issue an updated Drug Safety Communication.
- Obstetric and gynecological associations should update their guidance after reviewing all available research.
- The Acetaminophen Awareness Coalition (“Know Your Dose” Campaign) should add standardized warnings and specifically advise pregnant women to forgo APAP unless it’s medically indicated.
- All sales of APAP-containing medications should be accompanied by recommendations specifically for use in pregnancy. This information should include warning labels on packaging, and if possible, APAP should be sold only in pharmacies (as in France).
Mechanism of action
APAP is an endocrine disruptor (Neuroscientist. 2020 Sep 11. doi: 10.1177/1073858420952046). “Chemicals that disrupt the endocrine system are concerning because they can interfere with the activity of endogenous hormones that are essential for healthy neurological, urogenital, and reproductive development,” researchers wrote.
“The precise mechanism is not clear but its toxicity is thought to be due mainly to hormone disruption,” Dr. Bauer said in an interview.
Moreover, APAP readily crosses the placenta and blood–brain barrier, and changes in APAP metabolism during pregnancy might make women and their fetuses more vulnerable to its toxic effects. For instance, the molar dose fraction of APAP converted to the oxidative metabolite N-acetyl-p-benzoquinone imine increases during pregnancy. In addition to its hepatotoxicity, this poisonous byproduct is thought to be a genotoxin that increases DNA cleavage by acting on the enzyme topoisomerase II.
Asked for her perspective on the statement, Kjersti Aagaard, MD, PhD, a professor of obstetrics and gynecology at Baylor College of Medicine and Texas Children’s Hospital in Houston, called the expert panel’s statement thoughtful and comprehensive, but she urged caution in interpreting the role of acetaminophen.
The challenge in linking any commonly used medication to adverse effects and congenital defects, she said, is “teasing out an association from causation. Given the commonality of the use of acetaminophen with the relative rarity of the outcomes, it is clear that not all cases of exposure result in adverse outcomes.”
As for judicious use, she said, one would be to reduce a high fever, which can cause miscarriage, neural tube defects, and potential heart disease in adulthood. Acetaminophen is the drug of choice in this case since nonsteroidal anti-inflammatory drugs such as ibuprofen are not recommended owing to their known risks to the fetal heart.
Dr. Aagaard emphasized that while acetaminophen use is temporally associated with learning and behavioral problems, and urogenital disorders at birth in male infants such as like hypospadias, so is exposure to multiple environmental chemicals and pollutants, as well as climate change. “It would be a real mistake with real life implications if we associated any congenital disease or disorder with a commonly used medication with known benefits if the true causal link lies elsewhere.”
She said the precautionary statements fall into the time-honored therapeutic principle of first do no harm. “However, the call for research action must be undertaken earnestly and sincerely.”
According to Dr. Bauer, the statement’s essential take-home message is that “physicians should educate themselves and educate women about what we’re learning about the risks of acetaminophen in pregnancy.” Risk can be minimized by using the lowest effective dose for the shortest time and only when medically indicated. “Pregnant women should speak to their physicians about acetaminophen. It’s about empowerment and making smart decisions,” she said.
This study received no specific funding. Coauthor Dr. R.T. Mitchell is supported by a UK Research Institute fellowship.
Surge in new-onset tics in adults tied to COVID-19 stress
, new research suggests.
Results from a large, single-center study show several cases of tic-like movements and vocalizations with abrupt onset among older adolescents and adults during the pandemic. None had a previous diagnosis of a tic disorder. Among 10 patients, two were diagnosed with a purely functional movement disorder, four with an organic tic disorder, and four with both.
“Within our movement disorders clinic specifically ... we’ve been seeing an increased number of patients with an almost explosive onset of these tic-like movements and vocalizations later in life than what is typically seen with organic tic disorders and Tourette syndrome, which is typically in school-aged children,” said study investigator Caroline Olvera, MD, Rush University Medical Center, Chicago.
“Abrupt onset of symptoms can be seen in patients with tic disorders, although this is typically quoted as less than 10%, or even 5% is more characteristic of functional neurological disorders in general and also with psychogenic tics,” she added.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Anxiety, other psychiatric conditions
Tic disorders typically start in childhood. However, the researchers observed an increase in the number of patients with abrupt onset of tic-like movements and vocalizations later in life, which is more often characteristic of functional neurological disorders.
To examine the profile, associated conditions, and risk factors in this population, the investigators conducted a thorough chart review of patients attending movement disorder clinics between March 2020, when the COVID pandemic was officially declared, and March 2021.
Patients with acute onset of tics were identified using the International Classification of Diseases codes for behavioral tics, tic vocalizations, and Tourette syndrome.
The charts were then narrowed down to patients with no previous diagnosis of these conditions. Most patients were videotaped for assessment by the rest of the movement disorder neurologists in the practice. Since the end of the study inclusion period in March 2021, Dr. Olvera estimates that the clinic experienced a doubling or tripling of the number of similar patients.
In the study cohort of 10 patients, the median age at presentation was 19 years (range, 15-41 years), nine were female, the gender of the other one was unknown, and the duration of tics was 8 weeks (range, 1-24 weeks) by the time they were first seen in the clinic. Four patients reported having COVID infection before tic onset.
All exhibited motor tics and nine had vocal tics. Two were diagnosed with a purely functional neurologic disorder, four with only an organic tic disorder, and four with organic tics with a functional overlay.
“All patients, including those with organic tic disorders, had a history of anxiety and also reported worsening anxiety in the setting of the COVID pandemic,” Dr. Olvera said.
The majority of patients were on a psychotropic medication prior to coming to the clinic, and these were primarily for anxiety and depression. Three patients had a history of suicidality, often very severe and leading to hospitalization, she noted.
“In terms of our conclusions from the project, we feel that this phenotype of acute explosive onset of tic-like movements and vocalizations in this older population of adults, compared with typical organic tic disorders and Tourette syndrome, appears novel to the pandemic,” she said.
She cautioned that functional and organic tics share many characteristics and therefore may be difficult to differentiate.
COVID stress
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said the research highlights how clinicians’ understanding of particular diseases can be challenged during extraordinary events such as COVID-19 and the heightened stress it causes.
“I’m not surprised that these [disorders] might have had a spike during a stressful time as COVID,” he said.
Patients are “really scared and really anxious, they’re afraid to die, and they’re afraid that their life will be over. So they might express their psychological difficulty, their discomfort, with these calls for help that look like tics. But they’re not what we consider physiological or organic things,” he added.
Dr. Tagliati added that he doesn’t believe rapid tic onset in adults is not a complication of the coronavirus infection, but rather a consequence of psychological pressure brought on by the pandemic.
Treating underlying anxiety may be a useful approach, possibly with the support of psychiatrists, which in many cases is enough to relieve the conditions and overcome the symptoms, he noted.
However, at other times, it’s not that simple, he added. Sometimes patients “fall through the cracks between neurology and psychiatry,” Dr. Tagliati said.
Dr. Olvera and Dr. Tagliati have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large, single-center study show several cases of tic-like movements and vocalizations with abrupt onset among older adolescents and adults during the pandemic. None had a previous diagnosis of a tic disorder. Among 10 patients, two were diagnosed with a purely functional movement disorder, four with an organic tic disorder, and four with both.
“Within our movement disorders clinic specifically ... we’ve been seeing an increased number of patients with an almost explosive onset of these tic-like movements and vocalizations later in life than what is typically seen with organic tic disorders and Tourette syndrome, which is typically in school-aged children,” said study investigator Caroline Olvera, MD, Rush University Medical Center, Chicago.
“Abrupt onset of symptoms can be seen in patients with tic disorders, although this is typically quoted as less than 10%, or even 5% is more characteristic of functional neurological disorders in general and also with psychogenic tics,” she added.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Anxiety, other psychiatric conditions
Tic disorders typically start in childhood. However, the researchers observed an increase in the number of patients with abrupt onset of tic-like movements and vocalizations later in life, which is more often characteristic of functional neurological disorders.
To examine the profile, associated conditions, and risk factors in this population, the investigators conducted a thorough chart review of patients attending movement disorder clinics between March 2020, when the COVID pandemic was officially declared, and March 2021.
Patients with acute onset of tics were identified using the International Classification of Diseases codes for behavioral tics, tic vocalizations, and Tourette syndrome.
The charts were then narrowed down to patients with no previous diagnosis of these conditions. Most patients were videotaped for assessment by the rest of the movement disorder neurologists in the practice. Since the end of the study inclusion period in March 2021, Dr. Olvera estimates that the clinic experienced a doubling or tripling of the number of similar patients.
In the study cohort of 10 patients, the median age at presentation was 19 years (range, 15-41 years), nine were female, the gender of the other one was unknown, and the duration of tics was 8 weeks (range, 1-24 weeks) by the time they were first seen in the clinic. Four patients reported having COVID infection before tic onset.
All exhibited motor tics and nine had vocal tics. Two were diagnosed with a purely functional neurologic disorder, four with only an organic tic disorder, and four with organic tics with a functional overlay.
“All patients, including those with organic tic disorders, had a history of anxiety and also reported worsening anxiety in the setting of the COVID pandemic,” Dr. Olvera said.
The majority of patients were on a psychotropic medication prior to coming to the clinic, and these were primarily for anxiety and depression. Three patients had a history of suicidality, often very severe and leading to hospitalization, she noted.
“In terms of our conclusions from the project, we feel that this phenotype of acute explosive onset of tic-like movements and vocalizations in this older population of adults, compared with typical organic tic disorders and Tourette syndrome, appears novel to the pandemic,” she said.
She cautioned that functional and organic tics share many characteristics and therefore may be difficult to differentiate.
COVID stress
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said the research highlights how clinicians’ understanding of particular diseases can be challenged during extraordinary events such as COVID-19 and the heightened stress it causes.
“I’m not surprised that these [disorders] might have had a spike during a stressful time as COVID,” he said.
Patients are “really scared and really anxious, they’re afraid to die, and they’re afraid that their life will be over. So they might express their psychological difficulty, their discomfort, with these calls for help that look like tics. But they’re not what we consider physiological or organic things,” he added.
Dr. Tagliati added that he doesn’t believe rapid tic onset in adults is not a complication of the coronavirus infection, but rather a consequence of psychological pressure brought on by the pandemic.
Treating underlying anxiety may be a useful approach, possibly with the support of psychiatrists, which in many cases is enough to relieve the conditions and overcome the symptoms, he noted.
However, at other times, it’s not that simple, he added. Sometimes patients “fall through the cracks between neurology and psychiatry,” Dr. Tagliati said.
Dr. Olvera and Dr. Tagliati have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large, single-center study show several cases of tic-like movements and vocalizations with abrupt onset among older adolescents and adults during the pandemic. None had a previous diagnosis of a tic disorder. Among 10 patients, two were diagnosed with a purely functional movement disorder, four with an organic tic disorder, and four with both.
“Within our movement disorders clinic specifically ... we’ve been seeing an increased number of patients with an almost explosive onset of these tic-like movements and vocalizations later in life than what is typically seen with organic tic disorders and Tourette syndrome, which is typically in school-aged children,” said study investigator Caroline Olvera, MD, Rush University Medical Center, Chicago.
“Abrupt onset of symptoms can be seen in patients with tic disorders, although this is typically quoted as less than 10%, or even 5% is more characteristic of functional neurological disorders in general and also with psychogenic tics,” she added.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Anxiety, other psychiatric conditions
Tic disorders typically start in childhood. However, the researchers observed an increase in the number of patients with abrupt onset of tic-like movements and vocalizations later in life, which is more often characteristic of functional neurological disorders.
To examine the profile, associated conditions, and risk factors in this population, the investigators conducted a thorough chart review of patients attending movement disorder clinics between March 2020, when the COVID pandemic was officially declared, and March 2021.
Patients with acute onset of tics were identified using the International Classification of Diseases codes for behavioral tics, tic vocalizations, and Tourette syndrome.
The charts were then narrowed down to patients with no previous diagnosis of these conditions. Most patients were videotaped for assessment by the rest of the movement disorder neurologists in the practice. Since the end of the study inclusion period in March 2021, Dr. Olvera estimates that the clinic experienced a doubling or tripling of the number of similar patients.
In the study cohort of 10 patients, the median age at presentation was 19 years (range, 15-41 years), nine were female, the gender of the other one was unknown, and the duration of tics was 8 weeks (range, 1-24 weeks) by the time they were first seen in the clinic. Four patients reported having COVID infection before tic onset.
All exhibited motor tics and nine had vocal tics. Two were diagnosed with a purely functional neurologic disorder, four with only an organic tic disorder, and four with organic tics with a functional overlay.
“All patients, including those with organic tic disorders, had a history of anxiety and also reported worsening anxiety in the setting of the COVID pandemic,” Dr. Olvera said.
The majority of patients were on a psychotropic medication prior to coming to the clinic, and these were primarily for anxiety and depression. Three patients had a history of suicidality, often very severe and leading to hospitalization, she noted.
“In terms of our conclusions from the project, we feel that this phenotype of acute explosive onset of tic-like movements and vocalizations in this older population of adults, compared with typical organic tic disorders and Tourette syndrome, appears novel to the pandemic,” she said.
She cautioned that functional and organic tics share many characteristics and therefore may be difficult to differentiate.
