User login
White Hispanic Mohs patients less informed about skin cancer risks
White Hispanic adults report a lower quality of life and less knowledge of skin cancer and sun protection behaviors than white non-Hispanic adults, survey results of 175 adults with nonmelanoma skin cancer show.
“The incidence of nonmelanoma skin cancer (NMSC) is lower in Hispanics when compared to Caucasians, but a high index of suspicion is needed given ethnic differences in presentation,” wrote Ali Rajabi-Estarabadi, MD, of the University of Miami, and colleagues.
Hispanic patients with NMSC tend to be younger than non-Hispanic white patients, and their basal cell carcinomas are more likely to be pigmented, the investigators noted. Although previous research suggests ethnic disparities in NMSC, factors including sun safety knowledge and quality of life after diagnosis have not been well studied, they said.
With this in mind, the investigators conducted a survey of white Hispanics and non-Hispanics treated for NMSC. The results were published as a research letter in the Journal of the American Academy of Dermatology.
The investigators recruited 175 consecutive patients being treated for NMSC with Mohs surgery at a single center. The average age of the patients was 67 years; 58 identified as white Hispanic, 116 identified as white non-Hispanic.
White Hispanic patients had significantly lower skin cancer knowledge scores, compared with white non-Hispanics (P = .003). White Hispanics were significantly more likely than white non-Hispanics to report never wearing hats (39% vs. 12%) and never wearing sunglasses (26% vs. 9%) for sun protection.
The findings were limited by the study population that included only residents of South Florida. However, the results highlight the need for “targeted patient education initiatives to bridge ethnic disparities regarding cancer knowledge and ultimately improve [quality of life] among Hispanic skin cancer suffers,” the investigators concluded.
The study received no outside funding. The investigators declared no conflicts of interest.
SOURCE: Rajabi-Estarabadi A et al. J Am Acad Dermatol. 2020 Feb 4. doi: 10.1016/j.jaad.2020.01.063.
White Hispanic adults report a lower quality of life and less knowledge of skin cancer and sun protection behaviors than white non-Hispanic adults, survey results of 175 adults with nonmelanoma skin cancer show.
“The incidence of nonmelanoma skin cancer (NMSC) is lower in Hispanics when compared to Caucasians, but a high index of suspicion is needed given ethnic differences in presentation,” wrote Ali Rajabi-Estarabadi, MD, of the University of Miami, and colleagues.
Hispanic patients with NMSC tend to be younger than non-Hispanic white patients, and their basal cell carcinomas are more likely to be pigmented, the investigators noted. Although previous research suggests ethnic disparities in NMSC, factors including sun safety knowledge and quality of life after diagnosis have not been well studied, they said.
With this in mind, the investigators conducted a survey of white Hispanics and non-Hispanics treated for NMSC. The results were published as a research letter in the Journal of the American Academy of Dermatology.
The investigators recruited 175 consecutive patients being treated for NMSC with Mohs surgery at a single center. The average age of the patients was 67 years; 58 identified as white Hispanic, 116 identified as white non-Hispanic.
White Hispanic patients had significantly lower skin cancer knowledge scores, compared with white non-Hispanics (P = .003). White Hispanics were significantly more likely than white non-Hispanics to report never wearing hats (39% vs. 12%) and never wearing sunglasses (26% vs. 9%) for sun protection.
The findings were limited by the study population that included only residents of South Florida. However, the results highlight the need for “targeted patient education initiatives to bridge ethnic disparities regarding cancer knowledge and ultimately improve [quality of life] among Hispanic skin cancer suffers,” the investigators concluded.
The study received no outside funding. The investigators declared no conflicts of interest.
SOURCE: Rajabi-Estarabadi A et al. J Am Acad Dermatol. 2020 Feb 4. doi: 10.1016/j.jaad.2020.01.063.
White Hispanic adults report a lower quality of life and less knowledge of skin cancer and sun protection behaviors than white non-Hispanic adults, survey results of 175 adults with nonmelanoma skin cancer show.
“The incidence of nonmelanoma skin cancer (NMSC) is lower in Hispanics when compared to Caucasians, but a high index of suspicion is needed given ethnic differences in presentation,” wrote Ali Rajabi-Estarabadi, MD, of the University of Miami, and colleagues.
Hispanic patients with NMSC tend to be younger than non-Hispanic white patients, and their basal cell carcinomas are more likely to be pigmented, the investigators noted. Although previous research suggests ethnic disparities in NMSC, factors including sun safety knowledge and quality of life after diagnosis have not been well studied, they said.
With this in mind, the investigators conducted a survey of white Hispanics and non-Hispanics treated for NMSC. The results were published as a research letter in the Journal of the American Academy of Dermatology.
The investigators recruited 175 consecutive patients being treated for NMSC with Mohs surgery at a single center. The average age of the patients was 67 years; 58 identified as white Hispanic, 116 identified as white non-Hispanic.
White Hispanic patients had significantly lower skin cancer knowledge scores, compared with white non-Hispanics (P = .003). White Hispanics were significantly more likely than white non-Hispanics to report never wearing hats (39% vs. 12%) and never wearing sunglasses (26% vs. 9%) for sun protection.
The findings were limited by the study population that included only residents of South Florida. However, the results highlight the need for “targeted patient education initiatives to bridge ethnic disparities regarding cancer knowledge and ultimately improve [quality of life] among Hispanic skin cancer suffers,” the investigators concluded.
The study received no outside funding. The investigators declared no conflicts of interest.
SOURCE: Rajabi-Estarabadi A et al. J Am Acad Dermatol. 2020 Feb 4. doi: 10.1016/j.jaad.2020.01.063.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
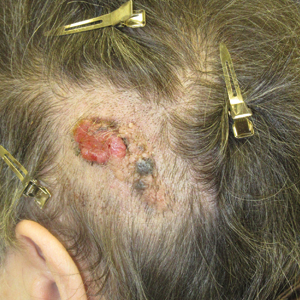
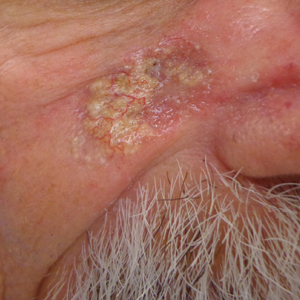
Friable Scalp Nodule
The Diagnosis: Adnexal Neoplasm Arising in a Nevus Sebaceus
Biopsy of the lesion showed a proliferation of basaloid-appearing cells with focal ductal differentiation and ulceration consistent with poroma (Figure 1). Due to the superficial nature of the biopsy, the pathologist recommended excision to ensure complete removal and to rule out a well-differentiated porocarcinoma. Excision of the lesion showed large basaloid aggregates with a hypercellular stroma and a surrounding papillomatous epidermis with well-developed sebaceous lobules consistent with a trichoblastoma and a nevus sebaceus, respectively (Figure 2). There also was evidence of poroma; however, there were no findings concerning for porocarcinoma, which could lead to metastasis (Figure 3).
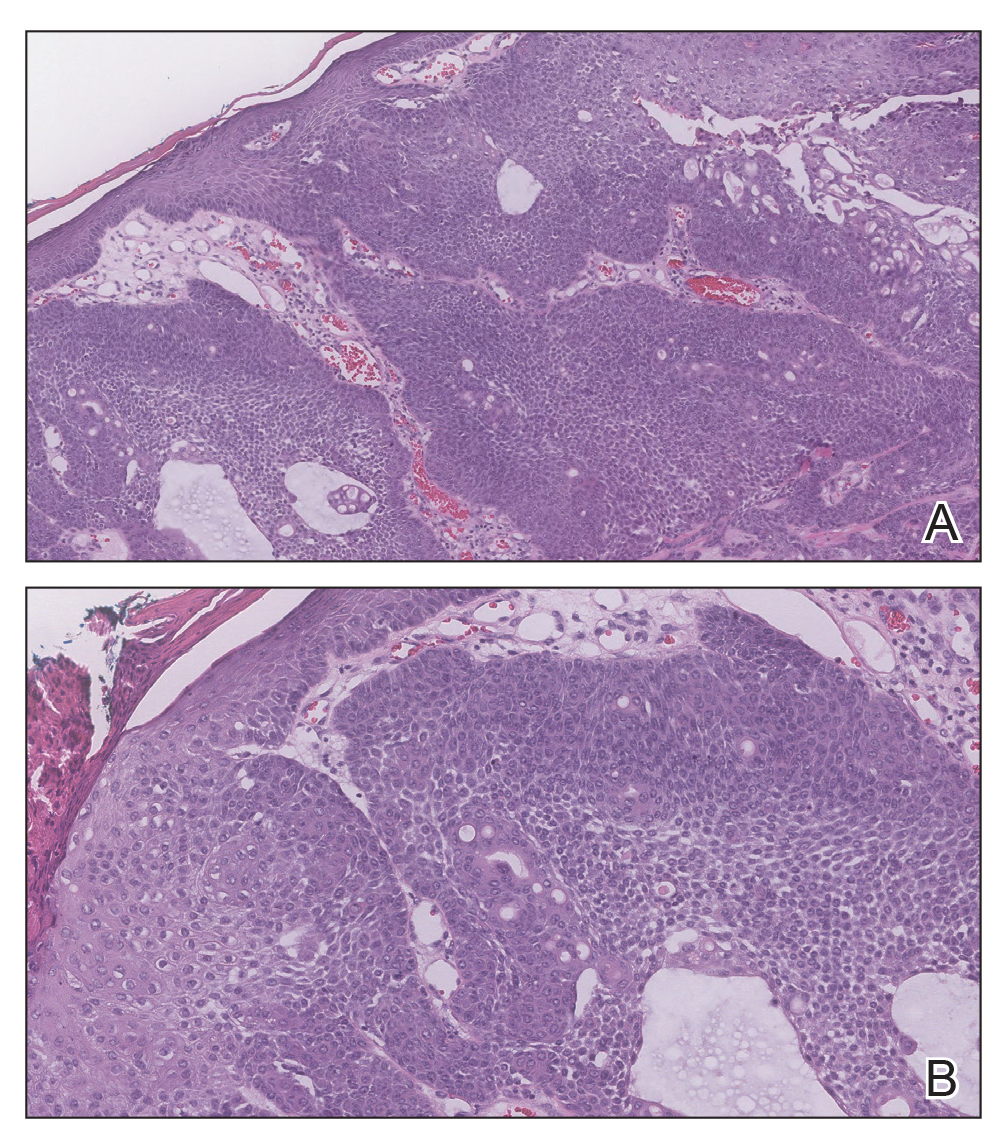
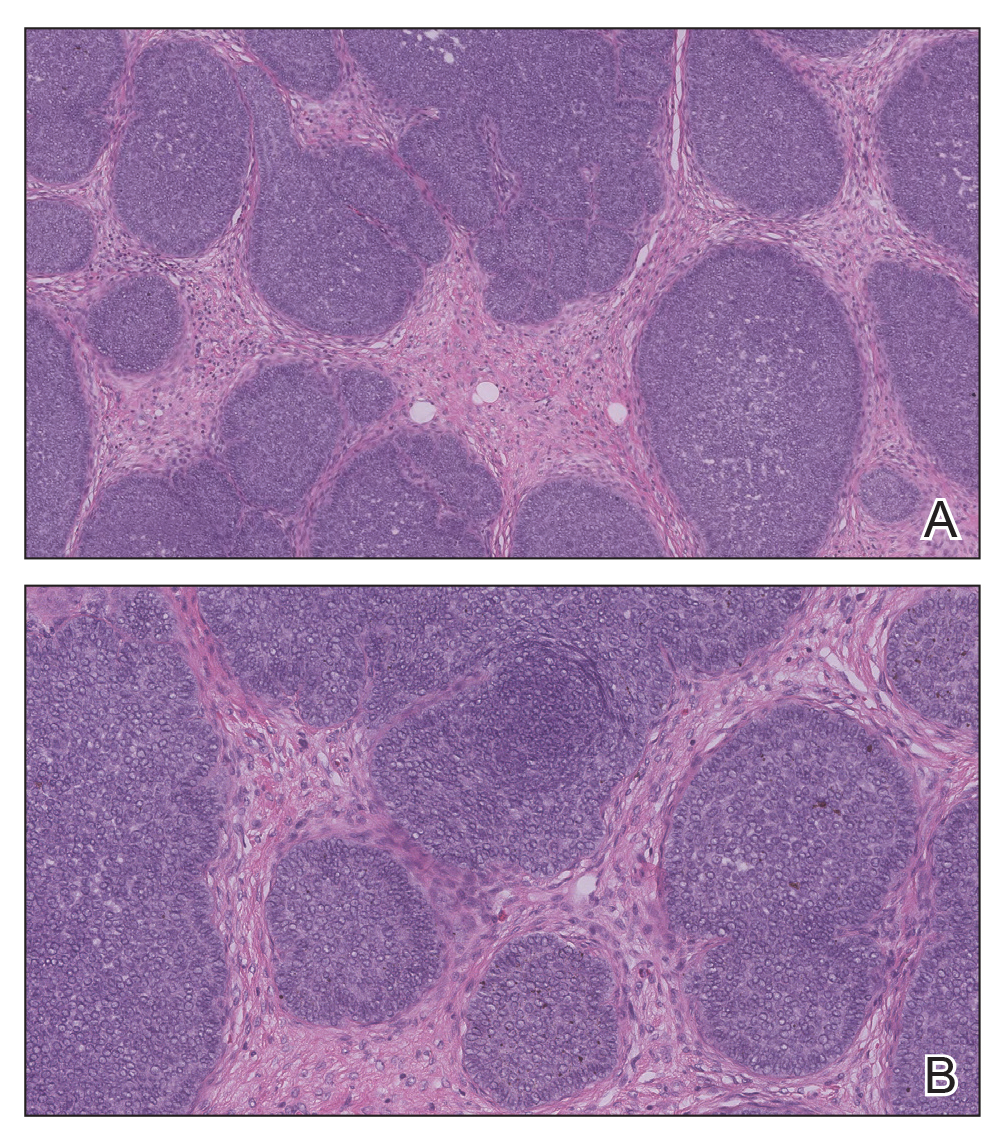
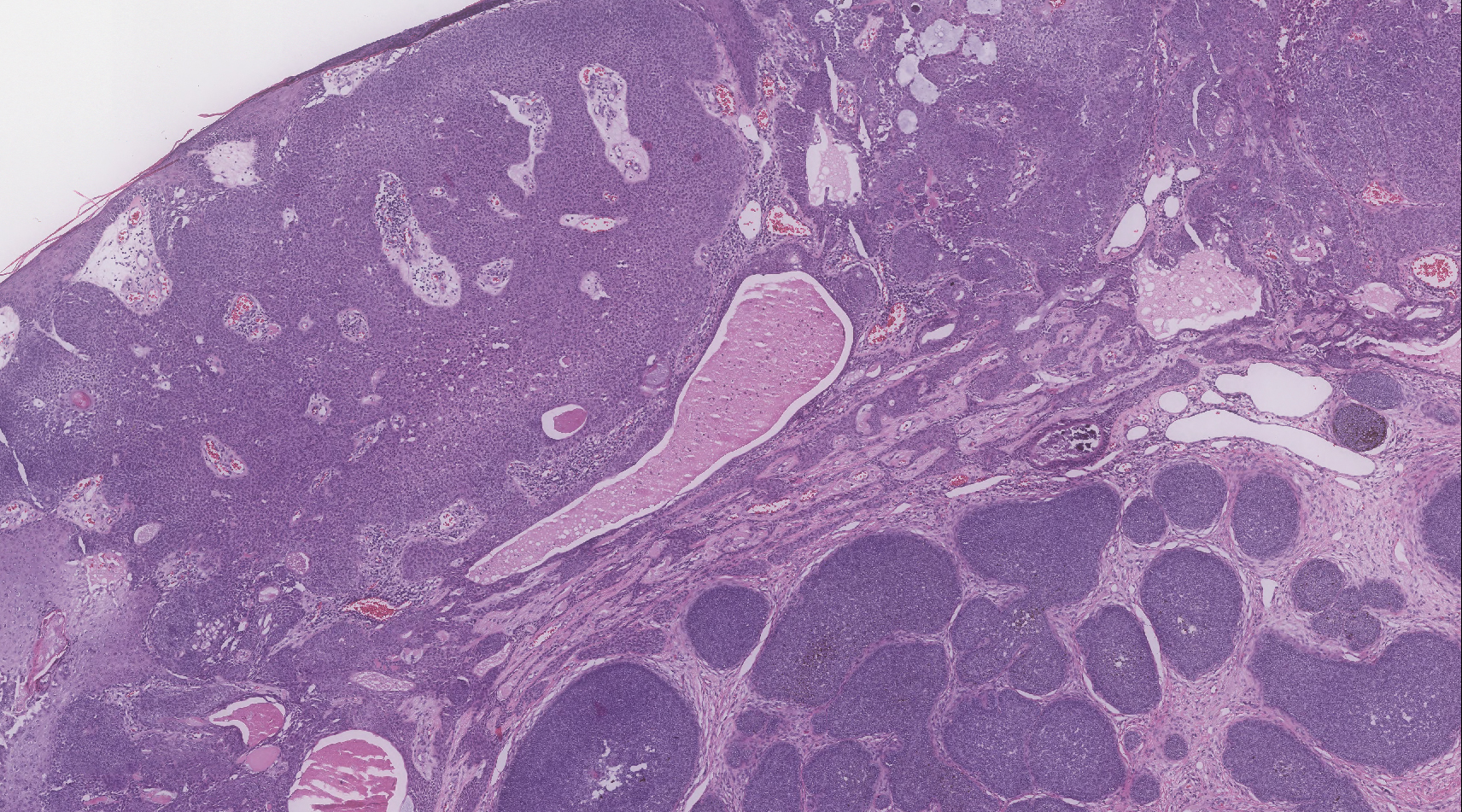
Nevus sebaceus is a benign, hamartomatous, congenital growth that occurs in approximately 1% of patients presenting to dermatology offices. It usually presents as a single asymptomatic plaque on the scalp (62.5%) or face (24.5%) that changes in morphology over its lifetime.1,2 In children, a nevus manifests as a yellowish, smooth, waxy skin lesion. As the sebaceous glands become more developed during adolescence, the lesion takes on more of a verrucous appearance and also can darken.
Although nevus sebaceus is benign, it may give rise to both benign and malignant neoplasms. In a 2014 study of 707 cases of nevus sebaceus, 21.4% developed secondary neoplasms, 88% of which were benign.2 The origins of these neoplasms can be epithelial, sebaceous, apocrine, and/or follicular. The 3 most common secondary neoplasms found in nevus sebaceus are trichoblastoma (34.7%), syringocystadenoma papilliferum (24.7%), and apocrine/eccrine adenoma (10%), all of which are benign.2 Trichoblastomas represent a type of hair follicle tumor. Malignant lesions manifest in approximately 2.5% of cases, with basal cell carcinoma (BCC) being the most common (5.3% of all neoplasms), followed by squamous cell carcinoma (2.7% of all neoplasms).2 Differentiating BCC from trichoblastoma can be difficult, but histologically BCCs usually have tumor stromal clefting while trichoblastomas do not.3 The incidence of secondary tumors in nevus sebaceus displays a strong correlation with age; thus, the highest proportion of neoplasms occur in adults.
Treatment of nevus sebaceus depends on the patient's age. In children, because of the low probability of secondary neoplasms, observation in lieu of surgical excision is a common approach. In adults, the approach typically is surgical excision or close follow-up, as there is a concern for secondary neoplasm and the potential for malignant degeneration.
A nevus sebaceus leading to 2 or more tumors within the same lesion is rare (seen in only 4.2% of lesions). The most common combination is trichoblastoma with syringocystadenoma papilliferum (0.6% of all cases).2 Poromas represent sweat gland tumors that usually appear on the soles (65%) or palms (10%).4 It is uncommon for these neoplasms to manifest on the scalp or within a nevus sebaceus. Three independent studies (N=596; N=707; N=450) did not report any occurrences of eccrine poroma.1,2,5 Eccrine poroma in conjunction with nodular trichoblastoma arising in a nevus sebaceus is unusual, and definitive excision should be strongly considered because of the possibility to develop a porocarcinoma.6
Atypical fibroxanthoma presents on sun-exposed areas as an exophytic nodule or plaque that frequently ulcerates. Pathology of this tumor shows a spindled cell proliferation that can stain positively for CD10 and procollagen 1. Basal cell carcinoma presents as a pearly papule or nodule displaying basaloid-appearing aggregates with tumor stromal clefting and can stain with Ber-EP4. Cylindromas typically present on the scalp as large rubbery-appearing plaques and nodules. Cylindromas usually present as a solitary tumor, but in the familial form there can be clusters of multiple nodules. Metastatic renal cell carcinoma frequently appears as a bleeding nodule on the scalp in patients with known renal cell cancer or as the initial presentation.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(pt 1):263-268.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Wang E, Lee JS, Kazakov DV. A rare combination of sebaceoma with carcinomatous change (sebaceous carcinoma), trichoblastoma, and poroma arising from a nevus sebaceus. J Cutan Pathol. 2013;40:676-682.
- Bae MI, Cho TH, Shin MK, et al. An unusual clinical presentation of eccrine poroma occurring on the auricle. Indian J Dermatol. 2015;60:523.
- Hsu MC, Liau JY, Hong JL, et al. Secondary neoplasms arising from nevus sebaceus: a retrospective study of 450 cases in Taiwan. J Dermatol. 2016;43:175-180.
- Takhan II, Domingo J. Metastasizing eccrine porocarcinoma developing in a sebaceous nevus of Jadassohn. report of a case. Arch Dermatol. 1985;121:413-415.
The Diagnosis: Adnexal Neoplasm Arising in a Nevus Sebaceus
Biopsy of the lesion showed a proliferation of basaloid-appearing cells with focal ductal differentiation and ulceration consistent with poroma (Figure 1). Due to the superficial nature of the biopsy, the pathologist recommended excision to ensure complete removal and to rule out a well-differentiated porocarcinoma. Excision of the lesion showed large basaloid aggregates with a hypercellular stroma and a surrounding papillomatous epidermis with well-developed sebaceous lobules consistent with a trichoblastoma and a nevus sebaceus, respectively (Figure 2). There also was evidence of poroma; however, there were no findings concerning for porocarcinoma, which could lead to metastasis (Figure 3).



Nevus sebaceus is a benign, hamartomatous, congenital growth that occurs in approximately 1% of patients presenting to dermatology offices. It usually presents as a single asymptomatic plaque on the scalp (62.5%) or face (24.5%) that changes in morphology over its lifetime.1,2 In children, a nevus manifests as a yellowish, smooth, waxy skin lesion. As the sebaceous glands become more developed during adolescence, the lesion takes on more of a verrucous appearance and also can darken.
Although nevus sebaceus is benign, it may give rise to both benign and malignant neoplasms. In a 2014 study of 707 cases of nevus sebaceus, 21.4% developed secondary neoplasms, 88% of which were benign.2 The origins of these neoplasms can be epithelial, sebaceous, apocrine, and/or follicular. The 3 most common secondary neoplasms found in nevus sebaceus are trichoblastoma (34.7%), syringocystadenoma papilliferum (24.7%), and apocrine/eccrine adenoma (10%), all of which are benign.2 Trichoblastomas represent a type of hair follicle tumor. Malignant lesions manifest in approximately 2.5% of cases, with basal cell carcinoma (BCC) being the most common (5.3% of all neoplasms), followed by squamous cell carcinoma (2.7% of all neoplasms).2 Differentiating BCC from trichoblastoma can be difficult, but histologically BCCs usually have tumor stromal clefting while trichoblastomas do not.3 The incidence of secondary tumors in nevus sebaceus displays a strong correlation with age; thus, the highest proportion of neoplasms occur in adults.
Treatment of nevus sebaceus depends on the patient's age. In children, because of the low probability of secondary neoplasms, observation in lieu of surgical excision is a common approach. In adults, the approach typically is surgical excision or close follow-up, as there is a concern for secondary neoplasm and the potential for malignant degeneration.
A nevus sebaceus leading to 2 or more tumors within the same lesion is rare (seen in only 4.2% of lesions). The most common combination is trichoblastoma with syringocystadenoma papilliferum (0.6% of all cases).2 Poromas represent sweat gland tumors that usually appear on the soles (65%) or palms (10%).4 It is uncommon for these neoplasms to manifest on the scalp or within a nevus sebaceus. Three independent studies (N=596; N=707; N=450) did not report any occurrences of eccrine poroma.1,2,5 Eccrine poroma in conjunction with nodular trichoblastoma arising in a nevus sebaceus is unusual, and definitive excision should be strongly considered because of the possibility to develop a porocarcinoma.6
Atypical fibroxanthoma presents on sun-exposed areas as an exophytic nodule or plaque that frequently ulcerates. Pathology of this tumor shows a spindled cell proliferation that can stain positively for CD10 and procollagen 1. Basal cell carcinoma presents as a pearly papule or nodule displaying basaloid-appearing aggregates with tumor stromal clefting and can stain with Ber-EP4. Cylindromas typically present on the scalp as large rubbery-appearing plaques and nodules. Cylindromas usually present as a solitary tumor, but in the familial form there can be clusters of multiple nodules. Metastatic renal cell carcinoma frequently appears as a bleeding nodule on the scalp in patients with known renal cell cancer or as the initial presentation.
The Diagnosis: Adnexal Neoplasm Arising in a Nevus Sebaceus
Biopsy of the lesion showed a proliferation of basaloid-appearing cells with focal ductal differentiation and ulceration consistent with poroma (Figure 1). Due to the superficial nature of the biopsy, the pathologist recommended excision to ensure complete removal and to rule out a well-differentiated porocarcinoma. Excision of the lesion showed large basaloid aggregates with a hypercellular stroma and a surrounding papillomatous epidermis with well-developed sebaceous lobules consistent with a trichoblastoma and a nevus sebaceus, respectively (Figure 2). There also was evidence of poroma; however, there were no findings concerning for porocarcinoma, which could lead to metastasis (Figure 3).