COVID stress
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said the research highlights how clinicians’ understanding of particular diseases can be challenged during extraordinary events such as COVID-19 and the heightened stress it causes.
“I’m not surprised that these [disorders] might have had a spike during a stressful time as COVID,” he said.
Patients are “really scared and really anxious, they’re afraid to die, and they’re afraid that their life will be over. So they might express their psychological difficulty, their discomfort, with these calls for help that look like tics. But they’re not what we consider physiological or organic things,” he added.
Dr. Tagliati added that he doesn’t believe rapid tic onset in adults is not a complication of the coronavirus infection, but rather a consequence of psychological pressure brought on by the pandemic.
Treating underlying anxiety may be a useful approach, possibly with the support of psychiatrists, which in many cases is enough to relieve the conditions and overcome the symptoms, he noted.
However, at other times, it’s not that simple, he added. Sometimes patients “fall through the cracks between neurology and psychiatry,” Dr. Tagliati said.
Dr. Olvera and Dr. Tagliati have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MDS VIRTUAL CONGRESS 2021
Parent-led intervention linked with decreased autism symptoms in at-risk infants
These findings, which were published in JAMA Pediatrics, were the first evidence worldwide that a preemptive intervention during infancy could lead to such a significant improvement in children’s social development, resulting in “three times fewer diagnoses of autism at age 3,” said lead author Andrew Whitehouse, PhD, in a statement.
“No trial of a preemptive infant intervention, applied prior to diagnosis, has to date shown such an effect to impact diagnostic outcomes – until now,” he said.
Study intervention is a nontraditonal approach
Dr. Whitehouse, who is professor of Autism Research at Telethon Kids and University of Western Australia, and Director of CliniKids in Perth, said the intervention is a departure from traditional approaches. “Traditionally, therapy seeks to train children to learn ‘typical’ behaviors,” he said in an email. “The difference of this therapy is that we help parents understand the unique abilities of their baby, and to use these strengths as a foundation for future development.”
Dr. Whitehouse’s study included 103 children (aged approximately 12 months), who displayed at least three of five behaviors indicating a high likelihood of ASD as defined by the Social Attention and Communication Surveillance–Revised (SACS-R) 12-month checklist. The infants were randomized to receive either usual care or the intervention, which is called the iBASIS–Video Interaction to Promote Positive Parenting (iBASIS-VIPP). Usual care was delivered by community physicians, whereas the intervention involved 10 sessions delivered at home by a trained therapist.
“The iBASIS-VIPP uses video-feedback as a means of helping parents recognize their baby’s communication cues so they can respond in a way that builds their social communication development,” Dr. Whitehouse explained in an interview. “The therapist then provides guidance to the parent as to how their baby is communicating with them, and they can communicate back to have back-and-forth conversations.”
“We know these back-and-forth conversations are crucial to support early social communication development, and are a precursor to more complex skills, such as verbal language,” he added.
Reassessment of the children at age 3 years showed a “small but enduring” benefit of the intervention, noted the authors. Children in the intervention group had a reduction in ASD symptom severity (P = .04), and reduced odds of ASD classification, compared with children receiving usual care (6.7% vs. 20.5%; odds ratio, 0.18; P = .02).
The findings provide “initial evidence of efficacy for a new clinical model that uses a specific developmentally focused intervention,” noted the authors. “The children falling below the diagnostic threshold still had developmental difficulties, but by working with each child’s unique differences, rather than trying to counter them, the therapy has effectively supported their development through the early childhood years,” noted Dr. Whitehouse in a statement.
Other research has shown benefits of new study approach
This is a “solid” study, “but, as acknowledged by the authors, the main effects are small in magnitude, and longer-term outcomes will be important to capture,” said Jessica Brian, PhD, C Psych, associate professor in the department of pediatrics at the University of Toronto, colead at the Autism Research Centre, and psychologist and clinician-investigator at Holland Bloorview Kids Rehab Hospital in Toronto.
Dr. Brian said she and her coauthors of a paper published in Autism Research and others have shown that the kind of approach used in the new study can be helpful for enhancing different areas of toddler development, but “the specific finding of reduced likelihood of a clinical ASD diagnosis is a bit different.”
The goal of reducing the likelihood of an ASD diagnosis “needs to be considered carefully, from the perspective of autism acceptance,” she added. “From an acceptance lens, the primary objective of early intervention in ASD might be better positioned as aiming to enhance or support a young child’s development, help them make developmental progress, build on their strengths, optimize outcomes, or reduce impairment. … I think the authors do a good job of balancing this perspective.”
New study shows value of parent-mediated interventions
Overall, Dr. Brian, who coauthored the Canadian Paediatric Society’s position statement on ASD diagnosis, lauded the findings as good news.
“It shows the value of using parent-mediated interventions, which are far less costly and are more resource-efficient than most therapist-delivered models.”
“In cases where parent-mediated approaches are made available to families prior to diagnosis, there is potential for strong effects, when the brain is most amenable to learning. Such models may also be an ideal fit before diagnosis, since they are less resource-intensive than therapist-delivered models, which may only be funded by governments once a diagnosis is confirmed,” she said.
“Finally, parent-mediated programs have the potential to support parents during what, for many families, is a particularly challenging time as they identify their child’s developmental differences or receive a diagnosis. Such programs have potential to increase parents’ confidence in parenting a young child with unique learning needs.”
What Dr. Brian thought was missing from the paper was acknowledgment that, “despite early developmental gains from parent-mediated interventions, it is likely that most children with ASD will need additional supports throughout development.”
This study was sponsored by the Telethon Kids Institute. Dr. Whitehouse reported no conflicts of interest. Dr. Brian codeveloped a parent-mediated intervention for toddlers with probable or confirmed ASD (the Social ABCs), for which she does not receive any royalties.
These findings, which were published in JAMA Pediatrics, were the first evidence worldwide that a preemptive intervention during infancy could lead to such a significant improvement in children’s social development, resulting in “three times fewer diagnoses of autism at age 3,” said lead author Andrew Whitehouse, PhD, in a statement.
“No trial of a preemptive infant intervention, applied prior to diagnosis, has to date shown such an effect to impact diagnostic outcomes – until now,” he said.
Study intervention is a nontraditonal approach
Dr. Whitehouse, who is professor of Autism Research at Telethon Kids and University of Western Australia, and Director of CliniKids in Perth, said the intervention is a departure from traditional approaches. “Traditionally, therapy seeks to train children to learn ‘typical’ behaviors,” he said in an email. “The difference of this therapy is that we help parents understand the unique abilities of their baby, and to use these strengths as a foundation for future development.”
Dr. Whitehouse’s study included 103 children (aged approximately 12 months), who displayed at least three of five behaviors indicating a high likelihood of ASD as defined by the Social Attention and Communication Surveillance–Revised (SACS-R) 12-month checklist. The infants were randomized to receive either usual care or the intervention, which is called the iBASIS–Video Interaction to Promote Positive Parenting (iBASIS-VIPP). Usual care was delivered by community physicians, whereas the intervention involved 10 sessions delivered at home by a trained therapist.
“The iBASIS-VIPP uses video-feedback as a means of helping parents recognize their baby’s communication cues so they can respond in a way that builds their social communication development,” Dr. Whitehouse explained in an interview. “The therapist then provides guidance to the parent as to how their baby is communicating with them, and they can communicate back to have back-and-forth conversations.”
“We know these back-and-forth conversations are crucial to support early social communication development, and are a precursor to more complex skills, such as verbal language,” he added.
Reassessment of the children at age 3 years showed a “small but enduring” benefit of the intervention, noted the authors. Children in the intervention group had a reduction in ASD symptom severity (P = .04), and reduced odds of ASD classification, compared with children receiving usual care (6.7% vs. 20.5%; odds ratio, 0.18; P = .02).
The findings provide “initial evidence of efficacy for a new clinical model that uses a specific developmentally focused intervention,” noted the authors. “The children falling below the diagnostic threshold still had developmental difficulties, but by working with each child’s unique differences, rather than trying to counter them, the therapy has effectively supported their development through the early childhood years,” noted Dr. Whitehouse in a statement.
Other research has shown benefits of new study approach
This is a “solid” study, “but, as acknowledged by the authors, the main effects are small in magnitude, and longer-term outcomes will be important to capture,” said Jessica Brian, PhD, C Psych, associate professor in the department of pediatrics at the University of Toronto, colead at the Autism Research Centre, and psychologist and clinician-investigator at Holland Bloorview Kids Rehab Hospital in Toronto.
Dr. Brian said she and her coauthors of a paper published in Autism Research and others have shown that the kind of approach used in the new study can be helpful for enhancing different areas of toddler development, but “the specific finding of reduced likelihood of a clinical ASD diagnosis is a bit different.”
The goal of reducing the likelihood of an ASD diagnosis “needs to be considered carefully, from the perspective of autism acceptance,” she added. “From an acceptance lens, the primary objective of early intervention in ASD might be better positioned as aiming to enhance or support a young child’s development, help them make developmental progress, build on their strengths, optimize outcomes, or reduce impairment. … I think the authors do a good job of balancing this perspective.”
New study shows value of parent-mediated interventions
Overall, Dr. Brian, who coauthored the Canadian Paediatric Society’s position statement on ASD diagnosis, lauded the findings as good news.
“It shows the value of using parent-mediated interventions, which are far less costly and are more resource-efficient than most therapist-delivered models.”
“In cases where parent-mediated approaches are made available to families prior to diagnosis, there is potential for strong effects, when the brain is most amenable to learning. Such models may also be an ideal fit before diagnosis, since they are less resource-intensive than therapist-delivered models, which may only be funded by governments once a diagnosis is confirmed,” she said.
“Finally, parent-mediated programs have the potential to support parents during what, for many families, is a particularly challenging time as they identify their child’s developmental differences or receive a diagnosis. Such programs have potential to increase parents’ confidence in parenting a young child with unique learning needs.”
What Dr. Brian thought was missing from the paper was acknowledgment that, “despite early developmental gains from parent-mediated interventions, it is likely that most children with ASD will need additional supports throughout development.”
This study was sponsored by the Telethon Kids Institute. Dr. Whitehouse reported no conflicts of interest. Dr. Brian codeveloped a parent-mediated intervention for toddlers with probable or confirmed ASD (the Social ABCs), for which she does not receive any royalties.
These findings, which were published in JAMA Pediatrics, were the first evidence worldwide that a preemptive intervention during infancy could lead to such a significant improvement in children’s social development, resulting in “three times fewer diagnoses of autism at age 3,” said lead author Andrew Whitehouse, PhD, in a statement.
“No trial of a preemptive infant intervention, applied prior to diagnosis, has to date shown such an effect to impact diagnostic outcomes – until now,” he said.
Study intervention is a nontraditonal approach
Dr. Whitehouse, who is professor of Autism Research at Telethon Kids and University of Western Australia, and Director of CliniKids in Perth, said the intervention is a departure from traditional approaches. “Traditionally, therapy seeks to train children to learn ‘typical’ behaviors,” he said in an email. “The difference of this therapy is that we help parents understand the unique abilities of their baby, and to use these strengths as a foundation for future development.”
Dr. Whitehouse’s study included 103 children (aged approximately 12 months), who displayed at least three of five behaviors indicating a high likelihood of ASD as defined by the Social Attention and Communication Surveillance–Revised (SACS-R) 12-month checklist. The infants were randomized to receive either usual care or the intervention, which is called the iBASIS–Video Interaction to Promote Positive Parenting (iBASIS-VIPP). Usual care was delivered by community physicians, whereas the intervention involved 10 sessions delivered at home by a trained therapist.
“The iBASIS-VIPP uses video-feedback as a means of helping parents recognize their baby’s communication cues so they can respond in a way that builds their social communication development,” Dr. Whitehouse explained in an interview. “The therapist then provides guidance to the parent as to how their baby is communicating with them, and they can communicate back to have back-and-forth conversations.”
“We know these back-and-forth conversations are crucial to support early social communication development, and are a precursor to more complex skills, such as verbal language,” he added.
Reassessment of the children at age 3 years showed a “small but enduring” benefit of the intervention, noted the authors. Children in the intervention group had a reduction in ASD symptom severity (P = .04), and reduced odds of ASD classification, compared with children receiving usual care (6.7% vs. 20.5%; odds ratio, 0.18; P = .02).
The findings provide “initial evidence of efficacy for a new clinical model that uses a specific developmentally focused intervention,” noted the authors. “The children falling below the diagnostic threshold still had developmental difficulties, but by working with each child’s unique differences, rather than trying to counter them, the therapy has effectively supported their development through the early childhood years,” noted Dr. Whitehouse in a statement.
Other research has shown benefits of new study approach
This is a “solid” study, “but, as acknowledged by the authors, the main effects are small in magnitude, and longer-term outcomes will be important to capture,” said Jessica Brian, PhD, C Psych, associate professor in the department of pediatrics at the University of Toronto, colead at the Autism Research Centre, and psychologist and clinician-investigator at Holland Bloorview Kids Rehab Hospital in Toronto.
Dr. Brian said she and her coauthors of a paper published in Autism Research and others have shown that the kind of approach used in the new study can be helpful for enhancing different areas of toddler development, but “the specific finding of reduced likelihood of a clinical ASD diagnosis is a bit different.”
The goal of reducing the likelihood of an ASD diagnosis “needs to be considered carefully, from the perspective of autism acceptance,” she added. “From an acceptance lens, the primary objective of early intervention in ASD might be better positioned as aiming to enhance or support a young child’s development, help them make developmental progress, build on their strengths, optimize outcomes, or reduce impairment. … I think the authors do a good job of balancing this perspective.”
New study shows value of parent-mediated interventions
Overall, Dr. Brian, who coauthored the Canadian Paediatric Society’s position statement on ASD diagnosis, lauded the findings as good news.
“It shows the value of using parent-mediated interventions, which are far less costly and are more resource-efficient than most therapist-delivered models.”
“In cases where parent-mediated approaches are made available to families prior to diagnosis, there is potential for strong effects, when the brain is most amenable to learning. Such models may also be an ideal fit before diagnosis, since they are less resource-intensive than therapist-delivered models, which may only be funded by governments once a diagnosis is confirmed,” she said.
“Finally, parent-mediated programs have the potential to support parents during what, for many families, is a particularly challenging time as they identify their child’s developmental differences or receive a diagnosis. Such programs have potential to increase parents’ confidence in parenting a young child with unique learning needs.”
What Dr. Brian thought was missing from the paper was acknowledgment that, “despite early developmental gains from parent-mediated interventions, it is likely that most children with ASD will need additional supports throughout development.”
This study was sponsored by the Telethon Kids Institute. Dr. Whitehouse reported no conflicts of interest. Dr. Brian codeveloped a parent-mediated intervention for toddlers with probable or confirmed ASD (the Social ABCs), for which she does not receive any royalties.
FROM JAMA PEDIATRICS
Higher than standard vitamin D dose provides no added benefits to children’s neurodevelopment
Prescribing higher doses of vitamin D may not provide any additional benefits to children’s brain development, a new study suggests.
New research published online in JAMA found that there were no differences in children’s developmental milestones or social-emotional problems when given a higher daily dose of 1,200 IU of vitamin D versus the standard dose of 400 IU.
Although past studies have looked into the relationship between vitamin D and neurodevelopment in children, the findings were inconsistent. A 2019 study published in Psychoneuroendocrinology found that vitamin D deficiency could be a biological risk factor for psychiatric disorders and that vitamin D acts as a neurosteroid with direct effect on brain development. However, a 2021 study published in Global Pediatric Health found no significant association between vitamin D levels and neurodevelopmental status in children at 2 years old.
Researchers of the current study said they expected to find a positive association between higher vitamin D levels and neurodevelopment.
“Our results highlight that the current recommendations, set forth mainly on the basis of bone health, also support healthy brain development,” said study author Kati Heinonen, PhD, associate professor of psychology and welfare sciences at Tampere (Finland) University. “Our results also point out that higher than currently recommended levels do not add to the benefits received from the vitamin D supplements.”
For the study, Dr. Heinonen and colleagues analyzed data from a double-blind, randomized clinical trial involving healthy infants born full-term between Jan. 1, 2013, and June 30, 2014, at a maternity hospital in Helsinki. They got follow-up information on 404 infants who were randomized to receive 400 IU of oral vitamin D supplements daily and 397 infants who received 1,200 IU of vitamin D supplements from 2 weeks to 24 months of age.
Researchers found no differences between the 400-IU group and the 1,200-IU group in the mean adjusted Ages and Stages Questionnaire total score at 12 months, a questionnaire that’s used to measure communication, problem solving, gross motor skills, fine motor skills, and personal and social skills. However, they did find that children receiving 1,200 IU of vitamin D supplementation had better developmental milestone scores in communication and problem-solving skills at 12 months.
Furthermore, they also found that higher vitamin D concentrations were associated with fewer sleeping problems at 24 months.
The researcher’s findings did not surprise Francis E. Rushton Jr., MD, a clinical professor of pediatrics at the University of South Carolina, Columbia, who was not involved in the study. “This study reveals that more is not always better,” Dr. Rushton said in an interview.
Dr. Rushton, who is also the medical director of the Quality Through Innovation in Pediatrics network, said other ways to enhance early brain development include initiatives like infant home visitation and language enrichment programs like Reach Out and Read.
Dr. Heinonen noted that the study’s findings might be different if it had been conducted on infants from a different country.
“We have to remember that the participants were from northern European countries where several food products are also fortified by vitamin D,” Dr. Heinonen explained. “Thus, direct recommendations of the amount of the supplementation given for children from 2 weeks to 2 years in other countries should not be done on the basis of our study.”
Researchers also observed that the children receiving 1,200 IU of vitamin D supplementation had a risk of scoring higher on the externalizing symptoms scale at 24 months, meaning these infants are more likely to lose their temper and become physically aggressive.
“We could not fully exclude potential disadvantageous effects of higher doses. Even if minimal, the potential nonbeneficial effects of higher than standard doses warrant further studies,” she said.
Researchers said more studies are needed that follow children up to school age and adolescence, when higher cognitive abilities develop, to understand the long-term outcomes of early vitamin D supplementation.
Prescribing higher doses of vitamin D may not provide any additional benefits to children’s brain development, a new study suggests.
New research published online in JAMA found that there were no differences in children’s developmental milestones or social-emotional problems when given a higher daily dose of 1,200 IU of vitamin D versus the standard dose of 400 IU.
Although past studies have looked into the relationship between vitamin D and neurodevelopment in children, the findings were inconsistent. A 2019 study published in Psychoneuroendocrinology found that vitamin D deficiency could be a biological risk factor for psychiatric disorders and that vitamin D acts as a neurosteroid with direct effect on brain development. However, a 2021 study published in Global Pediatric Health found no significant association between vitamin D levels and neurodevelopmental status in children at 2 years old.
Researchers of the current study said they expected to find a positive association between higher vitamin D levels and neurodevelopment.
“Our results highlight that the current recommendations, set forth mainly on the basis of bone health, also support healthy brain development,” said study author Kati Heinonen, PhD, associate professor of psychology and welfare sciences at Tampere (Finland) University. “Our results also point out that higher than currently recommended levels do not add to the benefits received from the vitamin D supplements.”
For the study, Dr. Heinonen and colleagues analyzed data from a double-blind, randomized clinical trial involving healthy infants born full-term between Jan. 1, 2013, and June 30, 2014, at a maternity hospital in Helsinki. They got follow-up information on 404 infants who were randomized to receive 400 IU of oral vitamin D supplements daily and 397 infants who received 1,200 IU of vitamin D supplements from 2 weeks to 24 months of age.
Researchers found no differences between the 400-IU group and the 1,200-IU group in the mean adjusted Ages and Stages Questionnaire total score at 12 months, a questionnaire that’s used to measure communication, problem solving, gross motor skills, fine motor skills, and personal and social skills. However, they did find that children receiving 1,200 IU of vitamin D supplementation had better developmental milestone scores in communication and problem-solving skills at 12 months.
Furthermore, they also found that higher vitamin D concentrations were associated with fewer sleeping problems at 24 months.
The researcher’s findings did not surprise Francis E. Rushton Jr., MD, a clinical professor of pediatrics at the University of South Carolina, Columbia, who was not involved in the study. “This study reveals that more is not always better,” Dr. Rushton said in an interview.
Dr. Rushton, who is also the medical director of the Quality Through Innovation in Pediatrics network, said other ways to enhance early brain development include initiatives like infant home visitation and language enrichment programs like Reach Out and Read.
Dr. Heinonen noted that the study’s findings might be different if it had been conducted on infants from a different country.
“We have to remember that the participants were from northern European countries where several food products are also fortified by vitamin D,” Dr. Heinonen explained. “Thus, direct recommendations of the amount of the supplementation given for children from 2 weeks to 2 years in other countries should not be done on the basis of our study.”
Researchers also observed that the children receiving 1,200 IU of vitamin D supplementation had a risk of scoring higher on the externalizing symptoms scale at 24 months, meaning these infants are more likely to lose their temper and become physically aggressive.
“We could not fully exclude potential disadvantageous effects of higher doses. Even if minimal, the potential nonbeneficial effects of higher than standard doses warrant further studies,” she said.
Researchers said more studies are needed that follow children up to school age and adolescence, when higher cognitive abilities develop, to understand the long-term outcomes of early vitamin D supplementation.
Prescribing higher doses of vitamin D may not provide any additional benefits to children’s brain development, a new study suggests.
New research published online in JAMA found that there were no differences in children’s developmental milestones or social-emotional problems when given a higher daily dose of 1,200 IU of vitamin D versus the standard dose of 400 IU.
Although past studies have looked into the relationship between vitamin D and neurodevelopment in children, the findings were inconsistent. A 2019 study published in Psychoneuroendocrinology found that vitamin D deficiency could be a biological risk factor for psychiatric disorders and that vitamin D acts as a neurosteroid with direct effect on brain development. However, a 2021 study published in Global Pediatric Health found no significant association between vitamin D levels and neurodevelopmental status in children at 2 years old.
Researchers of the current study said they expected to find a positive association between higher vitamin D levels and neurodevelopment.
“Our results highlight that the current recommendations, set forth mainly on the basis of bone health, also support healthy brain development,” said study author Kati Heinonen, PhD, associate professor of psychology and welfare sciences at Tampere (Finland) University. “Our results also point out that higher than currently recommended levels do not add to the benefits received from the vitamin D supplements.”
For the study, Dr. Heinonen and colleagues analyzed data from a double-blind, randomized clinical trial involving healthy infants born full-term between Jan. 1, 2013, and June 30, 2014, at a maternity hospital in Helsinki. They got follow-up information on 404 infants who were randomized to receive 400 IU of oral vitamin D supplements daily and 397 infants who received 1,200 IU of vitamin D supplements from 2 weeks to 24 months of age.
Researchers found no differences between the 400-IU group and the 1,200-IU group in the mean adjusted Ages and Stages Questionnaire total score at 12 months, a questionnaire that’s used to measure communication, problem solving, gross motor skills, fine motor skills, and personal and social skills. However, they did find that children receiving 1,200 IU of vitamin D supplementation had better developmental milestone scores in communication and problem-solving skills at 12 months.
Furthermore, they also found that higher vitamin D concentrations were associated with fewer sleeping problems at 24 months.
The researcher’s findings did not surprise Francis E. Rushton Jr., MD, a clinical professor of pediatrics at the University of South Carolina, Columbia, who was not involved in the study. “This study reveals that more is not always better,” Dr. Rushton said in an interview.
Dr. Rushton, who is also the medical director of the Quality Through Innovation in Pediatrics network, said other ways to enhance early brain development include initiatives like infant home visitation and language enrichment programs like Reach Out and Read.
Dr. Heinonen noted that the study’s findings might be different if it had been conducted on infants from a different country.
“We have to remember that the participants were from northern European countries where several food products are also fortified by vitamin D,” Dr. Heinonen explained. “Thus, direct recommendations of the amount of the supplementation given for children from 2 weeks to 2 years in other countries should not be done on the basis of our study.”
Researchers also observed that the children receiving 1,200 IU of vitamin D supplementation had a risk of scoring higher on the externalizing symptoms scale at 24 months, meaning these infants are more likely to lose their temper and become physically aggressive.
“We could not fully exclude potential disadvantageous effects of higher doses. Even if minimal, the potential nonbeneficial effects of higher than standard doses warrant further studies,” she said.
Researchers said more studies are needed that follow children up to school age and adolescence, when higher cognitive abilities develop, to understand the long-term outcomes of early vitamin D supplementation.
FROM JAMA
Assessing headache severity via migraine symptoms can help predict outcomes
according to an analysis of data from thousands of headache sufferers who recorded variables like pain and duration in a daily digital diary.
“Our hope is that this work serves as foundational basis for better understanding the complexity of headache as a symptom-based condition,” James S. McGinley, PhD, of Vector Psychometric Group in Chapel Hill, N.C., and coauthors wrote. The study was published in Cephalalgia.
To evaluate whether keeping track of daily headache features can produce a useful, predictive score, the researchers reviewed data from migraine patients that were collected via N1‑Headache, a commercial digital health platform. Ultimately, information from 4,380 adults with a self-reported migraine diagnosis was analyzed; the sample was 90% female and their mean age was 37 years. Study participants reported an average of 33 headaches per month over the last 3 months. Nine patient-reported variables were initially considered in calculating the Headache Day Severity (HDS) score: pain intensity, headache duration, aura, pulsating/throbbing pain, unilateral pain, pain aggravation by activity, nausea/vomiting, photophobia, and phonophobia.
After determining that unilateral pain was not a meaningful variable, the researchers’ model found that, for every 1 standard deviation increase in HDS, the patient’s odds of physician visit increased by 71% (odds ratio, 1.71; 95% confidence interval, 1.32-2.21) and the odds of an ED visit increased by 342% (OR, 4.42; 95% CI, 2.23-7.60). They also found that the likelihood of missed work or school increased by 190% (OR, 2.90; 95% CI, 2.56-3.29), the chances of missing household work increased by 237% (OR, 3.37; 95% CI, 3.06-3.72) and the odds of missing other leisure or social activity increased by 228% (OR, 3.28; 95% CI, 2.97-3.64).