Nevus sebaceus is a benign, hamartomatous, congenital growth that occurs in approximately 1% of patients presenting to dermatology offices. It usually presents as a single asymptomatic plaque on the scalp (62.5%) or face (24.5%) that changes in morphology over its lifetime.1,2 In children, a nevus manifests as a yellowish, smooth, waxy skin lesion. As the sebaceous glands become more developed during adolescence, the lesion takes on more of a verrucous appearance and also can darken.
Although nevus sebaceus is benign, it may give rise to both benign and malignant neoplasms. In a 2014 study of 707 cases of nevus sebaceus, 21.4% developed secondary neoplasms, 88% of which were benign.2 The origins of these neoplasms can be epithelial, sebaceous, apocrine, and/or follicular. The 3 most common secondary neoplasms found in nevus sebaceus are trichoblastoma (34.7%), syringocystadenoma papilliferum (24.7%), and apocrine/eccrine adenoma (10%), all of which are benign.2 Trichoblastomas represent a type of hair follicle tumor. Malignant lesions manifest in approximately 2.5% of cases, with basal cell carcinoma (BCC) being the most common (5.3% of all neoplasms), followed by squamous cell carcinoma (2.7% of all neoplasms).2 Differentiating BCC from trichoblastoma can be difficult, but histologically BCCs usually have tumor stromal clefting while trichoblastomas do not.3 The incidence of secondary tumors in nevus sebaceus displays a strong correlation with age; thus, the highest proportion of neoplasms occur in adults.
Treatment of nevus sebaceus depends on the patient's age. In children, because of the low probability of secondary neoplasms, observation in lieu of surgical excision is a common approach. In adults, the approach typically is surgical excision or close follow-up, as there is a concern for secondary neoplasm and the potential for malignant degeneration.
A nevus sebaceus leading to 2 or more tumors within the same lesion is rare (seen in only 4.2% of lesions). The most common combination is trichoblastoma with syringocystadenoma papilliferum (0.6% of all cases).2 Poromas represent sweat gland tumors that usually appear on the soles (65%) or palms (10%).4 It is uncommon for these neoplasms to manifest on the scalp or within a nevus sebaceus. Three independent studies (N=596; N=707; N=450) did not report any occurrences of eccrine poroma.1,2,5 Eccrine poroma in conjunction with nodular trichoblastoma arising in a nevus sebaceus is unusual, and definitive excision should be strongly considered because of the possibility to develop a porocarcinoma.6
Atypical fibroxanthoma presents on sun-exposed areas as an exophytic nodule or plaque that frequently ulcerates. Pathology of this tumor shows a spindled cell proliferation that can stain positively for CD10 and procollagen 1. Basal cell carcinoma presents as a pearly papule or nodule displaying basaloid-appearing aggregates with tumor stromal clefting and can stain with Ber-EP4. Cylindromas typically present on the scalp as large rubbery-appearing plaques and nodules. Cylindromas usually present as a solitary tumor, but in the familial form there can be clusters of multiple nodules. Metastatic renal cell carcinoma frequently appears as a bleeding nodule on the scalp in patients with known renal cell cancer or as the initial presentation.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(pt 1):263-268.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Wang E, Lee JS, Kazakov DV. A rare combination of sebaceoma with carcinomatous change (sebaceous carcinoma), trichoblastoma, and poroma arising from a nevus sebaceus. J Cutan Pathol. 2013;40:676-682.
- Bae MI, Cho TH, Shin MK, et al. An unusual clinical presentation of eccrine poroma occurring on the auricle. Indian J Dermatol. 2015;60:523.
- Hsu MC, Liau JY, Hong JL, et al. Secondary neoplasms arising from nevus sebaceus: a retrospective study of 450 cases in Taiwan. J Dermatol. 2016;43:175-180.
- Takhan II, Domingo J. Metastasizing eccrine porocarcinoma developing in a sebaceous nevus of Jadassohn. report of a case. Arch Dermatol. 1985;121:413-415.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(pt 1):263-268.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Wang E, Lee JS, Kazakov DV. A rare combination of sebaceoma with carcinomatous change (sebaceous carcinoma), trichoblastoma, and poroma arising from a nevus sebaceus. J Cutan Pathol. 2013;40:676-682.
- Bae MI, Cho TH, Shin MK, et al. An unusual clinical presentation of eccrine poroma occurring on the auricle. Indian J Dermatol. 2015;60:523.
- Hsu MC, Liau JY, Hong JL, et al. Secondary neoplasms arising from nevus sebaceus: a retrospective study of 450 cases in Taiwan. J Dermatol. 2016;43:175-180.
- Takhan II, Domingo J. Metastasizing eccrine porocarcinoma developing in a sebaceous nevus of Jadassohn. report of a case. Arch Dermatol. 1985;121:413-415.
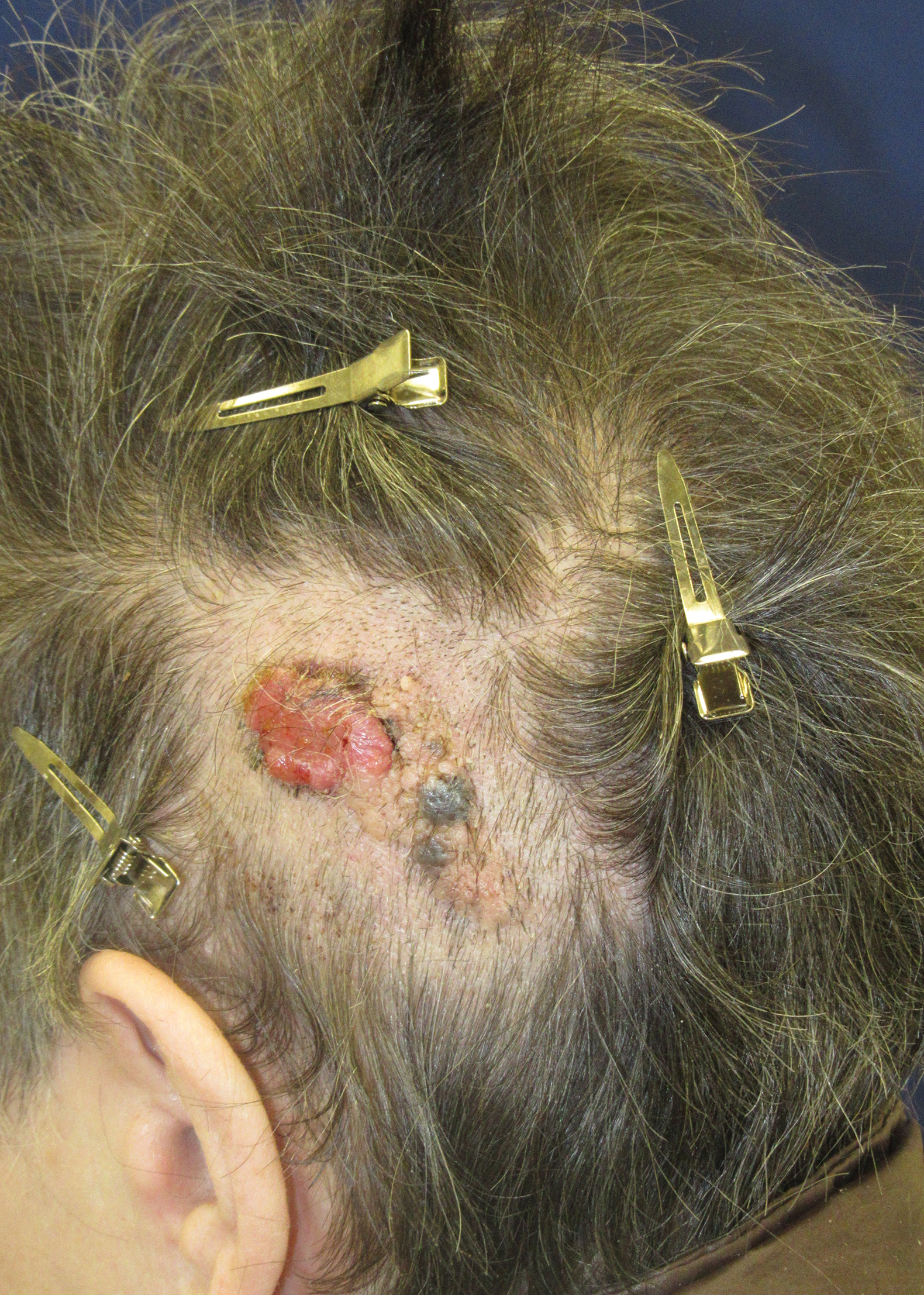
A 75-year-old woman presented with an enlarging plaque on the scalp of 5 years' duration. Physical examination revealed a 5.6.2 ×2.9-cm, tan-colored, verrucous plaque with an overlying pink friable nodule on the left occipital scalp. The lesion was not painful or pruritic, and the patient did not have any constitutional symptoms such as fever, night sweats, or weight loss. The patient denied prior tanning bed use and reported intermittent sun exposure over her lifetime. She denied any prior surgical intervention. There was no family history of similar lesions.
European marketing of Picato suspended while skin cancer risk reviewed
As a precaution, the European Medicines Agency (EMA) has recommended that patients stop using ingenol mebutate (Picato) while the agency continues to review the safety of the topical treatment, which is indicated for the treatment of actinic keratosis in Europe and the United States.
No such action has been taken in the United States.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) is reviewing data on skin cancer in patients treated with ingenol mebutate. In a trial comparing Picato and imiquimod, skin cancer was more common in the areas treated with Picato than in areas treated with imiquimod, the statement said.
“While uncertainties remain, the EMA said in a Jan. 17 news release. “The PRAC has therefore recommended suspending the medicine’s marketing authorization as a precaution and noted that alternative treatments are available.”
FDA is looking at the situation
LEO Pharma, the company that markets Picato, announced on Jan. 9 that it was initiating voluntary withdrawal of marketing authorization and possible voluntary withdrawal of Picato in the European Union (EU) and European Economic Area (EEA). The statement says, however, that “LEO Pharma has carefully reviewed the information received from PRAC, and the company disagrees with the ongoing assessment of PRAC.” There are “no additional safety data and it is LEO Pharma’s position that there is no evidence of a causal relationship or plausible mechanism hypothesis between the use of Picato and the development of skin malignancies.” An update added to the press release on Jan. 17 restates that the company disagrees with the assessment of PRAC.
“This matter does not affect Picato in the U.S., and there are no new developments in the [United States]. Picato continues to be available to patients in the U.S. We remain in dialogue with the U.S. Food and Drug Administration about Picato in the EU/EEA,” Rhonda Sciarra, associate director of global external communications for LEO Pharma, said in an email. “We remain committed to ensuring patient safety, rigorous pharmacovigilance monitoring, and transparency,” she added.
The FDA “is gathering data and information to investigate the safety concern related to Picato,” a spokesperson for the FDA told Dermatology News. “We are committed to sharing relevant findings when we have sufficient understanding of the situation and of what actions should be taken,” he added.
Examining the data
The EMA announcement described data about the risk of skin cancer in studies of Picato. A 3-year study in 484 patients found a higher incidence of skin malignancy with ingenol mebutate than with the comparator, imiquimod. In all, 3.3% of patients developed cancer in the ingenol mebutate group, compared with 0.4% in the comparator group.
In an 8-week vehicle-controlled trial in 1,262 patients, there were more skin tumors in patients who received ingenol mebutate than in those in the vehicle arm (1.0% vs. 0.1%).
In addition, according to the EMA statement, in four trials of a related ester that included 1,234 patients, a higher incidence of skin tumors occurred with the related drug, ingenol disoxate, than with a vehicle control (7.7% vs. 2.9%). PRAC considered these data because ingenol disoxate and ingenol mebutate are closely related, the EMA said.
“Health care professionals should stop prescribing Picato and consider different treatment options while authorities review the data,” according to the European agency. “Health care professionals should advise patients to be vigilant for any skin lesions developing and to seek medical advice promptly should any occur,” the statement adds.
Picato has been authorized in the EU since 2012, and the FDA approved Picato the same year. Patients have received about 2.8 million treatment courses in that time, according to the LEO Pharma press release.
As a precaution, the European Medicines Agency (EMA) has recommended that patients stop using ingenol mebutate (Picato) while the agency continues to review the safety of the topical treatment, which is indicated for the treatment of actinic keratosis in Europe and the United States.
No such action has been taken in the United States.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) is reviewing data on skin cancer in patients treated with ingenol mebutate. In a trial comparing Picato and imiquimod, skin cancer was more common in the areas treated with Picato than in areas treated with imiquimod, the statement said.
“While uncertainties remain, the EMA said in a Jan. 17 news release. “The PRAC has therefore recommended suspending the medicine’s marketing authorization as a precaution and noted that alternative treatments are available.”
FDA is looking at the situation
LEO Pharma, the company that markets Picato, announced on Jan. 9 that it was initiating voluntary withdrawal of marketing authorization and possible voluntary withdrawal of Picato in the European Union (EU) and European Economic Area (EEA). The statement says, however, that “LEO Pharma has carefully reviewed the information received from PRAC, and the company disagrees with the ongoing assessment of PRAC.” There are “no additional safety data and it is LEO Pharma’s position that there is no evidence of a causal relationship or plausible mechanism hypothesis between the use of Picato and the development of skin malignancies.” An update added to the press release on Jan. 17 restates that the company disagrees with the assessment of PRAC.
“This matter does not affect Picato in the U.S., and there are no new developments in the [United States]. Picato continues to be available to patients in the U.S. We remain in dialogue with the U.S. Food and Drug Administration about Picato in the EU/EEA,” Rhonda Sciarra, associate director of global external communications for LEO Pharma, said in an email. “We remain committed to ensuring patient safety, rigorous pharmacovigilance monitoring, and transparency,” she added.
The FDA “is gathering data and information to investigate the safety concern related to Picato,” a spokesperson for the FDA told Dermatology News. “We are committed to sharing relevant findings when we have sufficient understanding of the situation and of what actions should be taken,” he added.
Examining the data
The EMA announcement described data about the risk of skin cancer in studies of Picato. A 3-year study in 484 patients found a higher incidence of skin malignancy with ingenol mebutate than with the comparator, imiquimod. In all, 3.3% of patients developed cancer in the ingenol mebutate group, compared with 0.4% in the comparator group.
In an 8-week vehicle-controlled trial in 1,262 patients, there were more skin tumors in patients who received ingenol mebutate than in those in the vehicle arm (1.0% vs. 0.1%).
In addition, according to the EMA statement, in four trials of a related ester that included 1,234 patients, a higher incidence of skin tumors occurred with the related drug, ingenol disoxate, than with a vehicle control (7.7% vs. 2.9%). PRAC considered these data because ingenol disoxate and ingenol mebutate are closely related, the EMA said.
“Health care professionals should stop prescribing Picato and consider different treatment options while authorities review the data,” according to the European agency. “Health care professionals should advise patients to be vigilant for any skin lesions developing and to seek medical advice promptly should any occur,” the statement adds.
Picato has been authorized in the EU since 2012, and the FDA approved Picato the same year. Patients have received about 2.8 million treatment courses in that time, according to the LEO Pharma press release.
As a precaution, the European Medicines Agency (EMA) has recommended that patients stop using ingenol mebutate (Picato) while the agency continues to review the safety of the topical treatment, which is indicated for the treatment of actinic keratosis in Europe and the United States.
No such action has been taken in the United States.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) is reviewing data on skin cancer in patients treated with ingenol mebutate. In a trial comparing Picato and imiquimod, skin cancer was more common in the areas treated with Picato than in areas treated with imiquimod, the statement said.
“While uncertainties remain, the EMA said in a Jan. 17 news release. “The PRAC has therefore recommended suspending the medicine’s marketing authorization as a precaution and noted that alternative treatments are available.”
FDA is looking at the situation
LEO Pharma, the company that markets Picato, announced on Jan. 9 that it was initiating voluntary withdrawal of marketing authorization and possible voluntary withdrawal of Picato in the European Union (EU) and European Economic Area (EEA). The statement says, however, that “LEO Pharma has carefully reviewed the information received from PRAC, and the company disagrees with the ongoing assessment of PRAC.” There are “no additional safety data and it is LEO Pharma’s position that there is no evidence of a causal relationship or plausible mechanism hypothesis between the use of Picato and the development of skin malignancies.” An update added to the press release on Jan. 17 restates that the company disagrees with the assessment of PRAC.
“This matter does not affect Picato in the U.S., and there are no new developments in the [United States]. Picato continues to be available to patients in the U.S. We remain in dialogue with the U.S. Food and Drug Administration about Picato in the EU/EEA,” Rhonda Sciarra, associate director of global external communications for LEO Pharma, said in an email. “We remain committed to ensuring patient safety, rigorous pharmacovigilance monitoring, and transparency,” she added.
The FDA “is gathering data and information to investigate the safety concern related to Picato,” a spokesperson for the FDA told Dermatology News. “We are committed to sharing relevant findings when we have sufficient understanding of the situation and of what actions should be taken,” he added.
Examining the data
The EMA announcement described data about the risk of skin cancer in studies of Picato. A 3-year study in 484 patients found a higher incidence of skin malignancy with ingenol mebutate than with the comparator, imiquimod. In all, 3.3% of patients developed cancer in the ingenol mebutate group, compared with 0.4% in the comparator group.
In an 8-week vehicle-controlled trial in 1,262 patients, there were more skin tumors in patients who received ingenol mebutate than in those in the vehicle arm (1.0% vs. 0.1%).
In addition, according to the EMA statement, in four trials of a related ester that included 1,234 patients, a higher incidence of skin tumors occurred with the related drug, ingenol disoxate, than with a vehicle control (7.7% vs. 2.9%). PRAC considered these data because ingenol disoxate and ingenol mebutate are closely related, the EMA said.
“Health care professionals should stop prescribing Picato and consider different treatment options while authorities review the data,” according to the European agency. “Health care professionals should advise patients to be vigilant for any skin lesions developing and to seek medical advice promptly should any occur,” the statement adds.
Picato has been authorized in the EU since 2012, and the FDA approved Picato the same year. Patients have received about 2.8 million treatment courses in that time, according to the LEO Pharma press release.
The Ketogenic Diet and Dermatology: A Primer on Current Literature
The ketogenic diet has been therapeutically employed by physicians since the times of Hippocrates, primarily for its effect on the nervous system.1 The neurologic literature is inundated with the uses of this medicinal diet for applications in the treatment of epilepsy, neurodegenerative disease, malignancy, and enzyme deficiencies, among others.2 In recent years, physicians and scientists have moved to study the application of a ketogenic diet in the realms of cardiovascular disease,3 autoimmune disease,4 management of diabetes mellitus (DM) and obesity,3,5 and enhancement of sports and combat performance,6 all with promising results. Increased interest in alternative therapies among the lay population and the efficacy purported by many adherents has spurred intrigue by health care professionals. Over the last decade, there has seen a boom in so-called holistic approaches to health; included are the Paleo Diet, Primal Blueprint Diet, Bulletproof Diet, and the ketogenic/low-carbohydrate, high-fat diet. The benefits of ketones in these diets—through intermittent fasting or cyclical ketosis—–for cognitive enhancement, overall well-being, amelioration of chronic disease states, and increased health span have been promulgated to the lay population. But to date, there is a large gap in the literature on the applications of ketones as well as the ketogenic diet in dermatology and skin health and disease.
The aim of this article is not to summarize the uses of ketones and the ketogenic diet in dermatologic applications (because, unfortunately, those studies have not been undertaken) but to provide evidence from all available literature to support the need for targeted research and to encourage dermatologists to investigate ketones and their role in treating skin disease, primarily in an adjunctive manner. In doing so, a clearly medicinal diet may gain a foothold in the disease-treatment repertoire and among health-promoting agents of the dermatologist. Given the amount of capital being spent on health care, there is an ever-increasing need for low-cost, safe, and tolerable treatments that can be used for multiple disease processes and to promote health. We believe the ketogenic diet is such an adjunctive therapeutic option, as it has clearly been proven to be tolerable, safe, and efficacious for many people over the last millennia.
We conducted a PubMed search of articles indexed for MEDLINE using varying combinations of the terms ketones, ketogenic, skin, inflammation, metabolic, oxidation, dermatology, and dermatologic and found 12 articles. Herein, we summarize the relevant articles and the works cited by those articles.
Adverse Effects of the Ketogenic Diet
As with all medical therapies, the ketogenic diet is not without risk of adverse effects, which should be communicated at the outset of this article and with patients in the clinic. The only known absolute contraindications to a ketogenic diet are porphyria and pyruvate carboxylase deficiency secondary to underlying metabolic derangements.7 Certain metabolic cytopathies and carnitine deficiency are relative contraindications, and patients with these conditions should be cautiously placed on this diet and closely monitored. Dehydration, acidosis, lethargy, hypoglycemia, dyslipidemia, electrolyte imbalances, prurigo pigmentosa, and gastrointestinal distress may be an acute issue, but these effects are transient and can be managed. Chronic adverse effects are nephrolithiasis (there are recommended screening procedures for those at risk and prophylactic therapies, which is beyond the scope of this article) and weight loss.7
NLRP3 Inflammasome Suppression
Youm et al8 reported their findings in Nature Medicine that β-hydroxybutyrate, a ketone body that naturally circulates in the human body, specifically suppresses activity of the NLRP3 inflammasome. The NLRP3 inflammasome serves as the activating platform for IL-1β.8 Aberrant and elevated IL-1β levels cause or are associated with a number of dermatologic diseases—namely, the autoinflammatory syndromes (familial cold autoinflammatory syndrome, Muckle-Wells syndrome, neonatal-onset multisystemic disease/chronic infantile neurological cutaneous articular syndrome), hyperimmunoglobulinemia D with periodic fever syndrome, tumor necrosis factor–receptor associated periodic syndrome, juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, Sweet syndrome, Behçet disease, gout, sunburn and contact hypersensitivity, hidradenitis suppurativa, and metastatic melanoma.7 Clearly, the ketogenic diet may be employed in a therapeutic manner (though to what degree, we need further study) for these dermatologic conditions based on the interaction with the NRLP3 inflammasome and IL-1β.
Acne
A link between acne and diet has long been suspected, but a lack of well-controlled studies has caused only speculation to remain. Recent literature suggests that the effects of insulin may be a notable driver of acne through effects on sex hormones and subsequent effects on sebum production and inflammation. Cordain et al9 discuss the mechanism by which insulin can worsen acne in a valuable article, which Paoli et al10 later corroborated. Essentially, insulin propagates acne by 2 known mechanisms. First, an increase in serum insulin causes a rise in insulinlike growth factor 1 levels and a decrease in insulinlike growth factor binding protein 3 levels, which directly influences keratinocyte proliferation and reduces retinoic acid receptor/retinoid X receptor activity in the skin, causing hyperkeratinization and concomitant abnormal desquamation of the follicular epithelium.9,10 Second, this increase in insulinlike growth factor 1 and insulin causes a decrease in sex hormone–binding globulin and leads to increased androgen production and circulation in the skin, which causes an increase in sebum production. These factors combined with skin that is colonized with Cutibacterium acnes lead to an inflammatory response and the disease known as acne vulgaris.9,10 A ketogenic diet could help ameliorate acne because it results in very little insulin secretion, unlike the typical Western diet, which causes frequent large spikes in insulin levels. Furthermore, the anti-inflammatory effects of ketones would benefit the inflammatory nature of this disease.
DM and Diabetic Skin Disease
Diabetes mellitus carries with it the risk for skin diseases specific to the diabetic disease process, such as increased risk for bacterial and fungal infections, venous stasis, pruritus (secondary to poor circulation), acanthosis nigricans, diabetic dermopathy, necrobiosis lipoidica diabeticorum, digital sclerosis, and bullosis diabeticorum.11 It is well established that better control of DM results in better disease state outcomes.12 The ketogenic diet has shown itself to be a formidable and successful treatment in the diseases of carbohydrate intolerance (eg, metabolic syndrome, insulin resistance, type 2 DM) because of several known mechanisms, including less glucose entering the body and thus less fat deposition, end-product glycation, and free-radical production (discussed below); enhanced fat loss and metabolic efficiency; increased insulin sensitivity; and decreased inflammation.13 Lowering a patient’s insulin resistance through a ketogenic diet may help prevent or treat diabetic skin disease.
Dermatologic Malignancy
A ketogenic diet has been of interest in oncology research as an adjunctive therapy for several reasons: anti-inflammatory effects, antioxidation effects, possible effects on mammalian target of rapamycin (mTOR) regulation,7 and exploitation of the Warburg effect.14 One article discusses how mTOR, a cell-cycle regulator of particular importance in cancer biology, can be influenced by ketones both directly and indirectly through modulating the inflammatory response.7 It has been shown that suppressing mTOR activity limits and slows tumor growth and spread. Ketones also may prove to be a unique method of metabolically exploiting cancer physiology. The Warburg effect, which earned Otto Warburg the Nobel Prize in Physiology or Medicine in 1931, is the observation that cancerous cells produce adenosine triphosphate solely through aerobic glycolysis followed by lactic acid fermentation.14 This phenomenon is the basis of the positron emission tomography scan. There are several small studies of the effects of ketogenic diets on malignancy, and although none of these studies are of substantial size or control, they show that a ketogenic diet can halt or even reverse tumor growth.15 The hypothesis is that because cancer cells cannot metabolize ketones (but normal cells can), the Warburg effect can be taken advantage of through a ketogenic diet to aid in the treatment of malignant disease.14 If further studies find it a formidable treatment, it most certainly would be helpful for the dermatologist involved in the treatment of cutaneous cancers.
Oxidative Stress
Oxidative stress, a state brought about when reactive oxygen species (ROS) production exceeds the antioxidant capacity of the cell and causes damage, is known to be a central part of certain skin diseases (eg, acne, psoriasis, cutaneous malignancy, varicose ulcers, cutaneous allergic reactions, and drug-induced skin photosensitivity).7 There are 2 proven mechanisms by which a ketogenic diet can augment the body’s innate antioxidation capacity. First, ketones activate a potent antioxidant upregulating protein known as NRF2, which is bound in cytosol and remains inactive until activated by certain stimuli (ie, ketones).16 Migration to the nucleus causes transcriptional changes in DNA to upregulate, via a myriad of pathways, antioxidant production in the cell; most notably, it results in increased glutathione levels.17 NRF2 also targets several genes involved in chronic inflammatory skin diseases that cause an increase in the antioxidant capacity.18 As an aside, several foods encouraged on a ketogenic diet also activate NRF2 independently of ketones (eg, coffee, broccoli).19 Second, a ketogenic diet results in fewer produced ROS and an increase in the nicotinamide adenine dinucleotide ratio produced by the mitochondria; in short, it is a more efficient way of producing cellular energy while enhancing mitochondrial function. When fewer ROS are produced, there is less oxidative stress that needs to be attended to by the cell and less cellular damage. Feichtinger et al19 point out that mitochondrial inefficiency and dysfunction often are overlooked components in several skin diseases, and based on the studies discussed above, these diseases may be aided with a ketogenic diet.
Patient Applications
Clearly, a ketogenic diet is therapeutic, and there are many promising potential roles it may play in the treatment of a wide variety of health and disease states through hormonal normalization, antioxidant effects, anti-inflammatory effects, and improvement of metabolic risk factors. However, there are vast limitations to what is known about the ketogenic diet and how it might be employed, particularly by the dermatologist. First, the ketogenic diet lacks a firm definition. Although processed inflammatory vegetable oils and meats are low in carbohydrates and high in fat by definition, it is impossible to argue that they are healthy options for consumption and disease prevention and treatment. Second, nutrigenomics dictates that there must be an individual role in how the diet is employed (eg, patients who are lactose intolerant will need to stay away from dairy). Third, there are no clear proven clinical results from the ketogenic diet in the realm of dermatology. Fourth, as with everything, there are potential detrimental side effects of the ketogenic diet that must be considered for patients (though there are established screening procedures and prophylactic therapies that are beyond the scope of this article). Further, other diets have shown benefit for many other disease states and health promotion purposes (eg, the Mediterranean diet).20 We do not know yet if the avoidance of certain dietary factors such as processed carbohydrates and fats are more beneficial than adopting a state of ketosis at this time, and therefore we are not claiming superiority of one dietary approach over others that are proven to promote health.
Because there are no large-scale studies of the ketogenic diet, there is no verified standardization of initiating and monitoring it, though certain academic centers do have published methods of doing so.21 There are ample anecdotal methods of initiating, maintaining, and monitoring the ketogenic diet.22 In short, drastic restriction of carbohydrate intake and increased fat consumption are the staples of initiating the diet. Medium-chain triglyceride oil supplementation, coffee consumption, intermittent fasting, and low-level aerobic activity also are thought to aid in transition to a ketogenic state. As a result, a dermatologist may recommend that patients interested in this option begin by focusing on fat, fiber, and protein consumption while greatly reducing the amount of carbohydrates in the diet. Morning walks or more intense workouts for fitter patients should be encouraged. Consumption of serum ketone–enhancing foods (eg, coffee, medium-chain triglyceride oil, coconut products) also should be encouraged. A popular beverage known as Bulletproof coffee also may be of interest.23 A blood ketone meter can be used for biofeedback to reinforce these behaviors by aiming for proper β-hydroxybutyrate levels. Numerous companies and websites exist for supporting those patients wishing to pursue a ketogenic state, some hosted by physicians/researchers with others hosted by laypeople with an interest in the topic; discretion should be used as to the clinical and scientific accuracy of these sites. The dermatologist in particular can follow these patients and assess for changes in severity of skin disease, subjective well-being, need for medications and adjunctive therapies, and status of comorbid conditions.
For more information on the ketogenic diet, consider reading the works of the following physicians and researchers who all have been involved with or are currently conducting research in the medical use of ketones and ketogenic diets: David Perlmutter, MD; Thomas Seyfried, PhD; Dominic D’Agostino, PhD; Terry Wahls, MD; Jeff Volek, PhD; and Peter Attia, MD.
Conclusion
Based on the available data, there is potential for use of the ketogenic diet in an adjunctive manner for dermatologic applications, and studies should be undertaken to establish the efficacy or inefficacy of this diet as a preventive measure or treatment of skin disease. With the large push for complementary and alternative therapies over the last decade, particularly for skin disease, the time for research on the ketogenic diet is ripe. Over the coming years, it is our hope that larger clinical, randomized, controlled trials will be conducted for the benefit of dermatology patients worldwide.
- Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49:3-5.
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. 2015;2015:681289. doi:10.1155/2015/681289.
- Yancy WS, Foy M, Chalecki AM, et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2005;2:34.
- Phinney SD. Ketogenic diets and physical performance. Nutr Metab (Lond). 2004;1:2.
- J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of western civilization. Arch Dermatol
- Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- American Diabetes Association. Skin complications. http://www.diabetes.org/diabetes/complications/skin-complications. Accessed December 18, 2019.
- Greenapple R. Review of strategies to enhance outcomes for patients with type 2 diabetes: payers’ perspective. Am Health Drug Benefits. 2011;4:377-386.
- Paoli A, Rubini A, Volek JS, et al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789-796.
- Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963-970.
- Zhou W, Mukherjee P, Kiebish MA. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:5.
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960-14965.
- Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010:40:238-244.
- Vicente SJ, Ishimoto EY, Torres EA. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats.J Agric Food Chem. 2014;62:116-122.
- Feichtinger R, Sperl W, Bauer JW, et al. Mitochondrial dysfunction: a neglected component of skin diseases. Exp Dermatol. 2014;23:607-614.
- Brandhorst S, Longo VD. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res. 2019;124:952-965.
- Johns Hopkins Medicine. Ketogenic diet therapy for epilepsy. https://www.hopkinsmedicine.org/neurology_neurosurgery/
centers_clinics/epilepsy/pediatric_epilepsy/ketogenic_diet.html. Accessed December 18, 2019. - Bergqvist AG. Long-term monitoring of the ketogenic diet: do’s and don’ts. Epilepsy Res. 2012;100:261-266.
- Bulletproof. Bulletproof coffee: everything you want to know. https://blog.bulletproof.com/how-to-make-your-coffee-bulletproof-and-your-morning-too/. Accessed December 18, 2019.
The ketogenic diet has been therapeutically employed by physicians since the times of Hippocrates, primarily for its effect on the nervous system.1 The neurologic literature is inundated with the uses of this medicinal diet for applications in the treatment of epilepsy, neurodegenerative disease, malignancy, and enzyme deficiencies, among others.2 In recent years, physicians and scientists have moved to study the application of a ketogenic diet in the realms of cardiovascular disease,3 autoimmune disease,4 management of diabetes mellitus (DM) and obesity,3,5 and enhancement of sports and combat performance,6 all with promising results. Increased interest in alternative therapies among the lay population and the efficacy purported by many adherents has spurred intrigue by health care professionals. Over the last decade, there has seen a boom in so-called holistic approaches to health; included are the Paleo Diet, Primal Blueprint Diet, Bulletproof Diet, and the ketogenic/low-carbohydrate, high-fat diet. The benefits of ketones in these diets—through intermittent fasting or cyclical ketosis—–for cognitive enhancement, overall well-being, amelioration of chronic disease states, and increased health span have been promulgated to the lay population. But to date, there is a large gap in the literature on the applications of ketones as well as the ketogenic diet in dermatology and skin health and disease.
The aim of this article is not to summarize the uses of ketones and the ketogenic diet in dermatologic applications (because, unfortunately, those studies have not been undertaken) but to provide evidence from all available literature to support the need for targeted research and to encourage dermatologists to investigate ketones and their role in treating skin disease, primarily in an adjunctive manner. In doing so, a clearly medicinal diet may gain a foothold in the disease-treatment repertoire and among health-promoting agents of the dermatologist. Given the amount of capital being spent on health care, there is an ever-increasing need for low-cost, safe, and tolerable treatments that can be used for multiple disease processes and to promote health. We believe the ketogenic diet is such an adjunctive therapeutic option, as it has clearly been proven to be tolerable, safe, and efficacious for many people over the last millennia.
We conducted a PubMed search of articles indexed for MEDLINE using varying combinations of the terms ketones, ketogenic, skin, inflammation, metabolic, oxidation, dermatology, and dermatologic and found 12 articles. Herein, we summarize the relevant articles and the works cited by those articles.
Adverse Effects of the Ketogenic Diet
As with all medical therapies, the ketogenic diet is not without risk of adverse effects, which should be communicated at the outset of this article and with patients in the clinic. The only known absolute contraindications to a ketogenic diet are porphyria and pyruvate carboxylase deficiency secondary to underlying metabolic derangements.7 Certain metabolic cytopathies and carnitine deficiency are relative contraindications, and patients with these conditions should be cautiously placed on this diet and closely monitored. Dehydration, acidosis, lethargy, hypoglycemia, dyslipidemia, electrolyte imbalances, prurigo pigmentosa, and gastrointestinal distress may be an acute issue, but these effects are transient and can be managed. Chronic adverse effects are nephrolithiasis (there are recommended screening procedures for those at risk and prophylactic therapies, which is beyond the scope of this article) and weight loss.7
NLRP3 Inflammasome Suppression
Youm et al8 reported their findings in Nature Medicine that β-hydroxybutyrate, a ketone body that naturally circulates in the human body, specifically suppresses activity of the NLRP3 inflammasome. The NLRP3 inflammasome serves as the activating platform for IL-1β.8 Aberrant and elevated IL-1β levels cause or are associated with a number of dermatologic diseases—namely, the autoinflammatory syndromes (familial cold autoinflammatory syndrome, Muckle-Wells syndrome, neonatal-onset multisystemic disease/chronic infantile neurological cutaneous articular syndrome), hyperimmunoglobulinemia D with periodic fever syndrome, tumor necrosis factor–receptor associated periodic syndrome, juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, Sweet syndrome, Behçet disease, gout, sunburn and contact hypersensitivity, hidradenitis suppurativa, and metastatic melanoma.7 Clearly, the ketogenic diet may be employed in a therapeutic manner (though to what degree, we need further study) for these dermatologic conditions based on the interaction with the NRLP3 inflammasome and IL-1β.
Acne
A link between acne and diet has long been suspected, but a lack of well-controlled studies has caused only speculation to remain. Recent literature suggests that the effects of insulin may be a notable driver of acne through effects on sex hormones and subsequent effects on sebum production and inflammation. Cordain et al9 discuss the mechanism by which insulin can worsen acne in a valuable article, which Paoli et al10 later corroborated. Essentially, insulin propagates acne by 2 known mechanisms. First, an increase in serum insulin causes a rise in insulinlike growth factor 1 levels and a decrease in insulinlike growth factor binding protein 3 levels, which directly influences keratinocyte proliferation and reduces retinoic acid receptor/retinoid X receptor activity in the skin, causing hyperkeratinization and concomitant abnormal desquamation of the follicular epithelium.9,10 Second, this increase in insulinlike growth factor 1 and insulin causes a decrease in sex hormone–binding globulin and leads to increased androgen production and circulation in the skin, which causes an increase in sebum production. These factors combined with skin that is colonized with Cutibacterium acnes lead to an inflammatory response and the disease known as acne vulgaris.9,10 A ketogenic diet could help ameliorate acne because it results in very little insulin secretion, unlike the typical Western diet, which causes frequent large spikes in insulin levels. Furthermore, the anti-inflammatory effects of ketones would benefit the inflammatory nature of this disease.
DM and Diabetic Skin Disease
Diabetes mellitus carries with it the risk for skin diseases specific to the diabetic disease process, such as increased risk for bacterial and fungal infections, venous stasis, pruritus (secondary to poor circulation), acanthosis nigricans, diabetic dermopathy, necrobiosis lipoidica diabeticorum, digital sclerosis, and bullosis diabeticorum.11 It is well established that better control of DM results in better disease state outcomes.12 The ketogenic diet has shown itself to be a formidable and successful treatment in the diseases of carbohydrate intolerance (eg, metabolic syndrome, insulin resistance, type 2 DM) because of several known mechanisms, including less glucose entering the body and thus less fat deposition, end-product glycation, and free-radical production (discussed below); enhanced fat loss and metabolic efficiency; increased insulin sensitivity; and decreased inflammation.13 Lowering a patient’s insulin resistance through a ketogenic diet may help prevent or treat diabetic skin disease.
Dermatologic Malignancy
A ketogenic diet has been of interest in oncology research as an adjunctive therapy for several reasons: anti-inflammatory effects, antioxidation effects, possible effects on mammalian target of rapamycin (mTOR) regulation,7 and exploitation of the Warburg effect.14 One article discusses how mTOR, a cell-cycle regulator of particular importance in cancer biology, can be influenced by ketones both directly and indirectly through modulating the inflammatory response.7 It has been shown that suppressing mTOR activity limits and slows tumor growth and spread. Ketones also may prove to be a unique method of metabolically exploiting cancer physiology. The Warburg effect, which earned Otto Warburg the Nobel Prize in Physiology or Medicine in 1931, is the observation that cancerous cells produce adenosine triphosphate solely through aerobic glycolysis followed by lactic acid fermentation.14 This phenomenon is the basis of the positron emission tomography scan. There are several small studies of the effects of ketogenic diets on malignancy, and although none of these studies are of substantial size or control, they show that a ketogenic diet can halt or even reverse tumor growth.15 The hypothesis is that because cancer cells cannot metabolize ketones (but normal cells can), the Warburg effect can be taken advantage of through a ketogenic diet to aid in the treatment of malignant disease.14 If further studies find it a formidable treatment, it most certainly would be helpful for the dermatologist involved in the treatment of cutaneous cancers.
Oxidative Stress
Oxidative stress, a state brought about when reactive oxygen species (ROS) production exceeds the antioxidant capacity of the cell and causes damage, is known to be a central part of certain skin diseases (eg, acne, psoriasis, cutaneous malignancy, varicose ulcers, cutaneous allergic reactions, and drug-induced skin photosensitivity).7 There are 2 proven mechanisms by which a ketogenic diet can augment the body’s innate antioxidation capacity. First, ketones activate a potent antioxidant upregulating protein known as NRF2, which is bound in cytosol and remains inactive until activated by certain stimuli (ie, ketones).16 Migration to the nucleus causes transcriptional changes in DNA to upregulate, via a myriad of pathways, antioxidant production in the cell; most notably, it results in increased glutathione levels.17 NRF2 also targets several genes involved in chronic inflammatory skin diseases that cause an increase in the antioxidant capacity.18 As an aside, several foods encouraged on a ketogenic diet also activate NRF2 independently of ketones (eg, coffee, broccoli).19 Second, a ketogenic diet results in fewer produced ROS and an increase in the nicotinamide adenine dinucleotide ratio produced by the mitochondria; in short, it is a more efficient way of producing cellular energy while enhancing mitochondrial function. When fewer ROS are produced, there is less oxidative stress that needs to be attended to by the cell and less cellular damage. Feichtinger et al19 point out that mitochondrial inefficiency and dysfunction often are overlooked components in several skin diseases, and based on the studies discussed above, these diseases may be aided with a ketogenic diet.
Patient Applications
Clearly, a ketogenic diet is therapeutic, and there are many promising potential roles it may play in the treatment of a wide variety of health and disease states through hormonal normalization, antioxidant effects, anti-inflammatory effects, and improvement of metabolic risk factors. However, there are vast limitations to what is known about the ketogenic diet and how it might be employed, particularly by the dermatologist. First, the ketogenic diet lacks a firm definition. Although processed inflammatory vegetable oils and meats are low in carbohydrates and high in fat by definition, it is impossible to argue that they are healthy options for consumption and disease prevention and treatment. Second, nutrigenomics dictates that there must be an individual role in how the diet is employed (eg, patients who are lactose intolerant will need to stay away from dairy). Third, there are no clear proven clinical results from the ketogenic diet in the realm of dermatology. Fourth, as with everything, there are potential detrimental side effects of the ketogenic diet that must be considered for patients (though there are established screening procedures and prophylactic therapies that are beyond the scope of this article). Further, other diets have shown benefit for many other disease states and health promotion purposes (eg, the Mediterranean diet).20 We do not know yet if the avoidance of certain dietary factors such as processed carbohydrates and fats are more beneficial than adopting a state of ketosis at this time, and therefore we are not claiming superiority of one dietary approach over others that are proven to promote health.
Because there are no large-scale studies of the ketogenic diet, there is no verified standardization of initiating and monitoring it, though certain academic centers do have published methods of doing so.21 There are ample anecdotal methods of initiating, maintaining, and monitoring the ketogenic diet.22 In short, drastic restriction of carbohydrate intake and increased fat consumption are the staples of initiating the diet. Medium-chain triglyceride oil supplementation, coffee consumption, intermittent fasting, and low-level aerobic activity also are thought to aid in transition to a ketogenic state. As a result, a dermatologist may recommend that patients interested in this option begin by focusing on fat, fiber, and protein consumption while greatly reducing the amount of carbohydrates in the diet. Morning walks or more intense workouts for fitter patients should be encouraged. Consumption of serum ketone–enhancing foods (eg, coffee, medium-chain triglyceride oil, coconut products) also should be encouraged. A popular beverage known as Bulletproof coffee also may be of interest.23 A blood ketone meter can be used for biofeedback to reinforce these behaviors by aiming for proper β-hydroxybutyrate levels. Numerous companies and websites exist for supporting those patients wishing to pursue a ketogenic state, some hosted by physicians/researchers with others hosted by laypeople with an interest in the topic; discretion should be used as to the clinical and scientific accuracy of these sites. The dermatologist in particular can follow these patients and assess for changes in severity of skin disease, subjective well-being, need for medications and adjunctive therapies, and status of comorbid conditions.
For more information on the ketogenic diet, consider reading the works of the following physicians and researchers who all have been involved with or are currently conducting research in the medical use of ketones and ketogenic diets: David Perlmutter, MD; Thomas Seyfried, PhD; Dominic D’Agostino, PhD; Terry Wahls, MD; Jeff Volek, PhD; and Peter Attia, MD.
Conclusion
Based on the available data, there is potential for use of the ketogenic diet in an adjunctive manner for dermatologic applications, and studies should be undertaken to establish the efficacy or inefficacy of this diet as a preventive measure or treatment of skin disease. With the large push for complementary and alternative therapies over the last decade, particularly for skin disease, the time for research on the ketogenic diet is ripe. Over the coming years, it is our hope that larger clinical, randomized, controlled trials will be conducted for the benefit of dermatology patients worldwide.