Tracking multiple variables
“We encourage all of our patients to monitor their headaches; there are just too many variables to try to keep it in your head,” Robert Cowan, MD, professor of neurology and chief of the division of headache medicine at Stanford (Calif.) University, said in an interview. He referenced a previous study from the University of Washington where patients were asked to track their headaches; that data was then compared against their self-reported headaches at a quarterly physician visit.
“What they found was there was absolutely no correlation with reported frequency of headache at the visit and what was seen in the tracker,” he said. “If patients had a headache in the previous 3 days before their visit, they felt that their headaches were poorly controlled. If they hadn’t, they thought their headaches were under good control. So the value of tracking is pretty clear.”
He added that, while not every headache sufferer needs to track their daily routines and symptoms, once those symptoms interfere with your life on a day-to-day basis, it’s probably time to consider keeping tabs on yourself with a tool of some sort. And while this study’s calculated HDS score supports the idea of migraine’s complexity, it also leaves unanswered the question of how to treat patients with severe symptoms.
“Frequently,” he said, “we’ll see patients who say: ‘I can deal with the pain, but the nausea makes it impossible to work, or the light sensitivity makes it impossible to go outside.’ The big question within the headache community is, can you treat migraine and have it address the whole spectrum, from dizziness to light sensitivity to sound sensitivity to vertigo, or should you be going after individual symptoms? That’s a controversy that rages on; I think most of us go for a combination. We’re in a polypharmacy phase: ‘If nausea is a big problem, take this, but we also try to prevent the whole migraine complex, so take this as well.’ ”
The authors acknowledged their study’s limitations, including the inability to determine how many participants’ migraines were formally diagnosed by a trained medical professional and the lack of generalizability of data from a convenience sample, though they added that patients who independently track their own headaches “may be representative of those who would participate in a clinical trial.” In addition, as seven of the nine features were collected in N1‑Headache on a yes/no scale, they recognized that “increasing the number of response options for each item may improve our ability to measure HDS.”
The study was funded by Amgen through the Competitive Grant Program in Migraine Research. The authors declared several potential conflicts of interest, including receiving funding, research support, salary, and honoraria from various pharmaceutical companies.
according to an analysis of data from thousands of headache sufferers who recorded variables like pain and duration in a daily digital diary.
“Our hope is that this work serves as foundational basis for better understanding the complexity of headache as a symptom-based condition,” James S. McGinley, PhD, of Vector Psychometric Group in Chapel Hill, N.C., and coauthors wrote. The study was published in Cephalalgia.
To evaluate whether keeping track of daily headache features can produce a useful, predictive score, the researchers reviewed data from migraine patients that were collected via N1‑Headache, a commercial digital health platform. Ultimately, information from 4,380 adults with a self-reported migraine diagnosis was analyzed; the sample was 90% female and their mean age was 37 years. Study participants reported an average of 33 headaches per month over the last 3 months. Nine patient-reported variables were initially considered in calculating the Headache Day Severity (HDS) score: pain intensity, headache duration, aura, pulsating/throbbing pain, unilateral pain, pain aggravation by activity, nausea/vomiting, photophobia, and phonophobia.
After determining that unilateral pain was not a meaningful variable, the researchers’ model found that, for every 1 standard deviation increase in HDS, the patient’s odds of physician visit increased by 71% (odds ratio, 1.71; 95% confidence interval, 1.32-2.21) and the odds of an ED visit increased by 342% (OR, 4.42; 95% CI, 2.23-7.60). They also found that the likelihood of missed work or school increased by 190% (OR, 2.90; 95% CI, 2.56-3.29), the chances of missing household work increased by 237% (OR, 3.37; 95% CI, 3.06-3.72) and the odds of missing other leisure or social activity increased by 228% (OR, 3.28; 95% CI, 2.97-3.64).
Tracking multiple variables
“We encourage all of our patients to monitor their headaches; there are just too many variables to try to keep it in your head,” Robert Cowan, MD, professor of neurology and chief of the division of headache medicine at Stanford (Calif.) University, said in an interview. He referenced a previous study from the University of Washington where patients were asked to track their headaches; that data was then compared against their self-reported headaches at a quarterly physician visit.
“What they found was there was absolutely no correlation with reported frequency of headache at the visit and what was seen in the tracker,” he said. “If patients had a headache in the previous 3 days before their visit, they felt that their headaches were poorly controlled. If they hadn’t, they thought their headaches were under good control. So the value of tracking is pretty clear.”
He added that, while not every headache sufferer needs to track their daily routines and symptoms, once those symptoms interfere with your life on a day-to-day basis, it’s probably time to consider keeping tabs on yourself with a tool of some sort. And while this study’s calculated HDS score supports the idea of migraine’s complexity, it also leaves unanswered the question of how to treat patients with severe symptoms.
“Frequently,” he said, “we’ll see patients who say: ‘I can deal with the pain, but the nausea makes it impossible to work, or the light sensitivity makes it impossible to go outside.’ The big question within the headache community is, can you treat migraine and have it address the whole spectrum, from dizziness to light sensitivity to sound sensitivity to vertigo, or should you be going after individual symptoms? That’s a controversy that rages on; I think most of us go for a combination. We’re in a polypharmacy phase: ‘If nausea is a big problem, take this, but we also try to prevent the whole migraine complex, so take this as well.’ ”
The authors acknowledged their study’s limitations, including the inability to determine how many participants’ migraines were formally diagnosed by a trained medical professional and the lack of generalizability of data from a convenience sample, though they added that patients who independently track their own headaches “may be representative of those who would participate in a clinical trial.” In addition, as seven of the nine features were collected in N1‑Headache on a yes/no scale, they recognized that “increasing the number of response options for each item may improve our ability to measure HDS.”
The study was funded by Amgen through the Competitive Grant Program in Migraine Research. The authors declared several potential conflicts of interest, including receiving funding, research support, salary, and honoraria from various pharmaceutical companies.
according to an analysis of data from thousands of headache sufferers who recorded variables like pain and duration in a daily digital diary.
“Our hope is that this work serves as foundational basis for better understanding the complexity of headache as a symptom-based condition,” James S. McGinley, PhD, of Vector Psychometric Group in Chapel Hill, N.C., and coauthors wrote. The study was published in Cephalalgia.
To evaluate whether keeping track of daily headache features can produce a useful, predictive score, the researchers reviewed data from migraine patients that were collected via N1‑Headache, a commercial digital health platform. Ultimately, information from 4,380 adults with a self-reported migraine diagnosis was analyzed; the sample was 90% female and their mean age was 37 years. Study participants reported an average of 33 headaches per month over the last 3 months. Nine patient-reported variables were initially considered in calculating the Headache Day Severity (HDS) score: pain intensity, headache duration, aura, pulsating/throbbing pain, unilateral pain, pain aggravation by activity, nausea/vomiting, photophobia, and phonophobia.
After determining that unilateral pain was not a meaningful variable, the researchers’ model found that, for every 1 standard deviation increase in HDS, the patient’s odds of physician visit increased by 71% (odds ratio, 1.71; 95% confidence interval, 1.32-2.21) and the odds of an ED visit increased by 342% (OR, 4.42; 95% CI, 2.23-7.60). They also found that the likelihood of missed work or school increased by 190% (OR, 2.90; 95% CI, 2.56-3.29), the chances of missing household work increased by 237% (OR, 3.37; 95% CI, 3.06-3.72) and the odds of missing other leisure or social activity increased by 228% (OR, 3.28; 95% CI, 2.97-3.64).
Tracking multiple variables
“We encourage all of our patients to monitor their headaches; there are just too many variables to try to keep it in your head,” Robert Cowan, MD, professor of neurology and chief of the division of headache medicine at Stanford (Calif.) University, said in an interview. He referenced a previous study from the University of Washington where patients were asked to track their headaches; that data was then compared against their self-reported headaches at a quarterly physician visit.
“What they found was there was absolutely no correlation with reported frequency of headache at the visit and what was seen in the tracker,” he said. “If patients had a headache in the previous 3 days before their visit, they felt that their headaches were poorly controlled. If they hadn’t, they thought their headaches were under good control. So the value of tracking is pretty clear.”
He added that, while not every headache sufferer needs to track their daily routines and symptoms, once those symptoms interfere with your life on a day-to-day basis, it’s probably time to consider keeping tabs on yourself with a tool of some sort. And while this study’s calculated HDS score supports the idea of migraine’s complexity, it also leaves unanswered the question of how to treat patients with severe symptoms.
“Frequently,” he said, “we’ll see patients who say: ‘I can deal with the pain, but the nausea makes it impossible to work, or the light sensitivity makes it impossible to go outside.’ The big question within the headache community is, can you treat migraine and have it address the whole spectrum, from dizziness to light sensitivity to sound sensitivity to vertigo, or should you be going after individual symptoms? That’s a controversy that rages on; I think most of us go for a combination. We’re in a polypharmacy phase: ‘If nausea is a big problem, take this, but we also try to prevent the whole migraine complex, so take this as well.’ ”
The authors acknowledged their study’s limitations, including the inability to determine how many participants’ migraines were formally diagnosed by a trained medical professional and the lack of generalizability of data from a convenience sample, though they added that patients who independently track their own headaches “may be representative of those who would participate in a clinical trial.” In addition, as seven of the nine features were collected in N1‑Headache on a yes/no scale, they recognized that “increasing the number of response options for each item may improve our ability to measure HDS.”
The study was funded by Amgen through the Competitive Grant Program in Migraine Research. The authors declared several potential conflicts of interest, including receiving funding, research support, salary, and honoraria from various pharmaceutical companies.
FROM CEPHALALGIA
Aspirin and heparin increase bleeding risk during EVT
Treatment with acetylsalicylic acid (ASA) or heparin is associated with an increased risk for symptomatic intracranial hemorrhage (sICH) in patients with ischemic stroke who are undergoing endovascular therapy (EVT), new data show.
In this population, ASA and heparin are each associated with an approximately doubled risk for sICH when administered during EVT.
“We did not find any evidence for a beneficial effect on functional outcome,” investigator Wouter van der Steen, MD, research physician and PhD student at Erasmus University Medical Center, Rotterdam, the Netherlands, told this news organization. The possibility that a positive effect would be observed if the trial were continued was considered negligible, he added.
The researchers stopped the trial for safety reasons and recommend avoiding the evaluated dosages of both medications during EVT for ischemic stroke, said Dr. van der Steen.
He presented the findings from the MR CLEAN-MED trial at the European Stroke Organisation Conference (ESOC) 2021, which was held online.
Trial stopped for safety
Previous research has supported the safety and efficacy of EVT for ischemic stroke. Still, more than 30% of patients do not recover, despite fast and complete recanalization. Incomplete microvascular reperfusion (IMR) could explain this incomplete recovery, the researchers note.
Microthrombi, which occlude distal vessels, and neutrophil extracellular traps can cause IMR. This problem can be reduced through treatment with ASA, which has an antithrombotic effect, or with heparin, which dissolves neutrophil extracellular traps, they add. Although these drugs are associated with good clinical outcomes, they entail an increased risk for sICH.
The investigators conducted the multicenter, randomized controlled MR CLEAN-MED trial to evaluate the effect of intravenous (IV) ASA and heparin, alone or in combination, during EVT for acute ischemic stroke. Treatment was open label, but outcome assessment was blinded. Eligible participants were adults with a National Institutes of Health Stroke Scale (NIHSS) score of greater than or equal to 2 and an anterior circulation large-vessel occlusion for whom EVT could be initiated in fewer than 6 hours.
Investigators randomly assigned patients to receive or not to receive ASA. Within each of these two treatment groups, patients were randomly assigned to receive no heparin, low-dose heparin, or moderate-dose heparin.
ASA was given in a loading dose of 300 mg. Patients who were given low-dose heparin received a loading dose of 5,000 IU followed by 500 IU/h for 6 hours. Patients who received moderate-dose heparin were given a loading dose of 5,000 IU followed by 1,250 IU/h for 6 hours.
The study’s primary outcome was Modified Rankin Scale (mRS) score at 90 days. Secondary outcomes were NIHSS score at 24 hours, NIHSS score at 5 to 7 days, and recanalization grade at 24 hours on CT angiography or MRI. The primary safety outcomes were sICH and death within 90 days.
An independent, unblinded data and safety monitoring board (DSMB) assessed the risk for the primary safety outcomes throughout the trial. The board performed interim analyses of safety and efficacy for every 300 patients.
After the fourth safety assessment, the DSMB recommended that enrollment in the moderate-dose heparin arm be discontinued for safety reasons. Enrollment in other arms continued.
After the second interim analysis, the DSMB advised that the trial steering committee be unblinded to decide whether to stop or continue the trial. The steering committee decided to stop the trial for reasons of safety.
Increased risk for sICH
In all, 628 patients were included in the study. The ASA groups included 310 patients, and the no-ASA groups included 318 patients. In all, 332 participants received heparin, and 296 received no heparin.
The demographic characteristics were well balanced between groups. The population’s median age was 73 years, and about 53% were men. The median baseline NIHSS score was approximately 15. About 74% of patients received IV thrombolysis. The median baseline Alberta Stroke Program Early CT Scan score was 9.
The investigators observed a slight shift toward worse outcome in the ASA group, compared with the no-ASA group (adjusted OR, 0.91). In addition, the ASA group had a significantly increased risk for sICH, compared with the no-ASA group (14% vs. 7.2%; aOR, 1.95).