The ketogenic diet has been therapeutically employed by physicians since the times of Hippocrates, primarily for its effect on the nervous system.1 The neurologic literature is inundated with the uses of this medicinal diet for applications in the treatment of epilepsy, neurodegenerative disease, malignancy, and enzyme deficiencies, among others.2 In recent years, physicians and scientists have moved to study the application of a ketogenic diet in the realms of cardiovascular disease,3 autoimmune disease,4 management of diabetes mellitus (DM) and obesity,3,5 and enhancement of sports and combat performance,6 all with promising results. Increased interest in alternative therapies among the lay population and the efficacy purported by many adherents has spurred intrigue by health care professionals. Over the last decade, there has seen a boom in so-called holistic approaches to health; included are the Paleo Diet, Primal Blueprint Diet, Bulletproof Diet, and the ketogenic/low-carbohydrate, high-fat diet. The benefits of ketones in these diets—through intermittent fasting or cyclical ketosis—–for cognitive enhancement, overall well-being, amelioration of chronic disease states, and increased health span have been promulgated to the lay population. But to date, there is a large gap in the literature on the applications of ketones as well as the ketogenic diet in dermatology and skin health and disease.
The aim of this article is not to summarize the uses of ketones and the ketogenic diet in dermatologic applications (because, unfortunately, those studies have not been undertaken) but to provide evidence from all available literature to support the need for targeted research and to encourage dermatologists to investigate ketones and their role in treating skin disease, primarily in an adjunctive manner. In doing so, a clearly medicinal diet may gain a foothold in the disease-treatment repertoire and among health-promoting agents of the dermatologist. Given the amount of capital being spent on health care, there is an ever-increasing need for low-cost, safe, and tolerable treatments that can be used for multiple disease processes and to promote health. We believe the ketogenic diet is such an adjunctive therapeutic option, as it has clearly been proven to be tolerable, safe, and efficacious for many people over the last millennia.
We conducted a PubMed search of articles indexed for MEDLINE using varying combinations of the terms ketones, ketogenic, skin, inflammation, metabolic, oxidation, dermatology, and dermatologic and found 12 articles. Herein, we summarize the relevant articles and the works cited by those articles.
Adverse Effects of the Ketogenic Diet
As with all medical therapies, the ketogenic diet is not without risk of adverse effects, which should be communicated at the outset of this article and with patients in the clinic. The only known absolute contraindications to a ketogenic diet are porphyria and pyruvate carboxylase deficiency secondary to underlying metabolic derangements.7 Certain metabolic cytopathies and carnitine deficiency are relative contraindications, and patients with these conditions should be cautiously placed on this diet and closely monitored. Dehydration, acidosis, lethargy, hypoglycemia, dyslipidemia, electrolyte imbalances, prurigo pigmentosa, and gastrointestinal distress may be an acute issue, but these effects are transient and can be managed. Chronic adverse effects are nephrolithiasis (there are recommended screening procedures for those at risk and prophylactic therapies, which is beyond the scope of this article) and weight loss.7
NLRP3 Inflammasome Suppression
Youm et al8 reported their findings in Nature Medicine that β-hydroxybutyrate, a ketone body that naturally circulates in the human body, specifically suppresses activity of the NLRP3 inflammasome. The NLRP3 inflammasome serves as the activating platform for IL-1β.8 Aberrant and elevated IL-1β levels cause or are associated with a number of dermatologic diseases—namely, the autoinflammatory syndromes (familial cold autoinflammatory syndrome, Muckle-Wells syndrome, neonatal-onset multisystemic disease/chronic infantile neurological cutaneous articular syndrome), hyperimmunoglobulinemia D with periodic fever syndrome, tumor necrosis factor–receptor associated periodic syndrome, juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, Sweet syndrome, Behçet disease, gout, sunburn and contact hypersensitivity, hidradenitis suppurativa, and metastatic melanoma.7 Clearly, the ketogenic diet may be employed in a therapeutic manner (though to what degree, we need further study) for these dermatologic conditions based on the interaction with the NRLP3 inflammasome and IL-1β.
Acne
A link between acne and diet has long been suspected, but a lack of well-controlled studies has caused only speculation to remain. Recent literature suggests that the effects of insulin may be a notable driver of acne through effects on sex hormones and subsequent effects on sebum production and inflammation. Cordain et al9 discuss the mechanism by which insulin can worsen acne in a valuable article, which Paoli et al10 later corroborated. Essentially, insulin propagates acne by 2 known mechanisms. First, an increase in serum insulin causes a rise in insulinlike growth factor 1 levels and a decrease in insulinlike growth factor binding protein 3 levels, which directly influences keratinocyte proliferation and reduces retinoic acid receptor/retinoid X receptor activity in the skin, causing hyperkeratinization and concomitant abnormal desquamation of the follicular epithelium.9,10 Second, this increase in insulinlike growth factor 1 and insulin causes a decrease in sex hormone–binding globulin and leads to increased androgen production and circulation in the skin, which causes an increase in sebum production. These factors combined with skin that is colonized with Cutibacterium acnes lead to an inflammatory response and the disease known as acne vulgaris.9,10 A ketogenic diet could help ameliorate acne because it results in very little insulin secretion, unlike the typical Western diet, which causes frequent large spikes in insulin levels. Furthermore, the anti-inflammatory effects of ketones would benefit the inflammatory nature of this disease.
DM and Diabetic Skin Disease
Diabetes mellitus carries with it the risk for skin diseases specific to the diabetic disease process, such as increased risk for bacterial and fungal infections, venous stasis, pruritus (secondary to poor circulation), acanthosis nigricans, diabetic dermopathy, necrobiosis lipoidica diabeticorum, digital sclerosis, and bullosis diabeticorum.11 It is well established that better control of DM results in better disease state outcomes.12 The ketogenic diet has shown itself to be a formidable and successful treatment in the diseases of carbohydrate intolerance (eg, metabolic syndrome, insulin resistance, type 2 DM) because of several known mechanisms, including less glucose entering the body and thus less fat deposition, end-product glycation, and free-radical production (discussed below); enhanced fat loss and metabolic efficiency; increased insulin sensitivity; and decreased inflammation.13 Lowering a patient’s insulin resistance through a ketogenic diet may help prevent or treat diabetic skin disease.
Dermatologic Malignancy
A ketogenic diet has been of interest in oncology research as an adjunctive therapy for several reasons: anti-inflammatory effects, antioxidation effects, possible effects on mammalian target of rapamycin (mTOR) regulation,7 and exploitation of the Warburg effect.14 One article discusses how mTOR, a cell-cycle regulator of particular importance in cancer biology, can be influenced by ketones both directly and indirectly through modulating the inflammatory response.7 It has been shown that suppressing mTOR activity limits and slows tumor growth and spread. Ketones also may prove to be a unique method of metabolically exploiting cancer physiology. The Warburg effect, which earned Otto Warburg the Nobel Prize in Physiology or Medicine in 1931, is the observation that cancerous cells produce adenosine triphosphate solely through aerobic glycolysis followed by lactic acid fermentation.14 This phenomenon is the basis of the positron emission tomography scan. There are several small studies of the effects of ketogenic diets on malignancy, and although none of these studies are of substantial size or control, they show that a ketogenic diet can halt or even reverse tumor growth.15 The hypothesis is that because cancer cells cannot metabolize ketones (but normal cells can), the Warburg effect can be taken advantage of through a ketogenic diet to aid in the treatment of malignant disease.14 If further studies find it a formidable treatment, it most certainly would be helpful for the dermatologist involved in the treatment of cutaneous cancers.
Oxidative Stress
Oxidative stress, a state brought about when reactive oxygen species (ROS) production exceeds the antioxidant capacity of the cell and causes damage, is known to be a central part of certain skin diseases (eg, acne, psoriasis, cutaneous malignancy, varicose ulcers, cutaneous allergic reactions, and drug-induced skin photosensitivity).7 There are 2 proven mechanisms by which a ketogenic diet can augment the body’s innate antioxidation capacity. First, ketones activate a potent antioxidant upregulating protein known as NRF2, which is bound in cytosol and remains inactive until activated by certain stimuli (ie, ketones).16 Migration to the nucleus causes transcriptional changes in DNA to upregulate, via a myriad of pathways, antioxidant production in the cell; most notably, it results in increased glutathione levels.17 NRF2 also targets several genes involved in chronic inflammatory skin diseases that cause an increase in the antioxidant capacity.18 As an aside, several foods encouraged on a ketogenic diet also activate NRF2 independently of ketones (eg, coffee, broccoli).19 Second, a ketogenic diet results in fewer produced ROS and an increase in the nicotinamide adenine dinucleotide ratio produced by the mitochondria; in short, it is a more efficient way of producing cellular energy while enhancing mitochondrial function. When fewer ROS are produced, there is less oxidative stress that needs to be attended to by the cell and less cellular damage. Feichtinger et al19 point out that mitochondrial inefficiency and dysfunction often are overlooked components in several skin diseases, and based on the studies discussed above, these diseases may be aided with a ketogenic diet.
Patient Applications
Clearly, a ketogenic diet is therapeutic, and there are many promising potential roles it may play in the treatment of a wide variety of health and disease states through hormonal normalization, antioxidant effects, anti-inflammatory effects, and improvement of metabolic risk factors. However, there are vast limitations to what is known about the ketogenic diet and how it might be employed, particularly by the dermatologist. First, the ketogenic diet lacks a firm definition. Although processed inflammatory vegetable oils and meats are low in carbohydrates and high in fat by definition, it is impossible to argue that they are healthy options for consumption and disease prevention and treatment. Second, nutrigenomics dictates that there must be an individual role in how the diet is employed (eg, patients who are lactose intolerant will need to stay away from dairy). Third, there are no clear proven clinical results from the ketogenic diet in the realm of dermatology. Fourth, as with everything, there are potential detrimental side effects of the ketogenic diet that must be considered for patients (though there are established screening procedures and prophylactic therapies that are beyond the scope of this article). Further, other diets have shown benefit for many other disease states and health promotion purposes (eg, the Mediterranean diet).20 We do not know yet if the avoidance of certain dietary factors such as processed carbohydrates and fats are more beneficial than adopting a state of ketosis at this time, and therefore we are not claiming superiority of one dietary approach over others that are proven to promote health.
Because there are no large-scale studies of the ketogenic diet, there is no verified standardization of initiating and monitoring it, though certain academic centers do have published methods of doing so.21 There are ample anecdotal methods of initiating, maintaining, and monitoring the ketogenic diet.22 In short, drastic restriction of carbohydrate intake and increased fat consumption are the staples of initiating the diet. Medium-chain triglyceride oil supplementation, coffee consumption, intermittent fasting, and low-level aerobic activity also are thought to aid in transition to a ketogenic state. As a result, a dermatologist may recommend that patients interested in this option begin by focusing on fat, fiber, and protein consumption while greatly reducing the amount of carbohydrates in the diet. Morning walks or more intense workouts for fitter patients should be encouraged. Consumption of serum ketone–enhancing foods (eg, coffee, medium-chain triglyceride oil, coconut products) also should be encouraged. A popular beverage known as Bulletproof coffee also may be of interest.23 A blood ketone meter can be used for biofeedback to reinforce these behaviors by aiming for proper β-hydroxybutyrate levels. Numerous companies and websites exist for supporting those patients wishing to pursue a ketogenic state, some hosted by physicians/researchers with others hosted by laypeople with an interest in the topic; discretion should be used as to the clinical and scientific accuracy of these sites. The dermatologist in particular can follow these patients and assess for changes in severity of skin disease, subjective well-being, need for medications and adjunctive therapies, and status of comorbid conditions.
For more information on the ketogenic diet, consider reading the works of the following physicians and researchers who all have been involved with or are currently conducting research in the medical use of ketones and ketogenic diets: David Perlmutter, MD; Thomas Seyfried, PhD; Dominic D’Agostino, PhD; Terry Wahls, MD; Jeff Volek, PhD; and Peter Attia, MD.
Conclusion
Based on the available data, there is potential for use of the ketogenic diet in an adjunctive manner for dermatologic applications, and studies should be undertaken to establish the efficacy or inefficacy of this diet as a preventive measure or treatment of skin disease. With the large push for complementary and alternative therapies over the last decade, particularly for skin disease, the time for research on the ketogenic diet is ripe. Over the coming years, it is our hope that larger clinical, randomized, controlled trials will be conducted for the benefit of dermatology patients worldwide.
- Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49:3-5.
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. 2015;2015:681289. doi:10.1155/2015/681289.
- Yancy WS, Foy M, Chalecki AM, et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2005;2:34.
- Phinney SD. Ketogenic diets and physical performance. Nutr Metab (Lond). 2004;1:2.
- J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of western civilization. Arch Dermatol
- Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- American Diabetes Association. Skin complications. http://www.diabetes.org/diabetes/complications/skin-complications. Accessed December 18, 2019.
- Greenapple R. Review of strategies to enhance outcomes for patients with type 2 diabetes: payers’ perspective. Am Health Drug Benefits. 2011;4:377-386.
- Paoli A, Rubini A, Volek JS, et al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789-796.
- Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963-970.
- Zhou W, Mukherjee P, Kiebish MA. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:5.
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960-14965.
- Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010:40:238-244.
- Vicente SJ, Ishimoto EY, Torres EA. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats.J Agric Food Chem. 2014;62:116-122.
- Feichtinger R, Sperl W, Bauer JW, et al. Mitochondrial dysfunction: a neglected component of skin diseases. Exp Dermatol. 2014;23:607-614.
- Brandhorst S, Longo VD. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res. 2019;124:952-965.
- Johns Hopkins Medicine. Ketogenic diet therapy for epilepsy. https://www.hopkinsmedicine.org/neurology_neurosurgery/
centers_clinics/epilepsy/pediatric_epilepsy/ketogenic_diet.html. Accessed December 18, 2019. - Bergqvist AG. Long-term monitoring of the ketogenic diet: do’s and don’ts. Epilepsy Res. 2012;100:261-266.
- Bulletproof. Bulletproof coffee: everything you want to know. https://blog.bulletproof.com/how-to-make-your-coffee-bulletproof-and-your-morning-too/. Accessed December 18, 2019.
- Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49:3-5.
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. 2015;2015:681289. doi:10.1155/2015/681289.
- Yancy WS, Foy M, Chalecki AM, et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2005;2:34.
- Phinney SD. Ketogenic diets and physical performance. Nutr Metab (Lond). 2004;1:2.
- J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of western civilization. Arch Dermatol
- Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- American Diabetes Association. Skin complications. http://www.diabetes.org/diabetes/complications/skin-complications. Accessed December 18, 2019.
- Greenapple R. Review of strategies to enhance outcomes for patients with type 2 diabetes: payers’ perspective. Am Health Drug Benefits. 2011;4:377-386.
- Paoli A, Rubini A, Volek JS, et al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789-796.
- Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963-970.
- Zhou W, Mukherjee P, Kiebish MA. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:5.
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960-14965.
- Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010:40:238-244.
- Vicente SJ, Ishimoto EY, Torres EA. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats.J Agric Food Chem. 2014;62:116-122.
- Feichtinger R, Sperl W, Bauer JW, et al. Mitochondrial dysfunction: a neglected component of skin diseases. Exp Dermatol. 2014;23:607-614.
- Brandhorst S, Longo VD. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res. 2019;124:952-965.
- Johns Hopkins Medicine. Ketogenic diet therapy for epilepsy. https://www.hopkinsmedicine.org/neurology_neurosurgery/
centers_clinics/epilepsy/pediatric_epilepsy/ketogenic_diet.html. Accessed December 18, 2019. - Bergqvist AG. Long-term monitoring of the ketogenic diet: do’s and don’ts. Epilepsy Res. 2012;100:261-266.
- Bulletproof. Bulletproof coffee: everything you want to know. https://blog.bulletproof.com/how-to-make-your-coffee-bulletproof-and-your-morning-too/. Accessed December 18, 2019.
Practice Points
- The ketogenic diet has been employed since antiquity for varying ailments and has a good safety and efficacy profile if administered by a knowledgeable provider.
- New literature is showing promising potential roles for the ketogenic diet as an adjunctive therapy, particularly in the realm of inflammatory disorders, metabolic diseases, and malignancy.
- The dermatologist should be aware of this diet because it is gaining popularity with physicians and patients alike. Dermatologists also should know how it can potentially benefit a number of patients with dermatologic diseases based on small clinical trials, population studies, and basic science research.
New guideline provides recommendations for radiation therapy of basal cell, squamous cell cancers
who are not candidates for surgery, according to a new guideline from an American Society for Radiation Oncology task force.
“We hope that the dermatology community will find this guideline helpful, especially when it comes to defining clinical and pathological characteristics that may necessitate a discussion about the merits of postoperative radiation therapy,” said lead author Anna Likhacheva, MD, of the Sutter Medical Center in Sacramento, Calif., in an email. The guideline was published in Practical Radiation Oncology.
To address five key questions in regard to radiation therapy (RT) for the two most common skin cancers, the American Society for Radiation Oncology convened a task force of radiation, medical, and surgical oncologists; dermatopathologists; a radiation oncology resident; a medical physicist; and a dermatologist. They reviewed studies of adults with nonmetastatic, invasive basal cell carcinoma (BCC) or cutaneous squamous cell carcinoma (cSCC) that were published between May 1998 and June 2018, with the caveat that “there are limited, well-conducted modern randomized trials” in this area. As such, the majority of the recommendations have low to moderate quality of evidence designations.
“The conspicuous lack of prospective and randomized data should serve as a reminder to open clinical trials and collect outcomes data in a prospective fashion,” added Dr. Likhacheva, noting that “improving the quality of data on this topic will ultimately serve our common goal of improving patient outcomes.”
Their first recommendation was to strongly consider definitive RT as an alternative to surgery for BCC and cSCC, especially in areas where a surgical procedure would potentially compromise function or cosmesis. However, they did discourage its use in patients with genetic conditions associated with increased radiosensitivity.
Their second recommendation was to strongly consider postoperative radiation therapy for clinically or radiologically apparent gross perineural spread. They also strongly recommended PORT for cSCC patients with close or positive margins, with T3 or T4 tumors, or with desmoplastic or infiltrative tumors.
Their third recommendation was to strongly consider therapeutic lymphadenectomy followed by adjuvant RT in patients with cSCC or BCC that has metastasized to the regional lymph nodes. They also recommended definitive RT in medically inoperable patients with the same metastasized cSCC or BCC. In addition, patients with BCC or cSCC undergoing adjuvant RT after therapeutic lymphadenectomy were recommended a dose of 6,000-6,600 cGy, while patients with cSCC undergoing elective RT without a lymphadenectomy were recommended a dose of 5,000-5,400 cGy.
Their fourth recommendation focused on techniques and dose-fractionation schedules for RT in the definitive or postoperative setting. For patients with BCC and cSCC receiving definitive RT, the biologically effective dose (BED10) range for conventional fractionation – defined as 180-200 cGy/fraction – should be 70-93.5 and the BED10 range for hypofractionation – defined as 210-500 cGy/fraction – should be 56-88. For patients with BCC and cSCC receiving postoperative RT, the BED10 range for conventional fractionation should be 59.5-79.2 and the BED10 range for hypofractionation should be 56-70.2.
Finally, their fifth recommendation was to not add concurrent carboplatin to adjuvant RT in patients with resected, locally advanced cSCC. They also conditionally recommended adding concurrent drug therapies to definitive RT in patients with unresected, locally advanced cSCC.
Several of the authors reported receiving honoraria and travel expenses from medical and pharmaceutical companies, along with serving on their advisory boards. The others reported no conflicts of interest.
SOURCE: Likhacheva A et al. Pract Radiat Oncol. 2019 Dec 9. doi: 10.1016/j.prro.2019.10.014.
who are not candidates for surgery, according to a new guideline from an American Society for Radiation Oncology task force.
“We hope that the dermatology community will find this guideline helpful, especially when it comes to defining clinical and pathological characteristics that may necessitate a discussion about the merits of postoperative radiation therapy,” said lead author Anna Likhacheva, MD, of the Sutter Medical Center in Sacramento, Calif., in an email. The guideline was published in Practical Radiation Oncology.
To address five key questions in regard to radiation therapy (RT) for the two most common skin cancers, the American Society for Radiation Oncology convened a task force of radiation, medical, and surgical oncologists; dermatopathologists; a radiation oncology resident; a medical physicist; and a dermatologist. They reviewed studies of adults with nonmetastatic, invasive basal cell carcinoma (BCC) or cutaneous squamous cell carcinoma (cSCC) that were published between May 1998 and June 2018, with the caveat that “there are limited, well-conducted modern randomized trials” in this area. As such, the majority of the recommendations have low to moderate quality of evidence designations.
“The conspicuous lack of prospective and randomized data should serve as a reminder to open clinical trials and collect outcomes data in a prospective fashion,” added Dr. Likhacheva, noting that “improving the quality of data on this topic will ultimately serve our common goal of improving patient outcomes.”
Their first recommendation was to strongly consider definitive RT as an alternative to surgery for BCC and cSCC, especially in areas where a surgical procedure would potentially compromise function or cosmesis. However, they did discourage its use in patients with genetic conditions associated with increased radiosensitivity.
Their second recommendation was to strongly consider postoperative radiation therapy for clinically or radiologically apparent gross perineural spread. They also strongly recommended PORT for cSCC patients with close or positive margins, with T3 or T4 tumors, or with desmoplastic or infiltrative tumors.
Their third recommendation was to strongly consider therapeutic lymphadenectomy followed by adjuvant RT in patients with cSCC or BCC that has metastasized to the regional lymph nodes. They also recommended definitive RT in medically inoperable patients with the same metastasized cSCC or BCC. In addition, patients with BCC or cSCC undergoing adjuvant RT after therapeutic lymphadenectomy were recommended a dose of 6,000-6,600 cGy, while patients with cSCC undergoing elective RT without a lymphadenectomy were recommended a dose of 5,000-5,400 cGy.
Their fourth recommendation focused on techniques and dose-fractionation schedules for RT in the definitive or postoperative setting. For patients with BCC and cSCC receiving definitive RT, the biologically effective dose (BED10) range for conventional fractionation – defined as 180-200 cGy/fraction – should be 70-93.5 and the BED10 range for hypofractionation – defined as 210-500 cGy/fraction – should be 56-88. For patients with BCC and cSCC receiving postoperative RT, the BED10 range for conventional fractionation should be 59.5-79.2 and the BED10 range for hypofractionation should be 56-70.2.
Finally, their fifth recommendation was to not add concurrent carboplatin to adjuvant RT in patients with resected, locally advanced cSCC. They also conditionally recommended adding concurrent drug therapies to definitive RT in patients with unresected, locally advanced cSCC.
Several of the authors reported receiving honoraria and travel expenses from medical and pharmaceutical companies, along with serving on their advisory boards. The others reported no conflicts of interest.
SOURCE: Likhacheva A et al. Pract Radiat Oncol. 2019 Dec 9. doi: 10.1016/j.prro.2019.10.014.
who are not candidates for surgery, according to a new guideline from an American Society for Radiation Oncology task force.
“We hope that the dermatology community will find this guideline helpful, especially when it comes to defining clinical and pathological characteristics that may necessitate a discussion about the merits of postoperative radiation therapy,” said lead author Anna Likhacheva, MD, of the Sutter Medical Center in Sacramento, Calif., in an email. The guideline was published in Practical Radiation Oncology.
To address five key questions in regard to radiation therapy (RT) for the two most common skin cancers, the American Society for Radiation Oncology convened a task force of radiation, medical, and surgical oncologists; dermatopathologists; a radiation oncology resident; a medical physicist; and a dermatologist. They reviewed studies of adults with nonmetastatic, invasive basal cell carcinoma (BCC) or cutaneous squamous cell carcinoma (cSCC) that were published between May 1998 and June 2018, with the caveat that “there are limited, well-conducted modern randomized trials” in this area. As such, the majority of the recommendations have low to moderate quality of evidence designations.
“The conspicuous lack of prospective and randomized data should serve as a reminder to open clinical trials and collect outcomes data in a prospective fashion,” added Dr. Likhacheva, noting that “improving the quality of data on this topic will ultimately serve our common goal of improving patient outcomes.”
Their first recommendation was to strongly consider definitive RT as an alternative to surgery for BCC and cSCC, especially in areas where a surgical procedure would potentially compromise function or cosmesis. However, they did discourage its use in patients with genetic conditions associated with increased radiosensitivity.
Their second recommendation was to strongly consider postoperative radiation therapy for clinically or radiologically apparent gross perineural spread. They also strongly recommended PORT for cSCC patients with close or positive margins, with T3 or T4 tumors, or with desmoplastic or infiltrative tumors.
Their third recommendation was to strongly consider therapeutic lymphadenectomy followed by adjuvant RT in patients with cSCC or BCC that has metastasized to the regional lymph nodes. They also recommended definitive RT in medically inoperable patients with the same metastasized cSCC or BCC. In addition, patients with BCC or cSCC undergoing adjuvant RT after therapeutic lymphadenectomy were recommended a dose of 6,000-6,600 cGy, while patients with cSCC undergoing elective RT without a lymphadenectomy were recommended a dose of 5,000-5,400 cGy.
Their fourth recommendation focused on techniques and dose-fractionation schedules for RT in the definitive or postoperative setting. For patients with BCC and cSCC receiving definitive RT, the biologically effective dose (BED10) range for conventional fractionation – defined as 180-200 cGy/fraction – should be 70-93.5 and the BED10 range for hypofractionation – defined as 210-500 cGy/fraction – should be 56-88. For patients with BCC and cSCC receiving postoperative RT, the BED10 range for conventional fractionation should be 59.5-79.2 and the BED10 range for hypofractionation should be 56-70.2.
Finally, their fifth recommendation was to not add concurrent carboplatin to adjuvant RT in patients with resected, locally advanced cSCC. They also conditionally recommended adding concurrent drug therapies to definitive RT in patients with unresected, locally advanced cSCC.
Several of the authors reported receiving honoraria and travel expenses from medical and pharmaceutical companies, along with serving on their advisory boards. The others reported no conflicts of interest.
SOURCE: Likhacheva A et al. Pract Radiat Oncol. 2019 Dec 9. doi: 10.1016/j.prro.2019.10.014.
FROM PRACTICAL RADIATION ONCOLOGY
Clinical Characterization of Leukemia Cutis Presentation
Leukemia is a malignant, life-threatening neoplasm affecting the hematopoietic system. Extramedullary manifestations can occur in various organs, including skin.1 Skin findings in leukemia patients are common and varied, including pallor secondary to anemia, petechiae or ecchymoses due to thrombocytopenia, and skin manifestations of neutropenia and chemotherapy.2 When patients with leukemia develop skin lesions without leukemic infiltration, the resulting nonspecific cutaneous manifestations are known as leukemids.3 Specific cutaneous manifestations of leukemia resulting from direct invasion of leukemic cells into the epidermis, dermis, or subcutis are referred to as leukemia cutis (LC).2,3
Acute myeloid leukemia (AML) is the most common type of leukemia associated with LC, but LC also is seen in other leukemias with various frequencies.1 The lesions of LC can present anywhere on skin, though it has been reported that LC has a tendency to occur at sites of prior ongoing inflammation,2,4 most commonly the extremities, trunk, and face.2,5,6 LC lesions have a range of morphological findings and most commonly present as nodules, papules, and plaques.1,7
Most reports of LC in the literature are case reports or case series with small numbers of subjects.3,6,8 A study of LC patients (N=75) in Korea by Kang et al7 has been the only one to analyze clinical characteristics of LC since 2000.
The aim of this study was to further contribute to the knowledge of clinical characteristics of LC. Clinical patterns of 46 patients were analyzed to further characterize the presentation of LC and to compare our results with those in the literature.
Methods
We conducted a single-institution retrospective review of medical records of patients with LC diagnosed in the Department of Dermatology at Wake Forest School of Medicine (Winston-Salem, North Carolina) over a 17-year period (2001-2017). The study protocol was approved by the institutional review board of Wake Forest University School of Medicine (IRB No. 00054474). Patients had a leukemia diagnosis established by bone marrow biopsy. Patients were included in this analysis if they had ongoing active leukemia and a skin biopsy consistent with LC. Patients of all sexes and ages were included in the cohort. Patients were excluded if they presented only with nonspecific cutaneous lesions associated with leukemia (leukemids). After removing duplicate records from a total of 60 patients initially identified, 46 unique patients were included in this study.
Results
Demographics
Fifty-six percent (26/46) of patients were male. The average age at diagnosis of leukemia was 58 years (range, 8.5 months–84 years). Eighty-five percent of patients were white (39/46), 11% were black (5/46), 2% were Hispanic (1/46), and 2% were of unknown ethnicity (1/46).
Eighty percent (37/46) of patients with LC had AML; 3 of these patients had a prior diagnosis of chronic myeloid leukemia (CML) and 2 had myelodysplastic syndrome (MDS) that did not develop LC until after they had transitioned to AML. Other subtypes of leukemia in this patient population included acute lymphoblastic leukemia (ALL)(n=2), plasma cell leukemia (PCL)(n=2), undifferentiated leukemia (n=2), chronic lymphocytic leukemia (CLL)(n=1), myelodysplastic syndrome (n=1), and Burkitt-type leukemia (n=1).
Distribution and Morphology of LC Lesions
The clinical appearance of LC was widely variable in morphology and anatomic location (Table 1 and Figure). Eighty-four percent of LC occurrences involved more than one lesion (n=32); 14% were a solitary lesion (n=6). For the 2 patients who had 2 separate episodes of LC, the initial presentation of LC was multiple lesions; recurrent LC at relapse presented as a solitary lesion in both cases. Most LC lesions (77% [67/87]) occurred on the trunk or extremities; 23% (20/87) of LC lesions occurred on less common sites, such as the groin, face, hands, feet, and mucosa. Papules (38% [22/58]) and nodules (31% [18/58]) were the most common morphology; macules, plaques, and ulcers were observed less frequently. Clinical descriptions of LC lesions varied widely, with the most common descriptive characteristics being erythematous (57% [20/35]), violaceous (31% [11/35]), and asymptomatic (84% [32/38]). Rare descriptors included flesh colored, hyperpigmented, tender, pruritic, edema, crusting, and confluent erythematous.
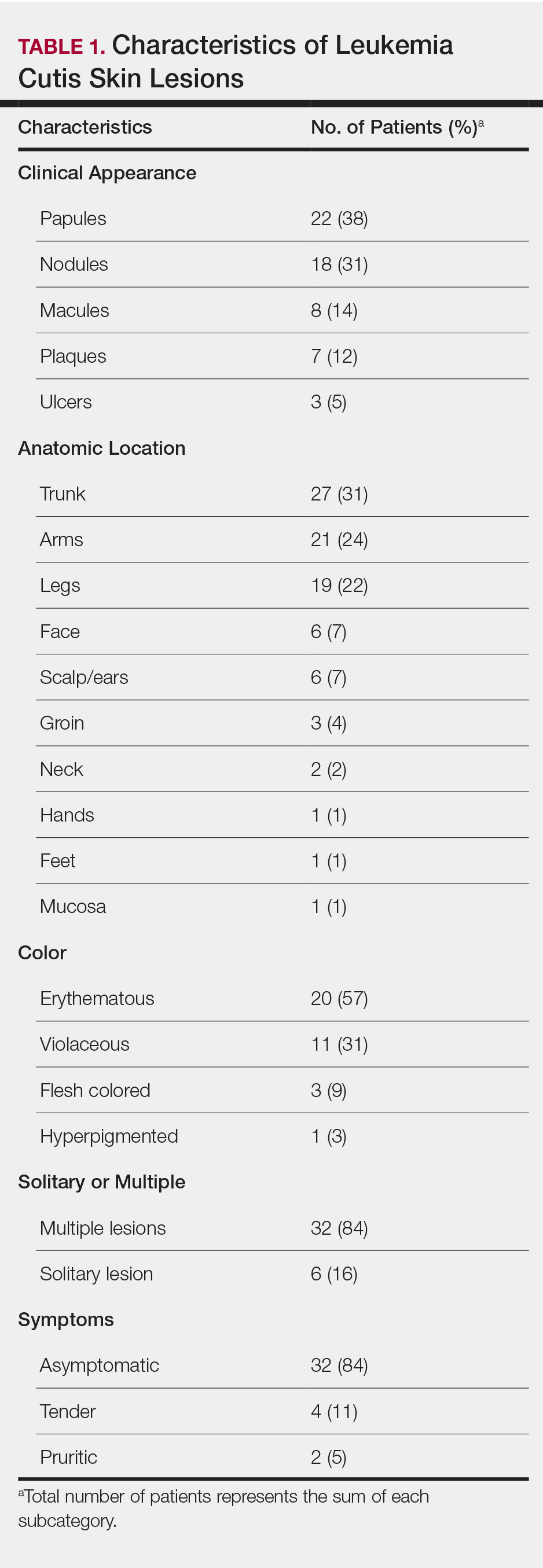
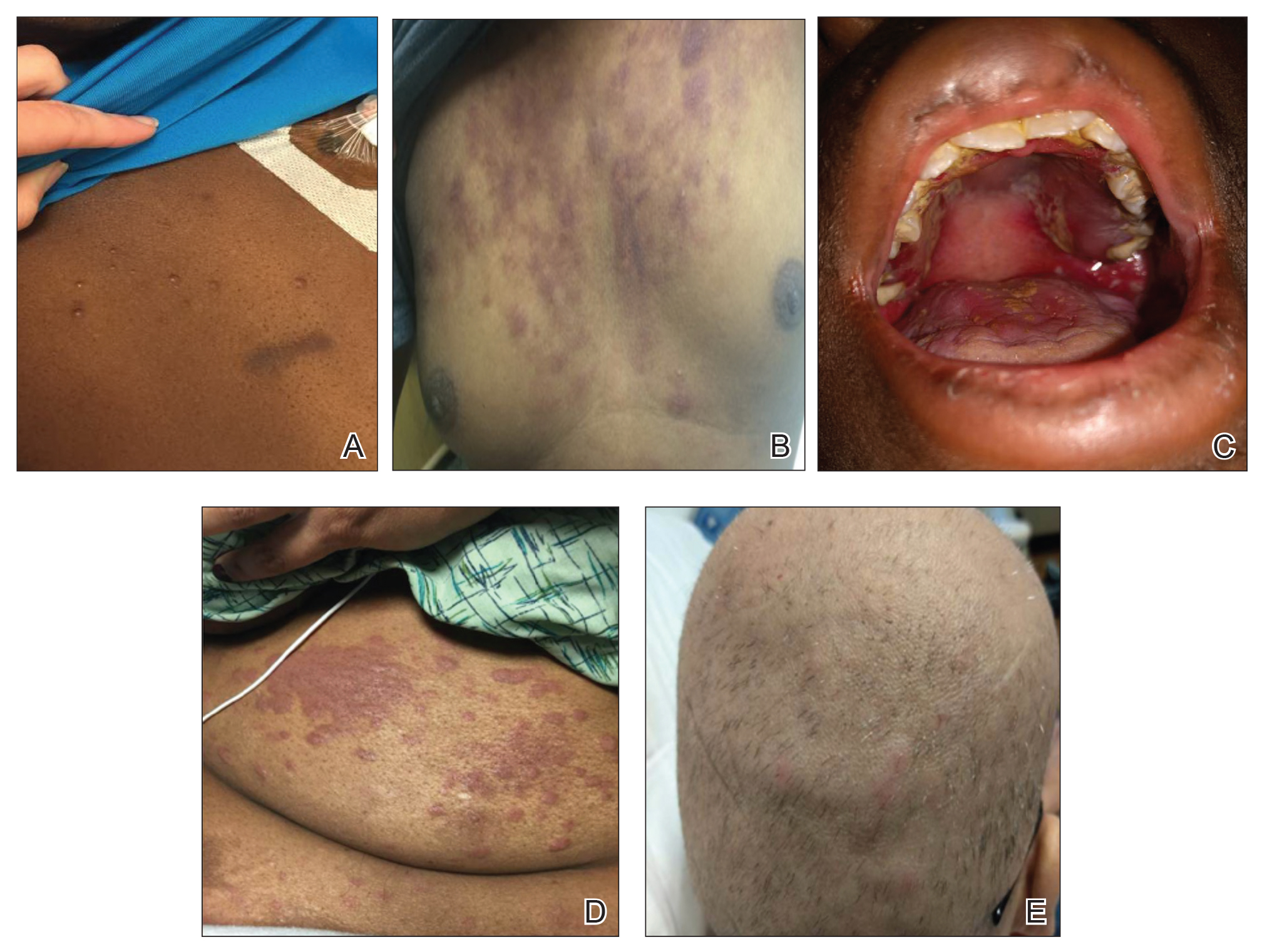
Interval Between Leukemia Diagnosis and LC Diagnosis
Approximately 59% (n=27) of patients had LC as a presenting finding of their leukemia (Table 2). Twenty-two percent (n=10) developed LC at the time of leukemia relapse; 20% (n=9) developed LC during consolidation or salvage chemotherapy. Two AML patients had recurrent episodes of LC both at initial presentation of leukemia and when AML relapsed. Two other AML patients received a diagnosis of LC at the same time as a negative concurrent bone marrow biopsy (ie, aleukemic LC). Mean duration between diagnosis of leukemia and diagnosis of LC was 0.4 months (CLL), 1.0 month (ALL), 4.7 months (AML), and 7.15 months (PCL). In cases of MDS and CML transformation to AML, the interval was 6.5 and 4.9 months, respectively.
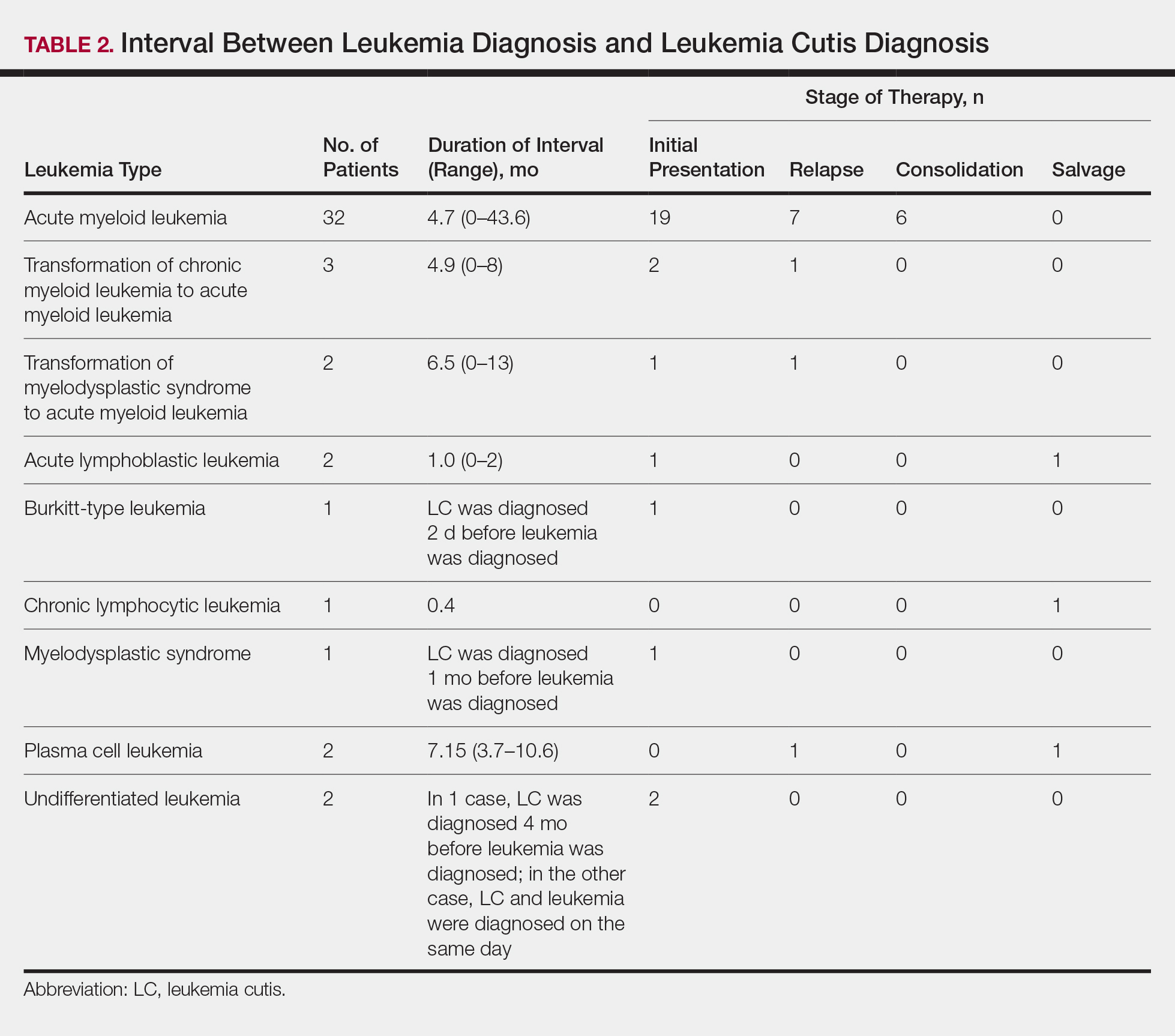
Interval Between LC Diagnosis and Death
As a whole, 17% (n=8) of patients were living at the time this article was written (eTable). Of patients who are still living, 10.9% (n=5) have AML. Looking at the cohort of patients with AML and LC, average age at AML diagnosis was 59.8 years. Average time from diagnosis of leukemia to death was 17.3 months (range, 0.6–49.6 months) for AML; 17.0 months (range, 10.0–24.0 months) for CML transformation to AML; 15.0 months (range, 12.0–18.0 months) for PCL; 14.75 months (range, 11.0–18.5 months) for undifferentiated leukemia; and 8.95 months (range, 4.2–13.7 months) for MDS transformation to AML. The interval between leukemia diagnosis and death was notably shorter for the CLL patient (4.0 months) and the deceased ALL patient (2.4 months). Mean duration between LC diagnosis and death was 11.7 months (AML), 11.2 months (undifferentiated leukemia), 9.9 months (CML transformation to AML), 2.75 months (PCL), and 2.4 months (MDS transformation to AML). The shortest intervals between LC diagnosis and death were seen in CLL (0.5 months) and ALL (0.4 months).
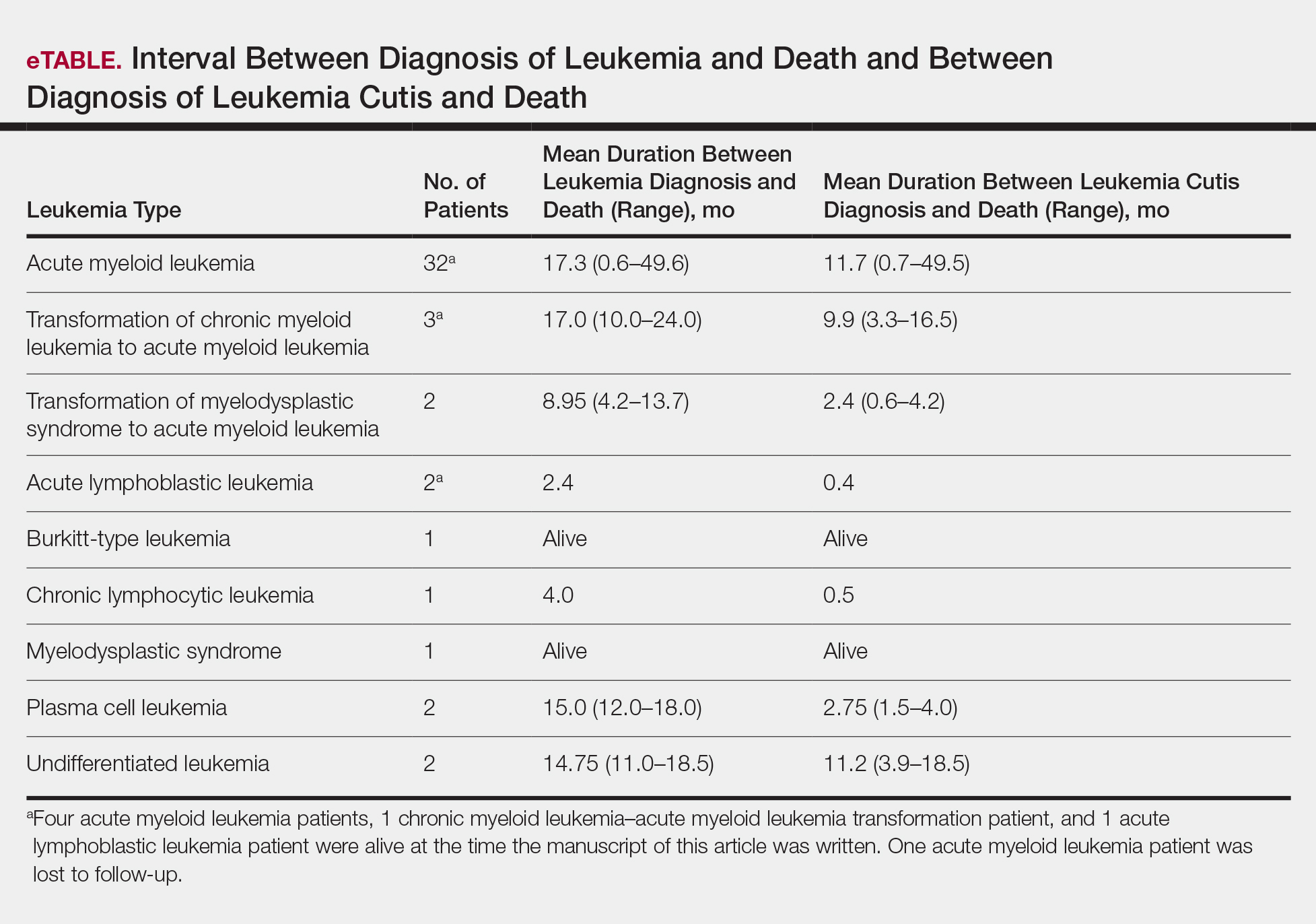
Discussion
Cutaneous manifestations are not uncommon in leukemia patients and can have a number of causes, including paraneoplastic cutaneous manifestations, such as pyoderma gangrenosum and Sweet syndrome; infection; cutaneous toxicities from antineoplastic agents; and LC.2 Leukemia cutis can be confused with other skin lesions in leukemia patients; diagnosis requires biopsy.2,9
We analyzed clinical characteristics and prognosis of 46 patients with LC over a 17-year period. To the best of our knowledge, this is the largest study of LC patients published in the United States. A similar study by Kang et al7 analyzed 75 patients in Korea; however, the incidence of LC among different types of leukemia in the Korean population cannot be applied to Western countries. We did compare the clinical characteristics of our cohort of patients to those reported by Kang et al7 and other studies including a smaller number of patients.
In this study, the male to female ratio was 1.3 to 1 compared to the 2:1 ratio reported by Kang et al.7 The mean age of leukemia diagnosis among our patients was 58 years, which is notably older than the mean age previously reported.7 In this cohort, 4 patients (8.7%) were 34 years or younger, including 1 infant aged 8.5 months; 24 (52.2%) were aged 35 to 64 years; and 18 (39.1%) were 65 years and older.
Consistent with other studies,2,5,7 the most common type of leukemia in patients who developed LC was AML (80%). Among AML patients, the mean age at AML diagnosis (59.8 years) was notably younger than the reported US average age of patients who had a diagnosis of AML (68 years).10 Gender breakdown was slightly different than US statistics: 63% of AML patients in our group were male, whereas AML is only slightly more common among men in the United States.10
Clinically, skin lesions observed most commonly were (in decreasing order) papules, nodules, macules, plaques, and ulcers. Papules (38%) were the most common lesion overall in our study, which differed from the Kang et al7 report in which nodules were the most common. Nodules (31%) were the second most common LC morphology among our patients. Among AML patients, papules were seen in 56% of patients (18/32); nodules were seen in 44% (14/32). The extremities (when combined together) were the most common location of LC lesions (46% [arms, 24%; legs, 22%]); the trunk was the second most common body region (31%). Our study did not find a difference among most common LC anatomic sites compared to other studies.5,7 Less common sites in our cohort included the head, scalp/ears, neck, hands, mucosa, and feet. All body sites were represented, including ocular and oral mucosa and groin, a finding that underscores the importance of complete and comprehensive skin examinations in this patient population. The terms erythematous and violaceous were used to describe the color of most lesions (88%), which commonly presented as multiple lesions (84%) and often were asymptomatic (84%).
It has been reported that, first, in most cases of LC, the condition develops in patients who have already been given a diagnosis of leukemia and, second, simultaneous manifestation of systemic leukemia and LC is less common.11,12 Leukemia cutis also can precede peripheral or bone marrow leukemia (known as aleukemic LC).1,13 Two AML patients (4.3% [2/46]) in this study met criteria for aleukemic LC because they had LC at the same time as negative bone marrow biopsy, which is consistent with a prior report that aleukemic LC can affect as many as 7% of patients.1 Our results differed slightly from prior studies in that most of our patients had LC as one of the presenting manifestations of their leukemia.3,7
Regardless of leukemia type, patients were likely to die within 1 year of LC diagnosis, on average, which is consistent with prior reports.7,11,12 However, the time between diagnosis of LC and death varied greatly among our patients (range, 12 days to 4.1 years). From 2007 to 2013, the 5-year relative survival rate overall for leukemia patients in the US population (by type) was 86.2% (CLL), 71.0% (ALL), 68.0% (CML), and 27.4% (AML).14 Compared to these national statistics, the relative survival rate in LC is poor, with patients who have AML surviving, on average, less than 8 months from time of leukemia diagnosis, whereas ALL and CLL patients survive less than 6 months.
When LC is a late presentation of B-cell CLL or when it presents as myeloid leukemia, blastic transformation (Richter syndrome), or T-cell CLL, it is occasionally associated with poor prognosis, though LC does not affect survival.15-17 In a study of the association of LC with survival in AML, 5-year survival among 62 AML patients with LC was 8.6%, shorter than 28.3% among the 186 matched patients with AML without LC.18 Similarly, the estimated 5-year survival for all patients with AML, according to Surveillance, Epidemiology, and End Results Program data (2007-2013), was 27.4%.14 Based on those results, LC might be a good prognostic indicator in patients with AML.
Conclusion
This study characterized the clinical presentation of LC, which is highly variable in appearance, symptoms, distribution, and stage of leukemia at presentation. In our study cohort, LC most commonly presented as asymptomatic erythematous or violaceous papules or nodules in older male AML patients at leukemia diagnosis. Given such wide variability, dermatologists and oncologists need to keep LC in the differential diagnosis for any new skin lesion and to have a low threshold for performing skin biopsy. Complete and thorough skin examinations should be performed on leukemia patients throughout the course of their disease to identify LC early so that treatment can be implemented in a timely fashion at initial diagnosis, first sign of relapse, or change in disease state.
- Wagner G, Fenchel K, Back W, et al. Leukemia cutis—epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012;10:27-36.
- Grunwald MR, McDonnell MH, Induru R, et al. Cutaneous manifestations in leukemia patients. Semin Oncol. 2016;43:359-365.
- Martínez-Leboráns L, Victoria-Martínez A, Torregrosa-Calatayu JL, et al. Leukemia cutis: a report of 17 cases and a review of literature. Actas Dermosifiliogr. 2016;107:e65-e69.
- Li L, Wang Y, Lian CG, et al. Clinical and pathological features of myeloid leukemia cutis. An Bras Dermatol. 2018;93:216-221.
- Paydas¸ S, Zorludemir S. Leukaemia cutis and leukaemic vasculitis. Br J Dermatol. 2000;143:773-779.
- Lee JI, Park HJ, Oh ST, et al. A case of leukemia cutis at the site of a prior catheter insertion. Ann Dermatol. 2009;21:193-196.
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci. 2013;28:614-619.
- Stern M, Halter J, Buser A, et al. Leukemia cutis preceding systemic relapse of acute myeloid leukemia. Int J Hematol. 2008;87:108-109.
- Patel LM, Maghari A, Schwartz RA, et al. Myeloid leukemia cutis in the setting of myelodysplastic syndrome: a crucial dermatological diagnosis. Int J Dermatol. 2012;51:383-388.
- American Cancer Society. Cancer Facts & Figures 2019. Atlanta, GA: American Cancer Society; 2019. http://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf. Accessed November 21, 2019.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994;13:223-230.
- Barzilai A, Lyakhovitsky A, Goldberg I, et al. Aleukemic monocytic leukemia cutis. Cutis. 2002;69:301-304
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER cancer statistics review (CSR) 1975-2014. Bethesda, MD: National Cancer Institute; April 2017. https://seer.cancer.gov/archive/csr/1975_2014/. Accessed November 21, 2019.
- Cerroni L, Zenahlik P, Höfler G, et al. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia: a clinicopathologic and prognostic study of 42 patients. Am J Surg Pathol. 1996;20:1000-1010.
- Colburn DE, Welch MA, Giles FJ. Skin infiltration with chronic lymphocytic leukemia is consistent with a good prognosis. Hematology. 2002;7:187-188.
- Ratnam KV, Khor CJ, Su WP. Leukemia cutis. Dermatol Clin. 1994;12:419-431.
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826-832.
Leukemia is a malignant, life-threatening neoplasm affecting the hematopoietic system. Extramedullary manifestations can occur in various organs, including skin.1 Skin findings in leukemia patients are common and varied, including pallor secondary to anemia, petechiae or ecchymoses due to thrombocytopenia, and skin manifestations of neutropenia and chemotherapy.2 When patients with leukemia develop skin lesions without leukemic infiltration, the resulting nonspecific cutaneous manifestations are known as leukemids.3 Specific cutaneous manifestations of leukemia resulting from direct invasion of leukemic cells into the epidermis, dermis, or subcutis are referred to as leukemia cutis (LC).2,3
Acute myeloid leukemia (AML) is the most common type of leukemia associated with LC, but LC also is seen in other leukemias with various frequencies.1 The lesions of LC can present anywhere on skin, though it has been reported that LC has a tendency to occur at sites of prior ongoing inflammation,2,4 most commonly the extremities, trunk, and face.2,5,6 LC lesions have a range of morphological findings and most commonly present as nodules, papules, and plaques.1,7
Most reports of LC in the literature are case reports or case series with small numbers of subjects.3,6,8 A study of LC patients (N=75) in Korea by Kang et al7 has been the only one to analyze clinical characteristics of LC since 2000.
The aim of this study was to further contribute to the knowledge of clinical characteristics of LC. Clinical patterns of 46 patients were analyzed to further characterize the presentation of LC and to compare our results with those in the literature.
Methods
We conducted a single-institution retrospective review of medical records of patients with LC diagnosed in the Department of Dermatology at Wake Forest School of Medicine (Winston-Salem, North Carolina) over a 17-year period (2001-2017). The study protocol was approved by the institutional review board of Wake Forest University School of Medicine (IRB No. 00054474). Patients had a leukemia diagnosis established by bone marrow biopsy. Patients were included in this analysis if they had ongoing active leukemia and a skin biopsy consistent with LC. Patients of all sexes and ages were included in the cohort. Patients were excluded if they presented only with nonspecific cutaneous lesions associated with leukemia (leukemids). After removing duplicate records from a total of 60 patients initially identified, 46 unique patients were included in this study.
Results
Demographics
Fifty-six percent (26/46) of patients were male. The average age at diagnosis of leukemia was 58 years (range, 8.5 months–84 years). Eighty-five percent of patients were white (39/46), 11% were black (5/46), 2% were Hispanic (1/46), and 2% were of unknown ethnicity (1/46).
Eighty percent (37/46) of patients with LC had AML; 3 of these patients had a prior diagnosis of chronic myeloid leukemia (CML) and 2 had myelodysplastic syndrome (MDS) that did not develop LC until after they had transitioned to AML. Other subtypes of leukemia in this patient population included acute lymphoblastic leukemia (ALL)(n=2), plasma cell leukemia (PCL)(n=2), undifferentiated leukemia (n=2), chronic lymphocytic leukemia (CLL)(n=1), myelodysplastic syndrome (n=1), and Burkitt-type leukemia (n=1).
Distribution and Morphology of LC Lesions
The clinical appearance of LC was widely variable in morphology and anatomic location (Table 1 and Figure). Eighty-four percent of LC occurrences involved more than one lesion (n=32); 14% were a solitary lesion (n=6). For the 2 patients who had 2 separate episodes of LC, the initial presentation of LC was multiple lesions; recurrent LC at relapse presented as a solitary lesion in both cases. Most LC lesions (77% [67/87]) occurred on the trunk or extremities; 23% (20/87) of LC lesions occurred on less common sites, such as the groin, face, hands, feet, and mucosa. Papules (38% [22/58]) and nodules (31% [18/58]) were the most common morphology; macules, plaques, and ulcers were observed less frequently. Clinical descriptions of LC lesions varied widely, with the most common descriptive characteristics being erythematous (57% [20/35]), violaceous (31% [11/35]), and asymptomatic (84% [32/38]). Rare descriptors included flesh colored, hyperpigmented, tender, pruritic, edema, crusting, and confluent erythematous.


Interval Between Leukemia Diagnosis and LC Diagnosis
Approximately 59% (n=27) of patients had LC as a presenting finding of their leukemia (Table 2). Twenty-two percent (n=10) developed LC at the time of leukemia relapse; 20% (n=9) developed LC during consolidation or salvage chemotherapy. Two AML patients had recurrent episodes of LC both at initial presentation of leukemia and when AML relapsed. Two other AML patients received a diagnosis of LC at the same time as a negative concurrent bone marrow biopsy (ie, aleukemic LC). Mean duration between diagnosis of leukemia and diagnosis of LC was 0.4 months (CLL), 1.0 month (ALL), 4.7 months (AML), and 7.15 months (PCL). In cases of MDS and CML transformation to AML, the interval was 6.5 and 4.9 months, respectively.

Interval Between LC Diagnosis and Death
As a whole, 17% (n=8) of patients were living at the time this article was written (eTable). Of patients who are still living, 10.9% (n=5) have AML. Looking at the cohort of patients with AML and LC, average age at AML diagnosis was 59.8 years. Average time from diagnosis of leukemia to death was 17.3 months (range, 0.6–49.6 months) for AML; 17.0 months (range, 10.0–24.0 months) for CML transformation to AML; 15.0 months (range, 12.0–18.0 months) for PCL; 14.75 months (range, 11.0–18.5 months) for undifferentiated leukemia; and 8.95 months (range, 4.2–13.7 months) for MDS transformation to AML. The interval between leukemia diagnosis and death was notably shorter for the CLL patient (4.0 months) and the deceased ALL patient (2.4 months). Mean duration between LC diagnosis and death was 11.7 months (AML), 11.2 months (undifferentiated leukemia), 9.9 months (CML transformation to AML), 2.75 months (PCL), and 2.4 months (MDS transformation to AML). The shortest intervals between LC diagnosis and death were seen in CLL (0.5 months) and ALL (0.4 months).