Patients in the ASA group were less likely to have good functional outcome (mRS of 0 to 2; aOR, 0.76), and the mortality rate tended to be higher.
The researchers found a nonsignificant shift toward a worse functional outcome in the heparin group, compared with the no-heparin group (aOR, 0.81). The risk for sICH was significantly higher in the heparin group, compared with the no-heparin group (13% vs. 7.4%; aOR, 2.00).
Patients in the heparin group were also less likely to have a good functional outcome (aOR, 0.78), and there was a nonsignificant increase in risk for death among those patients.
The rate of sICH was 11% in the group that received low-dose heparin; it was 26% in the group that received moderate-dose heparin (aOR, 6.05). The mortality rate was 23% in the low-dose group and 47% in the moderate-dose group (aOR, 5.45).
There was no significant interaction between ASA and heparin on the primary outcome and on sICH occurrence.
‘A unique trial’
“MR CLEAN-MED is a unique trial because it investigated a widely used treatment but until now without any proof of effectiveness,” said Dr. van der Steen. The researchers expect that their findings will have a strong impact on the management of patients with acute ischemic stroke. They suggest that the administration of antithrombotic agents during EVT be avoided.
“We consider it probable that the increased risk of sICH explains at least a part of the nonsignificant shift towards a worse functional outcome,” co-investigator Bob Roozenbeek, MD, PhD, a neurologist at the Erasmus Medical Center, said in an interview. “However, to make more definite statements, we will have to do more in-depth analyses.”
It remains unclear whether the periprocedural use of lower dosages of antithrombotic agents or of a single bolus of heparin could be safe and effective, said Dr. van der Steen.
To gain insight into these questions, the investigators will evaluate the effect of the medications and dosages examined in this trial on primary hemostasis and coagulation activity in the trial population. They also plan to examine the effect of primary hemostasis and coagulation activity on risk for sICH and functional outcome.
Enhancing the effectiveness of thrombectomy for acute ischemic stroke continues to be an important goal for stroke therapy, said Mark Fisher, MD, professor of neurology and pathology and laboratory medicine at the University of California, Irvine, who commented on the findings for this news organization.
At least three strategies are available: The use of ancillary antithrombotic medications, neuroprotection, and modulation of the vasoconstrictive properties of the microcirculation.
“Results of MR CLEAN-MED argue against the antithrombotic strategy,” said Dr. Fisher. “The alternate strategies remain viable, and results of interventions using those approaches are awaited with great interest.”
The study was funded by the CONTRAST consortium, which is supported by the Netherlands Cardiovascular Research Initiative and the Brain Foundation Netherlands. Funding also was provided by Stryker, Medtronic, and Cerenovus. Dr. van der Steen and Dr. Fisher have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment with acetylsalicylic acid (ASA) or heparin is associated with an increased risk for symptomatic intracranial hemorrhage (sICH) in patients with ischemic stroke who are undergoing endovascular therapy (EVT), new data show.
In this population, ASA and heparin are each associated with an approximately doubled risk for sICH when administered during EVT.
“We did not find any evidence for a beneficial effect on functional outcome,” investigator Wouter van der Steen, MD, research physician and PhD student at Erasmus University Medical Center, Rotterdam, the Netherlands, told this news organization. The possibility that a positive effect would be observed if the trial were continued was considered negligible, he added.
The researchers stopped the trial for safety reasons and recommend avoiding the evaluated dosages of both medications during EVT for ischemic stroke, said Dr. van der Steen.
He presented the findings from the MR CLEAN-MED trial at the European Stroke Organisation Conference (ESOC) 2021, which was held online.
Trial stopped for safety
Previous research has supported the safety and efficacy of EVT for ischemic stroke. Still, more than 30% of patients do not recover, despite fast and complete recanalization. Incomplete microvascular reperfusion (IMR) could explain this incomplete recovery, the researchers note.
Microthrombi, which occlude distal vessels, and neutrophil extracellular traps can cause IMR. This problem can be reduced through treatment with ASA, which has an antithrombotic effect, or with heparin, which dissolves neutrophil extracellular traps, they add. Although these drugs are associated with good clinical outcomes, they entail an increased risk for sICH.
The investigators conducted the multicenter, randomized controlled MR CLEAN-MED trial to evaluate the effect of intravenous (IV) ASA and heparin, alone or in combination, during EVT for acute ischemic stroke. Treatment was open label, but outcome assessment was blinded. Eligible participants were adults with a National Institutes of Health Stroke Scale (NIHSS) score of greater than or equal to 2 and an anterior circulation large-vessel occlusion for whom EVT could be initiated in fewer than 6 hours.
Investigators randomly assigned patients to receive or not to receive ASA. Within each of these two treatment groups, patients were randomly assigned to receive no heparin, low-dose heparin, or moderate-dose heparin.
ASA was given in a loading dose of 300 mg. Patients who were given low-dose heparin received a loading dose of 5,000 IU followed by 500 IU/h for 6 hours. Patients who received moderate-dose heparin were given a loading dose of 5,000 IU followed by 1,250 IU/h for 6 hours.
The study’s primary outcome was Modified Rankin Scale (mRS) score at 90 days. Secondary outcomes were NIHSS score at 24 hours, NIHSS score at 5 to 7 days, and recanalization grade at 24 hours on CT angiography or MRI. The primary safety outcomes were sICH and death within 90 days.
An independent, unblinded data and safety monitoring board (DSMB) assessed the risk for the primary safety outcomes throughout the trial. The board performed interim analyses of safety and efficacy for every 300 patients.
After the fourth safety assessment, the DSMB recommended that enrollment in the moderate-dose heparin arm be discontinued for safety reasons. Enrollment in other arms continued.
After the second interim analysis, the DSMB advised that the trial steering committee be unblinded to decide whether to stop or continue the trial. The steering committee decided to stop the trial for reasons of safety.
Increased risk for sICH
In all, 628 patients were included in the study. The ASA groups included 310 patients, and the no-ASA groups included 318 patients. In all, 332 participants received heparin, and 296 received no heparin.
The demographic characteristics were well balanced between groups. The population’s median age was 73 years, and about 53% were men. The median baseline NIHSS score was approximately 15. About 74% of patients received IV thrombolysis. The median baseline Alberta Stroke Program Early CT Scan score was 9.
The investigators observed a slight shift toward worse outcome in the ASA group, compared with the no-ASA group (adjusted OR, 0.91). In addition, the ASA group had a significantly increased risk for sICH, compared with the no-ASA group (14% vs. 7.2%; aOR, 1.95).
Patients in the ASA group were less likely to have good functional outcome (mRS of 0 to 2; aOR, 0.76), and the mortality rate tended to be higher.
The researchers found a nonsignificant shift toward a worse functional outcome in the heparin group, compared with the no-heparin group (aOR, 0.81). The risk for sICH was significantly higher in the heparin group, compared with the no-heparin group (13% vs. 7.4%; aOR, 2.00).
Patients in the heparin group were also less likely to have a good functional outcome (aOR, 0.78), and there was a nonsignificant increase in risk for death among those patients.
The rate of sICH was 11% in the group that received low-dose heparin; it was 26% in the group that received moderate-dose heparin (aOR, 6.05). The mortality rate was 23% in the low-dose group and 47% in the moderate-dose group (aOR, 5.45).
There was no significant interaction between ASA and heparin on the primary outcome and on sICH occurrence.
‘A unique trial’
“MR CLEAN-MED is a unique trial because it investigated a widely used treatment but until now without any proof of effectiveness,” said Dr. van der Steen. The researchers expect that their findings will have a strong impact on the management of patients with acute ischemic stroke. They suggest that the administration of antithrombotic agents during EVT be avoided.
“We consider it probable that the increased risk of sICH explains at least a part of the nonsignificant shift towards a worse functional outcome,” co-investigator Bob Roozenbeek, MD, PhD, a neurologist at the Erasmus Medical Center, said in an interview. “However, to make more definite statements, we will have to do more in-depth analyses.”
It remains unclear whether the periprocedural use of lower dosages of antithrombotic agents or of a single bolus of heparin could be safe and effective, said Dr. van der Steen.
To gain insight into these questions, the investigators will evaluate the effect of the medications and dosages examined in this trial on primary hemostasis and coagulation activity in the trial population. They also plan to examine the effect of primary hemostasis and coagulation activity on risk for sICH and functional outcome.
Enhancing the effectiveness of thrombectomy for acute ischemic stroke continues to be an important goal for stroke therapy, said Mark Fisher, MD, professor of neurology and pathology and laboratory medicine at the University of California, Irvine, who commented on the findings for this news organization.
At least three strategies are available: The use of ancillary antithrombotic medications, neuroprotection, and modulation of the vasoconstrictive properties of the microcirculation.
“Results of MR CLEAN-MED argue against the antithrombotic strategy,” said Dr. Fisher. “The alternate strategies remain viable, and results of interventions using those approaches are awaited with great interest.”
The study was funded by the CONTRAST consortium, which is supported by the Netherlands Cardiovascular Research Initiative and the Brain Foundation Netherlands. Funding also was provided by Stryker, Medtronic, and Cerenovus. Dr. van der Steen and Dr. Fisher have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment with acetylsalicylic acid (ASA) or heparin is associated with an increased risk for symptomatic intracranial hemorrhage (sICH) in patients with ischemic stroke who are undergoing endovascular therapy (EVT), new data show.
In this population, ASA and heparin are each associated with an approximately doubled risk for sICH when administered during EVT.
“We did not find any evidence for a beneficial effect on functional outcome,” investigator Wouter van der Steen, MD, research physician and PhD student at Erasmus University Medical Center, Rotterdam, the Netherlands, told this news organization. The possibility that a positive effect would be observed if the trial were continued was considered negligible, he added.
The researchers stopped the trial for safety reasons and recommend avoiding the evaluated dosages of both medications during EVT for ischemic stroke, said Dr. van der Steen.
He presented the findings from the MR CLEAN-MED trial at the European Stroke Organisation Conference (ESOC) 2021, which was held online.
Trial stopped for safety
Previous research has supported the safety and efficacy of EVT for ischemic stroke. Still, more than 30% of patients do not recover, despite fast and complete recanalization. Incomplete microvascular reperfusion (IMR) could explain this incomplete recovery, the researchers note.
Microthrombi, which occlude distal vessels, and neutrophil extracellular traps can cause IMR. This problem can be reduced through treatment with ASA, which has an antithrombotic effect, or with heparin, which dissolves neutrophil extracellular traps, they add. Although these drugs are associated with good clinical outcomes, they entail an increased risk for sICH.
The investigators conducted the multicenter, randomized controlled MR CLEAN-MED trial to evaluate the effect of intravenous (IV) ASA and heparin, alone or in combination, during EVT for acute ischemic stroke. Treatment was open label, but outcome assessment was blinded. Eligible participants were adults with a National Institutes of Health Stroke Scale (NIHSS) score of greater than or equal to 2 and an anterior circulation large-vessel occlusion for whom EVT could be initiated in fewer than 6 hours.
Investigators randomly assigned patients to receive or not to receive ASA. Within each of these two treatment groups, patients were randomly assigned to receive no heparin, low-dose heparin, or moderate-dose heparin.
ASA was given in a loading dose of 300 mg. Patients who were given low-dose heparin received a loading dose of 5,000 IU followed by 500 IU/h for 6 hours. Patients who received moderate-dose heparin were given a loading dose of 5,000 IU followed by 1,250 IU/h for 6 hours.
The study’s primary outcome was Modified Rankin Scale (mRS) score at 90 days. Secondary outcomes were NIHSS score at 24 hours, NIHSS score at 5 to 7 days, and recanalization grade at 24 hours on CT angiography or MRI. The primary safety outcomes were sICH and death within 90 days.
An independent, unblinded data and safety monitoring board (DSMB) assessed the risk for the primary safety outcomes throughout the trial. The board performed interim analyses of safety and efficacy for every 300 patients.
After the fourth safety assessment, the DSMB recommended that enrollment in the moderate-dose heparin arm be discontinued for safety reasons. Enrollment in other arms continued.
After the second interim analysis, the DSMB advised that the trial steering committee be unblinded to decide whether to stop or continue the trial. The steering committee decided to stop the trial for reasons of safety.
Increased risk for sICH
In all, 628 patients were included in the study. The ASA groups included 310 patients, and the no-ASA groups included 318 patients. In all, 332 participants received heparin, and 296 received no heparin.
The demographic characteristics were well balanced between groups. The population’s median age was 73 years, and about 53% were men. The median baseline NIHSS score was approximately 15. About 74% of patients received IV thrombolysis. The median baseline Alberta Stroke Program Early CT Scan score was 9.
The investigators observed a slight shift toward worse outcome in the ASA group, compared with the no-ASA group (adjusted OR, 0.91). In addition, the ASA group had a significantly increased risk for sICH, compared with the no-ASA group (14% vs. 7.2%; aOR, 1.95).
Patients in the ASA group were less likely to have good functional outcome (mRS of 0 to 2; aOR, 0.76), and the mortality rate tended to be higher.
The researchers found a nonsignificant shift toward a worse functional outcome in the heparin group, compared with the no-heparin group (aOR, 0.81). The risk for sICH was significantly higher in the heparin group, compared with the no-heparin group (13% vs. 7.4%; aOR, 2.00).