Discussion
Cutaneous manifestations are not uncommon in leukemia patients and can have a number of causes, including paraneoplastic cutaneous manifestations, such as pyoderma gangrenosum and Sweet syndrome; infection; cutaneous toxicities from antineoplastic agents; and LC.2 Leukemia cutis can be confused with other skin lesions in leukemia patients; diagnosis requires biopsy.2,9
We analyzed clinical characteristics and prognosis of 46 patients with LC over a 17-year period. To the best of our knowledge, this is the largest study of LC patients published in the United States. A similar study by Kang et al7 analyzed 75 patients in Korea; however, the incidence of LC among different types of leukemia in the Korean population cannot be applied to Western countries. We did compare the clinical characteristics of our cohort of patients to those reported by Kang et al7 and other studies including a smaller number of patients.
In this study, the male to female ratio was 1.3 to 1 compared to the 2:1 ratio reported by Kang et al.7 The mean age of leukemia diagnosis among our patients was 58 years, which is notably older than the mean age previously reported.7 In this cohort, 4 patients (8.7%) were 34 years or younger, including 1 infant aged 8.5 months; 24 (52.2%) were aged 35 to 64 years; and 18 (39.1%) were 65 years and older.
Consistent with other studies,2,5,7 the most common type of leukemia in patients who developed LC was AML (80%). Among AML patients, the mean age at AML diagnosis (59.8 years) was notably younger than the reported US average age of patients who had a diagnosis of AML (68 years).10 Gender breakdown was slightly different than US statistics: 63% of AML patients in our group were male, whereas AML is only slightly more common among men in the United States.10
Clinically, skin lesions observed most commonly were (in decreasing order) papules, nodules, macules, plaques, and ulcers. Papules (38%) were the most common lesion overall in our study, which differed from the Kang et al7 report in which nodules were the most common. Nodules (31%) were the second most common LC morphology among our patients. Among AML patients, papules were seen in 56% of patients (18/32); nodules were seen in 44% (14/32). The extremities (when combined together) were the most common location of LC lesions (46% [arms, 24%; legs, 22%]); the trunk was the second most common body region (31%). Our study did not find a difference among most common LC anatomic sites compared to other studies.5,7 Less common sites in our cohort included the head, scalp/ears, neck, hands, mucosa, and feet. All body sites were represented, including ocular and oral mucosa and groin, a finding that underscores the importance of complete and comprehensive skin examinations in this patient population. The terms erythematous and violaceous were used to describe the color of most lesions (88%), which commonly presented as multiple lesions (84%) and often were asymptomatic (84%).
It has been reported that, first, in most cases of LC, the condition develops in patients who have already been given a diagnosis of leukemia and, second, simultaneous manifestation of systemic leukemia and LC is less common.11,12 Leukemia cutis also can precede peripheral or bone marrow leukemia (known as aleukemic LC).1,13 Two AML patients (4.3% [2/46]) in this study met criteria for aleukemic LC because they had LC at the same time as negative bone marrow biopsy, which is consistent with a prior report that aleukemic LC can affect as many as 7% of patients.1 Our results differed slightly from prior studies in that most of our patients had LC as one of the presenting manifestations of their leukemia.3,7
Regardless of leukemia type, patients were likely to die within 1 year of LC diagnosis, on average, which is consistent with prior reports.7,11,12 However, the time between diagnosis of LC and death varied greatly among our patients (range, 12 days to 4.1 years). From 2007 to 2013, the 5-year relative survival rate overall for leukemia patients in the US population (by type) was 86.2% (CLL), 71.0% (ALL), 68.0% (CML), and 27.4% (AML).14 Compared to these national statistics, the relative survival rate in LC is poor, with patients who have AML surviving, on average, less than 8 months from time of leukemia diagnosis, whereas ALL and CLL patients survive less than 6 months.
When LC is a late presentation of B-cell CLL or when it presents as myeloid leukemia, blastic transformation (Richter syndrome), or T-cell CLL, it is occasionally associated with poor prognosis, though LC does not affect survival.15-17 In a study of the association of LC with survival in AML, 5-year survival among 62 AML patients with LC was 8.6%, shorter than 28.3% among the 186 matched patients with AML without LC.18 Similarly, the estimated 5-year survival for all patients with AML, according to Surveillance, Epidemiology, and End Results Program data (2007-2013), was 27.4%.14 Based on those results, LC might be a good prognostic indicator in patients with AML.
Conclusion
This study characterized the clinical presentation of LC, which is highly variable in appearance, symptoms, distribution, and stage of leukemia at presentation. In our study cohort, LC most commonly presented as asymptomatic erythematous or violaceous papules or nodules in older male AML patients at leukemia diagnosis. Given such wide variability, dermatologists and oncologists need to keep LC in the differential diagnosis for any new skin lesion and to have a low threshold for performing skin biopsy. Complete and thorough skin examinations should be performed on leukemia patients throughout the course of their disease to identify LC early so that treatment can be implemented in a timely fashion at initial diagnosis, first sign of relapse, or change in disease state.
Leukemia is a malignant, life-threatening neoplasm affecting the hematopoietic system. Extramedullary manifestations can occur in various organs, including skin.1 Skin findings in leukemia patients are common and varied, including pallor secondary to anemia, petechiae or ecchymoses due to thrombocytopenia, and skin manifestations of neutropenia and chemotherapy.2 When patients with leukemia develop skin lesions without leukemic infiltration, the resulting nonspecific cutaneous manifestations are known as leukemids.3 Specific cutaneous manifestations of leukemia resulting from direct invasion of leukemic cells into the epidermis, dermis, or subcutis are referred to as leukemia cutis (LC).2,3
Acute myeloid leukemia (AML) is the most common type of leukemia associated with LC, but LC also is seen in other leukemias with various frequencies.1 The lesions of LC can present anywhere on skin, though it has been reported that LC has a tendency to occur at sites of prior ongoing inflammation,2,4 most commonly the extremities, trunk, and face.2,5,6 LC lesions have a range of morphological findings and most commonly present as nodules, papules, and plaques.1,7
Most reports of LC in the literature are case reports or case series with small numbers of subjects.3,6,8 A study of LC patients (N=75) in Korea by Kang et al7 has been the only one to analyze clinical characteristics of LC since 2000.
The aim of this study was to further contribute to the knowledge of clinical characteristics of LC. Clinical patterns of 46 patients were analyzed to further characterize the presentation of LC and to compare our results with those in the literature.
Methods
We conducted a single-institution retrospective review of medical records of patients with LC diagnosed in the Department of Dermatology at Wake Forest School of Medicine (Winston-Salem, North Carolina) over a 17-year period (2001-2017). The study protocol was approved by the institutional review board of Wake Forest University School of Medicine (IRB No. 00054474). Patients had a leukemia diagnosis established by bone marrow biopsy. Patients were included in this analysis if they had ongoing active leukemia and a skin biopsy consistent with LC. Patients of all sexes and ages were included in the cohort. Patients were excluded if they presented only with nonspecific cutaneous lesions associated with leukemia (leukemids). After removing duplicate records from a total of 60 patients initially identified, 46 unique patients were included in this study.
Results
Demographics
Fifty-six percent (26/46) of patients were male. The average age at diagnosis of leukemia was 58 years (range, 8.5 months–84 years). Eighty-five percent of patients were white (39/46), 11% were black (5/46), 2% were Hispanic (1/46), and 2% were of unknown ethnicity (1/46).
Eighty percent (37/46) of patients with LC had AML; 3 of these patients had a prior diagnosis of chronic myeloid leukemia (CML) and 2 had myelodysplastic syndrome (MDS) that did not develop LC until after they had transitioned to AML. Other subtypes of leukemia in this patient population included acute lymphoblastic leukemia (ALL)(n=2), plasma cell leukemia (PCL)(n=2), undifferentiated leukemia (n=2), chronic lymphocytic leukemia (CLL)(n=1), myelodysplastic syndrome (n=1), and Burkitt-type leukemia (n=1).
Distribution and Morphology of LC Lesions
The clinical appearance of LC was widely variable in morphology and anatomic location (Table 1 and Figure). Eighty-four percent of LC occurrences involved more than one lesion (n=32); 14% were a solitary lesion (n=6). For the 2 patients who had 2 separate episodes of LC, the initial presentation of LC was multiple lesions; recurrent LC at relapse presented as a solitary lesion in both cases. Most LC lesions (77% [67/87]) occurred on the trunk or extremities; 23% (20/87) of LC lesions occurred on less common sites, such as the groin, face, hands, feet, and mucosa. Papules (38% [22/58]) and nodules (31% [18/58]) were the most common morphology; macules, plaques, and ulcers were observed less frequently. Clinical descriptions of LC lesions varied widely, with the most common descriptive characteristics being erythematous (57% [20/35]), violaceous (31% [11/35]), and asymptomatic (84% [32/38]). Rare descriptors included flesh colored, hyperpigmented, tender, pruritic, edema, crusting, and confluent erythematous.


Interval Between Leukemia Diagnosis and LC Diagnosis
Approximately 59% (n=27) of patients had LC as a presenting finding of their leukemia (Table 2). Twenty-two percent (n=10) developed LC at the time of leukemia relapse; 20% (n=9) developed LC during consolidation or salvage chemotherapy. Two AML patients had recurrent episodes of LC both at initial presentation of leukemia and when AML relapsed. Two other AML patients received a diagnosis of LC at the same time as a negative concurrent bone marrow biopsy (ie, aleukemic LC). Mean duration between diagnosis of leukemia and diagnosis of LC was 0.4 months (CLL), 1.0 month (ALL), 4.7 months (AML), and 7.15 months (PCL). In cases of MDS and CML transformation to AML, the interval was 6.5 and 4.9 months, respectively.

Interval Between LC Diagnosis and Death
As a whole, 17% (n=8) of patients were living at the time this article was written (eTable). Of patients who are still living, 10.9% (n=5) have AML. Looking at the cohort of patients with AML and LC, average age at AML diagnosis was 59.8 years. Average time from diagnosis of leukemia to death was 17.3 months (range, 0.6–49.6 months) for AML; 17.0 months (range, 10.0–24.0 months) for CML transformation to AML; 15.0 months (range, 12.0–18.0 months) for PCL; 14.75 months (range, 11.0–18.5 months) for undifferentiated leukemia; and 8.95 months (range, 4.2–13.7 months) for MDS transformation to AML. The interval between leukemia diagnosis and death was notably shorter for the CLL patient (4.0 months) and the deceased ALL patient (2.4 months). Mean duration between LC diagnosis and death was 11.7 months (AML), 11.2 months (undifferentiated leukemia), 9.9 months (CML transformation to AML), 2.75 months (PCL), and 2.4 months (MDS transformation to AML). The shortest intervals between LC diagnosis and death were seen in CLL (0.5 months) and ALL (0.4 months).

Discussion
Cutaneous manifestations are not uncommon in leukemia patients and can have a number of causes, including paraneoplastic cutaneous manifestations, such as pyoderma gangrenosum and Sweet syndrome; infection; cutaneous toxicities from antineoplastic agents; and LC.2 Leukemia cutis can be confused with other skin lesions in leukemia patients; diagnosis requires biopsy.2,9
We analyzed clinical characteristics and prognosis of 46 patients with LC over a 17-year period. To the best of our knowledge, this is the largest study of LC patients published in the United States. A similar study by Kang et al7 analyzed 75 patients in Korea; however, the incidence of LC among different types of leukemia in the Korean population cannot be applied to Western countries. We did compare the clinical characteristics of our cohort of patients to those reported by Kang et al7 and other studies including a smaller number of patients.
In this study, the male to female ratio was 1.3 to 1 compared to the 2:1 ratio reported by Kang et al.7 The mean age of leukemia diagnosis among our patients was 58 years, which is notably older than the mean age previously reported.7 In this cohort, 4 patients (8.7%) were 34 years or younger, including 1 infant aged 8.5 months; 24 (52.2%) were aged 35 to 64 years; and 18 (39.1%) were 65 years and older.
Consistent with other studies,2,5,7 the most common type of leukemia in patients who developed LC was AML (80%). Among AML patients, the mean age at AML diagnosis (59.8 years) was notably younger than the reported US average age of patients who had a diagnosis of AML (68 years).10 Gender breakdown was slightly different than US statistics: 63% of AML patients in our group were male, whereas AML is only slightly more common among men in the United States.10
Clinically, skin lesions observed most commonly were (in decreasing order) papules, nodules, macules, plaques, and ulcers. Papules (38%) were the most common lesion overall in our study, which differed from the Kang et al7 report in which nodules were the most common. Nodules (31%) were the second most common LC morphology among our patients. Among AML patients, papules were seen in 56% of patients (18/32); nodules were seen in 44% (14/32). The extremities (when combined together) were the most common location of LC lesions (46% [arms, 24%; legs, 22%]); the trunk was the second most common body region (31%). Our study did not find a difference among most common LC anatomic sites compared to other studies.5,7 Less common sites in our cohort included the head, scalp/ears, neck, hands, mucosa, and feet. All body sites were represented, including ocular and oral mucosa and groin, a finding that underscores the importance of complete and comprehensive skin examinations in this patient population. The terms erythematous and violaceous were used to describe the color of most lesions (88%), which commonly presented as multiple lesions (84%) and often were asymptomatic (84%).
It has been reported that, first, in most cases of LC, the condition develops in patients who have already been given a diagnosis of leukemia and, second, simultaneous manifestation of systemic leukemia and LC is less common.11,12 Leukemia cutis also can precede peripheral or bone marrow leukemia (known as aleukemic LC).1,13 Two AML patients (4.3% [2/46]) in this study met criteria for aleukemic LC because they had LC at the same time as negative bone marrow biopsy, which is consistent with a prior report that aleukemic LC can affect as many as 7% of patients.1 Our results differed slightly from prior studies in that most of our patients had LC as one of the presenting manifestations of their leukemia.3,7
Regardless of leukemia type, patients were likely to die within 1 year of LC diagnosis, on average, which is consistent with prior reports.7,11,12 However, the time between diagnosis of LC and death varied greatly among our patients (range, 12 days to 4.1 years). From 2007 to 2013, the 5-year relative survival rate overall for leukemia patients in the US population (by type) was 86.2% (CLL), 71.0% (ALL), 68.0% (CML), and 27.4% (AML).14 Compared to these national statistics, the relative survival rate in LC is poor, with patients who have AML surviving, on average, less than 8 months from time of leukemia diagnosis, whereas ALL and CLL patients survive less than 6 months.
When LC is a late presentation of B-cell CLL or when it presents as myeloid leukemia, blastic transformation (Richter syndrome), or T-cell CLL, it is occasionally associated with poor prognosis, though LC does not affect survival.15-17 In a study of the association of LC with survival in AML, 5-year survival among 62 AML patients with LC was 8.6%, shorter than 28.3% among the 186 matched patients with AML without LC.18 Similarly, the estimated 5-year survival for all patients with AML, according to Surveillance, Epidemiology, and End Results Program data (2007-2013), was 27.4%.14 Based on those results, LC might be a good prognostic indicator in patients with AML.
Conclusion
This study characterized the clinical presentation of LC, which is highly variable in appearance, symptoms, distribution, and stage of leukemia at presentation. In our study cohort, LC most commonly presented as asymptomatic erythematous or violaceous papules or nodules in older male AML patients at leukemia diagnosis. Given such wide variability, dermatologists and oncologists need to keep LC in the differential diagnosis for any new skin lesion and to have a low threshold for performing skin biopsy. Complete and thorough skin examinations should be performed on leukemia patients throughout the course of their disease to identify LC early so that treatment can be implemented in a timely fashion at initial diagnosis, first sign of relapse, or change in disease state.
- Wagner G, Fenchel K, Back W, et al. Leukemia cutis—epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012;10:27-36.
- Grunwald MR, McDonnell MH, Induru R, et al. Cutaneous manifestations in leukemia patients. Semin Oncol. 2016;43:359-365.
- Martínez-Leboráns L, Victoria-Martínez A, Torregrosa-Calatayu JL, et al. Leukemia cutis: a report of 17 cases and a review of literature. Actas Dermosifiliogr. 2016;107:e65-e69.
- Li L, Wang Y, Lian CG, et al. Clinical and pathological features of myeloid leukemia cutis. An Bras Dermatol. 2018;93:216-221.
- Paydas¸ S, Zorludemir S. Leukaemia cutis and leukaemic vasculitis. Br J Dermatol. 2000;143:773-779.
- Lee JI, Park HJ, Oh ST, et al. A case of leukemia cutis at the site of a prior catheter insertion. Ann Dermatol. 2009;21:193-196.
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci. 2013;28:614-619.
- Stern M, Halter J, Buser A, et al. Leukemia cutis preceding systemic relapse of acute myeloid leukemia. Int J Hematol. 2008;87:108-109.
- Patel LM, Maghari A, Schwartz RA, et al. Myeloid leukemia cutis in the setting of myelodysplastic syndrome: a crucial dermatological diagnosis. Int J Dermatol. 2012;51:383-388.
- American Cancer Society. Cancer Facts & Figures 2019. Atlanta, GA: American Cancer Society; 2019. http://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf. Accessed November 21, 2019.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994;13:223-230.
- Barzilai A, Lyakhovitsky A, Goldberg I, et al. Aleukemic monocytic leukemia cutis. Cutis. 2002;69:301-304
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER cancer statistics review (CSR) 1975-2014. Bethesda, MD: National Cancer Institute; April 2017. https://seer.cancer.gov/archive/csr/1975_2014/. Accessed November 21, 2019.
- Cerroni L, Zenahlik P, Höfler G, et al. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia: a clinicopathologic and prognostic study of 42 patients. Am J Surg Pathol. 1996;20:1000-1010.
- Colburn DE, Welch MA, Giles FJ. Skin infiltration with chronic lymphocytic leukemia is consistent with a good prognosis. Hematology. 2002;7:187-188.
- Ratnam KV, Khor CJ, Su WP. Leukemia cutis. Dermatol Clin. 1994;12:419-431.
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826-832.
- Wagner G, Fenchel K, Back W, et al. Leukemia cutis—epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012;10:27-36.
- Grunwald MR, McDonnell MH, Induru R, et al. Cutaneous manifestations in leukemia patients. Semin Oncol. 2016;43:359-365.
- Martínez-Leboráns L, Victoria-Martínez A, Torregrosa-Calatayu JL, et al. Leukemia cutis: a report of 17 cases and a review of literature. Actas Dermosifiliogr. 2016;107:e65-e69.
- Li L, Wang Y, Lian CG, et al. Clinical and pathological features of myeloid leukemia cutis. An Bras Dermatol. 2018;93:216-221.
- Paydas¸ S, Zorludemir S. Leukaemia cutis and leukaemic vasculitis. Br J Dermatol. 2000;143:773-779.
- Lee JI, Park HJ, Oh ST, et al. A case of leukemia cutis at the site of a prior catheter insertion. Ann Dermatol. 2009;21:193-196.
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci. 2013;28:614-619.
- Stern M, Halter J, Buser A, et al. Leukemia cutis preceding systemic relapse of acute myeloid leukemia. Int J Hematol. 2008;87:108-109.
- Patel LM, Maghari A, Schwartz RA, et al. Myeloid leukemia cutis in the setting of myelodysplastic syndrome: a crucial dermatological diagnosis. Int J Dermatol. 2012;51:383-388.
- American Cancer Society. Cancer Facts & Figures 2019. Atlanta, GA: American Cancer Society; 2019. http://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf. Accessed November 21, 2019.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994;13:223-230.
- Barzilai A, Lyakhovitsky A, Goldberg I, et al. Aleukemic monocytic leukemia cutis. Cutis. 2002;69:301-304
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER cancer statistics review (CSR) 1975-2014. Bethesda, MD: National Cancer Institute; April 2017. https://seer.cancer.gov/archive/csr/1975_2014/. Accessed November 21, 2019.
- Cerroni L, Zenahlik P, Höfler G, et al. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia: a clinicopathologic and prognostic study of 42 patients. Am J Surg Pathol. 1996;20:1000-1010.
- Colburn DE, Welch MA, Giles FJ. Skin infiltration with chronic lymphocytic leukemia is consistent with a good prognosis. Hematology. 2002;7:187-188.
- Ratnam KV, Khor CJ, Su WP. Leukemia cutis. Dermatol Clin. 1994;12:419-431.
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826-832.
Practice Points
- Complete and comprehensive skin examination is important in leukemia patients, as leukemia cutis (LC) lesions can present in all body sites including ocular and oral mucosa as well as the groin.
- Given the wide variability in appearance, symptoms, distribution, and stage of leukemia at presentation, dermatologists and oncologists need to keep LC in the differential diagnosis for any new skin lesion and to have a low threshold for performing skin biopsy.
- Performing thorough skin examination on leukemia patients throughout the course of their disease may help identify LC early so that treatment can be implemented in a timely fashion at initial diagnosis, first sign of relapse, or change in disease state.
Patient-Driven Management Using Same-Day Noninvasive Diagnosis and Complete Laser Treatment of Basal Cell Carcinomas: A Pilot Study
The increasing incidence of nonmelanoma skin cancer (NMSC) is a serious public health concern.1 Lesions often are identified on routine total-body examination, and there is a considerate burden on dermatologists to diagnose these lesions, which is both costly and results in a long wait time to see a specialist. Furthermore, standard care requires patients to attend multiple visits for the diagnosis and treatment of NMSC.
In recent decades, diagnosing basal cell carcinoma (BCC) has been facilitated by the handheld dermatoscope. The advent of dermoscopy has led to increased sensitivity and specificity of the NMSC diagnosis (estimated at 95%–99%) and has helped facilitate earlier diagnosis of BCC and reduce unnecessary biopsy of benign lesions.2-5 Dermoscopy also can be useful in monitoring response to treatment.5 Lesions that are detected early tend to be easier and less expensive to treat, a strong argument for the use of early detection techniques.6-8
More recently, in vivo reflectance confocal microscopy (RCM)(Vivascope 1500 [Caliber I.D.]) has become an acceptable means for confirming a BCC diagnosis, offering an alternative to tissue biopsy. Reflectance confocal microscopy can be reimbursed under Category I Current Procedural Terminology codes 96931 to 96936.9 Reflectance confocal microscopy is a noninvasive diagnostic technique that uses an 830-nm diode laser to enable visualization of a 0.5×0.5-mm patch of skin to a depth of 200 to 300 μm, which corresponds roughly to the papillary dermis. Reflectance confocal microscopy has the advantage of providing real-time diagnosis, enabling same-day treatment of BCC, and providing an efficient alternative to biopsy. Ultimately, these advantages are beneficial and time-saving for patients because biopsies can be painful; create a delay in diagnosis; and require further follow-up visits for treatment, which may be of importance to patients who have trouble attending multiple appointments.
Optical coherence tomography (OCT) is another noninvasive imaging device that is useful in BCC management. It uses an infrared broadband light source to visualize skin architecture to 2-mm deep with a 6×6-mm field of view.10 Although OCT does not offer the same cellular clarity as RCM, it allows visualization of a greater depth of skin and a wider field of view, making it a useful tool both in marginating NMSCs prior to treatment and monitoring response to treatment over time.11-16 Optical coherence tomography has demonstrated a high negative predictive value (92.1%) for BCC, which makes it useful for ruling out residual tumor in lesions undergoing management.17-19
With all available options, BCC management benefits from care that is tailored to the individual and the lesion, taking into account size and subtype because not every available treatment is appropriate. Lasers, including solid state, diode, dye, and gas types, are emerging as promising minimally invasive treatment modalities.20,21
Nonablative laser therapy with a pulsed dye laser (PDL) and fractional laser is an example; the principal investigator (PI) of this study (O.M.) recently reported a 95.70% clearance rate utilizing a PDL and fractional laser protocol.22 The 1064-nm Nd:YAG laser also has been used with PDL and as a stand-alone treatment. Jalian et al23 used PDL and the Nd:YAG laser on 13 BCC lesions, with a 58% (7/12) clearance rate after 4 treatments; all nonresponders were taking an anticoagulant, which inhibited the laser’s mechanism of action, according to the researchers.
Moskalik et al24 published a report of 3346 facial BCC lesions treated with pulsed Nd and pulsed Nd:YAG lasers, and included follow-up for as long as 5 years, with a 3.7% recurrence rate. Another report by Moskalik et al25 recorded a recurrence rate of 2.2% to 3.1% for BCCs that were followed for at least 5 years.
Ortiz et al26 reported use of the long-pulsed 1064-nm Nd:YAG laser to treat 13 lesions with biopsy-confirmed BCC on the trunk and extremities, with a 92% (12/13) clearance rate based on histologic analysis 1 month after laser treatment. In an expanded study of 31 patients by Ortiz et al,27 the histologic clearance rate was 90.3% (28/31)—also obtained after 1 month—after 1 Nd:YAG laser treatment, also treating lesions on the trunk and extremities. A further retrospective review of Nd:YAG laser treatment of BCC revealed a 100% clearance rate for 16 lesions (including lesions on the face) that were monitored for at least 6 months (mean duration, 9 months; range, 6–15 months).28 Optical coherence tomography imaging was used for one of the review’s lesions before and after treatment and suggested that the Nd:YAG laser works by selectively destroying the vasculature supplying BCC tumors while preserving surrounding healthy tissue.28
Apart from Moskalik et al,24,25 these studies are limited by a relatively short follow-up time to confirm tumor clearance. Prior studies utilizing the Nd:YAG laser to treat BCC are summarized in the eTable.
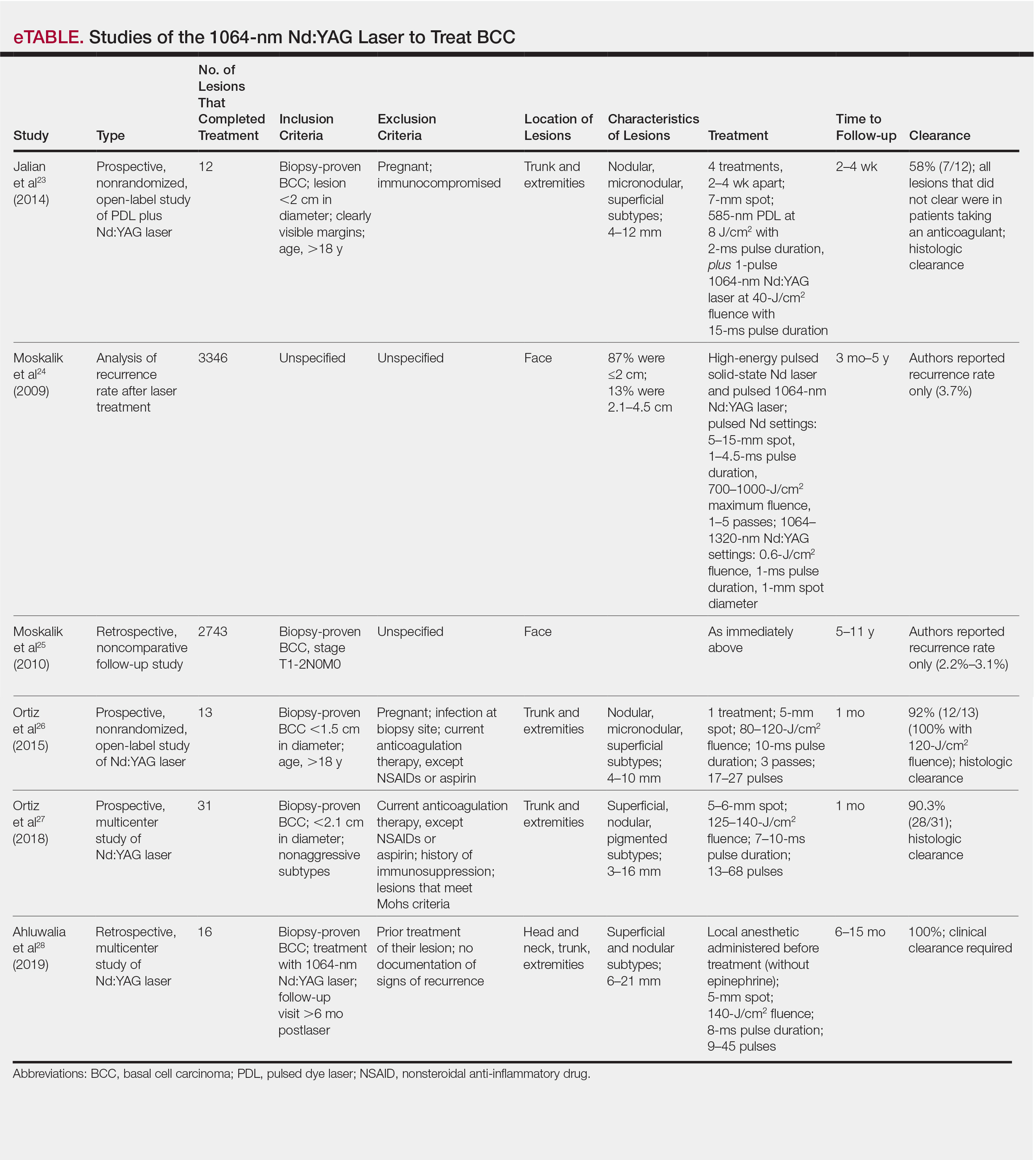
This pilot study describes a model of care that aims to alleviate some of the demand placed both on the specialty and on patients by utilizing a novel same-day approach to BCC management. We sought to evaluate management using noninvasive diagnosis with RCM; same-day laser treatment; and follow-up examination with clinical, dermoscopic, and noninvasive imaging using OCT. This method focuses on patient-driven health care from various perspectives. Patients are given real-time information about their diagnosis using RCM, leading to an increased level of information flow and immediate transparency regarding their diagnosis and management options. Patients also are receiving tailored care by incorporating noninvasive imaging and same-day laser treatment, allowing collaboration between patient and physician. Patients have more choices—to undergo surgical care; other at-home topical regimens; or laser management with potentially fewer visits, immediate results, a clearance rate similar to surgery, and improved cosmetic outcome.
Our study attempts to further evaluate the efficacy of the 1064-nm Nd:YAG laser in treating BCC while leveraging noninvasive imaging technology. The objective was to perform a retrospective review of medical records of a subgroup of patients with BCC diagnosed by RCM who were treated with the 1064-nm Nd:YAG laser and monitored for clearance using OCT imaging, in addition to clinical and dermoscopic examination. Similar to prior long-term Nd:YAG laser follow-up studies, we aimed to demonstrate the possibility of a minimally invasive BCC management approach—in our protocol, utilizing imaging instead of biopsy to facilitate long-term follow-up and by offering a model for patient-driven care.
Methods
Study Design
Institutional review board approval was received from Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects (New York, New York). We performed a retrospective review of medical records of patients diagnosed by RCM and treated with a 1064-nm Nd:YAG laser, as an alternative to surgery, at the Mount Sinai Faculty Practice Associates between March 2018 and August 2018. Included in this pilot study are 17 lesions in 16 patients.
Inclusion Criteria
Patients were enrolled based on the following criteria: BCCs diagnosed by clinical and dermoscopic examination followed by RCM imaging; treatment with the 1064-nm Nd:YAG laser, because of patients’ preference for this modality over surgery, superficial radiation therapy, topical regimens, and other laser therapies that require more visits; eligibility by PI included limited clinical ulceration or bleeding (or both) and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). The PI performed a detailed and thorough clinical and dermoscopic skin examination, enabling early detection of the BCCs. Basal cell carcinomas were not included if they exhibited rolled borders, visible ulceration, or oozing growths that allowed for treatment of less-advanced tumors. The PI utilized a clinical and dermoscopic color wheel algorithm to identify suspicious lesions combined with RCM for diagnostic confirmation.29
Two of 17 lesions that did not present as early lesions were included in the study due to patient refusal of surgery or radiation. We consider more advanced tumors to be exophytic, bleeding, crusting, nonhealing ulcerative growths. Patients who had received prior laser treatment with the PI’s PDL with fractional laser protocol with subsequent recurrence at the treatment site were included in the study. Lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor, were excluded.
Treatment Protocol
All patients attended the private clinic at Mount Sinai Hospital of a pigmented lesion expert (O.M.) for routine skin cancer screening. Patients with lesions suspicious for BCC—based on clinical and dermoscopic features—were offered tissue biopsy or RCM. Following diagnosis with RCM, treatment options were discussed, and patients were offered laser treatment when surgical options were declined. Topical treatment options were not emphasized because they require weeks of application to be effective and have been studied mainly in superficial BCC management.30,31
Patients with early lesions were offered either the PDL with fractional laser or Nd:YAG protocol, with their understanding that the Nd:YAG laser protocol would likely involve fewer treatments but a higher likelihood of residual hyperpigmentation or potential scarring (or both) than the more gentle PDL with fractional laser treatment.
All lesions on the face were premarginated using OCT by obtaining central scans and 4 additional scans—above, below, to the left, and to the right of the lesion—to ensure targeted laser treatment with desirable cosmetic results. Facial premargination scans were mandatory; however, patients with lesions on the trunk or extremities were offered the option to have pretreatment margination as an out-of-pocket expense. We did not require premargination of lesions on the body because of their location on less cosmetically critical areas. Most patients declined the optional scans.
This can be considered analogous to the situation in which more insurers reimburse Mohs surgery for cosmetically challenging areas such as the head and neck, while limiting reimbursement for treatment of lesions on the trunk and upper extremities to simple excision. Given cosmetic concerns on the head and neck compared to the body, some patients found it acceptable to have slightly increased dyschromia over a broader treatment area of non–cosmetically critical locations on the body.
Optical coherence tomography imaging was required for all anatomic locations at follow-up visits to detect residual disease or confirm clearance. All patients were given thorough information about the treatment, additional costs, treatment alternatives, potential adverse effects, and complications.
Clinical and dermoscopic images were obtained at every visit using a commercially available point-and-shoot digital single-lens reflex camera for clinical photographs, with an attached DermLite DL3N (3Gen) dermatoscope for all contact polarized dermoscopic photography.
Laser treatment was carried out with the 1064-nm Nd:YAG laser. Setting ranges were similar to previously published studies that used the 1064-nm Nd:YAG laser to treat BCCs (spot sizes, 5–6 mm; fluences, 125–140 J/cm2; pulse durations, 7–10 milliseconds).26-28 The exact settings and number of passes were tailored to the individual lesion based on skin type, anatomic location, extent of tumor involvement by depth (and margin on facial lesions), and posttreatment dermoscopic confirmation of clearance; additionally, for facial lesions, OCT confirmation of clearance.
Laser treatment was provided by the PI. Patients were instructed to apply a thick emollient (ie, formulation of petrolatum or 100% petrolatum) after treatment and until the area healed.
All tumors received 1 to 3 treatments at an interval of 1 to 2 months. The treatment end point was complete clearance, judged by absence of skin cancer clinically, dermoscopically, and on OCT scan. More specifically, the PI looked for vascular changes and echogenic changes on OCT consistent with tumor clearance as well as dermoscopic disappearance of recognized BCC features.
Patients were asked to return for follow-up visits 2 months after the final treatment to evaluate tumor clearance. They were asked to return subsequently every 6 to 12 months for routine care and long-term follow-up.
Results
Patient Characteristics
A total of 16 patients (6 female, 10 male) with 17 BCCs were included in this study. Mean age was 68 years (median, 71.5 years; range; 48–89 years). Mean lesion size was 7.1 mm (median, 6 mm; range, 3–15 mm). Eight lesions were on the face; 9 were on extrafacial sites. Two lesions had a history of laser treatment with the PI’s PDL with fractional laser treatment protocol and had locally recurred. Subtypes of lesions were not elicited by RCM.
Outcomes
Fourteen lesions (14/17 [82.4%]) required 1 treatment to achieve clearance, as confirmed clinically, dermoscopically, and by OCT scanning. One lesion on the back (1/17 [5.8%]) required 2 treatments (70 days between treatments). Two lesions (2/17 [11.8%]) required 3 treatments (time between treatments: 49 and 61 days [lesion 1]; 62 and 64 days [lesion 2]). Lesion 1 was on the face; lesion 2 was on the back. Mean time between last treatment and OCT clearance scan was 103 days (median, 64 days; range, 48–371 days).
Comment
Our study supports the notion that the 1064-nm Nd:YAG laser is a viable option for treating BCC. All (100%) lesions cleared, most (82.4%) with a single treatment. Of course, for patients who required more than 1 treatment (17.6%), we cannot make an argument for fewer patient visits because those patients had to return for multiple laser treatments, but they were able to avoid surgery, as they had wanted. Overall, our diagnostic approach utilizing RCM as opposed to traditional tissue biopsy meant that patients’ skin cancers were diagnosed and treated the same day.
A one-stop shop for diagnosis and treatment model has been reported by Kadouch et al32 as part of a randomized controlled trial in which patients were randomly assigned to receive standard care for BCC—biopsy followed by surgical excision—or RCM diagnosis followed by surgical excision. Their outcome was tumor-free margins after surgical treatment; the RCM approach was found to be noninferior to standard care.32 Our retrospective study differs, of course, in its laser treatment approach; however, both studies investigated a potentially more efficient pathway to BCC management, which becomes increasingly relevant given the rising incidence of NMSC.
A real-time, image-based diagnostic approach combined with laser treatment delivers patient-driven care, offering choice and convenience. It might be optimal for patients who have an extensive history of BCC, are poor surgical candidates, have difficulty with the logistics of the multiple visits required for surgical management, cannot (for practical reasons) spend multiple hours in office between Mohs stages, and do not want potentially disfiguring scars, making a minimally invasive treatment preferable.
As we found in our sample, not all patients are amenable to undergoing what is regarded now as the most definitive treatment—namely, surgical options. This subset of patients, whose lesions require more definitive treatment but who do not desire invasive management, need alternative approaches to BCC treatment. The present study proposes a model of patient-driven care that requires collaboration between physician and patient, offering more customized care that takes into account patient choice.
In our study, most patients had lesions that were detected early in their evolution; these lesions might be particularly amenable to laser management. The 2 resistant lesions in our set—requiring 3 treatments—appeared more aggressive clinically at initial evaluation but still had posttreatment outcomes with mild dyschromia similar to the lesions only treated once (Figure, A–D). Of those 2 lesions, the 9-mm lesion on the back (Figure, C and D) might have been larger than clinically apparent; in hindsight, it might have responded to a single treatment had it been premarginated. (An additional factor to have considered is the patient’s immunosuppressed status, which might have led to a more resistant lesion. Larger trials would help elucidate whether an immunosuppressed patient requires a different treatment approach, broader treatment area, OCT premargination regardless of anatomic location, or a greater number of treatments.) Nevertheless, the 2 aforementioned patients were offered treatment with the 1064-nm Nd:YAG laser because they refused surgery, radiation, and other more aggressive modalities. The patients were given advanced warning of an increased possibility of recurrence or nonclearance.
The lesion that required 2 treatments did not appear to be an aggressive subtype; however, it was considerably larger than most other treated lesions (1.5 cm)(Figure, E and F). In this patient, as with the others, we utilized milder (700–1000 J) fluence settings than those used in the Moskalik et al24 study; however, we were optimizing for patient comfort, overall downtime, and cosmetic outcomes.
Clearance in this study was assessed by OCT scanning. Scans were obtained 2 months after the last treatment to avoid detecting inflammation and early scar tissue. We opted not to perform biopsies to determine clearance, as done in prior studies, because we were investigating a fully nonsurgical protocol and wanted to enable patients to avoid surgical intervention, as they had requested. Clinical and dermoscopic examinations by a world expert in dermoscopy and OCT (O.M.) provided additional reassurance of lesion clearance.
Limitations
The retrospective study design with a limited sample size was a main limitation of our study. Our limited data suggest that there is value in further investigation and prospective trials of minimally invasive skin cancer management with the pulsed 1064-nm Nd:YAG laser.
Limitations or disadvantages of this nonablative laser treatment include dyschromia and minimal scarring. Furthermore, at fluence settings utilized, treatment can be painful. Without use of a local anesthetic, treatment is limited to what patients can tolerate.
The percentage of BCCs located on the body (53%) was higher in our study than in the general population, estimated in a study to be approximately 20%.33 This percentage might have been an effect of the larger Vivascope 1500 RCM probe, which made certain areas of the face difficult to access, therefore excluding certain facial lesions encountered in our practice from the initial noninvasive diagnosis.
Most lesions in our study have not been followed long-term; median noninvasive OCT follow-up was 64 days; however, the longest follow-up from our data set is longer than 1 year posttreatment (371 days). We have used OCT to establish clearance, which also will allow us to continue using imaging to monitor for changes that might indicate recurrence. Although OCT is not approved by the US Food and Drug Administration as a validated means of diagnosing and detecting BCC, numerous studies have suggested that this modality has high sensitivity (95.7%) and specificity (75.3%) for features of BCC as well as the more critical high negative predictive value (92.1%) for noninvasive management.22-24
Furthermore, setting up the lesions to be monitored long-term using OCT is likely to be more sensitive than monitoring lesions by clinical examination alone, as they have been followed in studies to date. In fact, an earlier study of 115 lesions by the PI found that utilizing OCT significantly improved sensitivity and specificity for detecting BCC (P<.01); improved diagnostic certainty by a factor of 4 compared to clinical examination alone; and improved overall diagnostic accuracy by 50% compared to clinical and dermoscopic examinations.19
Conclusion
Traditional approaches to BCC management usually involve multiple visits: the initial encounter, which might or might not include biopsy, and a return visit for more definitive management. Reflectance confocal microscopy enables live diagnosis and facilitates targeted same-day treatment of BCC. Our pilot study has contributed data to support the further investigation and use of the Nd:YAG laser to treat BCC in combination with early detection with noninvasive diagnosis for a more patient-driven approach. For some patients as well as for dermatologists, the potential for increased efficiency of same-day diagnosis and treatment might provide a clear advantage.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Menzies SW, Westerhoff K, Rabinovitz H, et al. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol. 2000;136:1012-1016.
- Altamura D, Menzies SW, Argenziano G, et al. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol. 2010;62:67-75.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Lallas A, Apalla Z, Ioannides D, et al. Dermoscopy in the diagnosis and management of basal cell carcinoma. Futur Oncol. 2015;11:2975-2984.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:202a10.
- Kauvar AN, Cronin T Jr, Roenigk R, et al; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41:550-571.
- Hoorens I, Vossaert K, Ongenae K, et al. Is early detection of basal cell carcinoma worthwhile? Systematic review based on the WHO criteria for screening. Br J Dermatol. 2016;174:1258-1265.
- Levine A, Markowitz O. In vivo reflectance confocal microscopy. Cutis. 2017;99:399-402.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer - a practical approach. Exp Dermatol. 2013;22:547-551.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- van Manen L, Dijkstra J, Boccara C, et al. The clinical usefulness of optical coherence tomography during cancer interventions. J Cancer Res Clin Oncol. 2018;144:1967-1990.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Markowitz O, Schwartz M. The use of noninvasive optical coherence tomography to monitor the treatment progress of vismodegib and imiquimod 5% cream in a transplant patient with advanced basal cell carcinoma of the nose. J Clin Aesthet Dermatol. 2016;9:37-41.
- Cheng HM, Guitera P. Systematic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Mirza FN, Khatri KA. The use of lasers in the treatment of skin cancer: a review. J Cosmet Laser Ther. 2017;19:451-458.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297.
- Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Moskalik K, Kozlow A, Demin E, et al. Powerful neodymium laser radiation for the treatment of facial carcinoma: 5 year follow-up data. Eur J Dermatol. 2010;20:738-742.
- Ortiz AE, Anderson RR, Avram MM. 1064 nm long-pulsed Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2015;47:106-110.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Papakostas D, Stockfleth E. Topical treatment of basal cell carcinoma with the immune response modifier imiquimod. Futur Oncol. 2015;11:2985-2990.
- Jansen MHE, Mosterd K, Arits AHMM, et al. Five-year results of a randomized controlled trial comparing effectiveness of photodynamic therapy, topical imiquimod, and topical 5-fluorouracil in patients with superficial basal cell carcinoma. J Invest Dermatol. 2018;138:527-533.
- Kadouch DJ, Elshot YS, Zupan-Kajcovski B, et al. One-stop-shop with confocal microscopy imaging vs. standard care for surgical treatment of basal cell carcinoma: an open-label, noninferiority, randomized controlled multicentre trial. Br J Dermatol. 2017;177:735-741.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
The increasing incidence of nonmelanoma skin cancer (NMSC) is a serious public health concern.1 Lesions often are identified on routine total-body examination, and there is a considerate burden on dermatologists to diagnose these lesions, which is both costly and results in a long wait time to see a specialist. Furthermore, standard care requires patients to attend multiple visits for the diagnosis and treatment of NMSC.
In recent decades, diagnosing basal cell carcinoma (BCC) has been facilitated by the handheld dermatoscope. The advent of dermoscopy has led to increased sensitivity and specificity of the NMSC diagnosis (estimated at 95%–99%) and has helped facilitate earlier diagnosis of BCC and reduce unnecessary biopsy of benign lesions.2-5 Dermoscopy also can be useful in monitoring response to treatment.5 Lesions that are detected early tend to be easier and less expensive to treat, a strong argument for the use of early detection techniques.6-8
More recently, in vivo reflectance confocal microscopy (RCM)(Vivascope 1500 [Caliber I.D.]) has become an acceptable means for confirming a BCC diagnosis, offering an alternative to tissue biopsy. Reflectance confocal microscopy can be reimbursed under Category I Current Procedural Terminology codes 96931 to 96936.9 Reflectance confocal microscopy is a noninvasive diagnostic technique that uses an 830-nm diode laser to enable visualization of a 0.5×0.5-mm patch of skin to a depth of 200 to 300 μm, which corresponds roughly to the papillary dermis. Reflectance confocal microscopy has the advantage of providing real-time diagnosis, enabling same-day treatment of BCC, and providing an efficient alternative to biopsy. Ultimately, these advantages are beneficial and time-saving for patients because biopsies can be painful; create a delay in diagnosis; and require further follow-up visits for treatment, which may be of importance to patients who have trouble attending multiple appointments.
Optical coherence tomography (OCT) is another noninvasive imaging device that is useful in BCC management. It uses an infrared broadband light source to visualize skin architecture to 2-mm deep with a 6×6-mm field of view.10 Although OCT does not offer the same cellular clarity as RCM, it allows visualization of a greater depth of skin and a wider field of view, making it a useful tool both in marginating NMSCs prior to treatment and monitoring response to treatment over time.11-16 Optical coherence tomography has demonstrated a high negative predictive value (92.1%) for BCC, which makes it useful for ruling out residual tumor in lesions undergoing management.17-19
With all available options, BCC management benefits from care that is tailored to the individual and the lesion, taking into account size and subtype because not every available treatment is appropriate. Lasers, including solid state, diode, dye, and gas types, are emerging as promising minimally invasive treatment modalities.20,21
Nonablative laser therapy with a pulsed dye laser (PDL) and fractional laser is an example; the principal investigator (PI) of this study (O.M.) recently reported a 95.70% clearance rate utilizing a PDL and fractional laser protocol.22 The 1064-nm Nd:YAG laser also has been used with PDL and as a stand-alone treatment. Jalian et al23 used PDL and the Nd:YAG laser on 13 BCC lesions, with a 58% (7/12) clearance rate after 4 treatments; all nonresponders were taking an anticoagulant, which inhibited the laser’s mechanism of action, according to the researchers.
Moskalik et al24 published a report of 3346 facial BCC lesions treated with pulsed Nd and pulsed Nd:YAG lasers, and included follow-up for as long as 5 years, with a 3.7% recurrence rate. Another report by Moskalik et al25 recorded a recurrence rate of 2.2% to 3.1% for BCCs that were followed for at least 5 years.
Ortiz et al26 reported use of the long-pulsed 1064-nm Nd:YAG laser to treat 13 lesions with biopsy-confirmed BCC on the trunk and extremities, with a 92% (12/13) clearance rate based on histologic analysis 1 month after laser treatment. In an expanded study of 31 patients by Ortiz et al,27 the histologic clearance rate was 90.3% (28/31)—also obtained after 1 month—after 1 Nd:YAG laser treatment, also treating lesions on the trunk and extremities. A further retrospective review of Nd:YAG laser treatment of BCC revealed a 100% clearance rate for 16 lesions (including lesions on the face) that were monitored for at least 6 months (mean duration, 9 months; range, 6–15 months).28 Optical coherence tomography imaging was used for one of the review’s lesions before and after treatment and suggested that the Nd:YAG laser works by selectively destroying the vasculature supplying BCC tumors while preserving surrounding healthy tissue.28
Apart from Moskalik et al,24,25 these studies are limited by a relatively short follow-up time to confirm tumor clearance. Prior studies utilizing the Nd:YAG laser to treat BCC are summarized in the eTable.