Patients in the heparin group were also less likely to have a good functional outcome (aOR, 0.78), and there was a nonsignificant increase in risk for death among those patients.
The rate of sICH was 11% in the group that received low-dose heparin; it was 26% in the group that received moderate-dose heparin (aOR, 6.05). The mortality rate was 23% in the low-dose group and 47% in the moderate-dose group (aOR, 5.45).
There was no significant interaction between ASA and heparin on the primary outcome and on sICH occurrence.
‘A unique trial’
“MR CLEAN-MED is a unique trial because it investigated a widely used treatment but until now without any proof of effectiveness,” said Dr. van der Steen. The researchers expect that their findings will have a strong impact on the management of patients with acute ischemic stroke. They suggest that the administration of antithrombotic agents during EVT be avoided.
“We consider it probable that the increased risk of sICH explains at least a part of the nonsignificant shift towards a worse functional outcome,” co-investigator Bob Roozenbeek, MD, PhD, a neurologist at the Erasmus Medical Center, said in an interview. “However, to make more definite statements, we will have to do more in-depth analyses.”
It remains unclear whether the periprocedural use of lower dosages of antithrombotic agents or of a single bolus of heparin could be safe and effective, said Dr. van der Steen.
To gain insight into these questions, the investigators will evaluate the effect of the medications and dosages examined in this trial on primary hemostasis and coagulation activity in the trial population. They also plan to examine the effect of primary hemostasis and coagulation activity on risk for sICH and functional outcome.
Enhancing the effectiveness of thrombectomy for acute ischemic stroke continues to be an important goal for stroke therapy, said Mark Fisher, MD, professor of neurology and pathology and laboratory medicine at the University of California, Irvine, who commented on the findings for this news organization.
At least three strategies are available: The use of ancillary antithrombotic medications, neuroprotection, and modulation of the vasoconstrictive properties of the microcirculation.
“Results of MR CLEAN-MED argue against the antithrombotic strategy,” said Dr. Fisher. “The alternate strategies remain viable, and results of interventions using those approaches are awaited with great interest.”
The study was funded by the CONTRAST consortium, which is supported by the Netherlands Cardiovascular Research Initiative and the Brain Foundation Netherlands. Funding also was provided by Stryker, Medtronic, and Cerenovus. Dr. van der Steen and Dr. Fisher have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ADHD a new risk factor for Alzheimer’s?
results from a large, multigenerational study show.
“The findings suggest there are common genetic and/or environmental contributions to the association between ADHD and dementia,” study investigator Zheng Chang, PhD, from the department of medical epidemiology and biostatistics at Karolinska Institute, Stockholm, said in a statement.
“There have been few studies previously on the link between ADHD and dementia, all with limited sample size,” Dr. Chang said in an interview.
“This is the first study to look at ADHD and dementia within extended families. It’s a large population-based study including over 2 million individuals and their over 5 million biological relatives,” he noted.
The study was published online Sept. 9, 2021, in the journal Alzheimer’s & Dementia.
Shared familial risk
The researchers identified roughly 2.1 million people born in Sweden between 1980 and 2001. Overall, 3.2% of the cohort had a diagnosis of ADHD.
Using national registries, they linked these individuals to more than 5 million of their biological relatives including parents, grandparents, uncles, and aunts and determined which of these relatives developed dementia over time.
In adjusted analyses, parents of individuals with ADHD had 34% higher risk for any dementia than parents of those without ADHD (hazard ratio, 1.34; 95% CI, 1.11-1.63).
The risk for AD, the most common type of dementia, was 55% higher in parents of individuals with ADHD (HR, 1.55; 95% CI, 1.26-1.89).
Individuals with ADHD were more likely to have parents with early-onset dementia rather than late-onset dementia. However, the absolute risk for dementia was low for the parent cohort: Only 0.17% of the parents were diagnosed with dementia during follow-up.
The association between ADHD and dementia was not as strong for second-degree relatives of individuals with ADHD. For example, grandparents of individuals with ADHD had a 10% increased risk for dementia, compared with grandparents of individuals without ADHD.
The finding of attenuated associations with decreasing genetic relatedness (parents > grandparents and uncles/aunts), points to shared familial risk between ADHD and AD, the researchers said.
There could be “undiscovered genetic variants that contribute to either traits or family-wide environmental risk factors, such as socioeconomic status, that may have an impact on the association,” Dr. Chang said in the news release.
“There are no direct clinical implications from this study, but research like this could lead to further research with goals for improved detection, prevention, and treatment,” he said in an interview.
More questions than answers
Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association that the way different brain diseases are linked “is a question the Alzheimer’s Association is often asked, and it is a part of our funding portfolio to get that question answered.”
This study looking at ADHD and dementia is “intriguing,” Dr. Snyder said, “because, right now, there is limited information available. That said, this is an association study; it shows that two things are somehow connected. Because of how the study was conducted, it does not – and cannot – prove causation,” Dr. Snyder said. “But it is interesting all the same. More research is needed to uncover specifically why and how these two diseases are related. That might eventually give us insight into how to manage risk or even improve treatment.”
The study was supported by grants from the Swedish Council for Health, Working Life and Welfare, the Swedish Research Council, the Swedish Brain Foundation, the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie, the Fredrik & Ingrid Thurings Stiftelse, and the Karolinska Institutet Research Foundation. Dr. Chang and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results from a large, multigenerational study show.
“The findings suggest there are common genetic and/or environmental contributions to the association between ADHD and dementia,” study investigator Zheng Chang, PhD, from the department of medical epidemiology and biostatistics at Karolinska Institute, Stockholm, said in a statement.
“There have been few studies previously on the link between ADHD and dementia, all with limited sample size,” Dr. Chang said in an interview.
“This is the first study to look at ADHD and dementia within extended families. It’s a large population-based study including over 2 million individuals and their over 5 million biological relatives,” he noted.
The study was published online Sept. 9, 2021, in the journal Alzheimer’s & Dementia.
Shared familial risk
The researchers identified roughly 2.1 million people born in Sweden between 1980 and 2001. Overall, 3.2% of the cohort had a diagnosis of ADHD.
Using national registries, they linked these individuals to more than 5 million of their biological relatives including parents, grandparents, uncles, and aunts and determined which of these relatives developed dementia over time.
In adjusted analyses, parents of individuals with ADHD had 34% higher risk for any dementia than parents of those without ADHD (hazard ratio, 1.34; 95% CI, 1.11-1.63).
The risk for AD, the most common type of dementia, was 55% higher in parents of individuals with ADHD (HR, 1.55; 95% CI, 1.26-1.89).
Individuals with ADHD were more likely to have parents with early-onset dementia rather than late-onset dementia. However, the absolute risk for dementia was low for the parent cohort: Only 0.17% of the parents were diagnosed with dementia during follow-up.
The association between ADHD and dementia was not as strong for second-degree relatives of individuals with ADHD. For example, grandparents of individuals with ADHD had a 10% increased risk for dementia, compared with grandparents of individuals without ADHD.
The finding of attenuated associations with decreasing genetic relatedness (parents > grandparents and uncles/aunts), points to shared familial risk between ADHD and AD, the researchers said.
There could be “undiscovered genetic variants that contribute to either traits or family-wide environmental risk factors, such as socioeconomic status, that may have an impact on the association,” Dr. Chang said in the news release.
“There are no direct clinical implications from this study, but research like this could lead to further research with goals for improved detection, prevention, and treatment,” he said in an interview.
More questions than answers
Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association that the way different brain diseases are linked “is a question the Alzheimer’s Association is often asked, and it is a part of our funding portfolio to get that question answered.”
This study looking at ADHD and dementia is “intriguing,” Dr. Snyder said, “because, right now, there is limited information available. That said, this is an association study; it shows that two things are somehow connected. Because of how the study was conducted, it does not – and cannot – prove causation,” Dr. Snyder said. “But it is interesting all the same. More research is needed to uncover specifically why and how these two diseases are related. That might eventually give us insight into how to manage risk or even improve treatment.”
The study was supported by grants from the Swedish Council for Health, Working Life and Welfare, the Swedish Research Council, the Swedish Brain Foundation, the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie, the Fredrik & Ingrid Thurings Stiftelse, and the Karolinska Institutet Research Foundation. Dr. Chang and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results from a large, multigenerational study show.
“The findings suggest there are common genetic and/or environmental contributions to the association between ADHD and dementia,” study investigator Zheng Chang, PhD, from the department of medical epidemiology and biostatistics at Karolinska Institute, Stockholm, said in a statement.
“There have been few studies previously on the link between ADHD and dementia, all with limited sample size,” Dr. Chang said in an interview.
“This is the first study to look at ADHD and dementia within extended families. It’s a large population-based study including over 2 million individuals and their over 5 million biological relatives,” he noted.
The study was published online Sept. 9, 2021, in the journal Alzheimer’s & Dementia.
Shared familial risk
The researchers identified roughly 2.1 million people born in Sweden between 1980 and 2001. Overall, 3.2% of the cohort had a diagnosis of ADHD.
Using national registries, they linked these individuals to more than 5 million of their biological relatives including parents, grandparents, uncles, and aunts and determined which of these relatives developed dementia over time.
In adjusted analyses, parents of individuals with ADHD had 34% higher risk for any dementia than parents of those without ADHD (hazard ratio, 1.34; 95% CI, 1.11-1.63).
The risk for AD, the most common type of dementia, was 55% higher in parents of individuals with ADHD (HR, 1.55; 95% CI, 1.26-1.89).
Individuals with ADHD were more likely to have parents with early-onset dementia rather than late-onset dementia. However, the absolute risk for dementia was low for the parent cohort: Only 0.17% of the parents were diagnosed with dementia during follow-up.
The association between ADHD and dementia was not as strong for second-degree relatives of individuals with ADHD. For example, grandparents of individuals with ADHD had a 10% increased risk for dementia, compared with grandparents of individuals without ADHD.
The finding of attenuated associations with decreasing genetic relatedness (parents > grandparents and uncles/aunts), points to shared familial risk between ADHD and AD, the researchers said.
There could be “undiscovered genetic variants that contribute to either traits or family-wide environmental risk factors, such as socioeconomic status, that may have an impact on the association,” Dr. Chang said in the news release.
“There are no direct clinical implications from this study, but research like this could lead to further research with goals for improved detection, prevention, and treatment,” he said in an interview.
More questions than answers
Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association that the way different brain diseases are linked “is a question the Alzheimer’s Association is often asked, and it is a part of our funding portfolio to get that question answered.”
This study looking at ADHD and dementia is “intriguing,” Dr. Snyder said, “because, right now, there is limited information available. That said, this is an association study; it shows that two things are somehow connected. Because of how the study was conducted, it does not – and cannot – prove causation,” Dr. Snyder said. “But it is interesting all the same. More research is needed to uncover specifically why and how these two diseases are related. That might eventually give us insight into how to manage risk or even improve treatment.”
The study was supported by grants from the Swedish Council for Health, Working Life and Welfare, the Swedish Research Council, the Swedish Brain Foundation, the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie, the Fredrik & Ingrid Thurings Stiftelse, and the Karolinska Institutet Research Foundation. Dr. Chang and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
78-year-old man • tail bone pain • unintended weight loss • history of diabetes and hypertension • Dx?
THE CASE
A 78-year-old man with a history of diabetes and hypertension was referred to the outpatient surgical office with a chief complaint of “tail bone pain” that had started after a fall a year earlier. The patient complained that the pain was worse when sitting and at nighttime. He also admitted to a 7-lb weight loss over the past 2 months without change in diet or appetite. He denied symptoms of incontinence, urinary retention, sharp stabbing pains in the lower extremities, night sweats, or anorexia.
The patient first visited an urgent care facility on the day after the fall because he was experiencing pain in his “tail bone” region while riding his lawn mower. A pelvic x-ray was performed at that time and showed no coccyx fracture. He received a steroid injection in the right sacroiliac joint, which provided some relief for a month. Throughout the course of the year, he was given 6 steroid injections into his sacroiliac joint by his primary care provider (PCP) and clinicians at his local urgent care facility. One year after the fall, the patient’s PCP ordered a computed tomography (CT) scan of the abdomen and pelvis, which revealed a 4.6 x 7.5–cm soft-tissue mass with bony destruction of the lower sacrum and coccyx that extended into the sacral and coccygeal canal (FIGURE 1).
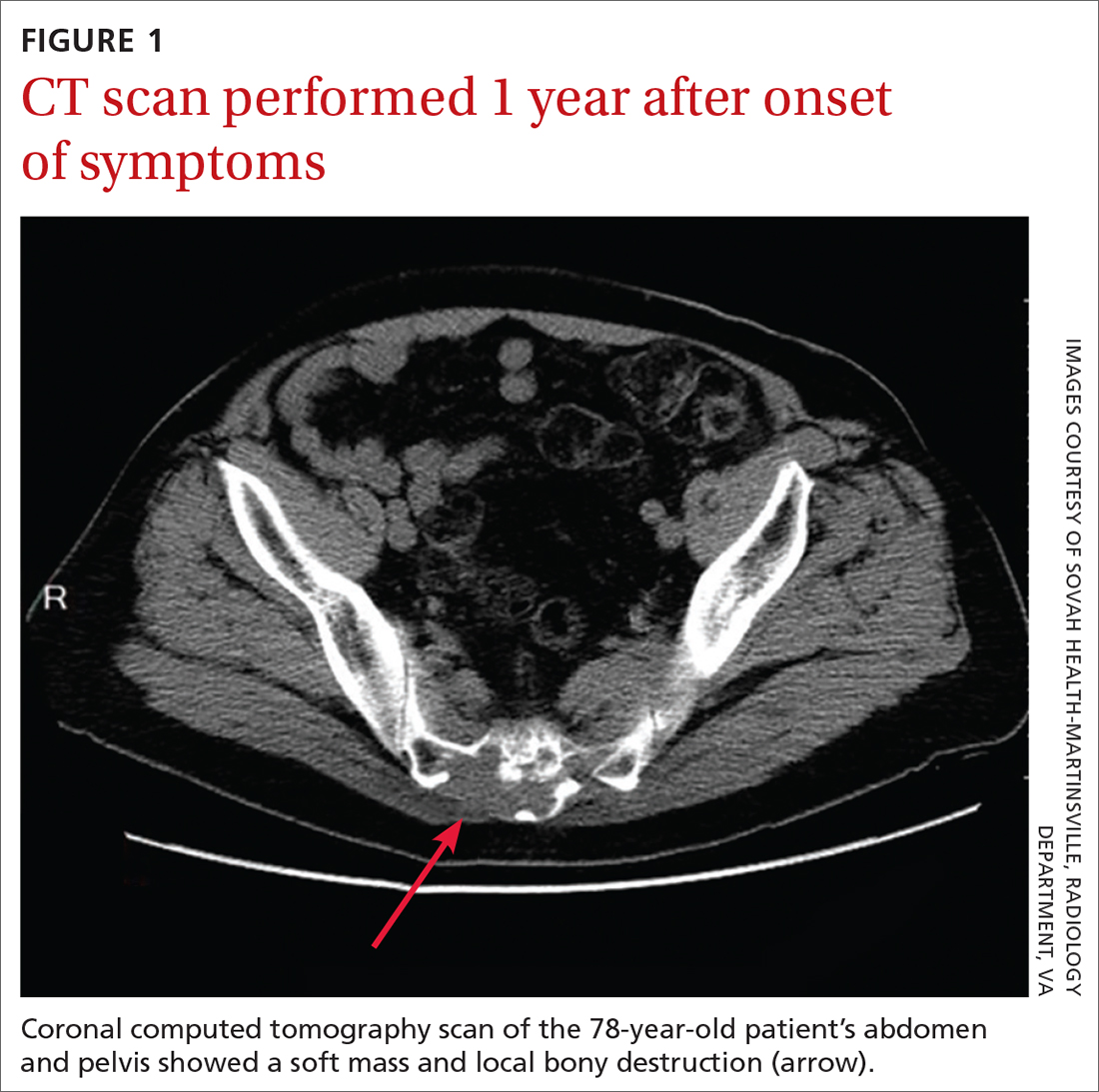
On exam in our surgical office, the patient was found to be alert and oriented. His neurologic exam was unremarkable, with an intact motor and sensory exam and no symptoms of cauda equina syndrome. During palpation over the lower sacrum and coccyx, both tenderness and a boggy, soft mass were observed. Nerve impingement was most likely caused by the size of the mass.
THE DIAGNOSIS
Biopsy revealed a large tan-gray, gelatinous, soft-tissue mass that was necrotizing through the lower sacrum. The diagnosis of a sacral chordoma was confirmed with magnetic resonance imaging of the pelvis, which demonstrated a 4.6 × 8.1–cm destructive expansile sacrococcygeal tumor with an exophytic soft-tissue component (FIGURE 2). The tumor also involved the piriformis and gluteus maximus muscles bilaterally.
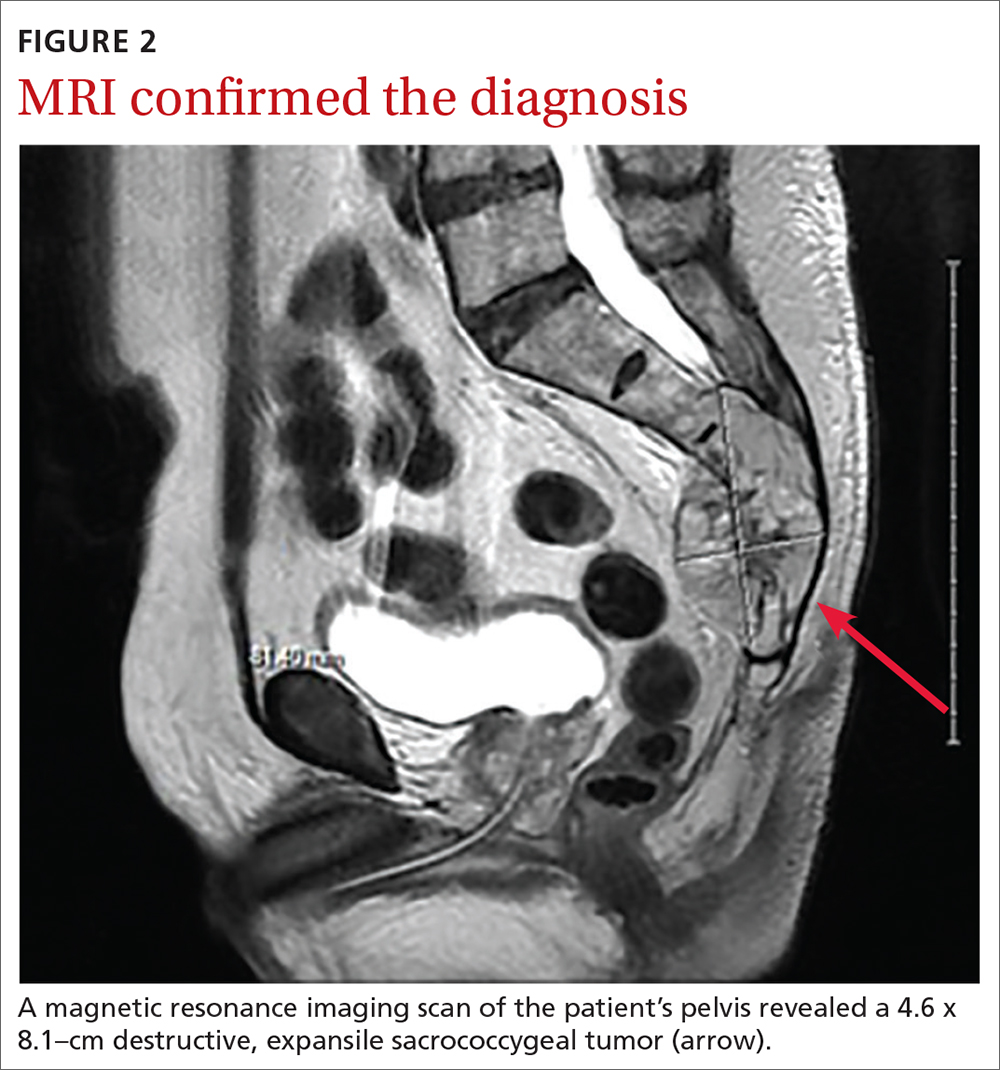
DISCUSSION
Chordomas are rare, malignant bone tumors that grow slowly and originate from embryonic remnants of the notochord.1 They are most commonly seen in the sacrococcygeal segment (50%) but are also seen in the spheno-occipital synchondrosis (30%-35%) and other spinal segments such as C2 and lumbar spine.2 Chordomas are typically seen in middle-aged patients, with sacral chordomas occurring predominantly in men compared to women (3:1).2
Slow to grow, slow to diagnose
The difficulty with diagnosing sacral chordomas lies in the tendency for these tumors to grow extremely slowly, making detection challenging due to a lack of symptoms in the early clinical course. Once the tumors cause noticeable symptoms, they are usually large and extensively locally invasive. As a result, most patients experience delayed diagnosis, with an average symptom duration of 2.3 years prior to diagnosis.3
Reexamining a common problem as a symptom of a rare condition
The most commonly manifesting symptom of sacral chordomas is lower back pain that is typically dull and worse with sitting.3,4 Since lower back pain is the leading cause of disability, it is difficult to determine when back pain is simply a benign consequence of aging or muscular pain and when it is, in fact, pathologic.5 A thorough history and physical are crucial in making the distinction.
Continue to: Clinical red flags...
Clinical red flags include pain with neurologic symptoms (including paresthesia, urinary or bowel disturbances, and weakness in the lower limbs), pain in the lower back with or without coccyx pain that persists and gradually worsens over time, and pain that fails to resolve.3 These symptoms are collectively strong indicators of underlying sacral pathology and should warrant further investigation, including a CT and MRI of the involved area.
Survival rate is improved by surgery
The gold standard for treatment of sacral chordomas is surgical resection with adequate margins, as these tumors are both radio- and chemo-insensitive.6 It is generally accepted that achieving a wide surgical margin is the most important predictor of survival and of reducing local recurrence in patients with sacrococcygeal chordoma.7-9
The survival rate varies after a posterior-only surgical approach; some studies cite the 5-year survival rate as 100% and others state the 7-year survival rate as 5%.4 The wide variation is likely due to small trial size, a lack of evidence, and how invasive the disease is at the time of surgery.
The recurrence rate 5 years after surgery is approximately 20%.4 The rate of urinary and fecal incontinence after surgery using a posterior-only approach is between 20% and 100%; some of this variation may be due to which spinal level is involved.4 If S3 is affected, there is almost always perineal anesthesia along with bowel and bladder incontinence.4
This patient was referred to Neurosurgery and underwent resection. He recovered well from surgery but suffered from some residual urinary incontinence. The patient did not receive chemotherapy or radiation, and further work-up revealed no evidence of metastasis.
Continue to: THE TAKEAWAY
THE TAKEAWAY
The diagnosis of sacral chordoma remains challenging. A history of clinical red flags, especially persistent lower back pain with neuropathy, should prompt an aggressive investigation to rule out underlying pathology. Other signs on physical exam could include urinary or bowel disturbances, weakness in the lower limbs, saddle anesthesia, new foot drop, and/or laxity of the anal sphincter.5 Early detection and surgical intervention are crucial for these patients to experience a better prognosis and preserve maximum function.
CORRESPONDENCE
Ginger Poulton, MD, 123 Hendersonville Road, Asheville, NC 28803; [email protected]
1. Zabel-du Bois A, Nikoghosyan A, Schwahofer A, et al. Intensity modulated radiotherapy in the management of sacral chordoma in primary versus recurrent disease. Radiother Oncol. 2010;97:408-412. doi: 10.1016/j.radonc.2010.10.008
2. Murphey MD, Andrews CL, Flemming DJ, et al. Primary tumors of the spine: radiologic pathologic correlation. Radiographics. 1996;1131-1158. doi: 10.1148/radiographics.16.5.8888395
3. Jeys L, Gibbins R, Evans G, et al. Sacral chordoma: a diagnosis not to be sat on? Int Orthopaedics. 2008;32:269-272. doi: 10.1007/s00264-006-0296-3
4. Pillai S, Govender, S. Sacral chordoma: a review of literature. J Orthop. 2018;15:679-684. doi: 10.1016/j.jor.2018.04.001
5. Traeger A, Buchbinder R, Harris I, et al. Diagnosis and management of low-back pain in primary care. CMAJ. 2017;189:E1386-E1395. doi: 10.1503/cmaj.170527
6. Walcott BP, Nahed BV, Mohyeldin A, et al. Chordoma: current concepts, management, and future directions. Lancet Oncol. 2012;13:e69-76. doi: 10.1016/S1470-2045(11)70337-0
7. Bergh P, Kindblom LG, Gunterberg B, et al. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000;88:2122-2134. doi: 10.1002/(sici)1097-0142(20000501)88:9<2122::aid-cncr19>3.0.co;2-1
8. Boriani S, Bandiera S, Biagini R, et al. Chordoma of the mobile spine: fifty years of experience. Spine. 2006;31:493-503. doi: 10.1097/01.brs.0000200038.30869.27
9. Hanna SA, Aston WJ, Briggs TW, et al. Sacral chordoma: can local recurrence after sacrectomy be predicted? Clin Orthop Relat Res. 2008;466:2217-2223. doi: 10.1007/s11999-008-0356-7
THE CASE
A 78-year-old man with a history of diabetes and hypertension was referred to the outpatient surgical office with a chief complaint of “tail bone pain” that had started after a fall a year earlier. The patient complained that the pain was worse when sitting and at nighttime. He also admitted to a 7-lb weight loss over the past 2 months without change in diet or appetite. He denied symptoms of incontinence, urinary retention, sharp stabbing pains in the lower extremities, night sweats, or anorexia.
The patient first visited an urgent care facility on the day after the fall because he was experiencing pain in his “tail bone” region while riding his lawn mower. A pelvic x-ray was performed at that time and showed no coccyx fracture. He received a steroid injection in the right sacroiliac joint, which provided some relief for a month. Throughout the course of the year, he was given 6 steroid injections into his sacroiliac joint by his primary care provider (PCP) and clinicians at his local urgent care facility. One year after the fall, the patient’s PCP ordered a computed tomography (CT) scan of the abdomen and pelvis, which revealed a 4.6 x 7.5–cm soft-tissue mass with bony destruction of the lower sacrum and coccyx that extended into the sacral and coccygeal canal (FIGURE 1).