This pilot study describes a model of care that aims to alleviate some of the demand placed both on the specialty and on patients by utilizing a novel same-day approach to BCC management. We sought to evaluate management using noninvasive diagnosis with RCM; same-day laser treatment; and follow-up examination with clinical, dermoscopic, and noninvasive imaging using OCT. This method focuses on patient-driven health care from various perspectives. Patients are given real-time information about their diagnosis using RCM, leading to an increased level of information flow and immediate transparency regarding their diagnosis and management options. Patients also are receiving tailored care by incorporating noninvasive imaging and same-day laser treatment, allowing collaboration between patient and physician. Patients have more choices—to undergo surgical care; other at-home topical regimens; or laser management with potentially fewer visits, immediate results, a clearance rate similar to surgery, and improved cosmetic outcome.
Our study attempts to further evaluate the efficacy of the 1064-nm Nd:YAG laser in treating BCC while leveraging noninvasive imaging technology. The objective was to perform a retrospective review of medical records of a subgroup of patients with BCC diagnosed by RCM who were treated with the 1064-nm Nd:YAG laser and monitored for clearance using OCT imaging, in addition to clinical and dermoscopic examination. Similar to prior long-term Nd:YAG laser follow-up studies, we aimed to demonstrate the possibility of a minimally invasive BCC management approach—in our protocol, utilizing imaging instead of biopsy to facilitate long-term follow-up and by offering a model for patient-driven care.
Methods
Study Design
Institutional review board approval was received from Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects (New York, New York). We performed a retrospective review of medical records of patients diagnosed by RCM and treated with a 1064-nm Nd:YAG laser, as an alternative to surgery, at the Mount Sinai Faculty Practice Associates between March 2018 and August 2018. Included in this pilot study are 17 lesions in 16 patients.
Inclusion Criteria
Patients were enrolled based on the following criteria: BCCs diagnosed by clinical and dermoscopic examination followed by RCM imaging; treatment with the 1064-nm Nd:YAG laser, because of patients’ preference for this modality over surgery, superficial radiation therapy, topical regimens, and other laser therapies that require more visits; eligibility by PI included limited clinical ulceration or bleeding (or both) and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). The PI performed a detailed and thorough clinical and dermoscopic skin examination, enabling early detection of the BCCs. Basal cell carcinomas were not included if they exhibited rolled borders, visible ulceration, or oozing growths that allowed for treatment of less-advanced tumors. The PI utilized a clinical and dermoscopic color wheel algorithm to identify suspicious lesions combined with RCM for diagnostic confirmation.29
Two of 17 lesions that did not present as early lesions were included in the study due to patient refusal of surgery or radiation. We consider more advanced tumors to be exophytic, bleeding, crusting, nonhealing ulcerative growths. Patients who had received prior laser treatment with the PI’s PDL with fractional laser protocol with subsequent recurrence at the treatment site were included in the study. Lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor, were excluded.
Treatment Protocol
All patients attended the private clinic at Mount Sinai Hospital of a pigmented lesion expert (O.M.) for routine skin cancer screening. Patients with lesions suspicious for BCC—based on clinical and dermoscopic features—were offered tissue biopsy or RCM. Following diagnosis with RCM, treatment options were discussed, and patients were offered laser treatment when surgical options were declined. Topical treatment options were not emphasized because they require weeks of application to be effective and have been studied mainly in superficial BCC management.30,31
Patients with early lesions were offered either the PDL with fractional laser or Nd:YAG protocol, with their understanding that the Nd:YAG laser protocol would likely involve fewer treatments but a higher likelihood of residual hyperpigmentation or potential scarring (or both) than the more gentle PDL with fractional laser treatment.
All lesions on the face were premarginated using OCT by obtaining central scans and 4 additional scans—above, below, to the left, and to the right of the lesion—to ensure targeted laser treatment with desirable cosmetic results. Facial premargination scans were mandatory; however, patients with lesions on the trunk or extremities were offered the option to have pretreatment margination as an out-of-pocket expense. We did not require premargination of lesions on the body because of their location on less cosmetically critical areas. Most patients declined the optional scans.
This can be considered analogous to the situation in which more insurers reimburse Mohs surgery for cosmetically challenging areas such as the head and neck, while limiting reimbursement for treatment of lesions on the trunk and upper extremities to simple excision. Given cosmetic concerns on the head and neck compared to the body, some patients found it acceptable to have slightly increased dyschromia over a broader treatment area of non–cosmetically critical locations on the body.
Optical coherence tomography imaging was required for all anatomic locations at follow-up visits to detect residual disease or confirm clearance. All patients were given thorough information about the treatment, additional costs, treatment alternatives, potential adverse effects, and complications.
Clinical and dermoscopic images were obtained at every visit using a commercially available point-and-shoot digital single-lens reflex camera for clinical photographs, with an attached DermLite DL3N (3Gen) dermatoscope for all contact polarized dermoscopic photography.
Laser treatment was carried out with the 1064-nm Nd:YAG laser. Setting ranges were similar to previously published studies that used the 1064-nm Nd:YAG laser to treat BCCs (spot sizes, 5–6 mm; fluences, 125–140 J/cm2; pulse durations, 7–10 milliseconds).26-28 The exact settings and number of passes were tailored to the individual lesion based on skin type, anatomic location, extent of tumor involvement by depth (and margin on facial lesions), and posttreatment dermoscopic confirmation of clearance; additionally, for facial lesions, OCT confirmation of clearance.
Laser treatment was provided by the PI. Patients were instructed to apply a thick emollient (ie, formulation of petrolatum or 100% petrolatum) after treatment and until the area healed.
All tumors received 1 to 3 treatments at an interval of 1 to 2 months. The treatment end point was complete clearance, judged by absence of skin cancer clinically, dermoscopically, and on OCT scan. More specifically, the PI looked for vascular changes and echogenic changes on OCT consistent with tumor clearance as well as dermoscopic disappearance of recognized BCC features.
Patients were asked to return for follow-up visits 2 months after the final treatment to evaluate tumor clearance. They were asked to return subsequently every 6 to 12 months for routine care and long-term follow-up.
Results
Patient Characteristics
A total of 16 patients (6 female, 10 male) with 17 BCCs were included in this study. Mean age was 68 years (median, 71.5 years; range; 48–89 years). Mean lesion size was 7.1 mm (median, 6 mm; range, 3–15 mm). Eight lesions were on the face; 9 were on extrafacial sites. Two lesions had a history of laser treatment with the PI’s PDL with fractional laser treatment protocol and had locally recurred. Subtypes of lesions were not elicited by RCM.
Outcomes
Fourteen lesions (14/17 [82.4%]) required 1 treatment to achieve clearance, as confirmed clinically, dermoscopically, and by OCT scanning. One lesion on the back (1/17 [5.8%]) required 2 treatments (70 days between treatments). Two lesions (2/17 [11.8%]) required 3 treatments (time between treatments: 49 and 61 days [lesion 1]; 62 and 64 days [lesion 2]). Lesion 1 was on the face; lesion 2 was on the back. Mean time between last treatment and OCT clearance scan was 103 days (median, 64 days; range, 48–371 days).
Comment
Our study supports the notion that the 1064-nm Nd:YAG laser is a viable option for treating BCC. All (100%) lesions cleared, most (82.4%) with a single treatment. Of course, for patients who required more than 1 treatment (17.6%), we cannot make an argument for fewer patient visits because those patients had to return for multiple laser treatments, but they were able to avoid surgery, as they had wanted. Overall, our diagnostic approach utilizing RCM as opposed to traditional tissue biopsy meant that patients’ skin cancers were diagnosed and treated the same day.
A one-stop shop for diagnosis and treatment model has been reported by Kadouch et al32 as part of a randomized controlled trial in which patients were randomly assigned to receive standard care for BCC—biopsy followed by surgical excision—or RCM diagnosis followed by surgical excision. Their outcome was tumor-free margins after surgical treatment; the RCM approach was found to be noninferior to standard care.32 Our retrospective study differs, of course, in its laser treatment approach; however, both studies investigated a potentially more efficient pathway to BCC management, which becomes increasingly relevant given the rising incidence of NMSC.
A real-time, image-based diagnostic approach combined with laser treatment delivers patient-driven care, offering choice and convenience. It might be optimal for patients who have an extensive history of BCC, are poor surgical candidates, have difficulty with the logistics of the multiple visits required for surgical management, cannot (for practical reasons) spend multiple hours in office between Mohs stages, and do not want potentially disfiguring scars, making a minimally invasive treatment preferable.
As we found in our sample, not all patients are amenable to undergoing what is regarded now as the most definitive treatment—namely, surgical options. This subset of patients, whose lesions require more definitive treatment but who do not desire invasive management, need alternative approaches to BCC treatment. The present study proposes a model of patient-driven care that requires collaboration between physician and patient, offering more customized care that takes into account patient choice.
In our study, most patients had lesions that were detected early in their evolution; these lesions might be particularly amenable to laser management. The 2 resistant lesions in our set—requiring 3 treatments—appeared more aggressive clinically at initial evaluation but still had posttreatment outcomes with mild dyschromia similar to the lesions only treated once (Figure, A–D). Of those 2 lesions, the 9-mm lesion on the back (Figure, C and D) might have been larger than clinically apparent; in hindsight, it might have responded to a single treatment had it been premarginated. (An additional factor to have considered is the patient’s immunosuppressed status, which might have led to a more resistant lesion. Larger trials would help elucidate whether an immunosuppressed patient requires a different treatment approach, broader treatment area, OCT premargination regardless of anatomic location, or a greater number of treatments.) Nevertheless, the 2 aforementioned patients were offered treatment with the 1064-nm Nd:YAG laser because they refused surgery, radiation, and other more aggressive modalities. The patients were given advanced warning of an increased possibility of recurrence or nonclearance.

The lesion that required 2 treatments did not appear to be an aggressive subtype; however, it was considerably larger than most other treated lesions (1.5 cm)(Figure, E and F). In this patient, as with the others, we utilized milder (700–1000 J) fluence settings than those used in the Moskalik et al24 study; however, we were optimizing for patient comfort, overall downtime, and cosmetic outcomes.
Clearance in this study was assessed by OCT scanning. Scans were obtained 2 months after the last treatment to avoid detecting inflammation and early scar tissue. We opted not to perform biopsies to determine clearance, as done in prior studies, because we were investigating a fully nonsurgical protocol and wanted to enable patients to avoid surgical intervention, as they had requested. Clinical and dermoscopic examinations by a world expert in dermoscopy and OCT (O.M.) provided additional reassurance of lesion clearance.
Limitations
The retrospective study design with a limited sample size was a main limitation of our study. Our limited data suggest that there is value in further investigation and prospective trials of minimally invasive skin cancer management with the pulsed 1064-nm Nd:YAG laser.
Limitations or disadvantages of this nonablative laser treatment include dyschromia and minimal scarring. Furthermore, at fluence settings utilized, treatment can be painful. Without use of a local anesthetic, treatment is limited to what patients can tolerate.
The percentage of BCCs located on the body (53%) was higher in our study than in the general population, estimated in a study to be approximately 20%.33 This percentage might have been an effect of the larger Vivascope 1500 RCM probe, which made certain areas of the face difficult to access, therefore excluding certain facial lesions encountered in our practice from the initial noninvasive diagnosis.
Most lesions in our study have not been followed long-term; median noninvasive OCT follow-up was 64 days; however, the longest follow-up from our data set is longer than 1 year posttreatment (371 days). We have used OCT to establish clearance, which also will allow us to continue using imaging to monitor for changes that might indicate recurrence. Although OCT is not approved by the US Food and Drug Administration as a validated means of diagnosing and detecting BCC, numerous studies have suggested that this modality has high sensitivity (95.7%) and specificity (75.3%) for features of BCC as well as the more critical high negative predictive value (92.1%) for noninvasive management.22-24
Furthermore, setting up the lesions to be monitored long-term using OCT is likely to be more sensitive than monitoring lesions by clinical examination alone, as they have been followed in studies to date. In fact, an earlier study of 115 lesions by the PI found that utilizing OCT significantly improved sensitivity and specificity for detecting BCC (P<.01); improved diagnostic certainty by a factor of 4 compared to clinical examination alone; and improved overall diagnostic accuracy by 50% compared to clinical and dermoscopic examinations.19
Conclusion
Traditional approaches to BCC management usually involve multiple visits: the initial encounter, which might or might not include biopsy, and a return visit for more definitive management. Reflectance confocal microscopy enables live diagnosis and facilitates targeted same-day treatment of BCC. Our pilot study has contributed data to support the further investigation and use of the Nd:YAG laser to treat BCC in combination with early detection with noninvasive diagnosis for a more patient-driven approach. For some patients as well as for dermatologists, the potential for increased efficiency of same-day diagnosis and treatment might provide a clear advantage.
The increasing incidence of nonmelanoma skin cancer (NMSC) is a serious public health concern.1 Lesions often are identified on routine total-body examination, and there is a considerate burden on dermatologists to diagnose these lesions, which is both costly and results in a long wait time to see a specialist. Furthermore, standard care requires patients to attend multiple visits for the diagnosis and treatment of NMSC.
In recent decades, diagnosing basal cell carcinoma (BCC) has been facilitated by the handheld dermatoscope. The advent of dermoscopy has led to increased sensitivity and specificity of the NMSC diagnosis (estimated at 95%–99%) and has helped facilitate earlier diagnosis of BCC and reduce unnecessary biopsy of benign lesions.2-5 Dermoscopy also can be useful in monitoring response to treatment.5 Lesions that are detected early tend to be easier and less expensive to treat, a strong argument for the use of early detection techniques.6-8
More recently, in vivo reflectance confocal microscopy (RCM)(Vivascope 1500 [Caliber I.D.]) has become an acceptable means for confirming a BCC diagnosis, offering an alternative to tissue biopsy. Reflectance confocal microscopy can be reimbursed under Category I Current Procedural Terminology codes 96931 to 96936.9 Reflectance confocal microscopy is a noninvasive diagnostic technique that uses an 830-nm diode laser to enable visualization of a 0.5×0.5-mm patch of skin to a depth of 200 to 300 μm, which corresponds roughly to the papillary dermis. Reflectance confocal microscopy has the advantage of providing real-time diagnosis, enabling same-day treatment of BCC, and providing an efficient alternative to biopsy. Ultimately, these advantages are beneficial and time-saving for patients because biopsies can be painful; create a delay in diagnosis; and require further follow-up visits for treatment, which may be of importance to patients who have trouble attending multiple appointments.
Optical coherence tomography (OCT) is another noninvasive imaging device that is useful in BCC management. It uses an infrared broadband light source to visualize skin architecture to 2-mm deep with a 6×6-mm field of view.10 Although OCT does not offer the same cellular clarity as RCM, it allows visualization of a greater depth of skin and a wider field of view, making it a useful tool both in marginating NMSCs prior to treatment and monitoring response to treatment over time.11-16 Optical coherence tomography has demonstrated a high negative predictive value (92.1%) for BCC, which makes it useful for ruling out residual tumor in lesions undergoing management.17-19
With all available options, BCC management benefits from care that is tailored to the individual and the lesion, taking into account size and subtype because not every available treatment is appropriate. Lasers, including solid state, diode, dye, and gas types, are emerging as promising minimally invasive treatment modalities.20,21
Nonablative laser therapy with a pulsed dye laser (PDL) and fractional laser is an example; the principal investigator (PI) of this study (O.M.) recently reported a 95.70% clearance rate utilizing a PDL and fractional laser protocol.22 The 1064-nm Nd:YAG laser also has been used with PDL and as a stand-alone treatment. Jalian et al23 used PDL and the Nd:YAG laser on 13 BCC lesions, with a 58% (7/12) clearance rate after 4 treatments; all nonresponders were taking an anticoagulant, which inhibited the laser’s mechanism of action, according to the researchers.
Moskalik et al24 published a report of 3346 facial BCC lesions treated with pulsed Nd and pulsed Nd:YAG lasers, and included follow-up for as long as 5 years, with a 3.7% recurrence rate. Another report by Moskalik et al25 recorded a recurrence rate of 2.2% to 3.1% for BCCs that were followed for at least 5 years.
Ortiz et al26 reported use of the long-pulsed 1064-nm Nd:YAG laser to treat 13 lesions with biopsy-confirmed BCC on the trunk and extremities, with a 92% (12/13) clearance rate based on histologic analysis 1 month after laser treatment. In an expanded study of 31 patients by Ortiz et al,27 the histologic clearance rate was 90.3% (28/31)—also obtained after 1 month—after 1 Nd:YAG laser treatment, also treating lesions on the trunk and extremities. A further retrospective review of Nd:YAG laser treatment of BCC revealed a 100% clearance rate for 16 lesions (including lesions on the face) that were monitored for at least 6 months (mean duration, 9 months; range, 6–15 months).28 Optical coherence tomography imaging was used for one of the review’s lesions before and after treatment and suggested that the Nd:YAG laser works by selectively destroying the vasculature supplying BCC tumors while preserving surrounding healthy tissue.28
Apart from Moskalik et al,24,25 these studies are limited by a relatively short follow-up time to confirm tumor clearance. Prior studies utilizing the Nd:YAG laser to treat BCC are summarized in the eTable.

This pilot study describes a model of care that aims to alleviate some of the demand placed both on the specialty and on patients by utilizing a novel same-day approach to BCC management. We sought to evaluate management using noninvasive diagnosis with RCM; same-day laser treatment; and follow-up examination with clinical, dermoscopic, and noninvasive imaging using OCT. This method focuses on patient-driven health care from various perspectives. Patients are given real-time information about their diagnosis using RCM, leading to an increased level of information flow and immediate transparency regarding their diagnosis and management options. Patients also are receiving tailored care by incorporating noninvasive imaging and same-day laser treatment, allowing collaboration between patient and physician. Patients have more choices—to undergo surgical care; other at-home topical regimens; or laser management with potentially fewer visits, immediate results, a clearance rate similar to surgery, and improved cosmetic outcome.
Our study attempts to further evaluate the efficacy of the 1064-nm Nd:YAG laser in treating BCC while leveraging noninvasive imaging technology. The objective was to perform a retrospective review of medical records of a subgroup of patients with BCC diagnosed by RCM who were treated with the 1064-nm Nd:YAG laser and monitored for clearance using OCT imaging, in addition to clinical and dermoscopic examination. Similar to prior long-term Nd:YAG laser follow-up studies, we aimed to demonstrate the possibility of a minimally invasive BCC management approach—in our protocol, utilizing imaging instead of biopsy to facilitate long-term follow-up and by offering a model for patient-driven care.
Methods
Study Design
Institutional review board approval was received from Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects (New York, New York). We performed a retrospective review of medical records of patients diagnosed by RCM and treated with a 1064-nm Nd:YAG laser, as an alternative to surgery, at the Mount Sinai Faculty Practice Associates between March 2018 and August 2018. Included in this pilot study are 17 lesions in 16 patients.
Inclusion Criteria
Patients were enrolled based on the following criteria: BCCs diagnosed by clinical and dermoscopic examination followed by RCM imaging; treatment with the 1064-nm Nd:YAG laser, because of patients’ preference for this modality over surgery, superficial radiation therapy, topical regimens, and other laser therapies that require more visits; eligibility by PI included limited clinical ulceration or bleeding (or both) and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). The PI performed a detailed and thorough clinical and dermoscopic skin examination, enabling early detection of the BCCs. Basal cell carcinomas were not included if they exhibited rolled borders, visible ulceration, or oozing growths that allowed for treatment of less-advanced tumors. The PI utilized a clinical and dermoscopic color wheel algorithm to identify suspicious lesions combined with RCM for diagnostic confirmation.29
Two of 17 lesions that did not present as early lesions were included in the study due to patient refusal of surgery or radiation. We consider more advanced tumors to be exophytic, bleeding, crusting, nonhealing ulcerative growths. Patients who had received prior laser treatment with the PI’s PDL with fractional laser protocol with subsequent recurrence at the treatment site were included in the study. Lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor, were excluded.
Treatment Protocol
All patients attended the private clinic at Mount Sinai Hospital of a pigmented lesion expert (O.M.) for routine skin cancer screening. Patients with lesions suspicious for BCC—based on clinical and dermoscopic features—were offered tissue biopsy or RCM. Following diagnosis with RCM, treatment options were discussed, and patients were offered laser treatment when surgical options were declined. Topical treatment options were not emphasized because they require weeks of application to be effective and have been studied mainly in superficial BCC management.30,31
Patients with early lesions were offered either the PDL with fractional laser or Nd:YAG protocol, with their understanding that the Nd:YAG laser protocol would likely involve fewer treatments but a higher likelihood of residual hyperpigmentation or potential scarring (or both) than the more gentle PDL with fractional laser treatment.
All lesions on the face were premarginated using OCT by obtaining central scans and 4 additional scans—above, below, to the left, and to the right of the lesion—to ensure targeted laser treatment with desirable cosmetic results. Facial premargination scans were mandatory; however, patients with lesions on the trunk or extremities were offered the option to have pretreatment margination as an out-of-pocket expense. We did not require premargination of lesions on the body because of their location on less cosmetically critical areas. Most patients declined the optional scans.
This can be considered analogous to the situation in which more insurers reimburse Mohs surgery for cosmetically challenging areas such as the head and neck, while limiting reimbursement for treatment of lesions on the trunk and upper extremities to simple excision. Given cosmetic concerns on the head and neck compared to the body, some patients found it acceptable to have slightly increased dyschromia over a broader treatment area of non–cosmetically critical locations on the body.
Optical coherence tomography imaging was required for all anatomic locations at follow-up visits to detect residual disease or confirm clearance. All patients were given thorough information about the treatment, additional costs, treatment alternatives, potential adverse effects, and complications.
Clinical and dermoscopic images were obtained at every visit using a commercially available point-and-shoot digital single-lens reflex camera for clinical photographs, with an attached DermLite DL3N (3Gen) dermatoscope for all contact polarized dermoscopic photography.
Laser treatment was carried out with the 1064-nm Nd:YAG laser. Setting ranges were similar to previously published studies that used the 1064-nm Nd:YAG laser to treat BCCs (spot sizes, 5–6 mm; fluences, 125–140 J/cm2; pulse durations, 7–10 milliseconds).26-28 The exact settings and number of passes were tailored to the individual lesion based on skin type, anatomic location, extent of tumor involvement by depth (and margin on facial lesions), and posttreatment dermoscopic confirmation of clearance; additionally, for facial lesions, OCT confirmation of clearance.
Laser treatment was provided by the PI. Patients were instructed to apply a thick emollient (ie, formulation of petrolatum or 100% petrolatum) after treatment and until the area healed.
All tumors received 1 to 3 treatments at an interval of 1 to 2 months. The treatment end point was complete clearance, judged by absence of skin cancer clinically, dermoscopically, and on OCT scan. More specifically, the PI looked for vascular changes and echogenic changes on OCT consistent with tumor clearance as well as dermoscopic disappearance of recognized BCC features.
Patients were asked to return for follow-up visits 2 months after the final treatment to evaluate tumor clearance. They were asked to return subsequently every 6 to 12 months for routine care and long-term follow-up.
Results
Patient Characteristics
A total of 16 patients (6 female, 10 male) with 17 BCCs were included in this study. Mean age was 68 years (median, 71.5 years; range; 48–89 years). Mean lesion size was 7.1 mm (median, 6 mm; range, 3–15 mm). Eight lesions were on the face; 9 were on extrafacial sites. Two lesions had a history of laser treatment with the PI’s PDL with fractional laser treatment protocol and had locally recurred. Subtypes of lesions were not elicited by RCM.
Outcomes
Fourteen lesions (14/17 [82.4%]) required 1 treatment to achieve clearance, as confirmed clinically, dermoscopically, and by OCT scanning. One lesion on the back (1/17 [5.8%]) required 2 treatments (70 days between treatments). Two lesions (2/17 [11.8%]) required 3 treatments (time between treatments: 49 and 61 days [lesion 1]; 62 and 64 days [lesion 2]). Lesion 1 was on the face; lesion 2 was on the back. Mean time between last treatment and OCT clearance scan was 103 days (median, 64 days; range, 48–371 days).
Comment
Our study supports the notion that the 1064-nm Nd:YAG laser is a viable option for treating BCC. All (100%) lesions cleared, most (82.4%) with a single treatment. Of course, for patients who required more than 1 treatment (17.6%), we cannot make an argument for fewer patient visits because those patients had to return for multiple laser treatments, but they were able to avoid surgery, as they had wanted. Overall, our diagnostic approach utilizing RCM as opposed to traditional tissue biopsy meant that patients’ skin cancers were diagnosed and treated the same day.
A one-stop shop for diagnosis and treatment model has been reported by Kadouch et al32 as part of a randomized controlled trial in which patients were randomly assigned to receive standard care for BCC—biopsy followed by surgical excision—or RCM diagnosis followed by surgical excision. Their outcome was tumor-free margins after surgical treatment; the RCM approach was found to be noninferior to standard care.32 Our retrospective study differs, of course, in its laser treatment approach; however, both studies investigated a potentially more efficient pathway to BCC management, which becomes increasingly relevant given the rising incidence of NMSC.
A real-time, image-based diagnostic approach combined with laser treatment delivers patient-driven care, offering choice and convenience. It might be optimal for patients who have an extensive history of BCC, are poor surgical candidates, have difficulty with the logistics of the multiple visits required for surgical management, cannot (for practical reasons) spend multiple hours in office between Mohs stages, and do not want potentially disfiguring scars, making a minimally invasive treatment preferable.
As we found in our sample, not all patients are amenable to undergoing what is regarded now as the most definitive treatment—namely, surgical options. This subset of patients, whose lesions require more definitive treatment but who do not desire invasive management, need alternative approaches to BCC treatment. The present study proposes a model of patient-driven care that requires collaboration between physician and patient, offering more customized care that takes into account patient choice.
In our study, most patients had lesions that were detected early in their evolution; these lesions might be particularly amenable to laser management. The 2 resistant lesions in our set—requiring 3 treatments—appeared more aggressive clinically at initial evaluation but still had posttreatment outcomes with mild dyschromia similar to the lesions only treated once (Figure, A–D). Of those 2 lesions, the 9-mm lesion on the back (Figure, C and D) might have been larger than clinically apparent; in hindsight, it might have responded to a single treatment had it been premarginated. (An additional factor to have considered is the patient’s immunosuppressed status, which might have led to a more resistant lesion. Larger trials would help elucidate whether an immunosuppressed patient requires a different treatment approach, broader treatment area, OCT premargination regardless of anatomic location, or a greater number of treatments.) Nevertheless, the 2 aforementioned patients were offered treatment with the 1064-nm Nd:YAG laser because they refused surgery, radiation, and other more aggressive modalities. The patients were given advanced warning of an increased possibility of recurrence or nonclearance.

The lesion that required 2 treatments did not appear to be an aggressive subtype; however, it was considerably larger than most other treated lesions (1.5 cm)(Figure, E and F). In this patient, as with the others, we utilized milder (700–1000 J) fluence settings than those used in the Moskalik et al24 study; however, we were optimizing for patient comfort, overall downtime, and cosmetic outcomes.
Clearance in this study was assessed by OCT scanning. Scans were obtained 2 months after the last treatment to avoid detecting inflammation and early scar tissue. We opted not to perform biopsies to determine clearance, as done in prior studies, because we were investigating a fully nonsurgical protocol and wanted to enable patients to avoid surgical intervention, as they had requested. Clinical and dermoscopic examinations by a world expert in dermoscopy and OCT (O.M.) provided additional reassurance of lesion clearance.
Limitations
The retrospective study design with a limited sample size was a main limitation of our study. Our limited data suggest that there is value in further investigation and prospective trials of minimally invasive skin cancer management with the pulsed 1064-nm Nd:YAG laser.
Limitations or disadvantages of this nonablative laser treatment include dyschromia and minimal scarring. Furthermore, at fluence settings utilized, treatment can be painful. Without use of a local anesthetic, treatment is limited to what patients can tolerate.
The percentage of BCCs located on the body (53%) was higher in our study than in the general population, estimated in a study to be approximately 20%.33 This percentage might have been an effect of the larger Vivascope 1500 RCM probe, which made certain areas of the face difficult to access, therefore excluding certain facial lesions encountered in our practice from the initial noninvasive diagnosis.
Most lesions in our study have not been followed long-term; median noninvasive OCT follow-up was 64 days; however, the longest follow-up from our data set is longer than 1 year posttreatment (371 days). We have used OCT to establish clearance, which also will allow us to continue using imaging to monitor for changes that might indicate recurrence. Although OCT is not approved by the US Food and Drug Administration as a validated means of diagnosing and detecting BCC, numerous studies have suggested that this modality has high sensitivity (95.7%) and specificity (75.3%) for features of BCC as well as the more critical high negative predictive value (92.1%) for noninvasive management.22-24
Furthermore, setting up the lesions to be monitored long-term using OCT is likely to be more sensitive than monitoring lesions by clinical examination alone, as they have been followed in studies to date. In fact, an earlier study of 115 lesions by the PI found that utilizing OCT significantly improved sensitivity and specificity for detecting BCC (P<.01); improved diagnostic certainty by a factor of 4 compared to clinical examination alone; and improved overall diagnostic accuracy by 50% compared to clinical and dermoscopic examinations.19
Conclusion
Traditional approaches to BCC management usually involve multiple visits: the initial encounter, which might or might not include biopsy, and a return visit for more definitive management. Reflectance confocal microscopy enables live diagnosis and facilitates targeted same-day treatment of BCC. Our pilot study has contributed data to support the further investigation and use of the Nd:YAG laser to treat BCC in combination with early detection with noninvasive diagnosis for a more patient-driven approach. For some patients as well as for dermatologists, the potential for increased efficiency of same-day diagnosis and treatment might provide a clear advantage.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Menzies SW, Westerhoff K, Rabinovitz H, et al. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol. 2000;136:1012-1016.
- Altamura D, Menzies SW, Argenziano G, et al. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol. 2010;62:67-75.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Lallas A, Apalla Z, Ioannides D, et al. Dermoscopy in the diagnosis and management of basal cell carcinoma. Futur Oncol. 2015;11:2975-2984.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:202a10.
- Kauvar AN, Cronin T Jr, Roenigk R, et al; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41:550-571.
- Hoorens I, Vossaert K, Ongenae K, et al. Is early detection of basal cell carcinoma worthwhile? Systematic review based on the WHO criteria for screening. Br J Dermatol. 2016;174:1258-1265.
- Levine A, Markowitz O. In vivo reflectance confocal microscopy. Cutis. 2017;99:399-402.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer - a practical approach. Exp Dermatol. 2013;22:547-551.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- van Manen L, Dijkstra J, Boccara C, et al. The clinical usefulness of optical coherence tomography during cancer interventions. J Cancer Res Clin Oncol. 2018;144:1967-1990.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Markowitz O, Schwartz M. The use of noninvasive optical coherence tomography to monitor the treatment progress of vismodegib and imiquimod 5% cream in a transplant patient with advanced basal cell carcinoma of the nose. J Clin Aesthet Dermatol. 2016;9:37-41.
- Cheng HM, Guitera P. Systematic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Mirza FN, Khatri KA. The use of lasers in the treatment of skin cancer: a review. J Cosmet Laser Ther. 2017;19:451-458.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297.
- Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Moskalik K, Kozlow A, Demin E, et al. Powerful neodymium laser radiation for the treatment of facial carcinoma: 5 year follow-up data. Eur J Dermatol. 2010;20:738-742.
- Ortiz AE, Anderson RR, Avram MM. 1064 nm long-pulsed Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2015;47:106-110.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Papakostas D, Stockfleth E. Topical treatment of basal cell carcinoma with the immune response modifier imiquimod. Futur Oncol. 2015;11:2985-2990.
- Jansen MHE, Mosterd K, Arits AHMM, et al. Five-year results of a randomized controlled trial comparing effectiveness of photodynamic therapy, topical imiquimod, and topical 5-fluorouracil in patients with superficial basal cell carcinoma. J Invest Dermatol. 2018;138:527-533.
- Kadouch DJ, Elshot YS, Zupan-Kajcovski B, et al. One-stop-shop with confocal microscopy imaging vs. standard care for surgical treatment of basal cell carcinoma: an open-label, noninferiority, randomized controlled multicentre trial. Br J Dermatol. 2017;177:735-741.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Menzies SW, Westerhoff K, Rabinovitz H, et al. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol. 2000;136:1012-1016.
- Altamura D, Menzies SW, Argenziano G, et al. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol. 2010;62:67-75.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Lallas A, Apalla Z, Ioannides D, et al. Dermoscopy in the diagnosis and management of basal cell carcinoma. Futur Oncol. 2015;11:2975-2984.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:202a10.
- Kauvar AN, Cronin T Jr, Roenigk R, et al; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41:550-571.
- Hoorens I, Vossaert K, Ongenae K, et al. Is early detection of basal cell carcinoma worthwhile? Systematic review based on the WHO criteria for screening. Br J Dermatol. 2016;174:1258-1265.
- Levine A, Markowitz O. In vivo reflectance confocal microscopy. Cutis. 2017;99:399-402.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer - a practical approach. Exp Dermatol. 2013;22:547-551.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- van Manen L, Dijkstra J, Boccara C, et al. The clinical usefulness of optical coherence tomography during cancer interventions. J Cancer Res Clin Oncol. 2018;144:1967-1990.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Markowitz O, Schwartz M. The use of noninvasive optical coherence tomography to monitor the treatment progress of vismodegib and imiquimod 5% cream in a transplant patient with advanced basal cell carcinoma of the nose. J Clin Aesthet Dermatol. 2016;9:37-41.
- Cheng HM, Guitera P. Systematic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Mirza FN, Khatri KA. The use of lasers in the treatment of skin cancer: a review. J Cosmet Laser Ther. 2017;19:451-458.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297.
- Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Moskalik K, Kozlow A, Demin E, et al. Powerful neodymium laser radiation for the treatment of facial carcinoma: 5 year follow-up data. Eur J Dermatol. 2010;20:738-742.
- Ortiz AE, Anderson RR, Avram MM. 1064 nm long-pulsed Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2015;47:106-110.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Papakostas D, Stockfleth E. Topical treatment of basal cell carcinoma with the immune response modifier imiquimod. Futur Oncol. 2015;11:2985-2990.
- Jansen MHE, Mosterd K, Arits AHMM, et al. Five-year results of a randomized controlled trial comparing effectiveness of photodynamic therapy, topical imiquimod, and topical 5-fluorouracil in patients with superficial basal cell carcinoma. J Invest Dermatol. 2018;138:527-533.
- Kadouch DJ, Elshot YS, Zupan-Kajcovski B, et al. One-stop-shop with confocal microscopy imaging vs. standard care for surgical treatment of basal cell carcinoma: an open-label, noninferiority, randomized controlled multicentre trial. Br J Dermatol. 2017;177:735-741.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
Practice Points
- Novel imaging modalities such as reflectance confocal microscopy and optical coherence tomography can be used to diagnose, monitor treatment response, and confirm clearance of basal cell carcinoma.
- Leveraging new imaging technologies can enable a streamlined patient experience, with same-day diagnosis and management of skin cancer.
- For patients who do not desire surgical management of nonmelanoma skin cancer, laser treatment with Nd:YAG is a promising emerging therapeutic option.
Kaposi Sarcoma in a Patient With Postpolio Syndrome
Kaposi sarcoma (KS) is a low-grade vascular tumor that is rare among the general US population, with an incidence rate of less than 1 per 100,000.1 The tumor is more common among certain groups of individuals due to geographic differences in the prevalence of KS-associated herpesvirus (also referred to as human herpesvirus 8) as well as host immune factors.2 Kaposi sarcoma often is defined by the patient's predisposing characteristics yielding the following distinct epidemiologic subtypes: (1) classic KS is a rare disease affecting older men of Mediterranean descent; (2) African KS is an endemic cancer with male predominance in sub-Saharan Africa; (3) AIDS-associated KS is an often aggressive AIDS-defining illness; and (4) iatrogenic KS occurs in patients on immunosuppressive therapy.3 When evaluating a patient without any of these risk factors, the clinical suspicion for KS may be low. We report a patient with postpolio syndrome (PPS) who presented with KS of the right leg, ankle, and foot.
A 77-year-old man with a distant history of paralytic poliomyelitis presented for an annual skin examination with concern for a new lesion on the right ankle. The patient had a history of PPS primarily affecting the right leg. Physical examination revealed residual weakness in an atrophic right lower extremity with a mottled appearance and mild pitting edema to the knee. Two red, dome-shaped, vascular papules were appreciated on the medial aspect of the right ankle (Figure 1), and a shave biopsy of the larger papule was performed. Microscopic examination of the biopsy specimen was consistent with KS (Figure 2). This patient had no history of human immunodeficiency virus or immunosuppressive therapy and was not of Mediterranean descent.
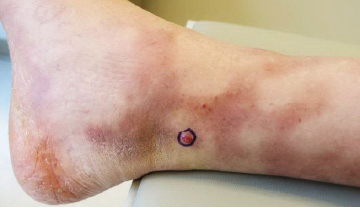
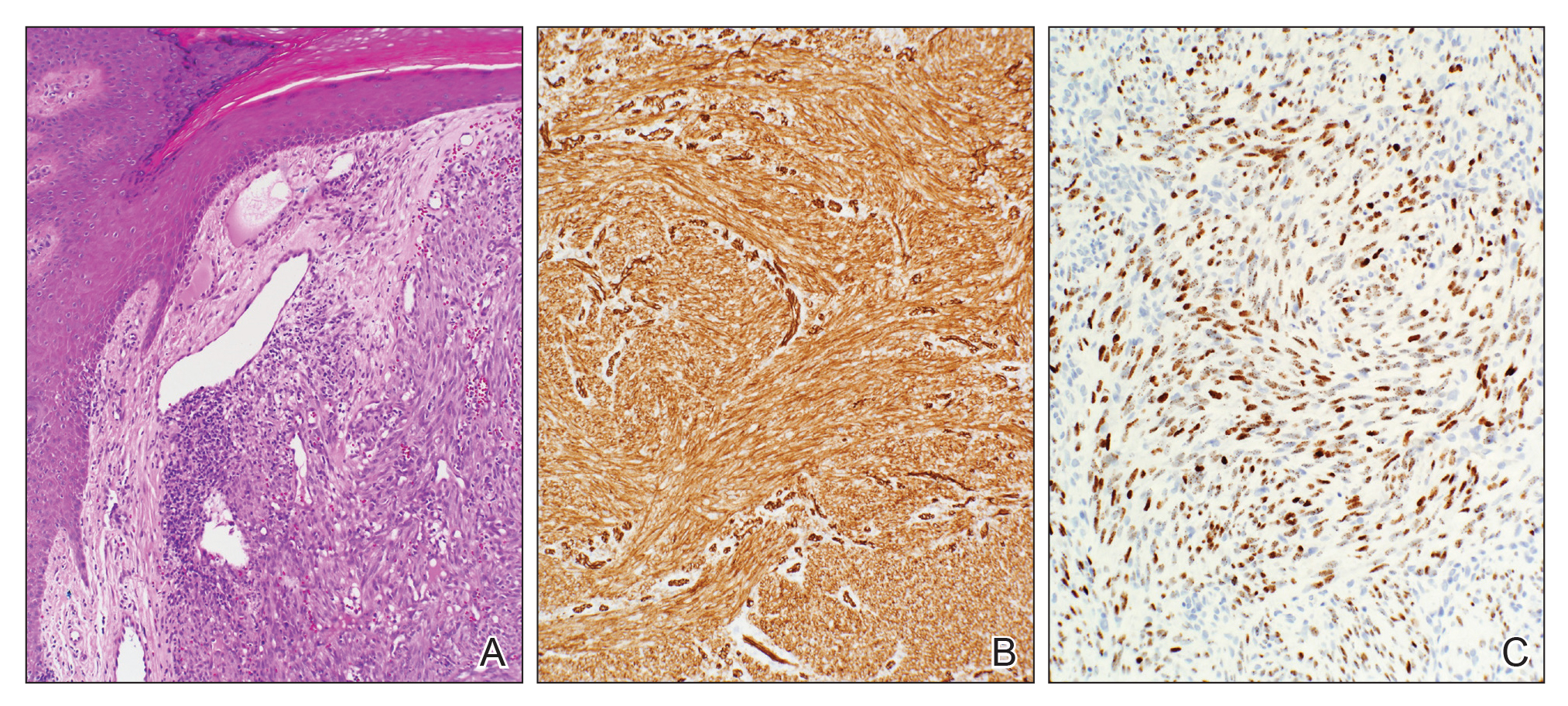
Because KS is a radiosensitive vascular neoplasm and radiation therapy (RT) alone can achieve local control,4 the patient was treated with 6 megaelectron-volt electron-beam RT. He received 30 Gy in 10 fractions to the affected area of the medial ankle. The patient tolerated RT well. Three weeks after completing treatment, he was found to have mild lichenification on the right medial ankle with no clinical evidence of disease. Four months later, he presented with multiple additional vascular papules on the right third toe and in the interdigital web space (Figure 3). Shave biopsy of one of these lesions was consistent with KS. Contrast computed tomography of the chest, abdomen, and pelvis was performed, revealing no evidence of metastatic disease. The patient was treated with 30 Gy in 15 fractions using opposed lateral 6 megaelectron-volt photon fields to the entire right lower extremity below the knee to treat all of the skin affected by the PPS. His posttreatment course was complicated by edema in the affected leg that resolved after daily pneumatic compression. He had no evidence of residual or recurrent disease 6 months after completing RT (Figure 4).
Cutaneous KS is a human herpesvirus 8-positive tumor of endothelial origin typically seen in older men of Mediterranean or African descent and among immunosuppressed patients.4 Our patient did not have any classic risk factors for KS, but his disease did arise in the setting of a right lower extremity that was notably affected by PPS. Postpolio syndrome is characterized by muscle atrophy due to denervation of the motor unit.5 Bruno et al6 found that such deficits in motor innervation could lead to impairments in venous outflow causing cutaneous venous congestion. Acroangiodermatitis clinically resembles KS but is a benign reactive vasoproliferative disorder and is well known to occur in the lower extremities as a sequela of chronic venous insufficiency.7 A case of bilateral lower extremity pseudo-KS was reported in a patient with notable PPS.8 A report of 2 patients describes KS arising in the setting of chronic venous insufficiency without any classic risk factors.9 Therefore, patients with PPS characterized by venous insufficiency may represent a population at increased risk for KS.
- Surveillance, Epidemiology, and End Results (SEER) Program. US Population Data--1969-2017. https://seer.cancer.gov/popdata/. Published January 2019. Accessed November 25, 2019.
- Uldrick TS, Whitby D. Update on KSHV epidemiology, kaposi sarcoma pathogenesis, and treatment of saposi sarcoma. Cancer Lett. 2011;305:150-162.
- Schwartz RA, Micali G, Nasca MR, et al. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008;59:179-206.
- Arnold HL, Odom RB, James WD, et al. Andrews' Diseases of the Skin: Clinical Dermatology. Philadelphia, PA: Saunders; 1990.
- Boyer FV, Tiffreau V, Rapin A, et al. Post-polio syndrome: pathophysiological hypotheses, diagnosis criteria, drug therapy. Ann Phys Rehabil Med. 2010;53:34-41.
- Bruno RL, Johnson JC, Berman WS. Vasomotor abnormalities as post-polio sequelae: functional and clinical implications. Orthopedics. 1985;8:865-869.
- Palmer B, Xia Y, Cho S, Lewis FS. Acroangiodermatitis secondary to chronic venous insufficiency. Cutis. 2010;86:239-240.
- Rotbart G. Kaposi's disease and venous insufficiency. Phlebologie. 1978;31:439-443.
- Que SK, DeFelice T, Abdulla FR, et al. Non-HIV-related kaposi sarcoma in 2 Hispanic patients arising in the setting of chronic venous insufficiency. Cutis. 2015;95:E30-E33.
Kaposi sarcoma (KS) is a low-grade vascular tumor that is rare among the general US population, with an incidence rate of less than 1 per 100,000.1 The tumor is more common among certain groups of individuals due to geographic differences in the prevalence of KS-associated herpesvirus (also referred to as human herpesvirus 8) as well as host immune factors.2 Kaposi sarcoma often is defined by the patient's predisposing characteristics yielding the following distinct epidemiologic subtypes: (1) classic KS is a rare disease affecting older men of Mediterranean descent; (2) African KS is an endemic cancer with male predominance in sub-Saharan Africa; (3) AIDS-associated KS is an often aggressive AIDS-defining illness; and (4) iatrogenic KS occurs in patients on immunosuppressive therapy.3 When evaluating a patient without any of these risk factors, the clinical suspicion for KS may be low. We report a patient with postpolio syndrome (PPS) who presented with KS of the right leg, ankle, and foot.
A 77-year-old man with a distant history of paralytic poliomyelitis presented for an annual skin examination with concern for a new lesion on the right ankle. The patient had a history of PPS primarily affecting the right leg. Physical examination revealed residual weakness in an atrophic right lower extremity with a mottled appearance and mild pitting edema to the knee. Two red, dome-shaped, vascular papules were appreciated on the medial aspect of the right ankle (Figure 1), and a shave biopsy of the larger papule was performed. Microscopic examination of the biopsy specimen was consistent with KS (Figure 2). This patient had no history of human immunodeficiency virus or immunosuppressive therapy and was not of Mediterranean descent.


Because KS is a radiosensitive vascular neoplasm and radiation therapy (RT) alone can achieve local control,4 the patient was treated with 6 megaelectron-volt electron-beam RT. He received 30 Gy in 10 fractions to the affected area of the medial ankle. The patient tolerated RT well. Three weeks after completing treatment, he was found to have mild lichenification on the right medial ankle with no clinical evidence of disease. Four months later, he presented with multiple additional vascular papules on the right third toe and in the interdigital web space (Figure 3). Shave biopsy of one of these lesions was consistent with KS. Contrast computed tomography of the chest, abdomen, and pelvis was performed, revealing no evidence of metastatic disease. The patient was treated with 30 Gy in 15 fractions using opposed lateral 6 megaelectron-volt photon fields to the entire right lower extremity below the knee to treat all of the skin affected by the PPS. His posttreatment course was complicated by edema in the affected leg that resolved after daily pneumatic compression. He had no evidence of residual or recurrent disease 6 months after completing RT (Figure 4).


Cutaneous KS is a human herpesvirus 8-positive tumor of endothelial origin typically seen in older men of Mediterranean or African descent and among immunosuppressed patients.4 Our patient did not have any classic risk factors for KS, but his disease did arise in the setting of a right lower extremity that was notably affected by PPS. Postpolio syndrome is characterized by muscle atrophy due to denervation of the motor unit.5 Bruno et al6 found that such deficits in motor innervation could lead to impairments in venous outflow causing cutaneous venous congestion. Acroangiodermatitis clinically resembles KS but is a benign reactive vasoproliferative disorder and is well known to occur in the lower extremities as a sequela of chronic venous insufficiency.7 A case of bilateral lower extremity pseudo-KS was reported in a patient with notable PPS.8 A report of 2 patients describes KS arising in the setting of chronic venous insufficiency without any classic risk factors.9 Therefore, patients with PPS characterized by venous insufficiency may represent a population at increased risk for KS.
Kaposi sarcoma (KS) is a low-grade vascular tumor that is rare among the general US population, with an incidence rate of less than 1 per 100,000.1 The tumor is more common among certain groups of individuals due to geographic differences in the prevalence of KS-associated herpesvirus (also referred to as human herpesvirus 8) as well as host immune factors.2 Kaposi sarcoma often is defined by the patient's predisposing characteristics yielding the following distinct epidemiologic subtypes: (1) classic KS is a rare disease affecting older men of Mediterranean descent; (2) African KS is an endemic cancer with male predominance in sub-Saharan Africa; (3) AIDS-associated KS is an often aggressive AIDS-defining illness; and (4) iatrogenic KS occurs in patients on immunosuppressive therapy.3 When evaluating a patient without any of these risk factors, the clinical suspicion for KS may be low. We report a patient with postpolio syndrome (PPS) who presented with KS of the right leg, ankle, and foot.
A 77-year-old man with a distant history of paralytic poliomyelitis presented for an annual skin examination with concern for a new lesion on the right ankle. The patient had a history of PPS primarily affecting the right leg. Physical examination revealed residual weakness in an atrophic right lower extremity with a mottled appearance and mild pitting edema to the knee. Two red, dome-shaped, vascular papules were appreciated on the medial aspect of the right ankle (Figure 1), and a shave biopsy of the larger papule was performed. Microscopic examination of the biopsy specimen was consistent with KS (Figure 2). This patient had no history of human immunodeficiency virus or immunosuppressive therapy and was not of Mediterranean descent.


Because KS is a radiosensitive vascular neoplasm and radiation therapy (RT) alone can achieve local control,4 the patient was treated with 6 megaelectron-volt electron-beam RT. He received 30 Gy in 10 fractions to the affected area of the medial ankle. The patient tolerated RT well. Three weeks after completing treatment, he was found to have mild lichenification on the right medial ankle with no clinical evidence of disease. Four months later, he presented with multiple additional vascular papules on the right third toe and in the interdigital web space (Figure 3). Shave biopsy of one of these lesions was consistent with KS. Contrast computed tomography of the chest, abdomen, and pelvis was performed, revealing no evidence of metastatic disease. The patient was treated with 30 Gy in 15 fractions using opposed lateral 6 megaelectron-volt photon fields to the entire right lower extremity below the knee to treat all of the skin affected by the PPS. His posttreatment course was complicated by edema in the affected leg that resolved after daily pneumatic compression. He had no evidence of residual or recurrent disease 6 months after completing RT (Figure 4).