On exam in our surgical office, the patient was found to be alert and oriented. His neurologic exam was unremarkable, with an intact motor and sensory exam and no symptoms of cauda equina syndrome. During palpation over the lower sacrum and coccyx, both tenderness and a boggy, soft mass were observed. Nerve impingement was most likely caused by the size of the mass.
THE DIAGNOSIS
Biopsy revealed a large tan-gray, gelatinous, soft-tissue mass that was necrotizing through the lower sacrum. The diagnosis of a sacral chordoma was confirmed with magnetic resonance imaging of the pelvis, which demonstrated a 4.6 × 8.1–cm destructive expansile sacrococcygeal tumor with an exophytic soft-tissue component (FIGURE 2). The tumor also involved the piriformis and gluteus maximus muscles bilaterally.

DISCUSSION
Chordomas are rare, malignant bone tumors that grow slowly and originate from embryonic remnants of the notochord.1 They are most commonly seen in the sacrococcygeal segment (50%) but are also seen in the spheno-occipital synchondrosis (30%-35%) and other spinal segments such as C2 and lumbar spine.2 Chordomas are typically seen in middle-aged patients, with sacral chordomas occurring predominantly in men compared to women (3:1).2
Slow to grow, slow to diagnose
The difficulty with diagnosing sacral chordomas lies in the tendency for these tumors to grow extremely slowly, making detection challenging due to a lack of symptoms in the early clinical course. Once the tumors cause noticeable symptoms, they are usually large and extensively locally invasive. As a result, most patients experience delayed diagnosis, with an average symptom duration of 2.3 years prior to diagnosis.3
Reexamining a common problem as a symptom of a rare condition
The most commonly manifesting symptom of sacral chordomas is lower back pain that is typically dull and worse with sitting.3,4 Since lower back pain is the leading cause of disability, it is difficult to determine when back pain is simply a benign consequence of aging or muscular pain and when it is, in fact, pathologic.5 A thorough history and physical are crucial in making the distinction.
Continue to: Clinical red flags...
Clinical red flags include pain with neurologic symptoms (including paresthesia, urinary or bowel disturbances, and weakness in the lower limbs), pain in the lower back with or without coccyx pain that persists and gradually worsens over time, and pain that fails to resolve.3 These symptoms are collectively strong indicators of underlying sacral pathology and should warrant further investigation, including a CT and MRI of the involved area.
Survival rate is improved by surgery
The gold standard for treatment of sacral chordomas is surgical resection with adequate margins, as these tumors are both radio- and chemo-insensitive.6 It is generally accepted that achieving a wide surgical margin is the most important predictor of survival and of reducing local recurrence in patients with sacrococcygeal chordoma.7-9
The survival rate varies after a posterior-only surgical approach; some studies cite the 5-year survival rate as 100% and others state the 7-year survival rate as 5%.4 The wide variation is likely due to small trial size, a lack of evidence, and how invasive the disease is at the time of surgery.
The recurrence rate 5 years after surgery is approximately 20%.4 The rate of urinary and fecal incontinence after surgery using a posterior-only approach is between 20% and 100%; some of this variation may be due to which spinal level is involved.4 If S3 is affected, there is almost always perineal anesthesia along with bowel and bladder incontinence.4
This patient was referred to Neurosurgery and underwent resection. He recovered well from surgery but suffered from some residual urinary incontinence. The patient did not receive chemotherapy or radiation, and further work-up revealed no evidence of metastasis.
Continue to: THE TAKEAWAY
THE TAKEAWAY
The diagnosis of sacral chordoma remains challenging. A history of clinical red flags, especially persistent lower back pain with neuropathy, should prompt an aggressive investigation to rule out underlying pathology. Other signs on physical exam could include urinary or bowel disturbances, weakness in the lower limbs, saddle anesthesia, new foot drop, and/or laxity of the anal sphincter.5 Early detection and surgical intervention are crucial for these patients to experience a better prognosis and preserve maximum function.
CORRESPONDENCE
Ginger Poulton, MD, 123 Hendersonville Road, Asheville, NC 28803; [email protected]
THE CASE
A 78-year-old man with a history of diabetes and hypertension was referred to the outpatient surgical office with a chief complaint of “tail bone pain” that had started after a fall a year earlier. The patient complained that the pain was worse when sitting and at nighttime. He also admitted to a 7-lb weight loss over the past 2 months without change in diet or appetite. He denied symptoms of incontinence, urinary retention, sharp stabbing pains in the lower extremities, night sweats, or anorexia.
The patient first visited an urgent care facility on the day after the fall because he was experiencing pain in his “tail bone” region while riding his lawn mower. A pelvic x-ray was performed at that time and showed no coccyx fracture. He received a steroid injection in the right sacroiliac joint, which provided some relief for a month. Throughout the course of the year, he was given 6 steroid injections into his sacroiliac joint by his primary care provider (PCP) and clinicians at his local urgent care facility. One year after the fall, the patient’s PCP ordered a computed tomography (CT) scan of the abdomen and pelvis, which revealed a 4.6 x 7.5–cm soft-tissue mass with bony destruction of the lower sacrum and coccyx that extended into the sacral and coccygeal canal (FIGURE 1).

On exam in our surgical office, the patient was found to be alert and oriented. His neurologic exam was unremarkable, with an intact motor and sensory exam and no symptoms of cauda equina syndrome. During palpation over the lower sacrum and coccyx, both tenderness and a boggy, soft mass were observed. Nerve impingement was most likely caused by the size of the mass.
THE DIAGNOSIS
Biopsy revealed a large tan-gray, gelatinous, soft-tissue mass that was necrotizing through the lower sacrum. The diagnosis of a sacral chordoma was confirmed with magnetic resonance imaging of the pelvis, which demonstrated a 4.6 × 8.1–cm destructive expansile sacrococcygeal tumor with an exophytic soft-tissue component (FIGURE 2). The tumor also involved the piriformis and gluteus maximus muscles bilaterally.

DISCUSSION
Chordomas are rare, malignant bone tumors that grow slowly and originate from embryonic remnants of the notochord.1 They are most commonly seen in the sacrococcygeal segment (50%) but are also seen in the spheno-occipital synchondrosis (30%-35%) and other spinal segments such as C2 and lumbar spine.2 Chordomas are typically seen in middle-aged patients, with sacral chordomas occurring predominantly in men compared to women (3:1).2
Slow to grow, slow to diagnose
The difficulty with diagnosing sacral chordomas lies in the tendency for these tumors to grow extremely slowly, making detection challenging due to a lack of symptoms in the early clinical course. Once the tumors cause noticeable symptoms, they are usually large and extensively locally invasive. As a result, most patients experience delayed diagnosis, with an average symptom duration of 2.3 years prior to diagnosis.3
Reexamining a common problem as a symptom of a rare condition
The most commonly manifesting symptom of sacral chordomas is lower back pain that is typically dull and worse with sitting.3,4 Since lower back pain is the leading cause of disability, it is difficult to determine when back pain is simply a benign consequence of aging or muscular pain and when it is, in fact, pathologic.5 A thorough history and physical are crucial in making the distinction.
Continue to: Clinical red flags...
Clinical red flags include pain with neurologic symptoms (including paresthesia, urinary or bowel disturbances, and weakness in the lower limbs), pain in the lower back with or without coccyx pain that persists and gradually worsens over time, and pain that fails to resolve.3 These symptoms are collectively strong indicators of underlying sacral pathology and should warrant further investigation, including a CT and MRI of the involved area.
Survival rate is improved by surgery
The gold standard for treatment of sacral chordomas is surgical resection with adequate margins, as these tumors are both radio- and chemo-insensitive.6 It is generally accepted that achieving a wide surgical margin is the most important predictor of survival and of reducing local recurrence in patients with sacrococcygeal chordoma.7-9
The survival rate varies after a posterior-only surgical approach; some studies cite the 5-year survival rate as 100% and others state the 7-year survival rate as 5%.4 The wide variation is likely due to small trial size, a lack of evidence, and how invasive the disease is at the time of surgery.
The recurrence rate 5 years after surgery is approximately 20%.4 The rate of urinary and fecal incontinence after surgery using a posterior-only approach is between 20% and 100%; some of this variation may be due to which spinal level is involved.4 If S3 is affected, there is almost always perineal anesthesia along with bowel and bladder incontinence.4
This patient was referred to Neurosurgery and underwent resection. He recovered well from surgery but suffered from some residual urinary incontinence. The patient did not receive chemotherapy or radiation, and further work-up revealed no evidence of metastasis.
Continue to: THE TAKEAWAY
THE TAKEAWAY
The diagnosis of sacral chordoma remains challenging. A history of clinical red flags, especially persistent lower back pain with neuropathy, should prompt an aggressive investigation to rule out underlying pathology. Other signs on physical exam could include urinary or bowel disturbances, weakness in the lower limbs, saddle anesthesia, new foot drop, and/or laxity of the anal sphincter.5 Early detection and surgical intervention are crucial for these patients to experience a better prognosis and preserve maximum function.
CORRESPONDENCE
Ginger Poulton, MD, 123 Hendersonville Road, Asheville, NC 28803; [email protected]
1. Zabel-du Bois A, Nikoghosyan A, Schwahofer A, et al. Intensity modulated radiotherapy in the management of sacral chordoma in primary versus recurrent disease. Radiother Oncol. 2010;97:408-412. doi: 10.1016/j.radonc.2010.10.008
2. Murphey MD, Andrews CL, Flemming DJ, et al. Primary tumors of the spine: radiologic pathologic correlation. Radiographics. 1996;1131-1158. doi: 10.1148/radiographics.16.5.8888395
3. Jeys L, Gibbins R, Evans G, et al. Sacral chordoma: a diagnosis not to be sat on? Int Orthopaedics. 2008;32:269-272. doi: 10.1007/s00264-006-0296-3
4. Pillai S, Govender, S. Sacral chordoma: a review of literature. J Orthop. 2018;15:679-684. doi: 10.1016/j.jor.2018.04.001
5. Traeger A, Buchbinder R, Harris I, et al. Diagnosis and management of low-back pain in primary care. CMAJ. 2017;189:E1386-E1395. doi: 10.1503/cmaj.170527
6. Walcott BP, Nahed BV, Mohyeldin A, et al. Chordoma: current concepts, management, and future directions. Lancet Oncol. 2012;13:e69-76. doi: 10.1016/S1470-2045(11)70337-0
7. Bergh P, Kindblom LG, Gunterberg B, et al. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000;88:2122-2134. doi: 10.1002/(sici)1097-0142(20000501)88:9<2122::aid-cncr19>3.0.co;2-1
8. Boriani S, Bandiera S, Biagini R, et al. Chordoma of the mobile spine: fifty years of experience. Spine. 2006;31:493-503. doi: 10.1097/01.brs.0000200038.30869.27
9. Hanna SA, Aston WJ, Briggs TW, et al. Sacral chordoma: can local recurrence after sacrectomy be predicted? Clin Orthop Relat Res. 2008;466:2217-2223. doi: 10.1007/s11999-008-0356-7
1. Zabel-du Bois A, Nikoghosyan A, Schwahofer A, et al. Intensity modulated radiotherapy in the management of sacral chordoma in primary versus recurrent disease. Radiother Oncol. 2010;97:408-412. doi: 10.1016/j.radonc.2010.10.008
2. Murphey MD, Andrews CL, Flemming DJ, et al. Primary tumors of the spine: radiologic pathologic correlation. Radiographics. 1996;1131-1158. doi: 10.1148/radiographics.16.5.8888395
3. Jeys L, Gibbins R, Evans G, et al. Sacral chordoma: a diagnosis not to be sat on? Int Orthopaedics. 2008;32:269-272. doi: 10.1007/s00264-006-0296-3
4. Pillai S, Govender, S. Sacral chordoma: a review of literature. J Orthop. 2018;15:679-684. doi: 10.1016/j.jor.2018.04.001
5. Traeger A, Buchbinder R, Harris I, et al. Diagnosis and management of low-back pain in primary care. CMAJ. 2017;189:E1386-E1395. doi: 10.1503/cmaj.170527
6. Walcott BP, Nahed BV, Mohyeldin A, et al. Chordoma: current concepts, management, and future directions. Lancet Oncol. 2012;13:e69-76. doi: 10.1016/S1470-2045(11)70337-0
7. Bergh P, Kindblom LG, Gunterberg B, et al. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000;88:2122-2134. doi: 10.1002/(sici)1097-0142(20000501)88:9<2122::aid-cncr19>3.0.co;2-1
8. Boriani S, Bandiera S, Biagini R, et al. Chordoma of the mobile spine: fifty years of experience. Spine. 2006;31:493-503. doi: 10.1097/01.brs.0000200038.30869.27
9. Hanna SA, Aston WJ, Briggs TW, et al. Sacral chordoma: can local recurrence after sacrectomy be predicted? Clin Orthop Relat Res. 2008;466:2217-2223. doi: 10.1007/s11999-008-0356-7