Cutaneous KS is a human herpesvirus 8-positive tumor of endothelial origin typically seen in older men of Mediterranean or African descent and among immunosuppressed patients.4 Our patient did not have any classic risk factors for KS, but his disease did arise in the setting of a right lower extremity that was notably affected by PPS. Postpolio syndrome is characterized by muscle atrophy due to denervation of the motor unit.5 Bruno et al6 found that such deficits in motor innervation could lead to impairments in venous outflow causing cutaneous venous congestion. Acroangiodermatitis clinically resembles KS but is a benign reactive vasoproliferative disorder and is well known to occur in the lower extremities as a sequela of chronic venous insufficiency.7 A case of bilateral lower extremity pseudo-KS was reported in a patient with notable PPS.8 A report of 2 patients describes KS arising in the setting of chronic venous insufficiency without any classic risk factors.9 Therefore, patients with PPS characterized by venous insufficiency may represent a population at increased risk for KS.
- Surveillance, Epidemiology, and End Results (SEER) Program. US Population Data--1969-2017. https://seer.cancer.gov/popdata/. Published January 2019. Accessed November 25, 2019.
- Uldrick TS, Whitby D. Update on KSHV epidemiology, kaposi sarcoma pathogenesis, and treatment of saposi sarcoma. Cancer Lett. 2011;305:150-162.
- Schwartz RA, Micali G, Nasca MR, et al. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008;59:179-206.
- Arnold HL, Odom RB, James WD, et al. Andrews' Diseases of the Skin: Clinical Dermatology. Philadelphia, PA: Saunders; 1990.
- Boyer FV, Tiffreau V, Rapin A, et al. Post-polio syndrome: pathophysiological hypotheses, diagnosis criteria, drug therapy. Ann Phys Rehabil Med. 2010;53:34-41.
- Bruno RL, Johnson JC, Berman WS. Vasomotor abnormalities as post-polio sequelae: functional and clinical implications. Orthopedics. 1985;8:865-869.
- Palmer B, Xia Y, Cho S, Lewis FS. Acroangiodermatitis secondary to chronic venous insufficiency. Cutis. 2010;86:239-240.
- Rotbart G. Kaposi's disease and venous insufficiency. Phlebologie. 1978;31:439-443.
- Que SK, DeFelice T, Abdulla FR, et al. Non-HIV-related kaposi sarcoma in 2 Hispanic patients arising in the setting of chronic venous insufficiency. Cutis. 2015;95:E30-E33.
- Surveillance, Epidemiology, and End Results (SEER) Program. US Population Data--1969-2017. https://seer.cancer.gov/popdata/. Published January 2019. Accessed November 25, 2019.
- Uldrick TS, Whitby D. Update on KSHV epidemiology, kaposi sarcoma pathogenesis, and treatment of saposi sarcoma. Cancer Lett. 2011;305:150-162.
- Schwartz RA, Micali G, Nasca MR, et al. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008;59:179-206.
- Arnold HL, Odom RB, James WD, et al. Andrews' Diseases of the Skin: Clinical Dermatology. Philadelphia, PA: Saunders; 1990.
- Boyer FV, Tiffreau V, Rapin A, et al. Post-polio syndrome: pathophysiological hypotheses, diagnosis criteria, drug therapy. Ann Phys Rehabil Med. 2010;53:34-41.
- Bruno RL, Johnson JC, Berman WS. Vasomotor abnormalities as post-polio sequelae: functional and clinical implications. Orthopedics. 1985;8:865-869.
- Palmer B, Xia Y, Cho S, Lewis FS. Acroangiodermatitis secondary to chronic venous insufficiency. Cutis. 2010;86:239-240.
- Rotbart G. Kaposi's disease and venous insufficiency. Phlebologie. 1978;31:439-443.
- Que SK, DeFelice T, Abdulla FR, et al. Non-HIV-related kaposi sarcoma in 2 Hispanic patients arising in the setting of chronic venous insufficiency. Cutis. 2015;95:E30-E33.
Practice Points
- Cutaneous Kaposi sarcoma (KS) is a human herpesvirus 8–positive tumor of endothelial origin typically seen in older men of Mediterranean or African descent and among immunosuppressed patients.
- In addition, patients with postpolio syndrome characterized by venous insufficiency may represent a population at increased risk for KS.
- Kaposi sarcoma is a radiosensitive vascular neoplasm, and radiation therapy can achieve local control.
New Plaques Arising at Site of Previously Excised Basal Cell Carcinoma
The Diagnosis: Actinic Comedonal Plaque
Histopathologic examination showed multiple small, keratin-filled cystic spaces in the superficial dermis lined by stratified squamous epithelium that keratinized with a granular layer with surrounding solar elastosis (Figure 1). These findings were consistent with an actinic comedonal plaque (ACP), a rare variant of Favre-Racouchot syndrome (FRS). Due to other cases of invasive squamous cell carcinomas developing within these lesions1 and the patient's history of basal cell carcinoma in the area, it was important to rule out malignancy and further confirm the diagnosis. Thus, an additional biopsy was obtained, which revealed no sign of cellular atypia. The patient was bothered by the appearance and texture of the lesion, so he elected to pursue treatment. Because of the lesion's moderate size and location, surgical excision was not recommended. He was treated with cryosurgery followed by tretinoin gel 0.025% for 3 months (Figure 2). At 1-year follow-up, there was no recurrence and an acceptable cosmetic outcome.
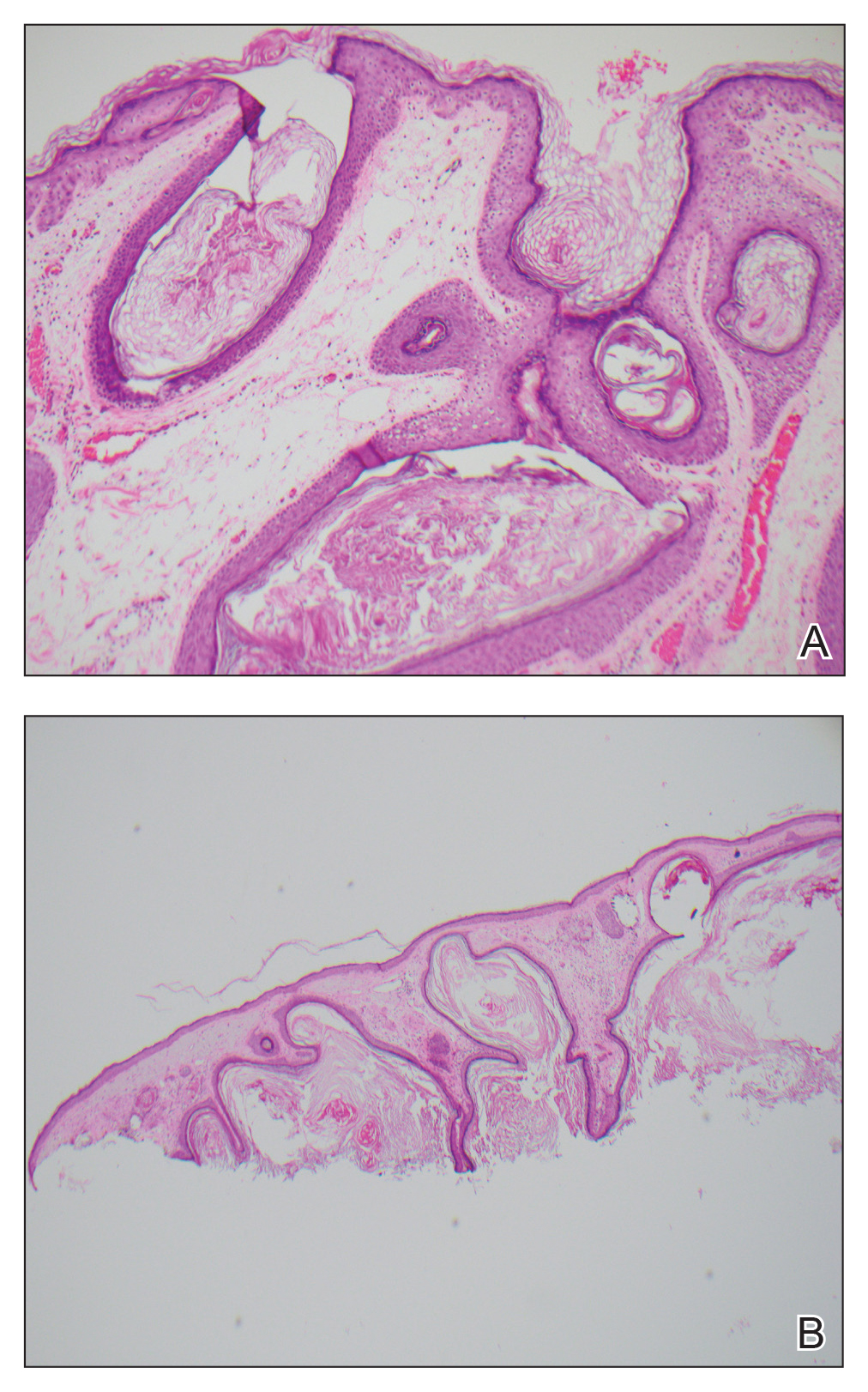
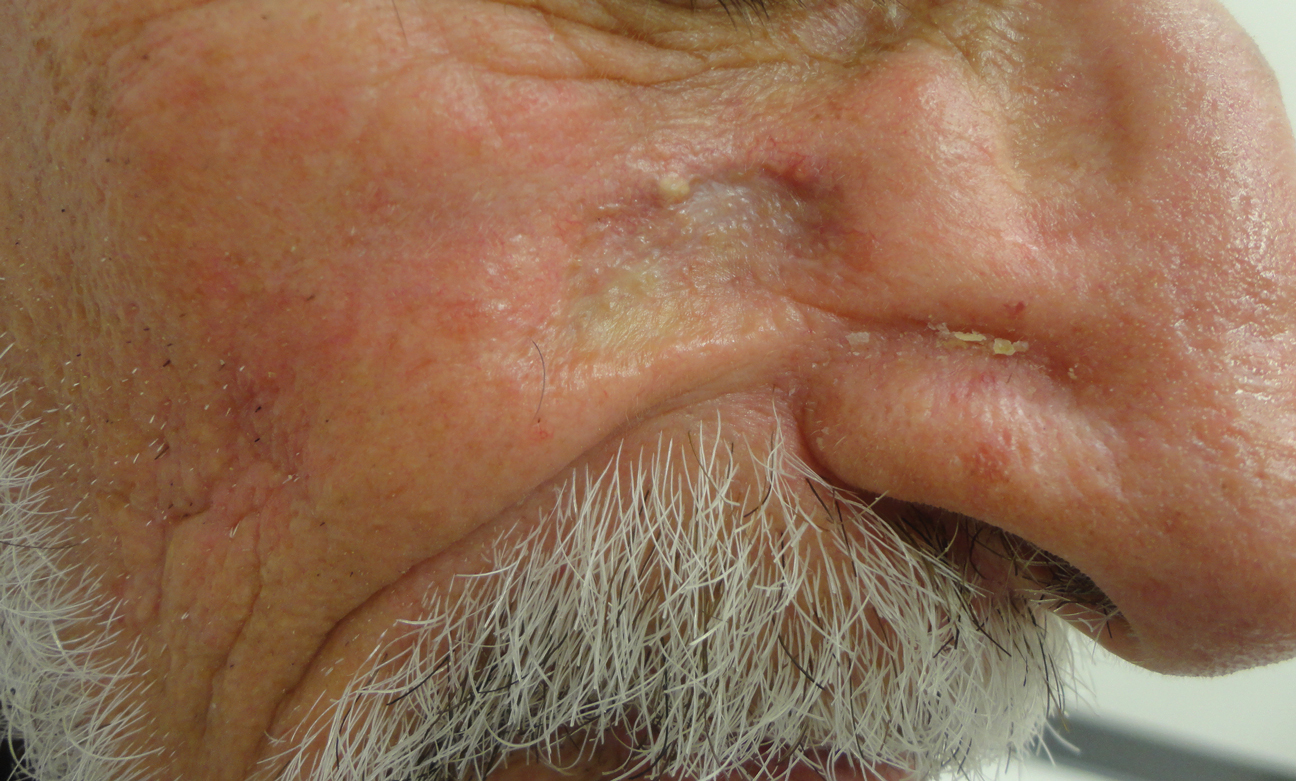
Actinic comedonal plaques are characterized by cysts, comedones, and papules that form well-defined yellow plaques on the skin after chronic sun exposure.2 First described in 1980 by Eastern and Martin,3 these lesions are most often found on the neck, thorax, nasal dorsum, helix, and forearms; they often are mistaken for basal cell carcinomas, chondrodermatitis nodularis chronica helicis, and amyloidosis.2,4
Similar to the cases described by Eastern and Martin,3 our patient presented with a solitary 2.8×1.6-cm yellowish and bumpy plaque that was growing on chronically sun-exposed skin of the medial right cheek. The patient's history of smoking and basal cell carcinoma removal led to the following differential diagnoses: recurrent malignancy, tumors of dermal appendages, and xanthelasmalike deposition within a scar, among others.
Histologic examination demonstrated keratin-filled cystic spaces in the superficial dermis, along with hyperkeratosis and solar elastosis. These findings were consistent with the original histologic descriptions of ACP that were cited as a rare ectopic form of FRS.5 Leeuwis-Fedorovich et al1 described multifocal squamous cell carcinomas arising in a FRS lesion in 2 cases. Additional biopsy was performed in our patient to rule out this possibility.
Aside from its role in dermatologic malignancy, exposure to UV light can lead to dilation of the sebaceous gland infundibulum and formation of comedones in various locations.2 Histologically, lesions of ACP feature dilated, keratin-filled follicles within a matrix of amorphous damaged collagen in the middle and lower dermis, along with elastosis and basophilic degeneration with fragmentation of collagen bundles in the upper dermis coated with flattened epidermis.2,3
Several clinical diagnoses were considered in the differential for our patient. In contrast to our patient's solitary lesion, classic FRS--nodular elastosis with cysts and comedones--is characterized by a diffuse yellowish hue of large open black comedones that are symmetrically distributed on the temporal and periorbital areas.6
Xanthoma has several clinical presentations. Plane xanthomas typically develop in skin folds, especially in palmar creases, while xanthelasma typically consists of yellow soft plaques on the eyelids and periorbital skin. Histologically, xanthomas contain lipid-laden macrophages, which were absent in our patient.7
Cutaneous amyloidosis can be characterized as macular, papular, and nodular. Nodular, the rarest subtype, commonly manifests on the face.8 However, its characteristic histologic features include atrophic epidermal changes with an amorphous, eosinophilic-appearing dermis due to amyloid deposition. These findings were absent in our case.
Recurrent neoplasm would be in the differential diagnosis of any solitary nodule arising within a previously treated site of malignancy, which was excluded by histologic examination of our patient.7
Topical tretinoin has been demonstrated as effective treatment of both FRS and ACP.2,9 Other effective treatment modalities include cryotherapy and photoprotection.9 For our patient, a combination of cryosurgery and topical tretinoin resulted in depression of the plaque and a good cosmetic outcome.
Acknowledgments
We thank the patient for granting permission to publish this article. We also are indebted to Morgan L. Wilson, MD (Springfield, Illinois), for his expert dermatopathologic evaluation in this case. He has received no compensation for his contribution.
- Leeuwis-Fedorovich NE, Starink M, van der Wal AC. Multifocal squamous cell carcinoma arising in a Favre-Racouchot lesion—report of two cases and review of the literature. J Dermatol Case Rep. 2015;9:103-106.
- Cardoso F, Nakandakari S, Zattar GA, et al. Actinic comedonal plaquevariant of Favre-Racouchot syndrome: report of two cases. An Bras Dermatol. 2015;90(suppl 1):185-187.
- Eastern JS, Martin S. Actinic comedonal plaque. J Am Acad Dermatol. 1980;3:633-636.
- Hauptman G, Kopf A, Rabinovitz HS, et al. The actinic comedonal plaque. Cutis. 1997;60:145-146.
- John SM, Hamm H. Actinic comedonal plaque—a rare ectopic form of the Favre-Racouchot syndrome. Clin Exp Dermatol. 1993;18:256-258.
- Sonthalia S, Arora R, Chhabra N, et al. Favre-Racouchot syndrome. Indian Dermatol Online J. 2014;5(suppl 2):S128-S129.
- Elder DE, Elenitsas R, Murphy GF, et al. Benign pigmented lesions and malignant melanoma. In: Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:699-790.
- Lee DY, Kim YJ, Lee JY, et al. Primary localized cutaneous nodular amyloidosis following local trauma. Ann Dermatol. 2011;23:515-518.
- Patterson WM, Fox MD, Schwartz RA. Favre-Racouchot disease. Int J Dermatol. 2004;43:167-169.
The Diagnosis: Actinic Comedonal Plaque
Histopathologic examination showed multiple small, keratin-filled cystic spaces in the superficial dermis lined by stratified squamous epithelium that keratinized with a granular layer with surrounding solar elastosis (Figure 1). These findings were consistent with an actinic comedonal plaque (ACP), a rare variant of Favre-Racouchot syndrome (FRS). Due to other cases of invasive squamous cell carcinomas developing within these lesions1 and the patient's history of basal cell carcinoma in the area, it was important to rule out malignancy and further confirm the diagnosis. Thus, an additional biopsy was obtained, which revealed no sign of cellular atypia. The patient was bothered by the appearance and texture of the lesion, so he elected to pursue treatment. Because of the lesion's moderate size and location, surgical excision was not recommended. He was treated with cryosurgery followed by tretinoin gel 0.025% for 3 months (Figure 2). At 1-year follow-up, there was no recurrence and an acceptable cosmetic outcome.


Actinic comedonal plaques are characterized by cysts, comedones, and papules that form well-defined yellow plaques on the skin after chronic sun exposure.2 First described in 1980 by Eastern and Martin,3 these lesions are most often found on the neck, thorax, nasal dorsum, helix, and forearms; they often are mistaken for basal cell carcinomas, chondrodermatitis nodularis chronica helicis, and amyloidosis.2,4
Similar to the cases described by Eastern and Martin,3 our patient presented with a solitary 2.8×1.6-cm yellowish and bumpy plaque that was growing on chronically sun-exposed skin of the medial right cheek. The patient's history of smoking and basal cell carcinoma removal led to the following differential diagnoses: recurrent malignancy, tumors of dermal appendages, and xanthelasmalike deposition within a scar, among others.
Histologic examination demonstrated keratin-filled cystic spaces in the superficial dermis, along with hyperkeratosis and solar elastosis. These findings were consistent with the original histologic descriptions of ACP that were cited as a rare ectopic form of FRS.5 Leeuwis-Fedorovich et al1 described multifocal squamous cell carcinomas arising in a FRS lesion in 2 cases. Additional biopsy was performed in our patient to rule out this possibility.
Aside from its role in dermatologic malignancy, exposure to UV light can lead to dilation of the sebaceous gland infundibulum and formation of comedones in various locations.2 Histologically, lesions of ACP feature dilated, keratin-filled follicles within a matrix of amorphous damaged collagen in the middle and lower dermis, along with elastosis and basophilic degeneration with fragmentation of collagen bundles in the upper dermis coated with flattened epidermis.2,3
Several clinical diagnoses were considered in the differential for our patient. In contrast to our patient's solitary lesion, classic FRS--nodular elastosis with cysts and comedones--is characterized by a diffuse yellowish hue of large open black comedones that are symmetrically distributed on the temporal and periorbital areas.6
Xanthoma has several clinical presentations. Plane xanthomas typically develop in skin folds, especially in palmar creases, while xanthelasma typically consists of yellow soft plaques on the eyelids and periorbital skin. Histologically, xanthomas contain lipid-laden macrophages, which were absent in our patient.7
Cutaneous amyloidosis can be characterized as macular, papular, and nodular. Nodular, the rarest subtype, commonly manifests on the face.8 However, its characteristic histologic features include atrophic epidermal changes with an amorphous, eosinophilic-appearing dermis due to amyloid deposition. These findings were absent in our case.
Recurrent neoplasm would be in the differential diagnosis of any solitary nodule arising within a previously treated site of malignancy, which was excluded by histologic examination of our patient.7
Topical tretinoin has been demonstrated as effective treatment of both FRS and ACP.2,9 Other effective treatment modalities include cryotherapy and photoprotection.9 For our patient, a combination of cryosurgery and topical tretinoin resulted in depression of the plaque and a good cosmetic outcome.
Acknowledgments
We thank the patient for granting permission to publish this article. We also are indebted to Morgan L. Wilson, MD (Springfield, Illinois), for his expert dermatopathologic evaluation in this case. He has received no compensation for his contribution.
The Diagnosis: Actinic Comedonal Plaque
Histopathologic examination showed multiple small, keratin-filled cystic spaces in the superficial dermis lined by stratified squamous epithelium that keratinized with a granular layer with surrounding solar elastosis (Figure 1). These findings were consistent with an actinic comedonal plaque (ACP), a rare variant of Favre-Racouchot syndrome (FRS). Due to other cases of invasive squamous cell carcinomas developing within these lesions1 and the patient's history of basal cell carcinoma in the area, it was important to rule out malignancy and further confirm the diagnosis. Thus, an additional biopsy was obtained, which revealed no sign of cellular atypia. The patient was bothered by the appearance and texture of the lesion, so he elected to pursue treatment. Because of the lesion's moderate size and location, surgical excision was not recommended. He was treated with cryosurgery followed by tretinoin gel 0.025% for 3 months (Figure 2). At 1-year follow-up, there was no recurrence and an acceptable cosmetic outcome.


Actinic comedonal plaques are characterized by cysts, comedones, and papules that form well-defined yellow plaques on the skin after chronic sun exposure.2 First described in 1980 by Eastern and Martin,3 these lesions are most often found on the neck, thorax, nasal dorsum, helix, and forearms; they often are mistaken for basal cell carcinomas, chondrodermatitis nodularis chronica helicis, and amyloidosis.2,4
Similar to the cases described by Eastern and Martin,3 our patient presented with a solitary 2.8×1.6-cm yellowish and bumpy plaque that was growing on chronically sun-exposed skin of the medial right cheek. The patient's history of smoking and basal cell carcinoma removal led to the following differential diagnoses: recurrent malignancy, tumors of dermal appendages, and xanthelasmalike deposition within a scar, among others.
Histologic examination demonstrated keratin-filled cystic spaces in the superficial dermis, along with hyperkeratosis and solar elastosis. These findings were consistent with the original histologic descriptions of ACP that were cited as a rare ectopic form of FRS.5 Leeuwis-Fedorovich et al1 described multifocal squamous cell carcinomas arising in a FRS lesion in 2 cases. Additional biopsy was performed in our patient to rule out this possibility.
Aside from its role in dermatologic malignancy, exposure to UV light can lead to dilation of the sebaceous gland infundibulum and formation of comedones in various locations.2 Histologically, lesions of ACP feature dilated, keratin-filled follicles within a matrix of amorphous damaged collagen in the middle and lower dermis, along with elastosis and basophilic degeneration with fragmentation of collagen bundles in the upper dermis coated with flattened epidermis.2,3
Several clinical diagnoses were considered in the differential for our patient. In contrast to our patient's solitary lesion, classic FRS--nodular elastosis with cysts and comedones--is characterized by a diffuse yellowish hue of large open black comedones that are symmetrically distributed on the temporal and periorbital areas.6
Xanthoma has several clinical presentations. Plane xanthomas typically develop in skin folds, especially in palmar creases, while xanthelasma typically consists of yellow soft plaques on the eyelids and periorbital skin. Histologically, xanthomas contain lipid-laden macrophages, which were absent in our patient.7
Cutaneous amyloidosis can be characterized as macular, papular, and nodular. Nodular, the rarest subtype, commonly manifests on the face.8 However, its characteristic histologic features include atrophic epidermal changes with an amorphous, eosinophilic-appearing dermis due to amyloid deposition. These findings were absent in our case.
Recurrent neoplasm would be in the differential diagnosis of any solitary nodule arising within a previously treated site of malignancy, which was excluded by histologic examination of our patient.7
Topical tretinoin has been demonstrated as effective treatment of both FRS and ACP.2,9 Other effective treatment modalities include cryotherapy and photoprotection.9 For our patient, a combination of cryosurgery and topical tretinoin resulted in depression of the plaque and a good cosmetic outcome.
Acknowledgments
We thank the patient for granting permission to publish this article. We also are indebted to Morgan L. Wilson, MD (Springfield, Illinois), for his expert dermatopathologic evaluation in this case. He has received no compensation for his contribution.
- Leeuwis-Fedorovich NE, Starink M, van der Wal AC. Multifocal squamous cell carcinoma arising in a Favre-Racouchot lesion—report of two cases and review of the literature. J Dermatol Case Rep. 2015;9:103-106.
- Cardoso F, Nakandakari S, Zattar GA, et al. Actinic comedonal plaquevariant of Favre-Racouchot syndrome: report of two cases. An Bras Dermatol. 2015;90(suppl 1):185-187.
- Eastern JS, Martin S. Actinic comedonal plaque. J Am Acad Dermatol. 1980;3:633-636.
- Hauptman G, Kopf A, Rabinovitz HS, et al. The actinic comedonal plaque. Cutis. 1997;60:145-146.
- John SM, Hamm H. Actinic comedonal plaque—a rare ectopic form of the Favre-Racouchot syndrome. Clin Exp Dermatol. 1993;18:256-258.
- Sonthalia S, Arora R, Chhabra N, et al. Favre-Racouchot syndrome. Indian Dermatol Online J. 2014;5(suppl 2):S128-S129.
- Elder DE, Elenitsas R, Murphy GF, et al. Benign pigmented lesions and malignant melanoma. In: Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:699-790.
- Lee DY, Kim YJ, Lee JY, et al. Primary localized cutaneous nodular amyloidosis following local trauma. Ann Dermatol. 2011;23:515-518.
- Patterson WM, Fox MD, Schwartz RA. Favre-Racouchot disease. Int J Dermatol. 2004;43:167-169.
- Leeuwis-Fedorovich NE, Starink M, van der Wal AC. Multifocal squamous cell carcinoma arising in a Favre-Racouchot lesion—report of two cases and review of the literature. J Dermatol Case Rep. 2015;9:103-106.
- Cardoso F, Nakandakari S, Zattar GA, et al. Actinic comedonal plaquevariant of Favre-Racouchot syndrome: report of two cases. An Bras Dermatol. 2015;90(suppl 1):185-187.
- Eastern JS, Martin S. Actinic comedonal plaque. J Am Acad Dermatol. 1980;3:633-636.
- Hauptman G, Kopf A, Rabinovitz HS, et al. The actinic comedonal plaque. Cutis. 1997;60:145-146.
- John SM, Hamm H. Actinic comedonal plaque—a rare ectopic form of the Favre-Racouchot syndrome. Clin Exp Dermatol. 1993;18:256-258.
- Sonthalia S, Arora R, Chhabra N, et al. Favre-Racouchot syndrome. Indian Dermatol Online J. 2014;5(suppl 2):S128-S129.
- Elder DE, Elenitsas R, Murphy GF, et al. Benign pigmented lesions and malignant melanoma. In: Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:699-790.
- Lee DY, Kim YJ, Lee JY, et al. Primary localized cutaneous nodular amyloidosis following local trauma. Ann Dermatol. 2011;23:515-518.
- Patterson WM, Fox MD, Schwartz RA. Favre-Racouchot disease. Int J Dermatol. 2004;43:167-169.
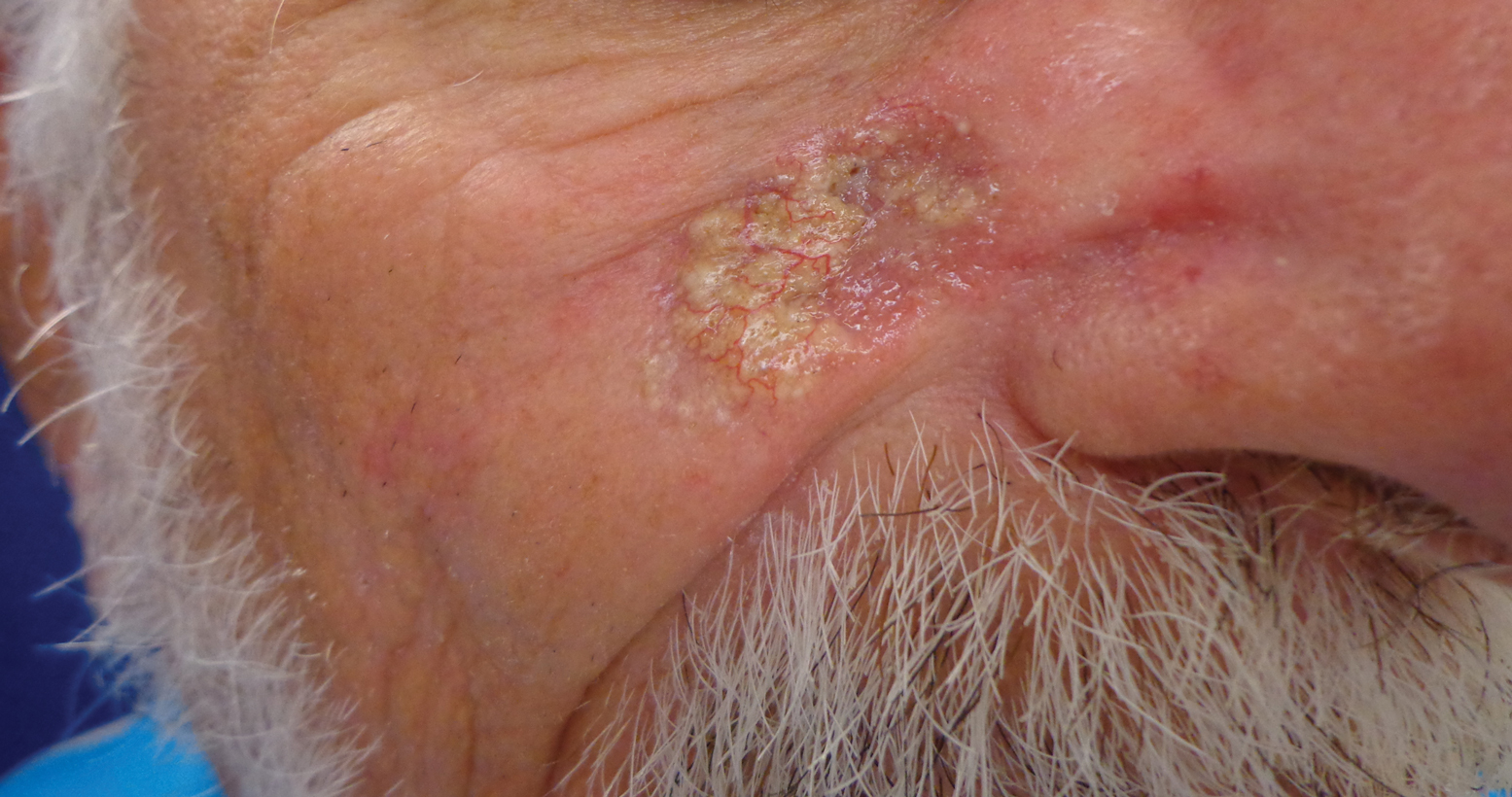
A 79-year-old man presented to dermatology with an enlarging bump on the right cheek. He reported a history of basal cell carcinoma on the medial right cheek that was removed 15 years prior with a resultant scar. Over the last 6 months, the area became more red and bumpy, and the lesion increased in size but was otherwise asymptomatic. The patient reported no history of other dermatologic conditions or skin cancer aside from the prior basal cell carcinoma. His medical history was notable for hypertension and hyperlipidemia. He had a history of smoking for many years (only recently quit) with extensive sun exposure in his lifetime as an outdoor worker. Physical examination revealed a 2.8.2 ×1.6-cm, pink, telangiectatic, and slightly depressed plaque, with the surface consisting of multiple white to yellowish coalescing papules. As part of the dermatologic workup, 4-mm punch and shave biopsies were obtained.
Serum test sheds light on Merkel cell carcinoma
LAS VEGAS – Merkel cell carcinoma, an extremely rare form of skin cancer, is often caused by a subclinical virus that routinely inhabits the skin. Now, a serum test of virus antibody levels is offering insight into the state of the disease, according to one dermatologist.
“If you have these antibodies, you have a better prognosis. You can follow those antibodies to test for recurrence or progression,” Isaac Brownell, MD, PhD, of the Dermatology Branch of the National Institutes of Health said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
The cancer appears in the skin’s Merkel cells, which contribute to our sense of touch by helping us to discriminate textures. “When you put your hand in your pocket, and you can tell the difference between the front and back of a quarter,” he said, “you’re using the Merkel cells in your fingertips.”
Only about 2,500 cases of Merkel cell carcinoma appear in the United States each year, Dr. Brownell said. It appears more often in elderly white patients, is more common in men than women, and is more likely among immunosuppressed patients, whose risk is increased 15- to 20-fold. Cases are more common in sunnier regions – at least in men – and lesions frequently appear on the head, face, and neck.
Five-year survival is estimated at 51% if the cancer is localized, according to a 2016 study of 9,387 cases that Dr. Brownell highlighted. But survival declines dramatically if it has spread to lymph nodes or distant sites (Ann Surg Oncol. 2016 Oct;23[11]:3564-71).
In recent years, researchers have linked 80% of Merkel cell carcinoma cases to the Merkel cell polyomavirus, he said. The virus normally inhabits our skin with no ill effects, he said. “We all have this virus on our skin. It’s everywhere, and even children have antibodies,” he said. But mutations can lead to Merkel cell carcinoma.
Does it matter if cases are polyomavirus positive or polyomavirus negative? Not really, Dr. Brownell said, since the presence of the virus doesn’t appear to affect overall prognosis. However, he said, serum antibody testing can be helpful in polyomavirus-positive patients because it offers insight into prognosis and tumor burden. For example, “if the baseline titer falls and then starts to go up, they’re likely to have a recurrence, and you’ll want to look out for that,” he said.
Dr. Brownell offered another bit of advice: Be prepared to respond to patients who worry that they have a contagious virus and could be a danger to others. The proper answer, he said, is this: “You don’t have to worry about infecting people. Your tumor is not making the virus, you’re not infectious, and we have the virus on us already.”
For more information about the antibody test, visit merkelcell.org/sero.
Dr. Brownell reported having no relevant disclosures. SDEF and this news organization are owned by the same parent company.
LAS VEGAS – Merkel cell carcinoma, an extremely rare form of skin cancer, is often caused by a subclinical virus that routinely inhabits the skin. Now, a serum test of virus antibody levels is offering insight into the state of the disease, according to one dermatologist.
“If you have these antibodies, you have a better prognosis. You can follow those antibodies to test for recurrence or progression,” Isaac Brownell, MD, PhD, of the Dermatology Branch of the National Institutes of Health said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
The cancer appears in the skin’s Merkel cells, which contribute to our sense of touch by helping us to discriminate textures. “When you put your hand in your pocket, and you can tell the difference between the front and back of a quarter,” he said, “you’re using the Merkel cells in your fingertips.”
Only about 2,500 cases of Merkel cell carcinoma appear in the United States each year, Dr. Brownell said. It appears more often in elderly white patients, is more common in men than women, and is more likely among immunosuppressed patients, whose risk is increased 15- to 20-fold. Cases are more common in sunnier regions – at least in men – and lesions frequently appear on the head, face, and neck.
Five-year survival is estimated at 51% if the cancer is localized, according to a 2016 study of 9,387 cases that Dr. Brownell highlighted. But survival declines dramatically if it has spread to lymph nodes or distant sites (Ann Surg Oncol. 2016 Oct;23[11]:3564-71).
In recent years, researchers have linked 80% of Merkel cell carcinoma cases to the Merkel cell polyomavirus, he said. The virus normally inhabits our skin with no ill effects, he said. “We all have this virus on our skin. It’s everywhere, and even children have antibodies,” he said. But mutations can lead to Merkel cell carcinoma.
Does it matter if cases are polyomavirus positive or polyomavirus negative? Not really, Dr. Brownell said, since the presence of the virus doesn’t appear to affect overall prognosis. However, he said, serum antibody testing can be helpful in polyomavirus-positive patients because it offers insight into prognosis and tumor burden. For example, “if the baseline titer falls and then starts to go up, they’re likely to have a recurrence, and you’ll want to look out for that,” he said.
Dr. Brownell offered another bit of advice: Be prepared to respond to patients who worry that they have a contagious virus and could be a danger to others. The proper answer, he said, is this: “You don’t have to worry about infecting people. Your tumor is not making the virus, you’re not infectious, and we have the virus on us already.”
For more information about the antibody test, visit merkelcell.org/sero.
Dr. Brownell reported having no relevant disclosures. SDEF and this news organization are owned by the same parent company.
LAS VEGAS – Merkel cell carcinoma, an extremely rare form of skin cancer, is often caused by a subclinical virus that routinely inhabits the skin. Now, a serum test of virus antibody levels is offering insight into the state of the disease, according to one dermatologist.
“If you have these antibodies, you have a better prognosis. You can follow those antibodies to test for recurrence or progression,” Isaac Brownell, MD, PhD, of the Dermatology Branch of the National Institutes of Health said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
The cancer appears in the skin’s Merkel cells, which contribute to our sense of touch by helping us to discriminate textures. “When you put your hand in your pocket, and you can tell the difference between the front and back of a quarter,” he said, “you’re using the Merkel cells in your fingertips.”
Only about 2,500 cases of Merkel cell carcinoma appear in the United States each year, Dr. Brownell said. It appears more often in elderly white patients, is more common in men than women, and is more likely among immunosuppressed patients, whose risk is increased 15- to 20-fold. Cases are more common in sunnier regions – at least in men – and lesions frequently appear on the head, face, and neck.
Five-year survival is estimated at 51% if the cancer is localized, according to a 2016 study of 9,387 cases that Dr. Brownell highlighted. But survival declines dramatically if it has spread to lymph nodes or distant sites (Ann Surg Oncol. 2016 Oct;23[11]:3564-71).
In recent years, researchers have linked 80% of Merkel cell carcinoma cases to the Merkel cell polyomavirus, he said. The virus normally inhabits our skin with no ill effects, he said. “We all have this virus on our skin. It’s everywhere, and even children have antibodies,” he said. But mutations can lead to Merkel cell carcinoma.
Does it matter if cases are polyomavirus positive or polyomavirus negative? Not really, Dr. Brownell said, since the presence of the virus doesn’t appear to affect overall prognosis. However, he said, serum antibody testing can be helpful in polyomavirus-positive patients because it offers insight into prognosis and tumor burden. For example, “if the baseline titer falls and then starts to go up, they’re likely to have a recurrence, and you’ll want to look out for that,” he said.
Dr. Brownell offered another bit of advice: Be prepared to respond to patients who worry that they have a contagious virus and could be a danger to others. The proper answer, he said, is this: “You don’t have to worry about infecting people. Your tumor is not making the virus, you’re not infectious, and we have the virus on us already.”
For more information about the antibody test, visit merkelcell.org/sero.
Dr. Brownell reported having no relevant disclosures. SDEF and this news organization are owned by the same parent company.
REPORTING FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR